User login
FDA approves first retinoid for OTC acne treatment
The Food and Drug Administration has approved adapalene gel 0.1% for over-the-counter use, making it the first retinoid for treating acne that will be available in the United States without a prescription.
The approval also makes the product, marketed as Differin Gel 0.1%, “the first new active ingredient for acne treatment for OTC use since the 1980s,” according to an FDA statement announcing the approval July 8. Differin Gel is approved for use in people aged 12 years and older.
The switch to OTC status was supported by postmarketing safety data; by consumer studies data, which included a label comprehension study, a self-selection study, and an “actual use” study; and data from a “maximal use” study submitted by the manufacturer.
“Overall, results from the consumer studies showed that consumers can understand the information on the OTC label, appropriately select whether the product is right for them, and use the product appropriately,” according to the FDA statement. “The maximal use trial, a study of absorption of the drug through acne-affected skin when applied daily over a large surface area (face, shoulders, upper back, and chest), demonstrated that absorption is limited, thus supporting safe use of Differin Gel 0.1% by people using it OTC.”
The FDA noted that “some other retinoid drugs have been shown to cause birth defects,” and it advises that women “who are pregnant, planning to become pregnant, or [are] breast-feeding” should ask a doctor before using the product.
“While there have been no adequate and well-controlled studies of Differin Gel 0.1% in pregnant women, there is no specific evidence that Differin Gel 0.1%, when used topically as directed, causes birth defects in humans,” according to the FDA.
Consumers should follow the OTC Drug Facts label “and consult with their health care providers if their symptoms do not improve,” the FDA added.
At an April 2016 meeting, the FDA’s nonprescription drugs advisory committee unanimously voted that the safety of adapalene gel 0.1% for OTC use for treating acne had been “adequately demonstrated.”
Galderma Laboratories markets Differin Gel 0.1%.
The Food and Drug Administration has approved adapalene gel 0.1% for over-the-counter use, making it the first retinoid for treating acne that will be available in the United States without a prescription.
The approval also makes the product, marketed as Differin Gel 0.1%, “the first new active ingredient for acne treatment for OTC use since the 1980s,” according to an FDA statement announcing the approval July 8. Differin Gel is approved for use in people aged 12 years and older.
The switch to OTC status was supported by postmarketing safety data; by consumer studies data, which included a label comprehension study, a self-selection study, and an “actual use” study; and data from a “maximal use” study submitted by the manufacturer.
“Overall, results from the consumer studies showed that consumers can understand the information on the OTC label, appropriately select whether the product is right for them, and use the product appropriately,” according to the FDA statement. “The maximal use trial, a study of absorption of the drug through acne-affected skin when applied daily over a large surface area (face, shoulders, upper back, and chest), demonstrated that absorption is limited, thus supporting safe use of Differin Gel 0.1% by people using it OTC.”
The FDA noted that “some other retinoid drugs have been shown to cause birth defects,” and it advises that women “who are pregnant, planning to become pregnant, or [are] breast-feeding” should ask a doctor before using the product.
“While there have been no adequate and well-controlled studies of Differin Gel 0.1% in pregnant women, there is no specific evidence that Differin Gel 0.1%, when used topically as directed, causes birth defects in humans,” according to the FDA.
Consumers should follow the OTC Drug Facts label “and consult with their health care providers if their symptoms do not improve,” the FDA added.
At an April 2016 meeting, the FDA’s nonprescription drugs advisory committee unanimously voted that the safety of adapalene gel 0.1% for OTC use for treating acne had been “adequately demonstrated.”
Galderma Laboratories markets Differin Gel 0.1%.
The Food and Drug Administration has approved adapalene gel 0.1% for over-the-counter use, making it the first retinoid for treating acne that will be available in the United States without a prescription.
The approval also makes the product, marketed as Differin Gel 0.1%, “the first new active ingredient for acne treatment for OTC use since the 1980s,” according to an FDA statement announcing the approval July 8. Differin Gel is approved for use in people aged 12 years and older.
The switch to OTC status was supported by postmarketing safety data; by consumer studies data, which included a label comprehension study, a self-selection study, and an “actual use” study; and data from a “maximal use” study submitted by the manufacturer.
“Overall, results from the consumer studies showed that consumers can understand the information on the OTC label, appropriately select whether the product is right for them, and use the product appropriately,” according to the FDA statement. “The maximal use trial, a study of absorption of the drug through acne-affected skin when applied daily over a large surface area (face, shoulders, upper back, and chest), demonstrated that absorption is limited, thus supporting safe use of Differin Gel 0.1% by people using it OTC.”
The FDA noted that “some other retinoid drugs have been shown to cause birth defects,” and it advises that women “who are pregnant, planning to become pregnant, or [are] breast-feeding” should ask a doctor before using the product.
“While there have been no adequate and well-controlled studies of Differin Gel 0.1% in pregnant women, there is no specific evidence that Differin Gel 0.1%, when used topically as directed, causes birth defects in humans,” according to the FDA.
Consumers should follow the OTC Drug Facts label “and consult with their health care providers if their symptoms do not improve,” the FDA added.
At an April 2016 meeting, the FDA’s nonprescription drugs advisory committee unanimously voted that the safety of adapalene gel 0.1% for OTC use for treating acne had been “adequately demonstrated.”
Galderma Laboratories markets Differin Gel 0.1%.
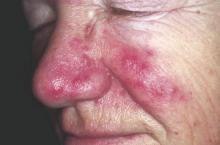
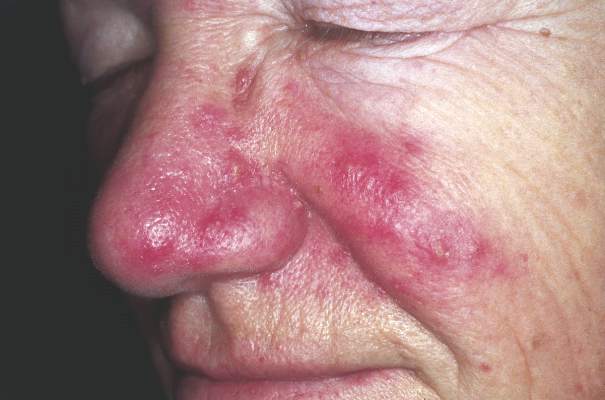
Using a Combination of Therapies to Manage Rosacea

What do your patients need to know at the first visit?
Patients need to understand that rosacea has no gender, age, or race predilection. It is caused by a personal and genetic proinflammatory predisposition. Rosacea patients seem to have a genetic predisposition to overproduce cathelicidins, a small antimicrobial peptide that is produced by the action of stratum corneum tryptic enzyme. They have more production and abnormal forms of cathelicidins produced by high levels of stratum corneum tryptic enzyme. This upregulation of inflammatory cathelicidins on the dermis is associated with vascular instability and exacerbated by triggers such as sunlight, hot drinks, spicy foods, stress, and rapid changing weather.
I divide the condition into proinflammatory predisposition, vascular instability with redness, Demodex infection of the hair follicle, and sebaceous gland overgrowth. More recently, the bacterium Bacillus oleronius was isolated from inside a Demodex mite and was found to produce molecules provoking an immune reaction in rosacea patients (Erbaguci and Ozgöztaşi). Other studies have shown that patients with varying types of rosacea react to the molecules produced by this bacterium, exposing it as a likely trigger for the condition (Li et al). What’s more, this bacterium is sensitive to the antibiotics used to treat rosacea.
What are your go-to treatments? What are the side effects?
For inflammation I prescribe anti-inflammatory (low dose) or antibacterial (high dose) doses of doxycycline and/or anti-inflammatory azelaic acid gel 15% twice daily after application of barrier repair topical hyaluronic acid. For the vascular component I use the temporary relief from the application of brimonidine gel 0.33% in the morning in addition to the topical given for inflammatory rosacea, and the more durable excel V (532 and 1064 nm) laser. Ultimately, topical ivermectin is prescribed for those patients who do not respond to previously mentioned treatments for coverage of Demodex infestation. For rhinophyma I offer a surgical approach and laser treatments; surgical removal of the excess glandular growth is followed by fractional ablative and nonablative treatments for scar reduction after surgery.
All patients should apply an inorganic sun protection factor 50+ sunblock with titanium dioxide and zinc oxide to prevent sunlight from being a trigger. All patients are encouraged to avoid triggers.
I try to prevent the potential side effects associated with rosacea treatments. For example, applying barrier repair hyaluronic acid before azelaic acid to prevent irritation and telling patients they might have vascular rebound phenomena with more redness after brimonidine application wears off. I also explain to patients that laser treatments induce temporary erythema and swelling that may last 3 days.
How do you keep patients compliant with treatment?
In general, my patients are compliant with their treatments, which I ascribe to the simplicity of a twice-daily regimen that is written for them. They understand that I design a treatment regimen for each individual patient based on his/her presentation.
What resources do you recommend to patients for more information?
I recommend web-based resources that can provide further assistance and information, such as the American Academy of Dermatology website (https://www.aad.org/public/diseases/acne-and-rosacea/rosacea), National Rosacea Society (www.rosacea.org), and specific disease foundations (eg, International Rosacea Foundation [www.internationalrosaceafoundation.org]).Suggested Readings
- Erbaguci Z, Ozgöztaşi O. The significance of Demodex folliculorum density in rosacea. Int J Dermatol. 1998;37:421-425.
- Li J, O’Reilly N, Sheha H, et al. Correlation between ocular Demodex infestation and serum immunoreactivity to Bacillus proteins in patients with facial rosacea. Ophthalmology. 2010;117:870-877.

What do your patients need to know at the first visit?
Patients need to understand that rosacea has no gender, age, or race predilection. It is caused by a personal and genetic proinflammatory predisposition. Rosacea patients seem to have a genetic predisposition to overproduce cathelicidins, a small antimicrobial peptide that is produced by the action of stratum corneum tryptic enzyme. They have more production and abnormal forms of cathelicidins produced by high levels of stratum corneum tryptic enzyme. This upregulation of inflammatory cathelicidins on the dermis is associated with vascular instability and exacerbated by triggers such as sunlight, hot drinks, spicy foods, stress, and rapid changing weather.
I divide the condition into proinflammatory predisposition, vascular instability with redness, Demodex infection of the hair follicle, and sebaceous gland overgrowth. More recently, the bacterium Bacillus oleronius was isolated from inside a Demodex mite and was found to produce molecules provoking an immune reaction in rosacea patients (Erbaguci and Ozgöztaşi). Other studies have shown that patients with varying types of rosacea react to the molecules produced by this bacterium, exposing it as a likely trigger for the condition (Li et al). What’s more, this bacterium is sensitive to the antibiotics used to treat rosacea.
What are your go-to treatments? What are the side effects?
For inflammation I prescribe anti-inflammatory (low dose) or antibacterial (high dose) doses of doxycycline and/or anti-inflammatory azelaic acid gel 15% twice daily after application of barrier repair topical hyaluronic acid. For the vascular component I use the temporary relief from the application of brimonidine gel 0.33% in the morning in addition to the topical given for inflammatory rosacea, and the more durable excel V (532 and 1064 nm) laser. Ultimately, topical ivermectin is prescribed for those patients who do not respond to previously mentioned treatments for coverage of Demodex infestation. For rhinophyma I offer a surgical approach and laser treatments; surgical removal of the excess glandular growth is followed by fractional ablative and nonablative treatments for scar reduction after surgery.
All patients should apply an inorganic sun protection factor 50+ sunblock with titanium dioxide and zinc oxide to prevent sunlight from being a trigger. All patients are encouraged to avoid triggers.
I try to prevent the potential side effects associated with rosacea treatments. For example, applying barrier repair hyaluronic acid before azelaic acid to prevent irritation and telling patients they might have vascular rebound phenomena with more redness after brimonidine application wears off. I also explain to patients that laser treatments induce temporary erythema and swelling that may last 3 days.
How do you keep patients compliant with treatment?
In general, my patients are compliant with their treatments, which I ascribe to the simplicity of a twice-daily regimen that is written for them. They understand that I design a treatment regimen for each individual patient based on his/her presentation.
What resources do you recommend to patients for more information?
I recommend web-based resources that can provide further assistance and information, such as the American Academy of Dermatology website (https://www.aad.org/public/diseases/acne-and-rosacea/rosacea), National Rosacea Society (www.rosacea.org), and specific disease foundations (eg, International Rosacea Foundation [www.internationalrosaceafoundation.org]).Suggested Readings
- Erbaguci Z, Ozgöztaşi O. The significance of Demodex folliculorum density in rosacea. Int J Dermatol. 1998;37:421-425.
- Li J, O’Reilly N, Sheha H, et al. Correlation between ocular Demodex infestation and serum immunoreactivity to Bacillus proteins in patients with facial rosacea. Ophthalmology. 2010;117:870-877.

What do your patients need to know at the first visit?
Patients need to understand that rosacea has no gender, age, or race predilection. It is caused by a personal and genetic proinflammatory predisposition. Rosacea patients seem to have a genetic predisposition to overproduce cathelicidins, a small antimicrobial peptide that is produced by the action of stratum corneum tryptic enzyme. They have more production and abnormal forms of cathelicidins produced by high levels of stratum corneum tryptic enzyme. This upregulation of inflammatory cathelicidins on the dermis is associated with vascular instability and exacerbated by triggers such as sunlight, hot drinks, spicy foods, stress, and rapid changing weather.
I divide the condition into proinflammatory predisposition, vascular instability with redness, Demodex infection of the hair follicle, and sebaceous gland overgrowth. More recently, the bacterium Bacillus oleronius was isolated from inside a Demodex mite and was found to produce molecules provoking an immune reaction in rosacea patients (Erbaguci and Ozgöztaşi). Other studies have shown that patients with varying types of rosacea react to the molecules produced by this bacterium, exposing it as a likely trigger for the condition (Li et al). What’s more, this bacterium is sensitive to the antibiotics used to treat rosacea.
What are your go-to treatments? What are the side effects?
For inflammation I prescribe anti-inflammatory (low dose) or antibacterial (high dose) doses of doxycycline and/or anti-inflammatory azelaic acid gel 15% twice daily after application of barrier repair topical hyaluronic acid. For the vascular component I use the temporary relief from the application of brimonidine gel 0.33% in the morning in addition to the topical given for inflammatory rosacea, and the more durable excel V (532 and 1064 nm) laser. Ultimately, topical ivermectin is prescribed for those patients who do not respond to previously mentioned treatments for coverage of Demodex infestation. For rhinophyma I offer a surgical approach and laser treatments; surgical removal of the excess glandular growth is followed by fractional ablative and nonablative treatments for scar reduction after surgery.
All patients should apply an inorganic sun protection factor 50+ sunblock with titanium dioxide and zinc oxide to prevent sunlight from being a trigger. All patients are encouraged to avoid triggers.
I try to prevent the potential side effects associated with rosacea treatments. For example, applying barrier repair hyaluronic acid before azelaic acid to prevent irritation and telling patients they might have vascular rebound phenomena with more redness after brimonidine application wears off. I also explain to patients that laser treatments induce temporary erythema and swelling that may last 3 days.
How do you keep patients compliant with treatment?
In general, my patients are compliant with their treatments, which I ascribe to the simplicity of a twice-daily regimen that is written for them. They understand that I design a treatment regimen for each individual patient based on his/her presentation.
What resources do you recommend to patients for more information?
I recommend web-based resources that can provide further assistance and information, such as the American Academy of Dermatology website (https://www.aad.org/public/diseases/acne-and-rosacea/rosacea), National Rosacea Society (www.rosacea.org), and specific disease foundations (eg, International Rosacea Foundation [www.internationalrosaceafoundation.org]).Suggested Readings
- Erbaguci Z, Ozgöztaşi O. The significance of Demodex folliculorum density in rosacea. Int J Dermatol. 1998;37:421-425.
- Li J, O’Reilly N, Sheha H, et al. Correlation between ocular Demodex infestation and serum immunoreactivity to Bacillus proteins in patients with facial rosacea. Ophthalmology. 2010;117:870-877.
Experts present first recommendations for treating acne fulminans
SCOTTSDALE, ARIZ. – Patients with acne fulminans should first begin corticosteroid monotherapy before adding isotretinoin, according to the first evidence-based consensus recommendations on this disease.
Antibiotics should not be used as first line treatment or monotherapy for acne fulminans, according to the experts who drafted the recommendations. Acne fulminans is an uncommon, understudied, and severe disorder that “typically manifests as an explosive worsening and ulceration of skin lesions, and can be associated with fever, bone pain, and other systemic symptoms,” noted panel co-chairs Dr. Andrea Zaenglein, professor of dermatology and pediatric dermatology, Pennsylvania State University, Hershey, and Dr. Sheila Friedlander, professor of medicine and pediatrics, University of California, San Diego, together with their associates.
The recommendations – based on a full literature review, a 5-hour audioconference, and two rounds of surveys to achieve consensus on these topics – were summarized in a poster presented at the annual meeting of the Society for Investigative Dermatology.
Until now, there have been no clear guidelines on the pathogenesis, treatment, and prevention of acne fulminans, according to the panelists.
Consensus definitions
“Acne fulminans is not just an extreme form of acne, but rather, a distinct, likely auto-inflammatory disorder,” the experts stated. Affected patients typically have an “abrupt, dramatic flare of inflammatory acne, with erosions, and with or without crusts, hemorrhagic nodules/plaques, and systemic findings.”
Systemic involvement is uncommon, but when present, includes fever, malaise, bone pain, arthralgias, erythema nodosum, and leukocytosis, they noted. Some patients also have anemia, an elevated erythrocyte sedimentation rate, and an increased C-reactive protein level. Radiography typically reveals osteolytic lesions of the sternum, clavicles, sacroiliac joints, and hips.
Acne fulminans is most often triggered by isotretinoin therapy, but can occur without it, the panel said. Isotretinoin-induced acne fulminans can have systemic involvement, but usually does not.
Corticosteroids
Patients should start corticosteroid monotherapy at a dose of 0.5 to 1.0 mg per kg per day, according to the recommendations. Patients with systemic involvement should receive steroids for at least 4 weeks, and other patients should continue steroids for at least 2 weeks and until all lesions have healed.
Oral corticosteroids should be tapered slowly over about 4 to 8 weeks, first by halving the dose to a physiologic dose each week, and then by dosing every other day for 2 weeks. Topical corticosteroids also can be used for eroded sites with granulation tissue, they noted.
Isotretinoin
Ironically, isotretinoin is both a treatment and a potential trigger of acne fulminans, and the recommendations included detailed guidance on its use.
Patients should wait at least 2 weeks after crusting resolves before starting isotretinoin, and should overlap isotretinoin with corticosteroids for at least 4 weeks, the experts emphasized. They recommended starting isotretinoin at 0.1 mg per kg per day, and waiting at least 2 months to increase this dose. Because patients clear at different rates, there is no universal optimal cumulative dose of isotretinoin, they noted.
If patients on isotretinoin develop flare, crusts, and erosions, they should halt treatment and either start corticosteroids, or increase the steroid dose to 1.0 mg per kg. If crusts and erosions persist, the panelists recommended considering cyclosporine, biologics, or dapsone.
If hemorrhagic crusts or erosions resolve after stopping isotretinoin, it can be restarted at the initial dose of 0.1 mg per kg and overlapped with steroids for 4 weeks.
If flares, crusts, and erosions begin when patients on isotretinoin are tapering corticosteroids, then steroids should be continued without tapering, isotretinoin should be stopped temporarily, and it should be restarted at 0.1 mg per kg after crusts and erosions have healed. This dose should be continued for 4 weeks, and then slowly increased as tolerated.
Antibiotics
Antibiotics are not useful as monotherapy for first-line therapy for acne fulminans, according to the recommendations. But to avoid isotretinoin-induced acne fulminans, the experts often pretreat patients with oral antibiotics before starting isotretinoin, and may continue oral antibiotics during the initial phase of isotretinoin treatment. However, there have been no prospective studies supporting this approach, they noted.
Other treatment considerations
Acne fulminans lesions should not be debrided, the experts emphasized. Acne fulminans associated with SAPHO (synovitis, acne, pustulosis, hyperostosis, and osteitis), PAPA (pyogenic arthritis, pyoderma gangrenosum, and acne), and PAPASH (pyogenic arthritis, pyoderma gangrenosum, acne, and hidradenitis suppurativa) has responded successfully to tumor necrosis factor–alpha inhibitors and interleukin-1 receptor antagonists, they noted. Pulsed dye laser also has been used to successfully treat acne fulminans, particularly when there is associated granulation tissue, they stated.
Reducing the risk of pseudotumor cerebri syndrome
Several drugs used to treat acne fulminans have been linked to pseudotumor cerebri syndrome, the experts cautioned. “Among the tetracyclines, minocycline carries the highest risk,” they noted. Both isotretinoin and corticosteroids have been linked to pseudotumor cerebri syndrome, but clinicians can reduce this risk by avoiding an abrupt steroid taper, they emphasized.
The panel included physicians from the University of California, San Diego; Pennsylvania State University, Hershey; State University of New York, Brooklyn; Cornell University, New York; Touro University California, Vallejo; the University of California, San Francisco; the University of Pennsylvania, Philadelphia; and Jefferson Medical College, Philadelphia. They did not disclose conflicts of interest.
SCOTTSDALE, ARIZ. – Patients with acne fulminans should first begin corticosteroid monotherapy before adding isotretinoin, according to the first evidence-based consensus recommendations on this disease.
Antibiotics should not be used as first line treatment or monotherapy for acne fulminans, according to the experts who drafted the recommendations. Acne fulminans is an uncommon, understudied, and severe disorder that “typically manifests as an explosive worsening and ulceration of skin lesions, and can be associated with fever, bone pain, and other systemic symptoms,” noted panel co-chairs Dr. Andrea Zaenglein, professor of dermatology and pediatric dermatology, Pennsylvania State University, Hershey, and Dr. Sheila Friedlander, professor of medicine and pediatrics, University of California, San Diego, together with their associates.
The recommendations – based on a full literature review, a 5-hour audioconference, and two rounds of surveys to achieve consensus on these topics – were summarized in a poster presented at the annual meeting of the Society for Investigative Dermatology.
Until now, there have been no clear guidelines on the pathogenesis, treatment, and prevention of acne fulminans, according to the panelists.
Consensus definitions
“Acne fulminans is not just an extreme form of acne, but rather, a distinct, likely auto-inflammatory disorder,” the experts stated. Affected patients typically have an “abrupt, dramatic flare of inflammatory acne, with erosions, and with or without crusts, hemorrhagic nodules/plaques, and systemic findings.”
Systemic involvement is uncommon, but when present, includes fever, malaise, bone pain, arthralgias, erythema nodosum, and leukocytosis, they noted. Some patients also have anemia, an elevated erythrocyte sedimentation rate, and an increased C-reactive protein level. Radiography typically reveals osteolytic lesions of the sternum, clavicles, sacroiliac joints, and hips.
Acne fulminans is most often triggered by isotretinoin therapy, but can occur without it, the panel said. Isotretinoin-induced acne fulminans can have systemic involvement, but usually does not.
Corticosteroids
Patients should start corticosteroid monotherapy at a dose of 0.5 to 1.0 mg per kg per day, according to the recommendations. Patients with systemic involvement should receive steroids for at least 4 weeks, and other patients should continue steroids for at least 2 weeks and until all lesions have healed.
Oral corticosteroids should be tapered slowly over about 4 to 8 weeks, first by halving the dose to a physiologic dose each week, and then by dosing every other day for 2 weeks. Topical corticosteroids also can be used for eroded sites with granulation tissue, they noted.
Isotretinoin
Ironically, isotretinoin is both a treatment and a potential trigger of acne fulminans, and the recommendations included detailed guidance on its use.
Patients should wait at least 2 weeks after crusting resolves before starting isotretinoin, and should overlap isotretinoin with corticosteroids for at least 4 weeks, the experts emphasized. They recommended starting isotretinoin at 0.1 mg per kg per day, and waiting at least 2 months to increase this dose. Because patients clear at different rates, there is no universal optimal cumulative dose of isotretinoin, they noted.
If patients on isotretinoin develop flare, crusts, and erosions, they should halt treatment and either start corticosteroids, or increase the steroid dose to 1.0 mg per kg. If crusts and erosions persist, the panelists recommended considering cyclosporine, biologics, or dapsone.
If hemorrhagic crusts or erosions resolve after stopping isotretinoin, it can be restarted at the initial dose of 0.1 mg per kg and overlapped with steroids for 4 weeks.
If flares, crusts, and erosions begin when patients on isotretinoin are tapering corticosteroids, then steroids should be continued without tapering, isotretinoin should be stopped temporarily, and it should be restarted at 0.1 mg per kg after crusts and erosions have healed. This dose should be continued for 4 weeks, and then slowly increased as tolerated.
Antibiotics
Antibiotics are not useful as monotherapy for first-line therapy for acne fulminans, according to the recommendations. But to avoid isotretinoin-induced acne fulminans, the experts often pretreat patients with oral antibiotics before starting isotretinoin, and may continue oral antibiotics during the initial phase of isotretinoin treatment. However, there have been no prospective studies supporting this approach, they noted.
Other treatment considerations
Acne fulminans lesions should not be debrided, the experts emphasized. Acne fulminans associated with SAPHO (synovitis, acne, pustulosis, hyperostosis, and osteitis), PAPA (pyogenic arthritis, pyoderma gangrenosum, and acne), and PAPASH (pyogenic arthritis, pyoderma gangrenosum, acne, and hidradenitis suppurativa) has responded successfully to tumor necrosis factor–alpha inhibitors and interleukin-1 receptor antagonists, they noted. Pulsed dye laser also has been used to successfully treat acne fulminans, particularly when there is associated granulation tissue, they stated.
Reducing the risk of pseudotumor cerebri syndrome
Several drugs used to treat acne fulminans have been linked to pseudotumor cerebri syndrome, the experts cautioned. “Among the tetracyclines, minocycline carries the highest risk,” they noted. Both isotretinoin and corticosteroids have been linked to pseudotumor cerebri syndrome, but clinicians can reduce this risk by avoiding an abrupt steroid taper, they emphasized.
The panel included physicians from the University of California, San Diego; Pennsylvania State University, Hershey; State University of New York, Brooklyn; Cornell University, New York; Touro University California, Vallejo; the University of California, San Francisco; the University of Pennsylvania, Philadelphia; and Jefferson Medical College, Philadelphia. They did not disclose conflicts of interest.
SCOTTSDALE, ARIZ. – Patients with acne fulminans should first begin corticosteroid monotherapy before adding isotretinoin, according to the first evidence-based consensus recommendations on this disease.
Antibiotics should not be used as first line treatment or monotherapy for acne fulminans, according to the experts who drafted the recommendations. Acne fulminans is an uncommon, understudied, and severe disorder that “typically manifests as an explosive worsening and ulceration of skin lesions, and can be associated with fever, bone pain, and other systemic symptoms,” noted panel co-chairs Dr. Andrea Zaenglein, professor of dermatology and pediatric dermatology, Pennsylvania State University, Hershey, and Dr. Sheila Friedlander, professor of medicine and pediatrics, University of California, San Diego, together with their associates.
The recommendations – based on a full literature review, a 5-hour audioconference, and two rounds of surveys to achieve consensus on these topics – were summarized in a poster presented at the annual meeting of the Society for Investigative Dermatology.
Until now, there have been no clear guidelines on the pathogenesis, treatment, and prevention of acne fulminans, according to the panelists.
Consensus definitions
“Acne fulminans is not just an extreme form of acne, but rather, a distinct, likely auto-inflammatory disorder,” the experts stated. Affected patients typically have an “abrupt, dramatic flare of inflammatory acne, with erosions, and with or without crusts, hemorrhagic nodules/plaques, and systemic findings.”
Systemic involvement is uncommon, but when present, includes fever, malaise, bone pain, arthralgias, erythema nodosum, and leukocytosis, they noted. Some patients also have anemia, an elevated erythrocyte sedimentation rate, and an increased C-reactive protein level. Radiography typically reveals osteolytic lesions of the sternum, clavicles, sacroiliac joints, and hips.
Acne fulminans is most often triggered by isotretinoin therapy, but can occur without it, the panel said. Isotretinoin-induced acne fulminans can have systemic involvement, but usually does not.
Corticosteroids
Patients should start corticosteroid monotherapy at a dose of 0.5 to 1.0 mg per kg per day, according to the recommendations. Patients with systemic involvement should receive steroids for at least 4 weeks, and other patients should continue steroids for at least 2 weeks and until all lesions have healed.
Oral corticosteroids should be tapered slowly over about 4 to 8 weeks, first by halving the dose to a physiologic dose each week, and then by dosing every other day for 2 weeks. Topical corticosteroids also can be used for eroded sites with granulation tissue, they noted.
Isotretinoin
Ironically, isotretinoin is both a treatment and a potential trigger of acne fulminans, and the recommendations included detailed guidance on its use.
Patients should wait at least 2 weeks after crusting resolves before starting isotretinoin, and should overlap isotretinoin with corticosteroids for at least 4 weeks, the experts emphasized. They recommended starting isotretinoin at 0.1 mg per kg per day, and waiting at least 2 months to increase this dose. Because patients clear at different rates, there is no universal optimal cumulative dose of isotretinoin, they noted.
If patients on isotretinoin develop flare, crusts, and erosions, they should halt treatment and either start corticosteroids, or increase the steroid dose to 1.0 mg per kg. If crusts and erosions persist, the panelists recommended considering cyclosporine, biologics, or dapsone.
If hemorrhagic crusts or erosions resolve after stopping isotretinoin, it can be restarted at the initial dose of 0.1 mg per kg and overlapped with steroids for 4 weeks.
If flares, crusts, and erosions begin when patients on isotretinoin are tapering corticosteroids, then steroids should be continued without tapering, isotretinoin should be stopped temporarily, and it should be restarted at 0.1 mg per kg after crusts and erosions have healed. This dose should be continued for 4 weeks, and then slowly increased as tolerated.
Antibiotics
Antibiotics are not useful as monotherapy for first-line therapy for acne fulminans, according to the recommendations. But to avoid isotretinoin-induced acne fulminans, the experts often pretreat patients with oral antibiotics before starting isotretinoin, and may continue oral antibiotics during the initial phase of isotretinoin treatment. However, there have been no prospective studies supporting this approach, they noted.
Other treatment considerations
Acne fulminans lesions should not be debrided, the experts emphasized. Acne fulminans associated with SAPHO (synovitis, acne, pustulosis, hyperostosis, and osteitis), PAPA (pyogenic arthritis, pyoderma gangrenosum, and acne), and PAPASH (pyogenic arthritis, pyoderma gangrenosum, acne, and hidradenitis suppurativa) has responded successfully to tumor necrosis factor–alpha inhibitors and interleukin-1 receptor antagonists, they noted. Pulsed dye laser also has been used to successfully treat acne fulminans, particularly when there is associated granulation tissue, they stated.
Reducing the risk of pseudotumor cerebri syndrome
Several drugs used to treat acne fulminans have been linked to pseudotumor cerebri syndrome, the experts cautioned. “Among the tetracyclines, minocycline carries the highest risk,” they noted. Both isotretinoin and corticosteroids have been linked to pseudotumor cerebri syndrome, but clinicians can reduce this risk by avoiding an abrupt steroid taper, they emphasized.
The panel included physicians from the University of California, San Diego; Pennsylvania State University, Hershey; State University of New York, Brooklyn; Cornell University, New York; Touro University California, Vallejo; the University of California, San Francisco; the University of Pennsylvania, Philadelphia; and Jefferson Medical College, Philadelphia. They did not disclose conflicts of interest.
AT THE 2016 SID ANNUAL MEETING
Rosacea patients have higher risk of dementia
Patients with rosacea have a higher risk of being diagnosed with certain forms of dementia, particularly Alzheimer’s disease (AD), according to Dr. Alexander Egeberg and his associates.
“We hypothesized that patients with rosacea may have increased risk of AD, because we have clinically observed a familial overrepresentation of AD in patients with rosacea, and because of the overlap of proinflammatory mediators between rosacea and AD,” wrote Dr. Egeberg of the National Allergy Research Center at the University of Copenhagen.
The researchers studied more than 5 million Danish citizens 18 years and older from Jan. 1, 1997, to Dec. 31, 2012. After excluding some people from the analysis for various reasons, including incomplete information, the cohort size was 5,591,718. Overall, 82,439 individuals had rosacea, and 29,193 of patients were diagnosed with AD.
“We found a significant association with dementia, and especially AD, in patients with rosacea,” the investigators wrote. They said the associations were strongest in people aged 60 or under at baseline, and “the results remained consistent in a range of sensitivity analyses and after adjustment for potential confounding factors.”
Future research might focus on symptoms of cognitive dysfunction in patients with rosacea, they wrote.
Find the study here.
Patients with rosacea have a higher risk of being diagnosed with certain forms of dementia, particularly Alzheimer’s disease (AD), according to Dr. Alexander Egeberg and his associates.
“We hypothesized that patients with rosacea may have increased risk of AD, because we have clinically observed a familial overrepresentation of AD in patients with rosacea, and because of the overlap of proinflammatory mediators between rosacea and AD,” wrote Dr. Egeberg of the National Allergy Research Center at the University of Copenhagen.
The researchers studied more than 5 million Danish citizens 18 years and older from Jan. 1, 1997, to Dec. 31, 2012. After excluding some people from the analysis for various reasons, including incomplete information, the cohort size was 5,591,718. Overall, 82,439 individuals had rosacea, and 29,193 of patients were diagnosed with AD.
“We found a significant association with dementia, and especially AD, in patients with rosacea,” the investigators wrote. They said the associations were strongest in people aged 60 or under at baseline, and “the results remained consistent in a range of sensitivity analyses and after adjustment for potential confounding factors.”
Future research might focus on symptoms of cognitive dysfunction in patients with rosacea, they wrote.
Find the study here.
Patients with rosacea have a higher risk of being diagnosed with certain forms of dementia, particularly Alzheimer’s disease (AD), according to Dr. Alexander Egeberg and his associates.
“We hypothesized that patients with rosacea may have increased risk of AD, because we have clinically observed a familial overrepresentation of AD in patients with rosacea, and because of the overlap of proinflammatory mediators between rosacea and AD,” wrote Dr. Egeberg of the National Allergy Research Center at the University of Copenhagen.
The researchers studied more than 5 million Danish citizens 18 years and older from Jan. 1, 1997, to Dec. 31, 2012. After excluding some people from the analysis for various reasons, including incomplete information, the cohort size was 5,591,718. Overall, 82,439 individuals had rosacea, and 29,193 of patients were diagnosed with AD.
“We found a significant association with dementia, and especially AD, in patients with rosacea,” the investigators wrote. They said the associations were strongest in people aged 60 or under at baseline, and “the results remained consistent in a range of sensitivity analyses and after adjustment for potential confounding factors.”
Future research might focus on symptoms of cognitive dysfunction in patients with rosacea, they wrote.
Find the study here.
FROM ANNALS OF NEUROLOGY
In rosacea, flushing and inflammation need to be addressed
WAIKOLOA, HAWAII – The topical vasoconstrictor brimonidine 0.33% gel brings impressive improvement in the facial redness component of rosacea, but it does nothing to address the inflammatory lesions of the disease, Dr. Joseph F. Fowler, Jr., said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Rebound flushing wasn’t seen in the 52-week, large, open-label safety study of brimonidine gel, but has been anecdotally reported since its approval. Flushing occurs 12-24 hours after application, and usually lasts a day or two, according to Dr. Fowler, who was the first author of several multicenter randomized trials of the therapy.
“The rebound phenomenon is not something that frightens me or others I know who use the medication routinely, because it’s not something that’s going to cause a long-term problem,” he declared. Indeed, once brimonidine gel (Mirvaso) has quelled the erythema, the inflammatory lesions will stand out even more prominently, noted Dr. Fowler, the conference co-director and a dermatologist at the University of Louisville. “You’ve got to do something about the inflammatory component.”
Two topical therapies that address the inflammatory component include ivermectin 1% cream (Soolantra), shown to reduce counts of papules and pustules in two 40-week extension studies of phase III trials (J Drugs Dermatol. 2014 Nov;13[11]:1380-6), and azelaic acid 15% foam (Finacea), which also achieved an impressive reduction in inflammatory lesion counts in a phase III trial (Cutis. 2015 Jul;96[1]:54-61).
Both agents were well tolerated in those studies. That’s a major treatment consideration because rosacea patients have elevated facial skin sensitivity and often can’t tolerate older off-label topical therapies because of stinging and burning, according to Dr. Fowler.
Some rosacea patients are so intolerant of topicals that they prefer oral therapy. For those patients, subantimicrobial-dose doxycycline is a good option, Dr. Fowler said.
In addition to its anti-inflammatory effects, topical ivermectin kills Demodex. While rosacea is an inflammatory disorder, not an infection, drugs like ivermectin and crotamiton 10% cream (Eurax) that kill Demodex also improve rosacea, he observed.
The initial irritation event rate with azelaic acid 15% foam in the phase III trial was in the 2%-5% range, and those events were short lived. Irritation is a much bigger problem with the older azelaic acid 15% gel, with event rates in the 15%-25% range.
Dr. Fowler has also had good results using topical calcineurin inhibitors off-label for rosacea. Pimecrolimus cream (Elidel) is in ongoing studies for treatment of seborrheic dermatitis, which often coexists with rosacea, he noted.
He reported serving as a consultant to half a dozen pharmaceutical companies, including Galderma, which markets ivermectin cream and brimonidine gel, and Bayer, which markets azelaic acid foam.
SDEF and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – The topical vasoconstrictor brimonidine 0.33% gel brings impressive improvement in the facial redness component of rosacea, but it does nothing to address the inflammatory lesions of the disease, Dr. Joseph F. Fowler, Jr., said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Rebound flushing wasn’t seen in the 52-week, large, open-label safety study of brimonidine gel, but has been anecdotally reported since its approval. Flushing occurs 12-24 hours after application, and usually lasts a day or two, according to Dr. Fowler, who was the first author of several multicenter randomized trials of the therapy.
“The rebound phenomenon is not something that frightens me or others I know who use the medication routinely, because it’s not something that’s going to cause a long-term problem,” he declared. Indeed, once brimonidine gel (Mirvaso) has quelled the erythema, the inflammatory lesions will stand out even more prominently, noted Dr. Fowler, the conference co-director and a dermatologist at the University of Louisville. “You’ve got to do something about the inflammatory component.”
Two topical therapies that address the inflammatory component include ivermectin 1% cream (Soolantra), shown to reduce counts of papules and pustules in two 40-week extension studies of phase III trials (J Drugs Dermatol. 2014 Nov;13[11]:1380-6), and azelaic acid 15% foam (Finacea), which also achieved an impressive reduction in inflammatory lesion counts in a phase III trial (Cutis. 2015 Jul;96[1]:54-61).
Both agents were well tolerated in those studies. That’s a major treatment consideration because rosacea patients have elevated facial skin sensitivity and often can’t tolerate older off-label topical therapies because of stinging and burning, according to Dr. Fowler.
Some rosacea patients are so intolerant of topicals that they prefer oral therapy. For those patients, subantimicrobial-dose doxycycline is a good option, Dr. Fowler said.
In addition to its anti-inflammatory effects, topical ivermectin kills Demodex. While rosacea is an inflammatory disorder, not an infection, drugs like ivermectin and crotamiton 10% cream (Eurax) that kill Demodex also improve rosacea, he observed.
The initial irritation event rate with azelaic acid 15% foam in the phase III trial was in the 2%-5% range, and those events were short lived. Irritation is a much bigger problem with the older azelaic acid 15% gel, with event rates in the 15%-25% range.
Dr. Fowler has also had good results using topical calcineurin inhibitors off-label for rosacea. Pimecrolimus cream (Elidel) is in ongoing studies for treatment of seborrheic dermatitis, which often coexists with rosacea, he noted.
He reported serving as a consultant to half a dozen pharmaceutical companies, including Galderma, which markets ivermectin cream and brimonidine gel, and Bayer, which markets azelaic acid foam.
SDEF and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – The topical vasoconstrictor brimonidine 0.33% gel brings impressive improvement in the facial redness component of rosacea, but it does nothing to address the inflammatory lesions of the disease, Dr. Joseph F. Fowler, Jr., said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Rebound flushing wasn’t seen in the 52-week, large, open-label safety study of brimonidine gel, but has been anecdotally reported since its approval. Flushing occurs 12-24 hours after application, and usually lasts a day or two, according to Dr. Fowler, who was the first author of several multicenter randomized trials of the therapy.
“The rebound phenomenon is not something that frightens me or others I know who use the medication routinely, because it’s not something that’s going to cause a long-term problem,” he declared. Indeed, once brimonidine gel (Mirvaso) has quelled the erythema, the inflammatory lesions will stand out even more prominently, noted Dr. Fowler, the conference co-director and a dermatologist at the University of Louisville. “You’ve got to do something about the inflammatory component.”
Two topical therapies that address the inflammatory component include ivermectin 1% cream (Soolantra), shown to reduce counts of papules and pustules in two 40-week extension studies of phase III trials (J Drugs Dermatol. 2014 Nov;13[11]:1380-6), and azelaic acid 15% foam (Finacea), which also achieved an impressive reduction in inflammatory lesion counts in a phase III trial (Cutis. 2015 Jul;96[1]:54-61).
Both agents were well tolerated in those studies. That’s a major treatment consideration because rosacea patients have elevated facial skin sensitivity and often can’t tolerate older off-label topical therapies because of stinging and burning, according to Dr. Fowler.
Some rosacea patients are so intolerant of topicals that they prefer oral therapy. For those patients, subantimicrobial-dose doxycycline is a good option, Dr. Fowler said.
In addition to its anti-inflammatory effects, topical ivermectin kills Demodex. While rosacea is an inflammatory disorder, not an infection, drugs like ivermectin and crotamiton 10% cream (Eurax) that kill Demodex also improve rosacea, he observed.
The initial irritation event rate with azelaic acid 15% foam in the phase III trial was in the 2%-5% range, and those events were short lived. Irritation is a much bigger problem with the older azelaic acid 15% gel, with event rates in the 15%-25% range.
Dr. Fowler has also had good results using topical calcineurin inhibitors off-label for rosacea. Pimecrolimus cream (Elidel) is in ongoing studies for treatment of seborrheic dermatitis, which often coexists with rosacea, he noted.
He reported serving as a consultant to half a dozen pharmaceutical companies, including Galderma, which markets ivermectin cream and brimonidine gel, and Bayer, which markets azelaic acid foam.
SDEF and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM THE SDEF HAWAII DERMATOLOGY SEMINAR
Smooth hair – an acne-causing epidemic
Do you ask your acne patients about which hair products they use? This common question has recently brought our attention to popular hair products that are causing an acne epidemic. Have we forgotten about “Pomade acne”? Well, it’s making a comeback. Originally described in ethnic women, new frizz-fighting hair products have resurged and so has pomade acne in all skin types and in both men and women.
Smoothing serums, heat styling sprays, leave-in products popularly known as “It’s-a-10,” “Biosilk,” “anti-frizz serums,” “heat-protectants,” “thermal setting sprays,” and “shine sprays,” contain silicone-derived ingredients and oils to control frizz, add shine, and detangle the hair. They work by smoothing the hair cuticle, and for women with difficult-to-manage hair, they have become an essential part of the daily beauty regimen.
Men are not in the clear either. Hair waxes and pomades used to style men’s hair contain greasy wax-based ingredients that also clog pores, trap bacteria, and cause inflammatory breakouts.
As a general rule in skin and body care, most products work well for what they are made to do, but when misused, they can cause mishaps. You wouldn’t moisturize your face with your hair serum would you? It seems obvious that this could cause some skin issues; however, most people will not think to correlate their acne breakouts with their hair products until we mention it. These products rub off on the face or on the pillow at night. In addition, the less we wash our hair, the more we are going to bed and getting the daytime products all over our pillowcases. Our faces are rolling around in oily, waxy, hair products all night.
Makeup is known to cause acne, and some of the makeups that are well known culprits contain the same ingredients as in hair products. Foundations, primers, and popular “BB” creams often contain cyclopentasiloxane and dimethicone. They serve a similar purpose: smoothing the skin and smoothing the hair. Both should be avoided in acne-prone patients.
Common culprits in hair products include PVP/DMAPA acrylates, cyclopentasiloxane, panthenol, dimethicone, silicone, Quaternium-70, oils, and petrolatum.
The only way to eliminate acne caused by hair products is to completely eliminate the hair product from the daily routine. However, if your patients can’t live without their hair products, here are some tips to share with them to reduce breakouts:
• Choose a hairstyle that keeps the hair away from the face, or wear hair up to avoid prolonged contact with the face, particularly while sleeping.
• Change pillowcase often (every day if possible), especially for side sleepers. Regardless of the fabric, pillowcases trap oil, dirt, and bacteria.
• Shower at night and sleep with clean hair and clean skin.
• Style hair before applying makeup. Wash hands thoroughly to remove all hair products before touching the skin.
• Cover the face prior to applying any hair sprays.
• Cover the hair at bedtime; however, tight head coverings can stimulate sweat and cause scalp breakouts.
As a general rule, any patient with difficult-to-control acne, recalcitrant acne, or acne in areas on the cheeks or hairline should eliminate these hair products in their daily routine or avoid skin contact with these products.
References
1. J Clin Aesthet Dermatol. 2010 Apr;3(4):24-38.
2. Arch Dermatol. 1972;106 (6):843-50.
3. J Am Acad Dermatol. 2003; 48:S127-33.
4. Arch Dermatol. 1970;101(5):580-584.
5. “Cosmetics in Dermatology,” Second Edition, by Zoe Diana Draelos (New York: Churchill Livingstone, 1995).
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub.
Do you ask your acne patients about which hair products they use? This common question has recently brought our attention to popular hair products that are causing an acne epidemic. Have we forgotten about “Pomade acne”? Well, it’s making a comeback. Originally described in ethnic women, new frizz-fighting hair products have resurged and so has pomade acne in all skin types and in both men and women.
Smoothing serums, heat styling sprays, leave-in products popularly known as “It’s-a-10,” “Biosilk,” “anti-frizz serums,” “heat-protectants,” “thermal setting sprays,” and “shine sprays,” contain silicone-derived ingredients and oils to control frizz, add shine, and detangle the hair. They work by smoothing the hair cuticle, and for women with difficult-to-manage hair, they have become an essential part of the daily beauty regimen.
Men are not in the clear either. Hair waxes and pomades used to style men’s hair contain greasy wax-based ingredients that also clog pores, trap bacteria, and cause inflammatory breakouts.
As a general rule in skin and body care, most products work well for what they are made to do, but when misused, they can cause mishaps. You wouldn’t moisturize your face with your hair serum would you? It seems obvious that this could cause some skin issues; however, most people will not think to correlate their acne breakouts with their hair products until we mention it. These products rub off on the face or on the pillow at night. In addition, the less we wash our hair, the more we are going to bed and getting the daytime products all over our pillowcases. Our faces are rolling around in oily, waxy, hair products all night.
Makeup is known to cause acne, and some of the makeups that are well known culprits contain the same ingredients as in hair products. Foundations, primers, and popular “BB” creams often contain cyclopentasiloxane and dimethicone. They serve a similar purpose: smoothing the skin and smoothing the hair. Both should be avoided in acne-prone patients.
Common culprits in hair products include PVP/DMAPA acrylates, cyclopentasiloxane, panthenol, dimethicone, silicone, Quaternium-70, oils, and petrolatum.
The only way to eliminate acne caused by hair products is to completely eliminate the hair product from the daily routine. However, if your patients can’t live without their hair products, here are some tips to share with them to reduce breakouts:
• Choose a hairstyle that keeps the hair away from the face, or wear hair up to avoid prolonged contact with the face, particularly while sleeping.
• Change pillowcase often (every day if possible), especially for side sleepers. Regardless of the fabric, pillowcases trap oil, dirt, and bacteria.
• Shower at night and sleep with clean hair and clean skin.
• Style hair before applying makeup. Wash hands thoroughly to remove all hair products before touching the skin.
• Cover the face prior to applying any hair sprays.
• Cover the hair at bedtime; however, tight head coverings can stimulate sweat and cause scalp breakouts.
As a general rule, any patient with difficult-to-control acne, recalcitrant acne, or acne in areas on the cheeks or hairline should eliminate these hair products in their daily routine or avoid skin contact with these products.
References
1. J Clin Aesthet Dermatol. 2010 Apr;3(4):24-38.
2. Arch Dermatol. 1972;106 (6):843-50.
3. J Am Acad Dermatol. 2003; 48:S127-33.
4. Arch Dermatol. 1970;101(5):580-584.
5. “Cosmetics in Dermatology,” Second Edition, by Zoe Diana Draelos (New York: Churchill Livingstone, 1995).
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub.
Do you ask your acne patients about which hair products they use? This common question has recently brought our attention to popular hair products that are causing an acne epidemic. Have we forgotten about “Pomade acne”? Well, it’s making a comeback. Originally described in ethnic women, new frizz-fighting hair products have resurged and so has pomade acne in all skin types and in both men and women.
Smoothing serums, heat styling sprays, leave-in products popularly known as “It’s-a-10,” “Biosilk,” “anti-frizz serums,” “heat-protectants,” “thermal setting sprays,” and “shine sprays,” contain silicone-derived ingredients and oils to control frizz, add shine, and detangle the hair. They work by smoothing the hair cuticle, and for women with difficult-to-manage hair, they have become an essential part of the daily beauty regimen.
Men are not in the clear either. Hair waxes and pomades used to style men’s hair contain greasy wax-based ingredients that also clog pores, trap bacteria, and cause inflammatory breakouts.
As a general rule in skin and body care, most products work well for what they are made to do, but when misused, they can cause mishaps. You wouldn’t moisturize your face with your hair serum would you? It seems obvious that this could cause some skin issues; however, most people will not think to correlate their acne breakouts with their hair products until we mention it. These products rub off on the face or on the pillow at night. In addition, the less we wash our hair, the more we are going to bed and getting the daytime products all over our pillowcases. Our faces are rolling around in oily, waxy, hair products all night.
Makeup is known to cause acne, and some of the makeups that are well known culprits contain the same ingredients as in hair products. Foundations, primers, and popular “BB” creams often contain cyclopentasiloxane and dimethicone. They serve a similar purpose: smoothing the skin and smoothing the hair. Both should be avoided in acne-prone patients.
Common culprits in hair products include PVP/DMAPA acrylates, cyclopentasiloxane, panthenol, dimethicone, silicone, Quaternium-70, oils, and petrolatum.
The only way to eliminate acne caused by hair products is to completely eliminate the hair product from the daily routine. However, if your patients can’t live without their hair products, here are some tips to share with them to reduce breakouts:
• Choose a hairstyle that keeps the hair away from the face, or wear hair up to avoid prolonged contact with the face, particularly while sleeping.
• Change pillowcase often (every day if possible), especially for side sleepers. Regardless of the fabric, pillowcases trap oil, dirt, and bacteria.
• Shower at night and sleep with clean hair and clean skin.
• Style hair before applying makeup. Wash hands thoroughly to remove all hair products before touching the skin.
• Cover the face prior to applying any hair sprays.
• Cover the hair at bedtime; however, tight head coverings can stimulate sweat and cause scalp breakouts.
As a general rule, any patient with difficult-to-control acne, recalcitrant acne, or acne in areas on the cheeks or hairline should eliminate these hair products in their daily routine or avoid skin contact with these products.
References
1. J Clin Aesthet Dermatol. 2010 Apr;3(4):24-38.
2. Arch Dermatol. 1972;106 (6):843-50.
3. J Am Acad Dermatol. 2003; 48:S127-33.
4. Arch Dermatol. 1970;101(5):580-584.
5. “Cosmetics in Dermatology,” Second Edition, by Zoe Diana Draelos (New York: Churchill Livingstone, 1995).
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub.
Rosacea linked to several autoimmune diseases in women
Rosacea in women is linked with an increased risk for a wide variety of autoimmune disorders including type 1 diabetes, celiac disease, multiple sclerosis, and rheumatoid arthritis, according to a large population-based case-control study.
The finding expands the association of rosacea and autoimmune diseases beyond the previously reported associations with type 1 diabetes and celiac disease, reported Dr. Alexander Egeberg of Herlev and Gentofte Hospital, University of Copenhagen, and colleagues (J Am Acad Dermatol. 2016;74:667-72.)
“Remember that the absolute risk is still low; having rosacea does not guarantee that patients will develop other autoimmune diseases, and vice versa,” Dr. Egeberg said in an interview. “The links may provide insight into the pathogenesis of these diseases, and it will be interesting to see if systemic treatment of rosacea also affects the autoimmune diseases and the other way around.”
Using Danish registered health records, the researchers found 6,759 patients with rosacea, and matched them by age and sex to 33,795 control subjects. About two-thirds of the patients were women.
Compared with their matched controls, people with rosacea were at least twice as likely to have type 1 diabetes, celiac disease, and rheumatoid arthritis (odds ratio 2.6 for type 1 diabetes, 2.0 for celiac disease, and 2.1 for rheumatoid arthritis) and 1.65 times as likely to have multiple sclerosis. The differences, with the exception of rheumatoid arthritis, were statistically significant only in women.
The researchers said the broader association with autoimmune comorbidities is “intriguing” and suggests the genetic component of rosacea could be stronger than previously assumed with autoimmune inflammatory pathways contributing to the disease course. Because distinct rosacea subtypes (erythematotelangiectatic, papulopustular, phymatous, and ocular) could be associated with specific comorbidities, future association studies should attempt to stratify for clinical subtypes of rosacea, they noted.
Rosacea in women is linked with an increased risk for a wide variety of autoimmune disorders including type 1 diabetes, celiac disease, multiple sclerosis, and rheumatoid arthritis, according to a large population-based case-control study.
The finding expands the association of rosacea and autoimmune diseases beyond the previously reported associations with type 1 diabetes and celiac disease, reported Dr. Alexander Egeberg of Herlev and Gentofte Hospital, University of Copenhagen, and colleagues (J Am Acad Dermatol. 2016;74:667-72.)
“Remember that the absolute risk is still low; having rosacea does not guarantee that patients will develop other autoimmune diseases, and vice versa,” Dr. Egeberg said in an interview. “The links may provide insight into the pathogenesis of these diseases, and it will be interesting to see if systemic treatment of rosacea also affects the autoimmune diseases and the other way around.”
Using Danish registered health records, the researchers found 6,759 patients with rosacea, and matched them by age and sex to 33,795 control subjects. About two-thirds of the patients were women.
Compared with their matched controls, people with rosacea were at least twice as likely to have type 1 diabetes, celiac disease, and rheumatoid arthritis (odds ratio 2.6 for type 1 diabetes, 2.0 for celiac disease, and 2.1 for rheumatoid arthritis) and 1.65 times as likely to have multiple sclerosis. The differences, with the exception of rheumatoid arthritis, were statistically significant only in women.
The researchers said the broader association with autoimmune comorbidities is “intriguing” and suggests the genetic component of rosacea could be stronger than previously assumed with autoimmune inflammatory pathways contributing to the disease course. Because distinct rosacea subtypes (erythematotelangiectatic, papulopustular, phymatous, and ocular) could be associated with specific comorbidities, future association studies should attempt to stratify for clinical subtypes of rosacea, they noted.
Rosacea in women is linked with an increased risk for a wide variety of autoimmune disorders including type 1 diabetes, celiac disease, multiple sclerosis, and rheumatoid arthritis, according to a large population-based case-control study.
The finding expands the association of rosacea and autoimmune diseases beyond the previously reported associations with type 1 diabetes and celiac disease, reported Dr. Alexander Egeberg of Herlev and Gentofte Hospital, University of Copenhagen, and colleagues (J Am Acad Dermatol. 2016;74:667-72.)
“Remember that the absolute risk is still low; having rosacea does not guarantee that patients will develop other autoimmune diseases, and vice versa,” Dr. Egeberg said in an interview. “The links may provide insight into the pathogenesis of these diseases, and it will be interesting to see if systemic treatment of rosacea also affects the autoimmune diseases and the other way around.”
Using Danish registered health records, the researchers found 6,759 patients with rosacea, and matched them by age and sex to 33,795 control subjects. About two-thirds of the patients were women.
Compared with their matched controls, people with rosacea were at least twice as likely to have type 1 diabetes, celiac disease, and rheumatoid arthritis (odds ratio 2.6 for type 1 diabetes, 2.0 for celiac disease, and 2.1 for rheumatoid arthritis) and 1.65 times as likely to have multiple sclerosis. The differences, with the exception of rheumatoid arthritis, were statistically significant only in women.
The researchers said the broader association with autoimmune comorbidities is “intriguing” and suggests the genetic component of rosacea could be stronger than previously assumed with autoimmune inflammatory pathways contributing to the disease course. Because distinct rosacea subtypes (erythematotelangiectatic, papulopustular, phymatous, and ocular) could be associated with specific comorbidities, future association studies should attempt to stratify for clinical subtypes of rosacea, they noted.
FROM JAAD
Key clinical point: Rosacea is linked with a broader spectrum of autoimmune diseases than previously recognized.
Major finding: Women with rosacea were more likely than were other women to have type 1 diabetes, celiac disease, multiple sclerosis, and rheumatoid arthritis.
Data source: A population-based case-control study of 6,759 patients with rosacea who were matched with 33,795 control subjects for age, sex, and calendar time.
Disclosures: Dr. Egeberg is employed by Pfizer. This research was performed independently through the authors’ academic university.
Standard incubation can miss P. acnes in infective endocarditis
AMSTERDAM – Accounting for less than 1% of cases, Propionibacterium acnes has been considered an uncommon cause of infective endocarditis.
But data presented at the annual congress of the European Society of Clinical Microbiology and Infectious Diseases suggest that the common anaerobe may be responsible for many more cases than is now believed. The bacteria are difficult to culture and grow very slowly, Dr. Jona Banzon said at the meeting. Incubating it for the standard 5 days may simply not be long enough.
“Due to this slow-growing nature, the standard incubation period may not be enough to detect it,” said Dr. Banzon, an infectious disease fellow at the Cleveland Clinic. “And since P. acnes is part of our commensal flora, it’s frequently a contaminate in culture, and we might be inappropriately dismissing it as such. In fact, we now wonder if this lack of extended incubation may be accounting for a significant proportion of negative blood cultures, and whether more prosthetic valve endocarditis than we think is actually being caused by P. acnes.”
Dr. Banzon presented a series of 23 cases included in the Cleveland Clinic Infective Endocarditis Registry from the period of 2007-2015. All had P. acnes confirmed as the causative organism. The group comprises 3.3% of the institution’s entire infective endocarditis registry, “making infective carditis with P. acnes already much more common than it is said to be in the literature,” she noted.
All of the cases were confirmed by any of the following standards:
• Two or more blood cultures positive for P. acnes.
• Two or more valve cultures positive for P. acnes.
• Two or more valve sequencing rests positive for P. acnes.
• At least two of the following: a positive blood culture, a positive valve culture or valve sequencing, or histopathologic demonstration of microorganism consistent with P. acnes.
Of the cohort, 22 had prosthetic valve endocarditis. One patient had endocarditis on a native valve. This is an important point, Dr. Banzon said.
The organism is being increasingly recognized for causing infections of prosthetic material, including shoulder joint infections and shunts, but it rarely seems to affect native tissue. The patient who had native valve endocarditis had experienced an episode of Staphylococcus aureus endocarditis about 18 months earlier. He was not treated surgically, and ended up with a damaged valve. After the initial illness, he was readmitted several times with symptoms of endocarditis, but all of his blood cultures were negative. This was attributed to the receipt of antibiotics. “When he was finally operated on, there were three valve sequencing specimens and all were positive for P. acnes,” Dr. Banzon said.
The patients in the cohort were a mean of 74 years old; about 75% were men. All of the cases were left-sided endocarditis, with the majority (74%) involving the aortic valve. Other sites included the mitral valve (18%), aortic plus mitral (4%), and aortic plus tricuspid (4%).
The most common predisposing factor was having a prosthetic valve (96%). Other factors included having a cardiac implantable device (17%), and a prior episode of infective endocarditis (13%). None of the patients had indwelling vascular catheters or used injectable drugs.
The cases presented with severe disease, Dr. Banzon said. Almost half (48%) had a perivalvular abscess at presentation. A third (35%) had valve dehiscence, and 35% had severe valvular regurgitation. There was a vegetation of more than 1 cm in 9% of cases.
Emboli were not uncommon; 17% had emboli in the central nervous system, and 17% had peripheral emboli. Two of these patients had kidney and spleen infarcts and two had acute arterial thromboembolism that required thrombectomy.
All of the patients underwent blood cultures; overall, 30% of the cultures were positive for P. acnes. But there were “striking differences” when the tests were broken down by incubation time, Dr. Banzon said.
Most of the cultures (16) were incubated for the standard of 5 days or less. Among these, 12.5% were positive. However, cultures on seven patients were incubated for more than 5 days and in this group, 71.4% were positive for P. acnes. The median time to positivity overall was 7 days, with a range of 3-9 days.
Other diagnostic methods were important in closing this gap, she said. Valve culturing was positive in 57% of the cases, while valve sequencing was positive in 95%. The median time to positive for valve culture was somewhat shorter than for blood culture (5.5 days).
In nine cases, no organism would have been identified without valve sequencing, Dr. Banzon said.
Because they presented with severe disease, almost all of the patients (22) underwent surgery as their intimal treatment. At the time of surgery, everyone was taking an antibiotic that covered P. acnes. Single-agent therapy was the definitive treatment for most, with vancomycin being most commonly employed (59%), followed by ceftriaxone (25%). A few patients had a combination of both drugs or a combination of vancomycin and rifampin. One patient took penicillin.
The single patient who was medically treated received 6 weeks of intravenous ceftriaxone. After 1 month, he was readmitted with blood cultures positive for P. acnes. He underwent surgery and a valve sequencing confirmed P. acnes as the infective agent.
There were two in-hospital deaths, but the rest of the patients were discharged on antibiotic therapy and recovered with no additional deaths or relapses.
The extended time P. acnes required to show in culture was enough for the Cleveland Clinic to reconsider incubation guidelines for the microorganism, Dr. Banzon said.
“There are enough cases taking 9 or 10 days that we now always hold these cultures for at least 10 days when we’re looking for P. acnes.”
She had no financial disclosures.
AMSTERDAM – Accounting for less than 1% of cases, Propionibacterium acnes has been considered an uncommon cause of infective endocarditis.
But data presented at the annual congress of the European Society of Clinical Microbiology and Infectious Diseases suggest that the common anaerobe may be responsible for many more cases than is now believed. The bacteria are difficult to culture and grow very slowly, Dr. Jona Banzon said at the meeting. Incubating it for the standard 5 days may simply not be long enough.
“Due to this slow-growing nature, the standard incubation period may not be enough to detect it,” said Dr. Banzon, an infectious disease fellow at the Cleveland Clinic. “And since P. acnes is part of our commensal flora, it’s frequently a contaminate in culture, and we might be inappropriately dismissing it as such. In fact, we now wonder if this lack of extended incubation may be accounting for a significant proportion of negative blood cultures, and whether more prosthetic valve endocarditis than we think is actually being caused by P. acnes.”
Dr. Banzon presented a series of 23 cases included in the Cleveland Clinic Infective Endocarditis Registry from the period of 2007-2015. All had P. acnes confirmed as the causative organism. The group comprises 3.3% of the institution’s entire infective endocarditis registry, “making infective carditis with P. acnes already much more common than it is said to be in the literature,” she noted.
All of the cases were confirmed by any of the following standards:
• Two or more blood cultures positive for P. acnes.
• Two or more valve cultures positive for P. acnes.
• Two or more valve sequencing rests positive for P. acnes.
• At least two of the following: a positive blood culture, a positive valve culture or valve sequencing, or histopathologic demonstration of microorganism consistent with P. acnes.
Of the cohort, 22 had prosthetic valve endocarditis. One patient had endocarditis on a native valve. This is an important point, Dr. Banzon said.
The organism is being increasingly recognized for causing infections of prosthetic material, including shoulder joint infections and shunts, but it rarely seems to affect native tissue. The patient who had native valve endocarditis had experienced an episode of Staphylococcus aureus endocarditis about 18 months earlier. He was not treated surgically, and ended up with a damaged valve. After the initial illness, he was readmitted several times with symptoms of endocarditis, but all of his blood cultures were negative. This was attributed to the receipt of antibiotics. “When he was finally operated on, there were three valve sequencing specimens and all were positive for P. acnes,” Dr. Banzon said.
The patients in the cohort were a mean of 74 years old; about 75% were men. All of the cases were left-sided endocarditis, with the majority (74%) involving the aortic valve. Other sites included the mitral valve (18%), aortic plus mitral (4%), and aortic plus tricuspid (4%).
The most common predisposing factor was having a prosthetic valve (96%). Other factors included having a cardiac implantable device (17%), and a prior episode of infective endocarditis (13%). None of the patients had indwelling vascular catheters or used injectable drugs.
The cases presented with severe disease, Dr. Banzon said. Almost half (48%) had a perivalvular abscess at presentation. A third (35%) had valve dehiscence, and 35% had severe valvular regurgitation. There was a vegetation of more than 1 cm in 9% of cases.
Emboli were not uncommon; 17% had emboli in the central nervous system, and 17% had peripheral emboli. Two of these patients had kidney and spleen infarcts and two had acute arterial thromboembolism that required thrombectomy.
All of the patients underwent blood cultures; overall, 30% of the cultures were positive for P. acnes. But there were “striking differences” when the tests were broken down by incubation time, Dr. Banzon said.
Most of the cultures (16) were incubated for the standard of 5 days or less. Among these, 12.5% were positive. However, cultures on seven patients were incubated for more than 5 days and in this group, 71.4% were positive for P. acnes. The median time to positivity overall was 7 days, with a range of 3-9 days.
Other diagnostic methods were important in closing this gap, she said. Valve culturing was positive in 57% of the cases, while valve sequencing was positive in 95%. The median time to positive for valve culture was somewhat shorter than for blood culture (5.5 days).
In nine cases, no organism would have been identified without valve sequencing, Dr. Banzon said.
Because they presented with severe disease, almost all of the patients (22) underwent surgery as their intimal treatment. At the time of surgery, everyone was taking an antibiotic that covered P. acnes. Single-agent therapy was the definitive treatment for most, with vancomycin being most commonly employed (59%), followed by ceftriaxone (25%). A few patients had a combination of both drugs or a combination of vancomycin and rifampin. One patient took penicillin.
The single patient who was medically treated received 6 weeks of intravenous ceftriaxone. After 1 month, he was readmitted with blood cultures positive for P. acnes. He underwent surgery and a valve sequencing confirmed P. acnes as the infective agent.
There were two in-hospital deaths, but the rest of the patients were discharged on antibiotic therapy and recovered with no additional deaths or relapses.
The extended time P. acnes required to show in culture was enough for the Cleveland Clinic to reconsider incubation guidelines for the microorganism, Dr. Banzon said.
“There are enough cases taking 9 or 10 days that we now always hold these cultures for at least 10 days when we’re looking for P. acnes.”
She had no financial disclosures.
AMSTERDAM – Accounting for less than 1% of cases, Propionibacterium acnes has been considered an uncommon cause of infective endocarditis.
But data presented at the annual congress of the European Society of Clinical Microbiology and Infectious Diseases suggest that the common anaerobe may be responsible for many more cases than is now believed. The bacteria are difficult to culture and grow very slowly, Dr. Jona Banzon said at the meeting. Incubating it for the standard 5 days may simply not be long enough.
“Due to this slow-growing nature, the standard incubation period may not be enough to detect it,” said Dr. Banzon, an infectious disease fellow at the Cleveland Clinic. “And since P. acnes is part of our commensal flora, it’s frequently a contaminate in culture, and we might be inappropriately dismissing it as such. In fact, we now wonder if this lack of extended incubation may be accounting for a significant proportion of negative blood cultures, and whether more prosthetic valve endocarditis than we think is actually being caused by P. acnes.”
Dr. Banzon presented a series of 23 cases included in the Cleveland Clinic Infective Endocarditis Registry from the period of 2007-2015. All had P. acnes confirmed as the causative organism. The group comprises 3.3% of the institution’s entire infective endocarditis registry, “making infective carditis with P. acnes already much more common than it is said to be in the literature,” she noted.
All of the cases were confirmed by any of the following standards:
• Two or more blood cultures positive for P. acnes.
• Two or more valve cultures positive for P. acnes.
• Two or more valve sequencing rests positive for P. acnes.
• At least two of the following: a positive blood culture, a positive valve culture or valve sequencing, or histopathologic demonstration of microorganism consistent with P. acnes.
Of the cohort, 22 had prosthetic valve endocarditis. One patient had endocarditis on a native valve. This is an important point, Dr. Banzon said.
The organism is being increasingly recognized for causing infections of prosthetic material, including shoulder joint infections and shunts, but it rarely seems to affect native tissue. The patient who had native valve endocarditis had experienced an episode of Staphylococcus aureus endocarditis about 18 months earlier. He was not treated surgically, and ended up with a damaged valve. After the initial illness, he was readmitted several times with symptoms of endocarditis, but all of his blood cultures were negative. This was attributed to the receipt of antibiotics. “When he was finally operated on, there were three valve sequencing specimens and all were positive for P. acnes,” Dr. Banzon said.
The patients in the cohort were a mean of 74 years old; about 75% were men. All of the cases were left-sided endocarditis, with the majority (74%) involving the aortic valve. Other sites included the mitral valve (18%), aortic plus mitral (4%), and aortic plus tricuspid (4%).
The most common predisposing factor was having a prosthetic valve (96%). Other factors included having a cardiac implantable device (17%), and a prior episode of infective endocarditis (13%). None of the patients had indwelling vascular catheters or used injectable drugs.
The cases presented with severe disease, Dr. Banzon said. Almost half (48%) had a perivalvular abscess at presentation. A third (35%) had valve dehiscence, and 35% had severe valvular regurgitation. There was a vegetation of more than 1 cm in 9% of cases.
Emboli were not uncommon; 17% had emboli in the central nervous system, and 17% had peripheral emboli. Two of these patients had kidney and spleen infarcts and two had acute arterial thromboembolism that required thrombectomy.
All of the patients underwent blood cultures; overall, 30% of the cultures were positive for P. acnes. But there were “striking differences” when the tests were broken down by incubation time, Dr. Banzon said.
Most of the cultures (16) were incubated for the standard of 5 days or less. Among these, 12.5% were positive. However, cultures on seven patients were incubated for more than 5 days and in this group, 71.4% were positive for P. acnes. The median time to positivity overall was 7 days, with a range of 3-9 days.
Other diagnostic methods were important in closing this gap, she said. Valve culturing was positive in 57% of the cases, while valve sequencing was positive in 95%. The median time to positive for valve culture was somewhat shorter than for blood culture (5.5 days).
In nine cases, no organism would have been identified without valve sequencing, Dr. Banzon said.
Because they presented with severe disease, almost all of the patients (22) underwent surgery as their intimal treatment. At the time of surgery, everyone was taking an antibiotic that covered P. acnes. Single-agent therapy was the definitive treatment for most, with vancomycin being most commonly employed (59%), followed by ceftriaxone (25%). A few patients had a combination of both drugs or a combination of vancomycin and rifampin. One patient took penicillin.
The single patient who was medically treated received 6 weeks of intravenous ceftriaxone. After 1 month, he was readmitted with blood cultures positive for P. acnes. He underwent surgery and a valve sequencing confirmed P. acnes as the infective agent.
There were two in-hospital deaths, but the rest of the patients were discharged on antibiotic therapy and recovered with no additional deaths or relapses.
The extended time P. acnes required to show in culture was enough for the Cleveland Clinic to reconsider incubation guidelines for the microorganism, Dr. Banzon said.
“There are enough cases taking 9 or 10 days that we now always hold these cultures for at least 10 days when we’re looking for P. acnes.”
She had no financial disclosures.
AT ECCMID 2016
Key clinical point: P. acnes’ slow growth may result in false negative blood cultures in infective endocarditis.
Major finding: Just 12% of blood cultures incubated for 5 days were positive, while 71% of those incubated for more than 5 days were positive.
Data source: The Cleveland Clinic Infective Endocarditis Registry.
Disclosures: Dr. Banzon had no financial disclosures.
Rosacea linked to increased Parkinson’s disease risk
Individuals with rosacea were nearly twice as likely to develop Parkinson’s disease as those without rosacea, based on an analysis of results of a nationwide cohort study of 5.4 million people conducted in Denmark. The findings were published online March 21 in JAMA Neurology.
Data from previous studies suggest a link between rosacea and Parkinson’s disease (PD), as both may stem from elevated activity of matrix metalloproteinases, wrote Dr. Alexander Egeberg of the University of Copenhagen, and his associates.
To explore the possible link between rosacea and Parkinson’s disease, the researchers reviewed data on about 5.4 million adults in a Danish database of all citizens aged 18 and older, over a 15-year period from January 1, 1997, to December 31, 2011. A total of 22,387 individuals were diagnosed with Parkinson’s disease and 68,053 were diagnosed with rosacea (JAMA Neurol. 2016 Mar 21. doi: 10.1001/jamaneurol.2016.0022).
The incidence of Parkinson’s disease was 7.62 per 10,000 person-years among rosacea patients vs. 3.54 per 10,000 person-years in the general population, the researchers noted.
Overall, the Parkinson’s disease risk was significantly higher among rosacea patients, with an adjusted incidence rate ratio (IRR) of 1.71 for rosacea patients compared with the general population. The IRR was even higher in patients with ocular rosacea (adjusted IRR, 2.03).
However, treatment with tetracycline appeared to reduce the Parkinson’s risk in the rosacea patients: After controlling for multiple variables, having filled a prescription for tetracycline was associated with a 2% drop in the risk of PD (adjusted IRR, 0.98).
A sensitivity analysis showed no dose-dependent relationship with disease severity; the IRRs for Parkinson’s disease in patients with mild rosacea and moderate to severe rosacea were 1.82 and 1.84, respectively. However, moderate to severe rosacea was defined as cases where tetracyclines were used, and the neuroprotective effect of tetracyclines might impact the relationship between rosacea and Parkinson’s disease, the researchers noted.
“It is tempting to speculate that, in patients with coexistent rosacea, Parkinson’s disease may display other phenotypic characteristics, and it is possible that rosacea-associated features, such as facial flushing, may contribute to support a Parkinson’s disease diagnosis at an early phase of the disease,” the researchers noted.
Limitations of the study include its observational design, which does not allow the establishment of causation, the researchers noted, and additional research is needed to confirm the observations and clinical consequences.
Lead author Dr. Egeberg was employed by Pfizer at the time of the study; one of the coauthors was supported by an unrestricted research scholarship from the Novo Nordisk Foundation.
An intriguing aspect of the study was the lack of disease severity impact on the incidence rate ratio (IRR) on Parkinson’s disease, Dr. Thomas S. Wingo wrote in an accompanying editorial. He added: “In other words, people with moderate to severe rosacea have the same IRR for PD as do those who have moderate disease. To explain this result, the authors hypothesized that a disease-severity effect might be blunted by the treatment of moderate to severe rosacea with tetracycline. The reason for this possible effect is that tetracycline is chemically similar to minocycline, which has shown evidence for exerting a protective effect in animal models of PD, although this effect has not been consistently seen.”
Dr. Wingo noted that the “intriguing finding that increased tetracycline use is associated with a small but appreciable reduction in the risk of PD should be further explored. Of particular interest would be to understand the temporal association between the use of tetracycline and effect on PD risk” (JAMA Neurol. 2016 Mar 21. doi: 10.1001/jamaneurol.2016.0291).
Dr. Wingo is a member of the departments of neurology and human genetics at Emory University, Atlanta. He had no financial conflicts to disclose.
An intriguing aspect of the study was the lack of disease severity impact on the incidence rate ratio (IRR) on Parkinson’s disease, Dr. Thomas S. Wingo wrote in an accompanying editorial. He added: “In other words, people with moderate to severe rosacea have the same IRR for PD as do those who have moderate disease. To explain this result, the authors hypothesized that a disease-severity effect might be blunted by the treatment of moderate to severe rosacea with tetracycline. The reason for this possible effect is that tetracycline is chemically similar to minocycline, which has shown evidence for exerting a protective effect in animal models of PD, although this effect has not been consistently seen.”
Dr. Wingo noted that the “intriguing finding that increased tetracycline use is associated with a small but appreciable reduction in the risk of PD should be further explored. Of particular interest would be to understand the temporal association between the use of tetracycline and effect on PD risk” (JAMA Neurol. 2016 Mar 21. doi: 10.1001/jamaneurol.2016.0291).
Dr. Wingo is a member of the departments of neurology and human genetics at Emory University, Atlanta. He had no financial conflicts to disclose.
An intriguing aspect of the study was the lack of disease severity impact on the incidence rate ratio (IRR) on Parkinson’s disease, Dr. Thomas S. Wingo wrote in an accompanying editorial. He added: “In other words, people with moderate to severe rosacea have the same IRR for PD as do those who have moderate disease. To explain this result, the authors hypothesized that a disease-severity effect might be blunted by the treatment of moderate to severe rosacea with tetracycline. The reason for this possible effect is that tetracycline is chemically similar to minocycline, which has shown evidence for exerting a protective effect in animal models of PD, although this effect has not been consistently seen.”
Dr. Wingo noted that the “intriguing finding that increased tetracycline use is associated with a small but appreciable reduction in the risk of PD should be further explored. Of particular interest would be to understand the temporal association between the use of tetracycline and effect on PD risk” (JAMA Neurol. 2016 Mar 21. doi: 10.1001/jamaneurol.2016.0291).
Dr. Wingo is a member of the departments of neurology and human genetics at Emory University, Atlanta. He had no financial conflicts to disclose.
Individuals with rosacea were nearly twice as likely to develop Parkinson’s disease as those without rosacea, based on an analysis of results of a nationwide cohort study of 5.4 million people conducted in Denmark. The findings were published online March 21 in JAMA Neurology.
Data from previous studies suggest a link between rosacea and Parkinson’s disease (PD), as both may stem from elevated activity of matrix metalloproteinases, wrote Dr. Alexander Egeberg of the University of Copenhagen, and his associates.
To explore the possible link between rosacea and Parkinson’s disease, the researchers reviewed data on about 5.4 million adults in a Danish database of all citizens aged 18 and older, over a 15-year period from January 1, 1997, to December 31, 2011. A total of 22,387 individuals were diagnosed with Parkinson’s disease and 68,053 were diagnosed with rosacea (JAMA Neurol. 2016 Mar 21. doi: 10.1001/jamaneurol.2016.0022).
The incidence of Parkinson’s disease was 7.62 per 10,000 person-years among rosacea patients vs. 3.54 per 10,000 person-years in the general population, the researchers noted.
Overall, the Parkinson’s disease risk was significantly higher among rosacea patients, with an adjusted incidence rate ratio (IRR) of 1.71 for rosacea patients compared with the general population. The IRR was even higher in patients with ocular rosacea (adjusted IRR, 2.03).
However, treatment with tetracycline appeared to reduce the Parkinson’s risk in the rosacea patients: After controlling for multiple variables, having filled a prescription for tetracycline was associated with a 2% drop in the risk of PD (adjusted IRR, 0.98).
A sensitivity analysis showed no dose-dependent relationship with disease severity; the IRRs for Parkinson’s disease in patients with mild rosacea and moderate to severe rosacea were 1.82 and 1.84, respectively. However, moderate to severe rosacea was defined as cases where tetracyclines were used, and the neuroprotective effect of tetracyclines might impact the relationship between rosacea and Parkinson’s disease, the researchers noted.
“It is tempting to speculate that, in patients with coexistent rosacea, Parkinson’s disease may display other phenotypic characteristics, and it is possible that rosacea-associated features, such as facial flushing, may contribute to support a Parkinson’s disease diagnosis at an early phase of the disease,” the researchers noted.
Limitations of the study include its observational design, which does not allow the establishment of causation, the researchers noted, and additional research is needed to confirm the observations and clinical consequences.
Lead author Dr. Egeberg was employed by Pfizer at the time of the study; one of the coauthors was supported by an unrestricted research scholarship from the Novo Nordisk Foundation.
Individuals with rosacea were nearly twice as likely to develop Parkinson’s disease as those without rosacea, based on an analysis of results of a nationwide cohort study of 5.4 million people conducted in Denmark. The findings were published online March 21 in JAMA Neurology.
Data from previous studies suggest a link between rosacea and Parkinson’s disease (PD), as both may stem from elevated activity of matrix metalloproteinases, wrote Dr. Alexander Egeberg of the University of Copenhagen, and his associates.
To explore the possible link between rosacea and Parkinson’s disease, the researchers reviewed data on about 5.4 million adults in a Danish database of all citizens aged 18 and older, over a 15-year period from January 1, 1997, to December 31, 2011. A total of 22,387 individuals were diagnosed with Parkinson’s disease and 68,053 were diagnosed with rosacea (JAMA Neurol. 2016 Mar 21. doi: 10.1001/jamaneurol.2016.0022).
The incidence of Parkinson’s disease was 7.62 per 10,000 person-years among rosacea patients vs. 3.54 per 10,000 person-years in the general population, the researchers noted.
Overall, the Parkinson’s disease risk was significantly higher among rosacea patients, with an adjusted incidence rate ratio (IRR) of 1.71 for rosacea patients compared with the general population. The IRR was even higher in patients with ocular rosacea (adjusted IRR, 2.03).
However, treatment with tetracycline appeared to reduce the Parkinson’s risk in the rosacea patients: After controlling for multiple variables, having filled a prescription for tetracycline was associated with a 2% drop in the risk of PD (adjusted IRR, 0.98).
A sensitivity analysis showed no dose-dependent relationship with disease severity; the IRRs for Parkinson’s disease in patients with mild rosacea and moderate to severe rosacea were 1.82 and 1.84, respectively. However, moderate to severe rosacea was defined as cases where tetracyclines were used, and the neuroprotective effect of tetracyclines might impact the relationship between rosacea and Parkinson’s disease, the researchers noted.
“It is tempting to speculate that, in patients with coexistent rosacea, Parkinson’s disease may display other phenotypic characteristics, and it is possible that rosacea-associated features, such as facial flushing, may contribute to support a Parkinson’s disease diagnosis at an early phase of the disease,” the researchers noted.
Limitations of the study include its observational design, which does not allow the establishment of causation, the researchers noted, and additional research is needed to confirm the observations and clinical consequences.
Lead author Dr. Egeberg was employed by Pfizer at the time of the study; one of the coauthors was supported by an unrestricted research scholarship from the Novo Nordisk Foundation.
FROM JAMA NEUROLOGY
Key clinical point: Rosacea was found to be an independent risk factor for Parkinson’s disease, an effect reduced by the use of tetracycline.
Major finding: The incidence of Parkinson’s disease was 7.62 per 10,000 person-years among people with rosacea vs. 3.54 per 10,000 person-years in the general population over a 15-year follow-up period.
Data source: The data come from a nationwide cohort study conducted in Denmark that included 5.4 million adults aged 18 years and older.
Disclosures: Lead author Dr. Alexander Egeberg was employed by Pfizer at the time of the study; a study coauthor was supported by an unrestricted research scholarship from the Novo Nordisk Foundation.
Primary care acne treatment reduces dermatology referrals
If primary care physicians initiated therapy in patients with mild to moderate acne, they could significantly decrease referrals to dermatologists, reduce patient waiting times, and cut health care costs, based on a study published online March 6 in JAMA Dermatology.
The researchers used retrospective data to model two treatment algorithms for initiating acne therapy based on evaluations of 253 dermatology referrals by primary care physicians at a single center. Half of the acne patients had not received any topical or systemic treatment prior to their referral to a dermatologist.
Two treatment algorithms were modeled for initiating acne therapy: In the first, the primary care physicians initiated topical acne treatments; in the second, they initiated topical treatments and systemic antibiotics.
If primary care physicians initiated topical acne therapy, the first algorithm predicted a 48% reduction in initial referrals to dermatologists, a 40% overall reduction in referrals, a mean 28.6-day reduction in delay to treatment, and an associated cost savings of $20.28 per patient.
Following the second algorithm entirely eliminated referrals for 72% of patients, reduced initial referrals by 86.7%, and reduced the mean delay to treatment by 27.9 days; the cost savings was $35.68 per patient, wrote Dr. Kristina J. Liu of the Harvard Combined Dermatology Residency Program and coauthors (JAMA Dermatol. 2016 March 6. doi: 10.1001/jamadermatol.2016.0183).
“Our findings are extracted from and applicable to patients who already have access to primary care clinicians. While patients can be efficiently treated by presenting directly to a dermatologist, in many health plans, a referral from primary care is a requisite step that precludes this possibility. Furthermore, 235 patients (93%) in our cohort were being managed by their primary care clinicians for other health conditions in addition to acne. Given these other health concerns, the patients in our study would likely access their primary care clinicians regardless of the problem of acne. Thus, the judicious use of primary care resources to manage acne as part of the holistic care of the patient can prove to be a time- and cost-saving strategy,” the authors wrote.
In addition, 32% of the referrals in the study were for acne plus another dermatologic issue, and such patients likely require dermatologic evaluation regardless of primary care clinician-driven acne management, the authors added.
One author declared employment with Walgreens Boots Alliance. No other conflicts of interest were declared.
If primary care physicians initiated therapy in patients with mild to moderate acne, they could significantly decrease referrals to dermatologists, reduce patient waiting times, and cut health care costs, based on a study published online March 6 in JAMA Dermatology.
The researchers used retrospective data to model two treatment algorithms for initiating acne therapy based on evaluations of 253 dermatology referrals by primary care physicians at a single center. Half of the acne patients had not received any topical or systemic treatment prior to their referral to a dermatologist.
Two treatment algorithms were modeled for initiating acne therapy: In the first, the primary care physicians initiated topical acne treatments; in the second, they initiated topical treatments and systemic antibiotics.
If primary care physicians initiated topical acne therapy, the first algorithm predicted a 48% reduction in initial referrals to dermatologists, a 40% overall reduction in referrals, a mean 28.6-day reduction in delay to treatment, and an associated cost savings of $20.28 per patient.
Following the second algorithm entirely eliminated referrals for 72% of patients, reduced initial referrals by 86.7%, and reduced the mean delay to treatment by 27.9 days; the cost savings was $35.68 per patient, wrote Dr. Kristina J. Liu of the Harvard Combined Dermatology Residency Program and coauthors (JAMA Dermatol. 2016 March 6. doi: 10.1001/jamadermatol.2016.0183).
“Our findings are extracted from and applicable to patients who already have access to primary care clinicians. While patients can be efficiently treated by presenting directly to a dermatologist, in many health plans, a referral from primary care is a requisite step that precludes this possibility. Furthermore, 235 patients (93%) in our cohort were being managed by their primary care clinicians for other health conditions in addition to acne. Given these other health concerns, the patients in our study would likely access their primary care clinicians regardless of the problem of acne. Thus, the judicious use of primary care resources to manage acne as part of the holistic care of the patient can prove to be a time- and cost-saving strategy,” the authors wrote.
In addition, 32% of the referrals in the study were for acne plus another dermatologic issue, and such patients likely require dermatologic evaluation regardless of primary care clinician-driven acne management, the authors added.
One author declared employment with Walgreens Boots Alliance. No other conflicts of interest were declared.
If primary care physicians initiated therapy in patients with mild to moderate acne, they could significantly decrease referrals to dermatologists, reduce patient waiting times, and cut health care costs, based on a study published online March 6 in JAMA Dermatology.
The researchers used retrospective data to model two treatment algorithms for initiating acne therapy based on evaluations of 253 dermatology referrals by primary care physicians at a single center. Half of the acne patients had not received any topical or systemic treatment prior to their referral to a dermatologist.
Two treatment algorithms were modeled for initiating acne therapy: In the first, the primary care physicians initiated topical acne treatments; in the second, they initiated topical treatments and systemic antibiotics.
If primary care physicians initiated topical acne therapy, the first algorithm predicted a 48% reduction in initial referrals to dermatologists, a 40% overall reduction in referrals, a mean 28.6-day reduction in delay to treatment, and an associated cost savings of $20.28 per patient.
Following the second algorithm entirely eliminated referrals for 72% of patients, reduced initial referrals by 86.7%, and reduced the mean delay to treatment by 27.9 days; the cost savings was $35.68 per patient, wrote Dr. Kristina J. Liu of the Harvard Combined Dermatology Residency Program and coauthors (JAMA Dermatol. 2016 March 6. doi: 10.1001/jamadermatol.2016.0183).
“Our findings are extracted from and applicable to patients who already have access to primary care clinicians. While patients can be efficiently treated by presenting directly to a dermatologist, in many health plans, a referral from primary care is a requisite step that precludes this possibility. Furthermore, 235 patients (93%) in our cohort were being managed by their primary care clinicians for other health conditions in addition to acne. Given these other health concerns, the patients in our study would likely access their primary care clinicians regardless of the problem of acne. Thus, the judicious use of primary care resources to manage acne as part of the holistic care of the patient can prove to be a time- and cost-saving strategy,” the authors wrote.
In addition, 32% of the referrals in the study were for acne plus another dermatologic issue, and such patients likely require dermatologic evaluation regardless of primary care clinician-driven acne management, the authors added.
One author declared employment with Walgreens Boots Alliance. No other conflicts of interest were declared.
FROM JAMA DERMATOLOGY
Key clinical point: If primary care physicians initiate treatment of mild to moderate acne, they could significantly reduce referrals to dermatologists and patient waiting times for treatment.
Major finding: Primary care treatment can reduce referrals to dermatologists for acne by up to 72%.
Data source: A retrospective study and modeling of 253 referrals.
Disclosures: One author declared employment with Walgreens Boots Alliance. No other conflicts of interest were declared.