User login
EULAR updates vaccination recommendations for autoimmune inflammatory rheumatic disease patients
Vaccination status should be reviewed annually for patients with autoimmune inflammatory rheumatic diseases, according to updated recommendations from the European League Against Rheumatism.
Patients with autoimmune inflammatory rheumatic diseases (AIIRD) are at increased risk for infections, and vaccination has been shown to reduce risk by “potentially translating into a lower rate of hospital admissions due to infections, emergency room visits, and the rate of invasive infectious diseases,” wrote Victoria Furer, MD, of Tel Aviv Sourasky Medical Center, and members of the task force that updated the recommendations, which were published in Annals of the Rheumatic Diseases.
However, AIIRD patients often go unvaccinated because of a lack of awareness or concerns about vaccine safety and efficacy, they said (Ann Rheum Dis. 2019 Aug 14. doi: 10.1136/annrheumdis-2019-215882).
The task force consisted of 21 experts, including patients, rheumatologists, immunologists, an infectious disease specialist, and health professionals in rheumatology representing eight countries. They evaluated data from four systematic literature reviews and developed nine recommendations based on six key principles.
“For each recommendation, the level of evidence for the incidence/prevalence of vaccine preventable infection in AIIRD, and efficacy/immunogenicity/safety of vaccination were stated, when available, followed by the strength of recommendation and the level of agreement,” the task force wrote.
These overarching principles start with an annual assessment of vaccination status by the AIIRD patient’s rheumatology team. Other principles include explanation of an individualized vaccination program to the patient as a foundation for joint decision-making, vaccinating patients during quiescent disease periods, vaccinating in advance of planned immunosuppression when possible, considering non-live vaccines for AIIRD patients also treated with systemic glucocorticoids and DMARDs, and considering live-attenuated vaccines with caution.
Several of the nine recommendations developed by the task force are modified from the previous recommendations issued in 2011. The task force made its recommendations with an eye toward optimizing individual risk stratification and avoiding “unnecessary” vaccination in AIIRD patients with low risk of infection as part of the update process. A notable change from the 2011 guidelines is the recommendation of both influenza and pneumococcal vaccinations for the majority of patients with AIIRD as opposed to all patients to emphasize the importance of individualized risk assessment, the task force noted.
The recommendations state that influenza vaccination and pneumococcal vaccination should be “strongly considered” for patients with AIIRD, and patients also should receive tetanus toxoid vaccination according to recommendations for the general population. However, clinicians should consider passive immunization for patients treated with B-cell depleting therapy, the task force wrote.
AIIRD patients at risk for hepatitis A and B should receive vaccinations for those diseases, with boosters or passive immunization if indicated, and high-risk patients may consider herpes zoster vaccination, according to the recommendations.
In addition, AIIRD patients – especially patients with systemic lupus erythematosus – should receive human papilloma virus vaccination according to recommendations for the general population, but AIIRD patients should avoid yellow fever vaccination, the task force stated. However, for AIIRD patients traveling to areas of yellow fever risk, “withholding immunosuppressive therapy to allow a safe vaccination or measuring serology in previously exposed patients may be considered.”
Finally, mothers treated with biologics during the second half of pregnancy should avoid live-attenuated vaccines for their newborns, and immunocompetent household members of AIIRD patients should be encouraged to follow national guidelines for routine vaccination with the exception of the oral polio vaccine, the task force concluded.
Vaccination status should be reviewed annually for patients with autoimmune inflammatory rheumatic diseases, according to updated recommendations from the European League Against Rheumatism.
Patients with autoimmune inflammatory rheumatic diseases (AIIRD) are at increased risk for infections, and vaccination has been shown to reduce risk by “potentially translating into a lower rate of hospital admissions due to infections, emergency room visits, and the rate of invasive infectious diseases,” wrote Victoria Furer, MD, of Tel Aviv Sourasky Medical Center, and members of the task force that updated the recommendations, which were published in Annals of the Rheumatic Diseases.
However, AIIRD patients often go unvaccinated because of a lack of awareness or concerns about vaccine safety and efficacy, they said (Ann Rheum Dis. 2019 Aug 14. doi: 10.1136/annrheumdis-2019-215882).
The task force consisted of 21 experts, including patients, rheumatologists, immunologists, an infectious disease specialist, and health professionals in rheumatology representing eight countries. They evaluated data from four systematic literature reviews and developed nine recommendations based on six key principles.
“For each recommendation, the level of evidence for the incidence/prevalence of vaccine preventable infection in AIIRD, and efficacy/immunogenicity/safety of vaccination were stated, when available, followed by the strength of recommendation and the level of agreement,” the task force wrote.
These overarching principles start with an annual assessment of vaccination status by the AIIRD patient’s rheumatology team. Other principles include explanation of an individualized vaccination program to the patient as a foundation for joint decision-making, vaccinating patients during quiescent disease periods, vaccinating in advance of planned immunosuppression when possible, considering non-live vaccines for AIIRD patients also treated with systemic glucocorticoids and DMARDs, and considering live-attenuated vaccines with caution.
Several of the nine recommendations developed by the task force are modified from the previous recommendations issued in 2011. The task force made its recommendations with an eye toward optimizing individual risk stratification and avoiding “unnecessary” vaccination in AIIRD patients with low risk of infection as part of the update process. A notable change from the 2011 guidelines is the recommendation of both influenza and pneumococcal vaccinations for the majority of patients with AIIRD as opposed to all patients to emphasize the importance of individualized risk assessment, the task force noted.
The recommendations state that influenza vaccination and pneumococcal vaccination should be “strongly considered” for patients with AIIRD, and patients also should receive tetanus toxoid vaccination according to recommendations for the general population. However, clinicians should consider passive immunization for patients treated with B-cell depleting therapy, the task force wrote.
AIIRD patients at risk for hepatitis A and B should receive vaccinations for those diseases, with boosters or passive immunization if indicated, and high-risk patients may consider herpes zoster vaccination, according to the recommendations.
In addition, AIIRD patients – especially patients with systemic lupus erythematosus – should receive human papilloma virus vaccination according to recommendations for the general population, but AIIRD patients should avoid yellow fever vaccination, the task force stated. However, for AIIRD patients traveling to areas of yellow fever risk, “withholding immunosuppressive therapy to allow a safe vaccination or measuring serology in previously exposed patients may be considered.”
Finally, mothers treated with biologics during the second half of pregnancy should avoid live-attenuated vaccines for their newborns, and immunocompetent household members of AIIRD patients should be encouraged to follow national guidelines for routine vaccination with the exception of the oral polio vaccine, the task force concluded.
Vaccination status should be reviewed annually for patients with autoimmune inflammatory rheumatic diseases, according to updated recommendations from the European League Against Rheumatism.
Patients with autoimmune inflammatory rheumatic diseases (AIIRD) are at increased risk for infections, and vaccination has been shown to reduce risk by “potentially translating into a lower rate of hospital admissions due to infections, emergency room visits, and the rate of invasive infectious diseases,” wrote Victoria Furer, MD, of Tel Aviv Sourasky Medical Center, and members of the task force that updated the recommendations, which were published in Annals of the Rheumatic Diseases.
However, AIIRD patients often go unvaccinated because of a lack of awareness or concerns about vaccine safety and efficacy, they said (Ann Rheum Dis. 2019 Aug 14. doi: 10.1136/annrheumdis-2019-215882).
The task force consisted of 21 experts, including patients, rheumatologists, immunologists, an infectious disease specialist, and health professionals in rheumatology representing eight countries. They evaluated data from four systematic literature reviews and developed nine recommendations based on six key principles.
“For each recommendation, the level of evidence for the incidence/prevalence of vaccine preventable infection in AIIRD, and efficacy/immunogenicity/safety of vaccination were stated, when available, followed by the strength of recommendation and the level of agreement,” the task force wrote.
These overarching principles start with an annual assessment of vaccination status by the AIIRD patient’s rheumatology team. Other principles include explanation of an individualized vaccination program to the patient as a foundation for joint decision-making, vaccinating patients during quiescent disease periods, vaccinating in advance of planned immunosuppression when possible, considering non-live vaccines for AIIRD patients also treated with systemic glucocorticoids and DMARDs, and considering live-attenuated vaccines with caution.
Several of the nine recommendations developed by the task force are modified from the previous recommendations issued in 2011. The task force made its recommendations with an eye toward optimizing individual risk stratification and avoiding “unnecessary” vaccination in AIIRD patients with low risk of infection as part of the update process. A notable change from the 2011 guidelines is the recommendation of both influenza and pneumococcal vaccinations for the majority of patients with AIIRD as opposed to all patients to emphasize the importance of individualized risk assessment, the task force noted.
The recommendations state that influenza vaccination and pneumococcal vaccination should be “strongly considered” for patients with AIIRD, and patients also should receive tetanus toxoid vaccination according to recommendations for the general population. However, clinicians should consider passive immunization for patients treated with B-cell depleting therapy, the task force wrote.
AIIRD patients at risk for hepatitis A and B should receive vaccinations for those diseases, with boosters or passive immunization if indicated, and high-risk patients may consider herpes zoster vaccination, according to the recommendations.
In addition, AIIRD patients – especially patients with systemic lupus erythematosus – should receive human papilloma virus vaccination according to recommendations for the general population, but AIIRD patients should avoid yellow fever vaccination, the task force stated. However, for AIIRD patients traveling to areas of yellow fever risk, “withholding immunosuppressive therapy to allow a safe vaccination or measuring serology in previously exposed patients may be considered.”
Finally, mothers treated with biologics during the second half of pregnancy should avoid live-attenuated vaccines for their newborns, and immunocompetent household members of AIIRD patients should be encouraged to follow national guidelines for routine vaccination with the exception of the oral polio vaccine, the task force concluded.
FROM ANNALS OF THE RHEUMATIC DISEASES
Digital health and big data: New tools for making the most of real-world evidence
LAKE BUENA VISTA, FLA. – Digital health technology is vastly expanding the real-world data pool for clinical and comparative effectiveness research, according to Jeffrey Curtis, MD.
The trick is to harness the power of that data to improve patient care and outcomes, and that can be achieved in part through linkage of data sources and through point-of-care access, Dr. Curtis, professor of medicine in the division of clinical immunology and rheumatology at the University of Alabama at Birmingham (UAB), said at the annual meeting of the Florida Society of Rheumatology.
“We want to take care of patients, but probably what you and I also want is to have real-world evidence ... evidence relevant for people [we] take care of on a day-to-day basis – not people in highly selected phase 3 or even phase 4 trials,” he said.
Real-world data, which gained particular cachet through the 21st Century Cures Act permitting the Food and Drug Administration to consider real-world evidence as part of the regulatory process and in post-marketing surveillance, includes information from electronic health records (EHRs), health plan claims, traditional registries, and mobile health and technology, explained Dr. Curtis, who also is codirector of the UAB Pharmacoepidemiology and Pharmacoeconomics Unit.
“And you and I want it because patients are different, and in medicine we only have about 20% of patients where there is direct evidence about what we should do,” he added. “Give me the trial that describes the 75-year-old African American smoker with diabetes and how well he does on biologic du jour; there’s no trial like that, and yet you and I need to make those kinds of decisions in light of patients’ comorbidities and other features.”
Generating real-world evidence, however, requires new approaches and new tools, he said, explaining that efficiency is key for applying the data in busy practices, as is compatibility with delivering an intervention and with randomization.
Imagine using the EHR at the point of care to look up what happened to “the last 10 patients like this” based on how they were treated by you or your colleagues, he said.
“That would be useful information to have. In fact, the day is not so far in the future where you could, perhaps, randomize within your EHR if you had a clinically important question that really needed an answer and a protocol attached,” he added.
Real-world data collection
Pragmatic trials offer one approach to garnering real-world data by addressing a simple question – usually with a hard outcome – using very few inclusion and exclusion criteria, Dr. Curtis said, describing the recently completed VERVE Zoster Vaccine trial.
He and his colleagues randomized 617 patients from 33 sites to look at the safety of the live-virus Zostavax herpes zoster vaccine in rheumatoid arthritis patients over age 50 years on any anti–tumor necrosis factor (anti-TNF) therapy. Half of the patients received saline, the other half received the vaccine, and no cases of varicella zoster occurred in either group.
“So, to the extent that half of 617 people with zero cases was reassuring, we now have some evidence where heretofore there was none,” he said, noting that those results will be presented at the 2019 American College of Rheumatology annual meeting. “But the focus of this talk is not on vaccination, it’s really on how we do real-world effectiveness or safety studies in a way that doesn’t slow us way down and doesn’t require some big research operation.”
One way is through efficient recruitment, and depending on how complicated the study is, qualified patients may be easily identifiable through the EHR. In fact, numerous tools are available to codify and search both structured and unstructured data, Dr. Curtis said, noting that he and his colleagues used the web-based i2b2 Query Tool for the VERVE study.
The study sites that did the best with recruiting had the ability to search their own EHRs for patients who met the inclusion criteria, and those patients were then invited to participate. A short video was created to educate those who were interested, and a “knowledge review” quiz was administered afterward to ensure informed consent, which was provided via digital signature.
Health plan and other “big data” can also be very useful for answering certain questions. One example is how soon biologics should be stopped before elective orthopedic surgery? Dr. Curtis and colleagues looked at this using claims data for nearly 4,300 patients undergoing elective hip or knee arthroplasty and found no evidence that administering infliximab within 4 weeks of surgery increased serious infection risk within 30 days or prosthetic joint infection within 1 year.
“Where else are you going to go run a prospective study of 4,300 elective hips and knees,” he said, stressing that it wouldn’t be easy.
Other sources that can help generate real-world effectiveness data include traditional or single-center registries and EHR-based registries.
“The EHR registries are, I think, the newest that many are part of in our field,” he said, noting that “a number of groups are aggregating that,” including the ACR RISE registry and some physician groups, for example.
“What we’re really after is to have a clinically integrated network and a learning health care environment,” he explained, adding that the goal is to develop care pathways.
The approach represents a shift from evidence-based practice to practice-based evidence, he noted.
“When you and I practice, we’re generating that evidence and now we just need to harness that data to get smarter to take care of patients,” he said, adding that the lack of randomization for much of these data isn’t necessarily a problem.
“Do you have to randomize? I would argue that you don’t necessarily have to randomize if the source of variability in how we treat patients is very related to patients’ characteristics,” he said.
If the evidence for a specific approach is weak, or a decision is based on physician preference, physician practice, or insurance company considerations instead of patient characteristics, randomization may not be necessary, he explained.
In fact, insurance company requirements often create “natural experiments” that can be used to help identify better practices. For example, if one only covers adalimumab for first-line TNF inhibition, and another has a “different fail-first policy and that’s not first line and everybody gets some other TNF inhibitor, then I can probably compare those quite reasonably,” he said.
“That’s a great setting where you might not need randomization.”
Of note, “having more data sometimes trumps smarter algorithms,” but that means finding and linking more data that “exist in the wild,” Dr. Curtis said.
Linking data sources
When he and his colleagues wanted to assess the cost of not achieving RA remission, no single data source provided all of the information they needed. They used both CORRONA registry data and health claims data to look at various outcome measures across disease activity categories and with adjustment for comorbidity clusters. They previously reported on the feasibility and validity of the approach.
“We’re currently doing another project where one of the local Blue Cross plans said ‘I’m interested to support you to see how efficient you are; we will donate or loan you our claims data [and] let you link it to your practice so you can actually tell us ... cost conditional on [a patient’s] disease activity,’ ” he said.
Another example involves a recent study looking at biomarker-based cardiovascular disease risk prediction in RA using data from nearly 31,000 Medicare patients linked with multibiomarker disease activity (MBDA) test results, with which they “basically built and validated a risk prediction model,” he said.
The point is that such data linkage provided tools for use at the point of care that can predict CVD risk using “some simple things that you and I have in our EHR,” he said. “But you couldn’t do this if you had to assemble a prospective cohort of tens of thousands of arthritis patients and then wait years for follow-up.”
Patient-reported outcomes collected at the point of care and by patients at home between visits, such as digital data collected via wearable technology, can provide additional information to help improve patient care and management.
“My interest is not to think about [these data sources] in isolation, but really to think about how we bring these together,” he said. “I’m interested in maximizing value for both patients and clinicians, and not having to pick only one of these data sources, but really to harness several of them if that’s what we need to take better care of patients and to answer important questions.”
Doing so is increasingly important given the workforce shortage in rheumatology, he noted.
“The point is that we’re going to need to be a whole lot more efficient as a field because there are going to be fewer of us even at a time when more of us are needed,” he said.
It’s a topic in which the ACR has shown a lot of interest, he said, noting that he cochaired a preconference course on mobile health technologies at the 2018 ACR annual meeting and is involved with a similar course on “big data” ahead of the 2019 meeting.
The thought of making use of the various digital health and “big data” sources can be overwhelming, but the key is to start with the question that needs an answer or the problem that needs to be solved.
“Don’t start with the data,” he explained. “Start with [asking] ... ‘What am I trying to do?’ ”
Dr. Curtis reported funding from the National Institute on Arthritis and Musculoskeletal and Skin Diseases and the Patient-Centered Outcomes Research Institute. He has also consulted for or received research grants from Amgen, AbbVie, Bristol-Myers Squibb, CORRONA, Lilly, Janssen, Myriad, Novartis, Roche, Pfizer, and Sanofi/Regeneron.
LAKE BUENA VISTA, FLA. – Digital health technology is vastly expanding the real-world data pool for clinical and comparative effectiveness research, according to Jeffrey Curtis, MD.
The trick is to harness the power of that data to improve patient care and outcomes, and that can be achieved in part through linkage of data sources and through point-of-care access, Dr. Curtis, professor of medicine in the division of clinical immunology and rheumatology at the University of Alabama at Birmingham (UAB), said at the annual meeting of the Florida Society of Rheumatology.
“We want to take care of patients, but probably what you and I also want is to have real-world evidence ... evidence relevant for people [we] take care of on a day-to-day basis – not people in highly selected phase 3 or even phase 4 trials,” he said.
Real-world data, which gained particular cachet through the 21st Century Cures Act permitting the Food and Drug Administration to consider real-world evidence as part of the regulatory process and in post-marketing surveillance, includes information from electronic health records (EHRs), health plan claims, traditional registries, and mobile health and technology, explained Dr. Curtis, who also is codirector of the UAB Pharmacoepidemiology and Pharmacoeconomics Unit.
“And you and I want it because patients are different, and in medicine we only have about 20% of patients where there is direct evidence about what we should do,” he added. “Give me the trial that describes the 75-year-old African American smoker with diabetes and how well he does on biologic du jour; there’s no trial like that, and yet you and I need to make those kinds of decisions in light of patients’ comorbidities and other features.”
Generating real-world evidence, however, requires new approaches and new tools, he said, explaining that efficiency is key for applying the data in busy practices, as is compatibility with delivering an intervention and with randomization.
Imagine using the EHR at the point of care to look up what happened to “the last 10 patients like this” based on how they were treated by you or your colleagues, he said.
“That would be useful information to have. In fact, the day is not so far in the future where you could, perhaps, randomize within your EHR if you had a clinically important question that really needed an answer and a protocol attached,” he added.
Real-world data collection
Pragmatic trials offer one approach to garnering real-world data by addressing a simple question – usually with a hard outcome – using very few inclusion and exclusion criteria, Dr. Curtis said, describing the recently completed VERVE Zoster Vaccine trial.
He and his colleagues randomized 617 patients from 33 sites to look at the safety of the live-virus Zostavax herpes zoster vaccine in rheumatoid arthritis patients over age 50 years on any anti–tumor necrosis factor (anti-TNF) therapy. Half of the patients received saline, the other half received the vaccine, and no cases of varicella zoster occurred in either group.
“So, to the extent that half of 617 people with zero cases was reassuring, we now have some evidence where heretofore there was none,” he said, noting that those results will be presented at the 2019 American College of Rheumatology annual meeting. “But the focus of this talk is not on vaccination, it’s really on how we do real-world effectiveness or safety studies in a way that doesn’t slow us way down and doesn’t require some big research operation.”
One way is through efficient recruitment, and depending on how complicated the study is, qualified patients may be easily identifiable through the EHR. In fact, numerous tools are available to codify and search both structured and unstructured data, Dr. Curtis said, noting that he and his colleagues used the web-based i2b2 Query Tool for the VERVE study.
The study sites that did the best with recruiting had the ability to search their own EHRs for patients who met the inclusion criteria, and those patients were then invited to participate. A short video was created to educate those who were interested, and a “knowledge review” quiz was administered afterward to ensure informed consent, which was provided via digital signature.
Health plan and other “big data” can also be very useful for answering certain questions. One example is how soon biologics should be stopped before elective orthopedic surgery? Dr. Curtis and colleagues looked at this using claims data for nearly 4,300 patients undergoing elective hip or knee arthroplasty and found no evidence that administering infliximab within 4 weeks of surgery increased serious infection risk within 30 days or prosthetic joint infection within 1 year.
“Where else are you going to go run a prospective study of 4,300 elective hips and knees,” he said, stressing that it wouldn’t be easy.
Other sources that can help generate real-world effectiveness data include traditional or single-center registries and EHR-based registries.
“The EHR registries are, I think, the newest that many are part of in our field,” he said, noting that “a number of groups are aggregating that,” including the ACR RISE registry and some physician groups, for example.
“What we’re really after is to have a clinically integrated network and a learning health care environment,” he explained, adding that the goal is to develop care pathways.
The approach represents a shift from evidence-based practice to practice-based evidence, he noted.
“When you and I practice, we’re generating that evidence and now we just need to harness that data to get smarter to take care of patients,” he said, adding that the lack of randomization for much of these data isn’t necessarily a problem.
“Do you have to randomize? I would argue that you don’t necessarily have to randomize if the source of variability in how we treat patients is very related to patients’ characteristics,” he said.
If the evidence for a specific approach is weak, or a decision is based on physician preference, physician practice, or insurance company considerations instead of patient characteristics, randomization may not be necessary, he explained.
In fact, insurance company requirements often create “natural experiments” that can be used to help identify better practices. For example, if one only covers adalimumab for first-line TNF inhibition, and another has a “different fail-first policy and that’s not first line and everybody gets some other TNF inhibitor, then I can probably compare those quite reasonably,” he said.
“That’s a great setting where you might not need randomization.”
Of note, “having more data sometimes trumps smarter algorithms,” but that means finding and linking more data that “exist in the wild,” Dr. Curtis said.
Linking data sources
When he and his colleagues wanted to assess the cost of not achieving RA remission, no single data source provided all of the information they needed. They used both CORRONA registry data and health claims data to look at various outcome measures across disease activity categories and with adjustment for comorbidity clusters. They previously reported on the feasibility and validity of the approach.
“We’re currently doing another project where one of the local Blue Cross plans said ‘I’m interested to support you to see how efficient you are; we will donate or loan you our claims data [and] let you link it to your practice so you can actually tell us ... cost conditional on [a patient’s] disease activity,’ ” he said.
Another example involves a recent study looking at biomarker-based cardiovascular disease risk prediction in RA using data from nearly 31,000 Medicare patients linked with multibiomarker disease activity (MBDA) test results, with which they “basically built and validated a risk prediction model,” he said.
The point is that such data linkage provided tools for use at the point of care that can predict CVD risk using “some simple things that you and I have in our EHR,” he said. “But you couldn’t do this if you had to assemble a prospective cohort of tens of thousands of arthritis patients and then wait years for follow-up.”
Patient-reported outcomes collected at the point of care and by patients at home between visits, such as digital data collected via wearable technology, can provide additional information to help improve patient care and management.
“My interest is not to think about [these data sources] in isolation, but really to think about how we bring these together,” he said. “I’m interested in maximizing value for both patients and clinicians, and not having to pick only one of these data sources, but really to harness several of them if that’s what we need to take better care of patients and to answer important questions.”
Doing so is increasingly important given the workforce shortage in rheumatology, he noted.
“The point is that we’re going to need to be a whole lot more efficient as a field because there are going to be fewer of us even at a time when more of us are needed,” he said.
It’s a topic in which the ACR has shown a lot of interest, he said, noting that he cochaired a preconference course on mobile health technologies at the 2018 ACR annual meeting and is involved with a similar course on “big data” ahead of the 2019 meeting.
The thought of making use of the various digital health and “big data” sources can be overwhelming, but the key is to start with the question that needs an answer or the problem that needs to be solved.
“Don’t start with the data,” he explained. “Start with [asking] ... ‘What am I trying to do?’ ”
Dr. Curtis reported funding from the National Institute on Arthritis and Musculoskeletal and Skin Diseases and the Patient-Centered Outcomes Research Institute. He has also consulted for or received research grants from Amgen, AbbVie, Bristol-Myers Squibb, CORRONA, Lilly, Janssen, Myriad, Novartis, Roche, Pfizer, and Sanofi/Regeneron.
LAKE BUENA VISTA, FLA. – Digital health technology is vastly expanding the real-world data pool for clinical and comparative effectiveness research, according to Jeffrey Curtis, MD.
The trick is to harness the power of that data to improve patient care and outcomes, and that can be achieved in part through linkage of data sources and through point-of-care access, Dr. Curtis, professor of medicine in the division of clinical immunology and rheumatology at the University of Alabama at Birmingham (UAB), said at the annual meeting of the Florida Society of Rheumatology.
“We want to take care of patients, but probably what you and I also want is to have real-world evidence ... evidence relevant for people [we] take care of on a day-to-day basis – not people in highly selected phase 3 or even phase 4 trials,” he said.
Real-world data, which gained particular cachet through the 21st Century Cures Act permitting the Food and Drug Administration to consider real-world evidence as part of the regulatory process and in post-marketing surveillance, includes information from electronic health records (EHRs), health plan claims, traditional registries, and mobile health and technology, explained Dr. Curtis, who also is codirector of the UAB Pharmacoepidemiology and Pharmacoeconomics Unit.
“And you and I want it because patients are different, and in medicine we only have about 20% of patients where there is direct evidence about what we should do,” he added. “Give me the trial that describes the 75-year-old African American smoker with diabetes and how well he does on biologic du jour; there’s no trial like that, and yet you and I need to make those kinds of decisions in light of patients’ comorbidities and other features.”
Generating real-world evidence, however, requires new approaches and new tools, he said, explaining that efficiency is key for applying the data in busy practices, as is compatibility with delivering an intervention and with randomization.
Imagine using the EHR at the point of care to look up what happened to “the last 10 patients like this” based on how they were treated by you or your colleagues, he said.
“That would be useful information to have. In fact, the day is not so far in the future where you could, perhaps, randomize within your EHR if you had a clinically important question that really needed an answer and a protocol attached,” he added.
Real-world data collection
Pragmatic trials offer one approach to garnering real-world data by addressing a simple question – usually with a hard outcome – using very few inclusion and exclusion criteria, Dr. Curtis said, describing the recently completed VERVE Zoster Vaccine trial.
He and his colleagues randomized 617 patients from 33 sites to look at the safety of the live-virus Zostavax herpes zoster vaccine in rheumatoid arthritis patients over age 50 years on any anti–tumor necrosis factor (anti-TNF) therapy. Half of the patients received saline, the other half received the vaccine, and no cases of varicella zoster occurred in either group.
“So, to the extent that half of 617 people with zero cases was reassuring, we now have some evidence where heretofore there was none,” he said, noting that those results will be presented at the 2019 American College of Rheumatology annual meeting. “But the focus of this talk is not on vaccination, it’s really on how we do real-world effectiveness or safety studies in a way that doesn’t slow us way down and doesn’t require some big research operation.”
One way is through efficient recruitment, and depending on how complicated the study is, qualified patients may be easily identifiable through the EHR. In fact, numerous tools are available to codify and search both structured and unstructured data, Dr. Curtis said, noting that he and his colleagues used the web-based i2b2 Query Tool for the VERVE study.
The study sites that did the best with recruiting had the ability to search their own EHRs for patients who met the inclusion criteria, and those patients were then invited to participate. A short video was created to educate those who were interested, and a “knowledge review” quiz was administered afterward to ensure informed consent, which was provided via digital signature.
Health plan and other “big data” can also be very useful for answering certain questions. One example is how soon biologics should be stopped before elective orthopedic surgery? Dr. Curtis and colleagues looked at this using claims data for nearly 4,300 patients undergoing elective hip or knee arthroplasty and found no evidence that administering infliximab within 4 weeks of surgery increased serious infection risk within 30 days or prosthetic joint infection within 1 year.
“Where else are you going to go run a prospective study of 4,300 elective hips and knees,” he said, stressing that it wouldn’t be easy.
Other sources that can help generate real-world effectiveness data include traditional or single-center registries and EHR-based registries.
“The EHR registries are, I think, the newest that many are part of in our field,” he said, noting that “a number of groups are aggregating that,” including the ACR RISE registry and some physician groups, for example.
“What we’re really after is to have a clinically integrated network and a learning health care environment,” he explained, adding that the goal is to develop care pathways.
The approach represents a shift from evidence-based practice to practice-based evidence, he noted.
“When you and I practice, we’re generating that evidence and now we just need to harness that data to get smarter to take care of patients,” he said, adding that the lack of randomization for much of these data isn’t necessarily a problem.
“Do you have to randomize? I would argue that you don’t necessarily have to randomize if the source of variability in how we treat patients is very related to patients’ characteristics,” he said.
If the evidence for a specific approach is weak, or a decision is based on physician preference, physician practice, or insurance company considerations instead of patient characteristics, randomization may not be necessary, he explained.
In fact, insurance company requirements often create “natural experiments” that can be used to help identify better practices. For example, if one only covers adalimumab for first-line TNF inhibition, and another has a “different fail-first policy and that’s not first line and everybody gets some other TNF inhibitor, then I can probably compare those quite reasonably,” he said.
“That’s a great setting where you might not need randomization.”
Of note, “having more data sometimes trumps smarter algorithms,” but that means finding and linking more data that “exist in the wild,” Dr. Curtis said.
Linking data sources
When he and his colleagues wanted to assess the cost of not achieving RA remission, no single data source provided all of the information they needed. They used both CORRONA registry data and health claims data to look at various outcome measures across disease activity categories and with adjustment for comorbidity clusters. They previously reported on the feasibility and validity of the approach.
“We’re currently doing another project where one of the local Blue Cross plans said ‘I’m interested to support you to see how efficient you are; we will donate or loan you our claims data [and] let you link it to your practice so you can actually tell us ... cost conditional on [a patient’s] disease activity,’ ” he said.
Another example involves a recent study looking at biomarker-based cardiovascular disease risk prediction in RA using data from nearly 31,000 Medicare patients linked with multibiomarker disease activity (MBDA) test results, with which they “basically built and validated a risk prediction model,” he said.
The point is that such data linkage provided tools for use at the point of care that can predict CVD risk using “some simple things that you and I have in our EHR,” he said. “But you couldn’t do this if you had to assemble a prospective cohort of tens of thousands of arthritis patients and then wait years for follow-up.”
Patient-reported outcomes collected at the point of care and by patients at home between visits, such as digital data collected via wearable technology, can provide additional information to help improve patient care and management.
“My interest is not to think about [these data sources] in isolation, but really to think about how we bring these together,” he said. “I’m interested in maximizing value for both patients and clinicians, and not having to pick only one of these data sources, but really to harness several of them if that’s what we need to take better care of patients and to answer important questions.”
Doing so is increasingly important given the workforce shortage in rheumatology, he noted.
“The point is that we’re going to need to be a whole lot more efficient as a field because there are going to be fewer of us even at a time when more of us are needed,” he said.
It’s a topic in which the ACR has shown a lot of interest, he said, noting that he cochaired a preconference course on mobile health technologies at the 2018 ACR annual meeting and is involved with a similar course on “big data” ahead of the 2019 meeting.
The thought of making use of the various digital health and “big data” sources can be overwhelming, but the key is to start with the question that needs an answer or the problem that needs to be solved.
“Don’t start with the data,” he explained. “Start with [asking] ... ‘What am I trying to do?’ ”
Dr. Curtis reported funding from the National Institute on Arthritis and Musculoskeletal and Skin Diseases and the Patient-Centered Outcomes Research Institute. He has also consulted for or received research grants from Amgen, AbbVie, Bristol-Myers Squibb, CORRONA, Lilly, Janssen, Myriad, Novartis, Roche, Pfizer, and Sanofi/Regeneron.
EXPERT ANALYSIS FROM FSR 2019
Analysis finds no mortality reductions with osteoporosis drugs
A paper published in JAMA Internal Medicine analyzed data from 38 randomized, placebo-controlled clinical trials of osteoporosis drugs involving a total of 101,642 participants.
“Studies have estimated that less than 30% of the mortality following hip and vertebral fractures may be attributed to the fracture itself and, therefore, potentially avoidable by preventing the fracture,” wrote Steven R. Cummings, MD, of the San Francisco Coordinating Center at the University of California, San Francisco, and colleagues. “Some studies have suggested that treatments for osteoporosis may directly reduce overall mortality rates in addition to decreasing fracture risk.”
Despite including a diversity of drugs including bisphosphonates, denosumab (Prolia), selective estrogen receptor modulators, parathyroid hormone analogues, odanacatib, and romosozumab (Evenity), the analysis found no significant association between receiving a drug treatment for osteoporosis and overall mortality.
The researchers did a separate analysis of the 21 clinical trials of bisphosphonate treatments, again finding no impact of the treatment on overall mortality. Similarly, analysis of six zoledronate clinical trials found no statistically significant impact on mortality, although the authors noted that there was some heterogeneity in the results. For example, two large trials found 28% and 35% reductions in mortality, however these effects were not seen in another other zoledronate trials.
An analysis limited to nitrogen-containing bisphosphonates (alendronate, risedronate, ibandronate, and zoledronate) showed a nonsignificant trend toward lower overall mortality, although this became even less statistically significant when trials of zoledronate were excluded.
“More data from placebo-controlled clinical trials of zoledronate therapy and mortality rates are needed to resolve whether treatment with zoledronate is associated with reduced mortality in addition to decreased fracture risk,” the authors wrote.
They added that the 25%-60% mortality reductions seen in previous observational were too large to be attributable solely to reductions in the risk of fracture, but were perhaps the result of unmeasured confounders that could have contributed to lower mortality.
“The apparent reduction in mortality may be an example of the ‘healthy adherer effect,’ which has been documented in studies reporting that participants who adhered to placebo treatment in clinical trials had lower mortality,” they wrote, citing data from the Women’s Health Study that showed 36% lower mortality in those who were at least 80% adherent to placebo.
“This effect is particularly applicable to observational studies of treatments for osteoporosis because only an estimated half of women taking oral drugs for the treatment of osteoporosis continued the regimen for 1 year, and even fewer continued longer,” they added.
They did note one limitation of their analysis was that it did not include a large clinical trial of the antiresorptive drug odanacatib, which was only available in abstract form at the time.
One author reported receiving grants and personal fees from a pharmaceutical company during the conduct of the study, and another reported receiving grants and personal fees outside the submitted work. No other conflicts of interest were reported.
SOURCE: Cummings SR et al. JAMA Intern Med. 2019 Aug 19. doi: 10.1001/jamainternmed.2019.2779.
A paper published in JAMA Internal Medicine analyzed data from 38 randomized, placebo-controlled clinical trials of osteoporosis drugs involving a total of 101,642 participants.
“Studies have estimated that less than 30% of the mortality following hip and vertebral fractures may be attributed to the fracture itself and, therefore, potentially avoidable by preventing the fracture,” wrote Steven R. Cummings, MD, of the San Francisco Coordinating Center at the University of California, San Francisco, and colleagues. “Some studies have suggested that treatments for osteoporosis may directly reduce overall mortality rates in addition to decreasing fracture risk.”
Despite including a diversity of drugs including bisphosphonates, denosumab (Prolia), selective estrogen receptor modulators, parathyroid hormone analogues, odanacatib, and romosozumab (Evenity), the analysis found no significant association between receiving a drug treatment for osteoporosis and overall mortality.
The researchers did a separate analysis of the 21 clinical trials of bisphosphonate treatments, again finding no impact of the treatment on overall mortality. Similarly, analysis of six zoledronate clinical trials found no statistically significant impact on mortality, although the authors noted that there was some heterogeneity in the results. For example, two large trials found 28% and 35% reductions in mortality, however these effects were not seen in another other zoledronate trials.
An analysis limited to nitrogen-containing bisphosphonates (alendronate, risedronate, ibandronate, and zoledronate) showed a nonsignificant trend toward lower overall mortality, although this became even less statistically significant when trials of zoledronate were excluded.
“More data from placebo-controlled clinical trials of zoledronate therapy and mortality rates are needed to resolve whether treatment with zoledronate is associated with reduced mortality in addition to decreased fracture risk,” the authors wrote.
They added that the 25%-60% mortality reductions seen in previous observational were too large to be attributable solely to reductions in the risk of fracture, but were perhaps the result of unmeasured confounders that could have contributed to lower mortality.
“The apparent reduction in mortality may be an example of the ‘healthy adherer effect,’ which has been documented in studies reporting that participants who adhered to placebo treatment in clinical trials had lower mortality,” they wrote, citing data from the Women’s Health Study that showed 36% lower mortality in those who were at least 80% adherent to placebo.
“This effect is particularly applicable to observational studies of treatments for osteoporosis because only an estimated half of women taking oral drugs for the treatment of osteoporosis continued the regimen for 1 year, and even fewer continued longer,” they added.
They did note one limitation of their analysis was that it did not include a large clinical trial of the antiresorptive drug odanacatib, which was only available in abstract form at the time.
One author reported receiving grants and personal fees from a pharmaceutical company during the conduct of the study, and another reported receiving grants and personal fees outside the submitted work. No other conflicts of interest were reported.
SOURCE: Cummings SR et al. JAMA Intern Med. 2019 Aug 19. doi: 10.1001/jamainternmed.2019.2779.
A paper published in JAMA Internal Medicine analyzed data from 38 randomized, placebo-controlled clinical trials of osteoporosis drugs involving a total of 101,642 participants.
“Studies have estimated that less than 30% of the mortality following hip and vertebral fractures may be attributed to the fracture itself and, therefore, potentially avoidable by preventing the fracture,” wrote Steven R. Cummings, MD, of the San Francisco Coordinating Center at the University of California, San Francisco, and colleagues. “Some studies have suggested that treatments for osteoporosis may directly reduce overall mortality rates in addition to decreasing fracture risk.”
Despite including a diversity of drugs including bisphosphonates, denosumab (Prolia), selective estrogen receptor modulators, parathyroid hormone analogues, odanacatib, and romosozumab (Evenity), the analysis found no significant association between receiving a drug treatment for osteoporosis and overall mortality.
The researchers did a separate analysis of the 21 clinical trials of bisphosphonate treatments, again finding no impact of the treatment on overall mortality. Similarly, analysis of six zoledronate clinical trials found no statistically significant impact on mortality, although the authors noted that there was some heterogeneity in the results. For example, two large trials found 28% and 35% reductions in mortality, however these effects were not seen in another other zoledronate trials.
An analysis limited to nitrogen-containing bisphosphonates (alendronate, risedronate, ibandronate, and zoledronate) showed a nonsignificant trend toward lower overall mortality, although this became even less statistically significant when trials of zoledronate were excluded.
“More data from placebo-controlled clinical trials of zoledronate therapy and mortality rates are needed to resolve whether treatment with zoledronate is associated with reduced mortality in addition to decreased fracture risk,” the authors wrote.
They added that the 25%-60% mortality reductions seen in previous observational were too large to be attributable solely to reductions in the risk of fracture, but were perhaps the result of unmeasured confounders that could have contributed to lower mortality.
“The apparent reduction in mortality may be an example of the ‘healthy adherer effect,’ which has been documented in studies reporting that participants who adhered to placebo treatment in clinical trials had lower mortality,” they wrote, citing data from the Women’s Health Study that showed 36% lower mortality in those who were at least 80% adherent to placebo.
“This effect is particularly applicable to observational studies of treatments for osteoporosis because only an estimated half of women taking oral drugs for the treatment of osteoporosis continued the regimen for 1 year, and even fewer continued longer,” they added.
They did note one limitation of their analysis was that it did not include a large clinical trial of the antiresorptive drug odanacatib, which was only available in abstract form at the time.
One author reported receiving grants and personal fees from a pharmaceutical company during the conduct of the study, and another reported receiving grants and personal fees outside the submitted work. No other conflicts of interest were reported.
SOURCE: Cummings SR et al. JAMA Intern Med. 2019 Aug 19. doi: 10.1001/jamainternmed.2019.2779.
FROM JAMA INTERNAL MEDICINE
FDA approves upadacitinib for rheumatoid arthritis
according to a release from its developer. The indication specifies that patients must have shown inadequate response or intolerance to methotrexate.
The approval is based on the SELECT program, which included 4,400 patients across five studies that tested the oral Janus kinase inhibitor in various settings, such as alone or with methotrexate. All primary and secondary endpoints were met in these trials. For example, among patients with inadequate response to methotrexate in one study, 68% of those treated with 15-mg upadacitinib monotherapy achieved 20% improvement in American College of Rheumatology response criteria (ACR20) at week 14 versus 41% of those who had continued on methotrexate. In another study of patients with in adequate response to methotrexate, 71% of those treated with upadacitinib/methotrexate combination therapy achieved ACR20 at week 12 versus 36% of those receiving placebo and methotrexate. Compared with other treatments, better rates of clinical remission and radiographic inhibition were seen with upadacitinib-based therapies, too.
Upadacitinib carries a boxed warning – the most serious warning the FDA issues – for risks of serious infection, malignancy, and thrombosis; these serious risks should be weighed against treatment benefits in any patients with increased risk for these problems or currently experiencing any of them. Concomitant use with other JAK inhibitors, biologic DMARDs, or with potent immunosuppressants is not recommended; its use also is not recommended for patients with severe hepatic impairment. The most common adverse reactions are upper respiratory tract infection, nausea, cough, and pyrexia. Live vaccines should be avoided with patients taking this drug, and patients who are breastfeeding should be advised against use of it.
More prescribing information can be found in the drug’s label, which can be found on the FDA’s website.
Upadacitinib is also being evaluated for treatment of other immune-mediated diseases.
according to a release from its developer. The indication specifies that patients must have shown inadequate response or intolerance to methotrexate.
The approval is based on the SELECT program, which included 4,400 patients across five studies that tested the oral Janus kinase inhibitor in various settings, such as alone or with methotrexate. All primary and secondary endpoints were met in these trials. For example, among patients with inadequate response to methotrexate in one study, 68% of those treated with 15-mg upadacitinib monotherapy achieved 20% improvement in American College of Rheumatology response criteria (ACR20) at week 14 versus 41% of those who had continued on methotrexate. In another study of patients with in adequate response to methotrexate, 71% of those treated with upadacitinib/methotrexate combination therapy achieved ACR20 at week 12 versus 36% of those receiving placebo and methotrexate. Compared with other treatments, better rates of clinical remission and radiographic inhibition were seen with upadacitinib-based therapies, too.
Upadacitinib carries a boxed warning – the most serious warning the FDA issues – for risks of serious infection, malignancy, and thrombosis; these serious risks should be weighed against treatment benefits in any patients with increased risk for these problems or currently experiencing any of them. Concomitant use with other JAK inhibitors, biologic DMARDs, or with potent immunosuppressants is not recommended; its use also is not recommended for patients with severe hepatic impairment. The most common adverse reactions are upper respiratory tract infection, nausea, cough, and pyrexia. Live vaccines should be avoided with patients taking this drug, and patients who are breastfeeding should be advised against use of it.
More prescribing information can be found in the drug’s label, which can be found on the FDA’s website.
Upadacitinib is also being evaluated for treatment of other immune-mediated diseases.
according to a release from its developer. The indication specifies that patients must have shown inadequate response or intolerance to methotrexate.
The approval is based on the SELECT program, which included 4,400 patients across five studies that tested the oral Janus kinase inhibitor in various settings, such as alone or with methotrexate. All primary and secondary endpoints were met in these trials. For example, among patients with inadequate response to methotrexate in one study, 68% of those treated with 15-mg upadacitinib monotherapy achieved 20% improvement in American College of Rheumatology response criteria (ACR20) at week 14 versus 41% of those who had continued on methotrexate. In another study of patients with in adequate response to methotrexate, 71% of those treated with upadacitinib/methotrexate combination therapy achieved ACR20 at week 12 versus 36% of those receiving placebo and methotrexate. Compared with other treatments, better rates of clinical remission and radiographic inhibition were seen with upadacitinib-based therapies, too.
Upadacitinib carries a boxed warning – the most serious warning the FDA issues – for risks of serious infection, malignancy, and thrombosis; these serious risks should be weighed against treatment benefits in any patients with increased risk for these problems or currently experiencing any of them. Concomitant use with other JAK inhibitors, biologic DMARDs, or with potent immunosuppressants is not recommended; its use also is not recommended for patients with severe hepatic impairment. The most common adverse reactions are upper respiratory tract infection, nausea, cough, and pyrexia. Live vaccines should be avoided with patients taking this drug, and patients who are breastfeeding should be advised against use of it.
More prescribing information can be found in the drug’s label, which can be found on the FDA’s website.
Upadacitinib is also being evaluated for treatment of other immune-mediated diseases.
Bisphosphonates improve BMD in pediatric rheumatic disease
Prophylactic treatment with bisphosphonates could significantly improve bone mineral density (BMD) in children and adolescents receiving steroids for chronic rheumatic disease, a study has found.
A paper published in EClinicalMedicine reported the outcomes of a multicenter, double-dummy, double-blind, placebo-controlled trial involving 217 patients who were receiving steroid therapy for juvenile idiopathic arthritis, juvenile systemic lupus erythematosus, juvenile dermatomyositis, or juvenile vasculitis. The patients were randomized to risedronate, alfacalcidol, or placebo, and all of the participants received 500 mg calcium and 400 IU vitamin D daily.
Lumbar spine and total body (less head) BMD increased in all groups, but the greatest increase was seen in patients treated with risedronate.
After 1 year, lumbar spine and total body (less head) BMD had increased in all groups, compared with baseline, but the greatest increase was seen in patients who had been treated with risedronate.
The lumbar spine areal BMD z score remained the same in the placebo group (−1.15 to −1.13), decreased from −0.96 to −1.00 in the alfacalcidol group, and increased from −0.99 to −0.75 in the risedronate group.
The change in z scores was significantly different between placebo and risedronate groups, and between risedronate and alfacalcidol groups, but not between placebo and alfacalcidol.
“The acquisition of adequate peak bone mass is not only important for the young person in reducing fracture risk but also has significant implications for the development of osteoporosis in later life, if peak bone mass is suboptimal,” wrote Madeleine Rooney, MBBCH, from the Queens University of Belfast, Northern Ireland, and associates.
There were no significant differences between the three groups in fracture rates. However, researchers were also able to compare Genant scores for vertebral fractures in 187 patients with pre- and posttreatment lateral spinal x-rays. That showed that the 54 patients in the placebo arm and 52 patients in the alfacalcidol arm had no change in their baseline Genant score of 0 (normal). However, although all 53 patients in the risedronate group had a Genant score of 0 at baseline, at 1-year follow-up, 2 patients had a Genant score of 1 (mild fracture), and 1 patient had a score of 3 (severe fracture).
In biochemical parameters, researchers saw a drop in parathyroid hormone in the placebo and alfacalcidol groups, but a rise in the risedronate group. However, the authors were not able to see any changes in bone markers that might have indicated which patients responded better to treatment.
Around 90% of participants in each group were also being treated with disease-modifying antirheumatic drugs. The rates of biologic use were 10.5% in the placebo group, 23.9% in the alfacalcidol group, and 10.1% in the risedronate group.
The researchers also noted a 7% higher rate of serious adverse events in the risedronate group, but emphasized that there were no differences in events related to the treatment.
In an accompanying editorial, Ian R. Reid, MBBCH, of the department of medicine, University of Auckland (New Zealand) noted that the study was an important step toward finding interventions for the prevention of steroid-induced bone loss in children. “The present study indicates that risedronate, and probably other potent bisphosphonates, can provide bone preservation in children and young people receiving therapeutic doses of glucocorticoid drugs, whereas alfacalcidol is without benefit. The targeted use of bisphosphonates in children and young people judged to be at significant fracture risk is appropriate. However, whether preventing loss of bone density will reduce fracture incidence remains to be established.”
The study was funded by Arthritis Research UK. No conflicts of interest were declared.
SOURCE: Rooney M et al. EClinicalMedicine. 2019 Jul 3. doi: 10.1016/j.eclinm.2019.06.004.
Prophylactic treatment with bisphosphonates could significantly improve bone mineral density (BMD) in children and adolescents receiving steroids for chronic rheumatic disease, a study has found.
A paper published in EClinicalMedicine reported the outcomes of a multicenter, double-dummy, double-blind, placebo-controlled trial involving 217 patients who were receiving steroid therapy for juvenile idiopathic arthritis, juvenile systemic lupus erythematosus, juvenile dermatomyositis, or juvenile vasculitis. The patients were randomized to risedronate, alfacalcidol, or placebo, and all of the participants received 500 mg calcium and 400 IU vitamin D daily.
Lumbar spine and total body (less head) BMD increased in all groups, but the greatest increase was seen in patients treated with risedronate.
After 1 year, lumbar spine and total body (less head) BMD had increased in all groups, compared with baseline, but the greatest increase was seen in patients who had been treated with risedronate.
The lumbar spine areal BMD z score remained the same in the placebo group (−1.15 to −1.13), decreased from −0.96 to −1.00 in the alfacalcidol group, and increased from −0.99 to −0.75 in the risedronate group.
The change in z scores was significantly different between placebo and risedronate groups, and between risedronate and alfacalcidol groups, but not between placebo and alfacalcidol.
“The acquisition of adequate peak bone mass is not only important for the young person in reducing fracture risk but also has significant implications for the development of osteoporosis in later life, if peak bone mass is suboptimal,” wrote Madeleine Rooney, MBBCH, from the Queens University of Belfast, Northern Ireland, and associates.
There were no significant differences between the three groups in fracture rates. However, researchers were also able to compare Genant scores for vertebral fractures in 187 patients with pre- and posttreatment lateral spinal x-rays. That showed that the 54 patients in the placebo arm and 52 patients in the alfacalcidol arm had no change in their baseline Genant score of 0 (normal). However, although all 53 patients in the risedronate group had a Genant score of 0 at baseline, at 1-year follow-up, 2 patients had a Genant score of 1 (mild fracture), and 1 patient had a score of 3 (severe fracture).
In biochemical parameters, researchers saw a drop in parathyroid hormone in the placebo and alfacalcidol groups, but a rise in the risedronate group. However, the authors were not able to see any changes in bone markers that might have indicated which patients responded better to treatment.
Around 90% of participants in each group were also being treated with disease-modifying antirheumatic drugs. The rates of biologic use were 10.5% in the placebo group, 23.9% in the alfacalcidol group, and 10.1% in the risedronate group.
The researchers also noted a 7% higher rate of serious adverse events in the risedronate group, but emphasized that there were no differences in events related to the treatment.
In an accompanying editorial, Ian R. Reid, MBBCH, of the department of medicine, University of Auckland (New Zealand) noted that the study was an important step toward finding interventions for the prevention of steroid-induced bone loss in children. “The present study indicates that risedronate, and probably other potent bisphosphonates, can provide bone preservation in children and young people receiving therapeutic doses of glucocorticoid drugs, whereas alfacalcidol is without benefit. The targeted use of bisphosphonates in children and young people judged to be at significant fracture risk is appropriate. However, whether preventing loss of bone density will reduce fracture incidence remains to be established.”
The study was funded by Arthritis Research UK. No conflicts of interest were declared.
SOURCE: Rooney M et al. EClinicalMedicine. 2019 Jul 3. doi: 10.1016/j.eclinm.2019.06.004.
Prophylactic treatment with bisphosphonates could significantly improve bone mineral density (BMD) in children and adolescents receiving steroids for chronic rheumatic disease, a study has found.
A paper published in EClinicalMedicine reported the outcomes of a multicenter, double-dummy, double-blind, placebo-controlled trial involving 217 patients who were receiving steroid therapy for juvenile idiopathic arthritis, juvenile systemic lupus erythematosus, juvenile dermatomyositis, or juvenile vasculitis. The patients were randomized to risedronate, alfacalcidol, or placebo, and all of the participants received 500 mg calcium and 400 IU vitamin D daily.
Lumbar spine and total body (less head) BMD increased in all groups, but the greatest increase was seen in patients treated with risedronate.
After 1 year, lumbar spine and total body (less head) BMD had increased in all groups, compared with baseline, but the greatest increase was seen in patients who had been treated with risedronate.
The lumbar spine areal BMD z score remained the same in the placebo group (−1.15 to −1.13), decreased from −0.96 to −1.00 in the alfacalcidol group, and increased from −0.99 to −0.75 in the risedronate group.
The change in z scores was significantly different between placebo and risedronate groups, and between risedronate and alfacalcidol groups, but not between placebo and alfacalcidol.
“The acquisition of adequate peak bone mass is not only important for the young person in reducing fracture risk but also has significant implications for the development of osteoporosis in later life, if peak bone mass is suboptimal,” wrote Madeleine Rooney, MBBCH, from the Queens University of Belfast, Northern Ireland, and associates.
There were no significant differences between the three groups in fracture rates. However, researchers were also able to compare Genant scores for vertebral fractures in 187 patients with pre- and posttreatment lateral spinal x-rays. That showed that the 54 patients in the placebo arm and 52 patients in the alfacalcidol arm had no change in their baseline Genant score of 0 (normal). However, although all 53 patients in the risedronate group had a Genant score of 0 at baseline, at 1-year follow-up, 2 patients had a Genant score of 1 (mild fracture), and 1 patient had a score of 3 (severe fracture).
In biochemical parameters, researchers saw a drop in parathyroid hormone in the placebo and alfacalcidol groups, but a rise in the risedronate group. However, the authors were not able to see any changes in bone markers that might have indicated which patients responded better to treatment.
Around 90% of participants in each group were also being treated with disease-modifying antirheumatic drugs. The rates of biologic use were 10.5% in the placebo group, 23.9% in the alfacalcidol group, and 10.1% in the risedronate group.
The researchers also noted a 7% higher rate of serious adverse events in the risedronate group, but emphasized that there were no differences in events related to the treatment.
In an accompanying editorial, Ian R. Reid, MBBCH, of the department of medicine, University of Auckland (New Zealand) noted that the study was an important step toward finding interventions for the prevention of steroid-induced bone loss in children. “The present study indicates that risedronate, and probably other potent bisphosphonates, can provide bone preservation in children and young people receiving therapeutic doses of glucocorticoid drugs, whereas alfacalcidol is without benefit. The targeted use of bisphosphonates in children and young people judged to be at significant fracture risk is appropriate. However, whether preventing loss of bone density will reduce fracture incidence remains to be established.”
The study was funded by Arthritis Research UK. No conflicts of interest were declared.
SOURCE: Rooney M et al. EClinicalMedicine. 2019 Jul 3. doi: 10.1016/j.eclinm.2019.06.004.
FROM ECLINICALMEDICINE
Criteria found largely interchangeable for classifying radiographic axSpA
, according to a comparative study first presented at the 2019 European League Against Rheumatism and now published.
“The major finding is that patients classified with one set of the criteria are essentially the same as those classified with the other,” according to Anne Boel, a researcher in the department of rheumatology at Leiden (the Netherlands) University Medical Center, and first author of the study.
The study addresses a controversy that has persisted since the introduction of ASAS criteria for defining axial spondyloarthritis (axSpA) with definite structural changes on conventional radiographs. It was unclear whether this ASAS diagnosis, called radiographic axSpA (r-axSpA), was the same as ankylosing spondylitis (AS) as defined by the older modified New York (mNY) criteria.
In this study, patients from eight cohorts were evaluated with the two classification sets. In addition to having radiographic sacroiliitis, all patients had to have back pain for at least 3 months, which is also mandatory for both classification sets.
Of the 3,434 fulfilling the ASAS criteria for r-axSpA, 96% fulfilled the mNY criteria for AS. Of the 3,882 meeting the mNY criteria for AS, 93% fulfilled the ASAS criteria for r-axSpA.
On the basis of this level of agreement, the authors called the terms r-axSpA and AS “interchangeable.” In the small proportion of cases when there was disagreement, the reason was considered to be minor and not to alter the conclusion that the disease entities are the same.
“Patients cannot be classified according to the ASAS criteria if they first develop back pain at age 45 years or older, so this is one difference between the two criteria sets that would affect classification,” Ms. Boel explained in an interview.
When tallied, 7% of the 4,041 patients with axSpA with radiographic sacroiliitis evaluated met only the mNY criteria, 3% met only the ASAS criteria, 89% met both sets of criteria, and 1% met neither, according to the published data.
Of those who met the mNY criteria but not the ASAS criteria, 99.7% would have potentially fulfilled the ASAS criteria for r-axSpA except for older age at onset. The remainder was attributed to an absence of inflammatory back pain or another spondyloarthritis feature.
Of the 3,434 patients fulfilling the ASAS criteria, 90% fulfilled the mNY criteria because of the presence of inflammatory back pain. Most of those without inflammatory back pain had a mobility restriction and so still met the mNY criteria. A small proportion without inflammatory back pain or mobility restriction fulfilled the ASAS criteria because of other SpA features.
The study resolves a persistent debate over whether AS patients identified by mNY criteria are the same as r-axSpA identified by ASAS criteria, according to the authors, reiterating that these data show that they can be considered the same disease.
This finding is particularly relevant when evaluating studies that have classified patients by either the mNY or the ASAS criteria.
This finding “has important implications for the axSpA research field,” the authors concluded. “Acknowledging that both criteria sets identify the same patients implies that older literature on AS and newer literature on r-axSpA can be directly compared.”
The study had no specific funding source. Ms. Boel reported having no potential conflicts of interest. Coauthors reported ties with pharmaceutical companies outside of this study.
SOURCE: Boel A et al. Ann Rheum Dis. 2019 Jul 30. doi: 10.1136/annrheumdis-2019-215707.
, according to a comparative study first presented at the 2019 European League Against Rheumatism and now published.
“The major finding is that patients classified with one set of the criteria are essentially the same as those classified with the other,” according to Anne Boel, a researcher in the department of rheumatology at Leiden (the Netherlands) University Medical Center, and first author of the study.
The study addresses a controversy that has persisted since the introduction of ASAS criteria for defining axial spondyloarthritis (axSpA) with definite structural changes on conventional radiographs. It was unclear whether this ASAS diagnosis, called radiographic axSpA (r-axSpA), was the same as ankylosing spondylitis (AS) as defined by the older modified New York (mNY) criteria.
In this study, patients from eight cohorts were evaluated with the two classification sets. In addition to having radiographic sacroiliitis, all patients had to have back pain for at least 3 months, which is also mandatory for both classification sets.
Of the 3,434 fulfilling the ASAS criteria for r-axSpA, 96% fulfilled the mNY criteria for AS. Of the 3,882 meeting the mNY criteria for AS, 93% fulfilled the ASAS criteria for r-axSpA.
On the basis of this level of agreement, the authors called the terms r-axSpA and AS “interchangeable.” In the small proportion of cases when there was disagreement, the reason was considered to be minor and not to alter the conclusion that the disease entities are the same.
“Patients cannot be classified according to the ASAS criteria if they first develop back pain at age 45 years or older, so this is one difference between the two criteria sets that would affect classification,” Ms. Boel explained in an interview.
When tallied, 7% of the 4,041 patients with axSpA with radiographic sacroiliitis evaluated met only the mNY criteria, 3% met only the ASAS criteria, 89% met both sets of criteria, and 1% met neither, according to the published data.
Of those who met the mNY criteria but not the ASAS criteria, 99.7% would have potentially fulfilled the ASAS criteria for r-axSpA except for older age at onset. The remainder was attributed to an absence of inflammatory back pain or another spondyloarthritis feature.
Of the 3,434 patients fulfilling the ASAS criteria, 90% fulfilled the mNY criteria because of the presence of inflammatory back pain. Most of those without inflammatory back pain had a mobility restriction and so still met the mNY criteria. A small proportion without inflammatory back pain or mobility restriction fulfilled the ASAS criteria because of other SpA features.
The study resolves a persistent debate over whether AS patients identified by mNY criteria are the same as r-axSpA identified by ASAS criteria, according to the authors, reiterating that these data show that they can be considered the same disease.
This finding is particularly relevant when evaluating studies that have classified patients by either the mNY or the ASAS criteria.
This finding “has important implications for the axSpA research field,” the authors concluded. “Acknowledging that both criteria sets identify the same patients implies that older literature on AS and newer literature on r-axSpA can be directly compared.”
The study had no specific funding source. Ms. Boel reported having no potential conflicts of interest. Coauthors reported ties with pharmaceutical companies outside of this study.
SOURCE: Boel A et al. Ann Rheum Dis. 2019 Jul 30. doi: 10.1136/annrheumdis-2019-215707.
, according to a comparative study first presented at the 2019 European League Against Rheumatism and now published.
“The major finding is that patients classified with one set of the criteria are essentially the same as those classified with the other,” according to Anne Boel, a researcher in the department of rheumatology at Leiden (the Netherlands) University Medical Center, and first author of the study.
The study addresses a controversy that has persisted since the introduction of ASAS criteria for defining axial spondyloarthritis (axSpA) with definite structural changes on conventional radiographs. It was unclear whether this ASAS diagnosis, called radiographic axSpA (r-axSpA), was the same as ankylosing spondylitis (AS) as defined by the older modified New York (mNY) criteria.
In this study, patients from eight cohorts were evaluated with the two classification sets. In addition to having radiographic sacroiliitis, all patients had to have back pain for at least 3 months, which is also mandatory for both classification sets.
Of the 3,434 fulfilling the ASAS criteria for r-axSpA, 96% fulfilled the mNY criteria for AS. Of the 3,882 meeting the mNY criteria for AS, 93% fulfilled the ASAS criteria for r-axSpA.
On the basis of this level of agreement, the authors called the terms r-axSpA and AS “interchangeable.” In the small proportion of cases when there was disagreement, the reason was considered to be minor and not to alter the conclusion that the disease entities are the same.
“Patients cannot be classified according to the ASAS criteria if they first develop back pain at age 45 years or older, so this is one difference between the two criteria sets that would affect classification,” Ms. Boel explained in an interview.
When tallied, 7% of the 4,041 patients with axSpA with radiographic sacroiliitis evaluated met only the mNY criteria, 3% met only the ASAS criteria, 89% met both sets of criteria, and 1% met neither, according to the published data.
Of those who met the mNY criteria but not the ASAS criteria, 99.7% would have potentially fulfilled the ASAS criteria for r-axSpA except for older age at onset. The remainder was attributed to an absence of inflammatory back pain or another spondyloarthritis feature.
Of the 3,434 patients fulfilling the ASAS criteria, 90% fulfilled the mNY criteria because of the presence of inflammatory back pain. Most of those without inflammatory back pain had a mobility restriction and so still met the mNY criteria. A small proportion without inflammatory back pain or mobility restriction fulfilled the ASAS criteria because of other SpA features.
The study resolves a persistent debate over whether AS patients identified by mNY criteria are the same as r-axSpA identified by ASAS criteria, according to the authors, reiterating that these data show that they can be considered the same disease.
This finding is particularly relevant when evaluating studies that have classified patients by either the mNY or the ASAS criteria.
This finding “has important implications for the axSpA research field,” the authors concluded. “Acknowledging that both criteria sets identify the same patients implies that older literature on AS and newer literature on r-axSpA can be directly compared.”
The study had no specific funding source. Ms. Boel reported having no potential conflicts of interest. Coauthors reported ties with pharmaceutical companies outside of this study.
SOURCE: Boel A et al. Ann Rheum Dis. 2019 Jul 30. doi: 10.1136/annrheumdis-2019-215707.
FROM ANNALS OF THE RHEUMATIC DISEASES
White and black patients have similar rates of giant cell arteritis
To determine the incidence of biopsy-proven GCA (BP-GCA) in a racially diverse cohort, Anna M. Gruener of Nottingham (England) University Hospitals NHS Trust and coauthors analyzed the medical records of more than 10 years of patients who underwent temporal artery biopsy at Johns Hopkins Wilmer Eye Institute in Baltimore. Of the 586 patients in the study, 167 (28.5%) were black, 382 (65.2%) were white, and 37 (6.3%) were other or unknown. The mean age was 70.5 years.
Of the 573 patients who were aged 50 years and older, 92 (16.1%) had a positive biopsy for BP-GCA; 14 were black (8.4% of all black patients), 75 were white (19.6% of all white patients), and 3 were other or unknown. The population-adjusted, age- and sex-standardized incidence rates per 100,000 were 3.1 (95% confidence interval, 1.0-5.2) for black patients and 3.6 (95% CI, 2.5-4.7) for white patients.
Overall, BP-GCA occurred more frequently in women than in men (incidence rate ratio, 1.9; 95% CI, 1.1-3.4; P = .03) but at similar levels in white and black patients (IRR, 1.2; 95% CI, 0.6-2.4; P = .66).
In an accompanying editorial, Michael K. Yoon, MD, and Joseph F. Rizzo III, MD, of Harvard Medical School, Boston, praised the researchers for conducting their study in a population with a large percentage of black patients, a noted weakness of earlier studies in this area (JAMA Ophthalmol. 2019 Aug 8. doi: 10.1001/jamaophthalmol.2019.2933). That said, the two doctors also recognized the limitations of the work done by Gruener et al., including relying on U.S. Census data to calculate adjusted incidence rates instead of local racial distribution and also the potentially problematic choice to count patients with healed arteritis as having BP-GCA.
Still, Dr. Yoon and Dr. Rizzo commended Gruener et al. for questioning previous findings on GCA rates. “Although the authors’ methods are imperfect,” they wrote, “the studies that had previously established a low incidence of GCA in black patients were also flawed in design.”
The study had no outside funding source, and no conflicts of interest were reported.
SOURCE: Gruener AM et al. JAMA Ophthalmol. 2019 Aug 8. doi: 10.1001/jamaophthalmol.2019.2919.
To determine the incidence of biopsy-proven GCA (BP-GCA) in a racially diverse cohort, Anna M. Gruener of Nottingham (England) University Hospitals NHS Trust and coauthors analyzed the medical records of more than 10 years of patients who underwent temporal artery biopsy at Johns Hopkins Wilmer Eye Institute in Baltimore. Of the 586 patients in the study, 167 (28.5%) were black, 382 (65.2%) were white, and 37 (6.3%) were other or unknown. The mean age was 70.5 years.
Of the 573 patients who were aged 50 years and older, 92 (16.1%) had a positive biopsy for BP-GCA; 14 were black (8.4% of all black patients), 75 were white (19.6% of all white patients), and 3 were other or unknown. The population-adjusted, age- and sex-standardized incidence rates per 100,000 were 3.1 (95% confidence interval, 1.0-5.2) for black patients and 3.6 (95% CI, 2.5-4.7) for white patients.
Overall, BP-GCA occurred more frequently in women than in men (incidence rate ratio, 1.9; 95% CI, 1.1-3.4; P = .03) but at similar levels in white and black patients (IRR, 1.2; 95% CI, 0.6-2.4; P = .66).
In an accompanying editorial, Michael K. Yoon, MD, and Joseph F. Rizzo III, MD, of Harvard Medical School, Boston, praised the researchers for conducting their study in a population with a large percentage of black patients, a noted weakness of earlier studies in this area (JAMA Ophthalmol. 2019 Aug 8. doi: 10.1001/jamaophthalmol.2019.2933). That said, the two doctors also recognized the limitations of the work done by Gruener et al., including relying on U.S. Census data to calculate adjusted incidence rates instead of local racial distribution and also the potentially problematic choice to count patients with healed arteritis as having BP-GCA.
Still, Dr. Yoon and Dr. Rizzo commended Gruener et al. for questioning previous findings on GCA rates. “Although the authors’ methods are imperfect,” they wrote, “the studies that had previously established a low incidence of GCA in black patients were also flawed in design.”
The study had no outside funding source, and no conflicts of interest were reported.
SOURCE: Gruener AM et al. JAMA Ophthalmol. 2019 Aug 8. doi: 10.1001/jamaophthalmol.2019.2919.
To determine the incidence of biopsy-proven GCA (BP-GCA) in a racially diverse cohort, Anna M. Gruener of Nottingham (England) University Hospitals NHS Trust and coauthors analyzed the medical records of more than 10 years of patients who underwent temporal artery biopsy at Johns Hopkins Wilmer Eye Institute in Baltimore. Of the 586 patients in the study, 167 (28.5%) were black, 382 (65.2%) were white, and 37 (6.3%) were other or unknown. The mean age was 70.5 years.
Of the 573 patients who were aged 50 years and older, 92 (16.1%) had a positive biopsy for BP-GCA; 14 were black (8.4% of all black patients), 75 were white (19.6% of all white patients), and 3 were other or unknown. The population-adjusted, age- and sex-standardized incidence rates per 100,000 were 3.1 (95% confidence interval, 1.0-5.2) for black patients and 3.6 (95% CI, 2.5-4.7) for white patients.
Overall, BP-GCA occurred more frequently in women than in men (incidence rate ratio, 1.9; 95% CI, 1.1-3.4; P = .03) but at similar levels in white and black patients (IRR, 1.2; 95% CI, 0.6-2.4; P = .66).
In an accompanying editorial, Michael K. Yoon, MD, and Joseph F. Rizzo III, MD, of Harvard Medical School, Boston, praised the researchers for conducting their study in a population with a large percentage of black patients, a noted weakness of earlier studies in this area (JAMA Ophthalmol. 2019 Aug 8. doi: 10.1001/jamaophthalmol.2019.2933). That said, the two doctors also recognized the limitations of the work done by Gruener et al., including relying on U.S. Census data to calculate adjusted incidence rates instead of local racial distribution and also the potentially problematic choice to count patients with healed arteritis as having BP-GCA.
Still, Dr. Yoon and Dr. Rizzo commended Gruener et al. for questioning previous findings on GCA rates. “Although the authors’ methods are imperfect,” they wrote, “the studies that had previously established a low incidence of GCA in black patients were also flawed in design.”
The study had no outside funding source, and no conflicts of interest were reported.
SOURCE: Gruener AM et al. JAMA Ophthalmol. 2019 Aug 8. doi: 10.1001/jamaophthalmol.2019.2919.
FROM JAMA OPHTHALMOLOGY
NSAIDs a significant mediator of cardiovascular risk in osteoarthritis
Writing in Arthritis & Rheumatology, researchers reported the outcomes of a longitudinal, population-based cohort study of 7,743 individuals with osteoarthritis patients and 23,229 age- and sex-matched controls without osteoarthritis.
“The prevailing hypothesis in the OA to CVD relationship has been that OA patients frequently take NSAIDs to control their pain and inflammation and that this may lead to them developing CVD,” wrote Mohammad Atiquzzaman, a PhD student at the University of British Columbia, Vancouver, and his coauthors. However they commented that no studies had so far examined this directly in patients with osteoarthritis.
Overall, people with osteoarthritis had a significant 23% higher risk of cardiovascular disease, compared with controls, after adjustment for factors such body mass index, hypertension, diabetes, hyperlipidemia, and socioeconomic status. They also had a 42% higher risk of congestive heart failure, 17% higher risk of ischemic heart disease, and 14% higher risk of stroke.
NSAID use was five times more common among people with osteoarthritis, and NSAIDs alone were associated with a greater than fourfold higher risk of cardiovascular disease, after adjusting for osteoarthritis and other potential confounders.
When the authors performed modeling to break down the effect of osteoarthritis on CVD risk into the direct effect of osteoarthritis itself and the indirect effect mediated by NSAID use, they concluded that 41% of the total effect of osteoarthritis on cardiovascular risk was mediated by NSAIDs. The effect of NSAIDs was particularly pronounced for stroke, in which cases they estimated that the drugs contributed to 64% of the increased in risk, and in ischemic heart disease, in which they contributed to 56% of the increased risk.
Subgroup analysis suggested that conventional NSAIDs were responsible for around 29% of the total increased risk of cardiovascular disease, while selective COX-2 inhibitors, or coxibs, such as celecoxib, lumiracoxib, rofecoxib, and valdecoxib mediated around 21%. For ischemic heart disease, conventional NSAIDs explained around 45% of the increased risk, while selective coxibs explained around 32% of the risk. Similarly, with congestive heart failure and stroke, the proportion of risk mediated by NSAIDs was higher for conventional NSAIDs, compared with coxibs.
The authors noted that while a number of previous studies have found osteoarthritis is an independent risk factor for cardiovascular disease, theirs was the first study to specifically examine the role that NSAIDs play in that increased risk.
However, they noted that their information on NSAID use was gleaned from prescription claims data, which did not include information on over-the-counter NSAID use. Their analysis was also unable to include information on family history of cardiovascular disease, smoking, and physical activity, which are important cardiovascular disease risk factors. They did observe that the rates of obesity were higher among the osteoarthritis group when compared with controls (29% vs. 20%), and hypertension and COPD were also more common among individuals with osteoarthritis.
There was no outside funding for the study, and the authors had no conflicts of interest to declare.
SOURCE: Atiquzzaman M et al. Arthritis Rheumatol. 2019 Aug 6. doi: 10.1002/art.41027
Writing in Arthritis & Rheumatology, researchers reported the outcomes of a longitudinal, population-based cohort study of 7,743 individuals with osteoarthritis patients and 23,229 age- and sex-matched controls without osteoarthritis.
“The prevailing hypothesis in the OA to CVD relationship has been that OA patients frequently take NSAIDs to control their pain and inflammation and that this may lead to them developing CVD,” wrote Mohammad Atiquzzaman, a PhD student at the University of British Columbia, Vancouver, and his coauthors. However they commented that no studies had so far examined this directly in patients with osteoarthritis.
Overall, people with osteoarthritis had a significant 23% higher risk of cardiovascular disease, compared with controls, after adjustment for factors such body mass index, hypertension, diabetes, hyperlipidemia, and socioeconomic status. They also had a 42% higher risk of congestive heart failure, 17% higher risk of ischemic heart disease, and 14% higher risk of stroke.
NSAID use was five times more common among people with osteoarthritis, and NSAIDs alone were associated with a greater than fourfold higher risk of cardiovascular disease, after adjusting for osteoarthritis and other potential confounders.
When the authors performed modeling to break down the effect of osteoarthritis on CVD risk into the direct effect of osteoarthritis itself and the indirect effect mediated by NSAID use, they concluded that 41% of the total effect of osteoarthritis on cardiovascular risk was mediated by NSAIDs. The effect of NSAIDs was particularly pronounced for stroke, in which cases they estimated that the drugs contributed to 64% of the increased in risk, and in ischemic heart disease, in which they contributed to 56% of the increased risk.
Subgroup analysis suggested that conventional NSAIDs were responsible for around 29% of the total increased risk of cardiovascular disease, while selective COX-2 inhibitors, or coxibs, such as celecoxib, lumiracoxib, rofecoxib, and valdecoxib mediated around 21%. For ischemic heart disease, conventional NSAIDs explained around 45% of the increased risk, while selective coxibs explained around 32% of the risk. Similarly, with congestive heart failure and stroke, the proportion of risk mediated by NSAIDs was higher for conventional NSAIDs, compared with coxibs.
The authors noted that while a number of previous studies have found osteoarthritis is an independent risk factor for cardiovascular disease, theirs was the first study to specifically examine the role that NSAIDs play in that increased risk.
However, they noted that their information on NSAID use was gleaned from prescription claims data, which did not include information on over-the-counter NSAID use. Their analysis was also unable to include information on family history of cardiovascular disease, smoking, and physical activity, which are important cardiovascular disease risk factors. They did observe that the rates of obesity were higher among the osteoarthritis group when compared with controls (29% vs. 20%), and hypertension and COPD were also more common among individuals with osteoarthritis.
There was no outside funding for the study, and the authors had no conflicts of interest to declare.
SOURCE: Atiquzzaman M et al. Arthritis Rheumatol. 2019 Aug 6. doi: 10.1002/art.41027
Writing in Arthritis & Rheumatology, researchers reported the outcomes of a longitudinal, population-based cohort study of 7,743 individuals with osteoarthritis patients and 23,229 age- and sex-matched controls without osteoarthritis.
“The prevailing hypothesis in the OA to CVD relationship has been that OA patients frequently take NSAIDs to control their pain and inflammation and that this may lead to them developing CVD,” wrote Mohammad Atiquzzaman, a PhD student at the University of British Columbia, Vancouver, and his coauthors. However they commented that no studies had so far examined this directly in patients with osteoarthritis.
Overall, people with osteoarthritis had a significant 23% higher risk of cardiovascular disease, compared with controls, after adjustment for factors such body mass index, hypertension, diabetes, hyperlipidemia, and socioeconomic status. They also had a 42% higher risk of congestive heart failure, 17% higher risk of ischemic heart disease, and 14% higher risk of stroke.
NSAID use was five times more common among people with osteoarthritis, and NSAIDs alone were associated with a greater than fourfold higher risk of cardiovascular disease, after adjusting for osteoarthritis and other potential confounders.
When the authors performed modeling to break down the effect of osteoarthritis on CVD risk into the direct effect of osteoarthritis itself and the indirect effect mediated by NSAID use, they concluded that 41% of the total effect of osteoarthritis on cardiovascular risk was mediated by NSAIDs. The effect of NSAIDs was particularly pronounced for stroke, in which cases they estimated that the drugs contributed to 64% of the increased in risk, and in ischemic heart disease, in which they contributed to 56% of the increased risk.
Subgroup analysis suggested that conventional NSAIDs were responsible for around 29% of the total increased risk of cardiovascular disease, while selective COX-2 inhibitors, or coxibs, such as celecoxib, lumiracoxib, rofecoxib, and valdecoxib mediated around 21%. For ischemic heart disease, conventional NSAIDs explained around 45% of the increased risk, while selective coxibs explained around 32% of the risk. Similarly, with congestive heart failure and stroke, the proportion of risk mediated by NSAIDs was higher for conventional NSAIDs, compared with coxibs.
The authors noted that while a number of previous studies have found osteoarthritis is an independent risk factor for cardiovascular disease, theirs was the first study to specifically examine the role that NSAIDs play in that increased risk.
However, they noted that their information on NSAID use was gleaned from prescription claims data, which did not include information on over-the-counter NSAID use. Their analysis was also unable to include information on family history of cardiovascular disease, smoking, and physical activity, which are important cardiovascular disease risk factors. They did observe that the rates of obesity were higher among the osteoarthritis group when compared with controls (29% vs. 20%), and hypertension and COPD were also more common among individuals with osteoarthritis.
There was no outside funding for the study, and the authors had no conflicts of interest to declare.
SOURCE: Atiquzzaman M et al. Arthritis Rheumatol. 2019 Aug 6. doi: 10.1002/art.41027
FROM ARTHRITIS & RHEUMATOLOGY
Click for Credit: Predicting preeclampsia; MI & stroke post-cancer Dx; more
Here are 5 articles from the August issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Smoking cessation could delay or prevent rheumatoid arthritis
To take the posttest, go to: https://bit.ly/2YguN2r
Expires February 22, 2020
2. No increased pregnancy loss risk for women conceiving soon after stillbirth
To take the posttest, go to: https://bit.ly/2ZnMaLc
Expires March 4, 2020
3. Total plasma tau correlates with dementia onset, Alzheimer’s disease
To take the posttest, go to: https://bit.ly/2YeglYV
Expires March 9, 2020
4. MI, strokes spike during 30 days after cancer diagnosis
To take the posttest, go to: https://bit.ly/2GCKZAv
Expires March 12, 2020
5. Combination model predicts imminent preeclampsia
To take the posttest, go to: https://bit.ly/2LTohrO
Expires February 21, 2020
Here are 5 articles from the August issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Smoking cessation could delay or prevent rheumatoid arthritis
To take the posttest, go to: https://bit.ly/2YguN2r
Expires February 22, 2020
2. No increased pregnancy loss risk for women conceiving soon after stillbirth
To take the posttest, go to: https://bit.ly/2ZnMaLc
Expires March 4, 2020
3. Total plasma tau correlates with dementia onset, Alzheimer’s disease
To take the posttest, go to: https://bit.ly/2YeglYV
Expires March 9, 2020
4. MI, strokes spike during 30 days after cancer diagnosis
To take the posttest, go to: https://bit.ly/2GCKZAv
Expires March 12, 2020
5. Combination model predicts imminent preeclampsia
To take the posttest, go to: https://bit.ly/2LTohrO
Expires February 21, 2020
Here are 5 articles from the August issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Smoking cessation could delay or prevent rheumatoid arthritis
To take the posttest, go to: https://bit.ly/2YguN2r
Expires February 22, 2020
2. No increased pregnancy loss risk for women conceiving soon after stillbirth
To take the posttest, go to: https://bit.ly/2ZnMaLc
Expires March 4, 2020
3. Total plasma tau correlates with dementia onset, Alzheimer’s disease
To take the posttest, go to: https://bit.ly/2YeglYV
Expires March 9, 2020
4. MI, strokes spike during 30 days after cancer diagnosis
To take the posttest, go to: https://bit.ly/2GCKZAv
Expires March 12, 2020
5. Combination model predicts imminent preeclampsia
To take the posttest, go to: https://bit.ly/2LTohrO
Expires February 21, 2020
Rheumatoid Arthritis: Therapeutic Strategies After Inadequate Response to Initial TNF Inhibitor Therapy
From the University of Iowa Hospitals and Clinics, Iowa City, IA.
Abstract
- Objective: To discuss the variability in response to tumor necrosis factor inhibitors (TNFis) observed in patients with rheumatoid arthritis (RA) and discuss therapeutic options for patients who do not respond to initial TNFi therapy.
- Methods: Review of the literature.
- Results: Optimal treatment of RA aims at achieving and then maintaining remission or low disease activity. In a patient with an inadequate response to initial biologic therapy, several therapeutic options exist. Current evidence supports TNFi dose escalation for only infliximab; optimization of concurrent conventional synthetic disease-modifying antirheumatic drug (csDMARD) or switching to a different csDMARD are other options. Cycling (switching to an alternative TNFi) and swapping (switching to a therapy with a different mode of action) strategies are other alternate approaches supported by many observational studies. While no head-to-head trials exist directly comparing the 2 strategies, data suggest superiority of the swapping strategy over the cycling approach. Also, several studies have shown that switching to a drug with a different mechanism of action is associated with higher treatment persistence and lower health care costs than TNFi cycling.
- Conclusion: Physicians have a growing list of treatment options to help their patients with RA achieve disease remission. The choice of best treatment for a given patient needs to be individualized, keeping in mind other factors, including comorbidities.
Keywords: biologics; rheumatoid arthritis; swapping strategy; cycling strategy; TNF inhibitors.
Following the discovery of tumor necrosis factor (TNF) as a proinflammatory cytokine 30 years ago, the use of TNF antagonists has revolutionized the treatment of rheumatoid arthritis (RA). Although TNF inhibitors (TNFIs) are frequently used as a first-line biologic disease-modifying antirheumatic drug (bDMARD), they are not uniformly efficacious in achieving remission in all patients with RA. This article highlights the reasons for such variability in observed response and discusses therapeutic options for patients who do not respond to TNFi therapy.
Case Presentation
A 60-year-old woman is evaluated in the clinic for complaints of pain in her hands, morning stiffness lasting 2 hours, and swelling in her wrists, all of which have been ongoing for 3 months. Physical exam reveals evidence of active inflammation, with synovitis in her second, third, and fourth metacarpophalangeal (MCP) and proximal interphalangeal (PIP) joints bilaterally, swelling over both wrists, and a weak grip. Inflammatory markers are elevated, and rheumatoid factor and anti-cyclic citrullinated peptide (anti-CCP) are both positive at high titer. Radiographs reveal evidence of small erosions at the third and fourth MCPs and PIPs bilaterally and periarticular osteopenia. The patient is diagnosed with seropositive, erosive RA based on history, physical exam, laboratory studies, and imaging. She is started on 20 mg of prednisone for acute treatment of her symptoms along with methotrexate, and, initially, her symptoms are well controlled. A few months after starting treatment, she develops voluminous diarrhea that necessitates cessation of methotrexate. Leflunomide also causes similar symptoms. The combination of sulfasalazine and hydroxychloroquine does not adequately control her symptoms, and ongoing use of low-dose glucocorticoids is required to improve functionality in all joints. Using the treat-to-target (T2T) strategy, adalimumab is initiated. However, she continues to report persistent swelling and pain and still requests oral glucocorticoids to help decrease inflammation. The 28-joint Disease Activity Score (DAS28) is 4.8, suggestive of moderate disease activity.
Why are TNFi agents sometimes ineffective?
The introduction of monoclonal antibodies and fusion proteins to block TNF and other cytokines was a remarkable development in the treatment of RA that revolutionized patient care. Despite the efficacy of TNFis, clinical response to these agents is not universal and only some patients achieve complete remission. In targeting the eventual goal of remission or low disease activity in patients with RA, the concept of “TNF failure” becomes extremely relevant. These inadequate responses to anti-TNF therapy may be due to primary failures, or complete lack of clinical response after initiation of the bDMARD, and secondary failures, or the loss of initially achieved clinical response to therapy. Other reasons for discontinuation of a given TNFi include partial disease control and intolerance to the medication (possible injection-site or infusion reactions). Keystone and Kavanaugh1 divided causes of failure of TNF agents into 2 broad categories: perceptual (related to natural variations in disease course like hormonal variation and physical and emotional stress) and pathophysiological failures (genetic variations, high body mass index, concomitant cigarette use).
Another important consideration in patients treated with a TNFi is the consequent formation of anti-drug antibodies (ADAs). TNFi agents are immunogenic and normally elicit an immune response. The appearance of such ADAs may reduce the bioavailability of free drug, resulting in a decreased clinical response,2 or may lead to serious adverse effects.
How common is discontinuation of the first TNFi?
Several studies have reported that the prevalence of primary failure, secondary failure, and intolerance to TNFis ranges from 30% to 40%.3-6 Female sex,7 concurrent prednisone use,8 high disease activity scores,6,8,9 and the absence of treatment with low-dose methotrexate7,8 have all been shown to be negative predictors of bDMARD retention and response.10
Are there any factors that predict TNFi failure?
There are no specific parameters to accurately predict responses to TNFI therapy.11 Several clinical and molecular biomarkers in synovium (initial TNF levels, macrophages, T cells)12 and peripheral blood (serum myeloid-related protein 8 and 14 complex levels,13 prealbumin, platelet factor 4, and S100A12)14 have been described as predictors of clinical response to TNFis, but their utility in clinical practice has not been established and the use of these markers has not yet been incorporated into clinical guidelines.
How is disease activity measured in patients with RA?
In 2010 an international expert consensus panel published treatment recommendations for RA that emphasized a T2T strategy of individualizing and escalating treatment to achieve the lowest disease activity or remission. In clinical practice, numerous tools are available to measure RA disease activity. Herein, we mention several that are most commonly used in clinical practice.
DAS28 combines single activity measures into an overall continuous measure of disease activity and has been endorsed by both the American College of Rheumatology (ACR) and European League Against Rheumatism (EULAR). It includes a 28-swollen joint count (SJC), 28-tender joint count (TJC), erythrocyte sedimentation rate (ESR; can also be calculated using C-reactive protein [CRP]), and a patient global assessment (PtGA). The cut-offs used for DAS28 interpretation are as follows: remission (< 2.6), low (≥ 2.6 but ≤ 3.2), moderate (> 3.2 but ≤ 5.1), or high (> 5.1).15 Some of the difficulties in using DAS28 in daily clinical practice include the need for a lab value and the time needed to perform the joint counts. Note also that due to the inclusion of ESR, which is influenced by age and other factors, DAS28 may underestimate remission in the elderly.
Another measure of RA disease activity is the Simplified Disease Activity Index (SDAI), which includes 28 SJC, 28 TJC, PtGA, provider global assessment (PrGA), and CRP in mg/dL. The level of disease activity using the SDAI is interpreted as: remission (SDAI ≤ 3.3), low (≥ 3.4 but ≤ 11), moderate (> 11 but ≤ 26), or high (> 26). The advantage of the SDAI is that a calculator or computer is not required for calculations. Another measure, the Clinical Disease Activity Index (CDAI), includes a 28 SJC, 28 TJC, PtGA, and PrGA. Because a laboratory value is not needed to calculate the CDAI, it is well-suited for use in clinical practice. When using the CDAI, the level of disease activity can be defined as remission (CDAI ≤ 2.8), low (> 2.8 but ≤ 10), moderate (> 10 but ≤ 22), or high (> 22). Again, as with the SDAI, a calculator or computer is not needed for calculations.
What are the alternative treatment options after first biologic failure?
In patients who have failed treatment with an initial biologic, usually a TNFi, the treating rheumatologist has the following options (Figure), with the best treatment strategy being driven by individualized patient and disease-related factors (Table 1 and Table 2):
- TNFi dose escalation
- Trial of an alternate TNFi agent (the “cycling” strategy)
- Optimization of therapy conjoined with a conventional synthetic DMARD (csDMARD)
- Use of a non-TNF biologic or targeted synthetic DMARD (the “swapping” strategy)
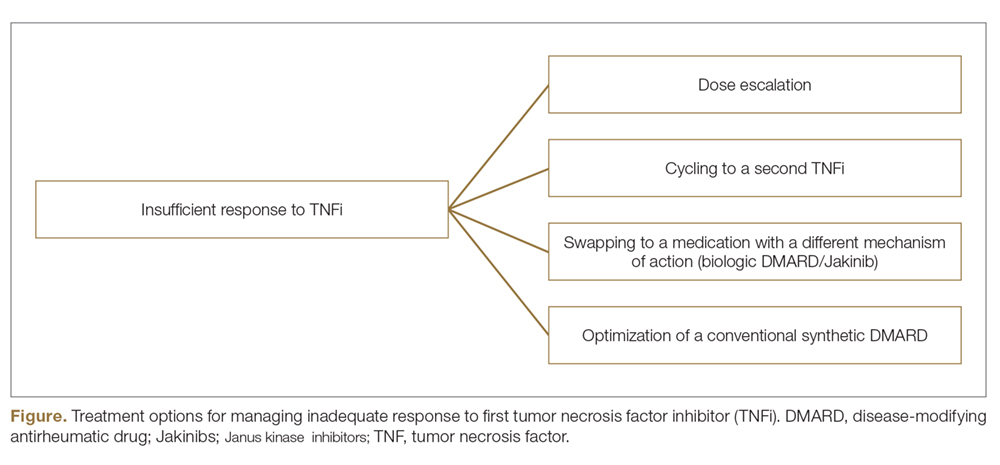
If all the listed strategies fail, the next step can be the addition of short-term, low-dose glucocorticoid therapy.
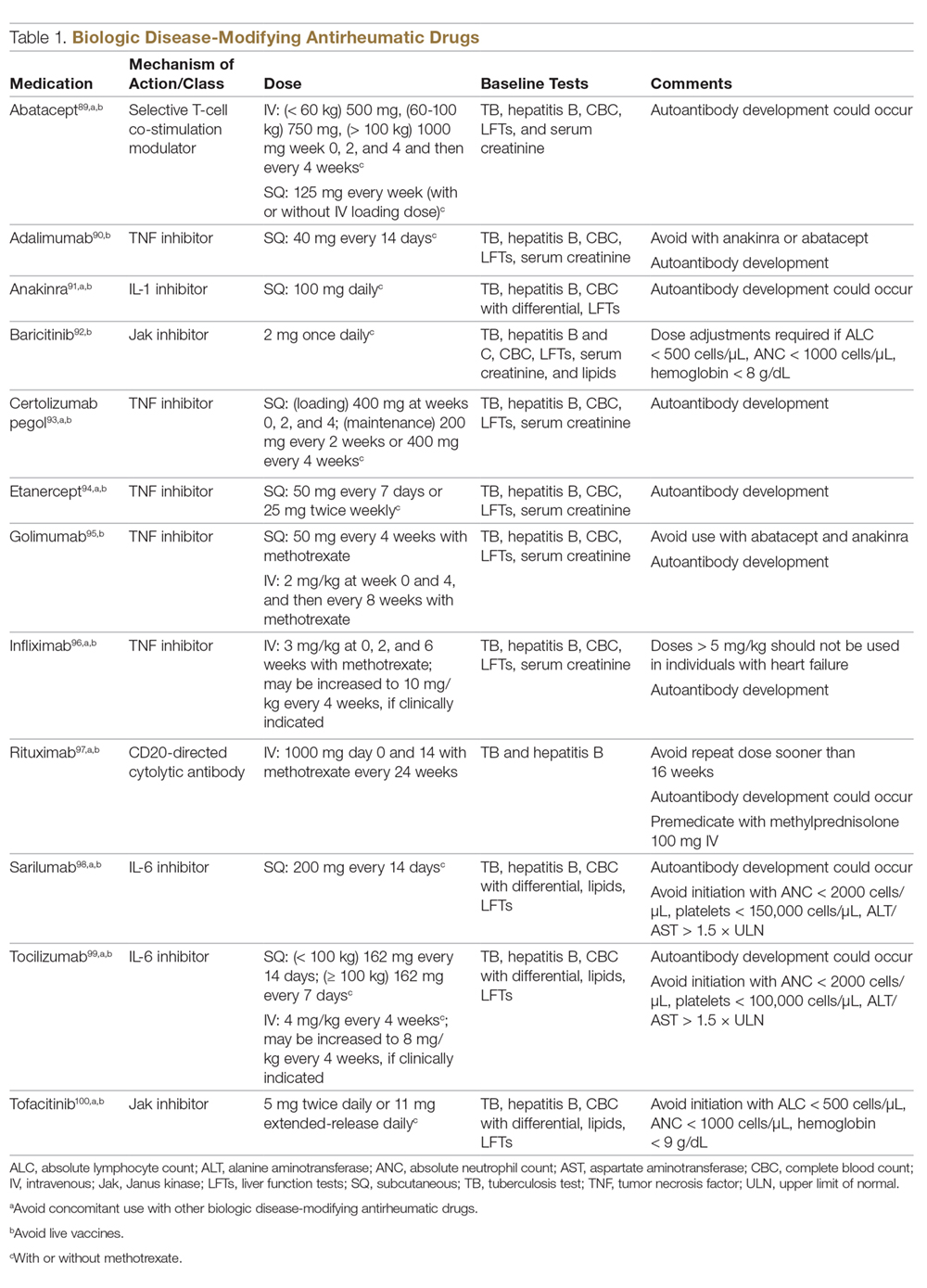
TNFi Dose Escalation
The available data have demonstrated the safety, efficacy, and cost-effectiveness of dose escalation in patients with RA receiving infliximab.16-18 The ATTRACT trial first demonstrated this, with greater clinical and radiographic improvements in those with higher trough serum concentrations, suggesting that doses higher than 3 mg/kg or more frequent than every 8 weeks may be needed for full response in some patients.19
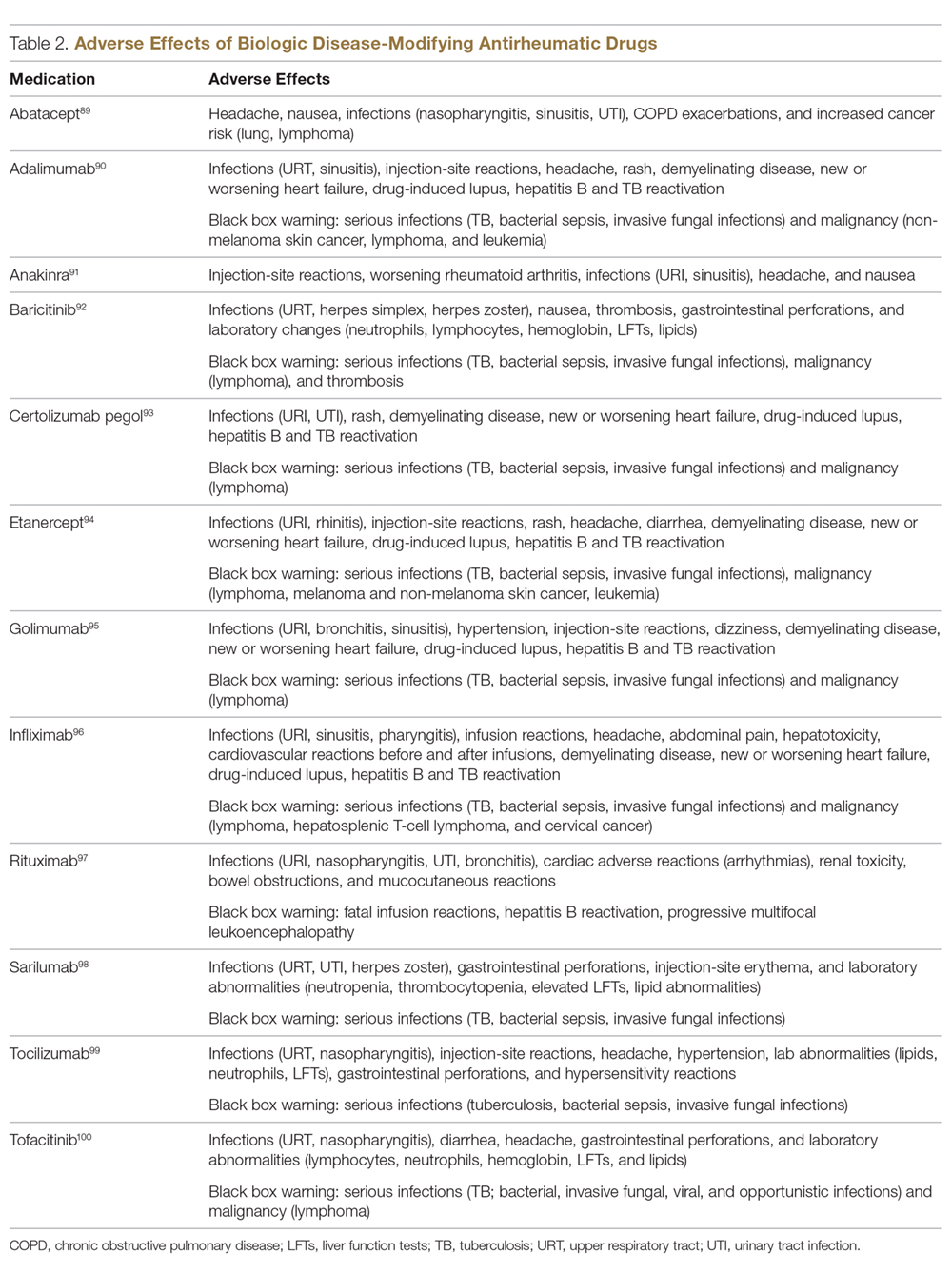
There is a lack of studies in RA patients to determine the most effective dose escalation strategy. A study in patients with Crohn disease showed that intensification to 10 mg/kg every 8 weeks (dose doubling) was at least as effective as 5 mg/kg every 4 weeks (halving interval) at 12 months.16 Due to greater patient and administration convenience of dose-doubling, this strategy may be preferred.17 A starting dose of 10 mg/kg every 8 weeks is not routinely recommended due to an increased risk of serious infection; these adverse events were not found when the dose was gradually increased, as clinically indicated, starting at 3 mg/kg.19,20 Further studies are needed to explore this approach in RA patients.
These results, however, have not been replicated with other TNFi agents. No significant clinical improvements were identified with etanercept 50 mg twice weekly,21 adalimumab 40 mg every week in the PREMIER trial,18 or certolizumab 400 mg every other week in an open-label extension phase of the RAPID 1 study.22 A Japanese study found significantly worse clinical outcomes with dose escalation of golimumab.23 Conversely, 2 studies found clinical benefits after escalating the tocilizumab dose, the first a real-world review from the Consortium of Rheumatology Researchers of North America (CORRONA) registry using the intravenous formulation,24 and the other the BREVACTA study utilizing subcutaneous tocilizumab.25 No studies to date have been published on dose escalation of abatacept in patients with RA who respond poorly. Overall, previous studies support dose escalation in individuals being treated with infliximab to improve clinical outcomes, but additional studies are needed for other bDMARDs.
Trial of an Alternate TNF Agent: The “Cycling” Strategy
Per the ACR/EULAR26,27 guidelines, all approved bDMARDs may be used without hierarchical positioning. However, after the failure of a TNFi agent, these guidelines do not provide specific advice about a preference between the “cycling” strategy (switching to an alternative TNFi) and “swapping” strategy (switching to a therapy with a different mode of action). Cycling might work for several reasons, including differences in the agents’ molecular structure, immunological mechanism of action, immunogenicity, and pharmacokinetics.28-30 The cycling strategy is a well-established approach adopted by more than 94% of practicing rheumatologists, according to a national survey,31 and its efficacy is supported by trials and additional observational studies.32-35
The greater clinical effectiveness of switching to infliximab compared with continuing with etanercept in patients with inadequate response to etanercept (n = 28) was suggested in the open-label OPPOSITE trial.36 Data from the GO-AFTER trial37 suggests that a greater proportion of patients with RA refractory to adalimumab, etanercept, or infliximab who were treated with golimumab achieved an ACR20 and ACR50 response compared with patients who received placebo, and this response persisted through 5 years.38 More recently, certolizumab pegol and adalimumab were compared head-to-head in the EXXELERATE trial.39 The results of this trial revealed the adequate efficacy of cycling to another TNFi after primary insufficient response to the first.
In studies from Finland and Sweden,35,40 it has been observed that a better response is achieved in patients in whom TNF failure was initially due to secondary failure or intolerance rather than primary failure. A post-hoc analysis of the results of the GO-AFTER trial41 and from a few observational studies35,40,42 revealed that switching from one TNFi to another, especially from a monoclonal antibody to a soluble receptor, was often more beneficial for RA patients than switching from a soluble receptor to a monoclonal antibody.
Optimization of Therapy Conjoined with csDMARDs
Methotrexate is one of the oldest and most effective csDMARDs available for the treatment of RA.43 The 2016 EULAR guidelines recommend the addition of methotrexate and/or other csDMARDs to potentiate the effect of bDMARDs.26 In the case of TNFi therapy, the observed synergistic effect between the monoclonal antibody and methotrexate may be explained by sustained suppression of ADA formation.44 In the TEMPO,45 PREMIER,18 and GO-BEFORE46 trials, the addition of methotrexate led to improved clinical and radiological outcomes in patients treated with etanercept, adalimumab, and golimumab,47 respectively. These findings were also demonstrated in several registries, where significant improvement in clinical response and retention rate of the TNFi agents was noted. Results have been replicated with non-TNFi bDMARDs, including abatacept48,49 and rituximab.50 Patients treated with interleukin (IL)-6 inhibitors in combination with methotrexate have shown significantly less radiographic progression compared to those treated with tocilizumab alone and those treated with monotherapy tocilizumab versus monotherapy methotrexate.51,52 Results possibly favor the use of IL-6 inhibitors alone in those who cannot tolerate or have contraindications to methotrexate.
An open prospective study by Cohen et al added methotrexate to the treatment regimens of individuals on bDMARD monotherapy with a primary failure and found favorable changes in ACR20 and DAS28 scores at 3 and 12 months and therapeutic biological response (ESR, CRP) at 3 months.53 Unlike monotherapy, in these situations methotrexate is known to be efficacious even at a lower dose, possibly at 7.5 mg to 10 mg per week. Some studies have shown that methotrexate administered parenterally may be more efficacious than when given orally.54-58
In clinical trials and observational studies, leflunomide, sulfasalazine, and hydroxychloroquine have been used as alternate csDMARDs added to the treatment regimen.59-62 There are, however, only 2 trials comparing the efficacy of methotrexate with that of other csDMARDs as concomitant treatment in patients with inadequate response to TNFi therapy. The RABBIT trial found a slight decrease in effectiveness with concomitant TNFi and leflunomide compared to TNFi/methotrexate, but overall each group had similar EULAR responses at 24 months.63 A study by De Stefano et al found comparable ACR20 and DAS28 responses among individuals receiving TNFis with methotrexate or leflunomide.61
The “Swapping” Strategy
The efficacy of the swapping strategy has been shown in 3 randomized clinical trials demonstrating the superiority of abatacept, tocilizumab, and rituximab in the treatment of individuals with RA refractory to TNFis. Tocilizumab was studied in the RADIATE64 trial, which involved 499 patients with inadequate response to 1 or more TNFi agents. The primary endpoint (24-week ACR20) was achieved by 50.0%, 30.4%, and 10.1% of patients in the 8 mg/kg, 4 mg/kg, and control groups, respectively (P < 0.001 for both tocilizumab groups versus placebo). The utility of abatacept as second-line therapy after initial TNF failure was evaluated in the ATTAIN65 study. Participants with an inadequate response to etanercept or infliximab were randomly assigned to receive either abatacept or placebo. ACR50 response rates after 6 months of treatment were 20.3% with abatacept and 3.8% with placebo (P < 0.001). The SWITCH-RA study,66 an observational study, compared rituximab to TNFis in 1112 participants with inadequate response to initial anti-TNF therapy. At 6 months, mean change in DAS28 was small but significantly greater for the rituximab group (–1.5 vs –1.1; P = 0.007). The difference in response rates was greatest among seropositive patients. These data suggest that rituximab has efficacy following TNFi failure, particularly for seropositive patients. Additionally, REFLEX67 is the sole randomized controlled trial in patients with insufficient response to TNFis that showed significant prevention of radiographic progression at week 56 in patients on rituximab compared to placebo (mean change from baseline in total Genant-modified Sharp score, 1.00 vs 2.31, respectively; P = 0.005).
One study randomly assigned 399 patients with active RA who had inadequate response to prior TNFi therapy to tofacitinib68 (5 mg twice daily or 10 mg twice daily) or placebo, both with methotrexate.6 After 3 months of treatment, ACR20 response rates (41.7% for 5 mg, 28.1% for 10 mg, 24.4% for placebo) and DAS28 remission rates (6.7% for 5 mg, 8.8% for 10 mg, 1.7% for placebo) were significantly greater among patients treated with tofacitinib compared to those treated with placebo. More recently, the RA-BEACON trial69 demonstrated a consistent, beneficial treatment effect of baricitinib in patients with insufficient response to 1 or more TNFis. In this trial, 527 patients with an inadequate response to bDMARDs were randomly assigned to receive baricitinib 2 mg or 4 mg daily or placebo for 24 weeks. A higher proportion of patients receiving baricitinib 4 mg had an ACR20 response at week 12 compared with those treated with placebo (55% vs 27%, P < 0.001), and patients receiving the 4-mg dose had significant improvements from baseline in DAS28 and Health Assessment Questionnaire–Disability Index scores (P < 0.001 for both comparisons).
To Cycle or to Swap?
Several observational studies (SCQM-RA,70 STURE,71 BSRBR,72 Favalli,43 MIRAR,73 SWITCH-RA,74 ROC72) have clearly demonstrated that the swapping strategy is favored over the cycling strategy. In the ROC study,72 patients were randomly assigned (based on physician discretion) to receive a non-TNF biologic or a TNFi. More patients in the non-TNF group than in the TNFi group showed low disease activity at week 24 (45% vs 28%; odds ratio [OR], 2.09; 95% confidence interval [CI], 1.27-3.43; P = 0.004) and at week 52 (41% vs 23%; OR, 2.26; 95% CI, 1.33-3.86; P = 0.003). The authors concluded that in patients having an insufficient response to TNFi therapy, a non-TNF biologic agent may be more effective than a second TNFi drug. Only a few studies75-77 have demonstrated similar results between the 2 strategies. Overall, the available evidence seems to suggest the superiority of the swapping over the cycling strategy.
An important clinical pearl to keep in mind is that both swapping and cycling strategies might theoretically increase the risk of infection; however, limited evidence is reported in the literature. In a large retrospective analysis78 of data on 4332 RA patients from a large US claims database, patients who had cycled between TNFi agents had a 30% to 40% increased risk of infection compared to patients treated with rituximab. Patients on infliximab had a 62% higher hazard of severe infections, and this has also been reported in an observational study.79 In another study,70 41% of 201 patients with RA followed between 1999 and 2013 who swapped to abatacept/rituximab or tocilizumab developed adverse events, as compared to 59% of those who switched to a second TNFi.
What are recent trends in the use of bDMARDs?
Currently, there are no specific guidelines or biomarkers available to facilitate selection of specific treatment from among the classes of biologics. With the development of several new drugs and regulatory approval of baricitinib, physicians now have several biologic options to treat patients. A recent large time-trend study80 deriving data from more than 200,000 patients with RA showed that etanercept remains the most frequently used agent for the treatment of RA; it also showed that the use of adalimumab and infliximab is decreasing, and that the use of newer agents, especially abatacept, golimumab, and certolizumab, has considerably risen in recent years. In this study, abatacept, rituximab, certolizumab, golimumab, tocilizumab, and tofacitinib accounted for 13.2%, 13.8%, 6.9%, 11.9%, and 7.5% switches from first TNFi therapy.
Jin et al81 studied factors associated with the choice of bDMARD for initial and subsequent use. They found that patients with commercial insurance had an 87% higher likelihood of initiating a bDMARD. In the Medicaid subgroup, African Americans had lower odds of initiating and switching bDMARDs than non-Hispanic whites. Prior use of steroids and nonbiologic DMARDs predicted both bDMARD initiation and subsequent switching. Etanercept, adalimumab, and infliximab were the most commonly used first- and second-line bDMARDS; patients on anakinra and golimumab were most likely to be switched to other bDMARDs.
Which treatment strategy is the most cost-effective?
Several studies have reported better treatment persistence rates among patients who are treated with the swapping strategy compared to the cycling strategy. In a retrospective analysis of claims data,82 the authors examined treatment persistence and health care costs in patients switching to biologics with a different mechanism of action or cycling to another TNFi. The mean cost was significantly lower among patients treated using the swapping strategy than among the TNFi cyclers, both for the total cost of care for RA and for the total cost of the targeted DMARDs in the first year after the change in therapy. The authors concluded that switching to a drug with a different mechanism of action is associated with higher treatment persistence and lower health care costs than TNFi cycling.
What about biosimilars?
Biosimilars are copies of already licensed biologics that are very similar to the biologics, but are made by different sponsors using independently derived cell lines and separately developed manufacturing processes.83 Regarding biosimilar use, EULAR26 states that biosimilar bDMARDs approved by the European Medicines Agency or US Food and Drug Administration have similar efficacy and safety as the originator bDMARDs, and recommends them as preferred agents if they are indeed appreciably cheaper than originator or other bDMARDs.
What are the novel treatment targets in RA?
New therapeutics for RA continue to be developed. One of the new agents is peficitinib (ASP015K), an oral, once-daily Janus kinase (Jak) inhibitor targeting Jak-1, Jak-2, and tyrosine kinase-2, with moderate selectivity for Jak-3. In a phase 2b trial, 100-mg and 150-mg doses of peficitinib achieved a statistically significant ACR20 response (48.3% and 56.3%) compared to placebo (29.4%) at 12 weeks.84
Given the benefit of targeting TNF-α and IL-17 in RA, a novel molecule (ABT-122) that targets both human TNF and IL-17 has been developed. Two phase 1 studies85 showed that dual neutralization of TNF and IL-17 with ABT-122 has characteristics acceptable for further exploration of therapeutic potential of this agent in TNF- and IL-17A–driven immune-mediated inflammatory diseases. Another novel drug is mavrilimumab, a human monoclonal antibody that targets granulocyte–macrophage colony-stimulating factor receptor α. A recent studyshowed that long-term treatment with mavrilimumab maintained response and was well-tolerated, with no increased incidence of treatment-emergent adverse events.86
Namilumab (AMG203) is an immunoglobulin G1 monoclonal antibody that binds with high affinity to the GM-CSF ligand. In a phase 1b, randomized, double-blind study (PRIORA)87 to assess namilumab in treating active, mild-to-moderate RA, significant improvement was seen in the DAS28-CRP score with namilumab (150 and 300 mg groups combined) compared with placebo at day 43 (P = 0.0117) and also 8 weeks after last dosing at day 99 (P = 0.0154). Adverse events were similar across different doses of namilumab and placebo, and included nasopharyngitis and exacerbation/worsening of RA. Another drug showing promise in RA is fosdagrocorat (PF-04171327), a potential dissociated agonist of the glucocorticoid receptor. A multicenter, double-blind, parallel-group, active- and placebo-controlled phase 2 study randomly assigned 86 patients to receive fosdagrocorat 10 mg, fosdagrocorat 25 mg, prednisone 5 mg, or placebo, all with stable background methotrexate therapy.88 Both fosdagrocorat doses demonstrated efficacy in improving signs and symptoms in RA patients, with manageable adverse events.
Case Conclusion
There are several available treatment options for the case patient. Based on the PREMIER trial, solely increasing the dose of adalimumab is unlikely to provide a therapeutic benefit. Adding low-dose methotrexate (possibly via a parenteral route because of patient-reported gastrointestinal discomfort) might provide some synergistic and therapeutic effect. However, because of primary failure with TNFi therapy, she may benefit from the initiation of a biologic with a different mechanism of action (ie, swapping strategy). Therapeutic options include tocilizumab, abatacept, rituximab, and the Jak inhibitors (tofacitinib and baricitinib).
Summary
The optimal treatment of RA aims at achieving, and then maintaining, remission or a low disease activity. The choice of best treatment must be individualized to the patient, keeping in mind other factors, including comorbidities like fibromyalgia, history of diverticulitis (prior to use of tocilizumab), history of chronic obstructive pulmonary disease (prior to the use of abatacept), malignancy, and the presence of risk factors for infections (age, diabetes, chronic bronchitis). In a patient with inadequate response to initial biologic therapy, several options exist for the rheumatologist. Current evidence supports TNFi dose escalation for only infliximab; optimization of concurrent csDMARD or switching to a different csDMARD are other options. Cycling and swapping are other alternate approaches supported by many observational studies. While no head-to-head trials exist comparing the 2 strategies, data suggest superiority of the swapping strategy over the cycling approach. With the continuing development of novel therapeutics in RA, physicians have a growing list of treatment options to help their patients achieve disease remission.
Corresponding author: Namrata Singh, MD, 200 Hawkins Drive, Iowa City, IA 52242.
Financial disclosures: None.
1. Keystone ED, Kavanaugh KA. What to do with TNF failures. Expert Opin Drug Saf. 2005;4:149-155.
2. Kalden JR, Schulze-Koops H. Immunogenicity and loss of response to TNF inhibitors: implications for rheumatoid arthritis treatment. Nat Rev Rheumatol. 2017;13:707-718.
3. Weinblatt ME, Kremer JM, Bankhurst AD, et al. A trial of etanercept, a recombinant tumor necrosis factor receptor:Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med. 1999;340:253-259.
4. Keystone EC, Kavanaugh AF, Sharp JT, et al. Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti-tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo-controlled, 52-week trial. Arthritis Rheum. 2004;50(5):1400-1411.
5. Lipsky PE, van der Heijde DM, St Clair EW, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N Engl J Med. 2000;343:1594-1602.
6. Finckh A, Simard JF, Gabay C, et al. Evidence for differential acquired drug resistance to anti-tumour necrosis factor agents in rheumatoid arthritis. Ann Rheum Dis. 2006;65:746-752.
7. Souto A, Maneiro JR, Gomez-Reino JJ. Rate of discontinuation and drug survival of biologic therapies in rheumatoid arthritis: a systematic review and meta-analysis of drug registries and health care databases. Rheumatology. 2016;55:523-534.
8. Hetland ML, Christensen IJ, Tarp U, et al. Direct comparison of treatment responses, remission rates, and drug adherence in patients with rheumatoid arthritis treated with adalimumab, etanercept, or infliximab: results from eight years of surveillance of clinical practice in the nationwide Danish DANBIO registry. Arthritis Rheum. 2010;62:22-32.
9. Gabay C, Riek M, Scherer A, et al. Effectiveness of biologic DMARDs in monotherapy versus in combination with synthetic DMARDs in rheumatoid arthritis: data from the Swiss Clinical Quality Management Registry. Rheumatology. 2015;54(9):1664-1672.
10. Ebina K, Hashimoto M, Yamamoto W, et al. Drug retention and discontinuation reasons between seven biologics in patients with rheumatoid arthritis-The ANSWER cohort study. PloS One. 2018;13:e0194130.
11. Wijbrandts CA, Tak PP. Prediction of response to targeted treatment in rheumatoid arthritis. Mayo Clin Proc. 2017;92:1129-1143.
12. Ulfgren AK, Andersson U, Engstrom M, et al. Systemic anti-tumor necrosis factor alpha therapy in rheumatoid arthritis down-regulates synovial tumor necrosis factor alpha synthesis. Arthritis Rheum. 2000;43:2391-2396.
13. Choi IY, Gerlag DM, Herenius MJ, et al. MRP8/14 serum levels as a strong predictor of response to biological treatments in patients with rheumatoid arthritis. Ann Rheum Dis. 2015;74:499-505.
14. Nguyen MVC, Baillet A, Romand X, et al. Prealbumin, platelet factor 4 and S100A12 combination at baseline predicts good response to TNF alpha inhibitors in rheumatoid arthritis. Joint Bone Spine. 2019;86:195-201.
15. Anderson JK, Zimmerman L, Caplan L, Michaud K. Measures of rheumatoid arthritis disease activity: Patient (PtGA) and Provider (PrGA) Global Assessment of Disease Activity, Disease Activity Score (DAS) and Disease Activity Score with 28-Joint Counts (DAS28), Simplified Disease Activity Index (SDAI), Clinical Disease Activity Index (CDAI), Patient Activity Score (PAS) and Patient Activity Score-II (PASII), Routine Assessment of Patient Index Data (RAPID), Rheumatoid Arthritis Disease Activity Index (RADAI) and Rheumatoid Arthritis Disease Activity Index-5 (RADAI-5), Chronic Arthritis Systemic Index (CASI), Patient-Based Disease Activity Score With ESR (PDAS1) and Patient-Based Disease Activity Score without ESR (PDAS2), and Mean Overall Index for Rheumatoid Arthritis (MOI-RA). Arthritis Care Res. 2011;63(suppl 11):S14-S36.
16. Katz L, Gisbert JP, Manoogian B, et al. Doubling the infliximab dose versus halving the infusion intervals in Crohn’s disease patients with loss of response. Inflamm Bowel Dis. 2012;18:2026-2033.
17. Durez P, Van den Bosch F, Corluy L, et al. A dose adjustment in patients with rheumatoid arthritis not optimally responding to a standard dose of infliximab of 3 mg/kg every 8 weeks can be effective: a Belgian prospective study. Rheumatology. 2005;44:465-468.
18. Breedveld FC, Weisman MH, Kavanaugh AF, et al. The PREMIER study: A multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 2006;54:26-37.
19. St Clair EW, Wagner CL, Fasanmade AA, et al. The relationship of serum infliximab concentrations to clinical improvement in rheumatoid arthritis: results from ATTRACT, a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2002;46:1451-1459.
20. Rahman MU, Strusberg I, Geusens P, et al. Double-blinded infliximab dose escalation in patients with rheumatoid arthritis. Ann Rheum Dis. 2007;66:1233-1238.
21. Weinblatt ME, Schiff MH, Ruderman EM, et al. Efficacy and safety of etanercept 50 mg twice a week in patients with rheumatoid arthritis who had a suboptimal response to etanercept 50 mg once a week: results of a multicenter, randomized, double-blind, active drug-controlled study. Arthritis Rheum. 2008;58:1921-1930.
22. Curtis JR, Chen L, Luijtens K, et al. Dose escalation of certolizumab pegol from 200 mg to 400 mg every other week provides no additional efficacy in rheumatoid arthritis: an analysis of individual patient-level data. Arthritis Rheum. 2011;63:2203-2208.
23. Okazaki M, Kobayashi H, Ishii Y, et al. Real-world treatment patterns for golimumab and concomitant medications in Japanese rheumatoid arthritis patients. Rheumatol Ther. 2018;5:185-201.
24. Pappas DA, John A, Curtis JR, et al. Dosing of intravenous tocilizumab in a real-world setting of rheumatoid arthritis: analyses from the Corrona Registry. Rheumatol Ther. 2016;3:103-115.
25. Kivitz A, Olech E, Borofsky MA, et al. Two-year efficacy and safety of subcutaneous tocilizumab in combination with disease-modifying antirheumatic drugs including escalation to weekly dosing in rheumatoid arthritis. J Rheumatol. 2018;45:456-464.
26. Smolen JS, Landewe R, Bijlsma J, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017;76:960-977.
27. Singh JA, Saag KG, Bridges SL Jr, et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res. 2016;68:1-25.
28. Tabrizi MA, Tseng CM, Roskos LK. Elimination mechanisms of therapeutic monoclonal antibodies. Drug Discov Today. 2006;11:81-88.
29. Keizer RJ, Huitema AD, Schellens JH, Beijnen JH. Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010;49:493-507.
30. Navarro Coy NC, Brown S, Bosworth A, et al. The ‘Switch’ study protocol: a randomised-controlled trial of switching to an alternative tumour-necrosis factor (TNF)-inhibitor drug or abatacept or rituximab in patients with rheumatoid arthritis who have failed an initial TNF-inhibitor drug. BMC Musculoskelet Disord. 2014;15:452.
31. Kamal KM, Madhavan SS, Hornsby JA, et al. Use of tumor necrosis factor inhibitors in rheumatoid arthritis: a national survey of practicing United States rheumatologists. Joint Bone Spine. 2006;73:718-724.
32. Gomez-Reino JJ, Carmona L, BIOBADASER Group. Switching TNF antagonists in patients with chronic arthritis: an observational study of 488 patients over a four-year period. Arthritis Res Ther. 2006;8:R29.
33. Caporali R, Sarzi-Puttini P, Atzeni F, et al. Switching TNF-alpha antagonists in rheumatoid arthritis: The experience of the LORHEN registry. Autoimmun Rev. 2010;9:465-469.
34. Iannone F, Trotta F, Monteccuco C, et al. Etanercept maintains the clinical benefit achieved by infliximab in patients with rheumatoid arthritis who discontinued infliximab because of side effects. Ann Rheum Dis. 2007;66:249-252.
35. Virkki LM, Valleala H, Takakubo Y, et al. Outcomes of switching anti-TNF drugs in rheumatoid arthritis—a study based on observational data from the Finnish Register of Biological Treatment (ROB-FIN). Clin Rheumatol. 2011;30:1447-1454.
36. Furst DE, Gaylis N, Bray V, et al. Open-label, pilot protocol of patients with rheumatoid arthritis who switch to infliximab after an incomplete response to etanercept: the opposite study. Ann Rheum Dis. 2007;66:893-899.
37. Smolen JS, Kay J, Doyle MK, et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumour necrosis factor alpha inhibitors (GO-AFTER study): a multicentre, randomised, double-blind, placebo-controlled, phase III trial. Lancet. 2009;374:210-221.
38. Smolen JS, Kay J, Doyle M, et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumor necrosis factor α inhibitors: findings with up to five years of treatment in the multicenter, randomized, double-blind, placebo-controlled, phase 3 GO-AFTER study. Arthritis Res Ther. 2015;17:14.
39. Smolen JS, Burmester G-R, Combe B, et al. Head-to-head comparison of certolizumab pegol versus adalimumab in rheumatoid arthritis: 2-year efficacy and safety results from the randomised EXXELERATE study. Lancet. 2016;388:2763-2774.
40. Chatzidionysiou K, Askling J, Eriksson J, et al. Effectiveness of TNF inhibitor switch in RA: results from the national Swedish register. Ann Rheum Dis. 2015;74:890.
41. Smolen JS, Kay J, Doyle M, et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumor necrosis factor alpha inhibitors: findings with up to five years of treatment in the multicenter, randomized, double-blind, placebo-controlled, phase 3 GO-AFTER study. Arthritis Res Ther. 2015;17:14.
42. Lequerré T, Farran É, Ménard J-F, et al. Switching from an anti-TNF monoclonal antibody to soluble TNF-receptor yields better results than vice versa: An observational retrospective study of 72 rheumatoid arthritis switchers. Joint Bone Spine. 2015;82:330-337.
43. Favalli EG, Biggioggero M, Meroni PL. Methotrexate for the treatment of rheumatoid arthritis in the biologic era: Still an “anchor” drug? Autoimmun Rev. 2014;13:1102-1108.
44. Kalden JR, Schulze-Koops H. Immunogenicity and loss of response to TNF inhibitors: implications for rheumatoid arthritis treatment. Nat Rev Rheumatol. 2017;13:707-718.
45. Klareskog L, van der Heijde D, de Jager JP, et al. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet. 2004;363:675-681.
46. Emery P, Fleischmann RM, Strusberg I, et al. Efficacy and safety of subcutaneous golimumab in methotrexate-naive patients with rheumatoid arthritis: five-year results of a randomized clinical trial. Arthritis Care Res. 2016;68:744-752.
47. Emery P, Fleischmann RM, Moreland LW, et al. Golimumab, a human anti-tumor necrosis factor alpha monoclonal antibody, injected subcutaneously every four weeks in methotrexate-naive patients with active rheumatoid arthritis: twenty-four-week results of a phase III, multicenter, randomized, double-blind, placebo-controlled study of golimumab before methotrexate as first-line therapy for early-onset rheumatoid arthritis. Arthritis Rheum. 2009;60:2272-2283.
48. Emery P, Burmester GR, Bykerk VP, et al. Evaluating drug-free remission with abatacept in early rheumatoid arthritis: results from the phase 3b, multicentre, randomised, active-controlled AVERT study of 24 months, with a 12-month, double-blind treatment period. Ann Rheum Dis. 2015;74:19-26.
49. Westhovens R, Robles M, Ximenes AC, et al. Clinical efficacy and safety of abatacept in methotrexate-naive patients with early rheumatoid arthritis and poor prognostic factors. Ann Rheum Dis. 2009;68:1870-1877.
50. Cohen SB, Emery P, Greenwald MW, et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: Results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 2006;54:2793-2806.
51. Burmester GR, Rigby WF, van Vollenhoven RF, et al. Tocilizumab in early progressive rheumatoid arthritis: FUNCTION, a randomised controlled trial. Ann Rheum Dis. 2016;75:1081-1091.
52. Bijlsma JWJ, Welsing PMJ, Woodworth TG, et al. Early rheumatoid arthritis treated with tocilizumab, methotrexate, or their combination (U-Act-Early): a multicentre, randomised, double-blind, double-dummy, strategy trial. Lancet. 2016;388:343-355.
53. Cohen JD, Zaltni S, Kaiser MJ, et al. Secondary addition of methotrexate to partial responders to etanercept alone is effective in severe rheumatoid arthritis. Ann Rheum Dis. 2004;63:209-210.
54. Hamilton RA, Kremer JM. Why intramuscular methotrexate may be more efficacious than oral dosing in patients with rheumatoid arthritis. Br J Rheumatol. 1997;36:86-90.
55. Hoekstra M, Haagsma C, Neef C, et al. Bioavailability of higher dose methotrexate comparing oral and subcutaneous administration in patients with rheumatoid arthritis. J Rheumatol. 2004;31:645-648.
56. Herman RA, Veng-Pedersen P, Hoffman J, et al. Pharmacokinetics of low-dose methotrexate in rheumatoid arthritis patients. J Pharm Sci. 1989;78:165-171.
57. Schiff MH, Jaffe JS, Freundlich B. Head-to-head, randomised, crossover study of oral versus subcutaneous methotrexate in patients with rheumatoid arthritis: drug-exposure limitations of oral methotrexate at doses ± 15 mg may be overcome with subcutaneous administration. Ann Rheum Dis. 2014;73:1549-1551.
58. Hazlewood GS, Thorne JC, Pope JE, et al. The comparative effectiveness of oral versus subcutaneous methotrexate for the treatment of early rheumatoid arthritis. Ann Rheum Dis. 2016;75:1003-1008.
59. O’Dell JR, Petersen K, Leff R, et al. Etanercept in combination with sulfasalazine, hydroxychloroquine, or gold in the treatment of rheumatoid arthritis. J Rheumatol. 2006;33:213-218.
60. Finckh A, Dehler S, Gabay C. The effectiveness of leflunomide as a co-therapy of tumour necrosis factor inhibitors in rheumatoid arthritis: a population-based study. Ann Rheum Dis. 2009;68:33-39.
61. De Stefano R, Frati E, Nargi F, et al. Comparison of combination therapies in the treatment of rheumatoid arthritis: leflunomide-anti-TNF-alpha versus methotrexate-anti-TNF-alpha. Clin Rheumatol. 2010;29:517-524.
62. Combe B, Codreanu C, Fiocco U, et al. Etanercept and sulfasalazine, alone and combined, in patients with active rheumatoid arthritis despite receiving sulfasalazine: a double-blind comparison. Ann Rheum Dis. 2006;65:1357-1362.
63. Strangfeld A, Hierse F, Kekow J, et al. Comparative effectiveness of tumour necrosis factor α inhibitors in combination with either methotrexate or leflunomide. Ann Rheum Dis. 2009;68:1856.
64. Emery P, Keystone E, Tony HP, et al. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann Rheum Dis. 2008;67:1516.
65. Genovese MC, Becker J-C, Schiff M, et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor α inhibition. N Engl J Med. 2005;353:1114-1123.
66. Emery P, Gottenberg JE, Rubbert-Roth A, et al. Rituximab versus an alternative TNF inhibitor in patients with rheumatoid arthritis who failed to respond to a single previous TNF inhibitor: SWITCH-RA, a global, observational, comparative effectiveness study. Ann Rheum Dis. 2015;74:979-984.
67. Keystone E, Emery P, Peterfy CG, et al. Rituximab inhibits structural joint damage in patients with rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitor therapies. Ann Rheum Dis. 2009;68:216.
68. Burmester GR, Blanco R, Charles-Schoeman C, et al. Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet. 2013;381:451-460.
69. Genovese MC, Kremer J, Zamani O, et al. Baricitinib in patients with refractory rheumatoid arthritis. N Engl J Med. 2016;374:1243-1252.
70. Favalli EG, Biggioggero M, Marchesoni A, Meroni PL. Survival on treatment with second-line biologic therapy: a cohort study comparing cycling and swap strategies. Rheumatology. 2014;53:1664-1668.
71. Harrold LR, Reed GW, Solomon DH, et al. Comparative effectiveness of abatacept versus tocilizumab in rheumatoid arthritis patients with prior TNFi exposure in the US Corrona registry. Arthritis Res Ther. 2016;18:280.
72. Gottenberg J, Brocq O, Perdriger A, et al. Non–TNF-targeted biologic vs a second anti-TNF drug to treat rheumatoid arthritis in patients with insufficient response to a first anti-TNF drug: A randomized clinical trial. JAMA. 2016;316:1172-1180.
73. Pascart T, Philippe P, Drumez E, et al. Comparative efficacy of tocilizumab, abatacept and rituximab after non-TNF inhibitor failure: results from a multicentre study. Int J Rheum Dis. 2016;19:1093-1102.
74. Akiyama M, Kaneko Y, Kondo H, Takeuchi T. Comparison of the clinical effectiveness of tumour necrosis factor inhibitors and abatacept after insufficient response to tocilizumab in patients with rheumatoid arthritis. Clin Rheumatol. 2016;35:2829-2834.
75. Schoels M, Aletaha D, Smolen JS, Wong JB. Comparative effectiveness and safety of biological treatment options after tumour necrosis factor α inhibitor failure in rheumatoid arthritis: systematic review and indirect pairwise meta-analysis. Ann Rheum Dis. 2012;71:1303.
76. Soliman MM, Hyrich KL, Lunt M, et al. Rituximab or a second anti-tumor necrosis factor therapy for rheumatoid arthritis patients who have failed their first anti-tumor necrosis factor therapy? Comparative analysis from the British Society for Rheumatology Biologics Register. Arthritis Care Res. 2012;64:1108-1115.
77. Chatzidionysiou K, Vollenhoven RF. Rituximab versus anti-TNF in patients who previously failed one TNF inhibitor in an observational cohort. Scand J Rheumatol. 2013;42:190-195.
78. Johnston SS, Turpcu A, Shi N, et al. Risk of infections in rheumatoid arthritis patients switching from anti-TNF agents to rituximab, abatacept, or another anti-TNF agent, a retrospective administrative claims analysis. Semim Arthritis Rheum. 2013;43:39-47.
79. Curtis JR, Xie F, Chen L, et al. The comparative risk of serious infections among rheumatoid arthritis patients starting or switching biological agents. Ann Rheum Dis. 2011;70:1401.
80. Desai RJ, Solomon DH, Jin Y, et al. Temporal trends in use of biologic DMARDs for rheumatoid arthritis in the United States: a cohort study of publicly and privately insured patients. J Manag Care Spec Pharm. 2017;23:809-814.
81. Jin Y, Desai RJ, Liu J, et al. Factors associated with initial or subsequent choice of biologic disease-modifying antirheumatic drugs for treatment of rheumatoid arthritis. Arthritis Res Ther. 2017;19:159.
82. Bonafede MMK, McMorrow D, Proudfoot C, et al. Treatment persistence and healthcare costs among patients with rheumatoid arthritis after a change in targeted therapy. Am Health Drug Benefits. 2018;11:192-202.
83. US Food and Drug Administration. Biosimilars are safe, effective treatment options. www.fda.gov/drugs/developmentapprovalprocess/howdrugsaredevelopedandapproved/approvalapplications/therapeuticbiologicapplications/biosimilars/. Accessed November 9, 2018.
84. Genovese MC, Greenwald M, Codding C, et al. Peficitinib, a JAK inhibitor, in combination with limited conventional synthetic disease-modifying antirheumatic drugs in the treatment of moderate-to-severe rheumatoid arthritis. Arthritis Rheumatol. 2017;69:932-942.
85. Fleischmann RM, Wagner F, Kivitz AJ, et al. Safety, tolerability, and pharmacodynamics of ABT-122, a tumor necrosis factor- and interleukin-17-targeted dual variable domain immunoglobulin, in patients with rheumatoid arthritis. Arthritis Rheumatol. 2017;69:2283-2291.
86. Burmester GR, McInnes IB, Kremer JM, et al. Mavrilimumab, a fully human granulocyte-macrophage colony-stimulating factor receptor alpha monoclonal antibody: long-term safety and efficacy in patients with rheumatoid arthritis. Arthritis Rheumatol. 2018;70:679-689.
87. Huizinga TW, Batalov A, Stoilov R, et al. Phase 1b randomized, double-blind study of namilumab, an anti-granulocyte macrophage colony-stimulating factor monoclonal antibody, in mild-to-moderate rheumatoid arthritis. Arthritis Res Ther. 2017;19:53.
88. Stock T, Fleishaker D, Wang X, et al. Improved disease activity with fosdagrocorat (PF-04171327), a partial agonist of the glucocorticoid receptor, in patients with rheumatoid arthritis: a Phase 2 randomized study. Int J Rheum Dis. 2017;20:960-970.
89. Orencia [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2013.
90. Humira[package insert]. North Chicago, IL: AbbVie; 2012.
91. Kineret [package insert]. Stockholm, Sweden: Sobi; 2012.
92. Olumiant [package insert]. Indianapolis, IN: Lilly USA, LLC; 2018.
93. Cimzia [package insert]. Smyrna, GA: UCB, Inc; 2008.
94. Enbrel [package insert]. Thousand Oaks, CA: Immunex Corporation; 1998.
95. Simponi [package insert]. Horsham, PA: Janssen Biotech, Inc; 2009.
96. Remicade [package insert]. Horsham, PA: Janssen Biotech, Inc; 1998.
97. Rituxan [package insert]. South San Francisco, CA: Genetech, Inc; 1997.
98. Kevzara [package insert]. Bridgewater, NJ: Sanofi-Aventis US LLC; 2018.
99. Actemra [package insert]. South San Francisco, CA: Genentech, Inc; 2013.
100. Xeljanz [package insert]. New York, NY: Pfizer Inc; 2016.
From the University of Iowa Hospitals and Clinics, Iowa City, IA.
Abstract
- Objective: To discuss the variability in response to tumor necrosis factor inhibitors (TNFis) observed in patients with rheumatoid arthritis (RA) and discuss therapeutic options for patients who do not respond to initial TNFi therapy.
- Methods: Review of the literature.
- Results: Optimal treatment of RA aims at achieving and then maintaining remission or low disease activity. In a patient with an inadequate response to initial biologic therapy, several therapeutic options exist. Current evidence supports TNFi dose escalation for only infliximab; optimization of concurrent conventional synthetic disease-modifying antirheumatic drug (csDMARD) or switching to a different csDMARD are other options. Cycling (switching to an alternative TNFi) and swapping (switching to a therapy with a different mode of action) strategies are other alternate approaches supported by many observational studies. While no head-to-head trials exist directly comparing the 2 strategies, data suggest superiority of the swapping strategy over the cycling approach. Also, several studies have shown that switching to a drug with a different mechanism of action is associated with higher treatment persistence and lower health care costs than TNFi cycling.
- Conclusion: Physicians have a growing list of treatment options to help their patients with RA achieve disease remission. The choice of best treatment for a given patient needs to be individualized, keeping in mind other factors, including comorbidities.
Keywords: biologics; rheumatoid arthritis; swapping strategy; cycling strategy; TNF inhibitors.
Following the discovery of tumor necrosis factor (TNF) as a proinflammatory cytokine 30 years ago, the use of TNF antagonists has revolutionized the treatment of rheumatoid arthritis (RA). Although TNF inhibitors (TNFIs) are frequently used as a first-line biologic disease-modifying antirheumatic drug (bDMARD), they are not uniformly efficacious in achieving remission in all patients with RA. This article highlights the reasons for such variability in observed response and discusses therapeutic options for patients who do not respond to TNFi therapy.
Case Presentation
A 60-year-old woman is evaluated in the clinic for complaints of pain in her hands, morning stiffness lasting 2 hours, and swelling in her wrists, all of which have been ongoing for 3 months. Physical exam reveals evidence of active inflammation, with synovitis in her second, third, and fourth metacarpophalangeal (MCP) and proximal interphalangeal (PIP) joints bilaterally, swelling over both wrists, and a weak grip. Inflammatory markers are elevated, and rheumatoid factor and anti-cyclic citrullinated peptide (anti-CCP) are both positive at high titer. Radiographs reveal evidence of small erosions at the third and fourth MCPs and PIPs bilaterally and periarticular osteopenia. The patient is diagnosed with seropositive, erosive RA based on history, physical exam, laboratory studies, and imaging. She is started on 20 mg of prednisone for acute treatment of her symptoms along with methotrexate, and, initially, her symptoms are well controlled. A few months after starting treatment, she develops voluminous diarrhea that necessitates cessation of methotrexate. Leflunomide also causes similar symptoms. The combination of sulfasalazine and hydroxychloroquine does not adequately control her symptoms, and ongoing use of low-dose glucocorticoids is required to improve functionality in all joints. Using the treat-to-target (T2T) strategy, adalimumab is initiated. However, she continues to report persistent swelling and pain and still requests oral glucocorticoids to help decrease inflammation. The 28-joint Disease Activity Score (DAS28) is 4.8, suggestive of moderate disease activity.
Why are TNFi agents sometimes ineffective?
The introduction of monoclonal antibodies and fusion proteins to block TNF and other cytokines was a remarkable development in the treatment of RA that revolutionized patient care. Despite the efficacy of TNFis, clinical response to these agents is not universal and only some patients achieve complete remission. In targeting the eventual goal of remission or low disease activity in patients with RA, the concept of “TNF failure” becomes extremely relevant. These inadequate responses to anti-TNF therapy may be due to primary failures, or complete lack of clinical response after initiation of the bDMARD, and secondary failures, or the loss of initially achieved clinical response to therapy. Other reasons for discontinuation of a given TNFi include partial disease control and intolerance to the medication (possible injection-site or infusion reactions). Keystone and Kavanaugh1 divided causes of failure of TNF agents into 2 broad categories: perceptual (related to natural variations in disease course like hormonal variation and physical and emotional stress) and pathophysiological failures (genetic variations, high body mass index, concomitant cigarette use).
Another important consideration in patients treated with a TNFi is the consequent formation of anti-drug antibodies (ADAs). TNFi agents are immunogenic and normally elicit an immune response. The appearance of such ADAs may reduce the bioavailability of free drug, resulting in a decreased clinical response,2 or may lead to serious adverse effects.
How common is discontinuation of the first TNFi?
Several studies have reported that the prevalence of primary failure, secondary failure, and intolerance to TNFis ranges from 30% to 40%.3-6 Female sex,7 concurrent prednisone use,8 high disease activity scores,6,8,9 and the absence of treatment with low-dose methotrexate7,8 have all been shown to be negative predictors of bDMARD retention and response.10
Are there any factors that predict TNFi failure?
There are no specific parameters to accurately predict responses to TNFI therapy.11 Several clinical and molecular biomarkers in synovium (initial TNF levels, macrophages, T cells)12 and peripheral blood (serum myeloid-related protein 8 and 14 complex levels,13 prealbumin, platelet factor 4, and S100A12)14 have been described as predictors of clinical response to TNFis, but their utility in clinical practice has not been established and the use of these markers has not yet been incorporated into clinical guidelines.
How is disease activity measured in patients with RA?
In 2010 an international expert consensus panel published treatment recommendations for RA that emphasized a T2T strategy of individualizing and escalating treatment to achieve the lowest disease activity or remission. In clinical practice, numerous tools are available to measure RA disease activity. Herein, we mention several that are most commonly used in clinical practice.
DAS28 combines single activity measures into an overall continuous measure of disease activity and has been endorsed by both the American College of Rheumatology (ACR) and European League Against Rheumatism (EULAR). It includes a 28-swollen joint count (SJC), 28-tender joint count (TJC), erythrocyte sedimentation rate (ESR; can also be calculated using C-reactive protein [CRP]), and a patient global assessment (PtGA). The cut-offs used for DAS28 interpretation are as follows: remission (< 2.6), low (≥ 2.6 but ≤ 3.2), moderate (> 3.2 but ≤ 5.1), or high (> 5.1).15 Some of the difficulties in using DAS28 in daily clinical practice include the need for a lab value and the time needed to perform the joint counts. Note also that due to the inclusion of ESR, which is influenced by age and other factors, DAS28 may underestimate remission in the elderly.
Another measure of RA disease activity is the Simplified Disease Activity Index (SDAI), which includes 28 SJC, 28 TJC, PtGA, provider global assessment (PrGA), and CRP in mg/dL. The level of disease activity using the SDAI is interpreted as: remission (SDAI ≤ 3.3), low (≥ 3.4 but ≤ 11), moderate (> 11 but ≤ 26), or high (> 26). The advantage of the SDAI is that a calculator or computer is not required for calculations. Another measure, the Clinical Disease Activity Index (CDAI), includes a 28 SJC, 28 TJC, PtGA, and PrGA. Because a laboratory value is not needed to calculate the CDAI, it is well-suited for use in clinical practice. When using the CDAI, the level of disease activity can be defined as remission (CDAI ≤ 2.8), low (> 2.8 but ≤ 10), moderate (> 10 but ≤ 22), or high (> 22). Again, as with the SDAI, a calculator or computer is not needed for calculations.
What are the alternative treatment options after first biologic failure?
In patients who have failed treatment with an initial biologic, usually a TNFi, the treating rheumatologist has the following options (Figure), with the best treatment strategy being driven by individualized patient and disease-related factors (Table 1 and Table 2):
- TNFi dose escalation
- Trial of an alternate TNFi agent (the “cycling” strategy)
- Optimization of therapy conjoined with a conventional synthetic DMARD (csDMARD)
- Use of a non-TNF biologic or targeted synthetic DMARD (the “swapping” strategy)

If all the listed strategies fail, the next step can be the addition of short-term, low-dose glucocorticoid therapy.

TNFi Dose Escalation
The available data have demonstrated the safety, efficacy, and cost-effectiveness of dose escalation in patients with RA receiving infliximab.16-18 The ATTRACT trial first demonstrated this, with greater clinical and radiographic improvements in those with higher trough serum concentrations, suggesting that doses higher than 3 mg/kg or more frequent than every 8 weeks may be needed for full response in some patients.19

There is a lack of studies in RA patients to determine the most effective dose escalation strategy. A study in patients with Crohn disease showed that intensification to 10 mg/kg every 8 weeks (dose doubling) was at least as effective as 5 mg/kg every 4 weeks (halving interval) at 12 months.16 Due to greater patient and administration convenience of dose-doubling, this strategy may be preferred.17 A starting dose of 10 mg/kg every 8 weeks is not routinely recommended due to an increased risk of serious infection; these adverse events were not found when the dose was gradually increased, as clinically indicated, starting at 3 mg/kg.19,20 Further studies are needed to explore this approach in RA patients.
These results, however, have not been replicated with other TNFi agents. No significant clinical improvements were identified with etanercept 50 mg twice weekly,21 adalimumab 40 mg every week in the PREMIER trial,18 or certolizumab 400 mg every other week in an open-label extension phase of the RAPID 1 study.22 A Japanese study found significantly worse clinical outcomes with dose escalation of golimumab.23 Conversely, 2 studies found clinical benefits after escalating the tocilizumab dose, the first a real-world review from the Consortium of Rheumatology Researchers of North America (CORRONA) registry using the intravenous formulation,24 and the other the BREVACTA study utilizing subcutaneous tocilizumab.25 No studies to date have been published on dose escalation of abatacept in patients with RA who respond poorly. Overall, previous studies support dose escalation in individuals being treated with infliximab to improve clinical outcomes, but additional studies are needed for other bDMARDs.
Trial of an Alternate TNF Agent: The “Cycling” Strategy
Per the ACR/EULAR26,27 guidelines, all approved bDMARDs may be used without hierarchical positioning. However, after the failure of a TNFi agent, these guidelines do not provide specific advice about a preference between the “cycling” strategy (switching to an alternative TNFi) and “swapping” strategy (switching to a therapy with a different mode of action). Cycling might work for several reasons, including differences in the agents’ molecular structure, immunological mechanism of action, immunogenicity, and pharmacokinetics.28-30 The cycling strategy is a well-established approach adopted by more than 94% of practicing rheumatologists, according to a national survey,31 and its efficacy is supported by trials and additional observational studies.32-35
The greater clinical effectiveness of switching to infliximab compared with continuing with etanercept in patients with inadequate response to etanercept (n = 28) was suggested in the open-label OPPOSITE trial.36 Data from the GO-AFTER trial37 suggests that a greater proportion of patients with RA refractory to adalimumab, etanercept, or infliximab who were treated with golimumab achieved an ACR20 and ACR50 response compared with patients who received placebo, and this response persisted through 5 years.38 More recently, certolizumab pegol and adalimumab were compared head-to-head in the EXXELERATE trial.39 The results of this trial revealed the adequate efficacy of cycling to another TNFi after primary insufficient response to the first.
In studies from Finland and Sweden,35,40 it has been observed that a better response is achieved in patients in whom TNF failure was initially due to secondary failure or intolerance rather than primary failure. A post-hoc analysis of the results of the GO-AFTER trial41 and from a few observational studies35,40,42 revealed that switching from one TNFi to another, especially from a monoclonal antibody to a soluble receptor, was often more beneficial for RA patients than switching from a soluble receptor to a monoclonal antibody.
Optimization of Therapy Conjoined with csDMARDs
Methotrexate is one of the oldest and most effective csDMARDs available for the treatment of RA.43 The 2016 EULAR guidelines recommend the addition of methotrexate and/or other csDMARDs to potentiate the effect of bDMARDs.26 In the case of TNFi therapy, the observed synergistic effect between the monoclonal antibody and methotrexate may be explained by sustained suppression of ADA formation.44 In the TEMPO,45 PREMIER,18 and GO-BEFORE46 trials, the addition of methotrexate led to improved clinical and radiological outcomes in patients treated with etanercept, adalimumab, and golimumab,47 respectively. These findings were also demonstrated in several registries, where significant improvement in clinical response and retention rate of the TNFi agents was noted. Results have been replicated with non-TNFi bDMARDs, including abatacept48,49 and rituximab.50 Patients treated with interleukin (IL)-6 inhibitors in combination with methotrexate have shown significantly less radiographic progression compared to those treated with tocilizumab alone and those treated with monotherapy tocilizumab versus monotherapy methotrexate.51,52 Results possibly favor the use of IL-6 inhibitors alone in those who cannot tolerate or have contraindications to methotrexate.
An open prospective study by Cohen et al added methotrexate to the treatment regimens of individuals on bDMARD monotherapy with a primary failure and found favorable changes in ACR20 and DAS28 scores at 3 and 12 months and therapeutic biological response (ESR, CRP) at 3 months.53 Unlike monotherapy, in these situations methotrexate is known to be efficacious even at a lower dose, possibly at 7.5 mg to 10 mg per week. Some studies have shown that methotrexate administered parenterally may be more efficacious than when given orally.54-58
In clinical trials and observational studies, leflunomide, sulfasalazine, and hydroxychloroquine have been used as alternate csDMARDs added to the treatment regimen.59-62 There are, however, only 2 trials comparing the efficacy of methotrexate with that of other csDMARDs as concomitant treatment in patients with inadequate response to TNFi therapy. The RABBIT trial found a slight decrease in effectiveness with concomitant TNFi and leflunomide compared to TNFi/methotrexate, but overall each group had similar EULAR responses at 24 months.63 A study by De Stefano et al found comparable ACR20 and DAS28 responses among individuals receiving TNFis with methotrexate or leflunomide.61
The “Swapping” Strategy
The efficacy of the swapping strategy has been shown in 3 randomized clinical trials demonstrating the superiority of abatacept, tocilizumab, and rituximab in the treatment of individuals with RA refractory to TNFis. Tocilizumab was studied in the RADIATE64 trial, which involved 499 patients with inadequate response to 1 or more TNFi agents. The primary endpoint (24-week ACR20) was achieved by 50.0%, 30.4%, and 10.1% of patients in the 8 mg/kg, 4 mg/kg, and control groups, respectively (P < 0.001 for both tocilizumab groups versus placebo). The utility of abatacept as second-line therapy after initial TNF failure was evaluated in the ATTAIN65 study. Participants with an inadequate response to etanercept or infliximab were randomly assigned to receive either abatacept or placebo. ACR50 response rates after 6 months of treatment were 20.3% with abatacept and 3.8% with placebo (P < 0.001). The SWITCH-RA study,66 an observational study, compared rituximab to TNFis in 1112 participants with inadequate response to initial anti-TNF therapy. At 6 months, mean change in DAS28 was small but significantly greater for the rituximab group (–1.5 vs –1.1; P = 0.007). The difference in response rates was greatest among seropositive patients. These data suggest that rituximab has efficacy following TNFi failure, particularly for seropositive patients. Additionally, REFLEX67 is the sole randomized controlled trial in patients with insufficient response to TNFis that showed significant prevention of radiographic progression at week 56 in patients on rituximab compared to placebo (mean change from baseline in total Genant-modified Sharp score, 1.00 vs 2.31, respectively; P = 0.005).
One study randomly assigned 399 patients with active RA who had inadequate response to prior TNFi therapy to tofacitinib68 (5 mg twice daily or 10 mg twice daily) or placebo, both with methotrexate.6 After 3 months of treatment, ACR20 response rates (41.7% for 5 mg, 28.1% for 10 mg, 24.4% for placebo) and DAS28 remission rates (6.7% for 5 mg, 8.8% for 10 mg, 1.7% for placebo) were significantly greater among patients treated with tofacitinib compared to those treated with placebo. More recently, the RA-BEACON trial69 demonstrated a consistent, beneficial treatment effect of baricitinib in patients with insufficient response to 1 or more TNFis. In this trial, 527 patients with an inadequate response to bDMARDs were randomly assigned to receive baricitinib 2 mg or 4 mg daily or placebo for 24 weeks. A higher proportion of patients receiving baricitinib 4 mg had an ACR20 response at week 12 compared with those treated with placebo (55% vs 27%, P < 0.001), and patients receiving the 4-mg dose had significant improvements from baseline in DAS28 and Health Assessment Questionnaire–Disability Index scores (P < 0.001 for both comparisons).
To Cycle or to Swap?
Several observational studies (SCQM-RA,70 STURE,71 BSRBR,72 Favalli,43 MIRAR,73 SWITCH-RA,74 ROC72) have clearly demonstrated that the swapping strategy is favored over the cycling strategy. In the ROC study,72 patients were randomly assigned (based on physician discretion) to receive a non-TNF biologic or a TNFi. More patients in the non-TNF group than in the TNFi group showed low disease activity at week 24 (45% vs 28%; odds ratio [OR], 2.09; 95% confidence interval [CI], 1.27-3.43; P = 0.004) and at week 52 (41% vs 23%; OR, 2.26; 95% CI, 1.33-3.86; P = 0.003). The authors concluded that in patients having an insufficient response to TNFi therapy, a non-TNF biologic agent may be more effective than a second TNFi drug. Only a few studies75-77 have demonstrated similar results between the 2 strategies. Overall, the available evidence seems to suggest the superiority of the swapping over the cycling strategy.
An important clinical pearl to keep in mind is that both swapping and cycling strategies might theoretically increase the risk of infection; however, limited evidence is reported in the literature. In a large retrospective analysis78 of data on 4332 RA patients from a large US claims database, patients who had cycled between TNFi agents had a 30% to 40% increased risk of infection compared to patients treated with rituximab. Patients on infliximab had a 62% higher hazard of severe infections, and this has also been reported in an observational study.79 In another study,70 41% of 201 patients with RA followed between 1999 and 2013 who swapped to abatacept/rituximab or tocilizumab developed adverse events, as compared to 59% of those who switched to a second TNFi.
What are recent trends in the use of bDMARDs?
Currently, there are no specific guidelines or biomarkers available to facilitate selection of specific treatment from among the classes of biologics. With the development of several new drugs and regulatory approval of baricitinib, physicians now have several biologic options to treat patients. A recent large time-trend study80 deriving data from more than 200,000 patients with RA showed that etanercept remains the most frequently used agent for the treatment of RA; it also showed that the use of adalimumab and infliximab is decreasing, and that the use of newer agents, especially abatacept, golimumab, and certolizumab, has considerably risen in recent years. In this study, abatacept, rituximab, certolizumab, golimumab, tocilizumab, and tofacitinib accounted for 13.2%, 13.8%, 6.9%, 11.9%, and 7.5% switches from first TNFi therapy.
Jin et al81 studied factors associated with the choice of bDMARD for initial and subsequent use. They found that patients with commercial insurance had an 87% higher likelihood of initiating a bDMARD. In the Medicaid subgroup, African Americans had lower odds of initiating and switching bDMARDs than non-Hispanic whites. Prior use of steroids and nonbiologic DMARDs predicted both bDMARD initiation and subsequent switching. Etanercept, adalimumab, and infliximab were the most commonly used first- and second-line bDMARDS; patients on anakinra and golimumab were most likely to be switched to other bDMARDs.
Which treatment strategy is the most cost-effective?
Several studies have reported better treatment persistence rates among patients who are treated with the swapping strategy compared to the cycling strategy. In a retrospective analysis of claims data,82 the authors examined treatment persistence and health care costs in patients switching to biologics with a different mechanism of action or cycling to another TNFi. The mean cost was significantly lower among patients treated using the swapping strategy than among the TNFi cyclers, both for the total cost of care for RA and for the total cost of the targeted DMARDs in the first year after the change in therapy. The authors concluded that switching to a drug with a different mechanism of action is associated with higher treatment persistence and lower health care costs than TNFi cycling.
What about biosimilars?
Biosimilars are copies of already licensed biologics that are very similar to the biologics, but are made by different sponsors using independently derived cell lines and separately developed manufacturing processes.83 Regarding biosimilar use, EULAR26 states that biosimilar bDMARDs approved by the European Medicines Agency or US Food and Drug Administration have similar efficacy and safety as the originator bDMARDs, and recommends them as preferred agents if they are indeed appreciably cheaper than originator or other bDMARDs.
What are the novel treatment targets in RA?
New therapeutics for RA continue to be developed. One of the new agents is peficitinib (ASP015K), an oral, once-daily Janus kinase (Jak) inhibitor targeting Jak-1, Jak-2, and tyrosine kinase-2, with moderate selectivity for Jak-3. In a phase 2b trial, 100-mg and 150-mg doses of peficitinib achieved a statistically significant ACR20 response (48.3% and 56.3%) compared to placebo (29.4%) at 12 weeks.84
Given the benefit of targeting TNF-α and IL-17 in RA, a novel molecule (ABT-122) that targets both human TNF and IL-17 has been developed. Two phase 1 studies85 showed that dual neutralization of TNF and IL-17 with ABT-122 has characteristics acceptable for further exploration of therapeutic potential of this agent in TNF- and IL-17A–driven immune-mediated inflammatory diseases. Another novel drug is mavrilimumab, a human monoclonal antibody that targets granulocyte–macrophage colony-stimulating factor receptor α. A recent studyshowed that long-term treatment with mavrilimumab maintained response and was well-tolerated, with no increased incidence of treatment-emergent adverse events.86
Namilumab (AMG203) is an immunoglobulin G1 monoclonal antibody that binds with high affinity to the GM-CSF ligand. In a phase 1b, randomized, double-blind study (PRIORA)87 to assess namilumab in treating active, mild-to-moderate RA, significant improvement was seen in the DAS28-CRP score with namilumab (150 and 300 mg groups combined) compared with placebo at day 43 (P = 0.0117) and also 8 weeks after last dosing at day 99 (P = 0.0154). Adverse events were similar across different doses of namilumab and placebo, and included nasopharyngitis and exacerbation/worsening of RA. Another drug showing promise in RA is fosdagrocorat (PF-04171327), a potential dissociated agonist of the glucocorticoid receptor. A multicenter, double-blind, parallel-group, active- and placebo-controlled phase 2 study randomly assigned 86 patients to receive fosdagrocorat 10 mg, fosdagrocorat 25 mg, prednisone 5 mg, or placebo, all with stable background methotrexate therapy.88 Both fosdagrocorat doses demonstrated efficacy in improving signs and symptoms in RA patients, with manageable adverse events.
Case Conclusion
There are several available treatment options for the case patient. Based on the PREMIER trial, solely increasing the dose of adalimumab is unlikely to provide a therapeutic benefit. Adding low-dose methotrexate (possibly via a parenteral route because of patient-reported gastrointestinal discomfort) might provide some synergistic and therapeutic effect. However, because of primary failure with TNFi therapy, she may benefit from the initiation of a biologic with a different mechanism of action (ie, swapping strategy). Therapeutic options include tocilizumab, abatacept, rituximab, and the Jak inhibitors (tofacitinib and baricitinib).
Summary
The optimal treatment of RA aims at achieving, and then maintaining, remission or a low disease activity. The choice of best treatment must be individualized to the patient, keeping in mind other factors, including comorbidities like fibromyalgia, history of diverticulitis (prior to use of tocilizumab), history of chronic obstructive pulmonary disease (prior to the use of abatacept), malignancy, and the presence of risk factors for infections (age, diabetes, chronic bronchitis). In a patient with inadequate response to initial biologic therapy, several options exist for the rheumatologist. Current evidence supports TNFi dose escalation for only infliximab; optimization of concurrent csDMARD or switching to a different csDMARD are other options. Cycling and swapping are other alternate approaches supported by many observational studies. While no head-to-head trials exist comparing the 2 strategies, data suggest superiority of the swapping strategy over the cycling approach. With the continuing development of novel therapeutics in RA, physicians have a growing list of treatment options to help their patients achieve disease remission.
Corresponding author: Namrata Singh, MD, 200 Hawkins Drive, Iowa City, IA 52242.
Financial disclosures: None.
From the University of Iowa Hospitals and Clinics, Iowa City, IA.
Abstract
- Objective: To discuss the variability in response to tumor necrosis factor inhibitors (TNFis) observed in patients with rheumatoid arthritis (RA) and discuss therapeutic options for patients who do not respond to initial TNFi therapy.
- Methods: Review of the literature.
- Results: Optimal treatment of RA aims at achieving and then maintaining remission or low disease activity. In a patient with an inadequate response to initial biologic therapy, several therapeutic options exist. Current evidence supports TNFi dose escalation for only infliximab; optimization of concurrent conventional synthetic disease-modifying antirheumatic drug (csDMARD) or switching to a different csDMARD are other options. Cycling (switching to an alternative TNFi) and swapping (switching to a therapy with a different mode of action) strategies are other alternate approaches supported by many observational studies. While no head-to-head trials exist directly comparing the 2 strategies, data suggest superiority of the swapping strategy over the cycling approach. Also, several studies have shown that switching to a drug with a different mechanism of action is associated with higher treatment persistence and lower health care costs than TNFi cycling.
- Conclusion: Physicians have a growing list of treatment options to help their patients with RA achieve disease remission. The choice of best treatment for a given patient needs to be individualized, keeping in mind other factors, including comorbidities.
Keywords: biologics; rheumatoid arthritis; swapping strategy; cycling strategy; TNF inhibitors.
Following the discovery of tumor necrosis factor (TNF) as a proinflammatory cytokine 30 years ago, the use of TNF antagonists has revolutionized the treatment of rheumatoid arthritis (RA). Although TNF inhibitors (TNFIs) are frequently used as a first-line biologic disease-modifying antirheumatic drug (bDMARD), they are not uniformly efficacious in achieving remission in all patients with RA. This article highlights the reasons for such variability in observed response and discusses therapeutic options for patients who do not respond to TNFi therapy.
Case Presentation
A 60-year-old woman is evaluated in the clinic for complaints of pain in her hands, morning stiffness lasting 2 hours, and swelling in her wrists, all of which have been ongoing for 3 months. Physical exam reveals evidence of active inflammation, with synovitis in her second, third, and fourth metacarpophalangeal (MCP) and proximal interphalangeal (PIP) joints bilaterally, swelling over both wrists, and a weak grip. Inflammatory markers are elevated, and rheumatoid factor and anti-cyclic citrullinated peptide (anti-CCP) are both positive at high titer. Radiographs reveal evidence of small erosions at the third and fourth MCPs and PIPs bilaterally and periarticular osteopenia. The patient is diagnosed with seropositive, erosive RA based on history, physical exam, laboratory studies, and imaging. She is started on 20 mg of prednisone for acute treatment of her symptoms along with methotrexate, and, initially, her symptoms are well controlled. A few months after starting treatment, she develops voluminous diarrhea that necessitates cessation of methotrexate. Leflunomide also causes similar symptoms. The combination of sulfasalazine and hydroxychloroquine does not adequately control her symptoms, and ongoing use of low-dose glucocorticoids is required to improve functionality in all joints. Using the treat-to-target (T2T) strategy, adalimumab is initiated. However, she continues to report persistent swelling and pain and still requests oral glucocorticoids to help decrease inflammation. The 28-joint Disease Activity Score (DAS28) is 4.8, suggestive of moderate disease activity.
Why are TNFi agents sometimes ineffective?
The introduction of monoclonal antibodies and fusion proteins to block TNF and other cytokines was a remarkable development in the treatment of RA that revolutionized patient care. Despite the efficacy of TNFis, clinical response to these agents is not universal and only some patients achieve complete remission. In targeting the eventual goal of remission or low disease activity in patients with RA, the concept of “TNF failure” becomes extremely relevant. These inadequate responses to anti-TNF therapy may be due to primary failures, or complete lack of clinical response after initiation of the bDMARD, and secondary failures, or the loss of initially achieved clinical response to therapy. Other reasons for discontinuation of a given TNFi include partial disease control and intolerance to the medication (possible injection-site or infusion reactions). Keystone and Kavanaugh1 divided causes of failure of TNF agents into 2 broad categories: perceptual (related to natural variations in disease course like hormonal variation and physical and emotional stress) and pathophysiological failures (genetic variations, high body mass index, concomitant cigarette use).
Another important consideration in patients treated with a TNFi is the consequent formation of anti-drug antibodies (ADAs). TNFi agents are immunogenic and normally elicit an immune response. The appearance of such ADAs may reduce the bioavailability of free drug, resulting in a decreased clinical response,2 or may lead to serious adverse effects.
How common is discontinuation of the first TNFi?
Several studies have reported that the prevalence of primary failure, secondary failure, and intolerance to TNFis ranges from 30% to 40%.3-6 Female sex,7 concurrent prednisone use,8 high disease activity scores,6,8,9 and the absence of treatment with low-dose methotrexate7,8 have all been shown to be negative predictors of bDMARD retention and response.10
Are there any factors that predict TNFi failure?
There are no specific parameters to accurately predict responses to TNFI therapy.11 Several clinical and molecular biomarkers in synovium (initial TNF levels, macrophages, T cells)12 and peripheral blood (serum myeloid-related protein 8 and 14 complex levels,13 prealbumin, platelet factor 4, and S100A12)14 have been described as predictors of clinical response to TNFis, but their utility in clinical practice has not been established and the use of these markers has not yet been incorporated into clinical guidelines.
How is disease activity measured in patients with RA?
In 2010 an international expert consensus panel published treatment recommendations for RA that emphasized a T2T strategy of individualizing and escalating treatment to achieve the lowest disease activity or remission. In clinical practice, numerous tools are available to measure RA disease activity. Herein, we mention several that are most commonly used in clinical practice.
DAS28 combines single activity measures into an overall continuous measure of disease activity and has been endorsed by both the American College of Rheumatology (ACR) and European League Against Rheumatism (EULAR). It includes a 28-swollen joint count (SJC), 28-tender joint count (TJC), erythrocyte sedimentation rate (ESR; can also be calculated using C-reactive protein [CRP]), and a patient global assessment (PtGA). The cut-offs used for DAS28 interpretation are as follows: remission (< 2.6), low (≥ 2.6 but ≤ 3.2), moderate (> 3.2 but ≤ 5.1), or high (> 5.1).15 Some of the difficulties in using DAS28 in daily clinical practice include the need for a lab value and the time needed to perform the joint counts. Note also that due to the inclusion of ESR, which is influenced by age and other factors, DAS28 may underestimate remission in the elderly.
Another measure of RA disease activity is the Simplified Disease Activity Index (SDAI), which includes 28 SJC, 28 TJC, PtGA, provider global assessment (PrGA), and CRP in mg/dL. The level of disease activity using the SDAI is interpreted as: remission (SDAI ≤ 3.3), low (≥ 3.4 but ≤ 11), moderate (> 11 but ≤ 26), or high (> 26). The advantage of the SDAI is that a calculator or computer is not required for calculations. Another measure, the Clinical Disease Activity Index (CDAI), includes a 28 SJC, 28 TJC, PtGA, and PrGA. Because a laboratory value is not needed to calculate the CDAI, it is well-suited for use in clinical practice. When using the CDAI, the level of disease activity can be defined as remission (CDAI ≤ 2.8), low (> 2.8 but ≤ 10), moderate (> 10 but ≤ 22), or high (> 22). Again, as with the SDAI, a calculator or computer is not needed for calculations.
What are the alternative treatment options after first biologic failure?
In patients who have failed treatment with an initial biologic, usually a TNFi, the treating rheumatologist has the following options (Figure), with the best treatment strategy being driven by individualized patient and disease-related factors (Table 1 and Table 2):
- TNFi dose escalation
- Trial of an alternate TNFi agent (the “cycling” strategy)
- Optimization of therapy conjoined with a conventional synthetic DMARD (csDMARD)
- Use of a non-TNF biologic or targeted synthetic DMARD (the “swapping” strategy)

If all the listed strategies fail, the next step can be the addition of short-term, low-dose glucocorticoid therapy.

TNFi Dose Escalation
The available data have demonstrated the safety, efficacy, and cost-effectiveness of dose escalation in patients with RA receiving infliximab.16-18 The ATTRACT trial first demonstrated this, with greater clinical and radiographic improvements in those with higher trough serum concentrations, suggesting that doses higher than 3 mg/kg or more frequent than every 8 weeks may be needed for full response in some patients.19

There is a lack of studies in RA patients to determine the most effective dose escalation strategy. A study in patients with Crohn disease showed that intensification to 10 mg/kg every 8 weeks (dose doubling) was at least as effective as 5 mg/kg every 4 weeks (halving interval) at 12 months.16 Due to greater patient and administration convenience of dose-doubling, this strategy may be preferred.17 A starting dose of 10 mg/kg every 8 weeks is not routinely recommended due to an increased risk of serious infection; these adverse events were not found when the dose was gradually increased, as clinically indicated, starting at 3 mg/kg.19,20 Further studies are needed to explore this approach in RA patients.
These results, however, have not been replicated with other TNFi agents. No significant clinical improvements were identified with etanercept 50 mg twice weekly,21 adalimumab 40 mg every week in the PREMIER trial,18 or certolizumab 400 mg every other week in an open-label extension phase of the RAPID 1 study.22 A Japanese study found significantly worse clinical outcomes with dose escalation of golimumab.23 Conversely, 2 studies found clinical benefits after escalating the tocilizumab dose, the first a real-world review from the Consortium of Rheumatology Researchers of North America (CORRONA) registry using the intravenous formulation,24 and the other the BREVACTA study utilizing subcutaneous tocilizumab.25 No studies to date have been published on dose escalation of abatacept in patients with RA who respond poorly. Overall, previous studies support dose escalation in individuals being treated with infliximab to improve clinical outcomes, but additional studies are needed for other bDMARDs.
Trial of an Alternate TNF Agent: The “Cycling” Strategy
Per the ACR/EULAR26,27 guidelines, all approved bDMARDs may be used without hierarchical positioning. However, after the failure of a TNFi agent, these guidelines do not provide specific advice about a preference between the “cycling” strategy (switching to an alternative TNFi) and “swapping” strategy (switching to a therapy with a different mode of action). Cycling might work for several reasons, including differences in the agents’ molecular structure, immunological mechanism of action, immunogenicity, and pharmacokinetics.28-30 The cycling strategy is a well-established approach adopted by more than 94% of practicing rheumatologists, according to a national survey,31 and its efficacy is supported by trials and additional observational studies.32-35
The greater clinical effectiveness of switching to infliximab compared with continuing with etanercept in patients with inadequate response to etanercept (n = 28) was suggested in the open-label OPPOSITE trial.36 Data from the GO-AFTER trial37 suggests that a greater proportion of patients with RA refractory to adalimumab, etanercept, or infliximab who were treated with golimumab achieved an ACR20 and ACR50 response compared with patients who received placebo, and this response persisted through 5 years.38 More recently, certolizumab pegol and adalimumab were compared head-to-head in the EXXELERATE trial.39 The results of this trial revealed the adequate efficacy of cycling to another TNFi after primary insufficient response to the first.
In studies from Finland and Sweden,35,40 it has been observed that a better response is achieved in patients in whom TNF failure was initially due to secondary failure or intolerance rather than primary failure. A post-hoc analysis of the results of the GO-AFTER trial41 and from a few observational studies35,40,42 revealed that switching from one TNFi to another, especially from a monoclonal antibody to a soluble receptor, was often more beneficial for RA patients than switching from a soluble receptor to a monoclonal antibody.
Optimization of Therapy Conjoined with csDMARDs
Methotrexate is one of the oldest and most effective csDMARDs available for the treatment of RA.43 The 2016 EULAR guidelines recommend the addition of methotrexate and/or other csDMARDs to potentiate the effect of bDMARDs.26 In the case of TNFi therapy, the observed synergistic effect between the monoclonal antibody and methotrexate may be explained by sustained suppression of ADA formation.44 In the TEMPO,45 PREMIER,18 and GO-BEFORE46 trials, the addition of methotrexate led to improved clinical and radiological outcomes in patients treated with etanercept, adalimumab, and golimumab,47 respectively. These findings were also demonstrated in several registries, where significant improvement in clinical response and retention rate of the TNFi agents was noted. Results have been replicated with non-TNFi bDMARDs, including abatacept48,49 and rituximab.50 Patients treated with interleukin (IL)-6 inhibitors in combination with methotrexate have shown significantly less radiographic progression compared to those treated with tocilizumab alone and those treated with monotherapy tocilizumab versus monotherapy methotrexate.51,52 Results possibly favor the use of IL-6 inhibitors alone in those who cannot tolerate or have contraindications to methotrexate.
An open prospective study by Cohen et al added methotrexate to the treatment regimens of individuals on bDMARD monotherapy with a primary failure and found favorable changes in ACR20 and DAS28 scores at 3 and 12 months and therapeutic biological response (ESR, CRP) at 3 months.53 Unlike monotherapy, in these situations methotrexate is known to be efficacious even at a lower dose, possibly at 7.5 mg to 10 mg per week. Some studies have shown that methotrexate administered parenterally may be more efficacious than when given orally.54-58
In clinical trials and observational studies, leflunomide, sulfasalazine, and hydroxychloroquine have been used as alternate csDMARDs added to the treatment regimen.59-62 There are, however, only 2 trials comparing the efficacy of methotrexate with that of other csDMARDs as concomitant treatment in patients with inadequate response to TNFi therapy. The RABBIT trial found a slight decrease in effectiveness with concomitant TNFi and leflunomide compared to TNFi/methotrexate, but overall each group had similar EULAR responses at 24 months.63 A study by De Stefano et al found comparable ACR20 and DAS28 responses among individuals receiving TNFis with methotrexate or leflunomide.61
The “Swapping” Strategy
The efficacy of the swapping strategy has been shown in 3 randomized clinical trials demonstrating the superiority of abatacept, tocilizumab, and rituximab in the treatment of individuals with RA refractory to TNFis. Tocilizumab was studied in the RADIATE64 trial, which involved 499 patients with inadequate response to 1 or more TNFi agents. The primary endpoint (24-week ACR20) was achieved by 50.0%, 30.4%, and 10.1% of patients in the 8 mg/kg, 4 mg/kg, and control groups, respectively (P < 0.001 for both tocilizumab groups versus placebo). The utility of abatacept as second-line therapy after initial TNF failure was evaluated in the ATTAIN65 study. Participants with an inadequate response to etanercept or infliximab were randomly assigned to receive either abatacept or placebo. ACR50 response rates after 6 months of treatment were 20.3% with abatacept and 3.8% with placebo (P < 0.001). The SWITCH-RA study,66 an observational study, compared rituximab to TNFis in 1112 participants with inadequate response to initial anti-TNF therapy. At 6 months, mean change in DAS28 was small but significantly greater for the rituximab group (–1.5 vs –1.1; P = 0.007). The difference in response rates was greatest among seropositive patients. These data suggest that rituximab has efficacy following TNFi failure, particularly for seropositive patients. Additionally, REFLEX67 is the sole randomized controlled trial in patients with insufficient response to TNFis that showed significant prevention of radiographic progression at week 56 in patients on rituximab compared to placebo (mean change from baseline in total Genant-modified Sharp score, 1.00 vs 2.31, respectively; P = 0.005).
One study randomly assigned 399 patients with active RA who had inadequate response to prior TNFi therapy to tofacitinib68 (5 mg twice daily or 10 mg twice daily) or placebo, both with methotrexate.6 After 3 months of treatment, ACR20 response rates (41.7% for 5 mg, 28.1% for 10 mg, 24.4% for placebo) and DAS28 remission rates (6.7% for 5 mg, 8.8% for 10 mg, 1.7% for placebo) were significantly greater among patients treated with tofacitinib compared to those treated with placebo. More recently, the RA-BEACON trial69 demonstrated a consistent, beneficial treatment effect of baricitinib in patients with insufficient response to 1 or more TNFis. In this trial, 527 patients with an inadequate response to bDMARDs were randomly assigned to receive baricitinib 2 mg or 4 mg daily or placebo for 24 weeks. A higher proportion of patients receiving baricitinib 4 mg had an ACR20 response at week 12 compared with those treated with placebo (55% vs 27%, P < 0.001), and patients receiving the 4-mg dose had significant improvements from baseline in DAS28 and Health Assessment Questionnaire–Disability Index scores (P < 0.001 for both comparisons).
To Cycle or to Swap?
Several observational studies (SCQM-RA,70 STURE,71 BSRBR,72 Favalli,43 MIRAR,73 SWITCH-RA,74 ROC72) have clearly demonstrated that the swapping strategy is favored over the cycling strategy. In the ROC study,72 patients were randomly assigned (based on physician discretion) to receive a non-TNF biologic or a TNFi. More patients in the non-TNF group than in the TNFi group showed low disease activity at week 24 (45% vs 28%; odds ratio [OR], 2.09; 95% confidence interval [CI], 1.27-3.43; P = 0.004) and at week 52 (41% vs 23%; OR, 2.26; 95% CI, 1.33-3.86; P = 0.003). The authors concluded that in patients having an insufficient response to TNFi therapy, a non-TNF biologic agent may be more effective than a second TNFi drug. Only a few studies75-77 have demonstrated similar results between the 2 strategies. Overall, the available evidence seems to suggest the superiority of the swapping over the cycling strategy.
An important clinical pearl to keep in mind is that both swapping and cycling strategies might theoretically increase the risk of infection; however, limited evidence is reported in the literature. In a large retrospective analysis78 of data on 4332 RA patients from a large US claims database, patients who had cycled between TNFi agents had a 30% to 40% increased risk of infection compared to patients treated with rituximab. Patients on infliximab had a 62% higher hazard of severe infections, and this has also been reported in an observational study.79 In another study,70 41% of 201 patients with RA followed between 1999 and 2013 who swapped to abatacept/rituximab or tocilizumab developed adverse events, as compared to 59% of those who switched to a second TNFi.
What are recent trends in the use of bDMARDs?
Currently, there are no specific guidelines or biomarkers available to facilitate selection of specific treatment from among the classes of biologics. With the development of several new drugs and regulatory approval of baricitinib, physicians now have several biologic options to treat patients. A recent large time-trend study80 deriving data from more than 200,000 patients with RA showed that etanercept remains the most frequently used agent for the treatment of RA; it also showed that the use of adalimumab and infliximab is decreasing, and that the use of newer agents, especially abatacept, golimumab, and certolizumab, has considerably risen in recent years. In this study, abatacept, rituximab, certolizumab, golimumab, tocilizumab, and tofacitinib accounted for 13.2%, 13.8%, 6.9%, 11.9%, and 7.5% switches from first TNFi therapy.
Jin et al81 studied factors associated with the choice of bDMARD for initial and subsequent use. They found that patients with commercial insurance had an 87% higher likelihood of initiating a bDMARD. In the Medicaid subgroup, African Americans had lower odds of initiating and switching bDMARDs than non-Hispanic whites. Prior use of steroids and nonbiologic DMARDs predicted both bDMARD initiation and subsequent switching. Etanercept, adalimumab, and infliximab were the most commonly used first- and second-line bDMARDS; patients on anakinra and golimumab were most likely to be switched to other bDMARDs.
Which treatment strategy is the most cost-effective?
Several studies have reported better treatment persistence rates among patients who are treated with the swapping strategy compared to the cycling strategy. In a retrospective analysis of claims data,82 the authors examined treatment persistence and health care costs in patients switching to biologics with a different mechanism of action or cycling to another TNFi. The mean cost was significantly lower among patients treated using the swapping strategy than among the TNFi cyclers, both for the total cost of care for RA and for the total cost of the targeted DMARDs in the first year after the change in therapy. The authors concluded that switching to a drug with a different mechanism of action is associated with higher treatment persistence and lower health care costs than TNFi cycling.
What about biosimilars?
Biosimilars are copies of already licensed biologics that are very similar to the biologics, but are made by different sponsors using independently derived cell lines and separately developed manufacturing processes.83 Regarding biosimilar use, EULAR26 states that biosimilar bDMARDs approved by the European Medicines Agency or US Food and Drug Administration have similar efficacy and safety as the originator bDMARDs, and recommends them as preferred agents if they are indeed appreciably cheaper than originator or other bDMARDs.
What are the novel treatment targets in RA?
New therapeutics for RA continue to be developed. One of the new agents is peficitinib (ASP015K), an oral, once-daily Janus kinase (Jak) inhibitor targeting Jak-1, Jak-2, and tyrosine kinase-2, with moderate selectivity for Jak-3. In a phase 2b trial, 100-mg and 150-mg doses of peficitinib achieved a statistically significant ACR20 response (48.3% and 56.3%) compared to placebo (29.4%) at 12 weeks.84
Given the benefit of targeting TNF-α and IL-17 in RA, a novel molecule (ABT-122) that targets both human TNF and IL-17 has been developed. Two phase 1 studies85 showed that dual neutralization of TNF and IL-17 with ABT-122 has characteristics acceptable for further exploration of therapeutic potential of this agent in TNF- and IL-17A–driven immune-mediated inflammatory diseases. Another novel drug is mavrilimumab, a human monoclonal antibody that targets granulocyte–macrophage colony-stimulating factor receptor α. A recent studyshowed that long-term treatment with mavrilimumab maintained response and was well-tolerated, with no increased incidence of treatment-emergent adverse events.86
Namilumab (AMG203) is an immunoglobulin G1 monoclonal antibody that binds with high affinity to the GM-CSF ligand. In a phase 1b, randomized, double-blind study (PRIORA)87 to assess namilumab in treating active, mild-to-moderate RA, significant improvement was seen in the DAS28-CRP score with namilumab (150 and 300 mg groups combined) compared with placebo at day 43 (P = 0.0117) and also 8 weeks after last dosing at day 99 (P = 0.0154). Adverse events were similar across different doses of namilumab and placebo, and included nasopharyngitis and exacerbation/worsening of RA. Another drug showing promise in RA is fosdagrocorat (PF-04171327), a potential dissociated agonist of the glucocorticoid receptor. A multicenter, double-blind, parallel-group, active- and placebo-controlled phase 2 study randomly assigned 86 patients to receive fosdagrocorat 10 mg, fosdagrocorat 25 mg, prednisone 5 mg, or placebo, all with stable background methotrexate therapy.88 Both fosdagrocorat doses demonstrated efficacy in improving signs and symptoms in RA patients, with manageable adverse events.
Case Conclusion
There are several available treatment options for the case patient. Based on the PREMIER trial, solely increasing the dose of adalimumab is unlikely to provide a therapeutic benefit. Adding low-dose methotrexate (possibly via a parenteral route because of patient-reported gastrointestinal discomfort) might provide some synergistic and therapeutic effect. However, because of primary failure with TNFi therapy, she may benefit from the initiation of a biologic with a different mechanism of action (ie, swapping strategy). Therapeutic options include tocilizumab, abatacept, rituximab, and the Jak inhibitors (tofacitinib and baricitinib).
Summary
The optimal treatment of RA aims at achieving, and then maintaining, remission or a low disease activity. The choice of best treatment must be individualized to the patient, keeping in mind other factors, including comorbidities like fibromyalgia, history of diverticulitis (prior to use of tocilizumab), history of chronic obstructive pulmonary disease (prior to the use of abatacept), malignancy, and the presence of risk factors for infections (age, diabetes, chronic bronchitis). In a patient with inadequate response to initial biologic therapy, several options exist for the rheumatologist. Current evidence supports TNFi dose escalation for only infliximab; optimization of concurrent csDMARD or switching to a different csDMARD are other options. Cycling and swapping are other alternate approaches supported by many observational studies. While no head-to-head trials exist comparing the 2 strategies, data suggest superiority of the swapping strategy over the cycling approach. With the continuing development of novel therapeutics in RA, physicians have a growing list of treatment options to help their patients achieve disease remission.
Corresponding author: Namrata Singh, MD, 200 Hawkins Drive, Iowa City, IA 52242.
Financial disclosures: None.
1. Keystone ED, Kavanaugh KA. What to do with TNF failures. Expert Opin Drug Saf. 2005;4:149-155.
2. Kalden JR, Schulze-Koops H. Immunogenicity and loss of response to TNF inhibitors: implications for rheumatoid arthritis treatment. Nat Rev Rheumatol. 2017;13:707-718.
3. Weinblatt ME, Kremer JM, Bankhurst AD, et al. A trial of etanercept, a recombinant tumor necrosis factor receptor:Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med. 1999;340:253-259.
4. Keystone EC, Kavanaugh AF, Sharp JT, et al. Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti-tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo-controlled, 52-week trial. Arthritis Rheum. 2004;50(5):1400-1411.
5. Lipsky PE, van der Heijde DM, St Clair EW, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N Engl J Med. 2000;343:1594-1602.
6. Finckh A, Simard JF, Gabay C, et al. Evidence for differential acquired drug resistance to anti-tumour necrosis factor agents in rheumatoid arthritis. Ann Rheum Dis. 2006;65:746-752.
7. Souto A, Maneiro JR, Gomez-Reino JJ. Rate of discontinuation and drug survival of biologic therapies in rheumatoid arthritis: a systematic review and meta-analysis of drug registries and health care databases. Rheumatology. 2016;55:523-534.
8. Hetland ML, Christensen IJ, Tarp U, et al. Direct comparison of treatment responses, remission rates, and drug adherence in patients with rheumatoid arthritis treated with adalimumab, etanercept, or infliximab: results from eight years of surveillance of clinical practice in the nationwide Danish DANBIO registry. Arthritis Rheum. 2010;62:22-32.
9. Gabay C, Riek M, Scherer A, et al. Effectiveness of biologic DMARDs in monotherapy versus in combination with synthetic DMARDs in rheumatoid arthritis: data from the Swiss Clinical Quality Management Registry. Rheumatology. 2015;54(9):1664-1672.
10. Ebina K, Hashimoto M, Yamamoto W, et al. Drug retention and discontinuation reasons between seven biologics in patients with rheumatoid arthritis-The ANSWER cohort study. PloS One. 2018;13:e0194130.
11. Wijbrandts CA, Tak PP. Prediction of response to targeted treatment in rheumatoid arthritis. Mayo Clin Proc. 2017;92:1129-1143.
12. Ulfgren AK, Andersson U, Engstrom M, et al. Systemic anti-tumor necrosis factor alpha therapy in rheumatoid arthritis down-regulates synovial tumor necrosis factor alpha synthesis. Arthritis Rheum. 2000;43:2391-2396.
13. Choi IY, Gerlag DM, Herenius MJ, et al. MRP8/14 serum levels as a strong predictor of response to biological treatments in patients with rheumatoid arthritis. Ann Rheum Dis. 2015;74:499-505.
14. Nguyen MVC, Baillet A, Romand X, et al. Prealbumin, platelet factor 4 and S100A12 combination at baseline predicts good response to TNF alpha inhibitors in rheumatoid arthritis. Joint Bone Spine. 2019;86:195-201.
15. Anderson JK, Zimmerman L, Caplan L, Michaud K. Measures of rheumatoid arthritis disease activity: Patient (PtGA) and Provider (PrGA) Global Assessment of Disease Activity, Disease Activity Score (DAS) and Disease Activity Score with 28-Joint Counts (DAS28), Simplified Disease Activity Index (SDAI), Clinical Disease Activity Index (CDAI), Patient Activity Score (PAS) and Patient Activity Score-II (PASII), Routine Assessment of Patient Index Data (RAPID), Rheumatoid Arthritis Disease Activity Index (RADAI) and Rheumatoid Arthritis Disease Activity Index-5 (RADAI-5), Chronic Arthritis Systemic Index (CASI), Patient-Based Disease Activity Score With ESR (PDAS1) and Patient-Based Disease Activity Score without ESR (PDAS2), and Mean Overall Index for Rheumatoid Arthritis (MOI-RA). Arthritis Care Res. 2011;63(suppl 11):S14-S36.
16. Katz L, Gisbert JP, Manoogian B, et al. Doubling the infliximab dose versus halving the infusion intervals in Crohn’s disease patients with loss of response. Inflamm Bowel Dis. 2012;18:2026-2033.
17. Durez P, Van den Bosch F, Corluy L, et al. A dose adjustment in patients with rheumatoid arthritis not optimally responding to a standard dose of infliximab of 3 mg/kg every 8 weeks can be effective: a Belgian prospective study. Rheumatology. 2005;44:465-468.
18. Breedveld FC, Weisman MH, Kavanaugh AF, et al. The PREMIER study: A multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 2006;54:26-37.
19. St Clair EW, Wagner CL, Fasanmade AA, et al. The relationship of serum infliximab concentrations to clinical improvement in rheumatoid arthritis: results from ATTRACT, a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2002;46:1451-1459.
20. Rahman MU, Strusberg I, Geusens P, et al. Double-blinded infliximab dose escalation in patients with rheumatoid arthritis. Ann Rheum Dis. 2007;66:1233-1238.
21. Weinblatt ME, Schiff MH, Ruderman EM, et al. Efficacy and safety of etanercept 50 mg twice a week in patients with rheumatoid arthritis who had a suboptimal response to etanercept 50 mg once a week: results of a multicenter, randomized, double-blind, active drug-controlled study. Arthritis Rheum. 2008;58:1921-1930.
22. Curtis JR, Chen L, Luijtens K, et al. Dose escalation of certolizumab pegol from 200 mg to 400 mg every other week provides no additional efficacy in rheumatoid arthritis: an analysis of individual patient-level data. Arthritis Rheum. 2011;63:2203-2208.
23. Okazaki M, Kobayashi H, Ishii Y, et al. Real-world treatment patterns for golimumab and concomitant medications in Japanese rheumatoid arthritis patients. Rheumatol Ther. 2018;5:185-201.
24. Pappas DA, John A, Curtis JR, et al. Dosing of intravenous tocilizumab in a real-world setting of rheumatoid arthritis: analyses from the Corrona Registry. Rheumatol Ther. 2016;3:103-115.
25. Kivitz A, Olech E, Borofsky MA, et al. Two-year efficacy and safety of subcutaneous tocilizumab in combination with disease-modifying antirheumatic drugs including escalation to weekly dosing in rheumatoid arthritis. J Rheumatol. 2018;45:456-464.
26. Smolen JS, Landewe R, Bijlsma J, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017;76:960-977.
27. Singh JA, Saag KG, Bridges SL Jr, et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res. 2016;68:1-25.
28. Tabrizi MA, Tseng CM, Roskos LK. Elimination mechanisms of therapeutic monoclonal antibodies. Drug Discov Today. 2006;11:81-88.
29. Keizer RJ, Huitema AD, Schellens JH, Beijnen JH. Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010;49:493-507.
30. Navarro Coy NC, Brown S, Bosworth A, et al. The ‘Switch’ study protocol: a randomised-controlled trial of switching to an alternative tumour-necrosis factor (TNF)-inhibitor drug or abatacept or rituximab in patients with rheumatoid arthritis who have failed an initial TNF-inhibitor drug. BMC Musculoskelet Disord. 2014;15:452.
31. Kamal KM, Madhavan SS, Hornsby JA, et al. Use of tumor necrosis factor inhibitors in rheumatoid arthritis: a national survey of practicing United States rheumatologists. Joint Bone Spine. 2006;73:718-724.
32. Gomez-Reino JJ, Carmona L, BIOBADASER Group. Switching TNF antagonists in patients with chronic arthritis: an observational study of 488 patients over a four-year period. Arthritis Res Ther. 2006;8:R29.
33. Caporali R, Sarzi-Puttini P, Atzeni F, et al. Switching TNF-alpha antagonists in rheumatoid arthritis: The experience of the LORHEN registry. Autoimmun Rev. 2010;9:465-469.
34. Iannone F, Trotta F, Monteccuco C, et al. Etanercept maintains the clinical benefit achieved by infliximab in patients with rheumatoid arthritis who discontinued infliximab because of side effects. Ann Rheum Dis. 2007;66:249-252.
35. Virkki LM, Valleala H, Takakubo Y, et al. Outcomes of switching anti-TNF drugs in rheumatoid arthritis—a study based on observational data from the Finnish Register of Biological Treatment (ROB-FIN). Clin Rheumatol. 2011;30:1447-1454.
36. Furst DE, Gaylis N, Bray V, et al. Open-label, pilot protocol of patients with rheumatoid arthritis who switch to infliximab after an incomplete response to etanercept: the opposite study. Ann Rheum Dis. 2007;66:893-899.
37. Smolen JS, Kay J, Doyle MK, et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumour necrosis factor alpha inhibitors (GO-AFTER study): a multicentre, randomised, double-blind, placebo-controlled, phase III trial. Lancet. 2009;374:210-221.
38. Smolen JS, Kay J, Doyle M, et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumor necrosis factor α inhibitors: findings with up to five years of treatment in the multicenter, randomized, double-blind, placebo-controlled, phase 3 GO-AFTER study. Arthritis Res Ther. 2015;17:14.
39. Smolen JS, Burmester G-R, Combe B, et al. Head-to-head comparison of certolizumab pegol versus adalimumab in rheumatoid arthritis: 2-year efficacy and safety results from the randomised EXXELERATE study. Lancet. 2016;388:2763-2774.
40. Chatzidionysiou K, Askling J, Eriksson J, et al. Effectiveness of TNF inhibitor switch in RA: results from the national Swedish register. Ann Rheum Dis. 2015;74:890.
41. Smolen JS, Kay J, Doyle M, et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumor necrosis factor alpha inhibitors: findings with up to five years of treatment in the multicenter, randomized, double-blind, placebo-controlled, phase 3 GO-AFTER study. Arthritis Res Ther. 2015;17:14.
42. Lequerré T, Farran É, Ménard J-F, et al. Switching from an anti-TNF monoclonal antibody to soluble TNF-receptor yields better results than vice versa: An observational retrospective study of 72 rheumatoid arthritis switchers. Joint Bone Spine. 2015;82:330-337.
43. Favalli EG, Biggioggero M, Meroni PL. Methotrexate for the treatment of rheumatoid arthritis in the biologic era: Still an “anchor” drug? Autoimmun Rev. 2014;13:1102-1108.
44. Kalden JR, Schulze-Koops H. Immunogenicity and loss of response to TNF inhibitors: implications for rheumatoid arthritis treatment. Nat Rev Rheumatol. 2017;13:707-718.
45. Klareskog L, van der Heijde D, de Jager JP, et al. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet. 2004;363:675-681.
46. Emery P, Fleischmann RM, Strusberg I, et al. Efficacy and safety of subcutaneous golimumab in methotrexate-naive patients with rheumatoid arthritis: five-year results of a randomized clinical trial. Arthritis Care Res. 2016;68:744-752.
47. Emery P, Fleischmann RM, Moreland LW, et al. Golimumab, a human anti-tumor necrosis factor alpha monoclonal antibody, injected subcutaneously every four weeks in methotrexate-naive patients with active rheumatoid arthritis: twenty-four-week results of a phase III, multicenter, randomized, double-blind, placebo-controlled study of golimumab before methotrexate as first-line therapy for early-onset rheumatoid arthritis. Arthritis Rheum. 2009;60:2272-2283.
48. Emery P, Burmester GR, Bykerk VP, et al. Evaluating drug-free remission with abatacept in early rheumatoid arthritis: results from the phase 3b, multicentre, randomised, active-controlled AVERT study of 24 months, with a 12-month, double-blind treatment period. Ann Rheum Dis. 2015;74:19-26.
49. Westhovens R, Robles M, Ximenes AC, et al. Clinical efficacy and safety of abatacept in methotrexate-naive patients with early rheumatoid arthritis and poor prognostic factors. Ann Rheum Dis. 2009;68:1870-1877.
50. Cohen SB, Emery P, Greenwald MW, et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: Results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 2006;54:2793-2806.
51. Burmester GR, Rigby WF, van Vollenhoven RF, et al. Tocilizumab in early progressive rheumatoid arthritis: FUNCTION, a randomised controlled trial. Ann Rheum Dis. 2016;75:1081-1091.
52. Bijlsma JWJ, Welsing PMJ, Woodworth TG, et al. Early rheumatoid arthritis treated with tocilizumab, methotrexate, or their combination (U-Act-Early): a multicentre, randomised, double-blind, double-dummy, strategy trial. Lancet. 2016;388:343-355.
53. Cohen JD, Zaltni S, Kaiser MJ, et al. Secondary addition of methotrexate to partial responders to etanercept alone is effective in severe rheumatoid arthritis. Ann Rheum Dis. 2004;63:209-210.
54. Hamilton RA, Kremer JM. Why intramuscular methotrexate may be more efficacious than oral dosing in patients with rheumatoid arthritis. Br J Rheumatol. 1997;36:86-90.
55. Hoekstra M, Haagsma C, Neef C, et al. Bioavailability of higher dose methotrexate comparing oral and subcutaneous administration in patients with rheumatoid arthritis. J Rheumatol. 2004;31:645-648.
56. Herman RA, Veng-Pedersen P, Hoffman J, et al. Pharmacokinetics of low-dose methotrexate in rheumatoid arthritis patients. J Pharm Sci. 1989;78:165-171.
57. Schiff MH, Jaffe JS, Freundlich B. Head-to-head, randomised, crossover study of oral versus subcutaneous methotrexate in patients with rheumatoid arthritis: drug-exposure limitations of oral methotrexate at doses ± 15 mg may be overcome with subcutaneous administration. Ann Rheum Dis. 2014;73:1549-1551.
58. Hazlewood GS, Thorne JC, Pope JE, et al. The comparative effectiveness of oral versus subcutaneous methotrexate for the treatment of early rheumatoid arthritis. Ann Rheum Dis. 2016;75:1003-1008.
59. O’Dell JR, Petersen K, Leff R, et al. Etanercept in combination with sulfasalazine, hydroxychloroquine, or gold in the treatment of rheumatoid arthritis. J Rheumatol. 2006;33:213-218.
60. Finckh A, Dehler S, Gabay C. The effectiveness of leflunomide as a co-therapy of tumour necrosis factor inhibitors in rheumatoid arthritis: a population-based study. Ann Rheum Dis. 2009;68:33-39.
61. De Stefano R, Frati E, Nargi F, et al. Comparison of combination therapies in the treatment of rheumatoid arthritis: leflunomide-anti-TNF-alpha versus methotrexate-anti-TNF-alpha. Clin Rheumatol. 2010;29:517-524.
62. Combe B, Codreanu C, Fiocco U, et al. Etanercept and sulfasalazine, alone and combined, in patients with active rheumatoid arthritis despite receiving sulfasalazine: a double-blind comparison. Ann Rheum Dis. 2006;65:1357-1362.
63. Strangfeld A, Hierse F, Kekow J, et al. Comparative effectiveness of tumour necrosis factor α inhibitors in combination with either methotrexate or leflunomide. Ann Rheum Dis. 2009;68:1856.
64. Emery P, Keystone E, Tony HP, et al. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann Rheum Dis. 2008;67:1516.
65. Genovese MC, Becker J-C, Schiff M, et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor α inhibition. N Engl J Med. 2005;353:1114-1123.
66. Emery P, Gottenberg JE, Rubbert-Roth A, et al. Rituximab versus an alternative TNF inhibitor in patients with rheumatoid arthritis who failed to respond to a single previous TNF inhibitor: SWITCH-RA, a global, observational, comparative effectiveness study. Ann Rheum Dis. 2015;74:979-984.
67. Keystone E, Emery P, Peterfy CG, et al. Rituximab inhibits structural joint damage in patients with rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitor therapies. Ann Rheum Dis. 2009;68:216.
68. Burmester GR, Blanco R, Charles-Schoeman C, et al. Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet. 2013;381:451-460.
69. Genovese MC, Kremer J, Zamani O, et al. Baricitinib in patients with refractory rheumatoid arthritis. N Engl J Med. 2016;374:1243-1252.
70. Favalli EG, Biggioggero M, Marchesoni A, Meroni PL. Survival on treatment with second-line biologic therapy: a cohort study comparing cycling and swap strategies. Rheumatology. 2014;53:1664-1668.
71. Harrold LR, Reed GW, Solomon DH, et al. Comparative effectiveness of abatacept versus tocilizumab in rheumatoid arthritis patients with prior TNFi exposure in the US Corrona registry. Arthritis Res Ther. 2016;18:280.
72. Gottenberg J, Brocq O, Perdriger A, et al. Non–TNF-targeted biologic vs a second anti-TNF drug to treat rheumatoid arthritis in patients with insufficient response to a first anti-TNF drug: A randomized clinical trial. JAMA. 2016;316:1172-1180.
73. Pascart T, Philippe P, Drumez E, et al. Comparative efficacy of tocilizumab, abatacept and rituximab after non-TNF inhibitor failure: results from a multicentre study. Int J Rheum Dis. 2016;19:1093-1102.
74. Akiyama M, Kaneko Y, Kondo H, Takeuchi T. Comparison of the clinical effectiveness of tumour necrosis factor inhibitors and abatacept after insufficient response to tocilizumab in patients with rheumatoid arthritis. Clin Rheumatol. 2016;35:2829-2834.
75. Schoels M, Aletaha D, Smolen JS, Wong JB. Comparative effectiveness and safety of biological treatment options after tumour necrosis factor α inhibitor failure in rheumatoid arthritis: systematic review and indirect pairwise meta-analysis. Ann Rheum Dis. 2012;71:1303.
76. Soliman MM, Hyrich KL, Lunt M, et al. Rituximab or a second anti-tumor necrosis factor therapy for rheumatoid arthritis patients who have failed their first anti-tumor necrosis factor therapy? Comparative analysis from the British Society for Rheumatology Biologics Register. Arthritis Care Res. 2012;64:1108-1115.
77. Chatzidionysiou K, Vollenhoven RF. Rituximab versus anti-TNF in patients who previously failed one TNF inhibitor in an observational cohort. Scand J Rheumatol. 2013;42:190-195.
78. Johnston SS, Turpcu A, Shi N, et al. Risk of infections in rheumatoid arthritis patients switching from anti-TNF agents to rituximab, abatacept, or another anti-TNF agent, a retrospective administrative claims analysis. Semim Arthritis Rheum. 2013;43:39-47.
79. Curtis JR, Xie F, Chen L, et al. The comparative risk of serious infections among rheumatoid arthritis patients starting or switching biological agents. Ann Rheum Dis. 2011;70:1401.
80. Desai RJ, Solomon DH, Jin Y, et al. Temporal trends in use of biologic DMARDs for rheumatoid arthritis in the United States: a cohort study of publicly and privately insured patients. J Manag Care Spec Pharm. 2017;23:809-814.
81. Jin Y, Desai RJ, Liu J, et al. Factors associated with initial or subsequent choice of biologic disease-modifying antirheumatic drugs for treatment of rheumatoid arthritis. Arthritis Res Ther. 2017;19:159.
82. Bonafede MMK, McMorrow D, Proudfoot C, et al. Treatment persistence and healthcare costs among patients with rheumatoid arthritis after a change in targeted therapy. Am Health Drug Benefits. 2018;11:192-202.
83. US Food and Drug Administration. Biosimilars are safe, effective treatment options. www.fda.gov/drugs/developmentapprovalprocess/howdrugsaredevelopedandapproved/approvalapplications/therapeuticbiologicapplications/biosimilars/. Accessed November 9, 2018.
84. Genovese MC, Greenwald M, Codding C, et al. Peficitinib, a JAK inhibitor, in combination with limited conventional synthetic disease-modifying antirheumatic drugs in the treatment of moderate-to-severe rheumatoid arthritis. Arthritis Rheumatol. 2017;69:932-942.
85. Fleischmann RM, Wagner F, Kivitz AJ, et al. Safety, tolerability, and pharmacodynamics of ABT-122, a tumor necrosis factor- and interleukin-17-targeted dual variable domain immunoglobulin, in patients with rheumatoid arthritis. Arthritis Rheumatol. 2017;69:2283-2291.
86. Burmester GR, McInnes IB, Kremer JM, et al. Mavrilimumab, a fully human granulocyte-macrophage colony-stimulating factor receptor alpha monoclonal antibody: long-term safety and efficacy in patients with rheumatoid arthritis. Arthritis Rheumatol. 2018;70:679-689.
87. Huizinga TW, Batalov A, Stoilov R, et al. Phase 1b randomized, double-blind study of namilumab, an anti-granulocyte macrophage colony-stimulating factor monoclonal antibody, in mild-to-moderate rheumatoid arthritis. Arthritis Res Ther. 2017;19:53.
88. Stock T, Fleishaker D, Wang X, et al. Improved disease activity with fosdagrocorat (PF-04171327), a partial agonist of the glucocorticoid receptor, in patients with rheumatoid arthritis: a Phase 2 randomized study. Int J Rheum Dis. 2017;20:960-970.
89. Orencia [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2013.
90. Humira[package insert]. North Chicago, IL: AbbVie; 2012.
91. Kineret [package insert]. Stockholm, Sweden: Sobi; 2012.
92. Olumiant [package insert]. Indianapolis, IN: Lilly USA, LLC; 2018.
93. Cimzia [package insert]. Smyrna, GA: UCB, Inc; 2008.
94. Enbrel [package insert]. Thousand Oaks, CA: Immunex Corporation; 1998.
95. Simponi [package insert]. Horsham, PA: Janssen Biotech, Inc; 2009.
96. Remicade [package insert]. Horsham, PA: Janssen Biotech, Inc; 1998.
97. Rituxan [package insert]. South San Francisco, CA: Genetech, Inc; 1997.
98. Kevzara [package insert]. Bridgewater, NJ: Sanofi-Aventis US LLC; 2018.
99. Actemra [package insert]. South San Francisco, CA: Genentech, Inc; 2013.
100. Xeljanz [package insert]. New York, NY: Pfizer Inc; 2016.
1. Keystone ED, Kavanaugh KA. What to do with TNF failures. Expert Opin Drug Saf. 2005;4:149-155.
2. Kalden JR, Schulze-Koops H. Immunogenicity and loss of response to TNF inhibitors: implications for rheumatoid arthritis treatment. Nat Rev Rheumatol. 2017;13:707-718.
3. Weinblatt ME, Kremer JM, Bankhurst AD, et al. A trial of etanercept, a recombinant tumor necrosis factor receptor:Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med. 1999;340:253-259.
4. Keystone EC, Kavanaugh AF, Sharp JT, et al. Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti-tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo-controlled, 52-week trial. Arthritis Rheum. 2004;50(5):1400-1411.
5. Lipsky PE, van der Heijde DM, St Clair EW, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N Engl J Med. 2000;343:1594-1602.
6. Finckh A, Simard JF, Gabay C, et al. Evidence for differential acquired drug resistance to anti-tumour necrosis factor agents in rheumatoid arthritis. Ann Rheum Dis. 2006;65:746-752.
7. Souto A, Maneiro JR, Gomez-Reino JJ. Rate of discontinuation and drug survival of biologic therapies in rheumatoid arthritis: a systematic review and meta-analysis of drug registries and health care databases. Rheumatology. 2016;55:523-534.
8. Hetland ML, Christensen IJ, Tarp U, et al. Direct comparison of treatment responses, remission rates, and drug adherence in patients with rheumatoid arthritis treated with adalimumab, etanercept, or infliximab: results from eight years of surveillance of clinical practice in the nationwide Danish DANBIO registry. Arthritis Rheum. 2010;62:22-32.
9. Gabay C, Riek M, Scherer A, et al. Effectiveness of biologic DMARDs in monotherapy versus in combination with synthetic DMARDs in rheumatoid arthritis: data from the Swiss Clinical Quality Management Registry. Rheumatology. 2015;54(9):1664-1672.
10. Ebina K, Hashimoto M, Yamamoto W, et al. Drug retention and discontinuation reasons between seven biologics in patients with rheumatoid arthritis-The ANSWER cohort study. PloS One. 2018;13:e0194130.
11. Wijbrandts CA, Tak PP. Prediction of response to targeted treatment in rheumatoid arthritis. Mayo Clin Proc. 2017;92:1129-1143.
12. Ulfgren AK, Andersson U, Engstrom M, et al. Systemic anti-tumor necrosis factor alpha therapy in rheumatoid arthritis down-regulates synovial tumor necrosis factor alpha synthesis. Arthritis Rheum. 2000;43:2391-2396.
13. Choi IY, Gerlag DM, Herenius MJ, et al. MRP8/14 serum levels as a strong predictor of response to biological treatments in patients with rheumatoid arthritis. Ann Rheum Dis. 2015;74:499-505.
14. Nguyen MVC, Baillet A, Romand X, et al. Prealbumin, platelet factor 4 and S100A12 combination at baseline predicts good response to TNF alpha inhibitors in rheumatoid arthritis. Joint Bone Spine. 2019;86:195-201.
15. Anderson JK, Zimmerman L, Caplan L, Michaud K. Measures of rheumatoid arthritis disease activity: Patient (PtGA) and Provider (PrGA) Global Assessment of Disease Activity, Disease Activity Score (DAS) and Disease Activity Score with 28-Joint Counts (DAS28), Simplified Disease Activity Index (SDAI), Clinical Disease Activity Index (CDAI), Patient Activity Score (PAS) and Patient Activity Score-II (PASII), Routine Assessment of Patient Index Data (RAPID), Rheumatoid Arthritis Disease Activity Index (RADAI) and Rheumatoid Arthritis Disease Activity Index-5 (RADAI-5), Chronic Arthritis Systemic Index (CASI), Patient-Based Disease Activity Score With ESR (PDAS1) and Patient-Based Disease Activity Score without ESR (PDAS2), and Mean Overall Index for Rheumatoid Arthritis (MOI-RA). Arthritis Care Res. 2011;63(suppl 11):S14-S36.
16. Katz L, Gisbert JP, Manoogian B, et al. Doubling the infliximab dose versus halving the infusion intervals in Crohn’s disease patients with loss of response. Inflamm Bowel Dis. 2012;18:2026-2033.
17. Durez P, Van den Bosch F, Corluy L, et al. A dose adjustment in patients with rheumatoid arthritis not optimally responding to a standard dose of infliximab of 3 mg/kg every 8 weeks can be effective: a Belgian prospective study. Rheumatology. 2005;44:465-468.
18. Breedveld FC, Weisman MH, Kavanaugh AF, et al. The PREMIER study: A multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 2006;54:26-37.
19. St Clair EW, Wagner CL, Fasanmade AA, et al. The relationship of serum infliximab concentrations to clinical improvement in rheumatoid arthritis: results from ATTRACT, a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2002;46:1451-1459.
20. Rahman MU, Strusberg I, Geusens P, et al. Double-blinded infliximab dose escalation in patients with rheumatoid arthritis. Ann Rheum Dis. 2007;66:1233-1238.
21. Weinblatt ME, Schiff MH, Ruderman EM, et al. Efficacy and safety of etanercept 50 mg twice a week in patients with rheumatoid arthritis who had a suboptimal response to etanercept 50 mg once a week: results of a multicenter, randomized, double-blind, active drug-controlled study. Arthritis Rheum. 2008;58:1921-1930.
22. Curtis JR, Chen L, Luijtens K, et al. Dose escalation of certolizumab pegol from 200 mg to 400 mg every other week provides no additional efficacy in rheumatoid arthritis: an analysis of individual patient-level data. Arthritis Rheum. 2011;63:2203-2208.
23. Okazaki M, Kobayashi H, Ishii Y, et al. Real-world treatment patterns for golimumab and concomitant medications in Japanese rheumatoid arthritis patients. Rheumatol Ther. 2018;5:185-201.
24. Pappas DA, John A, Curtis JR, et al. Dosing of intravenous tocilizumab in a real-world setting of rheumatoid arthritis: analyses from the Corrona Registry. Rheumatol Ther. 2016;3:103-115.
25. Kivitz A, Olech E, Borofsky MA, et al. Two-year efficacy and safety of subcutaneous tocilizumab in combination with disease-modifying antirheumatic drugs including escalation to weekly dosing in rheumatoid arthritis. J Rheumatol. 2018;45:456-464.
26. Smolen JS, Landewe R, Bijlsma J, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017;76:960-977.
27. Singh JA, Saag KG, Bridges SL Jr, et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res. 2016;68:1-25.
28. Tabrizi MA, Tseng CM, Roskos LK. Elimination mechanisms of therapeutic monoclonal antibodies. Drug Discov Today. 2006;11:81-88.
29. Keizer RJ, Huitema AD, Schellens JH, Beijnen JH. Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010;49:493-507.
30. Navarro Coy NC, Brown S, Bosworth A, et al. The ‘Switch’ study protocol: a randomised-controlled trial of switching to an alternative tumour-necrosis factor (TNF)-inhibitor drug or abatacept or rituximab in patients with rheumatoid arthritis who have failed an initial TNF-inhibitor drug. BMC Musculoskelet Disord. 2014;15:452.
31. Kamal KM, Madhavan SS, Hornsby JA, et al. Use of tumor necrosis factor inhibitors in rheumatoid arthritis: a national survey of practicing United States rheumatologists. Joint Bone Spine. 2006;73:718-724.
32. Gomez-Reino JJ, Carmona L, BIOBADASER Group. Switching TNF antagonists in patients with chronic arthritis: an observational study of 488 patients over a four-year period. Arthritis Res Ther. 2006;8:R29.
33. Caporali R, Sarzi-Puttini P, Atzeni F, et al. Switching TNF-alpha antagonists in rheumatoid arthritis: The experience of the LORHEN registry. Autoimmun Rev. 2010;9:465-469.
34. Iannone F, Trotta F, Monteccuco C, et al. Etanercept maintains the clinical benefit achieved by infliximab in patients with rheumatoid arthritis who discontinued infliximab because of side effects. Ann Rheum Dis. 2007;66:249-252.
35. Virkki LM, Valleala H, Takakubo Y, et al. Outcomes of switching anti-TNF drugs in rheumatoid arthritis—a study based on observational data from the Finnish Register of Biological Treatment (ROB-FIN). Clin Rheumatol. 2011;30:1447-1454.
36. Furst DE, Gaylis N, Bray V, et al. Open-label, pilot protocol of patients with rheumatoid arthritis who switch to infliximab after an incomplete response to etanercept: the opposite study. Ann Rheum Dis. 2007;66:893-899.
37. Smolen JS, Kay J, Doyle MK, et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumour necrosis factor alpha inhibitors (GO-AFTER study): a multicentre, randomised, double-blind, placebo-controlled, phase III trial. Lancet. 2009;374:210-221.
38. Smolen JS, Kay J, Doyle M, et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumor necrosis factor α inhibitors: findings with up to five years of treatment in the multicenter, randomized, double-blind, placebo-controlled, phase 3 GO-AFTER study. Arthritis Res Ther. 2015;17:14.
39. Smolen JS, Burmester G-R, Combe B, et al. Head-to-head comparison of certolizumab pegol versus adalimumab in rheumatoid arthritis: 2-year efficacy and safety results from the randomised EXXELERATE study. Lancet. 2016;388:2763-2774.
40. Chatzidionysiou K, Askling J, Eriksson J, et al. Effectiveness of TNF inhibitor switch in RA: results from the national Swedish register. Ann Rheum Dis. 2015;74:890.
41. Smolen JS, Kay J, Doyle M, et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumor necrosis factor alpha inhibitors: findings with up to five years of treatment in the multicenter, randomized, double-blind, placebo-controlled, phase 3 GO-AFTER study. Arthritis Res Ther. 2015;17:14.
42. Lequerré T, Farran É, Ménard J-F, et al. Switching from an anti-TNF monoclonal antibody to soluble TNF-receptor yields better results than vice versa: An observational retrospective study of 72 rheumatoid arthritis switchers. Joint Bone Spine. 2015;82:330-337.
43. Favalli EG, Biggioggero M, Meroni PL. Methotrexate for the treatment of rheumatoid arthritis in the biologic era: Still an “anchor” drug? Autoimmun Rev. 2014;13:1102-1108.
44. Kalden JR, Schulze-Koops H. Immunogenicity and loss of response to TNF inhibitors: implications for rheumatoid arthritis treatment. Nat Rev Rheumatol. 2017;13:707-718.
45. Klareskog L, van der Heijde D, de Jager JP, et al. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet. 2004;363:675-681.
46. Emery P, Fleischmann RM, Strusberg I, et al. Efficacy and safety of subcutaneous golimumab in methotrexate-naive patients with rheumatoid arthritis: five-year results of a randomized clinical trial. Arthritis Care Res. 2016;68:744-752.
47. Emery P, Fleischmann RM, Moreland LW, et al. Golimumab, a human anti-tumor necrosis factor alpha monoclonal antibody, injected subcutaneously every four weeks in methotrexate-naive patients with active rheumatoid arthritis: twenty-four-week results of a phase III, multicenter, randomized, double-blind, placebo-controlled study of golimumab before methotrexate as first-line therapy for early-onset rheumatoid arthritis. Arthritis Rheum. 2009;60:2272-2283.
48. Emery P, Burmester GR, Bykerk VP, et al. Evaluating drug-free remission with abatacept in early rheumatoid arthritis: results from the phase 3b, multicentre, randomised, active-controlled AVERT study of 24 months, with a 12-month, double-blind treatment period. Ann Rheum Dis. 2015;74:19-26.
49. Westhovens R, Robles M, Ximenes AC, et al. Clinical efficacy and safety of abatacept in methotrexate-naive patients with early rheumatoid arthritis and poor prognostic factors. Ann Rheum Dis. 2009;68:1870-1877.
50. Cohen SB, Emery P, Greenwald MW, et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: Results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 2006;54:2793-2806.
51. Burmester GR, Rigby WF, van Vollenhoven RF, et al. Tocilizumab in early progressive rheumatoid arthritis: FUNCTION, a randomised controlled trial. Ann Rheum Dis. 2016;75:1081-1091.
52. Bijlsma JWJ, Welsing PMJ, Woodworth TG, et al. Early rheumatoid arthritis treated with tocilizumab, methotrexate, or their combination (U-Act-Early): a multicentre, randomised, double-blind, double-dummy, strategy trial. Lancet. 2016;388:343-355.
53. Cohen JD, Zaltni S, Kaiser MJ, et al. Secondary addition of methotrexate to partial responders to etanercept alone is effective in severe rheumatoid arthritis. Ann Rheum Dis. 2004;63:209-210.
54. Hamilton RA, Kremer JM. Why intramuscular methotrexate may be more efficacious than oral dosing in patients with rheumatoid arthritis. Br J Rheumatol. 1997;36:86-90.
55. Hoekstra M, Haagsma C, Neef C, et al. Bioavailability of higher dose methotrexate comparing oral and subcutaneous administration in patients with rheumatoid arthritis. J Rheumatol. 2004;31:645-648.
56. Herman RA, Veng-Pedersen P, Hoffman J, et al. Pharmacokinetics of low-dose methotrexate in rheumatoid arthritis patients. J Pharm Sci. 1989;78:165-171.
57. Schiff MH, Jaffe JS, Freundlich B. Head-to-head, randomised, crossover study of oral versus subcutaneous methotrexate in patients with rheumatoid arthritis: drug-exposure limitations of oral methotrexate at doses ± 15 mg may be overcome with subcutaneous administration. Ann Rheum Dis. 2014;73:1549-1551.
58. Hazlewood GS, Thorne JC, Pope JE, et al. The comparative effectiveness of oral versus subcutaneous methotrexate for the treatment of early rheumatoid arthritis. Ann Rheum Dis. 2016;75:1003-1008.
59. O’Dell JR, Petersen K, Leff R, et al. Etanercept in combination with sulfasalazine, hydroxychloroquine, or gold in the treatment of rheumatoid arthritis. J Rheumatol. 2006;33:213-218.
60. Finckh A, Dehler S, Gabay C. The effectiveness of leflunomide as a co-therapy of tumour necrosis factor inhibitors in rheumatoid arthritis: a population-based study. Ann Rheum Dis. 2009;68:33-39.
61. De Stefano R, Frati E, Nargi F, et al. Comparison of combination therapies in the treatment of rheumatoid arthritis: leflunomide-anti-TNF-alpha versus methotrexate-anti-TNF-alpha. Clin Rheumatol. 2010;29:517-524.
62. Combe B, Codreanu C, Fiocco U, et al. Etanercept and sulfasalazine, alone and combined, in patients with active rheumatoid arthritis despite receiving sulfasalazine: a double-blind comparison. Ann Rheum Dis. 2006;65:1357-1362.
63. Strangfeld A, Hierse F, Kekow J, et al. Comparative effectiveness of tumour necrosis factor α inhibitors in combination with either methotrexate or leflunomide. Ann Rheum Dis. 2009;68:1856.
64. Emery P, Keystone E, Tony HP, et al. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann Rheum Dis. 2008;67:1516.
65. Genovese MC, Becker J-C, Schiff M, et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor α inhibition. N Engl J Med. 2005;353:1114-1123.
66. Emery P, Gottenberg JE, Rubbert-Roth A, et al. Rituximab versus an alternative TNF inhibitor in patients with rheumatoid arthritis who failed to respond to a single previous TNF inhibitor: SWITCH-RA, a global, observational, comparative effectiveness study. Ann Rheum Dis. 2015;74:979-984.
67. Keystone E, Emery P, Peterfy CG, et al. Rituximab inhibits structural joint damage in patients with rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitor therapies. Ann Rheum Dis. 2009;68:216.
68. Burmester GR, Blanco R, Charles-Schoeman C, et al. Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet. 2013;381:451-460.
69. Genovese MC, Kremer J, Zamani O, et al. Baricitinib in patients with refractory rheumatoid arthritis. N Engl J Med. 2016;374:1243-1252.
70. Favalli EG, Biggioggero M, Marchesoni A, Meroni PL. Survival on treatment with second-line biologic therapy: a cohort study comparing cycling and swap strategies. Rheumatology. 2014;53:1664-1668.
71. Harrold LR, Reed GW, Solomon DH, et al. Comparative effectiveness of abatacept versus tocilizumab in rheumatoid arthritis patients with prior TNFi exposure in the US Corrona registry. Arthritis Res Ther. 2016;18:280.
72. Gottenberg J, Brocq O, Perdriger A, et al. Non–TNF-targeted biologic vs a second anti-TNF drug to treat rheumatoid arthritis in patients with insufficient response to a first anti-TNF drug: A randomized clinical trial. JAMA. 2016;316:1172-1180.
73. Pascart T, Philippe P, Drumez E, et al. Comparative efficacy of tocilizumab, abatacept and rituximab after non-TNF inhibitor failure: results from a multicentre study. Int J Rheum Dis. 2016;19:1093-1102.
74. Akiyama M, Kaneko Y, Kondo H, Takeuchi T. Comparison of the clinical effectiveness of tumour necrosis factor inhibitors and abatacept after insufficient response to tocilizumab in patients with rheumatoid arthritis. Clin Rheumatol. 2016;35:2829-2834.
75. Schoels M, Aletaha D, Smolen JS, Wong JB. Comparative effectiveness and safety of biological treatment options after tumour necrosis factor α inhibitor failure in rheumatoid arthritis: systematic review and indirect pairwise meta-analysis. Ann Rheum Dis. 2012;71:1303.
76. Soliman MM, Hyrich KL, Lunt M, et al. Rituximab or a second anti-tumor necrosis factor therapy for rheumatoid arthritis patients who have failed their first anti-tumor necrosis factor therapy? Comparative analysis from the British Society for Rheumatology Biologics Register. Arthritis Care Res. 2012;64:1108-1115.
77. Chatzidionysiou K, Vollenhoven RF. Rituximab versus anti-TNF in patients who previously failed one TNF inhibitor in an observational cohort. Scand J Rheumatol. 2013;42:190-195.
78. Johnston SS, Turpcu A, Shi N, et al. Risk of infections in rheumatoid arthritis patients switching from anti-TNF agents to rituximab, abatacept, or another anti-TNF agent, a retrospective administrative claims analysis. Semim Arthritis Rheum. 2013;43:39-47.
79. Curtis JR, Xie F, Chen L, et al. The comparative risk of serious infections among rheumatoid arthritis patients starting or switching biological agents. Ann Rheum Dis. 2011;70:1401.
80. Desai RJ, Solomon DH, Jin Y, et al. Temporal trends in use of biologic DMARDs for rheumatoid arthritis in the United States: a cohort study of publicly and privately insured patients. J Manag Care Spec Pharm. 2017;23:809-814.
81. Jin Y, Desai RJ, Liu J, et al. Factors associated with initial or subsequent choice of biologic disease-modifying antirheumatic drugs for treatment of rheumatoid arthritis. Arthritis Res Ther. 2017;19:159.
82. Bonafede MMK, McMorrow D, Proudfoot C, et al. Treatment persistence and healthcare costs among patients with rheumatoid arthritis after a change in targeted therapy. Am Health Drug Benefits. 2018;11:192-202.
83. US Food and Drug Administration. Biosimilars are safe, effective treatment options. www.fda.gov/drugs/developmentapprovalprocess/howdrugsaredevelopedandapproved/approvalapplications/therapeuticbiologicapplications/biosimilars/. Accessed November 9, 2018.
84. Genovese MC, Greenwald M, Codding C, et al. Peficitinib, a JAK inhibitor, in combination with limited conventional synthetic disease-modifying antirheumatic drugs in the treatment of moderate-to-severe rheumatoid arthritis. Arthritis Rheumatol. 2017;69:932-942.
85. Fleischmann RM, Wagner F, Kivitz AJ, et al. Safety, tolerability, and pharmacodynamics of ABT-122, a tumor necrosis factor- and interleukin-17-targeted dual variable domain immunoglobulin, in patients with rheumatoid arthritis. Arthritis Rheumatol. 2017;69:2283-2291.
86. Burmester GR, McInnes IB, Kremer JM, et al. Mavrilimumab, a fully human granulocyte-macrophage colony-stimulating factor receptor alpha monoclonal antibody: long-term safety and efficacy in patients with rheumatoid arthritis. Arthritis Rheumatol. 2018;70:679-689.
87. Huizinga TW, Batalov A, Stoilov R, et al. Phase 1b randomized, double-blind study of namilumab, an anti-granulocyte macrophage colony-stimulating factor monoclonal antibody, in mild-to-moderate rheumatoid arthritis. Arthritis Res Ther. 2017;19:53.
88. Stock T, Fleishaker D, Wang X, et al. Improved disease activity with fosdagrocorat (PF-04171327), a partial agonist of the glucocorticoid receptor, in patients with rheumatoid arthritis: a Phase 2 randomized study. Int J Rheum Dis. 2017;20:960-970.
89. Orencia [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2013.
90. Humira[package insert]. North Chicago, IL: AbbVie; 2012.
91. Kineret [package insert]. Stockholm, Sweden: Sobi; 2012.
92. Olumiant [package insert]. Indianapolis, IN: Lilly USA, LLC; 2018.
93. Cimzia [package insert]. Smyrna, GA: UCB, Inc; 2008.
94. Enbrel [package insert]. Thousand Oaks, CA: Immunex Corporation; 1998.
95. Simponi [package insert]. Horsham, PA: Janssen Biotech, Inc; 2009.
96. Remicade [package insert]. Horsham, PA: Janssen Biotech, Inc; 1998.
97. Rituxan [package insert]. South San Francisco, CA: Genetech, Inc; 1997.
98. Kevzara [package insert]. Bridgewater, NJ: Sanofi-Aventis US LLC; 2018.
99. Actemra [package insert]. South San Francisco, CA: Genentech, Inc; 2013.
100. Xeljanz [package insert]. New York, NY: Pfizer Inc; 2016.