User login
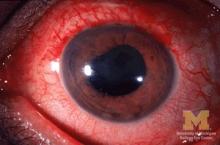
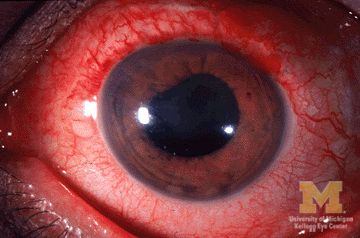
Many patients with acute anterior uveitis may have undiagnosed spondyloarthritis
More than half of patients with noninfectious acute anterior uveitis seen in ophthalmology clinics in a new cross-sectional study were found by rheumatologists to have spondyloarthritis (SpA), prompting the researchers to recommend referring “all patients with AAU reporting musculoskeletal symptoms to rheumatologists.”
The results also suggest that “rheumatologists should consider that SpA in AAU patients might present ‘atypically’ with no or mild back pain starting after the age of 45 years and lasting shorter than 3 months,” according to first author Judith Rademacher, MD, and colleagues at Charité–Universitätsmedizin Berlin, who published their work online in Arthritis & Rheumatology.
During July 2017–April 2021, the study team prospectively assessed 189 consecutive adult patients with noninfectious AAU at ophthalmology clinics in the Berlin area. The patients had rheumatologic examinations and underwent pelvic x-ray if they had back pain as well as MRI of sacroiliac joints regardless of back pain unless there was a contraindication. The patients had a mean age of nearly 41 years, and 54.5% were male.
Of the 189 patients with AAU, the researchers diagnosed SpA in 106, including 74 (70%) who had been previously undiagnosed. A total of 99 (93%) had predominately axial SpA, and 7 (7%) had peripheral SpA.
A multivariable logistic regression assessment found that male sex (odds ratio, 2.1; 95% confidence interval, 1.1-4.2), HLA-B27 positivity (OR, 6.3; 95% CI, 2.4-16.4), elevated C-reactive protein (OR, 4.8; 95% CI, 1.9-12.4), and psoriasis (OR, 12.5; 95% CI, 1.3-120.2) were significantly associated with SpA in patients with AAU. No ophthalmologic factors were significantly associated with SpA.
Among all patients, an adaptation of the Assessment of SpondyloArthritis International Society (ASAS) referral tool demonstrated lower specificity for SpA recognition than did the Dublin Uveitis Evaluation Tool (28% vs. 42%). The ASAS referral took had a slightly greater sensitivity than the Dublin Uveitis Evaluation Tool (80% vs. 78%).
“Taking into account only AAU patients without prior diagnosis of SpA, a rheumatologist would have to see 2.1 patients fulfilling the ASAS tool or 1.9 patients fulfilling the DUET to diagnose one patient with SpA. However, with both referral strategies more than 20% of SpA patients would have been missed,” the researchers wrote. “This might be due to an ‘unusual presentation’ of SpA in those patients as their back pain started more often after the age of 45 years, lasted shorter than 3 months and thus, ASAS classification criteria were less frequently fulfilled.”
The researchers acknowledged possible selection bias because 15 patients with an incomplete rheumatologic evaluation were excluded. MRI also was routinely done for sacroiliac joints alone, although it was possible for clinician to order spinal MRI. In addition, the researchers allowed patients with AAU into the study regardless of their current treatment, meaning that it may have been possible for some patients receiving biologic disease-modifying antirheumatic drugs to not be correctly identified as having SpA if the treatment improved their musculoskeletal symptoms.
Expert commentary
There are a number of diseases associated with SpA, including AAU, noted Kristine Kuhn, MD, PhD, an associate professor of medicine at the University of Colorado at Denver, Aurora, who was not involved in the study.
“As a rheumatologist, we are quite aware of [uveitis] as an association, and we are usually asking our patients about eye symptoms because of this association,” Dr. Kuhn said in an interview.
While just over half of the patients with AAU also met the criteria for SpA, “that doesn’t necessarily mean diagnosis per se because classification criteria are based on a series of features to homogenize a group of people for clinical research studies. So it doesn’t always align 100% with diagnosis, but it does give us an indication that a little of over half of people with anterior uveitis will have underlying spondyloarthritis and should be evaluated by a rheumatologist.”
Dr. Kuhn also highlighted the associations of male sex, HLA-B27 positivity, and concomitant presence of psoriasis. “I bring those up because I find those to be interesting associations. We have known those for years to be associated with axial spondyloarthritis, but when you look at the actual data, I would just put a little bit of caution to those conclusions.”
She pointed out that, although the link of male sex to SpA in patients with AAU was statistically significant, it is not a clinically meaningful association.
Dr. Kuhn also noted that caution should be used when interpreting the HLA-B27 positivity data. “The caution that I put there is that this was conducted in Germany, and we know that Northern European populations tend to be more enriched for HLA-B27 genes, so what that association would be in a more diverse population is unknown.
“I think ophthalmologists are really good when they see a patient with [acute]-onset anterior uveitis; they have a suspicion that there’s probably another systemic disease that they should be looking at. What this tells us as a physician community is that maybe we should lower the threshold for getting patients into rheumatology and looking at whether or not the patient has underlying spondyloarthritis,” she said.
AbbVie supported the study with an unrestricted research grant but had no role in the study design or in the collection, analysis, or interpretation of the data, the writing of the manuscript, or the decision to submit the manuscript for publication. Of the study’s 12 authors, 2 reported having no financial disclosures. All others reported financial relationships with pharmaceutical companies, including AbbVie.
More than half of patients with noninfectious acute anterior uveitis seen in ophthalmology clinics in a new cross-sectional study were found by rheumatologists to have spondyloarthritis (SpA), prompting the researchers to recommend referring “all patients with AAU reporting musculoskeletal symptoms to rheumatologists.”
The results also suggest that “rheumatologists should consider that SpA in AAU patients might present ‘atypically’ with no or mild back pain starting after the age of 45 years and lasting shorter than 3 months,” according to first author Judith Rademacher, MD, and colleagues at Charité–Universitätsmedizin Berlin, who published their work online in Arthritis & Rheumatology.
During July 2017–April 2021, the study team prospectively assessed 189 consecutive adult patients with noninfectious AAU at ophthalmology clinics in the Berlin area. The patients had rheumatologic examinations and underwent pelvic x-ray if they had back pain as well as MRI of sacroiliac joints regardless of back pain unless there was a contraindication. The patients had a mean age of nearly 41 years, and 54.5% were male.
Of the 189 patients with AAU, the researchers diagnosed SpA in 106, including 74 (70%) who had been previously undiagnosed. A total of 99 (93%) had predominately axial SpA, and 7 (7%) had peripheral SpA.
A multivariable logistic regression assessment found that male sex (odds ratio, 2.1; 95% confidence interval, 1.1-4.2), HLA-B27 positivity (OR, 6.3; 95% CI, 2.4-16.4), elevated C-reactive protein (OR, 4.8; 95% CI, 1.9-12.4), and psoriasis (OR, 12.5; 95% CI, 1.3-120.2) were significantly associated with SpA in patients with AAU. No ophthalmologic factors were significantly associated with SpA.
Among all patients, an adaptation of the Assessment of SpondyloArthritis International Society (ASAS) referral tool demonstrated lower specificity for SpA recognition than did the Dublin Uveitis Evaluation Tool (28% vs. 42%). The ASAS referral took had a slightly greater sensitivity than the Dublin Uveitis Evaluation Tool (80% vs. 78%).
“Taking into account only AAU patients without prior diagnosis of SpA, a rheumatologist would have to see 2.1 patients fulfilling the ASAS tool or 1.9 patients fulfilling the DUET to diagnose one patient with SpA. However, with both referral strategies more than 20% of SpA patients would have been missed,” the researchers wrote. “This might be due to an ‘unusual presentation’ of SpA in those patients as their back pain started more often after the age of 45 years, lasted shorter than 3 months and thus, ASAS classification criteria were less frequently fulfilled.”
The researchers acknowledged possible selection bias because 15 patients with an incomplete rheumatologic evaluation were excluded. MRI also was routinely done for sacroiliac joints alone, although it was possible for clinician to order spinal MRI. In addition, the researchers allowed patients with AAU into the study regardless of their current treatment, meaning that it may have been possible for some patients receiving biologic disease-modifying antirheumatic drugs to not be correctly identified as having SpA if the treatment improved their musculoskeletal symptoms.
Expert commentary
There are a number of diseases associated with SpA, including AAU, noted Kristine Kuhn, MD, PhD, an associate professor of medicine at the University of Colorado at Denver, Aurora, who was not involved in the study.
“As a rheumatologist, we are quite aware of [uveitis] as an association, and we are usually asking our patients about eye symptoms because of this association,” Dr. Kuhn said in an interview.
While just over half of the patients with AAU also met the criteria for SpA, “that doesn’t necessarily mean diagnosis per se because classification criteria are based on a series of features to homogenize a group of people for clinical research studies. So it doesn’t always align 100% with diagnosis, but it does give us an indication that a little of over half of people with anterior uveitis will have underlying spondyloarthritis and should be evaluated by a rheumatologist.”
Dr. Kuhn also highlighted the associations of male sex, HLA-B27 positivity, and concomitant presence of psoriasis. “I bring those up because I find those to be interesting associations. We have known those for years to be associated with axial spondyloarthritis, but when you look at the actual data, I would just put a little bit of caution to those conclusions.”
She pointed out that, although the link of male sex to SpA in patients with AAU was statistically significant, it is not a clinically meaningful association.
Dr. Kuhn also noted that caution should be used when interpreting the HLA-B27 positivity data. “The caution that I put there is that this was conducted in Germany, and we know that Northern European populations tend to be more enriched for HLA-B27 genes, so what that association would be in a more diverse population is unknown.
“I think ophthalmologists are really good when they see a patient with [acute]-onset anterior uveitis; they have a suspicion that there’s probably another systemic disease that they should be looking at. What this tells us as a physician community is that maybe we should lower the threshold for getting patients into rheumatology and looking at whether or not the patient has underlying spondyloarthritis,” she said.
AbbVie supported the study with an unrestricted research grant but had no role in the study design or in the collection, analysis, or interpretation of the data, the writing of the manuscript, or the decision to submit the manuscript for publication. Of the study’s 12 authors, 2 reported having no financial disclosures. All others reported financial relationships with pharmaceutical companies, including AbbVie.
More than half of patients with noninfectious acute anterior uveitis seen in ophthalmology clinics in a new cross-sectional study were found by rheumatologists to have spondyloarthritis (SpA), prompting the researchers to recommend referring “all patients with AAU reporting musculoskeletal symptoms to rheumatologists.”
The results also suggest that “rheumatologists should consider that SpA in AAU patients might present ‘atypically’ with no or mild back pain starting after the age of 45 years and lasting shorter than 3 months,” according to first author Judith Rademacher, MD, and colleagues at Charité–Universitätsmedizin Berlin, who published their work online in Arthritis & Rheumatology.
During July 2017–April 2021, the study team prospectively assessed 189 consecutive adult patients with noninfectious AAU at ophthalmology clinics in the Berlin area. The patients had rheumatologic examinations and underwent pelvic x-ray if they had back pain as well as MRI of sacroiliac joints regardless of back pain unless there was a contraindication. The patients had a mean age of nearly 41 years, and 54.5% were male.
Of the 189 patients with AAU, the researchers diagnosed SpA in 106, including 74 (70%) who had been previously undiagnosed. A total of 99 (93%) had predominately axial SpA, and 7 (7%) had peripheral SpA.
A multivariable logistic regression assessment found that male sex (odds ratio, 2.1; 95% confidence interval, 1.1-4.2), HLA-B27 positivity (OR, 6.3; 95% CI, 2.4-16.4), elevated C-reactive protein (OR, 4.8; 95% CI, 1.9-12.4), and psoriasis (OR, 12.5; 95% CI, 1.3-120.2) were significantly associated with SpA in patients with AAU. No ophthalmologic factors were significantly associated with SpA.
Among all patients, an adaptation of the Assessment of SpondyloArthritis International Society (ASAS) referral tool demonstrated lower specificity for SpA recognition than did the Dublin Uveitis Evaluation Tool (28% vs. 42%). The ASAS referral took had a slightly greater sensitivity than the Dublin Uveitis Evaluation Tool (80% vs. 78%).
“Taking into account only AAU patients without prior diagnosis of SpA, a rheumatologist would have to see 2.1 patients fulfilling the ASAS tool or 1.9 patients fulfilling the DUET to diagnose one patient with SpA. However, with both referral strategies more than 20% of SpA patients would have been missed,” the researchers wrote. “This might be due to an ‘unusual presentation’ of SpA in those patients as their back pain started more often after the age of 45 years, lasted shorter than 3 months and thus, ASAS classification criteria were less frequently fulfilled.”
The researchers acknowledged possible selection bias because 15 patients with an incomplete rheumatologic evaluation were excluded. MRI also was routinely done for sacroiliac joints alone, although it was possible for clinician to order spinal MRI. In addition, the researchers allowed patients with AAU into the study regardless of their current treatment, meaning that it may have been possible for some patients receiving biologic disease-modifying antirheumatic drugs to not be correctly identified as having SpA if the treatment improved their musculoskeletal symptoms.
Expert commentary
There are a number of diseases associated with SpA, including AAU, noted Kristine Kuhn, MD, PhD, an associate professor of medicine at the University of Colorado at Denver, Aurora, who was not involved in the study.
“As a rheumatologist, we are quite aware of [uveitis] as an association, and we are usually asking our patients about eye symptoms because of this association,” Dr. Kuhn said in an interview.
While just over half of the patients with AAU also met the criteria for SpA, “that doesn’t necessarily mean diagnosis per se because classification criteria are based on a series of features to homogenize a group of people for clinical research studies. So it doesn’t always align 100% with diagnosis, but it does give us an indication that a little of over half of people with anterior uveitis will have underlying spondyloarthritis and should be evaluated by a rheumatologist.”
Dr. Kuhn also highlighted the associations of male sex, HLA-B27 positivity, and concomitant presence of psoriasis. “I bring those up because I find those to be interesting associations. We have known those for years to be associated with axial spondyloarthritis, but when you look at the actual data, I would just put a little bit of caution to those conclusions.”
She pointed out that, although the link of male sex to SpA in patients with AAU was statistically significant, it is not a clinically meaningful association.
Dr. Kuhn also noted that caution should be used when interpreting the HLA-B27 positivity data. “The caution that I put there is that this was conducted in Germany, and we know that Northern European populations tend to be more enriched for HLA-B27 genes, so what that association would be in a more diverse population is unknown.
“I think ophthalmologists are really good when they see a patient with [acute]-onset anterior uveitis; they have a suspicion that there’s probably another systemic disease that they should be looking at. What this tells us as a physician community is that maybe we should lower the threshold for getting patients into rheumatology and looking at whether or not the patient has underlying spondyloarthritis,” she said.
AbbVie supported the study with an unrestricted research grant but had no role in the study design or in the collection, analysis, or interpretation of the data, the writing of the manuscript, or the decision to submit the manuscript for publication. Of the study’s 12 authors, 2 reported having no financial disclosures. All others reported financial relationships with pharmaceutical companies, including AbbVie.
FROM ARTHRITIS & RHEUMATOLOGY
Gout flares linked to transient jump in MI, stroke risk
There is evidence that gout and heart disease are mechanistically linked by inflammation and patients with gout are at elevated risk for cardiovascular disease (CVD). But do gout flares, on their own, affect short-term risk for CV events? A new analysis based on records from British medical practices suggests that might be the case.
Risk for myocardial infarction or stroke climbed in the weeks after individual gout flare-ups in the study’s more than 60,000 patients with a recent gout diagnosis. The jump in risk, significant but small in absolute terms, held for about 4 months in the case-control study before going away.
A sensitivity analysis that excluded patients who already had CVD when their gout was diagnosed yielded similar results.
The observational study isn’t able to show that gout flares themselves transiently raise the risk for MI or stroke, but it’s enough to send a cautionary message to physicians who care for patients with gout, rheumatologist Abhishek Abhishek, PhD, Nottingham (England) City Hospital, said in an interview.
In such patients who also have conditions like hypertension, diabetes, or dyslipidemia, or a history of heart disease, he said, it’s important “to manage risk factors really aggressively, knowing that when these patients have a gout flare, there’s a temporary increase in risk of a cardiovascular event.”
Managing their absolute CV risk – whether with drug therapy, lifestyle changes, or other interventions – should help limit the transient jump in risk for MI or stroke following a gout flare, proposed Dr. Abhishek, who is senior author on the study published in JAMA, with lead author Edoardo Cipolletta, MD, also from Nottingham City Hospital.
First robust evidence
The case-control study, which involved more than 60,000 patients with a recent gout diagnosis, some who went on to have MI or stroke, looked at rates of such events at different time intervals after gout flares. Those who experienced such events showed a more than 90% increased likelihood of a gout flare-up in the preceding 60 days, a greater than 50% chance of a flare between 60 and 120 days before the event, but no increased likelihood prior to 120 days before the event.
Such a link between gout flares and CV events “has been suspected but never proven,” observed rheumatologist Hyon K. Choi, MD, Harvard Medical School, Boston, who was not associated with the analysis. “This is the first time it has actually been shown in a robust way,” he said in an interview.
The study suggests a “likely causative relationship” between gout flares and CV events, but – as the published report noted – has limitations like any observational study, said Dr. Choi, who also directs the Gout & Crystal Arthropathy Center at Massachusetts General Hospital, Boston. “Hopefully, this can be replicated in other cohorts.”
The analysis controlled for a number of relevant potential confounders, he noted, but couldn’t account for all issues that could argue against gout flares as a direct cause of the MIs and strokes.
Gout attacks are a complex experience with a range of potential indirect effects on CV risk, Dr. Choi observed. They can immobilize patients, possibly raising their risk for thrombotic events, for example. They can be exceptionally painful, which causes stress and can lead to frequent or chronic use of glucocorticoids or NSAIDs, all of which can exacerbate high blood pressure and possibly worsen CV risk.
A unique insight
The timing of gout flares relative to acute vascular events hasn’t been fully explored, observed an accompanying editorial. The current study’s “unique insight,” it stated, “is that disease activity from gout was associated with an incremental increase in risk for acute vascular events during the time period immediately following the gout flare.”
Although the study is observational, a “large body of evidence from animal and human research, mechanistic insights, and clinical interventions” support an association between flares and vascular events and “make a causal link eminently reasonable,” stated the editorialists, Jeffrey L. Anderson, MD, and Kirk U. Knowlton, MD, both with Intermountain Medical Center, Salt Lake City, Utah.
The findings, they wrote, “should alert clinicians and patients to the increased cardiovascular risk in the weeks beginning after a gout flare and should focus attention on optimizing preventive measures.” Those can include “lifestyle measures and standard risk-factor control including adherence to diet, statins, anti-inflammatory drugs (e.g., aspirin, colchicine), smoking cessation, diabetic and blood pressure control, and antithrombotic medications as indicated.”
Dr. Choi said the current results argue for more liberal use of colchicine, and for preferring colchicine over other anti-inflammatories, in patients with gout and traditional CV risk factors, given multiple randomized trials supporting the drug’s use in such cases. “If you use colchicine, you are covering their heart disease risk as well as their gout. It’s two birds with one stone.”
Nested case-control study
The investigators accessed electronic health records from 96,153 patients with recently diagnosed gout in England from 1997 to 2020; the cohort’s mean age was about 76 years, and 69% of participants were men. They matched 10,475 patients with at least one CV event to 52,099 others who didn’t have such an event by age, sex, and time from gout diagnosis. In each matched set of patients, those not experiencing a CV event were assigned a flare-to-event interval based on their matching with patients who did experience such an event.
Those with CV events, compared with patients without an event, had a greater than 90% increased likelihood of experiencing a gout flare-up in the 60 days preceding the event, a more than 50% greater chance of a flare-up 60-120 days before the CV event, but no increased likelihood more than 120 days before the event.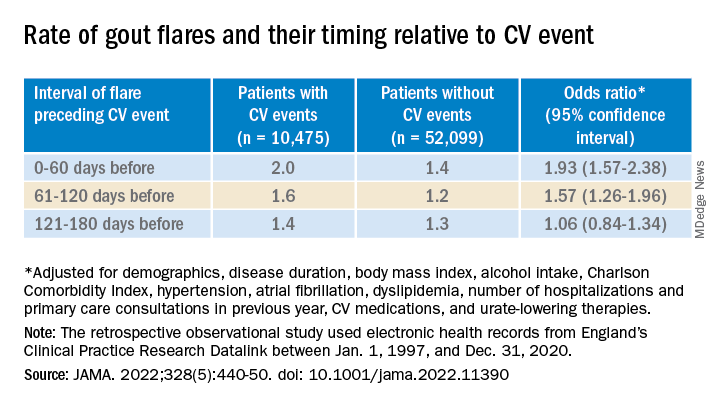
A self-controlled case series based on the same overall cohort with gout yielded similar results while sidestepping any potential for residual confounding, an inherent concern with any case–control analysis, the report notes. It involved 1,421 patients with one or more gout flare and at least one MI or stroke after the diagnosis of gout.
Among that cohort, the CV-event incidence rate ratio, adjusted for age and season of the year, by time interval after a gout flare, was 1.89 (95% confidence interval, 1.54-2.30) at 0-60 days, 1.64 (95% CI, 1.45-1.86) at 61-120 days, and1.29 (95% CI, 1.02-1.64) at 121-180 days.
Also similar, the report noted, were results of several sensitivity analyses, including one that excluded patients with confirmed CVD before their gout diagnosis; another that left out patients at low to moderate CV risk; and one that considered only gout flares treated with colchicine, corticosteroids, or NSAIDs.
The incremental CV event risks observed after flares in the study were small, which “has implications for both cost effectiveness and clinical relevance,” observed Dr. Anderson and Dr. Knowlton.
“An alternative to universal augmentation of cardiovascular risk prevention with therapies among patients with gout flares,” they wrote, would be “to further stratify risk by defining a group at highest near-term risk.” Such interventions could potentially be guided by markers of CV risk such as, for example, levels of high-sensitivity C-reactive protein or lipoprotein(a), or plaque burden on coronary-artery calcium scans.
Dr. Abhishek, Dr. Cipolletta, and the other authors reported no competing interests. Dr. Choi disclosed research support from Ironwood and Horizon; and consulting fees from Ironwood, Selecta, Horizon, Takeda, Kowa, and Vaxart. Dr. Anderson disclosed receiving grants to his institution from Novartis and Milestone.
A version of this article first appeared on Medscape.com.
There is evidence that gout and heart disease are mechanistically linked by inflammation and patients with gout are at elevated risk for cardiovascular disease (CVD). But do gout flares, on their own, affect short-term risk for CV events? A new analysis based on records from British medical practices suggests that might be the case.
Risk for myocardial infarction or stroke climbed in the weeks after individual gout flare-ups in the study’s more than 60,000 patients with a recent gout diagnosis. The jump in risk, significant but small in absolute terms, held for about 4 months in the case-control study before going away.
A sensitivity analysis that excluded patients who already had CVD when their gout was diagnosed yielded similar results.
The observational study isn’t able to show that gout flares themselves transiently raise the risk for MI or stroke, but it’s enough to send a cautionary message to physicians who care for patients with gout, rheumatologist Abhishek Abhishek, PhD, Nottingham (England) City Hospital, said in an interview.
In such patients who also have conditions like hypertension, diabetes, or dyslipidemia, or a history of heart disease, he said, it’s important “to manage risk factors really aggressively, knowing that when these patients have a gout flare, there’s a temporary increase in risk of a cardiovascular event.”
Managing their absolute CV risk – whether with drug therapy, lifestyle changes, or other interventions – should help limit the transient jump in risk for MI or stroke following a gout flare, proposed Dr. Abhishek, who is senior author on the study published in JAMA, with lead author Edoardo Cipolletta, MD, also from Nottingham City Hospital.
First robust evidence
The case-control study, which involved more than 60,000 patients with a recent gout diagnosis, some who went on to have MI or stroke, looked at rates of such events at different time intervals after gout flares. Those who experienced such events showed a more than 90% increased likelihood of a gout flare-up in the preceding 60 days, a greater than 50% chance of a flare between 60 and 120 days before the event, but no increased likelihood prior to 120 days before the event.
Such a link between gout flares and CV events “has been suspected but never proven,” observed rheumatologist Hyon K. Choi, MD, Harvard Medical School, Boston, who was not associated with the analysis. “This is the first time it has actually been shown in a robust way,” he said in an interview.
The study suggests a “likely causative relationship” between gout flares and CV events, but – as the published report noted – has limitations like any observational study, said Dr. Choi, who also directs the Gout & Crystal Arthropathy Center at Massachusetts General Hospital, Boston. “Hopefully, this can be replicated in other cohorts.”
The analysis controlled for a number of relevant potential confounders, he noted, but couldn’t account for all issues that could argue against gout flares as a direct cause of the MIs and strokes.
Gout attacks are a complex experience with a range of potential indirect effects on CV risk, Dr. Choi observed. They can immobilize patients, possibly raising their risk for thrombotic events, for example. They can be exceptionally painful, which causes stress and can lead to frequent or chronic use of glucocorticoids or NSAIDs, all of which can exacerbate high blood pressure and possibly worsen CV risk.
A unique insight
The timing of gout flares relative to acute vascular events hasn’t been fully explored, observed an accompanying editorial. The current study’s “unique insight,” it stated, “is that disease activity from gout was associated with an incremental increase in risk for acute vascular events during the time period immediately following the gout flare.”
Although the study is observational, a “large body of evidence from animal and human research, mechanistic insights, and clinical interventions” support an association between flares and vascular events and “make a causal link eminently reasonable,” stated the editorialists, Jeffrey L. Anderson, MD, and Kirk U. Knowlton, MD, both with Intermountain Medical Center, Salt Lake City, Utah.
The findings, they wrote, “should alert clinicians and patients to the increased cardiovascular risk in the weeks beginning after a gout flare and should focus attention on optimizing preventive measures.” Those can include “lifestyle measures and standard risk-factor control including adherence to diet, statins, anti-inflammatory drugs (e.g., aspirin, colchicine), smoking cessation, diabetic and blood pressure control, and antithrombotic medications as indicated.”
Dr. Choi said the current results argue for more liberal use of colchicine, and for preferring colchicine over other anti-inflammatories, in patients with gout and traditional CV risk factors, given multiple randomized trials supporting the drug’s use in such cases. “If you use colchicine, you are covering their heart disease risk as well as their gout. It’s two birds with one stone.”
Nested case-control study
The investigators accessed electronic health records from 96,153 patients with recently diagnosed gout in England from 1997 to 2020; the cohort’s mean age was about 76 years, and 69% of participants were men. They matched 10,475 patients with at least one CV event to 52,099 others who didn’t have such an event by age, sex, and time from gout diagnosis. In each matched set of patients, those not experiencing a CV event were assigned a flare-to-event interval based on their matching with patients who did experience such an event.
Those with CV events, compared with patients without an event, had a greater than 90% increased likelihood of experiencing a gout flare-up in the 60 days preceding the event, a more than 50% greater chance of a flare-up 60-120 days before the CV event, but no increased likelihood more than 120 days before the event.
A self-controlled case series based on the same overall cohort with gout yielded similar results while sidestepping any potential for residual confounding, an inherent concern with any case–control analysis, the report notes. It involved 1,421 patients with one or more gout flare and at least one MI or stroke after the diagnosis of gout.
Among that cohort, the CV-event incidence rate ratio, adjusted for age and season of the year, by time interval after a gout flare, was 1.89 (95% confidence interval, 1.54-2.30) at 0-60 days, 1.64 (95% CI, 1.45-1.86) at 61-120 days, and1.29 (95% CI, 1.02-1.64) at 121-180 days.
Also similar, the report noted, were results of several sensitivity analyses, including one that excluded patients with confirmed CVD before their gout diagnosis; another that left out patients at low to moderate CV risk; and one that considered only gout flares treated with colchicine, corticosteroids, or NSAIDs.
The incremental CV event risks observed after flares in the study were small, which “has implications for both cost effectiveness and clinical relevance,” observed Dr. Anderson and Dr. Knowlton.
“An alternative to universal augmentation of cardiovascular risk prevention with therapies among patients with gout flares,” they wrote, would be “to further stratify risk by defining a group at highest near-term risk.” Such interventions could potentially be guided by markers of CV risk such as, for example, levels of high-sensitivity C-reactive protein or lipoprotein(a), or plaque burden on coronary-artery calcium scans.
Dr. Abhishek, Dr. Cipolletta, and the other authors reported no competing interests. Dr. Choi disclosed research support from Ironwood and Horizon; and consulting fees from Ironwood, Selecta, Horizon, Takeda, Kowa, and Vaxart. Dr. Anderson disclosed receiving grants to his institution from Novartis and Milestone.
A version of this article first appeared on Medscape.com.
There is evidence that gout and heart disease are mechanistically linked by inflammation and patients with gout are at elevated risk for cardiovascular disease (CVD). But do gout flares, on their own, affect short-term risk for CV events? A new analysis based on records from British medical practices suggests that might be the case.
Risk for myocardial infarction or stroke climbed in the weeks after individual gout flare-ups in the study’s more than 60,000 patients with a recent gout diagnosis. The jump in risk, significant but small in absolute terms, held for about 4 months in the case-control study before going away.
A sensitivity analysis that excluded patients who already had CVD when their gout was diagnosed yielded similar results.
The observational study isn’t able to show that gout flares themselves transiently raise the risk for MI or stroke, but it’s enough to send a cautionary message to physicians who care for patients with gout, rheumatologist Abhishek Abhishek, PhD, Nottingham (England) City Hospital, said in an interview.
In such patients who also have conditions like hypertension, diabetes, or dyslipidemia, or a history of heart disease, he said, it’s important “to manage risk factors really aggressively, knowing that when these patients have a gout flare, there’s a temporary increase in risk of a cardiovascular event.”
Managing their absolute CV risk – whether with drug therapy, lifestyle changes, or other interventions – should help limit the transient jump in risk for MI or stroke following a gout flare, proposed Dr. Abhishek, who is senior author on the study published in JAMA, with lead author Edoardo Cipolletta, MD, also from Nottingham City Hospital.
First robust evidence
The case-control study, which involved more than 60,000 patients with a recent gout diagnosis, some who went on to have MI or stroke, looked at rates of such events at different time intervals after gout flares. Those who experienced such events showed a more than 90% increased likelihood of a gout flare-up in the preceding 60 days, a greater than 50% chance of a flare between 60 and 120 days before the event, but no increased likelihood prior to 120 days before the event.
Such a link between gout flares and CV events “has been suspected but never proven,” observed rheumatologist Hyon K. Choi, MD, Harvard Medical School, Boston, who was not associated with the analysis. “This is the first time it has actually been shown in a robust way,” he said in an interview.
The study suggests a “likely causative relationship” between gout flares and CV events, but – as the published report noted – has limitations like any observational study, said Dr. Choi, who also directs the Gout & Crystal Arthropathy Center at Massachusetts General Hospital, Boston. “Hopefully, this can be replicated in other cohorts.”
The analysis controlled for a number of relevant potential confounders, he noted, but couldn’t account for all issues that could argue against gout flares as a direct cause of the MIs and strokes.
Gout attacks are a complex experience with a range of potential indirect effects on CV risk, Dr. Choi observed. They can immobilize patients, possibly raising their risk for thrombotic events, for example. They can be exceptionally painful, which causes stress and can lead to frequent or chronic use of glucocorticoids or NSAIDs, all of which can exacerbate high blood pressure and possibly worsen CV risk.
A unique insight
The timing of gout flares relative to acute vascular events hasn’t been fully explored, observed an accompanying editorial. The current study’s “unique insight,” it stated, “is that disease activity from gout was associated with an incremental increase in risk for acute vascular events during the time period immediately following the gout flare.”
Although the study is observational, a “large body of evidence from animal and human research, mechanistic insights, and clinical interventions” support an association between flares and vascular events and “make a causal link eminently reasonable,” stated the editorialists, Jeffrey L. Anderson, MD, and Kirk U. Knowlton, MD, both with Intermountain Medical Center, Salt Lake City, Utah.
The findings, they wrote, “should alert clinicians and patients to the increased cardiovascular risk in the weeks beginning after a gout flare and should focus attention on optimizing preventive measures.” Those can include “lifestyle measures and standard risk-factor control including adherence to diet, statins, anti-inflammatory drugs (e.g., aspirin, colchicine), smoking cessation, diabetic and blood pressure control, and antithrombotic medications as indicated.”
Dr. Choi said the current results argue for more liberal use of colchicine, and for preferring colchicine over other anti-inflammatories, in patients with gout and traditional CV risk factors, given multiple randomized trials supporting the drug’s use in such cases. “If you use colchicine, you are covering their heart disease risk as well as their gout. It’s two birds with one stone.”
Nested case-control study
The investigators accessed electronic health records from 96,153 patients with recently diagnosed gout in England from 1997 to 2020; the cohort’s mean age was about 76 years, and 69% of participants were men. They matched 10,475 patients with at least one CV event to 52,099 others who didn’t have such an event by age, sex, and time from gout diagnosis. In each matched set of patients, those not experiencing a CV event were assigned a flare-to-event interval based on their matching with patients who did experience such an event.
Those with CV events, compared with patients without an event, had a greater than 90% increased likelihood of experiencing a gout flare-up in the 60 days preceding the event, a more than 50% greater chance of a flare-up 60-120 days before the CV event, but no increased likelihood more than 120 days before the event.
A self-controlled case series based on the same overall cohort with gout yielded similar results while sidestepping any potential for residual confounding, an inherent concern with any case–control analysis, the report notes. It involved 1,421 patients with one or more gout flare and at least one MI or stroke after the diagnosis of gout.
Among that cohort, the CV-event incidence rate ratio, adjusted for age and season of the year, by time interval after a gout flare, was 1.89 (95% confidence interval, 1.54-2.30) at 0-60 days, 1.64 (95% CI, 1.45-1.86) at 61-120 days, and1.29 (95% CI, 1.02-1.64) at 121-180 days.
Also similar, the report noted, were results of several sensitivity analyses, including one that excluded patients with confirmed CVD before their gout diagnosis; another that left out patients at low to moderate CV risk; and one that considered only gout flares treated with colchicine, corticosteroids, or NSAIDs.
The incremental CV event risks observed after flares in the study were small, which “has implications for both cost effectiveness and clinical relevance,” observed Dr. Anderson and Dr. Knowlton.
“An alternative to universal augmentation of cardiovascular risk prevention with therapies among patients with gout flares,” they wrote, would be “to further stratify risk by defining a group at highest near-term risk.” Such interventions could potentially be guided by markers of CV risk such as, for example, levels of high-sensitivity C-reactive protein or lipoprotein(a), or plaque burden on coronary-artery calcium scans.
Dr. Abhishek, Dr. Cipolletta, and the other authors reported no competing interests. Dr. Choi disclosed research support from Ironwood and Horizon; and consulting fees from Ironwood, Selecta, Horizon, Takeda, Kowa, and Vaxart. Dr. Anderson disclosed receiving grants to his institution from Novartis and Milestone.
A version of this article first appeared on Medscape.com.
FROM JAMA
Ustekinumab becomes second biologic approved for PsA in kids
The Food and Drug Administration has approved the dual interleukin-12 and IL-23 inhibitor ustekinumab (Stelara) for the treatment of juvenile psoriatic arthritis (jPsA) in patients aged 6 years and older, according to an Aug. 1 announcement from its manufacturer, Janssen.
The approval makes jPsA the sixth approved indication for ustekinumab, which include active psoriatic arthritis in adults, moderate to severe plaque psoriasis in both adults and children aged 6 years or older who are candidates for phototherapy or systemic therapy, moderately to severely active Crohn’s disease in adults, and moderately to severely active ulcerative colitis in adults.
In addition, ustekinumab is now the second biologic to be approved for jPsA, following the agency’s December 2021 approval of secukinumab (Cosentyx) to treat jPsA in children and adolescents aged 2 years and older as well as enthesitis-related arthritis in children and adolescents aged 4 years and older.
In pediatric patients, ustekinumab is administered as a subcutaneous injection dosed four times per year after two starter doses.
Ustekinumab’s approval is based on “an extrapolation of the established data and existing safety profile” of ustekinumab in multiple phase 3 studies in adult and pediatric patients with moderate to severe plaque psoriasis and adult patients with active PsA, according to Janssen.
“With the limited availability of pediatric patients for clinical trial inclusion, researchers can extrapolate data from trials with adults to determine the potential efficacy and tolerability of a treatment for a pediatric population,” according to the October 2021 announcement from the company that the Biologics License Application had been submitted to the FDA.
Juvenile arthritis occurs in an estimated 20-45 children per 100,000 in the United States, with about 5% of those children having jPsA, according to the National Psoriasis Foundation.
The prescribing information for ustekinumab includes specific warnings and areas of concern. The drug should not be administered to individuals with known hypersensitivity to ustekinumab. The drug may lower the ability of the immune system to fight infections and may increase risk of infections, sometimes serious, and a test for tuberculosis infection should be given before administration.
Patients taking ustekinumab should not be given a live vaccine, and their doctors should be informed if anyone in their household needs a live vaccine. They also should not receive the BCG vaccine during the 1 year before receiving the drug or 1 year after they stop taking it, according to Johnson & Johnson.
The most common adverse effects include nasal congestion, sore throat, runny nose, upper respiratory infections, fever, headache, tiredness, itching, nausea and vomiting, redness at the injection site, vaginal yeast infections, urinary tract infections, sinus infection, bronchitis, diarrhea, stomach pain, and joint pain.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved the dual interleukin-12 and IL-23 inhibitor ustekinumab (Stelara) for the treatment of juvenile psoriatic arthritis (jPsA) in patients aged 6 years and older, according to an Aug. 1 announcement from its manufacturer, Janssen.
The approval makes jPsA the sixth approved indication for ustekinumab, which include active psoriatic arthritis in adults, moderate to severe plaque psoriasis in both adults and children aged 6 years or older who are candidates for phototherapy or systemic therapy, moderately to severely active Crohn’s disease in adults, and moderately to severely active ulcerative colitis in adults.
In addition, ustekinumab is now the second biologic to be approved for jPsA, following the agency’s December 2021 approval of secukinumab (Cosentyx) to treat jPsA in children and adolescents aged 2 years and older as well as enthesitis-related arthritis in children and adolescents aged 4 years and older.
In pediatric patients, ustekinumab is administered as a subcutaneous injection dosed four times per year after two starter doses.
Ustekinumab’s approval is based on “an extrapolation of the established data and existing safety profile” of ustekinumab in multiple phase 3 studies in adult and pediatric patients with moderate to severe plaque psoriasis and adult patients with active PsA, according to Janssen.
“With the limited availability of pediatric patients for clinical trial inclusion, researchers can extrapolate data from trials with adults to determine the potential efficacy and tolerability of a treatment for a pediatric population,” according to the October 2021 announcement from the company that the Biologics License Application had been submitted to the FDA.
Juvenile arthritis occurs in an estimated 20-45 children per 100,000 in the United States, with about 5% of those children having jPsA, according to the National Psoriasis Foundation.
The prescribing information for ustekinumab includes specific warnings and areas of concern. The drug should not be administered to individuals with known hypersensitivity to ustekinumab. The drug may lower the ability of the immune system to fight infections and may increase risk of infections, sometimes serious, and a test for tuberculosis infection should be given before administration.
Patients taking ustekinumab should not be given a live vaccine, and their doctors should be informed if anyone in their household needs a live vaccine. They also should not receive the BCG vaccine during the 1 year before receiving the drug or 1 year after they stop taking it, according to Johnson & Johnson.
The most common adverse effects include nasal congestion, sore throat, runny nose, upper respiratory infections, fever, headache, tiredness, itching, nausea and vomiting, redness at the injection site, vaginal yeast infections, urinary tract infections, sinus infection, bronchitis, diarrhea, stomach pain, and joint pain.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved the dual interleukin-12 and IL-23 inhibitor ustekinumab (Stelara) for the treatment of juvenile psoriatic arthritis (jPsA) in patients aged 6 years and older, according to an Aug. 1 announcement from its manufacturer, Janssen.
The approval makes jPsA the sixth approved indication for ustekinumab, which include active psoriatic arthritis in adults, moderate to severe plaque psoriasis in both adults and children aged 6 years or older who are candidates for phototherapy or systemic therapy, moderately to severely active Crohn’s disease in adults, and moderately to severely active ulcerative colitis in adults.
In addition, ustekinumab is now the second biologic to be approved for jPsA, following the agency’s December 2021 approval of secukinumab (Cosentyx) to treat jPsA in children and adolescents aged 2 years and older as well as enthesitis-related arthritis in children and adolescents aged 4 years and older.
In pediatric patients, ustekinumab is administered as a subcutaneous injection dosed four times per year after two starter doses.
Ustekinumab’s approval is based on “an extrapolation of the established data and existing safety profile” of ustekinumab in multiple phase 3 studies in adult and pediatric patients with moderate to severe plaque psoriasis and adult patients with active PsA, according to Janssen.
“With the limited availability of pediatric patients for clinical trial inclusion, researchers can extrapolate data from trials with adults to determine the potential efficacy and tolerability of a treatment for a pediatric population,” according to the October 2021 announcement from the company that the Biologics License Application had been submitted to the FDA.
Juvenile arthritis occurs in an estimated 20-45 children per 100,000 in the United States, with about 5% of those children having jPsA, according to the National Psoriasis Foundation.
The prescribing information for ustekinumab includes specific warnings and areas of concern. The drug should not be administered to individuals with known hypersensitivity to ustekinumab. The drug may lower the ability of the immune system to fight infections and may increase risk of infections, sometimes serious, and a test for tuberculosis infection should be given before administration.
Patients taking ustekinumab should not be given a live vaccine, and their doctors should be informed if anyone in their household needs a live vaccine. They also should not receive the BCG vaccine during the 1 year before receiving the drug or 1 year after they stop taking it, according to Johnson & Johnson.
The most common adverse effects include nasal congestion, sore throat, runny nose, upper respiratory infections, fever, headache, tiredness, itching, nausea and vomiting, redness at the injection site, vaginal yeast infections, urinary tract infections, sinus infection, bronchitis, diarrhea, stomach pain, and joint pain.
A version of this article first appeared on Medscape.com.
FDA approves belimumab for children with lupus nephritis
The Food and Drug Administration has approved belimumab (Benlysta) for treating active lupus nephritis (LN) in children aged 5-17 years. The drug can now be used to treat adult and pediatric patients with systemic lupus erythematosus (SLE) and LN. The decision expands therapeutic options for the estimated 1.5 million Americans currently living with lupus.
“This approval marks a significant step forward in providing treatment options to these children at risk of incurring kidney damage early on in life,” Stevan W. Gibson, president and CEO of the Lupus Foundation of America, said in a press release issued by the manufacturer, GlaxoSmithKline. LN is a condition that sometimes develops in people with lupus. In LN, the autoimmune cells produced by the disease attack the kidney. Roughly 40% of people with SLE experience LN.
Damage to the kidneys causes the body to have difficulty processing waste and toxins. This can create a host of problems, including end-stage kidney disease, which may be treated only with dialysis or kidney transplant. These situations significantly increase mortality among people with lupus, especially children.
Prior to the approval, the only treatment pathway for children with active LN included immunosuppressants and corticosteroids. While they may be effective, use of these classes of drugs may come with many side effects, including susceptibility to other diseases and infections. Belimumab, by contrast, is a B-lymphocyte stimulator protein inhibitor. It inhibits the survival of B cells, which are thought to play a role in the disease’s pathophysiology.
Belimumab was first approved to treat patients with SLE in 2011. It was approved for children with SLE 8 years later. The drug’s indications were expanded to include adults with LN in 2020.
Organizations within the lupus research community have communicated their support of the FDA’s decision. “Our community has much to celebrate with the approval of the first and much-needed treatment for children with lupus nephritis,” Lupus Research Alliance President and CEO Kenneth M. Farber said in a release from the organization.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved belimumab (Benlysta) for treating active lupus nephritis (LN) in children aged 5-17 years. The drug can now be used to treat adult and pediatric patients with systemic lupus erythematosus (SLE) and LN. The decision expands therapeutic options for the estimated 1.5 million Americans currently living with lupus.
“This approval marks a significant step forward in providing treatment options to these children at risk of incurring kidney damage early on in life,” Stevan W. Gibson, president and CEO of the Lupus Foundation of America, said in a press release issued by the manufacturer, GlaxoSmithKline. LN is a condition that sometimes develops in people with lupus. In LN, the autoimmune cells produced by the disease attack the kidney. Roughly 40% of people with SLE experience LN.
Damage to the kidneys causes the body to have difficulty processing waste and toxins. This can create a host of problems, including end-stage kidney disease, which may be treated only with dialysis or kidney transplant. These situations significantly increase mortality among people with lupus, especially children.
Prior to the approval, the only treatment pathway for children with active LN included immunosuppressants and corticosteroids. While they may be effective, use of these classes of drugs may come with many side effects, including susceptibility to other diseases and infections. Belimumab, by contrast, is a B-lymphocyte stimulator protein inhibitor. It inhibits the survival of B cells, which are thought to play a role in the disease’s pathophysiology.
Belimumab was first approved to treat patients with SLE in 2011. It was approved for children with SLE 8 years later. The drug’s indications were expanded to include adults with LN in 2020.
Organizations within the lupus research community have communicated their support of the FDA’s decision. “Our community has much to celebrate with the approval of the first and much-needed treatment for children with lupus nephritis,” Lupus Research Alliance President and CEO Kenneth M. Farber said in a release from the organization.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved belimumab (Benlysta) for treating active lupus nephritis (LN) in children aged 5-17 years. The drug can now be used to treat adult and pediatric patients with systemic lupus erythematosus (SLE) and LN. The decision expands therapeutic options for the estimated 1.5 million Americans currently living with lupus.
“This approval marks a significant step forward in providing treatment options to these children at risk of incurring kidney damage early on in life,” Stevan W. Gibson, president and CEO of the Lupus Foundation of America, said in a press release issued by the manufacturer, GlaxoSmithKline. LN is a condition that sometimes develops in people with lupus. In LN, the autoimmune cells produced by the disease attack the kidney. Roughly 40% of people with SLE experience LN.
Damage to the kidneys causes the body to have difficulty processing waste and toxins. This can create a host of problems, including end-stage kidney disease, which may be treated only with dialysis or kidney transplant. These situations significantly increase mortality among people with lupus, especially children.
Prior to the approval, the only treatment pathway for children with active LN included immunosuppressants and corticosteroids. While they may be effective, use of these classes of drugs may come with many side effects, including susceptibility to other diseases and infections. Belimumab, by contrast, is a B-lymphocyte stimulator protein inhibitor. It inhibits the survival of B cells, which are thought to play a role in the disease’s pathophysiology.
Belimumab was first approved to treat patients with SLE in 2011. It was approved for children with SLE 8 years later. The drug’s indications were expanded to include adults with LN in 2020.
Organizations within the lupus research community have communicated their support of the FDA’s decision. “Our community has much to celebrate with the approval of the first and much-needed treatment for children with lupus nephritis,” Lupus Research Alliance President and CEO Kenneth M. Farber said in a release from the organization.
A version of this article first appeared on Medscape.com.
TNF inhibitor use for RA shows beneficial effect in pregnancy
Women with well-controlled rheumatoid arthritis who used a tumor necrosis factor (TNF) inhibitor during pregnancy gave birth to infants with higher birth weight than did other patients, without an increased risk of adverse outcomes, according to findings from a Dutch prospective cohort study published online in Annals of the Rheumatic Diseases.
The study involved 188 patients drawn from the ongoing Preconceptional Counseling in Active RA (PreCARA) study, which followed patients with inflammatory rheumatic diseases before and during pregnancy. Women enrolled in PreCARA were closely monitored and treated with a therapeutic approach that aimed to achieve minimal disease activity, which included the use of TNF inhibitors.
Much research on TNF inhibitors during pregnancy has been limited to the first trimester and focused primarily on congenital malformations. In addition, most previous studies evaluating TNF inhibitors during pregnancy involved patients with different underlying diseases, making it difficult to interpret the results.
Hieronymus T. W. Smeele, MD, and colleagues at Erasmus University Medical Center, Rotterdam, the Netherlands, evaluated participants every 3 months before pregnancy; then again in the first, second, and third trimesters; and at 6, 12, and 26 weeks post partum. At these visits, in addition to undergoing an examination of their joints, patients completed questionnaires and gave blood samples. Disease activity was determined using the Disease Activity Score in 28 joints. Twin births and diagnoses other than RA were excluded.
Bigger babies
The study found that use of TNF inhibitors during pregnancy (n = 92 women) did not increase the risk of birth defects or emergency cesarean sections. While RA is typically associated with small-for-gestational-age (SGA) birth weights, TNF inhibitors were associated with a significant increase in birth weight and fewer infants born SGA, even when the comparison was adjusted for confounders, such as disease activity. At the same time, TNF inhibitors were not associated with high birth weight or with infants who were large for gestational age (LGA).
The results showed that the effects were greatest when TNF inhibitors were used in the third trimester. However, teasing out the effects based on trimester is difficult because participants who used TNF inhibitors during the third trimester were likely to use them in the first and second trimester as well. The study’s authors pointed out that these results need to be replicated.
“The immune system is not only important in the pathogenesis of RA,” the study’s authors wrote, “but also for ensuring and maintaining a normal pregnancy.” They pointed out that many adverse outcomes of pregnancy that are thought to arise from inadequate development of the placenta, such as intrauterine growth restriction, SGA, and hypertensive disorders of pregnancy, can involve an increase in proinflammatory cytokines, such as TNF. “It is tempting to speculate that treatment with [TNF inhibitors] during pregnancy promotes placentation and thereby fetal growth and birth weight by changing the balance between proinflammatory and anti-inflammatory cytokines and by increasing the number and function of [regulatory T cells].” They also hypothesize that treatment with TNF inhibitors induces epigenetic changes in the fetus, which positively influence fetal growth.
Welcomed data
This is a well-done, interesting study that will add to the still-slim body of research on pregnancy in rheumatic diseases, Kevin Byram, MD, assistant professor of medicine in the division of rheumatology and immunology and associate director of the rheumatology training program at Vanderbilt University, Nashville, Tenn., told this news organization.
“Historically, pregnant women have been excluded from clinical trials, not just in rheumatoid arthritis, but in other rheumatic diseases, so we don’t have a lot of great data,” he said, adding that the more interesting part of the study was that it showed there was no increased risk of adverse outcomes. “I’m not sure what to make of the increased birth weight. It will be interesting to see if the hypothesis that there might be a role for this molecule in preventing low birth weight goes anywhere.”
The work was supported by the Dutch Arthritis Foundation. PreCARA is an investigator-initiated study that was financially supported by UCB. The authors declared no competing interests.
A version of this article first appeared on Medscape.com.
Women with well-controlled rheumatoid arthritis who used a tumor necrosis factor (TNF) inhibitor during pregnancy gave birth to infants with higher birth weight than did other patients, without an increased risk of adverse outcomes, according to findings from a Dutch prospective cohort study published online in Annals of the Rheumatic Diseases.
The study involved 188 patients drawn from the ongoing Preconceptional Counseling in Active RA (PreCARA) study, which followed patients with inflammatory rheumatic diseases before and during pregnancy. Women enrolled in PreCARA were closely monitored and treated with a therapeutic approach that aimed to achieve minimal disease activity, which included the use of TNF inhibitors.
Much research on TNF inhibitors during pregnancy has been limited to the first trimester and focused primarily on congenital malformations. In addition, most previous studies evaluating TNF inhibitors during pregnancy involved patients with different underlying diseases, making it difficult to interpret the results.
Hieronymus T. W. Smeele, MD, and colleagues at Erasmus University Medical Center, Rotterdam, the Netherlands, evaluated participants every 3 months before pregnancy; then again in the first, second, and third trimesters; and at 6, 12, and 26 weeks post partum. At these visits, in addition to undergoing an examination of their joints, patients completed questionnaires and gave blood samples. Disease activity was determined using the Disease Activity Score in 28 joints. Twin births and diagnoses other than RA were excluded.
Bigger babies
The study found that use of TNF inhibitors during pregnancy (n = 92 women) did not increase the risk of birth defects or emergency cesarean sections. While RA is typically associated with small-for-gestational-age (SGA) birth weights, TNF inhibitors were associated with a significant increase in birth weight and fewer infants born SGA, even when the comparison was adjusted for confounders, such as disease activity. At the same time, TNF inhibitors were not associated with high birth weight or with infants who were large for gestational age (LGA).
The results showed that the effects were greatest when TNF inhibitors were used in the third trimester. However, teasing out the effects based on trimester is difficult because participants who used TNF inhibitors during the third trimester were likely to use them in the first and second trimester as well. The study’s authors pointed out that these results need to be replicated.
“The immune system is not only important in the pathogenesis of RA,” the study’s authors wrote, “but also for ensuring and maintaining a normal pregnancy.” They pointed out that many adverse outcomes of pregnancy that are thought to arise from inadequate development of the placenta, such as intrauterine growth restriction, SGA, and hypertensive disorders of pregnancy, can involve an increase in proinflammatory cytokines, such as TNF. “It is tempting to speculate that treatment with [TNF inhibitors] during pregnancy promotes placentation and thereby fetal growth and birth weight by changing the balance between proinflammatory and anti-inflammatory cytokines and by increasing the number and function of [regulatory T cells].” They also hypothesize that treatment with TNF inhibitors induces epigenetic changes in the fetus, which positively influence fetal growth.
Welcomed data
This is a well-done, interesting study that will add to the still-slim body of research on pregnancy in rheumatic diseases, Kevin Byram, MD, assistant professor of medicine in the division of rheumatology and immunology and associate director of the rheumatology training program at Vanderbilt University, Nashville, Tenn., told this news organization.
“Historically, pregnant women have been excluded from clinical trials, not just in rheumatoid arthritis, but in other rheumatic diseases, so we don’t have a lot of great data,” he said, adding that the more interesting part of the study was that it showed there was no increased risk of adverse outcomes. “I’m not sure what to make of the increased birth weight. It will be interesting to see if the hypothesis that there might be a role for this molecule in preventing low birth weight goes anywhere.”
The work was supported by the Dutch Arthritis Foundation. PreCARA is an investigator-initiated study that was financially supported by UCB. The authors declared no competing interests.
A version of this article first appeared on Medscape.com.
Women with well-controlled rheumatoid arthritis who used a tumor necrosis factor (TNF) inhibitor during pregnancy gave birth to infants with higher birth weight than did other patients, without an increased risk of adverse outcomes, according to findings from a Dutch prospective cohort study published online in Annals of the Rheumatic Diseases.
The study involved 188 patients drawn from the ongoing Preconceptional Counseling in Active RA (PreCARA) study, which followed patients with inflammatory rheumatic diseases before and during pregnancy. Women enrolled in PreCARA were closely monitored and treated with a therapeutic approach that aimed to achieve minimal disease activity, which included the use of TNF inhibitors.
Much research on TNF inhibitors during pregnancy has been limited to the first trimester and focused primarily on congenital malformations. In addition, most previous studies evaluating TNF inhibitors during pregnancy involved patients with different underlying diseases, making it difficult to interpret the results.
Hieronymus T. W. Smeele, MD, and colleagues at Erasmus University Medical Center, Rotterdam, the Netherlands, evaluated participants every 3 months before pregnancy; then again in the first, second, and third trimesters; and at 6, 12, and 26 weeks post partum. At these visits, in addition to undergoing an examination of their joints, patients completed questionnaires and gave blood samples. Disease activity was determined using the Disease Activity Score in 28 joints. Twin births and diagnoses other than RA were excluded.
Bigger babies
The study found that use of TNF inhibitors during pregnancy (n = 92 women) did not increase the risk of birth defects or emergency cesarean sections. While RA is typically associated with small-for-gestational-age (SGA) birth weights, TNF inhibitors were associated with a significant increase in birth weight and fewer infants born SGA, even when the comparison was adjusted for confounders, such as disease activity. At the same time, TNF inhibitors were not associated with high birth weight or with infants who were large for gestational age (LGA).
The results showed that the effects were greatest when TNF inhibitors were used in the third trimester. However, teasing out the effects based on trimester is difficult because participants who used TNF inhibitors during the third trimester were likely to use them in the first and second trimester as well. The study’s authors pointed out that these results need to be replicated.
“The immune system is not only important in the pathogenesis of RA,” the study’s authors wrote, “but also for ensuring and maintaining a normal pregnancy.” They pointed out that many adverse outcomes of pregnancy that are thought to arise from inadequate development of the placenta, such as intrauterine growth restriction, SGA, and hypertensive disorders of pregnancy, can involve an increase in proinflammatory cytokines, such as TNF. “It is tempting to speculate that treatment with [TNF inhibitors] during pregnancy promotes placentation and thereby fetal growth and birth weight by changing the balance between proinflammatory and anti-inflammatory cytokines and by increasing the number and function of [regulatory T cells].” They also hypothesize that treatment with TNF inhibitors induces epigenetic changes in the fetus, which positively influence fetal growth.
Welcomed data
This is a well-done, interesting study that will add to the still-slim body of research on pregnancy in rheumatic diseases, Kevin Byram, MD, assistant professor of medicine in the division of rheumatology and immunology and associate director of the rheumatology training program at Vanderbilt University, Nashville, Tenn., told this news organization.
“Historically, pregnant women have been excluded from clinical trials, not just in rheumatoid arthritis, but in other rheumatic diseases, so we don’t have a lot of great data,” he said, adding that the more interesting part of the study was that it showed there was no increased risk of adverse outcomes. “I’m not sure what to make of the increased birth weight. It will be interesting to see if the hypothesis that there might be a role for this molecule in preventing low birth weight goes anywhere.”
The work was supported by the Dutch Arthritis Foundation. PreCARA is an investigator-initiated study that was financially supported by UCB. The authors declared no competing interests.
A version of this article first appeared on Medscape.com.
FROM ANNALS OF THE RHEUMATIC DISEASES
Vitamin D supplements do not lower risk of fractures
compared with placebo, according to results from an ancillary study of the Vitamin D and Omega-3 Trial (VITAL).
The data showed that taking 2,000 IU of supplemental vitamin D each day without coadministered calcium did not have a significant effect on nonvertebral fractures (hazard ratio, 0.97; P = .50), hip fractures (HR, 1.01; P = .96), or total fractures (HR, 0.98; P = .70), compared with taking placebo, among individuals who did not have osteoporosis, vitamin D deficiency, or low bone mass, report Meryl S. LeBoff, MD, a professor of medicine at Harvard Medical School and chief of the calcium and bone section at Brigham and Women’s Hospital, both in Boston, and colleagues.
The findings were published online in the New England Journal of Medicine.
Prior randomized, controlled trials have presented conflicting findings. Some have shown that there is some benefit to supplemental vitamin D, whereas others have shown no effect or even harm with regard to risk of fractures, Dr. LeBoff noted.
“Because of the conflicting data at the time, we tested this hypothesis in an effort to advance science and understanding of the effects of vitamin D on bone. In a previous study, we did not see an effect of supplemental vitamin D on bone density in a subcohort from the VITAL trial,” Dr. LeBoff said in an interview.
“We previously reported that vitamin D, about 2,000 units per day, did not increase bone density, nor did it affect bone structure, according to PQCT [peripheral quantitative CT]. So that was an indicator that since bone density is a surrogate marker of fractures, there may not be an effect on fractures,” she added.
These results should dispel any idea that vitamin D alone could significantly reduce fracture rates in the general population, noted Steven R. Cummings, MD, of the University of California, San Francisco, and Clifford Rosen, MD, of Maine Medical Center Research Institute, Scarborough, in an accompanying editorial.
“Adding those findings to previous reports from VITAL and other trials showing the lack of an effect for preventing numerous conditions suggests that providers should stop screening for 25-hydroxyvitamin D levels or recommending vitamin D supplements, and people should stop taking vitamin D supplements to prevent major diseases or extend life,” the editorialists wrote.
The researchers assessed 25,871 participants from all 50 states during a median follow-up time of 5.3 years. Participants were randomly assigned in a 1:1 ratio to receive placebo or vitamin D.
The mean age of the participants was 67.1 years; 50.6% of the study cohort were women, and 20.2% of the cohort were Black. Participants did not have low bone mass, vitamin D deficiency, or osteoporosis.
Participants agreed not to supplement their dietary intake with more than 1,200 mg of calcium each day and no more than 800 IU of vitamin D each day.
Participants filled out detailed surveys to evaluate baseline prescription drug use, demographic factors, medical history, and the consumption of supplements, such as fish oil, calcium, and vitamin D, during the run-in stage. Yearly surveys were used to assess side effects, adherence to the investigation protocol, falls, fractures, physical activity, osteoporosis and associated risk factors, onset of major illness, and the use of nontrial prescription drugs and supplements, such as vitamin D and calcium.
The researchers adjudicated incident fracture data using a centralized medical record review. To approximate the therapeutic effect in intention-to-treat analyses, they used proportional-hazard models.
Notably, outcomes were similar for the placebo and vitamin D groups with regard to incident kidney stones and hypercalcemia.
The effect of vitamin D supplementation was not modified by baseline parameters such as race or ethnicity, sex, body mass index, age, or blood 25-hydroxyvitamin D levels.
Dr. Cummings and Dr. Rosen pointed out that these findings, along with other VITAL trial data, show that no subgroups classified on the basis of baseline 25-hydroxyvitamin D levels, including those with levels less than 20 ng/mL, benefited from vitamin supplementation.
“There is no justification for measuring 25-hydroxyvitamin D in the general population or treating to a target serum level. A 25-hydroxyvitamin D level might be a useful diagnostic test for some patients with conditions that may be due to or that may cause severe deficiency,” the editorialists noted.
Except with regard to select patients, such as individuals living in nursing homes who have limited sun exposure, the use of the terms “vitamin D deficiency” and “vitamin D “insufficiency” should now be reevaluated, Dr. Rosen and Dr. Cummings wrote.
The study’s limitations include its assessment of only one dosage of vitamin D supplementation and a lack of adjustment for multiplicity, exploratory, parent trial, or secondary endpoints, the researchers noted.
The number of participants who had vitamin D deficiency was limited, owing to ethical and feasibility concerns regarding these patients. The data are not generalizable to individuals who are older and institutionalized or those who have osteomalacia or osteoporosis, the researchers wrote.
Expert commentary
“The interpretation of this [study] to me is that vitamin D is not for everybody,” said Baha Arafah, MD, professor of medicine at Case Western Reserve University and chief of the division of endocrinology at University Hospital, both in Cleveland, who was not involved in the study.
“This is not the final word; I would suggest that you don’t throw vitamin D at everybody. I would use markers of bone formation as a better measure to determine whether they need vitamin D or not, specifically looking at parathyroid hormone,” Dr. Arafah said in an interview.
Dr. Arafah pointed out that these data do not mean that clinicians should stop thinking about vitamin D altogether. “I think that would be the wrong message to read. If you read through the article, you will find that there are people who do need vitamin D; people who are deficient do need vitamin D. There’s no question that excessive or extreme vitamin D deficiency can lead to other things, specifically, osteomalacia, weak bones, [and] poor mineralization, so we are not totally out of the woods at this time.”
The ancillary study of the VITAL trial was sponsored by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Pharmavite donated the vitamin D 3 supplements used in the trial. Dr. LeBoff reported that she holds stock in Amgen. Cummings reported receiving personal fees and nonfinancial support from Amgen outside the submitted work. Dr. Rosen is associate editor of the New England Journal of Medicine. Dr. Arafah reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
compared with placebo, according to results from an ancillary study of the Vitamin D and Omega-3 Trial (VITAL).
The data showed that taking 2,000 IU of supplemental vitamin D each day without coadministered calcium did not have a significant effect on nonvertebral fractures (hazard ratio, 0.97; P = .50), hip fractures (HR, 1.01; P = .96), or total fractures (HR, 0.98; P = .70), compared with taking placebo, among individuals who did not have osteoporosis, vitamin D deficiency, or low bone mass, report Meryl S. LeBoff, MD, a professor of medicine at Harvard Medical School and chief of the calcium and bone section at Brigham and Women’s Hospital, both in Boston, and colleagues.
The findings were published online in the New England Journal of Medicine.
Prior randomized, controlled trials have presented conflicting findings. Some have shown that there is some benefit to supplemental vitamin D, whereas others have shown no effect or even harm with regard to risk of fractures, Dr. LeBoff noted.
“Because of the conflicting data at the time, we tested this hypothesis in an effort to advance science and understanding of the effects of vitamin D on bone. In a previous study, we did not see an effect of supplemental vitamin D on bone density in a subcohort from the VITAL trial,” Dr. LeBoff said in an interview.
“We previously reported that vitamin D, about 2,000 units per day, did not increase bone density, nor did it affect bone structure, according to PQCT [peripheral quantitative CT]. So that was an indicator that since bone density is a surrogate marker of fractures, there may not be an effect on fractures,” she added.
These results should dispel any idea that vitamin D alone could significantly reduce fracture rates in the general population, noted Steven R. Cummings, MD, of the University of California, San Francisco, and Clifford Rosen, MD, of Maine Medical Center Research Institute, Scarborough, in an accompanying editorial.
“Adding those findings to previous reports from VITAL and other trials showing the lack of an effect for preventing numerous conditions suggests that providers should stop screening for 25-hydroxyvitamin D levels or recommending vitamin D supplements, and people should stop taking vitamin D supplements to prevent major diseases or extend life,” the editorialists wrote.
The researchers assessed 25,871 participants from all 50 states during a median follow-up time of 5.3 years. Participants were randomly assigned in a 1:1 ratio to receive placebo or vitamin D.
The mean age of the participants was 67.1 years; 50.6% of the study cohort were women, and 20.2% of the cohort were Black. Participants did not have low bone mass, vitamin D deficiency, or osteoporosis.
Participants agreed not to supplement their dietary intake with more than 1,200 mg of calcium each day and no more than 800 IU of vitamin D each day.
Participants filled out detailed surveys to evaluate baseline prescription drug use, demographic factors, medical history, and the consumption of supplements, such as fish oil, calcium, and vitamin D, during the run-in stage. Yearly surveys were used to assess side effects, adherence to the investigation protocol, falls, fractures, physical activity, osteoporosis and associated risk factors, onset of major illness, and the use of nontrial prescription drugs and supplements, such as vitamin D and calcium.
The researchers adjudicated incident fracture data using a centralized medical record review. To approximate the therapeutic effect in intention-to-treat analyses, they used proportional-hazard models.
Notably, outcomes were similar for the placebo and vitamin D groups with regard to incident kidney stones and hypercalcemia.
The effect of vitamin D supplementation was not modified by baseline parameters such as race or ethnicity, sex, body mass index, age, or blood 25-hydroxyvitamin D levels.
Dr. Cummings and Dr. Rosen pointed out that these findings, along with other VITAL trial data, show that no subgroups classified on the basis of baseline 25-hydroxyvitamin D levels, including those with levels less than 20 ng/mL, benefited from vitamin supplementation.
“There is no justification for measuring 25-hydroxyvitamin D in the general population or treating to a target serum level. A 25-hydroxyvitamin D level might be a useful diagnostic test for some patients with conditions that may be due to or that may cause severe deficiency,” the editorialists noted.
Except with regard to select patients, such as individuals living in nursing homes who have limited sun exposure, the use of the terms “vitamin D deficiency” and “vitamin D “insufficiency” should now be reevaluated, Dr. Rosen and Dr. Cummings wrote.
The study’s limitations include its assessment of only one dosage of vitamin D supplementation and a lack of adjustment for multiplicity, exploratory, parent trial, or secondary endpoints, the researchers noted.
The number of participants who had vitamin D deficiency was limited, owing to ethical and feasibility concerns regarding these patients. The data are not generalizable to individuals who are older and institutionalized or those who have osteomalacia or osteoporosis, the researchers wrote.
Expert commentary
“The interpretation of this [study] to me is that vitamin D is not for everybody,” said Baha Arafah, MD, professor of medicine at Case Western Reserve University and chief of the division of endocrinology at University Hospital, both in Cleveland, who was not involved in the study.
“This is not the final word; I would suggest that you don’t throw vitamin D at everybody. I would use markers of bone formation as a better measure to determine whether they need vitamin D or not, specifically looking at parathyroid hormone,” Dr. Arafah said in an interview.
Dr. Arafah pointed out that these data do not mean that clinicians should stop thinking about vitamin D altogether. “I think that would be the wrong message to read. If you read through the article, you will find that there are people who do need vitamin D; people who are deficient do need vitamin D. There’s no question that excessive or extreme vitamin D deficiency can lead to other things, specifically, osteomalacia, weak bones, [and] poor mineralization, so we are not totally out of the woods at this time.”
The ancillary study of the VITAL trial was sponsored by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Pharmavite donated the vitamin D 3 supplements used in the trial. Dr. LeBoff reported that she holds stock in Amgen. Cummings reported receiving personal fees and nonfinancial support from Amgen outside the submitted work. Dr. Rosen is associate editor of the New England Journal of Medicine. Dr. Arafah reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
compared with placebo, according to results from an ancillary study of the Vitamin D and Omega-3 Trial (VITAL).
The data showed that taking 2,000 IU of supplemental vitamin D each day without coadministered calcium did not have a significant effect on nonvertebral fractures (hazard ratio, 0.97; P = .50), hip fractures (HR, 1.01; P = .96), or total fractures (HR, 0.98; P = .70), compared with taking placebo, among individuals who did not have osteoporosis, vitamin D deficiency, or low bone mass, report Meryl S. LeBoff, MD, a professor of medicine at Harvard Medical School and chief of the calcium and bone section at Brigham and Women’s Hospital, both in Boston, and colleagues.
The findings were published online in the New England Journal of Medicine.
Prior randomized, controlled trials have presented conflicting findings. Some have shown that there is some benefit to supplemental vitamin D, whereas others have shown no effect or even harm with regard to risk of fractures, Dr. LeBoff noted.
“Because of the conflicting data at the time, we tested this hypothesis in an effort to advance science and understanding of the effects of vitamin D on bone. In a previous study, we did not see an effect of supplemental vitamin D on bone density in a subcohort from the VITAL trial,” Dr. LeBoff said in an interview.
“We previously reported that vitamin D, about 2,000 units per day, did not increase bone density, nor did it affect bone structure, according to PQCT [peripheral quantitative CT]. So that was an indicator that since bone density is a surrogate marker of fractures, there may not be an effect on fractures,” she added.
These results should dispel any idea that vitamin D alone could significantly reduce fracture rates in the general population, noted Steven R. Cummings, MD, of the University of California, San Francisco, and Clifford Rosen, MD, of Maine Medical Center Research Institute, Scarborough, in an accompanying editorial.
“Adding those findings to previous reports from VITAL and other trials showing the lack of an effect for preventing numerous conditions suggests that providers should stop screening for 25-hydroxyvitamin D levels or recommending vitamin D supplements, and people should stop taking vitamin D supplements to prevent major diseases or extend life,” the editorialists wrote.
The researchers assessed 25,871 participants from all 50 states during a median follow-up time of 5.3 years. Participants were randomly assigned in a 1:1 ratio to receive placebo or vitamin D.
The mean age of the participants was 67.1 years; 50.6% of the study cohort were women, and 20.2% of the cohort were Black. Participants did not have low bone mass, vitamin D deficiency, or osteoporosis.
Participants agreed not to supplement their dietary intake with more than 1,200 mg of calcium each day and no more than 800 IU of vitamin D each day.
Participants filled out detailed surveys to evaluate baseline prescription drug use, demographic factors, medical history, and the consumption of supplements, such as fish oil, calcium, and vitamin D, during the run-in stage. Yearly surveys were used to assess side effects, adherence to the investigation protocol, falls, fractures, physical activity, osteoporosis and associated risk factors, onset of major illness, and the use of nontrial prescription drugs and supplements, such as vitamin D and calcium.
The researchers adjudicated incident fracture data using a centralized medical record review. To approximate the therapeutic effect in intention-to-treat analyses, they used proportional-hazard models.
Notably, outcomes were similar for the placebo and vitamin D groups with regard to incident kidney stones and hypercalcemia.
The effect of vitamin D supplementation was not modified by baseline parameters such as race or ethnicity, sex, body mass index, age, or blood 25-hydroxyvitamin D levels.
Dr. Cummings and Dr. Rosen pointed out that these findings, along with other VITAL trial data, show that no subgroups classified on the basis of baseline 25-hydroxyvitamin D levels, including those with levels less than 20 ng/mL, benefited from vitamin supplementation.
“There is no justification for measuring 25-hydroxyvitamin D in the general population or treating to a target serum level. A 25-hydroxyvitamin D level might be a useful diagnostic test for some patients with conditions that may be due to or that may cause severe deficiency,” the editorialists noted.
Except with regard to select patients, such as individuals living in nursing homes who have limited sun exposure, the use of the terms “vitamin D deficiency” and “vitamin D “insufficiency” should now be reevaluated, Dr. Rosen and Dr. Cummings wrote.
The study’s limitations include its assessment of only one dosage of vitamin D supplementation and a lack of adjustment for multiplicity, exploratory, parent trial, or secondary endpoints, the researchers noted.
The number of participants who had vitamin D deficiency was limited, owing to ethical and feasibility concerns regarding these patients. The data are not generalizable to individuals who are older and institutionalized or those who have osteomalacia or osteoporosis, the researchers wrote.
Expert commentary
“The interpretation of this [study] to me is that vitamin D is not for everybody,” said Baha Arafah, MD, professor of medicine at Case Western Reserve University and chief of the division of endocrinology at University Hospital, both in Cleveland, who was not involved in the study.
“This is not the final word; I would suggest that you don’t throw vitamin D at everybody. I would use markers of bone formation as a better measure to determine whether they need vitamin D or not, specifically looking at parathyroid hormone,” Dr. Arafah said in an interview.
Dr. Arafah pointed out that these data do not mean that clinicians should stop thinking about vitamin D altogether. “I think that would be the wrong message to read. If you read through the article, you will find that there are people who do need vitamin D; people who are deficient do need vitamin D. There’s no question that excessive or extreme vitamin D deficiency can lead to other things, specifically, osteomalacia, weak bones, [and] poor mineralization, so we are not totally out of the woods at this time.”
The ancillary study of the VITAL trial was sponsored by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Pharmavite donated the vitamin D 3 supplements used in the trial. Dr. LeBoff reported that she holds stock in Amgen. Cummings reported receiving personal fees and nonfinancial support from Amgen outside the submitted work. Dr. Rosen is associate editor of the New England Journal of Medicine. Dr. Arafah reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Questionnaire for patients with psoriasis might identify risk of axial involvement
Preliminary findings are encouraging
NEW YORK – A questionnaire-based screening tool appears to accelerate the time to diagnosis of axial involvement in patients presenting with psoriasis but no clinical signs of joint pain, according to a study called ATTRACT that was presented at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
The risk of a delayed diagnosis of an axial component in patients with psoriasis, meaning a delay in the underlying diagnosis of psoriatic arthritis (PsA), is substantial, according to Devis Benfaremo, MD, of the department of clinical and molecular science at Marche Polytechnic University, Ancona, Italy.
There is “no consensus for the best strategy to achieve early detection of joint disease” in patients presenting with psoriasis, but Dr. Benfaremo pointed out that missing axial involvement is a particular problem because it is far more likely than swollen joints to be missed on clinical examination.
While about one in three patients with psoriasis have or will develop psoriatic arthritis, according to the National Psoriasis Foundation, delays in diagnosis are common, according to Dr. Benfaremo. In patients with undiagnosed PsA characterized by axial involvement alone, subtle symptoms can be overlooked or attributed to other causes.
There are several screening questionnaires to detect joint symptoms in patients presenting with psoriasis, such as the five-question Psoriasis Epidemiology Screening Tool, but the questionnaire tested in the ATTRACT trial is focused on detecting axial involvement specifically. It was characterized as the first to do so.
In the ongoing ATTRACT study, 253 patients with psoriasis but no history of PsA or axial disease have been enrolled so far. In the study, patients are screened for PsA based on a patient-completed yes-or-no questionnaire, which takes only a few minutes to complete.
“It is a validated questionnaire for axial [spondyloarthritis], but we have adopted it for detection of psoriasis patients with PsA,” Dr. Benfaremo explained.
The questionnaire for axial spondyloarthritis (axSpA) was initially evaluated and validated by Fabian Proft, MD, head of the clinical trials unit at Charité Hospital, Berlin. In addition to a patient self-completed questionnaire, Dr. Proft and coinvestigators have also created a related questionnaire to be administered by physicians.
In the ATTRACT study, patients completed the questionnaire on an electronic device in the waiting room. Positive answers to specific questions about symptoms, which addressed back pain and joint function as well as joint symptoms, divided patients into three groups:
- Group A patients did not respond positively to any of the symptom questions that would prompt suspicion of axial disease. These represented about one-third of those screened so far.
- Group B patients were those who answered positively to at least two questions that related to a high suspicion of axial involvement. These represented 45% of patients.
- The remaining patients were placed in Group C, a category of intermediate risk based on positive responses to some, but not all, questions relating to axial symptoms.
Those in group B are being referred to rheumatology. Patients in group C are given “conditional” eligibility based on the presence of additional risk factors.
AxSpA screening tool ‘makes sense’ for potential use in PsA
The primary outcome of the ATTRACT trial is early identification of axial PsA. Correctly identifying patients with or without peripheral joint involvement is one of several secondary outcomes. The identification of patients who fulfill Assessment Spondyloarthritis International Society (ASAS) criteria for axSpA is another secondary outcome.
Of the 114 patients placed in group B and analyzed so far, 87 have completed an assessment by a rheumatologist with laboratory analyses and imaging, as well as a clinical examination.
Of those 87 assessed by a rheumatologist, 17 did not have either axial or peripheral inflammation. Another 19 were diagnosed with axial disease, including 14 who met ASAS criteria. A total of 10 were classified as having PsA with peripheral inflammation, according to Classification for Psoriatic Arthritis criteria, and 41 are still being considered for a diagnosis of axial or peripheral PsA on the basis of further workup.
“Among the patients with axial PsA, only 10% had elevated C-reactive protein levels,” according to Dr. Benfaremo, echoing previous evidence that inflammatory biomarkers by themselves have limited value for identifying psoriasis patients at high risk of joint involvement.
The findings are preliminary, but Dr. Benfaremo reported that the questionnaire is showing promise for the routine stratification of patients who should be considered for a rheumatology consultation.
If further analyses validate the clinical utility of these stratifications, there is the potential for a substantial acceleration to the diagnosis of PsA.
When contacted to comment about this work, Dr. Proft said that there is an important need for new strategies reduce delay in the diagnosis of PsA among patients presenting with psoriasis. He thinks the screening tool he developed for axSpA “makes sense” as a potential tool in PsA.
“If validated, this could be a very useful for earlier identification of PsA,” Dr. Proft said. He reiterated the importance of focusing on axial involvement.
“Previous screening tools have focused on symptoms of PsA more generally, but inflammation in the peripheral joints is something that you can easily see in most patients,” he said.
In addition to the patient-completed questionnaire and the physician-administered questionnaire, Dr. Proft has also evaluated an online self-referral tool for patients.
“If we can diagnose PsA earlier in the course of disease, we can start treatment earlier, prevent or delay joint damage, and potentially improve outcomes for patients,” Dr. Proft said. He considers this an important direction of research.
Dr. Benfaremo and Dr. Proft reported no potential conflicts of interest.
Preliminary findings are encouraging
Preliminary findings are encouraging
NEW YORK – A questionnaire-based screening tool appears to accelerate the time to diagnosis of axial involvement in patients presenting with psoriasis but no clinical signs of joint pain, according to a study called ATTRACT that was presented at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
The risk of a delayed diagnosis of an axial component in patients with psoriasis, meaning a delay in the underlying diagnosis of psoriatic arthritis (PsA), is substantial, according to Devis Benfaremo, MD, of the department of clinical and molecular science at Marche Polytechnic University, Ancona, Italy.
There is “no consensus for the best strategy to achieve early detection of joint disease” in patients presenting with psoriasis, but Dr. Benfaremo pointed out that missing axial involvement is a particular problem because it is far more likely than swollen joints to be missed on clinical examination.
While about one in three patients with psoriasis have or will develop psoriatic arthritis, according to the National Psoriasis Foundation, delays in diagnosis are common, according to Dr. Benfaremo. In patients with undiagnosed PsA characterized by axial involvement alone, subtle symptoms can be overlooked or attributed to other causes.
There are several screening questionnaires to detect joint symptoms in patients presenting with psoriasis, such as the five-question Psoriasis Epidemiology Screening Tool, but the questionnaire tested in the ATTRACT trial is focused on detecting axial involvement specifically. It was characterized as the first to do so.
In the ongoing ATTRACT study, 253 patients with psoriasis but no history of PsA or axial disease have been enrolled so far. In the study, patients are screened for PsA based on a patient-completed yes-or-no questionnaire, which takes only a few minutes to complete.
“It is a validated questionnaire for axial [spondyloarthritis], but we have adopted it for detection of psoriasis patients with PsA,” Dr. Benfaremo explained.
The questionnaire for axial spondyloarthritis (axSpA) was initially evaluated and validated by Fabian Proft, MD, head of the clinical trials unit at Charité Hospital, Berlin. In addition to a patient self-completed questionnaire, Dr. Proft and coinvestigators have also created a related questionnaire to be administered by physicians.
In the ATTRACT study, patients completed the questionnaire on an electronic device in the waiting room. Positive answers to specific questions about symptoms, which addressed back pain and joint function as well as joint symptoms, divided patients into three groups:
- Group A patients did not respond positively to any of the symptom questions that would prompt suspicion of axial disease. These represented about one-third of those screened so far.
- Group B patients were those who answered positively to at least two questions that related to a high suspicion of axial involvement. These represented 45% of patients.
- The remaining patients were placed in Group C, a category of intermediate risk based on positive responses to some, but not all, questions relating to axial symptoms.
Those in group B are being referred to rheumatology. Patients in group C are given “conditional” eligibility based on the presence of additional risk factors.
AxSpA screening tool ‘makes sense’ for potential use in PsA
The primary outcome of the ATTRACT trial is early identification of axial PsA. Correctly identifying patients with or without peripheral joint involvement is one of several secondary outcomes. The identification of patients who fulfill Assessment Spondyloarthritis International Society (ASAS) criteria for axSpA is another secondary outcome.
Of the 114 patients placed in group B and analyzed so far, 87 have completed an assessment by a rheumatologist with laboratory analyses and imaging, as well as a clinical examination.
Of those 87 assessed by a rheumatologist, 17 did not have either axial or peripheral inflammation. Another 19 were diagnosed with axial disease, including 14 who met ASAS criteria. A total of 10 were classified as having PsA with peripheral inflammation, according to Classification for Psoriatic Arthritis criteria, and 41 are still being considered for a diagnosis of axial or peripheral PsA on the basis of further workup.
“Among the patients with axial PsA, only 10% had elevated C-reactive protein levels,” according to Dr. Benfaremo, echoing previous evidence that inflammatory biomarkers by themselves have limited value for identifying psoriasis patients at high risk of joint involvement.
The findings are preliminary, but Dr. Benfaremo reported that the questionnaire is showing promise for the routine stratification of patients who should be considered for a rheumatology consultation.
If further analyses validate the clinical utility of these stratifications, there is the potential for a substantial acceleration to the diagnosis of PsA.
When contacted to comment about this work, Dr. Proft said that there is an important need for new strategies reduce delay in the diagnosis of PsA among patients presenting with psoriasis. He thinks the screening tool he developed for axSpA “makes sense” as a potential tool in PsA.
“If validated, this could be a very useful for earlier identification of PsA,” Dr. Proft said. He reiterated the importance of focusing on axial involvement.
“Previous screening tools have focused on symptoms of PsA more generally, but inflammation in the peripheral joints is something that you can easily see in most patients,” he said.
In addition to the patient-completed questionnaire and the physician-administered questionnaire, Dr. Proft has also evaluated an online self-referral tool for patients.
“If we can diagnose PsA earlier in the course of disease, we can start treatment earlier, prevent or delay joint damage, and potentially improve outcomes for patients,” Dr. Proft said. He considers this an important direction of research.
Dr. Benfaremo and Dr. Proft reported no potential conflicts of interest.
NEW YORK – A questionnaire-based screening tool appears to accelerate the time to diagnosis of axial involvement in patients presenting with psoriasis but no clinical signs of joint pain, according to a study called ATTRACT that was presented at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
The risk of a delayed diagnosis of an axial component in patients with psoriasis, meaning a delay in the underlying diagnosis of psoriatic arthritis (PsA), is substantial, according to Devis Benfaremo, MD, of the department of clinical and molecular science at Marche Polytechnic University, Ancona, Italy.
There is “no consensus for the best strategy to achieve early detection of joint disease” in patients presenting with psoriasis, but Dr. Benfaremo pointed out that missing axial involvement is a particular problem because it is far more likely than swollen joints to be missed on clinical examination.
While about one in three patients with psoriasis have or will develop psoriatic arthritis, according to the National Psoriasis Foundation, delays in diagnosis are common, according to Dr. Benfaremo. In patients with undiagnosed PsA characterized by axial involvement alone, subtle symptoms can be overlooked or attributed to other causes.
There are several screening questionnaires to detect joint symptoms in patients presenting with psoriasis, such as the five-question Psoriasis Epidemiology Screening Tool, but the questionnaire tested in the ATTRACT trial is focused on detecting axial involvement specifically. It was characterized as the first to do so.
In the ongoing ATTRACT study, 253 patients with psoriasis but no history of PsA or axial disease have been enrolled so far. In the study, patients are screened for PsA based on a patient-completed yes-or-no questionnaire, which takes only a few minutes to complete.
“It is a validated questionnaire for axial [spondyloarthritis], but we have adopted it for detection of psoriasis patients with PsA,” Dr. Benfaremo explained.
The questionnaire for axial spondyloarthritis (axSpA) was initially evaluated and validated by Fabian Proft, MD, head of the clinical trials unit at Charité Hospital, Berlin. In addition to a patient self-completed questionnaire, Dr. Proft and coinvestigators have also created a related questionnaire to be administered by physicians.
In the ATTRACT study, patients completed the questionnaire on an electronic device in the waiting room. Positive answers to specific questions about symptoms, which addressed back pain and joint function as well as joint symptoms, divided patients into three groups:
- Group A patients did not respond positively to any of the symptom questions that would prompt suspicion of axial disease. These represented about one-third of those screened so far.
- Group B patients were those who answered positively to at least two questions that related to a high suspicion of axial involvement. These represented 45% of patients.
- The remaining patients were placed in Group C, a category of intermediate risk based on positive responses to some, but not all, questions relating to axial symptoms.
Those in group B are being referred to rheumatology. Patients in group C are given “conditional” eligibility based on the presence of additional risk factors.
AxSpA screening tool ‘makes sense’ for potential use in PsA
The primary outcome of the ATTRACT trial is early identification of axial PsA. Correctly identifying patients with or without peripheral joint involvement is one of several secondary outcomes. The identification of patients who fulfill Assessment Spondyloarthritis International Society (ASAS) criteria for axSpA is another secondary outcome.
Of the 114 patients placed in group B and analyzed so far, 87 have completed an assessment by a rheumatologist with laboratory analyses and imaging, as well as a clinical examination.
Of those 87 assessed by a rheumatologist, 17 did not have either axial or peripheral inflammation. Another 19 were diagnosed with axial disease, including 14 who met ASAS criteria. A total of 10 were classified as having PsA with peripheral inflammation, according to Classification for Psoriatic Arthritis criteria, and 41 are still being considered for a diagnosis of axial or peripheral PsA on the basis of further workup.
“Among the patients with axial PsA, only 10% had elevated C-reactive protein levels,” according to Dr. Benfaremo, echoing previous evidence that inflammatory biomarkers by themselves have limited value for identifying psoriasis patients at high risk of joint involvement.
The findings are preliminary, but Dr. Benfaremo reported that the questionnaire is showing promise for the routine stratification of patients who should be considered for a rheumatology consultation.
If further analyses validate the clinical utility of these stratifications, there is the potential for a substantial acceleration to the diagnosis of PsA.
When contacted to comment about this work, Dr. Proft said that there is an important need for new strategies reduce delay in the diagnosis of PsA among patients presenting with psoriasis. He thinks the screening tool he developed for axSpA “makes sense” as a potential tool in PsA.
“If validated, this could be a very useful for earlier identification of PsA,” Dr. Proft said. He reiterated the importance of focusing on axial involvement.
“Previous screening tools have focused on symptoms of PsA more generally, but inflammation in the peripheral joints is something that you can easily see in most patients,” he said.
In addition to the patient-completed questionnaire and the physician-administered questionnaire, Dr. Proft has also evaluated an online self-referral tool for patients.
“If we can diagnose PsA earlier in the course of disease, we can start treatment earlier, prevent or delay joint damage, and potentially improve outcomes for patients,” Dr. Proft said. He considers this an important direction of research.
Dr. Benfaremo and Dr. Proft reported no potential conflicts of interest.
AT GRAPPA 2022
NAFLD strongly correlated with psoriasis, PsA; risk linked to severity
NEW YORK – – and probably in those with psoriatic arthritis (PsA) as well, according to a systematic review and meta-analysis presented at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
“Our findings imply that psoriatic patients should be screened with an ultrasonographic exam in cases where there are metabolic features that are associated with NAFLD,” reported Francesco Bellinato, MD, a researcher in the section of dermatology and venereology, University of Verona (Italy).
The data are strong. Of 76 nonduplicate publications found in the literature, the 11 observational studies included in the meta-analysis met stringent criteria, including a diagnosis of psoriasis and PsA based on objective criteria, NAFLD confirmed with liver biopsy or imaging, and odds rates calculated with 95% confidence intervals.
From these 11 studies, aggregate data were available for 249,333 psoriatic patients, of which 49% had NAFLD, and 1,491,402 were healthy controls. Among the controls, 36% had NAFLD. Four of the studies were from North America, four from Europe, and three from Asia.
In the pooled data, the risk of NAFLD among those with psoriasis relative to healthy controls fell just short of a twofold increase (odds ratio, 1.96; 95% CI, 1.70-2.26; P < .001). When stratified by studies that confirmed NAFLD by biopsy relative to ultrasonography, there was no significant heterogeneity.
Eight of the studies included an analysis of relative risk in the context of skin lesion severity defined by Psoriasis Area and Severity Index (PASI) score. Relative to those without NAFLD, psoriatic patients with NAFLD had a significant greater mean PASI score on a pooled weighted mean difference analysis (OR, 3.93; 95% CI, 2.01-5.84; P < .0001).
For PsA relative to no PsA in the five studies that compared risk between these two groups, the risk of NAFLD was again nearly twofold higher. This fell short of conventional definition of statistical significance, but it was associated with a strong trend (OR, 1.83; 95% CI, 0.98-3.43; P = .06).
The risk of NAFLD among patients with psoriasis was not found to vary significantly when assessed by univariable meta-regressions across numerous characteristics, such as sex and body mass index.
In one of the largest of the observational studies included in the meta-analysis by Alexis Ogdie, MD, associate professor of medicine and epidemiology at the University of Pennsylvania, Philadelphia, and colleagues, data were analyzed in more than 1.5 million patients, which included 54,251 patients with rheumatoid arthritis. While the hazard ratio of NAFLD was increased for both psoriasis (HR, 2.23) and PsA (HR, 2.11), it was not elevated in those with RA (HR, 0.96).
Risk by severity, possible mechanisms
This study also included an analysis of NAFLD risk according to psoriasis severity. While risk was still significant among those with mild disease (HR, 1.18; 95% CI, 1.07-1.30), it was almost twofold greater in those with moderate to severe psoriasis (HR, 2.23; 95% CI, 1.73-2.87).
Dr. Bellinato conceded that the mechanisms underlying the association between psoriasis and NAFLD are unknown, but he said “metaflammation” is suspected.
“The secretion of proinflammatory, prothrombotic, and oxidative stress mediators in both psoriatic skin and adipose tissue might act systemically and promote insulin resistance and other metabolic derangements that promote the development and progression of NAFLD,” Dr. Bellinato explained.
He thinks that noninvasive screening methods, such as currently used methods to calculate fibrosis score, might be useful for evaluating patients with psoriasis for NAFLD and referring them to a hepatologist when appropriate.
Given the strong association with NAFLD, Dr. Bellinato suggested that “the findings of this meta-analysis pave the way for novel, large, prospective, and histologically based studies.”
The association between psoriasis and NAFLD is clinically relevant, agreed Joel M. Gelfand, MD, vice-chair of clinical research and medical director of the clinical studies unit, department of dermatology, University of Pennsylvania, Philadelphia.
“It is not clear if psoriasis causes fatty liver disease or vice versa, but clinicians should be aware of this association,” he said in an interview. Dr. Gelfand was a coauthor of the study by Dr. Ogdie and colleagues and led another more recent population-based study that implicated methotrexate as a factor in psoriasis-related hepatotoxicity.
If NAFLD is identified in a patient with psoriasis, treatments are limited, but Dr. Gelfand suggested that patients should be made aware of the risk. “Clinicians should encourage patients with psoriasis to take measures to protect their liver, such as avoiding drinking alcohol to excess and trying to maintain a healthy body weight,” he said.
Dr. Bellinato reported no conflicts of interest. Dr. Gelfand has financial relationships with more than 10 pharmaceutical companies, including those that make therapies for psoriasis.
NEW YORK – – and probably in those with psoriatic arthritis (PsA) as well, according to a systematic review and meta-analysis presented at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
“Our findings imply that psoriatic patients should be screened with an ultrasonographic exam in cases where there are metabolic features that are associated with NAFLD,” reported Francesco Bellinato, MD, a researcher in the section of dermatology and venereology, University of Verona (Italy).
The data are strong. Of 76 nonduplicate publications found in the literature, the 11 observational studies included in the meta-analysis met stringent criteria, including a diagnosis of psoriasis and PsA based on objective criteria, NAFLD confirmed with liver biopsy or imaging, and odds rates calculated with 95% confidence intervals.
From these 11 studies, aggregate data were available for 249,333 psoriatic patients, of which 49% had NAFLD, and 1,491,402 were healthy controls. Among the controls, 36% had NAFLD. Four of the studies were from North America, four from Europe, and three from Asia.
In the pooled data, the risk of NAFLD among those with psoriasis relative to healthy controls fell just short of a twofold increase (odds ratio, 1.96; 95% CI, 1.70-2.26; P < .001). When stratified by studies that confirmed NAFLD by biopsy relative to ultrasonography, there was no significant heterogeneity.
Eight of the studies included an analysis of relative risk in the context of skin lesion severity defined by Psoriasis Area and Severity Index (PASI) score. Relative to those without NAFLD, psoriatic patients with NAFLD had a significant greater mean PASI score on a pooled weighted mean difference analysis (OR, 3.93; 95% CI, 2.01-5.84; P < .0001).
For PsA relative to no PsA in the five studies that compared risk between these two groups, the risk of NAFLD was again nearly twofold higher. This fell short of conventional definition of statistical significance, but it was associated with a strong trend (OR, 1.83; 95% CI, 0.98-3.43; P = .06).
The risk of NAFLD among patients with psoriasis was not found to vary significantly when assessed by univariable meta-regressions across numerous characteristics, such as sex and body mass index.
In one of the largest of the observational studies included in the meta-analysis by Alexis Ogdie, MD, associate professor of medicine and epidemiology at the University of Pennsylvania, Philadelphia, and colleagues, data were analyzed in more than 1.5 million patients, which included 54,251 patients with rheumatoid arthritis. While the hazard ratio of NAFLD was increased for both psoriasis (HR, 2.23) and PsA (HR, 2.11), it was not elevated in those with RA (HR, 0.96).
Risk by severity, possible mechanisms
This study also included an analysis of NAFLD risk according to psoriasis severity. While risk was still significant among those with mild disease (HR, 1.18; 95% CI, 1.07-1.30), it was almost twofold greater in those with moderate to severe psoriasis (HR, 2.23; 95% CI, 1.73-2.87).
Dr. Bellinato conceded that the mechanisms underlying the association between psoriasis and NAFLD are unknown, but he said “metaflammation” is suspected.
“The secretion of proinflammatory, prothrombotic, and oxidative stress mediators in both psoriatic skin and adipose tissue might act systemically and promote insulin resistance and other metabolic derangements that promote the development and progression of NAFLD,” Dr. Bellinato explained.
He thinks that noninvasive screening methods, such as currently used methods to calculate fibrosis score, might be useful for evaluating patients with psoriasis for NAFLD and referring them to a hepatologist when appropriate.
Given the strong association with NAFLD, Dr. Bellinato suggested that “the findings of this meta-analysis pave the way for novel, large, prospective, and histologically based studies.”
The association between psoriasis and NAFLD is clinically relevant, agreed Joel M. Gelfand, MD, vice-chair of clinical research and medical director of the clinical studies unit, department of dermatology, University of Pennsylvania, Philadelphia.
“It is not clear if psoriasis causes fatty liver disease or vice versa, but clinicians should be aware of this association,” he said in an interview. Dr. Gelfand was a coauthor of the study by Dr. Ogdie and colleagues and led another more recent population-based study that implicated methotrexate as a factor in psoriasis-related hepatotoxicity.
If NAFLD is identified in a patient with psoriasis, treatments are limited, but Dr. Gelfand suggested that patients should be made aware of the risk. “Clinicians should encourage patients with psoriasis to take measures to protect their liver, such as avoiding drinking alcohol to excess and trying to maintain a healthy body weight,” he said.
Dr. Bellinato reported no conflicts of interest. Dr. Gelfand has financial relationships with more than 10 pharmaceutical companies, including those that make therapies for psoriasis.
NEW YORK – – and probably in those with psoriatic arthritis (PsA) as well, according to a systematic review and meta-analysis presented at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
“Our findings imply that psoriatic patients should be screened with an ultrasonographic exam in cases where there are metabolic features that are associated with NAFLD,” reported Francesco Bellinato, MD, a researcher in the section of dermatology and venereology, University of Verona (Italy).
The data are strong. Of 76 nonduplicate publications found in the literature, the 11 observational studies included in the meta-analysis met stringent criteria, including a diagnosis of psoriasis and PsA based on objective criteria, NAFLD confirmed with liver biopsy or imaging, and odds rates calculated with 95% confidence intervals.
From these 11 studies, aggregate data were available for 249,333 psoriatic patients, of which 49% had NAFLD, and 1,491,402 were healthy controls. Among the controls, 36% had NAFLD. Four of the studies were from North America, four from Europe, and three from Asia.
In the pooled data, the risk of NAFLD among those with psoriasis relative to healthy controls fell just short of a twofold increase (odds ratio, 1.96; 95% CI, 1.70-2.26; P < .001). When stratified by studies that confirmed NAFLD by biopsy relative to ultrasonography, there was no significant heterogeneity.
Eight of the studies included an analysis of relative risk in the context of skin lesion severity defined by Psoriasis Area and Severity Index (PASI) score. Relative to those without NAFLD, psoriatic patients with NAFLD had a significant greater mean PASI score on a pooled weighted mean difference analysis (OR, 3.93; 95% CI, 2.01-5.84; P < .0001).
For PsA relative to no PsA in the five studies that compared risk between these two groups, the risk of NAFLD was again nearly twofold higher. This fell short of conventional definition of statistical significance, but it was associated with a strong trend (OR, 1.83; 95% CI, 0.98-3.43; P = .06).
The risk of NAFLD among patients with psoriasis was not found to vary significantly when assessed by univariable meta-regressions across numerous characteristics, such as sex and body mass index.
In one of the largest of the observational studies included in the meta-analysis by Alexis Ogdie, MD, associate professor of medicine and epidemiology at the University of Pennsylvania, Philadelphia, and colleagues, data were analyzed in more than 1.5 million patients, which included 54,251 patients with rheumatoid arthritis. While the hazard ratio of NAFLD was increased for both psoriasis (HR, 2.23) and PsA (HR, 2.11), it was not elevated in those with RA (HR, 0.96).
Risk by severity, possible mechanisms
This study also included an analysis of NAFLD risk according to psoriasis severity. While risk was still significant among those with mild disease (HR, 1.18; 95% CI, 1.07-1.30), it was almost twofold greater in those with moderate to severe psoriasis (HR, 2.23; 95% CI, 1.73-2.87).
Dr. Bellinato conceded that the mechanisms underlying the association between psoriasis and NAFLD are unknown, but he said “metaflammation” is suspected.
“The secretion of proinflammatory, prothrombotic, and oxidative stress mediators in both psoriatic skin and adipose tissue might act systemically and promote insulin resistance and other metabolic derangements that promote the development and progression of NAFLD,” Dr. Bellinato explained.
He thinks that noninvasive screening methods, such as currently used methods to calculate fibrosis score, might be useful for evaluating patients with psoriasis for NAFLD and referring them to a hepatologist when appropriate.
Given the strong association with NAFLD, Dr. Bellinato suggested that “the findings of this meta-analysis pave the way for novel, large, prospective, and histologically based studies.”
The association between psoriasis and NAFLD is clinically relevant, agreed Joel M. Gelfand, MD, vice-chair of clinical research and medical director of the clinical studies unit, department of dermatology, University of Pennsylvania, Philadelphia.
“It is not clear if psoriasis causes fatty liver disease or vice versa, but clinicians should be aware of this association,” he said in an interview. Dr. Gelfand was a coauthor of the study by Dr. Ogdie and colleagues and led another more recent population-based study that implicated methotrexate as a factor in psoriasis-related hepatotoxicity.
If NAFLD is identified in a patient with psoriasis, treatments are limited, but Dr. Gelfand suggested that patients should be made aware of the risk. “Clinicians should encourage patients with psoriasis to take measures to protect their liver, such as avoiding drinking alcohol to excess and trying to maintain a healthy body weight,” he said.
Dr. Bellinato reported no conflicts of interest. Dr. Gelfand has financial relationships with more than 10 pharmaceutical companies, including those that make therapies for psoriasis.
AT GRAPPA 2022
Methotrexate’s impact on COVID-19 vaccination: New insights made
Patients who take methotrexate for a variety of immune-mediated inflammatory diseases and pause taking the drug following receipt of a COVID-19 vaccine dose did not have a higher risk of disease flare and had higher antireceptor binding domain (anti-RBD) antibody titers and increased immunogenicity when compared with continuing the drug, three recent studies suggest.
In one study, British researchers examined the effects of a 2-week break in methotrexate therapy on anti-RBD titers following receipt of a third COVID-19 vaccine dose. In their paper published in The Lancet: Respiratory Medicine, they reported results from a randomized, open-label, superiority trial that suggested pausing the drug improved immunogenicity, compared with no break.
In two trials presented at the European Alliance of Associations for Rheumatology (EULAR) 2022 Congress, a team from India set out to determine whether holding methotrexate after receiving both doses of a COVID-19 vaccine, or holding it only after the second dose, was safe and effective. They found that pausing methotrexate only following the second dose contributed to a lower flare risk, and that patients had higher anti-RBD titers when holding methotrexate for 2 weeks following each dose.
Pausing methotrexate after booster
The 2-week methotrexate break and booster vaccine dose data in the Vaccine Response On Off Methotrexate (VROOM) trial showed that after a month, the geometric mean antispike 1 (S1)-RBD antibody titer was 10,798 U/mL (95% confidence interval [CI], 8,970-12,997) in the group that continued methotrexate and 22,750 U/mL (95% CI, 19,314-26,796) in the group that suspended methotrexate; the geometric mean ratio was 2.19 (P < .0001; mixed-effects model), reported Abhishek Abhishek, MD, PhD, professor of rheumatology at the University of Nottingham in Nottingham, England, and colleagues.
Prior research showed that stopping methotrexate therapy for 2 weeks following the seasonal influenza vaccine contributed to better vaccine immunity among patients with rheumatoid arthritis, but there was no impact of stopping the drug for up to 4 weeks before vaccination on vaccine-related immunity, the researchers noted.
It is crucial in maximizing long-lasting vaccine protection in people who are possibly susceptible through immune suppression at this point in the COVID-19 vaccination regimen, the study team noted.
“Evidence from this study will be useful for policymakers, national immunization advisory committees, and specialist societies formulating recommendations on the use of methotrexate around the time of COVID-19 vaccination. This evidence will help patients and clinicians make informed choices about the risks and benefits of interrupting methotrexate treatment around the time of COVID-19 vaccination, with implications for the potential to extend such approaches to other therapeutics,” they wrote.
In American College of Rheumatology (ACR) guidance for COVID-19 vaccination, the organization advised against using standard synthetic disease-modifying antirheumatic medicines such as methotrexate “for 1-2 weeks (as disease activity allows) after each COVID-19 vaccine dose,” given the at-risk population and public health concerns, Jeffrey A. Sparks, MD, MMSc, assistant professor of medicine and associate physician at Brigham and Women’s Hospital and Harvard Medical School, Boston, and Sara K. Tedeschi, MD, MPH, assistant professor of medicine at Harvard Medical School, noted in an accompanying editorial in The Lancet: Respiratory Medicine.
However, when the ACR developed this statement, there was only one trial involving patients with rheumatoid arthritis who paused methotrexate following seasonal influenza vaccination, the editorialists said.
“Although this finding adds to the evidence base to support interruption of methotrexate after vaccination, a shared decision process is needed to weigh the possible benefit of optimizing protection from COVID-19 and the possible risk of underlying disease flare,” they added.
Dr. Abhishek and colleagues assessed 254 patients with immune-mediated inflammatory disease from dermatology and rheumatology clinics across 26 hospitals in the United Kingdom. Participants had been diagnosed with systemic lupus erythematosus, rheumatoid arthritis, atopic dermatitis, polymyalgia rheumatica, axial spondyloarthritis, and psoriasis without or with arthritis. They had also been taking up to 25 mg of methotrexate per week for 3 months or longer and had received two doses of either the Pfizer/BioNTech BNT162b2 vaccine or AstraZeneca/Oxford viral vector vaccine. The booster dose was most often the Pfizer BNT162b2 vaccine (82%). The patients’ mean age was 59 years, with females comprising 61% of the cohort. Participants were randomly assigned 1:1 to either group.
Investigators performing laboratory analysis were masked to cohort assignment, and clinical research staff, data analysts, participants, and researchers were unmasked.
The elevated antibody response of patients who suspended methotrexate was the same across different kinds of immune-mediated inflammatory disease, primary vaccination platform, SARS-CoV-2 infection history, and age.
Notably, no intervention-associated adverse events were reported, the study team noted.
The conclusions that could be drawn from the booster-dose study were limited by the trial’s modest cohort size, the small number of patients in exploratory subgroup analyses, a lack of information about differences in prescription drug behavior, and early termination’s effect on the researchers’ ability to identify differences between subgroups and in secondary outcomes, the authors noted.
Other limitations included a lack of generalizability to patients with active disease who couldn’t stop therapy and were not included in the investigation, and participants were not blinded to what group they were in, the researchers said.
Expert commentary
This current study is consistent with other studies over the last several months showing that methotrexate harms both humoral and cell-mediated COVID-19 responses, noted Kevin Winthrop, MD, MPH, professor of infectious disease and public health at Oregon Health & Science University, Portland, who was not involved in the study. “And so now the new wave of studies are like this one, where they are holding methotrexate experimentally and seeing if it makes a difference,” he said.
“The one shortcoming of this study – and so far, the studies to date – is that no one has looked at whether the experimental hold has resulted in a change in T-cell responses, which ... we are [now] recognizing [the importance of] more and more in long-term protection, particularly in severe disease. Theoretically, holding [methotrexate] might help enhance T-cell responses, but that hasn’t been shown experimentally.”
Dr. Winthrop pointed out that one might get the same benefit from holding methotrexate for 1 week instead of 2 and that there likely is a reduced risk of flare-up from underlying autoimmune disease.
It is still not certain that this benefit extends to other vaccines, Dr. Winthrop noted. “It is probably true for most vaccines that if you hold methotrexate for 1 or 2 weeks, you might see some short-term benefit in responsiveness, but you don’t know that there is any clinical meaningfulness of this. That’s going to take other long-term studies. You don’t know how long this benefit lasts.”
Pausing methotrexate during initial COVID vaccine doses
Patients with either rheumatoid arthritis or psoriatic arthritis had higher anti-RBD antibody titers when methotrexate was stopped after both doses of the AstraZeneca vaccine, or simply after the second dose, than when methotrexate was continued, according to results from two single-center, randomized controlled trials called MIVAC I and II, Anu Sreekanth, MD, of Sree Sudheendra Medical Mission in Kochi, Kerala, India, and colleagues reported at EULAR 2022.
Results from MIVAC I indicated that there was a higher flare rate when methotrexate was stopped after both vaccine doses, but there was no difference in flare rate in MIVAC II when methotrexate was stopped only after the second dose as opposed to stopping it after both doses.
In the MIVAC I trial, 158 unvaccinated patients were randomized 1:1 to a cohort in which methotrexate was held for 2 weeks after both doses and a cohort in which methotrexate was continued despite the vaccine. In MIVAC II, 157 patients continued methotrexate while receiving the first vaccine dose. These patients were subsequently randomized either to continue or to stop methotrexate for 2 weeks following the second dose.
The findings from MIVAC I demonstrated the flare rate was lower in the methotrexate-continue group than in the methotrexate-pause group (8% vs. 25%; P = .005) and that the median anti-RBD titer was significantly higher for the methotrexate-pause group than the methotrexate-continue group (2,484 vs. 1,147; P = .001).
The results from MIVAC II trial indicated that there was no difference in flare rates between the two study groups (7.9% vs. 11.8%; P = .15). Yet, the median anti-RBD titer was significantly higher in the methotrexate-pause cohort than in the methotrexate-continue cohort (2,553 vs. 990; P = .001).
The report suggests there is a flare risk when methotrexate is stopped, Dr. Sreekanth noted. “It appears more logical to hold only after the second dose, as comparable anti-RBD titers are generated” with either approach, Dr. Sreekanth said.
Expert commentary: MIVAC I and II
Inés Colmegna, MD, associate professor at McGill University in Montreal, noted that it was intriguing that the risk of flares in MIVAC II is half of that reported after each of the doses of MIVAC I. “It is also worth emphasizing that despite the reported frequency of flares, the actual disease activity [as measured by the Disease Activity Score in 28 joints] in patients who did or did not withhold methotrexate was similar.
“MIVAC I and II have practical implications as they help to adequately inform patients about the risk and benefit trade of withholding methotrexate post–COVID-19 vaccination,” Dr. Colmegna told this news organization.
“Additional information would help to [further] interpret the findings of these studies, including whether any of the participants were taking any other DMARDs; data on the severity of the flares and functional impact; analysis of factors that predict the risk of flares, such as higher doses of methotrexate; [and change in] disease activity scores pre- and postvaccination,” Dr. Colmegna concluded.
Dr. Abhishek disclosed relationships with Springer, UpTodate, Oxford, Immunotec, AstraZeneca, Inflazome, NGM Biopharmaceuticals, Menarini Pharmaceuticals, and Cadila Pharmaceuticals. Dr. Abhishek is cochair of the ACR/EULAR CPPD Classification Criteria Working Group and the OMERACT CPPD Working Group. Dr. Sparks disclosed relationships with Gilead, Boehringer Ingelheim, Amgen, Bristol-Myers Squibb, and AbbVie, unrelated to this study. Dr. Tedeschi disclosed relationships with ModernaTx and NGM Biopharmaceuticals. Dr. Winthrop disclosed a research grant and serving as a scientific consultant for Pfizer. Dr. Sreekanth and Dr. Colmegna have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients who take methotrexate for a variety of immune-mediated inflammatory diseases and pause taking the drug following receipt of a COVID-19 vaccine dose did not have a higher risk of disease flare and had higher antireceptor binding domain (anti-RBD) antibody titers and increased immunogenicity when compared with continuing the drug, three recent studies suggest.
In one study, British researchers examined the effects of a 2-week break in methotrexate therapy on anti-RBD titers following receipt of a third COVID-19 vaccine dose. In their paper published in The Lancet: Respiratory Medicine, they reported results from a randomized, open-label, superiority trial that suggested pausing the drug improved immunogenicity, compared with no break.
In two trials presented at the European Alliance of Associations for Rheumatology (EULAR) 2022 Congress, a team from India set out to determine whether holding methotrexate after receiving both doses of a COVID-19 vaccine, or holding it only after the second dose, was safe and effective. They found that pausing methotrexate only following the second dose contributed to a lower flare risk, and that patients had higher anti-RBD titers when holding methotrexate for 2 weeks following each dose.
Pausing methotrexate after booster
The 2-week methotrexate break and booster vaccine dose data in the Vaccine Response On Off Methotrexate (VROOM) trial showed that after a month, the geometric mean antispike 1 (S1)-RBD antibody titer was 10,798 U/mL (95% confidence interval [CI], 8,970-12,997) in the group that continued methotrexate and 22,750 U/mL (95% CI, 19,314-26,796) in the group that suspended methotrexate; the geometric mean ratio was 2.19 (P < .0001; mixed-effects model), reported Abhishek Abhishek, MD, PhD, professor of rheumatology at the University of Nottingham in Nottingham, England, and colleagues.
Prior research showed that stopping methotrexate therapy for 2 weeks following the seasonal influenza vaccine contributed to better vaccine immunity among patients with rheumatoid arthritis, but there was no impact of stopping the drug for up to 4 weeks before vaccination on vaccine-related immunity, the researchers noted.
It is crucial in maximizing long-lasting vaccine protection in people who are possibly susceptible through immune suppression at this point in the COVID-19 vaccination regimen, the study team noted.
“Evidence from this study will be useful for policymakers, national immunization advisory committees, and specialist societies formulating recommendations on the use of methotrexate around the time of COVID-19 vaccination. This evidence will help patients and clinicians make informed choices about the risks and benefits of interrupting methotrexate treatment around the time of COVID-19 vaccination, with implications for the potential to extend such approaches to other therapeutics,” they wrote.
In American College of Rheumatology (ACR) guidance for COVID-19 vaccination, the organization advised against using standard synthetic disease-modifying antirheumatic medicines such as methotrexate “for 1-2 weeks (as disease activity allows) after each COVID-19 vaccine dose,” given the at-risk population and public health concerns, Jeffrey A. Sparks, MD, MMSc, assistant professor of medicine and associate physician at Brigham and Women’s Hospital and Harvard Medical School, Boston, and Sara K. Tedeschi, MD, MPH, assistant professor of medicine at Harvard Medical School, noted in an accompanying editorial in The Lancet: Respiratory Medicine.
However, when the ACR developed this statement, there was only one trial involving patients with rheumatoid arthritis who paused methotrexate following seasonal influenza vaccination, the editorialists said.
“Although this finding adds to the evidence base to support interruption of methotrexate after vaccination, a shared decision process is needed to weigh the possible benefit of optimizing protection from COVID-19 and the possible risk of underlying disease flare,” they added.
Dr. Abhishek and colleagues assessed 254 patients with immune-mediated inflammatory disease from dermatology and rheumatology clinics across 26 hospitals in the United Kingdom. Participants had been diagnosed with systemic lupus erythematosus, rheumatoid arthritis, atopic dermatitis, polymyalgia rheumatica, axial spondyloarthritis, and psoriasis without or with arthritis. They had also been taking up to 25 mg of methotrexate per week for 3 months or longer and had received two doses of either the Pfizer/BioNTech BNT162b2 vaccine or AstraZeneca/Oxford viral vector vaccine. The booster dose was most often the Pfizer BNT162b2 vaccine (82%). The patients’ mean age was 59 years, with females comprising 61% of the cohort. Participants were randomly assigned 1:1 to either group.
Investigators performing laboratory analysis were masked to cohort assignment, and clinical research staff, data analysts, participants, and researchers were unmasked.
The elevated antibody response of patients who suspended methotrexate was the same across different kinds of immune-mediated inflammatory disease, primary vaccination platform, SARS-CoV-2 infection history, and age.
Notably, no intervention-associated adverse events were reported, the study team noted.
The conclusions that could be drawn from the booster-dose study were limited by the trial’s modest cohort size, the small number of patients in exploratory subgroup analyses, a lack of information about differences in prescription drug behavior, and early termination’s effect on the researchers’ ability to identify differences between subgroups and in secondary outcomes, the authors noted.
Other limitations included a lack of generalizability to patients with active disease who couldn’t stop therapy and were not included in the investigation, and participants were not blinded to what group they were in, the researchers said.
Expert commentary
This current study is consistent with other studies over the last several months showing that methotrexate harms both humoral and cell-mediated COVID-19 responses, noted Kevin Winthrop, MD, MPH, professor of infectious disease and public health at Oregon Health & Science University, Portland, who was not involved in the study. “And so now the new wave of studies are like this one, where they are holding methotrexate experimentally and seeing if it makes a difference,” he said.
“The one shortcoming of this study – and so far, the studies to date – is that no one has looked at whether the experimental hold has resulted in a change in T-cell responses, which ... we are [now] recognizing [the importance of] more and more in long-term protection, particularly in severe disease. Theoretically, holding [methotrexate] might help enhance T-cell responses, but that hasn’t been shown experimentally.”
Dr. Winthrop pointed out that one might get the same benefit from holding methotrexate for 1 week instead of 2 and that there likely is a reduced risk of flare-up from underlying autoimmune disease.
It is still not certain that this benefit extends to other vaccines, Dr. Winthrop noted. “It is probably true for most vaccines that if you hold methotrexate for 1 or 2 weeks, you might see some short-term benefit in responsiveness, but you don’t know that there is any clinical meaningfulness of this. That’s going to take other long-term studies. You don’t know how long this benefit lasts.”
Pausing methotrexate during initial COVID vaccine doses
Patients with either rheumatoid arthritis or psoriatic arthritis had higher anti-RBD antibody titers when methotrexate was stopped after both doses of the AstraZeneca vaccine, or simply after the second dose, than when methotrexate was continued, according to results from two single-center, randomized controlled trials called MIVAC I and II, Anu Sreekanth, MD, of Sree Sudheendra Medical Mission in Kochi, Kerala, India, and colleagues reported at EULAR 2022.
Results from MIVAC I indicated that there was a higher flare rate when methotrexate was stopped after both vaccine doses, but there was no difference in flare rate in MIVAC II when methotrexate was stopped only after the second dose as opposed to stopping it after both doses.
In the MIVAC I trial, 158 unvaccinated patients were randomized 1:1 to a cohort in which methotrexate was held for 2 weeks after both doses and a cohort in which methotrexate was continued despite the vaccine. In MIVAC II, 157 patients continued methotrexate while receiving the first vaccine dose. These patients were subsequently randomized either to continue or to stop methotrexate for 2 weeks following the second dose.
The findings from MIVAC I demonstrated the flare rate was lower in the methotrexate-continue group than in the methotrexate-pause group (8% vs. 25%; P = .005) and that the median anti-RBD titer was significantly higher for the methotrexate-pause group than the methotrexate-continue group (2,484 vs. 1,147; P = .001).
The results from MIVAC II trial indicated that there was no difference in flare rates between the two study groups (7.9% vs. 11.8%; P = .15). Yet, the median anti-RBD titer was significantly higher in the methotrexate-pause cohort than in the methotrexate-continue cohort (2,553 vs. 990; P = .001).
The report suggests there is a flare risk when methotrexate is stopped, Dr. Sreekanth noted. “It appears more logical to hold only after the second dose, as comparable anti-RBD titers are generated” with either approach, Dr. Sreekanth said.
Expert commentary: MIVAC I and II
Inés Colmegna, MD, associate professor at McGill University in Montreal, noted that it was intriguing that the risk of flares in MIVAC II is half of that reported after each of the doses of MIVAC I. “It is also worth emphasizing that despite the reported frequency of flares, the actual disease activity [as measured by the Disease Activity Score in 28 joints] in patients who did or did not withhold methotrexate was similar.
“MIVAC I and II have practical implications as they help to adequately inform patients about the risk and benefit trade of withholding methotrexate post–COVID-19 vaccination,” Dr. Colmegna told this news organization.
“Additional information would help to [further] interpret the findings of these studies, including whether any of the participants were taking any other DMARDs; data on the severity of the flares and functional impact; analysis of factors that predict the risk of flares, such as higher doses of methotrexate; [and change in] disease activity scores pre- and postvaccination,” Dr. Colmegna concluded.
Dr. Abhishek disclosed relationships with Springer, UpTodate, Oxford, Immunotec, AstraZeneca, Inflazome, NGM Biopharmaceuticals, Menarini Pharmaceuticals, and Cadila Pharmaceuticals. Dr. Abhishek is cochair of the ACR/EULAR CPPD Classification Criteria Working Group and the OMERACT CPPD Working Group. Dr. Sparks disclosed relationships with Gilead, Boehringer Ingelheim, Amgen, Bristol-Myers Squibb, and AbbVie, unrelated to this study. Dr. Tedeschi disclosed relationships with ModernaTx and NGM Biopharmaceuticals. Dr. Winthrop disclosed a research grant and serving as a scientific consultant for Pfizer. Dr. Sreekanth and Dr. Colmegna have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients who take methotrexate for a variety of immune-mediated inflammatory diseases and pause taking the drug following receipt of a COVID-19 vaccine dose did not have a higher risk of disease flare and had higher antireceptor binding domain (anti-RBD) antibody titers and increased immunogenicity when compared with continuing the drug, three recent studies suggest.
In one study, British researchers examined the effects of a 2-week break in methotrexate therapy on anti-RBD titers following receipt of a third COVID-19 vaccine dose. In their paper published in The Lancet: Respiratory Medicine, they reported results from a randomized, open-label, superiority trial that suggested pausing the drug improved immunogenicity, compared with no break.
In two trials presented at the European Alliance of Associations for Rheumatology (EULAR) 2022 Congress, a team from India set out to determine whether holding methotrexate after receiving both doses of a COVID-19 vaccine, or holding it only after the second dose, was safe and effective. They found that pausing methotrexate only following the second dose contributed to a lower flare risk, and that patients had higher anti-RBD titers when holding methotrexate for 2 weeks following each dose.
Pausing methotrexate after booster
The 2-week methotrexate break and booster vaccine dose data in the Vaccine Response On Off Methotrexate (VROOM) trial showed that after a month, the geometric mean antispike 1 (S1)-RBD antibody titer was 10,798 U/mL (95% confidence interval [CI], 8,970-12,997) in the group that continued methotrexate and 22,750 U/mL (95% CI, 19,314-26,796) in the group that suspended methotrexate; the geometric mean ratio was 2.19 (P < .0001; mixed-effects model), reported Abhishek Abhishek, MD, PhD, professor of rheumatology at the University of Nottingham in Nottingham, England, and colleagues.
Prior research showed that stopping methotrexate therapy for 2 weeks following the seasonal influenza vaccine contributed to better vaccine immunity among patients with rheumatoid arthritis, but there was no impact of stopping the drug for up to 4 weeks before vaccination on vaccine-related immunity, the researchers noted.
It is crucial in maximizing long-lasting vaccine protection in people who are possibly susceptible through immune suppression at this point in the COVID-19 vaccination regimen, the study team noted.
“Evidence from this study will be useful for policymakers, national immunization advisory committees, and specialist societies formulating recommendations on the use of methotrexate around the time of COVID-19 vaccination. This evidence will help patients and clinicians make informed choices about the risks and benefits of interrupting methotrexate treatment around the time of COVID-19 vaccination, with implications for the potential to extend such approaches to other therapeutics,” they wrote.
In American College of Rheumatology (ACR) guidance for COVID-19 vaccination, the organization advised against using standard synthetic disease-modifying antirheumatic medicines such as methotrexate “for 1-2 weeks (as disease activity allows) after each COVID-19 vaccine dose,” given the at-risk population and public health concerns, Jeffrey A. Sparks, MD, MMSc, assistant professor of medicine and associate physician at Brigham and Women’s Hospital and Harvard Medical School, Boston, and Sara K. Tedeschi, MD, MPH, assistant professor of medicine at Harvard Medical School, noted in an accompanying editorial in The Lancet: Respiratory Medicine.
However, when the ACR developed this statement, there was only one trial involving patients with rheumatoid arthritis who paused methotrexate following seasonal influenza vaccination, the editorialists said.
“Although this finding adds to the evidence base to support interruption of methotrexate after vaccination, a shared decision process is needed to weigh the possible benefit of optimizing protection from COVID-19 and the possible risk of underlying disease flare,” they added.
Dr. Abhishek and colleagues assessed 254 patients with immune-mediated inflammatory disease from dermatology and rheumatology clinics across 26 hospitals in the United Kingdom. Participants had been diagnosed with systemic lupus erythematosus, rheumatoid arthritis, atopic dermatitis, polymyalgia rheumatica, axial spondyloarthritis, and psoriasis without or with arthritis. They had also been taking up to 25 mg of methotrexate per week for 3 months or longer and had received two doses of either the Pfizer/BioNTech BNT162b2 vaccine or AstraZeneca/Oxford viral vector vaccine. The booster dose was most often the Pfizer BNT162b2 vaccine (82%). The patients’ mean age was 59 years, with females comprising 61% of the cohort. Participants were randomly assigned 1:1 to either group.
Investigators performing laboratory analysis were masked to cohort assignment, and clinical research staff, data analysts, participants, and researchers were unmasked.
The elevated antibody response of patients who suspended methotrexate was the same across different kinds of immune-mediated inflammatory disease, primary vaccination platform, SARS-CoV-2 infection history, and age.
Notably, no intervention-associated adverse events were reported, the study team noted.
The conclusions that could be drawn from the booster-dose study were limited by the trial’s modest cohort size, the small number of patients in exploratory subgroup analyses, a lack of information about differences in prescription drug behavior, and early termination’s effect on the researchers’ ability to identify differences between subgroups and in secondary outcomes, the authors noted.
Other limitations included a lack of generalizability to patients with active disease who couldn’t stop therapy and were not included in the investigation, and participants were not blinded to what group they were in, the researchers said.
Expert commentary
This current study is consistent with other studies over the last several months showing that methotrexate harms both humoral and cell-mediated COVID-19 responses, noted Kevin Winthrop, MD, MPH, professor of infectious disease and public health at Oregon Health & Science University, Portland, who was not involved in the study. “And so now the new wave of studies are like this one, where they are holding methotrexate experimentally and seeing if it makes a difference,” he said.
“The one shortcoming of this study – and so far, the studies to date – is that no one has looked at whether the experimental hold has resulted in a change in T-cell responses, which ... we are [now] recognizing [the importance of] more and more in long-term protection, particularly in severe disease. Theoretically, holding [methotrexate] might help enhance T-cell responses, but that hasn’t been shown experimentally.”
Dr. Winthrop pointed out that one might get the same benefit from holding methotrexate for 1 week instead of 2 and that there likely is a reduced risk of flare-up from underlying autoimmune disease.
It is still not certain that this benefit extends to other vaccines, Dr. Winthrop noted. “It is probably true for most vaccines that if you hold methotrexate for 1 or 2 weeks, you might see some short-term benefit in responsiveness, but you don’t know that there is any clinical meaningfulness of this. That’s going to take other long-term studies. You don’t know how long this benefit lasts.”
Pausing methotrexate during initial COVID vaccine doses
Patients with either rheumatoid arthritis or psoriatic arthritis had higher anti-RBD antibody titers when methotrexate was stopped after both doses of the AstraZeneca vaccine, or simply after the second dose, than when methotrexate was continued, according to results from two single-center, randomized controlled trials called MIVAC I and II, Anu Sreekanth, MD, of Sree Sudheendra Medical Mission in Kochi, Kerala, India, and colleagues reported at EULAR 2022.
Results from MIVAC I indicated that there was a higher flare rate when methotrexate was stopped after both vaccine doses, but there was no difference in flare rate in MIVAC II when methotrexate was stopped only after the second dose as opposed to stopping it after both doses.
In the MIVAC I trial, 158 unvaccinated patients were randomized 1:1 to a cohort in which methotrexate was held for 2 weeks after both doses and a cohort in which methotrexate was continued despite the vaccine. In MIVAC II, 157 patients continued methotrexate while receiving the first vaccine dose. These patients were subsequently randomized either to continue or to stop methotrexate for 2 weeks following the second dose.
The findings from MIVAC I demonstrated the flare rate was lower in the methotrexate-continue group than in the methotrexate-pause group (8% vs. 25%; P = .005) and that the median anti-RBD titer was significantly higher for the methotrexate-pause group than the methotrexate-continue group (2,484 vs. 1,147; P = .001).
The results from MIVAC II trial indicated that there was no difference in flare rates between the two study groups (7.9% vs. 11.8%; P = .15). Yet, the median anti-RBD titer was significantly higher in the methotrexate-pause cohort than in the methotrexate-continue cohort (2,553 vs. 990; P = .001).
The report suggests there is a flare risk when methotrexate is stopped, Dr. Sreekanth noted. “It appears more logical to hold only after the second dose, as comparable anti-RBD titers are generated” with either approach, Dr. Sreekanth said.
Expert commentary: MIVAC I and II
Inés Colmegna, MD, associate professor at McGill University in Montreal, noted that it was intriguing that the risk of flares in MIVAC II is half of that reported after each of the doses of MIVAC I. “It is also worth emphasizing that despite the reported frequency of flares, the actual disease activity [as measured by the Disease Activity Score in 28 joints] in patients who did or did not withhold methotrexate was similar.
“MIVAC I and II have practical implications as they help to adequately inform patients about the risk and benefit trade of withholding methotrexate post–COVID-19 vaccination,” Dr. Colmegna told this news organization.
“Additional information would help to [further] interpret the findings of these studies, including whether any of the participants were taking any other DMARDs; data on the severity of the flares and functional impact; analysis of factors that predict the risk of flares, such as higher doses of methotrexate; [and change in] disease activity scores pre- and postvaccination,” Dr. Colmegna concluded.
Dr. Abhishek disclosed relationships with Springer, UpTodate, Oxford, Immunotec, AstraZeneca, Inflazome, NGM Biopharmaceuticals, Menarini Pharmaceuticals, and Cadila Pharmaceuticals. Dr. Abhishek is cochair of the ACR/EULAR CPPD Classification Criteria Working Group and the OMERACT CPPD Working Group. Dr. Sparks disclosed relationships with Gilead, Boehringer Ingelheim, Amgen, Bristol-Myers Squibb, and AbbVie, unrelated to this study. Dr. Tedeschi disclosed relationships with ModernaTx and NGM Biopharmaceuticals. Dr. Winthrop disclosed a research grant and serving as a scientific consultant for Pfizer. Dr. Sreekanth and Dr. Colmegna have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Benzbromarone tops febuxostat for gout?
Benzbromarone is not approved in the United States because of concerns of acute liver injury but is approved in several other countries, including China, Brazil, and New Zealand.
“The results suggest that low dosing of benzbromarone may warrant stronger consideration as a safe and effective therapy to achieve serum urate target in gout without moderate chronic kidney disease,” the study team writes.
“Benzbromarone is severely hepatotoxic in some individuals and unlikely to ever gain approval in the United States,” one of the study’s investigators, Robert Terkeltaub, MD, professor of medicine, University of California, San Diego, told this news organization.
However, this study “illustrates the value and impact of uricosuric therapy in general in gout, including potentially as an initial urate-lowering monotherapy strategy, and the sheer number of subjects reaching urate target with low-dose uricosuric monotherapy was impressive,” Dr. Terkeltaub said.
The study was published online in Arthritis & Rheumatology.
“Renal uric acid underexcretion is the chief mechanism driving hyperuricemia in gout, yet the standard urate-lowering therapy recommendation is first-line xanthine oxidase inhibition irrespective of the cause of hyperuricemia,” the study team explains in their article.
Their prospective, randomized, single-center, open-labeled trial was conducted at the Gout Clinic of the Affiliated Hospital of Qingdao University, China.
A total of 196 relatively young healthy men with gout and uric acid underexcretion were randomly assigned to receive low-dose benzbromarone (25 mg/d) or low-dose febuxostat (20 mg/d) for 12 weeks.
Renal uric acid underexcretion was defined as fractional excretion of urate less than 5.5% and uric acid excretion less than or equal to 600 mg/d/1.73 m2.
A “major aspect” of this comparative effectiveness trial was its specific focus on gout-associated renal uric acid underexcretion, where the uricosuric targeted the dominant abnormality promoting the hyperuricemia, Dr. Terkeltaub told this news organization.
In addition, all participants received daily urine alkalinization with oral sodium bicarbonate. “This is not always done in clinical practice, nor in clinical trials of uricosuric agents,” Dr. Terkeltaub said.
The results showed that more participants in the benzbromarone group achieved the serum urate target of less than 6 mg/dL, compared with those in the febuxostat group (primary endpoint, 61% vs. 32%, P < .001).
Adverse events, including gout flares and urolithiasis, did not differ significantly between the two groups, with the exception of more transaminase elevation in the febuxostat group (15% vs. 4%; P = .008).
“We did not find severe hepatotoxicity with low-dose benzbromarone, but ethnic background may affect drug responses, and severe hepatotoxicity of benzbromarone has rarely been reported in Asia,” the authors write.
The incidence of urolithiasis was numerically, but not significantly, higher in the benzbromarone group (5% vs. 2%).
This study found no significant changes in participants’ triglyceride levels, though a previous study suggested febuxostat could increase serum triglycerides.
The investigators caution that the study only included patients who had baseline serum urate levels ranging from 8.0 to 10 mg/dL, who were relatively young and with few comorbidities.
The authors further noted that the “... results may not be generalizable to patients with higher serum urate levels or impaired kidney function, as well [as] patients from other geographical regions, age, and ethnicity groups. The study only included men, and the findings may not be generalizable to women with gout.”
‘Very useful’ in select cases
Weighing in on the results, Valderilio Feijó Azevedo, MD, PhD, adjunct professor of rheumatology, Federal University of Paraná, Brazil, noted that in some specific clinical circumstances, benzbromarone has been “a very useful medication, alone or combined, to treat gout patients.”
“We have great experience with the drug in Brazil. However, it is not used to treat all patients. Patients must be very well-selected in our clinical practice,” Dr. Azevedo said in an interview.
“For most patients, benzbromarone is effective for those who have failed to achieve serum uric acid goals with allopurinol treatment. We do not use it to treat patients with asymptomatic hyperuricemia. In general, we avoid patients with hepatic dysfunction due to previous hepatotoxicity reports. In every patient, we do active monitoring of enzymes,” Dr. Azevedo explained.
“We also avoid using it in patients with severe kidney disease. However, we have used it in some patients with estimated glomerular filtration rate less than 30. We also avoid dosage over 200 mg per day. On average, we use 100 mg per day combined with allopurinol or alone,” said Dr. Azevedo, who was not involved with the study.
Also weighing in, Michael Pillinger, MD, rheumatologist at NYU Langone Health, noted that while benzbromarone is not used in the United States, “in many parts of the world, it is used and is felt to be effective.” Dr. Pillinger was not associated with this current research.
This study, Dr. Pillinger said, “does underline the fact that an alternative drug that lowers urate by promoting urate excretion, if it could gain [U.S. Food and Drug Association] approval and if it were safe, could present a viable new option for therapy.”
He added, “If one conclusion to the study is that determining the basis of hyperuricemia is helpful in guiding benzbromarone use, that implies an additional layer of effort for physicians and patients in a disease that is already notoriously known for patient noncompliance – and in a case where febuxostat and allopurinol will work for both overproducers and underexcreters and would not need this additional assessment.”
The study was sponsored by Shandong Provincial Key Research and Development Plan, the National Natural Science Foundation of China, and Shandong Provincial Science Foundation for Outstanding Youth Scholarship. Dr. Terkeltaub was supported by the National Institutes of Health and the VA Research Service. Dr. Terkeltaub has received research funding from AstraZeneca, and has consulted with Horizon, Selecta, SOBI, Dyve BioSciences, Fortress, AstraZeneca, Allena, Fortress Biotech, and LG Life Sciences. Dr. Azevedo and Dr. Pillinger have no reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Benzbromarone is not approved in the United States because of concerns of acute liver injury but is approved in several other countries, including China, Brazil, and New Zealand.
“The results suggest that low dosing of benzbromarone may warrant stronger consideration as a safe and effective therapy to achieve serum urate target in gout without moderate chronic kidney disease,” the study team writes.
“Benzbromarone is severely hepatotoxic in some individuals and unlikely to ever gain approval in the United States,” one of the study’s investigators, Robert Terkeltaub, MD, professor of medicine, University of California, San Diego, told this news organization.
However, this study “illustrates the value and impact of uricosuric therapy in general in gout, including potentially as an initial urate-lowering monotherapy strategy, and the sheer number of subjects reaching urate target with low-dose uricosuric monotherapy was impressive,” Dr. Terkeltaub said.
The study was published online in Arthritis & Rheumatology.
“Renal uric acid underexcretion is the chief mechanism driving hyperuricemia in gout, yet the standard urate-lowering therapy recommendation is first-line xanthine oxidase inhibition irrespective of the cause of hyperuricemia,” the study team explains in their article.
Their prospective, randomized, single-center, open-labeled trial was conducted at the Gout Clinic of the Affiliated Hospital of Qingdao University, China.
A total of 196 relatively young healthy men with gout and uric acid underexcretion were randomly assigned to receive low-dose benzbromarone (25 mg/d) or low-dose febuxostat (20 mg/d) for 12 weeks.
Renal uric acid underexcretion was defined as fractional excretion of urate less than 5.5% and uric acid excretion less than or equal to 600 mg/d/1.73 m2.
A “major aspect” of this comparative effectiveness trial was its specific focus on gout-associated renal uric acid underexcretion, where the uricosuric targeted the dominant abnormality promoting the hyperuricemia, Dr. Terkeltaub told this news organization.
In addition, all participants received daily urine alkalinization with oral sodium bicarbonate. “This is not always done in clinical practice, nor in clinical trials of uricosuric agents,” Dr. Terkeltaub said.
The results showed that more participants in the benzbromarone group achieved the serum urate target of less than 6 mg/dL, compared with those in the febuxostat group (primary endpoint, 61% vs. 32%, P < .001).
Adverse events, including gout flares and urolithiasis, did not differ significantly between the two groups, with the exception of more transaminase elevation in the febuxostat group (15% vs. 4%; P = .008).
“We did not find severe hepatotoxicity with low-dose benzbromarone, but ethnic background may affect drug responses, and severe hepatotoxicity of benzbromarone has rarely been reported in Asia,” the authors write.
The incidence of urolithiasis was numerically, but not significantly, higher in the benzbromarone group (5% vs. 2%).
This study found no significant changes in participants’ triglyceride levels, though a previous study suggested febuxostat could increase serum triglycerides.
The investigators caution that the study only included patients who had baseline serum urate levels ranging from 8.0 to 10 mg/dL, who were relatively young and with few comorbidities.
The authors further noted that the “... results may not be generalizable to patients with higher serum urate levels or impaired kidney function, as well [as] patients from other geographical regions, age, and ethnicity groups. The study only included men, and the findings may not be generalizable to women with gout.”
‘Very useful’ in select cases
Weighing in on the results, Valderilio Feijó Azevedo, MD, PhD, adjunct professor of rheumatology, Federal University of Paraná, Brazil, noted that in some specific clinical circumstances, benzbromarone has been “a very useful medication, alone or combined, to treat gout patients.”
“We have great experience with the drug in Brazil. However, it is not used to treat all patients. Patients must be very well-selected in our clinical practice,” Dr. Azevedo said in an interview.
“For most patients, benzbromarone is effective for those who have failed to achieve serum uric acid goals with allopurinol treatment. We do not use it to treat patients with asymptomatic hyperuricemia. In general, we avoid patients with hepatic dysfunction due to previous hepatotoxicity reports. In every patient, we do active monitoring of enzymes,” Dr. Azevedo explained.
“We also avoid using it in patients with severe kidney disease. However, we have used it in some patients with estimated glomerular filtration rate less than 30. We also avoid dosage over 200 mg per day. On average, we use 100 mg per day combined with allopurinol or alone,” said Dr. Azevedo, who was not involved with the study.
Also weighing in, Michael Pillinger, MD, rheumatologist at NYU Langone Health, noted that while benzbromarone is not used in the United States, “in many parts of the world, it is used and is felt to be effective.” Dr. Pillinger was not associated with this current research.
This study, Dr. Pillinger said, “does underline the fact that an alternative drug that lowers urate by promoting urate excretion, if it could gain [U.S. Food and Drug Association] approval and if it were safe, could present a viable new option for therapy.”
He added, “If one conclusion to the study is that determining the basis of hyperuricemia is helpful in guiding benzbromarone use, that implies an additional layer of effort for physicians and patients in a disease that is already notoriously known for patient noncompliance – and in a case where febuxostat and allopurinol will work for both overproducers and underexcreters and would not need this additional assessment.”
The study was sponsored by Shandong Provincial Key Research and Development Plan, the National Natural Science Foundation of China, and Shandong Provincial Science Foundation for Outstanding Youth Scholarship. Dr. Terkeltaub was supported by the National Institutes of Health and the VA Research Service. Dr. Terkeltaub has received research funding from AstraZeneca, and has consulted with Horizon, Selecta, SOBI, Dyve BioSciences, Fortress, AstraZeneca, Allena, Fortress Biotech, and LG Life Sciences. Dr. Azevedo and Dr. Pillinger have no reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Benzbromarone is not approved in the United States because of concerns of acute liver injury but is approved in several other countries, including China, Brazil, and New Zealand.
“The results suggest that low dosing of benzbromarone may warrant stronger consideration as a safe and effective therapy to achieve serum urate target in gout without moderate chronic kidney disease,” the study team writes.
“Benzbromarone is severely hepatotoxic in some individuals and unlikely to ever gain approval in the United States,” one of the study’s investigators, Robert Terkeltaub, MD, professor of medicine, University of California, San Diego, told this news organization.
However, this study “illustrates the value and impact of uricosuric therapy in general in gout, including potentially as an initial urate-lowering monotherapy strategy, and the sheer number of subjects reaching urate target with low-dose uricosuric monotherapy was impressive,” Dr. Terkeltaub said.
The study was published online in Arthritis & Rheumatology.
“Renal uric acid underexcretion is the chief mechanism driving hyperuricemia in gout, yet the standard urate-lowering therapy recommendation is first-line xanthine oxidase inhibition irrespective of the cause of hyperuricemia,” the study team explains in their article.
Their prospective, randomized, single-center, open-labeled trial was conducted at the Gout Clinic of the Affiliated Hospital of Qingdao University, China.
A total of 196 relatively young healthy men with gout and uric acid underexcretion were randomly assigned to receive low-dose benzbromarone (25 mg/d) or low-dose febuxostat (20 mg/d) for 12 weeks.
Renal uric acid underexcretion was defined as fractional excretion of urate less than 5.5% and uric acid excretion less than or equal to 600 mg/d/1.73 m2.
A “major aspect” of this comparative effectiveness trial was its specific focus on gout-associated renal uric acid underexcretion, where the uricosuric targeted the dominant abnormality promoting the hyperuricemia, Dr. Terkeltaub told this news organization.
In addition, all participants received daily urine alkalinization with oral sodium bicarbonate. “This is not always done in clinical practice, nor in clinical trials of uricosuric agents,” Dr. Terkeltaub said.
The results showed that more participants in the benzbromarone group achieved the serum urate target of less than 6 mg/dL, compared with those in the febuxostat group (primary endpoint, 61% vs. 32%, P < .001).
Adverse events, including gout flares and urolithiasis, did not differ significantly between the two groups, with the exception of more transaminase elevation in the febuxostat group (15% vs. 4%; P = .008).
“We did not find severe hepatotoxicity with low-dose benzbromarone, but ethnic background may affect drug responses, and severe hepatotoxicity of benzbromarone has rarely been reported in Asia,” the authors write.
The incidence of urolithiasis was numerically, but not significantly, higher in the benzbromarone group (5% vs. 2%).
This study found no significant changes in participants’ triglyceride levels, though a previous study suggested febuxostat could increase serum triglycerides.
The investigators caution that the study only included patients who had baseline serum urate levels ranging from 8.0 to 10 mg/dL, who were relatively young and with few comorbidities.
The authors further noted that the “... results may not be generalizable to patients with higher serum urate levels or impaired kidney function, as well [as] patients from other geographical regions, age, and ethnicity groups. The study only included men, and the findings may not be generalizable to women with gout.”
‘Very useful’ in select cases
Weighing in on the results, Valderilio Feijó Azevedo, MD, PhD, adjunct professor of rheumatology, Federal University of Paraná, Brazil, noted that in some specific clinical circumstances, benzbromarone has been “a very useful medication, alone or combined, to treat gout patients.”
“We have great experience with the drug in Brazil. However, it is not used to treat all patients. Patients must be very well-selected in our clinical practice,” Dr. Azevedo said in an interview.
“For most patients, benzbromarone is effective for those who have failed to achieve serum uric acid goals with allopurinol treatment. We do not use it to treat patients with asymptomatic hyperuricemia. In general, we avoid patients with hepatic dysfunction due to previous hepatotoxicity reports. In every patient, we do active monitoring of enzymes,” Dr. Azevedo explained.
“We also avoid using it in patients with severe kidney disease. However, we have used it in some patients with estimated glomerular filtration rate less than 30. We also avoid dosage over 200 mg per day. On average, we use 100 mg per day combined with allopurinol or alone,” said Dr. Azevedo, who was not involved with the study.
Also weighing in, Michael Pillinger, MD, rheumatologist at NYU Langone Health, noted that while benzbromarone is not used in the United States, “in many parts of the world, it is used and is felt to be effective.” Dr. Pillinger was not associated with this current research.
This study, Dr. Pillinger said, “does underline the fact that an alternative drug that lowers urate by promoting urate excretion, if it could gain [U.S. Food and Drug Association] approval and if it were safe, could present a viable new option for therapy.”
He added, “If one conclusion to the study is that determining the basis of hyperuricemia is helpful in guiding benzbromarone use, that implies an additional layer of effort for physicians and patients in a disease that is already notoriously known for patient noncompliance – and in a case where febuxostat and allopurinol will work for both overproducers and underexcreters and would not need this additional assessment.”
The study was sponsored by Shandong Provincial Key Research and Development Plan, the National Natural Science Foundation of China, and Shandong Provincial Science Foundation for Outstanding Youth Scholarship. Dr. Terkeltaub was supported by the National Institutes of Health and the VA Research Service. Dr. Terkeltaub has received research funding from AstraZeneca, and has consulted with Horizon, Selecta, SOBI, Dyve BioSciences, Fortress, AstraZeneca, Allena, Fortress Biotech, and LG Life Sciences. Dr. Azevedo and Dr. Pillinger have no reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.