User login
mIDH1 inhibitor ivosidenib improves progression-free survival in advanced cholangiocarcinoma
BARCELONA – Ivosidenib, a first-in-class, oral, small-molecule inhibitor of the mutant isocitrate dehydrogenase 1 (mIDH1) protein, significantly improved progression-free survival, compared with placebo, for the treatment of advanced cholangiocarcinoma in the global, randomized, phase 3 ClarIDHy trial.
A trend toward favorable overall survival was also seen in the pivotal double-blind trial, Ghassan K. Abou-Alfa, MD, of Memorial Sloan-Kettering Cancer Center, New York, reported at the European Society for Medical Oncology Congress.
Median progression-free survival (PFS) in 124 patients randomized to receive 500 mg of ivosidenib (IVO) once daily was 2.7 months, compared with 1.4 months in 61 patients who received placebo (hazard ratio, 0.37).
The primary study endpoint – PFS by central review – was reached, Dr. Abou-Alfa said, noting that the PFS rates at 6 and 12 months were 32% and 22% in the IVO arm, whereas none of the patients in the placebo arm were progression-free for 6 or more months.
Although the 1.3-month difference in PFS between the treatment and placebo arms “may seem short and some people may question whether this is clinically meaningful,” this outcome actually represents an important breakthrough for patients with a disease that has few treatment options, Angela Lamarca, MD, PhD, of the Christie NHS Foundation Trust, Manchester, England, explained in a press release about the study.
“A treatment that increases the chance of being free from progression by 32% at 6 months after starting treatment and that prolongs survival from 6 months with placebo to 10.8 months with ivosidenib, after adjusting for crossover, is definitely meaningful for our patients with cholangiocarcinoma and their families,” said Dr. Lamarca, representing the ESMO Press & Media Affairs Committee.
Indeed, the overall response rate in the IVO arm was 2.4%, representing three partial responses, and the stable disease rate was 50.8%. The overall response rate and stable disease rates in the placebo arm were 0% and 28%, Dr. Abou-Alfa said.
Median overall survival in the intent-to-treat population, including the 57% of placebo arm patients who crossed over to the treatment arm at the time of radiographic progression as allowed by study protocol, was 10.8 months, compared with 9.7 months in the placebo arm, respectively (HR, 0.69), Dr. Abou-Alfa said.
Crossover-adjusted overall survival was 6 months for the placebo arm (HR, 0.46) as assessed using rank-preserving structural failure time analysis, which suggested that the overall survival benefit would have been statistically significant had there been no crossover.
Study subjects had unresectable or metastatic mIDH1 cholangiocarcinoma, good performance status, and measurable disease. They had a median age of 62 years, and 68 were men. Most had intrahepatic disease (91%) and metastatic disease (92%), and 43% had two prior therapies, he noted.
They were randomized 2:1 to the treatment and placebo arms, respectively.
Since IVO targets mIDH1, which occurs in about 20% of patients with cholangiocarcinoma and results in production of the oncogenesis-promoting oncometabolite D-2-hydroxyglutarate, and since the IVO has shown encouraging activity in smaller prior studies, ClarIDHy was designed to evaluate it in the advanced mIDH1 cholangiocarcinoma setting because patients with this aggressive disease have generally poor prognosis and few treatment options beyond chemotherapy, he explained.
In addition to providing significant, clinically meaningful survival benefit, the treatment also was generally well tolerated, he noted.
Treatment-emergent adverse events occurring in more than 15% of patients in the IVO arm included nausea (32.1%), diarrhea (28.8%), fatigue (23.7%), cough (19.2%), abdominal pain (18.6%), ascites (18.6%), decreased appetite (17.3%), anemia (16.0%), and vomiting (16.0%). Grade 3 or higher adverse events were reported in 46% and 36% of the IVO and placebo patients, respectively.
The findings are notable, in part because of the unmet need and also because this is the first pivotal study demonstrating the clinical benefit of targeting mIDH1 in patients with advanced mIDH1 cholangiocarcinoma, he said, concluding that “these pivotal data demonstrate the clinical relevance and benefit of ivosidenib in mIDH1 cholangiocarcinoma ... [and] establish the role for genomic testing in this rare cancer with a high unmet need.”
He also said studies should investigate IVO in the first-line setting for IDH1-mutated cholangiocarcinoma, in addition to its use in combination therapy and as adjuvant therapy.
In the ESMO press release, Chris Verslype, MD, of University Hospital Leuven (Belgium) called the findings of this study unprecedented given the lack of treatment options in those who fail systemic therapy, which has led to very limited survival.
The findings are “very likely to change clinical practice” and “will, for sure, drive the further development of targeted therapy for the disease,” he said.
Despite being limited by the requirement that patients have good performance status after prior chemotherapy (which means the findings may not be representative of all patients), ClarIDHy is “still a strong study because of the randomization to placebo.”
“It showed a real effect,” he said.
ClarIDHy was funded by Agios Pharmaceuticals. Dr. Abou-Alfa reported both personal and institutional relationships with industry. These include advisory /consulting roles and research grants/funding from numerous pharmaceutical companies.
SOURCE: Abou-Alfa GK et al. ESMO 2019, Abstract LBA10-PR.
BARCELONA – Ivosidenib, a first-in-class, oral, small-molecule inhibitor of the mutant isocitrate dehydrogenase 1 (mIDH1) protein, significantly improved progression-free survival, compared with placebo, for the treatment of advanced cholangiocarcinoma in the global, randomized, phase 3 ClarIDHy trial.
A trend toward favorable overall survival was also seen in the pivotal double-blind trial, Ghassan K. Abou-Alfa, MD, of Memorial Sloan-Kettering Cancer Center, New York, reported at the European Society for Medical Oncology Congress.
Median progression-free survival (PFS) in 124 patients randomized to receive 500 mg of ivosidenib (IVO) once daily was 2.7 months, compared with 1.4 months in 61 patients who received placebo (hazard ratio, 0.37).
The primary study endpoint – PFS by central review – was reached, Dr. Abou-Alfa said, noting that the PFS rates at 6 and 12 months were 32% and 22% in the IVO arm, whereas none of the patients in the placebo arm were progression-free for 6 or more months.
Although the 1.3-month difference in PFS between the treatment and placebo arms “may seem short and some people may question whether this is clinically meaningful,” this outcome actually represents an important breakthrough for patients with a disease that has few treatment options, Angela Lamarca, MD, PhD, of the Christie NHS Foundation Trust, Manchester, England, explained in a press release about the study.
“A treatment that increases the chance of being free from progression by 32% at 6 months after starting treatment and that prolongs survival from 6 months with placebo to 10.8 months with ivosidenib, after adjusting for crossover, is definitely meaningful for our patients with cholangiocarcinoma and their families,” said Dr. Lamarca, representing the ESMO Press & Media Affairs Committee.
Indeed, the overall response rate in the IVO arm was 2.4%, representing three partial responses, and the stable disease rate was 50.8%. The overall response rate and stable disease rates in the placebo arm were 0% and 28%, Dr. Abou-Alfa said.
Median overall survival in the intent-to-treat population, including the 57% of placebo arm patients who crossed over to the treatment arm at the time of radiographic progression as allowed by study protocol, was 10.8 months, compared with 9.7 months in the placebo arm, respectively (HR, 0.69), Dr. Abou-Alfa said.
Crossover-adjusted overall survival was 6 months for the placebo arm (HR, 0.46) as assessed using rank-preserving structural failure time analysis, which suggested that the overall survival benefit would have been statistically significant had there been no crossover.
Study subjects had unresectable or metastatic mIDH1 cholangiocarcinoma, good performance status, and measurable disease. They had a median age of 62 years, and 68 were men. Most had intrahepatic disease (91%) and metastatic disease (92%), and 43% had two prior therapies, he noted.
They were randomized 2:1 to the treatment and placebo arms, respectively.
Since IVO targets mIDH1, which occurs in about 20% of patients with cholangiocarcinoma and results in production of the oncogenesis-promoting oncometabolite D-2-hydroxyglutarate, and since the IVO has shown encouraging activity in smaller prior studies, ClarIDHy was designed to evaluate it in the advanced mIDH1 cholangiocarcinoma setting because patients with this aggressive disease have generally poor prognosis and few treatment options beyond chemotherapy, he explained.
In addition to providing significant, clinically meaningful survival benefit, the treatment also was generally well tolerated, he noted.
Treatment-emergent adverse events occurring in more than 15% of patients in the IVO arm included nausea (32.1%), diarrhea (28.8%), fatigue (23.7%), cough (19.2%), abdominal pain (18.6%), ascites (18.6%), decreased appetite (17.3%), anemia (16.0%), and vomiting (16.0%). Grade 3 or higher adverse events were reported in 46% and 36% of the IVO and placebo patients, respectively.
The findings are notable, in part because of the unmet need and also because this is the first pivotal study demonstrating the clinical benefit of targeting mIDH1 in patients with advanced mIDH1 cholangiocarcinoma, he said, concluding that “these pivotal data demonstrate the clinical relevance and benefit of ivosidenib in mIDH1 cholangiocarcinoma ... [and] establish the role for genomic testing in this rare cancer with a high unmet need.”
He also said studies should investigate IVO in the first-line setting for IDH1-mutated cholangiocarcinoma, in addition to its use in combination therapy and as adjuvant therapy.
In the ESMO press release, Chris Verslype, MD, of University Hospital Leuven (Belgium) called the findings of this study unprecedented given the lack of treatment options in those who fail systemic therapy, which has led to very limited survival.
The findings are “very likely to change clinical practice” and “will, for sure, drive the further development of targeted therapy for the disease,” he said.
Despite being limited by the requirement that patients have good performance status after prior chemotherapy (which means the findings may not be representative of all patients), ClarIDHy is “still a strong study because of the randomization to placebo.”
“It showed a real effect,” he said.
ClarIDHy was funded by Agios Pharmaceuticals. Dr. Abou-Alfa reported both personal and institutional relationships with industry. These include advisory /consulting roles and research grants/funding from numerous pharmaceutical companies.
SOURCE: Abou-Alfa GK et al. ESMO 2019, Abstract LBA10-PR.
BARCELONA – Ivosidenib, a first-in-class, oral, small-molecule inhibitor of the mutant isocitrate dehydrogenase 1 (mIDH1) protein, significantly improved progression-free survival, compared with placebo, for the treatment of advanced cholangiocarcinoma in the global, randomized, phase 3 ClarIDHy trial.
A trend toward favorable overall survival was also seen in the pivotal double-blind trial, Ghassan K. Abou-Alfa, MD, of Memorial Sloan-Kettering Cancer Center, New York, reported at the European Society for Medical Oncology Congress.
Median progression-free survival (PFS) in 124 patients randomized to receive 500 mg of ivosidenib (IVO) once daily was 2.7 months, compared with 1.4 months in 61 patients who received placebo (hazard ratio, 0.37).
The primary study endpoint – PFS by central review – was reached, Dr. Abou-Alfa said, noting that the PFS rates at 6 and 12 months were 32% and 22% in the IVO arm, whereas none of the patients in the placebo arm were progression-free for 6 or more months.
Although the 1.3-month difference in PFS between the treatment and placebo arms “may seem short and some people may question whether this is clinically meaningful,” this outcome actually represents an important breakthrough for patients with a disease that has few treatment options, Angela Lamarca, MD, PhD, of the Christie NHS Foundation Trust, Manchester, England, explained in a press release about the study.
“A treatment that increases the chance of being free from progression by 32% at 6 months after starting treatment and that prolongs survival from 6 months with placebo to 10.8 months with ivosidenib, after adjusting for crossover, is definitely meaningful for our patients with cholangiocarcinoma and their families,” said Dr. Lamarca, representing the ESMO Press & Media Affairs Committee.
Indeed, the overall response rate in the IVO arm was 2.4%, representing three partial responses, and the stable disease rate was 50.8%. The overall response rate and stable disease rates in the placebo arm were 0% and 28%, Dr. Abou-Alfa said.
Median overall survival in the intent-to-treat population, including the 57% of placebo arm patients who crossed over to the treatment arm at the time of radiographic progression as allowed by study protocol, was 10.8 months, compared with 9.7 months in the placebo arm, respectively (HR, 0.69), Dr. Abou-Alfa said.
Crossover-adjusted overall survival was 6 months for the placebo arm (HR, 0.46) as assessed using rank-preserving structural failure time analysis, which suggested that the overall survival benefit would have been statistically significant had there been no crossover.
Study subjects had unresectable or metastatic mIDH1 cholangiocarcinoma, good performance status, and measurable disease. They had a median age of 62 years, and 68 were men. Most had intrahepatic disease (91%) and metastatic disease (92%), and 43% had two prior therapies, he noted.
They were randomized 2:1 to the treatment and placebo arms, respectively.
Since IVO targets mIDH1, which occurs in about 20% of patients with cholangiocarcinoma and results in production of the oncogenesis-promoting oncometabolite D-2-hydroxyglutarate, and since the IVO has shown encouraging activity in smaller prior studies, ClarIDHy was designed to evaluate it in the advanced mIDH1 cholangiocarcinoma setting because patients with this aggressive disease have generally poor prognosis and few treatment options beyond chemotherapy, he explained.
In addition to providing significant, clinically meaningful survival benefit, the treatment also was generally well tolerated, he noted.
Treatment-emergent adverse events occurring in more than 15% of patients in the IVO arm included nausea (32.1%), diarrhea (28.8%), fatigue (23.7%), cough (19.2%), abdominal pain (18.6%), ascites (18.6%), decreased appetite (17.3%), anemia (16.0%), and vomiting (16.0%). Grade 3 or higher adverse events were reported in 46% and 36% of the IVO and placebo patients, respectively.
The findings are notable, in part because of the unmet need and also because this is the first pivotal study demonstrating the clinical benefit of targeting mIDH1 in patients with advanced mIDH1 cholangiocarcinoma, he said, concluding that “these pivotal data demonstrate the clinical relevance and benefit of ivosidenib in mIDH1 cholangiocarcinoma ... [and] establish the role for genomic testing in this rare cancer with a high unmet need.”
He also said studies should investigate IVO in the first-line setting for IDH1-mutated cholangiocarcinoma, in addition to its use in combination therapy and as adjuvant therapy.
In the ESMO press release, Chris Verslype, MD, of University Hospital Leuven (Belgium) called the findings of this study unprecedented given the lack of treatment options in those who fail systemic therapy, which has led to very limited survival.
The findings are “very likely to change clinical practice” and “will, for sure, drive the further development of targeted therapy for the disease,” he said.
Despite being limited by the requirement that patients have good performance status after prior chemotherapy (which means the findings may not be representative of all patients), ClarIDHy is “still a strong study because of the randomization to placebo.”
“It showed a real effect,” he said.
ClarIDHy was funded by Agios Pharmaceuticals. Dr. Abou-Alfa reported both personal and institutional relationships with industry. These include advisory /consulting roles and research grants/funding from numerous pharmaceutical companies.
SOURCE: Abou-Alfa GK et al. ESMO 2019, Abstract LBA10-PR.
REPORTING FROM ESMO 2019
FDA approves rituximab to treat children with rare vasculitis
The Food and Drug Administration approved rituximab (Rituxan) by injection to treat granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA) in children 2 years of age and older in combination with glucocorticoid treatment, according to an FDA news release.
These rare forms of vasculitis damage small blood vessels through inflammation and can lead to serious organ failure, including lungs and kidneys.
The Genentech drug received priority review and an orphan drug designation based on the results of a pediatric clinical trial of 25 patients aged 6-17 years with active GPA or MPA who were treated with rituximab in an international multicenter, open-label, uncontrolled study. Patients in the trial were also given methylprednisolone prior to starting treatment.
The trial consisted of a 6-month remission induction phase where patients were treated only with rituximab and glucocorticoids. In addition, patients who had not achieved remission could receive additional treatment, including other therapies, at the discretion of the investigator, according to the FDA. By 6 months, 14 of the patients were in remission, and after 18 months, all 25 patients were in remission.
Rituximab contains a boxed warning regarding increased risks of fatal infusion reactions, potentially fatal severe skin and mouth reactions, hepatitis B virus reactivation that may cause serious or lethal liver problems, and progressive multifocal leukoencephalopathy, a rare, potentially lethal brain infection.
The trial was conducted and sponsored by F. Hoffmann-La Roche, which owns Genentech.
The Food and Drug Administration approved rituximab (Rituxan) by injection to treat granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA) in children 2 years of age and older in combination with glucocorticoid treatment, according to an FDA news release.
These rare forms of vasculitis damage small blood vessels through inflammation and can lead to serious organ failure, including lungs and kidneys.
The Genentech drug received priority review and an orphan drug designation based on the results of a pediatric clinical trial of 25 patients aged 6-17 years with active GPA or MPA who were treated with rituximab in an international multicenter, open-label, uncontrolled study. Patients in the trial were also given methylprednisolone prior to starting treatment.
The trial consisted of a 6-month remission induction phase where patients were treated only with rituximab and glucocorticoids. In addition, patients who had not achieved remission could receive additional treatment, including other therapies, at the discretion of the investigator, according to the FDA. By 6 months, 14 of the patients were in remission, and after 18 months, all 25 patients were in remission.
Rituximab contains a boxed warning regarding increased risks of fatal infusion reactions, potentially fatal severe skin and mouth reactions, hepatitis B virus reactivation that may cause serious or lethal liver problems, and progressive multifocal leukoencephalopathy, a rare, potentially lethal brain infection.
The trial was conducted and sponsored by F. Hoffmann-La Roche, which owns Genentech.
The Food and Drug Administration approved rituximab (Rituxan) by injection to treat granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA) in children 2 years of age and older in combination with glucocorticoid treatment, according to an FDA news release.
These rare forms of vasculitis damage small blood vessels through inflammation and can lead to serious organ failure, including lungs and kidneys.
The Genentech drug received priority review and an orphan drug designation based on the results of a pediatric clinical trial of 25 patients aged 6-17 years with active GPA or MPA who were treated with rituximab in an international multicenter, open-label, uncontrolled study. Patients in the trial were also given methylprednisolone prior to starting treatment.
The trial consisted of a 6-month remission induction phase where patients were treated only with rituximab and glucocorticoids. In addition, patients who had not achieved remission could receive additional treatment, including other therapies, at the discretion of the investigator, according to the FDA. By 6 months, 14 of the patients were in remission, and after 18 months, all 25 patients were in remission.
Rituximab contains a boxed warning regarding increased risks of fatal infusion reactions, potentially fatal severe skin and mouth reactions, hepatitis B virus reactivation that may cause serious or lethal liver problems, and progressive multifocal leukoencephalopathy, a rare, potentially lethal brain infection.
The trial was conducted and sponsored by F. Hoffmann-La Roche, which owns Genentech.
Anakinra treatment for pediatric ‘cytokine storms’: Does one size fit all?
The biologic drug anakinra appears to be effective in treating children with secondary hemophagocytic lymphohistiocytosis (sHLH)/macrophage activation syndrome (MAS), a dangerous “cytokine storm” that can emerge from infections, cancer, and rheumatic diseases.
Children with systematic juvenile idiopathic arthritis (sJIA) and sHLH/MAS are especially good candidates for treatment with the interleukin-1 receptor antagonist anakinra (Kineret), in whom its safety and benefits have been more widely explored than in pediatric patients with sHLH/MAS related to non-sJIA underlying conditions.
In a study published in Arthritis & Rheumatology, Esraa Eloseily, MD, and colleagues at the University of Alabama at Birmingham, looked at hospitalization records for 44 children (mean age, 10 years; n = 25 females) with sHLH/MAS. The children in the study had heterogeneous underlying conditions including leukemias, infections, and rheumatic diseases. About one-third of patients had no known rheumatic or autoimmune disorder.
Dr. Eloseily and colleagues found that early initiation of anakinra (within 5 days of hospitalization) was significantly associated with improved survival across the cohort, for which mortality was 27%. Thrombocytopenia (less than 100,000/mcL) and STXBP2 mutations were both seen significantly associated with mortality.
Patients with blood cancers – even those in remission at the time of treatment – did poorly. None of the three patients in the cohort with leukemia survived.
Importantly, no deaths were seen among the 13 patients with underlying SJIA who were treated with anakinra, suggesting particular benefit for this patient group.
“In addition to the 10% risk of developing overt MAS as part of sJIA, another 30%-40% of sJIA patients may have occult or subclinical MAS during a disease flare that can eventually lead to overt MAS,” Dr. Eloseily and colleagues wrote. “This association of MAS with sJIA suggested that anakinra would also be a valuable treatment for sJIA-MAS.”
The investigators acknowledged that their study was limited by its retrospective design and “nonuniform approach to therapy, lack of treatment controls, and variable follow-up period.” The authors also acknowledged the potential for selection bias favoring anakinra use in patients who are less severely ill.
In a comment accompanying Dr. Eloseily and colleagues’ study, Sarah Nikiforow, MD, PhD, of the Dana-Farber Cancer Institute in Boston, and Nancy Berliner, MD, of Brigham & Women’s Hospital in Boston, urged clinicians not to interpret the study results as supporting anakinra as “a carte blanche approach to hyperinflammatory syndromes.”
While the study supported the use of anakinra in sJIA with MAS or sHLH, “we posit that patients [with sHLH/MAS] in sepsis, cytokine release syndrome following chimeric antigen receptor T-cell therapy, and other hyperinflammatory syndromes still require individualized approaches to therapy,” Dr. Nikiforow and Dr. Berliner wrote, adding that, “in several studies and anecdotally in our institutional practice, cytotoxic chemotherapy was/is preferred over biologic agents in patients with evidence of more severe inflammatory activity.”
Outside sJIA, Dr. Nikiforow and Dr. Berliner wrote, “early anakinra therapy should be extended to treatment of other forms of sHLH with extreme caution. Specifically, the authors’ suggestion that cytotoxic therapy should be ‘considered’ only after anakinra therapy may be dangerous for some patients.”
Two of Dr. Eloseily’s coinvestigators reported financial and research support from Sobi, the manufacturer of anakinra. No other conflicts of interest were reported.
SOURCES: Eloseily E et al. Arthritis Rheumatol. 2019 Sep 12. doi: 10.1002/art.41103; Nikiforow S, Berliner N. Arthritis Rheumatol. 2019 Sep 16. doi: 10.1002/art.41106.
The biologic drug anakinra appears to be effective in treating children with secondary hemophagocytic lymphohistiocytosis (sHLH)/macrophage activation syndrome (MAS), a dangerous “cytokine storm” that can emerge from infections, cancer, and rheumatic diseases.
Children with systematic juvenile idiopathic arthritis (sJIA) and sHLH/MAS are especially good candidates for treatment with the interleukin-1 receptor antagonist anakinra (Kineret), in whom its safety and benefits have been more widely explored than in pediatric patients with sHLH/MAS related to non-sJIA underlying conditions.
In a study published in Arthritis & Rheumatology, Esraa Eloseily, MD, and colleagues at the University of Alabama at Birmingham, looked at hospitalization records for 44 children (mean age, 10 years; n = 25 females) with sHLH/MAS. The children in the study had heterogeneous underlying conditions including leukemias, infections, and rheumatic diseases. About one-third of patients had no known rheumatic or autoimmune disorder.
Dr. Eloseily and colleagues found that early initiation of anakinra (within 5 days of hospitalization) was significantly associated with improved survival across the cohort, for which mortality was 27%. Thrombocytopenia (less than 100,000/mcL) and STXBP2 mutations were both seen significantly associated with mortality.
Patients with blood cancers – even those in remission at the time of treatment – did poorly. None of the three patients in the cohort with leukemia survived.
Importantly, no deaths were seen among the 13 patients with underlying SJIA who were treated with anakinra, suggesting particular benefit for this patient group.
“In addition to the 10% risk of developing overt MAS as part of sJIA, another 30%-40% of sJIA patients may have occult or subclinical MAS during a disease flare that can eventually lead to overt MAS,” Dr. Eloseily and colleagues wrote. “This association of MAS with sJIA suggested that anakinra would also be a valuable treatment for sJIA-MAS.”
The investigators acknowledged that their study was limited by its retrospective design and “nonuniform approach to therapy, lack of treatment controls, and variable follow-up period.” The authors also acknowledged the potential for selection bias favoring anakinra use in patients who are less severely ill.
In a comment accompanying Dr. Eloseily and colleagues’ study, Sarah Nikiforow, MD, PhD, of the Dana-Farber Cancer Institute in Boston, and Nancy Berliner, MD, of Brigham & Women’s Hospital in Boston, urged clinicians not to interpret the study results as supporting anakinra as “a carte blanche approach to hyperinflammatory syndromes.”
While the study supported the use of anakinra in sJIA with MAS or sHLH, “we posit that patients [with sHLH/MAS] in sepsis, cytokine release syndrome following chimeric antigen receptor T-cell therapy, and other hyperinflammatory syndromes still require individualized approaches to therapy,” Dr. Nikiforow and Dr. Berliner wrote, adding that, “in several studies and anecdotally in our institutional practice, cytotoxic chemotherapy was/is preferred over biologic agents in patients with evidence of more severe inflammatory activity.”
Outside sJIA, Dr. Nikiforow and Dr. Berliner wrote, “early anakinra therapy should be extended to treatment of other forms of sHLH with extreme caution. Specifically, the authors’ suggestion that cytotoxic therapy should be ‘considered’ only after anakinra therapy may be dangerous for some patients.”
Two of Dr. Eloseily’s coinvestigators reported financial and research support from Sobi, the manufacturer of anakinra. No other conflicts of interest were reported.
SOURCES: Eloseily E et al. Arthritis Rheumatol. 2019 Sep 12. doi: 10.1002/art.41103; Nikiforow S, Berliner N. Arthritis Rheumatol. 2019 Sep 16. doi: 10.1002/art.41106.
The biologic drug anakinra appears to be effective in treating children with secondary hemophagocytic lymphohistiocytosis (sHLH)/macrophage activation syndrome (MAS), a dangerous “cytokine storm” that can emerge from infections, cancer, and rheumatic diseases.
Children with systematic juvenile idiopathic arthritis (sJIA) and sHLH/MAS are especially good candidates for treatment with the interleukin-1 receptor antagonist anakinra (Kineret), in whom its safety and benefits have been more widely explored than in pediatric patients with sHLH/MAS related to non-sJIA underlying conditions.
In a study published in Arthritis & Rheumatology, Esraa Eloseily, MD, and colleagues at the University of Alabama at Birmingham, looked at hospitalization records for 44 children (mean age, 10 years; n = 25 females) with sHLH/MAS. The children in the study had heterogeneous underlying conditions including leukemias, infections, and rheumatic diseases. About one-third of patients had no known rheumatic or autoimmune disorder.
Dr. Eloseily and colleagues found that early initiation of anakinra (within 5 days of hospitalization) was significantly associated with improved survival across the cohort, for which mortality was 27%. Thrombocytopenia (less than 100,000/mcL) and STXBP2 mutations were both seen significantly associated with mortality.
Patients with blood cancers – even those in remission at the time of treatment – did poorly. None of the three patients in the cohort with leukemia survived.
Importantly, no deaths were seen among the 13 patients with underlying SJIA who were treated with anakinra, suggesting particular benefit for this patient group.
“In addition to the 10% risk of developing overt MAS as part of sJIA, another 30%-40% of sJIA patients may have occult or subclinical MAS during a disease flare that can eventually lead to overt MAS,” Dr. Eloseily and colleagues wrote. “This association of MAS with sJIA suggested that anakinra would also be a valuable treatment for sJIA-MAS.”
The investigators acknowledged that their study was limited by its retrospective design and “nonuniform approach to therapy, lack of treatment controls, and variable follow-up period.” The authors also acknowledged the potential for selection bias favoring anakinra use in patients who are less severely ill.
In a comment accompanying Dr. Eloseily and colleagues’ study, Sarah Nikiforow, MD, PhD, of the Dana-Farber Cancer Institute in Boston, and Nancy Berliner, MD, of Brigham & Women’s Hospital in Boston, urged clinicians not to interpret the study results as supporting anakinra as “a carte blanche approach to hyperinflammatory syndromes.”
While the study supported the use of anakinra in sJIA with MAS or sHLH, “we posit that patients [with sHLH/MAS] in sepsis, cytokine release syndrome following chimeric antigen receptor T-cell therapy, and other hyperinflammatory syndromes still require individualized approaches to therapy,” Dr. Nikiforow and Dr. Berliner wrote, adding that, “in several studies and anecdotally in our institutional practice, cytotoxic chemotherapy was/is preferred over biologic agents in patients with evidence of more severe inflammatory activity.”
Outside sJIA, Dr. Nikiforow and Dr. Berliner wrote, “early anakinra therapy should be extended to treatment of other forms of sHLH with extreme caution. Specifically, the authors’ suggestion that cytotoxic therapy should be ‘considered’ only after anakinra therapy may be dangerous for some patients.”
Two of Dr. Eloseily’s coinvestigators reported financial and research support from Sobi, the manufacturer of anakinra. No other conflicts of interest were reported.
SOURCES: Eloseily E et al. Arthritis Rheumatol. 2019 Sep 12. doi: 10.1002/art.41103; Nikiforow S, Berliner N. Arthritis Rheumatol. 2019 Sep 16. doi: 10.1002/art.41106.
FROM ARTHRITIS & RHEUMATOLOGY
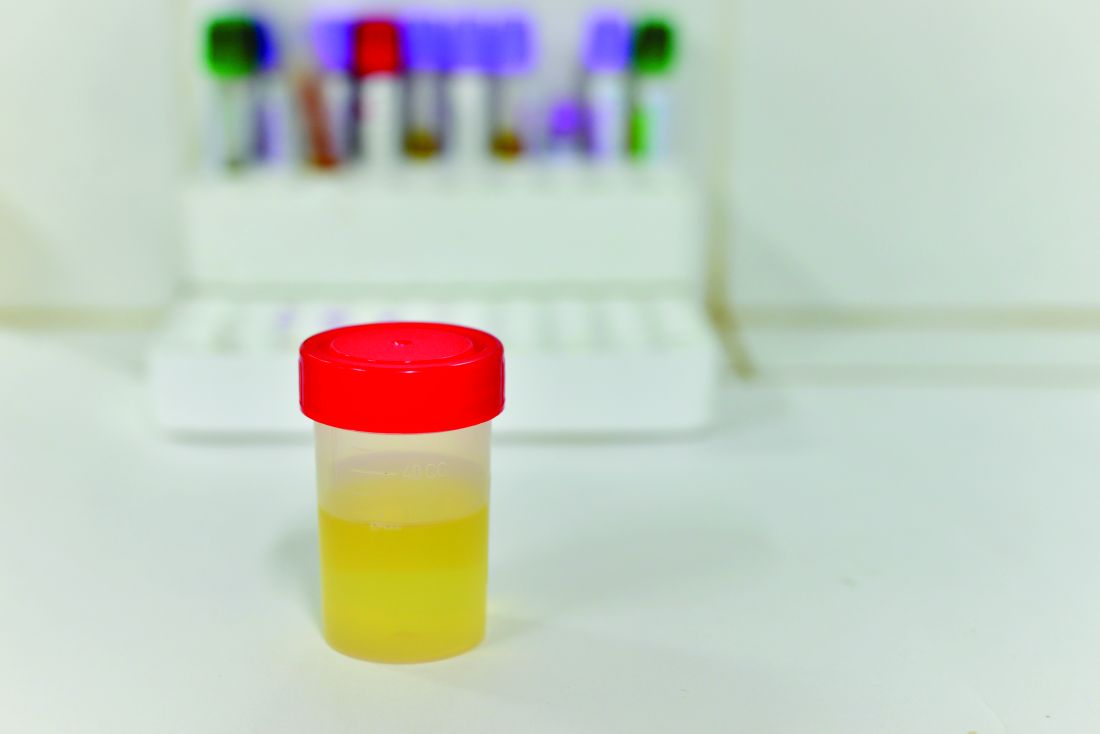
USPSTF: Screening pregnant women for asymptomatic bacteriuria cuts pyelonephritis risk
according to new recommendations set forth by the United States Preventive Services Task Force (USPSTF).
However, the investigating committee reported, there is evidence against screening nonpregnant women and adult men. In fact, the committee found “adequate” evidence of potential harm associated with treating asymptomatic bacteriuria in adults of both sexes, including adverse effects of antibiotics and on the microbiome.
The new document downgrades from A to B the group’s prior recommendation that urine culture screening for asymptomatic bacteriuria should be performed among pregnant women at 12-16 weeks’ gestation or at their first prenatal visit. The USPSTF recommendation to not screen nonpregnant adults retained its D rating, Jerome A. Leis, MD and Christine Soong, MD said in an accompanying editorial.
“Not screening or treating asymptomatic bacteriuria in this population has long been an ironclad recommendation endorsed by the Infectious Diseases Society of America, as well as numerous professional societies as part of the Choosing Wisely campaign,” wrote Dr. Leis of Sunnybrook Health Sciences Centre, Toronto, and Dr. Soong of the University of Toronto. “Restating this steadfast and pervasive recommendation may seem unremarkable and almost pedantic, yet it remains stubbornly disregarded by clinicians across multiple settings.”
The new recommendations were based on a review of 19 studies involving almost 8,500 pregnant and nonpregnant women, as well as a small number of adult men. Most were carried out in the 1960s or 1970s. The most recent ones were published in 2002 and 2015. The dearth of more recent data may have limited some conclusions and certainly highlighted the need for more research, said Jillian T. Henderson, PhD, chair of the committee assigned to investigate the evidence.
“Few studies of asymptomatic bacteriuria screening or treatment in pregnant populations have been conducted in the past 40 years,” wrote Dr. Henderson of Kaiser Permanente Northwest, Portland, and associates. “Historical evidence established asymptomatic bacteriuria screening and treatment as standard obstetric practice in the United States.” But these trials typically were less rigorous than modern studies, and the results are out of touch with modern clinical settings and treatment protocols, the team noted.
Additionally, Dr. Henderson and coauthors said, rates of pyelonephritis were about 10 times higher then than they are now. In the more recent studies, pyelonephritis rates in control groups were 2.2% and 2.5%; in most of the older studies, control group rates ranged from 33% to 36%.
In commissioning the investigation, the task force looked at the following four questions:
Does screening improve health outcomes?
Neither of two studies involving 5,289 women, one from Spain and one from Turkey, addressed this question in nonpregnant women; however, studies that looked at pregnant women generally found that screening did reduce the risk of pyelonephritis by about 70%. The investigators cautioned that these studies were out of date and perhaps methodologically flawed.
The only study that looked at newborn outcomes found no difference in birth weights or premature births between the screened and unscreened cohorts.
No study examined this question in nonpregnant women or men.
What are the harms of such screening?
A single study of 372 pregnant women described potential prenatal and perinatal harms associated with screening and treatment. It found a slight increase in congenital abnormalities in the screened cohort (1.6%), compared with those who were not screened (1.1%). However, those who were not screened were presumably not prescribed antibiotics.
Does treatment of screening-detected asymptomatic bacteriuria improve health outcomes?
Twelve trials of pregnant women (2,377) addressed this issue. All but two were conducted in the 1960s and 1970s. Treatment varied widely; sulfonamides were the most common, including the now discarded sulfamethazine and sulfadimethoxine. Dosages and duration of treatment also were considerably higher and longer than current practice.
In all but one study, there were higher rates of pyelonephritis in the control group. A pooled risk analysis indicated that treatment reduced the risk of pyelonephritis by nearly 80% (relative risk, 0.24).
Seven studies found higher rates of low birth weight in infants born to mothers who were treated, but two studies reported a significant reduction in the risk of low birth weight.
Among the six trials that examined perinatal mortality, none found significant associations with treatment.
Five studies examined treatment in nonpregnant women with screening-detected asymptomatic bacteriuria, and one included men as well. Of the four that reported the rate of symptomatic infection or pyelonephritis, none found a significant difference between treatment and control groups. The single study that included men also found no significant difference between treatment and control groups.
Among the three studies that focused on older adults, there also were no significant between-group differences in outcomes.
What harms are associated with treatment of screening-detected asymptomatic bacteriuria?
Seven studies comprised pregnant women. Five reported congenital malformations in the intervention and control groups. Overall, there were very few cases of malformations, with more – although not significantly more – in the control groups.
Evidence related to other infant and maternal harms was “sparsely and inconsistently reported,” Dr. Henderson and coauthors noted, “and there was a lack of evidence on long-term neonatal outcomes after antibiotic treatment of asymptomatic bacteriuria in pregnancy.”
Two studies listed maternal adverse events associated with different treatments including vaginitis and diarrhea with ampicillin and rashes and nausea with nalidixic acid.
In terms of nonpregnant women and men, four studies reported adverse events. None occurred with nitrofurantoin or trimethoprim treatment; however, one study that included daily treatment with ofloxacin noted that 6% withdrew because of adverse events – vertigo and gastrointestinal symptoms.
Treatments didn’t affect hematocrit, bilirubin, serum urea, or nitrogen, although some studies found a slight reduction in serum creatinine.
Although there’s a need for additional research into this question, the new recommendations provide a good reason to further reduce unnecessary antibiotic exposure, Lindsey E. Nicolle, MD, wrote in a second commentary.
These updated recommendations “contribute to the evolution of management of asymptomatic bacteriuria in healthy women,” wrote Dr. Nicolle of the University of Manitoba, Winnipeg. “However, questions remain about the risks and benefits of universal screening for and treatment of asymptomatic bacteriuria in pregnant women in the context of current clinical practice. The effects of changes in fetal-maternal care, of low- compared with high-risk pregnancies, and of health care access need to be understood. In the short term, application of current diagnostic recommendations for identification of persistent symptomatic bacteriuria with a second urine culture may provide an immediate opportunity to limit unnecessary antimicrobial use for some pregnant women.”
No conflicts of interest were reported by the USPSTF authors, nor by Dr. Leis, Dr. Soong, or Dr. Nicolle. The USPSTF report was funded by the Agency for Healthcare Research and Quality.
SOURCES: U.S. Preventive Services Task Force. JAMA. 2019;322(12):1188-94; Henderson JT et al. JAMA. 2019;322(12):1195-205; Leis JA and Soong C. JAMA. 2019. doi: 10.1001/jamainternmed.2019.4515; Nicolle LE. JAMA. 2019;322(12):1152-4.
according to new recommendations set forth by the United States Preventive Services Task Force (USPSTF).
However, the investigating committee reported, there is evidence against screening nonpregnant women and adult men. In fact, the committee found “adequate” evidence of potential harm associated with treating asymptomatic bacteriuria in adults of both sexes, including adverse effects of antibiotics and on the microbiome.
The new document downgrades from A to B the group’s prior recommendation that urine culture screening for asymptomatic bacteriuria should be performed among pregnant women at 12-16 weeks’ gestation or at their first prenatal visit. The USPSTF recommendation to not screen nonpregnant adults retained its D rating, Jerome A. Leis, MD and Christine Soong, MD said in an accompanying editorial.
“Not screening or treating asymptomatic bacteriuria in this population has long been an ironclad recommendation endorsed by the Infectious Diseases Society of America, as well as numerous professional societies as part of the Choosing Wisely campaign,” wrote Dr. Leis of Sunnybrook Health Sciences Centre, Toronto, and Dr. Soong of the University of Toronto. “Restating this steadfast and pervasive recommendation may seem unremarkable and almost pedantic, yet it remains stubbornly disregarded by clinicians across multiple settings.”
The new recommendations were based on a review of 19 studies involving almost 8,500 pregnant and nonpregnant women, as well as a small number of adult men. Most were carried out in the 1960s or 1970s. The most recent ones were published in 2002 and 2015. The dearth of more recent data may have limited some conclusions and certainly highlighted the need for more research, said Jillian T. Henderson, PhD, chair of the committee assigned to investigate the evidence.
“Few studies of asymptomatic bacteriuria screening or treatment in pregnant populations have been conducted in the past 40 years,” wrote Dr. Henderson of Kaiser Permanente Northwest, Portland, and associates. “Historical evidence established asymptomatic bacteriuria screening and treatment as standard obstetric practice in the United States.” But these trials typically were less rigorous than modern studies, and the results are out of touch with modern clinical settings and treatment protocols, the team noted.
Additionally, Dr. Henderson and coauthors said, rates of pyelonephritis were about 10 times higher then than they are now. In the more recent studies, pyelonephritis rates in control groups were 2.2% and 2.5%; in most of the older studies, control group rates ranged from 33% to 36%.
In commissioning the investigation, the task force looked at the following four questions:
Does screening improve health outcomes?
Neither of two studies involving 5,289 women, one from Spain and one from Turkey, addressed this question in nonpregnant women; however, studies that looked at pregnant women generally found that screening did reduce the risk of pyelonephritis by about 70%. The investigators cautioned that these studies were out of date and perhaps methodologically flawed.
The only study that looked at newborn outcomes found no difference in birth weights or premature births between the screened and unscreened cohorts.
No study examined this question in nonpregnant women or men.
What are the harms of such screening?
A single study of 372 pregnant women described potential prenatal and perinatal harms associated with screening and treatment. It found a slight increase in congenital abnormalities in the screened cohort (1.6%), compared with those who were not screened (1.1%). However, those who were not screened were presumably not prescribed antibiotics.
Does treatment of screening-detected asymptomatic bacteriuria improve health outcomes?
Twelve trials of pregnant women (2,377) addressed this issue. All but two were conducted in the 1960s and 1970s. Treatment varied widely; sulfonamides were the most common, including the now discarded sulfamethazine and sulfadimethoxine. Dosages and duration of treatment also were considerably higher and longer than current practice.
In all but one study, there were higher rates of pyelonephritis in the control group. A pooled risk analysis indicated that treatment reduced the risk of pyelonephritis by nearly 80% (relative risk, 0.24).
Seven studies found higher rates of low birth weight in infants born to mothers who were treated, but two studies reported a significant reduction in the risk of low birth weight.
Among the six trials that examined perinatal mortality, none found significant associations with treatment.
Five studies examined treatment in nonpregnant women with screening-detected asymptomatic bacteriuria, and one included men as well. Of the four that reported the rate of symptomatic infection or pyelonephritis, none found a significant difference between treatment and control groups. The single study that included men also found no significant difference between treatment and control groups.
Among the three studies that focused on older adults, there also were no significant between-group differences in outcomes.
What harms are associated with treatment of screening-detected asymptomatic bacteriuria?
Seven studies comprised pregnant women. Five reported congenital malformations in the intervention and control groups. Overall, there were very few cases of malformations, with more – although not significantly more – in the control groups.
Evidence related to other infant and maternal harms was “sparsely and inconsistently reported,” Dr. Henderson and coauthors noted, “and there was a lack of evidence on long-term neonatal outcomes after antibiotic treatment of asymptomatic bacteriuria in pregnancy.”
Two studies listed maternal adverse events associated with different treatments including vaginitis and diarrhea with ampicillin and rashes and nausea with nalidixic acid.
In terms of nonpregnant women and men, four studies reported adverse events. None occurred with nitrofurantoin or trimethoprim treatment; however, one study that included daily treatment with ofloxacin noted that 6% withdrew because of adverse events – vertigo and gastrointestinal symptoms.
Treatments didn’t affect hematocrit, bilirubin, serum urea, or nitrogen, although some studies found a slight reduction in serum creatinine.
Although there’s a need for additional research into this question, the new recommendations provide a good reason to further reduce unnecessary antibiotic exposure, Lindsey E. Nicolle, MD, wrote in a second commentary.
These updated recommendations “contribute to the evolution of management of asymptomatic bacteriuria in healthy women,” wrote Dr. Nicolle of the University of Manitoba, Winnipeg. “However, questions remain about the risks and benefits of universal screening for and treatment of asymptomatic bacteriuria in pregnant women in the context of current clinical practice. The effects of changes in fetal-maternal care, of low- compared with high-risk pregnancies, and of health care access need to be understood. In the short term, application of current diagnostic recommendations for identification of persistent symptomatic bacteriuria with a second urine culture may provide an immediate opportunity to limit unnecessary antimicrobial use for some pregnant women.”
No conflicts of interest were reported by the USPSTF authors, nor by Dr. Leis, Dr. Soong, or Dr. Nicolle. The USPSTF report was funded by the Agency for Healthcare Research and Quality.
SOURCES: U.S. Preventive Services Task Force. JAMA. 2019;322(12):1188-94; Henderson JT et al. JAMA. 2019;322(12):1195-205; Leis JA and Soong C. JAMA. 2019. doi: 10.1001/jamainternmed.2019.4515; Nicolle LE. JAMA. 2019;322(12):1152-4.
according to new recommendations set forth by the United States Preventive Services Task Force (USPSTF).
However, the investigating committee reported, there is evidence against screening nonpregnant women and adult men. In fact, the committee found “adequate” evidence of potential harm associated with treating asymptomatic bacteriuria in adults of both sexes, including adverse effects of antibiotics and on the microbiome.
The new document downgrades from A to B the group’s prior recommendation that urine culture screening for asymptomatic bacteriuria should be performed among pregnant women at 12-16 weeks’ gestation or at their first prenatal visit. The USPSTF recommendation to not screen nonpregnant adults retained its D rating, Jerome A. Leis, MD and Christine Soong, MD said in an accompanying editorial.
“Not screening or treating asymptomatic bacteriuria in this population has long been an ironclad recommendation endorsed by the Infectious Diseases Society of America, as well as numerous professional societies as part of the Choosing Wisely campaign,” wrote Dr. Leis of Sunnybrook Health Sciences Centre, Toronto, and Dr. Soong of the University of Toronto. “Restating this steadfast and pervasive recommendation may seem unremarkable and almost pedantic, yet it remains stubbornly disregarded by clinicians across multiple settings.”
The new recommendations were based on a review of 19 studies involving almost 8,500 pregnant and nonpregnant women, as well as a small number of adult men. Most were carried out in the 1960s or 1970s. The most recent ones were published in 2002 and 2015. The dearth of more recent data may have limited some conclusions and certainly highlighted the need for more research, said Jillian T. Henderson, PhD, chair of the committee assigned to investigate the evidence.
“Few studies of asymptomatic bacteriuria screening or treatment in pregnant populations have been conducted in the past 40 years,” wrote Dr. Henderson of Kaiser Permanente Northwest, Portland, and associates. “Historical evidence established asymptomatic bacteriuria screening and treatment as standard obstetric practice in the United States.” But these trials typically were less rigorous than modern studies, and the results are out of touch with modern clinical settings and treatment protocols, the team noted.
Additionally, Dr. Henderson and coauthors said, rates of pyelonephritis were about 10 times higher then than they are now. In the more recent studies, pyelonephritis rates in control groups were 2.2% and 2.5%; in most of the older studies, control group rates ranged from 33% to 36%.
In commissioning the investigation, the task force looked at the following four questions:
Does screening improve health outcomes?
Neither of two studies involving 5,289 women, one from Spain and one from Turkey, addressed this question in nonpregnant women; however, studies that looked at pregnant women generally found that screening did reduce the risk of pyelonephritis by about 70%. The investigators cautioned that these studies were out of date and perhaps methodologically flawed.
The only study that looked at newborn outcomes found no difference in birth weights or premature births between the screened and unscreened cohorts.
No study examined this question in nonpregnant women or men.
What are the harms of such screening?
A single study of 372 pregnant women described potential prenatal and perinatal harms associated with screening and treatment. It found a slight increase in congenital abnormalities in the screened cohort (1.6%), compared with those who were not screened (1.1%). However, those who were not screened were presumably not prescribed antibiotics.
Does treatment of screening-detected asymptomatic bacteriuria improve health outcomes?
Twelve trials of pregnant women (2,377) addressed this issue. All but two were conducted in the 1960s and 1970s. Treatment varied widely; sulfonamides were the most common, including the now discarded sulfamethazine and sulfadimethoxine. Dosages and duration of treatment also were considerably higher and longer than current practice.
In all but one study, there were higher rates of pyelonephritis in the control group. A pooled risk analysis indicated that treatment reduced the risk of pyelonephritis by nearly 80% (relative risk, 0.24).
Seven studies found higher rates of low birth weight in infants born to mothers who were treated, but two studies reported a significant reduction in the risk of low birth weight.
Among the six trials that examined perinatal mortality, none found significant associations with treatment.
Five studies examined treatment in nonpregnant women with screening-detected asymptomatic bacteriuria, and one included men as well. Of the four that reported the rate of symptomatic infection or pyelonephritis, none found a significant difference between treatment and control groups. The single study that included men also found no significant difference between treatment and control groups.
Among the three studies that focused on older adults, there also were no significant between-group differences in outcomes.
What harms are associated with treatment of screening-detected asymptomatic bacteriuria?
Seven studies comprised pregnant women. Five reported congenital malformations in the intervention and control groups. Overall, there were very few cases of malformations, with more – although not significantly more – in the control groups.
Evidence related to other infant and maternal harms was “sparsely and inconsistently reported,” Dr. Henderson and coauthors noted, “and there was a lack of evidence on long-term neonatal outcomes after antibiotic treatment of asymptomatic bacteriuria in pregnancy.”
Two studies listed maternal adverse events associated with different treatments including vaginitis and diarrhea with ampicillin and rashes and nausea with nalidixic acid.
In terms of nonpregnant women and men, four studies reported adverse events. None occurred with nitrofurantoin or trimethoprim treatment; however, one study that included daily treatment with ofloxacin noted that 6% withdrew because of adverse events – vertigo and gastrointestinal symptoms.
Treatments didn’t affect hematocrit, bilirubin, serum urea, or nitrogen, although some studies found a slight reduction in serum creatinine.
Although there’s a need for additional research into this question, the new recommendations provide a good reason to further reduce unnecessary antibiotic exposure, Lindsey E. Nicolle, MD, wrote in a second commentary.
These updated recommendations “contribute to the evolution of management of asymptomatic bacteriuria in healthy women,” wrote Dr. Nicolle of the University of Manitoba, Winnipeg. “However, questions remain about the risks and benefits of universal screening for and treatment of asymptomatic bacteriuria in pregnant women in the context of current clinical practice. The effects of changes in fetal-maternal care, of low- compared with high-risk pregnancies, and of health care access need to be understood. In the short term, application of current diagnostic recommendations for identification of persistent symptomatic bacteriuria with a second urine culture may provide an immediate opportunity to limit unnecessary antimicrobial use for some pregnant women.”
No conflicts of interest were reported by the USPSTF authors, nor by Dr. Leis, Dr. Soong, or Dr. Nicolle. The USPSTF report was funded by the Agency for Healthcare Research and Quality.
SOURCES: U.S. Preventive Services Task Force. JAMA. 2019;322(12):1188-94; Henderson JT et al. JAMA. 2019;322(12):1195-205; Leis JA and Soong C. JAMA. 2019. doi: 10.1001/jamainternmed.2019.4515; Nicolle LE. JAMA. 2019;322(12):1152-4.
FROM JAMA
Juvenile dermatomyositis derails growth and pubertal development
Children with juvenile dermatomyositis showed significant growth failure and pubertal delay, based on data from a longitudinal cohort study.
“Both the inflammatory activity of this severe chronic rheumatic disease and the well-known side effects of corticosteroid treatment may interfere with normal growth and pubertal development of children,” wrote Ellen Nordal, MD, of the University Hospital of Northern Norway, Tromsø, and colleagues.
The goal in treating juvenile dermatomyositis (JDM) is to achieve inactive disease and prevent permanent damage, but long-term data on growth and puberty in JDM patients are limited, they wrote.
In a study published in Arthritis Care & Research, the investigators reviewed data from 196 children and followed them for 2 years. The patients were part of the Paediatric Rheumatology International Trials Organisation (PRINTO) observational cohort study.
Overall, the researchers identified growth failure, height deflection, and/or delayed puberty in 94 children (48%) at the last study visit.
Growth failure was present at baseline in 17% of girls and 10% of boys. Over the 2-year study period, height deflection increased to 25% of girls and 31% of boys, but this change was not significant. Height deflection was defined as a change in the height z score of less than –0.25 per year from baseline. However, body mass index increased significantly from baseline during the study.
Catch-up growth had occurred by the final study visit in some patients, based on parent-adjusted z scores over time. Girls with a disease duration of 12 months or more showed no catch-up growth at 2 years and had significantly lower parent-adjusted height z scores.
In addition, the researchers observed a delay in the onset of puberty (including pubertal tempo and menarche) in approximately 36% of both boys and girls. However, neither growth failure nor height deflection was significantly associated with delayed puberty in either sex.
“In follow-up, clinicians should therefore be aware of both the pubertal development and the growth of the child, assess the milestones of development, and ensure that the children reach as much as possible of their genetic potential,” the researchers wrote.
The study participants were younger than 18 years at study enrollment, and all were in an active disease phase, defined as needing to start or receive a major dose increase of corticosteroids and/or immunosuppressants. Patients were assessed at baseline, at 6 months and/or at 12 months, and during a final visit at approximately 26 months. During the study, approximately half of the participants (50.5%) received methotrexate, 30 (15.3%) received cyclosporine A, 10 (5.1%) received cyclophosphamide, and 27 (13.8%) received intravenous immunoglobulin.
The study findings were limited by several factors, including the short follow-up period for assessing pubertal development and the inability to analyze any impact of corticosteroid use prior to the study, the researchers noted. However, “the overall frequency of growth failure was not significantly higher at the final study visit 2 years after baseline, indicating that the very high doses of corticosteroid treatment given during the study period is reasonably well tolerated with regards to growth,” they wrote. But monitoring remains essential, especially for children with previous growth failure or with disease onset early in pubertal development.
The study was supported by the European Union, Helse Nord Research grants, and by IRCCS Istituto Giannina Gaslini. Five authors of the study reported financial relationships with pharmaceutical companies.
SOURCE: Nordal E et al. Arthritis Care Res. 2019 Sep 10. doi: 10.1002/acr.24065.
Children with juvenile dermatomyositis showed significant growth failure and pubertal delay, based on data from a longitudinal cohort study.
“Both the inflammatory activity of this severe chronic rheumatic disease and the well-known side effects of corticosteroid treatment may interfere with normal growth and pubertal development of children,” wrote Ellen Nordal, MD, of the University Hospital of Northern Norway, Tromsø, and colleagues.
The goal in treating juvenile dermatomyositis (JDM) is to achieve inactive disease and prevent permanent damage, but long-term data on growth and puberty in JDM patients are limited, they wrote.
In a study published in Arthritis Care & Research, the investigators reviewed data from 196 children and followed them for 2 years. The patients were part of the Paediatric Rheumatology International Trials Organisation (PRINTO) observational cohort study.
Overall, the researchers identified growth failure, height deflection, and/or delayed puberty in 94 children (48%) at the last study visit.
Growth failure was present at baseline in 17% of girls and 10% of boys. Over the 2-year study period, height deflection increased to 25% of girls and 31% of boys, but this change was not significant. Height deflection was defined as a change in the height z score of less than –0.25 per year from baseline. However, body mass index increased significantly from baseline during the study.
Catch-up growth had occurred by the final study visit in some patients, based on parent-adjusted z scores over time. Girls with a disease duration of 12 months or more showed no catch-up growth at 2 years and had significantly lower parent-adjusted height z scores.
In addition, the researchers observed a delay in the onset of puberty (including pubertal tempo and menarche) in approximately 36% of both boys and girls. However, neither growth failure nor height deflection was significantly associated with delayed puberty in either sex.
“In follow-up, clinicians should therefore be aware of both the pubertal development and the growth of the child, assess the milestones of development, and ensure that the children reach as much as possible of their genetic potential,” the researchers wrote.
The study participants were younger than 18 years at study enrollment, and all were in an active disease phase, defined as needing to start or receive a major dose increase of corticosteroids and/or immunosuppressants. Patients were assessed at baseline, at 6 months and/or at 12 months, and during a final visit at approximately 26 months. During the study, approximately half of the participants (50.5%) received methotrexate, 30 (15.3%) received cyclosporine A, 10 (5.1%) received cyclophosphamide, and 27 (13.8%) received intravenous immunoglobulin.
The study findings were limited by several factors, including the short follow-up period for assessing pubertal development and the inability to analyze any impact of corticosteroid use prior to the study, the researchers noted. However, “the overall frequency of growth failure was not significantly higher at the final study visit 2 years after baseline, indicating that the very high doses of corticosteroid treatment given during the study period is reasonably well tolerated with regards to growth,” they wrote. But monitoring remains essential, especially for children with previous growth failure or with disease onset early in pubertal development.
The study was supported by the European Union, Helse Nord Research grants, and by IRCCS Istituto Giannina Gaslini. Five authors of the study reported financial relationships with pharmaceutical companies.
SOURCE: Nordal E et al. Arthritis Care Res. 2019 Sep 10. doi: 10.1002/acr.24065.
Children with juvenile dermatomyositis showed significant growth failure and pubertal delay, based on data from a longitudinal cohort study.
“Both the inflammatory activity of this severe chronic rheumatic disease and the well-known side effects of corticosteroid treatment may interfere with normal growth and pubertal development of children,” wrote Ellen Nordal, MD, of the University Hospital of Northern Norway, Tromsø, and colleagues.
The goal in treating juvenile dermatomyositis (JDM) is to achieve inactive disease and prevent permanent damage, but long-term data on growth and puberty in JDM patients are limited, they wrote.
In a study published in Arthritis Care & Research, the investigators reviewed data from 196 children and followed them for 2 years. The patients were part of the Paediatric Rheumatology International Trials Organisation (PRINTO) observational cohort study.
Overall, the researchers identified growth failure, height deflection, and/or delayed puberty in 94 children (48%) at the last study visit.
Growth failure was present at baseline in 17% of girls and 10% of boys. Over the 2-year study period, height deflection increased to 25% of girls and 31% of boys, but this change was not significant. Height deflection was defined as a change in the height z score of less than –0.25 per year from baseline. However, body mass index increased significantly from baseline during the study.
Catch-up growth had occurred by the final study visit in some patients, based on parent-adjusted z scores over time. Girls with a disease duration of 12 months or more showed no catch-up growth at 2 years and had significantly lower parent-adjusted height z scores.
In addition, the researchers observed a delay in the onset of puberty (including pubertal tempo and menarche) in approximately 36% of both boys and girls. However, neither growth failure nor height deflection was significantly associated with delayed puberty in either sex.
“In follow-up, clinicians should therefore be aware of both the pubertal development and the growth of the child, assess the milestones of development, and ensure that the children reach as much as possible of their genetic potential,” the researchers wrote.
The study participants were younger than 18 years at study enrollment, and all were in an active disease phase, defined as needing to start or receive a major dose increase of corticosteroids and/or immunosuppressants. Patients were assessed at baseline, at 6 months and/or at 12 months, and during a final visit at approximately 26 months. During the study, approximately half of the participants (50.5%) received methotrexate, 30 (15.3%) received cyclosporine A, 10 (5.1%) received cyclophosphamide, and 27 (13.8%) received intravenous immunoglobulin.
The study findings were limited by several factors, including the short follow-up period for assessing pubertal development and the inability to analyze any impact of corticosteroid use prior to the study, the researchers noted. However, “the overall frequency of growth failure was not significantly higher at the final study visit 2 years after baseline, indicating that the very high doses of corticosteroid treatment given during the study period is reasonably well tolerated with regards to growth,” they wrote. But monitoring remains essential, especially for children with previous growth failure or with disease onset early in pubertal development.
The study was supported by the European Union, Helse Nord Research grants, and by IRCCS Istituto Giannina Gaslini. Five authors of the study reported financial relationships with pharmaceutical companies.
SOURCE: Nordal E et al. Arthritis Care Res. 2019 Sep 10. doi: 10.1002/acr.24065.
FROM ARTHRITIS CARE & RESEARCH
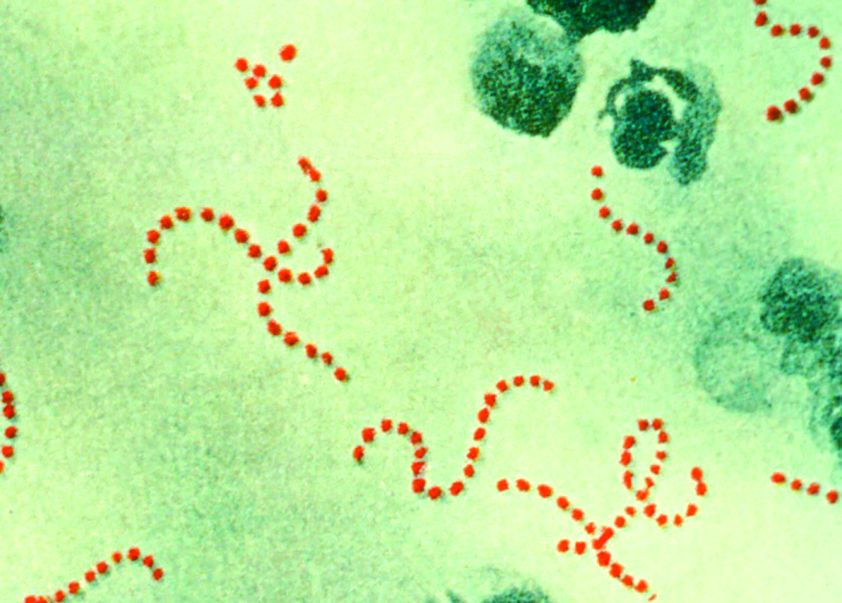
New genotype of S. pyrogenes found in rise of scarlet fever in U.K.
A new Streptococcus pyogenes genotype (designated M1UK) emerged in 2014 in England causing an increase in scarlet fever “unprecedented in modern times.” Researchers discovered that this new genotype became dominant during this increased period of scarlet fever. This new genotype was characterized by an increased production of streptococcal pyrogenic exotoxin A (SpeA, also known as scarlet fever or erythrogenic toxin A) compared to previous isolates, according to a report in The Lancet Infectious Diseases.
The researchers analyzed changes in S. pyogenes emm1 genotypes sampled from scarlet fever and invasive disease cases in 2014-2016. The emm1 gene encodes the cell surface M virulence protein and is used for serotyping S. pyogenes isolates. Using regional (northwest London) and national (England and Wales) data, they compared genomes of 135 noninvasive and 552 invasive emm1 isolates from 2009-2016 with 2,800 global emm1 sequences.
During the increase in scarlet fever and invasive disease, emm1 S. pyogenes upper respiratory tract isolates increased significantly in northwest London during the March to May periods over 3 years from 5% of isolates in 2014 to 19% isolates in 2015 to 33% isolates in 2016. Similarly, invasive emm1 isolates collected nationally in the same period increased from 31% of isolates in 2015 to 42% in 2016 (P less than .0001). Sequences of emm1 isolates from 2009-2016 showed emergence of a new emm1 lineage (designated M1UK), which could be genotypically distinguished from pandemic emm1 isolates (M1global) by 27 single-nucleotide polymorphisms. In addition, the median SpeA protein concentration was 9 times greater among M1UK isolates than among M1global isolates. By 2016, M1UK expanded nationally to comprise 84% of all emm1 genomes tested. Dataset analysis also identified single M1UK isolates present in Denmark and the United States.
“The expansion of such a lineage within the community reservoir of S. pyogenes might be sufficient to explain England’s recent increase in invasive infection. Further research to assess the likely effects of M1UK on infection transmissibility, treatment response, disease burden, and severity is required, coupled with consideration of public health interventions to limit transmission where appropriate,” Dr. Lynskey and colleagues concluded.
The authors reported that they had no disclosures.
SOURCE: Linskey NN et al. Lancet Infect Dis. 2019. doi: 10.1016/S1473-3099(19)30446-3.
A new Streptococcus pyogenes genotype (designated M1UK) emerged in 2014 in England causing an increase in scarlet fever “unprecedented in modern times.” Researchers discovered that this new genotype became dominant during this increased period of scarlet fever. This new genotype was characterized by an increased production of streptococcal pyrogenic exotoxin A (SpeA, also known as scarlet fever or erythrogenic toxin A) compared to previous isolates, according to a report in The Lancet Infectious Diseases.
The researchers analyzed changes in S. pyogenes emm1 genotypes sampled from scarlet fever and invasive disease cases in 2014-2016. The emm1 gene encodes the cell surface M virulence protein and is used for serotyping S. pyogenes isolates. Using regional (northwest London) and national (England and Wales) data, they compared genomes of 135 noninvasive and 552 invasive emm1 isolates from 2009-2016 with 2,800 global emm1 sequences.
During the increase in scarlet fever and invasive disease, emm1 S. pyogenes upper respiratory tract isolates increased significantly in northwest London during the March to May periods over 3 years from 5% of isolates in 2014 to 19% isolates in 2015 to 33% isolates in 2016. Similarly, invasive emm1 isolates collected nationally in the same period increased from 31% of isolates in 2015 to 42% in 2016 (P less than .0001). Sequences of emm1 isolates from 2009-2016 showed emergence of a new emm1 lineage (designated M1UK), which could be genotypically distinguished from pandemic emm1 isolates (M1global) by 27 single-nucleotide polymorphisms. In addition, the median SpeA protein concentration was 9 times greater among M1UK isolates than among M1global isolates. By 2016, M1UK expanded nationally to comprise 84% of all emm1 genomes tested. Dataset analysis also identified single M1UK isolates present in Denmark and the United States.
“The expansion of such a lineage within the community reservoir of S. pyogenes might be sufficient to explain England’s recent increase in invasive infection. Further research to assess the likely effects of M1UK on infection transmissibility, treatment response, disease burden, and severity is required, coupled with consideration of public health interventions to limit transmission where appropriate,” Dr. Lynskey and colleagues concluded.
The authors reported that they had no disclosures.
SOURCE: Linskey NN et al. Lancet Infect Dis. 2019. doi: 10.1016/S1473-3099(19)30446-3.
A new Streptococcus pyogenes genotype (designated M1UK) emerged in 2014 in England causing an increase in scarlet fever “unprecedented in modern times.” Researchers discovered that this new genotype became dominant during this increased period of scarlet fever. This new genotype was characterized by an increased production of streptococcal pyrogenic exotoxin A (SpeA, also known as scarlet fever or erythrogenic toxin A) compared to previous isolates, according to a report in The Lancet Infectious Diseases.
The researchers analyzed changes in S. pyogenes emm1 genotypes sampled from scarlet fever and invasive disease cases in 2014-2016. The emm1 gene encodes the cell surface M virulence protein and is used for serotyping S. pyogenes isolates. Using regional (northwest London) and national (England and Wales) data, they compared genomes of 135 noninvasive and 552 invasive emm1 isolates from 2009-2016 with 2,800 global emm1 sequences.
During the increase in scarlet fever and invasive disease, emm1 S. pyogenes upper respiratory tract isolates increased significantly in northwest London during the March to May periods over 3 years from 5% of isolates in 2014 to 19% isolates in 2015 to 33% isolates in 2016. Similarly, invasive emm1 isolates collected nationally in the same period increased from 31% of isolates in 2015 to 42% in 2016 (P less than .0001). Sequences of emm1 isolates from 2009-2016 showed emergence of a new emm1 lineage (designated M1UK), which could be genotypically distinguished from pandemic emm1 isolates (M1global) by 27 single-nucleotide polymorphisms. In addition, the median SpeA protein concentration was 9 times greater among M1UK isolates than among M1global isolates. By 2016, M1UK expanded nationally to comprise 84% of all emm1 genomes tested. Dataset analysis also identified single M1UK isolates present in Denmark and the United States.
“The expansion of such a lineage within the community reservoir of S. pyogenes might be sufficient to explain England’s recent increase in invasive infection. Further research to assess the likely effects of M1UK on infection transmissibility, treatment response, disease burden, and severity is required, coupled with consideration of public health interventions to limit transmission where appropriate,” Dr. Lynskey and colleagues concluded.
The authors reported that they had no disclosures.
SOURCE: Linskey NN et al. Lancet Infect Dis. 2019. doi: 10.1016/S1473-3099(19)30446-3.
FROM THE LANCET INFECTIOUS DISEASES
Key clinical point: An Streptococcus pyrogenes isolate with increased scarlet fever toxin production has become dominant.
Major finding: By 2016, M1UK expanded nationally to constitute 84% of all emm1 genomes tested.
Study details: Genomic comparison of 135 noninvasive and 552 invasive emm1 isolates from 2009-2016 with 2,800 global emm1 sequences.
Disclosures: The authors reported that they had no disclosures.
Source: Linskey NN et al. Lancet Infect Dis. 2019. doi: 10.1016/S1473-3099(19)30446-3.
Expert spotlights telltale clinical signs of xeroderma pigmentosum
AUSTIN, TEX. – If a child presents with acute (XP), a rare autosomal recessive disorder.
Other telltale symptoms of XP include the presence of skin cancer at an early age and a large number of skin cancers.
At the annual meeting of the Society for Pediatric Dermatology, John J. DiGiovanna, MD, described XP as a disorder of genomic instability, which has no cure. It’s caused by a mutation in genes XPA through XPG and the XP variant (XPV) gene. “The genome controls our genes, and UV rays damage DNA,” said Dr. DiGiovanna, who is a senior research physician at the National Cancer Institute’s Laboratory of Cancer and Biology and Genetics, Bethesda, Md. “This damage from UV radiation is similar to damage from chemical agents that form DNA adducts, such as cigarette smoke and certain chemotherapy agents such as cisplatinum.”
XP patients present with or without acute burning after minimal sun exposure, while children with both subtypes develop “freckling” by the time they reach 2 years of age. Dr. DiGiovanna pointed out that lentigo maligna lesions associated with XP resemble freckles at first glance, yet they vary in size, intensity, and border. Meanwhile, freckles in healthy patients are similar in size, are light tan in color, and have a regular border.
“The burning with minimal sun exposure that occurs during childhood leads to pigmentary changes, atrophy, xerosis, and telangiectasias,” he said. A follow-up analysis of 106 XP patients admitted to the National Institutes of Health between 1971 and 2009 found that patients were diagnosed with their first nonmelanoma skin cancer at a median age of 9 years, compared with age 67 among those in the general population (J Med Genet 2011 Mar;48[3]:168-76). “This is a 58-year decrease in age at risk, which is a 10,000-fold increase in skin cancer,” said Dr. DiGiovanna, who was one of the study authors.
Melanoma also occurs at an earlier age among XP patients – a median age of 22 years, compared with a median of 55 years in the general population. “In the general population, melanoma occurs at a younger age than nonmelanoma skin cancer, while in the XP population, melanoma occurs at an older age,” he said. “This is giving us a good biologic lesson that the melanoma induction mechanism must be different from nonmelanoma skin cancer.”
He recalled one XP patient who was followed by NIH researchers for 4 decades. She worked in a doctor’s office and drove a car, but developed progressive neurologic degeneration and died at the age of 40. “This was not due to unrepaired UV damage, but there are other agents which damage other neurons,” Dr. DiGiovanna explained. “Over time, what you get is a decrease in brain volume, an increase in the brain ventricles, and a loss of brain tissue. At postmortem examination, her brain was of infantile size, compared with that of an equivalent 40-year-old. This is a disease of neuronal loss, and it’s progressive. Only about 20%-25% of XP patients experience neural degeneration.”
Management of XP involves strict sun avoidance, including use of a portable UV meter and many layers of UV protection, including application of sunscreen, wearing protective clothing, sunglasses, hats, and face shields, and the use of UV-blocking window film, LED lights, and a vitamin D diet or oral supplementation. Affected individuals also require frequent skin monitoring by the patients and their family members, frequent dermatologic exams by clinicians, biopsy of suspicious lesions, removal of any skin cancers found, field treatments with agents such as 5-fluorouracil and imiquimod, and chemoprevention with oral retinoids for patients who are actively developing large numbers of new lesions (N Engl J Med. 1988 Jun23;318[25]:1633-7).
“Probably the most important thing you can do is refer them to patient support groups,” Dr. DiGiovanna said. “They are present in many countries and can help them manage the day-to-day issues of their condition.” Support groups based in North America include the XP Family Support Group, XP Society, and XP Grupo Luz De Esperanza.
Dr. DiGiovanna reported having no financial disclosures.
AUSTIN, TEX. – If a child presents with acute (XP), a rare autosomal recessive disorder.
Other telltale symptoms of XP include the presence of skin cancer at an early age and a large number of skin cancers.
At the annual meeting of the Society for Pediatric Dermatology, John J. DiGiovanna, MD, described XP as a disorder of genomic instability, which has no cure. It’s caused by a mutation in genes XPA through XPG and the XP variant (XPV) gene. “The genome controls our genes, and UV rays damage DNA,” said Dr. DiGiovanna, who is a senior research physician at the National Cancer Institute’s Laboratory of Cancer and Biology and Genetics, Bethesda, Md. “This damage from UV radiation is similar to damage from chemical agents that form DNA adducts, such as cigarette smoke and certain chemotherapy agents such as cisplatinum.”
XP patients present with or without acute burning after minimal sun exposure, while children with both subtypes develop “freckling” by the time they reach 2 years of age. Dr. DiGiovanna pointed out that lentigo maligna lesions associated with XP resemble freckles at first glance, yet they vary in size, intensity, and border. Meanwhile, freckles in healthy patients are similar in size, are light tan in color, and have a regular border.
“The burning with minimal sun exposure that occurs during childhood leads to pigmentary changes, atrophy, xerosis, and telangiectasias,” he said. A follow-up analysis of 106 XP patients admitted to the National Institutes of Health between 1971 and 2009 found that patients were diagnosed with their first nonmelanoma skin cancer at a median age of 9 years, compared with age 67 among those in the general population (J Med Genet 2011 Mar;48[3]:168-76). “This is a 58-year decrease in age at risk, which is a 10,000-fold increase in skin cancer,” said Dr. DiGiovanna, who was one of the study authors.
Melanoma also occurs at an earlier age among XP patients – a median age of 22 years, compared with a median of 55 years in the general population. “In the general population, melanoma occurs at a younger age than nonmelanoma skin cancer, while in the XP population, melanoma occurs at an older age,” he said. “This is giving us a good biologic lesson that the melanoma induction mechanism must be different from nonmelanoma skin cancer.”
He recalled one XP patient who was followed by NIH researchers for 4 decades. She worked in a doctor’s office and drove a car, but developed progressive neurologic degeneration and died at the age of 40. “This was not due to unrepaired UV damage, but there are other agents which damage other neurons,” Dr. DiGiovanna explained. “Over time, what you get is a decrease in brain volume, an increase in the brain ventricles, and a loss of brain tissue. At postmortem examination, her brain was of infantile size, compared with that of an equivalent 40-year-old. This is a disease of neuronal loss, and it’s progressive. Only about 20%-25% of XP patients experience neural degeneration.”
Management of XP involves strict sun avoidance, including use of a portable UV meter and many layers of UV protection, including application of sunscreen, wearing protective clothing, sunglasses, hats, and face shields, and the use of UV-blocking window film, LED lights, and a vitamin D diet or oral supplementation. Affected individuals also require frequent skin monitoring by the patients and their family members, frequent dermatologic exams by clinicians, biopsy of suspicious lesions, removal of any skin cancers found, field treatments with agents such as 5-fluorouracil and imiquimod, and chemoprevention with oral retinoids for patients who are actively developing large numbers of new lesions (N Engl J Med. 1988 Jun23;318[25]:1633-7).
“Probably the most important thing you can do is refer them to patient support groups,” Dr. DiGiovanna said. “They are present in many countries and can help them manage the day-to-day issues of their condition.” Support groups based in North America include the XP Family Support Group, XP Society, and XP Grupo Luz De Esperanza.
Dr. DiGiovanna reported having no financial disclosures.
AUSTIN, TEX. – If a child presents with acute (XP), a rare autosomal recessive disorder.
Other telltale symptoms of XP include the presence of skin cancer at an early age and a large number of skin cancers.
At the annual meeting of the Society for Pediatric Dermatology, John J. DiGiovanna, MD, described XP as a disorder of genomic instability, which has no cure. It’s caused by a mutation in genes XPA through XPG and the XP variant (XPV) gene. “The genome controls our genes, and UV rays damage DNA,” said Dr. DiGiovanna, who is a senior research physician at the National Cancer Institute’s Laboratory of Cancer and Biology and Genetics, Bethesda, Md. “This damage from UV radiation is similar to damage from chemical agents that form DNA adducts, such as cigarette smoke and certain chemotherapy agents such as cisplatinum.”
XP patients present with or without acute burning after minimal sun exposure, while children with both subtypes develop “freckling” by the time they reach 2 years of age. Dr. DiGiovanna pointed out that lentigo maligna lesions associated with XP resemble freckles at first glance, yet they vary in size, intensity, and border. Meanwhile, freckles in healthy patients are similar in size, are light tan in color, and have a regular border.
“The burning with minimal sun exposure that occurs during childhood leads to pigmentary changes, atrophy, xerosis, and telangiectasias,” he said. A follow-up analysis of 106 XP patients admitted to the National Institutes of Health between 1971 and 2009 found that patients were diagnosed with their first nonmelanoma skin cancer at a median age of 9 years, compared with age 67 among those in the general population (J Med Genet 2011 Mar;48[3]:168-76). “This is a 58-year decrease in age at risk, which is a 10,000-fold increase in skin cancer,” said Dr. DiGiovanna, who was one of the study authors.
Melanoma also occurs at an earlier age among XP patients – a median age of 22 years, compared with a median of 55 years in the general population. “In the general population, melanoma occurs at a younger age than nonmelanoma skin cancer, while in the XP population, melanoma occurs at an older age,” he said. “This is giving us a good biologic lesson that the melanoma induction mechanism must be different from nonmelanoma skin cancer.”
He recalled one XP patient who was followed by NIH researchers for 4 decades. She worked in a doctor’s office and drove a car, but developed progressive neurologic degeneration and died at the age of 40. “This was not due to unrepaired UV damage, but there are other agents which damage other neurons,” Dr. DiGiovanna explained. “Over time, what you get is a decrease in brain volume, an increase in the brain ventricles, and a loss of brain tissue. At postmortem examination, her brain was of infantile size, compared with that of an equivalent 40-year-old. This is a disease of neuronal loss, and it’s progressive. Only about 20%-25% of XP patients experience neural degeneration.”
Management of XP involves strict sun avoidance, including use of a portable UV meter and many layers of UV protection, including application of sunscreen, wearing protective clothing, sunglasses, hats, and face shields, and the use of UV-blocking window film, LED lights, and a vitamin D diet or oral supplementation. Affected individuals also require frequent skin monitoring by the patients and their family members, frequent dermatologic exams by clinicians, biopsy of suspicious lesions, removal of any skin cancers found, field treatments with agents such as 5-fluorouracil and imiquimod, and chemoprevention with oral retinoids for patients who are actively developing large numbers of new lesions (N Engl J Med. 1988 Jun23;318[25]:1633-7).
“Probably the most important thing you can do is refer them to patient support groups,” Dr. DiGiovanna said. “They are present in many countries and can help them manage the day-to-day issues of their condition.” Support groups based in North America include the XP Family Support Group, XP Society, and XP Grupo Luz De Esperanza.
Dr. DiGiovanna reported having no financial disclosures.
REPORTING FROM SPD 2019
Painful and Pruritic Erosions on the Back
The Diagnosis: Bullous Systemic Lupus Erythematosus
Bullous systemic lupus erythematosus (BSLE) is a rare blistering disease that affects patients with systemic lupus erythematosus (SLE). Our patient had a several-year history of SLE and was being managed by a rheumatologist. She was taking hydroxychloroquine at the time of the flare. Although BSLE tends to present in those with SLE that has already been diagnosed, BSLE has been reported as a possible initial manifestation of SLE.1
Bullous systemic lupus erythematosus is estimated to occur in less than 5% of patients with SLE and is more common in black women between the second and third decades of life,2 though it also can be seen in the pediatric population.3 The lesions of BSLE usually present as subepidermal blisters often located on the face, neck, and arms on an erythematous or possibly urticarial base. Although non-BSLE vesiculobullous eruptions may be seen in patients with SLE, BSLE is differentiated from these other eruptions by its appearance on sun-exposed and non-sun-exposed areas of the body, while other vesiculobullous eruptions associated with SLE typically are limited to sun-exposed sites.4
Due to its clinical presentation overlapping with several vesiculobullous conditions, a set of diagnostic criteria have been suggested for BSLE, including the following: (1) fulfillment of the American Rheumatism Association's criteria for SLE5; (2) a new-onset vesiculobullous eruption, primarily on sun-exposed skin; (3) histology showing a subepidermal blister with a predominantly neutrophilic infiltrate; (4) presence of IgG, IgA, IgM, and C3 at the basement membrane zone; (5) evidence of antibodies to type VII collagen; and (6) immunoelectron microscopy showing codistribution of immunoglobulin deposits with anchoring fibrils/type VII collagen. To meet the diagnosis of type I BSLE, all 6 criteria must be satisfied. To meet the diagnosis of type II BSLE, only criteria 1 to 4 need to be satisfied.6
Patients with BSLE may be presumed to have a different but clinically similar vesiculobullous condition (eg, bullous pemphigoid, cutaneous manifestations of SLE) and may be started on systemic corticosteroids. However, BSLE patients often do not show great improvement while on corticosteroids and may even flare shortly after beginning systemic corticosteroid treatment. The current treatment of choice for BSLE is dapsone, a sulfa drug that is thought to exhibit its anti-inflammatory properties via the inhibition of the alternative pathway of the complement system and through the inhibition of polymorphonuclear leukocyte functions.7 A response to dapsone helps differentiate BSLE from histopathologically and immunopathologically identical conditions such as epidermolysis bullosa acquisita.4 Bullous systemic lupus erythematosus can be differentiated from dermatitis herpetiformis with the presence of antigliadin and antitissue transglutaminase antibodies, which are found in the latter. Additionally, BSLE may show the presence of IgG and IgM deposition in addition to IgA deposition, as opposed to dermatitis herpetiformis where only IgA is found.8 The presence of these additional antibody depositions also help differentiate BSLE from linear IgA bullous dermatosis (LABD), as LABD will only have IgA depositions and often presents with an annular, crown of jewels-like appearance. Finally, there is a well-described phenomenon of LABD being drug induced, particularly after a course of vancomycin,9 and such an association with vancomycin has not been documented for BSLE.
Our patient was diagnosed with BSLE following the flare approximately 1.5 years prior to the current presentation. She had been started on dapsone 75 mg daily at that time and was taking 75 mg at the time of presentation. She was admitted and treated as an inpatient with high-dose (1 mg/kg) intravenous prednisone due to the extensive current flare.
- Fujimoto W, Hamada T, Yamada J, et al. Bullous systemic lupus erythematosus as an initial manifestation of SLE. J Dermatol. 2005;32:1021-1027.
- Miziara ID, Mahmoud A, Chagury AA, et al. Bullous systemic lupus erythematosus: case report. Int Arch Otorhinolaryngol. 2013;17:344-346.
- Tincopa M, Puttgen KB, Sule S, et al. Bullous lupus: an unusual initial presentation of systemic lupus erythematosus in an adolescent girl. Pediatr Dermatol. 2010;27:373-376.
- Grover C, Khurana A, Sharma S, et al. Bullous systemic lupus erythematosus. Indian J Dermatol. 2013;58:492.
- Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of RheumatologyClassification Criteria for Systemic Lupus Erythematosus [published online August 6, 2019]. Arthritis Rheumatol. 2019;71:1400-1412.
- Gammon WR, Briggaman RA. Bullous SLE: a phenotypically distinctive but immunologically heterogeneous bullous disorder. J Invest Dermatol. 1993;100:28S-34S.
- Duan L, Chen L, Zhong S, et al. Treatment of bullous systemic lupus erythematosus. J Immunol Res. 2015;2015:6.
- Barbosa WS, Rodarte CM, Guerra JG, et al. Bullous systemic lupus erythematosus: differential diagnosis with dermatitis herpetiformis. An Bras Dermatol. 2011;86(4 suppl 1):S92-S95.
- Yordanova I, Valtchev V, Gospodinov D, et al. IgA linear bullous dermatosis in childhood. J IMAB. 2015;21:1012-1014.
The Diagnosis: Bullous Systemic Lupus Erythematosus
Bullous systemic lupus erythematosus (BSLE) is a rare blistering disease that affects patients with systemic lupus erythematosus (SLE). Our patient had a several-year history of SLE and was being managed by a rheumatologist. She was taking hydroxychloroquine at the time of the flare. Although BSLE tends to present in those with SLE that has already been diagnosed, BSLE has been reported as a possible initial manifestation of SLE.1
Bullous systemic lupus erythematosus is estimated to occur in less than 5% of patients with SLE and is more common in black women between the second and third decades of life,2 though it also can be seen in the pediatric population.3 The lesions of BSLE usually present as subepidermal blisters often located on the face, neck, and arms on an erythematous or possibly urticarial base. Although non-BSLE vesiculobullous eruptions may be seen in patients with SLE, BSLE is differentiated from these other eruptions by its appearance on sun-exposed and non-sun-exposed areas of the body, while other vesiculobullous eruptions associated with SLE typically are limited to sun-exposed sites.4
Due to its clinical presentation overlapping with several vesiculobullous conditions, a set of diagnostic criteria have been suggested for BSLE, including the following: (1) fulfillment of the American Rheumatism Association's criteria for SLE5; (2) a new-onset vesiculobullous eruption, primarily on sun-exposed skin; (3) histology showing a subepidermal blister with a predominantly neutrophilic infiltrate; (4) presence of IgG, IgA, IgM, and C3 at the basement membrane zone; (5) evidence of antibodies to type VII collagen; and (6) immunoelectron microscopy showing codistribution of immunoglobulin deposits with anchoring fibrils/type VII collagen. To meet the diagnosis of type I BSLE, all 6 criteria must be satisfied. To meet the diagnosis of type II BSLE, only criteria 1 to 4 need to be satisfied.6
Patients with BSLE may be presumed to have a different but clinically similar vesiculobullous condition (eg, bullous pemphigoid, cutaneous manifestations of SLE) and may be started on systemic corticosteroids. However, BSLE patients often do not show great improvement while on corticosteroids and may even flare shortly after beginning systemic corticosteroid treatment. The current treatment of choice for BSLE is dapsone, a sulfa drug that is thought to exhibit its anti-inflammatory properties via the inhibition of the alternative pathway of the complement system and through the inhibition of polymorphonuclear leukocyte functions.7 A response to dapsone helps differentiate BSLE from histopathologically and immunopathologically identical conditions such as epidermolysis bullosa acquisita.4 Bullous systemic lupus erythematosus can be differentiated from dermatitis herpetiformis with the presence of antigliadin and antitissue transglutaminase antibodies, which are found in the latter. Additionally, BSLE may show the presence of IgG and IgM deposition in addition to IgA deposition, as opposed to dermatitis herpetiformis where only IgA is found.8 The presence of these additional antibody depositions also help differentiate BSLE from linear IgA bullous dermatosis (LABD), as LABD will only have IgA depositions and often presents with an annular, crown of jewels-like appearance. Finally, there is a well-described phenomenon of LABD being drug induced, particularly after a course of vancomycin,9 and such an association with vancomycin has not been documented for BSLE.
Our patient was diagnosed with BSLE following the flare approximately 1.5 years prior to the current presentation. She had been started on dapsone 75 mg daily at that time and was taking 75 mg at the time of presentation. She was admitted and treated as an inpatient with high-dose (1 mg/kg) intravenous prednisone due to the extensive current flare.
The Diagnosis: Bullous Systemic Lupus Erythematosus
Bullous systemic lupus erythematosus (BSLE) is a rare blistering disease that affects patients with systemic lupus erythematosus (SLE). Our patient had a several-year history of SLE and was being managed by a rheumatologist. She was taking hydroxychloroquine at the time of the flare. Although BSLE tends to present in those with SLE that has already been diagnosed, BSLE has been reported as a possible initial manifestation of SLE.1
Bullous systemic lupus erythematosus is estimated to occur in less than 5% of patients with SLE and is more common in black women between the second and third decades of life,2 though it also can be seen in the pediatric population.3 The lesions of BSLE usually present as subepidermal blisters often located on the face, neck, and arms on an erythematous or possibly urticarial base. Although non-BSLE vesiculobullous eruptions may be seen in patients with SLE, BSLE is differentiated from these other eruptions by its appearance on sun-exposed and non-sun-exposed areas of the body, while other vesiculobullous eruptions associated with SLE typically are limited to sun-exposed sites.4
Due to its clinical presentation overlapping with several vesiculobullous conditions, a set of diagnostic criteria have been suggested for BSLE, including the following: (1) fulfillment of the American Rheumatism Association's criteria for SLE5; (2) a new-onset vesiculobullous eruption, primarily on sun-exposed skin; (3) histology showing a subepidermal blister with a predominantly neutrophilic infiltrate; (4) presence of IgG, IgA, IgM, and C3 at the basement membrane zone; (5) evidence of antibodies to type VII collagen; and (6) immunoelectron microscopy showing codistribution of immunoglobulin deposits with anchoring fibrils/type VII collagen. To meet the diagnosis of type I BSLE, all 6 criteria must be satisfied. To meet the diagnosis of type II BSLE, only criteria 1 to 4 need to be satisfied.6
Patients with BSLE may be presumed to have a different but clinically similar vesiculobullous condition (eg, bullous pemphigoid, cutaneous manifestations of SLE) and may be started on systemic corticosteroids. However, BSLE patients often do not show great improvement while on corticosteroids and may even flare shortly after beginning systemic corticosteroid treatment. The current treatment of choice for BSLE is dapsone, a sulfa drug that is thought to exhibit its anti-inflammatory properties via the inhibition of the alternative pathway of the complement system and through the inhibition of polymorphonuclear leukocyte functions.7 A response to dapsone helps differentiate BSLE from histopathologically and immunopathologically identical conditions such as epidermolysis bullosa acquisita.4 Bullous systemic lupus erythematosus can be differentiated from dermatitis herpetiformis with the presence of antigliadin and antitissue transglutaminase antibodies, which are found in the latter. Additionally, BSLE may show the presence of IgG and IgM deposition in addition to IgA deposition, as opposed to dermatitis herpetiformis where only IgA is found.8 The presence of these additional antibody depositions also help differentiate BSLE from linear IgA bullous dermatosis (LABD), as LABD will only have IgA depositions and often presents with an annular, crown of jewels-like appearance. Finally, there is a well-described phenomenon of LABD being drug induced, particularly after a course of vancomycin,9 and such an association with vancomycin has not been documented for BSLE.
Our patient was diagnosed with BSLE following the flare approximately 1.5 years prior to the current presentation. She had been started on dapsone 75 mg daily at that time and was taking 75 mg at the time of presentation. She was admitted and treated as an inpatient with high-dose (1 mg/kg) intravenous prednisone due to the extensive current flare.
- Fujimoto W, Hamada T, Yamada J, et al. Bullous systemic lupus erythematosus as an initial manifestation of SLE. J Dermatol. 2005;32:1021-1027.
- Miziara ID, Mahmoud A, Chagury AA, et al. Bullous systemic lupus erythematosus: case report. Int Arch Otorhinolaryngol. 2013;17:344-346.
- Tincopa M, Puttgen KB, Sule S, et al. Bullous lupus: an unusual initial presentation of systemic lupus erythematosus in an adolescent girl. Pediatr Dermatol. 2010;27:373-376.
- Grover C, Khurana A, Sharma S, et al. Bullous systemic lupus erythematosus. Indian J Dermatol. 2013;58:492.
- Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of RheumatologyClassification Criteria for Systemic Lupus Erythematosus [published online August 6, 2019]. Arthritis Rheumatol. 2019;71:1400-1412.
- Gammon WR, Briggaman RA. Bullous SLE: a phenotypically distinctive but immunologically heterogeneous bullous disorder. J Invest Dermatol. 1993;100:28S-34S.
- Duan L, Chen L, Zhong S, et al. Treatment of bullous systemic lupus erythematosus. J Immunol Res. 2015;2015:6.
- Barbosa WS, Rodarte CM, Guerra JG, et al. Bullous systemic lupus erythematosus: differential diagnosis with dermatitis herpetiformis. An Bras Dermatol. 2011;86(4 suppl 1):S92-S95.
- Yordanova I, Valtchev V, Gospodinov D, et al. IgA linear bullous dermatosis in childhood. J IMAB. 2015;21:1012-1014.
- Fujimoto W, Hamada T, Yamada J, et al. Bullous systemic lupus erythematosus as an initial manifestation of SLE. J Dermatol. 2005;32:1021-1027.
- Miziara ID, Mahmoud A, Chagury AA, et al. Bullous systemic lupus erythematosus: case report. Int Arch Otorhinolaryngol. 2013;17:344-346.
- Tincopa M, Puttgen KB, Sule S, et al. Bullous lupus: an unusual initial presentation of systemic lupus erythematosus in an adolescent girl. Pediatr Dermatol. 2010;27:373-376.
- Grover C, Khurana A, Sharma S, et al. Bullous systemic lupus erythematosus. Indian J Dermatol. 2013;58:492.
- Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of RheumatologyClassification Criteria for Systemic Lupus Erythematosus [published online August 6, 2019]. Arthritis Rheumatol. 2019;71:1400-1412.
- Gammon WR, Briggaman RA. Bullous SLE: a phenotypically distinctive but immunologically heterogeneous bullous disorder. J Invest Dermatol. 1993;100:28S-34S.
- Duan L, Chen L, Zhong S, et al. Treatment of bullous systemic lupus erythematosus. J Immunol Res. 2015;2015:6.
- Barbosa WS, Rodarte CM, Guerra JG, et al. Bullous systemic lupus erythematosus: differential diagnosis with dermatitis herpetiformis. An Bras Dermatol. 2011;86(4 suppl 1):S92-S95.
- Yordanova I, Valtchev V, Gospodinov D, et al. IgA linear bullous dermatosis in childhood. J IMAB. 2015;21:1012-1014.
A 51-year-old black woman presented to the dermatology clinic with painful and pruritic erosions on the back, abdomen, neck, and arms of approximately 2 months' duration. The lesions started on the back and spread in a cephalocaudal manner. The patient denied any new changes in medication. Physical examination revealed large erosions with mild weeping of serosanguineous fluid on the back, abdomen, neck, and upper extremities. A few tense bullae were present on the dorsal aspect of the right hand. She had experienced a similar flare approximately 1.5 years prior to the current presentation. At that time, 2 shave biopsies from vesiculobullous lesions on the right side of the neck were sent for hematoxylin and eosin staining and direct immunofluorescence. Biopsy results showed a subepidermal blister that extended along the course of the hair follicle and was associated with an infiltrate of neutrophilic granulocytes that also extended along the course of the hair follicle. Direct immunofluorescence showed IgG and C3 deposition in the basement membrane zone extending along the floor of the blister where the epidermis was separated from the dermis.
Can we eradicate malaria by 2050?
A new report by members of the Lancet Commission on Malaria Eradication has called for ending malaria in Africa within a generation, specifically aiming at the year 2050.
The Lancet Commission on Malaria Eradication is a joint endeavor between The Lancet and the University of California, San Francisco, and was convened in 2017 to consider the feasibility and affordability of malaria eradication, as well as to identify priority actions for the achievement of the goal. Eradication was considered “a necessary one given the never-ending struggle against drug and insecticide resistance and the social and economic costs associated with a failure to eradicate.”
Between 2000 and 2017, the worldwide annual incidence of malaria declined by 36%, and the annual death rate declined by 60%, according to the report. In 2007, Bill and Melinda Gates proposed that controlling malaria was not enough and complete eradication was the only scientifically and ethically defensible objective. This goal was adopted by the World Health Organization and other interested parties, and by 2015, global strategies and a potential timeline for eradication were developed.
“Global progress has stalled since 2015 and the malaria community is now at a critical moment, faced with a decision to either temper its ambitions as it did in 1969 or recommit to an eradication goal,” according to the report.
In the report, the authors used new modeling analysis to estimate plausible scenarios for the distribution and intensity of malaria in 2030 and 2050. Socioeconomic and environmental trends, together with enhanced access to high-quality diagnosis, treatment, and vector control, could lead to a “world largely free of malaria” by 2050, but with pockets of low-level transmission persisting across a belt of Africa.
Current statistics lend weight to the promise of eventual eradication, according to the report.
Between 2000 and 2017, 20 countries – constituting about one-fifth of the 106 malaria-endemic countries in 2000 – eliminated malaria transmission within their borders, reporting zero indigenous malaria cases for at least 1 year. However, this was counterbalanced by the fact that between 2015 and 2017, 55 countries had an increase in cases, and 38 countries had an increase in deaths.
“The good news is that 38 countries had incidences of fewer than ten cases per 1,000 population in 2017, with 25 countries reporting fewer than one case per 1,000 population. The same 38 countries reported just 5% of total malaria deaths. Nearly all of these low-burden countries are actively working towards national and regional elimination goals of 2030 or earlier,” according to the report.
The analysis undertaken for the report consisted of the following four steps:
1. Development of a machine-learning model to capture associations between malaria endemicity data and a wide range of socioeconomic and environmental geospatial covariates.
2. Mapping of covariate estimates to the years 2030 and 2050 on the basis of projected global trends.
3. Application of the associations learned in the first step to projected covariates generated in the second step to estimate the possible future global landscape of malaria endemicity.
4. Use of a mathematical transmission model to explore the potential effect of differing levels of malaria interventions.
The report indicates that an annual spending of $6 billion or more is required, while the current global expenditure is approximately $4.3 billion. An additional investment of $2 billion per year is necessary, with a quarter of the funds coming from increased development assistance from external donors and the rest from government health spending in malaria-endemic countries, according to the report.
However, other areas of concern remain, including the current lack of effective and widely deployable outdoor biting technologies, though these are expected to be available within the next decade, according to the report.
In terms of the modeling used in the report, the authors noted that past performance does not “capture the effect of mass drug administration or mass chemoprevention because these interventions are either relatively new or have yet to be applied widely. These underestimates might be counteracted by the absence of drug or insecticide resistance from our projections,which result in overly optimistic estimates for the continued efficacy of current tools.”
The commission was launched in October 2017 by the Global Health Group at the University of California, San Francisco. The commission built on the 2010 Lancet Malaria Elimination Series, “which evaluated the operational, technical, and financial requirements for malaria elimination and helped shape and build early support for the eradication agenda,” according to the report.
SOURCE: Feachem RGA et al. Lancet. 2019 Sept 8. doi: 10.1016/S0140-6736(19)31139-0.
A new report by members of the Lancet Commission on Malaria Eradication has called for ending malaria in Africa within a generation, specifically aiming at the year 2050.
The Lancet Commission on Malaria Eradication is a joint endeavor between The Lancet and the University of California, San Francisco, and was convened in 2017 to consider the feasibility and affordability of malaria eradication, as well as to identify priority actions for the achievement of the goal. Eradication was considered “a necessary one given the never-ending struggle against drug and insecticide resistance and the social and economic costs associated with a failure to eradicate.”
Between 2000 and 2017, the worldwide annual incidence of malaria declined by 36%, and the annual death rate declined by 60%, according to the report. In 2007, Bill and Melinda Gates proposed that controlling malaria was not enough and complete eradication was the only scientifically and ethically defensible objective. This goal was adopted by the World Health Organization and other interested parties, and by 2015, global strategies and a potential timeline for eradication were developed.
“Global progress has stalled since 2015 and the malaria community is now at a critical moment, faced with a decision to either temper its ambitions as it did in 1969 or recommit to an eradication goal,” according to the report.
In the report, the authors used new modeling analysis to estimate plausible scenarios for the distribution and intensity of malaria in 2030 and 2050. Socioeconomic and environmental trends, together with enhanced access to high-quality diagnosis, treatment, and vector control, could lead to a “world largely free of malaria” by 2050, but with pockets of low-level transmission persisting across a belt of Africa.
Current statistics lend weight to the promise of eventual eradication, according to the report.
Between 2000 and 2017, 20 countries – constituting about one-fifth of the 106 malaria-endemic countries in 2000 – eliminated malaria transmission within their borders, reporting zero indigenous malaria cases for at least 1 year. However, this was counterbalanced by the fact that between 2015 and 2017, 55 countries had an increase in cases, and 38 countries had an increase in deaths.
“The good news is that 38 countries had incidences of fewer than ten cases per 1,000 population in 2017, with 25 countries reporting fewer than one case per 1,000 population. The same 38 countries reported just 5% of total malaria deaths. Nearly all of these low-burden countries are actively working towards national and regional elimination goals of 2030 or earlier,” according to the report.
The analysis undertaken for the report consisted of the following four steps:
1. Development of a machine-learning model to capture associations between malaria endemicity data and a wide range of socioeconomic and environmental geospatial covariates.
2. Mapping of covariate estimates to the years 2030 and 2050 on the basis of projected global trends.
3. Application of the associations learned in the first step to projected covariates generated in the second step to estimate the possible future global landscape of malaria endemicity.
4. Use of a mathematical transmission model to explore the potential effect of differing levels of malaria interventions.
The report indicates that an annual spending of $6 billion or more is required, while the current global expenditure is approximately $4.3 billion. An additional investment of $2 billion per year is necessary, with a quarter of the funds coming from increased development assistance from external donors and the rest from government health spending in malaria-endemic countries, according to the report.
However, other areas of concern remain, including the current lack of effective and widely deployable outdoor biting technologies, though these are expected to be available within the next decade, according to the report.
In terms of the modeling used in the report, the authors noted that past performance does not “capture the effect of mass drug administration or mass chemoprevention because these interventions are either relatively new or have yet to be applied widely. These underestimates might be counteracted by the absence of drug or insecticide resistance from our projections,which result in overly optimistic estimates for the continued efficacy of current tools.”
The commission was launched in October 2017 by the Global Health Group at the University of California, San Francisco. The commission built on the 2010 Lancet Malaria Elimination Series, “which evaluated the operational, technical, and financial requirements for malaria elimination and helped shape and build early support for the eradication agenda,” according to the report.
SOURCE: Feachem RGA et al. Lancet. 2019 Sept 8. doi: 10.1016/S0140-6736(19)31139-0.
A new report by members of the Lancet Commission on Malaria Eradication has called for ending malaria in Africa within a generation, specifically aiming at the year 2050.
The Lancet Commission on Malaria Eradication is a joint endeavor between The Lancet and the University of California, San Francisco, and was convened in 2017 to consider the feasibility and affordability of malaria eradication, as well as to identify priority actions for the achievement of the goal. Eradication was considered “a necessary one given the never-ending struggle against drug and insecticide resistance and the social and economic costs associated with a failure to eradicate.”
Between 2000 and 2017, the worldwide annual incidence of malaria declined by 36%, and the annual death rate declined by 60%, according to the report. In 2007, Bill and Melinda Gates proposed that controlling malaria was not enough and complete eradication was the only scientifically and ethically defensible objective. This goal was adopted by the World Health Organization and other interested parties, and by 2015, global strategies and a potential timeline for eradication were developed.
“Global progress has stalled since 2015 and the malaria community is now at a critical moment, faced with a decision to either temper its ambitions as it did in 1969 or recommit to an eradication goal,” according to the report.
In the report, the authors used new modeling analysis to estimate plausible scenarios for the distribution and intensity of malaria in 2030 and 2050. Socioeconomic and environmental trends, together with enhanced access to high-quality diagnosis, treatment, and vector control, could lead to a “world largely free of malaria” by 2050, but with pockets of low-level transmission persisting across a belt of Africa.
Current statistics lend weight to the promise of eventual eradication, according to the report.
Between 2000 and 2017, 20 countries – constituting about one-fifth of the 106 malaria-endemic countries in 2000 – eliminated malaria transmission within their borders, reporting zero indigenous malaria cases for at least 1 year. However, this was counterbalanced by the fact that between 2015 and 2017, 55 countries had an increase in cases, and 38 countries had an increase in deaths.
“The good news is that 38 countries had incidences of fewer than ten cases per 1,000 population in 2017, with 25 countries reporting fewer than one case per 1,000 population. The same 38 countries reported just 5% of total malaria deaths. Nearly all of these low-burden countries are actively working towards national and regional elimination goals of 2030 or earlier,” according to the report.
The analysis undertaken for the report consisted of the following four steps:
1. Development of a machine-learning model to capture associations between malaria endemicity data and a wide range of socioeconomic and environmental geospatial covariates.
2. Mapping of covariate estimates to the years 2030 and 2050 on the basis of projected global trends.
3. Application of the associations learned in the first step to projected covariates generated in the second step to estimate the possible future global landscape of malaria endemicity.
4. Use of a mathematical transmission model to explore the potential effect of differing levels of malaria interventions.
The report indicates that an annual spending of $6 billion or more is required, while the current global expenditure is approximately $4.3 billion. An additional investment of $2 billion per year is necessary, with a quarter of the funds coming from increased development assistance from external donors and the rest from government health spending in malaria-endemic countries, according to the report.
However, other areas of concern remain, including the current lack of effective and widely deployable outdoor biting technologies, though these are expected to be available within the next decade, according to the report.
In terms of the modeling used in the report, the authors noted that past performance does not “capture the effect of mass drug administration or mass chemoprevention because these interventions are either relatively new or have yet to be applied widely. These underestimates might be counteracted by the absence of drug or insecticide resistance from our projections,which result in overly optimistic estimates for the continued efficacy of current tools.”
The commission was launched in October 2017 by the Global Health Group at the University of California, San Francisco. The commission built on the 2010 Lancet Malaria Elimination Series, “which evaluated the operational, technical, and financial requirements for malaria elimination and helped shape and build early support for the eradication agenda,” according to the report.
SOURCE: Feachem RGA et al. Lancet. 2019 Sept 8. doi: 10.1016/S0140-6736(19)31139-0.
FROM THE LANCET
Disseminated Invasive Candidiasis in an Immunocompetent Host
Candida albicans (C albicans) is a normal commensal in the human gastrointestinal (GI) tract. In addition to localized infections in healthy human beings, dissemination with fatal outcome can occur in immunocompromised individuals.1
Invasive candidiasis (IC) due to C albicans is the most common nosocomial mycosis in the world and has 2 forms, candidemia and deep-seated tissue candidiasis, which can lead to multisystem organ failure.2 The deep-seated form may originate from nonhematogenous routes, such as introduction through a peritoneal catheter or ascending infection from cystitis.2 In addition, about 50% of primary candidemia cases lead to secondary deep-seated candidiasis; however, only about 40% of these cases show positive blood cultures. Since the window of opportunity for a positive culture is narrow, active candidemia may be missed.3,4
Once developed, the prognosis for IC is grim: Mortality is 40% regardless of therapy.2 IC typically occurs in immunocompromised hosts; IC in immunocompetent persons has rarely been reported.5,6 It is challenging to diagnose IC in the immunocompetent patients as 50% to 70% of the general population is naturally colonized by this organism, and when found, it is assumed to be mostly innocuous. Neutrophil-driven cell-mediated immunity associated with IL-1 and IL-17 response prevent fungal growth and dissemination, protecting the immunocompetent host.7
We report on a patient who showed no neutropenia or leukocytopenia but developed disseminated candidiasis. This report is one of the rare cases of full-blown disseminated candidiasis with lesions related to C albicans found in almost all of the important organs.
Case Presentation
A 67-year-old male patient with a history of hypertension, peripheral vascular disease, daily heavy alcohol consumption, and a 50-pack-year history of smoking developed gangrene of the left fifth toe. He underwent vascular surgery consultation with an aortogram/left lower extremity angiography that showed occlusion of the left external iliac artery as well as the left common femoral artery. It was decided to improve inflow in the common iliac artery by placing a bare metal stent and subsequent balloon dilatation before a right to left femoral to femoral artery bypass. The patient tolerated the procedure well and was discharged home.
Two days later, the patient was admitted to a US Department of Veterans Affairs (VA) complexity level 1a hospital with weakness and worsening pain in the left lower extremities. Examination revealed chronic ischemic changes in the feet bilaterally and evidence of dry gangrene in the left fifth toe requiring femoral bypass surgery. But poor nutritional status and cardiac status prevented pursuing a permanent solution.
Following completion of a stress echocardiogram, the patient developed shock with systolic blood pressure of 60 mm Hg, and atrial fibrillation (AF) with rapid ventricular rate (RVR). He was initially treated with IV fluid supplementation, vasopressor therapy, synchronized cardioversion, and IV amiodarone/anticoagulation therapy, due to his persistent AF with RVR. The patient was transferred to a tertiary care center for persistent hypothermia and received treatment with warm saline. After initial recovery with warm saline resuscitation, he had a prolonged, complicated hospital course in which he developed progressive respiratory failure requiring intubation and critical care support. He developed a right internal jugular deep venous thrombosis, heparin-induced thrombocytopenia, lower GI bleeding requiring emergent embolization by interventional radiology, inferior vena cava filter placement, renal failure requiring dialysis, small bowel obstruction secondary to right lower quadrant phlegmon and perforation requiring small bowel resection and end ileostomy. His antibiotic regimen included therapy with vancomycin and piperacillin-tazobactam.
He eventually recovered and was extubated and subsequently transferred back to the VA hospital where cefepime was initiated because of suspicion of a urinary tract infection and septicemia (urine cultures eventually grew C albicans). Over the subsequent 3 days, the patient’s renal output and hyperkalemia worsened, he also developed increased anion gap metabolic acidosis and was intubated again and placed on full mechanical ventilatory support. His blood cultures were negative, and sputum cultures revealed normal respiratory flora and 1+ C albicans. Infectious diseases consultation recommended an abdominal ultrasound, which revealed nonspecific findings. The antibiotic regimen was changed to daptomycin and piperacillin-tazobactam. A follow-up chest X-ray revealed a developing right lower lobe pneumonia and hilar prominence suggestive of lymphadenopathy. The patient’s clinical condition deteriorated, and he subsequently developed cardiac arrest; resuscitation was not successful and he expired.
Outcome and Follow-up
An autopsy disclosed the cause of death to be bilateral candida pneumonia, part of a disseminated (invasive) candidiasis, in a patient rendered vulnerable to such infection by peripheral vascular disease and renal insufficiency. Purulent inflammation was noted at the site of disarticulation of the left foot and confluent consolidation of the lower lobes of both lungs as well as focal consolidation of the middle lobe of the right lung. Examination of histologic sections, with staining both by routine method (hematoxylin and eosin) and the Grocott-Gömöri methenamine silver method for fungus, disclosed fungal forms (yeast and filamentous) in most tissues, including the lungs (Figure 1 A and B) and kidneys (Figure 1 C and D). The pulmonary sections in addition to massive inflammation showed macrophages with engulfed yeast (Figure 2 A) and a lymphatic channel, stuffed with yeast in an alveolar septum (Figure 2 B). These findings confirmed the antemortem presence of the fungus and the body’s response to it. Inflammation was noted around glomeruli overgrown by candida (Figure 1 C and D); fungi also were seen in capsular regions (not depicted). C albicans was present in the myocardium (Figure 1 E and F), brain, thyroid, and adrenal glands (Figure 3); the only organ without C albicans was the liver, either because invasion was truly absent here or because sampling had not managed to retrieve it.
Paraffin-embedded blocks of lung tissue, sent to the University of Washington Molecular Diagnosis Microbiology Laboratory for broad-range polymerase chain reaction (PCR) identification, were positive for C albicans after extraction of gDNA and conduction of PCR using internal transcribed spacer 1 and 2 specific primers.
Discussion
IC is rare among immunocompetent individuals, but C albicans can evolve into a fatal disseminated infection. We report an atypical case of IC, with profound pulmonary infection in a patient who died 1 month after hospitalization for lower extremity pain.
Cell-mediated immunity involving neutrophils and macrophages plays a major role in protection against candidiasis, while cytokines and chemokines involve regulating balanced immunity.1,2 A series of recent studies show that alcohol impairs neutrophil-mediated killing and phagocytic-mediated uptake of a pathogen in this process.8,9 As the patient chronically misused alcohol, his immune system may have experienced a subclinical immunosuppression, which would have become clinically relevant once C albicans was introduced systemically. Recent studies of bacterial pathogenesis and alcoholism strongly support this hypothesis.10,11
Most patients with the unusual diagnosis of candida pneumonia have had a background of malignancy or immunosuppressive factors (eg, administration of corticosteroids).12 In a series of 20 cases, 14 had sputum cultures positive for the organism, 6 had positive urine cultures, and 6 had positive blood cultures. Chest radiographs usually showed confluent bronchopneumonia. Five patients were diagnosed antemortem and treated with amphotericin B, but none survived.13 In the literature a positive blood culture or demonstration of yeast within pulmonary histiocytes has been considered proof of the pathogenicity of the fungus, as opposed to noninvasive colonization of the airways, a common occurrence in patients receiving mechanical ventilation.2
As previously discussed, blood cultures are often negative with invasive candidiasis, as the window of opportunity is short and may be missed. As shown in murine models, it is easy to miss a narrow window of candidemia, leading to false-negative blood cultures in clinical practice.14,15 Mouse model studies also have found that the window of candidemia is very short in disseminated candidiasis as a lethal IV dose of C albicans disappeared from blood within 48 hours of postinoculation.15 The biomarker of serum procalcitonin is a great diagnostic resource for the elimination of a likely bacterial sepsis, and conversely, the early suspicion of a fungemia, as serum procalcitonin would typically be elevated in a bacterial but not a fungal septicemia.16 The average cost per test is only about $30, and we recommend testing for serum procalcitonin as well as monitoring of serum lactate levels in cases of nonresponding septicemia.
The C albicans in this case may have been introduced hematogenously from the amputation site or through an ascending cystitis, or possibly have been derived from commensal flora in the GI tract. The iron supplementation provided to the patient may have promoted the growth and virulence of the candida; studies have shown that the kidneys assimilate increased levels of iron during disseminated candidiasis thus providing a more favorable site for colonization.17The presence of C albicans in a single collection of sputum or urine does not ordinarily indicate infection in an immunocompetent individual. Estimation of serum procalcitonin, a biomarker for bacterial infection and sepsis, might be useful if negative, for turning attention to a nonbacterial (such as, candida) source as the causative agent.18
Conclusion
C albicans can rarely cause disseminated disease in nonimmunocompromised critically ill patients. Low serum procalcitonin levels in a septic patient might indicate nonbacterial cause such as candidiasis. Even with disseminated candidiasis, blood cultures may remain negative.
1. Navarathna DH, Stein EV, Lessey-Morillon EC, Nayak D, Martin-Manso G, Roberts DD. CD47 promotes protective innate and adaptive immunity in a mouse model of disseminated candidiasis. PLoS One. 2015;10(5):e0128220.
2. Kullberg BJ, Arendrup MC. Invasive candidiasis. N Engl J Med. 2015;373(15):1445-1456.
3. Clancy CJ, Nguyen MH. Diagnosing invasive candidiasis. J Clin Microbiol. 2018;56(5):e01909-e01917.
4. Ericson EL, Klingspor L, Ullberg M, Ozenci V. Clinical comparison of the Bactec Mycosis IC/F, BacT/Alert FA, and BacT/Alert FN blood culture vials for the detection of candidemia. Diagn Microbiol Infect Dis. 2012;73(2):153-156.
5. Baum GL. The significance of Candida albicans in human sputum. N Engl J Med. 1960;263:70-73.
6. el-Ebiary M, Torres A, Fàbregas N, et al. Significance of the isolation of Candida species from respiratory samples in critically ill, non-neutropenic patients. An immediate postmortem histologic study. Am J Respir Crit Care Med. 1997;156(2, pt 1):583-590.
7. Altmeier S, Toska A, Sparber F, Teijeira A, Halin C, LeibundGut-Landmann S. IL-1 coordinates the neutrophil response to C. albicans in the oral mucosa. PLoS Pathog. 2016;12(9):e1005882.
8. Karavitis J, Kovacs EJ. Macrophage phagocytosis: effects of environmental pollutants, alcohol, cigarette smoke, and other external factors. J Leukoc Biol. 2011;90(6):1065-1078.
9. Chiu C-H, Wang Y-C, Yeh K-M, Lin J-C, Siu LK, Chang F-Y. Influence of ethanol concentration in the phagocytic function of neutrophils against Klebsiella pneumoniae isolates in an experimental model. J Microbiol Immunol Infect. 2018;51(1):64-69.
10. Khocht A, Schleifer S, Janal M, Keller S. Neutrophil function and periodontitis in alcohol-dependent males without medical disorders. J Int Acad Periodontol. 2013;15(3):68-74.
11. Gandhi JA, Ekhar VV, Asplund MB, et al. Alcohol enhances Acinetobacter baumannii-associated pneumonia and systemic dissemination by impairing neutrophil antimicrobial activity in a murine model of infection. PLoS One. 2014;9(4):e95707.
12. Mohsenifar Z, Chopra SK, Johnson BL, Simmons DH. Candida pneumonia: experience with 20 patients. West J Med. 1979;131(3):196-200.
13. Jones JM. Laboratory diagnosis of invasive candidiasis. Clin Microbiol Rev. 1990;3(1):32-45.
14. Clancy CJ, Nguyen MH. Finding the “missing 50%” of invasive candidiasis: how nonculture diagnostics will improve understanding of disease spectrum and transform patient care. Clin Infect Dis. 2013;56(9):1284-1292.
15. Kappe R, Mu¨ ller J. Rapid clearance of Candida albicans mannan antigens by liver and spleen in contrast to prolonged circulation of Cryptococcus neoformans antigens. J Clin Microbiol. 1991;29(8):1665-1669.
16. Balk RA, Kadri SS, Cao Z, Robinson SB, Lipkin C, Bozzette SA. Effect of procalcitonin testing on health-care utilization and costs in critically ill patients in the United States. Chest. 2017;151(1):23-33.
17. Potrykus J, Stead D, Maccallum DM, et al. Fungal iron availability during deep seated candidiasis is defined by a complex interplay involving systemic and local events. PLoS Pathog. 2013;9(10):e1003676.
18. Soni NJ, Samson DJ, Galaydick JL, Vats V, Pitrak DL, Aronson N. Procalcitonin-Guided Antibiotic Therapy. Rockville, MD: Agency for Healthcare Research and Quality (US); 2012.
Candida albicans (C albicans) is a normal commensal in the human gastrointestinal (GI) tract. In addition to localized infections in healthy human beings, dissemination with fatal outcome can occur in immunocompromised individuals.1
Invasive candidiasis (IC) due to C albicans is the most common nosocomial mycosis in the world and has 2 forms, candidemia and deep-seated tissue candidiasis, which can lead to multisystem organ failure.2 The deep-seated form may originate from nonhematogenous routes, such as introduction through a peritoneal catheter or ascending infection from cystitis.2 In addition, about 50% of primary candidemia cases lead to secondary deep-seated candidiasis; however, only about 40% of these cases show positive blood cultures. Since the window of opportunity for a positive culture is narrow, active candidemia may be missed.3,4
Once developed, the prognosis for IC is grim: Mortality is 40% regardless of therapy.2 IC typically occurs in immunocompromised hosts; IC in immunocompetent persons has rarely been reported.5,6 It is challenging to diagnose IC in the immunocompetent patients as 50% to 70% of the general population is naturally colonized by this organism, and when found, it is assumed to be mostly innocuous. Neutrophil-driven cell-mediated immunity associated with IL-1 and IL-17 response prevent fungal growth and dissemination, protecting the immunocompetent host.7
We report on a patient who showed no neutropenia or leukocytopenia but developed disseminated candidiasis. This report is one of the rare cases of full-blown disseminated candidiasis with lesions related to C albicans found in almost all of the important organs.
Case Presentation
A 67-year-old male patient with a history of hypertension, peripheral vascular disease, daily heavy alcohol consumption, and a 50-pack-year history of smoking developed gangrene of the left fifth toe. He underwent vascular surgery consultation with an aortogram/left lower extremity angiography that showed occlusion of the left external iliac artery as well as the left common femoral artery. It was decided to improve inflow in the common iliac artery by placing a bare metal stent and subsequent balloon dilatation before a right to left femoral to femoral artery bypass. The patient tolerated the procedure well and was discharged home.
Two days later, the patient was admitted to a US Department of Veterans Affairs (VA) complexity level 1a hospital with weakness and worsening pain in the left lower extremities. Examination revealed chronic ischemic changes in the feet bilaterally and evidence of dry gangrene in the left fifth toe requiring femoral bypass surgery. But poor nutritional status and cardiac status prevented pursuing a permanent solution.
Following completion of a stress echocardiogram, the patient developed shock with systolic blood pressure of 60 mm Hg, and atrial fibrillation (AF) with rapid ventricular rate (RVR). He was initially treated with IV fluid supplementation, vasopressor therapy, synchronized cardioversion, and IV amiodarone/anticoagulation therapy, due to his persistent AF with RVR. The patient was transferred to a tertiary care center for persistent hypothermia and received treatment with warm saline. After initial recovery with warm saline resuscitation, he had a prolonged, complicated hospital course in which he developed progressive respiratory failure requiring intubation and critical care support. He developed a right internal jugular deep venous thrombosis, heparin-induced thrombocytopenia, lower GI bleeding requiring emergent embolization by interventional radiology, inferior vena cava filter placement, renal failure requiring dialysis, small bowel obstruction secondary to right lower quadrant phlegmon and perforation requiring small bowel resection and end ileostomy. His antibiotic regimen included therapy with vancomycin and piperacillin-tazobactam.
He eventually recovered and was extubated and subsequently transferred back to the VA hospital where cefepime was initiated because of suspicion of a urinary tract infection and septicemia (urine cultures eventually grew C albicans). Over the subsequent 3 days, the patient’s renal output and hyperkalemia worsened, he also developed increased anion gap metabolic acidosis and was intubated again and placed on full mechanical ventilatory support. His blood cultures were negative, and sputum cultures revealed normal respiratory flora and 1+ C albicans. Infectious diseases consultation recommended an abdominal ultrasound, which revealed nonspecific findings. The antibiotic regimen was changed to daptomycin and piperacillin-tazobactam. A follow-up chest X-ray revealed a developing right lower lobe pneumonia and hilar prominence suggestive of lymphadenopathy. The patient’s clinical condition deteriorated, and he subsequently developed cardiac arrest; resuscitation was not successful and he expired.
Outcome and Follow-up
An autopsy disclosed the cause of death to be bilateral candida pneumonia, part of a disseminated (invasive) candidiasis, in a patient rendered vulnerable to such infection by peripheral vascular disease and renal insufficiency. Purulent inflammation was noted at the site of disarticulation of the left foot and confluent consolidation of the lower lobes of both lungs as well as focal consolidation of the middle lobe of the right lung. Examination of histologic sections, with staining both by routine method (hematoxylin and eosin) and the Grocott-Gömöri methenamine silver method for fungus, disclosed fungal forms (yeast and filamentous) in most tissues, including the lungs (Figure 1 A and B) and kidneys (Figure 1 C and D). The pulmonary sections in addition to massive inflammation showed macrophages with engulfed yeast (Figure 2 A) and a lymphatic channel, stuffed with yeast in an alveolar septum (Figure 2 B). These findings confirmed the antemortem presence of the fungus and the body’s response to it. Inflammation was noted around glomeruli overgrown by candida (Figure 1 C and D); fungi also were seen in capsular regions (not depicted). C albicans was present in the myocardium (Figure 1 E and F), brain, thyroid, and adrenal glands (Figure 3); the only organ without C albicans was the liver, either because invasion was truly absent here or because sampling had not managed to retrieve it.
Paraffin-embedded blocks of lung tissue, sent to the University of Washington Molecular Diagnosis Microbiology Laboratory for broad-range polymerase chain reaction (PCR) identification, were positive for C albicans after extraction of gDNA and conduction of PCR using internal transcribed spacer 1 and 2 specific primers.
Discussion
IC is rare among immunocompetent individuals, but C albicans can evolve into a fatal disseminated infection. We report an atypical case of IC, with profound pulmonary infection in a patient who died 1 month after hospitalization for lower extremity pain.
Cell-mediated immunity involving neutrophils and macrophages plays a major role in protection against candidiasis, while cytokines and chemokines involve regulating balanced immunity.1,2 A series of recent studies show that alcohol impairs neutrophil-mediated killing and phagocytic-mediated uptake of a pathogen in this process.8,9 As the patient chronically misused alcohol, his immune system may have experienced a subclinical immunosuppression, which would have become clinically relevant once C albicans was introduced systemically. Recent studies of bacterial pathogenesis and alcoholism strongly support this hypothesis.10,11
Most patients with the unusual diagnosis of candida pneumonia have had a background of malignancy or immunosuppressive factors (eg, administration of corticosteroids).12 In a series of 20 cases, 14 had sputum cultures positive for the organism, 6 had positive urine cultures, and 6 had positive blood cultures. Chest radiographs usually showed confluent bronchopneumonia. Five patients were diagnosed antemortem and treated with amphotericin B, but none survived.13 In the literature a positive blood culture or demonstration of yeast within pulmonary histiocytes has been considered proof of the pathogenicity of the fungus, as opposed to noninvasive colonization of the airways, a common occurrence in patients receiving mechanical ventilation.2
As previously discussed, blood cultures are often negative with invasive candidiasis, as the window of opportunity is short and may be missed. As shown in murine models, it is easy to miss a narrow window of candidemia, leading to false-negative blood cultures in clinical practice.14,15 Mouse model studies also have found that the window of candidemia is very short in disseminated candidiasis as a lethal IV dose of C albicans disappeared from blood within 48 hours of postinoculation.15 The biomarker of serum procalcitonin is a great diagnostic resource for the elimination of a likely bacterial sepsis, and conversely, the early suspicion of a fungemia, as serum procalcitonin would typically be elevated in a bacterial but not a fungal septicemia.16 The average cost per test is only about $30, and we recommend testing for serum procalcitonin as well as monitoring of serum lactate levels in cases of nonresponding septicemia.
The C albicans in this case may have been introduced hematogenously from the amputation site or through an ascending cystitis, or possibly have been derived from commensal flora in the GI tract. The iron supplementation provided to the patient may have promoted the growth and virulence of the candida; studies have shown that the kidneys assimilate increased levels of iron during disseminated candidiasis thus providing a more favorable site for colonization.17The presence of C albicans in a single collection of sputum or urine does not ordinarily indicate infection in an immunocompetent individual. Estimation of serum procalcitonin, a biomarker for bacterial infection and sepsis, might be useful if negative, for turning attention to a nonbacterial (such as, candida) source as the causative agent.18
Conclusion
C albicans can rarely cause disseminated disease in nonimmunocompromised critically ill patients. Low serum procalcitonin levels in a septic patient might indicate nonbacterial cause such as candidiasis. Even with disseminated candidiasis, blood cultures may remain negative.
Candida albicans (C albicans) is a normal commensal in the human gastrointestinal (GI) tract. In addition to localized infections in healthy human beings, dissemination with fatal outcome can occur in immunocompromised individuals.1
Invasive candidiasis (IC) due to C albicans is the most common nosocomial mycosis in the world and has 2 forms, candidemia and deep-seated tissue candidiasis, which can lead to multisystem organ failure.2 The deep-seated form may originate from nonhematogenous routes, such as introduction through a peritoneal catheter or ascending infection from cystitis.2 In addition, about 50% of primary candidemia cases lead to secondary deep-seated candidiasis; however, only about 40% of these cases show positive blood cultures. Since the window of opportunity for a positive culture is narrow, active candidemia may be missed.3,4
Once developed, the prognosis for IC is grim: Mortality is 40% regardless of therapy.2 IC typically occurs in immunocompromised hosts; IC in immunocompetent persons has rarely been reported.5,6 It is challenging to diagnose IC in the immunocompetent patients as 50% to 70% of the general population is naturally colonized by this organism, and when found, it is assumed to be mostly innocuous. Neutrophil-driven cell-mediated immunity associated with IL-1 and IL-17 response prevent fungal growth and dissemination, protecting the immunocompetent host.7
We report on a patient who showed no neutropenia or leukocytopenia but developed disseminated candidiasis. This report is one of the rare cases of full-blown disseminated candidiasis with lesions related to C albicans found in almost all of the important organs.
Case Presentation
A 67-year-old male patient with a history of hypertension, peripheral vascular disease, daily heavy alcohol consumption, and a 50-pack-year history of smoking developed gangrene of the left fifth toe. He underwent vascular surgery consultation with an aortogram/left lower extremity angiography that showed occlusion of the left external iliac artery as well as the left common femoral artery. It was decided to improve inflow in the common iliac artery by placing a bare metal stent and subsequent balloon dilatation before a right to left femoral to femoral artery bypass. The patient tolerated the procedure well and was discharged home.
Two days later, the patient was admitted to a US Department of Veterans Affairs (VA) complexity level 1a hospital with weakness and worsening pain in the left lower extremities. Examination revealed chronic ischemic changes in the feet bilaterally and evidence of dry gangrene in the left fifth toe requiring femoral bypass surgery. But poor nutritional status and cardiac status prevented pursuing a permanent solution.
Following completion of a stress echocardiogram, the patient developed shock with systolic blood pressure of 60 mm Hg, and atrial fibrillation (AF) with rapid ventricular rate (RVR). He was initially treated with IV fluid supplementation, vasopressor therapy, synchronized cardioversion, and IV amiodarone/anticoagulation therapy, due to his persistent AF with RVR. The patient was transferred to a tertiary care center for persistent hypothermia and received treatment with warm saline. After initial recovery with warm saline resuscitation, he had a prolonged, complicated hospital course in which he developed progressive respiratory failure requiring intubation and critical care support. He developed a right internal jugular deep venous thrombosis, heparin-induced thrombocytopenia, lower GI bleeding requiring emergent embolization by interventional radiology, inferior vena cava filter placement, renal failure requiring dialysis, small bowel obstruction secondary to right lower quadrant phlegmon and perforation requiring small bowel resection and end ileostomy. His antibiotic regimen included therapy with vancomycin and piperacillin-tazobactam.
He eventually recovered and was extubated and subsequently transferred back to the VA hospital where cefepime was initiated because of suspicion of a urinary tract infection and septicemia (urine cultures eventually grew C albicans). Over the subsequent 3 days, the patient’s renal output and hyperkalemia worsened, he also developed increased anion gap metabolic acidosis and was intubated again and placed on full mechanical ventilatory support. His blood cultures were negative, and sputum cultures revealed normal respiratory flora and 1+ C albicans. Infectious diseases consultation recommended an abdominal ultrasound, which revealed nonspecific findings. The antibiotic regimen was changed to daptomycin and piperacillin-tazobactam. A follow-up chest X-ray revealed a developing right lower lobe pneumonia and hilar prominence suggestive of lymphadenopathy. The patient’s clinical condition deteriorated, and he subsequently developed cardiac arrest; resuscitation was not successful and he expired.
Outcome and Follow-up
An autopsy disclosed the cause of death to be bilateral candida pneumonia, part of a disseminated (invasive) candidiasis, in a patient rendered vulnerable to such infection by peripheral vascular disease and renal insufficiency. Purulent inflammation was noted at the site of disarticulation of the left foot and confluent consolidation of the lower lobes of both lungs as well as focal consolidation of the middle lobe of the right lung. Examination of histologic sections, with staining both by routine method (hematoxylin and eosin) and the Grocott-Gömöri methenamine silver method for fungus, disclosed fungal forms (yeast and filamentous) in most tissues, including the lungs (Figure 1 A and B) and kidneys (Figure 1 C and D). The pulmonary sections in addition to massive inflammation showed macrophages with engulfed yeast (Figure 2 A) and a lymphatic channel, stuffed with yeast in an alveolar septum (Figure 2 B). These findings confirmed the antemortem presence of the fungus and the body’s response to it. Inflammation was noted around glomeruli overgrown by candida (Figure 1 C and D); fungi also were seen in capsular regions (not depicted). C albicans was present in the myocardium (Figure 1 E and F), brain, thyroid, and adrenal glands (Figure 3); the only organ without C albicans was the liver, either because invasion was truly absent here or because sampling had not managed to retrieve it.
Paraffin-embedded blocks of lung tissue, sent to the University of Washington Molecular Diagnosis Microbiology Laboratory for broad-range polymerase chain reaction (PCR) identification, were positive for C albicans after extraction of gDNA and conduction of PCR using internal transcribed spacer 1 and 2 specific primers.
Discussion
IC is rare among immunocompetent individuals, but C albicans can evolve into a fatal disseminated infection. We report an atypical case of IC, with profound pulmonary infection in a patient who died 1 month after hospitalization for lower extremity pain.
Cell-mediated immunity involving neutrophils and macrophages plays a major role in protection against candidiasis, while cytokines and chemokines involve regulating balanced immunity.1,2 A series of recent studies show that alcohol impairs neutrophil-mediated killing and phagocytic-mediated uptake of a pathogen in this process.8,9 As the patient chronically misused alcohol, his immune system may have experienced a subclinical immunosuppression, which would have become clinically relevant once C albicans was introduced systemically. Recent studies of bacterial pathogenesis and alcoholism strongly support this hypothesis.10,11
Most patients with the unusual diagnosis of candida pneumonia have had a background of malignancy or immunosuppressive factors (eg, administration of corticosteroids).12 In a series of 20 cases, 14 had sputum cultures positive for the organism, 6 had positive urine cultures, and 6 had positive blood cultures. Chest radiographs usually showed confluent bronchopneumonia. Five patients were diagnosed antemortem and treated with amphotericin B, but none survived.13 In the literature a positive blood culture or demonstration of yeast within pulmonary histiocytes has been considered proof of the pathogenicity of the fungus, as opposed to noninvasive colonization of the airways, a common occurrence in patients receiving mechanical ventilation.2
As previously discussed, blood cultures are often negative with invasive candidiasis, as the window of opportunity is short and may be missed. As shown in murine models, it is easy to miss a narrow window of candidemia, leading to false-negative blood cultures in clinical practice.14,15 Mouse model studies also have found that the window of candidemia is very short in disseminated candidiasis as a lethal IV dose of C albicans disappeared from blood within 48 hours of postinoculation.15 The biomarker of serum procalcitonin is a great diagnostic resource for the elimination of a likely bacterial sepsis, and conversely, the early suspicion of a fungemia, as serum procalcitonin would typically be elevated in a bacterial but not a fungal septicemia.16 The average cost per test is only about $30, and we recommend testing for serum procalcitonin as well as monitoring of serum lactate levels in cases of nonresponding septicemia.
The C albicans in this case may have been introduced hematogenously from the amputation site or through an ascending cystitis, or possibly have been derived from commensal flora in the GI tract. The iron supplementation provided to the patient may have promoted the growth and virulence of the candida; studies have shown that the kidneys assimilate increased levels of iron during disseminated candidiasis thus providing a more favorable site for colonization.17The presence of C albicans in a single collection of sputum or urine does not ordinarily indicate infection in an immunocompetent individual. Estimation of serum procalcitonin, a biomarker for bacterial infection and sepsis, might be useful if negative, for turning attention to a nonbacterial (such as, candida) source as the causative agent.18
Conclusion
C albicans can rarely cause disseminated disease in nonimmunocompromised critically ill patients. Low serum procalcitonin levels in a septic patient might indicate nonbacterial cause such as candidiasis. Even with disseminated candidiasis, blood cultures may remain negative.
1. Navarathna DH, Stein EV, Lessey-Morillon EC, Nayak D, Martin-Manso G, Roberts DD. CD47 promotes protective innate and adaptive immunity in a mouse model of disseminated candidiasis. PLoS One. 2015;10(5):e0128220.
2. Kullberg BJ, Arendrup MC. Invasive candidiasis. N Engl J Med. 2015;373(15):1445-1456.
3. Clancy CJ, Nguyen MH. Diagnosing invasive candidiasis. J Clin Microbiol. 2018;56(5):e01909-e01917.
4. Ericson EL, Klingspor L, Ullberg M, Ozenci V. Clinical comparison of the Bactec Mycosis IC/F, BacT/Alert FA, and BacT/Alert FN blood culture vials for the detection of candidemia. Diagn Microbiol Infect Dis. 2012;73(2):153-156.
5. Baum GL. The significance of Candida albicans in human sputum. N Engl J Med. 1960;263:70-73.
6. el-Ebiary M, Torres A, Fàbregas N, et al. Significance of the isolation of Candida species from respiratory samples in critically ill, non-neutropenic patients. An immediate postmortem histologic study. Am J Respir Crit Care Med. 1997;156(2, pt 1):583-590.
7. Altmeier S, Toska A, Sparber F, Teijeira A, Halin C, LeibundGut-Landmann S. IL-1 coordinates the neutrophil response to C. albicans in the oral mucosa. PLoS Pathog. 2016;12(9):e1005882.
8. Karavitis J, Kovacs EJ. Macrophage phagocytosis: effects of environmental pollutants, alcohol, cigarette smoke, and other external factors. J Leukoc Biol. 2011;90(6):1065-1078.
9. Chiu C-H, Wang Y-C, Yeh K-M, Lin J-C, Siu LK, Chang F-Y. Influence of ethanol concentration in the phagocytic function of neutrophils against Klebsiella pneumoniae isolates in an experimental model. J Microbiol Immunol Infect. 2018;51(1):64-69.
10. Khocht A, Schleifer S, Janal M, Keller S. Neutrophil function and periodontitis in alcohol-dependent males without medical disorders. J Int Acad Periodontol. 2013;15(3):68-74.
11. Gandhi JA, Ekhar VV, Asplund MB, et al. Alcohol enhances Acinetobacter baumannii-associated pneumonia and systemic dissemination by impairing neutrophil antimicrobial activity in a murine model of infection. PLoS One. 2014;9(4):e95707.
12. Mohsenifar Z, Chopra SK, Johnson BL, Simmons DH. Candida pneumonia: experience with 20 patients. West J Med. 1979;131(3):196-200.
13. Jones JM. Laboratory diagnosis of invasive candidiasis. Clin Microbiol Rev. 1990;3(1):32-45.
14. Clancy CJ, Nguyen MH. Finding the “missing 50%” of invasive candidiasis: how nonculture diagnostics will improve understanding of disease spectrum and transform patient care. Clin Infect Dis. 2013;56(9):1284-1292.
15. Kappe R, Mu¨ ller J. Rapid clearance of Candida albicans mannan antigens by liver and spleen in contrast to prolonged circulation of Cryptococcus neoformans antigens. J Clin Microbiol. 1991;29(8):1665-1669.
16. Balk RA, Kadri SS, Cao Z, Robinson SB, Lipkin C, Bozzette SA. Effect of procalcitonin testing on health-care utilization and costs in critically ill patients in the United States. Chest. 2017;151(1):23-33.
17. Potrykus J, Stead D, Maccallum DM, et al. Fungal iron availability during deep seated candidiasis is defined by a complex interplay involving systemic and local events. PLoS Pathog. 2013;9(10):e1003676.
18. Soni NJ, Samson DJ, Galaydick JL, Vats V, Pitrak DL, Aronson N. Procalcitonin-Guided Antibiotic Therapy. Rockville, MD: Agency for Healthcare Research and Quality (US); 2012.
1. Navarathna DH, Stein EV, Lessey-Morillon EC, Nayak D, Martin-Manso G, Roberts DD. CD47 promotes protective innate and adaptive immunity in a mouse model of disseminated candidiasis. PLoS One. 2015;10(5):e0128220.
2. Kullberg BJ, Arendrup MC. Invasive candidiasis. N Engl J Med. 2015;373(15):1445-1456.
3. Clancy CJ, Nguyen MH. Diagnosing invasive candidiasis. J Clin Microbiol. 2018;56(5):e01909-e01917.
4. Ericson EL, Klingspor L, Ullberg M, Ozenci V. Clinical comparison of the Bactec Mycosis IC/F, BacT/Alert FA, and BacT/Alert FN blood culture vials for the detection of candidemia. Diagn Microbiol Infect Dis. 2012;73(2):153-156.
5. Baum GL. The significance of Candida albicans in human sputum. N Engl J Med. 1960;263:70-73.
6. el-Ebiary M, Torres A, Fàbregas N, et al. Significance of the isolation of Candida species from respiratory samples in critically ill, non-neutropenic patients. An immediate postmortem histologic study. Am J Respir Crit Care Med. 1997;156(2, pt 1):583-590.
7. Altmeier S, Toska A, Sparber F, Teijeira A, Halin C, LeibundGut-Landmann S. IL-1 coordinates the neutrophil response to C. albicans in the oral mucosa. PLoS Pathog. 2016;12(9):e1005882.
8. Karavitis J, Kovacs EJ. Macrophage phagocytosis: effects of environmental pollutants, alcohol, cigarette smoke, and other external factors. J Leukoc Biol. 2011;90(6):1065-1078.
9. Chiu C-H, Wang Y-C, Yeh K-M, Lin J-C, Siu LK, Chang F-Y. Influence of ethanol concentration in the phagocytic function of neutrophils against Klebsiella pneumoniae isolates in an experimental model. J Microbiol Immunol Infect. 2018;51(1):64-69.
10. Khocht A, Schleifer S, Janal M, Keller S. Neutrophil function and periodontitis in alcohol-dependent males without medical disorders. J Int Acad Periodontol. 2013;15(3):68-74.
11. Gandhi JA, Ekhar VV, Asplund MB, et al. Alcohol enhances Acinetobacter baumannii-associated pneumonia and systemic dissemination by impairing neutrophil antimicrobial activity in a murine model of infection. PLoS One. 2014;9(4):e95707.
12. Mohsenifar Z, Chopra SK, Johnson BL, Simmons DH. Candida pneumonia: experience with 20 patients. West J Med. 1979;131(3):196-200.
13. Jones JM. Laboratory diagnosis of invasive candidiasis. Clin Microbiol Rev. 1990;3(1):32-45.
14. Clancy CJ, Nguyen MH. Finding the “missing 50%” of invasive candidiasis: how nonculture diagnostics will improve understanding of disease spectrum and transform patient care. Clin Infect Dis. 2013;56(9):1284-1292.
15. Kappe R, Mu¨ ller J. Rapid clearance of Candida albicans mannan antigens by liver and spleen in contrast to prolonged circulation of Cryptococcus neoformans antigens. J Clin Microbiol. 1991;29(8):1665-1669.
16. Balk RA, Kadri SS, Cao Z, Robinson SB, Lipkin C, Bozzette SA. Effect of procalcitonin testing on health-care utilization and costs in critically ill patients in the United States. Chest. 2017;151(1):23-33.
17. Potrykus J, Stead D, Maccallum DM, et al. Fungal iron availability during deep seated candidiasis is defined by a complex interplay involving systemic and local events. PLoS Pathog. 2013;9(10):e1003676.
18. Soni NJ, Samson DJ, Galaydick JL, Vats V, Pitrak DL, Aronson N. Procalcitonin-Guided Antibiotic Therapy. Rockville, MD: Agency for Healthcare Research and Quality (US); 2012.