User login
Medtronic recalls HawkOne directional atherectomy system
Medtronic has recalled 95,110 HawkOne Directional Atherectomy Systems because of the risk of the guidewire within the catheter moving downward or prolapsing during use, which may damage the tip of the catheter.
The U.S. Food and Drug Administration has identified this as a Class I recall, the most serious type, because of the potential for serious injury or death.
The HawkOne Directional Atherectomy system is used during procedures intended to remove blockage from peripheral arteries and improve blood flow.
If the guideline moves downward or prolapses during use, the “catheter tip may break off or separate, and this could lead to serious adverse events, including a tear along the inside wall of an artery (arterial dissection), a rupture or breakage of an artery (arterial rupture), decrease in blood flow to a part of the body because of a blocked artery (ischemia), and/or blood vessel complications that could require surgical repair and additional procedures to capture and remove the detached and/or migrated (embolized) tip,” the FDA says in a recall notice posted today on its website.
To date, there have been 55 injuries, no deaths, and 163 complaints reported for this device.
The recalled devices were distributed in the United States between Jan. 22, 2018 and Oct. 4, 2021. Product codes and lot numbers pertaining to the devices are listed on the FDA website.
Medtronic sent an urgent field safety notice to customers Dec. 6, 2021, requesting that they alert parties of the defect, review the instructions for use before using the device, and note the warnings and precautions listed in the letter.
Customers were also asked to complete the enclosed confirmation form and email to [email protected].
Health care providers can report adverse reactions or quality problems they experience using these devices to the FDA’s MedWatch program.
A version of this article first appeared on Medscape.com.
Medtronic has recalled 95,110 HawkOne Directional Atherectomy Systems because of the risk of the guidewire within the catheter moving downward or prolapsing during use, which may damage the tip of the catheter.
The U.S. Food and Drug Administration has identified this as a Class I recall, the most serious type, because of the potential for serious injury or death.
The HawkOne Directional Atherectomy system is used during procedures intended to remove blockage from peripheral arteries and improve blood flow.
If the guideline moves downward or prolapses during use, the “catheter tip may break off or separate, and this could lead to serious adverse events, including a tear along the inside wall of an artery (arterial dissection), a rupture or breakage of an artery (arterial rupture), decrease in blood flow to a part of the body because of a blocked artery (ischemia), and/or blood vessel complications that could require surgical repair and additional procedures to capture and remove the detached and/or migrated (embolized) tip,” the FDA says in a recall notice posted today on its website.
To date, there have been 55 injuries, no deaths, and 163 complaints reported for this device.
The recalled devices were distributed in the United States between Jan. 22, 2018 and Oct. 4, 2021. Product codes and lot numbers pertaining to the devices are listed on the FDA website.
Medtronic sent an urgent field safety notice to customers Dec. 6, 2021, requesting that they alert parties of the defect, review the instructions for use before using the device, and note the warnings and precautions listed in the letter.
Customers were also asked to complete the enclosed confirmation form and email to [email protected].
Health care providers can report adverse reactions or quality problems they experience using these devices to the FDA’s MedWatch program.
A version of this article first appeared on Medscape.com.
Medtronic has recalled 95,110 HawkOne Directional Atherectomy Systems because of the risk of the guidewire within the catheter moving downward or prolapsing during use, which may damage the tip of the catheter.
The U.S. Food and Drug Administration has identified this as a Class I recall, the most serious type, because of the potential for serious injury or death.
The HawkOne Directional Atherectomy system is used during procedures intended to remove blockage from peripheral arteries and improve blood flow.
If the guideline moves downward or prolapses during use, the “catheter tip may break off or separate, and this could lead to serious adverse events, including a tear along the inside wall of an artery (arterial dissection), a rupture or breakage of an artery (arterial rupture), decrease in blood flow to a part of the body because of a blocked artery (ischemia), and/or blood vessel complications that could require surgical repair and additional procedures to capture and remove the detached and/or migrated (embolized) tip,” the FDA says in a recall notice posted today on its website.
To date, there have been 55 injuries, no deaths, and 163 complaints reported for this device.
The recalled devices were distributed in the United States between Jan. 22, 2018 and Oct. 4, 2021. Product codes and lot numbers pertaining to the devices are listed on the FDA website.
Medtronic sent an urgent field safety notice to customers Dec. 6, 2021, requesting that they alert parties of the defect, review the instructions for use before using the device, and note the warnings and precautions listed in the letter.
Customers were also asked to complete the enclosed confirmation form and email to [email protected].
Health care providers can report adverse reactions or quality problems they experience using these devices to the FDA’s MedWatch program.
A version of this article first appeared on Medscape.com.
FFR-Guided or Angiography-Guided Nonculprit Lesion PCI in Patients With STEMI Without Cardiogenic Shock
Study Overview
Objective. To determine whether fractional flow reserve (FFR)-guided percutaneous coronary intervention (PCI) of nonculprit lesion in patients with ST-segment elevation myocardial infarction (STEMI) is superior to angiography-guided PCI.
Design. Multicenter randomized control trial blinded to outcome, conducted in 41 sites in France.
Setting and participants. A total of 1163 patients with STEMI and multivessel coronary disease, who had undergone successful PCI to the culprit lesion were randomized to either FFR-guided PCI or angiography-guided PCI for nonculprit lesions. Randomization was stratified according to the trial site and timing of the procedure (immediate or staged).
Main outcome measures. The primary outcome was a composite of death from any cause, nonfatal myocardial infarction (MI) or unplanned hospitalization leading to urgent revascularization at 1 year.
Main results. At 1 year, the primary outcome occurred in 32 of 586 patients (5.5%) in the FFR-guided group and in 24 of 577 (4.2%) in the angiography-guided group (hazard ratio [HR], 1.32; 95% CI, 0.78-2.23; P = .31). The rate of death (1.5% vs 1.7%), nonfatal MI (3.1% vs 1.7%), and unplanned hospitalization leading to urgent revascularization (3.1% vs 1.7%) were also similar between FFR-guided and angiography-guided groups.
Conclusion. Among patients with STEMI and multivessel disease who had undergone successful PCI of the culprit vessel, an FFR-guided strategy for complete revascularization was not superior to angiography-guided strategy for reducing death, MI, or urgent revascularization at 1 year.
Commentary
Patients presenting with STEMI often have multivessel disease.1 Recently, multiple studies have reported the benefit of nonculprit vessel revascularization in patients presenting with hemodynamically stable STEMI compared to culprit-only strategy including the most recent COMPLETE trial which showed reduction in death and MI.2-6 However, the previous studies have variable design in evaluating the nonculprit vessel, some utilized FFR guidance, while others used angiography guidance. Whether FFR-guided PCI of nonculprit vessel can improve outcome in patients presenting STEMI remains unknown.
In the FLOWER-MI study, Puymirat et al investigated the use of FFR compared to angiography-guided nonculprit vessel PCI. A total of 1163 patients presenting with STEMI and multivessel disease who had undergone successful PCI to the culprit vessel, were randomized to either FFR guidance or angiography guidance among 41 centers in France. The authors found that after 1 year, there was no difference in composite endpoint of death, nonfatal MI or unplanned hospitalization leading to urgent revascularization in the FFR-guided group compared to angiography-guided group (5.5% vs 4.2%, HR, 1.32; 95% CI, 0.678-2.23; P = .31). There was also no difference in individual components of primary outcomes or secondary outcomes such as rate of stent thrombosis, any revascularization, or hospitalization.
There are a few interesting points to consider in this study. Ever since the Fractional Flow Reserve vs Angiography for Multivessel Evaluation (FAME) trial reported the lower incidence of major adverse events in routine FFR measurement during PCI compared to angiography-guided PCI, physiological assessment has become the gold standard for treatment of stable ischemic heart disease.7 However, the results of the current FLOWER-MI trial were not consistent with the FAME trial and there are few possible reasons to consider.
First, the use of FFR in the setting of STEMI is less validated compared to stable ischemic heart disease.8 Microvascular dysfunction during the acute phase can affect the FFR reading and the lesion severity can be underestimated.8 Second, the rate of composite endpoint was much lower in this study compared to FAME despite using the same composite endpoint of death, nonfatal MI, and unplanned hospitalization leading to urgent revascularization. At 1 year, the incidence of primary outcome was 13.5% in the FFR-guided group compared to 18.6% in the angiography-guided group in the FAME study compared to 5.5% and 4.2% in the FLOWER-MI study, despite having a sicker population presenting with STEMI. This is likely due to improvement in the PCI techniques such as radial approach, imaging guidance, and advancement in medical therapy such as use of more potent antiplatelet therapy. With lower incidence of primary outcome, larger number of patients are needed to detect the difference in the composite outcome. Finally, the operators’ visual assessment may have been calibrated to the physiologic assessment as the operators are routinely using FFR assessment which may have diminished the benefit of FFR guidance seen in the early FAME study.
Another interesting finding from this study was that although the study protocol encouraged the operators to perform the nonculprit PCI in the same setting, only 4% had nonculprit PCI in the same setting and 96% of the patients underwent a staged PCI. The advantage of performing the nonculprit PCI on the same setting is to have 1 fewer procedure for the patient. On the other hand, the disadvantage of this approach includes prolongation of the index procedure, theoretically higher risk of complication during the acute phase and vasospasm leading to overestimation of the lesion severity. A recent analysis from the COMPLETE study did not show any difference when comparing staged PCI during the index hospitalization vs after discharge.9 The optimal timing of the staged PCI needs to be investigated in future studies.
A limitation of this study is the lower than expected incidence of clinical events decreasing the statistical power of the study. However, there was no signal that FFR-guided PCI is better compared to the angiography-guided group. In fact, the curve started to diverge at 6 months favoring the angiography-guided group. In addition, there was no core-lab analysis for completeness of revascularization.
Applications for Clinical Practice
In patients presenting with hemodynamically stable STEMI for undergoing nonculprit vessel PCI, both FFR-guided or angiography-guided strategies can be considered.
Financial disclosures: None.
1. Park DW, Clare RM, Schulte PJ, et al. Extent, location, and clinical significance of non-infarct-related coronary artery disease among patients with ST-elevation myocardial infarction. JAMA. 2014;312(19):2019-27. doi:10.1001/jama.2014.15095
2. Wald DS, Morris JK, Wald NJ, et al. Randomized trial of preventive angioplasty in myocardial infarction. N Engl J Med. 2013;369(12):1115-23. doi:10.1056/NEJMoa1305520
3. Gershlick AH, Khan JN, Kelly DJ, et al. Randomized trial of complete versus lesion-only revascularization in patients undergoing primary percutaneous coronary intervention for STEMI and multivessel disease: the CvLPRIT trial. J Am Coll Cardiol. 2015;65(10):963-72. doi:10.1016/j.jacc.2014.12.038
4. Engstrøm T, Kelbæk H, Helqvist S, et al. Complete revascularisation versus treatment of the culprit lesion only in patients with ST-segment elevation myocardial infarction and multivessel disease (DANAMI-3-PRIMULTI): an open-label, randomised controlled trial. Lancet. 2015;386(9994):665-71. doi:10.1016/s0140-6736(15)60648-1
5. Smits PC, Abdel-Wahab M, Neumann FJ, , et al. Fractional Flow Reserve-Guided Multivessel Angioplasty in Myocardial Infarction. N Engl J Med. 2017;376(13):1234-44. doi:10.1056/NEJMoa1701067
6. Mehta SR, Wood DA, Storey RF, et al. Complete Revascularization with Multivessel PCI for Myocardial Infarction. N Engl J Med. 2019;381(15):1411-21. doi:10.1056/NEJMoa1907775
7. Tonino PA, De Bruyne B, Pijls NH, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360(3):213-24. doi:10.1056/NEJMoa0807611
8. Thim T, van der Hoeven NW, Musto C, et al. Evaluation and Management of Nonculprit Lesions in STEMI. JACC Cardiovasc Interv. 2020;13(10):1145-54. doi:10.1016/j.jcin.2020.02.030
9. Wood DA, Cairns JA, Wang J, et al. Timing of Staged Nonculprit Artery Revascularization in Patients With ST-Segment Elevation Myocardial Infarction: COMPLETE Trial. J Am Coll Cardiol. 2019;74(22):2713-23. doi:10.1016/j.jacc.2019/09.051
Study Overview
Objective. To determine whether fractional flow reserve (FFR)-guided percutaneous coronary intervention (PCI) of nonculprit lesion in patients with ST-segment elevation myocardial infarction (STEMI) is superior to angiography-guided PCI.
Design. Multicenter randomized control trial blinded to outcome, conducted in 41 sites in France.
Setting and participants. A total of 1163 patients with STEMI and multivessel coronary disease, who had undergone successful PCI to the culprit lesion were randomized to either FFR-guided PCI or angiography-guided PCI for nonculprit lesions. Randomization was stratified according to the trial site and timing of the procedure (immediate or staged).
Main outcome measures. The primary outcome was a composite of death from any cause, nonfatal myocardial infarction (MI) or unplanned hospitalization leading to urgent revascularization at 1 year.
Main results. At 1 year, the primary outcome occurred in 32 of 586 patients (5.5%) in the FFR-guided group and in 24 of 577 (4.2%) in the angiography-guided group (hazard ratio [HR], 1.32; 95% CI, 0.78-2.23; P = .31). The rate of death (1.5% vs 1.7%), nonfatal MI (3.1% vs 1.7%), and unplanned hospitalization leading to urgent revascularization (3.1% vs 1.7%) were also similar between FFR-guided and angiography-guided groups.
Conclusion. Among patients with STEMI and multivessel disease who had undergone successful PCI of the culprit vessel, an FFR-guided strategy for complete revascularization was not superior to angiography-guided strategy for reducing death, MI, or urgent revascularization at 1 year.
Commentary
Patients presenting with STEMI often have multivessel disease.1 Recently, multiple studies have reported the benefit of nonculprit vessel revascularization in patients presenting with hemodynamically stable STEMI compared to culprit-only strategy including the most recent COMPLETE trial which showed reduction in death and MI.2-6 However, the previous studies have variable design in evaluating the nonculprit vessel, some utilized FFR guidance, while others used angiography guidance. Whether FFR-guided PCI of nonculprit vessel can improve outcome in patients presenting STEMI remains unknown.
In the FLOWER-MI study, Puymirat et al investigated the use of FFR compared to angiography-guided nonculprit vessel PCI. A total of 1163 patients presenting with STEMI and multivessel disease who had undergone successful PCI to the culprit vessel, were randomized to either FFR guidance or angiography guidance among 41 centers in France. The authors found that after 1 year, there was no difference in composite endpoint of death, nonfatal MI or unplanned hospitalization leading to urgent revascularization in the FFR-guided group compared to angiography-guided group (5.5% vs 4.2%, HR, 1.32; 95% CI, 0.678-2.23; P = .31). There was also no difference in individual components of primary outcomes or secondary outcomes such as rate of stent thrombosis, any revascularization, or hospitalization.
There are a few interesting points to consider in this study. Ever since the Fractional Flow Reserve vs Angiography for Multivessel Evaluation (FAME) trial reported the lower incidence of major adverse events in routine FFR measurement during PCI compared to angiography-guided PCI, physiological assessment has become the gold standard for treatment of stable ischemic heart disease.7 However, the results of the current FLOWER-MI trial were not consistent with the FAME trial and there are few possible reasons to consider.
First, the use of FFR in the setting of STEMI is less validated compared to stable ischemic heart disease.8 Microvascular dysfunction during the acute phase can affect the FFR reading and the lesion severity can be underestimated.8 Second, the rate of composite endpoint was much lower in this study compared to FAME despite using the same composite endpoint of death, nonfatal MI, and unplanned hospitalization leading to urgent revascularization. At 1 year, the incidence of primary outcome was 13.5% in the FFR-guided group compared to 18.6% in the angiography-guided group in the FAME study compared to 5.5% and 4.2% in the FLOWER-MI study, despite having a sicker population presenting with STEMI. This is likely due to improvement in the PCI techniques such as radial approach, imaging guidance, and advancement in medical therapy such as use of more potent antiplatelet therapy. With lower incidence of primary outcome, larger number of patients are needed to detect the difference in the composite outcome. Finally, the operators’ visual assessment may have been calibrated to the physiologic assessment as the operators are routinely using FFR assessment which may have diminished the benefit of FFR guidance seen in the early FAME study.
Another interesting finding from this study was that although the study protocol encouraged the operators to perform the nonculprit PCI in the same setting, only 4% had nonculprit PCI in the same setting and 96% of the patients underwent a staged PCI. The advantage of performing the nonculprit PCI on the same setting is to have 1 fewer procedure for the patient. On the other hand, the disadvantage of this approach includes prolongation of the index procedure, theoretically higher risk of complication during the acute phase and vasospasm leading to overestimation of the lesion severity. A recent analysis from the COMPLETE study did not show any difference when comparing staged PCI during the index hospitalization vs after discharge.9 The optimal timing of the staged PCI needs to be investigated in future studies.
A limitation of this study is the lower than expected incidence of clinical events decreasing the statistical power of the study. However, there was no signal that FFR-guided PCI is better compared to the angiography-guided group. In fact, the curve started to diverge at 6 months favoring the angiography-guided group. In addition, there was no core-lab analysis for completeness of revascularization.
Applications for Clinical Practice
In patients presenting with hemodynamically stable STEMI for undergoing nonculprit vessel PCI, both FFR-guided or angiography-guided strategies can be considered.
Financial disclosures: None.
Study Overview
Objective. To determine whether fractional flow reserve (FFR)-guided percutaneous coronary intervention (PCI) of nonculprit lesion in patients with ST-segment elevation myocardial infarction (STEMI) is superior to angiography-guided PCI.
Design. Multicenter randomized control trial blinded to outcome, conducted in 41 sites in France.
Setting and participants. A total of 1163 patients with STEMI and multivessel coronary disease, who had undergone successful PCI to the culprit lesion were randomized to either FFR-guided PCI or angiography-guided PCI for nonculprit lesions. Randomization was stratified according to the trial site and timing of the procedure (immediate or staged).
Main outcome measures. The primary outcome was a composite of death from any cause, nonfatal myocardial infarction (MI) or unplanned hospitalization leading to urgent revascularization at 1 year.
Main results. At 1 year, the primary outcome occurred in 32 of 586 patients (5.5%) in the FFR-guided group and in 24 of 577 (4.2%) in the angiography-guided group (hazard ratio [HR], 1.32; 95% CI, 0.78-2.23; P = .31). The rate of death (1.5% vs 1.7%), nonfatal MI (3.1% vs 1.7%), and unplanned hospitalization leading to urgent revascularization (3.1% vs 1.7%) were also similar between FFR-guided and angiography-guided groups.
Conclusion. Among patients with STEMI and multivessel disease who had undergone successful PCI of the culprit vessel, an FFR-guided strategy for complete revascularization was not superior to angiography-guided strategy for reducing death, MI, or urgent revascularization at 1 year.
Commentary
Patients presenting with STEMI often have multivessel disease.1 Recently, multiple studies have reported the benefit of nonculprit vessel revascularization in patients presenting with hemodynamically stable STEMI compared to culprit-only strategy including the most recent COMPLETE trial which showed reduction in death and MI.2-6 However, the previous studies have variable design in evaluating the nonculprit vessel, some utilized FFR guidance, while others used angiography guidance. Whether FFR-guided PCI of nonculprit vessel can improve outcome in patients presenting STEMI remains unknown.
In the FLOWER-MI study, Puymirat et al investigated the use of FFR compared to angiography-guided nonculprit vessel PCI. A total of 1163 patients presenting with STEMI and multivessel disease who had undergone successful PCI to the culprit vessel, were randomized to either FFR guidance or angiography guidance among 41 centers in France. The authors found that after 1 year, there was no difference in composite endpoint of death, nonfatal MI or unplanned hospitalization leading to urgent revascularization in the FFR-guided group compared to angiography-guided group (5.5% vs 4.2%, HR, 1.32; 95% CI, 0.678-2.23; P = .31). There was also no difference in individual components of primary outcomes or secondary outcomes such as rate of stent thrombosis, any revascularization, or hospitalization.
There are a few interesting points to consider in this study. Ever since the Fractional Flow Reserve vs Angiography for Multivessel Evaluation (FAME) trial reported the lower incidence of major adverse events in routine FFR measurement during PCI compared to angiography-guided PCI, physiological assessment has become the gold standard for treatment of stable ischemic heart disease.7 However, the results of the current FLOWER-MI trial were not consistent with the FAME trial and there are few possible reasons to consider.
First, the use of FFR in the setting of STEMI is less validated compared to stable ischemic heart disease.8 Microvascular dysfunction during the acute phase can affect the FFR reading and the lesion severity can be underestimated.8 Second, the rate of composite endpoint was much lower in this study compared to FAME despite using the same composite endpoint of death, nonfatal MI, and unplanned hospitalization leading to urgent revascularization. At 1 year, the incidence of primary outcome was 13.5% in the FFR-guided group compared to 18.6% in the angiography-guided group in the FAME study compared to 5.5% and 4.2% in the FLOWER-MI study, despite having a sicker population presenting with STEMI. This is likely due to improvement in the PCI techniques such as radial approach, imaging guidance, and advancement in medical therapy such as use of more potent antiplatelet therapy. With lower incidence of primary outcome, larger number of patients are needed to detect the difference in the composite outcome. Finally, the operators’ visual assessment may have been calibrated to the physiologic assessment as the operators are routinely using FFR assessment which may have diminished the benefit of FFR guidance seen in the early FAME study.
Another interesting finding from this study was that although the study protocol encouraged the operators to perform the nonculprit PCI in the same setting, only 4% had nonculprit PCI in the same setting and 96% of the patients underwent a staged PCI. The advantage of performing the nonculprit PCI on the same setting is to have 1 fewer procedure for the patient. On the other hand, the disadvantage of this approach includes prolongation of the index procedure, theoretically higher risk of complication during the acute phase and vasospasm leading to overestimation of the lesion severity. A recent analysis from the COMPLETE study did not show any difference when comparing staged PCI during the index hospitalization vs after discharge.9 The optimal timing of the staged PCI needs to be investigated in future studies.
A limitation of this study is the lower than expected incidence of clinical events decreasing the statistical power of the study. However, there was no signal that FFR-guided PCI is better compared to the angiography-guided group. In fact, the curve started to diverge at 6 months favoring the angiography-guided group. In addition, there was no core-lab analysis for completeness of revascularization.
Applications for Clinical Practice
In patients presenting with hemodynamically stable STEMI for undergoing nonculprit vessel PCI, both FFR-guided or angiography-guided strategies can be considered.
Financial disclosures: None.
1. Park DW, Clare RM, Schulte PJ, et al. Extent, location, and clinical significance of non-infarct-related coronary artery disease among patients with ST-elevation myocardial infarction. JAMA. 2014;312(19):2019-27. doi:10.1001/jama.2014.15095
2. Wald DS, Morris JK, Wald NJ, et al. Randomized trial of preventive angioplasty in myocardial infarction. N Engl J Med. 2013;369(12):1115-23. doi:10.1056/NEJMoa1305520
3. Gershlick AH, Khan JN, Kelly DJ, et al. Randomized trial of complete versus lesion-only revascularization in patients undergoing primary percutaneous coronary intervention for STEMI and multivessel disease: the CvLPRIT trial. J Am Coll Cardiol. 2015;65(10):963-72. doi:10.1016/j.jacc.2014.12.038
4. Engstrøm T, Kelbæk H, Helqvist S, et al. Complete revascularisation versus treatment of the culprit lesion only in patients with ST-segment elevation myocardial infarction and multivessel disease (DANAMI-3-PRIMULTI): an open-label, randomised controlled trial. Lancet. 2015;386(9994):665-71. doi:10.1016/s0140-6736(15)60648-1
5. Smits PC, Abdel-Wahab M, Neumann FJ, , et al. Fractional Flow Reserve-Guided Multivessel Angioplasty in Myocardial Infarction. N Engl J Med. 2017;376(13):1234-44. doi:10.1056/NEJMoa1701067
6. Mehta SR, Wood DA, Storey RF, et al. Complete Revascularization with Multivessel PCI for Myocardial Infarction. N Engl J Med. 2019;381(15):1411-21. doi:10.1056/NEJMoa1907775
7. Tonino PA, De Bruyne B, Pijls NH, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360(3):213-24. doi:10.1056/NEJMoa0807611
8. Thim T, van der Hoeven NW, Musto C, et al. Evaluation and Management of Nonculprit Lesions in STEMI. JACC Cardiovasc Interv. 2020;13(10):1145-54. doi:10.1016/j.jcin.2020.02.030
9. Wood DA, Cairns JA, Wang J, et al. Timing of Staged Nonculprit Artery Revascularization in Patients With ST-Segment Elevation Myocardial Infarction: COMPLETE Trial. J Am Coll Cardiol. 2019;74(22):2713-23. doi:10.1016/j.jacc.2019/09.051
1. Park DW, Clare RM, Schulte PJ, et al. Extent, location, and clinical significance of non-infarct-related coronary artery disease among patients with ST-elevation myocardial infarction. JAMA. 2014;312(19):2019-27. doi:10.1001/jama.2014.15095
2. Wald DS, Morris JK, Wald NJ, et al. Randomized trial of preventive angioplasty in myocardial infarction. N Engl J Med. 2013;369(12):1115-23. doi:10.1056/NEJMoa1305520
3. Gershlick AH, Khan JN, Kelly DJ, et al. Randomized trial of complete versus lesion-only revascularization in patients undergoing primary percutaneous coronary intervention for STEMI and multivessel disease: the CvLPRIT trial. J Am Coll Cardiol. 2015;65(10):963-72. doi:10.1016/j.jacc.2014.12.038
4. Engstrøm T, Kelbæk H, Helqvist S, et al. Complete revascularisation versus treatment of the culprit lesion only in patients with ST-segment elevation myocardial infarction and multivessel disease (DANAMI-3-PRIMULTI): an open-label, randomised controlled trial. Lancet. 2015;386(9994):665-71. doi:10.1016/s0140-6736(15)60648-1
5. Smits PC, Abdel-Wahab M, Neumann FJ, , et al. Fractional Flow Reserve-Guided Multivessel Angioplasty in Myocardial Infarction. N Engl J Med. 2017;376(13):1234-44. doi:10.1056/NEJMoa1701067
6. Mehta SR, Wood DA, Storey RF, et al. Complete Revascularization with Multivessel PCI for Myocardial Infarction. N Engl J Med. 2019;381(15):1411-21. doi:10.1056/NEJMoa1907775
7. Tonino PA, De Bruyne B, Pijls NH, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360(3):213-24. doi:10.1056/NEJMoa0807611
8. Thim T, van der Hoeven NW, Musto C, et al. Evaluation and Management of Nonculprit Lesions in STEMI. JACC Cardiovasc Interv. 2020;13(10):1145-54. doi:10.1016/j.jcin.2020.02.030
9. Wood DA, Cairns JA, Wang J, et al. Timing of Staged Nonculprit Artery Revascularization in Patients With ST-Segment Elevation Myocardial Infarction: COMPLETE Trial. J Am Coll Cardiol. 2019;74(22):2713-23. doi:10.1016/j.jacc.2019/09.051
Cell therapy promising as long-term limb-saving treatment in diabetes
Bone marrow derived autologous cell therapy (ACT) has been shown to significantly reduce the rate of major amputation at 5 years in people with diabetes who developed critical limb-threatening ischemia (CLTI).
In a study of 130 patients, 64% of 42 patients who were treated conservatively needed a major amputation at 5 years versus just 30% of 45 patients who had been treated with ACT (P = .011).
This compared favorably to the results seen with repeated percutaneous angioplasty (re-PTA), where just 20.9% of 43 patients underwent limb salvage (P = .002 vs. conservative therapy).
Furthermore, amputation-free survival was significantly longer in both active groups, Michal Dubský, MD, PhD, FRSPH, reported at the annual meeting of the European Association for the Study of Diabetes.
Dr. Dubský, of the Institute for Clinical and Experimental Medicine and Charles University in Prague, also reported that fewer patients who had undergone re-PTA or ACT than conservative treatment had died by 5 years (25.8% and 35.6%, respectively, vs. 61.9%), but that the difference was significant only for the revascularization procedure (P = .012).
Based on these findings, “we believe that autologous cell therapy seems to be an appropriate alternative to repeated PTA even for patients with no-option chronic limb-threatening ischemia,” he said.
“This is a very important area,” said Andrew J.M. Boulton, MBBS, MD, FRCP, who chaired the oral abstract presentation session during which the findings were presented.
“It is very difficult to get an evidence base from randomized studies in this area, because of the nature of the patients: They’re very sick and we all deal with them in our clinics very regularly,” added Dr. Boulton, professor of medicine within the division of diabetes, endocrinology and gastroenterology at the University of Manchester (England).
Dr. Boulton called the findings a “very important addition to what we know.”
New option for no-option CLTI
CLTI is associated with persistent pain at rest, ulcers, and gangrene, and can be the end result of longstanding peripheral arterial disease. Within the first year of presentation, there’s a 30% chance of having a major amputation and a 25% chance of dying.
Importantly, said Dr. Dubský, “there is a big difference in this diagnosis” between patients with diabetes and those without. For instance, CLTI is more diffuse in patients with diabetes than in those without, different arteries are affected and the sclerosis seen can be more rigid and “full of calcium.”
While surgery to improve blood flow is the standard of care, not everyone is suitable. Bypass surgery or endovascular procedures can be performed in only 40%-50% of patients, and even then a therapeutic effect may be seen in only a quarter of patients.
“We need some new therapeutic modalities for this diagnosis, and one of them could be autologous cell therapy,” said Dr. Dubský.
Study details
Dr. Dubský and coinvestigators consecutively recruited 130 patients with diabetic foot and CLTI who had been seen at their clinic over a 5-year period. Of these, 87 had not been eligible for standard revascularization and underwent ACT or were treated conservatively.
Of the patients who were not eligible for standard revascularization (‘no-option CLTI), 45 had undergone ACT and 42 had been treated conservatively. Dr. Dubský acknowledged that “his study was not prospective and randomized.”
All patients in the study had at least one unsuccessful revascularization procedure and diabetic foot ulcers, and low tissue oxygenation. The latter was defined as transcutaneous oxygen pressure (TcPO2) of below 30 mm Hg.
There were little differences in demographic characteristics between the treatment groups, the average age ranged from 62 to 67 years, there were more men (70%-80%) than women; most patients (90%) had type 2 diabetes for at least 20 years. There were similar rates of ischemic heart disease, hypertension, dialysis, and immunosuppressive therapy.
There were no differences in baseline values of TcPO2 between the groups, and similar improvements were seen in both the ACT and re-PTA groups versus conservative group.
ACT in practice
With such promising results, what about the practicalities of harvesting a patient’s bone marrow to make the ACT?
“Bone marrow harvesting usually takes about 20 minutes,” Dr. Dubský said. It then takes another 45 minutes to separate the cells and make the cell suspension, and then maybe another 10 minutes or so to administer this to the patient, which is done by injecting into the calf muscles and small muscles of the foot, aided by computed tomography. The whole process may take up to 2 hours, he said.
“Patients are under local or general anesthesia, so there is no pain during the procedure,” Dr. Dubský reassured. “Afterwards we sometimes see small hematoma[s], with low-intensity pain that responds well to usual analgesic therapy.”
Computed tomography was used to help guide the injections, which was advantageous, Dr. Boulton pointed out, because it was “less invasive than angioplasty in these very sick people with very distal lesions, many of whom already have renal problems.”
“It is surprising though, that everybody had re-PTA and not one had vascular surgery,” he suggested. Dr. Boulton added, however: “These are very important observations; they help us a lot in an area where there’s unlikely to be a full RCT.”
The next step in this research is to see if combining ACT and re-PTA could lead to even better results.
The study was funded by the Czech Republic Ministry of Health. Dr. Dubský had nothing to disclose. Dr. Boulton made no statement about his conflicts of interest.
Bone marrow derived autologous cell therapy (ACT) has been shown to significantly reduce the rate of major amputation at 5 years in people with diabetes who developed critical limb-threatening ischemia (CLTI).
In a study of 130 patients, 64% of 42 patients who were treated conservatively needed a major amputation at 5 years versus just 30% of 45 patients who had been treated with ACT (P = .011).
This compared favorably to the results seen with repeated percutaneous angioplasty (re-PTA), where just 20.9% of 43 patients underwent limb salvage (P = .002 vs. conservative therapy).
Furthermore, amputation-free survival was significantly longer in both active groups, Michal Dubský, MD, PhD, FRSPH, reported at the annual meeting of the European Association for the Study of Diabetes.
Dr. Dubský, of the Institute for Clinical and Experimental Medicine and Charles University in Prague, also reported that fewer patients who had undergone re-PTA or ACT than conservative treatment had died by 5 years (25.8% and 35.6%, respectively, vs. 61.9%), but that the difference was significant only for the revascularization procedure (P = .012).
Based on these findings, “we believe that autologous cell therapy seems to be an appropriate alternative to repeated PTA even for patients with no-option chronic limb-threatening ischemia,” he said.
“This is a very important area,” said Andrew J.M. Boulton, MBBS, MD, FRCP, who chaired the oral abstract presentation session during which the findings were presented.
“It is very difficult to get an evidence base from randomized studies in this area, because of the nature of the patients: They’re very sick and we all deal with them in our clinics very regularly,” added Dr. Boulton, professor of medicine within the division of diabetes, endocrinology and gastroenterology at the University of Manchester (England).
Dr. Boulton called the findings a “very important addition to what we know.”
New option for no-option CLTI
CLTI is associated with persistent pain at rest, ulcers, and gangrene, and can be the end result of longstanding peripheral arterial disease. Within the first year of presentation, there’s a 30% chance of having a major amputation and a 25% chance of dying.
Importantly, said Dr. Dubský, “there is a big difference in this diagnosis” between patients with diabetes and those without. For instance, CLTI is more diffuse in patients with diabetes than in those without, different arteries are affected and the sclerosis seen can be more rigid and “full of calcium.”
While surgery to improve blood flow is the standard of care, not everyone is suitable. Bypass surgery or endovascular procedures can be performed in only 40%-50% of patients, and even then a therapeutic effect may be seen in only a quarter of patients.
“We need some new therapeutic modalities for this diagnosis, and one of them could be autologous cell therapy,” said Dr. Dubský.
Study details
Dr. Dubský and coinvestigators consecutively recruited 130 patients with diabetic foot and CLTI who had been seen at their clinic over a 5-year period. Of these, 87 had not been eligible for standard revascularization and underwent ACT or were treated conservatively.
Of the patients who were not eligible for standard revascularization (‘no-option CLTI), 45 had undergone ACT and 42 had been treated conservatively. Dr. Dubský acknowledged that “his study was not prospective and randomized.”
All patients in the study had at least one unsuccessful revascularization procedure and diabetic foot ulcers, and low tissue oxygenation. The latter was defined as transcutaneous oxygen pressure (TcPO2) of below 30 mm Hg.
There were little differences in demographic characteristics between the treatment groups, the average age ranged from 62 to 67 years, there were more men (70%-80%) than women; most patients (90%) had type 2 diabetes for at least 20 years. There were similar rates of ischemic heart disease, hypertension, dialysis, and immunosuppressive therapy.
There were no differences in baseline values of TcPO2 between the groups, and similar improvements were seen in both the ACT and re-PTA groups versus conservative group.
ACT in practice
With such promising results, what about the practicalities of harvesting a patient’s bone marrow to make the ACT?
“Bone marrow harvesting usually takes about 20 minutes,” Dr. Dubský said. It then takes another 45 minutes to separate the cells and make the cell suspension, and then maybe another 10 minutes or so to administer this to the patient, which is done by injecting into the calf muscles and small muscles of the foot, aided by computed tomography. The whole process may take up to 2 hours, he said.
“Patients are under local or general anesthesia, so there is no pain during the procedure,” Dr. Dubský reassured. “Afterwards we sometimes see small hematoma[s], with low-intensity pain that responds well to usual analgesic therapy.”
Computed tomography was used to help guide the injections, which was advantageous, Dr. Boulton pointed out, because it was “less invasive than angioplasty in these very sick people with very distal lesions, many of whom already have renal problems.”
“It is surprising though, that everybody had re-PTA and not one had vascular surgery,” he suggested. Dr. Boulton added, however: “These are very important observations; they help us a lot in an area where there’s unlikely to be a full RCT.”
The next step in this research is to see if combining ACT and re-PTA could lead to even better results.
The study was funded by the Czech Republic Ministry of Health. Dr. Dubský had nothing to disclose. Dr. Boulton made no statement about his conflicts of interest.
Bone marrow derived autologous cell therapy (ACT) has been shown to significantly reduce the rate of major amputation at 5 years in people with diabetes who developed critical limb-threatening ischemia (CLTI).
In a study of 130 patients, 64% of 42 patients who were treated conservatively needed a major amputation at 5 years versus just 30% of 45 patients who had been treated with ACT (P = .011).
This compared favorably to the results seen with repeated percutaneous angioplasty (re-PTA), where just 20.9% of 43 patients underwent limb salvage (P = .002 vs. conservative therapy).
Furthermore, amputation-free survival was significantly longer in both active groups, Michal Dubský, MD, PhD, FRSPH, reported at the annual meeting of the European Association for the Study of Diabetes.
Dr. Dubský, of the Institute for Clinical and Experimental Medicine and Charles University in Prague, also reported that fewer patients who had undergone re-PTA or ACT than conservative treatment had died by 5 years (25.8% and 35.6%, respectively, vs. 61.9%), but that the difference was significant only for the revascularization procedure (P = .012).
Based on these findings, “we believe that autologous cell therapy seems to be an appropriate alternative to repeated PTA even for patients with no-option chronic limb-threatening ischemia,” he said.
“This is a very important area,” said Andrew J.M. Boulton, MBBS, MD, FRCP, who chaired the oral abstract presentation session during which the findings were presented.
“It is very difficult to get an evidence base from randomized studies in this area, because of the nature of the patients: They’re very sick and we all deal with them in our clinics very regularly,” added Dr. Boulton, professor of medicine within the division of diabetes, endocrinology and gastroenterology at the University of Manchester (England).
Dr. Boulton called the findings a “very important addition to what we know.”
New option for no-option CLTI
CLTI is associated with persistent pain at rest, ulcers, and gangrene, and can be the end result of longstanding peripheral arterial disease. Within the first year of presentation, there’s a 30% chance of having a major amputation and a 25% chance of dying.
Importantly, said Dr. Dubský, “there is a big difference in this diagnosis” between patients with diabetes and those without. For instance, CLTI is more diffuse in patients with diabetes than in those without, different arteries are affected and the sclerosis seen can be more rigid and “full of calcium.”
While surgery to improve blood flow is the standard of care, not everyone is suitable. Bypass surgery or endovascular procedures can be performed in only 40%-50% of patients, and even then a therapeutic effect may be seen in only a quarter of patients.
“We need some new therapeutic modalities for this diagnosis, and one of them could be autologous cell therapy,” said Dr. Dubský.
Study details
Dr. Dubský and coinvestigators consecutively recruited 130 patients with diabetic foot and CLTI who had been seen at their clinic over a 5-year period. Of these, 87 had not been eligible for standard revascularization and underwent ACT or were treated conservatively.
Of the patients who were not eligible for standard revascularization (‘no-option CLTI), 45 had undergone ACT and 42 had been treated conservatively. Dr. Dubský acknowledged that “his study was not prospective and randomized.”
All patients in the study had at least one unsuccessful revascularization procedure and diabetic foot ulcers, and low tissue oxygenation. The latter was defined as transcutaneous oxygen pressure (TcPO2) of below 30 mm Hg.
There were little differences in demographic characteristics between the treatment groups, the average age ranged from 62 to 67 years, there were more men (70%-80%) than women; most patients (90%) had type 2 diabetes for at least 20 years. There were similar rates of ischemic heart disease, hypertension, dialysis, and immunosuppressive therapy.
There were no differences in baseline values of TcPO2 between the groups, and similar improvements were seen in both the ACT and re-PTA groups versus conservative group.
ACT in practice
With such promising results, what about the practicalities of harvesting a patient’s bone marrow to make the ACT?
“Bone marrow harvesting usually takes about 20 minutes,” Dr. Dubský said. It then takes another 45 minutes to separate the cells and make the cell suspension, and then maybe another 10 minutes or so to administer this to the patient, which is done by injecting into the calf muscles and small muscles of the foot, aided by computed tomography. The whole process may take up to 2 hours, he said.
“Patients are under local or general anesthesia, so there is no pain during the procedure,” Dr. Dubský reassured. “Afterwards we sometimes see small hematoma[s], with low-intensity pain that responds well to usual analgesic therapy.”
Computed tomography was used to help guide the injections, which was advantageous, Dr. Boulton pointed out, because it was “less invasive than angioplasty in these very sick people with very distal lesions, many of whom already have renal problems.”
“It is surprising though, that everybody had re-PTA and not one had vascular surgery,” he suggested. Dr. Boulton added, however: “These are very important observations; they help us a lot in an area where there’s unlikely to be a full RCT.”
The next step in this research is to see if combining ACT and re-PTA could lead to even better results.
The study was funded by the Czech Republic Ministry of Health. Dr. Dubský had nothing to disclose. Dr. Boulton made no statement about his conflicts of interest.
FROM EASD 2021
Evaluation of a Digital Intervention for Hypertension Management in Primary Care Combining Self-monitoring of Blood Pressure With Guided Self-management
Study Overview
Objective. To evaluate whether a digital intervention comprising self-monitoring of blood pressure (BP) with reminders and predetermined drug changes combined with lifestyle change support resulted in lower systolic BP in people receiving treatment for hypertension that was poorly controlled, and whether this approach was cost effective.
Design. Unmasked randomized controlled trial.
Settings and participants. Eligible participants were identified from clinical codes recorded in the electronic health records of 76 collaborating general practices from the National Institute for Health Research Clinical Research Network, a United Kingdom government agency. The practices sent invitation letters to eligible participants to come to the clinic to establish eligibility, take consent, and collect baseline data via online questionnaires.
Eligible participants were aged 18 years or older with treated hypertension, a mean baseline BP reading of more than 140/90 mm Hg and were taking no more than 3 antihypertensive drugs. Participants also needed to be willing to self-monitor and have access to the internet (with support from a family member if needed). Exclusions included BP greater than 180/110 mm Hg, atrial fibrillation, hypertension not managed by their general practitioner, chronic kidney disease stage 4-5, postural hypotension (> 20 mm Hg systolic drop), an acute cardiovascular event in the previous 3 months, terminal disease, or another condition which in the opinion of their general practitioner made participation inappropriate.
Of the 11 399 invitation letters sent out, 1389 (12%) potential participants responded positively and were screened for eligibility. Those who declined to take part could optionally give their reasons, and responses were gained from 2426 of 10 010 (24%). The mean age of those who gave a reason for declining was 73 years. The most commonly selected reasons for declining were not having access to the internet (982, 41%), not wanting to participate in a research trial (617, 25%) or an internet study (543, 22%), and not wanting to change drugs (535, 22%). Of the 1389 screened, 734 were ineligible, and 33 did not complete baseline measures and randomization. The remaining 622 people who were randomized in a 1:1 ratio to receive the HOME BP intervention (n = 305) or usual care (n = 317).
Intervention vs usual care. The HOME BP intervention for the self-management of high BP consisted of an integrated patient and health care practitioner online digital intervention, BP self-monitoring (using an Omron M3 monitor), health care practitioner directed and supervised titration of antihypertensive drugs, and user-selected lifestyle modifications. Participants were advised via automated email reminders to take 2 morning BP readings for 7 days each month and to enter online each second reading. Mean home BP was calculated, accompanied by feedback of BP results to both patients and professionals with optional evidence-based lifestyle advice (for healthy eating, physical activity, losing weight if appropriate, and salt and alcohol reduction) and motivational support through practice nurses or health care assistances (using the CARE approach – congratulate, ask, reassure, encourage).
Participants allocated to usual care were not provided with self-monitoring equipment or the HOME BP intervention but had online access to the information provided in a patient leaflet for hypertension. This information comprised definitions of hypertension, causes, and brief guidance on treatment, including lifestyle changes and drugs. These participants received routine hypertension care that typically consisted of clinic BP monitoring to titrate drugs, with appointments and drug changes made at the discretion of the general practitioner. Participants were not prevented from self-monitoring, but data on self-monitoring practices were collected at the end of the trial from patients and practitioners.
Measures and analysis. The primary outcome measure was the difference in systolic BP at 12-month follow-up between the intervention and usual care groups (adjusting for baseline BP, practice, BP target levels, and sex). Secondary outcomes included systolic and diastolic BP at 6 and 12 months, weight, modified patient enablement instrument, drug adherence, health-related quality of life, and side effects from the symptoms section of an adjusted illness perceptions questionnaire. At trial, registration participants and general practitioners were asked about their use of self-monitoring in the usual care group.
The primary analysis used general linear modelling to compare systolic BP in the intervention and usual care groups at follow-up, adjusting for baseline BP, practice (as a random effect to take into account clustering), BP target levels, and sex. Analyses were on an intention-to-treat basis and used multiple imputation for missing data. Sensitivity analyses used complete cases and a repeated measures technique. Secondary analyses used similar techniques to assess differences between groups. A within-trial economic analysis estimated cost per unit reduction in systolic BP by using similar adjustments and multiple imputation for missing values. Repeated bootstrapping was used to estimate the probability of the intervention being cost-effective at different levels of willingness to pay per unit reduction in BP.
Main results. The intervention and usual care groups did not differ significantly – participants had a mean age of 66 years and mean baseline clinical BP of 151.6/85.3 mm Hg and 151.7/86.4 mm Hg (usual care and intervention, respectively). Most participants were White British (94%), just more than half were men, and the time since diagnosis averaged around 11 years. The most deprived group (based on the English Index of Multiple Deprivation) accounted for 63/622 (10%), with the least deprived group accounting for 326/622 (52%).
After 1 year, data were available from 552 participants (88.6%) with imputation for the remaining 70 participants (11.4%). Mean BP dropped from 151.7/86.4 to 138.4/80.2 mm Hg in the intervention group and from 151.6/85.3 to 141.8/79.8 mm Hg in the usual care group, giving a mean difference in systolic BP of −3.4 mm Hg (95% CI −6.1 to −0.8 mm Hg) and a mean difference in diastolic BP of −0.5 mm Hg (−1.9 to 0.9 mm Hg). Exploratory subgroup analyses suggested that participants aged 67 years or older had a smaller effect size than those younger than 67. Similarly, while the effect sizes in the standard and diabetes target groups were similar, those older than 80 years with a higher target of 145/85 mm Hg showed little evidence of benefit. Results for other subgroups, including sex, baseline BP, deprivation, and history of self-monitoring, were similar between groups.
Engagement with the digital intervention was high, with 281/305 (92%) participants completing the 2 core training sessions, 268/305 (88%) completing a week of practice BP readings, and 243/305 (80%) completing at least 3 weeks of BP entries. Furthermore, 214/305 (70%) were still monitoring in the last 3 months of participation. However, less than 1/3 of participants chose to register on 1 of the optional lifestyle change modules. In the usual care group, a post-hoc analysis after 12 months showed that 112/234 (47%) patients reported monitoring their own BP at home at least once per month during the trial.
The difference in mean cost per patient was £38 (US $51.30, €41.9; 95% CI £27 to £47), which along with the decrease in systolic BP, gave an incremental cost per mm Hg BP reduction of £11 (£6 to £29). Bootstrapping analysis showed the intervention had high (90%) probability of being cost-effective at willingness to pay above £20 per unit reduction. The probabilities of being cost-effective for the intervention against usual care were 87%, 93%, and 97% at thresholds of £20, £30, and £50, respectively.
Conclusion. The HOME BP digital intervention for the management of hypertension by using self-monitored BP led to better control of systolic BP after 1 year than usual care, with low incremental costs. Implementation in primary care will require integration into clinical workflows and consideration of people who are digitally excluded.
Commentary
Elevated BP, also known as hypertension, is the most important, modifiable risk factor for cardiovascular disease and mortality.1 Clinically significant effects and improvements in mortality can be achieved with relatively small reductions in BP levels. Long-established lifestyle modifications that effectively lower BP include weight loss, reduced sodium intake, increased physical activity, and limited alcohol intake. However, motivating patients to achieve lifestyle modifications is among the most difficult aspects of managing hypertension. Importantly, for individuals taking antihypertensive medication, lifestyle modification is recommended as adjunctive therapy to reduce BP. Given that target blood pressure levels are reached for less than half of adults, novel interventions are needed to improve BP control – in particular, individualized cognitive behavioral interventions are more likely to be effective than standardized, single-component interventions.
Guided self-management for hypertension as part of systematic, planned care offers the potential for improvements in adherence and in turn improved long-term patient outcomes.2 Self-management can encompass a wide range of behaviors in addition to medication titration and monitoring of symptoms, such as individuals’ ability to manage physical, psychosocial and lifestyle behaviors related to their condition.3 Digital interventions leveraging apps, software, and/or technologies in particular have the potential to support people in self-management, allow for remote monitoring, and enable personalized and adaptive strategies for chronic disease management.4-5 An example of a digital intervention in the context of guided self-management for hypertension can be a web-based program delivered by computer or phone that combines health information with decision support to help inform behavior change in patients and remote monitoring of patient status by health professionals. Well-designed digital interventions can effectively change patient health-related behaviors, improve patient knowledge and confidence for self-management of health, and lead to better health outcomes.6-7
This study adds to the literature as a large, randomized controlled trial evaluating the effectiveness of a digital intervention in the field of hypertension and with follow-up for a year. The authors highlight that relatively few studies have been performed that combine self-monitoring with a digitally delivered cointervention, and none has shown a major effect in an adequately powered trial over a year. Results from this study showed that HOME BP, a digital intervention enabling self-management of hypertension, including self-monitoring, titration based on self-monitored BP, lifestyle advice, and behavioral support for patients and health care professionals, resulted in a worthwhile reduction of systolic BP. In addition, this reduction was achieved at modest cost based on the within trial cost effectiveness analysis.
There are many important strengths of this study, especially related to the design and analysis strategy, and some limitations. This study was designed as a randomized controlled trial with a 1 year follow-up period, although participants were unmasked to the group they were randomized to, which may have impacted their behaviors while in the study. As the authors state, the study was not only adequately powered to detect a difference in blood pressure, but also over-recruitment ensured such an effect was not missed. Recruiting from a large number of general practices ensured generalizability in terms of health care professionals. Importantly, while study participants mostly identified as predominantly White and tended to be of higher socioeconomic status, this is representative of the aged population in England and Wales. Nevertheless, generalizability of findings from this study is still limited to the demographic characteristics of the study population. Other strengths included inclusion of intention-to-treat analysis, multiple imputation for missing data, sensitivity analysis, as well as economic analysis and cost effectiveness analysis.
Of note, results from the study are only attributable to the digital interventions used in this study (digital web-based with limited mechanisms of behavior change and engagement built-in) and thus should not be generalized to all digital interventions for managing hypertension. Also, as the authors highlight, the relative importance of the different parts of the digital intervention were unable to be distinguished, although this type of analysis is important in multicomponent interventions to better understand the most effective mechanism impacting change in the primary outcome.
Applications for Clinical Practice
Results of this study demonstrated that among participants being treated with hypertension, those engaged with the HOME BP digital intervention (combining self-monitoring of blood pressure with guided self-management) had better control of systolic BP after 1 year compared to participants receiving usual care. While these findings have important implications in the management of hypertension in health care systems, its integration into clinical workflow, sustainability, long-term clinical effectiveness, and effectiveness among diverse populations is unclear. However, clinicians can still encourage and support the use of evidence-based digital tools for patient self-monitoring of BP and guided-management of lifestyle modifications to lower BP. Additionally, clinicians can proactively propose incorporating evidence-based digital interventions like HOME BP into routine clinical practice guidelines.
Financial disclosures: None.
1. Samadian F, Dalili N, Jamalian A. Lifestyle Modifications to Prevent and Control Hypertension. Iran J Kidney Dis. 2016;10(5):237-263.
2. McLean G, Band R, Saunderson K, et al. Digital interventions to promote self-management in adults with hypertension systematic review and meta-analysis. J Hypertens. 2016;34(4):600-612. doi:10.1097/HJH.0000000000000859
3. Bodenheimer T, Lorig K, Holman H, Grumbach K. Patient self-management of chronic disease in primary care. JAMA. 2002 Nov 20;288(19):2469-2475. doi:10.1001/jama.288.19.2469
4. Morton K, Dennison L, May C, et al. Using digital interventions for self-management of chronic physical health conditions: A meta-ethnography review of published studies. Patient Educ Couns. 2017;100(4):616-635. doi:10.1016/j.ped.2016.10.019
5. Kario K. Management of Hypertension in the Digital Era: Small Wearable Monitoring Devices for Remote Blood Pressure Monitoring. Hypertension. 2020;76(3):640-650. doi:10.1161/HYPERTENSIONAHA.120.14742
6. Murray E, Burns J, See TS, et al. Interactive Health Communication Applications for people with chronic disease. Cochrane Database Syst Rev. 2005;(4):CD004274. doi:10.1002/14651858.CD004274.pub4
7. Webb TL, Joseph J, Yardley L, Michie S. Using the internet to promote health behavior change: a systematic review and meta-analysis of the impact of theoretical basis, use of behavior change techniques, and mode of delivery on efficacy. J Med Internet Res. 2010;12(1):e4. doi:10.2196/jmir.1376
Study Overview
Objective. To evaluate whether a digital intervention comprising self-monitoring of blood pressure (BP) with reminders and predetermined drug changes combined with lifestyle change support resulted in lower systolic BP in people receiving treatment for hypertension that was poorly controlled, and whether this approach was cost effective.
Design. Unmasked randomized controlled trial.
Settings and participants. Eligible participants were identified from clinical codes recorded in the electronic health records of 76 collaborating general practices from the National Institute for Health Research Clinical Research Network, a United Kingdom government agency. The practices sent invitation letters to eligible participants to come to the clinic to establish eligibility, take consent, and collect baseline data via online questionnaires.
Eligible participants were aged 18 years or older with treated hypertension, a mean baseline BP reading of more than 140/90 mm Hg and were taking no more than 3 antihypertensive drugs. Participants also needed to be willing to self-monitor and have access to the internet (with support from a family member if needed). Exclusions included BP greater than 180/110 mm Hg, atrial fibrillation, hypertension not managed by their general practitioner, chronic kidney disease stage 4-5, postural hypotension (> 20 mm Hg systolic drop), an acute cardiovascular event in the previous 3 months, terminal disease, or another condition which in the opinion of their general practitioner made participation inappropriate.
Of the 11 399 invitation letters sent out, 1389 (12%) potential participants responded positively and were screened for eligibility. Those who declined to take part could optionally give their reasons, and responses were gained from 2426 of 10 010 (24%). The mean age of those who gave a reason for declining was 73 years. The most commonly selected reasons for declining were not having access to the internet (982, 41%), not wanting to participate in a research trial (617, 25%) or an internet study (543, 22%), and not wanting to change drugs (535, 22%). Of the 1389 screened, 734 were ineligible, and 33 did not complete baseline measures and randomization. The remaining 622 people who were randomized in a 1:1 ratio to receive the HOME BP intervention (n = 305) or usual care (n = 317).
Intervention vs usual care. The HOME BP intervention for the self-management of high BP consisted of an integrated patient and health care practitioner online digital intervention, BP self-monitoring (using an Omron M3 monitor), health care practitioner directed and supervised titration of antihypertensive drugs, and user-selected lifestyle modifications. Participants were advised via automated email reminders to take 2 morning BP readings for 7 days each month and to enter online each second reading. Mean home BP was calculated, accompanied by feedback of BP results to both patients and professionals with optional evidence-based lifestyle advice (for healthy eating, physical activity, losing weight if appropriate, and salt and alcohol reduction) and motivational support through practice nurses or health care assistances (using the CARE approach – congratulate, ask, reassure, encourage).
Participants allocated to usual care were not provided with self-monitoring equipment or the HOME BP intervention but had online access to the information provided in a patient leaflet for hypertension. This information comprised definitions of hypertension, causes, and brief guidance on treatment, including lifestyle changes and drugs. These participants received routine hypertension care that typically consisted of clinic BP monitoring to titrate drugs, with appointments and drug changes made at the discretion of the general practitioner. Participants were not prevented from self-monitoring, but data on self-monitoring practices were collected at the end of the trial from patients and practitioners.
Measures and analysis. The primary outcome measure was the difference in systolic BP at 12-month follow-up between the intervention and usual care groups (adjusting for baseline BP, practice, BP target levels, and sex). Secondary outcomes included systolic and diastolic BP at 6 and 12 months, weight, modified patient enablement instrument, drug adherence, health-related quality of life, and side effects from the symptoms section of an adjusted illness perceptions questionnaire. At trial, registration participants and general practitioners were asked about their use of self-monitoring in the usual care group.
The primary analysis used general linear modelling to compare systolic BP in the intervention and usual care groups at follow-up, adjusting for baseline BP, practice (as a random effect to take into account clustering), BP target levels, and sex. Analyses were on an intention-to-treat basis and used multiple imputation for missing data. Sensitivity analyses used complete cases and a repeated measures technique. Secondary analyses used similar techniques to assess differences between groups. A within-trial economic analysis estimated cost per unit reduction in systolic BP by using similar adjustments and multiple imputation for missing values. Repeated bootstrapping was used to estimate the probability of the intervention being cost-effective at different levels of willingness to pay per unit reduction in BP.
Main results. The intervention and usual care groups did not differ significantly – participants had a mean age of 66 years and mean baseline clinical BP of 151.6/85.3 mm Hg and 151.7/86.4 mm Hg (usual care and intervention, respectively). Most participants were White British (94%), just more than half were men, and the time since diagnosis averaged around 11 years. The most deprived group (based on the English Index of Multiple Deprivation) accounted for 63/622 (10%), with the least deprived group accounting for 326/622 (52%).
After 1 year, data were available from 552 participants (88.6%) with imputation for the remaining 70 participants (11.4%). Mean BP dropped from 151.7/86.4 to 138.4/80.2 mm Hg in the intervention group and from 151.6/85.3 to 141.8/79.8 mm Hg in the usual care group, giving a mean difference in systolic BP of −3.4 mm Hg (95% CI −6.1 to −0.8 mm Hg) and a mean difference in diastolic BP of −0.5 mm Hg (−1.9 to 0.9 mm Hg). Exploratory subgroup analyses suggested that participants aged 67 years or older had a smaller effect size than those younger than 67. Similarly, while the effect sizes in the standard and diabetes target groups were similar, those older than 80 years with a higher target of 145/85 mm Hg showed little evidence of benefit. Results for other subgroups, including sex, baseline BP, deprivation, and history of self-monitoring, were similar between groups.
Engagement with the digital intervention was high, with 281/305 (92%) participants completing the 2 core training sessions, 268/305 (88%) completing a week of practice BP readings, and 243/305 (80%) completing at least 3 weeks of BP entries. Furthermore, 214/305 (70%) were still monitoring in the last 3 months of participation. However, less than 1/3 of participants chose to register on 1 of the optional lifestyle change modules. In the usual care group, a post-hoc analysis after 12 months showed that 112/234 (47%) patients reported monitoring their own BP at home at least once per month during the trial.
The difference in mean cost per patient was £38 (US $51.30, €41.9; 95% CI £27 to £47), which along with the decrease in systolic BP, gave an incremental cost per mm Hg BP reduction of £11 (£6 to £29). Bootstrapping analysis showed the intervention had high (90%) probability of being cost-effective at willingness to pay above £20 per unit reduction. The probabilities of being cost-effective for the intervention against usual care were 87%, 93%, and 97% at thresholds of £20, £30, and £50, respectively.
Conclusion. The HOME BP digital intervention for the management of hypertension by using self-monitored BP led to better control of systolic BP after 1 year than usual care, with low incremental costs. Implementation in primary care will require integration into clinical workflows and consideration of people who are digitally excluded.
Commentary
Elevated BP, also known as hypertension, is the most important, modifiable risk factor for cardiovascular disease and mortality.1 Clinically significant effects and improvements in mortality can be achieved with relatively small reductions in BP levels. Long-established lifestyle modifications that effectively lower BP include weight loss, reduced sodium intake, increased physical activity, and limited alcohol intake. However, motivating patients to achieve lifestyle modifications is among the most difficult aspects of managing hypertension. Importantly, for individuals taking antihypertensive medication, lifestyle modification is recommended as adjunctive therapy to reduce BP. Given that target blood pressure levels are reached for less than half of adults, novel interventions are needed to improve BP control – in particular, individualized cognitive behavioral interventions are more likely to be effective than standardized, single-component interventions.
Guided self-management for hypertension as part of systematic, planned care offers the potential for improvements in adherence and in turn improved long-term patient outcomes.2 Self-management can encompass a wide range of behaviors in addition to medication titration and monitoring of symptoms, such as individuals’ ability to manage physical, psychosocial and lifestyle behaviors related to their condition.3 Digital interventions leveraging apps, software, and/or technologies in particular have the potential to support people in self-management, allow for remote monitoring, and enable personalized and adaptive strategies for chronic disease management.4-5 An example of a digital intervention in the context of guided self-management for hypertension can be a web-based program delivered by computer or phone that combines health information with decision support to help inform behavior change in patients and remote monitoring of patient status by health professionals. Well-designed digital interventions can effectively change patient health-related behaviors, improve patient knowledge and confidence for self-management of health, and lead to better health outcomes.6-7
This study adds to the literature as a large, randomized controlled trial evaluating the effectiveness of a digital intervention in the field of hypertension and with follow-up for a year. The authors highlight that relatively few studies have been performed that combine self-monitoring with a digitally delivered cointervention, and none has shown a major effect in an adequately powered trial over a year. Results from this study showed that HOME BP, a digital intervention enabling self-management of hypertension, including self-monitoring, titration based on self-monitored BP, lifestyle advice, and behavioral support for patients and health care professionals, resulted in a worthwhile reduction of systolic BP. In addition, this reduction was achieved at modest cost based on the within trial cost effectiveness analysis.
There are many important strengths of this study, especially related to the design and analysis strategy, and some limitations. This study was designed as a randomized controlled trial with a 1 year follow-up period, although participants were unmasked to the group they were randomized to, which may have impacted their behaviors while in the study. As the authors state, the study was not only adequately powered to detect a difference in blood pressure, but also over-recruitment ensured such an effect was not missed. Recruiting from a large number of general practices ensured generalizability in terms of health care professionals. Importantly, while study participants mostly identified as predominantly White and tended to be of higher socioeconomic status, this is representative of the aged population in England and Wales. Nevertheless, generalizability of findings from this study is still limited to the demographic characteristics of the study population. Other strengths included inclusion of intention-to-treat analysis, multiple imputation for missing data, sensitivity analysis, as well as economic analysis and cost effectiveness analysis.
Of note, results from the study are only attributable to the digital interventions used in this study (digital web-based with limited mechanisms of behavior change and engagement built-in) and thus should not be generalized to all digital interventions for managing hypertension. Also, as the authors highlight, the relative importance of the different parts of the digital intervention were unable to be distinguished, although this type of analysis is important in multicomponent interventions to better understand the most effective mechanism impacting change in the primary outcome.
Applications for Clinical Practice
Results of this study demonstrated that among participants being treated with hypertension, those engaged with the HOME BP digital intervention (combining self-monitoring of blood pressure with guided self-management) had better control of systolic BP after 1 year compared to participants receiving usual care. While these findings have important implications in the management of hypertension in health care systems, its integration into clinical workflow, sustainability, long-term clinical effectiveness, and effectiveness among diverse populations is unclear. However, clinicians can still encourage and support the use of evidence-based digital tools for patient self-monitoring of BP and guided-management of lifestyle modifications to lower BP. Additionally, clinicians can proactively propose incorporating evidence-based digital interventions like HOME BP into routine clinical practice guidelines.
Financial disclosures: None.
Study Overview
Objective. To evaluate whether a digital intervention comprising self-monitoring of blood pressure (BP) with reminders and predetermined drug changes combined with lifestyle change support resulted in lower systolic BP in people receiving treatment for hypertension that was poorly controlled, and whether this approach was cost effective.
Design. Unmasked randomized controlled trial.
Settings and participants. Eligible participants were identified from clinical codes recorded in the electronic health records of 76 collaborating general practices from the National Institute for Health Research Clinical Research Network, a United Kingdom government agency. The practices sent invitation letters to eligible participants to come to the clinic to establish eligibility, take consent, and collect baseline data via online questionnaires.
Eligible participants were aged 18 years or older with treated hypertension, a mean baseline BP reading of more than 140/90 mm Hg and were taking no more than 3 antihypertensive drugs. Participants also needed to be willing to self-monitor and have access to the internet (with support from a family member if needed). Exclusions included BP greater than 180/110 mm Hg, atrial fibrillation, hypertension not managed by their general practitioner, chronic kidney disease stage 4-5, postural hypotension (> 20 mm Hg systolic drop), an acute cardiovascular event in the previous 3 months, terminal disease, or another condition which in the opinion of their general practitioner made participation inappropriate.
Of the 11 399 invitation letters sent out, 1389 (12%) potential participants responded positively and were screened for eligibility. Those who declined to take part could optionally give their reasons, and responses were gained from 2426 of 10 010 (24%). The mean age of those who gave a reason for declining was 73 years. The most commonly selected reasons for declining were not having access to the internet (982, 41%), not wanting to participate in a research trial (617, 25%) or an internet study (543, 22%), and not wanting to change drugs (535, 22%). Of the 1389 screened, 734 were ineligible, and 33 did not complete baseline measures and randomization. The remaining 622 people who were randomized in a 1:1 ratio to receive the HOME BP intervention (n = 305) or usual care (n = 317).
Intervention vs usual care. The HOME BP intervention for the self-management of high BP consisted of an integrated patient and health care practitioner online digital intervention, BP self-monitoring (using an Omron M3 monitor), health care practitioner directed and supervised titration of antihypertensive drugs, and user-selected lifestyle modifications. Participants were advised via automated email reminders to take 2 morning BP readings for 7 days each month and to enter online each second reading. Mean home BP was calculated, accompanied by feedback of BP results to both patients and professionals with optional evidence-based lifestyle advice (for healthy eating, physical activity, losing weight if appropriate, and salt and alcohol reduction) and motivational support through practice nurses or health care assistances (using the CARE approach – congratulate, ask, reassure, encourage).
Participants allocated to usual care were not provided with self-monitoring equipment or the HOME BP intervention but had online access to the information provided in a patient leaflet for hypertension. This information comprised definitions of hypertension, causes, and brief guidance on treatment, including lifestyle changes and drugs. These participants received routine hypertension care that typically consisted of clinic BP monitoring to titrate drugs, with appointments and drug changes made at the discretion of the general practitioner. Participants were not prevented from self-monitoring, but data on self-monitoring practices were collected at the end of the trial from patients and practitioners.
Measures and analysis. The primary outcome measure was the difference in systolic BP at 12-month follow-up between the intervention and usual care groups (adjusting for baseline BP, practice, BP target levels, and sex). Secondary outcomes included systolic and diastolic BP at 6 and 12 months, weight, modified patient enablement instrument, drug adherence, health-related quality of life, and side effects from the symptoms section of an adjusted illness perceptions questionnaire. At trial, registration participants and general practitioners were asked about their use of self-monitoring in the usual care group.
The primary analysis used general linear modelling to compare systolic BP in the intervention and usual care groups at follow-up, adjusting for baseline BP, practice (as a random effect to take into account clustering), BP target levels, and sex. Analyses were on an intention-to-treat basis and used multiple imputation for missing data. Sensitivity analyses used complete cases and a repeated measures technique. Secondary analyses used similar techniques to assess differences between groups. A within-trial economic analysis estimated cost per unit reduction in systolic BP by using similar adjustments and multiple imputation for missing values. Repeated bootstrapping was used to estimate the probability of the intervention being cost-effective at different levels of willingness to pay per unit reduction in BP.
Main results. The intervention and usual care groups did not differ significantly – participants had a mean age of 66 years and mean baseline clinical BP of 151.6/85.3 mm Hg and 151.7/86.4 mm Hg (usual care and intervention, respectively). Most participants were White British (94%), just more than half were men, and the time since diagnosis averaged around 11 years. The most deprived group (based on the English Index of Multiple Deprivation) accounted for 63/622 (10%), with the least deprived group accounting for 326/622 (52%).
After 1 year, data were available from 552 participants (88.6%) with imputation for the remaining 70 participants (11.4%). Mean BP dropped from 151.7/86.4 to 138.4/80.2 mm Hg in the intervention group and from 151.6/85.3 to 141.8/79.8 mm Hg in the usual care group, giving a mean difference in systolic BP of −3.4 mm Hg (95% CI −6.1 to −0.8 mm Hg) and a mean difference in diastolic BP of −0.5 mm Hg (−1.9 to 0.9 mm Hg). Exploratory subgroup analyses suggested that participants aged 67 years or older had a smaller effect size than those younger than 67. Similarly, while the effect sizes in the standard and diabetes target groups were similar, those older than 80 years with a higher target of 145/85 mm Hg showed little evidence of benefit. Results for other subgroups, including sex, baseline BP, deprivation, and history of self-monitoring, were similar between groups.
Engagement with the digital intervention was high, with 281/305 (92%) participants completing the 2 core training sessions, 268/305 (88%) completing a week of practice BP readings, and 243/305 (80%) completing at least 3 weeks of BP entries. Furthermore, 214/305 (70%) were still monitoring in the last 3 months of participation. However, less than 1/3 of participants chose to register on 1 of the optional lifestyle change modules. In the usual care group, a post-hoc analysis after 12 months showed that 112/234 (47%) patients reported monitoring their own BP at home at least once per month during the trial.
The difference in mean cost per patient was £38 (US $51.30, €41.9; 95% CI £27 to £47), which along with the decrease in systolic BP, gave an incremental cost per mm Hg BP reduction of £11 (£6 to £29). Bootstrapping analysis showed the intervention had high (90%) probability of being cost-effective at willingness to pay above £20 per unit reduction. The probabilities of being cost-effective for the intervention against usual care were 87%, 93%, and 97% at thresholds of £20, £30, and £50, respectively.
Conclusion. The HOME BP digital intervention for the management of hypertension by using self-monitored BP led to better control of systolic BP after 1 year than usual care, with low incremental costs. Implementation in primary care will require integration into clinical workflows and consideration of people who are digitally excluded.
Commentary
Elevated BP, also known as hypertension, is the most important, modifiable risk factor for cardiovascular disease and mortality.1 Clinically significant effects and improvements in mortality can be achieved with relatively small reductions in BP levels. Long-established lifestyle modifications that effectively lower BP include weight loss, reduced sodium intake, increased physical activity, and limited alcohol intake. However, motivating patients to achieve lifestyle modifications is among the most difficult aspects of managing hypertension. Importantly, for individuals taking antihypertensive medication, lifestyle modification is recommended as adjunctive therapy to reduce BP. Given that target blood pressure levels are reached for less than half of adults, novel interventions are needed to improve BP control – in particular, individualized cognitive behavioral interventions are more likely to be effective than standardized, single-component interventions.
Guided self-management for hypertension as part of systematic, planned care offers the potential for improvements in adherence and in turn improved long-term patient outcomes.2 Self-management can encompass a wide range of behaviors in addition to medication titration and monitoring of symptoms, such as individuals’ ability to manage physical, psychosocial and lifestyle behaviors related to their condition.3 Digital interventions leveraging apps, software, and/or technologies in particular have the potential to support people in self-management, allow for remote monitoring, and enable personalized and adaptive strategies for chronic disease management.4-5 An example of a digital intervention in the context of guided self-management for hypertension can be a web-based program delivered by computer or phone that combines health information with decision support to help inform behavior change in patients and remote monitoring of patient status by health professionals. Well-designed digital interventions can effectively change patient health-related behaviors, improve patient knowledge and confidence for self-management of health, and lead to better health outcomes.6-7
This study adds to the literature as a large, randomized controlled trial evaluating the effectiveness of a digital intervention in the field of hypertension and with follow-up for a year. The authors highlight that relatively few studies have been performed that combine self-monitoring with a digitally delivered cointervention, and none has shown a major effect in an adequately powered trial over a year. Results from this study showed that HOME BP, a digital intervention enabling self-management of hypertension, including self-monitoring, titration based on self-monitored BP, lifestyle advice, and behavioral support for patients and health care professionals, resulted in a worthwhile reduction of systolic BP. In addition, this reduction was achieved at modest cost based on the within trial cost effectiveness analysis.
There are many important strengths of this study, especially related to the design and analysis strategy, and some limitations. This study was designed as a randomized controlled trial with a 1 year follow-up period, although participants were unmasked to the group they were randomized to, which may have impacted their behaviors while in the study. As the authors state, the study was not only adequately powered to detect a difference in blood pressure, but also over-recruitment ensured such an effect was not missed. Recruiting from a large number of general practices ensured generalizability in terms of health care professionals. Importantly, while study participants mostly identified as predominantly White and tended to be of higher socioeconomic status, this is representative of the aged population in England and Wales. Nevertheless, generalizability of findings from this study is still limited to the demographic characteristics of the study population. Other strengths included inclusion of intention-to-treat analysis, multiple imputation for missing data, sensitivity analysis, as well as economic analysis and cost effectiveness analysis.
Of note, results from the study are only attributable to the digital interventions used in this study (digital web-based with limited mechanisms of behavior change and engagement built-in) and thus should not be generalized to all digital interventions for managing hypertension. Also, as the authors highlight, the relative importance of the different parts of the digital intervention were unable to be distinguished, although this type of analysis is important in multicomponent interventions to better understand the most effective mechanism impacting change in the primary outcome.
Applications for Clinical Practice
Results of this study demonstrated that among participants being treated with hypertension, those engaged with the HOME BP digital intervention (combining self-monitoring of blood pressure with guided self-management) had better control of systolic BP after 1 year compared to participants receiving usual care. While these findings have important implications in the management of hypertension in health care systems, its integration into clinical workflow, sustainability, long-term clinical effectiveness, and effectiveness among diverse populations is unclear. However, clinicians can still encourage and support the use of evidence-based digital tools for patient self-monitoring of BP and guided-management of lifestyle modifications to lower BP. Additionally, clinicians can proactively propose incorporating evidence-based digital interventions like HOME BP into routine clinical practice guidelines.
Financial disclosures: None.
1. Samadian F, Dalili N, Jamalian A. Lifestyle Modifications to Prevent and Control Hypertension. Iran J Kidney Dis. 2016;10(5):237-263.
2. McLean G, Band R, Saunderson K, et al. Digital interventions to promote self-management in adults with hypertension systematic review and meta-analysis. J Hypertens. 2016;34(4):600-612. doi:10.1097/HJH.0000000000000859
3. Bodenheimer T, Lorig K, Holman H, Grumbach K. Patient self-management of chronic disease in primary care. JAMA. 2002 Nov 20;288(19):2469-2475. doi:10.1001/jama.288.19.2469
4. Morton K, Dennison L, May C, et al. Using digital interventions for self-management of chronic physical health conditions: A meta-ethnography review of published studies. Patient Educ Couns. 2017;100(4):616-635. doi:10.1016/j.ped.2016.10.019
5. Kario K. Management of Hypertension in the Digital Era: Small Wearable Monitoring Devices for Remote Blood Pressure Monitoring. Hypertension. 2020;76(3):640-650. doi:10.1161/HYPERTENSIONAHA.120.14742
6. Murray E, Burns J, See TS, et al. Interactive Health Communication Applications for people with chronic disease. Cochrane Database Syst Rev. 2005;(4):CD004274. doi:10.1002/14651858.CD004274.pub4
7. Webb TL, Joseph J, Yardley L, Michie S. Using the internet to promote health behavior change: a systematic review and meta-analysis of the impact of theoretical basis, use of behavior change techniques, and mode of delivery on efficacy. J Med Internet Res. 2010;12(1):e4. doi:10.2196/jmir.1376
1. Samadian F, Dalili N, Jamalian A. Lifestyle Modifications to Prevent and Control Hypertension. Iran J Kidney Dis. 2016;10(5):237-263.
2. McLean G, Band R, Saunderson K, et al. Digital interventions to promote self-management in adults with hypertension systematic review and meta-analysis. J Hypertens. 2016;34(4):600-612. doi:10.1097/HJH.0000000000000859
3. Bodenheimer T, Lorig K, Holman H, Grumbach K. Patient self-management of chronic disease in primary care. JAMA. 2002 Nov 20;288(19):2469-2475. doi:10.1001/jama.288.19.2469
4. Morton K, Dennison L, May C, et al. Using digital interventions for self-management of chronic physical health conditions: A meta-ethnography review of published studies. Patient Educ Couns. 2017;100(4):616-635. doi:10.1016/j.ped.2016.10.019
5. Kario K. Management of Hypertension in the Digital Era: Small Wearable Monitoring Devices for Remote Blood Pressure Monitoring. Hypertension. 2020;76(3):640-650. doi:10.1161/HYPERTENSIONAHA.120.14742
6. Murray E, Burns J, See TS, et al. Interactive Health Communication Applications for people with chronic disease. Cochrane Database Syst Rev. 2005;(4):CD004274. doi:10.1002/14651858.CD004274.pub4
7. Webb TL, Joseph J, Yardley L, Michie S. Using the internet to promote health behavior change: a systematic review and meta-analysis of the impact of theoretical basis, use of behavior change techniques, and mode of delivery on efficacy. J Med Internet Res. 2010;12(1):e4. doi:10.2196/jmir.1376
Preoperative Advance Care Planning for Older Adults Undergoing High-Risk Surgery: An Essential but Underutilized Aspect of Clinical Care
Study Overview
Objective. The objectives of this study were to (1) quantify the frequency of preoperative advance care planning (ACP) discussion and documentation for older adults undergoing major surgery in a national sample, and (2) characterize how surgical patients and their family members considered ACP after postoperative complications.
Design. A secondary analysis of data from a multisite randomized clinical trial testing the effects of a question prompt list intervention (a Question Problem List [QPL] brochure with 11 questions) given to patients aged 60 years or older undergoing high-risk surgery on preoperative communication with their surgeons.
Setting and participants. This multisite randomized controlled trial involved 5 study sites that encompassed distinct US geographic areas, including University of Wisconsin Hospital and Clinics (UWHC), Madison; the University of California, San Francisco, Medical Center (UCSF); Oregon Health & Science University (OHSU), Portland; the University Hospital of Rutgers New Jersey Medical School (Rutgers), Newark; and the Brigham and Women’s Hospital (BWH), Boston, Massachusetts. The study enrolled 40 surgeons who routinely performed high-risk oncological or vascular surgery via purposeful sampling; patients aged 60 years or older with at least 1 comorbidity and an oncological or vascular problem that were treatable with high-risk surgery; and 1 invited family member per enrolled patient to participate in open-ended interviews postsurgery. High-risk surgery was defined as an operation that has a 30-day in-hospital mortality rate greater than or equal to 1%. Data were collected from June 1, 2016, to November 30, 2018.
Main outcome measures. The frequency of preoperative discussions and documentation of ACP was determined. For patients who had major surgery, any mention of ACP (ie, mention of advance directive [AD], health care power of attorney, or preference for limitations of life-sustaining treatments) by the surgeon, patient or family member during the audio recorded, transcribed, and coded preoperative consultation was counted. The presence of a written AD in the medical record at the time of the initial consultation, filed between the consultation and the date of surgery, or added postoperatively, was recorded using a standardized abstraction form. Postoperative treatments administered and complications experienced within 6 weeks after surgery were recorded. Open-ended interviews with patients who experienced significant postoperative complications (eg, prolonged hospitalization > 8 days, intensive care unit stay > 3 days) and their family members were conducted 6 weeks after surgery. Information ascertained during interviews focused on treatment decisions, postoperative experiences, and interpersonal relationships among patients, families, and clinicians. Transcripts of these interviews were then subjected to qualitative content analysis.
Main results. A total of 446 patients were enrolled in the primary study. Of these patients, 213 (122 men [57%]; 91 women [43%]; mean [SD] age, 72 [7] years) underwent major surgery. Only 13 (6.1%) of those who had major surgery had any discussion related to ACP in the preoperative consultation. In this cohort, 141 (66%) patients did not have an AD on file before undergoing major surgery. The presence of AD was not associated with age (60-69 years, 26 [31%]; 70-79 years, 31 [33%]; ≥ 80 years, 15 [42%]; P = .55), number of comorbidities (1, 35 [32%]; 2, 18 [33%]; ≥ 3, 19 [40%]; P = .62), or type of procedure (oncological, 53 [32%]; vascular, 19 [42%]; P = .22). Moreover, there was no difference in preoperative communication about ACP or documentation of an AD for patients who were mailed a QPL brochure compared to those who received usual care (intervention, 38 [35%]; usual care, 34 [33%]; P = .77). Rates of AD documentation were associated with individual study sites with BWH and UWHC having higher rates of documentation (20 [50%] and 27 [44%], respectively) compared to OHSU, UCSF, or Rutgers (7 [17%], 17 [35%], and 1 [5%], respectively). Analysis from the interviews indicated that patients and families felt unprepared for serious surgical complications and had varied interpretations of ACP. Patients with complications were enthusiastic about ACP but did not think it was important to discuss their preferences for life-sustaining treatments with their surgeon preoperatively.
Conclusion. Although surgeons and patients report that they believe ACP is important, preoperative discussion of patient preferences rarely occurs. This study found that the frequency of ACP discussions or AD documentations among older patients undergoing high-risk oncologic or vascular surgery was low. Interventions that are aimed to increase rates of preoperative ACP discussions should be implemented to help prepare patients and their families for difficult decisions in the setting of serious surgical complications and could help decrease postoperative conflicts that result from unclear patient care goals.
Commentary
Surgeons and patients approach surgical interventions with optimistic outlooks while simultaneously preparing for unintended adverse outcomes. For patients, preoperative ACP discussions ease the burden on their families and ensure their wishes and care goals are communicated. For surgeons, these discussions inform them how best to support the values of the patient. Therefore, it is unsurprising that preoperative ACP is viewed favorably by both groups. Given the consensus that ACP is important in the care of older adults undergoing high-risk surgery, one would assume that preoperative ACP discussion is a standard of practice among surgeons and their aging patients. However, in a secondary analysis of a randomized control trial testing a patient-mediated intervention to improve preoperative communication, Kalbfell et al1 showed that ACP discussions rarely take place prior to major surgery in older adults. This finding highlights the significant discrepancy between the belief that ACP is important, and the actual rate that it is practiced, in older patients undergoing high-risk surgery. This discordance is highly concerning because it suggests that surgeons who provide care to a very vulnerable subset of older patients may overlook an essential aspect of preoperative care and therefore lack a thorough and thoughtful understanding of the patient’s care goals. In practice, this omission can pose significant challenges associated with the surgeon and family’s decisions to use postoperative life-sustaining interventions or to manage unforeseen complications should a patient become unable to make medical decisions.
The barriers to conducting successful ACP discussions between surgeons and patients are multifactorial. Kalbfell et al1 highlighted several of these barriers, including lack of patient efficacy, physician attitudes, and institutional values in older adults who require major surgeries. The inadequacy of patient efficacy in preoperative ACP is illustrated by findings from the primary, multisite trial of QPL intervention conducted by Schwarze et al. Interestingly, the authors found that patients who did not receive QPL brochure had no ACP discussions, and that QPL implementation did not significantly improve discussion rates despite its intent to encourage these discussions.2 Possible explanations for this lack of engagement might be a lack of health literacy or patient efficacy in the study population. Qualitative data from the current study provided further evidence to support these explanations. For instance, some patients provided limited or incomplete information about their wishes for health care management while others felt it was unnecessary to have ACP discussions unless complications arose.1 However, the latter example counters the purpose of ACP which is to enable patients to make plans about future health care and not reactive to a medical complication or emergency.
Surgeons bear a large responsibility in providing treatments that are consistent with the care goals of the patient. Thus, surgeons play a crucial role in engaging, guiding, and facilitating ACP discussions with patients. This role is even more critical when patients are unable or unwilling to initiate care goal discussions. Physician attitudes towards ACP, therefore, greatly influence the effectiveness of these discussions. In a study of self-administered surveys by vascular, neurologic, and cardiothoracic surgeons, greater than 90% of respondents viewed postoperative life-supporting therapy as necessary, and 54% would decline to operate on patients with an AD limiting life-supporting therapy.3 Moreover, the same study showed that 52% of respondents reported discussing AD before surgery, a figure that exceeded the actual rates at which ACP discussions occur in many other studies. In the current study, Kalbfell et al1 also found that surgeons viewed ACP discussions largely in the context of AD creation and declined to investigate the full scope of patient preferences. These findings, when combined with other studies that indicate an incomplete understanding of ACP in some surgeons, suggest that not all physicians are able or willing to navigate these sometimes lengthy and difficult conversations with patients. This gap in practice provides opportunities for training in surgical specialties that center on optimizing preoperative ACP discussions to meet the care needs of older patients.
Institutional value and culture are important factors that impact physician behavior and the practice of ACP discussion. In the current study, the authors reported that the majority of ACP discussions were held by a minority of surgeons and that different institutions and study sites had vastly different rates of ACP documentation.1 These results are further supported by findings of large variations between physicians and hospitals in ACP reporting in hospitalized frail older adults.4 These variations in practices at different institutions suggest that it is possible to improve rates of preoperative ACP discussion. Reasons for these differences need to be further investigated in order to identify strategies, resources, or trainings required by medical institutions to support surgeons to carry out ACP discussions with patients undergoing high-risk surgeries.
The study conducted by Kalbfell et al1 has several strengths. For example, it included Spanish-speaking patients and the use of a Spanish version of the QPL intervention to account for cultural differences. The study also included multiple surgical specialties and institutions and captured a large and national sample, thus making its findings more generalizable. However, the lack of data on the duration of preoperative consultation visits in patients who completed ACP discussions poses a limitation to this study. This is relevant because surgeon availability to engage in lengthy ACP discussions may be limited due to busy clinical schedules. Additional data on the duration of preoperative visits inclusive of a thoughtfully conducted ACP discussion could help to modify clinical workflow to facilitate its uptake in surgical practices.
Applications for Clinical Practice
The findings from the current study indicate that patients and surgeons agree that preoperative ACP discussions are beneficial to the clinical care of older adults before high-risk surgeries. However, these important conversations do not occur frequently. Surgeons and health care institutions need to identify strategies to initiate, facilitate, and optimize productive preoperative ACP discussions to provide patient-centered care in vulnerable older surgical patients.
Financial disclosures: None.
1. Kalbfell E, Kata A, Buffington AS, et al. Frequency of Preoperative Advance Care Planning for Older Adults Undergoing High-risk Surgery: A Secondary Analysis of a Randomized Clinical Trial. JAMA Surg. 2021;156(7):e211521. doi:10.1001/jamasurg.2021.1521
2. Schwarze ML, Buffington A, Tucholka JL, et al. Effectiveness of a Question Prompt List Intervention for Older Patients Considering Major Surgery: A Multisite Randomized Clinical Trial. JAMA Surg. 2020;155(1):6-13. doi:10.1001/jamasurg.2019.3778
3. Redmann AJ, Brasel KJ, Alexander CG, Schwarze ML. Use of advance directives for high-risk operations: a national survey of surgeons. Ann Surgery. 2012;255(3):418-423. doi:10.1097/SLA.0b013e31823b6782
4. Hopkins SA, Bentley A, Phillips V, Barclay S. Advance care plans and hospitalized frail older adults: a systematic review. BMJ Support Palliat Care. 2020;10:164-174. doi:10.1136/bmjspcare-2019-002093
Study Overview
Objective. The objectives of this study were to (1) quantify the frequency of preoperative advance care planning (ACP) discussion and documentation for older adults undergoing major surgery in a national sample, and (2) characterize how surgical patients and their family members considered ACP after postoperative complications.
Design. A secondary analysis of data from a multisite randomized clinical trial testing the effects of a question prompt list intervention (a Question Problem List [QPL] brochure with 11 questions) given to patients aged 60 years or older undergoing high-risk surgery on preoperative communication with their surgeons.
Setting and participants. This multisite randomized controlled trial involved 5 study sites that encompassed distinct US geographic areas, including University of Wisconsin Hospital and Clinics (UWHC), Madison; the University of California, San Francisco, Medical Center (UCSF); Oregon Health & Science University (OHSU), Portland; the University Hospital of Rutgers New Jersey Medical School (Rutgers), Newark; and the Brigham and Women’s Hospital (BWH), Boston, Massachusetts. The study enrolled 40 surgeons who routinely performed high-risk oncological or vascular surgery via purposeful sampling; patients aged 60 years or older with at least 1 comorbidity and an oncological or vascular problem that were treatable with high-risk surgery; and 1 invited family member per enrolled patient to participate in open-ended interviews postsurgery. High-risk surgery was defined as an operation that has a 30-day in-hospital mortality rate greater than or equal to 1%. Data were collected from June 1, 2016, to November 30, 2018.
Main outcome measures. The frequency of preoperative discussions and documentation of ACP was determined. For patients who had major surgery, any mention of ACP (ie, mention of advance directive [AD], health care power of attorney, or preference for limitations of life-sustaining treatments) by the surgeon, patient or family member during the audio recorded, transcribed, and coded preoperative consultation was counted. The presence of a written AD in the medical record at the time of the initial consultation, filed between the consultation and the date of surgery, or added postoperatively, was recorded using a standardized abstraction form. Postoperative treatments administered and complications experienced within 6 weeks after surgery were recorded. Open-ended interviews with patients who experienced significant postoperative complications (eg, prolonged hospitalization > 8 days, intensive care unit stay > 3 days) and their family members were conducted 6 weeks after surgery. Information ascertained during interviews focused on treatment decisions, postoperative experiences, and interpersonal relationships among patients, families, and clinicians. Transcripts of these interviews were then subjected to qualitative content analysis.
Main results. A total of 446 patients were enrolled in the primary study. Of these patients, 213 (122 men [57%]; 91 women [43%]; mean [SD] age, 72 [7] years) underwent major surgery. Only 13 (6.1%) of those who had major surgery had any discussion related to ACP in the preoperative consultation. In this cohort, 141 (66%) patients did not have an AD on file before undergoing major surgery. The presence of AD was not associated with age (60-69 years, 26 [31%]; 70-79 years, 31 [33%]; ≥ 80 years, 15 [42%]; P = .55), number of comorbidities (1, 35 [32%]; 2, 18 [33%]; ≥ 3, 19 [40%]; P = .62), or type of procedure (oncological, 53 [32%]; vascular, 19 [42%]; P = .22). Moreover, there was no difference in preoperative communication about ACP or documentation of an AD for patients who were mailed a QPL brochure compared to those who received usual care (intervention, 38 [35%]; usual care, 34 [33%]; P = .77). Rates of AD documentation were associated with individual study sites with BWH and UWHC having higher rates of documentation (20 [50%] and 27 [44%], respectively) compared to OHSU, UCSF, or Rutgers (7 [17%], 17 [35%], and 1 [5%], respectively). Analysis from the interviews indicated that patients and families felt unprepared for serious surgical complications and had varied interpretations of ACP. Patients with complications were enthusiastic about ACP but did not think it was important to discuss their preferences for life-sustaining treatments with their surgeon preoperatively.
Conclusion. Although surgeons and patients report that they believe ACP is important, preoperative discussion of patient preferences rarely occurs. This study found that the frequency of ACP discussions or AD documentations among older patients undergoing high-risk oncologic or vascular surgery was low. Interventions that are aimed to increase rates of preoperative ACP discussions should be implemented to help prepare patients and their families for difficult decisions in the setting of serious surgical complications and could help decrease postoperative conflicts that result from unclear patient care goals.
Commentary
Surgeons and patients approach surgical interventions with optimistic outlooks while simultaneously preparing for unintended adverse outcomes. For patients, preoperative ACP discussions ease the burden on their families and ensure their wishes and care goals are communicated. For surgeons, these discussions inform them how best to support the values of the patient. Therefore, it is unsurprising that preoperative ACP is viewed favorably by both groups. Given the consensus that ACP is important in the care of older adults undergoing high-risk surgery, one would assume that preoperative ACP discussion is a standard of practice among surgeons and their aging patients. However, in a secondary analysis of a randomized control trial testing a patient-mediated intervention to improve preoperative communication, Kalbfell et al1 showed that ACP discussions rarely take place prior to major surgery in older adults. This finding highlights the significant discrepancy between the belief that ACP is important, and the actual rate that it is practiced, in older patients undergoing high-risk surgery. This discordance is highly concerning because it suggests that surgeons who provide care to a very vulnerable subset of older patients may overlook an essential aspect of preoperative care and therefore lack a thorough and thoughtful understanding of the patient’s care goals. In practice, this omission can pose significant challenges associated with the surgeon and family’s decisions to use postoperative life-sustaining interventions or to manage unforeseen complications should a patient become unable to make medical decisions.
The barriers to conducting successful ACP discussions between surgeons and patients are multifactorial. Kalbfell et al1 highlighted several of these barriers, including lack of patient efficacy, physician attitudes, and institutional values in older adults who require major surgeries. The inadequacy of patient efficacy in preoperative ACP is illustrated by findings from the primary, multisite trial of QPL intervention conducted by Schwarze et al. Interestingly, the authors found that patients who did not receive QPL brochure had no ACP discussions, and that QPL implementation did not significantly improve discussion rates despite its intent to encourage these discussions.2 Possible explanations for this lack of engagement might be a lack of health literacy or patient efficacy in the study population. Qualitative data from the current study provided further evidence to support these explanations. For instance, some patients provided limited or incomplete information about their wishes for health care management while others felt it was unnecessary to have ACP discussions unless complications arose.1 However, the latter example counters the purpose of ACP which is to enable patients to make plans about future health care and not reactive to a medical complication or emergency.
Surgeons bear a large responsibility in providing treatments that are consistent with the care goals of the patient. Thus, surgeons play a crucial role in engaging, guiding, and facilitating ACP discussions with patients. This role is even more critical when patients are unable or unwilling to initiate care goal discussions. Physician attitudes towards ACP, therefore, greatly influence the effectiveness of these discussions. In a study of self-administered surveys by vascular, neurologic, and cardiothoracic surgeons, greater than 90% of respondents viewed postoperative life-supporting therapy as necessary, and 54% would decline to operate on patients with an AD limiting life-supporting therapy.3 Moreover, the same study showed that 52% of respondents reported discussing AD before surgery, a figure that exceeded the actual rates at which ACP discussions occur in many other studies. In the current study, Kalbfell et al1 also found that surgeons viewed ACP discussions largely in the context of AD creation and declined to investigate the full scope of patient preferences. These findings, when combined with other studies that indicate an incomplete understanding of ACP in some surgeons, suggest that not all physicians are able or willing to navigate these sometimes lengthy and difficult conversations with patients. This gap in practice provides opportunities for training in surgical specialties that center on optimizing preoperative ACP discussions to meet the care needs of older patients.
Institutional value and culture are important factors that impact physician behavior and the practice of ACP discussion. In the current study, the authors reported that the majority of ACP discussions were held by a minority of surgeons and that different institutions and study sites had vastly different rates of ACP documentation.1 These results are further supported by findings of large variations between physicians and hospitals in ACP reporting in hospitalized frail older adults.4 These variations in practices at different institutions suggest that it is possible to improve rates of preoperative ACP discussion. Reasons for these differences need to be further investigated in order to identify strategies, resources, or trainings required by medical institutions to support surgeons to carry out ACP discussions with patients undergoing high-risk surgeries.
The study conducted by Kalbfell et al1 has several strengths. For example, it included Spanish-speaking patients and the use of a Spanish version of the QPL intervention to account for cultural differences. The study also included multiple surgical specialties and institutions and captured a large and national sample, thus making its findings more generalizable. However, the lack of data on the duration of preoperative consultation visits in patients who completed ACP discussions poses a limitation to this study. This is relevant because surgeon availability to engage in lengthy ACP discussions may be limited due to busy clinical schedules. Additional data on the duration of preoperative visits inclusive of a thoughtfully conducted ACP discussion could help to modify clinical workflow to facilitate its uptake in surgical practices.
Applications for Clinical Practice
The findings from the current study indicate that patients and surgeons agree that preoperative ACP discussions are beneficial to the clinical care of older adults before high-risk surgeries. However, these important conversations do not occur frequently. Surgeons and health care institutions need to identify strategies to initiate, facilitate, and optimize productive preoperative ACP discussions to provide patient-centered care in vulnerable older surgical patients.
Financial disclosures: None.
Study Overview
Objective. The objectives of this study were to (1) quantify the frequency of preoperative advance care planning (ACP) discussion and documentation for older adults undergoing major surgery in a national sample, and (2) characterize how surgical patients and their family members considered ACP after postoperative complications.
Design. A secondary analysis of data from a multisite randomized clinical trial testing the effects of a question prompt list intervention (a Question Problem List [QPL] brochure with 11 questions) given to patients aged 60 years or older undergoing high-risk surgery on preoperative communication with their surgeons.
Setting and participants. This multisite randomized controlled trial involved 5 study sites that encompassed distinct US geographic areas, including University of Wisconsin Hospital and Clinics (UWHC), Madison; the University of California, San Francisco, Medical Center (UCSF); Oregon Health & Science University (OHSU), Portland; the University Hospital of Rutgers New Jersey Medical School (Rutgers), Newark; and the Brigham and Women’s Hospital (BWH), Boston, Massachusetts. The study enrolled 40 surgeons who routinely performed high-risk oncological or vascular surgery via purposeful sampling; patients aged 60 years or older with at least 1 comorbidity and an oncological or vascular problem that were treatable with high-risk surgery; and 1 invited family member per enrolled patient to participate in open-ended interviews postsurgery. High-risk surgery was defined as an operation that has a 30-day in-hospital mortality rate greater than or equal to 1%. Data were collected from June 1, 2016, to November 30, 2018.
Main outcome measures. The frequency of preoperative discussions and documentation of ACP was determined. For patients who had major surgery, any mention of ACP (ie, mention of advance directive [AD], health care power of attorney, or preference for limitations of life-sustaining treatments) by the surgeon, patient or family member during the audio recorded, transcribed, and coded preoperative consultation was counted. The presence of a written AD in the medical record at the time of the initial consultation, filed between the consultation and the date of surgery, or added postoperatively, was recorded using a standardized abstraction form. Postoperative treatments administered and complications experienced within 6 weeks after surgery were recorded. Open-ended interviews with patients who experienced significant postoperative complications (eg, prolonged hospitalization > 8 days, intensive care unit stay > 3 days) and their family members were conducted 6 weeks after surgery. Information ascertained during interviews focused on treatment decisions, postoperative experiences, and interpersonal relationships among patients, families, and clinicians. Transcripts of these interviews were then subjected to qualitative content analysis.
Main results. A total of 446 patients were enrolled in the primary study. Of these patients, 213 (122 men [57%]; 91 women [43%]; mean [SD] age, 72 [7] years) underwent major surgery. Only 13 (6.1%) of those who had major surgery had any discussion related to ACP in the preoperative consultation. In this cohort, 141 (66%) patients did not have an AD on file before undergoing major surgery. The presence of AD was not associated with age (60-69 years, 26 [31%]; 70-79 years, 31 [33%]; ≥ 80 years, 15 [42%]; P = .55), number of comorbidities (1, 35 [32%]; 2, 18 [33%]; ≥ 3, 19 [40%]; P = .62), or type of procedure (oncological, 53 [32%]; vascular, 19 [42%]; P = .22). Moreover, there was no difference in preoperative communication about ACP or documentation of an AD for patients who were mailed a QPL brochure compared to those who received usual care (intervention, 38 [35%]; usual care, 34 [33%]; P = .77). Rates of AD documentation were associated with individual study sites with BWH and UWHC having higher rates of documentation (20 [50%] and 27 [44%], respectively) compared to OHSU, UCSF, or Rutgers (7 [17%], 17 [35%], and 1 [5%], respectively). Analysis from the interviews indicated that patients and families felt unprepared for serious surgical complications and had varied interpretations of ACP. Patients with complications were enthusiastic about ACP but did not think it was important to discuss their preferences for life-sustaining treatments with their surgeon preoperatively.
Conclusion. Although surgeons and patients report that they believe ACP is important, preoperative discussion of patient preferences rarely occurs. This study found that the frequency of ACP discussions or AD documentations among older patients undergoing high-risk oncologic or vascular surgery was low. Interventions that are aimed to increase rates of preoperative ACP discussions should be implemented to help prepare patients and their families for difficult decisions in the setting of serious surgical complications and could help decrease postoperative conflicts that result from unclear patient care goals.
Commentary
Surgeons and patients approach surgical interventions with optimistic outlooks while simultaneously preparing for unintended adverse outcomes. For patients, preoperative ACP discussions ease the burden on their families and ensure their wishes and care goals are communicated. For surgeons, these discussions inform them how best to support the values of the patient. Therefore, it is unsurprising that preoperative ACP is viewed favorably by both groups. Given the consensus that ACP is important in the care of older adults undergoing high-risk surgery, one would assume that preoperative ACP discussion is a standard of practice among surgeons and their aging patients. However, in a secondary analysis of a randomized control trial testing a patient-mediated intervention to improve preoperative communication, Kalbfell et al1 showed that ACP discussions rarely take place prior to major surgery in older adults. This finding highlights the significant discrepancy between the belief that ACP is important, and the actual rate that it is practiced, in older patients undergoing high-risk surgery. This discordance is highly concerning because it suggests that surgeons who provide care to a very vulnerable subset of older patients may overlook an essential aspect of preoperative care and therefore lack a thorough and thoughtful understanding of the patient’s care goals. In practice, this omission can pose significant challenges associated with the surgeon and family’s decisions to use postoperative life-sustaining interventions or to manage unforeseen complications should a patient become unable to make medical decisions.
The barriers to conducting successful ACP discussions between surgeons and patients are multifactorial. Kalbfell et al1 highlighted several of these barriers, including lack of patient efficacy, physician attitudes, and institutional values in older adults who require major surgeries. The inadequacy of patient efficacy in preoperative ACP is illustrated by findings from the primary, multisite trial of QPL intervention conducted by Schwarze et al. Interestingly, the authors found that patients who did not receive QPL brochure had no ACP discussions, and that QPL implementation did not significantly improve discussion rates despite its intent to encourage these discussions.2 Possible explanations for this lack of engagement might be a lack of health literacy or patient efficacy in the study population. Qualitative data from the current study provided further evidence to support these explanations. For instance, some patients provided limited or incomplete information about their wishes for health care management while others felt it was unnecessary to have ACP discussions unless complications arose.1 However, the latter example counters the purpose of ACP which is to enable patients to make plans about future health care and not reactive to a medical complication or emergency.
Surgeons bear a large responsibility in providing treatments that are consistent with the care goals of the patient. Thus, surgeons play a crucial role in engaging, guiding, and facilitating ACP discussions with patients. This role is even more critical when patients are unable or unwilling to initiate care goal discussions. Physician attitudes towards ACP, therefore, greatly influence the effectiveness of these discussions. In a study of self-administered surveys by vascular, neurologic, and cardiothoracic surgeons, greater than 90% of respondents viewed postoperative life-supporting therapy as necessary, and 54% would decline to operate on patients with an AD limiting life-supporting therapy.3 Moreover, the same study showed that 52% of respondents reported discussing AD before surgery, a figure that exceeded the actual rates at which ACP discussions occur in many other studies. In the current study, Kalbfell et al1 also found that surgeons viewed ACP discussions largely in the context of AD creation and declined to investigate the full scope of patient preferences. These findings, when combined with other studies that indicate an incomplete understanding of ACP in some surgeons, suggest that not all physicians are able or willing to navigate these sometimes lengthy and difficult conversations with patients. This gap in practice provides opportunities for training in surgical specialties that center on optimizing preoperative ACP discussions to meet the care needs of older patients.
Institutional value and culture are important factors that impact physician behavior and the practice of ACP discussion. In the current study, the authors reported that the majority of ACP discussions were held by a minority of surgeons and that different institutions and study sites had vastly different rates of ACP documentation.1 These results are further supported by findings of large variations between physicians and hospitals in ACP reporting in hospitalized frail older adults.4 These variations in practices at different institutions suggest that it is possible to improve rates of preoperative ACP discussion. Reasons for these differences need to be further investigated in order to identify strategies, resources, or trainings required by medical institutions to support surgeons to carry out ACP discussions with patients undergoing high-risk surgeries.
The study conducted by Kalbfell et al1 has several strengths. For example, it included Spanish-speaking patients and the use of a Spanish version of the QPL intervention to account for cultural differences. The study also included multiple surgical specialties and institutions and captured a large and national sample, thus making its findings more generalizable. However, the lack of data on the duration of preoperative consultation visits in patients who completed ACP discussions poses a limitation to this study. This is relevant because surgeon availability to engage in lengthy ACP discussions may be limited due to busy clinical schedules. Additional data on the duration of preoperative visits inclusive of a thoughtfully conducted ACP discussion could help to modify clinical workflow to facilitate its uptake in surgical practices.
Applications for Clinical Practice
The findings from the current study indicate that patients and surgeons agree that preoperative ACP discussions are beneficial to the clinical care of older adults before high-risk surgeries. However, these important conversations do not occur frequently. Surgeons and health care institutions need to identify strategies to initiate, facilitate, and optimize productive preoperative ACP discussions to provide patient-centered care in vulnerable older surgical patients.
Financial disclosures: None.
1. Kalbfell E, Kata A, Buffington AS, et al. Frequency of Preoperative Advance Care Planning for Older Adults Undergoing High-risk Surgery: A Secondary Analysis of a Randomized Clinical Trial. JAMA Surg. 2021;156(7):e211521. doi:10.1001/jamasurg.2021.1521
2. Schwarze ML, Buffington A, Tucholka JL, et al. Effectiveness of a Question Prompt List Intervention for Older Patients Considering Major Surgery: A Multisite Randomized Clinical Trial. JAMA Surg. 2020;155(1):6-13. doi:10.1001/jamasurg.2019.3778
3. Redmann AJ, Brasel KJ, Alexander CG, Schwarze ML. Use of advance directives for high-risk operations: a national survey of surgeons. Ann Surgery. 2012;255(3):418-423. doi:10.1097/SLA.0b013e31823b6782
4. Hopkins SA, Bentley A, Phillips V, Barclay S. Advance care plans and hospitalized frail older adults: a systematic review. BMJ Support Palliat Care. 2020;10:164-174. doi:10.1136/bmjspcare-2019-002093
1. Kalbfell E, Kata A, Buffington AS, et al. Frequency of Preoperative Advance Care Planning for Older Adults Undergoing High-risk Surgery: A Secondary Analysis of a Randomized Clinical Trial. JAMA Surg. 2021;156(7):e211521. doi:10.1001/jamasurg.2021.1521
2. Schwarze ML, Buffington A, Tucholka JL, et al. Effectiveness of a Question Prompt List Intervention for Older Patients Considering Major Surgery: A Multisite Randomized Clinical Trial. JAMA Surg. 2020;155(1):6-13. doi:10.1001/jamasurg.2019.3778
3. Redmann AJ, Brasel KJ, Alexander CG, Schwarze ML. Use of advance directives for high-risk operations: a national survey of surgeons. Ann Surgery. 2012;255(3):418-423. doi:10.1097/SLA.0b013e31823b6782
4. Hopkins SA, Bentley A, Phillips V, Barclay S. Advance care plans and hospitalized frail older adults: a systematic review. BMJ Support Palliat Care. 2020;10:164-174. doi:10.1136/bmjspcare-2019-002093
ACST-2: Carotid stenting, surgery on par in asymptomatic patients
Carotid artery stenting (CAS) and carotid endarterectomy (CEA) provided comparable outcomes over time in asymptomatic patients receiving good medical therapy in the largest trial to date of what to do with severe carotid artery narrowing that is yet to cause a stroke.
Among more than 3,600 patients, stenting and surgery performed by experienced physicians involved a 1.0% risk for causing disabling stroke or death within 30 days.
The annual rate of fatal or disabling strokes was about 0.5% with either procedure over an average 5 years’ follow-up – essentially halving the annual stroke risk had neither procedure been performed, according to Alison Halliday, MD, principal investigator of the Asymptomatic Carotid Surgery Trial-2 (ACST-2).
The results were reported Aug. 29 in a Hot Line session at the virtual annual congress of the European Society of Cardiology and published simultaneously online in The Lancet.
Session chair Gilles Montalescot, MD, Sorbonne University, Paris, noted that ACST-2 doubled the number of randomly assigned patients with asymptomatic carotid stenosis studied in previous trials, “so, a huge contribution to the evidence base in this field and apparently good news for both revascularization techniques.”
Thirty-day and 5-year outcomes
The trial was conducted in 33 countries between January 2008 and December 2020, enrolling 3,625 patients (70% were male; mean age, 70 years) with carotid stenosis of at least 60% on ultrasonography, in whom stenting or surgery was suitable but both the doctor and patient were “substantially uncertain” which procedure to prefer.
Among the 1,811 patients assigned to stenting, 87% underwent the procedure at a median of 14 days; 6% crossed over to surgery, typically because of a highly calcified lesion or a more tortuous carotid than anticipated; and 6% had no intervention.
Among the 1,814 patients assigned to surgery, 92% had the procedure at a median of 14 days; 3% crossed over to stenting, typically because of patient or doctor preference or reluctance to undergo general anesthesia; and 4% had no intervention.
Patients without complications who had stenting stayed on average 1 day less than did those undergoing surgery.
During an earlier press briefing, Dr. Halliday highlighted the need for procedural competency and said doctors had to submit a record of their CEA or CAS experience and, consistent with current guidelines, had to demonstrate an independently verified stroke or death rate of 6% or less for symptomatic patients and 3% or lower for asymptomatic patients.
The results showed the 30-day risk for death, myocardial infarction (MI), or any stroke was 3.9% with carotid stenting and 3.2% with surgery (P = .26).
But with stenting, there was a slightly higher risk for procedural nondisabling strokes (48 vs. 29; P = .03), including 15 strokes vs. 5 strokes, respectively, that left patients with no residual symptoms. This is “consistent with large, recent nationally representative registry data,” observed Dr. Halliday, of the University of Oxford (England).
For those undergoing surgery, cranial nerve palsies were reported in 5.4% vs. no patients undergoing stenting.
At 5 years, the nonprocedural fatal or disabling stroke rate was 2.5% in each group (rate ratio [RR], 0.98; P = .91), with any nonprocedural stroke occurring in 5.3% of patients with stenting vs. 4.5% with surgery (RR, 1.16; P = .33).
The investigators performed a meta-analysis combining the ACST-2 results with those of eight prior trials (four in asymptomatic and four in symptomatic patients) that yielded a similar nonsignificant result for any nonprocedural stroke (RR, 1.11; P = .21).
Based on the results from ACST-2 plus the major trials, stenting and surgery involve “similar risks and similar benefits,” Dr. Halliday concluded.
Discussant Marco Roffi, MD, University Hospital of Geneva, said, “In centers with documented expertise, carotid artery stenting should be offered as an alternative to carotid endarterectomy in patients with asymptomatic stenosis and suitable anatomy.”
While the trial provides “good news” for patients, he pointed out that a reduction in the sample size from 5,000 to 3,625 limited the statistical power and that enrollment over a long period of time may have introduced confounders, such as changes in equipment technique, and medical therapy.
Also, many centers enrolled few patients, raising the concern over low-volume centers and operators, Dr. Roffi said. “We know that 8% of the centers enrolled 39% of the patients,” and “information on the credentialing and experience of the interventionalists was limited.”
Further, a lack of systematic MI assessment may have favored the surgery group, and more recent developments in stenting with the potential of reducing periprocedural stroke were rarely used, such as proximal emboli protection in only 15% and double-layer stents in 11%.
Friedhelm Beyersdorf, MD, University Hospital of Freiburg, Germany, said that, as a vascular surgeon, he finds it understandable that there might be a higher incidence of nonfatal strokes when treating carotid stenosis with stents, given the vulnerability of these lesions.
“Nevertheless, the main conclusion from the entire study is that carotid artery treatment is extremely safe, it has to be done in order to avoid strokes, and, obviously, there seems to be an advantage for surgery in terms of nondisabling stroke,” he said.
Session chair Dr. Montalescot, however, said that what the study cannot address – and what was the subject of many online audience comments – is whether either intervention should be performed in these patients.
Unlike earlier trials comparing interventions to medical therapy, Dr. Halliday said ACST-2 enrolled patients for whom the decision had been made that revascularization was needed. In addition, 99%-100% were receiving antithrombotic therapy at baseline, 85%-90% were receiving antihypertensives, and about 85% were taking statins.
Longer-term follow-up should provide a better picture of the nonprocedural stroke risk, with patients asked annually about exactly what medications and doses they are taking, she said.
“We will have an enormous list of exactly what’s gone on and the intensity of that therapy, which is, of course, much more intense than when we carried out our first trial. But these were people in whom a procedure was thought to be necessary,” she noted.
When asked during the press conference which procedure she would choose, Dr. Halliday, a surgeon, observed that patient preference is important but that the nature of the lesion itself often determines the optimal choice.
“If you know the competence of the people doing it is equal, then the less invasive procedure – providing it has good long-term viability, and that’s why we’re following for 10 years – is the more important,” she added.
The study was funded by the UK Medical Research Council and Health Technology Assessment Programme. Dr. Halliday reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Carotid artery stenting (CAS) and carotid endarterectomy (CEA) provided comparable outcomes over time in asymptomatic patients receiving good medical therapy in the largest trial to date of what to do with severe carotid artery narrowing that is yet to cause a stroke.
Among more than 3,600 patients, stenting and surgery performed by experienced physicians involved a 1.0% risk for causing disabling stroke or death within 30 days.
The annual rate of fatal or disabling strokes was about 0.5% with either procedure over an average 5 years’ follow-up – essentially halving the annual stroke risk had neither procedure been performed, according to Alison Halliday, MD, principal investigator of the Asymptomatic Carotid Surgery Trial-2 (ACST-2).
The results were reported Aug. 29 in a Hot Line session at the virtual annual congress of the European Society of Cardiology and published simultaneously online in The Lancet.
Session chair Gilles Montalescot, MD, Sorbonne University, Paris, noted that ACST-2 doubled the number of randomly assigned patients with asymptomatic carotid stenosis studied in previous trials, “so, a huge contribution to the evidence base in this field and apparently good news for both revascularization techniques.”
Thirty-day and 5-year outcomes
The trial was conducted in 33 countries between January 2008 and December 2020, enrolling 3,625 patients (70% were male; mean age, 70 years) with carotid stenosis of at least 60% on ultrasonography, in whom stenting or surgery was suitable but both the doctor and patient were “substantially uncertain” which procedure to prefer.
Among the 1,811 patients assigned to stenting, 87% underwent the procedure at a median of 14 days; 6% crossed over to surgery, typically because of a highly calcified lesion or a more tortuous carotid than anticipated; and 6% had no intervention.
Among the 1,814 patients assigned to surgery, 92% had the procedure at a median of 14 days; 3% crossed over to stenting, typically because of patient or doctor preference or reluctance to undergo general anesthesia; and 4% had no intervention.
Patients without complications who had stenting stayed on average 1 day less than did those undergoing surgery.
During an earlier press briefing, Dr. Halliday highlighted the need for procedural competency and said doctors had to submit a record of their CEA or CAS experience and, consistent with current guidelines, had to demonstrate an independently verified stroke or death rate of 6% or less for symptomatic patients and 3% or lower for asymptomatic patients.
The results showed the 30-day risk for death, myocardial infarction (MI), or any stroke was 3.9% with carotid stenting and 3.2% with surgery (P = .26).
But with stenting, there was a slightly higher risk for procedural nondisabling strokes (48 vs. 29; P = .03), including 15 strokes vs. 5 strokes, respectively, that left patients with no residual symptoms. This is “consistent with large, recent nationally representative registry data,” observed Dr. Halliday, of the University of Oxford (England).
For those undergoing surgery, cranial nerve palsies were reported in 5.4% vs. no patients undergoing stenting.
At 5 years, the nonprocedural fatal or disabling stroke rate was 2.5% in each group (rate ratio [RR], 0.98; P = .91), with any nonprocedural stroke occurring in 5.3% of patients with stenting vs. 4.5% with surgery (RR, 1.16; P = .33).
The investigators performed a meta-analysis combining the ACST-2 results with those of eight prior trials (four in asymptomatic and four in symptomatic patients) that yielded a similar nonsignificant result for any nonprocedural stroke (RR, 1.11; P = .21).
Based on the results from ACST-2 plus the major trials, stenting and surgery involve “similar risks and similar benefits,” Dr. Halliday concluded.
Discussant Marco Roffi, MD, University Hospital of Geneva, said, “In centers with documented expertise, carotid artery stenting should be offered as an alternative to carotid endarterectomy in patients with asymptomatic stenosis and suitable anatomy.”
While the trial provides “good news” for patients, he pointed out that a reduction in the sample size from 5,000 to 3,625 limited the statistical power and that enrollment over a long period of time may have introduced confounders, such as changes in equipment technique, and medical therapy.
Also, many centers enrolled few patients, raising the concern over low-volume centers and operators, Dr. Roffi said. “We know that 8% of the centers enrolled 39% of the patients,” and “information on the credentialing and experience of the interventionalists was limited.”
Further, a lack of systematic MI assessment may have favored the surgery group, and more recent developments in stenting with the potential of reducing periprocedural stroke were rarely used, such as proximal emboli protection in only 15% and double-layer stents in 11%.
Friedhelm Beyersdorf, MD, University Hospital of Freiburg, Germany, said that, as a vascular surgeon, he finds it understandable that there might be a higher incidence of nonfatal strokes when treating carotid stenosis with stents, given the vulnerability of these lesions.
“Nevertheless, the main conclusion from the entire study is that carotid artery treatment is extremely safe, it has to be done in order to avoid strokes, and, obviously, there seems to be an advantage for surgery in terms of nondisabling stroke,” he said.
Session chair Dr. Montalescot, however, said that what the study cannot address – and what was the subject of many online audience comments – is whether either intervention should be performed in these patients.
Unlike earlier trials comparing interventions to medical therapy, Dr. Halliday said ACST-2 enrolled patients for whom the decision had been made that revascularization was needed. In addition, 99%-100% were receiving antithrombotic therapy at baseline, 85%-90% were receiving antihypertensives, and about 85% were taking statins.
Longer-term follow-up should provide a better picture of the nonprocedural stroke risk, with patients asked annually about exactly what medications and doses they are taking, she said.
“We will have an enormous list of exactly what’s gone on and the intensity of that therapy, which is, of course, much more intense than when we carried out our first trial. But these were people in whom a procedure was thought to be necessary,” she noted.
When asked during the press conference which procedure she would choose, Dr. Halliday, a surgeon, observed that patient preference is important but that the nature of the lesion itself often determines the optimal choice.
“If you know the competence of the people doing it is equal, then the less invasive procedure – providing it has good long-term viability, and that’s why we’re following for 10 years – is the more important,” she added.
The study was funded by the UK Medical Research Council and Health Technology Assessment Programme. Dr. Halliday reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Carotid artery stenting (CAS) and carotid endarterectomy (CEA) provided comparable outcomes over time in asymptomatic patients receiving good medical therapy in the largest trial to date of what to do with severe carotid artery narrowing that is yet to cause a stroke.
Among more than 3,600 patients, stenting and surgery performed by experienced physicians involved a 1.0% risk for causing disabling stroke or death within 30 days.
The annual rate of fatal or disabling strokes was about 0.5% with either procedure over an average 5 years’ follow-up – essentially halving the annual stroke risk had neither procedure been performed, according to Alison Halliday, MD, principal investigator of the Asymptomatic Carotid Surgery Trial-2 (ACST-2).
The results were reported Aug. 29 in a Hot Line session at the virtual annual congress of the European Society of Cardiology and published simultaneously online in The Lancet.
Session chair Gilles Montalescot, MD, Sorbonne University, Paris, noted that ACST-2 doubled the number of randomly assigned patients with asymptomatic carotid stenosis studied in previous trials, “so, a huge contribution to the evidence base in this field and apparently good news for both revascularization techniques.”
Thirty-day and 5-year outcomes
The trial was conducted in 33 countries between January 2008 and December 2020, enrolling 3,625 patients (70% were male; mean age, 70 years) with carotid stenosis of at least 60% on ultrasonography, in whom stenting or surgery was suitable but both the doctor and patient were “substantially uncertain” which procedure to prefer.
Among the 1,811 patients assigned to stenting, 87% underwent the procedure at a median of 14 days; 6% crossed over to surgery, typically because of a highly calcified lesion or a more tortuous carotid than anticipated; and 6% had no intervention.
Among the 1,814 patients assigned to surgery, 92% had the procedure at a median of 14 days; 3% crossed over to stenting, typically because of patient or doctor preference or reluctance to undergo general anesthesia; and 4% had no intervention.
Patients without complications who had stenting stayed on average 1 day less than did those undergoing surgery.
During an earlier press briefing, Dr. Halliday highlighted the need for procedural competency and said doctors had to submit a record of their CEA or CAS experience and, consistent with current guidelines, had to demonstrate an independently verified stroke or death rate of 6% or less for symptomatic patients and 3% or lower for asymptomatic patients.
The results showed the 30-day risk for death, myocardial infarction (MI), or any stroke was 3.9% with carotid stenting and 3.2% with surgery (P = .26).
But with stenting, there was a slightly higher risk for procedural nondisabling strokes (48 vs. 29; P = .03), including 15 strokes vs. 5 strokes, respectively, that left patients with no residual symptoms. This is “consistent with large, recent nationally representative registry data,” observed Dr. Halliday, of the University of Oxford (England).
For those undergoing surgery, cranial nerve palsies were reported in 5.4% vs. no patients undergoing stenting.
At 5 years, the nonprocedural fatal or disabling stroke rate was 2.5% in each group (rate ratio [RR], 0.98; P = .91), with any nonprocedural stroke occurring in 5.3% of patients with stenting vs. 4.5% with surgery (RR, 1.16; P = .33).
The investigators performed a meta-analysis combining the ACST-2 results with those of eight prior trials (four in asymptomatic and four in symptomatic patients) that yielded a similar nonsignificant result for any nonprocedural stroke (RR, 1.11; P = .21).
Based on the results from ACST-2 plus the major trials, stenting and surgery involve “similar risks and similar benefits,” Dr. Halliday concluded.
Discussant Marco Roffi, MD, University Hospital of Geneva, said, “In centers with documented expertise, carotid artery stenting should be offered as an alternative to carotid endarterectomy in patients with asymptomatic stenosis and suitable anatomy.”
While the trial provides “good news” for patients, he pointed out that a reduction in the sample size from 5,000 to 3,625 limited the statistical power and that enrollment over a long period of time may have introduced confounders, such as changes in equipment technique, and medical therapy.
Also, many centers enrolled few patients, raising the concern over low-volume centers and operators, Dr. Roffi said. “We know that 8% of the centers enrolled 39% of the patients,” and “information on the credentialing and experience of the interventionalists was limited.”
Further, a lack of systematic MI assessment may have favored the surgery group, and more recent developments in stenting with the potential of reducing periprocedural stroke were rarely used, such as proximal emboli protection in only 15% and double-layer stents in 11%.
Friedhelm Beyersdorf, MD, University Hospital of Freiburg, Germany, said that, as a vascular surgeon, he finds it understandable that there might be a higher incidence of nonfatal strokes when treating carotid stenosis with stents, given the vulnerability of these lesions.
“Nevertheless, the main conclusion from the entire study is that carotid artery treatment is extremely safe, it has to be done in order to avoid strokes, and, obviously, there seems to be an advantage for surgery in terms of nondisabling stroke,” he said.
Session chair Dr. Montalescot, however, said that what the study cannot address – and what was the subject of many online audience comments – is whether either intervention should be performed in these patients.
Unlike earlier trials comparing interventions to medical therapy, Dr. Halliday said ACST-2 enrolled patients for whom the decision had been made that revascularization was needed. In addition, 99%-100% were receiving antithrombotic therapy at baseline, 85%-90% were receiving antihypertensives, and about 85% were taking statins.
Longer-term follow-up should provide a better picture of the nonprocedural stroke risk, with patients asked annually about exactly what medications and doses they are taking, she said.
“We will have an enormous list of exactly what’s gone on and the intensity of that therapy, which is, of course, much more intense than when we carried out our first trial. But these were people in whom a procedure was thought to be necessary,” she noted.
When asked during the press conference which procedure she would choose, Dr. Halliday, a surgeon, observed that patient preference is important but that the nature of the lesion itself often determines the optimal choice.
“If you know the competence of the people doing it is equal, then the less invasive procedure – providing it has good long-term viability, and that’s why we’re following for 10 years – is the more important,” she added.
The study was funded by the UK Medical Research Council and Health Technology Assessment Programme. Dr. Halliday reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Frozen sections can guide biopsies for giant cell arteritis, but are they feasible?
Positive findings from frozen sections of a first temporal artery biopsy can effectively identify giant cell arteritis, ruling out in those cases the need to perform a second biopsy on the contralateral side and arguing against the use of simultaneous bilateral biopsies, according to results from a retrospective study of nearly 800 patients who underwent the procedure at the Mayo Clinic during 2010-2018.
Although temporal artery biopsy (TAB) remains the standard diagnostic test for giant cell arteritis (GCA), second TAB procedures are often performed in patients with a high level of suspicion for GCA, which may result in unnecessary treatments and complications, Devon A. Cohen, MD, of the Mayo Clinic, Rochester, Minn., and colleagues wrote. (Dr. Cohen is now a clinical fellow in ophthalmology at the Massachusetts Eye and Ear Infirmary.)
At the Mayo Clinic, TAB specimens are first examined with frozen sections at the time of the biopsy; this process, followed within days by formalin-fixed tissue permanent sections, is unique to Mayo. “A frozen section–guided sequential TAB is commonly performed, with the results of the first biopsy obtained within minutes, which determines the need for evaluation of the contralateral side,” the researchers said. However, the use of frozen sections to evaluate patients with GCA has not been well studied.
In a retrospective cohort study published in JAMA Ophthalmology, the researchers identified TAB patients aged 40 years and older who underwent TAB procedures between Jan. 1, 2010, and Dec. 1, 2018, at the Mayo Clinic. The average age of the patients was 72 years, and 41% were men.
Strong positive predictions from frozen sections
The researchers analyzed 1,162 TABs from 795 patients using frozen and permanent histologic sections.
Overall, 119 patients (15.0%) and 138 TABs had positive permanent section findings, and 103 (86.6%) of these patients also had positive frozen section findings, including 4 false positives and 20 false negatives. The frozen section specificity and sensitivity was 99.4% and 83.2%, respectively, for detecting inflammation suggestive of GCA, and the positive and negative predictive values were 96.1% and 96.6%, respectively. Positive and negative likelihood ratios for frozen section were 140.6 and 0.17, respectively.
In a multivariate analysis, the odds of a positive permanent section TAB significantly increased with age (odds ratio, 1.04), vision loss (OR, 2.72), diplopia (OR, 3.33), headache (OR, 2.32), weight loss (OR, 2.37), and anorexia (OR, 5.65).
A total of 60 patients underwent bilateral TABs, and 307 patients underwent bilateral frozen section–guided sequential TABs; the discordance rates based on permanent sections were 5.0% and 5.5%, respectively.
Those discordance rates are “an important result applying to everyone working with patients suspected for GCA,” Patricia Chévez-Barrios, MD, of Houston Methodist Hospital, wrote in an accompanying editorial. “This is on the low end of what was previously published (3%-40%) and supports the relative low need for bilateral synchronous TAB for the diagnosis of GCA.”
A key issue in GCA diagnosis is the need to confirm inflammation, Dr. Chévez-Barrios said. “The surgeon must obtain a significant portion of the artery, and the pathologist should review several sections and levels of the tissue to confidently say whether there is inflammation or no.”
Frozen sections can spare patients from second procedures
The findings suggest a role for frozen section to help to determine whether a unilateral or bilateral simultaneous TAB should be performed, the study authors noted.
“If the frozen section is positive on the first TAB, a contralateral TAB is deferred, given the very low false-positive rate (0.6%). However, if the frozen section does not align with the permanent section result, in particular if the frozen section is positive but permanent section is negative, the patient returns for a TAB on the contralateral side if the GCA suspicion remains high,” they said.
The use of frozen sections requires ideal conditions in order to be effective, Dr. Chévez-Barrios said. The Mayo Clinic approach “is only possible because of their appropriate hospital setting, the training of the histotechnologists, and the experience of the pathologists interpreting the stains and sections. For most pathology laboratories outside of the Mayo Clinic, frozen sections on arteries are the exception and are used only in specific scenarios.”
In addition, the American College of Rheumatology recommends that patients with a high suspicion of GCA should begin corticosteroids as soon as laboratory studies are obtained; “As a result, if a TAB is performed after treatment begins, the typical active pattern of inflammation in the artery changes,” Dr. Chévez-Barrios said. “This further challenges the diagnosis in a frozen section setting because of the need for immunohistochemistry.” Although frozen sections are feasible in specialized settings such as the Mayo Clinic, most patients receive adequate diagnosis and treatment based on permanent sections.
The study findings were limited by several factors including the use of data from patients at a single center and the unique setup of the Mayo Clinic to perform rapid processing of frozen sections, the researchers noted.
“Additionally, we acknowledge that there is controversy regarding the clinical interpretation of healed arteritis. At our institution, healed arteritis is interpreted in the context of patient clinical characteristics and radiographic findings, which may differ from other institutions and may impact the results of this study,” they said.
Overall, the results support the potential of frozen sections in guiding TAB, although “more studies with a comparative analysis of laboratory results, clinical symptoms, and patient demographic characteristics between positive and negative frozen and permanent TAB results are needed to confirm our findings,” they concluded.
The study received no outside funding. One author reported receiving grants from Eli Lilly and Kiniksa Pharmaceuticals as well as personal fees from Genentech-Roche and Sanofi. Dr. Chévez-Barrios had no financial conflicts to disclose.
Positive findings from frozen sections of a first temporal artery biopsy can effectively identify giant cell arteritis, ruling out in those cases the need to perform a second biopsy on the contralateral side and arguing against the use of simultaneous bilateral biopsies, according to results from a retrospective study of nearly 800 patients who underwent the procedure at the Mayo Clinic during 2010-2018.
Although temporal artery biopsy (TAB) remains the standard diagnostic test for giant cell arteritis (GCA), second TAB procedures are often performed in patients with a high level of suspicion for GCA, which may result in unnecessary treatments and complications, Devon A. Cohen, MD, of the Mayo Clinic, Rochester, Minn., and colleagues wrote. (Dr. Cohen is now a clinical fellow in ophthalmology at the Massachusetts Eye and Ear Infirmary.)
At the Mayo Clinic, TAB specimens are first examined with frozen sections at the time of the biopsy; this process, followed within days by formalin-fixed tissue permanent sections, is unique to Mayo. “A frozen section–guided sequential TAB is commonly performed, with the results of the first biopsy obtained within minutes, which determines the need for evaluation of the contralateral side,” the researchers said. However, the use of frozen sections to evaluate patients with GCA has not been well studied.
In a retrospective cohort study published in JAMA Ophthalmology, the researchers identified TAB patients aged 40 years and older who underwent TAB procedures between Jan. 1, 2010, and Dec. 1, 2018, at the Mayo Clinic. The average age of the patients was 72 years, and 41% were men.
Strong positive predictions from frozen sections
The researchers analyzed 1,162 TABs from 795 patients using frozen and permanent histologic sections.
Overall, 119 patients (15.0%) and 138 TABs had positive permanent section findings, and 103 (86.6%) of these patients also had positive frozen section findings, including 4 false positives and 20 false negatives. The frozen section specificity and sensitivity was 99.4% and 83.2%, respectively, for detecting inflammation suggestive of GCA, and the positive and negative predictive values were 96.1% and 96.6%, respectively. Positive and negative likelihood ratios for frozen section were 140.6 and 0.17, respectively.
In a multivariate analysis, the odds of a positive permanent section TAB significantly increased with age (odds ratio, 1.04), vision loss (OR, 2.72), diplopia (OR, 3.33), headache (OR, 2.32), weight loss (OR, 2.37), and anorexia (OR, 5.65).
A total of 60 patients underwent bilateral TABs, and 307 patients underwent bilateral frozen section–guided sequential TABs; the discordance rates based on permanent sections were 5.0% and 5.5%, respectively.
Those discordance rates are “an important result applying to everyone working with patients suspected for GCA,” Patricia Chévez-Barrios, MD, of Houston Methodist Hospital, wrote in an accompanying editorial. “This is on the low end of what was previously published (3%-40%) and supports the relative low need for bilateral synchronous TAB for the diagnosis of GCA.”
A key issue in GCA diagnosis is the need to confirm inflammation, Dr. Chévez-Barrios said. “The surgeon must obtain a significant portion of the artery, and the pathologist should review several sections and levels of the tissue to confidently say whether there is inflammation or no.”
Frozen sections can spare patients from second procedures
The findings suggest a role for frozen section to help to determine whether a unilateral or bilateral simultaneous TAB should be performed, the study authors noted.
“If the frozen section is positive on the first TAB, a contralateral TAB is deferred, given the very low false-positive rate (0.6%). However, if the frozen section does not align with the permanent section result, in particular if the frozen section is positive but permanent section is negative, the patient returns for a TAB on the contralateral side if the GCA suspicion remains high,” they said.
The use of frozen sections requires ideal conditions in order to be effective, Dr. Chévez-Barrios said. The Mayo Clinic approach “is only possible because of their appropriate hospital setting, the training of the histotechnologists, and the experience of the pathologists interpreting the stains and sections. For most pathology laboratories outside of the Mayo Clinic, frozen sections on arteries are the exception and are used only in specific scenarios.”
In addition, the American College of Rheumatology recommends that patients with a high suspicion of GCA should begin corticosteroids as soon as laboratory studies are obtained; “As a result, if a TAB is performed after treatment begins, the typical active pattern of inflammation in the artery changes,” Dr. Chévez-Barrios said. “This further challenges the diagnosis in a frozen section setting because of the need for immunohistochemistry.” Although frozen sections are feasible in specialized settings such as the Mayo Clinic, most patients receive adequate diagnosis and treatment based on permanent sections.
The study findings were limited by several factors including the use of data from patients at a single center and the unique setup of the Mayo Clinic to perform rapid processing of frozen sections, the researchers noted.
“Additionally, we acknowledge that there is controversy regarding the clinical interpretation of healed arteritis. At our institution, healed arteritis is interpreted in the context of patient clinical characteristics and radiographic findings, which may differ from other institutions and may impact the results of this study,” they said.
Overall, the results support the potential of frozen sections in guiding TAB, although “more studies with a comparative analysis of laboratory results, clinical symptoms, and patient demographic characteristics between positive and negative frozen and permanent TAB results are needed to confirm our findings,” they concluded.
The study received no outside funding. One author reported receiving grants from Eli Lilly and Kiniksa Pharmaceuticals as well as personal fees from Genentech-Roche and Sanofi. Dr. Chévez-Barrios had no financial conflicts to disclose.
Positive findings from frozen sections of a first temporal artery biopsy can effectively identify giant cell arteritis, ruling out in those cases the need to perform a second biopsy on the contralateral side and arguing against the use of simultaneous bilateral biopsies, according to results from a retrospective study of nearly 800 patients who underwent the procedure at the Mayo Clinic during 2010-2018.
Although temporal artery biopsy (TAB) remains the standard diagnostic test for giant cell arteritis (GCA), second TAB procedures are often performed in patients with a high level of suspicion for GCA, which may result in unnecessary treatments and complications, Devon A. Cohen, MD, of the Mayo Clinic, Rochester, Minn., and colleagues wrote. (Dr. Cohen is now a clinical fellow in ophthalmology at the Massachusetts Eye and Ear Infirmary.)
At the Mayo Clinic, TAB specimens are first examined with frozen sections at the time of the biopsy; this process, followed within days by formalin-fixed tissue permanent sections, is unique to Mayo. “A frozen section–guided sequential TAB is commonly performed, with the results of the first biopsy obtained within minutes, which determines the need for evaluation of the contralateral side,” the researchers said. However, the use of frozen sections to evaluate patients with GCA has not been well studied.
In a retrospective cohort study published in JAMA Ophthalmology, the researchers identified TAB patients aged 40 years and older who underwent TAB procedures between Jan. 1, 2010, and Dec. 1, 2018, at the Mayo Clinic. The average age of the patients was 72 years, and 41% were men.
Strong positive predictions from frozen sections
The researchers analyzed 1,162 TABs from 795 patients using frozen and permanent histologic sections.
Overall, 119 patients (15.0%) and 138 TABs had positive permanent section findings, and 103 (86.6%) of these patients also had positive frozen section findings, including 4 false positives and 20 false negatives. The frozen section specificity and sensitivity was 99.4% and 83.2%, respectively, for detecting inflammation suggestive of GCA, and the positive and negative predictive values were 96.1% and 96.6%, respectively. Positive and negative likelihood ratios for frozen section were 140.6 and 0.17, respectively.
In a multivariate analysis, the odds of a positive permanent section TAB significantly increased with age (odds ratio, 1.04), vision loss (OR, 2.72), diplopia (OR, 3.33), headache (OR, 2.32), weight loss (OR, 2.37), and anorexia (OR, 5.65).
A total of 60 patients underwent bilateral TABs, and 307 patients underwent bilateral frozen section–guided sequential TABs; the discordance rates based on permanent sections were 5.0% and 5.5%, respectively.
Those discordance rates are “an important result applying to everyone working with patients suspected for GCA,” Patricia Chévez-Barrios, MD, of Houston Methodist Hospital, wrote in an accompanying editorial. “This is on the low end of what was previously published (3%-40%) and supports the relative low need for bilateral synchronous TAB for the diagnosis of GCA.”
A key issue in GCA diagnosis is the need to confirm inflammation, Dr. Chévez-Barrios said. “The surgeon must obtain a significant portion of the artery, and the pathologist should review several sections and levels of the tissue to confidently say whether there is inflammation or no.”
Frozen sections can spare patients from second procedures
The findings suggest a role for frozen section to help to determine whether a unilateral or bilateral simultaneous TAB should be performed, the study authors noted.
“If the frozen section is positive on the first TAB, a contralateral TAB is deferred, given the very low false-positive rate (0.6%). However, if the frozen section does not align with the permanent section result, in particular if the frozen section is positive but permanent section is negative, the patient returns for a TAB on the contralateral side if the GCA suspicion remains high,” they said.
The use of frozen sections requires ideal conditions in order to be effective, Dr. Chévez-Barrios said. The Mayo Clinic approach “is only possible because of their appropriate hospital setting, the training of the histotechnologists, and the experience of the pathologists interpreting the stains and sections. For most pathology laboratories outside of the Mayo Clinic, frozen sections on arteries are the exception and are used only in specific scenarios.”
In addition, the American College of Rheumatology recommends that patients with a high suspicion of GCA should begin corticosteroids as soon as laboratory studies are obtained; “As a result, if a TAB is performed after treatment begins, the typical active pattern of inflammation in the artery changes,” Dr. Chévez-Barrios said. “This further challenges the diagnosis in a frozen section setting because of the need for immunohistochemistry.” Although frozen sections are feasible in specialized settings such as the Mayo Clinic, most patients receive adequate diagnosis and treatment based on permanent sections.
The study findings were limited by several factors including the use of data from patients at a single center and the unique setup of the Mayo Clinic to perform rapid processing of frozen sections, the researchers noted.
“Additionally, we acknowledge that there is controversy regarding the clinical interpretation of healed arteritis. At our institution, healed arteritis is interpreted in the context of patient clinical characteristics and radiographic findings, which may differ from other institutions and may impact the results of this study,” they said.
Overall, the results support the potential of frozen sections in guiding TAB, although “more studies with a comparative analysis of laboratory results, clinical symptoms, and patient demographic characteristics between positive and negative frozen and permanent TAB results are needed to confirm our findings,” they concluded.
The study received no outside funding. One author reported receiving grants from Eli Lilly and Kiniksa Pharmaceuticals as well as personal fees from Genentech-Roche and Sanofi. Dr. Chévez-Barrios had no financial conflicts to disclose.
FROM JAMA OPHTHALMOLOGY
Pharmacists’ Bleed Risk Tool and Treatment Preferences Prior to Initiating Anticoagulation in Patients With Nonvalvular Atrial Fibrillation: A Cross-Sectional Survey
From Nova Southeastern University College of Pharmacy, Fort Lauderdale, FL.
Abstract
- Objective: To determine pharmacists’ preferences in bleed risk tool (BRT) usage and gastroprotection when bleed risk was lower than or equal to stroke risk in patients with nonvalvular atrial fibrillation and who were candidates for oral anticoagulation therapy (warfarin or direct oral anticoagulants [DOACs]).
- Methods: A survey consisting of 4 domains (demographics, clinical experience, BRT usage, and treatment preferences based on cases where bleed risk was lower than or equal to stroke risk) was developed. The anonymous survey was disseminated via REDCap software to members of the American College of Clinical Pharmacy ambulatory care and cardiology Practice-based Research Networks. Descriptive statistics were calculated for all study variables and inferential statistics were employed as necessary.
- Results: Of 165 BRT users, 97% preferred HAS-BLED. When bleed risk was lower than stroke risk, 151 respondents chose either DOACs (65%) or warfarin (35%); 15% added gastroprotection. When bleed risk was equal to stroke risk, 141 respondents chose DOACs (50%), warfarin (45%), or aspirin (5%); 40% added gastroprotection.
- Conclusion: In addition to BRT usage, pharmacists were judicious in their recommendation to add gastroprotection and would consider doing so if there was a specific indication. As more than 80% of extracranial bleeds are gastrointestinal bleeds and most BRTs are nonspecific for predicting these bleeds, randomized, prospective studies stratified by HAS-BLED and stroke risk scores are needed to provide further guidance on the efficacy and safety of oral anticoagulation therapy with or without gastroprotection.
Keywords: NVAF; gastroprotection; proton pump inhibitors; warfarin; oral anticoagulants.
Management of patients with nonvalvular atrial fibrillation (NVAF) with oral anticoagulation therapy (OACT) requires constant attention to maintain a balance between preventing strokes and minimizing bleeds. Several validated bleed risk tools (BRTs) available for use in NVAF patients include HAS-BLED, HEMORR2HAGES, ATRIA, and mOBRI.1,2 A high bleed risk score is not a contraindication to OACT, but, prior to and throughout therapy, bleed risk should be assessed and modifiable risk factors addressed.3 While intraluminal gastrointestinal (GI) bleeds are not considered a critical bleed site, they are a common complication of chronic OACT and can result in hemodynamic compromise and permanent discontinuation of therapy.4,5 In 3233 patients with nonvariceal upper GI bleeds (2005-2016), the adjusted odds ratio of hospital admission, transfusion, and re-bleeding while on OACT (warfarin, heparin, or apixaban) was 3.48, 2.53, and 2.26, respectively.6 Addition of acid-suppressive therapy with a proton pump inhibitor (PPI) or histamine-2 receptor antagonist (H2RA) in NVAF patients at increased risk for upper GI bleeds and receiving OACT may result in fewer bleeds.7,8
Pharmacists play an integral part in managing patients on warfarin,9-11 and data on their role in managing patients receiving direct oral anticoagulants (DOACs) are increasing.12-16 Inpatient pharmacists actively participate in multidisciplinary collaborative teams and use clinical decision-support systems or enhanced monitoring to ensure safe prescribing of high-risk medications.12,15,16 Pharmacist-managed, outpatient-based anticoagulation services in patients on warfarin were associated with lower rates of bleeding and thromboembolic events and lower health care utilization versus routine care.17 However, it is unclear how pharmacists manage patients who are candidates for OACT but who may be at increased risk for upper GI bleeds. Using a US-based survey, the investigators sought to determine pharmacists’ preferences in BRT usage and gastroprotection when bleed risk was lower than or equal to stroke risk.
Methods
This cross-sectional study was conducted after receiving approval by Nova Southeastern University’s Institutional Review Board. The survey consisted of 16 items divided into 4 domains: demographics, clinical experience, use of BRTs, and treatment preferences based on cases where bleed risk was lower than or equal to stroke risk (Figure 1). Queries were multiple choice and allowed for free-text input when “Other” was selected. Licensed pharmacists ≥ 18 years of age who routinely provided care to patients with NVAF were eligible to participate in the study. Participants who reported using a BRT (users) completed all study domains, while participants who reported not using a BRT (nonusers) completed domains 1 through 3 only.
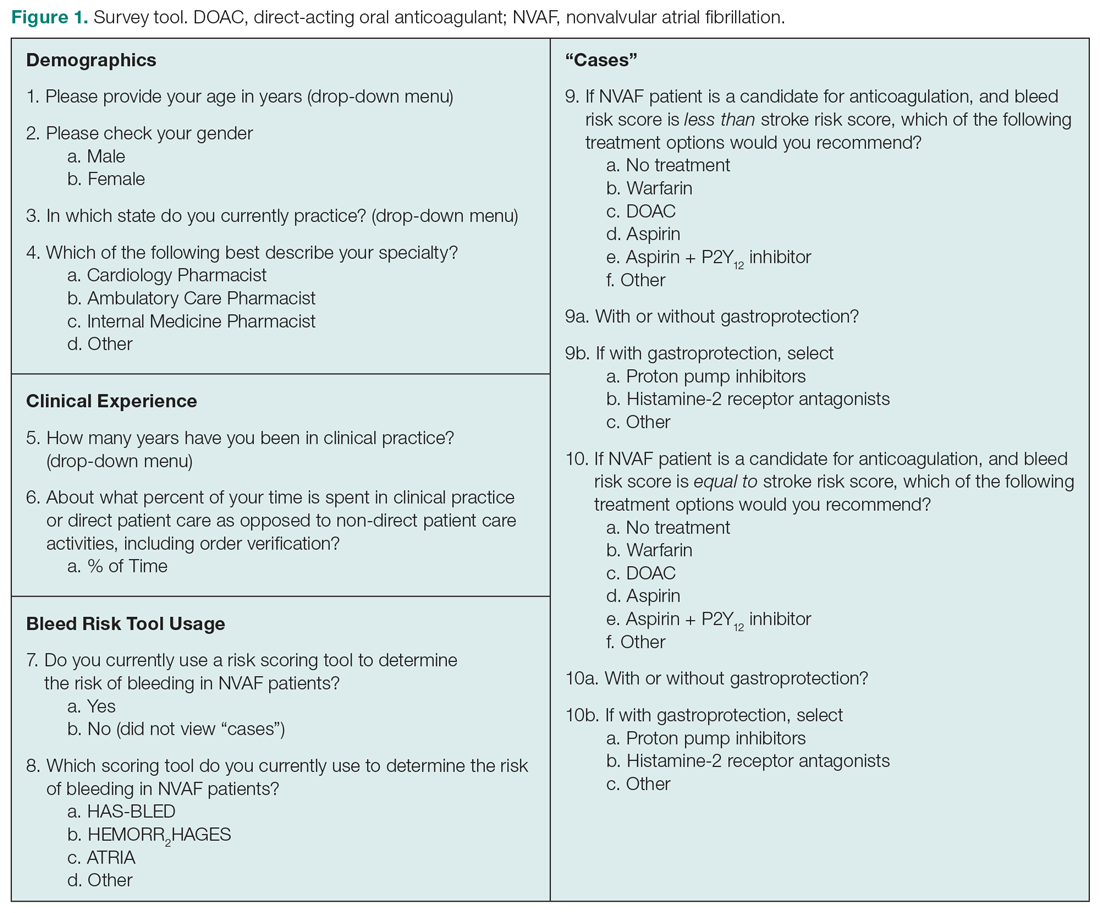
An invitation containing the survey link was sent to the American College of Clinical Pharmacy ambulatory care (n = 2237) and cardiology (n = 1318) pharmacists listed in the organization’s Practice-based Research Networks. The survey was administered in the United States between April and June 2016 via Research Electronic Data Capture (REDCap) software, a secure Web application for building and managing online surveys designed to support data collection for research studies.18
Survey responses were downloaded, and data were analyzed using NCSS 2019 Statistical Software, LLC (Kaysville, UT). Descriptive statistics were calculated for all study variables. Demographic and clinical experience data for the group that used a BRT versus the group that did not were compared using Pearson’s chi-square, ANOVA, or the Cochran-Armitage test for trends. Logistic regression with hierarchical forward selection with switching was used to identify predictors of drug selection and use of gastroprotection.
Results
Of 230 respondents who completed the survey (response rate 6.5%), 165 (72%) used a BRT and 65 (28%) did not. No significant differences were found for age, gender, duration in clinical practice, the percentage of time spent in patient care, or practice specialty between users and nonusers (Table). The median age of users was 32 years; 68% were females; the median duration in clinical practice was 6 years; 75% of their time was spent in clinical practice; and clinical settings included ambulatory care, cardiology, and internal medicine. A significant difference was found for practice region between users versus nonusers (P = 0.014). Respondents who managed more than 200 NVAF patients per year used a BRT more often than those who managed fewer than 100 NVAF patients per year (P = 0.001).
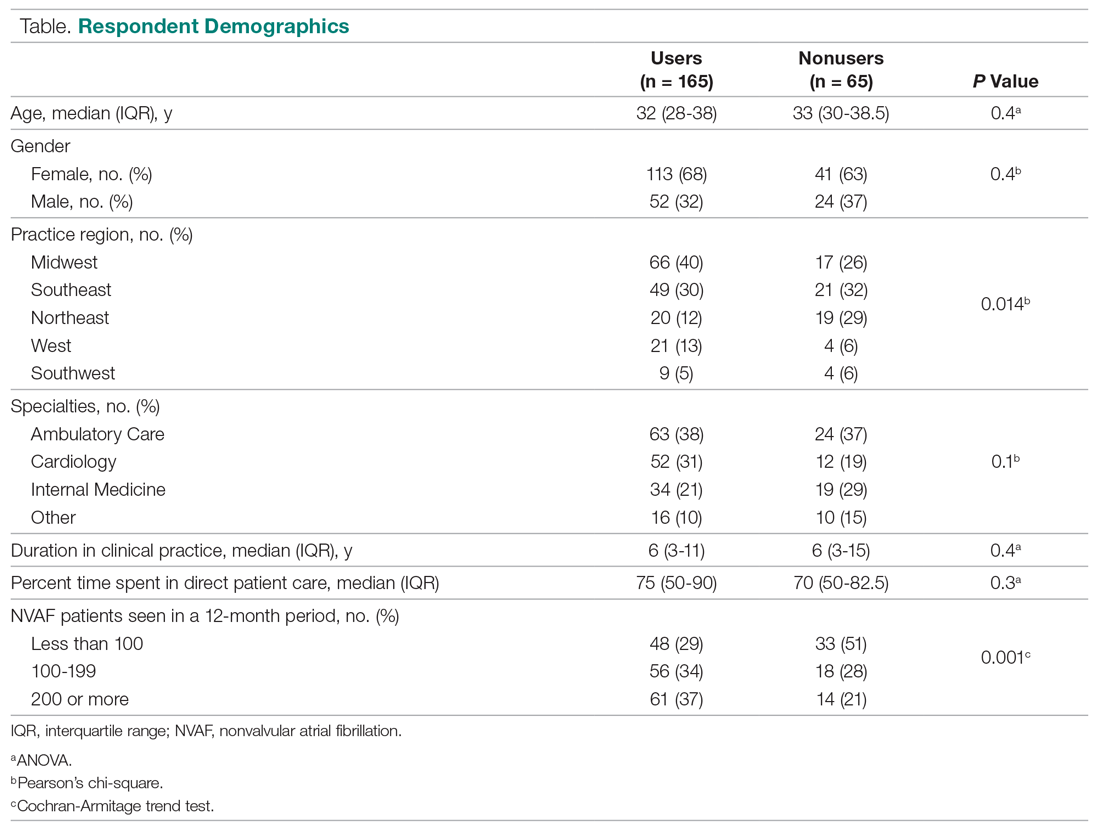
Of those who used a BRT, 97% utilized the HAS-BLED tool (n = 160). The remainder used HEMORR2HAGES (n = 3), ATRIA (n = 1), and mOBRI (n = 1). Reasons for choosing HAS-BLED included “familiarity/ease-of-use,” “preference by institution/clinical team,” and the fact that it was a “validated tool for NVAF.”
When bleed risk was lower than stroke risk, 151 of 165 users (92%) chose a treatment option (Figure 2). Of those, 65% chose a DOAC and 35% chose warfarin. Fourteen respondents chose “other” and explained that they “would initiate OACT after weighing patient factors and preferences.” When a DOAC was selected, 9% (n = 9) chose PPI co-therapy and 4% (n = 4) chose a H2RA. When warfarin was selected, 13% (n = 7) chose PPI co-therapy and 4% (n = 2) chose a H2RA. Respondents who chose gastroprotection did not provide reasons for doing so, but those who did not add it explained that they “would add gastroprotection only if patient is also on an NSAID or has a history of GI bleed” or cited “patient preference.” Specific to warfarin, some respondents would not add gastroprotection, as anticoagulation with warfarin is “easily reversed.”
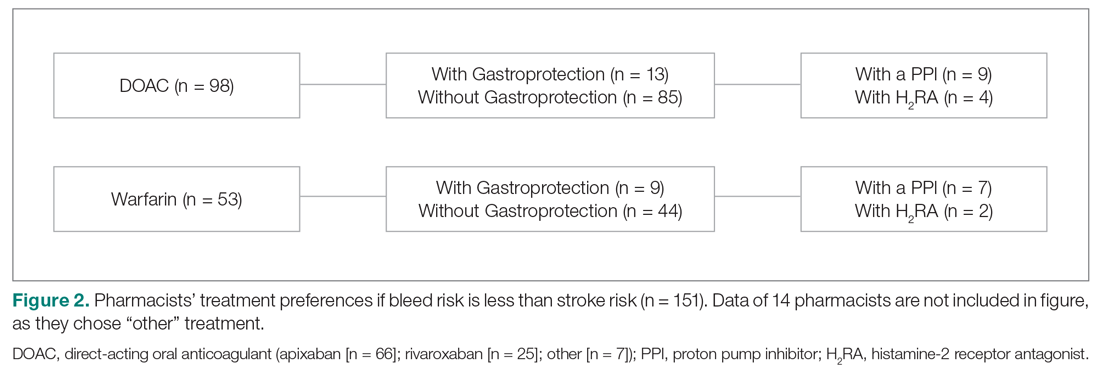
When bleed risk was equal to stroke risk, 141 of 165 users (85%) chose a treatment option (Figure 3). Fifty percent chose DOACs, 45% chose warfarin, and 5% chose aspirin.
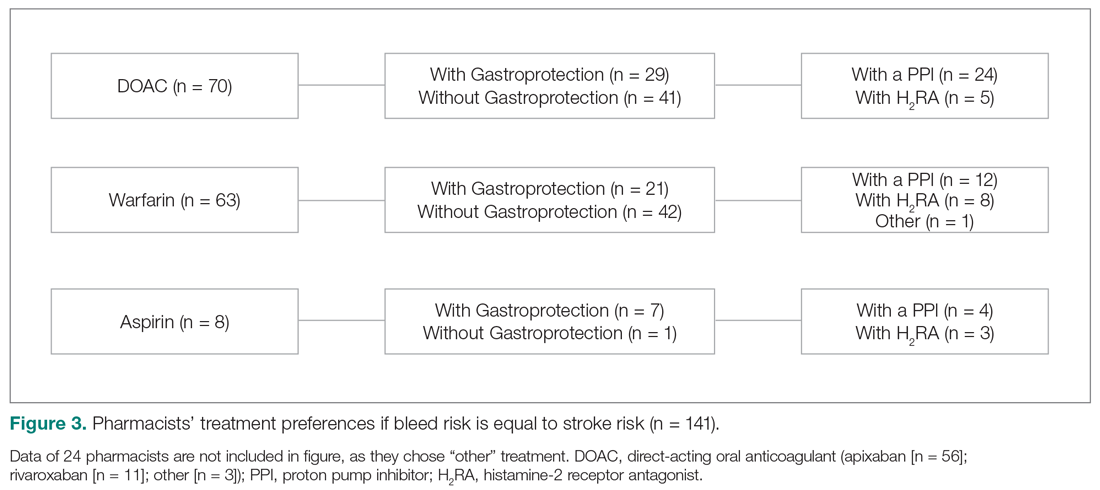
Of respondents who selected either a DOAC or warfarin, 38% (n = 50) also added gastroprotection (Figure 3). When a DOAC was selected, 34% (n = 24) favored PPI co-therapy and 7% (n = 5) chose a H2RA. When warfarin was selected, 19% (n = 12) favored PPI co-therapy, while 13% (n = 8) chose a H2RA. Rationale for choosing gastroprotection, regardless of OACT selection, included “stroke is more devastating, so if patient wants to continue treatment, but knew risks of bleeding were similar, would recommend gastroprotection to help minimize bleeding risk” and “patient-specific consideration.” Rationales for not choosing gastroprotection included “would add gastroprotection only if patient is on dual antiplatelet therapy or has another indication”; “in most patients, stroke risk outweighs bleed risk so no need for gastroprotection unless there is a stated reason”; “would use apixaban as has lowest bleeding rate of all DOACs in clinical trials”; and “gastroprotection has not been shown to be beneficial in large scale trials.”
Eight respondents chose aspirin because it was “easy and relatively low cost.” Twenty-four respondents chose “other” and explained that the choice of OACT depended on patient preference after they had discussed stroke and bleed risk with the patient and/or determined the etiology driving bleed risk.
Discussion
This is the first national survey exploring US pharmacists’ preferences in BRT usage and treatment based on bleed risk. Pharmacists preferred the HAS-BLED tool and considered patient-specific factors and evidence-based data when weighing the risk-benefit of OACT with or without gastroprotective therapy.
Similar to our findings, where three-quarters of pharmacists used a BRT, a recent Medscape/American College of Cardiology (ACC) survey reported that 74% of cardiologists used a BRT (eg, HAS-BLED) always/most of the time or sometimes to assess a patient’s overall risk of bleeding prior to initiating DOAC therapy; 27% never or rarely used a bleed risk score before prescribing DOACs.19 Although reasons for BRT preference were not provided, they may be similar to those reported by our respondents (ie, familiarity/ease-of-use). In both surveys, rationales for not using a BRT were not obtained, but possible reasons include lack of confidence with bleed risk calculators,20 inconsistent implementation of comprehensive assessments (stroke risk, bleed risk, and medication-related issues prior to decision-making),21 and nonspecific guideline recommendations.22
More recently, a network meta-analysis found that HAS-BLED and HEMORR2HAGES had modest but balanced sensitivity (
Although more than 80% of extracranial bleeds are GI bleeds,24 most BRTs are nonspecific for predicting GI bleeds. Indeed, one respondent used a spreadsheet with several BRTs to maximize treatment guidance for patients with multiple risk factors for strokes and bleeds. A comprehensive approach to determining factors that increase bleed risk should be adopted. These factors include age (HAS-BLED, HEMORR2HAGES, mOBRI, ATRIA); anemia (mOBRI, HEMORR2HAGES, ATRIA); hepatic/renal disease (HAS-BLED, HEMORR2HAGES, ATRIA, mOBRI); concomitant medications/alcohol use, including NSAIDs, corticosteroids, and antiplatelet therapy (HAS-BLED, HEMORR2HAGES); bleed history/rebleeding risk (HEMORR2HAGES, HAS-BLED, ATRIA); and GI bleeds (mOBRI).1,2 Additional risk factors for GI bleeds include being a tobacco smoker and/or being infected with Helicobacter pylori. A prospective cohort study that analyzed data from questionnaires completed by 99,359 individuals from the Copenhagen General Population Study reported that the multivariable adjusted hazard ratio for current smokers versus never smokers was 2.20 (95% CI, 1.84-2.62) for GI bleeds.25 Presence of H pylori should be investigated, with a subsequent eradication regimen implemented, as patients with warfarin-associated upper GI bleeds who were H pylori-positive had lower HAS-BLED scores versus those who were negative.26
When bleed risk was lower than stroke risk (eg, HAS-BLED < 3, CHA2DS2VASc ≥ 1), respondents appropriately initiated therapy with an OAC (predominantly apixaban); a small proportion also added gastroprotection. If the patient did not have any other GI bleed risk factors (eg, a previous GI bleed or on chronic antiplatelet or NSAID therapy), the choice of OACT depended on the attributes of each OAC and patient preference.27 Selection of warfarin was appropriate if cost, formulary restrictions, and availability of an inexpensive reversal agent were important concerns to patients and/or their health care providers. Rivaroxaban was selected because of its once-daily dosing and low risk for GI bleeding.
The recently published ARISTOPHANES study provides evidence that apixaban is an appropriate choice in patients with a HAS-BLED score < 3. In this retrospective observational study, more than 70% of patients received standard doses of DOACs (apixaban 5 mg, dabigatran 150 mg, or rivaroxaban 20 mg) and about 20% had a bleeding history, about 30% were on PPIs, less than 25% were on NSAIDs, and about 40% had a HAS-BLED score < 3. The study found that apixaban was more effective (reduced rates of ischemic or hemorrhagic strokes/systemic embolism) and safer (reduced rates of major GI bleed or intracranial bleed) than warfarin.28 Dabigatran and rivaroxaban were also more effective than warfarin for stroke prevention and had a lower risk for major intracranial bleed risk; while the risk of major GI bleed was similar between dabigatran and warfarin, major GI bleed risk was higher for rivaroxaban. When compared with each other, the 3 DOACs were effective at stroke prevention, with apixaban more effective than dabigatran and rivaroxaban; similar efficacy was noted for dabigatran versus rivaroxaban. Apixaban was associated with fewer GI bleeds versus dabigatran and rivaroxaban, but with similar intracranial bleed risks; dabigatran was associated with fewer GI bleeds but similar intracranial bleed risks versus rivaroxaban.28 Efficacy and safety findings from a subgroup analysis based on HAS-BLED scores < 3 and ≥ 3 were generally consistent with the main results.
When bleed risk was equal to stroke risk, the difficulty was determining how OACT in a patient at high stroke risk (CHA2DS2VASc score ≥ 2) and high bleed risk (HAS-BLED score ≥ 3) should be managed.
Another important finding was pharmacists’ uncertainty as to the effectiveness of PPIs in preventing GI bleeds in combination with DOACs. The data are conflicting. A meta-analysis of older studies (2007-2015) showed that PPIs (but not H2RAs) reduced the risk of upper GI bleeds in patients on warfarin but not for dabigatran
Limitations
Limitations of our survey included an overall low response rate,
Conclusion
In addition to applying BRTs in the management of NVAF patients, pharmacists considered patient-specific variables, prescriber preferences, and evidence-based guidance when recommending OACT with or without gastroprotection. To avoid suboptimal patient management, busy pharmacists should be granted time to attend continuing education programs describing optimal OACT selection and formulation of individualized, evidence-based plans to address modifiable risk factors for bleeding, including the appropriate use of gastroprotection. Randomized, prospective, long-term studies stratified by HAS-BLED and CHA2DS2VASc scores are needed to further clarify efficacy, safety, and cost-effectiveness of OACT, with and without PPIs, in patients who may be at risk for upper GI bleeds.
Acknowledgments:
Corresponding author: Devada Singh-Franco, PharmD, CDE, Nova Southeastern University College of Pharmacy, 3200 S University Drive, Fort Lauderdale, FL 33328; [email protected]
Disclosures: None.
Funding: The study was supported by Nova Southeastern University’s Health Professions Division Internal Research Grant.
1. Apostolakis S, Lane DA, Guo Y, et al. Performance of the HEMORR2HAGES, ATRIA, and HAS-BLED bleeding risk–prediction scores in patients with atrial fibrillation undergoing anticoagulation. J Am Coll Cardiol. 2012;60:861-867.
2. Chang G, Xie Q, Ma L, et al. Accuracy of HAS-BLED and other bleeding risk assessment tools in predicting major bleeding events in atrial fibrillation: A network meta-analysis. J Thromb Haemost. 2020;18:791-801.
3. Ding WY, Harrison SL, Lane DA, Lip GYH. Considerations when choosing an appropriate bleeding risk assessment tool for patients with atrial fibrillation. J Thromb Haemost. 2020;18:788-790.
4. Lauffenburger JC, Rhoney DH, Farley JF, et al. Predictors of gastrointestinal bleeding among patients with atrial fibrillation after initiating dabigatran therapy. Pharmacotherapy. 2015;35:560-568.
5. Tomaselli GF, Mahaffey KW, Cuker A, et al. 2020 ACC Expert Consensus Decision Pathway on Management of Bleeding in Patients on Oral Anticoagulants: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2020;76:594-622.
6. Taha A, McCloskey C, Craigen T, Angerson W. Antiplatelet versus anticoagulant effects in non-variceal upper gastrointestinal bleeding. Gut. 2019;68(suppl 2):A152.
7. Chan EW, Lau WC, Leung WK, et al. Prevention of dabigatran-related gastrointestinal bleeding with gastroprotective agents: A population-based study. Gastroenterology. 2015;149:586-595.
8. Ray WA, Chung CP, Murray KT, et al. Association of oral anticoagulants and proton pump inhibitor cotherapy with hospitalization for upper gastrointestinal tract bleeding. JAMA. 2018;320:2221-2230.
9. Brunetti L, Lee S-M, Doherty N, et al. Impact of warfarin discharge education program on hospital readmission and treatment costs. Int J Clin Pharm. 2018;40:721-729.
10. Hasan SS, Kow CS, Curley LE, et al. Economic evaluation of prescribing conventional and newer oral anticoagulants in older adults. Expert Rev Pharmacoecon Outcomes Res. 2018;18:371-377.
11. Phelps E, Delate T, Witt DM, et al. Effect of increased time in the therapeutic range on atrial fibrillation outcomes within a centralized anticoagulation service. Thromb Res. 2018;163:54-59.
12. Ahuja T, Raco V, Papadopoulos J, Green D. Antithrombotic stewardship: Assessing use of computerized clinical decision support tools to enhance safe prescribing of direct oral anticoagulants in hospitalized patients. J Patient Saf. 2018 Sep 25. [Epub ahead of print]
13. Leef GC, Perino AC, Askari M, et al. Appropriateness of direct oral anticoagulant dosing in patients with atrial fibrillation: Insights from the Veterans Health Administration. J Pharm Pract. 2020;33:647-653.
14. Papastergiou J, Kheir N, Ladova K, et al. Pharmacists’ confidence when providing pharmaceutical care on anticoagulants, a multinational survey. Int J Clin Pharm. 2017;39:1282-1290.
15. Perlman A, Horwitz E, Hirsh-Raccah B, et al. Clinical pharmacist led hospital-wide direct oral anticoagulant stewardship program. Isr J Health Policy Res. 2019;8:19.
16. Uppuluri EM, McComb MN, Shapiro NL. Implementation of a direct oral anticoagulation screening service at a large academic medical center provided by a pharmacist-managed antithrombosis clinic as a method to expand antithrombotic stewardship efforts. J Pharm Pract. 2020;33:271-275.
17. Manzoor BS, Cheng W-H, Lee JC, et al. Quality of pharmacist-managed anticoagulation therapy in long-term ambulatory settings: A systematic review. Ann Pharmacother. 2017;51:1122-1137.
18. Harris PA, Taylor R, Thielke R, et al. Research Electronic Data Capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-381.
19. Brooks M. AF management: Are clinicians in agreement? Medscape. May 30, 2019. Accessed December 29, 2020. https://www.medscape.com/viewarticle/913386
20. Amroze A, Mazor K, Crawford S, et al. Survey of confidence in use of stroke and bleeding risk calculators, knowledge of anticoagulants, and comfort with prescription of anticoagulation in challenging scenarios: SUPPORT-AF II study. J Thromb Thrombolysis. 2019;48:629-637.
21. Wang Y, Bajorek B. Decision-making around antithrombotics for stroke prevention in atrial fibrillation: the health professionals’ views. Int J Clin Pharm. 2016;38:985-995.
22. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2014;130:e199-e267.
23. January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019;74:104-132.
24. Anghel L, Sascu R, Trifan A, et al. Non-vitamin K antagonist oral anticoagulants and the gastrointestinal bleeding risk in real-world studies. J Clin Med. 2020;9:1398.
25. Langsted A, Nordestgaard BG. Smoking is associated with increased risk of major bleeding: a prospective cohort study. Thromb Haemost. 2019;119:39-47.
26. Faye AS, Hung KW, Cheng K, et al. HAS-BLED scores underestimate gastrointestinal bleeding risk among those with H. pylori. Am J Gastroenterol. 2019;114:S364.
27. Fawzy AM, Yang W-Y, Lip GY. Safety of direct oral anticoagulants in real-world clinical practice: translating the trials to everyday clinical management. Expert Opin Drug Saf. 2019;18:187-209.
28. Lip GYH, Keshishian A, Li X, et al. Effectiveness and safety of oral anticoagulants among nonvalvular atrial fibrillation patients. Stroke. 2018;49:2933-2944.
29. Abraham NS, Singh S, Alexander GC, et al. Comparative risk of gastrointestinal bleeding with dabigatran, rivaroxaban, and warfarin: population based cohort study. BMJ. 2015;350:h1857.
30. Holster IL, Valkhoff VE, Kuipers EJ, Tjwa E. New oral anticoagulants increase risk for gastrointestinal bleeding: a systematic review and meta-analysis. Gastroenterology. 2013;145:105-112.
31. Sherwood MW, Nessel CC, Hellkamp AS, et al. Gastrointestinal bleeding in patients with atrial fibrillation treated with rivaroxaban or warfarin: ROCKET AF Trial. J Am Coll Cardiol. 2015;66:2271-2281.
32. Di Minno A, Spadarella G, Spadarella E, et al. Gastrointestinal bleeding in patients receiving oral anticoagulation: Current treatment and pharmacological perspectives. Thromb Res. 2015;136:1074-1081.
33. Abraham NS, Hlatky MA, Antman EM, et al. ACCF/ACG/AHA 2010 Expert Consensus Document on the Concomitant Use of Proton Pump Inhibitors and Thienopyridines: A Focused Update of the ACCF/ACG/AHA 2008 Expert Consensus Document on Reducing the Gastrointestinal Risks of Antiplatelet Therapy and NSAID Use. Circulation. 2010;122:2619-2633.
34. Bhatt DL, Scheiman J, Abraham NS, et al. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2008;52:1502-1517.
35. Lanza FL, Chan FK, Quigley EM. Guidelines for prevention of NSAID-related ulcer complications. Am J Gastroenterol. 2009;104:728-738.
36. Bang CS, Joo MK, Kim BW, et al. The role of acid suppressants in the prevention of anticoagulant-related gastrointestinal bleeding: a systematic review and meta-analysis. Gut Liver. 2020;14:57-66.
37. Farrell B, Pottie K, Thompson W, et al. Deprescribing proton pump inhibitors: Evidence-based clinical practice guideline. Can Fam Physician. 2017;63:354-364.
38. Fossmark R, Martinsen TC, Waldum HL. Adverse effects of proton pump inhibitors—evidence and plausibility. Int J Mol Sci. 2019;20:5203.
39. Haastrup PF, Thompson W, Sondergaard J, Jarbol DE. Side effects of long-term proton pump inhibitor use: A review. Basic Clin Pharmacol Toxicol. 2018;123:114-121.
40. Wong JM, Maddox TM, Kennedy K, Shaw RE. Comparing major bleeding risk in outpatients with atrial fibrillation or flutter by oral anticoagulant type (from the National Cardiovascular Disease Registry’s Practice Innovation and Clinical Excellence Registry). Am J Cardiol. 2020;125:1500-1507.
41. Nagata N, Niikura R, Aoki T, et al. Effect of proton-pump inhibitors on the risk of lower gastrointestinal bleeding associated with NSAIDs, aspirin, clopidogrel, and warfarin. J Gastroenterol. 2015;50:1079-1086.
From Nova Southeastern University College of Pharmacy, Fort Lauderdale, FL.
Abstract
- Objective: To determine pharmacists’ preferences in bleed risk tool (BRT) usage and gastroprotection when bleed risk was lower than or equal to stroke risk in patients with nonvalvular atrial fibrillation and who were candidates for oral anticoagulation therapy (warfarin or direct oral anticoagulants [DOACs]).
- Methods: A survey consisting of 4 domains (demographics, clinical experience, BRT usage, and treatment preferences based on cases where bleed risk was lower than or equal to stroke risk) was developed. The anonymous survey was disseminated via REDCap software to members of the American College of Clinical Pharmacy ambulatory care and cardiology Practice-based Research Networks. Descriptive statistics were calculated for all study variables and inferential statistics were employed as necessary.
- Results: Of 165 BRT users, 97% preferred HAS-BLED. When bleed risk was lower than stroke risk, 151 respondents chose either DOACs (65%) or warfarin (35%); 15% added gastroprotection. When bleed risk was equal to stroke risk, 141 respondents chose DOACs (50%), warfarin (45%), or aspirin (5%); 40% added gastroprotection.
- Conclusion: In addition to BRT usage, pharmacists were judicious in their recommendation to add gastroprotection and would consider doing so if there was a specific indication. As more than 80% of extracranial bleeds are gastrointestinal bleeds and most BRTs are nonspecific for predicting these bleeds, randomized, prospective studies stratified by HAS-BLED and stroke risk scores are needed to provide further guidance on the efficacy and safety of oral anticoagulation therapy with or without gastroprotection.
Keywords: NVAF; gastroprotection; proton pump inhibitors; warfarin; oral anticoagulants.
Management of patients with nonvalvular atrial fibrillation (NVAF) with oral anticoagulation therapy (OACT) requires constant attention to maintain a balance between preventing strokes and minimizing bleeds. Several validated bleed risk tools (BRTs) available for use in NVAF patients include HAS-BLED, HEMORR2HAGES, ATRIA, and mOBRI.1,2 A high bleed risk score is not a contraindication to OACT, but, prior to and throughout therapy, bleed risk should be assessed and modifiable risk factors addressed.3 While intraluminal gastrointestinal (GI) bleeds are not considered a critical bleed site, they are a common complication of chronic OACT and can result in hemodynamic compromise and permanent discontinuation of therapy.4,5 In 3233 patients with nonvariceal upper GI bleeds (2005-2016), the adjusted odds ratio of hospital admission, transfusion, and re-bleeding while on OACT (warfarin, heparin, or apixaban) was 3.48, 2.53, and 2.26, respectively.6 Addition of acid-suppressive therapy with a proton pump inhibitor (PPI) or histamine-2 receptor antagonist (H2RA) in NVAF patients at increased risk for upper GI bleeds and receiving OACT may result in fewer bleeds.7,8
Pharmacists play an integral part in managing patients on warfarin,9-11 and data on their role in managing patients receiving direct oral anticoagulants (DOACs) are increasing.12-16 Inpatient pharmacists actively participate in multidisciplinary collaborative teams and use clinical decision-support systems or enhanced monitoring to ensure safe prescribing of high-risk medications.12,15,16 Pharmacist-managed, outpatient-based anticoagulation services in patients on warfarin were associated with lower rates of bleeding and thromboembolic events and lower health care utilization versus routine care.17 However, it is unclear how pharmacists manage patients who are candidates for OACT but who may be at increased risk for upper GI bleeds. Using a US-based survey, the investigators sought to determine pharmacists’ preferences in BRT usage and gastroprotection when bleed risk was lower than or equal to stroke risk.
Methods
This cross-sectional study was conducted after receiving approval by Nova Southeastern University’s Institutional Review Board. The survey consisted of 16 items divided into 4 domains: demographics, clinical experience, use of BRTs, and treatment preferences based on cases where bleed risk was lower than or equal to stroke risk (Figure 1). Queries were multiple choice and allowed for free-text input when “Other” was selected. Licensed pharmacists ≥ 18 years of age who routinely provided care to patients with NVAF were eligible to participate in the study. Participants who reported using a BRT (users) completed all study domains, while participants who reported not using a BRT (nonusers) completed domains 1 through 3 only.

An invitation containing the survey link was sent to the American College of Clinical Pharmacy ambulatory care (n = 2237) and cardiology (n = 1318) pharmacists listed in the organization’s Practice-based Research Networks. The survey was administered in the United States between April and June 2016 via Research Electronic Data Capture (REDCap) software, a secure Web application for building and managing online surveys designed to support data collection for research studies.18
Survey responses were downloaded, and data were analyzed using NCSS 2019 Statistical Software, LLC (Kaysville, UT). Descriptive statistics were calculated for all study variables. Demographic and clinical experience data for the group that used a BRT versus the group that did not were compared using Pearson’s chi-square, ANOVA, or the Cochran-Armitage test for trends. Logistic regression with hierarchical forward selection with switching was used to identify predictors of drug selection and use of gastroprotection.
Results
Of 230 respondents who completed the survey (response rate 6.5%), 165 (72%) used a BRT and 65 (28%) did not. No significant differences were found for age, gender, duration in clinical practice, the percentage of time spent in patient care, or practice specialty between users and nonusers (Table). The median age of users was 32 years; 68% were females; the median duration in clinical practice was 6 years; 75% of their time was spent in clinical practice; and clinical settings included ambulatory care, cardiology, and internal medicine. A significant difference was found for practice region between users versus nonusers (P = 0.014). Respondents who managed more than 200 NVAF patients per year used a BRT more often than those who managed fewer than 100 NVAF patients per year (P = 0.001).

Of those who used a BRT, 97% utilized the HAS-BLED tool (n = 160). The remainder used HEMORR2HAGES (n = 3), ATRIA (n = 1), and mOBRI (n = 1). Reasons for choosing HAS-BLED included “familiarity/ease-of-use,” “preference by institution/clinical team,” and the fact that it was a “validated tool for NVAF.”
When bleed risk was lower than stroke risk, 151 of 165 users (92%) chose a treatment option (Figure 2). Of those, 65% chose a DOAC and 35% chose warfarin. Fourteen respondents chose “other” and explained that they “would initiate OACT after weighing patient factors and preferences.” When a DOAC was selected, 9% (n = 9) chose PPI co-therapy and 4% (n = 4) chose a H2RA. When warfarin was selected, 13% (n = 7) chose PPI co-therapy and 4% (n = 2) chose a H2RA. Respondents who chose gastroprotection did not provide reasons for doing so, but those who did not add it explained that they “would add gastroprotection only if patient is also on an NSAID or has a history of GI bleed” or cited “patient preference.” Specific to warfarin, some respondents would not add gastroprotection, as anticoagulation with warfarin is “easily reversed.”

When bleed risk was equal to stroke risk, 141 of 165 users (85%) chose a treatment option (Figure 3). Fifty percent chose DOACs, 45% chose warfarin, and 5% chose aspirin.

Of respondents who selected either a DOAC or warfarin, 38% (n = 50) also added gastroprotection (Figure 3). When a DOAC was selected, 34% (n = 24) favored PPI co-therapy and 7% (n = 5) chose a H2RA. When warfarin was selected, 19% (n = 12) favored PPI co-therapy, while 13% (n = 8) chose a H2RA. Rationale for choosing gastroprotection, regardless of OACT selection, included “stroke is more devastating, so if patient wants to continue treatment, but knew risks of bleeding were similar, would recommend gastroprotection to help minimize bleeding risk” and “patient-specific consideration.” Rationales for not choosing gastroprotection included “would add gastroprotection only if patient is on dual antiplatelet therapy or has another indication”; “in most patients, stroke risk outweighs bleed risk so no need for gastroprotection unless there is a stated reason”; “would use apixaban as has lowest bleeding rate of all DOACs in clinical trials”; and “gastroprotection has not been shown to be beneficial in large scale trials.”
Eight respondents chose aspirin because it was “easy and relatively low cost.” Twenty-four respondents chose “other” and explained that the choice of OACT depended on patient preference after they had discussed stroke and bleed risk with the patient and/or determined the etiology driving bleed risk.
Discussion
This is the first national survey exploring US pharmacists’ preferences in BRT usage and treatment based on bleed risk. Pharmacists preferred the HAS-BLED tool and considered patient-specific factors and evidence-based data when weighing the risk-benefit of OACT with or without gastroprotective therapy.
Similar to our findings, where three-quarters of pharmacists used a BRT, a recent Medscape/American College of Cardiology (ACC) survey reported that 74% of cardiologists used a BRT (eg, HAS-BLED) always/most of the time or sometimes to assess a patient’s overall risk of bleeding prior to initiating DOAC therapy; 27% never or rarely used a bleed risk score before prescribing DOACs.19 Although reasons for BRT preference were not provided, they may be similar to those reported by our respondents (ie, familiarity/ease-of-use). In both surveys, rationales for not using a BRT were not obtained, but possible reasons include lack of confidence with bleed risk calculators,20 inconsistent implementation of comprehensive assessments (stroke risk, bleed risk, and medication-related issues prior to decision-making),21 and nonspecific guideline recommendations.22
More recently, a network meta-analysis found that HAS-BLED and HEMORR2HAGES had modest but balanced sensitivity (
Although more than 80% of extracranial bleeds are GI bleeds,24 most BRTs are nonspecific for predicting GI bleeds. Indeed, one respondent used a spreadsheet with several BRTs to maximize treatment guidance for patients with multiple risk factors for strokes and bleeds. A comprehensive approach to determining factors that increase bleed risk should be adopted. These factors include age (HAS-BLED, HEMORR2HAGES, mOBRI, ATRIA); anemia (mOBRI, HEMORR2HAGES, ATRIA); hepatic/renal disease (HAS-BLED, HEMORR2HAGES, ATRIA, mOBRI); concomitant medications/alcohol use, including NSAIDs, corticosteroids, and antiplatelet therapy (HAS-BLED, HEMORR2HAGES); bleed history/rebleeding risk (HEMORR2HAGES, HAS-BLED, ATRIA); and GI bleeds (mOBRI).1,2 Additional risk factors for GI bleeds include being a tobacco smoker and/or being infected with Helicobacter pylori. A prospective cohort study that analyzed data from questionnaires completed by 99,359 individuals from the Copenhagen General Population Study reported that the multivariable adjusted hazard ratio for current smokers versus never smokers was 2.20 (95% CI, 1.84-2.62) for GI bleeds.25 Presence of H pylori should be investigated, with a subsequent eradication regimen implemented, as patients with warfarin-associated upper GI bleeds who were H pylori-positive had lower HAS-BLED scores versus those who were negative.26
When bleed risk was lower than stroke risk (eg, HAS-BLED < 3, CHA2DS2VASc ≥ 1), respondents appropriately initiated therapy with an OAC (predominantly apixaban); a small proportion also added gastroprotection. If the patient did not have any other GI bleed risk factors (eg, a previous GI bleed or on chronic antiplatelet or NSAID therapy), the choice of OACT depended on the attributes of each OAC and patient preference.27 Selection of warfarin was appropriate if cost, formulary restrictions, and availability of an inexpensive reversal agent were important concerns to patients and/or their health care providers. Rivaroxaban was selected because of its once-daily dosing and low risk for GI bleeding.
The recently published ARISTOPHANES study provides evidence that apixaban is an appropriate choice in patients with a HAS-BLED score < 3. In this retrospective observational study, more than 70% of patients received standard doses of DOACs (apixaban 5 mg, dabigatran 150 mg, or rivaroxaban 20 mg) and about 20% had a bleeding history, about 30% were on PPIs, less than 25% were on NSAIDs, and about 40% had a HAS-BLED score < 3. The study found that apixaban was more effective (reduced rates of ischemic or hemorrhagic strokes/systemic embolism) and safer (reduced rates of major GI bleed or intracranial bleed) than warfarin.28 Dabigatran and rivaroxaban were also more effective than warfarin for stroke prevention and had a lower risk for major intracranial bleed risk; while the risk of major GI bleed was similar between dabigatran and warfarin, major GI bleed risk was higher for rivaroxaban. When compared with each other, the 3 DOACs were effective at stroke prevention, with apixaban more effective than dabigatran and rivaroxaban; similar efficacy was noted for dabigatran versus rivaroxaban. Apixaban was associated with fewer GI bleeds versus dabigatran and rivaroxaban, but with similar intracranial bleed risks; dabigatran was associated with fewer GI bleeds but similar intracranial bleed risks versus rivaroxaban.28 Efficacy and safety findings from a subgroup analysis based on HAS-BLED scores < 3 and ≥ 3 were generally consistent with the main results.
When bleed risk was equal to stroke risk, the difficulty was determining how OACT in a patient at high stroke risk (CHA2DS2VASc score ≥ 2) and high bleed risk (HAS-BLED score ≥ 3) should be managed.
Another important finding was pharmacists’ uncertainty as to the effectiveness of PPIs in preventing GI bleeds in combination with DOACs. The data are conflicting. A meta-analysis of older studies (2007-2015) showed that PPIs (but not H2RAs) reduced the risk of upper GI bleeds in patients on warfarin but not for dabigatran
Limitations
Limitations of our survey included an overall low response rate,
Conclusion
In addition to applying BRTs in the management of NVAF patients, pharmacists considered patient-specific variables, prescriber preferences, and evidence-based guidance when recommending OACT with or without gastroprotection. To avoid suboptimal patient management, busy pharmacists should be granted time to attend continuing education programs describing optimal OACT selection and formulation of individualized, evidence-based plans to address modifiable risk factors for bleeding, including the appropriate use of gastroprotection. Randomized, prospective, long-term studies stratified by HAS-BLED and CHA2DS2VASc scores are needed to further clarify efficacy, safety, and cost-effectiveness of OACT, with and without PPIs, in patients who may be at risk for upper GI bleeds.
Acknowledgments:
Corresponding author: Devada Singh-Franco, PharmD, CDE, Nova Southeastern University College of Pharmacy, 3200 S University Drive, Fort Lauderdale, FL 33328; [email protected]
Disclosures: None.
Funding: The study was supported by Nova Southeastern University’s Health Professions Division Internal Research Grant.
From Nova Southeastern University College of Pharmacy, Fort Lauderdale, FL.
Abstract
- Objective: To determine pharmacists’ preferences in bleed risk tool (BRT) usage and gastroprotection when bleed risk was lower than or equal to stroke risk in patients with nonvalvular atrial fibrillation and who were candidates for oral anticoagulation therapy (warfarin or direct oral anticoagulants [DOACs]).
- Methods: A survey consisting of 4 domains (demographics, clinical experience, BRT usage, and treatment preferences based on cases where bleed risk was lower than or equal to stroke risk) was developed. The anonymous survey was disseminated via REDCap software to members of the American College of Clinical Pharmacy ambulatory care and cardiology Practice-based Research Networks. Descriptive statistics were calculated for all study variables and inferential statistics were employed as necessary.
- Results: Of 165 BRT users, 97% preferred HAS-BLED. When bleed risk was lower than stroke risk, 151 respondents chose either DOACs (65%) or warfarin (35%); 15% added gastroprotection. When bleed risk was equal to stroke risk, 141 respondents chose DOACs (50%), warfarin (45%), or aspirin (5%); 40% added gastroprotection.
- Conclusion: In addition to BRT usage, pharmacists were judicious in their recommendation to add gastroprotection and would consider doing so if there was a specific indication. As more than 80% of extracranial bleeds are gastrointestinal bleeds and most BRTs are nonspecific for predicting these bleeds, randomized, prospective studies stratified by HAS-BLED and stroke risk scores are needed to provide further guidance on the efficacy and safety of oral anticoagulation therapy with or without gastroprotection.
Keywords: NVAF; gastroprotection; proton pump inhibitors; warfarin; oral anticoagulants.
Management of patients with nonvalvular atrial fibrillation (NVAF) with oral anticoagulation therapy (OACT) requires constant attention to maintain a balance between preventing strokes and minimizing bleeds. Several validated bleed risk tools (BRTs) available for use in NVAF patients include HAS-BLED, HEMORR2HAGES, ATRIA, and mOBRI.1,2 A high bleed risk score is not a contraindication to OACT, but, prior to and throughout therapy, bleed risk should be assessed and modifiable risk factors addressed.3 While intraluminal gastrointestinal (GI) bleeds are not considered a critical bleed site, they are a common complication of chronic OACT and can result in hemodynamic compromise and permanent discontinuation of therapy.4,5 In 3233 patients with nonvariceal upper GI bleeds (2005-2016), the adjusted odds ratio of hospital admission, transfusion, and re-bleeding while on OACT (warfarin, heparin, or apixaban) was 3.48, 2.53, and 2.26, respectively.6 Addition of acid-suppressive therapy with a proton pump inhibitor (PPI) or histamine-2 receptor antagonist (H2RA) in NVAF patients at increased risk for upper GI bleeds and receiving OACT may result in fewer bleeds.7,8
Pharmacists play an integral part in managing patients on warfarin,9-11 and data on their role in managing patients receiving direct oral anticoagulants (DOACs) are increasing.12-16 Inpatient pharmacists actively participate in multidisciplinary collaborative teams and use clinical decision-support systems or enhanced monitoring to ensure safe prescribing of high-risk medications.12,15,16 Pharmacist-managed, outpatient-based anticoagulation services in patients on warfarin were associated with lower rates of bleeding and thromboembolic events and lower health care utilization versus routine care.17 However, it is unclear how pharmacists manage patients who are candidates for OACT but who may be at increased risk for upper GI bleeds. Using a US-based survey, the investigators sought to determine pharmacists’ preferences in BRT usage and gastroprotection when bleed risk was lower than or equal to stroke risk.
Methods
This cross-sectional study was conducted after receiving approval by Nova Southeastern University’s Institutional Review Board. The survey consisted of 16 items divided into 4 domains: demographics, clinical experience, use of BRTs, and treatment preferences based on cases where bleed risk was lower than or equal to stroke risk (Figure 1). Queries were multiple choice and allowed for free-text input when “Other” was selected. Licensed pharmacists ≥ 18 years of age who routinely provided care to patients with NVAF were eligible to participate in the study. Participants who reported using a BRT (users) completed all study domains, while participants who reported not using a BRT (nonusers) completed domains 1 through 3 only.

An invitation containing the survey link was sent to the American College of Clinical Pharmacy ambulatory care (n = 2237) and cardiology (n = 1318) pharmacists listed in the organization’s Practice-based Research Networks. The survey was administered in the United States between April and June 2016 via Research Electronic Data Capture (REDCap) software, a secure Web application for building and managing online surveys designed to support data collection for research studies.18
Survey responses were downloaded, and data were analyzed using NCSS 2019 Statistical Software, LLC (Kaysville, UT). Descriptive statistics were calculated for all study variables. Demographic and clinical experience data for the group that used a BRT versus the group that did not were compared using Pearson’s chi-square, ANOVA, or the Cochran-Armitage test for trends. Logistic regression with hierarchical forward selection with switching was used to identify predictors of drug selection and use of gastroprotection.
Results
Of 230 respondents who completed the survey (response rate 6.5%), 165 (72%) used a BRT and 65 (28%) did not. No significant differences were found for age, gender, duration in clinical practice, the percentage of time spent in patient care, or practice specialty between users and nonusers (Table). The median age of users was 32 years; 68% were females; the median duration in clinical practice was 6 years; 75% of their time was spent in clinical practice; and clinical settings included ambulatory care, cardiology, and internal medicine. A significant difference was found for practice region between users versus nonusers (P = 0.014). Respondents who managed more than 200 NVAF patients per year used a BRT more often than those who managed fewer than 100 NVAF patients per year (P = 0.001).

Of those who used a BRT, 97% utilized the HAS-BLED tool (n = 160). The remainder used HEMORR2HAGES (n = 3), ATRIA (n = 1), and mOBRI (n = 1). Reasons for choosing HAS-BLED included “familiarity/ease-of-use,” “preference by institution/clinical team,” and the fact that it was a “validated tool for NVAF.”
When bleed risk was lower than stroke risk, 151 of 165 users (92%) chose a treatment option (Figure 2). Of those, 65% chose a DOAC and 35% chose warfarin. Fourteen respondents chose “other” and explained that they “would initiate OACT after weighing patient factors and preferences.” When a DOAC was selected, 9% (n = 9) chose PPI co-therapy and 4% (n = 4) chose a H2RA. When warfarin was selected, 13% (n = 7) chose PPI co-therapy and 4% (n = 2) chose a H2RA. Respondents who chose gastroprotection did not provide reasons for doing so, but those who did not add it explained that they “would add gastroprotection only if patient is also on an NSAID or has a history of GI bleed” or cited “patient preference.” Specific to warfarin, some respondents would not add gastroprotection, as anticoagulation with warfarin is “easily reversed.”

When bleed risk was equal to stroke risk, 141 of 165 users (85%) chose a treatment option (Figure 3). Fifty percent chose DOACs, 45% chose warfarin, and 5% chose aspirin.

Of respondents who selected either a DOAC or warfarin, 38% (n = 50) also added gastroprotection (Figure 3). When a DOAC was selected, 34% (n = 24) favored PPI co-therapy and 7% (n = 5) chose a H2RA. When warfarin was selected, 19% (n = 12) favored PPI co-therapy, while 13% (n = 8) chose a H2RA. Rationale for choosing gastroprotection, regardless of OACT selection, included “stroke is more devastating, so if patient wants to continue treatment, but knew risks of bleeding were similar, would recommend gastroprotection to help minimize bleeding risk” and “patient-specific consideration.” Rationales for not choosing gastroprotection included “would add gastroprotection only if patient is on dual antiplatelet therapy or has another indication”; “in most patients, stroke risk outweighs bleed risk so no need for gastroprotection unless there is a stated reason”; “would use apixaban as has lowest bleeding rate of all DOACs in clinical trials”; and “gastroprotection has not been shown to be beneficial in large scale trials.”
Eight respondents chose aspirin because it was “easy and relatively low cost.” Twenty-four respondents chose “other” and explained that the choice of OACT depended on patient preference after they had discussed stroke and bleed risk with the patient and/or determined the etiology driving bleed risk.
Discussion
This is the first national survey exploring US pharmacists’ preferences in BRT usage and treatment based on bleed risk. Pharmacists preferred the HAS-BLED tool and considered patient-specific factors and evidence-based data when weighing the risk-benefit of OACT with or without gastroprotective therapy.
Similar to our findings, where three-quarters of pharmacists used a BRT, a recent Medscape/American College of Cardiology (ACC) survey reported that 74% of cardiologists used a BRT (eg, HAS-BLED) always/most of the time or sometimes to assess a patient’s overall risk of bleeding prior to initiating DOAC therapy; 27% never or rarely used a bleed risk score before prescribing DOACs.19 Although reasons for BRT preference were not provided, they may be similar to those reported by our respondents (ie, familiarity/ease-of-use). In both surveys, rationales for not using a BRT were not obtained, but possible reasons include lack of confidence with bleed risk calculators,20 inconsistent implementation of comprehensive assessments (stroke risk, bleed risk, and medication-related issues prior to decision-making),21 and nonspecific guideline recommendations.22
More recently, a network meta-analysis found that HAS-BLED and HEMORR2HAGES had modest but balanced sensitivity (
Although more than 80% of extracranial bleeds are GI bleeds,24 most BRTs are nonspecific for predicting GI bleeds. Indeed, one respondent used a spreadsheet with several BRTs to maximize treatment guidance for patients with multiple risk factors for strokes and bleeds. A comprehensive approach to determining factors that increase bleed risk should be adopted. These factors include age (HAS-BLED, HEMORR2HAGES, mOBRI, ATRIA); anemia (mOBRI, HEMORR2HAGES, ATRIA); hepatic/renal disease (HAS-BLED, HEMORR2HAGES, ATRIA, mOBRI); concomitant medications/alcohol use, including NSAIDs, corticosteroids, and antiplatelet therapy (HAS-BLED, HEMORR2HAGES); bleed history/rebleeding risk (HEMORR2HAGES, HAS-BLED, ATRIA); and GI bleeds (mOBRI).1,2 Additional risk factors for GI bleeds include being a tobacco smoker and/or being infected with Helicobacter pylori. A prospective cohort study that analyzed data from questionnaires completed by 99,359 individuals from the Copenhagen General Population Study reported that the multivariable adjusted hazard ratio for current smokers versus never smokers was 2.20 (95% CI, 1.84-2.62) for GI bleeds.25 Presence of H pylori should be investigated, with a subsequent eradication regimen implemented, as patients with warfarin-associated upper GI bleeds who were H pylori-positive had lower HAS-BLED scores versus those who were negative.26
When bleed risk was lower than stroke risk (eg, HAS-BLED < 3, CHA2DS2VASc ≥ 1), respondents appropriately initiated therapy with an OAC (predominantly apixaban); a small proportion also added gastroprotection. If the patient did not have any other GI bleed risk factors (eg, a previous GI bleed or on chronic antiplatelet or NSAID therapy), the choice of OACT depended on the attributes of each OAC and patient preference.27 Selection of warfarin was appropriate if cost, formulary restrictions, and availability of an inexpensive reversal agent were important concerns to patients and/or their health care providers. Rivaroxaban was selected because of its once-daily dosing and low risk for GI bleeding.
The recently published ARISTOPHANES study provides evidence that apixaban is an appropriate choice in patients with a HAS-BLED score < 3. In this retrospective observational study, more than 70% of patients received standard doses of DOACs (apixaban 5 mg, dabigatran 150 mg, or rivaroxaban 20 mg) and about 20% had a bleeding history, about 30% were on PPIs, less than 25% were on NSAIDs, and about 40% had a HAS-BLED score < 3. The study found that apixaban was more effective (reduced rates of ischemic or hemorrhagic strokes/systemic embolism) and safer (reduced rates of major GI bleed or intracranial bleed) than warfarin.28 Dabigatran and rivaroxaban were also more effective than warfarin for stroke prevention and had a lower risk for major intracranial bleed risk; while the risk of major GI bleed was similar between dabigatran and warfarin, major GI bleed risk was higher for rivaroxaban. When compared with each other, the 3 DOACs were effective at stroke prevention, with apixaban more effective than dabigatran and rivaroxaban; similar efficacy was noted for dabigatran versus rivaroxaban. Apixaban was associated with fewer GI bleeds versus dabigatran and rivaroxaban, but with similar intracranial bleed risks; dabigatran was associated with fewer GI bleeds but similar intracranial bleed risks versus rivaroxaban.28 Efficacy and safety findings from a subgroup analysis based on HAS-BLED scores < 3 and ≥ 3 were generally consistent with the main results.
When bleed risk was equal to stroke risk, the difficulty was determining how OACT in a patient at high stroke risk (CHA2DS2VASc score ≥ 2) and high bleed risk (HAS-BLED score ≥ 3) should be managed.
Another important finding was pharmacists’ uncertainty as to the effectiveness of PPIs in preventing GI bleeds in combination with DOACs. The data are conflicting. A meta-analysis of older studies (2007-2015) showed that PPIs (but not H2RAs) reduced the risk of upper GI bleeds in patients on warfarin but not for dabigatran
Limitations
Limitations of our survey included an overall low response rate,
Conclusion
In addition to applying BRTs in the management of NVAF patients, pharmacists considered patient-specific variables, prescriber preferences, and evidence-based guidance when recommending OACT with or without gastroprotection. To avoid suboptimal patient management, busy pharmacists should be granted time to attend continuing education programs describing optimal OACT selection and formulation of individualized, evidence-based plans to address modifiable risk factors for bleeding, including the appropriate use of gastroprotection. Randomized, prospective, long-term studies stratified by HAS-BLED and CHA2DS2VASc scores are needed to further clarify efficacy, safety, and cost-effectiveness of OACT, with and without PPIs, in patients who may be at risk for upper GI bleeds.
Acknowledgments:
Corresponding author: Devada Singh-Franco, PharmD, CDE, Nova Southeastern University College of Pharmacy, 3200 S University Drive, Fort Lauderdale, FL 33328; [email protected]
Disclosures: None.
Funding: The study was supported by Nova Southeastern University’s Health Professions Division Internal Research Grant.
1. Apostolakis S, Lane DA, Guo Y, et al. Performance of the HEMORR2HAGES, ATRIA, and HAS-BLED bleeding risk–prediction scores in patients with atrial fibrillation undergoing anticoagulation. J Am Coll Cardiol. 2012;60:861-867.
2. Chang G, Xie Q, Ma L, et al. Accuracy of HAS-BLED and other bleeding risk assessment tools in predicting major bleeding events in atrial fibrillation: A network meta-analysis. J Thromb Haemost. 2020;18:791-801.
3. Ding WY, Harrison SL, Lane DA, Lip GYH. Considerations when choosing an appropriate bleeding risk assessment tool for patients with atrial fibrillation. J Thromb Haemost. 2020;18:788-790.
4. Lauffenburger JC, Rhoney DH, Farley JF, et al. Predictors of gastrointestinal bleeding among patients with atrial fibrillation after initiating dabigatran therapy. Pharmacotherapy. 2015;35:560-568.
5. Tomaselli GF, Mahaffey KW, Cuker A, et al. 2020 ACC Expert Consensus Decision Pathway on Management of Bleeding in Patients on Oral Anticoagulants: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2020;76:594-622.
6. Taha A, McCloskey C, Craigen T, Angerson W. Antiplatelet versus anticoagulant effects in non-variceal upper gastrointestinal bleeding. Gut. 2019;68(suppl 2):A152.
7. Chan EW, Lau WC, Leung WK, et al. Prevention of dabigatran-related gastrointestinal bleeding with gastroprotective agents: A population-based study. Gastroenterology. 2015;149:586-595.
8. Ray WA, Chung CP, Murray KT, et al. Association of oral anticoagulants and proton pump inhibitor cotherapy with hospitalization for upper gastrointestinal tract bleeding. JAMA. 2018;320:2221-2230.
9. Brunetti L, Lee S-M, Doherty N, et al. Impact of warfarin discharge education program on hospital readmission and treatment costs. Int J Clin Pharm. 2018;40:721-729.
10. Hasan SS, Kow CS, Curley LE, et al. Economic evaluation of prescribing conventional and newer oral anticoagulants in older adults. Expert Rev Pharmacoecon Outcomes Res. 2018;18:371-377.
11. Phelps E, Delate T, Witt DM, et al. Effect of increased time in the therapeutic range on atrial fibrillation outcomes within a centralized anticoagulation service. Thromb Res. 2018;163:54-59.
12. Ahuja T, Raco V, Papadopoulos J, Green D. Antithrombotic stewardship: Assessing use of computerized clinical decision support tools to enhance safe prescribing of direct oral anticoagulants in hospitalized patients. J Patient Saf. 2018 Sep 25. [Epub ahead of print]
13. Leef GC, Perino AC, Askari M, et al. Appropriateness of direct oral anticoagulant dosing in patients with atrial fibrillation: Insights from the Veterans Health Administration. J Pharm Pract. 2020;33:647-653.
14. Papastergiou J, Kheir N, Ladova K, et al. Pharmacists’ confidence when providing pharmaceutical care on anticoagulants, a multinational survey. Int J Clin Pharm. 2017;39:1282-1290.
15. Perlman A, Horwitz E, Hirsh-Raccah B, et al. Clinical pharmacist led hospital-wide direct oral anticoagulant stewardship program. Isr J Health Policy Res. 2019;8:19.
16. Uppuluri EM, McComb MN, Shapiro NL. Implementation of a direct oral anticoagulation screening service at a large academic medical center provided by a pharmacist-managed antithrombosis clinic as a method to expand antithrombotic stewardship efforts. J Pharm Pract. 2020;33:271-275.
17. Manzoor BS, Cheng W-H, Lee JC, et al. Quality of pharmacist-managed anticoagulation therapy in long-term ambulatory settings: A systematic review. Ann Pharmacother. 2017;51:1122-1137.
18. Harris PA, Taylor R, Thielke R, et al. Research Electronic Data Capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-381.
19. Brooks M. AF management: Are clinicians in agreement? Medscape. May 30, 2019. Accessed December 29, 2020. https://www.medscape.com/viewarticle/913386
20. Amroze A, Mazor K, Crawford S, et al. Survey of confidence in use of stroke and bleeding risk calculators, knowledge of anticoagulants, and comfort with prescription of anticoagulation in challenging scenarios: SUPPORT-AF II study. J Thromb Thrombolysis. 2019;48:629-637.
21. Wang Y, Bajorek B. Decision-making around antithrombotics for stroke prevention in atrial fibrillation: the health professionals’ views. Int J Clin Pharm. 2016;38:985-995.
22. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2014;130:e199-e267.
23. January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019;74:104-132.
24. Anghel L, Sascu R, Trifan A, et al. Non-vitamin K antagonist oral anticoagulants and the gastrointestinal bleeding risk in real-world studies. J Clin Med. 2020;9:1398.
25. Langsted A, Nordestgaard BG. Smoking is associated with increased risk of major bleeding: a prospective cohort study. Thromb Haemost. 2019;119:39-47.
26. Faye AS, Hung KW, Cheng K, et al. HAS-BLED scores underestimate gastrointestinal bleeding risk among those with H. pylori. Am J Gastroenterol. 2019;114:S364.
27. Fawzy AM, Yang W-Y, Lip GY. Safety of direct oral anticoagulants in real-world clinical practice: translating the trials to everyday clinical management. Expert Opin Drug Saf. 2019;18:187-209.
28. Lip GYH, Keshishian A, Li X, et al. Effectiveness and safety of oral anticoagulants among nonvalvular atrial fibrillation patients. Stroke. 2018;49:2933-2944.
29. Abraham NS, Singh S, Alexander GC, et al. Comparative risk of gastrointestinal bleeding with dabigatran, rivaroxaban, and warfarin: population based cohort study. BMJ. 2015;350:h1857.
30. Holster IL, Valkhoff VE, Kuipers EJ, Tjwa E. New oral anticoagulants increase risk for gastrointestinal bleeding: a systematic review and meta-analysis. Gastroenterology. 2013;145:105-112.
31. Sherwood MW, Nessel CC, Hellkamp AS, et al. Gastrointestinal bleeding in patients with atrial fibrillation treated with rivaroxaban or warfarin: ROCKET AF Trial. J Am Coll Cardiol. 2015;66:2271-2281.
32. Di Minno A, Spadarella G, Spadarella E, et al. Gastrointestinal bleeding in patients receiving oral anticoagulation: Current treatment and pharmacological perspectives. Thromb Res. 2015;136:1074-1081.
33. Abraham NS, Hlatky MA, Antman EM, et al. ACCF/ACG/AHA 2010 Expert Consensus Document on the Concomitant Use of Proton Pump Inhibitors and Thienopyridines: A Focused Update of the ACCF/ACG/AHA 2008 Expert Consensus Document on Reducing the Gastrointestinal Risks of Antiplatelet Therapy and NSAID Use. Circulation. 2010;122:2619-2633.
34. Bhatt DL, Scheiman J, Abraham NS, et al. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2008;52:1502-1517.
35. Lanza FL, Chan FK, Quigley EM. Guidelines for prevention of NSAID-related ulcer complications. Am J Gastroenterol. 2009;104:728-738.
36. Bang CS, Joo MK, Kim BW, et al. The role of acid suppressants in the prevention of anticoagulant-related gastrointestinal bleeding: a systematic review and meta-analysis. Gut Liver. 2020;14:57-66.
37. Farrell B, Pottie K, Thompson W, et al. Deprescribing proton pump inhibitors: Evidence-based clinical practice guideline. Can Fam Physician. 2017;63:354-364.
38. Fossmark R, Martinsen TC, Waldum HL. Adverse effects of proton pump inhibitors—evidence and plausibility. Int J Mol Sci. 2019;20:5203.
39. Haastrup PF, Thompson W, Sondergaard J, Jarbol DE. Side effects of long-term proton pump inhibitor use: A review. Basic Clin Pharmacol Toxicol. 2018;123:114-121.
40. Wong JM, Maddox TM, Kennedy K, Shaw RE. Comparing major bleeding risk in outpatients with atrial fibrillation or flutter by oral anticoagulant type (from the National Cardiovascular Disease Registry’s Practice Innovation and Clinical Excellence Registry). Am J Cardiol. 2020;125:1500-1507.
41. Nagata N, Niikura R, Aoki T, et al. Effect of proton-pump inhibitors on the risk of lower gastrointestinal bleeding associated with NSAIDs, aspirin, clopidogrel, and warfarin. J Gastroenterol. 2015;50:1079-1086.
1. Apostolakis S, Lane DA, Guo Y, et al. Performance of the HEMORR2HAGES, ATRIA, and HAS-BLED bleeding risk–prediction scores in patients with atrial fibrillation undergoing anticoagulation. J Am Coll Cardiol. 2012;60:861-867.
2. Chang G, Xie Q, Ma L, et al. Accuracy of HAS-BLED and other bleeding risk assessment tools in predicting major bleeding events in atrial fibrillation: A network meta-analysis. J Thromb Haemost. 2020;18:791-801.
3. Ding WY, Harrison SL, Lane DA, Lip GYH. Considerations when choosing an appropriate bleeding risk assessment tool for patients with atrial fibrillation. J Thromb Haemost. 2020;18:788-790.
4. Lauffenburger JC, Rhoney DH, Farley JF, et al. Predictors of gastrointestinal bleeding among patients with atrial fibrillation after initiating dabigatran therapy. Pharmacotherapy. 2015;35:560-568.
5. Tomaselli GF, Mahaffey KW, Cuker A, et al. 2020 ACC Expert Consensus Decision Pathway on Management of Bleeding in Patients on Oral Anticoagulants: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2020;76:594-622.
6. Taha A, McCloskey C, Craigen T, Angerson W. Antiplatelet versus anticoagulant effects in non-variceal upper gastrointestinal bleeding. Gut. 2019;68(suppl 2):A152.
7. Chan EW, Lau WC, Leung WK, et al. Prevention of dabigatran-related gastrointestinal bleeding with gastroprotective agents: A population-based study. Gastroenterology. 2015;149:586-595.
8. Ray WA, Chung CP, Murray KT, et al. Association of oral anticoagulants and proton pump inhibitor cotherapy with hospitalization for upper gastrointestinal tract bleeding. JAMA. 2018;320:2221-2230.
9. Brunetti L, Lee S-M, Doherty N, et al. Impact of warfarin discharge education program on hospital readmission and treatment costs. Int J Clin Pharm. 2018;40:721-729.
10. Hasan SS, Kow CS, Curley LE, et al. Economic evaluation of prescribing conventional and newer oral anticoagulants in older adults. Expert Rev Pharmacoecon Outcomes Res. 2018;18:371-377.
11. Phelps E, Delate T, Witt DM, et al. Effect of increased time in the therapeutic range on atrial fibrillation outcomes within a centralized anticoagulation service. Thromb Res. 2018;163:54-59.
12. Ahuja T, Raco V, Papadopoulos J, Green D. Antithrombotic stewardship: Assessing use of computerized clinical decision support tools to enhance safe prescribing of direct oral anticoagulants in hospitalized patients. J Patient Saf. 2018 Sep 25. [Epub ahead of print]
13. Leef GC, Perino AC, Askari M, et al. Appropriateness of direct oral anticoagulant dosing in patients with atrial fibrillation: Insights from the Veterans Health Administration. J Pharm Pract. 2020;33:647-653.
14. Papastergiou J, Kheir N, Ladova K, et al. Pharmacists’ confidence when providing pharmaceutical care on anticoagulants, a multinational survey. Int J Clin Pharm. 2017;39:1282-1290.
15. Perlman A, Horwitz E, Hirsh-Raccah B, et al. Clinical pharmacist led hospital-wide direct oral anticoagulant stewardship program. Isr J Health Policy Res. 2019;8:19.
16. Uppuluri EM, McComb MN, Shapiro NL. Implementation of a direct oral anticoagulation screening service at a large academic medical center provided by a pharmacist-managed antithrombosis clinic as a method to expand antithrombotic stewardship efforts. J Pharm Pract. 2020;33:271-275.
17. Manzoor BS, Cheng W-H, Lee JC, et al. Quality of pharmacist-managed anticoagulation therapy in long-term ambulatory settings: A systematic review. Ann Pharmacother. 2017;51:1122-1137.
18. Harris PA, Taylor R, Thielke R, et al. Research Electronic Data Capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-381.
19. Brooks M. AF management: Are clinicians in agreement? Medscape. May 30, 2019. Accessed December 29, 2020. https://www.medscape.com/viewarticle/913386
20. Amroze A, Mazor K, Crawford S, et al. Survey of confidence in use of stroke and bleeding risk calculators, knowledge of anticoagulants, and comfort with prescription of anticoagulation in challenging scenarios: SUPPORT-AF II study. J Thromb Thrombolysis. 2019;48:629-637.
21. Wang Y, Bajorek B. Decision-making around antithrombotics for stroke prevention in atrial fibrillation: the health professionals’ views. Int J Clin Pharm. 2016;38:985-995.
22. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2014;130:e199-e267.
23. January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019;74:104-132.
24. Anghel L, Sascu R, Trifan A, et al. Non-vitamin K antagonist oral anticoagulants and the gastrointestinal bleeding risk in real-world studies. J Clin Med. 2020;9:1398.
25. Langsted A, Nordestgaard BG. Smoking is associated with increased risk of major bleeding: a prospective cohort study. Thromb Haemost. 2019;119:39-47.
26. Faye AS, Hung KW, Cheng K, et al. HAS-BLED scores underestimate gastrointestinal bleeding risk among those with H. pylori. Am J Gastroenterol. 2019;114:S364.
27. Fawzy AM, Yang W-Y, Lip GY. Safety of direct oral anticoagulants in real-world clinical practice: translating the trials to everyday clinical management. Expert Opin Drug Saf. 2019;18:187-209.
28. Lip GYH, Keshishian A, Li X, et al. Effectiveness and safety of oral anticoagulants among nonvalvular atrial fibrillation patients. Stroke. 2018;49:2933-2944.
29. Abraham NS, Singh S, Alexander GC, et al. Comparative risk of gastrointestinal bleeding with dabigatran, rivaroxaban, and warfarin: population based cohort study. BMJ. 2015;350:h1857.
30. Holster IL, Valkhoff VE, Kuipers EJ, Tjwa E. New oral anticoagulants increase risk for gastrointestinal bleeding: a systematic review and meta-analysis. Gastroenterology. 2013;145:105-112.
31. Sherwood MW, Nessel CC, Hellkamp AS, et al. Gastrointestinal bleeding in patients with atrial fibrillation treated with rivaroxaban or warfarin: ROCKET AF Trial. J Am Coll Cardiol. 2015;66:2271-2281.
32. Di Minno A, Spadarella G, Spadarella E, et al. Gastrointestinal bleeding in patients receiving oral anticoagulation: Current treatment and pharmacological perspectives. Thromb Res. 2015;136:1074-1081.
33. Abraham NS, Hlatky MA, Antman EM, et al. ACCF/ACG/AHA 2010 Expert Consensus Document on the Concomitant Use of Proton Pump Inhibitors and Thienopyridines: A Focused Update of the ACCF/ACG/AHA 2008 Expert Consensus Document on Reducing the Gastrointestinal Risks of Antiplatelet Therapy and NSAID Use. Circulation. 2010;122:2619-2633.
34. Bhatt DL, Scheiman J, Abraham NS, et al. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2008;52:1502-1517.
35. Lanza FL, Chan FK, Quigley EM. Guidelines for prevention of NSAID-related ulcer complications. Am J Gastroenterol. 2009;104:728-738.
36. Bang CS, Joo MK, Kim BW, et al. The role of acid suppressants in the prevention of anticoagulant-related gastrointestinal bleeding: a systematic review and meta-analysis. Gut Liver. 2020;14:57-66.
37. Farrell B, Pottie K, Thompson W, et al. Deprescribing proton pump inhibitors: Evidence-based clinical practice guideline. Can Fam Physician. 2017;63:354-364.
38. Fossmark R, Martinsen TC, Waldum HL. Adverse effects of proton pump inhibitors—evidence and plausibility. Int J Mol Sci. 2019;20:5203.
39. Haastrup PF, Thompson W, Sondergaard J, Jarbol DE. Side effects of long-term proton pump inhibitor use: A review. Basic Clin Pharmacol Toxicol. 2018;123:114-121.
40. Wong JM, Maddox TM, Kennedy K, Shaw RE. Comparing major bleeding risk in outpatients with atrial fibrillation or flutter by oral anticoagulant type (from the National Cardiovascular Disease Registry’s Practice Innovation and Clinical Excellence Registry). Am J Cardiol. 2020;125:1500-1507.
41. Nagata N, Niikura R, Aoki T, et al. Effect of proton-pump inhibitors on the risk of lower gastrointestinal bleeding associated with NSAIDs, aspirin, clopidogrel, and warfarin. J Gastroenterol. 2015;50:1079-1086.
Timing of Complete Revascularization in Patients With STEMI
Study Overview
Objective. To determine the effect of the timing of nonculprit-lesion percutaneous coronary intervention (PCI) on outcomes in patients with ST-segment elevation myocardial infarction (STEMI).
Design. Planned substudy of an international, multicenter, randomized controlled trial blinded to outcome.
Setting and participants. Among 4041 patients with STEMI who had multivessel coronary disease, randomization to nonculprit PCI versus culprit-only PCI was stratified according to intended timing of nonculprit lesion PCI. A total of 2702 patients with intended timing of nonculprit PCI during the index hospitalization and 1339 patients with intended timing of nonculprit PCI after the index hospitalization within 45 days were included.
Main outcome measures. The first co-primary endpoint was a composite of cardiovascular (CV) death or myocardial infarction (MI).
Main results. In both groups, the composite endpoint of CV death or MI was reduced with complete revascularization compared to the culprit-only strategy (index hospitalization: hazard ratio [HR], 0.77, 95% confidence interval [CI], 0.59-1.00; after hospital discharge: HR, 0.69, 95% CI, 0.49-0.97; interaction, P = 0.62). Landmark analyses demonstrated a HR of 0.86 (95% CI, 0.59-1.24) during the first 45 days and 0.69 (95% CI,0.54-0.89) from 45 days to the end of follow-up for intended nonculprit lesion PCI versus culprit-lesion-only PCI.
Conclusion. Among patients with STEMI and multivessel disease, the benefit of complete revascularization over culprit-lesion-only PCI was consistent, irrespective of the investigator-determined timing of staged nonculprit lesion intervention.
Commentary
Patients presenting with STEMI often have multivessel disease.1 Although the question of whether to revascularize the nonculprit vessel has been controversial, multiple contemporary studies have reported benefit of nonculprit-vessel revascularization compared to the culprit-only strategy.2-5 Compared to these previous medium-sized randomized controlled trials that included ischemia-driven revascularization as a composite endpoint, the COMPETE trial was unique in that it enrolled a large number of patients and reported a benefit in hard outcomes of a composite of CV death or MI.6
As the previous studies point toward the benefit of complete revascularization in patients presenting with STEMI, another important question has been the optimal timing of nonculprit vessel revascularization. Operators have 3 possible options: during the index procedure as primary PCI, as a staged procedure during the index admission, or as a staged procedure as an outpatient following discharge. Timing of nonculprit PCI has been inconsistent in the previous studies. For example, in the PRAMI trial, nonculprit PCI was performed during the index procedure,2 while in the CvPRIT and COMPARE ACUTE trials, the nonculprit PCI was performed during the index procedure or as a staged procedure during the same admission at the operator’s discretion.3,5
In this context, the COMPLETE investigators report their findings of the prespecified substudy regarding the timing of staged nonculprit vessel PCI. In the COMPLETE trial, 4041 patients were stratified by intended timing of nonculprit lesion PCI (2702 patients during index hospitalization, 1339 after discharge), which was predetermined by the operator prior to the randomization. Among the patients with intended staged nonculprit PCI during index hospitalization, the incidence of the first co-primary outcome of CV death or MI was 2.7% per year in patients with complete revascularization, as compared to 3.5% per year in patients with culprit-lesion only PCI (HR, 0.77; 95% CI, 0.59-1.00). Similarly, in patients with intended nonculprit PCI after the index hospitalization, the incidence of the first co-primary outcome of CV death or MI was 2.7% per year in patients randomized to complete revascularization, as compared to 3.9% per year in patients with culprit-lesion-only PCI (HR, 0.69; 95% CI, 0.49-0.97). These findings were similar for the second co-primary outcome of CV death, MI, or ischemia-driven revascularization (3.0% vs 6.6% per year for intended timing of nonculprit PCI during index admission, and 3.1% vs 5.4% per year for intended timing of nonculprit PCI after discharge, both favoring complete revascularization).
The investigators also performed a landmark analysis before and after 45 days of randomization. Within the first 45 days, CV death or MI occurred in 2.5% of the complete revascularization group and 3.0% of the culprit-lesion-only PCI group (HR, 0.86; 95% CI, 0.59-1.24). On the other hand, during the interval from 45 days to the end of the study, CV death or MI occurred in 5.5% in the complete revascularization group and 7.8% in the culprit-lesion-only group (HR, 0.69; 95% CI, 0.54-0.89).
There were a number of strengths of the COMPLETE study, as we have previously described, such as multiple patients enrolled, contemporary therapy with high use of radial access, mandated use of fractional flow reserve for 50% to 69% stenosis lesions, and low cross-over rate.7 In addition, the current substudy is unique and important, as it was the first study to systematically evaluate the timing of the staged PCI. In addition to their finding of consistent benefit between staged procedure before or after discharge, the results from their landmark analysis suggest that the benefit of complete revascularization accumulates over the long term rather than the short term.
The main limitation of the COMPLETE study is that it was not adequately powered to find statistical differences in each subgroup studied. In addition, since all nonculprit PCIs were staged in this study, nonculprit PCI performed during the index procedure cannot be assessed.
Nevertheless, the finding of similar benefit of complete revascularization regardless of the timing of the staged PCI has clinical implication for practicing interventional cardiologists and patients presenting with STEMI. For example, if the patient presents with hemodynamically stable STEMI on a Friday, the patient can potentially be safely discharged over the weekend and return for a staged PCI as an outpatient instead of staying extra days for an inpatient staged PCI. Whether this approach may improve the patient satisfaction and hospital resource utilization will require further study.
Applications for Clinical Practice
In patients presenting with hemodynamically stable STEMI, staged complete revascularization can be performed during the admission or after discharge within 45 days.
—Taishi Hirai, MD
1. Park DW, Clare RM, Schulte PJ, et al. Extent, location, and clinical significance of non-infarct-related coronary artery disease among patients with ST-elevation myocardial infarction. JAMA. 2014;312:2019-2027.
2. Wald DS, Morris JK, Wald NJ, et al. Randomized trial of preventive angioplasty in myocardial infarction. N Engl J Med. 2013;369:1115-1123.
3. Gershlick AH, Khan JN, Kelly DJ, et al. Randomized trial of complete versus lesion-only revascularization in patients undergoing primary percutaneous coronary intervention for STEMI and multivessel disease: the CvLPRIT trial. J Am Coll Cardiol. 2015;65:963-972.
4. Engstrom T, Kelbaek H, Helqvist S, et al. Complete revascularisation versus treatment of the culprit lesion only in patients with ST-segment elevation myocardial infarction and multivessel disease (DANAMI-3-PRIMULTI): an open-label, randomised controlled trial. Lancet. 2015;386(9994):665-671.
5. Smits PC, Abdel-Wahab M, Neumann FJ, et al. Fractional flow reserve-guided multivessel angioplasty in myocardial infarction. N Engl J Med. 2017;376:1234-1244.
6. Mehta SR, Wood DA, Storey RF, et al. Complete revascularization with multivessel pci for myocardial infarction. N Engl J Med. 2019;381:1411-1421.
7. Hirai T, Blair JEA. Nonculprit lesion PCI strategies in patients with STEMI without cardiogenic shock. J Clin Outcomes Management. 2020;27:7-9.
Study Overview
Objective. To determine the effect of the timing of nonculprit-lesion percutaneous coronary intervention (PCI) on outcomes in patients with ST-segment elevation myocardial infarction (STEMI).
Design. Planned substudy of an international, multicenter, randomized controlled trial blinded to outcome.
Setting and participants. Among 4041 patients with STEMI who had multivessel coronary disease, randomization to nonculprit PCI versus culprit-only PCI was stratified according to intended timing of nonculprit lesion PCI. A total of 2702 patients with intended timing of nonculprit PCI during the index hospitalization and 1339 patients with intended timing of nonculprit PCI after the index hospitalization within 45 days were included.
Main outcome measures. The first co-primary endpoint was a composite of cardiovascular (CV) death or myocardial infarction (MI).
Main results. In both groups, the composite endpoint of CV death or MI was reduced with complete revascularization compared to the culprit-only strategy (index hospitalization: hazard ratio [HR], 0.77, 95% confidence interval [CI], 0.59-1.00; after hospital discharge: HR, 0.69, 95% CI, 0.49-0.97; interaction, P = 0.62). Landmark analyses demonstrated a HR of 0.86 (95% CI, 0.59-1.24) during the first 45 days and 0.69 (95% CI,0.54-0.89) from 45 days to the end of follow-up for intended nonculprit lesion PCI versus culprit-lesion-only PCI.
Conclusion. Among patients with STEMI and multivessel disease, the benefit of complete revascularization over culprit-lesion-only PCI was consistent, irrespective of the investigator-determined timing of staged nonculprit lesion intervention.
Commentary
Patients presenting with STEMI often have multivessel disease.1 Although the question of whether to revascularize the nonculprit vessel has been controversial, multiple contemporary studies have reported benefit of nonculprit-vessel revascularization compared to the culprit-only strategy.2-5 Compared to these previous medium-sized randomized controlled trials that included ischemia-driven revascularization as a composite endpoint, the COMPETE trial was unique in that it enrolled a large number of patients and reported a benefit in hard outcomes of a composite of CV death or MI.6
As the previous studies point toward the benefit of complete revascularization in patients presenting with STEMI, another important question has been the optimal timing of nonculprit vessel revascularization. Operators have 3 possible options: during the index procedure as primary PCI, as a staged procedure during the index admission, or as a staged procedure as an outpatient following discharge. Timing of nonculprit PCI has been inconsistent in the previous studies. For example, in the PRAMI trial, nonculprit PCI was performed during the index procedure,2 while in the CvPRIT and COMPARE ACUTE trials, the nonculprit PCI was performed during the index procedure or as a staged procedure during the same admission at the operator’s discretion.3,5
In this context, the COMPLETE investigators report their findings of the prespecified substudy regarding the timing of staged nonculprit vessel PCI. In the COMPLETE trial, 4041 patients were stratified by intended timing of nonculprit lesion PCI (2702 patients during index hospitalization, 1339 after discharge), which was predetermined by the operator prior to the randomization. Among the patients with intended staged nonculprit PCI during index hospitalization, the incidence of the first co-primary outcome of CV death or MI was 2.7% per year in patients with complete revascularization, as compared to 3.5% per year in patients with culprit-lesion only PCI (HR, 0.77; 95% CI, 0.59-1.00). Similarly, in patients with intended nonculprit PCI after the index hospitalization, the incidence of the first co-primary outcome of CV death or MI was 2.7% per year in patients randomized to complete revascularization, as compared to 3.9% per year in patients with culprit-lesion-only PCI (HR, 0.69; 95% CI, 0.49-0.97). These findings were similar for the second co-primary outcome of CV death, MI, or ischemia-driven revascularization (3.0% vs 6.6% per year for intended timing of nonculprit PCI during index admission, and 3.1% vs 5.4% per year for intended timing of nonculprit PCI after discharge, both favoring complete revascularization).
The investigators also performed a landmark analysis before and after 45 days of randomization. Within the first 45 days, CV death or MI occurred in 2.5% of the complete revascularization group and 3.0% of the culprit-lesion-only PCI group (HR, 0.86; 95% CI, 0.59-1.24). On the other hand, during the interval from 45 days to the end of the study, CV death or MI occurred in 5.5% in the complete revascularization group and 7.8% in the culprit-lesion-only group (HR, 0.69; 95% CI, 0.54-0.89).
There were a number of strengths of the COMPLETE study, as we have previously described, such as multiple patients enrolled, contemporary therapy with high use of radial access, mandated use of fractional flow reserve for 50% to 69% stenosis lesions, and low cross-over rate.7 In addition, the current substudy is unique and important, as it was the first study to systematically evaluate the timing of the staged PCI. In addition to their finding of consistent benefit between staged procedure before or after discharge, the results from their landmark analysis suggest that the benefit of complete revascularization accumulates over the long term rather than the short term.
The main limitation of the COMPLETE study is that it was not adequately powered to find statistical differences in each subgroup studied. In addition, since all nonculprit PCIs were staged in this study, nonculprit PCI performed during the index procedure cannot be assessed.
Nevertheless, the finding of similar benefit of complete revascularization regardless of the timing of the staged PCI has clinical implication for practicing interventional cardiologists and patients presenting with STEMI. For example, if the patient presents with hemodynamically stable STEMI on a Friday, the patient can potentially be safely discharged over the weekend and return for a staged PCI as an outpatient instead of staying extra days for an inpatient staged PCI. Whether this approach may improve the patient satisfaction and hospital resource utilization will require further study.
Applications for Clinical Practice
In patients presenting with hemodynamically stable STEMI, staged complete revascularization can be performed during the admission or after discharge within 45 days.
—Taishi Hirai, MD
Study Overview
Objective. To determine the effect of the timing of nonculprit-lesion percutaneous coronary intervention (PCI) on outcomes in patients with ST-segment elevation myocardial infarction (STEMI).
Design. Planned substudy of an international, multicenter, randomized controlled trial blinded to outcome.
Setting and participants. Among 4041 patients with STEMI who had multivessel coronary disease, randomization to nonculprit PCI versus culprit-only PCI was stratified according to intended timing of nonculprit lesion PCI. A total of 2702 patients with intended timing of nonculprit PCI during the index hospitalization and 1339 patients with intended timing of nonculprit PCI after the index hospitalization within 45 days were included.
Main outcome measures. The first co-primary endpoint was a composite of cardiovascular (CV) death or myocardial infarction (MI).
Main results. In both groups, the composite endpoint of CV death or MI was reduced with complete revascularization compared to the culprit-only strategy (index hospitalization: hazard ratio [HR], 0.77, 95% confidence interval [CI], 0.59-1.00; after hospital discharge: HR, 0.69, 95% CI, 0.49-0.97; interaction, P = 0.62). Landmark analyses demonstrated a HR of 0.86 (95% CI, 0.59-1.24) during the first 45 days and 0.69 (95% CI,0.54-0.89) from 45 days to the end of follow-up for intended nonculprit lesion PCI versus culprit-lesion-only PCI.
Conclusion. Among patients with STEMI and multivessel disease, the benefit of complete revascularization over culprit-lesion-only PCI was consistent, irrespective of the investigator-determined timing of staged nonculprit lesion intervention.
Commentary
Patients presenting with STEMI often have multivessel disease.1 Although the question of whether to revascularize the nonculprit vessel has been controversial, multiple contemporary studies have reported benefit of nonculprit-vessel revascularization compared to the culprit-only strategy.2-5 Compared to these previous medium-sized randomized controlled trials that included ischemia-driven revascularization as a composite endpoint, the COMPETE trial was unique in that it enrolled a large number of patients and reported a benefit in hard outcomes of a composite of CV death or MI.6
As the previous studies point toward the benefit of complete revascularization in patients presenting with STEMI, another important question has been the optimal timing of nonculprit vessel revascularization. Operators have 3 possible options: during the index procedure as primary PCI, as a staged procedure during the index admission, or as a staged procedure as an outpatient following discharge. Timing of nonculprit PCI has been inconsistent in the previous studies. For example, in the PRAMI trial, nonculprit PCI was performed during the index procedure,2 while in the CvPRIT and COMPARE ACUTE trials, the nonculprit PCI was performed during the index procedure or as a staged procedure during the same admission at the operator’s discretion.3,5
In this context, the COMPLETE investigators report their findings of the prespecified substudy regarding the timing of staged nonculprit vessel PCI. In the COMPLETE trial, 4041 patients were stratified by intended timing of nonculprit lesion PCI (2702 patients during index hospitalization, 1339 after discharge), which was predetermined by the operator prior to the randomization. Among the patients with intended staged nonculprit PCI during index hospitalization, the incidence of the first co-primary outcome of CV death or MI was 2.7% per year in patients with complete revascularization, as compared to 3.5% per year in patients with culprit-lesion only PCI (HR, 0.77; 95% CI, 0.59-1.00). Similarly, in patients with intended nonculprit PCI after the index hospitalization, the incidence of the first co-primary outcome of CV death or MI was 2.7% per year in patients randomized to complete revascularization, as compared to 3.9% per year in patients with culprit-lesion-only PCI (HR, 0.69; 95% CI, 0.49-0.97). These findings were similar for the second co-primary outcome of CV death, MI, or ischemia-driven revascularization (3.0% vs 6.6% per year for intended timing of nonculprit PCI during index admission, and 3.1% vs 5.4% per year for intended timing of nonculprit PCI after discharge, both favoring complete revascularization).
The investigators also performed a landmark analysis before and after 45 days of randomization. Within the first 45 days, CV death or MI occurred in 2.5% of the complete revascularization group and 3.0% of the culprit-lesion-only PCI group (HR, 0.86; 95% CI, 0.59-1.24). On the other hand, during the interval from 45 days to the end of the study, CV death or MI occurred in 5.5% in the complete revascularization group and 7.8% in the culprit-lesion-only group (HR, 0.69; 95% CI, 0.54-0.89).
There were a number of strengths of the COMPLETE study, as we have previously described, such as multiple patients enrolled, contemporary therapy with high use of radial access, mandated use of fractional flow reserve for 50% to 69% stenosis lesions, and low cross-over rate.7 In addition, the current substudy is unique and important, as it was the first study to systematically evaluate the timing of the staged PCI. In addition to their finding of consistent benefit between staged procedure before or after discharge, the results from their landmark analysis suggest that the benefit of complete revascularization accumulates over the long term rather than the short term.
The main limitation of the COMPLETE study is that it was not adequately powered to find statistical differences in each subgroup studied. In addition, since all nonculprit PCIs were staged in this study, nonculprit PCI performed during the index procedure cannot be assessed.
Nevertheless, the finding of similar benefit of complete revascularization regardless of the timing of the staged PCI has clinical implication for practicing interventional cardiologists and patients presenting with STEMI. For example, if the patient presents with hemodynamically stable STEMI on a Friday, the patient can potentially be safely discharged over the weekend and return for a staged PCI as an outpatient instead of staying extra days for an inpatient staged PCI. Whether this approach may improve the patient satisfaction and hospital resource utilization will require further study.
Applications for Clinical Practice
In patients presenting with hemodynamically stable STEMI, staged complete revascularization can be performed during the admission or after discharge within 45 days.
—Taishi Hirai, MD
1. Park DW, Clare RM, Schulte PJ, et al. Extent, location, and clinical significance of non-infarct-related coronary artery disease among patients with ST-elevation myocardial infarction. JAMA. 2014;312:2019-2027.
2. Wald DS, Morris JK, Wald NJ, et al. Randomized trial of preventive angioplasty in myocardial infarction. N Engl J Med. 2013;369:1115-1123.
3. Gershlick AH, Khan JN, Kelly DJ, et al. Randomized trial of complete versus lesion-only revascularization in patients undergoing primary percutaneous coronary intervention for STEMI and multivessel disease: the CvLPRIT trial. J Am Coll Cardiol. 2015;65:963-972.
4. Engstrom T, Kelbaek H, Helqvist S, et al. Complete revascularisation versus treatment of the culprit lesion only in patients with ST-segment elevation myocardial infarction and multivessel disease (DANAMI-3-PRIMULTI): an open-label, randomised controlled trial. Lancet. 2015;386(9994):665-671.
5. Smits PC, Abdel-Wahab M, Neumann FJ, et al. Fractional flow reserve-guided multivessel angioplasty in myocardial infarction. N Engl J Med. 2017;376:1234-1244.
6. Mehta SR, Wood DA, Storey RF, et al. Complete revascularization with multivessel pci for myocardial infarction. N Engl J Med. 2019;381:1411-1421.
7. Hirai T, Blair JEA. Nonculprit lesion PCI strategies in patients with STEMI without cardiogenic shock. J Clin Outcomes Management. 2020;27:7-9.
1. Park DW, Clare RM, Schulte PJ, et al. Extent, location, and clinical significance of non-infarct-related coronary artery disease among patients with ST-elevation myocardial infarction. JAMA. 2014;312:2019-2027.
2. Wald DS, Morris JK, Wald NJ, et al. Randomized trial of preventive angioplasty in myocardial infarction. N Engl J Med. 2013;369:1115-1123.
3. Gershlick AH, Khan JN, Kelly DJ, et al. Randomized trial of complete versus lesion-only revascularization in patients undergoing primary percutaneous coronary intervention for STEMI and multivessel disease: the CvLPRIT trial. J Am Coll Cardiol. 2015;65:963-972.
4. Engstrom T, Kelbaek H, Helqvist S, et al. Complete revascularisation versus treatment of the culprit lesion only in patients with ST-segment elevation myocardial infarction and multivessel disease (DANAMI-3-PRIMULTI): an open-label, randomised controlled trial. Lancet. 2015;386(9994):665-671.
5. Smits PC, Abdel-Wahab M, Neumann FJ, et al. Fractional flow reserve-guided multivessel angioplasty in myocardial infarction. N Engl J Med. 2017;376:1234-1244.
6. Mehta SR, Wood DA, Storey RF, et al. Complete revascularization with multivessel pci for myocardial infarction. N Engl J Med. 2019;381:1411-1421.
7. Hirai T, Blair JEA. Nonculprit lesion PCI strategies in patients with STEMI without cardiogenic shock. J Clin Outcomes Management. 2020;27:7-9.
Health Care Disparities Among Adolescents and Adults With Sickle Cell Disease: A Community-Based Needs Assessment to Inform Intervention Strategies
From the University of California San Francisco (Dr. Treadwell, Dr. Hessler, Yumei Chen, Swapandeep Mushiana, Dr. Potter, and Dr. Vichinsky), the University of California Los Angeles (Dr. Jacob), and the University of California Berkeley (Alex Chen).
Abstract
- Objective: Adolescents and adults with sickle cell disease (SCD) face pervasive disparities in health resources and outcomes. We explored barriers to and facilitators of care to identify opportunities to support implementation of evidence-based interventions aimed at improving care quality for patients with SCD.
- Methods: We engaged a representative sample of adolescents and adults with SCD (n = 58), health care providers (n = 51), and community stakeholders (health care administrators and community-based organization leads (n = 5) in Northern California in a community-based needs assessment. We conducted group interviews separately with participant groups to obtain in-depth perspectives. Adolescents and adults with SCD completed validated measures of pain interference, quality of care, self-efficacy, and barriers to care. Providers and community stakeholders completed surveys about barriers to SCD care.
- Results: We triangulated qualitative and quantitative data and found that participants with SCD (mean age, 31 ± 8.6 years), providers, and community stakeholders emphasized the social and emotional burden of SCD as barriers. Concrete barriers agreed upon included insurance and lack of resources for addressing pain impact. Adolescents and adults with SCD identified provider issues (lack of knowledge, implicit bias), transportation, and limited social support as barriers. Negative encounters with the health care system contributed to 84% of adolescents and adults with SCD reporting they chose to manage severe pain at home. Providers focused on structural barriers: lack of access to care guidelines, comfort level with and knowledge of SCD management, and poor care coordination.
- Conclusion: Strategies for improving access to compassionate, evidence-based quality care, as well as strategies for minimizing the burden of having SCD, are warranted for this medically complex population.
Keywords: barriers to care; quality of care; care access; care coordination.
Sickle cell disease (SCD), an inherited chronic medical condition, affects about 100,000 individuals in the United States, a population that is predominantly African American.1 These individuals experience multiple serious and life-threatening complications, most frequently recurrent vaso-occlusive pain episodes,2 and they require interactions with multidisciplinary specialists from childhood. Because of advances in treatments, the majority are reaching adulthood; however, there is a dearth of adult health care providers with the training and expertise to manage their complex medical needs.3 Other concrete barriers to adequate SCD care include insurance and distance to comprehensive SCD centers.4,5
Social, behavioral, and emotional factors may also contribute to challenges with SCD management. SCD may limit daily functional abilities and lead to diminished overall quality of life.6,7 Some adolescents and adults may require high doses of opioids, which contributes to health care providers’ perceptions that there is a high prevalence of drug addiction in the population.8,9 These providers express negative attitudes towards adults with SCD, and, consequently, delay medication administration when it is acutely needed and provide otherwise suboptimal treatment.8,10,11 Adult care providers may also be uncomfortable with prescribing and managing disease-modifying therapies (blood transfusion, hydroxyurea) that have established efficacy.12-17
As 1 of 8 programs funded by the National Heart, Lung, and Blood Institute’s (NHLBI) Sickle Cell Disease Implementation Consortium (SCDIC), we are using implementation science to reduce barriers to care and improve quality of care and health care outcomes in SCD.18,19 Given that adolescents and adults with SCD experience high mortality, severe pain, and progressive decline in their ability to function day to day, and also face lack of access to knowledgeable, compassionate providers in primary and emergency settings, the SCDIC focuses on individuals aged 15 to 45 years.6,8,9,11,12
Our regional SCDIC program, the Sickle Cell Care Coordination Initiative (SCCCI), brings together researchers, clinicians, adolescents, and adults with SCD and their families, dedicated community members, policy makers, and administrators to identify and address barriers to health care within 5 counties in Northern California. One of our first steps was to conduct a community-based needs assessment, designed to inform implementation of evidence-based interventions, accounting for unique contextual factors in our region.
Conceptual Framework for Improving Medical Practice
Our needs assessment is guided by Solberg’s Conceptual Framework for Improving Medical Practice (Figure 1).20 Consistent with the overarching principles of the SCDIC, this conceptual framework focuses on the inadequate implementation of evidence-based guidelines, and on the need to first understand multifactorial facilitators and barriers to guideline implementation in order to effect change. The framework identifies 3 main elements that must be present to ensure improvements in quality-of-care processes and patient outcomes: priority, change process capability, and care process content. Priority refers to ample resource allocation for the specific change, as well as freedom from competing priorities for those implementing the change. Change process capability includes strong, effective leadership, adequate infrastructure for managing change (including resources and time), change management skills at all levels, and an established clinical information system. Care process content refers to context and systems-level changes, such as delivery system redesign as needed, support for self-management to lessen the impact of the disease, and decision support.21-23
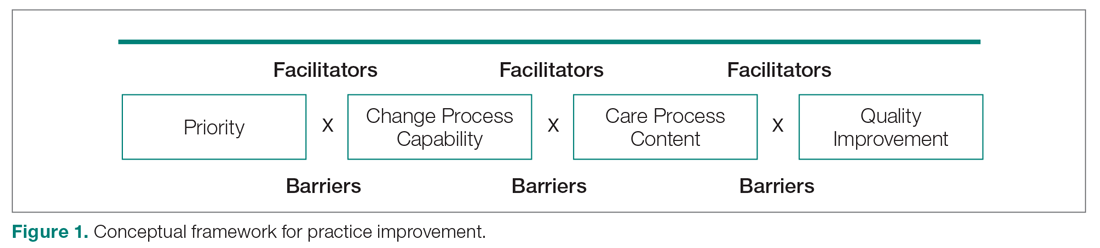
The purpose of our community-based needs assessment was to evaluate barriers to care and quality of care in SCD, within Solberg’s conceptual model for improving medical practice. The specific aims were to evaluate access and barriers to care (eg, lack of provider expertise and training, health care system barriers such as poor care coordination and provider communication); evaluate quality of care; and assess patient needs related to pain, pain interference, self-efficacy, and self-management for adolescents and adults with SCD. We gathered the perspectives of a representative community of adolescents and adults with SCD, their providers, and community stakeholders in order to examine barriers, quality of life and care, and patient experiences in our region.
Methods
Design
In this cross-sectional study, adolescents and adults with SCD, their providers, and community stakeholders participated in group or individual qualitative interviews and completed surveys between October 2017 and March 2018.
Setting and Sample
Recruitment flyers were posted on a regional SCD-focused website, and clinical providers or a study coordinator introduced information about the needs assessment to potential participants with SCD during clinic visits at the participating centers. Participants with SCD were eligible if they had any diagnosis of SCD, were aged 15 to 48 years, and received health services within 5 Northern California counties (Alameda, Contra Costa, Sacramento, San Francisco, and Solano). They were excluded if they did not have a SCD diagnosis or had not received health services within the catchment area. As the project proceeded, participants were asked to refer other adolescents and adults with SCD for the interviews and surveys (snowball sampling). Our goal was to recruit 50 adolescents and adults with SCD into the study, aiming for 10 representatives from each county.
Providers and community stakeholders were recruited via emails, letters and informational flyers. We engaged our partner, the Sickle Cell Data Collection Program,2 to generate a list of providers and institutions that had seen patients with SCD in primary, emergency, or inpatient settings in the region. We contacted these institutions to describe the SCCCI and invite participation in the needs assessment. We also invited community-based organization leads and health care administrators who worked with SCD to participate. Providers accessed confidential surveys via a secure link on the study website or completed paper versions. Common data collected across providers included demographics and descriptions of practice settings.
Participants were eligible to be part of the study if they were health care providers (physicians and nurses) representing hematology, primary care, family medicine, internal medicine, or emergency medicine; ancillary staff (social work, psychology, child life); or leaders or administrators of clinical or sickle cell community-based organizations in Northern California (recruitment goal of n = 50). Providers were excluded if they practiced in specialties other than those noted or did not practice within the region.
Data Collection Procedures
After providing assent/consent, participating adolescents and adults with SCD took part in individual and group interviews and completed survey questionnaires. All procedures were conducted in a private space in the sickle cell center or community. Adolescents and adults with SCD completed the survey questionnaire on a tablet, with responses recorded directly in a REDCap (Research Electronic Data Capture) database,24 or on a paper version. Interviews lasted 60 (individual) to 90 (group) minutes, while survey completion time was 20 to 25 minutes. Each participant received a gift card upon completion as an expression of appreciation. All procedures were approved by the institutional review boards of the participating health care facilities.
Group and Individual Interviews
Participants with SCD and providers were invited to participate in a semi-structured qualitative interview prior to being presented with the surveys. Adolescents and adults with SCD were interviewed about barriers to care, quality of care, and pain-related experiences. Providers were asked about barriers to care and treatments. Interview guides were modified for community-based organization leaders and health care administrators who did not provide clinical services. Interview guides can be found in the Appendix. Interviews were conducted by research coordinators trained in qualitative research methods by the first author (MT). As appropriate with semi-structured interviews, the interviewers could word questions spontaneously, change the order of questions for ease of flow of conversation, and inform simultaneous coding of interviews with new themes as those might arise, as long as they touched on all topics within the interview guide.25 The interview guides were written, per qualitative research standards, based on the aims and purpose of the research,26 and were informed by existing literature on access and barriers to care in SCD, quality of care, and the needs of individuals with SCD, including in relation to impact of the disease, self-efficacy, and self-management.
Interviewees participated in either individual or group interviews, but not both. The decision for which type of interview an individual participated in was based on 2 factors: if there were not comparable participants for group interviews (eg, health care administrator and community-based organization lead), these interviews were done individually; and given that we were drawing participants from a 5-county area in Northern California, scheduling was challenging for individuals with SCD with regard to aligning schedules and traveling to a central location where the group interviews were conducted. Provider group interviews were easier to arrange because we could schedule them at the same time as regularly scheduled meetings at the participants’ health care institutions.
Interview Data Gathering and Analysis
Digital recordings of the interviews were cleaned of any participant identifying data and sent for transcription to an outside service. Transcripts were reviewed for completeness and imported into NVivo (www.qsrinternational.com), a qualitative data management program.
A thematic content analysis and deductive and inductive approaches were used to analyze the verbatim transcripts generated from the interviews. The research team was trained in the use of NVivo software to facilitate the coding process. A deductive coding scheme was initially used based on existing concepts in the literature regarding challenges to optimal SCD care, with new codes added as the thematic content analyses progressed. The initial coding, pattern coding, and use of displays to examine the relationships between different categories were conducted simultaneously.27,28 Using the constant comparative method, new concepts from participants with SCD and providers could be incorporated into subsequent interviews with other participants. For this study, the only additional concepts added were in relation to participant recruitment and retention in the SCDIC Registry. Research team members coded transcripts separately and came together weekly, constantly comparing codes and developing the consensus coding scheme. Where differences between coders existed, code meanings were discussed and clarified until consensus was reached.29
Quantitative data were analyzed using SPSS (v. 25, Chicago, IL). Descriptive statistics (means, standard deviations, frequencies, percentages) were used to summarize demographics (eg, age, gender, and race), economic status, and type of SCD. No systematic differences were detected from cases with missing values. Scale reliabilities (ie, Cronbach α) were evaluated for self-report measures.
Measurement
Adolescents and adults with SCD completed items from the PhenX Toolkit (consensus measures for Phenotypes and eXposures), assessing sociodemographics (age, sex, race, ethnicity, educational attainment, occupation, marital status, annual income, insurance), and clinical characteristics (sickle cell diagnosis and emergency department [ED] and hospital utilization for pain).30
Pain Interference Short Form (Patient-Reported Outcomes Measurement Information System [PROMIS]). The Pain Interference Form consists of 8 items that assess the degree to which pain interfered with day-to-day activities in the previous 7 days at home, including impacts on social, cognitive, emotional, and physical functioning; household chores and recreational activities; sleep; and enjoyment in life. Reliability and validity of the PROMIS Pain Interference Scale has been demonstrated, with strong negative correlations with Physical Function Scales (r = 0.717, P < 0.01), indicating that higher scores are associated with lower function (β = 0.707, P < 0.001).31 The Cronbach α estimate for the other items on the pain interference scale was 0.99. Validity analysis indicated strong correlations with pain-related domains: BPI Interference Subscale (rho = 0.90), SF-36 Bodily Pain Subscale (rho = –0.84), and 0–10 Numerical Rating of Pain Intensity (rho = 0.48).32
Adult Sickle Cell Quality of Life Measurement Information System (ASCQ-Me) Quality of Care (QOC). ASCQ-Me QOC consists of 27 items that measure the quality of care that adults with SCD have received from health care providers.33 There are 3 composites: provider communication (quality of patient and provider communication), ED care (quality of care in the ED), and access (to routine and emergency care). Internal consistency reliability for all 3 composites is greater than 0.70. Strong correlations of the provider communication composite with overall ratings of routine care (r = 0.65) and overall provider ratings (r = 0.83) provided evidence of construct validity. Similarly, the ED care composite was strongly correlated with overall ratings of QOC in the ED, and the access composite was highly correlated with overall evaluations of ED care (r = 0.70). Access, provider interaction, and ED care composites were reliable (Cronbach α, 0.70–0.83) and correlated with ratings of global care (r = 0.32–0.83), further indicating construct validity.33
Sickle Cell Self-Efficacy Scale (SCSES). The SCSES is a 9-item, self-administered questionnaire measuring perceptions of the ability to manage day-to-day issues resulting from SCD. SCSES items are scored on a 5-point scale ranging from Not sure at all (1) to Very sure (5). Individual item responses are summed to give an overall score, with higher scores indicating greater self-efficacy. The SCSES has acceptable reliability (r = 0.45, P < 0.001) and validity (α = 0.89).34,35
Sickle Cell Disease Barriers Checklist. This checklist consists of 53 items organized into 8 categories: insurance, transportation, accommodations and accessibility, provider knowledge and attitudes, social support, individual barriers such as forgetting or difficulties understanding instructions, emotional barriers (fear, anger), and disease-related barriers. Participants check applicable barriers, with a total score range of 0 to 53 and higher scores indicating more barriers to care. The SCD Barriers Checklist has demonstrated face validity and test-retest reliability (Pearson r = 0.74, P < 0.05).5
ED Provider Checklist. The ED provider survey is a checklist of 14 statements pertaining to issues regarding patient care, with which the provider rates level of agreement. Items representing the attitudes and beliefs of providers towards patients with SCD are rated on a Likert-type scale, with level of agreement indicated as 1 (strongly disagree) to 6 (strongly agree). The positive attitudes subscale consists of 4 items (Cronbach α= 0.85), and the negative attitudes subscale consists of 6 items (Cronbach α = 0.89). The Red-Flag Behaviors subscale includes 4 items that indicate behavior concerns about drug-seeking, such as requesting specific narcotics and changing behavior when the provider walks in.8,36,37
Sickle cell and primary care providers also completed a survey consisting of sets of items compiled from existing provider surveys; this survey consisted of a list of 16 barriers to using opioids, which the providers rated on a 5-point Likert-type scale (1, not a barrier; 5, complete barrier).13,16,38 Providers indicated their level of experience with caring for patients with SCD; care provided, such as routine health screenings; and comfort level with providing preventive care, managing comorbidities, and managing acute and chronic pain. Providers were asked what potential facilitators might improve care for patients with SCD, including higher reimbursement, case management services, access to pain management specialists, and access to clinical decision-support tools. Providers responded to specific questions about management with hydroxyurea (eg, criteria for, barriers to, and comfort level with prescribing).39 The surveys are included in the Appendix.
Triangulation
Data from the interviews and surveys were triangulated to enhance understanding of results generated from the different data sources.40 Convergence of findings, different facets of the same phenomenon, or new perspectives were examined.
Results
Qualitative Data
Adolescents and adults with SCD (n = 55) and health care providers and community stakeholders (n = 56) participated in group or individual interviews to help us gain an in-depth understanding of the needs and barriers related to SCD care in our 5-county region. Participants with SCD described their experiences, which included stigma, racism, labeling, and, consequently, stress. They also identified barriers such as lack of transportation, challenges with insurance, and lack of access to providers who were competent with pain management. They reported that having SCD in a health care system that was unable to meet their needs was burdensome.
Barriers to Care and Treatments. Adolescents and adults indicated that SCD and its sequelae posed significant barriers to health care. Feelings of tiredness and pain make it more difficult for them to seek care. The emotional burden of SCD (fear and anger) was a frequently cited barrier, which was fueled by previous negative encounters with the health care system. All adolescents and adults with SCD reported that they knew of stigma in relation to seeking pain management that was pervasive and long-standing, and the majority reported they had directly experienced stigma. They reported that being labeled as “drug-seekers” was typical when in the ED for pain management. Participants articulated unconscious bias or overt racism among providers: “people with sickle cell are Black ... and Black pain is never as valuable as White pain” (25-year-old male). Respondents with SCD described challenges to the credibility of their pain reports in the ED. They reported that ED providers expressed doubts regarding the existence and/or severity of their pain, consequently creating a feeling of disrespect for patients seeking pain relief. The issue of stigma was mentioned by only 2 of 56 providers during their interviews.
Lack of Access to Knowledgeable, Compassionate Providers. Lack of access to knowledgeable care providers was another prevalent theme expressed by adolescents and adults with SCD. Frustration occurred when providers did not have knowledge of SCD and its management, particularly pain assessment. Adolescents and adults with SCD noted the lack of compassion among providers: “I’ve been kicked out of the hospital because they felt like okay, well we gave you enough medication, you should be all right” (29-year-old female). Providers specifically mentioned lack of compassion and knowledge as barriers to SCD care much less often during their interviews compared with the adolescents and adults with SCD.
Health Care System Barriers. Patient participants often expressed concerns about concrete and structural aspects of care. Getting to their appointments was a challenge for half of the interviewees, as they either did not have access to a vehicle or could not afford to travel the needed distance to obtain quality care. Even when hospitals were accessible by public transportation, those with excruciating pain understandably preferred a more comfortable and private way to travel: “I would like to change that, something that will be much easier, convenient for sickle cell patients that do suffer with pain, that they don’t have to travel always to see the doctor” (30-year-old male).
Insurance and other financial barriers also played an important role in influencing decisions to seek health care services. Medical expenses were not covered, or co-pays were too high. The Medicaid managed care system could prevent access to knowledgeable providers who were not within network. Such a lack of access discouraged some adolescents and adults with SCD from seeking acute and preventive care.
Transition From Pediatric to Adult Care. Interviewees with SCD expressed distress about the gap between pediatric and adult care. They described how they had a long-standing relationship with their medical providers, who were familiar with their medical background and history from childhood. Adolescent interviewees reported an understanding of their own pain management as well as adherence to and satisfaction with their individualized pain plans. However, adults noted that satisfaction plummeted with increasing age due to the limited number of experienced adult SCD providers, which was compounded by negative experiences (stigma, racism, drug-seeking label).
One interviewee emphasized the difficulty of finding knowledgeable providers after transition: “When you’re a pediatric sickle cell [patient], you have the doctors there every step of the way, but not with adult sickle cell… I know when I first transitioned I never felt more alone in my life… you look at that ER doctor kind of with the same mindset as you would your hematologist who just hand walked you through everything. And adult care providers were a lot more blunt and cold and they’re like… ‘I don’t know; I’m not really educated in sickle cell.’” A sickle cell provider shared his insight about the problem of transitioning: “I think it’s particularly challenging because we, as a community, don’t really set them up for success. It’s different from other chronic conditions [in that] it’s much harder to find an adult sickle cell provider. There’s not a lot of adult hematologists that will take care of our adult patients, and so I know statistically, there’s like a drop-down in the overall outcomes of our kids after they age out of our pediatric program.”
Self-Management, Supporting Hydroxyurea Use. Interview participants with SCD reported using a variety of methods to manage pain at home and chose to go to the ED only when the pain became intolerable. Patients and providers expressed awareness of different resources for managing pain at home, yet they also indicated that these resources have not been consolidated in an accessible way for patients and families. Some resources cited included heat therapy, acupuncture, meditation, medical marijuana, virtual reality devices, and pain medications other than opioids.
Patients and providers expressed the need for increasing awareness and education about hydroxyurea. Many interview participants with SCD were concerned about side effects, multiple visits with a provider during dose titration, and ongoing laboratory monitoring. They also expressed difficulties with scheduling multiple appointments, depending on access to transportation and limited provider clinic hours. They were aware of strategies for improving adherence with hydroxyurea, including setting phone alarms, educating family members about hydroxyurea, and eliciting family support, but expressed needing help to consistently implement these strategies.
Safe Opioid Prescribing. Adult care providers expressed concerns about safe opioid prescribing for patients with SCD. They were reluctant to prescribe opioid doses needed to adequately control SCD pain. Providers expressed uncertainty and fear or concern about medical/legal liability or about their judgment about what’s safe and not safe for patients with chronic use/very high doses of opioids. “I know we’re in like this opiate epidemic here in this country but I feel like these patients don’t really fit under that umbrella that the problem is coming from so [I am] just trying to learn more about how to take care of them.”
Care Coordination and Provider Communication. Adolescents and adults with SCD reported having positive experiences—good communication, established trust, and compassionate care—with their usual providers. However, they perceived that ED physicians and nurses did not really care about them. Both interviewees with SCD and providers recognized the importance of good communication in all settings as the key to overcoming barriers to receiving quality care. All agreed on the importance of using individual pain plans so that all providers, especially ED providers, can be more at ease with treating adolescents and adults with SCD.
Quantitative Data: Adolescents and Adults With SCD
Fifty-eight adolescents and adults with SCD (aged 15 to 48 years) completed the survey. Three additional individuals who did not complete the interview completed the survey. Reasons for not completing the interview included scheduling challenges (n = 2) or a sickle cell pain episode (n = 1). The average age of participants was 31 years ± 8.6, more than half (57%) were female, and the majority (93%) were African American (Table 1). Most (71%) had never been married. Half (50%) had some college or an associate degree, and 40% were employed and reported an annual household income of less than $30,000. Insurance coverage was predominantly Medi-Cal (Medicaid, 69%). The majority of participants resided in Alameda (34.5%) or Contra Costa (21%) counties. The majority of sickle cell care was received in Alameda County, whether outpatient (52%), inpatient (40%), or ED care (41%). The majority (71%) had a diagnosis of SCD hemoglobin SS.
Pain. More than one-third of individuals with SCD reported 1 or 2 ED visits for pain in the previous 6 months (34%), and more than 3 hospitalizations (36%) related to pain in the previous year (Table 2). The majority (85%) reported having severe pain at home in the previous 6 months that they did not seek health care for, consistent with their reports in the qualitative interviews. More than half (59%) reported 4 or more of these severe pain episodes that led to inability to perform daily activities for 1 week or more. While pain interference on the PROMIS Pain Interference Short Form on average (T-score, 59.6 ± 8.6) was similar to that of the general population (T-score, 50 ± 10), a higher proportion of patients with SCD reported pain interference compared with the general population. The mean self-efficacy (confidence in ability to manage complications of SCD) score on the SCSES of 30.0 ± 7.3 (range, 9–45) was similar to that of other adults with SCD (mean, 32.2 ± 7.0). Twenty-five percent of the present sample had a low self-efficacy score (< 25).
Barriers to Care and Treatments. Consistent with the qualitative data, SCD-related symptoms such as tiredness (64%) and pain (62%) were reported most often as barriers to care (Table 3). Emotions (> 25%) such as worry/fear, frustration/anger, and lack of confidence were other important barriers to care. Provider knowledge and attitudes were cited next most often, with 38% of the sample indicating “Providers accuse me of drug-seeking” and “It is hard for me to find a provider who has enough experiences with or knowledge about SCD.” Participants expressed that they were not believed when in pain and “I am treated differently from other patients.” Almost half of respondents cited “I am not seen quickly enough when I am in pain” as a barrier to their care.
Consistent with the qualitative data, transportation barriers (not having a vehicle, costs of transportation, public transit not easy to get to) were cited by 55% of participants. About half of participants reported that insurance was an important barrier, with high co-pays and medications and other services not covered. In addition, gathering approvals was a long and fragmented process, particularly for consultations among providers (hematology, primary care provider, pain specialist). Furthermore, insurance provided limited choices about location for services.
Participants reported social support system burnout (22%), help needed with daily activities (21%), and social isolation or generally not having enough support (33%) as ongoing barriers. Difficulties were encountered with self-management (eg, taking medications on time or making follow-up appointments, 19%), with 22% of participants finding the health care system confusing or hard to understand. Thirty percent reported “Places for me to go to learn how to stay well are not close by or easy to get to.” ”Worry about side effects” (33%) was a common barrier to hydroxyurea use. Participants described “forgetting to take the medicine,” “tried before but it did not work,” “heard scary things” about hydroxyurea, and “not interested in taking another medicine” as barriers.
Quality of Care. More than half (51%) of the 53 participants who had accessed health care in the previous year rated their overall health care as poor on the ASCQ-Me QOC measure. This was significantly higher compared to the reports from more than 47,000 adults with Medicaid in 2017 (16%),41 and to the 2008-2009 report from 556 adults with SCD from across the United States (37%, Figure 2).33 The major contributor to these poor ratings for participants in our sample was low satisfaction with ED care.
Sixty percent of the 42 participants who had accessed ED care in the past year indicated “never” or “sometimes” to the question “When you went to the ED for care, how often did you get it as soon as you wanted?” compared with only 16% of the 2017 adult Medicaid population responding (n = 25,789) (Figure 3). Forty-seven percent of those with an ED visit indicated that, in the previous 12 months, they had been made to wait “more than 2 hours before receiving treatment for acute pain in the ED.” However, in the previous 12 months, 39% reported that their wait time in the ED had been only “between five minutes and one hour.”
On the ASCQ-Me QOC Access to Care composite measure, 33% of 42 participants responding reported they were seen at a routine appointment as soon as they would have liked. This is significantly lower compared to 56% of the adult Medicaid population responding to the same question. Reports of provider communication (Provider Communication composite) for adolescents and adults with SCD were comparable to reports of adults with SCD from the ASCQ-Me field test,33 but adults with Medicaid reported higher ratings of quality communication behaviors (Figure 4).33,41 Nearly 60% of both groups with SCD reported that providers “always” performed quality communication behaviors—listened carefully, spent enough time, treated them with respect, and explained things well—compared with more than 70% of adults with Medicaid.
Participants from all counties reported the same number of barriers to care on average (3.3 ± 2.1). Adolescents and adults who reported more barriers to care also reported lower satisfaction with care (r = –0.47, P < 0.01) and less confidence in their ability to manage their SCD (self-efficacy, r = – 0.36, P < 0.05). Female participants reported more barriers to care on average compared with male participants (2.6 ± 2.4 vs 1.4 ± 2.0, P = 0.05). Participants with higher self-efficacy reported lower pain ratings (r = –0.47, P < 0.001).
Quantitative Data: Health Care Providers
Providers (n = 56) and community stakeholders (2 leaders of community-based organizations and 3 health care administrators) were interviewed, with 29 also completing the survey. The reason for not completing (n = 22) was not having the time once the interview was complete. A link to the survey was sent to any provider not completing at the time of the interview, with 2 follow-up reminders. The majority of providers were between the ages of 31 and 50 years (46.4%), female (71.4%), and white (66.1%) (Table 4). None were of Hispanic, Latinx, or Spanish origin. Thirty-six were physicians (64.3%), and 16 were allied health professionals (28.6%). Of the 56 providers, 32 indicated they had expertise caring for patients with SCD (57.1%), 14 were ED providers (25%), and 5 were primary care providers. Most of the providers practiced in an urban setting (91.1%).
Barriers to Care: ED Provider Perspectives. Nine of 14 ED providers interviewed completed the survey on their perspectives regarding barriers to care in the ED, difficulty with follow-ups, ED training resources, and pain control for patients with SCD. ED providers (n = 8) indicated that “provider attitudes” were a barrier to care delivery in the ED for patients with SCD. Some providers (n = 7) indicated that “implicit bias,” “opioid epidemic,” “concern about addiction,” and “patient behavior” were barriers. Respondents indicated that “overcrowding” (n = 6) and “lack of care pathway/protocol” (n = 5) were barriers. When asked to express their level of agreement with statements about SCD care in the ED, respondents disagreed/strongly disagreed (n = 5) that they were “able to make a follow-up appointment” with a sickle cell specialist or primary care provider upon discharge from the ED, and others disagreed/strongly disagreed (n = 4) that they were able to make a “referral to a case management program.”
ED training and resources. Providers agreed/strongly agreed (n = 8) that they had the knowledge and training to care for patients with SCD, that they had access to needed medications, and that they had access to knowledgeable nursing staff with expertise in SCD care. All 9 ED providers indicated that they had sufficient physician/provider staffing to provide good pain management to persons with SCD in the ED.
Pain control in the ED. Seven ED providers indicated that their ED used individualized dosing protocols to treat sickle cell pain, and 5 respondents indicated their ED had a protocol for treating sickle cell pain. Surprisingly, only 3 indicated that they were aware of the NHLBI recommendations for the treatment of vaso-occlusive pain.
Barriers to Care: Primary Care Provider Perspectives. Twenty providers completed the SCD provider section of the survey, including 17 multidisciplinary SCD providers from 4 sickle cell special care centers and 3 community primary care providers. Of the 20, 12 were primary care providers for patients with SCD (Table 4).
Patient needs. Six primary care providers indicated that the medical needs of patients with SCD were being met, but none indicated that the behavioral health or mental health needs were being met.
Managing SCD comorbidities. Five primary care providers indicated they were very comfortable providing preventive ambulatory care to patients with SCD. Six indicated they were very comfortable managing acute pain episodes, but none were very comfortable managing comorbidities such as pulmonary hypertension, diabetes, or chronic pain.
Barriers to opioid use. Only 3 of 12 providers reviewing a list of 15 potential barriers to the use of opioids for SCD pain management indicated a perceived lack of efficacy of opioids, development of tolerance and dependence, and concerns about community perceptions as barriers. Two providers selected potential for diversion as a moderate barrier to opioid use.
Barriers to hydroxyurea use. Eight of 12 providers indicated that the common reasons that patients/families refuse hydroxyurea were “worry about side effects”; 7 chose “don’t want to take another medicine,” and 6 chose “worry about carcinogenic potential.” Others (n = 10) indicated that “patient/family adherence with hydroxyurea” and “patient/family adherence with required blood tests” were important barriers to hydroxyurea use. Eight of the 12 providers indicated that they were comfortable with managing hydroxyurea in patients with SCD.
Care redesign. Twenty SCD and primary care providers completed the Care Redesign section of the survey. Respondents (n = 11) indicated that they would see more patients with SCD if they had accessible case management services available without charge or if patient access to transportation to clinic was also available. Ten indicated that they would see more patients with SCD if they had an accessible community health worker (who understands patient’s/family’s social situation) and access to a pain management specialist on call to answer questions and who would manage chronic pain. All (n = 20) were willing to see more patients with SCD in their practices. Most reported that a clinical decision-support tool for SCD treatment (n = 13) and avoidance of complications (n = 12) would be useful.
Discussion
We evaluated access and barriers to care, quality of care, care coordination, and provider communication from the perspectives of adolescents and adults with SCD, their care providers, and community stakeholders, within the Solberg conceptual model for quality improvement. We found that barriers within the care process content domain (context and systems) were most salient for this population of adolescents and adults with SCD, with lack of provider knowledge and poor attitudes toward adolescents and adults with SCD, particularly in the ED, cited consistently by participant groups. Stigmatization and lack of provider compassion that affected the quality of care were particularly problematic. These findings are consistent with previous reports.42,43 Adult health care (particularly ED) provider biases and negative attitudes have been recognized as major barriers to optimal pain management in SCD.8,11,44,45 Interestingly, ED providers in our needs assessment indicated that they felt they had the training and resources to manage patients with SCD. However, only a few actually reported knowing about the NHLBI recommendations for the treatment of vaso-occlusive pain.
Within the care process content domain, we also found that SCD-related complications and associated emotions (fear, worry, anxiety), compounded by lack of access to knowledgeable and compassionate providers, pose a significant burden. Negative encounters with the health care system contributed to a striking 84% of patient participants choosing to manage severe pain at home, with pain seriously interfering with their ability to function on a daily basis. ED providers agreed that provider attitudes and implicit bias pose important barriers to care for adolescents and adults with SCD. Adolescents and adults with SCD wanted, and understood the need, to enhance self-management skills. Both they and their providers agreed that barriers to hydroxyurea uptake included worries about potential side effects, challenges with adherence to repeated laboratory testing, and support with remembering to take the medicine. However, providers uniformly expressed that access to behavioral and mental health services were, if not nonexistent, impossible to access.
Participants with SCD and their providers reported infrastructural challenges (change process capability), as manifested in limitations with accessing acute and preventive care due to transportation- and insurance- related issues. There were health system barriers that were particularly encountered during the transition from pediatric to adult care. These findings are consistent with previous reports that have found fewer interdisciplinary services available in the adult care settings compared with pediatrics.46,47 Furthermore, adult care providers were less willing to accept adults with SCD because of the complexity of their management, for which the providers did not have the necessary expertise.3,48-50 In addition, both adolescents and adults with SCD and primary care providers highlighted the inadequacies of the current system in addressing the chronic pain needs of this population. Linking back to the Solberg conceptual framework, our needs assessment results confirm the important role of establishing SCD care as a priority within a health care system—this requires leadership and vision. The vision and priorities must be implemented by effective health care teams. Multilevel approaches or interventions, when implemented, will lead to the desired outcomes.
Findings from our needs assessment within our 5-county region mirror needs assessment results from the broader consortium.51 The SCDIC has prioritized developing an intervention that addresses the challenges identified within the care process domain by directly enhancing provider access to patient individualized care plans in the electronic health record in the ED. Importantly, ED providers will be asked to view a short video that directly challenges bias and stigma in the ED. Previous studies have indeed found that attitudes can be improved by providers viewing short video segments of adults with SCD discussing their experiences.36,52 This ED protocol will be one of the interventions that we will roll out in Northern California, given the significance of negative ED encounters reported by needs assessment participants. An additional feature of the intervention is a script for adults with SCD that guides them through introducing their individualized pain plan to their ED providers, thereby enhancing their self-efficacy in a situation that has been so overwhelmingly challenging.
We will implement a second SCDIC intervention that utilizes a mobile app to support self-management on the part of the patient, by supporting motivation and adherence with hydroxyurea.53 A companion app supports hydroxyurea guideline adherence on the part of the provider, in keeping with one of our findings that providers are in need of decision-support tools. Elements of the intervention also align with our findings related to the importance of a support system in managing SCD, in that participants will identify a supportive partner who will play a specific role in supporting their adherence with hydroxyurea.
On our local level, we have, by necessity, partnered with leaders and community stakeholders throughout the region to ensure that these interventions to improve SCD care are prioritized. Grant funds provide initial resources for the SCDIC interventions, but our partnering health care administrators and medical directors must ensure that participating ED and hematology providers are free from competing priorities in order to implement the changes. We have partnered with a SCD community-based organization that is designing additional educational presentations for local emergency medicine providers, with the goal to bring to life very personal stories of bias and stigma within the EDs that directly contribute to decisions to avoid ED care despite severe symptoms.
Although we attempted to obtain samples of adolescents and adults with SCD and their providers that were representative across the 5-county region, the larger proportion of respondents were from 1 county. We did not assess concerns of age- and race-matched adults in our catchment area, so we cannot definitively say that our findings are unique to SCD. However, our results are consistent with findings from the national sample of adults with SCD who participated in the ASCQ-Me field test, and with results from the SCDIC needs assessment.33,51 Interviews and surveys are subject to self-report bias and, therefore, may or may not reflect the actual behaviors or thoughts of participants. Confidence is increased in our results given the triangulation of expressed concerns across participant groups and across data collection strategies. The majority of adolescents and adults with SCD (95%) completed both the interview and survey, while 64% of ED providers interviewed completed the survey, compared with 54% of SCD specialists and primary care providers. These response rates are more than acceptable within the realm of survey response rates.54,55
Although we encourage examining issues with care delivery within the conceptual framework for quality improvement presented, we recognize that grant funding allowed us to conduct an in-depth needs assessment that might not be feasible in other settings. Still, we would like readers to understand the importance of gathering data for improvement in a systematic manner across a range of participant groups, to ultimately inform the development of interventions and provide for evaluation of outcomes as a result of the interventions. This is particularly important for a disease, such as SCD, that is both medically and sociopolitically complex.
Conclusion
Our needs assessment brought into focus the multiple factors contributing to the disparities in health care experienced by adolescents and adults with SCD on our local level, and within the context of inequities in health resources and outcomes on the national level. We propose solutions that include specific interventions developed by a consortium of SCD and implementation science experts. We utilize a quality improvement framework to ensure that the elements of the interventions also address the barriers identified by our local providers and patients that are unique to our community. The pervasive challenges in SCD care, coupled with its medical complexities, may seem insurmountable, but our survey and qualitative results provide us with a road map for the way forward.
Acknowledgments: The authors thank the adolescents and adults with sickle cell disease, the providers, and the community stakeholders who completed the interviews and surveys. The authors also acknowledge the SCCCI co-investigators for their contributions to this project, including Michael Bell, MD, Ward Hagar, MD, Christine Hoehner, FNP, Kimberly Major, MSW, Anne Marsh, MD, Lynne Neumayr, MD, and Ted Wun, MD. We also thank Kamilah Bailey, Jameelah Hodge, Jennifer Kim, Michael Rowland, Adria Stauber, Amber Fearon, and Shanda Robertson, and the Sickle Cell Data Collection Program for their contributions.
Corresponding author: Marsha J. Treadwell, PhD, University of California San Francisco Benioff Children’s Hospital Oakland, 747 52nd St., Oakland, CA 94609; [email protected].
Financial disclosures: None.
Funding/support: This work was supported by grant # 1U01HL134007 from the National Heart, Lung, and Blood Institute to the University of California San Francisco Benioff Children’s Hospital Oakland.
1. Hassell KL. Population Estimates of sickle cell disease in the U.S. Am J Prev Med. 2010; 38:S512-S521.
2. Data & Statistics on Sickle Cell Disease. Centers for Disease Control and Prevention website. www.cdc.gov/ncbddd/sicklecell/data.html. Accessed March 25, 2020.
3. Inusa BPD, Stewart CE, Mathurin-Charles S, et al. Paediatric to adult transition care for patients with sickle cell disease: a global perspective. Lancet Haematol. 2020;7:e329-e341.
4. Smith SK, Johnston J, Rutherford C, et al. Identifying social-behavioral health needs of adults with sickle cell disease in the emergency department. J Emerg Nurs. 2017;43:444-450.
5. Treadwell MJ, Barreda F, Kaur K, et al. Emotional distress, barriers to care, and health-related quality of life in sickle cell disease. J Clin Outcomes Manag. 2015;22:8-17.
6. Treadwell MJ, Hassell K, Levine R, et al. Adult Sickle Cell Quality-of-Life Measurement Information System (ASCQ-Me): conceptual model based on review of the literature and formative research. Clin J Pain. 2014;30:902-914.
7. Rizio AA, Bhor M, Lin X, et al. The relationship between frequency and severity of vaso-occlusive crises and health-related quality of life and work productivity in adults with sickle cell disease. Qual Life Res. 2020;29:1533-1547.
8. Freiermuth CE, Haywood C, Silva S, et al. Attitudes toward patients with sickle cell disease in a multicenter sample of emergency department providers. Adv Emerg Nurs J. 2014;36:335-347.
9. Jenerette CM, Brewer C. Health-related stigma in young adults with sickle cell disease. J Natl Med Assoc. 2010;102:1050-1055.
10. Lazio MP, Costello HH, Courtney DM, et al. A comparison of analgesic management for emergency department patients with sickle cell disease and renal colic. Clin J Pain. 2010;26:199-205.
11. Haywood C, Tanabe P, Naik R, et al. The impact of race and disease on sickle cell patient wait times in the emergency department. Am J Emerg Med. 2013;31:651-656.
12. Haywood C, Beach MC, Lanzkron S, et al. A systematic review of barriers and interventions to improve appropriate use of therapies for sickle cell disease. J Natl Med Assoc. 2009;101:1022-1033.
13. Mainous AG, Tanner RJ, Harle CA, et al. Attitudes toward management of sickle cell disease and its complications: a national survey of academic family physicians. Anemia. 2015;2015:1-6.
14. Yawn BP, Buchanan GR, Afenyi-Annan AN, et al. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA. 2014;312:1033.
15. Lunyera J, Jonassaint C, Jonassaint J, et al. Attitudes of primary care physicians toward sickle cell disease care, guidelines, and comanaging hydroxyurea with a specialist. J Prim Care Community Health. 2017;8:37-40.
16. Whiteman LN, Haywood C, Lanzkron S, et al. Primary care providers’ comfort levels in caring for patients with sickle cell disease. South Med J. 2015;108:531-536.
17. Wong TE, Brandow AM, Lim W, Lottenberg R. Update on the use of hydroxyurea therapy in sickle cell disease. Blood. 2014;124:3850-4004.
18. DiMartino LD, Baumann AA, Hsu LL, et al. The sickle cell disease implementation consortium: Translating evidence-based guidelines into practice for sickle cell disease. Am J Hematol. 2018;93:E391-E395.
19. King AA, Baumann AA. Sickle cell disease and implementation science: A partnership to accelerate advances. Pediatr Blood Cancer. 2017;64:e26649.
20. Solberg LI. Improving medical practice: a conceptual framework. Ann Fam Med. 2007;5:251-256.
21. Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness. J Am Med Assoc. 2002;288:5.
22. Bodenheimer T. Interventions to improve chronic illness care: evaluating their effectiveness. Dis Manag. 2003;6:63-71.
23. Tsai AC, Morton SC, Mangione CM, Keeler EB. A meta-analysis of interventions to improve care for chronic illnesses. Am J Manag Care. 2005;11:478-488.
24. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-381.
25. Kallio H, Pietilä A-M, Johnson M, et al. Systematic methodological review: developing a framework for a qualitative semi-structured interview guide. J Adv Nurs. 2016;72:2954-2965.
26. Clarke V, Braun V. Successful Qualitative Research: A Practical Guide for Beginners. First. Thousand Oaks, CA: Sage; 2013.
27. Hsieh H-F, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15:1277-1288.
28. Creswell JW, Hanson WE, Clark Plano VL, et al. Qualitative research designs: selection and implementation. Couns Psychol. 2007;35:236-264.
29. Miles MB, Huberman AM, Saldana J. Qualitative Data Analysis A Methods Sourcebook. 4th ed. Thousand Oaks, CA: Sage; 2019.
30. Eckman JR, Hassell KL, Huggins W, et al. Standard measures for sickle cell disease research: the PhenX Toolkit sickle cell disease collections. Blood Adv. 2017; 1: 2703-2711.
31. Kendall R, Wagner B, Brodke D, et al. The relationship of PROMIS pain interference and physical function scales. Pain Med. 2018;19:1720-1724.
32. Amtmann D, Cook KF, Jensen MP, et al. Development of a PROMIS item bank to measure pain interference. Pain. 2010;150:173-182.
33. Evensen CT, Treadwell MJ, Keller S, et al. Quality of care in sickle cell disease: Cross-sectional study and development of a measure for adults reporting on ambulatory and emergency department care. Medicine (Baltimore). 2016;95:e4528.
34. Edwards R, Telfair J, Cecil H, et al. Reliability and validity of a self-efficacy instrument specific to sickle cell disease. Behav Res Ther. 2000;38:951-963.
35. Edwards R, Telfair J, Cecil H, et al. Self-efficacy as a predictor of adult adjustment to sickle cell disease: one-year outcomes. Psychosom Med. 2001;63:850-858.
36. Puri Singh A, Haywood C, Beach MC, et al. Improving emergency providers’ attitudes toward sickle cell patients in pain. J Pain Symptom Manage. 2016;51:628-632.e3.
37. Glassberg JA, Tanabe P, Chow A, et al. Emergency provider analgesic practices and attitudes towards patients with sickle cell disease. Ann Emerg Med. 2013;62:293-302.e10.
38. Grahmann PH, Jackson KC 2nd, Lipman AG. Clinician beliefs about opioid use and barriers in chronic nonmalignant pain [published correction appears in J Pain Palliat Care Pharmacother. 2004;18:145-6]. J Pain Palliat Care Pharmacother. 2004;18:7-28.
39. Brandow AM, Panepinto JA. Hydroxyurea use in sickle cell disease: the battle with low prescription rates, poor patient compliance and fears of toxicities. Expert Rev Hematol. 2010;3:255-260.
40. Fielding N. Triangulation and mixed methods designs: data integration with new research technologies. J Mixed Meth Res. 2012;6:124-136.
41. 2017 CAHPS Health Plan Survey Chartbook. Agency for Healthcare Research and Quality website. www.ahrq.gov/cahps/cahps-database/comparative-data/2017-health-plan-chartbook/results-enrollee-population.html. Accessed September 8, 2020.
42. Bulgin D, Tanabe P, Jenerette C. Stigma of sickle cell disease: a systematic review. Issues Ment Health Nurs. 2018;1-11.
43. Wakefield EO, Zempsky WT, Puhl RM, et al. Conceptualizing pain-related stigma in adolescent chronic pain: a literature review and preliminary focus group findings. PAIN Rep. 2018;3:e679.
44. Nelson SC, Hackman HW. Race matters: Perceptions of race and racism in a sickle cell center. Pediatr Blood Cancer. 2013;60:451-454.
45. Dyal BW, Abudawood K, Schoppee TM, et al. Reflections of healthcare experiences of african americans with sickle cell disease or cancer: a qualitative study. Cancer Nurs. 2019;10.1097/NCC.0000000000000750.
46. Renedo A. Not being heard: barriers to high quality unplanned hospital care during young people’s transition to adult services - evidence from ‘this sickle cell life’ research. BMC Health Serv Res. 2019;19:876.
47. Ballas S, Vichinsky E. Is the medical home for adult patients with sickle cell disease a reality or an illusion? Hemoglobin. 2015;39:130-133.
48. Hankins JS, Osarogiagbon R, Adams-Graves P, et al. A transition pilot program for adolescents with sickle cell disease. J Pediatr Health Care. 2012;26 e45-e49.
49. Smith WR, Sisler IY, Johnson S, et al. Lessons learned from building a pediatric-to-adult sickle cell transition program. South Med J. 2019;112:190-197.
50. Lanzkron S, Sawicki GS, Hassell KL, et al. Transition to adulthood and adult health care for patients with sickle cell disease or cystic fibrosis: Current practices and research priorities. J Clin Transl Sci. 2018;2:334-342.
51. Kanter J, Gibson R, Lawrence RH, et al. Perceptions of US adolescents and adults with sickle cell disease on their quality of care. JAMA Netw Open. 2020;3:e206016.
52. Haywood C, Lanzkron S, Hughes MT, et al. A video-intervention to improve clinician attitudes toward patients with sickle cell disease: the results of a randomized experiment. J Gen Intern Med. 2011;26:518-523.
53. Hankins JS, Shah N, DiMartino L, et al. Integration of mobile health into sickle cell disease care to increase hydroxyurea utilization: protocol for an efficacy and implementation study. JMIR Res Protoc. 2020;9:e16319.
54. Fan W, Yan Z. Factors affecting response rates of the web survey: A systematic review. Comput Hum Behav. 2010;26:132-139.
55. Millar MM, Dillman DA. Improving response to web and mixed-mode surveys. Public Opin Q. 2011;75:249-269.
From the University of California San Francisco (Dr. Treadwell, Dr. Hessler, Yumei Chen, Swapandeep Mushiana, Dr. Potter, and Dr. Vichinsky), the University of California Los Angeles (Dr. Jacob), and the University of California Berkeley (Alex Chen).
Abstract
- Objective: Adolescents and adults with sickle cell disease (SCD) face pervasive disparities in health resources and outcomes. We explored barriers to and facilitators of care to identify opportunities to support implementation of evidence-based interventions aimed at improving care quality for patients with SCD.
- Methods: We engaged a representative sample of adolescents and adults with SCD (n = 58), health care providers (n = 51), and community stakeholders (health care administrators and community-based organization leads (n = 5) in Northern California in a community-based needs assessment. We conducted group interviews separately with participant groups to obtain in-depth perspectives. Adolescents and adults with SCD completed validated measures of pain interference, quality of care, self-efficacy, and barriers to care. Providers and community stakeholders completed surveys about barriers to SCD care.
- Results: We triangulated qualitative and quantitative data and found that participants with SCD (mean age, 31 ± 8.6 years), providers, and community stakeholders emphasized the social and emotional burden of SCD as barriers. Concrete barriers agreed upon included insurance and lack of resources for addressing pain impact. Adolescents and adults with SCD identified provider issues (lack of knowledge, implicit bias), transportation, and limited social support as barriers. Negative encounters with the health care system contributed to 84% of adolescents and adults with SCD reporting they chose to manage severe pain at home. Providers focused on structural barriers: lack of access to care guidelines, comfort level with and knowledge of SCD management, and poor care coordination.
- Conclusion: Strategies for improving access to compassionate, evidence-based quality care, as well as strategies for minimizing the burden of having SCD, are warranted for this medically complex population.
Keywords: barriers to care; quality of care; care access; care coordination.
Sickle cell disease (SCD), an inherited chronic medical condition, affects about 100,000 individuals in the United States, a population that is predominantly African American.1 These individuals experience multiple serious and life-threatening complications, most frequently recurrent vaso-occlusive pain episodes,2 and they require interactions with multidisciplinary specialists from childhood. Because of advances in treatments, the majority are reaching adulthood; however, there is a dearth of adult health care providers with the training and expertise to manage their complex medical needs.3 Other concrete barriers to adequate SCD care include insurance and distance to comprehensive SCD centers.4,5
Social, behavioral, and emotional factors may also contribute to challenges with SCD management. SCD may limit daily functional abilities and lead to diminished overall quality of life.6,7 Some adolescents and adults may require high doses of opioids, which contributes to health care providers’ perceptions that there is a high prevalence of drug addiction in the population.8,9 These providers express negative attitudes towards adults with SCD, and, consequently, delay medication administration when it is acutely needed and provide otherwise suboptimal treatment.8,10,11 Adult care providers may also be uncomfortable with prescribing and managing disease-modifying therapies (blood transfusion, hydroxyurea) that have established efficacy.12-17
As 1 of 8 programs funded by the National Heart, Lung, and Blood Institute’s (NHLBI) Sickle Cell Disease Implementation Consortium (SCDIC), we are using implementation science to reduce barriers to care and improve quality of care and health care outcomes in SCD.18,19 Given that adolescents and adults with SCD experience high mortality, severe pain, and progressive decline in their ability to function day to day, and also face lack of access to knowledgeable, compassionate providers in primary and emergency settings, the SCDIC focuses on individuals aged 15 to 45 years.6,8,9,11,12
Our regional SCDIC program, the Sickle Cell Care Coordination Initiative (SCCCI), brings together researchers, clinicians, adolescents, and adults with SCD and their families, dedicated community members, policy makers, and administrators to identify and address barriers to health care within 5 counties in Northern California. One of our first steps was to conduct a community-based needs assessment, designed to inform implementation of evidence-based interventions, accounting for unique contextual factors in our region.
Conceptual Framework for Improving Medical Practice
Our needs assessment is guided by Solberg’s Conceptual Framework for Improving Medical Practice (Figure 1).20 Consistent with the overarching principles of the SCDIC, this conceptual framework focuses on the inadequate implementation of evidence-based guidelines, and on the need to first understand multifactorial facilitators and barriers to guideline implementation in order to effect change. The framework identifies 3 main elements that must be present to ensure improvements in quality-of-care processes and patient outcomes: priority, change process capability, and care process content. Priority refers to ample resource allocation for the specific change, as well as freedom from competing priorities for those implementing the change. Change process capability includes strong, effective leadership, adequate infrastructure for managing change (including resources and time), change management skills at all levels, and an established clinical information system. Care process content refers to context and systems-level changes, such as delivery system redesign as needed, support for self-management to lessen the impact of the disease, and decision support.21-23

The purpose of our community-based needs assessment was to evaluate barriers to care and quality of care in SCD, within Solberg’s conceptual model for improving medical practice. The specific aims were to evaluate access and barriers to care (eg, lack of provider expertise and training, health care system barriers such as poor care coordination and provider communication); evaluate quality of care; and assess patient needs related to pain, pain interference, self-efficacy, and self-management for adolescents and adults with SCD. We gathered the perspectives of a representative community of adolescents and adults with SCD, their providers, and community stakeholders in order to examine barriers, quality of life and care, and patient experiences in our region.
Methods
Design
In this cross-sectional study, adolescents and adults with SCD, their providers, and community stakeholders participated in group or individual qualitative interviews and completed surveys between October 2017 and March 2018.
Setting and Sample
Recruitment flyers were posted on a regional SCD-focused website, and clinical providers or a study coordinator introduced information about the needs assessment to potential participants with SCD during clinic visits at the participating centers. Participants with SCD were eligible if they had any diagnosis of SCD, were aged 15 to 48 years, and received health services within 5 Northern California counties (Alameda, Contra Costa, Sacramento, San Francisco, and Solano). They were excluded if they did not have a SCD diagnosis or had not received health services within the catchment area. As the project proceeded, participants were asked to refer other adolescents and adults with SCD for the interviews and surveys (snowball sampling). Our goal was to recruit 50 adolescents and adults with SCD into the study, aiming for 10 representatives from each county.
Providers and community stakeholders were recruited via emails, letters and informational flyers. We engaged our partner, the Sickle Cell Data Collection Program,2 to generate a list of providers and institutions that had seen patients with SCD in primary, emergency, or inpatient settings in the region. We contacted these institutions to describe the SCCCI and invite participation in the needs assessment. We also invited community-based organization leads and health care administrators who worked with SCD to participate. Providers accessed confidential surveys via a secure link on the study website or completed paper versions. Common data collected across providers included demographics and descriptions of practice settings.
Participants were eligible to be part of the study if they were health care providers (physicians and nurses) representing hematology, primary care, family medicine, internal medicine, or emergency medicine; ancillary staff (social work, psychology, child life); or leaders or administrators of clinical or sickle cell community-based organizations in Northern California (recruitment goal of n = 50). Providers were excluded if they practiced in specialties other than those noted or did not practice within the region.
Data Collection Procedures
After providing assent/consent, participating adolescents and adults with SCD took part in individual and group interviews and completed survey questionnaires. All procedures were conducted in a private space in the sickle cell center or community. Adolescents and adults with SCD completed the survey questionnaire on a tablet, with responses recorded directly in a REDCap (Research Electronic Data Capture) database,24 or on a paper version. Interviews lasted 60 (individual) to 90 (group) minutes, while survey completion time was 20 to 25 minutes. Each participant received a gift card upon completion as an expression of appreciation. All procedures were approved by the institutional review boards of the participating health care facilities.
Group and Individual Interviews
Participants with SCD and providers were invited to participate in a semi-structured qualitative interview prior to being presented with the surveys. Adolescents and adults with SCD were interviewed about barriers to care, quality of care, and pain-related experiences. Providers were asked about barriers to care and treatments. Interview guides were modified for community-based organization leaders and health care administrators who did not provide clinical services. Interview guides can be found in the Appendix. Interviews were conducted by research coordinators trained in qualitative research methods by the first author (MT). As appropriate with semi-structured interviews, the interviewers could word questions spontaneously, change the order of questions for ease of flow of conversation, and inform simultaneous coding of interviews with new themes as those might arise, as long as they touched on all topics within the interview guide.25 The interview guides were written, per qualitative research standards, based on the aims and purpose of the research,26 and were informed by existing literature on access and barriers to care in SCD, quality of care, and the needs of individuals with SCD, including in relation to impact of the disease, self-efficacy, and self-management.
Interviewees participated in either individual or group interviews, but not both. The decision for which type of interview an individual participated in was based on 2 factors: if there were not comparable participants for group interviews (eg, health care administrator and community-based organization lead), these interviews were done individually; and given that we were drawing participants from a 5-county area in Northern California, scheduling was challenging for individuals with SCD with regard to aligning schedules and traveling to a central location where the group interviews were conducted. Provider group interviews were easier to arrange because we could schedule them at the same time as regularly scheduled meetings at the participants’ health care institutions.
Interview Data Gathering and Analysis
Digital recordings of the interviews were cleaned of any participant identifying data and sent for transcription to an outside service. Transcripts were reviewed for completeness and imported into NVivo (www.qsrinternational.com), a qualitative data management program.
A thematic content analysis and deductive and inductive approaches were used to analyze the verbatim transcripts generated from the interviews. The research team was trained in the use of NVivo software to facilitate the coding process. A deductive coding scheme was initially used based on existing concepts in the literature regarding challenges to optimal SCD care, with new codes added as the thematic content analyses progressed. The initial coding, pattern coding, and use of displays to examine the relationships between different categories were conducted simultaneously.27,28 Using the constant comparative method, new concepts from participants with SCD and providers could be incorporated into subsequent interviews with other participants. For this study, the only additional concepts added were in relation to participant recruitment and retention in the SCDIC Registry. Research team members coded transcripts separately and came together weekly, constantly comparing codes and developing the consensus coding scheme. Where differences between coders existed, code meanings were discussed and clarified until consensus was reached.29
Quantitative data were analyzed using SPSS (v. 25, Chicago, IL). Descriptive statistics (means, standard deviations, frequencies, percentages) were used to summarize demographics (eg, age, gender, and race), economic status, and type of SCD. No systematic differences were detected from cases with missing values. Scale reliabilities (ie, Cronbach α) were evaluated for self-report measures.
Measurement
Adolescents and adults with SCD completed items from the PhenX Toolkit (consensus measures for Phenotypes and eXposures), assessing sociodemographics (age, sex, race, ethnicity, educational attainment, occupation, marital status, annual income, insurance), and clinical characteristics (sickle cell diagnosis and emergency department [ED] and hospital utilization for pain).30
Pain Interference Short Form (Patient-Reported Outcomes Measurement Information System [PROMIS]). The Pain Interference Form consists of 8 items that assess the degree to which pain interfered with day-to-day activities in the previous 7 days at home, including impacts on social, cognitive, emotional, and physical functioning; household chores and recreational activities; sleep; and enjoyment in life. Reliability and validity of the PROMIS Pain Interference Scale has been demonstrated, with strong negative correlations with Physical Function Scales (r = 0.717, P < 0.01), indicating that higher scores are associated with lower function (β = 0.707, P < 0.001).31 The Cronbach α estimate for the other items on the pain interference scale was 0.99. Validity analysis indicated strong correlations with pain-related domains: BPI Interference Subscale (rho = 0.90), SF-36 Bodily Pain Subscale (rho = –0.84), and 0–10 Numerical Rating of Pain Intensity (rho = 0.48).32
Adult Sickle Cell Quality of Life Measurement Information System (ASCQ-Me) Quality of Care (QOC). ASCQ-Me QOC consists of 27 items that measure the quality of care that adults with SCD have received from health care providers.33 There are 3 composites: provider communication (quality of patient and provider communication), ED care (quality of care in the ED), and access (to routine and emergency care). Internal consistency reliability for all 3 composites is greater than 0.70. Strong correlations of the provider communication composite with overall ratings of routine care (r = 0.65) and overall provider ratings (r = 0.83) provided evidence of construct validity. Similarly, the ED care composite was strongly correlated with overall ratings of QOC in the ED, and the access composite was highly correlated with overall evaluations of ED care (r = 0.70). Access, provider interaction, and ED care composites were reliable (Cronbach α, 0.70–0.83) and correlated with ratings of global care (r = 0.32–0.83), further indicating construct validity.33
Sickle Cell Self-Efficacy Scale (SCSES). The SCSES is a 9-item, self-administered questionnaire measuring perceptions of the ability to manage day-to-day issues resulting from SCD. SCSES items are scored on a 5-point scale ranging from Not sure at all (1) to Very sure (5). Individual item responses are summed to give an overall score, with higher scores indicating greater self-efficacy. The SCSES has acceptable reliability (r = 0.45, P < 0.001) and validity (α = 0.89).34,35
Sickle Cell Disease Barriers Checklist. This checklist consists of 53 items organized into 8 categories: insurance, transportation, accommodations and accessibility, provider knowledge and attitudes, social support, individual barriers such as forgetting or difficulties understanding instructions, emotional barriers (fear, anger), and disease-related barriers. Participants check applicable barriers, with a total score range of 0 to 53 and higher scores indicating more barriers to care. The SCD Barriers Checklist has demonstrated face validity and test-retest reliability (Pearson r = 0.74, P < 0.05).5
ED Provider Checklist. The ED provider survey is a checklist of 14 statements pertaining to issues regarding patient care, with which the provider rates level of agreement. Items representing the attitudes and beliefs of providers towards patients with SCD are rated on a Likert-type scale, with level of agreement indicated as 1 (strongly disagree) to 6 (strongly agree). The positive attitudes subscale consists of 4 items (Cronbach α= 0.85), and the negative attitudes subscale consists of 6 items (Cronbach α = 0.89). The Red-Flag Behaviors subscale includes 4 items that indicate behavior concerns about drug-seeking, such as requesting specific narcotics and changing behavior when the provider walks in.8,36,37
Sickle cell and primary care providers also completed a survey consisting of sets of items compiled from existing provider surveys; this survey consisted of a list of 16 barriers to using opioids, which the providers rated on a 5-point Likert-type scale (1, not a barrier; 5, complete barrier).13,16,38 Providers indicated their level of experience with caring for patients with SCD; care provided, such as routine health screenings; and comfort level with providing preventive care, managing comorbidities, and managing acute and chronic pain. Providers were asked what potential facilitators might improve care for patients with SCD, including higher reimbursement, case management services, access to pain management specialists, and access to clinical decision-support tools. Providers responded to specific questions about management with hydroxyurea (eg, criteria for, barriers to, and comfort level with prescribing).39 The surveys are included in the Appendix.
Triangulation
Data from the interviews and surveys were triangulated to enhance understanding of results generated from the different data sources.40 Convergence of findings, different facets of the same phenomenon, or new perspectives were examined.
Results
Qualitative Data
Adolescents and adults with SCD (n = 55) and health care providers and community stakeholders (n = 56) participated in group or individual interviews to help us gain an in-depth understanding of the needs and barriers related to SCD care in our 5-county region. Participants with SCD described their experiences, which included stigma, racism, labeling, and, consequently, stress. They also identified barriers such as lack of transportation, challenges with insurance, and lack of access to providers who were competent with pain management. They reported that having SCD in a health care system that was unable to meet their needs was burdensome.
Barriers to Care and Treatments. Adolescents and adults indicated that SCD and its sequelae posed significant barriers to health care. Feelings of tiredness and pain make it more difficult for them to seek care. The emotional burden of SCD (fear and anger) was a frequently cited barrier, which was fueled by previous negative encounters with the health care system. All adolescents and adults with SCD reported that they knew of stigma in relation to seeking pain management that was pervasive and long-standing, and the majority reported they had directly experienced stigma. They reported that being labeled as “drug-seekers” was typical when in the ED for pain management. Participants articulated unconscious bias or overt racism among providers: “people with sickle cell are Black ... and Black pain is never as valuable as White pain” (25-year-old male). Respondents with SCD described challenges to the credibility of their pain reports in the ED. They reported that ED providers expressed doubts regarding the existence and/or severity of their pain, consequently creating a feeling of disrespect for patients seeking pain relief. The issue of stigma was mentioned by only 2 of 56 providers during their interviews.
Lack of Access to Knowledgeable, Compassionate Providers. Lack of access to knowledgeable care providers was another prevalent theme expressed by adolescents and adults with SCD. Frustration occurred when providers did not have knowledge of SCD and its management, particularly pain assessment. Adolescents and adults with SCD noted the lack of compassion among providers: “I’ve been kicked out of the hospital because they felt like okay, well we gave you enough medication, you should be all right” (29-year-old female). Providers specifically mentioned lack of compassion and knowledge as barriers to SCD care much less often during their interviews compared with the adolescents and adults with SCD.
Health Care System Barriers. Patient participants often expressed concerns about concrete and structural aspects of care. Getting to their appointments was a challenge for half of the interviewees, as they either did not have access to a vehicle or could not afford to travel the needed distance to obtain quality care. Even when hospitals were accessible by public transportation, those with excruciating pain understandably preferred a more comfortable and private way to travel: “I would like to change that, something that will be much easier, convenient for sickle cell patients that do suffer with pain, that they don’t have to travel always to see the doctor” (30-year-old male).
Insurance and other financial barriers also played an important role in influencing decisions to seek health care services. Medical expenses were not covered, or co-pays were too high. The Medicaid managed care system could prevent access to knowledgeable providers who were not within network. Such a lack of access discouraged some adolescents and adults with SCD from seeking acute and preventive care.
Transition From Pediatric to Adult Care. Interviewees with SCD expressed distress about the gap between pediatric and adult care. They described how they had a long-standing relationship with their medical providers, who were familiar with their medical background and history from childhood. Adolescent interviewees reported an understanding of their own pain management as well as adherence to and satisfaction with their individualized pain plans. However, adults noted that satisfaction plummeted with increasing age due to the limited number of experienced adult SCD providers, which was compounded by negative experiences (stigma, racism, drug-seeking label).
One interviewee emphasized the difficulty of finding knowledgeable providers after transition: “When you’re a pediatric sickle cell [patient], you have the doctors there every step of the way, but not with adult sickle cell… I know when I first transitioned I never felt more alone in my life… you look at that ER doctor kind of with the same mindset as you would your hematologist who just hand walked you through everything. And adult care providers were a lot more blunt and cold and they’re like… ‘I don’t know; I’m not really educated in sickle cell.’” A sickle cell provider shared his insight about the problem of transitioning: “I think it’s particularly challenging because we, as a community, don’t really set them up for success. It’s different from other chronic conditions [in that] it’s much harder to find an adult sickle cell provider. There’s not a lot of adult hematologists that will take care of our adult patients, and so I know statistically, there’s like a drop-down in the overall outcomes of our kids after they age out of our pediatric program.”
Self-Management, Supporting Hydroxyurea Use. Interview participants with SCD reported using a variety of methods to manage pain at home and chose to go to the ED only when the pain became intolerable. Patients and providers expressed awareness of different resources for managing pain at home, yet they also indicated that these resources have not been consolidated in an accessible way for patients and families. Some resources cited included heat therapy, acupuncture, meditation, medical marijuana, virtual reality devices, and pain medications other than opioids.
Patients and providers expressed the need for increasing awareness and education about hydroxyurea. Many interview participants with SCD were concerned about side effects, multiple visits with a provider during dose titration, and ongoing laboratory monitoring. They also expressed difficulties with scheduling multiple appointments, depending on access to transportation and limited provider clinic hours. They were aware of strategies for improving adherence with hydroxyurea, including setting phone alarms, educating family members about hydroxyurea, and eliciting family support, but expressed needing help to consistently implement these strategies.
Safe Opioid Prescribing. Adult care providers expressed concerns about safe opioid prescribing for patients with SCD. They were reluctant to prescribe opioid doses needed to adequately control SCD pain. Providers expressed uncertainty and fear or concern about medical/legal liability or about their judgment about what’s safe and not safe for patients with chronic use/very high doses of opioids. “I know we’re in like this opiate epidemic here in this country but I feel like these patients don’t really fit under that umbrella that the problem is coming from so [I am] just trying to learn more about how to take care of them.”
Care Coordination and Provider Communication. Adolescents and adults with SCD reported having positive experiences—good communication, established trust, and compassionate care—with their usual providers. However, they perceived that ED physicians and nurses did not really care about them. Both interviewees with SCD and providers recognized the importance of good communication in all settings as the key to overcoming barriers to receiving quality care. All agreed on the importance of using individual pain plans so that all providers, especially ED providers, can be more at ease with treating adolescents and adults with SCD.
Quantitative Data: Adolescents and Adults With SCD
Fifty-eight adolescents and adults with SCD (aged 15 to 48 years) completed the survey. Three additional individuals who did not complete the interview completed the survey. Reasons for not completing the interview included scheduling challenges (n = 2) or a sickle cell pain episode (n = 1). The average age of participants was 31 years ± 8.6, more than half (57%) were female, and the majority (93%) were African American (Table 1). Most (71%) had never been married. Half (50%) had some college or an associate degree, and 40% were employed and reported an annual household income of less than $30,000. Insurance coverage was predominantly Medi-Cal (Medicaid, 69%). The majority of participants resided in Alameda (34.5%) or Contra Costa (21%) counties. The majority of sickle cell care was received in Alameda County, whether outpatient (52%), inpatient (40%), or ED care (41%). The majority (71%) had a diagnosis of SCD hemoglobin SS.
Pain. More than one-third of individuals with SCD reported 1 or 2 ED visits for pain in the previous 6 months (34%), and more than 3 hospitalizations (36%) related to pain in the previous year (Table 2). The majority (85%) reported having severe pain at home in the previous 6 months that they did not seek health care for, consistent with their reports in the qualitative interviews. More than half (59%) reported 4 or more of these severe pain episodes that led to inability to perform daily activities for 1 week or more. While pain interference on the PROMIS Pain Interference Short Form on average (T-score, 59.6 ± 8.6) was similar to that of the general population (T-score, 50 ± 10), a higher proportion of patients with SCD reported pain interference compared with the general population. The mean self-efficacy (confidence in ability to manage complications of SCD) score on the SCSES of 30.0 ± 7.3 (range, 9–45) was similar to that of other adults with SCD (mean, 32.2 ± 7.0). Twenty-five percent of the present sample had a low self-efficacy score (< 25).
Barriers to Care and Treatments. Consistent with the qualitative data, SCD-related symptoms such as tiredness (64%) and pain (62%) were reported most often as barriers to care (Table 3). Emotions (> 25%) such as worry/fear, frustration/anger, and lack of confidence were other important barriers to care. Provider knowledge and attitudes were cited next most often, with 38% of the sample indicating “Providers accuse me of drug-seeking” and “It is hard for me to find a provider who has enough experiences with or knowledge about SCD.” Participants expressed that they were not believed when in pain and “I am treated differently from other patients.” Almost half of respondents cited “I am not seen quickly enough when I am in pain” as a barrier to their care.

Consistent with the qualitative data, transportation barriers (not having a vehicle, costs of transportation, public transit not easy to get to) were cited by 55% of participants. About half of participants reported that insurance was an important barrier, with high co-pays and medications and other services not covered. In addition, gathering approvals was a long and fragmented process, particularly for consultations among providers (hematology, primary care provider, pain specialist). Furthermore, insurance provided limited choices about location for services.
Participants reported social support system burnout (22%), help needed with daily activities (21%), and social isolation or generally not having enough support (33%) as ongoing barriers. Difficulties were encountered with self-management (eg, taking medications on time or making follow-up appointments, 19%), with 22% of participants finding the health care system confusing or hard to understand. Thirty percent reported “Places for me to go to learn how to stay well are not close by or easy to get to.” ”Worry about side effects” (33%) was a common barrier to hydroxyurea use. Participants described “forgetting to take the medicine,” “tried before but it did not work,” “heard scary things” about hydroxyurea, and “not interested in taking another medicine” as barriers.
Quality of Care. More than half (51%) of the 53 participants who had accessed health care in the previous year rated their overall health care as poor on the ASCQ-Me QOC measure. This was significantly higher compared to the reports from more than 47,000 adults with Medicaid in 2017 (16%),41 and to the 2008-2009 report from 556 adults with SCD from across the United States (37%, Figure 2).33 The major contributor to these poor ratings for participants in our sample was low satisfaction with ED care.

Sixty percent of the 42 participants who had accessed ED care in the past year indicated “never” or “sometimes” to the question “When you went to the ED for care, how often did you get it as soon as you wanted?” compared with only 16% of the 2017 adult Medicaid population responding (n = 25,789) (Figure 3). Forty-seven percent of those with an ED visit indicated that, in the previous 12 months, they had been made to wait “more than 2 hours before receiving treatment for acute pain in the ED.” However, in the previous 12 months, 39% reported that their wait time in the ED had been only “between five minutes and one hour.”

On the ASCQ-Me QOC Access to Care composite measure, 33% of 42 participants responding reported they were seen at a routine appointment as soon as they would have liked. This is significantly lower compared to 56% of the adult Medicaid population responding to the same question. Reports of provider communication (Provider Communication composite) for adolescents and adults with SCD were comparable to reports of adults with SCD from the ASCQ-Me field test,33 but adults with Medicaid reported higher ratings of quality communication behaviors (Figure 4).33,41 Nearly 60% of both groups with SCD reported that providers “always” performed quality communication behaviors—listened carefully, spent enough time, treated them with respect, and explained things well—compared with more than 70% of adults with Medicaid.

Participants from all counties reported the same number of barriers to care on average (3.3 ± 2.1). Adolescents and adults who reported more barriers to care also reported lower satisfaction with care (r = –0.47, P < 0.01) and less confidence in their ability to manage their SCD (self-efficacy, r = – 0.36, P < 0.05). Female participants reported more barriers to care on average compared with male participants (2.6 ± 2.4 vs 1.4 ± 2.0, P = 0.05). Participants with higher self-efficacy reported lower pain ratings (r = –0.47, P < 0.001).
Quantitative Data: Health Care Providers
Providers (n = 56) and community stakeholders (2 leaders of community-based organizations and 3 health care administrators) were interviewed, with 29 also completing the survey. The reason for not completing (n = 22) was not having the time once the interview was complete. A link to the survey was sent to any provider not completing at the time of the interview, with 2 follow-up reminders. The majority of providers were between the ages of 31 and 50 years (46.4%), female (71.4%), and white (66.1%) (Table 4). None were of Hispanic, Latinx, or Spanish origin. Thirty-six were physicians (64.3%), and 16 were allied health professionals (28.6%). Of the 56 providers, 32 indicated they had expertise caring for patients with SCD (57.1%), 14 were ED providers (25%), and 5 were primary care providers. Most of the providers practiced in an urban setting (91.1%).

Barriers to Care: ED Provider Perspectives. Nine of 14 ED providers interviewed completed the survey on their perspectives regarding barriers to care in the ED, difficulty with follow-ups, ED training resources, and pain control for patients with SCD. ED providers (n = 8) indicated that “provider attitudes” were a barrier to care delivery in the ED for patients with SCD. Some providers (n = 7) indicated that “implicit bias,” “opioid epidemic,” “concern about addiction,” and “patient behavior” were barriers. Respondents indicated that “overcrowding” (n = 6) and “lack of care pathway/protocol” (n = 5) were barriers. When asked to express their level of agreement with statements about SCD care in the ED, respondents disagreed/strongly disagreed (n = 5) that they were “able to make a follow-up appointment” with a sickle cell specialist or primary care provider upon discharge from the ED, and others disagreed/strongly disagreed (n = 4) that they were able to make a “referral to a case management program.”
ED training and resources. Providers agreed/strongly agreed (n = 8) that they had the knowledge and training to care for patients with SCD, that they had access to needed medications, and that they had access to knowledgeable nursing staff with expertise in SCD care. All 9 ED providers indicated that they had sufficient physician/provider staffing to provide good pain management to persons with SCD in the ED.
Pain control in the ED. Seven ED providers indicated that their ED used individualized dosing protocols to treat sickle cell pain, and 5 respondents indicated their ED had a protocol for treating sickle cell pain. Surprisingly, only 3 indicated that they were aware of the NHLBI recommendations for the treatment of vaso-occlusive pain.
Barriers to Care: Primary Care Provider Perspectives. Twenty providers completed the SCD provider section of the survey, including 17 multidisciplinary SCD providers from 4 sickle cell special care centers and 3 community primary care providers. Of the 20, 12 were primary care providers for patients with SCD (Table 4).
Patient needs. Six primary care providers indicated that the medical needs of patients with SCD were being met, but none indicated that the behavioral health or mental health needs were being met.
Managing SCD comorbidities. Five primary care providers indicated they were very comfortable providing preventive ambulatory care to patients with SCD. Six indicated they were very comfortable managing acute pain episodes, but none were very comfortable managing comorbidities such as pulmonary hypertension, diabetes, or chronic pain.
Barriers to opioid use. Only 3 of 12 providers reviewing a list of 15 potential barriers to the use of opioids for SCD pain management indicated a perceived lack of efficacy of opioids, development of tolerance and dependence, and concerns about community perceptions as barriers. Two providers selected potential for diversion as a moderate barrier to opioid use.
Barriers to hydroxyurea use. Eight of 12 providers indicated that the common reasons that patients/families refuse hydroxyurea were “worry about side effects”; 7 chose “don’t want to take another medicine,” and 6 chose “worry about carcinogenic potential.” Others (n = 10) indicated that “patient/family adherence with hydroxyurea” and “patient/family adherence with required blood tests” were important barriers to hydroxyurea use. Eight of the 12 providers indicated that they were comfortable with managing hydroxyurea in patients with SCD.
Care redesign. Twenty SCD and primary care providers completed the Care Redesign section of the survey. Respondents (n = 11) indicated that they would see more patients with SCD if they had accessible case management services available without charge or if patient access to transportation to clinic was also available. Ten indicated that they would see more patients with SCD if they had an accessible community health worker (who understands patient’s/family’s social situation) and access to a pain management specialist on call to answer questions and who would manage chronic pain. All (n = 20) were willing to see more patients with SCD in their practices. Most reported that a clinical decision-support tool for SCD treatment (n = 13) and avoidance of complications (n = 12) would be useful.
Discussion
We evaluated access and barriers to care, quality of care, care coordination, and provider communication from the perspectives of adolescents and adults with SCD, their care providers, and community stakeholders, within the Solberg conceptual model for quality improvement. We found that barriers within the care process content domain (context and systems) were most salient for this population of adolescents and adults with SCD, with lack of provider knowledge and poor attitudes toward adolescents and adults with SCD, particularly in the ED, cited consistently by participant groups. Stigmatization and lack of provider compassion that affected the quality of care were particularly problematic. These findings are consistent with previous reports.42,43 Adult health care (particularly ED) provider biases and negative attitudes have been recognized as major barriers to optimal pain management in SCD.8,11,44,45 Interestingly, ED providers in our needs assessment indicated that they felt they had the training and resources to manage patients with SCD. However, only a few actually reported knowing about the NHLBI recommendations for the treatment of vaso-occlusive pain.
Within the care process content domain, we also found that SCD-related complications and associated emotions (fear, worry, anxiety), compounded by lack of access to knowledgeable and compassionate providers, pose a significant burden. Negative encounters with the health care system contributed to a striking 84% of patient participants choosing to manage severe pain at home, with pain seriously interfering with their ability to function on a daily basis. ED providers agreed that provider attitudes and implicit bias pose important barriers to care for adolescents and adults with SCD. Adolescents and adults with SCD wanted, and understood the need, to enhance self-management skills. Both they and their providers agreed that barriers to hydroxyurea uptake included worries about potential side effects, challenges with adherence to repeated laboratory testing, and support with remembering to take the medicine. However, providers uniformly expressed that access to behavioral and mental health services were, if not nonexistent, impossible to access.
Participants with SCD and their providers reported infrastructural challenges (change process capability), as manifested in limitations with accessing acute and preventive care due to transportation- and insurance- related issues. There were health system barriers that were particularly encountered during the transition from pediatric to adult care. These findings are consistent with previous reports that have found fewer interdisciplinary services available in the adult care settings compared with pediatrics.46,47 Furthermore, adult care providers were less willing to accept adults with SCD because of the complexity of their management, for which the providers did not have the necessary expertise.3,48-50 In addition, both adolescents and adults with SCD and primary care providers highlighted the inadequacies of the current system in addressing the chronic pain needs of this population. Linking back to the Solberg conceptual framework, our needs assessment results confirm the important role of establishing SCD care as a priority within a health care system—this requires leadership and vision. The vision and priorities must be implemented by effective health care teams. Multilevel approaches or interventions, when implemented, will lead to the desired outcomes.
Findings from our needs assessment within our 5-county region mirror needs assessment results from the broader consortium.51 The SCDIC has prioritized developing an intervention that addresses the challenges identified within the care process domain by directly enhancing provider access to patient individualized care plans in the electronic health record in the ED. Importantly, ED providers will be asked to view a short video that directly challenges bias and stigma in the ED. Previous studies have indeed found that attitudes can be improved by providers viewing short video segments of adults with SCD discussing their experiences.36,52 This ED protocol will be one of the interventions that we will roll out in Northern California, given the significance of negative ED encounters reported by needs assessment participants. An additional feature of the intervention is a script for adults with SCD that guides them through introducing their individualized pain plan to their ED providers, thereby enhancing their self-efficacy in a situation that has been so overwhelmingly challenging.
We will implement a second SCDIC intervention that utilizes a mobile app to support self-management on the part of the patient, by supporting motivation and adherence with hydroxyurea.53 A companion app supports hydroxyurea guideline adherence on the part of the provider, in keeping with one of our findings that providers are in need of decision-support tools. Elements of the intervention also align with our findings related to the importance of a support system in managing SCD, in that participants will identify a supportive partner who will play a specific role in supporting their adherence with hydroxyurea.
On our local level, we have, by necessity, partnered with leaders and community stakeholders throughout the region to ensure that these interventions to improve SCD care are prioritized. Grant funds provide initial resources for the SCDIC interventions, but our partnering health care administrators and medical directors must ensure that participating ED and hematology providers are free from competing priorities in order to implement the changes. We have partnered with a SCD community-based organization that is designing additional educational presentations for local emergency medicine providers, with the goal to bring to life very personal stories of bias and stigma within the EDs that directly contribute to decisions to avoid ED care despite severe symptoms.
Although we attempted to obtain samples of adolescents and adults with SCD and their providers that were representative across the 5-county region, the larger proportion of respondents were from 1 county. We did not assess concerns of age- and race-matched adults in our catchment area, so we cannot definitively say that our findings are unique to SCD. However, our results are consistent with findings from the national sample of adults with SCD who participated in the ASCQ-Me field test, and with results from the SCDIC needs assessment.33,51 Interviews and surveys are subject to self-report bias and, therefore, may or may not reflect the actual behaviors or thoughts of participants. Confidence is increased in our results given the triangulation of expressed concerns across participant groups and across data collection strategies. The majority of adolescents and adults with SCD (95%) completed both the interview and survey, while 64% of ED providers interviewed completed the survey, compared with 54% of SCD specialists and primary care providers. These response rates are more than acceptable within the realm of survey response rates.54,55
Although we encourage examining issues with care delivery within the conceptual framework for quality improvement presented, we recognize that grant funding allowed us to conduct an in-depth needs assessment that might not be feasible in other settings. Still, we would like readers to understand the importance of gathering data for improvement in a systematic manner across a range of participant groups, to ultimately inform the development of interventions and provide for evaluation of outcomes as a result of the interventions. This is particularly important for a disease, such as SCD, that is both medically and sociopolitically complex.
Conclusion
Our needs assessment brought into focus the multiple factors contributing to the disparities in health care experienced by adolescents and adults with SCD on our local level, and within the context of inequities in health resources and outcomes on the national level. We propose solutions that include specific interventions developed by a consortium of SCD and implementation science experts. We utilize a quality improvement framework to ensure that the elements of the interventions also address the barriers identified by our local providers and patients that are unique to our community. The pervasive challenges in SCD care, coupled with its medical complexities, may seem insurmountable, but our survey and qualitative results provide us with a road map for the way forward.
Acknowledgments: The authors thank the adolescents and adults with sickle cell disease, the providers, and the community stakeholders who completed the interviews and surveys. The authors also acknowledge the SCCCI co-investigators for their contributions to this project, including Michael Bell, MD, Ward Hagar, MD, Christine Hoehner, FNP, Kimberly Major, MSW, Anne Marsh, MD, Lynne Neumayr, MD, and Ted Wun, MD. We also thank Kamilah Bailey, Jameelah Hodge, Jennifer Kim, Michael Rowland, Adria Stauber, Amber Fearon, and Shanda Robertson, and the Sickle Cell Data Collection Program for their contributions.
Corresponding author: Marsha J. Treadwell, PhD, University of California San Francisco Benioff Children’s Hospital Oakland, 747 52nd St., Oakland, CA 94609; [email protected].
Financial disclosures: None.
Funding/support: This work was supported by grant # 1U01HL134007 from the National Heart, Lung, and Blood Institute to the University of California San Francisco Benioff Children’s Hospital Oakland.
From the University of California San Francisco (Dr. Treadwell, Dr. Hessler, Yumei Chen, Swapandeep Mushiana, Dr. Potter, and Dr. Vichinsky), the University of California Los Angeles (Dr. Jacob), and the University of California Berkeley (Alex Chen).
Abstract
- Objective: Adolescents and adults with sickle cell disease (SCD) face pervasive disparities in health resources and outcomes. We explored barriers to and facilitators of care to identify opportunities to support implementation of evidence-based interventions aimed at improving care quality for patients with SCD.
- Methods: We engaged a representative sample of adolescents and adults with SCD (n = 58), health care providers (n = 51), and community stakeholders (health care administrators and community-based organization leads (n = 5) in Northern California in a community-based needs assessment. We conducted group interviews separately with participant groups to obtain in-depth perspectives. Adolescents and adults with SCD completed validated measures of pain interference, quality of care, self-efficacy, and barriers to care. Providers and community stakeholders completed surveys about barriers to SCD care.
- Results: We triangulated qualitative and quantitative data and found that participants with SCD (mean age, 31 ± 8.6 years), providers, and community stakeholders emphasized the social and emotional burden of SCD as barriers. Concrete barriers agreed upon included insurance and lack of resources for addressing pain impact. Adolescents and adults with SCD identified provider issues (lack of knowledge, implicit bias), transportation, and limited social support as barriers. Negative encounters with the health care system contributed to 84% of adolescents and adults with SCD reporting they chose to manage severe pain at home. Providers focused on structural barriers: lack of access to care guidelines, comfort level with and knowledge of SCD management, and poor care coordination.
- Conclusion: Strategies for improving access to compassionate, evidence-based quality care, as well as strategies for minimizing the burden of having SCD, are warranted for this medically complex population.
Keywords: barriers to care; quality of care; care access; care coordination.
Sickle cell disease (SCD), an inherited chronic medical condition, affects about 100,000 individuals in the United States, a population that is predominantly African American.1 These individuals experience multiple serious and life-threatening complications, most frequently recurrent vaso-occlusive pain episodes,2 and they require interactions with multidisciplinary specialists from childhood. Because of advances in treatments, the majority are reaching adulthood; however, there is a dearth of adult health care providers with the training and expertise to manage their complex medical needs.3 Other concrete barriers to adequate SCD care include insurance and distance to comprehensive SCD centers.4,5
Social, behavioral, and emotional factors may also contribute to challenges with SCD management. SCD may limit daily functional abilities and lead to diminished overall quality of life.6,7 Some adolescents and adults may require high doses of opioids, which contributes to health care providers’ perceptions that there is a high prevalence of drug addiction in the population.8,9 These providers express negative attitudes towards adults with SCD, and, consequently, delay medication administration when it is acutely needed and provide otherwise suboptimal treatment.8,10,11 Adult care providers may also be uncomfortable with prescribing and managing disease-modifying therapies (blood transfusion, hydroxyurea) that have established efficacy.12-17
As 1 of 8 programs funded by the National Heart, Lung, and Blood Institute’s (NHLBI) Sickle Cell Disease Implementation Consortium (SCDIC), we are using implementation science to reduce barriers to care and improve quality of care and health care outcomes in SCD.18,19 Given that adolescents and adults with SCD experience high mortality, severe pain, and progressive decline in their ability to function day to day, and also face lack of access to knowledgeable, compassionate providers in primary and emergency settings, the SCDIC focuses on individuals aged 15 to 45 years.6,8,9,11,12
Our regional SCDIC program, the Sickle Cell Care Coordination Initiative (SCCCI), brings together researchers, clinicians, adolescents, and adults with SCD and their families, dedicated community members, policy makers, and administrators to identify and address barriers to health care within 5 counties in Northern California. One of our first steps was to conduct a community-based needs assessment, designed to inform implementation of evidence-based interventions, accounting for unique contextual factors in our region.
Conceptual Framework for Improving Medical Practice
Our needs assessment is guided by Solberg’s Conceptual Framework for Improving Medical Practice (Figure 1).20 Consistent with the overarching principles of the SCDIC, this conceptual framework focuses on the inadequate implementation of evidence-based guidelines, and on the need to first understand multifactorial facilitators and barriers to guideline implementation in order to effect change. The framework identifies 3 main elements that must be present to ensure improvements in quality-of-care processes and patient outcomes: priority, change process capability, and care process content. Priority refers to ample resource allocation for the specific change, as well as freedom from competing priorities for those implementing the change. Change process capability includes strong, effective leadership, adequate infrastructure for managing change (including resources and time), change management skills at all levels, and an established clinical information system. Care process content refers to context and systems-level changes, such as delivery system redesign as needed, support for self-management to lessen the impact of the disease, and decision support.21-23

The purpose of our community-based needs assessment was to evaluate barriers to care and quality of care in SCD, within Solberg’s conceptual model for improving medical practice. The specific aims were to evaluate access and barriers to care (eg, lack of provider expertise and training, health care system barriers such as poor care coordination and provider communication); evaluate quality of care; and assess patient needs related to pain, pain interference, self-efficacy, and self-management for adolescents and adults with SCD. We gathered the perspectives of a representative community of adolescents and adults with SCD, their providers, and community stakeholders in order to examine barriers, quality of life and care, and patient experiences in our region.
Methods
Design
In this cross-sectional study, adolescents and adults with SCD, their providers, and community stakeholders participated in group or individual qualitative interviews and completed surveys between October 2017 and March 2018.
Setting and Sample
Recruitment flyers were posted on a regional SCD-focused website, and clinical providers or a study coordinator introduced information about the needs assessment to potential participants with SCD during clinic visits at the participating centers. Participants with SCD were eligible if they had any diagnosis of SCD, were aged 15 to 48 years, and received health services within 5 Northern California counties (Alameda, Contra Costa, Sacramento, San Francisco, and Solano). They were excluded if they did not have a SCD diagnosis or had not received health services within the catchment area. As the project proceeded, participants were asked to refer other adolescents and adults with SCD for the interviews and surveys (snowball sampling). Our goal was to recruit 50 adolescents and adults with SCD into the study, aiming for 10 representatives from each county.
Providers and community stakeholders were recruited via emails, letters and informational flyers. We engaged our partner, the Sickle Cell Data Collection Program,2 to generate a list of providers and institutions that had seen patients with SCD in primary, emergency, or inpatient settings in the region. We contacted these institutions to describe the SCCCI and invite participation in the needs assessment. We also invited community-based organization leads and health care administrators who worked with SCD to participate. Providers accessed confidential surveys via a secure link on the study website or completed paper versions. Common data collected across providers included demographics and descriptions of practice settings.
Participants were eligible to be part of the study if they were health care providers (physicians and nurses) representing hematology, primary care, family medicine, internal medicine, or emergency medicine; ancillary staff (social work, psychology, child life); or leaders or administrators of clinical or sickle cell community-based organizations in Northern California (recruitment goal of n = 50). Providers were excluded if they practiced in specialties other than those noted or did not practice within the region.
Data Collection Procedures
After providing assent/consent, participating adolescents and adults with SCD took part in individual and group interviews and completed survey questionnaires. All procedures were conducted in a private space in the sickle cell center or community. Adolescents and adults with SCD completed the survey questionnaire on a tablet, with responses recorded directly in a REDCap (Research Electronic Data Capture) database,24 or on a paper version. Interviews lasted 60 (individual) to 90 (group) minutes, while survey completion time was 20 to 25 minutes. Each participant received a gift card upon completion as an expression of appreciation. All procedures were approved by the institutional review boards of the participating health care facilities.
Group and Individual Interviews
Participants with SCD and providers were invited to participate in a semi-structured qualitative interview prior to being presented with the surveys. Adolescents and adults with SCD were interviewed about barriers to care, quality of care, and pain-related experiences. Providers were asked about barriers to care and treatments. Interview guides were modified for community-based organization leaders and health care administrators who did not provide clinical services. Interview guides can be found in the Appendix. Interviews were conducted by research coordinators trained in qualitative research methods by the first author (MT). As appropriate with semi-structured interviews, the interviewers could word questions spontaneously, change the order of questions for ease of flow of conversation, and inform simultaneous coding of interviews with new themes as those might arise, as long as they touched on all topics within the interview guide.25 The interview guides were written, per qualitative research standards, based on the aims and purpose of the research,26 and were informed by existing literature on access and barriers to care in SCD, quality of care, and the needs of individuals with SCD, including in relation to impact of the disease, self-efficacy, and self-management.
Interviewees participated in either individual or group interviews, but not both. The decision for which type of interview an individual participated in was based on 2 factors: if there were not comparable participants for group interviews (eg, health care administrator and community-based organization lead), these interviews were done individually; and given that we were drawing participants from a 5-county area in Northern California, scheduling was challenging for individuals with SCD with regard to aligning schedules and traveling to a central location where the group interviews were conducted. Provider group interviews were easier to arrange because we could schedule them at the same time as regularly scheduled meetings at the participants’ health care institutions.
Interview Data Gathering and Analysis
Digital recordings of the interviews were cleaned of any participant identifying data and sent for transcription to an outside service. Transcripts were reviewed for completeness and imported into NVivo (www.qsrinternational.com), a qualitative data management program.
A thematic content analysis and deductive and inductive approaches were used to analyze the verbatim transcripts generated from the interviews. The research team was trained in the use of NVivo software to facilitate the coding process. A deductive coding scheme was initially used based on existing concepts in the literature regarding challenges to optimal SCD care, with new codes added as the thematic content analyses progressed. The initial coding, pattern coding, and use of displays to examine the relationships between different categories were conducted simultaneously.27,28 Using the constant comparative method, new concepts from participants with SCD and providers could be incorporated into subsequent interviews with other participants. For this study, the only additional concepts added were in relation to participant recruitment and retention in the SCDIC Registry. Research team members coded transcripts separately and came together weekly, constantly comparing codes and developing the consensus coding scheme. Where differences between coders existed, code meanings were discussed and clarified until consensus was reached.29
Quantitative data were analyzed using SPSS (v. 25, Chicago, IL). Descriptive statistics (means, standard deviations, frequencies, percentages) were used to summarize demographics (eg, age, gender, and race), economic status, and type of SCD. No systematic differences were detected from cases with missing values. Scale reliabilities (ie, Cronbach α) were evaluated for self-report measures.
Measurement
Adolescents and adults with SCD completed items from the PhenX Toolkit (consensus measures for Phenotypes and eXposures), assessing sociodemographics (age, sex, race, ethnicity, educational attainment, occupation, marital status, annual income, insurance), and clinical characteristics (sickle cell diagnosis and emergency department [ED] and hospital utilization for pain).30
Pain Interference Short Form (Patient-Reported Outcomes Measurement Information System [PROMIS]). The Pain Interference Form consists of 8 items that assess the degree to which pain interfered with day-to-day activities in the previous 7 days at home, including impacts on social, cognitive, emotional, and physical functioning; household chores and recreational activities; sleep; and enjoyment in life. Reliability and validity of the PROMIS Pain Interference Scale has been demonstrated, with strong negative correlations with Physical Function Scales (r = 0.717, P < 0.01), indicating that higher scores are associated with lower function (β = 0.707, P < 0.001).31 The Cronbach α estimate for the other items on the pain interference scale was 0.99. Validity analysis indicated strong correlations with pain-related domains: BPI Interference Subscale (rho = 0.90), SF-36 Bodily Pain Subscale (rho = –0.84), and 0–10 Numerical Rating of Pain Intensity (rho = 0.48).32
Adult Sickle Cell Quality of Life Measurement Information System (ASCQ-Me) Quality of Care (QOC). ASCQ-Me QOC consists of 27 items that measure the quality of care that adults with SCD have received from health care providers.33 There are 3 composites: provider communication (quality of patient and provider communication), ED care (quality of care in the ED), and access (to routine and emergency care). Internal consistency reliability for all 3 composites is greater than 0.70. Strong correlations of the provider communication composite with overall ratings of routine care (r = 0.65) and overall provider ratings (r = 0.83) provided evidence of construct validity. Similarly, the ED care composite was strongly correlated with overall ratings of QOC in the ED, and the access composite was highly correlated with overall evaluations of ED care (r = 0.70). Access, provider interaction, and ED care composites were reliable (Cronbach α, 0.70–0.83) and correlated with ratings of global care (r = 0.32–0.83), further indicating construct validity.33
Sickle Cell Self-Efficacy Scale (SCSES). The SCSES is a 9-item, self-administered questionnaire measuring perceptions of the ability to manage day-to-day issues resulting from SCD. SCSES items are scored on a 5-point scale ranging from Not sure at all (1) to Very sure (5). Individual item responses are summed to give an overall score, with higher scores indicating greater self-efficacy. The SCSES has acceptable reliability (r = 0.45, P < 0.001) and validity (α = 0.89).34,35
Sickle Cell Disease Barriers Checklist. This checklist consists of 53 items organized into 8 categories: insurance, transportation, accommodations and accessibility, provider knowledge and attitudes, social support, individual barriers such as forgetting or difficulties understanding instructions, emotional barriers (fear, anger), and disease-related barriers. Participants check applicable barriers, with a total score range of 0 to 53 and higher scores indicating more barriers to care. The SCD Barriers Checklist has demonstrated face validity and test-retest reliability (Pearson r = 0.74, P < 0.05).5
ED Provider Checklist. The ED provider survey is a checklist of 14 statements pertaining to issues regarding patient care, with which the provider rates level of agreement. Items representing the attitudes and beliefs of providers towards patients with SCD are rated on a Likert-type scale, with level of agreement indicated as 1 (strongly disagree) to 6 (strongly agree). The positive attitudes subscale consists of 4 items (Cronbach α= 0.85), and the negative attitudes subscale consists of 6 items (Cronbach α = 0.89). The Red-Flag Behaviors subscale includes 4 items that indicate behavior concerns about drug-seeking, such as requesting specific narcotics and changing behavior when the provider walks in.8,36,37
Sickle cell and primary care providers also completed a survey consisting of sets of items compiled from existing provider surveys; this survey consisted of a list of 16 barriers to using opioids, which the providers rated on a 5-point Likert-type scale (1, not a barrier; 5, complete barrier).13,16,38 Providers indicated their level of experience with caring for patients with SCD; care provided, such as routine health screenings; and comfort level with providing preventive care, managing comorbidities, and managing acute and chronic pain. Providers were asked what potential facilitators might improve care for patients with SCD, including higher reimbursement, case management services, access to pain management specialists, and access to clinical decision-support tools. Providers responded to specific questions about management with hydroxyurea (eg, criteria for, barriers to, and comfort level with prescribing).39 The surveys are included in the Appendix.
Triangulation
Data from the interviews and surveys were triangulated to enhance understanding of results generated from the different data sources.40 Convergence of findings, different facets of the same phenomenon, or new perspectives were examined.
Results
Qualitative Data
Adolescents and adults with SCD (n = 55) and health care providers and community stakeholders (n = 56) participated in group or individual interviews to help us gain an in-depth understanding of the needs and barriers related to SCD care in our 5-county region. Participants with SCD described their experiences, which included stigma, racism, labeling, and, consequently, stress. They also identified barriers such as lack of transportation, challenges with insurance, and lack of access to providers who were competent with pain management. They reported that having SCD in a health care system that was unable to meet their needs was burdensome.
Barriers to Care and Treatments. Adolescents and adults indicated that SCD and its sequelae posed significant barriers to health care. Feelings of tiredness and pain make it more difficult for them to seek care. The emotional burden of SCD (fear and anger) was a frequently cited barrier, which was fueled by previous negative encounters with the health care system. All adolescents and adults with SCD reported that they knew of stigma in relation to seeking pain management that was pervasive and long-standing, and the majority reported they had directly experienced stigma. They reported that being labeled as “drug-seekers” was typical when in the ED for pain management. Participants articulated unconscious bias or overt racism among providers: “people with sickle cell are Black ... and Black pain is never as valuable as White pain” (25-year-old male). Respondents with SCD described challenges to the credibility of their pain reports in the ED. They reported that ED providers expressed doubts regarding the existence and/or severity of their pain, consequently creating a feeling of disrespect for patients seeking pain relief. The issue of stigma was mentioned by only 2 of 56 providers during their interviews.
Lack of Access to Knowledgeable, Compassionate Providers. Lack of access to knowledgeable care providers was another prevalent theme expressed by adolescents and adults with SCD. Frustration occurred when providers did not have knowledge of SCD and its management, particularly pain assessment. Adolescents and adults with SCD noted the lack of compassion among providers: “I’ve been kicked out of the hospital because they felt like okay, well we gave you enough medication, you should be all right” (29-year-old female). Providers specifically mentioned lack of compassion and knowledge as barriers to SCD care much less often during their interviews compared with the adolescents and adults with SCD.
Health Care System Barriers. Patient participants often expressed concerns about concrete and structural aspects of care. Getting to their appointments was a challenge for half of the interviewees, as they either did not have access to a vehicle or could not afford to travel the needed distance to obtain quality care. Even when hospitals were accessible by public transportation, those with excruciating pain understandably preferred a more comfortable and private way to travel: “I would like to change that, something that will be much easier, convenient for sickle cell patients that do suffer with pain, that they don’t have to travel always to see the doctor” (30-year-old male).
Insurance and other financial barriers also played an important role in influencing decisions to seek health care services. Medical expenses were not covered, or co-pays were too high. The Medicaid managed care system could prevent access to knowledgeable providers who were not within network. Such a lack of access discouraged some adolescents and adults with SCD from seeking acute and preventive care.
Transition From Pediatric to Adult Care. Interviewees with SCD expressed distress about the gap between pediatric and adult care. They described how they had a long-standing relationship with their medical providers, who were familiar with their medical background and history from childhood. Adolescent interviewees reported an understanding of their own pain management as well as adherence to and satisfaction with their individualized pain plans. However, adults noted that satisfaction plummeted with increasing age due to the limited number of experienced adult SCD providers, which was compounded by negative experiences (stigma, racism, drug-seeking label).
One interviewee emphasized the difficulty of finding knowledgeable providers after transition: “When you’re a pediatric sickle cell [patient], you have the doctors there every step of the way, but not with adult sickle cell… I know when I first transitioned I never felt more alone in my life… you look at that ER doctor kind of with the same mindset as you would your hematologist who just hand walked you through everything. And adult care providers were a lot more blunt and cold and they’re like… ‘I don’t know; I’m not really educated in sickle cell.’” A sickle cell provider shared his insight about the problem of transitioning: “I think it’s particularly challenging because we, as a community, don’t really set them up for success. It’s different from other chronic conditions [in that] it’s much harder to find an adult sickle cell provider. There’s not a lot of adult hematologists that will take care of our adult patients, and so I know statistically, there’s like a drop-down in the overall outcomes of our kids after they age out of our pediatric program.”
Self-Management, Supporting Hydroxyurea Use. Interview participants with SCD reported using a variety of methods to manage pain at home and chose to go to the ED only when the pain became intolerable. Patients and providers expressed awareness of different resources for managing pain at home, yet they also indicated that these resources have not been consolidated in an accessible way for patients and families. Some resources cited included heat therapy, acupuncture, meditation, medical marijuana, virtual reality devices, and pain medications other than opioids.
Patients and providers expressed the need for increasing awareness and education about hydroxyurea. Many interview participants with SCD were concerned about side effects, multiple visits with a provider during dose titration, and ongoing laboratory monitoring. They also expressed difficulties with scheduling multiple appointments, depending on access to transportation and limited provider clinic hours. They were aware of strategies for improving adherence with hydroxyurea, including setting phone alarms, educating family members about hydroxyurea, and eliciting family support, but expressed needing help to consistently implement these strategies.
Safe Opioid Prescribing. Adult care providers expressed concerns about safe opioid prescribing for patients with SCD. They were reluctant to prescribe opioid doses needed to adequately control SCD pain. Providers expressed uncertainty and fear or concern about medical/legal liability or about their judgment about what’s safe and not safe for patients with chronic use/very high doses of opioids. “I know we’re in like this opiate epidemic here in this country but I feel like these patients don’t really fit under that umbrella that the problem is coming from so [I am] just trying to learn more about how to take care of them.”
Care Coordination and Provider Communication. Adolescents and adults with SCD reported having positive experiences—good communication, established trust, and compassionate care—with their usual providers. However, they perceived that ED physicians and nurses did not really care about them. Both interviewees with SCD and providers recognized the importance of good communication in all settings as the key to overcoming barriers to receiving quality care. All agreed on the importance of using individual pain plans so that all providers, especially ED providers, can be more at ease with treating adolescents and adults with SCD.
Quantitative Data: Adolescents and Adults With SCD
Fifty-eight adolescents and adults with SCD (aged 15 to 48 years) completed the survey. Three additional individuals who did not complete the interview completed the survey. Reasons for not completing the interview included scheduling challenges (n = 2) or a sickle cell pain episode (n = 1). The average age of participants was 31 years ± 8.6, more than half (57%) were female, and the majority (93%) were African American (Table 1). Most (71%) had never been married. Half (50%) had some college or an associate degree, and 40% were employed and reported an annual household income of less than $30,000. Insurance coverage was predominantly Medi-Cal (Medicaid, 69%). The majority of participants resided in Alameda (34.5%) or Contra Costa (21%) counties. The majority of sickle cell care was received in Alameda County, whether outpatient (52%), inpatient (40%), or ED care (41%). The majority (71%) had a diagnosis of SCD hemoglobin SS.
Pain. More than one-third of individuals with SCD reported 1 or 2 ED visits for pain in the previous 6 months (34%), and more than 3 hospitalizations (36%) related to pain in the previous year (Table 2). The majority (85%) reported having severe pain at home in the previous 6 months that they did not seek health care for, consistent with their reports in the qualitative interviews. More than half (59%) reported 4 or more of these severe pain episodes that led to inability to perform daily activities for 1 week or more. While pain interference on the PROMIS Pain Interference Short Form on average (T-score, 59.6 ± 8.6) was similar to that of the general population (T-score, 50 ± 10), a higher proportion of patients with SCD reported pain interference compared with the general population. The mean self-efficacy (confidence in ability to manage complications of SCD) score on the SCSES of 30.0 ± 7.3 (range, 9–45) was similar to that of other adults with SCD (mean, 32.2 ± 7.0). Twenty-five percent of the present sample had a low self-efficacy score (< 25).
Barriers to Care and Treatments. Consistent with the qualitative data, SCD-related symptoms such as tiredness (64%) and pain (62%) were reported most often as barriers to care (Table 3). Emotions (> 25%) such as worry/fear, frustration/anger, and lack of confidence were other important barriers to care. Provider knowledge and attitudes were cited next most often, with 38% of the sample indicating “Providers accuse me of drug-seeking” and “It is hard for me to find a provider who has enough experiences with or knowledge about SCD.” Participants expressed that they were not believed when in pain and “I am treated differently from other patients.” Almost half of respondents cited “I am not seen quickly enough when I am in pain” as a barrier to their care.

Consistent with the qualitative data, transportation barriers (not having a vehicle, costs of transportation, public transit not easy to get to) were cited by 55% of participants. About half of participants reported that insurance was an important barrier, with high co-pays and medications and other services not covered. In addition, gathering approvals was a long and fragmented process, particularly for consultations among providers (hematology, primary care provider, pain specialist). Furthermore, insurance provided limited choices about location for services.
Participants reported social support system burnout (22%), help needed with daily activities (21%), and social isolation or generally not having enough support (33%) as ongoing barriers. Difficulties were encountered with self-management (eg, taking medications on time or making follow-up appointments, 19%), with 22% of participants finding the health care system confusing or hard to understand. Thirty percent reported “Places for me to go to learn how to stay well are not close by or easy to get to.” ”Worry about side effects” (33%) was a common barrier to hydroxyurea use. Participants described “forgetting to take the medicine,” “tried before but it did not work,” “heard scary things” about hydroxyurea, and “not interested in taking another medicine” as barriers.
Quality of Care. More than half (51%) of the 53 participants who had accessed health care in the previous year rated their overall health care as poor on the ASCQ-Me QOC measure. This was significantly higher compared to the reports from more than 47,000 adults with Medicaid in 2017 (16%),41 and to the 2008-2009 report from 556 adults with SCD from across the United States (37%, Figure 2).33 The major contributor to these poor ratings for participants in our sample was low satisfaction with ED care.

Sixty percent of the 42 participants who had accessed ED care in the past year indicated “never” or “sometimes” to the question “When you went to the ED for care, how often did you get it as soon as you wanted?” compared with only 16% of the 2017 adult Medicaid population responding (n = 25,789) (Figure 3). Forty-seven percent of those with an ED visit indicated that, in the previous 12 months, they had been made to wait “more than 2 hours before receiving treatment for acute pain in the ED.” However, in the previous 12 months, 39% reported that their wait time in the ED had been only “between five minutes and one hour.”

On the ASCQ-Me QOC Access to Care composite measure, 33% of 42 participants responding reported they were seen at a routine appointment as soon as they would have liked. This is significantly lower compared to 56% of the adult Medicaid population responding to the same question. Reports of provider communication (Provider Communication composite) for adolescents and adults with SCD were comparable to reports of adults with SCD from the ASCQ-Me field test,33 but adults with Medicaid reported higher ratings of quality communication behaviors (Figure 4).33,41 Nearly 60% of both groups with SCD reported that providers “always” performed quality communication behaviors—listened carefully, spent enough time, treated them with respect, and explained things well—compared with more than 70% of adults with Medicaid.

Participants from all counties reported the same number of barriers to care on average (3.3 ± 2.1). Adolescents and adults who reported more barriers to care also reported lower satisfaction with care (r = –0.47, P < 0.01) and less confidence in their ability to manage their SCD (self-efficacy, r = – 0.36, P < 0.05). Female participants reported more barriers to care on average compared with male participants (2.6 ± 2.4 vs 1.4 ± 2.0, P = 0.05). Participants with higher self-efficacy reported lower pain ratings (r = –0.47, P < 0.001).
Quantitative Data: Health Care Providers
Providers (n = 56) and community stakeholders (2 leaders of community-based organizations and 3 health care administrators) were interviewed, with 29 also completing the survey. The reason for not completing (n = 22) was not having the time once the interview was complete. A link to the survey was sent to any provider not completing at the time of the interview, with 2 follow-up reminders. The majority of providers were between the ages of 31 and 50 years (46.4%), female (71.4%), and white (66.1%) (Table 4). None were of Hispanic, Latinx, or Spanish origin. Thirty-six were physicians (64.3%), and 16 were allied health professionals (28.6%). Of the 56 providers, 32 indicated they had expertise caring for patients with SCD (57.1%), 14 were ED providers (25%), and 5 were primary care providers. Most of the providers practiced in an urban setting (91.1%).

Barriers to Care: ED Provider Perspectives. Nine of 14 ED providers interviewed completed the survey on their perspectives regarding barriers to care in the ED, difficulty with follow-ups, ED training resources, and pain control for patients with SCD. ED providers (n = 8) indicated that “provider attitudes” were a barrier to care delivery in the ED for patients with SCD. Some providers (n = 7) indicated that “implicit bias,” “opioid epidemic,” “concern about addiction,” and “patient behavior” were barriers. Respondents indicated that “overcrowding” (n = 6) and “lack of care pathway/protocol” (n = 5) were barriers. When asked to express their level of agreement with statements about SCD care in the ED, respondents disagreed/strongly disagreed (n = 5) that they were “able to make a follow-up appointment” with a sickle cell specialist or primary care provider upon discharge from the ED, and others disagreed/strongly disagreed (n = 4) that they were able to make a “referral to a case management program.”
ED training and resources. Providers agreed/strongly agreed (n = 8) that they had the knowledge and training to care for patients with SCD, that they had access to needed medications, and that they had access to knowledgeable nursing staff with expertise in SCD care. All 9 ED providers indicated that they had sufficient physician/provider staffing to provide good pain management to persons with SCD in the ED.
Pain control in the ED. Seven ED providers indicated that their ED used individualized dosing protocols to treat sickle cell pain, and 5 respondents indicated their ED had a protocol for treating sickle cell pain. Surprisingly, only 3 indicated that they were aware of the NHLBI recommendations for the treatment of vaso-occlusive pain.
Barriers to Care: Primary Care Provider Perspectives. Twenty providers completed the SCD provider section of the survey, including 17 multidisciplinary SCD providers from 4 sickle cell special care centers and 3 community primary care providers. Of the 20, 12 were primary care providers for patients with SCD (Table 4).
Patient needs. Six primary care providers indicated that the medical needs of patients with SCD were being met, but none indicated that the behavioral health or mental health needs were being met.
Managing SCD comorbidities. Five primary care providers indicated they were very comfortable providing preventive ambulatory care to patients with SCD. Six indicated they were very comfortable managing acute pain episodes, but none were very comfortable managing comorbidities such as pulmonary hypertension, diabetes, or chronic pain.
Barriers to opioid use. Only 3 of 12 providers reviewing a list of 15 potential barriers to the use of opioids for SCD pain management indicated a perceived lack of efficacy of opioids, development of tolerance and dependence, and concerns about community perceptions as barriers. Two providers selected potential for diversion as a moderate barrier to opioid use.
Barriers to hydroxyurea use. Eight of 12 providers indicated that the common reasons that patients/families refuse hydroxyurea were “worry about side effects”; 7 chose “don’t want to take another medicine,” and 6 chose “worry about carcinogenic potential.” Others (n = 10) indicated that “patient/family adherence with hydroxyurea” and “patient/family adherence with required blood tests” were important barriers to hydroxyurea use. Eight of the 12 providers indicated that they were comfortable with managing hydroxyurea in patients with SCD.
Care redesign. Twenty SCD and primary care providers completed the Care Redesign section of the survey. Respondents (n = 11) indicated that they would see more patients with SCD if they had accessible case management services available without charge or if patient access to transportation to clinic was also available. Ten indicated that they would see more patients with SCD if they had an accessible community health worker (who understands patient’s/family’s social situation) and access to a pain management specialist on call to answer questions and who would manage chronic pain. All (n = 20) were willing to see more patients with SCD in their practices. Most reported that a clinical decision-support tool for SCD treatment (n = 13) and avoidance of complications (n = 12) would be useful.
Discussion
We evaluated access and barriers to care, quality of care, care coordination, and provider communication from the perspectives of adolescents and adults with SCD, their care providers, and community stakeholders, within the Solberg conceptual model for quality improvement. We found that barriers within the care process content domain (context and systems) were most salient for this population of adolescents and adults with SCD, with lack of provider knowledge and poor attitudes toward adolescents and adults with SCD, particularly in the ED, cited consistently by participant groups. Stigmatization and lack of provider compassion that affected the quality of care were particularly problematic. These findings are consistent with previous reports.42,43 Adult health care (particularly ED) provider biases and negative attitudes have been recognized as major barriers to optimal pain management in SCD.8,11,44,45 Interestingly, ED providers in our needs assessment indicated that they felt they had the training and resources to manage patients with SCD. However, only a few actually reported knowing about the NHLBI recommendations for the treatment of vaso-occlusive pain.
Within the care process content domain, we also found that SCD-related complications and associated emotions (fear, worry, anxiety), compounded by lack of access to knowledgeable and compassionate providers, pose a significant burden. Negative encounters with the health care system contributed to a striking 84% of patient participants choosing to manage severe pain at home, with pain seriously interfering with their ability to function on a daily basis. ED providers agreed that provider attitudes and implicit bias pose important barriers to care for adolescents and adults with SCD. Adolescents and adults with SCD wanted, and understood the need, to enhance self-management skills. Both they and their providers agreed that barriers to hydroxyurea uptake included worries about potential side effects, challenges with adherence to repeated laboratory testing, and support with remembering to take the medicine. However, providers uniformly expressed that access to behavioral and mental health services were, if not nonexistent, impossible to access.
Participants with SCD and their providers reported infrastructural challenges (change process capability), as manifested in limitations with accessing acute and preventive care due to transportation- and insurance- related issues. There were health system barriers that were particularly encountered during the transition from pediatric to adult care. These findings are consistent with previous reports that have found fewer interdisciplinary services available in the adult care settings compared with pediatrics.46,47 Furthermore, adult care providers were less willing to accept adults with SCD because of the complexity of their management, for which the providers did not have the necessary expertise.3,48-50 In addition, both adolescents and adults with SCD and primary care providers highlighted the inadequacies of the current system in addressing the chronic pain needs of this population. Linking back to the Solberg conceptual framework, our needs assessment results confirm the important role of establishing SCD care as a priority within a health care system—this requires leadership and vision. The vision and priorities must be implemented by effective health care teams. Multilevel approaches or interventions, when implemented, will lead to the desired outcomes.
Findings from our needs assessment within our 5-county region mirror needs assessment results from the broader consortium.51 The SCDIC has prioritized developing an intervention that addresses the challenges identified within the care process domain by directly enhancing provider access to patient individualized care plans in the electronic health record in the ED. Importantly, ED providers will be asked to view a short video that directly challenges bias and stigma in the ED. Previous studies have indeed found that attitudes can be improved by providers viewing short video segments of adults with SCD discussing their experiences.36,52 This ED protocol will be one of the interventions that we will roll out in Northern California, given the significance of negative ED encounters reported by needs assessment participants. An additional feature of the intervention is a script for adults with SCD that guides them through introducing their individualized pain plan to their ED providers, thereby enhancing their self-efficacy in a situation that has been so overwhelmingly challenging.
We will implement a second SCDIC intervention that utilizes a mobile app to support self-management on the part of the patient, by supporting motivation and adherence with hydroxyurea.53 A companion app supports hydroxyurea guideline adherence on the part of the provider, in keeping with one of our findings that providers are in need of decision-support tools. Elements of the intervention also align with our findings related to the importance of a support system in managing SCD, in that participants will identify a supportive partner who will play a specific role in supporting their adherence with hydroxyurea.
On our local level, we have, by necessity, partnered with leaders and community stakeholders throughout the region to ensure that these interventions to improve SCD care are prioritized. Grant funds provide initial resources for the SCDIC interventions, but our partnering health care administrators and medical directors must ensure that participating ED and hematology providers are free from competing priorities in order to implement the changes. We have partnered with a SCD community-based organization that is designing additional educational presentations for local emergency medicine providers, with the goal to bring to life very personal stories of bias and stigma within the EDs that directly contribute to decisions to avoid ED care despite severe symptoms.
Although we attempted to obtain samples of adolescents and adults with SCD and their providers that were representative across the 5-county region, the larger proportion of respondents were from 1 county. We did not assess concerns of age- and race-matched adults in our catchment area, so we cannot definitively say that our findings are unique to SCD. However, our results are consistent with findings from the national sample of adults with SCD who participated in the ASCQ-Me field test, and with results from the SCDIC needs assessment.33,51 Interviews and surveys are subject to self-report bias and, therefore, may or may not reflect the actual behaviors or thoughts of participants. Confidence is increased in our results given the triangulation of expressed concerns across participant groups and across data collection strategies. The majority of adolescents and adults with SCD (95%) completed both the interview and survey, while 64% of ED providers interviewed completed the survey, compared with 54% of SCD specialists and primary care providers. These response rates are more than acceptable within the realm of survey response rates.54,55
Although we encourage examining issues with care delivery within the conceptual framework for quality improvement presented, we recognize that grant funding allowed us to conduct an in-depth needs assessment that might not be feasible in other settings. Still, we would like readers to understand the importance of gathering data for improvement in a systematic manner across a range of participant groups, to ultimately inform the development of interventions and provide for evaluation of outcomes as a result of the interventions. This is particularly important for a disease, such as SCD, that is both medically and sociopolitically complex.
Conclusion
Our needs assessment brought into focus the multiple factors contributing to the disparities in health care experienced by adolescents and adults with SCD on our local level, and within the context of inequities in health resources and outcomes on the national level. We propose solutions that include specific interventions developed by a consortium of SCD and implementation science experts. We utilize a quality improvement framework to ensure that the elements of the interventions also address the barriers identified by our local providers and patients that are unique to our community. The pervasive challenges in SCD care, coupled with its medical complexities, may seem insurmountable, but our survey and qualitative results provide us with a road map for the way forward.
Acknowledgments: The authors thank the adolescents and adults with sickle cell disease, the providers, and the community stakeholders who completed the interviews and surveys. The authors also acknowledge the SCCCI co-investigators for their contributions to this project, including Michael Bell, MD, Ward Hagar, MD, Christine Hoehner, FNP, Kimberly Major, MSW, Anne Marsh, MD, Lynne Neumayr, MD, and Ted Wun, MD. We also thank Kamilah Bailey, Jameelah Hodge, Jennifer Kim, Michael Rowland, Adria Stauber, Amber Fearon, and Shanda Robertson, and the Sickle Cell Data Collection Program for their contributions.
Corresponding author: Marsha J. Treadwell, PhD, University of California San Francisco Benioff Children’s Hospital Oakland, 747 52nd St., Oakland, CA 94609; [email protected].
Financial disclosures: None.
Funding/support: This work was supported by grant # 1U01HL134007 from the National Heart, Lung, and Blood Institute to the University of California San Francisco Benioff Children’s Hospital Oakland.
1. Hassell KL. Population Estimates of sickle cell disease in the U.S. Am J Prev Med. 2010; 38:S512-S521.
2. Data & Statistics on Sickle Cell Disease. Centers for Disease Control and Prevention website. www.cdc.gov/ncbddd/sicklecell/data.html. Accessed March 25, 2020.
3. Inusa BPD, Stewart CE, Mathurin-Charles S, et al. Paediatric to adult transition care for patients with sickle cell disease: a global perspective. Lancet Haematol. 2020;7:e329-e341.
4. Smith SK, Johnston J, Rutherford C, et al. Identifying social-behavioral health needs of adults with sickle cell disease in the emergency department. J Emerg Nurs. 2017;43:444-450.
5. Treadwell MJ, Barreda F, Kaur K, et al. Emotional distress, barriers to care, and health-related quality of life in sickle cell disease. J Clin Outcomes Manag. 2015;22:8-17.
6. Treadwell MJ, Hassell K, Levine R, et al. Adult Sickle Cell Quality-of-Life Measurement Information System (ASCQ-Me): conceptual model based on review of the literature and formative research. Clin J Pain. 2014;30:902-914.
7. Rizio AA, Bhor M, Lin X, et al. The relationship between frequency and severity of vaso-occlusive crises and health-related quality of life and work productivity in adults with sickle cell disease. Qual Life Res. 2020;29:1533-1547.
8. Freiermuth CE, Haywood C, Silva S, et al. Attitudes toward patients with sickle cell disease in a multicenter sample of emergency department providers. Adv Emerg Nurs J. 2014;36:335-347.
9. Jenerette CM, Brewer C. Health-related stigma in young adults with sickle cell disease. J Natl Med Assoc. 2010;102:1050-1055.
10. Lazio MP, Costello HH, Courtney DM, et al. A comparison of analgesic management for emergency department patients with sickle cell disease and renal colic. Clin J Pain. 2010;26:199-205.
11. Haywood C, Tanabe P, Naik R, et al. The impact of race and disease on sickle cell patient wait times in the emergency department. Am J Emerg Med. 2013;31:651-656.
12. Haywood C, Beach MC, Lanzkron S, et al. A systematic review of barriers and interventions to improve appropriate use of therapies for sickle cell disease. J Natl Med Assoc. 2009;101:1022-1033.
13. Mainous AG, Tanner RJ, Harle CA, et al. Attitudes toward management of sickle cell disease and its complications: a national survey of academic family physicians. Anemia. 2015;2015:1-6.
14. Yawn BP, Buchanan GR, Afenyi-Annan AN, et al. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA. 2014;312:1033.
15. Lunyera J, Jonassaint C, Jonassaint J, et al. Attitudes of primary care physicians toward sickle cell disease care, guidelines, and comanaging hydroxyurea with a specialist. J Prim Care Community Health. 2017;8:37-40.
16. Whiteman LN, Haywood C, Lanzkron S, et al. Primary care providers’ comfort levels in caring for patients with sickle cell disease. South Med J. 2015;108:531-536.
17. Wong TE, Brandow AM, Lim W, Lottenberg R. Update on the use of hydroxyurea therapy in sickle cell disease. Blood. 2014;124:3850-4004.
18. DiMartino LD, Baumann AA, Hsu LL, et al. The sickle cell disease implementation consortium: Translating evidence-based guidelines into practice for sickle cell disease. Am J Hematol. 2018;93:E391-E395.
19. King AA, Baumann AA. Sickle cell disease and implementation science: A partnership to accelerate advances. Pediatr Blood Cancer. 2017;64:e26649.
20. Solberg LI. Improving medical practice: a conceptual framework. Ann Fam Med. 2007;5:251-256.
21. Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness. J Am Med Assoc. 2002;288:5.
22. Bodenheimer T. Interventions to improve chronic illness care: evaluating their effectiveness. Dis Manag. 2003;6:63-71.
23. Tsai AC, Morton SC, Mangione CM, Keeler EB. A meta-analysis of interventions to improve care for chronic illnesses. Am J Manag Care. 2005;11:478-488.
24. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-381.
25. Kallio H, Pietilä A-M, Johnson M, et al. Systematic methodological review: developing a framework for a qualitative semi-structured interview guide. J Adv Nurs. 2016;72:2954-2965.
26. Clarke V, Braun V. Successful Qualitative Research: A Practical Guide for Beginners. First. Thousand Oaks, CA: Sage; 2013.
27. Hsieh H-F, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15:1277-1288.
28. Creswell JW, Hanson WE, Clark Plano VL, et al. Qualitative research designs: selection and implementation. Couns Psychol. 2007;35:236-264.
29. Miles MB, Huberman AM, Saldana J. Qualitative Data Analysis A Methods Sourcebook. 4th ed. Thousand Oaks, CA: Sage; 2019.
30. Eckman JR, Hassell KL, Huggins W, et al. Standard measures for sickle cell disease research: the PhenX Toolkit sickle cell disease collections. Blood Adv. 2017; 1: 2703-2711.
31. Kendall R, Wagner B, Brodke D, et al. The relationship of PROMIS pain interference and physical function scales. Pain Med. 2018;19:1720-1724.
32. Amtmann D, Cook KF, Jensen MP, et al. Development of a PROMIS item bank to measure pain interference. Pain. 2010;150:173-182.
33. Evensen CT, Treadwell MJ, Keller S, et al. Quality of care in sickle cell disease: Cross-sectional study and development of a measure for adults reporting on ambulatory and emergency department care. Medicine (Baltimore). 2016;95:e4528.
34. Edwards R, Telfair J, Cecil H, et al. Reliability and validity of a self-efficacy instrument specific to sickle cell disease. Behav Res Ther. 2000;38:951-963.
35. Edwards R, Telfair J, Cecil H, et al. Self-efficacy as a predictor of adult adjustment to sickle cell disease: one-year outcomes. Psychosom Med. 2001;63:850-858.
36. Puri Singh A, Haywood C, Beach MC, et al. Improving emergency providers’ attitudes toward sickle cell patients in pain. J Pain Symptom Manage. 2016;51:628-632.e3.
37. Glassberg JA, Tanabe P, Chow A, et al. Emergency provider analgesic practices and attitudes towards patients with sickle cell disease. Ann Emerg Med. 2013;62:293-302.e10.
38. Grahmann PH, Jackson KC 2nd, Lipman AG. Clinician beliefs about opioid use and barriers in chronic nonmalignant pain [published correction appears in J Pain Palliat Care Pharmacother. 2004;18:145-6]. J Pain Palliat Care Pharmacother. 2004;18:7-28.
39. Brandow AM, Panepinto JA. Hydroxyurea use in sickle cell disease: the battle with low prescription rates, poor patient compliance and fears of toxicities. Expert Rev Hematol. 2010;3:255-260.
40. Fielding N. Triangulation and mixed methods designs: data integration with new research technologies. J Mixed Meth Res. 2012;6:124-136.
41. 2017 CAHPS Health Plan Survey Chartbook. Agency for Healthcare Research and Quality website. www.ahrq.gov/cahps/cahps-database/comparative-data/2017-health-plan-chartbook/results-enrollee-population.html. Accessed September 8, 2020.
42. Bulgin D, Tanabe P, Jenerette C. Stigma of sickle cell disease: a systematic review. Issues Ment Health Nurs. 2018;1-11.
43. Wakefield EO, Zempsky WT, Puhl RM, et al. Conceptualizing pain-related stigma in adolescent chronic pain: a literature review and preliminary focus group findings. PAIN Rep. 2018;3:e679.
44. Nelson SC, Hackman HW. Race matters: Perceptions of race and racism in a sickle cell center. Pediatr Blood Cancer. 2013;60:451-454.
45. Dyal BW, Abudawood K, Schoppee TM, et al. Reflections of healthcare experiences of african americans with sickle cell disease or cancer: a qualitative study. Cancer Nurs. 2019;10.1097/NCC.0000000000000750.
46. Renedo A. Not being heard: barriers to high quality unplanned hospital care during young people’s transition to adult services - evidence from ‘this sickle cell life’ research. BMC Health Serv Res. 2019;19:876.
47. Ballas S, Vichinsky E. Is the medical home for adult patients with sickle cell disease a reality or an illusion? Hemoglobin. 2015;39:130-133.
48. Hankins JS, Osarogiagbon R, Adams-Graves P, et al. A transition pilot program for adolescents with sickle cell disease. J Pediatr Health Care. 2012;26 e45-e49.
49. Smith WR, Sisler IY, Johnson S, et al. Lessons learned from building a pediatric-to-adult sickle cell transition program. South Med J. 2019;112:190-197.
50. Lanzkron S, Sawicki GS, Hassell KL, et al. Transition to adulthood and adult health care for patients with sickle cell disease or cystic fibrosis: Current practices and research priorities. J Clin Transl Sci. 2018;2:334-342.
51. Kanter J, Gibson R, Lawrence RH, et al. Perceptions of US adolescents and adults with sickle cell disease on their quality of care. JAMA Netw Open. 2020;3:e206016.
52. Haywood C, Lanzkron S, Hughes MT, et al. A video-intervention to improve clinician attitudes toward patients with sickle cell disease: the results of a randomized experiment. J Gen Intern Med. 2011;26:518-523.
53. Hankins JS, Shah N, DiMartino L, et al. Integration of mobile health into sickle cell disease care to increase hydroxyurea utilization: protocol for an efficacy and implementation study. JMIR Res Protoc. 2020;9:e16319.
54. Fan W, Yan Z. Factors affecting response rates of the web survey: A systematic review. Comput Hum Behav. 2010;26:132-139.
55. Millar MM, Dillman DA. Improving response to web and mixed-mode surveys. Public Opin Q. 2011;75:249-269.
1. Hassell KL. Population Estimates of sickle cell disease in the U.S. Am J Prev Med. 2010; 38:S512-S521.
2. Data & Statistics on Sickle Cell Disease. Centers for Disease Control and Prevention website. www.cdc.gov/ncbddd/sicklecell/data.html. Accessed March 25, 2020.
3. Inusa BPD, Stewart CE, Mathurin-Charles S, et al. Paediatric to adult transition care for patients with sickle cell disease: a global perspective. Lancet Haematol. 2020;7:e329-e341.
4. Smith SK, Johnston J, Rutherford C, et al. Identifying social-behavioral health needs of adults with sickle cell disease in the emergency department. J Emerg Nurs. 2017;43:444-450.
5. Treadwell MJ, Barreda F, Kaur K, et al. Emotional distress, barriers to care, and health-related quality of life in sickle cell disease. J Clin Outcomes Manag. 2015;22:8-17.
6. Treadwell MJ, Hassell K, Levine R, et al. Adult Sickle Cell Quality-of-Life Measurement Information System (ASCQ-Me): conceptual model based on review of the literature and formative research. Clin J Pain. 2014;30:902-914.
7. Rizio AA, Bhor M, Lin X, et al. The relationship between frequency and severity of vaso-occlusive crises and health-related quality of life and work productivity in adults with sickle cell disease. Qual Life Res. 2020;29:1533-1547.
8. Freiermuth CE, Haywood C, Silva S, et al. Attitudes toward patients with sickle cell disease in a multicenter sample of emergency department providers. Adv Emerg Nurs J. 2014;36:335-347.
9. Jenerette CM, Brewer C. Health-related stigma in young adults with sickle cell disease. J Natl Med Assoc. 2010;102:1050-1055.
10. Lazio MP, Costello HH, Courtney DM, et al. A comparison of analgesic management for emergency department patients with sickle cell disease and renal colic. Clin J Pain. 2010;26:199-205.
11. Haywood C, Tanabe P, Naik R, et al. The impact of race and disease on sickle cell patient wait times in the emergency department. Am J Emerg Med. 2013;31:651-656.
12. Haywood C, Beach MC, Lanzkron S, et al. A systematic review of barriers and interventions to improve appropriate use of therapies for sickle cell disease. J Natl Med Assoc. 2009;101:1022-1033.
13. Mainous AG, Tanner RJ, Harle CA, et al. Attitudes toward management of sickle cell disease and its complications: a national survey of academic family physicians. Anemia. 2015;2015:1-6.
14. Yawn BP, Buchanan GR, Afenyi-Annan AN, et al. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA. 2014;312:1033.
15. Lunyera J, Jonassaint C, Jonassaint J, et al. Attitudes of primary care physicians toward sickle cell disease care, guidelines, and comanaging hydroxyurea with a specialist. J Prim Care Community Health. 2017;8:37-40.
16. Whiteman LN, Haywood C, Lanzkron S, et al. Primary care providers’ comfort levels in caring for patients with sickle cell disease. South Med J. 2015;108:531-536.
17. Wong TE, Brandow AM, Lim W, Lottenberg R. Update on the use of hydroxyurea therapy in sickle cell disease. Blood. 2014;124:3850-4004.
18. DiMartino LD, Baumann AA, Hsu LL, et al. The sickle cell disease implementation consortium: Translating evidence-based guidelines into practice for sickle cell disease. Am J Hematol. 2018;93:E391-E395.
19. King AA, Baumann AA. Sickle cell disease and implementation science: A partnership to accelerate advances. Pediatr Blood Cancer. 2017;64:e26649.
20. Solberg LI. Improving medical practice: a conceptual framework. Ann Fam Med. 2007;5:251-256.
21. Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness. J Am Med Assoc. 2002;288:5.
22. Bodenheimer T. Interventions to improve chronic illness care: evaluating their effectiveness. Dis Manag. 2003;6:63-71.
23. Tsai AC, Morton SC, Mangione CM, Keeler EB. A meta-analysis of interventions to improve care for chronic illnesses. Am J Manag Care. 2005;11:478-488.
24. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-381.
25. Kallio H, Pietilä A-M, Johnson M, et al. Systematic methodological review: developing a framework for a qualitative semi-structured interview guide. J Adv Nurs. 2016;72:2954-2965.
26. Clarke V, Braun V. Successful Qualitative Research: A Practical Guide for Beginners. First. Thousand Oaks, CA: Sage; 2013.
27. Hsieh H-F, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15:1277-1288.
28. Creswell JW, Hanson WE, Clark Plano VL, et al. Qualitative research designs: selection and implementation. Couns Psychol. 2007;35:236-264.
29. Miles MB, Huberman AM, Saldana J. Qualitative Data Analysis A Methods Sourcebook. 4th ed. Thousand Oaks, CA: Sage; 2019.
30. Eckman JR, Hassell KL, Huggins W, et al. Standard measures for sickle cell disease research: the PhenX Toolkit sickle cell disease collections. Blood Adv. 2017; 1: 2703-2711.
31. Kendall R, Wagner B, Brodke D, et al. The relationship of PROMIS pain interference and physical function scales. Pain Med. 2018;19:1720-1724.
32. Amtmann D, Cook KF, Jensen MP, et al. Development of a PROMIS item bank to measure pain interference. Pain. 2010;150:173-182.
33. Evensen CT, Treadwell MJ, Keller S, et al. Quality of care in sickle cell disease: Cross-sectional study and development of a measure for adults reporting on ambulatory and emergency department care. Medicine (Baltimore). 2016;95:e4528.
34. Edwards R, Telfair J, Cecil H, et al. Reliability and validity of a self-efficacy instrument specific to sickle cell disease. Behav Res Ther. 2000;38:951-963.
35. Edwards R, Telfair J, Cecil H, et al. Self-efficacy as a predictor of adult adjustment to sickle cell disease: one-year outcomes. Psychosom Med. 2001;63:850-858.
36. Puri Singh A, Haywood C, Beach MC, et al. Improving emergency providers’ attitudes toward sickle cell patients in pain. J Pain Symptom Manage. 2016;51:628-632.e3.
37. Glassberg JA, Tanabe P, Chow A, et al. Emergency provider analgesic practices and attitudes towards patients with sickle cell disease. Ann Emerg Med. 2013;62:293-302.e10.
38. Grahmann PH, Jackson KC 2nd, Lipman AG. Clinician beliefs about opioid use and barriers in chronic nonmalignant pain [published correction appears in J Pain Palliat Care Pharmacother. 2004;18:145-6]. J Pain Palliat Care Pharmacother. 2004;18:7-28.
39. Brandow AM, Panepinto JA. Hydroxyurea use in sickle cell disease: the battle with low prescription rates, poor patient compliance and fears of toxicities. Expert Rev Hematol. 2010;3:255-260.
40. Fielding N. Triangulation and mixed methods designs: data integration with new research technologies. J Mixed Meth Res. 2012;6:124-136.
41. 2017 CAHPS Health Plan Survey Chartbook. Agency for Healthcare Research and Quality website. www.ahrq.gov/cahps/cahps-database/comparative-data/2017-health-plan-chartbook/results-enrollee-population.html. Accessed September 8, 2020.
42. Bulgin D, Tanabe P, Jenerette C. Stigma of sickle cell disease: a systematic review. Issues Ment Health Nurs. 2018;1-11.
43. Wakefield EO, Zempsky WT, Puhl RM, et al. Conceptualizing pain-related stigma in adolescent chronic pain: a literature review and preliminary focus group findings. PAIN Rep. 2018;3:e679.
44. Nelson SC, Hackman HW. Race matters: Perceptions of race and racism in a sickle cell center. Pediatr Blood Cancer. 2013;60:451-454.
45. Dyal BW, Abudawood K, Schoppee TM, et al. Reflections of healthcare experiences of african americans with sickle cell disease or cancer: a qualitative study. Cancer Nurs. 2019;10.1097/NCC.0000000000000750.
46. Renedo A. Not being heard: barriers to high quality unplanned hospital care during young people’s transition to adult services - evidence from ‘this sickle cell life’ research. BMC Health Serv Res. 2019;19:876.
47. Ballas S, Vichinsky E. Is the medical home for adult patients with sickle cell disease a reality or an illusion? Hemoglobin. 2015;39:130-133.
48. Hankins JS, Osarogiagbon R, Adams-Graves P, et al. A transition pilot program for adolescents with sickle cell disease. J Pediatr Health Care. 2012;26 e45-e49.
49. Smith WR, Sisler IY, Johnson S, et al. Lessons learned from building a pediatric-to-adult sickle cell transition program. South Med J. 2019;112:190-197.
50. Lanzkron S, Sawicki GS, Hassell KL, et al. Transition to adulthood and adult health care for patients with sickle cell disease or cystic fibrosis: Current practices and research priorities. J Clin Transl Sci. 2018;2:334-342.
51. Kanter J, Gibson R, Lawrence RH, et al. Perceptions of US adolescents and adults with sickle cell disease on their quality of care. JAMA Netw Open. 2020;3:e206016.
52. Haywood C, Lanzkron S, Hughes MT, et al. A video-intervention to improve clinician attitudes toward patients with sickle cell disease: the results of a randomized experiment. J Gen Intern Med. 2011;26:518-523.
53. Hankins JS, Shah N, DiMartino L, et al. Integration of mobile health into sickle cell disease care to increase hydroxyurea utilization: protocol for an efficacy and implementation study. JMIR Res Protoc. 2020;9:e16319.
54. Fan W, Yan Z. Factors affecting response rates of the web survey: A systematic review. Comput Hum Behav. 2010;26:132-139.
55. Millar MM, Dillman DA. Improving response to web and mixed-mode surveys. Public Opin Q. 2011;75:249-269.