User login
Expert calls for paradigm shift in lab monitoring of some dermatology drugs
From time to time, Joslyn Kirby, MD, asks other physicians about their experience with certain medications used in dermatology, especially when something new hits the market.
“Sometimes I get an answer like, ‘The last time I used that medicine, my patient needed a liver transplant,’ ” Dr. Kirby, associate professor of dermatology, Penn State University, Hershey, said during the Orlando Dermatology Aesthetic and Clinical Conference. “It’s typically a story of something rare, uncommon, and awful. The challenge with an anecdote is that for all its power, it has a lower level of evidence. But it sticks with us and influences us more than a better level of evidence because it’s a situation and a story that we might relate to.”
Dr. Kirby said that when she thinks about managing side effects from drugs used in dermatology, it usually relates to something common and low-risk such as sore, dry skin with isotretinoin use. In contrast, if there is an uncommon but serious side effect, then mitigation rather than management is key. “I want to mitigate the risk – meaning warn my patient about it or be careful about how I select my patients when it is a serious side effect that happens infrequently,” she said. “The worst combination is a frequent and severe side effect. That is something we should avoid, for sure.”
Isotretinoin
But another aspect of prescribing a new drug for patients can be less clear-cut, Dr. Kirby continued, such as the rationale for routine lab monitoring. She began by discussing one of her male patients with moderate to severe acne. After he failed oral antibiotics and topical retinoids, she recommended isotretinoin, which carries a risk of hypertriglyceridemia-associated pancreatitis. “Early in my career, I was getting a lot of monthly labs in patients on this drug that were totally normal and not influencing my practice,” Dr. Kirby recalled. “We’ve seen studies coming out on isotretinoin lab monitoring, showing us that we can keep our patients safe and that we really don’t need to be checking labs as often, because lab changes are infrequent.”
In one of those studies, researchers evaluated 1,863 patients treated with isotretinoin for acne between Jan. 1, 2008, and June 30, 2017 (J Am Acad Dermatol. 2020 Jan;82[1]:72-9).Over time, fewer than 1% of patients screened developed grade 3 or greater triglyceride testing abnormalities, while fewer than 0.5% developed liver function testing (LFT) abnormalities. Authors of a separate systematic review concluded that for patients on isotretinoin therapy without elevated baseline triglycerides, or risk thereof, monitoring triglycerides is of little value (Br J Dermatol. 2017 Oct;177[4]:960-6). Of the 25 patients in the analysis who developed pancreatitis on isotretinoin, only 3 had elevated triglycerides at baseline.
“I was taught that I need to check triglycerides frequently due to the risk of pancreatitis developing with isotretinoin use,” Dr. Kirby said. “Lipid changes on therapy are expected, but they tend to peak early, meaning the first 3 months of treatment when we’re ramping up from a starting dose to a maintenance dose. It’s rare for somebody to be a late bloomer, meaning that they have totally normal labs in the first 3 months and then suddenly develop an abnormality. People are either going to demonstrate an abnormality early or not have one at all.”
When Dr. Kirby starts patients on isotretinoin, she orders baseline LFTs and a lipid panel and repeats them 60 days later. “If everything is fine or only mildly high, we don’t do more testing, only a review of systems,” she said. “This is valuable to our patients because fear of needles and fainting peak during adolescence.”
Spironolactone
The clinical use of regularly monitoring potassium levels in young women taking spironolactone for acne has also been questioned. The drug has been linked to an increased risk for hyperkalemia, but the prevalence is unclear. “I got a lot of normal potassium levels in these patients [when] I was in training and I really questioned, ‘Why am I doing this? What is the rationale?’ ” Dr. Kirby said.
In a study that informed her own practice, researchers reviewed the rate of hyperkalemia in 974 healthy young women taking spironolactone for acne or for an endocrine disorder with associated acne between Dec. 1, 2000, and March 31, 2014 (JAMA Dermatol. 2015 Sep;151[9]:941-4). Of the total of 1,802 serum potassium measurements taken during treatment, 13 (0.72%) were mildly elevated levels and none of the patients had a potassium level above 5.5 mEq/L. Retesting within 1 to 3 weeks in 6 of 13 patients with elevated levels found that potassium levels were normal. “The recommendation for spironolactone in healthy women is not to check the potassium level,” Dr. Kirby said, adding that she does counsel patients about the risk of breast tenderness (which can occur 5% to 40% of the time) and spotting (which can occur in 10% to 20% of patients). Gynecomastia can occur in 10% to 30% of men, which is one of the reasons she does not use spironolactone in male patients.
TB testing and biologics
Whether or not to test for TB in patients with psoriasis taking biologic therapies represents another conundrum, she continued. Patients taking biologics are at risk of reactivation of latent TB infection, but in her experience, package inserts contain language like “perform TB testing at baseline, then periodically,” or “use at baseline, then with active TB symptoms,” and “after treatment is discontinued.”
“What the inserts didn’t recommend was to perform TB testing every year, which is what my routine had been,” Dr. Kirby said. “In the United States, thankfully we don’t have a lot of TB.” In a study that informed her own practice, researchers at a single academic medical center retrospectively reviewed the TB seroconversion rate among 316 patients treated with second-generation biologics (J Am Acad Dermatol. 2020 Oct 1;S0190-9622[20]32676-1. doi: 10.1016/j.jaad.2020.09.075). It found that only six patients (2%) converted and had a positive TB test later during treatment with the biologic. “Of these six people, all had grown up outside the U.S., had traveled outside of the U.S., or were in a group living situation,” said Dr. Kirby, who was not affiliated with the study.
“This informs our rationale for how we can do this testing. If insurance requires it every year, fine. But if they don’t, I ask patients about travel, about their living situation, and how they’re feeling. If everything’s going great, I don’t order TB testing. I do favor the interferon-gamma release assays because they’re a lot more effective than PPDs [purified protein derivative skin tests]. Also, PPDs are difficult for patients who have a low rate of returning to have that test read.”
Terbinafine for onychomycosis
Dr. Kirby also discussed the rationale for ordering regular LFTs in patients taking terbinafine for onychomycosis. “There is a risk of drug-induced liver injury from taking terbinafine, but it’s rare,” she said. “Can we be thoughtful about which patients we expose?”
Evidence suggests that patients with hyperkeratosis greater than 2 mm, with nail matrix involvement, with 50% or more of the nail involved, or having concomitant peripheral vascular disease and diabetes are recalcitrant to treatment with terbinafine
(J Am Acad Dermatol. 2019 Apr;80[4]:853-67). “If we can frame this risk, then we can frame it for our patients,” she said. “We’re more likely to cause liver injury with an antibiotic. When it comes to an oral antifungal, itraconazole is more likely than terbinafine to cause liver injury. The rate of liver injury with terbinafine is only about 2 out of 100,000. It’s five times more likely with itraconazole and 21 times more likely with Augmentin.”
She recommends obtaining a baseline LFT in patients starting terbinafine therapy “to make sure their liver is normal from the start.” In addition, she advised, “let them know that there is a TB seroconversion risk of about 1 in 50,000 people, and that if it happens there would be symptomatic changes. They would maybe notice pruritus and have a darkening in their urine, and they’d have some flu-like symptoms, which would mean stop the drug and get some care.”
Dr. Kirby emphasized that a patient’s propensity for developing drug-induced liver injury from terbinafine use is not predictable from LFT monitoring. “What you’re more likely to find is an asymptomatic LFT rise in about 1% of people,” she said.
She disclosed that she has received honoraria from AbbVie, ChemoCentryx, Incyte, Janssen, Novartis, and UCB Pharma.
From time to time, Joslyn Kirby, MD, asks other physicians about their experience with certain medications used in dermatology, especially when something new hits the market.
“Sometimes I get an answer like, ‘The last time I used that medicine, my patient needed a liver transplant,’ ” Dr. Kirby, associate professor of dermatology, Penn State University, Hershey, said during the Orlando Dermatology Aesthetic and Clinical Conference. “It’s typically a story of something rare, uncommon, and awful. The challenge with an anecdote is that for all its power, it has a lower level of evidence. But it sticks with us and influences us more than a better level of evidence because it’s a situation and a story that we might relate to.”
Dr. Kirby said that when she thinks about managing side effects from drugs used in dermatology, it usually relates to something common and low-risk such as sore, dry skin with isotretinoin use. In contrast, if there is an uncommon but serious side effect, then mitigation rather than management is key. “I want to mitigate the risk – meaning warn my patient about it or be careful about how I select my patients when it is a serious side effect that happens infrequently,” she said. “The worst combination is a frequent and severe side effect. That is something we should avoid, for sure.”
Isotretinoin
But another aspect of prescribing a new drug for patients can be less clear-cut, Dr. Kirby continued, such as the rationale for routine lab monitoring. She began by discussing one of her male patients with moderate to severe acne. After he failed oral antibiotics and topical retinoids, she recommended isotretinoin, which carries a risk of hypertriglyceridemia-associated pancreatitis. “Early in my career, I was getting a lot of monthly labs in patients on this drug that were totally normal and not influencing my practice,” Dr. Kirby recalled. “We’ve seen studies coming out on isotretinoin lab monitoring, showing us that we can keep our patients safe and that we really don’t need to be checking labs as often, because lab changes are infrequent.”
In one of those studies, researchers evaluated 1,863 patients treated with isotretinoin for acne between Jan. 1, 2008, and June 30, 2017 (J Am Acad Dermatol. 2020 Jan;82[1]:72-9).Over time, fewer than 1% of patients screened developed grade 3 or greater triglyceride testing abnormalities, while fewer than 0.5% developed liver function testing (LFT) abnormalities. Authors of a separate systematic review concluded that for patients on isotretinoin therapy without elevated baseline triglycerides, or risk thereof, monitoring triglycerides is of little value (Br J Dermatol. 2017 Oct;177[4]:960-6). Of the 25 patients in the analysis who developed pancreatitis on isotretinoin, only 3 had elevated triglycerides at baseline.
“I was taught that I need to check triglycerides frequently due to the risk of pancreatitis developing with isotretinoin use,” Dr. Kirby said. “Lipid changes on therapy are expected, but they tend to peak early, meaning the first 3 months of treatment when we’re ramping up from a starting dose to a maintenance dose. It’s rare for somebody to be a late bloomer, meaning that they have totally normal labs in the first 3 months and then suddenly develop an abnormality. People are either going to demonstrate an abnormality early or not have one at all.”
When Dr. Kirby starts patients on isotretinoin, she orders baseline LFTs and a lipid panel and repeats them 60 days later. “If everything is fine or only mildly high, we don’t do more testing, only a review of systems,” she said. “This is valuable to our patients because fear of needles and fainting peak during adolescence.”
Spironolactone
The clinical use of regularly monitoring potassium levels in young women taking spironolactone for acne has also been questioned. The drug has been linked to an increased risk for hyperkalemia, but the prevalence is unclear. “I got a lot of normal potassium levels in these patients [when] I was in training and I really questioned, ‘Why am I doing this? What is the rationale?’ ” Dr. Kirby said.
In a study that informed her own practice, researchers reviewed the rate of hyperkalemia in 974 healthy young women taking spironolactone for acne or for an endocrine disorder with associated acne between Dec. 1, 2000, and March 31, 2014 (JAMA Dermatol. 2015 Sep;151[9]:941-4). Of the total of 1,802 serum potassium measurements taken during treatment, 13 (0.72%) were mildly elevated levels and none of the patients had a potassium level above 5.5 mEq/L. Retesting within 1 to 3 weeks in 6 of 13 patients with elevated levels found that potassium levels were normal. “The recommendation for spironolactone in healthy women is not to check the potassium level,” Dr. Kirby said, adding that she does counsel patients about the risk of breast tenderness (which can occur 5% to 40% of the time) and spotting (which can occur in 10% to 20% of patients). Gynecomastia can occur in 10% to 30% of men, which is one of the reasons she does not use spironolactone in male patients.
TB testing and biologics
Whether or not to test for TB in patients with psoriasis taking biologic therapies represents another conundrum, she continued. Patients taking biologics are at risk of reactivation of latent TB infection, but in her experience, package inserts contain language like “perform TB testing at baseline, then periodically,” or “use at baseline, then with active TB symptoms,” and “after treatment is discontinued.”
“What the inserts didn’t recommend was to perform TB testing every year, which is what my routine had been,” Dr. Kirby said. “In the United States, thankfully we don’t have a lot of TB.” In a study that informed her own practice, researchers at a single academic medical center retrospectively reviewed the TB seroconversion rate among 316 patients treated with second-generation biologics (J Am Acad Dermatol. 2020 Oct 1;S0190-9622[20]32676-1. doi: 10.1016/j.jaad.2020.09.075). It found that only six patients (2%) converted and had a positive TB test later during treatment with the biologic. “Of these six people, all had grown up outside the U.S., had traveled outside of the U.S., or were in a group living situation,” said Dr. Kirby, who was not affiliated with the study.
“This informs our rationale for how we can do this testing. If insurance requires it every year, fine. But if they don’t, I ask patients about travel, about their living situation, and how they’re feeling. If everything’s going great, I don’t order TB testing. I do favor the interferon-gamma release assays because they’re a lot more effective than PPDs [purified protein derivative skin tests]. Also, PPDs are difficult for patients who have a low rate of returning to have that test read.”
Terbinafine for onychomycosis
Dr. Kirby also discussed the rationale for ordering regular LFTs in patients taking terbinafine for onychomycosis. “There is a risk of drug-induced liver injury from taking terbinafine, but it’s rare,” she said. “Can we be thoughtful about which patients we expose?”
Evidence suggests that patients with hyperkeratosis greater than 2 mm, with nail matrix involvement, with 50% or more of the nail involved, or having concomitant peripheral vascular disease and diabetes are recalcitrant to treatment with terbinafine
(J Am Acad Dermatol. 2019 Apr;80[4]:853-67). “If we can frame this risk, then we can frame it for our patients,” she said. “We’re more likely to cause liver injury with an antibiotic. When it comes to an oral antifungal, itraconazole is more likely than terbinafine to cause liver injury. The rate of liver injury with terbinafine is only about 2 out of 100,000. It’s five times more likely with itraconazole and 21 times more likely with Augmentin.”
She recommends obtaining a baseline LFT in patients starting terbinafine therapy “to make sure their liver is normal from the start.” In addition, she advised, “let them know that there is a TB seroconversion risk of about 1 in 50,000 people, and that if it happens there would be symptomatic changes. They would maybe notice pruritus and have a darkening in their urine, and they’d have some flu-like symptoms, which would mean stop the drug and get some care.”
Dr. Kirby emphasized that a patient’s propensity for developing drug-induced liver injury from terbinafine use is not predictable from LFT monitoring. “What you’re more likely to find is an asymptomatic LFT rise in about 1% of people,” she said.
She disclosed that she has received honoraria from AbbVie, ChemoCentryx, Incyte, Janssen, Novartis, and UCB Pharma.
From time to time, Joslyn Kirby, MD, asks other physicians about their experience with certain medications used in dermatology, especially when something new hits the market.
“Sometimes I get an answer like, ‘The last time I used that medicine, my patient needed a liver transplant,’ ” Dr. Kirby, associate professor of dermatology, Penn State University, Hershey, said during the Orlando Dermatology Aesthetic and Clinical Conference. “It’s typically a story of something rare, uncommon, and awful. The challenge with an anecdote is that for all its power, it has a lower level of evidence. But it sticks with us and influences us more than a better level of evidence because it’s a situation and a story that we might relate to.”
Dr. Kirby said that when she thinks about managing side effects from drugs used in dermatology, it usually relates to something common and low-risk such as sore, dry skin with isotretinoin use. In contrast, if there is an uncommon but serious side effect, then mitigation rather than management is key. “I want to mitigate the risk – meaning warn my patient about it or be careful about how I select my patients when it is a serious side effect that happens infrequently,” she said. “The worst combination is a frequent and severe side effect. That is something we should avoid, for sure.”
Isotretinoin
But another aspect of prescribing a new drug for patients can be less clear-cut, Dr. Kirby continued, such as the rationale for routine lab monitoring. She began by discussing one of her male patients with moderate to severe acne. After he failed oral antibiotics and topical retinoids, she recommended isotretinoin, which carries a risk of hypertriglyceridemia-associated pancreatitis. “Early in my career, I was getting a lot of monthly labs in patients on this drug that were totally normal and not influencing my practice,” Dr. Kirby recalled. “We’ve seen studies coming out on isotretinoin lab monitoring, showing us that we can keep our patients safe and that we really don’t need to be checking labs as often, because lab changes are infrequent.”
In one of those studies, researchers evaluated 1,863 patients treated with isotretinoin for acne between Jan. 1, 2008, and June 30, 2017 (J Am Acad Dermatol. 2020 Jan;82[1]:72-9).Over time, fewer than 1% of patients screened developed grade 3 or greater triglyceride testing abnormalities, while fewer than 0.5% developed liver function testing (LFT) abnormalities. Authors of a separate systematic review concluded that for patients on isotretinoin therapy without elevated baseline triglycerides, or risk thereof, monitoring triglycerides is of little value (Br J Dermatol. 2017 Oct;177[4]:960-6). Of the 25 patients in the analysis who developed pancreatitis on isotretinoin, only 3 had elevated triglycerides at baseline.
“I was taught that I need to check triglycerides frequently due to the risk of pancreatitis developing with isotretinoin use,” Dr. Kirby said. “Lipid changes on therapy are expected, but they tend to peak early, meaning the first 3 months of treatment when we’re ramping up from a starting dose to a maintenance dose. It’s rare for somebody to be a late bloomer, meaning that they have totally normal labs in the first 3 months and then suddenly develop an abnormality. People are either going to demonstrate an abnormality early or not have one at all.”
When Dr. Kirby starts patients on isotretinoin, she orders baseline LFTs and a lipid panel and repeats them 60 days later. “If everything is fine or only mildly high, we don’t do more testing, only a review of systems,” she said. “This is valuable to our patients because fear of needles and fainting peak during adolescence.”
Spironolactone
The clinical use of regularly monitoring potassium levels in young women taking spironolactone for acne has also been questioned. The drug has been linked to an increased risk for hyperkalemia, but the prevalence is unclear. “I got a lot of normal potassium levels in these patients [when] I was in training and I really questioned, ‘Why am I doing this? What is the rationale?’ ” Dr. Kirby said.
In a study that informed her own practice, researchers reviewed the rate of hyperkalemia in 974 healthy young women taking spironolactone for acne or for an endocrine disorder with associated acne between Dec. 1, 2000, and March 31, 2014 (JAMA Dermatol. 2015 Sep;151[9]:941-4). Of the total of 1,802 serum potassium measurements taken during treatment, 13 (0.72%) were mildly elevated levels and none of the patients had a potassium level above 5.5 mEq/L. Retesting within 1 to 3 weeks in 6 of 13 patients with elevated levels found that potassium levels were normal. “The recommendation for spironolactone in healthy women is not to check the potassium level,” Dr. Kirby said, adding that she does counsel patients about the risk of breast tenderness (which can occur 5% to 40% of the time) and spotting (which can occur in 10% to 20% of patients). Gynecomastia can occur in 10% to 30% of men, which is one of the reasons she does not use spironolactone in male patients.
TB testing and biologics
Whether or not to test for TB in patients with psoriasis taking biologic therapies represents another conundrum, she continued. Patients taking biologics are at risk of reactivation of latent TB infection, but in her experience, package inserts contain language like “perform TB testing at baseline, then periodically,” or “use at baseline, then with active TB symptoms,” and “after treatment is discontinued.”
“What the inserts didn’t recommend was to perform TB testing every year, which is what my routine had been,” Dr. Kirby said. “In the United States, thankfully we don’t have a lot of TB.” In a study that informed her own practice, researchers at a single academic medical center retrospectively reviewed the TB seroconversion rate among 316 patients treated with second-generation biologics (J Am Acad Dermatol. 2020 Oct 1;S0190-9622[20]32676-1. doi: 10.1016/j.jaad.2020.09.075). It found that only six patients (2%) converted and had a positive TB test later during treatment with the biologic. “Of these six people, all had grown up outside the U.S., had traveled outside of the U.S., or were in a group living situation,” said Dr. Kirby, who was not affiliated with the study.
“This informs our rationale for how we can do this testing. If insurance requires it every year, fine. But if they don’t, I ask patients about travel, about their living situation, and how they’re feeling. If everything’s going great, I don’t order TB testing. I do favor the interferon-gamma release assays because they’re a lot more effective than PPDs [purified protein derivative skin tests]. Also, PPDs are difficult for patients who have a low rate of returning to have that test read.”
Terbinafine for onychomycosis
Dr. Kirby also discussed the rationale for ordering regular LFTs in patients taking terbinafine for onychomycosis. “There is a risk of drug-induced liver injury from taking terbinafine, but it’s rare,” she said. “Can we be thoughtful about which patients we expose?”
Evidence suggests that patients with hyperkeratosis greater than 2 mm, with nail matrix involvement, with 50% or more of the nail involved, or having concomitant peripheral vascular disease and diabetes are recalcitrant to treatment with terbinafine
(J Am Acad Dermatol. 2019 Apr;80[4]:853-67). “If we can frame this risk, then we can frame it for our patients,” she said. “We’re more likely to cause liver injury with an antibiotic. When it comes to an oral antifungal, itraconazole is more likely than terbinafine to cause liver injury. The rate of liver injury with terbinafine is only about 2 out of 100,000. It’s five times more likely with itraconazole and 21 times more likely with Augmentin.”
She recommends obtaining a baseline LFT in patients starting terbinafine therapy “to make sure their liver is normal from the start.” In addition, she advised, “let them know that there is a TB seroconversion risk of about 1 in 50,000 people, and that if it happens there would be symptomatic changes. They would maybe notice pruritus and have a darkening in their urine, and they’d have some flu-like symptoms, which would mean stop the drug and get some care.”
Dr. Kirby emphasized that a patient’s propensity for developing drug-induced liver injury from terbinafine use is not predictable from LFT monitoring. “What you’re more likely to find is an asymptomatic LFT rise in about 1% of people,” she said.
She disclosed that she has received honoraria from AbbVie, ChemoCentryx, Incyte, Janssen, Novartis, and UCB Pharma.
FROM ODAC 2021
Anybody for a nanobody? Novel psoriasis therapy impresses in phase 2b
in a phase 2b randomized trial, Kim A. Papp, MD, PhD, reported at the annual congress of the European Academy of Dermatology and Venereology.
A nanobody is a tiny antibody fragment with a much smaller molecular weight than the monoclonal antibodies utilized today in treating psoriasis or atopic dermatitis. The sonelokinab nanobody, derived from animals in the camel family, is a recombinant sequence-optimized nanobody specific for human IL-17F, IL-17A, the heterodimer IL-17A/F, and serum albumin. The binding to serum albumin give sonelokinab a lengthy half-life of 10-12 hours, which may be therapeutically relevant, explained Dr. Papp, president and founder of Probity Medical Research in Waterloo, Ont.
He presented the 24-week results of a multicenter, double-blind, double-dummy randomized trial including 313 North American and European adults with an average 18-year history of psoriasis and a baseline Psoriasis Area and Severity Index (PASI) score of about 21. They were randomized to one of six treatment arms for the first 12 weeks: subcutaneous injection of sonelokinab at 30, 60, or 120 mg at weeks 0, 2, 4, and 8; enhanced–loading-dose sonelokinab at 120 mg every 2 weeks through week 10; the IL-17A inhibitor secukinumab (Cosentyx) at its standard dosing as an active comparator; or placebo. Data analysis was by rigorous nonresponder imputation, meaning anyone who didn’t complete the study was scored as a nonresponder.
“This yields a conservative data analysis somewhat biased against sonelokinab,” the dermatologist pointed out.
The primary outcome in the trial was the week-12 rate of an Investigator’s Global Assessment score of 0 or 1, indicative of clear or almost clear skin. This was achieved in 88.2% of patients in the highest-dose arm of sonelokinab. That group also had a week-12 PASI 90 response rate of 76.5% and a PASI 100 response rate of 33.3%. By comparison, patients on standard-dose secukinumab had a less robust week-12 IGA 0/1 rate of 77.4%, a PASI 90 of 64.2%, and a PASI 100 of 28.3%. Of note, however, this secukinumab performance was better than seen in the 30-mg sonelokinab group, and comparable to outcomes with 60 mg of sonelokinab.
Dose escalation was performed from weeks 12-24. Patients with a week-12 IGA score greater than 1 after being on sonelokinab at 30 or 60 mg were upgraded to 120 mg at week 12 and again every 4 weeks thereafter. Placebo-treated controls were switched to 120 mg at weeks 12, 14, 16, and every 4 weeks thereafter. The group on the enhanced–loading-dose sonelokinab moved to 120 mg every 4 weeks, while those who had gotten four doses of sonelokinab at 120 mg during the first 12 weeks were switched to 120 mg every 8 weeks. The secukinumab group remained on the approved dosing through week 24.
At week 24, superior outcomes were seen in the enhanced–loading-dose sonelokinab group, with an IGA 0/1 response rate of 94.2%, a PASI 90 of 90.4%, and a PASI 100 of 56.9%. The corresponding week-24 rates in patients on 120 mg of sonelokinab every 8 weeks from week 12 on were 80.4%, 79.2%, and 40.4%, outcomes similar to those seen with secukinumab.
The rapidity of response to sonelokinab at 120 mg was striking, with approximately one-third of treated patients achieving a PASI 90 response by week 4.
“This could reflect the smaller molecular profile. There is possibly rapid increased absorption or bioavailability, quicker time to achieving serum half-life, better penetration into target tissue, and perhaps more effective engagement at the target. All of those things are possibilities. These are things that are yet to be explored, but it’s very enticing to see that uncharacteristically rapid initial response. It’s all very gratifying – and tantalizing,” Dr. Papp said in response to an audience question.
The safety profile of sonelokinab was reassuring. The most common adverse events were nasopharyngitis in 13.5% of patients and pruritus in 6.7%, with most cases being mild or moderate. As with other IL-17 blockers, there was an increase in oral candidiasis. This side effect appeared to occur in dose-dependent fashion: The incidence was zero in the 30-mg group, 1.9% with 60 mg, 3.8% with sonelokinab at 120 mg without an enhanced loading dose, and 5.9% with the enhanced loading dose.
The study was conducted by Avillion in partnership with Merck. Dr. Papp reported receiving research funding from and serving as a consultant to those and numerous other pharmaceutical companies.
in a phase 2b randomized trial, Kim A. Papp, MD, PhD, reported at the annual congress of the European Academy of Dermatology and Venereology.
A nanobody is a tiny antibody fragment with a much smaller molecular weight than the monoclonal antibodies utilized today in treating psoriasis or atopic dermatitis. The sonelokinab nanobody, derived from animals in the camel family, is a recombinant sequence-optimized nanobody specific for human IL-17F, IL-17A, the heterodimer IL-17A/F, and serum albumin. The binding to serum albumin give sonelokinab a lengthy half-life of 10-12 hours, which may be therapeutically relevant, explained Dr. Papp, president and founder of Probity Medical Research in Waterloo, Ont.
He presented the 24-week results of a multicenter, double-blind, double-dummy randomized trial including 313 North American and European adults with an average 18-year history of psoriasis and a baseline Psoriasis Area and Severity Index (PASI) score of about 21. They were randomized to one of six treatment arms for the first 12 weeks: subcutaneous injection of sonelokinab at 30, 60, or 120 mg at weeks 0, 2, 4, and 8; enhanced–loading-dose sonelokinab at 120 mg every 2 weeks through week 10; the IL-17A inhibitor secukinumab (Cosentyx) at its standard dosing as an active comparator; or placebo. Data analysis was by rigorous nonresponder imputation, meaning anyone who didn’t complete the study was scored as a nonresponder.
“This yields a conservative data analysis somewhat biased against sonelokinab,” the dermatologist pointed out.
The primary outcome in the trial was the week-12 rate of an Investigator’s Global Assessment score of 0 or 1, indicative of clear or almost clear skin. This was achieved in 88.2% of patients in the highest-dose arm of sonelokinab. That group also had a week-12 PASI 90 response rate of 76.5% and a PASI 100 response rate of 33.3%. By comparison, patients on standard-dose secukinumab had a less robust week-12 IGA 0/1 rate of 77.4%, a PASI 90 of 64.2%, and a PASI 100 of 28.3%. Of note, however, this secukinumab performance was better than seen in the 30-mg sonelokinab group, and comparable to outcomes with 60 mg of sonelokinab.
Dose escalation was performed from weeks 12-24. Patients with a week-12 IGA score greater than 1 after being on sonelokinab at 30 or 60 mg were upgraded to 120 mg at week 12 and again every 4 weeks thereafter. Placebo-treated controls were switched to 120 mg at weeks 12, 14, 16, and every 4 weeks thereafter. The group on the enhanced–loading-dose sonelokinab moved to 120 mg every 4 weeks, while those who had gotten four doses of sonelokinab at 120 mg during the first 12 weeks were switched to 120 mg every 8 weeks. The secukinumab group remained on the approved dosing through week 24.
At week 24, superior outcomes were seen in the enhanced–loading-dose sonelokinab group, with an IGA 0/1 response rate of 94.2%, a PASI 90 of 90.4%, and a PASI 100 of 56.9%. The corresponding week-24 rates in patients on 120 mg of sonelokinab every 8 weeks from week 12 on were 80.4%, 79.2%, and 40.4%, outcomes similar to those seen with secukinumab.
The rapidity of response to sonelokinab at 120 mg was striking, with approximately one-third of treated patients achieving a PASI 90 response by week 4.
“This could reflect the smaller molecular profile. There is possibly rapid increased absorption or bioavailability, quicker time to achieving serum half-life, better penetration into target tissue, and perhaps more effective engagement at the target. All of those things are possibilities. These are things that are yet to be explored, but it’s very enticing to see that uncharacteristically rapid initial response. It’s all very gratifying – and tantalizing,” Dr. Papp said in response to an audience question.
The safety profile of sonelokinab was reassuring. The most common adverse events were nasopharyngitis in 13.5% of patients and pruritus in 6.7%, with most cases being mild or moderate. As with other IL-17 blockers, there was an increase in oral candidiasis. This side effect appeared to occur in dose-dependent fashion: The incidence was zero in the 30-mg group, 1.9% with 60 mg, 3.8% with sonelokinab at 120 mg without an enhanced loading dose, and 5.9% with the enhanced loading dose.
The study was conducted by Avillion in partnership with Merck. Dr. Papp reported receiving research funding from and serving as a consultant to those and numerous other pharmaceutical companies.
in a phase 2b randomized trial, Kim A. Papp, MD, PhD, reported at the annual congress of the European Academy of Dermatology and Venereology.
A nanobody is a tiny antibody fragment with a much smaller molecular weight than the monoclonal antibodies utilized today in treating psoriasis or atopic dermatitis. The sonelokinab nanobody, derived from animals in the camel family, is a recombinant sequence-optimized nanobody specific for human IL-17F, IL-17A, the heterodimer IL-17A/F, and serum albumin. The binding to serum albumin give sonelokinab a lengthy half-life of 10-12 hours, which may be therapeutically relevant, explained Dr. Papp, president and founder of Probity Medical Research in Waterloo, Ont.
He presented the 24-week results of a multicenter, double-blind, double-dummy randomized trial including 313 North American and European adults with an average 18-year history of psoriasis and a baseline Psoriasis Area and Severity Index (PASI) score of about 21. They were randomized to one of six treatment arms for the first 12 weeks: subcutaneous injection of sonelokinab at 30, 60, or 120 mg at weeks 0, 2, 4, and 8; enhanced–loading-dose sonelokinab at 120 mg every 2 weeks through week 10; the IL-17A inhibitor secukinumab (Cosentyx) at its standard dosing as an active comparator; or placebo. Data analysis was by rigorous nonresponder imputation, meaning anyone who didn’t complete the study was scored as a nonresponder.
“This yields a conservative data analysis somewhat biased against sonelokinab,” the dermatologist pointed out.
The primary outcome in the trial was the week-12 rate of an Investigator’s Global Assessment score of 0 or 1, indicative of clear or almost clear skin. This was achieved in 88.2% of patients in the highest-dose arm of sonelokinab. That group also had a week-12 PASI 90 response rate of 76.5% and a PASI 100 response rate of 33.3%. By comparison, patients on standard-dose secukinumab had a less robust week-12 IGA 0/1 rate of 77.4%, a PASI 90 of 64.2%, and a PASI 100 of 28.3%. Of note, however, this secukinumab performance was better than seen in the 30-mg sonelokinab group, and comparable to outcomes with 60 mg of sonelokinab.
Dose escalation was performed from weeks 12-24. Patients with a week-12 IGA score greater than 1 after being on sonelokinab at 30 or 60 mg were upgraded to 120 mg at week 12 and again every 4 weeks thereafter. Placebo-treated controls were switched to 120 mg at weeks 12, 14, 16, and every 4 weeks thereafter. The group on the enhanced–loading-dose sonelokinab moved to 120 mg every 4 weeks, while those who had gotten four doses of sonelokinab at 120 mg during the first 12 weeks were switched to 120 mg every 8 weeks. The secukinumab group remained on the approved dosing through week 24.
At week 24, superior outcomes were seen in the enhanced–loading-dose sonelokinab group, with an IGA 0/1 response rate of 94.2%, a PASI 90 of 90.4%, and a PASI 100 of 56.9%. The corresponding week-24 rates in patients on 120 mg of sonelokinab every 8 weeks from week 12 on were 80.4%, 79.2%, and 40.4%, outcomes similar to those seen with secukinumab.
The rapidity of response to sonelokinab at 120 mg was striking, with approximately one-third of treated patients achieving a PASI 90 response by week 4.
“This could reflect the smaller molecular profile. There is possibly rapid increased absorption or bioavailability, quicker time to achieving serum half-life, better penetration into target tissue, and perhaps more effective engagement at the target. All of those things are possibilities. These are things that are yet to be explored, but it’s very enticing to see that uncharacteristically rapid initial response. It’s all very gratifying – and tantalizing,” Dr. Papp said in response to an audience question.
The safety profile of sonelokinab was reassuring. The most common adverse events were nasopharyngitis in 13.5% of patients and pruritus in 6.7%, with most cases being mild or moderate. As with other IL-17 blockers, there was an increase in oral candidiasis. This side effect appeared to occur in dose-dependent fashion: The incidence was zero in the 30-mg group, 1.9% with 60 mg, 3.8% with sonelokinab at 120 mg without an enhanced loading dose, and 5.9% with the enhanced loading dose.
The study was conducted by Avillion in partnership with Merck. Dr. Papp reported receiving research funding from and serving as a consultant to those and numerous other pharmaceutical companies.
FROM the eadv congress
In head-to-head trial, two biologics differ markedly for control of psoriasis
with other biologics, according to data from two simultaneously published trials, one of which was a head-to-head comparison with ustekinumab.
In the head-to-head trial called BE VIVID, which included a placebo arm, there was a large advantage of bimekizumab over ustekinumab, a biologic that targets IL-12 and IL-23 and is approved for treating psoriasis, for both coprimary endpoints, according to a multinational group of investigators led by Kristian Reich, MD, PhD, professor of dermatology at the University Medical Center, Hamburg-Eppendorf, Germany.
The proportion of patients with skin clearance was not only greater but faster, “with responses observed after one dose,” Dr. Reich and coinvestigators reported.
The data from the BE VIVID trial was published simultaneously with the BE READY trial, which was placebo-controlled but did not include an active comparator.
Evaluated at week 16, the coprimary endpoints in both studies were skin clearance as measured by a Psoriasis Area Severity Index greater than 90% (PASI 90) and Investigators Global Assessment (IGA) score of 0 (clear) or 1 (almost clear).
In BE VIVID, 567 patients were randomized in 11 countries, including the United States. The dose of bimekizumab was 320 mg administered subcutaneously every 4 weeks. In a randomization scheme of 4:2:1, half as many patients (163) were randomized to ustekinumab (Stelara), which was administered in weight-based dosing of 45 mg or 90 mg at enrollment, at 4 weeks, and then every 12 weeks. The placebo arm had 83 patients. All were switched to bimekizumab at 16 weeks.
At week 16, PASI 90 was achieved in 85% of patients randomized to bimekizumab, compared with 50% of patients randomized to ustekinumab (P < .0001). The rate in the placebo group was 5%.
The bimekizumab advantage for an IGA response of 0 or 1 was of similar magnitude, relative to ustekinumab (84% vs. 53%; P < .0001) and placebo (5%). All secondary efficacy endpoints, such as PASI 90 at week 12 (85% vs. 44%) and PASI 100 at week 16 (59% vs. 21%), favored bimekizumab over ustekinumab.
In the BE READY trial, which evaluated the same dose and schedule of bimekizumab, the rates of PASI 90 at week 16 were 91% and 1% (P < .0001) for the experimental arm and placebo, respectively. The proportion of patients with an IGA score of 0 or 1 were 93% and 1% (P < .0001), respectively.
In BE READY, patients who achieved PASI 90 at week 16 were reallocated to receive bimekizumab every 4 weeks, bimekizumab every 8 weeks (also 320 mg), or placebo. Both schedules of bimekizumab maintained responses through week 56, according to the authors, led by Kenneth B. Gordon, MD, professor and chair of dermatology, Medical College of Wisconsin, Milwaukee.
In both trials, safety was evaluated over the first 16 weeks as well as over a subsequent maintenance period, which extended to 52 weeks in BE VIVID and 56 weeks in BE READY. For bimekizumab, oral candidiasis was the most common treatment-related adverse event. In BE VIVID, this adverse event was reported in 9% of bimekizumab patients, compared with 0% of either the ustekinumab or placebo groups, up to week 16. Out to week 52, the rates were 15% in the bimekizumab group and 1% in the ustekinumab group.
In the BE READY trial, the rates of oral candidiasis were 6% and 0% for bimekizumab and placebo, respectively, through week 16. Over the maintenance periods, the rates were 9% and 11% for the every-8-week and every-4-week doses, respectively.
Discontinuation for adverse events was not higher on bimekizumab than placebo in either trial, nor was the proportion of serious treatment-emergent adverse events.
Nevertheless, the potential for adverse events was a key part of the discussion regarding the future role of bimekizumab, if approved, in an editorial that accompanied the publication of these studies.
“Bimekizumab might be our most effective biologic for psoriasis yet,” coauthors, William W. Huang, MD, PhD, associate professor of dermatology, and Steven R. Feldman, MD, PhD, professor of dermatology, both at Wake Forest University, Winston-Salem, NC, wrote in the editorial. “If the goal of psoriasis treatment is complete clearance, bimekizumab seems like a good option from an efficacy perspective.”
However, they noted that other IL-17 blockers, like secukinumab (Cosentyx) and brodalumab (Siliq), have been associated with risks, including the development of inflammatory bowel disease. In addition to the oral candidiasis seen in the BE VIVID and BE READY trials, they cautioned that other issues might arise with longer follow-up and greater numbers of patients exposed to this therapy.
In an interview, Dr. Feldman said adequately informed patients might be willing to accept these risks for the potential of greater efficacy, but he emphasized the need for appropriate warnings and education.
“We have a lot of very good treatments that offer patients an excellent chance of an excellent outcome – treatments that have been around and in use in large numbers of people for years,” Dr. Feldman said. “Unless the doctor and patient felt strongly about the need to use this new, perhaps more potent option, I would be personally inclined to use treatment with well-established safety profiles first.”
The senior author of the BE VIVID trial, Mark Lebwohl, MD, dean for clinical therapeutics and professor of dermatology, at the Icahn School of Medicine at Mount Sinai, New York, disagreed. He acknowledged that other agents targeting IL-17 have been associated with IBD, but risk of IBD is already elevated in patients with psoriasis and the risk appears to be lower with bimekizumab relative to prior agents in this class.
“Bimekizumab has now been studied in thousands of patients over several years. We can say with support from a sizable amount of data that IBD is very uncommon,” he said. While oral candidiasis is associated with bimekizumab, it is “easy to treat.”
Asked specifically if he will consider using bimekizumab as a first-line agent in psoriasis patients who are candidates for a biologic, Dr. Lebwohl said he would. Based on the evidence that this agent is more effective than other options and has manageable side effects, he believes it will be an important new treatment option.
Dr. Reich, Dr. Lebwohl, Dr. Gordon, and Dr. Feldman have financial relationships with multiple companies that produce therapies for psoriasis, including UCB Pharma, the sponsor of these studies.
with other biologics, according to data from two simultaneously published trials, one of which was a head-to-head comparison with ustekinumab.
In the head-to-head trial called BE VIVID, which included a placebo arm, there was a large advantage of bimekizumab over ustekinumab, a biologic that targets IL-12 and IL-23 and is approved for treating psoriasis, for both coprimary endpoints, according to a multinational group of investigators led by Kristian Reich, MD, PhD, professor of dermatology at the University Medical Center, Hamburg-Eppendorf, Germany.
The proportion of patients with skin clearance was not only greater but faster, “with responses observed after one dose,” Dr. Reich and coinvestigators reported.
The data from the BE VIVID trial was published simultaneously with the BE READY trial, which was placebo-controlled but did not include an active comparator.
Evaluated at week 16, the coprimary endpoints in both studies were skin clearance as measured by a Psoriasis Area Severity Index greater than 90% (PASI 90) and Investigators Global Assessment (IGA) score of 0 (clear) or 1 (almost clear).
In BE VIVID, 567 patients were randomized in 11 countries, including the United States. The dose of bimekizumab was 320 mg administered subcutaneously every 4 weeks. In a randomization scheme of 4:2:1, half as many patients (163) were randomized to ustekinumab (Stelara), which was administered in weight-based dosing of 45 mg or 90 mg at enrollment, at 4 weeks, and then every 12 weeks. The placebo arm had 83 patients. All were switched to bimekizumab at 16 weeks.
At week 16, PASI 90 was achieved in 85% of patients randomized to bimekizumab, compared with 50% of patients randomized to ustekinumab (P < .0001). The rate in the placebo group was 5%.
The bimekizumab advantage for an IGA response of 0 or 1 was of similar magnitude, relative to ustekinumab (84% vs. 53%; P < .0001) and placebo (5%). All secondary efficacy endpoints, such as PASI 90 at week 12 (85% vs. 44%) and PASI 100 at week 16 (59% vs. 21%), favored bimekizumab over ustekinumab.
In the BE READY trial, which evaluated the same dose and schedule of bimekizumab, the rates of PASI 90 at week 16 were 91% and 1% (P < .0001) for the experimental arm and placebo, respectively. The proportion of patients with an IGA score of 0 or 1 were 93% and 1% (P < .0001), respectively.
In BE READY, patients who achieved PASI 90 at week 16 were reallocated to receive bimekizumab every 4 weeks, bimekizumab every 8 weeks (also 320 mg), or placebo. Both schedules of bimekizumab maintained responses through week 56, according to the authors, led by Kenneth B. Gordon, MD, professor and chair of dermatology, Medical College of Wisconsin, Milwaukee.
In both trials, safety was evaluated over the first 16 weeks as well as over a subsequent maintenance period, which extended to 52 weeks in BE VIVID and 56 weeks in BE READY. For bimekizumab, oral candidiasis was the most common treatment-related adverse event. In BE VIVID, this adverse event was reported in 9% of bimekizumab patients, compared with 0% of either the ustekinumab or placebo groups, up to week 16. Out to week 52, the rates were 15% in the bimekizumab group and 1% in the ustekinumab group.
In the BE READY trial, the rates of oral candidiasis were 6% and 0% for bimekizumab and placebo, respectively, through week 16. Over the maintenance periods, the rates were 9% and 11% for the every-8-week and every-4-week doses, respectively.
Discontinuation for adverse events was not higher on bimekizumab than placebo in either trial, nor was the proportion of serious treatment-emergent adverse events.
Nevertheless, the potential for adverse events was a key part of the discussion regarding the future role of bimekizumab, if approved, in an editorial that accompanied the publication of these studies.
“Bimekizumab might be our most effective biologic for psoriasis yet,” coauthors, William W. Huang, MD, PhD, associate professor of dermatology, and Steven R. Feldman, MD, PhD, professor of dermatology, both at Wake Forest University, Winston-Salem, NC, wrote in the editorial. “If the goal of psoriasis treatment is complete clearance, bimekizumab seems like a good option from an efficacy perspective.”
However, they noted that other IL-17 blockers, like secukinumab (Cosentyx) and brodalumab (Siliq), have been associated with risks, including the development of inflammatory bowel disease. In addition to the oral candidiasis seen in the BE VIVID and BE READY trials, they cautioned that other issues might arise with longer follow-up and greater numbers of patients exposed to this therapy.
In an interview, Dr. Feldman said adequately informed patients might be willing to accept these risks for the potential of greater efficacy, but he emphasized the need for appropriate warnings and education.
“We have a lot of very good treatments that offer patients an excellent chance of an excellent outcome – treatments that have been around and in use in large numbers of people for years,” Dr. Feldman said. “Unless the doctor and patient felt strongly about the need to use this new, perhaps more potent option, I would be personally inclined to use treatment with well-established safety profiles first.”
The senior author of the BE VIVID trial, Mark Lebwohl, MD, dean for clinical therapeutics and professor of dermatology, at the Icahn School of Medicine at Mount Sinai, New York, disagreed. He acknowledged that other agents targeting IL-17 have been associated with IBD, but risk of IBD is already elevated in patients with psoriasis and the risk appears to be lower with bimekizumab relative to prior agents in this class.
“Bimekizumab has now been studied in thousands of patients over several years. We can say with support from a sizable amount of data that IBD is very uncommon,” he said. While oral candidiasis is associated with bimekizumab, it is “easy to treat.”
Asked specifically if he will consider using bimekizumab as a first-line agent in psoriasis patients who are candidates for a biologic, Dr. Lebwohl said he would. Based on the evidence that this agent is more effective than other options and has manageable side effects, he believes it will be an important new treatment option.
Dr. Reich, Dr. Lebwohl, Dr. Gordon, and Dr. Feldman have financial relationships with multiple companies that produce therapies for psoriasis, including UCB Pharma, the sponsor of these studies.
with other biologics, according to data from two simultaneously published trials, one of which was a head-to-head comparison with ustekinumab.
In the head-to-head trial called BE VIVID, which included a placebo arm, there was a large advantage of bimekizumab over ustekinumab, a biologic that targets IL-12 and IL-23 and is approved for treating psoriasis, for both coprimary endpoints, according to a multinational group of investigators led by Kristian Reich, MD, PhD, professor of dermatology at the University Medical Center, Hamburg-Eppendorf, Germany.
The proportion of patients with skin clearance was not only greater but faster, “with responses observed after one dose,” Dr. Reich and coinvestigators reported.
The data from the BE VIVID trial was published simultaneously with the BE READY trial, which was placebo-controlled but did not include an active comparator.
Evaluated at week 16, the coprimary endpoints in both studies were skin clearance as measured by a Psoriasis Area Severity Index greater than 90% (PASI 90) and Investigators Global Assessment (IGA) score of 0 (clear) or 1 (almost clear).
In BE VIVID, 567 patients were randomized in 11 countries, including the United States. The dose of bimekizumab was 320 mg administered subcutaneously every 4 weeks. In a randomization scheme of 4:2:1, half as many patients (163) were randomized to ustekinumab (Stelara), which was administered in weight-based dosing of 45 mg or 90 mg at enrollment, at 4 weeks, and then every 12 weeks. The placebo arm had 83 patients. All were switched to bimekizumab at 16 weeks.
At week 16, PASI 90 was achieved in 85% of patients randomized to bimekizumab, compared with 50% of patients randomized to ustekinumab (P < .0001). The rate in the placebo group was 5%.
The bimekizumab advantage for an IGA response of 0 or 1 was of similar magnitude, relative to ustekinumab (84% vs. 53%; P < .0001) and placebo (5%). All secondary efficacy endpoints, such as PASI 90 at week 12 (85% vs. 44%) and PASI 100 at week 16 (59% vs. 21%), favored bimekizumab over ustekinumab.
In the BE READY trial, which evaluated the same dose and schedule of bimekizumab, the rates of PASI 90 at week 16 were 91% and 1% (P < .0001) for the experimental arm and placebo, respectively. The proportion of patients with an IGA score of 0 or 1 were 93% and 1% (P < .0001), respectively.
In BE READY, patients who achieved PASI 90 at week 16 were reallocated to receive bimekizumab every 4 weeks, bimekizumab every 8 weeks (also 320 mg), or placebo. Both schedules of bimekizumab maintained responses through week 56, according to the authors, led by Kenneth B. Gordon, MD, professor and chair of dermatology, Medical College of Wisconsin, Milwaukee.
In both trials, safety was evaluated over the first 16 weeks as well as over a subsequent maintenance period, which extended to 52 weeks in BE VIVID and 56 weeks in BE READY. For bimekizumab, oral candidiasis was the most common treatment-related adverse event. In BE VIVID, this adverse event was reported in 9% of bimekizumab patients, compared with 0% of either the ustekinumab or placebo groups, up to week 16. Out to week 52, the rates were 15% in the bimekizumab group and 1% in the ustekinumab group.
In the BE READY trial, the rates of oral candidiasis were 6% and 0% for bimekizumab and placebo, respectively, through week 16. Over the maintenance periods, the rates were 9% and 11% for the every-8-week and every-4-week doses, respectively.
Discontinuation for adverse events was not higher on bimekizumab than placebo in either trial, nor was the proportion of serious treatment-emergent adverse events.
Nevertheless, the potential for adverse events was a key part of the discussion regarding the future role of bimekizumab, if approved, in an editorial that accompanied the publication of these studies.
“Bimekizumab might be our most effective biologic for psoriasis yet,” coauthors, William W. Huang, MD, PhD, associate professor of dermatology, and Steven R. Feldman, MD, PhD, professor of dermatology, both at Wake Forest University, Winston-Salem, NC, wrote in the editorial. “If the goal of psoriasis treatment is complete clearance, bimekizumab seems like a good option from an efficacy perspective.”
However, they noted that other IL-17 blockers, like secukinumab (Cosentyx) and brodalumab (Siliq), have been associated with risks, including the development of inflammatory bowel disease. In addition to the oral candidiasis seen in the BE VIVID and BE READY trials, they cautioned that other issues might arise with longer follow-up and greater numbers of patients exposed to this therapy.
In an interview, Dr. Feldman said adequately informed patients might be willing to accept these risks for the potential of greater efficacy, but he emphasized the need for appropriate warnings and education.
“We have a lot of very good treatments that offer patients an excellent chance of an excellent outcome – treatments that have been around and in use in large numbers of people for years,” Dr. Feldman said. “Unless the doctor and patient felt strongly about the need to use this new, perhaps more potent option, I would be personally inclined to use treatment with well-established safety profiles first.”
The senior author of the BE VIVID trial, Mark Lebwohl, MD, dean for clinical therapeutics and professor of dermatology, at the Icahn School of Medicine at Mount Sinai, New York, disagreed. He acknowledged that other agents targeting IL-17 have been associated with IBD, but risk of IBD is already elevated in patients with psoriasis and the risk appears to be lower with bimekizumab relative to prior agents in this class.
“Bimekizumab has now been studied in thousands of patients over several years. We can say with support from a sizable amount of data that IBD is very uncommon,” he said. While oral candidiasis is associated with bimekizumab, it is “easy to treat.”
Asked specifically if he will consider using bimekizumab as a first-line agent in psoriasis patients who are candidates for a biologic, Dr. Lebwohl said he would. Based on the evidence that this agent is more effective than other options and has manageable side effects, he believes it will be an important new treatment option.
Dr. Reich, Dr. Lebwohl, Dr. Gordon, and Dr. Feldman have financial relationships with multiple companies that produce therapies for psoriasis, including UCB Pharma, the sponsor of these studies.
FROM THE LANCET
Home Phototherapy During the COVID-19 Pandemic
Office-based phototherapy practices have closed or are operating below capacity because of the coronavirus disease 2019 (COVID-19) pandemic.1 Social distancing measures to reduce virus transmission are a significant driving factor.1-3 In the age of biologics, other options requiring fewer patient visits are available, such as UVB phototherapy. UV phototherapy is considered first line when more than 10% of the body surface area is affected.4 Although phototherapy often is performed in the office, it also may be delivered at home.2 Home-based phototherapy is safe, effective, and similar in cost to office-based phototherapy.4 Currently, there are limited COVID-19–specific guidelines for home-based phototherapy.
The risks and sequelae of COVID-19 are still being investigated, with cases varying by location. As such, local and national public health recommendations are evolving. Dermatologists must make individualized decisions about practice services, as local restrictions differ. As office-based phototherapy services may struggle to implement mitigation strategies, home-based phototherapy is an increasingly viable treatment option.1,4,5 Patient benefits of home therapy include improved treatment compliance; greater patient satisfaction; reduced travel/waiting time; and reduced long-term cost, including co-pays, depending on insurance coverage.2,4
We aim to provide recommendations on home-based phototherapy during the pandemic. Throughout the decision-making process, careful consideration of safety, risks, benefits, and treatment options for physicians, staff, and patients will be vital to the successful implementation of home-based phototherapy. Our recommendations are based on maximizing benefits and minimizing risks.
Considerations for Physicians
Physicians should take the following steps when assessing if home phototherapy is an option for each patient.1,2,4
• Determine patient eligibility for phototherapy treatment if currently not on phototherapy
• Carefully review patient and provider requirements for home phototherapy supplier
• Review patient history of treatment compliance
• Determine insurance coverage and consider exclusion criteria
• Review prior treatments
• Provide education on side effects
• Provide education on signs of adequate treatment response
• Indicate the type of UV light and unit on the prescription
• Consider whether the patient is in the maintenance or initiation phase when providing recommendations
• Work with the supplier if the light therapy unit is denied by submitting an appeal or prescribing a different unit
• Follow up with telemedicine to assess treatment effectiveness and monitor for adverse effects
Considerations for Patients
Counsel patients to weigh the risks and benefits of home phototherapy prescription and usage.1,2,4
• Evaluate cost
• Carefully review patient and provider requirements for home phototherapy supplier
• Ensure a complete understanding of treatment schedule
• Properly utilize protective equipment (eg, genital shields for men, eye shields for all)
• Avoid sharing phototherapy units with household members
• Disinfect and maintain units
• Maintain proper ventilation of spaces
• Maintain treatment log
• Attend follow-up
Treatment Alternatives
For patients with severe psoriasis, there are alternative treatments to office and home phototherapy. Biologics, immunosuppressive therapies, and other treatment options may be considered on a case-by-case basis.3,4,6 Currently, recommendations for the risk of COVID-19 with biologics or systemic immunosuppressive therapies remains inconsistent and should be carefully considered when providing alternative treatments.7-11
Final Thoughts
As restrictions are lifted according to local public health measures, prepandemic office phototherapy practices may resume operations. Home phototherapy is a practical and effective alternative for treatment of psoriasis when access to the office setting is limited.
- Lim HW, Feldman SR, Van Voorhees AS, et al. Recommendations for phototherapy during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:287-288.
Anderson KL, Feldman SR. A guide to prescribing home phototherapy for patients with psoriasis: the appropriate patient, the type of unit, the treatment regimen, and the potential obstacles. J Am Acad Dermatol. 2015;72:868.E1-878.E1. - Palmore TN, Smith BA. Coronavirus disease 2019 (COVID-19): infection control in health care and home settings. UpToDate. Updated January 7, 2021. Accessed January 25, 2021.https://www.uptodate.com/contents/coronavirus-disease-2019-covid-19-infection-control-in-health-care-and-home-settings
- Koek MB, Buskens E, van Weelden H, et al. Home versus outpatient ultraviolet B phototherapy for mild to severe psoriasis: pragmatic multicentre randomised controlled non-inferiority trial (PLUTO study). BMJ. 2009;338:b1542.
- Sadeghinia A, Daneshpazhooh M. Immunosuppressive drugs for patients with psoriasis during the COVID-19 pandemic era. a review [published online November 3, 2020]. Dermatol Ther. 2020:E14498. doi:10.1111/dth.14498
- Damiani G, Pacifico A, Bragazzi NL, et al. Biologics increase the risk of SARS-CoV-2 infection and hospitalization, but not ICU admission and death: real-life data from a large cohort during red-zone declaration. Dermatol Ther. 2020;33:E13475.
- Lebwohl M, Rivera-Oyola R, Murrell DF. Should biologics for psoriasis be interrupted in the era of COVID-19? J Am Acad Dermatol. 2020;82:1217-1218.
- Mehta P, Ciurtin C, Scully M, et al. JAK inhibitors in COVID-19: the need for vigilance regarding increased inherent thrombotic risk. Eur Respir J. 2020;56:2001919.
- Walz L, Cohen AJ, Rebaza AP, et al. JAK-inhibitor and type I interferon ability to produce favorable clinical outcomes in COVID-19 patients: a systematic review and meta-analysis. BMC Infect Dis. 2021;21:47.
- Carugno A, Gambini DM, Raponi F, et al. COVID-19 and biologics for psoriasis: a high-epidemic area experience-Bergamo, Lombardy, Italy. J Am Acad Dermatol. 2020;83:292-294.
- Gisondi P, Piaserico S, Naldi L, et al. Incidence rates of hospitalization and death from COVID-19 in patients with psoriasis receiving biological treatment: a Northern Italy experience [published online November 5, 2020]. J Allergy Clin Immunol. doi:10.1016/j.jaci.2020.10.032
Office-based phototherapy practices have closed or are operating below capacity because of the coronavirus disease 2019 (COVID-19) pandemic.1 Social distancing measures to reduce virus transmission are a significant driving factor.1-3 In the age of biologics, other options requiring fewer patient visits are available, such as UVB phototherapy. UV phototherapy is considered first line when more than 10% of the body surface area is affected.4 Although phototherapy often is performed in the office, it also may be delivered at home.2 Home-based phototherapy is safe, effective, and similar in cost to office-based phototherapy.4 Currently, there are limited COVID-19–specific guidelines for home-based phototherapy.
The risks and sequelae of COVID-19 are still being investigated, with cases varying by location. As such, local and national public health recommendations are evolving. Dermatologists must make individualized decisions about practice services, as local restrictions differ. As office-based phototherapy services may struggle to implement mitigation strategies, home-based phototherapy is an increasingly viable treatment option.1,4,5 Patient benefits of home therapy include improved treatment compliance; greater patient satisfaction; reduced travel/waiting time; and reduced long-term cost, including co-pays, depending on insurance coverage.2,4
We aim to provide recommendations on home-based phototherapy during the pandemic. Throughout the decision-making process, careful consideration of safety, risks, benefits, and treatment options for physicians, staff, and patients will be vital to the successful implementation of home-based phototherapy. Our recommendations are based on maximizing benefits and minimizing risks.
Considerations for Physicians
Physicians should take the following steps when assessing if home phototherapy is an option for each patient.1,2,4
• Determine patient eligibility for phototherapy treatment if currently not on phototherapy
• Carefully review patient and provider requirements for home phototherapy supplier
• Review patient history of treatment compliance
• Determine insurance coverage and consider exclusion criteria
• Review prior treatments
• Provide education on side effects
• Provide education on signs of adequate treatment response
• Indicate the type of UV light and unit on the prescription
• Consider whether the patient is in the maintenance or initiation phase when providing recommendations
• Work with the supplier if the light therapy unit is denied by submitting an appeal or prescribing a different unit
• Follow up with telemedicine to assess treatment effectiveness and monitor for adverse effects
Considerations for Patients
Counsel patients to weigh the risks and benefits of home phototherapy prescription and usage.1,2,4
• Evaluate cost
• Carefully review patient and provider requirements for home phototherapy supplier
• Ensure a complete understanding of treatment schedule
• Properly utilize protective equipment (eg, genital shields for men, eye shields for all)
• Avoid sharing phototherapy units with household members
• Disinfect and maintain units
• Maintain proper ventilation of spaces
• Maintain treatment log
• Attend follow-up
Treatment Alternatives
For patients with severe psoriasis, there are alternative treatments to office and home phototherapy. Biologics, immunosuppressive therapies, and other treatment options may be considered on a case-by-case basis.3,4,6 Currently, recommendations for the risk of COVID-19 with biologics or systemic immunosuppressive therapies remains inconsistent and should be carefully considered when providing alternative treatments.7-11
Final Thoughts
As restrictions are lifted according to local public health measures, prepandemic office phototherapy practices may resume operations. Home phototherapy is a practical and effective alternative for treatment of psoriasis when access to the office setting is limited.
Office-based phototherapy practices have closed or are operating below capacity because of the coronavirus disease 2019 (COVID-19) pandemic.1 Social distancing measures to reduce virus transmission are a significant driving factor.1-3 In the age of biologics, other options requiring fewer patient visits are available, such as UVB phototherapy. UV phototherapy is considered first line when more than 10% of the body surface area is affected.4 Although phototherapy often is performed in the office, it also may be delivered at home.2 Home-based phototherapy is safe, effective, and similar in cost to office-based phototherapy.4 Currently, there are limited COVID-19–specific guidelines for home-based phototherapy.
The risks and sequelae of COVID-19 are still being investigated, with cases varying by location. As such, local and national public health recommendations are evolving. Dermatologists must make individualized decisions about practice services, as local restrictions differ. As office-based phototherapy services may struggle to implement mitigation strategies, home-based phototherapy is an increasingly viable treatment option.1,4,5 Patient benefits of home therapy include improved treatment compliance; greater patient satisfaction; reduced travel/waiting time; and reduced long-term cost, including co-pays, depending on insurance coverage.2,4
We aim to provide recommendations on home-based phototherapy during the pandemic. Throughout the decision-making process, careful consideration of safety, risks, benefits, and treatment options for physicians, staff, and patients will be vital to the successful implementation of home-based phototherapy. Our recommendations are based on maximizing benefits and minimizing risks.
Considerations for Physicians
Physicians should take the following steps when assessing if home phototherapy is an option for each patient.1,2,4
• Determine patient eligibility for phototherapy treatment if currently not on phototherapy
• Carefully review patient and provider requirements for home phototherapy supplier
• Review patient history of treatment compliance
• Determine insurance coverage and consider exclusion criteria
• Review prior treatments
• Provide education on side effects
• Provide education on signs of adequate treatment response
• Indicate the type of UV light and unit on the prescription
• Consider whether the patient is in the maintenance or initiation phase when providing recommendations
• Work with the supplier if the light therapy unit is denied by submitting an appeal or prescribing a different unit
• Follow up with telemedicine to assess treatment effectiveness and monitor for adverse effects
Considerations for Patients
Counsel patients to weigh the risks and benefits of home phototherapy prescription and usage.1,2,4
• Evaluate cost
• Carefully review patient and provider requirements for home phototherapy supplier
• Ensure a complete understanding of treatment schedule
• Properly utilize protective equipment (eg, genital shields for men, eye shields for all)
• Avoid sharing phototherapy units with household members
• Disinfect and maintain units
• Maintain proper ventilation of spaces
• Maintain treatment log
• Attend follow-up
Treatment Alternatives
For patients with severe psoriasis, there are alternative treatments to office and home phototherapy. Biologics, immunosuppressive therapies, and other treatment options may be considered on a case-by-case basis.3,4,6 Currently, recommendations for the risk of COVID-19 with biologics or systemic immunosuppressive therapies remains inconsistent and should be carefully considered when providing alternative treatments.7-11
Final Thoughts
As restrictions are lifted according to local public health measures, prepandemic office phototherapy practices may resume operations. Home phototherapy is a practical and effective alternative for treatment of psoriasis when access to the office setting is limited.
- Lim HW, Feldman SR, Van Voorhees AS, et al. Recommendations for phototherapy during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:287-288.
Anderson KL, Feldman SR. A guide to prescribing home phototherapy for patients with psoriasis: the appropriate patient, the type of unit, the treatment regimen, and the potential obstacles. J Am Acad Dermatol. 2015;72:868.E1-878.E1. - Palmore TN, Smith BA. Coronavirus disease 2019 (COVID-19): infection control in health care and home settings. UpToDate. Updated January 7, 2021. Accessed January 25, 2021.https://www.uptodate.com/contents/coronavirus-disease-2019-covid-19-infection-control-in-health-care-and-home-settings
- Koek MB, Buskens E, van Weelden H, et al. Home versus outpatient ultraviolet B phototherapy for mild to severe psoriasis: pragmatic multicentre randomised controlled non-inferiority trial (PLUTO study). BMJ. 2009;338:b1542.
- Sadeghinia A, Daneshpazhooh M. Immunosuppressive drugs for patients with psoriasis during the COVID-19 pandemic era. a review [published online November 3, 2020]. Dermatol Ther. 2020:E14498. doi:10.1111/dth.14498
- Damiani G, Pacifico A, Bragazzi NL, et al. Biologics increase the risk of SARS-CoV-2 infection and hospitalization, but not ICU admission and death: real-life data from a large cohort during red-zone declaration. Dermatol Ther. 2020;33:E13475.
- Lebwohl M, Rivera-Oyola R, Murrell DF. Should biologics for psoriasis be interrupted in the era of COVID-19? J Am Acad Dermatol. 2020;82:1217-1218.
- Mehta P, Ciurtin C, Scully M, et al. JAK inhibitors in COVID-19: the need for vigilance regarding increased inherent thrombotic risk. Eur Respir J. 2020;56:2001919.
- Walz L, Cohen AJ, Rebaza AP, et al. JAK-inhibitor and type I interferon ability to produce favorable clinical outcomes in COVID-19 patients: a systematic review and meta-analysis. BMC Infect Dis. 2021;21:47.
- Carugno A, Gambini DM, Raponi F, et al. COVID-19 and biologics for psoriasis: a high-epidemic area experience-Bergamo, Lombardy, Italy. J Am Acad Dermatol. 2020;83:292-294.
- Gisondi P, Piaserico S, Naldi L, et al. Incidence rates of hospitalization and death from COVID-19 in patients with psoriasis receiving biological treatment: a Northern Italy experience [published online November 5, 2020]. J Allergy Clin Immunol. doi:10.1016/j.jaci.2020.10.032
- Lim HW, Feldman SR, Van Voorhees AS, et al. Recommendations for phototherapy during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:287-288.
Anderson KL, Feldman SR. A guide to prescribing home phototherapy for patients with psoriasis: the appropriate patient, the type of unit, the treatment regimen, and the potential obstacles. J Am Acad Dermatol. 2015;72:868.E1-878.E1. - Palmore TN, Smith BA. Coronavirus disease 2019 (COVID-19): infection control in health care and home settings. UpToDate. Updated January 7, 2021. Accessed January 25, 2021.https://www.uptodate.com/contents/coronavirus-disease-2019-covid-19-infection-control-in-health-care-and-home-settings
- Koek MB, Buskens E, van Weelden H, et al. Home versus outpatient ultraviolet B phototherapy for mild to severe psoriasis: pragmatic multicentre randomised controlled non-inferiority trial (PLUTO study). BMJ. 2009;338:b1542.
- Sadeghinia A, Daneshpazhooh M. Immunosuppressive drugs for patients with psoriasis during the COVID-19 pandemic era. a review [published online November 3, 2020]. Dermatol Ther. 2020:E14498. doi:10.1111/dth.14498
- Damiani G, Pacifico A, Bragazzi NL, et al. Biologics increase the risk of SARS-CoV-2 infection and hospitalization, but not ICU admission and death: real-life data from a large cohort during red-zone declaration. Dermatol Ther. 2020;33:E13475.
- Lebwohl M, Rivera-Oyola R, Murrell DF. Should biologics for psoriasis be interrupted in the era of COVID-19? J Am Acad Dermatol. 2020;82:1217-1218.
- Mehta P, Ciurtin C, Scully M, et al. JAK inhibitors in COVID-19: the need for vigilance regarding increased inherent thrombotic risk. Eur Respir J. 2020;56:2001919.
- Walz L, Cohen AJ, Rebaza AP, et al. JAK-inhibitor and type I interferon ability to produce favorable clinical outcomes in COVID-19 patients: a systematic review and meta-analysis. BMC Infect Dis. 2021;21:47.
- Carugno A, Gambini DM, Raponi F, et al. COVID-19 and biologics for psoriasis: a high-epidemic area experience-Bergamo, Lombardy, Italy. J Am Acad Dermatol. 2020;83:292-294.
- Gisondi P, Piaserico S, Naldi L, et al. Incidence rates of hospitalization and death from COVID-19 in patients with psoriasis receiving biological treatment: a Northern Italy experience [published online November 5, 2020]. J Allergy Clin Immunol. doi:10.1016/j.jaci.2020.10.032
Practice Points
- Home phototherapy is a safe and effective option for patients with psoriasis during the coronavirus disease 2019 (COVID-19) pandemic.
- Although a consensus has not been reached with systemic immunosuppressive therapies for patients with psoriasis and the risk of COVID-19, we continue to recommend caution and careful monitoring of clinical outcomes for patients.
Translating the 2020 AAD-NPF Guidelines of Care for the Management of Psoriasis With Systemic Nonbiologics to Clinical Practice
Psoriasis is a chronic relapsing skin condition characterized by keratinocyte hyperproliferation and a chronic inflammatory cascade. Therefore, controlling inflammatory responses with systemic medications is beneficial in managing psoriatic lesions and their accompanying symptoms, especially in disease inadequately controlled by topicals. Ease of drug administration and treatment availability are benefits that systemic nonbiologic therapies may have over biologic therapies.
In 2020, the American Academy of Dermatology (AAD) and the National Psoriasis Foundation (NPF) published guidelines for managing psoriasis in adults with systemic nonbiologic therapies.1 Dosing, efficacy, toxicity, drug-related interactions, and contraindications are addressed alongside evidence-based treatment recommendations. This review addresses current recommendations for systemic nonbiologics in psoriasis with a focus on the treatments approved by the US Food and Drug Administration (FDA): acitretin, apremilast, cyclosporine, and methotrexate (eTable). Fumaric acid esters and tofacitinib are FDA approved for psoriatic arthritis but not for plaque psoriasis. Additional long-term safety analyses of tofacitinib for plaque psoriasis were requested by the FDA. Dimethyl fumarate is approved by the European Medicines Agency for treatment of psoriasis and is among the first-line systemic treatments used in Germany.2
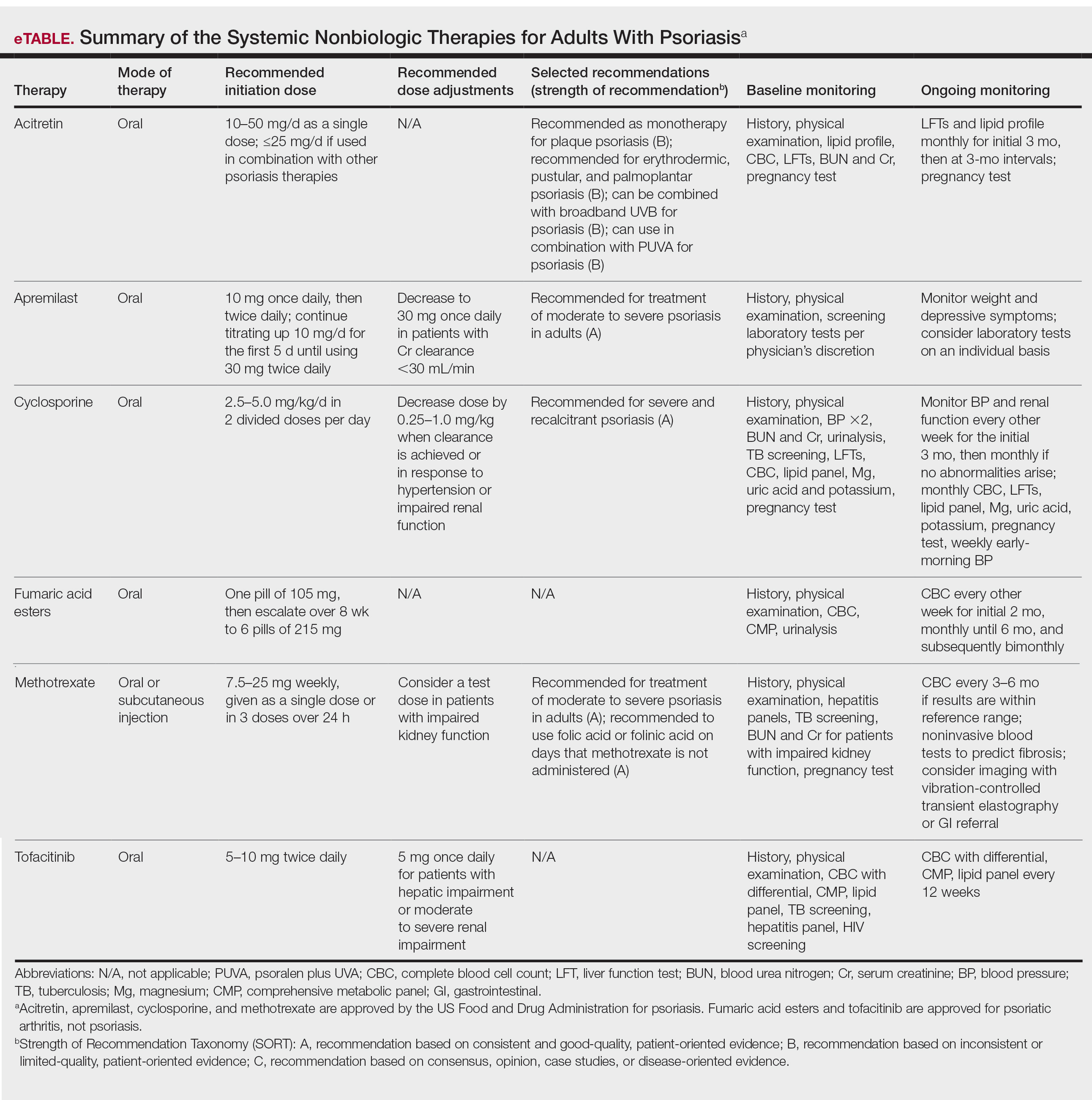
Selecting a Systemic Nonbiologic Agent
Methotrexate and apremilast have a strength level A recommendation for treating moderate to severe psoriasis in adults. However, methotrexate is less effective than biologic agents, including adalimumab and infliximab, for cutaneous psoriasis. Methotrexate is believed to improve psoriasis because of its direct immunosuppressive effect and inhibition of lymphoid cell proliferation. It typically is administered orally but can be administered subcutaneously for decreased gastrointestinal (GI) adverse effects. Compliance with close laboratory monitoring and lifestyle modifications, such as contraceptive use (because of teratogenicity) and alcohol cessation (because of the risk of liver damage) are essential in patients using methotrexate.
Apremilast, the most recently FDA-approved oral systemic medication for psoriasis, inhibits phosphodiesterase 4, subsequently decreasing inflammatory responses involving helper T cells TH1 and TH17 as well as type 1 interferon pathways. Apremilast is particularly effective in treating psoriasis with scalp and palmoplantar involvement.3 Additionally, it has an encouraging safety profile and is favorable in patients with multiple comorbidities.
Among the 4 oral agents, cyclosporine has the quickest onset of effect and has a strength level A recommendation for treating severe and recalcitrant psoriasis. Because of its high-risk profile, it is recommended for short periods of time, acute flares, or during transitions to safer long-term treatment. Patients with multiple comorbidities should avoid cyclosporine as a treatment option.
Acitretin, an FDA-approved oral retinoid, is an optimal treatment option for immunosuppressed patients or patients with HIV on antiretroviral therapy because it is not immunosuppressive.4 Unlike cyclosporine, acitretin is less helpful for acute flares because it takes 3 to 6 months to reach peak therapeutic response for treating plaque psoriasis. Similar to cyclosporine, acitretin can be recommended for severe psoriatic variants of erythrodermic, generalized pustular, and palmoplantar psoriasis. Acitretin has been reported to be more effective and have a more rapid onset of action in erythrodermic and pustular psoriasis than in plaque psoriasis.5
Patient Comorbidities
Psoriatic arthritis (PsA) is a common comorbidity that affects treatment choice. Patients with coexisting PsA could be treated with apremilast, as it is approved for both psoriasis and PsA. In a phase 3 randomized, controlled trial, American College of Rheumatology (ACR) 20 response at weeks 16 and 52 was achieved by significantly more patients on apremilast at 20 mg twice daily (BID)(P=.0166) or 30 mg BID (P=.0001) than placebo.6 Although not FDA approved for PsA, methotrexate has been shown to improve concomitant PsA of the peripheral joints in patients with psoriasis. Furthermore, a trial of methotrexate has shown considerable improvements in PsA symptoms in patients with psoriasis—a 62.7% decrease in proportion of patients with dactylitis, 25.7% decrease in enthesitis, and improvements in ACR outcomes (ACR20 in 40.8%, ACR50 in 18.8%, and ACR70 in 8.6%, with 22.4% achieving minimal disease activity).7
Prior to starting a systemic medication for psoriasis, it is necessary to discuss effects on pregnancy and fertility. Pregnancy is an absolute contraindication for methotrexate and acitretin use because of the drugs’ teratogenicity. Fetal death and fetal abnormalities have been reported with methotrexate use in pregnant women.8 Bone, central nervous system, auditory, ocular, and cardiovascular fetal abnormalities have been reported with maternal acitretin use.9 Breastfeeding also is an absolute contraindication for methotrexate use, as methotrexate passes into breastmilk in small quantities. Patients taking acitretin also are strongly discouraged from nursing because of the long half-life (168 days) of etretinate, a reverse metabolism product of acitretin that is increased in the presence of alcohol. Women should wait 3 months after discontinuing methotrexate for complete drug clearance before conceiving compared to 3 years in women who have discontinued acitretin.8,10 Men also are recommended to wait 3 months after discontinuing methotrexate before attempting to conceive, as its effect on male spermatogenesis and teratogenicity is unclear. Acitretin has no documented teratogenic effect in men. For women planning to become pregnant, apremilast and cyclosporine can be continued throughout pregnancy on an individual basis. The benefit of apremilast should be weighed against its potential risk to the fetus. There is no evidence of teratogenicity of apremilast at doses of 20 mg/kg daily.11 Current research regarding cyclosporine use in pregnancy only exists in transplant patients and has revealed higher rates of prematurity and lower birth weight without teratogenic effects.10,12 The risks and benefits of continuing cyclosporine while nursing should be evaluated, as cyclosporine (and ethanol-methanol components used in some formulations) is detectable in breast milk.
Drug Contraindications
Hypersensitivity to a specific systemic nonbiologic medication is a contraindication to its use and is an absolute contraindication for methotrexate. Other absolute contraindications to methotrexate are pregnancy and nursing, alcoholism, alcoholic liver disease, chronic liver disease, immunodeficiency, and cytopenia. Contraindications to acitretin include pregnancy, severely impaired liver and kidney function, and chronic abnormally elevated lipid levels. There are no additional contraindications for apremilast, but patients must be informed of the risk for depression before initiating therapy. Cyclosporine is contraindicated in patients with prior psoralen plus UVA (PUVA) treatment or radiation therapy, abnormal renal function, uncontrolled hypertension, uncontrolled and active infections, and a history of systemic malignancy. Live vaccines should be avoided in patients on cyclosporine, and caution is advised when cyclosporine is prescribed for patients with poorly controlled diabetes.
Pretreatment Screening
Because of drug interactions, a detailed medication history is essential prior to starting any systemic medication for psoriasis. Apremilast and cyclosporine are metabolized by cytochrome P450 and therefore are more susceptible to drug-related interactions. Cyclosporine use can affect levels of other medications that are metabolized by cytochrome P450, such as statins, calcium channel blockers, and warfarin. Similarly, acitretin’s metabolism is affected by drugs that interfere with cytochrome P450. Additionally, screening laboratory tests are needed before initiating systemic nonbiologic agents for psoriasis, with the exception of apremilast.
Prior to initiating methotrexate treatment, patients may require tuberculosis (TB), hepatitis B, and hepatitis C screening tests, depending on their risk factors. A baseline liver fibrosis assessment is recommended because of the potential of hepatotoxicity in patients receiving methotrexate. Noninvasive serology tests utilized to evaluate the presence of pre-existing liver disease include Fibrosis-4, FibroMeter, FibroSure, and Hepascore. Patients with impaired renal function have an increased predisposition to methotrexate-induced hematologic toxicity. Thus, it is necessary to administer a test dose of methotrexate in these patients followed by a complete blood cell count (CBC) 5 to 7 days later. An unremarkable CBC after the test dose suggests the absence of myelosuppression, and methotrexate dosage can be increased weekly. Patients on methotrexate also must receive folate supplementation to reduce the risk for adverse effects during treatment.
Patients considering cyclosporine must undergo screening for family and personal history of renal disease. Prior to initiating treatment, patients require 2 blood pressure measurements, hepatitis screening, TB screening, urinalysis, serum creatinine (Cr), blood urea nitrogen (BUN), CBC, potassium and magnesium levels, uric acid levels, lipid profile, bilirubin, and liver function tests (LFTs). A pregnancy test also is warranted for women of childbearing potential (WOCP).
Patients receiving acitretin should receive screening laboratory tests consisting of fasting cholesterol and triglycerides, CBC, renal function tests, LFTs, and a pregnancy test, if applicable.
After baseline evaluations, the selected oral systemic can be initiated using specific dosing regimens to ensure optimal drug efficacy and reduce incidence of adverse effects (eTable).
Monitoring During Active Treatment
Physicians need to counsel patients on potential adverse effects of their medications. Because of its relatively safe profile among the systemic nonbiologic agents, apremilast requires the least monitoring during treatment. There is no required routine laboratory monitoring for patients using apremilast, though testing may be pursued at the clinician’s discretion. However, weight should be regularly measured in patients on apremilast. In a phase 3 clinical trial of patients with psoriasis, 12% of patients on apremilast experienced a 5% to 10% weight loss compared to 5% of patients on placebo.11,13 Thus, it is recommended that physicians consider discontinuing apremilast in patients with a weight loss of more than 5% from baseline, especially if it may lead to other unfavorable health effects. Because depression is reported among 1% of patients on apremilast, close monitoring for new or worsening symptoms of depression should be performed during treatment.11,13 To avoid common GI side effects, apremilast is initiated at 10 mg/d and is increased by 10 mg/d over the first 5 days to a final dose of 30 mg BID. Elderly patients in particular should be cautioned about the risk of dehydration associated with GI side effects. Patients with severe renal impairment (Cr clearance, <30 mL/min) should use apremilast at a dosage of 30 mg once daily.
For patients on methotrexate, laboratory monitoring is essential after each dose increase. It also is important for physicians to obtain regular blood work to assess for hematologic abnormalities and hepatoxicity. Patients with risk factors such as renal insufficiency, increased age, hypoalbuminemia, alcohol abuse and alcoholic liver disease, and methotrexate dosing errors, as well as those prone to drug-related interactions, must be monitored closely for pancytopenia.14,15 The protocol for screening for methotrexate-induced hepatotoxicity during treatment depends on patient risk factors. Risk factors for hepatoxicity include history of or current alcohol abuse, abnormal LFTs, personal or family history of liver disease, diabetes, obesity, use of other hepatotoxic drugs, and hyperlipidemia.16 In patients without blood work abnormalities, CBC and LFTs can be performed every 3 to 6 months. Patients with abnormally elevated LFTs require repeat blood work every 2 to 4 weeks. Persistent elevations in LFTs require further evaluation by a GI specialist. After a cumulative dose of 3.5 to 4 g, patients should receive a GI referral and further studies (such as vibration-controlled transient elastography or liver biopsy) to assess for liver fibrosis. Patients with signs of stage 3 liver fibrosis are recommended to discontinue methotrexate and switch to another medication for psoriasis. For patients with impaired renal function, periodic BUN and Cr monitoring are needed. Common adverse effects of methotrexate include diarrhea, nausea, and anorexia, which can be mitigated by taking methotrexate with food or lowering the dosage.8 Patients on methotrexate should be monitored for rare but potential risks of infection and reactivation of latent TB, hepatitis, and lymphoma. To reduce the incidence of methotrexate toxicity from drug interactions, a review of current medications at each follow-up visit is recommended.
Nephrotoxicity and hypertension are the most common adverse effects of cyclosporine. It is important to monitor BUN and Cr biweekly for the initial 3 months, then at monthly intervals if there are no persistent abnormalities. Patients also must receive monthly CBC, potassium and magnesium levels, uric acid levels, lipid panel, serum bilirubin, and LFTs to monitor for adverse effects.17 Physicians should obtain regular pregnancy tests in WOCP. Weekly monitoring of early-morning blood pressure is recommended for patients on cyclosporine to detect early cyclosporine-induced nephrotoxicity. Hypertension on 2 separate occasions warrants a reduction in cyclosporine dosage or an addition of a calcium channel blocker for blood pressure control. Dose reduction also should be performed in patients with an increase in Cr above baseline greater than 25%.17 If Cr level is persistently elevated or if blood pressure does not normalize to lower than 140/90 after dose reduction, cyclosporine should be immediately discontinued. Patients on cyclosporine for more than a year warrant an annual estimation of glomerular filtration rate because of irreversible kidney damage associated with long-term use. A systematic review of patients treated with cyclosporine for more than 2 years found that at least 50% of patients experienced a 30% increase in Cr above baseline.18
Patients taking acitretin should be monitored for hyperlipidemia, the most common laboratory abnormality seen in 25% to 50% of patients.19 Fasting lipid panel and LFTs should be performed monthly for the initial 3 months on acitretin, then at 3-month intervals. Lifestyle changes should be encouraged to reduce hyperlipidemia, and fibrates may be given to treat elevated triglyceride levels, the most common type of hyperlipidemia seen with acitretin. Acitretin-induced toxic hepatitis is a rare occurrence that warrants immediate discontinuation of the medication.20 Monthly pregnancy tests must be performed in WOCP.
Combination Therapy
For apremilast, there is anecdotal evidence supporting its use in conjunction with phototherapy or biologics in some cases, but no high-quality data.21 On the other hand, using combination therapy with other systemic therapies can reduce adverse effects and decrease the amount of medication needed to achieve psoriasis clearance. Methotrexate used with etanercept, for example, has been more effective than methotrexate monotherapy in treating psoriasis, which has been attributed to a methotrexate-mediated reduction in the production of antidrug antibodies.22,23
Methotrexate, cyclosporine, and acitretin have synergistic effects when used with phototherapy. Narrowband UVB (NB-UVB) phototherapy combined with methotrexate is more effective in clearing psoriasis than methotrexate or NB-UVB phototherapy alone. Similarly, acitretin and PUVA combination therapy is more effective than acitretin or PUVA phototherapy alone. Combination regimens of acitretin and broadband UVB phototherapy, acitretin and NB-UVB phototherapy, and acitretin and PUVA phototherapy also have been more effective than individual modalities alone. Combination therapy reduces the cumulative doses of both therapies and reduces the frequency and duration of phototherapy needed for psoriatic clearance.24 In acitretin combination therapy with UVB phototherapy, the recommended regimen is 2 weeks of acitretin monotherapy followed by UVB phototherapy. For patients with an inadequate response to UVB phototherapy, the UVB dose can be reduced by 30% to 50%, and acitretin 25 mg/d can be added to phototherapy treatment. Acitretin-UVB combination therapy has been shown to reduce the risk of UVB-induced erythema seen in UVB monotherapy. Similarly, the risk of squamous cell carcinoma is reduced in acitretin-PUVA combination therapy compared to PUVA monotherapy.25
The timing of phototherapy in combination with systemic nonbiologic agents is critical. Phototherapy used simultaneously with cyclosporine is contraindicated owing to increased risk of photocarcinogenesis, whereas phototherapy used in sequence with cyclosporine is well tolerated and effective. Furthermore, cyclosporine 3 mg/kg/d for 4 weeks followed by a rapid cyclosporine taper and initiation of NB-UVB phototherapy demonstrated resolution of psoriasis with fewer NB-UVB treatments and less UVB exposure than NB-UVB therapy alone.26
Final Thoughts
The FDA-approved systemic nonbiologic agents are accessible and effective treatment options for adults with widespread or inadequately controlled psoriasis. Selecting the ideal therapy requires careful consideration of medication toxicity, contraindications, monitoring requirements, and patient comorbidities. The AAD-NPF guidelines guide dermatologists in prescribing systemic nonbiologic treatments in adults with psoriasis. Utilizing these recommendations in combination with clinician judgment will help patients achieve safe and optimal psoriasis clearance.
- Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J Am Acad Dermatol. 2020;82:1445-1486.
- Mrowietz U, Barker J, Boehncke WH, et al. Clinical use of dimethyl fumarate in moderate-to-severe plaque-type psoriasis: a European expert consensus. J Eur Acad Dermatol Venereol. 2018;32(suppl 3):3-14.
- Van Voorhees AS, Gold LS, Lebwohl M, et al. Efficacy and safety of apremilast in patients with moderate to severe plaque psoriasis of the scalp: results of a phase 3b, multicenter, randomized, placebo-controlled, double-blind study. J Am Acad Dermatol. 2020;83:96-103.
- Buccheri L, Katchen BR, Karter AJ, et al. Acitretin therapy is effective for psoriasis associated with human immunodeficiency virus infection. Arch Dermatol. 1997;133:711-715.
- Ormerod AD, Campalani E, Goodfield MJD. British Association of Dermatologists guidelines on the efficacy and use of acitretin in dermatology. Br J Dermatol. 2010;162:952-963.
- Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. Longterm (52-week) results of a phase III randomized, controlled trial of apremilast in patients with psoriatic arthritis. J Rheumatol. 2015;42:479-488.
- Coates LC, Aslam T, Al Balushi F, et al. Comparison of three screening tools to detect psoriatic arthritis in patients with psoriasis (CONTEST study). Br J Dermatol. 2013;168:802-807.
- Antares Pharma, Inc. Otrexup PFS (methotrexate) [package insert]. US Food and Drug Administration website. Revised June 2019. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/204824s009lbl.pdf
- David M, Hodak E, Lowe NJ. Adverse effects of retinoids. Med Toxicol Adverse Drug Exp. 1988;3:273-288.
- Stiefel Laboratories, Inc. Soriatane (acitretin) [package insert]. US Food and Drug Administration website. Revised September 2017. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/019821s028lbl.pdf
- Celgene Corporation. Otezla (apremilast) [package insert]. US Food and Drug Administration website. Revised March 2014. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/205437s000lbl.pdf
- Ghanem ME, El-Baghdadi LA, Badawy AM, et al. Pregnancy outcome after renal allograft transplantation: 15 years experience. Eur J Obstet Gynecol Reprod Biol. 2005;121:178-181.
- Zerilli T, Ocheretyaner E. Apremilast (Otezla): A new oral treatment for adults with psoriasis and psoriatic arthritis. P T. 2015;40:495-500.
- Kivity S, Zafrir Y, Loebstein R, et al. Clinical characteristics and risk factors for low dose methotrexate toxicity: a cohort of 28 patients. Autoimmun Rev. 2014;13:1109-1113.
- Boffa MJ, Chalmers RJ. Methotrexate for psoriasis. Clin Exp Dermatol. 1996;21:399-408.
- Rosenberg P, Urwitz H, Johannesson A, et al. Psoriasis patients with diabetes type 2 are at high risk of developing liver fibrosis during methotrexate treatment. J Hepatol. 2007;46:1111-1118.
- Novartis Pharmaceuticals Corporation. Sandimmune (cyclosporine) [package insert]. US Food and Drug Administration website. Published 2015. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/050573s041,050574s051,050625s055lbl.pdf
- Maza A, Montaudie H, Sbidian E, et al. Oral cyclosporin in psoriasis: a systematic review on treatment modalities, risk of kidney toxicity and evidence for use in non-plaque psoriasis. J Eur Acad Dermatol Venereol. 2011;25(suppl 2):19-27.
- Yamauchi PS, Rizk D, Kormilli T, et al. Systemic retinoids. In: Weinstein GD, Gottlieb AB, eds. Therapy of Moderate-to-Severe Psoriasis. Marcel Dekker; 2003:137-150.
- van Ditzhuijsen TJ, van Haelst UJ, van Dooren-Greebe RJ, et al. Severe hepatotoxic reaction with progression to cirrhosis after use of a novel retinoid (acitretin). J Hepatol. 1990;11:185-188.
- AbuHilal M, Walsh S, Shear N. Use of apremilast in combination with other therapies for treatment of chronic plaque psoriasis: a retrospective study. J Cutan Med Surg. 2016;20:313-316.
- Gottlieb AB, Langley RG, Strober BE, et al. A randomized, double-blind, placebo-controlled study to evaluate the addition of methotrexate to etanercept in patients with moderate to severe plaque psoriasis. Br J Dermatol. 2012;167:649-657.
- Cronstein BN. Methotrexate BAFFles anti-drug antibodies. Nat Rev Rheumatol. 2018;14:505-506.
- Lebwohl M, Drake L, Menter A, et al. Consensus conference: acitretin in combination with UVB or PUVA in the treatment of psoriasis. J Am Acad Dermatol. 2001;45:544-553.
- Nijsten TE, Stern RS. Oral retinoid use reduces cutaneous squamous cell carcinoma risk in patients with psoriasis treated with psoralen-UVA: a nested cohort study. J Am Acad Dermatol. 2003;49:644-650.
- Calzavara-Pinton P, Leone G, Venturini M, et al. A comparative non randomized study of narrow-band (NB) (312 +/- 2 nm) UVB phototherapy versus sequential therapy with oral administration of low-dose cyclosporin A and NB-UVB phototherapy in patients with severe psoriasis vulgaris. Eur J Dermatol. 2005;15:470-473.
Psoriasis is a chronic relapsing skin condition characterized by keratinocyte hyperproliferation and a chronic inflammatory cascade. Therefore, controlling inflammatory responses with systemic medications is beneficial in managing psoriatic lesions and their accompanying symptoms, especially in disease inadequately controlled by topicals. Ease of drug administration and treatment availability are benefits that systemic nonbiologic therapies may have over biologic therapies.
In 2020, the American Academy of Dermatology (AAD) and the National Psoriasis Foundation (NPF) published guidelines for managing psoriasis in adults with systemic nonbiologic therapies.1 Dosing, efficacy, toxicity, drug-related interactions, and contraindications are addressed alongside evidence-based treatment recommendations. This review addresses current recommendations for systemic nonbiologics in psoriasis with a focus on the treatments approved by the US Food and Drug Administration (FDA): acitretin, apremilast, cyclosporine, and methotrexate (eTable). Fumaric acid esters and tofacitinib are FDA approved for psoriatic arthritis but not for plaque psoriasis. Additional long-term safety analyses of tofacitinib for plaque psoriasis were requested by the FDA. Dimethyl fumarate is approved by the European Medicines Agency for treatment of psoriasis and is among the first-line systemic treatments used in Germany.2

Selecting a Systemic Nonbiologic Agent
Methotrexate and apremilast have a strength level A recommendation for treating moderate to severe psoriasis in adults. However, methotrexate is less effective than biologic agents, including adalimumab and infliximab, for cutaneous psoriasis. Methotrexate is believed to improve psoriasis because of its direct immunosuppressive effect and inhibition of lymphoid cell proliferation. It typically is administered orally but can be administered subcutaneously for decreased gastrointestinal (GI) adverse effects. Compliance with close laboratory monitoring and lifestyle modifications, such as contraceptive use (because of teratogenicity) and alcohol cessation (because of the risk of liver damage) are essential in patients using methotrexate.
Apremilast, the most recently FDA-approved oral systemic medication for psoriasis, inhibits phosphodiesterase 4, subsequently decreasing inflammatory responses involving helper T cells TH1 and TH17 as well as type 1 interferon pathways. Apremilast is particularly effective in treating psoriasis with scalp and palmoplantar involvement.3 Additionally, it has an encouraging safety profile and is favorable in patients with multiple comorbidities.
Among the 4 oral agents, cyclosporine has the quickest onset of effect and has a strength level A recommendation for treating severe and recalcitrant psoriasis. Because of its high-risk profile, it is recommended for short periods of time, acute flares, or during transitions to safer long-term treatment. Patients with multiple comorbidities should avoid cyclosporine as a treatment option.
Acitretin, an FDA-approved oral retinoid, is an optimal treatment option for immunosuppressed patients or patients with HIV on antiretroviral therapy because it is not immunosuppressive.4 Unlike cyclosporine, acitretin is less helpful for acute flares because it takes 3 to 6 months to reach peak therapeutic response for treating plaque psoriasis. Similar to cyclosporine, acitretin can be recommended for severe psoriatic variants of erythrodermic, generalized pustular, and palmoplantar psoriasis. Acitretin has been reported to be more effective and have a more rapid onset of action in erythrodermic and pustular psoriasis than in plaque psoriasis.5
Patient Comorbidities
Psoriatic arthritis (PsA) is a common comorbidity that affects treatment choice. Patients with coexisting PsA could be treated with apremilast, as it is approved for both psoriasis and PsA. In a phase 3 randomized, controlled trial, American College of Rheumatology (ACR) 20 response at weeks 16 and 52 was achieved by significantly more patients on apremilast at 20 mg twice daily (BID)(P=.0166) or 30 mg BID (P=.0001) than placebo.6 Although not FDA approved for PsA, methotrexate has been shown to improve concomitant PsA of the peripheral joints in patients with psoriasis. Furthermore, a trial of methotrexate has shown considerable improvements in PsA symptoms in patients with psoriasis—a 62.7% decrease in proportion of patients with dactylitis, 25.7% decrease in enthesitis, and improvements in ACR outcomes (ACR20 in 40.8%, ACR50 in 18.8%, and ACR70 in 8.6%, with 22.4% achieving minimal disease activity).7
Prior to starting a systemic medication for psoriasis, it is necessary to discuss effects on pregnancy and fertility. Pregnancy is an absolute contraindication for methotrexate and acitretin use because of the drugs’ teratogenicity. Fetal death and fetal abnormalities have been reported with methotrexate use in pregnant women.8 Bone, central nervous system, auditory, ocular, and cardiovascular fetal abnormalities have been reported with maternal acitretin use.9 Breastfeeding also is an absolute contraindication for methotrexate use, as methotrexate passes into breastmilk in small quantities. Patients taking acitretin also are strongly discouraged from nursing because of the long half-life (168 days) of etretinate, a reverse metabolism product of acitretin that is increased in the presence of alcohol. Women should wait 3 months after discontinuing methotrexate for complete drug clearance before conceiving compared to 3 years in women who have discontinued acitretin.8,10 Men also are recommended to wait 3 months after discontinuing methotrexate before attempting to conceive, as its effect on male spermatogenesis and teratogenicity is unclear. Acitretin has no documented teratogenic effect in men. For women planning to become pregnant, apremilast and cyclosporine can be continued throughout pregnancy on an individual basis. The benefit of apremilast should be weighed against its potential risk to the fetus. There is no evidence of teratogenicity of apremilast at doses of 20 mg/kg daily.11 Current research regarding cyclosporine use in pregnancy only exists in transplant patients and has revealed higher rates of prematurity and lower birth weight without teratogenic effects.10,12 The risks and benefits of continuing cyclosporine while nursing should be evaluated, as cyclosporine (and ethanol-methanol components used in some formulations) is detectable in breast milk.
Drug Contraindications
Hypersensitivity to a specific systemic nonbiologic medication is a contraindication to its use and is an absolute contraindication for methotrexate. Other absolute contraindications to methotrexate are pregnancy and nursing, alcoholism, alcoholic liver disease, chronic liver disease, immunodeficiency, and cytopenia. Contraindications to acitretin include pregnancy, severely impaired liver and kidney function, and chronic abnormally elevated lipid levels. There are no additional contraindications for apremilast, but patients must be informed of the risk for depression before initiating therapy. Cyclosporine is contraindicated in patients with prior psoralen plus UVA (PUVA) treatment or radiation therapy, abnormal renal function, uncontrolled hypertension, uncontrolled and active infections, and a history of systemic malignancy. Live vaccines should be avoided in patients on cyclosporine, and caution is advised when cyclosporine is prescribed for patients with poorly controlled diabetes.
Pretreatment Screening
Because of drug interactions, a detailed medication history is essential prior to starting any systemic medication for psoriasis. Apremilast and cyclosporine are metabolized by cytochrome P450 and therefore are more susceptible to drug-related interactions. Cyclosporine use can affect levels of other medications that are metabolized by cytochrome P450, such as statins, calcium channel blockers, and warfarin. Similarly, acitretin’s metabolism is affected by drugs that interfere with cytochrome P450. Additionally, screening laboratory tests are needed before initiating systemic nonbiologic agents for psoriasis, with the exception of apremilast.
Prior to initiating methotrexate treatment, patients may require tuberculosis (TB), hepatitis B, and hepatitis C screening tests, depending on their risk factors. A baseline liver fibrosis assessment is recommended because of the potential of hepatotoxicity in patients receiving methotrexate. Noninvasive serology tests utilized to evaluate the presence of pre-existing liver disease include Fibrosis-4, FibroMeter, FibroSure, and Hepascore. Patients with impaired renal function have an increased predisposition to methotrexate-induced hematologic toxicity. Thus, it is necessary to administer a test dose of methotrexate in these patients followed by a complete blood cell count (CBC) 5 to 7 days later. An unremarkable CBC after the test dose suggests the absence of myelosuppression, and methotrexate dosage can be increased weekly. Patients on methotrexate also must receive folate supplementation to reduce the risk for adverse effects during treatment.
Patients considering cyclosporine must undergo screening for family and personal history of renal disease. Prior to initiating treatment, patients require 2 blood pressure measurements, hepatitis screening, TB screening, urinalysis, serum creatinine (Cr), blood urea nitrogen (BUN), CBC, potassium and magnesium levels, uric acid levels, lipid profile, bilirubin, and liver function tests (LFTs). A pregnancy test also is warranted for women of childbearing potential (WOCP).
Patients receiving acitretin should receive screening laboratory tests consisting of fasting cholesterol and triglycerides, CBC, renal function tests, LFTs, and a pregnancy test, if applicable.
After baseline evaluations, the selected oral systemic can be initiated using specific dosing regimens to ensure optimal drug efficacy and reduce incidence of adverse effects (eTable).
Monitoring During Active Treatment
Physicians need to counsel patients on potential adverse effects of their medications. Because of its relatively safe profile among the systemic nonbiologic agents, apremilast requires the least monitoring during treatment. There is no required routine laboratory monitoring for patients using apremilast, though testing may be pursued at the clinician’s discretion. However, weight should be regularly measured in patients on apremilast. In a phase 3 clinical trial of patients with psoriasis, 12% of patients on apremilast experienced a 5% to 10% weight loss compared to 5% of patients on placebo.11,13 Thus, it is recommended that physicians consider discontinuing apremilast in patients with a weight loss of more than 5% from baseline, especially if it may lead to other unfavorable health effects. Because depression is reported among 1% of patients on apremilast, close monitoring for new or worsening symptoms of depression should be performed during treatment.11,13 To avoid common GI side effects, apremilast is initiated at 10 mg/d and is increased by 10 mg/d over the first 5 days to a final dose of 30 mg BID. Elderly patients in particular should be cautioned about the risk of dehydration associated with GI side effects. Patients with severe renal impairment (Cr clearance, <30 mL/min) should use apremilast at a dosage of 30 mg once daily.
For patients on methotrexate, laboratory monitoring is essential after each dose increase. It also is important for physicians to obtain regular blood work to assess for hematologic abnormalities and hepatoxicity. Patients with risk factors such as renal insufficiency, increased age, hypoalbuminemia, alcohol abuse and alcoholic liver disease, and methotrexate dosing errors, as well as those prone to drug-related interactions, must be monitored closely for pancytopenia.14,15 The protocol for screening for methotrexate-induced hepatotoxicity during treatment depends on patient risk factors. Risk factors for hepatoxicity include history of or current alcohol abuse, abnormal LFTs, personal or family history of liver disease, diabetes, obesity, use of other hepatotoxic drugs, and hyperlipidemia.16 In patients without blood work abnormalities, CBC and LFTs can be performed every 3 to 6 months. Patients with abnormally elevated LFTs require repeat blood work every 2 to 4 weeks. Persistent elevations in LFTs require further evaluation by a GI specialist. After a cumulative dose of 3.5 to 4 g, patients should receive a GI referral and further studies (such as vibration-controlled transient elastography or liver biopsy) to assess for liver fibrosis. Patients with signs of stage 3 liver fibrosis are recommended to discontinue methotrexate and switch to another medication for psoriasis. For patients with impaired renal function, periodic BUN and Cr monitoring are needed. Common adverse effects of methotrexate include diarrhea, nausea, and anorexia, which can be mitigated by taking methotrexate with food or lowering the dosage.8 Patients on methotrexate should be monitored for rare but potential risks of infection and reactivation of latent TB, hepatitis, and lymphoma. To reduce the incidence of methotrexate toxicity from drug interactions, a review of current medications at each follow-up visit is recommended.
Nephrotoxicity and hypertension are the most common adverse effects of cyclosporine. It is important to monitor BUN and Cr biweekly for the initial 3 months, then at monthly intervals if there are no persistent abnormalities. Patients also must receive monthly CBC, potassium and magnesium levels, uric acid levels, lipid panel, serum bilirubin, and LFTs to monitor for adverse effects.17 Physicians should obtain regular pregnancy tests in WOCP. Weekly monitoring of early-morning blood pressure is recommended for patients on cyclosporine to detect early cyclosporine-induced nephrotoxicity. Hypertension on 2 separate occasions warrants a reduction in cyclosporine dosage or an addition of a calcium channel blocker for blood pressure control. Dose reduction also should be performed in patients with an increase in Cr above baseline greater than 25%.17 If Cr level is persistently elevated or if blood pressure does not normalize to lower than 140/90 after dose reduction, cyclosporine should be immediately discontinued. Patients on cyclosporine for more than a year warrant an annual estimation of glomerular filtration rate because of irreversible kidney damage associated with long-term use. A systematic review of patients treated with cyclosporine for more than 2 years found that at least 50% of patients experienced a 30% increase in Cr above baseline.18
Patients taking acitretin should be monitored for hyperlipidemia, the most common laboratory abnormality seen in 25% to 50% of patients.19 Fasting lipid panel and LFTs should be performed monthly for the initial 3 months on acitretin, then at 3-month intervals. Lifestyle changes should be encouraged to reduce hyperlipidemia, and fibrates may be given to treat elevated triglyceride levels, the most common type of hyperlipidemia seen with acitretin. Acitretin-induced toxic hepatitis is a rare occurrence that warrants immediate discontinuation of the medication.20 Monthly pregnancy tests must be performed in WOCP.
Combination Therapy
For apremilast, there is anecdotal evidence supporting its use in conjunction with phototherapy or biologics in some cases, but no high-quality data.21 On the other hand, using combination therapy with other systemic therapies can reduce adverse effects and decrease the amount of medication needed to achieve psoriasis clearance. Methotrexate used with etanercept, for example, has been more effective than methotrexate monotherapy in treating psoriasis, which has been attributed to a methotrexate-mediated reduction in the production of antidrug antibodies.22,23
Methotrexate, cyclosporine, and acitretin have synergistic effects when used with phototherapy. Narrowband UVB (NB-UVB) phototherapy combined with methotrexate is more effective in clearing psoriasis than methotrexate or NB-UVB phototherapy alone. Similarly, acitretin and PUVA combination therapy is more effective than acitretin or PUVA phototherapy alone. Combination regimens of acitretin and broadband UVB phototherapy, acitretin and NB-UVB phototherapy, and acitretin and PUVA phototherapy also have been more effective than individual modalities alone. Combination therapy reduces the cumulative doses of both therapies and reduces the frequency and duration of phototherapy needed for psoriatic clearance.24 In acitretin combination therapy with UVB phototherapy, the recommended regimen is 2 weeks of acitretin monotherapy followed by UVB phototherapy. For patients with an inadequate response to UVB phototherapy, the UVB dose can be reduced by 30% to 50%, and acitretin 25 mg/d can be added to phototherapy treatment. Acitretin-UVB combination therapy has been shown to reduce the risk of UVB-induced erythema seen in UVB monotherapy. Similarly, the risk of squamous cell carcinoma is reduced in acitretin-PUVA combination therapy compared to PUVA monotherapy.25
The timing of phototherapy in combination with systemic nonbiologic agents is critical. Phototherapy used simultaneously with cyclosporine is contraindicated owing to increased risk of photocarcinogenesis, whereas phototherapy used in sequence with cyclosporine is well tolerated and effective. Furthermore, cyclosporine 3 mg/kg/d for 4 weeks followed by a rapid cyclosporine taper and initiation of NB-UVB phototherapy demonstrated resolution of psoriasis with fewer NB-UVB treatments and less UVB exposure than NB-UVB therapy alone.26
Final Thoughts
The FDA-approved systemic nonbiologic agents are accessible and effective treatment options for adults with widespread or inadequately controlled psoriasis. Selecting the ideal therapy requires careful consideration of medication toxicity, contraindications, monitoring requirements, and patient comorbidities. The AAD-NPF guidelines guide dermatologists in prescribing systemic nonbiologic treatments in adults with psoriasis. Utilizing these recommendations in combination with clinician judgment will help patients achieve safe and optimal psoriasis clearance.
Psoriasis is a chronic relapsing skin condition characterized by keratinocyte hyperproliferation and a chronic inflammatory cascade. Therefore, controlling inflammatory responses with systemic medications is beneficial in managing psoriatic lesions and their accompanying symptoms, especially in disease inadequately controlled by topicals. Ease of drug administration and treatment availability are benefits that systemic nonbiologic therapies may have over biologic therapies.
In 2020, the American Academy of Dermatology (AAD) and the National Psoriasis Foundation (NPF) published guidelines for managing psoriasis in adults with systemic nonbiologic therapies.1 Dosing, efficacy, toxicity, drug-related interactions, and contraindications are addressed alongside evidence-based treatment recommendations. This review addresses current recommendations for systemic nonbiologics in psoriasis with a focus on the treatments approved by the US Food and Drug Administration (FDA): acitretin, apremilast, cyclosporine, and methotrexate (eTable). Fumaric acid esters and tofacitinib are FDA approved for psoriatic arthritis but not for plaque psoriasis. Additional long-term safety analyses of tofacitinib for plaque psoriasis were requested by the FDA. Dimethyl fumarate is approved by the European Medicines Agency for treatment of psoriasis and is among the first-line systemic treatments used in Germany.2

Selecting a Systemic Nonbiologic Agent
Methotrexate and apremilast have a strength level A recommendation for treating moderate to severe psoriasis in adults. However, methotrexate is less effective than biologic agents, including adalimumab and infliximab, for cutaneous psoriasis. Methotrexate is believed to improve psoriasis because of its direct immunosuppressive effect and inhibition of lymphoid cell proliferation. It typically is administered orally but can be administered subcutaneously for decreased gastrointestinal (GI) adverse effects. Compliance with close laboratory monitoring and lifestyle modifications, such as contraceptive use (because of teratogenicity) and alcohol cessation (because of the risk of liver damage) are essential in patients using methotrexate.
Apremilast, the most recently FDA-approved oral systemic medication for psoriasis, inhibits phosphodiesterase 4, subsequently decreasing inflammatory responses involving helper T cells TH1 and TH17 as well as type 1 interferon pathways. Apremilast is particularly effective in treating psoriasis with scalp and palmoplantar involvement.3 Additionally, it has an encouraging safety profile and is favorable in patients with multiple comorbidities.
Among the 4 oral agents, cyclosporine has the quickest onset of effect and has a strength level A recommendation for treating severe and recalcitrant psoriasis. Because of its high-risk profile, it is recommended for short periods of time, acute flares, or during transitions to safer long-term treatment. Patients with multiple comorbidities should avoid cyclosporine as a treatment option.
Acitretin, an FDA-approved oral retinoid, is an optimal treatment option for immunosuppressed patients or patients with HIV on antiretroviral therapy because it is not immunosuppressive.4 Unlike cyclosporine, acitretin is less helpful for acute flares because it takes 3 to 6 months to reach peak therapeutic response for treating plaque psoriasis. Similar to cyclosporine, acitretin can be recommended for severe psoriatic variants of erythrodermic, generalized pustular, and palmoplantar psoriasis. Acitretin has been reported to be more effective and have a more rapid onset of action in erythrodermic and pustular psoriasis than in plaque psoriasis.5
Patient Comorbidities
Psoriatic arthritis (PsA) is a common comorbidity that affects treatment choice. Patients with coexisting PsA could be treated with apremilast, as it is approved for both psoriasis and PsA. In a phase 3 randomized, controlled trial, American College of Rheumatology (ACR) 20 response at weeks 16 and 52 was achieved by significantly more patients on apremilast at 20 mg twice daily (BID)(P=.0166) or 30 mg BID (P=.0001) than placebo.6 Although not FDA approved for PsA, methotrexate has been shown to improve concomitant PsA of the peripheral joints in patients with psoriasis. Furthermore, a trial of methotrexate has shown considerable improvements in PsA symptoms in patients with psoriasis—a 62.7% decrease in proportion of patients with dactylitis, 25.7% decrease in enthesitis, and improvements in ACR outcomes (ACR20 in 40.8%, ACR50 in 18.8%, and ACR70 in 8.6%, with 22.4% achieving minimal disease activity).7
Prior to starting a systemic medication for psoriasis, it is necessary to discuss effects on pregnancy and fertility. Pregnancy is an absolute contraindication for methotrexate and acitretin use because of the drugs’ teratogenicity. Fetal death and fetal abnormalities have been reported with methotrexate use in pregnant women.8 Bone, central nervous system, auditory, ocular, and cardiovascular fetal abnormalities have been reported with maternal acitretin use.9 Breastfeeding also is an absolute contraindication for methotrexate use, as methotrexate passes into breastmilk in small quantities. Patients taking acitretin also are strongly discouraged from nursing because of the long half-life (168 days) of etretinate, a reverse metabolism product of acitretin that is increased in the presence of alcohol. Women should wait 3 months after discontinuing methotrexate for complete drug clearance before conceiving compared to 3 years in women who have discontinued acitretin.8,10 Men also are recommended to wait 3 months after discontinuing methotrexate before attempting to conceive, as its effect on male spermatogenesis and teratogenicity is unclear. Acitretin has no documented teratogenic effect in men. For women planning to become pregnant, apremilast and cyclosporine can be continued throughout pregnancy on an individual basis. The benefit of apremilast should be weighed against its potential risk to the fetus. There is no evidence of teratogenicity of apremilast at doses of 20 mg/kg daily.11 Current research regarding cyclosporine use in pregnancy only exists in transplant patients and has revealed higher rates of prematurity and lower birth weight without teratogenic effects.10,12 The risks and benefits of continuing cyclosporine while nursing should be evaluated, as cyclosporine (and ethanol-methanol components used in some formulations) is detectable in breast milk.
Drug Contraindications
Hypersensitivity to a specific systemic nonbiologic medication is a contraindication to its use and is an absolute contraindication for methotrexate. Other absolute contraindications to methotrexate are pregnancy and nursing, alcoholism, alcoholic liver disease, chronic liver disease, immunodeficiency, and cytopenia. Contraindications to acitretin include pregnancy, severely impaired liver and kidney function, and chronic abnormally elevated lipid levels. There are no additional contraindications for apremilast, but patients must be informed of the risk for depression before initiating therapy. Cyclosporine is contraindicated in patients with prior psoralen plus UVA (PUVA) treatment or radiation therapy, abnormal renal function, uncontrolled hypertension, uncontrolled and active infections, and a history of systemic malignancy. Live vaccines should be avoided in patients on cyclosporine, and caution is advised when cyclosporine is prescribed for patients with poorly controlled diabetes.
Pretreatment Screening
Because of drug interactions, a detailed medication history is essential prior to starting any systemic medication for psoriasis. Apremilast and cyclosporine are metabolized by cytochrome P450 and therefore are more susceptible to drug-related interactions. Cyclosporine use can affect levels of other medications that are metabolized by cytochrome P450, such as statins, calcium channel blockers, and warfarin. Similarly, acitretin’s metabolism is affected by drugs that interfere with cytochrome P450. Additionally, screening laboratory tests are needed before initiating systemic nonbiologic agents for psoriasis, with the exception of apremilast.
Prior to initiating methotrexate treatment, patients may require tuberculosis (TB), hepatitis B, and hepatitis C screening tests, depending on their risk factors. A baseline liver fibrosis assessment is recommended because of the potential of hepatotoxicity in patients receiving methotrexate. Noninvasive serology tests utilized to evaluate the presence of pre-existing liver disease include Fibrosis-4, FibroMeter, FibroSure, and Hepascore. Patients with impaired renal function have an increased predisposition to methotrexate-induced hematologic toxicity. Thus, it is necessary to administer a test dose of methotrexate in these patients followed by a complete blood cell count (CBC) 5 to 7 days later. An unremarkable CBC after the test dose suggests the absence of myelosuppression, and methotrexate dosage can be increased weekly. Patients on methotrexate also must receive folate supplementation to reduce the risk for adverse effects during treatment.
Patients considering cyclosporine must undergo screening for family and personal history of renal disease. Prior to initiating treatment, patients require 2 blood pressure measurements, hepatitis screening, TB screening, urinalysis, serum creatinine (Cr), blood urea nitrogen (BUN), CBC, potassium and magnesium levels, uric acid levels, lipid profile, bilirubin, and liver function tests (LFTs). A pregnancy test also is warranted for women of childbearing potential (WOCP).
Patients receiving acitretin should receive screening laboratory tests consisting of fasting cholesterol and triglycerides, CBC, renal function tests, LFTs, and a pregnancy test, if applicable.
After baseline evaluations, the selected oral systemic can be initiated using specific dosing regimens to ensure optimal drug efficacy and reduce incidence of adverse effects (eTable).
Monitoring During Active Treatment
Physicians need to counsel patients on potential adverse effects of their medications. Because of its relatively safe profile among the systemic nonbiologic agents, apremilast requires the least monitoring during treatment. There is no required routine laboratory monitoring for patients using apremilast, though testing may be pursued at the clinician’s discretion. However, weight should be regularly measured in patients on apremilast. In a phase 3 clinical trial of patients with psoriasis, 12% of patients on apremilast experienced a 5% to 10% weight loss compared to 5% of patients on placebo.11,13 Thus, it is recommended that physicians consider discontinuing apremilast in patients with a weight loss of more than 5% from baseline, especially if it may lead to other unfavorable health effects. Because depression is reported among 1% of patients on apremilast, close monitoring for new or worsening symptoms of depression should be performed during treatment.11,13 To avoid common GI side effects, apremilast is initiated at 10 mg/d and is increased by 10 mg/d over the first 5 days to a final dose of 30 mg BID. Elderly patients in particular should be cautioned about the risk of dehydration associated with GI side effects. Patients with severe renal impairment (Cr clearance, <30 mL/min) should use apremilast at a dosage of 30 mg once daily.
For patients on methotrexate, laboratory monitoring is essential after each dose increase. It also is important for physicians to obtain regular blood work to assess for hematologic abnormalities and hepatoxicity. Patients with risk factors such as renal insufficiency, increased age, hypoalbuminemia, alcohol abuse and alcoholic liver disease, and methotrexate dosing errors, as well as those prone to drug-related interactions, must be monitored closely for pancytopenia.14,15 The protocol for screening for methotrexate-induced hepatotoxicity during treatment depends on patient risk factors. Risk factors for hepatoxicity include history of or current alcohol abuse, abnormal LFTs, personal or family history of liver disease, diabetes, obesity, use of other hepatotoxic drugs, and hyperlipidemia.16 In patients without blood work abnormalities, CBC and LFTs can be performed every 3 to 6 months. Patients with abnormally elevated LFTs require repeat blood work every 2 to 4 weeks. Persistent elevations in LFTs require further evaluation by a GI specialist. After a cumulative dose of 3.5 to 4 g, patients should receive a GI referral and further studies (such as vibration-controlled transient elastography or liver biopsy) to assess for liver fibrosis. Patients with signs of stage 3 liver fibrosis are recommended to discontinue methotrexate and switch to another medication for psoriasis. For patients with impaired renal function, periodic BUN and Cr monitoring are needed. Common adverse effects of methotrexate include diarrhea, nausea, and anorexia, which can be mitigated by taking methotrexate with food or lowering the dosage.8 Patients on methotrexate should be monitored for rare but potential risks of infection and reactivation of latent TB, hepatitis, and lymphoma. To reduce the incidence of methotrexate toxicity from drug interactions, a review of current medications at each follow-up visit is recommended.
Nephrotoxicity and hypertension are the most common adverse effects of cyclosporine. It is important to monitor BUN and Cr biweekly for the initial 3 months, then at monthly intervals if there are no persistent abnormalities. Patients also must receive monthly CBC, potassium and magnesium levels, uric acid levels, lipid panel, serum bilirubin, and LFTs to monitor for adverse effects.17 Physicians should obtain regular pregnancy tests in WOCP. Weekly monitoring of early-morning blood pressure is recommended for patients on cyclosporine to detect early cyclosporine-induced nephrotoxicity. Hypertension on 2 separate occasions warrants a reduction in cyclosporine dosage or an addition of a calcium channel blocker for blood pressure control. Dose reduction also should be performed in patients with an increase in Cr above baseline greater than 25%.17 If Cr level is persistently elevated or if blood pressure does not normalize to lower than 140/90 after dose reduction, cyclosporine should be immediately discontinued. Patients on cyclosporine for more than a year warrant an annual estimation of glomerular filtration rate because of irreversible kidney damage associated with long-term use. A systematic review of patients treated with cyclosporine for more than 2 years found that at least 50% of patients experienced a 30% increase in Cr above baseline.18
Patients taking acitretin should be monitored for hyperlipidemia, the most common laboratory abnormality seen in 25% to 50% of patients.19 Fasting lipid panel and LFTs should be performed monthly for the initial 3 months on acitretin, then at 3-month intervals. Lifestyle changes should be encouraged to reduce hyperlipidemia, and fibrates may be given to treat elevated triglyceride levels, the most common type of hyperlipidemia seen with acitretin. Acitretin-induced toxic hepatitis is a rare occurrence that warrants immediate discontinuation of the medication.20 Monthly pregnancy tests must be performed in WOCP.
Combination Therapy
For apremilast, there is anecdotal evidence supporting its use in conjunction with phototherapy or biologics in some cases, but no high-quality data.21 On the other hand, using combination therapy with other systemic therapies can reduce adverse effects and decrease the amount of medication needed to achieve psoriasis clearance. Methotrexate used with etanercept, for example, has been more effective than methotrexate monotherapy in treating psoriasis, which has been attributed to a methotrexate-mediated reduction in the production of antidrug antibodies.22,23
Methotrexate, cyclosporine, and acitretin have synergistic effects when used with phototherapy. Narrowband UVB (NB-UVB) phototherapy combined with methotrexate is more effective in clearing psoriasis than methotrexate or NB-UVB phototherapy alone. Similarly, acitretin and PUVA combination therapy is more effective than acitretin or PUVA phototherapy alone. Combination regimens of acitretin and broadband UVB phototherapy, acitretin and NB-UVB phototherapy, and acitretin and PUVA phototherapy also have been more effective than individual modalities alone. Combination therapy reduces the cumulative doses of both therapies and reduces the frequency and duration of phototherapy needed for psoriatic clearance.24 In acitretin combination therapy with UVB phototherapy, the recommended regimen is 2 weeks of acitretin monotherapy followed by UVB phototherapy. For patients with an inadequate response to UVB phototherapy, the UVB dose can be reduced by 30% to 50%, and acitretin 25 mg/d can be added to phototherapy treatment. Acitretin-UVB combination therapy has been shown to reduce the risk of UVB-induced erythema seen in UVB monotherapy. Similarly, the risk of squamous cell carcinoma is reduced in acitretin-PUVA combination therapy compared to PUVA monotherapy.25
The timing of phototherapy in combination with systemic nonbiologic agents is critical. Phototherapy used simultaneously with cyclosporine is contraindicated owing to increased risk of photocarcinogenesis, whereas phototherapy used in sequence with cyclosporine is well tolerated and effective. Furthermore, cyclosporine 3 mg/kg/d for 4 weeks followed by a rapid cyclosporine taper and initiation of NB-UVB phototherapy demonstrated resolution of psoriasis with fewer NB-UVB treatments and less UVB exposure than NB-UVB therapy alone.26
Final Thoughts
The FDA-approved systemic nonbiologic agents are accessible and effective treatment options for adults with widespread or inadequately controlled psoriasis. Selecting the ideal therapy requires careful consideration of medication toxicity, contraindications, monitoring requirements, and patient comorbidities. The AAD-NPF guidelines guide dermatologists in prescribing systemic nonbiologic treatments in adults with psoriasis. Utilizing these recommendations in combination with clinician judgment will help patients achieve safe and optimal psoriasis clearance.
- Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J Am Acad Dermatol. 2020;82:1445-1486.
- Mrowietz U, Barker J, Boehncke WH, et al. Clinical use of dimethyl fumarate in moderate-to-severe plaque-type psoriasis: a European expert consensus. J Eur Acad Dermatol Venereol. 2018;32(suppl 3):3-14.
- Van Voorhees AS, Gold LS, Lebwohl M, et al. Efficacy and safety of apremilast in patients with moderate to severe plaque psoriasis of the scalp: results of a phase 3b, multicenter, randomized, placebo-controlled, double-blind study. J Am Acad Dermatol. 2020;83:96-103.
- Buccheri L, Katchen BR, Karter AJ, et al. Acitretin therapy is effective for psoriasis associated with human immunodeficiency virus infection. Arch Dermatol. 1997;133:711-715.
- Ormerod AD, Campalani E, Goodfield MJD. British Association of Dermatologists guidelines on the efficacy and use of acitretin in dermatology. Br J Dermatol. 2010;162:952-963.
- Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. Longterm (52-week) results of a phase III randomized, controlled trial of apremilast in patients with psoriatic arthritis. J Rheumatol. 2015;42:479-488.
- Coates LC, Aslam T, Al Balushi F, et al. Comparison of three screening tools to detect psoriatic arthritis in patients with psoriasis (CONTEST study). Br J Dermatol. 2013;168:802-807.
- Antares Pharma, Inc. Otrexup PFS (methotrexate) [package insert]. US Food and Drug Administration website. Revised June 2019. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/204824s009lbl.pdf
- David M, Hodak E, Lowe NJ. Adverse effects of retinoids. Med Toxicol Adverse Drug Exp. 1988;3:273-288.
- Stiefel Laboratories, Inc. Soriatane (acitretin) [package insert]. US Food and Drug Administration website. Revised September 2017. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/019821s028lbl.pdf
- Celgene Corporation. Otezla (apremilast) [package insert]. US Food and Drug Administration website. Revised March 2014. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/205437s000lbl.pdf
- Ghanem ME, El-Baghdadi LA, Badawy AM, et al. Pregnancy outcome after renal allograft transplantation: 15 years experience. Eur J Obstet Gynecol Reprod Biol. 2005;121:178-181.
- Zerilli T, Ocheretyaner E. Apremilast (Otezla): A new oral treatment for adults with psoriasis and psoriatic arthritis. P T. 2015;40:495-500.
- Kivity S, Zafrir Y, Loebstein R, et al. Clinical characteristics and risk factors for low dose methotrexate toxicity: a cohort of 28 patients. Autoimmun Rev. 2014;13:1109-1113.
- Boffa MJ, Chalmers RJ. Methotrexate for psoriasis. Clin Exp Dermatol. 1996;21:399-408.
- Rosenberg P, Urwitz H, Johannesson A, et al. Psoriasis patients with diabetes type 2 are at high risk of developing liver fibrosis during methotrexate treatment. J Hepatol. 2007;46:1111-1118.
- Novartis Pharmaceuticals Corporation. Sandimmune (cyclosporine) [package insert]. US Food and Drug Administration website. Published 2015. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/050573s041,050574s051,050625s055lbl.pdf
- Maza A, Montaudie H, Sbidian E, et al. Oral cyclosporin in psoriasis: a systematic review on treatment modalities, risk of kidney toxicity and evidence for use in non-plaque psoriasis. J Eur Acad Dermatol Venereol. 2011;25(suppl 2):19-27.
- Yamauchi PS, Rizk D, Kormilli T, et al. Systemic retinoids. In: Weinstein GD, Gottlieb AB, eds. Therapy of Moderate-to-Severe Psoriasis. Marcel Dekker; 2003:137-150.
- van Ditzhuijsen TJ, van Haelst UJ, van Dooren-Greebe RJ, et al. Severe hepatotoxic reaction with progression to cirrhosis after use of a novel retinoid (acitretin). J Hepatol. 1990;11:185-188.
- AbuHilal M, Walsh S, Shear N. Use of apremilast in combination with other therapies for treatment of chronic plaque psoriasis: a retrospective study. J Cutan Med Surg. 2016;20:313-316.
- Gottlieb AB, Langley RG, Strober BE, et al. A randomized, double-blind, placebo-controlled study to evaluate the addition of methotrexate to etanercept in patients with moderate to severe plaque psoriasis. Br J Dermatol. 2012;167:649-657.
- Cronstein BN. Methotrexate BAFFles anti-drug antibodies. Nat Rev Rheumatol. 2018;14:505-506.
- Lebwohl M, Drake L, Menter A, et al. Consensus conference: acitretin in combination with UVB or PUVA in the treatment of psoriasis. J Am Acad Dermatol. 2001;45:544-553.
- Nijsten TE, Stern RS. Oral retinoid use reduces cutaneous squamous cell carcinoma risk in patients with psoriasis treated with psoralen-UVA: a nested cohort study. J Am Acad Dermatol. 2003;49:644-650.
- Calzavara-Pinton P, Leone G, Venturini M, et al. A comparative non randomized study of narrow-band (NB) (312 +/- 2 nm) UVB phototherapy versus sequential therapy with oral administration of low-dose cyclosporin A and NB-UVB phototherapy in patients with severe psoriasis vulgaris. Eur J Dermatol. 2005;15:470-473.
- Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J Am Acad Dermatol. 2020;82:1445-1486.
- Mrowietz U, Barker J, Boehncke WH, et al. Clinical use of dimethyl fumarate in moderate-to-severe plaque-type psoriasis: a European expert consensus. J Eur Acad Dermatol Venereol. 2018;32(suppl 3):3-14.
- Van Voorhees AS, Gold LS, Lebwohl M, et al. Efficacy and safety of apremilast in patients with moderate to severe plaque psoriasis of the scalp: results of a phase 3b, multicenter, randomized, placebo-controlled, double-blind study. J Am Acad Dermatol. 2020;83:96-103.
- Buccheri L, Katchen BR, Karter AJ, et al. Acitretin therapy is effective for psoriasis associated with human immunodeficiency virus infection. Arch Dermatol. 1997;133:711-715.
- Ormerod AD, Campalani E, Goodfield MJD. British Association of Dermatologists guidelines on the efficacy and use of acitretin in dermatology. Br J Dermatol. 2010;162:952-963.
- Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. Longterm (52-week) results of a phase III randomized, controlled trial of apremilast in patients with psoriatic arthritis. J Rheumatol. 2015;42:479-488.
- Coates LC, Aslam T, Al Balushi F, et al. Comparison of three screening tools to detect psoriatic arthritis in patients with psoriasis (CONTEST study). Br J Dermatol. 2013;168:802-807.
- Antares Pharma, Inc. Otrexup PFS (methotrexate) [package insert]. US Food and Drug Administration website. Revised June 2019. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/204824s009lbl.pdf
- David M, Hodak E, Lowe NJ. Adverse effects of retinoids. Med Toxicol Adverse Drug Exp. 1988;3:273-288.
- Stiefel Laboratories, Inc. Soriatane (acitretin) [package insert]. US Food and Drug Administration website. Revised September 2017. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/019821s028lbl.pdf
- Celgene Corporation. Otezla (apremilast) [package insert]. US Food and Drug Administration website. Revised March 2014. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/205437s000lbl.pdf
- Ghanem ME, El-Baghdadi LA, Badawy AM, et al. Pregnancy outcome after renal allograft transplantation: 15 years experience. Eur J Obstet Gynecol Reprod Biol. 2005;121:178-181.
- Zerilli T, Ocheretyaner E. Apremilast (Otezla): A new oral treatment for adults with psoriasis and psoriatic arthritis. P T. 2015;40:495-500.
- Kivity S, Zafrir Y, Loebstein R, et al. Clinical characteristics and risk factors for low dose methotrexate toxicity: a cohort of 28 patients. Autoimmun Rev. 2014;13:1109-1113.
- Boffa MJ, Chalmers RJ. Methotrexate for psoriasis. Clin Exp Dermatol. 1996;21:399-408.
- Rosenberg P, Urwitz H, Johannesson A, et al. Psoriasis patients with diabetes type 2 are at high risk of developing liver fibrosis during methotrexate treatment. J Hepatol. 2007;46:1111-1118.
- Novartis Pharmaceuticals Corporation. Sandimmune (cyclosporine) [package insert]. US Food and Drug Administration website. Published 2015. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/050573s041,050574s051,050625s055lbl.pdf
- Maza A, Montaudie H, Sbidian E, et al. Oral cyclosporin in psoriasis: a systematic review on treatment modalities, risk of kidney toxicity and evidence for use in non-plaque psoriasis. J Eur Acad Dermatol Venereol. 2011;25(suppl 2):19-27.
- Yamauchi PS, Rizk D, Kormilli T, et al. Systemic retinoids. In: Weinstein GD, Gottlieb AB, eds. Therapy of Moderate-to-Severe Psoriasis. Marcel Dekker; 2003:137-150.
- van Ditzhuijsen TJ, van Haelst UJ, van Dooren-Greebe RJ, et al. Severe hepatotoxic reaction with progression to cirrhosis after use of a novel retinoid (acitretin). J Hepatol. 1990;11:185-188.
- AbuHilal M, Walsh S, Shear N. Use of apremilast in combination with other therapies for treatment of chronic plaque psoriasis: a retrospective study. J Cutan Med Surg. 2016;20:313-316.
- Gottlieb AB, Langley RG, Strober BE, et al. A randomized, double-blind, placebo-controlled study to evaluate the addition of methotrexate to etanercept in patients with moderate to severe plaque psoriasis. Br J Dermatol. 2012;167:649-657.
- Cronstein BN. Methotrexate BAFFles anti-drug antibodies. Nat Rev Rheumatol. 2018;14:505-506.
- Lebwohl M, Drake L, Menter A, et al. Consensus conference: acitretin in combination with UVB or PUVA in the treatment of psoriasis. J Am Acad Dermatol. 2001;45:544-553.
- Nijsten TE, Stern RS. Oral retinoid use reduces cutaneous squamous cell carcinoma risk in patients with psoriasis treated with psoralen-UVA: a nested cohort study. J Am Acad Dermatol. 2003;49:644-650.
- Calzavara-Pinton P, Leone G, Venturini M, et al. A comparative non randomized study of narrow-band (NB) (312 +/- 2 nm) UVB phototherapy versus sequential therapy with oral administration of low-dose cyclosporin A and NB-UVB phototherapy in patients with severe psoriasis vulgaris. Eur J Dermatol. 2005;15:470-473.
Practice Points
- Systemic nonbiologic therapies are effective treatments for adults with psoriasis. The benefits of these treatments include ease of administration and the ability to control widespread disease.
- When selecting a therapy, a thorough evaluation of patient characteristics and commitment to lifestyle adjustments is necessary, including careful consideration in women of childbearing potential and those with plans of starting a family.
- Regular drug monitoring and patient follow-up is crucial to ensure safe dosing adjustments and to mitigate potential adverse effects.
Brodalumab in an Organ Transplant Recipient With Psoriasis
The treatment landscape for psoriasis has evolved rapidly over the last decade. Biologic therapies have demonstrated robust efficacy and acceptable safety profiles among many patients with moderate to severe plaque psoriasis. However, the use of biologics among immunocompromised patients with psoriasis rarely is discussed in the literature. As new biologics for psoriasis are being developed, a critical gap exists in the literature regarding the safety and efficacy of these medications in immunocompromised patients. Per American Academy of Dermatology–National Psoriasis Foundation guidelines, caution should be exercised when using biologics in patients with immunocompromising conditions.1 In organ transplant recipients, the potential risks of combining systemic medications used for organ transplantation and biologic treatments for psoriasis are unknown.2
In the posttransplant period, the immunosuppressive regimens for transplantation likely will improve psoriasis. However, patients with organ transplant and psoriasis still experience flares that can be challenging to treat.3 Prior treatment modalities to prevent psoriasis flares in organ transplant recipients have relied largely on topical therapies, posttransplant immunosuppressive medications (eg, cyclosporine, tacrolimus, mycophenolate mofetil) that prevent graft rejection, and systemic corticosteroids. We report a case of a 50-year-old man with a recent history of liver transplantation who presented with severe plaque psoriasis and psoriatic arthritis.
Case Report
A 50-year-old man presented to the dermatology clinic with moderate to severe plaque psoriasis and psoriatic arthritis that had been present for 15 years. His plaque psoriasis covered approximately 40% of the body surface area, including the scalp, trunk, arms, and legs. In addition, he had diffuse joint pain in the hands and feet; a radiograph revealed active psoriatic arthritis involving the joints of the fingers and toes.
One year prior to presentation to our dermatology clinic, the patient underwent an an orthotopic liver transplant for history of Child-Pugh class C liver cirrhosis secondary to untreated hepatitis C virus (HCV) and alcohol use that was complicated by hepatocellular carcinoma. He acquired a high-risk donor liver that was HCV positive with HCV genotype 1a. Starting 2 months after the transplant, he underwent 12 weeks of treatment for HCV with glecaprevir-pibrentasvir. Once his HCV treatment course was completed, he achieved a sustained virologic response with an undetectable viral load. To prevent transplant rejection, he was on chronic immunosuppression with tacrolimus, a calcineurin inhibitor, and mycophenolate mofetil, an inhibitor of inosine monophosphate dehydrogenase whose action leads to decreased proliferation of T cells and B cells.
The patient’s psoriasis initially was treated with triamcinolone acetonide ointment 0.1% applied twice daily to the psoriasis lesions for 1 year by another dermatologist. However, his psoriasis progressed to involve 40% of the body surface area. Following our evaluation 1 year posttransplant, the patient was started on subcutaneous brodalumab 210 mg at weeks 0, 1, and 2, then every 2 weeks thereafter. Approximately 10 weeks after initiation of brodalumab, the patient’s psoriasis was completely clear, and he was asymptomatic from psoriatic arthritis. The patient’s improvement persisted at 6 months, and his liver enzymes, including alkaline phosphatase, total bilirubin, alanine transaminase, and aspartate transaminase, continued to be within reference range. To date, there has been no evidence of posttransplant complications such as graft-vs-host disease, serious infections, or skin cancers.
Comment
Increased Risk for Infection and Malignancies in Transplant Patients
Transplant patients are on immunosuppressive regimens that increase their risk for infection and malignancies. For example, high doses of immunosuppresants predispose these patients to reactivation of viral infections, including BK and JC viruses.4 In addition, the incidence of squamous cell carcinoma is 65- to 250-fold higher in transplant patients compared to the general population.5 The risk for Merkel cell carcinoma is increased after solid organ transplantation compared to the general population.6 Importantly, transplant patients have a higher mortality from skin cancers than other types of cancers, including breast and colon cancer.7
Psoriasis in Organ Transplant Recipients
Psoriasis is a chronic, immune-mediated, inflammatory disease with a prevalence of approximately 3% in the United States.8 Approximately one-third of patients with psoriasis develop psoriatic arthritis.9 Organ transplant recipients with psoriasis and psoriatic arthritis represent a unique patient population whereby their use of chronic immunosuppressive medications to prevent graft rejection may put them at risk for developing infections and malignancies.
Special Considerations for Brodalumab
Brodalumab is an immunomodulatory biologic that binds to and inhibits IL-17RA, thereby inhibiting the actions of IL-17A, F, E, and C.2 The blockade of IL-17RA by brodalumab has been shown to result in reversal of psoriatic phenotype and gene expression patterns.10 Brodalumab was chosen as the treatment in our patient because it has a rapid onset of action, sustained efficacy, and an acceptable safety profile.11 Brodalumab is well tolerated, with approximately 60% of patients achieving clearance long-term.12 Candidal infections can occur in patients with brodalumab, but the rates are low and they are reversible with antifungal treatment.13 The increased mucocutaneous candidal infections are consistent with medications whose mechanism of action is IL-17 inhibition.14,15 The most common adverse reactions found were nasopharyngitis and headache.16 The causal link between brodalumab and suicidality has not been established.17
The use of brodalumab for psoriasis in organ transplant recipients has not been previously reported in the literature. A few case reports have been published on the successful use of etanercept and ixekizumab as biologic treatment options for psoriasis in transplant patients.18-23 In addition to choosing an appropriate biologic for psoriasis in transplant patients, transplant providers may evaluate the choice of immunosuppression regimen for the organ transplant in the context of psoriasis. In a retrospective analysis of liver transplant patients with psoriasis, Foroncewicz et al3 found cyclosporine, which was used as an antirejection immunosuppressive agent in the posttransplant period, to be more effective than tacrolimus in treating recurrent psoriasis in liver transplant recipients.
Our case illustrates one example of the successful use of brodalumab in a patient with a solid organ transplant. Our patient’s psoriasis and symptoms of psoriatic arthritis greatly improved after initiation of brodalumab. In the posttransplant period, the patient did not develop graft-vs-host disease, infections, malignancies, depression, or suicidal ideation while taking brodalumab.
Conclusion
It is important that the patient, dermatology team, and transplant team work together to navigate the challenges and relatively unknown landscape of psoriasis treatment in organ transplant recipients. As the number of organ transplant recipients continues to increase, this issue will become more clinically relevant. Case reports and future prospective studies will continue to inform us regarding the role of biologics in psoriasis treatment posttransplantation.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072.
- Prussick R, Wu JJ, Armstrong AW, et al. Psoriasis in solid organ transplant patients: best practice recommendations from The Medical Board of the National Psoriasis Foundation. J Dermatol Treat. 2018;29:329-333.
- Foroncewicz B, Mucha K, Lerut J, et al. Cyclosporine is superior to tacrolimus in liver transplant recipients with recurrent psoriasis. Ann Transplant. 2014;19:427-433.
- Boukoum H, Nahdi I, Sahtout W, et al. BK and JC virus infections in healthy patients compared to kidney transplant recipients in Tunisia. Microbial Pathogenesis. 2016;97:204-208.
- Bouwes Bavinck JN, Euvrard S, Naldi L, et al. Keratotic skin lesions and other risk factors are associated with skin cancer in organ-transplant recipients: a case-control study in The Netherlands, United Kingdom, Germany, France, and Italy. J Invest Dermatol. 2007;127:1647-1656.
- Clark CA, Robbins HA, Tatalovich Z, et al. Risk of Merkel cell carcinoma after transplant. Clin Oncol. 2019;31:779-788.
- Lakhani NA, Saraiya M, Thompson TD, et al. Total body skin examination for skin cancer screening among U.S. adults from 2000 to 2010. Prev Med. 2014;61:75-80.
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512-516.
- Alinaghi F, Calov M, Kristensen LE, et al. Prevalence of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol. 2019;80:251-265.
- Russell CB, Rand H, Bigler J, et al. Gene expression profiles normalized in psoriatic skin by treatment with brodalumab, a human anti-IL-17 receptor monoclonal antibody. J Immunol. 2014;192:3828-3836.
- Foulkes AC, Warren RB. Brodalumab in psoriasis: evidence to date and clinical potential. Drugs Context. 2019;8:212570. doi:10.7573/dic.212570
- Puig L, Lebwohl M, Bachelez H, et al. Long-term efficacy and safety of brodalumab in the treatment of psoriasis: 120-week results from the randomized, double-blind, placebo- and active comparator-controlled phase 3 AMAGINE-2 trial. J Am Acad Dermatol. 2020;82:352-359.
- Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab and ustekinumab in psoriasis. N Engl J Med. 2015;373:1318-1328.
- Conti HR, Shen F, Nayyar N, et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med. 2009;206:299-311.
- Puel A, Cypowyj S, Bustamante J, et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science. 2011;332:65-68.
- Farahnik B, Beroukhim B, Abrouk M, et al. Brodalumab for the treatment of psoriasis: a review of Phase III trials. Dermatol Ther. 2016;6:111-124.
- Lebwohl MG, Papp KA, Marangell LB, et al. Psychiatric adverse events during treatment with brodalumab: analysis of psoriasis clinical trials. J Am Acad Dermatol. 2018;78:81-89.
- DeSimone C, Perino F, Caldarola G, et al. Treatment of psoriasis with etanercept in immunocompromised patients: two case reports. J Int Med Res. 2016;44:67-71.
- Madankumar R, Teperman LW, Stein JA. Use of etanercept for psoriasis in a liver transplant recipient. JAAD Case Rep. 2015;1:S36-S37.
- Collazo MH, González JR, Torres EA. Etanercept therapy for psoriasis in a patient with concomitant hepatitis C and liver transplant. P R Health Sci J. 2008;27:346-347.
- Hoover WD. Etanercept therapy for severe plaque psoriasis in a patient who underwent a liver transplant. Cutis. 2007;80:211-214.
- Brokalaki EI, Voshege N, Witzke O, et al. Treatment of severe psoriasis with etanercept in a pancreas-kidney transplant recipient. Transplant Proc. 2012;44:2776-2777.
- Lora V, Graceffa D, De Felice C, et al. Treatment of severe psoriasis with ixekizumab in a liver transplant recipient with concomitant hepatitis B virus infection. Dermatol Ther. 2019;32:E12909.
The treatment landscape for psoriasis has evolved rapidly over the last decade. Biologic therapies have demonstrated robust efficacy and acceptable safety profiles among many patients with moderate to severe plaque psoriasis. However, the use of biologics among immunocompromised patients with psoriasis rarely is discussed in the literature. As new biologics for psoriasis are being developed, a critical gap exists in the literature regarding the safety and efficacy of these medications in immunocompromised patients. Per American Academy of Dermatology–National Psoriasis Foundation guidelines, caution should be exercised when using biologics in patients with immunocompromising conditions.1 In organ transplant recipients, the potential risks of combining systemic medications used for organ transplantation and biologic treatments for psoriasis are unknown.2
In the posttransplant period, the immunosuppressive regimens for transplantation likely will improve psoriasis. However, patients with organ transplant and psoriasis still experience flares that can be challenging to treat.3 Prior treatment modalities to prevent psoriasis flares in organ transplant recipients have relied largely on topical therapies, posttransplant immunosuppressive medications (eg, cyclosporine, tacrolimus, mycophenolate mofetil) that prevent graft rejection, and systemic corticosteroids. We report a case of a 50-year-old man with a recent history of liver transplantation who presented with severe plaque psoriasis and psoriatic arthritis.
Case Report
A 50-year-old man presented to the dermatology clinic with moderate to severe plaque psoriasis and psoriatic arthritis that had been present for 15 years. His plaque psoriasis covered approximately 40% of the body surface area, including the scalp, trunk, arms, and legs. In addition, he had diffuse joint pain in the hands and feet; a radiograph revealed active psoriatic arthritis involving the joints of the fingers and toes.
One year prior to presentation to our dermatology clinic, the patient underwent an an orthotopic liver transplant for history of Child-Pugh class C liver cirrhosis secondary to untreated hepatitis C virus (HCV) and alcohol use that was complicated by hepatocellular carcinoma. He acquired a high-risk donor liver that was HCV positive with HCV genotype 1a. Starting 2 months after the transplant, he underwent 12 weeks of treatment for HCV with glecaprevir-pibrentasvir. Once his HCV treatment course was completed, he achieved a sustained virologic response with an undetectable viral load. To prevent transplant rejection, he was on chronic immunosuppression with tacrolimus, a calcineurin inhibitor, and mycophenolate mofetil, an inhibitor of inosine monophosphate dehydrogenase whose action leads to decreased proliferation of T cells and B cells.
The patient’s psoriasis initially was treated with triamcinolone acetonide ointment 0.1% applied twice daily to the psoriasis lesions for 1 year by another dermatologist. However, his psoriasis progressed to involve 40% of the body surface area. Following our evaluation 1 year posttransplant, the patient was started on subcutaneous brodalumab 210 mg at weeks 0, 1, and 2, then every 2 weeks thereafter. Approximately 10 weeks after initiation of brodalumab, the patient’s psoriasis was completely clear, and he was asymptomatic from psoriatic arthritis. The patient’s improvement persisted at 6 months, and his liver enzymes, including alkaline phosphatase, total bilirubin, alanine transaminase, and aspartate transaminase, continued to be within reference range. To date, there has been no evidence of posttransplant complications such as graft-vs-host disease, serious infections, or skin cancers.
Comment
Increased Risk for Infection and Malignancies in Transplant Patients
Transplant patients are on immunosuppressive regimens that increase their risk for infection and malignancies. For example, high doses of immunosuppresants predispose these patients to reactivation of viral infections, including BK and JC viruses.4 In addition, the incidence of squamous cell carcinoma is 65- to 250-fold higher in transplant patients compared to the general population.5 The risk for Merkel cell carcinoma is increased after solid organ transplantation compared to the general population.6 Importantly, transplant patients have a higher mortality from skin cancers than other types of cancers, including breast and colon cancer.7
Psoriasis in Organ Transplant Recipients
Psoriasis is a chronic, immune-mediated, inflammatory disease with a prevalence of approximately 3% in the United States.8 Approximately one-third of patients with psoriasis develop psoriatic arthritis.9 Organ transplant recipients with psoriasis and psoriatic arthritis represent a unique patient population whereby their use of chronic immunosuppressive medications to prevent graft rejection may put them at risk for developing infections and malignancies.
Special Considerations for Brodalumab
Brodalumab is an immunomodulatory biologic that binds to and inhibits IL-17RA, thereby inhibiting the actions of IL-17A, F, E, and C.2 The blockade of IL-17RA by brodalumab has been shown to result in reversal of psoriatic phenotype and gene expression patterns.10 Brodalumab was chosen as the treatment in our patient because it has a rapid onset of action, sustained efficacy, and an acceptable safety profile.11 Brodalumab is well tolerated, with approximately 60% of patients achieving clearance long-term.12 Candidal infections can occur in patients with brodalumab, but the rates are low and they are reversible with antifungal treatment.13 The increased mucocutaneous candidal infections are consistent with medications whose mechanism of action is IL-17 inhibition.14,15 The most common adverse reactions found were nasopharyngitis and headache.16 The causal link between brodalumab and suicidality has not been established.17
The use of brodalumab for psoriasis in organ transplant recipients has not been previously reported in the literature. A few case reports have been published on the successful use of etanercept and ixekizumab as biologic treatment options for psoriasis in transplant patients.18-23 In addition to choosing an appropriate biologic for psoriasis in transplant patients, transplant providers may evaluate the choice of immunosuppression regimen for the organ transplant in the context of psoriasis. In a retrospective analysis of liver transplant patients with psoriasis, Foroncewicz et al3 found cyclosporine, which was used as an antirejection immunosuppressive agent in the posttransplant period, to be more effective than tacrolimus in treating recurrent psoriasis in liver transplant recipients.
Our case illustrates one example of the successful use of brodalumab in a patient with a solid organ transplant. Our patient’s psoriasis and symptoms of psoriatic arthritis greatly improved after initiation of brodalumab. In the posttransplant period, the patient did not develop graft-vs-host disease, infections, malignancies, depression, or suicidal ideation while taking brodalumab.
Conclusion
It is important that the patient, dermatology team, and transplant team work together to navigate the challenges and relatively unknown landscape of psoriasis treatment in organ transplant recipients. As the number of organ transplant recipients continues to increase, this issue will become more clinically relevant. Case reports and future prospective studies will continue to inform us regarding the role of biologics in psoriasis treatment posttransplantation.
The treatment landscape for psoriasis has evolved rapidly over the last decade. Biologic therapies have demonstrated robust efficacy and acceptable safety profiles among many patients with moderate to severe plaque psoriasis. However, the use of biologics among immunocompromised patients with psoriasis rarely is discussed in the literature. As new biologics for psoriasis are being developed, a critical gap exists in the literature regarding the safety and efficacy of these medications in immunocompromised patients. Per American Academy of Dermatology–National Psoriasis Foundation guidelines, caution should be exercised when using biologics in patients with immunocompromising conditions.1 In organ transplant recipients, the potential risks of combining systemic medications used for organ transplantation and biologic treatments for psoriasis are unknown.2
In the posttransplant period, the immunosuppressive regimens for transplantation likely will improve psoriasis. However, patients with organ transplant and psoriasis still experience flares that can be challenging to treat.3 Prior treatment modalities to prevent psoriasis flares in organ transplant recipients have relied largely on topical therapies, posttransplant immunosuppressive medications (eg, cyclosporine, tacrolimus, mycophenolate mofetil) that prevent graft rejection, and systemic corticosteroids. We report a case of a 50-year-old man with a recent history of liver transplantation who presented with severe plaque psoriasis and psoriatic arthritis.
Case Report
A 50-year-old man presented to the dermatology clinic with moderate to severe plaque psoriasis and psoriatic arthritis that had been present for 15 years. His plaque psoriasis covered approximately 40% of the body surface area, including the scalp, trunk, arms, and legs. In addition, he had diffuse joint pain in the hands and feet; a radiograph revealed active psoriatic arthritis involving the joints of the fingers and toes.
One year prior to presentation to our dermatology clinic, the patient underwent an an orthotopic liver transplant for history of Child-Pugh class C liver cirrhosis secondary to untreated hepatitis C virus (HCV) and alcohol use that was complicated by hepatocellular carcinoma. He acquired a high-risk donor liver that was HCV positive with HCV genotype 1a. Starting 2 months after the transplant, he underwent 12 weeks of treatment for HCV with glecaprevir-pibrentasvir. Once his HCV treatment course was completed, he achieved a sustained virologic response with an undetectable viral load. To prevent transplant rejection, he was on chronic immunosuppression with tacrolimus, a calcineurin inhibitor, and mycophenolate mofetil, an inhibitor of inosine monophosphate dehydrogenase whose action leads to decreased proliferation of T cells and B cells.
The patient’s psoriasis initially was treated with triamcinolone acetonide ointment 0.1% applied twice daily to the psoriasis lesions for 1 year by another dermatologist. However, his psoriasis progressed to involve 40% of the body surface area. Following our evaluation 1 year posttransplant, the patient was started on subcutaneous brodalumab 210 mg at weeks 0, 1, and 2, then every 2 weeks thereafter. Approximately 10 weeks after initiation of brodalumab, the patient’s psoriasis was completely clear, and he was asymptomatic from psoriatic arthritis. The patient’s improvement persisted at 6 months, and his liver enzymes, including alkaline phosphatase, total bilirubin, alanine transaminase, and aspartate transaminase, continued to be within reference range. To date, there has been no evidence of posttransplant complications such as graft-vs-host disease, serious infections, or skin cancers.
Comment
Increased Risk for Infection and Malignancies in Transplant Patients
Transplant patients are on immunosuppressive regimens that increase their risk for infection and malignancies. For example, high doses of immunosuppresants predispose these patients to reactivation of viral infections, including BK and JC viruses.4 In addition, the incidence of squamous cell carcinoma is 65- to 250-fold higher in transplant patients compared to the general population.5 The risk for Merkel cell carcinoma is increased after solid organ transplantation compared to the general population.6 Importantly, transplant patients have a higher mortality from skin cancers than other types of cancers, including breast and colon cancer.7
Psoriasis in Organ Transplant Recipients
Psoriasis is a chronic, immune-mediated, inflammatory disease with a prevalence of approximately 3% in the United States.8 Approximately one-third of patients with psoriasis develop psoriatic arthritis.9 Organ transplant recipients with psoriasis and psoriatic arthritis represent a unique patient population whereby their use of chronic immunosuppressive medications to prevent graft rejection may put them at risk for developing infections and malignancies.
Special Considerations for Brodalumab
Brodalumab is an immunomodulatory biologic that binds to and inhibits IL-17RA, thereby inhibiting the actions of IL-17A, F, E, and C.2 The blockade of IL-17RA by brodalumab has been shown to result in reversal of psoriatic phenotype and gene expression patterns.10 Brodalumab was chosen as the treatment in our patient because it has a rapid onset of action, sustained efficacy, and an acceptable safety profile.11 Brodalumab is well tolerated, with approximately 60% of patients achieving clearance long-term.12 Candidal infections can occur in patients with brodalumab, but the rates are low and they are reversible with antifungal treatment.13 The increased mucocutaneous candidal infections are consistent with medications whose mechanism of action is IL-17 inhibition.14,15 The most common adverse reactions found were nasopharyngitis and headache.16 The causal link between brodalumab and suicidality has not been established.17
The use of brodalumab for psoriasis in organ transplant recipients has not been previously reported in the literature. A few case reports have been published on the successful use of etanercept and ixekizumab as biologic treatment options for psoriasis in transplant patients.18-23 In addition to choosing an appropriate biologic for psoriasis in transplant patients, transplant providers may evaluate the choice of immunosuppression regimen for the organ transplant in the context of psoriasis. In a retrospective analysis of liver transplant patients with psoriasis, Foroncewicz et al3 found cyclosporine, which was used as an antirejection immunosuppressive agent in the posttransplant period, to be more effective than tacrolimus in treating recurrent psoriasis in liver transplant recipients.
Our case illustrates one example of the successful use of brodalumab in a patient with a solid organ transplant. Our patient’s psoriasis and symptoms of psoriatic arthritis greatly improved after initiation of brodalumab. In the posttransplant period, the patient did not develop graft-vs-host disease, infections, malignancies, depression, or suicidal ideation while taking brodalumab.
Conclusion
It is important that the patient, dermatology team, and transplant team work together to navigate the challenges and relatively unknown landscape of psoriasis treatment in organ transplant recipients. As the number of organ transplant recipients continues to increase, this issue will become more clinically relevant. Case reports and future prospective studies will continue to inform us regarding the role of biologics in psoriasis treatment posttransplantation.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072.
- Prussick R, Wu JJ, Armstrong AW, et al. Psoriasis in solid organ transplant patients: best practice recommendations from The Medical Board of the National Psoriasis Foundation. J Dermatol Treat. 2018;29:329-333.
- Foroncewicz B, Mucha K, Lerut J, et al. Cyclosporine is superior to tacrolimus in liver transplant recipients with recurrent psoriasis. Ann Transplant. 2014;19:427-433.
- Boukoum H, Nahdi I, Sahtout W, et al. BK and JC virus infections in healthy patients compared to kidney transplant recipients in Tunisia. Microbial Pathogenesis. 2016;97:204-208.
- Bouwes Bavinck JN, Euvrard S, Naldi L, et al. Keratotic skin lesions and other risk factors are associated with skin cancer in organ-transplant recipients: a case-control study in The Netherlands, United Kingdom, Germany, France, and Italy. J Invest Dermatol. 2007;127:1647-1656.
- Clark CA, Robbins HA, Tatalovich Z, et al. Risk of Merkel cell carcinoma after transplant. Clin Oncol. 2019;31:779-788.
- Lakhani NA, Saraiya M, Thompson TD, et al. Total body skin examination for skin cancer screening among U.S. adults from 2000 to 2010. Prev Med. 2014;61:75-80.
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512-516.
- Alinaghi F, Calov M, Kristensen LE, et al. Prevalence of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol. 2019;80:251-265.
- Russell CB, Rand H, Bigler J, et al. Gene expression profiles normalized in psoriatic skin by treatment with brodalumab, a human anti-IL-17 receptor monoclonal antibody. J Immunol. 2014;192:3828-3836.
- Foulkes AC, Warren RB. Brodalumab in psoriasis: evidence to date and clinical potential. Drugs Context. 2019;8:212570. doi:10.7573/dic.212570
- Puig L, Lebwohl M, Bachelez H, et al. Long-term efficacy and safety of brodalumab in the treatment of psoriasis: 120-week results from the randomized, double-blind, placebo- and active comparator-controlled phase 3 AMAGINE-2 trial. J Am Acad Dermatol. 2020;82:352-359.
- Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab and ustekinumab in psoriasis. N Engl J Med. 2015;373:1318-1328.
- Conti HR, Shen F, Nayyar N, et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med. 2009;206:299-311.
- Puel A, Cypowyj S, Bustamante J, et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science. 2011;332:65-68.
- Farahnik B, Beroukhim B, Abrouk M, et al. Brodalumab for the treatment of psoriasis: a review of Phase III trials. Dermatol Ther. 2016;6:111-124.
- Lebwohl MG, Papp KA, Marangell LB, et al. Psychiatric adverse events during treatment with brodalumab: analysis of psoriasis clinical trials. J Am Acad Dermatol. 2018;78:81-89.
- DeSimone C, Perino F, Caldarola G, et al. Treatment of psoriasis with etanercept in immunocompromised patients: two case reports. J Int Med Res. 2016;44:67-71.
- Madankumar R, Teperman LW, Stein JA. Use of etanercept for psoriasis in a liver transplant recipient. JAAD Case Rep. 2015;1:S36-S37.
- Collazo MH, González JR, Torres EA. Etanercept therapy for psoriasis in a patient with concomitant hepatitis C and liver transplant. P R Health Sci J. 2008;27:346-347.
- Hoover WD. Etanercept therapy for severe plaque psoriasis in a patient who underwent a liver transplant. Cutis. 2007;80:211-214.
- Brokalaki EI, Voshege N, Witzke O, et al. Treatment of severe psoriasis with etanercept in a pancreas-kidney transplant recipient. Transplant Proc. 2012;44:2776-2777.
- Lora V, Graceffa D, De Felice C, et al. Treatment of severe psoriasis with ixekizumab in a liver transplant recipient with concomitant hepatitis B virus infection. Dermatol Ther. 2019;32:E12909.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072.
- Prussick R, Wu JJ, Armstrong AW, et al. Psoriasis in solid organ transplant patients: best practice recommendations from The Medical Board of the National Psoriasis Foundation. J Dermatol Treat. 2018;29:329-333.
- Foroncewicz B, Mucha K, Lerut J, et al. Cyclosporine is superior to tacrolimus in liver transplant recipients with recurrent psoriasis. Ann Transplant. 2014;19:427-433.
- Boukoum H, Nahdi I, Sahtout W, et al. BK and JC virus infections in healthy patients compared to kidney transplant recipients in Tunisia. Microbial Pathogenesis. 2016;97:204-208.
- Bouwes Bavinck JN, Euvrard S, Naldi L, et al. Keratotic skin lesions and other risk factors are associated with skin cancer in organ-transplant recipients: a case-control study in The Netherlands, United Kingdom, Germany, France, and Italy. J Invest Dermatol. 2007;127:1647-1656.
- Clark CA, Robbins HA, Tatalovich Z, et al. Risk of Merkel cell carcinoma after transplant. Clin Oncol. 2019;31:779-788.
- Lakhani NA, Saraiya M, Thompson TD, et al. Total body skin examination for skin cancer screening among U.S. adults from 2000 to 2010. Prev Med. 2014;61:75-80.
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512-516.
- Alinaghi F, Calov M, Kristensen LE, et al. Prevalence of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol. 2019;80:251-265.
- Russell CB, Rand H, Bigler J, et al. Gene expression profiles normalized in psoriatic skin by treatment with brodalumab, a human anti-IL-17 receptor monoclonal antibody. J Immunol. 2014;192:3828-3836.
- Foulkes AC, Warren RB. Brodalumab in psoriasis: evidence to date and clinical potential. Drugs Context. 2019;8:212570. doi:10.7573/dic.212570
- Puig L, Lebwohl M, Bachelez H, et al. Long-term efficacy and safety of brodalumab in the treatment of psoriasis: 120-week results from the randomized, double-blind, placebo- and active comparator-controlled phase 3 AMAGINE-2 trial. J Am Acad Dermatol. 2020;82:352-359.
- Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab and ustekinumab in psoriasis. N Engl J Med. 2015;373:1318-1328.
- Conti HR, Shen F, Nayyar N, et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med. 2009;206:299-311.
- Puel A, Cypowyj S, Bustamante J, et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science. 2011;332:65-68.
- Farahnik B, Beroukhim B, Abrouk M, et al. Brodalumab for the treatment of psoriasis: a review of Phase III trials. Dermatol Ther. 2016;6:111-124.
- Lebwohl MG, Papp KA, Marangell LB, et al. Psychiatric adverse events during treatment with brodalumab: analysis of psoriasis clinical trials. J Am Acad Dermatol. 2018;78:81-89.
- DeSimone C, Perino F, Caldarola G, et al. Treatment of psoriasis with etanercept in immunocompromised patients: two case reports. J Int Med Res. 2016;44:67-71.
- Madankumar R, Teperman LW, Stein JA. Use of etanercept for psoriasis in a liver transplant recipient. JAAD Case Rep. 2015;1:S36-S37.
- Collazo MH, González JR, Torres EA. Etanercept therapy for psoriasis in a patient with concomitant hepatitis C and liver transplant. P R Health Sci J. 2008;27:346-347.
- Hoover WD. Etanercept therapy for severe plaque psoriasis in a patient who underwent a liver transplant. Cutis. 2007;80:211-214.
- Brokalaki EI, Voshege N, Witzke O, et al. Treatment of severe psoriasis with etanercept in a pancreas-kidney transplant recipient. Transplant Proc. 2012;44:2776-2777.
- Lora V, Graceffa D, De Felice C, et al. Treatment of severe psoriasis with ixekizumab in a liver transplant recipient with concomitant hepatitis B virus infection. Dermatol Ther. 2019;32:E12909.
Practice Points
- Immunocompromised patients, such as organ transplant recipients, require careful benefit-risk consideration when selecting a systemic agent for psoriasis.
- Brodalumab, an IL-17RA antagonist, was used to treat a patient with psoriasis who had undergone solid organ transplant with excellent response and good tolerability.
- Further studies are needed to evaluate the benefits and risks of using biologic treatments in patients with psoriasis who are organ transplant recipients.
Unilateral Verrucous Psoriasis
Case Report
An 80-year-old man with a history of hypertension and coronary artery disease presented to the dermatology clinic with a rash characterized by multiple asymptomatic plaques with overlying verrucous nodules on the left side of the abdomen, back, and leg (Figure 1). He reported that these “growths” appeared 20 years prior to presentation, shortly after coronary artery bypass surgery with a saphenous vein graft. The patient initially was given a diagnosis of verruca vulgaris and then biopsy-proven psoriasis later that year. At that time, he refused systemic treatment and was treated instead with triamcinolone acetonide ointment, with periodic surgical removal of bothersome lesions.
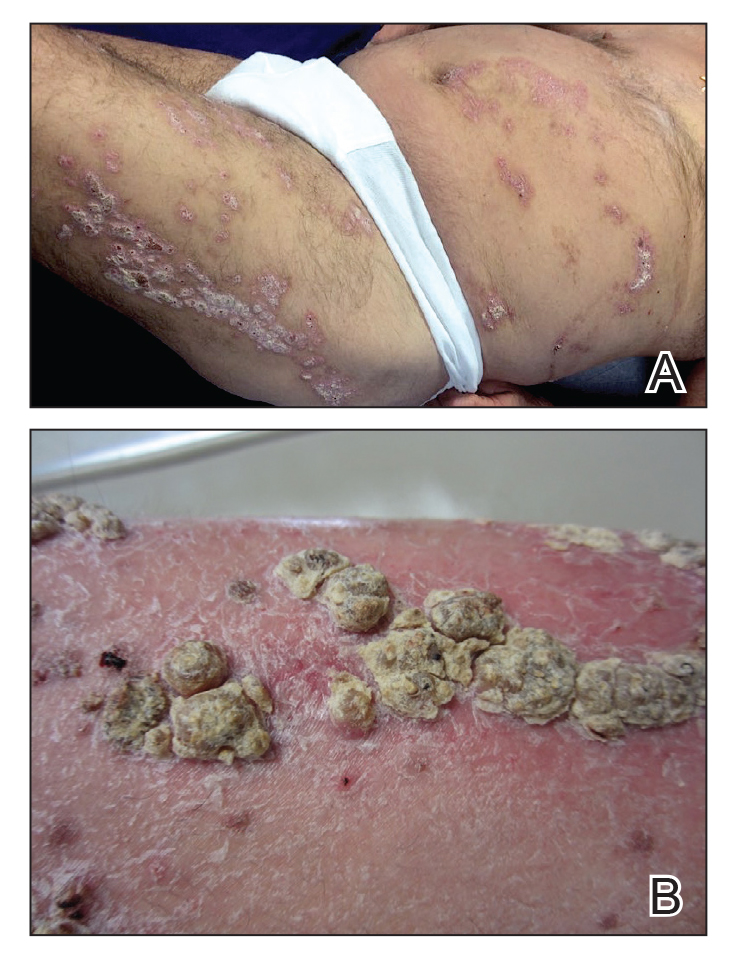
At the current presentation, physical examination revealed many hyperkeratotic, yellow-gray, verrucous nodules overlying scaly, erythematous, sharply demarcated plaques, exclusively on the left side of the body, including the left side of the abdomen, back, and leg. The differential diagnosis included linear psoriasis and inflammatory linear verrucous epidermal nevus (ILVEN).
Skin biopsy showed irregular psoriasiform epidermal hyperplasia with acanthosis, hyperkeratosis, and papillomatosis, with convergence of the rete ridges, known as buttressing (Figure 2A). There were tortuous dilated blood vessels in the dermal papillae, epidermal neutrophils at the tip of the suprapapillary plates, and Munro microabscesses in the stratum corneum (Figure 2B). Koilocytes were absent, and periodic acid–Schiff staining was negative. Taken together, clinical and histologic features led to a diagnosis of unilateral verrucous psoriasis.
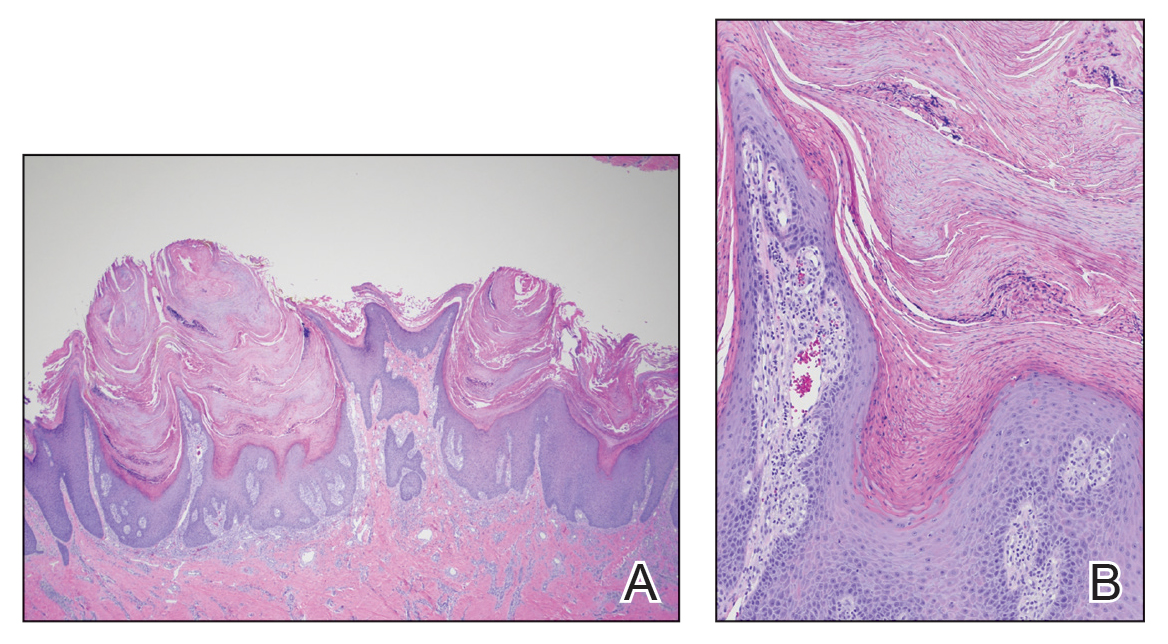
Comment
Presentation and Histology
Verrucous psoriasis is a variant of psoriasis that presents with wartlike clinical features and overlapping histologic features of verruca and psoriasis. It typically arises in patients with established psoriasis but can occur de novo.
Histologic features of verrucous psoriasis include epidermal hyperplasia with acanthosis, papillomatosis, and epidermal buttressing.1 It has been hypothesized that notable hyperkeratosis observed in these lesions is induced by repeat trauma to the extremities in patients with established psoriasis or by anoxia from conditions that predispose to poor circulation, such as diabetes mellitus and pulmonary disease.1,2
Pathogenesis
Most reported cases of verrucous psoriasis arose atop pre-existing psoriasis lesions.3,4 The relevance of our patient’s verrucous psoriasis to his prior coronary artery bypass surgery with saphenous vein graft is unknown; however, the distribution of lesions, timing of psoriasis onset in relation to the surgical procedure, and recent data proposing a role for neuropeptide responses to nerve injury in the development of psoriasis, taken together, provide an argument for a role for surgical trauma in the development of our patient’s condition.
Treatment
Although verrucous psoriasis presents both diagnostic and therapeutic challenges, there are some reports of improvement with topical or intralesional corticosteroids in combination with keratolytics,3 coal tar,5 and oral methotrexate.6 In addition, there are rare reports of successful treatment with biologics. A case report showed successful resolution with adalimumab,4 and a case of erythrodermic verrucous psoriasis showed moderate improvement with ustekinumab after other failed treatments.7
Differential Diagnosis
Psoriasis typically presents in a symmetric distribution, with rare reported cases of unilateral distribution. Two cases of unilateral psoriasis arising after a surgical procedure have been reported, one after mastectomy and the other after neurosurgery.8,9 Other cases of unilateral psoriasis are reported to have arisen in adolescents and young adults idiopathically.
A case of linear psoriasis arising in the distribution of the sciatic nerve in a patient with radiculopathy implicated tumor necrosis factor α, neuropeptides, and nerve growth factor released in response to compression as possible etiologic agents.10 However, none of the reported cases of linear psoriasis, or reported cases of unilateral psoriasis, exhibited verrucous features clinically or histologically. In our patient, distribution of the lesions appeared less typically blaschkoid than in linear psoriasis, and the presence of exophytic wartlike growths throughout the lesions was not characteristic of linear psoriasis.
Late-adulthood onset in this patient in addition to the absence of typical histologic features of ILVEN, including alternating orthokeratosis and parakeratosis,11 make a diagnosis of ILVEN less likely; ILVEN can be distinguished from linear psoriasis based on later age of onset and responsiveness to antipsoriatic therapy of linear psoriasis.12
Conclusion
We describe a unique presentation of an already rare variant of psoriasis that can be difficult to diagnose clinically. The unilateral distribution of lesions in this patient can create further diagnostic confusion with other entities, such as ILVEN and linear psoriasis, though it can be distinguished from those diseases based on histologic features. Our aim is that this report improves recognition of this unusual presentation of verrucous psoriasis in clinical settings and decreases delays in diagnosis and treatment.
- Khalil FK, Keehn CA, Saeed S, et al. Verrucous psoriasis: a distinctive clinicopathologic variant of psoriasis. Am J Dermatopathol. 2005;27:204-207.
- Wakamatsu K, Naniwa K, Hagiya Y, et al. Psoriasis verrucosa. J Dermatol. 2010;37:1060-1062.
- Monroe HR, Hillman JD, Chiu MW. A case of verrucous psoriasis. Dermatol Online J. 2011;17:10.
- Maejima H, Katayama C, Watarai A, et al. A case of psoriasis verrucosa successfully treated with adalimumab. J Drugs Dermatol. 2012;11:E74-E75.
- Erkek E, Bozdog˘an O. Annular verrucous psoriasis with exaggerated papillomatosis. Am J Dermatopathol. 2001;23:133-135.
- Hall L, Marks V, Tyler W. Verrucous psoriasis: a clinical and histopathologic mimicker of verruca vulgaris. J Am Acad Dermatol. 2013;68(4 suppl 1):AB218.
- Curtis AR, Yosipovitch G. Erythrodermic verrucous psoriasis. J Dermatolog Treat. 2012;23:215-218.
- Kim M, Jung JY, Na SY, et al. Unilateral psoriasis in a woman with ipsilateral post-mastectomy lymphedema. Ann Dermatol. 2011;23(suppl 3):S303-S305.
- Reyter I, Woodley D. Widespread unilateral plaques in a 68-year-old woman after neurosurgery. Arch Dermatol. 2004;140:1531-1536.
- Galluzzo M, Talamonti M, Di Stefani A, et al. Linear psoriasis following the typical distribution of the sciatic nerve. J Dermatol Case Rep. 2015;9:6-11.
- Sengupta S, Das JK, Gangopadhyay A. Naevoid psoriasis and ILVEN: same coin, two faces? Indian J Dermatol. 2012;57:489-491.
- Morag C, Metzker A. Inflammatory linear verrucous epidermal nevus: report of seven new cases and review of the literature. Pediatr Dermatol. 1985;3:15-18.
Case Report
An 80-year-old man with a history of hypertension and coronary artery disease presented to the dermatology clinic with a rash characterized by multiple asymptomatic plaques with overlying verrucous nodules on the left side of the abdomen, back, and leg (Figure 1). He reported that these “growths” appeared 20 years prior to presentation, shortly after coronary artery bypass surgery with a saphenous vein graft. The patient initially was given a diagnosis of verruca vulgaris and then biopsy-proven psoriasis later that year. At that time, he refused systemic treatment and was treated instead with triamcinolone acetonide ointment, with periodic surgical removal of bothersome lesions.

At the current presentation, physical examination revealed many hyperkeratotic, yellow-gray, verrucous nodules overlying scaly, erythematous, sharply demarcated plaques, exclusively on the left side of the body, including the left side of the abdomen, back, and leg. The differential diagnosis included linear psoriasis and inflammatory linear verrucous epidermal nevus (ILVEN).
Skin biopsy showed irregular psoriasiform epidermal hyperplasia with acanthosis, hyperkeratosis, and papillomatosis, with convergence of the rete ridges, known as buttressing (Figure 2A). There were tortuous dilated blood vessels in the dermal papillae, epidermal neutrophils at the tip of the suprapapillary plates, and Munro microabscesses in the stratum corneum (Figure 2B). Koilocytes were absent, and periodic acid–Schiff staining was negative. Taken together, clinical and histologic features led to a diagnosis of unilateral verrucous psoriasis.

Comment
Presentation and Histology
Verrucous psoriasis is a variant of psoriasis that presents with wartlike clinical features and overlapping histologic features of verruca and psoriasis. It typically arises in patients with established psoriasis but can occur de novo.
Histologic features of verrucous psoriasis include epidermal hyperplasia with acanthosis, papillomatosis, and epidermal buttressing.1 It has been hypothesized that notable hyperkeratosis observed in these lesions is induced by repeat trauma to the extremities in patients with established psoriasis or by anoxia from conditions that predispose to poor circulation, such as diabetes mellitus and pulmonary disease.1,2
Pathogenesis
Most reported cases of verrucous psoriasis arose atop pre-existing psoriasis lesions.3,4 The relevance of our patient’s verrucous psoriasis to his prior coronary artery bypass surgery with saphenous vein graft is unknown; however, the distribution of lesions, timing of psoriasis onset in relation to the surgical procedure, and recent data proposing a role for neuropeptide responses to nerve injury in the development of psoriasis, taken together, provide an argument for a role for surgical trauma in the development of our patient’s condition.
Treatment
Although verrucous psoriasis presents both diagnostic and therapeutic challenges, there are some reports of improvement with topical or intralesional corticosteroids in combination with keratolytics,3 coal tar,5 and oral methotrexate.6 In addition, there are rare reports of successful treatment with biologics. A case report showed successful resolution with adalimumab,4 and a case of erythrodermic verrucous psoriasis showed moderate improvement with ustekinumab after other failed treatments.7
Differential Diagnosis
Psoriasis typically presents in a symmetric distribution, with rare reported cases of unilateral distribution. Two cases of unilateral psoriasis arising after a surgical procedure have been reported, one after mastectomy and the other after neurosurgery.8,9 Other cases of unilateral psoriasis are reported to have arisen in adolescents and young adults idiopathically.
A case of linear psoriasis arising in the distribution of the sciatic nerve in a patient with radiculopathy implicated tumor necrosis factor α, neuropeptides, and nerve growth factor released in response to compression as possible etiologic agents.10 However, none of the reported cases of linear psoriasis, or reported cases of unilateral psoriasis, exhibited verrucous features clinically or histologically. In our patient, distribution of the lesions appeared less typically blaschkoid than in linear psoriasis, and the presence of exophytic wartlike growths throughout the lesions was not characteristic of linear psoriasis.
Late-adulthood onset in this patient in addition to the absence of typical histologic features of ILVEN, including alternating orthokeratosis and parakeratosis,11 make a diagnosis of ILVEN less likely; ILVEN can be distinguished from linear psoriasis based on later age of onset and responsiveness to antipsoriatic therapy of linear psoriasis.12
Conclusion
We describe a unique presentation of an already rare variant of psoriasis that can be difficult to diagnose clinically. The unilateral distribution of lesions in this patient can create further diagnostic confusion with other entities, such as ILVEN and linear psoriasis, though it can be distinguished from those diseases based on histologic features. Our aim is that this report improves recognition of this unusual presentation of verrucous psoriasis in clinical settings and decreases delays in diagnosis and treatment.
Case Report
An 80-year-old man with a history of hypertension and coronary artery disease presented to the dermatology clinic with a rash characterized by multiple asymptomatic plaques with overlying verrucous nodules on the left side of the abdomen, back, and leg (Figure 1). He reported that these “growths” appeared 20 years prior to presentation, shortly after coronary artery bypass surgery with a saphenous vein graft. The patient initially was given a diagnosis of verruca vulgaris and then biopsy-proven psoriasis later that year. At that time, he refused systemic treatment and was treated instead with triamcinolone acetonide ointment, with periodic surgical removal of bothersome lesions.

At the current presentation, physical examination revealed many hyperkeratotic, yellow-gray, verrucous nodules overlying scaly, erythematous, sharply demarcated plaques, exclusively on the left side of the body, including the left side of the abdomen, back, and leg. The differential diagnosis included linear psoriasis and inflammatory linear verrucous epidermal nevus (ILVEN).
Skin biopsy showed irregular psoriasiform epidermal hyperplasia with acanthosis, hyperkeratosis, and papillomatosis, with convergence of the rete ridges, known as buttressing (Figure 2A). There were tortuous dilated blood vessels in the dermal papillae, epidermal neutrophils at the tip of the suprapapillary plates, and Munro microabscesses in the stratum corneum (Figure 2B). Koilocytes were absent, and periodic acid–Schiff staining was negative. Taken together, clinical and histologic features led to a diagnosis of unilateral verrucous psoriasis.

Comment
Presentation and Histology
Verrucous psoriasis is a variant of psoriasis that presents with wartlike clinical features and overlapping histologic features of verruca and psoriasis. It typically arises in patients with established psoriasis but can occur de novo.
Histologic features of verrucous psoriasis include epidermal hyperplasia with acanthosis, papillomatosis, and epidermal buttressing.1 It has been hypothesized that notable hyperkeratosis observed in these lesions is induced by repeat trauma to the extremities in patients with established psoriasis or by anoxia from conditions that predispose to poor circulation, such as diabetes mellitus and pulmonary disease.1,2
Pathogenesis
Most reported cases of verrucous psoriasis arose atop pre-existing psoriasis lesions.3,4 The relevance of our patient’s verrucous psoriasis to his prior coronary artery bypass surgery with saphenous vein graft is unknown; however, the distribution of lesions, timing of psoriasis onset in relation to the surgical procedure, and recent data proposing a role for neuropeptide responses to nerve injury in the development of psoriasis, taken together, provide an argument for a role for surgical trauma in the development of our patient’s condition.
Treatment
Although verrucous psoriasis presents both diagnostic and therapeutic challenges, there are some reports of improvement with topical or intralesional corticosteroids in combination with keratolytics,3 coal tar,5 and oral methotrexate.6 In addition, there are rare reports of successful treatment with biologics. A case report showed successful resolution with adalimumab,4 and a case of erythrodermic verrucous psoriasis showed moderate improvement with ustekinumab after other failed treatments.7
Differential Diagnosis
Psoriasis typically presents in a symmetric distribution, with rare reported cases of unilateral distribution. Two cases of unilateral psoriasis arising after a surgical procedure have been reported, one after mastectomy and the other after neurosurgery.8,9 Other cases of unilateral psoriasis are reported to have arisen in adolescents and young adults idiopathically.
A case of linear psoriasis arising in the distribution of the sciatic nerve in a patient with radiculopathy implicated tumor necrosis factor α, neuropeptides, and nerve growth factor released in response to compression as possible etiologic agents.10 However, none of the reported cases of linear psoriasis, or reported cases of unilateral psoriasis, exhibited verrucous features clinically or histologically. In our patient, distribution of the lesions appeared less typically blaschkoid than in linear psoriasis, and the presence of exophytic wartlike growths throughout the lesions was not characteristic of linear psoriasis.
Late-adulthood onset in this patient in addition to the absence of typical histologic features of ILVEN, including alternating orthokeratosis and parakeratosis,11 make a diagnosis of ILVEN less likely; ILVEN can be distinguished from linear psoriasis based on later age of onset and responsiveness to antipsoriatic therapy of linear psoriasis.12
Conclusion
We describe a unique presentation of an already rare variant of psoriasis that can be difficult to diagnose clinically. The unilateral distribution of lesions in this patient can create further diagnostic confusion with other entities, such as ILVEN and linear psoriasis, though it can be distinguished from those diseases based on histologic features. Our aim is that this report improves recognition of this unusual presentation of verrucous psoriasis in clinical settings and decreases delays in diagnosis and treatment.
- Khalil FK, Keehn CA, Saeed S, et al. Verrucous psoriasis: a distinctive clinicopathologic variant of psoriasis. Am J Dermatopathol. 2005;27:204-207.
- Wakamatsu K, Naniwa K, Hagiya Y, et al. Psoriasis verrucosa. J Dermatol. 2010;37:1060-1062.
- Monroe HR, Hillman JD, Chiu MW. A case of verrucous psoriasis. Dermatol Online J. 2011;17:10.
- Maejima H, Katayama C, Watarai A, et al. A case of psoriasis verrucosa successfully treated with adalimumab. J Drugs Dermatol. 2012;11:E74-E75.
- Erkek E, Bozdog˘an O. Annular verrucous psoriasis with exaggerated papillomatosis. Am J Dermatopathol. 2001;23:133-135.
- Hall L, Marks V, Tyler W. Verrucous psoriasis: a clinical and histopathologic mimicker of verruca vulgaris. J Am Acad Dermatol. 2013;68(4 suppl 1):AB218.
- Curtis AR, Yosipovitch G. Erythrodermic verrucous psoriasis. J Dermatolog Treat. 2012;23:215-218.
- Kim M, Jung JY, Na SY, et al. Unilateral psoriasis in a woman with ipsilateral post-mastectomy lymphedema. Ann Dermatol. 2011;23(suppl 3):S303-S305.
- Reyter I, Woodley D. Widespread unilateral plaques in a 68-year-old woman after neurosurgery. Arch Dermatol. 2004;140:1531-1536.
- Galluzzo M, Talamonti M, Di Stefani A, et al. Linear psoriasis following the typical distribution of the sciatic nerve. J Dermatol Case Rep. 2015;9:6-11.
- Sengupta S, Das JK, Gangopadhyay A. Naevoid psoriasis and ILVEN: same coin, two faces? Indian J Dermatol. 2012;57:489-491.
- Morag C, Metzker A. Inflammatory linear verrucous epidermal nevus: report of seven new cases and review of the literature. Pediatr Dermatol. 1985;3:15-18.
- Khalil FK, Keehn CA, Saeed S, et al. Verrucous psoriasis: a distinctive clinicopathologic variant of psoriasis. Am J Dermatopathol. 2005;27:204-207.
- Wakamatsu K, Naniwa K, Hagiya Y, et al. Psoriasis verrucosa. J Dermatol. 2010;37:1060-1062.
- Monroe HR, Hillman JD, Chiu MW. A case of verrucous psoriasis. Dermatol Online J. 2011;17:10.
- Maejima H, Katayama C, Watarai A, et al. A case of psoriasis verrucosa successfully treated with adalimumab. J Drugs Dermatol. 2012;11:E74-E75.
- Erkek E, Bozdog˘an O. Annular verrucous psoriasis with exaggerated papillomatosis. Am J Dermatopathol. 2001;23:133-135.
- Hall L, Marks V, Tyler W. Verrucous psoriasis: a clinical and histopathologic mimicker of verruca vulgaris. J Am Acad Dermatol. 2013;68(4 suppl 1):AB218.
- Curtis AR, Yosipovitch G. Erythrodermic verrucous psoriasis. J Dermatolog Treat. 2012;23:215-218.
- Kim M, Jung JY, Na SY, et al. Unilateral psoriasis in a woman with ipsilateral post-mastectomy lymphedema. Ann Dermatol. 2011;23(suppl 3):S303-S305.
- Reyter I, Woodley D. Widespread unilateral plaques in a 68-year-old woman after neurosurgery. Arch Dermatol. 2004;140:1531-1536.
- Galluzzo M, Talamonti M, Di Stefani A, et al. Linear psoriasis following the typical distribution of the sciatic nerve. J Dermatol Case Rep. 2015;9:6-11.
- Sengupta S, Das JK, Gangopadhyay A. Naevoid psoriasis and ILVEN: same coin, two faces? Indian J Dermatol. 2012;57:489-491.
- Morag C, Metzker A. Inflammatory linear verrucous epidermal nevus: report of seven new cases and review of the literature. Pediatr Dermatol. 1985;3:15-18.
Practice Points
- Verrucous psoriasis is a rare variant of psoriasis characterized by hypertrophic verrucous papules and plaques on an erythematous base.
- Histologically, verrucous psoriasis presents with overlapping features of verruca and psoriasis.
- Although psoriasis typically presents in a symmetric distribution, unilateral psoriasis can occur either de novo in younger patients or after surgical trauma in older patients.
Guselkumab maintains psoriasis efficacy long after discontinuation
Fully half of patients with moderate to severe psoriasis who achieve complete clearance after their first four doses of guselkumab (Tremfya) continue to maintain a PASI 90 response nearly 6 months after withdrawal of the biologic, according to a post hoc analysis of the pivotal phase 3 VOYAGE 2 trial.
“That’s impressive maintenance of efficacy,” said Curdin Conrad, MD, who presented the data at the virtual annual congress of the European Academy of Dermatology and Venereology.
“These findings are reassuring when you have to interrupt guselkumab therapy: For example, due to acute infection, pregnancy, or surgery. But it might also help when considering in the future a flexible dosing interval, particularly for patients who had complete clearance,” added Dr. Conrad, professor of dermatology and head of the polyclinic and the Center of Excellence for Psoriasis at Lausanne (Switzerland) University Hospital.
The intriguing implication from VOYAGE 2 that guselkumab might lend itself to flexible dosing featuring lengthy drug-free intervals is being prospectively examined in the ongoing phase 3b GUIDE trial. This is a double-blind, placebo-controlled trial including 888 French and German patients with moderate to severe psoriasis and a study hypothesis that those who have a Psoriasis Area and Severity Index score of 0 at weeks 20 and 28 in response to on-label dosing – the so-called ‘super responders’ – will maintain disease control until week 68 if their dosing is reduced to 100 mg of guselkumab every 16 weeks instead of the standard 8-week intervals.
Dr. Conrad reported that in VOYAGE 2, 106 patients on standard-dose guselkumab who had a PASI score of 0 at weeks 20 and 28 were randomized to discontinue the interleukin-23 inhibitor after receiving their fourth dose at week 20. It took 25 weeks for 50% of them to lose their PASI 90 response as defined by regression to a PASI score of 1 or greater. Using a less stringent definition of maintenance of efficacy, the super responders’ median time off guselkumab until reaching a PASI score of 3 or more was 30.7 weeks, with a median of 35.4 weeks to a PASI score of 5 or more.
In addition, 34 other VOYAGE 2 participants who were almost clear on guselkumab at weeks 20 and 28, with a PASI score of more than 0 but less than 1, were randomized to guselkumab withdrawal after their week-20 dose. Median time to loss of their PASI 90 response was shorter than that of the super responders – not surprising since their mean PASI score when the biologic was halted was 0.5, rather than 0 as for the super responders. But Dr. Conrad said the maintenance of response was still impressive: A median of 16.2 weeks to reach a PASI score of 1 or more, 27.2 weeks for a PASI 3, and 33.7 weeks for a PASI score of 5.
He reported receiving research funding from and serving as a scientific adviser to Janssen, the study sponsor, as well as to more than a dozen other pharmaceutical companies.
Fully half of patients with moderate to severe psoriasis who achieve complete clearance after their first four doses of guselkumab (Tremfya) continue to maintain a PASI 90 response nearly 6 months after withdrawal of the biologic, according to a post hoc analysis of the pivotal phase 3 VOYAGE 2 trial.
“That’s impressive maintenance of efficacy,” said Curdin Conrad, MD, who presented the data at the virtual annual congress of the European Academy of Dermatology and Venereology.
“These findings are reassuring when you have to interrupt guselkumab therapy: For example, due to acute infection, pregnancy, or surgery. But it might also help when considering in the future a flexible dosing interval, particularly for patients who had complete clearance,” added Dr. Conrad, professor of dermatology and head of the polyclinic and the Center of Excellence for Psoriasis at Lausanne (Switzerland) University Hospital.
The intriguing implication from VOYAGE 2 that guselkumab might lend itself to flexible dosing featuring lengthy drug-free intervals is being prospectively examined in the ongoing phase 3b GUIDE trial. This is a double-blind, placebo-controlled trial including 888 French and German patients with moderate to severe psoriasis and a study hypothesis that those who have a Psoriasis Area and Severity Index score of 0 at weeks 20 and 28 in response to on-label dosing – the so-called ‘super responders’ – will maintain disease control until week 68 if their dosing is reduced to 100 mg of guselkumab every 16 weeks instead of the standard 8-week intervals.
Dr. Conrad reported that in VOYAGE 2, 106 patients on standard-dose guselkumab who had a PASI score of 0 at weeks 20 and 28 were randomized to discontinue the interleukin-23 inhibitor after receiving their fourth dose at week 20. It took 25 weeks for 50% of them to lose their PASI 90 response as defined by regression to a PASI score of 1 or greater. Using a less stringent definition of maintenance of efficacy, the super responders’ median time off guselkumab until reaching a PASI score of 3 or more was 30.7 weeks, with a median of 35.4 weeks to a PASI score of 5 or more.
In addition, 34 other VOYAGE 2 participants who were almost clear on guselkumab at weeks 20 and 28, with a PASI score of more than 0 but less than 1, were randomized to guselkumab withdrawal after their week-20 dose. Median time to loss of their PASI 90 response was shorter than that of the super responders – not surprising since their mean PASI score when the biologic was halted was 0.5, rather than 0 as for the super responders. But Dr. Conrad said the maintenance of response was still impressive: A median of 16.2 weeks to reach a PASI score of 1 or more, 27.2 weeks for a PASI 3, and 33.7 weeks for a PASI score of 5.
He reported receiving research funding from and serving as a scientific adviser to Janssen, the study sponsor, as well as to more than a dozen other pharmaceutical companies.
Fully half of patients with moderate to severe psoriasis who achieve complete clearance after their first four doses of guselkumab (Tremfya) continue to maintain a PASI 90 response nearly 6 months after withdrawal of the biologic, according to a post hoc analysis of the pivotal phase 3 VOYAGE 2 trial.
“That’s impressive maintenance of efficacy,” said Curdin Conrad, MD, who presented the data at the virtual annual congress of the European Academy of Dermatology and Venereology.
“These findings are reassuring when you have to interrupt guselkumab therapy: For example, due to acute infection, pregnancy, or surgery. But it might also help when considering in the future a flexible dosing interval, particularly for patients who had complete clearance,” added Dr. Conrad, professor of dermatology and head of the polyclinic and the Center of Excellence for Psoriasis at Lausanne (Switzerland) University Hospital.
The intriguing implication from VOYAGE 2 that guselkumab might lend itself to flexible dosing featuring lengthy drug-free intervals is being prospectively examined in the ongoing phase 3b GUIDE trial. This is a double-blind, placebo-controlled trial including 888 French and German patients with moderate to severe psoriasis and a study hypothesis that those who have a Psoriasis Area and Severity Index score of 0 at weeks 20 and 28 in response to on-label dosing – the so-called ‘super responders’ – will maintain disease control until week 68 if their dosing is reduced to 100 mg of guselkumab every 16 weeks instead of the standard 8-week intervals.
Dr. Conrad reported that in VOYAGE 2, 106 patients on standard-dose guselkumab who had a PASI score of 0 at weeks 20 and 28 were randomized to discontinue the interleukin-23 inhibitor after receiving their fourth dose at week 20. It took 25 weeks for 50% of them to lose their PASI 90 response as defined by regression to a PASI score of 1 or greater. Using a less stringent definition of maintenance of efficacy, the super responders’ median time off guselkumab until reaching a PASI score of 3 or more was 30.7 weeks, with a median of 35.4 weeks to a PASI score of 5 or more.
In addition, 34 other VOYAGE 2 participants who were almost clear on guselkumab at weeks 20 and 28, with a PASI score of more than 0 but less than 1, were randomized to guselkumab withdrawal after their week-20 dose. Median time to loss of their PASI 90 response was shorter than that of the super responders – not surprising since their mean PASI score when the biologic was halted was 0.5, rather than 0 as for the super responders. But Dr. Conrad said the maintenance of response was still impressive: A median of 16.2 weeks to reach a PASI score of 1 or more, 27.2 weeks for a PASI 3, and 33.7 weeks for a PASI score of 5.
He reported receiving research funding from and serving as a scientific adviser to Janssen, the study sponsor, as well as to more than a dozen other pharmaceutical companies.
FROM THE EADV CONGRESS
Dermatologist survey spotlights psoriasis care deficiencies in reproductive-age women
In a , Jenny Murase, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
“In Germany, the UK, and the United States, dermatologists face challenges in discussing pregnancy and child-bearing aspiration with women of reproductive age, in recommending compatible treatments during pregnancy, and engaging patients in the shared decision-making process. These challenges may exist due to suboptimal knowledge, skills, confidence, and attitude in respective areas of care,” said Dr. Murase, a dermatologist at the University of California, San Francisco, and coeditor-in-chief of the International Journal of Women’s Dermatology.
These shortcomings were documented in a survey, which began with Dr. Murase and her coinvestigators conducting detailed, 45-minute-long, semistructured telephone interviews with 24 dermatologists in the three countries. Those interviews provided the basis for subsequent development of a 20-minute online survey on psoriasis and pregnancy completed by 167 American, German, and UK dermatologists. The survey incorporated multiple choice questions and quantitative rating scales.
“Participants expressed challenges engaging in family planning counseling and reproductive health care as part of risk assessments for psoriasis,” Dr. Murase said.
Among the key findings:
- Forty-seven percent of respondents considered their knowledge of the impact of psoriasis on women’s reproductive health to be suboptimal. This knowledge gap was most common among American dermatologists, 59% of whom rated themselves as having suboptimal knowledge, and least common among German practitioners, only 27% of whom reported deficiencies in this area.
Fifty percent of dermatologists rated themselves as having suboptimal skills in discussing contraceptive methods with their psoriasis patients of childbearing potential.
- Forty-eight percent of respondents – and 59% of the American dermatologists – indicated they prefer to leave pregnancy-related discussions to ob.gyns.
- Fifty-five percent of dermatologists had only limited knowledge of the safety data and indications for prescribing biologic therapies before, during, and after pregnancy. Respondents gave themselves an average score of 58 out of 100 in terms of their confidence in prescribing biologics during pregnancy, compared to 74 out of 100 when prescribing before or after pregnancy.
- Forty-eight percent of participants indicated they had suboptimal skills in helping patients counter obstacles to treatment adherence.
Consideration of treatment of psoriasis in pregnancy requires balancing potential medication risks to the fetus versus the possible maternal and fetal harms of under- or nontreatment of their chronic inflammatory skin disease. It’s a matter that calls for shared decision-making between dermatologist and patient. But the survey showed that shared decision-making was often poorly integrated into clinical practice. Ninety-seven percent of the U.S. dermatologists were unaware of the existence of shared decision-making practice guidelines or models, as were 80% of UK respondents and 85% of the Germans. Of the relatively few dermatologists who were aware of such guidance, nearly half dismissed it as inapplicable to their clinical practice. More than one-third of respondents admitted having suboptimal skills in assessing their patients’ desired level of involvement in medical decisions. And one-third of the German dermatologists and roughly one-quarter of those from the United States and United Kingdom reported feeling pressure to make treatment decisions quickly and without patient input.
Dr. Murase added that the survey findings make a strong case for future interventions designed to help dermatologists appreciate the value of shared decision-making and develop the requisite patient-engagement skills. Dr. Murase reported serving as a paid consultant to UCB Pharma, which funded the survey via an educational grant.
In a , Jenny Murase, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
“In Germany, the UK, and the United States, dermatologists face challenges in discussing pregnancy and child-bearing aspiration with women of reproductive age, in recommending compatible treatments during pregnancy, and engaging patients in the shared decision-making process. These challenges may exist due to suboptimal knowledge, skills, confidence, and attitude in respective areas of care,” said Dr. Murase, a dermatologist at the University of California, San Francisco, and coeditor-in-chief of the International Journal of Women’s Dermatology.
These shortcomings were documented in a survey, which began with Dr. Murase and her coinvestigators conducting detailed, 45-minute-long, semistructured telephone interviews with 24 dermatologists in the three countries. Those interviews provided the basis for subsequent development of a 20-minute online survey on psoriasis and pregnancy completed by 167 American, German, and UK dermatologists. The survey incorporated multiple choice questions and quantitative rating scales.
“Participants expressed challenges engaging in family planning counseling and reproductive health care as part of risk assessments for psoriasis,” Dr. Murase said.
Among the key findings:
- Forty-seven percent of respondents considered their knowledge of the impact of psoriasis on women’s reproductive health to be suboptimal. This knowledge gap was most common among American dermatologists, 59% of whom rated themselves as having suboptimal knowledge, and least common among German practitioners, only 27% of whom reported deficiencies in this area.
Fifty percent of dermatologists rated themselves as having suboptimal skills in discussing contraceptive methods with their psoriasis patients of childbearing potential.
- Forty-eight percent of respondents – and 59% of the American dermatologists – indicated they prefer to leave pregnancy-related discussions to ob.gyns.
- Fifty-five percent of dermatologists had only limited knowledge of the safety data and indications for prescribing biologic therapies before, during, and after pregnancy. Respondents gave themselves an average score of 58 out of 100 in terms of their confidence in prescribing biologics during pregnancy, compared to 74 out of 100 when prescribing before or after pregnancy.
- Forty-eight percent of participants indicated they had suboptimal skills in helping patients counter obstacles to treatment adherence.
Consideration of treatment of psoriasis in pregnancy requires balancing potential medication risks to the fetus versus the possible maternal and fetal harms of under- or nontreatment of their chronic inflammatory skin disease. It’s a matter that calls for shared decision-making between dermatologist and patient. But the survey showed that shared decision-making was often poorly integrated into clinical practice. Ninety-seven percent of the U.S. dermatologists were unaware of the existence of shared decision-making practice guidelines or models, as were 80% of UK respondents and 85% of the Germans. Of the relatively few dermatologists who were aware of such guidance, nearly half dismissed it as inapplicable to their clinical practice. More than one-third of respondents admitted having suboptimal skills in assessing their patients’ desired level of involvement in medical decisions. And one-third of the German dermatologists and roughly one-quarter of those from the United States and United Kingdom reported feeling pressure to make treatment decisions quickly and without patient input.
Dr. Murase added that the survey findings make a strong case for future interventions designed to help dermatologists appreciate the value of shared decision-making and develop the requisite patient-engagement skills. Dr. Murase reported serving as a paid consultant to UCB Pharma, which funded the survey via an educational grant.
In a , Jenny Murase, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
“In Germany, the UK, and the United States, dermatologists face challenges in discussing pregnancy and child-bearing aspiration with women of reproductive age, in recommending compatible treatments during pregnancy, and engaging patients in the shared decision-making process. These challenges may exist due to suboptimal knowledge, skills, confidence, and attitude in respective areas of care,” said Dr. Murase, a dermatologist at the University of California, San Francisco, and coeditor-in-chief of the International Journal of Women’s Dermatology.
These shortcomings were documented in a survey, which began with Dr. Murase and her coinvestigators conducting detailed, 45-minute-long, semistructured telephone interviews with 24 dermatologists in the three countries. Those interviews provided the basis for subsequent development of a 20-minute online survey on psoriasis and pregnancy completed by 167 American, German, and UK dermatologists. The survey incorporated multiple choice questions and quantitative rating scales.
“Participants expressed challenges engaging in family planning counseling and reproductive health care as part of risk assessments for psoriasis,” Dr. Murase said.
Among the key findings:
- Forty-seven percent of respondents considered their knowledge of the impact of psoriasis on women’s reproductive health to be suboptimal. This knowledge gap was most common among American dermatologists, 59% of whom rated themselves as having suboptimal knowledge, and least common among German practitioners, only 27% of whom reported deficiencies in this area.
Fifty percent of dermatologists rated themselves as having suboptimal skills in discussing contraceptive methods with their psoriasis patients of childbearing potential.
- Forty-eight percent of respondents – and 59% of the American dermatologists – indicated they prefer to leave pregnancy-related discussions to ob.gyns.
- Fifty-five percent of dermatologists had only limited knowledge of the safety data and indications for prescribing biologic therapies before, during, and after pregnancy. Respondents gave themselves an average score of 58 out of 100 in terms of their confidence in prescribing biologics during pregnancy, compared to 74 out of 100 when prescribing before or after pregnancy.
- Forty-eight percent of participants indicated they had suboptimal skills in helping patients counter obstacles to treatment adherence.
Consideration of treatment of psoriasis in pregnancy requires balancing potential medication risks to the fetus versus the possible maternal and fetal harms of under- or nontreatment of their chronic inflammatory skin disease. It’s a matter that calls for shared decision-making between dermatologist and patient. But the survey showed that shared decision-making was often poorly integrated into clinical practice. Ninety-seven percent of the U.S. dermatologists were unaware of the existence of shared decision-making practice guidelines or models, as were 80% of UK respondents and 85% of the Germans. Of the relatively few dermatologists who were aware of such guidance, nearly half dismissed it as inapplicable to their clinical practice. More than one-third of respondents admitted having suboptimal skills in assessing their patients’ desired level of involvement in medical decisions. And one-third of the German dermatologists and roughly one-quarter of those from the United States and United Kingdom reported feeling pressure to make treatment decisions quickly and without patient input.
Dr. Murase added that the survey findings make a strong case for future interventions designed to help dermatologists appreciate the value of shared decision-making and develop the requisite patient-engagement skills. Dr. Murase reported serving as a paid consultant to UCB Pharma, which funded the survey via an educational grant.
FROM THE EADV CONGRESS
Deucravacitinib offers biologic-like psoriasis efficacy in oral form
and a range of other chronic inflammatory diseases, Bruce E. Strober, MD, PhD, said at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually this year.
Deucravacitinib solely blocks tyrosine kinase 2 (TYK2) signaling without touching Janus kinase (JAK) 1, 2, or 3. In so doing, it inhibits several cytokines important for inflammation: interleukin-12, IL-13, and interferon-alpha and -beta. Yet it doesn’t affect the numerous pathways mediated by JAKs 1-3, many of which relate to growth and development of cell lineages, including production of erythropoietin, thrombopoietin, granulocyte-macrophage colony-stimulating factor, prolactin, growth hormone, and leptin. These deucravacitinib characteristics should translate into fewer off-target side effects than with oral JAK inhibitors.
“The promise of TYK2 inhibition that’s brought to you by deucravacitinib is there will be no laboratory monitoring and the effects will be narrow in blocking inflammation,” said Dr. Strober, a dermatologist at Yale University, New Haven, Conn., and in private practice in Cromwell, Conn.
He highlighted the positive results of a randomized, phase 2, dose-ranging study conducted in 267 patients with moderate or severe plaque psoriasis. Participants had an average baseline Psoriasis Area and Severity Index (PASI) score of 19, with a Dermatology Life Quality Index score of about 12. At the top dose of 12 mg once daily, 75% of patients achieved a PASI 75 response at week 12, and 44% reached a PASI 90, as did 69% and 44%, respectively, who were on deucravacitinib at 3 mg twice daily. Those are collective efficacy numbers similar to adalimumab (Humira) or ustekinumab (Stelara).
Deucravacitinib may provide efficacy “like one of our second-tier biological therapies, yet it will be oral,” Dr. Strober commented.
Importantly, no laboratory abnormalities were detected in this trial. Only mild side effects were documented, most prominently acne, which occurred in dose-dependent fashion in 2% of patients on 3 mg of deucravacitinib twice daily and 4% at 12 mg once daily.
“The treatment of the acne that is elicited by this drug is yet to be fully described, but I’m sure we’ll learn the best approaches, given that acne is in our wheel house,” the dermatologist added.
Bristol-Myers Squibb has announced positive results from the pivotal phase 3 POETYK PSO-1 trial. Deucravacitinib at 6 mg once daily met both of its coprimary efficacy endpoints in the study, which included 666 patients with moderate to severe psoriasis. The TYK 2 inhibitor demonstrated superiority to both placebo and oral apremilast (Otezla) at week 16. The company said the safety profile was consistent with the phase 2 results, and that the full details of the phase 3 trial will be presented next year at a major medical meeting.
In addition, positive phase 2 results were reported for deucravacitinib in the treatment of psoriatic arthritis in a randomized trial presented at the fall 2020 meeting of the American College of Rheumatology. Deucravacitinib is also under study for lupus and inflammatory bowel disease.
Dr. Strober, an active clinical trialist, reported serving as a consultant to more than two dozen pharmaceutical companies, including Bristol-Myers Squibb.
MedscapeLive and this news organization are owned by the same parent company.
and a range of other chronic inflammatory diseases, Bruce E. Strober, MD, PhD, said at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually this year.
Deucravacitinib solely blocks tyrosine kinase 2 (TYK2) signaling without touching Janus kinase (JAK) 1, 2, or 3. In so doing, it inhibits several cytokines important for inflammation: interleukin-12, IL-13, and interferon-alpha and -beta. Yet it doesn’t affect the numerous pathways mediated by JAKs 1-3, many of which relate to growth and development of cell lineages, including production of erythropoietin, thrombopoietin, granulocyte-macrophage colony-stimulating factor, prolactin, growth hormone, and leptin. These deucravacitinib characteristics should translate into fewer off-target side effects than with oral JAK inhibitors.
“The promise of TYK2 inhibition that’s brought to you by deucravacitinib is there will be no laboratory monitoring and the effects will be narrow in blocking inflammation,” said Dr. Strober, a dermatologist at Yale University, New Haven, Conn., and in private practice in Cromwell, Conn.
He highlighted the positive results of a randomized, phase 2, dose-ranging study conducted in 267 patients with moderate or severe plaque psoriasis. Participants had an average baseline Psoriasis Area and Severity Index (PASI) score of 19, with a Dermatology Life Quality Index score of about 12. At the top dose of 12 mg once daily, 75% of patients achieved a PASI 75 response at week 12, and 44% reached a PASI 90, as did 69% and 44%, respectively, who were on deucravacitinib at 3 mg twice daily. Those are collective efficacy numbers similar to adalimumab (Humira) or ustekinumab (Stelara).
Deucravacitinib may provide efficacy “like one of our second-tier biological therapies, yet it will be oral,” Dr. Strober commented.
Importantly, no laboratory abnormalities were detected in this trial. Only mild side effects were documented, most prominently acne, which occurred in dose-dependent fashion in 2% of patients on 3 mg of deucravacitinib twice daily and 4% at 12 mg once daily.
“The treatment of the acne that is elicited by this drug is yet to be fully described, but I’m sure we’ll learn the best approaches, given that acne is in our wheel house,” the dermatologist added.
Bristol-Myers Squibb has announced positive results from the pivotal phase 3 POETYK PSO-1 trial. Deucravacitinib at 6 mg once daily met both of its coprimary efficacy endpoints in the study, which included 666 patients with moderate to severe psoriasis. The TYK 2 inhibitor demonstrated superiority to both placebo and oral apremilast (Otezla) at week 16. The company said the safety profile was consistent with the phase 2 results, and that the full details of the phase 3 trial will be presented next year at a major medical meeting.
In addition, positive phase 2 results were reported for deucravacitinib in the treatment of psoriatic arthritis in a randomized trial presented at the fall 2020 meeting of the American College of Rheumatology. Deucravacitinib is also under study for lupus and inflammatory bowel disease.
Dr. Strober, an active clinical trialist, reported serving as a consultant to more than two dozen pharmaceutical companies, including Bristol-Myers Squibb.
MedscapeLive and this news organization are owned by the same parent company.
and a range of other chronic inflammatory diseases, Bruce E. Strober, MD, PhD, said at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually this year.
Deucravacitinib solely blocks tyrosine kinase 2 (TYK2) signaling without touching Janus kinase (JAK) 1, 2, or 3. In so doing, it inhibits several cytokines important for inflammation: interleukin-12, IL-13, and interferon-alpha and -beta. Yet it doesn’t affect the numerous pathways mediated by JAKs 1-3, many of which relate to growth and development of cell lineages, including production of erythropoietin, thrombopoietin, granulocyte-macrophage colony-stimulating factor, prolactin, growth hormone, and leptin. These deucravacitinib characteristics should translate into fewer off-target side effects than with oral JAK inhibitors.
“The promise of TYK2 inhibition that’s brought to you by deucravacitinib is there will be no laboratory monitoring and the effects will be narrow in blocking inflammation,” said Dr. Strober, a dermatologist at Yale University, New Haven, Conn., and in private practice in Cromwell, Conn.
He highlighted the positive results of a randomized, phase 2, dose-ranging study conducted in 267 patients with moderate or severe plaque psoriasis. Participants had an average baseline Psoriasis Area and Severity Index (PASI) score of 19, with a Dermatology Life Quality Index score of about 12. At the top dose of 12 mg once daily, 75% of patients achieved a PASI 75 response at week 12, and 44% reached a PASI 90, as did 69% and 44%, respectively, who were on deucravacitinib at 3 mg twice daily. Those are collective efficacy numbers similar to adalimumab (Humira) or ustekinumab (Stelara).
Deucravacitinib may provide efficacy “like one of our second-tier biological therapies, yet it will be oral,” Dr. Strober commented.
Importantly, no laboratory abnormalities were detected in this trial. Only mild side effects were documented, most prominently acne, which occurred in dose-dependent fashion in 2% of patients on 3 mg of deucravacitinib twice daily and 4% at 12 mg once daily.
“The treatment of the acne that is elicited by this drug is yet to be fully described, but I’m sure we’ll learn the best approaches, given that acne is in our wheel house,” the dermatologist added.
Bristol-Myers Squibb has announced positive results from the pivotal phase 3 POETYK PSO-1 trial. Deucravacitinib at 6 mg once daily met both of its coprimary efficacy endpoints in the study, which included 666 patients with moderate to severe psoriasis. The TYK 2 inhibitor demonstrated superiority to both placebo and oral apremilast (Otezla) at week 16. The company said the safety profile was consistent with the phase 2 results, and that the full details of the phase 3 trial will be presented next year at a major medical meeting.
In addition, positive phase 2 results were reported for deucravacitinib in the treatment of psoriatic arthritis in a randomized trial presented at the fall 2020 meeting of the American College of Rheumatology. Deucravacitinib is also under study for lupus and inflammatory bowel disease.
Dr. Strober, an active clinical trialist, reported serving as a consultant to more than two dozen pharmaceutical companies, including Bristol-Myers Squibb.
MedscapeLive and this news organization are owned by the same parent company.
FROM MEDSCAPELIVE LAS VEGAS DERMATOLOGY SEMINAR