User login
VIDEO: Recognizing the systemic impact of rosacea
LAS VEGAS – At its core, “we understand that rosacea is an inflammatory disease,” Linda Stein Gold, MD, said at the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
As with psoriasis, she added, “we understand that rosacea might have some systemic implications.”
In fact, data suggest that cardiovascular disease is independently associated with rosacea after controlling for multiple variables, she noted. “So we now believe it’s important to get the inflammation under control, not just for the skin, but for the body as a whole,” Dr. Stein Gold, director of dermatology research, Henry Ford Health System, Detroit, said in a video interview.
She disclosed receiving research support, serving as a consultant, serving on the speakers bureau, and/or serving on the scientific advisory board for multiple pharmaceutical companies.
SDEF and this organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LAS VEGAS – At its core, “we understand that rosacea is an inflammatory disease,” Linda Stein Gold, MD, said at the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
As with psoriasis, she added, “we understand that rosacea might have some systemic implications.”
In fact, data suggest that cardiovascular disease is independently associated with rosacea after controlling for multiple variables, she noted. “So we now believe it’s important to get the inflammation under control, not just for the skin, but for the body as a whole,” Dr. Stein Gold, director of dermatology research, Henry Ford Health System, Detroit, said in a video interview.
She disclosed receiving research support, serving as a consultant, serving on the speakers bureau, and/or serving on the scientific advisory board for multiple pharmaceutical companies.
SDEF and this organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
LAS VEGAS – At its core, “we understand that rosacea is an inflammatory disease,” Linda Stein Gold, MD, said at the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
As with psoriasis, she added, “we understand that rosacea might have some systemic implications.”
In fact, data suggest that cardiovascular disease is independently associated with rosacea after controlling for multiple variables, she noted. “So we now believe it’s important to get the inflammation under control, not just for the skin, but for the body as a whole,” Dr. Stein Gold, director of dermatology research, Henry Ford Health System, Detroit, said in a video interview.
She disclosed receiving research support, serving as a consultant, serving on the speakers bureau, and/or serving on the scientific advisory board for multiple pharmaceutical companies.
SDEF and this organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
EXPERT ANALYSIS AT SDEF LAS VEGAS DERMATOLOGY SEMINAR
Cosmetic Corner: Dermatologists Weigh in on OTC Rosacea Treatments
To improve patient care and outcomes, leading dermatologists offered their recommendations on OTC rosacea treatments. Consideration must be given to:
- Eucerin Redness Relief
Beiersdorf Inc
Recommended by Gary Goldenberg, MD, New York, New York
- Rosaliac CC Cream
La Roche-Posay Laboratoire Dermatologique
“This product provides hydration and an even tint to correct and reduce the erythema of rosacea. It is made with both titanium dioxide and organic UV filters to give broad-spectrum coverage with SPF 30.”—Cherise Mizrahi-Levi, DO, New York, New York
- Vanicream
Pharmaceutical Specialties, Inc.
“I like Vanicream products since they are mild and hypoallergenic.”—Gary Goldenberg, MD, New York, New York
Cutis invites readers to send us their recommendations. Cleansing devices, skin-lightening agents, and self-tanners will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
To improve patient care and outcomes, leading dermatologists offered their recommendations on OTC rosacea treatments. Consideration must be given to:
- Eucerin Redness Relief
Beiersdorf Inc
Recommended by Gary Goldenberg, MD, New York, New York
- Rosaliac CC Cream
La Roche-Posay Laboratoire Dermatologique
“This product provides hydration and an even tint to correct and reduce the erythema of rosacea. It is made with both titanium dioxide and organic UV filters to give broad-spectrum coverage with SPF 30.”—Cherise Mizrahi-Levi, DO, New York, New York
- Vanicream
Pharmaceutical Specialties, Inc.
“I like Vanicream products since they are mild and hypoallergenic.”—Gary Goldenberg, MD, New York, New York
Cutis invites readers to send us their recommendations. Cleansing devices, skin-lightening agents, and self-tanners will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
To improve patient care and outcomes, leading dermatologists offered their recommendations on OTC rosacea treatments. Consideration must be given to:
- Eucerin Redness Relief
Beiersdorf Inc
Recommended by Gary Goldenberg, MD, New York, New York
- Rosaliac CC Cream
La Roche-Posay Laboratoire Dermatologique
“This product provides hydration and an even tint to correct and reduce the erythema of rosacea. It is made with both titanium dioxide and organic UV filters to give broad-spectrum coverage with SPF 30.”—Cherise Mizrahi-Levi, DO, New York, New York
- Vanicream
Pharmaceutical Specialties, Inc.
“I like Vanicream products since they are mild and hypoallergenic.”—Gary Goldenberg, MD, New York, New York
Cutis invites readers to send us their recommendations. Cleansing devices, skin-lightening agents, and self-tanners will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
NRS awards grants for rosacea studies
The National Rosacea Society (NRS) is awarding funding to three new studies and continuing funding for two ongoing studies on topics that include the impact of epigenetics on rosacea, the society announced.
The first study, by Dr. Luis Garza at John Hopkins University, Baltimore, will examine epigenetic lesions in rosacea. Epigenetics, the study of how DNA can be modified to act in certain ways, “may be responsible for why rosacea persists even though keratinocytes … slough off and are replaced every 2 months,” the NRS said in a written statement.
The second study, by Dr. Wenqing Li of Brown University, Providence, R.I., will use data from the Nurses’ Health Study II to examine how hormone use and hormone levels during menopause and pregnancy affect the risk of developing rosacea.
The third study, by Dr. Anna Di Nardo of the University of California, San Diego, and her associates, is looking at “whether the release of cathelicidin antimicrobial peptides, key players in the body’s normal innate immune system response, is central to the connection between the nervous system and skin inflammation through the activation of mast cells in rosacea,” the NRS statement noted.
Ongoing studies that also are receiving funding include work by Dr. Gideon Smith of Massachusetts General Hospital, Boston, and his associates, who are examining the risk of vascular disorders in people with rosacea, and a study by Dr. Lori Lee Stohl of Cornell University, New York, who is researching how stress-related biochemicals can increase mast-cell count.
“Research supported by the NRS has led to important insights into the physiology of the disorder, providing an essential foundation for developing new and better treatments. In addition, our growing knowledge is now pointing toward potentially meaningful connections between rosacea and other systemic illnesses,” Dr. Martin Steinhoff, chairman of dermatology and director of the Charles Institute of Dermatology, University College, Dublin, and a member of the NRS Medical Advisory Board, said in the statement.
Find the full NRS statement on the society’s website.
The National Rosacea Society (NRS) is awarding funding to three new studies and continuing funding for two ongoing studies on topics that include the impact of epigenetics on rosacea, the society announced.
The first study, by Dr. Luis Garza at John Hopkins University, Baltimore, will examine epigenetic lesions in rosacea. Epigenetics, the study of how DNA can be modified to act in certain ways, “may be responsible for why rosacea persists even though keratinocytes … slough off and are replaced every 2 months,” the NRS said in a written statement.
The second study, by Dr. Wenqing Li of Brown University, Providence, R.I., will use data from the Nurses’ Health Study II to examine how hormone use and hormone levels during menopause and pregnancy affect the risk of developing rosacea.
The third study, by Dr. Anna Di Nardo of the University of California, San Diego, and her associates, is looking at “whether the release of cathelicidin antimicrobial peptides, key players in the body’s normal innate immune system response, is central to the connection between the nervous system and skin inflammation through the activation of mast cells in rosacea,” the NRS statement noted.
Ongoing studies that also are receiving funding include work by Dr. Gideon Smith of Massachusetts General Hospital, Boston, and his associates, who are examining the risk of vascular disorders in people with rosacea, and a study by Dr. Lori Lee Stohl of Cornell University, New York, who is researching how stress-related biochemicals can increase mast-cell count.
“Research supported by the NRS has led to important insights into the physiology of the disorder, providing an essential foundation for developing new and better treatments. In addition, our growing knowledge is now pointing toward potentially meaningful connections between rosacea and other systemic illnesses,” Dr. Martin Steinhoff, chairman of dermatology and director of the Charles Institute of Dermatology, University College, Dublin, and a member of the NRS Medical Advisory Board, said in the statement.
Find the full NRS statement on the society’s website.
The National Rosacea Society (NRS) is awarding funding to three new studies and continuing funding for two ongoing studies on topics that include the impact of epigenetics on rosacea, the society announced.
The first study, by Dr. Luis Garza at John Hopkins University, Baltimore, will examine epigenetic lesions in rosacea. Epigenetics, the study of how DNA can be modified to act in certain ways, “may be responsible for why rosacea persists even though keratinocytes … slough off and are replaced every 2 months,” the NRS said in a written statement.
The second study, by Dr. Wenqing Li of Brown University, Providence, R.I., will use data from the Nurses’ Health Study II to examine how hormone use and hormone levels during menopause and pregnancy affect the risk of developing rosacea.
The third study, by Dr. Anna Di Nardo of the University of California, San Diego, and her associates, is looking at “whether the release of cathelicidin antimicrobial peptides, key players in the body’s normal innate immune system response, is central to the connection between the nervous system and skin inflammation through the activation of mast cells in rosacea,” the NRS statement noted.
Ongoing studies that also are receiving funding include work by Dr. Gideon Smith of Massachusetts General Hospital, Boston, and his associates, who are examining the risk of vascular disorders in people with rosacea, and a study by Dr. Lori Lee Stohl of Cornell University, New York, who is researching how stress-related biochemicals can increase mast-cell count.
“Research supported by the NRS has led to important insights into the physiology of the disorder, providing an essential foundation for developing new and better treatments. In addition, our growing knowledge is now pointing toward potentially meaningful connections between rosacea and other systemic illnesses,” Dr. Martin Steinhoff, chairman of dermatology and director of the Charles Institute of Dermatology, University College, Dublin, and a member of the NRS Medical Advisory Board, said in the statement.
Find the full NRS statement on the society’s website.
Experts outline phenotype approach to rosacea
A phenotype approach should be used to diagnose and manage rosacea, according to an expert panel that included 17 dermatologists from North America, Europe, Asia, Africa, and South America.
“As individual treatments do not address multiple features simultaneously, consideration of specific phenotypical issues facilitates individualized optimization of rosacea,” the panel concluded. As individual presentations of rosacea can span more than one of the currently defined disease subtypes, and vary widely in severity, dermatologists have long expressed a need to move to a phenotype-based system for diagnosis and classification.
The goal of the panel was “to establish international consensus on diagnosis and severity determination to improve outcomes” for people with rosacea (Br J Dermatol. 2016 Oct 8. doi: 10.1111/bjd.15122).
Jerry L. Tan, MD, of the University of Western Ontario, Windsor, and coauthors, explained why they considered a transition to the phenotype-based approach important: “Subtype classification may not fully cover the range of clinical presentations and is likely to confound severity assessment, whereas a phenotype-based approach could improve patient outcomes by addressing an individual patient’s clinical presentation and concerns.”
The panel identified two phenotypes as independently diagnostic of rosacea: persistent, centrofacial erythema associated with periodic intensification, and phymatous changes. Flushing or transient erythema, telangiectasia, inflammatory lesions, and ocular manifestations – the other phenotypes identified in the study – were not considered individually diagnostic.
Severity measurements for each phenotype were defined with a high degree of consensus, and the panel agreed that the severity of each feature should be rated independently and not grouped into subtype. For flushing or transient erythema, for example, the panel recommended that clinicians consider the intensity and frequency of episodes along with the area of involvement. For phymatous changes, inflammation, skin thickening, and deformation were identified as the key severity measures.
Although the investigators acknowledged that their expert consensus was the product of clinical opinion in the absence of extensive evidence, they cited as one of the study’s strengths its broad expert representation across geographical regions, where rosacea presentations may differ. Erythema and telangiectasia, Dr. Tan and colleagues wrote, “may not be visible in skin phototypes V and VI, an issue that may be overcome with experience and appropriate history taking.” They added that “other techniques, including skin biopsy, can also be considered for diagnostic support.” They recommended the development of new validated scales to be used in darker-skinned patients.
The panel also identified the psychosocial impact of rosacea as one severely understudied area of rosacea, and advocated the development of a new research tool that would assess psychological comorbidities. The proposed tool, they wrote, “should go beyond those currently available and assess the psychosocial impact for all major phenotypes.” The only rosacea-specific quality of life scoring measure, RosaQoL, contains notable deficiencies, they noted, including a lack of a measure for phymatous changes.
“Since clinicians and patients often have disparate views of disease,” the researchers wrote, “objective and practical tools based on individual presenting features are likely to be of value in setting treatment targets and monitoring treatment progress for patients with rosacea.”
The panel included three ophthalmologists from Germany and the United States; their recommendations were considered exploratory.
The study, which consisted of both electronic surveys and in-person meetings, was funded by Galderma. Twelve of its coauthors, including Dr. Tan, disclosed financial relationships with manufacturers.
A phenotype approach should be used to diagnose and manage rosacea, according to an expert panel that included 17 dermatologists from North America, Europe, Asia, Africa, and South America.
“As individual treatments do not address multiple features simultaneously, consideration of specific phenotypical issues facilitates individualized optimization of rosacea,” the panel concluded. As individual presentations of rosacea can span more than one of the currently defined disease subtypes, and vary widely in severity, dermatologists have long expressed a need to move to a phenotype-based system for diagnosis and classification.
The goal of the panel was “to establish international consensus on diagnosis and severity determination to improve outcomes” for people with rosacea (Br J Dermatol. 2016 Oct 8. doi: 10.1111/bjd.15122).
Jerry L. Tan, MD, of the University of Western Ontario, Windsor, and coauthors, explained why they considered a transition to the phenotype-based approach important: “Subtype classification may not fully cover the range of clinical presentations and is likely to confound severity assessment, whereas a phenotype-based approach could improve patient outcomes by addressing an individual patient’s clinical presentation and concerns.”
The panel identified two phenotypes as independently diagnostic of rosacea: persistent, centrofacial erythema associated with periodic intensification, and phymatous changes. Flushing or transient erythema, telangiectasia, inflammatory lesions, and ocular manifestations – the other phenotypes identified in the study – were not considered individually diagnostic.
Severity measurements for each phenotype were defined with a high degree of consensus, and the panel agreed that the severity of each feature should be rated independently and not grouped into subtype. For flushing or transient erythema, for example, the panel recommended that clinicians consider the intensity and frequency of episodes along with the area of involvement. For phymatous changes, inflammation, skin thickening, and deformation were identified as the key severity measures.
Although the investigators acknowledged that their expert consensus was the product of clinical opinion in the absence of extensive evidence, they cited as one of the study’s strengths its broad expert representation across geographical regions, where rosacea presentations may differ. Erythema and telangiectasia, Dr. Tan and colleagues wrote, “may not be visible in skin phototypes V and VI, an issue that may be overcome with experience and appropriate history taking.” They added that “other techniques, including skin biopsy, can also be considered for diagnostic support.” They recommended the development of new validated scales to be used in darker-skinned patients.
The panel also identified the psychosocial impact of rosacea as one severely understudied area of rosacea, and advocated the development of a new research tool that would assess psychological comorbidities. The proposed tool, they wrote, “should go beyond those currently available and assess the psychosocial impact for all major phenotypes.” The only rosacea-specific quality of life scoring measure, RosaQoL, contains notable deficiencies, they noted, including a lack of a measure for phymatous changes.
“Since clinicians and patients often have disparate views of disease,” the researchers wrote, “objective and practical tools based on individual presenting features are likely to be of value in setting treatment targets and monitoring treatment progress for patients with rosacea.”
The panel included three ophthalmologists from Germany and the United States; their recommendations were considered exploratory.
The study, which consisted of both electronic surveys and in-person meetings, was funded by Galderma. Twelve of its coauthors, including Dr. Tan, disclosed financial relationships with manufacturers.
A phenotype approach should be used to diagnose and manage rosacea, according to an expert panel that included 17 dermatologists from North America, Europe, Asia, Africa, and South America.
“As individual treatments do not address multiple features simultaneously, consideration of specific phenotypical issues facilitates individualized optimization of rosacea,” the panel concluded. As individual presentations of rosacea can span more than one of the currently defined disease subtypes, and vary widely in severity, dermatologists have long expressed a need to move to a phenotype-based system for diagnosis and classification.
The goal of the panel was “to establish international consensus on diagnosis and severity determination to improve outcomes” for people with rosacea (Br J Dermatol. 2016 Oct 8. doi: 10.1111/bjd.15122).
Jerry L. Tan, MD, of the University of Western Ontario, Windsor, and coauthors, explained why they considered a transition to the phenotype-based approach important: “Subtype classification may not fully cover the range of clinical presentations and is likely to confound severity assessment, whereas a phenotype-based approach could improve patient outcomes by addressing an individual patient’s clinical presentation and concerns.”
The panel identified two phenotypes as independently diagnostic of rosacea: persistent, centrofacial erythema associated with periodic intensification, and phymatous changes. Flushing or transient erythema, telangiectasia, inflammatory lesions, and ocular manifestations – the other phenotypes identified in the study – were not considered individually diagnostic.
Severity measurements for each phenotype were defined with a high degree of consensus, and the panel agreed that the severity of each feature should be rated independently and not grouped into subtype. For flushing or transient erythema, for example, the panel recommended that clinicians consider the intensity and frequency of episodes along with the area of involvement. For phymatous changes, inflammation, skin thickening, and deformation were identified as the key severity measures.
Although the investigators acknowledged that their expert consensus was the product of clinical opinion in the absence of extensive evidence, they cited as one of the study’s strengths its broad expert representation across geographical regions, where rosacea presentations may differ. Erythema and telangiectasia, Dr. Tan and colleagues wrote, “may not be visible in skin phototypes V and VI, an issue that may be overcome with experience and appropriate history taking.” They added that “other techniques, including skin biopsy, can also be considered for diagnostic support.” They recommended the development of new validated scales to be used in darker-skinned patients.
The panel also identified the psychosocial impact of rosacea as one severely understudied area of rosacea, and advocated the development of a new research tool that would assess psychological comorbidities. The proposed tool, they wrote, “should go beyond those currently available and assess the psychosocial impact for all major phenotypes.” The only rosacea-specific quality of life scoring measure, RosaQoL, contains notable deficiencies, they noted, including a lack of a measure for phymatous changes.
“Since clinicians and patients often have disparate views of disease,” the researchers wrote, “objective and practical tools based on individual presenting features are likely to be of value in setting treatment targets and monitoring treatment progress for patients with rosacea.”
The panel included three ophthalmologists from Germany and the United States; their recommendations were considered exploratory.
The study, which consisted of both electronic surveys and in-person meetings, was funded by Galderma. Twelve of its coauthors, including Dr. Tan, disclosed financial relationships with manufacturers.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
Key clinical point: Rosacea diagnosis, severity grading, and management should be based on disease phenotypes, which can span more than one of the currently recognized subtypes.
Major finding: Persistent centrofacial erythema with periodic intensification, and phymatous changes, are two phenotypes independently diagnostic of rosacea
Data source: An expert panel of 17 dermatologists from North America, Europe, Asia, Africa, and South America.
Disclosures: Galderma sponsored the study, for which all authors received honoraria; 12 disclosed additional funding from Galderma or other manufacturers.
Patient-Reported Outcomes of Azelaic Acid Foam 15% for Patients With Papulopustular Rosacea: Secondary Efficacy Results From a Randomized, Controlled, Double-blind, Phase 3 Trial
Rosacea is a chronic inflammatory disorder that may negatively impact patients’ quality of life (QOL).1,2 Papulopustular rosacea (PPR) is characterized by centrofacial inflammatory lesions and erythema as well as burning and stinging secondary to skin barrier dysfunction.3-5 Increasing rosacea severity is associated with greater rates of anxiety and depression and lower QOL6 as well as low self-esteem and feelings of embarrassment.7,8 Accordingly, assessing patient perceptions of rosacea treatments is necessary for understanding its impact on patient health.6,9
The Rosacea International Expert Group has emphasized the need to incorporate patient assessments of disease severity and QOL when developing therapeutic strategies for rosacea.7 Ease of use, sensory experience, and patient preference also are important dimensions in the evaluation of topical medications, as attributes of specific formulations may affect usability, adherence, and efficacy.10,11
An azelaic acid (AzA) 15% foam formulation, which was approved by the US Food and Drug Administration in 2015, was developed to deliver AzA in a vehicle designed to improve treatment experience in patients with mild to moderate PPR.12 Results from a clinical trial demonstrated superiority of AzA foam to vehicle foam for primary end points that included therapeutic success rate and change in inflammatory lesion count.13,14 Secondary end points assessed in the current analysis included patient perception of product usability, efficacy, and effect on QOL. These patient-reported outcome (PRO) results are reported here.
Methods
Study Design
The design of this phase 3 multicenter, randomized, double-blind, vehicle-controlled, parallel-group clinical trial was described in more detail in an earlier report.13 This study was approved by all appropriate institutional review boards. Eligible participants were 18 years and older with moderate or severe PPR, 12 to 50 inflammatory lesions, and persistent erythema with or without telangiectasia. Exclusion criteria included known nonresponse to AzA, current or prior use (within 6 weeks of randomization) of noninvestigational products to treat rosacea, and presence of other dermatoses that could interfere with rosacea evaluation.
Participants were randomized into the AzA foam or vehicle group (1:1 ratio). The study medication (0.5 g) or vehicle foam was applied twice daily to the entire face until the end of treatment (EoT) at 12 weeks. Efficacy and safety parameters were evaluated at baseline and at 4, 8, and 12 weeks of treatment, and at a follow-up visit 4 weeks after EoT (week 16).
Results for the coprimary efficacy end points—therapeutic success rate according to investigator global assessment and nominal change in inflammatory lesion count—were previously reported,13 as well as secondary efficacy outcomes including change in inflammatory lesion count, therapeutic response rate, and change in erythema rating.14
Patient-Reported Secondary Efficacy Outcomes
The secondary PRO end points were patient-reported global assessment of treatment response (rated as excellent, good, fair, none, or worse), global assessment of tolerability (rated as excellent, good, acceptable despite minor irritation, less acceptable due to continuous irritation, not acceptable, or no opinion), and opinion on cosmetic acceptability and practicability of product use in areas adjacent to the hairline (rated as very good, good, satisfactory, poor, or no opinion).
Additionally, QOL was measured by 3 validated standardized PRO tools, including the Rosacea Quality of Life Index (RosaQOL),15 the EuroQOL 5-dimension 5-level questionnaire (EQ-5D-5L),16 and the Dermatology Life Quality Index (DLQI). The RosaQOL is a rosacea-specific instrument assessing 3 constructs: (1) symptom, (2) emotion, and (3) function. The EQ-5D-5L questionnaire measures overall health status and comprises 5 constructs: (1) mobility, (2) self-care, (3) usual activities, (4) pain/discomfort, and (5) anxiety/depression. The DLQI is a general, dermatology-oriented instrument categorized into 6 constructs: (1) symptoms and feelings, (2) daily activities, (3) leisure, (4) work and school, (5) personal relationships, and (6) treatment.
Statistical Analyses
Patient-reported outcomes were analyzed in an exploratory manner and evaluated at EoT relative to baseline. Self-reported global assessment of treatment response and change in RosaQOL, EQ-5D-5L, and DLQI scores between AzA foam and vehicle foam groups were evaluated using the Wilcoxon rank sum test. Categorical change in the number of participants achieving an increase of 5 or more points in overall DLQI score was evaluated using a χ2 test.
Safety
Safety was analyzed for all randomized patients who were dispensed any study medication. All analyses were performed using SAS version 9.2.
Results
Of the 961 participants included in the study, 483 were randomized to receive AzA foam and 478 were randomized to receive vehicle foam. The mean age was 51.5 years, and the majority of participants were female (73.0%) and white (95.5%)(Table). At baseline, 834 (86.8%) participants had moderate PPR and 127 (13.2%) had severe PPR. The mean inflammatory lesion count (SD) was 21.4 (8.9). No significant differences in baseline characteristics were observed between treatment groups.

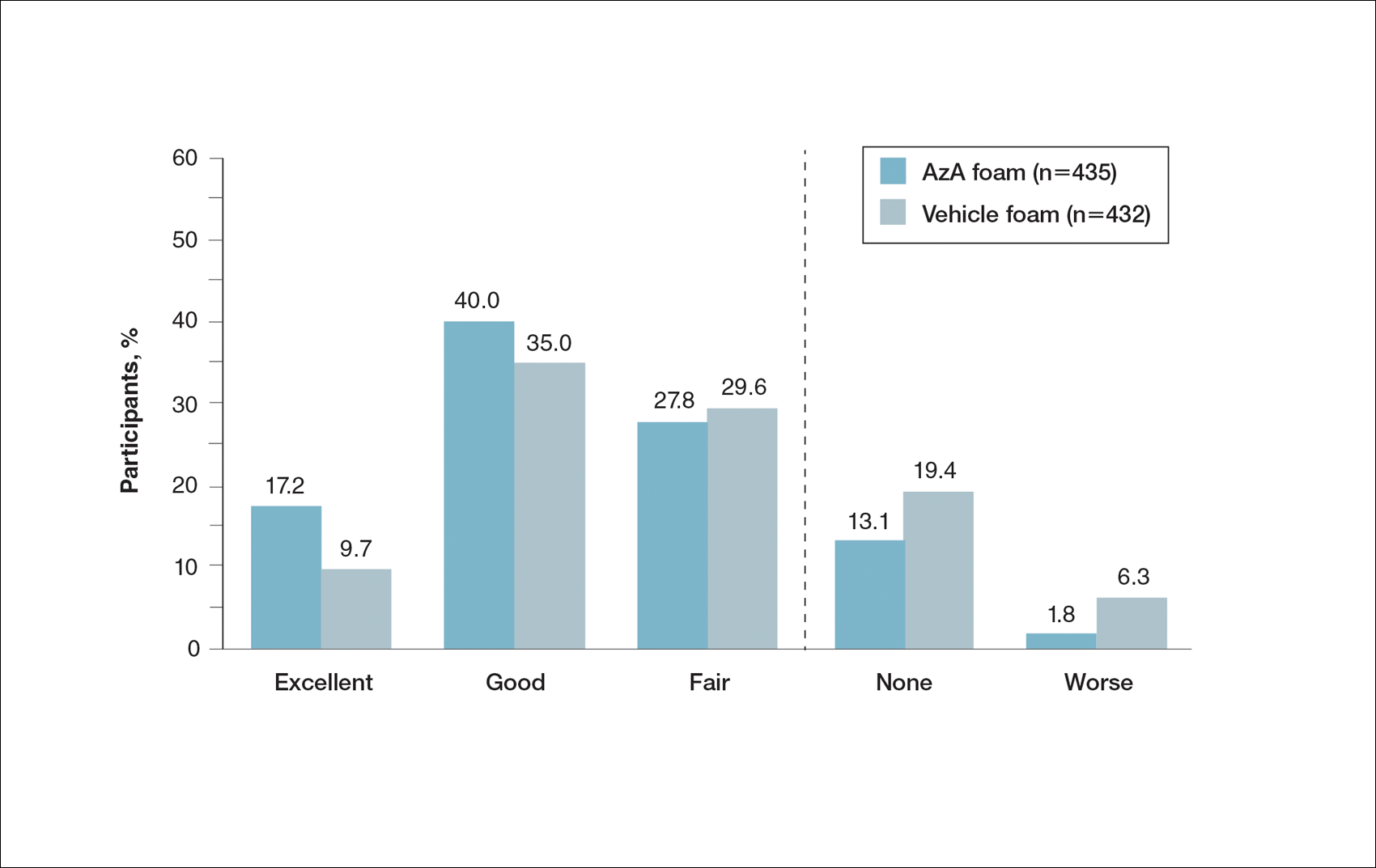
Patient-reported global assessment of treatment response differed between treatment groups at EoT (P<.001)(Figure 1). Higher ratings of treatment response were reported among the AzA foam group (excellent, 17.2%; good, 40.0%) versus vehicle foam (excellent, 9.7%; good, 35.0%). The number of participants reporting no treatment response was 13.1% in the AzA foam group, with 1.8% reporting worsening of their condition, while 19.4% of participants in the vehicle foam group reported no response, with 6.3% reporting worsening of their condition (Figure 1).
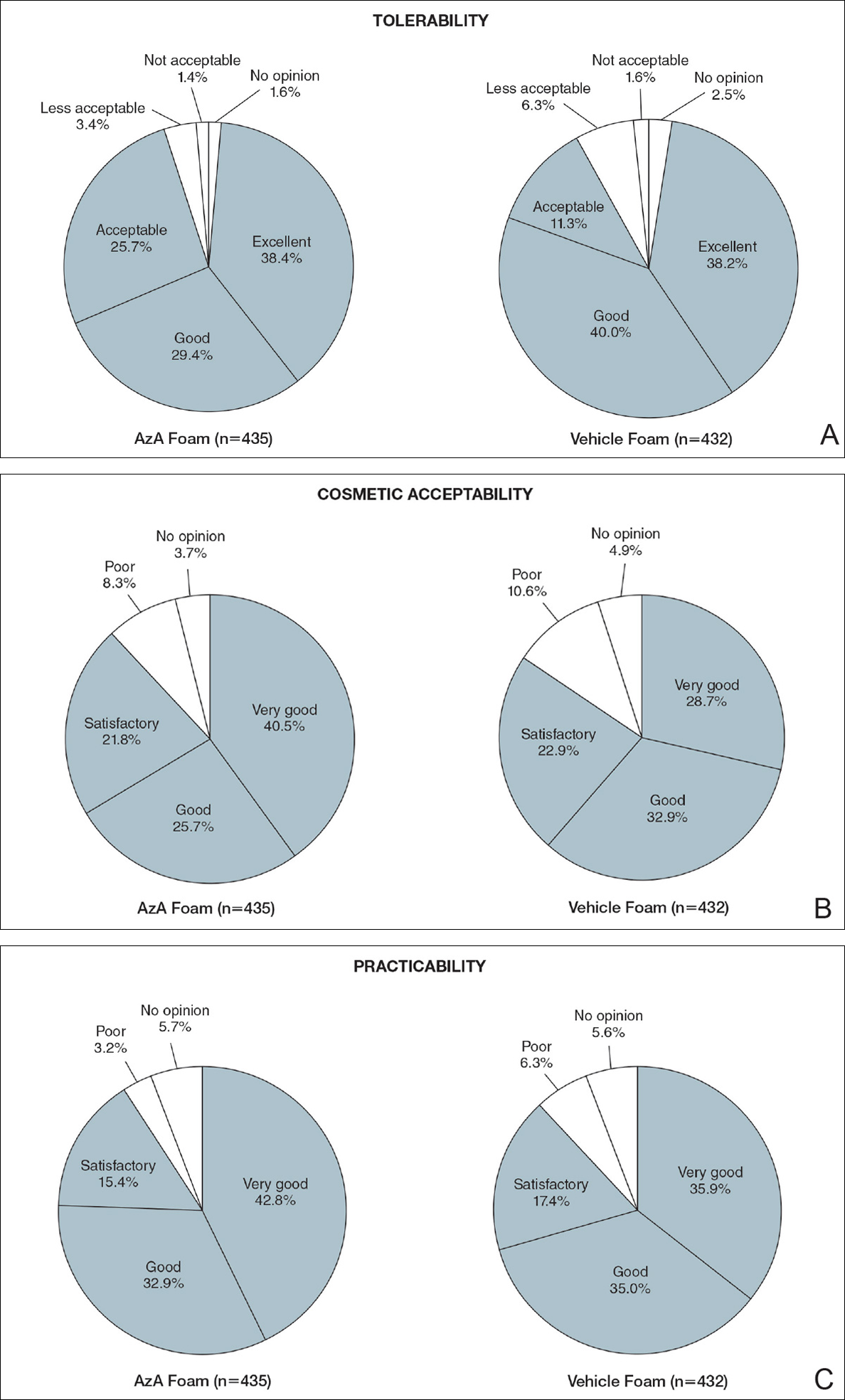
Tolerability was rated excellent or good in 67.8% of the AzA foam group versus 78.2% of the vehicle foam group (Figure 2A). Approximately 38.4% of the AzA foam group versus 38.2% of the vehicle foam group rated treatment tolerability as excellent, while 93.5% of the AzA foam group rated tolerability as acceptable, good, or excellent compared with 89.5% of the vehicle foam group. Only 1.4% of participants in the AzA foam group indicated that treatment was not acceptable due to irritation. In addition, a greater proportion of the AzA foam group reported cosmetic acceptability as very good versus the vehicle foam group (40.5% vs 28.7%)(Figure 2B), with two-thirds reporting cosmetic acceptability as very good or good. Practicability of product use in areas adjacent to the hairline was rated very good by substantial proportions of both the AzA foam and vehicle foam groups (42.8% vs 35.9%)(Figure 2C).
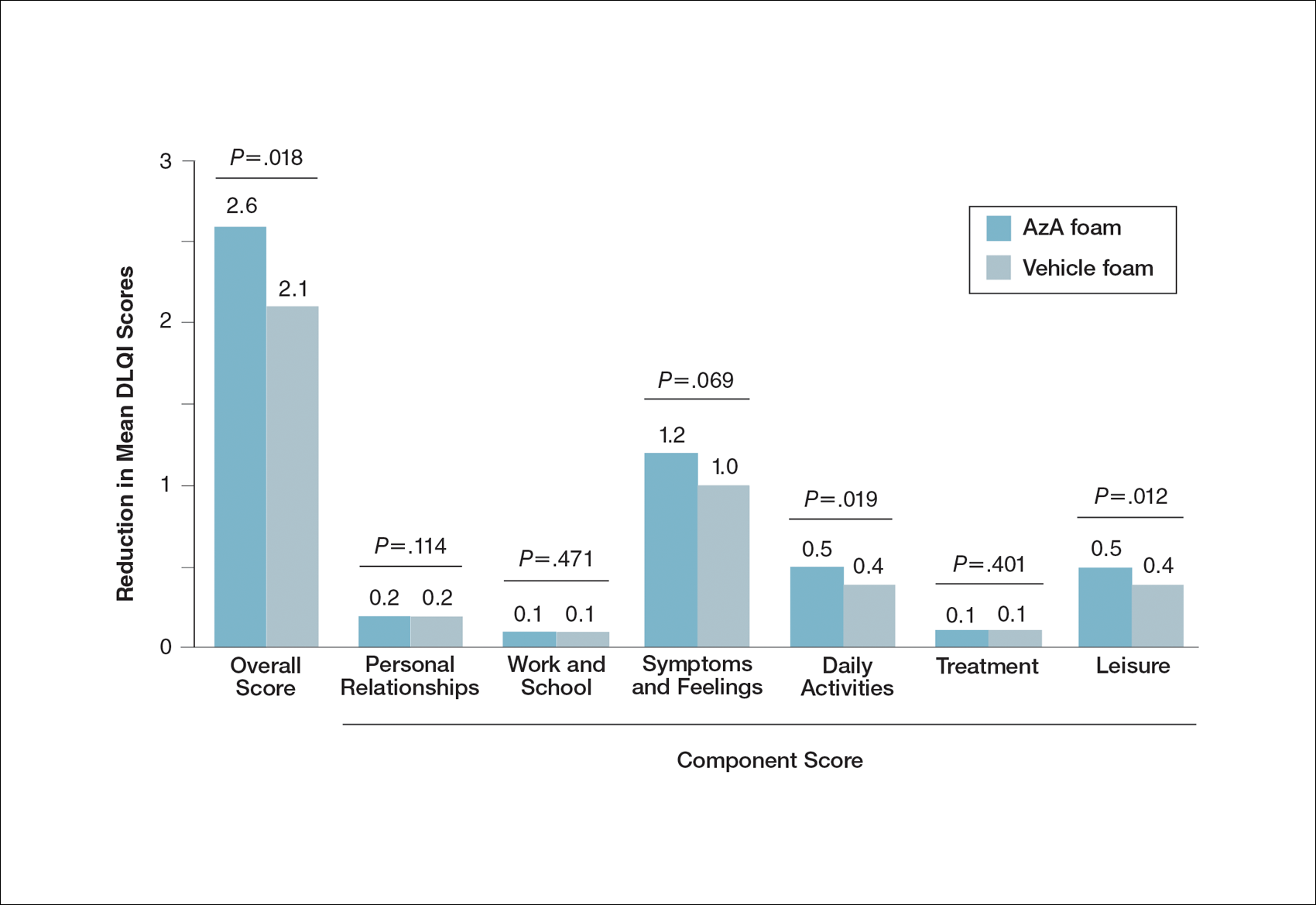
At baseline, average disease burden was moderate according to mean overall DLQI scores (SD) for the AzA foam (5.4 [4.8]) and vehicle foam (5.4 [4.9]) groups. Mean overall DLQI scores improved at EoT, with greater improvement occurring in the AzA foam group (2.6 vs 2.1; P=.018)(Figure 3). A larger proportion of participants in the AzA foam group versus the vehicle foam group also achieved a 5-point or more improvement in overall DLQI score (24.6% vs 19.0%; P=.047). Changes in specific DLQI subscore components were either balanced or in favor of the AzA foam group, including daily activities (0.5 vs 0.4; P=.019), symptoms and feelings (1.2 vs 1.0; P=.069), and leisure (0.5 vs 0.4; P=.012). Specific DLQI items with differences in scores between treatment groups from baseline included the following questions: Over the last week, how embarrassed or self-conscious have you been because of your skin? (P<.001); Over the last week, how much has your skin interfered with you going shopping or looking after your home or garden? (P=.005); Over the last week, how much has your skin affected any social or leisure activities? (P=.040); Over the last week, how much has your skin created problems with your partner or any of your close friends or relatives? (P=.001). Differences between treatment groups favored the AzA foam group for each of these items.
Participants in the AzA foam and vehicle foam groups also showed improvement in RosaQOL scores at EoT (6.8 vs 6.4; P=.67), while EQ-5D-5L scores changed minimally from baseline (0.006 vs 0.007; P=.50).
Safety
The incidence of drug-related adverse events (AEs) was greater in the AzA foam group versus the vehicle foam group (7.7% vs 4.8%). Drug-related AEs occurring in 1% of the AzA foam group were application-site pain including tenderness, stinging, and burning (3.5% for AzA foam vs 1.3% for vehicle foam); application-site pruritus (1.4% vs 0.4%); and application-site dryness (1.0% vs 0.6%). One drug-related AE of severe intensity—application-site dermatitis—occurred in the vehicle foam group; all other drug-related AEs were mild or moderate.14 More detailed safety results are described in a previous report.13
Comment
The PRO outcome data reported here are consistent with previously reported statistically significant improvements in investigator-assessed primary end points for the treatment of PPR with AzA foam.13,14 The data demonstrate that AzA foam benefits both clinical and patient-oriented dimensions of rosacea disease burden and suggest an association between positive treatment response and improved QOL.
Specifically, patient evaluation of treatment response to AzA foam was highly favorable, with 57.2% reporting excellent or good response and 85.1% reporting positive response overall. Recognizing the relapsing-remitting course of PPR, only 1.8% of the AzA foam group experienced worsening of disease at EoT.
The DLQI and RosaQOL instruments revealed notable improvements in QOL from baseline for both treatment groups. Although no significant differences in RosaQOL scores were observed between groups at EoT, significant differences in DLQI scores were detected. Almost one-quarter of participants in the AzA foam group achieved at least a 5-point improvement in DLQI score, exceeding the 4-point threshold for clinically meaningful change.17 Although little change in EQ-5D-5L scores was observed at EoT for both groups with no between-group differences, this finding is not unexpected, as this instrument assesses QOL dimensions such as loss of function, mobility, and ability to wash or dress, which are unlikely to be compromised in most rosacea patients.
Our results also underscore the importance of vehicle in the treatment of compromised skin. Studies of topical treatments for other dermatoses suggest that vehicle properties may reduce disease severity and improve QOL independent of active ingredients.10,18 For example, ease of application, minimal residue, and less time spent in application may explain the superiority of foam to other vehicles in the treatment of psoriasis.18 Our data demonstrating high cosmetic favorability of AzA foam are consistent with these prior observations. Increased tolerability of foam formulations also may affect response to treatment, in part by supporting adherence.18 Most participants receiving AzA foam described tolerability as excellent or good, and the discontinuation rate was low (1.2% of participants in the AzA foam group left the study due to AEs) in the setting of near-complete dosage administration (97% of expected doses applied).13
Conclusion
These results indicate that use of AzA foam as well as its novel vehicle results in high patient satisfaction and improved QOL. Although additional research is necessary to further delineate the relationship between PROs and other measures of clinical efficacy, our data demonstrate a positive treatment experience as perceived by patients that parallels the clinical efficacy of AzA foam for the treatment of PPR.13,14
Acknowledgment
Editorial support through inVentiv Medical Communications (New York, New York) was provided by Bayer Pharmaceuticals.
- Cardwell LA, Farhangian ME, Alinia H, et al. Psychological disorders associated with rosacea: analysis of unscripted comments. J Dermatol Surg. 2015;19:99-103.
- Moustafa F, Lewallen RS, Feldman SR. The psychological impact of rosacea and the influence of current management options. J Am Acad Dermatol. 2014;71:973-980.
- Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Yamasaki K, Gallo RL. The molecular pathology of rosacea. J Dermatol Sci. 2009;55:77-81.
- Del Rosso JQ. Advances in understanding and managing rosacea: part 1: connecting the dots between pathophysiological mechanisms and common clinical features of rosacea with emphasis on vascular changes and facial erythema. J Clin Aesthet Dermatol. 2012;5:16-25.
- Bohm D, Schwanitz P, Stock Gissendanner S, et al. Symptom severity and psychological sequelae in rosacea: results of a survey. Psychol Health Med. 2014;19:586-591.
- Elewski BE, Draelos Z, Dreno B, et al. Rosacea—global diversity and optimized outcome: proposed international consensus from the Rosacea International Expert Group. J Eur Acad Dermatol Venereol. 2011;25:188-200.
- Dirschka T, Micali G, Papadopoulos L, et al. Perceptions on the psychological impact of facial erythema associated with rosacea: results of international survey [published online May 29, 2015]. Dermatol Ther (Heidelb). 2015;5:117-127.
- Abram K, Silm H, Maaroos HI, et al. Subjective disease perception and symptoms of depression in relation to healthcare-seeking behaviour in patients with rosacea. Acta Derm Venereol. 2009;89:488-491.
- Stein L. Clinical studies of a new vehicle formulation for topical corticosteroids in the treatment of psoriasis. J Am Acad Dermatol. 2005;53(1, suppl 1):S39-S49.
- Yentzer BA, Camacho FT, Young T, et al. Good adherence and early efficacy using desonide hydrogel for atopic dermatitis: results from a program addressing patient compliance. J Drugs Dermatol. 2010;9:324-329.
- Finacea (azelaic acid) foam 15% [package insert]. Whippany, NJ: Bayer Pharmaceuticals; 2015.
- Draelos ZD, Elewski BE, Harper JC, et al. A phase 3 randomized, double-blind, vehicle-controlled trial of azelaic acid foam 15% in the treatment of papulopustular rosacea. Cutis. 2015;96:54-61.
- Solomon JA, Tyring S, Staedtler G, et al. Investigator-reported efficacy of azelaic acid foam 15% in patients with papulopustular rosacea: secondary efficacy outcomes from a randomized, controlled, double-blind, phase 3 trial. Cutis. 2016;98:187-194.
- Nicholson K, Abramova L, Chren MM, et al. A pilot quality-of-life instrument for acne rosacea. J Am Acad Dermatol. 2007;57:213-221.
- Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20:1727-1736.
- Basra MK, Salek MS, Camilleri L, et al. Determining the minimal clinically important difference and responsiveness of the Dermatology Life Quality Index (DLQI): further data. Dermatology. 2015;230:27-33.
- Bergstrom KG, Arambula K, Kimball AB. Medication formulation affects quality of life: a randomized single-blind study of clobetasol propionate foam 0.05% compared with a combined program of clobetasol cream 0.05% and solution 0.05% for the treatment of psoriasis. Cutis. 2003;72:407-411.
Rosacea is a chronic inflammatory disorder that may negatively impact patients’ quality of life (QOL).1,2 Papulopustular rosacea (PPR) is characterized by centrofacial inflammatory lesions and erythema as well as burning and stinging secondary to skin barrier dysfunction.3-5 Increasing rosacea severity is associated with greater rates of anxiety and depression and lower QOL6 as well as low self-esteem and feelings of embarrassment.7,8 Accordingly, assessing patient perceptions of rosacea treatments is necessary for understanding its impact on patient health.6,9
The Rosacea International Expert Group has emphasized the need to incorporate patient assessments of disease severity and QOL when developing therapeutic strategies for rosacea.7 Ease of use, sensory experience, and patient preference also are important dimensions in the evaluation of topical medications, as attributes of specific formulations may affect usability, adherence, and efficacy.10,11
An azelaic acid (AzA) 15% foam formulation, which was approved by the US Food and Drug Administration in 2015, was developed to deliver AzA in a vehicle designed to improve treatment experience in patients with mild to moderate PPR.12 Results from a clinical trial demonstrated superiority of AzA foam to vehicle foam for primary end points that included therapeutic success rate and change in inflammatory lesion count.13,14 Secondary end points assessed in the current analysis included patient perception of product usability, efficacy, and effect on QOL. These patient-reported outcome (PRO) results are reported here.
Methods
Study Design
The design of this phase 3 multicenter, randomized, double-blind, vehicle-controlled, parallel-group clinical trial was described in more detail in an earlier report.13 This study was approved by all appropriate institutional review boards. Eligible participants were 18 years and older with moderate or severe PPR, 12 to 50 inflammatory lesions, and persistent erythema with or without telangiectasia. Exclusion criteria included known nonresponse to AzA, current or prior use (within 6 weeks of randomization) of noninvestigational products to treat rosacea, and presence of other dermatoses that could interfere with rosacea evaluation.
Participants were randomized into the AzA foam or vehicle group (1:1 ratio). The study medication (0.5 g) or vehicle foam was applied twice daily to the entire face until the end of treatment (EoT) at 12 weeks. Efficacy and safety parameters were evaluated at baseline and at 4, 8, and 12 weeks of treatment, and at a follow-up visit 4 weeks after EoT (week 16).
Results for the coprimary efficacy end points—therapeutic success rate according to investigator global assessment and nominal change in inflammatory lesion count—were previously reported,13 as well as secondary efficacy outcomes including change in inflammatory lesion count, therapeutic response rate, and change in erythema rating.14
Patient-Reported Secondary Efficacy Outcomes
The secondary PRO end points were patient-reported global assessment of treatment response (rated as excellent, good, fair, none, or worse), global assessment of tolerability (rated as excellent, good, acceptable despite minor irritation, less acceptable due to continuous irritation, not acceptable, or no opinion), and opinion on cosmetic acceptability and practicability of product use in areas adjacent to the hairline (rated as very good, good, satisfactory, poor, or no opinion).
Additionally, QOL was measured by 3 validated standardized PRO tools, including the Rosacea Quality of Life Index (RosaQOL),15 the EuroQOL 5-dimension 5-level questionnaire (EQ-5D-5L),16 and the Dermatology Life Quality Index (DLQI). The RosaQOL is a rosacea-specific instrument assessing 3 constructs: (1) symptom, (2) emotion, and (3) function. The EQ-5D-5L questionnaire measures overall health status and comprises 5 constructs: (1) mobility, (2) self-care, (3) usual activities, (4) pain/discomfort, and (5) anxiety/depression. The DLQI is a general, dermatology-oriented instrument categorized into 6 constructs: (1) symptoms and feelings, (2) daily activities, (3) leisure, (4) work and school, (5) personal relationships, and (6) treatment.
Statistical Analyses
Patient-reported outcomes were analyzed in an exploratory manner and evaluated at EoT relative to baseline. Self-reported global assessment of treatment response and change in RosaQOL, EQ-5D-5L, and DLQI scores between AzA foam and vehicle foam groups were evaluated using the Wilcoxon rank sum test. Categorical change in the number of participants achieving an increase of 5 or more points in overall DLQI score was evaluated using a χ2 test.
Safety
Safety was analyzed for all randomized patients who were dispensed any study medication. All analyses were performed using SAS version 9.2.
Results
Of the 961 participants included in the study, 483 were randomized to receive AzA foam and 478 were randomized to receive vehicle foam. The mean age was 51.5 years, and the majority of participants were female (73.0%) and white (95.5%)(Table). At baseline, 834 (86.8%) participants had moderate PPR and 127 (13.2%) had severe PPR. The mean inflammatory lesion count (SD) was 21.4 (8.9). No significant differences in baseline characteristics were observed between treatment groups.

Patient-reported global assessment of treatment response differed between treatment groups at EoT (P<.001)(Figure 1). Higher ratings of treatment response were reported among the AzA foam group (excellent, 17.2%; good, 40.0%) versus vehicle foam (excellent, 9.7%; good, 35.0%). The number of participants reporting no treatment response was 13.1% in the AzA foam group, with 1.8% reporting worsening of their condition, while 19.4% of participants in the vehicle foam group reported no response, with 6.3% reporting worsening of their condition (Figure 1).

Tolerability was rated excellent or good in 67.8% of the AzA foam group versus 78.2% of the vehicle foam group (Figure 2A). Approximately 38.4% of the AzA foam group versus 38.2% of the vehicle foam group rated treatment tolerability as excellent, while 93.5% of the AzA foam group rated tolerability as acceptable, good, or excellent compared with 89.5% of the vehicle foam group. Only 1.4% of participants in the AzA foam group indicated that treatment was not acceptable due to irritation. In addition, a greater proportion of the AzA foam group reported cosmetic acceptability as very good versus the vehicle foam group (40.5% vs 28.7%)(Figure 2B), with two-thirds reporting cosmetic acceptability as very good or good. Practicability of product use in areas adjacent to the hairline was rated very good by substantial proportions of both the AzA foam and vehicle foam groups (42.8% vs 35.9%)(Figure 2C).

At baseline, average disease burden was moderate according to mean overall DLQI scores (SD) for the AzA foam (5.4 [4.8]) and vehicle foam (5.4 [4.9]) groups. Mean overall DLQI scores improved at EoT, with greater improvement occurring in the AzA foam group (2.6 vs 2.1; P=.018)(Figure 3). A larger proportion of participants in the AzA foam group versus the vehicle foam group also achieved a 5-point or more improvement in overall DLQI score (24.6% vs 19.0%; P=.047). Changes in specific DLQI subscore components were either balanced or in favor of the AzA foam group, including daily activities (0.5 vs 0.4; P=.019), symptoms and feelings (1.2 vs 1.0; P=.069), and leisure (0.5 vs 0.4; P=.012). Specific DLQI items with differences in scores between treatment groups from baseline included the following questions: Over the last week, how embarrassed or self-conscious have you been because of your skin? (P<.001); Over the last week, how much has your skin interfered with you going shopping or looking after your home or garden? (P=.005); Over the last week, how much has your skin affected any social or leisure activities? (P=.040); Over the last week, how much has your skin created problems with your partner or any of your close friends or relatives? (P=.001). Differences between treatment groups favored the AzA foam group for each of these items.

Participants in the AzA foam and vehicle foam groups also showed improvement in RosaQOL scores at EoT (6.8 vs 6.4; P=.67), while EQ-5D-5L scores changed minimally from baseline (0.006 vs 0.007; P=.50).
Safety
The incidence of drug-related adverse events (AEs) was greater in the AzA foam group versus the vehicle foam group (7.7% vs 4.8%). Drug-related AEs occurring in 1% of the AzA foam group were application-site pain including tenderness, stinging, and burning (3.5% for AzA foam vs 1.3% for vehicle foam); application-site pruritus (1.4% vs 0.4%); and application-site dryness (1.0% vs 0.6%). One drug-related AE of severe intensity—application-site dermatitis—occurred in the vehicle foam group; all other drug-related AEs were mild or moderate.14 More detailed safety results are described in a previous report.13
Comment
The PRO outcome data reported here are consistent with previously reported statistically significant improvements in investigator-assessed primary end points for the treatment of PPR with AzA foam.13,14 The data demonstrate that AzA foam benefits both clinical and patient-oriented dimensions of rosacea disease burden and suggest an association between positive treatment response and improved QOL.
Specifically, patient evaluation of treatment response to AzA foam was highly favorable, with 57.2% reporting excellent or good response and 85.1% reporting positive response overall. Recognizing the relapsing-remitting course of PPR, only 1.8% of the AzA foam group experienced worsening of disease at EoT.
The DLQI and RosaQOL instruments revealed notable improvements in QOL from baseline for both treatment groups. Although no significant differences in RosaQOL scores were observed between groups at EoT, significant differences in DLQI scores were detected. Almost one-quarter of participants in the AzA foam group achieved at least a 5-point improvement in DLQI score, exceeding the 4-point threshold for clinically meaningful change.17 Although little change in EQ-5D-5L scores was observed at EoT for both groups with no between-group differences, this finding is not unexpected, as this instrument assesses QOL dimensions such as loss of function, mobility, and ability to wash or dress, which are unlikely to be compromised in most rosacea patients.
Our results also underscore the importance of vehicle in the treatment of compromised skin. Studies of topical treatments for other dermatoses suggest that vehicle properties may reduce disease severity and improve QOL independent of active ingredients.10,18 For example, ease of application, minimal residue, and less time spent in application may explain the superiority of foam to other vehicles in the treatment of psoriasis.18 Our data demonstrating high cosmetic favorability of AzA foam are consistent with these prior observations. Increased tolerability of foam formulations also may affect response to treatment, in part by supporting adherence.18 Most participants receiving AzA foam described tolerability as excellent or good, and the discontinuation rate was low (1.2% of participants in the AzA foam group left the study due to AEs) in the setting of near-complete dosage administration (97% of expected doses applied).13
Conclusion
These results indicate that use of AzA foam as well as its novel vehicle results in high patient satisfaction and improved QOL. Although additional research is necessary to further delineate the relationship between PROs and other measures of clinical efficacy, our data demonstrate a positive treatment experience as perceived by patients that parallels the clinical efficacy of AzA foam for the treatment of PPR.13,14
Acknowledgment
Editorial support through inVentiv Medical Communications (New York, New York) was provided by Bayer Pharmaceuticals.
Rosacea is a chronic inflammatory disorder that may negatively impact patients’ quality of life (QOL).1,2 Papulopustular rosacea (PPR) is characterized by centrofacial inflammatory lesions and erythema as well as burning and stinging secondary to skin barrier dysfunction.3-5 Increasing rosacea severity is associated with greater rates of anxiety and depression and lower QOL6 as well as low self-esteem and feelings of embarrassment.7,8 Accordingly, assessing patient perceptions of rosacea treatments is necessary for understanding its impact on patient health.6,9
The Rosacea International Expert Group has emphasized the need to incorporate patient assessments of disease severity and QOL when developing therapeutic strategies for rosacea.7 Ease of use, sensory experience, and patient preference also are important dimensions in the evaluation of topical medications, as attributes of specific formulations may affect usability, adherence, and efficacy.10,11
An azelaic acid (AzA) 15% foam formulation, which was approved by the US Food and Drug Administration in 2015, was developed to deliver AzA in a vehicle designed to improve treatment experience in patients with mild to moderate PPR.12 Results from a clinical trial demonstrated superiority of AzA foam to vehicle foam for primary end points that included therapeutic success rate and change in inflammatory lesion count.13,14 Secondary end points assessed in the current analysis included patient perception of product usability, efficacy, and effect on QOL. These patient-reported outcome (PRO) results are reported here.
Methods
Study Design
The design of this phase 3 multicenter, randomized, double-blind, vehicle-controlled, parallel-group clinical trial was described in more detail in an earlier report.13 This study was approved by all appropriate institutional review boards. Eligible participants were 18 years and older with moderate or severe PPR, 12 to 50 inflammatory lesions, and persistent erythema with or without telangiectasia. Exclusion criteria included known nonresponse to AzA, current or prior use (within 6 weeks of randomization) of noninvestigational products to treat rosacea, and presence of other dermatoses that could interfere with rosacea evaluation.
Participants were randomized into the AzA foam or vehicle group (1:1 ratio). The study medication (0.5 g) or vehicle foam was applied twice daily to the entire face until the end of treatment (EoT) at 12 weeks. Efficacy and safety parameters were evaluated at baseline and at 4, 8, and 12 weeks of treatment, and at a follow-up visit 4 weeks after EoT (week 16).
Results for the coprimary efficacy end points—therapeutic success rate according to investigator global assessment and nominal change in inflammatory lesion count—were previously reported,13 as well as secondary efficacy outcomes including change in inflammatory lesion count, therapeutic response rate, and change in erythema rating.14
Patient-Reported Secondary Efficacy Outcomes
The secondary PRO end points were patient-reported global assessment of treatment response (rated as excellent, good, fair, none, or worse), global assessment of tolerability (rated as excellent, good, acceptable despite minor irritation, less acceptable due to continuous irritation, not acceptable, or no opinion), and opinion on cosmetic acceptability and practicability of product use in areas adjacent to the hairline (rated as very good, good, satisfactory, poor, or no opinion).
Additionally, QOL was measured by 3 validated standardized PRO tools, including the Rosacea Quality of Life Index (RosaQOL),15 the EuroQOL 5-dimension 5-level questionnaire (EQ-5D-5L),16 and the Dermatology Life Quality Index (DLQI). The RosaQOL is a rosacea-specific instrument assessing 3 constructs: (1) symptom, (2) emotion, and (3) function. The EQ-5D-5L questionnaire measures overall health status and comprises 5 constructs: (1) mobility, (2) self-care, (3) usual activities, (4) pain/discomfort, and (5) anxiety/depression. The DLQI is a general, dermatology-oriented instrument categorized into 6 constructs: (1) symptoms and feelings, (2) daily activities, (3) leisure, (4) work and school, (5) personal relationships, and (6) treatment.
Statistical Analyses
Patient-reported outcomes were analyzed in an exploratory manner and evaluated at EoT relative to baseline. Self-reported global assessment of treatment response and change in RosaQOL, EQ-5D-5L, and DLQI scores between AzA foam and vehicle foam groups were evaluated using the Wilcoxon rank sum test. Categorical change in the number of participants achieving an increase of 5 or more points in overall DLQI score was evaluated using a χ2 test.
Safety
Safety was analyzed for all randomized patients who were dispensed any study medication. All analyses were performed using SAS version 9.2.
Results
Of the 961 participants included in the study, 483 were randomized to receive AzA foam and 478 were randomized to receive vehicle foam. The mean age was 51.5 years, and the majority of participants were female (73.0%) and white (95.5%)(Table). At baseline, 834 (86.8%) participants had moderate PPR and 127 (13.2%) had severe PPR. The mean inflammatory lesion count (SD) was 21.4 (8.9). No significant differences in baseline characteristics were observed between treatment groups.

Patient-reported global assessment of treatment response differed between treatment groups at EoT (P<.001)(Figure 1). Higher ratings of treatment response were reported among the AzA foam group (excellent, 17.2%; good, 40.0%) versus vehicle foam (excellent, 9.7%; good, 35.0%). The number of participants reporting no treatment response was 13.1% in the AzA foam group, with 1.8% reporting worsening of their condition, while 19.4% of participants in the vehicle foam group reported no response, with 6.3% reporting worsening of their condition (Figure 1).

Tolerability was rated excellent or good in 67.8% of the AzA foam group versus 78.2% of the vehicle foam group (Figure 2A). Approximately 38.4% of the AzA foam group versus 38.2% of the vehicle foam group rated treatment tolerability as excellent, while 93.5% of the AzA foam group rated tolerability as acceptable, good, or excellent compared with 89.5% of the vehicle foam group. Only 1.4% of participants in the AzA foam group indicated that treatment was not acceptable due to irritation. In addition, a greater proportion of the AzA foam group reported cosmetic acceptability as very good versus the vehicle foam group (40.5% vs 28.7%)(Figure 2B), with two-thirds reporting cosmetic acceptability as very good or good. Practicability of product use in areas adjacent to the hairline was rated very good by substantial proportions of both the AzA foam and vehicle foam groups (42.8% vs 35.9%)(Figure 2C).

At baseline, average disease burden was moderate according to mean overall DLQI scores (SD) for the AzA foam (5.4 [4.8]) and vehicle foam (5.4 [4.9]) groups. Mean overall DLQI scores improved at EoT, with greater improvement occurring in the AzA foam group (2.6 vs 2.1; P=.018)(Figure 3). A larger proportion of participants in the AzA foam group versus the vehicle foam group also achieved a 5-point or more improvement in overall DLQI score (24.6% vs 19.0%; P=.047). Changes in specific DLQI subscore components were either balanced or in favor of the AzA foam group, including daily activities (0.5 vs 0.4; P=.019), symptoms and feelings (1.2 vs 1.0; P=.069), and leisure (0.5 vs 0.4; P=.012). Specific DLQI items with differences in scores between treatment groups from baseline included the following questions: Over the last week, how embarrassed or self-conscious have you been because of your skin? (P<.001); Over the last week, how much has your skin interfered with you going shopping or looking after your home or garden? (P=.005); Over the last week, how much has your skin affected any social or leisure activities? (P=.040); Over the last week, how much has your skin created problems with your partner or any of your close friends or relatives? (P=.001). Differences between treatment groups favored the AzA foam group for each of these items.

Participants in the AzA foam and vehicle foam groups also showed improvement in RosaQOL scores at EoT (6.8 vs 6.4; P=.67), while EQ-5D-5L scores changed minimally from baseline (0.006 vs 0.007; P=.50).
Safety
The incidence of drug-related adverse events (AEs) was greater in the AzA foam group versus the vehicle foam group (7.7% vs 4.8%). Drug-related AEs occurring in 1% of the AzA foam group were application-site pain including tenderness, stinging, and burning (3.5% for AzA foam vs 1.3% for vehicle foam); application-site pruritus (1.4% vs 0.4%); and application-site dryness (1.0% vs 0.6%). One drug-related AE of severe intensity—application-site dermatitis—occurred in the vehicle foam group; all other drug-related AEs were mild or moderate.14 More detailed safety results are described in a previous report.13
Comment
The PRO outcome data reported here are consistent with previously reported statistically significant improvements in investigator-assessed primary end points for the treatment of PPR with AzA foam.13,14 The data demonstrate that AzA foam benefits both clinical and patient-oriented dimensions of rosacea disease burden and suggest an association between positive treatment response and improved QOL.
Specifically, patient evaluation of treatment response to AzA foam was highly favorable, with 57.2% reporting excellent or good response and 85.1% reporting positive response overall. Recognizing the relapsing-remitting course of PPR, only 1.8% of the AzA foam group experienced worsening of disease at EoT.
The DLQI and RosaQOL instruments revealed notable improvements in QOL from baseline for both treatment groups. Although no significant differences in RosaQOL scores were observed between groups at EoT, significant differences in DLQI scores were detected. Almost one-quarter of participants in the AzA foam group achieved at least a 5-point improvement in DLQI score, exceeding the 4-point threshold for clinically meaningful change.17 Although little change in EQ-5D-5L scores was observed at EoT for both groups with no between-group differences, this finding is not unexpected, as this instrument assesses QOL dimensions such as loss of function, mobility, and ability to wash or dress, which are unlikely to be compromised in most rosacea patients.
Our results also underscore the importance of vehicle in the treatment of compromised skin. Studies of topical treatments for other dermatoses suggest that vehicle properties may reduce disease severity and improve QOL independent of active ingredients.10,18 For example, ease of application, minimal residue, and less time spent in application may explain the superiority of foam to other vehicles in the treatment of psoriasis.18 Our data demonstrating high cosmetic favorability of AzA foam are consistent with these prior observations. Increased tolerability of foam formulations also may affect response to treatment, in part by supporting adherence.18 Most participants receiving AzA foam described tolerability as excellent or good, and the discontinuation rate was low (1.2% of participants in the AzA foam group left the study due to AEs) in the setting of near-complete dosage administration (97% of expected doses applied).13
Conclusion
These results indicate that use of AzA foam as well as its novel vehicle results in high patient satisfaction and improved QOL. Although additional research is necessary to further delineate the relationship between PROs and other measures of clinical efficacy, our data demonstrate a positive treatment experience as perceived by patients that parallels the clinical efficacy of AzA foam for the treatment of PPR.13,14
Acknowledgment
Editorial support through inVentiv Medical Communications (New York, New York) was provided by Bayer Pharmaceuticals.
- Cardwell LA, Farhangian ME, Alinia H, et al. Psychological disorders associated with rosacea: analysis of unscripted comments. J Dermatol Surg. 2015;19:99-103.
- Moustafa F, Lewallen RS, Feldman SR. The psychological impact of rosacea and the influence of current management options. J Am Acad Dermatol. 2014;71:973-980.
- Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Yamasaki K, Gallo RL. The molecular pathology of rosacea. J Dermatol Sci. 2009;55:77-81.
- Del Rosso JQ. Advances in understanding and managing rosacea: part 1: connecting the dots between pathophysiological mechanisms and common clinical features of rosacea with emphasis on vascular changes and facial erythema. J Clin Aesthet Dermatol. 2012;5:16-25.
- Bohm D, Schwanitz P, Stock Gissendanner S, et al. Symptom severity and psychological sequelae in rosacea: results of a survey. Psychol Health Med. 2014;19:586-591.
- Elewski BE, Draelos Z, Dreno B, et al. Rosacea—global diversity and optimized outcome: proposed international consensus from the Rosacea International Expert Group. J Eur Acad Dermatol Venereol. 2011;25:188-200.
- Dirschka T, Micali G, Papadopoulos L, et al. Perceptions on the psychological impact of facial erythema associated with rosacea: results of international survey [published online May 29, 2015]. Dermatol Ther (Heidelb). 2015;5:117-127.
- Abram K, Silm H, Maaroos HI, et al. Subjective disease perception and symptoms of depression in relation to healthcare-seeking behaviour in patients with rosacea. Acta Derm Venereol. 2009;89:488-491.
- Stein L. Clinical studies of a new vehicle formulation for topical corticosteroids in the treatment of psoriasis. J Am Acad Dermatol. 2005;53(1, suppl 1):S39-S49.
- Yentzer BA, Camacho FT, Young T, et al. Good adherence and early efficacy using desonide hydrogel for atopic dermatitis: results from a program addressing patient compliance. J Drugs Dermatol. 2010;9:324-329.
- Finacea (azelaic acid) foam 15% [package insert]. Whippany, NJ: Bayer Pharmaceuticals; 2015.
- Draelos ZD, Elewski BE, Harper JC, et al. A phase 3 randomized, double-blind, vehicle-controlled trial of azelaic acid foam 15% in the treatment of papulopustular rosacea. Cutis. 2015;96:54-61.
- Solomon JA, Tyring S, Staedtler G, et al. Investigator-reported efficacy of azelaic acid foam 15% in patients with papulopustular rosacea: secondary efficacy outcomes from a randomized, controlled, double-blind, phase 3 trial. Cutis. 2016;98:187-194.
- Nicholson K, Abramova L, Chren MM, et al. A pilot quality-of-life instrument for acne rosacea. J Am Acad Dermatol. 2007;57:213-221.
- Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20:1727-1736.
- Basra MK, Salek MS, Camilleri L, et al. Determining the minimal clinically important difference and responsiveness of the Dermatology Life Quality Index (DLQI): further data. Dermatology. 2015;230:27-33.
- Bergstrom KG, Arambula K, Kimball AB. Medication formulation affects quality of life: a randomized single-blind study of clobetasol propionate foam 0.05% compared with a combined program of clobetasol cream 0.05% and solution 0.05% for the treatment of psoriasis. Cutis. 2003;72:407-411.
- Cardwell LA, Farhangian ME, Alinia H, et al. Psychological disorders associated with rosacea: analysis of unscripted comments. J Dermatol Surg. 2015;19:99-103.
- Moustafa F, Lewallen RS, Feldman SR. The psychological impact of rosacea and the influence of current management options. J Am Acad Dermatol. 2014;71:973-980.
- Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Yamasaki K, Gallo RL. The molecular pathology of rosacea. J Dermatol Sci. 2009;55:77-81.
- Del Rosso JQ. Advances in understanding and managing rosacea: part 1: connecting the dots between pathophysiological mechanisms and common clinical features of rosacea with emphasis on vascular changes and facial erythema. J Clin Aesthet Dermatol. 2012;5:16-25.
- Bohm D, Schwanitz P, Stock Gissendanner S, et al. Symptom severity and psychological sequelae in rosacea: results of a survey. Psychol Health Med. 2014;19:586-591.
- Elewski BE, Draelos Z, Dreno B, et al. Rosacea—global diversity and optimized outcome: proposed international consensus from the Rosacea International Expert Group. J Eur Acad Dermatol Venereol. 2011;25:188-200.
- Dirschka T, Micali G, Papadopoulos L, et al. Perceptions on the psychological impact of facial erythema associated with rosacea: results of international survey [published online May 29, 2015]. Dermatol Ther (Heidelb). 2015;5:117-127.
- Abram K, Silm H, Maaroos HI, et al. Subjective disease perception and symptoms of depression in relation to healthcare-seeking behaviour in patients with rosacea. Acta Derm Venereol. 2009;89:488-491.
- Stein L. Clinical studies of a new vehicle formulation for topical corticosteroids in the treatment of psoriasis. J Am Acad Dermatol. 2005;53(1, suppl 1):S39-S49.
- Yentzer BA, Camacho FT, Young T, et al. Good adherence and early efficacy using desonide hydrogel for atopic dermatitis: results from a program addressing patient compliance. J Drugs Dermatol. 2010;9:324-329.
- Finacea (azelaic acid) foam 15% [package insert]. Whippany, NJ: Bayer Pharmaceuticals; 2015.
- Draelos ZD, Elewski BE, Harper JC, et al. A phase 3 randomized, double-blind, vehicle-controlled trial of azelaic acid foam 15% in the treatment of papulopustular rosacea. Cutis. 2015;96:54-61.
- Solomon JA, Tyring S, Staedtler G, et al. Investigator-reported efficacy of azelaic acid foam 15% in patients with papulopustular rosacea: secondary efficacy outcomes from a randomized, controlled, double-blind, phase 3 trial. Cutis. 2016;98:187-194.
- Nicholson K, Abramova L, Chren MM, et al. A pilot quality-of-life instrument for acne rosacea. J Am Acad Dermatol. 2007;57:213-221.
- Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20:1727-1736.
- Basra MK, Salek MS, Camilleri L, et al. Determining the minimal clinically important difference and responsiveness of the Dermatology Life Quality Index (DLQI): further data. Dermatology. 2015;230:27-33.
- Bergstrom KG, Arambula K, Kimball AB. Medication formulation affects quality of life: a randomized single-blind study of clobetasol propionate foam 0.05% compared with a combined program of clobetasol cream 0.05% and solution 0.05% for the treatment of psoriasis. Cutis. 2003;72:407-411.
Practice Points
- Patient perceptions of treatment are an important consideration in developing topical therapeutic strategies for papulopustular rosacea.
- A novel hydrophilic foam formulation of azelaic acid (AzA) provided substantial benefits in patient-reported measures of treatment response and quality of life.
- Patients reported high levels of satisfaction with the usability, tolerability, and practicability of AzA foam.
- The positive treatment experience described by patients parallels investigator-reported measures of clinical efficacy reported elsewhere.
Investigator-Reported Efficacy of Azelaic Acid Foam 15% in Patients With Papulopustular Rosacea: Secondary Efficacy Outcomes From a Randomized, Controlled, Double-blind, Phase 3 Trial
Papulopustular rosacea (PPR) is characterized by centrofacial papules, pustules, erythema, and occasionally telangiectasia.1,2 A myriad of factors, including genetic predisposition3 and environmental triggers,4 have been associated with dysregulated inflammatory responses,5 contributing to the disease pathogenesis and symptoms. Inflammation associated with PPR may decrease skin barrier function, increase transepidermal water loss, and reduce stratum corneum hydration,6,7 resulting in heightened skin sensitivity, pain, burning, and/or stinging.5,8
Azelaic acid (AzA), which historically has only been available in gel or cream formulations, is well established for the treatment of rosacea9; however, these formulations have been associated with application-site adverse events (AEs)(eg, burning, erythema, irritation), limited cosmetic acceptability, and reduced compliance or efficacy.10
For select skin conditions, active agents delivered in foam vehicles may offer superior tolerability with improved outcomes.11 An AzA foam 15% formulation was approved for the treatment of mild to moderate PPR. Primary outcomes from a phase 3 trial demonstrated the efficacy and safety of AzA foam in improving inflammatory lesion counts (ILCs) and disease severity in participants with PPR. The trial also evaluated additional secondary end points, including the effect of AzA foam on erythema, inflammatory lesions, treatment response, and other manifestations of PPR.12 The current study evaluated investigator-reported efficacy outcomes for these secondary end points for AzA foam 15% versus vehicle foam.
Methods
Study Design
This phase 3 multicenter, randomized, double-blind, vehicle-controlled, parallel-group clinical trial was conducted from September 2012 to January 2014 at 48 US study centers comparing the efficacy of AzA foam versus vehicle foam in patients with PPR. Eligible participants were 18 years and older with PPR rated as moderate or severe according to investigator global assessment (IGA), plus 12 to 50 inflammatory lesions and persistent erythema with or without telangiectasia. Exclusion criteria included known nonresponse to AzA, current or prior use (within 6 weeks of randomization) of noninvestigational products to treat rosacea, and presence of other dermatoses that could interfere with rosacea evaluation.
Participants were randomized into the AzA foam or vehicle group (1:1 ratio). The study medication was applied in 0.5-g doses twice daily until the end of treatment (EoT) at 12 weeks. Efficacy and safety parameters were evaluated at baseline and at 4, 8, and 12 weeks of treatment, and at a follow-up visit 4 weeks after EoT (week 16).
Results for the coprimary efficacy end points—therapeutic success rate according to IGA and nominal change in ILC—were previously reported.12
Investigator-Reported Secondary Efficacy Outcomes
The secondary efficacy end points were grouped change in erythema rating, grouped change in telangiectasia rating, grouped change in IGA score, therapeutic response rate according to IGA, percentage change in ILC from baseline, and facial skin color rating at EoT.
Grouped change for all secondary end points was measured as improved, no change, or worsened relative to baseline. For grouped change in erythema and telangiectasia ratings, a participant was considered improved if the rating at the postbaseline visit was lower than the baseline rating, no change if the postbaseline and baseline ratings were identical, and worsened if the postbaseline rating was higher than at baseline. For grouped change in IGA score, a participant was considered improved if a responder showed at least a 1-step improvement postbaseline compared to baseline, no change if postbaseline and baseline ratings were identical, and worsened if the postbaseline rating was higher than at baseline.
For the therapeutic response rate, a participant was considered a treatment responder if the IGA score improved from baseline and resulted in clear, minimal, or mild disease severity at EoT.
Safety
Adverse events also were assessed.
Statistical Analyses
Secondary efficacy and safety end points were assessed for all randomized participants who were dispensed the study medication. Missing data were imputed using last observation carried forward.
For the percentage change in ILC from baseline, therapeutic response rate, and grouped change in erythema rating, confirmatory analyses were conducted in a hierarchical manner (in the order listed), with testing stopped as soon as a null hypothesis of superior treatment effect could not be rejected. Analyses without significance level were exploratory. The Cochran-Mantel-Haenszel van Elteren test stratified by study center was used for grouped change in erythema rating (1-tailed, 2.5%) and IGA score (2-tailed, 5%); Wilcoxon rank sum tests also were performed. Percentage change in ILC from baseline was evaluated using the Student t test and F test of analysis of covariance (1-tailed, 2.5%). Therapeutic response rate was evaluated using the Cochran-Mantel-Haenszel van Elteren test stratified by study center and the Pearson χ2 test. Facial skin color and grouped change in telangiectasia rating were evaluated using the Wilcoxon rank sum test.
Adverse events beginning or worsening after the first dose of the study drug were considered treatment emergent and were coded using the Medical Dictionary for Regulatory Activities (MedDRA) Version 16.1. Statistical analyses were performed using SAS software version 9.2.
Results
Study Participants
The study included 961 total participants; 483 were randomized to the AzA foam group and 478 to the vehicle group (Figure 1). Overall, 803 participants completed follow-up; however, week 16 results for the efficacy outcomes include data for 4 additional patients (2 per study arm) who did not formally meet all requirements for follow-up completion. The mean age was 51.5 years, and the majority of the participants were white and female (Table 1). Most participants (86.8%) had moderate PPR at baseline, with the remaining rated as having severe disease (13.2%). The majority (76.4%) had more than 14 inflammatory lesions with moderate (76.4%) or severe (15.1%) erythema at baseline.

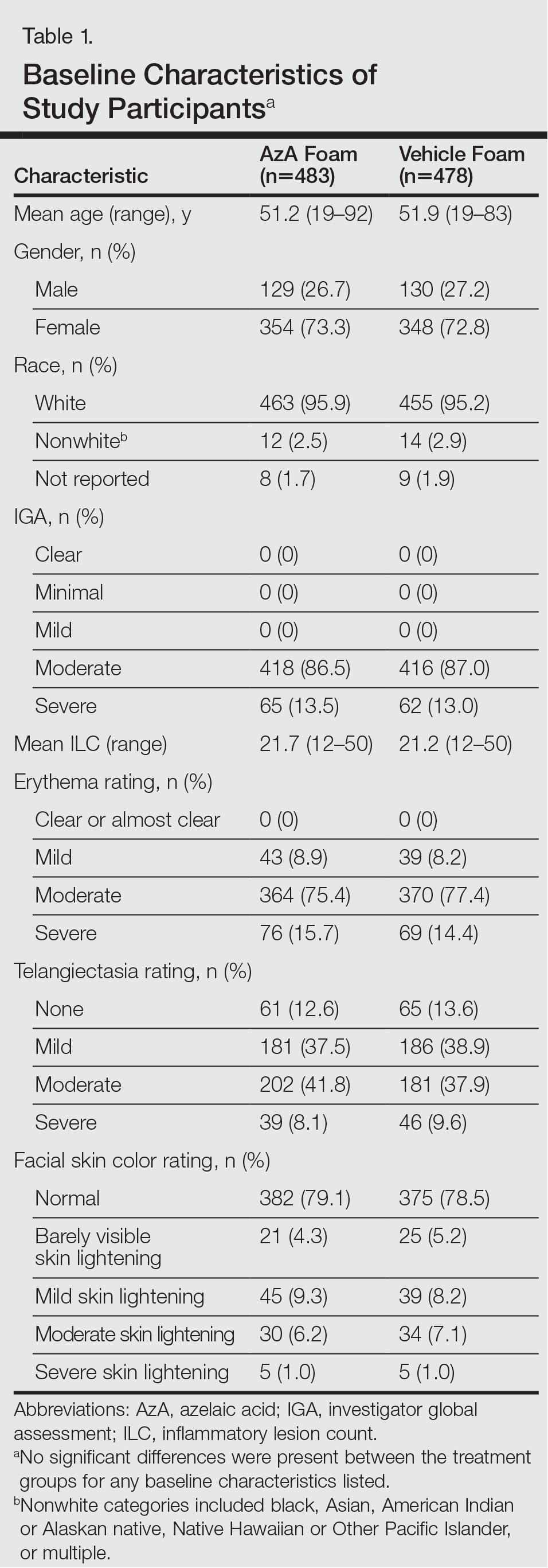
Efficacy
Significantly more participants in the AzA group than in the vehicle group showed an improved erythema rating at EoT (61.5% vs 51.3%; P<.001)(Figure 2), with more participants in the AzA group showing improvement at weeks 4 (P=.022) and 8 (P=.002).

A significantly greater mean percentage reduction in ILC from baseline to EoT was observed in the AzA group versus the vehicle group (61.6% vs 50.8%; P<.001)(Figure 3), and between-group differences were observed at week 4 (P<.001), week 8 (P=.003), and week 16 (end of study/follow-up)(P=.002).

A significantly higher proportion of participants treated with AzA foam versus vehicle were considered responders at week 12/EoT (66.3% vs 54.4%; P<.001)(Figure 4). Differences in responder rate also were observed at week 4 (P=.026) and week 8 (P=.026).

No study drug was administered between week 12/EoT and week 16/follow-up; last observation carried forward was not applied to week 16/follow-up analysis. AzA indicates azelaic acid; IGA, investigator global assessment.
Differences in grouped change in IGA score were observed between groups at every evaluation during the treatment phase (Figure 5). Specifically, IGA score was improved at week 12/EoT relative to baseline in 71.2% of participants in the AzA group versus 58.8% in the vehicle group (P<.001).

For grouped change in telangiectasia rating at EoT, the majority of participants in both treatment groups showed no change (Table 2). Regarding facial skin color, the majority of participants in both the AzA and vehicle treatment groups (80.1% and 78.7%, respectively) showed normal skin color compared to nontreated skin EoT; no between-group differences were detected for facial skin color rating (P=.315, Wilcoxon rank sum test).
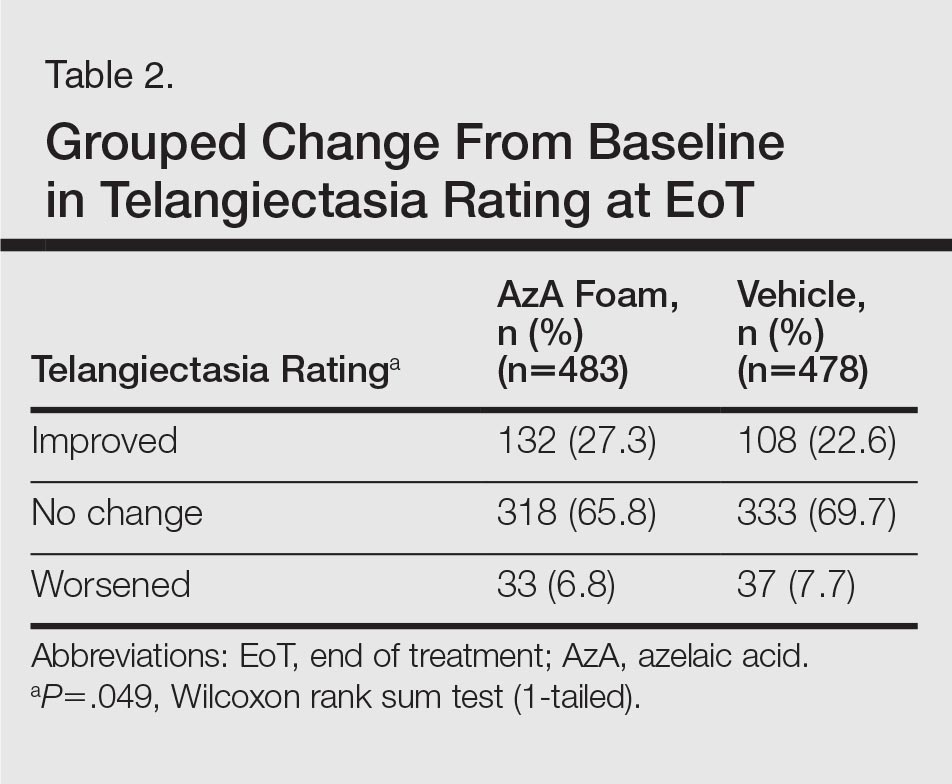
Safety
The incidence of drug-related AEs was greater in the AzA group than the vehicle group (7.7% vs 4.8%)(Table 3). Drug-related AEs occurring in at least 1% of the AzA group were pain at application site (eg, tenderness, stinging, burning)(AzA group, 3.5%; vehicle group, 1.3%), application-site pruritus (1.4% vs 0.4%), and application-site dryness (1.0% vs 0.6%). A single drug-related AE of severe intensity (ie, application-site dermatitis) was observed in the vehicle group; all other drug-related AEs were mild or moderate. The incidence of withdrawals due to AEs was lower in the AzA group than the vehicle group (1.2% vs 2.5%). This AE profile correlated with a treatment compliance (the percentage of expected doses that were actually administered) of 97.0% in the AzA group and 95.9% in the vehicle group. One participant in the vehicle group died due to head trauma unrelated to administration of the study drug.
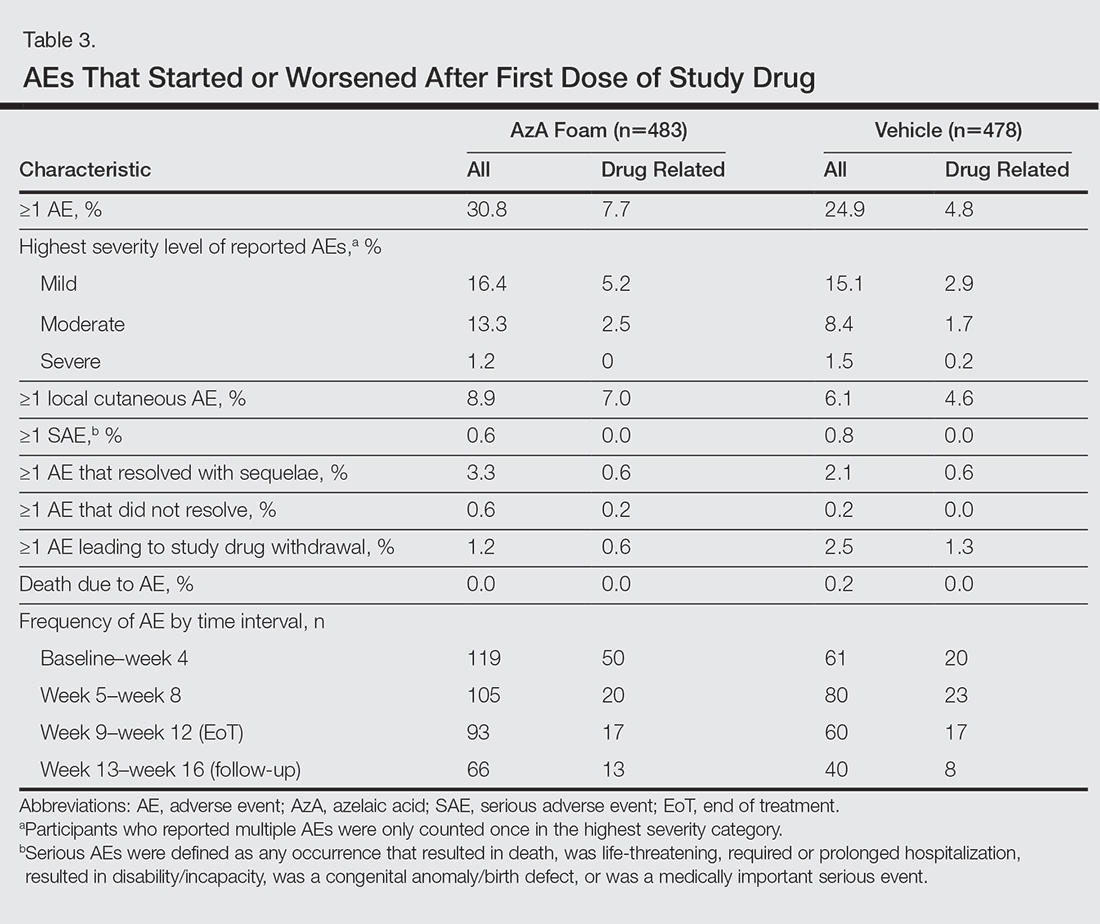
Comment
The results of this study further support the efficacy of AzA foam for the treatment of PPR. The percentage reduction in ILC was consistent with nominal decreases in ILC, a coprimary efficacy end point of this study.12 Almost two-thirds of participants treated with AzA foam achieved a therapeutic response, indicating that many participants who did not strictly achieve the primary outcome of therapeutic success nevertheless attained notable reductions in disease severity. The number of participants who showed any improvement on the IGA scale increased throughout the course of treatment (63.8% AzA foam vs 55.0% vehicle at week 8) up to EoT (71.2% vs 58.8%)(Figure 5). In addition, the number of participants showing any improvement at week 8 (63.8% AzA foam vs 55.0% vehicle)(Figure 5) was comparable to the number of participants achieving therapeutic response at week 12/EoT (66.3% vs 54.4%)(Figure 4). These data suggest that increasing time of treatment increases the likelihood of achieving better results.
Erythema also appeared to respond to AzA foam, with 10.2% more participants in the AzA group demonstrating improvement at week 12/EoT compared to vehicle. The difference in grouped change in erythema rating also was statistically significant and favored AzA foam, sustained up to 4 weeks after EoT.
The outcomes for percentage change in ILC, therapeutic response rate, and grouped change in erythema rating consequently led to the rejection of all 3 null hypotheses in hierarchical confirmatory analyses, underscoring the benefits of AzA foam treatment.
The therapeutic effects of AzA foam were apparent at the first postbaseline evaluation and persisted throughout treatment. Differences favoring AzA foam were observed at every on-treatment evaluation for grouped change in erythema rating, percentage change in ILC, therapeutic response rate, and grouped change in IGA score. Symptoms showed minimal resurgence after treatment cessation, and there were no signs of disease flare-up within the 4 weeks of observational follow-up. In addition, the percentage reduction in ILC remained higher in the AzA foam group during follow-up.
These results also show that AzA foam was well tolerated with a low incidence of discontinuation because of drug-related AEs. No serious drug-related AEs were reported for this study or in the preceding phase 2 trial.12,13 Although not directly evaluated, the low incidence of cutaneous AEs suggests that AzA foam may be better tolerated than prior formulations of AzA14,15 and correlates with high compliance observed during the study.12 Azelaic acid foam appeared to have minimal to no effect on skin color, with more than 88% of participants reporting barely visible or no skin lightening.
Interestingly, the vehicle foam showed appreciable efficacy independent of AzA. Improvements in erythema were recorded in approximately half of the vehicle group at week 12/EoT. A similar proportion attained a therapeutic response, and ILC was reduced by 50.8% at week 12/EoT. Comparable results also were evident in the vehicle group for the primary end points of this study.12 Vehicles in dermatologic trials frequently exert effects on diseased skin16,17 via a skin care regimen effect (eg, moisturization and other vehicle-related effects that may improve skin barrier integrity and function) and thus should not be regarded as placebo controls. The mechanism underlying this efficacy may be due to the impact of vehicle composition on skin barrier integrity and transepidermal water loss.18 The hydrophilic emulsion or other constituents of AzA foam (eg, fatty alcohols) may play a role.
A notable strength of our study is detailed clinical characterization using carefully chosen parameters and preplanned analyses that complement the primary end points. As the latter are often driven by regulatory requirements, opportunities to characterize other outcomes of interest to clinicians may be missed. The additional analyses reported here hopefully will aid dermatologists in both assessing the role of AzA foam in the treatment armamentarium for PPR and counseling patients.
Because participants with lighter skin pigmentation dominated our study population, the impact of AzA foam among patients with darker skin complexions is unknown. Although AzA is unlikely to cause hypopigmentation in normal undiseased skin, patients should be monitored for early signs of hypopigmentation.19,20 Our data also do not allow assessment of the differential effect, if any, of AzA foam on erythema of different etiologies in PPR, as corresponding information was not collected in the trial.
Conclusion
Azelaic acid foam 15% combines a well-established treatment of PPR with new vehicle technology to deliver effective therapy across multiple disease dimensions. In addition, the vehicle foam appears to demonstrate inherent therapeutic properties independent of AzA. The availability of this novel, efficacious, and well-tolerated option for PPR has the potential to improve patient care, reduce disease burden, and minimize unnecessary costs through increased tolerability and compliance.21
Acknowledgment
Editorial support through inVentiv Medical Communications (New York, New York) was provided by Bayer Pharmaceuticals.
- Tan J, Berg M. Rosacea: current state of epidemiology. J Am Acad Dermatol. 2013;69(6, suppl 1):S27-S35.
- Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Chang AL, Raber I, Xu J, et al. Assessment of the genetic basis of rosacea by genome-wide association study. J Invest Dermatol. 2015;135:1548-1555.
- Abram K, Silm H, Maaroos HI, et al. Risk factors associated with rosacea. J Eur Acad Dermatol Venereol. 2010;24:565-571.
- Yamasaki K, Di Nardo A, Bardan A, et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med. 2007;13:975-980.
- Yamasaki K, Kanada K, Macleod DT, et al. TLR2 expression is increased in rosacea and stimulates enhanced serine protease production by keratinocytes. J Invest Dermatol. 2011;131:688-697.
- Darlenski R, Kazandjieva J, Tsankov N, et al. Acute irritant threshold correlates with barrier function, skin hydration and contact hypersensitivity in atopic dermatitis and rosacea. Exp Dermatol. 2013;22:752-753.
- Del Rosso JQ, Levin J. The clinical relevance of maintaining the functional integrity of the stratum corneum in both healthy and disease-affected skin. J Clin Aesthet Dermatol. 2011;4:22-42.
- van Zuuren EJ, Kramer SF, Carter BR, et al. Effective and evidence-based management strategies for rosacea: summary of a Cochrane systematic review. Br J Dermatol. 2011;165:760-781.
- Tan X, Feldman SR, Chang J, et al. Topical drug delivery systems in dermatology: a review of patient adherence issues. Expert Opin Drug Deliv. 2012;9:1263-1271.
- Stein L. Clinical studies of a new vehicle formulation for topical corticosteroids in the treatment of psoriasis. J Am Acad Dermatol. 2005;53(1, suppl 1):S39-S49.
- Draelos ZD, Elewski BE, Harper JC, et al. A phase 3 randomized, double-blind, vehicle-controlled trial of azelaic acid foam 15% in the treatment of papulopustular rosacea. Cutis. 2015;96:54-61.
- Draelos ZD, Elewski B, Staedtler G, et al. Azelaic acid foam 15% in the treatment of papulopustular rosacea: a randomized, double-blind, vehicle-controlled study. Cutis. 2013;92:306-317.
- Finacea gel [package insert]. Whippany, NJ: Bayer HealthCare Pharmaceuticals Inc; 2016.
- Elewski BE, Fleischer AB Jr, Pariser DM. A comparison of 15% azelaic acid gel and 0.75% metronidazole gel in the topical treatment of papulopustular rosacea: results of a randomized trial. Arch Dermatol. 2003;139:1444-1450.
- Daniels R, Knie U. Galenics of dermal products—vehicles, properties and drug release. J Dtsch Dermatol Ges. 2007;5:367-383.
- Shamsudin N, Fleischer AB Jr. Vehicle or placebo? Investigators use incorrect terminology in randomized controlled trials half of the time: a systematic review of randomized controlled trials published in three major dermatology journals. J Drugs Dermatol. 2010;9:1221-1226.
- Del Rosso JQ, Thiboutot D, Gallo R, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 2: a status report on topical agents. Cutis. 2013;92:277-284.
- Finacea foam [package insert]. Whippany, NJ: Bayer HealthCare Pharmaceuticals Inc; 2015.
- Solano F, Briganti S, Picardo M, et al. Hypopigmenting agents: an updated review on biological, chemical and clinical aspects. Pigment Cell Res. 2006;19:550-571.
- Hammarstrom B, Wessling A, Nilsson JL. Pharmaceutical care for patients with skin diseases: a campaign year at Swedish pharmacies. J Clin Pharm Ther. 1995;20:327-334.
Papulopustular rosacea (PPR) is characterized by centrofacial papules, pustules, erythema, and occasionally telangiectasia.1,2 A myriad of factors, including genetic predisposition3 and environmental triggers,4 have been associated with dysregulated inflammatory responses,5 contributing to the disease pathogenesis and symptoms. Inflammation associated with PPR may decrease skin barrier function, increase transepidermal water loss, and reduce stratum corneum hydration,6,7 resulting in heightened skin sensitivity, pain, burning, and/or stinging.5,8
Azelaic acid (AzA), which historically has only been available in gel or cream formulations, is well established for the treatment of rosacea9; however, these formulations have been associated with application-site adverse events (AEs)(eg, burning, erythema, irritation), limited cosmetic acceptability, and reduced compliance or efficacy.10
For select skin conditions, active agents delivered in foam vehicles may offer superior tolerability with improved outcomes.11 An AzA foam 15% formulation was approved for the treatment of mild to moderate PPR. Primary outcomes from a phase 3 trial demonstrated the efficacy and safety of AzA foam in improving inflammatory lesion counts (ILCs) and disease severity in participants with PPR. The trial also evaluated additional secondary end points, including the effect of AzA foam on erythema, inflammatory lesions, treatment response, and other manifestations of PPR.12 The current study evaluated investigator-reported efficacy outcomes for these secondary end points for AzA foam 15% versus vehicle foam.
Methods
Study Design
This phase 3 multicenter, randomized, double-blind, vehicle-controlled, parallel-group clinical trial was conducted from September 2012 to January 2014 at 48 US study centers comparing the efficacy of AzA foam versus vehicle foam in patients with PPR. Eligible participants were 18 years and older with PPR rated as moderate or severe according to investigator global assessment (IGA), plus 12 to 50 inflammatory lesions and persistent erythema with or without telangiectasia. Exclusion criteria included known nonresponse to AzA, current or prior use (within 6 weeks of randomization) of noninvestigational products to treat rosacea, and presence of other dermatoses that could interfere with rosacea evaluation.
Participants were randomized into the AzA foam or vehicle group (1:1 ratio). The study medication was applied in 0.5-g doses twice daily until the end of treatment (EoT) at 12 weeks. Efficacy and safety parameters were evaluated at baseline and at 4, 8, and 12 weeks of treatment, and at a follow-up visit 4 weeks after EoT (week 16).
Results for the coprimary efficacy end points—therapeutic success rate according to IGA and nominal change in ILC—were previously reported.12
Investigator-Reported Secondary Efficacy Outcomes
The secondary efficacy end points were grouped change in erythema rating, grouped change in telangiectasia rating, grouped change in IGA score, therapeutic response rate according to IGA, percentage change in ILC from baseline, and facial skin color rating at EoT.
Grouped change for all secondary end points was measured as improved, no change, or worsened relative to baseline. For grouped change in erythema and telangiectasia ratings, a participant was considered improved if the rating at the postbaseline visit was lower than the baseline rating, no change if the postbaseline and baseline ratings were identical, and worsened if the postbaseline rating was higher than at baseline. For grouped change in IGA score, a participant was considered improved if a responder showed at least a 1-step improvement postbaseline compared to baseline, no change if postbaseline and baseline ratings were identical, and worsened if the postbaseline rating was higher than at baseline.
For the therapeutic response rate, a participant was considered a treatment responder if the IGA score improved from baseline and resulted in clear, minimal, or mild disease severity at EoT.
Safety
Adverse events also were assessed.
Statistical Analyses
Secondary efficacy and safety end points were assessed for all randomized participants who were dispensed the study medication. Missing data were imputed using last observation carried forward.
For the percentage change in ILC from baseline, therapeutic response rate, and grouped change in erythema rating, confirmatory analyses were conducted in a hierarchical manner (in the order listed), with testing stopped as soon as a null hypothesis of superior treatment effect could not be rejected. Analyses without significance level were exploratory. The Cochran-Mantel-Haenszel van Elteren test stratified by study center was used for grouped change in erythema rating (1-tailed, 2.5%) and IGA score (2-tailed, 5%); Wilcoxon rank sum tests also were performed. Percentage change in ILC from baseline was evaluated using the Student t test and F test of analysis of covariance (1-tailed, 2.5%). Therapeutic response rate was evaluated using the Cochran-Mantel-Haenszel van Elteren test stratified by study center and the Pearson χ2 test. Facial skin color and grouped change in telangiectasia rating were evaluated using the Wilcoxon rank sum test.
Adverse events beginning or worsening after the first dose of the study drug were considered treatment emergent and were coded using the Medical Dictionary for Regulatory Activities (MedDRA) Version 16.1. Statistical analyses were performed using SAS software version 9.2.
Results
Study Participants
The study included 961 total participants; 483 were randomized to the AzA foam group and 478 to the vehicle group (Figure 1). Overall, 803 participants completed follow-up; however, week 16 results for the efficacy outcomes include data for 4 additional patients (2 per study arm) who did not formally meet all requirements for follow-up completion. The mean age was 51.5 years, and the majority of the participants were white and female (Table 1). Most participants (86.8%) had moderate PPR at baseline, with the remaining rated as having severe disease (13.2%). The majority (76.4%) had more than 14 inflammatory lesions with moderate (76.4%) or severe (15.1%) erythema at baseline.


Efficacy
Significantly more participants in the AzA group than in the vehicle group showed an improved erythema rating at EoT (61.5% vs 51.3%; P<.001)(Figure 2), with more participants in the AzA group showing improvement at weeks 4 (P=.022) and 8 (P=.002).

A significantly greater mean percentage reduction in ILC from baseline to EoT was observed in the AzA group versus the vehicle group (61.6% vs 50.8%; P<.001)(Figure 3), and between-group differences were observed at week 4 (P<.001), week 8 (P=.003), and week 16 (end of study/follow-up)(P=.002).

A significantly higher proportion of participants treated with AzA foam versus vehicle were considered responders at week 12/EoT (66.3% vs 54.4%; P<.001)(Figure 4). Differences in responder rate also were observed at week 4 (P=.026) and week 8 (P=.026).

No study drug was administered between week 12/EoT and week 16/follow-up; last observation carried forward was not applied to week 16/follow-up analysis. AzA indicates azelaic acid; IGA, investigator global assessment.
Differences in grouped change in IGA score were observed between groups at every evaluation during the treatment phase (Figure 5). Specifically, IGA score was improved at week 12/EoT relative to baseline in 71.2% of participants in the AzA group versus 58.8% in the vehicle group (P<.001).

For grouped change in telangiectasia rating at EoT, the majority of participants in both treatment groups showed no change (Table 2). Regarding facial skin color, the majority of participants in both the AzA and vehicle treatment groups (80.1% and 78.7%, respectively) showed normal skin color compared to nontreated skin EoT; no between-group differences were detected for facial skin color rating (P=.315, Wilcoxon rank sum test).

Safety
The incidence of drug-related AEs was greater in the AzA group than the vehicle group (7.7% vs 4.8%)(Table 3). Drug-related AEs occurring in at least 1% of the AzA group were pain at application site (eg, tenderness, stinging, burning)(AzA group, 3.5%; vehicle group, 1.3%), application-site pruritus (1.4% vs 0.4%), and application-site dryness (1.0% vs 0.6%). A single drug-related AE of severe intensity (ie, application-site dermatitis) was observed in the vehicle group; all other drug-related AEs were mild or moderate. The incidence of withdrawals due to AEs was lower in the AzA group than the vehicle group (1.2% vs 2.5%). This AE profile correlated with a treatment compliance (the percentage of expected doses that were actually administered) of 97.0% in the AzA group and 95.9% in the vehicle group. One participant in the vehicle group died due to head trauma unrelated to administration of the study drug.

Comment
The results of this study further support the efficacy of AzA foam for the treatment of PPR. The percentage reduction in ILC was consistent with nominal decreases in ILC, a coprimary efficacy end point of this study.12 Almost two-thirds of participants treated with AzA foam achieved a therapeutic response, indicating that many participants who did not strictly achieve the primary outcome of therapeutic success nevertheless attained notable reductions in disease severity. The number of participants who showed any improvement on the IGA scale increased throughout the course of treatment (63.8% AzA foam vs 55.0% vehicle at week 8) up to EoT (71.2% vs 58.8%)(Figure 5). In addition, the number of participants showing any improvement at week 8 (63.8% AzA foam vs 55.0% vehicle)(Figure 5) was comparable to the number of participants achieving therapeutic response at week 12/EoT (66.3% vs 54.4%)(Figure 4). These data suggest that increasing time of treatment increases the likelihood of achieving better results.
Erythema also appeared to respond to AzA foam, with 10.2% more participants in the AzA group demonstrating improvement at week 12/EoT compared to vehicle. The difference in grouped change in erythema rating also was statistically significant and favored AzA foam, sustained up to 4 weeks after EoT.
The outcomes for percentage change in ILC, therapeutic response rate, and grouped change in erythema rating consequently led to the rejection of all 3 null hypotheses in hierarchical confirmatory analyses, underscoring the benefits of AzA foam treatment.
The therapeutic effects of AzA foam were apparent at the first postbaseline evaluation and persisted throughout treatment. Differences favoring AzA foam were observed at every on-treatment evaluation for grouped change in erythema rating, percentage change in ILC, therapeutic response rate, and grouped change in IGA score. Symptoms showed minimal resurgence after treatment cessation, and there were no signs of disease flare-up within the 4 weeks of observational follow-up. In addition, the percentage reduction in ILC remained higher in the AzA foam group during follow-up.
These results also show that AzA foam was well tolerated with a low incidence of discontinuation because of drug-related AEs. No serious drug-related AEs were reported for this study or in the preceding phase 2 trial.12,13 Although not directly evaluated, the low incidence of cutaneous AEs suggests that AzA foam may be better tolerated than prior formulations of AzA14,15 and correlates with high compliance observed during the study.12 Azelaic acid foam appeared to have minimal to no effect on skin color, with more than 88% of participants reporting barely visible or no skin lightening.
Interestingly, the vehicle foam showed appreciable efficacy independent of AzA. Improvements in erythema were recorded in approximately half of the vehicle group at week 12/EoT. A similar proportion attained a therapeutic response, and ILC was reduced by 50.8% at week 12/EoT. Comparable results also were evident in the vehicle group for the primary end points of this study.12 Vehicles in dermatologic trials frequently exert effects on diseased skin16,17 via a skin care regimen effect (eg, moisturization and other vehicle-related effects that may improve skin barrier integrity and function) and thus should not be regarded as placebo controls. The mechanism underlying this efficacy may be due to the impact of vehicle composition on skin barrier integrity and transepidermal water loss.18 The hydrophilic emulsion or other constituents of AzA foam (eg, fatty alcohols) may play a role.
A notable strength of our study is detailed clinical characterization using carefully chosen parameters and preplanned analyses that complement the primary end points. As the latter are often driven by regulatory requirements, opportunities to characterize other outcomes of interest to clinicians may be missed. The additional analyses reported here hopefully will aid dermatologists in both assessing the role of AzA foam in the treatment armamentarium for PPR and counseling patients.
Because participants with lighter skin pigmentation dominated our study population, the impact of AzA foam among patients with darker skin complexions is unknown. Although AzA is unlikely to cause hypopigmentation in normal undiseased skin, patients should be monitored for early signs of hypopigmentation.19,20 Our data also do not allow assessment of the differential effect, if any, of AzA foam on erythema of different etiologies in PPR, as corresponding information was not collected in the trial.
Conclusion
Azelaic acid foam 15% combines a well-established treatment of PPR with new vehicle technology to deliver effective therapy across multiple disease dimensions. In addition, the vehicle foam appears to demonstrate inherent therapeutic properties independent of AzA. The availability of this novel, efficacious, and well-tolerated option for PPR has the potential to improve patient care, reduce disease burden, and minimize unnecessary costs through increased tolerability and compliance.21
Acknowledgment
Editorial support through inVentiv Medical Communications (New York, New York) was provided by Bayer Pharmaceuticals.
Papulopustular rosacea (PPR) is characterized by centrofacial papules, pustules, erythema, and occasionally telangiectasia.1,2 A myriad of factors, including genetic predisposition3 and environmental triggers,4 have been associated with dysregulated inflammatory responses,5 contributing to the disease pathogenesis and symptoms. Inflammation associated with PPR may decrease skin barrier function, increase transepidermal water loss, and reduce stratum corneum hydration,6,7 resulting in heightened skin sensitivity, pain, burning, and/or stinging.5,8
Azelaic acid (AzA), which historically has only been available in gel or cream formulations, is well established for the treatment of rosacea9; however, these formulations have been associated with application-site adverse events (AEs)(eg, burning, erythema, irritation), limited cosmetic acceptability, and reduced compliance or efficacy.10
For select skin conditions, active agents delivered in foam vehicles may offer superior tolerability with improved outcomes.11 An AzA foam 15% formulation was approved for the treatment of mild to moderate PPR. Primary outcomes from a phase 3 trial demonstrated the efficacy and safety of AzA foam in improving inflammatory lesion counts (ILCs) and disease severity in participants with PPR. The trial also evaluated additional secondary end points, including the effect of AzA foam on erythema, inflammatory lesions, treatment response, and other manifestations of PPR.12 The current study evaluated investigator-reported efficacy outcomes for these secondary end points for AzA foam 15% versus vehicle foam.
Methods
Study Design
This phase 3 multicenter, randomized, double-blind, vehicle-controlled, parallel-group clinical trial was conducted from September 2012 to January 2014 at 48 US study centers comparing the efficacy of AzA foam versus vehicle foam in patients with PPR. Eligible participants were 18 years and older with PPR rated as moderate or severe according to investigator global assessment (IGA), plus 12 to 50 inflammatory lesions and persistent erythema with or without telangiectasia. Exclusion criteria included known nonresponse to AzA, current or prior use (within 6 weeks of randomization) of noninvestigational products to treat rosacea, and presence of other dermatoses that could interfere with rosacea evaluation.
Participants were randomized into the AzA foam or vehicle group (1:1 ratio). The study medication was applied in 0.5-g doses twice daily until the end of treatment (EoT) at 12 weeks. Efficacy and safety parameters were evaluated at baseline and at 4, 8, and 12 weeks of treatment, and at a follow-up visit 4 weeks after EoT (week 16).
Results for the coprimary efficacy end points—therapeutic success rate according to IGA and nominal change in ILC—were previously reported.12
Investigator-Reported Secondary Efficacy Outcomes
The secondary efficacy end points were grouped change in erythema rating, grouped change in telangiectasia rating, grouped change in IGA score, therapeutic response rate according to IGA, percentage change in ILC from baseline, and facial skin color rating at EoT.
Grouped change for all secondary end points was measured as improved, no change, or worsened relative to baseline. For grouped change in erythema and telangiectasia ratings, a participant was considered improved if the rating at the postbaseline visit was lower than the baseline rating, no change if the postbaseline and baseline ratings were identical, and worsened if the postbaseline rating was higher than at baseline. For grouped change in IGA score, a participant was considered improved if a responder showed at least a 1-step improvement postbaseline compared to baseline, no change if postbaseline and baseline ratings were identical, and worsened if the postbaseline rating was higher than at baseline.
For the therapeutic response rate, a participant was considered a treatment responder if the IGA score improved from baseline and resulted in clear, minimal, or mild disease severity at EoT.
Safety
Adverse events also were assessed.
Statistical Analyses
Secondary efficacy and safety end points were assessed for all randomized participants who were dispensed the study medication. Missing data were imputed using last observation carried forward.
For the percentage change in ILC from baseline, therapeutic response rate, and grouped change in erythema rating, confirmatory analyses were conducted in a hierarchical manner (in the order listed), with testing stopped as soon as a null hypothesis of superior treatment effect could not be rejected. Analyses without significance level were exploratory. The Cochran-Mantel-Haenszel van Elteren test stratified by study center was used for grouped change in erythema rating (1-tailed, 2.5%) and IGA score (2-tailed, 5%); Wilcoxon rank sum tests also were performed. Percentage change in ILC from baseline was evaluated using the Student t test and F test of analysis of covariance (1-tailed, 2.5%). Therapeutic response rate was evaluated using the Cochran-Mantel-Haenszel van Elteren test stratified by study center and the Pearson χ2 test. Facial skin color and grouped change in telangiectasia rating were evaluated using the Wilcoxon rank sum test.
Adverse events beginning or worsening after the first dose of the study drug were considered treatment emergent and were coded using the Medical Dictionary for Regulatory Activities (MedDRA) Version 16.1. Statistical analyses were performed using SAS software version 9.2.
Results
Study Participants
The study included 961 total participants; 483 were randomized to the AzA foam group and 478 to the vehicle group (Figure 1). Overall, 803 participants completed follow-up; however, week 16 results for the efficacy outcomes include data for 4 additional patients (2 per study arm) who did not formally meet all requirements for follow-up completion. The mean age was 51.5 years, and the majority of the participants were white and female (Table 1). Most participants (86.8%) had moderate PPR at baseline, with the remaining rated as having severe disease (13.2%). The majority (76.4%) had more than 14 inflammatory lesions with moderate (76.4%) or severe (15.1%) erythema at baseline.


Efficacy
Significantly more participants in the AzA group than in the vehicle group showed an improved erythema rating at EoT (61.5% vs 51.3%; P<.001)(Figure 2), with more participants in the AzA group showing improvement at weeks 4 (P=.022) and 8 (P=.002).

A significantly greater mean percentage reduction in ILC from baseline to EoT was observed in the AzA group versus the vehicle group (61.6% vs 50.8%; P<.001)(Figure 3), and between-group differences were observed at week 4 (P<.001), week 8 (P=.003), and week 16 (end of study/follow-up)(P=.002).

A significantly higher proportion of participants treated with AzA foam versus vehicle were considered responders at week 12/EoT (66.3% vs 54.4%; P<.001)(Figure 4). Differences in responder rate also were observed at week 4 (P=.026) and week 8 (P=.026).

No study drug was administered between week 12/EoT and week 16/follow-up; last observation carried forward was not applied to week 16/follow-up analysis. AzA indicates azelaic acid; IGA, investigator global assessment.
Differences in grouped change in IGA score were observed between groups at every evaluation during the treatment phase (Figure 5). Specifically, IGA score was improved at week 12/EoT relative to baseline in 71.2% of participants in the AzA group versus 58.8% in the vehicle group (P<.001).

For grouped change in telangiectasia rating at EoT, the majority of participants in both treatment groups showed no change (Table 2). Regarding facial skin color, the majority of participants in both the AzA and vehicle treatment groups (80.1% and 78.7%, respectively) showed normal skin color compared to nontreated skin EoT; no between-group differences were detected for facial skin color rating (P=.315, Wilcoxon rank sum test).

Safety
The incidence of drug-related AEs was greater in the AzA group than the vehicle group (7.7% vs 4.8%)(Table 3). Drug-related AEs occurring in at least 1% of the AzA group were pain at application site (eg, tenderness, stinging, burning)(AzA group, 3.5%; vehicle group, 1.3%), application-site pruritus (1.4% vs 0.4%), and application-site dryness (1.0% vs 0.6%). A single drug-related AE of severe intensity (ie, application-site dermatitis) was observed in the vehicle group; all other drug-related AEs were mild or moderate. The incidence of withdrawals due to AEs was lower in the AzA group than the vehicle group (1.2% vs 2.5%). This AE profile correlated with a treatment compliance (the percentage of expected doses that were actually administered) of 97.0% in the AzA group and 95.9% in the vehicle group. One participant in the vehicle group died due to head trauma unrelated to administration of the study drug.

Comment
The results of this study further support the efficacy of AzA foam for the treatment of PPR. The percentage reduction in ILC was consistent with nominal decreases in ILC, a coprimary efficacy end point of this study.12 Almost two-thirds of participants treated with AzA foam achieved a therapeutic response, indicating that many participants who did not strictly achieve the primary outcome of therapeutic success nevertheless attained notable reductions in disease severity. The number of participants who showed any improvement on the IGA scale increased throughout the course of treatment (63.8% AzA foam vs 55.0% vehicle at week 8) up to EoT (71.2% vs 58.8%)(Figure 5). In addition, the number of participants showing any improvement at week 8 (63.8% AzA foam vs 55.0% vehicle)(Figure 5) was comparable to the number of participants achieving therapeutic response at week 12/EoT (66.3% vs 54.4%)(Figure 4). These data suggest that increasing time of treatment increases the likelihood of achieving better results.
Erythema also appeared to respond to AzA foam, with 10.2% more participants in the AzA group demonstrating improvement at week 12/EoT compared to vehicle. The difference in grouped change in erythema rating also was statistically significant and favored AzA foam, sustained up to 4 weeks after EoT.
The outcomes for percentage change in ILC, therapeutic response rate, and grouped change in erythema rating consequently led to the rejection of all 3 null hypotheses in hierarchical confirmatory analyses, underscoring the benefits of AzA foam treatment.
The therapeutic effects of AzA foam were apparent at the first postbaseline evaluation and persisted throughout treatment. Differences favoring AzA foam were observed at every on-treatment evaluation for grouped change in erythema rating, percentage change in ILC, therapeutic response rate, and grouped change in IGA score. Symptoms showed minimal resurgence after treatment cessation, and there were no signs of disease flare-up within the 4 weeks of observational follow-up. In addition, the percentage reduction in ILC remained higher in the AzA foam group during follow-up.
These results also show that AzA foam was well tolerated with a low incidence of discontinuation because of drug-related AEs. No serious drug-related AEs were reported for this study or in the preceding phase 2 trial.12,13 Although not directly evaluated, the low incidence of cutaneous AEs suggests that AzA foam may be better tolerated than prior formulations of AzA14,15 and correlates with high compliance observed during the study.12 Azelaic acid foam appeared to have minimal to no effect on skin color, with more than 88% of participants reporting barely visible or no skin lightening.
Interestingly, the vehicle foam showed appreciable efficacy independent of AzA. Improvements in erythema were recorded in approximately half of the vehicle group at week 12/EoT. A similar proportion attained a therapeutic response, and ILC was reduced by 50.8% at week 12/EoT. Comparable results also were evident in the vehicle group for the primary end points of this study.12 Vehicles in dermatologic trials frequently exert effects on diseased skin16,17 via a skin care regimen effect (eg, moisturization and other vehicle-related effects that may improve skin barrier integrity and function) and thus should not be regarded as placebo controls. The mechanism underlying this efficacy may be due to the impact of vehicle composition on skin barrier integrity and transepidermal water loss.18 The hydrophilic emulsion or other constituents of AzA foam (eg, fatty alcohols) may play a role.
A notable strength of our study is detailed clinical characterization using carefully chosen parameters and preplanned analyses that complement the primary end points. As the latter are often driven by regulatory requirements, opportunities to characterize other outcomes of interest to clinicians may be missed. The additional analyses reported here hopefully will aid dermatologists in both assessing the role of AzA foam in the treatment armamentarium for PPR and counseling patients.
Because participants with lighter skin pigmentation dominated our study population, the impact of AzA foam among patients with darker skin complexions is unknown. Although AzA is unlikely to cause hypopigmentation in normal undiseased skin, patients should be monitored for early signs of hypopigmentation.19,20 Our data also do not allow assessment of the differential effect, if any, of AzA foam on erythema of different etiologies in PPR, as corresponding information was not collected in the trial.
Conclusion
Azelaic acid foam 15% combines a well-established treatment of PPR with new vehicle technology to deliver effective therapy across multiple disease dimensions. In addition, the vehicle foam appears to demonstrate inherent therapeutic properties independent of AzA. The availability of this novel, efficacious, and well-tolerated option for PPR has the potential to improve patient care, reduce disease burden, and minimize unnecessary costs through increased tolerability and compliance.21
Acknowledgment
Editorial support through inVentiv Medical Communications (New York, New York) was provided by Bayer Pharmaceuticals.
- Tan J, Berg M. Rosacea: current state of epidemiology. J Am Acad Dermatol. 2013;69(6, suppl 1):S27-S35.
- Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Chang AL, Raber I, Xu J, et al. Assessment of the genetic basis of rosacea by genome-wide association study. J Invest Dermatol. 2015;135:1548-1555.
- Abram K, Silm H, Maaroos HI, et al. Risk factors associated with rosacea. J Eur Acad Dermatol Venereol. 2010;24:565-571.
- Yamasaki K, Di Nardo A, Bardan A, et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med. 2007;13:975-980.
- Yamasaki K, Kanada K, Macleod DT, et al. TLR2 expression is increased in rosacea and stimulates enhanced serine protease production by keratinocytes. J Invest Dermatol. 2011;131:688-697.
- Darlenski R, Kazandjieva J, Tsankov N, et al. Acute irritant threshold correlates with barrier function, skin hydration and contact hypersensitivity in atopic dermatitis and rosacea. Exp Dermatol. 2013;22:752-753.
- Del Rosso JQ, Levin J. The clinical relevance of maintaining the functional integrity of the stratum corneum in both healthy and disease-affected skin. J Clin Aesthet Dermatol. 2011;4:22-42.
- van Zuuren EJ, Kramer SF, Carter BR, et al. Effective and evidence-based management strategies for rosacea: summary of a Cochrane systematic review. Br J Dermatol. 2011;165:760-781.
- Tan X, Feldman SR, Chang J, et al. Topical drug delivery systems in dermatology: a review of patient adherence issues. Expert Opin Drug Deliv. 2012;9:1263-1271.
- Stein L. Clinical studies of a new vehicle formulation for topical corticosteroids in the treatment of psoriasis. J Am Acad Dermatol. 2005;53(1, suppl 1):S39-S49.
- Draelos ZD, Elewski BE, Harper JC, et al. A phase 3 randomized, double-blind, vehicle-controlled trial of azelaic acid foam 15% in the treatment of papulopustular rosacea. Cutis. 2015;96:54-61.
- Draelos ZD, Elewski B, Staedtler G, et al. Azelaic acid foam 15% in the treatment of papulopustular rosacea: a randomized, double-blind, vehicle-controlled study. Cutis. 2013;92:306-317.
- Finacea gel [package insert]. Whippany, NJ: Bayer HealthCare Pharmaceuticals Inc; 2016.
- Elewski BE, Fleischer AB Jr, Pariser DM. A comparison of 15% azelaic acid gel and 0.75% metronidazole gel in the topical treatment of papulopustular rosacea: results of a randomized trial. Arch Dermatol. 2003;139:1444-1450.
- Daniels R, Knie U. Galenics of dermal products—vehicles, properties and drug release. J Dtsch Dermatol Ges. 2007;5:367-383.
- Shamsudin N, Fleischer AB Jr. Vehicle or placebo? Investigators use incorrect terminology in randomized controlled trials half of the time: a systematic review of randomized controlled trials published in three major dermatology journals. J Drugs Dermatol. 2010;9:1221-1226.
- Del Rosso JQ, Thiboutot D, Gallo R, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 2: a status report on topical agents. Cutis. 2013;92:277-284.
- Finacea foam [package insert]. Whippany, NJ: Bayer HealthCare Pharmaceuticals Inc; 2015.
- Solano F, Briganti S, Picardo M, et al. Hypopigmenting agents: an updated review on biological, chemical and clinical aspects. Pigment Cell Res. 2006;19:550-571.
- Hammarstrom B, Wessling A, Nilsson JL. Pharmaceutical care for patients with skin diseases: a campaign year at Swedish pharmacies. J Clin Pharm Ther. 1995;20:327-334.
- Tan J, Berg M. Rosacea: current state of epidemiology. J Am Acad Dermatol. 2013;69(6, suppl 1):S27-S35.
- Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Chang AL, Raber I, Xu J, et al. Assessment of the genetic basis of rosacea by genome-wide association study. J Invest Dermatol. 2015;135:1548-1555.
- Abram K, Silm H, Maaroos HI, et al. Risk factors associated with rosacea. J Eur Acad Dermatol Venereol. 2010;24:565-571.
- Yamasaki K, Di Nardo A, Bardan A, et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med. 2007;13:975-980.
- Yamasaki K, Kanada K, Macleod DT, et al. TLR2 expression is increased in rosacea and stimulates enhanced serine protease production by keratinocytes. J Invest Dermatol. 2011;131:688-697.
- Darlenski R, Kazandjieva J, Tsankov N, et al. Acute irritant threshold correlates with barrier function, skin hydration and contact hypersensitivity in atopic dermatitis and rosacea. Exp Dermatol. 2013;22:752-753.
- Del Rosso JQ, Levin J. The clinical relevance of maintaining the functional integrity of the stratum corneum in both healthy and disease-affected skin. J Clin Aesthet Dermatol. 2011;4:22-42.
- van Zuuren EJ, Kramer SF, Carter BR, et al. Effective and evidence-based management strategies for rosacea: summary of a Cochrane systematic review. Br J Dermatol. 2011;165:760-781.
- Tan X, Feldman SR, Chang J, et al. Topical drug delivery systems in dermatology: a review of patient adherence issues. Expert Opin Drug Deliv. 2012;9:1263-1271.
- Stein L. Clinical studies of a new vehicle formulation for topical corticosteroids in the treatment of psoriasis. J Am Acad Dermatol. 2005;53(1, suppl 1):S39-S49.
- Draelos ZD, Elewski BE, Harper JC, et al. A phase 3 randomized, double-blind, vehicle-controlled trial of azelaic acid foam 15% in the treatment of papulopustular rosacea. Cutis. 2015;96:54-61.
- Draelos ZD, Elewski B, Staedtler G, et al. Azelaic acid foam 15% in the treatment of papulopustular rosacea: a randomized, double-blind, vehicle-controlled study. Cutis. 2013;92:306-317.
- Finacea gel [package insert]. Whippany, NJ: Bayer HealthCare Pharmaceuticals Inc; 2016.
- Elewski BE, Fleischer AB Jr, Pariser DM. A comparison of 15% azelaic acid gel and 0.75% metronidazole gel in the topical treatment of papulopustular rosacea: results of a randomized trial. Arch Dermatol. 2003;139:1444-1450.
- Daniels R, Knie U. Galenics of dermal products—vehicles, properties and drug release. J Dtsch Dermatol Ges. 2007;5:367-383.
- Shamsudin N, Fleischer AB Jr. Vehicle or placebo? Investigators use incorrect terminology in randomized controlled trials half of the time: a systematic review of randomized controlled trials published in three major dermatology journals. J Drugs Dermatol. 2010;9:1221-1226.
- Del Rosso JQ, Thiboutot D, Gallo R, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 2: a status report on topical agents. Cutis. 2013;92:277-284.
- Finacea foam [package insert]. Whippany, NJ: Bayer HealthCare Pharmaceuticals Inc; 2015.
- Solano F, Briganti S, Picardo M, et al. Hypopigmenting agents: an updated review on biological, chemical and clinical aspects. Pigment Cell Res. 2006;19:550-571.
- Hammarstrom B, Wessling A, Nilsson JL. Pharmaceutical care for patients with skin diseases: a campaign year at Swedish pharmacies. J Clin Pharm Ther. 1995;20:327-334.
Practice Points
- Papulopustular rosacea (PPR) is a common chronic inflammatory dermatosis.
- A novel hydrophilic foam formulation of azelaic acid (AzA) was approved for the treatment of PPR.
- In addition to effectively treating papules and pustules, AzA foam also may reduce rosacea-associated erythema.
- The unique AzA foam vehicle may improve epidermal barrier integrity and function, thereby offering patients a distinct topical approach to rosacea management.
Combined OCs remain a good choice for teen acne
MINNEAPOLIS – Whether a young female patient has a refractory flare of inflammatory acne, or has a condition that can predispose to androgen excess, using a hormonal approach can be an effective management tool for controlling adolescent acne.
During a presentation at the annual meeting of the Society for Pediatric Dermatology, Dr. Diane Thiboutot outlined tips and tricks for optimizing hormonal therapy for acne in teens, and referred to the new acne treatment guidelines from the American Academy of Dermatology, which clarify when to treat with hormones, which to choose, and when further testing might be indicated.
The full range of hormonal therapy options for acne can include oral contraceptives, which block ovarian hormone production; antiandrogens such as spironolactone, and the less commonly used flutamide, which blocks the effects of androgen on the skin; and glucocorticoids, which block adrenal production.
The 2016 guidelines recommend oral contraceptives as an effective treatment for inflammatory acne in females (J Am Acad Dermatol. 2016 May;74[5]; 945-973.e33). Combined oral contraceptives (COCs) reduce serum androgens, and reduce free testosterone by increasing sex hormone binding globulin production, thus reducing sebum production. “The only things that really decrease sebum are oral contraceptives in women, and isotretinoin,” said Dr. Thiboutot, professor of dermatology at Penn State University, Hershey.
For most female adolescents with acne, hormonal testing is not indicated. The AAD guidelines recommend laboratory evaluation for younger patients with acne who have clinical signs of androgen excess, such as early onset body odor and axillary and/or pubic hair, accelerated growth, advanced bone age, or early genital maturation. Just obtaining a hand film for bone age and mapping growth against a growth chart can be a good initial screening tool when considering whether to perform hormonal testing, she noted.
For postpubertal females in whom polycystic ovary syndrome (PCOS) or other hyperandrogenic states are suspected, hormonal testing is indicated in the presence of the clinical signs of infrequent menses and infertility, hirsutism, truncal obesity, androgenetic alopecia, polycystic ovaries, or clitoromegaly.
In searching for an endocrine disorder, Dr. Thiboutot recommends checking total and free testosterone, luteinizing hormone/follicle stimulating hormone ratio, 17-hydroxyprogesterone levels, and dehydroepiandrosterone (DHEA-S) levels. These tests should be performed at least 6 weeks after the patient has been off hormonal contraception, and should be done during the menstrual period, or during the week prior to menses, in order to avoid ovulation-related hormonal changes.
Lab findings consistent with congenital adrenal hyperplasia include elevated serum DHEA-S, together with elevated 17-hydroxyprogesterone or testosterone. A PCOS diagnosis can be made in adolescent females if there is clinical or laboratory evidence of hyperandrogenism with concomitant persistent oligomenorrhea.
Acne related to hyperandrogenism may respond well to oral contraceptives, but COCs can also be an effective alternative to repeated courses of isotretinoin and antibiotics, as well as an effective adjunct to topical therapy, Dr. Thiboutot said.
When beginning a patient on oral contraceptives, it’s not necessary to perform a pelvic exam or obtain a Pap smear before initiating the COC, but it is important to obtain a thorough medical history and an accurate blood pressure measurement at the outset, she noted. The World Health Organization (WHO) has established recommendations outlining contraindications to COC use, also identifying populations in whom COCs should be used with caution, and who should be monitored.
Headaches are a condition frequently seen among healthy teens and young women, and one for which the WHO advises caution. There are concerns that women with migraines may be at increased risk of stroke if they take COCs, but the overall risk is low, and the American College of Obstetricians and Gynecologists (ACOG) advises that COCs can be considered for women younger than 35 with migraines if they have no focal neurologic signs, are nonsmokers, and are otherwise healthy, Dr. Thiboutot added.
A large Food and Drug Administration–sponsored retrospective cohort study examined the risk of venous thromboembolism in contraceptive users. In April 2012, the FDA concluded that though the risk of blood clots may be higher for those on hormonal contraception methods than for those who are not using them, the risk of blood clots during pregnancy and the postpartum period is higher than the thromboembolism risk for contraceptive users.
Regarding the potential for antibiotics to reduce contraceptive efficacy, Dr. Thiboutot said,“it’s okay to use oral contraceptives with antibiotics. There’s a lot of misunderstanding about antibiotics and combined oral contraceptives.” She cited an ACOG practice bulletin that reported that only rifampin has been shown to reduce serum steroid levels when taken with oral contraceptives (Obstet Gynecol. 2006 Jun;107[6]:1453-72).
According to the 2016 AAD guidelines, the use of oral glucocorticoids may be appropriate over the short term when initiating therapy for severe inflammatory acne. “Pharmacokinetic studies have not demonstrated decreased oral contraceptive levels with common antibiotics,” Dr. Thiboutot said.
Spironolactone, according to the new guidelines, is useful for acne in select females. Spironolactone is an androgen receptor and 5a-reductase blocker, and its antiandrogen effects can improve acne. Many patients do well with 25-50 mg twice daily, though breast tenderness and menstrual irregularities are commonly seen side effects, she noted. If a woman taking spironolactone becomes pregnant, there’s a risk of hypospadias for a male fetus.
Though spironolactone carries a boxed warning because of tumorigenicity observed in animal studies, Dr. Thiboutot said that a large Danish study searched for any association between breast, uterine, or ovarian cancers and spironolactone use. Among the 2.3 million women studied, no increased association was seen (Cancer Epidemiol. 2013 Dec;37:870-5).
She also noted that there’s “low usefulness in monitoring potassium levels in young healthy women on spironolactone.” She cited a study that compared 974 healthy young women taking spironolactone with 1,165 women who were not on spironolactone, which found that the hyperkalemia rate of 0.72% among those on spironolactone was equivalent to the 0.76% baseline rate of hyperkalemia in the young, healthy female population (JAMA Dermatol. 2015;151[9];941-944).
Oral corticosteroids for acne, Dr. Thiboutot said, should be reserved to quiet a severe bout of inflammatory acne while standard therapies are being initiated.
She reported being an investigator or a consultant for a number of pharmaceutical companies.
On Twitter @karioakes
MINNEAPOLIS – Whether a young female patient has a refractory flare of inflammatory acne, or has a condition that can predispose to androgen excess, using a hormonal approach can be an effective management tool for controlling adolescent acne.
During a presentation at the annual meeting of the Society for Pediatric Dermatology, Dr. Diane Thiboutot outlined tips and tricks for optimizing hormonal therapy for acne in teens, and referred to the new acne treatment guidelines from the American Academy of Dermatology, which clarify when to treat with hormones, which to choose, and when further testing might be indicated.
The full range of hormonal therapy options for acne can include oral contraceptives, which block ovarian hormone production; antiandrogens such as spironolactone, and the less commonly used flutamide, which blocks the effects of androgen on the skin; and glucocorticoids, which block adrenal production.
The 2016 guidelines recommend oral contraceptives as an effective treatment for inflammatory acne in females (J Am Acad Dermatol. 2016 May;74[5]; 945-973.e33). Combined oral contraceptives (COCs) reduce serum androgens, and reduce free testosterone by increasing sex hormone binding globulin production, thus reducing sebum production. “The only things that really decrease sebum are oral contraceptives in women, and isotretinoin,” said Dr. Thiboutot, professor of dermatology at Penn State University, Hershey.
For most female adolescents with acne, hormonal testing is not indicated. The AAD guidelines recommend laboratory evaluation for younger patients with acne who have clinical signs of androgen excess, such as early onset body odor and axillary and/or pubic hair, accelerated growth, advanced bone age, or early genital maturation. Just obtaining a hand film for bone age and mapping growth against a growth chart can be a good initial screening tool when considering whether to perform hormonal testing, she noted.
For postpubertal females in whom polycystic ovary syndrome (PCOS) or other hyperandrogenic states are suspected, hormonal testing is indicated in the presence of the clinical signs of infrequent menses and infertility, hirsutism, truncal obesity, androgenetic alopecia, polycystic ovaries, or clitoromegaly.
In searching for an endocrine disorder, Dr. Thiboutot recommends checking total and free testosterone, luteinizing hormone/follicle stimulating hormone ratio, 17-hydroxyprogesterone levels, and dehydroepiandrosterone (DHEA-S) levels. These tests should be performed at least 6 weeks after the patient has been off hormonal contraception, and should be done during the menstrual period, or during the week prior to menses, in order to avoid ovulation-related hormonal changes.
Lab findings consistent with congenital adrenal hyperplasia include elevated serum DHEA-S, together with elevated 17-hydroxyprogesterone or testosterone. A PCOS diagnosis can be made in adolescent females if there is clinical or laboratory evidence of hyperandrogenism with concomitant persistent oligomenorrhea.
Acne related to hyperandrogenism may respond well to oral contraceptives, but COCs can also be an effective alternative to repeated courses of isotretinoin and antibiotics, as well as an effective adjunct to topical therapy, Dr. Thiboutot said.
When beginning a patient on oral contraceptives, it’s not necessary to perform a pelvic exam or obtain a Pap smear before initiating the COC, but it is important to obtain a thorough medical history and an accurate blood pressure measurement at the outset, she noted. The World Health Organization (WHO) has established recommendations outlining contraindications to COC use, also identifying populations in whom COCs should be used with caution, and who should be monitored.
Headaches are a condition frequently seen among healthy teens and young women, and one for which the WHO advises caution. There are concerns that women with migraines may be at increased risk of stroke if they take COCs, but the overall risk is low, and the American College of Obstetricians and Gynecologists (ACOG) advises that COCs can be considered for women younger than 35 with migraines if they have no focal neurologic signs, are nonsmokers, and are otherwise healthy, Dr. Thiboutot added.
A large Food and Drug Administration–sponsored retrospective cohort study examined the risk of venous thromboembolism in contraceptive users. In April 2012, the FDA concluded that though the risk of blood clots may be higher for those on hormonal contraception methods than for those who are not using them, the risk of blood clots during pregnancy and the postpartum period is higher than the thromboembolism risk for contraceptive users.
Regarding the potential for antibiotics to reduce contraceptive efficacy, Dr. Thiboutot said,“it’s okay to use oral contraceptives with antibiotics. There’s a lot of misunderstanding about antibiotics and combined oral contraceptives.” She cited an ACOG practice bulletin that reported that only rifampin has been shown to reduce serum steroid levels when taken with oral contraceptives (Obstet Gynecol. 2006 Jun;107[6]:1453-72).
According to the 2016 AAD guidelines, the use of oral glucocorticoids may be appropriate over the short term when initiating therapy for severe inflammatory acne. “Pharmacokinetic studies have not demonstrated decreased oral contraceptive levels with common antibiotics,” Dr. Thiboutot said.
Spironolactone, according to the new guidelines, is useful for acne in select females. Spironolactone is an androgen receptor and 5a-reductase blocker, and its antiandrogen effects can improve acne. Many patients do well with 25-50 mg twice daily, though breast tenderness and menstrual irregularities are commonly seen side effects, she noted. If a woman taking spironolactone becomes pregnant, there’s a risk of hypospadias for a male fetus.
Though spironolactone carries a boxed warning because of tumorigenicity observed in animal studies, Dr. Thiboutot said that a large Danish study searched for any association between breast, uterine, or ovarian cancers and spironolactone use. Among the 2.3 million women studied, no increased association was seen (Cancer Epidemiol. 2013 Dec;37:870-5).
She also noted that there’s “low usefulness in monitoring potassium levels in young healthy women on spironolactone.” She cited a study that compared 974 healthy young women taking spironolactone with 1,165 women who were not on spironolactone, which found that the hyperkalemia rate of 0.72% among those on spironolactone was equivalent to the 0.76% baseline rate of hyperkalemia in the young, healthy female population (JAMA Dermatol. 2015;151[9];941-944).
Oral corticosteroids for acne, Dr. Thiboutot said, should be reserved to quiet a severe bout of inflammatory acne while standard therapies are being initiated.
She reported being an investigator or a consultant for a number of pharmaceutical companies.
On Twitter @karioakes
MINNEAPOLIS – Whether a young female patient has a refractory flare of inflammatory acne, or has a condition that can predispose to androgen excess, using a hormonal approach can be an effective management tool for controlling adolescent acne.
During a presentation at the annual meeting of the Society for Pediatric Dermatology, Dr. Diane Thiboutot outlined tips and tricks for optimizing hormonal therapy for acne in teens, and referred to the new acne treatment guidelines from the American Academy of Dermatology, which clarify when to treat with hormones, which to choose, and when further testing might be indicated.
The full range of hormonal therapy options for acne can include oral contraceptives, which block ovarian hormone production; antiandrogens such as spironolactone, and the less commonly used flutamide, which blocks the effects of androgen on the skin; and glucocorticoids, which block adrenal production.
The 2016 guidelines recommend oral contraceptives as an effective treatment for inflammatory acne in females (J Am Acad Dermatol. 2016 May;74[5]; 945-973.e33). Combined oral contraceptives (COCs) reduce serum androgens, and reduce free testosterone by increasing sex hormone binding globulin production, thus reducing sebum production. “The only things that really decrease sebum are oral contraceptives in women, and isotretinoin,” said Dr. Thiboutot, professor of dermatology at Penn State University, Hershey.
For most female adolescents with acne, hormonal testing is not indicated. The AAD guidelines recommend laboratory evaluation for younger patients with acne who have clinical signs of androgen excess, such as early onset body odor and axillary and/or pubic hair, accelerated growth, advanced bone age, or early genital maturation. Just obtaining a hand film for bone age and mapping growth against a growth chart can be a good initial screening tool when considering whether to perform hormonal testing, she noted.
For postpubertal females in whom polycystic ovary syndrome (PCOS) or other hyperandrogenic states are suspected, hormonal testing is indicated in the presence of the clinical signs of infrequent menses and infertility, hirsutism, truncal obesity, androgenetic alopecia, polycystic ovaries, or clitoromegaly.
In searching for an endocrine disorder, Dr. Thiboutot recommends checking total and free testosterone, luteinizing hormone/follicle stimulating hormone ratio, 17-hydroxyprogesterone levels, and dehydroepiandrosterone (DHEA-S) levels. These tests should be performed at least 6 weeks after the patient has been off hormonal contraception, and should be done during the menstrual period, or during the week prior to menses, in order to avoid ovulation-related hormonal changes.
Lab findings consistent with congenital adrenal hyperplasia include elevated serum DHEA-S, together with elevated 17-hydroxyprogesterone or testosterone. A PCOS diagnosis can be made in adolescent females if there is clinical or laboratory evidence of hyperandrogenism with concomitant persistent oligomenorrhea.
Acne related to hyperandrogenism may respond well to oral contraceptives, but COCs can also be an effective alternative to repeated courses of isotretinoin and antibiotics, as well as an effective adjunct to topical therapy, Dr. Thiboutot said.
When beginning a patient on oral contraceptives, it’s not necessary to perform a pelvic exam or obtain a Pap smear before initiating the COC, but it is important to obtain a thorough medical history and an accurate blood pressure measurement at the outset, she noted. The World Health Organization (WHO) has established recommendations outlining contraindications to COC use, also identifying populations in whom COCs should be used with caution, and who should be monitored.
Headaches are a condition frequently seen among healthy teens and young women, and one for which the WHO advises caution. There are concerns that women with migraines may be at increased risk of stroke if they take COCs, but the overall risk is low, and the American College of Obstetricians and Gynecologists (ACOG) advises that COCs can be considered for women younger than 35 with migraines if they have no focal neurologic signs, are nonsmokers, and are otherwise healthy, Dr. Thiboutot added.
A large Food and Drug Administration–sponsored retrospective cohort study examined the risk of venous thromboembolism in contraceptive users. In April 2012, the FDA concluded that though the risk of blood clots may be higher for those on hormonal contraception methods than for those who are not using them, the risk of blood clots during pregnancy and the postpartum period is higher than the thromboembolism risk for contraceptive users.
Regarding the potential for antibiotics to reduce contraceptive efficacy, Dr. Thiboutot said,“it’s okay to use oral contraceptives with antibiotics. There’s a lot of misunderstanding about antibiotics and combined oral contraceptives.” She cited an ACOG practice bulletin that reported that only rifampin has been shown to reduce serum steroid levels when taken with oral contraceptives (Obstet Gynecol. 2006 Jun;107[6]:1453-72).
According to the 2016 AAD guidelines, the use of oral glucocorticoids may be appropriate over the short term when initiating therapy for severe inflammatory acne. “Pharmacokinetic studies have not demonstrated decreased oral contraceptive levels with common antibiotics,” Dr. Thiboutot said.
Spironolactone, according to the new guidelines, is useful for acne in select females. Spironolactone is an androgen receptor and 5a-reductase blocker, and its antiandrogen effects can improve acne. Many patients do well with 25-50 mg twice daily, though breast tenderness and menstrual irregularities are commonly seen side effects, she noted. If a woman taking spironolactone becomes pregnant, there’s a risk of hypospadias for a male fetus.
Though spironolactone carries a boxed warning because of tumorigenicity observed in animal studies, Dr. Thiboutot said that a large Danish study searched for any association between breast, uterine, or ovarian cancers and spironolactone use. Among the 2.3 million women studied, no increased association was seen (Cancer Epidemiol. 2013 Dec;37:870-5).
She also noted that there’s “low usefulness in monitoring potassium levels in young healthy women on spironolactone.” She cited a study that compared 974 healthy young women taking spironolactone with 1,165 women who were not on spironolactone, which found that the hyperkalemia rate of 0.72% among those on spironolactone was equivalent to the 0.76% baseline rate of hyperkalemia in the young, healthy female population (JAMA Dermatol. 2015;151[9];941-944).
Oral corticosteroids for acne, Dr. Thiboutot said, should be reserved to quiet a severe bout of inflammatory acne while standard therapies are being initiated.
She reported being an investigator or a consultant for a number of pharmaceutical companies.
On Twitter @karioakes
EXPERT ANALYSIS FROM THE SPD ANNUAL MEETING
Expert shares new insights on the pathophysiology of rosacea
NEWPORT BEACH, CALIF. – In the clinical opinion of Richard L. Gallo, MD, PhD, current nomenclature for the diagnosis of rosacea could use a makeover.
“Currently, we’re still operating with an almost 20-year-old set of diagnostic subtypes of rosacea,” Dr. Gallo said at the annual meeting of the Pacific Dermatologic Association. He plans to participate in consensus meeting of experts who will convene this fall in an effort to update and modify these diagnostic criteria.
According to current nomenclature, subtype 1 is erythematotelangiectatic rosacea characterized by facial redness; subtype 2 is papulopustular, marked by bumps and pimples; subtype 3 is phymatous, characterized by enlargement of the nose, and subtype 4 is ocular, marked by eye irritation. Dr. Gallo pointed out that it’s rare to see just one of these subtypes in rosacea patients, with the exception of the erythematotelangiectatic rosacea (ETR). “There is a large population with ETR alone,” he said.” Most patients with the papulopustular subtype have aspects of ETR. There is a mix of subtypes of rosacea and we clearly need to modify our diagnostic criteria.”
Secondary rosacea features may include burning or stinging, plaque, dry appearance and scale, edema, ocular manifestations, peripheral location, and phymatous changes. Work by several researchers in recent years has shed light on the pathophysiology of rosacea. “We’re learning that there are many aspects to this disease that both trigger it and result in progression of the disease,” said Dr. Gallo, professor and chair of the department of dermatology at the University of California, San Diego. “It seems to have biological triggers that exist both in the environment and initiate from internal sources. We’re understanding more about the nature of those, at least specific molecules externally from microbes and so forth. Internally we understand more about the unique inflammatory signals.”
For example, he and other researchers began to look at the innate immune system patients with rosacea and identified LL37, a multifunction peptide that plays a role in a number of skin diseases, as something that can promote inflammation (Nat Med. 2007;13[8]:975-80). “It also promotes the vascular changes [that occur with the disease],” Dr. Gallo said. “We’re now learning how a dysregulation of enzymes in the skin contributes to making too much of these types of peptides. Therefore, treatment approaches that might modify enzymatic activity become useful.” Researchers have also discovered that some of the innate recognition molecules like toll-like receptor 2 (TLR2) are overexpressed in rosacea patients. “Similarly, in terms of the vascular signals, a number of labs are identifying some of the newer vascular transmitters that seem to be uniquely elevated in rosacea, so there’s great reason to be optimistic that given the increased specificity and understanding of what uniquely makes this disease happen, we’ll be able to target it in a safe way.”
A number of published studies have supported these notions, including an analysis of 275 twin pairs (JAMA Dermatol. 2015;151[11]:1213-9). The researchers found that compared with fraternal twins, identical twins had a higher association of National Rosacea Society scores (P = .04), “supporting the concept that there are fundamental genetic factors that are influencing disease,” Dr. Gallo said. Environmental factors found to be associated with rosacea include lifetime UV exposure, smoking, obesity, and alcohol use.
In an assessment of the genetic basis of rosacea by genome-wide association study, researchers identified one confirmed single-nucleotide polymorphism that could be associated with rosacea (J Invest Dermatol. 2015;135[6]:1548-55). It was located in an intergenic region between HLA-DRA and BTNL2, “which is consistent with the overall concept that there is perhaps a genetic abnormality that is leading to increased amino modulation of difficulties,” Dr. Gallo said. For another recent study, researchers analyzed 14 randomized or case control trials involving rosacea patients (Int J Med Sci. 2015;12[5]:387-96). They concluded that vasculature, chronic inflammatory responses, environmental triggers, food and chemicals ingested, and microorganisms either alone or in combination are responsible for rosacea.
Dr. Gallo went on to highlight findings from several studies that link rosacea to an increased risk for certain comorbidities. One, a nationwide case-control study from Taiwan that comprised 35,553 rosacea patients, found that the disease was significantly associated with a risk of certain cardiovascular comorbidities (J Acad Dermatol. 2015;73:249-54). These included dyslipidemia (OR 1.41), coronary artery disease (OR 1.35), and hypertension (OR 1.17). A separate analysis, based 93,314 participants in the Nurse’s Health Study, found that rosacea was significantly associated with a risk of coronary artery disease (OR 2.2). The researchers also observed that comorbidities seemed to increase with duration of the disease (Clin Gastroenterol Hepatol. 2016;14[2]:220-5). Another smaller case-control study of 65 patients and 65 controls found an increased risk with rosacea for coronary artery disease, hyperlipidemia, hypertension, and gastroesophageal reflux disease (J Am Acad Dermatol. 2015;73[4]:604-8).
A recent analysis of 75,088 participants in the Nurses’ Health Study found that women with rosacea faced an increased risk of thyroid cancer (HR 1.59) and basal cell carcinoma (HR 1.50; Br J Cancer 2015;113[3]:520-3). Rosacea may also impact one’s risk for developing certain neurological conditions. One study found an increased risk for dementia (HR 1.42) and Alzheimer’s disease (HR 1.92; Ann Neurol. 2016;79:921-8), while another found an increased risk for Parkinson’s disease (an adjusted incident ratio of 1.71 in patients with rosacea, compared with the referent population; JAMA Neurol. 2016;73[5]:529-34).
As for therapy, a recent Cochrane systematic review found strong evidence supporting benefits of several therapies over placebo, including metronidazole, azelaic acid, brimonidine, tetracycline, doxycycline 40 mg, ivermectin, and isotretinoin (Br J Dermatol. 2015;173[3]:651-2). A separate, 7-year retrospective study of 275 adults with rosacea published online in The Journal of Dermatology on Oct. 28, 2015, found that patients with the PPR subtype had a better overall prognosis, compared with their counterparts with the other subtypes. Overall, the median time to complete remission was 56 months. Complete remission was achieved in 46% of those with PPR subtype, compared with 19% of those with mixed subtype and 11% of those with ETR subtype.
Dr. Gallo disclosed that he has received research grants from the National Institutes of Health, Allergan, L’Oreal, Colgate-Palmolive, Regeneron, GSK, Galderma, and Bayer. He is a consultant for Allergan, Colgate-Palmolive, Sente, Matrisys, Dermata, Alnylam, Abbvie, Roche, and Promius.
NEWPORT BEACH, CALIF. – In the clinical opinion of Richard L. Gallo, MD, PhD, current nomenclature for the diagnosis of rosacea could use a makeover.
“Currently, we’re still operating with an almost 20-year-old set of diagnostic subtypes of rosacea,” Dr. Gallo said at the annual meeting of the Pacific Dermatologic Association. He plans to participate in consensus meeting of experts who will convene this fall in an effort to update and modify these diagnostic criteria.
According to current nomenclature, subtype 1 is erythematotelangiectatic rosacea characterized by facial redness; subtype 2 is papulopustular, marked by bumps and pimples; subtype 3 is phymatous, characterized by enlargement of the nose, and subtype 4 is ocular, marked by eye irritation. Dr. Gallo pointed out that it’s rare to see just one of these subtypes in rosacea patients, with the exception of the erythematotelangiectatic rosacea (ETR). “There is a large population with ETR alone,” he said.” Most patients with the papulopustular subtype have aspects of ETR. There is a mix of subtypes of rosacea and we clearly need to modify our diagnostic criteria.”
Secondary rosacea features may include burning or stinging, plaque, dry appearance and scale, edema, ocular manifestations, peripheral location, and phymatous changes. Work by several researchers in recent years has shed light on the pathophysiology of rosacea. “We’re learning that there are many aspects to this disease that both trigger it and result in progression of the disease,” said Dr. Gallo, professor and chair of the department of dermatology at the University of California, San Diego. “It seems to have biological triggers that exist both in the environment and initiate from internal sources. We’re understanding more about the nature of those, at least specific molecules externally from microbes and so forth. Internally we understand more about the unique inflammatory signals.”
For example, he and other researchers began to look at the innate immune system patients with rosacea and identified LL37, a multifunction peptide that plays a role in a number of skin diseases, as something that can promote inflammation (Nat Med. 2007;13[8]:975-80). “It also promotes the vascular changes [that occur with the disease],” Dr. Gallo said. “We’re now learning how a dysregulation of enzymes in the skin contributes to making too much of these types of peptides. Therefore, treatment approaches that might modify enzymatic activity become useful.” Researchers have also discovered that some of the innate recognition molecules like toll-like receptor 2 (TLR2) are overexpressed in rosacea patients. “Similarly, in terms of the vascular signals, a number of labs are identifying some of the newer vascular transmitters that seem to be uniquely elevated in rosacea, so there’s great reason to be optimistic that given the increased specificity and understanding of what uniquely makes this disease happen, we’ll be able to target it in a safe way.”
A number of published studies have supported these notions, including an analysis of 275 twin pairs (JAMA Dermatol. 2015;151[11]:1213-9). The researchers found that compared with fraternal twins, identical twins had a higher association of National Rosacea Society scores (P = .04), “supporting the concept that there are fundamental genetic factors that are influencing disease,” Dr. Gallo said. Environmental factors found to be associated with rosacea include lifetime UV exposure, smoking, obesity, and alcohol use.
In an assessment of the genetic basis of rosacea by genome-wide association study, researchers identified one confirmed single-nucleotide polymorphism that could be associated with rosacea (J Invest Dermatol. 2015;135[6]:1548-55). It was located in an intergenic region between HLA-DRA and BTNL2, “which is consistent with the overall concept that there is perhaps a genetic abnormality that is leading to increased amino modulation of difficulties,” Dr. Gallo said. For another recent study, researchers analyzed 14 randomized or case control trials involving rosacea patients (Int J Med Sci. 2015;12[5]:387-96). They concluded that vasculature, chronic inflammatory responses, environmental triggers, food and chemicals ingested, and microorganisms either alone or in combination are responsible for rosacea.
Dr. Gallo went on to highlight findings from several studies that link rosacea to an increased risk for certain comorbidities. One, a nationwide case-control study from Taiwan that comprised 35,553 rosacea patients, found that the disease was significantly associated with a risk of certain cardiovascular comorbidities (J Acad Dermatol. 2015;73:249-54). These included dyslipidemia (OR 1.41), coronary artery disease (OR 1.35), and hypertension (OR 1.17). A separate analysis, based 93,314 participants in the Nurse’s Health Study, found that rosacea was significantly associated with a risk of coronary artery disease (OR 2.2). The researchers also observed that comorbidities seemed to increase with duration of the disease (Clin Gastroenterol Hepatol. 2016;14[2]:220-5). Another smaller case-control study of 65 patients and 65 controls found an increased risk with rosacea for coronary artery disease, hyperlipidemia, hypertension, and gastroesophageal reflux disease (J Am Acad Dermatol. 2015;73[4]:604-8).
A recent analysis of 75,088 participants in the Nurses’ Health Study found that women with rosacea faced an increased risk of thyroid cancer (HR 1.59) and basal cell carcinoma (HR 1.50; Br J Cancer 2015;113[3]:520-3). Rosacea may also impact one’s risk for developing certain neurological conditions. One study found an increased risk for dementia (HR 1.42) and Alzheimer’s disease (HR 1.92; Ann Neurol. 2016;79:921-8), while another found an increased risk for Parkinson’s disease (an adjusted incident ratio of 1.71 in patients with rosacea, compared with the referent population; JAMA Neurol. 2016;73[5]:529-34).
As for therapy, a recent Cochrane systematic review found strong evidence supporting benefits of several therapies over placebo, including metronidazole, azelaic acid, brimonidine, tetracycline, doxycycline 40 mg, ivermectin, and isotretinoin (Br J Dermatol. 2015;173[3]:651-2). A separate, 7-year retrospective study of 275 adults with rosacea published online in The Journal of Dermatology on Oct. 28, 2015, found that patients with the PPR subtype had a better overall prognosis, compared with their counterparts with the other subtypes. Overall, the median time to complete remission was 56 months. Complete remission was achieved in 46% of those with PPR subtype, compared with 19% of those with mixed subtype and 11% of those with ETR subtype.
Dr. Gallo disclosed that he has received research grants from the National Institutes of Health, Allergan, L’Oreal, Colgate-Palmolive, Regeneron, GSK, Galderma, and Bayer. He is a consultant for Allergan, Colgate-Palmolive, Sente, Matrisys, Dermata, Alnylam, Abbvie, Roche, and Promius.
NEWPORT BEACH, CALIF. – In the clinical opinion of Richard L. Gallo, MD, PhD, current nomenclature for the diagnosis of rosacea could use a makeover.
“Currently, we’re still operating with an almost 20-year-old set of diagnostic subtypes of rosacea,” Dr. Gallo said at the annual meeting of the Pacific Dermatologic Association. He plans to participate in consensus meeting of experts who will convene this fall in an effort to update and modify these diagnostic criteria.
According to current nomenclature, subtype 1 is erythematotelangiectatic rosacea characterized by facial redness; subtype 2 is papulopustular, marked by bumps and pimples; subtype 3 is phymatous, characterized by enlargement of the nose, and subtype 4 is ocular, marked by eye irritation. Dr. Gallo pointed out that it’s rare to see just one of these subtypes in rosacea patients, with the exception of the erythematotelangiectatic rosacea (ETR). “There is a large population with ETR alone,” he said.” Most patients with the papulopustular subtype have aspects of ETR. There is a mix of subtypes of rosacea and we clearly need to modify our diagnostic criteria.”
Secondary rosacea features may include burning or stinging, plaque, dry appearance and scale, edema, ocular manifestations, peripheral location, and phymatous changes. Work by several researchers in recent years has shed light on the pathophysiology of rosacea. “We’re learning that there are many aspects to this disease that both trigger it and result in progression of the disease,” said Dr. Gallo, professor and chair of the department of dermatology at the University of California, San Diego. “It seems to have biological triggers that exist both in the environment and initiate from internal sources. We’re understanding more about the nature of those, at least specific molecules externally from microbes and so forth. Internally we understand more about the unique inflammatory signals.”
For example, he and other researchers began to look at the innate immune system patients with rosacea and identified LL37, a multifunction peptide that plays a role in a number of skin diseases, as something that can promote inflammation (Nat Med. 2007;13[8]:975-80). “It also promotes the vascular changes [that occur with the disease],” Dr. Gallo said. “We’re now learning how a dysregulation of enzymes in the skin contributes to making too much of these types of peptides. Therefore, treatment approaches that might modify enzymatic activity become useful.” Researchers have also discovered that some of the innate recognition molecules like toll-like receptor 2 (TLR2) are overexpressed in rosacea patients. “Similarly, in terms of the vascular signals, a number of labs are identifying some of the newer vascular transmitters that seem to be uniquely elevated in rosacea, so there’s great reason to be optimistic that given the increased specificity and understanding of what uniquely makes this disease happen, we’ll be able to target it in a safe way.”
A number of published studies have supported these notions, including an analysis of 275 twin pairs (JAMA Dermatol. 2015;151[11]:1213-9). The researchers found that compared with fraternal twins, identical twins had a higher association of National Rosacea Society scores (P = .04), “supporting the concept that there are fundamental genetic factors that are influencing disease,” Dr. Gallo said. Environmental factors found to be associated with rosacea include lifetime UV exposure, smoking, obesity, and alcohol use.
In an assessment of the genetic basis of rosacea by genome-wide association study, researchers identified one confirmed single-nucleotide polymorphism that could be associated with rosacea (J Invest Dermatol. 2015;135[6]:1548-55). It was located in an intergenic region between HLA-DRA and BTNL2, “which is consistent with the overall concept that there is perhaps a genetic abnormality that is leading to increased amino modulation of difficulties,” Dr. Gallo said. For another recent study, researchers analyzed 14 randomized or case control trials involving rosacea patients (Int J Med Sci. 2015;12[5]:387-96). They concluded that vasculature, chronic inflammatory responses, environmental triggers, food and chemicals ingested, and microorganisms either alone or in combination are responsible for rosacea.
Dr. Gallo went on to highlight findings from several studies that link rosacea to an increased risk for certain comorbidities. One, a nationwide case-control study from Taiwan that comprised 35,553 rosacea patients, found that the disease was significantly associated with a risk of certain cardiovascular comorbidities (J Acad Dermatol. 2015;73:249-54). These included dyslipidemia (OR 1.41), coronary artery disease (OR 1.35), and hypertension (OR 1.17). A separate analysis, based 93,314 participants in the Nurse’s Health Study, found that rosacea was significantly associated with a risk of coronary artery disease (OR 2.2). The researchers also observed that comorbidities seemed to increase with duration of the disease (Clin Gastroenterol Hepatol. 2016;14[2]:220-5). Another smaller case-control study of 65 patients and 65 controls found an increased risk with rosacea for coronary artery disease, hyperlipidemia, hypertension, and gastroesophageal reflux disease (J Am Acad Dermatol. 2015;73[4]:604-8).
A recent analysis of 75,088 participants in the Nurses’ Health Study found that women with rosacea faced an increased risk of thyroid cancer (HR 1.59) and basal cell carcinoma (HR 1.50; Br J Cancer 2015;113[3]:520-3). Rosacea may also impact one’s risk for developing certain neurological conditions. One study found an increased risk for dementia (HR 1.42) and Alzheimer’s disease (HR 1.92; Ann Neurol. 2016;79:921-8), while another found an increased risk for Parkinson’s disease (an adjusted incident ratio of 1.71 in patients with rosacea, compared with the referent population; JAMA Neurol. 2016;73[5]:529-34).
As for therapy, a recent Cochrane systematic review found strong evidence supporting benefits of several therapies over placebo, including metronidazole, azelaic acid, brimonidine, tetracycline, doxycycline 40 mg, ivermectin, and isotretinoin (Br J Dermatol. 2015;173[3]:651-2). A separate, 7-year retrospective study of 275 adults with rosacea published online in The Journal of Dermatology on Oct. 28, 2015, found that patients with the PPR subtype had a better overall prognosis, compared with their counterparts with the other subtypes. Overall, the median time to complete remission was 56 months. Complete remission was achieved in 46% of those with PPR subtype, compared with 19% of those with mixed subtype and 11% of those with ETR subtype.
Dr. Gallo disclosed that he has received research grants from the National Institutes of Health, Allergan, L’Oreal, Colgate-Palmolive, Regeneron, GSK, Galderma, and Bayer. He is a consultant for Allergan, Colgate-Palmolive, Sente, Matrisys, Dermata, Alnylam, Abbvie, Roche, and Promius.
EXPERT ANALYSIS AT PDA 2016
Study identifies link between rosacea and several GI disorders
A Danish population-based cohort study identified a significant association between patients who have rosacea and their risk of having certain other gastrointestinal diseases – specifically, celiac disease, Crohn’s disease, ulcerative colitis, and irritable bowel syndrome.
“While a co-occurrence of rosacea and gastrointestinal disorders has previously been evaluated, the topic remains controversial,” wrote the authors, led by Alexander Egeberg, MD, of the department of dermatology and allergy, Herlev and Gentofte Hospital, Hellerup, Denmark (Br J Dermatol. 2016 Aug 8. doi: 10.1111/bjd.14930).
Dr. Egeberg and his coinvestigators conducted a nationwide cohort study of adults aged 18 years and older from national administrative registers, starting on Jan. 1, 2008, through Dec. 31, 2012. In total, 49,475 rosacea patients were included, with 4,312,213 individuals from the general population who were used as controls. The outcomes were any occurrences of celiac disease (CeD), Crohn’s disease (CD), ulcerative colitis (UC), and irritable bowel syndrome (IBS), Helicobactor pylori (HP) infection, and small intestinal bacterial overgrowth that occurred during the study period, conditions that were chosen “due to their potential mechanistic and pathogenic overlap with rosacea,” they wrote.
At baseline, the prevalence of CeD, CD, UC, HP infection, small intestinal bacterial overgrowth, and IBS was significantly higher among the patients with rosacea, compared with the controls.
Adjusted hazard ratios showed a significant association between patients with rosacea and one of the following GI diagnoses: CeD (Hazard ratio, 1.46), CD (HR, 1.45), UC (HR, 1.19), and IBS (HR, 1.34). However, no significant association was found between rosacea and HP infection or small intestinal bacterial overgrowth.
“The findings from this study raise an important question about the pathogenic overlap between the studied gastrointestinal disorders and rosacea,” Dr. Egeberg and his coauthors wrote. Most of the outcomes examined in the study, they noted, “carry several autoimmune characteristics and, although speculative, it is possible that shared autoimmune susceptibility may provide a link between rosacea and the examined gastrointestinal disorders.”
No funding was received for this study. Dr. Egeberg and his coauthors reported no relevant financial disclosures.
A Danish population-based cohort study identified a significant association between patients who have rosacea and their risk of having certain other gastrointestinal diseases – specifically, celiac disease, Crohn’s disease, ulcerative colitis, and irritable bowel syndrome.
“While a co-occurrence of rosacea and gastrointestinal disorders has previously been evaluated, the topic remains controversial,” wrote the authors, led by Alexander Egeberg, MD, of the department of dermatology and allergy, Herlev and Gentofte Hospital, Hellerup, Denmark (Br J Dermatol. 2016 Aug 8. doi: 10.1111/bjd.14930).
Dr. Egeberg and his coinvestigators conducted a nationwide cohort study of adults aged 18 years and older from national administrative registers, starting on Jan. 1, 2008, through Dec. 31, 2012. In total, 49,475 rosacea patients were included, with 4,312,213 individuals from the general population who were used as controls. The outcomes were any occurrences of celiac disease (CeD), Crohn’s disease (CD), ulcerative colitis (UC), and irritable bowel syndrome (IBS), Helicobactor pylori (HP) infection, and small intestinal bacterial overgrowth that occurred during the study period, conditions that were chosen “due to their potential mechanistic and pathogenic overlap with rosacea,” they wrote.
At baseline, the prevalence of CeD, CD, UC, HP infection, small intestinal bacterial overgrowth, and IBS was significantly higher among the patients with rosacea, compared with the controls.
Adjusted hazard ratios showed a significant association between patients with rosacea and one of the following GI diagnoses: CeD (Hazard ratio, 1.46), CD (HR, 1.45), UC (HR, 1.19), and IBS (HR, 1.34). However, no significant association was found between rosacea and HP infection or small intestinal bacterial overgrowth.
“The findings from this study raise an important question about the pathogenic overlap between the studied gastrointestinal disorders and rosacea,” Dr. Egeberg and his coauthors wrote. Most of the outcomes examined in the study, they noted, “carry several autoimmune characteristics and, although speculative, it is possible that shared autoimmune susceptibility may provide a link between rosacea and the examined gastrointestinal disorders.”
No funding was received for this study. Dr. Egeberg and his coauthors reported no relevant financial disclosures.
A Danish population-based cohort study identified a significant association between patients who have rosacea and their risk of having certain other gastrointestinal diseases – specifically, celiac disease, Crohn’s disease, ulcerative colitis, and irritable bowel syndrome.
“While a co-occurrence of rosacea and gastrointestinal disorders has previously been evaluated, the topic remains controversial,” wrote the authors, led by Alexander Egeberg, MD, of the department of dermatology and allergy, Herlev and Gentofte Hospital, Hellerup, Denmark (Br J Dermatol. 2016 Aug 8. doi: 10.1111/bjd.14930).
Dr. Egeberg and his coinvestigators conducted a nationwide cohort study of adults aged 18 years and older from national administrative registers, starting on Jan. 1, 2008, through Dec. 31, 2012. In total, 49,475 rosacea patients were included, with 4,312,213 individuals from the general population who were used as controls. The outcomes were any occurrences of celiac disease (CeD), Crohn’s disease (CD), ulcerative colitis (UC), and irritable bowel syndrome (IBS), Helicobactor pylori (HP) infection, and small intestinal bacterial overgrowth that occurred during the study period, conditions that were chosen “due to their potential mechanistic and pathogenic overlap with rosacea,” they wrote.
At baseline, the prevalence of CeD, CD, UC, HP infection, small intestinal bacterial overgrowth, and IBS was significantly higher among the patients with rosacea, compared with the controls.
Adjusted hazard ratios showed a significant association between patients with rosacea and one of the following GI diagnoses: CeD (Hazard ratio, 1.46), CD (HR, 1.45), UC (HR, 1.19), and IBS (HR, 1.34). However, no significant association was found between rosacea and HP infection or small intestinal bacterial overgrowth.
“The findings from this study raise an important question about the pathogenic overlap between the studied gastrointestinal disorders and rosacea,” Dr. Egeberg and his coauthors wrote. Most of the outcomes examined in the study, they noted, “carry several autoimmune characteristics and, although speculative, it is possible that shared autoimmune susceptibility may provide a link between rosacea and the examined gastrointestinal disorders.”
No funding was received for this study. Dr. Egeberg and his coauthors reported no relevant financial disclosures.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
Key clinical point: There is an association between rosacea and certain gastrointestinal diseases, but the specific link between the two is not clear.
Major finding: People with rosacea had a significantly increased risk of having celiac disease, Crohn’s disease, ulcerative colitis, and irritable bowel syndrome.
Data source: A population-based case-control study in Denmark compared the risks of several GI disorders in 49,475 people with rosacea and 4,312,213 controls without rosacea.
Disclosures: No funding was received for this study. The authors reported no relevant financial disclosures.
Rosacea responds well to laser, IPL therapies
BOSTON – Patients with rosacea, particularly the erythrotelangiectatic form, are considered good candidates for treatment with lasers and light therapies, but for acne, treatments with these therapies are still in the development stage.
For acne, treatments that are being studied include those that target the sebaceous glands, according to Mathew M. Avram, MD, who spoke about laser and light therapies for acne and rosacea at the American Academy of Dermatology summer meeting.
Light therapies for rosacea
Oxyhemoglobin in the blood absorbs light from lasers at wavelengths of about 595 nm (pulsed dye laser) and 532 nm (KTP laser), creating heat that helps destroy capillaries that contribute to the appearance of rosacea. Over a period of 3-4 weeks, the vessels are resorbed, and facial redness diminishes.
Patients with rosacea that are expected to do best with laser therapy are those with telangiectasia. Laser therapy is also effective for background redness but will be less effective for people with the papules associated with rosacea and “almost not effective at all for preventing flushing,” Dr. Avram said in an interview at the meeting.
Intense pulsed light (IPL) is another modality for treating rosacea. As with lasers, the mode of action is heating of certain structures and chromophores, causing their destruction and resorption, but unlike lasers, IPL output is broad spectrum and can be modified using filters.
With IPL, “basically, the endpoint that you want to see is transient purpural change, just a fleeting period of some black and blue, or if you’re treating vessels, you want to see vessel clearance when you’re firing the laser or the intense pulsed light,” said Dr. Avram, director of the Massachusetts General Hospital Dermatology Laser & Cosmetic Center, Boston.
On-screen settings of laser or IPL devices are essential, “but ultimately, if you want to have an effective treatment you really have to see what’s happening to your target … you need to pay attention to clinical endpoints, which is seeing that black and blue or that vessel clearance, not just paying attention to what’s on the screen.”
With IPL, too much pressure can compress vessels and blanch the skin, resulting in a less effective treatment, he added. He noted that tissue graying, whitening, or contraction indicates overly aggressive treatment, with the risk of scarring.
Certain factors can reduce the efficacy of IPL treatments of rosacea. Dr. Avram recommended against treating tanned skin and pointed out that anemic patients may benefit less from this approach since less hemoglobin presents a less-absorptive target for the treatments. He also advised particular caution when treating darker skin phototypes. But the most common factor that may make these treatments less effective is when a patient is on any type of anticoagulant, including NSAIDs or warfarin (Coumadin), because the mechanism of action is immediate microvascular hemorrhage, thrombosis, and eventual resorption.
For best results, Dr. Avram advises “appropriate overlap with the laser” to get an even and uniform improvement in the redness, with about a 15% overlap. Spacing laser spots too far apart can result in “foot printing,” the appearance of clearance in the areas of the laser pulse, but not in areas immediately around it “so it looks almost polka dotted.” After treatments, all patients should avoid the sun, he added.
What’s ahead for acne treatment
Until now, laser and light-based treatments for acne, “have provided inconsistent benefits for patients and all too often disappointing results,” Dr. Avram said in the interview. But several developments on the horizon may offer more effective therapies based on completely different technologies than are currently available. “Each of these therapies will be targeting the sebaceous glands in order to provide improved treatment for acne.”
One is a cryolysis device that uses cooling to selectively target lipids in the sebaceous glands. (Cryolysis is similar to cryolipolysis, which uses cooling to target fat cells.) The lipid-filled adipocytes are more sensitive to cold than is the water-rich dermis, thereby preserving the surrounding structures. There are also laser wavelengths that are absorbed by lipids, one of which is at about 1720 nm. In this case, heating rather than cooling targets the lipids.
Another technology in development is the use of nanoparticles coated with elements such as gold that are massaged through the skin into the sebaceous glands. Laser treatment with multiple different wavelengths targets the nanoparticles, heating them within the sebaceous glands, resulting in improvements in acne. In this case, treatment does not depend on the absorption spectrum of lipids. Clinical trials of this approach are now underway.
It is too early to tell which of these technologies is going to be effective or what potential side effects may occur. “However, the exciting news is that there will be multiple different technologies” designed to improve acne by targeting the sebaceous gland, and there is “the promise of potentially more effective noninvasive treatments that don’t require topical medications or oral medications,” Dr. Avram said.
For these potential new treatments, some objective outcome measure is needed to judge their efficacy. Right now, all that can be said is that they target the sebaceous gland, and clinical work still needs to be done to determine whether they will be effective, the degree of effectiveness, and how to optimize treatment, he noted.
Dr. Avram reported financial relationships with Cytrellis Biosystems, Invasix, Kythera, Masters of Aesthetics, Sciton, Zalea, and Zeltiq Aesthetics.
BOSTON – Patients with rosacea, particularly the erythrotelangiectatic form, are considered good candidates for treatment with lasers and light therapies, but for acne, treatments with these therapies are still in the development stage.
For acne, treatments that are being studied include those that target the sebaceous glands, according to Mathew M. Avram, MD, who spoke about laser and light therapies for acne and rosacea at the American Academy of Dermatology summer meeting.
Light therapies for rosacea
Oxyhemoglobin in the blood absorbs light from lasers at wavelengths of about 595 nm (pulsed dye laser) and 532 nm (KTP laser), creating heat that helps destroy capillaries that contribute to the appearance of rosacea. Over a period of 3-4 weeks, the vessels are resorbed, and facial redness diminishes.
Patients with rosacea that are expected to do best with laser therapy are those with telangiectasia. Laser therapy is also effective for background redness but will be less effective for people with the papules associated with rosacea and “almost not effective at all for preventing flushing,” Dr. Avram said in an interview at the meeting.
Intense pulsed light (IPL) is another modality for treating rosacea. As with lasers, the mode of action is heating of certain structures and chromophores, causing their destruction and resorption, but unlike lasers, IPL output is broad spectrum and can be modified using filters.
With IPL, “basically, the endpoint that you want to see is transient purpural change, just a fleeting period of some black and blue, or if you’re treating vessels, you want to see vessel clearance when you’re firing the laser or the intense pulsed light,” said Dr. Avram, director of the Massachusetts General Hospital Dermatology Laser & Cosmetic Center, Boston.
On-screen settings of laser or IPL devices are essential, “but ultimately, if you want to have an effective treatment you really have to see what’s happening to your target … you need to pay attention to clinical endpoints, which is seeing that black and blue or that vessel clearance, not just paying attention to what’s on the screen.”
With IPL, too much pressure can compress vessels and blanch the skin, resulting in a less effective treatment, he added. He noted that tissue graying, whitening, or contraction indicates overly aggressive treatment, with the risk of scarring.
Certain factors can reduce the efficacy of IPL treatments of rosacea. Dr. Avram recommended against treating tanned skin and pointed out that anemic patients may benefit less from this approach since less hemoglobin presents a less-absorptive target for the treatments. He also advised particular caution when treating darker skin phototypes. But the most common factor that may make these treatments less effective is when a patient is on any type of anticoagulant, including NSAIDs or warfarin (Coumadin), because the mechanism of action is immediate microvascular hemorrhage, thrombosis, and eventual resorption.
For best results, Dr. Avram advises “appropriate overlap with the laser” to get an even and uniform improvement in the redness, with about a 15% overlap. Spacing laser spots too far apart can result in “foot printing,” the appearance of clearance in the areas of the laser pulse, but not in areas immediately around it “so it looks almost polka dotted.” After treatments, all patients should avoid the sun, he added.
What’s ahead for acne treatment
Until now, laser and light-based treatments for acne, “have provided inconsistent benefits for patients and all too often disappointing results,” Dr. Avram said in the interview. But several developments on the horizon may offer more effective therapies based on completely different technologies than are currently available. “Each of these therapies will be targeting the sebaceous glands in order to provide improved treatment for acne.”
One is a cryolysis device that uses cooling to selectively target lipids in the sebaceous glands. (Cryolysis is similar to cryolipolysis, which uses cooling to target fat cells.) The lipid-filled adipocytes are more sensitive to cold than is the water-rich dermis, thereby preserving the surrounding structures. There are also laser wavelengths that are absorbed by lipids, one of which is at about 1720 nm. In this case, heating rather than cooling targets the lipids.
Another technology in development is the use of nanoparticles coated with elements such as gold that are massaged through the skin into the sebaceous glands. Laser treatment with multiple different wavelengths targets the nanoparticles, heating them within the sebaceous glands, resulting in improvements in acne. In this case, treatment does not depend on the absorption spectrum of lipids. Clinical trials of this approach are now underway.
It is too early to tell which of these technologies is going to be effective or what potential side effects may occur. “However, the exciting news is that there will be multiple different technologies” designed to improve acne by targeting the sebaceous gland, and there is “the promise of potentially more effective noninvasive treatments that don’t require topical medications or oral medications,” Dr. Avram said.
For these potential new treatments, some objective outcome measure is needed to judge their efficacy. Right now, all that can be said is that they target the sebaceous gland, and clinical work still needs to be done to determine whether they will be effective, the degree of effectiveness, and how to optimize treatment, he noted.
Dr. Avram reported financial relationships with Cytrellis Biosystems, Invasix, Kythera, Masters of Aesthetics, Sciton, Zalea, and Zeltiq Aesthetics.
BOSTON – Patients with rosacea, particularly the erythrotelangiectatic form, are considered good candidates for treatment with lasers and light therapies, but for acne, treatments with these therapies are still in the development stage.
For acne, treatments that are being studied include those that target the sebaceous glands, according to Mathew M. Avram, MD, who spoke about laser and light therapies for acne and rosacea at the American Academy of Dermatology summer meeting.
Light therapies for rosacea
Oxyhemoglobin in the blood absorbs light from lasers at wavelengths of about 595 nm (pulsed dye laser) and 532 nm (KTP laser), creating heat that helps destroy capillaries that contribute to the appearance of rosacea. Over a period of 3-4 weeks, the vessels are resorbed, and facial redness diminishes.
Patients with rosacea that are expected to do best with laser therapy are those with telangiectasia. Laser therapy is also effective for background redness but will be less effective for people with the papules associated with rosacea and “almost not effective at all for preventing flushing,” Dr. Avram said in an interview at the meeting.
Intense pulsed light (IPL) is another modality for treating rosacea. As with lasers, the mode of action is heating of certain structures and chromophores, causing their destruction and resorption, but unlike lasers, IPL output is broad spectrum and can be modified using filters.
With IPL, “basically, the endpoint that you want to see is transient purpural change, just a fleeting period of some black and blue, or if you’re treating vessels, you want to see vessel clearance when you’re firing the laser or the intense pulsed light,” said Dr. Avram, director of the Massachusetts General Hospital Dermatology Laser & Cosmetic Center, Boston.
On-screen settings of laser or IPL devices are essential, “but ultimately, if you want to have an effective treatment you really have to see what’s happening to your target … you need to pay attention to clinical endpoints, which is seeing that black and blue or that vessel clearance, not just paying attention to what’s on the screen.”
With IPL, too much pressure can compress vessels and blanch the skin, resulting in a less effective treatment, he added. He noted that tissue graying, whitening, or contraction indicates overly aggressive treatment, with the risk of scarring.
Certain factors can reduce the efficacy of IPL treatments of rosacea. Dr. Avram recommended against treating tanned skin and pointed out that anemic patients may benefit less from this approach since less hemoglobin presents a less-absorptive target for the treatments. He also advised particular caution when treating darker skin phototypes. But the most common factor that may make these treatments less effective is when a patient is on any type of anticoagulant, including NSAIDs or warfarin (Coumadin), because the mechanism of action is immediate microvascular hemorrhage, thrombosis, and eventual resorption.
For best results, Dr. Avram advises “appropriate overlap with the laser” to get an even and uniform improvement in the redness, with about a 15% overlap. Spacing laser spots too far apart can result in “foot printing,” the appearance of clearance in the areas of the laser pulse, but not in areas immediately around it “so it looks almost polka dotted.” After treatments, all patients should avoid the sun, he added.
What’s ahead for acne treatment
Until now, laser and light-based treatments for acne, “have provided inconsistent benefits for patients and all too often disappointing results,” Dr. Avram said in the interview. But several developments on the horizon may offer more effective therapies based on completely different technologies than are currently available. “Each of these therapies will be targeting the sebaceous glands in order to provide improved treatment for acne.”
One is a cryolysis device that uses cooling to selectively target lipids in the sebaceous glands. (Cryolysis is similar to cryolipolysis, which uses cooling to target fat cells.) The lipid-filled adipocytes are more sensitive to cold than is the water-rich dermis, thereby preserving the surrounding structures. There are also laser wavelengths that are absorbed by lipids, one of which is at about 1720 nm. In this case, heating rather than cooling targets the lipids.
Another technology in development is the use of nanoparticles coated with elements such as gold that are massaged through the skin into the sebaceous glands. Laser treatment with multiple different wavelengths targets the nanoparticles, heating them within the sebaceous glands, resulting in improvements in acne. In this case, treatment does not depend on the absorption spectrum of lipids. Clinical trials of this approach are now underway.
It is too early to tell which of these technologies is going to be effective or what potential side effects may occur. “However, the exciting news is that there will be multiple different technologies” designed to improve acne by targeting the sebaceous gland, and there is “the promise of potentially more effective noninvasive treatments that don’t require topical medications or oral medications,” Dr. Avram said.
For these potential new treatments, some objective outcome measure is needed to judge their efficacy. Right now, all that can be said is that they target the sebaceous gland, and clinical work still needs to be done to determine whether they will be effective, the degree of effectiveness, and how to optimize treatment, he noted.
Dr. Avram reported financial relationships with Cytrellis Biosystems, Invasix, Kythera, Masters of Aesthetics, Sciton, Zalea, and Zeltiq Aesthetics.
EXPERT ANALYSIS FROM THE AAD SUMMER ACADEMY 2016







