User login
National Psoriasis Foundation recommends some stop methotrexate for 2 weeks after J&J vaccine
The , Joel M. Gelfand, MD, said at Innovations in Dermatology: Virtual Spring Conference 2021.
The new guidance states: “Patients 60 or older who have at least one comorbidity associated with an increased risk for poor COVID-19 outcomes, and who are taking methotrexate with well-controlled psoriatic disease, may, in consultation with their prescriber, consider holding it for 2 weeks after receiving the Ad26.COV2.S [Johnson & Johnson] vaccine in order to potentially improve vaccine response.”
The key word here is “potentially.” There is no hard evidence that a 2-week hold on methotrexate after receiving the killed adenovirus vaccine will actually provide a clinically meaningful benefit. But it’s a hypothetical possibility. The rationale stems from a small randomized trial conducted in South Korea several years ago in which patients with rheumatoid arthritis were assigned to hold or continue their methotrexate for the first 2 weeks after receiving an inactivated-virus influenza vaccine. The antibody response to the vaccine was better in those who temporarily halted their methotrexate, explained Dr. Gelfand, cochair of the NPF COVID-19 Task Force and professor of dermatology and of epidemiology at the University of Pennsylvania, Philadelphia.
“If you have a patient on methotrexate who’s 60 or older and whose psoriasis is completely controlled and quiescent and the patient is concerned about how well the vaccine is going to work, this is a reasonable thing to consider in someone who’s at higher risk for poor outcomes if they get infected,” he said.
If the informed patient wants to continue on methotrexate without interruption, that’s fine, too, in light of the lack of compelling evidence on this issue, the dermatologist added at the conference, sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
The NPF task force does not extend the recommendation to consider holding methotrexate in recipients of the mRNA-based Moderna and Pfizer vaccines because of their very different mechanisms of action. Nor is it recommended to hold biologic agents after receiving any of the available COVID-19 vaccines. Studies have shown no altered immunologic response to influenza or pneumococcal vaccines in patients who continued on tumor necrosis factor inhibitors or interleukin-17 inhibitors. The interleukin-23 inhibitors haven’t been studied in this regard.
The task force recommends that most psoriasis patients should continue on treatment throughout the pandemic, and newly diagnosed patients should commence appropriate therapy as if there was no pandemic.
“We’ve learned that many patients who stopped their treatment for psoriatic disease early in the pandemic came to regret that decision because their psoriasis flared and got worse and required reinstitution of therapy,” Dr. Gelfand said. “The current data is largely reassuring that if there is an effect of our therapies on the risk of COVID, it must be rather small and therefore unlikely to be clinically meaningful for our patients.”
Dr. Gelfand reported serving as a consultant to and recipient of institutional research grants from Pfizer and numerous other pharmaceutical companies.
MedscapeLIVE and this news organization are owned by the same parent company.
The , Joel M. Gelfand, MD, said at Innovations in Dermatology: Virtual Spring Conference 2021.
The new guidance states: “Patients 60 or older who have at least one comorbidity associated with an increased risk for poor COVID-19 outcomes, and who are taking methotrexate with well-controlled psoriatic disease, may, in consultation with their prescriber, consider holding it for 2 weeks after receiving the Ad26.COV2.S [Johnson & Johnson] vaccine in order to potentially improve vaccine response.”
The key word here is “potentially.” There is no hard evidence that a 2-week hold on methotrexate after receiving the killed adenovirus vaccine will actually provide a clinically meaningful benefit. But it’s a hypothetical possibility. The rationale stems from a small randomized trial conducted in South Korea several years ago in which patients with rheumatoid arthritis were assigned to hold or continue their methotrexate for the first 2 weeks after receiving an inactivated-virus influenza vaccine. The antibody response to the vaccine was better in those who temporarily halted their methotrexate, explained Dr. Gelfand, cochair of the NPF COVID-19 Task Force and professor of dermatology and of epidemiology at the University of Pennsylvania, Philadelphia.
“If you have a patient on methotrexate who’s 60 or older and whose psoriasis is completely controlled and quiescent and the patient is concerned about how well the vaccine is going to work, this is a reasonable thing to consider in someone who’s at higher risk for poor outcomes if they get infected,” he said.
If the informed patient wants to continue on methotrexate without interruption, that’s fine, too, in light of the lack of compelling evidence on this issue, the dermatologist added at the conference, sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
The NPF task force does not extend the recommendation to consider holding methotrexate in recipients of the mRNA-based Moderna and Pfizer vaccines because of their very different mechanisms of action. Nor is it recommended to hold biologic agents after receiving any of the available COVID-19 vaccines. Studies have shown no altered immunologic response to influenza or pneumococcal vaccines in patients who continued on tumor necrosis factor inhibitors or interleukin-17 inhibitors. The interleukin-23 inhibitors haven’t been studied in this regard.
The task force recommends that most psoriasis patients should continue on treatment throughout the pandemic, and newly diagnosed patients should commence appropriate therapy as if there was no pandemic.
“We’ve learned that many patients who stopped their treatment for psoriatic disease early in the pandemic came to regret that decision because their psoriasis flared and got worse and required reinstitution of therapy,” Dr. Gelfand said. “The current data is largely reassuring that if there is an effect of our therapies on the risk of COVID, it must be rather small and therefore unlikely to be clinically meaningful for our patients.”
Dr. Gelfand reported serving as a consultant to and recipient of institutional research grants from Pfizer and numerous other pharmaceutical companies.
MedscapeLIVE and this news organization are owned by the same parent company.
The , Joel M. Gelfand, MD, said at Innovations in Dermatology: Virtual Spring Conference 2021.
The new guidance states: “Patients 60 or older who have at least one comorbidity associated with an increased risk for poor COVID-19 outcomes, and who are taking methotrexate with well-controlled psoriatic disease, may, in consultation with their prescriber, consider holding it for 2 weeks after receiving the Ad26.COV2.S [Johnson & Johnson] vaccine in order to potentially improve vaccine response.”
The key word here is “potentially.” There is no hard evidence that a 2-week hold on methotrexate after receiving the killed adenovirus vaccine will actually provide a clinically meaningful benefit. But it’s a hypothetical possibility. The rationale stems from a small randomized trial conducted in South Korea several years ago in which patients with rheumatoid arthritis were assigned to hold or continue their methotrexate for the first 2 weeks after receiving an inactivated-virus influenza vaccine. The antibody response to the vaccine was better in those who temporarily halted their methotrexate, explained Dr. Gelfand, cochair of the NPF COVID-19 Task Force and professor of dermatology and of epidemiology at the University of Pennsylvania, Philadelphia.
“If you have a patient on methotrexate who’s 60 or older and whose psoriasis is completely controlled and quiescent and the patient is concerned about how well the vaccine is going to work, this is a reasonable thing to consider in someone who’s at higher risk for poor outcomes if they get infected,” he said.
If the informed patient wants to continue on methotrexate without interruption, that’s fine, too, in light of the lack of compelling evidence on this issue, the dermatologist added at the conference, sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
The NPF task force does not extend the recommendation to consider holding methotrexate in recipients of the mRNA-based Moderna and Pfizer vaccines because of their very different mechanisms of action. Nor is it recommended to hold biologic agents after receiving any of the available COVID-19 vaccines. Studies have shown no altered immunologic response to influenza or pneumococcal vaccines in patients who continued on tumor necrosis factor inhibitors or interleukin-17 inhibitors. The interleukin-23 inhibitors haven’t been studied in this regard.
The task force recommends that most psoriasis patients should continue on treatment throughout the pandemic, and newly diagnosed patients should commence appropriate therapy as if there was no pandemic.
“We’ve learned that many patients who stopped their treatment for psoriatic disease early in the pandemic came to regret that decision because their psoriasis flared and got worse and required reinstitution of therapy,” Dr. Gelfand said. “The current data is largely reassuring that if there is an effect of our therapies on the risk of COVID, it must be rather small and therefore unlikely to be clinically meaningful for our patients.”
Dr. Gelfand reported serving as a consultant to and recipient of institutional research grants from Pfizer and numerous other pharmaceutical companies.
MedscapeLIVE and this news organization are owned by the same parent company.
FROM INNOVATIONS IN DERMATOLOGY
Will psoriasis patients embrace proactive topical therapy?
Long-term proactive topical management of plaque psoriasis with twice-weekly calcipotriene/betamethasone dipropionate foam has been shown in a high-quality randomized trial to be more effective than conventional reactive management – but will patients go for it?
Bruce E. Strober, MD, PhD, has his doubts, and he shared them with Linda Stein Gold, MD, after she presented updated results from the 52-week PSO-LONG trial at Innovations in Dermatology: Virtual Spring Conference 2021.
. And while they did so in this study with an assist in the form of monthly office visits and nudging from investigators, in real-world clinical practice that’s unlikely to happen, according to Dr. Strober, of Yale University, New Haven, Conn.
“It makes sense to do what’s being done in this study, there’s no doubt, but I’m concerned about adherence and whether patients are really going to do it,” he said.
“Adherence is going to be everything here, and you know patients don’t like to apply topicals to their body. Once they’re clear they’re just going to walk away from the topical,” Dr. Strober predicted.
Dr. Stein Gold countered: “When a study goes on for a full year, it starts to reflect real life.”
Moreover, the PSO-LONG trial provides the first high-quality evidence physicians can share with patients demonstrating that proactive management pays off in terms of fewer relapses and more time in remission over the long haul, added Dr. Stein Gold, director of dermatology clinical research at the Henry Ford Health System in Detroit.
PSO-LONG was a double-blind, international, phase 3 study including 545 adults with plaque psoriasis who had clear or almost-clear skin after 4 weeks of once-daily calcipotriene 0.005%/betamethasone dipropionate 0.064% (Cal/BD) foam (Enstilar), and were then randomized to twice-weekly proactive management or to a reactive approach involving application of vehicle on the same twice-weekly schedule. Relapses resulted in rescue therapy with 4 weeks of once-daily Cal/BD foam.
The primary endpoint was the median time to first relapse: 56 days with the proactive approach, a significant improvement over the 30 days with the reactive approach. Over the course of 52 weeks, the proactive group spent an additional 41 days in remission, compared with the reactive group. Patients randomized to twice-weekly Cal/BD foam averaged 3.1 relapses per year, compared with 4.8 with reactive management. The side-effect profiles in the two study arms were similar.
Mean Physician Global Assessment scores and Psoriasis Area and Activity Index scores for the proactive group clearly separated from the reactive group by week 4, with those differences maintained throughout the year. The area under the curve for distribution for the Physician Global Assessment score was 15% lower in the proactive group, and 20% lower for the modified PASI score.
“These results suggest that proactive management – a concept that’s been used for atopic dermatitis – could be applied to patients with psoriasis to prolong remission,” Dr. Stein Gold concluded at the conference, sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
Asked how confident she is that patients in the real world truly will do this, Dr. Stein Gold replied: “You know, I don’t know. We hope so. Now we can tell them we actually have some data that supports treating the cleared areas. And it’s only twice a week, separated on Mondays and Thursdays.”
“I take a much more reactive approach,” Dr. Strober said. “I advise patients to get back in there with their topical steroid as soon as they see any signs of recurrence.
He added that he’s eager to see if a proactive management approach such as the one that was successful in PSO-LONG is also beneficial using some of the promising topical agents with nonsteroidal mechanisms of action, which are advancing through the developmental pipeline.
Late in 2020, the Food and Drug Administration approved an expanded indication for Cal/BD foam, which includes the PSO-LONG data on the efficacy and safety of long-term twice-weekly therapy in adults in product labeling. The combination spray/foam was previously approved by the FDA as once-daily therapy in psoriasis patients aged 12 years and older, but only for up to 4 weeks because of safety concerns regarding longer use of the potent topical steroid as daily therapy.
The PSO-LONG trial was funded by LEO Pharma. Dr. Stein Gold reported serving as a paid investigator and/or consultant to LEO and numerous other pharmaceutical companies. Dr. Strober, reported serving as a consultant to more than two dozen pharmaceutical companies. MedscapeLIVE! and this news organization are owned by the same parent company.
Long-term proactive topical management of plaque psoriasis with twice-weekly calcipotriene/betamethasone dipropionate foam has been shown in a high-quality randomized trial to be more effective than conventional reactive management – but will patients go for it?
Bruce E. Strober, MD, PhD, has his doubts, and he shared them with Linda Stein Gold, MD, after she presented updated results from the 52-week PSO-LONG trial at Innovations in Dermatology: Virtual Spring Conference 2021.
. And while they did so in this study with an assist in the form of monthly office visits and nudging from investigators, in real-world clinical practice that’s unlikely to happen, according to Dr. Strober, of Yale University, New Haven, Conn.
“It makes sense to do what’s being done in this study, there’s no doubt, but I’m concerned about adherence and whether patients are really going to do it,” he said.
“Adherence is going to be everything here, and you know patients don’t like to apply topicals to their body. Once they’re clear they’re just going to walk away from the topical,” Dr. Strober predicted.
Dr. Stein Gold countered: “When a study goes on for a full year, it starts to reflect real life.”
Moreover, the PSO-LONG trial provides the first high-quality evidence physicians can share with patients demonstrating that proactive management pays off in terms of fewer relapses and more time in remission over the long haul, added Dr. Stein Gold, director of dermatology clinical research at the Henry Ford Health System in Detroit.
PSO-LONG was a double-blind, international, phase 3 study including 545 adults with plaque psoriasis who had clear or almost-clear skin after 4 weeks of once-daily calcipotriene 0.005%/betamethasone dipropionate 0.064% (Cal/BD) foam (Enstilar), and were then randomized to twice-weekly proactive management or to a reactive approach involving application of vehicle on the same twice-weekly schedule. Relapses resulted in rescue therapy with 4 weeks of once-daily Cal/BD foam.
The primary endpoint was the median time to first relapse: 56 days with the proactive approach, a significant improvement over the 30 days with the reactive approach. Over the course of 52 weeks, the proactive group spent an additional 41 days in remission, compared with the reactive group. Patients randomized to twice-weekly Cal/BD foam averaged 3.1 relapses per year, compared with 4.8 with reactive management. The side-effect profiles in the two study arms were similar.
Mean Physician Global Assessment scores and Psoriasis Area and Activity Index scores for the proactive group clearly separated from the reactive group by week 4, with those differences maintained throughout the year. The area under the curve for distribution for the Physician Global Assessment score was 15% lower in the proactive group, and 20% lower for the modified PASI score.
“These results suggest that proactive management – a concept that’s been used for atopic dermatitis – could be applied to patients with psoriasis to prolong remission,” Dr. Stein Gold concluded at the conference, sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
Asked how confident she is that patients in the real world truly will do this, Dr. Stein Gold replied: “You know, I don’t know. We hope so. Now we can tell them we actually have some data that supports treating the cleared areas. And it’s only twice a week, separated on Mondays and Thursdays.”
“I take a much more reactive approach,” Dr. Strober said. “I advise patients to get back in there with their topical steroid as soon as they see any signs of recurrence.
He added that he’s eager to see if a proactive management approach such as the one that was successful in PSO-LONG is also beneficial using some of the promising topical agents with nonsteroidal mechanisms of action, which are advancing through the developmental pipeline.
Late in 2020, the Food and Drug Administration approved an expanded indication for Cal/BD foam, which includes the PSO-LONG data on the efficacy and safety of long-term twice-weekly therapy in adults in product labeling. The combination spray/foam was previously approved by the FDA as once-daily therapy in psoriasis patients aged 12 years and older, but only for up to 4 weeks because of safety concerns regarding longer use of the potent topical steroid as daily therapy.
The PSO-LONG trial was funded by LEO Pharma. Dr. Stein Gold reported serving as a paid investigator and/or consultant to LEO and numerous other pharmaceutical companies. Dr. Strober, reported serving as a consultant to more than two dozen pharmaceutical companies. MedscapeLIVE! and this news organization are owned by the same parent company.
Long-term proactive topical management of plaque psoriasis with twice-weekly calcipotriene/betamethasone dipropionate foam has been shown in a high-quality randomized trial to be more effective than conventional reactive management – but will patients go for it?
Bruce E. Strober, MD, PhD, has his doubts, and he shared them with Linda Stein Gold, MD, after she presented updated results from the 52-week PSO-LONG trial at Innovations in Dermatology: Virtual Spring Conference 2021.
. And while they did so in this study with an assist in the form of monthly office visits and nudging from investigators, in real-world clinical practice that’s unlikely to happen, according to Dr. Strober, of Yale University, New Haven, Conn.
“It makes sense to do what’s being done in this study, there’s no doubt, but I’m concerned about adherence and whether patients are really going to do it,” he said.
“Adherence is going to be everything here, and you know patients don’t like to apply topicals to their body. Once they’re clear they’re just going to walk away from the topical,” Dr. Strober predicted.
Dr. Stein Gold countered: “When a study goes on for a full year, it starts to reflect real life.”
Moreover, the PSO-LONG trial provides the first high-quality evidence physicians can share with patients demonstrating that proactive management pays off in terms of fewer relapses and more time in remission over the long haul, added Dr. Stein Gold, director of dermatology clinical research at the Henry Ford Health System in Detroit.
PSO-LONG was a double-blind, international, phase 3 study including 545 adults with plaque psoriasis who had clear or almost-clear skin after 4 weeks of once-daily calcipotriene 0.005%/betamethasone dipropionate 0.064% (Cal/BD) foam (Enstilar), and were then randomized to twice-weekly proactive management or to a reactive approach involving application of vehicle on the same twice-weekly schedule. Relapses resulted in rescue therapy with 4 weeks of once-daily Cal/BD foam.
The primary endpoint was the median time to first relapse: 56 days with the proactive approach, a significant improvement over the 30 days with the reactive approach. Over the course of 52 weeks, the proactive group spent an additional 41 days in remission, compared with the reactive group. Patients randomized to twice-weekly Cal/BD foam averaged 3.1 relapses per year, compared with 4.8 with reactive management. The side-effect profiles in the two study arms were similar.
Mean Physician Global Assessment scores and Psoriasis Area and Activity Index scores for the proactive group clearly separated from the reactive group by week 4, with those differences maintained throughout the year. The area under the curve for distribution for the Physician Global Assessment score was 15% lower in the proactive group, and 20% lower for the modified PASI score.
“These results suggest that proactive management – a concept that’s been used for atopic dermatitis – could be applied to patients with psoriasis to prolong remission,” Dr. Stein Gold concluded at the conference, sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
Asked how confident she is that patients in the real world truly will do this, Dr. Stein Gold replied: “You know, I don’t know. We hope so. Now we can tell them we actually have some data that supports treating the cleared areas. And it’s only twice a week, separated on Mondays and Thursdays.”
“I take a much more reactive approach,” Dr. Strober said. “I advise patients to get back in there with their topical steroid as soon as they see any signs of recurrence.
He added that he’s eager to see if a proactive management approach such as the one that was successful in PSO-LONG is also beneficial using some of the promising topical agents with nonsteroidal mechanisms of action, which are advancing through the developmental pipeline.
Late in 2020, the Food and Drug Administration approved an expanded indication for Cal/BD foam, which includes the PSO-LONG data on the efficacy and safety of long-term twice-weekly therapy in adults in product labeling. The combination spray/foam was previously approved by the FDA as once-daily therapy in psoriasis patients aged 12 years and older, but only for up to 4 weeks because of safety concerns regarding longer use of the potent topical steroid as daily therapy.
The PSO-LONG trial was funded by LEO Pharma. Dr. Stein Gold reported serving as a paid investigator and/or consultant to LEO and numerous other pharmaceutical companies. Dr. Strober, reported serving as a consultant to more than two dozen pharmaceutical companies. MedscapeLIVE! and this news organization are owned by the same parent company.
FROM INNOVATIONS IN DERMATOLOGY
Treatment of Generalized Pustular Psoriasis of Pregnancy With Infliximab
Generalized pustular psoriasis of pregnancy (GPPP), formerly known as impetigo herpetiformis, is a rare dermatosis that causes maternal and fetal morbidity and mortality. It is characterized by widespread, circular, erythematous plaques with pustules at the periphery.1 Conventional first-line treatment includes systemic corticosteroids and cyclosporine. The National Psoriasis Foundation Medical Board also has included infliximab among the first-line treatment options for GPPP.2 Herein, we report a case of GPPP treated with infliximab at 30 weeks’ gestation and during the postpartum period.
Case Report
A 22-year-old woman was admitted to our inpatient clinic at 20 weeks’ gestation in her second pregnancy for evaluation of cutaneous eruptions covering the entire body. The lesions first appeared 3 to 4 days prior to her admission and dramatically progressed. She had a history of psoriasis vulgaris diagnosed during her first pregnancy 2 years prior that was treated with topical steroids throughout the pregnancy and methotrexate during lactation for a total of 11 months. She then was started on cyclosporine, which she used for 6 months due to ineffectiveness of the methotrexate, but she stopped treatment 4 months before the second pregnancy.
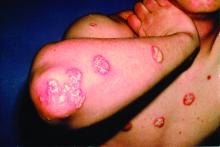
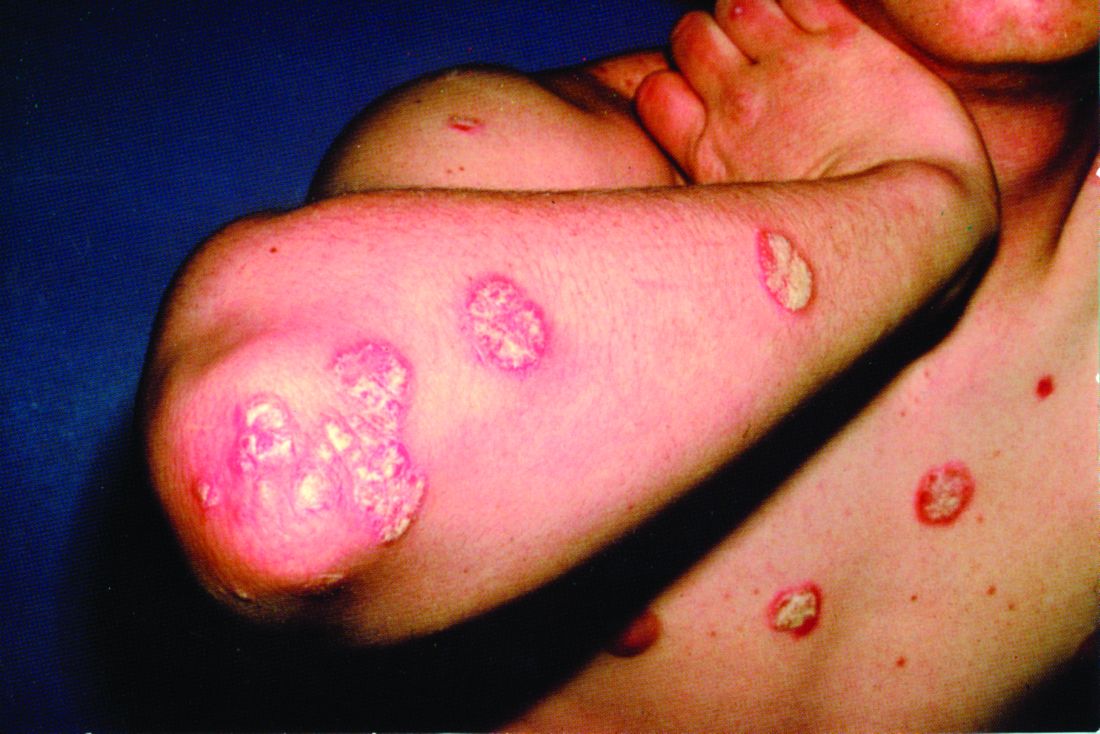
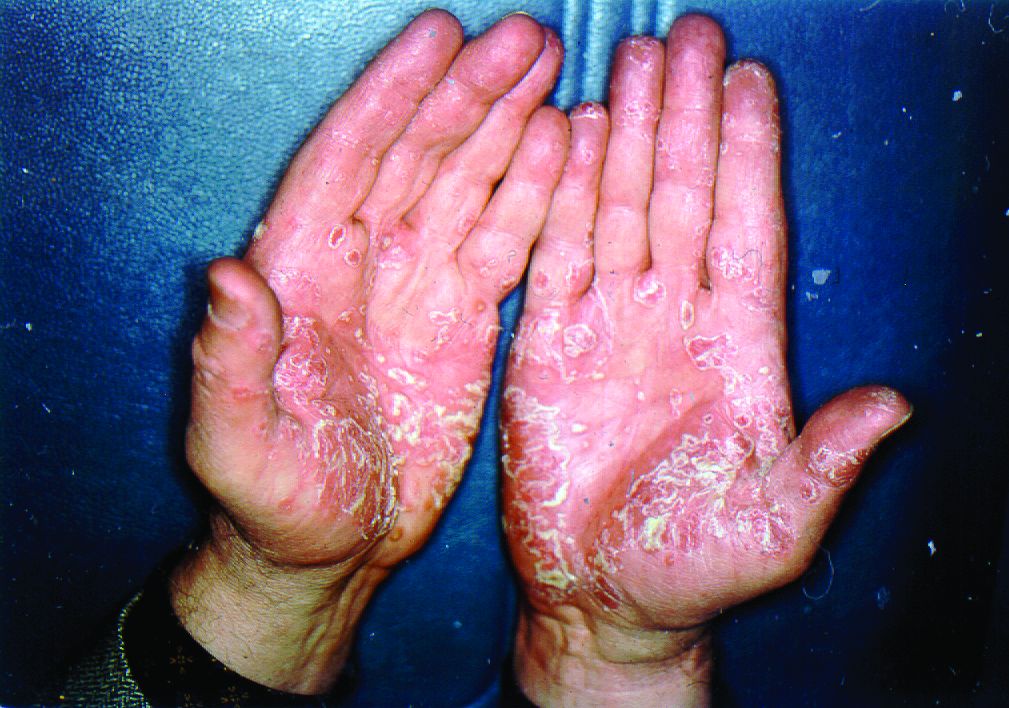
At the current presentation, physical examination revealed erythroderma and widespread pustules on the chest, abdomen, arms, and legs, including the intertriginous regions, that tended to coalesce and form lakes of pus over an erythematous base (Figure 1). The mucosae were normal. She exhibited a low blood pressure (85/50 mmHg) and high body temperature (102 °F [38.9 °C]). Routine laboratory examination revealed anemia and a normal leukocyte count. Her erythrocyte sedimentation rate (57 mm/h [reference range, <20 mm/h]) and C-reactive protein level (102 mg/L [reference range, <6 mg/L]) were elevated, whereas total calcium (8.11 mg/dL [reference range, 8.2–10.6 mg/dL]) and albumin (3.15 g/dL [reference range, >4.0 g/dL]) levels were low.
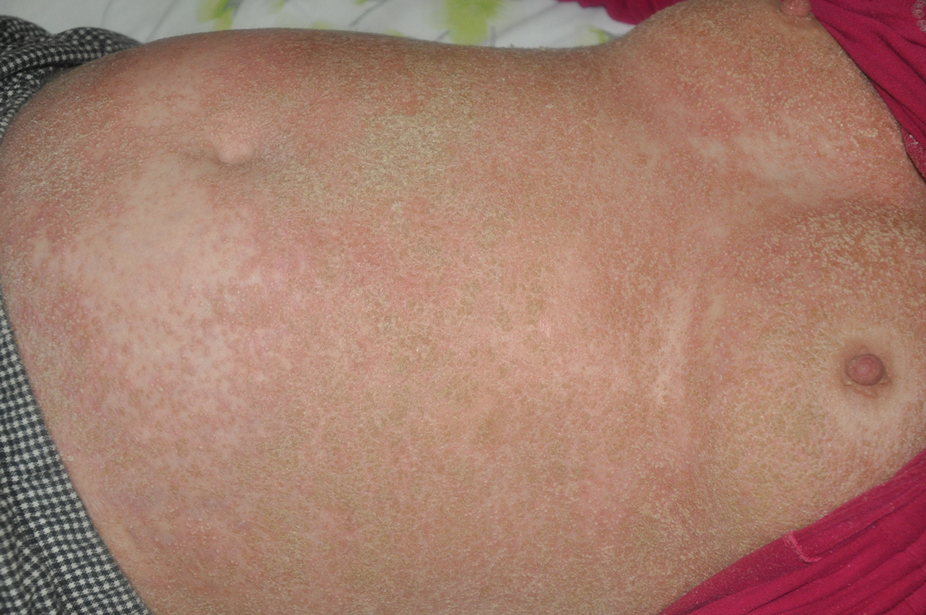
Empirical intravenous piperacillin/tazobactam was started due to hypotension, high fever, and elevated C-reactive protein levels; however, treatment was stopped after 4 days when microbiological cultures taken from blood and pustules revealed no bacterial growth, and therefore the fever was assumed to be caused by erythroderma. A skin biopsy before the start of topical and systemic treatment revealed changes consistent with GPPP.
Because her disease was extensive, systemic methylprednisolone 1.5 mg/kg once daily was started, and the dose was increased up to 2.5 mg/kg once daily on the tenth day of treatment to control new crops of eruptions. The dose was tapered to 2 mg/kg once daily when the lesions subsided 4 weeks into the treatment. The patient was discharged after 7 weeks at 27 weeks’ gestation.
Twelve days later, the patient was readmitted to the clinic in an erythrodermic state. The lesions were not controlled with increased doses of systemic corticosteroids. Treatment with cyclosporine was considered, but the patient refused; thus, infliximab treatment was planned. Isoniazid 300 mg once daily was started due to a risk of latent Mycobacterium tuberculosis infection revealed by a tuberculosis blood test. Other evaluations revealed no contraindications, and an infusion of infliximab 300 mg (5 mg/kg) was administered at 30 weeks’ gestation. There was visible improvement in the erythroderma and pustular lesions within the same day of treatment, and the lesions were completely cleared within 2 days of the infusion. The methylprednisolone dose was reduced to 1.5 mg/kg once daily.
Three days after treatment with infliximab, lesions with yellow encrustation appeared in the perioral region and on the oral mucosa and left ear. She was diagnosed with an oral herpes infection. Oral valacyclovir 1 g twice daily and topical mupirocin were started and the lesions subsided within 1 week. Twelve days after the infliximab infusion, new pustular lesions appeared, and a second infusion of infliximab was administered 13 days after the first, which cleared all lesions within 48 hours.
The patient’s methylprednisolone dose was tapered and stopped prior to delivery at 34 weeks’ gestation—2 weeks after the second dose of infliximab—as she did not have any new skin eruptions. A third infliximab infusion that normally would have occurred 4 weeks after the second treatment was postponed for a Cesarean section scheduled at 36 weeks’ gestation due to suspected intrauterine growth retardation. The patient stayed at the hospital until delivery without any new skin lesions. The gross and histopathologic examination of the placenta was normal. The neonate weighed 4.8 lb at birth and had neonatal jaundice that resolved spontaneously within 10 days but was otherwise healthy.
The patient returned to the clinic 3 weeks postpartum with a few pustules on erythematous plaques on the chest, abdomen, and back. At this time, she received a third infusion of infliximab 8 weeks after the second dose. For the past 5 years, the patient has been undergoing infliximab maintenance treatment, which she receives at the hospital every 8 weeks with excellent response. She has had no further pregnancies to date.
Comment
Generalized pustular psoriasis of pregnancy is a rare condition that typically occurs in the third trimester but also can start in the first and second trimesters. It may result in maternal and fetal morbidity by causing fluid and electrolyte imbalance and/or placental insufficiency, resulting in an increased risk for fetal abnormalities, stillbirth, and neonatal death.3 In subsequent pregnancies, GPPP has been observed to recur at an earlier gestational age with a more severe presentation.1,3
Generalized pustular psoriasis of pregnancy usually involves an eruption that begins symmetrically in the intertriginous areas and spreads to the rest of the body. The lesions present as erythematous annular plaques with pustules on the periphery and desquamation in the center due to older pustules.1,3 The mucous membranes also may be involved with erosive and exfoliative plaques, and there may be nail involvement. Patients often present with systemic symptoms such as fever, malaise, diarrhea, and vomiting.1 Laboratory investigations may reveal neutrophilic leukocytosis, high erythrocyte sedimentation rate, hypocalcemia, and hypoalbuminemia.4 Cultures from blood and pustules show no bacterial growth. A skin biopsy is helpful in diagnosis, with features similar to generalized pustular psoriasis, demonstrating spongiform pustules containing neutrophils, lymphocytic and neutrophilic infiltrates in the papillary dermis, and negative direct immunofluorescence.3
The differential diagnosis of GPPP includes subcorneal pustular dermatosis, dermatitis herpetiformis, herpes gestationis, impetigo, and acute generalized exanthematous pustulosis.1,3 Due to concerns of fetal implications, treatment options in GPPP are somewhat limited; however, the condition requires treatment because it may result in unfavorable pregnancy outcomes. Topical corticosteroids may be an option for limited disease.5,6 Systemic corticosteroids (eg, prednisone 60–80 mg/d) were previously considered as first-line agents, although they have shown limited efficacy in our case as well as in other case reports.7 Their ineffectiveness and risk for flare-up after dose tapering should be kept in mind when starting GPPP patients on systemic corticosteroids. Systemic cyclosporine (2–3 mg/kg/d) may be added to increase the efficacy of systemic steroids, which was done in several cases in literature.1,6,8 Although cyclosporine has been classified as a pregnancy category C drug, an analysis of pregnancy outcomes of 629 renal transplant patients revealed no association with adverse pregnancy outcomes compared to the general population and no increase in fetal malformations.9 Therefore, cyclosporine is a safe treatment option and was classified as a first-line drug for GPPP in a 2012 review by the National Psoriasis Foundation Medical Board.2 Narrowband UVB also has been reported to be used for the treatment of GPPP.10 Methotrexate and retinoids have been used in cases with lesions that persisted postpartum.1
Anti–tumor necrosis factor (TNF) α agents are another effective option for treatment of GPPP. Anti-TNF agents are classified as pregnancy category B due to results showing that anti-mouse TNF-α monoclonal antibodies did not cause embryotoxicity or teratogenicity in pregnant mice.11 Although Carter et al12 published a review of US Food and Drug Administration data on pregnant women receiving anti-TNF treatment and concluded that these agents were associated with the VACTERL group of malformations (vertebral defects, anal atresia, cardiac defect, tracheoesophageal fistula with esophageal atresia, cardiac defects, renal and limb anomalies), no such association was found in further studies. A 2014 study showed no difference in the rate of major malformations in infants born to women who were treated with anti-TNF drugs compared to the disease-matched group not treated with these agents and pregnant women counselled for nonteratogenic exposure.13 The same study detected an increase in preterm and low-birth-weight deliveries and suggested this might be caused by the increased severity of disease in patients requiring anti-TNF medication. The British Society of Rheumatology Biologics Register published data on pregnancy outcomes in 130 rheumatoid arthritis patients who had been exposed to anti-TNF agents.14 The results suggested an increased rate of spontaneous abortions in women exposed to anti-TNF treatment around the time of conception, especially in those taking these medications together with methotrexate or leflunomide; however, results also indicated that disease activity may have had an impact on the rate of spontaneous abortions in these patients. In a 2013 review of 462 women with inflammatory bowel disease who had been exposed to anti-TNF agents during pregnancy, the investigators concluded that pregnancy outcomes and the rate of congenital anomalies did not significantly differ from other inflammatory bowel disease patients not receiving anti-TNF drugs or the general population.15
In 2012, the National Board of the National Psoriasis Foundation put infliximab amongst the first-line treatment modalities for GPPP.2 In one case of GPPP in which the eruption persisted after delivery, the patient was treated with infliximab 7 weeks postpartum due to failure to control the disease with prednisolone 60 mg daily and cyclosporine 7.5 mg/kg daily. Unlike our patient, this patient was only started on an infliximab regimen after delivery.16 In another case reported in 2010, the patient was started on infliximab during the postpartum period of her first pregnancy following a pustular flare of previously diagnosed plaque psoriasis (not a generalized pustular psoriasis, as in our case).17 As a good response was obtained, infliximab treatment was continued in the patient throughout her second pregnancy.
Our case is unique in that infliximab was started during pregnancy because of intractable disease leading to systemic symptoms. Our patient showed an excellent response to infliximab after a 10-week disease course with repeated flare-ups and impairment to her overall condition. Delivery occurred at 36 weeks’ gestation due to suspected intrauterine growth retardation; however, the neonate was born with a 5-minute APGAR score of 10 and required no special medical care, which suggests that the low birth weight was constitutional due to the patient’s small frame (her height was 4 ft 11 in). The breast milk of patients with inflammatory bowel disease has been detected to contain very small amounts of infliximab (101 ng/mL, about 1/200 of the therapeutic blood level).18 Considering the large molecular weight of this agent and possible proteolysis in the stomach and intestines, infliximab is unlikely to affect the neonate.15 Thus, we encouraged our patient to breastfeed her baby. A case of fatal disseminated Bacille-Calmette-Guérin infection in an infant whose mother received infliximab treatment during pregnancy has been reported.19 It has been suggested that live vaccines should be avoided in neonates exposed to anti-TNF agents at least for the first 6 months of life or until the agent is no longer detectable in their blood.15 We therefore informed our patient’s family practitioner about this data.
Conclusion
We report a case of infliximab treatment for GPPP that was continued during the postpartum period. Infliximab was an effective treatment option in our patient with no detected serious adverse events and may be considered in other cases of GPPP that are not responsive to systemic steroids. However, further studies are warranted to evaluate the safety and efficacy of infliximab treatment for GPPP and psoriasis in pregnancy.
- Lerhoff S, Pomeranz MK. Specific dermatoses of pregnancy and their treatment. Dermatol Ther. 2013;26:274-284.
- Robinson A, Van Voorhees AS, Hsu S, et al. Treatment of pustular psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:279-288.
- Oumeish OY, Parish JL. Impetigo herpetiformis. Clin Dermatol. 2006;24:101-104.
- Gao QQ, Xi MR, Yao Q. Impetigo herpetiformis during pregnancy: a case report and literature review. Dermatology. 2013;226:35-40.
- Bae YS, Van Voorhees AS, Hsu S, et al. Review of treatment options for psoriasis in pregnant or lactating women: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:459-477.
- Shaw CJ, Wu P, Sriemevan A. First trimester impetigo herpetiformis in multiparous female successfully treated with oral cyclosporine [published May 12, 2011]. BMJ Case Rep. doi:10.1136/bcr.02.2011.3915
- Hazarika D. Generalized pustular psoriasis of pregnancy successfully treated with cyclosporine. Indian J Dermatol Venereol Leprol. 2009;75:638.
- Luan L, Han S, Zhang Z, et al. Personal treatment experience for severe generalized pustular psoriasis of pregnancy: two case reports. Dermatol Ther. 2014;27:174-177.
- Lamarque V, Leleu MF, Monka C, et al. Analysis of 629 pregnancy outcomes in transplant recipients treated with Sandimmun. Transplant Proc. 1997;29:2480.
- Bozdag K, Ozturk S, Ermete M. A case of recurrent impetigo herpetiformis treated with systemic corticosteroids and narrowband UVB. Cutan Ocul Toxicol. 2012;31:67-69.
- Treacy G. Using an analogous monoclonal antibody to evaluate the reproductive and chronic toxicity potential for a humanized anti-TNF alpha monoclonal antibody. Hum Exp Toxicol. 2000;19:226-228.
- Carter JD, Ladhani A, Ricca LR, et al. A safety assessment of tumor necrosis factor antagonists during pregnancy: a review of the Food and Drug Administration database. J Rheumatol. 2009;36:635-641.
- Diav-Citrin O, Otcheretianski-Volodarsky A, Shechtman S, et al. Pregnancy outcome following gestational exposure to TNF-alpha-inhibitors: a prospective, comparative, observational study. Reprod Toxicol. 2014;43:78-84.
- Verstappen SM, King Y, Watson KD, et al. Anti-TNF therapies and pregnancy: outcome of 130 pregnancies in the British Society for Rheumatology Biologics Register. Ann Rheum Dis. 2011;70:823-826.
- Gisbert JP, Chaparro M. Safety of anti-TNF agents during pregnancy and breastfeeding in women with inflammatory bowel disease. Am J Gastroenterol. 2013;108:1426-1438.
- Sheth N, Greenblatt DT, Acland K, et al. Generalized pustular psoriasis of pregnancy treated with infliximab. Clin Exp Dermatol. 2009;34:521-522.
- Puig L, Barco D, Alomar A. Treatment of psoriasis with anti-TNF drugs during pregnancy: case report and review of the literature. Dermatology. 2010;220:71-76.
- Ben-Horin S, Yavzori M, Kopylov U, et al. Detection of infliximab in breast milk of nursing mothers with inflammatory bowel disease. J Crohns Colitis. 2011;5:555-558.
- Cheent K, Nolan J, Shariq S, et al. Case report: fatal case of disseminated BCG infection in an infant born to a mother taking infliximab for Crohn’s disease. J Crohns Colitis. 2010;4:603-605.
Generalized pustular psoriasis of pregnancy (GPPP), formerly known as impetigo herpetiformis, is a rare dermatosis that causes maternal and fetal morbidity and mortality. It is characterized by widespread, circular, erythematous plaques with pustules at the periphery.1 Conventional first-line treatment includes systemic corticosteroids and cyclosporine. The National Psoriasis Foundation Medical Board also has included infliximab among the first-line treatment options for GPPP.2 Herein, we report a case of GPPP treated with infliximab at 30 weeks’ gestation and during the postpartum period.
Case Report
A 22-year-old woman was admitted to our inpatient clinic at 20 weeks’ gestation in her second pregnancy for evaluation of cutaneous eruptions covering the entire body. The lesions first appeared 3 to 4 days prior to her admission and dramatically progressed. She had a history of psoriasis vulgaris diagnosed during her first pregnancy 2 years prior that was treated with topical steroids throughout the pregnancy and methotrexate during lactation for a total of 11 months. She then was started on cyclosporine, which she used for 6 months due to ineffectiveness of the methotrexate, but she stopped treatment 4 months before the second pregnancy.
At the current presentation, physical examination revealed erythroderma and widespread pustules on the chest, abdomen, arms, and legs, including the intertriginous regions, that tended to coalesce and form lakes of pus over an erythematous base (Figure 1). The mucosae were normal. She exhibited a low blood pressure (85/50 mmHg) and high body temperature (102 °F [38.9 °C]). Routine laboratory examination revealed anemia and a normal leukocyte count. Her erythrocyte sedimentation rate (57 mm/h [reference range, <20 mm/h]) and C-reactive protein level (102 mg/L [reference range, <6 mg/L]) were elevated, whereas total calcium (8.11 mg/dL [reference range, 8.2–10.6 mg/dL]) and albumin (3.15 g/dL [reference range, >4.0 g/dL]) levels were low.

Empirical intravenous piperacillin/tazobactam was started due to hypotension, high fever, and elevated C-reactive protein levels; however, treatment was stopped after 4 days when microbiological cultures taken from blood and pustules revealed no bacterial growth, and therefore the fever was assumed to be caused by erythroderma. A skin biopsy before the start of topical and systemic treatment revealed changes consistent with GPPP.
Because her disease was extensive, systemic methylprednisolone 1.5 mg/kg once daily was started, and the dose was increased up to 2.5 mg/kg once daily on the tenth day of treatment to control new crops of eruptions. The dose was tapered to 2 mg/kg once daily when the lesions subsided 4 weeks into the treatment. The patient was discharged after 7 weeks at 27 weeks’ gestation.
Twelve days later, the patient was readmitted to the clinic in an erythrodermic state. The lesions were not controlled with increased doses of systemic corticosteroids. Treatment with cyclosporine was considered, but the patient refused; thus, infliximab treatment was planned. Isoniazid 300 mg once daily was started due to a risk of latent Mycobacterium tuberculosis infection revealed by a tuberculosis blood test. Other evaluations revealed no contraindications, and an infusion of infliximab 300 mg (5 mg/kg) was administered at 30 weeks’ gestation. There was visible improvement in the erythroderma and pustular lesions within the same day of treatment, and the lesions were completely cleared within 2 days of the infusion. The methylprednisolone dose was reduced to 1.5 mg/kg once daily.
Three days after treatment with infliximab, lesions with yellow encrustation appeared in the perioral region and on the oral mucosa and left ear. She was diagnosed with an oral herpes infection. Oral valacyclovir 1 g twice daily and topical mupirocin were started and the lesions subsided within 1 week. Twelve days after the infliximab infusion, new pustular lesions appeared, and a second infusion of infliximab was administered 13 days after the first, which cleared all lesions within 48 hours.
The patient’s methylprednisolone dose was tapered and stopped prior to delivery at 34 weeks’ gestation—2 weeks after the second dose of infliximab—as she did not have any new skin eruptions. A third infliximab infusion that normally would have occurred 4 weeks after the second treatment was postponed for a Cesarean section scheduled at 36 weeks’ gestation due to suspected intrauterine growth retardation. The patient stayed at the hospital until delivery without any new skin lesions. The gross and histopathologic examination of the placenta was normal. The neonate weighed 4.8 lb at birth and had neonatal jaundice that resolved spontaneously within 10 days but was otherwise healthy.
The patient returned to the clinic 3 weeks postpartum with a few pustules on erythematous plaques on the chest, abdomen, and back. At this time, she received a third infusion of infliximab 8 weeks after the second dose. For the past 5 years, the patient has been undergoing infliximab maintenance treatment, which she receives at the hospital every 8 weeks with excellent response. She has had no further pregnancies to date.
Comment
Generalized pustular psoriasis of pregnancy is a rare condition that typically occurs in the third trimester but also can start in the first and second trimesters. It may result in maternal and fetal morbidity by causing fluid and electrolyte imbalance and/or placental insufficiency, resulting in an increased risk for fetal abnormalities, stillbirth, and neonatal death.3 In subsequent pregnancies, GPPP has been observed to recur at an earlier gestational age with a more severe presentation.1,3
Generalized pustular psoriasis of pregnancy usually involves an eruption that begins symmetrically in the intertriginous areas and spreads to the rest of the body. The lesions present as erythematous annular plaques with pustules on the periphery and desquamation in the center due to older pustules.1,3 The mucous membranes also may be involved with erosive and exfoliative plaques, and there may be nail involvement. Patients often present with systemic symptoms such as fever, malaise, diarrhea, and vomiting.1 Laboratory investigations may reveal neutrophilic leukocytosis, high erythrocyte sedimentation rate, hypocalcemia, and hypoalbuminemia.4 Cultures from blood and pustules show no bacterial growth. A skin biopsy is helpful in diagnosis, with features similar to generalized pustular psoriasis, demonstrating spongiform pustules containing neutrophils, lymphocytic and neutrophilic infiltrates in the papillary dermis, and negative direct immunofluorescence.3
The differential diagnosis of GPPP includes subcorneal pustular dermatosis, dermatitis herpetiformis, herpes gestationis, impetigo, and acute generalized exanthematous pustulosis.1,3 Due to concerns of fetal implications, treatment options in GPPP are somewhat limited; however, the condition requires treatment because it may result in unfavorable pregnancy outcomes. Topical corticosteroids may be an option for limited disease.5,6 Systemic corticosteroids (eg, prednisone 60–80 mg/d) were previously considered as first-line agents, although they have shown limited efficacy in our case as well as in other case reports.7 Their ineffectiveness and risk for flare-up after dose tapering should be kept in mind when starting GPPP patients on systemic corticosteroids. Systemic cyclosporine (2–3 mg/kg/d) may be added to increase the efficacy of systemic steroids, which was done in several cases in literature.1,6,8 Although cyclosporine has been classified as a pregnancy category C drug, an analysis of pregnancy outcomes of 629 renal transplant patients revealed no association with adverse pregnancy outcomes compared to the general population and no increase in fetal malformations.9 Therefore, cyclosporine is a safe treatment option and was classified as a first-line drug for GPPP in a 2012 review by the National Psoriasis Foundation Medical Board.2 Narrowband UVB also has been reported to be used for the treatment of GPPP.10 Methotrexate and retinoids have been used in cases with lesions that persisted postpartum.1
Anti–tumor necrosis factor (TNF) α agents are another effective option for treatment of GPPP. Anti-TNF agents are classified as pregnancy category B due to results showing that anti-mouse TNF-α monoclonal antibodies did not cause embryotoxicity or teratogenicity in pregnant mice.11 Although Carter et al12 published a review of US Food and Drug Administration data on pregnant women receiving anti-TNF treatment and concluded that these agents were associated with the VACTERL group of malformations (vertebral defects, anal atresia, cardiac defect, tracheoesophageal fistula with esophageal atresia, cardiac defects, renal and limb anomalies), no such association was found in further studies. A 2014 study showed no difference in the rate of major malformations in infants born to women who were treated with anti-TNF drugs compared to the disease-matched group not treated with these agents and pregnant women counselled for nonteratogenic exposure.13 The same study detected an increase in preterm and low-birth-weight deliveries and suggested this might be caused by the increased severity of disease in patients requiring anti-TNF medication. The British Society of Rheumatology Biologics Register published data on pregnancy outcomes in 130 rheumatoid arthritis patients who had been exposed to anti-TNF agents.14 The results suggested an increased rate of spontaneous abortions in women exposed to anti-TNF treatment around the time of conception, especially in those taking these medications together with methotrexate or leflunomide; however, results also indicated that disease activity may have had an impact on the rate of spontaneous abortions in these patients. In a 2013 review of 462 women with inflammatory bowel disease who had been exposed to anti-TNF agents during pregnancy, the investigators concluded that pregnancy outcomes and the rate of congenital anomalies did not significantly differ from other inflammatory bowel disease patients not receiving anti-TNF drugs or the general population.15
In 2012, the National Board of the National Psoriasis Foundation put infliximab amongst the first-line treatment modalities for GPPP.2 In one case of GPPP in which the eruption persisted after delivery, the patient was treated with infliximab 7 weeks postpartum due to failure to control the disease with prednisolone 60 mg daily and cyclosporine 7.5 mg/kg daily. Unlike our patient, this patient was only started on an infliximab regimen after delivery.16 In another case reported in 2010, the patient was started on infliximab during the postpartum period of her first pregnancy following a pustular flare of previously diagnosed plaque psoriasis (not a generalized pustular psoriasis, as in our case).17 As a good response was obtained, infliximab treatment was continued in the patient throughout her second pregnancy.
Our case is unique in that infliximab was started during pregnancy because of intractable disease leading to systemic symptoms. Our patient showed an excellent response to infliximab after a 10-week disease course with repeated flare-ups and impairment to her overall condition. Delivery occurred at 36 weeks’ gestation due to suspected intrauterine growth retardation; however, the neonate was born with a 5-minute APGAR score of 10 and required no special medical care, which suggests that the low birth weight was constitutional due to the patient’s small frame (her height was 4 ft 11 in). The breast milk of patients with inflammatory bowel disease has been detected to contain very small amounts of infliximab (101 ng/mL, about 1/200 of the therapeutic blood level).18 Considering the large molecular weight of this agent and possible proteolysis in the stomach and intestines, infliximab is unlikely to affect the neonate.15 Thus, we encouraged our patient to breastfeed her baby. A case of fatal disseminated Bacille-Calmette-Guérin infection in an infant whose mother received infliximab treatment during pregnancy has been reported.19 It has been suggested that live vaccines should be avoided in neonates exposed to anti-TNF agents at least for the first 6 months of life or until the agent is no longer detectable in their blood.15 We therefore informed our patient’s family practitioner about this data.
Conclusion
We report a case of infliximab treatment for GPPP that was continued during the postpartum period. Infliximab was an effective treatment option in our patient with no detected serious adverse events and may be considered in other cases of GPPP that are not responsive to systemic steroids. However, further studies are warranted to evaluate the safety and efficacy of infliximab treatment for GPPP and psoriasis in pregnancy.
Generalized pustular psoriasis of pregnancy (GPPP), formerly known as impetigo herpetiformis, is a rare dermatosis that causes maternal and fetal morbidity and mortality. It is characterized by widespread, circular, erythematous plaques with pustules at the periphery.1 Conventional first-line treatment includes systemic corticosteroids and cyclosporine. The National Psoriasis Foundation Medical Board also has included infliximab among the first-line treatment options for GPPP.2 Herein, we report a case of GPPP treated with infliximab at 30 weeks’ gestation and during the postpartum period.
Case Report
A 22-year-old woman was admitted to our inpatient clinic at 20 weeks’ gestation in her second pregnancy for evaluation of cutaneous eruptions covering the entire body. The lesions first appeared 3 to 4 days prior to her admission and dramatically progressed. She had a history of psoriasis vulgaris diagnosed during her first pregnancy 2 years prior that was treated with topical steroids throughout the pregnancy and methotrexate during lactation for a total of 11 months. She then was started on cyclosporine, which she used for 6 months due to ineffectiveness of the methotrexate, but she stopped treatment 4 months before the second pregnancy.
At the current presentation, physical examination revealed erythroderma and widespread pustules on the chest, abdomen, arms, and legs, including the intertriginous regions, that tended to coalesce and form lakes of pus over an erythematous base (Figure 1). The mucosae were normal. She exhibited a low blood pressure (85/50 mmHg) and high body temperature (102 °F [38.9 °C]). Routine laboratory examination revealed anemia and a normal leukocyte count. Her erythrocyte sedimentation rate (57 mm/h [reference range, <20 mm/h]) and C-reactive protein level (102 mg/L [reference range, <6 mg/L]) were elevated, whereas total calcium (8.11 mg/dL [reference range, 8.2–10.6 mg/dL]) and albumin (3.15 g/dL [reference range, >4.0 g/dL]) levels were low.

Empirical intravenous piperacillin/tazobactam was started due to hypotension, high fever, and elevated C-reactive protein levels; however, treatment was stopped after 4 days when microbiological cultures taken from blood and pustules revealed no bacterial growth, and therefore the fever was assumed to be caused by erythroderma. A skin biopsy before the start of topical and systemic treatment revealed changes consistent with GPPP.
Because her disease was extensive, systemic methylprednisolone 1.5 mg/kg once daily was started, and the dose was increased up to 2.5 mg/kg once daily on the tenth day of treatment to control new crops of eruptions. The dose was tapered to 2 mg/kg once daily when the lesions subsided 4 weeks into the treatment. The patient was discharged after 7 weeks at 27 weeks’ gestation.
Twelve days later, the patient was readmitted to the clinic in an erythrodermic state. The lesions were not controlled with increased doses of systemic corticosteroids. Treatment with cyclosporine was considered, but the patient refused; thus, infliximab treatment was planned. Isoniazid 300 mg once daily was started due to a risk of latent Mycobacterium tuberculosis infection revealed by a tuberculosis blood test. Other evaluations revealed no contraindications, and an infusion of infliximab 300 mg (5 mg/kg) was administered at 30 weeks’ gestation. There was visible improvement in the erythroderma and pustular lesions within the same day of treatment, and the lesions were completely cleared within 2 days of the infusion. The methylprednisolone dose was reduced to 1.5 mg/kg once daily.
Three days after treatment with infliximab, lesions with yellow encrustation appeared in the perioral region and on the oral mucosa and left ear. She was diagnosed with an oral herpes infection. Oral valacyclovir 1 g twice daily and topical mupirocin were started and the lesions subsided within 1 week. Twelve days after the infliximab infusion, new pustular lesions appeared, and a second infusion of infliximab was administered 13 days after the first, which cleared all lesions within 48 hours.
The patient’s methylprednisolone dose was tapered and stopped prior to delivery at 34 weeks’ gestation—2 weeks after the second dose of infliximab—as she did not have any new skin eruptions. A third infliximab infusion that normally would have occurred 4 weeks after the second treatment was postponed for a Cesarean section scheduled at 36 weeks’ gestation due to suspected intrauterine growth retardation. The patient stayed at the hospital until delivery without any new skin lesions. The gross and histopathologic examination of the placenta was normal. The neonate weighed 4.8 lb at birth and had neonatal jaundice that resolved spontaneously within 10 days but was otherwise healthy.
The patient returned to the clinic 3 weeks postpartum with a few pustules on erythematous plaques on the chest, abdomen, and back. At this time, she received a third infusion of infliximab 8 weeks after the second dose. For the past 5 years, the patient has been undergoing infliximab maintenance treatment, which she receives at the hospital every 8 weeks with excellent response. She has had no further pregnancies to date.
Comment
Generalized pustular psoriasis of pregnancy is a rare condition that typically occurs in the third trimester but also can start in the first and second trimesters. It may result in maternal and fetal morbidity by causing fluid and electrolyte imbalance and/or placental insufficiency, resulting in an increased risk for fetal abnormalities, stillbirth, and neonatal death.3 In subsequent pregnancies, GPPP has been observed to recur at an earlier gestational age with a more severe presentation.1,3
Generalized pustular psoriasis of pregnancy usually involves an eruption that begins symmetrically in the intertriginous areas and spreads to the rest of the body. The lesions present as erythematous annular plaques with pustules on the periphery and desquamation in the center due to older pustules.1,3 The mucous membranes also may be involved with erosive and exfoliative plaques, and there may be nail involvement. Patients often present with systemic symptoms such as fever, malaise, diarrhea, and vomiting.1 Laboratory investigations may reveal neutrophilic leukocytosis, high erythrocyte sedimentation rate, hypocalcemia, and hypoalbuminemia.4 Cultures from blood and pustules show no bacterial growth. A skin biopsy is helpful in diagnosis, with features similar to generalized pustular psoriasis, demonstrating spongiform pustules containing neutrophils, lymphocytic and neutrophilic infiltrates in the papillary dermis, and negative direct immunofluorescence.3
The differential diagnosis of GPPP includes subcorneal pustular dermatosis, dermatitis herpetiformis, herpes gestationis, impetigo, and acute generalized exanthematous pustulosis.1,3 Due to concerns of fetal implications, treatment options in GPPP are somewhat limited; however, the condition requires treatment because it may result in unfavorable pregnancy outcomes. Topical corticosteroids may be an option for limited disease.5,6 Systemic corticosteroids (eg, prednisone 60–80 mg/d) were previously considered as first-line agents, although they have shown limited efficacy in our case as well as in other case reports.7 Their ineffectiveness and risk for flare-up after dose tapering should be kept in mind when starting GPPP patients on systemic corticosteroids. Systemic cyclosporine (2–3 mg/kg/d) may be added to increase the efficacy of systemic steroids, which was done in several cases in literature.1,6,8 Although cyclosporine has been classified as a pregnancy category C drug, an analysis of pregnancy outcomes of 629 renal transplant patients revealed no association with adverse pregnancy outcomes compared to the general population and no increase in fetal malformations.9 Therefore, cyclosporine is a safe treatment option and was classified as a first-line drug for GPPP in a 2012 review by the National Psoriasis Foundation Medical Board.2 Narrowband UVB also has been reported to be used for the treatment of GPPP.10 Methotrexate and retinoids have been used in cases with lesions that persisted postpartum.1
Anti–tumor necrosis factor (TNF) α agents are another effective option for treatment of GPPP. Anti-TNF agents are classified as pregnancy category B due to results showing that anti-mouse TNF-α monoclonal antibodies did not cause embryotoxicity or teratogenicity in pregnant mice.11 Although Carter et al12 published a review of US Food and Drug Administration data on pregnant women receiving anti-TNF treatment and concluded that these agents were associated with the VACTERL group of malformations (vertebral defects, anal atresia, cardiac defect, tracheoesophageal fistula with esophageal atresia, cardiac defects, renal and limb anomalies), no such association was found in further studies. A 2014 study showed no difference in the rate of major malformations in infants born to women who were treated with anti-TNF drugs compared to the disease-matched group not treated with these agents and pregnant women counselled for nonteratogenic exposure.13 The same study detected an increase in preterm and low-birth-weight deliveries and suggested this might be caused by the increased severity of disease in patients requiring anti-TNF medication. The British Society of Rheumatology Biologics Register published data on pregnancy outcomes in 130 rheumatoid arthritis patients who had been exposed to anti-TNF agents.14 The results suggested an increased rate of spontaneous abortions in women exposed to anti-TNF treatment around the time of conception, especially in those taking these medications together with methotrexate or leflunomide; however, results also indicated that disease activity may have had an impact on the rate of spontaneous abortions in these patients. In a 2013 review of 462 women with inflammatory bowel disease who had been exposed to anti-TNF agents during pregnancy, the investigators concluded that pregnancy outcomes and the rate of congenital anomalies did not significantly differ from other inflammatory bowel disease patients not receiving anti-TNF drugs or the general population.15
In 2012, the National Board of the National Psoriasis Foundation put infliximab amongst the first-line treatment modalities for GPPP.2 In one case of GPPP in which the eruption persisted after delivery, the patient was treated with infliximab 7 weeks postpartum due to failure to control the disease with prednisolone 60 mg daily and cyclosporine 7.5 mg/kg daily. Unlike our patient, this patient was only started on an infliximab regimen after delivery.16 In another case reported in 2010, the patient was started on infliximab during the postpartum period of her first pregnancy following a pustular flare of previously diagnosed plaque psoriasis (not a generalized pustular psoriasis, as in our case).17 As a good response was obtained, infliximab treatment was continued in the patient throughout her second pregnancy.
Our case is unique in that infliximab was started during pregnancy because of intractable disease leading to systemic symptoms. Our patient showed an excellent response to infliximab after a 10-week disease course with repeated flare-ups and impairment to her overall condition. Delivery occurred at 36 weeks’ gestation due to suspected intrauterine growth retardation; however, the neonate was born with a 5-minute APGAR score of 10 and required no special medical care, which suggests that the low birth weight was constitutional due to the patient’s small frame (her height was 4 ft 11 in). The breast milk of patients with inflammatory bowel disease has been detected to contain very small amounts of infliximab (101 ng/mL, about 1/200 of the therapeutic blood level).18 Considering the large molecular weight of this agent and possible proteolysis in the stomach and intestines, infliximab is unlikely to affect the neonate.15 Thus, we encouraged our patient to breastfeed her baby. A case of fatal disseminated Bacille-Calmette-Guérin infection in an infant whose mother received infliximab treatment during pregnancy has been reported.19 It has been suggested that live vaccines should be avoided in neonates exposed to anti-TNF agents at least for the first 6 months of life or until the agent is no longer detectable in their blood.15 We therefore informed our patient’s family practitioner about this data.
Conclusion
We report a case of infliximab treatment for GPPP that was continued during the postpartum period. Infliximab was an effective treatment option in our patient with no detected serious adverse events and may be considered in other cases of GPPP that are not responsive to systemic steroids. However, further studies are warranted to evaluate the safety and efficacy of infliximab treatment for GPPP and psoriasis in pregnancy.
- Lerhoff S, Pomeranz MK. Specific dermatoses of pregnancy and their treatment. Dermatol Ther. 2013;26:274-284.
- Robinson A, Van Voorhees AS, Hsu S, et al. Treatment of pustular psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:279-288.
- Oumeish OY, Parish JL. Impetigo herpetiformis. Clin Dermatol. 2006;24:101-104.
- Gao QQ, Xi MR, Yao Q. Impetigo herpetiformis during pregnancy: a case report and literature review. Dermatology. 2013;226:35-40.
- Bae YS, Van Voorhees AS, Hsu S, et al. Review of treatment options for psoriasis in pregnant or lactating women: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:459-477.
- Shaw CJ, Wu P, Sriemevan A. First trimester impetigo herpetiformis in multiparous female successfully treated with oral cyclosporine [published May 12, 2011]. BMJ Case Rep. doi:10.1136/bcr.02.2011.3915
- Hazarika D. Generalized pustular psoriasis of pregnancy successfully treated with cyclosporine. Indian J Dermatol Venereol Leprol. 2009;75:638.
- Luan L, Han S, Zhang Z, et al. Personal treatment experience for severe generalized pustular psoriasis of pregnancy: two case reports. Dermatol Ther. 2014;27:174-177.
- Lamarque V, Leleu MF, Monka C, et al. Analysis of 629 pregnancy outcomes in transplant recipients treated with Sandimmun. Transplant Proc. 1997;29:2480.
- Bozdag K, Ozturk S, Ermete M. A case of recurrent impetigo herpetiformis treated with systemic corticosteroids and narrowband UVB. Cutan Ocul Toxicol. 2012;31:67-69.
- Treacy G. Using an analogous monoclonal antibody to evaluate the reproductive and chronic toxicity potential for a humanized anti-TNF alpha monoclonal antibody. Hum Exp Toxicol. 2000;19:226-228.
- Carter JD, Ladhani A, Ricca LR, et al. A safety assessment of tumor necrosis factor antagonists during pregnancy: a review of the Food and Drug Administration database. J Rheumatol. 2009;36:635-641.
- Diav-Citrin O, Otcheretianski-Volodarsky A, Shechtman S, et al. Pregnancy outcome following gestational exposure to TNF-alpha-inhibitors: a prospective, comparative, observational study. Reprod Toxicol. 2014;43:78-84.
- Verstappen SM, King Y, Watson KD, et al. Anti-TNF therapies and pregnancy: outcome of 130 pregnancies in the British Society for Rheumatology Biologics Register. Ann Rheum Dis. 2011;70:823-826.
- Gisbert JP, Chaparro M. Safety of anti-TNF agents during pregnancy and breastfeeding in women with inflammatory bowel disease. Am J Gastroenterol. 2013;108:1426-1438.
- Sheth N, Greenblatt DT, Acland K, et al. Generalized pustular psoriasis of pregnancy treated with infliximab. Clin Exp Dermatol. 2009;34:521-522.
- Puig L, Barco D, Alomar A. Treatment of psoriasis with anti-TNF drugs during pregnancy: case report and review of the literature. Dermatology. 2010;220:71-76.
- Ben-Horin S, Yavzori M, Kopylov U, et al. Detection of infliximab in breast milk of nursing mothers with inflammatory bowel disease. J Crohns Colitis. 2011;5:555-558.
- Cheent K, Nolan J, Shariq S, et al. Case report: fatal case of disseminated BCG infection in an infant born to a mother taking infliximab for Crohn’s disease. J Crohns Colitis. 2010;4:603-605.
- Lerhoff S, Pomeranz MK. Specific dermatoses of pregnancy and their treatment. Dermatol Ther. 2013;26:274-284.
- Robinson A, Van Voorhees AS, Hsu S, et al. Treatment of pustular psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:279-288.
- Oumeish OY, Parish JL. Impetigo herpetiformis. Clin Dermatol. 2006;24:101-104.
- Gao QQ, Xi MR, Yao Q. Impetigo herpetiformis during pregnancy: a case report and literature review. Dermatology. 2013;226:35-40.
- Bae YS, Van Voorhees AS, Hsu S, et al. Review of treatment options for psoriasis in pregnant or lactating women: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:459-477.
- Shaw CJ, Wu P, Sriemevan A. First trimester impetigo herpetiformis in multiparous female successfully treated with oral cyclosporine [published May 12, 2011]. BMJ Case Rep. doi:10.1136/bcr.02.2011.3915
- Hazarika D. Generalized pustular psoriasis of pregnancy successfully treated with cyclosporine. Indian J Dermatol Venereol Leprol. 2009;75:638.
- Luan L, Han S, Zhang Z, et al. Personal treatment experience for severe generalized pustular psoriasis of pregnancy: two case reports. Dermatol Ther. 2014;27:174-177.
- Lamarque V, Leleu MF, Monka C, et al. Analysis of 629 pregnancy outcomes in transplant recipients treated with Sandimmun. Transplant Proc. 1997;29:2480.
- Bozdag K, Ozturk S, Ermete M. A case of recurrent impetigo herpetiformis treated with systemic corticosteroids and narrowband UVB. Cutan Ocul Toxicol. 2012;31:67-69.
- Treacy G. Using an analogous monoclonal antibody to evaluate the reproductive and chronic toxicity potential for a humanized anti-TNF alpha monoclonal antibody. Hum Exp Toxicol. 2000;19:226-228.
- Carter JD, Ladhani A, Ricca LR, et al. A safety assessment of tumor necrosis factor antagonists during pregnancy: a review of the Food and Drug Administration database. J Rheumatol. 2009;36:635-641.
- Diav-Citrin O, Otcheretianski-Volodarsky A, Shechtman S, et al. Pregnancy outcome following gestational exposure to TNF-alpha-inhibitors: a prospective, comparative, observational study. Reprod Toxicol. 2014;43:78-84.
- Verstappen SM, King Y, Watson KD, et al. Anti-TNF therapies and pregnancy: outcome of 130 pregnancies in the British Society for Rheumatology Biologics Register. Ann Rheum Dis. 2011;70:823-826.
- Gisbert JP, Chaparro M. Safety of anti-TNF agents during pregnancy and breastfeeding in women with inflammatory bowel disease. Am J Gastroenterol. 2013;108:1426-1438.
- Sheth N, Greenblatt DT, Acland K, et al. Generalized pustular psoriasis of pregnancy treated with infliximab. Clin Exp Dermatol. 2009;34:521-522.
- Puig L, Barco D, Alomar A. Treatment of psoriasis with anti-TNF drugs during pregnancy: case report and review of the literature. Dermatology. 2010;220:71-76.
- Ben-Horin S, Yavzori M, Kopylov U, et al. Detection of infliximab in breast milk of nursing mothers with inflammatory bowel disease. J Crohns Colitis. 2011;5:555-558.
- Cheent K, Nolan J, Shariq S, et al. Case report: fatal case of disseminated BCG infection in an infant born to a mother taking infliximab for Crohn’s disease. J Crohns Colitis. 2010;4:603-605.
Practice Points
- Generalized pustular psoriasis of pregnancy (GPPP) is a rare and severe condition that may lead to complications in both the mother and the fetus. Effective treatment with low impact on the fetus is essential.
- Infliximab, among other biologic agents, may be considered for the rapid clearing of skin lesions in GPPP.
To improve psoriatic arthritis outcomes, address common comorbidities
Only about 30% or fewer of patients with psoriatic arthritis (PsA) on therapy achieve disease remission by any definition. One reason for this may be inadequate attention to common comorbid conditions, Alexis Ogdie, MD, MSCE, declared at the 2021 Rheumatology Winter Clinical Symposium.
“I believe that addressing off-target aspects of disease is really important to improving the patient experience of their disease. We might need to target these directly in order to improve outcomes,” said Dr. Ogdie, a rheumatologist and epidemiologist at the University of Pennsylvania, Philadelphia, who coauthored the current American College of Rheumatology/National Psoriasis Foundation PsA guidelines.
Since rheumatologists are by now well informed about the increased cardiovascular risk associated with PsA, she focused on two common comorbidities that get less attention, both of which are associated with worse clinical outcomes in PsA: obesity and mental health issues.
Anxiety and depression
Dr. Ogdie was first author of a large, population-based, longitudinal cohort study of cause-specific mortality in 8,706 U.K. patients with PsA, 41,752 with RA, and more than 81,000 controls. Particularly striking was the finding of elevated mortality because of suicide in the rheumatic disease patients: a 203% increased risk in the PsA population, compared with the general population, and a 147% greater risk in patients with RA.
Overall, 30%-40% of PsA patients have comorbid depression and/or anxiety.
“That’s pretty striking. It’s also true for rheumatoid arthritis and axial spondyloarthritis. And if you’re depressed, you’re much less likely to respond to therapy in the way that we are measuring response to therapy,” Dr. Ogdie said.
Her approach to screening for depression and anxiety in her PsA patients, and indeed in all her other patients, is to begin by normalizing the topic, explaining to them that these affective disorders are common among patients with these disorders. She lets her patients know they can talk to her about it. And she informs them that, while effective treatment of their rheumatic disease may improve their depression or anxiety, managing those is also important for improving their disease. Additionally, understanding whether depression is present is important prior to prescribing certain medications. Apremilast (Otezla), for example, can worsen preexisting depression.
“Ask about signs and symptoms of depression,” Dr. Ogdie urged her colleagues. “I do this at every single visit in my review of symptoms. This is one I don’t skip. I ask: ‘Do you have any symptoms of depression or anxiety?’ ”
Structured evidence-based screening tools, many of which are well suited for completion during a patient’s preappointment check-in survey, include the Patient Health Questionnaire–2, the PHQ-9, the Patient-Reported Outcomes Measure Information System–10, PROMIS–Depression, and Routine Assessment of Patient Index Data 3.
“I also really like the PROMIS-29. It covers many domains of interest: depression and anxiety, sleep, fatigue, pain, physical function. It gives a lot of information about what’s going on in a patient’s life right now,” according to the rheumatologist.
The main thing is to regularly screen for anxiety and depression and then refer symptomatic patients for further assessment and treatment. This is not something that all rheumatologists have been trained to do.
Obesity
Dr. Ogdie was lead author of a national CORRONA Registry study which concluded that obese patients with PsA were only half as likely to achieve remission on a tumor necrosis factor (TNF) inhibitor, compared with nonobese patients. She believes the same holds true for all other types of therapy: Across the board, obesity is associated with a poor response. And obesity is much more common in PsA patients than the general population in every age group. Moreover, obesity is associated with risk factors for cardiovascular disease and is associated with fatty liver disease, two other major comorbid conditions in the PsA population.
The CORRONA Registry findings are supportive of an earlier Italian prospective, observational study of 135 obese and an equal number of normal-weight PsA patients, all of whom started on a TNF inhibitor and were followed for 24 months. In a multivariate-adjusted analysis, obesity was independently associated with a 390% higher risk of not achieving minimal disease activity.
The same Italian group subsequently conducted a prospective dietary intervention study in 138 overweight or obese patients with PsA starting anti-TNF therapy. A total of 59% of participants randomized to either of the two dietary interventions experienced at least a 5% weight loss at 6 months. The key study finding: Compared with the subjects with less than 5% weight loss, those with 5%-10% weight loss were 275% more likely to achieve minimal disease activity at 6 months, and in those with greater than 10% weight loss the likelihood of attaining minimal disease activity increased by 567%.
“We’re talking about a disease where treatments tested in clinical trials have odds ratios in the 1.2 range, compared with other therapies, so this is a really striking difference,” she observed.
Several studies have demonstrated that obesity in psoriasis patients is a risk factor for developing PsA. Recently, U.K. investigators took things a step further, reporting in a huge observational study that obese or overweight psoriasis patients who reduced their body mass index over a 10-year period had a corresponding reduction in the risk of developing PsA, compared with overweight or obese psoriasis patients whose BMI remained steady over the same period.
What’s needed now is access to programs to help patients with PsA lose weight. Health insurers are often unwilling to provide coverage. “We have a really tough time getting the patients in to see a nutritionist unless they’re willing to pay out of pocket,” Dr. Ogdie said.
Physical activity is an important element in successful weight loss. It also is recommended in practice guidelines for patients with inflammatory arthritis because of its salutary effects on disease activity scores, pain and stiffness, sleep, and quality of life. But a recent survey conducted by Dr. Ogdie and coworkers concluded that patients with PsA and other forms of inflammatory arthritis don’t receive much exercise guidance from their rheumatologists. About 60% of subjects were inactive. Those who were physically active typically engaged in aerobic exercise but were much less likely to do the other guideline-recommended forms of exercise, namely flexibility, balance, and resistance training. The patients’ report of low engagement of their physicians “suggests an opportunity for more prescriptive exercise discussions,” according to the investigators.
Diabetes, a critical risk factor for cardiovascular disease, occurs at an increased incidence in PsA. This was demonstrated in a U.K. cohort study coauthored by Dr. Ogdie. The study, which included nearly 4,200 individuals with PsA, concluded that they had a 43% greater incidence of diabetes than the general population in an analysis adjusted for body mass index, smoking, alcohol use, and demographics.
New-onset diabetes can be readily picked up by rheumatologists based upon the laboratory work they often order at patient office visits, or during their review of symptoms, she noted, and added that the U.S. Preventive Services Task Force recommends ordering a hemoglobin A1c test every 3 years.
Dr. Ogdie reported receiving research grants and/or consulting fees from numerous pharmaceutical companies. Her research is also funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the Rheumatology Research Foundation, and the National Psoriasis Foundation.
Only about 30% or fewer of patients with psoriatic arthritis (PsA) on therapy achieve disease remission by any definition. One reason for this may be inadequate attention to common comorbid conditions, Alexis Ogdie, MD, MSCE, declared at the 2021 Rheumatology Winter Clinical Symposium.
“I believe that addressing off-target aspects of disease is really important to improving the patient experience of their disease. We might need to target these directly in order to improve outcomes,” said Dr. Ogdie, a rheumatologist and epidemiologist at the University of Pennsylvania, Philadelphia, who coauthored the current American College of Rheumatology/National Psoriasis Foundation PsA guidelines.
Since rheumatologists are by now well informed about the increased cardiovascular risk associated with PsA, she focused on two common comorbidities that get less attention, both of which are associated with worse clinical outcomes in PsA: obesity and mental health issues.
Anxiety and depression
Dr. Ogdie was first author of a large, population-based, longitudinal cohort study of cause-specific mortality in 8,706 U.K. patients with PsA, 41,752 with RA, and more than 81,000 controls. Particularly striking was the finding of elevated mortality because of suicide in the rheumatic disease patients: a 203% increased risk in the PsA population, compared with the general population, and a 147% greater risk in patients with RA.
Overall, 30%-40% of PsA patients have comorbid depression and/or anxiety.
“That’s pretty striking. It’s also true for rheumatoid arthritis and axial spondyloarthritis. And if you’re depressed, you’re much less likely to respond to therapy in the way that we are measuring response to therapy,” Dr. Ogdie said.
Her approach to screening for depression and anxiety in her PsA patients, and indeed in all her other patients, is to begin by normalizing the topic, explaining to them that these affective disorders are common among patients with these disorders. She lets her patients know they can talk to her about it. And she informs them that, while effective treatment of their rheumatic disease may improve their depression or anxiety, managing those is also important for improving their disease. Additionally, understanding whether depression is present is important prior to prescribing certain medications. Apremilast (Otezla), for example, can worsen preexisting depression.
“Ask about signs and symptoms of depression,” Dr. Ogdie urged her colleagues. “I do this at every single visit in my review of symptoms. This is one I don’t skip. I ask: ‘Do you have any symptoms of depression or anxiety?’ ”
Structured evidence-based screening tools, many of which are well suited for completion during a patient’s preappointment check-in survey, include the Patient Health Questionnaire–2, the PHQ-9, the Patient-Reported Outcomes Measure Information System–10, PROMIS–Depression, and Routine Assessment of Patient Index Data 3.
“I also really like the PROMIS-29. It covers many domains of interest: depression and anxiety, sleep, fatigue, pain, physical function. It gives a lot of information about what’s going on in a patient’s life right now,” according to the rheumatologist.
The main thing is to regularly screen for anxiety and depression and then refer symptomatic patients for further assessment and treatment. This is not something that all rheumatologists have been trained to do.
Obesity
Dr. Ogdie was lead author of a national CORRONA Registry study which concluded that obese patients with PsA were only half as likely to achieve remission on a tumor necrosis factor (TNF) inhibitor, compared with nonobese patients. She believes the same holds true for all other types of therapy: Across the board, obesity is associated with a poor response. And obesity is much more common in PsA patients than the general population in every age group. Moreover, obesity is associated with risk factors for cardiovascular disease and is associated with fatty liver disease, two other major comorbid conditions in the PsA population.
The CORRONA Registry findings are supportive of an earlier Italian prospective, observational study of 135 obese and an equal number of normal-weight PsA patients, all of whom started on a TNF inhibitor and were followed for 24 months. In a multivariate-adjusted analysis, obesity was independently associated with a 390% higher risk of not achieving minimal disease activity.
The same Italian group subsequently conducted a prospective dietary intervention study in 138 overweight or obese patients with PsA starting anti-TNF therapy. A total of 59% of participants randomized to either of the two dietary interventions experienced at least a 5% weight loss at 6 months. The key study finding: Compared with the subjects with less than 5% weight loss, those with 5%-10% weight loss were 275% more likely to achieve minimal disease activity at 6 months, and in those with greater than 10% weight loss the likelihood of attaining minimal disease activity increased by 567%.
“We’re talking about a disease where treatments tested in clinical trials have odds ratios in the 1.2 range, compared with other therapies, so this is a really striking difference,” she observed.
Several studies have demonstrated that obesity in psoriasis patients is a risk factor for developing PsA. Recently, U.K. investigators took things a step further, reporting in a huge observational study that obese or overweight psoriasis patients who reduced their body mass index over a 10-year period had a corresponding reduction in the risk of developing PsA, compared with overweight or obese psoriasis patients whose BMI remained steady over the same period.
What’s needed now is access to programs to help patients with PsA lose weight. Health insurers are often unwilling to provide coverage. “We have a really tough time getting the patients in to see a nutritionist unless they’re willing to pay out of pocket,” Dr. Ogdie said.
Physical activity is an important element in successful weight loss. It also is recommended in practice guidelines for patients with inflammatory arthritis because of its salutary effects on disease activity scores, pain and stiffness, sleep, and quality of life. But a recent survey conducted by Dr. Ogdie and coworkers concluded that patients with PsA and other forms of inflammatory arthritis don’t receive much exercise guidance from their rheumatologists. About 60% of subjects were inactive. Those who were physically active typically engaged in aerobic exercise but were much less likely to do the other guideline-recommended forms of exercise, namely flexibility, balance, and resistance training. The patients’ report of low engagement of their physicians “suggests an opportunity for more prescriptive exercise discussions,” according to the investigators.
Diabetes, a critical risk factor for cardiovascular disease, occurs at an increased incidence in PsA. This was demonstrated in a U.K. cohort study coauthored by Dr. Ogdie. The study, which included nearly 4,200 individuals with PsA, concluded that they had a 43% greater incidence of diabetes than the general population in an analysis adjusted for body mass index, smoking, alcohol use, and demographics.
New-onset diabetes can be readily picked up by rheumatologists based upon the laboratory work they often order at patient office visits, or during their review of symptoms, she noted, and added that the U.S. Preventive Services Task Force recommends ordering a hemoglobin A1c test every 3 years.
Dr. Ogdie reported receiving research grants and/or consulting fees from numerous pharmaceutical companies. Her research is also funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the Rheumatology Research Foundation, and the National Psoriasis Foundation.
Only about 30% or fewer of patients with psoriatic arthritis (PsA) on therapy achieve disease remission by any definition. One reason for this may be inadequate attention to common comorbid conditions, Alexis Ogdie, MD, MSCE, declared at the 2021 Rheumatology Winter Clinical Symposium.
“I believe that addressing off-target aspects of disease is really important to improving the patient experience of their disease. We might need to target these directly in order to improve outcomes,” said Dr. Ogdie, a rheumatologist and epidemiologist at the University of Pennsylvania, Philadelphia, who coauthored the current American College of Rheumatology/National Psoriasis Foundation PsA guidelines.
Since rheumatologists are by now well informed about the increased cardiovascular risk associated with PsA, she focused on two common comorbidities that get less attention, both of which are associated with worse clinical outcomes in PsA: obesity and mental health issues.
Anxiety and depression
Dr. Ogdie was first author of a large, population-based, longitudinal cohort study of cause-specific mortality in 8,706 U.K. patients with PsA, 41,752 with RA, and more than 81,000 controls. Particularly striking was the finding of elevated mortality because of suicide in the rheumatic disease patients: a 203% increased risk in the PsA population, compared with the general population, and a 147% greater risk in patients with RA.
Overall, 30%-40% of PsA patients have comorbid depression and/or anxiety.
“That’s pretty striking. It’s also true for rheumatoid arthritis and axial spondyloarthritis. And if you’re depressed, you’re much less likely to respond to therapy in the way that we are measuring response to therapy,” Dr. Ogdie said.
Her approach to screening for depression and anxiety in her PsA patients, and indeed in all her other patients, is to begin by normalizing the topic, explaining to them that these affective disorders are common among patients with these disorders. She lets her patients know they can talk to her about it. And she informs them that, while effective treatment of their rheumatic disease may improve their depression or anxiety, managing those is also important for improving their disease. Additionally, understanding whether depression is present is important prior to prescribing certain medications. Apremilast (Otezla), for example, can worsen preexisting depression.
“Ask about signs and symptoms of depression,” Dr. Ogdie urged her colleagues. “I do this at every single visit in my review of symptoms. This is one I don’t skip. I ask: ‘Do you have any symptoms of depression or anxiety?’ ”
Structured evidence-based screening tools, many of which are well suited for completion during a patient’s preappointment check-in survey, include the Patient Health Questionnaire–2, the PHQ-9, the Patient-Reported Outcomes Measure Information System–10, PROMIS–Depression, and Routine Assessment of Patient Index Data 3.
“I also really like the PROMIS-29. It covers many domains of interest: depression and anxiety, sleep, fatigue, pain, physical function. It gives a lot of information about what’s going on in a patient’s life right now,” according to the rheumatologist.
The main thing is to regularly screen for anxiety and depression and then refer symptomatic patients for further assessment and treatment. This is not something that all rheumatologists have been trained to do.
Obesity
Dr. Ogdie was lead author of a national CORRONA Registry study which concluded that obese patients with PsA were only half as likely to achieve remission on a tumor necrosis factor (TNF) inhibitor, compared with nonobese patients. She believes the same holds true for all other types of therapy: Across the board, obesity is associated with a poor response. And obesity is much more common in PsA patients than the general population in every age group. Moreover, obesity is associated with risk factors for cardiovascular disease and is associated with fatty liver disease, two other major comorbid conditions in the PsA population.
The CORRONA Registry findings are supportive of an earlier Italian prospective, observational study of 135 obese and an equal number of normal-weight PsA patients, all of whom started on a TNF inhibitor and were followed for 24 months. In a multivariate-adjusted analysis, obesity was independently associated with a 390% higher risk of not achieving minimal disease activity.
The same Italian group subsequently conducted a prospective dietary intervention study in 138 overweight or obese patients with PsA starting anti-TNF therapy. A total of 59% of participants randomized to either of the two dietary interventions experienced at least a 5% weight loss at 6 months. The key study finding: Compared with the subjects with less than 5% weight loss, those with 5%-10% weight loss were 275% more likely to achieve minimal disease activity at 6 months, and in those with greater than 10% weight loss the likelihood of attaining minimal disease activity increased by 567%.
“We’re talking about a disease where treatments tested in clinical trials have odds ratios in the 1.2 range, compared with other therapies, so this is a really striking difference,” she observed.
Several studies have demonstrated that obesity in psoriasis patients is a risk factor for developing PsA. Recently, U.K. investigators took things a step further, reporting in a huge observational study that obese or overweight psoriasis patients who reduced their body mass index over a 10-year period had a corresponding reduction in the risk of developing PsA, compared with overweight or obese psoriasis patients whose BMI remained steady over the same period.
What’s needed now is access to programs to help patients with PsA lose weight. Health insurers are often unwilling to provide coverage. “We have a really tough time getting the patients in to see a nutritionist unless they’re willing to pay out of pocket,” Dr. Ogdie said.
Physical activity is an important element in successful weight loss. It also is recommended in practice guidelines for patients with inflammatory arthritis because of its salutary effects on disease activity scores, pain and stiffness, sleep, and quality of life. But a recent survey conducted by Dr. Ogdie and coworkers concluded that patients with PsA and other forms of inflammatory arthritis don’t receive much exercise guidance from their rheumatologists. About 60% of subjects were inactive. Those who were physically active typically engaged in aerobic exercise but were much less likely to do the other guideline-recommended forms of exercise, namely flexibility, balance, and resistance training. The patients’ report of low engagement of their physicians “suggests an opportunity for more prescriptive exercise discussions,” according to the investigators.
Diabetes, a critical risk factor for cardiovascular disease, occurs at an increased incidence in PsA. This was demonstrated in a U.K. cohort study coauthored by Dr. Ogdie. The study, which included nearly 4,200 individuals with PsA, concluded that they had a 43% greater incidence of diabetes than the general population in an analysis adjusted for body mass index, smoking, alcohol use, and demographics.
New-onset diabetes can be readily picked up by rheumatologists based upon the laboratory work they often order at patient office visits, or during their review of symptoms, she noted, and added that the U.S. Preventive Services Task Force recommends ordering a hemoglobin A1c test every 3 years.
Dr. Ogdie reported receiving research grants and/or consulting fees from numerous pharmaceutical companies. Her research is also funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the Rheumatology Research Foundation, and the National Psoriasis Foundation.
FROM RWCS 2021
Recent psoriasis pathophysiology insights carry treatment implications
at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually.
Among these unexpected developments was the startling finding that skin inflammation in mild psoriasis is at least as great as in severe disease; evidence that psoriasis may actually be an autoimmune disease rather than a nonspecific immune-mediated disease; and the newly appreciated importance of interleukin-19 (IL-19) in keratinocyte proliferation, according to Dr. Gordon, professor and chair of the department of dermatology at the Medical College of Wisconsin, Milwaukee.
“Our understanding of the pathophysiology of psoriasis is still a work in progress,” the dermatologist observed.
Immunoregulatory deficits in mild vs. severe psoriasis
Conventional wisdom has held that mild psoriasis as defined by limited affected body surface area involves less skin inflammation than more extensive severe psoriasis, so less-potent topical therapies are appropriate. Not so, according to Dr. Gordon, who highlighted work by James G. Krueger, MD, PhD, head of the laboratory of investigative dermatology at Rockefeller University, New York, and coinvestigators. They demonstrated that overall skin inflammation expressed as the sum of T-cell activation and IL-19-mediated epidermal responses didn’t differ in lesions of mild as compared with severe psoriasis. Indeed, mild skin lesions featured a greater number of T-cells, stronger expression of proinflammatory cytokine IL-17A, and greater expression of the central psoriasis transcriptome. The big difference between skin lesions of mild versus severe psoriasis was that severe psoriasis was characterized by strikingly weaker expression of immunoregulatory genes, including programmed death-ligand 1 (PD-L1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA4), than that of mild lesions.
The implication is that IL-17-targeted therapies may be of benefit in a much larger segment of the psoriatic population: namely, those with mild disease, who comprise the majority of psoriasis patients by a wide margin, according to the investigators.
Dr. Gordon concurs.
“The primary problem in psoriasis is not so much the inflammatory activity, but the ability to turn off the activity,” he explained. “That implies that if a patient wants to get clear or have significant improvement in disease, you can’t use a less effective medication just because they have less amount of disease. You’re going to need to treat it just as aggressively because the great majority of our medications block the proinflammatory pathways.”
The deficit in immunoregulatory action identified by Dr. Krueger and colleagues in patients with severe disease could provide a novel therapeutic target. If the deficient immunoregulation could be boosted, it might achieve disease control without need for continuous anti-inflammatory therapy.
Autoimmunity in psoriasis
“When I started work in psoriasis, we always thought there would be a common antigen for the immune process in the disease. We never found it. So for that reason, we sort of put it aside and called psoriasis a nonspecific immune-mediated disease,” Dr. Gordon recalled.
That view is being reexamined. “While we’re not completely certain, there is now some evidence that there might be autoimmunity in psoriasis,” he said.
He cited work by an international team of investigators who identified the cathelicidin antimicrobial peptide LL37 as being overexpressed in psoriatic skin, where it appears to serve as a T-cell autoantigen. LL37-specific CD4+ and CD8+ T-cells are skin homing: They can infiltrate lesional skin, where they produce interferon-gamma and proinflammatory Th17 cytokines. The investigators reported that levels of circulating LL37-specific T cells correlated with disease activity such that they were found in three-quarters of patients with moderate to severe plaque psoriasis.
“As LL37 is able to activate innate immune cells and break innate tolerance to self-nucleic acids, it represents an even more appealing target to treat psoriasis. Therapeutic targeting of LL37-specific T cells may provide new avenues to prevent or treat psoriasis without inducing indiscriminate immunosuppression,” the investigators concluded.
Similarly, German investigators have identified ADAMTS-like protein 5 (ADAMTSL5) as an autoantigen specific for melanocytes in psoriasis patients who possess the central psoriasis risk gene, known as HLA-C*06:02, which is present in two-thirds of patients with psoriasis. They proposed that their newly recognized autoimmune pathway may explain how HLA-C*06.02 predisposes to psoriasis.
Growing clinical relevance of IL-19
It’s now well-established that IL-17 is the pivotal force driving the changes in keratinocytes that define the visible expressions of psoriasis, including plaque scale and thickness, which are due to abnormal keratinocyte maturation and proliferation, respectively. Less well appreciated is the fact that IL-17-activated keratinocytes produce IL-19, which feeds back and further stimulates keratinocyte proliferation.
In light of mounting evidence that IL-19 plays an important role in the pathogenesis of psoriasis and that naked eye assessment of visible psoriasis may not reflect the true extent of inflammation, Brian J. Nickoloff, MD, PhD, and coworkers at Lilly Research Laboratories have developed a novel serum IL-19 immunoassay that appears to provide a much-needed objective biomarker of disease activity in psoriasis patients. They demonstrated that serum IL-19 levels correlated with Psoriasis Area and Severity Index scores, and that treatment with the anti-IL-17A biologic ixekizumab (Taltz) led to rapid reduction of IL-19 down to a normal level.
Moreover, following withdrawal of ixekizumab, IL-19 levels rose prior to clinical relapse, then dropped again in response to retreatment. The hope is that this assay will serve as an accurate tool for assessment of response to therapy.
Dr. Gordon reported receiving research funding and/or honoraria from more than a dozen pharmaceutical companies involved in psoriasis therapy.
MedscapeLive and this news organization are owned by the same parent company.
at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually.
Among these unexpected developments was the startling finding that skin inflammation in mild psoriasis is at least as great as in severe disease; evidence that psoriasis may actually be an autoimmune disease rather than a nonspecific immune-mediated disease; and the newly appreciated importance of interleukin-19 (IL-19) in keratinocyte proliferation, according to Dr. Gordon, professor and chair of the department of dermatology at the Medical College of Wisconsin, Milwaukee.
“Our understanding of the pathophysiology of psoriasis is still a work in progress,” the dermatologist observed.
Immunoregulatory deficits in mild vs. severe psoriasis
Conventional wisdom has held that mild psoriasis as defined by limited affected body surface area involves less skin inflammation than more extensive severe psoriasis, so less-potent topical therapies are appropriate. Not so, according to Dr. Gordon, who highlighted work by James G. Krueger, MD, PhD, head of the laboratory of investigative dermatology at Rockefeller University, New York, and coinvestigators. They demonstrated that overall skin inflammation expressed as the sum of T-cell activation and IL-19-mediated epidermal responses didn’t differ in lesions of mild as compared with severe psoriasis. Indeed, mild skin lesions featured a greater number of T-cells, stronger expression of proinflammatory cytokine IL-17A, and greater expression of the central psoriasis transcriptome. The big difference between skin lesions of mild versus severe psoriasis was that severe psoriasis was characterized by strikingly weaker expression of immunoregulatory genes, including programmed death-ligand 1 (PD-L1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA4), than that of mild lesions.
The implication is that IL-17-targeted therapies may be of benefit in a much larger segment of the psoriatic population: namely, those with mild disease, who comprise the majority of psoriasis patients by a wide margin, according to the investigators.
Dr. Gordon concurs.
“The primary problem in psoriasis is not so much the inflammatory activity, but the ability to turn off the activity,” he explained. “That implies that if a patient wants to get clear or have significant improvement in disease, you can’t use a less effective medication just because they have less amount of disease. You’re going to need to treat it just as aggressively because the great majority of our medications block the proinflammatory pathways.”
The deficit in immunoregulatory action identified by Dr. Krueger and colleagues in patients with severe disease could provide a novel therapeutic target. If the deficient immunoregulation could be boosted, it might achieve disease control without need for continuous anti-inflammatory therapy.
Autoimmunity in psoriasis
“When I started work in psoriasis, we always thought there would be a common antigen for the immune process in the disease. We never found it. So for that reason, we sort of put it aside and called psoriasis a nonspecific immune-mediated disease,” Dr. Gordon recalled.
That view is being reexamined. “While we’re not completely certain, there is now some evidence that there might be autoimmunity in psoriasis,” he said.
He cited work by an international team of investigators who identified the cathelicidin antimicrobial peptide LL37 as being overexpressed in psoriatic skin, where it appears to serve as a T-cell autoantigen. LL37-specific CD4+ and CD8+ T-cells are skin homing: They can infiltrate lesional skin, where they produce interferon-gamma and proinflammatory Th17 cytokines. The investigators reported that levels of circulating LL37-specific T cells correlated with disease activity such that they were found in three-quarters of patients with moderate to severe plaque psoriasis.
“As LL37 is able to activate innate immune cells and break innate tolerance to self-nucleic acids, it represents an even more appealing target to treat psoriasis. Therapeutic targeting of LL37-specific T cells may provide new avenues to prevent or treat psoriasis without inducing indiscriminate immunosuppression,” the investigators concluded.
Similarly, German investigators have identified ADAMTS-like protein 5 (ADAMTSL5) as an autoantigen specific for melanocytes in psoriasis patients who possess the central psoriasis risk gene, known as HLA-C*06:02, which is present in two-thirds of patients with psoriasis. They proposed that their newly recognized autoimmune pathway may explain how HLA-C*06.02 predisposes to psoriasis.
Growing clinical relevance of IL-19
It’s now well-established that IL-17 is the pivotal force driving the changes in keratinocytes that define the visible expressions of psoriasis, including plaque scale and thickness, which are due to abnormal keratinocyte maturation and proliferation, respectively. Less well appreciated is the fact that IL-17-activated keratinocytes produce IL-19, which feeds back and further stimulates keratinocyte proliferation.
In light of mounting evidence that IL-19 plays an important role in the pathogenesis of psoriasis and that naked eye assessment of visible psoriasis may not reflect the true extent of inflammation, Brian J. Nickoloff, MD, PhD, and coworkers at Lilly Research Laboratories have developed a novel serum IL-19 immunoassay that appears to provide a much-needed objective biomarker of disease activity in psoriasis patients. They demonstrated that serum IL-19 levels correlated with Psoriasis Area and Severity Index scores, and that treatment with the anti-IL-17A biologic ixekizumab (Taltz) led to rapid reduction of IL-19 down to a normal level.
Moreover, following withdrawal of ixekizumab, IL-19 levels rose prior to clinical relapse, then dropped again in response to retreatment. The hope is that this assay will serve as an accurate tool for assessment of response to therapy.
Dr. Gordon reported receiving research funding and/or honoraria from more than a dozen pharmaceutical companies involved in psoriasis therapy.
MedscapeLive and this news organization are owned by the same parent company.
at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually.
Among these unexpected developments was the startling finding that skin inflammation in mild psoriasis is at least as great as in severe disease; evidence that psoriasis may actually be an autoimmune disease rather than a nonspecific immune-mediated disease; and the newly appreciated importance of interleukin-19 (IL-19) in keratinocyte proliferation, according to Dr. Gordon, professor and chair of the department of dermatology at the Medical College of Wisconsin, Milwaukee.
“Our understanding of the pathophysiology of psoriasis is still a work in progress,” the dermatologist observed.
Immunoregulatory deficits in mild vs. severe psoriasis
Conventional wisdom has held that mild psoriasis as defined by limited affected body surface area involves less skin inflammation than more extensive severe psoriasis, so less-potent topical therapies are appropriate. Not so, according to Dr. Gordon, who highlighted work by James G. Krueger, MD, PhD, head of the laboratory of investigative dermatology at Rockefeller University, New York, and coinvestigators. They demonstrated that overall skin inflammation expressed as the sum of T-cell activation and IL-19-mediated epidermal responses didn’t differ in lesions of mild as compared with severe psoriasis. Indeed, mild skin lesions featured a greater number of T-cells, stronger expression of proinflammatory cytokine IL-17A, and greater expression of the central psoriasis transcriptome. The big difference between skin lesions of mild versus severe psoriasis was that severe psoriasis was characterized by strikingly weaker expression of immunoregulatory genes, including programmed death-ligand 1 (PD-L1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA4), than that of mild lesions.
The implication is that IL-17-targeted therapies may be of benefit in a much larger segment of the psoriatic population: namely, those with mild disease, who comprise the majority of psoriasis patients by a wide margin, according to the investigators.
Dr. Gordon concurs.
“The primary problem in psoriasis is not so much the inflammatory activity, but the ability to turn off the activity,” he explained. “That implies that if a patient wants to get clear or have significant improvement in disease, you can’t use a less effective medication just because they have less amount of disease. You’re going to need to treat it just as aggressively because the great majority of our medications block the proinflammatory pathways.”
The deficit in immunoregulatory action identified by Dr. Krueger and colleagues in patients with severe disease could provide a novel therapeutic target. If the deficient immunoregulation could be boosted, it might achieve disease control without need for continuous anti-inflammatory therapy.
Autoimmunity in psoriasis
“When I started work in psoriasis, we always thought there would be a common antigen for the immune process in the disease. We never found it. So for that reason, we sort of put it aside and called psoriasis a nonspecific immune-mediated disease,” Dr. Gordon recalled.
That view is being reexamined. “While we’re not completely certain, there is now some evidence that there might be autoimmunity in psoriasis,” he said.
He cited work by an international team of investigators who identified the cathelicidin antimicrobial peptide LL37 as being overexpressed in psoriatic skin, where it appears to serve as a T-cell autoantigen. LL37-specific CD4+ and CD8+ T-cells are skin homing: They can infiltrate lesional skin, where they produce interferon-gamma and proinflammatory Th17 cytokines. The investigators reported that levels of circulating LL37-specific T cells correlated with disease activity such that they were found in three-quarters of patients with moderate to severe plaque psoriasis.
“As LL37 is able to activate innate immune cells and break innate tolerance to self-nucleic acids, it represents an even more appealing target to treat psoriasis. Therapeutic targeting of LL37-specific T cells may provide new avenues to prevent or treat psoriasis without inducing indiscriminate immunosuppression,” the investigators concluded.
Similarly, German investigators have identified ADAMTS-like protein 5 (ADAMTSL5) as an autoantigen specific for melanocytes in psoriasis patients who possess the central psoriasis risk gene, known as HLA-C*06:02, which is present in two-thirds of patients with psoriasis. They proposed that their newly recognized autoimmune pathway may explain how HLA-C*06.02 predisposes to psoriasis.
Growing clinical relevance of IL-19
It’s now well-established that IL-17 is the pivotal force driving the changes in keratinocytes that define the visible expressions of psoriasis, including plaque scale and thickness, which are due to abnormal keratinocyte maturation and proliferation, respectively. Less well appreciated is the fact that IL-17-activated keratinocytes produce IL-19, which feeds back and further stimulates keratinocyte proliferation.
In light of mounting evidence that IL-19 plays an important role in the pathogenesis of psoriasis and that naked eye assessment of visible psoriasis may not reflect the true extent of inflammation, Brian J. Nickoloff, MD, PhD, and coworkers at Lilly Research Laboratories have developed a novel serum IL-19 immunoassay that appears to provide a much-needed objective biomarker of disease activity in psoriasis patients. They demonstrated that serum IL-19 levels correlated with Psoriasis Area and Severity Index scores, and that treatment with the anti-IL-17A biologic ixekizumab (Taltz) led to rapid reduction of IL-19 down to a normal level.
Moreover, following withdrawal of ixekizumab, IL-19 levels rose prior to clinical relapse, then dropped again in response to retreatment. The hope is that this assay will serve as an accurate tool for assessment of response to therapy.
Dr. Gordon reported receiving research funding and/or honoraria from more than a dozen pharmaceutical companies involved in psoriasis therapy.
MedscapeLive and this news organization are owned by the same parent company.
FROM MEDSCAPELIVE LAS VEGAS DERMATOLOGY SEMINAR
Sudden Cardiac Death in a Young Patient With Psoriasis
To the Editor:
The evolution in the understanding of psoriasis and psoriatic arthritis has unfolded many new facets of this immune-mediated inflammatory disease. Once considered to be just a cutaneous disease, psoriasis is not actually confined to skin but can involve almost any other system of the body. Cardiovascular morbidity and mortality are the major concerns in patients with psoriasis. We report the sudden death of a young man with severe psoriasis.
A 31-year-old man was admitted for severe psoriasis with pustular exacerbation (Figures 1A and 1B). He had moderate to severe unstable disease during the last 8 years and was managed with oral methotrexate (0.3–0.5 mg/kg/wk). He was not compliant with treatment, which led to multiple relapses. There was no personal or family history of risk factors for cardiovascular events (CVEs). At the time of present hospitalization, his vital parameters were normal. Physical examination revealed erythematous scaly plaques on more than 75% of the body surface area. Multiple pustules also were noted, often coalescing to form plaques (Figure 1C). Baseline investigations consisting of complete blood cell count, lipid profile, liver and renal functions, and chest radiography were within reference range. Baseline electrocardiogram (ECG) at admission was unremarkable (Figure 2A), except for sinus tachycardia. Low-voltage complexes in limb leads were appreciated as well as a corrected QT interval of 420 milliseconds (within reference range). Echocardiography was normal (visual ejection fraction of 60%).
The patient was unable to tolerate methotrexate due to excessive nausea; he was started on oral acitretin 25 mg once daily. There was no improvement in psoriasis over the following week, and he reported mild upper abdominal discomfort. He did not have any chest pain or dyspnea, and his pulse and blood pressure were normal. Serum electrolytes, liver function, lipid profile, and an ultrasound of the abdomen revealed no abnormalities. A repeat ECG showed no changes, and cardiac biomarkers were not elevated. Two days later, the patient collapsed while still in the hospital. A cardiac monitor and ECG showed ventricular tachycardia (VT)(Figure 2B); however, serum electrolytes, calcium, magnesium, and phosphorus levels were within reference range. Aggressive resuscitative measures including multiple attempts at cardioversion with up to 200 J (biphasic) and intravenous amiodarone infusion failed to revive the patient, and he died.
Proinflammatory cytokines such as IL-6 and tumor necrosis factor α are increased in young people with ventricular arrhythmias who have no evidence of myocardial injury (MI), suggesting an inflammatory background is involved.1 Psoriasis, a common immune-mediated inflammatory disease, has a chronic state of systemic inflammation with notably higher serum levels of tumor necrosis factor α, IFN-γ, IL-6, IL-8, IL-12, and IL-18 compared to controls.2 This inflammation is not confined to skin but can involve blood vessels, joints, and the liver, as demonstrated by increased fluorodeoxyglucose uptake.3 It also seems to exert its influence on supraventricular beat development in patients with psoriasis who do not have a history of CVEs.4 Tumor necrosis factor α is one of the major cytokines playing a role in the inflammatory process of psoriasis. Studies have shown serum levels of tumor necrosis factor α to correlate with the clinical symptoms of heart failure and to supraventricular arrhythmia in animal models.4 Various extreme CVEs can be an expression of this ongoing dynamic process. It would be interesting to know which specific factors among these inflammatory cytokines lead to rhythm irregularities.
Another theory is that young patients may experience micro-MI during the disease course. These small infarcted areas may act as aberrant pulse generators or lead to conduction disturbances. One study found increased correct QT interval dispersion, a predictor of ventricular arrhythmias, to be associated with psoriasis.5 A nationwide population-based matched cohort study by Chiu et al6 revealed that patients with psoriasis have a higher risk for arrhythmia independent of traditional cardiovascular risk factors. Our patient also had severe unstable psoriasis for 8 years that may have led to increased accumulation of proarrhythmogenic cytokines in the heart and could have led to VT.
Acitretin as a potential cause of sudden cardiac death remains a possibility in our case; however, the exact mechanism leading to such sudden arrhythmia is lacking. Acitretin is known to increase serum triglycerides and cholesterol, specifically by shifting high-density lipoproteins to low-density lipoproteins, thereby increasing the risk for CVE. However, it takes time for such derangement to occur, eventually leading to CVE. Mittal et al7 reported a psoriasis patient who died secondary to MI after 5 days of low-dose acitretin. Lack of evidence makes acitretin a less likely cause of mortality.
We present a case of sudden cardiac death secondary to VT in a young patient with psoriasis and no other traditional cardiovascular risk factors. This case highlights the importance of being vigilant for adverse CVEs such as arrhythmia in psoriatic patients, especially in younger patients with severe unstable disease.
- Kowalewski M, Urban M, Mroczko B, et al. Proinflammatory cytokines (IL-6, TNF-alpha) and cardiac troponin I (cTnI) in serum of young people with ventricular arrhythmias. Pol Arch Med Wewn. 2002;108:647-651.
- Arican O, Aral M, Sasmaz S, et al. Serum levels of TNF-alpha, IFN-gamma, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005;2005:273-279.
- Mehta NN, Yu Y, Saboury B, et al. Systemic and vascular inflammation in patients with moderate to severe psoriasis as measured by [18F]-fluorodeoxyglucose positron emission tomography-computed tomography (FDG-PET/CT): a pilot study. Arch Dermatol. 2011;147:1031-1039.
- Markuszeski L, Bissinger A, Janusz I, et al. Heart rate and arrhythmia in patients with psoriasis vulgaris. Arch Med Res. 2007;38:64-69.
- Simsek H, Sahin M, Akyol A, et al. Increased risk of atrial and ventricular arrhythmia in long-lasting psoriasis patients. ScientificWorldJournal. 2013;2013:901215.
- Chiu HY, Chang WL, Huang WF, et al. Increased risk of arrhythmia in patients with psoriatic disease: a nationwide population-based matched cohort study. J Am Acad Dermatol. 2015;73:429-438.
- Mittal R, Malhotra S, Pandhi P, et al. Efficacy and safety of combination acitretin and pioglitazone therapy in patients with moderate to severe chronic plaque-type psoriasis: a randomized, double-blind, placebo-controlled clinical trial. Arch Dermatol. 2009;145:387-393.
To the Editor:
The evolution in the understanding of psoriasis and psoriatic arthritis has unfolded many new facets of this immune-mediated inflammatory disease. Once considered to be just a cutaneous disease, psoriasis is not actually confined to skin but can involve almost any other system of the body. Cardiovascular morbidity and mortality are the major concerns in patients with psoriasis. We report the sudden death of a young man with severe psoriasis.
A 31-year-old man was admitted for severe psoriasis with pustular exacerbation (Figures 1A and 1B). He had moderate to severe unstable disease during the last 8 years and was managed with oral methotrexate (0.3–0.5 mg/kg/wk). He was not compliant with treatment, which led to multiple relapses. There was no personal or family history of risk factors for cardiovascular events (CVEs). At the time of present hospitalization, his vital parameters were normal. Physical examination revealed erythematous scaly plaques on more than 75% of the body surface area. Multiple pustules also were noted, often coalescing to form plaques (Figure 1C). Baseline investigations consisting of complete blood cell count, lipid profile, liver and renal functions, and chest radiography were within reference range. Baseline electrocardiogram (ECG) at admission was unremarkable (Figure 2A), except for sinus tachycardia. Low-voltage complexes in limb leads were appreciated as well as a corrected QT interval of 420 milliseconds (within reference range). Echocardiography was normal (visual ejection fraction of 60%).


The patient was unable to tolerate methotrexate due to excessive nausea; he was started on oral acitretin 25 mg once daily. There was no improvement in psoriasis over the following week, and he reported mild upper abdominal discomfort. He did not have any chest pain or dyspnea, and his pulse and blood pressure were normal. Serum electrolytes, liver function, lipid profile, and an ultrasound of the abdomen revealed no abnormalities. A repeat ECG showed no changes, and cardiac biomarkers were not elevated. Two days later, the patient collapsed while still in the hospital. A cardiac monitor and ECG showed ventricular tachycardia (VT)(Figure 2B); however, serum electrolytes, calcium, magnesium, and phosphorus levels were within reference range. Aggressive resuscitative measures including multiple attempts at cardioversion with up to 200 J (biphasic) and intravenous amiodarone infusion failed to revive the patient, and he died.
Proinflammatory cytokines such as IL-6 and tumor necrosis factor α are increased in young people with ventricular arrhythmias who have no evidence of myocardial injury (MI), suggesting an inflammatory background is involved.1 Psoriasis, a common immune-mediated inflammatory disease, has a chronic state of systemic inflammation with notably higher serum levels of tumor necrosis factor α, IFN-γ, IL-6, IL-8, IL-12, and IL-18 compared to controls.2 This inflammation is not confined to skin but can involve blood vessels, joints, and the liver, as demonstrated by increased fluorodeoxyglucose uptake.3 It also seems to exert its influence on supraventricular beat development in patients with psoriasis who do not have a history of CVEs.4 Tumor necrosis factor α is one of the major cytokines playing a role in the inflammatory process of psoriasis. Studies have shown serum levels of tumor necrosis factor α to correlate with the clinical symptoms of heart failure and to supraventricular arrhythmia in animal models.4 Various extreme CVEs can be an expression of this ongoing dynamic process. It would be interesting to know which specific factors among these inflammatory cytokines lead to rhythm irregularities.
Another theory is that young patients may experience micro-MI during the disease course. These small infarcted areas may act as aberrant pulse generators or lead to conduction disturbances. One study found increased correct QT interval dispersion, a predictor of ventricular arrhythmias, to be associated with psoriasis.5 A nationwide population-based matched cohort study by Chiu et al6 revealed that patients with psoriasis have a higher risk for arrhythmia independent of traditional cardiovascular risk factors. Our patient also had severe unstable psoriasis for 8 years that may have led to increased accumulation of proarrhythmogenic cytokines in the heart and could have led to VT.
Acitretin as a potential cause of sudden cardiac death remains a possibility in our case; however, the exact mechanism leading to such sudden arrhythmia is lacking. Acitretin is known to increase serum triglycerides and cholesterol, specifically by shifting high-density lipoproteins to low-density lipoproteins, thereby increasing the risk for CVE. However, it takes time for such derangement to occur, eventually leading to CVE. Mittal et al7 reported a psoriasis patient who died secondary to MI after 5 days of low-dose acitretin. Lack of evidence makes acitretin a less likely cause of mortality.
We present a case of sudden cardiac death secondary to VT in a young patient with psoriasis and no other traditional cardiovascular risk factors. This case highlights the importance of being vigilant for adverse CVEs such as arrhythmia in psoriatic patients, especially in younger patients with severe unstable disease.
To the Editor:
The evolution in the understanding of psoriasis and psoriatic arthritis has unfolded many new facets of this immune-mediated inflammatory disease. Once considered to be just a cutaneous disease, psoriasis is not actually confined to skin but can involve almost any other system of the body. Cardiovascular morbidity and mortality are the major concerns in patients with psoriasis. We report the sudden death of a young man with severe psoriasis.
A 31-year-old man was admitted for severe psoriasis with pustular exacerbation (Figures 1A and 1B). He had moderate to severe unstable disease during the last 8 years and was managed with oral methotrexate (0.3–0.5 mg/kg/wk). He was not compliant with treatment, which led to multiple relapses. There was no personal or family history of risk factors for cardiovascular events (CVEs). At the time of present hospitalization, his vital parameters were normal. Physical examination revealed erythematous scaly plaques on more than 75% of the body surface area. Multiple pustules also were noted, often coalescing to form plaques (Figure 1C). Baseline investigations consisting of complete blood cell count, lipid profile, liver and renal functions, and chest radiography were within reference range. Baseline electrocardiogram (ECG) at admission was unremarkable (Figure 2A), except for sinus tachycardia. Low-voltage complexes in limb leads were appreciated as well as a corrected QT interval of 420 milliseconds (within reference range). Echocardiography was normal (visual ejection fraction of 60%).


The patient was unable to tolerate methotrexate due to excessive nausea; he was started on oral acitretin 25 mg once daily. There was no improvement in psoriasis over the following week, and he reported mild upper abdominal discomfort. He did not have any chest pain or dyspnea, and his pulse and blood pressure were normal. Serum electrolytes, liver function, lipid profile, and an ultrasound of the abdomen revealed no abnormalities. A repeat ECG showed no changes, and cardiac biomarkers were not elevated. Two days later, the patient collapsed while still in the hospital. A cardiac monitor and ECG showed ventricular tachycardia (VT)(Figure 2B); however, serum electrolytes, calcium, magnesium, and phosphorus levels were within reference range. Aggressive resuscitative measures including multiple attempts at cardioversion with up to 200 J (biphasic) and intravenous amiodarone infusion failed to revive the patient, and he died.
Proinflammatory cytokines such as IL-6 and tumor necrosis factor α are increased in young people with ventricular arrhythmias who have no evidence of myocardial injury (MI), suggesting an inflammatory background is involved.1 Psoriasis, a common immune-mediated inflammatory disease, has a chronic state of systemic inflammation with notably higher serum levels of tumor necrosis factor α, IFN-γ, IL-6, IL-8, IL-12, and IL-18 compared to controls.2 This inflammation is not confined to skin but can involve blood vessels, joints, and the liver, as demonstrated by increased fluorodeoxyglucose uptake.3 It also seems to exert its influence on supraventricular beat development in patients with psoriasis who do not have a history of CVEs.4 Tumor necrosis factor α is one of the major cytokines playing a role in the inflammatory process of psoriasis. Studies have shown serum levels of tumor necrosis factor α to correlate with the clinical symptoms of heart failure and to supraventricular arrhythmia in animal models.4 Various extreme CVEs can be an expression of this ongoing dynamic process. It would be interesting to know which specific factors among these inflammatory cytokines lead to rhythm irregularities.
Another theory is that young patients may experience micro-MI during the disease course. These small infarcted areas may act as aberrant pulse generators or lead to conduction disturbances. One study found increased correct QT interval dispersion, a predictor of ventricular arrhythmias, to be associated with psoriasis.5 A nationwide population-based matched cohort study by Chiu et al6 revealed that patients with psoriasis have a higher risk for arrhythmia independent of traditional cardiovascular risk factors. Our patient also had severe unstable psoriasis for 8 years that may have led to increased accumulation of proarrhythmogenic cytokines in the heart and could have led to VT.
Acitretin as a potential cause of sudden cardiac death remains a possibility in our case; however, the exact mechanism leading to such sudden arrhythmia is lacking. Acitretin is known to increase serum triglycerides and cholesterol, specifically by shifting high-density lipoproteins to low-density lipoproteins, thereby increasing the risk for CVE. However, it takes time for such derangement to occur, eventually leading to CVE. Mittal et al7 reported a psoriasis patient who died secondary to MI after 5 days of low-dose acitretin. Lack of evidence makes acitretin a less likely cause of mortality.
We present a case of sudden cardiac death secondary to VT in a young patient with psoriasis and no other traditional cardiovascular risk factors. This case highlights the importance of being vigilant for adverse CVEs such as arrhythmia in psoriatic patients, especially in younger patients with severe unstable disease.
- Kowalewski M, Urban M, Mroczko B, et al. Proinflammatory cytokines (IL-6, TNF-alpha) and cardiac troponin I (cTnI) in serum of young people with ventricular arrhythmias. Pol Arch Med Wewn. 2002;108:647-651.
- Arican O, Aral M, Sasmaz S, et al. Serum levels of TNF-alpha, IFN-gamma, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005;2005:273-279.
- Mehta NN, Yu Y, Saboury B, et al. Systemic and vascular inflammation in patients with moderate to severe psoriasis as measured by [18F]-fluorodeoxyglucose positron emission tomography-computed tomography (FDG-PET/CT): a pilot study. Arch Dermatol. 2011;147:1031-1039.
- Markuszeski L, Bissinger A, Janusz I, et al. Heart rate and arrhythmia in patients with psoriasis vulgaris. Arch Med Res. 2007;38:64-69.
- Simsek H, Sahin M, Akyol A, et al. Increased risk of atrial and ventricular arrhythmia in long-lasting psoriasis patients. ScientificWorldJournal. 2013;2013:901215.
- Chiu HY, Chang WL, Huang WF, et al. Increased risk of arrhythmia in patients with psoriatic disease: a nationwide population-based matched cohort study. J Am Acad Dermatol. 2015;73:429-438.
- Mittal R, Malhotra S, Pandhi P, et al. Efficacy and safety of combination acitretin and pioglitazone therapy in patients with moderate to severe chronic plaque-type psoriasis: a randomized, double-blind, placebo-controlled clinical trial. Arch Dermatol. 2009;145:387-393.
- Kowalewski M, Urban M, Mroczko B, et al. Proinflammatory cytokines (IL-6, TNF-alpha) and cardiac troponin I (cTnI) in serum of young people with ventricular arrhythmias. Pol Arch Med Wewn. 2002;108:647-651.
- Arican O, Aral M, Sasmaz S, et al. Serum levels of TNF-alpha, IFN-gamma, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005;2005:273-279.
- Mehta NN, Yu Y, Saboury B, et al. Systemic and vascular inflammation in patients with moderate to severe psoriasis as measured by [18F]-fluorodeoxyglucose positron emission tomography-computed tomography (FDG-PET/CT): a pilot study. Arch Dermatol. 2011;147:1031-1039.
- Markuszeski L, Bissinger A, Janusz I, et al. Heart rate and arrhythmia in patients with psoriasis vulgaris. Arch Med Res. 2007;38:64-69.
- Simsek H, Sahin M, Akyol A, et al. Increased risk of atrial and ventricular arrhythmia in long-lasting psoriasis patients. ScientificWorldJournal. 2013;2013:901215.
- Chiu HY, Chang WL, Huang WF, et al. Increased risk of arrhythmia in patients with psoriatic disease: a nationwide population-based matched cohort study. J Am Acad Dermatol. 2015;73:429-438.
- Mittal R, Malhotra S, Pandhi P, et al. Efficacy and safety of combination acitretin and pioglitazone therapy in patients with moderate to severe chronic plaque-type psoriasis: a randomized, double-blind, placebo-controlled clinical trial. Arch Dermatol. 2009;145:387-393.
Practice Points
- Low-grade chronic inflammation in patients with psoriasis can lead to vascular inflammation, which can further lead to the development of major adverse cardiovascular events (CVEs) and arrhythmia.
- The need for a multidisciplinary approach and close monitoring of cardiovascular risk factors in patients with psoriasis to prevent a CVE is vital.
- Baseline electrocardiogram and biomarkers for cardiovascular disease also should be performed in young patients with severe or unstable psoriasis.
Methotrexate-associated hepatotoxicity risk differs between psoriasis, PsA, and RA patients
, in a large population-based study published in the Journal of the American Academy of Dermatology.
“These findings suggest that conservative liver monitoring is warranted in patients receiving methotrexate for psoriatic disease,” particularly psoriasis, the investigators concluded.
Joel M. Gelfand, MD, professor of dermatology at the University of Pennsylvania, Philadelphia, and colleagues performed a population-based cohort study of patients in Denmark in a hospital clinic with psoriasis, PsA, or RA who received methotrexate between 1997 and 2015; they compared rates of mild liver disease, moderate to severe liver disease, cirrhosis, and cirrhosis-related hospitalization between the groups.
In total, 5,687 patients with psoriasis, 6,520 patients with PsA, and 28,030 patients with RA met inclusion criteria: receiving one or more methotrexate prescriptions or having been dispensed methotrexate at the hospital clinic during the study period. Patients with RA tended to be older (mean, 59.7 years) and the group consisted of more women (71.6%) than the psoriasis patients (47.7 years; 45.3% women) or PsA patients (50.7 years; 57.3% women). In the groups, 17.9% to 23.5% had a history of smoking, and 2.8% to 7.4% had a history of alcohol abuse; the rates of diabetes were between 7.0% and 8.3%, and hyperlipidemia or statin use between 13.6% and 16.4%.
The average weekly methotrexate dose was similar in the three patient groups (a mean of 19.2-19.9 mg). However, the duration of methotrexate use among patients with RA was longer (a mean of 72.1 weeks) compared with the PsA (56.3 weeks) and psoriasis (43.0 weeks) groups. In addition, 50% of the patients in the RA group discontinued treatment after 80 months, 50% in the PsA group discontinued after 54 months, and 50% of patients with psoriasis discontinued after 26 months.
Patients with RA also had a higher cumulative methotrexate dose (a mean of 4.0 g) compared with PsA (3.0 g) and psoriasis (2.1) groups.
When the researchers looked at the incidence rate (IR) for the different categories of liver disease, they found the following differences:
- Mild liver disease: The IR per 1,000 person-years for patients with psoriasis was 4.22 per 1,000 person-years (95% confidence interval, 3.61-4.91), compared with 2.39 per 1,000 person-years (95% CI, 1.95-2.91) for patients with PsA, and 1.39 per 1,000 person-years (95% CI, 1.25-1.55) for patients with RA.
- Moderate to severe liver disease: The IR for patients with psoriasis was 0.98 per 1,000 person years (95% CI, 0.70-1.33), compared with 0.51 (95% CI, 0.32-0.77) for patients with PsA, and 0.46 (95% CI, 0.37-0.55) for patients with RA.
- Cirrhosis: The IR for patients with psoriasis was 1.89 per 1,000 person years (95% CI, 1.49-2.37), compared with 0.84 (95% CI, 0.59-1.16) for patients with PsA, and 0.42 (95% CI, 0.34-0.51) for patients with RA.
- Cirrhosis-related hospitalization: This was the least common outcome, with an IR per 1,000 person years of 0.73 (95% CI, 0.49-1.05) for patients with psoriasis, 0.32 (95% CI, 0.18-0.54) for patients with PsA, and 0.22 (95% CI, 0.17-0.29) for patients with RA.
When results were adjusted with Cox regression analyses, the psoriasis group had a significantly increased risk compared with the RA group with regard to mild liver disease (hazard ratio, 2.22; 95% CI, 1.81-2.72), moderate to-severe liver disease (HR, 1.56; 95% CI, 1.05-2.31), cirrhosis (HR, 3.38; 95% CI, 2.44-4.68), and cirrhosis-related hospitalization (HR, 2.25; 95% CI, 1.37-3.69). Compared with patients with RA, patients with PsA had a significantly increased risk of mild liver disease (HR, 1.27; 95% CI, 1.01-1.60) and cirrhosis (HR, 1.63; 95% CI, 1.10-2.42), but not moderate to severe liver disease or hospitalizations related to cirrhosis.
The researchers noted it is unclear why there was a difference in risk between the three groups of patients.
“While such differences in hepatotoxicity risk were previously attributed to differences in rates of alcoholism, obesity, diabetes, and other comorbidities between the disease populations, our study finds that the underlying disease influences liver disease risk independent of age, sex, smoking, alcohol use, diabetes, hyperlipidemia, overall comorbidity, and weekly methotrexate dose,” wrote Dr. Gelfand and colleagues.
As far as they know, their study “ is one of the first and largest population-based studies to directly compare” liver disease in these three groups of patients on methotrexate, they wrote, noting that earlier studies were smaller and frequently used indirect hepatic injury measures.
Limitations of the study included the inability to account for disease severity as well as the potential for disease misclassification, surveillance bias, and confounding by unmeasured variables such as body mass index. Further, the results do not show whether “liver disease is attributed to methotrexate use, the underlying disease, or a combination of both,” the researchers noted.
Four authors report relationships in the form of consultancies, continuing medical information payments, deputy editor positions, fellowship support, individual or spousal honoraria, patents, research grants, and/or speaker positions with various pharmaceutical companies, medical journals, societies, and other organizations; two authors had no disclosures. There was no funding source.
, in a large population-based study published in the Journal of the American Academy of Dermatology.
“These findings suggest that conservative liver monitoring is warranted in patients receiving methotrexate for psoriatic disease,” particularly psoriasis, the investigators concluded.
Joel M. Gelfand, MD, professor of dermatology at the University of Pennsylvania, Philadelphia, and colleagues performed a population-based cohort study of patients in Denmark in a hospital clinic with psoriasis, PsA, or RA who received methotrexate between 1997 and 2015; they compared rates of mild liver disease, moderate to severe liver disease, cirrhosis, and cirrhosis-related hospitalization between the groups.
In total, 5,687 patients with psoriasis, 6,520 patients with PsA, and 28,030 patients with RA met inclusion criteria: receiving one or more methotrexate prescriptions or having been dispensed methotrexate at the hospital clinic during the study period. Patients with RA tended to be older (mean, 59.7 years) and the group consisted of more women (71.6%) than the psoriasis patients (47.7 years; 45.3% women) or PsA patients (50.7 years; 57.3% women). In the groups, 17.9% to 23.5% had a history of smoking, and 2.8% to 7.4% had a history of alcohol abuse; the rates of diabetes were between 7.0% and 8.3%, and hyperlipidemia or statin use between 13.6% and 16.4%.
The average weekly methotrexate dose was similar in the three patient groups (a mean of 19.2-19.9 mg). However, the duration of methotrexate use among patients with RA was longer (a mean of 72.1 weeks) compared with the PsA (56.3 weeks) and psoriasis (43.0 weeks) groups. In addition, 50% of the patients in the RA group discontinued treatment after 80 months, 50% in the PsA group discontinued after 54 months, and 50% of patients with psoriasis discontinued after 26 months.
Patients with RA also had a higher cumulative methotrexate dose (a mean of 4.0 g) compared with PsA (3.0 g) and psoriasis (2.1) groups.
When the researchers looked at the incidence rate (IR) for the different categories of liver disease, they found the following differences:
- Mild liver disease: The IR per 1,000 person-years for patients with psoriasis was 4.22 per 1,000 person-years (95% confidence interval, 3.61-4.91), compared with 2.39 per 1,000 person-years (95% CI, 1.95-2.91) for patients with PsA, and 1.39 per 1,000 person-years (95% CI, 1.25-1.55) for patients with RA.
- Moderate to severe liver disease: The IR for patients with psoriasis was 0.98 per 1,000 person years (95% CI, 0.70-1.33), compared with 0.51 (95% CI, 0.32-0.77) for patients with PsA, and 0.46 (95% CI, 0.37-0.55) for patients with RA.
- Cirrhosis: The IR for patients with psoriasis was 1.89 per 1,000 person years (95% CI, 1.49-2.37), compared with 0.84 (95% CI, 0.59-1.16) for patients with PsA, and 0.42 (95% CI, 0.34-0.51) for patients with RA.
- Cirrhosis-related hospitalization: This was the least common outcome, with an IR per 1,000 person years of 0.73 (95% CI, 0.49-1.05) for patients with psoriasis, 0.32 (95% CI, 0.18-0.54) for patients with PsA, and 0.22 (95% CI, 0.17-0.29) for patients with RA.
When results were adjusted with Cox regression analyses, the psoriasis group had a significantly increased risk compared with the RA group with regard to mild liver disease (hazard ratio, 2.22; 95% CI, 1.81-2.72), moderate to-severe liver disease (HR, 1.56; 95% CI, 1.05-2.31), cirrhosis (HR, 3.38; 95% CI, 2.44-4.68), and cirrhosis-related hospitalization (HR, 2.25; 95% CI, 1.37-3.69). Compared with patients with RA, patients with PsA had a significantly increased risk of mild liver disease (HR, 1.27; 95% CI, 1.01-1.60) and cirrhosis (HR, 1.63; 95% CI, 1.10-2.42), but not moderate to severe liver disease or hospitalizations related to cirrhosis.
The researchers noted it is unclear why there was a difference in risk between the three groups of patients.
“While such differences in hepatotoxicity risk were previously attributed to differences in rates of alcoholism, obesity, diabetes, and other comorbidities between the disease populations, our study finds that the underlying disease influences liver disease risk independent of age, sex, smoking, alcohol use, diabetes, hyperlipidemia, overall comorbidity, and weekly methotrexate dose,” wrote Dr. Gelfand and colleagues.
As far as they know, their study “ is one of the first and largest population-based studies to directly compare” liver disease in these three groups of patients on methotrexate, they wrote, noting that earlier studies were smaller and frequently used indirect hepatic injury measures.
Limitations of the study included the inability to account for disease severity as well as the potential for disease misclassification, surveillance bias, and confounding by unmeasured variables such as body mass index. Further, the results do not show whether “liver disease is attributed to methotrexate use, the underlying disease, or a combination of both,” the researchers noted.
Four authors report relationships in the form of consultancies, continuing medical information payments, deputy editor positions, fellowship support, individual or spousal honoraria, patents, research grants, and/or speaker positions with various pharmaceutical companies, medical journals, societies, and other organizations; two authors had no disclosures. There was no funding source.
, in a large population-based study published in the Journal of the American Academy of Dermatology.
“These findings suggest that conservative liver monitoring is warranted in patients receiving methotrexate for psoriatic disease,” particularly psoriasis, the investigators concluded.
Joel M. Gelfand, MD, professor of dermatology at the University of Pennsylvania, Philadelphia, and colleagues performed a population-based cohort study of patients in Denmark in a hospital clinic with psoriasis, PsA, or RA who received methotrexate between 1997 and 2015; they compared rates of mild liver disease, moderate to severe liver disease, cirrhosis, and cirrhosis-related hospitalization between the groups.
In total, 5,687 patients with psoriasis, 6,520 patients with PsA, and 28,030 patients with RA met inclusion criteria: receiving one or more methotrexate prescriptions or having been dispensed methotrexate at the hospital clinic during the study period. Patients with RA tended to be older (mean, 59.7 years) and the group consisted of more women (71.6%) than the psoriasis patients (47.7 years; 45.3% women) or PsA patients (50.7 years; 57.3% women). In the groups, 17.9% to 23.5% had a history of smoking, and 2.8% to 7.4% had a history of alcohol abuse; the rates of diabetes were between 7.0% and 8.3%, and hyperlipidemia or statin use between 13.6% and 16.4%.
The average weekly methotrexate dose was similar in the three patient groups (a mean of 19.2-19.9 mg). However, the duration of methotrexate use among patients with RA was longer (a mean of 72.1 weeks) compared with the PsA (56.3 weeks) and psoriasis (43.0 weeks) groups. In addition, 50% of the patients in the RA group discontinued treatment after 80 months, 50% in the PsA group discontinued after 54 months, and 50% of patients with psoriasis discontinued after 26 months.
Patients with RA also had a higher cumulative methotrexate dose (a mean of 4.0 g) compared with PsA (3.0 g) and psoriasis (2.1) groups.
When the researchers looked at the incidence rate (IR) for the different categories of liver disease, they found the following differences:
- Mild liver disease: The IR per 1,000 person-years for patients with psoriasis was 4.22 per 1,000 person-years (95% confidence interval, 3.61-4.91), compared with 2.39 per 1,000 person-years (95% CI, 1.95-2.91) for patients with PsA, and 1.39 per 1,000 person-years (95% CI, 1.25-1.55) for patients with RA.
- Moderate to severe liver disease: The IR for patients with psoriasis was 0.98 per 1,000 person years (95% CI, 0.70-1.33), compared with 0.51 (95% CI, 0.32-0.77) for patients with PsA, and 0.46 (95% CI, 0.37-0.55) for patients with RA.
- Cirrhosis: The IR for patients with psoriasis was 1.89 per 1,000 person years (95% CI, 1.49-2.37), compared with 0.84 (95% CI, 0.59-1.16) for patients with PsA, and 0.42 (95% CI, 0.34-0.51) for patients with RA.
- Cirrhosis-related hospitalization: This was the least common outcome, with an IR per 1,000 person years of 0.73 (95% CI, 0.49-1.05) for patients with psoriasis, 0.32 (95% CI, 0.18-0.54) for patients with PsA, and 0.22 (95% CI, 0.17-0.29) for patients with RA.
When results were adjusted with Cox regression analyses, the psoriasis group had a significantly increased risk compared with the RA group with regard to mild liver disease (hazard ratio, 2.22; 95% CI, 1.81-2.72), moderate to-severe liver disease (HR, 1.56; 95% CI, 1.05-2.31), cirrhosis (HR, 3.38; 95% CI, 2.44-4.68), and cirrhosis-related hospitalization (HR, 2.25; 95% CI, 1.37-3.69). Compared with patients with RA, patients with PsA had a significantly increased risk of mild liver disease (HR, 1.27; 95% CI, 1.01-1.60) and cirrhosis (HR, 1.63; 95% CI, 1.10-2.42), but not moderate to severe liver disease or hospitalizations related to cirrhosis.
The researchers noted it is unclear why there was a difference in risk between the three groups of patients.
“While such differences in hepatotoxicity risk were previously attributed to differences in rates of alcoholism, obesity, diabetes, and other comorbidities between the disease populations, our study finds that the underlying disease influences liver disease risk independent of age, sex, smoking, alcohol use, diabetes, hyperlipidemia, overall comorbidity, and weekly methotrexate dose,” wrote Dr. Gelfand and colleagues.
As far as they know, their study “ is one of the first and largest population-based studies to directly compare” liver disease in these three groups of patients on methotrexate, they wrote, noting that earlier studies were smaller and frequently used indirect hepatic injury measures.
Limitations of the study included the inability to account for disease severity as well as the potential for disease misclassification, surveillance bias, and confounding by unmeasured variables such as body mass index. Further, the results do not show whether “liver disease is attributed to methotrexate use, the underlying disease, or a combination of both,” the researchers noted.
Four authors report relationships in the form of consultancies, continuing medical information payments, deputy editor positions, fellowship support, individual or spousal honoraria, patents, research grants, and/or speaker positions with various pharmaceutical companies, medical journals, societies, and other organizations; two authors had no disclosures. There was no funding source.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Pandemic puts patients with psoriatic disease off seeking medical help
More than half of respondents to a recent survey looking at how the COVID-19 pandemic has affected people with psoriasis or psoriatic arthritis (PsA) said that they had avoided seeking medical care in person with a doctor or at a hospital.
Moreover, around a quarter had their appointment with a rheumatologist canceled, rescheduled, or conducted virtually. Another 1 in 10 had their treatment plan disrupted, and 6% had to change or stop treatment entirely.
The mental health impact of living with these conditions during the pandemic was also notable, said Rachael Manion, the executive director of the Canadian Association of Psoriasis Patients (CAPP), which conducted the survey in collaboration with the Canadian Psoriasis Network (CPN) and Unmasking Psoriasis.
“It’s important to know that there have been a lot of different impacts of the pandemic on people living with psoriatic arthritis and psoriasis. Mental health in particular has had a really big hit as a result,” she said at the Canadian Arthritis Research Conference: Research with Impact.
“About half of the people who responded to our survey noted that their mental health was ‘worse’ or ‘much worse’ during the pandemic,” she said at the meeting, which was sponsored by the Arthritis Society, the Canadian Rheumatology Association, and Canada’s Institute of Musculoskeletal Health and Arthritis. Anxiety and feelings of isolation were reported by a respective 57% and 58% of respondents, and 40% reported depression.
“We can compare that to our earlier information around depression,” Ms. Manion said, which showed that, prior to the pandemic, 24% of people with psoriasis and 23% of those with PsA had said they experienced depression.
“What I found alarming looking at these results was that about a third of people were experiencing despair. Now that’s a really big, scary, overwhelming emotion that has a lot of burden on your mental health,” Ms. Manion said.
Despite the substantial effects on mental health, only 29% of respondents said they had been able to access mental health services during the pandemic.
To look at the impact of the COVID-19 pandemic on the psoriasis and PsA community in Canada, three patient advocacy groups – CAPP, CPN, and Unmasking Psoriasis – codeveloped a survey to look at the disease experience before and after the start of the COVID-19 pandemic. The survey was performed once, with 830 respondents providing information on their lives with psoriasis or PsA in the months before the start of the pandemic and at the time they were surveyed in September and October 2020.
Most of the survey respondents lived in Ontario, Quebec, British Columbia, or Alberta, although other provinces or territories were represented. Almost all respondents (96%) had psoriasis, and 60% also had PsA.
Pre-COVID, nearly half (49%) of patients said that they had not been seen by a rheumatologist, and 39% had not seen a dermatologist for treatment. Asked why, 56% and 27%, respectively, had not been referred, 9% and 15% said they had no specialist located nearby, and 7% and 10% stated that the wait list was too long.
“This tells us that there’s a lot more work that can be done and a lot more education of general practitioners and family medicine professionals about the benefits and the value of specialized care for psoriatic arthritis,” Ms. Manion suggested.
Before the pandemic, joint pain was occurring in 88% of patients, stiffness in 71%, and joint swelling in 67%. Disease flares or sudden periods of worsening occurred on a daily basis for 17%, and around one in five (21%) experienced multiple flares every month.
Prepandemic data also highlighted the negative impact that living with psoriasis or PsA has on people’s ability to sleep, interactions and intimacy with others, and on their school or work lives.
During the pandemic, around a quarter (26%) of respondents said they had worse or much worse access to employment, as well as its benefits such as a stable income (24%). A minority of respondent also described worse access to prescription medication (15%) and over-the-counter medication (13%).
“There are all kinds of things going on for patients in our community: changes to their work, changes to their drug coverage, their ability to sleep and sleep well, their mental health, and their ability to access care and treatments as part of their disease management,” Ms. Manion said.
Her final message to health care professionals was: “I just want to encourage you to continue to check in with your patients about what their experiences have been during the pandemic, and to really consider those impacts as you’re working with them to manage their disease.”
The survey received funding support from AbbVie, Bausch Health, Boehringer Ingelheim, Janssen, LEO Pharma, and Novartis.
More than half of respondents to a recent survey looking at how the COVID-19 pandemic has affected people with psoriasis or psoriatic arthritis (PsA) said that they had avoided seeking medical care in person with a doctor or at a hospital.
Moreover, around a quarter had their appointment with a rheumatologist canceled, rescheduled, or conducted virtually. Another 1 in 10 had their treatment plan disrupted, and 6% had to change or stop treatment entirely.
The mental health impact of living with these conditions during the pandemic was also notable, said Rachael Manion, the executive director of the Canadian Association of Psoriasis Patients (CAPP), which conducted the survey in collaboration with the Canadian Psoriasis Network (CPN) and Unmasking Psoriasis.
“It’s important to know that there have been a lot of different impacts of the pandemic on people living with psoriatic arthritis and psoriasis. Mental health in particular has had a really big hit as a result,” she said at the Canadian Arthritis Research Conference: Research with Impact.
“About half of the people who responded to our survey noted that their mental health was ‘worse’ or ‘much worse’ during the pandemic,” she said at the meeting, which was sponsored by the Arthritis Society, the Canadian Rheumatology Association, and Canada’s Institute of Musculoskeletal Health and Arthritis. Anxiety and feelings of isolation were reported by a respective 57% and 58% of respondents, and 40% reported depression.
“We can compare that to our earlier information around depression,” Ms. Manion said, which showed that, prior to the pandemic, 24% of people with psoriasis and 23% of those with PsA had said they experienced depression.
“What I found alarming looking at these results was that about a third of people were experiencing despair. Now that’s a really big, scary, overwhelming emotion that has a lot of burden on your mental health,” Ms. Manion said.
Despite the substantial effects on mental health, only 29% of respondents said they had been able to access mental health services during the pandemic.
To look at the impact of the COVID-19 pandemic on the psoriasis and PsA community in Canada, three patient advocacy groups – CAPP, CPN, and Unmasking Psoriasis – codeveloped a survey to look at the disease experience before and after the start of the COVID-19 pandemic. The survey was performed once, with 830 respondents providing information on their lives with psoriasis or PsA in the months before the start of the pandemic and at the time they were surveyed in September and October 2020.
Most of the survey respondents lived in Ontario, Quebec, British Columbia, or Alberta, although other provinces or territories were represented. Almost all respondents (96%) had psoriasis, and 60% also had PsA.
Pre-COVID, nearly half (49%) of patients said that they had not been seen by a rheumatologist, and 39% had not seen a dermatologist for treatment. Asked why, 56% and 27%, respectively, had not been referred, 9% and 15% said they had no specialist located nearby, and 7% and 10% stated that the wait list was too long.
“This tells us that there’s a lot more work that can be done and a lot more education of general practitioners and family medicine professionals about the benefits and the value of specialized care for psoriatic arthritis,” Ms. Manion suggested.
Before the pandemic, joint pain was occurring in 88% of patients, stiffness in 71%, and joint swelling in 67%. Disease flares or sudden periods of worsening occurred on a daily basis for 17%, and around one in five (21%) experienced multiple flares every month.
Prepandemic data also highlighted the negative impact that living with psoriasis or PsA has on people’s ability to sleep, interactions and intimacy with others, and on their school or work lives.
During the pandemic, around a quarter (26%) of respondents said they had worse or much worse access to employment, as well as its benefits such as a stable income (24%). A minority of respondent also described worse access to prescription medication (15%) and over-the-counter medication (13%).
“There are all kinds of things going on for patients in our community: changes to their work, changes to their drug coverage, their ability to sleep and sleep well, their mental health, and their ability to access care and treatments as part of their disease management,” Ms. Manion said.
Her final message to health care professionals was: “I just want to encourage you to continue to check in with your patients about what their experiences have been during the pandemic, and to really consider those impacts as you’re working with them to manage their disease.”
The survey received funding support from AbbVie, Bausch Health, Boehringer Ingelheim, Janssen, LEO Pharma, and Novartis.
More than half of respondents to a recent survey looking at how the COVID-19 pandemic has affected people with psoriasis or psoriatic arthritis (PsA) said that they had avoided seeking medical care in person with a doctor or at a hospital.
Moreover, around a quarter had their appointment with a rheumatologist canceled, rescheduled, or conducted virtually. Another 1 in 10 had their treatment plan disrupted, and 6% had to change or stop treatment entirely.
The mental health impact of living with these conditions during the pandemic was also notable, said Rachael Manion, the executive director of the Canadian Association of Psoriasis Patients (CAPP), which conducted the survey in collaboration with the Canadian Psoriasis Network (CPN) and Unmasking Psoriasis.
“It’s important to know that there have been a lot of different impacts of the pandemic on people living with psoriatic arthritis and psoriasis. Mental health in particular has had a really big hit as a result,” she said at the Canadian Arthritis Research Conference: Research with Impact.
“About half of the people who responded to our survey noted that their mental health was ‘worse’ or ‘much worse’ during the pandemic,” she said at the meeting, which was sponsored by the Arthritis Society, the Canadian Rheumatology Association, and Canada’s Institute of Musculoskeletal Health and Arthritis. Anxiety and feelings of isolation were reported by a respective 57% and 58% of respondents, and 40% reported depression.
“We can compare that to our earlier information around depression,” Ms. Manion said, which showed that, prior to the pandemic, 24% of people with psoriasis and 23% of those with PsA had said they experienced depression.
“What I found alarming looking at these results was that about a third of people were experiencing despair. Now that’s a really big, scary, overwhelming emotion that has a lot of burden on your mental health,” Ms. Manion said.
Despite the substantial effects on mental health, only 29% of respondents said they had been able to access mental health services during the pandemic.
To look at the impact of the COVID-19 pandemic on the psoriasis and PsA community in Canada, three patient advocacy groups – CAPP, CPN, and Unmasking Psoriasis – codeveloped a survey to look at the disease experience before and after the start of the COVID-19 pandemic. The survey was performed once, with 830 respondents providing information on their lives with psoriasis or PsA in the months before the start of the pandemic and at the time they were surveyed in September and October 2020.
Most of the survey respondents lived in Ontario, Quebec, British Columbia, or Alberta, although other provinces or territories were represented. Almost all respondents (96%) had psoriasis, and 60% also had PsA.
Pre-COVID, nearly half (49%) of patients said that they had not been seen by a rheumatologist, and 39% had not seen a dermatologist for treatment. Asked why, 56% and 27%, respectively, had not been referred, 9% and 15% said they had no specialist located nearby, and 7% and 10% stated that the wait list was too long.
“This tells us that there’s a lot more work that can be done and a lot more education of general practitioners and family medicine professionals about the benefits and the value of specialized care for psoriatic arthritis,” Ms. Manion suggested.
Before the pandemic, joint pain was occurring in 88% of patients, stiffness in 71%, and joint swelling in 67%. Disease flares or sudden periods of worsening occurred on a daily basis for 17%, and around one in five (21%) experienced multiple flares every month.
Prepandemic data also highlighted the negative impact that living with psoriasis or PsA has on people’s ability to sleep, interactions and intimacy with others, and on their school or work lives.
During the pandemic, around a quarter (26%) of respondents said they had worse or much worse access to employment, as well as its benefits such as a stable income (24%). A minority of respondent also described worse access to prescription medication (15%) and over-the-counter medication (13%).
“There are all kinds of things going on for patients in our community: changes to their work, changes to their drug coverage, their ability to sleep and sleep well, their mental health, and their ability to access care and treatments as part of their disease management,” Ms. Manion said.
Her final message to health care professionals was: “I just want to encourage you to continue to check in with your patients about what their experiences have been during the pandemic, and to really consider those impacts as you’re working with them to manage their disease.”
The survey received funding support from AbbVie, Bausch Health, Boehringer Ingelheim, Janssen, LEO Pharma, and Novartis.
FROM CARC 2021
Cumulative exposure to high-potency topical steroid doses drives osteoporosis fractures
In support of previously published case reports, in a dose-response relationship.
In a stepwise manner, the hazard ratios for major osteoporotic fracture (MOF) were found to start climbing incrementally for those with a cumulative topical steroid dose equivalent of more than 500 g of mometasone furoate when compared with exposure of 200-499 g, according to the team of investigators from the University of Copenhagen.
“Use of these drugs is very common, and we found an estimated population-attributable risk of as much as 4.3%,” the investigators reported in the study, published in JAMA Dermatology.
The retrospective cohort study drew data from the Danish National Patient Registry, which covers 99% of the country’s population. It was linked to the Danish National Prescription Registry, which captures data on pharmacy-dispensed medications. Data collected from the beginning of 2003 to the end of 2017 were evaluated.
Exposures to potent or very potent topical corticosteroids were converted into a single standard with potency equivalent to 1 mg/g of mometasone furoate. Four strata of exposure were compared to a reference exposure of 200-499 g. These were 500-999 g, 1,000-1,999 g, 2,000-9,999 g, and 10,000 g or greater.
For the first strata, the small increased risk for MOF did not reach significance (HR, 1.01; 95% confidence interval, 0.99-1.03), but each of the others did. These climbed from a 5% greater risk (HR 1.05 95% CI 1.02-1.08) for a cumulative exposure of 1,000 to 1,999 g, to a 10% greater risk (HR, 1.10; 95% CI, 1.07-1.13) for a cumulative exposure of 2,000-9,999 g, and finally to a 27% greater risk (HR, 1.27; 95% CI, 1.19-1.35) for a cumulative exposure of 10,000 g or higher.
The study included more than 700,000 individuals exposed to topical mometasone at a potency equivalent of 200 g or more over the study period. The reference group (200-499 g) was the largest (317,907 individuals). The first strata (500-999 g) included 186,359 patients; the second (1,000-1,999 g), 111,203 patients; the third (2,000-9,999 g), 94,334 patients; and the fifth (10,000 g or more), 13,448 patients.
“A 3% increase in the relative risk of osteoporosis and MOF was observed per doubling of the TCS dose,” according to the investigators.
Patients exposed to doses of high-potency topical steroids that put them at risk of MOF is limited but substantial, according to the senior author, Alexander Egeberg, MD, PhD, of the department of dermatology and allergy at Herlev and Gentofte Hospital, Copenhagen.
“It is true that the risk is modest for the average user of topical steroids,” Dr. Egeberg said in an interview. However, despite the fact that topical steroids are intended for short-term use, “2% of all our users had been exposed to the equivalent of 10,000 g of mometasone, which mean 100 tubes of 100 g.”
If the other two strata at significantly increased risk of MOF (greater than 1,000 g) are included, an additional 28% of all users are facing the potential for clinically significant osteoporosis, according to the Danish data.
The adverse effect of steroids on bone metabolism has been established previously, and several studies have linked systemic corticosteroid exposure, including inhaled corticosteroids, with increased risk of osteoporotic fracture. For example, one study showed that patients with chronic obstructive pulmonary disease on daily inhaled doses of the equivalent of fluticasone at or above 1,000 mcg for more than 4 years had about a 10% increased risk of MOF relative to those not exposed.
The data associate topical steroids with increased risk of osteoporotic fracture, but Dr. Egeberg said osteoporosis is not the only reason to use topical steroids prudently.
“It is important to keep in mind that osteoporosis and fractures are at the extreme end of the side-effect profile and that other side effects, such as striae formation, skin thinning, and dysregulated diabetes, can occur with much lower quantities of topical steroids,” Dr. Egeberg said
For avoiding this risk, “there are no specific cutoffs” recommended for topical steroids in current guidelines, but dermatologists should be aware that many of the indications for topical steroids, such as psoriasis and atopic dermatitis, involve skin with an impaired barrier function, exposing patients to an increased likelihood of absorption, according to Dr. Egeberg.
“A general rule of thumb that we use is that, if a patient with persistent disease activity requires a new prescription of the equivalent of 100 g mometasone every 1-2 months, it might be worth considering if there is a suitable alternative,” Dr. Egeberg said.
In an accompanying editorial, Rebecca D. Jackson, MD, of the division of endocrinology, diabetes, and metabolism in the department of internal medicine at Ohio State University, Columbus, agreed that no guidelines specific to avoiding the risks of topical corticosteroids are currently available, but she advised clinicians to be considering these risks nonetheless. In general, she suggested that topical steroids, like oral steroids, should be used at “the lowest dose for the shortest duration necessary to manage the underlying medical condition.”
The correlation between topical corticosteroids and increased risk of osteoporotic fracture, although not established previously in a large study, is not surprising, according to Victoria Werth, MD, chief of dermatology at the Philadelphia Veterans Affairs Hospital and professor of dermatology at the University of Pennsylvania, also in Philadelphia.
“Systemic absorption of potent topical steroids has previously been demonstrated with a rapid decrease in serum cortisol levels,” Dr. Werth said in an interview. She indicated that concern about the risk of osteoporosis imposed by use of potent steroids over large body surface areas is appropriate.
To minimize this risk, “it is reasonable to use the lowest dose of steroid possible and to try to substitute other medications when possible,” she said.
Dr. Egeberg reported financial relationships with Abbvie, Almirall, Bristol-Myers Squibb, Dermavant Sciences, Galderma, Janssen Pharmaceuticals, Eli Lilly, Novartis, Pfizer, Samsung, Bioepis, and UCB. Five authors had disclosures related to some of those pharmaceutical companies and/or others. Dr. Jackson had no disclosures.
In support of previously published case reports, in a dose-response relationship.
In a stepwise manner, the hazard ratios for major osteoporotic fracture (MOF) were found to start climbing incrementally for those with a cumulative topical steroid dose equivalent of more than 500 g of mometasone furoate when compared with exposure of 200-499 g, according to the team of investigators from the University of Copenhagen.
“Use of these drugs is very common, and we found an estimated population-attributable risk of as much as 4.3%,” the investigators reported in the study, published in JAMA Dermatology.
The retrospective cohort study drew data from the Danish National Patient Registry, which covers 99% of the country’s population. It was linked to the Danish National Prescription Registry, which captures data on pharmacy-dispensed medications. Data collected from the beginning of 2003 to the end of 2017 were evaluated.
Exposures to potent or very potent topical corticosteroids were converted into a single standard with potency equivalent to 1 mg/g of mometasone furoate. Four strata of exposure were compared to a reference exposure of 200-499 g. These were 500-999 g, 1,000-1,999 g, 2,000-9,999 g, and 10,000 g or greater.
For the first strata, the small increased risk for MOF did not reach significance (HR, 1.01; 95% confidence interval, 0.99-1.03), but each of the others did. These climbed from a 5% greater risk (HR 1.05 95% CI 1.02-1.08) for a cumulative exposure of 1,000 to 1,999 g, to a 10% greater risk (HR, 1.10; 95% CI, 1.07-1.13) for a cumulative exposure of 2,000-9,999 g, and finally to a 27% greater risk (HR, 1.27; 95% CI, 1.19-1.35) for a cumulative exposure of 10,000 g or higher.
The study included more than 700,000 individuals exposed to topical mometasone at a potency equivalent of 200 g or more over the study period. The reference group (200-499 g) was the largest (317,907 individuals). The first strata (500-999 g) included 186,359 patients; the second (1,000-1,999 g), 111,203 patients; the third (2,000-9,999 g), 94,334 patients; and the fifth (10,000 g or more), 13,448 patients.
“A 3% increase in the relative risk of osteoporosis and MOF was observed per doubling of the TCS dose,” according to the investigators.
Patients exposed to doses of high-potency topical steroids that put them at risk of MOF is limited but substantial, according to the senior author, Alexander Egeberg, MD, PhD, of the department of dermatology and allergy at Herlev and Gentofte Hospital, Copenhagen.
“It is true that the risk is modest for the average user of topical steroids,” Dr. Egeberg said in an interview. However, despite the fact that topical steroids are intended for short-term use, “2% of all our users had been exposed to the equivalent of 10,000 g of mometasone, which mean 100 tubes of 100 g.”
If the other two strata at significantly increased risk of MOF (greater than 1,000 g) are included, an additional 28% of all users are facing the potential for clinically significant osteoporosis, according to the Danish data.
The adverse effect of steroids on bone metabolism has been established previously, and several studies have linked systemic corticosteroid exposure, including inhaled corticosteroids, with increased risk of osteoporotic fracture. For example, one study showed that patients with chronic obstructive pulmonary disease on daily inhaled doses of the equivalent of fluticasone at or above 1,000 mcg for more than 4 years had about a 10% increased risk of MOF relative to those not exposed.
The data associate topical steroids with increased risk of osteoporotic fracture, but Dr. Egeberg said osteoporosis is not the only reason to use topical steroids prudently.
“It is important to keep in mind that osteoporosis and fractures are at the extreme end of the side-effect profile and that other side effects, such as striae formation, skin thinning, and dysregulated diabetes, can occur with much lower quantities of topical steroids,” Dr. Egeberg said
For avoiding this risk, “there are no specific cutoffs” recommended for topical steroids in current guidelines, but dermatologists should be aware that many of the indications for topical steroids, such as psoriasis and atopic dermatitis, involve skin with an impaired barrier function, exposing patients to an increased likelihood of absorption, according to Dr. Egeberg.
“A general rule of thumb that we use is that, if a patient with persistent disease activity requires a new prescription of the equivalent of 100 g mometasone every 1-2 months, it might be worth considering if there is a suitable alternative,” Dr. Egeberg said.
In an accompanying editorial, Rebecca D. Jackson, MD, of the division of endocrinology, diabetes, and metabolism in the department of internal medicine at Ohio State University, Columbus, agreed that no guidelines specific to avoiding the risks of topical corticosteroids are currently available, but she advised clinicians to be considering these risks nonetheless. In general, she suggested that topical steroids, like oral steroids, should be used at “the lowest dose for the shortest duration necessary to manage the underlying medical condition.”
The correlation between topical corticosteroids and increased risk of osteoporotic fracture, although not established previously in a large study, is not surprising, according to Victoria Werth, MD, chief of dermatology at the Philadelphia Veterans Affairs Hospital and professor of dermatology at the University of Pennsylvania, also in Philadelphia.
“Systemic absorption of potent topical steroids has previously been demonstrated with a rapid decrease in serum cortisol levels,” Dr. Werth said in an interview. She indicated that concern about the risk of osteoporosis imposed by use of potent steroids over large body surface areas is appropriate.
To minimize this risk, “it is reasonable to use the lowest dose of steroid possible and to try to substitute other medications when possible,” she said.
Dr. Egeberg reported financial relationships with Abbvie, Almirall, Bristol-Myers Squibb, Dermavant Sciences, Galderma, Janssen Pharmaceuticals, Eli Lilly, Novartis, Pfizer, Samsung, Bioepis, and UCB. Five authors had disclosures related to some of those pharmaceutical companies and/or others. Dr. Jackson had no disclosures.
In support of previously published case reports, in a dose-response relationship.
In a stepwise manner, the hazard ratios for major osteoporotic fracture (MOF) were found to start climbing incrementally for those with a cumulative topical steroid dose equivalent of more than 500 g of mometasone furoate when compared with exposure of 200-499 g, according to the team of investigators from the University of Copenhagen.
“Use of these drugs is very common, and we found an estimated population-attributable risk of as much as 4.3%,” the investigators reported in the study, published in JAMA Dermatology.
The retrospective cohort study drew data from the Danish National Patient Registry, which covers 99% of the country’s population. It was linked to the Danish National Prescription Registry, which captures data on pharmacy-dispensed medications. Data collected from the beginning of 2003 to the end of 2017 were evaluated.
Exposures to potent or very potent topical corticosteroids were converted into a single standard with potency equivalent to 1 mg/g of mometasone furoate. Four strata of exposure were compared to a reference exposure of 200-499 g. These were 500-999 g, 1,000-1,999 g, 2,000-9,999 g, and 10,000 g or greater.
For the first strata, the small increased risk for MOF did not reach significance (HR, 1.01; 95% confidence interval, 0.99-1.03), but each of the others did. These climbed from a 5% greater risk (HR 1.05 95% CI 1.02-1.08) for a cumulative exposure of 1,000 to 1,999 g, to a 10% greater risk (HR, 1.10; 95% CI, 1.07-1.13) for a cumulative exposure of 2,000-9,999 g, and finally to a 27% greater risk (HR, 1.27; 95% CI, 1.19-1.35) for a cumulative exposure of 10,000 g or higher.
The study included more than 700,000 individuals exposed to topical mometasone at a potency equivalent of 200 g or more over the study period. The reference group (200-499 g) was the largest (317,907 individuals). The first strata (500-999 g) included 186,359 patients; the second (1,000-1,999 g), 111,203 patients; the third (2,000-9,999 g), 94,334 patients; and the fifth (10,000 g or more), 13,448 patients.
“A 3% increase in the relative risk of osteoporosis and MOF was observed per doubling of the TCS dose,” according to the investigators.
Patients exposed to doses of high-potency topical steroids that put them at risk of MOF is limited but substantial, according to the senior author, Alexander Egeberg, MD, PhD, of the department of dermatology and allergy at Herlev and Gentofte Hospital, Copenhagen.
“It is true that the risk is modest for the average user of topical steroids,” Dr. Egeberg said in an interview. However, despite the fact that topical steroids are intended for short-term use, “2% of all our users had been exposed to the equivalent of 10,000 g of mometasone, which mean 100 tubes of 100 g.”
If the other two strata at significantly increased risk of MOF (greater than 1,000 g) are included, an additional 28% of all users are facing the potential for clinically significant osteoporosis, according to the Danish data.
The adverse effect of steroids on bone metabolism has been established previously, and several studies have linked systemic corticosteroid exposure, including inhaled corticosteroids, with increased risk of osteoporotic fracture. For example, one study showed that patients with chronic obstructive pulmonary disease on daily inhaled doses of the equivalent of fluticasone at or above 1,000 mcg for more than 4 years had about a 10% increased risk of MOF relative to those not exposed.
The data associate topical steroids with increased risk of osteoporotic fracture, but Dr. Egeberg said osteoporosis is not the only reason to use topical steroids prudently.
“It is important to keep in mind that osteoporosis and fractures are at the extreme end of the side-effect profile and that other side effects, such as striae formation, skin thinning, and dysregulated diabetes, can occur with much lower quantities of topical steroids,” Dr. Egeberg said
For avoiding this risk, “there are no specific cutoffs” recommended for topical steroids in current guidelines, but dermatologists should be aware that many of the indications for topical steroids, such as psoriasis and atopic dermatitis, involve skin with an impaired barrier function, exposing patients to an increased likelihood of absorption, according to Dr. Egeberg.
“A general rule of thumb that we use is that, if a patient with persistent disease activity requires a new prescription of the equivalent of 100 g mometasone every 1-2 months, it might be worth considering if there is a suitable alternative,” Dr. Egeberg said.
In an accompanying editorial, Rebecca D. Jackson, MD, of the division of endocrinology, diabetes, and metabolism in the department of internal medicine at Ohio State University, Columbus, agreed that no guidelines specific to avoiding the risks of topical corticosteroids are currently available, but she advised clinicians to be considering these risks nonetheless. In general, she suggested that topical steroids, like oral steroids, should be used at “the lowest dose for the shortest duration necessary to manage the underlying medical condition.”
The correlation between topical corticosteroids and increased risk of osteoporotic fracture, although not established previously in a large study, is not surprising, according to Victoria Werth, MD, chief of dermatology at the Philadelphia Veterans Affairs Hospital and professor of dermatology at the University of Pennsylvania, also in Philadelphia.
“Systemic absorption of potent topical steroids has previously been demonstrated with a rapid decrease in serum cortisol levels,” Dr. Werth said in an interview. She indicated that concern about the risk of osteoporosis imposed by use of potent steroids over large body surface areas is appropriate.
To minimize this risk, “it is reasonable to use the lowest dose of steroid possible and to try to substitute other medications when possible,” she said.
Dr. Egeberg reported financial relationships with Abbvie, Almirall, Bristol-Myers Squibb, Dermavant Sciences, Galderma, Janssen Pharmaceuticals, Eli Lilly, Novartis, Pfizer, Samsung, Bioepis, and UCB. Five authors had disclosures related to some of those pharmaceutical companies and/or others. Dr. Jackson had no disclosures.
FROM JAMA DERMATOLOGY
Psoriasis registry study finds normal pregnancy outcomes
according to one of the largest studies to examine the issue to date.
However, “pregnancy-specific registries that include a larger number of pregnant women with psoriasis ... are needed to more fully characterize the association between psoriasis and treatment and birth outcomes,” acknowledged first author Alexa B. Kimball, MD, MPH, professor of dermatology, Harvard Medical School, Boston, and colleagues.
The cohort study, published in JAMA Dermatology, used data from the Psoriasis Longitudinal Assessment and Registry (PSOLAR), which “is not a pregnancy specific registry, and medical history is captured only at baseline,” they noted.
Their findings showed pregnancy outcomes such as spontaneous abortion, neonatal problems, and congenital anomalies among women with moderate to severe psoriasis were similar to rates in the general U.S. population, and are “consistent with previously reported data,” they reported. “And pregnancy outcomes for women exposed to biologics were similar to those for women with exposure to nonbiologics.”
The study “provides further reassurance that the biologics appear safe at least related to pregnancy outcomes,” commented Jenny Murase, MD, associate professor of dermatology at the University of California, San Francisco, who was not involved in the study. In an interview, she noted that the study “did not examine any potential immunosuppression of the fetus in the first 6 months of life,” which she described as “the heart of the concern, more than whether or not the psoriasis or the biologic affects the pregnancy itself.”
The study used data from the PSOLAR registry collected from June 20, 2007, to Aug.23, 2019, which included 2,224 women of childbearing age (18-45 years) who were collectively followed up for 12,929 patient-years. Among these women, 220 had 298 pregnancies, with 244 live births (81.9%).
“Birth outcomes among all 244 births included 231 healthy newborns (94.7%), 10 infants with a neonatal problem (4.1%), 1 stillbirth (0.4%), and 2 congenital anomalies (0.8%),” the authors reported.
There were also 41 spontaneous abortions (13.8%), and 13 elective terminations (4.4%). “No elective terminations were known to derive from a congenital anomaly or other medical issue,” they added.
Among the documented pregnancies, 252 occurred in women with exposure to biologic therapy either before or during pregnancy, including 168 (56.4%) during the prenatal period, while 46 pregnancies occurred in women with no exposure to biologic therapy.
Dr. Murase, director of medical consultative dermatology for the Palo Alto Foundation Medical Group in Mountain View, Calif., said that a more detailed comparison of the different psoriasis treatments, as well as the offspring outcomes during the first 6 months of life, might offer some further important insight,.
Infants born after exposure to infliximab “and potentially other anti–tumor necrosis factor–alpha agents during the third trimester may be unable to develop an appropriate immune response to live vaccines,” she and her coauthors cautioned in a letter published in 2011, which referred to a case of an infant with disseminated bacillus Calmette-Guérin infection, whose mother had received infliximab for Crohn’s disease throughout pregnancy.
Dr. Murase pointed out that, in the registry study, exposures to certolizumab, which is pegylated and does not cross the placental barrier, were not separated from other cases. It is important to consider “the cross over late in the second trimester and especially third trimester as the infant is getting the ‘antibody boost’ from the mother as it gets ready to set foot in this world and needs the maternal antibodies to prepare its immune system. If the IgG biologics cross third trimester and immunosuppress the infant ... then I think a medication that does not cross the placental barrier is important to consider.”
The study was sponsored by Janssen Scientific Affairs. Dr. Kimball’s disclosures included serving as a consultant and investigator for companies that included AbbVie, Bristol-Myers Squibb, and Janssen; several other authors also had disclosures related to multiple pharmaceutical companies. Dr. Murase’s disclosures included serving as a consultant for Dermira, UCB Pharma, Sanofi, Ferndale, and Regeneron.
according to one of the largest studies to examine the issue to date.
However, “pregnancy-specific registries that include a larger number of pregnant women with psoriasis ... are needed to more fully characterize the association between psoriasis and treatment and birth outcomes,” acknowledged first author Alexa B. Kimball, MD, MPH, professor of dermatology, Harvard Medical School, Boston, and colleagues.
The cohort study, published in JAMA Dermatology, used data from the Psoriasis Longitudinal Assessment and Registry (PSOLAR), which “is not a pregnancy specific registry, and medical history is captured only at baseline,” they noted.
Their findings showed pregnancy outcomes such as spontaneous abortion, neonatal problems, and congenital anomalies among women with moderate to severe psoriasis were similar to rates in the general U.S. population, and are “consistent with previously reported data,” they reported. “And pregnancy outcomes for women exposed to biologics were similar to those for women with exposure to nonbiologics.”
The study “provides further reassurance that the biologics appear safe at least related to pregnancy outcomes,” commented Jenny Murase, MD, associate professor of dermatology at the University of California, San Francisco, who was not involved in the study. In an interview, she noted that the study “did not examine any potential immunosuppression of the fetus in the first 6 months of life,” which she described as “the heart of the concern, more than whether or not the psoriasis or the biologic affects the pregnancy itself.”
The study used data from the PSOLAR registry collected from June 20, 2007, to Aug.23, 2019, which included 2,224 women of childbearing age (18-45 years) who were collectively followed up for 12,929 patient-years. Among these women, 220 had 298 pregnancies, with 244 live births (81.9%).
“Birth outcomes among all 244 births included 231 healthy newborns (94.7%), 10 infants with a neonatal problem (4.1%), 1 stillbirth (0.4%), and 2 congenital anomalies (0.8%),” the authors reported.
There were also 41 spontaneous abortions (13.8%), and 13 elective terminations (4.4%). “No elective terminations were known to derive from a congenital anomaly or other medical issue,” they added.
Among the documented pregnancies, 252 occurred in women with exposure to biologic therapy either before or during pregnancy, including 168 (56.4%) during the prenatal period, while 46 pregnancies occurred in women with no exposure to biologic therapy.
Dr. Murase, director of medical consultative dermatology for the Palo Alto Foundation Medical Group in Mountain View, Calif., said that a more detailed comparison of the different psoriasis treatments, as well as the offspring outcomes during the first 6 months of life, might offer some further important insight,.
Infants born after exposure to infliximab “and potentially other anti–tumor necrosis factor–alpha agents during the third trimester may be unable to develop an appropriate immune response to live vaccines,” she and her coauthors cautioned in a letter published in 2011, which referred to a case of an infant with disseminated bacillus Calmette-Guérin infection, whose mother had received infliximab for Crohn’s disease throughout pregnancy.
Dr. Murase pointed out that, in the registry study, exposures to certolizumab, which is pegylated and does not cross the placental barrier, were not separated from other cases. It is important to consider “the cross over late in the second trimester and especially third trimester as the infant is getting the ‘antibody boost’ from the mother as it gets ready to set foot in this world and needs the maternal antibodies to prepare its immune system. If the IgG biologics cross third trimester and immunosuppress the infant ... then I think a medication that does not cross the placental barrier is important to consider.”
The study was sponsored by Janssen Scientific Affairs. Dr. Kimball’s disclosures included serving as a consultant and investigator for companies that included AbbVie, Bristol-Myers Squibb, and Janssen; several other authors also had disclosures related to multiple pharmaceutical companies. Dr. Murase’s disclosures included serving as a consultant for Dermira, UCB Pharma, Sanofi, Ferndale, and Regeneron.
according to one of the largest studies to examine the issue to date.
However, “pregnancy-specific registries that include a larger number of pregnant women with psoriasis ... are needed to more fully characterize the association between psoriasis and treatment and birth outcomes,” acknowledged first author Alexa B. Kimball, MD, MPH, professor of dermatology, Harvard Medical School, Boston, and colleagues.
The cohort study, published in JAMA Dermatology, used data from the Psoriasis Longitudinal Assessment and Registry (PSOLAR), which “is not a pregnancy specific registry, and medical history is captured only at baseline,” they noted.
Their findings showed pregnancy outcomes such as spontaneous abortion, neonatal problems, and congenital anomalies among women with moderate to severe psoriasis were similar to rates in the general U.S. population, and are “consistent with previously reported data,” they reported. “And pregnancy outcomes for women exposed to biologics were similar to those for women with exposure to nonbiologics.”
The study “provides further reassurance that the biologics appear safe at least related to pregnancy outcomes,” commented Jenny Murase, MD, associate professor of dermatology at the University of California, San Francisco, who was not involved in the study. In an interview, she noted that the study “did not examine any potential immunosuppression of the fetus in the first 6 months of life,” which she described as “the heart of the concern, more than whether or not the psoriasis or the biologic affects the pregnancy itself.”
The study used data from the PSOLAR registry collected from June 20, 2007, to Aug.23, 2019, which included 2,224 women of childbearing age (18-45 years) who were collectively followed up for 12,929 patient-years. Among these women, 220 had 298 pregnancies, with 244 live births (81.9%).
“Birth outcomes among all 244 births included 231 healthy newborns (94.7%), 10 infants with a neonatal problem (4.1%), 1 stillbirth (0.4%), and 2 congenital anomalies (0.8%),” the authors reported.
There were also 41 spontaneous abortions (13.8%), and 13 elective terminations (4.4%). “No elective terminations were known to derive from a congenital anomaly or other medical issue,” they added.
Among the documented pregnancies, 252 occurred in women with exposure to biologic therapy either before or during pregnancy, including 168 (56.4%) during the prenatal period, while 46 pregnancies occurred in women with no exposure to biologic therapy.
Dr. Murase, director of medical consultative dermatology for the Palo Alto Foundation Medical Group in Mountain View, Calif., said that a more detailed comparison of the different psoriasis treatments, as well as the offspring outcomes during the first 6 months of life, might offer some further important insight,.
Infants born after exposure to infliximab “and potentially other anti–tumor necrosis factor–alpha agents during the third trimester may be unable to develop an appropriate immune response to live vaccines,” she and her coauthors cautioned in a letter published in 2011, which referred to a case of an infant with disseminated bacillus Calmette-Guérin infection, whose mother had received infliximab for Crohn’s disease throughout pregnancy.
Dr. Murase pointed out that, in the registry study, exposures to certolizumab, which is pegylated and does not cross the placental barrier, were not separated from other cases. It is important to consider “the cross over late in the second trimester and especially third trimester as the infant is getting the ‘antibody boost’ from the mother as it gets ready to set foot in this world and needs the maternal antibodies to prepare its immune system. If the IgG biologics cross third trimester and immunosuppress the infant ... then I think a medication that does not cross the placental barrier is important to consider.”
The study was sponsored by Janssen Scientific Affairs. Dr. Kimball’s disclosures included serving as a consultant and investigator for companies that included AbbVie, Bristol-Myers Squibb, and Janssen; several other authors also had disclosures related to multiple pharmaceutical companies. Dr. Murase’s disclosures included serving as a consultant for Dermira, UCB Pharma, Sanofi, Ferndale, and Regeneron.
FROM JAMA DERMATOLOGY