User login
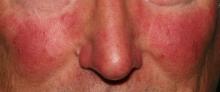
Rosacea research reveals advances, promising therapies
MIAMI – Management of rosacea continues to challenge dermatologists and patients alike, although new advances and recent studies shine a light on promising new therapies to target this inflammatory skin condition.
Linda Stein Gold, MD, who directs dermatology clinical trials at the Henry Ford Hospital in Detroit, shared new information about the pathophysiology of rosacea and the controversial associations with cardiovascular disease and addressed the rosacea “genes versus environment” etiology question at the Orlando Dermatology Aesthetic and Clinical Conference.
The topical vasoconstrictor of cutaneous vasculature, oxymetazoline hydrochloride cream 1%, showed a statistically significant improvement in erythema, compared with vehicle only in people with rosacea in a phase III study, Dr. Stein Gold said. The outcome was strict, requiring both physician and patient assessment of at least a two-point improvement on the Erythema Assessment Scale. Investigators observed responses over 12 hours on the same day. “It’s actually kind of fun to do these studies,” she added. “You get to see what happens with patients across a whole day.”
A long-term analysis showed the efficacy of oxymetazoline “actually increased over the course of 52 weeks,” Dr. Stein Gold said. A total of 43% of participants experienced a two-grade improvement in erythema during this time. The agent was generally well tolerated, with dermatitis, pruritus, and headaches the most common treatment-related adverse events reported. (In January, the Food and Drug Administration approved oxymetazoline cream for the treatment of “persistent facial erythema associated with rosacea in adults.”)
Sometimes, a new formulation can make a difference in terms of treatment tolerability, a major consideration for patients with rosacea, Dr. Stein Gold said. Recent evidence suggests azelaic acid foam, 15% (Finacea), approved by the FDA in 2015, provides a well-tolerated option with only 6.2% of patients experiencing any application site pain, compared with 1.5% on vehicle alone, she added.
Cardiovascular comorbidities
“We’ve heard a lot about psoriasis and cardiovascular comorbidities, and we worry that other skin diseases may have similar associations,” Dr. Stein Gold said. New revelations in the pathogenesis of rosacea suggest a comparable association, she added, including findings related to matrix metalloproteinases (MMPs). MMPs have a key role in rosacea, for example, and are also important in the pathogenesis of cardiovascular disease, she noted. Several studies have confirmed this association as well as other links, including to Parkinson’s disease.
Although these studies support associations, more evidence is needed to prove any causal relationship between rosacea and other conditions where inflammation plays a prominent role, she added.
Translating findings into action
Given this emerging evidence, “what are we going to do about it?” Dr. Stein Gold asked attendees at the meeting. Research suggests tetracycline might be protective, she said, because this antibiotic can inhibit MMP activity. In a retrospective cohort study, investigators discovered rosacea patients on tetracycline therapy were at lower risk for developing vascular disease (J Invest Dermatol. 2014 Aug;134[8]:2267-9).
Nature or nurture?
Researchers and clinicians frequently debate the precise etiology of rosacea and whether the underlying causes are primarily genetic versus environmental. Investigators conducted a twin cohort study to find a more concrete answer, specifically looking at identical and fraternal twin pairs to determine how much genetics or environment likely contributes to factors on the National Rosacea Society grading system (JAMA Dermatol. 2015 Nov;151[11]:1213-9).
“The bottom line is it’s really about half and half – about half were associated with genetics, the other half with environment,” Dr. Stein Gold said.
No matter what the etiology, it’s important to diagnose and treat rosacea, Dr. Stein Gold said. Although patients tend to be middle-aged white women, the condition is not limited to this patient population, and “you have to think about it to diagnose it in skin of color,” she added.
Rosacea, which has a high emotional impact, presents an opportunity for dermatologists to improve quality of life, Dr. Stein Gold said. “When people walk around with papules and pustules, [other] people think there is something wrong with them.”
Dr. Stein Gold disclosed that she is a consultant, member of the advisory boards and speaker’s bureaus for, and receives research grants from Galderma, Leo, Novan, Valeant, Novartis, Celgene and Allergan. She is also a consultant, advisory board member, and receives research grants from Dermira and Foamix. She is a consultant to Sol-Gel, Promis, Anacor, and Medimetriks. She is on the advisory board for Promis.
MIAMI – Management of rosacea continues to challenge dermatologists and patients alike, although new advances and recent studies shine a light on promising new therapies to target this inflammatory skin condition.
Linda Stein Gold, MD, who directs dermatology clinical trials at the Henry Ford Hospital in Detroit, shared new information about the pathophysiology of rosacea and the controversial associations with cardiovascular disease and addressed the rosacea “genes versus environment” etiology question at the Orlando Dermatology Aesthetic and Clinical Conference.
The topical vasoconstrictor of cutaneous vasculature, oxymetazoline hydrochloride cream 1%, showed a statistically significant improvement in erythema, compared with vehicle only in people with rosacea in a phase III study, Dr. Stein Gold said. The outcome was strict, requiring both physician and patient assessment of at least a two-point improvement on the Erythema Assessment Scale. Investigators observed responses over 12 hours on the same day. “It’s actually kind of fun to do these studies,” she added. “You get to see what happens with patients across a whole day.”
A long-term analysis showed the efficacy of oxymetazoline “actually increased over the course of 52 weeks,” Dr. Stein Gold said. A total of 43% of participants experienced a two-grade improvement in erythema during this time. The agent was generally well tolerated, with dermatitis, pruritus, and headaches the most common treatment-related adverse events reported. (In January, the Food and Drug Administration approved oxymetazoline cream for the treatment of “persistent facial erythema associated with rosacea in adults.”)
Sometimes, a new formulation can make a difference in terms of treatment tolerability, a major consideration for patients with rosacea, Dr. Stein Gold said. Recent evidence suggests azelaic acid foam, 15% (Finacea), approved by the FDA in 2015, provides a well-tolerated option with only 6.2% of patients experiencing any application site pain, compared with 1.5% on vehicle alone, she added.
Cardiovascular comorbidities
“We’ve heard a lot about psoriasis and cardiovascular comorbidities, and we worry that other skin diseases may have similar associations,” Dr. Stein Gold said. New revelations in the pathogenesis of rosacea suggest a comparable association, she added, including findings related to matrix metalloproteinases (MMPs). MMPs have a key role in rosacea, for example, and are also important in the pathogenesis of cardiovascular disease, she noted. Several studies have confirmed this association as well as other links, including to Parkinson’s disease.
Although these studies support associations, more evidence is needed to prove any causal relationship between rosacea and other conditions where inflammation plays a prominent role, she added.
Translating findings into action
Given this emerging evidence, “what are we going to do about it?” Dr. Stein Gold asked attendees at the meeting. Research suggests tetracycline might be protective, she said, because this antibiotic can inhibit MMP activity. In a retrospective cohort study, investigators discovered rosacea patients on tetracycline therapy were at lower risk for developing vascular disease (J Invest Dermatol. 2014 Aug;134[8]:2267-9).
Nature or nurture?
Researchers and clinicians frequently debate the precise etiology of rosacea and whether the underlying causes are primarily genetic versus environmental. Investigators conducted a twin cohort study to find a more concrete answer, specifically looking at identical and fraternal twin pairs to determine how much genetics or environment likely contributes to factors on the National Rosacea Society grading system (JAMA Dermatol. 2015 Nov;151[11]:1213-9).
“The bottom line is it’s really about half and half – about half were associated with genetics, the other half with environment,” Dr. Stein Gold said.
No matter what the etiology, it’s important to diagnose and treat rosacea, Dr. Stein Gold said. Although patients tend to be middle-aged white women, the condition is not limited to this patient population, and “you have to think about it to diagnose it in skin of color,” she added.
Rosacea, which has a high emotional impact, presents an opportunity for dermatologists to improve quality of life, Dr. Stein Gold said. “When people walk around with papules and pustules, [other] people think there is something wrong with them.”
Dr. Stein Gold disclosed that she is a consultant, member of the advisory boards and speaker’s bureaus for, and receives research grants from Galderma, Leo, Novan, Valeant, Novartis, Celgene and Allergan. She is also a consultant, advisory board member, and receives research grants from Dermira and Foamix. She is a consultant to Sol-Gel, Promis, Anacor, and Medimetriks. She is on the advisory board for Promis.
MIAMI – Management of rosacea continues to challenge dermatologists and patients alike, although new advances and recent studies shine a light on promising new therapies to target this inflammatory skin condition.
Linda Stein Gold, MD, who directs dermatology clinical trials at the Henry Ford Hospital in Detroit, shared new information about the pathophysiology of rosacea and the controversial associations with cardiovascular disease and addressed the rosacea “genes versus environment” etiology question at the Orlando Dermatology Aesthetic and Clinical Conference.
The topical vasoconstrictor of cutaneous vasculature, oxymetazoline hydrochloride cream 1%, showed a statistically significant improvement in erythema, compared with vehicle only in people with rosacea in a phase III study, Dr. Stein Gold said. The outcome was strict, requiring both physician and patient assessment of at least a two-point improvement on the Erythema Assessment Scale. Investigators observed responses over 12 hours on the same day. “It’s actually kind of fun to do these studies,” she added. “You get to see what happens with patients across a whole day.”
A long-term analysis showed the efficacy of oxymetazoline “actually increased over the course of 52 weeks,” Dr. Stein Gold said. A total of 43% of participants experienced a two-grade improvement in erythema during this time. The agent was generally well tolerated, with dermatitis, pruritus, and headaches the most common treatment-related adverse events reported. (In January, the Food and Drug Administration approved oxymetazoline cream for the treatment of “persistent facial erythema associated with rosacea in adults.”)
Sometimes, a new formulation can make a difference in terms of treatment tolerability, a major consideration for patients with rosacea, Dr. Stein Gold said. Recent evidence suggests azelaic acid foam, 15% (Finacea), approved by the FDA in 2015, provides a well-tolerated option with only 6.2% of patients experiencing any application site pain, compared with 1.5% on vehicle alone, she added.
Cardiovascular comorbidities
“We’ve heard a lot about psoriasis and cardiovascular comorbidities, and we worry that other skin diseases may have similar associations,” Dr. Stein Gold said. New revelations in the pathogenesis of rosacea suggest a comparable association, she added, including findings related to matrix metalloproteinases (MMPs). MMPs have a key role in rosacea, for example, and are also important in the pathogenesis of cardiovascular disease, she noted. Several studies have confirmed this association as well as other links, including to Parkinson’s disease.
Although these studies support associations, more evidence is needed to prove any causal relationship between rosacea and other conditions where inflammation plays a prominent role, she added.
Translating findings into action
Given this emerging evidence, “what are we going to do about it?” Dr. Stein Gold asked attendees at the meeting. Research suggests tetracycline might be protective, she said, because this antibiotic can inhibit MMP activity. In a retrospective cohort study, investigators discovered rosacea patients on tetracycline therapy were at lower risk for developing vascular disease (J Invest Dermatol. 2014 Aug;134[8]:2267-9).
Nature or nurture?
Researchers and clinicians frequently debate the precise etiology of rosacea and whether the underlying causes are primarily genetic versus environmental. Investigators conducted a twin cohort study to find a more concrete answer, specifically looking at identical and fraternal twin pairs to determine how much genetics or environment likely contributes to factors on the National Rosacea Society grading system (JAMA Dermatol. 2015 Nov;151[11]:1213-9).
“The bottom line is it’s really about half and half – about half were associated with genetics, the other half with environment,” Dr. Stein Gold said.
No matter what the etiology, it’s important to diagnose and treat rosacea, Dr. Stein Gold said. Although patients tend to be middle-aged white women, the condition is not limited to this patient population, and “you have to think about it to diagnose it in skin of color,” she added.
Rosacea, which has a high emotional impact, presents an opportunity for dermatologists to improve quality of life, Dr. Stein Gold said. “When people walk around with papules and pustules, [other] people think there is something wrong with them.”
Dr. Stein Gold disclosed that she is a consultant, member of the advisory boards and speaker’s bureaus for, and receives research grants from Galderma, Leo, Novan, Valeant, Novartis, Celgene and Allergan. She is also a consultant, advisory board member, and receives research grants from Dermira and Foamix. She is a consultant to Sol-Gel, Promis, Anacor, and Medimetriks. She is on the advisory board for Promis.
EXPERT ANALYSIS FROM ODAC 2017
Cost of Diagnosing Psoriasis and Rosacea for Dermatologists Versus Primary Care Physicians
Growing incentives to control health care costs may cause accountable care organizations (ACOs) to reconsider how diseases are best managed. Few studies have examined the cost difference between primary care providers (PCPs) and specialists in managing the same disease. Limited data have suggested that management of some diseases by a PCP may be less costly compared to a specialist1,2; however, it is not clear if this finding extends to skin disease. This study sought to assess the cost of seeing a dermatologist versus a PCP for diagnosis of the common skin diseases psoriasis and rosacea.
Methods
Patient data were obtained from the Humana database, a large commercial data set for claims and reimbursed costs encompassing 18,162,539 patients covered between January 2007 and December 2014. Our study population consisted of 3,944,465 patients with claims that included International Classification of Diseases, Ninth Revision (ICD-9), codes for dermatological diagnoses (680.0–709.9). We searched by ICD-9 code for US patients with primary diagnoses of psoriasis (696.1) and rosacea (695.3). We narrowed the search to include patients aged 30 to 64 years, as the diagnoses for these diseases are most common in patients older than 30 years. Patients who were older than 64 years were not included in the study, as most are covered by Medicare and therefore costs covered by Humana in this age group would not be as representative as in younger age groups. Total and average diagnosis-related costs per patient were compared between dermatologists and PCPs. Diagnosis-related costs encompassed physician reimbursement; laboratory and imaging costs, including skin biopsies; inpatient hospitalization cost; and any other charge that could be coded or billed by providers and reimbursed by the insurance company. To be eligible for reimbursement from Humana, dermatologists and PCPs must be registered with the insurer according to specialty board certification and practice credentialing, and they are reimbursed differently based on specialty. Drug costs, which would possibly skew the data toward providers using more expensive systemic medications (ie, dermatologists), were not included in this study, as the discussion is better reserved for long-term management of disease rather than diagnosis-related costs. All diagnoses of psoriasis were included in the study, which likely includes all severities of psoriasis, though we did not have the ability to further break down these diagnoses by severity.
Results
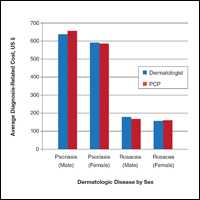
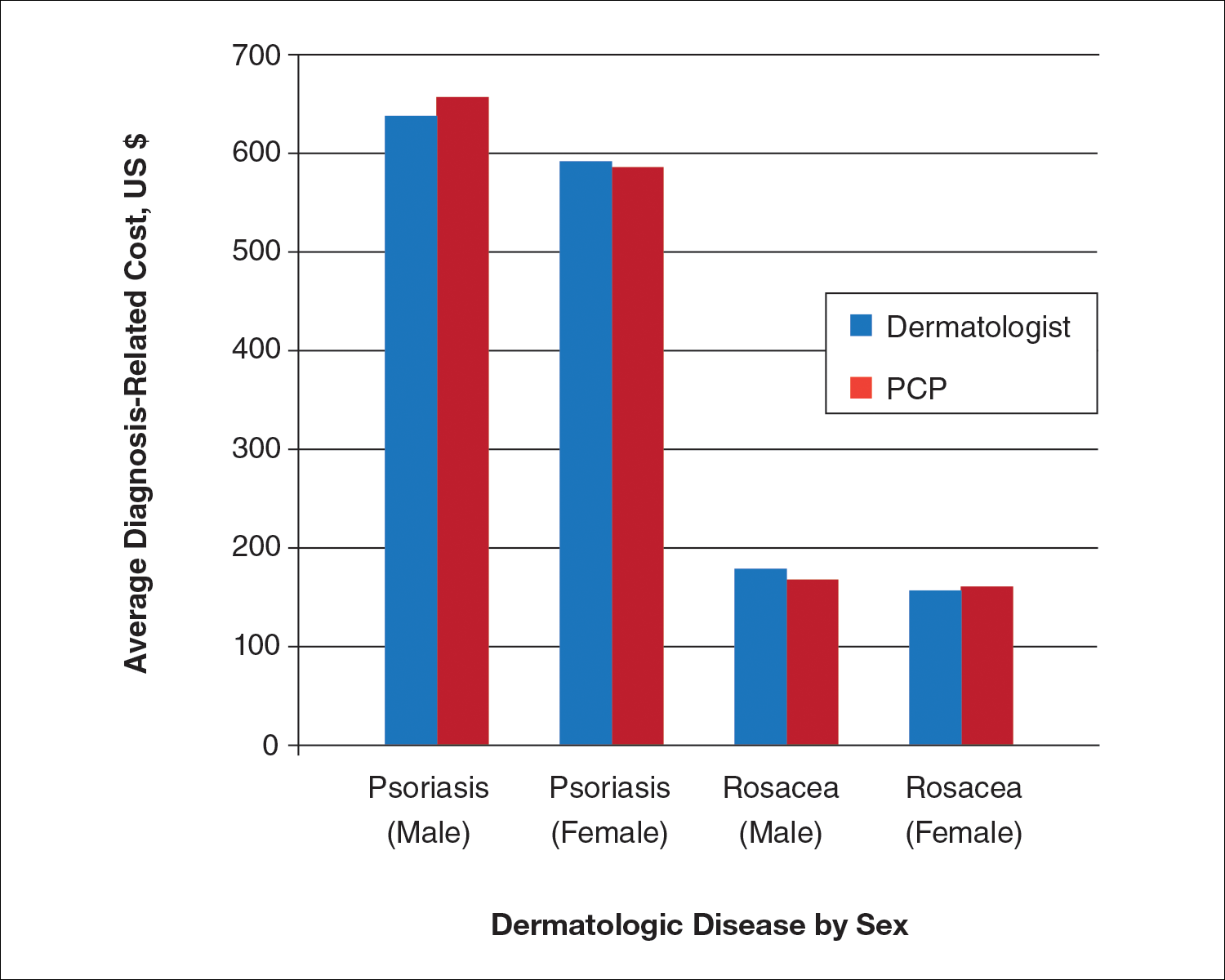
We identified 30,217 psoriasis patients and 37,561 rosacea patients. Of those patients with a primary diagnosis of psoriasis, 26,112 (86%) were seen by a dermatologist and 4105 (14%) were seen by a PCP (Table). Of those patients with a primary diagnosis of rosacea, 34,694 (92%) were seen by a dermatologist and 2867 (8%) were seen by a PCP (Table). There was little difference in the average diagnosis-related cost per patient for psoriasis in males (dermatologists, $638; PCPs, $657) versus females (dermatologists, $592; PCPs, $586) or between specialties (Figure). Findings were similar for rosacea in males (dermatologists, $179; PCPs, $168) versus females (dermatologists, $157; PCPs, $161). For these skin diseases, i

Comment
For the management of common skin disorders such as psoriasis and rosacea, there is little cost difference in seeing a dermatologist versus a PCP. Through extensive training and repeated exposure to many skin diseases, dermatologists are expected to be more comfortable in diagnosing and managing psoriasis and rosacea. Compared to PCPs, dermatologists have demonstrated increased diagnostic accuracy and efficiency when examining pigmented lesions and other dermatologic diseases in several studies.3-6 Although the current study shows that diagnosis-related costs for psoriasis and rosacea are essentially equal between dermatologists and PCPs, it actually may be less expensive for patients to see a dermatologist, as unnecessary tests, biopsies, or medications are more likely to be ordered/prescribed when there is less clinical diagnostic certainty.7,8 Additionally, seeing a PCP for diagnosis of a skin disease may be inefficient if subsequent referral to a dermatologist is needed, a common scenario that occurs when patients see a PCP for skin conditions.9
Our study had limitations, which is typical of a study using a claims database. We used ICD-9 codes recorded in patients’ medical claims to determine diagnosis of psoriasis and rosacea; therefore, our study and data are subject to coding errors. We could not assess the severity of disease, only the presence of disease. Further confirmation of diagnosis could have been made through searching for a second ICD-9 code in the patient’s history. Our data also are from a limited time period and may not represent costs from other time periods.
Conclusion
Given the lack of cost difference between both specialties, we conclude that ACOs should consider encouraging patients to seek care for dermatologic diseases by dermatologists who generally are more accurate and efficient skin diagnosticians, particularly if there is a shortage of PCPs within the ACO network.
- Wimo A, Religa D, Spångberg K, et al. Costs of diagnosing dementia: results from SveDem, the Swedish Dementia Registry. Int J Geriatr Psychiatry. 2013;28:1039-1044.
- Grunfeld E, Fitzpatrick R, Mant D, et al. Comparison of breast cancer patient satisfaction with follow-up in primary care versus specialist care: results from a randomized controlled trial. Br J Gen Pract. 1999;49:705-710.
- Chen SC, Pennie ML, Kolm P, et al. Diagnosing and managing cutaneous pigmented lesions: primary care physicians versus dermatologists. J Gen Intern Med. 2006;21:678-682.
- Federman D, Hogan D, Taylor JR, et al. A comparison of diagnosis, evaluation, and treatment of patients with dermatologic disorders. J Am Acad Dermatol. 1995;32:726-729.
- Feldman SR, Fleischer AB, Young AC, et al. Time-efficiency of nondermatologists compared with dermatologists in the care of skin disease. J Am Acad Dermatol. 1999;40:194-199.
- Feldman SR, Peterson SR, Fleischer AB Jr. Dermatologists meet the primary care standard for first contact management of skin disease. J Am Acad Dermatol. 1998;39(2, pt 1):182-186.
- Smith ES, Fleischer AB, Feldman SR. Nondermatologists are more likely than dermatologists to prescribe antifungal/corticosteroid products: an analysis of office visits for cutaneous fungal infections, 1990-1994. J Am Acad Dermatol. 1998;39:43-47.
- Shaffer MP, Feldman SR, Fleischer AB. Use of clotrimazole/betamethasone diproprionate by family physicians. Fam Med. 2000;32:561-565.
- Feldman SR, Fleischer AB, Chen JG. The gatekeeper model is inefficient for the delivery of dermatologic services. J Am Acad Dermatol. 1999;40:426-432.
Growing incentives to control health care costs may cause accountable care organizations (ACOs) to reconsider how diseases are best managed. Few studies have examined the cost difference between primary care providers (PCPs) and specialists in managing the same disease. Limited data have suggested that management of some diseases by a PCP may be less costly compared to a specialist1,2; however, it is not clear if this finding extends to skin disease. This study sought to assess the cost of seeing a dermatologist versus a PCP for diagnosis of the common skin diseases psoriasis and rosacea.
Methods
Patient data were obtained from the Humana database, a large commercial data set for claims and reimbursed costs encompassing 18,162,539 patients covered between January 2007 and December 2014. Our study population consisted of 3,944,465 patients with claims that included International Classification of Diseases, Ninth Revision (ICD-9), codes for dermatological diagnoses (680.0–709.9). We searched by ICD-9 code for US patients with primary diagnoses of psoriasis (696.1) and rosacea (695.3). We narrowed the search to include patients aged 30 to 64 years, as the diagnoses for these diseases are most common in patients older than 30 years. Patients who were older than 64 years were not included in the study, as most are covered by Medicare and therefore costs covered by Humana in this age group would not be as representative as in younger age groups. Total and average diagnosis-related costs per patient were compared between dermatologists and PCPs. Diagnosis-related costs encompassed physician reimbursement; laboratory and imaging costs, including skin biopsies; inpatient hospitalization cost; and any other charge that could be coded or billed by providers and reimbursed by the insurance company. To be eligible for reimbursement from Humana, dermatologists and PCPs must be registered with the insurer according to specialty board certification and practice credentialing, and they are reimbursed differently based on specialty. Drug costs, which would possibly skew the data toward providers using more expensive systemic medications (ie, dermatologists), were not included in this study, as the discussion is better reserved for long-term management of disease rather than diagnosis-related costs. All diagnoses of psoriasis were included in the study, which likely includes all severities of psoriasis, though we did not have the ability to further break down these diagnoses by severity.
Results
We identified 30,217 psoriasis patients and 37,561 rosacea patients. Of those patients with a primary diagnosis of psoriasis, 26,112 (86%) were seen by a dermatologist and 4105 (14%) were seen by a PCP (Table). Of those patients with a primary diagnosis of rosacea, 34,694 (92%) were seen by a dermatologist and 2867 (8%) were seen by a PCP (Table). There was little difference in the average diagnosis-related cost per patient for psoriasis in males (dermatologists, $638; PCPs, $657) versus females (dermatologists, $592; PCPs, $586) or between specialties (Figure). Findings were similar for rosacea in males (dermatologists, $179; PCPs, $168) versus females (dermatologists, $157; PCPs, $161). For these skin diseases, i


Comment
For the management of common skin disorders such as psoriasis and rosacea, there is little cost difference in seeing a dermatologist versus a PCP. Through extensive training and repeated exposure to many skin diseases, dermatologists are expected to be more comfortable in diagnosing and managing psoriasis and rosacea. Compared to PCPs, dermatologists have demonstrated increased diagnostic accuracy and efficiency when examining pigmented lesions and other dermatologic diseases in several studies.3-6 Although the current study shows that diagnosis-related costs for psoriasis and rosacea are essentially equal between dermatologists and PCPs, it actually may be less expensive for patients to see a dermatologist, as unnecessary tests, biopsies, or medications are more likely to be ordered/prescribed when there is less clinical diagnostic certainty.7,8 Additionally, seeing a PCP for diagnosis of a skin disease may be inefficient if subsequent referral to a dermatologist is needed, a common scenario that occurs when patients see a PCP for skin conditions.9
Our study had limitations, which is typical of a study using a claims database. We used ICD-9 codes recorded in patients’ medical claims to determine diagnosis of psoriasis and rosacea; therefore, our study and data are subject to coding errors. We could not assess the severity of disease, only the presence of disease. Further confirmation of diagnosis could have been made through searching for a second ICD-9 code in the patient’s history. Our data also are from a limited time period and may not represent costs from other time periods.
Conclusion
Given the lack of cost difference between both specialties, we conclude that ACOs should consider encouraging patients to seek care for dermatologic diseases by dermatologists who generally are more accurate and efficient skin diagnosticians, particularly if there is a shortage of PCPs within the ACO network.
Growing incentives to control health care costs may cause accountable care organizations (ACOs) to reconsider how diseases are best managed. Few studies have examined the cost difference between primary care providers (PCPs) and specialists in managing the same disease. Limited data have suggested that management of some diseases by a PCP may be less costly compared to a specialist1,2; however, it is not clear if this finding extends to skin disease. This study sought to assess the cost of seeing a dermatologist versus a PCP for diagnosis of the common skin diseases psoriasis and rosacea.
Methods
Patient data were obtained from the Humana database, a large commercial data set for claims and reimbursed costs encompassing 18,162,539 patients covered between January 2007 and December 2014. Our study population consisted of 3,944,465 patients with claims that included International Classification of Diseases, Ninth Revision (ICD-9), codes for dermatological diagnoses (680.0–709.9). We searched by ICD-9 code for US patients with primary diagnoses of psoriasis (696.1) and rosacea (695.3). We narrowed the search to include patients aged 30 to 64 years, as the diagnoses for these diseases are most common in patients older than 30 years. Patients who were older than 64 years were not included in the study, as most are covered by Medicare and therefore costs covered by Humana in this age group would not be as representative as in younger age groups. Total and average diagnosis-related costs per patient were compared between dermatologists and PCPs. Diagnosis-related costs encompassed physician reimbursement; laboratory and imaging costs, including skin biopsies; inpatient hospitalization cost; and any other charge that could be coded or billed by providers and reimbursed by the insurance company. To be eligible for reimbursement from Humana, dermatologists and PCPs must be registered with the insurer according to specialty board certification and practice credentialing, and they are reimbursed differently based on specialty. Drug costs, which would possibly skew the data toward providers using more expensive systemic medications (ie, dermatologists), were not included in this study, as the discussion is better reserved for long-term management of disease rather than diagnosis-related costs. All diagnoses of psoriasis were included in the study, which likely includes all severities of psoriasis, though we did not have the ability to further break down these diagnoses by severity.
Results
We identified 30,217 psoriasis patients and 37,561 rosacea patients. Of those patients with a primary diagnosis of psoriasis, 26,112 (86%) were seen by a dermatologist and 4105 (14%) were seen by a PCP (Table). Of those patients with a primary diagnosis of rosacea, 34,694 (92%) were seen by a dermatologist and 2867 (8%) were seen by a PCP (Table). There was little difference in the average diagnosis-related cost per patient for psoriasis in males (dermatologists, $638; PCPs, $657) versus females (dermatologists, $592; PCPs, $586) or between specialties (Figure). Findings were similar for rosacea in males (dermatologists, $179; PCPs, $168) versus females (dermatologists, $157; PCPs, $161). For these skin diseases, i


Comment
For the management of common skin disorders such as psoriasis and rosacea, there is little cost difference in seeing a dermatologist versus a PCP. Through extensive training and repeated exposure to many skin diseases, dermatologists are expected to be more comfortable in diagnosing and managing psoriasis and rosacea. Compared to PCPs, dermatologists have demonstrated increased diagnostic accuracy and efficiency when examining pigmented lesions and other dermatologic diseases in several studies.3-6 Although the current study shows that diagnosis-related costs for psoriasis and rosacea are essentially equal between dermatologists and PCPs, it actually may be less expensive for patients to see a dermatologist, as unnecessary tests, biopsies, or medications are more likely to be ordered/prescribed when there is less clinical diagnostic certainty.7,8 Additionally, seeing a PCP for diagnosis of a skin disease may be inefficient if subsequent referral to a dermatologist is needed, a common scenario that occurs when patients see a PCP for skin conditions.9
Our study had limitations, which is typical of a study using a claims database. We used ICD-9 codes recorded in patients’ medical claims to determine diagnosis of psoriasis and rosacea; therefore, our study and data are subject to coding errors. We could not assess the severity of disease, only the presence of disease. Further confirmation of diagnosis could have been made through searching for a second ICD-9 code in the patient’s history. Our data also are from a limited time period and may not represent costs from other time periods.
Conclusion
Given the lack of cost difference between both specialties, we conclude that ACOs should consider encouraging patients to seek care for dermatologic diseases by dermatologists who generally are more accurate and efficient skin diagnosticians, particularly if there is a shortage of PCPs within the ACO network.
- Wimo A, Religa D, Spångberg K, et al. Costs of diagnosing dementia: results from SveDem, the Swedish Dementia Registry. Int J Geriatr Psychiatry. 2013;28:1039-1044.
- Grunfeld E, Fitzpatrick R, Mant D, et al. Comparison of breast cancer patient satisfaction with follow-up in primary care versus specialist care: results from a randomized controlled trial. Br J Gen Pract. 1999;49:705-710.
- Chen SC, Pennie ML, Kolm P, et al. Diagnosing and managing cutaneous pigmented lesions: primary care physicians versus dermatologists. J Gen Intern Med. 2006;21:678-682.
- Federman D, Hogan D, Taylor JR, et al. A comparison of diagnosis, evaluation, and treatment of patients with dermatologic disorders. J Am Acad Dermatol. 1995;32:726-729.
- Feldman SR, Fleischer AB, Young AC, et al. Time-efficiency of nondermatologists compared with dermatologists in the care of skin disease. J Am Acad Dermatol. 1999;40:194-199.
- Feldman SR, Peterson SR, Fleischer AB Jr. Dermatologists meet the primary care standard for first contact management of skin disease. J Am Acad Dermatol. 1998;39(2, pt 1):182-186.
- Smith ES, Fleischer AB, Feldman SR. Nondermatologists are more likely than dermatologists to prescribe antifungal/corticosteroid products: an analysis of office visits for cutaneous fungal infections, 1990-1994. J Am Acad Dermatol. 1998;39:43-47.
- Shaffer MP, Feldman SR, Fleischer AB. Use of clotrimazole/betamethasone diproprionate by family physicians. Fam Med. 2000;32:561-565.
- Feldman SR, Fleischer AB, Chen JG. The gatekeeper model is inefficient for the delivery of dermatologic services. J Am Acad Dermatol. 1999;40:426-432.
- Wimo A, Religa D, Spångberg K, et al. Costs of diagnosing dementia: results from SveDem, the Swedish Dementia Registry. Int J Geriatr Psychiatry. 2013;28:1039-1044.
- Grunfeld E, Fitzpatrick R, Mant D, et al. Comparison of breast cancer patient satisfaction with follow-up in primary care versus specialist care: results from a randomized controlled trial. Br J Gen Pract. 1999;49:705-710.
- Chen SC, Pennie ML, Kolm P, et al. Diagnosing and managing cutaneous pigmented lesions: primary care physicians versus dermatologists. J Gen Intern Med. 2006;21:678-682.
- Federman D, Hogan D, Taylor JR, et al. A comparison of diagnosis, evaluation, and treatment of patients with dermatologic disorders. J Am Acad Dermatol. 1995;32:726-729.
- Feldman SR, Fleischer AB, Young AC, et al. Time-efficiency of nondermatologists compared with dermatologists in the care of skin disease. J Am Acad Dermatol. 1999;40:194-199.
- Feldman SR, Peterson SR, Fleischer AB Jr. Dermatologists meet the primary care standard for first contact management of skin disease. J Am Acad Dermatol. 1998;39(2, pt 1):182-186.
- Smith ES, Fleischer AB, Feldman SR. Nondermatologists are more likely than dermatologists to prescribe antifungal/corticosteroid products: an analysis of office visits for cutaneous fungal infections, 1990-1994. J Am Acad Dermatol. 1998;39:43-47.
- Shaffer MP, Feldman SR, Fleischer AB. Use of clotrimazole/betamethasone diproprionate by family physicians. Fam Med. 2000;32:561-565.
- Feldman SR, Fleischer AB, Chen JG. The gatekeeper model is inefficient for the delivery of dermatologic services. J Am Acad Dermatol. 1999;40:426-432.
Practice Points
- Growing health care costs are causing accountable care organizations (ACOs) to reconsider how to best manage skin disease.
- There is little difference in average diagnosis-related cost between primary care physicians and dermatologists in diagnosing psoriasis or rosacea.
- With diagnosis costs essentially equal and increased dermatologist diagnostic accuracy, ACOs may encourage skin disease to be managed by dermatologists.
VIDEO: Oxymetazoline approval expands options for rosacea
WAILEA, Hawaii – The Food and Drug Administration approval of oxymetazoline 1% cream for the background erythema of rosacea is big news for dermatologists and patients, according to Linda Stein Gold, MD, director of dermatology research, Henry Ford Health System, Detroit.
In studies, patients showed a two grade improvement in baseline erythema, and erythema reduction that lasted for 9-12 hours in many patients, said Dr. Stein Gold in a video interview, who was involved in the clinical trials.
“We have safety data that lasts up to an entire year, with no new safety signals,” and the incidence of exacerbation of erythema was rare, she added in a video interview at the meeting provided by Global Academy for Medical Education/Skin Disease Education Foundation. Oxymetazoline 1% cream, which will be marketed as Rhofade by Allergan, was approved in January 2017 for the “topical treatment of persistent facial erythema associated with rosacea in adults.”
Dr. Stein Gold disclosed relationships with several companies, including Galderma, Leo, Novan, Valeant, Dermira, Novartis, Celgene, Allergan, Foamix, Anacor, Medimetriks, Sol-Gel, and Promius.
SDEF and this news organization are owned by the same parent organization.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WAILEA, Hawaii – The Food and Drug Administration approval of oxymetazoline 1% cream for the background erythema of rosacea is big news for dermatologists and patients, according to Linda Stein Gold, MD, director of dermatology research, Henry Ford Health System, Detroit.
In studies, patients showed a two grade improvement in baseline erythema, and erythema reduction that lasted for 9-12 hours in many patients, said Dr. Stein Gold in a video interview, who was involved in the clinical trials.
“We have safety data that lasts up to an entire year, with no new safety signals,” and the incidence of exacerbation of erythema was rare, she added in a video interview at the meeting provided by Global Academy for Medical Education/Skin Disease Education Foundation. Oxymetazoline 1% cream, which will be marketed as Rhofade by Allergan, was approved in January 2017 for the “topical treatment of persistent facial erythema associated with rosacea in adults.”
Dr. Stein Gold disclosed relationships with several companies, including Galderma, Leo, Novan, Valeant, Dermira, Novartis, Celgene, Allergan, Foamix, Anacor, Medimetriks, Sol-Gel, and Promius.
SDEF and this news organization are owned by the same parent organization.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WAILEA, Hawaii – The Food and Drug Administration approval of oxymetazoline 1% cream for the background erythema of rosacea is big news for dermatologists and patients, according to Linda Stein Gold, MD, director of dermatology research, Henry Ford Health System, Detroit.
In studies, patients showed a two grade improvement in baseline erythema, and erythema reduction that lasted for 9-12 hours in many patients, said Dr. Stein Gold in a video interview, who was involved in the clinical trials.
“We have safety data that lasts up to an entire year, with no new safety signals,” and the incidence of exacerbation of erythema was rare, she added in a video interview at the meeting provided by Global Academy for Medical Education/Skin Disease Education Foundation. Oxymetazoline 1% cream, which will be marketed as Rhofade by Allergan, was approved in January 2017 for the “topical treatment of persistent facial erythema associated with rosacea in adults.”
Dr. Stein Gold disclosed relationships with several companies, including Galderma, Leo, Novan, Valeant, Dermira, Novartis, Celgene, Allergan, Foamix, Anacor, Medimetriks, Sol-Gel, and Promius.
SDEF and this news organization are owned by the same parent organization.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT SDEF HAWAII DERMATOLOGY SEMINAR
VIDEO: Spironolactone holds its own for treating women’s acne
WAILEA, Hawaii – While spironolactone is an old drug, it remains a safe and effective treatment option for acne in women, according to Julie Harper, MD, of the University of Alabama, Birmingham.
In a video interview, Dr. Harper said that she usually does not choose spironolactone as a first-line drug, “but more often than not this is a drug that is an add-on to other therapies” that have been tried. She tends to start with a low dose and titrates up based on side effects. The drug has been tested in men, but Dr. Harper cited a Japanese study that discontinued a male treatment arm because men developed gynecomastia.
In her opinion, it isn’t always necessary to routinely check potassium levels in patients on spironolactone. “In the vast majority of patients, I do not check labs routinely” before starting spironolactone, she said at the meeting, provided by Global Academy for Medical Education/Skin Disease Education Foundation. But she noted that some physicians feel more comfortable checking baseline labs.
Dr. Harper disclosed financial relationships with Allergan, Galderma, BioPharmX, La Roche-Posay, Promius, Valeant, and Bayer.
SDEF and this news organization are owned by the same parent organization.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WAILEA, Hawaii – While spironolactone is an old drug, it remains a safe and effective treatment option for acne in women, according to Julie Harper, MD, of the University of Alabama, Birmingham.
In a video interview, Dr. Harper said that she usually does not choose spironolactone as a first-line drug, “but more often than not this is a drug that is an add-on to other therapies” that have been tried. She tends to start with a low dose and titrates up based on side effects. The drug has been tested in men, but Dr. Harper cited a Japanese study that discontinued a male treatment arm because men developed gynecomastia.
In her opinion, it isn’t always necessary to routinely check potassium levels in patients on spironolactone. “In the vast majority of patients, I do not check labs routinely” before starting spironolactone, she said at the meeting, provided by Global Academy for Medical Education/Skin Disease Education Foundation. But she noted that some physicians feel more comfortable checking baseline labs.
Dr. Harper disclosed financial relationships with Allergan, Galderma, BioPharmX, La Roche-Posay, Promius, Valeant, and Bayer.
SDEF and this news organization are owned by the same parent organization.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WAILEA, Hawaii – While spironolactone is an old drug, it remains a safe and effective treatment option for acne in women, according to Julie Harper, MD, of the University of Alabama, Birmingham.
In a video interview, Dr. Harper said that she usually does not choose spironolactone as a first-line drug, “but more often than not this is a drug that is an add-on to other therapies” that have been tried. She tends to start with a low dose and titrates up based on side effects. The drug has been tested in men, but Dr. Harper cited a Japanese study that discontinued a male treatment arm because men developed gynecomastia.
In her opinion, it isn’t always necessary to routinely check potassium levels in patients on spironolactone. “In the vast majority of patients, I do not check labs routinely” before starting spironolactone, she said at the meeting, provided by Global Academy for Medical Education/Skin Disease Education Foundation. But she noted that some physicians feel more comfortable checking baseline labs.
Dr. Harper disclosed financial relationships with Allergan, Galderma, BioPharmX, La Roche-Posay, Promius, Valeant, and Bayer.
SDEF and this news organization are owned by the same parent organization.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT SDEF HAWAII DERMATOLOGY SEMINAR
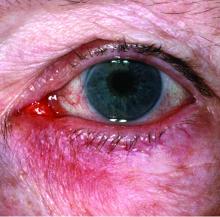
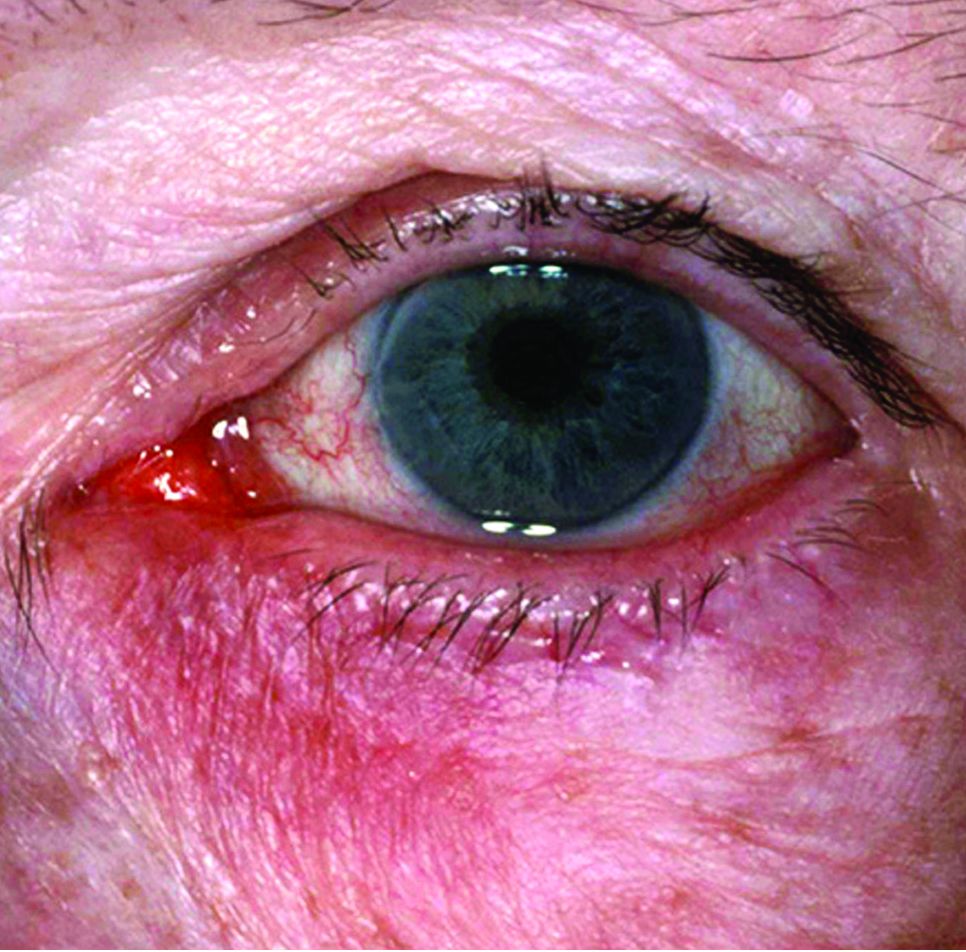
FDA approves topical oxymetazoline for rosacea
A topical cream containing the vasoconstrictor oxymetazoline has been approved by the Food and Drug Administration to treat symptoms of rosacea, its manufacturer announced.
Oxymetazoline hydrochloride cream 1%, which will be marketed as Rhofade by Allergan, is indicated for the treatment of “persistent facial erythema associated with rosacea in adults.” While nasal sprays containing a lower concentration of oxymetazoline HCl, an alpha1A-adrenoceptor agonist, have been used off label for a decade, this is the first time this ingredient has been harnessed to formulate an approved rosacea treatment.
Safety results from three pooled trials showed 2% of patients in the active treatment arms (489 people) had treatment-site dermatitis, and 1% had worsening of rosacea symptoms, pruritus, or pain. The vehicle cream groups (483 people) experienced similar rates of pruritus but negligible rates of other adverse effects, according to the prescribing information.
Brimonidine (Mirvaso) is another topical treatment approved by the FDA for treating rosacea, and its active ingredient is also an alpha-adrenergic agonist that works on the cutaneous microvasculature. However, there are differences in the two agents’ activity. Oxymetazoline acts on alpha1A receptors and brimonidine on alpha2 receptors. There have been reports of rebound erythema more severe than at baseline with brimonidine, and its manufacturer, Galderma, acknowledges the phenomenon in patient labeling.
When Allergan announced the FDA application for oxymetazoline in May 2016, it issued a press statement, describing oxymetazoline as a “sympathomimetic agonist that is selective for the alpha1A adrenoceptor or over other alpha1 adrenoceptors and nonselective for the alpha2 adrenoceptors.”In a 1-year open label trial of oxymetazoline (440 people), 3% of patients had worsening inflammatory lesions of rosacea, according to the prescribing information for oxymetazoline HCl 1%.
A topical cream containing the vasoconstrictor oxymetazoline has been approved by the Food and Drug Administration to treat symptoms of rosacea, its manufacturer announced.
Oxymetazoline hydrochloride cream 1%, which will be marketed as Rhofade by Allergan, is indicated for the treatment of “persistent facial erythema associated with rosacea in adults.” While nasal sprays containing a lower concentration of oxymetazoline HCl, an alpha1A-adrenoceptor agonist, have been used off label for a decade, this is the first time this ingredient has been harnessed to formulate an approved rosacea treatment.
Safety results from three pooled trials showed 2% of patients in the active treatment arms (489 people) had treatment-site dermatitis, and 1% had worsening of rosacea symptoms, pruritus, or pain. The vehicle cream groups (483 people) experienced similar rates of pruritus but negligible rates of other adverse effects, according to the prescribing information.
Brimonidine (Mirvaso) is another topical treatment approved by the FDA for treating rosacea, and its active ingredient is also an alpha-adrenergic agonist that works on the cutaneous microvasculature. However, there are differences in the two agents’ activity. Oxymetazoline acts on alpha1A receptors and brimonidine on alpha2 receptors. There have been reports of rebound erythema more severe than at baseline with brimonidine, and its manufacturer, Galderma, acknowledges the phenomenon in patient labeling.
When Allergan announced the FDA application for oxymetazoline in May 2016, it issued a press statement, describing oxymetazoline as a “sympathomimetic agonist that is selective for the alpha1A adrenoceptor or over other alpha1 adrenoceptors and nonselective for the alpha2 adrenoceptors.”In a 1-year open label trial of oxymetazoline (440 people), 3% of patients had worsening inflammatory lesions of rosacea, according to the prescribing information for oxymetazoline HCl 1%.
A topical cream containing the vasoconstrictor oxymetazoline has been approved by the Food and Drug Administration to treat symptoms of rosacea, its manufacturer announced.
Oxymetazoline hydrochloride cream 1%, which will be marketed as Rhofade by Allergan, is indicated for the treatment of “persistent facial erythema associated with rosacea in adults.” While nasal sprays containing a lower concentration of oxymetazoline HCl, an alpha1A-adrenoceptor agonist, have been used off label for a decade, this is the first time this ingredient has been harnessed to formulate an approved rosacea treatment.
Safety results from three pooled trials showed 2% of patients in the active treatment arms (489 people) had treatment-site dermatitis, and 1% had worsening of rosacea symptoms, pruritus, or pain. The vehicle cream groups (483 people) experienced similar rates of pruritus but negligible rates of other adverse effects, according to the prescribing information.
Brimonidine (Mirvaso) is another topical treatment approved by the FDA for treating rosacea, and its active ingredient is also an alpha-adrenergic agonist that works on the cutaneous microvasculature. However, there are differences in the two agents’ activity. Oxymetazoline acts on alpha1A receptors and brimonidine on alpha2 receptors. There have been reports of rebound erythema more severe than at baseline with brimonidine, and its manufacturer, Galderma, acknowledges the phenomenon in patient labeling.
When Allergan announced the FDA application for oxymetazoline in May 2016, it issued a press statement, describing oxymetazoline as a “sympathomimetic agonist that is selective for the alpha1A adrenoceptor or over other alpha1 adrenoceptors and nonselective for the alpha2 adrenoceptors.”In a 1-year open label trial of oxymetazoline (440 people), 3% of patients had worsening inflammatory lesions of rosacea, according to the prescribing information for oxymetazoline HCl 1%.
Topical treatments for rosacea to be reviewed at this year’s meeting
This year’s SDEF Hawaii Dermatology Seminar is set to showcase the latest research and expert tips, advice, and perspective on a range of topics.
Linda Stein Gold, MD, director of dermatology research, Henry Ford Health System, Detroit, and a codirector of the meeting, shared a taste of the topics she will be presenting.
In the field of rosacea, “topical ivermectin has proven to be a very effective and safe treatment,” she said in an interview. “On the horizon we will have a new treatment for the erythema of rosacea which seems very well tolerated. Also, topical minocycline foam appears to be very promising in phase II studies.”
See our video interview with Dr. Stein Gold from last year’s Hawaii Dermatology Seminar, where she discussed new approaches for the topical treatment of acne that are on the horizon.
The Hawaii Dermatology Seminar is provided by Global Academy for Medical Education/Skin Disease Education Foundation. SDEF and this news organization are owned by the same parent company. It is being held in Maui, Jan. 29-Feb. 3.
Dr. Stein Gold disclosed relationships with companies including Galderma, Leo, Novan, Valeant, Dermira, Novartis, Celgene, Allergan, Foamix, and Promius.
This year’s SDEF Hawaii Dermatology Seminar is set to showcase the latest research and expert tips, advice, and perspective on a range of topics.
Linda Stein Gold, MD, director of dermatology research, Henry Ford Health System, Detroit, and a codirector of the meeting, shared a taste of the topics she will be presenting.
In the field of rosacea, “topical ivermectin has proven to be a very effective and safe treatment,” she said in an interview. “On the horizon we will have a new treatment for the erythema of rosacea which seems very well tolerated. Also, topical minocycline foam appears to be very promising in phase II studies.”
See our video interview with Dr. Stein Gold from last year’s Hawaii Dermatology Seminar, where she discussed new approaches for the topical treatment of acne that are on the horizon.
The Hawaii Dermatology Seminar is provided by Global Academy for Medical Education/Skin Disease Education Foundation. SDEF and this news organization are owned by the same parent company. It is being held in Maui, Jan. 29-Feb. 3.
Dr. Stein Gold disclosed relationships with companies including Galderma, Leo, Novan, Valeant, Dermira, Novartis, Celgene, Allergan, Foamix, and Promius.
This year’s SDEF Hawaii Dermatology Seminar is set to showcase the latest research and expert tips, advice, and perspective on a range of topics.
Linda Stein Gold, MD, director of dermatology research, Henry Ford Health System, Detroit, and a codirector of the meeting, shared a taste of the topics she will be presenting.
In the field of rosacea, “topical ivermectin has proven to be a very effective and safe treatment,” she said in an interview. “On the horizon we will have a new treatment for the erythema of rosacea which seems very well tolerated. Also, topical minocycline foam appears to be very promising in phase II studies.”
See our video interview with Dr. Stein Gold from last year’s Hawaii Dermatology Seminar, where she discussed new approaches for the topical treatment of acne that are on the horizon.
The Hawaii Dermatology Seminar is provided by Global Academy for Medical Education/Skin Disease Education Foundation. SDEF and this news organization are owned by the same parent company. It is being held in Maui, Jan. 29-Feb. 3.
Dr. Stein Gold disclosed relationships with companies including Galderma, Leo, Novan, Valeant, Dermira, Novartis, Celgene, Allergan, Foamix, and Promius.
A sweet new solution for rosacea
VIENNA – A medical-grade topical honey product proved safe and effective for the treatment of rosacea in a randomized, placebo-controlled clinical trial, Brigitte Dreno, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
The product, known as Honevo, is a cream consisting of 90% New Zealand kanuka honey and 10% glycerine. It is applied twice daily as a face mask. Honevo is designed to wash off easily and is less sticky than honey.
The primary outcome in the trial was at least a 2-point improvement from baseline on the 7-point Investigator Global Assessment of Rosacea Severity Score (IGA-RSS) as assessed by blinded investigators. This outcome, which reflects a clinical improvement from severe to moderate or from moderate to mild, was achieved in 34% of the Honevo group, compared with 17% of controls. Significant improvement in the honey group was seen after 2 weeks.
Rosacea resolved in 13% of the Honevo group and in 3% of controls, based on a week 8 IGA-RSS of zero, noted Dr. Dreno, professor and chair of the department of dermatology at the University of Nantes (France).
The investigators, from the Medical Research Institute of New Zealand and the University of Otago in Wellington, observed that the study outcomes look at least as good as the results of placebo-controlled studies of topical metronidazole or azelaic cream. They plan to conduct randomized, head-to-head comparative trials of those prescription drugs versus Honevo, which is an OTC product.
The mechanism of action of kanuka honey in treating rosacea is believed to involve its previously reported antibacterial and anti-inflammatory effects, according to the investigators (BMJ Open. 2015 Jun 24;5[6]:e007651. doi: 10.1136/bmjopen-2015-007651).
The researchers noted that many rosacea patients aren’t interested in long-term antibiotic therapy. They want a natural product that doesn’t contribute to the global antibiotic resistance problem and is available OTC. And Honevo is one of the few natural or complementary medicine therapies backed by data from a rigorous clinical trial, in this case one registered in the Australian New Zealand Clinical Trials Registry (ACTRN12614000004662).
Dr. Dreno wasn’t involved in the study but included it in a talk in which she examined the strengths and weaknesses of current rosacea therapies. She is waiting for a confirmatory study before she incorporates Honevo in her own treatment algorithm. She said that she also would like to see studies examining whether combining the topical honey product with prescription drugs for rosacea provides synergistic efficacy.
HoneyLab, which funded the clinical trial and markets Honevo, ships the product to customers worldwide from its New Zealand headquarters.
VIENNA – A medical-grade topical honey product proved safe and effective for the treatment of rosacea in a randomized, placebo-controlled clinical trial, Brigitte Dreno, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
The product, known as Honevo, is a cream consisting of 90% New Zealand kanuka honey and 10% glycerine. It is applied twice daily as a face mask. Honevo is designed to wash off easily and is less sticky than honey.
The primary outcome in the trial was at least a 2-point improvement from baseline on the 7-point Investigator Global Assessment of Rosacea Severity Score (IGA-RSS) as assessed by blinded investigators. This outcome, which reflects a clinical improvement from severe to moderate or from moderate to mild, was achieved in 34% of the Honevo group, compared with 17% of controls. Significant improvement in the honey group was seen after 2 weeks.
Rosacea resolved in 13% of the Honevo group and in 3% of controls, based on a week 8 IGA-RSS of zero, noted Dr. Dreno, professor and chair of the department of dermatology at the University of Nantes (France).
The investigators, from the Medical Research Institute of New Zealand and the University of Otago in Wellington, observed that the study outcomes look at least as good as the results of placebo-controlled studies of topical metronidazole or azelaic cream. They plan to conduct randomized, head-to-head comparative trials of those prescription drugs versus Honevo, which is an OTC product.
The mechanism of action of kanuka honey in treating rosacea is believed to involve its previously reported antibacterial and anti-inflammatory effects, according to the investigators (BMJ Open. 2015 Jun 24;5[6]:e007651. doi: 10.1136/bmjopen-2015-007651).
The researchers noted that many rosacea patients aren’t interested in long-term antibiotic therapy. They want a natural product that doesn’t contribute to the global antibiotic resistance problem and is available OTC. And Honevo is one of the few natural or complementary medicine therapies backed by data from a rigorous clinical trial, in this case one registered in the Australian New Zealand Clinical Trials Registry (ACTRN12614000004662).
Dr. Dreno wasn’t involved in the study but included it in a talk in which she examined the strengths and weaknesses of current rosacea therapies. She is waiting for a confirmatory study before she incorporates Honevo in her own treatment algorithm. She said that she also would like to see studies examining whether combining the topical honey product with prescription drugs for rosacea provides synergistic efficacy.
HoneyLab, which funded the clinical trial and markets Honevo, ships the product to customers worldwide from its New Zealand headquarters.
VIENNA – A medical-grade topical honey product proved safe and effective for the treatment of rosacea in a randomized, placebo-controlled clinical trial, Brigitte Dreno, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
The product, known as Honevo, is a cream consisting of 90% New Zealand kanuka honey and 10% glycerine. It is applied twice daily as a face mask. Honevo is designed to wash off easily and is less sticky than honey.
The primary outcome in the trial was at least a 2-point improvement from baseline on the 7-point Investigator Global Assessment of Rosacea Severity Score (IGA-RSS) as assessed by blinded investigators. This outcome, which reflects a clinical improvement from severe to moderate or from moderate to mild, was achieved in 34% of the Honevo group, compared with 17% of controls. Significant improvement in the honey group was seen after 2 weeks.
Rosacea resolved in 13% of the Honevo group and in 3% of controls, based on a week 8 IGA-RSS of zero, noted Dr. Dreno, professor and chair of the department of dermatology at the University of Nantes (France).
The investigators, from the Medical Research Institute of New Zealand and the University of Otago in Wellington, observed that the study outcomes look at least as good as the results of placebo-controlled studies of topical metronidazole or azelaic cream. They plan to conduct randomized, head-to-head comparative trials of those prescription drugs versus Honevo, which is an OTC product.
The mechanism of action of kanuka honey in treating rosacea is believed to involve its previously reported antibacterial and anti-inflammatory effects, according to the investigators (BMJ Open. 2015 Jun 24;5[6]:e007651. doi: 10.1136/bmjopen-2015-007651).
The researchers noted that many rosacea patients aren’t interested in long-term antibiotic therapy. They want a natural product that doesn’t contribute to the global antibiotic resistance problem and is available OTC. And Honevo is one of the few natural or complementary medicine therapies backed by data from a rigorous clinical trial, in this case one registered in the Australian New Zealand Clinical Trials Registry (ACTRN12614000004662).
Dr. Dreno wasn’t involved in the study but included it in a talk in which she examined the strengths and weaknesses of current rosacea therapies. She is waiting for a confirmatory study before she incorporates Honevo in her own treatment algorithm. She said that she also would like to see studies examining whether combining the topical honey product with prescription drugs for rosacea provides synergistic efficacy.
HoneyLab, which funded the clinical trial and markets Honevo, ships the product to customers worldwide from its New Zealand headquarters.
AT THE EADV CONGRESS
Key clinical point:
Major finding: 34% of rosacea patients experienced clinically meaningful improvement in response to twice-daily kanuka honey face masks, a rate twice that in controls.
Data source: This was a randomized, placebo-controlled, single-blind, 8-week clinical trial involving 138 adults with rosacea.
Disclosures: The presenter reported having no financial conflicts of interest regarding the study.
‘Anxiety sensitivity’ tied to psychodermatologic disorders
Adult patients who experience stress in the form of “anxiety sensitivity” are more likely to develop psychodermatological conditions than those that are not psychodermatological, a cross-sectional study of 115 participants shows.
“The results suggest that [anxiety sensitivity] interventions combined with dermatology treatments may be beneficial for psychodermatological patients,” wrote Laura J. Dixon, PhD, of the University of Mississippi Medical Center, Jackson, and her associates. “There is strong evidence that cognitive-behavioral therapy significantly reduces [anxiety sensitivity] through strategies such as psychoeducation, interoceptive exposure, and cognitive therapy.”
Dr. Dixon and her associates recruited 123 dermatologic patients aged 18-83 years over 30 weeks through three outpatient university dermatology clinics in Central Mississippi. Sixty-five percent of the participants were white, 33% were black, 1% were Asian, and 1% were Native American; 65% were female. Most of the patients were married and living with their spouses. The final sample of participants comprised 63 psychodermatological patients and 52 nonpsychodermatological patients (Psychosomatics. 2016;57:498-504).
The investigators assessed general anxiety symptoms using the 7-item depression, anxiety, and stress subscale (DASS-A) from the 21-item version of the questionnaire (DASS-21). Anxiety sensitivity – which refers to the “extent of beliefs that anxiety symptoms or arousal can have harmful consequences” (Turk Psikiyatri Derg. 2011 Fall;22[3]:187-93) – was measured using the Anxiety Sensitivity Index–3 (ASI-3, an 18-item self-report instrument that assesses physical manifestations of anxiety, such as blushing and fast heart beating.
Psychodermatological conditions were classified as disorders that might be rooted in or made worse by psychological, behavioral, or stress-related factors. Conditions in this category include acne, alopecia, atopic dermatitis, eczema, hidradenitis, prurigo, psoriasis, and rosacea. Dermatologic conditions not tied to psychological factors and classified as biologically based include brittle fingernails, cysts, keloids, rashes, skin cancer, skin lesions, spider veins, and warts, reported Dr. Dixon.
No significant differences were observed on the DASS-A scores between the two groups.
The mean scores of psychodermatological patients on the ASI-3 were significantly higher than the scores of patients with nonpsychodermatological conditions (21.1 vs. 13.7; P = .013). In fact, Dr. Dixon and her associates found that “each 1-unit increment in the ASI-3 social subscale score was associated with a 12.7% increased odds of patients having a psychodermatological condition.”
“Taken together, these results are supported by existing theoretical models of psychodermatological disorders that highlight the importance of stress among patients with certain dermatological conditions,” the researchers wrote.
One of the authors, dermatologist Robert T. Brodell, disclosed receiving honoraria from Allergan, Galderma Laboratories, and PharmaDerm; he also disclosed receiving consultant fees and performing clinical trials for other pharmaceutical companies. Neither Dr. Dixon nor any of the other authors declared relevant financial disclosures.
[email protected]
On Twitter @ginalhenderson
Adult patients who experience stress in the form of “anxiety sensitivity” are more likely to develop psychodermatological conditions than those that are not psychodermatological, a cross-sectional study of 115 participants shows.
“The results suggest that [anxiety sensitivity] interventions combined with dermatology treatments may be beneficial for psychodermatological patients,” wrote Laura J. Dixon, PhD, of the University of Mississippi Medical Center, Jackson, and her associates. “There is strong evidence that cognitive-behavioral therapy significantly reduces [anxiety sensitivity] through strategies such as psychoeducation, interoceptive exposure, and cognitive therapy.”
Dr. Dixon and her associates recruited 123 dermatologic patients aged 18-83 years over 30 weeks through three outpatient university dermatology clinics in Central Mississippi. Sixty-five percent of the participants were white, 33% were black, 1% were Asian, and 1% were Native American; 65% were female. Most of the patients were married and living with their spouses. The final sample of participants comprised 63 psychodermatological patients and 52 nonpsychodermatological patients (Psychosomatics. 2016;57:498-504).
The investigators assessed general anxiety symptoms using the 7-item depression, anxiety, and stress subscale (DASS-A) from the 21-item version of the questionnaire (DASS-21). Anxiety sensitivity – which refers to the “extent of beliefs that anxiety symptoms or arousal can have harmful consequences” (Turk Psikiyatri Derg. 2011 Fall;22[3]:187-93) – was measured using the Anxiety Sensitivity Index–3 (ASI-3, an 18-item self-report instrument that assesses physical manifestations of anxiety, such as blushing and fast heart beating.
Psychodermatological conditions were classified as disorders that might be rooted in or made worse by psychological, behavioral, or stress-related factors. Conditions in this category include acne, alopecia, atopic dermatitis, eczema, hidradenitis, prurigo, psoriasis, and rosacea. Dermatologic conditions not tied to psychological factors and classified as biologically based include brittle fingernails, cysts, keloids, rashes, skin cancer, skin lesions, spider veins, and warts, reported Dr. Dixon.
No significant differences were observed on the DASS-A scores between the two groups.
The mean scores of psychodermatological patients on the ASI-3 were significantly higher than the scores of patients with nonpsychodermatological conditions (21.1 vs. 13.7; P = .013). In fact, Dr. Dixon and her associates found that “each 1-unit increment in the ASI-3 social subscale score was associated with a 12.7% increased odds of patients having a psychodermatological condition.”
“Taken together, these results are supported by existing theoretical models of psychodermatological disorders that highlight the importance of stress among patients with certain dermatological conditions,” the researchers wrote.
One of the authors, dermatologist Robert T. Brodell, disclosed receiving honoraria from Allergan, Galderma Laboratories, and PharmaDerm; he also disclosed receiving consultant fees and performing clinical trials for other pharmaceutical companies. Neither Dr. Dixon nor any of the other authors declared relevant financial disclosures.
[email protected]
On Twitter @ginalhenderson
Adult patients who experience stress in the form of “anxiety sensitivity” are more likely to develop psychodermatological conditions than those that are not psychodermatological, a cross-sectional study of 115 participants shows.
“The results suggest that [anxiety sensitivity] interventions combined with dermatology treatments may be beneficial for psychodermatological patients,” wrote Laura J. Dixon, PhD, of the University of Mississippi Medical Center, Jackson, and her associates. “There is strong evidence that cognitive-behavioral therapy significantly reduces [anxiety sensitivity] through strategies such as psychoeducation, interoceptive exposure, and cognitive therapy.”
Dr. Dixon and her associates recruited 123 dermatologic patients aged 18-83 years over 30 weeks through three outpatient university dermatology clinics in Central Mississippi. Sixty-five percent of the participants were white, 33% were black, 1% were Asian, and 1% were Native American; 65% were female. Most of the patients were married and living with their spouses. The final sample of participants comprised 63 psychodermatological patients and 52 nonpsychodermatological patients (Psychosomatics. 2016;57:498-504).
The investigators assessed general anxiety symptoms using the 7-item depression, anxiety, and stress subscale (DASS-A) from the 21-item version of the questionnaire (DASS-21). Anxiety sensitivity – which refers to the “extent of beliefs that anxiety symptoms or arousal can have harmful consequences” (Turk Psikiyatri Derg. 2011 Fall;22[3]:187-93) – was measured using the Anxiety Sensitivity Index–3 (ASI-3, an 18-item self-report instrument that assesses physical manifestations of anxiety, such as blushing and fast heart beating.
Psychodermatological conditions were classified as disorders that might be rooted in or made worse by psychological, behavioral, or stress-related factors. Conditions in this category include acne, alopecia, atopic dermatitis, eczema, hidradenitis, prurigo, psoriasis, and rosacea. Dermatologic conditions not tied to psychological factors and classified as biologically based include brittle fingernails, cysts, keloids, rashes, skin cancer, skin lesions, spider veins, and warts, reported Dr. Dixon.
No significant differences were observed on the DASS-A scores between the two groups.
The mean scores of psychodermatological patients on the ASI-3 were significantly higher than the scores of patients with nonpsychodermatological conditions (21.1 vs. 13.7; P = .013). In fact, Dr. Dixon and her associates found that “each 1-unit increment in the ASI-3 social subscale score was associated with a 12.7% increased odds of patients having a psychodermatological condition.”
“Taken together, these results are supported by existing theoretical models of psychodermatological disorders that highlight the importance of stress among patients with certain dermatological conditions,” the researchers wrote.
One of the authors, dermatologist Robert T. Brodell, disclosed receiving honoraria from Allergan, Galderma Laboratories, and PharmaDerm; he also disclosed receiving consultant fees and performing clinical trials for other pharmaceutical companies. Neither Dr. Dixon nor any of the other authors declared relevant financial disclosures.
[email protected]
On Twitter @ginalhenderson
Rosacea improved with fractional microneedling radiofrequency therapy
Fractional microneedling radiofrequency (FMR) therapy resulted in modest but clinically significant improvements in the appearance and inflammation of rosacea in a small study of patients with mild to moderate rosacea.
The treatment delivers bipolar radiofrequency energy to the dermis via an array of microneedles, without damaging the epidermis, noted Seon Yong Park, MD, and colleagues from the department of dermatology at Seoul (South Korea) National University. It has previously been associated with clinical and histological improvements in acne-associated postinflammatory erythema and is used in the treatment of cutaneous wrinkles. The authors said that, as far as they know, this is the first study to evaluate the use of FMR in patients with rosacea.
In the prospective, single-blind, randomized, split-face clinical study, 21 patients (20 females, 1 male) with mild to moderate rosacea were treated with two FMR sessions, 4 weeks apart, then assessed 4, 8, and 12 weeks after the second session. The mean age of the patients was 43 years; they had Fitzpatrick skin type III (13 patients) or IV (8 patients), and rosacea was considered mild in 12 patients and moderate in 9 patients at baseline.
Researchers saw clinical improvements in 17 (81%) of the patients on the treated side; these patients had a mean improvement in the Investigator’s Global Assessment (IGA) score of 2.47 by week 12, representing about a 20% improvement (Dermatol Surg. 2016 Dec;42[12]:1362-9).
In the group overall, mean IGA scores at weeks 4, 8, and 12 were 1.05, 1.57, and 2.00 for the treated side, compared with 0.29, 0.38, and 0.38, respectively, for the untreated side, which, the authors wrote, indicated that “there was modest but statistically significant improvements in the treated side.”
Photometric measurements of redness showed significant reductions on the treated side, compared with the untreated side and baseline, with reductions in the erythema index of 11.9%, 10.7%, and 13.6% at week 4, 8, and 12, respectively.
Histological assessment showed reduced dermal inflammation, significant reductions in average mast cell count, and an overall decrease in immunohistochemical intensity in the treated skin 8 weeks after treatment. Similarly, there were significant decreases in markers of angiogenesis, inflammation, innate immunity, and neuroimmunity on the treated side, compared with baseline.
“Fractional microneedling radiofrequency was slightly more effective in reducing erythema in patients with PPR [papulopustular rosacea] than in those with ETR [erythematotelangiectatic rosacea], suggesting that inflammatory lesions, such as papules and pustules, could be more effectively treated with this device,” the authors wrote. “This result agreed with reports showing that FMR is effective in treating inflammatory acne.”
No serious adverse effects were reported, although 19 patients (90.5%) experienced mild pain during the procedure and 17 (81%) had mild erythema that lasted for up to 5 days. Patients also reported less itching, heat, burning, or pricking on the treated side, which showed that the treatment was effective in controlling the symptoms of rosacea, Dr. Park and associates said.
The study was supported by the SNUH Research Fund and National Research Foundation of Korea. The authors, who are also in the acne and rosacea research laboratory, Seoul National University Hospital, had no conflicts to disclose.
Fractional microneedling radiofrequency (FMR) therapy resulted in modest but clinically significant improvements in the appearance and inflammation of rosacea in a small study of patients with mild to moderate rosacea.
The treatment delivers bipolar radiofrequency energy to the dermis via an array of microneedles, without damaging the epidermis, noted Seon Yong Park, MD, and colleagues from the department of dermatology at Seoul (South Korea) National University. It has previously been associated with clinical and histological improvements in acne-associated postinflammatory erythema and is used in the treatment of cutaneous wrinkles. The authors said that, as far as they know, this is the first study to evaluate the use of FMR in patients with rosacea.
In the prospective, single-blind, randomized, split-face clinical study, 21 patients (20 females, 1 male) with mild to moderate rosacea were treated with two FMR sessions, 4 weeks apart, then assessed 4, 8, and 12 weeks after the second session. The mean age of the patients was 43 years; they had Fitzpatrick skin type III (13 patients) or IV (8 patients), and rosacea was considered mild in 12 patients and moderate in 9 patients at baseline.
Researchers saw clinical improvements in 17 (81%) of the patients on the treated side; these patients had a mean improvement in the Investigator’s Global Assessment (IGA) score of 2.47 by week 12, representing about a 20% improvement (Dermatol Surg. 2016 Dec;42[12]:1362-9).
In the group overall, mean IGA scores at weeks 4, 8, and 12 were 1.05, 1.57, and 2.00 for the treated side, compared with 0.29, 0.38, and 0.38, respectively, for the untreated side, which, the authors wrote, indicated that “there was modest but statistically significant improvements in the treated side.”
Photometric measurements of redness showed significant reductions on the treated side, compared with the untreated side and baseline, with reductions in the erythema index of 11.9%, 10.7%, and 13.6% at week 4, 8, and 12, respectively.
Histological assessment showed reduced dermal inflammation, significant reductions in average mast cell count, and an overall decrease in immunohistochemical intensity in the treated skin 8 weeks after treatment. Similarly, there were significant decreases in markers of angiogenesis, inflammation, innate immunity, and neuroimmunity on the treated side, compared with baseline.
“Fractional microneedling radiofrequency was slightly more effective in reducing erythema in patients with PPR [papulopustular rosacea] than in those with ETR [erythematotelangiectatic rosacea], suggesting that inflammatory lesions, such as papules and pustules, could be more effectively treated with this device,” the authors wrote. “This result agreed with reports showing that FMR is effective in treating inflammatory acne.”
No serious adverse effects were reported, although 19 patients (90.5%) experienced mild pain during the procedure and 17 (81%) had mild erythema that lasted for up to 5 days. Patients also reported less itching, heat, burning, or pricking on the treated side, which showed that the treatment was effective in controlling the symptoms of rosacea, Dr. Park and associates said.
The study was supported by the SNUH Research Fund and National Research Foundation of Korea. The authors, who are also in the acne and rosacea research laboratory, Seoul National University Hospital, had no conflicts to disclose.
Fractional microneedling radiofrequency (FMR) therapy resulted in modest but clinically significant improvements in the appearance and inflammation of rosacea in a small study of patients with mild to moderate rosacea.
The treatment delivers bipolar radiofrequency energy to the dermis via an array of microneedles, without damaging the epidermis, noted Seon Yong Park, MD, and colleagues from the department of dermatology at Seoul (South Korea) National University. It has previously been associated with clinical and histological improvements in acne-associated postinflammatory erythema and is used in the treatment of cutaneous wrinkles. The authors said that, as far as they know, this is the first study to evaluate the use of FMR in patients with rosacea.
In the prospective, single-blind, randomized, split-face clinical study, 21 patients (20 females, 1 male) with mild to moderate rosacea were treated with two FMR sessions, 4 weeks apart, then assessed 4, 8, and 12 weeks after the second session. The mean age of the patients was 43 years; they had Fitzpatrick skin type III (13 patients) or IV (8 patients), and rosacea was considered mild in 12 patients and moderate in 9 patients at baseline.
Researchers saw clinical improvements in 17 (81%) of the patients on the treated side; these patients had a mean improvement in the Investigator’s Global Assessment (IGA) score of 2.47 by week 12, representing about a 20% improvement (Dermatol Surg. 2016 Dec;42[12]:1362-9).
In the group overall, mean IGA scores at weeks 4, 8, and 12 were 1.05, 1.57, and 2.00 for the treated side, compared with 0.29, 0.38, and 0.38, respectively, for the untreated side, which, the authors wrote, indicated that “there was modest but statistically significant improvements in the treated side.”
Photometric measurements of redness showed significant reductions on the treated side, compared with the untreated side and baseline, with reductions in the erythema index of 11.9%, 10.7%, and 13.6% at week 4, 8, and 12, respectively.
Histological assessment showed reduced dermal inflammation, significant reductions in average mast cell count, and an overall decrease in immunohistochemical intensity in the treated skin 8 weeks after treatment. Similarly, there were significant decreases in markers of angiogenesis, inflammation, innate immunity, and neuroimmunity on the treated side, compared with baseline.
“Fractional microneedling radiofrequency was slightly more effective in reducing erythema in patients with PPR [papulopustular rosacea] than in those with ETR [erythematotelangiectatic rosacea], suggesting that inflammatory lesions, such as papules and pustules, could be more effectively treated with this device,” the authors wrote. “This result agreed with reports showing that FMR is effective in treating inflammatory acne.”
No serious adverse effects were reported, although 19 patients (90.5%) experienced mild pain during the procedure and 17 (81%) had mild erythema that lasted for up to 5 days. Patients also reported less itching, heat, burning, or pricking on the treated side, which showed that the treatment was effective in controlling the symptoms of rosacea, Dr. Park and associates said.
The study was supported by the SNUH Research Fund and National Research Foundation of Korea. The authors, who are also in the acne and rosacea research laboratory, Seoul National University Hospital, had no conflicts to disclose.
FROM DERMATOLOGIC SURGERY
Key clinical point: Fractional microneedling radiofrequency therapy may achieve modest but clinically significant improvements in the appearance and inflammation of rosacea.
Major finding: Fractional microneedling radiofrequency therapy was associated with clinical improvements in 81% of treated patients.
Data source: A prospective, single-blind, randomized, split-face clinical trial in 21 patients with mild to moderate rosacea.
Disclosures: The study was supported by the SNUH Research Fund and National Research Foundation of Korea. No conflicts of interest were declared.
Ocular rosacea remains a stubborn foe
Few skin disorders have the power to devastate lives like ocular rosacea, a painful condition that disrupts vision and can lead to blindness, according to ophthalmologist Edward Wladis, MD.
“Patients really suffer from this diagnosis,” said Dr. Wladis, who practices in Slingerlands, N.Y.
Charles Slonim, MD, an ophthalmologist who practices in Tampa, Fla., put it this way: “We control the condition more than 50 percent of the time, but frequently patients go into periods of remission only to have a recurrence or exacerbation of their ocular rosacea.”
Dr. Wladis coauthored a 2013 report that examined treatments for ocular rosacea, which noted that estimates of the proportion of people with rosacea in the United States who develop ocular rosacea vary, ranging from 58% to 72% (US Ophthalmic Review, 2013;6[2]:86-8).
“Ocular rosacea is one of the subtypes of this disease of cutaneous inflammation,” Dr. Wladis said. “Once the skin becomes so severely inflamed, the glands that lubricate the eye become damaged, and the tear film evaporates rapidly. As a result, patients complain of the effects of a dry ocular surface, and they suffer from blurred vision, tearing, pain, and problems with glare.”
Dr. Slonim suggests that dermatologists refer rosacea patients to an ophthalmologist if they present with any eye symptom, such as dryness, burning, or itching, foreign body sensation in one or both eyes, or chronic redness of either the eyes or the eyelid margins. “They should be seen should be seen by an ophthalmologist to rule out ocular rosacea,” he said. “The ophthalmologist’s ability to look at the eye and eyelids under high magnification – a slit lamp examination – gives us an advantage in the diagnosis of ocular rosacea.”
If these patients do have ocular rosacea, their prognosis is unclear. “Unfortunately, many of our treatments haven’t been carefully vetted,” Dr. Wladis said.
He tends to begin with simpler treatments to heal the ocular surface, such as artificial tears and plugs in the tear drainage ducts to keep tears from leaving the eye quickly. Eyelid scrubs and warm compresses can also be helpful, he said, along with suggestions about lifestyle modifications to avoid the triggers that may exacerbate rosacea.
If those treatments fail, antibiotics are an option.
A 2015 Cochrane Review of studies of rosacea treatments suggested that for treating ocular rosacea, cyclosporine 0.05% ophthalmic emulsion “appeared to be more effective than artificial tears” (Cochrane Database Syst Rev. 2015 Apr 28;[3]:CD003262). And a 2015 study of 38 patients with ocular rosacea concluded that topical cyclosporine was significantly more effective in relieving symptoms and in the treatment of eyelid signs, compared with oral doxycycline (Int J Ophthalmol. 2015 Jun 18;8[3]:544-9).
Antibiotics seem to improve the eyelid’s health, “although some studies have documented that the cornea often doesn’t benefit from antibiotics, and patients’ visual acuity may not improve,” Dr. Wladis said.
Ophthalmologists also may prescribe nonsteroidal and steroidal anti-inflammatory drops, Dr. Slonim added, although “the use of topical ophthalmic steroids do carry the risk of secondary glaucoma with increased intraocular pressures.”
There are even more alternatives. “Dietary modification with omega-3 fatty acids appears to benefit the quality of the tear film,” Dr. Wladis said, referring to the results of a prospective, placebo-controlled, double-blind, randomized trial of patients with dry eye (Int J Ophthalmol. 2013 Dec 18;[6]:811-6). “Intraductal meibomian gland probing and intense pulsed light therapy have both been shown to improve ocular surface–related quality of life, although these treatments are relatively invasive and can rapidly become quite expensive for the patient.”
What’s on the horizon? Researchers have “started to unlock the mysteries of rosacea at the cellular level,” Dr. Wladis said. “Our efforts have recently focused on the cellular changes in the skin of rosacea patients. Using several methods, we assayed the activation of a wide variety of signals within the cells of the skin of these patients and found a consistent elevation of two specific signals.”
The researchers were especially pleased, he said, “that these signals appear to be activated in the outer layers of the skin, meaning that a topical preparation could be developed to selectively suppress these cell signals to turn off the disease without interfering with normal skin structure and function and without the side effects of oral or intravenous medications.”
His team is now working on a topical medication. “Ideally,” he noted, “future clinicians will be able to shift their focus from nonspecific therapies like antibiotics and steroids to really powerful, meaningful cellular therapeutics.”
Dr. Slonim reported no relevant disclosures. Dr. Wladis shares a provisional patent for the use of topical kinase inhibitors in the management of rosacea and recently co-started a biotechnology company called Praxis Biotechnology that aims to develop and test therapies for the condition. He serves as a consultant for both Bausch & Lomb and Valeant Pharmaceuticals.
Few skin disorders have the power to devastate lives like ocular rosacea, a painful condition that disrupts vision and can lead to blindness, according to ophthalmologist Edward Wladis, MD.
“Patients really suffer from this diagnosis,” said Dr. Wladis, who practices in Slingerlands, N.Y.
Charles Slonim, MD, an ophthalmologist who practices in Tampa, Fla., put it this way: “We control the condition more than 50 percent of the time, but frequently patients go into periods of remission only to have a recurrence or exacerbation of their ocular rosacea.”
Dr. Wladis coauthored a 2013 report that examined treatments for ocular rosacea, which noted that estimates of the proportion of people with rosacea in the United States who develop ocular rosacea vary, ranging from 58% to 72% (US Ophthalmic Review, 2013;6[2]:86-8).
“Ocular rosacea is one of the subtypes of this disease of cutaneous inflammation,” Dr. Wladis said. “Once the skin becomes so severely inflamed, the glands that lubricate the eye become damaged, and the tear film evaporates rapidly. As a result, patients complain of the effects of a dry ocular surface, and they suffer from blurred vision, tearing, pain, and problems with glare.”
Dr. Slonim suggests that dermatologists refer rosacea patients to an ophthalmologist if they present with any eye symptom, such as dryness, burning, or itching, foreign body sensation in one or both eyes, or chronic redness of either the eyes or the eyelid margins. “They should be seen should be seen by an ophthalmologist to rule out ocular rosacea,” he said. “The ophthalmologist’s ability to look at the eye and eyelids under high magnification – a slit lamp examination – gives us an advantage in the diagnosis of ocular rosacea.”
If these patients do have ocular rosacea, their prognosis is unclear. “Unfortunately, many of our treatments haven’t been carefully vetted,” Dr. Wladis said.
He tends to begin with simpler treatments to heal the ocular surface, such as artificial tears and plugs in the tear drainage ducts to keep tears from leaving the eye quickly. Eyelid scrubs and warm compresses can also be helpful, he said, along with suggestions about lifestyle modifications to avoid the triggers that may exacerbate rosacea.
If those treatments fail, antibiotics are an option.
A 2015 Cochrane Review of studies of rosacea treatments suggested that for treating ocular rosacea, cyclosporine 0.05% ophthalmic emulsion “appeared to be more effective than artificial tears” (Cochrane Database Syst Rev. 2015 Apr 28;[3]:CD003262). And a 2015 study of 38 patients with ocular rosacea concluded that topical cyclosporine was significantly more effective in relieving symptoms and in the treatment of eyelid signs, compared with oral doxycycline (Int J Ophthalmol. 2015 Jun 18;8[3]:544-9).
Antibiotics seem to improve the eyelid’s health, “although some studies have documented that the cornea often doesn’t benefit from antibiotics, and patients’ visual acuity may not improve,” Dr. Wladis said.
Ophthalmologists also may prescribe nonsteroidal and steroidal anti-inflammatory drops, Dr. Slonim added, although “the use of topical ophthalmic steroids do carry the risk of secondary glaucoma with increased intraocular pressures.”
There are even more alternatives. “Dietary modification with omega-3 fatty acids appears to benefit the quality of the tear film,” Dr. Wladis said, referring to the results of a prospective, placebo-controlled, double-blind, randomized trial of patients with dry eye (Int J Ophthalmol. 2013 Dec 18;[6]:811-6). “Intraductal meibomian gland probing and intense pulsed light therapy have both been shown to improve ocular surface–related quality of life, although these treatments are relatively invasive and can rapidly become quite expensive for the patient.”
What’s on the horizon? Researchers have “started to unlock the mysteries of rosacea at the cellular level,” Dr. Wladis said. “Our efforts have recently focused on the cellular changes in the skin of rosacea patients. Using several methods, we assayed the activation of a wide variety of signals within the cells of the skin of these patients and found a consistent elevation of two specific signals.”
The researchers were especially pleased, he said, “that these signals appear to be activated in the outer layers of the skin, meaning that a topical preparation could be developed to selectively suppress these cell signals to turn off the disease without interfering with normal skin structure and function and without the side effects of oral or intravenous medications.”
His team is now working on a topical medication. “Ideally,” he noted, “future clinicians will be able to shift their focus from nonspecific therapies like antibiotics and steroids to really powerful, meaningful cellular therapeutics.”
Dr. Slonim reported no relevant disclosures. Dr. Wladis shares a provisional patent for the use of topical kinase inhibitors in the management of rosacea and recently co-started a biotechnology company called Praxis Biotechnology that aims to develop and test therapies for the condition. He serves as a consultant for both Bausch & Lomb and Valeant Pharmaceuticals.
Few skin disorders have the power to devastate lives like ocular rosacea, a painful condition that disrupts vision and can lead to blindness, according to ophthalmologist Edward Wladis, MD.
“Patients really suffer from this diagnosis,” said Dr. Wladis, who practices in Slingerlands, N.Y.
Charles Slonim, MD, an ophthalmologist who practices in Tampa, Fla., put it this way: “We control the condition more than 50 percent of the time, but frequently patients go into periods of remission only to have a recurrence or exacerbation of their ocular rosacea.”
Dr. Wladis coauthored a 2013 report that examined treatments for ocular rosacea, which noted that estimates of the proportion of people with rosacea in the United States who develop ocular rosacea vary, ranging from 58% to 72% (US Ophthalmic Review, 2013;6[2]:86-8).
“Ocular rosacea is one of the subtypes of this disease of cutaneous inflammation,” Dr. Wladis said. “Once the skin becomes so severely inflamed, the glands that lubricate the eye become damaged, and the tear film evaporates rapidly. As a result, patients complain of the effects of a dry ocular surface, and they suffer from blurred vision, tearing, pain, and problems with glare.”
Dr. Slonim suggests that dermatologists refer rosacea patients to an ophthalmologist if they present with any eye symptom, such as dryness, burning, or itching, foreign body sensation in one or both eyes, or chronic redness of either the eyes or the eyelid margins. “They should be seen should be seen by an ophthalmologist to rule out ocular rosacea,” he said. “The ophthalmologist’s ability to look at the eye and eyelids under high magnification – a slit lamp examination – gives us an advantage in the diagnosis of ocular rosacea.”
If these patients do have ocular rosacea, their prognosis is unclear. “Unfortunately, many of our treatments haven’t been carefully vetted,” Dr. Wladis said.
He tends to begin with simpler treatments to heal the ocular surface, such as artificial tears and plugs in the tear drainage ducts to keep tears from leaving the eye quickly. Eyelid scrubs and warm compresses can also be helpful, he said, along with suggestions about lifestyle modifications to avoid the triggers that may exacerbate rosacea.
If those treatments fail, antibiotics are an option.
A 2015 Cochrane Review of studies of rosacea treatments suggested that for treating ocular rosacea, cyclosporine 0.05% ophthalmic emulsion “appeared to be more effective than artificial tears” (Cochrane Database Syst Rev. 2015 Apr 28;[3]:CD003262). And a 2015 study of 38 patients with ocular rosacea concluded that topical cyclosporine was significantly more effective in relieving symptoms and in the treatment of eyelid signs, compared with oral doxycycline (Int J Ophthalmol. 2015 Jun 18;8[3]:544-9).
Antibiotics seem to improve the eyelid’s health, “although some studies have documented that the cornea often doesn’t benefit from antibiotics, and patients’ visual acuity may not improve,” Dr. Wladis said.
Ophthalmologists also may prescribe nonsteroidal and steroidal anti-inflammatory drops, Dr. Slonim added, although “the use of topical ophthalmic steroids do carry the risk of secondary glaucoma with increased intraocular pressures.”
There are even more alternatives. “Dietary modification with omega-3 fatty acids appears to benefit the quality of the tear film,” Dr. Wladis said, referring to the results of a prospective, placebo-controlled, double-blind, randomized trial of patients with dry eye (Int J Ophthalmol. 2013 Dec 18;[6]:811-6). “Intraductal meibomian gland probing and intense pulsed light therapy have both been shown to improve ocular surface–related quality of life, although these treatments are relatively invasive and can rapidly become quite expensive for the patient.”
What’s on the horizon? Researchers have “started to unlock the mysteries of rosacea at the cellular level,” Dr. Wladis said. “Our efforts have recently focused on the cellular changes in the skin of rosacea patients. Using several methods, we assayed the activation of a wide variety of signals within the cells of the skin of these patients and found a consistent elevation of two specific signals.”
The researchers were especially pleased, he said, “that these signals appear to be activated in the outer layers of the skin, meaning that a topical preparation could be developed to selectively suppress these cell signals to turn off the disease without interfering with normal skin structure and function and without the side effects of oral or intravenous medications.”
His team is now working on a topical medication. “Ideally,” he noted, “future clinicians will be able to shift their focus from nonspecific therapies like antibiotics and steroids to really powerful, meaningful cellular therapeutics.”
Dr. Slonim reported no relevant disclosures. Dr. Wladis shares a provisional patent for the use of topical kinase inhibitors in the management of rosacea and recently co-started a biotechnology company called Praxis Biotechnology that aims to develop and test therapies for the condition. He serves as a consultant for both Bausch & Lomb and Valeant Pharmaceuticals.