User login
Mechanical ventilation in children tied to slightly lower IQ
Children who survive an episode of acute respiratory failure that requires invasive mechanical ventilation may be at risk for slightly lower long-term neurocognitive function, new research suggests.
Investigators found lower IQs in children without previous neurocognitive problems who survived pediatric intensive care unit admission for acute respiratory failure, compared with their biological siblings.
Although this magnitude of difference was small on average, more than twice as many patients as siblings had an IQ of ≤85, and children hospitalized at the youngest ages did worse than their siblings.
“Children surviving acute respiratory failure may benefit from routine evaluation of neurocognitive function after hospital discharge and may require serial evaluation to identify deficits that emerge over the course of child’s continued development to facilitate early intervention to prevent disability and optimize school performance,” study investigator R. Scott Watson, MD, MPH, professor of pediatrics, University of Washington, Seattle, told this news organization.
The study was published online March 1 in JAMA.
Unknown long-term effects
“Approximately 23,700 U.S. children undergo invasive mechanical ventilation for acute respiratory failure annually, with unknown long-term effects on neurocognitive function,” the authors write.
“With improvements in pediatric critical care over the past several decades, critical illness–associated mortality has improved dramatically [but] as survivorship has increased, we are starting to learn that many patients and their families suffer from long-term morbidity associated with the illness and its treatment,” said Dr. Watson, who is the associate division chief, pediatric critical care medicine, Seattle Children’s Hospital, Center for Child Health, Behavior, and Development.
Animal studies “have found that some sedative medications commonly used to keep children safe during mechanical ventilation may have detrimental neurologic effects, particularly in the developing brain,” Dr. Watson added.
To gain a better understanding of this potential association, the researchers turned to a subset of participants in the previously conducted Randomized Evaluation of Sedation Titration for Respiratory Failure (RESTORE) trial of pediatric patients receiving mechanical ventilation for acute respiratory failure.
For the current study (RESTORE-Cognition), multiple domains of neurocognitive function were assessed 3-8 years after hospital discharge in trial patients who did not have a history of neurocognitive dysfunction, as well as matched, healthy siblings.
To be included in the study, the children had to be ≤8 years old at trial enrollment, have a Pediatric Cerebral Performance Category (PCPC) score of 1 (normal) prior to PICU admission, and have no worse than moderate neurocognitive dysfunction at PICU discharge.
Siblings of enrolled patients were required to be between 4 and 16 years old at the time of neurocognitive testing, have a PCPC score of 1, have the same biological parents as the patient, and live with the patient.
The primary outcome was IQ, estimated by the age-appropriate Vocabulary and Block Design subtests of the Wechsler Intelligence Scale. Secondary outcomes included attention, processing speed, learning and memory, visuospatial skills, motor skills, language, and executive function. Enough time was allowed after hospitalization “for transient deficits to resolve and longer-lasting neurocognitive sequelae to manifest.”
‘Uncertain’ clinical importance
Of the 121 sibling pairs (67% non-Hispanic White, 47% from families in which one or both parents worked full-time), 116 were included in the primary outcome analysis, and 66-19 were included in analyses of secondary outcomes.
Patients had been in the PICU at a median (interquartile range [IQR]) age of 1.0 (0.2-3.2) years and had received a median of 5.5 (3.1-7.7) days of invasive mechanical ventilation.
The median age at testing for patients and matched siblings was 6.6 (5.4-9.1) and 8.4 (7.0-10.2) years, respectively. Interviews with parents and testing of patients were conducted a median (IQR) of 3.8 (3.2-5.2) and 5.2 (4.3-6.1) years, respectively, after hospitalization.
The most common etiologies of respiratory failure were bronchiolitis and asthma and pneumonia (44% and 37%, respectively). Beyond respiratory failure, most patients (72%) also had experienced multiple organ dysfunction syndrome.
Patients had a lower mean estimated IQ, compared with the matched siblings (101.5 vs. 104.3; mean difference, –2.8 [95% confidence interval, –5.4 to –0.2]), and more patients than siblings had an estimated IQ of ≤5 but not of ≤70.
Patients also had significantly lower scores on nonverbal memory, visuospatial skills, and fine motor control (mean differences, –0.9 [–1.6 to –0.3]; –0.9 [–1.8 to –.1]; and –-3.1 [–4.9 to –1.4], respectively), compared with matched siblings. They also had significantly higher scores on processing speed (mean difference, 4.4 [0.2-8.5]). There were no significant differences in the other secondary outcomes.
Differences in scores between patients and siblings varied significantly by age at hospitalization in several tests – for example, Block Design scores in patients were lower than those of siblings for patients hospitalized at <1 year old, versus those hospitalized between ages 4 and 8 years.
“When adjusting for patient age at PICU admission, patient age at testing, sibling age at testing, and duration between hospital discharge and testing, the difference in estimated IQ between patients and siblings remained statistically significantly different,” the authors note.
The investigators point out several limitations, including the fact that “little is known about sibling outcomes after critical illness, nor about whether parenting of siblings or child development differs based on birth order or on relationship between patient critical illness and the birth of siblings. ... If siblings also incur negative effects related to the critical illness, differences between critically ill children and the control siblings would be blunted.”
Despite the statistical significance of the difference between the patients and the matched controls, ultimately, the magnitude of the difference was “small and of uncertain clinical importance,” the authors conclude.
Filling a research gap
Commenting on the findings, Alexandre T. Rotta, MD, professor of pediatrics and chief of the division of pediatric critical care medicine, Duke University Medical Center, Durham, N.C., said the study “addresses an important yet vastly understudied gap: long-term neurocognitive morbidity in children exposed to critical care.”
Dr. Rotta, who is also a coauthor of an accompanying editorial, noted that the fact that the “vast majority of children with an IQ significantly lower than their siblings were under the age of 4 years suggests that the developing immature brain may be particularly susceptible to the effects of critical illness and therapies required to treat it.”
The study “underscores the need to include assessments of long-term morbidity as part of any future trial evaluating interventions in pediatric critical care,” he added.
The study was supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development for RESTORE-Cognition and by grants for the RESTORE trial from the National Heart, Lung, and Blood Institute and the National Institute of Nursing Research, National Institutes of Health. Dr. Watson and coauthors report no relevant financial relationships. Dr. Rotta has received personal fees from Vapotherm for lecturing and development of educational materials and from Breas US for participation in a scientific advisory board, as well as royalties from Elsevier for editorial work outside the submitted work. His coauthor reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Children who survive an episode of acute respiratory failure that requires invasive mechanical ventilation may be at risk for slightly lower long-term neurocognitive function, new research suggests.
Investigators found lower IQs in children without previous neurocognitive problems who survived pediatric intensive care unit admission for acute respiratory failure, compared with their biological siblings.
Although this magnitude of difference was small on average, more than twice as many patients as siblings had an IQ of ≤85, and children hospitalized at the youngest ages did worse than their siblings.
“Children surviving acute respiratory failure may benefit from routine evaluation of neurocognitive function after hospital discharge and may require serial evaluation to identify deficits that emerge over the course of child’s continued development to facilitate early intervention to prevent disability and optimize school performance,” study investigator R. Scott Watson, MD, MPH, professor of pediatrics, University of Washington, Seattle, told this news organization.
The study was published online March 1 in JAMA.
Unknown long-term effects
“Approximately 23,700 U.S. children undergo invasive mechanical ventilation for acute respiratory failure annually, with unknown long-term effects on neurocognitive function,” the authors write.
“With improvements in pediatric critical care over the past several decades, critical illness–associated mortality has improved dramatically [but] as survivorship has increased, we are starting to learn that many patients and their families suffer from long-term morbidity associated with the illness and its treatment,” said Dr. Watson, who is the associate division chief, pediatric critical care medicine, Seattle Children’s Hospital, Center for Child Health, Behavior, and Development.
Animal studies “have found that some sedative medications commonly used to keep children safe during mechanical ventilation may have detrimental neurologic effects, particularly in the developing brain,” Dr. Watson added.
To gain a better understanding of this potential association, the researchers turned to a subset of participants in the previously conducted Randomized Evaluation of Sedation Titration for Respiratory Failure (RESTORE) trial of pediatric patients receiving mechanical ventilation for acute respiratory failure.
For the current study (RESTORE-Cognition), multiple domains of neurocognitive function were assessed 3-8 years after hospital discharge in trial patients who did not have a history of neurocognitive dysfunction, as well as matched, healthy siblings.
To be included in the study, the children had to be ≤8 years old at trial enrollment, have a Pediatric Cerebral Performance Category (PCPC) score of 1 (normal) prior to PICU admission, and have no worse than moderate neurocognitive dysfunction at PICU discharge.
Siblings of enrolled patients were required to be between 4 and 16 years old at the time of neurocognitive testing, have a PCPC score of 1, have the same biological parents as the patient, and live with the patient.
The primary outcome was IQ, estimated by the age-appropriate Vocabulary and Block Design subtests of the Wechsler Intelligence Scale. Secondary outcomes included attention, processing speed, learning and memory, visuospatial skills, motor skills, language, and executive function. Enough time was allowed after hospitalization “for transient deficits to resolve and longer-lasting neurocognitive sequelae to manifest.”
‘Uncertain’ clinical importance
Of the 121 sibling pairs (67% non-Hispanic White, 47% from families in which one or both parents worked full-time), 116 were included in the primary outcome analysis, and 66-19 were included in analyses of secondary outcomes.
Patients had been in the PICU at a median (interquartile range [IQR]) age of 1.0 (0.2-3.2) years and had received a median of 5.5 (3.1-7.7) days of invasive mechanical ventilation.
The median age at testing for patients and matched siblings was 6.6 (5.4-9.1) and 8.4 (7.0-10.2) years, respectively. Interviews with parents and testing of patients were conducted a median (IQR) of 3.8 (3.2-5.2) and 5.2 (4.3-6.1) years, respectively, after hospitalization.
The most common etiologies of respiratory failure were bronchiolitis and asthma and pneumonia (44% and 37%, respectively). Beyond respiratory failure, most patients (72%) also had experienced multiple organ dysfunction syndrome.
Patients had a lower mean estimated IQ, compared with the matched siblings (101.5 vs. 104.3; mean difference, –2.8 [95% confidence interval, –5.4 to –0.2]), and more patients than siblings had an estimated IQ of ≤5 but not of ≤70.
Patients also had significantly lower scores on nonverbal memory, visuospatial skills, and fine motor control (mean differences, –0.9 [–1.6 to –0.3]; –0.9 [–1.8 to –.1]; and –-3.1 [–4.9 to –1.4], respectively), compared with matched siblings. They also had significantly higher scores on processing speed (mean difference, 4.4 [0.2-8.5]). There were no significant differences in the other secondary outcomes.
Differences in scores between patients and siblings varied significantly by age at hospitalization in several tests – for example, Block Design scores in patients were lower than those of siblings for patients hospitalized at <1 year old, versus those hospitalized between ages 4 and 8 years.
“When adjusting for patient age at PICU admission, patient age at testing, sibling age at testing, and duration between hospital discharge and testing, the difference in estimated IQ between patients and siblings remained statistically significantly different,” the authors note.
The investigators point out several limitations, including the fact that “little is known about sibling outcomes after critical illness, nor about whether parenting of siblings or child development differs based on birth order or on relationship between patient critical illness and the birth of siblings. ... If siblings also incur negative effects related to the critical illness, differences between critically ill children and the control siblings would be blunted.”
Despite the statistical significance of the difference between the patients and the matched controls, ultimately, the magnitude of the difference was “small and of uncertain clinical importance,” the authors conclude.
Filling a research gap
Commenting on the findings, Alexandre T. Rotta, MD, professor of pediatrics and chief of the division of pediatric critical care medicine, Duke University Medical Center, Durham, N.C., said the study “addresses an important yet vastly understudied gap: long-term neurocognitive morbidity in children exposed to critical care.”
Dr. Rotta, who is also a coauthor of an accompanying editorial, noted that the fact that the “vast majority of children with an IQ significantly lower than their siblings were under the age of 4 years suggests that the developing immature brain may be particularly susceptible to the effects of critical illness and therapies required to treat it.”
The study “underscores the need to include assessments of long-term morbidity as part of any future trial evaluating interventions in pediatric critical care,” he added.
The study was supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development for RESTORE-Cognition and by grants for the RESTORE trial from the National Heart, Lung, and Blood Institute and the National Institute of Nursing Research, National Institutes of Health. Dr. Watson and coauthors report no relevant financial relationships. Dr. Rotta has received personal fees from Vapotherm for lecturing and development of educational materials and from Breas US for participation in a scientific advisory board, as well as royalties from Elsevier for editorial work outside the submitted work. His coauthor reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Children who survive an episode of acute respiratory failure that requires invasive mechanical ventilation may be at risk for slightly lower long-term neurocognitive function, new research suggests.
Investigators found lower IQs in children without previous neurocognitive problems who survived pediatric intensive care unit admission for acute respiratory failure, compared with their biological siblings.
Although this magnitude of difference was small on average, more than twice as many patients as siblings had an IQ of ≤85, and children hospitalized at the youngest ages did worse than their siblings.
“Children surviving acute respiratory failure may benefit from routine evaluation of neurocognitive function after hospital discharge and may require serial evaluation to identify deficits that emerge over the course of child’s continued development to facilitate early intervention to prevent disability and optimize school performance,” study investigator R. Scott Watson, MD, MPH, professor of pediatrics, University of Washington, Seattle, told this news organization.
The study was published online March 1 in JAMA.
Unknown long-term effects
“Approximately 23,700 U.S. children undergo invasive mechanical ventilation for acute respiratory failure annually, with unknown long-term effects on neurocognitive function,” the authors write.
“With improvements in pediatric critical care over the past several decades, critical illness–associated mortality has improved dramatically [but] as survivorship has increased, we are starting to learn that many patients and their families suffer from long-term morbidity associated with the illness and its treatment,” said Dr. Watson, who is the associate division chief, pediatric critical care medicine, Seattle Children’s Hospital, Center for Child Health, Behavior, and Development.
Animal studies “have found that some sedative medications commonly used to keep children safe during mechanical ventilation may have detrimental neurologic effects, particularly in the developing brain,” Dr. Watson added.
To gain a better understanding of this potential association, the researchers turned to a subset of participants in the previously conducted Randomized Evaluation of Sedation Titration for Respiratory Failure (RESTORE) trial of pediatric patients receiving mechanical ventilation for acute respiratory failure.
For the current study (RESTORE-Cognition), multiple domains of neurocognitive function were assessed 3-8 years after hospital discharge in trial patients who did not have a history of neurocognitive dysfunction, as well as matched, healthy siblings.
To be included in the study, the children had to be ≤8 years old at trial enrollment, have a Pediatric Cerebral Performance Category (PCPC) score of 1 (normal) prior to PICU admission, and have no worse than moderate neurocognitive dysfunction at PICU discharge.
Siblings of enrolled patients were required to be between 4 and 16 years old at the time of neurocognitive testing, have a PCPC score of 1, have the same biological parents as the patient, and live with the patient.
The primary outcome was IQ, estimated by the age-appropriate Vocabulary and Block Design subtests of the Wechsler Intelligence Scale. Secondary outcomes included attention, processing speed, learning and memory, visuospatial skills, motor skills, language, and executive function. Enough time was allowed after hospitalization “for transient deficits to resolve and longer-lasting neurocognitive sequelae to manifest.”
‘Uncertain’ clinical importance
Of the 121 sibling pairs (67% non-Hispanic White, 47% from families in which one or both parents worked full-time), 116 were included in the primary outcome analysis, and 66-19 were included in analyses of secondary outcomes.
Patients had been in the PICU at a median (interquartile range [IQR]) age of 1.0 (0.2-3.2) years and had received a median of 5.5 (3.1-7.7) days of invasive mechanical ventilation.
The median age at testing for patients and matched siblings was 6.6 (5.4-9.1) and 8.4 (7.0-10.2) years, respectively. Interviews with parents and testing of patients were conducted a median (IQR) of 3.8 (3.2-5.2) and 5.2 (4.3-6.1) years, respectively, after hospitalization.
The most common etiologies of respiratory failure were bronchiolitis and asthma and pneumonia (44% and 37%, respectively). Beyond respiratory failure, most patients (72%) also had experienced multiple organ dysfunction syndrome.
Patients had a lower mean estimated IQ, compared with the matched siblings (101.5 vs. 104.3; mean difference, –2.8 [95% confidence interval, –5.4 to –0.2]), and more patients than siblings had an estimated IQ of ≤5 but not of ≤70.
Patients also had significantly lower scores on nonverbal memory, visuospatial skills, and fine motor control (mean differences, –0.9 [–1.6 to –0.3]; –0.9 [–1.8 to –.1]; and –-3.1 [–4.9 to –1.4], respectively), compared with matched siblings. They also had significantly higher scores on processing speed (mean difference, 4.4 [0.2-8.5]). There were no significant differences in the other secondary outcomes.
Differences in scores between patients and siblings varied significantly by age at hospitalization in several tests – for example, Block Design scores in patients were lower than those of siblings for patients hospitalized at <1 year old, versus those hospitalized between ages 4 and 8 years.
“When adjusting for patient age at PICU admission, patient age at testing, sibling age at testing, and duration between hospital discharge and testing, the difference in estimated IQ between patients and siblings remained statistically significantly different,” the authors note.
The investigators point out several limitations, including the fact that “little is known about sibling outcomes after critical illness, nor about whether parenting of siblings or child development differs based on birth order or on relationship between patient critical illness and the birth of siblings. ... If siblings also incur negative effects related to the critical illness, differences between critically ill children and the control siblings would be blunted.”
Despite the statistical significance of the difference between the patients and the matched controls, ultimately, the magnitude of the difference was “small and of uncertain clinical importance,” the authors conclude.
Filling a research gap
Commenting on the findings, Alexandre T. Rotta, MD, professor of pediatrics and chief of the division of pediatric critical care medicine, Duke University Medical Center, Durham, N.C., said the study “addresses an important yet vastly understudied gap: long-term neurocognitive morbidity in children exposed to critical care.”
Dr. Rotta, who is also a coauthor of an accompanying editorial, noted that the fact that the “vast majority of children with an IQ significantly lower than their siblings were under the age of 4 years suggests that the developing immature brain may be particularly susceptible to the effects of critical illness and therapies required to treat it.”
The study “underscores the need to include assessments of long-term morbidity as part of any future trial evaluating interventions in pediatric critical care,” he added.
The study was supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development for RESTORE-Cognition and by grants for the RESTORE trial from the National Heart, Lung, and Blood Institute and the National Institute of Nursing Research, National Institutes of Health. Dr. Watson and coauthors report no relevant financial relationships. Dr. Rotta has received personal fees from Vapotherm for lecturing and development of educational materials and from Breas US for participation in a scientific advisory board, as well as royalties from Elsevier for editorial work outside the submitted work. His coauthor reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA
Nirsevimab protects healthy infants from RSV
A single injection of the experimental agent nirsevimab ahead of respiratory syncytial virus (RSV) season protects healthy infants from lower respiratory tract infections associated with the pathogen, according to the results of a phase 3 study.
A previously published trial showed that a single dose of nirsevimab was effective in preterm infants. The ability to protect all babies from RSV, which causes bronchiolitis and pneumonia and is a leading cause of hospitalization for this age group, “would be a paradigm shift in the approach to this disease,” William Muller, MD, PhD, of the Lurie Children’s Hospital of Chicago and a coauthor of the study, said in a statement.
The primary endpoint of the study was medically attended lower respiratory tract infections linked to RSV. The single injection of nirsevimab was associated with a 74.5% reduction in such infections (P < .001), according to Dr. Muller’s group, who published their findings March 2 in the New England Journal of Medicine.
Nirsevimab, a monoclonal antibody to the RSV fusion protein being developed by AstraZeneca and Sanofi, has an extended half-life, which may allow one dose to confer protection throughout a season. The only approved option to prevent RSV, palivizumab (Synagis), is used for high-risk infants, and five injections are needed to cover a viral season.
Nearly 1,500 infants in more than 20 countries studied
To assess the effectiveness of nirsevimab in late-preterm and term infants, investigators at 160 sites randomly assigned 1,490 babies born at a gestational age of at least 35 weeks to receive an intramuscular injection of nirsevimab or placebo.
During the 150 days after injection, medically attended RSV-associated lower respiratory tract infections occurred in 12 of 994 infants who received nirsevimab, compared with 25 of 496 babies who received placebo (1.2% vs. 5%).
Six of 994 infants who received nirsevimab were hospitalized for RSV-associated lower respiratory tract infections, compared with 8 of 496 infants in the placebo group (0.6% vs. 1.6%; P = .07). The proportion of children hospitalized for any respiratory illness as a result of RSV was 0.9% among those who received nirsevimab, compared with 2.2% among those who received placebo.
Serious adverse events occurred in 6.8% of the nirsevimab group and 7.3% of the placebo group. None of these events, including three deaths in the nirsevimab group, was considered related to nirsevimab or placebo, according to the researchers. One infant who received nirsevimab had a generalized macular rash without systemic features that did not require treatment and resolved in 20 days, they said.
Antidrug antibodies were detected in 6.1% of the nirsevimab group and in 1.1% of the placebo group. These antidrug antibodies tended to develop later and did not affect nirsevimab pharmacokinetics during the RSV season, the researchers reported. How they might affect subsequent doses of nirsevimab is not known, they added.
In a separate report in the journal, researcher Joseph Domachowske, MD, SUNY Upstate Medical University, Syracuse, New York, and colleagues described safety results from an ongoing study of nirsevimab that includes infants with congenital heart disease, chronic lung disease, and prematurity.
In this trial, infants received nirsevimab or palivizumab, and the treatments appeared to have similar safety profiles, the authors reported.
Other approaches to RSV protection include passive antibodies acquired from maternal vaccination in pregnancy and active vaccination of infants.
The publication follows news last month that GlaxoSmithKline is pausing a maternal RSV vaccine trial, which “had the same goal of protecting babies against severe RSV infection,” said Louis Bont, MD, PhD, with University Medical Center Utrecht, the Netherlands.
RSV infection is one of the deadliest diseases during infancy, and the nirsevimab trial, conducted in more than 20 countries, is “gamechanging,” Dr. Bont told this news organization. Still, researchers will need to monitor for RSV resistance to this treatment, he said.
Whether nirsevimab prevents the development of reactive airway disease and asthma is another open question, he said.
“Finally, we need to keep in mind that RSV mortality is almost limited to the developing world, and it is unlikely that this novel drug will become available to these countries in the coming years,” Dr. Bont said. “Nevertheless, nirsevimab has the potential to seriously decrease the annual overwhelming number of RSV infected babies.”
Nirsevimab may have advantages in low- and middle-income countries, including its potential to be incorporated into established immunization programs and to be given seasonally, said Amy Sarah Ginsburg, MD, MPH, of the University of Washington, Seattle. “However, cost remains a significant factor, as does susceptibility to pathogen escape,” she said.
MedImmune/AstraZeneca and Sanofi funded the nirsevimab studies. UMC Utrecht has received research grants and fees for advisory work from AstraZeneca for RSV-related work by Bont.
A version of this article first appeared on Medscape.com.
A single injection of the experimental agent nirsevimab ahead of respiratory syncytial virus (RSV) season protects healthy infants from lower respiratory tract infections associated with the pathogen, according to the results of a phase 3 study.
A previously published trial showed that a single dose of nirsevimab was effective in preterm infants. The ability to protect all babies from RSV, which causes bronchiolitis and pneumonia and is a leading cause of hospitalization for this age group, “would be a paradigm shift in the approach to this disease,” William Muller, MD, PhD, of the Lurie Children’s Hospital of Chicago and a coauthor of the study, said in a statement.
The primary endpoint of the study was medically attended lower respiratory tract infections linked to RSV. The single injection of nirsevimab was associated with a 74.5% reduction in such infections (P < .001), according to Dr. Muller’s group, who published their findings March 2 in the New England Journal of Medicine.
Nirsevimab, a monoclonal antibody to the RSV fusion protein being developed by AstraZeneca and Sanofi, has an extended half-life, which may allow one dose to confer protection throughout a season. The only approved option to prevent RSV, palivizumab (Synagis), is used for high-risk infants, and five injections are needed to cover a viral season.
Nearly 1,500 infants in more than 20 countries studied
To assess the effectiveness of nirsevimab in late-preterm and term infants, investigators at 160 sites randomly assigned 1,490 babies born at a gestational age of at least 35 weeks to receive an intramuscular injection of nirsevimab or placebo.
During the 150 days after injection, medically attended RSV-associated lower respiratory tract infections occurred in 12 of 994 infants who received nirsevimab, compared with 25 of 496 babies who received placebo (1.2% vs. 5%).
Six of 994 infants who received nirsevimab were hospitalized for RSV-associated lower respiratory tract infections, compared with 8 of 496 infants in the placebo group (0.6% vs. 1.6%; P = .07). The proportion of children hospitalized for any respiratory illness as a result of RSV was 0.9% among those who received nirsevimab, compared with 2.2% among those who received placebo.
Serious adverse events occurred in 6.8% of the nirsevimab group and 7.3% of the placebo group. None of these events, including three deaths in the nirsevimab group, was considered related to nirsevimab or placebo, according to the researchers. One infant who received nirsevimab had a generalized macular rash without systemic features that did not require treatment and resolved in 20 days, they said.
Antidrug antibodies were detected in 6.1% of the nirsevimab group and in 1.1% of the placebo group. These antidrug antibodies tended to develop later and did not affect nirsevimab pharmacokinetics during the RSV season, the researchers reported. How they might affect subsequent doses of nirsevimab is not known, they added.
In a separate report in the journal, researcher Joseph Domachowske, MD, SUNY Upstate Medical University, Syracuse, New York, and colleagues described safety results from an ongoing study of nirsevimab that includes infants with congenital heart disease, chronic lung disease, and prematurity.
In this trial, infants received nirsevimab or palivizumab, and the treatments appeared to have similar safety profiles, the authors reported.
Other approaches to RSV protection include passive antibodies acquired from maternal vaccination in pregnancy and active vaccination of infants.
The publication follows news last month that GlaxoSmithKline is pausing a maternal RSV vaccine trial, which “had the same goal of protecting babies against severe RSV infection,” said Louis Bont, MD, PhD, with University Medical Center Utrecht, the Netherlands.
RSV infection is one of the deadliest diseases during infancy, and the nirsevimab trial, conducted in more than 20 countries, is “gamechanging,” Dr. Bont told this news organization. Still, researchers will need to monitor for RSV resistance to this treatment, he said.
Whether nirsevimab prevents the development of reactive airway disease and asthma is another open question, he said.
“Finally, we need to keep in mind that RSV mortality is almost limited to the developing world, and it is unlikely that this novel drug will become available to these countries in the coming years,” Dr. Bont said. “Nevertheless, nirsevimab has the potential to seriously decrease the annual overwhelming number of RSV infected babies.”
Nirsevimab may have advantages in low- and middle-income countries, including its potential to be incorporated into established immunization programs and to be given seasonally, said Amy Sarah Ginsburg, MD, MPH, of the University of Washington, Seattle. “However, cost remains a significant factor, as does susceptibility to pathogen escape,” she said.
MedImmune/AstraZeneca and Sanofi funded the nirsevimab studies. UMC Utrecht has received research grants and fees for advisory work from AstraZeneca for RSV-related work by Bont.
A version of this article first appeared on Medscape.com.
A single injection of the experimental agent nirsevimab ahead of respiratory syncytial virus (RSV) season protects healthy infants from lower respiratory tract infections associated with the pathogen, according to the results of a phase 3 study.
A previously published trial showed that a single dose of nirsevimab was effective in preterm infants. The ability to protect all babies from RSV, which causes bronchiolitis and pneumonia and is a leading cause of hospitalization for this age group, “would be a paradigm shift in the approach to this disease,” William Muller, MD, PhD, of the Lurie Children’s Hospital of Chicago and a coauthor of the study, said in a statement.
The primary endpoint of the study was medically attended lower respiratory tract infections linked to RSV. The single injection of nirsevimab was associated with a 74.5% reduction in such infections (P < .001), according to Dr. Muller’s group, who published their findings March 2 in the New England Journal of Medicine.
Nirsevimab, a monoclonal antibody to the RSV fusion protein being developed by AstraZeneca and Sanofi, has an extended half-life, which may allow one dose to confer protection throughout a season. The only approved option to prevent RSV, palivizumab (Synagis), is used for high-risk infants, and five injections are needed to cover a viral season.
Nearly 1,500 infants in more than 20 countries studied
To assess the effectiveness of nirsevimab in late-preterm and term infants, investigators at 160 sites randomly assigned 1,490 babies born at a gestational age of at least 35 weeks to receive an intramuscular injection of nirsevimab or placebo.
During the 150 days after injection, medically attended RSV-associated lower respiratory tract infections occurred in 12 of 994 infants who received nirsevimab, compared with 25 of 496 babies who received placebo (1.2% vs. 5%).
Six of 994 infants who received nirsevimab were hospitalized for RSV-associated lower respiratory tract infections, compared with 8 of 496 infants in the placebo group (0.6% vs. 1.6%; P = .07). The proportion of children hospitalized for any respiratory illness as a result of RSV was 0.9% among those who received nirsevimab, compared with 2.2% among those who received placebo.
Serious adverse events occurred in 6.8% of the nirsevimab group and 7.3% of the placebo group. None of these events, including three deaths in the nirsevimab group, was considered related to nirsevimab or placebo, according to the researchers. One infant who received nirsevimab had a generalized macular rash without systemic features that did not require treatment and resolved in 20 days, they said.
Antidrug antibodies were detected in 6.1% of the nirsevimab group and in 1.1% of the placebo group. These antidrug antibodies tended to develop later and did not affect nirsevimab pharmacokinetics during the RSV season, the researchers reported. How they might affect subsequent doses of nirsevimab is not known, they added.
In a separate report in the journal, researcher Joseph Domachowske, MD, SUNY Upstate Medical University, Syracuse, New York, and colleagues described safety results from an ongoing study of nirsevimab that includes infants with congenital heart disease, chronic lung disease, and prematurity.
In this trial, infants received nirsevimab or palivizumab, and the treatments appeared to have similar safety profiles, the authors reported.
Other approaches to RSV protection include passive antibodies acquired from maternal vaccination in pregnancy and active vaccination of infants.
The publication follows news last month that GlaxoSmithKline is pausing a maternal RSV vaccine trial, which “had the same goal of protecting babies against severe RSV infection,” said Louis Bont, MD, PhD, with University Medical Center Utrecht, the Netherlands.
RSV infection is one of the deadliest diseases during infancy, and the nirsevimab trial, conducted in more than 20 countries, is “gamechanging,” Dr. Bont told this news organization. Still, researchers will need to monitor for RSV resistance to this treatment, he said.
Whether nirsevimab prevents the development of reactive airway disease and asthma is another open question, he said.
“Finally, we need to keep in mind that RSV mortality is almost limited to the developing world, and it is unlikely that this novel drug will become available to these countries in the coming years,” Dr. Bont said. “Nevertheless, nirsevimab has the potential to seriously decrease the annual overwhelming number of RSV infected babies.”
Nirsevimab may have advantages in low- and middle-income countries, including its potential to be incorporated into established immunization programs and to be given seasonally, said Amy Sarah Ginsburg, MD, MPH, of the University of Washington, Seattle. “However, cost remains a significant factor, as does susceptibility to pathogen escape,” she said.
MedImmune/AstraZeneca and Sanofi funded the nirsevimab studies. UMC Utrecht has received research grants and fees for advisory work from AstraZeneca for RSV-related work by Bont.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Preliminary Observations of Veterans Without HIV Who Have Mycobacterium avium Complex Pulmonary Disease
Nontuberculous Mycobacterium (NTM) is a ubiquitous organism known to cause a variety of infections in susceptible hosts; however, pulmonary infection is the most common. Mycobacterium avium complex (MAC) is the most prevalent cause of NTM-related pulmonary disease (NTM-PD) and is associated with underlying structural lung disease, such as chronic obstructive pulmonary disease (COPD) and noncystic fibrosis bronchiectasis.1-3
Diagnosis of NTM-PD requires (1) symptoms or radiographic abnormality; and (2) at least 2 sputum cultures positive with the same organism or at least 1 positive culture result on bronchoscopy (wash, lavage, or biopsy).1 Notably, the natural history of untreated NTM-PD varies, though even mild disease may progress substantially.4-6 Progressive disease is more likely to occur in those with a positive smear or more extensive radiographic findings at the initial diagnosis.7 A nationwide Medicare-based study showed that patients with NTM-PD had a higher rate of all-cause mortality than did patients without NTM-PD.8 In a study of 123 patients from Taiwan with MAC-PD, lack of treatment was an independent predictor of mortality.9 Given the risk of progressive morbidity and mortality, recent guidelines recommend initiation of a susceptibility driven, macrolide-based, 3-drug treatment regimen over watchful waiting.10
MAC-PD is increasingly recognized among US veterans.11,12 The Jesse Brown Veterans Affairs Medical Center (JBVAMC) in south/west Chicago serves a large, predominantly Black male population of veterans many of whom are socioeconomically underresourced, and half are aged ≥ 65 years. We observed that initiation of guideline-directed therapy in veterans with MAC-PD at JBVAMC varied among health care professionals (HCPs) in the pulmonary clinic. Therefore, the purpose of this retrospective study was to describe and compare the characteristics of veterans without HIV were diagnosed with MAC-PD and managed at JBVAMC.
Methods
The hospital microbiology department identified veterans diagnosed with NTM at JBVAMC between October 2008 and July 2019. Veterans included in the study were considered to have MAC-PD per American Thoracic Society (ATS)/Infectious Diseases Society of America (ISDA) guidelines and those diagnosed with HIV were excluded from analysis. The electronic health record (EHR) was queried for pertinent demographics, smoking history, comorbidities, and symptoms at the time of a positive mycobacterial culture. Computed tomography (CT) and pulmonary function tests (PFTs) performed within 1 year of diagnosis were included. PFTs were assessed in accordance with Global Initiative for Obstructive Lung Disease (GOLD) criteria, with normal forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC) values defined as ≥ 80% and a normal FEV1/FVC ratio defined as ≥ 70. The diffusion capacity of lung for carbon monoxide (DLCO) was assessed per 2017 European Respiratory Society (ERS) technical standards and was considered reduced if below the lower limit of normal.13 Information regarding treatment decisions, initiation, and cessation were collected. All-cause mortality was recorded if available in the EHR at the time of data collection.
Statistical analysis was performed using Mann-Whitney U and Fisher exact tests where appropriate. P < .05 was considered statistically significant. The study was approved by the JBVAMC Institutional Review Board.
Results
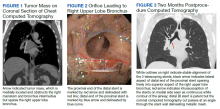
We identified 43 veterans who had a positive culture for MAC; however, only 19 veterans met the diagnostic criteria for MAC-PD and were included in the study (Table). The cohort included predominantly Black and male veterans with a median age of 74 years at time of diagnosis (range, 45-92). Sixteen veterans had underlying lung disease (84.2%), and 16 (84.2%) were current or former smokers. Common comorbidities included COPD, obstructive sleep apnea, gastroesophageal reflux disease, and lung cancer. Respiratory symptoms were reported in 17 veterans (89.5%), 15 (78.9%) had a chronic cough, and 10 (52.6%) had dyspnea. Fifteen veterans had a chest CT scan within 1 year of diagnosis: A nodular and tree-in-bud pattern was most commonly found in 13 (86.7%) of veterans. Thirteen veterans had PFTs within 1 year of MAC-PD diagnosis, of whom 6 had a restrictive pattern with percent predicted FVC < 80%, and 9 had evidence of obstruction with FEV1/FVC < 70. DLCO was below the lower limit of normal in 18 veterans. Finally, 6 veterans were deceased at the time of the study.
Of the 19 veterans, guideline-directed, combination antimycobacterial therapy for MAC-PD was initiated in only 10 (52.6%) patients due to presence of symptoms and/or imaging abnormalities. Treatment was deferred due to improved symptoms, concern for adverse events (AEs), or lost to follow-up. Five veterans stopped treatment prematurely due to AEs, lost to follow-up, or all-cause mortality. Assessment of differences between treated and untreated groups revealed no significant difference in race, sex, age, body mass index (BMI), symptom presence, or chest CT abnormalities. There was no statistically significant difference in all-cause mortality (40% and 22.2% in treated and untreated group, respectively).
To further understand the differences of this cohort, the 13 veterans alive at time of the study were compared with the 6 who had since died of all-cause mortality. No statistically significant differences were found.
Discussion
Consistent with previous reports in the literature, veterans in our cohort were predominantly current or former smoking males with underlying COPD and bronchiectasis.1-3,11,12 Chest CT findings varied: Most veterans presented not only with nodules and tree-in-bud opacities, but also a high frequency of fibrosis and emphysema. PFTs revealed a variety of obstruction and restrictive patterns, and most veterans had a reduced DLCO, though it is unclear whether this is reflective of underlying emphysema, fibrosis, or an alternative cardiopulmonary disease.13,14
While underlying structural lung disease may have been a risk factor for MAC-PD in this cohort, the contribution of environmental and domiciliary factors in metropolitan Chicago neighborhoods is unknown. JBVAMC serves an underresourced population who live in the west and south Chicago neighborhoods. Household factors, ambient and indoor air pollution, and potential contamination of the water supply and surface soil may contribute to the prevalence of MAC-PD in this group.15-19 Further studies are warranted to characterize MAC-PD and its treatment in veterans without HIV who reside in underresourced urban communities in the US.
Recent ATS, European Society of Clinical Microbiology and Infectious Diseases, and IDSA guidelines recommend combination antimycobacterial therapy for patients who meet clinical, radiographic, and microbiologic criteria for the diagnosis of MAC-PD.10 Patients who meet these diagnostic criteria, particularly patients with smear positivity or fibrocavitary disease, should be treated because of risk of unfavorable outcomes.15,20-22 However, we found that the initiation of guideline-recommended antimycobacterial therapy in veterans without HIV with MAC-PD were inconsistent among HCPs. The reasons underlying this phenomenon were not apparent beyond cited reasons for treatment initiation or deference. Despite this inconsistency, there was no clear difference in age, BMI, symptom burden, radiographic abnormality, or all-cause mortality between treatment groups. Existing studies support slow but substantial progression of untreated MAC-PD, and while treatment prevents deterioration of the disease, it does not prevent progression of bronchiectasis.6 The natural history of MAC-PD in this veteran cohort has yet to be fully elucidated. Furthermore, the 50% treatment dropout rate was higher than previously reported rates (11-33%).5 However, the small number of veterans in this study precludes meaningful comparison with similar reports in the literature.
Limitations
The limitations of this small, single-center, retrospective study prevent a robust, generalizable comparison between groups. Further studies are warranted to characterize MAC-PD and its treatment in veterans without HIV who reside in underresourced urban communities in the US.24-26
Conclusions
These data suggest that clinical, imaging, and treatment attributes of MAC-PD in veterans without HIV who reside in metropolitan Chicago are heterogeneous and are associated with a relatively high mortality rate. Although there was no difference in the attributes or outcomes of veterans who did and did not initiate treatment despite current recommendations, further studies are needed to better explore these relationships.
1. Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/ IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases [published correction appears in Am J Respir Crit Care Med. 2007 Apr 1;175(7):744-5. Dosage error in article text]. Am J Respir Crit Care Med. 2007;175(4):367-416. doi:10.1164/rccm.200604-571ST
2. Prevots DR, Shaw PA, Strickland D, et al. Nontuberculous mycobacterial lung disease prevalence at four integrated health care delivery systems. Am J Respir Crit Care Med. 2010;182(7):970-976. doi:10.1164/rccm.201002-0310OC
3. Winthrop KL, Marras TK, Adjemian J, Zhang H, Wang P, Zhang Q. Incidence and prevalence of nontuberculous mycobacterial lung disease in a large U.S. managed care health plan, 2008-2015. Ann Am Thorac Soc. 2020;17(2):178-185. doi:10.1513/AnnalsATS.201804-236OC
4. Field SK, Fisher D, Cowie RL. Mycobacterium avium complex pulmonary disease in patients without HIV infection. Chest. 2004;126(2):566-581. doi:10.1378/chest.126.2.566
5. Kimizuka Y, Hoshino Y, Nishimura T, et al. Retrospective evaluation of natural course in mild cases of Mycobacterium avium complex pulmonary disease. PLoS One. 2019;14(4):e0216034. Published 2019 Apr 25. doi:10.1371/journal.pone.0216034
6. Kotilainen H, Valtonen V, Tukiainen P, Poussa T, Eskola J, Järvinen A. Clinical findings in relation to mortality in nontuberculous mycobacterial infections: patients with Mycobacterium avium complex have better survival than patients with other mycobacteria. Eur J Clin Microbiol Infect Dis. 2015;34(9):1909-1918. doi:10.1007/s10096-015-2432-8.
7. Hwang JA, Kim S, Jo KW, Shim TS. Natural history of Mycobacterium avium complex lung disease in untreated patients with stable course. Eur Respir J. 2017;49(3):1600537. Published 2017 Mar 8. doi:10.1183/13993003.00537-2016
8. Adjemian J, Olivier KN, Seitz AE, Holland SM, Prevots DR. Prevalence of nontuberculous mycobacterial lung disease in U.S. Medicare beneficiaries. Am J Respir Crit Care Med. 2012;185(8):881-886. doi:10.1164/rccm.201111-2016OC
9. Wang PH, Pan SW, Shu CC, et al. Clinical course and risk factors of mortality in Mycobacterium avium complex lung disease without initial treatment. Respir Med. 2020;171:106070. doi:10.1016/j.rmed.2020.106070
10. Daley CL, Iaccarino JM, Lange C, et al. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ ERS/ESCMID/IDSA Clinical Practice Guideline [published correction appears in Clin Infect Dis. 2020 Dec 31;71(11):3023]. Clin Infect Dis. 2020;71(4):e1-e36. doi:10.1093/cid/ciaa241
11. Mirsaeidi M, Hadid W, Ericsoussi B, Rodgers D, Sadikot RT. Non-tuberculous mycobacterial disease is common in patients with non-cystic fibrosis bronchiectasis. Int J Infect Dis. 2013;17(11):e1000-e1004. doi:10.1016/j.ijid.2013.03.018
12. Oda G, Winters MA, Pacheco SM, et al. Clusters of nontuberculous mycobacteria linked to water sources at three Veterans Affairs medical centers. Infect Control Hosp Epidemiol. 2020;41(3):320-330. doi:10.1017/ice.2019.342
13. Stanojevic S, Graham BL, Cooper BG, et al. Official ERS technical standards: Global Lung Function Initiative reference values for the carbon monoxide transfer factor for Caucasians [published correction appears in Eur Respir J. 2020 Oct 15;56(4):]. Eur Respir J. 2017;50(3):1700010. Published 2017 Sep 11. doi:10.1183/13993003.00010-2017
14. Macintyre N, Crapo RO, Viegi G, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005;26(4):720-735. doi:10.1183/09031936.05.00034905
15. Chalmers JD, Balavoine C, Castellotti PF, et al. European Respiratory Society International Congress, Madrid, 2019: nontuberculous mycobacterial pulmonary disease highlights. ERJ Open Res. 2020;6(4):00317-2020. Published 2020 Oct 19. doi:10.1183/23120541.00317-2020
16. Hamilton LA, Falkinham JO. Aerosolization of Mycobacterium avium and Mycobacterium abscessus from a household ultrasonic humidifier. J Med Microbiol. 2018;67(10):1491-1495. doi:10.1099/jmm.0.000822
17. Hannah CE, Ford BA, Chung J, Ince D, Wanat KA. Characteristics of nontuberculous mycobacterial infections at a midwestern tertiary hospital: a retrospective study of 365 patients. Open Forum Infect Dis. 2020;7(6):ofaa173. Published 2020 May 25. doi:10.1093/ofid/ofaa173
18. Rautiala S, Torvinen E, Torkko P, et al. Potentially pathogenic, slow-growing mycobacteria released into workplace air during the remediation of buildings. J Occup Environ Hyg. 2004;1(1):1-6. doi:10.1080/15459620490250008
19. Tzou CL, Dirac MA, Becker AL, et al. Association between Mycobacterium avium complex pulmonary disease and mycobacteria in home water and soil. Ann Am Thorac Soc. 2020;17(1):57-62. doi:10.1513/AnnalsATS.201812-915OC
20. Daley CL, Winthrop KL. Mycobacterium avium complex: addressing gaps in diagnosis and management. J Infect Dis. 2020;222(suppl 4):S199-S211. doi:10.1093/infdis/jiaa354 21. Kwon BS, Lee JH, Koh Y, et al. The natural history of noncavitary nodular bronchiectatic Mycobacterium avium complex lung disease. Respir Med. 2019;150:45-50. doi:10.1016/j.rmed.2019.02.007
22. Nasiri MJ, Ebrahimi G, Arefzadeh S, Zamani S, Nikpor Z, Mirsaeidi M. Antibiotic therapy success rate in pulmonary Mycobacterium avium complex: a systematic review and meta-analysis. Expert Rev Anti Infect Ther. 2020;18(3):263- 273. doi:10.1080/14787210.2020.1720650
23. Diel R, Lipman M, Hoefsloot W. High mortality in patients with Mycobacterium avium complex lung disease: a systematic review. BMC Infect Dis. 2018;18(1):206. Published 2018 May 3. doi:10.1186/s12879-018-3113-x
24. Marras TK, Prevots DR, Jamieson FB, Winthrop KL; Pulmonary MAC Outcomes Group. Opinions differ by expertise in Mycobacterium avium complex disease. Ann Am Thorac Soc. 2014;11(1):17-22. doi:10.1513/AnnalsATS.201305-136OC
25. Plotinsky RN, Talbot EA, von Reyn CF. Proposed definitions for epidemiologic and clinical studies of Mycobacterium avium complex pulmonary disease. PLoS One. 2013;8(11):e77385. Published 2013 Nov 12. doi:10.1371/journal.pone.0077385
26. Swenson C, Zerbe CS, Fennelly K. Host variability in NTM disease: implications for research needs. Front Microbiol. 2018;9:2901. Published 2018 Dec 3. doi:10.3389/fmicb.2018.02901
Nontuberculous Mycobacterium (NTM) is a ubiquitous organism known to cause a variety of infections in susceptible hosts; however, pulmonary infection is the most common. Mycobacterium avium complex (MAC) is the most prevalent cause of NTM-related pulmonary disease (NTM-PD) and is associated with underlying structural lung disease, such as chronic obstructive pulmonary disease (COPD) and noncystic fibrosis bronchiectasis.1-3
Diagnosis of NTM-PD requires (1) symptoms or radiographic abnormality; and (2) at least 2 sputum cultures positive with the same organism or at least 1 positive culture result on bronchoscopy (wash, lavage, or biopsy).1 Notably, the natural history of untreated NTM-PD varies, though even mild disease may progress substantially.4-6 Progressive disease is more likely to occur in those with a positive smear or more extensive radiographic findings at the initial diagnosis.7 A nationwide Medicare-based study showed that patients with NTM-PD had a higher rate of all-cause mortality than did patients without NTM-PD.8 In a study of 123 patients from Taiwan with MAC-PD, lack of treatment was an independent predictor of mortality.9 Given the risk of progressive morbidity and mortality, recent guidelines recommend initiation of a susceptibility driven, macrolide-based, 3-drug treatment regimen over watchful waiting.10
MAC-PD is increasingly recognized among US veterans.11,12 The Jesse Brown Veterans Affairs Medical Center (JBVAMC) in south/west Chicago serves a large, predominantly Black male population of veterans many of whom are socioeconomically underresourced, and half are aged ≥ 65 years. We observed that initiation of guideline-directed therapy in veterans with MAC-PD at JBVAMC varied among health care professionals (HCPs) in the pulmonary clinic. Therefore, the purpose of this retrospective study was to describe and compare the characteristics of veterans without HIV were diagnosed with MAC-PD and managed at JBVAMC.
Methods
The hospital microbiology department identified veterans diagnosed with NTM at JBVAMC between October 2008 and July 2019. Veterans included in the study were considered to have MAC-PD per American Thoracic Society (ATS)/Infectious Diseases Society of America (ISDA) guidelines and those diagnosed with HIV were excluded from analysis. The electronic health record (EHR) was queried for pertinent demographics, smoking history, comorbidities, and symptoms at the time of a positive mycobacterial culture. Computed tomography (CT) and pulmonary function tests (PFTs) performed within 1 year of diagnosis were included. PFTs were assessed in accordance with Global Initiative for Obstructive Lung Disease (GOLD) criteria, with normal forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC) values defined as ≥ 80% and a normal FEV1/FVC ratio defined as ≥ 70. The diffusion capacity of lung for carbon monoxide (DLCO) was assessed per 2017 European Respiratory Society (ERS) technical standards and was considered reduced if below the lower limit of normal.13 Information regarding treatment decisions, initiation, and cessation were collected. All-cause mortality was recorded if available in the EHR at the time of data collection.
Statistical analysis was performed using Mann-Whitney U and Fisher exact tests where appropriate. P < .05 was considered statistically significant. The study was approved by the JBVAMC Institutional Review Board.
Results
We identified 43 veterans who had a positive culture for MAC; however, only 19 veterans met the diagnostic criteria for MAC-PD and were included in the study (Table). The cohort included predominantly Black and male veterans with a median age of 74 years at time of diagnosis (range, 45-92). Sixteen veterans had underlying lung disease (84.2%), and 16 (84.2%) were current or former smokers. Common comorbidities included COPD, obstructive sleep apnea, gastroesophageal reflux disease, and lung cancer. Respiratory symptoms were reported in 17 veterans (89.5%), 15 (78.9%) had a chronic cough, and 10 (52.6%) had dyspnea. Fifteen veterans had a chest CT scan within 1 year of diagnosis: A nodular and tree-in-bud pattern was most commonly found in 13 (86.7%) of veterans. Thirteen veterans had PFTs within 1 year of MAC-PD diagnosis, of whom 6 had a restrictive pattern with percent predicted FVC < 80%, and 9 had evidence of obstruction with FEV1/FVC < 70. DLCO was below the lower limit of normal in 18 veterans. Finally, 6 veterans were deceased at the time of the study.
Of the 19 veterans, guideline-directed, combination antimycobacterial therapy for MAC-PD was initiated in only 10 (52.6%) patients due to presence of symptoms and/or imaging abnormalities. Treatment was deferred due to improved symptoms, concern for adverse events (AEs), or lost to follow-up. Five veterans stopped treatment prematurely due to AEs, lost to follow-up, or all-cause mortality. Assessment of differences between treated and untreated groups revealed no significant difference in race, sex, age, body mass index (BMI), symptom presence, or chest CT abnormalities. There was no statistically significant difference in all-cause mortality (40% and 22.2% in treated and untreated group, respectively).
To further understand the differences of this cohort, the 13 veterans alive at time of the study were compared with the 6 who had since died of all-cause mortality. No statistically significant differences were found.
Discussion
Consistent with previous reports in the literature, veterans in our cohort were predominantly current or former smoking males with underlying COPD and bronchiectasis.1-3,11,12 Chest CT findings varied: Most veterans presented not only with nodules and tree-in-bud opacities, but also a high frequency of fibrosis and emphysema. PFTs revealed a variety of obstruction and restrictive patterns, and most veterans had a reduced DLCO, though it is unclear whether this is reflective of underlying emphysema, fibrosis, or an alternative cardiopulmonary disease.13,14
While underlying structural lung disease may have been a risk factor for MAC-PD in this cohort, the contribution of environmental and domiciliary factors in metropolitan Chicago neighborhoods is unknown. JBVAMC serves an underresourced population who live in the west and south Chicago neighborhoods. Household factors, ambient and indoor air pollution, and potential contamination of the water supply and surface soil may contribute to the prevalence of MAC-PD in this group.15-19 Further studies are warranted to characterize MAC-PD and its treatment in veterans without HIV who reside in underresourced urban communities in the US.
Recent ATS, European Society of Clinical Microbiology and Infectious Diseases, and IDSA guidelines recommend combination antimycobacterial therapy for patients who meet clinical, radiographic, and microbiologic criteria for the diagnosis of MAC-PD.10 Patients who meet these diagnostic criteria, particularly patients with smear positivity or fibrocavitary disease, should be treated because of risk of unfavorable outcomes.15,20-22 However, we found that the initiation of guideline-recommended antimycobacterial therapy in veterans without HIV with MAC-PD were inconsistent among HCPs. The reasons underlying this phenomenon were not apparent beyond cited reasons for treatment initiation or deference. Despite this inconsistency, there was no clear difference in age, BMI, symptom burden, radiographic abnormality, or all-cause mortality between treatment groups. Existing studies support slow but substantial progression of untreated MAC-PD, and while treatment prevents deterioration of the disease, it does not prevent progression of bronchiectasis.6 The natural history of MAC-PD in this veteran cohort has yet to be fully elucidated. Furthermore, the 50% treatment dropout rate was higher than previously reported rates (11-33%).5 However, the small number of veterans in this study precludes meaningful comparison with similar reports in the literature.
Limitations
The limitations of this small, single-center, retrospective study prevent a robust, generalizable comparison between groups. Further studies are warranted to characterize MAC-PD and its treatment in veterans without HIV who reside in underresourced urban communities in the US.24-26
Conclusions
These data suggest that clinical, imaging, and treatment attributes of MAC-PD in veterans without HIV who reside in metropolitan Chicago are heterogeneous and are associated with a relatively high mortality rate. Although there was no difference in the attributes or outcomes of veterans who did and did not initiate treatment despite current recommendations, further studies are needed to better explore these relationships.
Nontuberculous Mycobacterium (NTM) is a ubiquitous organism known to cause a variety of infections in susceptible hosts; however, pulmonary infection is the most common. Mycobacterium avium complex (MAC) is the most prevalent cause of NTM-related pulmonary disease (NTM-PD) and is associated with underlying structural lung disease, such as chronic obstructive pulmonary disease (COPD) and noncystic fibrosis bronchiectasis.1-3
Diagnosis of NTM-PD requires (1) symptoms or radiographic abnormality; and (2) at least 2 sputum cultures positive with the same organism or at least 1 positive culture result on bronchoscopy (wash, lavage, or biopsy).1 Notably, the natural history of untreated NTM-PD varies, though even mild disease may progress substantially.4-6 Progressive disease is more likely to occur in those with a positive smear or more extensive radiographic findings at the initial diagnosis.7 A nationwide Medicare-based study showed that patients with NTM-PD had a higher rate of all-cause mortality than did patients without NTM-PD.8 In a study of 123 patients from Taiwan with MAC-PD, lack of treatment was an independent predictor of mortality.9 Given the risk of progressive morbidity and mortality, recent guidelines recommend initiation of a susceptibility driven, macrolide-based, 3-drug treatment regimen over watchful waiting.10
MAC-PD is increasingly recognized among US veterans.11,12 The Jesse Brown Veterans Affairs Medical Center (JBVAMC) in south/west Chicago serves a large, predominantly Black male population of veterans many of whom are socioeconomically underresourced, and half are aged ≥ 65 years. We observed that initiation of guideline-directed therapy in veterans with MAC-PD at JBVAMC varied among health care professionals (HCPs) in the pulmonary clinic. Therefore, the purpose of this retrospective study was to describe and compare the characteristics of veterans without HIV were diagnosed with MAC-PD and managed at JBVAMC.
Methods
The hospital microbiology department identified veterans diagnosed with NTM at JBVAMC between October 2008 and July 2019. Veterans included in the study were considered to have MAC-PD per American Thoracic Society (ATS)/Infectious Diseases Society of America (ISDA) guidelines and those diagnosed with HIV were excluded from analysis. The electronic health record (EHR) was queried for pertinent demographics, smoking history, comorbidities, and symptoms at the time of a positive mycobacterial culture. Computed tomography (CT) and pulmonary function tests (PFTs) performed within 1 year of diagnosis were included. PFTs were assessed in accordance with Global Initiative for Obstructive Lung Disease (GOLD) criteria, with normal forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC) values defined as ≥ 80% and a normal FEV1/FVC ratio defined as ≥ 70. The diffusion capacity of lung for carbon monoxide (DLCO) was assessed per 2017 European Respiratory Society (ERS) technical standards and was considered reduced if below the lower limit of normal.13 Information regarding treatment decisions, initiation, and cessation were collected. All-cause mortality was recorded if available in the EHR at the time of data collection.
Statistical analysis was performed using Mann-Whitney U and Fisher exact tests where appropriate. P < .05 was considered statistically significant. The study was approved by the JBVAMC Institutional Review Board.
Results
We identified 43 veterans who had a positive culture for MAC; however, only 19 veterans met the diagnostic criteria for MAC-PD and were included in the study (Table). The cohort included predominantly Black and male veterans with a median age of 74 years at time of diagnosis (range, 45-92). Sixteen veterans had underlying lung disease (84.2%), and 16 (84.2%) were current or former smokers. Common comorbidities included COPD, obstructive sleep apnea, gastroesophageal reflux disease, and lung cancer. Respiratory symptoms were reported in 17 veterans (89.5%), 15 (78.9%) had a chronic cough, and 10 (52.6%) had dyspnea. Fifteen veterans had a chest CT scan within 1 year of diagnosis: A nodular and tree-in-bud pattern was most commonly found in 13 (86.7%) of veterans. Thirteen veterans had PFTs within 1 year of MAC-PD diagnosis, of whom 6 had a restrictive pattern with percent predicted FVC < 80%, and 9 had evidence of obstruction with FEV1/FVC < 70. DLCO was below the lower limit of normal in 18 veterans. Finally, 6 veterans were deceased at the time of the study.
Of the 19 veterans, guideline-directed, combination antimycobacterial therapy for MAC-PD was initiated in only 10 (52.6%) patients due to presence of symptoms and/or imaging abnormalities. Treatment was deferred due to improved symptoms, concern for adverse events (AEs), or lost to follow-up. Five veterans stopped treatment prematurely due to AEs, lost to follow-up, or all-cause mortality. Assessment of differences between treated and untreated groups revealed no significant difference in race, sex, age, body mass index (BMI), symptom presence, or chest CT abnormalities. There was no statistically significant difference in all-cause mortality (40% and 22.2% in treated and untreated group, respectively).
To further understand the differences of this cohort, the 13 veterans alive at time of the study were compared with the 6 who had since died of all-cause mortality. No statistically significant differences were found.
Discussion
Consistent with previous reports in the literature, veterans in our cohort were predominantly current or former smoking males with underlying COPD and bronchiectasis.1-3,11,12 Chest CT findings varied: Most veterans presented not only with nodules and tree-in-bud opacities, but also a high frequency of fibrosis and emphysema. PFTs revealed a variety of obstruction and restrictive patterns, and most veterans had a reduced DLCO, though it is unclear whether this is reflective of underlying emphysema, fibrosis, or an alternative cardiopulmonary disease.13,14
While underlying structural lung disease may have been a risk factor for MAC-PD in this cohort, the contribution of environmental and domiciliary factors in metropolitan Chicago neighborhoods is unknown. JBVAMC serves an underresourced population who live in the west and south Chicago neighborhoods. Household factors, ambient and indoor air pollution, and potential contamination of the water supply and surface soil may contribute to the prevalence of MAC-PD in this group.15-19 Further studies are warranted to characterize MAC-PD and its treatment in veterans without HIV who reside in underresourced urban communities in the US.
Recent ATS, European Society of Clinical Microbiology and Infectious Diseases, and IDSA guidelines recommend combination antimycobacterial therapy for patients who meet clinical, radiographic, and microbiologic criteria for the diagnosis of MAC-PD.10 Patients who meet these diagnostic criteria, particularly patients with smear positivity or fibrocavitary disease, should be treated because of risk of unfavorable outcomes.15,20-22 However, we found that the initiation of guideline-recommended antimycobacterial therapy in veterans without HIV with MAC-PD were inconsistent among HCPs. The reasons underlying this phenomenon were not apparent beyond cited reasons for treatment initiation or deference. Despite this inconsistency, there was no clear difference in age, BMI, symptom burden, radiographic abnormality, or all-cause mortality between treatment groups. Existing studies support slow but substantial progression of untreated MAC-PD, and while treatment prevents deterioration of the disease, it does not prevent progression of bronchiectasis.6 The natural history of MAC-PD in this veteran cohort has yet to be fully elucidated. Furthermore, the 50% treatment dropout rate was higher than previously reported rates (11-33%).5 However, the small number of veterans in this study precludes meaningful comparison with similar reports in the literature.
Limitations
The limitations of this small, single-center, retrospective study prevent a robust, generalizable comparison between groups. Further studies are warranted to characterize MAC-PD and its treatment in veterans without HIV who reside in underresourced urban communities in the US.24-26
Conclusions
These data suggest that clinical, imaging, and treatment attributes of MAC-PD in veterans without HIV who reside in metropolitan Chicago are heterogeneous and are associated with a relatively high mortality rate. Although there was no difference in the attributes or outcomes of veterans who did and did not initiate treatment despite current recommendations, further studies are needed to better explore these relationships.
1. Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/ IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases [published correction appears in Am J Respir Crit Care Med. 2007 Apr 1;175(7):744-5. Dosage error in article text]. Am J Respir Crit Care Med. 2007;175(4):367-416. doi:10.1164/rccm.200604-571ST
2. Prevots DR, Shaw PA, Strickland D, et al. Nontuberculous mycobacterial lung disease prevalence at four integrated health care delivery systems. Am J Respir Crit Care Med. 2010;182(7):970-976. doi:10.1164/rccm.201002-0310OC
3. Winthrop KL, Marras TK, Adjemian J, Zhang H, Wang P, Zhang Q. Incidence and prevalence of nontuberculous mycobacterial lung disease in a large U.S. managed care health plan, 2008-2015. Ann Am Thorac Soc. 2020;17(2):178-185. doi:10.1513/AnnalsATS.201804-236OC
4. Field SK, Fisher D, Cowie RL. Mycobacterium avium complex pulmonary disease in patients without HIV infection. Chest. 2004;126(2):566-581. doi:10.1378/chest.126.2.566
5. Kimizuka Y, Hoshino Y, Nishimura T, et al. Retrospective evaluation of natural course in mild cases of Mycobacterium avium complex pulmonary disease. PLoS One. 2019;14(4):e0216034. Published 2019 Apr 25. doi:10.1371/journal.pone.0216034
6. Kotilainen H, Valtonen V, Tukiainen P, Poussa T, Eskola J, Järvinen A. Clinical findings in relation to mortality in nontuberculous mycobacterial infections: patients with Mycobacterium avium complex have better survival than patients with other mycobacteria. Eur J Clin Microbiol Infect Dis. 2015;34(9):1909-1918. doi:10.1007/s10096-015-2432-8.
7. Hwang JA, Kim S, Jo KW, Shim TS. Natural history of Mycobacterium avium complex lung disease in untreated patients with stable course. Eur Respir J. 2017;49(3):1600537. Published 2017 Mar 8. doi:10.1183/13993003.00537-2016
8. Adjemian J, Olivier KN, Seitz AE, Holland SM, Prevots DR. Prevalence of nontuberculous mycobacterial lung disease in U.S. Medicare beneficiaries. Am J Respir Crit Care Med. 2012;185(8):881-886. doi:10.1164/rccm.201111-2016OC
9. Wang PH, Pan SW, Shu CC, et al. Clinical course and risk factors of mortality in Mycobacterium avium complex lung disease without initial treatment. Respir Med. 2020;171:106070. doi:10.1016/j.rmed.2020.106070
10. Daley CL, Iaccarino JM, Lange C, et al. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ ERS/ESCMID/IDSA Clinical Practice Guideline [published correction appears in Clin Infect Dis. 2020 Dec 31;71(11):3023]. Clin Infect Dis. 2020;71(4):e1-e36. doi:10.1093/cid/ciaa241
11. Mirsaeidi M, Hadid W, Ericsoussi B, Rodgers D, Sadikot RT. Non-tuberculous mycobacterial disease is common in patients with non-cystic fibrosis bronchiectasis. Int J Infect Dis. 2013;17(11):e1000-e1004. doi:10.1016/j.ijid.2013.03.018
12. Oda G, Winters MA, Pacheco SM, et al. Clusters of nontuberculous mycobacteria linked to water sources at three Veterans Affairs medical centers. Infect Control Hosp Epidemiol. 2020;41(3):320-330. doi:10.1017/ice.2019.342
13. Stanojevic S, Graham BL, Cooper BG, et al. Official ERS technical standards: Global Lung Function Initiative reference values for the carbon monoxide transfer factor for Caucasians [published correction appears in Eur Respir J. 2020 Oct 15;56(4):]. Eur Respir J. 2017;50(3):1700010. Published 2017 Sep 11. doi:10.1183/13993003.00010-2017
14. Macintyre N, Crapo RO, Viegi G, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005;26(4):720-735. doi:10.1183/09031936.05.00034905
15. Chalmers JD, Balavoine C, Castellotti PF, et al. European Respiratory Society International Congress, Madrid, 2019: nontuberculous mycobacterial pulmonary disease highlights. ERJ Open Res. 2020;6(4):00317-2020. Published 2020 Oct 19. doi:10.1183/23120541.00317-2020
16. Hamilton LA, Falkinham JO. Aerosolization of Mycobacterium avium and Mycobacterium abscessus from a household ultrasonic humidifier. J Med Microbiol. 2018;67(10):1491-1495. doi:10.1099/jmm.0.000822
17. Hannah CE, Ford BA, Chung J, Ince D, Wanat KA. Characteristics of nontuberculous mycobacterial infections at a midwestern tertiary hospital: a retrospective study of 365 patients. Open Forum Infect Dis. 2020;7(6):ofaa173. Published 2020 May 25. doi:10.1093/ofid/ofaa173
18. Rautiala S, Torvinen E, Torkko P, et al. Potentially pathogenic, slow-growing mycobacteria released into workplace air during the remediation of buildings. J Occup Environ Hyg. 2004;1(1):1-6. doi:10.1080/15459620490250008
19. Tzou CL, Dirac MA, Becker AL, et al. Association between Mycobacterium avium complex pulmonary disease and mycobacteria in home water and soil. Ann Am Thorac Soc. 2020;17(1):57-62. doi:10.1513/AnnalsATS.201812-915OC
20. Daley CL, Winthrop KL. Mycobacterium avium complex: addressing gaps in diagnosis and management. J Infect Dis. 2020;222(suppl 4):S199-S211. doi:10.1093/infdis/jiaa354 21. Kwon BS, Lee JH, Koh Y, et al. The natural history of noncavitary nodular bronchiectatic Mycobacterium avium complex lung disease. Respir Med. 2019;150:45-50. doi:10.1016/j.rmed.2019.02.007
22. Nasiri MJ, Ebrahimi G, Arefzadeh S, Zamani S, Nikpor Z, Mirsaeidi M. Antibiotic therapy success rate in pulmonary Mycobacterium avium complex: a systematic review and meta-analysis. Expert Rev Anti Infect Ther. 2020;18(3):263- 273. doi:10.1080/14787210.2020.1720650
23. Diel R, Lipman M, Hoefsloot W. High mortality in patients with Mycobacterium avium complex lung disease: a systematic review. BMC Infect Dis. 2018;18(1):206. Published 2018 May 3. doi:10.1186/s12879-018-3113-x
24. Marras TK, Prevots DR, Jamieson FB, Winthrop KL; Pulmonary MAC Outcomes Group. Opinions differ by expertise in Mycobacterium avium complex disease. Ann Am Thorac Soc. 2014;11(1):17-22. doi:10.1513/AnnalsATS.201305-136OC
25. Plotinsky RN, Talbot EA, von Reyn CF. Proposed definitions for epidemiologic and clinical studies of Mycobacterium avium complex pulmonary disease. PLoS One. 2013;8(11):e77385. Published 2013 Nov 12. doi:10.1371/journal.pone.0077385
26. Swenson C, Zerbe CS, Fennelly K. Host variability in NTM disease: implications for research needs. Front Microbiol. 2018;9:2901. Published 2018 Dec 3. doi:10.3389/fmicb.2018.02901
1. Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/ IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases [published correction appears in Am J Respir Crit Care Med. 2007 Apr 1;175(7):744-5. Dosage error in article text]. Am J Respir Crit Care Med. 2007;175(4):367-416. doi:10.1164/rccm.200604-571ST
2. Prevots DR, Shaw PA, Strickland D, et al. Nontuberculous mycobacterial lung disease prevalence at four integrated health care delivery systems. Am J Respir Crit Care Med. 2010;182(7):970-976. doi:10.1164/rccm.201002-0310OC
3. Winthrop KL, Marras TK, Adjemian J, Zhang H, Wang P, Zhang Q. Incidence and prevalence of nontuberculous mycobacterial lung disease in a large U.S. managed care health plan, 2008-2015. Ann Am Thorac Soc. 2020;17(2):178-185. doi:10.1513/AnnalsATS.201804-236OC
4. Field SK, Fisher D, Cowie RL. Mycobacterium avium complex pulmonary disease in patients without HIV infection. Chest. 2004;126(2):566-581. doi:10.1378/chest.126.2.566
5. Kimizuka Y, Hoshino Y, Nishimura T, et al. Retrospective evaluation of natural course in mild cases of Mycobacterium avium complex pulmonary disease. PLoS One. 2019;14(4):e0216034. Published 2019 Apr 25. doi:10.1371/journal.pone.0216034
6. Kotilainen H, Valtonen V, Tukiainen P, Poussa T, Eskola J, Järvinen A. Clinical findings in relation to mortality in nontuberculous mycobacterial infections: patients with Mycobacterium avium complex have better survival than patients with other mycobacteria. Eur J Clin Microbiol Infect Dis. 2015;34(9):1909-1918. doi:10.1007/s10096-015-2432-8.
7. Hwang JA, Kim S, Jo KW, Shim TS. Natural history of Mycobacterium avium complex lung disease in untreated patients with stable course. Eur Respir J. 2017;49(3):1600537. Published 2017 Mar 8. doi:10.1183/13993003.00537-2016
8. Adjemian J, Olivier KN, Seitz AE, Holland SM, Prevots DR. Prevalence of nontuberculous mycobacterial lung disease in U.S. Medicare beneficiaries. Am J Respir Crit Care Med. 2012;185(8):881-886. doi:10.1164/rccm.201111-2016OC
9. Wang PH, Pan SW, Shu CC, et al. Clinical course and risk factors of mortality in Mycobacterium avium complex lung disease without initial treatment. Respir Med. 2020;171:106070. doi:10.1016/j.rmed.2020.106070
10. Daley CL, Iaccarino JM, Lange C, et al. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ ERS/ESCMID/IDSA Clinical Practice Guideline [published correction appears in Clin Infect Dis. 2020 Dec 31;71(11):3023]. Clin Infect Dis. 2020;71(4):e1-e36. doi:10.1093/cid/ciaa241
11. Mirsaeidi M, Hadid W, Ericsoussi B, Rodgers D, Sadikot RT. Non-tuberculous mycobacterial disease is common in patients with non-cystic fibrosis bronchiectasis. Int J Infect Dis. 2013;17(11):e1000-e1004. doi:10.1016/j.ijid.2013.03.018
12. Oda G, Winters MA, Pacheco SM, et al. Clusters of nontuberculous mycobacteria linked to water sources at three Veterans Affairs medical centers. Infect Control Hosp Epidemiol. 2020;41(3):320-330. doi:10.1017/ice.2019.342
13. Stanojevic S, Graham BL, Cooper BG, et al. Official ERS technical standards: Global Lung Function Initiative reference values for the carbon monoxide transfer factor for Caucasians [published correction appears in Eur Respir J. 2020 Oct 15;56(4):]. Eur Respir J. 2017;50(3):1700010. Published 2017 Sep 11. doi:10.1183/13993003.00010-2017
14. Macintyre N, Crapo RO, Viegi G, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005;26(4):720-735. doi:10.1183/09031936.05.00034905
15. Chalmers JD, Balavoine C, Castellotti PF, et al. European Respiratory Society International Congress, Madrid, 2019: nontuberculous mycobacterial pulmonary disease highlights. ERJ Open Res. 2020;6(4):00317-2020. Published 2020 Oct 19. doi:10.1183/23120541.00317-2020
16. Hamilton LA, Falkinham JO. Aerosolization of Mycobacterium avium and Mycobacterium abscessus from a household ultrasonic humidifier. J Med Microbiol. 2018;67(10):1491-1495. doi:10.1099/jmm.0.000822
17. Hannah CE, Ford BA, Chung J, Ince D, Wanat KA. Characteristics of nontuberculous mycobacterial infections at a midwestern tertiary hospital: a retrospective study of 365 patients. Open Forum Infect Dis. 2020;7(6):ofaa173. Published 2020 May 25. doi:10.1093/ofid/ofaa173
18. Rautiala S, Torvinen E, Torkko P, et al. Potentially pathogenic, slow-growing mycobacteria released into workplace air during the remediation of buildings. J Occup Environ Hyg. 2004;1(1):1-6. doi:10.1080/15459620490250008
19. Tzou CL, Dirac MA, Becker AL, et al. Association between Mycobacterium avium complex pulmonary disease and mycobacteria in home water and soil. Ann Am Thorac Soc. 2020;17(1):57-62. doi:10.1513/AnnalsATS.201812-915OC
20. Daley CL, Winthrop KL. Mycobacterium avium complex: addressing gaps in diagnosis and management. J Infect Dis. 2020;222(suppl 4):S199-S211. doi:10.1093/infdis/jiaa354 21. Kwon BS, Lee JH, Koh Y, et al. The natural history of noncavitary nodular bronchiectatic Mycobacterium avium complex lung disease. Respir Med. 2019;150:45-50. doi:10.1016/j.rmed.2019.02.007
22. Nasiri MJ, Ebrahimi G, Arefzadeh S, Zamani S, Nikpor Z, Mirsaeidi M. Antibiotic therapy success rate in pulmonary Mycobacterium avium complex: a systematic review and meta-analysis. Expert Rev Anti Infect Ther. 2020;18(3):263- 273. doi:10.1080/14787210.2020.1720650
23. Diel R, Lipman M, Hoefsloot W. High mortality in patients with Mycobacterium avium complex lung disease: a systematic review. BMC Infect Dis. 2018;18(1):206. Published 2018 May 3. doi:10.1186/s12879-018-3113-x
24. Marras TK, Prevots DR, Jamieson FB, Winthrop KL; Pulmonary MAC Outcomes Group. Opinions differ by expertise in Mycobacterium avium complex disease. Ann Am Thorac Soc. 2014;11(1):17-22. doi:10.1513/AnnalsATS.201305-136OC
25. Plotinsky RN, Talbot EA, von Reyn CF. Proposed definitions for epidemiologic and clinical studies of Mycobacterium avium complex pulmonary disease. PLoS One. 2013;8(11):e77385. Published 2013 Nov 12. doi:10.1371/journal.pone.0077385
26. Swenson C, Zerbe CS, Fennelly K. Host variability in NTM disease: implications for research needs. Front Microbiol. 2018;9:2901. Published 2018 Dec 3. doi:10.3389/fmicb.2018.02901
Telescoping Stents to Maintain a 3-Way Patency of the Airway
There are several malignant and nonmalignant conditions that can lead to central airway obstruction (CAO) resulting in lobar collapse. The clinical consequences range from significant dyspnea to respiratory failure. Airway stenting has been used to maintain patency of obstructed airways and relieve symptoms. Before lung cancer screening became more common, approximately 10% of lung cancers at presentation had evidence of CAO.1
On occasion, an endobronchial malignancy involves the right mainstem (RMS) bronchus near the orifice of the right upper lobe (RUL).2 Such strategically located lesions pose a challenge to relieve the RMS obstruction through stenting, securing airway patency into the bronchus intermedius (BI) while avoiding obstruction of the RUL bronchus. The use of endobronchial silicone stents, hybrid covered stents, as well as self-expanding metal stents (SEMS) is an established mode of relieving CAO due to malignant disease.3 We reviewed the literature for approaches that were available before and after the date of the index case reported here.
Case Presentation
A 65-year-old veteran with a history of smoking presented to a US Department of Veterans Affairs Medical Center (VAMC) in 2011, with hemoptysis of 2-week duration. Computed tomography (CT) of the chest revealed a 5.3 × 4.2 × 6.5 cm right mediastinal mass and a 3.0 × 2.8 × 3 cm right hilar mass. Flexible bronchoscopy revealed > 80% occlusion of the RMS and BI due to a medially located mass sparing the RUL orifice, which was patent (Figure 1). Airways distal to the BI were free of disease. Endobronchial biopsies revealed poorly differentiated non-small cell carcinoma of the lung. The patient was referred to the interventional pulmonary service for further airway management.
Under general anesthesia and through a size-9 endotracheal tube, piecemeal debulking of the mass using a cryoprobe was performed. Argon photocoagulation (APC) was used to control bleeding. Balloon bronchoplasty was performed next with pulmonary Boston Scientific CRE balloon at the BI and the RMS bronchus. Under fluoroscopic guidance, a 12 × 30 mm self-expanding hybrid Merit Medical AERO stent was placed distally into the BI. Next, a 14 × 30 mm AERO stent was placed proximally in the RMS bronchus with its distal end telescoped into the smaller distal stent for a distance of 3 to 4 mm at a slanted angle. The overlap was deliberately performed at the level of RUL takeoff. Forcing the distal end of the proximal larger stent into a smaller stent created mechanical stress. The angled alignment channeled this mechanical stress so that the distal end of the proximal stent flared open laterally into the RUL orifice to allow for ventilation (Figure 2). On follow-up 6 months later, all 3 airways remained patent with stents in place (Figure 3).
The patient returned to the VAMC and underwent chemotherapy with carboplatin and paclitaxel cycles that were completed in May 2012, as well as completing 6300 centigray (cGy) of radiation to the area. This led to regression of the tumor permitting removal of the proximal stent in October 2012. Unfortunately, upon follow-up in July 2013, a hypermetabolic lesion in the right upper posterior chest was noted to be eroding the third rib. Biopsy proved it to be poorly differentiated non-small cell lung cancer. Palliative external beam radiation was used to treat this lesion with a total of 3780 cGy completed by the end of August 2013.
Sadly, the patient was admitted later in 2013 with worsening cough and shortness of breath. Chest and abdominal CTs showed an increase in the size of the right apical mass, and mediastinal lymphadenopathy, as well as innumerable nodules in the left lung. The mass had recurred and extended distal to the stent into the lower and middle lobes. New liver nodule and lytic lesion within left ischial tuberosity, T12, L1, and S1 vertebral bodies were noted. The pulmonary service reached out to us via email and we recommended either additional chemoradiotherapy or palliative care. At that point the tumor was widespread and resistant to therapy. It extended beyond the central airways making airway debulking futile. Stents are palliative in nature and we believed that the initial stenting allowed the patient to get chemoradiation by improving functional status through preventing collapse of the right lung. As a result, the patient had about 19 months of a remission period with quality of life. The patient ultimately died under the care of palliative care in inpatient hospice setting.
Literature Review
A literature review revealed multiple approaches to preserving a 3-way patent airway at the takeoff of the RUL (Table). One approach to alleviating such an obstruction favors placing a straight silicone stent from the RMS into the BI, closing off the orifice of the RUL (Figure 4A).4 However, this entails sacrificing ventilation of the RUL. An alternative suggested by Peled and colleagues was carried out successfully in 3 patients. After placing a stent to relieve the obstruction, a Nd:YAG laser is used to create a window in the stent in proximity to the RUL orifice, which allows preservation or ventilations to the RUL (Figure 4B).5
A third effective approach utilizes silicone Y stents, which are usually employed for relief of obstruction at the level of the main carina.6,7 Instead of deploying them at the main carina, they would be deployed at the secondary carina, which the RUL makes with the BI, often with customized cutting for adjustment of the stent limbs to the appropriate size of the RUL and BI (Figure 4C). This approach has been successfully used to maintain RUL ventilation.2
A fourth technique involves using an Oki stent, a dedicated bifurcated silicone stent, which was first described in 2013. It is designed for the RMS bronchus around the RUL and BI bifurcation, enabling the stent to maintain airway patency in the right lung without affecting the trachea and carina (Figure 4D). The arm located in the RUL prevents migration.8 A fifth technique involves deploying a precisely selected Oki stent specially modified based on a printed 3-dimensional (3D) model of the airways after computer-aided simulation.9A sixth technique employs de novo custom printing stents based on 3D models of the tracheobronchial tree constructed based on CT imaging. This approach creates more accurately fitting stents.1
Discussion
The RUL contributes roughly 5 to 10% of the total oxygenation capacity of the lung.10 In patients with lung cancer and limited pulmonary reserve, preserving ventilation to the RUL can be clinically important. The chosen method to relieve endobronchial obstruction depends on several variables, including expertise, ability of the patient to undergo general anesthesia for rigid or flexible bronchoscopy, stent availability, and airway anatomy.
This case illustrates a new method to deal with lesions close to the RUL orifice. This maneuver may not be possible with all types of stents. AERO stents are fully covered (Figure 4E). In contrast, stents that are uncovered at both distal ends, such as a Boston Scientific Ultraflex stent, may not be adequate for such a maneuver. Intercalating uncovered ends of SEMS may allow for tumor in-growth through the uncovered metal mesh near the RUL orifice and may paradoxically compromise both the RUL and BI. The diameter of AERO stents is slightly larger at its ends.11 This helps prevent migration, which in this case maintained the crucial overlap of the stents. On the other hand, use of AERO stents may be associated with a higher risk of infection.12 Precise measurements of the airway diameter are essential given the difference in internal and external stent diameter with silicone stents.
Silicone stents migrate more readily than SEMS and may not be well suited for the procedure we performed. In our case, we wished to maintain ventilation for the RUL; hence, we elected not to bypass it with a silicone stent. We did not have access to a YAG. Moreover, laser carries more energy than APC. Nd:YAG laser has been reported to cause airway fire when used with silicone stents.13 Several authors have reported the use of silicone Y stents at the primary or secondary carina to preserve luminal patency.6,7 Airway anatomy and the angle of the Y may require modification of these stents prior to their use. Cutting stents may compromise their integrity. The bifurcating limb prevents migration which can be a significant concern with the tubular silicone stents. An important consideration for patients in advanced stages of malignancy is that placement of such stent requires undergoing general anesthesia and rigid bronchoscopy, unlike with AERO and metal stents that can be deployed with fiberoptic bronchoscopy under moderate sedation. As such, we did not elect to use a silicone Y stent. Accumulation of secretions or formation of granulation tissue at the orifices can result in recurrence of obstruction.14
Advances in 3D printing seem to be the future of customized airway stenting. This could help clinicians overcome the challenges of improperly sized stents and distorted airway anatomy. Cases have reported successful use of 3D-printed patient-specific airway prostheses.15,16 However, their use is not common practice, as there is a limited amount of materials that are flexible, biocompatible, and approved by the US Food and Drug Administration (FDA) for medical use. Infection control is another layer of consideration in such stents. Standardization of materials and regulation of personalized devices and their cleansing protocols is neccesary.17 At the time of this case, Oki stents and 3D printing were not available in the market. This report provides a viable alternative to use AERO stents for this maneuver.
Conclusions
Patients presenting with malignant CAO near the RUL require a personalized approach to treatment, considering their overall health, functional status, nature and location of CAO, and degree of symptoms. Once a decision is made to stent the airway, careful assessment of airway anatomy, delineation of obstruction, available expertise, and types of stents available needs to be made to preserve ventilation to the nondiseased RUL. Airway stents are expensive and need to be used wisely for palliation and allowing for a quality life while the patient receives more definitive targeted therapy.
Acknowledgments
The authors would like to gratefully acknowledge Dr Jenny Kim, who referred the patient to the interventional service and helped obtain consent for publishing the case.
1. Criner GJ, Eberhardt R, Fernandez-Bussy S, et al. Interventional bronchoscopy. Am J Respir Crit Care Med. 2020;202(1):29-50. doi:10.1164/rccm.201907-1292SO
2. Oki M, Saka H, Kitagawa C, Kogure Y. Silicone y-stent placement on the carina between bronchus to the right upper lobe and bronchus intermedius. Ann Thorac Surg. 2009;87(3):971-974. doi:10.1016/j.athoracsur.2008.06.049
3. Ernst A, Feller-Kopman D, Becker HD, Mehta AC. Central airway obstruction. Am J Respir Crit Care Med. 2004;169(12):1278-1297. doi:10.1164/rccm.200210-1181SO
4. Liu Y-H, Wu Y-C, Hsieh M-J, Ko P-J. Straight bronchial stent placement across the right upper lobe bronchus: A simple alternative for the management of airway obstruction around the carina and right main bronchus. J Thorac Cardiovasc Surg. 2011;141(1):303-305.e1.doi:10.1016/j.jtcvs.2010.06.015
5. Peled N, Shitrit D, Bendayan D, Kramer MR. Right upper lobe ‘window’ in right main bronchus stenting. Eur J Cardiothorac Surg. 2006;30(4):680-682. doi:10.1016/j.ejcts.2006.07.020
6. Dumon J-F, Dumon MC. Dumon-Novatech Y-stents: a four-year experience with 50 tracheobronchial tumors involving the carina. J Bronchol. 2000;7(1):26-32 doi:10.1097/00128594-200007000-00005
7. Dutau H, Toutblanc B, Lamb C, Seijo L. Use of the Dumon Y-stent in the management of malignant disease involving the carina: a retrospective review of 86 patients. Chest. 2004;126(3):951-958. doi:10.1378/chest.126.3.951
8. Dalar L, Abul Y. Safety and efficacy of Oki stenting used to treat obstructions in the right mainstem bronchus. J Bronchol Interv Pulmonol. 2018;25(3):212-217. doi:10.1097/LBR.0000000000000486
9. Guibert N, Moreno B, Plat G, Didier A, Mazieres J, Hermant C. Stenting of complex malignant central-airway obstruction guided by a three-dimensional printed model of the airways. Ann Thorac Surg. 2017;103(4):e357-e359. doi:10.1016/j.athoracsur.2016.09.082
10. Win T, Tasker AD, Groves AM, et al. Ventilation-perfusion scintigraphy to predict postoperative pulmonary function in lung cancer patients undergoing pneumonectomy. AJR Am J Roentgenol. 2006;187(5):1260-1265. doi:10.2214/AJR.04.1973
11. Mehta AC. AERO self-expanding hybrid stent for airway stenosis. Expert Rev Med Devices. 2008;5(5):553-557. doi:10.1586/17434440.5.5.553
12. Ost DE, Shah AM, Lei X, et al. Respiratory infections increase the risk of granulation tissue formation following airway stenting in patients with malignant airway obstruction. Chest. 2012;141(6):1473-1481. doi:10.1378/chest.11-2005
13. Scherer TA. Nd-YAG laser ignition of silicone endobronchial stents. Chest. 2000;117(5):1449-1454. doi:10.1378/chest.117.5.1449
14. Folch E, Keyes C. Airway stents. Ann Cardiothorac Surg. 2018;7(2):273-283. doi:10.21037/acs.2018.03.08
15. Cheng GZ, Folch E, Brik R, et al. Three-dimensional modeled T-tube design and insertion in a patient with tracheal dehiscence. Chest. 2015;148(4):e106-e108. doi:10.1378/chest.15-0240
16. Tam MD, Laycock SD, Jayne D, Babar J, Noble B. 3-D printouts of the tracheobronchial tree generated from CT images as an aid to management in a case of tracheobronchial chondromalacia caused by relapsing polychondritis. J Radiol Case Rep. 2013;7(8):34-43. Published 2013 Aug 1. doi:10.3941/jrcr.v7i8.1390
17. Alraiyes AH, Avasarala SK, Machuzak MS, Gildea TR. 3D printing for airway disease. AME Med J. 2019;4:14. doi:10.21037/amj.2019.01.05
There are several malignant and nonmalignant conditions that can lead to central airway obstruction (CAO) resulting in lobar collapse. The clinical consequences range from significant dyspnea to respiratory failure. Airway stenting has been used to maintain patency of obstructed airways and relieve symptoms. Before lung cancer screening became more common, approximately 10% of lung cancers at presentation had evidence of CAO.1
On occasion, an endobronchial malignancy involves the right mainstem (RMS) bronchus near the orifice of the right upper lobe (RUL).2 Such strategically located lesions pose a challenge to relieve the RMS obstruction through stenting, securing airway patency into the bronchus intermedius (BI) while avoiding obstruction of the RUL bronchus. The use of endobronchial silicone stents, hybrid covered stents, as well as self-expanding metal stents (SEMS) is an established mode of relieving CAO due to malignant disease.3 We reviewed the literature for approaches that were available before and after the date of the index case reported here.
Case Presentation
A 65-year-old veteran with a history of smoking presented to a US Department of Veterans Affairs Medical Center (VAMC) in 2011, with hemoptysis of 2-week duration. Computed tomography (CT) of the chest revealed a 5.3 × 4.2 × 6.5 cm right mediastinal mass and a 3.0 × 2.8 × 3 cm right hilar mass. Flexible bronchoscopy revealed > 80% occlusion of the RMS and BI due to a medially located mass sparing the RUL orifice, which was patent (Figure 1). Airways distal to the BI were free of disease. Endobronchial biopsies revealed poorly differentiated non-small cell carcinoma of the lung. The patient was referred to the interventional pulmonary service for further airway management.
Under general anesthesia and through a size-9 endotracheal tube, piecemeal debulking of the mass using a cryoprobe was performed. Argon photocoagulation (APC) was used to control bleeding. Balloon bronchoplasty was performed next with pulmonary Boston Scientific CRE balloon at the BI and the RMS bronchus. Under fluoroscopic guidance, a 12 × 30 mm self-expanding hybrid Merit Medical AERO stent was placed distally into the BI. Next, a 14 × 30 mm AERO stent was placed proximally in the RMS bronchus with its distal end telescoped into the smaller distal stent for a distance of 3 to 4 mm at a slanted angle. The overlap was deliberately performed at the level of RUL takeoff. Forcing the distal end of the proximal larger stent into a smaller stent created mechanical stress. The angled alignment channeled this mechanical stress so that the distal end of the proximal stent flared open laterally into the RUL orifice to allow for ventilation (Figure 2). On follow-up 6 months later, all 3 airways remained patent with stents in place (Figure 3).
The patient returned to the VAMC and underwent chemotherapy with carboplatin and paclitaxel cycles that were completed in May 2012, as well as completing 6300 centigray (cGy) of radiation to the area. This led to regression of the tumor permitting removal of the proximal stent in October 2012. Unfortunately, upon follow-up in July 2013, a hypermetabolic lesion in the right upper posterior chest was noted to be eroding the third rib. Biopsy proved it to be poorly differentiated non-small cell lung cancer. Palliative external beam radiation was used to treat this lesion with a total of 3780 cGy completed by the end of August 2013.
Sadly, the patient was admitted later in 2013 with worsening cough and shortness of breath. Chest and abdominal CTs showed an increase in the size of the right apical mass, and mediastinal lymphadenopathy, as well as innumerable nodules in the left lung. The mass had recurred and extended distal to the stent into the lower and middle lobes. New liver nodule and lytic lesion within left ischial tuberosity, T12, L1, and S1 vertebral bodies were noted. The pulmonary service reached out to us via email and we recommended either additional chemoradiotherapy or palliative care. At that point the tumor was widespread and resistant to therapy. It extended beyond the central airways making airway debulking futile. Stents are palliative in nature and we believed that the initial stenting allowed the patient to get chemoradiation by improving functional status through preventing collapse of the right lung. As a result, the patient had about 19 months of a remission period with quality of life. The patient ultimately died under the care of palliative care in inpatient hospice setting.
Literature Review
A literature review revealed multiple approaches to preserving a 3-way patent airway at the takeoff of the RUL (Table). One approach to alleviating such an obstruction favors placing a straight silicone stent from the RMS into the BI, closing off the orifice of the RUL (Figure 4A).4 However, this entails sacrificing ventilation of the RUL. An alternative suggested by Peled and colleagues was carried out successfully in 3 patients. After placing a stent to relieve the obstruction, a Nd:YAG laser is used to create a window in the stent in proximity to the RUL orifice, which allows preservation or ventilations to the RUL (Figure 4B).5
A third effective approach utilizes silicone Y stents, which are usually employed for relief of obstruction at the level of the main carina.6,7 Instead of deploying them at the main carina, they would be deployed at the secondary carina, which the RUL makes with the BI, often with customized cutting for adjustment of the stent limbs to the appropriate size of the RUL and BI (Figure 4C). This approach has been successfully used to maintain RUL ventilation.2
A fourth technique involves using an Oki stent, a dedicated bifurcated silicone stent, which was first described in 2013. It is designed for the RMS bronchus around the RUL and BI bifurcation, enabling the stent to maintain airway patency in the right lung without affecting the trachea and carina (Figure 4D). The arm located in the RUL prevents migration.8 A fifth technique involves deploying a precisely selected Oki stent specially modified based on a printed 3-dimensional (3D) model of the airways after computer-aided simulation.9A sixth technique employs de novo custom printing stents based on 3D models of the tracheobronchial tree constructed based on CT imaging. This approach creates more accurately fitting stents.1
Discussion
The RUL contributes roughly 5 to 10% of the total oxygenation capacity of the lung.10 In patients with lung cancer and limited pulmonary reserve, preserving ventilation to the RUL can be clinically important. The chosen method to relieve endobronchial obstruction depends on several variables, including expertise, ability of the patient to undergo general anesthesia for rigid or flexible bronchoscopy, stent availability, and airway anatomy.
This case illustrates a new method to deal with lesions close to the RUL orifice. This maneuver may not be possible with all types of stents. AERO stents are fully covered (Figure 4E). In contrast, stents that are uncovered at both distal ends, such as a Boston Scientific Ultraflex stent, may not be adequate for such a maneuver. Intercalating uncovered ends of SEMS may allow for tumor in-growth through the uncovered metal mesh near the RUL orifice and may paradoxically compromise both the RUL and BI. The diameter of AERO stents is slightly larger at its ends.11 This helps prevent migration, which in this case maintained the crucial overlap of the stents. On the other hand, use of AERO stents may be associated with a higher risk of infection.12 Precise measurements of the airway diameter are essential given the difference in internal and external stent diameter with silicone stents.
Silicone stents migrate more readily than SEMS and may not be well suited for the procedure we performed. In our case, we wished to maintain ventilation for the RUL; hence, we elected not to bypass it with a silicone stent. We did not have access to a YAG. Moreover, laser carries more energy than APC. Nd:YAG laser has been reported to cause airway fire when used with silicone stents.13 Several authors have reported the use of silicone Y stents at the primary or secondary carina to preserve luminal patency.6,7 Airway anatomy and the angle of the Y may require modification of these stents prior to their use. Cutting stents may compromise their integrity. The bifurcating limb prevents migration which can be a significant concern with the tubular silicone stents. An important consideration for patients in advanced stages of malignancy is that placement of such stent requires undergoing general anesthesia and rigid bronchoscopy, unlike with AERO and metal stents that can be deployed with fiberoptic bronchoscopy under moderate sedation. As such, we did not elect to use a silicone Y stent. Accumulation of secretions or formation of granulation tissue at the orifices can result in recurrence of obstruction.14
Advances in 3D printing seem to be the future of customized airway stenting. This could help clinicians overcome the challenges of improperly sized stents and distorted airway anatomy. Cases have reported successful use of 3D-printed patient-specific airway prostheses.15,16 However, their use is not common practice, as there is a limited amount of materials that are flexible, biocompatible, and approved by the US Food and Drug Administration (FDA) for medical use. Infection control is another layer of consideration in such stents. Standardization of materials and regulation of personalized devices and their cleansing protocols is neccesary.17 At the time of this case, Oki stents and 3D printing were not available in the market. This report provides a viable alternative to use AERO stents for this maneuver.
Conclusions
Patients presenting with malignant CAO near the RUL require a personalized approach to treatment, considering their overall health, functional status, nature and location of CAO, and degree of symptoms. Once a decision is made to stent the airway, careful assessment of airway anatomy, delineation of obstruction, available expertise, and types of stents available needs to be made to preserve ventilation to the nondiseased RUL. Airway stents are expensive and need to be used wisely for palliation and allowing for a quality life while the patient receives more definitive targeted therapy.
Acknowledgments
The authors would like to gratefully acknowledge Dr Jenny Kim, who referred the patient to the interventional service and helped obtain consent for publishing the case.
There are several malignant and nonmalignant conditions that can lead to central airway obstruction (CAO) resulting in lobar collapse. The clinical consequences range from significant dyspnea to respiratory failure. Airway stenting has been used to maintain patency of obstructed airways and relieve symptoms. Before lung cancer screening became more common, approximately 10% of lung cancers at presentation had evidence of CAO.1
On occasion, an endobronchial malignancy involves the right mainstem (RMS) bronchus near the orifice of the right upper lobe (RUL).2 Such strategically located lesions pose a challenge to relieve the RMS obstruction through stenting, securing airway patency into the bronchus intermedius (BI) while avoiding obstruction of the RUL bronchus. The use of endobronchial silicone stents, hybrid covered stents, as well as self-expanding metal stents (SEMS) is an established mode of relieving CAO due to malignant disease.3 We reviewed the literature for approaches that were available before and after the date of the index case reported here.
Case Presentation
A 65-year-old veteran with a history of smoking presented to a US Department of Veterans Affairs Medical Center (VAMC) in 2011, with hemoptysis of 2-week duration. Computed tomography (CT) of the chest revealed a 5.3 × 4.2 × 6.5 cm right mediastinal mass and a 3.0 × 2.8 × 3 cm right hilar mass. Flexible bronchoscopy revealed > 80% occlusion of the RMS and BI due to a medially located mass sparing the RUL orifice, which was patent (Figure 1). Airways distal to the BI were free of disease. Endobronchial biopsies revealed poorly differentiated non-small cell carcinoma of the lung. The patient was referred to the interventional pulmonary service for further airway management.
Under general anesthesia and through a size-9 endotracheal tube, piecemeal debulking of the mass using a cryoprobe was performed. Argon photocoagulation (APC) was used to control bleeding. Balloon bronchoplasty was performed next with pulmonary Boston Scientific CRE balloon at the BI and the RMS bronchus. Under fluoroscopic guidance, a 12 × 30 mm self-expanding hybrid Merit Medical AERO stent was placed distally into the BI. Next, a 14 × 30 mm AERO stent was placed proximally in the RMS bronchus with its distal end telescoped into the smaller distal stent for a distance of 3 to 4 mm at a slanted angle. The overlap was deliberately performed at the level of RUL takeoff. Forcing the distal end of the proximal larger stent into a smaller stent created mechanical stress. The angled alignment channeled this mechanical stress so that the distal end of the proximal stent flared open laterally into the RUL orifice to allow for ventilation (Figure 2). On follow-up 6 months later, all 3 airways remained patent with stents in place (Figure 3).
The patient returned to the VAMC and underwent chemotherapy with carboplatin and paclitaxel cycles that were completed in May 2012, as well as completing 6300 centigray (cGy) of radiation to the area. This led to regression of the tumor permitting removal of the proximal stent in October 2012. Unfortunately, upon follow-up in July 2013, a hypermetabolic lesion in the right upper posterior chest was noted to be eroding the third rib. Biopsy proved it to be poorly differentiated non-small cell lung cancer. Palliative external beam radiation was used to treat this lesion with a total of 3780 cGy completed by the end of August 2013.
Sadly, the patient was admitted later in 2013 with worsening cough and shortness of breath. Chest and abdominal CTs showed an increase in the size of the right apical mass, and mediastinal lymphadenopathy, as well as innumerable nodules in the left lung. The mass had recurred and extended distal to the stent into the lower and middle lobes. New liver nodule and lytic lesion within left ischial tuberosity, T12, L1, and S1 vertebral bodies were noted. The pulmonary service reached out to us via email and we recommended either additional chemoradiotherapy or palliative care. At that point the tumor was widespread and resistant to therapy. It extended beyond the central airways making airway debulking futile. Stents are palliative in nature and we believed that the initial stenting allowed the patient to get chemoradiation by improving functional status through preventing collapse of the right lung. As a result, the patient had about 19 months of a remission period with quality of life. The patient ultimately died under the care of palliative care in inpatient hospice setting.
Literature Review
A literature review revealed multiple approaches to preserving a 3-way patent airway at the takeoff of the RUL (Table). One approach to alleviating such an obstruction favors placing a straight silicone stent from the RMS into the BI, closing off the orifice of the RUL (Figure 4A).4 However, this entails sacrificing ventilation of the RUL. An alternative suggested by Peled and colleagues was carried out successfully in 3 patients. After placing a stent to relieve the obstruction, a Nd:YAG laser is used to create a window in the stent in proximity to the RUL orifice, which allows preservation or ventilations to the RUL (Figure 4B).5
A third effective approach utilizes silicone Y stents, which are usually employed for relief of obstruction at the level of the main carina.6,7 Instead of deploying them at the main carina, they would be deployed at the secondary carina, which the RUL makes with the BI, often with customized cutting for adjustment of the stent limbs to the appropriate size of the RUL and BI (Figure 4C). This approach has been successfully used to maintain RUL ventilation.2
A fourth technique involves using an Oki stent, a dedicated bifurcated silicone stent, which was first described in 2013. It is designed for the RMS bronchus around the RUL and BI bifurcation, enabling the stent to maintain airway patency in the right lung without affecting the trachea and carina (Figure 4D). The arm located in the RUL prevents migration.8 A fifth technique involves deploying a precisely selected Oki stent specially modified based on a printed 3-dimensional (3D) model of the airways after computer-aided simulation.9A sixth technique employs de novo custom printing stents based on 3D models of the tracheobronchial tree constructed based on CT imaging. This approach creates more accurately fitting stents.1
Discussion
The RUL contributes roughly 5 to 10% of the total oxygenation capacity of the lung.10 In patients with lung cancer and limited pulmonary reserve, preserving ventilation to the RUL can be clinically important. The chosen method to relieve endobronchial obstruction depends on several variables, including expertise, ability of the patient to undergo general anesthesia for rigid or flexible bronchoscopy, stent availability, and airway anatomy.
This case illustrates a new method to deal with lesions close to the RUL orifice. This maneuver may not be possible with all types of stents. AERO stents are fully covered (Figure 4E). In contrast, stents that are uncovered at both distal ends, such as a Boston Scientific Ultraflex stent, may not be adequate for such a maneuver. Intercalating uncovered ends of SEMS may allow for tumor in-growth through the uncovered metal mesh near the RUL orifice and may paradoxically compromise both the RUL and BI. The diameter of AERO stents is slightly larger at its ends.11 This helps prevent migration, which in this case maintained the crucial overlap of the stents. On the other hand, use of AERO stents may be associated with a higher risk of infection.12 Precise measurements of the airway diameter are essential given the difference in internal and external stent diameter with silicone stents.
Silicone stents migrate more readily than SEMS and may not be well suited for the procedure we performed. In our case, we wished to maintain ventilation for the RUL; hence, we elected not to bypass it with a silicone stent. We did not have access to a YAG. Moreover, laser carries more energy than APC. Nd:YAG laser has been reported to cause airway fire when used with silicone stents.13 Several authors have reported the use of silicone Y stents at the primary or secondary carina to preserve luminal patency.6,7 Airway anatomy and the angle of the Y may require modification of these stents prior to their use. Cutting stents may compromise their integrity. The bifurcating limb prevents migration which can be a significant concern with the tubular silicone stents. An important consideration for patients in advanced stages of malignancy is that placement of such stent requires undergoing general anesthesia and rigid bronchoscopy, unlike with AERO and metal stents that can be deployed with fiberoptic bronchoscopy under moderate sedation. As such, we did not elect to use a silicone Y stent. Accumulation of secretions or formation of granulation tissue at the orifices can result in recurrence of obstruction.14
Advances in 3D printing seem to be the future of customized airway stenting. This could help clinicians overcome the challenges of improperly sized stents and distorted airway anatomy. Cases have reported successful use of 3D-printed patient-specific airway prostheses.15,16 However, their use is not common practice, as there is a limited amount of materials that are flexible, biocompatible, and approved by the US Food and Drug Administration (FDA) for medical use. Infection control is another layer of consideration in such stents. Standardization of materials and regulation of personalized devices and their cleansing protocols is neccesary.17 At the time of this case, Oki stents and 3D printing were not available in the market. This report provides a viable alternative to use AERO stents for this maneuver.
Conclusions
Patients presenting with malignant CAO near the RUL require a personalized approach to treatment, considering their overall health, functional status, nature and location of CAO, and degree of symptoms. Once a decision is made to stent the airway, careful assessment of airway anatomy, delineation of obstruction, available expertise, and types of stents available needs to be made to preserve ventilation to the nondiseased RUL. Airway stents are expensive and need to be used wisely for palliation and allowing for a quality life while the patient receives more definitive targeted therapy.
Acknowledgments
The authors would like to gratefully acknowledge Dr Jenny Kim, who referred the patient to the interventional service and helped obtain consent for publishing the case.
1. Criner GJ, Eberhardt R, Fernandez-Bussy S, et al. Interventional bronchoscopy. Am J Respir Crit Care Med. 2020;202(1):29-50. doi:10.1164/rccm.201907-1292SO
2. Oki M, Saka H, Kitagawa C, Kogure Y. Silicone y-stent placement on the carina between bronchus to the right upper lobe and bronchus intermedius. Ann Thorac Surg. 2009;87(3):971-974. doi:10.1016/j.athoracsur.2008.06.049
3. Ernst A, Feller-Kopman D, Becker HD, Mehta AC. Central airway obstruction. Am J Respir Crit Care Med. 2004;169(12):1278-1297. doi:10.1164/rccm.200210-1181SO
4. Liu Y-H, Wu Y-C, Hsieh M-J, Ko P-J. Straight bronchial stent placement across the right upper lobe bronchus: A simple alternative for the management of airway obstruction around the carina and right main bronchus. J Thorac Cardiovasc Surg. 2011;141(1):303-305.e1.doi:10.1016/j.jtcvs.2010.06.015
5. Peled N, Shitrit D, Bendayan D, Kramer MR. Right upper lobe ‘window’ in right main bronchus stenting. Eur J Cardiothorac Surg. 2006;30(4):680-682. doi:10.1016/j.ejcts.2006.07.020
6. Dumon J-F, Dumon MC. Dumon-Novatech Y-stents: a four-year experience with 50 tracheobronchial tumors involving the carina. J Bronchol. 2000;7(1):26-32 doi:10.1097/00128594-200007000-00005
7. Dutau H, Toutblanc B, Lamb C, Seijo L. Use of the Dumon Y-stent in the management of malignant disease involving the carina: a retrospective review of 86 patients. Chest. 2004;126(3):951-958. doi:10.1378/chest.126.3.951
8. Dalar L, Abul Y. Safety and efficacy of Oki stenting used to treat obstructions in the right mainstem bronchus. J Bronchol Interv Pulmonol. 2018;25(3):212-217. doi:10.1097/LBR.0000000000000486
9. Guibert N, Moreno B, Plat G, Didier A, Mazieres J, Hermant C. Stenting of complex malignant central-airway obstruction guided by a three-dimensional printed model of the airways. Ann Thorac Surg. 2017;103(4):e357-e359. doi:10.1016/j.athoracsur.2016.09.082
10. Win T, Tasker AD, Groves AM, et al. Ventilation-perfusion scintigraphy to predict postoperative pulmonary function in lung cancer patients undergoing pneumonectomy. AJR Am J Roentgenol. 2006;187(5):1260-1265. doi:10.2214/AJR.04.1973
11. Mehta AC. AERO self-expanding hybrid stent for airway stenosis. Expert Rev Med Devices. 2008;5(5):553-557. doi:10.1586/17434440.5.5.553
12. Ost DE, Shah AM, Lei X, et al. Respiratory infections increase the risk of granulation tissue formation following airway stenting in patients with malignant airway obstruction. Chest. 2012;141(6):1473-1481. doi:10.1378/chest.11-2005
13. Scherer TA. Nd-YAG laser ignition of silicone endobronchial stents. Chest. 2000;117(5):1449-1454. doi:10.1378/chest.117.5.1449
14. Folch E, Keyes C. Airway stents. Ann Cardiothorac Surg. 2018;7(2):273-283. doi:10.21037/acs.2018.03.08
15. Cheng GZ, Folch E, Brik R, et al. Three-dimensional modeled T-tube design and insertion in a patient with tracheal dehiscence. Chest. 2015;148(4):e106-e108. doi:10.1378/chest.15-0240
16. Tam MD, Laycock SD, Jayne D, Babar J, Noble B. 3-D printouts of the tracheobronchial tree generated from CT images as an aid to management in a case of tracheobronchial chondromalacia caused by relapsing polychondritis. J Radiol Case Rep. 2013;7(8):34-43. Published 2013 Aug 1. doi:10.3941/jrcr.v7i8.1390
17. Alraiyes AH, Avasarala SK, Machuzak MS, Gildea TR. 3D printing for airway disease. AME Med J. 2019;4:14. doi:10.21037/amj.2019.01.05
1. Criner GJ, Eberhardt R, Fernandez-Bussy S, et al. Interventional bronchoscopy. Am J Respir Crit Care Med. 2020;202(1):29-50. doi:10.1164/rccm.201907-1292SO
2. Oki M, Saka H, Kitagawa C, Kogure Y. Silicone y-stent placement on the carina between bronchus to the right upper lobe and bronchus intermedius. Ann Thorac Surg. 2009;87(3):971-974. doi:10.1016/j.athoracsur.2008.06.049
3. Ernst A, Feller-Kopman D, Becker HD, Mehta AC. Central airway obstruction. Am J Respir Crit Care Med. 2004;169(12):1278-1297. doi:10.1164/rccm.200210-1181SO
4. Liu Y-H, Wu Y-C, Hsieh M-J, Ko P-J. Straight bronchial stent placement across the right upper lobe bronchus: A simple alternative for the management of airway obstruction around the carina and right main bronchus. J Thorac Cardiovasc Surg. 2011;141(1):303-305.e1.doi:10.1016/j.jtcvs.2010.06.015
5. Peled N, Shitrit D, Bendayan D, Kramer MR. Right upper lobe ‘window’ in right main bronchus stenting. Eur J Cardiothorac Surg. 2006;30(4):680-682. doi:10.1016/j.ejcts.2006.07.020
6. Dumon J-F, Dumon MC. Dumon-Novatech Y-stents: a four-year experience with 50 tracheobronchial tumors involving the carina. J Bronchol. 2000;7(1):26-32 doi:10.1097/00128594-200007000-00005
7. Dutau H, Toutblanc B, Lamb C, Seijo L. Use of the Dumon Y-stent in the management of malignant disease involving the carina: a retrospective review of 86 patients. Chest. 2004;126(3):951-958. doi:10.1378/chest.126.3.951
8. Dalar L, Abul Y. Safety and efficacy of Oki stenting used to treat obstructions in the right mainstem bronchus. J Bronchol Interv Pulmonol. 2018;25(3):212-217. doi:10.1097/LBR.0000000000000486
9. Guibert N, Moreno B, Plat G, Didier A, Mazieres J, Hermant C. Stenting of complex malignant central-airway obstruction guided by a three-dimensional printed model of the airways. Ann Thorac Surg. 2017;103(4):e357-e359. doi:10.1016/j.athoracsur.2016.09.082
10. Win T, Tasker AD, Groves AM, et al. Ventilation-perfusion scintigraphy to predict postoperative pulmonary function in lung cancer patients undergoing pneumonectomy. AJR Am J Roentgenol. 2006;187(5):1260-1265. doi:10.2214/AJR.04.1973
11. Mehta AC. AERO self-expanding hybrid stent for airway stenosis. Expert Rev Med Devices. 2008;5(5):553-557. doi:10.1586/17434440.5.5.553
12. Ost DE, Shah AM, Lei X, et al. Respiratory infections increase the risk of granulation tissue formation following airway stenting in patients with malignant airway obstruction. Chest. 2012;141(6):1473-1481. doi:10.1378/chest.11-2005
13. Scherer TA. Nd-YAG laser ignition of silicone endobronchial stents. Chest. 2000;117(5):1449-1454. doi:10.1378/chest.117.5.1449
14. Folch E, Keyes C. Airway stents. Ann Cardiothorac Surg. 2018;7(2):273-283. doi:10.21037/acs.2018.03.08
15. Cheng GZ, Folch E, Brik R, et al. Three-dimensional modeled T-tube design and insertion in a patient with tracheal dehiscence. Chest. 2015;148(4):e106-e108. doi:10.1378/chest.15-0240
16. Tam MD, Laycock SD, Jayne D, Babar J, Noble B. 3-D printouts of the tracheobronchial tree generated from CT images as an aid to management in a case of tracheobronchial chondromalacia caused by relapsing polychondritis. J Radiol Case Rep. 2013;7(8):34-43. Published 2013 Aug 1. doi:10.3941/jrcr.v7i8.1390
17. Alraiyes AH, Avasarala SK, Machuzak MS, Gildea TR. 3D printing for airway disease. AME Med J. 2019;4:14. doi:10.21037/amj.2019.01.05
Honoring Dr. Paul Farmer: Dr. Serena Koenig shares her memories of working with him
Infectious disease specialist and humanitarian, Paul Edward Farmer, MD, PhD, who cofounded Partners In Health, died suddenly on Feb. 21. To celebrate his life, this news organization interviewed Serena Koenig, MD, MPH, who met Dr. Farmer when she was an internal medicine resident at Brigham and Women’s Hospital. Dr. Koenig had worked closely with Dr. Farmer ever since they met.
Q. Can you please share one of your best memories of Dr. Farmer?
Dr. Serena Koenig: Paul and some other incredible colleagues at Partners IN Health (PIH) had started the HIV Equity Initiative, which was one of the first programs in the world to provide free, comprehensive treatment for HIV. This was at the time when millions of people in Africa were dying of HIV and many experts said it was not feasible to treat HIV in a poor country, because it was too complicated and expensive. Paul took me on some home visits with patients who had what he called the Lazarus effect, coming back from death’s door from advanced AIDS to vigorous health on antiretroviral therapy. I had just started working in Haiti with Paul and PIH, and I felt the enormous magnitude of what he was doing.
Q. What aspects of him and his work do you find most admirable?
Dr. Koenig: I most admired Paul’s humanity, his belief that every person matters and has the right to high-quality health care, and his vision of global health equity.
He said: “The idea that some lives matter less is the root of all that is wrong with the world.” Paul lived this philosophy. He has spoken extensively about harms of socialization for scarcity on behalf of those who are poor, leading policy makers to decisions regarding the feasibility of treating some diseases, but not others.
He said in an interview with the Harvard Gazette in 2018: “The most compelling thing to fight socialization for scarcity on behalf of others is health system strengthening. Health systems that integrate prevention and quality care.”
A few weeks ago, I asked him his thoughts about the high-level resources we have invested in some patients who have needed specialty care over the years, and he said: “No way that we should waste all of our emotional energy responding only to those constant, nagging critics that it’s not cost effective, not feasible, not sustainable, not even prudent. Because you know what they would have done if it was their child or family member.”
Q. When did you first meet Dr. Farmer, and what inspired you to work with him?
Dr. Koenig: When I was an internal medicine resident at the Brigham, Paul and I bonded over the care of one of my clinic patients who I followed very closely, and who was admitted to his inpatient service.
Like everyone else who has worked with Paul, I was touched by his kindness and warmth.
A couple of years later, he asked me to help him raise money to bring a young man named Wilnot from Haiti to the Brigham for an aortic valve replacement. After we raised the money, he asked me to go to Haiti to help Wilnot get his medical visa and to escort him to Boston.
That short trip to Haiti had an enormous impact on my life. I was shattered to see the poverty that the people of Haiti were enduring – and in a country a short plane flight from Miami.
Shortly after this, Paul asked me to help him find treatment for another patient, a young boy named John, who presented with neck masses that were later diagnosed as nasopharyngeal carcinoma.
It took us some time to make the diagnosis and then to arrange free care at Mass General.
When I returned to Haiti with two PIH colleagues to help John get a visa and escort him back to Boston, we found that John’s condition was much worse. We ended up medically evacuating him to Boston, because he was too sick for a commercial flight.
Tracy Kidder wrote about this heartbreaking experience in the book “Mountains Beyond Mountains.”
Throughout all of these experiences, I was deeply impressed with Paul’s commitment to do whatever it took to provide the best care for patients, as if they were members of his own family. He said “Tout Moun Se Moun” (Haitian Creole for “every person is a person”), and I could tell that he meant it.
Q. How did you collaborate with him professionally?
Dr. Koenig: I spent the first few years after residency working with Paul and Partners In Health. Initially, I served as a liaison between PIH in Haiti and the Brigham, bringing several more patients to Boston for care, and arranging specialty surgical trips to Haiti.
Later, when HIV funding became available from the Global Fund for HIV, Tuberculosis, and Malaria, I moved to rural Haiti to provide treatment for patients with HIV and/or TB at one of the first PIH expansion sites. We treated many patients with advanced stages of HIV and/or TB, and many of them recovered remarkably quickly with antiretroviral therapy.
When I returned to Boston to complete an infectious disease fellowship I switched my focus to conducting clinical research to improve HIV and TB treatment outcomes. Paul emailed his mentor and friend, Jean “Bill” Pape, the director of a Haitian NGO called GHESKIO (Haitian Group for the Study of Kaposi’s Sarcoma and Opportunistic Infections), which is an internationally celebrated center of excellence in HIV-related research and clinical care, to ask if I could collaborate with them.
Ever since that time, I have been based between the Brigham’s division of global health equity, which was led by Paul, and GHESKIO.
Paul was very supportive of our research, which aims to improve health service delivery and treatment regimens for HIV and TB.
Q. What lessons do you think other physicians can learn from him?
Dr. Koenig: As Joia Mukherjee, chief medical officer of Partners In Health, has said, Paul left us a roadmap. He wrote many books, and he was very eloquent in expressing his philosophy about equity and justice in numerous interviews. This is relevant not only for international sites, but in the United States as well, with our major disparities in health outcomes by race, geography, and socioeconomic status.
No one will be able to replace Paul, but he left us with a vision of what is achievable.
Dr. Koenig is associate physician, Brigham and Women’s Hospital, Boston, with faculty appointments in the divisions of global health equity and infectious diseases. She is also associate professor at Harvard Medical School.
Infectious disease specialist and humanitarian, Paul Edward Farmer, MD, PhD, who cofounded Partners In Health, died suddenly on Feb. 21. To celebrate his life, this news organization interviewed Serena Koenig, MD, MPH, who met Dr. Farmer when she was an internal medicine resident at Brigham and Women’s Hospital. Dr. Koenig had worked closely with Dr. Farmer ever since they met.
Q. Can you please share one of your best memories of Dr. Farmer?
Dr. Serena Koenig: Paul and some other incredible colleagues at Partners IN Health (PIH) had started the HIV Equity Initiative, which was one of the first programs in the world to provide free, comprehensive treatment for HIV. This was at the time when millions of people in Africa were dying of HIV and many experts said it was not feasible to treat HIV in a poor country, because it was too complicated and expensive. Paul took me on some home visits with patients who had what he called the Lazarus effect, coming back from death’s door from advanced AIDS to vigorous health on antiretroviral therapy. I had just started working in Haiti with Paul and PIH, and I felt the enormous magnitude of what he was doing.
Q. What aspects of him and his work do you find most admirable?
Dr. Koenig: I most admired Paul’s humanity, his belief that every person matters and has the right to high-quality health care, and his vision of global health equity.
He said: “The idea that some lives matter less is the root of all that is wrong with the world.” Paul lived this philosophy. He has spoken extensively about harms of socialization for scarcity on behalf of those who are poor, leading policy makers to decisions regarding the feasibility of treating some diseases, but not others.
He said in an interview with the Harvard Gazette in 2018: “The most compelling thing to fight socialization for scarcity on behalf of others is health system strengthening. Health systems that integrate prevention and quality care.”
A few weeks ago, I asked him his thoughts about the high-level resources we have invested in some patients who have needed specialty care over the years, and he said: “No way that we should waste all of our emotional energy responding only to those constant, nagging critics that it’s not cost effective, not feasible, not sustainable, not even prudent. Because you know what they would have done if it was their child or family member.”
Q. When did you first meet Dr. Farmer, and what inspired you to work with him?
Dr. Koenig: When I was an internal medicine resident at the Brigham, Paul and I bonded over the care of one of my clinic patients who I followed very closely, and who was admitted to his inpatient service.
Like everyone else who has worked with Paul, I was touched by his kindness and warmth.
A couple of years later, he asked me to help him raise money to bring a young man named Wilnot from Haiti to the Brigham for an aortic valve replacement. After we raised the money, he asked me to go to Haiti to help Wilnot get his medical visa and to escort him to Boston.
That short trip to Haiti had an enormous impact on my life. I was shattered to see the poverty that the people of Haiti were enduring – and in a country a short plane flight from Miami.
Shortly after this, Paul asked me to help him find treatment for another patient, a young boy named John, who presented with neck masses that were later diagnosed as nasopharyngeal carcinoma.
It took us some time to make the diagnosis and then to arrange free care at Mass General.
When I returned to Haiti with two PIH colleagues to help John get a visa and escort him back to Boston, we found that John’s condition was much worse. We ended up medically evacuating him to Boston, because he was too sick for a commercial flight.
Tracy Kidder wrote about this heartbreaking experience in the book “Mountains Beyond Mountains.”
Throughout all of these experiences, I was deeply impressed with Paul’s commitment to do whatever it took to provide the best care for patients, as if they were members of his own family. He said “Tout Moun Se Moun” (Haitian Creole for “every person is a person”), and I could tell that he meant it.
Q. How did you collaborate with him professionally?
Dr. Koenig: I spent the first few years after residency working with Paul and Partners In Health. Initially, I served as a liaison between PIH in Haiti and the Brigham, bringing several more patients to Boston for care, and arranging specialty surgical trips to Haiti.
Later, when HIV funding became available from the Global Fund for HIV, Tuberculosis, and Malaria, I moved to rural Haiti to provide treatment for patients with HIV and/or TB at one of the first PIH expansion sites. We treated many patients with advanced stages of HIV and/or TB, and many of them recovered remarkably quickly with antiretroviral therapy.
When I returned to Boston to complete an infectious disease fellowship I switched my focus to conducting clinical research to improve HIV and TB treatment outcomes. Paul emailed his mentor and friend, Jean “Bill” Pape, the director of a Haitian NGO called GHESKIO (Haitian Group for the Study of Kaposi’s Sarcoma and Opportunistic Infections), which is an internationally celebrated center of excellence in HIV-related research and clinical care, to ask if I could collaborate with them.
Ever since that time, I have been based between the Brigham’s division of global health equity, which was led by Paul, and GHESKIO.
Paul was very supportive of our research, which aims to improve health service delivery and treatment regimens for HIV and TB.
Q. What lessons do you think other physicians can learn from him?
Dr. Koenig: As Joia Mukherjee, chief medical officer of Partners In Health, has said, Paul left us a roadmap. He wrote many books, and he was very eloquent in expressing his philosophy about equity and justice in numerous interviews. This is relevant not only for international sites, but in the United States as well, with our major disparities in health outcomes by race, geography, and socioeconomic status.
No one will be able to replace Paul, but he left us with a vision of what is achievable.
Dr. Koenig is associate physician, Brigham and Women’s Hospital, Boston, with faculty appointments in the divisions of global health equity and infectious diseases. She is also associate professor at Harvard Medical School.
Infectious disease specialist and humanitarian, Paul Edward Farmer, MD, PhD, who cofounded Partners In Health, died suddenly on Feb. 21. To celebrate his life, this news organization interviewed Serena Koenig, MD, MPH, who met Dr. Farmer when she was an internal medicine resident at Brigham and Women’s Hospital. Dr. Koenig had worked closely with Dr. Farmer ever since they met.
Q. Can you please share one of your best memories of Dr. Farmer?
Dr. Serena Koenig: Paul and some other incredible colleagues at Partners IN Health (PIH) had started the HIV Equity Initiative, which was one of the first programs in the world to provide free, comprehensive treatment for HIV. This was at the time when millions of people in Africa were dying of HIV and many experts said it was not feasible to treat HIV in a poor country, because it was too complicated and expensive. Paul took me on some home visits with patients who had what he called the Lazarus effect, coming back from death’s door from advanced AIDS to vigorous health on antiretroviral therapy. I had just started working in Haiti with Paul and PIH, and I felt the enormous magnitude of what he was doing.
Q. What aspects of him and his work do you find most admirable?
Dr. Koenig: I most admired Paul’s humanity, his belief that every person matters and has the right to high-quality health care, and his vision of global health equity.
He said: “The idea that some lives matter less is the root of all that is wrong with the world.” Paul lived this philosophy. He has spoken extensively about harms of socialization for scarcity on behalf of those who are poor, leading policy makers to decisions regarding the feasibility of treating some diseases, but not others.
He said in an interview with the Harvard Gazette in 2018: “The most compelling thing to fight socialization for scarcity on behalf of others is health system strengthening. Health systems that integrate prevention and quality care.”
A few weeks ago, I asked him his thoughts about the high-level resources we have invested in some patients who have needed specialty care over the years, and he said: “No way that we should waste all of our emotional energy responding only to those constant, nagging critics that it’s not cost effective, not feasible, not sustainable, not even prudent. Because you know what they would have done if it was their child or family member.”
Q. When did you first meet Dr. Farmer, and what inspired you to work with him?
Dr. Koenig: When I was an internal medicine resident at the Brigham, Paul and I bonded over the care of one of my clinic patients who I followed very closely, and who was admitted to his inpatient service.
Like everyone else who has worked with Paul, I was touched by his kindness and warmth.
A couple of years later, he asked me to help him raise money to bring a young man named Wilnot from Haiti to the Brigham for an aortic valve replacement. After we raised the money, he asked me to go to Haiti to help Wilnot get his medical visa and to escort him to Boston.
That short trip to Haiti had an enormous impact on my life. I was shattered to see the poverty that the people of Haiti were enduring – and in a country a short plane flight from Miami.
Shortly after this, Paul asked me to help him find treatment for another patient, a young boy named John, who presented with neck masses that were later diagnosed as nasopharyngeal carcinoma.
It took us some time to make the diagnosis and then to arrange free care at Mass General.
When I returned to Haiti with two PIH colleagues to help John get a visa and escort him back to Boston, we found that John’s condition was much worse. We ended up medically evacuating him to Boston, because he was too sick for a commercial flight.
Tracy Kidder wrote about this heartbreaking experience in the book “Mountains Beyond Mountains.”
Throughout all of these experiences, I was deeply impressed with Paul’s commitment to do whatever it took to provide the best care for patients, as if they were members of his own family. He said “Tout Moun Se Moun” (Haitian Creole for “every person is a person”), and I could tell that he meant it.
Q. How did you collaborate with him professionally?
Dr. Koenig: I spent the first few years after residency working with Paul and Partners In Health. Initially, I served as a liaison between PIH in Haiti and the Brigham, bringing several more patients to Boston for care, and arranging specialty surgical trips to Haiti.
Later, when HIV funding became available from the Global Fund for HIV, Tuberculosis, and Malaria, I moved to rural Haiti to provide treatment for patients with HIV and/or TB at one of the first PIH expansion sites. We treated many patients with advanced stages of HIV and/or TB, and many of them recovered remarkably quickly with antiretroviral therapy.
When I returned to Boston to complete an infectious disease fellowship I switched my focus to conducting clinical research to improve HIV and TB treatment outcomes. Paul emailed his mentor and friend, Jean “Bill” Pape, the director of a Haitian NGO called GHESKIO (Haitian Group for the Study of Kaposi’s Sarcoma and Opportunistic Infections), which is an internationally celebrated center of excellence in HIV-related research and clinical care, to ask if I could collaborate with them.
Ever since that time, I have been based between the Brigham’s division of global health equity, which was led by Paul, and GHESKIO.
Paul was very supportive of our research, which aims to improve health service delivery and treatment regimens for HIV and TB.
Q. What lessons do you think other physicians can learn from him?
Dr. Koenig: As Joia Mukherjee, chief medical officer of Partners In Health, has said, Paul left us a roadmap. He wrote many books, and he was very eloquent in expressing his philosophy about equity and justice in numerous interviews. This is relevant not only for international sites, but in the United States as well, with our major disparities in health outcomes by race, geography, and socioeconomic status.
No one will be able to replace Paul, but he left us with a vision of what is achievable.
Dr. Koenig is associate physician, Brigham and Women’s Hospital, Boston, with faculty appointments in the divisions of global health equity and infectious diseases. She is also associate professor at Harvard Medical School.
Analysis questions tocilizumab in ventilated COVID patients
A new statistical analysis of an existing meta-analysis reaffirms a finding that hospitalized patients with COVID-19 who are on simple oxygen or noninvasive ventilation can benefit from treatment with the arthritis drug tocilizumab (Actemra) in conjunction with corticosteroids. But the report also casts doubt on the effectiveness of tocilizumab in patients who are on ventilators.
“Clinicians should prescribe steroids and tocilizumab for hospitalized patients needing simple oxygen or noninvasive ventilation,” epidemiologist and study coauthor James (Jay) Brophy, MD, PhD, of McGill University, Montreal, said in an interview. “Further research is required to answer the question of whether tocilizumab is beneficial in patients requiring invasive ventilation, and consideration of participation in further tocilizumab studies seems reasonable.”
The new analysis was published Feb. 28, 2022, in JAMA Network Open.
The initial meta-analysis, published in 2021 in JAMA, was conducted by the WHO Rapid Evidence Appraisal for COVID-19 Therapies Working Group. It analyzed the results of 27 randomized trials that explored the use of interleukin-6 antagonists, including tocilizumab, and found that “28-day all-cause mortality was lower among patients who received IL-6 antagonists, compared with those who received usual care or placebo (summary odds ratio, 0.86). The summary ORs for the association of IL-6 antagonist treatment with 28-day all-cause mortality were 0.78 with concomitant administration of corticosteroids versus 1.09 without administration of corticosteroids.”
For the new report, researchers conducted a Bayesian statistical analysis of 15 studies within the meta-analysis that specifically examined the use of the rheumatoid arthritis drug tocilizumab. “Bayesian analysis allows one to make direct probability statements regarding the exact magnitude and the certainty of any benefit,” Dr. Brophy said. “This provides clinicians with the information they require to make well-informed decisions.”
The analysis estimated that the probability of a “clinically meaningful association” (absolute mortality risk difference, >1%) because of use of tocilizumab was higher than 95% in patients receiving simple oxygen and higher than 90% in those receiving noninvasive ventilation. But the probability was only about 67% higher in those receiving invasive mechanical ventilation.
Also, the researchers estimated that about 72% of future tocilizumab studies in patients on invasive mechanical ventilation would show a benefit.
The new analysis findings don’t add much to existing knowledge, said nephrologist David E. Leaf, MD, MMSc, of Harvard Medical School, Boston, who’s studied tocilizumab in COVID-19.
“The signal seems to be consistent that there is a greater benefit of tocilizumab in less ill patients than those who are more ill – e.g., those who are receiving invasive mechanical ventilation,” Dr. Leaf said in an interview. “This is interesting because in clinical practice the opposite approach is often undertaken, with tocilizumab use only being used in the sickest patients, even though the patients most likely to benefit seem to be those who are less ill.”
Clinically, he said, “hospitalized patients with COVID-19 should receive tocilizumab unless they have a clear contraindication and assuming it can be administered relatively early in their disease course. Earlier administration, before the onset of irreversible organ injury, is likely to have greater benefit.”
Dr. Leaf also noted it’s unknown whether the drug is helpful in several groups – patients presenting later in the course of COVID-19 illness, patients with additional infections, and immunocompromised patients.
It’s also not clear if tocilizumab benefits patients with lower levels of C-reactive protein, Shruti Gupta, MD, MPH, a nephrologist at Brigham and Women’s Hospital in Boston, said in an interview. The RECOVERY trial, for example, limited subjects to those with C-reactive protein of at least 75 mg/L.
Dr. Leaf and Dr. Gupta coauthored a 2021 cohort study analyzing mortality rates in patients with COVID-19 who were treated with tocilizumab versus those who were not.
No study funding was reported. Dr. Brophy, Dr. Leaf, and Dr. Gupta disclosed no relevant financial relationships. One study author reported participating in one of the randomized clinical trials included in the analysis.
A version of this article first appeared on Medscape.com.
A new statistical analysis of an existing meta-analysis reaffirms a finding that hospitalized patients with COVID-19 who are on simple oxygen or noninvasive ventilation can benefit from treatment with the arthritis drug tocilizumab (Actemra) in conjunction with corticosteroids. But the report also casts doubt on the effectiveness of tocilizumab in patients who are on ventilators.
“Clinicians should prescribe steroids and tocilizumab for hospitalized patients needing simple oxygen or noninvasive ventilation,” epidemiologist and study coauthor James (Jay) Brophy, MD, PhD, of McGill University, Montreal, said in an interview. “Further research is required to answer the question of whether tocilizumab is beneficial in patients requiring invasive ventilation, and consideration of participation in further tocilizumab studies seems reasonable.”
The new analysis was published Feb. 28, 2022, in JAMA Network Open.
The initial meta-analysis, published in 2021 in JAMA, was conducted by the WHO Rapid Evidence Appraisal for COVID-19 Therapies Working Group. It analyzed the results of 27 randomized trials that explored the use of interleukin-6 antagonists, including tocilizumab, and found that “28-day all-cause mortality was lower among patients who received IL-6 antagonists, compared with those who received usual care or placebo (summary odds ratio, 0.86). The summary ORs for the association of IL-6 antagonist treatment with 28-day all-cause mortality were 0.78 with concomitant administration of corticosteroids versus 1.09 without administration of corticosteroids.”
For the new report, researchers conducted a Bayesian statistical analysis of 15 studies within the meta-analysis that specifically examined the use of the rheumatoid arthritis drug tocilizumab. “Bayesian analysis allows one to make direct probability statements regarding the exact magnitude and the certainty of any benefit,” Dr. Brophy said. “This provides clinicians with the information they require to make well-informed decisions.”
The analysis estimated that the probability of a “clinically meaningful association” (absolute mortality risk difference, >1%) because of use of tocilizumab was higher than 95% in patients receiving simple oxygen and higher than 90% in those receiving noninvasive ventilation. But the probability was only about 67% higher in those receiving invasive mechanical ventilation.
Also, the researchers estimated that about 72% of future tocilizumab studies in patients on invasive mechanical ventilation would show a benefit.
The new analysis findings don’t add much to existing knowledge, said nephrologist David E. Leaf, MD, MMSc, of Harvard Medical School, Boston, who’s studied tocilizumab in COVID-19.
“The signal seems to be consistent that there is a greater benefit of tocilizumab in less ill patients than those who are more ill – e.g., those who are receiving invasive mechanical ventilation,” Dr. Leaf said in an interview. “This is interesting because in clinical practice the opposite approach is often undertaken, with tocilizumab use only being used in the sickest patients, even though the patients most likely to benefit seem to be those who are less ill.”
Clinically, he said, “hospitalized patients with COVID-19 should receive tocilizumab unless they have a clear contraindication and assuming it can be administered relatively early in their disease course. Earlier administration, before the onset of irreversible organ injury, is likely to have greater benefit.”
Dr. Leaf also noted it’s unknown whether the drug is helpful in several groups – patients presenting later in the course of COVID-19 illness, patients with additional infections, and immunocompromised patients.
It’s also not clear if tocilizumab benefits patients with lower levels of C-reactive protein, Shruti Gupta, MD, MPH, a nephrologist at Brigham and Women’s Hospital in Boston, said in an interview. The RECOVERY trial, for example, limited subjects to those with C-reactive protein of at least 75 mg/L.
Dr. Leaf and Dr. Gupta coauthored a 2021 cohort study analyzing mortality rates in patients with COVID-19 who were treated with tocilizumab versus those who were not.
No study funding was reported. Dr. Brophy, Dr. Leaf, and Dr. Gupta disclosed no relevant financial relationships. One study author reported participating in one of the randomized clinical trials included in the analysis.
A version of this article first appeared on Medscape.com.
A new statistical analysis of an existing meta-analysis reaffirms a finding that hospitalized patients with COVID-19 who are on simple oxygen or noninvasive ventilation can benefit from treatment with the arthritis drug tocilizumab (Actemra) in conjunction with corticosteroids. But the report also casts doubt on the effectiveness of tocilizumab in patients who are on ventilators.
“Clinicians should prescribe steroids and tocilizumab for hospitalized patients needing simple oxygen or noninvasive ventilation,” epidemiologist and study coauthor James (Jay) Brophy, MD, PhD, of McGill University, Montreal, said in an interview. “Further research is required to answer the question of whether tocilizumab is beneficial in patients requiring invasive ventilation, and consideration of participation in further tocilizumab studies seems reasonable.”
The new analysis was published Feb. 28, 2022, in JAMA Network Open.
The initial meta-analysis, published in 2021 in JAMA, was conducted by the WHO Rapid Evidence Appraisal for COVID-19 Therapies Working Group. It analyzed the results of 27 randomized trials that explored the use of interleukin-6 antagonists, including tocilizumab, and found that “28-day all-cause mortality was lower among patients who received IL-6 antagonists, compared with those who received usual care or placebo (summary odds ratio, 0.86). The summary ORs for the association of IL-6 antagonist treatment with 28-day all-cause mortality were 0.78 with concomitant administration of corticosteroids versus 1.09 without administration of corticosteroids.”
For the new report, researchers conducted a Bayesian statistical analysis of 15 studies within the meta-analysis that specifically examined the use of the rheumatoid arthritis drug tocilizumab. “Bayesian analysis allows one to make direct probability statements regarding the exact magnitude and the certainty of any benefit,” Dr. Brophy said. “This provides clinicians with the information they require to make well-informed decisions.”
The analysis estimated that the probability of a “clinically meaningful association” (absolute mortality risk difference, >1%) because of use of tocilizumab was higher than 95% in patients receiving simple oxygen and higher than 90% in those receiving noninvasive ventilation. But the probability was only about 67% higher in those receiving invasive mechanical ventilation.
Also, the researchers estimated that about 72% of future tocilizumab studies in patients on invasive mechanical ventilation would show a benefit.
The new analysis findings don’t add much to existing knowledge, said nephrologist David E. Leaf, MD, MMSc, of Harvard Medical School, Boston, who’s studied tocilizumab in COVID-19.
“The signal seems to be consistent that there is a greater benefit of tocilizumab in less ill patients than those who are more ill – e.g., those who are receiving invasive mechanical ventilation,” Dr. Leaf said in an interview. “This is interesting because in clinical practice the opposite approach is often undertaken, with tocilizumab use only being used in the sickest patients, even though the patients most likely to benefit seem to be those who are less ill.”
Clinically, he said, “hospitalized patients with COVID-19 should receive tocilizumab unless they have a clear contraindication and assuming it can be administered relatively early in their disease course. Earlier administration, before the onset of irreversible organ injury, is likely to have greater benefit.”
Dr. Leaf also noted it’s unknown whether the drug is helpful in several groups – patients presenting later in the course of COVID-19 illness, patients with additional infections, and immunocompromised patients.
It’s also not clear if tocilizumab benefits patients with lower levels of C-reactive protein, Shruti Gupta, MD, MPH, a nephrologist at Brigham and Women’s Hospital in Boston, said in an interview. The RECOVERY trial, for example, limited subjects to those with C-reactive protein of at least 75 mg/L.
Dr. Leaf and Dr. Gupta coauthored a 2021 cohort study analyzing mortality rates in patients with COVID-19 who were treated with tocilizumab versus those who were not.
No study funding was reported. Dr. Brophy, Dr. Leaf, and Dr. Gupta disclosed no relevant financial relationships. One study author reported participating in one of the randomized clinical trials included in the analysis.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Some physicians still lack access to COVID-19 vaccines
It would be overused and trite to say that the pandemic has drastically altered all of our lives and will cause lasting impact on how we function in society and medicine for years to come. While it seems that the current trend of the latest Omicron variant is on the downslope, the path to get to this point has been fraught with challenges that have struck at the very core of our society. As a primary care physician on the front lines seeing COVID patients, I have had to deal with not only the disease but the politics around it. I practice in Florida, and I still cannot give COVID vaccines in my office.
I am a firm believer in the ability for physicians to be able to give all the necessary adult vaccines and provide them for their patients. The COVID vaccine exacerbated a majorly flawed system that further increased the health care disparities in the country. The current vaccine system for the majority of adult vaccines involves the physician’s being able to directly purchase supplies from the vaccine manufacturer, administer them to the patients, and be reimbursed.
Third parties can purchase vaccines at lower rates than those for physicians
The Affordable Care Act mandates that all vaccines approved by the Advisory Committee on Immunization Practices (ACIP) at the Centers for Disease Control and Prevention must be covered. This allows for better access to care as physicians will be able to purchase, store, and deliver vaccines to their patients. The fallacy in this system is that third parties get involved and rebates or incentives are given to these groups to purchase vaccines at a rate lower than those for physicians.
In addition, many organizations can get access to vaccines before physicians and at a lower cost. That system was flawed to begin with and created a deterrent for access to care and physician involvement in the vaccination process. This was worsened by different states being given the ability to decide how vaccines would be distributed for COVID.
Many pharmacies were able to give out COVID vaccines while many physician offices still have not received access to any of the vaccines. One of the major safety issues with this is that no physicians were involved in the administration of the vaccine, and it is unclear what training was given to the individuals injecting that vaccine. Finally, different places were interpreting the recommendations from ACIP on their own and not necessarily following the appropriate guidelines. All of these factors have further widened the health care disparity gap and made it difficult to provide the COVID vaccines in doctors’ offices.
Recommended next steps, solutions to problem
The question is what to do about this. The most important thing is to get the vaccines in arms so they can save lives. In addition, doctors need to be able to get the vaccines in their offices.
Many patients trust their physicians to advise them on what to do regarding health care. The majority of patients want to know if they should get the vaccine and ask for counseling. Physicians answering patients’ questions about vaccines is an important step in overcoming vaccine hesitancy.
Also, doctors need to be informed and supportive of the vaccine process.
The next step is the governmental aspect with those in power making sure that vaccines are accessible to all. Even if the vaccine cannot be given in the office, doctors should still be recommending that patients receive them. Plus, doctors should take every opportunity to ask about what vaccines their patients have received and encourage their patients to get vaccinated.
The COVID-19 vaccines are safe and effective and have been monitored for safety more than any other vaccine. There are multiple systems in place to look for any signals that could indicate an issue was caused by a COVID-19 vaccine. These vaccines can be administered with other vaccines, and there is a great opportunity for physicians to encourage patients to receive these life-saving vaccines.
While it may seem that the COVID-19 case counts are on the downslope, the importance of continuing to vaccinate is predicated on the very real concern that the disease is still circulating and the unvaccinated are still at risk for severe infection.
Dr. Goldman is immediate past governor of the Florida chapter of the American College of Physicians, a regent for the American College of Physicians, vice-president of the Florida Medical Association, and president of the Florida Medical Association Political Action Committee. You can reach Dr. Goldman at [email protected].
It would be overused and trite to say that the pandemic has drastically altered all of our lives and will cause lasting impact on how we function in society and medicine for years to come. While it seems that the current trend of the latest Omicron variant is on the downslope, the path to get to this point has been fraught with challenges that have struck at the very core of our society. As a primary care physician on the front lines seeing COVID patients, I have had to deal with not only the disease but the politics around it. I practice in Florida, and I still cannot give COVID vaccines in my office.
I am a firm believer in the ability for physicians to be able to give all the necessary adult vaccines and provide them for their patients. The COVID vaccine exacerbated a majorly flawed system that further increased the health care disparities in the country. The current vaccine system for the majority of adult vaccines involves the physician’s being able to directly purchase supplies from the vaccine manufacturer, administer them to the patients, and be reimbursed.
Third parties can purchase vaccines at lower rates than those for physicians
The Affordable Care Act mandates that all vaccines approved by the Advisory Committee on Immunization Practices (ACIP) at the Centers for Disease Control and Prevention must be covered. This allows for better access to care as physicians will be able to purchase, store, and deliver vaccines to their patients. The fallacy in this system is that third parties get involved and rebates or incentives are given to these groups to purchase vaccines at a rate lower than those for physicians.
In addition, many organizations can get access to vaccines before physicians and at a lower cost. That system was flawed to begin with and created a deterrent for access to care and physician involvement in the vaccination process. This was worsened by different states being given the ability to decide how vaccines would be distributed for COVID.
Many pharmacies were able to give out COVID vaccines while many physician offices still have not received access to any of the vaccines. One of the major safety issues with this is that no physicians were involved in the administration of the vaccine, and it is unclear what training was given to the individuals injecting that vaccine. Finally, different places were interpreting the recommendations from ACIP on their own and not necessarily following the appropriate guidelines. All of these factors have further widened the health care disparity gap and made it difficult to provide the COVID vaccines in doctors’ offices.
Recommended next steps, solutions to problem
The question is what to do about this. The most important thing is to get the vaccines in arms so they can save lives. In addition, doctors need to be able to get the vaccines in their offices.
Many patients trust their physicians to advise them on what to do regarding health care. The majority of patients want to know if they should get the vaccine and ask for counseling. Physicians answering patients’ questions about vaccines is an important step in overcoming vaccine hesitancy.
Also, doctors need to be informed and supportive of the vaccine process.
The next step is the governmental aspect with those in power making sure that vaccines are accessible to all. Even if the vaccine cannot be given in the office, doctors should still be recommending that patients receive them. Plus, doctors should take every opportunity to ask about what vaccines their patients have received and encourage their patients to get vaccinated.
The COVID-19 vaccines are safe and effective and have been monitored for safety more than any other vaccine. There are multiple systems in place to look for any signals that could indicate an issue was caused by a COVID-19 vaccine. These vaccines can be administered with other vaccines, and there is a great opportunity for physicians to encourage patients to receive these life-saving vaccines.
While it may seem that the COVID-19 case counts are on the downslope, the importance of continuing to vaccinate is predicated on the very real concern that the disease is still circulating and the unvaccinated are still at risk for severe infection.
Dr. Goldman is immediate past governor of the Florida chapter of the American College of Physicians, a regent for the American College of Physicians, vice-president of the Florida Medical Association, and president of the Florida Medical Association Political Action Committee. You can reach Dr. Goldman at [email protected].
It would be overused and trite to say that the pandemic has drastically altered all of our lives and will cause lasting impact on how we function in society and medicine for years to come. While it seems that the current trend of the latest Omicron variant is on the downslope, the path to get to this point has been fraught with challenges that have struck at the very core of our society. As a primary care physician on the front lines seeing COVID patients, I have had to deal with not only the disease but the politics around it. I practice in Florida, and I still cannot give COVID vaccines in my office.
I am a firm believer in the ability for physicians to be able to give all the necessary adult vaccines and provide them for their patients. The COVID vaccine exacerbated a majorly flawed system that further increased the health care disparities in the country. The current vaccine system for the majority of adult vaccines involves the physician’s being able to directly purchase supplies from the vaccine manufacturer, administer them to the patients, and be reimbursed.
Third parties can purchase vaccines at lower rates than those for physicians
The Affordable Care Act mandates that all vaccines approved by the Advisory Committee on Immunization Practices (ACIP) at the Centers for Disease Control and Prevention must be covered. This allows for better access to care as physicians will be able to purchase, store, and deliver vaccines to their patients. The fallacy in this system is that third parties get involved and rebates or incentives are given to these groups to purchase vaccines at a rate lower than those for physicians.
In addition, many organizations can get access to vaccines before physicians and at a lower cost. That system was flawed to begin with and created a deterrent for access to care and physician involvement in the vaccination process. This was worsened by different states being given the ability to decide how vaccines would be distributed for COVID.
Many pharmacies were able to give out COVID vaccines while many physician offices still have not received access to any of the vaccines. One of the major safety issues with this is that no physicians were involved in the administration of the vaccine, and it is unclear what training was given to the individuals injecting that vaccine. Finally, different places were interpreting the recommendations from ACIP on their own and not necessarily following the appropriate guidelines. All of these factors have further widened the health care disparity gap and made it difficult to provide the COVID vaccines in doctors’ offices.
Recommended next steps, solutions to problem
The question is what to do about this. The most important thing is to get the vaccines in arms so they can save lives. In addition, doctors need to be able to get the vaccines in their offices.
Many patients trust their physicians to advise them on what to do regarding health care. The majority of patients want to know if they should get the vaccine and ask for counseling. Physicians answering patients’ questions about vaccines is an important step in overcoming vaccine hesitancy.
Also, doctors need to be informed and supportive of the vaccine process.
The next step is the governmental aspect with those in power making sure that vaccines are accessible to all. Even if the vaccine cannot be given in the office, doctors should still be recommending that patients receive them. Plus, doctors should take every opportunity to ask about what vaccines their patients have received and encourage their patients to get vaccinated.
The COVID-19 vaccines are safe and effective and have been monitored for safety more than any other vaccine. There are multiple systems in place to look for any signals that could indicate an issue was caused by a COVID-19 vaccine. These vaccines can be administered with other vaccines, and there is a great opportunity for physicians to encourage patients to receive these life-saving vaccines.
While it may seem that the COVID-19 case counts are on the downslope, the importance of continuing to vaccinate is predicated on the very real concern that the disease is still circulating and the unvaccinated are still at risk for severe infection.
Dr. Goldman is immediate past governor of the Florida chapter of the American College of Physicians, a regent for the American College of Physicians, vice-president of the Florida Medical Association, and president of the Florida Medical Association Political Action Committee. You can reach Dr. Goldman at [email protected].
Nasal microbiota show promise as polyp predictor
A study of the nasal microbiome helped researchers predict recurrent polyps in chronic rhinosinusitis patients with more than 90% accuracy, based on data from 85 individuals.
Chronic rhinosinusitis with nasal polyps (CRSwNP) has a significant impact on patient quality of life, but the underlying mechanism of the disease has not been well studied, and treatment options remain limited, wrote Yan Zhao, MD, of Capital Medical University, Beijing, and study coauthors.
Previous research has shown that nasal microbiome composition differs in patients with and without asthma, and some studies suggest that changes in microbiota could contribute to CRSwNP, the authors wrote. The researchers wondered if features of the nasal microbiome can predict the recurrence of nasal polyps after endoscopic sinus surgery and serve as a potential treatment target.
In a study in Allergy, the researchers examined nasal swab samples from 85 adults with CRSwNP who underwent endoscopic sinus surgery between August 2014 and March 2016 at a single center in China. The researchers performed bacterial analysis and gene sequencing on all samples.
The patients ranged in age from 18-73 years, with a mean age of 46 years, and included 64 men and 21 women. The primary outcome was recurrence of polyps. Of the total, 39 individuals had recurrence, and 46 did not.
When the researchers compared microbiota from swab samples of recurrent and nonrecurrent patients, they found differences in composition based on bacterial genus abundance. “Campylobacter, Bdellovibrio, and Aggregatibacter, among others, were more abundant in swabs from CRSwNP recurrence samples, whereas Actinobacillus, Gemella, and Moraxella were more abundant in non-recurrence samples,” they wrote.
The researchers then tested their theory that distinct nasal microbiota could be a predictive marker of risk for future nasal polyp recurrence. They used a training set of 48 samples and constructed models from nasal microbiota alone, clinical features alone, and both together.
The regression model identified Porphyromonas, Bacteroides, Moryella, Aggregatibacter, Butyrivibrio, Shewanella, Pseudoxanthomonas, Friedmanniella, Limnobacter, and Curvibacter as the most important taxa that distinguished recurrence from nonrecurrence in the specimens. When the model was validated, the area under the curve was 0.914, yielding a predictor of nasal polyp recurrence with 91.4% accuracy.
“It is highly likely that proteins, nucleic acids, and other small molecules produced by nasal microbiota are associated with the progression of CRSwNP,” the researchers noted in their discussion of the findings. “Further, the nasal microbiota could maintain a stable community environment through the secretion of various chemical compounds and/or inflammatory factors, thus playing a central role in the development of CRSwNP.”
The study findings were limited by several factors, including the analysis of nasal flora only at the genus level in the screening phase, the use only of bioinformatic analysis for recurrence prediction, and the inclusion only of subjects from a single center, the researchers noted. Future studies should combine predictors to increase accuracy and include deeper sequencing, they said. However, the results support data from previous studies and suggest a strategy to meet the need for predictors of recurrence in CRSwNP, they concluded.
“There is a critical need to understand the role of the upper airway microbiome in different phenotypes of CRS,” said Emily K. Cope, PhD, assistant director at the Pathogen and Microbiome Institute, Northern Arizona University, Flagstaff, in an interview. “This was one of the first studies to evaluate the predictive power of the microbiome in recurrence of a common CRS phenotype – CRS with nasal polyps,” she said. “Importantly, the researchers were able to predict recurrence of polyps prior to the disease manifestation,” she noted.
“Given the nascent state of current upper airway microbiome research, I was surprised that they were able to predict polyp recurrence prior to disease manifestation,” Dr. Cope said. “This is exciting, and I can imagine a future where we use microbiome data to understand risk for disease.”
What is the take-home message for clinicians? Although the immediate clinical implications are limited, Dr. Cope expressed enthusiasm for additional research. “At this point, there’s not a lot we can do without validation studies, but this study is promising. I hope we can understand the mechanism that an altered microbiome might drive (or be a result of) polyposis,” she said.
The study was supported by the National Natural Science Foundation of China, the program for the Changjiang scholars and innovative research team, the Beijing Bai-Qian-Wan talent project, the Public Welfare Development and Reform Pilot Project, the National Science and Technology Major Project, and the CAMS Innovation Fund for Medical Sciences. The researchers and Dr. Cope disclosed no financial conflicts.
A version of this article first appeared on Medscape.com.
A study of the nasal microbiome helped researchers predict recurrent polyps in chronic rhinosinusitis patients with more than 90% accuracy, based on data from 85 individuals.
Chronic rhinosinusitis with nasal polyps (CRSwNP) has a significant impact on patient quality of life, but the underlying mechanism of the disease has not been well studied, and treatment options remain limited, wrote Yan Zhao, MD, of Capital Medical University, Beijing, and study coauthors.
Previous research has shown that nasal microbiome composition differs in patients with and without asthma, and some studies suggest that changes in microbiota could contribute to CRSwNP, the authors wrote. The researchers wondered if features of the nasal microbiome can predict the recurrence of nasal polyps after endoscopic sinus surgery and serve as a potential treatment target.
In a study in Allergy, the researchers examined nasal swab samples from 85 adults with CRSwNP who underwent endoscopic sinus surgery between August 2014 and March 2016 at a single center in China. The researchers performed bacterial analysis and gene sequencing on all samples.
The patients ranged in age from 18-73 years, with a mean age of 46 years, and included 64 men and 21 women. The primary outcome was recurrence of polyps. Of the total, 39 individuals had recurrence, and 46 did not.
When the researchers compared microbiota from swab samples of recurrent and nonrecurrent patients, they found differences in composition based on bacterial genus abundance. “Campylobacter, Bdellovibrio, and Aggregatibacter, among others, were more abundant in swabs from CRSwNP recurrence samples, whereas Actinobacillus, Gemella, and Moraxella were more abundant in non-recurrence samples,” they wrote.
The researchers then tested their theory that distinct nasal microbiota could be a predictive marker of risk for future nasal polyp recurrence. They used a training set of 48 samples and constructed models from nasal microbiota alone, clinical features alone, and both together.
The regression model identified Porphyromonas, Bacteroides, Moryella, Aggregatibacter, Butyrivibrio, Shewanella, Pseudoxanthomonas, Friedmanniella, Limnobacter, and Curvibacter as the most important taxa that distinguished recurrence from nonrecurrence in the specimens. When the model was validated, the area under the curve was 0.914, yielding a predictor of nasal polyp recurrence with 91.4% accuracy.
“It is highly likely that proteins, nucleic acids, and other small molecules produced by nasal microbiota are associated with the progression of CRSwNP,” the researchers noted in their discussion of the findings. “Further, the nasal microbiota could maintain a stable community environment through the secretion of various chemical compounds and/or inflammatory factors, thus playing a central role in the development of CRSwNP.”
The study findings were limited by several factors, including the analysis of nasal flora only at the genus level in the screening phase, the use only of bioinformatic analysis for recurrence prediction, and the inclusion only of subjects from a single center, the researchers noted. Future studies should combine predictors to increase accuracy and include deeper sequencing, they said. However, the results support data from previous studies and suggest a strategy to meet the need for predictors of recurrence in CRSwNP, they concluded.
“There is a critical need to understand the role of the upper airway microbiome in different phenotypes of CRS,” said Emily K. Cope, PhD, assistant director at the Pathogen and Microbiome Institute, Northern Arizona University, Flagstaff, in an interview. “This was one of the first studies to evaluate the predictive power of the microbiome in recurrence of a common CRS phenotype – CRS with nasal polyps,” she said. “Importantly, the researchers were able to predict recurrence of polyps prior to the disease manifestation,” she noted.
“Given the nascent state of current upper airway microbiome research, I was surprised that they were able to predict polyp recurrence prior to disease manifestation,” Dr. Cope said. “This is exciting, and I can imagine a future where we use microbiome data to understand risk for disease.”
What is the take-home message for clinicians? Although the immediate clinical implications are limited, Dr. Cope expressed enthusiasm for additional research. “At this point, there’s not a lot we can do without validation studies, but this study is promising. I hope we can understand the mechanism that an altered microbiome might drive (or be a result of) polyposis,” she said.
The study was supported by the National Natural Science Foundation of China, the program for the Changjiang scholars and innovative research team, the Beijing Bai-Qian-Wan talent project, the Public Welfare Development and Reform Pilot Project, the National Science and Technology Major Project, and the CAMS Innovation Fund for Medical Sciences. The researchers and Dr. Cope disclosed no financial conflicts.
A version of this article first appeared on Medscape.com.
A study of the nasal microbiome helped researchers predict recurrent polyps in chronic rhinosinusitis patients with more than 90% accuracy, based on data from 85 individuals.
Chronic rhinosinusitis with nasal polyps (CRSwNP) has a significant impact on patient quality of life, but the underlying mechanism of the disease has not been well studied, and treatment options remain limited, wrote Yan Zhao, MD, of Capital Medical University, Beijing, and study coauthors.
Previous research has shown that nasal microbiome composition differs in patients with and without asthma, and some studies suggest that changes in microbiota could contribute to CRSwNP, the authors wrote. The researchers wondered if features of the nasal microbiome can predict the recurrence of nasal polyps after endoscopic sinus surgery and serve as a potential treatment target.
In a study in Allergy, the researchers examined nasal swab samples from 85 adults with CRSwNP who underwent endoscopic sinus surgery between August 2014 and March 2016 at a single center in China. The researchers performed bacterial analysis and gene sequencing on all samples.
The patients ranged in age from 18-73 years, with a mean age of 46 years, and included 64 men and 21 women. The primary outcome was recurrence of polyps. Of the total, 39 individuals had recurrence, and 46 did not.
When the researchers compared microbiota from swab samples of recurrent and nonrecurrent patients, they found differences in composition based on bacterial genus abundance. “Campylobacter, Bdellovibrio, and Aggregatibacter, among others, were more abundant in swabs from CRSwNP recurrence samples, whereas Actinobacillus, Gemella, and Moraxella were more abundant in non-recurrence samples,” they wrote.
The researchers then tested their theory that distinct nasal microbiota could be a predictive marker of risk for future nasal polyp recurrence. They used a training set of 48 samples and constructed models from nasal microbiota alone, clinical features alone, and both together.
The regression model identified Porphyromonas, Bacteroides, Moryella, Aggregatibacter, Butyrivibrio, Shewanella, Pseudoxanthomonas, Friedmanniella, Limnobacter, and Curvibacter as the most important taxa that distinguished recurrence from nonrecurrence in the specimens. When the model was validated, the area under the curve was 0.914, yielding a predictor of nasal polyp recurrence with 91.4% accuracy.
“It is highly likely that proteins, nucleic acids, and other small molecules produced by nasal microbiota are associated with the progression of CRSwNP,” the researchers noted in their discussion of the findings. “Further, the nasal microbiota could maintain a stable community environment through the secretion of various chemical compounds and/or inflammatory factors, thus playing a central role in the development of CRSwNP.”
The study findings were limited by several factors, including the analysis of nasal flora only at the genus level in the screening phase, the use only of bioinformatic analysis for recurrence prediction, and the inclusion only of subjects from a single center, the researchers noted. Future studies should combine predictors to increase accuracy and include deeper sequencing, they said. However, the results support data from previous studies and suggest a strategy to meet the need for predictors of recurrence in CRSwNP, they concluded.
“There is a critical need to understand the role of the upper airway microbiome in different phenotypes of CRS,” said Emily K. Cope, PhD, assistant director at the Pathogen and Microbiome Institute, Northern Arizona University, Flagstaff, in an interview. “This was one of the first studies to evaluate the predictive power of the microbiome in recurrence of a common CRS phenotype – CRS with nasal polyps,” she said. “Importantly, the researchers were able to predict recurrence of polyps prior to the disease manifestation,” she noted.
“Given the nascent state of current upper airway microbiome research, I was surprised that they were able to predict polyp recurrence prior to disease manifestation,” Dr. Cope said. “This is exciting, and I can imagine a future where we use microbiome data to understand risk for disease.”
What is the take-home message for clinicians? Although the immediate clinical implications are limited, Dr. Cope expressed enthusiasm for additional research. “At this point, there’s not a lot we can do without validation studies, but this study is promising. I hope we can understand the mechanism that an altered microbiome might drive (or be a result of) polyposis,” she said.
The study was supported by the National Natural Science Foundation of China, the program for the Changjiang scholars and innovative research team, the Beijing Bai-Qian-Wan talent project, the Public Welfare Development and Reform Pilot Project, the National Science and Technology Major Project, and the CAMS Innovation Fund for Medical Sciences. The researchers and Dr. Cope disclosed no financial conflicts.
A version of this article first appeared on Medscape.com.
Legionnaires’ disease shows steady increase in U.S. over 15+ years
Legionnaires’ disease (LD) in the United States appears to be on an upswing that started in 2003, according to a study from the Centers for Disease Control and Prevention.
The reasons for this increased incidence are unclear, the researchers write in Emerging Infectious Diseases.
“The findings revealed a rising national trend in cases, widening racial disparities between Black or African American persons and White persons, and an increasing geographic focus in the Middle Atlantic, the East North Central, and New England,” lead author Albert E. Barskey, MPH, an epidemiologist in CDC’s Division of Bacterial Diseases, Atlanta, said in an email.
“Legionnaires’ disease cannot be diagnosed based on clinical features alone, and studies estimate that it is underdiagnosed, perhaps by 50%,” he added. “Our findings may serve to heighten clinicians’ awareness of this severe pneumonia’s etiology, so with an earlier correct diagnosis, appropriate treatment can be rendered sooner.”
Mr. Barskey and his coauthors at CDC – mathematical statistician Gordana Derado, PhD, and epidemiologist Chris Edens, PhD – used surveillance data to investigate the incidence of LD in the U.S. over time. They compared LD incidence in 2018 with average incidence between 1992 and 2002. The incidence data, from over 80,000 LD cases, were age-standardized using the 2005 U.S. standard population as the reference.
The researchers analyzed LD data reported to CDC by the 50 states, New York City, and Washington, D.C., through the National Notifiable Diseases Surveillance System. They performed regression analysis to identify the optimal year when population parameters changed, and for most analyses, they compared 1992-2002 data with 2003-2018 data.
Legionnaires’ disease up in various groups
- The overall age-standardized average incidence grew from 0.48 per 100,000 people during 1992-2002 to 2.71 per 100,000 in 2018 (incidence risk ratio, 5.67; 95% confidence interval, 5.52-5.83).
- LD incidence more than quintupled for people over 34 years of age, with the largest relative increase in those over 85 (RR, 6.50; 95% CI, 5.82-7.27).
- Incidence in men increased slightly more (RR, 5.86; 95% CI, 5.67-6.05) than in women (RR, 5.29; 95% CI, 5.06-5.53).
- Over the years, the racial disparity in incidence grew markedly. Incidence in Black persons increased from 0.47 to 5.21 per 100,000 (RR, 11.04; 95% CI, 10.39-11.73), compared with an increase from 0.37 to 1.99 per 100,000 in White persons (RR, 5.30; 95% CI, 5.12-5.49).
- The relative increase in incidence was highest in the Northeast (RR, 7.04; 95% CI, 6.70-7.40), followed by the Midwest (RR, 6.13; 95% CI, 5.85-6.42), the South (RR, 5.97; 95% CI, 5.67-6.29), and the West (RR, 3.39; 95% CI, 3.11-3.68).
Most LD cases occurred in summer or fall, and the seasonal pattern became more pronounced over time. The average of 57.8% of cases between June and November during 1992-2002 grew to 68.9% in 2003-2018.
Although the study “was hindered by incomplete race and ethnicity data,” Mr. Barskey said, “its breadth was a strength.”
Consider legionella in your diagnosis
In an interview, Paul G. Auwaerter, MD, a professor of medicine and the clinical director of the Division of Infectious Diseases at Johns Hopkins University School of Medicine, Baltimore, said he was not surprised by the results. “CDC has been reporting increased incidence of Legionnaires’ disease from water source outbreaks over the years. As a clinician, I very much depend on epidemiologic trends to help me understand the patient in front of me.
“The key point is that there’s more of it around, so consider it in your diagnosis,” he advised.
“Physicians are increasingly beginning to consider Legionella. Because LD is difficult to diagnose by traditional methods such as culture, they may use a PCR test,” said Dr. Auwaerter, who was not involved in the study. “Legionella needs antibiotics that differ a bit from traditional antibiotics used to treat bacterial pneumonia, so a correct diagnosis can inform a more directed therapy.”
“Why the incidence is increasing is the big question, and the authors nicely outline a litany of things,” he said.
The authors and Dr. Auwaerter proposed a number of possible contributing factors to the increased incidence:
- an aging population
- aging municipal and residential water sources that may harbor more organisms
- racial disparities and poverty
- underlying conditions, including diabetes, end-stage renal disease, and some cancers
- occupations in transportation, repair, cleaning services, and construction
- weather patterns
- improved surveillance and reporting
“Why Legionella appears in some locations more than others has not been explained,” Dr. Auwaerter added. “For example, Pittsburgh always seemed to have much more Legionella than Baltimore.”
Mr. Barskey and his team are planning further research into racial disparities and links between weather and climate and Legionnaires’ disease.
The authors are employees of CDC. Dr. Auwaerter has disclosed no relevant financial realtionships.
A version of this article first appeared on Medscape.com.
Legionnaires’ disease (LD) in the United States appears to be on an upswing that started in 2003, according to a study from the Centers for Disease Control and Prevention.
The reasons for this increased incidence are unclear, the researchers write in Emerging Infectious Diseases.
“The findings revealed a rising national trend in cases, widening racial disparities between Black or African American persons and White persons, and an increasing geographic focus in the Middle Atlantic, the East North Central, and New England,” lead author Albert E. Barskey, MPH, an epidemiologist in CDC’s Division of Bacterial Diseases, Atlanta, said in an email.
“Legionnaires’ disease cannot be diagnosed based on clinical features alone, and studies estimate that it is underdiagnosed, perhaps by 50%,” he added. “Our findings may serve to heighten clinicians’ awareness of this severe pneumonia’s etiology, so with an earlier correct diagnosis, appropriate treatment can be rendered sooner.”
Mr. Barskey and his coauthors at CDC – mathematical statistician Gordana Derado, PhD, and epidemiologist Chris Edens, PhD – used surveillance data to investigate the incidence of LD in the U.S. over time. They compared LD incidence in 2018 with average incidence between 1992 and 2002. The incidence data, from over 80,000 LD cases, were age-standardized using the 2005 U.S. standard population as the reference.
The researchers analyzed LD data reported to CDC by the 50 states, New York City, and Washington, D.C., through the National Notifiable Diseases Surveillance System. They performed regression analysis to identify the optimal year when population parameters changed, and for most analyses, they compared 1992-2002 data with 2003-2018 data.
Legionnaires’ disease up in various groups
- The overall age-standardized average incidence grew from 0.48 per 100,000 people during 1992-2002 to 2.71 per 100,000 in 2018 (incidence risk ratio, 5.67; 95% confidence interval, 5.52-5.83).
- LD incidence more than quintupled for people over 34 years of age, with the largest relative increase in those over 85 (RR, 6.50; 95% CI, 5.82-7.27).
- Incidence in men increased slightly more (RR, 5.86; 95% CI, 5.67-6.05) than in women (RR, 5.29; 95% CI, 5.06-5.53).
- Over the years, the racial disparity in incidence grew markedly. Incidence in Black persons increased from 0.47 to 5.21 per 100,000 (RR, 11.04; 95% CI, 10.39-11.73), compared with an increase from 0.37 to 1.99 per 100,000 in White persons (RR, 5.30; 95% CI, 5.12-5.49).
- The relative increase in incidence was highest in the Northeast (RR, 7.04; 95% CI, 6.70-7.40), followed by the Midwest (RR, 6.13; 95% CI, 5.85-6.42), the South (RR, 5.97; 95% CI, 5.67-6.29), and the West (RR, 3.39; 95% CI, 3.11-3.68).
Most LD cases occurred in summer or fall, and the seasonal pattern became more pronounced over time. The average of 57.8% of cases between June and November during 1992-2002 grew to 68.9% in 2003-2018.
Although the study “was hindered by incomplete race and ethnicity data,” Mr. Barskey said, “its breadth was a strength.”
Consider legionella in your diagnosis
In an interview, Paul G. Auwaerter, MD, a professor of medicine and the clinical director of the Division of Infectious Diseases at Johns Hopkins University School of Medicine, Baltimore, said he was not surprised by the results. “CDC has been reporting increased incidence of Legionnaires’ disease from water source outbreaks over the years. As a clinician, I very much depend on epidemiologic trends to help me understand the patient in front of me.
“The key point is that there’s more of it around, so consider it in your diagnosis,” he advised.
“Physicians are increasingly beginning to consider Legionella. Because LD is difficult to diagnose by traditional methods such as culture, they may use a PCR test,” said Dr. Auwaerter, who was not involved in the study. “Legionella needs antibiotics that differ a bit from traditional antibiotics used to treat bacterial pneumonia, so a correct diagnosis can inform a more directed therapy.”
“Why the incidence is increasing is the big question, and the authors nicely outline a litany of things,” he said.
The authors and Dr. Auwaerter proposed a number of possible contributing factors to the increased incidence:
- an aging population
- aging municipal and residential water sources that may harbor more organisms
- racial disparities and poverty
- underlying conditions, including diabetes, end-stage renal disease, and some cancers
- occupations in transportation, repair, cleaning services, and construction
- weather patterns
- improved surveillance and reporting
“Why Legionella appears in some locations more than others has not been explained,” Dr. Auwaerter added. “For example, Pittsburgh always seemed to have much more Legionella than Baltimore.”
Mr. Barskey and his team are planning further research into racial disparities and links between weather and climate and Legionnaires’ disease.
The authors are employees of CDC. Dr. Auwaerter has disclosed no relevant financial realtionships.
A version of this article first appeared on Medscape.com.
Legionnaires’ disease (LD) in the United States appears to be on an upswing that started in 2003, according to a study from the Centers for Disease Control and Prevention.
The reasons for this increased incidence are unclear, the researchers write in Emerging Infectious Diseases.
“The findings revealed a rising national trend in cases, widening racial disparities between Black or African American persons and White persons, and an increasing geographic focus in the Middle Atlantic, the East North Central, and New England,” lead author Albert E. Barskey, MPH, an epidemiologist in CDC’s Division of Bacterial Diseases, Atlanta, said in an email.
“Legionnaires’ disease cannot be diagnosed based on clinical features alone, and studies estimate that it is underdiagnosed, perhaps by 50%,” he added. “Our findings may serve to heighten clinicians’ awareness of this severe pneumonia’s etiology, so with an earlier correct diagnosis, appropriate treatment can be rendered sooner.”
Mr. Barskey and his coauthors at CDC – mathematical statistician Gordana Derado, PhD, and epidemiologist Chris Edens, PhD – used surveillance data to investigate the incidence of LD in the U.S. over time. They compared LD incidence in 2018 with average incidence between 1992 and 2002. The incidence data, from over 80,000 LD cases, were age-standardized using the 2005 U.S. standard population as the reference.
The researchers analyzed LD data reported to CDC by the 50 states, New York City, and Washington, D.C., through the National Notifiable Diseases Surveillance System. They performed regression analysis to identify the optimal year when population parameters changed, and for most analyses, they compared 1992-2002 data with 2003-2018 data.
Legionnaires’ disease up in various groups
- The overall age-standardized average incidence grew from 0.48 per 100,000 people during 1992-2002 to 2.71 per 100,000 in 2018 (incidence risk ratio, 5.67; 95% confidence interval, 5.52-5.83).
- LD incidence more than quintupled for people over 34 years of age, with the largest relative increase in those over 85 (RR, 6.50; 95% CI, 5.82-7.27).
- Incidence in men increased slightly more (RR, 5.86; 95% CI, 5.67-6.05) than in women (RR, 5.29; 95% CI, 5.06-5.53).
- Over the years, the racial disparity in incidence grew markedly. Incidence in Black persons increased from 0.47 to 5.21 per 100,000 (RR, 11.04; 95% CI, 10.39-11.73), compared with an increase from 0.37 to 1.99 per 100,000 in White persons (RR, 5.30; 95% CI, 5.12-5.49).
- The relative increase in incidence was highest in the Northeast (RR, 7.04; 95% CI, 6.70-7.40), followed by the Midwest (RR, 6.13; 95% CI, 5.85-6.42), the South (RR, 5.97; 95% CI, 5.67-6.29), and the West (RR, 3.39; 95% CI, 3.11-3.68).
Most LD cases occurred in summer or fall, and the seasonal pattern became more pronounced over time. The average of 57.8% of cases between June and November during 1992-2002 grew to 68.9% in 2003-2018.
Although the study “was hindered by incomplete race and ethnicity data,” Mr. Barskey said, “its breadth was a strength.”
Consider legionella in your diagnosis
In an interview, Paul G. Auwaerter, MD, a professor of medicine and the clinical director of the Division of Infectious Diseases at Johns Hopkins University School of Medicine, Baltimore, said he was not surprised by the results. “CDC has been reporting increased incidence of Legionnaires’ disease from water source outbreaks over the years. As a clinician, I very much depend on epidemiologic trends to help me understand the patient in front of me.
“The key point is that there’s more of it around, so consider it in your diagnosis,” he advised.
“Physicians are increasingly beginning to consider Legionella. Because LD is difficult to diagnose by traditional methods such as culture, they may use a PCR test,” said Dr. Auwaerter, who was not involved in the study. “Legionella needs antibiotics that differ a bit from traditional antibiotics used to treat bacterial pneumonia, so a correct diagnosis can inform a more directed therapy.”
“Why the incidence is increasing is the big question, and the authors nicely outline a litany of things,” he said.
The authors and Dr. Auwaerter proposed a number of possible contributing factors to the increased incidence:
- an aging population
- aging municipal and residential water sources that may harbor more organisms
- racial disparities and poverty
- underlying conditions, including diabetes, end-stage renal disease, and some cancers
- occupations in transportation, repair, cleaning services, and construction
- weather patterns
- improved surveillance and reporting
“Why Legionella appears in some locations more than others has not been explained,” Dr. Auwaerter added. “For example, Pittsburgh always seemed to have much more Legionella than Baltimore.”
Mr. Barskey and his team are planning further research into racial disparities and links between weather and climate and Legionnaires’ disease.
The authors are employees of CDC. Dr. Auwaerter has disclosed no relevant financial realtionships.
A version of this article first appeared on Medscape.com.
Geography hampers access to lung cancer screening
a recent analysis shows.
That percentage, although quite small, still translates to more than 750,000 individuals who are eligible to receive lung cancer screening but live at least 40 miles from a facility.
Overall, a larger proportion of eligible individuals in rural areas had no access to a facility, but a greater number of people in urban areas had no access, especially at shorter distances.
Understanding access issues is important given that “lung cancer screening with low-dose computed tomography scanning (LDCT) reduces mortality among high-risk adults, ... [but] annual screening rates remain low,” write study authors Liora Sahar, PhD, of the American Cancer Society in Atlanta, and colleagues.
The study was published online Feb. 15 in the journal Cancer.
It expands on a previous report, which found that “less than 6% of those 55 to 79 years of age do not have access to registry screening facilities”.
The new analysis incorporates the most recent guidelines from the U.S. Preventive Services Task Force, which lowered the screening age to 50 years and compares access across urban and rural areas.
Dr. Sahar and colleagues calculated the distances from population centers to screening facilities and estimated the number of individuals living within different distances of those facilities – 10, 20, 40, 50, and 100 miles. Geographical subdivisions, or census tracts, were also classified along a spectrum of rural to urban.
The authors found that, overall, about 14.8 million people aged 50-80 years are eligible for lung cancer screening, and 5.1% of that population – or 753,038 individuals – do not live within 40 miles of a facility and have no access to screening.
The proportion of people affected by access issues varies by geographic location. For eligible people living 40 miles or more from a facility, almost 25% (n = 287,803) in rural counties had no access, compared with 1.6% (n = 195,120) in metropolitan areas.
At greater distances to facilities (50 and 100 miles), these proportions diminish. In rural counties, for instance, 16% of eligible individuals (n = 186,401) living 50 or more miles away and 2.8% (n = 33,504) living 100 or more miles away had no access to a facility.
Not surprisingly, across all distances, “there is a significantly higher percentage of rural residents who do not have access to facilities in comparison with those in urban settings,” the authors write. “There are fewer facilities in rural areas, so residents need to travel longer distances to reach a facility.”
Notably, however, distance to a facility was not necessarily the greatest barrier to screening. The authors found a greater number of eligible individuals living in or close to urban areas were not getting screening when facilities were 10 miles away – more than 2.8 million in metropolitan areas versus just over 1 million in rural areas.
“The total number of individuals with no access in urban areas exceeds that of rural individuals, particularly at shorter distances ... [which] reveals an additional underserved population.”
Identifying geographic areas with greater access issues can help researchers address barriers to screening and improve uptake.
“Areas and local pockets with persistently low or no access across short and long distances should be considered for tailored interventions, such as implementing mobile units, repurposing existing imaging or health facilities, and adding appropriate navigation, radiology, and screening program staff to better support the communities,” the authors conclude.
The study was supported in part by the National Lung Cancer Roundtable. Coauthor Debra S. Dyer, MD, serves on the clinical advisory board for computer software company Imidex and on the GO2 Foundation scientific advisory board; she also serves as a consultant for Lung Ambition Alliance. Coauthor Ella A. Kazerooni, MD, reports past participation on the Bristol Myers Squibb Foundation advisory board. The other authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a recent analysis shows.
That percentage, although quite small, still translates to more than 750,000 individuals who are eligible to receive lung cancer screening but live at least 40 miles from a facility.
Overall, a larger proportion of eligible individuals in rural areas had no access to a facility, but a greater number of people in urban areas had no access, especially at shorter distances.
Understanding access issues is important given that “lung cancer screening with low-dose computed tomography scanning (LDCT) reduces mortality among high-risk adults, ... [but] annual screening rates remain low,” write study authors Liora Sahar, PhD, of the American Cancer Society in Atlanta, and colleagues.
The study was published online Feb. 15 in the journal Cancer.
It expands on a previous report, which found that “less than 6% of those 55 to 79 years of age do not have access to registry screening facilities”.
The new analysis incorporates the most recent guidelines from the U.S. Preventive Services Task Force, which lowered the screening age to 50 years and compares access across urban and rural areas.
Dr. Sahar and colleagues calculated the distances from population centers to screening facilities and estimated the number of individuals living within different distances of those facilities – 10, 20, 40, 50, and 100 miles. Geographical subdivisions, or census tracts, were also classified along a spectrum of rural to urban.
The authors found that, overall, about 14.8 million people aged 50-80 years are eligible for lung cancer screening, and 5.1% of that population – or 753,038 individuals – do not live within 40 miles of a facility and have no access to screening.
The proportion of people affected by access issues varies by geographic location. For eligible people living 40 miles or more from a facility, almost 25% (n = 287,803) in rural counties had no access, compared with 1.6% (n = 195,120) in metropolitan areas.
At greater distances to facilities (50 and 100 miles), these proportions diminish. In rural counties, for instance, 16% of eligible individuals (n = 186,401) living 50 or more miles away and 2.8% (n = 33,504) living 100 or more miles away had no access to a facility.
Not surprisingly, across all distances, “there is a significantly higher percentage of rural residents who do not have access to facilities in comparison with those in urban settings,” the authors write. “There are fewer facilities in rural areas, so residents need to travel longer distances to reach a facility.”
Notably, however, distance to a facility was not necessarily the greatest barrier to screening. The authors found a greater number of eligible individuals living in or close to urban areas were not getting screening when facilities were 10 miles away – more than 2.8 million in metropolitan areas versus just over 1 million in rural areas.
“The total number of individuals with no access in urban areas exceeds that of rural individuals, particularly at shorter distances ... [which] reveals an additional underserved population.”
Identifying geographic areas with greater access issues can help researchers address barriers to screening and improve uptake.
“Areas and local pockets with persistently low or no access across short and long distances should be considered for tailored interventions, such as implementing mobile units, repurposing existing imaging or health facilities, and adding appropriate navigation, radiology, and screening program staff to better support the communities,” the authors conclude.
The study was supported in part by the National Lung Cancer Roundtable. Coauthor Debra S. Dyer, MD, serves on the clinical advisory board for computer software company Imidex and on the GO2 Foundation scientific advisory board; she also serves as a consultant for Lung Ambition Alliance. Coauthor Ella A. Kazerooni, MD, reports past participation on the Bristol Myers Squibb Foundation advisory board. The other authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a recent analysis shows.
That percentage, although quite small, still translates to more than 750,000 individuals who are eligible to receive lung cancer screening but live at least 40 miles from a facility.
Overall, a larger proportion of eligible individuals in rural areas had no access to a facility, but a greater number of people in urban areas had no access, especially at shorter distances.
Understanding access issues is important given that “lung cancer screening with low-dose computed tomography scanning (LDCT) reduces mortality among high-risk adults, ... [but] annual screening rates remain low,” write study authors Liora Sahar, PhD, of the American Cancer Society in Atlanta, and colleagues.
The study was published online Feb. 15 in the journal Cancer.
It expands on a previous report, which found that “less than 6% of those 55 to 79 years of age do not have access to registry screening facilities”.
The new analysis incorporates the most recent guidelines from the U.S. Preventive Services Task Force, which lowered the screening age to 50 years and compares access across urban and rural areas.
Dr. Sahar and colleagues calculated the distances from population centers to screening facilities and estimated the number of individuals living within different distances of those facilities – 10, 20, 40, 50, and 100 miles. Geographical subdivisions, or census tracts, were also classified along a spectrum of rural to urban.
The authors found that, overall, about 14.8 million people aged 50-80 years are eligible for lung cancer screening, and 5.1% of that population – or 753,038 individuals – do not live within 40 miles of a facility and have no access to screening.
The proportion of people affected by access issues varies by geographic location. For eligible people living 40 miles or more from a facility, almost 25% (n = 287,803) in rural counties had no access, compared with 1.6% (n = 195,120) in metropolitan areas.
At greater distances to facilities (50 and 100 miles), these proportions diminish. In rural counties, for instance, 16% of eligible individuals (n = 186,401) living 50 or more miles away and 2.8% (n = 33,504) living 100 or more miles away had no access to a facility.
Not surprisingly, across all distances, “there is a significantly higher percentage of rural residents who do not have access to facilities in comparison with those in urban settings,” the authors write. “There are fewer facilities in rural areas, so residents need to travel longer distances to reach a facility.”
Notably, however, distance to a facility was not necessarily the greatest barrier to screening. The authors found a greater number of eligible individuals living in or close to urban areas were not getting screening when facilities were 10 miles away – more than 2.8 million in metropolitan areas versus just over 1 million in rural areas.
“The total number of individuals with no access in urban areas exceeds that of rural individuals, particularly at shorter distances ... [which] reveals an additional underserved population.”
Identifying geographic areas with greater access issues can help researchers address barriers to screening and improve uptake.
“Areas and local pockets with persistently low or no access across short and long distances should be considered for tailored interventions, such as implementing mobile units, repurposing existing imaging or health facilities, and adding appropriate navigation, radiology, and screening program staff to better support the communities,” the authors conclude.
The study was supported in part by the National Lung Cancer Roundtable. Coauthor Debra S. Dyer, MD, serves on the clinical advisory board for computer software company Imidex and on the GO2 Foundation scientific advisory board; she also serves as a consultant for Lung Ambition Alliance. Coauthor Ella A. Kazerooni, MD, reports past participation on the Bristol Myers Squibb Foundation advisory board. The other authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CANCER