User login
Cardiotoxicity after checkpoint inhibitor treatment seen early, linked to elevated biomarkers
PHILADELPHIA – , and occurred more frequently in patients with elevated biomarkers, in a retrospective cohort study reported at the annual scientific meeting of the Heart Failure Society of America.
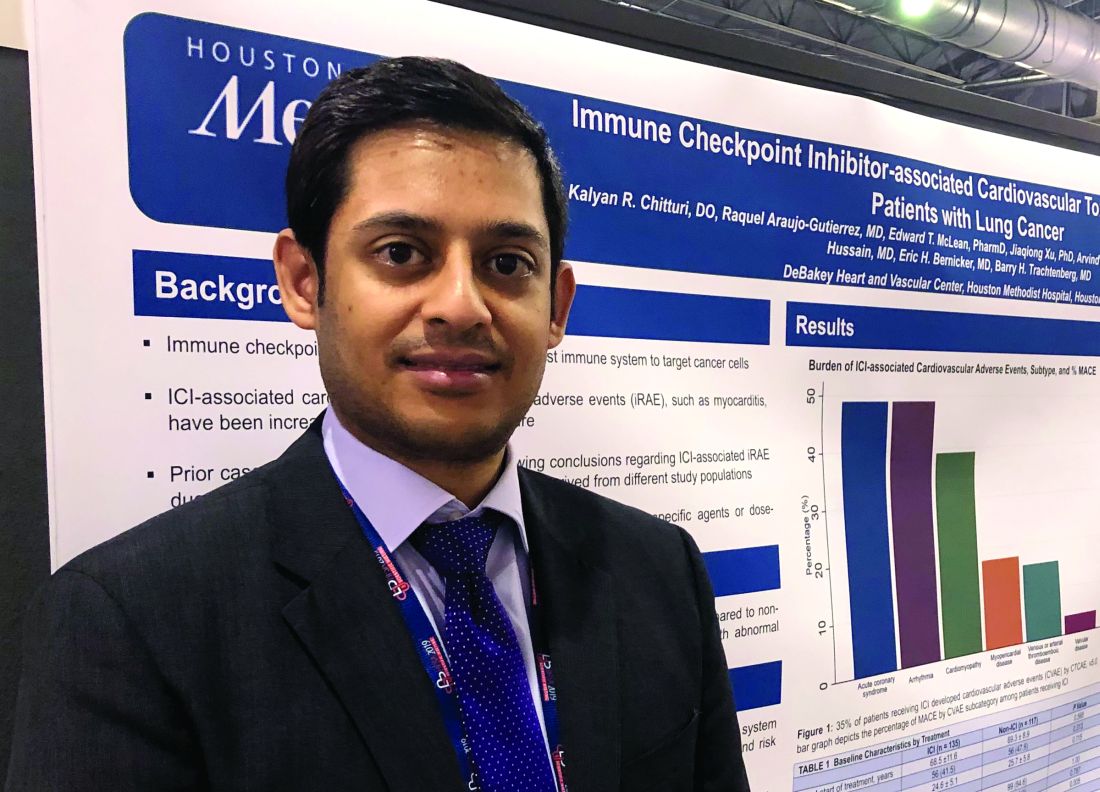
The findings support monitoring of cardiac biomarkers in the initial phase of checkpoint inhibitor treatment to identify patient at high cardiac risk, according to Kalyan R. Chitturi, DO, a resident physician with the DeBakey Heart and Vascular Center, Houston Methodist Hospital, who presented the results.
“It’s the early period that warrants the closest monitoring, as within the first 30-40 days, there’s higher risk,” Dr. Chitturi said in an interview. “When there was a biomarker elevation, it markedly increased the risk of MACE, warranting a closer vigilance during that time period.”
The retrospective study conducted by Dr. Chitturi and colleagues included a total of 252 patients with lung cancer who had been treated at one of seven different sites in Houston Methodist Cancer Center between Aug. 1, 2015, and Aug. 1, 2018.
Immune checkpoint inhibitors did not significantly increase the risk of MACE, compared with other lung cancer therapies, with incidences of 13.3% and 10.3%, respectively (P = .632), the investigators found.
However, MACE did occur earlier in the checkpoint inhibitor group, at a median time to event of 40 days, compared with 118 days in the patients not treated with checkpoint inhibitors, they found.
Risk of MACE with checkpoint inhibitor treatment was increased in patients with elevated troponin (hazard ratio, 2.48; 95% confidence interval, 1.18-5.21; P = .017) or elevated brain natriuretic peptide (HR, 5.77; 95% CI, 2.70-12.35; P less than .001), according to multivariate logistic regression analysis results.
These results suggest biomarkers such as cardiac troponin and brain natriuretic peptide are warranted to monitor patients in the early phase of checkpoint inhibitor treatment, according to Dr. Chitturi. “In the cost-benefit ratio of often-lethal MACE, it’s well worth it to collect these,” he said in the interview.
The results corroborate findings from some other recent studies, he noted. These include a recent study that linked elevated serum troponin to myocarditis in patients treated with immune checkpoint inhibitors (J Am Coll Cardiol. 2018 Apr 24;71[16]:1755-64).
Dr. Chitturi and coauthors reported no disclosures related to their presentation at the HFSA meeting.
SOURCE: Chitturi KR et al. HFSA 2019, Abstract 127.
PHILADELPHIA – , and occurred more frequently in patients with elevated biomarkers, in a retrospective cohort study reported at the annual scientific meeting of the Heart Failure Society of America.
The findings support monitoring of cardiac biomarkers in the initial phase of checkpoint inhibitor treatment to identify patient at high cardiac risk, according to Kalyan R. Chitturi, DO, a resident physician with the DeBakey Heart and Vascular Center, Houston Methodist Hospital, who presented the results.
“It’s the early period that warrants the closest monitoring, as within the first 30-40 days, there’s higher risk,” Dr. Chitturi said in an interview. “When there was a biomarker elevation, it markedly increased the risk of MACE, warranting a closer vigilance during that time period.”
The retrospective study conducted by Dr. Chitturi and colleagues included a total of 252 patients with lung cancer who had been treated at one of seven different sites in Houston Methodist Cancer Center between Aug. 1, 2015, and Aug. 1, 2018.
Immune checkpoint inhibitors did not significantly increase the risk of MACE, compared with other lung cancer therapies, with incidences of 13.3% and 10.3%, respectively (P = .632), the investigators found.
However, MACE did occur earlier in the checkpoint inhibitor group, at a median time to event of 40 days, compared with 118 days in the patients not treated with checkpoint inhibitors, they found.
Risk of MACE with checkpoint inhibitor treatment was increased in patients with elevated troponin (hazard ratio, 2.48; 95% confidence interval, 1.18-5.21; P = .017) or elevated brain natriuretic peptide (HR, 5.77; 95% CI, 2.70-12.35; P less than .001), according to multivariate logistic regression analysis results.
These results suggest biomarkers such as cardiac troponin and brain natriuretic peptide are warranted to monitor patients in the early phase of checkpoint inhibitor treatment, according to Dr. Chitturi. “In the cost-benefit ratio of often-lethal MACE, it’s well worth it to collect these,” he said in the interview.
The results corroborate findings from some other recent studies, he noted. These include a recent study that linked elevated serum troponin to myocarditis in patients treated with immune checkpoint inhibitors (J Am Coll Cardiol. 2018 Apr 24;71[16]:1755-64).
Dr. Chitturi and coauthors reported no disclosures related to their presentation at the HFSA meeting.
SOURCE: Chitturi KR et al. HFSA 2019, Abstract 127.
PHILADELPHIA – , and occurred more frequently in patients with elevated biomarkers, in a retrospective cohort study reported at the annual scientific meeting of the Heart Failure Society of America.
The findings support monitoring of cardiac biomarkers in the initial phase of checkpoint inhibitor treatment to identify patient at high cardiac risk, according to Kalyan R. Chitturi, DO, a resident physician with the DeBakey Heart and Vascular Center, Houston Methodist Hospital, who presented the results.
“It’s the early period that warrants the closest monitoring, as within the first 30-40 days, there’s higher risk,” Dr. Chitturi said in an interview. “When there was a biomarker elevation, it markedly increased the risk of MACE, warranting a closer vigilance during that time period.”
The retrospective study conducted by Dr. Chitturi and colleagues included a total of 252 patients with lung cancer who had been treated at one of seven different sites in Houston Methodist Cancer Center between Aug. 1, 2015, and Aug. 1, 2018.
Immune checkpoint inhibitors did not significantly increase the risk of MACE, compared with other lung cancer therapies, with incidences of 13.3% and 10.3%, respectively (P = .632), the investigators found.
However, MACE did occur earlier in the checkpoint inhibitor group, at a median time to event of 40 days, compared with 118 days in the patients not treated with checkpoint inhibitors, they found.
Risk of MACE with checkpoint inhibitor treatment was increased in patients with elevated troponin (hazard ratio, 2.48; 95% confidence interval, 1.18-5.21; P = .017) or elevated brain natriuretic peptide (HR, 5.77; 95% CI, 2.70-12.35; P less than .001), according to multivariate logistic regression analysis results.
These results suggest biomarkers such as cardiac troponin and brain natriuretic peptide are warranted to monitor patients in the early phase of checkpoint inhibitor treatment, according to Dr. Chitturi. “In the cost-benefit ratio of often-lethal MACE, it’s well worth it to collect these,” he said in the interview.
The results corroborate findings from some other recent studies, he noted. These include a recent study that linked elevated serum troponin to myocarditis in patients treated with immune checkpoint inhibitors (J Am Coll Cardiol. 2018 Apr 24;71[16]:1755-64).
Dr. Chitturi and coauthors reported no disclosures related to their presentation at the HFSA meeting.
SOURCE: Chitturi KR et al. HFSA 2019, Abstract 127.
REPORTING FROM HFSA 2019
CDC reports most vaping lung disease linked to THC-containing cartridges
and most products used were prepackaged, prefilled cartridges, according to new data released by the Centers for Disease Control and Prevention.
The majority of these products (66%) were THC-containing cartridges marketed under the brand name Dank. Dank cartridges are available at legal dispensaries and online in areas where they are legal. The Dank company posted a statement on its website warning buyers about fake cartridges and showing images of genuine cartridges. However, 89% of the cartridges were obtained on the street, from dealers, online, or from friends or social contacts, Jennifer Layden, MD, of the Illinois Department of Public Health said during a CDC telebriefing.
The illness was first recognized in Wisconsin and Illinois. Marijuana is illegal in Wisconsin; Illinois licensed recreational marijuana in 2009.
Other commonalties among cases have also emerged, Anne Schuchat, MD, deputy director of CDC, said during the call. More than two-thirds of the 805 confirmed or probable cases were male, and the median age was 23 years. The illness crosses age barriers, she said. About 62% were 18-24 years of age, and 54% under age 25. However, among the 12 deaths so far reported, the median age was 50 years. The age range was wide, from 27 to 71 years. Dr. Schuchat said data about medical comorbidities potentially linking the deaths is not yet available, although it is part of the ongoing investigation.
Other clinical commonalities included intensive use of THC-containing products and, in a small number of cases, concomitant use of benzodiazepenes, opioids, and narcotics.
Cases have now emerged in 46 states and in the U.S. Virgin Islands, although the number reported each week is dropping. However, this decrease may not represent a drop in newly occurring cases, but instead reflect delays in clinical recognition or reporting to local health departments, Dr. Schuchat said.
Regardless of the recent decline in reported cases, she said, the epidemic is serious, far reaching, and ongoing.
“I want to stress that this is a serious, life-threatening disease occurring mostly in otherwise healthy young people. These illnesses and deaths are occurring in the context of a dynamic marketplace with mix of products with mixes of ingredients, including potentially illicit substances. Users don’t know what’s in them and cannot tell from the ingredients listed on the packaging.”
Dr. Schuchat drew her data from two reports issued in the Morbidity and Mortality Weekly Report: a national case update by Peter A. Briss, MD, chair of CDC’s Lung Injury Response Epidemiology/Surveillance Group, and colleagues, and a regional report coauthored by Dr. Layden of cases in Illinois and Wisconsin.
In the national report, 514 patients self-reported their history of e-cigarette and vaping use. Among those, 395 (76.9%) reported using THC-containing products, and 292 (56.8%) reported using nicotine-containing products in the 30 days preceding symptom onset. Almost half (210; 40.9%) reported using both THC- and nicotine-containing products.
But there appeared to be no clear pattern of use, said Dr. Briss, who also participated in the briefing. More than a third (185; 36.0%) reported exclusive use of THC-containing products, and 82 (16.0%) reported exclusive use of nicotine-containing products.
The regional report added additional details.
Among the 86 patients who self-reported details, there were 234 unique cases of e-cigarette or THC vaping in 87 brands.
“Patients reported using numerous products and brands,” Dr. Layden noted. “Those who reported using THC products used an average of 2.1 different products and those who reported using nicotine products used about 1.3 different ones. Some patients reported using up to seven different brands, and these were used at least daily and sometimes numerous times in the day.”
According to the MMWR regional report, among the urinary THC screens obtained for 32 patients, “29 (91%) were positive for THC. One of these patients reported smoking combustible marijuana. Urinary THC levels for four patients who reported using THC-containing products exceeded 400 ng/ml, indicating intensive use of THC or THC-containing products.”
About 40% of THC users and 65% of nicotine-product users reported using the product at least five times a day; 52% said they used combustible marijuana in addition to the vapes, and 24% reported also smoking combustible tobacco.
There was a very low level of concomitant drug use. Two patients reported using LSD; one reported misusing dextroamphetamine-amphetamine (Adderall), and one reported misusing oxycodone. Two tested positive for benzodiazepines and opioids, and one each for only benzodiazepines, only opioids, only amphetamines. One patient screened positive for unidentified narcotics.
and most products used were prepackaged, prefilled cartridges, according to new data released by the Centers for Disease Control and Prevention.
The majority of these products (66%) were THC-containing cartridges marketed under the brand name Dank. Dank cartridges are available at legal dispensaries and online in areas where they are legal. The Dank company posted a statement on its website warning buyers about fake cartridges and showing images of genuine cartridges. However, 89% of the cartridges were obtained on the street, from dealers, online, or from friends or social contacts, Jennifer Layden, MD, of the Illinois Department of Public Health said during a CDC telebriefing.
The illness was first recognized in Wisconsin and Illinois. Marijuana is illegal in Wisconsin; Illinois licensed recreational marijuana in 2009.
Other commonalties among cases have also emerged, Anne Schuchat, MD, deputy director of CDC, said during the call. More than two-thirds of the 805 confirmed or probable cases were male, and the median age was 23 years. The illness crosses age barriers, she said. About 62% were 18-24 years of age, and 54% under age 25. However, among the 12 deaths so far reported, the median age was 50 years. The age range was wide, from 27 to 71 years. Dr. Schuchat said data about medical comorbidities potentially linking the deaths is not yet available, although it is part of the ongoing investigation.
Other clinical commonalities included intensive use of THC-containing products and, in a small number of cases, concomitant use of benzodiazepenes, opioids, and narcotics.
Cases have now emerged in 46 states and in the U.S. Virgin Islands, although the number reported each week is dropping. However, this decrease may not represent a drop in newly occurring cases, but instead reflect delays in clinical recognition or reporting to local health departments, Dr. Schuchat said.
Regardless of the recent decline in reported cases, she said, the epidemic is serious, far reaching, and ongoing.
“I want to stress that this is a serious, life-threatening disease occurring mostly in otherwise healthy young people. These illnesses and deaths are occurring in the context of a dynamic marketplace with mix of products with mixes of ingredients, including potentially illicit substances. Users don’t know what’s in them and cannot tell from the ingredients listed on the packaging.”
Dr. Schuchat drew her data from two reports issued in the Morbidity and Mortality Weekly Report: a national case update by Peter A. Briss, MD, chair of CDC’s Lung Injury Response Epidemiology/Surveillance Group, and colleagues, and a regional report coauthored by Dr. Layden of cases in Illinois and Wisconsin.
In the national report, 514 patients self-reported their history of e-cigarette and vaping use. Among those, 395 (76.9%) reported using THC-containing products, and 292 (56.8%) reported using nicotine-containing products in the 30 days preceding symptom onset. Almost half (210; 40.9%) reported using both THC- and nicotine-containing products.
But there appeared to be no clear pattern of use, said Dr. Briss, who also participated in the briefing. More than a third (185; 36.0%) reported exclusive use of THC-containing products, and 82 (16.0%) reported exclusive use of nicotine-containing products.
The regional report added additional details.
Among the 86 patients who self-reported details, there were 234 unique cases of e-cigarette or THC vaping in 87 brands.
“Patients reported using numerous products and brands,” Dr. Layden noted. “Those who reported using THC products used an average of 2.1 different products and those who reported using nicotine products used about 1.3 different ones. Some patients reported using up to seven different brands, and these were used at least daily and sometimes numerous times in the day.”
According to the MMWR regional report, among the urinary THC screens obtained for 32 patients, “29 (91%) were positive for THC. One of these patients reported smoking combustible marijuana. Urinary THC levels for four patients who reported using THC-containing products exceeded 400 ng/ml, indicating intensive use of THC or THC-containing products.”
About 40% of THC users and 65% of nicotine-product users reported using the product at least five times a day; 52% said they used combustible marijuana in addition to the vapes, and 24% reported also smoking combustible tobacco.
There was a very low level of concomitant drug use. Two patients reported using LSD; one reported misusing dextroamphetamine-amphetamine (Adderall), and one reported misusing oxycodone. Two tested positive for benzodiazepines and opioids, and one each for only benzodiazepines, only opioids, only amphetamines. One patient screened positive for unidentified narcotics.
and most products used were prepackaged, prefilled cartridges, according to new data released by the Centers for Disease Control and Prevention.
The majority of these products (66%) were THC-containing cartridges marketed under the brand name Dank. Dank cartridges are available at legal dispensaries and online in areas where they are legal. The Dank company posted a statement on its website warning buyers about fake cartridges and showing images of genuine cartridges. However, 89% of the cartridges were obtained on the street, from dealers, online, or from friends or social contacts, Jennifer Layden, MD, of the Illinois Department of Public Health said during a CDC telebriefing.
The illness was first recognized in Wisconsin and Illinois. Marijuana is illegal in Wisconsin; Illinois licensed recreational marijuana in 2009.
Other commonalties among cases have also emerged, Anne Schuchat, MD, deputy director of CDC, said during the call. More than two-thirds of the 805 confirmed or probable cases were male, and the median age was 23 years. The illness crosses age barriers, she said. About 62% were 18-24 years of age, and 54% under age 25. However, among the 12 deaths so far reported, the median age was 50 years. The age range was wide, from 27 to 71 years. Dr. Schuchat said data about medical comorbidities potentially linking the deaths is not yet available, although it is part of the ongoing investigation.
Other clinical commonalities included intensive use of THC-containing products and, in a small number of cases, concomitant use of benzodiazepenes, opioids, and narcotics.
Cases have now emerged in 46 states and in the U.S. Virgin Islands, although the number reported each week is dropping. However, this decrease may not represent a drop in newly occurring cases, but instead reflect delays in clinical recognition or reporting to local health departments, Dr. Schuchat said.
Regardless of the recent decline in reported cases, she said, the epidemic is serious, far reaching, and ongoing.
“I want to stress that this is a serious, life-threatening disease occurring mostly in otherwise healthy young people. These illnesses and deaths are occurring in the context of a dynamic marketplace with mix of products with mixes of ingredients, including potentially illicit substances. Users don’t know what’s in them and cannot tell from the ingredients listed on the packaging.”
Dr. Schuchat drew her data from two reports issued in the Morbidity and Mortality Weekly Report: a national case update by Peter A. Briss, MD, chair of CDC’s Lung Injury Response Epidemiology/Surveillance Group, and colleagues, and a regional report coauthored by Dr. Layden of cases in Illinois and Wisconsin.
In the national report, 514 patients self-reported their history of e-cigarette and vaping use. Among those, 395 (76.9%) reported using THC-containing products, and 292 (56.8%) reported using nicotine-containing products in the 30 days preceding symptom onset. Almost half (210; 40.9%) reported using both THC- and nicotine-containing products.
But there appeared to be no clear pattern of use, said Dr. Briss, who also participated in the briefing. More than a third (185; 36.0%) reported exclusive use of THC-containing products, and 82 (16.0%) reported exclusive use of nicotine-containing products.
The regional report added additional details.
Among the 86 patients who self-reported details, there were 234 unique cases of e-cigarette or THC vaping in 87 brands.
“Patients reported using numerous products and brands,” Dr. Layden noted. “Those who reported using THC products used an average of 2.1 different products and those who reported using nicotine products used about 1.3 different ones. Some patients reported using up to seven different brands, and these were used at least daily and sometimes numerous times in the day.”
According to the MMWR regional report, among the urinary THC screens obtained for 32 patients, “29 (91%) were positive for THC. One of these patients reported smoking combustible marijuana. Urinary THC levels for four patients who reported using THC-containing products exceeded 400 ng/ml, indicating intensive use of THC or THC-containing products.”
About 40% of THC users and 65% of nicotine-product users reported using the product at least five times a day; 52% said they used combustible marijuana in addition to the vapes, and 24% reported also smoking combustible tobacco.
There was a very low level of concomitant drug use. Two patients reported using LSD; one reported misusing dextroamphetamine-amphetamine (Adderall), and one reported misusing oxycodone. Two tested positive for benzodiazepines and opioids, and one each for only benzodiazepines, only opioids, only amphetamines. One patient screened positive for unidentified narcotics.
Click for Credit: Psoriasis relief; Stress & CV problems; more
Here are 5 articles from the October issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Bronchiolitis is a feared complication of connective tissue disease
To take the posttest, go to: https://bit.ly/2klWpRb
Expires April 8, 2020
2. Stress incontinence surgery improves sexual dysfunction
To take the posttest, go to: https://bit.ly/2m0wb71
Expires April 10, 2020
3. Survey finds psoriasis patients seek relief with alternative therapies
To take the posttest, go to: https://bit.ly/2lZZDtO
Expires April 10, 2020
4. New data further suggest that stress does a number on the CV system
To take the posttest, go to: https://bit.ly/2lR31ax
Expires April 11, 2020
5. Rate of objects ingested by young children increased over last two decades
To take the posttest, go to: https://bit.ly/2mmYptb
Expires April 12, 2020
Here are 5 articles from the October issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Bronchiolitis is a feared complication of connective tissue disease
To take the posttest, go to: https://bit.ly/2klWpRb
Expires April 8, 2020
2. Stress incontinence surgery improves sexual dysfunction
To take the posttest, go to: https://bit.ly/2m0wb71
Expires April 10, 2020
3. Survey finds psoriasis patients seek relief with alternative therapies
To take the posttest, go to: https://bit.ly/2lZZDtO
Expires April 10, 2020
4. New data further suggest that stress does a number on the CV system
To take the posttest, go to: https://bit.ly/2lR31ax
Expires April 11, 2020
5. Rate of objects ingested by young children increased over last two decades
To take the posttest, go to: https://bit.ly/2mmYptb
Expires April 12, 2020
Here are 5 articles from the October issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Bronchiolitis is a feared complication of connective tissue disease
To take the posttest, go to: https://bit.ly/2klWpRb
Expires April 8, 2020
2. Stress incontinence surgery improves sexual dysfunction
To take the posttest, go to: https://bit.ly/2m0wb71
Expires April 10, 2020
3. Survey finds psoriasis patients seek relief with alternative therapies
To take the posttest, go to: https://bit.ly/2lZZDtO
Expires April 10, 2020
4. New data further suggest that stress does a number on the CV system
To take the posttest, go to: https://bit.ly/2lR31ax
Expires April 11, 2020
5. Rate of objects ingested by young children increased over last two decades
To take the posttest, go to: https://bit.ly/2mmYptb
Expires April 12, 2020
Step-up therapy with glucocorticoids benefits black children with asthma
based on data from 280 children aged 5-11 years with at least one grandparent identified as black.
Previous studies have suggested that long-acting beta2-agonists (LABAs) may be more effective for patients with poorly controlled asthma, but such step-up therapy has not been well studied in black patients, wrote Michael E. Wechsler, MD, of National Jewish Health, Denver, and colleagues.
In a study published in the New England Journal of Medicine, the researchers reported results of two parallel BARD (Best African American Response to Asthma Drugs) trials conducted at nine centers between January 2014 and March 2016 of individuals with poorly controlled asthma. One trial included 280 children aged 5-11 years (average age, 8.5 years); the second trial included adolescents aged 12 years and older and adults (average age, 37 years) who had family backgrounds that were similar to those of the children.
The researchers randomized the children to four groups to compare the following protocols: doubling the dose of a glucocorticoid (fluticasone propionate) to a dose of 100 mcg, twice daily (the double-fluticasone group); doubling the dose of fluticasone to 100 mcg and adding 50 mcg of the LABA salmeterol (the salmeterol/double-fluticasone group); quintupling the dose of fluticasone to 250 mcg (the quintuple-fluticasone group); or quintupling the dose of fluticasone to 250 mcg and adding 50 mcg of salmeterol (the salmeterol/quintuple-fluticasone group). The trial consisted of a four-way crossover design with each treatment period lasting 14 weeks.
The primary outcome was a composite measure including asthma exacerbations, asthma control days, and percentage of predicted forced expiratory volume in the first second at the end of each treatment.
Overall, a superior response occurred in 53% of the salmeterol/double-fluticasone group, 41% of the double-fluticasone group, 43% of the salmeterol/quintuple fluticasone group, and 47% of the quintuple-fluticasone group.
The superior response was 46% for both groups when the researchers compared a quintupled dose of fluticasone propionate (250 mcg) with a two step–up strategy of adding salmeterol at a dose of 50 mcg and increasing the dose of fluticasone to 100 mcg.
“In contrast to black adults and white persons of all ages, almost half the children who had at least one grandparent who identified as black and who had poorly controlled asthma had a superior response to an increased dose of an inhaled glucocorticoid over the addition of a LABA,” Dr. Wechsler and coauthors wrote. No more than 12% of the children in any treatment group did not have a superior response. No significant differences in reports of respiratory tract infections or pneumonia were seen between the groups. Children younger than 8 years showed a decrease in the ratio of urinary cortisol to creatinine with an increased dose of inhaled glucocorticoids.
In the adolescent and adult study using the same treatment protocols, 20%-25% of patients did not have a differential outcome between treatments. “In adolescents and adults, the addition of a LABA was more likely to produce superior responses than increasing the dose of an inhaled glucocorticoid,” Dr. Wechsler and coauthors wrote.
The study findings were limited by several factors, including the inability to assess long-term effects on growth and inability to detect biomarkers associated with responses to specific therapies, the researchers noted. However, the results suggest that black children with poorly controlled asthma can benefit from additional inhaled glucocorticoids, and larger studies are needed to identify the best treatment for this patient population.
The study was supported by the National Heart, Lung, and Blood Institute. Dr. Wechsler reported relationships with companies including AstraZeneca, Equillium, Genentech, GlaxoSmithKline, Mylan, Novartis, Regeneron, resTORbio, Sanofi, and others. Coauthors identified relationships with numerous pharmaceutical companies.
SOURCE: Wechsler ME et al. N Engl J Med. 2019 Sep 25. doi: 10.1056/NEJMoa1905560.
based on data from 280 children aged 5-11 years with at least one grandparent identified as black.
Previous studies have suggested that long-acting beta2-agonists (LABAs) may be more effective for patients with poorly controlled asthma, but such step-up therapy has not been well studied in black patients, wrote Michael E. Wechsler, MD, of National Jewish Health, Denver, and colleagues.
In a study published in the New England Journal of Medicine, the researchers reported results of two parallel BARD (Best African American Response to Asthma Drugs) trials conducted at nine centers between January 2014 and March 2016 of individuals with poorly controlled asthma. One trial included 280 children aged 5-11 years (average age, 8.5 years); the second trial included adolescents aged 12 years and older and adults (average age, 37 years) who had family backgrounds that were similar to those of the children.
The researchers randomized the children to four groups to compare the following protocols: doubling the dose of a glucocorticoid (fluticasone propionate) to a dose of 100 mcg, twice daily (the double-fluticasone group); doubling the dose of fluticasone to 100 mcg and adding 50 mcg of the LABA salmeterol (the salmeterol/double-fluticasone group); quintupling the dose of fluticasone to 250 mcg (the quintuple-fluticasone group); or quintupling the dose of fluticasone to 250 mcg and adding 50 mcg of salmeterol (the salmeterol/quintuple-fluticasone group). The trial consisted of a four-way crossover design with each treatment period lasting 14 weeks.
The primary outcome was a composite measure including asthma exacerbations, asthma control days, and percentage of predicted forced expiratory volume in the first second at the end of each treatment.
Overall, a superior response occurred in 53% of the salmeterol/double-fluticasone group, 41% of the double-fluticasone group, 43% of the salmeterol/quintuple fluticasone group, and 47% of the quintuple-fluticasone group.
The superior response was 46% for both groups when the researchers compared a quintupled dose of fluticasone propionate (250 mcg) with a two step–up strategy of adding salmeterol at a dose of 50 mcg and increasing the dose of fluticasone to 100 mcg.
“In contrast to black adults and white persons of all ages, almost half the children who had at least one grandparent who identified as black and who had poorly controlled asthma had a superior response to an increased dose of an inhaled glucocorticoid over the addition of a LABA,” Dr. Wechsler and coauthors wrote. No more than 12% of the children in any treatment group did not have a superior response. No significant differences in reports of respiratory tract infections or pneumonia were seen between the groups. Children younger than 8 years showed a decrease in the ratio of urinary cortisol to creatinine with an increased dose of inhaled glucocorticoids.
In the adolescent and adult study using the same treatment protocols, 20%-25% of patients did not have a differential outcome between treatments. “In adolescents and adults, the addition of a LABA was more likely to produce superior responses than increasing the dose of an inhaled glucocorticoid,” Dr. Wechsler and coauthors wrote.
The study findings were limited by several factors, including the inability to assess long-term effects on growth and inability to detect biomarkers associated with responses to specific therapies, the researchers noted. However, the results suggest that black children with poorly controlled asthma can benefit from additional inhaled glucocorticoids, and larger studies are needed to identify the best treatment for this patient population.
The study was supported by the National Heart, Lung, and Blood Institute. Dr. Wechsler reported relationships with companies including AstraZeneca, Equillium, Genentech, GlaxoSmithKline, Mylan, Novartis, Regeneron, resTORbio, Sanofi, and others. Coauthors identified relationships with numerous pharmaceutical companies.
SOURCE: Wechsler ME et al. N Engl J Med. 2019 Sep 25. doi: 10.1056/NEJMoa1905560.
based on data from 280 children aged 5-11 years with at least one grandparent identified as black.
Previous studies have suggested that long-acting beta2-agonists (LABAs) may be more effective for patients with poorly controlled asthma, but such step-up therapy has not been well studied in black patients, wrote Michael E. Wechsler, MD, of National Jewish Health, Denver, and colleagues.
In a study published in the New England Journal of Medicine, the researchers reported results of two parallel BARD (Best African American Response to Asthma Drugs) trials conducted at nine centers between January 2014 and March 2016 of individuals with poorly controlled asthma. One trial included 280 children aged 5-11 years (average age, 8.5 years); the second trial included adolescents aged 12 years and older and adults (average age, 37 years) who had family backgrounds that were similar to those of the children.
The researchers randomized the children to four groups to compare the following protocols: doubling the dose of a glucocorticoid (fluticasone propionate) to a dose of 100 mcg, twice daily (the double-fluticasone group); doubling the dose of fluticasone to 100 mcg and adding 50 mcg of the LABA salmeterol (the salmeterol/double-fluticasone group); quintupling the dose of fluticasone to 250 mcg (the quintuple-fluticasone group); or quintupling the dose of fluticasone to 250 mcg and adding 50 mcg of salmeterol (the salmeterol/quintuple-fluticasone group). The trial consisted of a four-way crossover design with each treatment period lasting 14 weeks.
The primary outcome was a composite measure including asthma exacerbations, asthma control days, and percentage of predicted forced expiratory volume in the first second at the end of each treatment.
Overall, a superior response occurred in 53% of the salmeterol/double-fluticasone group, 41% of the double-fluticasone group, 43% of the salmeterol/quintuple fluticasone group, and 47% of the quintuple-fluticasone group.
The superior response was 46% for both groups when the researchers compared a quintupled dose of fluticasone propionate (250 mcg) with a two step–up strategy of adding salmeterol at a dose of 50 mcg and increasing the dose of fluticasone to 100 mcg.
“In contrast to black adults and white persons of all ages, almost half the children who had at least one grandparent who identified as black and who had poorly controlled asthma had a superior response to an increased dose of an inhaled glucocorticoid over the addition of a LABA,” Dr. Wechsler and coauthors wrote. No more than 12% of the children in any treatment group did not have a superior response. No significant differences in reports of respiratory tract infections or pneumonia were seen between the groups. Children younger than 8 years showed a decrease in the ratio of urinary cortisol to creatinine with an increased dose of inhaled glucocorticoids.
In the adolescent and adult study using the same treatment protocols, 20%-25% of patients did not have a differential outcome between treatments. “In adolescents and adults, the addition of a LABA was more likely to produce superior responses than increasing the dose of an inhaled glucocorticoid,” Dr. Wechsler and coauthors wrote.
The study findings were limited by several factors, including the inability to assess long-term effects on growth and inability to detect biomarkers associated with responses to specific therapies, the researchers noted. However, the results suggest that black children with poorly controlled asthma can benefit from additional inhaled glucocorticoids, and larger studies are needed to identify the best treatment for this patient population.
The study was supported by the National Heart, Lung, and Blood Institute. Dr. Wechsler reported relationships with companies including AstraZeneca, Equillium, Genentech, GlaxoSmithKline, Mylan, Novartis, Regeneron, resTORbio, Sanofi, and others. Coauthors identified relationships with numerous pharmaceutical companies.
SOURCE: Wechsler ME et al. N Engl J Med. 2019 Sep 25. doi: 10.1056/NEJMoa1905560.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Black children with poorly controlled asthma had a superior response to stepped increases in inhaled glucocorticoids, compared with stepped increases in long-acting beta2-agonists.
Major finding: A total of 46% of black children with poorly controlled asthma improved when inhaled glucocorticoids were increased.
Study details: The data come from the BARD trials, a pair of prospective, randomized, double-blind trials including 280 children aged 5-11 years with at least one grandparent identified as black and 294 adolescents and adults who had family backgrounds that were similar to the children.
Disclosures: The study was supported by the National Heart, Lung, and Blood Institute. Dr. Wechsler reported relationships with companies including AstraZeneca, Equillium, Genentech, GlaxoSmithKline, Mylan, Novartis, Regeneron, resTORbio, Sanofi, and others. Coauthors identified relationships with numerous pharmaceutical companies.
Source: Wechsler ME et al. N Engl J Med. 2019 Sep 25. doi: 10.1056/NEJMoa1905560.
Acting FDA commissioner issues remarks on ENDS, vaping illnesses
Norman E. Sharpless, MD, acting commissioner of the Food and Drug Administration, has issued remarks that were prepared for a testimony before a U.S. House Energy and Commerce subcommittee on FDA regulation of electronic nicotine delivery systems and investigation of vaping illnesses.
Dr. Sharpless’s statement focuses on two priorities: the continuing investigation into the cause of lung injury associated with the use of vaping products and the FDA’s ongoing efforts to address an epidemic of youth use of electronic nicotine delivery systems (ENDS). In regards to lung injuries associated with vaping, Dr. Sharpless said that the FDA and Centers for Disease Control and Prevention, in conjunction with state partners, have been investigating the outbreak and noted that, while most cases have involved tetrahydrocannabinol, there is as of yet no common cause or product linked to all cases.
The FDA is not currently pursuing any legal action against personal usage of vaping products, but “if we determine that someone is manufacturing or distributing illicit, adulterated vaping products that caused illness and death for personal profit, we would consider that to be a criminal act,” Dr. Sharpless said.
Research from the 2018 and 2019 National Youth Tobacco Surveys has indicated that ENDS usage among youth has risen dramatically in recent years, Dr. Sharpless continued in the statement. The FDA has pursued several courses of action, including the issue of a warning letter to Juul for marketing unauthorized modified-risk tobacco products to children.
However, he noted, youth e-cigarette use continues to rise, which is the reason for the FDA’s intention to finalize a compliance policy related to flavored ENDS, a policy supported by President TRump.
“FDA is not ‘banning’ flavors, as has been described in some news reports. Rather, FDA intends to enforce existing law that limits the marketing of such products,” Dr. Sharpless said. “This policy would not mean that flavored e-cigarettes could never be marketed. If a company can show through an application to FDA that a specific product meets the standard set forth by Congress, then the FDA would authorize that ENDS product for sale.”
Find the full statement on the FDA website.
Norman E. Sharpless, MD, acting commissioner of the Food and Drug Administration, has issued remarks that were prepared for a testimony before a U.S. House Energy and Commerce subcommittee on FDA regulation of electronic nicotine delivery systems and investigation of vaping illnesses.
Dr. Sharpless’s statement focuses on two priorities: the continuing investigation into the cause of lung injury associated with the use of vaping products and the FDA’s ongoing efforts to address an epidemic of youth use of electronic nicotine delivery systems (ENDS). In regards to lung injuries associated with vaping, Dr. Sharpless said that the FDA and Centers for Disease Control and Prevention, in conjunction with state partners, have been investigating the outbreak and noted that, while most cases have involved tetrahydrocannabinol, there is as of yet no common cause or product linked to all cases.
The FDA is not currently pursuing any legal action against personal usage of vaping products, but “if we determine that someone is manufacturing or distributing illicit, adulterated vaping products that caused illness and death for personal profit, we would consider that to be a criminal act,” Dr. Sharpless said.
Research from the 2018 and 2019 National Youth Tobacco Surveys has indicated that ENDS usage among youth has risen dramatically in recent years, Dr. Sharpless continued in the statement. The FDA has pursued several courses of action, including the issue of a warning letter to Juul for marketing unauthorized modified-risk tobacco products to children.
However, he noted, youth e-cigarette use continues to rise, which is the reason for the FDA’s intention to finalize a compliance policy related to flavored ENDS, a policy supported by President TRump.
“FDA is not ‘banning’ flavors, as has been described in some news reports. Rather, FDA intends to enforce existing law that limits the marketing of such products,” Dr. Sharpless said. “This policy would not mean that flavored e-cigarettes could never be marketed. If a company can show through an application to FDA that a specific product meets the standard set forth by Congress, then the FDA would authorize that ENDS product for sale.”
Find the full statement on the FDA website.
Norman E. Sharpless, MD, acting commissioner of the Food and Drug Administration, has issued remarks that were prepared for a testimony before a U.S. House Energy and Commerce subcommittee on FDA regulation of electronic nicotine delivery systems and investigation of vaping illnesses.
Dr. Sharpless’s statement focuses on two priorities: the continuing investigation into the cause of lung injury associated with the use of vaping products and the FDA’s ongoing efforts to address an epidemic of youth use of electronic nicotine delivery systems (ENDS). In regards to lung injuries associated with vaping, Dr. Sharpless said that the FDA and Centers for Disease Control and Prevention, in conjunction with state partners, have been investigating the outbreak and noted that, while most cases have involved tetrahydrocannabinol, there is as of yet no common cause or product linked to all cases.
The FDA is not currently pursuing any legal action against personal usage of vaping products, but “if we determine that someone is manufacturing or distributing illicit, adulterated vaping products that caused illness and death for personal profit, we would consider that to be a criminal act,” Dr. Sharpless said.
Research from the 2018 and 2019 National Youth Tobacco Surveys has indicated that ENDS usage among youth has risen dramatically in recent years, Dr. Sharpless continued in the statement. The FDA has pursued several courses of action, including the issue of a warning letter to Juul for marketing unauthorized modified-risk tobacco products to children.
However, he noted, youth e-cigarette use continues to rise, which is the reason for the FDA’s intention to finalize a compliance policy related to flavored ENDS, a policy supported by President TRump.
“FDA is not ‘banning’ flavors, as has been described in some news reports. Rather, FDA intends to enforce existing law that limits the marketing of such products,” Dr. Sharpless said. “This policy would not mean that flavored e-cigarettes could never be marketed. If a company can show through an application to FDA that a specific product meets the standard set forth by Congress, then the FDA would authorize that ENDS product for sale.”
Find the full statement on the FDA website.
PACIFIC: Patterns of lung cancer progression suggest role for local ablative therapy
Most patients with stage III non–small cell lung cancer (NSCLC) who have distant progression on standard therapy typically have one or two new lesions, often in the same organ, which suggests a role for local ablative therapy, according to investigators.
This conclusion was drawn from an exploratory analysis of the phase 3 PACIFIC trial, which previously showed that durvalumab prolonged survival among patients with NSCLC who did not progress after chemoradiotherapy, which turned the trial protocol into a new standard of care.
At the annual meeting of the American Society for Radiation Oncology, coauthor Andreas Rimner, MD, of the Memorial Sloan Kettering Cancer Center in New York presented findings.
“There were always questions regarding detailed patterns of failure and disease progression in [the PACIFIC] trial,” Dr. Rimner said. “This study ... focuses on these patterns of failure, including the type of first progression in the patients on the PACIFIC trial.”
During the trial, 713 patients with NSCLC were randomized in a 2:1 ratio to receive either durvalumab or placebo. After a median follow-up of 25.2 months, the superiority of durvalumab was clear, with a lower rate of progression (45.4% vs. 64.6%).
But the present analysis dug deeper into this finding by dividing patients into three groups based on site or sites of first progression: local (intrathoracic) progression only, distant (extrathoracic) progression only, or simultaneously local and distant progression. Scans were reviewed by an independent radiologist who was not involved in the original PACIFIC trial. In addition to spatial data, the investigators reported times until progression.
Regardless of site, durvalumab was associated with a longer time until progression or death. Although comparative values were not reached for distant or simultaneous spread, median time until local progression or death was reportable, at 25.2 months in the durvalumab group versus with 9.2 months in the placebo group.
These values were available, in part, because local spread was the most common type of progression: It occurred in 80.6% of patients who progressed on durvalumab and 74.5% of progressors in the placebo group.
Durvalumab reduced the rate of progression across the three spatial categories, compared with placebo, including local only (36.6% vs. 48.1%, respectively), distant only (6.9% vs. 13.1%), and simultaneously local and distant (1.9% vs. 3.4%). This means that, at first progression, new distant lesions were found in 8.8% of patients treated with durvalumab, compared with 16.5% of those treated with placebo. Of note, approximately two-thirds of patients with distant progression had only one or two distant lesions, often confined to one organ, most commonly the brain. This pattern of progression was observed in both treatment arms.
According to Dr. Rimner, this finding is clinically relevant because it suggests a potential role for local ablative therapy.
Expert perspective on the analysis was provided by Benjamin Movsas, MD, chair of radiation oncology at the Henry Ford Cancer Institute in Detroit.
“The PACIFIC trial has really transformed the standard of care for patients with locally advanced, inoperable non–small cell lung cancer by adding immunotherapy to the prior standard of care combining chemotherapy and radiation, and this has shown a dramatic improvement in survival,” Dr. Movsas said.
“By adding the immunotherapy durvalumab, you can reduce risk of local failure, you can reduce the risk of distant failure, and interestingly enough, when patients do fail distantly, and this is true in both arms, they tended to fail in only one or two spots, which is encouraging because that suggests maybe a window of opportunity to treat those one or two spots, and we have newer technologies that allow us to consider that. So we really have a new paradigm.”
The study was funded by AstraZeneca. The investigators disclosed additional relationships with Merck, Nanobiotix, Boehringer Ingelheim, and others.
SOURCE: Rimner A et al. ASTRO 2019, Abstract LBA6.
Most patients with stage III non–small cell lung cancer (NSCLC) who have distant progression on standard therapy typically have one or two new lesions, often in the same organ, which suggests a role for local ablative therapy, according to investigators.
This conclusion was drawn from an exploratory analysis of the phase 3 PACIFIC trial, which previously showed that durvalumab prolonged survival among patients with NSCLC who did not progress after chemoradiotherapy, which turned the trial protocol into a new standard of care.
At the annual meeting of the American Society for Radiation Oncology, coauthor Andreas Rimner, MD, of the Memorial Sloan Kettering Cancer Center in New York presented findings.
“There were always questions regarding detailed patterns of failure and disease progression in [the PACIFIC] trial,” Dr. Rimner said. “This study ... focuses on these patterns of failure, including the type of first progression in the patients on the PACIFIC trial.”
During the trial, 713 patients with NSCLC were randomized in a 2:1 ratio to receive either durvalumab or placebo. After a median follow-up of 25.2 months, the superiority of durvalumab was clear, with a lower rate of progression (45.4% vs. 64.6%).
But the present analysis dug deeper into this finding by dividing patients into three groups based on site or sites of first progression: local (intrathoracic) progression only, distant (extrathoracic) progression only, or simultaneously local and distant progression. Scans were reviewed by an independent radiologist who was not involved in the original PACIFIC trial. In addition to spatial data, the investigators reported times until progression.
Regardless of site, durvalumab was associated with a longer time until progression or death. Although comparative values were not reached for distant or simultaneous spread, median time until local progression or death was reportable, at 25.2 months in the durvalumab group versus with 9.2 months in the placebo group.
These values were available, in part, because local spread was the most common type of progression: It occurred in 80.6% of patients who progressed on durvalumab and 74.5% of progressors in the placebo group.
Durvalumab reduced the rate of progression across the three spatial categories, compared with placebo, including local only (36.6% vs. 48.1%, respectively), distant only (6.9% vs. 13.1%), and simultaneously local and distant (1.9% vs. 3.4%). This means that, at first progression, new distant lesions were found in 8.8% of patients treated with durvalumab, compared with 16.5% of those treated with placebo. Of note, approximately two-thirds of patients with distant progression had only one or two distant lesions, often confined to one organ, most commonly the brain. This pattern of progression was observed in both treatment arms.
According to Dr. Rimner, this finding is clinically relevant because it suggests a potential role for local ablative therapy.
Expert perspective on the analysis was provided by Benjamin Movsas, MD, chair of radiation oncology at the Henry Ford Cancer Institute in Detroit.
“The PACIFIC trial has really transformed the standard of care for patients with locally advanced, inoperable non–small cell lung cancer by adding immunotherapy to the prior standard of care combining chemotherapy and radiation, and this has shown a dramatic improvement in survival,” Dr. Movsas said.
“By adding the immunotherapy durvalumab, you can reduce risk of local failure, you can reduce the risk of distant failure, and interestingly enough, when patients do fail distantly, and this is true in both arms, they tended to fail in only one or two spots, which is encouraging because that suggests maybe a window of opportunity to treat those one or two spots, and we have newer technologies that allow us to consider that. So we really have a new paradigm.”
The study was funded by AstraZeneca. The investigators disclosed additional relationships with Merck, Nanobiotix, Boehringer Ingelheim, and others.
SOURCE: Rimner A et al. ASTRO 2019, Abstract LBA6.
Most patients with stage III non–small cell lung cancer (NSCLC) who have distant progression on standard therapy typically have one or two new lesions, often in the same organ, which suggests a role for local ablative therapy, according to investigators.
This conclusion was drawn from an exploratory analysis of the phase 3 PACIFIC trial, which previously showed that durvalumab prolonged survival among patients with NSCLC who did not progress after chemoradiotherapy, which turned the trial protocol into a new standard of care.
At the annual meeting of the American Society for Radiation Oncology, coauthor Andreas Rimner, MD, of the Memorial Sloan Kettering Cancer Center in New York presented findings.
“There were always questions regarding detailed patterns of failure and disease progression in [the PACIFIC] trial,” Dr. Rimner said. “This study ... focuses on these patterns of failure, including the type of first progression in the patients on the PACIFIC trial.”
During the trial, 713 patients with NSCLC were randomized in a 2:1 ratio to receive either durvalumab or placebo. After a median follow-up of 25.2 months, the superiority of durvalumab was clear, with a lower rate of progression (45.4% vs. 64.6%).
But the present analysis dug deeper into this finding by dividing patients into three groups based on site or sites of first progression: local (intrathoracic) progression only, distant (extrathoracic) progression only, or simultaneously local and distant progression. Scans were reviewed by an independent radiologist who was not involved in the original PACIFIC trial. In addition to spatial data, the investigators reported times until progression.
Regardless of site, durvalumab was associated with a longer time until progression or death. Although comparative values were not reached for distant or simultaneous spread, median time until local progression or death was reportable, at 25.2 months in the durvalumab group versus with 9.2 months in the placebo group.
These values were available, in part, because local spread was the most common type of progression: It occurred in 80.6% of patients who progressed on durvalumab and 74.5% of progressors in the placebo group.
Durvalumab reduced the rate of progression across the three spatial categories, compared with placebo, including local only (36.6% vs. 48.1%, respectively), distant only (6.9% vs. 13.1%), and simultaneously local and distant (1.9% vs. 3.4%). This means that, at first progression, new distant lesions were found in 8.8% of patients treated with durvalumab, compared with 16.5% of those treated with placebo. Of note, approximately two-thirds of patients with distant progression had only one or two distant lesions, often confined to one organ, most commonly the brain. This pattern of progression was observed in both treatment arms.
According to Dr. Rimner, this finding is clinically relevant because it suggests a potential role for local ablative therapy.
Expert perspective on the analysis was provided by Benjamin Movsas, MD, chair of radiation oncology at the Henry Ford Cancer Institute in Detroit.
“The PACIFIC trial has really transformed the standard of care for patients with locally advanced, inoperable non–small cell lung cancer by adding immunotherapy to the prior standard of care combining chemotherapy and radiation, and this has shown a dramatic improvement in survival,” Dr. Movsas said.
“By adding the immunotherapy durvalumab, you can reduce risk of local failure, you can reduce the risk of distant failure, and interestingly enough, when patients do fail distantly, and this is true in both arms, they tended to fail in only one or two spots, which is encouraging because that suggests maybe a window of opportunity to treat those one or two spots, and we have newer technologies that allow us to consider that. So we really have a new paradigm.”
The study was funded by AstraZeneca. The investigators disclosed additional relationships with Merck, Nanobiotix, Boehringer Ingelheim, and others.
SOURCE: Rimner A et al. ASTRO 2019, Abstract LBA6.
REPORTING FROM ASTRO 2019
Key clinical point: Most patients with stage 3 non–small cell lung cancer (NSCLC) who have distant progression on standard therapy typically have one or two new lesions, often in the same organ, which suggests a role for local ablative therapy.
Major finding: Approximately two-thirds of patients with distant progression had one or two new lesions.
Study details: An exploratory analysis of patterns of progression in the phase 3 PACIFIC trial, which involved 713 patients with stage III NSCLC that had not progressed after chemoradiotherapy.
Disclosures: The study was funded by AstraZeneca. The investigators disclosed additional relationships with Merck, Nanobiotix, Boehringer Ingelheim, and others.
Source: Rimner A et al. ASTRO 2019, Abstract LBA6.
PRAGMA-CF shows disease progression of cystic fibrosis in children
reported Nynke R. Bouma, BSc, and colleagues.
“Even though bronchiectasis is present in 60% to 80% of children with CF in school age, the extent and severity of bronchiectasis in preschool children are generally lower ... however, diffuse airway abnormalities such as airway wall thickening and mucus plugging are observed in many preschool children. It is hypothesized that these preschool airway changes reflect diffuse airway disease that eventually will result in bronchiectasis in school age,” they noted.
The PRAGMA-CF image scoring system can measure airway disease and can also be used to monitor disease progression, noted Ms. Bouma of Sophia Children’s Hospital, Rotterdam, and colleagues. The study was published in Pediatric Pulmonology. PRAGMA-CF is a composite score of airway wall thickening, mucus plugging, and bronchiectasis as percent disease (%disease). “In preschool children, %disease measured by PRAGMA-CF on chest CT allows quantification of early clinically relevant morphological features of CF airway disease and it is associated with later school-age bronchiectasis,” the team wrote. “These findings support the use of %disease as a clinically relevant outcome measure in early CF lung disease.”
The team conducted a prospective cohort study of 61 children (mean age 4 years) with cystic fibrosis, following them for a mean of 5 years. A total of 122 CT scans were available from this group, in addition to spirometry data and cystic fibrosis quality of life scores.
From preschool age to school age, the %disease on PRAGMA-CF increased significantly, from a mean of 0.7% to 1.73%. Scores on another composite measuring tool (%MUPAT, a composite score of airway wall thickening and mucus plugging) went from 0.46 to 0.58 – not a significant difference.
A multivariate analysis corrected for age in each school group and the type of scanner used to acquire the images. That analysis determined that each 1% increase in %disease at preschool age resulted in an increase of 1.18% of bronchiectasis at school age.
A cross-sectional analysis of the group at school age found significant associations between the %disease and percent of forced expiratory volume and the cystic fibrosis quality of life score.
At least one pulmonary exacerbation requiring intravenous antibiotics occurred in 19 of the patients. However, the investigators didn’t find any significant interactions between the %disease in preschool and these exacerbations..
“These findings are in line with previous studies in school‐aged children that showed that mucus plugging is associated with inflammation and airway wall thickening, and that these are thought to be risk factors for later bronchiectasis,” they concluded. “On the basis of our findings, we suggest that %disease and %MUPAT could be used as a clinically relevant outcome measure in clinical studies in preschool patients with cystic fibrosis, as these measures predict later bronchiectasis. Percent disease may be preferred as it captures all the principal features of CF airways disease including bronchiectasis.”
Ms. Bouma had no financial disclosures.
SOURCE: Bouma NR et al. Pediatr Pulmonol. 2019 Sep 9 doi: 10.1002/ppul.24498; Rosenow et al. Am J Respir Crit Care Med. 2015 May 15. doi: 10.1164/rccm.201501-0061OC.
reported Nynke R. Bouma, BSc, and colleagues.
“Even though bronchiectasis is present in 60% to 80% of children with CF in school age, the extent and severity of bronchiectasis in preschool children are generally lower ... however, diffuse airway abnormalities such as airway wall thickening and mucus plugging are observed in many preschool children. It is hypothesized that these preschool airway changes reflect diffuse airway disease that eventually will result in bronchiectasis in school age,” they noted.
The PRAGMA-CF image scoring system can measure airway disease and can also be used to monitor disease progression, noted Ms. Bouma of Sophia Children’s Hospital, Rotterdam, and colleagues. The study was published in Pediatric Pulmonology. PRAGMA-CF is a composite score of airway wall thickening, mucus plugging, and bronchiectasis as percent disease (%disease). “In preschool children, %disease measured by PRAGMA-CF on chest CT allows quantification of early clinically relevant morphological features of CF airway disease and it is associated with later school-age bronchiectasis,” the team wrote. “These findings support the use of %disease as a clinically relevant outcome measure in early CF lung disease.”
The team conducted a prospective cohort study of 61 children (mean age 4 years) with cystic fibrosis, following them for a mean of 5 years. A total of 122 CT scans were available from this group, in addition to spirometry data and cystic fibrosis quality of life scores.
From preschool age to school age, the %disease on PRAGMA-CF increased significantly, from a mean of 0.7% to 1.73%. Scores on another composite measuring tool (%MUPAT, a composite score of airway wall thickening and mucus plugging) went from 0.46 to 0.58 – not a significant difference.
A multivariate analysis corrected for age in each school group and the type of scanner used to acquire the images. That analysis determined that each 1% increase in %disease at preschool age resulted in an increase of 1.18% of bronchiectasis at school age.
A cross-sectional analysis of the group at school age found significant associations between the %disease and percent of forced expiratory volume and the cystic fibrosis quality of life score.
At least one pulmonary exacerbation requiring intravenous antibiotics occurred in 19 of the patients. However, the investigators didn’t find any significant interactions between the %disease in preschool and these exacerbations..
“These findings are in line with previous studies in school‐aged children that showed that mucus plugging is associated with inflammation and airway wall thickening, and that these are thought to be risk factors for later bronchiectasis,” they concluded. “On the basis of our findings, we suggest that %disease and %MUPAT could be used as a clinically relevant outcome measure in clinical studies in preschool patients with cystic fibrosis, as these measures predict later bronchiectasis. Percent disease may be preferred as it captures all the principal features of CF airways disease including bronchiectasis.”
Ms. Bouma had no financial disclosures.
SOURCE: Bouma NR et al. Pediatr Pulmonol. 2019 Sep 9 doi: 10.1002/ppul.24498; Rosenow et al. Am J Respir Crit Care Med. 2015 May 15. doi: 10.1164/rccm.201501-0061OC.
reported Nynke R. Bouma, BSc, and colleagues.
“Even though bronchiectasis is present in 60% to 80% of children with CF in school age, the extent and severity of bronchiectasis in preschool children are generally lower ... however, diffuse airway abnormalities such as airway wall thickening and mucus plugging are observed in many preschool children. It is hypothesized that these preschool airway changes reflect diffuse airway disease that eventually will result in bronchiectasis in school age,” they noted.
The PRAGMA-CF image scoring system can measure airway disease and can also be used to monitor disease progression, noted Ms. Bouma of Sophia Children’s Hospital, Rotterdam, and colleagues. The study was published in Pediatric Pulmonology. PRAGMA-CF is a composite score of airway wall thickening, mucus plugging, and bronchiectasis as percent disease (%disease). “In preschool children, %disease measured by PRAGMA-CF on chest CT allows quantification of early clinically relevant morphological features of CF airway disease and it is associated with later school-age bronchiectasis,” the team wrote. “These findings support the use of %disease as a clinically relevant outcome measure in early CF lung disease.”
The team conducted a prospective cohort study of 61 children (mean age 4 years) with cystic fibrosis, following them for a mean of 5 years. A total of 122 CT scans were available from this group, in addition to spirometry data and cystic fibrosis quality of life scores.
From preschool age to school age, the %disease on PRAGMA-CF increased significantly, from a mean of 0.7% to 1.73%. Scores on another composite measuring tool (%MUPAT, a composite score of airway wall thickening and mucus plugging) went from 0.46 to 0.58 – not a significant difference.
A multivariate analysis corrected for age in each school group and the type of scanner used to acquire the images. That analysis determined that each 1% increase in %disease at preschool age resulted in an increase of 1.18% of bronchiectasis at school age.
A cross-sectional analysis of the group at school age found significant associations between the %disease and percent of forced expiratory volume and the cystic fibrosis quality of life score.
At least one pulmonary exacerbation requiring intravenous antibiotics occurred in 19 of the patients. However, the investigators didn’t find any significant interactions between the %disease in preschool and these exacerbations..
“These findings are in line with previous studies in school‐aged children that showed that mucus plugging is associated with inflammation and airway wall thickening, and that these are thought to be risk factors for later bronchiectasis,” they concluded. “On the basis of our findings, we suggest that %disease and %MUPAT could be used as a clinically relevant outcome measure in clinical studies in preschool patients with cystic fibrosis, as these measures predict later bronchiectasis. Percent disease may be preferred as it captures all the principal features of CF airways disease including bronchiectasis.”
Ms. Bouma had no financial disclosures.
SOURCE: Bouma NR et al. Pediatr Pulmonol. 2019 Sep 9 doi: 10.1002/ppul.24498; Rosenow et al. Am J Respir Crit Care Med. 2015 May 15. doi: 10.1164/rccm.201501-0061OC.
FROM PEDIATRIC PULMONOLOGY
Many institutions exceed recommended radiation doses during lung cancer screening
according to a study published in JAMA Internal Medicine.
Various institutional characteristics, such as allowing any radiologist to establish CT scan protocols, are associated with a greater likelihood of using higher radiation doses. “Dose optimization practices may benefit from being tailored to specific practice types, as well as different organizational structures, to have a higher likelihood of meeting dose guidelines,” wrote Joshua Demb, PhD, MPH, a cancer epidemiologist at the University of California, San Diego, and colleagues.
Lung cancer screening benefits patients when low-dose CT is used, but not when high-dose CT is used, because radiation from higher doses may cause as many cancers as are detected by screening. The Centers for Medicare & Medicaid Services require institutions to use low-dose techniques and participate in a dose registry to be reimbursed for lung cancer screening. The American College of Radiology recommends that lung cancer screening scans have a volume CT dose index (CTDIvol) of 3 mGy or lower and an effective dose (ED) of 1 millisieverts (mSv) or lower.
A prospective study of registry data
Dr. Demb and colleagues conducted a study to describe CT radiation doses for lung cancer screening in current practice and to identify the factors that explain variation in doses between institutions. They prospectively collected lung cancer screening examination dose metrics from 2016 to 2017 at U.S. institutions participating in the University of California, San Francisco, International Dose Registry. Eligible institutions performed a minimum of 24 lung cancer screening scans during the study period. At baseline, the investigators surveyed institutions about their characteristics (for example, how they perform and oversee CT). Dr. Demb and colleagues estimated mixed-effects linear and logistic regression models using forward variable selection. They conducted their analysis between 2018 and 2019.
The researchers chose four outcome measures. The first was mean CTDIvol, reflecting the average radiation dose per slice. The second was mean ED, reflecting the total dose received and estimated future cancer risk. The third was the proportion of CT scans using radiation doses above ACR benchmarks. The fourth was the proportion of CT scans using radiation doses above the 75th percentile of registry doses (CTDIvol greater than 2.7 mGy and ED greater than 1.4 mSv).
Institutional characteristics associated with radiation dose
Dr. Demb and colleagues collected data from 72 institutions about 12,529 patients undergoing CT scans for lung cancer screening. Approximately 58% of patients were men, and the patients’ median age was 65 years. The mean CTDIvol, adjusted for patient size, was 2.4 mGy. The mean ED for lung cancer screening, adjusted for chest diameter, was 1.2 mSv.
A total of 15 institutions (21%) had a median adjusted CTDIvol value higher than the ACR guideline, and 47 (65%) had a median adjusted ED higher than the ACR guideline. Approximately 18% of CT scans had a CTDIvol higher than guidelines, and 50% had an ED higher than ACR guidelines.
Institutions that permitted any radiologist to establish CT protocols had 44% higher mean CTDIvol and 27% higher mean ED, compared with institutions that restricted who could establish protocols. Institutions that permitted any radiologist to establish protocols also had higher odds of conducting examinations that exceeded ACR CTDIvol guidelines (odds ratio, 12.0) and of being in the 75th percentile of the registry CTDIvol (OR, 19.0) or ED (OR, 8.5) values.
In contrast, having lead radiologists establish CT protocols resulted in lower odds of using doses that exceeded ACR ED guidelines (OR, 0.01). Employing external, rather than internal, medical physicists was associated with increased odds of exceeding ACR CTDIvol guidelines (OR, 6.1). Having medical physicists establish protocols was associated with decreased odds of exceeding the 75th percentile of the registry CTDIvol (OR, 0.09) values. Institutions that updated protocols as needed, rather than annually, had 27% higher mean CTDIvol.
“Although we cannot establish causality in this observational study, our results suggest that considering these factors (for example, allowing only lead radiologists to establish protocols) could have a meaningful impact on dose, and could be important areas to develop interventions to optimize doses of CT protocols” the investigators wrote.
The Patient Centered Outcomes Research Institute and the National Institutes of Health supported this research. The authors reported no conflicts of interest.
SOURCE: Demb J et al. JAMA Intern Med. 2019 Sep 23. doi: 10.1001/jamainternmed.2019.3893.
according to a study published in JAMA Internal Medicine.
Various institutional characteristics, such as allowing any radiologist to establish CT scan protocols, are associated with a greater likelihood of using higher radiation doses. “Dose optimization practices may benefit from being tailored to specific practice types, as well as different organizational structures, to have a higher likelihood of meeting dose guidelines,” wrote Joshua Demb, PhD, MPH, a cancer epidemiologist at the University of California, San Diego, and colleagues.
Lung cancer screening benefits patients when low-dose CT is used, but not when high-dose CT is used, because radiation from higher doses may cause as many cancers as are detected by screening. The Centers for Medicare & Medicaid Services require institutions to use low-dose techniques and participate in a dose registry to be reimbursed for lung cancer screening. The American College of Radiology recommends that lung cancer screening scans have a volume CT dose index (CTDIvol) of 3 mGy or lower and an effective dose (ED) of 1 millisieverts (mSv) or lower.
A prospective study of registry data
Dr. Demb and colleagues conducted a study to describe CT radiation doses for lung cancer screening in current practice and to identify the factors that explain variation in doses between institutions. They prospectively collected lung cancer screening examination dose metrics from 2016 to 2017 at U.S. institutions participating in the University of California, San Francisco, International Dose Registry. Eligible institutions performed a minimum of 24 lung cancer screening scans during the study period. At baseline, the investigators surveyed institutions about their characteristics (for example, how they perform and oversee CT). Dr. Demb and colleagues estimated mixed-effects linear and logistic regression models using forward variable selection. They conducted their analysis between 2018 and 2019.
The researchers chose four outcome measures. The first was mean CTDIvol, reflecting the average radiation dose per slice. The second was mean ED, reflecting the total dose received and estimated future cancer risk. The third was the proportion of CT scans using radiation doses above ACR benchmarks. The fourth was the proportion of CT scans using radiation doses above the 75th percentile of registry doses (CTDIvol greater than 2.7 mGy and ED greater than 1.4 mSv).
Institutional characteristics associated with radiation dose
Dr. Demb and colleagues collected data from 72 institutions about 12,529 patients undergoing CT scans for lung cancer screening. Approximately 58% of patients were men, and the patients’ median age was 65 years. The mean CTDIvol, adjusted for patient size, was 2.4 mGy. The mean ED for lung cancer screening, adjusted for chest diameter, was 1.2 mSv.
A total of 15 institutions (21%) had a median adjusted CTDIvol value higher than the ACR guideline, and 47 (65%) had a median adjusted ED higher than the ACR guideline. Approximately 18% of CT scans had a CTDIvol higher than guidelines, and 50% had an ED higher than ACR guidelines.
Institutions that permitted any radiologist to establish CT protocols had 44% higher mean CTDIvol and 27% higher mean ED, compared with institutions that restricted who could establish protocols. Institutions that permitted any radiologist to establish protocols also had higher odds of conducting examinations that exceeded ACR CTDIvol guidelines (odds ratio, 12.0) and of being in the 75th percentile of the registry CTDIvol (OR, 19.0) or ED (OR, 8.5) values.
In contrast, having lead radiologists establish CT protocols resulted in lower odds of using doses that exceeded ACR ED guidelines (OR, 0.01). Employing external, rather than internal, medical physicists was associated with increased odds of exceeding ACR CTDIvol guidelines (OR, 6.1). Having medical physicists establish protocols was associated with decreased odds of exceeding the 75th percentile of the registry CTDIvol (OR, 0.09) values. Institutions that updated protocols as needed, rather than annually, had 27% higher mean CTDIvol.
“Although we cannot establish causality in this observational study, our results suggest that considering these factors (for example, allowing only lead radiologists to establish protocols) could have a meaningful impact on dose, and could be important areas to develop interventions to optimize doses of CT protocols” the investigators wrote.
The Patient Centered Outcomes Research Institute and the National Institutes of Health supported this research. The authors reported no conflicts of interest.
SOURCE: Demb J et al. JAMA Intern Med. 2019 Sep 23. doi: 10.1001/jamainternmed.2019.3893.
according to a study published in JAMA Internal Medicine.
Various institutional characteristics, such as allowing any radiologist to establish CT scan protocols, are associated with a greater likelihood of using higher radiation doses. “Dose optimization practices may benefit from being tailored to specific practice types, as well as different organizational structures, to have a higher likelihood of meeting dose guidelines,” wrote Joshua Demb, PhD, MPH, a cancer epidemiologist at the University of California, San Diego, and colleagues.
Lung cancer screening benefits patients when low-dose CT is used, but not when high-dose CT is used, because radiation from higher doses may cause as many cancers as are detected by screening. The Centers for Medicare & Medicaid Services require institutions to use low-dose techniques and participate in a dose registry to be reimbursed for lung cancer screening. The American College of Radiology recommends that lung cancer screening scans have a volume CT dose index (CTDIvol) of 3 mGy or lower and an effective dose (ED) of 1 millisieverts (mSv) or lower.
A prospective study of registry data
Dr. Demb and colleagues conducted a study to describe CT radiation doses for lung cancer screening in current practice and to identify the factors that explain variation in doses between institutions. They prospectively collected lung cancer screening examination dose metrics from 2016 to 2017 at U.S. institutions participating in the University of California, San Francisco, International Dose Registry. Eligible institutions performed a minimum of 24 lung cancer screening scans during the study period. At baseline, the investigators surveyed institutions about their characteristics (for example, how they perform and oversee CT). Dr. Demb and colleagues estimated mixed-effects linear and logistic regression models using forward variable selection. They conducted their analysis between 2018 and 2019.
The researchers chose four outcome measures. The first was mean CTDIvol, reflecting the average radiation dose per slice. The second was mean ED, reflecting the total dose received and estimated future cancer risk. The third was the proportion of CT scans using radiation doses above ACR benchmarks. The fourth was the proportion of CT scans using radiation doses above the 75th percentile of registry doses (CTDIvol greater than 2.7 mGy and ED greater than 1.4 mSv).
Institutional characteristics associated with radiation dose
Dr. Demb and colleagues collected data from 72 institutions about 12,529 patients undergoing CT scans for lung cancer screening. Approximately 58% of patients were men, and the patients’ median age was 65 years. The mean CTDIvol, adjusted for patient size, was 2.4 mGy. The mean ED for lung cancer screening, adjusted for chest diameter, was 1.2 mSv.
A total of 15 institutions (21%) had a median adjusted CTDIvol value higher than the ACR guideline, and 47 (65%) had a median adjusted ED higher than the ACR guideline. Approximately 18% of CT scans had a CTDIvol higher than guidelines, and 50% had an ED higher than ACR guidelines.
Institutions that permitted any radiologist to establish CT protocols had 44% higher mean CTDIvol and 27% higher mean ED, compared with institutions that restricted who could establish protocols. Institutions that permitted any radiologist to establish protocols also had higher odds of conducting examinations that exceeded ACR CTDIvol guidelines (odds ratio, 12.0) and of being in the 75th percentile of the registry CTDIvol (OR, 19.0) or ED (OR, 8.5) values.
In contrast, having lead radiologists establish CT protocols resulted in lower odds of using doses that exceeded ACR ED guidelines (OR, 0.01). Employing external, rather than internal, medical physicists was associated with increased odds of exceeding ACR CTDIvol guidelines (OR, 6.1). Having medical physicists establish protocols was associated with decreased odds of exceeding the 75th percentile of the registry CTDIvol (OR, 0.09) values. Institutions that updated protocols as needed, rather than annually, had 27% higher mean CTDIvol.
“Although we cannot establish causality in this observational study, our results suggest that considering these factors (for example, allowing only lead radiologists to establish protocols) could have a meaningful impact on dose, and could be important areas to develop interventions to optimize doses of CT protocols” the investigators wrote.
The Patient Centered Outcomes Research Institute and the National Institutes of Health supported this research. The authors reported no conflicts of interest.
SOURCE: Demb J et al. JAMA Intern Med. 2019 Sep 23. doi: 10.1001/jamainternmed.2019.3893.
FROM JAMA INTERNAL MEDICINE
Key clinical point: A significant proportion of institutions exceed guideline-recommended dose levels for CT screening for lung cancer.
Major finding: About 21% of institutions have median volume CT dose index above American College of Radiology guidelines, and 65% have median effective dose above ACR guidelines.
Study details: A prospective study of data for 12,529 patients undergoing screening at 72 institutions.
Disclosures: The Patient Centered Outcomes Research Institute and the National Institutes of Health supported this research. The authors reported no conflicts of interest.
Source: Demb J et al. JAMA Intern Med. 2019 Sep 23. doi: 10.1001/jamainternmed.2019.3893.
New guideline conditionally recommends long-term home NIV for COPD patients
from a European Respiratory Society task force.
“Our recommendations, based on the best available evidence, can guide the management of chronic hypercapnic respiratory failure in COPD patients aimed at improving patient outcomes,” wrote Begum Ergan, MD, of Dokuz Eylul University, Izmir, Turkey, and coauthors. The guideline was published in the European Respiratory Journal.
To provide insight into the clinical application of LTH-NIV, the European Respiratory Society convened a task force of 20 clinicians, methodologists, and experts. Their four recommendations were developed based on the GRADE (Grading, Recommendation, Assessment, Development and Evaluation) methodology.
The first recommendation was to use LTH-NIV for patients with chronic stable hypercapnic COPD. Though an analysis of randomized, controlled trials showed little effect on mortality or hospitalizations, pooled analyses showed that NIV may decrease dyspnea scores (standardized mean difference, –0.51; 95% confidence interval, –0.06 to –0.95) and increase health-related quality of life (SMD, 0.49; 95% CI, –0.01 to 0.98).
The second was to use LTH-NIV in patients with COPD following a life-threatening episode of acute hypercapnic respiratory failure requiring acute NIV, if hypercapnia persists. Though it was not associated with a reduction in mortality (risk ratio, 0.92; 95% CI, 0.67-1.25), it was found to potentially reduce exacerbations (SMD, 0.19; 95% CI, –0.40 to 0.01) and hospitalizations (RR, 0.61; 95% CI, 0.30-1.24).
The third was to titrate LTH-NIV to normalize or reduce PaCO2 levels in patients with COPD. While this recommendation was issued with a very low certainty of evidence, it was driven by the “minimal potential harms of targeted PaCO2 reduction.”
The fourth was to use fixed pressure support mode as first-choice ventilator mode in patients with COPD using LTH-NIV. The six trials on this subject did not provide insight into long-term outcomes, nor were there significant improvements seen in health-related quality of life, sleep quality, or exercise tolerance. As such, it was also issued with a very low certainty of evidence.
The authors acknowledged all four recommendations as weak and conditional, “due to limitations in the certainty of the available evidence.” As such, they noted that their recommendations “require consideration of individual preferences, resource considerations, technical expertise, and clinical circumstances prior to implementation in clinical practice.”
The authors reported numerous disclosures, including receiving grants and personal fees from various medical supply companies.
SOURCE: Ergan B et al. Eur Respir J. 2019 Aug 29. doi: 10.1183/13993003.01003-2019.
from a European Respiratory Society task force.
“Our recommendations, based on the best available evidence, can guide the management of chronic hypercapnic respiratory failure in COPD patients aimed at improving patient outcomes,” wrote Begum Ergan, MD, of Dokuz Eylul University, Izmir, Turkey, and coauthors. The guideline was published in the European Respiratory Journal.
To provide insight into the clinical application of LTH-NIV, the European Respiratory Society convened a task force of 20 clinicians, methodologists, and experts. Their four recommendations were developed based on the GRADE (Grading, Recommendation, Assessment, Development and Evaluation) methodology.
The first recommendation was to use LTH-NIV for patients with chronic stable hypercapnic COPD. Though an analysis of randomized, controlled trials showed little effect on mortality or hospitalizations, pooled analyses showed that NIV may decrease dyspnea scores (standardized mean difference, –0.51; 95% confidence interval, –0.06 to –0.95) and increase health-related quality of life (SMD, 0.49; 95% CI, –0.01 to 0.98).
The second was to use LTH-NIV in patients with COPD following a life-threatening episode of acute hypercapnic respiratory failure requiring acute NIV, if hypercapnia persists. Though it was not associated with a reduction in mortality (risk ratio, 0.92; 95% CI, 0.67-1.25), it was found to potentially reduce exacerbations (SMD, 0.19; 95% CI, –0.40 to 0.01) and hospitalizations (RR, 0.61; 95% CI, 0.30-1.24).
The third was to titrate LTH-NIV to normalize or reduce PaCO2 levels in patients with COPD. While this recommendation was issued with a very low certainty of evidence, it was driven by the “minimal potential harms of targeted PaCO2 reduction.”
The fourth was to use fixed pressure support mode as first-choice ventilator mode in patients with COPD using LTH-NIV. The six trials on this subject did not provide insight into long-term outcomes, nor were there significant improvements seen in health-related quality of life, sleep quality, or exercise tolerance. As such, it was also issued with a very low certainty of evidence.
The authors acknowledged all four recommendations as weak and conditional, “due to limitations in the certainty of the available evidence.” As such, they noted that their recommendations “require consideration of individual preferences, resource considerations, technical expertise, and clinical circumstances prior to implementation in clinical practice.”
The authors reported numerous disclosures, including receiving grants and personal fees from various medical supply companies.
SOURCE: Ergan B et al. Eur Respir J. 2019 Aug 29. doi: 10.1183/13993003.01003-2019.
from a European Respiratory Society task force.
“Our recommendations, based on the best available evidence, can guide the management of chronic hypercapnic respiratory failure in COPD patients aimed at improving patient outcomes,” wrote Begum Ergan, MD, of Dokuz Eylul University, Izmir, Turkey, and coauthors. The guideline was published in the European Respiratory Journal.
To provide insight into the clinical application of LTH-NIV, the European Respiratory Society convened a task force of 20 clinicians, methodologists, and experts. Their four recommendations were developed based on the GRADE (Grading, Recommendation, Assessment, Development and Evaluation) methodology.
The first recommendation was to use LTH-NIV for patients with chronic stable hypercapnic COPD. Though an analysis of randomized, controlled trials showed little effect on mortality or hospitalizations, pooled analyses showed that NIV may decrease dyspnea scores (standardized mean difference, –0.51; 95% confidence interval, –0.06 to –0.95) and increase health-related quality of life (SMD, 0.49; 95% CI, –0.01 to 0.98).
The second was to use LTH-NIV in patients with COPD following a life-threatening episode of acute hypercapnic respiratory failure requiring acute NIV, if hypercapnia persists. Though it was not associated with a reduction in mortality (risk ratio, 0.92; 95% CI, 0.67-1.25), it was found to potentially reduce exacerbations (SMD, 0.19; 95% CI, –0.40 to 0.01) and hospitalizations (RR, 0.61; 95% CI, 0.30-1.24).
The third was to titrate LTH-NIV to normalize or reduce PaCO2 levels in patients with COPD. While this recommendation was issued with a very low certainty of evidence, it was driven by the “minimal potential harms of targeted PaCO2 reduction.”
The fourth was to use fixed pressure support mode as first-choice ventilator mode in patients with COPD using LTH-NIV. The six trials on this subject did not provide insight into long-term outcomes, nor were there significant improvements seen in health-related quality of life, sleep quality, or exercise tolerance. As such, it was also issued with a very low certainty of evidence.
The authors acknowledged all four recommendations as weak and conditional, “due to limitations in the certainty of the available evidence.” As such, they noted that their recommendations “require consideration of individual preferences, resource considerations, technical expertise, and clinical circumstances prior to implementation in clinical practice.”
The authors reported numerous disclosures, including receiving grants and personal fees from various medical supply companies.
SOURCE: Ergan B et al. Eur Respir J. 2019 Aug 29. doi: 10.1183/13993003.01003-2019.
FROM THE EUROPEAN RESPIRATORY JOURNAL