User login
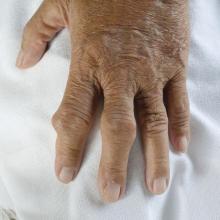
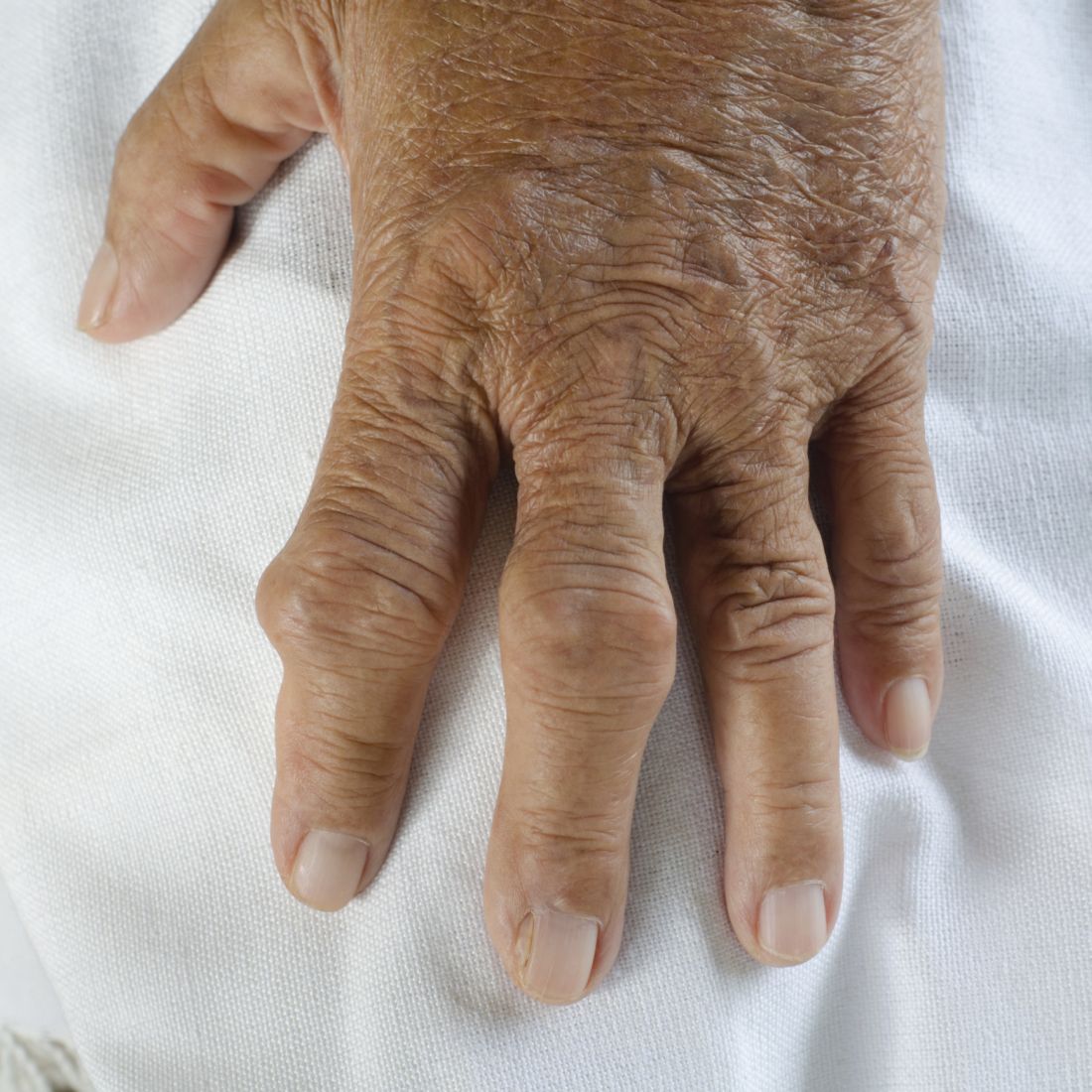
Physicians urged to write indications on drug scripts as methotrexate users face new barriers with SCOTUS decision
.
The Court’s 5-4 decision in Dobbs v. Jackson Women’s Health Organization, which halted abortion procedures across the country, also appears to be affecting certain drug regimens. Reports have emerged that pharmacies are denying access to methotrexate (MTX), a drug often used in patients with arthritis or cancer, as well as psoriasis and other skin diseases. In very high doses, MTX it is used to terminate an ectopic pregnancy after miscarriage. The drug can also lead to birth defects.
“It’s happening all over,” Donald Miller, PharmD, professor of pharmacy practice at North Dakota State University, Fargo, said in an interview. “Pharmacists are reluctant to dispense it, and rheumatologists are reluctant to prescribe it because they’re afraid of going to jail.”
Becky Schwartz, a patient who takes MTX for lupus, recently tweeted that her physician’s office stopped prescribing the drug because it is considered an abortifacient. “I had care that made my disabled life easier, and [the Supreme Court] took that from me,” Ms. Schwartz wrote.
Prior to the Supreme Court’s ruling, physicians were concerned about the impact an overturning of the 1973 law would have on patient access to MTX and other prescription medications with abortifacient properties. Doctors in general are becoming afraid of prescribing anything that’s a teratogen, said Dr. Miller.
MTX is used far more often for autoimmune disease than as an abortifacient, said rheumatologist Kristen Young, MD, clinical assistant professor at the University of Arizona College of Medicine, Phoenix. It’s a slippery slope if states reacting to the Supreme Court ruling start regulating oral abortifacients, she added. Specifically, this will have a significant impact on patients with rheumatic disease.
Texas pharmacies target two drugs
MTX denials have caught the attention of health care organizations. “Uncertainty in financial and criminal liability for health care professionals in certain state laws and regulations are possibly compromising continuity of care and access [to] medications proven to be safe and effective by the Food and Drug Administration for these indications,” warned the American Pharmacists Association (APhA) in a statement to this news organization.
The APhA said that it was monitoring this situation to assess the effect on patients and pharmacists.
The Arthritis Foundation was made aware of challenges from patients in accessing their MTX prescription for managing their arthritis and shared a statement on the Foundation’s website.
In Texas, pharmacists can refuse to fill scripts for misoprostol and MTX, a combination used for medical abortions. According to the foundation, “Already there are reports that people in Texas who miscarry or take methotrexate for arthritis [are] having trouble getting their prescriptions filled.”
MTX, approved by the FDA in 1985, “is the absolute cornerstone of rheumatoid arthritis. We cannot deny our patients this incredibly valuable drug,” said John Reveille, MD, vice-chair for the department of medicine at the University of Texas McGovern School of Medicine and a member of the Arthritis Foundation expert panel, in an interview.
“While it’s true that methotrexate can be lethal to the fetus, misoprostol is much more likely to cause a spontaneous abortion, and the combination is especially effective,” he said.
“If you look at Cochrane clinical studies, the dose of misoprostol contained in certain combinations with NSAIDs [nonsteroidal anti-inflammatory drugs] can induce spontaneous abortions. It’s surprising that pharmacists are targeting methotrexate, an essential drug in arthritis treatment, when there are medications available that do not have this benefit that can by themselves cause loss of the fetus, such as mifepristone,” added Dr. Reveille.
The Dobbs ruling could also affect the ability of oncologists to provide lifesaving cancer care, according to Jason Westin, MD, an oncologist at the University of Texas MD Anderson Cancer Center in the department of lymphoma and myeloma.
“We have heard of medications with multiple indications, such as methotrexate, not being dispensed by pharmacies due to confusion regarding the intended use and potential consequences for the health care team,” he said in an interview.
Conflicting laws pose challenges for physicians
In North Dakota, inconsistencies in several laws are making it difficult for physicians and pharmacists to make decisions. “Lots of confusion can result when people pass laws against abortion. There’s sometimes no insight into the ramifications of those laws,” said Dr. Miller.
North Dakota approved a trigger law several years ago that makes abortion illegal 30 days after an overturning of Roe. However, another law that regulates abortion conflicts with the trigger law. “Some of the language will need clarification in the next legislative session,” he said.
APhA and other pharmacy associations strongly favor not interfering with the doctor- or pharmacist-patient relationship. The law needs to defer to appropriate care between doctor and patient, said Dr. Miller. State pharmacy associations in North Dakota are working with legislatures to clarify any exceptions in the law, he added.
Arizona lawmakers are trying to reconcile two abortion laws on the books. One, based on an 1864 territorial law, deems abortion illegal. In addition, a newly approved law bans abortions after 15 weeks. The latter will go into effect in September 2022. In both laws, a risk to the mother’s life is the only exception for abortion, said Dr. Young.
Denials aren’t widespread
Not all doctors are seeing MTX denials, but they’re worried about the future. “To date, we have not encountered difficulty in obtaining methotrexate based upon state abortion restrictions but are concerned that this could occur and result in dangerous delays in care,” said Dr. Westin.
Dr. Reveille, who practices rheumatology in Houston, has not yet received any complaints from patients. Things may be different in more rural parts of Texas, where pharmacists could be denying prescriptions based on religious issues, he offered.
It’s a little soon to see what repercussions may result from the Supreme Court ruling and state actions, said Dr. Reveille. “In Texas, we’re a bit ahead of the tidal wave.”
Access problems also haven’t shown up at the university clinic where Dr. Young practices. “In Arizona, it’s unclear if there would be a legal basis to refuse a person methotrexate on the basis that it can be used as an abortifacient,” she said.
Specificity is key in writing Rx scripts
Physicians can make things easier for patients by writing the indication and dose for the drug on the prescription slip. For example, a 10-mg script for MTX is not going to be used for an abortion, said Dr. Miller.
Rheumatologists in Texas have been doing this for some time, even before the Supreme Court ruling, said Fehmida Zahabi, MD, FACR, president of the Society of Texas Association of Rheumatology. For MTX prescriptions in premenopausal women, “patients are told their doctor needs to call the pharmacist. In the small print, we are asked to give a diagnosis to make sure we aren’t using it to terminate pregnancies,” said Dr. Zahabi.
She further noted that if the diagnosis is already indicated on the script, pharmacies generally won’t give patients a hard time.
Patients can also ask their physicians for a letter of medical necessity that confirms a drug’s use for a specific medical condition.
Mail order is another option if a local pharmacy won’t fill a prescription, said Dr. Miller. “This is legal unless a state makes it illegal to send an abortifacient across state lines,” he added.
Many medications used in rheumatic diseases are harmful in pregnancy, and it’s important to routinely discuss pregnancy risk and planning in the rheumatology clinic, said Dr. Young. This should include a thorough discussion and referral for long-acting reversible contraception in most cases, she suggested.
Actions at the federal, state level
President Joe Biden recently signed an executive order prompting federal regulators to protect access to medication abortions, among other steps to safeguard access to reproductive services.
In a statement on Twitter, the American College of Rheumatology (ACR) said that it was “ ... following this issue closely to determine if rheumatology providers and patients are experiencing any widespread difficulty accessing methotrexate or if any initial disruptions are potentially temporary and due to the independent actions of pharmacists trying to figure out what is and isn’t allowed where they practice.”
ACR has assembled a task force of medical and policy experts to determine the best course of action for patients.
The Arthritis Foundation also continues to monitor the situation, encouraging patients to call its hotline, said Steven Schultz, director of state legislative affairs, in an interview.
“We are analyzing how medication abortion could cause confusion on the part of providers or pharmacists dispensing the medication and what this means for specific patients,” said Mr. Schultz. Through a survey, the foundation hopes to get a better idea of what’s going on in the states at a macro level.
This may take some time, as states go through a process of lawsuits, injunctions, or coming into session to do something that may affect access to MTX, said Mr. Schultz.
Being involved in local advocacy is more important than ever, stressed Dr. Young. “Additionally, being plugged into what the ACR and other advocacy groups are doing on the national level is helpful as well to know the status of these medication access issues.”
Rheumatologists have a unique voice in this discussion, she added. “We guide our patients to stability for a safe pregnancy, and even with careful planning, we see patients who become critically ill during pregnancy and require lifesaving treatment, which at times can mean an abortion is necessary.”
Oncologists also advocate for their patients on a regular basis to make sure they have access to the care they need, said Dr. Westin. This situation with Roe is no different, he added. “We will continue to use our unique expertise to advocate for policies that assure access to high-quality, evidence-based care – and to help our patients overcome barriers that may interfere.”
Dr. Reveille participated on an advisory board with Eli Lilly in October 2021.
A version of this article first appeared on Medscape.com.
.
The Court’s 5-4 decision in Dobbs v. Jackson Women’s Health Organization, which halted abortion procedures across the country, also appears to be affecting certain drug regimens. Reports have emerged that pharmacies are denying access to methotrexate (MTX), a drug often used in patients with arthritis or cancer, as well as psoriasis and other skin diseases. In very high doses, MTX it is used to terminate an ectopic pregnancy after miscarriage. The drug can also lead to birth defects.
“It’s happening all over,” Donald Miller, PharmD, professor of pharmacy practice at North Dakota State University, Fargo, said in an interview. “Pharmacists are reluctant to dispense it, and rheumatologists are reluctant to prescribe it because they’re afraid of going to jail.”
Becky Schwartz, a patient who takes MTX for lupus, recently tweeted that her physician’s office stopped prescribing the drug because it is considered an abortifacient. “I had care that made my disabled life easier, and [the Supreme Court] took that from me,” Ms. Schwartz wrote.
Prior to the Supreme Court’s ruling, physicians were concerned about the impact an overturning of the 1973 law would have on patient access to MTX and other prescription medications with abortifacient properties. Doctors in general are becoming afraid of prescribing anything that’s a teratogen, said Dr. Miller.
MTX is used far more often for autoimmune disease than as an abortifacient, said rheumatologist Kristen Young, MD, clinical assistant professor at the University of Arizona College of Medicine, Phoenix. It’s a slippery slope if states reacting to the Supreme Court ruling start regulating oral abortifacients, she added. Specifically, this will have a significant impact on patients with rheumatic disease.
Texas pharmacies target two drugs
MTX denials have caught the attention of health care organizations. “Uncertainty in financial and criminal liability for health care professionals in certain state laws and regulations are possibly compromising continuity of care and access [to] medications proven to be safe and effective by the Food and Drug Administration for these indications,” warned the American Pharmacists Association (APhA) in a statement to this news organization.
The APhA said that it was monitoring this situation to assess the effect on patients and pharmacists.
The Arthritis Foundation was made aware of challenges from patients in accessing their MTX prescription for managing their arthritis and shared a statement on the Foundation’s website.
In Texas, pharmacists can refuse to fill scripts for misoprostol and MTX, a combination used for medical abortions. According to the foundation, “Already there are reports that people in Texas who miscarry or take methotrexate for arthritis [are] having trouble getting their prescriptions filled.”
MTX, approved by the FDA in 1985, “is the absolute cornerstone of rheumatoid arthritis. We cannot deny our patients this incredibly valuable drug,” said John Reveille, MD, vice-chair for the department of medicine at the University of Texas McGovern School of Medicine and a member of the Arthritis Foundation expert panel, in an interview.
“While it’s true that methotrexate can be lethal to the fetus, misoprostol is much more likely to cause a spontaneous abortion, and the combination is especially effective,” he said.
“If you look at Cochrane clinical studies, the dose of misoprostol contained in certain combinations with NSAIDs [nonsteroidal anti-inflammatory drugs] can induce spontaneous abortions. It’s surprising that pharmacists are targeting methotrexate, an essential drug in arthritis treatment, when there are medications available that do not have this benefit that can by themselves cause loss of the fetus, such as mifepristone,” added Dr. Reveille.
The Dobbs ruling could also affect the ability of oncologists to provide lifesaving cancer care, according to Jason Westin, MD, an oncologist at the University of Texas MD Anderson Cancer Center in the department of lymphoma and myeloma.
“We have heard of medications with multiple indications, such as methotrexate, not being dispensed by pharmacies due to confusion regarding the intended use and potential consequences for the health care team,” he said in an interview.
Conflicting laws pose challenges for physicians
In North Dakota, inconsistencies in several laws are making it difficult for physicians and pharmacists to make decisions. “Lots of confusion can result when people pass laws against abortion. There’s sometimes no insight into the ramifications of those laws,” said Dr. Miller.
North Dakota approved a trigger law several years ago that makes abortion illegal 30 days after an overturning of Roe. However, another law that regulates abortion conflicts with the trigger law. “Some of the language will need clarification in the next legislative session,” he said.
APhA and other pharmacy associations strongly favor not interfering with the doctor- or pharmacist-patient relationship. The law needs to defer to appropriate care between doctor and patient, said Dr. Miller. State pharmacy associations in North Dakota are working with legislatures to clarify any exceptions in the law, he added.
Arizona lawmakers are trying to reconcile two abortion laws on the books. One, based on an 1864 territorial law, deems abortion illegal. In addition, a newly approved law bans abortions after 15 weeks. The latter will go into effect in September 2022. In both laws, a risk to the mother’s life is the only exception for abortion, said Dr. Young.
Denials aren’t widespread
Not all doctors are seeing MTX denials, but they’re worried about the future. “To date, we have not encountered difficulty in obtaining methotrexate based upon state abortion restrictions but are concerned that this could occur and result in dangerous delays in care,” said Dr. Westin.
Dr. Reveille, who practices rheumatology in Houston, has not yet received any complaints from patients. Things may be different in more rural parts of Texas, where pharmacists could be denying prescriptions based on religious issues, he offered.
It’s a little soon to see what repercussions may result from the Supreme Court ruling and state actions, said Dr. Reveille. “In Texas, we’re a bit ahead of the tidal wave.”
Access problems also haven’t shown up at the university clinic where Dr. Young practices. “In Arizona, it’s unclear if there would be a legal basis to refuse a person methotrexate on the basis that it can be used as an abortifacient,” she said.
Specificity is key in writing Rx scripts
Physicians can make things easier for patients by writing the indication and dose for the drug on the prescription slip. For example, a 10-mg script for MTX is not going to be used for an abortion, said Dr. Miller.
Rheumatologists in Texas have been doing this for some time, even before the Supreme Court ruling, said Fehmida Zahabi, MD, FACR, president of the Society of Texas Association of Rheumatology. For MTX prescriptions in premenopausal women, “patients are told their doctor needs to call the pharmacist. In the small print, we are asked to give a diagnosis to make sure we aren’t using it to terminate pregnancies,” said Dr. Zahabi.
She further noted that if the diagnosis is already indicated on the script, pharmacies generally won’t give patients a hard time.
Patients can also ask their physicians for a letter of medical necessity that confirms a drug’s use for a specific medical condition.
Mail order is another option if a local pharmacy won’t fill a prescription, said Dr. Miller. “This is legal unless a state makes it illegal to send an abortifacient across state lines,” he added.
Many medications used in rheumatic diseases are harmful in pregnancy, and it’s important to routinely discuss pregnancy risk and planning in the rheumatology clinic, said Dr. Young. This should include a thorough discussion and referral for long-acting reversible contraception in most cases, she suggested.
Actions at the federal, state level
President Joe Biden recently signed an executive order prompting federal regulators to protect access to medication abortions, among other steps to safeguard access to reproductive services.
In a statement on Twitter, the American College of Rheumatology (ACR) said that it was “ ... following this issue closely to determine if rheumatology providers and patients are experiencing any widespread difficulty accessing methotrexate or if any initial disruptions are potentially temporary and due to the independent actions of pharmacists trying to figure out what is and isn’t allowed where they practice.”
ACR has assembled a task force of medical and policy experts to determine the best course of action for patients.
The Arthritis Foundation also continues to monitor the situation, encouraging patients to call its hotline, said Steven Schultz, director of state legislative affairs, in an interview.
“We are analyzing how medication abortion could cause confusion on the part of providers or pharmacists dispensing the medication and what this means for specific patients,” said Mr. Schultz. Through a survey, the foundation hopes to get a better idea of what’s going on in the states at a macro level.
This may take some time, as states go through a process of lawsuits, injunctions, or coming into session to do something that may affect access to MTX, said Mr. Schultz.
Being involved in local advocacy is more important than ever, stressed Dr. Young. “Additionally, being plugged into what the ACR and other advocacy groups are doing on the national level is helpful as well to know the status of these medication access issues.”
Rheumatologists have a unique voice in this discussion, she added. “We guide our patients to stability for a safe pregnancy, and even with careful planning, we see patients who become critically ill during pregnancy and require lifesaving treatment, which at times can mean an abortion is necessary.”
Oncologists also advocate for their patients on a regular basis to make sure they have access to the care they need, said Dr. Westin. This situation with Roe is no different, he added. “We will continue to use our unique expertise to advocate for policies that assure access to high-quality, evidence-based care – and to help our patients overcome barriers that may interfere.”
Dr. Reveille participated on an advisory board with Eli Lilly in October 2021.
A version of this article first appeared on Medscape.com.
.
The Court’s 5-4 decision in Dobbs v. Jackson Women’s Health Organization, which halted abortion procedures across the country, also appears to be affecting certain drug regimens. Reports have emerged that pharmacies are denying access to methotrexate (MTX), a drug often used in patients with arthritis or cancer, as well as psoriasis and other skin diseases. In very high doses, MTX it is used to terminate an ectopic pregnancy after miscarriage. The drug can also lead to birth defects.
“It’s happening all over,” Donald Miller, PharmD, professor of pharmacy practice at North Dakota State University, Fargo, said in an interview. “Pharmacists are reluctant to dispense it, and rheumatologists are reluctant to prescribe it because they’re afraid of going to jail.”
Becky Schwartz, a patient who takes MTX for lupus, recently tweeted that her physician’s office stopped prescribing the drug because it is considered an abortifacient. “I had care that made my disabled life easier, and [the Supreme Court] took that from me,” Ms. Schwartz wrote.
Prior to the Supreme Court’s ruling, physicians were concerned about the impact an overturning of the 1973 law would have on patient access to MTX and other prescription medications with abortifacient properties. Doctors in general are becoming afraid of prescribing anything that’s a teratogen, said Dr. Miller.
MTX is used far more often for autoimmune disease than as an abortifacient, said rheumatologist Kristen Young, MD, clinical assistant professor at the University of Arizona College of Medicine, Phoenix. It’s a slippery slope if states reacting to the Supreme Court ruling start regulating oral abortifacients, she added. Specifically, this will have a significant impact on patients with rheumatic disease.
Texas pharmacies target two drugs
MTX denials have caught the attention of health care organizations. “Uncertainty in financial and criminal liability for health care professionals in certain state laws and regulations are possibly compromising continuity of care and access [to] medications proven to be safe and effective by the Food and Drug Administration for these indications,” warned the American Pharmacists Association (APhA) in a statement to this news organization.
The APhA said that it was monitoring this situation to assess the effect on patients and pharmacists.
The Arthritis Foundation was made aware of challenges from patients in accessing their MTX prescription for managing their arthritis and shared a statement on the Foundation’s website.
In Texas, pharmacists can refuse to fill scripts for misoprostol and MTX, a combination used for medical abortions. According to the foundation, “Already there are reports that people in Texas who miscarry or take methotrexate for arthritis [are] having trouble getting their prescriptions filled.”
MTX, approved by the FDA in 1985, “is the absolute cornerstone of rheumatoid arthritis. We cannot deny our patients this incredibly valuable drug,” said John Reveille, MD, vice-chair for the department of medicine at the University of Texas McGovern School of Medicine and a member of the Arthritis Foundation expert panel, in an interview.
“While it’s true that methotrexate can be lethal to the fetus, misoprostol is much more likely to cause a spontaneous abortion, and the combination is especially effective,” he said.
“If you look at Cochrane clinical studies, the dose of misoprostol contained in certain combinations with NSAIDs [nonsteroidal anti-inflammatory drugs] can induce spontaneous abortions. It’s surprising that pharmacists are targeting methotrexate, an essential drug in arthritis treatment, when there are medications available that do not have this benefit that can by themselves cause loss of the fetus, such as mifepristone,” added Dr. Reveille.
The Dobbs ruling could also affect the ability of oncologists to provide lifesaving cancer care, according to Jason Westin, MD, an oncologist at the University of Texas MD Anderson Cancer Center in the department of lymphoma and myeloma.
“We have heard of medications with multiple indications, such as methotrexate, not being dispensed by pharmacies due to confusion regarding the intended use and potential consequences for the health care team,” he said in an interview.
Conflicting laws pose challenges for physicians
In North Dakota, inconsistencies in several laws are making it difficult for physicians and pharmacists to make decisions. “Lots of confusion can result when people pass laws against abortion. There’s sometimes no insight into the ramifications of those laws,” said Dr. Miller.
North Dakota approved a trigger law several years ago that makes abortion illegal 30 days after an overturning of Roe. However, another law that regulates abortion conflicts with the trigger law. “Some of the language will need clarification in the next legislative session,” he said.
APhA and other pharmacy associations strongly favor not interfering with the doctor- or pharmacist-patient relationship. The law needs to defer to appropriate care between doctor and patient, said Dr. Miller. State pharmacy associations in North Dakota are working with legislatures to clarify any exceptions in the law, he added.
Arizona lawmakers are trying to reconcile two abortion laws on the books. One, based on an 1864 territorial law, deems abortion illegal. In addition, a newly approved law bans abortions after 15 weeks. The latter will go into effect in September 2022. In both laws, a risk to the mother’s life is the only exception for abortion, said Dr. Young.
Denials aren’t widespread
Not all doctors are seeing MTX denials, but they’re worried about the future. “To date, we have not encountered difficulty in obtaining methotrexate based upon state abortion restrictions but are concerned that this could occur and result in dangerous delays in care,” said Dr. Westin.
Dr. Reveille, who practices rheumatology in Houston, has not yet received any complaints from patients. Things may be different in more rural parts of Texas, where pharmacists could be denying prescriptions based on religious issues, he offered.
It’s a little soon to see what repercussions may result from the Supreme Court ruling and state actions, said Dr. Reveille. “In Texas, we’re a bit ahead of the tidal wave.”
Access problems also haven’t shown up at the university clinic where Dr. Young practices. “In Arizona, it’s unclear if there would be a legal basis to refuse a person methotrexate on the basis that it can be used as an abortifacient,” she said.
Specificity is key in writing Rx scripts
Physicians can make things easier for patients by writing the indication and dose for the drug on the prescription slip. For example, a 10-mg script for MTX is not going to be used for an abortion, said Dr. Miller.
Rheumatologists in Texas have been doing this for some time, even before the Supreme Court ruling, said Fehmida Zahabi, MD, FACR, president of the Society of Texas Association of Rheumatology. For MTX prescriptions in premenopausal women, “patients are told their doctor needs to call the pharmacist. In the small print, we are asked to give a diagnosis to make sure we aren’t using it to terminate pregnancies,” said Dr. Zahabi.
She further noted that if the diagnosis is already indicated on the script, pharmacies generally won’t give patients a hard time.
Patients can also ask their physicians for a letter of medical necessity that confirms a drug’s use for a specific medical condition.
Mail order is another option if a local pharmacy won’t fill a prescription, said Dr. Miller. “This is legal unless a state makes it illegal to send an abortifacient across state lines,” he added.
Many medications used in rheumatic diseases are harmful in pregnancy, and it’s important to routinely discuss pregnancy risk and planning in the rheumatology clinic, said Dr. Young. This should include a thorough discussion and referral for long-acting reversible contraception in most cases, she suggested.
Actions at the federal, state level
President Joe Biden recently signed an executive order prompting federal regulators to protect access to medication abortions, among other steps to safeguard access to reproductive services.
In a statement on Twitter, the American College of Rheumatology (ACR) said that it was “ ... following this issue closely to determine if rheumatology providers and patients are experiencing any widespread difficulty accessing methotrexate or if any initial disruptions are potentially temporary and due to the independent actions of pharmacists trying to figure out what is and isn’t allowed where they practice.”
ACR has assembled a task force of medical and policy experts to determine the best course of action for patients.
The Arthritis Foundation also continues to monitor the situation, encouraging patients to call its hotline, said Steven Schultz, director of state legislative affairs, in an interview.
“We are analyzing how medication abortion could cause confusion on the part of providers or pharmacists dispensing the medication and what this means for specific patients,” said Mr. Schultz. Through a survey, the foundation hopes to get a better idea of what’s going on in the states at a macro level.
This may take some time, as states go through a process of lawsuits, injunctions, or coming into session to do something that may affect access to MTX, said Mr. Schultz.
Being involved in local advocacy is more important than ever, stressed Dr. Young. “Additionally, being plugged into what the ACR and other advocacy groups are doing on the national level is helpful as well to know the status of these medication access issues.”
Rheumatologists have a unique voice in this discussion, she added. “We guide our patients to stability for a safe pregnancy, and even with careful planning, we see patients who become critically ill during pregnancy and require lifesaving treatment, which at times can mean an abortion is necessary.”
Oncologists also advocate for their patients on a regular basis to make sure they have access to the care they need, said Dr. Westin. This situation with Roe is no different, he added. “We will continue to use our unique expertise to advocate for policies that assure access to high-quality, evidence-based care – and to help our patients overcome barriers that may interfere.”
Dr. Reveille participated on an advisory board with Eli Lilly in October 2021.
A version of this article first appeared on Medscape.com.
FDA approves combination pegloticase and methotrexate for refractory gout
Pegloticase, which has been available for 12 years, is a pegylated uric acid specific enzyme that lowers sUA by converting it to allantoin.
Though pegloticase is effective in treating chronic gout in patients refractory to conventional treatment, approximately 92% of patients develop antibodies against the drug, resulting in reduced efficacy.
Based on the immunomodulatory effects of methotrexate, researchers of the randomized, placebo-controlled MIRROR trial sought to determine whether combination treatment of pegloticase with methotrexate (multiple brands) would prevent the development of anti-drug antibodies.
Findings from the phase 4 trial found that co-administration of pegloticase and methotrexate reduced the formation of new anti-PEG antibodies. In the group receiving methotrexate and pegloticase, 23.2% (22 out of 95) of patients had an increase in anti-PEG antibodies, compared with 50% (24 of 48) in the pegloticase plus placebo group, according to a recent company press release.
Nearly three-quarters (71%) of participants in the group pretreated with methotrexate, followed by combination pegloticase-methotrexate, had sUA levels that dopped to below 6 mg/dL during the 52-week study. By comparison, 38.5% of participants in the pegloticase and placebo group reached the endpoint. Though gout flare occurred in both groups, methotrexate did not appear to increase the risk for adverse events or gout flare.
The study, led by John Botson, MD, RPh, CCD, a rheumatologist in Anchorage, Alaska, concluded that these measurements demonstrated a significant improvement from traditional pegloticase-only treatment of gout. “This trial confirms not only improved efficacy but improved safety in patients treated with pegloticase in combination with methotrexate 15 mg orally once weekly,” Dr. Botson said last month in an interview with this news organization.
The study was funded by Horizon. Dr. Botson reports receiving research support from Horizon and Radius Health and speaker fees from AbbVie, Amgen, Aurinia, ChemoCentryx, Horizon, Eli Lilly, and Novartis.
A version of this article first appeared on Medscape.com.
Pegloticase, which has been available for 12 years, is a pegylated uric acid specific enzyme that lowers sUA by converting it to allantoin.
Though pegloticase is effective in treating chronic gout in patients refractory to conventional treatment, approximately 92% of patients develop antibodies against the drug, resulting in reduced efficacy.
Based on the immunomodulatory effects of methotrexate, researchers of the randomized, placebo-controlled MIRROR trial sought to determine whether combination treatment of pegloticase with methotrexate (multiple brands) would prevent the development of anti-drug antibodies.
Findings from the phase 4 trial found that co-administration of pegloticase and methotrexate reduced the formation of new anti-PEG antibodies. In the group receiving methotrexate and pegloticase, 23.2% (22 out of 95) of patients had an increase in anti-PEG antibodies, compared with 50% (24 of 48) in the pegloticase plus placebo group, according to a recent company press release.
Nearly three-quarters (71%) of participants in the group pretreated with methotrexate, followed by combination pegloticase-methotrexate, had sUA levels that dopped to below 6 mg/dL during the 52-week study. By comparison, 38.5% of participants in the pegloticase and placebo group reached the endpoint. Though gout flare occurred in both groups, methotrexate did not appear to increase the risk for adverse events or gout flare.
The study, led by John Botson, MD, RPh, CCD, a rheumatologist in Anchorage, Alaska, concluded that these measurements demonstrated a significant improvement from traditional pegloticase-only treatment of gout. “This trial confirms not only improved efficacy but improved safety in patients treated with pegloticase in combination with methotrexate 15 mg orally once weekly,” Dr. Botson said last month in an interview with this news organization.
The study was funded by Horizon. Dr. Botson reports receiving research support from Horizon and Radius Health and speaker fees from AbbVie, Amgen, Aurinia, ChemoCentryx, Horizon, Eli Lilly, and Novartis.
A version of this article first appeared on Medscape.com.
Pegloticase, which has been available for 12 years, is a pegylated uric acid specific enzyme that lowers sUA by converting it to allantoin.
Though pegloticase is effective in treating chronic gout in patients refractory to conventional treatment, approximately 92% of patients develop antibodies against the drug, resulting in reduced efficacy.
Based on the immunomodulatory effects of methotrexate, researchers of the randomized, placebo-controlled MIRROR trial sought to determine whether combination treatment of pegloticase with methotrexate (multiple brands) would prevent the development of anti-drug antibodies.
Findings from the phase 4 trial found that co-administration of pegloticase and methotrexate reduced the formation of new anti-PEG antibodies. In the group receiving methotrexate and pegloticase, 23.2% (22 out of 95) of patients had an increase in anti-PEG antibodies, compared with 50% (24 of 48) in the pegloticase plus placebo group, according to a recent company press release.
Nearly three-quarters (71%) of participants in the group pretreated with methotrexate, followed by combination pegloticase-methotrexate, had sUA levels that dopped to below 6 mg/dL during the 52-week study. By comparison, 38.5% of participants in the pegloticase and placebo group reached the endpoint. Though gout flare occurred in both groups, methotrexate did not appear to increase the risk for adverse events or gout flare.
The study, led by John Botson, MD, RPh, CCD, a rheumatologist in Anchorage, Alaska, concluded that these measurements demonstrated a significant improvement from traditional pegloticase-only treatment of gout. “This trial confirms not only improved efficacy but improved safety in patients treated with pegloticase in combination with methotrexate 15 mg orally once weekly,” Dr. Botson said last month in an interview with this news organization.
The study was funded by Horizon. Dr. Botson reports receiving research support from Horizon and Radius Health and speaker fees from AbbVie, Amgen, Aurinia, ChemoCentryx, Horizon, Eli Lilly, and Novartis.
A version of this article first appeared on Medscape.com.
Zoster vaccination does not appear to increase flare risk in patients with immune-mediated inflammatory disease
, according to research published in Arthritis & Rheumatology.
The authors of the study noted that individuals with IMIDs are at increased risk for herpes zoster and related complications, including postherpetic neuralgia, and that vaccination has been recommended for certain groups of patients with rheumatoid arthritis, inflammatory bowel disease, and psoriasis, by the American College of Rheumatology and other professional organizations for individuals aged 50 and older.
The study investigators used medical claims from IBM MarketScan, which provided data on patients aged 50-64 years, and data from the Centers for Medicare and Medicaid Services’ Medicare on patients aged 65 and older.
They defined presumed flares in three ways: hospitalization/emergency department visits for IMIDs, steroid treatment with a short-acting oral glucocorticoid, or treatment with a parenteral glucocorticoid injection. The investigators conducted a self-controlled case series (SCCS) analysis to examine any temporal link between the RZV and disease flares.
Among enrollees with IMIDs, 14.8% of the 55,654 patients in the MarketScan database and 43.2% of the 160,545 patients in the Medicare database received at least one dose of RZV during 2018-2019. The two-dose series completion within 6 months was 76.6% in the MarketScan group (age range, 50-64 years) and 85.4% among Medicare enrollees (age range, 65 years and older). In the SCCS analysis, 10% and 13% of patients developed flares in the control group as compared to 9%, and 11%-12% in the risk window following one or two doses of RZV among MarketScan and Medicare enrollees, respectively.
Based on these findings, the investigators concluded there was no statistically significant increase in flares subsequent to RZV administration for any IMID in either patients aged 50-64 years or patients aged 65 years and older following the first dose or second dose.
Nilanjana Bose, MD, a rheumatologist with Lonestar Rheumatology, Houston, Texas, who was not involved with the study, said that the research addresses a topic where there is uneasiness, namely vaccination in patients with IMIDs.
“Anytime you are vaccinating a patient with an autoimmune disease, especially one on a biologic, you always worry about the risk of flares,” said Dr. Bose. “Any time you tamper with the immune system, there is a risk of flares.”
The study serves as a clarification for the primary care setting, said Dr. Bose. “A lot of the time, the shingles vaccine is administered not by rheumatology but by primary care or through the pharmacy,” she said. “This study puts them [primary care physicians] at ease.”
Findings from the study reflect that most RZV vaccinations were administered in pharmacies.
One of the weaknesses of the study is that the investigators did not include patients younger than 50 years old, said Dr. Bose. “It would have been nice if they could have looked at younger patients,” she said. “We try to vaccinate all our [immunocompromised] adult patients, even the younger ones, because they are also at risk for shingles.”
Given that there are increasing options of medical therapies in rheumatology that are immunomodulatory, the subject of vaccination for patients is often one of discussion, added Dr. Bose.
Arthur Kavanaugh, MD, professor of medicine, University of California San Diego (UCSD), La Jolla, Calif., and director of the Center for Innovative Therapy in the UCSD Division of Rheumatology, Allergy, and Immunology, told this news organization that a strength of the study is its large numbers of patients but noted the shortcoming of using claims data. “Claims data has inherent limitations, such as the lack of detailed granular data on the patients,” wrote Dr. Kavanaugh, who was not involved with the study. He described this investigation as “really about the first evidence that I am aware of addressing this issue.”
No funding source was listed. One author disclosed having received research grants and consulting fees received from Pfizer and GSK for unrelated work; the other authors had no disclosures. Dr. Bose and Dr. Kavanaugh had no relevant disclosures.
, according to research published in Arthritis & Rheumatology.
The authors of the study noted that individuals with IMIDs are at increased risk for herpes zoster and related complications, including postherpetic neuralgia, and that vaccination has been recommended for certain groups of patients with rheumatoid arthritis, inflammatory bowel disease, and psoriasis, by the American College of Rheumatology and other professional organizations for individuals aged 50 and older.
The study investigators used medical claims from IBM MarketScan, which provided data on patients aged 50-64 years, and data from the Centers for Medicare and Medicaid Services’ Medicare on patients aged 65 and older.
They defined presumed flares in three ways: hospitalization/emergency department visits for IMIDs, steroid treatment with a short-acting oral glucocorticoid, or treatment with a parenteral glucocorticoid injection. The investigators conducted a self-controlled case series (SCCS) analysis to examine any temporal link between the RZV and disease flares.
Among enrollees with IMIDs, 14.8% of the 55,654 patients in the MarketScan database and 43.2% of the 160,545 patients in the Medicare database received at least one dose of RZV during 2018-2019. The two-dose series completion within 6 months was 76.6% in the MarketScan group (age range, 50-64 years) and 85.4% among Medicare enrollees (age range, 65 years and older). In the SCCS analysis, 10% and 13% of patients developed flares in the control group as compared to 9%, and 11%-12% in the risk window following one or two doses of RZV among MarketScan and Medicare enrollees, respectively.
Based on these findings, the investigators concluded there was no statistically significant increase in flares subsequent to RZV administration for any IMID in either patients aged 50-64 years or patients aged 65 years and older following the first dose or second dose.
Nilanjana Bose, MD, a rheumatologist with Lonestar Rheumatology, Houston, Texas, who was not involved with the study, said that the research addresses a topic where there is uneasiness, namely vaccination in patients with IMIDs.
“Anytime you are vaccinating a patient with an autoimmune disease, especially one on a biologic, you always worry about the risk of flares,” said Dr. Bose. “Any time you tamper with the immune system, there is a risk of flares.”
The study serves as a clarification for the primary care setting, said Dr. Bose. “A lot of the time, the shingles vaccine is administered not by rheumatology but by primary care or through the pharmacy,” she said. “This study puts them [primary care physicians] at ease.”
Findings from the study reflect that most RZV vaccinations were administered in pharmacies.
One of the weaknesses of the study is that the investigators did not include patients younger than 50 years old, said Dr. Bose. “It would have been nice if they could have looked at younger patients,” she said. “We try to vaccinate all our [immunocompromised] adult patients, even the younger ones, because they are also at risk for shingles.”
Given that there are increasing options of medical therapies in rheumatology that are immunomodulatory, the subject of vaccination for patients is often one of discussion, added Dr. Bose.
Arthur Kavanaugh, MD, professor of medicine, University of California San Diego (UCSD), La Jolla, Calif., and director of the Center for Innovative Therapy in the UCSD Division of Rheumatology, Allergy, and Immunology, told this news organization that a strength of the study is its large numbers of patients but noted the shortcoming of using claims data. “Claims data has inherent limitations, such as the lack of detailed granular data on the patients,” wrote Dr. Kavanaugh, who was not involved with the study. He described this investigation as “really about the first evidence that I am aware of addressing this issue.”
No funding source was listed. One author disclosed having received research grants and consulting fees received from Pfizer and GSK for unrelated work; the other authors had no disclosures. Dr. Bose and Dr. Kavanaugh had no relevant disclosures.
, according to research published in Arthritis & Rheumatology.
The authors of the study noted that individuals with IMIDs are at increased risk for herpes zoster and related complications, including postherpetic neuralgia, and that vaccination has been recommended for certain groups of patients with rheumatoid arthritis, inflammatory bowel disease, and psoriasis, by the American College of Rheumatology and other professional organizations for individuals aged 50 and older.
The study investigators used medical claims from IBM MarketScan, which provided data on patients aged 50-64 years, and data from the Centers for Medicare and Medicaid Services’ Medicare on patients aged 65 and older.
They defined presumed flares in three ways: hospitalization/emergency department visits for IMIDs, steroid treatment with a short-acting oral glucocorticoid, or treatment with a parenteral glucocorticoid injection. The investigators conducted a self-controlled case series (SCCS) analysis to examine any temporal link between the RZV and disease flares.
Among enrollees with IMIDs, 14.8% of the 55,654 patients in the MarketScan database and 43.2% of the 160,545 patients in the Medicare database received at least one dose of RZV during 2018-2019. The two-dose series completion within 6 months was 76.6% in the MarketScan group (age range, 50-64 years) and 85.4% among Medicare enrollees (age range, 65 years and older). In the SCCS analysis, 10% and 13% of patients developed flares in the control group as compared to 9%, and 11%-12% in the risk window following one or two doses of RZV among MarketScan and Medicare enrollees, respectively.
Based on these findings, the investigators concluded there was no statistically significant increase in flares subsequent to RZV administration for any IMID in either patients aged 50-64 years or patients aged 65 years and older following the first dose or second dose.
Nilanjana Bose, MD, a rheumatologist with Lonestar Rheumatology, Houston, Texas, who was not involved with the study, said that the research addresses a topic where there is uneasiness, namely vaccination in patients with IMIDs.
“Anytime you are vaccinating a patient with an autoimmune disease, especially one on a biologic, you always worry about the risk of flares,” said Dr. Bose. “Any time you tamper with the immune system, there is a risk of flares.”
The study serves as a clarification for the primary care setting, said Dr. Bose. “A lot of the time, the shingles vaccine is administered not by rheumatology but by primary care or through the pharmacy,” she said. “This study puts them [primary care physicians] at ease.”
Findings from the study reflect that most RZV vaccinations were administered in pharmacies.
One of the weaknesses of the study is that the investigators did not include patients younger than 50 years old, said Dr. Bose. “It would have been nice if they could have looked at younger patients,” she said. “We try to vaccinate all our [immunocompromised] adult patients, even the younger ones, because they are also at risk for shingles.”
Given that there are increasing options of medical therapies in rheumatology that are immunomodulatory, the subject of vaccination for patients is often one of discussion, added Dr. Bose.
Arthur Kavanaugh, MD, professor of medicine, University of California San Diego (UCSD), La Jolla, Calif., and director of the Center for Innovative Therapy in the UCSD Division of Rheumatology, Allergy, and Immunology, told this news organization that a strength of the study is its large numbers of patients but noted the shortcoming of using claims data. “Claims data has inherent limitations, such as the lack of detailed granular data on the patients,” wrote Dr. Kavanaugh, who was not involved with the study. He described this investigation as “really about the first evidence that I am aware of addressing this issue.”
No funding source was listed. One author disclosed having received research grants and consulting fees received from Pfizer and GSK for unrelated work; the other authors had no disclosures. Dr. Bose and Dr. Kavanaugh had no relevant disclosures.
Large study reaffirms rare risk of TNF inhibitor–induced psoriasis in patients with RA, IBD
according to a new study published in JAMA Dermatology.
Despite this finding, the authors of the large Danish nationwide cohort study noted that TNFi-induced psoriasis is still a rare adverse event. “Practitioners and patients should be aware and observant of the potential for TNFi-associated psoriasis during TNFi treatment but keep in mind that the absolute risk appears to be low,” David Thein, MB, of the department of dermatology at Bispebjerg Hospital, University of Copenhagen, and colleagues wrote in the study.
They analyzed 109,085 patients with RA and IBD enrolled in Danish national registries between 1995 and 2018 without a previous diagnosis of psoriasis, who received either TNFi (20,910 patients) or conventional treatments (108,024 patients) and were followed for 5 years. They were a mean of 50 years old when they started treatment, 62% were women, with 87.8% of patients in the TNFi group receiving prior conventional therapy and 1% of patients in the conventional therapy group receiving prior TNFi treatment.
The investigators assessed the risk of developing any psoriasis, nonpustular psoriasis, and pustular psoriasis in the two groups using ICD-10 codes as well as a record of two consecutive prescriptions for topical vitamin D analogs.
Overall, 1,471 patients (1.4%) developed psoriasis of any type; 1,332 had non-pustular psoriasis, 127 had palmoplantar pustulosis, and 12 had generalized pustulosis.
The incidence rate of developing any psoriasis was 3.0 per 1,000 patient-years (95% confidence interval, 2.9-3.2) for patients receiving conventional therapy and 7.8 per 1,000 patient-years (95% CI, 7.5-8.9) for patients receiving TNFi treatment. Compared with conventional treatment, the risk of developing nonpustular psoriasis was twofold higher among patients receiving TNFi treatment (hazard ratio, 2.12; 95% CI, 1.87-2.40; P < .001). The risk of developing pustular psoriasis was more than sixfold higher among those on a TNFi (HR, 6.50; 95% CI, 4.60-9.23; P < .001).
Dr. Thein and colleagues estimated that the exposure needed to harm 1 additional patient was 241 patient-years for any psoriasis type, 342 patient-years for nonpustular psoriasis, and 909 patient-years for pustular psoriasis, with an estimated absolute risk difference of 5 per 1,000 patient-years.
Best evidence to date on risk
Asked to comment on the study findings, Anthony Fernandez, MD, PhD, director of medical dermatology at the Cleveland Clinic, said that he applauded the researchers for performing this well-designed study to determine the risk of TNF inhibitor–induced psoriasis in patients with RA and IBD.
The strengths of the study include excluding patients with a history of psoriasis to rule out disease recurrence and having a large comparator group of patients with IBD and RA who were taking medications other than TNF inhibitors, while one limitation was the potential accuracy of the ICD-10 codes used as the basis for diagnosing psoriasis. “It’s probably closer to the truth of what the true risk is compared to studies done in the past,” he said in an interview.
Dr. Fernandez noted that the results aren’t likely to change how dermatologists, rheumatologists, or gastroenterologists practice, but the message to stay the course in initially treating TNFi-induced psoriasis also holds value. “We don’t need to change anything in our clinical practice when it comes to TNF-alpha inhibitors.”
For patients with RA or IBD who develop TNFi-induced psoriasis with disease that is well controlled with TNFi treatment, keeping them on that treatment is a priority, Dr. Fernandez explained. “The first and foremost goal is, if the TNF inhibitor is working very well to control the disease that it was prescribed for, then you exhaust your efforts to try to control the psoriasis and allow those patients to stay on the TNF inhibitor.”
In his experience, most patients with RA and IBD who develop TNFi-induced psoriasis are controlled with topical medications. Switching to another TNFi is not recommended, he noted, as patients are “likely to have that reaction with any TNF inhibitor.”
However, Dr. Fernandez said that won’t be an option for all patients with RA and IBD. “In some patients you do simply have to stop the TNF inhibitor” and try an alternative treatment with a different mechanism of action.
The cause of TNFi-induced psoriasis is still not well understood. “There certainly is evidence to support that interferon alpha production by plasmacytoid dendritic cells is playing some role in this phenomenon,” but there is “more to the story” and unanswered questions remain, Dr. Fernandez said.
What’s most interesting about this phenomenon, he added, is that “patients can develop it at any time when exposed to a TNF inhibitor.” For instance, most patients develop drug reactions within 2-3 weeks of starting a treatment, but TNFi-induced psoriasis can appear after a single dose or several years after initiating treatment.
“Why so few patients, and why is there such variability in terms of how long they’re on the TNF inhibitor before the reaction occurs?” he asked. “That really points to ... some other trigger besides exposure to the TNF inhibitor needed for the initiation of this reaction.”
He noted that it would be valuable to identify triggers – or the most likely triggers – which would be challenging, but could “potentially impact clinical practice.”
The authors reported personal and institutional relationships in the form of personal and institutional research grants, honoraria, personal fees, investigator fees paid to university, consultancies, and speaker’s bureau positions for a variety of pharmaceutical companies, data companies, hospitals, and foundations. Dr. Fernandez reported he has nonbranded speaking, consulting, and research relationships with AbbVie and Novartis; and is a consultant for UCB, Bristol-Myers Squibb, and Boehringer Ingelheim on related products.
according to a new study published in JAMA Dermatology.
Despite this finding, the authors of the large Danish nationwide cohort study noted that TNFi-induced psoriasis is still a rare adverse event. “Practitioners and patients should be aware and observant of the potential for TNFi-associated psoriasis during TNFi treatment but keep in mind that the absolute risk appears to be low,” David Thein, MB, of the department of dermatology at Bispebjerg Hospital, University of Copenhagen, and colleagues wrote in the study.
They analyzed 109,085 patients with RA and IBD enrolled in Danish national registries between 1995 and 2018 without a previous diagnosis of psoriasis, who received either TNFi (20,910 patients) or conventional treatments (108,024 patients) and were followed for 5 years. They were a mean of 50 years old when they started treatment, 62% were women, with 87.8% of patients in the TNFi group receiving prior conventional therapy and 1% of patients in the conventional therapy group receiving prior TNFi treatment.
The investigators assessed the risk of developing any psoriasis, nonpustular psoriasis, and pustular psoriasis in the two groups using ICD-10 codes as well as a record of two consecutive prescriptions for topical vitamin D analogs.
Overall, 1,471 patients (1.4%) developed psoriasis of any type; 1,332 had non-pustular psoriasis, 127 had palmoplantar pustulosis, and 12 had generalized pustulosis.
The incidence rate of developing any psoriasis was 3.0 per 1,000 patient-years (95% confidence interval, 2.9-3.2) for patients receiving conventional therapy and 7.8 per 1,000 patient-years (95% CI, 7.5-8.9) for patients receiving TNFi treatment. Compared with conventional treatment, the risk of developing nonpustular psoriasis was twofold higher among patients receiving TNFi treatment (hazard ratio, 2.12; 95% CI, 1.87-2.40; P < .001). The risk of developing pustular psoriasis was more than sixfold higher among those on a TNFi (HR, 6.50; 95% CI, 4.60-9.23; P < .001).
Dr. Thein and colleagues estimated that the exposure needed to harm 1 additional patient was 241 patient-years for any psoriasis type, 342 patient-years for nonpustular psoriasis, and 909 patient-years for pustular psoriasis, with an estimated absolute risk difference of 5 per 1,000 patient-years.
Best evidence to date on risk
Asked to comment on the study findings, Anthony Fernandez, MD, PhD, director of medical dermatology at the Cleveland Clinic, said that he applauded the researchers for performing this well-designed study to determine the risk of TNF inhibitor–induced psoriasis in patients with RA and IBD.
The strengths of the study include excluding patients with a history of psoriasis to rule out disease recurrence and having a large comparator group of patients with IBD and RA who were taking medications other than TNF inhibitors, while one limitation was the potential accuracy of the ICD-10 codes used as the basis for diagnosing psoriasis. “It’s probably closer to the truth of what the true risk is compared to studies done in the past,” he said in an interview.
Dr. Fernandez noted that the results aren’t likely to change how dermatologists, rheumatologists, or gastroenterologists practice, but the message to stay the course in initially treating TNFi-induced psoriasis also holds value. “We don’t need to change anything in our clinical practice when it comes to TNF-alpha inhibitors.”
For patients with RA or IBD who develop TNFi-induced psoriasis with disease that is well controlled with TNFi treatment, keeping them on that treatment is a priority, Dr. Fernandez explained. “The first and foremost goal is, if the TNF inhibitor is working very well to control the disease that it was prescribed for, then you exhaust your efforts to try to control the psoriasis and allow those patients to stay on the TNF inhibitor.”
In his experience, most patients with RA and IBD who develop TNFi-induced psoriasis are controlled with topical medications. Switching to another TNFi is not recommended, he noted, as patients are “likely to have that reaction with any TNF inhibitor.”
However, Dr. Fernandez said that won’t be an option for all patients with RA and IBD. “In some patients you do simply have to stop the TNF inhibitor” and try an alternative treatment with a different mechanism of action.
The cause of TNFi-induced psoriasis is still not well understood. “There certainly is evidence to support that interferon alpha production by plasmacytoid dendritic cells is playing some role in this phenomenon,” but there is “more to the story” and unanswered questions remain, Dr. Fernandez said.
What’s most interesting about this phenomenon, he added, is that “patients can develop it at any time when exposed to a TNF inhibitor.” For instance, most patients develop drug reactions within 2-3 weeks of starting a treatment, but TNFi-induced psoriasis can appear after a single dose or several years after initiating treatment.
“Why so few patients, and why is there such variability in terms of how long they’re on the TNF inhibitor before the reaction occurs?” he asked. “That really points to ... some other trigger besides exposure to the TNF inhibitor needed for the initiation of this reaction.”
He noted that it would be valuable to identify triggers – or the most likely triggers – which would be challenging, but could “potentially impact clinical practice.”
The authors reported personal and institutional relationships in the form of personal and institutional research grants, honoraria, personal fees, investigator fees paid to university, consultancies, and speaker’s bureau positions for a variety of pharmaceutical companies, data companies, hospitals, and foundations. Dr. Fernandez reported he has nonbranded speaking, consulting, and research relationships with AbbVie and Novartis; and is a consultant for UCB, Bristol-Myers Squibb, and Boehringer Ingelheim on related products.
according to a new study published in JAMA Dermatology.
Despite this finding, the authors of the large Danish nationwide cohort study noted that TNFi-induced psoriasis is still a rare adverse event. “Practitioners and patients should be aware and observant of the potential for TNFi-associated psoriasis during TNFi treatment but keep in mind that the absolute risk appears to be low,” David Thein, MB, of the department of dermatology at Bispebjerg Hospital, University of Copenhagen, and colleagues wrote in the study.
They analyzed 109,085 patients with RA and IBD enrolled in Danish national registries between 1995 and 2018 without a previous diagnosis of psoriasis, who received either TNFi (20,910 patients) or conventional treatments (108,024 patients) and were followed for 5 years. They were a mean of 50 years old when they started treatment, 62% were women, with 87.8% of patients in the TNFi group receiving prior conventional therapy and 1% of patients in the conventional therapy group receiving prior TNFi treatment.
The investigators assessed the risk of developing any psoriasis, nonpustular psoriasis, and pustular psoriasis in the two groups using ICD-10 codes as well as a record of two consecutive prescriptions for topical vitamin D analogs.
Overall, 1,471 patients (1.4%) developed psoriasis of any type; 1,332 had non-pustular psoriasis, 127 had palmoplantar pustulosis, and 12 had generalized pustulosis.
The incidence rate of developing any psoriasis was 3.0 per 1,000 patient-years (95% confidence interval, 2.9-3.2) for patients receiving conventional therapy and 7.8 per 1,000 patient-years (95% CI, 7.5-8.9) for patients receiving TNFi treatment. Compared with conventional treatment, the risk of developing nonpustular psoriasis was twofold higher among patients receiving TNFi treatment (hazard ratio, 2.12; 95% CI, 1.87-2.40; P < .001). The risk of developing pustular psoriasis was more than sixfold higher among those on a TNFi (HR, 6.50; 95% CI, 4.60-9.23; P < .001).
Dr. Thein and colleagues estimated that the exposure needed to harm 1 additional patient was 241 patient-years for any psoriasis type, 342 patient-years for nonpustular psoriasis, and 909 patient-years for pustular psoriasis, with an estimated absolute risk difference of 5 per 1,000 patient-years.
Best evidence to date on risk
Asked to comment on the study findings, Anthony Fernandez, MD, PhD, director of medical dermatology at the Cleveland Clinic, said that he applauded the researchers for performing this well-designed study to determine the risk of TNF inhibitor–induced psoriasis in patients with RA and IBD.
The strengths of the study include excluding patients with a history of psoriasis to rule out disease recurrence and having a large comparator group of patients with IBD and RA who were taking medications other than TNF inhibitors, while one limitation was the potential accuracy of the ICD-10 codes used as the basis for diagnosing psoriasis. “It’s probably closer to the truth of what the true risk is compared to studies done in the past,” he said in an interview.
Dr. Fernandez noted that the results aren’t likely to change how dermatologists, rheumatologists, or gastroenterologists practice, but the message to stay the course in initially treating TNFi-induced psoriasis also holds value. “We don’t need to change anything in our clinical practice when it comes to TNF-alpha inhibitors.”
For patients with RA or IBD who develop TNFi-induced psoriasis with disease that is well controlled with TNFi treatment, keeping them on that treatment is a priority, Dr. Fernandez explained. “The first and foremost goal is, if the TNF inhibitor is working very well to control the disease that it was prescribed for, then you exhaust your efforts to try to control the psoriasis and allow those patients to stay on the TNF inhibitor.”
In his experience, most patients with RA and IBD who develop TNFi-induced psoriasis are controlled with topical medications. Switching to another TNFi is not recommended, he noted, as patients are “likely to have that reaction with any TNF inhibitor.”
However, Dr. Fernandez said that won’t be an option for all patients with RA and IBD. “In some patients you do simply have to stop the TNF inhibitor” and try an alternative treatment with a different mechanism of action.
The cause of TNFi-induced psoriasis is still not well understood. “There certainly is evidence to support that interferon alpha production by plasmacytoid dendritic cells is playing some role in this phenomenon,” but there is “more to the story” and unanswered questions remain, Dr. Fernandez said.
What’s most interesting about this phenomenon, he added, is that “patients can develop it at any time when exposed to a TNF inhibitor.” For instance, most patients develop drug reactions within 2-3 weeks of starting a treatment, but TNFi-induced psoriasis can appear after a single dose or several years after initiating treatment.
“Why so few patients, and why is there such variability in terms of how long they’re on the TNF inhibitor before the reaction occurs?” he asked. “That really points to ... some other trigger besides exposure to the TNF inhibitor needed for the initiation of this reaction.”
He noted that it would be valuable to identify triggers – or the most likely triggers – which would be challenging, but could “potentially impact clinical practice.”
The authors reported personal and institutional relationships in the form of personal and institutional research grants, honoraria, personal fees, investigator fees paid to university, consultancies, and speaker’s bureau positions for a variety of pharmaceutical companies, data companies, hospitals, and foundations. Dr. Fernandez reported he has nonbranded speaking, consulting, and research relationships with AbbVie and Novartis; and is a consultant for UCB, Bristol-Myers Squibb, and Boehringer Ingelheim on related products.
FROM JAMA DERMATOLOGY
Hydroxychloroquine risk found in some older patients with RA
Hydroxychloroquine should be initiated with caution in older patients with rheumatoid arthritis who also have heart failure or are at risk for it, say the authors of a study suggesting that the drug could increase their risk for major adverse cardiovascular events (MACE), compared with methotrexate.
A cohort study published online in the Journal of the American College of Cardiology looked at outcomes in 54,462 patients with RA aged 65 years or older and not previously treated with disease-modifying antirheumatic drugs. Half were initiated on methotrexate and half on hydroxychloroquine, making 27,231 propensity-matched pairs.
Across the entire cohort, hydroxychloroquine was not associated with a higher risk for sudden cardiac arrest, ventricular arrhythmia, or MACE, compared with methotrexate. When broken down into individual cardiovascular events, the data suggested a statistically significant 17% increase in the risk for cardiovascular mortality and 10% increase in all-cause mortality with hydroxychloroquine, although there were no differences in the risks for myocardial infarction or stroke.
However, a subgroup analysis revealed a significant 30% increase in the risk for MACE among patients starting hydroxychloroquine who also had a history of heart failure, compared with patients taking methotrexate. The researchers found no difference between the two drugs in patients without a history of heart failure. The study also suggested an overall 41% increase in the risk for hospitalization with heart failure with hydroxychloroquine, regardless of heart failure history.
Hydroxychloroquine was also associated with a 34% increase in the risk for cardiovascular mortality, a 22% increase in the risk for all-cause mortality, and a 74% increase in the risk for MI.
The lead author of the study, Elvira D’Andrea, MD, PhD, of Brigham and Women’s Hospital and Harvard Medical School in Boston, said that hydroxychloroquine is used as a first-line treatment for RA, but there was limited evidence on its cardiovascular risks. The pandemic in particular shined a spotlight on these concerns and prompted the researchers to extend their original prepandemic study to encompass additional cardiovascular outcomes.
“The emerging concerns on its cardiovascular safety in early 2020 has led the rheumatological community, and patients regularly taking hydroxychloroquine for rheumatoid arthritis, to confusion,” Dr. D’Andrea said in an interview.
She advised that clinicians be cautious when initiating hydroxychloroquine in older patients with existing heart failure or who have risk factors for it. “Although heart failure is a known concern for hydroxychloroquine use, these findings helped to clarify the relationship between the use of hydroxychloroquine or methotrexate and heart failure. Clinicians should pay careful attention to clinical manifestations of cardiomyopathy or heart failure in older patients with rheumatoid arthritis treated with hydroxychloroquine.”
Hydroxychloroquine is associated with cardiotoxicity, particularly cardiomyopathy, which may help precipitate MACE or heart failure exacerbations in patients who already have deterioration of their cardiac tissue, the authors suggested.
Short follow-up period leaves risk attribution under question
In an accompanying editorial, Elizabeth Blair Solow, MD, and Bonnie L. Bermas, MD, of the University of Texas Southwestern Medical Center, Dallas, commented that the lack of an increased risk for arrhythmic events or MACE in the overall cohort taking hydroxychloroquine was reassuring. They also suggested the subgroup analysis findings among patients with preexisting heart failure were still “exploratory and hypothesis-generating” and should be interpreted with caution.
They noted that the follow-up time of the study was relatively short – a median of 209 days – given that hydroxychloroquine does not reach a steady-state level for 6 months.
“Evidence to date suggests cardiomyopathy from HCQ [hydroxychloroquine] takes years to develop, many months beyond the exposures described here, bringing into question as to whether HCQ itself increased HF hospitalizations,” the editorialists wrote.
The editorial also raised the question of whether the association observed in the study was related to a possible cardioprotective effect of methotrexate, given that previous studies have suggested this effect in older patients with RA.
The study authors did an exploratory analysis comparing hydroxychloroquine with sulfasalazine, which appeared to support their main findings of a possible cardiovascular effect of hydroxychloroquine. However, they qualified this by pointing out that the analysis involved small numbers of patients.
Senior investigator Seoyoung C. Kim, MD, ScD, of Brigham and Women’s Hospital and Harvard Medical School, both in Boston, also noted that the study only looked at outcomes in patients aged 65 years and older.
“It would be clinically important to further examine the cardiovascular safety of hydroxychloroquine versus methotrexate in a younger population with rheumatic conditions,” she said.
The study was supported by the National Institutes of Health, Brigham and Women’s Hospital, and Harvard Medical School. Four authors declared unrelated research grants from the pharmaceutical sector, with one also declaring stock options and consulting work with the pharmaceutical sector. No other conflicts of interest were declared.
A version of this article first appeared on Medscape.com.
Hydroxychloroquine should be initiated with caution in older patients with rheumatoid arthritis who also have heart failure or are at risk for it, say the authors of a study suggesting that the drug could increase their risk for major adverse cardiovascular events (MACE), compared with methotrexate.
A cohort study published online in the Journal of the American College of Cardiology looked at outcomes in 54,462 patients with RA aged 65 years or older and not previously treated with disease-modifying antirheumatic drugs. Half were initiated on methotrexate and half on hydroxychloroquine, making 27,231 propensity-matched pairs.
Across the entire cohort, hydroxychloroquine was not associated with a higher risk for sudden cardiac arrest, ventricular arrhythmia, or MACE, compared with methotrexate. When broken down into individual cardiovascular events, the data suggested a statistically significant 17% increase in the risk for cardiovascular mortality and 10% increase in all-cause mortality with hydroxychloroquine, although there were no differences in the risks for myocardial infarction or stroke.
However, a subgroup analysis revealed a significant 30% increase in the risk for MACE among patients starting hydroxychloroquine who also had a history of heart failure, compared with patients taking methotrexate. The researchers found no difference between the two drugs in patients without a history of heart failure. The study also suggested an overall 41% increase in the risk for hospitalization with heart failure with hydroxychloroquine, regardless of heart failure history.
Hydroxychloroquine was also associated with a 34% increase in the risk for cardiovascular mortality, a 22% increase in the risk for all-cause mortality, and a 74% increase in the risk for MI.
The lead author of the study, Elvira D’Andrea, MD, PhD, of Brigham and Women’s Hospital and Harvard Medical School in Boston, said that hydroxychloroquine is used as a first-line treatment for RA, but there was limited evidence on its cardiovascular risks. The pandemic in particular shined a spotlight on these concerns and prompted the researchers to extend their original prepandemic study to encompass additional cardiovascular outcomes.
“The emerging concerns on its cardiovascular safety in early 2020 has led the rheumatological community, and patients regularly taking hydroxychloroquine for rheumatoid arthritis, to confusion,” Dr. D’Andrea said in an interview.
She advised that clinicians be cautious when initiating hydroxychloroquine in older patients with existing heart failure or who have risk factors for it. “Although heart failure is a known concern for hydroxychloroquine use, these findings helped to clarify the relationship between the use of hydroxychloroquine or methotrexate and heart failure. Clinicians should pay careful attention to clinical manifestations of cardiomyopathy or heart failure in older patients with rheumatoid arthritis treated with hydroxychloroquine.”
Hydroxychloroquine is associated with cardiotoxicity, particularly cardiomyopathy, which may help precipitate MACE or heart failure exacerbations in patients who already have deterioration of their cardiac tissue, the authors suggested.
Short follow-up period leaves risk attribution under question
In an accompanying editorial, Elizabeth Blair Solow, MD, and Bonnie L. Bermas, MD, of the University of Texas Southwestern Medical Center, Dallas, commented that the lack of an increased risk for arrhythmic events or MACE in the overall cohort taking hydroxychloroquine was reassuring. They also suggested the subgroup analysis findings among patients with preexisting heart failure were still “exploratory and hypothesis-generating” and should be interpreted with caution.
They noted that the follow-up time of the study was relatively short – a median of 209 days – given that hydroxychloroquine does not reach a steady-state level for 6 months.
“Evidence to date suggests cardiomyopathy from HCQ [hydroxychloroquine] takes years to develop, many months beyond the exposures described here, bringing into question as to whether HCQ itself increased HF hospitalizations,” the editorialists wrote.
The editorial also raised the question of whether the association observed in the study was related to a possible cardioprotective effect of methotrexate, given that previous studies have suggested this effect in older patients with RA.
The study authors did an exploratory analysis comparing hydroxychloroquine with sulfasalazine, which appeared to support their main findings of a possible cardiovascular effect of hydroxychloroquine. However, they qualified this by pointing out that the analysis involved small numbers of patients.
Senior investigator Seoyoung C. Kim, MD, ScD, of Brigham and Women’s Hospital and Harvard Medical School, both in Boston, also noted that the study only looked at outcomes in patients aged 65 years and older.
“It would be clinically important to further examine the cardiovascular safety of hydroxychloroquine versus methotrexate in a younger population with rheumatic conditions,” she said.
The study was supported by the National Institutes of Health, Brigham and Women’s Hospital, and Harvard Medical School. Four authors declared unrelated research grants from the pharmaceutical sector, with one also declaring stock options and consulting work with the pharmaceutical sector. No other conflicts of interest were declared.
A version of this article first appeared on Medscape.com.
Hydroxychloroquine should be initiated with caution in older patients with rheumatoid arthritis who also have heart failure or are at risk for it, say the authors of a study suggesting that the drug could increase their risk for major adverse cardiovascular events (MACE), compared with methotrexate.
A cohort study published online in the Journal of the American College of Cardiology looked at outcomes in 54,462 patients with RA aged 65 years or older and not previously treated with disease-modifying antirheumatic drugs. Half were initiated on methotrexate and half on hydroxychloroquine, making 27,231 propensity-matched pairs.
Across the entire cohort, hydroxychloroquine was not associated with a higher risk for sudden cardiac arrest, ventricular arrhythmia, or MACE, compared with methotrexate. When broken down into individual cardiovascular events, the data suggested a statistically significant 17% increase in the risk for cardiovascular mortality and 10% increase in all-cause mortality with hydroxychloroquine, although there were no differences in the risks for myocardial infarction or stroke.
However, a subgroup analysis revealed a significant 30% increase in the risk for MACE among patients starting hydroxychloroquine who also had a history of heart failure, compared with patients taking methotrexate. The researchers found no difference between the two drugs in patients without a history of heart failure. The study also suggested an overall 41% increase in the risk for hospitalization with heart failure with hydroxychloroquine, regardless of heart failure history.
Hydroxychloroquine was also associated with a 34% increase in the risk for cardiovascular mortality, a 22% increase in the risk for all-cause mortality, and a 74% increase in the risk for MI.
The lead author of the study, Elvira D’Andrea, MD, PhD, of Brigham and Women’s Hospital and Harvard Medical School in Boston, said that hydroxychloroquine is used as a first-line treatment for RA, but there was limited evidence on its cardiovascular risks. The pandemic in particular shined a spotlight on these concerns and prompted the researchers to extend their original prepandemic study to encompass additional cardiovascular outcomes.
“The emerging concerns on its cardiovascular safety in early 2020 has led the rheumatological community, and patients regularly taking hydroxychloroquine for rheumatoid arthritis, to confusion,” Dr. D’Andrea said in an interview.
She advised that clinicians be cautious when initiating hydroxychloroquine in older patients with existing heart failure or who have risk factors for it. “Although heart failure is a known concern for hydroxychloroquine use, these findings helped to clarify the relationship between the use of hydroxychloroquine or methotrexate and heart failure. Clinicians should pay careful attention to clinical manifestations of cardiomyopathy or heart failure in older patients with rheumatoid arthritis treated with hydroxychloroquine.”
Hydroxychloroquine is associated with cardiotoxicity, particularly cardiomyopathy, which may help precipitate MACE or heart failure exacerbations in patients who already have deterioration of their cardiac tissue, the authors suggested.
Short follow-up period leaves risk attribution under question
In an accompanying editorial, Elizabeth Blair Solow, MD, and Bonnie L. Bermas, MD, of the University of Texas Southwestern Medical Center, Dallas, commented that the lack of an increased risk for arrhythmic events or MACE in the overall cohort taking hydroxychloroquine was reassuring. They also suggested the subgroup analysis findings among patients with preexisting heart failure were still “exploratory and hypothesis-generating” and should be interpreted with caution.
They noted that the follow-up time of the study was relatively short – a median of 209 days – given that hydroxychloroquine does not reach a steady-state level for 6 months.
“Evidence to date suggests cardiomyopathy from HCQ [hydroxychloroquine] takes years to develop, many months beyond the exposures described here, bringing into question as to whether HCQ itself increased HF hospitalizations,” the editorialists wrote.
The editorial also raised the question of whether the association observed in the study was related to a possible cardioprotective effect of methotrexate, given that previous studies have suggested this effect in older patients with RA.
The study authors did an exploratory analysis comparing hydroxychloroquine with sulfasalazine, which appeared to support their main findings of a possible cardiovascular effect of hydroxychloroquine. However, they qualified this by pointing out that the analysis involved small numbers of patients.
Senior investigator Seoyoung C. Kim, MD, ScD, of Brigham and Women’s Hospital and Harvard Medical School, both in Boston, also noted that the study only looked at outcomes in patients aged 65 years and older.
“It would be clinically important to further examine the cardiovascular safety of hydroxychloroquine versus methotrexate in a younger population with rheumatic conditions,” she said.
The study was supported by the National Institutes of Health, Brigham and Women’s Hospital, and Harvard Medical School. Four authors declared unrelated research grants from the pharmaceutical sector, with one also declaring stock options and consulting work with the pharmaceutical sector. No other conflicts of interest were declared.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Treat-to-target strategy with tapering proves effective in PsA and axSpA
Aiming for a disease activity target while reducing biologic therapy could be a winning approach for patients with psoriatic arthritis (PsA) and axial spondyloarthritis (axSpA), according to the results of a new study presented at the annual European Congress of Rheumatology.
The findings show that a treat-to-target (T2T) strategy with tapering using a tumor necrosis factor (TNF) inhibitor produces results that are noninferior to a T2T strategy that doesn’t include tapering in these patients.
“Our study has for the first time shown that a treat-to-target tapering strategy is just as good as full-dose continuation, while reducing medication use substantially,” first author Celia Michielsens, MD, a PhD student and researcher at Sint Maartenskliniek in Nijmegen, the Netherlands, said in an interview before her presentation of the study during an oral abstract session at the congress. “Stepwise tapering is also better than fixed-dose reduction or discontinuation, since it is much more individualized.”
The study is now published in Annals of the Rheumatic Diseases.
In the randomized, controlled, open-label, noninferiority study, researchers enrolled patients with PsA or axSpA who were using a TNF inhibitor such as etanercept, adalimumab, or infliximab, and had stable low disease activity for at least 6 months. Patients needed to have a Psoriatic Arthritis Disease Activity Score (PASDAS) of 3.2 or less, or an Ankylosing Spondylitis Disease Activity Score (ASDAS) of at 2.1 or less. In cases of flare, patients were treated with NSAIDs and/or glucorticoids, and if they still had not reached low disease activity after a month, their previous TNF inhibitor dose was reinstated to the last effective interval or dosage, which was maintained throughout the study period. When the patient was already using a full TNF-inhibitor dose or if dose adjustment did not suffice, patients were switched to another biologic or targeted synthetic disease-modifying antirheumatic drug (DMARD).
Participants were randomized, from January 2019 to June 2021, to a tapering or a nontapering T2T strategy in a 2:1 fashion. Then researchers then followed them for 12 months and aimed to determine if the tapering strategy proved noninferior to not tapering within a predefined 20% margin for noninferiority, which Dr. Michielsens said was derived from other studies and what her group determined to be “an acceptable risk.”
Results show strategy is ‘feasible in daily clinical care’
A total of 81 patients – 42 with PsA and 39 with axSpA – were in the group with tapering, and 41 were in the group without tapering: 22 with PsA and 19 with axSpA.
At 12 months, researchers found that 69% of the patients in the group with tapering had low disease activity, measured via the PASDAS and ASDAS, compared with 73% in patients who did not taper. And those in the tapering group saw their medication use dramatically reduced. At the 12-month mark, they were taking just 53% of the defined daily dose for maintenance, compared with 91% of the defined daily dose for the group that didn’t taper.
The researchers were able to successfully taper 72% of the patients in the tapering group, with 28% of them discontinuing their TNF-inhibitor medication entirely. The incidence of flares was 85% in the tapering group and 78% in the nontapering group, a nonsignificant difference (P = .32).
The start of a new medication or an increase in use of an existing medication was more frequent in the tapering group, and significantly so for NSAIDs. An increase in NSAID use was seen in 54% of the tapering group and in just 24% of the nontapering group (P = .002).
Conventional synthetic DMARD use went up in the tapering group, compared with the nontapering group, but this was only among the PsA patients and the change in use was not statistically significant. There were also more frequent increases in glucocorticoid use in the tapering group, compared with the nontapering group, but this was not significant.
Dr. Michielsens said the findings show the value of an individualized approach in treating patients with PsA or axSpA.
“Our study – and those [studies] in rheumatoid arthritis earlier – deliver the highest quality of evidence that disease activity–guided dose personalization can, and in fact should, be used in clinical practice,” she said. “Our pragmatic treat-to-target tapering strategy is feasible in daily clinical care, although treat-to-target using PASDAS and ASDAS needs some implementation. In shared decision-making with patients, a 50% reduction in TNFi use is obtainable, while maintaining low disease activity.”
The increase in the use of NSAIDs is something to be aware of, but it is “not concerning,” Dr. Michielsens added. She pointed out that the NSAID use was typically temporary, used when flares arose, and that the drugs are effective, safe, and inexpensive. She also noted that the use of TNF blockers decreased more than the use of NSAIDs increased.
“This seems a perfectly acceptable trade-off that can be discussed with your patient,” she said.
The 12-month duration of the study is likely long enough to show that the tapering strategy works, Dr. Michielsens said. In rheumatoid arthritis studies, for example, differences in strategies didn’t change after 1 year.
“That said, we are doing an observational extension study to provide more insights in the long-term effects of this treat-to-target strategy,” she said. “At the end of this summer, all patients will have completed their extended follow-up period – a 12-month observational period – so hopefully we can present the results next year at EULAR.”
This study received funding from ReumaNederland. Dr. Michielsens did not have any financial interests to disclose. Two coauthors reported financial relationships with numerous pharmaceutical companies.
Aiming for a disease activity target while reducing biologic therapy could be a winning approach for patients with psoriatic arthritis (PsA) and axial spondyloarthritis (axSpA), according to the results of a new study presented at the annual European Congress of Rheumatology.
The findings show that a treat-to-target (T2T) strategy with tapering using a tumor necrosis factor (TNF) inhibitor produces results that are noninferior to a T2T strategy that doesn’t include tapering in these patients.
“Our study has for the first time shown that a treat-to-target tapering strategy is just as good as full-dose continuation, while reducing medication use substantially,” first author Celia Michielsens, MD, a PhD student and researcher at Sint Maartenskliniek in Nijmegen, the Netherlands, said in an interview before her presentation of the study during an oral abstract session at the congress. “Stepwise tapering is also better than fixed-dose reduction or discontinuation, since it is much more individualized.”
The study is now published in Annals of the Rheumatic Diseases.
In the randomized, controlled, open-label, noninferiority study, researchers enrolled patients with PsA or axSpA who were using a TNF inhibitor such as etanercept, adalimumab, or infliximab, and had stable low disease activity for at least 6 months. Patients needed to have a Psoriatic Arthritis Disease Activity Score (PASDAS) of 3.2 or less, or an Ankylosing Spondylitis Disease Activity Score (ASDAS) of at 2.1 or less. In cases of flare, patients were treated with NSAIDs and/or glucorticoids, and if they still had not reached low disease activity after a month, their previous TNF inhibitor dose was reinstated to the last effective interval or dosage, which was maintained throughout the study period. When the patient was already using a full TNF-inhibitor dose or if dose adjustment did not suffice, patients were switched to another biologic or targeted synthetic disease-modifying antirheumatic drug (DMARD).
Participants were randomized, from January 2019 to June 2021, to a tapering or a nontapering T2T strategy in a 2:1 fashion. Then researchers then followed them for 12 months and aimed to determine if the tapering strategy proved noninferior to not tapering within a predefined 20% margin for noninferiority, which Dr. Michielsens said was derived from other studies and what her group determined to be “an acceptable risk.”
Results show strategy is ‘feasible in daily clinical care’
A total of 81 patients – 42 with PsA and 39 with axSpA – were in the group with tapering, and 41 were in the group without tapering: 22 with PsA and 19 with axSpA.
At 12 months, researchers found that 69% of the patients in the group with tapering had low disease activity, measured via the PASDAS and ASDAS, compared with 73% in patients who did not taper. And those in the tapering group saw their medication use dramatically reduced. At the 12-month mark, they were taking just 53% of the defined daily dose for maintenance, compared with 91% of the defined daily dose for the group that didn’t taper.
The researchers were able to successfully taper 72% of the patients in the tapering group, with 28% of them discontinuing their TNF-inhibitor medication entirely. The incidence of flares was 85% in the tapering group and 78% in the nontapering group, a nonsignificant difference (P = .32).
The start of a new medication or an increase in use of an existing medication was more frequent in the tapering group, and significantly so for NSAIDs. An increase in NSAID use was seen in 54% of the tapering group and in just 24% of the nontapering group (P = .002).
Conventional synthetic DMARD use went up in the tapering group, compared with the nontapering group, but this was only among the PsA patients and the change in use was not statistically significant. There were also more frequent increases in glucocorticoid use in the tapering group, compared with the nontapering group, but this was not significant.
Dr. Michielsens said the findings show the value of an individualized approach in treating patients with PsA or axSpA.
“Our study – and those [studies] in rheumatoid arthritis earlier – deliver the highest quality of evidence that disease activity–guided dose personalization can, and in fact should, be used in clinical practice,” she said. “Our pragmatic treat-to-target tapering strategy is feasible in daily clinical care, although treat-to-target using PASDAS and ASDAS needs some implementation. In shared decision-making with patients, a 50% reduction in TNFi use is obtainable, while maintaining low disease activity.”
The increase in the use of NSAIDs is something to be aware of, but it is “not concerning,” Dr. Michielsens added. She pointed out that the NSAID use was typically temporary, used when flares arose, and that the drugs are effective, safe, and inexpensive. She also noted that the use of TNF blockers decreased more than the use of NSAIDs increased.
“This seems a perfectly acceptable trade-off that can be discussed with your patient,” she said.
The 12-month duration of the study is likely long enough to show that the tapering strategy works, Dr. Michielsens said. In rheumatoid arthritis studies, for example, differences in strategies didn’t change after 1 year.
“That said, we are doing an observational extension study to provide more insights in the long-term effects of this treat-to-target strategy,” she said. “At the end of this summer, all patients will have completed their extended follow-up period – a 12-month observational period – so hopefully we can present the results next year at EULAR.”
This study received funding from ReumaNederland. Dr. Michielsens did not have any financial interests to disclose. Two coauthors reported financial relationships with numerous pharmaceutical companies.
Aiming for a disease activity target while reducing biologic therapy could be a winning approach for patients with psoriatic arthritis (PsA) and axial spondyloarthritis (axSpA), according to the results of a new study presented at the annual European Congress of Rheumatology.
The findings show that a treat-to-target (T2T) strategy with tapering using a tumor necrosis factor (TNF) inhibitor produces results that are noninferior to a T2T strategy that doesn’t include tapering in these patients.
“Our study has for the first time shown that a treat-to-target tapering strategy is just as good as full-dose continuation, while reducing medication use substantially,” first author Celia Michielsens, MD, a PhD student and researcher at Sint Maartenskliniek in Nijmegen, the Netherlands, said in an interview before her presentation of the study during an oral abstract session at the congress. “Stepwise tapering is also better than fixed-dose reduction or discontinuation, since it is much more individualized.”
The study is now published in Annals of the Rheumatic Diseases.
In the randomized, controlled, open-label, noninferiority study, researchers enrolled patients with PsA or axSpA who were using a TNF inhibitor such as etanercept, adalimumab, or infliximab, and had stable low disease activity for at least 6 months. Patients needed to have a Psoriatic Arthritis Disease Activity Score (PASDAS) of 3.2 or less, or an Ankylosing Spondylitis Disease Activity Score (ASDAS) of at 2.1 or less. In cases of flare, patients were treated with NSAIDs and/or glucorticoids, and if they still had not reached low disease activity after a month, their previous TNF inhibitor dose was reinstated to the last effective interval or dosage, which was maintained throughout the study period. When the patient was already using a full TNF-inhibitor dose or if dose adjustment did not suffice, patients were switched to another biologic or targeted synthetic disease-modifying antirheumatic drug (DMARD).
Participants were randomized, from January 2019 to June 2021, to a tapering or a nontapering T2T strategy in a 2:1 fashion. Then researchers then followed them for 12 months and aimed to determine if the tapering strategy proved noninferior to not tapering within a predefined 20% margin for noninferiority, which Dr. Michielsens said was derived from other studies and what her group determined to be “an acceptable risk.”
Results show strategy is ‘feasible in daily clinical care’
A total of 81 patients – 42 with PsA and 39 with axSpA – were in the group with tapering, and 41 were in the group without tapering: 22 with PsA and 19 with axSpA.
At 12 months, researchers found that 69% of the patients in the group with tapering had low disease activity, measured via the PASDAS and ASDAS, compared with 73% in patients who did not taper. And those in the tapering group saw their medication use dramatically reduced. At the 12-month mark, they were taking just 53% of the defined daily dose for maintenance, compared with 91% of the defined daily dose for the group that didn’t taper.
The researchers were able to successfully taper 72% of the patients in the tapering group, with 28% of them discontinuing their TNF-inhibitor medication entirely. The incidence of flares was 85% in the tapering group and 78% in the nontapering group, a nonsignificant difference (P = .32).
The start of a new medication or an increase in use of an existing medication was more frequent in the tapering group, and significantly so for NSAIDs. An increase in NSAID use was seen in 54% of the tapering group and in just 24% of the nontapering group (P = .002).
Conventional synthetic DMARD use went up in the tapering group, compared with the nontapering group, but this was only among the PsA patients and the change in use was not statistically significant. There were also more frequent increases in glucocorticoid use in the tapering group, compared with the nontapering group, but this was not significant.
Dr. Michielsens said the findings show the value of an individualized approach in treating patients with PsA or axSpA.
“Our study – and those [studies] in rheumatoid arthritis earlier – deliver the highest quality of evidence that disease activity–guided dose personalization can, and in fact should, be used in clinical practice,” she said. “Our pragmatic treat-to-target tapering strategy is feasible in daily clinical care, although treat-to-target using PASDAS and ASDAS needs some implementation. In shared decision-making with patients, a 50% reduction in TNFi use is obtainable, while maintaining low disease activity.”
The increase in the use of NSAIDs is something to be aware of, but it is “not concerning,” Dr. Michielsens added. She pointed out that the NSAID use was typically temporary, used when flares arose, and that the drugs are effective, safe, and inexpensive. She also noted that the use of TNF blockers decreased more than the use of NSAIDs increased.
“This seems a perfectly acceptable trade-off that can be discussed with your patient,” she said.
The 12-month duration of the study is likely long enough to show that the tapering strategy works, Dr. Michielsens said. In rheumatoid arthritis studies, for example, differences in strategies didn’t change after 1 year.
“That said, we are doing an observational extension study to provide more insights in the long-term effects of this treat-to-target strategy,” she said. “At the end of this summer, all patients will have completed their extended follow-up period – a 12-month observational period – so hopefully we can present the results next year at EULAR.”
This study received funding from ReumaNederland. Dr. Michielsens did not have any financial interests to disclose. Two coauthors reported financial relationships with numerous pharmaceutical companies.
FROM THE EULAR 2022 CONGRESS
Alcohol consumption habits can predict gout tophi
The more years a person drinks alcohol, the kind of alcohol consumed, and the amount consumed can help to predict gout tophi, researchers say in a newly published paper in Arthritis Care and Research.
The study, led by Lin Han, PhD, of the gout laboratory, Shandong provincial clinical research center for immune diseases and gout, Affiliated Hospital of Qingdao (China) University, helps clarify the already-established relationship between alcohol consumption and gout tophi.
Additionally, the effects of drinking alcohol on ultrasound (US)–detected tophi and subcutaneous tophi (subtophi) were evaluated separately for the first time in this work, the authors say.
Tophi may be underdiagnosed because they are hard to find with only a physical exam. US can help with early detection, especially with small clusters of crystals or those found deep in the tissues, and offers good diagnostic accuracy with high specificity.
“Unlike subtophi, which represent long-term subcutaneous MSU [monosodium urate] deposition over many years, US-detected tophi represent the early stage of tophi in both intra- and extra-articular settings,” the authors write.
This cross-sectional study in China included 554 patients with gout who had joint ultrasound and physical exams through the Affiliated Hospital of Qingdao University. Physicians gathered medical histories using the Biobank Information Management System.
Physicians also tracked alcohol consumption patterns through the biobank information, which included answers to a detailed drinking questionnaire.
Patients were classified as either nondrinkers (no history of drinking; n = 141), former drinkers (n = 60), or current regular drinkers (n = 353). Current regular drinkers were asked further questions about their drinking patterns, including how long they have been drinking, type of alcohol they drink, and how much and how often they drink. In China, the average drink is considered to contain 10 g of alcohol, according to the World Health Organization.
Results from US and clinically detected tophi
Compared with nondrinkers, excessive drinkers (more than 70 g/week); long-term drinkers (at least 10 years), and spirits drinkers had a greater proportion, size, and number of US-detected tophi and subtophi (all P < .05).
After adjusting for confounders, the researchers found that excessive drinking was significantly associated with having US-detected tophi (odds ratio, 1.79) and subtophi (OR, 2.00). Similar associations were found for consumption of alcohol for at least 10 years (OR, 1.96 for US-detected tophi; OR, 2.17 for sub-tophi) and drinking spirits (OR, 1.81 for US-detected tophi; OR, 2.10 for subtophi). All comparisons were P < .05.
Among patients who already have US-detected tophi or subtophi, moderate drinking (70 g/week or less) was linked with larger or multiple tophi (all P < .05).
Angelo Gaffo, MD, section chief of rheumatology at the Birmingham VA Medical Center and associate professor of medicine in the division of rheumatology at the University of Alabama at Birmingham, said in an interview that the results are likely generalizable.
“I wouldn’t expect them to be specific to the Chinese population,” he said.
Most of the 554 patients were male (97.8%) and had no family history of gout (79.8%). The median duration of gout was 4 years, and the average age was 45.1 years.
Dr. Gaffo noted the population age was fairly young and the average duration of gout in these patients was fairly short. He also noted most had small tophi that were detected only by ultrasound and small numbers of tophi overall.
“I would like to see how these results will replicate in a population that has had gout for, say, 10 years on average,” he said.
Dr. Gaffo says he explores alcohol history with his patients with gout. If they are frequent drinkers, he encourages them to cut back.
“At the very least,” he said, “you have to restrict your intake to no more than 1-2 servings per week,” he said. “For some patients, even minimal amounts of alcohol intake can be associated with the development of flares.”
Still, research like this, he says, can help physicians point to evidence in their advice to patients about alcohol use.
He noted that the authors found the association between different types of alcohol and tophi was independent of serum urate level.
“That surprised me,” Dr. Gaffo said. “That’s a very unique finding.”
This work was supported by grants from the National Natural Science Foundation of China, the Natural Science Foundation of Shandong Province, Qingdao applied basic research project, National College Students’ Innovation and Entrepreneurship Training Program, and Shandong Provincial Science Foundation for Outstanding Youth Scholars.
The authors of the study and Dr. Gaffo report no relevant financial relationships.
The more years a person drinks alcohol, the kind of alcohol consumed, and the amount consumed can help to predict gout tophi, researchers say in a newly published paper in Arthritis Care and Research.
The study, led by Lin Han, PhD, of the gout laboratory, Shandong provincial clinical research center for immune diseases and gout, Affiliated Hospital of Qingdao (China) University, helps clarify the already-established relationship between alcohol consumption and gout tophi.
Additionally, the effects of drinking alcohol on ultrasound (US)–detected tophi and subcutaneous tophi (subtophi) were evaluated separately for the first time in this work, the authors say.
Tophi may be underdiagnosed because they are hard to find with only a physical exam. US can help with early detection, especially with small clusters of crystals or those found deep in the tissues, and offers good diagnostic accuracy with high specificity.
“Unlike subtophi, which represent long-term subcutaneous MSU [monosodium urate] deposition over many years, US-detected tophi represent the early stage of tophi in both intra- and extra-articular settings,” the authors write.
This cross-sectional study in China included 554 patients with gout who had joint ultrasound and physical exams through the Affiliated Hospital of Qingdao University. Physicians gathered medical histories using the Biobank Information Management System.
Physicians also tracked alcohol consumption patterns through the biobank information, which included answers to a detailed drinking questionnaire.
Patients were classified as either nondrinkers (no history of drinking; n = 141), former drinkers (n = 60), or current regular drinkers (n = 353). Current regular drinkers were asked further questions about their drinking patterns, including how long they have been drinking, type of alcohol they drink, and how much and how often they drink. In China, the average drink is considered to contain 10 g of alcohol, according to the World Health Organization.
Results from US and clinically detected tophi
Compared with nondrinkers, excessive drinkers (more than 70 g/week); long-term drinkers (at least 10 years), and spirits drinkers had a greater proportion, size, and number of US-detected tophi and subtophi (all P < .05).
After adjusting for confounders, the researchers found that excessive drinking was significantly associated with having US-detected tophi (odds ratio, 1.79) and subtophi (OR, 2.00). Similar associations were found for consumption of alcohol for at least 10 years (OR, 1.96 for US-detected tophi; OR, 2.17 for sub-tophi) and drinking spirits (OR, 1.81 for US-detected tophi; OR, 2.10 for subtophi). All comparisons were P < .05.
Among patients who already have US-detected tophi or subtophi, moderate drinking (70 g/week or less) was linked with larger or multiple tophi (all P < .05).
Angelo Gaffo, MD, section chief of rheumatology at the Birmingham VA Medical Center and associate professor of medicine in the division of rheumatology at the University of Alabama at Birmingham, said in an interview that the results are likely generalizable.
“I wouldn’t expect them to be specific to the Chinese population,” he said.
Most of the 554 patients were male (97.8%) and had no family history of gout (79.8%). The median duration of gout was 4 years, and the average age was 45.1 years.
Dr. Gaffo noted the population age was fairly young and the average duration of gout in these patients was fairly short. He also noted most had small tophi that were detected only by ultrasound and small numbers of tophi overall.
“I would like to see how these results will replicate in a population that has had gout for, say, 10 years on average,” he said.
Dr. Gaffo says he explores alcohol history with his patients with gout. If they are frequent drinkers, he encourages them to cut back.
“At the very least,” he said, “you have to restrict your intake to no more than 1-2 servings per week,” he said. “For some patients, even minimal amounts of alcohol intake can be associated with the development of flares.”
Still, research like this, he says, can help physicians point to evidence in their advice to patients about alcohol use.
He noted that the authors found the association between different types of alcohol and tophi was independent of serum urate level.
“That surprised me,” Dr. Gaffo said. “That’s a very unique finding.”
This work was supported by grants from the National Natural Science Foundation of China, the Natural Science Foundation of Shandong Province, Qingdao applied basic research project, National College Students’ Innovation and Entrepreneurship Training Program, and Shandong Provincial Science Foundation for Outstanding Youth Scholars.
The authors of the study and Dr. Gaffo report no relevant financial relationships.
The more years a person drinks alcohol, the kind of alcohol consumed, and the amount consumed can help to predict gout tophi, researchers say in a newly published paper in Arthritis Care and Research.
The study, led by Lin Han, PhD, of the gout laboratory, Shandong provincial clinical research center for immune diseases and gout, Affiliated Hospital of Qingdao (China) University, helps clarify the already-established relationship between alcohol consumption and gout tophi.
Additionally, the effects of drinking alcohol on ultrasound (US)–detected tophi and subcutaneous tophi (subtophi) were evaluated separately for the first time in this work, the authors say.
Tophi may be underdiagnosed because they are hard to find with only a physical exam. US can help with early detection, especially with small clusters of crystals or those found deep in the tissues, and offers good diagnostic accuracy with high specificity.
“Unlike subtophi, which represent long-term subcutaneous MSU [monosodium urate] deposition over many years, US-detected tophi represent the early stage of tophi in both intra- and extra-articular settings,” the authors write.
This cross-sectional study in China included 554 patients with gout who had joint ultrasound and physical exams through the Affiliated Hospital of Qingdao University. Physicians gathered medical histories using the Biobank Information Management System.
Physicians also tracked alcohol consumption patterns through the biobank information, which included answers to a detailed drinking questionnaire.
Patients were classified as either nondrinkers (no history of drinking; n = 141), former drinkers (n = 60), or current regular drinkers (n = 353). Current regular drinkers were asked further questions about their drinking patterns, including how long they have been drinking, type of alcohol they drink, and how much and how often they drink. In China, the average drink is considered to contain 10 g of alcohol, according to the World Health Organization.
Results from US and clinically detected tophi
Compared with nondrinkers, excessive drinkers (more than 70 g/week); long-term drinkers (at least 10 years), and spirits drinkers had a greater proportion, size, and number of US-detected tophi and subtophi (all P < .05).
After adjusting for confounders, the researchers found that excessive drinking was significantly associated with having US-detected tophi (odds ratio, 1.79) and subtophi (OR, 2.00). Similar associations were found for consumption of alcohol for at least 10 years (OR, 1.96 for US-detected tophi; OR, 2.17 for sub-tophi) and drinking spirits (OR, 1.81 for US-detected tophi; OR, 2.10 for subtophi). All comparisons were P < .05.
Among patients who already have US-detected tophi or subtophi, moderate drinking (70 g/week or less) was linked with larger or multiple tophi (all P < .05).
Angelo Gaffo, MD, section chief of rheumatology at the Birmingham VA Medical Center and associate professor of medicine in the division of rheumatology at the University of Alabama at Birmingham, said in an interview that the results are likely generalizable.
“I wouldn’t expect them to be specific to the Chinese population,” he said.
Most of the 554 patients were male (97.8%) and had no family history of gout (79.8%). The median duration of gout was 4 years, and the average age was 45.1 years.
Dr. Gaffo noted the population age was fairly young and the average duration of gout in these patients was fairly short. He also noted most had small tophi that were detected only by ultrasound and small numbers of tophi overall.
“I would like to see how these results will replicate in a population that has had gout for, say, 10 years on average,” he said.
Dr. Gaffo says he explores alcohol history with his patients with gout. If they are frequent drinkers, he encourages them to cut back.
“At the very least,” he said, “you have to restrict your intake to no more than 1-2 servings per week,” he said. “For some patients, even minimal amounts of alcohol intake can be associated with the development of flares.”
Still, research like this, he says, can help physicians point to evidence in their advice to patients about alcohol use.
He noted that the authors found the association between different types of alcohol and tophi was independent of serum urate level.
“That surprised me,” Dr. Gaffo said. “That’s a very unique finding.”
This work was supported by grants from the National Natural Science Foundation of China, the Natural Science Foundation of Shandong Province, Qingdao applied basic research project, National College Students’ Innovation and Entrepreneurship Training Program, and Shandong Provincial Science Foundation for Outstanding Youth Scholars.
The authors of the study and Dr. Gaffo report no relevant financial relationships.
FROM ARTHRITIS CARE AND RESEARCH
Add AFib to noncardiac surgery risk evaluation: New support
Practice has gone back and forth on whether atrial fibrillation (AFib) should be considered in the preoperative cardiovascular risk (CV) evaluation of patients slated for noncardiac surgery, and the Revised Cardiac Risk Index (RCRI), currently widely used as an assessment tool, doesn’t include the arrhythmia.
But consideration of preexisting AFib along with the RCRI predicted 30-day mortality more sharply than the RCRI alone in an analysis of data covering several million patients slated for such procedures.
Indeed, AFib emerged as a significant, independent risk factor for a number of bad postoperative outcomes. Mortality within a month of the procedure climbed about 30% for patients with AFib before the noncardiac surgery. Their 30-day risks for stroke and for heart failure hospitalization went up similarly.
The addition of AFib to the RCRI significantly improved its ability to discriminate 30-day postoperative risk levels regardless of age, sex, and type of noncardiac surgery, Amgad Mentias, MD, Cleveland Clinic, told this news organization. And “it was able to correctly up-classify patients to high risk, if AFib was there, and it was able to down-classify some patients to lower risk if it wasn’t there.”
“I think [the findings] are convincing evidence that atrial fib should at least be part of the thought process for the surgical team and the medical team taking care of the patient,” said Dr. Mentias, who is senior author on the study published in the Journal of the American College of Cardiology, with lead author Sameer Prasada, MD, also of the Cleveland Clinic.
The results “call for incorporating AFib as a risk factor in perioperative risk scores for cardiovascular morbidity and mortality,” the published report states.
Supraventricular arrhythmias had been part of the Goldman Risk Index once widely used preoperatively to assess cardiac risk before practice adopted the RCRI in the past decade, observe Anne B. Curtis, MD, and Sai Krishna C. Korada, MD, University at Buffalo, New York, in an accompanying editorial.
The current findings “demonstrate improved prediction of adverse postsurgical outcomes” from supplementing the RCRI with AFib, they write. Given associations between preexisting AFib and serious cardiac events, “it is time to ‘re-revise’ the RCRI and acknowledge the importance of AFib in predicting adverse outcomes” after noncardiac surgery.
The new findings, however, aren’t all straightforward. In one result that remains a bit of a head-scratcher, postoperative risk of myocardial infarction (MI) in patients with preexisting AFib went in the opposite direction of risk for death and other CV outcomes, falling by almost 20%.
That is “hard to explain with the available data,” the report states, but “the use of anticoagulation, whether oral or parenteral (as a bridge therapy in the perioperative period), is a plausible explanation” given the frequent role of thrombosis in triggering MIs.
Consistent with such a mechanism, the group argues, the MI risk reduction was seen primarily among patients with AFib and a CHA2DS2-VASc score of 2 or higher – that is, those at highest risk for stroke and therefore most likely to be on oral anticoagulation. The MI risk reduction wasn’t seen in such patients with a CHA2DS2-VASc score of 0 or 1.
“I think that’s part of the explanation, that anticoagulation can reduce risk of MI. But it’s not the whole explanation,” Dr. Mentias said in an interview. If it were the sole mechanism, he said, then the same oral anticoagulation that protected against MI should have also cut the postoperative stroke risk. Yet that risk climbed 40% among patients with preexisting AFib.
The analysis started with 8.6 million Medicare patients with planned noncardiac surgery, seen from 2015 to 2019, of whom 16.4% had preexisting AFib. Propensity matching for demographics, urgency and type of surgery, CHA2DS2-VASc score, and RCRI index created two cohorts for comparison: 1.13 million patients with and 1.92 million without preexisting AFib.
Preexisting AFib was associated with a higher 30-day risk for death from any cause, the primary endpoint being 8.3% versus 5.8% for those without such AFib (P < .001), for an odds ratio of 1.31 (95% confidence interval, 1.30-1.32).
Corresponding 30-day ORs for other events, all significant at P < .001, were:
- 1.31 (95% CI, 1.30-1.33) for heart failure
- 1.40 (95% CI, 1.37-1.43) for stroke
- 1.59 (95% CI, 1.43-1.75) for systemic embolism
- 1.14 (95% CI, 1.13-1.16) for major bleeding
- 0.81 (95% CI, 0.79-0.82) for MI
Those with preexisting AFib also had longer hospitalizations at a median 5 days, compared with 4 days for those without such AFib (P < .001).
The study has the limitations of most any retrospective cohort analysis. Other limitations, the report notes, include lack of information on any antiarrhythmic meds given during hospitalization or type of AFib.
For example, AFib that is permanent – compared with paroxysmal or persistent – may be associated with more atrial fibrosis, greater atrial dilatation, “and probably higher pressures inside the heart,” Dr. Mentias observed.
“That’s not always the case, but that’s the notion. So presumably people with persistent or permanent atrial fib would have more advanced heart disease, and that could imply more risk. But we did not have that kind of data.”
Dr. Mentias and Dr. Prasada report no relevant financial relationships; disclosures for the other authors are in the report. Dr. Curtis discloses serving on advisory boards for Abbott, Janssen Pharmaceuticals, Sanofi, and Milestone Pharmaceuticals; receiving honoraria for speaking from Medtronic and Zoll; and serving on a data-monitoring board for Medtronic. Dr. Korada reports he has no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Practice has gone back and forth on whether atrial fibrillation (AFib) should be considered in the preoperative cardiovascular risk (CV) evaluation of patients slated for noncardiac surgery, and the Revised Cardiac Risk Index (RCRI), currently widely used as an assessment tool, doesn’t include the arrhythmia.
But consideration of preexisting AFib along with the RCRI predicted 30-day mortality more sharply than the RCRI alone in an analysis of data covering several million patients slated for such procedures.
Indeed, AFib emerged as a significant, independent risk factor for a number of bad postoperative outcomes. Mortality within a month of the procedure climbed about 30% for patients with AFib before the noncardiac surgery. Their 30-day risks for stroke and for heart failure hospitalization went up similarly.
The addition of AFib to the RCRI significantly improved its ability to discriminate 30-day postoperative risk levels regardless of age, sex, and type of noncardiac surgery, Amgad Mentias, MD, Cleveland Clinic, told this news organization. And “it was able to correctly up-classify patients to high risk, if AFib was there, and it was able to down-classify some patients to lower risk if it wasn’t there.”
“I think [the findings] are convincing evidence that atrial fib should at least be part of the thought process for the surgical team and the medical team taking care of the patient,” said Dr. Mentias, who is senior author on the study published in the Journal of the American College of Cardiology, with lead author Sameer Prasada, MD, also of the Cleveland Clinic.
The results “call for incorporating AFib as a risk factor in perioperative risk scores for cardiovascular morbidity and mortality,” the published report states.
Supraventricular arrhythmias had been part of the Goldman Risk Index once widely used preoperatively to assess cardiac risk before practice adopted the RCRI in the past decade, observe Anne B. Curtis, MD, and Sai Krishna C. Korada, MD, University at Buffalo, New York, in an accompanying editorial.
The current findings “demonstrate improved prediction of adverse postsurgical outcomes” from supplementing the RCRI with AFib, they write. Given associations between preexisting AFib and serious cardiac events, “it is time to ‘re-revise’ the RCRI and acknowledge the importance of AFib in predicting adverse outcomes” after noncardiac surgery.
The new findings, however, aren’t all straightforward. In one result that remains a bit of a head-scratcher, postoperative risk of myocardial infarction (MI) in patients with preexisting AFib went in the opposite direction of risk for death and other CV outcomes, falling by almost 20%.
That is “hard to explain with the available data,” the report states, but “the use of anticoagulation, whether oral or parenteral (as a bridge therapy in the perioperative period), is a plausible explanation” given the frequent role of thrombosis in triggering MIs.
Consistent with such a mechanism, the group argues, the MI risk reduction was seen primarily among patients with AFib and a CHA2DS2-VASc score of 2 or higher – that is, those at highest risk for stroke and therefore most likely to be on oral anticoagulation. The MI risk reduction wasn’t seen in such patients with a CHA2DS2-VASc score of 0 or 1.
“I think that’s part of the explanation, that anticoagulation can reduce risk of MI. But it’s not the whole explanation,” Dr. Mentias said in an interview. If it were the sole mechanism, he said, then the same oral anticoagulation that protected against MI should have also cut the postoperative stroke risk. Yet that risk climbed 40% among patients with preexisting AFib.
The analysis started with 8.6 million Medicare patients with planned noncardiac surgery, seen from 2015 to 2019, of whom 16.4% had preexisting AFib. Propensity matching for demographics, urgency and type of surgery, CHA2DS2-VASc score, and RCRI index created two cohorts for comparison: 1.13 million patients with and 1.92 million without preexisting AFib.
Preexisting AFib was associated with a higher 30-day risk for death from any cause, the primary endpoint being 8.3% versus 5.8% for those without such AFib (P < .001), for an odds ratio of 1.31 (95% confidence interval, 1.30-1.32).
Corresponding 30-day ORs for other events, all significant at P < .001, were:
- 1.31 (95% CI, 1.30-1.33) for heart failure
- 1.40 (95% CI, 1.37-1.43) for stroke
- 1.59 (95% CI, 1.43-1.75) for systemic embolism
- 1.14 (95% CI, 1.13-1.16) for major bleeding
- 0.81 (95% CI, 0.79-0.82) for MI
Those with preexisting AFib also had longer hospitalizations at a median 5 days, compared with 4 days for those without such AFib (P < .001).
The study has the limitations of most any retrospective cohort analysis. Other limitations, the report notes, include lack of information on any antiarrhythmic meds given during hospitalization or type of AFib.
For example, AFib that is permanent – compared with paroxysmal or persistent – may be associated with more atrial fibrosis, greater atrial dilatation, “and probably higher pressures inside the heart,” Dr. Mentias observed.
“That’s not always the case, but that’s the notion. So presumably people with persistent or permanent atrial fib would have more advanced heart disease, and that could imply more risk. But we did not have that kind of data.”
Dr. Mentias and Dr. Prasada report no relevant financial relationships; disclosures for the other authors are in the report. Dr. Curtis discloses serving on advisory boards for Abbott, Janssen Pharmaceuticals, Sanofi, and Milestone Pharmaceuticals; receiving honoraria for speaking from Medtronic and Zoll; and serving on a data-monitoring board for Medtronic. Dr. Korada reports he has no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Practice has gone back and forth on whether atrial fibrillation (AFib) should be considered in the preoperative cardiovascular risk (CV) evaluation of patients slated for noncardiac surgery, and the Revised Cardiac Risk Index (RCRI), currently widely used as an assessment tool, doesn’t include the arrhythmia.
But consideration of preexisting AFib along with the RCRI predicted 30-day mortality more sharply than the RCRI alone in an analysis of data covering several million patients slated for such procedures.
Indeed, AFib emerged as a significant, independent risk factor for a number of bad postoperative outcomes. Mortality within a month of the procedure climbed about 30% for patients with AFib before the noncardiac surgery. Their 30-day risks for stroke and for heart failure hospitalization went up similarly.
The addition of AFib to the RCRI significantly improved its ability to discriminate 30-day postoperative risk levels regardless of age, sex, and type of noncardiac surgery, Amgad Mentias, MD, Cleveland Clinic, told this news organization. And “it was able to correctly up-classify patients to high risk, if AFib was there, and it was able to down-classify some patients to lower risk if it wasn’t there.”
“I think [the findings] are convincing evidence that atrial fib should at least be part of the thought process for the surgical team and the medical team taking care of the patient,” said Dr. Mentias, who is senior author on the study published in the Journal of the American College of Cardiology, with lead author Sameer Prasada, MD, also of the Cleveland Clinic.
The results “call for incorporating AFib as a risk factor in perioperative risk scores for cardiovascular morbidity and mortality,” the published report states.
Supraventricular arrhythmias had been part of the Goldman Risk Index once widely used preoperatively to assess cardiac risk before practice adopted the RCRI in the past decade, observe Anne B. Curtis, MD, and Sai Krishna C. Korada, MD, University at Buffalo, New York, in an accompanying editorial.
The current findings “demonstrate improved prediction of adverse postsurgical outcomes” from supplementing the RCRI with AFib, they write. Given associations between preexisting AFib and serious cardiac events, “it is time to ‘re-revise’ the RCRI and acknowledge the importance of AFib in predicting adverse outcomes” after noncardiac surgery.
The new findings, however, aren’t all straightforward. In one result that remains a bit of a head-scratcher, postoperative risk of myocardial infarction (MI) in patients with preexisting AFib went in the opposite direction of risk for death and other CV outcomes, falling by almost 20%.
That is “hard to explain with the available data,” the report states, but “the use of anticoagulation, whether oral or parenteral (as a bridge therapy in the perioperative period), is a plausible explanation” given the frequent role of thrombosis in triggering MIs.
Consistent with such a mechanism, the group argues, the MI risk reduction was seen primarily among patients with AFib and a CHA2DS2-VASc score of 2 or higher – that is, those at highest risk for stroke and therefore most likely to be on oral anticoagulation. The MI risk reduction wasn’t seen in such patients with a CHA2DS2-VASc score of 0 or 1.
“I think that’s part of the explanation, that anticoagulation can reduce risk of MI. But it’s not the whole explanation,” Dr. Mentias said in an interview. If it were the sole mechanism, he said, then the same oral anticoagulation that protected against MI should have also cut the postoperative stroke risk. Yet that risk climbed 40% among patients with preexisting AFib.
The analysis started with 8.6 million Medicare patients with planned noncardiac surgery, seen from 2015 to 2019, of whom 16.4% had preexisting AFib. Propensity matching for demographics, urgency and type of surgery, CHA2DS2-VASc score, and RCRI index created two cohorts for comparison: 1.13 million patients with and 1.92 million without preexisting AFib.
Preexisting AFib was associated with a higher 30-day risk for death from any cause, the primary endpoint being 8.3% versus 5.8% for those without such AFib (P < .001), for an odds ratio of 1.31 (95% confidence interval, 1.30-1.32).
Corresponding 30-day ORs for other events, all significant at P < .001, were:
- 1.31 (95% CI, 1.30-1.33) for heart failure
- 1.40 (95% CI, 1.37-1.43) for stroke
- 1.59 (95% CI, 1.43-1.75) for systemic embolism
- 1.14 (95% CI, 1.13-1.16) for major bleeding
- 0.81 (95% CI, 0.79-0.82) for MI
Those with preexisting AFib also had longer hospitalizations at a median 5 days, compared with 4 days for those without such AFib (P < .001).
The study has the limitations of most any retrospective cohort analysis. Other limitations, the report notes, include lack of information on any antiarrhythmic meds given during hospitalization or type of AFib.
For example, AFib that is permanent – compared with paroxysmal or persistent – may be associated with more atrial fibrosis, greater atrial dilatation, “and probably higher pressures inside the heart,” Dr. Mentias observed.
“That’s not always the case, but that’s the notion. So presumably people with persistent or permanent atrial fib would have more advanced heart disease, and that could imply more risk. But we did not have that kind of data.”
Dr. Mentias and Dr. Prasada report no relevant financial relationships; disclosures for the other authors are in the report. Dr. Curtis discloses serving on advisory boards for Abbott, Janssen Pharmaceuticals, Sanofi, and Milestone Pharmaceuticals; receiving honoraria for speaking from Medtronic and Zoll; and serving on a data-monitoring board for Medtronic. Dr. Korada reports he has no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Osteoporosis risk rises with air pollution levels
COPENHAGEN – Chronic exposure to high levels of particulate matter (PM) air pollution 2.5 mcm (PM2.5) or larger, and 10 mcm (PM10) or larger, in size is associated with a significantly higher likelihood of having osteoporosis, according to research presented at the annual European Congress of Rheumatology.
The results of the 7-year longitudinal study carried out across Italy from 2013 to 2019 dovetail with other recent published accounts from the same team of Italian researchers, led by Giovanni Adami, MD, of the rheumatology unit at the University of Verona (Italy). In addition to the current report presented at EULAR 2022, Dr. Adami and associates have reported an increased risk of flares of both rheumatoid arthritis and psoriasis following periods of elevated pollution, as well as an overall elevated risk for autoimmune diseases with higher concentrations of PM2.5 and PM10.
The pathogenesis of osteoporosis is thought to involve both genetic and environmental input, such as smoking, which is itself environmental air pollution, Dr. Adami said. The biological rationale for why air pollution might contribute to risk for osteoporosis comes from studies showing that exposure to indoor air pollution from biomass combustion raises serum levels of RANKL (receptor activator of nuclear factor-kappa ligand 1) but lowers serum osteoprotegerin – suggesting an increased risk of bone resorption – and that toxic metals such as lead, cadmium, mercury, and aluminum accumulate in the skeleton and negatively affect bone health.
In their study, Dr. Adami and colleagues found that, overall, the average exposure during the period 2013-2019 across Italy was 16.0 mcg/m3 for PM2.5 and 25.0 mcg/m3 for PM10.
“I can tell you that [25.0 mcg/m3 for PM10] is a very high exposure. It’s not very good for your health,” Dr. Adami said.
Data on more than 59,000 Italian women
Dr. Adami and colleagues used clinical characteristics and densitometric data from Italy’s osteoporosis fracture risk and osteoporosis screening reimbursement tool known as DeFRAcalc79, which has amassed variables from more than 59,000 women across the country. They used long-term average PM concentrations across Italy during 2013-2019 that were obtained from the Italian Institute for Environmental Protection and Research’s 617 air quality stations in 110 Italian provinces. The researchers linked individuals to a PM exposure value determined from the average concentration of urban, rural, and near-traffic stations in each person’s province of residence.
For 59,950 women across Italy who were at high risk for fracture, the researchers found 64.5% with bone mineral density that was defined as osteoporotic. At PM10 concentrations of 30 mcg/m3 or greater, there was a significantly higher likelihood of osteoporosis at both the femoral neck (odds ratio, 1.15) and lumbar spine (OR, 1.17).
The likelihood of osteoporosis was slightly greater with PM2.5 at concentrations of 25 mcg/m3 or more at the femoral neck (OR, 1.22) and lumbar spine (OR, 1.18). These comparisons were adjusted for age, body mass index (BMI), presence of prevalent fragility fractures, family history of osteoporosis, menopause, glucocorticoid use, comorbidities, and for residency in northern, central, or southern Italy.
Both thresholds of PM10 > 30 mcg/m3 and PM2.5 > 25 mcg/m3 “are considered safe … by the World Health Organization,” Dr. Adami pointed out.
“If you live in a place where the chronic exposure is less than 30 mcg/m3, you probably have slightly lower risk of osteoporosis as compared to those who live in a highly industrialized, polluted zone,” he explained.
“The cortical bone – femoral neck – seemed to be more susceptible, compared to trabecular bone, which is the lumbar spine. We have no idea why this is true, but we might speculate that somehow chronic inflammation like the [kind] seen in rheumatoid arthritis might be responsible for cortical bone impairment and not trabecular bone impairment,” Dr. Adami said.
One audience member, Kenneth Poole, BM, PhD, senior lecturer and honorary consultant in Metabolic Bone Disease and Rheumatology at the University of Cambridge (England), asked whether it was possible to account for the possibility of confounding caused by areas with dense housing in places where the particulate matter would be highest, and where residents may be less active and use stairs less often.
Dr. Adami noted that confounding is indeed a possibility, but he said Italy is unique in that its most polluted area – the Po River valley – is also its most wealthy area and in general has less crowded living situations with a healthier population, which could have attenuated, rather than reinforced, the results.
Does air pollution have an immunologic effect?
In interviews with this news organization, session comoderators Filipe Araújo, MD, and Irene Bultink, MD, PhD, said that the growth in evidence for the impact of air pollution on risk for, and severity of, various diseases suggests air pollution might have an immunologic effect.
“I think it’s very important to point this out. I also think it’s very hard to rule out confounding, because when you’re living in a city with crowded housing you may not walk or ride your bike but instead go by car or metro, and [the lifestyle is different],” said Dr. Bultink of Amsterdam University Medical Centers.
“It stresses that these diseases [that are associated with air pollution] although they are different in their pathophysiology, it points toward the systemic nature of rheumatic diseases, including osteoporosis,” said Dr. Araújo of Hospital Cuf Cascais (Portugal) and Hospital Ortopédico de Sant’Ana, Parede, Portugal.
The study was independently supported.Dr. Adami disclosed being a shareholder of Galapagos and Theramex.
A version of this article first appeared on Medscape.com.
COPENHAGEN – Chronic exposure to high levels of particulate matter (PM) air pollution 2.5 mcm (PM2.5) or larger, and 10 mcm (PM10) or larger, in size is associated with a significantly higher likelihood of having osteoporosis, according to research presented at the annual European Congress of Rheumatology.
The results of the 7-year longitudinal study carried out across Italy from 2013 to 2019 dovetail with other recent published accounts from the same team of Italian researchers, led by Giovanni Adami, MD, of the rheumatology unit at the University of Verona (Italy). In addition to the current report presented at EULAR 2022, Dr. Adami and associates have reported an increased risk of flares of both rheumatoid arthritis and psoriasis following periods of elevated pollution, as well as an overall elevated risk for autoimmune diseases with higher concentrations of PM2.5 and PM10.
The pathogenesis of osteoporosis is thought to involve both genetic and environmental input, such as smoking, which is itself environmental air pollution, Dr. Adami said. The biological rationale for why air pollution might contribute to risk for osteoporosis comes from studies showing that exposure to indoor air pollution from biomass combustion raises serum levels of RANKL (receptor activator of nuclear factor-kappa ligand 1) but lowers serum osteoprotegerin – suggesting an increased risk of bone resorption – and that toxic metals such as lead, cadmium, mercury, and aluminum accumulate in the skeleton and negatively affect bone health.
In their study, Dr. Adami and colleagues found that, overall, the average exposure during the period 2013-2019 across Italy was 16.0 mcg/m3 for PM2.5 and 25.0 mcg/m3 for PM10.
“I can tell you that [25.0 mcg/m3 for PM10] is a very high exposure. It’s not very good for your health,” Dr. Adami said.
Data on more than 59,000 Italian women
Dr. Adami and colleagues used clinical characteristics and densitometric data from Italy’s osteoporosis fracture risk and osteoporosis screening reimbursement tool known as DeFRAcalc79, which has amassed variables from more than 59,000 women across the country. They used long-term average PM concentrations across Italy during 2013-2019 that were obtained from the Italian Institute for Environmental Protection and Research’s 617 air quality stations in 110 Italian provinces. The researchers linked individuals to a PM exposure value determined from the average concentration of urban, rural, and near-traffic stations in each person’s province of residence.
For 59,950 women across Italy who were at high risk for fracture, the researchers found 64.5% with bone mineral density that was defined as osteoporotic. At PM10 concentrations of 30 mcg/m3 or greater, there was a significantly higher likelihood of osteoporosis at both the femoral neck (odds ratio, 1.15) and lumbar spine (OR, 1.17).
The likelihood of osteoporosis was slightly greater with PM2.5 at concentrations of 25 mcg/m3 or more at the femoral neck (OR, 1.22) and lumbar spine (OR, 1.18). These comparisons were adjusted for age, body mass index (BMI), presence of prevalent fragility fractures, family history of osteoporosis, menopause, glucocorticoid use, comorbidities, and for residency in northern, central, or southern Italy.
Both thresholds of PM10 > 30 mcg/m3 and PM2.5 > 25 mcg/m3 “are considered safe … by the World Health Organization,” Dr. Adami pointed out.
“If you live in a place where the chronic exposure is less than 30 mcg/m3, you probably have slightly lower risk of osteoporosis as compared to those who live in a highly industrialized, polluted zone,” he explained.
“The cortical bone – femoral neck – seemed to be more susceptible, compared to trabecular bone, which is the lumbar spine. We have no idea why this is true, but we might speculate that somehow chronic inflammation like the [kind] seen in rheumatoid arthritis might be responsible for cortical bone impairment and not trabecular bone impairment,” Dr. Adami said.
One audience member, Kenneth Poole, BM, PhD, senior lecturer and honorary consultant in Metabolic Bone Disease and Rheumatology at the University of Cambridge (England), asked whether it was possible to account for the possibility of confounding caused by areas with dense housing in places where the particulate matter would be highest, and where residents may be less active and use stairs less often.
Dr. Adami noted that confounding is indeed a possibility, but he said Italy is unique in that its most polluted area – the Po River valley – is also its most wealthy area and in general has less crowded living situations with a healthier population, which could have attenuated, rather than reinforced, the results.
Does air pollution have an immunologic effect?
In interviews with this news organization, session comoderators Filipe Araújo, MD, and Irene Bultink, MD, PhD, said that the growth in evidence for the impact of air pollution on risk for, and severity of, various diseases suggests air pollution might have an immunologic effect.
“I think it’s very important to point this out. I also think it’s very hard to rule out confounding, because when you’re living in a city with crowded housing you may not walk or ride your bike but instead go by car or metro, and [the lifestyle is different],” said Dr. Bultink of Amsterdam University Medical Centers.
“It stresses that these diseases [that are associated with air pollution] although they are different in their pathophysiology, it points toward the systemic nature of rheumatic diseases, including osteoporosis,” said Dr. Araújo of Hospital Cuf Cascais (Portugal) and Hospital Ortopédico de Sant’Ana, Parede, Portugal.
The study was independently supported.Dr. Adami disclosed being a shareholder of Galapagos and Theramex.
A version of this article first appeared on Medscape.com.
COPENHAGEN – Chronic exposure to high levels of particulate matter (PM) air pollution 2.5 mcm (PM2.5) or larger, and 10 mcm (PM10) or larger, in size is associated with a significantly higher likelihood of having osteoporosis, according to research presented at the annual European Congress of Rheumatology.
The results of the 7-year longitudinal study carried out across Italy from 2013 to 2019 dovetail with other recent published accounts from the same team of Italian researchers, led by Giovanni Adami, MD, of the rheumatology unit at the University of Verona (Italy). In addition to the current report presented at EULAR 2022, Dr. Adami and associates have reported an increased risk of flares of both rheumatoid arthritis and psoriasis following periods of elevated pollution, as well as an overall elevated risk for autoimmune diseases with higher concentrations of PM2.5 and PM10.
The pathogenesis of osteoporosis is thought to involve both genetic and environmental input, such as smoking, which is itself environmental air pollution, Dr. Adami said. The biological rationale for why air pollution might contribute to risk for osteoporosis comes from studies showing that exposure to indoor air pollution from biomass combustion raises serum levels of RANKL (receptor activator of nuclear factor-kappa ligand 1) but lowers serum osteoprotegerin – suggesting an increased risk of bone resorption – and that toxic metals such as lead, cadmium, mercury, and aluminum accumulate in the skeleton and negatively affect bone health.
In their study, Dr. Adami and colleagues found that, overall, the average exposure during the period 2013-2019 across Italy was 16.0 mcg/m3 for PM2.5 and 25.0 mcg/m3 for PM10.
“I can tell you that [25.0 mcg/m3 for PM10] is a very high exposure. It’s not very good for your health,” Dr. Adami said.
Data on more than 59,000 Italian women
Dr. Adami and colleagues used clinical characteristics and densitometric data from Italy’s osteoporosis fracture risk and osteoporosis screening reimbursement tool known as DeFRAcalc79, which has amassed variables from more than 59,000 women across the country. They used long-term average PM concentrations across Italy during 2013-2019 that were obtained from the Italian Institute for Environmental Protection and Research’s 617 air quality stations in 110 Italian provinces. The researchers linked individuals to a PM exposure value determined from the average concentration of urban, rural, and near-traffic stations in each person’s province of residence.
For 59,950 women across Italy who were at high risk for fracture, the researchers found 64.5% with bone mineral density that was defined as osteoporotic. At PM10 concentrations of 30 mcg/m3 or greater, there was a significantly higher likelihood of osteoporosis at both the femoral neck (odds ratio, 1.15) and lumbar spine (OR, 1.17).
The likelihood of osteoporosis was slightly greater with PM2.5 at concentrations of 25 mcg/m3 or more at the femoral neck (OR, 1.22) and lumbar spine (OR, 1.18). These comparisons were adjusted for age, body mass index (BMI), presence of prevalent fragility fractures, family history of osteoporosis, menopause, glucocorticoid use, comorbidities, and for residency in northern, central, or southern Italy.
Both thresholds of PM10 > 30 mcg/m3 and PM2.5 > 25 mcg/m3 “are considered safe … by the World Health Organization,” Dr. Adami pointed out.
“If you live in a place where the chronic exposure is less than 30 mcg/m3, you probably have slightly lower risk of osteoporosis as compared to those who live in a highly industrialized, polluted zone,” he explained.
“The cortical bone – femoral neck – seemed to be more susceptible, compared to trabecular bone, which is the lumbar spine. We have no idea why this is true, but we might speculate that somehow chronic inflammation like the [kind] seen in rheumatoid arthritis might be responsible for cortical bone impairment and not trabecular bone impairment,” Dr. Adami said.
One audience member, Kenneth Poole, BM, PhD, senior lecturer and honorary consultant in Metabolic Bone Disease and Rheumatology at the University of Cambridge (England), asked whether it was possible to account for the possibility of confounding caused by areas with dense housing in places where the particulate matter would be highest, and where residents may be less active and use stairs less often.
Dr. Adami noted that confounding is indeed a possibility, but he said Italy is unique in that its most polluted area – the Po River valley – is also its most wealthy area and in general has less crowded living situations with a healthier population, which could have attenuated, rather than reinforced, the results.
Does air pollution have an immunologic effect?
In interviews with this news organization, session comoderators Filipe Araújo, MD, and Irene Bultink, MD, PhD, said that the growth in evidence for the impact of air pollution on risk for, and severity of, various diseases suggests air pollution might have an immunologic effect.
“I think it’s very important to point this out. I also think it’s very hard to rule out confounding, because when you’re living in a city with crowded housing you may not walk or ride your bike but instead go by car or metro, and [the lifestyle is different],” said Dr. Bultink of Amsterdam University Medical Centers.
“It stresses that these diseases [that are associated with air pollution] although they are different in their pathophysiology, it points toward the systemic nature of rheumatic diseases, including osteoporosis,” said Dr. Araújo of Hospital Cuf Cascais (Portugal) and Hospital Ortopédico de Sant’Ana, Parede, Portugal.
The study was independently supported.Dr. Adami disclosed being a shareholder of Galapagos and Theramex.
A version of this article first appeared on Medscape.com.
AT THE EULAR 2022 CONGRESS
Bimekizumab calms psoriatic arthritis in phase 3 ‘BE’ trials
COPENHAGEN – For patients with active psoriatic arthritis for whom tumor necrosis factor (TNF) inhibitors failed to produce an adequate response, use of the dual interleukin-17 (IL-17) inhibitor bimekizumab (Bimzelx) was associated with significant improvement in joint, skin, and health-related quality-of-life parameters, compared with placebo, reported investigators in the phase 3, double-blind, randomized BE COMPLETE trial.
The primary endpoint, which was the percentage of patients who had 50% improvement in American College of Rheumatology response criteria (ACR50) at 16 weeks, was achieved in 43.4% of patients assigned to receive bimekizumab 160 mg every 4 weeks, compared with 6.8% among patients who received placebo, reported Joseph F. Merola, MD, a dermatologist and rheumatologist at Brigham and Women’s Hospital in Boston.
“The high-level and exciting take-home [message is] that BE COMPLETE did meet all primary and all ranked secondary endpoints at week 16,” he said at the annual European Congress of Rheumatology.
Also at the congress, Iain McInnes, MD, PhD, of the Institute of Infection, Immunity, and Inflammation at the University of Glasgow, Scotland, presented data from a second phase 3, double-blind, randomized trial called BE OPTIMAL that showed similar benefits for patients with psoriatic arthritis who had not previously received biologic disease-modifying antirheumatic drugs.
“This is a new mode of action, inhibiting two cytokines simultaneously,” he said in a late-breaking oral abstract session.
As previously reported by this news organization, use of bimekizumab led to rapid reductions in signs and symptoms of radiographic axial spondyloarthritis in the phase 3 trial called BE MOBILE 2.
Bimekizumab is a monoclonal immunoglobulin G1 antibody that selectively inhibits IL-17A and IL-17F. It is approved in the European Union for treating adults with moderate to severe plaque psoriasis.
BE COMPLETE efficacy
Inclusion criteria comprised adult-onset psoriatic arthritis meeting Classification Criteria for Psoriatic Arthritis (CASPAR) for at least 6 months; tender and swollen joint counts of at least 3/68; one or more active psoriatic lesions; and/or a documented history of psoriasis characterized by intolerance to one or two TNF inhibitors or failure of TNF inhibitors. Patients were randomly assigned in a 2:1 ratio to receive either bimekizumab 160 mg every 4 weeks (n = 267) or placebo (n = 133) for 16 weeks.
Some participants are being followed in the extension BE VITAL study, which will evaluate response to treatment and long-term safety. Patients who do enroll in the extension study will be followed for safety for a period of 20 weeks after the last dose.
As noted before, the trial met its primary endpoint of a significant improvement over placebo in ACR50 (hazard ratio, 11.1; P < .001).
In addition, the trial met all ranked secondary endpoints, including the Health Assessment Questionnaire–Disability Index change from baseline, 90% improvement in the Psoriasis Area and Severity Index (PASI90), Short-Form 36-Item Health Survey, and minimal disease activity (P < .001 for all comparisons).
Improvement with bimekizumab was rapid; curves began to separate from placebo by week 4, Dr. Merola said.
BE OPTIMAL efficacy
In this study, which had the same eligibility criteria as BE COMPLETE, patients were randomly assigned in a 2:3:1 ratio to receive 16 weeks of treatment with either placebo, bimekizumab 160 mg every 4 weeks, or adalimumab 40 mg every 2 weeks as a reference treatment.
This trial also met its primary and ranked secondary endpoints, which were similar to those of BE COMPLETE but also included measures of pooled resolution of enthesitis and dactylitis and change from baseline in van der Heijde modified total Sharp score (P < .001 for all comparisons).
In all, 43.9% of patients who received bimekizumab and 45.7% who received adalimumab achieved ACR50 at week 16, compared with 10% of patients who received placebo. The difference between the placebo and bimekizumab groups was significant (P < .001).
Safety
More patients who received the two active agents in this trial had treatment-emergent adverse events (TEAEs) in comparison with those in the placebo arm, but the incidence of serious TEAEs was less than 2% in each arm.
The most frequent events were nasopharyngitis, upper respiratory tract infection, headache, diarrhea, and hypertension.
Patients tolerated bimekizumab well, and there were no unexpected safety signals, Dr. McInnes said.
Clues to efficacy
In the question-and-answer session following Dr. McInnes’ presentation, Ronald Van Vollenhoven, MD, of the University of Amsterdam, said, “I have a question that is sort of generic in studies of psoriatic arthritis, so it does not only apply to this study, but the skin responses seem to be excellent – PASI90 sounds wonderful – but given that this is the case, is it reasonable to claim that the study is double-blinded in respect to the joints?”
Dr. McInnes replied that while he has considered this conundrum for many years in trials of drugs for psoriatic arthritis, “it doesn’t seem to be a major determinant of the outcome.”
The studies were supported by UCB Pharma. Dr. Merola and Dr. McInnes have consulted for UCB and other pharmaceutical companies that market drugs for psoriatic arthritis and psoriasis. Dr. Van Vollenhoven has received research support, has consulted for, and has spoken on behalf of UCB and other pharmaceutical companies.
A version of this article first appeared on Medscape.com.
COPENHAGEN – For patients with active psoriatic arthritis for whom tumor necrosis factor (TNF) inhibitors failed to produce an adequate response, use of the dual interleukin-17 (IL-17) inhibitor bimekizumab (Bimzelx) was associated with significant improvement in joint, skin, and health-related quality-of-life parameters, compared with placebo, reported investigators in the phase 3, double-blind, randomized BE COMPLETE trial.
The primary endpoint, which was the percentage of patients who had 50% improvement in American College of Rheumatology response criteria (ACR50) at 16 weeks, was achieved in 43.4% of patients assigned to receive bimekizumab 160 mg every 4 weeks, compared with 6.8% among patients who received placebo, reported Joseph F. Merola, MD, a dermatologist and rheumatologist at Brigham and Women’s Hospital in Boston.
“The high-level and exciting take-home [message is] that BE COMPLETE did meet all primary and all ranked secondary endpoints at week 16,” he said at the annual European Congress of Rheumatology.
Also at the congress, Iain McInnes, MD, PhD, of the Institute of Infection, Immunity, and Inflammation at the University of Glasgow, Scotland, presented data from a second phase 3, double-blind, randomized trial called BE OPTIMAL that showed similar benefits for patients with psoriatic arthritis who had not previously received biologic disease-modifying antirheumatic drugs.
“This is a new mode of action, inhibiting two cytokines simultaneously,” he said in a late-breaking oral abstract session.
As previously reported by this news organization, use of bimekizumab led to rapid reductions in signs and symptoms of radiographic axial spondyloarthritis in the phase 3 trial called BE MOBILE 2.
Bimekizumab is a monoclonal immunoglobulin G1 antibody that selectively inhibits IL-17A and IL-17F. It is approved in the European Union for treating adults with moderate to severe plaque psoriasis.
BE COMPLETE efficacy
Inclusion criteria comprised adult-onset psoriatic arthritis meeting Classification Criteria for Psoriatic Arthritis (CASPAR) for at least 6 months; tender and swollen joint counts of at least 3/68; one or more active psoriatic lesions; and/or a documented history of psoriasis characterized by intolerance to one or two TNF inhibitors or failure of TNF inhibitors. Patients were randomly assigned in a 2:1 ratio to receive either bimekizumab 160 mg every 4 weeks (n = 267) or placebo (n = 133) for 16 weeks.
Some participants are being followed in the extension BE VITAL study, which will evaluate response to treatment and long-term safety. Patients who do enroll in the extension study will be followed for safety for a period of 20 weeks after the last dose.
As noted before, the trial met its primary endpoint of a significant improvement over placebo in ACR50 (hazard ratio, 11.1; P < .001).
In addition, the trial met all ranked secondary endpoints, including the Health Assessment Questionnaire–Disability Index change from baseline, 90% improvement in the Psoriasis Area and Severity Index (PASI90), Short-Form 36-Item Health Survey, and minimal disease activity (P < .001 for all comparisons).
Improvement with bimekizumab was rapid; curves began to separate from placebo by week 4, Dr. Merola said.
BE OPTIMAL efficacy
In this study, which had the same eligibility criteria as BE COMPLETE, patients were randomly assigned in a 2:3:1 ratio to receive 16 weeks of treatment with either placebo, bimekizumab 160 mg every 4 weeks, or adalimumab 40 mg every 2 weeks as a reference treatment.
This trial also met its primary and ranked secondary endpoints, which were similar to those of BE COMPLETE but also included measures of pooled resolution of enthesitis and dactylitis and change from baseline in van der Heijde modified total Sharp score (P < .001 for all comparisons).
In all, 43.9% of patients who received bimekizumab and 45.7% who received adalimumab achieved ACR50 at week 16, compared with 10% of patients who received placebo. The difference between the placebo and bimekizumab groups was significant (P < .001).
Safety
More patients who received the two active agents in this trial had treatment-emergent adverse events (TEAEs) in comparison with those in the placebo arm, but the incidence of serious TEAEs was less than 2% in each arm.
The most frequent events were nasopharyngitis, upper respiratory tract infection, headache, diarrhea, and hypertension.
Patients tolerated bimekizumab well, and there were no unexpected safety signals, Dr. McInnes said.
Clues to efficacy
In the question-and-answer session following Dr. McInnes’ presentation, Ronald Van Vollenhoven, MD, of the University of Amsterdam, said, “I have a question that is sort of generic in studies of psoriatic arthritis, so it does not only apply to this study, but the skin responses seem to be excellent – PASI90 sounds wonderful – but given that this is the case, is it reasonable to claim that the study is double-blinded in respect to the joints?”
Dr. McInnes replied that while he has considered this conundrum for many years in trials of drugs for psoriatic arthritis, “it doesn’t seem to be a major determinant of the outcome.”
The studies were supported by UCB Pharma. Dr. Merola and Dr. McInnes have consulted for UCB and other pharmaceutical companies that market drugs for psoriatic arthritis and psoriasis. Dr. Van Vollenhoven has received research support, has consulted for, and has spoken on behalf of UCB and other pharmaceutical companies.
A version of this article first appeared on Medscape.com.
COPENHAGEN – For patients with active psoriatic arthritis for whom tumor necrosis factor (TNF) inhibitors failed to produce an adequate response, use of the dual interleukin-17 (IL-17) inhibitor bimekizumab (Bimzelx) was associated with significant improvement in joint, skin, and health-related quality-of-life parameters, compared with placebo, reported investigators in the phase 3, double-blind, randomized BE COMPLETE trial.
The primary endpoint, which was the percentage of patients who had 50% improvement in American College of Rheumatology response criteria (ACR50) at 16 weeks, was achieved in 43.4% of patients assigned to receive bimekizumab 160 mg every 4 weeks, compared with 6.8% among patients who received placebo, reported Joseph F. Merola, MD, a dermatologist and rheumatologist at Brigham and Women’s Hospital in Boston.
“The high-level and exciting take-home [message is] that BE COMPLETE did meet all primary and all ranked secondary endpoints at week 16,” he said at the annual European Congress of Rheumatology.
Also at the congress, Iain McInnes, MD, PhD, of the Institute of Infection, Immunity, and Inflammation at the University of Glasgow, Scotland, presented data from a second phase 3, double-blind, randomized trial called BE OPTIMAL that showed similar benefits for patients with psoriatic arthritis who had not previously received biologic disease-modifying antirheumatic drugs.
“This is a new mode of action, inhibiting two cytokines simultaneously,” he said in a late-breaking oral abstract session.
As previously reported by this news organization, use of bimekizumab led to rapid reductions in signs and symptoms of radiographic axial spondyloarthritis in the phase 3 trial called BE MOBILE 2.
Bimekizumab is a monoclonal immunoglobulin G1 antibody that selectively inhibits IL-17A and IL-17F. It is approved in the European Union for treating adults with moderate to severe plaque psoriasis.
BE COMPLETE efficacy
Inclusion criteria comprised adult-onset psoriatic arthritis meeting Classification Criteria for Psoriatic Arthritis (CASPAR) for at least 6 months; tender and swollen joint counts of at least 3/68; one or more active psoriatic lesions; and/or a documented history of psoriasis characterized by intolerance to one or two TNF inhibitors or failure of TNF inhibitors. Patients were randomly assigned in a 2:1 ratio to receive either bimekizumab 160 mg every 4 weeks (n = 267) or placebo (n = 133) for 16 weeks.
Some participants are being followed in the extension BE VITAL study, which will evaluate response to treatment and long-term safety. Patients who do enroll in the extension study will be followed for safety for a period of 20 weeks after the last dose.
As noted before, the trial met its primary endpoint of a significant improvement over placebo in ACR50 (hazard ratio, 11.1; P < .001).
In addition, the trial met all ranked secondary endpoints, including the Health Assessment Questionnaire–Disability Index change from baseline, 90% improvement in the Psoriasis Area and Severity Index (PASI90), Short-Form 36-Item Health Survey, and minimal disease activity (P < .001 for all comparisons).
Improvement with bimekizumab was rapid; curves began to separate from placebo by week 4, Dr. Merola said.
BE OPTIMAL efficacy
In this study, which had the same eligibility criteria as BE COMPLETE, patients were randomly assigned in a 2:3:1 ratio to receive 16 weeks of treatment with either placebo, bimekizumab 160 mg every 4 weeks, or adalimumab 40 mg every 2 weeks as a reference treatment.
This trial also met its primary and ranked secondary endpoints, which were similar to those of BE COMPLETE but also included measures of pooled resolution of enthesitis and dactylitis and change from baseline in van der Heijde modified total Sharp score (P < .001 for all comparisons).
In all, 43.9% of patients who received bimekizumab and 45.7% who received adalimumab achieved ACR50 at week 16, compared with 10% of patients who received placebo. The difference between the placebo and bimekizumab groups was significant (P < .001).
Safety
More patients who received the two active agents in this trial had treatment-emergent adverse events (TEAEs) in comparison with those in the placebo arm, but the incidence of serious TEAEs was less than 2% in each arm.
The most frequent events were nasopharyngitis, upper respiratory tract infection, headache, diarrhea, and hypertension.
Patients tolerated bimekizumab well, and there were no unexpected safety signals, Dr. McInnes said.
Clues to efficacy
In the question-and-answer session following Dr. McInnes’ presentation, Ronald Van Vollenhoven, MD, of the University of Amsterdam, said, “I have a question that is sort of generic in studies of psoriatic arthritis, so it does not only apply to this study, but the skin responses seem to be excellent – PASI90 sounds wonderful – but given that this is the case, is it reasonable to claim that the study is double-blinded in respect to the joints?”
Dr. McInnes replied that while he has considered this conundrum for many years in trials of drugs for psoriatic arthritis, “it doesn’t seem to be a major determinant of the outcome.”
The studies were supported by UCB Pharma. Dr. Merola and Dr. McInnes have consulted for UCB and other pharmaceutical companies that market drugs for psoriatic arthritis and psoriasis. Dr. Van Vollenhoven has received research support, has consulted for, and has spoken on behalf of UCB and other pharmaceutical companies.
A version of this article first appeared on Medscape.com.
AT THE EULAR 2022 CONGRESS