User login
Rosacea: Expert recommends treating erythema, papules/pustules simultaneously
The results of a recently published trial helps answer one of the toughest questions in rosacea treatment: when patients present with both papules/pustules and erythema, which problem do you treat first?
In the past, Hilary Baldwin, MD, tended to target papules and pustules first, usually with ivermectin cream (Soolantra) and, when warranted, anti-inflammatory doses of doxycycline. Going after the erythema first and making the skin paler could make the cherry red inflammatory lesions stand out even more, she said.
In the study, one group was put on ivermectin for 12 weeks, with the vasoconstrictor brimonidine 0.33% (Mirvaso topical gel) added after 4 weeks to help with the erythema; and another group was treated with both ivermectin 1% cream and brimonidine daily for the entire 12 weeks. The third group received vehicles of both applied every day for 12 weeks (J Drugs Dermatol. 2017 Sep 1;16[9]:909-16).
“What they found was that both the combinations worked better than ivermectin alone,” and that treatment with both agents for the full 12 weeks worked best, with no increased risk of irritation or worsening of erythema than when brimonidine was brought in after 4 weeks of ivermectin, said Dr. Baldwin, a clinical associate professor of dermatology at Rutgers Robert Wood Johnson Medical School, New Brunswick, N.J., said in an interview. The vasoconstriction might have somehow helped with the papules and pustules, she noted.
The lesions might have looked more prominent “for the week or two it took for the ivermectin to kick in, but the patients didn’t care. They were happier campers by virtue of treating both aspects of their rosacea at the same time,” she added.
The new kid on the block for vasoconstriction – oxymetazoline (Rhofade cream) – appears to be gentler than brimonidine. “It takes a little bit longer to reach peak effect, and the peak doesn’t give you quite as much vasoconstriction as brimonidine, which for some people is a good thing,” she said. “Perhaps they’re a little bit too white with brimonidine. For other people who are bright red, oxymetazoline might not be enough. I think there’s a place for both drugs.”
Both vasoconstrictors might actually make erythema temporarily worse; it’s a known side effect. Dr. Baldwin has her patients try them for the first time when they’re at home and don’t have any important impending social engagements, just in case. “I like to give a tube of each one and say, ‘use one on one side of your face and the other on the other side and see which makes you happier.’ ”
Some patients can get away with “a really low dose and be completely cleared,” she commented. “I have some fully controlled on 10 mg twice weekly. As long as there’s no pregnancy risk, there’s no reason you can’t do this almost indefinitely.”
This publication and the Global Academy for Medical Education are both owned by Frontline Medical News. Dr. Baldwin is a speaker and advisor for Allergan, Galderma, and Valeant; and is an investigator for Dermira, Galderma, Novan, and Valeant.
The results of a recently published trial helps answer one of the toughest questions in rosacea treatment: when patients present with both papules/pustules and erythema, which problem do you treat first?
In the past, Hilary Baldwin, MD, tended to target papules and pustules first, usually with ivermectin cream (Soolantra) and, when warranted, anti-inflammatory doses of doxycycline. Going after the erythema first and making the skin paler could make the cherry red inflammatory lesions stand out even more, she said.
In the study, one group was put on ivermectin for 12 weeks, with the vasoconstrictor brimonidine 0.33% (Mirvaso topical gel) added after 4 weeks to help with the erythema; and another group was treated with both ivermectin 1% cream and brimonidine daily for the entire 12 weeks. The third group received vehicles of both applied every day for 12 weeks (J Drugs Dermatol. 2017 Sep 1;16[9]:909-16).
“What they found was that both the combinations worked better than ivermectin alone,” and that treatment with both agents for the full 12 weeks worked best, with no increased risk of irritation or worsening of erythema than when brimonidine was brought in after 4 weeks of ivermectin, said Dr. Baldwin, a clinical associate professor of dermatology at Rutgers Robert Wood Johnson Medical School, New Brunswick, N.J., said in an interview. The vasoconstriction might have somehow helped with the papules and pustules, she noted.
The lesions might have looked more prominent “for the week or two it took for the ivermectin to kick in, but the patients didn’t care. They were happier campers by virtue of treating both aspects of their rosacea at the same time,” she added.
The new kid on the block for vasoconstriction – oxymetazoline (Rhofade cream) – appears to be gentler than brimonidine. “It takes a little bit longer to reach peak effect, and the peak doesn’t give you quite as much vasoconstriction as brimonidine, which for some people is a good thing,” she said. “Perhaps they’re a little bit too white with brimonidine. For other people who are bright red, oxymetazoline might not be enough. I think there’s a place for both drugs.”
Both vasoconstrictors might actually make erythema temporarily worse; it’s a known side effect. Dr. Baldwin has her patients try them for the first time when they’re at home and don’t have any important impending social engagements, just in case. “I like to give a tube of each one and say, ‘use one on one side of your face and the other on the other side and see which makes you happier.’ ”
Some patients can get away with “a really low dose and be completely cleared,” she commented. “I have some fully controlled on 10 mg twice weekly. As long as there’s no pregnancy risk, there’s no reason you can’t do this almost indefinitely.”
This publication and the Global Academy for Medical Education are both owned by Frontline Medical News. Dr. Baldwin is a speaker and advisor for Allergan, Galderma, and Valeant; and is an investigator for Dermira, Galderma, Novan, and Valeant.
The results of a recently published trial helps answer one of the toughest questions in rosacea treatment: when patients present with both papules/pustules and erythema, which problem do you treat first?
In the past, Hilary Baldwin, MD, tended to target papules and pustules first, usually with ivermectin cream (Soolantra) and, when warranted, anti-inflammatory doses of doxycycline. Going after the erythema first and making the skin paler could make the cherry red inflammatory lesions stand out even more, she said.
In the study, one group was put on ivermectin for 12 weeks, with the vasoconstrictor brimonidine 0.33% (Mirvaso topical gel) added after 4 weeks to help with the erythema; and another group was treated with both ivermectin 1% cream and brimonidine daily for the entire 12 weeks. The third group received vehicles of both applied every day for 12 weeks (J Drugs Dermatol. 2017 Sep 1;16[9]:909-16).
“What they found was that both the combinations worked better than ivermectin alone,” and that treatment with both agents for the full 12 weeks worked best, with no increased risk of irritation or worsening of erythema than when brimonidine was brought in after 4 weeks of ivermectin, said Dr. Baldwin, a clinical associate professor of dermatology at Rutgers Robert Wood Johnson Medical School, New Brunswick, N.J., said in an interview. The vasoconstriction might have somehow helped with the papules and pustules, she noted.
The lesions might have looked more prominent “for the week or two it took for the ivermectin to kick in, but the patients didn’t care. They were happier campers by virtue of treating both aspects of their rosacea at the same time,” she added.
The new kid on the block for vasoconstriction – oxymetazoline (Rhofade cream) – appears to be gentler than brimonidine. “It takes a little bit longer to reach peak effect, and the peak doesn’t give you quite as much vasoconstriction as brimonidine, which for some people is a good thing,” she said. “Perhaps they’re a little bit too white with brimonidine. For other people who are bright red, oxymetazoline might not be enough. I think there’s a place for both drugs.”
Both vasoconstrictors might actually make erythema temporarily worse; it’s a known side effect. Dr. Baldwin has her patients try them for the first time when they’re at home and don’t have any important impending social engagements, just in case. “I like to give a tube of each one and say, ‘use one on one side of your face and the other on the other side and see which makes you happier.’ ”
Some patients can get away with “a really low dose and be completely cleared,” she commented. “I have some fully controlled on 10 mg twice weekly. As long as there’s no pregnancy risk, there’s no reason you can’t do this almost indefinitely.”
This publication and the Global Academy for Medical Education are both owned by Frontline Medical News. Dr. Baldwin is a speaker and advisor for Allergan, Galderma, and Valeant; and is an investigator for Dermira, Galderma, Novan, and Valeant.
EXPERT ANALYSIS FROM THE COASTAL DERMATOLOGY SYMPOSIUM
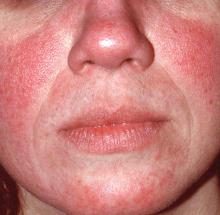
Clues to rosacea in patients of skin of color
NEW YORK – A middle-aged patient with Fitzpatrick type V skin comes to the office with a 10-year history of “breakouts” on her face. When asked about topical treatments, she reports that “everything burns or stings,” and says she just has very sensitive skin. What could this be?
Rosacea may often be missed in skin of color, said Dr. Alexis, speaking at the summer meeting of the American Academy of Dermatology. “It’s reportedly rare in darker skin types, especially in blacks,” and as a result, dermatologists and patients alike have a low index of suspicion for the diagnosis, he noted.
Also, rosacea looks different on darker skin than it does on lighter skin, which is featured in much of the dermatology teaching material. “In richly pigmented [Fitzpatrick] type VI skin, the erythema of rosacea can be masked,” said Dr. Alexis, chairman of the department of dermatology at Mount Sinai St. Luke’s and at Mount Sinai West, both in New York.
Dermatologists, in this case, may have to do some detective work: looking at the distribution of the lesions, thinking about trigger factors from the patient history, and noting the lack of comedones – although the patient has “pimples.” Patient complaints that they are sensitive to almost all topical products and experience stinging with “everything” is another very good clue that the patient may have rosacea.
Keep rosacea on the differential for this picture, advised Dr. Alexis. In skin of color, “rosacea may not be as rare as previously thought – less common, maybe – but not rare.”
Dr. Alexis reported financial relationships with multiple pharmaceutical companies.
[email protected]
On Twitter @karioakes
NEW YORK – A middle-aged patient with Fitzpatrick type V skin comes to the office with a 10-year history of “breakouts” on her face. When asked about topical treatments, she reports that “everything burns or stings,” and says she just has very sensitive skin. What could this be?
Rosacea may often be missed in skin of color, said Dr. Alexis, speaking at the summer meeting of the American Academy of Dermatology. “It’s reportedly rare in darker skin types, especially in blacks,” and as a result, dermatologists and patients alike have a low index of suspicion for the diagnosis, he noted.
Also, rosacea looks different on darker skin than it does on lighter skin, which is featured in much of the dermatology teaching material. “In richly pigmented [Fitzpatrick] type VI skin, the erythema of rosacea can be masked,” said Dr. Alexis, chairman of the department of dermatology at Mount Sinai St. Luke’s and at Mount Sinai West, both in New York.
Dermatologists, in this case, may have to do some detective work: looking at the distribution of the lesions, thinking about trigger factors from the patient history, and noting the lack of comedones – although the patient has “pimples.” Patient complaints that they are sensitive to almost all topical products and experience stinging with “everything” is another very good clue that the patient may have rosacea.
Keep rosacea on the differential for this picture, advised Dr. Alexis. In skin of color, “rosacea may not be as rare as previously thought – less common, maybe – but not rare.”
Dr. Alexis reported financial relationships with multiple pharmaceutical companies.
[email protected]
On Twitter @karioakes
NEW YORK – A middle-aged patient with Fitzpatrick type V skin comes to the office with a 10-year history of “breakouts” on her face. When asked about topical treatments, she reports that “everything burns or stings,” and says she just has very sensitive skin. What could this be?
Rosacea may often be missed in skin of color, said Dr. Alexis, speaking at the summer meeting of the American Academy of Dermatology. “It’s reportedly rare in darker skin types, especially in blacks,” and as a result, dermatologists and patients alike have a low index of suspicion for the diagnosis, he noted.
Also, rosacea looks different on darker skin than it does on lighter skin, which is featured in much of the dermatology teaching material. “In richly pigmented [Fitzpatrick] type VI skin, the erythema of rosacea can be masked,” said Dr. Alexis, chairman of the department of dermatology at Mount Sinai St. Luke’s and at Mount Sinai West, both in New York.
Dermatologists, in this case, may have to do some detective work: looking at the distribution of the lesions, thinking about trigger factors from the patient history, and noting the lack of comedones – although the patient has “pimples.” Patient complaints that they are sensitive to almost all topical products and experience stinging with “everything” is another very good clue that the patient may have rosacea.
Keep rosacea on the differential for this picture, advised Dr. Alexis. In skin of color, “rosacea may not be as rare as previously thought – less common, maybe – but not rare.”
Dr. Alexis reported financial relationships with multiple pharmaceutical companies.
[email protected]
On Twitter @karioakes
EXPERT ANALYSIS FROM THE 2017 AAD SUMMER MEETING
Cosmeceuticals and Alternative Therapies for Rosacea
What do your patients need to know?
Vascular instability associated with rosacea is exacerbated by triggers such as sunlight, hot drinks, spicy foods, stress, and rapid changing weather, which make patients flush and blush, increase the appearance of telangiectasia, and disrupt the normal skin barrier. Because the patients feel on fire, an anti-inflammatory approach is indicated. The regimen I recommend includes mild cleansers, barrier repair creams and supplements, antioxidants (topical and oral), and sun protection, all without parabens and harsh chemicals. I always recommend a product that I dispense at the office and another one of similar effectiveness that can be found over-the-counter.
What are your go-to treatments?
Cleansing is indispensable to maintain the normal flow in and out of the skin. I recommend mild cleansers without potentially sensitizing agents such as propylene glycol or parabens. It also should have calming agents (eg, fruit extracts) that remove the contaminants from the skin surface without stripping the important layers of lipids that constitute the barrier of the skin as well as ingredients (eg, prebiotics) that promote the healthy skin biome. Selenium in thermal spring water has free radical scavenging and anti-inflammatory properties as well as protection against heavy metals.
After cleansing, I recommend a product to repair, maintain, and improve the barrier of the skin. A healthy skin barrier has an equal ratio of cholesterol, ceramides, and free fatty acids, the building blocks of the skin. In a barrier repair cream I look for ingredients that stop and prevent damaging inflammation, improve the skin's natural ability to repair and heal (eg, niacinamide), and protect against environmental insults. It should contain petrolatum and/or dimethicone to form a protective barrier on the skin to seal in moisture.
Oral niacinamide should be taken as a photoprotective agent. Oral supplementation (500 mg twice daily) is effective in reducing skin cancer. Because UV light is a trigger factor, oral photoprotection is recommended.
Topical antioxidants also are important. Free radical formation has been documented even in photoprotected skin. These free radicals have been implicated in skin cancer development and metalloproteinase production and are triggers of rosacea. As a result, I advise my patients to apply topical encapsulated vitamin C every night. The encapsulated form prevents oxidation of the product before application. In addition, I recommend oral vitamin C (1 g daily) and vitamin E (400 U daily).
For sun protection I recommend sunblocks with titanium dioxide and zinc oxide for total UVA and UVB protection. If the patient has a darker skin type, sun protection should contain iron oxide. Chemical agents can cause irritation, photocontact dermatitis, and exacerbation of rosacea symptoms. Daily application of sun protection with reapplication every 2 hours is reinforced.
What holistic therapies do you recommend?
Stress reduction activities, including yoga, relaxation, massages, and meditation, can help. Oral consumption of trigger factors is discouraged. Antioxidant green tea is recommended instead of caffeinated beverages.
Suggested Readings
Baldwin HE, Bathia ND, Friedman A, et al. The role of cutaneous microbiota harmony in maintaining functional skin barrier. J Drugs Dermatol. 2017;16:12-18.
Celerier P, Richard A, Litoux P, et al. Modulatory effects of selenium and strontium salts on keratinocyte-derived inflammatory cytokines. Arch Dermatol Res. 1995;287:680-682.
Chen AC, Martin AJ, Choy B, et al. A phase 3 randomized trial of nicotinamide for skin cancer chemoprevention. N Engl J Med. 2015;373:1618-1626.
Jones D. Reactive oxygen species and rosacea. Cutis. 2004;74(suppl 3):17-20.
What do your patients need to know?
Vascular instability associated with rosacea is exacerbated by triggers such as sunlight, hot drinks, spicy foods, stress, and rapid changing weather, which make patients flush and blush, increase the appearance of telangiectasia, and disrupt the normal skin barrier. Because the patients feel on fire, an anti-inflammatory approach is indicated. The regimen I recommend includes mild cleansers, barrier repair creams and supplements, antioxidants (topical and oral), and sun protection, all without parabens and harsh chemicals. I always recommend a product that I dispense at the office and another one of similar effectiveness that can be found over-the-counter.
What are your go-to treatments?
Cleansing is indispensable to maintain the normal flow in and out of the skin. I recommend mild cleansers without potentially sensitizing agents such as propylene glycol or parabens. It also should have calming agents (eg, fruit extracts) that remove the contaminants from the skin surface without stripping the important layers of lipids that constitute the barrier of the skin as well as ingredients (eg, prebiotics) that promote the healthy skin biome. Selenium in thermal spring water has free radical scavenging and anti-inflammatory properties as well as protection against heavy metals.
After cleansing, I recommend a product to repair, maintain, and improve the barrier of the skin. A healthy skin barrier has an equal ratio of cholesterol, ceramides, and free fatty acids, the building blocks of the skin. In a barrier repair cream I look for ingredients that stop and prevent damaging inflammation, improve the skin's natural ability to repair and heal (eg, niacinamide), and protect against environmental insults. It should contain petrolatum and/or dimethicone to form a protective barrier on the skin to seal in moisture.
Oral niacinamide should be taken as a photoprotective agent. Oral supplementation (500 mg twice daily) is effective in reducing skin cancer. Because UV light is a trigger factor, oral photoprotection is recommended.
Topical antioxidants also are important. Free radical formation has been documented even in photoprotected skin. These free radicals have been implicated in skin cancer development and metalloproteinase production and are triggers of rosacea. As a result, I advise my patients to apply topical encapsulated vitamin C every night. The encapsulated form prevents oxidation of the product before application. In addition, I recommend oral vitamin C (1 g daily) and vitamin E (400 U daily).
For sun protection I recommend sunblocks with titanium dioxide and zinc oxide for total UVA and UVB protection. If the patient has a darker skin type, sun protection should contain iron oxide. Chemical agents can cause irritation, photocontact dermatitis, and exacerbation of rosacea symptoms. Daily application of sun protection with reapplication every 2 hours is reinforced.
What holistic therapies do you recommend?
Stress reduction activities, including yoga, relaxation, massages, and meditation, can help. Oral consumption of trigger factors is discouraged. Antioxidant green tea is recommended instead of caffeinated beverages.
Suggested Readings
Baldwin HE, Bathia ND, Friedman A, et al. The role of cutaneous microbiota harmony in maintaining functional skin barrier. J Drugs Dermatol. 2017;16:12-18.
Celerier P, Richard A, Litoux P, et al. Modulatory effects of selenium and strontium salts on keratinocyte-derived inflammatory cytokines. Arch Dermatol Res. 1995;287:680-682.
Chen AC, Martin AJ, Choy B, et al. A phase 3 randomized trial of nicotinamide for skin cancer chemoprevention. N Engl J Med. 2015;373:1618-1626.
Jones D. Reactive oxygen species and rosacea. Cutis. 2004;74(suppl 3):17-20.
What do your patients need to know?
Vascular instability associated with rosacea is exacerbated by triggers such as sunlight, hot drinks, spicy foods, stress, and rapid changing weather, which make patients flush and blush, increase the appearance of telangiectasia, and disrupt the normal skin barrier. Because the patients feel on fire, an anti-inflammatory approach is indicated. The regimen I recommend includes mild cleansers, barrier repair creams and supplements, antioxidants (topical and oral), and sun protection, all without parabens and harsh chemicals. I always recommend a product that I dispense at the office and another one of similar effectiveness that can be found over-the-counter.
What are your go-to treatments?
Cleansing is indispensable to maintain the normal flow in and out of the skin. I recommend mild cleansers without potentially sensitizing agents such as propylene glycol or parabens. It also should have calming agents (eg, fruit extracts) that remove the contaminants from the skin surface without stripping the important layers of lipids that constitute the barrier of the skin as well as ingredients (eg, prebiotics) that promote the healthy skin biome. Selenium in thermal spring water has free radical scavenging and anti-inflammatory properties as well as protection against heavy metals.
After cleansing, I recommend a product to repair, maintain, and improve the barrier of the skin. A healthy skin barrier has an equal ratio of cholesterol, ceramides, and free fatty acids, the building blocks of the skin. In a barrier repair cream I look for ingredients that stop and prevent damaging inflammation, improve the skin's natural ability to repair and heal (eg, niacinamide), and protect against environmental insults. It should contain petrolatum and/or dimethicone to form a protective barrier on the skin to seal in moisture.
Oral niacinamide should be taken as a photoprotective agent. Oral supplementation (500 mg twice daily) is effective in reducing skin cancer. Because UV light is a trigger factor, oral photoprotection is recommended.
Topical antioxidants also are important. Free radical formation has been documented even in photoprotected skin. These free radicals have been implicated in skin cancer development and metalloproteinase production and are triggers of rosacea. As a result, I advise my patients to apply topical encapsulated vitamin C every night. The encapsulated form prevents oxidation of the product before application. In addition, I recommend oral vitamin C (1 g daily) and vitamin E (400 U daily).
For sun protection I recommend sunblocks with titanium dioxide and zinc oxide for total UVA and UVB protection. If the patient has a darker skin type, sun protection should contain iron oxide. Chemical agents can cause irritation, photocontact dermatitis, and exacerbation of rosacea symptoms. Daily application of sun protection with reapplication every 2 hours is reinforced.
What holistic therapies do you recommend?
Stress reduction activities, including yoga, relaxation, massages, and meditation, can help. Oral consumption of trigger factors is discouraged. Antioxidant green tea is recommended instead of caffeinated beverages.
Suggested Readings
Baldwin HE, Bathia ND, Friedman A, et al. The role of cutaneous microbiota harmony in maintaining functional skin barrier. J Drugs Dermatol. 2017;16:12-18.
Celerier P, Richard A, Litoux P, et al. Modulatory effects of selenium and strontium salts on keratinocyte-derived inflammatory cytokines. Arch Dermatol Res. 1995;287:680-682.
Chen AC, Martin AJ, Choy B, et al. A phase 3 randomized trial of nicotinamide for skin cancer chemoprevention. N Engl J Med. 2015;373:1618-1626.
Jones D. Reactive oxygen species and rosacea. Cutis. 2004;74(suppl 3):17-20.
Lack of Significant Anti-inflammatory Activity With Clindamycin in the Treatment of Rosacea: Results of 2 Randomized, Vehicle-Controlled Trials
Rosacea is a chronic inflammatory skin disease characterized by central facial erythema with or without intermittent papules and pustules (described as the inflammatory lesions of rosacea). Although twice-daily clindamycin 1% solution or gel has been used in the treatment of acne, few studies have investigated the use of clindamycin in rosacea.1,2 In one study comparing twice-daily clindamycin lotion 1% with oral tetracycline in 43 rosacea patients, clindamycin was found to be superior in the eradication of pustules.3 A combination therapy of clindamycin 1% and benzoyl peroxide 5% was found to be more effective than the vehicle in inflammatory lesions and erythema of rosacea in a 12-week randomized controlled trial; however, a definitive advantage over US Food and Drug Administration-approved topical agents used to treat papulopustular rosacea was not established.4,5 Two further studies evaluated clindamycin phosphate 1.2%-tretinoin 0.025% combination gel in the treatment of rosacea, but only 1 showed any effect on papulopustular lesions.6-8 The objective of the studies reported here was to evaluate the efficacy and safety of clindamycin in the treatment of patients with moderate to severe rosacea.
Methods
Study Design
Two multicenter (study A, 20 centers; study B, 10 centers), randomized, investigator-blinded, vehicle-controlled studies were conducted in the United States between 1999 and 2002 in accordance with the Declaration of Helsinki, International Conference on Harmonisation Good Clinical Practice guidelines, and local regulatory requirements. The studies were reviewed and approved by the respective institutional review boards, and all participants provided written informed consent.
In study A, moderate to severe rosacea patients with erythema, telangiectasia, and at least 8 inflammatory lesions were randomized to receive clindamycin cream 1% or vehicle cream once (in the evening) or twice daily (in the morning and evening) or clindamycin cream 0.3% once daily (in the evening) for 12 weeks (1:1:1:1:1 ratio). All study treatments were supplied in identical tubes with blinded labels.
In study B, patients with moderate to severe rosacea and at least 8 inflammatory lesions were randomized in a 1:1 ratio with instructions to apply clindamycin gel 1% or vehicle gel to the affected areas twice daily (morning and evening) for 12 weeks.
Efficacy Evaluation
Evaluations were performed at baseline and weeks 2, 4, 8, and 12 on the intention-to-treat population with the last observation carried forward.
Efficacy assessments in both studies included inflammatory lesion counts (papules and pustules) of 5 facial regions--forehead, chin, nose, right cheek, left cheek--counted separately and then combined to give the total inflammatory lesion count (both studies), as well as improvement in the investigator global rosacea severity score (0=none/clear; 1=mild, detectable erythema with ≤7 papules/pustules; 2=moderate, prominent erythema with ≥8 papules/pustules; 3=severe, intense erythema with ≥10 to <50 papules/pustules; 3.5 [study A] or 4 [study B]=very severe, intense erythema with >50 papules/pustules). In study B, the proportion of participants dichotomized to success (a score of 0 [none/clear] or 1 [mild/almost clear]) or failure (a score of ≥2) on the 5-point investigator global rosacea severity scale at week 12 was evaluated. In study A, investigator global improvement assessment at week 12, based on photographs taken at baseline, was graded on a 7-point scale (from -1 [worse], 0 [no change], and 1 [minimal improvement] to 5 [clear]). In both studies, erythema severity was graded on a 7-point scale in increments of 0.5 (from 0=no erythema to 3.5=very severe redness, very intense redness). Skin irritation also was graded as none, mild, moderate, or severe.
Safety Evaluation
Safety was assessed by the incidence of adverse events (AEs).
Statistical Analysis
Studies were powered assuming 60% reduction in inflammatory lesion counts with active and 40% with vehicle, based on historical data from a prior study with metronidazole cream 0.75% versus vehicle; 64 participants were required in each treatment group to detect this effect using a 2-sided t test (α=.017). Pairwise comparisons (clindamycin vs respective vehicle) were performed using the Cochran-Mantel-Haenszel test for combined lesion count percentage change.
Results
Participant Disposition and Baseline Characteristics
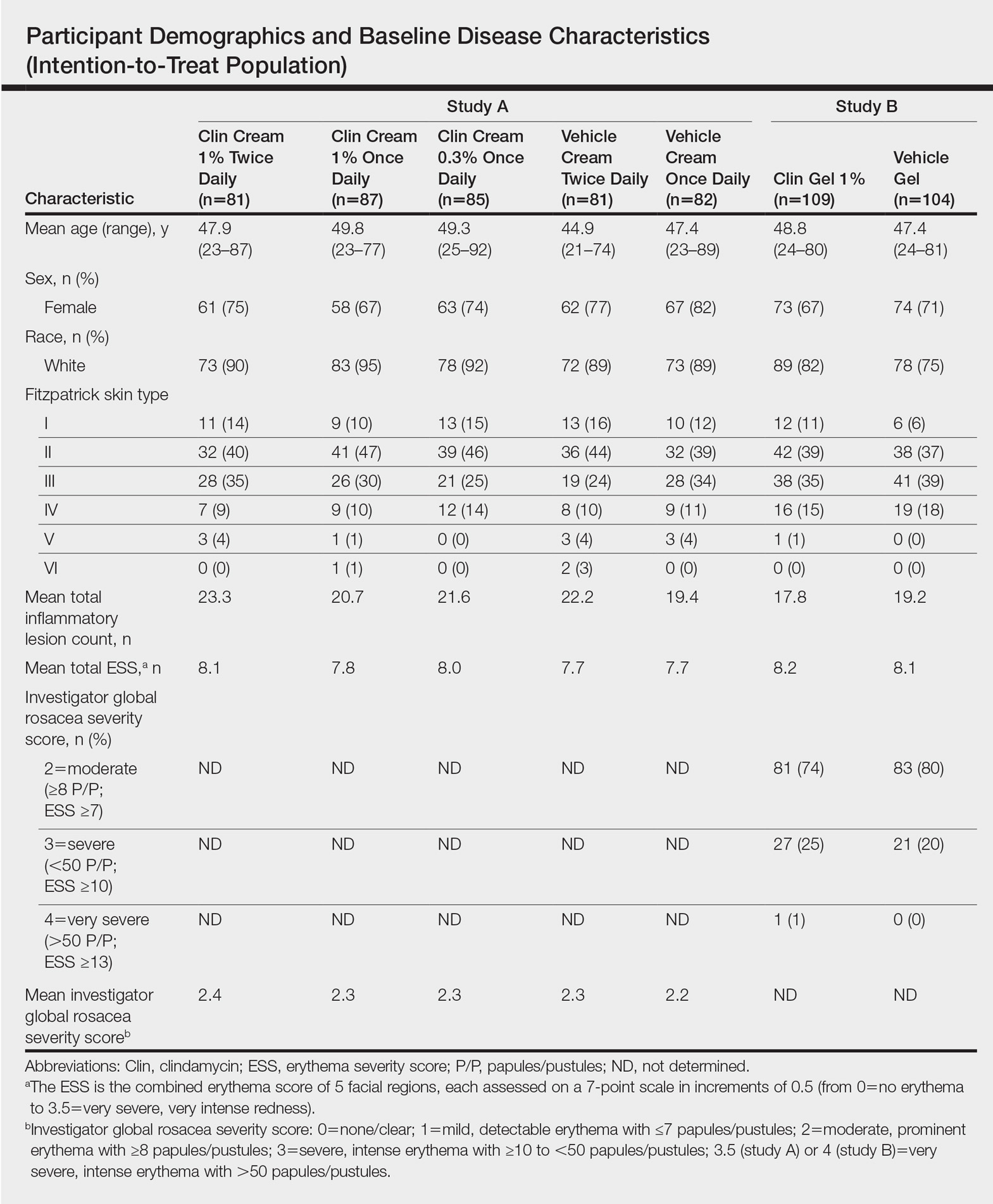
Overall, a total of 629 participants were randomized across both studies. In study A, a total of 416 participants were randomized into 5 treatment arms, with 369 participants (88.7%) completing the study; 47 (11.3%) participants discontinued study A, mainly due to participant request (19/47 [40.4%]) or lost to follow-up (11/47 [23.4%]). In study B, a total of 213 participants were randomized to receive either clindamycin gel 1% (n=109 [51.2%]) twice daily or vehicle gel (n=104 [48.8%]) twice daily, with 193 participants (90.6%) completing the study; 20 (9.4%) participants discontinued study B, mainly due to participant request (6/20 [30%]) or lost to follow-up (4/20 [20%]). Participants in studies A and B were similar in demographics and baseline disease characteristics (Table). The majority of participants were white females.
Efficacy
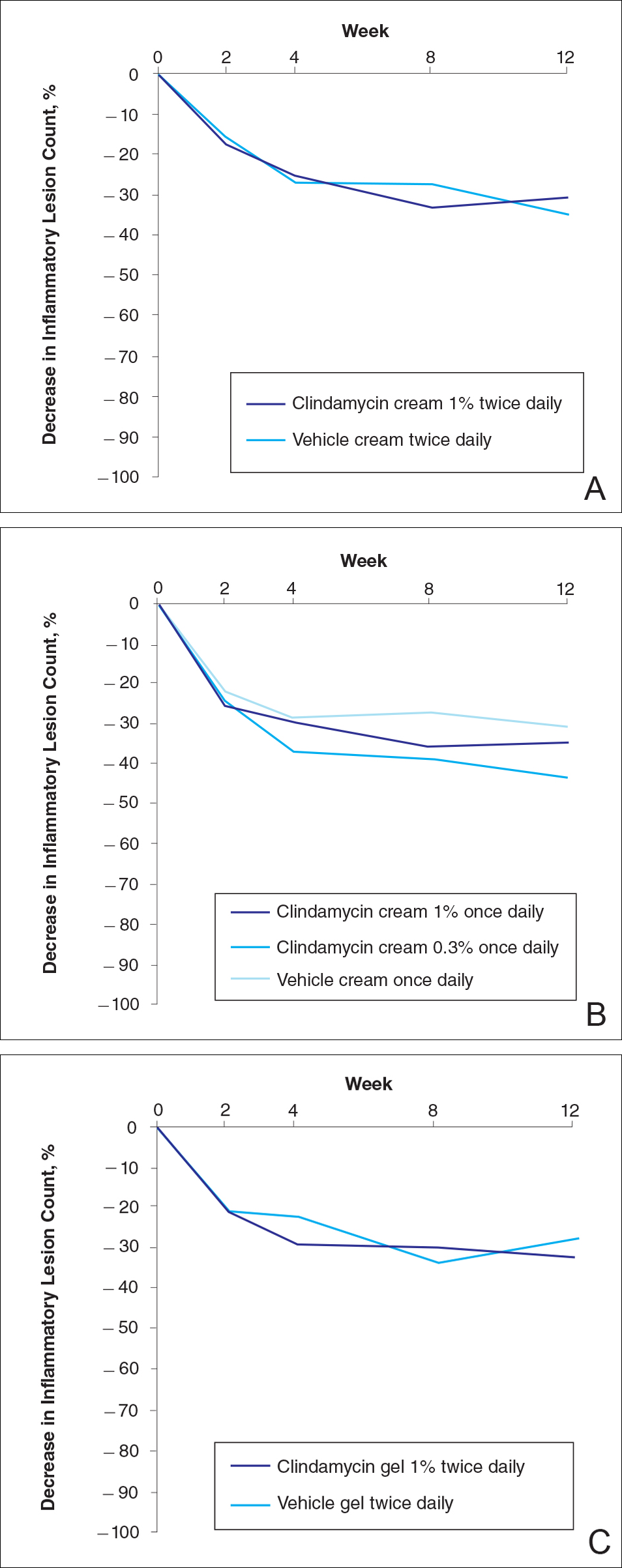
No statistically significant difference was observed in all pairwise comparisons (clindamycin cream twice daily vs vehicle twice daily, clindamycin cream once daily vs vehicle once daily, clindamycin gel vs vehicle gel) for the primary end point of mean percentage change from baseline in inflammatory lesion counts at week 12 (Figure 1; P>.5 for all pairwise comparisons).
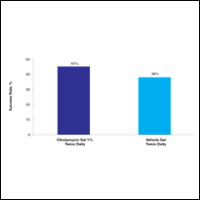
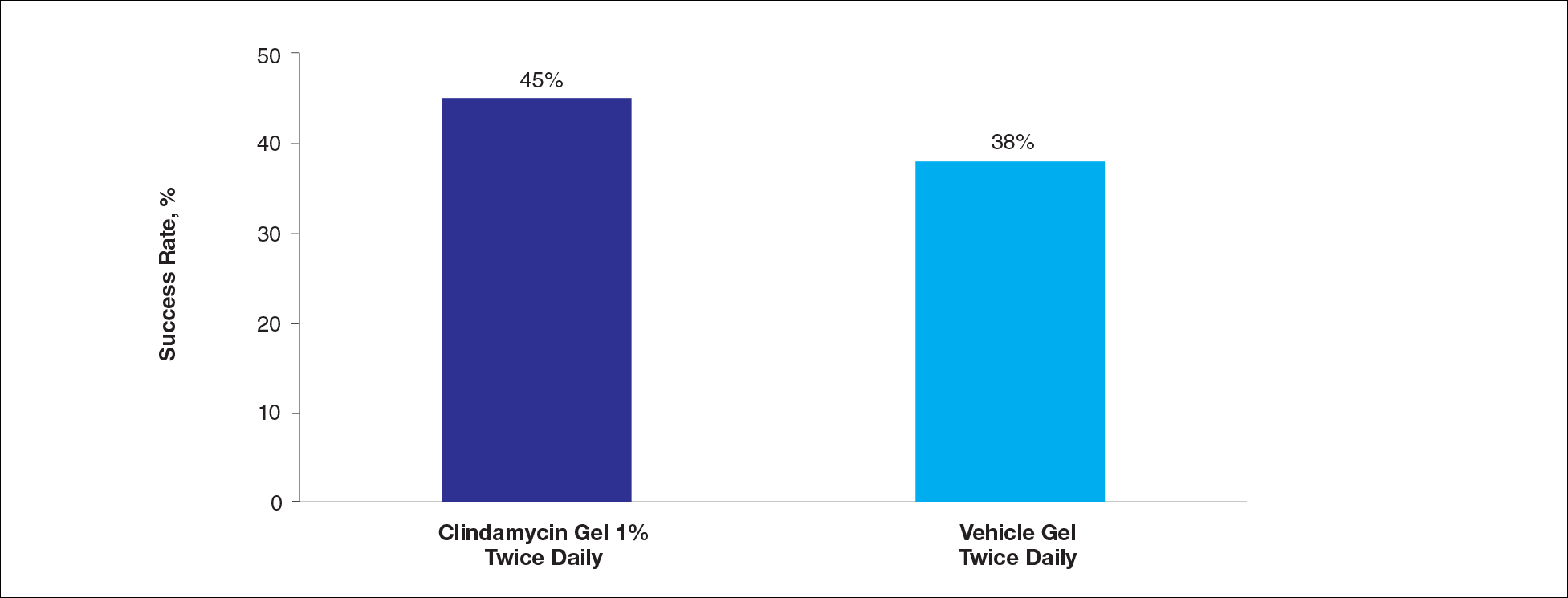
At week 12, the proportion of participants in study B deemed as a success (none/clear or mild/almost clear [investigator global rosacea severity score of 0 or 1]) in the clindamycin gel 1% and vehicle gel groups were 45% versus 38%, respectively (P=.347) (Figure 2).
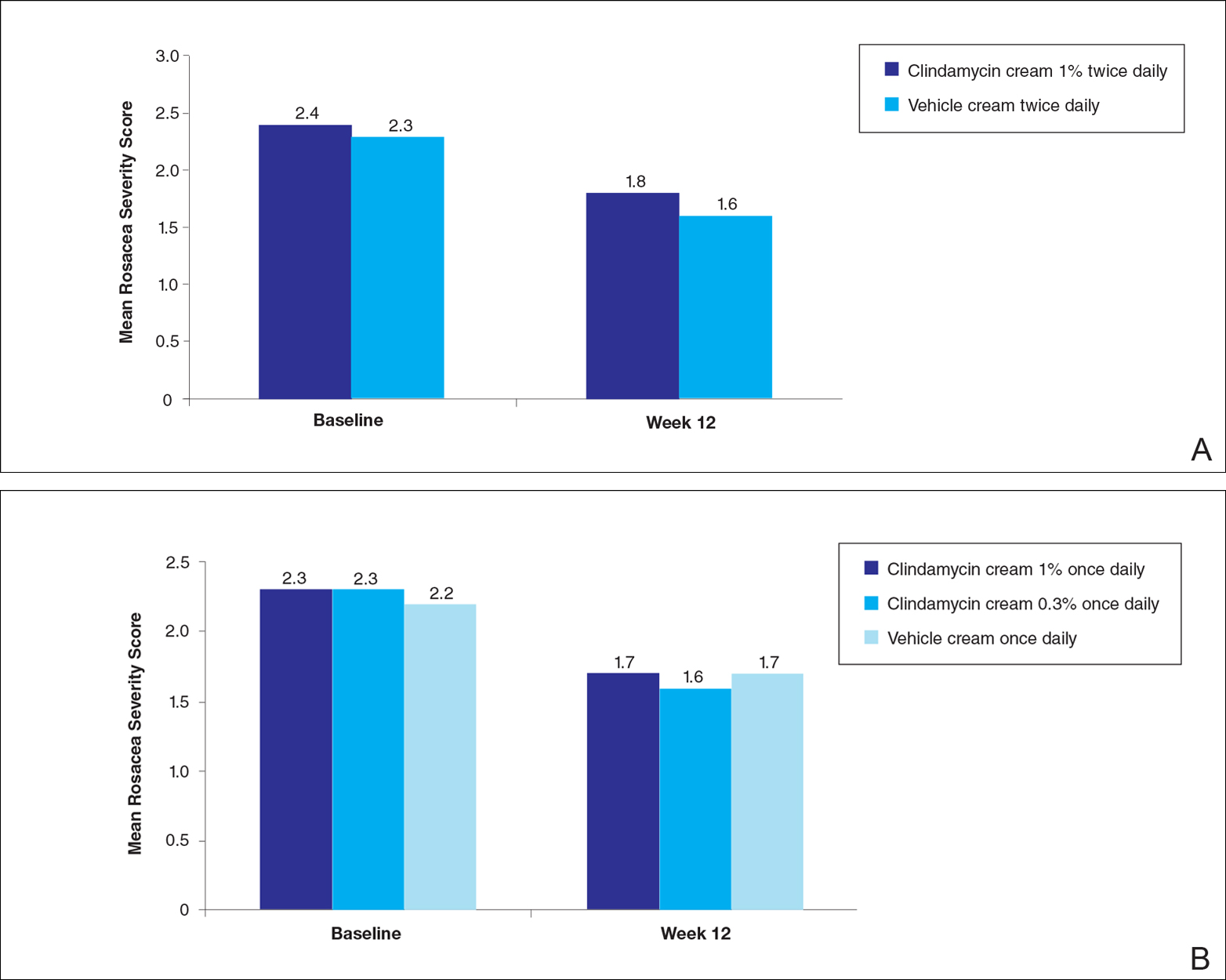
For the secondary end point of mean investigator global rosacea severity assessment at week 12 (study A), there were no significant differences between the active and vehicle control groups (P>.5 for all pairwise comparisons)(Figure 3). Also, the proportion of participants with at least a moderate investigator global improvement assessment from baseline to week 12 ranged from 45% for clindamycin cream 1% twice daily to 56% for clindamycin cream 0.3% cream once daily and from 45% for vehicle cream once daily to 51% for vehicle cream twice daily (P>.5 for all pairwise comparisons).
There were no significant differences in the mean total erythema severity scores at week 12 for clindamycin cream 1% twice daily versus vehicle cream twice daily (6.3 vs 6.0; P>.5), clindamycin cream 1% once daily versus vehicle cream once daily (6.2 vs 6.0; P>.5), clindamycin cream 0.3% once daily versus vehicle cream once daily (5.9 vs 6.0; P>.5), and clindamycin gel 1% twice daily versus vehicle gel twice daily (6.7 vs 6.2; P>.5).
There were no relevant differences between any of the clindamycin cream groups and their respective vehicle group at week 12 for skin irritation, including desquamation, edema, dryness, pruritus, and stinging/burning.
Safety
In study A, the majority of AEs in all 5 treatment arms were nondermatologic, mild in intensity, and not considered to be related to the study treatment by the investigator. Overall, 12 participants had AEs considered by the investigator as possibly or probably related to the study treatment: 4.9% in the clindamycin cream 1% twice daily group, 4.6% in the clindamycin cream 1% once daily group, 3.7% in the vehicle cream twice daily group, 1.2% in the clindamycin cream 0.3% once daily group, and 0% in the vehicle cream once daily group. Two treatment-related AEs led to treatment discontinuation, including dermatitis in 1 participant from the clindamycin cream 1% once daily group and contact dermatitis in 1 participant from the clindamycin cream 1% twice daily group.
Comment
No evidence of increased efficacy over the respective vehicles was observed with clindamycin cream or gel, whatever the regimen, in the treatment of rosacea patients in either of these well-designed and well-powered, blinded studies. Slight improvements in the various efficacy criteria were observed, even in the vehicle groups, highlighting the importance of using a good basic skin care regimen in the management of rosacea.9 In contrast to our observations of lack of efficacy in the treatment of rosacea, clinical efficacy of clindamycin has been demonstrated in acne,10-12 albeit with low efficacy for clindamycin monotherapy.13 It is noteworthy that oral or topical antibiotics are no longer recommended as monotherapy for acne to prevent and minimize antibiotic resistance and to preserve the therapeutic value of antibiotics.14
Acne and rosacea are both chronic inflammatory disorders of the skin associated with papules and pustules, and they share some common inflammatory patterns.15-19 Furthermore, the intrinsic anti-inflammatory activity of clindamycin in addition to its antibiotic effects has been suggested by some authors as the main reason for treating acne with clindamycin.20 However, the relative contributions of antibacterial and/or anti-inflammatory properties remain to be fully elucidated, and evidence for direct anti-inflammatory effects of clindamycin remains heterogeneous.21,22 Several pathophysiological factors have been implicated in acne, including hormonal effects, abnormal keratinocyte function, increased sebum production, and microbial components (eg, hypercolonization of the skin follicles by Propionibacterium acnes).23,24 The antibiotic activity of clindamycin against P acnes may be the key factor responsible for the clinical effects in acne.25,26 Although clindamycin may have anti-inflammatory effects in acne via a different inflammatory pathway not shared by rosacea, a purely antibiotic mechanism of action of clindamycin also could explain why we observed no evidence of efficacy in the treatment of rosacea, as no causative bacterial component has been clearly demonstrated in rosacea.27
Conclusion
In these studies, clindamycin cream 0.3% once daily, clindamycin cream 1% once or twice daily, and clindamycin gel 1% twice daily were all well tolerated; however, they were no more effective than the vehicles in the treatment of moderate to severe rosacea.
Acknowledgment
The authors would like to thank Helen Simpson, PhD, of Galderma R&D (Sophia Antipolis, France), for editorial and medical writing assistance.
- Whitney KM, Ditre CM. Anti-inflammatory properties of clindamycin: a review of its use in the treatment of acne vulgaris. Clinical Medicine Insights: Dermatology. 2011;4:27-41.
- Mays RM, Gordon RA, Wilson JM, et al. New antibiotic therapies for acne and rosacea. Dermatol Ther. 2012;25:23-37.
- Wilkin JK, DeWitt S. Treatment of rosacea: topical clindamycin versus oral tetracycline. Int J Dermatol. 1993;32:65-67.
- Breneman D, Savin R, VandePol C, et al. Double-blind, randomized, vehicle-controlled clinical trial of once-daily benzoyl peroxide/clindamycin topical gel in the treatment of patients with moderate to severe rosacea. Int J Dermatol. 2004;43:381-387.
- Leyden JJ, Thiboutot D, Shalita A. Photographic review of results from a clinical study comparing benzoyl peroxide 5%/clindamycin 1% topical gel with vehicle in the treatment of rosacea. Cutis. 2004;73(6 suppl):11-17.
- Chang AL, Alora-Palli M, Lima XT, et al. A randomized, double-blind, placebo-controlled, pilot study to assess the efficacy and safety of clindamycin 1.2% and tretinoin 0.025% combination gel for the treatment of acne rosacea over 12 weeks. J Drugs Dermatol. 2012;11:333-339.
- Freeman SA, Moon SD, Spencer JM. Clindamycin phosphate 1.2% and tretinoin 0.025% gel for rosacea: summary of a placebo-controlled, double-blind trial. J Drugs Dermatol. 2012;11:1410-1414.
- van Zuuren EJ, Fedorowicz Z, Carter B, et al. Interventions for rosacea. Cochrane Database Syst Rev. 2015;4:CD003262.
- Laquieze S, Czernielewski J, Baltas E. Beneficial use of Cetaphil moisturizing cream as part of a daily skin care regimen for individuals with rosacea. J Dermatolog Treat. 2007;18:158-162.
- Lookingbill DP, Chalker DK, Lindholm JS, et al. Treatment of acne with a combination clindamycin/benzoyl peroxide gel compared with clindamycin gel, benzoyl peroxide gel and vehicle gel: combined results of two double-blind investigations. J Am Acad Dermatol. 1997;37:590-595.
- Alirezaï M, Gerlach B, Horvath A, et al. Results of a randomised, multicentre study comparing a new water-based gel of clindamycin 1% versus clindamycin 1% topical solution in the treatment of acne vulgaris. Eur J Dermatol. 2005;15:274-278.
- Jarratt MT, Brundage T. Efficacy and safety of clindamycin-tretinoin gel versus clindamycin or tretinoin alone in acne vulgaris: a randomized, double-blind, vehicle-controlled study. J Drugs Dermatol. 2012;11:318-326.
- Benzaclin. Med Library website. http://medlibrary.org/lib/rx/meds/benzaclin-3. Updated May 8, 2013. Accessed January 24, 2017.
- Walsh TR, Efthimiou J, Dréno B. Systematic review of antibiotic resistance in acne: an increasing topical and oral threat. Lancet Infect Dis. 2016;16:E23-E33.
- Jeremy AH, Holland DB, Roberts SG, et al. Inflammatory events are involved in acne lesion initiation. J Invest Dermatol. 2003;121:20-27.
- Kircik LH. Re-evaluating treatment targets in acne vulgaris: adapting to a new understanding of pathophysiology. J Drugs Dermatol. 2014;13:S57-S60.
- Salzer S, Kresse S, Hirai Y, et al. Cathelicidin peptide LL-37 increases UVB-triggered inflammasome activation: possible implications for rosacea. J Dermatol Sci. 2014;76:173-179.
- Buhl T, Sulk M, Nowak P, et al. Molecular and morphological characterization of inflammatory infiltrate in rosacea reveals activation of Th1/Th17 pathways. J Invest Dermatol. 2015;135:2198-2208.
- Kistowska M, Meier B, Proust T, et al. Propionibacterium acnes promotes Th17 and Th17/Th1 responses in acne patients. J Invest Dermatol. 2015;135:110-118.
- Zeichner JA. Inflammatory acne treatment: review of current and new topical therapeutic options. J Drugs Dermatol. 2016;15(1 suppl 1):S11-S16.
- Nakano T, Hiramatsu K, Kishi K, et al. Clindamycin modulates inflammatory-cytokine induction in lipopolysaccharide-stimulated mouse peritoneal macrophages. Antimicrob Agents Chemother. 2003;47:363-367.
- Orman KL, English BK. Effects of antibiotic class on the macrophage inflammatory response to Streptococcus pneumoniae. J Infect Dis. 2000;182:1561-1565.
- Taylor M, Gonzalez M, Porter R. Pathways to inflammation: acne pathophysiology. Eur J Dermatol. 2011;21:323-333.
- Del Rosso JQ, Kircik LH. The sequence of inflammation, relevant biomarkers, and the pathogenesis of acne vulgaris: what does recent research show and what does it mean to the clinician? J Drugs Dermatol. 2013;12(8 suppl):S109-S115.
- Leyden J, Kaidbey K, Levy SF. The combination formulation of clindamycin 1% plus benzoyl peroxide 5% versus 3 different formulations of topical clindamycin alone in the reduction of Propionibacterium acnes. an in vivo comparative study. Am J Clin Dermatol. 2001;2:263-266.
- Wang WL, Everett ED, Johnson M, et al. Susceptibility of Propionibacterium acnes to seventeen antibiotics. Antimicrob Agents Chemother. 1977;11:171-173.
- Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013;69(6 suppl 1):S15-S26.
Rosacea is a chronic inflammatory skin disease characterized by central facial erythema with or without intermittent papules and pustules (described as the inflammatory lesions of rosacea). Although twice-daily clindamycin 1% solution or gel has been used in the treatment of acne, few studies have investigated the use of clindamycin in rosacea.1,2 In one study comparing twice-daily clindamycin lotion 1% with oral tetracycline in 43 rosacea patients, clindamycin was found to be superior in the eradication of pustules.3 A combination therapy of clindamycin 1% and benzoyl peroxide 5% was found to be more effective than the vehicle in inflammatory lesions and erythema of rosacea in a 12-week randomized controlled trial; however, a definitive advantage over US Food and Drug Administration-approved topical agents used to treat papulopustular rosacea was not established.4,5 Two further studies evaluated clindamycin phosphate 1.2%-tretinoin 0.025% combination gel in the treatment of rosacea, but only 1 showed any effect on papulopustular lesions.6-8 The objective of the studies reported here was to evaluate the efficacy and safety of clindamycin in the treatment of patients with moderate to severe rosacea.
Methods
Study Design
Two multicenter (study A, 20 centers; study B, 10 centers), randomized, investigator-blinded, vehicle-controlled studies were conducted in the United States between 1999 and 2002 in accordance with the Declaration of Helsinki, International Conference on Harmonisation Good Clinical Practice guidelines, and local regulatory requirements. The studies were reviewed and approved by the respective institutional review boards, and all participants provided written informed consent.
In study A, moderate to severe rosacea patients with erythema, telangiectasia, and at least 8 inflammatory lesions were randomized to receive clindamycin cream 1% or vehicle cream once (in the evening) or twice daily (in the morning and evening) or clindamycin cream 0.3% once daily (in the evening) for 12 weeks (1:1:1:1:1 ratio). All study treatments were supplied in identical tubes with blinded labels.
In study B, patients with moderate to severe rosacea and at least 8 inflammatory lesions were randomized in a 1:1 ratio with instructions to apply clindamycin gel 1% or vehicle gel to the affected areas twice daily (morning and evening) for 12 weeks.
Efficacy Evaluation
Evaluations were performed at baseline and weeks 2, 4, 8, and 12 on the intention-to-treat population with the last observation carried forward.
Efficacy assessments in both studies included inflammatory lesion counts (papules and pustules) of 5 facial regions--forehead, chin, nose, right cheek, left cheek--counted separately and then combined to give the total inflammatory lesion count (both studies), as well as improvement in the investigator global rosacea severity score (0=none/clear; 1=mild, detectable erythema with ≤7 papules/pustules; 2=moderate, prominent erythema with ≥8 papules/pustules; 3=severe, intense erythema with ≥10 to <50 papules/pustules; 3.5 [study A] or 4 [study B]=very severe, intense erythema with >50 papules/pustules). In study B, the proportion of participants dichotomized to success (a score of 0 [none/clear] or 1 [mild/almost clear]) or failure (a score of ≥2) on the 5-point investigator global rosacea severity scale at week 12 was evaluated. In study A, investigator global improvement assessment at week 12, based on photographs taken at baseline, was graded on a 7-point scale (from -1 [worse], 0 [no change], and 1 [minimal improvement] to 5 [clear]). In both studies, erythema severity was graded on a 7-point scale in increments of 0.5 (from 0=no erythema to 3.5=very severe redness, very intense redness). Skin irritation also was graded as none, mild, moderate, or severe.
Safety Evaluation
Safety was assessed by the incidence of adverse events (AEs).
Statistical Analysis
Studies were powered assuming 60% reduction in inflammatory lesion counts with active and 40% with vehicle, based on historical data from a prior study with metronidazole cream 0.75% versus vehicle; 64 participants were required in each treatment group to detect this effect using a 2-sided t test (α=.017). Pairwise comparisons (clindamycin vs respective vehicle) were performed using the Cochran-Mantel-Haenszel test for combined lesion count percentage change.
Results
Participant Disposition and Baseline Characteristics
Overall, a total of 629 participants were randomized across both studies. In study A, a total of 416 participants were randomized into 5 treatment arms, with 369 participants (88.7%) completing the study; 47 (11.3%) participants discontinued study A, mainly due to participant request (19/47 [40.4%]) or lost to follow-up (11/47 [23.4%]). In study B, a total of 213 participants were randomized to receive either clindamycin gel 1% (n=109 [51.2%]) twice daily or vehicle gel (n=104 [48.8%]) twice daily, with 193 participants (90.6%) completing the study; 20 (9.4%) participants discontinued study B, mainly due to participant request (6/20 [30%]) or lost to follow-up (4/20 [20%]). Participants in studies A and B were similar in demographics and baseline disease characteristics (Table). The majority of participants were white females.

Efficacy
No statistically significant difference was observed in all pairwise comparisons (clindamycin cream twice daily vs vehicle twice daily, clindamycin cream once daily vs vehicle once daily, clindamycin gel vs vehicle gel) for the primary end point of mean percentage change from baseline in inflammatory lesion counts at week 12 (Figure 1; P>.5 for all pairwise comparisons).

At week 12, the proportion of participants in study B deemed as a success (none/clear or mild/almost clear [investigator global rosacea severity score of 0 or 1]) in the clindamycin gel 1% and vehicle gel groups were 45% versus 38%, respectively (P=.347) (Figure 2).

For the secondary end point of mean investigator global rosacea severity assessment at week 12 (study A), there were no significant differences between the active and vehicle control groups (P>.5 for all pairwise comparisons)(Figure 3). Also, the proportion of participants with at least a moderate investigator global improvement assessment from baseline to week 12 ranged from 45% for clindamycin cream 1% twice daily to 56% for clindamycin cream 0.3% cream once daily and from 45% for vehicle cream once daily to 51% for vehicle cream twice daily (P>.5 for all pairwise comparisons).

There were no significant differences in the mean total erythema severity scores at week 12 for clindamycin cream 1% twice daily versus vehicle cream twice daily (6.3 vs 6.0; P>.5), clindamycin cream 1% once daily versus vehicle cream once daily (6.2 vs 6.0; P>.5), clindamycin cream 0.3% once daily versus vehicle cream once daily (5.9 vs 6.0; P>.5), and clindamycin gel 1% twice daily versus vehicle gel twice daily (6.7 vs 6.2; P>.5).
There were no relevant differences between any of the clindamycin cream groups and their respective vehicle group at week 12 for skin irritation, including desquamation, edema, dryness, pruritus, and stinging/burning.
Safety
In study A, the majority of AEs in all 5 treatment arms were nondermatologic, mild in intensity, and not considered to be related to the study treatment by the investigator. Overall, 12 participants had AEs considered by the investigator as possibly or probably related to the study treatment: 4.9% in the clindamycin cream 1% twice daily group, 4.6% in the clindamycin cream 1% once daily group, 3.7% in the vehicle cream twice daily group, 1.2% in the clindamycin cream 0.3% once daily group, and 0% in the vehicle cream once daily group. Two treatment-related AEs led to treatment discontinuation, including dermatitis in 1 participant from the clindamycin cream 1% once daily group and contact dermatitis in 1 participant from the clindamycin cream 1% twice daily group.
Comment
No evidence of increased efficacy over the respective vehicles was observed with clindamycin cream or gel, whatever the regimen, in the treatment of rosacea patients in either of these well-designed and well-powered, blinded studies. Slight improvements in the various efficacy criteria were observed, even in the vehicle groups, highlighting the importance of using a good basic skin care regimen in the management of rosacea.9 In contrast to our observations of lack of efficacy in the treatment of rosacea, clinical efficacy of clindamycin has been demonstrated in acne,10-12 albeit with low efficacy for clindamycin monotherapy.13 It is noteworthy that oral or topical antibiotics are no longer recommended as monotherapy for acne to prevent and minimize antibiotic resistance and to preserve the therapeutic value of antibiotics.14
Acne and rosacea are both chronic inflammatory disorders of the skin associated with papules and pustules, and they share some common inflammatory patterns.15-19 Furthermore, the intrinsic anti-inflammatory activity of clindamycin in addition to its antibiotic effects has been suggested by some authors as the main reason for treating acne with clindamycin.20 However, the relative contributions of antibacterial and/or anti-inflammatory properties remain to be fully elucidated, and evidence for direct anti-inflammatory effects of clindamycin remains heterogeneous.21,22 Several pathophysiological factors have been implicated in acne, including hormonal effects, abnormal keratinocyte function, increased sebum production, and microbial components (eg, hypercolonization of the skin follicles by Propionibacterium acnes).23,24 The antibiotic activity of clindamycin against P acnes may be the key factor responsible for the clinical effects in acne.25,26 Although clindamycin may have anti-inflammatory effects in acne via a different inflammatory pathway not shared by rosacea, a purely antibiotic mechanism of action of clindamycin also could explain why we observed no evidence of efficacy in the treatment of rosacea, as no causative bacterial component has been clearly demonstrated in rosacea.27
Conclusion
In these studies, clindamycin cream 0.3% once daily, clindamycin cream 1% once or twice daily, and clindamycin gel 1% twice daily were all well tolerated; however, they were no more effective than the vehicles in the treatment of moderate to severe rosacea.
Acknowledgment
The authors would like to thank Helen Simpson, PhD, of Galderma R&D (Sophia Antipolis, France), for editorial and medical writing assistance.
Rosacea is a chronic inflammatory skin disease characterized by central facial erythema with or without intermittent papules and pustules (described as the inflammatory lesions of rosacea). Although twice-daily clindamycin 1% solution or gel has been used in the treatment of acne, few studies have investigated the use of clindamycin in rosacea.1,2 In one study comparing twice-daily clindamycin lotion 1% with oral tetracycline in 43 rosacea patients, clindamycin was found to be superior in the eradication of pustules.3 A combination therapy of clindamycin 1% and benzoyl peroxide 5% was found to be more effective than the vehicle in inflammatory lesions and erythema of rosacea in a 12-week randomized controlled trial; however, a definitive advantage over US Food and Drug Administration-approved topical agents used to treat papulopustular rosacea was not established.4,5 Two further studies evaluated clindamycin phosphate 1.2%-tretinoin 0.025% combination gel in the treatment of rosacea, but only 1 showed any effect on papulopustular lesions.6-8 The objective of the studies reported here was to evaluate the efficacy and safety of clindamycin in the treatment of patients with moderate to severe rosacea.
Methods
Study Design
Two multicenter (study A, 20 centers; study B, 10 centers), randomized, investigator-blinded, vehicle-controlled studies were conducted in the United States between 1999 and 2002 in accordance with the Declaration of Helsinki, International Conference on Harmonisation Good Clinical Practice guidelines, and local regulatory requirements. The studies were reviewed and approved by the respective institutional review boards, and all participants provided written informed consent.
In study A, moderate to severe rosacea patients with erythema, telangiectasia, and at least 8 inflammatory lesions were randomized to receive clindamycin cream 1% or vehicle cream once (in the evening) or twice daily (in the morning and evening) or clindamycin cream 0.3% once daily (in the evening) for 12 weeks (1:1:1:1:1 ratio). All study treatments were supplied in identical tubes with blinded labels.
In study B, patients with moderate to severe rosacea and at least 8 inflammatory lesions were randomized in a 1:1 ratio with instructions to apply clindamycin gel 1% or vehicle gel to the affected areas twice daily (morning and evening) for 12 weeks.
Efficacy Evaluation
Evaluations were performed at baseline and weeks 2, 4, 8, and 12 on the intention-to-treat population with the last observation carried forward.
Efficacy assessments in both studies included inflammatory lesion counts (papules and pustules) of 5 facial regions--forehead, chin, nose, right cheek, left cheek--counted separately and then combined to give the total inflammatory lesion count (both studies), as well as improvement in the investigator global rosacea severity score (0=none/clear; 1=mild, detectable erythema with ≤7 papules/pustules; 2=moderate, prominent erythema with ≥8 papules/pustules; 3=severe, intense erythema with ≥10 to <50 papules/pustules; 3.5 [study A] or 4 [study B]=very severe, intense erythema with >50 papules/pustules). In study B, the proportion of participants dichotomized to success (a score of 0 [none/clear] or 1 [mild/almost clear]) or failure (a score of ≥2) on the 5-point investigator global rosacea severity scale at week 12 was evaluated. In study A, investigator global improvement assessment at week 12, based on photographs taken at baseline, was graded on a 7-point scale (from -1 [worse], 0 [no change], and 1 [minimal improvement] to 5 [clear]). In both studies, erythema severity was graded on a 7-point scale in increments of 0.5 (from 0=no erythema to 3.5=very severe redness, very intense redness). Skin irritation also was graded as none, mild, moderate, or severe.
Safety Evaluation
Safety was assessed by the incidence of adverse events (AEs).
Statistical Analysis
Studies were powered assuming 60% reduction in inflammatory lesion counts with active and 40% with vehicle, based on historical data from a prior study with metronidazole cream 0.75% versus vehicle; 64 participants were required in each treatment group to detect this effect using a 2-sided t test (α=.017). Pairwise comparisons (clindamycin vs respective vehicle) were performed using the Cochran-Mantel-Haenszel test for combined lesion count percentage change.
Results
Participant Disposition and Baseline Characteristics
Overall, a total of 629 participants were randomized across both studies. In study A, a total of 416 participants were randomized into 5 treatment arms, with 369 participants (88.7%) completing the study; 47 (11.3%) participants discontinued study A, mainly due to participant request (19/47 [40.4%]) or lost to follow-up (11/47 [23.4%]). In study B, a total of 213 participants were randomized to receive either clindamycin gel 1% (n=109 [51.2%]) twice daily or vehicle gel (n=104 [48.8%]) twice daily, with 193 participants (90.6%) completing the study; 20 (9.4%) participants discontinued study B, mainly due to participant request (6/20 [30%]) or lost to follow-up (4/20 [20%]). Participants in studies A and B were similar in demographics and baseline disease characteristics (Table). The majority of participants were white females.

Efficacy
No statistically significant difference was observed in all pairwise comparisons (clindamycin cream twice daily vs vehicle twice daily, clindamycin cream once daily vs vehicle once daily, clindamycin gel vs vehicle gel) for the primary end point of mean percentage change from baseline in inflammatory lesion counts at week 12 (Figure 1; P>.5 for all pairwise comparisons).

At week 12, the proportion of participants in study B deemed as a success (none/clear or mild/almost clear [investigator global rosacea severity score of 0 or 1]) in the clindamycin gel 1% and vehicle gel groups were 45% versus 38%, respectively (P=.347) (Figure 2).

For the secondary end point of mean investigator global rosacea severity assessment at week 12 (study A), there were no significant differences between the active and vehicle control groups (P>.5 for all pairwise comparisons)(Figure 3). Also, the proportion of participants with at least a moderate investigator global improvement assessment from baseline to week 12 ranged from 45% for clindamycin cream 1% twice daily to 56% for clindamycin cream 0.3% cream once daily and from 45% for vehicle cream once daily to 51% for vehicle cream twice daily (P>.5 for all pairwise comparisons).

There were no significant differences in the mean total erythema severity scores at week 12 for clindamycin cream 1% twice daily versus vehicle cream twice daily (6.3 vs 6.0; P>.5), clindamycin cream 1% once daily versus vehicle cream once daily (6.2 vs 6.0; P>.5), clindamycin cream 0.3% once daily versus vehicle cream once daily (5.9 vs 6.0; P>.5), and clindamycin gel 1% twice daily versus vehicle gel twice daily (6.7 vs 6.2; P>.5).
There were no relevant differences between any of the clindamycin cream groups and their respective vehicle group at week 12 for skin irritation, including desquamation, edema, dryness, pruritus, and stinging/burning.
Safety
In study A, the majority of AEs in all 5 treatment arms were nondermatologic, mild in intensity, and not considered to be related to the study treatment by the investigator. Overall, 12 participants had AEs considered by the investigator as possibly or probably related to the study treatment: 4.9% in the clindamycin cream 1% twice daily group, 4.6% in the clindamycin cream 1% once daily group, 3.7% in the vehicle cream twice daily group, 1.2% in the clindamycin cream 0.3% once daily group, and 0% in the vehicle cream once daily group. Two treatment-related AEs led to treatment discontinuation, including dermatitis in 1 participant from the clindamycin cream 1% once daily group and contact dermatitis in 1 participant from the clindamycin cream 1% twice daily group.
Comment
No evidence of increased efficacy over the respective vehicles was observed with clindamycin cream or gel, whatever the regimen, in the treatment of rosacea patients in either of these well-designed and well-powered, blinded studies. Slight improvements in the various efficacy criteria were observed, even in the vehicle groups, highlighting the importance of using a good basic skin care regimen in the management of rosacea.9 In contrast to our observations of lack of efficacy in the treatment of rosacea, clinical efficacy of clindamycin has been demonstrated in acne,10-12 albeit with low efficacy for clindamycin monotherapy.13 It is noteworthy that oral or topical antibiotics are no longer recommended as monotherapy for acne to prevent and minimize antibiotic resistance and to preserve the therapeutic value of antibiotics.14
Acne and rosacea are both chronic inflammatory disorders of the skin associated with papules and pustules, and they share some common inflammatory patterns.15-19 Furthermore, the intrinsic anti-inflammatory activity of clindamycin in addition to its antibiotic effects has been suggested by some authors as the main reason for treating acne with clindamycin.20 However, the relative contributions of antibacterial and/or anti-inflammatory properties remain to be fully elucidated, and evidence for direct anti-inflammatory effects of clindamycin remains heterogeneous.21,22 Several pathophysiological factors have been implicated in acne, including hormonal effects, abnormal keratinocyte function, increased sebum production, and microbial components (eg, hypercolonization of the skin follicles by Propionibacterium acnes).23,24 The antibiotic activity of clindamycin against P acnes may be the key factor responsible for the clinical effects in acne.25,26 Although clindamycin may have anti-inflammatory effects in acne via a different inflammatory pathway not shared by rosacea, a purely antibiotic mechanism of action of clindamycin also could explain why we observed no evidence of efficacy in the treatment of rosacea, as no causative bacterial component has been clearly demonstrated in rosacea.27
Conclusion
In these studies, clindamycin cream 0.3% once daily, clindamycin cream 1% once or twice daily, and clindamycin gel 1% twice daily were all well tolerated; however, they were no more effective than the vehicles in the treatment of moderate to severe rosacea.
Acknowledgment
The authors would like to thank Helen Simpson, PhD, of Galderma R&D (Sophia Antipolis, France), for editorial and medical writing assistance.
- Whitney KM, Ditre CM. Anti-inflammatory properties of clindamycin: a review of its use in the treatment of acne vulgaris. Clinical Medicine Insights: Dermatology. 2011;4:27-41.
- Mays RM, Gordon RA, Wilson JM, et al. New antibiotic therapies for acne and rosacea. Dermatol Ther. 2012;25:23-37.
- Wilkin JK, DeWitt S. Treatment of rosacea: topical clindamycin versus oral tetracycline. Int J Dermatol. 1993;32:65-67.
- Breneman D, Savin R, VandePol C, et al. Double-blind, randomized, vehicle-controlled clinical trial of once-daily benzoyl peroxide/clindamycin topical gel in the treatment of patients with moderate to severe rosacea. Int J Dermatol. 2004;43:381-387.
- Leyden JJ, Thiboutot D, Shalita A. Photographic review of results from a clinical study comparing benzoyl peroxide 5%/clindamycin 1% topical gel with vehicle in the treatment of rosacea. Cutis. 2004;73(6 suppl):11-17.
- Chang AL, Alora-Palli M, Lima XT, et al. A randomized, double-blind, placebo-controlled, pilot study to assess the efficacy and safety of clindamycin 1.2% and tretinoin 0.025% combination gel for the treatment of acne rosacea over 12 weeks. J Drugs Dermatol. 2012;11:333-339.
- Freeman SA, Moon SD, Spencer JM. Clindamycin phosphate 1.2% and tretinoin 0.025% gel for rosacea: summary of a placebo-controlled, double-blind trial. J Drugs Dermatol. 2012;11:1410-1414.
- van Zuuren EJ, Fedorowicz Z, Carter B, et al. Interventions for rosacea. Cochrane Database Syst Rev. 2015;4:CD003262.
- Laquieze S, Czernielewski J, Baltas E. Beneficial use of Cetaphil moisturizing cream as part of a daily skin care regimen for individuals with rosacea. J Dermatolog Treat. 2007;18:158-162.
- Lookingbill DP, Chalker DK, Lindholm JS, et al. Treatment of acne with a combination clindamycin/benzoyl peroxide gel compared with clindamycin gel, benzoyl peroxide gel and vehicle gel: combined results of two double-blind investigations. J Am Acad Dermatol. 1997;37:590-595.
- Alirezaï M, Gerlach B, Horvath A, et al. Results of a randomised, multicentre study comparing a new water-based gel of clindamycin 1% versus clindamycin 1% topical solution in the treatment of acne vulgaris. Eur J Dermatol. 2005;15:274-278.
- Jarratt MT, Brundage T. Efficacy and safety of clindamycin-tretinoin gel versus clindamycin or tretinoin alone in acne vulgaris: a randomized, double-blind, vehicle-controlled study. J Drugs Dermatol. 2012;11:318-326.
- Benzaclin. Med Library website. http://medlibrary.org/lib/rx/meds/benzaclin-3. Updated May 8, 2013. Accessed January 24, 2017.
- Walsh TR, Efthimiou J, Dréno B. Systematic review of antibiotic resistance in acne: an increasing topical and oral threat. Lancet Infect Dis. 2016;16:E23-E33.
- Jeremy AH, Holland DB, Roberts SG, et al. Inflammatory events are involved in acne lesion initiation. J Invest Dermatol. 2003;121:20-27.
- Kircik LH. Re-evaluating treatment targets in acne vulgaris: adapting to a new understanding of pathophysiology. J Drugs Dermatol. 2014;13:S57-S60.
- Salzer S, Kresse S, Hirai Y, et al. Cathelicidin peptide LL-37 increases UVB-triggered inflammasome activation: possible implications for rosacea. J Dermatol Sci. 2014;76:173-179.
- Buhl T, Sulk M, Nowak P, et al. Molecular and morphological characterization of inflammatory infiltrate in rosacea reveals activation of Th1/Th17 pathways. J Invest Dermatol. 2015;135:2198-2208.
- Kistowska M, Meier B, Proust T, et al. Propionibacterium acnes promotes Th17 and Th17/Th1 responses in acne patients. J Invest Dermatol. 2015;135:110-118.
- Zeichner JA. Inflammatory acne treatment: review of current and new topical therapeutic options. J Drugs Dermatol. 2016;15(1 suppl 1):S11-S16.
- Nakano T, Hiramatsu K, Kishi K, et al. Clindamycin modulates inflammatory-cytokine induction in lipopolysaccharide-stimulated mouse peritoneal macrophages. Antimicrob Agents Chemother. 2003;47:363-367.
- Orman KL, English BK. Effects of antibiotic class on the macrophage inflammatory response to Streptococcus pneumoniae. J Infect Dis. 2000;182:1561-1565.
- Taylor M, Gonzalez M, Porter R. Pathways to inflammation: acne pathophysiology. Eur J Dermatol. 2011;21:323-333.
- Del Rosso JQ, Kircik LH. The sequence of inflammation, relevant biomarkers, and the pathogenesis of acne vulgaris: what does recent research show and what does it mean to the clinician? J Drugs Dermatol. 2013;12(8 suppl):S109-S115.
- Leyden J, Kaidbey K, Levy SF. The combination formulation of clindamycin 1% plus benzoyl peroxide 5% versus 3 different formulations of topical clindamycin alone in the reduction of Propionibacterium acnes. an in vivo comparative study. Am J Clin Dermatol. 2001;2:263-266.
- Wang WL, Everett ED, Johnson M, et al. Susceptibility of Propionibacterium acnes to seventeen antibiotics. Antimicrob Agents Chemother. 1977;11:171-173.
- Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013;69(6 suppl 1):S15-S26.
- Whitney KM, Ditre CM. Anti-inflammatory properties of clindamycin: a review of its use in the treatment of acne vulgaris. Clinical Medicine Insights: Dermatology. 2011;4:27-41.
- Mays RM, Gordon RA, Wilson JM, et al. New antibiotic therapies for acne and rosacea. Dermatol Ther. 2012;25:23-37.
- Wilkin JK, DeWitt S. Treatment of rosacea: topical clindamycin versus oral tetracycline. Int J Dermatol. 1993;32:65-67.
- Breneman D, Savin R, VandePol C, et al. Double-blind, randomized, vehicle-controlled clinical trial of once-daily benzoyl peroxide/clindamycin topical gel in the treatment of patients with moderate to severe rosacea. Int J Dermatol. 2004;43:381-387.
- Leyden JJ, Thiboutot D, Shalita A. Photographic review of results from a clinical study comparing benzoyl peroxide 5%/clindamycin 1% topical gel with vehicle in the treatment of rosacea. Cutis. 2004;73(6 suppl):11-17.
- Chang AL, Alora-Palli M, Lima XT, et al. A randomized, double-blind, placebo-controlled, pilot study to assess the efficacy and safety of clindamycin 1.2% and tretinoin 0.025% combination gel for the treatment of acne rosacea over 12 weeks. J Drugs Dermatol. 2012;11:333-339.
- Freeman SA, Moon SD, Spencer JM. Clindamycin phosphate 1.2% and tretinoin 0.025% gel for rosacea: summary of a placebo-controlled, double-blind trial. J Drugs Dermatol. 2012;11:1410-1414.
- van Zuuren EJ, Fedorowicz Z, Carter B, et al. Interventions for rosacea. Cochrane Database Syst Rev. 2015;4:CD003262.
- Laquieze S, Czernielewski J, Baltas E. Beneficial use of Cetaphil moisturizing cream as part of a daily skin care regimen for individuals with rosacea. J Dermatolog Treat. 2007;18:158-162.
- Lookingbill DP, Chalker DK, Lindholm JS, et al. Treatment of acne with a combination clindamycin/benzoyl peroxide gel compared with clindamycin gel, benzoyl peroxide gel and vehicle gel: combined results of two double-blind investigations. J Am Acad Dermatol. 1997;37:590-595.
- Alirezaï M, Gerlach B, Horvath A, et al. Results of a randomised, multicentre study comparing a new water-based gel of clindamycin 1% versus clindamycin 1% topical solution in the treatment of acne vulgaris. Eur J Dermatol. 2005;15:274-278.
- Jarratt MT, Brundage T. Efficacy and safety of clindamycin-tretinoin gel versus clindamycin or tretinoin alone in acne vulgaris: a randomized, double-blind, vehicle-controlled study. J Drugs Dermatol. 2012;11:318-326.
- Benzaclin. Med Library website. http://medlibrary.org/lib/rx/meds/benzaclin-3. Updated May 8, 2013. Accessed January 24, 2017.
- Walsh TR, Efthimiou J, Dréno B. Systematic review of antibiotic resistance in acne: an increasing topical and oral threat. Lancet Infect Dis. 2016;16:E23-E33.
- Jeremy AH, Holland DB, Roberts SG, et al. Inflammatory events are involved in acne lesion initiation. J Invest Dermatol. 2003;121:20-27.
- Kircik LH. Re-evaluating treatment targets in acne vulgaris: adapting to a new understanding of pathophysiology. J Drugs Dermatol. 2014;13:S57-S60.
- Salzer S, Kresse S, Hirai Y, et al. Cathelicidin peptide LL-37 increases UVB-triggered inflammasome activation: possible implications for rosacea. J Dermatol Sci. 2014;76:173-179.
- Buhl T, Sulk M, Nowak P, et al. Molecular and morphological characterization of inflammatory infiltrate in rosacea reveals activation of Th1/Th17 pathways. J Invest Dermatol. 2015;135:2198-2208.
- Kistowska M, Meier B, Proust T, et al. Propionibacterium acnes promotes Th17 and Th17/Th1 responses in acne patients. J Invest Dermatol. 2015;135:110-118.
- Zeichner JA. Inflammatory acne treatment: review of current and new topical therapeutic options. J Drugs Dermatol. 2016;15(1 suppl 1):S11-S16.
- Nakano T, Hiramatsu K, Kishi K, et al. Clindamycin modulates inflammatory-cytokine induction in lipopolysaccharide-stimulated mouse peritoneal macrophages. Antimicrob Agents Chemother. 2003;47:363-367.
- Orman KL, English BK. Effects of antibiotic class on the macrophage inflammatory response to Streptococcus pneumoniae. J Infect Dis. 2000;182:1561-1565.
- Taylor M, Gonzalez M, Porter R. Pathways to inflammation: acne pathophysiology. Eur J Dermatol. 2011;21:323-333.
- Del Rosso JQ, Kircik LH. The sequence of inflammation, relevant biomarkers, and the pathogenesis of acne vulgaris: what does recent research show and what does it mean to the clinician? J Drugs Dermatol. 2013;12(8 suppl):S109-S115.
- Leyden J, Kaidbey K, Levy SF. The combination formulation of clindamycin 1% plus benzoyl peroxide 5% versus 3 different formulations of topical clindamycin alone in the reduction of Propionibacterium acnes. an in vivo comparative study. Am J Clin Dermatol. 2001;2:263-266.
- Wang WL, Everett ED, Johnson M, et al. Susceptibility of Propionibacterium acnes to seventeen antibiotics. Antimicrob Agents Chemother. 1977;11:171-173.
- Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013;69(6 suppl 1):S15-S26.
Practice Points
- Clindamycin cream 0.3% and 1% and clindamycin gel 1% were no more effective than their respective vehicles in the treatment of moderate to severe rosacea.
- Clindamycin may have no intrinsic anti-inflammatory activity in rosacea.
Cosmetic Corner: Dermatologists Weigh in on Redness-Reducing Products
To improve patient care and outcomes, leading dermatologists offered their recommendations on redness-reducing products. Consideration must be given to:
- Avène Antirougeurs FORT Relief Concentrate
Pierre Fabre Dermo-Cosmetique USA
“This formula has medical-grade ruscus extract to support microcirculation and soothe skin reactivity and redness, as well as soothing Avène Thermal Spring Water.” — Jeannette Graf, MD, New York, New York
- Eucerin Redness Relief
Beiersdorf Inc
“Eucerin’s Redness Relief product line has worked well for some of my patients.” — Gary Goldenberg, MD, New York, New York
- Redness Solutions Daily Protective Base Broad Spectrum SPF 15
Clinique Laboratories, LLC
“This oil-free makeup primer has a sheer green tint that camouflages redness while also protecting from UV rays.” — Shari Lipner, MD, PhD, New York, New York
Cutis invites readers to send us their recommendations. Athlete’s foot treatments, cleansing devices, and men’s products will be featured in upcoming editions of Cosmetic Corner. Please email your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
To improve patient care and outcomes, leading dermatologists offered their recommendations on redness-reducing products. Consideration must be given to:
- Avène Antirougeurs FORT Relief Concentrate
Pierre Fabre Dermo-Cosmetique USA
“This formula has medical-grade ruscus extract to support microcirculation and soothe skin reactivity and redness, as well as soothing Avène Thermal Spring Water.” — Jeannette Graf, MD, New York, New York
- Eucerin Redness Relief
Beiersdorf Inc
“Eucerin’s Redness Relief product line has worked well for some of my patients.” — Gary Goldenberg, MD, New York, New York
- Redness Solutions Daily Protective Base Broad Spectrum SPF 15
Clinique Laboratories, LLC
“This oil-free makeup primer has a sheer green tint that camouflages redness while also protecting from UV rays.” — Shari Lipner, MD, PhD, New York, New York
Cutis invites readers to send us their recommendations. Athlete’s foot treatments, cleansing devices, and men’s products will be featured in upcoming editions of Cosmetic Corner. Please email your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
To improve patient care and outcomes, leading dermatologists offered their recommendations on redness-reducing products. Consideration must be given to:
- Avène Antirougeurs FORT Relief Concentrate
Pierre Fabre Dermo-Cosmetique USA
“This formula has medical-grade ruscus extract to support microcirculation and soothe skin reactivity and redness, as well as soothing Avène Thermal Spring Water.” — Jeannette Graf, MD, New York, New York
- Eucerin Redness Relief
Beiersdorf Inc
“Eucerin’s Redness Relief product line has worked well for some of my patients.” — Gary Goldenberg, MD, New York, New York
- Redness Solutions Daily Protective Base Broad Spectrum SPF 15
Clinique Laboratories, LLC
“This oil-free makeup primer has a sheer green tint that camouflages redness while also protecting from UV rays.” — Shari Lipner, MD, PhD, New York, New York
Cutis invites readers to send us their recommendations. Athlete’s foot treatments, cleansing devices, and men’s products will be featured in upcoming editions of Cosmetic Corner. Please email your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
Rosacea Treatment Schema: An Update
When tasked with outlining updated therapy regimens for rosacea, specific patient vignettes come to mind.
A 53-year-old male golfer presents with years of central facial flushing, prominent telangiectases, erythema, and scattered pink papules. He attempted various over-the-counter topical products indicated for acne, such as salicylic acid scrub and benzoyl peroxide cream, with no improvement and much irritation. Recently, his wife has been helping him apply redness-concealing makeup in the morning and over-the-counter hydrocortisone cream in the evening, which has been slightly helpful.
This patient’s rosacea could conceivably be labeled under the papulopustular rosacea subtype; however, the conventional categories are fluid with subtype overlap and imprecise diagnostic criteria. He also seemed to display features of the erythematotelangiectatic subtype, perhaps with underlying photodamage as well as steroid rebound erythema and/or atrophy.1 Nevertheless, it is a common presentation, and certain baseline tenets should be applied. First, all steroid products and irritants (eg, benzoyl peroxide and salicylic acid ingredients, any scrub vehicle) should be discontinued. Education about avoidance of triggers (ie, sun, heat, spicy food, alcohol, stress is paramount. Because barrier inadequacy is a recent insight into rosacea pathogenesis, mild syndet- or lipid-free cleansers, daily sunscreen, and evening emollients dictate baseline skin care, as does meticulous situation-specific sun protection.2,3 The papular component and immediate erythema in and around the papules can be managed topically (prior to sunscreen or emollient application) with metronidazole gel or cream up to twice daily, ivermectin cream once daily, or azelaic acid gel or foam up to twice daily. Oral doxycycline 40 mg (delayed release) on an empty stomach or 50 mg (immediate release) with food to avert antimicrobial dosing and antibiotic resistance also could be considered if topical therapy is inadequate or irritating, though gastrointestinal comorbidities with rosacea also should be delineated before initiating oral antibiotics.4-6 (Management of this patient’s nonlesional fixed erythema, telangiectases, and flushing is discussed after the next vignette.)
What if a woman presented in a similar fashion as above, only without papules? Her family physician prescribed metronidazole gel twice daily for years with no improvement in flushing, redness, or telangiectases.
Background erythema in rosacea often is persistent with trigger-specific intensification, with or without episodic facial flushing; undoubtedly, these symptoms can be difficult to compartmentalize depending on the clarity of the patient’s history and frequency of clinic visits. The aforementioned baseline skin care and sun-protection regimen applies, and newer topical agents such as α-adrenergics (daily oxymetazoline cream or brimonidine gel) may be considered for persistent erythema; however, irritant potential and rebound erythema are common.7-9 Topical therapies such as metronidazole gel, as in this case, are inadequate for persistent background erythema or flushing. Persistent erythema and telangiectases can be reduced with pulsed dye laser or intense pulsed light modalities, particularly following conservative management of acute inflammation.5 Episodic flushing is poorly controlled with the above tactics, but anecdotally, topical or oral α-adrenergics or oral nonselective beta-blockers could be considered; the latter is also applicable to migraine therapy, which is perhaps comorbid with rosacea.5,10
A 35-year-old Hispanic woman states that the scalp, forehead, and cheeks have been flaky, pink, and pruritic for years. She saw several aestheticians for it and the admixed “acne” on the face, receiving salicylic acid chemical peels with no improvement and much dyspigmentation.
Although underreported, the commingling of rosacea with seborrheic dermatitis is common, perhaps with mutual Demodex mite overpopulation, assigning topical therapies to its management such as daily ivermectin cream or steroid-sparing pimecrolimus cream for inflammatory papules and scaly regions of the face and scalp.11-13 Further, this case exemplifies the increasing incidence and awareness of rosacea in darker skin types, along with its postinflammatory pigmentary perturbations, which necessitate repeated education about barrier control and sun protection.14
A 72-year-old male farmer presents with his wife whoinsists that his nose has been increasing in size for years; she procures a prior driver’s license photograph as proof. She also notes that he has been snoring at night and having more trouble breathing while working outdoors. The patient had not noticed.
Phymatous rosacea may exist as an additional feature of any rosacea subtype or as a singular finding, presenting as actively inflamed, fibrotic/noninflamed, or both. Management, particularly if inflamed, involves baseline gentle skin care and sun protection, avoidance of rosacea triggers, and implementation of oral therapy such as doxycycline or isotretinoin. Many cases, particularly those with a fibrotic component, warrant surgical methods such as fractionated CO2 laser or Shaw scalpel surgical sculpting. These cases frequently demonstrate varying degrees of airway compromise, validating surgery as a legitimate medical, not merely cosmetic, presentation.5,15
Final Thoughts
The Table, constructed as a concise therapy compendium by the ROSacea COnsensus (ROSCO) international panel of dermatologists and ophthalmologists, outlines data-driven and expert experience-based therapies for rosacea.5 This panel asserts that phenotypical features, not rigid subtypes, oblige patient-specific treatment schema. Also, as these cases outline, an evolving understanding of rosacea’s multifaceted pathogenesis, assorted presentations, and frequent pitfalls in daily skin care and initial management require individualized care.
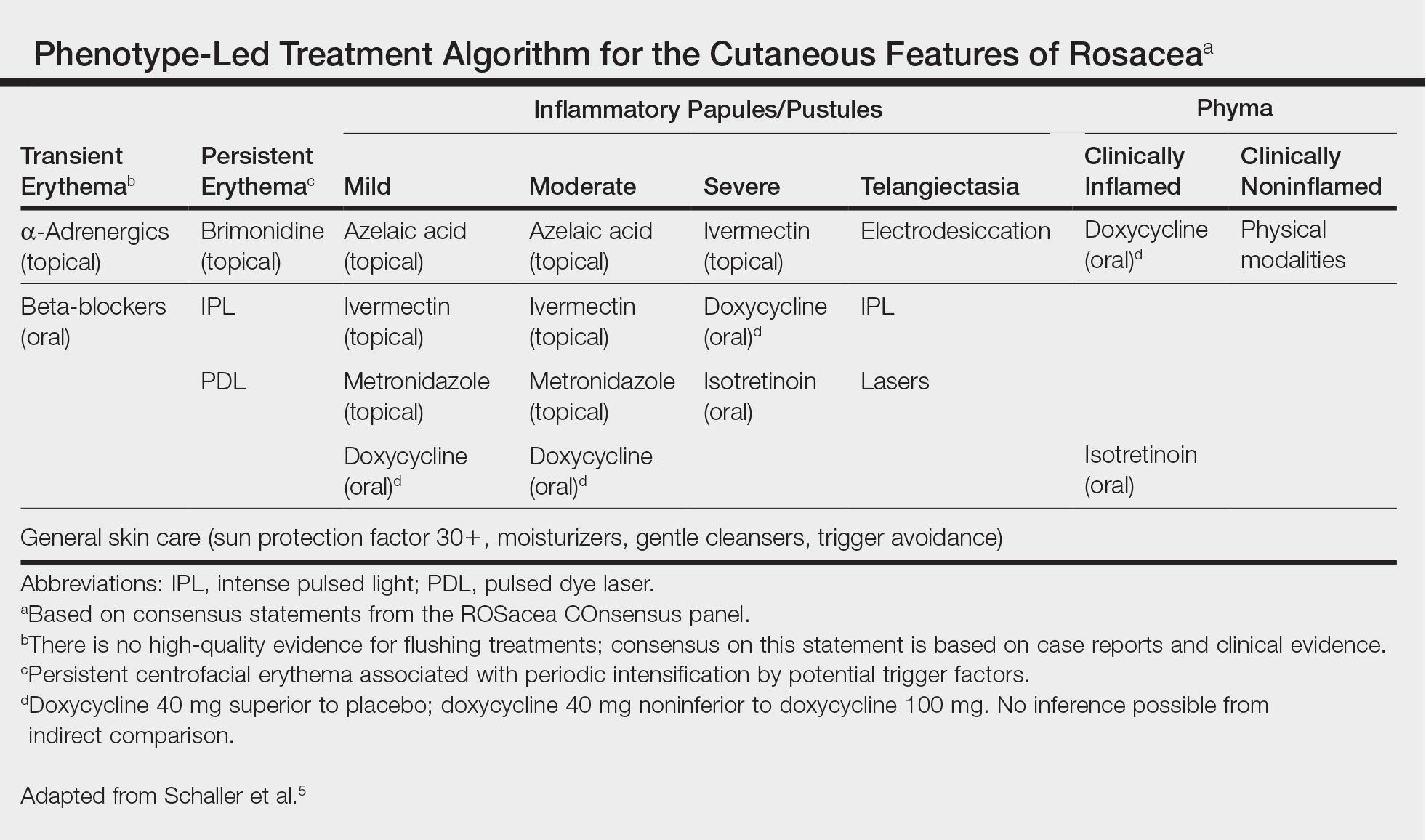
- Tan J, Steinhoff M, Berg M, et al; Rosacea International Study Group. Shortcomings in rosacea diagnosis and classification. Br J Dermatol. 2017;176:197-199.
- Levin J, Miller R. A guide to the ingredients and potential benefits of over-the-counter cleansers and moisturizers for rosacea patients. J Clin Aesthet Dermatol. 2011;4:31-49.
- Del Rosso JQ. Adjunctive skin care in the management of rosacea: cleansers, moisturizers, and photoprotectants. Cutis. 2005;75(suppl 3):17-21;discussion 33-36.
- van Zuuren EJ, Fedorowicz Z. Interventions for rosacea: abridged updated Cochrane systematic review including GRADE assessments [published online August 30, 2015]. Br J Dermatol. 2015;173:651-662.
- Schaller M, Almeida LM, Bewley A, et al. Rosacea treatment update: recommendations from the global ROSacea COnsensus (ROSCO) panel. Br J Dermatol. 2017;176:465-471.
- Egeberg A, Weinstock LB, Thvssen EP, et al. Rosacea and gastrointestinal disorders: a population-based cohort study. Br J Dermatol. 2017;176:100-106.
- Layton AM, Schaller M, Homey B, et al. Brimonidine gel 0.33% rapidly improves patient-reported outcomes by controlling facial erythema of rosacea: a randomized, double-blind, vehicle-controlled study. J Eur Acad Dermatol Venereol. 2015;29:2405-2410.
- Docherty JR, Steinhoff M, Lorton D, et al. Multidisciplinary consideration of potential pathophysiologic mechanisms of paradoxical erythema with topical brimonidine therapy [published online August 25, 2016]. Adv Ther. 2016;33:1885-1895.
- Shanler SD, Ondo AL. Successful treatment of the erythema and flushing of rosacea using a topically applied selective alpha1-adrenergic receptor agonist, oxymetazoline. Arch Dermatol. 2007;143:1369-1371.
- Egeberg A, Ashina M, Gaist D, et al. Prevalence and risk of migraine in patients with rosacea: a population-based cohort study. J Am Acad Dermatol. 2017;76:454-458.
- Zhao YE, Peng Y, Wang XL, et al. Facial dermatosis associated with Demodex: a case-control study. J Zhejiang Univ Sci B. 2011;12:1008-1015.
- Siddiqui K, Stein Gold L, Gill J. The efficacy, safety, and tolerability of ivermectin compared with current topical treatments for the inflammatory lesions of rosacea: a network meta-analysis. Springerplus. 2016;5:1151. doi: 10.1186/s40064-016-2819-8.
- Kim MB, Kim GW, Park HJ, et al. Pimecrolimus 1% cream for the treatment of rosacea. J Dermatol. 2011;38:1135-1139.
- Al-Dabagh A, Davis SA, McMichael AJ, et al. Rosacea in skin of color: not a rare diagnosis. Dermatol Online J. 2014;20. pii:13030/qt1mv9r0ss.
- Little SC, Stucker FJ, Compton A, et al. Nuances in the management of rhinophyma. Facial Plast Surg. 2012;28:231-237.
When tasked with outlining updated therapy regimens for rosacea, specific patient vignettes come to mind.
A 53-year-old male golfer presents with years of central facial flushing, prominent telangiectases, erythema, and scattered pink papules. He attempted various over-the-counter topical products indicated for acne, such as salicylic acid scrub and benzoyl peroxide cream, with no improvement and much irritation. Recently, his wife has been helping him apply redness-concealing makeup in the morning and over-the-counter hydrocortisone cream in the evening, which has been slightly helpful.
This patient’s rosacea could conceivably be labeled under the papulopustular rosacea subtype; however, the conventional categories are fluid with subtype overlap and imprecise diagnostic criteria. He also seemed to display features of the erythematotelangiectatic subtype, perhaps with underlying photodamage as well as steroid rebound erythema and/or atrophy.1 Nevertheless, it is a common presentation, and certain baseline tenets should be applied. First, all steroid products and irritants (eg, benzoyl peroxide and salicylic acid ingredients, any scrub vehicle) should be discontinued. Education about avoidance of triggers (ie, sun, heat, spicy food, alcohol, stress is paramount. Because barrier inadequacy is a recent insight into rosacea pathogenesis, mild syndet- or lipid-free cleansers, daily sunscreen, and evening emollients dictate baseline skin care, as does meticulous situation-specific sun protection.2,3 The papular component and immediate erythema in and around the papules can be managed topically (prior to sunscreen or emollient application) with metronidazole gel or cream up to twice daily, ivermectin cream once daily, or azelaic acid gel or foam up to twice daily. Oral doxycycline 40 mg (delayed release) on an empty stomach or 50 mg (immediate release) with food to avert antimicrobial dosing and antibiotic resistance also could be considered if topical therapy is inadequate or irritating, though gastrointestinal comorbidities with rosacea also should be delineated before initiating oral antibiotics.4-6 (Management of this patient’s nonlesional fixed erythema, telangiectases, and flushing is discussed after the next vignette.)
What if a woman presented in a similar fashion as above, only without papules? Her family physician prescribed metronidazole gel twice daily for years with no improvement in flushing, redness, or telangiectases.
Background erythema in rosacea often is persistent with trigger-specific intensification, with or without episodic facial flushing; undoubtedly, these symptoms can be difficult to compartmentalize depending on the clarity of the patient’s history and frequency of clinic visits. The aforementioned baseline skin care and sun-protection regimen applies, and newer topical agents such as α-adrenergics (daily oxymetazoline cream or brimonidine gel) may be considered for persistent erythema; however, irritant potential and rebound erythema are common.7-9 Topical therapies such as metronidazole gel, as in this case, are inadequate for persistent background erythema or flushing. Persistent erythema and telangiectases can be reduced with pulsed dye laser or intense pulsed light modalities, particularly following conservative management of acute inflammation.5 Episodic flushing is poorly controlled with the above tactics, but anecdotally, topical or oral α-adrenergics or oral nonselective beta-blockers could be considered; the latter is also applicable to migraine therapy, which is perhaps comorbid with rosacea.5,10
A 35-year-old Hispanic woman states that the scalp, forehead, and cheeks have been flaky, pink, and pruritic for years. She saw several aestheticians for it and the admixed “acne” on the face, receiving salicylic acid chemical peels with no improvement and much dyspigmentation.
Although underreported, the commingling of rosacea with seborrheic dermatitis is common, perhaps with mutual Demodex mite overpopulation, assigning topical therapies to its management such as daily ivermectin cream or steroid-sparing pimecrolimus cream for inflammatory papules and scaly regions of the face and scalp.11-13 Further, this case exemplifies the increasing incidence and awareness of rosacea in darker skin types, along with its postinflammatory pigmentary perturbations, which necessitate repeated education about barrier control and sun protection.14
A 72-year-old male farmer presents with his wife whoinsists that his nose has been increasing in size for years; she procures a prior driver’s license photograph as proof. She also notes that he has been snoring at night and having more trouble breathing while working outdoors. The patient had not noticed.
Phymatous rosacea may exist as an additional feature of any rosacea subtype or as a singular finding, presenting as actively inflamed, fibrotic/noninflamed, or both. Management, particularly if inflamed, involves baseline gentle skin care and sun protection, avoidance of rosacea triggers, and implementation of oral therapy such as doxycycline or isotretinoin. Many cases, particularly those with a fibrotic component, warrant surgical methods such as fractionated CO2 laser or Shaw scalpel surgical sculpting. These cases frequently demonstrate varying degrees of airway compromise, validating surgery as a legitimate medical, not merely cosmetic, presentation.5,15
Final Thoughts
The Table, constructed as a concise therapy compendium by the ROSacea COnsensus (ROSCO) international panel of dermatologists and ophthalmologists, outlines data-driven and expert experience-based therapies for rosacea.5 This panel asserts that phenotypical features, not rigid subtypes, oblige patient-specific treatment schema. Also, as these cases outline, an evolving understanding of rosacea’s multifaceted pathogenesis, assorted presentations, and frequent pitfalls in daily skin care and initial management require individualized care.

When tasked with outlining updated therapy regimens for rosacea, specific patient vignettes come to mind.
A 53-year-old male golfer presents with years of central facial flushing, prominent telangiectases, erythema, and scattered pink papules. He attempted various over-the-counter topical products indicated for acne, such as salicylic acid scrub and benzoyl peroxide cream, with no improvement and much irritation. Recently, his wife has been helping him apply redness-concealing makeup in the morning and over-the-counter hydrocortisone cream in the evening, which has been slightly helpful.
This patient’s rosacea could conceivably be labeled under the papulopustular rosacea subtype; however, the conventional categories are fluid with subtype overlap and imprecise diagnostic criteria. He also seemed to display features of the erythematotelangiectatic subtype, perhaps with underlying photodamage as well as steroid rebound erythema and/or atrophy.1 Nevertheless, it is a common presentation, and certain baseline tenets should be applied. First, all steroid products and irritants (eg, benzoyl peroxide and salicylic acid ingredients, any scrub vehicle) should be discontinued. Education about avoidance of triggers (ie, sun, heat, spicy food, alcohol, stress is paramount. Because barrier inadequacy is a recent insight into rosacea pathogenesis, mild syndet- or lipid-free cleansers, daily sunscreen, and evening emollients dictate baseline skin care, as does meticulous situation-specific sun protection.2,3 The papular component and immediate erythema in and around the papules can be managed topically (prior to sunscreen or emollient application) with metronidazole gel or cream up to twice daily, ivermectin cream once daily, or azelaic acid gel or foam up to twice daily. Oral doxycycline 40 mg (delayed release) on an empty stomach or 50 mg (immediate release) with food to avert antimicrobial dosing and antibiotic resistance also could be considered if topical therapy is inadequate or irritating, though gastrointestinal comorbidities with rosacea also should be delineated before initiating oral antibiotics.4-6 (Management of this patient’s nonlesional fixed erythema, telangiectases, and flushing is discussed after the next vignette.)
What if a woman presented in a similar fashion as above, only without papules? Her family physician prescribed metronidazole gel twice daily for years with no improvement in flushing, redness, or telangiectases.
Background erythema in rosacea often is persistent with trigger-specific intensification, with or without episodic facial flushing; undoubtedly, these symptoms can be difficult to compartmentalize depending on the clarity of the patient’s history and frequency of clinic visits. The aforementioned baseline skin care and sun-protection regimen applies, and newer topical agents such as α-adrenergics (daily oxymetazoline cream or brimonidine gel) may be considered for persistent erythema; however, irritant potential and rebound erythema are common.7-9 Topical therapies such as metronidazole gel, as in this case, are inadequate for persistent background erythema or flushing. Persistent erythema and telangiectases can be reduced with pulsed dye laser or intense pulsed light modalities, particularly following conservative management of acute inflammation.5 Episodic flushing is poorly controlled with the above tactics, but anecdotally, topical or oral α-adrenergics or oral nonselective beta-blockers could be considered; the latter is also applicable to migraine therapy, which is perhaps comorbid with rosacea.5,10
A 35-year-old Hispanic woman states that the scalp, forehead, and cheeks have been flaky, pink, and pruritic for years. She saw several aestheticians for it and the admixed “acne” on the face, receiving salicylic acid chemical peels with no improvement and much dyspigmentation.
Although underreported, the commingling of rosacea with seborrheic dermatitis is common, perhaps with mutual Demodex mite overpopulation, assigning topical therapies to its management such as daily ivermectin cream or steroid-sparing pimecrolimus cream for inflammatory papules and scaly regions of the face and scalp.11-13 Further, this case exemplifies the increasing incidence and awareness of rosacea in darker skin types, along with its postinflammatory pigmentary perturbations, which necessitate repeated education about barrier control and sun protection.14
A 72-year-old male farmer presents with his wife whoinsists that his nose has been increasing in size for years; she procures a prior driver’s license photograph as proof. She also notes that he has been snoring at night and having more trouble breathing while working outdoors. The patient had not noticed.
Phymatous rosacea may exist as an additional feature of any rosacea subtype or as a singular finding, presenting as actively inflamed, fibrotic/noninflamed, or both. Management, particularly if inflamed, involves baseline gentle skin care and sun protection, avoidance of rosacea triggers, and implementation of oral therapy such as doxycycline or isotretinoin. Many cases, particularly those with a fibrotic component, warrant surgical methods such as fractionated CO2 laser or Shaw scalpel surgical sculpting. These cases frequently demonstrate varying degrees of airway compromise, validating surgery as a legitimate medical, not merely cosmetic, presentation.5,15
Final Thoughts
The Table, constructed as a concise therapy compendium by the ROSacea COnsensus (ROSCO) international panel of dermatologists and ophthalmologists, outlines data-driven and expert experience-based therapies for rosacea.5 This panel asserts that phenotypical features, not rigid subtypes, oblige patient-specific treatment schema. Also, as these cases outline, an evolving understanding of rosacea’s multifaceted pathogenesis, assorted presentations, and frequent pitfalls in daily skin care and initial management require individualized care.

- Tan J, Steinhoff M, Berg M, et al; Rosacea International Study Group. Shortcomings in rosacea diagnosis and classification. Br J Dermatol. 2017;176:197-199.
- Levin J, Miller R. A guide to the ingredients and potential benefits of over-the-counter cleansers and moisturizers for rosacea patients. J Clin Aesthet Dermatol. 2011;4:31-49.
- Del Rosso JQ. Adjunctive skin care in the management of rosacea: cleansers, moisturizers, and photoprotectants. Cutis. 2005;75(suppl 3):17-21;discussion 33-36.
- van Zuuren EJ, Fedorowicz Z. Interventions for rosacea: abridged updated Cochrane systematic review including GRADE assessments [published online August 30, 2015]. Br J Dermatol. 2015;173:651-662.
- Schaller M, Almeida LM, Bewley A, et al. Rosacea treatment update: recommendations from the global ROSacea COnsensus (ROSCO) panel. Br J Dermatol. 2017;176:465-471.
- Egeberg A, Weinstock LB, Thvssen EP, et al. Rosacea and gastrointestinal disorders: a population-based cohort study. Br J Dermatol. 2017;176:100-106.
- Layton AM, Schaller M, Homey B, et al. Brimonidine gel 0.33% rapidly improves patient-reported outcomes by controlling facial erythema of rosacea: a randomized, double-blind, vehicle-controlled study. J Eur Acad Dermatol Venereol. 2015;29:2405-2410.
- Docherty JR, Steinhoff M, Lorton D, et al. Multidisciplinary consideration of potential pathophysiologic mechanisms of paradoxical erythema with topical brimonidine therapy [published online August 25, 2016]. Adv Ther. 2016;33:1885-1895.
- Shanler SD, Ondo AL. Successful treatment of the erythema and flushing of rosacea using a topically applied selective alpha1-adrenergic receptor agonist, oxymetazoline. Arch Dermatol. 2007;143:1369-1371.
- Egeberg A, Ashina M, Gaist D, et al. Prevalence and risk of migraine in patients with rosacea: a population-based cohort study. J Am Acad Dermatol. 2017;76:454-458.
- Zhao YE, Peng Y, Wang XL, et al. Facial dermatosis associated with Demodex: a case-control study. J Zhejiang Univ Sci B. 2011;12:1008-1015.
- Siddiqui K, Stein Gold L, Gill J. The efficacy, safety, and tolerability of ivermectin compared with current topical treatments for the inflammatory lesions of rosacea: a network meta-analysis. Springerplus. 2016;5:1151. doi: 10.1186/s40064-016-2819-8.
- Kim MB, Kim GW, Park HJ, et al. Pimecrolimus 1% cream for the treatment of rosacea. J Dermatol. 2011;38:1135-1139.
- Al-Dabagh A, Davis SA, McMichael AJ, et al. Rosacea in skin of color: not a rare diagnosis. Dermatol Online J. 2014;20. pii:13030/qt1mv9r0ss.
- Little SC, Stucker FJ, Compton A, et al. Nuances in the management of rhinophyma. Facial Plast Surg. 2012;28:231-237.
- Tan J, Steinhoff M, Berg M, et al; Rosacea International Study Group. Shortcomings in rosacea diagnosis and classification. Br J Dermatol. 2017;176:197-199.
- Levin J, Miller R. A guide to the ingredients and potential benefits of over-the-counter cleansers and moisturizers for rosacea patients. J Clin Aesthet Dermatol. 2011;4:31-49.
- Del Rosso JQ. Adjunctive skin care in the management of rosacea: cleansers, moisturizers, and photoprotectants. Cutis. 2005;75(suppl 3):17-21;discussion 33-36.
- van Zuuren EJ, Fedorowicz Z. Interventions for rosacea: abridged updated Cochrane systematic review including GRADE assessments [published online August 30, 2015]. Br J Dermatol. 2015;173:651-662.
- Schaller M, Almeida LM, Bewley A, et al. Rosacea treatment update: recommendations from the global ROSacea COnsensus (ROSCO) panel. Br J Dermatol. 2017;176:465-471.
- Egeberg A, Weinstock LB, Thvssen EP, et al. Rosacea and gastrointestinal disorders: a population-based cohort study. Br J Dermatol. 2017;176:100-106.
- Layton AM, Schaller M, Homey B, et al. Brimonidine gel 0.33% rapidly improves patient-reported outcomes by controlling facial erythema of rosacea: a randomized, double-blind, vehicle-controlled study. J Eur Acad Dermatol Venereol. 2015;29:2405-2410.
- Docherty JR, Steinhoff M, Lorton D, et al. Multidisciplinary consideration of potential pathophysiologic mechanisms of paradoxical erythema with topical brimonidine therapy [published online August 25, 2016]. Adv Ther. 2016;33:1885-1895.
- Shanler SD, Ondo AL. Successful treatment of the erythema and flushing of rosacea using a topically applied selective alpha1-adrenergic receptor agonist, oxymetazoline. Arch Dermatol. 2007;143:1369-1371.
- Egeberg A, Ashina M, Gaist D, et al. Prevalence and risk of migraine in patients with rosacea: a population-based cohort study. J Am Acad Dermatol. 2017;76:454-458.
- Zhao YE, Peng Y, Wang XL, et al. Facial dermatosis associated with Demodex: a case-control study. J Zhejiang Univ Sci B. 2011;12:1008-1015.
- Siddiqui K, Stein Gold L, Gill J. The efficacy, safety, and tolerability of ivermectin compared with current topical treatments for the inflammatory lesions of rosacea: a network meta-analysis. Springerplus. 2016;5:1151. doi: 10.1186/s40064-016-2819-8.
- Kim MB, Kim GW, Park HJ, et al. Pimecrolimus 1% cream for the treatment of rosacea. J Dermatol. 2011;38:1135-1139.
- Al-Dabagh A, Davis SA, McMichael AJ, et al. Rosacea in skin of color: not a rare diagnosis. Dermatol Online J. 2014;20. pii:13030/qt1mv9r0ss.
- Little SC, Stucker FJ, Compton A, et al. Nuances in the management of rhinophyma. Facial Plast Surg. 2012;28:231-237.
Dermatologist calls for paradigm shift on treating ocular rosacea
A partnership between ophthalmologists and dermatologists holds the potential to produce a better treatment for ocular rosacea, one of the few conditions that brings the two specialties together.
“Hopefully, the paradigm eventually shifts from treating symptoms and signs of ocular rosacea with oral antibiotics or reflexive referral to ophthalmology to approaching cutaneous treatment first,” said dermatologist Kara Capriotti, MD, who has been working with a team of ophthalmologists to develop a treatment that focuses on ocular rosacea as a “lid disease,” instead of as a primary ocular surface problem.
Eye scrubs, artificial tears, stress relief measures, and antibiotics are among the treatments used for the condition, which, however, can be treatment-resistant.
The key, she believes, is to move from a focus on ocular symptoms to an emphasis on lid and skin signs associated with ocular rosacea. “Almost all of the ophthalmology approaches to the disease start at the ocular surface, and these surface treatments are always a little bit [or very] toxic to the ocular surface,” she said. “By treating the lid signs first through a topical approach through the closed lid, we can eliminate a lot of the ocular surface symptoms without ever touching the ocular surface, thus preventing a lot of the associated toxicity and side effects you commonly see with topical ophthalmics.”
Dr. Capriotti and her colleagues have developed a topical treatment that contains dimethyl sulfoxide (DMSO) combined with dilute povidone iodine, which is applied to the lid margin of the closed eye.
In 2015, they published a case report describing the successful use of the treatment for a 78-year-old man with rosacea blepharoconjunctivitis, who had failed oral and topical treatments. He was treated with povidone iodine 10% solution in a DMSO vehicle, which was compounded into a topical gel by a pharmacy and administered twice a day, rubbed onto the lash line and eyelid.
After one week, “remarkable improvements were noted,” with reversal of much of the conjunctivitis, anterior lid erythema, and thickening, they wrote. One month later, after once daily application, “not only were the initial improvements conserved, but the posterior lid margin vessels and telangiectasias had begun to attenuate and involute. Moreover, meibomian capping was no longer present, secretions were less viscous, and tear break-up time normalized.”
DMSO is a skin penetration agent that is rarely used in ophthalmology and povidone iodine is a biocidal agent used in eye care, they pointed out in the case report. “This novel therapy may warrant further investigation in randomized, controlled clinical trials,” they concluded (Ophthalmol Ther. 2015 Dec;4[2]:143-50).
There are plans to start a clinical trial later in 2017, according to Dr. Capriottis, the cofounder of Veloce Biopharma, a company that is developing this product.
Until more specific treatments are available, what can dermatologists do now to improve their care of patients with ocular rosacea? “First and foremost, you have to be comfortable looking at the eyelids and ruling out anything that could be masquerading as blepharitis,” Dr. Capriotti commented. “We have a big disadvantage in dermatology because we don’t use slit-lamp biomicroscopy, but we are pretty good at looking at skin. If the patient has a lot of extraocular rosacea signs, I definitely think dermatologists can manage the prescription of a topical for the lids and evaluate the response.”
She cautioned, however, that referrals are in order in several situations: cases that are severe, those that involve the eye only, and those with which ocular symptoms seem to outweigh the lid signs.
Dr. Capriotti is also the senior vice president of dermatology at Veloce. In the study published in 2015, she and her three coauthors disclosed having financial interest in ALC Therapeutics, a company that was dissolved and restructured into Veloce.
A partnership between ophthalmologists and dermatologists holds the potential to produce a better treatment for ocular rosacea, one of the few conditions that brings the two specialties together.
“Hopefully, the paradigm eventually shifts from treating symptoms and signs of ocular rosacea with oral antibiotics or reflexive referral to ophthalmology to approaching cutaneous treatment first,” said dermatologist Kara Capriotti, MD, who has been working with a team of ophthalmologists to develop a treatment that focuses on ocular rosacea as a “lid disease,” instead of as a primary ocular surface problem.
Eye scrubs, artificial tears, stress relief measures, and antibiotics are among the treatments used for the condition, which, however, can be treatment-resistant.
The key, she believes, is to move from a focus on ocular symptoms to an emphasis on lid and skin signs associated with ocular rosacea. “Almost all of the ophthalmology approaches to the disease start at the ocular surface, and these surface treatments are always a little bit [or very] toxic to the ocular surface,” she said. “By treating the lid signs first through a topical approach through the closed lid, we can eliminate a lot of the ocular surface symptoms without ever touching the ocular surface, thus preventing a lot of the associated toxicity and side effects you commonly see with topical ophthalmics.”
Dr. Capriotti and her colleagues have developed a topical treatment that contains dimethyl sulfoxide (DMSO) combined with dilute povidone iodine, which is applied to the lid margin of the closed eye.
In 2015, they published a case report describing the successful use of the treatment for a 78-year-old man with rosacea blepharoconjunctivitis, who had failed oral and topical treatments. He was treated with povidone iodine 10% solution in a DMSO vehicle, which was compounded into a topical gel by a pharmacy and administered twice a day, rubbed onto the lash line and eyelid.
After one week, “remarkable improvements were noted,” with reversal of much of the conjunctivitis, anterior lid erythema, and thickening, they wrote. One month later, after once daily application, “not only were the initial improvements conserved, but the posterior lid margin vessels and telangiectasias had begun to attenuate and involute. Moreover, meibomian capping was no longer present, secretions were less viscous, and tear break-up time normalized.”
DMSO is a skin penetration agent that is rarely used in ophthalmology and povidone iodine is a biocidal agent used in eye care, they pointed out in the case report. “This novel therapy may warrant further investigation in randomized, controlled clinical trials,” they concluded (Ophthalmol Ther. 2015 Dec;4[2]:143-50).
There are plans to start a clinical trial later in 2017, according to Dr. Capriottis, the cofounder of Veloce Biopharma, a company that is developing this product.
Until more specific treatments are available, what can dermatologists do now to improve their care of patients with ocular rosacea? “First and foremost, you have to be comfortable looking at the eyelids and ruling out anything that could be masquerading as blepharitis,” Dr. Capriotti commented. “We have a big disadvantage in dermatology because we don’t use slit-lamp biomicroscopy, but we are pretty good at looking at skin. If the patient has a lot of extraocular rosacea signs, I definitely think dermatologists can manage the prescription of a topical for the lids and evaluate the response.”
She cautioned, however, that referrals are in order in several situations: cases that are severe, those that involve the eye only, and those with which ocular symptoms seem to outweigh the lid signs.
Dr. Capriotti is also the senior vice president of dermatology at Veloce. In the study published in 2015, she and her three coauthors disclosed having financial interest in ALC Therapeutics, a company that was dissolved and restructured into Veloce.
A partnership between ophthalmologists and dermatologists holds the potential to produce a better treatment for ocular rosacea, one of the few conditions that brings the two specialties together.
“Hopefully, the paradigm eventually shifts from treating symptoms and signs of ocular rosacea with oral antibiotics or reflexive referral to ophthalmology to approaching cutaneous treatment first,” said dermatologist Kara Capriotti, MD, who has been working with a team of ophthalmologists to develop a treatment that focuses on ocular rosacea as a “lid disease,” instead of as a primary ocular surface problem.
Eye scrubs, artificial tears, stress relief measures, and antibiotics are among the treatments used for the condition, which, however, can be treatment-resistant.
The key, she believes, is to move from a focus on ocular symptoms to an emphasis on lid and skin signs associated with ocular rosacea. “Almost all of the ophthalmology approaches to the disease start at the ocular surface, and these surface treatments are always a little bit [or very] toxic to the ocular surface,” she said. “By treating the lid signs first through a topical approach through the closed lid, we can eliminate a lot of the ocular surface symptoms without ever touching the ocular surface, thus preventing a lot of the associated toxicity and side effects you commonly see with topical ophthalmics.”
Dr. Capriotti and her colleagues have developed a topical treatment that contains dimethyl sulfoxide (DMSO) combined with dilute povidone iodine, which is applied to the lid margin of the closed eye.
In 2015, they published a case report describing the successful use of the treatment for a 78-year-old man with rosacea blepharoconjunctivitis, who had failed oral and topical treatments. He was treated with povidone iodine 10% solution in a DMSO vehicle, which was compounded into a topical gel by a pharmacy and administered twice a day, rubbed onto the lash line and eyelid.
After one week, “remarkable improvements were noted,” with reversal of much of the conjunctivitis, anterior lid erythema, and thickening, they wrote. One month later, after once daily application, “not only were the initial improvements conserved, but the posterior lid margin vessels and telangiectasias had begun to attenuate and involute. Moreover, meibomian capping was no longer present, secretions were less viscous, and tear break-up time normalized.”
DMSO is a skin penetration agent that is rarely used in ophthalmology and povidone iodine is a biocidal agent used in eye care, they pointed out in the case report. “This novel therapy may warrant further investigation in randomized, controlled clinical trials,” they concluded (Ophthalmol Ther. 2015 Dec;4[2]:143-50).
There are plans to start a clinical trial later in 2017, according to Dr. Capriottis, the cofounder of Veloce Biopharma, a company that is developing this product.
Until more specific treatments are available, what can dermatologists do now to improve their care of patients with ocular rosacea? “First and foremost, you have to be comfortable looking at the eyelids and ruling out anything that could be masquerading as blepharitis,” Dr. Capriotti commented. “We have a big disadvantage in dermatology because we don’t use slit-lamp biomicroscopy, but we are pretty good at looking at skin. If the patient has a lot of extraocular rosacea signs, I definitely think dermatologists can manage the prescription of a topical for the lids and evaluate the response.”
She cautioned, however, that referrals are in order in several situations: cases that are severe, those that involve the eye only, and those with which ocular symptoms seem to outweigh the lid signs.
Dr. Capriotti is also the senior vice president of dermatology at Veloce. In the study published in 2015, she and her three coauthors disclosed having financial interest in ALC Therapeutics, a company that was dissolved and restructured into Veloce.
Topical tretinoin resolves inflammatory symptoms in rosacea, in small study
SYDNEY, AUSTRALIA – Treatment with topical tretinoin resulted in complete resolution of rosacea symptoms in a significant number of patients, in a small retrospective study presented at the annual meeting of the Australasian College of Dermatologists.
“As an intermediary step between topical antibiotics and oral isotretinoin, we propose that topical tretinoin may be effective in the management and reduction of rosacea symptoms,” Emily Forward, MD, of the University of Sydney, said at the meeting. There has been recent discussion regarding the use of low-dose isotretinoin in the treatment of rosacea, but safety with long-term use is an issue, she noted.
More than 80% of patients had complete or excellent resolution of papules and pustules, with only one patient showing no benefit. Of the patients with erythema as the primary feature of their rosacea, 42% achieved complete resolution, 33% achieved excellent resolution, 17% achieved a good response, and 8% showed no benefit, Dr. Forward reported.
Among patients with telangiectasia, 40% achieved complete resolution, while 37% of those with flushing achieved complete resolution.
Topical tretinoin should be considered among the treatment options for rosacea “as it is effective, well tolerated, and has synergistic benefits in the prevention of photoaging,” Dr. Forward said. The ideal patient candidate would be someone with inflammatory features such as papules, pustules, or erythema, she added.
No patients experienced worsening of their symptoms with treatment, although one patient stopped treatment because of adverse effects. Dr. Forward also stressed that tretinoin is a known teratogen, so it should not be used during pregnancy or breastfeeding, or in patients trying to conceive.
In an interview, Dr. Forward said that she and her associates were surprised at the degree of improvement with topical tretinoin, particularly for erythema symptoms. However, she said it was important to educate patients about how to use topical tretinoin. “You use a pea-sized amount, at night, and in the beginning we advise them to use it every second or third day, and if they tolerate it they can increase the amount,” she said.
No conflicts of interest were declared.
SYDNEY, AUSTRALIA – Treatment with topical tretinoin resulted in complete resolution of rosacea symptoms in a significant number of patients, in a small retrospective study presented at the annual meeting of the Australasian College of Dermatologists.
“As an intermediary step between topical antibiotics and oral isotretinoin, we propose that topical tretinoin may be effective in the management and reduction of rosacea symptoms,” Emily Forward, MD, of the University of Sydney, said at the meeting. There has been recent discussion regarding the use of low-dose isotretinoin in the treatment of rosacea, but safety with long-term use is an issue, she noted.
More than 80% of patients had complete or excellent resolution of papules and pustules, with only one patient showing no benefit. Of the patients with erythema as the primary feature of their rosacea, 42% achieved complete resolution, 33% achieved excellent resolution, 17% achieved a good response, and 8% showed no benefit, Dr. Forward reported.
Among patients with telangiectasia, 40% achieved complete resolution, while 37% of those with flushing achieved complete resolution.
Topical tretinoin should be considered among the treatment options for rosacea “as it is effective, well tolerated, and has synergistic benefits in the prevention of photoaging,” Dr. Forward said. The ideal patient candidate would be someone with inflammatory features such as papules, pustules, or erythema, she added.
No patients experienced worsening of their symptoms with treatment, although one patient stopped treatment because of adverse effects. Dr. Forward also stressed that tretinoin is a known teratogen, so it should not be used during pregnancy or breastfeeding, or in patients trying to conceive.
In an interview, Dr. Forward said that she and her associates were surprised at the degree of improvement with topical tretinoin, particularly for erythema symptoms. However, she said it was important to educate patients about how to use topical tretinoin. “You use a pea-sized amount, at night, and in the beginning we advise them to use it every second or third day, and if they tolerate it they can increase the amount,” she said.
No conflicts of interest were declared.
SYDNEY, AUSTRALIA – Treatment with topical tretinoin resulted in complete resolution of rosacea symptoms in a significant number of patients, in a small retrospective study presented at the annual meeting of the Australasian College of Dermatologists.
“As an intermediary step between topical antibiotics and oral isotretinoin, we propose that topical tretinoin may be effective in the management and reduction of rosacea symptoms,” Emily Forward, MD, of the University of Sydney, said at the meeting. There has been recent discussion regarding the use of low-dose isotretinoin in the treatment of rosacea, but safety with long-term use is an issue, she noted.
More than 80% of patients had complete or excellent resolution of papules and pustules, with only one patient showing no benefit. Of the patients with erythema as the primary feature of their rosacea, 42% achieved complete resolution, 33% achieved excellent resolution, 17% achieved a good response, and 8% showed no benefit, Dr. Forward reported.
Among patients with telangiectasia, 40% achieved complete resolution, while 37% of those with flushing achieved complete resolution.
Topical tretinoin should be considered among the treatment options for rosacea “as it is effective, well tolerated, and has synergistic benefits in the prevention of photoaging,” Dr. Forward said. The ideal patient candidate would be someone with inflammatory features such as papules, pustules, or erythema, she added.
No patients experienced worsening of their symptoms with treatment, although one patient stopped treatment because of adverse effects. Dr. Forward also stressed that tretinoin is a known teratogen, so it should not be used during pregnancy or breastfeeding, or in patients trying to conceive.
In an interview, Dr. Forward said that she and her associates were surprised at the degree of improvement with topical tretinoin, particularly for erythema symptoms. However, she said it was important to educate patients about how to use topical tretinoin. “You use a pea-sized amount, at night, and in the beginning we advise them to use it every second or third day, and if they tolerate it they can increase the amount,” she said.
No conflicts of interest were declared.
AT ACDASM 2017
Key clinical point: Topical tretinoin may be useful in treating erythema and the inflammatory symptoms of rosacea.
Major finding: More than 80% of patients with rosacea had complete or excellent resolution of papules and pustules with topical tretinoin 0.05%.
Data source: A retrospective study of 25 patients with mild to severe rosacea.
Disclosures: No conflicts of interest were declared.
Some data support botulinum toxin for psoriasis and rosacea
ORLANDO – Botulinum toxin may have a place in treating psoriasis and rosacea.
There is not a huge body of literature supporting the use of neuromodulators for these conditions, but a smattering of case reports have shown positive results and some clinicians are exploring their off label use, Erin Gilbert, MD, said at the annual meeting of the American Academy of Dermatology.
Her own interest was originally piqued when she began working with Nicole Ward, PhD, director of the morphology core of the Skin Diseases Research Center in the department of dermatology at Case Western Reserve University, Cleveland, who developed a transgenic mouse model of psoriasis. Dr. Ward discovered that transecting the thoracic-level cutaneous nerves at their entry site into back skin resulted in rapid and significant changes in the psoriatic phenotype (J Invest Dermatol. 2011 Jul;131[7]:1530–8). These included decreases of up to 40% in various immune cell populations and a 30% improvement in acanthosis relative to sham surgery sites on the same animals.
This gave rise to a new thought, Dr. Gilbert said. Could chemical denervation produce similar improvements?
Using the same mouse model, she and Dr. Ward evaluated the effect of injecting botulinum neurotoxin A (BoNT-A) 9 units/kg diluted in 1 ml saline at one site, and saline control at another site (J Invest Dermatol. 2012 Jul;132[7]:1927–30). The mice were euthanized at 2 and 6 weeks after treatment. The results were similar to those of the surgical denervation: At 6 weeks, a 25% reduction in acanthosis was observed relative to the control site, with decreases in immune cells and inflammatory markers.
BoNT-A inhibits the release of neurotransmitters by cleaving the SPAP25 protein, an inhibitor of acetylcholine, at the neuromuscular junction. This is the root of the toxin’s ability to relax muscle spasm and decrease hyperhidrosis. The investigators also suggested that BoNT-A inhibits nerve-derived release of calcitonin gene-related peptide and substance P – important peptides in pain and itch sensation.
Dr. Gilbert and Dr. Ward also published a case report in which abobotulinumtoxinA was used off label to treat a recalcitrant psoriatic plaque in a 75-year-old woman (J Drugs Dermatol. 2014;13[11]:1407-8).
“This patient had psoriatic plaques concentrated on her trunk, arms, buttocks, and legs. She had been using strong topical corticosteroids for quite a long time with incomplete relief. I asked her to withdraw from all steroids for 3 months and then treated one lesion.”
The treated plaque was on the patient’s buttock. Dr. Gilbert injected a total of 30 units of abobotulinumtoxinA intradermally at eight points, about 1 cm apart. Within 3 weeks, there was complete remission of that plaque, sustained for 7 months. During this time, new lesions formed on other areas of her body. At 8 months, the treated plaque returned in the same place.
“Some of my patients had been completely recalcitrant to other therapies, and, following off label injection with neuromodulators, they have had life-changing results. In my experience, the key to consistently successful treatment is using adequate doses of toxin.”
This practice is supported by case reports in 2012 and 2015 (J Drugs Dermatol. 2012;11[12]:e76-e79; Dermatology 2015;230:299-301). Some investigators seem to think that, along with the anti-inflammatory and neurotransmitter effects, the toxin alters vascular tone.
Dr. Gilbert acknowledged that these treatments are expensive and cannot, in the case of psoriasis, be used in disseminated disease. However, she said that, for many patients, the relief is so profound and the benefit so long-lasting, that the expense is worth it. An argument in favor of this approach is that, where effective, BoNT-A could be used as a steroid-sparing agent and one that might reduce the need for systemic therapies.
“I will tell you that, sometimes, we get only partial relief and still need adjunctive therapies. Ultimately, neuromodulators may be especially useful for psoriatic plaques that are of cosmetic concern, such as those in the scalp or on the face. Limitations to their use include cost, the need for further studies, and safety concerns, such as muscle weakness.”
Dr. Gilbert had no relevant financial disclosures.
[email protected]
On Twitter @alz_gal
ORLANDO – Botulinum toxin may have a place in treating psoriasis and rosacea.
There is not a huge body of literature supporting the use of neuromodulators for these conditions, but a smattering of case reports have shown positive results and some clinicians are exploring their off label use, Erin Gilbert, MD, said at the annual meeting of the American Academy of Dermatology.
Her own interest was originally piqued when she began working with Nicole Ward, PhD, director of the morphology core of the Skin Diseases Research Center in the department of dermatology at Case Western Reserve University, Cleveland, who developed a transgenic mouse model of psoriasis. Dr. Ward discovered that transecting the thoracic-level cutaneous nerves at their entry site into back skin resulted in rapid and significant changes in the psoriatic phenotype (J Invest Dermatol. 2011 Jul;131[7]:1530–8). These included decreases of up to 40% in various immune cell populations and a 30% improvement in acanthosis relative to sham surgery sites on the same animals.
This gave rise to a new thought, Dr. Gilbert said. Could chemical denervation produce similar improvements?
Using the same mouse model, she and Dr. Ward evaluated the effect of injecting botulinum neurotoxin A (BoNT-A) 9 units/kg diluted in 1 ml saline at one site, and saline control at another site (J Invest Dermatol. 2012 Jul;132[7]:1927–30). The mice were euthanized at 2 and 6 weeks after treatment. The results were similar to those of the surgical denervation: At 6 weeks, a 25% reduction in acanthosis was observed relative to the control site, with decreases in immune cells and inflammatory markers.
BoNT-A inhibits the release of neurotransmitters by cleaving the SPAP25 protein, an inhibitor of acetylcholine, at the neuromuscular junction. This is the root of the toxin’s ability to relax muscle spasm and decrease hyperhidrosis. The investigators also suggested that BoNT-A inhibits nerve-derived release of calcitonin gene-related peptide and substance P – important peptides in pain and itch sensation.
Dr. Gilbert and Dr. Ward also published a case report in which abobotulinumtoxinA was used off label to treat a recalcitrant psoriatic plaque in a 75-year-old woman (J Drugs Dermatol. 2014;13[11]:1407-8).
“This patient had psoriatic plaques concentrated on her trunk, arms, buttocks, and legs. She had been using strong topical corticosteroids for quite a long time with incomplete relief. I asked her to withdraw from all steroids for 3 months and then treated one lesion.”
The treated plaque was on the patient’s buttock. Dr. Gilbert injected a total of 30 units of abobotulinumtoxinA intradermally at eight points, about 1 cm apart. Within 3 weeks, there was complete remission of that plaque, sustained for 7 months. During this time, new lesions formed on other areas of her body. At 8 months, the treated plaque returned in the same place.
“Some of my patients had been completely recalcitrant to other therapies, and, following off label injection with neuromodulators, they have had life-changing results. In my experience, the key to consistently successful treatment is using adequate doses of toxin.”
This practice is supported by case reports in 2012 and 2015 (J Drugs Dermatol. 2012;11[12]:e76-e79; Dermatology 2015;230:299-301). Some investigators seem to think that, along with the anti-inflammatory and neurotransmitter effects, the toxin alters vascular tone.
Dr. Gilbert acknowledged that these treatments are expensive and cannot, in the case of psoriasis, be used in disseminated disease. However, she said that, for many patients, the relief is so profound and the benefit so long-lasting, that the expense is worth it. An argument in favor of this approach is that, where effective, BoNT-A could be used as a steroid-sparing agent and one that might reduce the need for systemic therapies.
“I will tell you that, sometimes, we get only partial relief and still need adjunctive therapies. Ultimately, neuromodulators may be especially useful for psoriatic plaques that are of cosmetic concern, such as those in the scalp or on the face. Limitations to their use include cost, the need for further studies, and safety concerns, such as muscle weakness.”
Dr. Gilbert had no relevant financial disclosures.
[email protected]
On Twitter @alz_gal
ORLANDO – Botulinum toxin may have a place in treating psoriasis and rosacea.
There is not a huge body of literature supporting the use of neuromodulators for these conditions, but a smattering of case reports have shown positive results and some clinicians are exploring their off label use, Erin Gilbert, MD, said at the annual meeting of the American Academy of Dermatology.
Her own interest was originally piqued when she began working with Nicole Ward, PhD, director of the morphology core of the Skin Diseases Research Center in the department of dermatology at Case Western Reserve University, Cleveland, who developed a transgenic mouse model of psoriasis. Dr. Ward discovered that transecting the thoracic-level cutaneous nerves at their entry site into back skin resulted in rapid and significant changes in the psoriatic phenotype (J Invest Dermatol. 2011 Jul;131[7]:1530–8). These included decreases of up to 40% in various immune cell populations and a 30% improvement in acanthosis relative to sham surgery sites on the same animals.
This gave rise to a new thought, Dr. Gilbert said. Could chemical denervation produce similar improvements?
Using the same mouse model, she and Dr. Ward evaluated the effect of injecting botulinum neurotoxin A (BoNT-A) 9 units/kg diluted in 1 ml saline at one site, and saline control at another site (J Invest Dermatol. 2012 Jul;132[7]:1927–30). The mice were euthanized at 2 and 6 weeks after treatment. The results were similar to those of the surgical denervation: At 6 weeks, a 25% reduction in acanthosis was observed relative to the control site, with decreases in immune cells and inflammatory markers.
BoNT-A inhibits the release of neurotransmitters by cleaving the SPAP25 protein, an inhibitor of acetylcholine, at the neuromuscular junction. This is the root of the toxin’s ability to relax muscle spasm and decrease hyperhidrosis. The investigators also suggested that BoNT-A inhibits nerve-derived release of calcitonin gene-related peptide and substance P – important peptides in pain and itch sensation.
Dr. Gilbert and Dr. Ward also published a case report in which abobotulinumtoxinA was used off label to treat a recalcitrant psoriatic plaque in a 75-year-old woman (J Drugs Dermatol. 2014;13[11]:1407-8).
“This patient had psoriatic plaques concentrated on her trunk, arms, buttocks, and legs. She had been using strong topical corticosteroids for quite a long time with incomplete relief. I asked her to withdraw from all steroids for 3 months and then treated one lesion.”
The treated plaque was on the patient’s buttock. Dr. Gilbert injected a total of 30 units of abobotulinumtoxinA intradermally at eight points, about 1 cm apart. Within 3 weeks, there was complete remission of that plaque, sustained for 7 months. During this time, new lesions formed on other areas of her body. At 8 months, the treated plaque returned in the same place.
“Some of my patients had been completely recalcitrant to other therapies, and, following off label injection with neuromodulators, they have had life-changing results. In my experience, the key to consistently successful treatment is using adequate doses of toxin.”
This practice is supported by case reports in 2012 and 2015 (J Drugs Dermatol. 2012;11[12]:e76-e79; Dermatology 2015;230:299-301). Some investigators seem to think that, along with the anti-inflammatory and neurotransmitter effects, the toxin alters vascular tone.
Dr. Gilbert acknowledged that these treatments are expensive and cannot, in the case of psoriasis, be used in disseminated disease. However, she said that, for many patients, the relief is so profound and the benefit so long-lasting, that the expense is worth it. An argument in favor of this approach is that, where effective, BoNT-A could be used as a steroid-sparing agent and one that might reduce the need for systemic therapies.
“I will tell you that, sometimes, we get only partial relief and still need adjunctive therapies. Ultimately, neuromodulators may be especially useful for psoriatic plaques that are of cosmetic concern, such as those in the scalp or on the face. Limitations to their use include cost, the need for further studies, and safety concerns, such as muscle weakness.”
Dr. Gilbert had no relevant financial disclosures.
[email protected]
On Twitter @alz_gal
EXPERT ANALYSIS FROM AAD 17
Acne, rosacea prescriptions cost more when prescribed by dermatologists
PORTLAND, ORE. – Dermatologists have plenty of room to improve when it comes to choosing cost-effective medications for acne or rosacea, based on the results of a large retrospective analysis of Medicare claims data.
Patients with acne or rosacea consistently paid more for topical retinoids, topical antibiotics, and oral tetracyclines when the prescriber was a dermatologist instead of a family or internal medicine physician, Myron Zhang reported at the annual meeting of the Society for Investigative Dermatology.
“There is a large potential for reducing expenditures on health care for patients with acne and rosacea by choosing more generics and less expensive options within drug classes,” said Mr. Zhang, a medical student at the Ohio State University, Columbus, who conducted the study with colleagues there and at Northwestern University in Chicago.
Treating acne and rosacea falls under the purview of both primary and specialty outpatient care, but, researchers had not broken down costs of prescriptions for these conditions by provider type. To help fill that gap, Mr. Zhang and his colleagues retrospectively analyzed all Medicare drug claims for topical retinoids, topical antibiotics, isotretinoin, and oral tetracycline-class antibiotics used to treat acne and rosacea in the United States in 2008 and 2010.
Medicare claims for these prescriptions added up to $65 million in 2008 and $74 million in 2004, Mr. Zhang said. Although most generics either dropped in cost or rose by small amounts in that 2-year span, many brand name prescriptions rose by between 30% and 70%. “Specialist prescriptions were associated with higher brand name usage, a greater variety of medications, and higher costs,” Mr. Zhang said.
Patients paid an average of about $2-$3 more for a topical retinoid, $3-$4 more for oral tetracycline, and $1 more for a topical antibiotic prescribed by a specialist instead of a primary care physician.
This finding might indicate that dermatologists are more comfortable prescribing a greater variety of medications for acne and rosacea, such as tazarotene, azelaic acid, and sulfacetamide, while primary care physicians might stick to a narrower range of medicines, Mr. Zhang said. However, the prescribing behavior of dermatologists could also reflect “external factors,” such as increased contact with representatives from pharmaceutical companies, he added.
“As always, prescription costs need to be weighed against patient preference, compliance, and quality of care,” he said. “More outcomes research is needed to understand the comparative efficacy of generic and brand name treatments.”
The study included both rosacea and acne so that the researchers could capture the total costs of medications such as tretinoin, which is used to treat both conditions. Approximately 80% of topical retinoid prescriptions were for tretinoin, about 50% of topical antibiotic prescriptions were for metronidazole, while about 27% were for clindamycin. About half of oral antibiotic prescriptions were for tetracycline, while about 40% were for minocycline.
Mr. Zhang reported having no conflicts of interest.
PORTLAND, ORE. – Dermatologists have plenty of room to improve when it comes to choosing cost-effective medications for acne or rosacea, based on the results of a large retrospective analysis of Medicare claims data.
Patients with acne or rosacea consistently paid more for topical retinoids, topical antibiotics, and oral tetracyclines when the prescriber was a dermatologist instead of a family or internal medicine physician, Myron Zhang reported at the annual meeting of the Society for Investigative Dermatology.
“There is a large potential for reducing expenditures on health care for patients with acne and rosacea by choosing more generics and less expensive options within drug classes,” said Mr. Zhang, a medical student at the Ohio State University, Columbus, who conducted the study with colleagues there and at Northwestern University in Chicago.
Treating acne and rosacea falls under the purview of both primary and specialty outpatient care, but, researchers had not broken down costs of prescriptions for these conditions by provider type. To help fill that gap, Mr. Zhang and his colleagues retrospectively analyzed all Medicare drug claims for topical retinoids, topical antibiotics, isotretinoin, and oral tetracycline-class antibiotics used to treat acne and rosacea in the United States in 2008 and 2010.
Medicare claims for these prescriptions added up to $65 million in 2008 and $74 million in 2004, Mr. Zhang said. Although most generics either dropped in cost or rose by small amounts in that 2-year span, many brand name prescriptions rose by between 30% and 70%. “Specialist prescriptions were associated with higher brand name usage, a greater variety of medications, and higher costs,” Mr. Zhang said.
Patients paid an average of about $2-$3 more for a topical retinoid, $3-$4 more for oral tetracycline, and $1 more for a topical antibiotic prescribed by a specialist instead of a primary care physician.
This finding might indicate that dermatologists are more comfortable prescribing a greater variety of medications for acne and rosacea, such as tazarotene, azelaic acid, and sulfacetamide, while primary care physicians might stick to a narrower range of medicines, Mr. Zhang said. However, the prescribing behavior of dermatologists could also reflect “external factors,” such as increased contact with representatives from pharmaceutical companies, he added.
“As always, prescription costs need to be weighed against patient preference, compliance, and quality of care,” he said. “More outcomes research is needed to understand the comparative efficacy of generic and brand name treatments.”
The study included both rosacea and acne so that the researchers could capture the total costs of medications such as tretinoin, which is used to treat both conditions. Approximately 80% of topical retinoid prescriptions were for tretinoin, about 50% of topical antibiotic prescriptions were for metronidazole, while about 27% were for clindamycin. About half of oral antibiotic prescriptions were for tetracycline, while about 40% were for minocycline.
Mr. Zhang reported having no conflicts of interest.
PORTLAND, ORE. – Dermatologists have plenty of room to improve when it comes to choosing cost-effective medications for acne or rosacea, based on the results of a large retrospective analysis of Medicare claims data.
Patients with acne or rosacea consistently paid more for topical retinoids, topical antibiotics, and oral tetracyclines when the prescriber was a dermatologist instead of a family or internal medicine physician, Myron Zhang reported at the annual meeting of the Society for Investigative Dermatology.
“There is a large potential for reducing expenditures on health care for patients with acne and rosacea by choosing more generics and less expensive options within drug classes,” said Mr. Zhang, a medical student at the Ohio State University, Columbus, who conducted the study with colleagues there and at Northwestern University in Chicago.
Treating acne and rosacea falls under the purview of both primary and specialty outpatient care, but, researchers had not broken down costs of prescriptions for these conditions by provider type. To help fill that gap, Mr. Zhang and his colleagues retrospectively analyzed all Medicare drug claims for topical retinoids, topical antibiotics, isotretinoin, and oral tetracycline-class antibiotics used to treat acne and rosacea in the United States in 2008 and 2010.
Medicare claims for these prescriptions added up to $65 million in 2008 and $74 million in 2004, Mr. Zhang said. Although most generics either dropped in cost or rose by small amounts in that 2-year span, many brand name prescriptions rose by between 30% and 70%. “Specialist prescriptions were associated with higher brand name usage, a greater variety of medications, and higher costs,” Mr. Zhang said.
Patients paid an average of about $2-$3 more for a topical retinoid, $3-$4 more for oral tetracycline, and $1 more for a topical antibiotic prescribed by a specialist instead of a primary care physician.
This finding might indicate that dermatologists are more comfortable prescribing a greater variety of medications for acne and rosacea, such as tazarotene, azelaic acid, and sulfacetamide, while primary care physicians might stick to a narrower range of medicines, Mr. Zhang said. However, the prescribing behavior of dermatologists could also reflect “external factors,” such as increased contact with representatives from pharmaceutical companies, he added.
“As always, prescription costs need to be weighed against patient preference, compliance, and quality of care,” he said. “More outcomes research is needed to understand the comparative efficacy of generic and brand name treatments.”
The study included both rosacea and acne so that the researchers could capture the total costs of medications such as tretinoin, which is used to treat both conditions. Approximately 80% of topical retinoid prescriptions were for tretinoin, about 50% of topical antibiotic prescriptions were for metronidazole, while about 27% were for clindamycin. About half of oral antibiotic prescriptions were for tetracycline, while about 40% were for minocycline.
Mr. Zhang reported having no conflicts of interest.
At SID 2017
Key clinical point:
Major finding: Patients paid an average of $2-$3 more for a topical retinoid, $3-$4 more for an oral tetracycline-class antibiotic, and $1 more for a topical antibiotic prescribed by a dermatologist, compared with prescriptions from a primary care physician.
Data source: A retrospective analysis of Medicare prescription claims for oral antibiotics, topical antibiotics, and topical retinoids for acne and rosacea in 2008 and 2010.
Disclosures: Mr. Zhang reported having no conflicts of interest.