User login
Exogenous boosting against shingles not as robust as thought
Exposure to children with chickenpox reduces the incidence of shingles in adults 33% over 2 years, and 27% out to 20 years, according to British investigators.
Being exposed to children with illness due to varicella infection acts as an “exogenous booster” in adults who had chickenpox themselves as children, making shingles less likely, they explained in a BMJ article.
Although that’s good news, it’s been reported previously that exposure to children with chickenpox confers complete protection against shingles in adults for years afterward.
The finding matters in the United Kingdom because varicella vaccine is not part of the pediatric immunization schedule. The United States is the only country that mandates two shots as a requirement for children to attend school.
The United Kingdom, however, is reconsidering its policy. In the past, the exogenous booster idea has been one of the arguments used against mandating the vaccine for children; the concern is that preventing chickenpox in children – and subsequent reexposure to herpes zoster in adults – would kick off a costly wave of shingles in adults.
The study results “are themselves unable to justify for or against specific vaccination schedules, but they do suggest that revised mathematical models are required to estimate the impact of varicella vaccination, with the updated assumption that exogenous boosting is incomplete and only reduces the risk of zoster by about 30%,” noted the investigators, led by Harriet Forbes of the London School of Hygiene and Tropical Medicine.
The researchers identified 9,604 adults with a shingles diagnosis during 1997-2018 who at some point lived with a child who had chickenpox. Data came from the U.K. Clinical Practice Research Datalink, a general practice database.
They then looked at the incidence of shingles within 20 years of exposure to the sick child and compared it with the incidence before exposure and after 20 years, by which time the exogenous booster is thought to wear off. It was a self-controlled case series analysis, “a relatively novel epidemiological study design where individuals act as their own controls. Comparisons are made within individuals rather than between individuals as in a cohort or case control study,” Ms. Forbes and colleagues explained.
After adjustment for age, calendar time, and season, they found that in the 2 years after household exposure to a child with varicella, adults were 33% less likely to develop zoster (incidence ratio 0.67, 95% confidence interval 0.62-0.73), and 27% less likely from 10 to 20 years (IR 0.73, CI 0.62-0.87). The boosting effect appeared to be stronger in men.
“Exogenous boosting provides some protection from the risk of herpes zoster, but not complete immunity, as assumed by previous cost effectiveness estimates of varicella immunization,” the researchers said.
More than two-thirds of the adults with shingles were women, which fits with previous reports. Median age of exposure to a child with varicella was 38 years.
Ms. Forbes and colleagues noted that “the study design required patients with zoster to be living with a child with varicella, therefore the study cohort is younger than a general population with zoster. ... However, when we restricted our analysis to adults aged 50 and older at exposure to varicella, a similar pattern of association was observed, with no evidence of effect modification by age. This suggests that although the median age of our study cohort ... was low, the findings can be generalized to older people.”
There was no external funding for the work, and the lead investigator had no relevant financial disclosures. One investigator reported research grants from GSK and Merck, both makers of chickenpox and shingles vaccines.
SOURCE: Forbes H et al. BMJ. 2020 Jan 22;368:l6987.
Exposure to children with chickenpox reduces the incidence of shingles in adults 33% over 2 years, and 27% out to 20 years, according to British investigators.
Being exposed to children with illness due to varicella infection acts as an “exogenous booster” in adults who had chickenpox themselves as children, making shingles less likely, they explained in a BMJ article.
Although that’s good news, it’s been reported previously that exposure to children with chickenpox confers complete protection against shingles in adults for years afterward.
The finding matters in the United Kingdom because varicella vaccine is not part of the pediatric immunization schedule. The United States is the only country that mandates two shots as a requirement for children to attend school.
The United Kingdom, however, is reconsidering its policy. In the past, the exogenous booster idea has been one of the arguments used against mandating the vaccine for children; the concern is that preventing chickenpox in children – and subsequent reexposure to herpes zoster in adults – would kick off a costly wave of shingles in adults.
The study results “are themselves unable to justify for or against specific vaccination schedules, but they do suggest that revised mathematical models are required to estimate the impact of varicella vaccination, with the updated assumption that exogenous boosting is incomplete and only reduces the risk of zoster by about 30%,” noted the investigators, led by Harriet Forbes of the London School of Hygiene and Tropical Medicine.
The researchers identified 9,604 adults with a shingles diagnosis during 1997-2018 who at some point lived with a child who had chickenpox. Data came from the U.K. Clinical Practice Research Datalink, a general practice database.
They then looked at the incidence of shingles within 20 years of exposure to the sick child and compared it with the incidence before exposure and after 20 years, by which time the exogenous booster is thought to wear off. It was a self-controlled case series analysis, “a relatively novel epidemiological study design where individuals act as their own controls. Comparisons are made within individuals rather than between individuals as in a cohort or case control study,” Ms. Forbes and colleagues explained.
After adjustment for age, calendar time, and season, they found that in the 2 years after household exposure to a child with varicella, adults were 33% less likely to develop zoster (incidence ratio 0.67, 95% confidence interval 0.62-0.73), and 27% less likely from 10 to 20 years (IR 0.73, CI 0.62-0.87). The boosting effect appeared to be stronger in men.
“Exogenous boosting provides some protection from the risk of herpes zoster, but not complete immunity, as assumed by previous cost effectiveness estimates of varicella immunization,” the researchers said.
More than two-thirds of the adults with shingles were women, which fits with previous reports. Median age of exposure to a child with varicella was 38 years.
Ms. Forbes and colleagues noted that “the study design required patients with zoster to be living with a child with varicella, therefore the study cohort is younger than a general population with zoster. ... However, when we restricted our analysis to adults aged 50 and older at exposure to varicella, a similar pattern of association was observed, with no evidence of effect modification by age. This suggests that although the median age of our study cohort ... was low, the findings can be generalized to older people.”
There was no external funding for the work, and the lead investigator had no relevant financial disclosures. One investigator reported research grants from GSK and Merck, both makers of chickenpox and shingles vaccines.
SOURCE: Forbes H et al. BMJ. 2020 Jan 22;368:l6987.
Exposure to children with chickenpox reduces the incidence of shingles in adults 33% over 2 years, and 27% out to 20 years, according to British investigators.
Being exposed to children with illness due to varicella infection acts as an “exogenous booster” in adults who had chickenpox themselves as children, making shingles less likely, they explained in a BMJ article.
Although that’s good news, it’s been reported previously that exposure to children with chickenpox confers complete protection against shingles in adults for years afterward.
The finding matters in the United Kingdom because varicella vaccine is not part of the pediatric immunization schedule. The United States is the only country that mandates two shots as a requirement for children to attend school.
The United Kingdom, however, is reconsidering its policy. In the past, the exogenous booster idea has been one of the arguments used against mandating the vaccine for children; the concern is that preventing chickenpox in children – and subsequent reexposure to herpes zoster in adults – would kick off a costly wave of shingles in adults.
The study results “are themselves unable to justify for or against specific vaccination schedules, but they do suggest that revised mathematical models are required to estimate the impact of varicella vaccination, with the updated assumption that exogenous boosting is incomplete and only reduces the risk of zoster by about 30%,” noted the investigators, led by Harriet Forbes of the London School of Hygiene and Tropical Medicine.
The researchers identified 9,604 adults with a shingles diagnosis during 1997-2018 who at some point lived with a child who had chickenpox. Data came from the U.K. Clinical Practice Research Datalink, a general practice database.
They then looked at the incidence of shingles within 20 years of exposure to the sick child and compared it with the incidence before exposure and after 20 years, by which time the exogenous booster is thought to wear off. It was a self-controlled case series analysis, “a relatively novel epidemiological study design where individuals act as their own controls. Comparisons are made within individuals rather than between individuals as in a cohort or case control study,” Ms. Forbes and colleagues explained.
After adjustment for age, calendar time, and season, they found that in the 2 years after household exposure to a child with varicella, adults were 33% less likely to develop zoster (incidence ratio 0.67, 95% confidence interval 0.62-0.73), and 27% less likely from 10 to 20 years (IR 0.73, CI 0.62-0.87). The boosting effect appeared to be stronger in men.
“Exogenous boosting provides some protection from the risk of herpes zoster, but not complete immunity, as assumed by previous cost effectiveness estimates of varicella immunization,” the researchers said.
More than two-thirds of the adults with shingles were women, which fits with previous reports. Median age of exposure to a child with varicella was 38 years.
Ms. Forbes and colleagues noted that “the study design required patients with zoster to be living with a child with varicella, therefore the study cohort is younger than a general population with zoster. ... However, when we restricted our analysis to adults aged 50 and older at exposure to varicella, a similar pattern of association was observed, with no evidence of effect modification by age. This suggests that although the median age of our study cohort ... was low, the findings can be generalized to older people.”
There was no external funding for the work, and the lead investigator had no relevant financial disclosures. One investigator reported research grants from GSK and Merck, both makers of chickenpox and shingles vaccines.
SOURCE: Forbes H et al. BMJ. 2020 Jan 22;368:l6987.
FROM BMJ
Why is AOM frequency decreasing in the pneumococcal conjugate vaccine era?
In 2000, pneumococcal conjugate vaccine 7 (PCV7) was introduced in the United States, and in 2010, PCV13 was introduced. When each of those vaccines were used, they reduced acute otitis media (AOM) incidence caused by the pneumococcal types included in the vaccines. In the time frame of those vaccine introductions, about one-third of AOM cases occurred because of pneumococci and half of those cases occurred because of strains expressing the serotypes in the two formulations of the vaccines. Efficacy is about 70% for AOM prevention for PCVs. The math matches clinical trial results that have shown about an 11%-12% reduction of all AOM attributable to PCVs. However, our group continues to do tympanocentesis to track the etiology of AOM, and we have reported that elimination of strains of pneumococci expressing capsular types included in the PCVs has been followed by emergence of replacement strains of pneumococci that express non-PCV capsules. We also have shown that Haemophilus influenzae has increased proportionally as a cause of AOM and is the most frequent cause of recurrent AOM. So what else is going on?
My colleague, Stephen I. Pelton, MD, – another ID Consult columnist – is a coauthor of a paper along with Ron Dagan, MD; Lauren Bakaletz, PhD; and Robert Cohen, MD, (all major figures in pneumococcal disease or AOM) that was published in Lancet Infectious Diseases (Dagan R et al. Lancet Infect Dis. 2016 Apr;16[4]:480-92.). They gathered evidence suggesting that prevention of early AOM episodes caused by pneumococci expressing PCV serotypes resulted in a reduction of subsequent complex cases caused by nonvaccine serotypes and other otopathogens. Thus, PCVs may have an impact on AOM indirectly attributable to vaccination.
However, the American Academy of Pediatrics made several recommendations in the 2004 and 2013 guidelines for diagnosis and management of AOM that had a remarkable impact in reducing the frequency that this infection is diagnosed and treated as well. The recommendations included:
- Stricter diagnostic criteria in 2004 that became more strict in 2013 requiring bulging of the eardrum.
- Introduction of “watchful waiting” as an option in management that possibly led to no antibiotic treatment.
- Introduction of delayed prescription of antibiotic when diagnosis was uncertain that possibly led to no antibiotic treatment.
- Endorsement of specific antibiotics with the greatest anticipated efficacy taking into consideration spectrum of activity, safety, and costs.
In the same general time frame, a second development occurred: The Centers for Disease Control and Prevention launched a national campaign to reduce unnecessary and inappropriate antibiotic use in an effort to reduce rising antibiotic resistance among bacteria. The public media and professional communication campaign emphasized that antibiotic treatment carried with it risks that should be considered by patients and clinicians.
Because of the AAP and CDC recommendations, clinicians diagnosed AOM less frequently, and they treated it less frequently. Parents of children took note of the fact that their children with viral upper respiratory infections suspected to have AOM were diagnosed with AOM less often; even when a diagnosis was made, an antibiotic was prescribed less often. Therefore, parents brought their children to clinicians less often when their child had a viral upper respiratory infections or when they suspected AOM.
In addition, guidelines endorsed specific antibiotics that had better efficacy in treatment of AOM. Therefore, when clinicians did treat the infection with antibiotics, they used more effective drugs resulting in fewer treatment failures. This gives the impression of less-frequent AOM as well.
Both universal PCV use and universal influenza vaccine use have been endorsed in recent years, and uptake of that recommendation has increased over time. Clinical trials have shown that influenza is a common virus associated with secondary bacterial AOM.
Lastly, returning to antibiotic use, we now increasingly appreciate the adverse effect on the natural microbiome of the nasopharynx and gut when antibiotics are given. Natural resistance provided by commensals is disrupted when antibiotics are given. This may allow otopathogens to colonize the nasopharynx more readily, an effect that may last for months after a single antibiotic course. We also appreciate more that the microbiome modulates our immune system favorably, so antibiotics that disrupt the microbiome may have an adverse effect on innate or adaptive immunity as well. These adverse consequences of antibiotic use on microbiome and immunity are reduced when less antibiotics are given to children, as has been occurring over the past 2 decades.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He said he had no relevent financial disclosures. Email him at [email protected].
In 2000, pneumococcal conjugate vaccine 7 (PCV7) was introduced in the United States, and in 2010, PCV13 was introduced. When each of those vaccines were used, they reduced acute otitis media (AOM) incidence caused by the pneumococcal types included in the vaccines. In the time frame of those vaccine introductions, about one-third of AOM cases occurred because of pneumococci and half of those cases occurred because of strains expressing the serotypes in the two formulations of the vaccines. Efficacy is about 70% for AOM prevention for PCVs. The math matches clinical trial results that have shown about an 11%-12% reduction of all AOM attributable to PCVs. However, our group continues to do tympanocentesis to track the etiology of AOM, and we have reported that elimination of strains of pneumococci expressing capsular types included in the PCVs has been followed by emergence of replacement strains of pneumococci that express non-PCV capsules. We also have shown that Haemophilus influenzae has increased proportionally as a cause of AOM and is the most frequent cause of recurrent AOM. So what else is going on?
My colleague, Stephen I. Pelton, MD, – another ID Consult columnist – is a coauthor of a paper along with Ron Dagan, MD; Lauren Bakaletz, PhD; and Robert Cohen, MD, (all major figures in pneumococcal disease or AOM) that was published in Lancet Infectious Diseases (Dagan R et al. Lancet Infect Dis. 2016 Apr;16[4]:480-92.). They gathered evidence suggesting that prevention of early AOM episodes caused by pneumococci expressing PCV serotypes resulted in a reduction of subsequent complex cases caused by nonvaccine serotypes and other otopathogens. Thus, PCVs may have an impact on AOM indirectly attributable to vaccination.
However, the American Academy of Pediatrics made several recommendations in the 2004 and 2013 guidelines for diagnosis and management of AOM that had a remarkable impact in reducing the frequency that this infection is diagnosed and treated as well. The recommendations included:
- Stricter diagnostic criteria in 2004 that became more strict in 2013 requiring bulging of the eardrum.
- Introduction of “watchful waiting” as an option in management that possibly led to no antibiotic treatment.
- Introduction of delayed prescription of antibiotic when diagnosis was uncertain that possibly led to no antibiotic treatment.
- Endorsement of specific antibiotics with the greatest anticipated efficacy taking into consideration spectrum of activity, safety, and costs.
In the same general time frame, a second development occurred: The Centers for Disease Control and Prevention launched a national campaign to reduce unnecessary and inappropriate antibiotic use in an effort to reduce rising antibiotic resistance among bacteria. The public media and professional communication campaign emphasized that antibiotic treatment carried with it risks that should be considered by patients and clinicians.
Because of the AAP and CDC recommendations, clinicians diagnosed AOM less frequently, and they treated it less frequently. Parents of children took note of the fact that their children with viral upper respiratory infections suspected to have AOM were diagnosed with AOM less often; even when a diagnosis was made, an antibiotic was prescribed less often. Therefore, parents brought their children to clinicians less often when their child had a viral upper respiratory infections or when they suspected AOM.
In addition, guidelines endorsed specific antibiotics that had better efficacy in treatment of AOM. Therefore, when clinicians did treat the infection with antibiotics, they used more effective drugs resulting in fewer treatment failures. This gives the impression of less-frequent AOM as well.
Both universal PCV use and universal influenza vaccine use have been endorsed in recent years, and uptake of that recommendation has increased over time. Clinical trials have shown that influenza is a common virus associated with secondary bacterial AOM.
Lastly, returning to antibiotic use, we now increasingly appreciate the adverse effect on the natural microbiome of the nasopharynx and gut when antibiotics are given. Natural resistance provided by commensals is disrupted when antibiotics are given. This may allow otopathogens to colonize the nasopharynx more readily, an effect that may last for months after a single antibiotic course. We also appreciate more that the microbiome modulates our immune system favorably, so antibiotics that disrupt the microbiome may have an adverse effect on innate or adaptive immunity as well. These adverse consequences of antibiotic use on microbiome and immunity are reduced when less antibiotics are given to children, as has been occurring over the past 2 decades.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He said he had no relevent financial disclosures. Email him at [email protected].
In 2000, pneumococcal conjugate vaccine 7 (PCV7) was introduced in the United States, and in 2010, PCV13 was introduced. When each of those vaccines were used, they reduced acute otitis media (AOM) incidence caused by the pneumococcal types included in the vaccines. In the time frame of those vaccine introductions, about one-third of AOM cases occurred because of pneumococci and half of those cases occurred because of strains expressing the serotypes in the two formulations of the vaccines. Efficacy is about 70% for AOM prevention for PCVs. The math matches clinical trial results that have shown about an 11%-12% reduction of all AOM attributable to PCVs. However, our group continues to do tympanocentesis to track the etiology of AOM, and we have reported that elimination of strains of pneumococci expressing capsular types included in the PCVs has been followed by emergence of replacement strains of pneumococci that express non-PCV capsules. We also have shown that Haemophilus influenzae has increased proportionally as a cause of AOM and is the most frequent cause of recurrent AOM. So what else is going on?
My colleague, Stephen I. Pelton, MD, – another ID Consult columnist – is a coauthor of a paper along with Ron Dagan, MD; Lauren Bakaletz, PhD; and Robert Cohen, MD, (all major figures in pneumococcal disease or AOM) that was published in Lancet Infectious Diseases (Dagan R et al. Lancet Infect Dis. 2016 Apr;16[4]:480-92.). They gathered evidence suggesting that prevention of early AOM episodes caused by pneumococci expressing PCV serotypes resulted in a reduction of subsequent complex cases caused by nonvaccine serotypes and other otopathogens. Thus, PCVs may have an impact on AOM indirectly attributable to vaccination.
However, the American Academy of Pediatrics made several recommendations in the 2004 and 2013 guidelines for diagnosis and management of AOM that had a remarkable impact in reducing the frequency that this infection is diagnosed and treated as well. The recommendations included:
- Stricter diagnostic criteria in 2004 that became more strict in 2013 requiring bulging of the eardrum.
- Introduction of “watchful waiting” as an option in management that possibly led to no antibiotic treatment.
- Introduction of delayed prescription of antibiotic when diagnosis was uncertain that possibly led to no antibiotic treatment.
- Endorsement of specific antibiotics with the greatest anticipated efficacy taking into consideration spectrum of activity, safety, and costs.
In the same general time frame, a second development occurred: The Centers for Disease Control and Prevention launched a national campaign to reduce unnecessary and inappropriate antibiotic use in an effort to reduce rising antibiotic resistance among bacteria. The public media and professional communication campaign emphasized that antibiotic treatment carried with it risks that should be considered by patients and clinicians.
Because of the AAP and CDC recommendations, clinicians diagnosed AOM less frequently, and they treated it less frequently. Parents of children took note of the fact that their children with viral upper respiratory infections suspected to have AOM were diagnosed with AOM less often; even when a diagnosis was made, an antibiotic was prescribed less often. Therefore, parents brought their children to clinicians less often when their child had a viral upper respiratory infections or when they suspected AOM.
In addition, guidelines endorsed specific antibiotics that had better efficacy in treatment of AOM. Therefore, when clinicians did treat the infection with antibiotics, they used more effective drugs resulting in fewer treatment failures. This gives the impression of less-frequent AOM as well.
Both universal PCV use and universal influenza vaccine use have been endorsed in recent years, and uptake of that recommendation has increased over time. Clinical trials have shown that influenza is a common virus associated with secondary bacterial AOM.
Lastly, returning to antibiotic use, we now increasingly appreciate the adverse effect on the natural microbiome of the nasopharynx and gut when antibiotics are given. Natural resistance provided by commensals is disrupted when antibiotics are given. This may allow otopathogens to colonize the nasopharynx more readily, an effect that may last for months after a single antibiotic course. We also appreciate more that the microbiome modulates our immune system favorably, so antibiotics that disrupt the microbiome may have an adverse effect on innate or adaptive immunity as well. These adverse consequences of antibiotic use on microbiome and immunity are reduced when less antibiotics are given to children, as has been occurring over the past 2 decades.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He said he had no relevent financial disclosures. Email him at [email protected].
Despite PCV, pediatric asthma patients face pneumococcal risks
Even on-time pneumococcal vaccines don’t completely protect children with asthma from developing invasive pneumococcal disease, a meta-analysis has determined.
Despite receiving pneumococcal valent 7, 10, or 13, children with asthma were still almost twice as likely to develop the disease as were children without asthma, Jose A. Castro-Rodriguez, MD, PhD, and colleagues reported in Pediatrics (2020 Jan. doi: 10.1542/peds.2019-1200). None of the studies included rates for those who received the pneumococcal polysaccharide vaccine (PPSV23).
“For the first time, this meta-analysis reveals 90% increased odds of invasive pneumococcal disease (IPD) among [vaccinated] children with asthma,” said Dr. Castro-Rodriguez, of Pontificia Universidad Católica de Chile, Santiago, and colleagues. “If confirmed, these findings will bear clinical and public health importance,” they noted, because guidelines now recommend PPSV23 after age 2 in children with asthma only if they’re treated with prolonged high-dose oral corticosteroids.
However, because the analysis comprised only four studies, the authors cautioned that the results aren’t enough to justify changes to practice recommendations.
Asthma treatment with inhaled corticosteroids (ICS) may be driving the increased risk, Dr. Castro-Rodriguez and his coauthors suggested. ICS deposition in the oropharynx could boost oropharyngeal candidiasis risk by weakening the mucosal immune response, the researchers noted. And that same process may be at work with Streptococcus pneumoniae.
A prior study found that children with asthma who received ICS for at least 1 month were almost four times more likely to have oropharyngeal colonization by S. pneumoniae as were those who didn’t get the drugs. Thus, a higher carrier rate of S. pneumoniae in the oropharynx, along with asthma’s impaired airway clearance, might increase the risk of pneumococcal diseases, the investigators explained.
Dr. Castro-Rodriguez and colleagues analyzed four studies with more than 4,000 cases and controls, and about 26 million person-years of follow-up.
Rates and risks of IPD in the four studies were as follows:
- Among those with IPD, 27% had asthma, with 18% of those without, an adjusted odds ratio (aOR) of 1.8.
- In a European of patients who received at least 3 doses of PCV7, IPD rates per 100,000 person-years for 5-year-olds were 11.6 for children with asthma and 7.3 for those without. For 5- to 17-year-olds with and without asthma, the rates were 2.3 and 1.6, respectively.
- In 2001, a Korean found an aOR of 2.08 for IPD in children with asthma, compared with those without. In 2010, the aOR was 3.26. No vaccine types were reported in the study.
- of IPD were 3.7 per 100,000 person-years for children with asthma, compared with 2.5 for healthy controls – an adjusted relative risk of 1.5.
The pooled estimate of the four studies revealed an aOR of 1.9 for IPD among children with asthma, compared with those without, Dr. Castro-Rodriguez and his team concluded.
None of the studies reported hospital admissions, mortality, length of hospital stay, intensive care admission, invasive respiratory support, or additional medication use.
One, however, did find asthma severity was significantly associated with increasing IPD treatment costs per 100,000 person-years: $72,581 for healthy controls, compared with $100,020 for children with mild asthma, $172,002 for moderate asthma, and $638,452 for severe asthma.
In addition, treating all-cause pneumonia was more expensive in children with asthma. For all-cause pneumonia, the researchers found that estimated costs per 100,000 person-years for mild, moderate, and severe asthma were $7.5 million, $14.6 million, and $46.8 million, respectively, compared with $1.7 million for healthy controls.
The authors had no relevant financial disclosures.
SOURCE: Castro-Rodriguez J et al. Pediatrics. 2020 Jan. doi: 10.1542/peds.2019-1200.
The meta-analysis contains some important lessons for pediatricians, Tina Q. Tan, MD, wrote in an accompanying editorial.
“First, asthma remains a risk factor for invasive pneumococcal disease and pneumococcal pneumonia, even in the era of widespread use of PCV,” Dr. Tan noted. “Second, it is important that all patients, especially those with asthma, are receiving their vaccinations on time and, most notably, are up to date on their pneumococcal vaccinations. This will provide the best protection against pneumococcal infections and their complications for pediatric patients with asthma.”
Pneumococcal conjugate vaccines (PCV) have impressively decreased rates of invasive pneumococcal disease (IPD) and pneumonia in children in the United States, Dr. Tan explained. Overall, incidence dropped from 95 cases per 100,000 person-years in 1998 to only 9 cases per 100,000 in 2016.
In addition, the incidence of IPD caused by 13-valent PCV serotypes fell, from 88 cases per 100,000 in 1998 to 2 cases per 100,000 in 2016.
The threat is not over, however.
“IPD still remains a leading cause of morbidity and mortality in the United States and worldwide,” Dr. Tan cautioned. “In 2017, the CDC’s Active Bacterial Core surveillance network reported that there were 31,000 cases of IPD (meningitis, bacteremia, and bacteremic pneumonia) and 3,590 deaths, of which 147 cases and 9 deaths occurred in children younger than 5 years of age.”
Dr. Tan is a professor of pediatrics at Northwestern University, Chicago. Her comments appear in Pediatrics 2020 Jan. doi: 10.1542/peds.2019-3360 .
The meta-analysis contains some important lessons for pediatricians, Tina Q. Tan, MD, wrote in an accompanying editorial.
“First, asthma remains a risk factor for invasive pneumococcal disease and pneumococcal pneumonia, even in the era of widespread use of PCV,” Dr. Tan noted. “Second, it is important that all patients, especially those with asthma, are receiving their vaccinations on time and, most notably, are up to date on their pneumococcal vaccinations. This will provide the best protection against pneumococcal infections and their complications for pediatric patients with asthma.”
Pneumococcal conjugate vaccines (PCV) have impressively decreased rates of invasive pneumococcal disease (IPD) and pneumonia in children in the United States, Dr. Tan explained. Overall, incidence dropped from 95 cases per 100,000 person-years in 1998 to only 9 cases per 100,000 in 2016.
In addition, the incidence of IPD caused by 13-valent PCV serotypes fell, from 88 cases per 100,000 in 1998 to 2 cases per 100,000 in 2016.
The threat is not over, however.
“IPD still remains a leading cause of morbidity and mortality in the United States and worldwide,” Dr. Tan cautioned. “In 2017, the CDC’s Active Bacterial Core surveillance network reported that there were 31,000 cases of IPD (meningitis, bacteremia, and bacteremic pneumonia) and 3,590 deaths, of which 147 cases and 9 deaths occurred in children younger than 5 years of age.”
Dr. Tan is a professor of pediatrics at Northwestern University, Chicago. Her comments appear in Pediatrics 2020 Jan. doi: 10.1542/peds.2019-3360 .
The meta-analysis contains some important lessons for pediatricians, Tina Q. Tan, MD, wrote in an accompanying editorial.
“First, asthma remains a risk factor for invasive pneumococcal disease and pneumococcal pneumonia, even in the era of widespread use of PCV,” Dr. Tan noted. “Second, it is important that all patients, especially those with asthma, are receiving their vaccinations on time and, most notably, are up to date on their pneumococcal vaccinations. This will provide the best protection against pneumococcal infections and their complications for pediatric patients with asthma.”
Pneumococcal conjugate vaccines (PCV) have impressively decreased rates of invasive pneumococcal disease (IPD) and pneumonia in children in the United States, Dr. Tan explained. Overall, incidence dropped from 95 cases per 100,000 person-years in 1998 to only 9 cases per 100,000 in 2016.
In addition, the incidence of IPD caused by 13-valent PCV serotypes fell, from 88 cases per 100,000 in 1998 to 2 cases per 100,000 in 2016.
The threat is not over, however.
“IPD still remains a leading cause of morbidity and mortality in the United States and worldwide,” Dr. Tan cautioned. “In 2017, the CDC’s Active Bacterial Core surveillance network reported that there were 31,000 cases of IPD (meningitis, bacteremia, and bacteremic pneumonia) and 3,590 deaths, of which 147 cases and 9 deaths occurred in children younger than 5 years of age.”
Dr. Tan is a professor of pediatrics at Northwestern University, Chicago. Her comments appear in Pediatrics 2020 Jan. doi: 10.1542/peds.2019-3360 .
Even on-time pneumococcal vaccines don’t completely protect children with asthma from developing invasive pneumococcal disease, a meta-analysis has determined.
Despite receiving pneumococcal valent 7, 10, or 13, children with asthma were still almost twice as likely to develop the disease as were children without asthma, Jose A. Castro-Rodriguez, MD, PhD, and colleagues reported in Pediatrics (2020 Jan. doi: 10.1542/peds.2019-1200). None of the studies included rates for those who received the pneumococcal polysaccharide vaccine (PPSV23).
“For the first time, this meta-analysis reveals 90% increased odds of invasive pneumococcal disease (IPD) among [vaccinated] children with asthma,” said Dr. Castro-Rodriguez, of Pontificia Universidad Católica de Chile, Santiago, and colleagues. “If confirmed, these findings will bear clinical and public health importance,” they noted, because guidelines now recommend PPSV23 after age 2 in children with asthma only if they’re treated with prolonged high-dose oral corticosteroids.
However, because the analysis comprised only four studies, the authors cautioned that the results aren’t enough to justify changes to practice recommendations.
Asthma treatment with inhaled corticosteroids (ICS) may be driving the increased risk, Dr. Castro-Rodriguez and his coauthors suggested. ICS deposition in the oropharynx could boost oropharyngeal candidiasis risk by weakening the mucosal immune response, the researchers noted. And that same process may be at work with Streptococcus pneumoniae.
A prior study found that children with asthma who received ICS for at least 1 month were almost four times more likely to have oropharyngeal colonization by S. pneumoniae as were those who didn’t get the drugs. Thus, a higher carrier rate of S. pneumoniae in the oropharynx, along with asthma’s impaired airway clearance, might increase the risk of pneumococcal diseases, the investigators explained.
Dr. Castro-Rodriguez and colleagues analyzed four studies with more than 4,000 cases and controls, and about 26 million person-years of follow-up.
Rates and risks of IPD in the four studies were as follows:
- Among those with IPD, 27% had asthma, with 18% of those without, an adjusted odds ratio (aOR) of 1.8.
- In a European of patients who received at least 3 doses of PCV7, IPD rates per 100,000 person-years for 5-year-olds were 11.6 for children with asthma and 7.3 for those without. For 5- to 17-year-olds with and without asthma, the rates were 2.3 and 1.6, respectively.
- In 2001, a Korean found an aOR of 2.08 for IPD in children with asthma, compared with those without. In 2010, the aOR was 3.26. No vaccine types were reported in the study.
- of IPD were 3.7 per 100,000 person-years for children with asthma, compared with 2.5 for healthy controls – an adjusted relative risk of 1.5.
The pooled estimate of the four studies revealed an aOR of 1.9 for IPD among children with asthma, compared with those without, Dr. Castro-Rodriguez and his team concluded.
None of the studies reported hospital admissions, mortality, length of hospital stay, intensive care admission, invasive respiratory support, or additional medication use.
One, however, did find asthma severity was significantly associated with increasing IPD treatment costs per 100,000 person-years: $72,581 for healthy controls, compared with $100,020 for children with mild asthma, $172,002 for moderate asthma, and $638,452 for severe asthma.
In addition, treating all-cause pneumonia was more expensive in children with asthma. For all-cause pneumonia, the researchers found that estimated costs per 100,000 person-years for mild, moderate, and severe asthma were $7.5 million, $14.6 million, and $46.8 million, respectively, compared with $1.7 million for healthy controls.
The authors had no relevant financial disclosures.
SOURCE: Castro-Rodriguez J et al. Pediatrics. 2020 Jan. doi: 10.1542/peds.2019-1200.
Even on-time pneumococcal vaccines don’t completely protect children with asthma from developing invasive pneumococcal disease, a meta-analysis has determined.
Despite receiving pneumococcal valent 7, 10, or 13, children with asthma were still almost twice as likely to develop the disease as were children without asthma, Jose A. Castro-Rodriguez, MD, PhD, and colleagues reported in Pediatrics (2020 Jan. doi: 10.1542/peds.2019-1200). None of the studies included rates for those who received the pneumococcal polysaccharide vaccine (PPSV23).
“For the first time, this meta-analysis reveals 90% increased odds of invasive pneumococcal disease (IPD) among [vaccinated] children with asthma,” said Dr. Castro-Rodriguez, of Pontificia Universidad Católica de Chile, Santiago, and colleagues. “If confirmed, these findings will bear clinical and public health importance,” they noted, because guidelines now recommend PPSV23 after age 2 in children with asthma only if they’re treated with prolonged high-dose oral corticosteroids.
However, because the analysis comprised only four studies, the authors cautioned that the results aren’t enough to justify changes to practice recommendations.
Asthma treatment with inhaled corticosteroids (ICS) may be driving the increased risk, Dr. Castro-Rodriguez and his coauthors suggested. ICS deposition in the oropharynx could boost oropharyngeal candidiasis risk by weakening the mucosal immune response, the researchers noted. And that same process may be at work with Streptococcus pneumoniae.
A prior study found that children with asthma who received ICS for at least 1 month were almost four times more likely to have oropharyngeal colonization by S. pneumoniae as were those who didn’t get the drugs. Thus, a higher carrier rate of S. pneumoniae in the oropharynx, along with asthma’s impaired airway clearance, might increase the risk of pneumococcal diseases, the investigators explained.
Dr. Castro-Rodriguez and colleagues analyzed four studies with more than 4,000 cases and controls, and about 26 million person-years of follow-up.
Rates and risks of IPD in the four studies were as follows:
- Among those with IPD, 27% had asthma, with 18% of those without, an adjusted odds ratio (aOR) of 1.8.
- In a European of patients who received at least 3 doses of PCV7, IPD rates per 100,000 person-years for 5-year-olds were 11.6 for children with asthma and 7.3 for those without. For 5- to 17-year-olds with and without asthma, the rates were 2.3 and 1.6, respectively.
- In 2001, a Korean found an aOR of 2.08 for IPD in children with asthma, compared with those without. In 2010, the aOR was 3.26. No vaccine types were reported in the study.
- of IPD were 3.7 per 100,000 person-years for children with asthma, compared with 2.5 for healthy controls – an adjusted relative risk of 1.5.
The pooled estimate of the four studies revealed an aOR of 1.9 for IPD among children with asthma, compared with those without, Dr. Castro-Rodriguez and his team concluded.
None of the studies reported hospital admissions, mortality, length of hospital stay, intensive care admission, invasive respiratory support, or additional medication use.
One, however, did find asthma severity was significantly associated with increasing IPD treatment costs per 100,000 person-years: $72,581 for healthy controls, compared with $100,020 for children with mild asthma, $172,002 for moderate asthma, and $638,452 for severe asthma.
In addition, treating all-cause pneumonia was more expensive in children with asthma. For all-cause pneumonia, the researchers found that estimated costs per 100,000 person-years for mild, moderate, and severe asthma were $7.5 million, $14.6 million, and $46.8 million, respectively, compared with $1.7 million for healthy controls.
The authors had no relevant financial disclosures.
SOURCE: Castro-Rodriguez J et al. Pediatrics. 2020 Jan. doi: 10.1542/peds.2019-1200.
FROM PEDIATRICS
ID Consult: It’s not necessarily over when measles infection clears
As I write, I imagine readers groaning at yet another measles story. But in early November 2019, in Portland, Oregon, Judy Guzman-Cottrill, DO, recently was groaning at yet another measles case.
Dr. Guzman-Cottrill, a pediatric infectious diseases specialist at Doernbecher Children’s Hospital, recently shared details provided by the local health department:
An unimmunized child developed measles while traveling outside the county. The child may have exposed others at Portland International Airport, a medical center in Vancouver, and potentially at another children’s hospital in the area.
As of Nov. 7, 2019, 1,261 cases of measles from 31 states had been reported to the Centers for Disease Control and Prevention – more cases in a single year since 1992. The case in Portland added at least one to that total, although public officials warned that additional cases could occur Nov. 18th through Dec. 9 (given the incubation period). Like the child in Oregon, most of the individuals who developed measles nationwide in 2019 were unimmunized. At press time, from Jan. 1 to Dec. 5, 2019, 1,276 individual cases of measles have been confirmed in 31 states; CDC released measles reports monthly.
The reasons for refusal of measles vaccine vary, but historically, some parents have made a calculated risk. Measles is rare. Most children are vaccinated. My child will be protected by herd immunity. In some communities, that is no longer true, as we have seen in 2019.
Other parents have decided – erroneously – that measles infection is less risky than measles vaccine. We need to be able to tell them the facts. Thirty percent of individuals who contract measles will develop at least one complication, according to the Centers for Disease Control and Prevention. One in four will be hospitalized. While death from acute measles infection is uncommon, children remain at risk for sequelae months or years after the initial infection.
For example, measles is known to suppress the immune system, an effect that lasts for months or years after the initial infection. Practically, this means that once a child recovers from acute measles infection, he or she has an increased susceptibility to other infections that may last for years. Two studies published late in 2019 described the immune “amnesia” that occurs following measles infection. Essentially, the immune system forgets how to fight other pathogens, leaving children vulnerable to potentially life-threatening infections.
Michael Mina, MD, of the Harvard T.H. Chan School of Public Health, Boston, and colleagues measured the effects of measles infection on the immune system by studying blood samples taken from 77 unimmunized children in the Netherlands before and after measles infection.1 Two months after recovery from mild measles, children had lost a median of 33 % (range, 12%-73%) of preexisting antibodies against a range of common viruses and bacteria. The median loss was 40% after severe measles (range 11% to 62%). Similar changes were not observed after measles vaccine.
A second group of researchers led by Velislava N. Petrova, PhD, of the Wellcome Sanger Institute in Cambridge, England, investigated genetic changes in 26 unvaccinated children from the Netherlands who previously had measles. They found that measles infection reduced the diversity of immune cells available to recognize and fight infections and depleted memory B cells, essentially returning the immune to a more immature state.2
Parents also need to know that children who develop measles are at risk for noninfectious complications.
Yes, SSPE is a rare, but it is not as rare as we once thought. In 2017, investigators in California described 17 cases of SSPE identified in that state between 1998 and 2005.3 The incidence of SSPE was 1 in 1,367 for children less than 5 years at the time of measles infection and 1 in 609 for children less than 12 months when they contracted the virus.
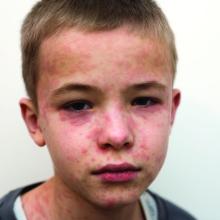
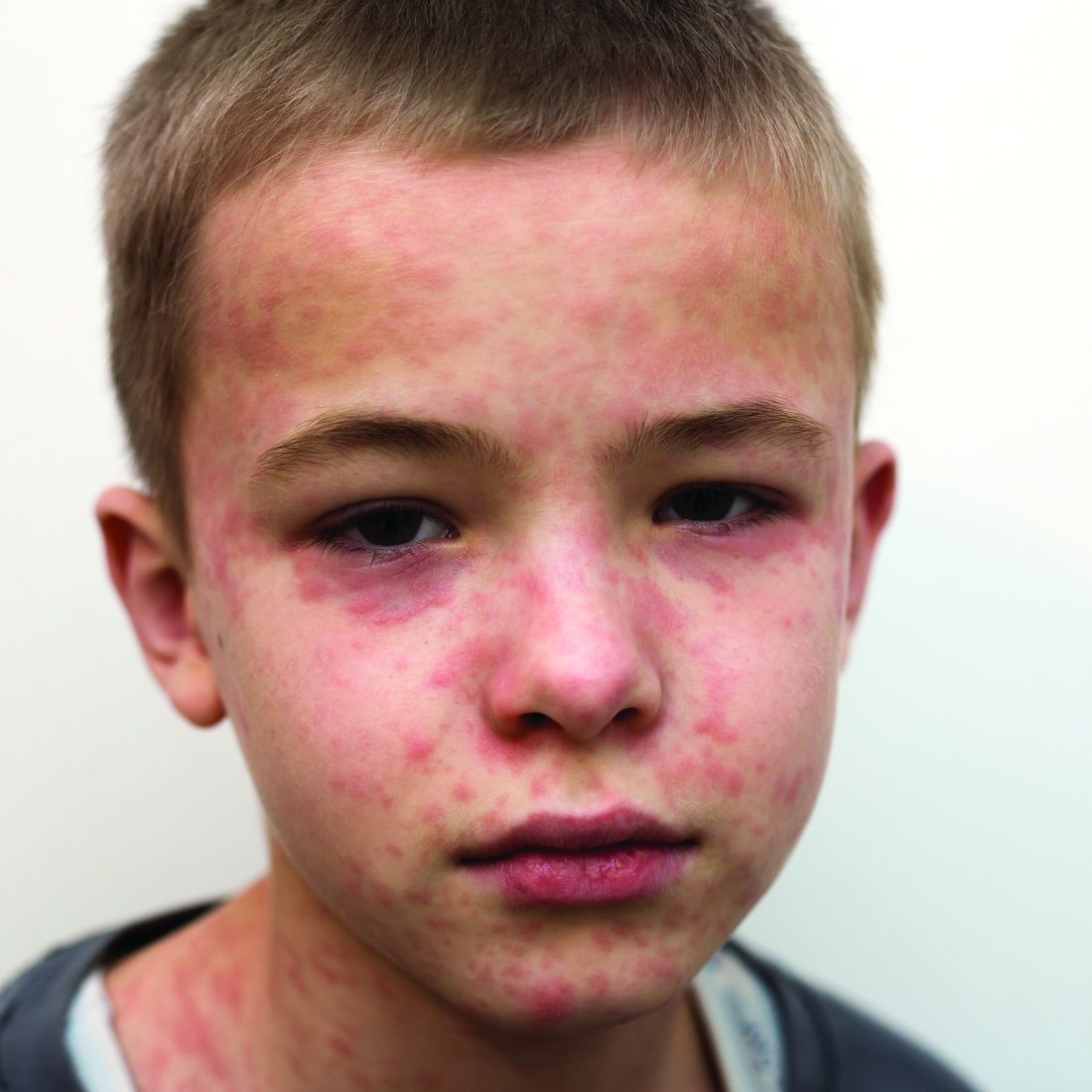
Dr. Guzman-Cottrill has seen a case of SSPE, and she hopes to never see another one. “He had been a healthy 11-year-old boy,” she recalled. “He played soccer and basketball and did well in school.” In the beginning, his symptoms were insidious and nonspecific, Dr. Guzman-Cottrill and colleagues wrote in a 2016 issue of Morbidity and Mortality Weekly Report.4 He started to struggle in school. He dozed off in the middle of meals. He started to drop things. Over a 4-month period, the boy developed progressive spasticity, became unable to eat or drink, and could no longer recognize or communicate with his family. “That’s when I met him,” Dr. Guzman-Cottrill said. “It was heartbreaking, and there was very little we could do for him except give the family a diagnosis. He eventually died in hospice care, nearly 4 years after his symptoms began.”
The boy had been infected with measles at 1 year of age while living in the Philippines. Dr. Guzman-Cottrill emphasized that this family had not refused measles immunization. The child had received a measles vaccine at 8 months of age, but a single vaccine at such a young age wasn’t enough to protect him.
We can hope for change in 2020, including improved immunization rates and a decline in measles cases. If that happens, measles will no longer be a hot topic in the news. We’ll likely never know what happens to the children infected in 2019, those who are facing the current cold and flu season with impaired immune systems. A decade or more will pass before we’ll know if anyone develops SSPE. For now, all we can do is wait … and worry.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville, Ky., and Norton Children’s Hospital, also in Louisville. Dr. Bryant had no relevant financial disclosures. Email her at [email protected].
References
1. Science. 2019 Nov 1;366:599-606.
2. Science Immunology. 2019 Nov 1;4:eaay6125.
As I write, I imagine readers groaning at yet another measles story. But in early November 2019, in Portland, Oregon, Judy Guzman-Cottrill, DO, recently was groaning at yet another measles case.
Dr. Guzman-Cottrill, a pediatric infectious diseases specialist at Doernbecher Children’s Hospital, recently shared details provided by the local health department:
An unimmunized child developed measles while traveling outside the county. The child may have exposed others at Portland International Airport, a medical center in Vancouver, and potentially at another children’s hospital in the area.
As of Nov. 7, 2019, 1,261 cases of measles from 31 states had been reported to the Centers for Disease Control and Prevention – more cases in a single year since 1992. The case in Portland added at least one to that total, although public officials warned that additional cases could occur Nov. 18th through Dec. 9 (given the incubation period). Like the child in Oregon, most of the individuals who developed measles nationwide in 2019 were unimmunized. At press time, from Jan. 1 to Dec. 5, 2019, 1,276 individual cases of measles have been confirmed in 31 states; CDC released measles reports monthly.
The reasons for refusal of measles vaccine vary, but historically, some parents have made a calculated risk. Measles is rare. Most children are vaccinated. My child will be protected by herd immunity. In some communities, that is no longer true, as we have seen in 2019.
Other parents have decided – erroneously – that measles infection is less risky than measles vaccine. We need to be able to tell them the facts. Thirty percent of individuals who contract measles will develop at least one complication, according to the Centers for Disease Control and Prevention. One in four will be hospitalized. While death from acute measles infection is uncommon, children remain at risk for sequelae months or years after the initial infection.
For example, measles is known to suppress the immune system, an effect that lasts for months or years after the initial infection. Practically, this means that once a child recovers from acute measles infection, he or she has an increased susceptibility to other infections that may last for years. Two studies published late in 2019 described the immune “amnesia” that occurs following measles infection. Essentially, the immune system forgets how to fight other pathogens, leaving children vulnerable to potentially life-threatening infections.
Michael Mina, MD, of the Harvard T.H. Chan School of Public Health, Boston, and colleagues measured the effects of measles infection on the immune system by studying blood samples taken from 77 unimmunized children in the Netherlands before and after measles infection.1 Two months after recovery from mild measles, children had lost a median of 33 % (range, 12%-73%) of preexisting antibodies against a range of common viruses and bacteria. The median loss was 40% after severe measles (range 11% to 62%). Similar changes were not observed after measles vaccine.
A second group of researchers led by Velislava N. Petrova, PhD, of the Wellcome Sanger Institute in Cambridge, England, investigated genetic changes in 26 unvaccinated children from the Netherlands who previously had measles. They found that measles infection reduced the diversity of immune cells available to recognize and fight infections and depleted memory B cells, essentially returning the immune to a more immature state.2
Parents also need to know that children who develop measles are at risk for noninfectious complications.
Yes, SSPE is a rare, but it is not as rare as we once thought. In 2017, investigators in California described 17 cases of SSPE identified in that state between 1998 and 2005.3 The incidence of SSPE was 1 in 1,367 for children less than 5 years at the time of measles infection and 1 in 609 for children less than 12 months when they contracted the virus.
Dr. Guzman-Cottrill has seen a case of SSPE, and she hopes to never see another one. “He had been a healthy 11-year-old boy,” she recalled. “He played soccer and basketball and did well in school.” In the beginning, his symptoms were insidious and nonspecific, Dr. Guzman-Cottrill and colleagues wrote in a 2016 issue of Morbidity and Mortality Weekly Report.4 He started to struggle in school. He dozed off in the middle of meals. He started to drop things. Over a 4-month period, the boy developed progressive spasticity, became unable to eat or drink, and could no longer recognize or communicate with his family. “That’s when I met him,” Dr. Guzman-Cottrill said. “It was heartbreaking, and there was very little we could do for him except give the family a diagnosis. He eventually died in hospice care, nearly 4 years after his symptoms began.”
The boy had been infected with measles at 1 year of age while living in the Philippines. Dr. Guzman-Cottrill emphasized that this family had not refused measles immunization. The child had received a measles vaccine at 8 months of age, but a single vaccine at such a young age wasn’t enough to protect him.
We can hope for change in 2020, including improved immunization rates and a decline in measles cases. If that happens, measles will no longer be a hot topic in the news. We’ll likely never know what happens to the children infected in 2019, those who are facing the current cold and flu season with impaired immune systems. A decade or more will pass before we’ll know if anyone develops SSPE. For now, all we can do is wait … and worry.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville, Ky., and Norton Children’s Hospital, also in Louisville. Dr. Bryant had no relevant financial disclosures. Email her at [email protected].
References
1. Science. 2019 Nov 1;366:599-606.
2. Science Immunology. 2019 Nov 1;4:eaay6125.
As I write, I imagine readers groaning at yet another measles story. But in early November 2019, in Portland, Oregon, Judy Guzman-Cottrill, DO, recently was groaning at yet another measles case.
Dr. Guzman-Cottrill, a pediatric infectious diseases specialist at Doernbecher Children’s Hospital, recently shared details provided by the local health department:
An unimmunized child developed measles while traveling outside the county. The child may have exposed others at Portland International Airport, a medical center in Vancouver, and potentially at another children’s hospital in the area.
As of Nov. 7, 2019, 1,261 cases of measles from 31 states had been reported to the Centers for Disease Control and Prevention – more cases in a single year since 1992. The case in Portland added at least one to that total, although public officials warned that additional cases could occur Nov. 18th through Dec. 9 (given the incubation period). Like the child in Oregon, most of the individuals who developed measles nationwide in 2019 were unimmunized. At press time, from Jan. 1 to Dec. 5, 2019, 1,276 individual cases of measles have been confirmed in 31 states; CDC released measles reports monthly.
The reasons for refusal of measles vaccine vary, but historically, some parents have made a calculated risk. Measles is rare. Most children are vaccinated. My child will be protected by herd immunity. In some communities, that is no longer true, as we have seen in 2019.
Other parents have decided – erroneously – that measles infection is less risky than measles vaccine. We need to be able to tell them the facts. Thirty percent of individuals who contract measles will develop at least one complication, according to the Centers for Disease Control and Prevention. One in four will be hospitalized. While death from acute measles infection is uncommon, children remain at risk for sequelae months or years after the initial infection.
For example, measles is known to suppress the immune system, an effect that lasts for months or years after the initial infection. Practically, this means that once a child recovers from acute measles infection, he or she has an increased susceptibility to other infections that may last for years. Two studies published late in 2019 described the immune “amnesia” that occurs following measles infection. Essentially, the immune system forgets how to fight other pathogens, leaving children vulnerable to potentially life-threatening infections.
Michael Mina, MD, of the Harvard T.H. Chan School of Public Health, Boston, and colleagues measured the effects of measles infection on the immune system by studying blood samples taken from 77 unimmunized children in the Netherlands before and after measles infection.1 Two months after recovery from mild measles, children had lost a median of 33 % (range, 12%-73%) of preexisting antibodies against a range of common viruses and bacteria. The median loss was 40% after severe measles (range 11% to 62%). Similar changes were not observed after measles vaccine.
A second group of researchers led by Velislava N. Petrova, PhD, of the Wellcome Sanger Institute in Cambridge, England, investigated genetic changes in 26 unvaccinated children from the Netherlands who previously had measles. They found that measles infection reduced the diversity of immune cells available to recognize and fight infections and depleted memory B cells, essentially returning the immune to a more immature state.2
Parents also need to know that children who develop measles are at risk for noninfectious complications.
Yes, SSPE is a rare, but it is not as rare as we once thought. In 2017, investigators in California described 17 cases of SSPE identified in that state between 1998 and 2005.3 The incidence of SSPE was 1 in 1,367 for children less than 5 years at the time of measles infection and 1 in 609 for children less than 12 months when they contracted the virus.
Dr. Guzman-Cottrill has seen a case of SSPE, and she hopes to never see another one. “He had been a healthy 11-year-old boy,” she recalled. “He played soccer and basketball and did well in school.” In the beginning, his symptoms were insidious and nonspecific, Dr. Guzman-Cottrill and colleagues wrote in a 2016 issue of Morbidity and Mortality Weekly Report.4 He started to struggle in school. He dozed off in the middle of meals. He started to drop things. Over a 4-month period, the boy developed progressive spasticity, became unable to eat or drink, and could no longer recognize or communicate with his family. “That’s when I met him,” Dr. Guzman-Cottrill said. “It was heartbreaking, and there was very little we could do for him except give the family a diagnosis. He eventually died in hospice care, nearly 4 years after his symptoms began.”
The boy had been infected with measles at 1 year of age while living in the Philippines. Dr. Guzman-Cottrill emphasized that this family had not refused measles immunization. The child had received a measles vaccine at 8 months of age, but a single vaccine at such a young age wasn’t enough to protect him.
We can hope for change in 2020, including improved immunization rates and a decline in measles cases. If that happens, measles will no longer be a hot topic in the news. We’ll likely never know what happens to the children infected in 2019, those who are facing the current cold and flu season with impaired immune systems. A decade or more will pass before we’ll know if anyone develops SSPE. For now, all we can do is wait … and worry.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville, Ky., and Norton Children’s Hospital, also in Louisville. Dr. Bryant had no relevant financial disclosures. Email her at [email protected].
References
1. Science. 2019 Nov 1;366:599-606.
2. Science Immunology. 2019 Nov 1;4:eaay6125.
Bringing the HPV vaccination rate into line with other adolescent immunizations
Overall adolescent vaccination coverage is improving in the United States.1 But for adolescents up to 15 years of age, there’s a large gap between the rate of vaccination for human papillomavirus (HPV) and the higher rates of coverage for tetanus, diphtheria, and acellular pertussis (Tdap) and meningococcal conjugate (MenACWY) vaccines.1 Adopting or refining practice customs reviewed in this article can increase HPV vaccination rates and continue to improve coverage of all vaccines recommended by the Advisory Committee on Immunization Practices (ACIP) for adolescents between the ages of 11 and 12.
The evolution of ACIP’s HPV vaccine recommendations
Before December 2016, ACIP recommended a 3-dose HPV series for all adolescents between the ages of 11 and 12, given on a 0, 1-2, and 6-month schedule.2 The series could be started at 9 years of age. It could be administered to females as old as 26 years, and to males through 21 years (or ages 22-26 years for those who wish to be vaccinated, who have certain medical conditions, or who are included in special populations—ie, gay and bisexual men, men who have sex with men, immunocompromised men, men with human immunodeficiency virus [HIV], and transgender men).
In 2016, ACIP revised its recommendation for adolescents who initiate vaccination before their 15th birthday: a 2-dose schedule is adequate, with the second dose given 6 to 12 months after the first dose. For those who initiate vaccination on or after their 15th birthday, and for those with certain medical conditions, the recommendation remains 3 doses on a 0, 1-2, and 6-month schedule.3
As of August 2019,4 ACIP now recommends that all women and men receive catch-up HPV vaccination through age 26. For individuals 27 to 45 years of age who have not been adequately vaccinated, HPV vaccine may be given based on shared clinical decision making with their physician.
How are we doing?
Overall, adolescent vaccination coverage is improving in the United States (see “Vaccination goals from ACIP and Healthy People 2020”1,5,6), but the rate of improvement of HPV coverage is lower than that for Tdap and MenACWY coverage by age 15 years (although completion of the MenACWY vaccine series is low). From 2015 to 2016, coverage increased for 1 or more doses of Tdap, from 86.4% to 88% among 17-year olds (87.9% for 15-year olds), and coverage for 1 or more doses of MenACWY increased from 81.7% to 83.5% among 17-year olds (80.4% among 15-year olds).1 Both Tdap and MenACWY coverage rates have surpassed Healthy People 2020 goals of 80%, and the focus now is on maintenance of coverage. Data from the 2016 National Immunization Survey (NIS)-Teen show that completion of the HPV vaccine series (applying updated HPV vaccine recommendations retrospectively) increased to 45.4% for 15-year-olds,1 still far below the Healthy People 2020 goal of 80%. Completion rates for 2 or more doses of MenACWY also increased from 33.3% to 39.1%.1
SIDEBAR
Vaccination goals from ACIP and Healthy People 2020
The Advisory Committee on Immunization Practices (ACIP) recommends that adolescents routinely receive several vaccines between the ages of 11 and 12 years: an annual influenza vaccine, Tdap, the first dose of MenACWY, and initiation of the HPV series. ACIP also advises a booster dose of MenACWY at age 16 years, and teens and young adults (16-23 years) also may be vaccinated with a multidose serogroup B meningococcal vaccine, preferably before age 18. For those adolescents not up to date with their childhood vaccines, ACIP recommends the following catch-up vaccinations: measles, mumps, rubella (MMR, 2 doses); hepatitis B (HepB, 3 doses); and varicella (VAR, 2 doses).5
Healthy People 2020. In December 2010, the US Department of Health and Human Services released Healthy People 2020, a wide-ranging initiative on health promotion and disease prevention that includes 10-year objectives of increasing coverage with Tdap, at least one dose of MenACWY, and completion of the HPV series among 80% of those ages 13 to 15 years.6 This initiative reflects extensive feedback from more than 2000 organizations and authorities in public health and prevention at federal, state, and local levels—as well as from the public. Adolescent vaccination coverage is estimated by the Centers for Disease Control and Prevention using data from the National Immunization Survey (NIS)-Teen annual survey conducted among parents and guardians of adolescents ages 13 to 17 years.1
Common barriers to improved vaccine coverage
Barriers to improved vaccination rates include a lack of regular assessment of vaccine status; limited use of electronic records, tools, and immunization registries; lack of health care provider knowledge on current vaccine recommendations; vaccine costs; missed opportunities; and patient/parent refusals.7,8 The Community Preventive Services Task Force outlines several well-established evidence-based ways that administrators and physicians can counter these barriers:
- give a strong recommendation to vaccinate,9,10
- incorporate an audit/feedback mechanism for health care providers who vaccinate,9,11
- use electronic alerts to remind health care providers to vaccinate,9,12
- use your state’s electronic immunization information systems (IIS),7,13
- appoint a vaccine practice team/vaccine champion,9,14 and
- implement standing orders and reminder/recall systems.7,9,15
The passage of the Affordable Care Act (ACA)—which mandates that certain preventive services, including ACIP-recommended immunizations, be covered as part of basic care at no cost-sharing—reduces the once-common financial barrier to vaccine uptake.16 A key contributor to low uptake of HPV vaccination by adolescents is parental refusal.17
Continue to: The threats posed by HPV
The threats posed by HPV
HPV infections are the most commonly transmitted infections in the United States and nearly all men and women will be exposed to one or more types of HPV at some point in their lives. Current data show that 79 million Americans, most in their late teens and early 20s, are infected with HPV, and about 14 million people in the United States become newly infected each year.18 HPV is a DNA tumor virus that causes epithelial proliferation at cutaneous and mucosal surfaces.
There are more than 100 types of the virus,19 including more than 40 strains that infect the human genital tract. Of the latter 40 strains, there are oncogenic or high-risk types and non-oncogenic or low-risk types.20 HPV infection with high-risk types causes cervical, vaginal, and vulvar cancers in women; penile cancers in men; and oropharyngeal and anal cancers in both men and women. Low-risk HPV types cause genital warts in both men and women.21 The current available HPV vaccine in the United States is a 9-valent vaccine (9vHPV) that replaces the former 2- and 4-valent HPV vaccines and includes immunogenic coverage against high-risk HPV types 16, 18, 31, 33, 45, 52, and 58; and low-risk types 6 and 11.22
Centers for Disease Control and Prevention (CDC) data from 2010 to 2014 show that approximately 23,700 women and approximately 17,300 men in the United States developed HPV-associated cancer. Most common in women are cervical cancers and in men, oropharyngeal cancers (cancers of the back of the throat, base of the tongue, and tonsils). Using population-based data to genotype HPV types from cancer tissues, the CDC reports that HPV is responsible for about 90% of cervical and anal cancers, 70% of oropharyngeal, vaginal, and vulvar cancers, and 60% of penile cancers.23 A significant percentage of these cancers could potentially be prevented by receipt of 9vHPV.23,24
Make adolescent immunization a high priority
Anticipate opportunities to vaccinate and take steps to make your immunization and scheduling processes more prominent. With HPV specifically, you can strongly advocate for vaccination, address parental misgivings and educate them using clear communication styles, and acquire knowledge to answer concerns about potential vaccine adverse effects.
Every visit is an opportunity to vaccinate. The American Academy of Family Physicians and The American Academy of Pediatrics recommend that adolescents have annual preventive visits for screening, immunizations, and assessment and counseling for risky behaviors. However, many adolescents do not present annually for preventive visits, and fewer than half of adolescents receive regular preventive care.
Continue to: Missed opportunities for the HPV vaccine
Missed opportunities for the HPV vaccine. One study showed that at least 86% of unvaccinated adolescents had missed opportunities to receive HPV vaccine.29 A study of 14,588 adolescent girls from January 2010 through August 2015 showed that HPV vaccine was given at only 37.1% of visits in which MenACWY or Tdap vaccines were administered.30 The rate of HPV vaccination was just 26% during well adolescent visits, and 41.8% during all other primary care visits.30 Every adolescent health care visit—including visits for acute care, chronic care, follow-up, or office-based procedures—is an opportunity to review vaccination status.
Give vaccines concomitantly (simultaneously or same-day). ACIP counsels that minor illnesses, such as mild upper respiratory infections with or without low-grade fever, are not contraindications to routine vaccination.30 Also, the safety of simultaneous vaccine administration, often a concern of both parents and health care providers, has been well established. Each vaccine’s immunogenicity and safety profile are maintained when given concomitantly with other vaccines, and fewer visits are needed to complete an adolescent’s vaccination status.31,32
Immediately schedule follow up visits and use reminder/recall systems. Parents of adolescents who opt for HPV vaccination are not always aware of the timing of the 2- or 3-dose schedule and may not even be aware that more than 1 dose of vaccine is recommended.
A qualitative study of pediatric primary care providers and parents/guardians of adolescent patients showed that for HPV vaccination series completion, 65% of parents/guardians expected to be reminded of any needed doses, while 52% of the pediatric primary care providers relied on parents to schedule subsequent immunizations, and often the HPV series was not completed.33 Higher completion rates of the HPV vaccination series were achieved when follow-up appointments were scheduled at checkout for the 2nd or 3rd vaccine dose after initiation of HPV vaccination.33 The use of patient reminder/recall systems using telephone calls or mailings (phone usage is more effective than mailings) is also shown to improve vaccination completion rates.34
Recommend HPV vaccination clearly and resolutely
In a cross-sectional survey of 800 parents of adolescents ages 9 to 14 years, HPV vaccine was deemed the least likely vaccine to have been “very strongly” recommended by their health care provider, compared with the strength of recommendations for influenza, Tdap, and MenACWY vaccines.35 The strength of a health care provider’s recommendation to vaccinate is the single most influential factor in vaccine uptake.10,36,37 Most family physicians self-report “always recommending standard pediatric vaccines”; however, only a minority are following ACIP recommendations.38 A national study reported that only about two-thirds of parents who received HPV vaccine recommendations perceived a high level of health care provider endorsement.39 The takeaway point: Give a clear, unambiguous, strong recommendation to vaccinate with HPV to prevent infection; cervical, oropharyngeal, and other cancers; and genital warts.
Continue to: Tell parents why the timing is important
Tell parents why the timing is important. Inform parents that the HPV vaccine must be administered while their child is young (before the adolescent’s first sexual contact) to ensure the most robust immune response to the vaccine.40 Unsolicited explanations about sexual activity need not be offered when discussing HPV vaccination, as it is fair to assume that sexual contact is a reality for nearly all people in their adolescent or adult life; and by extension, most sexually active people will likely have exposure to HPV at some time in their lives. By adulthood, sexual activity is nearly universal: The National Longitudinal Study of Adolescent Health showed that only about 3% of participants tracked since adolescence reported no sexual experience by (average age) 28.5 years.41
How you say it matters. Many pediatricians and family physicians report recommending HPV vaccine inconsistently, behind schedule, or without urgency,42 sending mixed messages by failing to endorse HPV vaccination strongly, failing to differentiate it from other vaccines, and presenting it as an “optional” vaccine that could be delayed.43 Physicians and other health care providers who begin conversations about HPV vaccine by saying that the adolescent is “due” for the vaccine show higher vaccine recommendation quality scores than those who give unsolicited information about the vaccine, elicit questions before recommendation, or present the vaccine as an “option.”42 Parents who are “on the fence” may hesitate and decline HPV vaccination with a halfhearted recommendation.44
“Your child is due for his/her Tdap, HPV, influenza, and meningococcal vaccinations to prevent potentially devastating disease and several cancers. I highly recommend all 4 vaccinations today” is more persuasive than, “I recommend your child receive his/her Tdap, meningococcal, and influenza vaccines. And we can also discuss the HPV vaccine.”
Direct presumptive language that assumes vaccine delivery is associated with higher odds of HPV vaccine acceptance and same-day agreement to vaccination than is an open-ended participatory conversational style.45 Saying, “I believe in the importance of this cancer-preventing vaccine for your child” is more persuasive than saying, “What do you think about starting the HPV vaccination series today?”46
Don’t give up when parents initially refuse HPV vaccinations for their adolescents. Parents’ decisions about HPV vaccination may change over time. Repeated positive recommendations and counseling for HPV vaccination over multiple visits have been shown in a large multivariable analysis to increase parent acceptance of HPV vaccination: 45% of parents reported secondary acceptance of HPV vaccination, and an additional 24% intended to vaccinate in the next 12 months.47 Combining a presumptive communication style with motivational interviewing and a fact sheet has contributed to higher clinician-perceived levels of parental HPV vaccine acceptance and increased vaccination rates.48
Continue to: Know how to address parents' concerns about safety
Know how to address parents’ concerns about safety
Be prepared to discuss and answer parents’ questions or concerns regarding any vaccine, especially the HPV vaccine. Social networks are important in parents’ vaccination decision-making,49 and they may seek information from such sources as Twitter, Facebook, Google, and YouTube, where misinformation may be disseminated. A quantitative analysis of 560 YouTube videos relaying a false link between vaccines and autism or other serious adverse effects on children were uploaded between December 2007 and July 2017, with a peak of 224 videos uploaded in the first 7 months of 2017.50 Most were negative in tone and dispensed misinformation.50
The National Vaccine Information Center (NVIC) is an organization that takes a skeptical view of the US government and pharmaceutical companies. NVIC is widely criticized by scientists and leaders in vaccine science and public health as spreading false information on the risks of vaccines and, specifically, that HPV vaccination causes chronic disease. NVIC reports that receipt of HPV vaccine may increase the risk for cervical cancer and death.51 Pediatrician and vaccine researcher Dr. Paul Offit, interviewed by The Lancet in response to NVIC and other anti-vaccine groups’ messages, stated: “anti-vaccination organizations are unequivocally threatening public health.”52
Describe the robust safety-monitoring system. The CDC is aware of public concern about the safety of HPV vaccine. Ongoing monitoring of vaccine safety and studies conducted by the CDC, the Food and Drug Administration (FDA), and other organizations has documented a reassuring safety record since the vaccine’s introduction in 2006.53 Assure parents that the Vaccine Adverse Event Reporting System (VAERS) summary of 7244 reports following 9vHPV vaccination (December 1, 2014 – December 31, 2017) showed that most (97%) reports were nonserious: No new safety signals or unexpected patterns were observed, confirming consistency of the safety profile of 9vHPV with data from pre-licensure trials and post-licensure data on 4vHPV.54
Acknowledge the usually mild, transient potential risks of HPV vaccination as reported to VAERS: local injection site symptoms such as pain, redness, or swelling in the arm where the injection was given (most common adverse effect), dizziness, fainting, headache, nausea, and fever.53 Point out that fainting after vaccination is common in adolescents55 and that the CDC and ACIP recommend observation of adolescents for 15 minutes following HPV vaccination.56 Consider this 15-minute observation period after adolescent receipt of any vaccine to be part of standard practice in your vaccination setting.56
Contest unfounded views. Other common parental concerns about effects of HPV vaccine include supposed promotion of promiscuity, increased incidence of premature ovarian failure or insufficiency (POI), and increased risk of Guillain-Barré Syndrome (GBS), often propagated through published reports, media coverage, Web sites, and social media. Assure worried parents that many studies have shown that receipt of the vaccine is safe and does not lead to initiation of sexual activity or promiscuity, and, in fact, safer sexual health practices have been observed following vaccination.57-59
Continue to: A large longitudinal...
A large longitudinal adolescent health survey administered in British Columbia looked at sexual health behaviors and risk factors in adolescent girls before and after receipt of HPV vaccination (2003, 2008, 2013).59 Results showed no significant change in the reported number of sexual partners (2003-2013), increased reported use of contraception and condoms, and lower pregnancy rates.59 There is no evidence that HPV vaccines cause reproductive problems in women53; a review of VAERS reports from 2009 through 2015 did not detect any safety concerns for POI or other reproductive problems in females.60 A 2018 population-based study of nearly 200,000 women observed no increase of POI following receipt of HPV vaccination.61 In addition, several recent studies have shown no increased risk for GBS following receipt of HPV vaccine.62-64
CORRESPONDENCE
Pamela G. Rockwell, DO, FAAFP, 24 Frank Lloyd Wright Drive, SPC 5795, Room 2300, Lobby H, Ann Arbor, MI 48105; [email protected].
1. Walker TY, Elam-Evans LD, Singleton JA, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:874-882.
2. Markowitz LE, Dunne EF, Saraiya M, et al. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2014;63:1-30.
3. Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2016;65:1405-1408.
4. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702.
5. Robinson CL, Romero JR, Kempe A, et al. Advisory Committee on Immunization Practices (ACIP) Child/Adolescent Immunization Work Group. Advisory Committee on Immunization Practices recommended immunization schedules for persons aged 18 years or younger—United States, 2017. MMWR Morb Mortal Wkly Rep. 2017;66:134-135.
6. US Department of Health and Human Services Office of Disease Prevention and Health Promotion. Healthy People 2020. www.healthypeople.gov/node/4654/data_details. Accessed December 4, 2019.
7. Rockwell PG. What you can do to improve adult immunization rates. J Fam Pract. 2015;64:625-633.
8. Kimmel Sr, Burns IT, Wolfe RM, et al. Addressing immunization barriers, benefits, and risks. J Fam Pract. 2007;56:S61-S69.
9. Briss PA, Zaza S, Pappaioanou M, et al. Developing an evidence-based guide to community preventive services-methods. The Task Force on Community Preventive Services. Am J Prev Med. 2000;18:35-43.
10. Ylitalo KR, Lee H, Mehta NK. Health care provider recommendation, human papillomavirus vaccination, and race/ethnicity in the U.S. National Immunization Survey. Am J Public Health. 2013;103:164-169.
11. National Center for Immunization and Respiratory Diseases. General recommendations on immunization—recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2011;60:1-64.
12. Klatt TE, Hopp E. Effect of a best-practice alert on the rate of influenza vaccination of pregnant women. Obstet Gynecol. 2012;119:301-305.
13. Jones KL, Hammer AL, Swenson C, et al. Improving adult immunization rates in primary care clinics. Nurs Econ. 2008;26:404-407.
14. Hainer BL. Vaccine administration: making the process more efficient in your practice. Fam Pract Manag. 2007;14:48-53.
15. Task Force on Community Preventive Services. Recommendations regarding interventions to improve vaccination coverage in children, adolescents, and adults. Am J Prev Med. 2000;18(suppl 1):92-96.
16. US Department of Health and Human Services. Preventive care. www.hhs.gov/healthcare/about-the-aca/preventive-care/index.html. Accessed December 4, 2019.
17. Gilkey MB, Calo WA, Marciniak, MW, et al. Parents who refuse or delay HPV vaccine: differences in vaccination behavior, beliefs, and clinical communication preferences. Hum Vaccin Immunother. 2017;13:680-686.
18. CDC. Genital HPV infection—fact sheet. www.cdc.gov/std/hpv/stdfact-hpv.htm. Accessed December 4, 2019.
19. WHO. Human papillomavirus (HPV) and cervical cancer. www.who.int/news-room/fact-sheets/detail/human-papillomavirus-(hpv)-and-cervical-cancer. Accessed December 4, 2019.
20. Muñoz N, Bosch FX, de Sanjosé S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518-527.
21. Viens LJ, Henley SJ, Watson M, et al. Human papillomavirus-associated cancers—United States, 2008–2012. MMWR Morb Mortal Wkly Rep. 2016;65:661-666.
22. CDC. Luxembourg A. Program summary and new 9-valent HPV vaccine trial data. Presented at the Advisory Committee on Immunization Practices (ACIP), October 30, 2014. Atlanta, Ga. 2014. www.cdc.gov/vaccines/acip/meetings/downloads/min-archive/min-2014-10.pdf. Accessed December 4, 2019.
23. CDC. HPV and cancer. www.cdc.gov/cancer/hpv/statistics/cases.htm. Accessed December 4, 2019.
24. Lowy DR, Schiller JT. Reducing HPV-associated cancer globally. Cancer Prev Res (Phila). 2012;5:18-23.
25. Rand CM, Goldstein NPN. Patterns of primary care physician visits for US adolescents in 2014: implications for vaccination. Acad Pediatr. 2018;18:S72-S78.
26. Taylor JL, Aalsma MC, Gilbert AL, et al. Perspectives of family medicine physicians on the importance of adolescent preventive care: a multivariate analysis. BMC Fam Pract. 2016;17:4.
27. Harris SK, Aalsma MC, Weitzman ER, et al. Research on clinical preventive services for adolescents and young adults: Where are we and where do we need to go? J Adolesc Health. 2017;60:249-260.
28. Gilkey MB, Moss JL, McRee AL, et al. Do correlates of HPV vaccine initiation differ between adolescent boys and girls? Vaccine. 2012;30:5928-5934.
29. Espinosa CM, Marshall GS, Woods CR, et al. Missed opportunities for human papillomavirus vaccine initiation in an insured adolescent female population. J Pediatric Infect Dis Soc. 2017;6:360-365.
30. CDC. Update: Vaccine side effects, adverse reactions, contraindications, and precautions. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 1996;45:1-35.
31. Moss JL, Reiter PL, Brewer NT. Concomitant adolescent vaccination in the U.S., 2007-2012. Am J Prev Med. 2016;51:693-705.
32. Noronha AS, Markowitz LE, Dunne EF. Systematic review of human papillomavirus vaccine coadministration. Vaccine. 2014;32:2670-2674.
33. Perkins RB, Chigurupati NL, Apte G, et al. Why don’t adolescents finish the HPV vaccine series? A qualitative study of parents and providers. Hum Vaccin Immunother. 2016;12:1528-1535.
34. Jacobson Vann JC, Szilagyi P. Patient reminder and patient recall systems to improve immunization rates. Cochrane Database Syst Rev. 2005;(3):CD003941.
35. Dempsey AF, O’Leary ST. Human papillomavirus vaccination: narrative review of studies on how providers’ vaccine communication affects attitudes and uptake. Acad Pediatr. 2018;18:S23-S27.
36. Rosenthal SL, Weiss TW, Zimet GD, et al. Predictors of HPV vaccine uptake among women aged 19–26: importance of a physician’s recommendation. Vaccine. 2011;29:890-895.
37. Gargano LM, Herbert NL, Painter JE, et al. Impact of a physician recommendation and parental immunization attitudes on receipt or intention to receive adolescent vaccines. Hum Vaccin Immunother. 2013;9:2627-2633.
38. Bonville CA, Domachowske JB, Cibula DA, et al. Immunization attitudes and practices among family medicine providers. Hum Vaccin Immunother. 2017;13:2646-2653.
39. Wilson R, Brown DR, Boothe MA, et al. Knowledge and acceptability of the HPV vaccine among ethnically diverse black women. J Immigr Minor Health. 2013;15:747-757.
40. Iversen O, Miranda MJ, Ulied A, et al. Immunogenicity of the 9-valent HPV vaccine using 2-dose regimens in girls and boys vs a 3-dose regimen in women. JAMA. 2016;316:2411–2421.
41. Haydon AA, Cheng MM, Herring AH, et al. Prevalence and predictors of sexual inexperience in adulthood. Arch Sex Behav. 2014;43:221-230.
42. Gilkey MB, Malo TL, Shah PD, et al. Quality of physician communication about human papillomavirus vaccine: findings from a national survey. Cancer Epidemiol Biomarkers Prev. 2015;24:1673-1679.
43. Gilkey MB, McRee AL. Provider communication about HPV vaccination: a systemic review. Hum Vaccin Immunother. 2016;12:1454-1468.
44. American Academy of Family Physicians. Strong recommendation to vaccinate against HPV is key to boosting uptake. www.aafp.org/news/health-of-the-public/20140212hpv-vaccltr.html. Accessed December 4, 2019.
45. Sturm L, Donahue K, Kasting M, et al. Pediatrician-parent conversations about human papillomavirus vaccination: an analysis of audio recordings. J Adolesc Health. 2017;61:246-251.
46. Malo TL, Gilkey MB, Hall ME, et al. Messages to motivate human papillomavirus vaccination: national studies of parents and physicians. Cancer Epidemiol Biomarkers Prev. 2016;25:1383-1391.
47. Kornides ML, McRee AL, Gilkey MB. Parents who decline HPV vaccination: Who later accepts and why? Acad Pediatr. 2018;18:S37-S43.
48. Reno JE, Thomas J, Pyrzanowski J, et al. Examining strategies for improving healthcare providers’ communication about adolescent HPV vaccination: evaluation of secondary outcomes in a randomized controlled trial. Hum Vaccin Immunother. 2018;15:1592-1598.
49. Brunson EK. The impact of social networks on parents’ vaccination decisions. Pediatrics. 2013;131:e1397-e1404.
50. Donzelli G, Palomba G, Federigi L, et al. Misinformation on vaccination: a quantitative analysis of YouTube videos. Hum Vaccin Immunother. 2018;14:1654-1659.
51. National Vaccine Information Center. Human papillomavirus (HPV) disease and vaccine information. www.nvic.org/Vaccines-and-Diseases/hpv.aspx. Accessed December 4, 2019.
52. Shetty P. Experts concerned about vaccination backlash. Lancet. 2010; 375:970-971.
53. CDC. Frequently asked questions about HPV vaccine safety. www.cdc.gov/vaccinesafety/vaccines/hpv/hpv-safety-faqs.html. Accessed December 4, 2019.
54. Arana J, Su J, Lewis P, et al. Post-licensure surveillance of 9-valent human papillomavirus vaccine (9vHPV) in the Vaccine Adverse Event Reporting System (VAERS), United States, 2014-2017. https://idsa.confex.com/idsa/2018/webprogram/Paper69618.html. Accessed December 4, 2019.
55. Braun MM, Patriarca PA, Ellenberg SS. Syncope after immunization. Arch Ped Adolesc Med. 1997;151:255-259.
56. Kroger AT, Duchin J, Vázquez M. General best practice guidelines for immunization. Best practices guidance of the Advisory Committee on Immunization Practices (ACIP). www.cdc.gov/vaccines/hcp/acip-recs/general-recs/index.html. Accessed December 4, 2019.
57. Hansen BT. No evidence that HPV vaccination leads to sexual risk compensation. Hum Vaccin Immunother. 2016;12:1451-1453.
58. Smith LM, Kaufman JS, Strumpf EC, et al. Effect of human papillomavirus (HPV) vaccination on clinical indicators of sexual behaviour among adolescent girls: the Ontario Grade 8 HPV Vaccine Cohort Study. CMAJ. 2015;187:E74-81.
59. Ogilvie GS, Phan F, Pederson HN, et al. Population-level sexual behaviours in adolescent girls before and after introduction of the human papillomavirus vaccine (2003-2013). CMAJ. 2018;190:E1221-E1226.
60. Arana JE, Harrington T, Cano M, et al. Post-licensure safety monitoring of quadrivalent human papillomavirus vaccine in the Vaccine Adverse Event Reporting System (VAERS), 2009-2015. Vaccine. 2018;36:1781-1788.
61. Naleway AL, Mittendorf KF, Irving SA, et al. Primary ovarian insufficiency and adolescent vaccination. Pediatrics. 2018;142. pii: e20190943.
62. Deceuninck G, Sauvageau C, Gilca V, et al. Absence of association between Guillain-Barré syndrome hospitalizations and HPV-vaccine. Expert Rev Vaccines. 2018;17:99-102.
63. Mouchet J, Salvo F, Raschi E, et al. Human papillomavirus vaccine and demyelinating diseases – a systematic review and meta-analysis. Pharmacol Res. 2018;132:108-118.
64. Gee J, Sukumaran L, Weinstraub E, et al. Risk of Guillain-Barre Syndrome following quadrivalent human papillomavirus vaccine in the Vaccine Safety Datalink. Vaccine. 2017;35:5756-5758.
Overall adolescent vaccination coverage is improving in the United States.1 But for adolescents up to 15 years of age, there’s a large gap between the rate of vaccination for human papillomavirus (HPV) and the higher rates of coverage for tetanus, diphtheria, and acellular pertussis (Tdap) and meningococcal conjugate (MenACWY) vaccines.1 Adopting or refining practice customs reviewed in this article can increase HPV vaccination rates and continue to improve coverage of all vaccines recommended by the Advisory Committee on Immunization Practices (ACIP) for adolescents between the ages of 11 and 12.
The evolution of ACIP’s HPV vaccine recommendations
Before December 2016, ACIP recommended a 3-dose HPV series for all adolescents between the ages of 11 and 12, given on a 0, 1-2, and 6-month schedule.2 The series could be started at 9 years of age. It could be administered to females as old as 26 years, and to males through 21 years (or ages 22-26 years for those who wish to be vaccinated, who have certain medical conditions, or who are included in special populations—ie, gay and bisexual men, men who have sex with men, immunocompromised men, men with human immunodeficiency virus [HIV], and transgender men).
In 2016, ACIP revised its recommendation for adolescents who initiate vaccination before their 15th birthday: a 2-dose schedule is adequate, with the second dose given 6 to 12 months after the first dose. For those who initiate vaccination on or after their 15th birthday, and for those with certain medical conditions, the recommendation remains 3 doses on a 0, 1-2, and 6-month schedule.3
As of August 2019,4 ACIP now recommends that all women and men receive catch-up HPV vaccination through age 26. For individuals 27 to 45 years of age who have not been adequately vaccinated, HPV vaccine may be given based on shared clinical decision making with their physician.
How are we doing?
Overall, adolescent vaccination coverage is improving in the United States (see “Vaccination goals from ACIP and Healthy People 2020”1,5,6), but the rate of improvement of HPV coverage is lower than that for Tdap and MenACWY coverage by age 15 years (although completion of the MenACWY vaccine series is low). From 2015 to 2016, coverage increased for 1 or more doses of Tdap, from 86.4% to 88% among 17-year olds (87.9% for 15-year olds), and coverage for 1 or more doses of MenACWY increased from 81.7% to 83.5% among 17-year olds (80.4% among 15-year olds).1 Both Tdap and MenACWY coverage rates have surpassed Healthy People 2020 goals of 80%, and the focus now is on maintenance of coverage. Data from the 2016 National Immunization Survey (NIS)-Teen show that completion of the HPV vaccine series (applying updated HPV vaccine recommendations retrospectively) increased to 45.4% for 15-year-olds,1 still far below the Healthy People 2020 goal of 80%. Completion rates for 2 or more doses of MenACWY also increased from 33.3% to 39.1%.1
SIDEBAR
Vaccination goals from ACIP and Healthy People 2020
The Advisory Committee on Immunization Practices (ACIP) recommends that adolescents routinely receive several vaccines between the ages of 11 and 12 years: an annual influenza vaccine, Tdap, the first dose of MenACWY, and initiation of the HPV series. ACIP also advises a booster dose of MenACWY at age 16 years, and teens and young adults (16-23 years) also may be vaccinated with a multidose serogroup B meningococcal vaccine, preferably before age 18. For those adolescents not up to date with their childhood vaccines, ACIP recommends the following catch-up vaccinations: measles, mumps, rubella (MMR, 2 doses); hepatitis B (HepB, 3 doses); and varicella (VAR, 2 doses).5
Healthy People 2020. In December 2010, the US Department of Health and Human Services released Healthy People 2020, a wide-ranging initiative on health promotion and disease prevention that includes 10-year objectives of increasing coverage with Tdap, at least one dose of MenACWY, and completion of the HPV series among 80% of those ages 13 to 15 years.6 This initiative reflects extensive feedback from more than 2000 organizations and authorities in public health and prevention at federal, state, and local levels—as well as from the public. Adolescent vaccination coverage is estimated by the Centers for Disease Control and Prevention using data from the National Immunization Survey (NIS)-Teen annual survey conducted among parents and guardians of adolescents ages 13 to 17 years.1
Common barriers to improved vaccine coverage
Barriers to improved vaccination rates include a lack of regular assessment of vaccine status; limited use of electronic records, tools, and immunization registries; lack of health care provider knowledge on current vaccine recommendations; vaccine costs; missed opportunities; and patient/parent refusals.7,8 The Community Preventive Services Task Force outlines several well-established evidence-based ways that administrators and physicians can counter these barriers:
- give a strong recommendation to vaccinate,9,10
- incorporate an audit/feedback mechanism for health care providers who vaccinate,9,11
- use electronic alerts to remind health care providers to vaccinate,9,12
- use your state’s electronic immunization information systems (IIS),7,13
- appoint a vaccine practice team/vaccine champion,9,14 and
- implement standing orders and reminder/recall systems.7,9,15
The passage of the Affordable Care Act (ACA)—which mandates that certain preventive services, including ACIP-recommended immunizations, be covered as part of basic care at no cost-sharing—reduces the once-common financial barrier to vaccine uptake.16 A key contributor to low uptake of HPV vaccination by adolescents is parental refusal.17
Continue to: The threats posed by HPV
The threats posed by HPV
HPV infections are the most commonly transmitted infections in the United States and nearly all men and women will be exposed to one or more types of HPV at some point in their lives. Current data show that 79 million Americans, most in their late teens and early 20s, are infected with HPV, and about 14 million people in the United States become newly infected each year.18 HPV is a DNA tumor virus that causes epithelial proliferation at cutaneous and mucosal surfaces.
There are more than 100 types of the virus,19 including more than 40 strains that infect the human genital tract. Of the latter 40 strains, there are oncogenic or high-risk types and non-oncogenic or low-risk types.20 HPV infection with high-risk types causes cervical, vaginal, and vulvar cancers in women; penile cancers in men; and oropharyngeal and anal cancers in both men and women. Low-risk HPV types cause genital warts in both men and women.21 The current available HPV vaccine in the United States is a 9-valent vaccine (9vHPV) that replaces the former 2- and 4-valent HPV vaccines and includes immunogenic coverage against high-risk HPV types 16, 18, 31, 33, 45, 52, and 58; and low-risk types 6 and 11.22
Centers for Disease Control and Prevention (CDC) data from 2010 to 2014 show that approximately 23,700 women and approximately 17,300 men in the United States developed HPV-associated cancer. Most common in women are cervical cancers and in men, oropharyngeal cancers (cancers of the back of the throat, base of the tongue, and tonsils). Using population-based data to genotype HPV types from cancer tissues, the CDC reports that HPV is responsible for about 90% of cervical and anal cancers, 70% of oropharyngeal, vaginal, and vulvar cancers, and 60% of penile cancers.23 A significant percentage of these cancers could potentially be prevented by receipt of 9vHPV.23,24
Make adolescent immunization a high priority
Anticipate opportunities to vaccinate and take steps to make your immunization and scheduling processes more prominent. With HPV specifically, you can strongly advocate for vaccination, address parental misgivings and educate them using clear communication styles, and acquire knowledge to answer concerns about potential vaccine adverse effects.
Every visit is an opportunity to vaccinate. The American Academy of Family Physicians and The American Academy of Pediatrics recommend that adolescents have annual preventive visits for screening, immunizations, and assessment and counseling for risky behaviors. However, many adolescents do not present annually for preventive visits, and fewer than half of adolescents receive regular preventive care.
Continue to: Missed opportunities for the HPV vaccine
Missed opportunities for the HPV vaccine. One study showed that at least 86% of unvaccinated adolescents had missed opportunities to receive HPV vaccine.29 A study of 14,588 adolescent girls from January 2010 through August 2015 showed that HPV vaccine was given at only 37.1% of visits in which MenACWY or Tdap vaccines were administered.30 The rate of HPV vaccination was just 26% during well adolescent visits, and 41.8% during all other primary care visits.30 Every adolescent health care visit—including visits for acute care, chronic care, follow-up, or office-based procedures—is an opportunity to review vaccination status.
Give vaccines concomitantly (simultaneously or same-day). ACIP counsels that minor illnesses, such as mild upper respiratory infections with or without low-grade fever, are not contraindications to routine vaccination.30 Also, the safety of simultaneous vaccine administration, often a concern of both parents and health care providers, has been well established. Each vaccine’s immunogenicity and safety profile are maintained when given concomitantly with other vaccines, and fewer visits are needed to complete an adolescent’s vaccination status.31,32
Immediately schedule follow up visits and use reminder/recall systems. Parents of adolescents who opt for HPV vaccination are not always aware of the timing of the 2- or 3-dose schedule and may not even be aware that more than 1 dose of vaccine is recommended.
A qualitative study of pediatric primary care providers and parents/guardians of adolescent patients showed that for HPV vaccination series completion, 65% of parents/guardians expected to be reminded of any needed doses, while 52% of the pediatric primary care providers relied on parents to schedule subsequent immunizations, and often the HPV series was not completed.33 Higher completion rates of the HPV vaccination series were achieved when follow-up appointments were scheduled at checkout for the 2nd or 3rd vaccine dose after initiation of HPV vaccination.33 The use of patient reminder/recall systems using telephone calls or mailings (phone usage is more effective than mailings) is also shown to improve vaccination completion rates.34
Recommend HPV vaccination clearly and resolutely
In a cross-sectional survey of 800 parents of adolescents ages 9 to 14 years, HPV vaccine was deemed the least likely vaccine to have been “very strongly” recommended by their health care provider, compared with the strength of recommendations for influenza, Tdap, and MenACWY vaccines.35 The strength of a health care provider’s recommendation to vaccinate is the single most influential factor in vaccine uptake.10,36,37 Most family physicians self-report “always recommending standard pediatric vaccines”; however, only a minority are following ACIP recommendations.38 A national study reported that only about two-thirds of parents who received HPV vaccine recommendations perceived a high level of health care provider endorsement.39 The takeaway point: Give a clear, unambiguous, strong recommendation to vaccinate with HPV to prevent infection; cervical, oropharyngeal, and other cancers; and genital warts.
Continue to: Tell parents why the timing is important
Tell parents why the timing is important. Inform parents that the HPV vaccine must be administered while their child is young (before the adolescent’s first sexual contact) to ensure the most robust immune response to the vaccine.40 Unsolicited explanations about sexual activity need not be offered when discussing HPV vaccination, as it is fair to assume that sexual contact is a reality for nearly all people in their adolescent or adult life; and by extension, most sexually active people will likely have exposure to HPV at some time in their lives. By adulthood, sexual activity is nearly universal: The National Longitudinal Study of Adolescent Health showed that only about 3% of participants tracked since adolescence reported no sexual experience by (average age) 28.5 years.41
How you say it matters. Many pediatricians and family physicians report recommending HPV vaccine inconsistently, behind schedule, or without urgency,42 sending mixed messages by failing to endorse HPV vaccination strongly, failing to differentiate it from other vaccines, and presenting it as an “optional” vaccine that could be delayed.43 Physicians and other health care providers who begin conversations about HPV vaccine by saying that the adolescent is “due” for the vaccine show higher vaccine recommendation quality scores than those who give unsolicited information about the vaccine, elicit questions before recommendation, or present the vaccine as an “option.”42 Parents who are “on the fence” may hesitate and decline HPV vaccination with a halfhearted recommendation.44
“Your child is due for his/her Tdap, HPV, influenza, and meningococcal vaccinations to prevent potentially devastating disease and several cancers. I highly recommend all 4 vaccinations today” is more persuasive than, “I recommend your child receive his/her Tdap, meningococcal, and influenza vaccines. And we can also discuss the HPV vaccine.”
Direct presumptive language that assumes vaccine delivery is associated with higher odds of HPV vaccine acceptance and same-day agreement to vaccination than is an open-ended participatory conversational style.45 Saying, “I believe in the importance of this cancer-preventing vaccine for your child” is more persuasive than saying, “What do you think about starting the HPV vaccination series today?”46
Don’t give up when parents initially refuse HPV vaccinations for their adolescents. Parents’ decisions about HPV vaccination may change over time. Repeated positive recommendations and counseling for HPV vaccination over multiple visits have been shown in a large multivariable analysis to increase parent acceptance of HPV vaccination: 45% of parents reported secondary acceptance of HPV vaccination, and an additional 24% intended to vaccinate in the next 12 months.47 Combining a presumptive communication style with motivational interviewing and a fact sheet has contributed to higher clinician-perceived levels of parental HPV vaccine acceptance and increased vaccination rates.48
Continue to: Know how to address parents' concerns about safety
Know how to address parents’ concerns about safety
Be prepared to discuss and answer parents’ questions or concerns regarding any vaccine, especially the HPV vaccine. Social networks are important in parents’ vaccination decision-making,49 and they may seek information from such sources as Twitter, Facebook, Google, and YouTube, where misinformation may be disseminated. A quantitative analysis of 560 YouTube videos relaying a false link between vaccines and autism or other serious adverse effects on children were uploaded between December 2007 and July 2017, with a peak of 224 videos uploaded in the first 7 months of 2017.50 Most were negative in tone and dispensed misinformation.50
The National Vaccine Information Center (NVIC) is an organization that takes a skeptical view of the US government and pharmaceutical companies. NVIC is widely criticized by scientists and leaders in vaccine science and public health as spreading false information on the risks of vaccines and, specifically, that HPV vaccination causes chronic disease. NVIC reports that receipt of HPV vaccine may increase the risk for cervical cancer and death.51 Pediatrician and vaccine researcher Dr. Paul Offit, interviewed by The Lancet in response to NVIC and other anti-vaccine groups’ messages, stated: “anti-vaccination organizations are unequivocally threatening public health.”52
Describe the robust safety-monitoring system. The CDC is aware of public concern about the safety of HPV vaccine. Ongoing monitoring of vaccine safety and studies conducted by the CDC, the Food and Drug Administration (FDA), and other organizations has documented a reassuring safety record since the vaccine’s introduction in 2006.53 Assure parents that the Vaccine Adverse Event Reporting System (VAERS) summary of 7244 reports following 9vHPV vaccination (December 1, 2014 – December 31, 2017) showed that most (97%) reports were nonserious: No new safety signals or unexpected patterns were observed, confirming consistency of the safety profile of 9vHPV with data from pre-licensure trials and post-licensure data on 4vHPV.54
Acknowledge the usually mild, transient potential risks of HPV vaccination as reported to VAERS: local injection site symptoms such as pain, redness, or swelling in the arm where the injection was given (most common adverse effect), dizziness, fainting, headache, nausea, and fever.53 Point out that fainting after vaccination is common in adolescents55 and that the CDC and ACIP recommend observation of adolescents for 15 minutes following HPV vaccination.56 Consider this 15-minute observation period after adolescent receipt of any vaccine to be part of standard practice in your vaccination setting.56
Contest unfounded views. Other common parental concerns about effects of HPV vaccine include supposed promotion of promiscuity, increased incidence of premature ovarian failure or insufficiency (POI), and increased risk of Guillain-Barré Syndrome (GBS), often propagated through published reports, media coverage, Web sites, and social media. Assure worried parents that many studies have shown that receipt of the vaccine is safe and does not lead to initiation of sexual activity or promiscuity, and, in fact, safer sexual health practices have been observed following vaccination.57-59
Continue to: A large longitudinal...
A large longitudinal adolescent health survey administered in British Columbia looked at sexual health behaviors and risk factors in adolescent girls before and after receipt of HPV vaccination (2003, 2008, 2013).59 Results showed no significant change in the reported number of sexual partners (2003-2013), increased reported use of contraception and condoms, and lower pregnancy rates.59 There is no evidence that HPV vaccines cause reproductive problems in women53; a review of VAERS reports from 2009 through 2015 did not detect any safety concerns for POI or other reproductive problems in females.60 A 2018 population-based study of nearly 200,000 women observed no increase of POI following receipt of HPV vaccination.61 In addition, several recent studies have shown no increased risk for GBS following receipt of HPV vaccine.62-64
CORRESPONDENCE
Pamela G. Rockwell, DO, FAAFP, 24 Frank Lloyd Wright Drive, SPC 5795, Room 2300, Lobby H, Ann Arbor, MI 48105; [email protected].
Overall adolescent vaccination coverage is improving in the United States.1 But for adolescents up to 15 years of age, there’s a large gap between the rate of vaccination for human papillomavirus (HPV) and the higher rates of coverage for tetanus, diphtheria, and acellular pertussis (Tdap) and meningococcal conjugate (MenACWY) vaccines.1 Adopting or refining practice customs reviewed in this article can increase HPV vaccination rates and continue to improve coverage of all vaccines recommended by the Advisory Committee on Immunization Practices (ACIP) for adolescents between the ages of 11 and 12.
The evolution of ACIP’s HPV vaccine recommendations
Before December 2016, ACIP recommended a 3-dose HPV series for all adolescents between the ages of 11 and 12, given on a 0, 1-2, and 6-month schedule.2 The series could be started at 9 years of age. It could be administered to females as old as 26 years, and to males through 21 years (or ages 22-26 years for those who wish to be vaccinated, who have certain medical conditions, or who are included in special populations—ie, gay and bisexual men, men who have sex with men, immunocompromised men, men with human immunodeficiency virus [HIV], and transgender men).
In 2016, ACIP revised its recommendation for adolescents who initiate vaccination before their 15th birthday: a 2-dose schedule is adequate, with the second dose given 6 to 12 months after the first dose. For those who initiate vaccination on or after their 15th birthday, and for those with certain medical conditions, the recommendation remains 3 doses on a 0, 1-2, and 6-month schedule.3
As of August 2019,4 ACIP now recommends that all women and men receive catch-up HPV vaccination through age 26. For individuals 27 to 45 years of age who have not been adequately vaccinated, HPV vaccine may be given based on shared clinical decision making with their physician.
How are we doing?
Overall, adolescent vaccination coverage is improving in the United States (see “Vaccination goals from ACIP and Healthy People 2020”1,5,6), but the rate of improvement of HPV coverage is lower than that for Tdap and MenACWY coverage by age 15 years (although completion of the MenACWY vaccine series is low). From 2015 to 2016, coverage increased for 1 or more doses of Tdap, from 86.4% to 88% among 17-year olds (87.9% for 15-year olds), and coverage for 1 or more doses of MenACWY increased from 81.7% to 83.5% among 17-year olds (80.4% among 15-year olds).1 Both Tdap and MenACWY coverage rates have surpassed Healthy People 2020 goals of 80%, and the focus now is on maintenance of coverage. Data from the 2016 National Immunization Survey (NIS)-Teen show that completion of the HPV vaccine series (applying updated HPV vaccine recommendations retrospectively) increased to 45.4% for 15-year-olds,1 still far below the Healthy People 2020 goal of 80%. Completion rates for 2 or more doses of MenACWY also increased from 33.3% to 39.1%.1
SIDEBAR
Vaccination goals from ACIP and Healthy People 2020
The Advisory Committee on Immunization Practices (ACIP) recommends that adolescents routinely receive several vaccines between the ages of 11 and 12 years: an annual influenza vaccine, Tdap, the first dose of MenACWY, and initiation of the HPV series. ACIP also advises a booster dose of MenACWY at age 16 years, and teens and young adults (16-23 years) also may be vaccinated with a multidose serogroup B meningococcal vaccine, preferably before age 18. For those adolescents not up to date with their childhood vaccines, ACIP recommends the following catch-up vaccinations: measles, mumps, rubella (MMR, 2 doses); hepatitis B (HepB, 3 doses); and varicella (VAR, 2 doses).5
Healthy People 2020. In December 2010, the US Department of Health and Human Services released Healthy People 2020, a wide-ranging initiative on health promotion and disease prevention that includes 10-year objectives of increasing coverage with Tdap, at least one dose of MenACWY, and completion of the HPV series among 80% of those ages 13 to 15 years.6 This initiative reflects extensive feedback from more than 2000 organizations and authorities in public health and prevention at federal, state, and local levels—as well as from the public. Adolescent vaccination coverage is estimated by the Centers for Disease Control and Prevention using data from the National Immunization Survey (NIS)-Teen annual survey conducted among parents and guardians of adolescents ages 13 to 17 years.1
Common barriers to improved vaccine coverage
Barriers to improved vaccination rates include a lack of regular assessment of vaccine status; limited use of electronic records, tools, and immunization registries; lack of health care provider knowledge on current vaccine recommendations; vaccine costs; missed opportunities; and patient/parent refusals.7,8 The Community Preventive Services Task Force outlines several well-established evidence-based ways that administrators and physicians can counter these barriers:
- give a strong recommendation to vaccinate,9,10
- incorporate an audit/feedback mechanism for health care providers who vaccinate,9,11
- use electronic alerts to remind health care providers to vaccinate,9,12
- use your state’s electronic immunization information systems (IIS),7,13
- appoint a vaccine practice team/vaccine champion,9,14 and
- implement standing orders and reminder/recall systems.7,9,15
The passage of the Affordable Care Act (ACA)—which mandates that certain preventive services, including ACIP-recommended immunizations, be covered as part of basic care at no cost-sharing—reduces the once-common financial barrier to vaccine uptake.16 A key contributor to low uptake of HPV vaccination by adolescents is parental refusal.17
Continue to: The threats posed by HPV
The threats posed by HPV
HPV infections are the most commonly transmitted infections in the United States and nearly all men and women will be exposed to one or more types of HPV at some point in their lives. Current data show that 79 million Americans, most in their late teens and early 20s, are infected with HPV, and about 14 million people in the United States become newly infected each year.18 HPV is a DNA tumor virus that causes epithelial proliferation at cutaneous and mucosal surfaces.
There are more than 100 types of the virus,19 including more than 40 strains that infect the human genital tract. Of the latter 40 strains, there are oncogenic or high-risk types and non-oncogenic or low-risk types.20 HPV infection with high-risk types causes cervical, vaginal, and vulvar cancers in women; penile cancers in men; and oropharyngeal and anal cancers in both men and women. Low-risk HPV types cause genital warts in both men and women.21 The current available HPV vaccine in the United States is a 9-valent vaccine (9vHPV) that replaces the former 2- and 4-valent HPV vaccines and includes immunogenic coverage against high-risk HPV types 16, 18, 31, 33, 45, 52, and 58; and low-risk types 6 and 11.22
Centers for Disease Control and Prevention (CDC) data from 2010 to 2014 show that approximately 23,700 women and approximately 17,300 men in the United States developed HPV-associated cancer. Most common in women are cervical cancers and in men, oropharyngeal cancers (cancers of the back of the throat, base of the tongue, and tonsils). Using population-based data to genotype HPV types from cancer tissues, the CDC reports that HPV is responsible for about 90% of cervical and anal cancers, 70% of oropharyngeal, vaginal, and vulvar cancers, and 60% of penile cancers.23 A significant percentage of these cancers could potentially be prevented by receipt of 9vHPV.23,24
Make adolescent immunization a high priority
Anticipate opportunities to vaccinate and take steps to make your immunization and scheduling processes more prominent. With HPV specifically, you can strongly advocate for vaccination, address parental misgivings and educate them using clear communication styles, and acquire knowledge to answer concerns about potential vaccine adverse effects.
Every visit is an opportunity to vaccinate. The American Academy of Family Physicians and The American Academy of Pediatrics recommend that adolescents have annual preventive visits for screening, immunizations, and assessment and counseling for risky behaviors. However, many adolescents do not present annually for preventive visits, and fewer than half of adolescents receive regular preventive care.
Continue to: Missed opportunities for the HPV vaccine
Missed opportunities for the HPV vaccine. One study showed that at least 86% of unvaccinated adolescents had missed opportunities to receive HPV vaccine.29 A study of 14,588 adolescent girls from January 2010 through August 2015 showed that HPV vaccine was given at only 37.1% of visits in which MenACWY or Tdap vaccines were administered.30 The rate of HPV vaccination was just 26% during well adolescent visits, and 41.8% during all other primary care visits.30 Every adolescent health care visit—including visits for acute care, chronic care, follow-up, or office-based procedures—is an opportunity to review vaccination status.
Give vaccines concomitantly (simultaneously or same-day). ACIP counsels that minor illnesses, such as mild upper respiratory infections with or without low-grade fever, are not contraindications to routine vaccination.30 Also, the safety of simultaneous vaccine administration, often a concern of both parents and health care providers, has been well established. Each vaccine’s immunogenicity and safety profile are maintained when given concomitantly with other vaccines, and fewer visits are needed to complete an adolescent’s vaccination status.31,32
Immediately schedule follow up visits and use reminder/recall systems. Parents of adolescents who opt for HPV vaccination are not always aware of the timing of the 2- or 3-dose schedule and may not even be aware that more than 1 dose of vaccine is recommended.
A qualitative study of pediatric primary care providers and parents/guardians of adolescent patients showed that for HPV vaccination series completion, 65% of parents/guardians expected to be reminded of any needed doses, while 52% of the pediatric primary care providers relied on parents to schedule subsequent immunizations, and often the HPV series was not completed.33 Higher completion rates of the HPV vaccination series were achieved when follow-up appointments were scheduled at checkout for the 2nd or 3rd vaccine dose after initiation of HPV vaccination.33 The use of patient reminder/recall systems using telephone calls or mailings (phone usage is more effective than mailings) is also shown to improve vaccination completion rates.34
Recommend HPV vaccination clearly and resolutely
In a cross-sectional survey of 800 parents of adolescents ages 9 to 14 years, HPV vaccine was deemed the least likely vaccine to have been “very strongly” recommended by their health care provider, compared with the strength of recommendations for influenza, Tdap, and MenACWY vaccines.35 The strength of a health care provider’s recommendation to vaccinate is the single most influential factor in vaccine uptake.10,36,37 Most family physicians self-report “always recommending standard pediatric vaccines”; however, only a minority are following ACIP recommendations.38 A national study reported that only about two-thirds of parents who received HPV vaccine recommendations perceived a high level of health care provider endorsement.39 The takeaway point: Give a clear, unambiguous, strong recommendation to vaccinate with HPV to prevent infection; cervical, oropharyngeal, and other cancers; and genital warts.
Continue to: Tell parents why the timing is important
Tell parents why the timing is important. Inform parents that the HPV vaccine must be administered while their child is young (before the adolescent’s first sexual contact) to ensure the most robust immune response to the vaccine.40 Unsolicited explanations about sexual activity need not be offered when discussing HPV vaccination, as it is fair to assume that sexual contact is a reality for nearly all people in their adolescent or adult life; and by extension, most sexually active people will likely have exposure to HPV at some time in their lives. By adulthood, sexual activity is nearly universal: The National Longitudinal Study of Adolescent Health showed that only about 3% of participants tracked since adolescence reported no sexual experience by (average age) 28.5 years.41
How you say it matters. Many pediatricians and family physicians report recommending HPV vaccine inconsistently, behind schedule, or without urgency,42 sending mixed messages by failing to endorse HPV vaccination strongly, failing to differentiate it from other vaccines, and presenting it as an “optional” vaccine that could be delayed.43 Physicians and other health care providers who begin conversations about HPV vaccine by saying that the adolescent is “due” for the vaccine show higher vaccine recommendation quality scores than those who give unsolicited information about the vaccine, elicit questions before recommendation, or present the vaccine as an “option.”42 Parents who are “on the fence” may hesitate and decline HPV vaccination with a halfhearted recommendation.44
“Your child is due for his/her Tdap, HPV, influenza, and meningococcal vaccinations to prevent potentially devastating disease and several cancers. I highly recommend all 4 vaccinations today” is more persuasive than, “I recommend your child receive his/her Tdap, meningococcal, and influenza vaccines. And we can also discuss the HPV vaccine.”
Direct presumptive language that assumes vaccine delivery is associated with higher odds of HPV vaccine acceptance and same-day agreement to vaccination than is an open-ended participatory conversational style.45 Saying, “I believe in the importance of this cancer-preventing vaccine for your child” is more persuasive than saying, “What do you think about starting the HPV vaccination series today?”46
Don’t give up when parents initially refuse HPV vaccinations for their adolescents. Parents’ decisions about HPV vaccination may change over time. Repeated positive recommendations and counseling for HPV vaccination over multiple visits have been shown in a large multivariable analysis to increase parent acceptance of HPV vaccination: 45% of parents reported secondary acceptance of HPV vaccination, and an additional 24% intended to vaccinate in the next 12 months.47 Combining a presumptive communication style with motivational interviewing and a fact sheet has contributed to higher clinician-perceived levels of parental HPV vaccine acceptance and increased vaccination rates.48
Continue to: Know how to address parents' concerns about safety
Know how to address parents’ concerns about safety
Be prepared to discuss and answer parents’ questions or concerns regarding any vaccine, especially the HPV vaccine. Social networks are important in parents’ vaccination decision-making,49 and they may seek information from such sources as Twitter, Facebook, Google, and YouTube, where misinformation may be disseminated. A quantitative analysis of 560 YouTube videos relaying a false link between vaccines and autism or other serious adverse effects on children were uploaded between December 2007 and July 2017, with a peak of 224 videos uploaded in the first 7 months of 2017.50 Most were negative in tone and dispensed misinformation.50
The National Vaccine Information Center (NVIC) is an organization that takes a skeptical view of the US government and pharmaceutical companies. NVIC is widely criticized by scientists and leaders in vaccine science and public health as spreading false information on the risks of vaccines and, specifically, that HPV vaccination causes chronic disease. NVIC reports that receipt of HPV vaccine may increase the risk for cervical cancer and death.51 Pediatrician and vaccine researcher Dr. Paul Offit, interviewed by The Lancet in response to NVIC and other anti-vaccine groups’ messages, stated: “anti-vaccination organizations are unequivocally threatening public health.”52
Describe the robust safety-monitoring system. The CDC is aware of public concern about the safety of HPV vaccine. Ongoing monitoring of vaccine safety and studies conducted by the CDC, the Food and Drug Administration (FDA), and other organizations has documented a reassuring safety record since the vaccine’s introduction in 2006.53 Assure parents that the Vaccine Adverse Event Reporting System (VAERS) summary of 7244 reports following 9vHPV vaccination (December 1, 2014 – December 31, 2017) showed that most (97%) reports were nonserious: No new safety signals or unexpected patterns were observed, confirming consistency of the safety profile of 9vHPV with data from pre-licensure trials and post-licensure data on 4vHPV.54
Acknowledge the usually mild, transient potential risks of HPV vaccination as reported to VAERS: local injection site symptoms such as pain, redness, or swelling in the arm where the injection was given (most common adverse effect), dizziness, fainting, headache, nausea, and fever.53 Point out that fainting after vaccination is common in adolescents55 and that the CDC and ACIP recommend observation of adolescents for 15 minutes following HPV vaccination.56 Consider this 15-minute observation period after adolescent receipt of any vaccine to be part of standard practice in your vaccination setting.56
Contest unfounded views. Other common parental concerns about effects of HPV vaccine include supposed promotion of promiscuity, increased incidence of premature ovarian failure or insufficiency (POI), and increased risk of Guillain-Barré Syndrome (GBS), often propagated through published reports, media coverage, Web sites, and social media. Assure worried parents that many studies have shown that receipt of the vaccine is safe and does not lead to initiation of sexual activity or promiscuity, and, in fact, safer sexual health practices have been observed following vaccination.57-59
Continue to: A large longitudinal...
A large longitudinal adolescent health survey administered in British Columbia looked at sexual health behaviors and risk factors in adolescent girls before and after receipt of HPV vaccination (2003, 2008, 2013).59 Results showed no significant change in the reported number of sexual partners (2003-2013), increased reported use of contraception and condoms, and lower pregnancy rates.59 There is no evidence that HPV vaccines cause reproductive problems in women53; a review of VAERS reports from 2009 through 2015 did not detect any safety concerns for POI or other reproductive problems in females.60 A 2018 population-based study of nearly 200,000 women observed no increase of POI following receipt of HPV vaccination.61 In addition, several recent studies have shown no increased risk for GBS following receipt of HPV vaccine.62-64
CORRESPONDENCE
Pamela G. Rockwell, DO, FAAFP, 24 Frank Lloyd Wright Drive, SPC 5795, Room 2300, Lobby H, Ann Arbor, MI 48105; [email protected].
1. Walker TY, Elam-Evans LD, Singleton JA, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:874-882.
2. Markowitz LE, Dunne EF, Saraiya M, et al. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2014;63:1-30.
3. Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2016;65:1405-1408.
4. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702.
5. Robinson CL, Romero JR, Kempe A, et al. Advisory Committee on Immunization Practices (ACIP) Child/Adolescent Immunization Work Group. Advisory Committee on Immunization Practices recommended immunization schedules for persons aged 18 years or younger—United States, 2017. MMWR Morb Mortal Wkly Rep. 2017;66:134-135.
6. US Department of Health and Human Services Office of Disease Prevention and Health Promotion. Healthy People 2020. www.healthypeople.gov/node/4654/data_details. Accessed December 4, 2019.
7. Rockwell PG. What you can do to improve adult immunization rates. J Fam Pract. 2015;64:625-633.
8. Kimmel Sr, Burns IT, Wolfe RM, et al. Addressing immunization barriers, benefits, and risks. J Fam Pract. 2007;56:S61-S69.
9. Briss PA, Zaza S, Pappaioanou M, et al. Developing an evidence-based guide to community preventive services-methods. The Task Force on Community Preventive Services. Am J Prev Med. 2000;18:35-43.
10. Ylitalo KR, Lee H, Mehta NK. Health care provider recommendation, human papillomavirus vaccination, and race/ethnicity in the U.S. National Immunization Survey. Am J Public Health. 2013;103:164-169.
11. National Center for Immunization and Respiratory Diseases. General recommendations on immunization—recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2011;60:1-64.
12. Klatt TE, Hopp E. Effect of a best-practice alert on the rate of influenza vaccination of pregnant women. Obstet Gynecol. 2012;119:301-305.
13. Jones KL, Hammer AL, Swenson C, et al. Improving adult immunization rates in primary care clinics. Nurs Econ. 2008;26:404-407.
14. Hainer BL. Vaccine administration: making the process more efficient in your practice. Fam Pract Manag. 2007;14:48-53.
15. Task Force on Community Preventive Services. Recommendations regarding interventions to improve vaccination coverage in children, adolescents, and adults. Am J Prev Med. 2000;18(suppl 1):92-96.
16. US Department of Health and Human Services. Preventive care. www.hhs.gov/healthcare/about-the-aca/preventive-care/index.html. Accessed December 4, 2019.
17. Gilkey MB, Calo WA, Marciniak, MW, et al. Parents who refuse or delay HPV vaccine: differences in vaccination behavior, beliefs, and clinical communication preferences. Hum Vaccin Immunother. 2017;13:680-686.
18. CDC. Genital HPV infection—fact sheet. www.cdc.gov/std/hpv/stdfact-hpv.htm. Accessed December 4, 2019.
19. WHO. Human papillomavirus (HPV) and cervical cancer. www.who.int/news-room/fact-sheets/detail/human-papillomavirus-(hpv)-and-cervical-cancer. Accessed December 4, 2019.
20. Muñoz N, Bosch FX, de Sanjosé S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518-527.
21. Viens LJ, Henley SJ, Watson M, et al. Human papillomavirus-associated cancers—United States, 2008–2012. MMWR Morb Mortal Wkly Rep. 2016;65:661-666.
22. CDC. Luxembourg A. Program summary and new 9-valent HPV vaccine trial data. Presented at the Advisory Committee on Immunization Practices (ACIP), October 30, 2014. Atlanta, Ga. 2014. www.cdc.gov/vaccines/acip/meetings/downloads/min-archive/min-2014-10.pdf. Accessed December 4, 2019.
23. CDC. HPV and cancer. www.cdc.gov/cancer/hpv/statistics/cases.htm. Accessed December 4, 2019.
24. Lowy DR, Schiller JT. Reducing HPV-associated cancer globally. Cancer Prev Res (Phila). 2012;5:18-23.
25. Rand CM, Goldstein NPN. Patterns of primary care physician visits for US adolescents in 2014: implications for vaccination. Acad Pediatr. 2018;18:S72-S78.
26. Taylor JL, Aalsma MC, Gilbert AL, et al. Perspectives of family medicine physicians on the importance of adolescent preventive care: a multivariate analysis. BMC Fam Pract. 2016;17:4.
27. Harris SK, Aalsma MC, Weitzman ER, et al. Research on clinical preventive services for adolescents and young adults: Where are we and where do we need to go? J Adolesc Health. 2017;60:249-260.
28. Gilkey MB, Moss JL, McRee AL, et al. Do correlates of HPV vaccine initiation differ between adolescent boys and girls? Vaccine. 2012;30:5928-5934.
29. Espinosa CM, Marshall GS, Woods CR, et al. Missed opportunities for human papillomavirus vaccine initiation in an insured adolescent female population. J Pediatric Infect Dis Soc. 2017;6:360-365.
30. CDC. Update: Vaccine side effects, adverse reactions, contraindications, and precautions. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 1996;45:1-35.
31. Moss JL, Reiter PL, Brewer NT. Concomitant adolescent vaccination in the U.S., 2007-2012. Am J Prev Med. 2016;51:693-705.
32. Noronha AS, Markowitz LE, Dunne EF. Systematic review of human papillomavirus vaccine coadministration. Vaccine. 2014;32:2670-2674.
33. Perkins RB, Chigurupati NL, Apte G, et al. Why don’t adolescents finish the HPV vaccine series? A qualitative study of parents and providers. Hum Vaccin Immunother. 2016;12:1528-1535.
34. Jacobson Vann JC, Szilagyi P. Patient reminder and patient recall systems to improve immunization rates. Cochrane Database Syst Rev. 2005;(3):CD003941.
35. Dempsey AF, O’Leary ST. Human papillomavirus vaccination: narrative review of studies on how providers’ vaccine communication affects attitudes and uptake. Acad Pediatr. 2018;18:S23-S27.
36. Rosenthal SL, Weiss TW, Zimet GD, et al. Predictors of HPV vaccine uptake among women aged 19–26: importance of a physician’s recommendation. Vaccine. 2011;29:890-895.
37. Gargano LM, Herbert NL, Painter JE, et al. Impact of a physician recommendation and parental immunization attitudes on receipt or intention to receive adolescent vaccines. Hum Vaccin Immunother. 2013;9:2627-2633.
38. Bonville CA, Domachowske JB, Cibula DA, et al. Immunization attitudes and practices among family medicine providers. Hum Vaccin Immunother. 2017;13:2646-2653.
39. Wilson R, Brown DR, Boothe MA, et al. Knowledge and acceptability of the HPV vaccine among ethnically diverse black women. J Immigr Minor Health. 2013;15:747-757.
40. Iversen O, Miranda MJ, Ulied A, et al. Immunogenicity of the 9-valent HPV vaccine using 2-dose regimens in girls and boys vs a 3-dose regimen in women. JAMA. 2016;316:2411–2421.
41. Haydon AA, Cheng MM, Herring AH, et al. Prevalence and predictors of sexual inexperience in adulthood. Arch Sex Behav. 2014;43:221-230.
42. Gilkey MB, Malo TL, Shah PD, et al. Quality of physician communication about human papillomavirus vaccine: findings from a national survey. Cancer Epidemiol Biomarkers Prev. 2015;24:1673-1679.
43. Gilkey MB, McRee AL. Provider communication about HPV vaccination: a systemic review. Hum Vaccin Immunother. 2016;12:1454-1468.
44. American Academy of Family Physicians. Strong recommendation to vaccinate against HPV is key to boosting uptake. www.aafp.org/news/health-of-the-public/20140212hpv-vaccltr.html. Accessed December 4, 2019.
45. Sturm L, Donahue K, Kasting M, et al. Pediatrician-parent conversations about human papillomavirus vaccination: an analysis of audio recordings. J Adolesc Health. 2017;61:246-251.
46. Malo TL, Gilkey MB, Hall ME, et al. Messages to motivate human papillomavirus vaccination: national studies of parents and physicians. Cancer Epidemiol Biomarkers Prev. 2016;25:1383-1391.
47. Kornides ML, McRee AL, Gilkey MB. Parents who decline HPV vaccination: Who later accepts and why? Acad Pediatr. 2018;18:S37-S43.
48. Reno JE, Thomas J, Pyrzanowski J, et al. Examining strategies for improving healthcare providers’ communication about adolescent HPV vaccination: evaluation of secondary outcomes in a randomized controlled trial. Hum Vaccin Immunother. 2018;15:1592-1598.
49. Brunson EK. The impact of social networks on parents’ vaccination decisions. Pediatrics. 2013;131:e1397-e1404.
50. Donzelli G, Palomba G, Federigi L, et al. Misinformation on vaccination: a quantitative analysis of YouTube videos. Hum Vaccin Immunother. 2018;14:1654-1659.
51. National Vaccine Information Center. Human papillomavirus (HPV) disease and vaccine information. www.nvic.org/Vaccines-and-Diseases/hpv.aspx. Accessed December 4, 2019.
52. Shetty P. Experts concerned about vaccination backlash. Lancet. 2010; 375:970-971.
53. CDC. Frequently asked questions about HPV vaccine safety. www.cdc.gov/vaccinesafety/vaccines/hpv/hpv-safety-faqs.html. Accessed December 4, 2019.
54. Arana J, Su J, Lewis P, et al. Post-licensure surveillance of 9-valent human papillomavirus vaccine (9vHPV) in the Vaccine Adverse Event Reporting System (VAERS), United States, 2014-2017. https://idsa.confex.com/idsa/2018/webprogram/Paper69618.html. Accessed December 4, 2019.
55. Braun MM, Patriarca PA, Ellenberg SS. Syncope after immunization. Arch Ped Adolesc Med. 1997;151:255-259.
56. Kroger AT, Duchin J, Vázquez M. General best practice guidelines for immunization. Best practices guidance of the Advisory Committee on Immunization Practices (ACIP). www.cdc.gov/vaccines/hcp/acip-recs/general-recs/index.html. Accessed December 4, 2019.
57. Hansen BT. No evidence that HPV vaccination leads to sexual risk compensation. Hum Vaccin Immunother. 2016;12:1451-1453.
58. Smith LM, Kaufman JS, Strumpf EC, et al. Effect of human papillomavirus (HPV) vaccination on clinical indicators of sexual behaviour among adolescent girls: the Ontario Grade 8 HPV Vaccine Cohort Study. CMAJ. 2015;187:E74-81.
59. Ogilvie GS, Phan F, Pederson HN, et al. Population-level sexual behaviours in adolescent girls before and after introduction of the human papillomavirus vaccine (2003-2013). CMAJ. 2018;190:E1221-E1226.
60. Arana JE, Harrington T, Cano M, et al. Post-licensure safety monitoring of quadrivalent human papillomavirus vaccine in the Vaccine Adverse Event Reporting System (VAERS), 2009-2015. Vaccine. 2018;36:1781-1788.
61. Naleway AL, Mittendorf KF, Irving SA, et al. Primary ovarian insufficiency and adolescent vaccination. Pediatrics. 2018;142. pii: e20190943.
62. Deceuninck G, Sauvageau C, Gilca V, et al. Absence of association between Guillain-Barré syndrome hospitalizations and HPV-vaccine. Expert Rev Vaccines. 2018;17:99-102.
63. Mouchet J, Salvo F, Raschi E, et al. Human papillomavirus vaccine and demyelinating diseases – a systematic review and meta-analysis. Pharmacol Res. 2018;132:108-118.
64. Gee J, Sukumaran L, Weinstraub E, et al. Risk of Guillain-Barre Syndrome following quadrivalent human papillomavirus vaccine in the Vaccine Safety Datalink. Vaccine. 2017;35:5756-5758.
1. Walker TY, Elam-Evans LD, Singleton JA, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:874-882.
2. Markowitz LE, Dunne EF, Saraiya M, et al. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2014;63:1-30.
3. Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2016;65:1405-1408.
4. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702.
5. Robinson CL, Romero JR, Kempe A, et al. Advisory Committee on Immunization Practices (ACIP) Child/Adolescent Immunization Work Group. Advisory Committee on Immunization Practices recommended immunization schedules for persons aged 18 years or younger—United States, 2017. MMWR Morb Mortal Wkly Rep. 2017;66:134-135.
6. US Department of Health and Human Services Office of Disease Prevention and Health Promotion. Healthy People 2020. www.healthypeople.gov/node/4654/data_details. Accessed December 4, 2019.
7. Rockwell PG. What you can do to improve adult immunization rates. J Fam Pract. 2015;64:625-633.
8. Kimmel Sr, Burns IT, Wolfe RM, et al. Addressing immunization barriers, benefits, and risks. J Fam Pract. 2007;56:S61-S69.
9. Briss PA, Zaza S, Pappaioanou M, et al. Developing an evidence-based guide to community preventive services-methods. The Task Force on Community Preventive Services. Am J Prev Med. 2000;18:35-43.
10. Ylitalo KR, Lee H, Mehta NK. Health care provider recommendation, human papillomavirus vaccination, and race/ethnicity in the U.S. National Immunization Survey. Am J Public Health. 2013;103:164-169.
11. National Center for Immunization and Respiratory Diseases. General recommendations on immunization—recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2011;60:1-64.
12. Klatt TE, Hopp E. Effect of a best-practice alert on the rate of influenza vaccination of pregnant women. Obstet Gynecol. 2012;119:301-305.
13. Jones KL, Hammer AL, Swenson C, et al. Improving adult immunization rates in primary care clinics. Nurs Econ. 2008;26:404-407.
14. Hainer BL. Vaccine administration: making the process more efficient in your practice. Fam Pract Manag. 2007;14:48-53.
15. Task Force on Community Preventive Services. Recommendations regarding interventions to improve vaccination coverage in children, adolescents, and adults. Am J Prev Med. 2000;18(suppl 1):92-96.
16. US Department of Health and Human Services. Preventive care. www.hhs.gov/healthcare/about-the-aca/preventive-care/index.html. Accessed December 4, 2019.
17. Gilkey MB, Calo WA, Marciniak, MW, et al. Parents who refuse or delay HPV vaccine: differences in vaccination behavior, beliefs, and clinical communication preferences. Hum Vaccin Immunother. 2017;13:680-686.
18. CDC. Genital HPV infection—fact sheet. www.cdc.gov/std/hpv/stdfact-hpv.htm. Accessed December 4, 2019.
19. WHO. Human papillomavirus (HPV) and cervical cancer. www.who.int/news-room/fact-sheets/detail/human-papillomavirus-(hpv)-and-cervical-cancer. Accessed December 4, 2019.
20. Muñoz N, Bosch FX, de Sanjosé S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518-527.
21. Viens LJ, Henley SJ, Watson M, et al. Human papillomavirus-associated cancers—United States, 2008–2012. MMWR Morb Mortal Wkly Rep. 2016;65:661-666.
22. CDC. Luxembourg A. Program summary and new 9-valent HPV vaccine trial data. Presented at the Advisory Committee on Immunization Practices (ACIP), October 30, 2014. Atlanta, Ga. 2014. www.cdc.gov/vaccines/acip/meetings/downloads/min-archive/min-2014-10.pdf. Accessed December 4, 2019.
23. CDC. HPV and cancer. www.cdc.gov/cancer/hpv/statistics/cases.htm. Accessed December 4, 2019.
24. Lowy DR, Schiller JT. Reducing HPV-associated cancer globally. Cancer Prev Res (Phila). 2012;5:18-23.
25. Rand CM, Goldstein NPN. Patterns of primary care physician visits for US adolescents in 2014: implications for vaccination. Acad Pediatr. 2018;18:S72-S78.
26. Taylor JL, Aalsma MC, Gilbert AL, et al. Perspectives of family medicine physicians on the importance of adolescent preventive care: a multivariate analysis. BMC Fam Pract. 2016;17:4.
27. Harris SK, Aalsma MC, Weitzman ER, et al. Research on clinical preventive services for adolescents and young adults: Where are we and where do we need to go? J Adolesc Health. 2017;60:249-260.
28. Gilkey MB, Moss JL, McRee AL, et al. Do correlates of HPV vaccine initiation differ between adolescent boys and girls? Vaccine. 2012;30:5928-5934.
29. Espinosa CM, Marshall GS, Woods CR, et al. Missed opportunities for human papillomavirus vaccine initiation in an insured adolescent female population. J Pediatric Infect Dis Soc. 2017;6:360-365.
30. CDC. Update: Vaccine side effects, adverse reactions, contraindications, and precautions. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 1996;45:1-35.
31. Moss JL, Reiter PL, Brewer NT. Concomitant adolescent vaccination in the U.S., 2007-2012. Am J Prev Med. 2016;51:693-705.
32. Noronha AS, Markowitz LE, Dunne EF. Systematic review of human papillomavirus vaccine coadministration. Vaccine. 2014;32:2670-2674.
33. Perkins RB, Chigurupati NL, Apte G, et al. Why don’t adolescents finish the HPV vaccine series? A qualitative study of parents and providers. Hum Vaccin Immunother. 2016;12:1528-1535.
34. Jacobson Vann JC, Szilagyi P. Patient reminder and patient recall systems to improve immunization rates. Cochrane Database Syst Rev. 2005;(3):CD003941.
35. Dempsey AF, O’Leary ST. Human papillomavirus vaccination: narrative review of studies on how providers’ vaccine communication affects attitudes and uptake. Acad Pediatr. 2018;18:S23-S27.
36. Rosenthal SL, Weiss TW, Zimet GD, et al. Predictors of HPV vaccine uptake among women aged 19–26: importance of a physician’s recommendation. Vaccine. 2011;29:890-895.
37. Gargano LM, Herbert NL, Painter JE, et al. Impact of a physician recommendation and parental immunization attitudes on receipt or intention to receive adolescent vaccines. Hum Vaccin Immunother. 2013;9:2627-2633.
38. Bonville CA, Domachowske JB, Cibula DA, et al. Immunization attitudes and practices among family medicine providers. Hum Vaccin Immunother. 2017;13:2646-2653.
39. Wilson R, Brown DR, Boothe MA, et al. Knowledge and acceptability of the HPV vaccine among ethnically diverse black women. J Immigr Minor Health. 2013;15:747-757.
40. Iversen O, Miranda MJ, Ulied A, et al. Immunogenicity of the 9-valent HPV vaccine using 2-dose regimens in girls and boys vs a 3-dose regimen in women. JAMA. 2016;316:2411–2421.
41. Haydon AA, Cheng MM, Herring AH, et al. Prevalence and predictors of sexual inexperience in adulthood. Arch Sex Behav. 2014;43:221-230.
42. Gilkey MB, Malo TL, Shah PD, et al. Quality of physician communication about human papillomavirus vaccine: findings from a national survey. Cancer Epidemiol Biomarkers Prev. 2015;24:1673-1679.
43. Gilkey MB, McRee AL. Provider communication about HPV vaccination: a systemic review. Hum Vaccin Immunother. 2016;12:1454-1468.
44. American Academy of Family Physicians. Strong recommendation to vaccinate against HPV is key to boosting uptake. www.aafp.org/news/health-of-the-public/20140212hpv-vaccltr.html. Accessed December 4, 2019.
45. Sturm L, Donahue K, Kasting M, et al. Pediatrician-parent conversations about human papillomavirus vaccination: an analysis of audio recordings. J Adolesc Health. 2017;61:246-251.
46. Malo TL, Gilkey MB, Hall ME, et al. Messages to motivate human papillomavirus vaccination: national studies of parents and physicians. Cancer Epidemiol Biomarkers Prev. 2016;25:1383-1391.
47. Kornides ML, McRee AL, Gilkey MB. Parents who decline HPV vaccination: Who later accepts and why? Acad Pediatr. 2018;18:S37-S43.
48. Reno JE, Thomas J, Pyrzanowski J, et al. Examining strategies for improving healthcare providers’ communication about adolescent HPV vaccination: evaluation of secondary outcomes in a randomized controlled trial. Hum Vaccin Immunother. 2018;15:1592-1598.
49. Brunson EK. The impact of social networks on parents’ vaccination decisions. Pediatrics. 2013;131:e1397-e1404.
50. Donzelli G, Palomba G, Federigi L, et al. Misinformation on vaccination: a quantitative analysis of YouTube videos. Hum Vaccin Immunother. 2018;14:1654-1659.
51. National Vaccine Information Center. Human papillomavirus (HPV) disease and vaccine information. www.nvic.org/Vaccines-and-Diseases/hpv.aspx. Accessed December 4, 2019.
52. Shetty P. Experts concerned about vaccination backlash. Lancet. 2010; 375:970-971.
53. CDC. Frequently asked questions about HPV vaccine safety. www.cdc.gov/vaccinesafety/vaccines/hpv/hpv-safety-faqs.html. Accessed December 4, 2019.
54. Arana J, Su J, Lewis P, et al. Post-licensure surveillance of 9-valent human papillomavirus vaccine (9vHPV) in the Vaccine Adverse Event Reporting System (VAERS), United States, 2014-2017. https://idsa.confex.com/idsa/2018/webprogram/Paper69618.html. Accessed December 4, 2019.
55. Braun MM, Patriarca PA, Ellenberg SS. Syncope after immunization. Arch Ped Adolesc Med. 1997;151:255-259.
56. Kroger AT, Duchin J, Vázquez M. General best practice guidelines for immunization. Best practices guidance of the Advisory Committee on Immunization Practices (ACIP). www.cdc.gov/vaccines/hcp/acip-recs/general-recs/index.html. Accessed December 4, 2019.
57. Hansen BT. No evidence that HPV vaccination leads to sexual risk compensation. Hum Vaccin Immunother. 2016;12:1451-1453.
58. Smith LM, Kaufman JS, Strumpf EC, et al. Effect of human papillomavirus (HPV) vaccination on clinical indicators of sexual behaviour among adolescent girls: the Ontario Grade 8 HPV Vaccine Cohort Study. CMAJ. 2015;187:E74-81.
59. Ogilvie GS, Phan F, Pederson HN, et al. Population-level sexual behaviours in adolescent girls before and after introduction of the human papillomavirus vaccine (2003-2013). CMAJ. 2018;190:E1221-E1226.
60. Arana JE, Harrington T, Cano M, et al. Post-licensure safety monitoring of quadrivalent human papillomavirus vaccine in the Vaccine Adverse Event Reporting System (VAERS), 2009-2015. Vaccine. 2018;36:1781-1788.
61. Naleway AL, Mittendorf KF, Irving SA, et al. Primary ovarian insufficiency and adolescent vaccination. Pediatrics. 2018;142. pii: e20190943.
62. Deceuninck G, Sauvageau C, Gilca V, et al. Absence of association between Guillain-Barré syndrome hospitalizations and HPV-vaccine. Expert Rev Vaccines. 2018;17:99-102.
63. Mouchet J, Salvo F, Raschi E, et al. Human papillomavirus vaccine and demyelinating diseases – a systematic review and meta-analysis. Pharmacol Res. 2018;132:108-118.
64. Gee J, Sukumaran L, Weinstraub E, et al. Risk of Guillain-Barre Syndrome following quadrivalent human papillomavirus vaccine in the Vaccine Safety Datalink. Vaccine. 2017;35:5756-5758.
From The Journal of Family Practice | 2019;68(10):E1-E7.
PRACTICE RECOMMENDATIONS
› Review vaccination status at every adolescent health care visit. C
› Give a clear, unambiguous, strong recommendation to vaccinate with human papillomavirus (HPV) to prevent infection; cervical, oropharyngeal, and other cancers; and genital warts. A
› Schedule follow-up appointments at checkout following initiation of HPV vaccination to help ensure completion of the series. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Many Americans planning to avoid flu vaccination
As the 2019-20 flu season got underway, more than half of American adults had not yet been vaccinated, according to a survey from the research organization NORC at the University of Chicago.
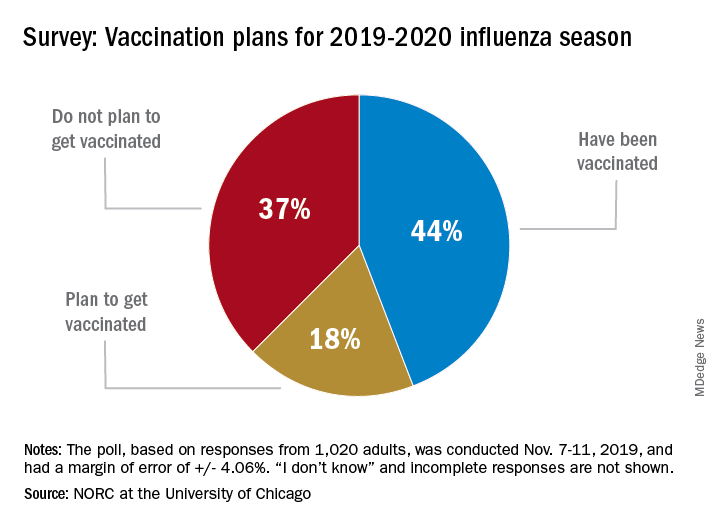
Only 44% of the 1,020 adults surveyed said that they had already received the vaccine as of Nov. 7-11, when the poll was conducted. Another the NORC reported. About 1% of those surveyed said they didn’t know or skipped the question.
Age was a strong determinant of vaccination status: 35% of those aged 18-29 years had gotten their flu shot, along with 36% of respondents aged 30-44 years and 34% of those aged 45- 59 years, compared with 65% of those aged 60 years and older. Of the respondents with children under age 18 years, 43% said that they were not planning to have the children vaccinated, the NORC said.
Concern about side effects, mentioned by 37% of those who were not planning to get vaccinated, was the most common reason given to avoid a flu shot, followed by belief that the vaccine doesn’t work very well (36%) and “never get the flu” (26%), the survey results showed.
“Widespread misconceptions exist regarding the safety and efficacy of flu shots. Because of the way the flu spreads in a community, failing to get a vaccination not only puts you at risk but also others for whom the consequences of the flu can be severe. Policymakers should focus on changing erroneous beliefs about immunizing against the flu,” said Caitlin Oppenheimer, who is senior vice president of public health research for the NORC, which has conducted the National Immunization Survey for the Centers for Disease Control and Prevention since 2005.
As the 2019-20 flu season got underway, more than half of American adults had not yet been vaccinated, according to a survey from the research organization NORC at the University of Chicago.

Only 44% of the 1,020 adults surveyed said that they had already received the vaccine as of Nov. 7-11, when the poll was conducted. Another the NORC reported. About 1% of those surveyed said they didn’t know or skipped the question.
Age was a strong determinant of vaccination status: 35% of those aged 18-29 years had gotten their flu shot, along with 36% of respondents aged 30-44 years and 34% of those aged 45- 59 years, compared with 65% of those aged 60 years and older. Of the respondents with children under age 18 years, 43% said that they were not planning to have the children vaccinated, the NORC said.
Concern about side effects, mentioned by 37% of those who were not planning to get vaccinated, was the most common reason given to avoid a flu shot, followed by belief that the vaccine doesn’t work very well (36%) and “never get the flu” (26%), the survey results showed.
“Widespread misconceptions exist regarding the safety and efficacy of flu shots. Because of the way the flu spreads in a community, failing to get a vaccination not only puts you at risk but also others for whom the consequences of the flu can be severe. Policymakers should focus on changing erroneous beliefs about immunizing against the flu,” said Caitlin Oppenheimer, who is senior vice president of public health research for the NORC, which has conducted the National Immunization Survey for the Centers for Disease Control and Prevention since 2005.
As the 2019-20 flu season got underway, more than half of American adults had not yet been vaccinated, according to a survey from the research organization NORC at the University of Chicago.

Only 44% of the 1,020 adults surveyed said that they had already received the vaccine as of Nov. 7-11, when the poll was conducted. Another the NORC reported. About 1% of those surveyed said they didn’t know or skipped the question.
Age was a strong determinant of vaccination status: 35% of those aged 18-29 years had gotten their flu shot, along with 36% of respondents aged 30-44 years and 34% of those aged 45- 59 years, compared with 65% of those aged 60 years and older. Of the respondents with children under age 18 years, 43% said that they were not planning to have the children vaccinated, the NORC said.
Concern about side effects, mentioned by 37% of those who were not planning to get vaccinated, was the most common reason given to avoid a flu shot, followed by belief that the vaccine doesn’t work very well (36%) and “never get the flu” (26%), the survey results showed.
“Widespread misconceptions exist regarding the safety and efficacy of flu shots. Because of the way the flu spreads in a community, failing to get a vaccination not only puts you at risk but also others for whom the consequences of the flu can be severe. Policymakers should focus on changing erroneous beliefs about immunizing against the flu,” said Caitlin Oppenheimer, who is senior vice president of public health research for the NORC, which has conducted the National Immunization Survey for the Centers for Disease Control and Prevention since 2005.
Pneumococcal conjugate vaccine update
Two pneumococcal vaccines are licensed for use in the United States: the 13-valent pneumococcal conjugate vaccine (PCV13 [Prevnar 13, Wyeth]) and the 23-valent pneumococcal polysaccharide vaccine (PPSV23 [Pneumovax, Merck]). The recommendations for using these vaccines in adults ages ≥ 19 years are arguably among the most complicated and confusing of all vaccine recommendations made by the Advisory Committee on Immunization Practices (ACIP).
In June 2019, things got even more complicated with ACIP’s unusual decision to change the previous recommendation on the routine use of PCV13 in adults ≥ 65 years. The new recommendation states that PCV13 should be used in immunocompetent older adults only after individual clinical decision making. The recommendation for routine use of PPSV23 remains unchanged. This Practice Alert explains the reasoning behind this change and its practical implications.
How we got to where we are now
Nearly 20 years ago, PCV was introduced into the child immunization schedule in the United States as a 7-valent vaccine (PCV7). In 2010, it was modified to include 13 antigens. And in 2012, the use of PCV13 was expanded to include adults with immunocompromising conditions.1 In 2014, PCV13 was recommended as an addition to PPSV23 for adults ≥ 65 years.2 However, with this recommendation, ACIP noted that the incidence of invasive pneumococcal disease in the elderly had been declining since the introduction of PCV7 use in children in the year 2000 (FIGURE 13), presumably due to the decreased transmission of pneumococcal infections from children to older adults.
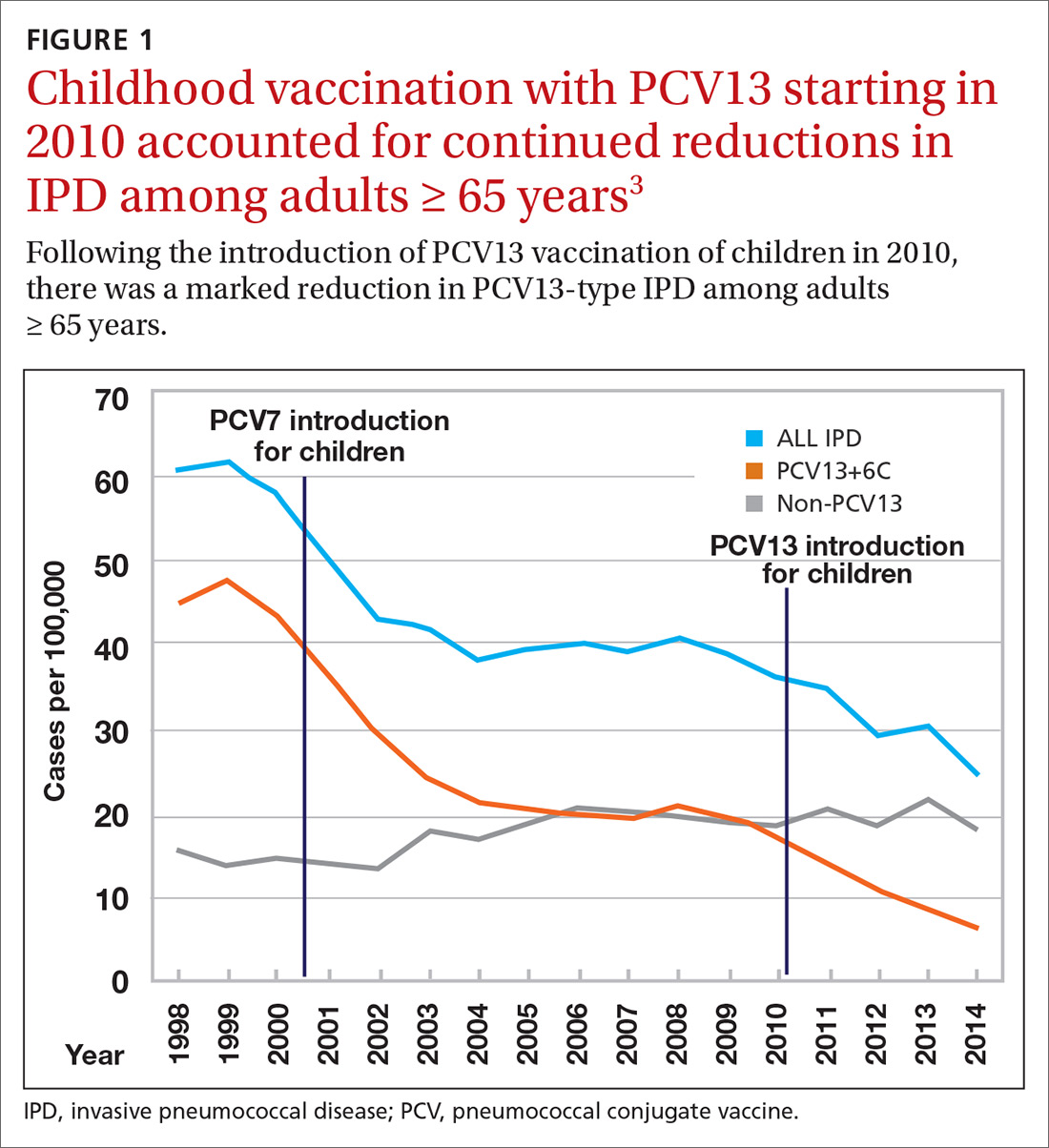
Because it was unclear in 2014 how much added benefit PCV13 would offer older adults, ACIP voted to restudy the issue after 4 years. At the June 2019 ACIP meeting, the results of an interim analysis were presented. ACIP concluded that routine use of PCV13 in immunocompetent adults ≥ 65 years adds little population-wide public health benefit given the vaccine’s routine use among children and immunocompromised adults (FIGURE 23).
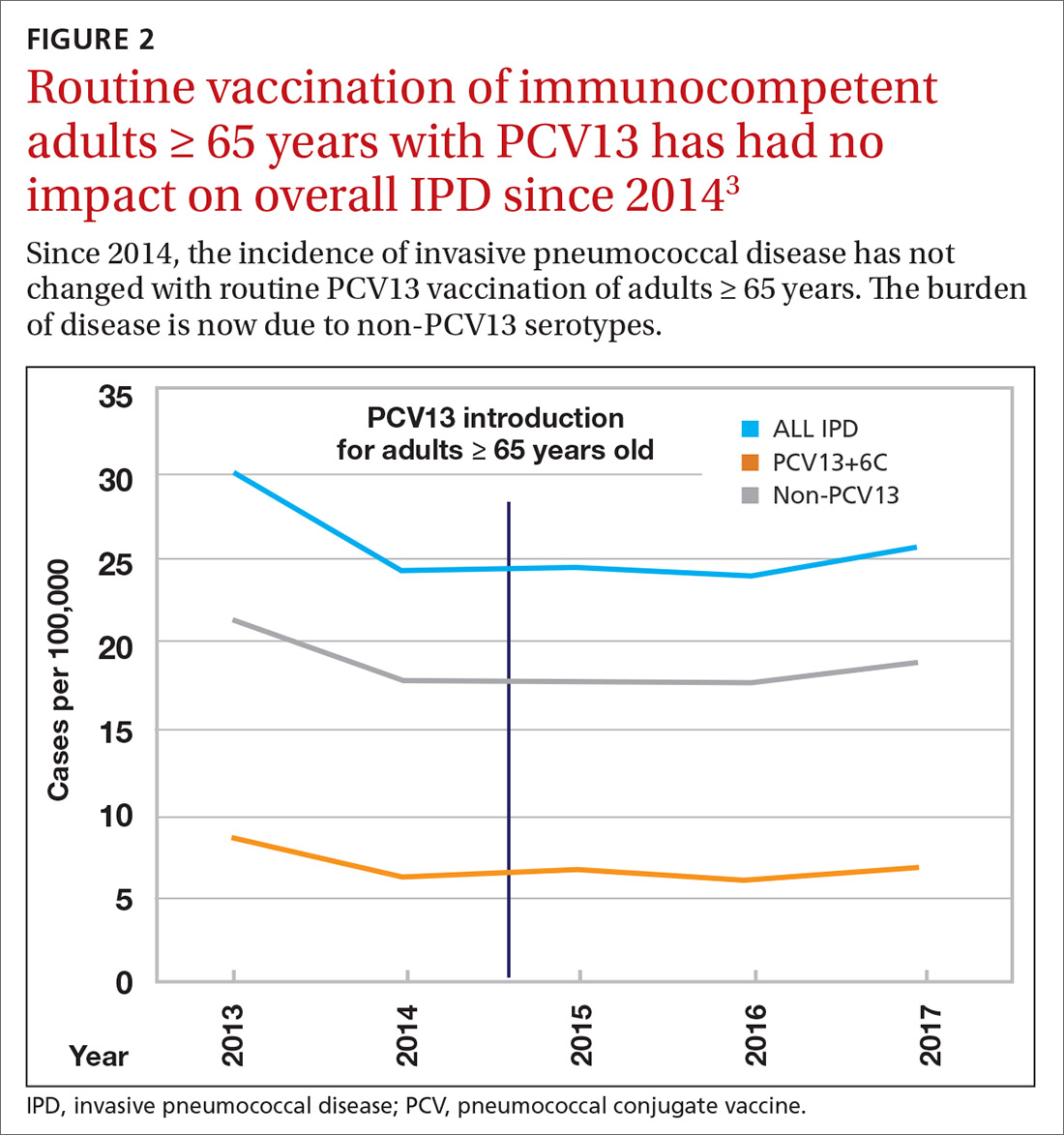
ACIP had 3 options in formulating its recommendations.
- Recommend the vaccine for routine use universally or among designated high-risk groups.
- Do not recommend the vaccine.
- Recommend the vaccine only for specific patients after individualized clinical decision making.
The last option—the one ACIP decided on—applies when a safe and immunogenic vaccine has been approved by the Food and Drug Administration and may be beneficial for (or desired by) individuals even though it does not meet criteria for routine universal or targeted use.
Practical issues
ACIP recommendations for the use of PCV13 and PPSV23 in adults vary according to 3 categories of health status: immunocompetent patients with underlying medical conditions; those with functional or anatomic asplenia; and immunocompromised individuals (TABLE1). Those in the latter 2 categories should receive both PCV13 and PPSV23 and be revaccinated once with PPSV23 before the age of 65 (given 5 years after the first dose). For immunocompetent individuals with underlying medical conditions, only those with cerebral spinal fluid leaks or cochlear implants should receive both PCV13 and PPSV23, although revaccination with PPSV23 before the age of 65 is not recommended.
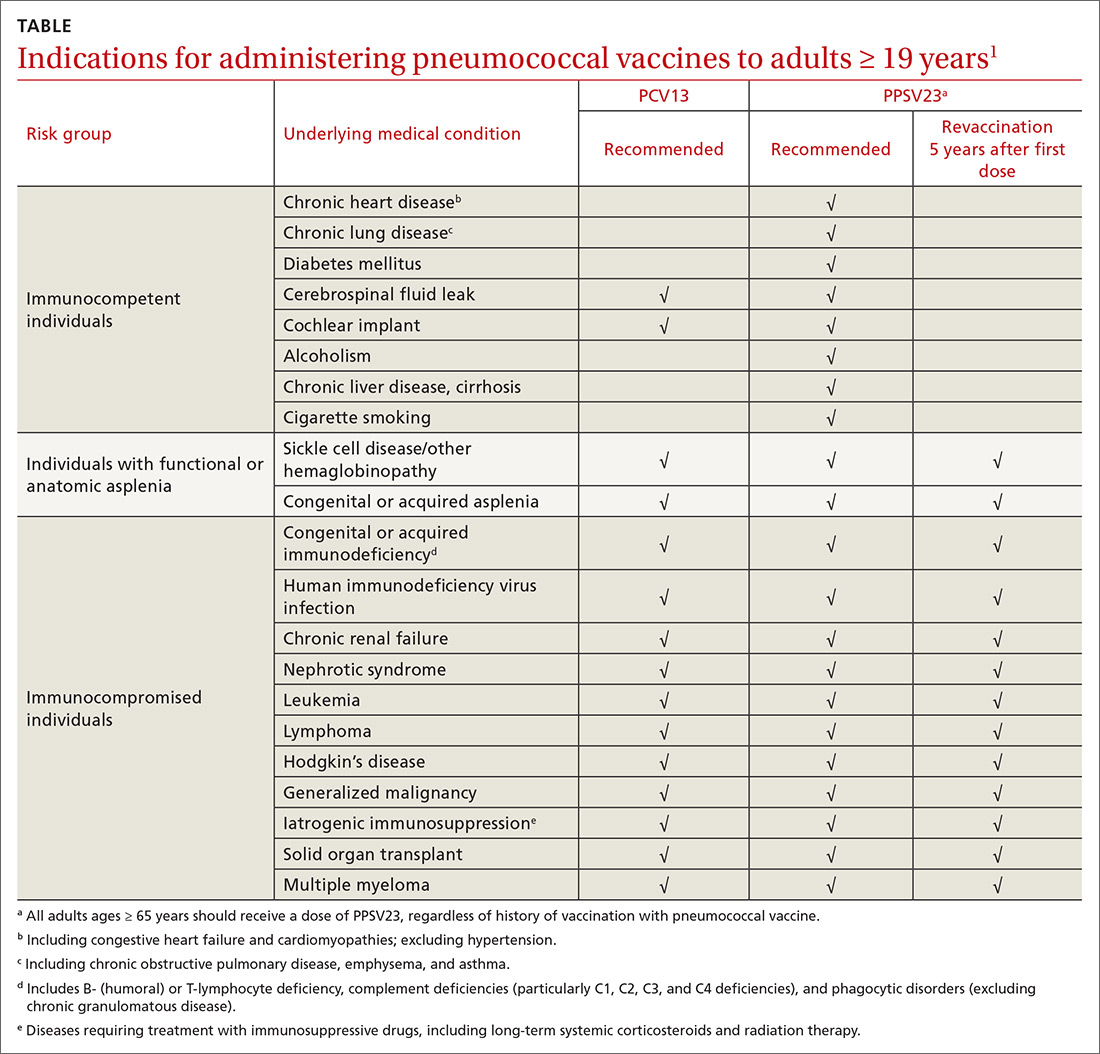
Continue to: Prior to the recent change...
Prior to the recent change, ACIP recommended both PCV13 and PPSV23 for those ≥ 65 years. Now, PCV13 is not recommended routinely for immunocompetent adults ≥ 65 years; however, individuals in this age group who have chronic underlying medical conditions may receive PCV13 after consulting with their physician. PPSV23 is still recommended for all adults in this age group. Recommendations for those with immunocompromising conditions are also unchanged.
3 sentences summarize change in vaccine intervals. Another source of confusion is the recommended intervals in administering the 2 vaccines when both are indicated. The current guidance has been simplified and can be summarized in 3 sentences4:
- When both PCV13 and PPSV23 are indicated, give PCV13 before PPSV23.
- For patients ≥ 65 years, separate the vaccines by 12 months or more—regardless of which vaccine is administered first.
- For patients who are 19 to 64 years of age, separate the vaccines by ≥ 8 weeks.
Advice on repeating the PPSV23 vaccine also can be summarized in 3 sentences1:
- When a repeat PPSV23 dose is indicated, give it at least 5 years after the first dose.
- Administer no more than 2 doses before age 65.
- For an individual older than 65, only 1 dose should be administered and it should be done at least 5 years after a previous PPSV23 dose.
1. CDC. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2012;61:816-819.
2. Tomczyk S, Bennett NM, Stoecker C, et al. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2014;63:822-825.
3. Matanock A. Considerations for PCV13 use among adults ≥65 years old and a summary of the evidence to recommendations framework. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-06/Pneumococcal-2-Matanock-508.pdf. Accessed December 5, 2019.
4. Kobayashi M, Bennett NM, Gierke R, et al. Intervals between PCV13 and PPSV23 vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2015; 64:944-947.
Two pneumococcal vaccines are licensed for use in the United States: the 13-valent pneumococcal conjugate vaccine (PCV13 [Prevnar 13, Wyeth]) and the 23-valent pneumococcal polysaccharide vaccine (PPSV23 [Pneumovax, Merck]). The recommendations for using these vaccines in adults ages ≥ 19 years are arguably among the most complicated and confusing of all vaccine recommendations made by the Advisory Committee on Immunization Practices (ACIP).
In June 2019, things got even more complicated with ACIP’s unusual decision to change the previous recommendation on the routine use of PCV13 in adults ≥ 65 years. The new recommendation states that PCV13 should be used in immunocompetent older adults only after individual clinical decision making. The recommendation for routine use of PPSV23 remains unchanged. This Practice Alert explains the reasoning behind this change and its practical implications.
How we got to where we are now
Nearly 20 years ago, PCV was introduced into the child immunization schedule in the United States as a 7-valent vaccine (PCV7). In 2010, it was modified to include 13 antigens. And in 2012, the use of PCV13 was expanded to include adults with immunocompromising conditions.1 In 2014, PCV13 was recommended as an addition to PPSV23 for adults ≥ 65 years.2 However, with this recommendation, ACIP noted that the incidence of invasive pneumococcal disease in the elderly had been declining since the introduction of PCV7 use in children in the year 2000 (FIGURE 13), presumably due to the decreased transmission of pneumococcal infections from children to older adults.

Because it was unclear in 2014 how much added benefit PCV13 would offer older adults, ACIP voted to restudy the issue after 4 years. At the June 2019 ACIP meeting, the results of an interim analysis were presented. ACIP concluded that routine use of PCV13 in immunocompetent adults ≥ 65 years adds little population-wide public health benefit given the vaccine’s routine use among children and immunocompromised adults (FIGURE 23).

ACIP had 3 options in formulating its recommendations.
- Recommend the vaccine for routine use universally or among designated high-risk groups.
- Do not recommend the vaccine.
- Recommend the vaccine only for specific patients after individualized clinical decision making.
The last option—the one ACIP decided on—applies when a safe and immunogenic vaccine has been approved by the Food and Drug Administration and may be beneficial for (or desired by) individuals even though it does not meet criteria for routine universal or targeted use.
Practical issues
ACIP recommendations for the use of PCV13 and PPSV23 in adults vary according to 3 categories of health status: immunocompetent patients with underlying medical conditions; those with functional or anatomic asplenia; and immunocompromised individuals (TABLE1). Those in the latter 2 categories should receive both PCV13 and PPSV23 and be revaccinated once with PPSV23 before the age of 65 (given 5 years after the first dose). For immunocompetent individuals with underlying medical conditions, only those with cerebral spinal fluid leaks or cochlear implants should receive both PCV13 and PPSV23, although revaccination with PPSV23 before the age of 65 is not recommended.

Continue to: Prior to the recent change...
Prior to the recent change, ACIP recommended both PCV13 and PPSV23 for those ≥ 65 years. Now, PCV13 is not recommended routinely for immunocompetent adults ≥ 65 years; however, individuals in this age group who have chronic underlying medical conditions may receive PCV13 after consulting with their physician. PPSV23 is still recommended for all adults in this age group. Recommendations for those with immunocompromising conditions are also unchanged.
3 sentences summarize change in vaccine intervals. Another source of confusion is the recommended intervals in administering the 2 vaccines when both are indicated. The current guidance has been simplified and can be summarized in 3 sentences4:
- When both PCV13 and PPSV23 are indicated, give PCV13 before PPSV23.
- For patients ≥ 65 years, separate the vaccines by 12 months or more—regardless of which vaccine is administered first.
- For patients who are 19 to 64 years of age, separate the vaccines by ≥ 8 weeks.
Advice on repeating the PPSV23 vaccine also can be summarized in 3 sentences1:
- When a repeat PPSV23 dose is indicated, give it at least 5 years after the first dose.
- Administer no more than 2 doses before age 65.
- For an individual older than 65, only 1 dose should be administered and it should be done at least 5 years after a previous PPSV23 dose.
Two pneumococcal vaccines are licensed for use in the United States: the 13-valent pneumococcal conjugate vaccine (PCV13 [Prevnar 13, Wyeth]) and the 23-valent pneumococcal polysaccharide vaccine (PPSV23 [Pneumovax, Merck]). The recommendations for using these vaccines in adults ages ≥ 19 years are arguably among the most complicated and confusing of all vaccine recommendations made by the Advisory Committee on Immunization Practices (ACIP).
In June 2019, things got even more complicated with ACIP’s unusual decision to change the previous recommendation on the routine use of PCV13 in adults ≥ 65 years. The new recommendation states that PCV13 should be used in immunocompetent older adults only after individual clinical decision making. The recommendation for routine use of PPSV23 remains unchanged. This Practice Alert explains the reasoning behind this change and its practical implications.
How we got to where we are now
Nearly 20 years ago, PCV was introduced into the child immunization schedule in the United States as a 7-valent vaccine (PCV7). In 2010, it was modified to include 13 antigens. And in 2012, the use of PCV13 was expanded to include adults with immunocompromising conditions.1 In 2014, PCV13 was recommended as an addition to PPSV23 for adults ≥ 65 years.2 However, with this recommendation, ACIP noted that the incidence of invasive pneumococcal disease in the elderly had been declining since the introduction of PCV7 use in children in the year 2000 (FIGURE 13), presumably due to the decreased transmission of pneumococcal infections from children to older adults.

Because it was unclear in 2014 how much added benefit PCV13 would offer older adults, ACIP voted to restudy the issue after 4 years. At the June 2019 ACIP meeting, the results of an interim analysis were presented. ACIP concluded that routine use of PCV13 in immunocompetent adults ≥ 65 years adds little population-wide public health benefit given the vaccine’s routine use among children and immunocompromised adults (FIGURE 23).

ACIP had 3 options in formulating its recommendations.
- Recommend the vaccine for routine use universally or among designated high-risk groups.
- Do not recommend the vaccine.
- Recommend the vaccine only for specific patients after individualized clinical decision making.
The last option—the one ACIP decided on—applies when a safe and immunogenic vaccine has been approved by the Food and Drug Administration and may be beneficial for (or desired by) individuals even though it does not meet criteria for routine universal or targeted use.
Practical issues
ACIP recommendations for the use of PCV13 and PPSV23 in adults vary according to 3 categories of health status: immunocompetent patients with underlying medical conditions; those with functional or anatomic asplenia; and immunocompromised individuals (TABLE1). Those in the latter 2 categories should receive both PCV13 and PPSV23 and be revaccinated once with PPSV23 before the age of 65 (given 5 years after the first dose). For immunocompetent individuals with underlying medical conditions, only those with cerebral spinal fluid leaks or cochlear implants should receive both PCV13 and PPSV23, although revaccination with PPSV23 before the age of 65 is not recommended.

Continue to: Prior to the recent change...
Prior to the recent change, ACIP recommended both PCV13 and PPSV23 for those ≥ 65 years. Now, PCV13 is not recommended routinely for immunocompetent adults ≥ 65 years; however, individuals in this age group who have chronic underlying medical conditions may receive PCV13 after consulting with their physician. PPSV23 is still recommended for all adults in this age group. Recommendations for those with immunocompromising conditions are also unchanged.
3 sentences summarize change in vaccine intervals. Another source of confusion is the recommended intervals in administering the 2 vaccines when both are indicated. The current guidance has been simplified and can be summarized in 3 sentences4:
- When both PCV13 and PPSV23 are indicated, give PCV13 before PPSV23.
- For patients ≥ 65 years, separate the vaccines by 12 months or more—regardless of which vaccine is administered first.
- For patients who are 19 to 64 years of age, separate the vaccines by ≥ 8 weeks.
Advice on repeating the PPSV23 vaccine also can be summarized in 3 sentences1:
- When a repeat PPSV23 dose is indicated, give it at least 5 years after the first dose.
- Administer no more than 2 doses before age 65.
- For an individual older than 65, only 1 dose should be administered and it should be done at least 5 years after a previous PPSV23 dose.
1. CDC. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2012;61:816-819.
2. Tomczyk S, Bennett NM, Stoecker C, et al. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2014;63:822-825.
3. Matanock A. Considerations for PCV13 use among adults ≥65 years old and a summary of the evidence to recommendations framework. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-06/Pneumococcal-2-Matanock-508.pdf. Accessed December 5, 2019.
4. Kobayashi M, Bennett NM, Gierke R, et al. Intervals between PCV13 and PPSV23 vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2015; 64:944-947.
1. CDC. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2012;61:816-819.
2. Tomczyk S, Bennett NM, Stoecker C, et al. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2014;63:822-825.
3. Matanock A. Considerations for PCV13 use among adults ≥65 years old and a summary of the evidence to recommendations framework. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-06/Pneumococcal-2-Matanock-508.pdf. Accessed December 5, 2019.
4. Kobayashi M, Bennett NM, Gierke R, et al. Intervals between PCV13 and PPSV23 vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2015; 64:944-947.
How to respond to flu vaccine doubters
The benefits of influenza vaccination are clear to those in the medical community. Yet misinformation and unfounded fears continue to discourage some people from getting a flu shot. During the 2018–2019 influenza season, only 45% of US adults and 63% of children were vaccinated.1
‘IT DOESN’T WORK FOR MANY PEOPLE’
Multiple studies have shown that the flu vaccine prevents millions of flu cases and flu-related doctor’s visits each year. During the 2016–2017 flu season, flu vaccine prevented an estimated 5.3 million influenza cases, 2.6 million influenza-associated medical visits, and 85,000 influenza-associated hospitalizations.2
Several viral and host factors affect vaccine effectiveness. In seasons when the vaccine viruses have matched circulating strains, flu vaccine has been shown to reduce the following:
- The risk of having to go to the doctor with flu by 40% to 60%
- Children’s risk of flu-related death and intensive care unit (ICU) admission by 74%
- The risk in adults of flu-associated hospitalizations by 40% and ICU admission by 82%
- The rate of cardiac events in people with heart disease
- Hospitalizations in people with diabetes or underlying chronic lung disease.3
In people hospitalized with influenza despite receiving the flu vaccine for the season, studies have shown that receiving the flu vaccine shortens the average duration of hospitalization, reduces the chance of ICU admission by 59%, shortens the duration of ICU stay by 4 days, and reduces deaths.3
‘IT TARGETS THE WRONG VIRUS’
Selecting an effective influenza vaccine is a challenge. Every year, the World Health Organization and the CDC decide on the influenza strains expected to circulate in the upcoming flu season in the Northern Hemisphere, based on data for circulating strains in the Southern Hemisphere. This decision takes place about 7 months before the expected onset of the flu season. Flu viruses may mutate between the time the decision is made and the time the vaccine is administered (as well as after the flu season starts). Also, vaccine production in eggs needs time, which is why this decision must be made several months ahead of the flu season.
Vaccine effectiveness varies by virus serotype. Vaccines are typically less effective against influenza A H3N2 viruses than against influenza A H1N1 and influenza B viruses. Effectiveness also varies from season to season depending on how close the vaccine serotypes match the circulating serotypes, but some effectiveness is retained even in seasons when some of the serotypes don’t match circulating viruses. For example, in the 2017–2018 season, when the influenza A H3N2 vaccine serotype did not match the circulating serotype, the overall effectiveness in preventing medically attended, laboratory-confirmed influenza virus infection was 36%.5
A universal flu vaccine that does not need to be updated annually is the ultimate solution, but according to the National Institute of Allergy and Infectious Diseases, such a vaccine is likely several years away.6
‘IT MAKES PEOPLE SICK’
Pain at the injection site of a flu shot occurs in 10% to 65% of people, lasts less than 2 days, and does not usually interfere with daily activities.7
Systemic symptoms such as fever, malaise, and myalgia may occur in people who have had no previous exposure to the influenza virus antigens in the vaccine, particularly in children. In adults, the frequency of systemic symptoms after the flu shot is similar to that with placebo.
The Vaccine Adverse Event Reporting System, which has been capturing data since 1990, shows that the influenza vaccine accounted for 5.7% of people who developed malaise after receiving any vaccine.8
The injectable inactivated influenza vaccine cannot biologically cause an influenza virus-related illness, since the inactivated vaccine viruses can elicit a protective immune response but cannot replicate. The nasal live-attenuated flu vaccine can in theory cause acute illness in the person receiving it, but because it is cold-adapted, it multiplies only in the colder environment of the nasal epithelium, not in the lower airways where the temperature is higher. Consequently, the vaccine virus triggers immunity by multiplying in the nose, but doesn’t infect the lungs.
From 10% to 50% of people who receive the nasal live-attenuated vaccine develop runny nose, wheezing, headache, vomiting, muscle aches, fever, sore throat, or cough shortly after receiving the vaccine, but these symptoms are usually mild and short-lived.
The most common reactions people have to flu vaccines are considerably less severe than the symptoms caused by actual flu illness.
While influenza illness results in natural immunity to the specific viral serotype causing it, this illness results in hospitalization in 2% and is fatal in 0.16% of people. Influenza vaccine results in immunity to the serotypes included in the vaccine, and multiple studies have not found a causal relationship between vaccination and death.9
‘IT CAUSES GUILLAIN-BARRÉ SYNDROME’
In the United States, 3,000 to 6,000 people per year develop Guillain-Barré syndrome, or 1 to 2 of every 100,000, which translates to 80 to 160 cases per week.10 While the exact cause of Guillain-Barré syndrome is unknown, about two-thirds of people have an acute diarrheal or respiratory illness within 3 months before the onset of symptoms. In 1976, the estimated attributable risk of influenza vaccine-related Guillain-Barré syndrome in the US adult population was 1 case per 100,000 in the 6 weeks after vaccination.11 Studies in subsequent influenza seasons have not shown similar findings.12 In fact, one study showed that the risk of developing Guillain-Barré syndrome was 15 times higher after influenza illness than after influenza vaccination.13
Since 5% to 15% of the US population develop symptomatic influenza annually,14 the decision to vaccinate with respect to the risk of Guillain-Barré syndrome should be obvious: vaccinate. The correct question to ask before influenza vaccination should be, “Have you previously developed Guillain-Barré syndrome within 6 weeks after receiving the flu vaccine?” If the answer is yes, the CDC considers this a caution, not a contraindication against receiving the influenza vaccine, since the benefit may still outweigh the risk.
‘I GOT THE FLU SHOT AND STILL GOT SICK’
The flu vaccine does not prevent illnesses caused by other viruses or bacteria that can make people sick during flu season. Influenza, the common cold, and streptococcal pharyngitis can have similar symptoms that make it difficult for patients—and, frequently, even healthcare providers—to distinguish between these illnesses with certainty.
One study suggested that influenza vaccine recipients had an increased risk of virologically confirmed noninfluenza respiratory viral infections,15 citing the phenomenon of virus interference that was described in the 1940s16 as a potential explanation. In essence, people protected against influenza by the vaccine may lack temporary nonspecific immunity against other respiratory viruses. However, these findings have not been replicated in subsequent studies.17
Viral gastroenteritis, mistakenly called “stomach flu,” is also not prevented by influenza vaccination.
‘I’M ALLERGIC TO EGGS’
The prevalence of egg allergy in US children is 0.5% to 2.5%.18 Most outgrow it by school age, but in one-third, the allergy persists into adulthood.
In general, people who can eat lightly cooked eggs (eg, scrambled eggs) without a reaction are unlikely to be allergic. On the other hand, the fact that egg-allergic people may tolerate egg included in baked products does not exclude the possibility of egg allergy. Egg allergy can be confirmed by a consistent medical history of adverse reaction to eggs and egg-containing foods, in addition to skin or blood testing for immunoglobulin E directed against egg proteins.19
Most currently available influenza vaccines are prepared by propagation of virus in embryonated eggs and so may contain trace amounts of egg proteins such as ovalbumin, with the exception of the inactivated quadrivalent recombinant influenza vaccine (Flublok) and the inactivated quadrivalent cell culture-based vaccine (Flucelvax).
The ACIP recommends that persons with a history of urticaria (hives) after exposure to eggs should receive any licensed, recommended influenza vaccine that is otherwise appropriate for their age and health status. Persons who report having angioedema, respiratory distress, lightheadedness, or recurrent vomiting, or who required epinephrine or another emergency medical intervention after exposure to eggs, should receive the influenza vaccine in an inpatient or outpatient medical setting under the supervision of a healthcare provider who is able to recognize and manage severe allergic reactions.
A history of severe allergic reaction such as anaphylaxis to a previous dose of any influenza vaccine, regardless of the vaccine component (including eggs) suspected of being responsible for the reaction, is a contraindication to influenza vaccination. The ACIP recommends that vaccine providers consider observing patients for 15 minutes after administration of any vaccine (regardless of history of egg allergy) to decrease the risk of injury should syncope occur.20
‘I DON’T WANT TO PUT POISONOUS MERCURY IN MY BODY’
A process of biomagnification of methylmercury occurs when humans eat large fish that have eaten smaller fish. Thus, larger fish such as shark can be hazardous for women who are or may become pregnant, for nursing mothers, and for young children, while smaller fish such as herring are relatively safe.
As a precautionary measure, thimerosal was taken out of childhood vaccines in the United States in 2001. Thimerosal-free influenza vaccine formulations include the nasal live-attenuated flu vaccine, the inactivated quadrivalent recombinant influenza vaccine, and the inactivated quadrivalent cell culture-based vaccine.
‘I DON’T LIKE NEEDLES’
At least 10% of US adults have aichmophobia, the fear of sharp objects including needles.22 Vasovagal syncope is the most common manifestation. Behavioral therapy, topical anesthetics, and systemic anxiolytics have variable efficacy in treating needle phobia. For those who are absolutely averse to needles, the nasal flu vaccine is an appropriate alternative.
‘I DON’T WANT TO TAKE ANYTHING THAT CAN MESS WITH MY OTHER MEDICATIONS’
Some immunosuppressive medications may decrease influenza vaccine immunogenicity. Concomitant administration of the inactivated influenza vaccine with other vaccines is safe and does not alter immunogenicity of other vaccines.1 The live-attenuated influenza vaccine is contraindicated in children and adolescents taking aspirin or other salicylates due to the risk of Reye syndrome.
‘I’M AFRAID IT WILL TRIGGER AN IMMUNE RESPONSE THAT WILL MAKE MY ASTHMA WORSE’
A recent systematic review and meta-analysis showed that the inactivated influenza vaccine is not associated with asthma exacerbation.23 However, the nasal live-attenuated influenza vaccine is contraindicated in children 2 to 4 years old who have asthma and should be used with caution in persons with asthma 5 years old and older. In the systematic review, influenza vaccine prevented 59% to 78% of asthma attacks leading to emergency visits or hospitalization.23 In other immune-mediated diseases such as rheumatoid arthritis, influenza vaccine does not precipitate exacerbations.24
‘I HAD AN ORGAN TRANSPLANT, AND I’M AFRAID THE FLU SHOT WILL CAUSE ORGAN REJECTION’
A study of 51,730 kidney transplant recipients found that receipt of the inactivated influenza vaccine in the first year after transplant was associated with a lower risk of subsequent allograft loss (adjusted hazard ratio 0.77; 95% confidence interval 0.69–0.85; P < .001) and death (adjusted hazard ratio 0.82; 95% confidence interval 0.76–0.89; P < .001).25 In the same study, although acute rejection in the first year was not associated with influenza vaccination, influenza infection in the first year was associated with rejection (odds ratio 1.58; 95% confidence interval 1.10–2.26; P < 0.001), but not with graft loss or death. Solid organ transplant recipients should receive the inactivated influenza vaccine starting 3 months after transplant.26
Influenza vaccination has not been shown to precipitate graft-vs-host disease in hematopoietic stem cell transplant recipients. These patients should also receive the inactivated influenza vaccine starting 3 to 6 months after transplant.27
The nasal live-attenuated influenza vaccine is contraindicated in these immunocompromised patients.
‘I’M PREGNANT, AND I DON’T WANT TO EXPOSE MY UNBORN BABY TO ANYTHING POTENTIALLY HARMFUL’
The morbidity and mortality risk from influenza is high in children under 2 years old because of low immunogenicity to flu vaccine. This is particularly true in children younger than 6 months, but the vaccine is not recommended in this population. The best way to protect infants is for all household members to be vaccinated against the flu.
Equally important, morbidity and mortality risk from influenza is much higher in pregnant women than in the general population. Many studies have shown the value of influenza vaccination during pregnancy for both mothers and their infants. A recently published study showed that 18% of infants who developed influenza required hospitalization.28 In that study, prenatal and postpartum maternal influenza vaccination decreased the odds of influenza in infants by 61% and 53%, respectively. Another study showed that vaccine effectiveness did not vary by gestational age at vaccination.29 A post hoc analysis of an influenza vaccination study in pregnant women suggested that the vaccine was also associated with decreased rates of pertussis in these women.30
Healthcare providers should try to understand the public’s misconceptions31 about seasonal influenza and influenza vaccines in order to best address them.
- Centers for Disease Control and Prevention (CDC). Flu vaccination coverage, United States, 2018–19 influenza season. www.cdc.gov/flu/fluvaxview/coverage-1819estimates.htm. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). Immunogenicity, efficacy, and effectiveness of influenza vaccines. www.cdc.gov/flu/professionals/acip/immunogenicity.htm. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). What are the benefits of flu vaccination? www.cdc.gov/flu/prevent/vaccine-benefits.htm. Accessed November 13, 2019.
- Grohskopf LA, Alyanak E, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2019–20 influenza season. MMWR Recomm Rep 2019; 68(3):1–21. doi:10.15585/mmwr.rr6803a1
- Flannery B, Chung JR, Belongia EA, et al. Interim estimates of 2017–18 seasonal influenza vaccine effectiveness—United States, February 2018. MMWR Morb Mortal Wkly Rep 2018; 67(6):180–185. doi:10.15585/mmwr.mm6706a2
- Erbelding EJ, Post DJ, Stemmy EJ, et al. A universal influenza vaccine: the strategic plan for the National Institute of Allergy and Infectious Diseases. J Infect Dis 2018; 218(3):347–354. doi:10.1093/infdis/jiy103
- Centers for Disease Control and Prevention (CDC). Seasonal influenza vaccine safety: a summary for clinicians. www.cdc.gov/flu/professionals/vaccination/vaccine_safety.htm. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). About the Vaccine Adverse Event Reporting System (VAERS). https://wonder.cdc.gov/vaers.html. Accessed November 13, 2019.
- Miller ER, Moro PL, Cano M, Shimabukuro TT. Deaths following vaccination: what does the evidence show? Vaccine 2015; 33(29):3288–3292. doi:10.1016/j.vaccine.2015.05.023
- Centers for Disease Control and Prevention (CDC). Guillain-Barré syndrome and flu vaccine. www.cdc.gov/flu/prevent/guillainbarre.htm. Accessed November 13, 2019.
- Schonberger LB, Bregman DJ, Sullivan-Bolyai JZ, et al. Guillain-Barre syndrome following vaccination in the national influenza immunization program, United States, 1976–1977. Am J Epidemiol 1979; 110(2):105–123. doi:10.1093/oxfordjournals.aje.a112795
- Baxter R, Bakshi N, Fireman B, et al. Lack of association of Guillain-Barré syndrome with vaccinations. Clin Infect Dis 2013; 57(2):197–204. doi:10.1093/cid/cit222
- Kwong JC, Vasa PP, Campitelli MA, et al. Risk of Guillain-Barré syndrome after seasonal influenza vaccination and influenza health-care encounters: a self-controlled study. Lancet Infect Dis 2013; 13(9):769–776. doi:10.1016/S1473-3099(13)70104-X
- Centers for Disease Control and Prevention (CDC). Disease burden of influenza. www.cdc.gov/flu/about/burden/index.html. Accessed November 13, 2019.
- Cowling BJ, Fang VJ, Nishiura H, et al. Increased risk of noninfluenza respiratory virus infections associated with receipt of inactivated influenza vaccine. Clin Infect Dis 2012; 54(12):1778–1783. doi:10.1093/cid/cis307
- Henle W, Henle G. Interference of inactive virus with the propagation of virus of influenza. Science 1943; 98(2534):87–89. doi:10.1126/science.98.2534.87
- Sundaram ME, McClure DL, VanWormer JJ, Friedrich TC, Meece JK, Belongia EA. Influenza vaccination is not associated with detection of noninfluenza respiratory viruses in seasonal studies of influenza vaccine effectiveness. Clin Infect Dis 2013; 57(6):789–793. doi:10.1093/cid/cit379
- Caubet JC, Wang J. Current understanding of egg allergy. Pediatr Clin North Am 2011; 58(2):427–443. doi:10.1016/j.pcl.2011.02.014
- Erlewyn-Lajeunesse M, Brathwaite N, Lucas JS, Warner JO. Recommendations for the administration of influenza vaccine in children allergic to egg. BMJ 2009; 339:b3680. doi:10.1136/bmj.b3680
- Ezeanolue E, Harriman K, Hunter P, Kroger A, Pellegrini C. General Best Practice Guidelines for Immunization. Best Practices Guidance of the Advisory Committee on Immunization Practices (ACIP). https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/downloads/general-recs.pdf. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). Thimerosal in vaccines. www.cdc.gov/vaccinesafety/concerns/thimerosal/index.html. Accessed November 13, 2019.
- Hamilton JG. Needle phobia: a neglected diagnosis. J Fam Pract 1995; 41(2):169–175. pmid:7636457
- Vasileiou E, Sheikh A, Butler C, et al. Effectiveness of influenza vaccines in asthma: a systematic review and meta-analysis. Clin Infect Dis 2017; 65(8):1388–1395. doi:10.1093/cid/cix524
- Fomin I, Caspi D, Levy V, et al. Vaccination against influenza in rheumatoid arthritis: the effect of disease modifying drugs, including TNF alpha blockers. Ann Rheum Dis 2006; 65(2):191–194. doi:10.1136/ard.2005.036434
- Hurst FP, Lee JJ, Jindal RM, Agodoa LY, Abbott KC. Outcomes associated with influenza vaccination in the first year after kidney transplantation. Clin J Am Soc Nephrol 2011; 6(5):1192–1197. doi:10.2215/CJN.05430610
- Chong PP, Handler L, Weber DJ. A systematic review of safety and immunogenicity of influenza vaccination strategies in solid organ transplant recipients. Clin Infect Dis 2018; 66(11):1802–1811. doi:10.1093/cid/cix1081
- Ljungman P, Avetisyan G. Influenza vaccination in hematopoietic SCT recipients. Bone Marrow Transplant 2008; 42(10):637–641. doi:10.1038/bmt.2008.264
- Ohfuji S, Deguchi M, Tachibana D, et al; Osaka Pregnant Women Influenza Study Group. Protective effect of maternal influenza vaccination on influenza in their infants: a prospective cohort study. J Infect Dis 2018; 217(6):878–886. doi:10.1093/infdis/jix629
- Katz J, Englund JA, Steinhoff MC, et al. Impact of timing of influenza vaccination in pregnancy on transplacental antibody transfer, influenza incidence, and birth outcomes: a randomized trial in rural Nepal. Clin Infect Dis 2018; 67(3):334–340. doi:10.1093/cid/ciy090
- Nunes MC, Cutland CL, Madhi SA. Influenza vaccination during pregnancy and protection against pertussis. N Engl J Med 2018; 378(13):1257–1258. doi:10.1056/NEJMc1705208
- Centers for Disease Control and Prevention (CDC). Misconceptions about seasonal flu and flu vaccines. www.cdc.gov/flu/prevent/misconceptions.htm. Accessed November 13, 2019.
The benefits of influenza vaccination are clear to those in the medical community. Yet misinformation and unfounded fears continue to discourage some people from getting a flu shot. During the 2018–2019 influenza season, only 45% of US adults and 63% of children were vaccinated.1
‘IT DOESN’T WORK FOR MANY PEOPLE’
Multiple studies have shown that the flu vaccine prevents millions of flu cases and flu-related doctor’s visits each year. During the 2016–2017 flu season, flu vaccine prevented an estimated 5.3 million influenza cases, 2.6 million influenza-associated medical visits, and 85,000 influenza-associated hospitalizations.2
Several viral and host factors affect vaccine effectiveness. In seasons when the vaccine viruses have matched circulating strains, flu vaccine has been shown to reduce the following:
- The risk of having to go to the doctor with flu by 40% to 60%
- Children’s risk of flu-related death and intensive care unit (ICU) admission by 74%
- The risk in adults of flu-associated hospitalizations by 40% and ICU admission by 82%
- The rate of cardiac events in people with heart disease
- Hospitalizations in people with diabetes or underlying chronic lung disease.3
In people hospitalized with influenza despite receiving the flu vaccine for the season, studies have shown that receiving the flu vaccine shortens the average duration of hospitalization, reduces the chance of ICU admission by 59%, shortens the duration of ICU stay by 4 days, and reduces deaths.3
‘IT TARGETS THE WRONG VIRUS’
Selecting an effective influenza vaccine is a challenge. Every year, the World Health Organization and the CDC decide on the influenza strains expected to circulate in the upcoming flu season in the Northern Hemisphere, based on data for circulating strains in the Southern Hemisphere. This decision takes place about 7 months before the expected onset of the flu season. Flu viruses may mutate between the time the decision is made and the time the vaccine is administered (as well as after the flu season starts). Also, vaccine production in eggs needs time, which is why this decision must be made several months ahead of the flu season.
Vaccine effectiveness varies by virus serotype. Vaccines are typically less effective against influenza A H3N2 viruses than against influenza A H1N1 and influenza B viruses. Effectiveness also varies from season to season depending on how close the vaccine serotypes match the circulating serotypes, but some effectiveness is retained even in seasons when some of the serotypes don’t match circulating viruses. For example, in the 2017–2018 season, when the influenza A H3N2 vaccine serotype did not match the circulating serotype, the overall effectiveness in preventing medically attended, laboratory-confirmed influenza virus infection was 36%.5
A universal flu vaccine that does not need to be updated annually is the ultimate solution, but according to the National Institute of Allergy and Infectious Diseases, such a vaccine is likely several years away.6
‘IT MAKES PEOPLE SICK’
Pain at the injection site of a flu shot occurs in 10% to 65% of people, lasts less than 2 days, and does not usually interfere with daily activities.7
Systemic symptoms such as fever, malaise, and myalgia may occur in people who have had no previous exposure to the influenza virus antigens in the vaccine, particularly in children. In adults, the frequency of systemic symptoms after the flu shot is similar to that with placebo.
The Vaccine Adverse Event Reporting System, which has been capturing data since 1990, shows that the influenza vaccine accounted for 5.7% of people who developed malaise after receiving any vaccine.8
The injectable inactivated influenza vaccine cannot biologically cause an influenza virus-related illness, since the inactivated vaccine viruses can elicit a protective immune response but cannot replicate. The nasal live-attenuated flu vaccine can in theory cause acute illness in the person receiving it, but because it is cold-adapted, it multiplies only in the colder environment of the nasal epithelium, not in the lower airways where the temperature is higher. Consequently, the vaccine virus triggers immunity by multiplying in the nose, but doesn’t infect the lungs.
From 10% to 50% of people who receive the nasal live-attenuated vaccine develop runny nose, wheezing, headache, vomiting, muscle aches, fever, sore throat, or cough shortly after receiving the vaccine, but these symptoms are usually mild and short-lived.
The most common reactions people have to flu vaccines are considerably less severe than the symptoms caused by actual flu illness.
While influenza illness results in natural immunity to the specific viral serotype causing it, this illness results in hospitalization in 2% and is fatal in 0.16% of people. Influenza vaccine results in immunity to the serotypes included in the vaccine, and multiple studies have not found a causal relationship between vaccination and death.9
‘IT CAUSES GUILLAIN-BARRÉ SYNDROME’
In the United States, 3,000 to 6,000 people per year develop Guillain-Barré syndrome, or 1 to 2 of every 100,000, which translates to 80 to 160 cases per week.10 While the exact cause of Guillain-Barré syndrome is unknown, about two-thirds of people have an acute diarrheal or respiratory illness within 3 months before the onset of symptoms. In 1976, the estimated attributable risk of influenza vaccine-related Guillain-Barré syndrome in the US adult population was 1 case per 100,000 in the 6 weeks after vaccination.11 Studies in subsequent influenza seasons have not shown similar findings.12 In fact, one study showed that the risk of developing Guillain-Barré syndrome was 15 times higher after influenza illness than after influenza vaccination.13
Since 5% to 15% of the US population develop symptomatic influenza annually,14 the decision to vaccinate with respect to the risk of Guillain-Barré syndrome should be obvious: vaccinate. The correct question to ask before influenza vaccination should be, “Have you previously developed Guillain-Barré syndrome within 6 weeks after receiving the flu vaccine?” If the answer is yes, the CDC considers this a caution, not a contraindication against receiving the influenza vaccine, since the benefit may still outweigh the risk.
‘I GOT THE FLU SHOT AND STILL GOT SICK’
The flu vaccine does not prevent illnesses caused by other viruses or bacteria that can make people sick during flu season. Influenza, the common cold, and streptococcal pharyngitis can have similar symptoms that make it difficult for patients—and, frequently, even healthcare providers—to distinguish between these illnesses with certainty.
One study suggested that influenza vaccine recipients had an increased risk of virologically confirmed noninfluenza respiratory viral infections,15 citing the phenomenon of virus interference that was described in the 1940s16 as a potential explanation. In essence, people protected against influenza by the vaccine may lack temporary nonspecific immunity against other respiratory viruses. However, these findings have not been replicated in subsequent studies.17
Viral gastroenteritis, mistakenly called “stomach flu,” is also not prevented by influenza vaccination.
‘I’M ALLERGIC TO EGGS’
The prevalence of egg allergy in US children is 0.5% to 2.5%.18 Most outgrow it by school age, but in one-third, the allergy persists into adulthood.
In general, people who can eat lightly cooked eggs (eg, scrambled eggs) without a reaction are unlikely to be allergic. On the other hand, the fact that egg-allergic people may tolerate egg included in baked products does not exclude the possibility of egg allergy. Egg allergy can be confirmed by a consistent medical history of adverse reaction to eggs and egg-containing foods, in addition to skin or blood testing for immunoglobulin E directed against egg proteins.19
Most currently available influenza vaccines are prepared by propagation of virus in embryonated eggs and so may contain trace amounts of egg proteins such as ovalbumin, with the exception of the inactivated quadrivalent recombinant influenza vaccine (Flublok) and the inactivated quadrivalent cell culture-based vaccine (Flucelvax).
The ACIP recommends that persons with a history of urticaria (hives) after exposure to eggs should receive any licensed, recommended influenza vaccine that is otherwise appropriate for their age and health status. Persons who report having angioedema, respiratory distress, lightheadedness, or recurrent vomiting, or who required epinephrine or another emergency medical intervention after exposure to eggs, should receive the influenza vaccine in an inpatient or outpatient medical setting under the supervision of a healthcare provider who is able to recognize and manage severe allergic reactions.
A history of severe allergic reaction such as anaphylaxis to a previous dose of any influenza vaccine, regardless of the vaccine component (including eggs) suspected of being responsible for the reaction, is a contraindication to influenza vaccination. The ACIP recommends that vaccine providers consider observing patients for 15 minutes after administration of any vaccine (regardless of history of egg allergy) to decrease the risk of injury should syncope occur.20
‘I DON’T WANT TO PUT POISONOUS MERCURY IN MY BODY’
A process of biomagnification of methylmercury occurs when humans eat large fish that have eaten smaller fish. Thus, larger fish such as shark can be hazardous for women who are or may become pregnant, for nursing mothers, and for young children, while smaller fish such as herring are relatively safe.
As a precautionary measure, thimerosal was taken out of childhood vaccines in the United States in 2001. Thimerosal-free influenza vaccine formulations include the nasal live-attenuated flu vaccine, the inactivated quadrivalent recombinant influenza vaccine, and the inactivated quadrivalent cell culture-based vaccine.
‘I DON’T LIKE NEEDLES’
At least 10% of US adults have aichmophobia, the fear of sharp objects including needles.22 Vasovagal syncope is the most common manifestation. Behavioral therapy, topical anesthetics, and systemic anxiolytics have variable efficacy in treating needle phobia. For those who are absolutely averse to needles, the nasal flu vaccine is an appropriate alternative.
‘I DON’T WANT TO TAKE ANYTHING THAT CAN MESS WITH MY OTHER MEDICATIONS’
Some immunosuppressive medications may decrease influenza vaccine immunogenicity. Concomitant administration of the inactivated influenza vaccine with other vaccines is safe and does not alter immunogenicity of other vaccines.1 The live-attenuated influenza vaccine is contraindicated in children and adolescents taking aspirin or other salicylates due to the risk of Reye syndrome.
‘I’M AFRAID IT WILL TRIGGER AN IMMUNE RESPONSE THAT WILL MAKE MY ASTHMA WORSE’
A recent systematic review and meta-analysis showed that the inactivated influenza vaccine is not associated with asthma exacerbation.23 However, the nasal live-attenuated influenza vaccine is contraindicated in children 2 to 4 years old who have asthma and should be used with caution in persons with asthma 5 years old and older. In the systematic review, influenza vaccine prevented 59% to 78% of asthma attacks leading to emergency visits or hospitalization.23 In other immune-mediated diseases such as rheumatoid arthritis, influenza vaccine does not precipitate exacerbations.24
‘I HAD AN ORGAN TRANSPLANT, AND I’M AFRAID THE FLU SHOT WILL CAUSE ORGAN REJECTION’
A study of 51,730 kidney transplant recipients found that receipt of the inactivated influenza vaccine in the first year after transplant was associated with a lower risk of subsequent allograft loss (adjusted hazard ratio 0.77; 95% confidence interval 0.69–0.85; P < .001) and death (adjusted hazard ratio 0.82; 95% confidence interval 0.76–0.89; P < .001).25 In the same study, although acute rejection in the first year was not associated with influenza vaccination, influenza infection in the first year was associated with rejection (odds ratio 1.58; 95% confidence interval 1.10–2.26; P < 0.001), but not with graft loss or death. Solid organ transplant recipients should receive the inactivated influenza vaccine starting 3 months after transplant.26
Influenza vaccination has not been shown to precipitate graft-vs-host disease in hematopoietic stem cell transplant recipients. These patients should also receive the inactivated influenza vaccine starting 3 to 6 months after transplant.27
The nasal live-attenuated influenza vaccine is contraindicated in these immunocompromised patients.
‘I’M PREGNANT, AND I DON’T WANT TO EXPOSE MY UNBORN BABY TO ANYTHING POTENTIALLY HARMFUL’
The morbidity and mortality risk from influenza is high in children under 2 years old because of low immunogenicity to flu vaccine. This is particularly true in children younger than 6 months, but the vaccine is not recommended in this population. The best way to protect infants is for all household members to be vaccinated against the flu.
Equally important, morbidity and mortality risk from influenza is much higher in pregnant women than in the general population. Many studies have shown the value of influenza vaccination during pregnancy for both mothers and their infants. A recently published study showed that 18% of infants who developed influenza required hospitalization.28 In that study, prenatal and postpartum maternal influenza vaccination decreased the odds of influenza in infants by 61% and 53%, respectively. Another study showed that vaccine effectiveness did not vary by gestational age at vaccination.29 A post hoc analysis of an influenza vaccination study in pregnant women suggested that the vaccine was also associated with decreased rates of pertussis in these women.30
Healthcare providers should try to understand the public’s misconceptions31 about seasonal influenza and influenza vaccines in order to best address them.
The benefits of influenza vaccination are clear to those in the medical community. Yet misinformation and unfounded fears continue to discourage some people from getting a flu shot. During the 2018–2019 influenza season, only 45% of US adults and 63% of children were vaccinated.1
‘IT DOESN’T WORK FOR MANY PEOPLE’
Multiple studies have shown that the flu vaccine prevents millions of flu cases and flu-related doctor’s visits each year. During the 2016–2017 flu season, flu vaccine prevented an estimated 5.3 million influenza cases, 2.6 million influenza-associated medical visits, and 85,000 influenza-associated hospitalizations.2
Several viral and host factors affect vaccine effectiveness. In seasons when the vaccine viruses have matched circulating strains, flu vaccine has been shown to reduce the following:
- The risk of having to go to the doctor with flu by 40% to 60%
- Children’s risk of flu-related death and intensive care unit (ICU) admission by 74%
- The risk in adults of flu-associated hospitalizations by 40% and ICU admission by 82%
- The rate of cardiac events in people with heart disease
- Hospitalizations in people with diabetes or underlying chronic lung disease.3
In people hospitalized with influenza despite receiving the flu vaccine for the season, studies have shown that receiving the flu vaccine shortens the average duration of hospitalization, reduces the chance of ICU admission by 59%, shortens the duration of ICU stay by 4 days, and reduces deaths.3
‘IT TARGETS THE WRONG VIRUS’
Selecting an effective influenza vaccine is a challenge. Every year, the World Health Organization and the CDC decide on the influenza strains expected to circulate in the upcoming flu season in the Northern Hemisphere, based on data for circulating strains in the Southern Hemisphere. This decision takes place about 7 months before the expected onset of the flu season. Flu viruses may mutate between the time the decision is made and the time the vaccine is administered (as well as after the flu season starts). Also, vaccine production in eggs needs time, which is why this decision must be made several months ahead of the flu season.
Vaccine effectiveness varies by virus serotype. Vaccines are typically less effective against influenza A H3N2 viruses than against influenza A H1N1 and influenza B viruses. Effectiveness also varies from season to season depending on how close the vaccine serotypes match the circulating serotypes, but some effectiveness is retained even in seasons when some of the serotypes don’t match circulating viruses. For example, in the 2017–2018 season, when the influenza A H3N2 vaccine serotype did not match the circulating serotype, the overall effectiveness in preventing medically attended, laboratory-confirmed influenza virus infection was 36%.5
A universal flu vaccine that does not need to be updated annually is the ultimate solution, but according to the National Institute of Allergy and Infectious Diseases, such a vaccine is likely several years away.6
‘IT MAKES PEOPLE SICK’
Pain at the injection site of a flu shot occurs in 10% to 65% of people, lasts less than 2 days, and does not usually interfere with daily activities.7
Systemic symptoms such as fever, malaise, and myalgia may occur in people who have had no previous exposure to the influenza virus antigens in the vaccine, particularly in children. In adults, the frequency of systemic symptoms after the flu shot is similar to that with placebo.
The Vaccine Adverse Event Reporting System, which has been capturing data since 1990, shows that the influenza vaccine accounted for 5.7% of people who developed malaise after receiving any vaccine.8
The injectable inactivated influenza vaccine cannot biologically cause an influenza virus-related illness, since the inactivated vaccine viruses can elicit a protective immune response but cannot replicate. The nasal live-attenuated flu vaccine can in theory cause acute illness in the person receiving it, but because it is cold-adapted, it multiplies only in the colder environment of the nasal epithelium, not in the lower airways where the temperature is higher. Consequently, the vaccine virus triggers immunity by multiplying in the nose, but doesn’t infect the lungs.
From 10% to 50% of people who receive the nasal live-attenuated vaccine develop runny nose, wheezing, headache, vomiting, muscle aches, fever, sore throat, or cough shortly after receiving the vaccine, but these symptoms are usually mild and short-lived.
The most common reactions people have to flu vaccines are considerably less severe than the symptoms caused by actual flu illness.
While influenza illness results in natural immunity to the specific viral serotype causing it, this illness results in hospitalization in 2% and is fatal in 0.16% of people. Influenza vaccine results in immunity to the serotypes included in the vaccine, and multiple studies have not found a causal relationship between vaccination and death.9
‘IT CAUSES GUILLAIN-BARRÉ SYNDROME’
In the United States, 3,000 to 6,000 people per year develop Guillain-Barré syndrome, or 1 to 2 of every 100,000, which translates to 80 to 160 cases per week.10 While the exact cause of Guillain-Barré syndrome is unknown, about two-thirds of people have an acute diarrheal or respiratory illness within 3 months before the onset of symptoms. In 1976, the estimated attributable risk of influenza vaccine-related Guillain-Barré syndrome in the US adult population was 1 case per 100,000 in the 6 weeks after vaccination.11 Studies in subsequent influenza seasons have not shown similar findings.12 In fact, one study showed that the risk of developing Guillain-Barré syndrome was 15 times higher after influenza illness than after influenza vaccination.13
Since 5% to 15% of the US population develop symptomatic influenza annually,14 the decision to vaccinate with respect to the risk of Guillain-Barré syndrome should be obvious: vaccinate. The correct question to ask before influenza vaccination should be, “Have you previously developed Guillain-Barré syndrome within 6 weeks after receiving the flu vaccine?” If the answer is yes, the CDC considers this a caution, not a contraindication against receiving the influenza vaccine, since the benefit may still outweigh the risk.
‘I GOT THE FLU SHOT AND STILL GOT SICK’
The flu vaccine does not prevent illnesses caused by other viruses or bacteria that can make people sick during flu season. Influenza, the common cold, and streptococcal pharyngitis can have similar symptoms that make it difficult for patients—and, frequently, even healthcare providers—to distinguish between these illnesses with certainty.
One study suggested that influenza vaccine recipients had an increased risk of virologically confirmed noninfluenza respiratory viral infections,15 citing the phenomenon of virus interference that was described in the 1940s16 as a potential explanation. In essence, people protected against influenza by the vaccine may lack temporary nonspecific immunity against other respiratory viruses. However, these findings have not been replicated in subsequent studies.17
Viral gastroenteritis, mistakenly called “stomach flu,” is also not prevented by influenza vaccination.
‘I’M ALLERGIC TO EGGS’
The prevalence of egg allergy in US children is 0.5% to 2.5%.18 Most outgrow it by school age, but in one-third, the allergy persists into adulthood.
In general, people who can eat lightly cooked eggs (eg, scrambled eggs) without a reaction are unlikely to be allergic. On the other hand, the fact that egg-allergic people may tolerate egg included in baked products does not exclude the possibility of egg allergy. Egg allergy can be confirmed by a consistent medical history of adverse reaction to eggs and egg-containing foods, in addition to skin or blood testing for immunoglobulin E directed against egg proteins.19
Most currently available influenza vaccines are prepared by propagation of virus in embryonated eggs and so may contain trace amounts of egg proteins such as ovalbumin, with the exception of the inactivated quadrivalent recombinant influenza vaccine (Flublok) and the inactivated quadrivalent cell culture-based vaccine (Flucelvax).
The ACIP recommends that persons with a history of urticaria (hives) after exposure to eggs should receive any licensed, recommended influenza vaccine that is otherwise appropriate for their age and health status. Persons who report having angioedema, respiratory distress, lightheadedness, or recurrent vomiting, or who required epinephrine or another emergency medical intervention after exposure to eggs, should receive the influenza vaccine in an inpatient or outpatient medical setting under the supervision of a healthcare provider who is able to recognize and manage severe allergic reactions.
A history of severe allergic reaction such as anaphylaxis to a previous dose of any influenza vaccine, regardless of the vaccine component (including eggs) suspected of being responsible for the reaction, is a contraindication to influenza vaccination. The ACIP recommends that vaccine providers consider observing patients for 15 minutes after administration of any vaccine (regardless of history of egg allergy) to decrease the risk of injury should syncope occur.20
‘I DON’T WANT TO PUT POISONOUS MERCURY IN MY BODY’
A process of biomagnification of methylmercury occurs when humans eat large fish that have eaten smaller fish. Thus, larger fish such as shark can be hazardous for women who are or may become pregnant, for nursing mothers, and for young children, while smaller fish such as herring are relatively safe.
As a precautionary measure, thimerosal was taken out of childhood vaccines in the United States in 2001. Thimerosal-free influenza vaccine formulations include the nasal live-attenuated flu vaccine, the inactivated quadrivalent recombinant influenza vaccine, and the inactivated quadrivalent cell culture-based vaccine.
‘I DON’T LIKE NEEDLES’
At least 10% of US adults have aichmophobia, the fear of sharp objects including needles.22 Vasovagal syncope is the most common manifestation. Behavioral therapy, topical anesthetics, and systemic anxiolytics have variable efficacy in treating needle phobia. For those who are absolutely averse to needles, the nasal flu vaccine is an appropriate alternative.
‘I DON’T WANT TO TAKE ANYTHING THAT CAN MESS WITH MY OTHER MEDICATIONS’
Some immunosuppressive medications may decrease influenza vaccine immunogenicity. Concomitant administration of the inactivated influenza vaccine with other vaccines is safe and does not alter immunogenicity of other vaccines.1 The live-attenuated influenza vaccine is contraindicated in children and adolescents taking aspirin or other salicylates due to the risk of Reye syndrome.
‘I’M AFRAID IT WILL TRIGGER AN IMMUNE RESPONSE THAT WILL MAKE MY ASTHMA WORSE’
A recent systematic review and meta-analysis showed that the inactivated influenza vaccine is not associated with asthma exacerbation.23 However, the nasal live-attenuated influenza vaccine is contraindicated in children 2 to 4 years old who have asthma and should be used with caution in persons with asthma 5 years old and older. In the systematic review, influenza vaccine prevented 59% to 78% of asthma attacks leading to emergency visits or hospitalization.23 In other immune-mediated diseases such as rheumatoid arthritis, influenza vaccine does not precipitate exacerbations.24
‘I HAD AN ORGAN TRANSPLANT, AND I’M AFRAID THE FLU SHOT WILL CAUSE ORGAN REJECTION’
A study of 51,730 kidney transplant recipients found that receipt of the inactivated influenza vaccine in the first year after transplant was associated with a lower risk of subsequent allograft loss (adjusted hazard ratio 0.77; 95% confidence interval 0.69–0.85; P < .001) and death (adjusted hazard ratio 0.82; 95% confidence interval 0.76–0.89; P < .001).25 In the same study, although acute rejection in the first year was not associated with influenza vaccination, influenza infection in the first year was associated with rejection (odds ratio 1.58; 95% confidence interval 1.10–2.26; P < 0.001), but not with graft loss or death. Solid organ transplant recipients should receive the inactivated influenza vaccine starting 3 months after transplant.26
Influenza vaccination has not been shown to precipitate graft-vs-host disease in hematopoietic stem cell transplant recipients. These patients should also receive the inactivated influenza vaccine starting 3 to 6 months after transplant.27
The nasal live-attenuated influenza vaccine is contraindicated in these immunocompromised patients.
‘I’M PREGNANT, AND I DON’T WANT TO EXPOSE MY UNBORN BABY TO ANYTHING POTENTIALLY HARMFUL’
The morbidity and mortality risk from influenza is high in children under 2 years old because of low immunogenicity to flu vaccine. This is particularly true in children younger than 6 months, but the vaccine is not recommended in this population. The best way to protect infants is for all household members to be vaccinated against the flu.
Equally important, morbidity and mortality risk from influenza is much higher in pregnant women than in the general population. Many studies have shown the value of influenza vaccination during pregnancy for both mothers and their infants. A recently published study showed that 18% of infants who developed influenza required hospitalization.28 In that study, prenatal and postpartum maternal influenza vaccination decreased the odds of influenza in infants by 61% and 53%, respectively. Another study showed that vaccine effectiveness did not vary by gestational age at vaccination.29 A post hoc analysis of an influenza vaccination study in pregnant women suggested that the vaccine was also associated with decreased rates of pertussis in these women.30
Healthcare providers should try to understand the public’s misconceptions31 about seasonal influenza and influenza vaccines in order to best address them.
- Centers for Disease Control and Prevention (CDC). Flu vaccination coverage, United States, 2018–19 influenza season. www.cdc.gov/flu/fluvaxview/coverage-1819estimates.htm. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). Immunogenicity, efficacy, and effectiveness of influenza vaccines. www.cdc.gov/flu/professionals/acip/immunogenicity.htm. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). What are the benefits of flu vaccination? www.cdc.gov/flu/prevent/vaccine-benefits.htm. Accessed November 13, 2019.
- Grohskopf LA, Alyanak E, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2019–20 influenza season. MMWR Recomm Rep 2019; 68(3):1–21. doi:10.15585/mmwr.rr6803a1
- Flannery B, Chung JR, Belongia EA, et al. Interim estimates of 2017–18 seasonal influenza vaccine effectiveness—United States, February 2018. MMWR Morb Mortal Wkly Rep 2018; 67(6):180–185. doi:10.15585/mmwr.mm6706a2
- Erbelding EJ, Post DJ, Stemmy EJ, et al. A universal influenza vaccine: the strategic plan for the National Institute of Allergy and Infectious Diseases. J Infect Dis 2018; 218(3):347–354. doi:10.1093/infdis/jiy103
- Centers for Disease Control and Prevention (CDC). Seasonal influenza vaccine safety: a summary for clinicians. www.cdc.gov/flu/professionals/vaccination/vaccine_safety.htm. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). About the Vaccine Adverse Event Reporting System (VAERS). https://wonder.cdc.gov/vaers.html. Accessed November 13, 2019.
- Miller ER, Moro PL, Cano M, Shimabukuro TT. Deaths following vaccination: what does the evidence show? Vaccine 2015; 33(29):3288–3292. doi:10.1016/j.vaccine.2015.05.023
- Centers for Disease Control and Prevention (CDC). Guillain-Barré syndrome and flu vaccine. www.cdc.gov/flu/prevent/guillainbarre.htm. Accessed November 13, 2019.
- Schonberger LB, Bregman DJ, Sullivan-Bolyai JZ, et al. Guillain-Barre syndrome following vaccination in the national influenza immunization program, United States, 1976–1977. Am J Epidemiol 1979; 110(2):105–123. doi:10.1093/oxfordjournals.aje.a112795
- Baxter R, Bakshi N, Fireman B, et al. Lack of association of Guillain-Barré syndrome with vaccinations. Clin Infect Dis 2013; 57(2):197–204. doi:10.1093/cid/cit222
- Kwong JC, Vasa PP, Campitelli MA, et al. Risk of Guillain-Barré syndrome after seasonal influenza vaccination and influenza health-care encounters: a self-controlled study. Lancet Infect Dis 2013; 13(9):769–776. doi:10.1016/S1473-3099(13)70104-X
- Centers for Disease Control and Prevention (CDC). Disease burden of influenza. www.cdc.gov/flu/about/burden/index.html. Accessed November 13, 2019.
- Cowling BJ, Fang VJ, Nishiura H, et al. Increased risk of noninfluenza respiratory virus infections associated with receipt of inactivated influenza vaccine. Clin Infect Dis 2012; 54(12):1778–1783. doi:10.1093/cid/cis307
- Henle W, Henle G. Interference of inactive virus with the propagation of virus of influenza. Science 1943; 98(2534):87–89. doi:10.1126/science.98.2534.87
- Sundaram ME, McClure DL, VanWormer JJ, Friedrich TC, Meece JK, Belongia EA. Influenza vaccination is not associated with detection of noninfluenza respiratory viruses in seasonal studies of influenza vaccine effectiveness. Clin Infect Dis 2013; 57(6):789–793. doi:10.1093/cid/cit379
- Caubet JC, Wang J. Current understanding of egg allergy. Pediatr Clin North Am 2011; 58(2):427–443. doi:10.1016/j.pcl.2011.02.014
- Erlewyn-Lajeunesse M, Brathwaite N, Lucas JS, Warner JO. Recommendations for the administration of influenza vaccine in children allergic to egg. BMJ 2009; 339:b3680. doi:10.1136/bmj.b3680
- Ezeanolue E, Harriman K, Hunter P, Kroger A, Pellegrini C. General Best Practice Guidelines for Immunization. Best Practices Guidance of the Advisory Committee on Immunization Practices (ACIP). https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/downloads/general-recs.pdf. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). Thimerosal in vaccines. www.cdc.gov/vaccinesafety/concerns/thimerosal/index.html. Accessed November 13, 2019.
- Hamilton JG. Needle phobia: a neglected diagnosis. J Fam Pract 1995; 41(2):169–175. pmid:7636457
- Vasileiou E, Sheikh A, Butler C, et al. Effectiveness of influenza vaccines in asthma: a systematic review and meta-analysis. Clin Infect Dis 2017; 65(8):1388–1395. doi:10.1093/cid/cix524
- Fomin I, Caspi D, Levy V, et al. Vaccination against influenza in rheumatoid arthritis: the effect of disease modifying drugs, including TNF alpha blockers. Ann Rheum Dis 2006; 65(2):191–194. doi:10.1136/ard.2005.036434
- Hurst FP, Lee JJ, Jindal RM, Agodoa LY, Abbott KC. Outcomes associated with influenza vaccination in the first year after kidney transplantation. Clin J Am Soc Nephrol 2011; 6(5):1192–1197. doi:10.2215/CJN.05430610
- Chong PP, Handler L, Weber DJ. A systematic review of safety and immunogenicity of influenza vaccination strategies in solid organ transplant recipients. Clin Infect Dis 2018; 66(11):1802–1811. doi:10.1093/cid/cix1081
- Ljungman P, Avetisyan G. Influenza vaccination in hematopoietic SCT recipients. Bone Marrow Transplant 2008; 42(10):637–641. doi:10.1038/bmt.2008.264
- Ohfuji S, Deguchi M, Tachibana D, et al; Osaka Pregnant Women Influenza Study Group. Protective effect of maternal influenza vaccination on influenza in their infants: a prospective cohort study. J Infect Dis 2018; 217(6):878–886. doi:10.1093/infdis/jix629
- Katz J, Englund JA, Steinhoff MC, et al. Impact of timing of influenza vaccination in pregnancy on transplacental antibody transfer, influenza incidence, and birth outcomes: a randomized trial in rural Nepal. Clin Infect Dis 2018; 67(3):334–340. doi:10.1093/cid/ciy090
- Nunes MC, Cutland CL, Madhi SA. Influenza vaccination during pregnancy and protection against pertussis. N Engl J Med 2018; 378(13):1257–1258. doi:10.1056/NEJMc1705208
- Centers for Disease Control and Prevention (CDC). Misconceptions about seasonal flu and flu vaccines. www.cdc.gov/flu/prevent/misconceptions.htm. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). Flu vaccination coverage, United States, 2018–19 influenza season. www.cdc.gov/flu/fluvaxview/coverage-1819estimates.htm. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). Immunogenicity, efficacy, and effectiveness of influenza vaccines. www.cdc.gov/flu/professionals/acip/immunogenicity.htm. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). What are the benefits of flu vaccination? www.cdc.gov/flu/prevent/vaccine-benefits.htm. Accessed November 13, 2019.
- Grohskopf LA, Alyanak E, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2019–20 influenza season. MMWR Recomm Rep 2019; 68(3):1–21. doi:10.15585/mmwr.rr6803a1
- Flannery B, Chung JR, Belongia EA, et al. Interim estimates of 2017–18 seasonal influenza vaccine effectiveness—United States, February 2018. MMWR Morb Mortal Wkly Rep 2018; 67(6):180–185. doi:10.15585/mmwr.mm6706a2
- Erbelding EJ, Post DJ, Stemmy EJ, et al. A universal influenza vaccine: the strategic plan for the National Institute of Allergy and Infectious Diseases. J Infect Dis 2018; 218(3):347–354. doi:10.1093/infdis/jiy103
- Centers for Disease Control and Prevention (CDC). Seasonal influenza vaccine safety: a summary for clinicians. www.cdc.gov/flu/professionals/vaccination/vaccine_safety.htm. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). About the Vaccine Adverse Event Reporting System (VAERS). https://wonder.cdc.gov/vaers.html. Accessed November 13, 2019.
- Miller ER, Moro PL, Cano M, Shimabukuro TT. Deaths following vaccination: what does the evidence show? Vaccine 2015; 33(29):3288–3292. doi:10.1016/j.vaccine.2015.05.023
- Centers for Disease Control and Prevention (CDC). Guillain-Barré syndrome and flu vaccine. www.cdc.gov/flu/prevent/guillainbarre.htm. Accessed November 13, 2019.
- Schonberger LB, Bregman DJ, Sullivan-Bolyai JZ, et al. Guillain-Barre syndrome following vaccination in the national influenza immunization program, United States, 1976–1977. Am J Epidemiol 1979; 110(2):105–123. doi:10.1093/oxfordjournals.aje.a112795
- Baxter R, Bakshi N, Fireman B, et al. Lack of association of Guillain-Barré syndrome with vaccinations. Clin Infect Dis 2013; 57(2):197–204. doi:10.1093/cid/cit222
- Kwong JC, Vasa PP, Campitelli MA, et al. Risk of Guillain-Barré syndrome after seasonal influenza vaccination and influenza health-care encounters: a self-controlled study. Lancet Infect Dis 2013; 13(9):769–776. doi:10.1016/S1473-3099(13)70104-X
- Centers for Disease Control and Prevention (CDC). Disease burden of influenza. www.cdc.gov/flu/about/burden/index.html. Accessed November 13, 2019.
- Cowling BJ, Fang VJ, Nishiura H, et al. Increased risk of noninfluenza respiratory virus infections associated with receipt of inactivated influenza vaccine. Clin Infect Dis 2012; 54(12):1778–1783. doi:10.1093/cid/cis307
- Henle W, Henle G. Interference of inactive virus with the propagation of virus of influenza. Science 1943; 98(2534):87–89. doi:10.1126/science.98.2534.87
- Sundaram ME, McClure DL, VanWormer JJ, Friedrich TC, Meece JK, Belongia EA. Influenza vaccination is not associated with detection of noninfluenza respiratory viruses in seasonal studies of influenza vaccine effectiveness. Clin Infect Dis 2013; 57(6):789–793. doi:10.1093/cid/cit379
- Caubet JC, Wang J. Current understanding of egg allergy. Pediatr Clin North Am 2011; 58(2):427–443. doi:10.1016/j.pcl.2011.02.014
- Erlewyn-Lajeunesse M, Brathwaite N, Lucas JS, Warner JO. Recommendations for the administration of influenza vaccine in children allergic to egg. BMJ 2009; 339:b3680. doi:10.1136/bmj.b3680
- Ezeanolue E, Harriman K, Hunter P, Kroger A, Pellegrini C. General Best Practice Guidelines for Immunization. Best Practices Guidance of the Advisory Committee on Immunization Practices (ACIP). https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/downloads/general-recs.pdf. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). Thimerosal in vaccines. www.cdc.gov/vaccinesafety/concerns/thimerosal/index.html. Accessed November 13, 2019.
- Hamilton JG. Needle phobia: a neglected diagnosis. J Fam Pract 1995; 41(2):169–175. pmid:7636457
- Vasileiou E, Sheikh A, Butler C, et al. Effectiveness of influenza vaccines in asthma: a systematic review and meta-analysis. Clin Infect Dis 2017; 65(8):1388–1395. doi:10.1093/cid/cix524
- Fomin I, Caspi D, Levy V, et al. Vaccination against influenza in rheumatoid arthritis: the effect of disease modifying drugs, including TNF alpha blockers. Ann Rheum Dis 2006; 65(2):191–194. doi:10.1136/ard.2005.036434
- Hurst FP, Lee JJ, Jindal RM, Agodoa LY, Abbott KC. Outcomes associated with influenza vaccination in the first year after kidney transplantation. Clin J Am Soc Nephrol 2011; 6(5):1192–1197. doi:10.2215/CJN.05430610
- Chong PP, Handler L, Weber DJ. A systematic review of safety and immunogenicity of influenza vaccination strategies in solid organ transplant recipients. Clin Infect Dis 2018; 66(11):1802–1811. doi:10.1093/cid/cix1081
- Ljungman P, Avetisyan G. Influenza vaccination in hematopoietic SCT recipients. Bone Marrow Transplant 2008; 42(10):637–641. doi:10.1038/bmt.2008.264
- Ohfuji S, Deguchi M, Tachibana D, et al; Osaka Pregnant Women Influenza Study Group. Protective effect of maternal influenza vaccination on influenza in their infants: a prospective cohort study. J Infect Dis 2018; 217(6):878–886. doi:10.1093/infdis/jix629
- Katz J, Englund JA, Steinhoff MC, et al. Impact of timing of influenza vaccination in pregnancy on transplacental antibody transfer, influenza incidence, and birth outcomes: a randomized trial in rural Nepal. Clin Infect Dis 2018; 67(3):334–340. doi:10.1093/cid/ciy090
- Nunes MC, Cutland CL, Madhi SA. Influenza vaccination during pregnancy and protection against pertussis. N Engl J Med 2018; 378(13):1257–1258. doi:10.1056/NEJMc1705208
- Centers for Disease Control and Prevention (CDC). Misconceptions about seasonal flu and flu vaccines. www.cdc.gov/flu/prevent/misconceptions.htm. Accessed November 13, 2019.
Two national analyses confirm safety of 9vHPV vaccine
most of which cannot be definitively tied to the vaccine, according to two large studies published simultaneously in Pediatrics.
“The body of evidence on the safety of 9vHPV now includes prelicensure clinical trial data on 15,000 study subjects, reassuring results from postlicensure near real-time sequential monitoring by the Centers for Disease Control and Prevention’s Vaccine Safety Datalink, on approximately 839 000 doses administered, and our review of VAERS [Vaccine Adverse Event Reporting System] reports over a 3-year period, during which time approximately 28 million doses were distributed in the United States,” Tom T. Shimabukuro, MD, and colleagues reported in Pediatrics.
James G. Donahue, PhD, and colleagues, authors of the Vaccine Safety Datalink study published in the same issue, concluded much the same thing.
The new numbers bolster extant safety data on the vaccine, which was approved in 2015, wrote Dr. Donahue, an epidemiologist at the Marshfield (Wis.) Clinic Research Institute, and coauthors. “With this large observational study, we contribute reassuring postlicensure data that will help bolster the safety profile of 9vHPV. Although we detected several unexpected potential safety signals, none were confirmed after further evaluation.”
The Vaccine Safety Datalink study of 838,991 doses looked for safety signals in a prespecified group of potential events, including anaphylaxis, appendicitis, Guillain-Barré syndrome, chronic inflammatory demyelinating polyneuropathy, pancreatitis, seizures, stroke, and venous thromboembolism.
Dr. Donahue and coauthors used real-time vaccination data and time-matched historical controls to evaluate any changes in expected disease rates, compared with those occurring in vaccine recipients.
Most doses in the study (76%) were given to children aged 9-17 years, with 48% going to girls. The remaining 24% of doses were given to persons aged 18-26 years, with 64% going to women.
The analysis found potential safety signals in allergic reactions (43 cases), appendicitis (30 cases), pancreatitis (8 cases), and syncope (67). None of these were confirmed after further investigation.
“The safety profile of 9vHPV is favorable and comparable to that of its predecessor, 4vHPV,” Dr. Donahue and associates concluded.
The VAERS analysis was similarly reassuring. It examined all reported adverse events, not predetermined events.
Among 28 million doses, there were 7,244 adverse event reports – a rate of about 1 event per 7 million doses. Of these, 97% were nonserious, wrote Dr. Shimabukuro, deputy director of the CDC’s Immunization Safety Office, and colleagues.
The vaccine manufacturer submitted 64% of these to VAERS; health care providers submitted 27%. Adverse events were reported from postvaccine day 0 to 2 years afterward. 9vHPV was the only vaccine given in 75% of reports. Coadministered vaccines included meningococcal conjugate (1,028); tetanus and diphtheria (Td) or Tdap (673); and hepatitis A (434).
There were nine reports of anaphylaxis (five males, four females); 9vHPV was the only vaccine administered in five cases. Three reports involved coadministration of meningococcal vaccine, two with hepatitis A, one with TDaP, and one with varicella.
There were eight reports of Guillain-Barré.
There were 17 reports of postural orthostatic tachycardia syndrome, most of which (71%) did not meet diagnostic criteria. Five cases, however, did.
One possible case of complex regional pain syndrome was reported in a 13-year-old girl with comorbid anxiety.
There were two reports of acute disseminated encephalomyelitis, both in boys. There were no reports of transverse myelitis or chronic inflammatory demyelinating polyneuropathy.
Seven vaccine recipients died after vaccination. Five of these reports did not contain medical information or any proof-of-death confirmation. The other two were verified by autopsy. A 14-year-old girl who received a flu vaccination with 9vHPV died of a thoracic aorta dissection 7 days postvaccination. The other death was a 16-year-old boy who received a concurrent hepatitis A vaccine. Four days later, he died of a cerebellar hemorrhage.
“We did not identify any unusual or unexpected safety concerns in our review of 9vHPV reports to the VAERS; most (97%) reports were nonserious, and adverse events were analogous to those observed in the prelicensure clinical trials,” Dr. Shimabukuro and associates concluded.
Neither Dr. Shimabukuro nor Dr. Donahue had financial disclosures. Dr. Donahue’s study was funded by the Centers for Disease Control and Prevention. One coauthor had ties to several pharmaceutical companies. Dr. Shimabukuro’s study had no external funding. One coauthor is employed by Merck, but was not at the time of the study.
SOURCES: Shimabukuro T et al. Pediatrics. 2019 Nov 1. doi: 10.1542/peds.2019-1791; Donahue J et al. Pediatrics. 2019 Nov 1. doi: 10.1542/peds.2019-1808.
most of which cannot be definitively tied to the vaccine, according to two large studies published simultaneously in Pediatrics.
“The body of evidence on the safety of 9vHPV now includes prelicensure clinical trial data on 15,000 study subjects, reassuring results from postlicensure near real-time sequential monitoring by the Centers for Disease Control and Prevention’s Vaccine Safety Datalink, on approximately 839 000 doses administered, and our review of VAERS [Vaccine Adverse Event Reporting System] reports over a 3-year period, during which time approximately 28 million doses were distributed in the United States,” Tom T. Shimabukuro, MD, and colleagues reported in Pediatrics.
James G. Donahue, PhD, and colleagues, authors of the Vaccine Safety Datalink study published in the same issue, concluded much the same thing.
The new numbers bolster extant safety data on the vaccine, which was approved in 2015, wrote Dr. Donahue, an epidemiologist at the Marshfield (Wis.) Clinic Research Institute, and coauthors. “With this large observational study, we contribute reassuring postlicensure data that will help bolster the safety profile of 9vHPV. Although we detected several unexpected potential safety signals, none were confirmed after further evaluation.”
The Vaccine Safety Datalink study of 838,991 doses looked for safety signals in a prespecified group of potential events, including anaphylaxis, appendicitis, Guillain-Barré syndrome, chronic inflammatory demyelinating polyneuropathy, pancreatitis, seizures, stroke, and venous thromboembolism.
Dr. Donahue and coauthors used real-time vaccination data and time-matched historical controls to evaluate any changes in expected disease rates, compared with those occurring in vaccine recipients.
Most doses in the study (76%) were given to children aged 9-17 years, with 48% going to girls. The remaining 24% of doses were given to persons aged 18-26 years, with 64% going to women.
The analysis found potential safety signals in allergic reactions (43 cases), appendicitis (30 cases), pancreatitis (8 cases), and syncope (67). None of these were confirmed after further investigation.
“The safety profile of 9vHPV is favorable and comparable to that of its predecessor, 4vHPV,” Dr. Donahue and associates concluded.
The VAERS analysis was similarly reassuring. It examined all reported adverse events, not predetermined events.
Among 28 million doses, there were 7,244 adverse event reports – a rate of about 1 event per 7 million doses. Of these, 97% were nonserious, wrote Dr. Shimabukuro, deputy director of the CDC’s Immunization Safety Office, and colleagues.
The vaccine manufacturer submitted 64% of these to VAERS; health care providers submitted 27%. Adverse events were reported from postvaccine day 0 to 2 years afterward. 9vHPV was the only vaccine given in 75% of reports. Coadministered vaccines included meningococcal conjugate (1,028); tetanus and diphtheria (Td) or Tdap (673); and hepatitis A (434).
There were nine reports of anaphylaxis (five males, four females); 9vHPV was the only vaccine administered in five cases. Three reports involved coadministration of meningococcal vaccine, two with hepatitis A, one with TDaP, and one with varicella.
There were eight reports of Guillain-Barré.
There were 17 reports of postural orthostatic tachycardia syndrome, most of which (71%) did not meet diagnostic criteria. Five cases, however, did.
One possible case of complex regional pain syndrome was reported in a 13-year-old girl with comorbid anxiety.
There were two reports of acute disseminated encephalomyelitis, both in boys. There were no reports of transverse myelitis or chronic inflammatory demyelinating polyneuropathy.
Seven vaccine recipients died after vaccination. Five of these reports did not contain medical information or any proof-of-death confirmation. The other two were verified by autopsy. A 14-year-old girl who received a flu vaccination with 9vHPV died of a thoracic aorta dissection 7 days postvaccination. The other death was a 16-year-old boy who received a concurrent hepatitis A vaccine. Four days later, he died of a cerebellar hemorrhage.
“We did not identify any unusual or unexpected safety concerns in our review of 9vHPV reports to the VAERS; most (97%) reports were nonserious, and adverse events were analogous to those observed in the prelicensure clinical trials,” Dr. Shimabukuro and associates concluded.
Neither Dr. Shimabukuro nor Dr. Donahue had financial disclosures. Dr. Donahue’s study was funded by the Centers for Disease Control and Prevention. One coauthor had ties to several pharmaceutical companies. Dr. Shimabukuro’s study had no external funding. One coauthor is employed by Merck, but was not at the time of the study.
SOURCES: Shimabukuro T et al. Pediatrics. 2019 Nov 1. doi: 10.1542/peds.2019-1791; Donahue J et al. Pediatrics. 2019 Nov 1. doi: 10.1542/peds.2019-1808.
most of which cannot be definitively tied to the vaccine, according to two large studies published simultaneously in Pediatrics.
“The body of evidence on the safety of 9vHPV now includes prelicensure clinical trial data on 15,000 study subjects, reassuring results from postlicensure near real-time sequential monitoring by the Centers for Disease Control and Prevention’s Vaccine Safety Datalink, on approximately 839 000 doses administered, and our review of VAERS [Vaccine Adverse Event Reporting System] reports over a 3-year period, during which time approximately 28 million doses were distributed in the United States,” Tom T. Shimabukuro, MD, and colleagues reported in Pediatrics.
James G. Donahue, PhD, and colleagues, authors of the Vaccine Safety Datalink study published in the same issue, concluded much the same thing.
The new numbers bolster extant safety data on the vaccine, which was approved in 2015, wrote Dr. Donahue, an epidemiologist at the Marshfield (Wis.) Clinic Research Institute, and coauthors. “With this large observational study, we contribute reassuring postlicensure data that will help bolster the safety profile of 9vHPV. Although we detected several unexpected potential safety signals, none were confirmed after further evaluation.”
The Vaccine Safety Datalink study of 838,991 doses looked for safety signals in a prespecified group of potential events, including anaphylaxis, appendicitis, Guillain-Barré syndrome, chronic inflammatory demyelinating polyneuropathy, pancreatitis, seizures, stroke, and venous thromboembolism.
Dr. Donahue and coauthors used real-time vaccination data and time-matched historical controls to evaluate any changes in expected disease rates, compared with those occurring in vaccine recipients.
Most doses in the study (76%) were given to children aged 9-17 years, with 48% going to girls. The remaining 24% of doses were given to persons aged 18-26 years, with 64% going to women.
The analysis found potential safety signals in allergic reactions (43 cases), appendicitis (30 cases), pancreatitis (8 cases), and syncope (67). None of these were confirmed after further investigation.
“The safety profile of 9vHPV is favorable and comparable to that of its predecessor, 4vHPV,” Dr. Donahue and associates concluded.
The VAERS analysis was similarly reassuring. It examined all reported adverse events, not predetermined events.
Among 28 million doses, there were 7,244 adverse event reports – a rate of about 1 event per 7 million doses. Of these, 97% were nonserious, wrote Dr. Shimabukuro, deputy director of the CDC’s Immunization Safety Office, and colleagues.
The vaccine manufacturer submitted 64% of these to VAERS; health care providers submitted 27%. Adverse events were reported from postvaccine day 0 to 2 years afterward. 9vHPV was the only vaccine given in 75% of reports. Coadministered vaccines included meningococcal conjugate (1,028); tetanus and diphtheria (Td) or Tdap (673); and hepatitis A (434).
There were nine reports of anaphylaxis (five males, four females); 9vHPV was the only vaccine administered in five cases. Three reports involved coadministration of meningococcal vaccine, two with hepatitis A, one with TDaP, and one with varicella.
There were eight reports of Guillain-Barré.
There were 17 reports of postural orthostatic tachycardia syndrome, most of which (71%) did not meet diagnostic criteria. Five cases, however, did.
One possible case of complex regional pain syndrome was reported in a 13-year-old girl with comorbid anxiety.
There were two reports of acute disseminated encephalomyelitis, both in boys. There were no reports of transverse myelitis or chronic inflammatory demyelinating polyneuropathy.
Seven vaccine recipients died after vaccination. Five of these reports did not contain medical information or any proof-of-death confirmation. The other two were verified by autopsy. A 14-year-old girl who received a flu vaccination with 9vHPV died of a thoracic aorta dissection 7 days postvaccination. The other death was a 16-year-old boy who received a concurrent hepatitis A vaccine. Four days later, he died of a cerebellar hemorrhage.
“We did not identify any unusual or unexpected safety concerns in our review of 9vHPV reports to the VAERS; most (97%) reports were nonserious, and adverse events were analogous to those observed in the prelicensure clinical trials,” Dr. Shimabukuro and associates concluded.
Neither Dr. Shimabukuro nor Dr. Donahue had financial disclosures. Dr. Donahue’s study was funded by the Centers for Disease Control and Prevention. One coauthor had ties to several pharmaceutical companies. Dr. Shimabukuro’s study had no external funding. One coauthor is employed by Merck, but was not at the time of the study.
SOURCES: Shimabukuro T et al. Pediatrics. 2019 Nov 1. doi: 10.1542/peds.2019-1791; Donahue J et al. Pediatrics. 2019 Nov 1. doi: 10.1542/peds.2019-1808.
FROM PEDIATRICS
Key clinical point: Postlicensure studies confirm the safety of the 9vHPV vaccine.
Major finding: The adverse event rate is 1 in 7 million doses. Most of these events were not definitively tied to the vaccine.
Study details: The two studies covered all doses given in the United States since vaccine approval in 2015.
Disclosures: Neither Dr. Shimabukuro nor Dr. Donahue had financial disclosures. Dr. Donahue’s study was funded by the Centers for Disease Control and Prevention. One coauthor on his study had ties to several pharmaceutical companies. Dr. Shimabukuro’s study had no external funding. One coauthor is employed by Merck, but was not at the time of the study.
Sources: Shimabukuro T et al. Pediatrics. 2019 Nov 1. doi: 10.1542/peds.2019-1791; Donahue J et al. Pediatrics. 2019 Nov 1. doi: 10.1542/peds.2019-1808.
Newborns’ maternal protection against measles wanes within 6 months
according to new research.
In fact, most of the 196 infants’ maternal measles antibodies had dropped below the protective threshold by 3 months of age – well before the recommended age of 12-15 months for the first dose of MMR vaccine.
The odds of inadequate protection doubled for each additional month of age, Michelle Science, MD, of the University of Toronto and associates reported in Pediatrics.
“The widening gap between loss of maternal antibodies and measles vaccination described in our study leaves infants vulnerable to measles for much of their infancy and highlights the need for further research to support public health policy,” Dr. Science and colleagues wrote.
The findings are not surprising for a setting in which measles has been eliminated and align with results from past research, Huong Q. McLean, PhD, MPH, of the Marshfield (Wis.) Clinic Research Institute and Walter A. Orenstein, MD, of Emory University in Atlanta wrote in an accompanying editorial (Pediatrics. 2019 Nov 21. doi: 10.1542/peds.2019-2541).
However, this susceptibility prior to receiving the MMR has taken on a new significance more recently, Dr. McLean and Dr. Orenstein suggested.
“In light of increasing measles outbreaks during the past year reaching levels not recorded in the United States since 1992 and increased measles elsewhere, coupled with the risk of severe illness in infants, there is increased concern regarding the protection of infants against measles,” the editorialists wrote.
Dr. Science and colleagues tested serum samples from 196 term infants, all under 12 months old, for antibodies against measles. The sera had been previously collected at a single tertiary care center in Ontario for clinical testing and then stored. Measles has been eliminated in Canada since 1998.
The researchers randomly selected 25 samples for each of eight different age groups: up to 30 days old; 1 month (31-60 days); 2 months (61-89 days); 3 months (90-119 days); 4 months; 5 months; 6-9 months; and 9-11 months.
Just over half the babies (56%) were male, and 35% had an underlying condition, but none had conditions that might affect antibody levels. The conditions were primarily a developmental delay or otherwise affecting the central nervous system, liver, or gastrointestinal function. Mean maternal age was 32 years.
To ensure high test sensitivity, the researchers used the plaque-reduction neutralization test (PRNT) to test for measles-neutralizing antibodies instead of using enzyme-linked immunosorbent assay (ELISA) because “ELISA sensitivity decreases as antibody titers decrease,” Dr. Science and colleagues wrote. They used a neutralization titer of less than 192 mIU/mL as the threshold for protection against measles.
When the researchers calculated the predicted standardized mean antibody titer for infants with a mother aged 32 years, they determined their mean to be 541 mIU/mL at 1 month, 142 mIU/mL at 3 months (below the measles threshold of susceptibility of 192 mIU/mL) , and 64 mIU/mL at 6 months. None of the infants had measles antibodies above the protective threshold at 6 months old, the authors noted.
Children’s odds of susceptibility to measles doubled for each additional month of age, after adjustment for infant sex and maternal age (odds ratio, 2.13). Children’s likelihood of susceptibility to measles modestly increased as maternal age increased in 5-year increments from 25 to 40 years.
Children with an underlying conditions had greater susceptibility to measles (83%), compared with those without a comorbidity (68%, P = .03). No difference in susceptibility existed between males and females or based on gestational age at birth (ranging from 37 to 41 weeks).
The Advisory Committee on Immunization Practices permits measles vaccination “as early as 6 months for infants who plan to travel internationally, infants with ongoing risk for exposure during measles outbreaks and as postexposure prophylaxis,” Dr. McLean and Dr. Orenstein noted in their editorial.
They discussed the rationale for various changes in the recommended schedule for measles immunization, based on changes in epidemiology of the disease and improved understanding of the immune response to vaccination since the vaccine became available in 1963. Then they posed the question of whether the recommendation should be revised again.
“Ideally, the schedule should minimize the risk of measles and its complications and optimize vaccine-induced protection,” Dr. McLean and Dr. Orenstein wrote.
They argued that the evidence cannot currently support changing the first MMR dose to a younger age because measles incidence in the United States remains extremely low outside of the extraordinary outbreaks in 2014 and 2019. Further, infants under 12 months of age make up less than 15% of measles cases during outbreaks, and unvaccinated people make up more than 70% of cases.
Rather, they stated, this new study emphasizes the importance of following the current schedule, with consideration of an earlier schedule only warranted during outbreaks.
“Health care providers must work to maintain high levels of coverage with 2 doses of MMR among vaccine-eligible populations and minimize pockets of susceptibility to prevent transmission to infants and prevent reestablishment of endemic transmission,” they concluded.
The research was funded by the Public Health Ontario Project Initiation Fund. The authors had no relevant financial disclosures. The editorialists had no external funding and no relevant financial disclosures.
SOURCE: Science M et al. Pediatrics. 2019 Nov 21. doi: 10.1542/peds.2019-0630.
according to new research.
In fact, most of the 196 infants’ maternal measles antibodies had dropped below the protective threshold by 3 months of age – well before the recommended age of 12-15 months for the first dose of MMR vaccine.
The odds of inadequate protection doubled for each additional month of age, Michelle Science, MD, of the University of Toronto and associates reported in Pediatrics.
“The widening gap between loss of maternal antibodies and measles vaccination described in our study leaves infants vulnerable to measles for much of their infancy and highlights the need for further research to support public health policy,” Dr. Science and colleagues wrote.
The findings are not surprising for a setting in which measles has been eliminated and align with results from past research, Huong Q. McLean, PhD, MPH, of the Marshfield (Wis.) Clinic Research Institute and Walter A. Orenstein, MD, of Emory University in Atlanta wrote in an accompanying editorial (Pediatrics. 2019 Nov 21. doi: 10.1542/peds.2019-2541).
However, this susceptibility prior to receiving the MMR has taken on a new significance more recently, Dr. McLean and Dr. Orenstein suggested.
“In light of increasing measles outbreaks during the past year reaching levels not recorded in the United States since 1992 and increased measles elsewhere, coupled with the risk of severe illness in infants, there is increased concern regarding the protection of infants against measles,” the editorialists wrote.
Dr. Science and colleagues tested serum samples from 196 term infants, all under 12 months old, for antibodies against measles. The sera had been previously collected at a single tertiary care center in Ontario for clinical testing and then stored. Measles has been eliminated in Canada since 1998.
The researchers randomly selected 25 samples for each of eight different age groups: up to 30 days old; 1 month (31-60 days); 2 months (61-89 days); 3 months (90-119 days); 4 months; 5 months; 6-9 months; and 9-11 months.
Just over half the babies (56%) were male, and 35% had an underlying condition, but none had conditions that might affect antibody levels. The conditions were primarily a developmental delay or otherwise affecting the central nervous system, liver, or gastrointestinal function. Mean maternal age was 32 years.
To ensure high test sensitivity, the researchers used the plaque-reduction neutralization test (PRNT) to test for measles-neutralizing antibodies instead of using enzyme-linked immunosorbent assay (ELISA) because “ELISA sensitivity decreases as antibody titers decrease,” Dr. Science and colleagues wrote. They used a neutralization titer of less than 192 mIU/mL as the threshold for protection against measles.
When the researchers calculated the predicted standardized mean antibody titer for infants with a mother aged 32 years, they determined their mean to be 541 mIU/mL at 1 month, 142 mIU/mL at 3 months (below the measles threshold of susceptibility of 192 mIU/mL) , and 64 mIU/mL at 6 months. None of the infants had measles antibodies above the protective threshold at 6 months old, the authors noted.
Children’s odds of susceptibility to measles doubled for each additional month of age, after adjustment for infant sex and maternal age (odds ratio, 2.13). Children’s likelihood of susceptibility to measles modestly increased as maternal age increased in 5-year increments from 25 to 40 years.
Children with an underlying conditions had greater susceptibility to measles (83%), compared with those without a comorbidity (68%, P = .03). No difference in susceptibility existed between males and females or based on gestational age at birth (ranging from 37 to 41 weeks).
The Advisory Committee on Immunization Practices permits measles vaccination “as early as 6 months for infants who plan to travel internationally, infants with ongoing risk for exposure during measles outbreaks and as postexposure prophylaxis,” Dr. McLean and Dr. Orenstein noted in their editorial.
They discussed the rationale for various changes in the recommended schedule for measles immunization, based on changes in epidemiology of the disease and improved understanding of the immune response to vaccination since the vaccine became available in 1963. Then they posed the question of whether the recommendation should be revised again.
“Ideally, the schedule should minimize the risk of measles and its complications and optimize vaccine-induced protection,” Dr. McLean and Dr. Orenstein wrote.
They argued that the evidence cannot currently support changing the first MMR dose to a younger age because measles incidence in the United States remains extremely low outside of the extraordinary outbreaks in 2014 and 2019. Further, infants under 12 months of age make up less than 15% of measles cases during outbreaks, and unvaccinated people make up more than 70% of cases.
Rather, they stated, this new study emphasizes the importance of following the current schedule, with consideration of an earlier schedule only warranted during outbreaks.
“Health care providers must work to maintain high levels of coverage with 2 doses of MMR among vaccine-eligible populations and minimize pockets of susceptibility to prevent transmission to infants and prevent reestablishment of endemic transmission,” they concluded.
The research was funded by the Public Health Ontario Project Initiation Fund. The authors had no relevant financial disclosures. The editorialists had no external funding and no relevant financial disclosures.
SOURCE: Science M et al. Pediatrics. 2019 Nov 21. doi: 10.1542/peds.2019-0630.
according to new research.
In fact, most of the 196 infants’ maternal measles antibodies had dropped below the protective threshold by 3 months of age – well before the recommended age of 12-15 months for the first dose of MMR vaccine.
The odds of inadequate protection doubled for each additional month of age, Michelle Science, MD, of the University of Toronto and associates reported in Pediatrics.
“The widening gap between loss of maternal antibodies and measles vaccination described in our study leaves infants vulnerable to measles for much of their infancy and highlights the need for further research to support public health policy,” Dr. Science and colleagues wrote.
The findings are not surprising for a setting in which measles has been eliminated and align with results from past research, Huong Q. McLean, PhD, MPH, of the Marshfield (Wis.) Clinic Research Institute and Walter A. Orenstein, MD, of Emory University in Atlanta wrote in an accompanying editorial (Pediatrics. 2019 Nov 21. doi: 10.1542/peds.2019-2541).
However, this susceptibility prior to receiving the MMR has taken on a new significance more recently, Dr. McLean and Dr. Orenstein suggested.
“In light of increasing measles outbreaks during the past year reaching levels not recorded in the United States since 1992 and increased measles elsewhere, coupled with the risk of severe illness in infants, there is increased concern regarding the protection of infants against measles,” the editorialists wrote.
Dr. Science and colleagues tested serum samples from 196 term infants, all under 12 months old, for antibodies against measles. The sera had been previously collected at a single tertiary care center in Ontario for clinical testing and then stored. Measles has been eliminated in Canada since 1998.
The researchers randomly selected 25 samples for each of eight different age groups: up to 30 days old; 1 month (31-60 days); 2 months (61-89 days); 3 months (90-119 days); 4 months; 5 months; 6-9 months; and 9-11 months.
Just over half the babies (56%) were male, and 35% had an underlying condition, but none had conditions that might affect antibody levels. The conditions were primarily a developmental delay or otherwise affecting the central nervous system, liver, or gastrointestinal function. Mean maternal age was 32 years.
To ensure high test sensitivity, the researchers used the plaque-reduction neutralization test (PRNT) to test for measles-neutralizing antibodies instead of using enzyme-linked immunosorbent assay (ELISA) because “ELISA sensitivity decreases as antibody titers decrease,” Dr. Science and colleagues wrote. They used a neutralization titer of less than 192 mIU/mL as the threshold for protection against measles.
When the researchers calculated the predicted standardized mean antibody titer for infants with a mother aged 32 years, they determined their mean to be 541 mIU/mL at 1 month, 142 mIU/mL at 3 months (below the measles threshold of susceptibility of 192 mIU/mL) , and 64 mIU/mL at 6 months. None of the infants had measles antibodies above the protective threshold at 6 months old, the authors noted.
Children’s odds of susceptibility to measles doubled for each additional month of age, after adjustment for infant sex and maternal age (odds ratio, 2.13). Children’s likelihood of susceptibility to measles modestly increased as maternal age increased in 5-year increments from 25 to 40 years.
Children with an underlying conditions had greater susceptibility to measles (83%), compared with those without a comorbidity (68%, P = .03). No difference in susceptibility existed between males and females or based on gestational age at birth (ranging from 37 to 41 weeks).
The Advisory Committee on Immunization Practices permits measles vaccination “as early as 6 months for infants who plan to travel internationally, infants with ongoing risk for exposure during measles outbreaks and as postexposure prophylaxis,” Dr. McLean and Dr. Orenstein noted in their editorial.
They discussed the rationale for various changes in the recommended schedule for measles immunization, based on changes in epidemiology of the disease and improved understanding of the immune response to vaccination since the vaccine became available in 1963. Then they posed the question of whether the recommendation should be revised again.
“Ideally, the schedule should minimize the risk of measles and its complications and optimize vaccine-induced protection,” Dr. McLean and Dr. Orenstein wrote.
They argued that the evidence cannot currently support changing the first MMR dose to a younger age because measles incidence in the United States remains extremely low outside of the extraordinary outbreaks in 2014 and 2019. Further, infants under 12 months of age make up less than 15% of measles cases during outbreaks, and unvaccinated people make up more than 70% of cases.
Rather, they stated, this new study emphasizes the importance of following the current schedule, with consideration of an earlier schedule only warranted during outbreaks.
“Health care providers must work to maintain high levels of coverage with 2 doses of MMR among vaccine-eligible populations and minimize pockets of susceptibility to prevent transmission to infants and prevent reestablishment of endemic transmission,” they concluded.
The research was funded by the Public Health Ontario Project Initiation Fund. The authors had no relevant financial disclosures. The editorialists had no external funding and no relevant financial disclosures.
SOURCE: Science M et al. Pediatrics. 2019 Nov 21. doi: 10.1542/peds.2019-0630.
FROM PEDIATRICS
Key clinical point: Infants’ maternal measles antibodies fell below protective levels by 6 months old.
Major finding: Infants were twice as likely not to have protective immunity against measles for each month of age after birth (odds ratio, 2.13).
Study details: The findings are based on measles antibody testing of 196 serum samples from infants born in a tertiary care center in Ontario.
Disclosures: The research was funded by the Public Health Ontario Project Initiation Fund. The authors had no relevant financial disclosures.
Source: Science M et al. Pediatrics. 2019 Nov 21. doi: 10.1542/peds.2019-0630.