User login
Thrombectomy benefits stroke with large core volumes: SELECT2 trial results
in a major international trial, which is expected to lead to a change in clinical practice and the way in which systems of stroke care are organized.
The results of the SELECT2 trial, which was conducted in sites in the United States, Canada, Europe, Australia, and New Zealand, showed that endovascular thrombectomy plus medical care resulted in better clinical outcomes than medical care alone in patients with a large ischemic core who presented within 24 hours after the time they were last known to be well.
The results of the SELECT2 trial were presented at the International Stroke Conference by Amrou Sarraj, MD. Dr. Sarraj is professor of neurology at University Hospitals Cleveland Medical Center–Case Western Reserve University in Ohio. The study was also simultaneously published online in the New England Journal of Medicine.
A similar trial conducted in China, the ANGEL-ASPECT trial, was also presented at the same ISC session and showed very similar results.
These two trials add to another Japanese study reported last year, the RESCUE-JAPAN LIMIT trial, also showing benefit of thrombectomy in patients with large core strokes.
Dr. Sarraj concluded that the results of these three trials together “unequivocally demonstrate the benefit of endovascular thrombectomy in patients with large ischemic core.”
A clear benefit
Approximately 20% of large-vessel occlusion strokes have a large core, but these patients have not been considered candidates for endovascular thrombectomy because of concerns about potential reperfusion injury in necrotic brain tissue, resulting in an increased risk of hemorrhage, edema, disability, and death.
This has resulted in uncertainty about how to manage these patients with a core infarct, Dr. Sarraj noted at the conference presented by the American Stroke Association, a division of the American Heart Association.
The SELECT2 trial involved patients with stroke as a result of occlusion of the internal carotid artery or the first segment of the middle cerebral artery. Patients had a large ischemic core volume, defined as an ASPECTS (Alberta Stroke Program Early Computed Tomography Score) of 3-5, or a core volume of at least 50 mL on imaging. They were randomly assigned to endovascular thrombectomy plus medical care or to medical care alone.
The trial was aiming to enroll 560 patients but was stopped early for efficacy after 178 patients had been assigned to the thrombectomy group and 174 to the medical-care group.
The primary outcome – the generalized odds ratio for a shift in the distribution of modified Rankin scale scores toward better outcomes in favor of thrombectomy was 1.51 (P < .001).
“This translates into a 60% probability of achieving a better functional outcome in patients receiving thrombectomy, with a number needed to treat of five. That means five patients need to be treated with thrombectomy for one to achieve a better functional outcome,” Dr. Sarraj stated.
The secondary outcome of functional independence at 90 days (a score on the modified Rankin scale of 0-2) occurred in 20% of the patients in the thrombectomy group and 7% in the medical-care group (relative risk, 2.97), with a number needed to treat of seven.
Independent ambulation (a score on the modified Rankin Scale of 0-3) at 90 days occurred in 37.9% of the patients in the thrombectomy group and in 18.7% of the patients in the medical-care group (relative risk, 2.06), with a number needed to treat of five.
Mortality was similar in the two groups.
The results for other secondary outcomes were generally in the same direction as those of the primary analysis, with the possible exception of early neurologic improvement, the authors reported.
The incidence of symptomatic intracranial hemorrhage was low in both trial groups, occurring in one patient in the thrombectomy group and two in the medical care group.
The investigators pointed out that previous studies have reported rates of symptomatic intracranial hemorrhage in patients with large ischemic core lesions that are higher than those in this trial. “Therefore, the low percentage of patients with symptomatic intracranial hemorrhage observed in both trial groups was unexpected.”
Approximately 20% of the patients in the thrombectomy group had complications associated with the procedure. In the thrombectomy group, arterial access-site complications occurred in 5 patients, dissection in 10, cerebral vessel perforation in 7, and transient vasospasm in 11.
Early neurologic worsening, defined as an increase of 4 or more points on the National Institutes of Health Stroke Scale (NIHSS), occurred in 24.7% in the thrombectomy group and in 15.5% in the medical-care group (relative risk, 1.59).
In a post-hoc analysis, “from which no conclusions can be drawn,” the authors reported, early neurologic worsening was associated with worse functional outcomes at 90 days, and patients who had neurologic worsening had larger ischemic core lesions at baseline (median volume, 107 mL) versus 77 mL among patients without neurologic worsening.
They noted that a potential cause of deterioration in some of these patients was brain edema associated with reperfusion. However, they emphasize that overall, endovascular thrombectomy was associated with better outcomes than medical care alone.
“Two-thirds of patients had core infarct sizes more than 70 mL, and one-third of patients had core infarct sizes of more than 100 mL, but even in patients with large and very large core volumes, thrombectomy was superior to medical care alone,” Dr. Sarraj said.
This will ‘change practice’
In a comment, ISC 2023 chair Tudor Jovin, MD, Cooper Neurological Institute, Cherry Hill, N.J., said: “This trial shows that even patients with a large core infarct who we would not have treated with thrombectomy in the past, actually do benefit from this procedure. And the surprise is that the benefit is nearly to the same extent as that in patients with smaller core infarcts. That is going to change practice.”
Dr. Jovin said that these results should not only change the selection of patients for thrombectomy, but they should also change systems of care. “Because the systems of care now are based around excluding these patients with large infarcts. We won’t need to do that in future.”
He elaborated: “I think imaging has held us back to be honest. We can exclude hemorrhage with a plain CT scan. Then after this, the biggest piece of information we need from imaging is the size of the infarct. We were concerned that we might hurt the patient if the infarct was large. Outside hospitals had to do advanced imaging before deciding whether to transfer patients for thrombectomy. These are all sources of delays.
“I am very pleased to see these results, and I hope to see a much more simplified triage of patients that will be more liberal to patients with the large infarcts,” he added.
Also commenting, Joseph Broderick, MD, professor of neurology and director of the Neuroscience Institute at the University of Cincinnati, said the results were “robust and important.”
He said the results of the SELECT2 trial, along with the other two similar trials, “will change practice and extend endovascular therapy to more patients with severe strokes.”
But Dr. Broderick believes imaging will still be necessary to exclude patients with ASPECTS scores of 0-2, who were not included in these trials. “These are patients who have very large areas of clear hypodensity on the baseline image (brain already dying or dead). These patients do not benefit from reperfusion with lytic drugs or endovascular therapy,” he noted.
‘Welcome news’
In an editorial accompanying the print publication of the two new studies, Pierre Fayad, MD, University of Nebraska Medical Center, Omaha, points out that all three trials of thrombectomy in patients with large core infarct strokes “showed remarkably similar results” despite differences in design, patient selection, thrombolytic treatment and dose, geographic location, and imaging criteria.
“Together, the trials provide reassuring information from more than a thousand patients with large ischemic strokes in different medical systems that will probably lead to changes in patterns of care delivery.”
Dr. Fayad said it is reasonable to suggest that endovascular thrombectomy be offered to patients with large strokes if they arrive in a timely fashion at a center that is capable of performing the procedure, and if the patients have an ASPECTS value of 3-5 or an ischemic core volume of 50 mL or greater.
Higher rates of good outcomes may be anticipated if this treatment is performed, despite increased risks of symptomatic hemorrhage, edema, neurologic worsening, and hemicraniectomy, he noted.
“Patients and families should be made aware of the limitations of treatment and the anticipated residual neurologic deficits resulting from the large infarction. The improved chance of independent walking and the ability to perform other daily activities in patients with the most severe strokes is welcome news for patients and for the field of stroke treatment,” he concluded.
The SELECT2 trial was supported by an investigator-initiated grant from Stryker Neurovascular to University Hospitals Cleveland Medical Center and the University of Texas McGovern Medical School.
A version of this article first appeared on Medscape.com.
in a major international trial, which is expected to lead to a change in clinical practice and the way in which systems of stroke care are organized.
The results of the SELECT2 trial, which was conducted in sites in the United States, Canada, Europe, Australia, and New Zealand, showed that endovascular thrombectomy plus medical care resulted in better clinical outcomes than medical care alone in patients with a large ischemic core who presented within 24 hours after the time they were last known to be well.
The results of the SELECT2 trial were presented at the International Stroke Conference by Amrou Sarraj, MD. Dr. Sarraj is professor of neurology at University Hospitals Cleveland Medical Center–Case Western Reserve University in Ohio. The study was also simultaneously published online in the New England Journal of Medicine.
A similar trial conducted in China, the ANGEL-ASPECT trial, was also presented at the same ISC session and showed very similar results.
These two trials add to another Japanese study reported last year, the RESCUE-JAPAN LIMIT trial, also showing benefit of thrombectomy in patients with large core strokes.
Dr. Sarraj concluded that the results of these three trials together “unequivocally demonstrate the benefit of endovascular thrombectomy in patients with large ischemic core.”
A clear benefit
Approximately 20% of large-vessel occlusion strokes have a large core, but these patients have not been considered candidates for endovascular thrombectomy because of concerns about potential reperfusion injury in necrotic brain tissue, resulting in an increased risk of hemorrhage, edema, disability, and death.
This has resulted in uncertainty about how to manage these patients with a core infarct, Dr. Sarraj noted at the conference presented by the American Stroke Association, a division of the American Heart Association.
The SELECT2 trial involved patients with stroke as a result of occlusion of the internal carotid artery or the first segment of the middle cerebral artery. Patients had a large ischemic core volume, defined as an ASPECTS (Alberta Stroke Program Early Computed Tomography Score) of 3-5, or a core volume of at least 50 mL on imaging. They were randomly assigned to endovascular thrombectomy plus medical care or to medical care alone.
The trial was aiming to enroll 560 patients but was stopped early for efficacy after 178 patients had been assigned to the thrombectomy group and 174 to the medical-care group.
The primary outcome – the generalized odds ratio for a shift in the distribution of modified Rankin scale scores toward better outcomes in favor of thrombectomy was 1.51 (P < .001).
“This translates into a 60% probability of achieving a better functional outcome in patients receiving thrombectomy, with a number needed to treat of five. That means five patients need to be treated with thrombectomy for one to achieve a better functional outcome,” Dr. Sarraj stated.
The secondary outcome of functional independence at 90 days (a score on the modified Rankin scale of 0-2) occurred in 20% of the patients in the thrombectomy group and 7% in the medical-care group (relative risk, 2.97), with a number needed to treat of seven.
Independent ambulation (a score on the modified Rankin Scale of 0-3) at 90 days occurred in 37.9% of the patients in the thrombectomy group and in 18.7% of the patients in the medical-care group (relative risk, 2.06), with a number needed to treat of five.
Mortality was similar in the two groups.
The results for other secondary outcomes were generally in the same direction as those of the primary analysis, with the possible exception of early neurologic improvement, the authors reported.
The incidence of symptomatic intracranial hemorrhage was low in both trial groups, occurring in one patient in the thrombectomy group and two in the medical care group.
The investigators pointed out that previous studies have reported rates of symptomatic intracranial hemorrhage in patients with large ischemic core lesions that are higher than those in this trial. “Therefore, the low percentage of patients with symptomatic intracranial hemorrhage observed in both trial groups was unexpected.”
Approximately 20% of the patients in the thrombectomy group had complications associated with the procedure. In the thrombectomy group, arterial access-site complications occurred in 5 patients, dissection in 10, cerebral vessel perforation in 7, and transient vasospasm in 11.
Early neurologic worsening, defined as an increase of 4 or more points on the National Institutes of Health Stroke Scale (NIHSS), occurred in 24.7% in the thrombectomy group and in 15.5% in the medical-care group (relative risk, 1.59).
In a post-hoc analysis, “from which no conclusions can be drawn,” the authors reported, early neurologic worsening was associated with worse functional outcomes at 90 days, and patients who had neurologic worsening had larger ischemic core lesions at baseline (median volume, 107 mL) versus 77 mL among patients without neurologic worsening.
They noted that a potential cause of deterioration in some of these patients was brain edema associated with reperfusion. However, they emphasize that overall, endovascular thrombectomy was associated with better outcomes than medical care alone.
“Two-thirds of patients had core infarct sizes more than 70 mL, and one-third of patients had core infarct sizes of more than 100 mL, but even in patients with large and very large core volumes, thrombectomy was superior to medical care alone,” Dr. Sarraj said.
This will ‘change practice’
In a comment, ISC 2023 chair Tudor Jovin, MD, Cooper Neurological Institute, Cherry Hill, N.J., said: “This trial shows that even patients with a large core infarct who we would not have treated with thrombectomy in the past, actually do benefit from this procedure. And the surprise is that the benefit is nearly to the same extent as that in patients with smaller core infarcts. That is going to change practice.”
Dr. Jovin said that these results should not only change the selection of patients for thrombectomy, but they should also change systems of care. “Because the systems of care now are based around excluding these patients with large infarcts. We won’t need to do that in future.”
He elaborated: “I think imaging has held us back to be honest. We can exclude hemorrhage with a plain CT scan. Then after this, the biggest piece of information we need from imaging is the size of the infarct. We were concerned that we might hurt the patient if the infarct was large. Outside hospitals had to do advanced imaging before deciding whether to transfer patients for thrombectomy. These are all sources of delays.
“I am very pleased to see these results, and I hope to see a much more simplified triage of patients that will be more liberal to patients with the large infarcts,” he added.
Also commenting, Joseph Broderick, MD, professor of neurology and director of the Neuroscience Institute at the University of Cincinnati, said the results were “robust and important.”
He said the results of the SELECT2 trial, along with the other two similar trials, “will change practice and extend endovascular therapy to more patients with severe strokes.”
But Dr. Broderick believes imaging will still be necessary to exclude patients with ASPECTS scores of 0-2, who were not included in these trials. “These are patients who have very large areas of clear hypodensity on the baseline image (brain already dying or dead). These patients do not benefit from reperfusion with lytic drugs or endovascular therapy,” he noted.
‘Welcome news’
In an editorial accompanying the print publication of the two new studies, Pierre Fayad, MD, University of Nebraska Medical Center, Omaha, points out that all three trials of thrombectomy in patients with large core infarct strokes “showed remarkably similar results” despite differences in design, patient selection, thrombolytic treatment and dose, geographic location, and imaging criteria.
“Together, the trials provide reassuring information from more than a thousand patients with large ischemic strokes in different medical systems that will probably lead to changes in patterns of care delivery.”
Dr. Fayad said it is reasonable to suggest that endovascular thrombectomy be offered to patients with large strokes if they arrive in a timely fashion at a center that is capable of performing the procedure, and if the patients have an ASPECTS value of 3-5 or an ischemic core volume of 50 mL or greater.
Higher rates of good outcomes may be anticipated if this treatment is performed, despite increased risks of symptomatic hemorrhage, edema, neurologic worsening, and hemicraniectomy, he noted.
“Patients and families should be made aware of the limitations of treatment and the anticipated residual neurologic deficits resulting from the large infarction. The improved chance of independent walking and the ability to perform other daily activities in patients with the most severe strokes is welcome news for patients and for the field of stroke treatment,” he concluded.
The SELECT2 trial was supported by an investigator-initiated grant from Stryker Neurovascular to University Hospitals Cleveland Medical Center and the University of Texas McGovern Medical School.
A version of this article first appeared on Medscape.com.
in a major international trial, which is expected to lead to a change in clinical practice and the way in which systems of stroke care are organized.
The results of the SELECT2 trial, which was conducted in sites in the United States, Canada, Europe, Australia, and New Zealand, showed that endovascular thrombectomy plus medical care resulted in better clinical outcomes than medical care alone in patients with a large ischemic core who presented within 24 hours after the time they were last known to be well.
The results of the SELECT2 trial were presented at the International Stroke Conference by Amrou Sarraj, MD. Dr. Sarraj is professor of neurology at University Hospitals Cleveland Medical Center–Case Western Reserve University in Ohio. The study was also simultaneously published online in the New England Journal of Medicine.
A similar trial conducted in China, the ANGEL-ASPECT trial, was also presented at the same ISC session and showed very similar results.
These two trials add to another Japanese study reported last year, the RESCUE-JAPAN LIMIT trial, also showing benefit of thrombectomy in patients with large core strokes.
Dr. Sarraj concluded that the results of these three trials together “unequivocally demonstrate the benefit of endovascular thrombectomy in patients with large ischemic core.”
A clear benefit
Approximately 20% of large-vessel occlusion strokes have a large core, but these patients have not been considered candidates for endovascular thrombectomy because of concerns about potential reperfusion injury in necrotic brain tissue, resulting in an increased risk of hemorrhage, edema, disability, and death.
This has resulted in uncertainty about how to manage these patients with a core infarct, Dr. Sarraj noted at the conference presented by the American Stroke Association, a division of the American Heart Association.
The SELECT2 trial involved patients with stroke as a result of occlusion of the internal carotid artery or the first segment of the middle cerebral artery. Patients had a large ischemic core volume, defined as an ASPECTS (Alberta Stroke Program Early Computed Tomography Score) of 3-5, or a core volume of at least 50 mL on imaging. They were randomly assigned to endovascular thrombectomy plus medical care or to medical care alone.
The trial was aiming to enroll 560 patients but was stopped early for efficacy after 178 patients had been assigned to the thrombectomy group and 174 to the medical-care group.
The primary outcome – the generalized odds ratio for a shift in the distribution of modified Rankin scale scores toward better outcomes in favor of thrombectomy was 1.51 (P < .001).
“This translates into a 60% probability of achieving a better functional outcome in patients receiving thrombectomy, with a number needed to treat of five. That means five patients need to be treated with thrombectomy for one to achieve a better functional outcome,” Dr. Sarraj stated.
The secondary outcome of functional independence at 90 days (a score on the modified Rankin scale of 0-2) occurred in 20% of the patients in the thrombectomy group and 7% in the medical-care group (relative risk, 2.97), with a number needed to treat of seven.
Independent ambulation (a score on the modified Rankin Scale of 0-3) at 90 days occurred in 37.9% of the patients in the thrombectomy group and in 18.7% of the patients in the medical-care group (relative risk, 2.06), with a number needed to treat of five.
Mortality was similar in the two groups.
The results for other secondary outcomes were generally in the same direction as those of the primary analysis, with the possible exception of early neurologic improvement, the authors reported.
The incidence of symptomatic intracranial hemorrhage was low in both trial groups, occurring in one patient in the thrombectomy group and two in the medical care group.
The investigators pointed out that previous studies have reported rates of symptomatic intracranial hemorrhage in patients with large ischemic core lesions that are higher than those in this trial. “Therefore, the low percentage of patients with symptomatic intracranial hemorrhage observed in both trial groups was unexpected.”
Approximately 20% of the patients in the thrombectomy group had complications associated with the procedure. In the thrombectomy group, arterial access-site complications occurred in 5 patients, dissection in 10, cerebral vessel perforation in 7, and transient vasospasm in 11.
Early neurologic worsening, defined as an increase of 4 or more points on the National Institutes of Health Stroke Scale (NIHSS), occurred in 24.7% in the thrombectomy group and in 15.5% in the medical-care group (relative risk, 1.59).
In a post-hoc analysis, “from which no conclusions can be drawn,” the authors reported, early neurologic worsening was associated with worse functional outcomes at 90 days, and patients who had neurologic worsening had larger ischemic core lesions at baseline (median volume, 107 mL) versus 77 mL among patients without neurologic worsening.
They noted that a potential cause of deterioration in some of these patients was brain edema associated with reperfusion. However, they emphasize that overall, endovascular thrombectomy was associated with better outcomes than medical care alone.
“Two-thirds of patients had core infarct sizes more than 70 mL, and one-third of patients had core infarct sizes of more than 100 mL, but even in patients with large and very large core volumes, thrombectomy was superior to medical care alone,” Dr. Sarraj said.
This will ‘change practice’
In a comment, ISC 2023 chair Tudor Jovin, MD, Cooper Neurological Institute, Cherry Hill, N.J., said: “This trial shows that even patients with a large core infarct who we would not have treated with thrombectomy in the past, actually do benefit from this procedure. And the surprise is that the benefit is nearly to the same extent as that in patients with smaller core infarcts. That is going to change practice.”
Dr. Jovin said that these results should not only change the selection of patients for thrombectomy, but they should also change systems of care. “Because the systems of care now are based around excluding these patients with large infarcts. We won’t need to do that in future.”
He elaborated: “I think imaging has held us back to be honest. We can exclude hemorrhage with a plain CT scan. Then after this, the biggest piece of information we need from imaging is the size of the infarct. We were concerned that we might hurt the patient if the infarct was large. Outside hospitals had to do advanced imaging before deciding whether to transfer patients for thrombectomy. These are all sources of delays.
“I am very pleased to see these results, and I hope to see a much more simplified triage of patients that will be more liberal to patients with the large infarcts,” he added.
Also commenting, Joseph Broderick, MD, professor of neurology and director of the Neuroscience Institute at the University of Cincinnati, said the results were “robust and important.”
He said the results of the SELECT2 trial, along with the other two similar trials, “will change practice and extend endovascular therapy to more patients with severe strokes.”
But Dr. Broderick believes imaging will still be necessary to exclude patients with ASPECTS scores of 0-2, who were not included in these trials. “These are patients who have very large areas of clear hypodensity on the baseline image (brain already dying or dead). These patients do not benefit from reperfusion with lytic drugs or endovascular therapy,” he noted.
‘Welcome news’
In an editorial accompanying the print publication of the two new studies, Pierre Fayad, MD, University of Nebraska Medical Center, Omaha, points out that all three trials of thrombectomy in patients with large core infarct strokes “showed remarkably similar results” despite differences in design, patient selection, thrombolytic treatment and dose, geographic location, and imaging criteria.
“Together, the trials provide reassuring information from more than a thousand patients with large ischemic strokes in different medical systems that will probably lead to changes in patterns of care delivery.”
Dr. Fayad said it is reasonable to suggest that endovascular thrombectomy be offered to patients with large strokes if they arrive in a timely fashion at a center that is capable of performing the procedure, and if the patients have an ASPECTS value of 3-5 or an ischemic core volume of 50 mL or greater.
Higher rates of good outcomes may be anticipated if this treatment is performed, despite increased risks of symptomatic hemorrhage, edema, neurologic worsening, and hemicraniectomy, he noted.
“Patients and families should be made aware of the limitations of treatment and the anticipated residual neurologic deficits resulting from the large infarction. The improved chance of independent walking and the ability to perform other daily activities in patients with the most severe strokes is welcome news for patients and for the field of stroke treatment,” he concluded.
The SELECT2 trial was supported by an investigator-initiated grant from Stryker Neurovascular to University Hospitals Cleveland Medical Center and the University of Texas McGovern Medical School.
A version of this article first appeared on Medscape.com.
FROM ISC 2023
Blood pressure lowering after thrombectomy may be harmful
, new research suggests. Preliminary results of a new study showed that using an antihypertensive drug to target systolic blood pressure to below 160 mm Hg or 140 mm Hg in these patients may not be beneficial, and may even be harmful.
“This line of inquiry is probably not worth pursuing,” said stroke neurologist Eva A. Mistry, MBBS, MSCI, assistant professor of clinical neurology and rehabilitation medicine, University of Cincinnati.
Following current blood pressure guidelines in these patients (so targeting blood pressure under 180/105 mm Hg) “is probably reasonable,” unless the patient’s systolic blood pressure goes above 180, Dr. Mistry said. “Artificially trying to lower it may result in harm, at least in terms of the disability outcome.”
The findings were presented at the 2023 International Stroke Conference presented by the American Stroke Association, a division of the American Heart Association.
Endovascular therapy has become standard of care for patients with large vessel occlusion after studies showed “massive benefit,” yet about 50% of patients remain disabled or die at 90 days, Dr. Mistry said.
“We have been on the quest to understand if there’s something we can do to improve these outcomes.”
One approach could be optimizing medical management. Previous observational studies showed that higher blood pressure values after thrombectomy are associated with worse outcomes.
Taking it forward
“We wanted to take that forward in a randomized inquiry to see first with this trial if [artificially] lowering blood pressure using medications is safe, and preliminarily understand if it could be efficacious in a larger trial,” she said.
This blood pressure–lowering strategy is already practiced in some centers. A nationwide survey conducted by Dr. Mistry and her colleagues showed a wide range of targets, with some institutions aiming it as low as under 120 mm Hg after thrombectomy, which she found “surprising.”
The Blood pressure after Endovascular Stroke Treatment-II (BEST-II) study included 120 ischemic stroke patients at three stroke centers, mean age 70 years and 57% female, who had undergone endovascular treatment. They were randomized to one of three target blood pressure groups: 180 mm Hg or under, less than 160 mm Hg, or under 140 mm Hg.
To lower blood pressure, researchers used intravenous nicardipine, a calcium channel blocker, as a first line. This was started within 1 hour of the endovascular treatment and given for 24 hours if the patient’s systolic blood pressure was above the target of their group.
In the highest target group (≤180 mm Hg), the average systolic blood pressure reached 129 mm Hg. In the middle target group (<160 mm Hg), the average systolic blood pressure was 131 mm Hg, and in the lowest target group (<140 mm Hg), systolic blood pressure was lowered to an average of 123 mm Hg.
Mean infarct volumes
At 36 hours, the mean adjusted infarct volume was slightly lower in the lowest blood pressure target group (32.4), compared with the other groups (46.4 for the 180 mm Hg group and 50.7 for the under-160 mm Hg group).
“Based on a model or a slope that would be associated with serial lowering of blood pressure targets, we found the point estimate of the effect size was slightly in the direction of benefit of lower blood pressure targets in terms of lower infarct volume,” Dr. Mistry said.
But this was not conclusive. While the point estimate was in the direction of benefit, Dr. Mistry stressed that the trial design doesn’t “definitely rule out” the possibility of harm.
Researchers also measured functional status at 90 days with the modified Rankin Scale (mRS). They found that the utility-weighted mRS was slightly lower in the lowest blood pressure target group (0.507), compared with the higher target groups (0.584 and 0.475, respectively, for the 180 mm Hg and under-160 mm Hg groups).
“The effect size was slightly in the direction of harm,” Dr. Mistry said. “To me, that means there might be safety issues associated with the lower blood pressure target.”
Probably futile
The results suggest that studying this issue further is probably futile. “If lowering blood pressure improves outcomes, that improvement is fairly marginal, and there are trends that suggest that, in fact, it might be harmful,” Dr. Mistry said. Her researcher team “believes it would not be the wisest decision” to pursue this strategy any further in a phase 3 study, she said.
“We wanted to understand whether or not we should spend millions of dollars to do a thousand-patient or two thousand-patient trial, and the answer to that is probably not.”
And there are other therapeutics “we can test that might be more promising than this approach,” she added.
In the meantime, Dr. Mistry stressed that clinicians should be cautious about automatically lowering blood pressure in this patient population and that decisions to target lower levels should be done on an individual basis.
Timely and important
In a comment, Karen Furie, MD, MPH, chair of neurology, Brown University, Providence, R.I., said that the study is “timely and important,” given the uncertainty about management of blood pressure after opening the vessel again using endovascular treatment.
“We already knew that letting the blood pressure go very high after reperfusion was bad, and this study shows that lowering it may also pose a risk, and I think that’s an important message for the community.”
The results send a cautionary message to clinicians but do not provide definitive evidence, she added. “Perhaps in the future we will have a better understanding of what the optimal range is.”
Dr. Furie stressed that this was a small pilot study and conclusions are “guarded.”
“I think the authors didn’t want to overinterpret the results so they ended up concluding that because the final disability might have been worse in the patients who had their blood pressure significantly lowered, recommending that as an approach across the board is sort of discouraged.”
Instead, the authors indicated that there may be factors such as degree of recanalization, size of the infarct, or other patient-specific factors “that would dictate where you target blood pressures,” Dr. Furie said.
The study was funded by the National Institutes of Health/National Institute of Neurological Disorders and Stroke. Mistry receives funding from the Patient-Centered Outcomes Research Institute and compensation from the American Heart Association for editorial activities, and is a consultant for RapidAI. Dr. Furie has declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests. Preliminary results of a new study showed that using an antihypertensive drug to target systolic blood pressure to below 160 mm Hg or 140 mm Hg in these patients may not be beneficial, and may even be harmful.
“This line of inquiry is probably not worth pursuing,” said stroke neurologist Eva A. Mistry, MBBS, MSCI, assistant professor of clinical neurology and rehabilitation medicine, University of Cincinnati.
Following current blood pressure guidelines in these patients (so targeting blood pressure under 180/105 mm Hg) “is probably reasonable,” unless the patient’s systolic blood pressure goes above 180, Dr. Mistry said. “Artificially trying to lower it may result in harm, at least in terms of the disability outcome.”
The findings were presented at the 2023 International Stroke Conference presented by the American Stroke Association, a division of the American Heart Association.
Endovascular therapy has become standard of care for patients with large vessel occlusion after studies showed “massive benefit,” yet about 50% of patients remain disabled or die at 90 days, Dr. Mistry said.
“We have been on the quest to understand if there’s something we can do to improve these outcomes.”
One approach could be optimizing medical management. Previous observational studies showed that higher blood pressure values after thrombectomy are associated with worse outcomes.
Taking it forward
“We wanted to take that forward in a randomized inquiry to see first with this trial if [artificially] lowering blood pressure using medications is safe, and preliminarily understand if it could be efficacious in a larger trial,” she said.
This blood pressure–lowering strategy is already practiced in some centers. A nationwide survey conducted by Dr. Mistry and her colleagues showed a wide range of targets, with some institutions aiming it as low as under 120 mm Hg after thrombectomy, which she found “surprising.”
The Blood pressure after Endovascular Stroke Treatment-II (BEST-II) study included 120 ischemic stroke patients at three stroke centers, mean age 70 years and 57% female, who had undergone endovascular treatment. They were randomized to one of three target blood pressure groups: 180 mm Hg or under, less than 160 mm Hg, or under 140 mm Hg.
To lower blood pressure, researchers used intravenous nicardipine, a calcium channel blocker, as a first line. This was started within 1 hour of the endovascular treatment and given for 24 hours if the patient’s systolic blood pressure was above the target of their group.
In the highest target group (≤180 mm Hg), the average systolic blood pressure reached 129 mm Hg. In the middle target group (<160 mm Hg), the average systolic blood pressure was 131 mm Hg, and in the lowest target group (<140 mm Hg), systolic blood pressure was lowered to an average of 123 mm Hg.
Mean infarct volumes
At 36 hours, the mean adjusted infarct volume was slightly lower in the lowest blood pressure target group (32.4), compared with the other groups (46.4 for the 180 mm Hg group and 50.7 for the under-160 mm Hg group).
“Based on a model or a slope that would be associated with serial lowering of blood pressure targets, we found the point estimate of the effect size was slightly in the direction of benefit of lower blood pressure targets in terms of lower infarct volume,” Dr. Mistry said.
But this was not conclusive. While the point estimate was in the direction of benefit, Dr. Mistry stressed that the trial design doesn’t “definitely rule out” the possibility of harm.
Researchers also measured functional status at 90 days with the modified Rankin Scale (mRS). They found that the utility-weighted mRS was slightly lower in the lowest blood pressure target group (0.507), compared with the higher target groups (0.584 and 0.475, respectively, for the 180 mm Hg and under-160 mm Hg groups).
“The effect size was slightly in the direction of harm,” Dr. Mistry said. “To me, that means there might be safety issues associated with the lower blood pressure target.”
Probably futile
The results suggest that studying this issue further is probably futile. “If lowering blood pressure improves outcomes, that improvement is fairly marginal, and there are trends that suggest that, in fact, it might be harmful,” Dr. Mistry said. Her researcher team “believes it would not be the wisest decision” to pursue this strategy any further in a phase 3 study, she said.
“We wanted to understand whether or not we should spend millions of dollars to do a thousand-patient or two thousand-patient trial, and the answer to that is probably not.”
And there are other therapeutics “we can test that might be more promising than this approach,” she added.
In the meantime, Dr. Mistry stressed that clinicians should be cautious about automatically lowering blood pressure in this patient population and that decisions to target lower levels should be done on an individual basis.
Timely and important
In a comment, Karen Furie, MD, MPH, chair of neurology, Brown University, Providence, R.I., said that the study is “timely and important,” given the uncertainty about management of blood pressure after opening the vessel again using endovascular treatment.
“We already knew that letting the blood pressure go very high after reperfusion was bad, and this study shows that lowering it may also pose a risk, and I think that’s an important message for the community.”
The results send a cautionary message to clinicians but do not provide definitive evidence, she added. “Perhaps in the future we will have a better understanding of what the optimal range is.”
Dr. Furie stressed that this was a small pilot study and conclusions are “guarded.”
“I think the authors didn’t want to overinterpret the results so they ended up concluding that because the final disability might have been worse in the patients who had their blood pressure significantly lowered, recommending that as an approach across the board is sort of discouraged.”
Instead, the authors indicated that there may be factors such as degree of recanalization, size of the infarct, or other patient-specific factors “that would dictate where you target blood pressures,” Dr. Furie said.
The study was funded by the National Institutes of Health/National Institute of Neurological Disorders and Stroke. Mistry receives funding from the Patient-Centered Outcomes Research Institute and compensation from the American Heart Association for editorial activities, and is a consultant for RapidAI. Dr. Furie has declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests. Preliminary results of a new study showed that using an antihypertensive drug to target systolic blood pressure to below 160 mm Hg or 140 mm Hg in these patients may not be beneficial, and may even be harmful.
“This line of inquiry is probably not worth pursuing,” said stroke neurologist Eva A. Mistry, MBBS, MSCI, assistant professor of clinical neurology and rehabilitation medicine, University of Cincinnati.
Following current blood pressure guidelines in these patients (so targeting blood pressure under 180/105 mm Hg) “is probably reasonable,” unless the patient’s systolic blood pressure goes above 180, Dr. Mistry said. “Artificially trying to lower it may result in harm, at least in terms of the disability outcome.”
The findings were presented at the 2023 International Stroke Conference presented by the American Stroke Association, a division of the American Heart Association.
Endovascular therapy has become standard of care for patients with large vessel occlusion after studies showed “massive benefit,” yet about 50% of patients remain disabled or die at 90 days, Dr. Mistry said.
“We have been on the quest to understand if there’s something we can do to improve these outcomes.”
One approach could be optimizing medical management. Previous observational studies showed that higher blood pressure values after thrombectomy are associated with worse outcomes.
Taking it forward
“We wanted to take that forward in a randomized inquiry to see first with this trial if [artificially] lowering blood pressure using medications is safe, and preliminarily understand if it could be efficacious in a larger trial,” she said.
This blood pressure–lowering strategy is already practiced in some centers. A nationwide survey conducted by Dr. Mistry and her colleagues showed a wide range of targets, with some institutions aiming it as low as under 120 mm Hg after thrombectomy, which she found “surprising.”
The Blood pressure after Endovascular Stroke Treatment-II (BEST-II) study included 120 ischemic stroke patients at three stroke centers, mean age 70 years and 57% female, who had undergone endovascular treatment. They were randomized to one of three target blood pressure groups: 180 mm Hg or under, less than 160 mm Hg, or under 140 mm Hg.
To lower blood pressure, researchers used intravenous nicardipine, a calcium channel blocker, as a first line. This was started within 1 hour of the endovascular treatment and given for 24 hours if the patient’s systolic blood pressure was above the target of their group.
In the highest target group (≤180 mm Hg), the average systolic blood pressure reached 129 mm Hg. In the middle target group (<160 mm Hg), the average systolic blood pressure was 131 mm Hg, and in the lowest target group (<140 mm Hg), systolic blood pressure was lowered to an average of 123 mm Hg.
Mean infarct volumes
At 36 hours, the mean adjusted infarct volume was slightly lower in the lowest blood pressure target group (32.4), compared with the other groups (46.4 for the 180 mm Hg group and 50.7 for the under-160 mm Hg group).
“Based on a model or a slope that would be associated with serial lowering of blood pressure targets, we found the point estimate of the effect size was slightly in the direction of benefit of lower blood pressure targets in terms of lower infarct volume,” Dr. Mistry said.
But this was not conclusive. While the point estimate was in the direction of benefit, Dr. Mistry stressed that the trial design doesn’t “definitely rule out” the possibility of harm.
Researchers also measured functional status at 90 days with the modified Rankin Scale (mRS). They found that the utility-weighted mRS was slightly lower in the lowest blood pressure target group (0.507), compared with the higher target groups (0.584 and 0.475, respectively, for the 180 mm Hg and under-160 mm Hg groups).
“The effect size was slightly in the direction of harm,” Dr. Mistry said. “To me, that means there might be safety issues associated with the lower blood pressure target.”
Probably futile
The results suggest that studying this issue further is probably futile. “If lowering blood pressure improves outcomes, that improvement is fairly marginal, and there are trends that suggest that, in fact, it might be harmful,” Dr. Mistry said. Her researcher team “believes it would not be the wisest decision” to pursue this strategy any further in a phase 3 study, she said.
“We wanted to understand whether or not we should spend millions of dollars to do a thousand-patient or two thousand-patient trial, and the answer to that is probably not.”
And there are other therapeutics “we can test that might be more promising than this approach,” she added.
In the meantime, Dr. Mistry stressed that clinicians should be cautious about automatically lowering blood pressure in this patient population and that decisions to target lower levels should be done on an individual basis.
Timely and important
In a comment, Karen Furie, MD, MPH, chair of neurology, Brown University, Providence, R.I., said that the study is “timely and important,” given the uncertainty about management of blood pressure after opening the vessel again using endovascular treatment.
“We already knew that letting the blood pressure go very high after reperfusion was bad, and this study shows that lowering it may also pose a risk, and I think that’s an important message for the community.”
The results send a cautionary message to clinicians but do not provide definitive evidence, she added. “Perhaps in the future we will have a better understanding of what the optimal range is.”
Dr. Furie stressed that this was a small pilot study and conclusions are “guarded.”
“I think the authors didn’t want to overinterpret the results so they ended up concluding that because the final disability might have been worse in the patients who had their blood pressure significantly lowered, recommending that as an approach across the board is sort of discouraged.”
Instead, the authors indicated that there may be factors such as degree of recanalization, size of the infarct, or other patient-specific factors “that would dictate where you target blood pressures,” Dr. Furie said.
The study was funded by the National Institutes of Health/National Institute of Neurological Disorders and Stroke. Mistry receives funding from the Patient-Centered Outcomes Research Institute and compensation from the American Heart Association for editorial activities, and is a consultant for RapidAI. Dr. Furie has declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ISC 2023
STROKE AF at 3 years: High AFib rate after atherosclerotic stroke
In the STROKE AF study, among patients who had a stroke presumably caused by atherosclerosis, the rate of atrial fibrillation (AFib) was almost 22% at 3 years, as detected by continuous monitoring.
The 3-year results from the study were presented by Lee H. Schwamm, MD, of Massachusetts General Hospital, Boston, at the International Stroke Conference presented by the American Stroke Association, a division of the American Heart Association.
Dr. Schwamm said the high rate of AFib detection in this study suggests that continuous monitoring for AFib should be considered for a larger population of stroke patients, rather than just those with cryptogenic stroke.
“We found a much higher rate of AF[ib] than we expected in this population of patients who have had an atherosclerotic stroke,” Dr. Schwamm said in an interview.
“These AF[ib] occurrences were found by a device, so they are known as ‘device-documented AF[ib].’ The patient is not generally aware of symptoms, but 67% of the AF[ib] episodes lasted for more than 1 hour, showing that this is not trivial AF[ib]. This is meaningful AF[ib],” he said.
Dr. Schwamm said the major question is whether these cases of AFib that are detected with a device warrant treatment with anticoagulation. He noted that, in this study, clinicians decided to provide anticoagulation to 70%-80% of patients in whom AFib was detected.
“If we think it deserves treatment, then we have to look for it. And if we care about finding AF[ib], we have no choice but to monitor continuously,” he said.
“If this data doesn’t convince you that AF[ib] is present in this population, I don’t think any data will. Because it is consistent, it accumulates over time and looks remarkably similar to a set of data that we have all become very comfortable with – the CRYSTAL-AF study in patients with cryptogenic stroke,” he stated.
Dr. Schwamm noted that the STROKE AF trial was not based on the cause of the index stroke; rather, it was asking whether there are risk factors that could contribute to the 25% stroke recurrence rate in this population that are not covered in current guidelines.
“I’m really trying to move away from the anchor that I was trained in, which is to figure out the cause of the last stroke to help decide how to prevent the next stroke, towards more of a probabilistic model – of what is all the information I have at my disposal and how do I act on it to prevent the next stroke? We have to start thinking differently about building models for future stroke risk and determining therapy based on that,” he commented.
Changing practice
ISC 2023 program chair Tudor Jovin, MD, Cooper Neurological Institute, Cherry Hill, N.J., and moderator of the session at which the results were presented, discussed the STROKE AF results in a highlights presentation.
“To me as clinician, these results are even more relevant than those at 12 months,” Dr. Jovin said. “The lesson I took is that AF[ib] is even more prevalent than we thought. The burden of AF[ib] is significant in these patients, and it doesn’t seem to be limited to a particular time. These are very thought-provoking results which are going to change clinical practice. I think the threshold for long-term monitoring will be lower.”
Comoderator Lauren Sansing, MD, Yale University, New Haven, Conn., added: “This study shows that the longer we monitor, the more patients with AF[ib] we are likely to pick up. And because in two-thirds of patients with AF[ib], it lasted longer than 1 hour, I do believe this was clinically relevant AF[ib]. The question now is, do we monitor everyone? I think it puts the burden on us to search for AF[ib] in our patients.”
In his presentation, Dr. Schwamm explained that, on the basis of the CRYSTAL-AF study, insertable cardiac monitoring devices are frequently used to identify poststroke AFib in patients with cryptogenic stroke. In the device-monitored arm of that study, AFib was detected in 12.4% of patients over 12 months versus 2.0% in the control arm.
“However, we don’t know how often AF[ib] is detected in other presumed stroke types – largely those due to atherosclerosis,” he said.
He pointed out that, at present, long-term monitoring post stroke for the detection of AFib is not currently recommended for patients with ischemic stroke, owing to presumed small-vessel occlusion or large-artery atherosclerosis.
“In these patients, we are not suspecting AF[ib] because we believe the cause of the stroke was not embolic. But we wanted to investigate what the AF[ib] risk is in these patients, who often have multiple stroke risk factors,” he said.
The trial enrolled 496 patients at 33 centers in the United States. Eligible patients were aged 60 years or older or aged 50-59 years with at least one additional stroke risk factor and had an index stroke that was attributed to large-artery or small-vessel disease. Patients were randomly assigned either to continuous monitoring with the Reveal LINQ device (Medtronic) or to the control arm following site-specific standard of care for AFib detection.
Dr. Schwamm noted that usual care for these patients normally involves monitoring for just a few days while in hospital, but this picks up less than 5% of AFib occurrences.
Baseline characteristics of patients in the STROKE AF study showed that the enrolled population was at high risk for stroke, with a CHADSVASC score of 5. But the index strokes were generally small; the median National Institutes of Health Stroke Scale score was 2.
Results at 12 months, reported 2 years ago, showed a 12.5% incidence of AFib with continuous monitoring versus 1.8% with standard of care (hazard ratio, 7.7; P < .001), rates similar to that found in the CRYSTAL-AF study.
By 3 years, the rate of detected AFib had risen to 21.7% in the continuous monitoring arm versus 2.4% in the control arm (HR, 10.0; P < .001).
“At 12 months, we were seven times more likely to detect AF[ib] with continuous monitoring in these patients, and by 3 years, it was 10 times more likely that AF would be detected with continuous monitoring. I think we’ve settled the question of the best way to find AF[ib] in these patients – it is with an inserted device,” Dr. Schwamm said.
“We have also shown that this is not a transient rise in AFib after the stroke which then diminishes over the next few years. It is a continuous and progressive detection of AF[ib].”
Dr. Schwamm pointed out that 88% of the recorded AFib episodes were asymptomatic. “So relying on patients self-reporting symptoms when deciding who to monitor is unreliable and not a sensible strategy.”
The median time to the first adjudicated AFib episode at 12-month follow-up was 99 days; at the 3-year follow-up, it was 284 days.
“This shows that 30 days of monitoring with an external patch is not sufficient to exclude the presence of AF[ib]. And this really argues for a strategy of immediate insertion of cardiac monitor placement if your goal is to look for AF[ib],” Dr. Schwamm commented.
Is this clinically relevant AFib?
Dr. Schwamm acknowledged that there is a question of whether device-detected AFib should be thought about in the same way as clinically detected AFib with respect to future stroke risk.
He noted that, in this study, 67.4% of patients for whom AFib was detected by continuous monitoring (31 of 46 patients) had at least one episode of AFib that lasted more than 1 hour.
“This is not a trivial little squiggle of something on an EKG which then goes away. This is of significant duration that the cardiologist who adjudicated these rhythm strips felt confident was AF[ib].”
He added: “AF[ib] lasting more than 1 hour crosses the threshold for most practitioners I know to feel confident in treating the patient with anticoagulation. If it was symptomatic AF, this wouldn’t even be a question.”
Dr. Schwamm made the point that device-detected A AFib F has been accepted as worthy of treatment in patients after cryptogenic stroke.
“If we are honest with ourselves and if we have no hesitation in starting anticoagulation in a patient with cryptogenic stroke who has had device-detected AF 6 months later, should we decide that if the patient has had a lacunar stroke, we can ignore that same device-detected fibrillation?”
He put forward the idea that, at some level, all stroke is cryptogenic. “We never know for sure what the cause was. We have hypotheses, we have associations, but we don’t really know. So how much should we weigh that presumptive etiology in terms of how we interpret a rhythm disturbance of fibrillation?”
When looking for predictors of AFib in this study, the investigators found that patients were more likely to have an episode of AFib detected if they had one of the four following risk factors: congestive heart failure, left atrial enlargement, obesity, or QRS prolongation.
“In patients with any one of those four factors, 30% of those had device-detected AF[ib]. These are same predictors of AF[ib] that we are all accustomed to,” Dr. Schwamm said.
Shared decision-making
Dr. Schwamm said in an interview that, in his practice, for these patients, the decision as to whether to use continuous monitoring is made with the patient through shared decision-making.
“We discuss the chance that they could have AF[ib], and I suggest that it might be worth looking for it, but there are factors to be considered. There is a cost to the device, and reimbursement may depend on insurance coverage. Also, some patients may have strong feelings about having the chip implanted in their body.”
He says implanting the chip is easy. “It takes longer to check in at the front desk than to put the device in. It is injected under the skin. It just needs two stitches and a Band-Aid.” The device connects with a smartphone, and the results are interpreted by a cardiologist.
Dr. Schwamm pointed out that the optimal antithrombotic regimen for these patients in whom AFib is detected remains uncertain and should be the focus of future research.
“Do we just stick to antiplatelet therapy or advance to anticoagulation? In moving to an anticoagulant, are we providing less effective prevention for the atherosclerotic stroke risk at the expense of reducing the AF[ib]-related stroke risk? That may be a reasonable trade-off because we know the disability from AF[ib]-associated stroke is much higher.
“Or perhaps the optimal therapy is aspirin plus low-dose anticoagulant? Or left atrial appendage closure and an antiplatelet for patients at a higher risk of bleeding?” he said. “These are the really important questions we need to start asking.”
He added that he hopes a future study will address these questions, but he noted that it would have to be a large study, that it would have to first identify these patients and then randomly assign them to anticoagulation or to no treatment. “That is quite a major undertaking.”
In the highlights presentation, Dr. Jovin said he was uncertain of which of these patients in whom AFib is detected would benefit from anticoagulation. He said he would also like to see a randomized trial on this. But he added: “This would be challenging, as there is the issue of whether there would be equipoise to allow us to randomize to a placebo.”
Dr. Sansing agreed. “I think it would be a hard sell. I would have to think carefully about randomizing a patient to anticoagulation therapy or no therapy who has been found to have AF[ib].”
Dr. Schwamm noted that the current STROKE-AF study was not designed or powered to detect differences in stroke recurrence rates and that there was no difference in stroke recurrence rates between the two arms. There was also no randomization with regard to treatment; choice of medication was left to the discretion of the treating physician.
But he noted that only for 3 of the 34 patients with recurrent stroke in the continuous-monitor arm was AFib detected prior to the recurrent stroke, and only one of those three was receiving anticoagulation at the time of the recurrent stroke.
“These strokes were occurring in patients who did not have device-detected AF[ib],” Dr. Schwamm said. “This is because the population in this study were loaded with stroke risk factors and are at risk of recurrent stroke, but we don’t have the opportunity in this study to really understand the significance of the recurrent strokes.”
The STROKE AF trial was funded by Medtronic. Dr. Schwamm is a consultant to Medtronic.
A version of this article originally appeared on Medscape.com.
In the STROKE AF study, among patients who had a stroke presumably caused by atherosclerosis, the rate of atrial fibrillation (AFib) was almost 22% at 3 years, as detected by continuous monitoring.
The 3-year results from the study were presented by Lee H. Schwamm, MD, of Massachusetts General Hospital, Boston, at the International Stroke Conference presented by the American Stroke Association, a division of the American Heart Association.
Dr. Schwamm said the high rate of AFib detection in this study suggests that continuous monitoring for AFib should be considered for a larger population of stroke patients, rather than just those with cryptogenic stroke.
“We found a much higher rate of AF[ib] than we expected in this population of patients who have had an atherosclerotic stroke,” Dr. Schwamm said in an interview.
“These AF[ib] occurrences were found by a device, so they are known as ‘device-documented AF[ib].’ The patient is not generally aware of symptoms, but 67% of the AF[ib] episodes lasted for more than 1 hour, showing that this is not trivial AF[ib]. This is meaningful AF[ib],” he said.
Dr. Schwamm said the major question is whether these cases of AFib that are detected with a device warrant treatment with anticoagulation. He noted that, in this study, clinicians decided to provide anticoagulation to 70%-80% of patients in whom AFib was detected.
“If we think it deserves treatment, then we have to look for it. And if we care about finding AF[ib], we have no choice but to monitor continuously,” he said.
“If this data doesn’t convince you that AF[ib] is present in this population, I don’t think any data will. Because it is consistent, it accumulates over time and looks remarkably similar to a set of data that we have all become very comfortable with – the CRYSTAL-AF study in patients with cryptogenic stroke,” he stated.
Dr. Schwamm noted that the STROKE AF trial was not based on the cause of the index stroke; rather, it was asking whether there are risk factors that could contribute to the 25% stroke recurrence rate in this population that are not covered in current guidelines.
“I’m really trying to move away from the anchor that I was trained in, which is to figure out the cause of the last stroke to help decide how to prevent the next stroke, towards more of a probabilistic model – of what is all the information I have at my disposal and how do I act on it to prevent the next stroke? We have to start thinking differently about building models for future stroke risk and determining therapy based on that,” he commented.
Changing practice
ISC 2023 program chair Tudor Jovin, MD, Cooper Neurological Institute, Cherry Hill, N.J., and moderator of the session at which the results were presented, discussed the STROKE AF results in a highlights presentation.
“To me as clinician, these results are even more relevant than those at 12 months,” Dr. Jovin said. “The lesson I took is that AF[ib] is even more prevalent than we thought. The burden of AF[ib] is significant in these patients, and it doesn’t seem to be limited to a particular time. These are very thought-provoking results which are going to change clinical practice. I think the threshold for long-term monitoring will be lower.”
Comoderator Lauren Sansing, MD, Yale University, New Haven, Conn., added: “This study shows that the longer we monitor, the more patients with AF[ib] we are likely to pick up. And because in two-thirds of patients with AF[ib], it lasted longer than 1 hour, I do believe this was clinically relevant AF[ib]. The question now is, do we monitor everyone? I think it puts the burden on us to search for AF[ib] in our patients.”
In his presentation, Dr. Schwamm explained that, on the basis of the CRYSTAL-AF study, insertable cardiac monitoring devices are frequently used to identify poststroke AFib in patients with cryptogenic stroke. In the device-monitored arm of that study, AFib was detected in 12.4% of patients over 12 months versus 2.0% in the control arm.
“However, we don’t know how often AF[ib] is detected in other presumed stroke types – largely those due to atherosclerosis,” he said.
He pointed out that, at present, long-term monitoring post stroke for the detection of AFib is not currently recommended for patients with ischemic stroke, owing to presumed small-vessel occlusion or large-artery atherosclerosis.
“In these patients, we are not suspecting AF[ib] because we believe the cause of the stroke was not embolic. But we wanted to investigate what the AF[ib] risk is in these patients, who often have multiple stroke risk factors,” he said.
The trial enrolled 496 patients at 33 centers in the United States. Eligible patients were aged 60 years or older or aged 50-59 years with at least one additional stroke risk factor and had an index stroke that was attributed to large-artery or small-vessel disease. Patients were randomly assigned either to continuous monitoring with the Reveal LINQ device (Medtronic) or to the control arm following site-specific standard of care for AFib detection.
Dr. Schwamm noted that usual care for these patients normally involves monitoring for just a few days while in hospital, but this picks up less than 5% of AFib occurrences.
Baseline characteristics of patients in the STROKE AF study showed that the enrolled population was at high risk for stroke, with a CHADSVASC score of 5. But the index strokes were generally small; the median National Institutes of Health Stroke Scale score was 2.
Results at 12 months, reported 2 years ago, showed a 12.5% incidence of AFib with continuous monitoring versus 1.8% with standard of care (hazard ratio, 7.7; P < .001), rates similar to that found in the CRYSTAL-AF study.
By 3 years, the rate of detected AFib had risen to 21.7% in the continuous monitoring arm versus 2.4% in the control arm (HR, 10.0; P < .001).
“At 12 months, we were seven times more likely to detect AF[ib] with continuous monitoring in these patients, and by 3 years, it was 10 times more likely that AF would be detected with continuous monitoring. I think we’ve settled the question of the best way to find AF[ib] in these patients – it is with an inserted device,” Dr. Schwamm said.
“We have also shown that this is not a transient rise in AFib after the stroke which then diminishes over the next few years. It is a continuous and progressive detection of AF[ib].”
Dr. Schwamm pointed out that 88% of the recorded AFib episodes were asymptomatic. “So relying on patients self-reporting symptoms when deciding who to monitor is unreliable and not a sensible strategy.”
The median time to the first adjudicated AFib episode at 12-month follow-up was 99 days; at the 3-year follow-up, it was 284 days.
“This shows that 30 days of monitoring with an external patch is not sufficient to exclude the presence of AF[ib]. And this really argues for a strategy of immediate insertion of cardiac monitor placement if your goal is to look for AF[ib],” Dr. Schwamm commented.
Is this clinically relevant AFib?
Dr. Schwamm acknowledged that there is a question of whether device-detected AFib should be thought about in the same way as clinically detected AFib with respect to future stroke risk.
He noted that, in this study, 67.4% of patients for whom AFib was detected by continuous monitoring (31 of 46 patients) had at least one episode of AFib that lasted more than 1 hour.
“This is not a trivial little squiggle of something on an EKG which then goes away. This is of significant duration that the cardiologist who adjudicated these rhythm strips felt confident was AF[ib].”
He added: “AF[ib] lasting more than 1 hour crosses the threshold for most practitioners I know to feel confident in treating the patient with anticoagulation. If it was symptomatic AF, this wouldn’t even be a question.”
Dr. Schwamm made the point that device-detected A AFib F has been accepted as worthy of treatment in patients after cryptogenic stroke.
“If we are honest with ourselves and if we have no hesitation in starting anticoagulation in a patient with cryptogenic stroke who has had device-detected AF 6 months later, should we decide that if the patient has had a lacunar stroke, we can ignore that same device-detected fibrillation?”
He put forward the idea that, at some level, all stroke is cryptogenic. “We never know for sure what the cause was. We have hypotheses, we have associations, but we don’t really know. So how much should we weigh that presumptive etiology in terms of how we interpret a rhythm disturbance of fibrillation?”
When looking for predictors of AFib in this study, the investigators found that patients were more likely to have an episode of AFib detected if they had one of the four following risk factors: congestive heart failure, left atrial enlargement, obesity, or QRS prolongation.
“In patients with any one of those four factors, 30% of those had device-detected AF[ib]. These are same predictors of AF[ib] that we are all accustomed to,” Dr. Schwamm said.
Shared decision-making
Dr. Schwamm said in an interview that, in his practice, for these patients, the decision as to whether to use continuous monitoring is made with the patient through shared decision-making.
“We discuss the chance that they could have AF[ib], and I suggest that it might be worth looking for it, but there are factors to be considered. There is a cost to the device, and reimbursement may depend on insurance coverage. Also, some patients may have strong feelings about having the chip implanted in their body.”
He says implanting the chip is easy. “It takes longer to check in at the front desk than to put the device in. It is injected under the skin. It just needs two stitches and a Band-Aid.” The device connects with a smartphone, and the results are interpreted by a cardiologist.
Dr. Schwamm pointed out that the optimal antithrombotic regimen for these patients in whom AFib is detected remains uncertain and should be the focus of future research.
“Do we just stick to antiplatelet therapy or advance to anticoagulation? In moving to an anticoagulant, are we providing less effective prevention for the atherosclerotic stroke risk at the expense of reducing the AF[ib]-related stroke risk? That may be a reasonable trade-off because we know the disability from AF[ib]-associated stroke is much higher.
“Or perhaps the optimal therapy is aspirin plus low-dose anticoagulant? Or left atrial appendage closure and an antiplatelet for patients at a higher risk of bleeding?” he said. “These are the really important questions we need to start asking.”
He added that he hopes a future study will address these questions, but he noted that it would have to be a large study, that it would have to first identify these patients and then randomly assign them to anticoagulation or to no treatment. “That is quite a major undertaking.”
In the highlights presentation, Dr. Jovin said he was uncertain of which of these patients in whom AFib is detected would benefit from anticoagulation. He said he would also like to see a randomized trial on this. But he added: “This would be challenging, as there is the issue of whether there would be equipoise to allow us to randomize to a placebo.”
Dr. Sansing agreed. “I think it would be a hard sell. I would have to think carefully about randomizing a patient to anticoagulation therapy or no therapy who has been found to have AF[ib].”
Dr. Schwamm noted that the current STROKE-AF study was not designed or powered to detect differences in stroke recurrence rates and that there was no difference in stroke recurrence rates between the two arms. There was also no randomization with regard to treatment; choice of medication was left to the discretion of the treating physician.
But he noted that only for 3 of the 34 patients with recurrent stroke in the continuous-monitor arm was AFib detected prior to the recurrent stroke, and only one of those three was receiving anticoagulation at the time of the recurrent stroke.
“These strokes were occurring in patients who did not have device-detected AF[ib],” Dr. Schwamm said. “This is because the population in this study were loaded with stroke risk factors and are at risk of recurrent stroke, but we don’t have the opportunity in this study to really understand the significance of the recurrent strokes.”
The STROKE AF trial was funded by Medtronic. Dr. Schwamm is a consultant to Medtronic.
A version of this article originally appeared on Medscape.com.
In the STROKE AF study, among patients who had a stroke presumably caused by atherosclerosis, the rate of atrial fibrillation (AFib) was almost 22% at 3 years, as detected by continuous monitoring.
The 3-year results from the study were presented by Lee H. Schwamm, MD, of Massachusetts General Hospital, Boston, at the International Stroke Conference presented by the American Stroke Association, a division of the American Heart Association.
Dr. Schwamm said the high rate of AFib detection in this study suggests that continuous monitoring for AFib should be considered for a larger population of stroke patients, rather than just those with cryptogenic stroke.
“We found a much higher rate of AF[ib] than we expected in this population of patients who have had an atherosclerotic stroke,” Dr. Schwamm said in an interview.
“These AF[ib] occurrences were found by a device, so they are known as ‘device-documented AF[ib].’ The patient is not generally aware of symptoms, but 67% of the AF[ib] episodes lasted for more than 1 hour, showing that this is not trivial AF[ib]. This is meaningful AF[ib],” he said.
Dr. Schwamm said the major question is whether these cases of AFib that are detected with a device warrant treatment with anticoagulation. He noted that, in this study, clinicians decided to provide anticoagulation to 70%-80% of patients in whom AFib was detected.
“If we think it deserves treatment, then we have to look for it. And if we care about finding AF[ib], we have no choice but to monitor continuously,” he said.
“If this data doesn’t convince you that AF[ib] is present in this population, I don’t think any data will. Because it is consistent, it accumulates over time and looks remarkably similar to a set of data that we have all become very comfortable with – the CRYSTAL-AF study in patients with cryptogenic stroke,” he stated.
Dr. Schwamm noted that the STROKE AF trial was not based on the cause of the index stroke; rather, it was asking whether there are risk factors that could contribute to the 25% stroke recurrence rate in this population that are not covered in current guidelines.
“I’m really trying to move away from the anchor that I was trained in, which is to figure out the cause of the last stroke to help decide how to prevent the next stroke, towards more of a probabilistic model – of what is all the information I have at my disposal and how do I act on it to prevent the next stroke? We have to start thinking differently about building models for future stroke risk and determining therapy based on that,” he commented.
Changing practice
ISC 2023 program chair Tudor Jovin, MD, Cooper Neurological Institute, Cherry Hill, N.J., and moderator of the session at which the results were presented, discussed the STROKE AF results in a highlights presentation.
“To me as clinician, these results are even more relevant than those at 12 months,” Dr. Jovin said. “The lesson I took is that AF[ib] is even more prevalent than we thought. The burden of AF[ib] is significant in these patients, and it doesn’t seem to be limited to a particular time. These are very thought-provoking results which are going to change clinical practice. I think the threshold for long-term monitoring will be lower.”
Comoderator Lauren Sansing, MD, Yale University, New Haven, Conn., added: “This study shows that the longer we monitor, the more patients with AF[ib] we are likely to pick up. And because in two-thirds of patients with AF[ib], it lasted longer than 1 hour, I do believe this was clinically relevant AF[ib]. The question now is, do we monitor everyone? I think it puts the burden on us to search for AF[ib] in our patients.”
In his presentation, Dr. Schwamm explained that, on the basis of the CRYSTAL-AF study, insertable cardiac monitoring devices are frequently used to identify poststroke AFib in patients with cryptogenic stroke. In the device-monitored arm of that study, AFib was detected in 12.4% of patients over 12 months versus 2.0% in the control arm.
“However, we don’t know how often AF[ib] is detected in other presumed stroke types – largely those due to atherosclerosis,” he said.
He pointed out that, at present, long-term monitoring post stroke for the detection of AFib is not currently recommended for patients with ischemic stroke, owing to presumed small-vessel occlusion or large-artery atherosclerosis.
“In these patients, we are not suspecting AF[ib] because we believe the cause of the stroke was not embolic. But we wanted to investigate what the AF[ib] risk is in these patients, who often have multiple stroke risk factors,” he said.
The trial enrolled 496 patients at 33 centers in the United States. Eligible patients were aged 60 years or older or aged 50-59 years with at least one additional stroke risk factor and had an index stroke that was attributed to large-artery or small-vessel disease. Patients were randomly assigned either to continuous monitoring with the Reveal LINQ device (Medtronic) or to the control arm following site-specific standard of care for AFib detection.
Dr. Schwamm noted that usual care for these patients normally involves monitoring for just a few days while in hospital, but this picks up less than 5% of AFib occurrences.
Baseline characteristics of patients in the STROKE AF study showed that the enrolled population was at high risk for stroke, with a CHADSVASC score of 5. But the index strokes were generally small; the median National Institutes of Health Stroke Scale score was 2.
Results at 12 months, reported 2 years ago, showed a 12.5% incidence of AFib with continuous monitoring versus 1.8% with standard of care (hazard ratio, 7.7; P < .001), rates similar to that found in the CRYSTAL-AF study.
By 3 years, the rate of detected AFib had risen to 21.7% in the continuous monitoring arm versus 2.4% in the control arm (HR, 10.0; P < .001).
“At 12 months, we were seven times more likely to detect AF[ib] with continuous monitoring in these patients, and by 3 years, it was 10 times more likely that AF would be detected with continuous monitoring. I think we’ve settled the question of the best way to find AF[ib] in these patients – it is with an inserted device,” Dr. Schwamm said.
“We have also shown that this is not a transient rise in AFib after the stroke which then diminishes over the next few years. It is a continuous and progressive detection of AF[ib].”
Dr. Schwamm pointed out that 88% of the recorded AFib episodes were asymptomatic. “So relying on patients self-reporting symptoms when deciding who to monitor is unreliable and not a sensible strategy.”
The median time to the first adjudicated AFib episode at 12-month follow-up was 99 days; at the 3-year follow-up, it was 284 days.
“This shows that 30 days of monitoring with an external patch is not sufficient to exclude the presence of AF[ib]. And this really argues for a strategy of immediate insertion of cardiac monitor placement if your goal is to look for AF[ib],” Dr. Schwamm commented.
Is this clinically relevant AFib?
Dr. Schwamm acknowledged that there is a question of whether device-detected AFib should be thought about in the same way as clinically detected AFib with respect to future stroke risk.
He noted that, in this study, 67.4% of patients for whom AFib was detected by continuous monitoring (31 of 46 patients) had at least one episode of AFib that lasted more than 1 hour.
“This is not a trivial little squiggle of something on an EKG which then goes away. This is of significant duration that the cardiologist who adjudicated these rhythm strips felt confident was AF[ib].”
He added: “AF[ib] lasting more than 1 hour crosses the threshold for most practitioners I know to feel confident in treating the patient with anticoagulation. If it was symptomatic AF, this wouldn’t even be a question.”
Dr. Schwamm made the point that device-detected A AFib F has been accepted as worthy of treatment in patients after cryptogenic stroke.
“If we are honest with ourselves and if we have no hesitation in starting anticoagulation in a patient with cryptogenic stroke who has had device-detected AF 6 months later, should we decide that if the patient has had a lacunar stroke, we can ignore that same device-detected fibrillation?”
He put forward the idea that, at some level, all stroke is cryptogenic. “We never know for sure what the cause was. We have hypotheses, we have associations, but we don’t really know. So how much should we weigh that presumptive etiology in terms of how we interpret a rhythm disturbance of fibrillation?”
When looking for predictors of AFib in this study, the investigators found that patients were more likely to have an episode of AFib detected if they had one of the four following risk factors: congestive heart failure, left atrial enlargement, obesity, or QRS prolongation.
“In patients with any one of those four factors, 30% of those had device-detected AF[ib]. These are same predictors of AF[ib] that we are all accustomed to,” Dr. Schwamm said.
Shared decision-making
Dr. Schwamm said in an interview that, in his practice, for these patients, the decision as to whether to use continuous monitoring is made with the patient through shared decision-making.
“We discuss the chance that they could have AF[ib], and I suggest that it might be worth looking for it, but there are factors to be considered. There is a cost to the device, and reimbursement may depend on insurance coverage. Also, some patients may have strong feelings about having the chip implanted in their body.”
He says implanting the chip is easy. “It takes longer to check in at the front desk than to put the device in. It is injected under the skin. It just needs two stitches and a Band-Aid.” The device connects with a smartphone, and the results are interpreted by a cardiologist.
Dr. Schwamm pointed out that the optimal antithrombotic regimen for these patients in whom AFib is detected remains uncertain and should be the focus of future research.
“Do we just stick to antiplatelet therapy or advance to anticoagulation? In moving to an anticoagulant, are we providing less effective prevention for the atherosclerotic stroke risk at the expense of reducing the AF[ib]-related stroke risk? That may be a reasonable trade-off because we know the disability from AF[ib]-associated stroke is much higher.
“Or perhaps the optimal therapy is aspirin plus low-dose anticoagulant? Or left atrial appendage closure and an antiplatelet for patients at a higher risk of bleeding?” he said. “These are the really important questions we need to start asking.”
He added that he hopes a future study will address these questions, but he noted that it would have to be a large study, that it would have to first identify these patients and then randomly assign them to anticoagulation or to no treatment. “That is quite a major undertaking.”
In the highlights presentation, Dr. Jovin said he was uncertain of which of these patients in whom AFib is detected would benefit from anticoagulation. He said he would also like to see a randomized trial on this. But he added: “This would be challenging, as there is the issue of whether there would be equipoise to allow us to randomize to a placebo.”
Dr. Sansing agreed. “I think it would be a hard sell. I would have to think carefully about randomizing a patient to anticoagulation therapy or no therapy who has been found to have AF[ib].”
Dr. Schwamm noted that the current STROKE-AF study was not designed or powered to detect differences in stroke recurrence rates and that there was no difference in stroke recurrence rates between the two arms. There was also no randomization with regard to treatment; choice of medication was left to the discretion of the treating physician.
But he noted that only for 3 of the 34 patients with recurrent stroke in the continuous-monitor arm was AFib detected prior to the recurrent stroke, and only one of those three was receiving anticoagulation at the time of the recurrent stroke.
“These strokes were occurring in patients who did not have device-detected AF[ib],” Dr. Schwamm said. “This is because the population in this study were loaded with stroke risk factors and are at risk of recurrent stroke, but we don’t have the opportunity in this study to really understand the significance of the recurrent strokes.”
The STROKE AF trial was funded by Medtronic. Dr. Schwamm is a consultant to Medtronic.
A version of this article originally appeared on Medscape.com.
FROM ISC 2023
Novel neuroprotective agent promising in stroke
preliminary results of a first-in-human study show.
The findings illustrate that it is possible to improve outcomes for stroke patients “not only with reperfusion therapy but with neuroprotectants,” study author Macarena Hernandez, PhD, associate professor, University Complutense, Madrid, told this news organization.
Dr. Hernandez said she hopes these positive results will spur investigation into other neuroprotective agents.
The findings were presented at the International Stroke Conference presented by the American Stroke Association, a division of the American Heart Association.
Best doses
The study investigated ApTOLL, which blocks the TOLL-like receptor 4 (TLR4) that induces inflammation after a stroke. Previous studies found that ApTOLL protected brain tissue in animal models of stroke.
The phase 1B part of the study found no safety issues and determined the best two doses to be used in phase 2A were 0.05 mg/kg and 0.2 mg/kg.
The analysis included 139 patients at 14 centers in Spain and France (mean age, about 70 years; 42% women) who had a large-vessel occlusion and were eligible for endovascular therapy.
“Our aim was to have a very homogeneous population” to try to replicate in humans what had worked in animals, another study author, Marc Ribó, MD, interventional neurologist, Hospital Vall d’Hebron, Barcelona, told this news organization.
Study participants had an Alberta Stroke Program Early CT Score (ASPECTS) of 5-10, and estimated infarct core volume on CT-perfusion was 5-70 mL. All were treated within 6 hours of stroke onset.
Researchers randomly assigned patients to receive the low dose of the drug, the high dose of the drug, or placebo. The drug was administered intravenously over a 30-minute period just prior to the groin puncture for the thrombectomy procedure.
“So, the drug had already started to work when they underwent the usual standard practice, the thrombectomy,” said Dr. Ribó.
Those who were eligible also received tissue plasminogen activator.
The primary endpoint was safety, including death, symptomatic intracranial hemorrhage (SICH), and recurrent stroke.
Lower mortality
At 90 days, there was a statistically significant lower mortality rate in the high-dose group, compared with the group that received placebo (4.76% vs. 18.18%).
The mortality rate was 26.19% in the low-dose group, but Dr. Ribó stressed that this dose was a quarter of the higher dose and so performed “much more like placebo.”
The higher dose also yielded a better SICH outcome (4.76% of patients vs. 7.27% for placebo and 7.14% for the lower dose). And it was superior in terms of brain edema (2.4% of the population vs. 7.3% for the placebo and 4.8% for the low-dose groups).
About 7.1% of the high-dose group, 3.7% of the placebo group, and 4.8% of the low-dose group had a recurrent transient ischemic attack or stroke.
A secondary efficacy endpoint was infarct volume on MRI at 72 hours. Here, for the higher-dose group, mean infarct volume was reduced, compared with the patients who received placebo (–29.31 cc; 90% confidence interval, –49.28 to –9.34).
This higher dose was also superior for the secondary outcome of National Institutes of Health Stroke Scale score at 72 hours and for the disability outcome on the modified Rankin Score (mRS).
Clear shift in disability
“There was a clear shift toward less disability across levels of the mRS score in the high-dose group at 90 days,” said Dr. Ribó.
He added that he and his colleagues are “very happy” with these results, as they reflect “a consistency” of outcomes.
“We observed that the infarct volumes were lower in the high-dose group, and that led to a significant lower NIH score, meaning less clinical neurological symptoms at 72 hours, and finally, this led to less disability at 90 days.”
These results are “very exciting,” Dr. Hernandez added. “This is the first neuroprotectant that has demonstrated this acute effect in reducing deaths, in reducing the infarct volume and improving functionality long-term in patients treated with the higher dose.”
Dr. Ribó noted the treatment would eventually be used in addition to reperfusion therapy. “It’s not competing with reperfusion treatment; it’s an additional layer” of treatment.
Although it would initially be offered only to patients eligible for thrombectomy, researchers will explore the drug’s effectiveness for other stroke patients, said Dr. Ribó. “We wanted to secure this indication, and from there, progressively expand to other profiles of stroke patients, and even to patients with intracranial hemorrhage.”
The study confirmed the safety of the drug. “There were no safety issues at all,” said Dr. Ribó. “We were initially concerned that an anti-inflammatory in these patients could lead to higher rates of infections, but this was absolutely not the case.”
The next step is to confirm the effects in a larger, multicenter study, which is planned to launch at the end of this year, said Dr. Hernandez.
‘Very robust results’
In a comment, Philip B. Gorelick, MD, professor of neurology, Northwestern University, Chicago, said that, while this was a small early-phase study, the results are “very robust.”
“The authors demonstrated proof of a neuroprotective effect; they showed at 90 days that the death rates were substantially reduced by about four times – 4% vs. 18% – and the size of the damaged tissue at about 72 hours was reduced by 40%,” said Dr. Gorelick, who did not participate in the study.
He also noted that the disability was “less pronounced” at 90 days in the 0.2 mg/kg group.
“So overall, these are very encouraging results,” said Dr. Gorelick. “We have had a lot of difficulty finding neuroprotectant drugs that work, and this drug, in combination with endovascular therapy, seems to be very promising.”
However, he stressed the drug “is not ready for prime-time practice.”
“The proof in the pudding will be in the large-scale main phase 3 trials,” he added.
The study was funded by aptaTargets. Dr. Hernandez is chief scientific officer at aptaTargets. Dr. Ribó is an adviser at AptaTargets; a consultant at Medtronic; has ownership interest in Anaconda and NoraHealth; is a consultant for Cerenovus and Philips; and has stock options at Methink. Dr. Gorelick has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
preliminary results of a first-in-human study show.
The findings illustrate that it is possible to improve outcomes for stroke patients “not only with reperfusion therapy but with neuroprotectants,” study author Macarena Hernandez, PhD, associate professor, University Complutense, Madrid, told this news organization.
Dr. Hernandez said she hopes these positive results will spur investigation into other neuroprotective agents.
The findings were presented at the International Stroke Conference presented by the American Stroke Association, a division of the American Heart Association.
Best doses
The study investigated ApTOLL, which blocks the TOLL-like receptor 4 (TLR4) that induces inflammation after a stroke. Previous studies found that ApTOLL protected brain tissue in animal models of stroke.
The phase 1B part of the study found no safety issues and determined the best two doses to be used in phase 2A were 0.05 mg/kg and 0.2 mg/kg.
The analysis included 139 patients at 14 centers in Spain and France (mean age, about 70 years; 42% women) who had a large-vessel occlusion and were eligible for endovascular therapy.
“Our aim was to have a very homogeneous population” to try to replicate in humans what had worked in animals, another study author, Marc Ribó, MD, interventional neurologist, Hospital Vall d’Hebron, Barcelona, told this news organization.
Study participants had an Alberta Stroke Program Early CT Score (ASPECTS) of 5-10, and estimated infarct core volume on CT-perfusion was 5-70 mL. All were treated within 6 hours of stroke onset.
Researchers randomly assigned patients to receive the low dose of the drug, the high dose of the drug, or placebo. The drug was administered intravenously over a 30-minute period just prior to the groin puncture for the thrombectomy procedure.
“So, the drug had already started to work when they underwent the usual standard practice, the thrombectomy,” said Dr. Ribó.
Those who were eligible also received tissue plasminogen activator.
The primary endpoint was safety, including death, symptomatic intracranial hemorrhage (SICH), and recurrent stroke.
Lower mortality
At 90 days, there was a statistically significant lower mortality rate in the high-dose group, compared with the group that received placebo (4.76% vs. 18.18%).
The mortality rate was 26.19% in the low-dose group, but Dr. Ribó stressed that this dose was a quarter of the higher dose and so performed “much more like placebo.”
The higher dose also yielded a better SICH outcome (4.76% of patients vs. 7.27% for placebo and 7.14% for the lower dose). And it was superior in terms of brain edema (2.4% of the population vs. 7.3% for the placebo and 4.8% for the low-dose groups).
About 7.1% of the high-dose group, 3.7% of the placebo group, and 4.8% of the low-dose group had a recurrent transient ischemic attack or stroke.
A secondary efficacy endpoint was infarct volume on MRI at 72 hours. Here, for the higher-dose group, mean infarct volume was reduced, compared with the patients who received placebo (–29.31 cc; 90% confidence interval, –49.28 to –9.34).
This higher dose was also superior for the secondary outcome of National Institutes of Health Stroke Scale score at 72 hours and for the disability outcome on the modified Rankin Score (mRS).
Clear shift in disability
“There was a clear shift toward less disability across levels of the mRS score in the high-dose group at 90 days,” said Dr. Ribó.
He added that he and his colleagues are “very happy” with these results, as they reflect “a consistency” of outcomes.
“We observed that the infarct volumes were lower in the high-dose group, and that led to a significant lower NIH score, meaning less clinical neurological symptoms at 72 hours, and finally, this led to less disability at 90 days.”
These results are “very exciting,” Dr. Hernandez added. “This is the first neuroprotectant that has demonstrated this acute effect in reducing deaths, in reducing the infarct volume and improving functionality long-term in patients treated with the higher dose.”
Dr. Ribó noted the treatment would eventually be used in addition to reperfusion therapy. “It’s not competing with reperfusion treatment; it’s an additional layer” of treatment.
Although it would initially be offered only to patients eligible for thrombectomy, researchers will explore the drug’s effectiveness for other stroke patients, said Dr. Ribó. “We wanted to secure this indication, and from there, progressively expand to other profiles of stroke patients, and even to patients with intracranial hemorrhage.”
The study confirmed the safety of the drug. “There were no safety issues at all,” said Dr. Ribó. “We were initially concerned that an anti-inflammatory in these patients could lead to higher rates of infections, but this was absolutely not the case.”
The next step is to confirm the effects in a larger, multicenter study, which is planned to launch at the end of this year, said Dr. Hernandez.
‘Very robust results’
In a comment, Philip B. Gorelick, MD, professor of neurology, Northwestern University, Chicago, said that, while this was a small early-phase study, the results are “very robust.”
“The authors demonstrated proof of a neuroprotective effect; they showed at 90 days that the death rates were substantially reduced by about four times – 4% vs. 18% – and the size of the damaged tissue at about 72 hours was reduced by 40%,” said Dr. Gorelick, who did not participate in the study.
He also noted that the disability was “less pronounced” at 90 days in the 0.2 mg/kg group.
“So overall, these are very encouraging results,” said Dr. Gorelick. “We have had a lot of difficulty finding neuroprotectant drugs that work, and this drug, in combination with endovascular therapy, seems to be very promising.”
However, he stressed the drug “is not ready for prime-time practice.”
“The proof in the pudding will be in the large-scale main phase 3 trials,” he added.
The study was funded by aptaTargets. Dr. Hernandez is chief scientific officer at aptaTargets. Dr. Ribó is an adviser at AptaTargets; a consultant at Medtronic; has ownership interest in Anaconda and NoraHealth; is a consultant for Cerenovus and Philips; and has stock options at Methink. Dr. Gorelick has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
preliminary results of a first-in-human study show.
The findings illustrate that it is possible to improve outcomes for stroke patients “not only with reperfusion therapy but with neuroprotectants,” study author Macarena Hernandez, PhD, associate professor, University Complutense, Madrid, told this news organization.
Dr. Hernandez said she hopes these positive results will spur investigation into other neuroprotective agents.
The findings were presented at the International Stroke Conference presented by the American Stroke Association, a division of the American Heart Association.
Best doses
The study investigated ApTOLL, which blocks the TOLL-like receptor 4 (TLR4) that induces inflammation after a stroke. Previous studies found that ApTOLL protected brain tissue in animal models of stroke.
The phase 1B part of the study found no safety issues and determined the best two doses to be used in phase 2A were 0.05 mg/kg and 0.2 mg/kg.
The analysis included 139 patients at 14 centers in Spain and France (mean age, about 70 years; 42% women) who had a large-vessel occlusion and were eligible for endovascular therapy.
“Our aim was to have a very homogeneous population” to try to replicate in humans what had worked in animals, another study author, Marc Ribó, MD, interventional neurologist, Hospital Vall d’Hebron, Barcelona, told this news organization.
Study participants had an Alberta Stroke Program Early CT Score (ASPECTS) of 5-10, and estimated infarct core volume on CT-perfusion was 5-70 mL. All were treated within 6 hours of stroke onset.
Researchers randomly assigned patients to receive the low dose of the drug, the high dose of the drug, or placebo. The drug was administered intravenously over a 30-minute period just prior to the groin puncture for the thrombectomy procedure.
“So, the drug had already started to work when they underwent the usual standard practice, the thrombectomy,” said Dr. Ribó.
Those who were eligible also received tissue plasminogen activator.
The primary endpoint was safety, including death, symptomatic intracranial hemorrhage (SICH), and recurrent stroke.
Lower mortality
At 90 days, there was a statistically significant lower mortality rate in the high-dose group, compared with the group that received placebo (4.76% vs. 18.18%).
The mortality rate was 26.19% in the low-dose group, but Dr. Ribó stressed that this dose was a quarter of the higher dose and so performed “much more like placebo.”
The higher dose also yielded a better SICH outcome (4.76% of patients vs. 7.27% for placebo and 7.14% for the lower dose). And it was superior in terms of brain edema (2.4% of the population vs. 7.3% for the placebo and 4.8% for the low-dose groups).
About 7.1% of the high-dose group, 3.7% of the placebo group, and 4.8% of the low-dose group had a recurrent transient ischemic attack or stroke.
A secondary efficacy endpoint was infarct volume on MRI at 72 hours. Here, for the higher-dose group, mean infarct volume was reduced, compared with the patients who received placebo (–29.31 cc; 90% confidence interval, –49.28 to –9.34).
This higher dose was also superior for the secondary outcome of National Institutes of Health Stroke Scale score at 72 hours and for the disability outcome on the modified Rankin Score (mRS).
Clear shift in disability
“There was a clear shift toward less disability across levels of the mRS score in the high-dose group at 90 days,” said Dr. Ribó.
He added that he and his colleagues are “very happy” with these results, as they reflect “a consistency” of outcomes.
“We observed that the infarct volumes were lower in the high-dose group, and that led to a significant lower NIH score, meaning less clinical neurological symptoms at 72 hours, and finally, this led to less disability at 90 days.”
These results are “very exciting,” Dr. Hernandez added. “This is the first neuroprotectant that has demonstrated this acute effect in reducing deaths, in reducing the infarct volume and improving functionality long-term in patients treated with the higher dose.”
Dr. Ribó noted the treatment would eventually be used in addition to reperfusion therapy. “It’s not competing with reperfusion treatment; it’s an additional layer” of treatment.
Although it would initially be offered only to patients eligible for thrombectomy, researchers will explore the drug’s effectiveness for other stroke patients, said Dr. Ribó. “We wanted to secure this indication, and from there, progressively expand to other profiles of stroke patients, and even to patients with intracranial hemorrhage.”
The study confirmed the safety of the drug. “There were no safety issues at all,” said Dr. Ribó. “We were initially concerned that an anti-inflammatory in these patients could lead to higher rates of infections, but this was absolutely not the case.”
The next step is to confirm the effects in a larger, multicenter study, which is planned to launch at the end of this year, said Dr. Hernandez.
‘Very robust results’
In a comment, Philip B. Gorelick, MD, professor of neurology, Northwestern University, Chicago, said that, while this was a small early-phase study, the results are “very robust.”
“The authors demonstrated proof of a neuroprotective effect; they showed at 90 days that the death rates were substantially reduced by about four times – 4% vs. 18% – and the size of the damaged tissue at about 72 hours was reduced by 40%,” said Dr. Gorelick, who did not participate in the study.
He also noted that the disability was “less pronounced” at 90 days in the 0.2 mg/kg group.
“So overall, these are very encouraging results,” said Dr. Gorelick. “We have had a lot of difficulty finding neuroprotectant drugs that work, and this drug, in combination with endovascular therapy, seems to be very promising.”
However, he stressed the drug “is not ready for prime-time practice.”
“The proof in the pudding will be in the large-scale main phase 3 trials,” he added.
The study was funded by aptaTargets. Dr. Hernandez is chief scientific officer at aptaTargets. Dr. Ribó is an adviser at AptaTargets; a consultant at Medtronic; has ownership interest in Anaconda and NoraHealth; is a consultant for Cerenovus and Philips; and has stock options at Methink. Dr. Gorelick has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ISC 2023
Canadian guidance recommends reducing alcohol consumption
“Drinking less is better,” says the guidance, which replaces Canada’s 2011 Low-Risk Drinking Guidelines (LRDGs).
Developed in consultation with an executive committee from federal, provincial, and territorial governments; national organizations; three scientific expert panels; and an internal evidence review working group, the guidance presents the following findings:
- Consuming no drinks per week has benefits, such as better health and better sleep, and it’s the only safe option during pregnancy.
- Consuming one or two standard drinks weekly will likely not have alcohol-related consequences.
- Three to six drinks raise the risk of developing breast, colon, and other cancers.
- Seven or more increase the risk of heart disease or stroke.
- Each additional drink “radically increases” the risk of these health consequences.
“Alcohol is more harmful than was previously thought and is a key component of the health of your patients,” Adam Sherk, PhD, a scientist at the Canadian Institute for Substance Use Research at the University of Victoria (B.C.), and a member of the scientific expert panel that contributed to the guidance, said in an interview. “Display and discuss the new guidance with your patients with the main message that drinking less is better.”
Peter Butt, MD, a clinical associate professor at the University of Saskatchewan, Saskatoon, and cochair of the guidance project, said in an interview: “The World Health Organization has identified over 200 ICD-coded conditions associated with alcohol use. This creates many opportunities to inquire into quantity and frequency of alcohol use, relate it to the patient’s health and well-being, and provide advice on reduction.”
“Canada’s Guidance on Alcohol and Health: Final Report” and a related infographic were published online Jan. 17.
Continuum of risk
The impetus for the new guidance came from the fact that “our 2011 LRDGs were no longer current, and there was emerging evidence that people drinking within those levels were coming to harm,” said Dr. Butt.
That evidence indicates that alcohol causes at least seven types of cancer, mostly of the breast or colon; is a risk factor for most types of heart disease; and is a main cause of liver disease. Evidence also indicates that avoiding drinking to the point of intoxication will reduce people’s risk of perpetrating alcohol-related violence.
Responding to the need to accurately quantify the risk, the guidance defines a “standard” drink as 12 oz of beer, cooler, or cider (5% alcohol); 5 oz of wine (12% alcohol); and 1.5 oz of spirits such as whiskey, vodka, or gin (40% alcohol).
Using different mortality risk thresholds, the project’s experts developed the following continuum of risk:
- Low for individuals who consume two standard drinks or fewer per week
- Moderate for those who consume from three to six standard drinks per week
- Increasingly high for those who consume seven standard drinks or more per week
The guidance makes the following observations:
- Consuming more than two standard drinks per drinking occasion is associated with an increased risk of harms to self and others, including injuries and violence.
- When pregnant or trying to get pregnant, no amount of alcohol is safe.
- When breastfeeding, not drinking is safest.
- Above the upper limit of the moderate risk zone, health risks increase more steeply for females than males.
- Far more injuries, violence, and deaths result from men’s alcohol use, especially for per occasion drinking, than from women’s alcohol use.
- Young people should delay alcohol use for as long as possible.
- Individuals should not start to use alcohol or increase their alcohol use for health benefits.
- Any reduction in alcohol use is beneficial.
Other national guidelines
“Countries that haven’t updated their alcohol use guidelines recently should do so, as the evidence regarding alcohol and health has advanced considerably in the past 10 years,” said Dr. Sherk. He acknowledged that “any time health guidance changes substantially, it’s reasonable to expect a period of readjustment.”
“Some will be resistant,” Dr. Butt agreed. “Some professionals will need more education than others on the health effects of alcohol. Some patients will also be more invested in drinking than others. The harm-reduction, risk-zone approach should assist in the process of engaging patients and helping them reduce over time.
“Just as we benefited from the updates done in the United Kingdom, France, and especially Australia, so also researchers elsewhere will critique our work and our approach and make their own decisions on how best to communicate with their public,” Dr. Butt said. He noted that Canada’s contributions regarding the association between alcohol and violence, as well as their sex/gender approach to the evidence, “may influence the next country’s review.”
Commenting on whether the United States should consider changing its guidance, Timothy Brennan, MD, MPH, chief of clinical services for the Addiction Institute of Mount Sinai Health System in New York, said in an interview, “A lot of people will be surprised at the recommended limits on alcohol. Most think that they can have one or two glasses of alcohol per day and not have any increased risk to their health. I think the Canadians deserve credit for putting themselves out there.”
Dr. Brennan said there will “certainly be pushback by the drinking lobby, which is very strong both in the U.S. and in Canada.” In fact, the national trade group Beer Canada was recently quoted as stating that it still supports the 2011 guidelines and that the updating process lacked full transparency and expert technical peer review.
Nevertheless, Dr. Brennan said, “it’s overwhelmingly clear that alcohol affects a ton of different parts of our body, so limiting the amount of alcohol we take in is always going to be a good thing. The Canadian graphic is great because it color-codes the risk. I recommend that clinicians put it up in their offices and begin quantifying the units of alcohol that are going into a patient’s body each day.”
A version of this article originally appeared on Medscape.com.
“Drinking less is better,” says the guidance, which replaces Canada’s 2011 Low-Risk Drinking Guidelines (LRDGs).
Developed in consultation with an executive committee from federal, provincial, and territorial governments; national organizations; three scientific expert panels; and an internal evidence review working group, the guidance presents the following findings:
- Consuming no drinks per week has benefits, such as better health and better sleep, and it’s the only safe option during pregnancy.
- Consuming one or two standard drinks weekly will likely not have alcohol-related consequences.
- Three to six drinks raise the risk of developing breast, colon, and other cancers.
- Seven or more increase the risk of heart disease or stroke.
- Each additional drink “radically increases” the risk of these health consequences.
“Alcohol is more harmful than was previously thought and is a key component of the health of your patients,” Adam Sherk, PhD, a scientist at the Canadian Institute for Substance Use Research at the University of Victoria (B.C.), and a member of the scientific expert panel that contributed to the guidance, said in an interview. “Display and discuss the new guidance with your patients with the main message that drinking less is better.”
Peter Butt, MD, a clinical associate professor at the University of Saskatchewan, Saskatoon, and cochair of the guidance project, said in an interview: “The World Health Organization has identified over 200 ICD-coded conditions associated with alcohol use. This creates many opportunities to inquire into quantity and frequency of alcohol use, relate it to the patient’s health and well-being, and provide advice on reduction.”
“Canada’s Guidance on Alcohol and Health: Final Report” and a related infographic were published online Jan. 17.
Continuum of risk
The impetus for the new guidance came from the fact that “our 2011 LRDGs were no longer current, and there was emerging evidence that people drinking within those levels were coming to harm,” said Dr. Butt.
That evidence indicates that alcohol causes at least seven types of cancer, mostly of the breast or colon; is a risk factor for most types of heart disease; and is a main cause of liver disease. Evidence also indicates that avoiding drinking to the point of intoxication will reduce people’s risk of perpetrating alcohol-related violence.
Responding to the need to accurately quantify the risk, the guidance defines a “standard” drink as 12 oz of beer, cooler, or cider (5% alcohol); 5 oz of wine (12% alcohol); and 1.5 oz of spirits such as whiskey, vodka, or gin (40% alcohol).
Using different mortality risk thresholds, the project’s experts developed the following continuum of risk:
- Low for individuals who consume two standard drinks or fewer per week
- Moderate for those who consume from three to six standard drinks per week
- Increasingly high for those who consume seven standard drinks or more per week
The guidance makes the following observations:
- Consuming more than two standard drinks per drinking occasion is associated with an increased risk of harms to self and others, including injuries and violence.
- When pregnant or trying to get pregnant, no amount of alcohol is safe.
- When breastfeeding, not drinking is safest.
- Above the upper limit of the moderate risk zone, health risks increase more steeply for females than males.
- Far more injuries, violence, and deaths result from men’s alcohol use, especially for per occasion drinking, than from women’s alcohol use.
- Young people should delay alcohol use for as long as possible.
- Individuals should not start to use alcohol or increase their alcohol use for health benefits.
- Any reduction in alcohol use is beneficial.
Other national guidelines
“Countries that haven’t updated their alcohol use guidelines recently should do so, as the evidence regarding alcohol and health has advanced considerably in the past 10 years,” said Dr. Sherk. He acknowledged that “any time health guidance changes substantially, it’s reasonable to expect a period of readjustment.”
“Some will be resistant,” Dr. Butt agreed. “Some professionals will need more education than others on the health effects of alcohol. Some patients will also be more invested in drinking than others. The harm-reduction, risk-zone approach should assist in the process of engaging patients and helping them reduce over time.
“Just as we benefited from the updates done in the United Kingdom, France, and especially Australia, so also researchers elsewhere will critique our work and our approach and make their own decisions on how best to communicate with their public,” Dr. Butt said. He noted that Canada’s contributions regarding the association between alcohol and violence, as well as their sex/gender approach to the evidence, “may influence the next country’s review.”
Commenting on whether the United States should consider changing its guidance, Timothy Brennan, MD, MPH, chief of clinical services for the Addiction Institute of Mount Sinai Health System in New York, said in an interview, “A lot of people will be surprised at the recommended limits on alcohol. Most think that they can have one or two glasses of alcohol per day and not have any increased risk to their health. I think the Canadians deserve credit for putting themselves out there.”
Dr. Brennan said there will “certainly be pushback by the drinking lobby, which is very strong both in the U.S. and in Canada.” In fact, the national trade group Beer Canada was recently quoted as stating that it still supports the 2011 guidelines and that the updating process lacked full transparency and expert technical peer review.
Nevertheless, Dr. Brennan said, “it’s overwhelmingly clear that alcohol affects a ton of different parts of our body, so limiting the amount of alcohol we take in is always going to be a good thing. The Canadian graphic is great because it color-codes the risk. I recommend that clinicians put it up in their offices and begin quantifying the units of alcohol that are going into a patient’s body each day.”
A version of this article originally appeared on Medscape.com.
“Drinking less is better,” says the guidance, which replaces Canada’s 2011 Low-Risk Drinking Guidelines (LRDGs).
Developed in consultation with an executive committee from federal, provincial, and territorial governments; national organizations; three scientific expert panels; and an internal evidence review working group, the guidance presents the following findings:
- Consuming no drinks per week has benefits, such as better health and better sleep, and it’s the only safe option during pregnancy.
- Consuming one or two standard drinks weekly will likely not have alcohol-related consequences.
- Three to six drinks raise the risk of developing breast, colon, and other cancers.
- Seven or more increase the risk of heart disease or stroke.
- Each additional drink “radically increases” the risk of these health consequences.
“Alcohol is more harmful than was previously thought and is a key component of the health of your patients,” Adam Sherk, PhD, a scientist at the Canadian Institute for Substance Use Research at the University of Victoria (B.C.), and a member of the scientific expert panel that contributed to the guidance, said in an interview. “Display and discuss the new guidance with your patients with the main message that drinking less is better.”
Peter Butt, MD, a clinical associate professor at the University of Saskatchewan, Saskatoon, and cochair of the guidance project, said in an interview: “The World Health Organization has identified over 200 ICD-coded conditions associated with alcohol use. This creates many opportunities to inquire into quantity and frequency of alcohol use, relate it to the patient’s health and well-being, and provide advice on reduction.”
“Canada’s Guidance on Alcohol and Health: Final Report” and a related infographic were published online Jan. 17.
Continuum of risk
The impetus for the new guidance came from the fact that “our 2011 LRDGs were no longer current, and there was emerging evidence that people drinking within those levels were coming to harm,” said Dr. Butt.
That evidence indicates that alcohol causes at least seven types of cancer, mostly of the breast or colon; is a risk factor for most types of heart disease; and is a main cause of liver disease. Evidence also indicates that avoiding drinking to the point of intoxication will reduce people’s risk of perpetrating alcohol-related violence.
Responding to the need to accurately quantify the risk, the guidance defines a “standard” drink as 12 oz of beer, cooler, or cider (5% alcohol); 5 oz of wine (12% alcohol); and 1.5 oz of spirits such as whiskey, vodka, or gin (40% alcohol).
Using different mortality risk thresholds, the project’s experts developed the following continuum of risk:
- Low for individuals who consume two standard drinks or fewer per week
- Moderate for those who consume from three to six standard drinks per week
- Increasingly high for those who consume seven standard drinks or more per week
The guidance makes the following observations:
- Consuming more than two standard drinks per drinking occasion is associated with an increased risk of harms to self and others, including injuries and violence.
- When pregnant or trying to get pregnant, no amount of alcohol is safe.
- When breastfeeding, not drinking is safest.
- Above the upper limit of the moderate risk zone, health risks increase more steeply for females than males.
- Far more injuries, violence, and deaths result from men’s alcohol use, especially for per occasion drinking, than from women’s alcohol use.
- Young people should delay alcohol use for as long as possible.
- Individuals should not start to use alcohol or increase their alcohol use for health benefits.
- Any reduction in alcohol use is beneficial.
Other national guidelines
“Countries that haven’t updated their alcohol use guidelines recently should do so, as the evidence regarding alcohol and health has advanced considerably in the past 10 years,” said Dr. Sherk. He acknowledged that “any time health guidance changes substantially, it’s reasonable to expect a period of readjustment.”
“Some will be resistant,” Dr. Butt agreed. “Some professionals will need more education than others on the health effects of alcohol. Some patients will also be more invested in drinking than others. The harm-reduction, risk-zone approach should assist in the process of engaging patients and helping them reduce over time.
“Just as we benefited from the updates done in the United Kingdom, France, and especially Australia, so also researchers elsewhere will critique our work and our approach and make their own decisions on how best to communicate with their public,” Dr. Butt said. He noted that Canada’s contributions regarding the association between alcohol and violence, as well as their sex/gender approach to the evidence, “may influence the next country’s review.”
Commenting on whether the United States should consider changing its guidance, Timothy Brennan, MD, MPH, chief of clinical services for the Addiction Institute of Mount Sinai Health System in New York, said in an interview, “A lot of people will be surprised at the recommended limits on alcohol. Most think that they can have one or two glasses of alcohol per day and not have any increased risk to their health. I think the Canadians deserve credit for putting themselves out there.”
Dr. Brennan said there will “certainly be pushback by the drinking lobby, which is very strong both in the U.S. and in Canada.” In fact, the national trade group Beer Canada was recently quoted as stating that it still supports the 2011 guidelines and that the updating process lacked full transparency and expert technical peer review.
Nevertheless, Dr. Brennan said, “it’s overwhelmingly clear that alcohol affects a ton of different parts of our body, so limiting the amount of alcohol we take in is always going to be a good thing. The Canadian graphic is great because it color-codes the risk. I recommend that clinicians put it up in their offices and begin quantifying the units of alcohol that are going into a patient’s body each day.”
A version of this article originally appeared on Medscape.com.
AHA scientific statement on rapid evaluation for suspected TIA
TIAs are “warning shots” of a future stroke and require emergency evaluation, Hardik Amin, MD, chair of the writing committee and medical stroke director, Yale New Haven (Conn.) Hospital, said in an AHA podcast.
A key aim of the scientific statement is to help clinicians properly risk-stratify patients with suspected TIA and determine which patients need to be admitted to the hospital and which patients might be safely discharged as long as proper and prompt follow-up has been arranged, Dr. Amin explained.
The statement, published online in the journal Stroke, addresses “how we can identify and be confident in diagnosing a TIA patient and what might suggest an alternative diagnosis,” he added.
Diagnostic challenge
It’s estimated that nearly one in five people who suffer a TIA will have a full-blown stroke within 3 months; close to half of these strokes will happen within 2 days.
The challenge with TIAs is that they can be tough to diagnose because many patients no longer have symptoms when they arrive at the emergency department. There is also no confirmatory test. Limited resources and access to stroke specialists in rural centers may exacerbate these challenges, the authors noted.
The statement pointed out that the F.A.S.T. acronym for stroke symptoms (Face drooping, Arm weakness, Speech difficulty, Time to call 911) can also be used to identify a TIA – even if the symptoms resolve.
The statement also provided guidance on how to tell the difference between a TIA and a TIA mimic.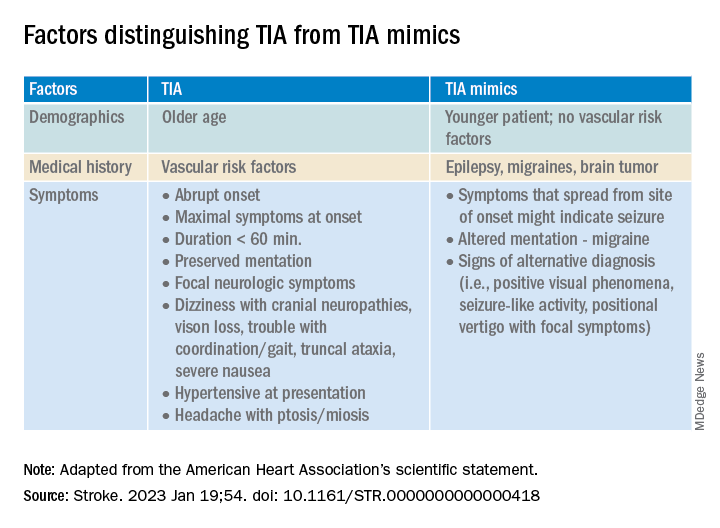
If available, a noncontrast head CT (NCCT) scan should be done initially in the emergency department to evaluate for subacute ischemia, hemorrhage, or mass lesion. Although the sensitivity of NCCT to detect an acute infarct is low, NCCT is useful for ruling out TIA mimics, the writing group said.
Multimodal brain MRI is the “preferred” method to evaluate for acute ischemic infarct and ideally should be obtained within 24 hours of symptom onset, and in most centers will follow an NCCT.
“When MRI cannot be obtained acutely to definitively distinguish TIA from stroke, it remains reasonable to make a clinical diagnosis of TIA in the ED on the basis of a negative NCCT and symptom resolution within 24 hours,” the authors said.
“A potential next step would be hospital admission for MRI, comprehensive workup, and neurology consultation. Other options might include transferring patients to a facility with advanced imaging and vascular neurology expertise or arranging a timely (ideally < 24 hours) outpatient MRI,” they advised.
The statement also provides guidance on the advantages, limitations, and considerations of Doppler ultrasonography, CT angiography, and magnetic resonance angiography for TIA assessment.
Once TIA is diagnosed, a cardiac work-up is advised because of the potential for heart-related factors to cause a TIA.
An individual’s risk of future stroke after TIA can be rapidly assessed using the ABCD2 score, which stratifies patients into low, medium, and high risk based on age, blood pressure, clinical features, duration of symptoms, and diabetes.
“It is up to each center to use the resources available and create a pathway to ensure successful management and disposition of patients with TIA, with the ultimate goal of reducing the risk of future stroke,” the authors concluded.
This scientific statement was prepared by the volunteer writing group on behalf of the American Heart Association’s Emergency Neurovascular Care Committee of the Stroke Council and the Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists, and it is endorsed by the American Association of Neurological Surgeons/Congress of Neurological Surgeons.
A version of this article first appeared on Medscape.com.
TIAs are “warning shots” of a future stroke and require emergency evaluation, Hardik Amin, MD, chair of the writing committee and medical stroke director, Yale New Haven (Conn.) Hospital, said in an AHA podcast.
A key aim of the scientific statement is to help clinicians properly risk-stratify patients with suspected TIA and determine which patients need to be admitted to the hospital and which patients might be safely discharged as long as proper and prompt follow-up has been arranged, Dr. Amin explained.
The statement, published online in the journal Stroke, addresses “how we can identify and be confident in diagnosing a TIA patient and what might suggest an alternative diagnosis,” he added.
Diagnostic challenge
It’s estimated that nearly one in five people who suffer a TIA will have a full-blown stroke within 3 months; close to half of these strokes will happen within 2 days.
The challenge with TIAs is that they can be tough to diagnose because many patients no longer have symptoms when they arrive at the emergency department. There is also no confirmatory test. Limited resources and access to stroke specialists in rural centers may exacerbate these challenges, the authors noted.
The statement pointed out that the F.A.S.T. acronym for stroke symptoms (Face drooping, Arm weakness, Speech difficulty, Time to call 911) can also be used to identify a TIA – even if the symptoms resolve.
The statement also provided guidance on how to tell the difference between a TIA and a TIA mimic.
If available, a noncontrast head CT (NCCT) scan should be done initially in the emergency department to evaluate for subacute ischemia, hemorrhage, or mass lesion. Although the sensitivity of NCCT to detect an acute infarct is low, NCCT is useful for ruling out TIA mimics, the writing group said.
Multimodal brain MRI is the “preferred” method to evaluate for acute ischemic infarct and ideally should be obtained within 24 hours of symptom onset, and in most centers will follow an NCCT.
“When MRI cannot be obtained acutely to definitively distinguish TIA from stroke, it remains reasonable to make a clinical diagnosis of TIA in the ED on the basis of a negative NCCT and symptom resolution within 24 hours,” the authors said.
“A potential next step would be hospital admission for MRI, comprehensive workup, and neurology consultation. Other options might include transferring patients to a facility with advanced imaging and vascular neurology expertise or arranging a timely (ideally < 24 hours) outpatient MRI,” they advised.
The statement also provides guidance on the advantages, limitations, and considerations of Doppler ultrasonography, CT angiography, and magnetic resonance angiography for TIA assessment.
Once TIA is diagnosed, a cardiac work-up is advised because of the potential for heart-related factors to cause a TIA.
An individual’s risk of future stroke after TIA can be rapidly assessed using the ABCD2 score, which stratifies patients into low, medium, and high risk based on age, blood pressure, clinical features, duration of symptoms, and diabetes.
“It is up to each center to use the resources available and create a pathway to ensure successful management and disposition of patients with TIA, with the ultimate goal of reducing the risk of future stroke,” the authors concluded.
This scientific statement was prepared by the volunteer writing group on behalf of the American Heart Association’s Emergency Neurovascular Care Committee of the Stroke Council and the Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists, and it is endorsed by the American Association of Neurological Surgeons/Congress of Neurological Surgeons.
A version of this article first appeared on Medscape.com.
TIAs are “warning shots” of a future stroke and require emergency evaluation, Hardik Amin, MD, chair of the writing committee and medical stroke director, Yale New Haven (Conn.) Hospital, said in an AHA podcast.
A key aim of the scientific statement is to help clinicians properly risk-stratify patients with suspected TIA and determine which patients need to be admitted to the hospital and which patients might be safely discharged as long as proper and prompt follow-up has been arranged, Dr. Amin explained.
The statement, published online in the journal Stroke, addresses “how we can identify and be confident in diagnosing a TIA patient and what might suggest an alternative diagnosis,” he added.
Diagnostic challenge
It’s estimated that nearly one in five people who suffer a TIA will have a full-blown stroke within 3 months; close to half of these strokes will happen within 2 days.
The challenge with TIAs is that they can be tough to diagnose because many patients no longer have symptoms when they arrive at the emergency department. There is also no confirmatory test. Limited resources and access to stroke specialists in rural centers may exacerbate these challenges, the authors noted.
The statement pointed out that the F.A.S.T. acronym for stroke symptoms (Face drooping, Arm weakness, Speech difficulty, Time to call 911) can also be used to identify a TIA – even if the symptoms resolve.
The statement also provided guidance on how to tell the difference between a TIA and a TIA mimic.
If available, a noncontrast head CT (NCCT) scan should be done initially in the emergency department to evaluate for subacute ischemia, hemorrhage, or mass lesion. Although the sensitivity of NCCT to detect an acute infarct is low, NCCT is useful for ruling out TIA mimics, the writing group said.
Multimodal brain MRI is the “preferred” method to evaluate for acute ischemic infarct and ideally should be obtained within 24 hours of symptom onset, and in most centers will follow an NCCT.
“When MRI cannot be obtained acutely to definitively distinguish TIA from stroke, it remains reasonable to make a clinical diagnosis of TIA in the ED on the basis of a negative NCCT and symptom resolution within 24 hours,” the authors said.
“A potential next step would be hospital admission for MRI, comprehensive workup, and neurology consultation. Other options might include transferring patients to a facility with advanced imaging and vascular neurology expertise or arranging a timely (ideally < 24 hours) outpatient MRI,” they advised.
The statement also provides guidance on the advantages, limitations, and considerations of Doppler ultrasonography, CT angiography, and magnetic resonance angiography for TIA assessment.
Once TIA is diagnosed, a cardiac work-up is advised because of the potential for heart-related factors to cause a TIA.
An individual’s risk of future stroke after TIA can be rapidly assessed using the ABCD2 score, which stratifies patients into low, medium, and high risk based on age, blood pressure, clinical features, duration of symptoms, and diabetes.
“It is up to each center to use the resources available and create a pathway to ensure successful management and disposition of patients with TIA, with the ultimate goal of reducing the risk of future stroke,” the authors concluded.
This scientific statement was prepared by the volunteer writing group on behalf of the American Heart Association’s Emergency Neurovascular Care Committee of the Stroke Council and the Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists, and it is endorsed by the American Association of Neurological Surgeons/Congress of Neurological Surgeons.
A version of this article first appeared on Medscape.com.
FROM STROKE
Renowned stroke expert Ralph L. Sacco, MD, dies
Ralph L. Sacco, MD, the first neurologist to serve as president of the American Heart Association and the only physician to serve as president of both the AHA and the American Academy of Neurology, died Jan. 17 at the age of 65.
He died of a brain tumor at his home in Amagansett, N.Y., according to an obituary published in Neurology, Circulation, and Stroke.
“Ralph was one of a kind,” Nancy Brown, chief executive officer for the AHA and American Stroke Association, said in a statement. “His leadership was unparalleled, and his warm, generous heart and care transcended his research and clinic to every person fortunate to meet him and likely become a friend,” Ms. Brown said.
In a tweet, Natalia S. Rost, MD, professor of neurology at Harvard Medical School, Boston, called him, “a dear friend, an inspiring colleague, a generous mentor, an astute scientist, a consummate advocate for brain health worldwide.”
Dedicated to improving stroke care
Dr. Sacco was chair of the University of Miami Miller School of Medicine in the department of neurology; the Olemberg Family Chair in Neurological Disorders; professor of neurology, public health sciences, human genetics, and neurosurgery; executive director of the Evelyn F. McKnight Brain Institute; director and multi-principal investigator of the Miami Clinical and Translational Science Institute; and senior associate dean for clinical and translational science.
Dr. Sacco was a population-based researcher in the field of cerebrovascular diseases.
As founder of the Northern Manhattan Study, he paved the way for examining the differences in stroke risk related to race, ethnicity, sex, and neighborhood, and realizing the impact of modifiable lifestyle behaviors, such as alcohol consumption and physical activity, on stroke risk.
Dr. Sacco’s work led to more targeted stroke prevention programs and his “drive and dedication fueled changes that improved stroke research and fostered the development of targeted stroke care delivery, ultimately improving stroke recovery and post-stroke quality of life for many,” the AHA statement said.
Dr. Sacco was also founder and executive director of the Florida Stroke Registry, which consists of 167 Florida stroke centers. He was a member of the National Academy of Medicine.
In an obituary written by Orly Avitzur, MD, current president of the AAN, she notes that he “was the only physician to have become both the president of the AHA (2010-2011) and the AAN (2017-2019), positions that reflected the respect and admiration of professional colleagues earned over the years.”
During his tenure as AAN president, Dr. Sacco led an initiative to ensure that academic neurology, from department chairs to professors to students, knew about the abundance of academy resources available to them, the AAN noted in a statement.
Dr. Sacco was a “strong proponent of enlarging the neurology workforce through the academic pipeline and promoted the concept of the ‘newrologist’ to get people excited in careers in neurology, moving beyond just diagnosis and treatments to include interventions, preventative care, and the future of regenerative care,” the AAN said.
Dr. Sacco received numerous awards throughout his career, most recently the AHA 2022 Distinguished Scientist award. He also received the 2015 Gold Heart Award, the 2011 Distinguished National Leadership Award, and the 2006 William Feinberg Award.
In addition to his husband, Scott Dutcher, Dr. Sacco is survived by his father, Anthony P. Sacco, and his father’s wife, Rosemary; and his four siblings and their families, along with many nieces and nephews.
A version of this article first appeared on Medscape.com.
Ralph L. Sacco, MD, the first neurologist to serve as president of the American Heart Association and the only physician to serve as president of both the AHA and the American Academy of Neurology, died Jan. 17 at the age of 65.
He died of a brain tumor at his home in Amagansett, N.Y., according to an obituary published in Neurology, Circulation, and Stroke.
“Ralph was one of a kind,” Nancy Brown, chief executive officer for the AHA and American Stroke Association, said in a statement. “His leadership was unparalleled, and his warm, generous heart and care transcended his research and clinic to every person fortunate to meet him and likely become a friend,” Ms. Brown said.
In a tweet, Natalia S. Rost, MD, professor of neurology at Harvard Medical School, Boston, called him, “a dear friend, an inspiring colleague, a generous mentor, an astute scientist, a consummate advocate for brain health worldwide.”
Dedicated to improving stroke care
Dr. Sacco was chair of the University of Miami Miller School of Medicine in the department of neurology; the Olemberg Family Chair in Neurological Disorders; professor of neurology, public health sciences, human genetics, and neurosurgery; executive director of the Evelyn F. McKnight Brain Institute; director and multi-principal investigator of the Miami Clinical and Translational Science Institute; and senior associate dean for clinical and translational science.
Dr. Sacco was a population-based researcher in the field of cerebrovascular diseases.
As founder of the Northern Manhattan Study, he paved the way for examining the differences in stroke risk related to race, ethnicity, sex, and neighborhood, and realizing the impact of modifiable lifestyle behaviors, such as alcohol consumption and physical activity, on stroke risk.
Dr. Sacco’s work led to more targeted stroke prevention programs and his “drive and dedication fueled changes that improved stroke research and fostered the development of targeted stroke care delivery, ultimately improving stroke recovery and post-stroke quality of life for many,” the AHA statement said.
Dr. Sacco was also founder and executive director of the Florida Stroke Registry, which consists of 167 Florida stroke centers. He was a member of the National Academy of Medicine.
In an obituary written by Orly Avitzur, MD, current president of the AAN, she notes that he “was the only physician to have become both the president of the AHA (2010-2011) and the AAN (2017-2019), positions that reflected the respect and admiration of professional colleagues earned over the years.”
During his tenure as AAN president, Dr. Sacco led an initiative to ensure that academic neurology, from department chairs to professors to students, knew about the abundance of academy resources available to them, the AAN noted in a statement.
Dr. Sacco was a “strong proponent of enlarging the neurology workforce through the academic pipeline and promoted the concept of the ‘newrologist’ to get people excited in careers in neurology, moving beyond just diagnosis and treatments to include interventions, preventative care, and the future of regenerative care,” the AAN said.
Dr. Sacco received numerous awards throughout his career, most recently the AHA 2022 Distinguished Scientist award. He also received the 2015 Gold Heart Award, the 2011 Distinguished National Leadership Award, and the 2006 William Feinberg Award.
In addition to his husband, Scott Dutcher, Dr. Sacco is survived by his father, Anthony P. Sacco, and his father’s wife, Rosemary; and his four siblings and their families, along with many nieces and nephews.
A version of this article first appeared on Medscape.com.
Ralph L. Sacco, MD, the first neurologist to serve as president of the American Heart Association and the only physician to serve as president of both the AHA and the American Academy of Neurology, died Jan. 17 at the age of 65.
He died of a brain tumor at his home in Amagansett, N.Y., according to an obituary published in Neurology, Circulation, and Stroke.
“Ralph was one of a kind,” Nancy Brown, chief executive officer for the AHA and American Stroke Association, said in a statement. “His leadership was unparalleled, and his warm, generous heart and care transcended his research and clinic to every person fortunate to meet him and likely become a friend,” Ms. Brown said.
In a tweet, Natalia S. Rost, MD, professor of neurology at Harvard Medical School, Boston, called him, “a dear friend, an inspiring colleague, a generous mentor, an astute scientist, a consummate advocate for brain health worldwide.”
Dedicated to improving stroke care
Dr. Sacco was chair of the University of Miami Miller School of Medicine in the department of neurology; the Olemberg Family Chair in Neurological Disorders; professor of neurology, public health sciences, human genetics, and neurosurgery; executive director of the Evelyn F. McKnight Brain Institute; director and multi-principal investigator of the Miami Clinical and Translational Science Institute; and senior associate dean for clinical and translational science.
Dr. Sacco was a population-based researcher in the field of cerebrovascular diseases.
As founder of the Northern Manhattan Study, he paved the way for examining the differences in stroke risk related to race, ethnicity, sex, and neighborhood, and realizing the impact of modifiable lifestyle behaviors, such as alcohol consumption and physical activity, on stroke risk.
Dr. Sacco’s work led to more targeted stroke prevention programs and his “drive and dedication fueled changes that improved stroke research and fostered the development of targeted stroke care delivery, ultimately improving stroke recovery and post-stroke quality of life for many,” the AHA statement said.
Dr. Sacco was also founder and executive director of the Florida Stroke Registry, which consists of 167 Florida stroke centers. He was a member of the National Academy of Medicine.
In an obituary written by Orly Avitzur, MD, current president of the AAN, she notes that he “was the only physician to have become both the president of the AHA (2010-2011) and the AAN (2017-2019), positions that reflected the respect and admiration of professional colleagues earned over the years.”
During his tenure as AAN president, Dr. Sacco led an initiative to ensure that academic neurology, from department chairs to professors to students, knew about the abundance of academy resources available to them, the AAN noted in a statement.
Dr. Sacco was a “strong proponent of enlarging the neurology workforce through the academic pipeline and promoted the concept of the ‘newrologist’ to get people excited in careers in neurology, moving beyond just diagnosis and treatments to include interventions, preventative care, and the future of regenerative care,” the AAN said.
Dr. Sacco received numerous awards throughout his career, most recently the AHA 2022 Distinguished Scientist award. He also received the 2015 Gold Heart Award, the 2011 Distinguished National Leadership Award, and the 2006 William Feinberg Award.
In addition to his husband, Scott Dutcher, Dr. Sacco is survived by his father, Anthony P. Sacco, and his father’s wife, Rosemary; and his four siblings and their families, along with many nieces and nephews.
A version of this article first appeared on Medscape.com.
Possible bivalent vaccine link to strokes in people over 65
who got the shot, the Centers for Disease Control and Prevention and the Food and Drug Administration said in a joint news release.
The release did not recommend people change their vaccine practices, saying the database finding probably did not represent a “true clinical risk.” The CDC said everybody, including people over 65, should stay up to date on their COVID vaccines, including the bivalent booster.
The news release said the Vaccine Safety Datalink (VSD), “a near real-time surveillance system,” raised a safety concern about the Pfizer/BioNTech booster.
“Rapid-response investigation of the signal in the VSD raised a question of whether people 65 and older who have received the Pfizer-BioNTech COVID-19 Vaccine, Bivalent were more likely to have an ischemic stroke in the 21 days following vaccination compared with days 22-44 following vaccination,” the news release said.
Ischemic strokes are blockages of blood to the brain, often caused by blood clots.
“Although the totality of the data currently suggests that it is very unlikely that the signal in VSD (Vaccine Safety Datalink) represents a true clinical risk, we believe it is important to share this information with the public, as we have in the past, when one of our safety monitoring systems detects a signal,” the release said.
No higher likelihood of strokes linked to the Pfizer bivalent vaccine had been found by Pfizer/BioNTech, the Department of Veterans Affairs, the Vaccine Adverse Event Reporting System maintained by the CDC and the FDA, or other agencies that monitor reactions of vaccines, the news release said. No safety issues about strokes have been identified with the Moderna bivalent vaccine.
CNN, citing a CDC official, reported that about 550,000 seniors who got Pfizer bivalent boosters were tracked by the VSD, and 130 of them had strokes within 3 weeks of getting the shot. None of those 130 people died, CNN said. The official spoke on the condition of anonymity because they weren’t authorized to share the data.
The issue will be discussed at the January meeting of the FDA’s Vaccines and Related Biological Products Advisory Committee.
In a joint statement, Pfizer and BioNTech said: “Neither Pfizer and BioNTech nor the CDC or FDA have observed similar findings across numerous other monitoring systems in the U.S. and globally and there is no evidence to conclude that ischemic stroke is associated with the use of the companies’ COVID-19 vaccines.”
Bivalent boosters contain two strains of vaccine – one to protect against the original COVID-19 virus and another targeting Omicron subvariants.
A version of this article first appeared on WebMD.com.
who got the shot, the Centers for Disease Control and Prevention and the Food and Drug Administration said in a joint news release.
The release did not recommend people change their vaccine practices, saying the database finding probably did not represent a “true clinical risk.” The CDC said everybody, including people over 65, should stay up to date on their COVID vaccines, including the bivalent booster.
The news release said the Vaccine Safety Datalink (VSD), “a near real-time surveillance system,” raised a safety concern about the Pfizer/BioNTech booster.
“Rapid-response investigation of the signal in the VSD raised a question of whether people 65 and older who have received the Pfizer-BioNTech COVID-19 Vaccine, Bivalent were more likely to have an ischemic stroke in the 21 days following vaccination compared with days 22-44 following vaccination,” the news release said.
Ischemic strokes are blockages of blood to the brain, often caused by blood clots.
“Although the totality of the data currently suggests that it is very unlikely that the signal in VSD (Vaccine Safety Datalink) represents a true clinical risk, we believe it is important to share this information with the public, as we have in the past, when one of our safety monitoring systems detects a signal,” the release said.
No higher likelihood of strokes linked to the Pfizer bivalent vaccine had been found by Pfizer/BioNTech, the Department of Veterans Affairs, the Vaccine Adverse Event Reporting System maintained by the CDC and the FDA, or other agencies that monitor reactions of vaccines, the news release said. No safety issues about strokes have been identified with the Moderna bivalent vaccine.
CNN, citing a CDC official, reported that about 550,000 seniors who got Pfizer bivalent boosters were tracked by the VSD, and 130 of them had strokes within 3 weeks of getting the shot. None of those 130 people died, CNN said. The official spoke on the condition of anonymity because they weren’t authorized to share the data.
The issue will be discussed at the January meeting of the FDA’s Vaccines and Related Biological Products Advisory Committee.
In a joint statement, Pfizer and BioNTech said: “Neither Pfizer and BioNTech nor the CDC or FDA have observed similar findings across numerous other monitoring systems in the U.S. and globally and there is no evidence to conclude that ischemic stroke is associated with the use of the companies’ COVID-19 vaccines.”
Bivalent boosters contain two strains of vaccine – one to protect against the original COVID-19 virus and another targeting Omicron subvariants.
A version of this article first appeared on WebMD.com.
who got the shot, the Centers for Disease Control and Prevention and the Food and Drug Administration said in a joint news release.
The release did not recommend people change their vaccine practices, saying the database finding probably did not represent a “true clinical risk.” The CDC said everybody, including people over 65, should stay up to date on their COVID vaccines, including the bivalent booster.
The news release said the Vaccine Safety Datalink (VSD), “a near real-time surveillance system,” raised a safety concern about the Pfizer/BioNTech booster.
“Rapid-response investigation of the signal in the VSD raised a question of whether people 65 and older who have received the Pfizer-BioNTech COVID-19 Vaccine, Bivalent were more likely to have an ischemic stroke in the 21 days following vaccination compared with days 22-44 following vaccination,” the news release said.
Ischemic strokes are blockages of blood to the brain, often caused by blood clots.
“Although the totality of the data currently suggests that it is very unlikely that the signal in VSD (Vaccine Safety Datalink) represents a true clinical risk, we believe it is important to share this information with the public, as we have in the past, when one of our safety monitoring systems detects a signal,” the release said.
No higher likelihood of strokes linked to the Pfizer bivalent vaccine had been found by Pfizer/BioNTech, the Department of Veterans Affairs, the Vaccine Adverse Event Reporting System maintained by the CDC and the FDA, or other agencies that monitor reactions of vaccines, the news release said. No safety issues about strokes have been identified with the Moderna bivalent vaccine.
CNN, citing a CDC official, reported that about 550,000 seniors who got Pfizer bivalent boosters were tracked by the VSD, and 130 of them had strokes within 3 weeks of getting the shot. None of those 130 people died, CNN said. The official spoke on the condition of anonymity because they weren’t authorized to share the data.
The issue will be discussed at the January meeting of the FDA’s Vaccines and Related Biological Products Advisory Committee.
In a joint statement, Pfizer and BioNTech said: “Neither Pfizer and BioNTech nor the CDC or FDA have observed similar findings across numerous other monitoring systems in the U.S. and globally and there is no evidence to conclude that ischemic stroke is associated with the use of the companies’ COVID-19 vaccines.”
Bivalent boosters contain two strains of vaccine – one to protect against the original COVID-19 virus and another targeting Omicron subvariants.
A version of this article first appeared on WebMD.com.
After PCI, 1-month beats 12-month DAPT in high-risk patients
Replacing dual-antiplatelet therapy (DAPT) with clopidogrel alone 1 month after percutaneous intervention (PCI) offers a lower risk of bleeding with comparable protection against cardiovascular events, according to two subgroup analyses of the Japanese STOPDAPT-2 and STOPDAPT-2 ACS trials.
The objective of these two analyses was to evaluate whether there was a benefit-to-risk ratio advantage for those who entered the study with high bleeding risk or who had undergone a complex PCI. Overall, bleeding risk was reduced without a major increase in cardiovascular events regardless of subgroup, according to results published by a multicenter group of Japanese investigators.
In this substudy, like the previously published studies from which the data were drawn, the primary endpoint was a composite of cardiovascular death, myocardial infarction, definite stent thrombosis, stroke, and Thrombolysis In Myocardial Infarction bleeding (major or minor).
The proportion of patients in the 1-month and 12-month DAPT groups reaching this composite endpoint at 1 year was not significantly different among patients stratified by baseline bleeding risk or by PCI complexity, according to a multicenter group of authors led by Takeshi Kimura, MD, department of cardiovascular medicine, Kyoto University.
Shortened DAPT is focus of multiple trials
The new analysis, published in JACC Asia, is a follow-up to the 2019 STOPDAPT-2 trial, published in JAMA, and the 2022 STOPDAPT-2 ACS trial, published in JAMA Cardiology. The first tested 1- versus 12-month DAPT in PCI patients receiving a drug-eluting stent. The second study compared the same strategies in patients undergoing PCI to treat an acute coronary syndrome (ACS).
Both studies were conducted in Japan. DAPT consisted of the P2Y12 receptor inhibitor clopidogrel plus aspirin. The experimental arm received this regimen for 1 month followed by clopidogrel monotherapy. The control arm remained on DAPT for 12 months.
The study is potentially important because it addresses the challenge of finding “the sweet spot of antiplatelet therapy in East Asian patients,” according to the coauthors of an accompanying editorial in the same issue of JACC Asia.
Previous data suggest East Asians have a higher risk of bleeding but lower anti-ischemic benefits from DAPT therapy, explained the coauthors, Antonio Greco, MD and Davide Capodanno, MD, PhD, both from the University of Catania (Italy). They praised the effort to explore this question.
In the STOPDAPT-2 trial, the shortened DAPT regimen was associated with a significantly lower rate of a composite endpoint of cardiovascular and bleeding events than standard DAPT, meeting criteria for superiority as well as noninferiority. In the STOPDAPT-2 ACS trial, shortened DAPT failed to achieve noninferiority to standard DAPT because of an increase in cardiovascular events despite a reduction in bleeding events.
Neither of these studies specifically compared shortened to standard DAPT in patients with high bleeding risk or in patients who underwent complex PCI, which are among the most common patient groups in which to consider a modified DAPT regimen. To do this, two new substudies were performed with the combined data from 5,997 patients in the two STOPDAPT-2 trials.
Two candidate groups for shortened DAPT evaluated
In the first substudy, the 1,893 patients who met criteria for high bleeding risk were compared with the 4,104 who did not. In those with a high risk of bleeding, the proportion reaching a primary endpoint at 1 year was lower, but not significantly different, for those on 1-month versus standard DAPT (5.01% vs. 5.14%). This was also true in those without an elevated bleeding risk (1.90% vs. 2.02%).
In the second substudy, 999 patients who had a complex PCI, defined by such characteristics as implantation of at least three stents or chronic total occlusion in the target lesions, were compared with the 4,998 who did not. Again, the primary endpoint was lower in both those who had a complex PCI (3.15% vs. 4.07%) and those who did not (2.78% vs. 2.82%).
Not surprisingly, patients with a high bleeding risk benefited from a substantially lower risk of bleeding events on the 1-month DAPT regimen (0.66% vs. 2.27%). The cost was a higher risk of cardiovascular events (4.35% vs. 3.52%), but this difference did not reach significance. Those without an elevated bleeding risk also had a lower risk of bleeding events (0.43% vs. 0.85%) but a higher risk of cardiovascular events (1.56% vs. 1.22%). Again, differences were nonsignificant. In the substudy evaluating DAPT duration in relation to complex PCI, the rate of cardiovascular events at 1 year in those treated with short versus 12-month DAPT was nearly identical (2.53% vs. 2.52%). In the non–complex PCI patients, event rates were nonsignificantly greater on the shortened DAPT regimen (2.38% vs. 1.86%), but the bleeding rate was lower on shortened DAPT whether PCI had been complex (0.63% vs. 1.75%) or not (0.48% vs. 1.22%).
In the absence of any major signal that complex PCI benefited from longer duration DAPT, “complex PCI might not be an appropriate determinant for DAPT durations,” according to Dr. Kimura and coinvestigators.
Study data might not be generalizable
Dr. Greco and Dr. Capodanno pointed out that there are differences between patients and PCI practices in Japan relative to other areas of the world, limiting the generalizability of these findings even if the question is relevant.
“This is an approach that might be suggested for patients at high bleeding risk who have the characteristics of the patients enrolled in the STOPDAPT-2 trials,” Dr. Capodanno said in an interview. In his own PCI practice treating ACS patients, “I would not feel safe enough with clopidogrel monotherapy after only 1 month.”
He considers the ACS population to have a particularly “delicate bleeding-ischemia trade-off,” which is why he thinks this question is relevant and needs to be explored further in additional populations. However, he might design trials differently in his own practice setting. For example, he would at the very least be interested in testing a more potent P2Y12 inhibitor such as ticagrelor when considering a single antiplatelet agent after a limited course of DAPT.
One message from this study is that “bleeding risk trumps PCI complexity,” according to Deepak L. Bhatt, MD, who recently assumed the position of director of Mount Sinai Heart in New York. He liked the approach the investigators took to address a complex and relevant clinical issue, but he also expressed reservations about the clinical applicability of this subgroup analysis.
“We really need more data before uniformly shortening DAPT duration in all patients,” Dr. Bhatt said in an interview. He considers this a hot clinical issue that is likely to generate more trials. He hopes these will provide more definitive evidence of when and how DAPT duration can be reduced. Overall, he anticipates progress toward tailoring therapy in specific populations in order to achieve the best risk-to-benefit balance.
Dr. Kimura has financial relationships with Boston Scientific, Daiichi Sankyo, Sanofi, Terumo, and Abbott Medical Japan, which provided funding for the STOPDAPT-2 and STOPDAPT-2 ACS trials. Dr. Capodanno reported financial relationships with Amgen, Arena, Chiesi, Daiichi Sakyo, Sanofi Aventis, and Terumo. Dr. Bhatt reported financial relationships with more than 20 pharmaceutical companies, including Abbott Medical.
Replacing dual-antiplatelet therapy (DAPT) with clopidogrel alone 1 month after percutaneous intervention (PCI) offers a lower risk of bleeding with comparable protection against cardiovascular events, according to two subgroup analyses of the Japanese STOPDAPT-2 and STOPDAPT-2 ACS trials.
The objective of these two analyses was to evaluate whether there was a benefit-to-risk ratio advantage for those who entered the study with high bleeding risk or who had undergone a complex PCI. Overall, bleeding risk was reduced without a major increase in cardiovascular events regardless of subgroup, according to results published by a multicenter group of Japanese investigators.
In this substudy, like the previously published studies from which the data were drawn, the primary endpoint was a composite of cardiovascular death, myocardial infarction, definite stent thrombosis, stroke, and Thrombolysis In Myocardial Infarction bleeding (major or minor).
The proportion of patients in the 1-month and 12-month DAPT groups reaching this composite endpoint at 1 year was not significantly different among patients stratified by baseline bleeding risk or by PCI complexity, according to a multicenter group of authors led by Takeshi Kimura, MD, department of cardiovascular medicine, Kyoto University.
Shortened DAPT is focus of multiple trials
The new analysis, published in JACC Asia, is a follow-up to the 2019 STOPDAPT-2 trial, published in JAMA, and the 2022 STOPDAPT-2 ACS trial, published in JAMA Cardiology. The first tested 1- versus 12-month DAPT in PCI patients receiving a drug-eluting stent. The second study compared the same strategies in patients undergoing PCI to treat an acute coronary syndrome (ACS).
Both studies were conducted in Japan. DAPT consisted of the P2Y12 receptor inhibitor clopidogrel plus aspirin. The experimental arm received this regimen for 1 month followed by clopidogrel monotherapy. The control arm remained on DAPT for 12 months.
The study is potentially important because it addresses the challenge of finding “the sweet spot of antiplatelet therapy in East Asian patients,” according to the coauthors of an accompanying editorial in the same issue of JACC Asia.
Previous data suggest East Asians have a higher risk of bleeding but lower anti-ischemic benefits from DAPT therapy, explained the coauthors, Antonio Greco, MD and Davide Capodanno, MD, PhD, both from the University of Catania (Italy). They praised the effort to explore this question.
In the STOPDAPT-2 trial, the shortened DAPT regimen was associated with a significantly lower rate of a composite endpoint of cardiovascular and bleeding events than standard DAPT, meeting criteria for superiority as well as noninferiority. In the STOPDAPT-2 ACS trial, shortened DAPT failed to achieve noninferiority to standard DAPT because of an increase in cardiovascular events despite a reduction in bleeding events.
Neither of these studies specifically compared shortened to standard DAPT in patients with high bleeding risk or in patients who underwent complex PCI, which are among the most common patient groups in which to consider a modified DAPT regimen. To do this, two new substudies were performed with the combined data from 5,997 patients in the two STOPDAPT-2 trials.
Two candidate groups for shortened DAPT evaluated
In the first substudy, the 1,893 patients who met criteria for high bleeding risk were compared with the 4,104 who did not. In those with a high risk of bleeding, the proportion reaching a primary endpoint at 1 year was lower, but not significantly different, for those on 1-month versus standard DAPT (5.01% vs. 5.14%). This was also true in those without an elevated bleeding risk (1.90% vs. 2.02%).
In the second substudy, 999 patients who had a complex PCI, defined by such characteristics as implantation of at least three stents or chronic total occlusion in the target lesions, were compared with the 4,998 who did not. Again, the primary endpoint was lower in both those who had a complex PCI (3.15% vs. 4.07%) and those who did not (2.78% vs. 2.82%).
Not surprisingly, patients with a high bleeding risk benefited from a substantially lower risk of bleeding events on the 1-month DAPT regimen (0.66% vs. 2.27%). The cost was a higher risk of cardiovascular events (4.35% vs. 3.52%), but this difference did not reach significance. Those without an elevated bleeding risk also had a lower risk of bleeding events (0.43% vs. 0.85%) but a higher risk of cardiovascular events (1.56% vs. 1.22%). Again, differences were nonsignificant. In the substudy evaluating DAPT duration in relation to complex PCI, the rate of cardiovascular events at 1 year in those treated with short versus 12-month DAPT was nearly identical (2.53% vs. 2.52%). In the non–complex PCI patients, event rates were nonsignificantly greater on the shortened DAPT regimen (2.38% vs. 1.86%), but the bleeding rate was lower on shortened DAPT whether PCI had been complex (0.63% vs. 1.75%) or not (0.48% vs. 1.22%).
In the absence of any major signal that complex PCI benefited from longer duration DAPT, “complex PCI might not be an appropriate determinant for DAPT durations,” according to Dr. Kimura and coinvestigators.
Study data might not be generalizable
Dr. Greco and Dr. Capodanno pointed out that there are differences between patients and PCI practices in Japan relative to other areas of the world, limiting the generalizability of these findings even if the question is relevant.
“This is an approach that might be suggested for patients at high bleeding risk who have the characteristics of the patients enrolled in the STOPDAPT-2 trials,” Dr. Capodanno said in an interview. In his own PCI practice treating ACS patients, “I would not feel safe enough with clopidogrel monotherapy after only 1 month.”
He considers the ACS population to have a particularly “delicate bleeding-ischemia trade-off,” which is why he thinks this question is relevant and needs to be explored further in additional populations. However, he might design trials differently in his own practice setting. For example, he would at the very least be interested in testing a more potent P2Y12 inhibitor such as ticagrelor when considering a single antiplatelet agent after a limited course of DAPT.
One message from this study is that “bleeding risk trumps PCI complexity,” according to Deepak L. Bhatt, MD, who recently assumed the position of director of Mount Sinai Heart in New York. He liked the approach the investigators took to address a complex and relevant clinical issue, but he also expressed reservations about the clinical applicability of this subgroup analysis.
“We really need more data before uniformly shortening DAPT duration in all patients,” Dr. Bhatt said in an interview. He considers this a hot clinical issue that is likely to generate more trials. He hopes these will provide more definitive evidence of when and how DAPT duration can be reduced. Overall, he anticipates progress toward tailoring therapy in specific populations in order to achieve the best risk-to-benefit balance.
Dr. Kimura has financial relationships with Boston Scientific, Daiichi Sankyo, Sanofi, Terumo, and Abbott Medical Japan, which provided funding for the STOPDAPT-2 and STOPDAPT-2 ACS trials. Dr. Capodanno reported financial relationships with Amgen, Arena, Chiesi, Daiichi Sakyo, Sanofi Aventis, and Terumo. Dr. Bhatt reported financial relationships with more than 20 pharmaceutical companies, including Abbott Medical.
Replacing dual-antiplatelet therapy (DAPT) with clopidogrel alone 1 month after percutaneous intervention (PCI) offers a lower risk of bleeding with comparable protection against cardiovascular events, according to two subgroup analyses of the Japanese STOPDAPT-2 and STOPDAPT-2 ACS trials.
The objective of these two analyses was to evaluate whether there was a benefit-to-risk ratio advantage for those who entered the study with high bleeding risk or who had undergone a complex PCI. Overall, bleeding risk was reduced without a major increase in cardiovascular events regardless of subgroup, according to results published by a multicenter group of Japanese investigators.
In this substudy, like the previously published studies from which the data were drawn, the primary endpoint was a composite of cardiovascular death, myocardial infarction, definite stent thrombosis, stroke, and Thrombolysis In Myocardial Infarction bleeding (major or minor).
The proportion of patients in the 1-month and 12-month DAPT groups reaching this composite endpoint at 1 year was not significantly different among patients stratified by baseline bleeding risk or by PCI complexity, according to a multicenter group of authors led by Takeshi Kimura, MD, department of cardiovascular medicine, Kyoto University.
Shortened DAPT is focus of multiple trials
The new analysis, published in JACC Asia, is a follow-up to the 2019 STOPDAPT-2 trial, published in JAMA, and the 2022 STOPDAPT-2 ACS trial, published in JAMA Cardiology. The first tested 1- versus 12-month DAPT in PCI patients receiving a drug-eluting stent. The second study compared the same strategies in patients undergoing PCI to treat an acute coronary syndrome (ACS).
Both studies were conducted in Japan. DAPT consisted of the P2Y12 receptor inhibitor clopidogrel plus aspirin. The experimental arm received this regimen for 1 month followed by clopidogrel monotherapy. The control arm remained on DAPT for 12 months.
The study is potentially important because it addresses the challenge of finding “the sweet spot of antiplatelet therapy in East Asian patients,” according to the coauthors of an accompanying editorial in the same issue of JACC Asia.
Previous data suggest East Asians have a higher risk of bleeding but lower anti-ischemic benefits from DAPT therapy, explained the coauthors, Antonio Greco, MD and Davide Capodanno, MD, PhD, both from the University of Catania (Italy). They praised the effort to explore this question.
In the STOPDAPT-2 trial, the shortened DAPT regimen was associated with a significantly lower rate of a composite endpoint of cardiovascular and bleeding events than standard DAPT, meeting criteria for superiority as well as noninferiority. In the STOPDAPT-2 ACS trial, shortened DAPT failed to achieve noninferiority to standard DAPT because of an increase in cardiovascular events despite a reduction in bleeding events.
Neither of these studies specifically compared shortened to standard DAPT in patients with high bleeding risk or in patients who underwent complex PCI, which are among the most common patient groups in which to consider a modified DAPT regimen. To do this, two new substudies were performed with the combined data from 5,997 patients in the two STOPDAPT-2 trials.
Two candidate groups for shortened DAPT evaluated
In the first substudy, the 1,893 patients who met criteria for high bleeding risk were compared with the 4,104 who did not. In those with a high risk of bleeding, the proportion reaching a primary endpoint at 1 year was lower, but not significantly different, for those on 1-month versus standard DAPT (5.01% vs. 5.14%). This was also true in those without an elevated bleeding risk (1.90% vs. 2.02%).
In the second substudy, 999 patients who had a complex PCI, defined by such characteristics as implantation of at least three stents or chronic total occlusion in the target lesions, were compared with the 4,998 who did not. Again, the primary endpoint was lower in both those who had a complex PCI (3.15% vs. 4.07%) and those who did not (2.78% vs. 2.82%).
Not surprisingly, patients with a high bleeding risk benefited from a substantially lower risk of bleeding events on the 1-month DAPT regimen (0.66% vs. 2.27%). The cost was a higher risk of cardiovascular events (4.35% vs. 3.52%), but this difference did not reach significance. Those without an elevated bleeding risk also had a lower risk of bleeding events (0.43% vs. 0.85%) but a higher risk of cardiovascular events (1.56% vs. 1.22%). Again, differences were nonsignificant. In the substudy evaluating DAPT duration in relation to complex PCI, the rate of cardiovascular events at 1 year in those treated with short versus 12-month DAPT was nearly identical (2.53% vs. 2.52%). In the non–complex PCI patients, event rates were nonsignificantly greater on the shortened DAPT regimen (2.38% vs. 1.86%), but the bleeding rate was lower on shortened DAPT whether PCI had been complex (0.63% vs. 1.75%) or not (0.48% vs. 1.22%).
In the absence of any major signal that complex PCI benefited from longer duration DAPT, “complex PCI might not be an appropriate determinant for DAPT durations,” according to Dr. Kimura and coinvestigators.
Study data might not be generalizable
Dr. Greco and Dr. Capodanno pointed out that there are differences between patients and PCI practices in Japan relative to other areas of the world, limiting the generalizability of these findings even if the question is relevant.
“This is an approach that might be suggested for patients at high bleeding risk who have the characteristics of the patients enrolled in the STOPDAPT-2 trials,” Dr. Capodanno said in an interview. In his own PCI practice treating ACS patients, “I would not feel safe enough with clopidogrel monotherapy after only 1 month.”
He considers the ACS population to have a particularly “delicate bleeding-ischemia trade-off,” which is why he thinks this question is relevant and needs to be explored further in additional populations. However, he might design trials differently in his own practice setting. For example, he would at the very least be interested in testing a more potent P2Y12 inhibitor such as ticagrelor when considering a single antiplatelet agent after a limited course of DAPT.
One message from this study is that “bleeding risk trumps PCI complexity,” according to Deepak L. Bhatt, MD, who recently assumed the position of director of Mount Sinai Heart in New York. He liked the approach the investigators took to address a complex and relevant clinical issue, but he also expressed reservations about the clinical applicability of this subgroup analysis.
“We really need more data before uniformly shortening DAPT duration in all patients,” Dr. Bhatt said in an interview. He considers this a hot clinical issue that is likely to generate more trials. He hopes these will provide more definitive evidence of when and how DAPT duration can be reduced. Overall, he anticipates progress toward tailoring therapy in specific populations in order to achieve the best risk-to-benefit balance.
Dr. Kimura has financial relationships with Boston Scientific, Daiichi Sankyo, Sanofi, Terumo, and Abbott Medical Japan, which provided funding for the STOPDAPT-2 and STOPDAPT-2 ACS trials. Dr. Capodanno reported financial relationships with Amgen, Arena, Chiesi, Daiichi Sakyo, Sanofi Aventis, and Terumo. Dr. Bhatt reported financial relationships with more than 20 pharmaceutical companies, including Abbott Medical.
FROM JACC ASIA
Transition to Tenecteplase From t-PA for Acute Ischemic Stroke at Walter Reed National Military Medical Center
Tissue plasminogen activator (t-PA) has been the standard IV thrombolytic used in acute ischemic stroke treatment since its US Food and Drug Administration (FDA) approval in 1995. Trials have established this drug’s efficacy in the treatment of acute ischemic stroke and the appropriate patient population for therapy.1-3 Published guidelines and experiences have made clear that a written protocol with extensive personnel training is important to deliver this care properly.4
Tenecteplase has been available for use in the treatment of acute myocardial infarction (MI) and studied in acute ischemic strokes since 2000. Recent large multicenter trials have suggested tenecteplase may work better than t-PA in the recanalization of large vessel occlusions (LVOs) and have provided guidance on proper dosing in acute ischemic stroke victims.5-8 Compared with t-PA, tenecteplase has a longer half-life, is more fibrin specific (causing less coagulopathy), and is more resistant to endogenous plasminogen activator inhibitor.9,10 Using tenecteplase for acute ischemic stroke is simpler as a single dose bolus rather than a bolus followed by a 1-hour infusion with t-PA. Immediate mechanical thrombectomy for LVO is less complicated without the 1-hour t-PA infusion.5,6 Tenecteplase use also allows for nonthrombectomy hospitals to accelerate transfer times for patients who need thrombectomy following thrombolysis by eliminating the need for critical care nurse–staffed ambulances for interfacility transfer.11 Tenecteplase also is cheaper: Tenecteplase costs $3748 per vial, whereas t-PA costs $5800 per vial equating to roughly a $2000 savings per patient.12,13 Finally, the pharmacy formulary is simplified by using a single thrombolytic agent for both cardiac and neurologic emergencies.
Tenecteplase does have some drawbacks to consider. Currently, tenecteplase is not approved by the FDA for the indication of acute ischemic stroke, though the drug is endorsed by the American Heart Association stroke guidelines of 2019 as an alternative to t-PA.14 There is no stroke-specific preparation of the drug, leading to potential dosing errors. Therefore, a systematic process to safely transition from t-PA to tenecteplase for acute ischemic stroke was undertaken at Walter Reed National Military Medical Center (WRNMMC) in Bethesda, Maryland. Here, we report the process required in making a complex switch in thrombolytic medication along with the potential benefits of making this transition.
OBSERVATIONS
The process to implement tenecteplase required extensive training and education for staff physicians, nurses, pharmacists, radiologists, trainees, and the rapid response team. Our institution administered IV thrombolytic drugs up to 25 times annually to acute ischemic stroke victims, meaning we had to train personnel extensively and repeatedly.
In preparation for the transition to tenecteplase, hospital leadership gathered staff for multidisciplinary administrative meetings that included neurology, emergency medicine, intensive care, pharmacy, radiology, and nursing departments. The purpose of these meetings was to establish a standard operating procedure (SOP) to ensure a safe transition. This process began in May 2020 and involved regular meetings to draft and revise our SOP. Additionally, several leadership and training sessions were held over a 6-month period. Stroke boxes were developed that contained the required evaluation tools, consent forms, medications (tenecteplase and treatments for known complications), dosing cards, and instructions. Final approval of the updated acute ischemic stroke hospital policy was obtained in November 2020 and signed by the above departments.
All inclusion and exclusion criteria were determined to be the same for tenecteplase as they were for t-PA with the notable exception that the WAKE-UP trial protocol would not be supported until further evidence became available.9 The results of the WAKE-UP trial had previously been used at WRNMMC to justify administration of t-PA in patients who awoke with symptoms of acute ischemic stroke, the last known well was unclear or > 4.5 hours, and for whom a magnetic resonance imaging (MRI) of the brain could be obtained rapidly. Based on the WAKE-UP trial, if the MRI scan of the brain in these patients demonstrated restricted diffusion without fluid attenuated inversion recovery (FLAIR) signal changes (diffusion-weighted [DWI]-FLAIR mismatch sign), this indicated that the stroke had likely occurred recently, and it was safe to administer t-PA. This allowed for administration of t-PA outside the standard treatment window of 4.5 hours from last known well, especially in the cases of patients who awoke with symptoms.
Since safety data are not yet available for the use of tenecteplase in this fashion, the WAKE-UP trial protocol was not used as an inclusion criterion. The informed consent form was modified, and the following scenarios were outlined: (1) If the patient or surrogate is immediately available to consent, paper consent will be documented with the additional note that tenecteplase is being used off-label; and (2) If the patient cannot consent and a surrogate is not immediately available, the medicine will be used emergently as long as the neurology resident and attending physicians agree.15
Risk mitigation was considered carefully. The stroke box described above is stocked and maintained by the pharmacy as we have transitioned to using designated pharmacists for the storage and preparation of tenecteplase. We highly recommend the use of designated pharmacists or emergency department pharmacists in this manner to avoid dosing errors.7,16 Since the current pharmacy-provided tenecteplase bottle contains twice the maximum dose indicated for ischemic stroke, only a 5 mL syringe is included in the stroke box to ensure a maximum dose of 25 mg is drawn up after reconstitution. Dosing card charts were made like existing dosing card charts for t-PA to quickly calculate the 0.25 mg/kg dose. In training, the difference in dosing in ischemic stroke was emphasized. Finally, pharmacy has taken responsibility for dosing the medication during stroke codes.
Any medical personnel at WRNMMC can initiate a stroke code by sending a page to the neurology consult service (Figure).
TRANSITION AND RESULTS
From November 2020 to December 2021, 10 patients have been treated in total at WRNMMC (Table).
CONCLUSIONS
The available evidence supports the transition from t-PA to tenecteplase for acute ischemic stroke. The successful transition required months of preparation involving multidisciplinary meetings between neurology, nursing, pharmacy, radiology, rapid response teams, critical care, and emergency medicine departments. Safeguards must be implemented to avoid a tenecteplase dosing error that can lead to potentially life-threatening adverse effects. The results at WRNMMC thus far are promising for safety and efficacy. Several process improvements are planned: a hospital-wide overhead page will accompany the direct page to neurology; other team members, including radiology and pharmacy, will be included on the acute stroke alert; and a stroke-specific paging application will be implemented to better track real-time stroke metrics and improve flow. These measures mirror processes that are occurring in institutions that treat acute stroke patients.
1. Lees KR, Bluhmki E, von Kummer R, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010;375(9727):1695-1703. doi:10.1016/S0140-6736(10)60491-6
2. National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333(24):1581- 1587. doi:10.1056/NEJM199512143332401
3. Hacke W, Donnan G, Fieschi C, et al. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004;363(9411):768-774. doi:10.1016/S0140-6736(04)15692-4
4. Jauch EC, Saver JL, Adams HP Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870-947. doi:10.1161/STR.0b013e318284056a
5. Campbell B, Mitchell P, Churilov L, et al. Tenecteplase versus alteplase before thrombectomy for ischemic stroke. N Engl J Med. 2018;378(17):1573-1582. doi:10.1056/nejmoa1716405
6. Yang P, Zhang Y, Zhang L, et al. Endovascular thrombectomy with or without intravenous alteplase in acute stroke. N Engl J Med. 2020;382(21):1981-1993. doi:10.1056/NEJMoa2001123
7. Menon BK, Buck BH, Singh N, et al. Intravenous tenecteplase compared with alteplase for acute ischaemic stroke in Canada (AcT): a pragmatic, multicentre, open-label, registry-linked, randomised, controlled, noninferiority trial. Lancet. 2022;400(10347):161-169. doi:10.1016/S0140-6736(22)01054-6
8. Campbell BCV, Mitchell PJ, Churilov L, et al. Effect of intravenous tenecteplase dose on cerebral reperfusion before thrombectomy in patients with large vessel occlusion ischemic stroke: the EXTEND-IA TNK part 2 randomized clinical trial. JAMA. 2020;323(13):1257- 1265. doi:10.1001/jama.2020.1511
9. Warach SJ, Dula AN, Milling TJ Jr. Tenecteplase thrombolysis for acute ischemic stroke. Stroke. 2020;51(11):3440- 3451. doi:10.1161/STROKEAHA.120.029749
10. Huang X, Moreton FC, Kalladka D, et al. Coagulation and fibrinolytic activity of tenecteplase and alteplase in acute ischemic stroke. Stroke. 2015;46(12):3543-3546. doi:10.1161/STROKEAHA.115.011290
11. Burgos AM, Saver JL. Evidence that tenecteplase is noninferior to alteplase for acute ischemic stroke: meta-analysis of 5 randomized trials. Stroke. 2019;50(8):2156-2162. doi:10.1161/STROKEAHA.119.025080
12. Potla N, Ganti L. Tenecteplase vs. alteplase for acute ischemic stroke: a systematic review. Int J Emerg Med. 2022;15(1). doi:10.1186/s12245-021-00399-w
13. Warach SJ, Winegar A, Ottenbacher A, Miller C, Gibson D. Abstract WMP52: reduced hospital costs for ischemic stroke treated with tenecteplase. Stroke. 2022;53(suppl 1):AWMP52. doi:10.1161/str.53.suppl_1.WMP52
14. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344-e418. doi:10.1161/str.0000000000000211
15. Faris H, Dewar B, Dowlatshahi D, et al. Ethical justification for deferral of consent in the AcT trial for acute ischemic stroke. Stroke. 2022;53(7):2420-2423. doi:10.1161/strokeaha.122.038760
16. Kvistad CE, Næss H, Helleberg BH, et al. Tenecteplase versus alteplase for the management of acute ischaemic stroke in Norway (NOR-TEST 2, part A): a phase 3, randomised, open-label, blinded endpoint, non-inferiority trial. Lancet Neurol. 2022;21(6):511-519. doi:10.1016/S1474-4422(22)00124-7
Tissue plasminogen activator (t-PA) has been the standard IV thrombolytic used in acute ischemic stroke treatment since its US Food and Drug Administration (FDA) approval in 1995. Trials have established this drug’s efficacy in the treatment of acute ischemic stroke and the appropriate patient population for therapy.1-3 Published guidelines and experiences have made clear that a written protocol with extensive personnel training is important to deliver this care properly.4
Tenecteplase has been available for use in the treatment of acute myocardial infarction (MI) and studied in acute ischemic strokes since 2000. Recent large multicenter trials have suggested tenecteplase may work better than t-PA in the recanalization of large vessel occlusions (LVOs) and have provided guidance on proper dosing in acute ischemic stroke victims.5-8 Compared with t-PA, tenecteplase has a longer half-life, is more fibrin specific (causing less coagulopathy), and is more resistant to endogenous plasminogen activator inhibitor.9,10 Using tenecteplase for acute ischemic stroke is simpler as a single dose bolus rather than a bolus followed by a 1-hour infusion with t-PA. Immediate mechanical thrombectomy for LVO is less complicated without the 1-hour t-PA infusion.5,6 Tenecteplase use also allows for nonthrombectomy hospitals to accelerate transfer times for patients who need thrombectomy following thrombolysis by eliminating the need for critical care nurse–staffed ambulances for interfacility transfer.11 Tenecteplase also is cheaper: Tenecteplase costs $3748 per vial, whereas t-PA costs $5800 per vial equating to roughly a $2000 savings per patient.12,13 Finally, the pharmacy formulary is simplified by using a single thrombolytic agent for both cardiac and neurologic emergencies.
Tenecteplase does have some drawbacks to consider. Currently, tenecteplase is not approved by the FDA for the indication of acute ischemic stroke, though the drug is endorsed by the American Heart Association stroke guidelines of 2019 as an alternative to t-PA.14 There is no stroke-specific preparation of the drug, leading to potential dosing errors. Therefore, a systematic process to safely transition from t-PA to tenecteplase for acute ischemic stroke was undertaken at Walter Reed National Military Medical Center (WRNMMC) in Bethesda, Maryland. Here, we report the process required in making a complex switch in thrombolytic medication along with the potential benefits of making this transition.
OBSERVATIONS
The process to implement tenecteplase required extensive training and education for staff physicians, nurses, pharmacists, radiologists, trainees, and the rapid response team. Our institution administered IV thrombolytic drugs up to 25 times annually to acute ischemic stroke victims, meaning we had to train personnel extensively and repeatedly.
In preparation for the transition to tenecteplase, hospital leadership gathered staff for multidisciplinary administrative meetings that included neurology, emergency medicine, intensive care, pharmacy, radiology, and nursing departments. The purpose of these meetings was to establish a standard operating procedure (SOP) to ensure a safe transition. This process began in May 2020 and involved regular meetings to draft and revise our SOP. Additionally, several leadership and training sessions were held over a 6-month period. Stroke boxes were developed that contained the required evaluation tools, consent forms, medications (tenecteplase and treatments for known complications), dosing cards, and instructions. Final approval of the updated acute ischemic stroke hospital policy was obtained in November 2020 and signed by the above departments.
All inclusion and exclusion criteria were determined to be the same for tenecteplase as they were for t-PA with the notable exception that the WAKE-UP trial protocol would not be supported until further evidence became available.9 The results of the WAKE-UP trial had previously been used at WRNMMC to justify administration of t-PA in patients who awoke with symptoms of acute ischemic stroke, the last known well was unclear or > 4.5 hours, and for whom a magnetic resonance imaging (MRI) of the brain could be obtained rapidly. Based on the WAKE-UP trial, if the MRI scan of the brain in these patients demonstrated restricted diffusion without fluid attenuated inversion recovery (FLAIR) signal changes (diffusion-weighted [DWI]-FLAIR mismatch sign), this indicated that the stroke had likely occurred recently, and it was safe to administer t-PA. This allowed for administration of t-PA outside the standard treatment window of 4.5 hours from last known well, especially in the cases of patients who awoke with symptoms.
Since safety data are not yet available for the use of tenecteplase in this fashion, the WAKE-UP trial protocol was not used as an inclusion criterion. The informed consent form was modified, and the following scenarios were outlined: (1) If the patient or surrogate is immediately available to consent, paper consent will be documented with the additional note that tenecteplase is being used off-label; and (2) If the patient cannot consent and a surrogate is not immediately available, the medicine will be used emergently as long as the neurology resident and attending physicians agree.15
Risk mitigation was considered carefully. The stroke box described above is stocked and maintained by the pharmacy as we have transitioned to using designated pharmacists for the storage and preparation of tenecteplase. We highly recommend the use of designated pharmacists or emergency department pharmacists in this manner to avoid dosing errors.7,16 Since the current pharmacy-provided tenecteplase bottle contains twice the maximum dose indicated for ischemic stroke, only a 5 mL syringe is included in the stroke box to ensure a maximum dose of 25 mg is drawn up after reconstitution. Dosing card charts were made like existing dosing card charts for t-PA to quickly calculate the 0.25 mg/kg dose. In training, the difference in dosing in ischemic stroke was emphasized. Finally, pharmacy has taken responsibility for dosing the medication during stroke codes.
Any medical personnel at WRNMMC can initiate a stroke code by sending a page to the neurology consult service (Figure).
TRANSITION AND RESULTS
From November 2020 to December 2021, 10 patients have been treated in total at WRNMMC (Table).
CONCLUSIONS
The available evidence supports the transition from t-PA to tenecteplase for acute ischemic stroke. The successful transition required months of preparation involving multidisciplinary meetings between neurology, nursing, pharmacy, radiology, rapid response teams, critical care, and emergency medicine departments. Safeguards must be implemented to avoid a tenecteplase dosing error that can lead to potentially life-threatening adverse effects. The results at WRNMMC thus far are promising for safety and efficacy. Several process improvements are planned: a hospital-wide overhead page will accompany the direct page to neurology; other team members, including radiology and pharmacy, will be included on the acute stroke alert; and a stroke-specific paging application will be implemented to better track real-time stroke metrics and improve flow. These measures mirror processes that are occurring in institutions that treat acute stroke patients.
Tissue plasminogen activator (t-PA) has been the standard IV thrombolytic used in acute ischemic stroke treatment since its US Food and Drug Administration (FDA) approval in 1995. Trials have established this drug’s efficacy in the treatment of acute ischemic stroke and the appropriate patient population for therapy.1-3 Published guidelines and experiences have made clear that a written protocol with extensive personnel training is important to deliver this care properly.4
Tenecteplase has been available for use in the treatment of acute myocardial infarction (MI) and studied in acute ischemic strokes since 2000. Recent large multicenter trials have suggested tenecteplase may work better than t-PA in the recanalization of large vessel occlusions (LVOs) and have provided guidance on proper dosing in acute ischemic stroke victims.5-8 Compared with t-PA, tenecteplase has a longer half-life, is more fibrin specific (causing less coagulopathy), and is more resistant to endogenous plasminogen activator inhibitor.9,10 Using tenecteplase for acute ischemic stroke is simpler as a single dose bolus rather than a bolus followed by a 1-hour infusion with t-PA. Immediate mechanical thrombectomy for LVO is less complicated without the 1-hour t-PA infusion.5,6 Tenecteplase use also allows for nonthrombectomy hospitals to accelerate transfer times for patients who need thrombectomy following thrombolysis by eliminating the need for critical care nurse–staffed ambulances for interfacility transfer.11 Tenecteplase also is cheaper: Tenecteplase costs $3748 per vial, whereas t-PA costs $5800 per vial equating to roughly a $2000 savings per patient.12,13 Finally, the pharmacy formulary is simplified by using a single thrombolytic agent for both cardiac and neurologic emergencies.
Tenecteplase does have some drawbacks to consider. Currently, tenecteplase is not approved by the FDA for the indication of acute ischemic stroke, though the drug is endorsed by the American Heart Association stroke guidelines of 2019 as an alternative to t-PA.14 There is no stroke-specific preparation of the drug, leading to potential dosing errors. Therefore, a systematic process to safely transition from t-PA to tenecteplase for acute ischemic stroke was undertaken at Walter Reed National Military Medical Center (WRNMMC) in Bethesda, Maryland. Here, we report the process required in making a complex switch in thrombolytic medication along with the potential benefits of making this transition.
OBSERVATIONS
The process to implement tenecteplase required extensive training and education for staff physicians, nurses, pharmacists, radiologists, trainees, and the rapid response team. Our institution administered IV thrombolytic drugs up to 25 times annually to acute ischemic stroke victims, meaning we had to train personnel extensively and repeatedly.
In preparation for the transition to tenecteplase, hospital leadership gathered staff for multidisciplinary administrative meetings that included neurology, emergency medicine, intensive care, pharmacy, radiology, and nursing departments. The purpose of these meetings was to establish a standard operating procedure (SOP) to ensure a safe transition. This process began in May 2020 and involved regular meetings to draft and revise our SOP. Additionally, several leadership and training sessions were held over a 6-month period. Stroke boxes were developed that contained the required evaluation tools, consent forms, medications (tenecteplase and treatments for known complications), dosing cards, and instructions. Final approval of the updated acute ischemic stroke hospital policy was obtained in November 2020 and signed by the above departments.
All inclusion and exclusion criteria were determined to be the same for tenecteplase as they were for t-PA with the notable exception that the WAKE-UP trial protocol would not be supported until further evidence became available.9 The results of the WAKE-UP trial had previously been used at WRNMMC to justify administration of t-PA in patients who awoke with symptoms of acute ischemic stroke, the last known well was unclear or > 4.5 hours, and for whom a magnetic resonance imaging (MRI) of the brain could be obtained rapidly. Based on the WAKE-UP trial, if the MRI scan of the brain in these patients demonstrated restricted diffusion without fluid attenuated inversion recovery (FLAIR) signal changes (diffusion-weighted [DWI]-FLAIR mismatch sign), this indicated that the stroke had likely occurred recently, and it was safe to administer t-PA. This allowed for administration of t-PA outside the standard treatment window of 4.5 hours from last known well, especially in the cases of patients who awoke with symptoms.
Since safety data are not yet available for the use of tenecteplase in this fashion, the WAKE-UP trial protocol was not used as an inclusion criterion. The informed consent form was modified, and the following scenarios were outlined: (1) If the patient or surrogate is immediately available to consent, paper consent will be documented with the additional note that tenecteplase is being used off-label; and (2) If the patient cannot consent and a surrogate is not immediately available, the medicine will be used emergently as long as the neurology resident and attending physicians agree.15
Risk mitigation was considered carefully. The stroke box described above is stocked and maintained by the pharmacy as we have transitioned to using designated pharmacists for the storage and preparation of tenecteplase. We highly recommend the use of designated pharmacists or emergency department pharmacists in this manner to avoid dosing errors.7,16 Since the current pharmacy-provided tenecteplase bottle contains twice the maximum dose indicated for ischemic stroke, only a 5 mL syringe is included in the stroke box to ensure a maximum dose of 25 mg is drawn up after reconstitution. Dosing card charts were made like existing dosing card charts for t-PA to quickly calculate the 0.25 mg/kg dose. In training, the difference in dosing in ischemic stroke was emphasized. Finally, pharmacy has taken responsibility for dosing the medication during stroke codes.
Any medical personnel at WRNMMC can initiate a stroke code by sending a page to the neurology consult service (Figure).
TRANSITION AND RESULTS
From November 2020 to December 2021, 10 patients have been treated in total at WRNMMC (Table).
CONCLUSIONS
The available evidence supports the transition from t-PA to tenecteplase for acute ischemic stroke. The successful transition required months of preparation involving multidisciplinary meetings between neurology, nursing, pharmacy, radiology, rapid response teams, critical care, and emergency medicine departments. Safeguards must be implemented to avoid a tenecteplase dosing error that can lead to potentially life-threatening adverse effects. The results at WRNMMC thus far are promising for safety and efficacy. Several process improvements are planned: a hospital-wide overhead page will accompany the direct page to neurology; other team members, including radiology and pharmacy, will be included on the acute stroke alert; and a stroke-specific paging application will be implemented to better track real-time stroke metrics and improve flow. These measures mirror processes that are occurring in institutions that treat acute stroke patients.
1. Lees KR, Bluhmki E, von Kummer R, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010;375(9727):1695-1703. doi:10.1016/S0140-6736(10)60491-6
2. National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333(24):1581- 1587. doi:10.1056/NEJM199512143332401
3. Hacke W, Donnan G, Fieschi C, et al. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004;363(9411):768-774. doi:10.1016/S0140-6736(04)15692-4
4. Jauch EC, Saver JL, Adams HP Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870-947. doi:10.1161/STR.0b013e318284056a
5. Campbell B, Mitchell P, Churilov L, et al. Tenecteplase versus alteplase before thrombectomy for ischemic stroke. N Engl J Med. 2018;378(17):1573-1582. doi:10.1056/nejmoa1716405
6. Yang P, Zhang Y, Zhang L, et al. Endovascular thrombectomy with or without intravenous alteplase in acute stroke. N Engl J Med. 2020;382(21):1981-1993. doi:10.1056/NEJMoa2001123
7. Menon BK, Buck BH, Singh N, et al. Intravenous tenecteplase compared with alteplase for acute ischaemic stroke in Canada (AcT): a pragmatic, multicentre, open-label, registry-linked, randomised, controlled, noninferiority trial. Lancet. 2022;400(10347):161-169. doi:10.1016/S0140-6736(22)01054-6
8. Campbell BCV, Mitchell PJ, Churilov L, et al. Effect of intravenous tenecteplase dose on cerebral reperfusion before thrombectomy in patients with large vessel occlusion ischemic stroke: the EXTEND-IA TNK part 2 randomized clinical trial. JAMA. 2020;323(13):1257- 1265. doi:10.1001/jama.2020.1511
9. Warach SJ, Dula AN, Milling TJ Jr. Tenecteplase thrombolysis for acute ischemic stroke. Stroke. 2020;51(11):3440- 3451. doi:10.1161/STROKEAHA.120.029749
10. Huang X, Moreton FC, Kalladka D, et al. Coagulation and fibrinolytic activity of tenecteplase and alteplase in acute ischemic stroke. Stroke. 2015;46(12):3543-3546. doi:10.1161/STROKEAHA.115.011290
11. Burgos AM, Saver JL. Evidence that tenecteplase is noninferior to alteplase for acute ischemic stroke: meta-analysis of 5 randomized trials. Stroke. 2019;50(8):2156-2162. doi:10.1161/STROKEAHA.119.025080
12. Potla N, Ganti L. Tenecteplase vs. alteplase for acute ischemic stroke: a systematic review. Int J Emerg Med. 2022;15(1). doi:10.1186/s12245-021-00399-w
13. Warach SJ, Winegar A, Ottenbacher A, Miller C, Gibson D. Abstract WMP52: reduced hospital costs for ischemic stroke treated with tenecteplase. Stroke. 2022;53(suppl 1):AWMP52. doi:10.1161/str.53.suppl_1.WMP52
14. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344-e418. doi:10.1161/str.0000000000000211
15. Faris H, Dewar B, Dowlatshahi D, et al. Ethical justification for deferral of consent in the AcT trial for acute ischemic stroke. Stroke. 2022;53(7):2420-2423. doi:10.1161/strokeaha.122.038760
16. Kvistad CE, Næss H, Helleberg BH, et al. Tenecteplase versus alteplase for the management of acute ischaemic stroke in Norway (NOR-TEST 2, part A): a phase 3, randomised, open-label, blinded endpoint, non-inferiority trial. Lancet Neurol. 2022;21(6):511-519. doi:10.1016/S1474-4422(22)00124-7
1. Lees KR, Bluhmki E, von Kummer R, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010;375(9727):1695-1703. doi:10.1016/S0140-6736(10)60491-6
2. National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333(24):1581- 1587. doi:10.1056/NEJM199512143332401
3. Hacke W, Donnan G, Fieschi C, et al. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004;363(9411):768-774. doi:10.1016/S0140-6736(04)15692-4
4. Jauch EC, Saver JL, Adams HP Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870-947. doi:10.1161/STR.0b013e318284056a
5. Campbell B, Mitchell P, Churilov L, et al. Tenecteplase versus alteplase before thrombectomy for ischemic stroke. N Engl J Med. 2018;378(17):1573-1582. doi:10.1056/nejmoa1716405
6. Yang P, Zhang Y, Zhang L, et al. Endovascular thrombectomy with or without intravenous alteplase in acute stroke. N Engl J Med. 2020;382(21):1981-1993. doi:10.1056/NEJMoa2001123
7. Menon BK, Buck BH, Singh N, et al. Intravenous tenecteplase compared with alteplase for acute ischaemic stroke in Canada (AcT): a pragmatic, multicentre, open-label, registry-linked, randomised, controlled, noninferiority trial. Lancet. 2022;400(10347):161-169. doi:10.1016/S0140-6736(22)01054-6
8. Campbell BCV, Mitchell PJ, Churilov L, et al. Effect of intravenous tenecteplase dose on cerebral reperfusion before thrombectomy in patients with large vessel occlusion ischemic stroke: the EXTEND-IA TNK part 2 randomized clinical trial. JAMA. 2020;323(13):1257- 1265. doi:10.1001/jama.2020.1511
9. Warach SJ, Dula AN, Milling TJ Jr. Tenecteplase thrombolysis for acute ischemic stroke. Stroke. 2020;51(11):3440- 3451. doi:10.1161/STROKEAHA.120.029749
10. Huang X, Moreton FC, Kalladka D, et al. Coagulation and fibrinolytic activity of tenecteplase and alteplase in acute ischemic stroke. Stroke. 2015;46(12):3543-3546. doi:10.1161/STROKEAHA.115.011290
11. Burgos AM, Saver JL. Evidence that tenecteplase is noninferior to alteplase for acute ischemic stroke: meta-analysis of 5 randomized trials. Stroke. 2019;50(8):2156-2162. doi:10.1161/STROKEAHA.119.025080
12. Potla N, Ganti L. Tenecteplase vs. alteplase for acute ischemic stroke: a systematic review. Int J Emerg Med. 2022;15(1). doi:10.1186/s12245-021-00399-w
13. Warach SJ, Winegar A, Ottenbacher A, Miller C, Gibson D. Abstract WMP52: reduced hospital costs for ischemic stroke treated with tenecteplase. Stroke. 2022;53(suppl 1):AWMP52. doi:10.1161/str.53.suppl_1.WMP52
14. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344-e418. doi:10.1161/str.0000000000000211
15. Faris H, Dewar B, Dowlatshahi D, et al. Ethical justification for deferral of consent in the AcT trial for acute ischemic stroke. Stroke. 2022;53(7):2420-2423. doi:10.1161/strokeaha.122.038760
16. Kvistad CE, Næss H, Helleberg BH, et al. Tenecteplase versus alteplase for the management of acute ischaemic stroke in Norway (NOR-TEST 2, part A): a phase 3, randomised, open-label, blinded endpoint, non-inferiority trial. Lancet Neurol. 2022;21(6):511-519. doi:10.1016/S1474-4422(22)00124-7



