User login
A Multidisciplinary Ambulation Protocol to Reduce Postoperative Venous Thromboembolism After Colorectal Surgery
From the Department of Surgery, Washington University School of Medicine, St. Louis, MO.
Abstract
Background: Patients undergoing colorectal surgery are at high risk for postoperative venous thromboembolism (VTE). Early ambulation has been encouraged to lower rates of VTE, but evidence demonstrating its effectiveness outside of a bundle is limited.
Objective: To create a multidisciplinary ambulation protocol in an effort to reduce postoperative VTE.
Methods: A single-center, retrospective, comparative study of patients who underwent colectomy or proctectomy was conducted. Outcomes of patients operated on prior to protocol implementation were compared with a cohort after implementation. The intervention studied was the implementation of a multidisciplinary ambulation protocol. The primary endpoint was postoperative VTE.
Results: There was no difference between the pre-intervention group (n = 1762) and the postintervention group (n = 253) in terms of sex, race, origin, emergency status, operative time, and the majority of medical comorbidities (with the exception of smoking status and congestive heart failure). After the protocol was implemented, ambulation rates on postoperative days 0, 1, and 2 improved from 36.4%, 47.3%, and 50.2% to 36.8%, 74.7%, and 82.6%, respectively The VTE rate in the pre-intervention group was 2.7% versus a rate of 0.4% in the postintervention group (P = 0.02).
Conclusion: Creation of an ambulation protocol is associated with a significant reduction in VTE. Commitment from patients, families, nurses, physician extenders, and physicians is critical to the success of the program.
Keywords: VTE; pulmonary embolism; deep vein thrombosis; postoperative; quality improvement.
Postoperative venous thromboembolism (VTE) is a significant source of morbidity, mortality, and cost.1,2 Colorectal surgery patients are at particularly high risk for VTE due to positioning during surgery, pelvic dissection, and other conditions often found in these patients, such as cancer and inflammatory bowel disease.3 A National Surgical Quality Improvement Program (NSQIP) analysis demonstrated an overall rate of VTE in colorectal surgery patients of 2.4%, although other studies have demonstrated rates up to 9%, even in those receiving appropriate chemoprophylaxis.4-6 Many of these VTEs occur in the postdischarge setting. In a NSQIP study of colorectal surgery patients, the rate of VTE between discharge and 30 days was 0.47%.7 The cost burdenfor a postoperative VTE has been estimated to be more than $18,000.8
Studies from NSQIP have identified multiple factors associated with VTE in colorectal surgery patients, but NSQIP does not record ambulation as a standard variable.9 Multiple strategies have been implemented to reduce postoperative VTE. Often, these studies focus on increasing compliance with appropriate chemoprophylaxis, risk stratification, or bundling multiple strategies.10,11 However, despite the fact that postsurgical ambulation is widely encouraged and recommended by the American Society of Colon and Rectal Surgeons clinical practice guidelines, there is little evidence demonstrating the role of ambulation alone in the reduction of VTE.4,12 The purpose of this study was to create a multidisciplinary protocol to increase postoperative ambulation and evaluate its effect on VTE.
Methods
Setting
This study was conducted at a single academic tertiary care center.
Patients and Outcome Measures
All patients undergoing colectomy or proctectomy by surgeons in the section of colon and rectal surgery at a single institution between January 2011 and March 2017 were included. Colectomy and proctectomy were defined by CPT codes 44140, 44141, 44143, 44144, 44145, 44146, 44147, 44150, 44151, 44155, 44156, 44157, 44158, 44160, 44204, 44205, 44206, 44207, 44208, 44210, 44211, 44212, 44213, 45110, 45111, 45112, 45113, 45114, 45116, 45119, 45120, 45121, 45123, 45126, 45160, 45395, and 45397. The primary outcome of VTE within 30 days, including deep venous thrombosis (DVT) and pulmonary embolism (PE), was measured using institution-specific data from NSQIP in both the pre-intervention and postintervention setting. The occurrence of both DVT and PE in 1 patient was counted as a single event of VTE. Ambulation rate on postoperative day (POD) 0, 1, and 2 was calculated by NSQIP in the pre-intervention setting (our institution-specific NSQIP recorded ambulation data for an unrelated project) and by review of the electronic health record in the postintervention setting, as this institution-specific variable was no longer being collected. Ambulation was defined as getting out of bed and taking at least 1 step. The threshold for ambulating each day was once on POD 0 and twice on PODs 1 and 2. Patients with missing ambulation data were excluded from the analysis. Both prior to and throughout the intervention, all patients were given VTE chemoprophylaxis with either low-dose unfractionated heparin or low-molecular-weight heparin prior to induction of anesthesia, with chemoprophylaxis extending an additional 21 days after discharge (unless specifically contraindicated); sequential compression devices; and standard orders to ambulate 3 times daily from POD 0 as part of the standard Enhanced Recovery After Surgery protocol.
Analysis
Statistical analysis was performed using univariate analysis. Chi-square test and univariate logistic regression were used to determine the association between ambulation rates and VTE in the pre-intervention group. Chi-square test was also used to compare ambulation and VTE rates between the pre-intervention and postintervention groups. Plan-Do-Study-Act (PDSA) cycle fidelity (the degree to which a PDSA cycle is carried out in accordance with the guiding principles of its use) was measured by recording the ambulation rates both before and after the intervention.13 Statistical analysis was performed using SAS Version 9.4 (SAS Institute, Cary, NC). This study was reviewed by the Washington University School of Medicine Institutional Review Board and deemed to be quality improvement, not human subjects research, and therefore did not require formal approval.
Baseline Outcome Rates
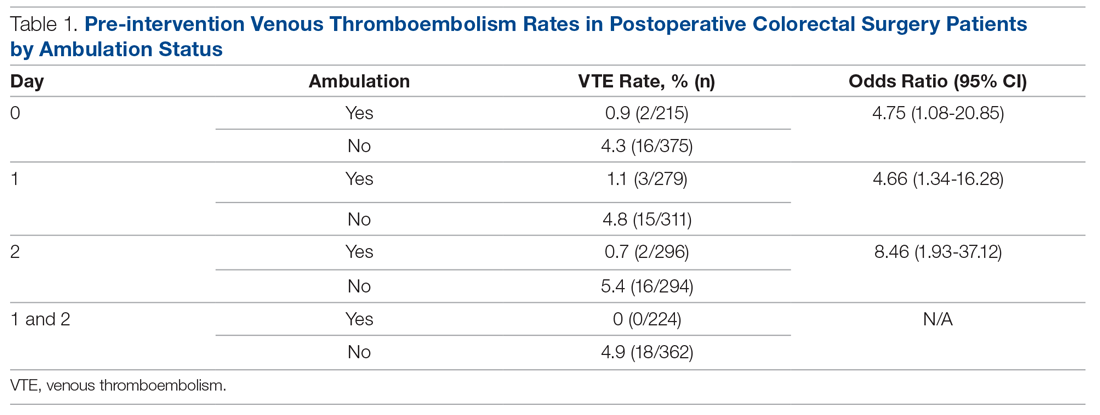
A total of 1762 patients were identified during the pre-intervention period. The overall VTE rate in the pre-intervention group was 2.7% (n = 48), with 39 DVTs (2.2%) and 13 PEs (0.7%). Pre-intervention ambulation data were available on 590 patients. Baseline ambulation rates on PODs 0, 1, and 2 were 36.4% (213/590), 47.3% (279/590), and 50.2% (296/590), respectively. Patients who did not ambulate on POD 0 had a VTE rate of 4.3%, as compared to 0.9% in those who did ambulate (Table 1). Patients who did not ambulate twice on POD 1 had a VTE rate of 4.8%, compared to 1.1% in those who did ambulate (odds ratio [OR], 4.66; 95% confidence interval [CI], 1.34 to 16.28). Patients who did not ambulate twice on POD 2 had a VTE rate of 5.4%, compared to 0.7% in those who did. Finally, those who ambulated twice on both PODs 1 and 2 had a 0% rate of VTE, compared to 4.9% in those who did not ambulate on both PODs.
Ambulation Protocol
After baseline outcome rates had been established, a multidisciplinary team of medical assistants, nurses, nurse practitioners, and physicians worked together to identify all processes that involved postoperative ambulation. Given the significant differences in VTE rates between patients who ambulated and those that did not, we created a multidisciplinary ambulation protocol using the PDSA method.14 Multiple points of patient contact were chosen for intervention, and the ambulation protocol was implemented in June 2018 and continued for 7 months.
Patients were observed from their initial office visit with a surgeon, during the preoperative education encounter, and in the operating room and on the surgical ward until discharge. Representatives from multiple disciplines who encountered patients at various times in the process, including medical assistants, patient care technicians, nurses, nurse practitioners, physical therapists, and physicians, participated in a kick-off meeting to identify difficulties they encounter when encouraging patient ambulation. The following 4 areas were identified.
Barriers to Patient Ambulation
Patient Expectations. Patients did not appear to have a clear expectation of what their ambulation goals were postoperatively, despite the fact that each patient is given an operative pathway booklet that includes their goals for each day, including ambulation. The consensus was that patients were overwhelmed with the amount of information and, oftentimes, the severity of their diagnosis, so the information regarding ambulation was not retained. Nurses commented that patients frequently stated that they did not think their surgeon wanted them to get out of bed postoperatively.
Electronic Orders. There was confusion within the nursing staff regarding orders in the electronic health record compared to physician expectations. Orders stated patients should ambulate 3 times daily, but did not specify on which postoperative day this should start. Often, nursing verbal sign-out from the post-anesthesia care unit (PACU) would be an order for bedrest, despite no clear origin of this order. This created confusion among the nursing staff as to what the appropriate ambulation orders should be.
Nursing Workflow. The initial state of the nursing workflow was not conducive to evaluating for, or assisting with, ambulation. With no set time to assist and evaluate patients for ambulation, it turned into a task nurses needed to accomplish when they had extra time. With increasing demands of charting in the electronic health record, nurses often had to skip ambulation in order to accomplish other tasks.
Family Expectations. In addition to patient expectations, family members often had expectations that were not congruent with the planned postoperative course. Nurses stated family members would often tell them that they did not feel that their family member should be ambulating so soon after surgery. Often these family members had not attended preoperative education sessions with the patient. This was compounded by the uncertainty among the nursing staff regarding what exactly the ambulation orders were.
Interventions
Targeted interventions were created to address these 4 barriers to ambulation identified by staff.
Preoperative Education. Although all elective patients received a printed operative pathway booklet describing daily goals, including ambulation, patients still did not have a sufficient understanding of what was expected of them. The education session was modified to increase the time spent on both the expectation for and the rationale behind ambulation. That section of the education session ended with a verbal commitment and read-back of the expectations for ambulation by the patient.
Clarification of Electronic Orders. Postoperative orders within the colorectal standard pathway were changed, including specific time frames and frequency, to match the information provided in the patient education booklet. These orders were for ambulation within 4 hours of arrival to the floor, and the orders also noted that no patient should be on bedrest unless explicitly stated. From POD 1, all patients were to ambulate at least twice daily for the remainder of the hospital stay (patients were encouraged to walk 4 times daily, but we set a minimum expectation of twice daily for the order set). These orders were clarified with in-person meetings with the nursing staff and leadership from the PACU and the colorectal surgical ward.
Adjusted Nursing Workflow. Nurses were interviewed and asked to create a plan regarding how they could better incorporate ambulation into their daily workflow. Ambulation assessment was incorporated into the twice-per-shift recording of vital signs and patient safety assessment. This was recorded into the electronic health record at the same time as the patients’ vital signs. This allowed nurses to keep track of which patients would need extra assistance in ambulation and which patients were doing well on their own with the assistance of family. It also helped focus the resources of physical therapy and the single ambulation technician on the floor and to assist patients who needed more assistance.
Creation of Ambulation Encouragement Signs. The authors discovered that despite patients being told preoperatively about ambulation expectations, friends and family are not always included in these conversations. As nurses frequently cited both patients and family as reasons patients thought they should not walk, multiple signs inviting patients to take an active role in their recovery by ambulating were created and placed around the unit. The signs outlined the expectations of being out of bed and taking at least 1 step on the day of surgery and walking at least 4 times per day thereafter. In addition, we addressed frequently asked questions around issues such as walking with intravenous poles and urinary catheters. The posters were signed by all staff colorectal surgeons.
Results
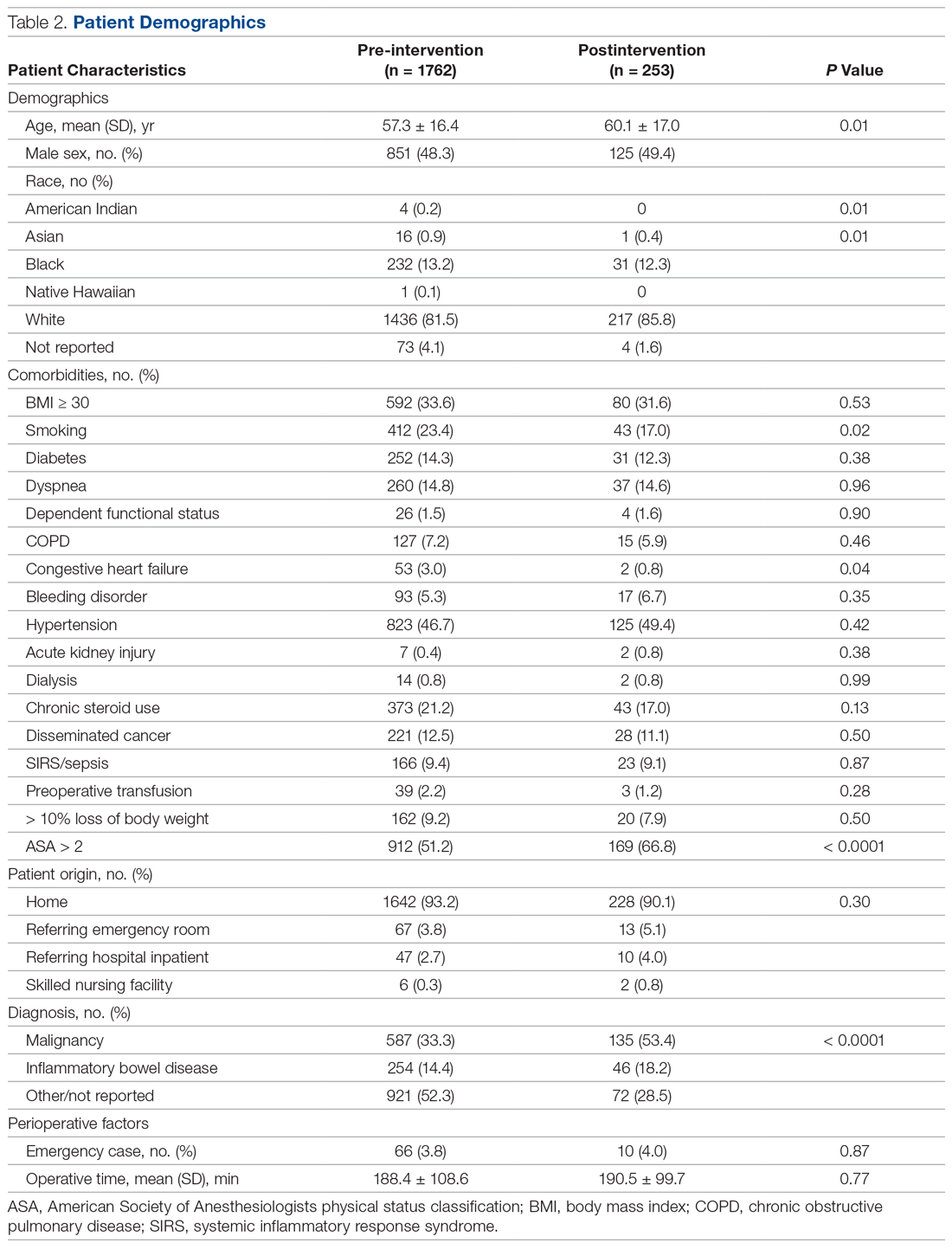
Over the course of 7 months (June 2018 to December 2018), 253 postintervention patients were identified (Table 2). There was no difference between the pre-intervention group (n = 1762) and the postintervention group in terms of sex, race, origin, emergency status, operative time, and the majority of medical comorbidities (with the exception of smoking status and congestive heart failure). The postintervention group was slightly older (60 versus 57 years) and had a higher percentage of patients with an American Society of Anesthesiologists physical status score greater than 2 (66.8% versus 51.2%). The postintervention group also had higher rates of both malignancy (53.4% versus 33.3%) and inflammatory bowel disease (18.2% versus 14.4%).
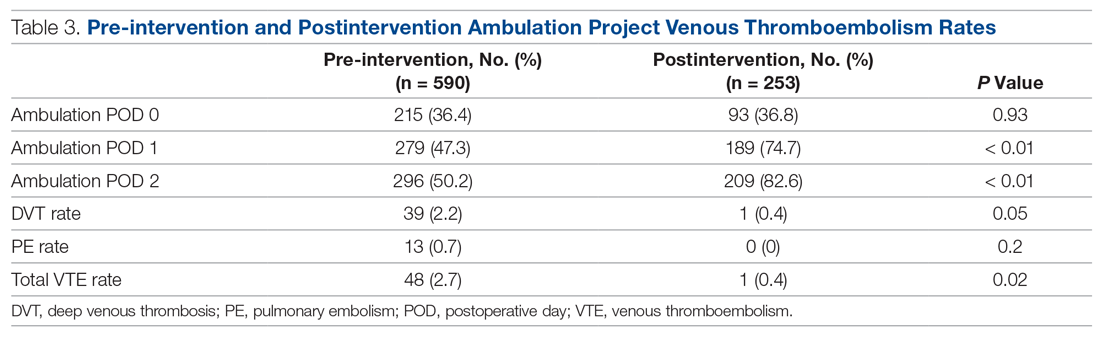
The fidelity of the PDSA cycle was measured by pre-intervention and postintervention ambulation rates. Ambulation rates on POD 0, 1, and 2 improved from 36.4%, 47.3%, and 50.2% to 36.8%, 74.7%, and 82.6%, respectively (Table 3). The VTE rate decreased from 2.7% to 0.4% (P = 0.02), with 1 DVT and 0 PEs. It should be noted that the only patient who developed a VTE postintervention did not ambulate on PODs 0, 1, or 2.
Discussion
Postoperative VTE is a severe complication for postoperative colorectal surgery patients. Previous studies have demonstrated that increasing ambulation is associated with a lower rate of overall complications, and, when incorporated into a bundle, is associated with decreased rates of VTE.11,15 However, this is the first study to our knowledge demonstrating that creation of an ambulation protocol alone is associated with a decrease in VTE.
Analysis of pre-intervention data demonstrated a strong association between ambulation and an absence of VTE. No patient who ambulated on PODs 0, 1, and 2 developed a VTE. Based on those results, we moved forward with creating the ambulation protocol. While ambulation stayed stable on POD 0, there were 60% and 65% increases on PODs 1 and 2, respectively. Nurses cited late arrival to the floor for second and third start cases as the primary difficulty in getting patients to ambulate more on POD 0.
We believe the key to the success of the ambulation protocol was its multidisciplinary nature. Certainly, the easiest way to create an ambulation protocol is to change the postoperative orders to state patients must walk 4 times per day. However, if the nursing staff is unable or unwilling to carry out these orders, the orders serve little purpose. In order to make lasting changes, all stakeholders in the process must be identified. In our case, stakeholders included surgery and nursing leadership, surgeons, nurse practitioners, nurses, medical assistants, physical therapists, patient care technicians, and patients. This is where we utilized kaizen, a core principle of Lean methodology that empowers employees at the level of the work being carried out to propose ideas for improvement.16 From the beginning of the patient experience, the health care practitioners who were carrying out each step of the process were best able to identify the problems and create solutions. In addition, stakeholders were given regular updates regarding how their efforts were increasing ambulation rates and the results at the end of the study period.
This study also demonstrates that, in a health care system increasingly focused on both quality and cost, significant improvements in quality can be made without increasing cost or resource utilization. Early in the process, it was proposed that the only way to increase the ambulation rate would be to increase the number of physical therapists, nurses, and nursing assistants. However, after identifying the root causes of the problem, the solutions had more to do with improving workflow and fixing problem areas identified by the staff.
In addition to having a positive effect on the outcome studied, collaborative projects such as this between physicians and nurses may lead to increased nursing job satisfaction. A meta-analysis of 31 studies identified nurse-physician collaboration and autonomy as 2 factors that correlate most strongly with nursing satisfaction.17 A Cochrane review also suggests that practice-based interprofessional collaboration may lead to improved health care processes and outcomes.18
This study has several limitations. Pre-intervention ambulation rates were abstracted from institution-specific NSQIP data, and missing data were excluded from analysis. Also, due to the retrospective collection of the pre-intervention data, the distance of ambulation could not be quantified. The bar for ambulation is low, as patients were only required to get out of bed and walk 1 step. However, we feel that getting out of bed and taking even 1 step is substantially better than complete bedrest. It is likely that once patients cross the threshold of taking 1 step, they are more likely to ambulate. An area of future study may be to more precisely define the relationship between the quantity of ambulation in steps and its effect on VTE. Finally, we acknowledge that while there is no direct increase in costs, implementing an ambulation protocol does take time from all who participate in the project.
Conclusion
Creation of an ambulation protocol is associated with a decrease in postoperative VTE rates in colorectal surgery patients. A multidisciplinary approach is critical to identify the underlying problems and propose effective solutions. Further studies are required to better correlate the distance of ambulation and its effect on VTE. However, this study shows that even a minimum of 1 step is associated with decreased VTE rates.
Corresponding author: Aneel Damle, MD, MBA, Colon & Rectal Surgery Associates, 3433 Broadway St. NE, Suite 115, Minneapolis, MN 55413; [email protected].
Financial disclosures: None.
1. Gangireddy C, Rectenwald JR, Upchurch GR, et al. Risk factors and clinical impact of postoperative symptomatic venous thromboembolism. J Vasc Surg. 2007;45:341-342.
2. Newhook TE, LaPar DJ, Walters DM, et al. Impact of postoperative venous thromboembolism on postoperative morbidity, mortality, and resource utilization after hepatectomy. Am Surg. 2015;81:1216-1223.
3. Bergqvist D. Venous thromboembolism: a review of risk and prevention in colorectal surgery patients. Dis Colon Rectum. 2006;49:1620-1628.
4. Fleming F, Gaertner W, Ternent CA, et al. The American society of colon and rectal surgeons clinical practice guideline for the prevention of venous thromboembolic disease in colorectal surgery. Dis Colon Rectum. 2018;61:14-20.
5. McLeod RS, Geerts WH, Sniderman KW, et al. Canadian Colorectal Surgery DVT Prophylaxis Trial investigators. Subcutaneous heparin versus low-molecular-weight heparin as thromboprophylaxis in patients undergoing colorectal surgery: results of the Canadian colorectal DV prophylaxis trial: a randomized, double-blind trial. Ann Surg. 2001;233:438-444.
6. Shapiro R, Vogel JD, Kiran RP. Risk of postoperative venous thromboembolism after laparoscopic and open colorectal surgery: an additional benefit of the minimally invasive approach? Dis Colon Rectum. 2011;54:1496-1502.
7. Dimick JB, Chen SL, Taheri PA, et al. Hospital costs associated with surgical complications: a report from the private-sector National Surgical Quality Improvement Program. J Am Coll Surg. 2004;199:531-537.
8. Fleming FJ, Kim MJ, Salloum RM, et al. How much do we need to worry about venous thromboembolism after hospital discharge? A study of colorectal surgery patients using the National Surgical Quality Improvement Program database. Dis Colon Rectum. 2010;53:1355-1360.
9. ACS NSQIP. User guide for the 2016 ACS NSQIP participant use data file (PUF). 2017. www.facs.org/~/media/files/quality%20programs/nsqip/nsqip_puf_userguide_2016.ashx Accessed July 10, 2020.
10. Caprini JA. Risk assessment as a guide for the prevention of the many faces of venous thromboembolism. Am J Surg. 2010;199(1 Suppl):S3-S10.
11. Cassidy MR, Rosenkranz P, McAney D. Reducing postoperative venous thromboembolism complications with a standardized risk-stratified prophylaxis protocol and mobilization protocol. J Am Coll Surg. 2014;218:1095-1104.
12. Lau BD, Streiff MB, Kraus PS, et al. No evidence to support ambulation for reducing postoperative venous thromboembolism. J Am Coll Surg. 2014;219:1101-1103.
13. McNicholas C, Lennox L, Woodcock T, et al. Evolving quality improvement support strategies to improve Plan–Do–Study–Act cycle fidelity: a retrospective mixed-methods study. BMJ Qual Saf. 2019;28:356-365.
14. Taylor MJ, McNicholas C, Nicolay C, et al. Systematic review of the application of the plan–do–study–act method to improve quality in healthcare. BMC Qual Saf. 2014;23:290-298.
15. Nevo Y, Shaltiel T, Constantini N, et al. Effect of ambulation and physical activity on postoperative complications. J Am Coll Surg. 2016;223(Suppl 1):S61.
16. Mazzocato P, Stenfors-Hayes T, von Thiele Schwarz U, et al. Kaizen practice in healthcare: a qualitative analysis of hospital employees’ suggestions for improvement. BMJ Open. 2016;6:e012256.
17. Zangaro GA, Soeken KL. A meta-analysis of studies of nurses’ job satisfaction. Res Nursing Health. 2007;30:445-458.
18. Reeves S, Pelone F, Harrison R, et al. Interprofessional collaboration to improve professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2017;6(6):CD000072.
From the Department of Surgery, Washington University School of Medicine, St. Louis, MO.
Abstract
Background: Patients undergoing colorectal surgery are at high risk for postoperative venous thromboembolism (VTE). Early ambulation has been encouraged to lower rates of VTE, but evidence demonstrating its effectiveness outside of a bundle is limited.
Objective: To create a multidisciplinary ambulation protocol in an effort to reduce postoperative VTE.
Methods: A single-center, retrospective, comparative study of patients who underwent colectomy or proctectomy was conducted. Outcomes of patients operated on prior to protocol implementation were compared with a cohort after implementation. The intervention studied was the implementation of a multidisciplinary ambulation protocol. The primary endpoint was postoperative VTE.
Results: There was no difference between the pre-intervention group (n = 1762) and the postintervention group (n = 253) in terms of sex, race, origin, emergency status, operative time, and the majority of medical comorbidities (with the exception of smoking status and congestive heart failure). After the protocol was implemented, ambulation rates on postoperative days 0, 1, and 2 improved from 36.4%, 47.3%, and 50.2% to 36.8%, 74.7%, and 82.6%, respectively The VTE rate in the pre-intervention group was 2.7% versus a rate of 0.4% in the postintervention group (P = 0.02).
Conclusion: Creation of an ambulation protocol is associated with a significant reduction in VTE. Commitment from patients, families, nurses, physician extenders, and physicians is critical to the success of the program.
Keywords: VTE; pulmonary embolism; deep vein thrombosis; postoperative; quality improvement.
Postoperative venous thromboembolism (VTE) is a significant source of morbidity, mortality, and cost.1,2 Colorectal surgery patients are at particularly high risk for VTE due to positioning during surgery, pelvic dissection, and other conditions often found in these patients, such as cancer and inflammatory bowel disease.3 A National Surgical Quality Improvement Program (NSQIP) analysis demonstrated an overall rate of VTE in colorectal surgery patients of 2.4%, although other studies have demonstrated rates up to 9%, even in those receiving appropriate chemoprophylaxis.4-6 Many of these VTEs occur in the postdischarge setting. In a NSQIP study of colorectal surgery patients, the rate of VTE between discharge and 30 days was 0.47%.7 The cost burdenfor a postoperative VTE has been estimated to be more than $18,000.8
Studies from NSQIP have identified multiple factors associated with VTE in colorectal surgery patients, but NSQIP does not record ambulation as a standard variable.9 Multiple strategies have been implemented to reduce postoperative VTE. Often, these studies focus on increasing compliance with appropriate chemoprophylaxis, risk stratification, or bundling multiple strategies.10,11 However, despite the fact that postsurgical ambulation is widely encouraged and recommended by the American Society of Colon and Rectal Surgeons clinical practice guidelines, there is little evidence demonstrating the role of ambulation alone in the reduction of VTE.4,12 The purpose of this study was to create a multidisciplinary protocol to increase postoperative ambulation and evaluate its effect on VTE.
Methods
Setting
This study was conducted at a single academic tertiary care center.
Patients and Outcome Measures
All patients undergoing colectomy or proctectomy by surgeons in the section of colon and rectal surgery at a single institution between January 2011 and March 2017 were included. Colectomy and proctectomy were defined by CPT codes 44140, 44141, 44143, 44144, 44145, 44146, 44147, 44150, 44151, 44155, 44156, 44157, 44158, 44160, 44204, 44205, 44206, 44207, 44208, 44210, 44211, 44212, 44213, 45110, 45111, 45112, 45113, 45114, 45116, 45119, 45120, 45121, 45123, 45126, 45160, 45395, and 45397. The primary outcome of VTE within 30 days, including deep venous thrombosis (DVT) and pulmonary embolism (PE), was measured using institution-specific data from NSQIP in both the pre-intervention and postintervention setting. The occurrence of both DVT and PE in 1 patient was counted as a single event of VTE. Ambulation rate on postoperative day (POD) 0, 1, and 2 was calculated by NSQIP in the pre-intervention setting (our institution-specific NSQIP recorded ambulation data for an unrelated project) and by review of the electronic health record in the postintervention setting, as this institution-specific variable was no longer being collected. Ambulation was defined as getting out of bed and taking at least 1 step. The threshold for ambulating each day was once on POD 0 and twice on PODs 1 and 2. Patients with missing ambulation data were excluded from the analysis. Both prior to and throughout the intervention, all patients were given VTE chemoprophylaxis with either low-dose unfractionated heparin or low-molecular-weight heparin prior to induction of anesthesia, with chemoprophylaxis extending an additional 21 days after discharge (unless specifically contraindicated); sequential compression devices; and standard orders to ambulate 3 times daily from POD 0 as part of the standard Enhanced Recovery After Surgery protocol.
Analysis
Statistical analysis was performed using univariate analysis. Chi-square test and univariate logistic regression were used to determine the association between ambulation rates and VTE in the pre-intervention group. Chi-square test was also used to compare ambulation and VTE rates between the pre-intervention and postintervention groups. Plan-Do-Study-Act (PDSA) cycle fidelity (the degree to which a PDSA cycle is carried out in accordance with the guiding principles of its use) was measured by recording the ambulation rates both before and after the intervention.13 Statistical analysis was performed using SAS Version 9.4 (SAS Institute, Cary, NC). This study was reviewed by the Washington University School of Medicine Institutional Review Board and deemed to be quality improvement, not human subjects research, and therefore did not require formal approval.
Baseline Outcome Rates
A total of 1762 patients were identified during the pre-intervention period. The overall VTE rate in the pre-intervention group was 2.7% (n = 48), with 39 DVTs (2.2%) and 13 PEs (0.7%). Pre-intervention ambulation data were available on 590 patients. Baseline ambulation rates on PODs 0, 1, and 2 were 36.4% (213/590), 47.3% (279/590), and 50.2% (296/590), respectively. Patients who did not ambulate on POD 0 had a VTE rate of 4.3%, as compared to 0.9% in those who did ambulate (Table 1). Patients who did not ambulate twice on POD 1 had a VTE rate of 4.8%, compared to 1.1% in those who did ambulate (odds ratio [OR], 4.66; 95% confidence interval [CI], 1.34 to 16.28). Patients who did not ambulate twice on POD 2 had a VTE rate of 5.4%, compared to 0.7% in those who did. Finally, those who ambulated twice on both PODs 1 and 2 had a 0% rate of VTE, compared to 4.9% in those who did not ambulate on both PODs.

Ambulation Protocol
After baseline outcome rates had been established, a multidisciplinary team of medical assistants, nurses, nurse practitioners, and physicians worked together to identify all processes that involved postoperative ambulation. Given the significant differences in VTE rates between patients who ambulated and those that did not, we created a multidisciplinary ambulation protocol using the PDSA method.14 Multiple points of patient contact were chosen for intervention, and the ambulation protocol was implemented in June 2018 and continued for 7 months.
Patients were observed from their initial office visit with a surgeon, during the preoperative education encounter, and in the operating room and on the surgical ward until discharge. Representatives from multiple disciplines who encountered patients at various times in the process, including medical assistants, patient care technicians, nurses, nurse practitioners, physical therapists, and physicians, participated in a kick-off meeting to identify difficulties they encounter when encouraging patient ambulation. The following 4 areas were identified.
Barriers to Patient Ambulation
Patient Expectations. Patients did not appear to have a clear expectation of what their ambulation goals were postoperatively, despite the fact that each patient is given an operative pathway booklet that includes their goals for each day, including ambulation. The consensus was that patients were overwhelmed with the amount of information and, oftentimes, the severity of their diagnosis, so the information regarding ambulation was not retained. Nurses commented that patients frequently stated that they did not think their surgeon wanted them to get out of bed postoperatively.
Electronic Orders. There was confusion within the nursing staff regarding orders in the electronic health record compared to physician expectations. Orders stated patients should ambulate 3 times daily, but did not specify on which postoperative day this should start. Often, nursing verbal sign-out from the post-anesthesia care unit (PACU) would be an order for bedrest, despite no clear origin of this order. This created confusion among the nursing staff as to what the appropriate ambulation orders should be.
Nursing Workflow. The initial state of the nursing workflow was not conducive to evaluating for, or assisting with, ambulation. With no set time to assist and evaluate patients for ambulation, it turned into a task nurses needed to accomplish when they had extra time. With increasing demands of charting in the electronic health record, nurses often had to skip ambulation in order to accomplish other tasks.
Family Expectations. In addition to patient expectations, family members often had expectations that were not congruent with the planned postoperative course. Nurses stated family members would often tell them that they did not feel that their family member should be ambulating so soon after surgery. Often these family members had not attended preoperative education sessions with the patient. This was compounded by the uncertainty among the nursing staff regarding what exactly the ambulation orders were.
Interventions
Targeted interventions were created to address these 4 barriers to ambulation identified by staff.
Preoperative Education. Although all elective patients received a printed operative pathway booklet describing daily goals, including ambulation, patients still did not have a sufficient understanding of what was expected of them. The education session was modified to increase the time spent on both the expectation for and the rationale behind ambulation. That section of the education session ended with a verbal commitment and read-back of the expectations for ambulation by the patient.
Clarification of Electronic Orders. Postoperative orders within the colorectal standard pathway were changed, including specific time frames and frequency, to match the information provided in the patient education booklet. These orders were for ambulation within 4 hours of arrival to the floor, and the orders also noted that no patient should be on bedrest unless explicitly stated. From POD 1, all patients were to ambulate at least twice daily for the remainder of the hospital stay (patients were encouraged to walk 4 times daily, but we set a minimum expectation of twice daily for the order set). These orders were clarified with in-person meetings with the nursing staff and leadership from the PACU and the colorectal surgical ward.
Adjusted Nursing Workflow. Nurses were interviewed and asked to create a plan regarding how they could better incorporate ambulation into their daily workflow. Ambulation assessment was incorporated into the twice-per-shift recording of vital signs and patient safety assessment. This was recorded into the electronic health record at the same time as the patients’ vital signs. This allowed nurses to keep track of which patients would need extra assistance in ambulation and which patients were doing well on their own with the assistance of family. It also helped focus the resources of physical therapy and the single ambulation technician on the floor and to assist patients who needed more assistance.
Creation of Ambulation Encouragement Signs. The authors discovered that despite patients being told preoperatively about ambulation expectations, friends and family are not always included in these conversations. As nurses frequently cited both patients and family as reasons patients thought they should not walk, multiple signs inviting patients to take an active role in their recovery by ambulating were created and placed around the unit. The signs outlined the expectations of being out of bed and taking at least 1 step on the day of surgery and walking at least 4 times per day thereafter. In addition, we addressed frequently asked questions around issues such as walking with intravenous poles and urinary catheters. The posters were signed by all staff colorectal surgeons.
Results
Over the course of 7 months (June 2018 to December 2018), 253 postintervention patients were identified (Table 2). There was no difference between the pre-intervention group (n = 1762) and the postintervention group in terms of sex, race, origin, emergency status, operative time, and the majority of medical comorbidities (with the exception of smoking status and congestive heart failure). The postintervention group was slightly older (60 versus 57 years) and had a higher percentage of patients with an American Society of Anesthesiologists physical status score greater than 2 (66.8% versus 51.2%). The postintervention group also had higher rates of both malignancy (53.4% versus 33.3%) and inflammatory bowel disease (18.2% versus 14.4%).

The fidelity of the PDSA cycle was measured by pre-intervention and postintervention ambulation rates. Ambulation rates on POD 0, 1, and 2 improved from 36.4%, 47.3%, and 50.2% to 36.8%, 74.7%, and 82.6%, respectively (Table 3). The VTE rate decreased from 2.7% to 0.4% (P = 0.02), with 1 DVT and 0 PEs. It should be noted that the only patient who developed a VTE postintervention did not ambulate on PODs 0, 1, or 2.

Discussion
Postoperative VTE is a severe complication for postoperative colorectal surgery patients. Previous studies have demonstrated that increasing ambulation is associated with a lower rate of overall complications, and, when incorporated into a bundle, is associated with decreased rates of VTE.11,15 However, this is the first study to our knowledge demonstrating that creation of an ambulation protocol alone is associated with a decrease in VTE.
Analysis of pre-intervention data demonstrated a strong association between ambulation and an absence of VTE. No patient who ambulated on PODs 0, 1, and 2 developed a VTE. Based on those results, we moved forward with creating the ambulation protocol. While ambulation stayed stable on POD 0, there were 60% and 65% increases on PODs 1 and 2, respectively. Nurses cited late arrival to the floor for second and third start cases as the primary difficulty in getting patients to ambulate more on POD 0.
We believe the key to the success of the ambulation protocol was its multidisciplinary nature. Certainly, the easiest way to create an ambulation protocol is to change the postoperative orders to state patients must walk 4 times per day. However, if the nursing staff is unable or unwilling to carry out these orders, the orders serve little purpose. In order to make lasting changes, all stakeholders in the process must be identified. In our case, stakeholders included surgery and nursing leadership, surgeons, nurse practitioners, nurses, medical assistants, physical therapists, patient care technicians, and patients. This is where we utilized kaizen, a core principle of Lean methodology that empowers employees at the level of the work being carried out to propose ideas for improvement.16 From the beginning of the patient experience, the health care practitioners who were carrying out each step of the process were best able to identify the problems and create solutions. In addition, stakeholders were given regular updates regarding how their efforts were increasing ambulation rates and the results at the end of the study period.
This study also demonstrates that, in a health care system increasingly focused on both quality and cost, significant improvements in quality can be made without increasing cost or resource utilization. Early in the process, it was proposed that the only way to increase the ambulation rate would be to increase the number of physical therapists, nurses, and nursing assistants. However, after identifying the root causes of the problem, the solutions had more to do with improving workflow and fixing problem areas identified by the staff.
In addition to having a positive effect on the outcome studied, collaborative projects such as this between physicians and nurses may lead to increased nursing job satisfaction. A meta-analysis of 31 studies identified nurse-physician collaboration and autonomy as 2 factors that correlate most strongly with nursing satisfaction.17 A Cochrane review also suggests that practice-based interprofessional collaboration may lead to improved health care processes and outcomes.18
This study has several limitations. Pre-intervention ambulation rates were abstracted from institution-specific NSQIP data, and missing data were excluded from analysis. Also, due to the retrospective collection of the pre-intervention data, the distance of ambulation could not be quantified. The bar for ambulation is low, as patients were only required to get out of bed and walk 1 step. However, we feel that getting out of bed and taking even 1 step is substantially better than complete bedrest. It is likely that once patients cross the threshold of taking 1 step, they are more likely to ambulate. An area of future study may be to more precisely define the relationship between the quantity of ambulation in steps and its effect on VTE. Finally, we acknowledge that while there is no direct increase in costs, implementing an ambulation protocol does take time from all who participate in the project.
Conclusion
Creation of an ambulation protocol is associated with a decrease in postoperative VTE rates in colorectal surgery patients. A multidisciplinary approach is critical to identify the underlying problems and propose effective solutions. Further studies are required to better correlate the distance of ambulation and its effect on VTE. However, this study shows that even a minimum of 1 step is associated with decreased VTE rates.
Corresponding author: Aneel Damle, MD, MBA, Colon & Rectal Surgery Associates, 3433 Broadway St. NE, Suite 115, Minneapolis, MN 55413; [email protected].
Financial disclosures: None.
From the Department of Surgery, Washington University School of Medicine, St. Louis, MO.
Abstract
Background: Patients undergoing colorectal surgery are at high risk for postoperative venous thromboembolism (VTE). Early ambulation has been encouraged to lower rates of VTE, but evidence demonstrating its effectiveness outside of a bundle is limited.
Objective: To create a multidisciplinary ambulation protocol in an effort to reduce postoperative VTE.
Methods: A single-center, retrospective, comparative study of patients who underwent colectomy or proctectomy was conducted. Outcomes of patients operated on prior to protocol implementation were compared with a cohort after implementation. The intervention studied was the implementation of a multidisciplinary ambulation protocol. The primary endpoint was postoperative VTE.
Results: There was no difference between the pre-intervention group (n = 1762) and the postintervention group (n = 253) in terms of sex, race, origin, emergency status, operative time, and the majority of medical comorbidities (with the exception of smoking status and congestive heart failure). After the protocol was implemented, ambulation rates on postoperative days 0, 1, and 2 improved from 36.4%, 47.3%, and 50.2% to 36.8%, 74.7%, and 82.6%, respectively The VTE rate in the pre-intervention group was 2.7% versus a rate of 0.4% in the postintervention group (P = 0.02).
Conclusion: Creation of an ambulation protocol is associated with a significant reduction in VTE. Commitment from patients, families, nurses, physician extenders, and physicians is critical to the success of the program.
Keywords: VTE; pulmonary embolism; deep vein thrombosis; postoperative; quality improvement.
Postoperative venous thromboembolism (VTE) is a significant source of morbidity, mortality, and cost.1,2 Colorectal surgery patients are at particularly high risk for VTE due to positioning during surgery, pelvic dissection, and other conditions often found in these patients, such as cancer and inflammatory bowel disease.3 A National Surgical Quality Improvement Program (NSQIP) analysis demonstrated an overall rate of VTE in colorectal surgery patients of 2.4%, although other studies have demonstrated rates up to 9%, even in those receiving appropriate chemoprophylaxis.4-6 Many of these VTEs occur in the postdischarge setting. In a NSQIP study of colorectal surgery patients, the rate of VTE between discharge and 30 days was 0.47%.7 The cost burdenfor a postoperative VTE has been estimated to be more than $18,000.8
Studies from NSQIP have identified multiple factors associated with VTE in colorectal surgery patients, but NSQIP does not record ambulation as a standard variable.9 Multiple strategies have been implemented to reduce postoperative VTE. Often, these studies focus on increasing compliance with appropriate chemoprophylaxis, risk stratification, or bundling multiple strategies.10,11 However, despite the fact that postsurgical ambulation is widely encouraged and recommended by the American Society of Colon and Rectal Surgeons clinical practice guidelines, there is little evidence demonstrating the role of ambulation alone in the reduction of VTE.4,12 The purpose of this study was to create a multidisciplinary protocol to increase postoperative ambulation and evaluate its effect on VTE.
Methods
Setting
This study was conducted at a single academic tertiary care center.
Patients and Outcome Measures
All patients undergoing colectomy or proctectomy by surgeons in the section of colon and rectal surgery at a single institution between January 2011 and March 2017 were included. Colectomy and proctectomy were defined by CPT codes 44140, 44141, 44143, 44144, 44145, 44146, 44147, 44150, 44151, 44155, 44156, 44157, 44158, 44160, 44204, 44205, 44206, 44207, 44208, 44210, 44211, 44212, 44213, 45110, 45111, 45112, 45113, 45114, 45116, 45119, 45120, 45121, 45123, 45126, 45160, 45395, and 45397. The primary outcome of VTE within 30 days, including deep venous thrombosis (DVT) and pulmonary embolism (PE), was measured using institution-specific data from NSQIP in both the pre-intervention and postintervention setting. The occurrence of both DVT and PE in 1 patient was counted as a single event of VTE. Ambulation rate on postoperative day (POD) 0, 1, and 2 was calculated by NSQIP in the pre-intervention setting (our institution-specific NSQIP recorded ambulation data for an unrelated project) and by review of the electronic health record in the postintervention setting, as this institution-specific variable was no longer being collected. Ambulation was defined as getting out of bed and taking at least 1 step. The threshold for ambulating each day was once on POD 0 and twice on PODs 1 and 2. Patients with missing ambulation data were excluded from the analysis. Both prior to and throughout the intervention, all patients were given VTE chemoprophylaxis with either low-dose unfractionated heparin or low-molecular-weight heparin prior to induction of anesthesia, with chemoprophylaxis extending an additional 21 days after discharge (unless specifically contraindicated); sequential compression devices; and standard orders to ambulate 3 times daily from POD 0 as part of the standard Enhanced Recovery After Surgery protocol.
Analysis
Statistical analysis was performed using univariate analysis. Chi-square test and univariate logistic regression were used to determine the association between ambulation rates and VTE in the pre-intervention group. Chi-square test was also used to compare ambulation and VTE rates between the pre-intervention and postintervention groups. Plan-Do-Study-Act (PDSA) cycle fidelity (the degree to which a PDSA cycle is carried out in accordance with the guiding principles of its use) was measured by recording the ambulation rates both before and after the intervention.13 Statistical analysis was performed using SAS Version 9.4 (SAS Institute, Cary, NC). This study was reviewed by the Washington University School of Medicine Institutional Review Board and deemed to be quality improvement, not human subjects research, and therefore did not require formal approval.
Baseline Outcome Rates
A total of 1762 patients were identified during the pre-intervention period. The overall VTE rate in the pre-intervention group was 2.7% (n = 48), with 39 DVTs (2.2%) and 13 PEs (0.7%). Pre-intervention ambulation data were available on 590 patients. Baseline ambulation rates on PODs 0, 1, and 2 were 36.4% (213/590), 47.3% (279/590), and 50.2% (296/590), respectively. Patients who did not ambulate on POD 0 had a VTE rate of 4.3%, as compared to 0.9% in those who did ambulate (Table 1). Patients who did not ambulate twice on POD 1 had a VTE rate of 4.8%, compared to 1.1% in those who did ambulate (odds ratio [OR], 4.66; 95% confidence interval [CI], 1.34 to 16.28). Patients who did not ambulate twice on POD 2 had a VTE rate of 5.4%, compared to 0.7% in those who did. Finally, those who ambulated twice on both PODs 1 and 2 had a 0% rate of VTE, compared to 4.9% in those who did not ambulate on both PODs.

Ambulation Protocol
After baseline outcome rates had been established, a multidisciplinary team of medical assistants, nurses, nurse practitioners, and physicians worked together to identify all processes that involved postoperative ambulation. Given the significant differences in VTE rates between patients who ambulated and those that did not, we created a multidisciplinary ambulation protocol using the PDSA method.14 Multiple points of patient contact were chosen for intervention, and the ambulation protocol was implemented in June 2018 and continued for 7 months.
Patients were observed from their initial office visit with a surgeon, during the preoperative education encounter, and in the operating room and on the surgical ward until discharge. Representatives from multiple disciplines who encountered patients at various times in the process, including medical assistants, patient care technicians, nurses, nurse practitioners, physical therapists, and physicians, participated in a kick-off meeting to identify difficulties they encounter when encouraging patient ambulation. The following 4 areas were identified.
Barriers to Patient Ambulation
Patient Expectations. Patients did not appear to have a clear expectation of what their ambulation goals were postoperatively, despite the fact that each patient is given an operative pathway booklet that includes their goals for each day, including ambulation. The consensus was that patients were overwhelmed with the amount of information and, oftentimes, the severity of their diagnosis, so the information regarding ambulation was not retained. Nurses commented that patients frequently stated that they did not think their surgeon wanted them to get out of bed postoperatively.
Electronic Orders. There was confusion within the nursing staff regarding orders in the electronic health record compared to physician expectations. Orders stated patients should ambulate 3 times daily, but did not specify on which postoperative day this should start. Often, nursing verbal sign-out from the post-anesthesia care unit (PACU) would be an order for bedrest, despite no clear origin of this order. This created confusion among the nursing staff as to what the appropriate ambulation orders should be.
Nursing Workflow. The initial state of the nursing workflow was not conducive to evaluating for, or assisting with, ambulation. With no set time to assist and evaluate patients for ambulation, it turned into a task nurses needed to accomplish when they had extra time. With increasing demands of charting in the electronic health record, nurses often had to skip ambulation in order to accomplish other tasks.
Family Expectations. In addition to patient expectations, family members often had expectations that were not congruent with the planned postoperative course. Nurses stated family members would often tell them that they did not feel that their family member should be ambulating so soon after surgery. Often these family members had not attended preoperative education sessions with the patient. This was compounded by the uncertainty among the nursing staff regarding what exactly the ambulation orders were.
Interventions
Targeted interventions were created to address these 4 barriers to ambulation identified by staff.
Preoperative Education. Although all elective patients received a printed operative pathway booklet describing daily goals, including ambulation, patients still did not have a sufficient understanding of what was expected of them. The education session was modified to increase the time spent on both the expectation for and the rationale behind ambulation. That section of the education session ended with a verbal commitment and read-back of the expectations for ambulation by the patient.
Clarification of Electronic Orders. Postoperative orders within the colorectal standard pathway were changed, including specific time frames and frequency, to match the information provided in the patient education booklet. These orders were for ambulation within 4 hours of arrival to the floor, and the orders also noted that no patient should be on bedrest unless explicitly stated. From POD 1, all patients were to ambulate at least twice daily for the remainder of the hospital stay (patients were encouraged to walk 4 times daily, but we set a minimum expectation of twice daily for the order set). These orders were clarified with in-person meetings with the nursing staff and leadership from the PACU and the colorectal surgical ward.
Adjusted Nursing Workflow. Nurses were interviewed and asked to create a plan regarding how they could better incorporate ambulation into their daily workflow. Ambulation assessment was incorporated into the twice-per-shift recording of vital signs and patient safety assessment. This was recorded into the electronic health record at the same time as the patients’ vital signs. This allowed nurses to keep track of which patients would need extra assistance in ambulation and which patients were doing well on their own with the assistance of family. It also helped focus the resources of physical therapy and the single ambulation technician on the floor and to assist patients who needed more assistance.
Creation of Ambulation Encouragement Signs. The authors discovered that despite patients being told preoperatively about ambulation expectations, friends and family are not always included in these conversations. As nurses frequently cited both patients and family as reasons patients thought they should not walk, multiple signs inviting patients to take an active role in their recovery by ambulating were created and placed around the unit. The signs outlined the expectations of being out of bed and taking at least 1 step on the day of surgery and walking at least 4 times per day thereafter. In addition, we addressed frequently asked questions around issues such as walking with intravenous poles and urinary catheters. The posters were signed by all staff colorectal surgeons.
Results
Over the course of 7 months (June 2018 to December 2018), 253 postintervention patients were identified (Table 2). There was no difference between the pre-intervention group (n = 1762) and the postintervention group in terms of sex, race, origin, emergency status, operative time, and the majority of medical comorbidities (with the exception of smoking status and congestive heart failure). The postintervention group was slightly older (60 versus 57 years) and had a higher percentage of patients with an American Society of Anesthesiologists physical status score greater than 2 (66.8% versus 51.2%). The postintervention group also had higher rates of both malignancy (53.4% versus 33.3%) and inflammatory bowel disease (18.2% versus 14.4%).

The fidelity of the PDSA cycle was measured by pre-intervention and postintervention ambulation rates. Ambulation rates on POD 0, 1, and 2 improved from 36.4%, 47.3%, and 50.2% to 36.8%, 74.7%, and 82.6%, respectively (Table 3). The VTE rate decreased from 2.7% to 0.4% (P = 0.02), with 1 DVT and 0 PEs. It should be noted that the only patient who developed a VTE postintervention did not ambulate on PODs 0, 1, or 2.

Discussion
Postoperative VTE is a severe complication for postoperative colorectal surgery patients. Previous studies have demonstrated that increasing ambulation is associated with a lower rate of overall complications, and, when incorporated into a bundle, is associated with decreased rates of VTE.11,15 However, this is the first study to our knowledge demonstrating that creation of an ambulation protocol alone is associated with a decrease in VTE.
Analysis of pre-intervention data demonstrated a strong association between ambulation and an absence of VTE. No patient who ambulated on PODs 0, 1, and 2 developed a VTE. Based on those results, we moved forward with creating the ambulation protocol. While ambulation stayed stable on POD 0, there were 60% and 65% increases on PODs 1 and 2, respectively. Nurses cited late arrival to the floor for second and third start cases as the primary difficulty in getting patients to ambulate more on POD 0.
We believe the key to the success of the ambulation protocol was its multidisciplinary nature. Certainly, the easiest way to create an ambulation protocol is to change the postoperative orders to state patients must walk 4 times per day. However, if the nursing staff is unable or unwilling to carry out these orders, the orders serve little purpose. In order to make lasting changes, all stakeholders in the process must be identified. In our case, stakeholders included surgery and nursing leadership, surgeons, nurse practitioners, nurses, medical assistants, physical therapists, patient care technicians, and patients. This is where we utilized kaizen, a core principle of Lean methodology that empowers employees at the level of the work being carried out to propose ideas for improvement.16 From the beginning of the patient experience, the health care practitioners who were carrying out each step of the process were best able to identify the problems and create solutions. In addition, stakeholders were given regular updates regarding how their efforts were increasing ambulation rates and the results at the end of the study period.
This study also demonstrates that, in a health care system increasingly focused on both quality and cost, significant improvements in quality can be made without increasing cost or resource utilization. Early in the process, it was proposed that the only way to increase the ambulation rate would be to increase the number of physical therapists, nurses, and nursing assistants. However, after identifying the root causes of the problem, the solutions had more to do with improving workflow and fixing problem areas identified by the staff.
In addition to having a positive effect on the outcome studied, collaborative projects such as this between physicians and nurses may lead to increased nursing job satisfaction. A meta-analysis of 31 studies identified nurse-physician collaboration and autonomy as 2 factors that correlate most strongly with nursing satisfaction.17 A Cochrane review also suggests that practice-based interprofessional collaboration may lead to improved health care processes and outcomes.18
This study has several limitations. Pre-intervention ambulation rates were abstracted from institution-specific NSQIP data, and missing data were excluded from analysis. Also, due to the retrospective collection of the pre-intervention data, the distance of ambulation could not be quantified. The bar for ambulation is low, as patients were only required to get out of bed and walk 1 step. However, we feel that getting out of bed and taking even 1 step is substantially better than complete bedrest. It is likely that once patients cross the threshold of taking 1 step, they are more likely to ambulate. An area of future study may be to more precisely define the relationship between the quantity of ambulation in steps and its effect on VTE. Finally, we acknowledge that while there is no direct increase in costs, implementing an ambulation protocol does take time from all who participate in the project.
Conclusion
Creation of an ambulation protocol is associated with a decrease in postoperative VTE rates in colorectal surgery patients. A multidisciplinary approach is critical to identify the underlying problems and propose effective solutions. Further studies are required to better correlate the distance of ambulation and its effect on VTE. However, this study shows that even a minimum of 1 step is associated with decreased VTE rates.
Corresponding author: Aneel Damle, MD, MBA, Colon & Rectal Surgery Associates, 3433 Broadway St. NE, Suite 115, Minneapolis, MN 55413; [email protected].
Financial disclosures: None.
1. Gangireddy C, Rectenwald JR, Upchurch GR, et al. Risk factors and clinical impact of postoperative symptomatic venous thromboembolism. J Vasc Surg. 2007;45:341-342.
2. Newhook TE, LaPar DJ, Walters DM, et al. Impact of postoperative venous thromboembolism on postoperative morbidity, mortality, and resource utilization after hepatectomy. Am Surg. 2015;81:1216-1223.
3. Bergqvist D. Venous thromboembolism: a review of risk and prevention in colorectal surgery patients. Dis Colon Rectum. 2006;49:1620-1628.
4. Fleming F, Gaertner W, Ternent CA, et al. The American society of colon and rectal surgeons clinical practice guideline for the prevention of venous thromboembolic disease in colorectal surgery. Dis Colon Rectum. 2018;61:14-20.
5. McLeod RS, Geerts WH, Sniderman KW, et al. Canadian Colorectal Surgery DVT Prophylaxis Trial investigators. Subcutaneous heparin versus low-molecular-weight heparin as thromboprophylaxis in patients undergoing colorectal surgery: results of the Canadian colorectal DV prophylaxis trial: a randomized, double-blind trial. Ann Surg. 2001;233:438-444.
6. Shapiro R, Vogel JD, Kiran RP. Risk of postoperative venous thromboembolism after laparoscopic and open colorectal surgery: an additional benefit of the minimally invasive approach? Dis Colon Rectum. 2011;54:1496-1502.
7. Dimick JB, Chen SL, Taheri PA, et al. Hospital costs associated with surgical complications: a report from the private-sector National Surgical Quality Improvement Program. J Am Coll Surg. 2004;199:531-537.
8. Fleming FJ, Kim MJ, Salloum RM, et al. How much do we need to worry about venous thromboembolism after hospital discharge? A study of colorectal surgery patients using the National Surgical Quality Improvement Program database. Dis Colon Rectum. 2010;53:1355-1360.
9. ACS NSQIP. User guide for the 2016 ACS NSQIP participant use data file (PUF). 2017. www.facs.org/~/media/files/quality%20programs/nsqip/nsqip_puf_userguide_2016.ashx Accessed July 10, 2020.
10. Caprini JA. Risk assessment as a guide for the prevention of the many faces of venous thromboembolism. Am J Surg. 2010;199(1 Suppl):S3-S10.
11. Cassidy MR, Rosenkranz P, McAney D. Reducing postoperative venous thromboembolism complications with a standardized risk-stratified prophylaxis protocol and mobilization protocol. J Am Coll Surg. 2014;218:1095-1104.
12. Lau BD, Streiff MB, Kraus PS, et al. No evidence to support ambulation for reducing postoperative venous thromboembolism. J Am Coll Surg. 2014;219:1101-1103.
13. McNicholas C, Lennox L, Woodcock T, et al. Evolving quality improvement support strategies to improve Plan–Do–Study–Act cycle fidelity: a retrospective mixed-methods study. BMJ Qual Saf. 2019;28:356-365.
14. Taylor MJ, McNicholas C, Nicolay C, et al. Systematic review of the application of the plan–do–study–act method to improve quality in healthcare. BMC Qual Saf. 2014;23:290-298.
15. Nevo Y, Shaltiel T, Constantini N, et al. Effect of ambulation and physical activity on postoperative complications. J Am Coll Surg. 2016;223(Suppl 1):S61.
16. Mazzocato P, Stenfors-Hayes T, von Thiele Schwarz U, et al. Kaizen practice in healthcare: a qualitative analysis of hospital employees’ suggestions for improvement. BMJ Open. 2016;6:e012256.
17. Zangaro GA, Soeken KL. A meta-analysis of studies of nurses’ job satisfaction. Res Nursing Health. 2007;30:445-458.
18. Reeves S, Pelone F, Harrison R, et al. Interprofessional collaboration to improve professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2017;6(6):CD000072.
1. Gangireddy C, Rectenwald JR, Upchurch GR, et al. Risk factors and clinical impact of postoperative symptomatic venous thromboembolism. J Vasc Surg. 2007;45:341-342.
2. Newhook TE, LaPar DJ, Walters DM, et al. Impact of postoperative venous thromboembolism on postoperative morbidity, mortality, and resource utilization after hepatectomy. Am Surg. 2015;81:1216-1223.
3. Bergqvist D. Venous thromboembolism: a review of risk and prevention in colorectal surgery patients. Dis Colon Rectum. 2006;49:1620-1628.
4. Fleming F, Gaertner W, Ternent CA, et al. The American society of colon and rectal surgeons clinical practice guideline for the prevention of venous thromboembolic disease in colorectal surgery. Dis Colon Rectum. 2018;61:14-20.
5. McLeod RS, Geerts WH, Sniderman KW, et al. Canadian Colorectal Surgery DVT Prophylaxis Trial investigators. Subcutaneous heparin versus low-molecular-weight heparin as thromboprophylaxis in patients undergoing colorectal surgery: results of the Canadian colorectal DV prophylaxis trial: a randomized, double-blind trial. Ann Surg. 2001;233:438-444.
6. Shapiro R, Vogel JD, Kiran RP. Risk of postoperative venous thromboembolism after laparoscopic and open colorectal surgery: an additional benefit of the minimally invasive approach? Dis Colon Rectum. 2011;54:1496-1502.
7. Dimick JB, Chen SL, Taheri PA, et al. Hospital costs associated with surgical complications: a report from the private-sector National Surgical Quality Improvement Program. J Am Coll Surg. 2004;199:531-537.
8. Fleming FJ, Kim MJ, Salloum RM, et al. How much do we need to worry about venous thromboembolism after hospital discharge? A study of colorectal surgery patients using the National Surgical Quality Improvement Program database. Dis Colon Rectum. 2010;53:1355-1360.
9. ACS NSQIP. User guide for the 2016 ACS NSQIP participant use data file (PUF). 2017. www.facs.org/~/media/files/quality%20programs/nsqip/nsqip_puf_userguide_2016.ashx Accessed July 10, 2020.
10. Caprini JA. Risk assessment as a guide for the prevention of the many faces of venous thromboembolism. Am J Surg. 2010;199(1 Suppl):S3-S10.
11. Cassidy MR, Rosenkranz P, McAney D. Reducing postoperative venous thromboembolism complications with a standardized risk-stratified prophylaxis protocol and mobilization protocol. J Am Coll Surg. 2014;218:1095-1104.
12. Lau BD, Streiff MB, Kraus PS, et al. No evidence to support ambulation for reducing postoperative venous thromboembolism. J Am Coll Surg. 2014;219:1101-1103.
13. McNicholas C, Lennox L, Woodcock T, et al. Evolving quality improvement support strategies to improve Plan–Do–Study–Act cycle fidelity: a retrospective mixed-methods study. BMJ Qual Saf. 2019;28:356-365.
14. Taylor MJ, McNicholas C, Nicolay C, et al. Systematic review of the application of the plan–do–study–act method to improve quality in healthcare. BMC Qual Saf. 2014;23:290-298.
15. Nevo Y, Shaltiel T, Constantini N, et al. Effect of ambulation and physical activity on postoperative complications. J Am Coll Surg. 2016;223(Suppl 1):S61.
16. Mazzocato P, Stenfors-Hayes T, von Thiele Schwarz U, et al. Kaizen practice in healthcare: a qualitative analysis of hospital employees’ suggestions for improvement. BMJ Open. 2016;6:e012256.
17. Zangaro GA, Soeken KL. A meta-analysis of studies of nurses’ job satisfaction. Res Nursing Health. 2007;30:445-458.
18. Reeves S, Pelone F, Harrison R, et al. Interprofessional collaboration to improve professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2017;6(6):CD000072.
Dapagliflozin Improves Cardiovascular Outcomes in Patients With Heart Failure and Reduced Ejection Fraction
Study Overview
Objective. To evaluate the effects of dapagliflozin in patients with heart failure with reduced ejection fraction in the presence or absence of type 2 diabetes.
Design. Multicenter, international, double-blind, prospective, randomized, controlled trial.
Setting and participants. Adult patients with symptomatic heart failure with an ejection fraction of 40% or less and elevated heart failure biomarkers who were already on appropriate guideline-directed therapies were eligible for the study.
Intervention. A total of 4744 patients were randomly assigned to receive dapagliflozin (10 mg once daily) or placebo, in addition to recommended therapy. Randomization was stratified by the presence or absence of type 2 diabetes.
Main outcome measures. The primary outcome was the composite of a first episode of worsening heart failure (hospitalization or urgent intravenous therapy) or cardiovascular death.
Main results. Median follow-up was 18.2 months; during this time, the primary outcome occurred in 16.3% (386 of 2373) of patients in the dapagliflozin group and in 21.2% (502 of 2371) of patients in the placebo group (hazard ratio [HR], 0.74; 95% confidence interval [CI], 0.65-0.85; P < 0.001). In the dapagliflozin group, 237 patients (10.0%) experienced a first worsening heart failure event, as compared with 326 patients (13.7%) in the placebo group (HR, 0.70; 95% CI, 0.59-0.83). The dapagliflozin group hadlower rates of death from cardiovascular causes (9.6% vs 11.5%; HR, 0.82; 95% CI, 0.69-0.98) and from any causes (11.6% vs 13.9%; HR, 0.83; 95% CI, 0.71-0.97), compared to the placebo group. Findings in patients with diabetes were similar to those in patients without diabetes.
Conclusion. Among patients with heart failure and a reduced ejection fraction, the risk of worsening heart failure or death from cardiovascular causes was lower among those who received dapagliflozin than among those who received placebo, regardless of the presence or absence of diabetes.
Commentary
Inhibitors of sodium-glucose cotransporter 2 (SGLT-2) are a novel class of diabetic medication that decrease renal glucose reabsorption, thereby increasing urinary glucose excretion. In several large clinical trials of these medications for patients with diabetes, which were designed to meet the regulatory requirements for cardiovascular safety in novel diabetic agents, investigators unexpectedly found that SGLT-2 inhibitors were associated with a reduction in cardiovascular events, driven by a reduction in heart failure hospitalizations. The results of EMPA-REG OUTCOME, the first of these trials, showed significantly lower risks of both death from any cause and hospitalization for heart failure in patients treated with empagliflozin.1 This improvement in cardiovascular outcomes was subsequently confirmed as a class effect of SGLT-2 inhibitors in the CANVAS Program (canagliflozin) and DECLARE TIMI 58 (dapagliflozin) trials.2,3
While these trials were designed for patients with type 2 diabetes who had either established cardiovascular disease or multiple risk factors for it, most patients did not have heart failure at baseline. Accordingly, despite a signal toward benefit of SGLT-2 inhibitors in patients with heart failure, the trials were not powered to test the hypothesis that SGLT-2 inhibitors benefit patients with heart failure, regardless of diabetes status. Therefore, McMurray et al designed the DAPA-HF trial to investigate whether SGLT-2 inhibitors can improve cardiovascular outcomes in patients with heart failure with reduced ejection fraction, with or without diabetes. The trial included 4744 patients with heart failure with reduced ejection fraction, who were randomly assigned to dapagliflozin 10 mg once daily or placebo, atop guideline-directed heart failure therapy, with randomization stratified by presence or absence of type 2 diabetes. Investigators found that the composite primary outcome, a first episode of worsening heart failure or cardiovascular death, occurred less frequently in patients in the dapagliflozin group compared to the placebo group (16.3% vs 21.2%; HR, 0.74; 95% CI, 0.65-0.85; P < 0.001). Individual components of the primary outcome and death from any cause were all significantly lower, and heart failure–related quality of life was significantly improved in the dapagliflozin group compared to placebo.
DAPA-HF was the first randomized study to investigate the effect of SGLT-2 inhibitors on patients with heart failure regardless of the presence of diabetes. In addition to the reduction in the above-mentioned primary and secondary endpoints, the study yielded other important findings worth noting. First, the consistent benefit of dapagliflozin on cardiovascular outcomes in patients with and without diabetes suggests that the cardioprotective effect of dapagliflozin is independent of its glucose-lowering effect. Prior studies have proposed alternative mechanisms, such as diuretic function and related hemodynamic actions, effects on myocardial metabolism, ion transporters, fibrosis, adipokines, vascular function, and the preservation of renal function. Future studies are needed to fully understand the likely pleiotropic effects of this class of medication on patients with heart failure. Second, there was no difference in the safety endpoints between the groups, including renal adverse events and major hypoglycemia, implying dapagliflozin is as safe as placebo.
There are a few limitations of this trial. First, as the authors point out, the study included mostly white males—less than 5% of participants were African Americans—and the finding may not be generalizable to all patient populations. Second, although all patients were already treated with guideline-directed heart failure therapy, only 10% of patients were on sacubitril–valsartan, which is more effective than renin–angiotensin system blockade alone at reducing the incidence of hospitalization for heart failure and death from cardiovascular causes. Also, mineralocorticoid receptor blockers were used in only 70% of the population. Finally, since the doses were not provided, whether patients were on the maximal tolerated dose of heart failure therapy prior to enrollment is unclear.
Based on the results of the DAPA-HF trial, the Food and Drug Administration approved dapagliflozin for the treatment of heart failure with reduced ejection fraction on May 5, 2020. This is the first diabetic drug approved for the treatment of heart failure.
Applications for Clinical Practice
SGLT-2 inhibitors represent a fourth class of medication that patients with heart failure with reduced ejection fraction should be initiated on, in addition to beta blocker, ACE inhibitor/angiotensin receptor blocker/neprilysin inhibitor, and mineralocorticoid receptor blocker. SGLT-2 inhibitors may be especially applicable in patients with heart failure with reduced ejection fraction and relative hypotension, as these agents are not associated with a significant blood-pressure-lowering effect, which can often limit our ability to initiate or uptitrate the other main 3 classes of guideline-directed medical therapy.
—Rie Hirai, MD, Fukui Kosei Hospital, Fukui, Japan
—Taishi Hirai, MD, University of Missouri Medical Center, Columbia, MO
—Timothy Fendler, MD, St. Luke’s Mid America Heart Institute, Kansas City, MO
1. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
2. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644-657.
3. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347-357.
Study Overview
Objective. To evaluate the effects of dapagliflozin in patients with heart failure with reduced ejection fraction in the presence or absence of type 2 diabetes.
Design. Multicenter, international, double-blind, prospective, randomized, controlled trial.
Setting and participants. Adult patients with symptomatic heart failure with an ejection fraction of 40% or less and elevated heart failure biomarkers who were already on appropriate guideline-directed therapies were eligible for the study.
Intervention. A total of 4744 patients were randomly assigned to receive dapagliflozin (10 mg once daily) or placebo, in addition to recommended therapy. Randomization was stratified by the presence or absence of type 2 diabetes.
Main outcome measures. The primary outcome was the composite of a first episode of worsening heart failure (hospitalization or urgent intravenous therapy) or cardiovascular death.
Main results. Median follow-up was 18.2 months; during this time, the primary outcome occurred in 16.3% (386 of 2373) of patients in the dapagliflozin group and in 21.2% (502 of 2371) of patients in the placebo group (hazard ratio [HR], 0.74; 95% confidence interval [CI], 0.65-0.85; P < 0.001). In the dapagliflozin group, 237 patients (10.0%) experienced a first worsening heart failure event, as compared with 326 patients (13.7%) in the placebo group (HR, 0.70; 95% CI, 0.59-0.83). The dapagliflozin group hadlower rates of death from cardiovascular causes (9.6% vs 11.5%; HR, 0.82; 95% CI, 0.69-0.98) and from any causes (11.6% vs 13.9%; HR, 0.83; 95% CI, 0.71-0.97), compared to the placebo group. Findings in patients with diabetes were similar to those in patients without diabetes.
Conclusion. Among patients with heart failure and a reduced ejection fraction, the risk of worsening heart failure or death from cardiovascular causes was lower among those who received dapagliflozin than among those who received placebo, regardless of the presence or absence of diabetes.
Commentary
Inhibitors of sodium-glucose cotransporter 2 (SGLT-2) are a novel class of diabetic medication that decrease renal glucose reabsorption, thereby increasing urinary glucose excretion. In several large clinical trials of these medications for patients with diabetes, which were designed to meet the regulatory requirements for cardiovascular safety in novel diabetic agents, investigators unexpectedly found that SGLT-2 inhibitors were associated with a reduction in cardiovascular events, driven by a reduction in heart failure hospitalizations. The results of EMPA-REG OUTCOME, the first of these trials, showed significantly lower risks of both death from any cause and hospitalization for heart failure in patients treated with empagliflozin.1 This improvement in cardiovascular outcomes was subsequently confirmed as a class effect of SGLT-2 inhibitors in the CANVAS Program (canagliflozin) and DECLARE TIMI 58 (dapagliflozin) trials.2,3
While these trials were designed for patients with type 2 diabetes who had either established cardiovascular disease or multiple risk factors for it, most patients did not have heart failure at baseline. Accordingly, despite a signal toward benefit of SGLT-2 inhibitors in patients with heart failure, the trials were not powered to test the hypothesis that SGLT-2 inhibitors benefit patients with heart failure, regardless of diabetes status. Therefore, McMurray et al designed the DAPA-HF trial to investigate whether SGLT-2 inhibitors can improve cardiovascular outcomes in patients with heart failure with reduced ejection fraction, with or without diabetes. The trial included 4744 patients with heart failure with reduced ejection fraction, who were randomly assigned to dapagliflozin 10 mg once daily or placebo, atop guideline-directed heart failure therapy, with randomization stratified by presence or absence of type 2 diabetes. Investigators found that the composite primary outcome, a first episode of worsening heart failure or cardiovascular death, occurred less frequently in patients in the dapagliflozin group compared to the placebo group (16.3% vs 21.2%; HR, 0.74; 95% CI, 0.65-0.85; P < 0.001). Individual components of the primary outcome and death from any cause were all significantly lower, and heart failure–related quality of life was significantly improved in the dapagliflozin group compared to placebo.
DAPA-HF was the first randomized study to investigate the effect of SGLT-2 inhibitors on patients with heart failure regardless of the presence of diabetes. In addition to the reduction in the above-mentioned primary and secondary endpoints, the study yielded other important findings worth noting. First, the consistent benefit of dapagliflozin on cardiovascular outcomes in patients with and without diabetes suggests that the cardioprotective effect of dapagliflozin is independent of its glucose-lowering effect. Prior studies have proposed alternative mechanisms, such as diuretic function and related hemodynamic actions, effects on myocardial metabolism, ion transporters, fibrosis, adipokines, vascular function, and the preservation of renal function. Future studies are needed to fully understand the likely pleiotropic effects of this class of medication on patients with heart failure. Second, there was no difference in the safety endpoints between the groups, including renal adverse events and major hypoglycemia, implying dapagliflozin is as safe as placebo.
There are a few limitations of this trial. First, as the authors point out, the study included mostly white males—less than 5% of participants were African Americans—and the finding may not be generalizable to all patient populations. Second, although all patients were already treated with guideline-directed heart failure therapy, only 10% of patients were on sacubitril–valsartan, which is more effective than renin–angiotensin system blockade alone at reducing the incidence of hospitalization for heart failure and death from cardiovascular causes. Also, mineralocorticoid receptor blockers were used in only 70% of the population. Finally, since the doses were not provided, whether patients were on the maximal tolerated dose of heart failure therapy prior to enrollment is unclear.
Based on the results of the DAPA-HF trial, the Food and Drug Administration approved dapagliflozin for the treatment of heart failure with reduced ejection fraction on May 5, 2020. This is the first diabetic drug approved for the treatment of heart failure.
Applications for Clinical Practice
SGLT-2 inhibitors represent a fourth class of medication that patients with heart failure with reduced ejection fraction should be initiated on, in addition to beta blocker, ACE inhibitor/angiotensin receptor blocker/neprilysin inhibitor, and mineralocorticoid receptor blocker. SGLT-2 inhibitors may be especially applicable in patients with heart failure with reduced ejection fraction and relative hypotension, as these agents are not associated with a significant blood-pressure-lowering effect, which can often limit our ability to initiate or uptitrate the other main 3 classes of guideline-directed medical therapy.
—Rie Hirai, MD, Fukui Kosei Hospital, Fukui, Japan
—Taishi Hirai, MD, University of Missouri Medical Center, Columbia, MO
—Timothy Fendler, MD, St. Luke’s Mid America Heart Institute, Kansas City, MO
Study Overview
Objective. To evaluate the effects of dapagliflozin in patients with heart failure with reduced ejection fraction in the presence or absence of type 2 diabetes.
Design. Multicenter, international, double-blind, prospective, randomized, controlled trial.
Setting and participants. Adult patients with symptomatic heart failure with an ejection fraction of 40% or less and elevated heart failure biomarkers who were already on appropriate guideline-directed therapies were eligible for the study.
Intervention. A total of 4744 patients were randomly assigned to receive dapagliflozin (10 mg once daily) or placebo, in addition to recommended therapy. Randomization was stratified by the presence or absence of type 2 diabetes.
Main outcome measures. The primary outcome was the composite of a first episode of worsening heart failure (hospitalization or urgent intravenous therapy) or cardiovascular death.
Main results. Median follow-up was 18.2 months; during this time, the primary outcome occurred in 16.3% (386 of 2373) of patients in the dapagliflozin group and in 21.2% (502 of 2371) of patients in the placebo group (hazard ratio [HR], 0.74; 95% confidence interval [CI], 0.65-0.85; P < 0.001). In the dapagliflozin group, 237 patients (10.0%) experienced a first worsening heart failure event, as compared with 326 patients (13.7%) in the placebo group (HR, 0.70; 95% CI, 0.59-0.83). The dapagliflozin group hadlower rates of death from cardiovascular causes (9.6% vs 11.5%; HR, 0.82; 95% CI, 0.69-0.98) and from any causes (11.6% vs 13.9%; HR, 0.83; 95% CI, 0.71-0.97), compared to the placebo group. Findings in patients with diabetes were similar to those in patients without diabetes.
Conclusion. Among patients with heart failure and a reduced ejection fraction, the risk of worsening heart failure or death from cardiovascular causes was lower among those who received dapagliflozin than among those who received placebo, regardless of the presence or absence of diabetes.
Commentary
Inhibitors of sodium-glucose cotransporter 2 (SGLT-2) are a novel class of diabetic medication that decrease renal glucose reabsorption, thereby increasing urinary glucose excretion. In several large clinical trials of these medications for patients with diabetes, which were designed to meet the regulatory requirements for cardiovascular safety in novel diabetic agents, investigators unexpectedly found that SGLT-2 inhibitors were associated with a reduction in cardiovascular events, driven by a reduction in heart failure hospitalizations. The results of EMPA-REG OUTCOME, the first of these trials, showed significantly lower risks of both death from any cause and hospitalization for heart failure in patients treated with empagliflozin.1 This improvement in cardiovascular outcomes was subsequently confirmed as a class effect of SGLT-2 inhibitors in the CANVAS Program (canagliflozin) and DECLARE TIMI 58 (dapagliflozin) trials.2,3
While these trials were designed for patients with type 2 diabetes who had either established cardiovascular disease or multiple risk factors for it, most patients did not have heart failure at baseline. Accordingly, despite a signal toward benefit of SGLT-2 inhibitors in patients with heart failure, the trials were not powered to test the hypothesis that SGLT-2 inhibitors benefit patients with heart failure, regardless of diabetes status. Therefore, McMurray et al designed the DAPA-HF trial to investigate whether SGLT-2 inhibitors can improve cardiovascular outcomes in patients with heart failure with reduced ejection fraction, with or without diabetes. The trial included 4744 patients with heart failure with reduced ejection fraction, who were randomly assigned to dapagliflozin 10 mg once daily or placebo, atop guideline-directed heart failure therapy, with randomization stratified by presence or absence of type 2 diabetes. Investigators found that the composite primary outcome, a first episode of worsening heart failure or cardiovascular death, occurred less frequently in patients in the dapagliflozin group compared to the placebo group (16.3% vs 21.2%; HR, 0.74; 95% CI, 0.65-0.85; P < 0.001). Individual components of the primary outcome and death from any cause were all significantly lower, and heart failure–related quality of life was significantly improved in the dapagliflozin group compared to placebo.
DAPA-HF was the first randomized study to investigate the effect of SGLT-2 inhibitors on patients with heart failure regardless of the presence of diabetes. In addition to the reduction in the above-mentioned primary and secondary endpoints, the study yielded other important findings worth noting. First, the consistent benefit of dapagliflozin on cardiovascular outcomes in patients with and without diabetes suggests that the cardioprotective effect of dapagliflozin is independent of its glucose-lowering effect. Prior studies have proposed alternative mechanisms, such as diuretic function and related hemodynamic actions, effects on myocardial metabolism, ion transporters, fibrosis, adipokines, vascular function, and the preservation of renal function. Future studies are needed to fully understand the likely pleiotropic effects of this class of medication on patients with heart failure. Second, there was no difference in the safety endpoints between the groups, including renal adverse events and major hypoglycemia, implying dapagliflozin is as safe as placebo.
There are a few limitations of this trial. First, as the authors point out, the study included mostly white males—less than 5% of participants were African Americans—and the finding may not be generalizable to all patient populations. Second, although all patients were already treated with guideline-directed heart failure therapy, only 10% of patients were on sacubitril–valsartan, which is more effective than renin–angiotensin system blockade alone at reducing the incidence of hospitalization for heart failure and death from cardiovascular causes. Also, mineralocorticoid receptor blockers were used in only 70% of the population. Finally, since the doses were not provided, whether patients were on the maximal tolerated dose of heart failure therapy prior to enrollment is unclear.
Based on the results of the DAPA-HF trial, the Food and Drug Administration approved dapagliflozin for the treatment of heart failure with reduced ejection fraction on May 5, 2020. This is the first diabetic drug approved for the treatment of heart failure.
Applications for Clinical Practice
SGLT-2 inhibitors represent a fourth class of medication that patients with heart failure with reduced ejection fraction should be initiated on, in addition to beta blocker, ACE inhibitor/angiotensin receptor blocker/neprilysin inhibitor, and mineralocorticoid receptor blocker. SGLT-2 inhibitors may be especially applicable in patients with heart failure with reduced ejection fraction and relative hypotension, as these agents are not associated with a significant blood-pressure-lowering effect, which can often limit our ability to initiate or uptitrate the other main 3 classes of guideline-directed medical therapy.
—Rie Hirai, MD, Fukui Kosei Hospital, Fukui, Japan
—Taishi Hirai, MD, University of Missouri Medical Center, Columbia, MO
—Timothy Fendler, MD, St. Luke’s Mid America Heart Institute, Kansas City, MO
1. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
2. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644-657.
3. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347-357.
1. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
2. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644-657.
3. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347-357.
Team-Based Hypertension Management in Outpatient Settings
From Western University of Health Sciences College of Pharmacy, Department of Pharmacy Practice and Administration, Pomona, CA.
Abstract
- Objective: To review the current literature regarding the clinical effectiveness and cost-effectiveness of implementing hypertension team-based care (TBC) interventions in the outpatient setting, and discuss challenges to implementation.
- Methods: A literature review was conducted of meta-analyses, systematic reviews, and randomized controlled trials comparing TBC models to usual care for hypertension management.
- Results: Compared to usual care, TBC models have demonstrated greater blood pressure reductions and improved blood pressure control rates. Evidence was strongest for models involving nurses and pharmacists whose roles included medication management, patient education and counseling, coordination of care and follow-up, population health management, and performance measurement with quality improvement. Although TBC results in an increase in health care costs, the overall long-term benefits support the cost-effectiveness of these models over usual care. The most common barriers to TBC implementation include underutilization of technology, stakeholder engagement, and reimbursement issues.
- Conclusion: Hypertension TBC models have been shown to be clinically effective and cost-effective, but continued research comparing different models is warranted to determine which combination of health professionals and interventions is most impactful and cost-effective in practice. An implementation science approach, in which TBC models unique to each organization’s situation are created, will be useful to identify and overcome challenges and provide a solid foundation for sustainment.
Keywords: blood pressure; pharmacist; nurse; nurse practitioner; cost-effectiveness; team-based care.
Approximately 1 in 3 US adults—or about 100 million people—have high blood pressure, and only about half (48%) have their blood pressure under control.1 Effective blood pressure management has been shown to decrease the incidence of stroke, heart attack, and heart failure.2-4 The American College of Cardiology/American Heart Association (ACC/AHA) 2017 blood pressure guidelines recommended lower thresholds for diagnosing hypertension and initiating antihypertensive medication, and intensified the blood pressure goal to less than 130/80 mm Hg.5 Changing practice standards to more intensive blood pressure goals requires significant adjustments by clinicians and health care systems. In fact, new guideline uptake is often delayed, ignored, or sparsely applied.6 Due to this dramatic change in hypertension practice standards, the ACC/AHA guidelines support interdisciplinary team-based care (TBC) for hypertension management.5,7 Additionally, the Centers for Disease Control and Prevention (CDC) and the Community Preventive Services Task Force (CPSTF) promote TBC to improve blood pressure control in their initiatives to prevent heart disease and stroke.8,9
The National Academy of Medicine defines TBC as “the provision of health services to individuals, families, and/or their communities by at least 2 healthcare providers who work collaboratively with patients and their caregivers—to the extent preferred by each patient—to accomplish shared goals within and across settings to achieve coordinated, high-quality care.”10 Specific goals for TBC in hypertension treatment are listed in Table 1, and a checklist of key elements of TBC to consider before implementation are presented in Table 2.
TBC has been shown to have many advantages, including increased access to care due to expanded hours of operation and shorter wait times.11 Team-based models also provide effective and efficient delivery of patient education, behavioral health care, and care coordination.12-14 Patients are more likely to receive high-quality care when multiple providers, each with varied expertise, are on the health care team.11,15 Furthermore, clinicians report improved professional job satisfaction related to their ability to practice in environments where they are encouraged to work at the top of their licenses.16 Consequently, TBC has been accepted as a vital part of the patient-centered medical home (PCMH) model.17-19 Standards set by the National Committee for Quality Assurance (NCQA) include TBC as a requirement health systems must meet in order to achieve the highest level of PCMH recognition. While a team-based approach offers substantial benefits and is recognized as a marker of quality, implementation has presented various challenges, and the sustainability of these models in care settings has been questioned.20

In this article, we review the current literature regarding the clinical effectiveness and cost-effectiveness of implementing hypertension TBC interventions in the outpatient setting. We also discuss the challenges and opportunities of implementing this strategy in health systems and community settings in the United States.
Evidence of Impact and Effectiveness
Various models of hypertension TBC have been shown to increase the proportion of individuals with controlled blood pressure and to lead to a reduction in both systolic (SBP) and diastolic blood pressure (DBP), resulting in a strong recommendation for TBC approaches by the 2017 ACC/AHA blood pressure guidelines.5,21-25 There is great diversity in the types of hypertension treatment models studied, with few utilizing physician specialists and most utilizing nonphysician providers, such as community health workers, physician assistants, nurses, nurse practitioners, dietitians, social workers, and pharmacists.22,26-29 These professionals share duties of hypertension management with primary care physicians to reduce the burden of responsibility for care on any single provider type. TBC is patient-centered, and typically includes interprofessional collaboration, treatment algorithms, adherence counseling, frequent follow-up, home blood pressure monitoring, and patient self-management education.
Numerous studies have supported implementation of TBC in recent years. A systematic review and meta-analysis of 100 trials of hypertension TBC involving 55,920 patients concluded that the most effective blood pressure–lowering strategies use multilevel, multicomponent approaches to address barriers to hypertension control. Nonphysician providers are often involved in measuring blood pressure, ordering and assessing laboratory tests, and titrating medications.30 Compared with usual care, TBC with physician medication titration resulted in reductions in mean SBP and DBP (6.2 mm Hg and 2.7 mm Hg, respectively), while TBC with nonphysician medication titration also resulted in reductions in mean SBP and DBP (7.1 mm Hg and 3.1 mm Hg, respectively). Nurses and pharmacists are specifically mentioned by the 2017 ACC/AHA blood pressure guidelines as essential members of the hypertension treatment team.5 Randomized controlled trials (RCTs) and meta-analyses of TBC involving nurse or pharmacist interventions demonstrated greater reductions in SBP and/or greater attainment of blood pressure goals compared to usual care.21,26,31,32 The literature supports the roles of nurses and pharmacists in hypertension management in all aspects of care, including medication management, patient education and counseling, coordination of care and follow-up, population health management, and performance measurement with quality improvement.33
Nurses
Nurses are commonly part of TBC hypertension management programs. One meta-analysis and systematic review of international RCTs compared nurse, nurse prescriber (United Kingdom), and nurse practitioner interventions for hypertension with usual care. Interventions that included a stepped treatment algorithm and nurse prescribing showed greater reductions in SBP (8.2 mm Hg and 8.9 mm Hg, respectively) compared to usual care.31 Similarly, models that utilized telephone monitoring demonstrated greater achievement of blood pressure targets, while those that involved home monitoring showed significant reductions in blood pressure. Another international meta-analysis and systematic review of 11 nurse-led interventions in hypertensive patients with diabetes demonstrated a 5.8 mm Hg mean decrease in SBP compared to physician-led care. However, nurse-led care was not superior in achievement of study targets.34
A recent meta-analysis and systematic review, performed by Shaw and colleagues, sought to determine whether nurse-led protocols are effective for outpatient management of adults with diabetes, hypertension, and hyperlipidemia. All of the included studies involved a registered nurse who titrated medications by following a protocol, and most were RCTs comparing the nurse protocols to usual care. Overall, mean SBP and DBP decreased by 3.86 mm Hg and 1.56 mm Hg, respectively, while blood glucose and lipid levels were also reduced compared to usual care.24
Limited RCT data have been published since the Shaw et al meta-analysis. A single-blind RCT was performed in an urban community health care center in China among patients with uncontrolled blood pressure (SBP ≥ 140 mm Hg and/or DBP ≥ 90 mm Hg).35 The study group received care via a nurse-led model, which included a delivery design system, decision support, clinical information system, and self-management support, and the control group received usual care. At 12 weeks, patients in the study group had significantly lower blood pressure than control patients, with mean SBP/DBP reduction of 14.37/7.43 mm Hg and 5.10/2.69 mm Hg, respectively (P < 0.01). Improved medication adherence and increased patient satisfaction were other benefits of the nurse-led model.
Nurse case managers (NCM) also play a critical role in hypertension management, coordinating health care services to meet patient health needs. Ogedegbe sought to evaluate the comparative effectiveness of home blood pressure telemonitoring (HBPTM)+NCM versus HBPTM alone on SBP reduction in black and Hispanic stroke survivors.36,37 NCMs evaluated patient profiles, counseled patients on target lifestyle behaviors, and reviewed home blood pressure data. At 6 months, SBP declined by 13.63 mm Hg from baseline in the HBPTM+NCM group and 6.31 mm Hg in the HBPTM alone group (P < 0.0001). At 12 months, SBP in the HBPTM+NCM group declined by 14.76 mm Hg, while blood pressure in the HBPTM alone group declined by 5.53 mm Hg (P < 0.0001).
Pharmacists
Clinical pharmacists are also widely utilized in TBC models for hypertension management. Typical models involve pharmacists entering into collaborative practice agreements with physicians, leading to optimization of medications, avoidance of adverse drug events, and transitional care activities focusing on medication reconciliation and patient education in outpatient settings.30,38 The largest and most recent meta-analysis of pharmacist interventions, conducted in 2014 by Santschi et al,23 combined 2 previous systematic reviews to include a total of 39 RCTs with 14,224 patients.32,39 Pharmacist interventions included patient education, recommendations to physicians, and medication management. Compared with usual care, pharmacist interventions showed greater reductions in SBP (7.6 mm Hg) and DBP (3.9 mm Hg).23
Numerous studies substantiating the impact of pharmacist interventions on clinical outcomes have heavily influenced clinical practice and guideline development. Carter et al conducted a prospective, multi-state, cluster-randomized trial in 32 primary care clinics to evaluate whether clinics randomized to receive the pharmacist-physician collaborative care model (PPCCM) achieved better blood pressure outcomes versus clinics randomized to usual care.25 Investigators enrolled 625 patients with uncontrolled hypertension, 50% of whom had a prior diagnosis of diabetes mellitus or chronic kidney disease. The primary outcome of blood pressure control at 9 months in the intervention clinics compared to the control clinics was 43% and 34%, respectively (P = 0.059). The difference in mean SBP/DBP between the intervention and control clinics for all patients at 9 months was −6.1/−2.9 mm Hg. In a post-hoc analysis of patients with chronic kidney disease and diabetes, the pharmacist-intervention group had a significantly greater mean SBP reduction and higher blood pressure control rates compared to usual care at 9 months.40
A pre-specified secondary analysis from the Carter et al study determined that, in patients from racial minority groups, the mean SBP was 7.3 mm Hg lower in those who received the intervention compared to those in the control group (P = 0.0042).41 In patients with less than 12 years of education, those in the intervention group had a mean SBP 8.1 mm Hg lower than the SBP of those in the control group (P = 0.0001). Similar reductions in blood pressure occurred in patients with low income, Medicaid beneficiaries, or those without insurance. This study demonstrated that pharmacist interventions reduced racial and socioeconomic disparities in blood pressure treatment.
Other studies of pharmacist interventions in underserved populations have yielded positive results. In a retrospective review of uninsured patients, blood pressure control rates in a pharmacist-driven primary care clinic ranked in the 90th percentile of NCQA benchmarks, and was superior to the 2013 reported mean for commercial insurers.42 Similarly, another retrospective cohort study of a PPCCM on time to goal blood pressure in uninsured patients with hypertension showed the median time to blood pressure goal was 36 days in the PPCCM cohort versus 259 days in usual care cohorts (P < 0.001).43 A post-hoc analysis revealed the mean time-in-therapeutic blood pressure range was 46.2% ± 24.3% in the PPCCM group and 24.8% ± 27.4% in the usual care group (P < 0.0001). The blood pressure control rates at 12 months were 89% in the PPCCM group compared with 50% in the usual care group (P < 0.0001).44
Tsuyuki et al conducted the RxACTION study, a multicenter RCT evaluating the effectiveness of enhanced pharmacist care versus usual care in 23 Canadian community pharmacies and outpatient clinics following a 6-month intervention.45 Enhanced pharmacy services included pharmacist assessment of and counseling about cardiovascular disease risk and blood pressure control, review of current antihypertensive medications, and prescribing/titrating drug therapy, as needed, through independent prescriptive authority. Compared to the usual care group (n = 67), the intervention group had a reduction in SBP of 6.6 mm Hg (P = 0.006) and in DBP of 3.2 mm Hg (P = 0.01). This study expanded the pharmacists’ scope of practice, showing evidence for enhancing pharmacist roles on the hypertension care team. Tsuyuki et al also conducted the RxEACH randomized trial, which evaluated community pharmacist cardiovascular risk reduction interventions and showed an improvement in SBP and DBP, with reported results comparable to RxACTION.46
Victor et al conducted the landmark Black Barbershop Study, a cluster RCT involving 319 non-Hispanic black male patients with hypertension from 52 black-owned barbershops.47,48 Barbershops were assigned to 1 of 2 groups. The control group consisted of barbers who encouraged lifestyle modifications and made referrals to primary care providers. The intervention group had pharmacists who met regularly with participants at the barbershops and measured blood pressure, encouraged lifestyle changes, and prescribed drug therapy under collaborative practice agreements with physicians. Both groups demonstrated improvements in blood pressure outcomes, but the intervention group showed greater improvement in SBP and achievement of blood pressure goals compared to the control group. The results in the intervention group proved sustainable over the course of a year, even after the frequency of pharmacists’ visits was reduced. At 6 months, the mean SBP fell by 27.0 mm Hg (to 125.8 mm Hg) in the intervention group, as compared to a 9.3 mm Hg (to 145.4 mm Hg) reduction in the control group (P < 0.001), and blood pressure less than 130/80 mm Hg was achieved among 63.6% of the participants in the intervention group versus 11.7% in the control group (P < 0.001).
This community-level trial brought pharmacists to the barbershop and made them an essential part of the health care team through the endorsement of the barber, who the participants trusted and with whom they had a relationship. Long-standing issues related to distrust of the medical profession by this population were addressed, and trusted community barbershops were utilized as safe spaces for health care delivery. Health care professionals should consider utilizing community locations that other minority populations perceive as social centers and safe places, to reduce health disparities and barriers to care. However, models that bring care to patients need further economic and feasibility evaluations.
Other Health Care Professionals and Future Studies
In addition to models led by nurses and pharmacists, studies have also assessed models of TBC incorporating other health care professionals, including registered dietitians, medical assistants, community health workers, and health coaches (NCT02674464).49,50 Ongoing studies are also looking at the impact of TBC on underserved communities (NCT02674464, NCT03504124). Involving a variety of health care professionals with different communities and populations in TBC studies is warranted to determine the optimal settings in which to utilize different skill sets.
The Impress Study involves nurses who are assessing lifestyle risk and developing an action plan according to a standardized procedure, which may be advantageous given the degree of heterogeneity found in other TBC models.51 There are also studies underway or recently published that compare different components of TBC in order to determine which combination of TBC elements is preferred. Some of these have shown the benefits of using clinical decision-support systems (through a guideline-based treatment protocol) or training programs with ongoing support.52,53 Continued research comparing different TBC models is needed to determine which combination of health professionals and interventions is most impactful in practice.
Cost-Effectiveness
According to the CDC, TBC in hypertension management has proven to be cost-effective.54 Systematic reviews and meta-analyses assessing the cost-effectiveness of TBC in hypertension management have been conducted.26,27,29,55-58 While the general consensus supports this approach as being cost-effective, these determinations are based on studies that are widely heterogeneous. In each of these studies, different types of costs are taken into account when determining cost-effectiveness. The range of costs can be quite wide, depending on how they are calculated, making it difficult to determine the true cost-effectiveness of different TBC models.
Intervention cost is represented by the amount of money spent to implement and maintain the intervention beyond the cost of usual care or the cost without the intervention. For TBC, intervention cost consists of personnel resources such as provider time, patient time, and non-personnel resources, including rent and utilities. Studies show that intervention costs for TBC can range from $35 to $1350 per person per year (mean, $618; median, $428).27,56 One analysis, based on 20 studies comparing TBC to usual care, calculated an intervention cost of $284 per person per year,55 while another study showed an intervention cost of $525 per enrollee per year.56 Intervention cost can vary by the type of provider that is used, the amount of time spent per patient, and the setting where services are provided. Overall, the intervention cost of implementing TBC for hypertension management is consistently higher than the cost of usual care.
Health care cost is another factor to consider. It is the difference in the cost of health care products and services that are utilized in the process of TBC, as compared to care that is provided in the absence of TBC. Health care costs include the costs associated with hospitalizations, outpatient visits, emergency room visits, and medications. One study estimated a median health care cost of hypertension TBC of $65 per person per year.55 Overall, studies evaluating the impact of TBC for hypertension management on health care costs were mixed, with some showing that TBC resulted in an increase in health care cost, and others showing a savings compared to usual care.58 The variability in health care costs was due to the different number of health care components and comorbidities of the patients included in the studies. Also, study duration affected the estimated health care costs of TBC. Most studies did not assess long-term health care cost savings that could be achieved from prolonged blood pressure control.58 When considering both intervention and health care cost, Jacob et al estimated that TBC increased overall net cost by a median value of $329 per person per year.55 While some studies did attribute an overall reduction in health care costs to TBC for hypertension management, on average, team-based models increased health care costs compared to usual care.27,29,55,58,59
However, health care costs do not take into account the long-term reductions in morbidity and mortality or increased quality-adjusted life years (QALY) that result from improved blood pressure control attributed to TBC. In most cost-effectiveness studies, an intervention is considered to be cost-effective if the cost per QALY gained is less than the accepted threshold of $50,000.55 One study estimated that the cost per QALY of TBC in hypertension management is $4763,55,60 while another study estimated a median cost per QALY of $9716 to $13,992.55 A systematic review of 34 international studies estimated the median cost per QALY to be $13,986, ranging from $6683 to $58,610.57 The wide range in cost can be attributed to the variability in interventions, health outcomes used to measure effectiveness, and the settings and countries where the studies were conducted. In another study, a TBC intervention involving pharmacists resulted in a cost per QALY of $26,800.61 The intervention was found to be cost-effective for higher-risk patients, defined as those having diabetes, a smoking history, dyslipidemia, or obesity. For patients who did not have these risk factors, the cost per QALY increased to $43,330.61 Thus, the patient population should be considered before implementing a TBC model. Furthermore, the increased use of technology, allowing for more efficient provision of services and communication between providers, could reduce intervention costs and lead to increased cost efficacy in these models.
The variation in the models used for TBC makes it difficult to draw conclusions on the cost-effectiveness of these interventions. Although it is apparent that TBC in general is cost-effective, more studies are needed comparing different team-based models to determine which specific ones are most cost-effective.
Challenges to Implementation of Team-Based Care
Recognizing and addressing the challenges inherent to a TBC approach is important to the sustainability of such a model within various settings and institutions. Numerous studies conducted on team-based models have identified common challenges that appear to be consistent across multiple settings. These challenges can be categorized as financial, provider-specific, and technology.
Financial Barriers
Although studies have demonstrated the cost-effectiveness of controlling hypertension and preventing serious complications, health systems are still confronted with the challenge of covering the cost for TBC implementation and maintenance.29 The 2 main financial barriers for TBC services are stakeholder engagement and reimbursement for services. According to Kennelty et al, stakeholder engagement is key to the sustainability of the service.27 However, decisions by stakeholders on cost are influenced by many factors, which include available funds, perceived value, and estimates for return on investment. Additionally, interventions must align with the organization’s mission and vision and be feasible to implement, and organizations must have the capacity for administrative support.29 These various financial decisions may greatly influence the sustainability of a TBC model.
The reimbursement challenges for individual providers are an additional barrier to the sustainability of the service. In the United States, most providers are reimbursed via fee-for-service payment plans, but these plans do not reimburse all clinical providers because they are not all recognized as licensed providers.62,63 For example, pharmacists are not recognized by the Centers for Medicare & Medicaid Services as licensed health care providers, which limits their ability to be reimbursed for clinical services provided outside of a traditional dispensing role. Furthermore, state laws determine the services nonphysician providers can offer and how they are recognized for reimbursement by tertiary payers. For instance, pharmacist roles, such as ordering labs and modifying or prescribing medication regimens, vary greatly between states.7,63,64
Financial barriers are a major challenge facing the sustainability of a TBC hypertension service, so including all stakeholders in the decision-making process may improve the organization’s ability to sustain the service.
Provider-Specific Barriers
Notable barriers that are attributed to providers include lack of knowledge, lack of time, lack of initiative to change blood pressure medications, and inability to reach intensive blood pressure goals set in guidelines.29 Studies such as the SPRINT trial have significantly impacted clinical guideline cut-offs for blood pressure, but reaching the intensive blood pressure goals from clinical trials is difficult to emulate in clinical practice.65 In a typical clinical setting, providers may lack the confidence to make adjustments in therapy based on a single blood pressure measurement, and clinical inertia, defined as failure of health care providers to modify therapy when indicated,66 may contribute to the inability to achieve blood pressure goals. Many factors contribute to clinical inertia, including lack of knowledge, time, or clinical protocols on how to modify therapy, causing providers to delay clinical decisions. Implementing site-specific protocols and utilizing hypertension specialist health care professionals in TBC can address the barriers contributing to clinical inertia.
Technology Barriers
A common barrier in a variety of services, but especially prevalent in a TBC service, is access to an electronic health record (EHR) for all providers treating the patient. Some providers who are not directly tied to the same clinical site as the patient’s primary care provider may not have adequate access to the full EHR. For example, pharmacists who are managing hypertension in a TBC model in a community pharmacy may have access only to health information from prescription records. Patient interviews may not provide the pharmacist with adequate information about laboratory results, vitals, and other medical information and history for the patient, making it difficult for the pharmacist to make a proper recommendation for treatment.27 Depending on the setting, communication between providers may be a barrier in achieving optimal outcomes, especially when providers do not have access to a shared medical record.
In addition, patients often lack access to technology used to manage hypertension. Many new technologies exist that aid patients in managing their blood pressure, such as smart phone applications to track blood pressure readings and alarms to remind patients to take their medications. Studies have shown that telemonitoring of blood pressure measurements and management of hypertension, especially in combination with TBC, is effective and reduces costs compared to usual care.67 However, the lack of equal access to the various technologies available may inhibit the success of a TBC hypertension program. Patients may lack access, knowledge, or financial means to utilize the various methods available for managing their hypertension electronically.29
Conclusion
Incorporating nonphysician providers into the health care team for the treatment of hypertension has proven to be more effective than usual care and has been recognized by recent guidelines as a best practice approach to achieving blood pressure goals. Multiple studies have demonstrated that TBC utilizing nurses and pharmacists can improve blood pressure management. While adding members to the team increases health care costs, the long-term benefits of achieving optimal blood pressure goals contribute to the overall cost-effectiveness of TBC strategies over usual care. However, comparisons between different TBC models are warranted to determine which combination of health care professionals and/or interventions is most effective. Cost-analysis estimates are difficult to compare due to widely varied methodology and variance in the models that have been employed. Studies must consider pathways to overcoming reimbursement issues, provider-specific challenges, and technology barriers. Follow-up and monitoring after initiation of drug therapy for hypertension control should include systematic strategies to help improve blood pressure, including use of home blood pressure monitoring, TBC, and telehealth strategies. Future implementation science approaches to hypertension TBC models within specific clinic settings will be useful to identify and overcome challenges and will help to determine the populations who will benefit most, allowing for greater success in sustaining TBC models.
Corresponding author: Shawn R. Smith, PharmD, 309 E. 2nd Street, Pomona, CA 91766; [email protected].
Financial disclosures: None.
1. Fryar CD, Ostchega Y, Hales CM, et al. Hypertension prevalence and control among adults: United States, 2015–2016. NCHS Data Brief. 2017(289):1-8.
2. Ambrosius WT, Sink KM, Foy CG, et al. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: The Systolic Blood Pressure Intervention Trial (SPRINT). Clin Trials. 2014;11:532-546.
3. Lawes CM, Bennett DA, Feigin VL, Rodgers A. Blood pressure and stroke: an overview of published reviews. Stroke. 2017;35:776-785.
4. Zanchetti A, Thomopoulos C, Parati G. Randomized controlled trials of blood pressure lowering in hypertension: A critical reappraisal. Circ Res. 2015;116:1058-1073.
5. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/ AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127-e248.
6. Grol R. Successes and failures in the implementation of evidence-based guidelines for clinical practice. Med Care. 2001;39:II46-II54.
7. Brush JE, Handberg EM, Biga C, et al. 2015 ACC health policy statement on cardiovascular team-based care and the role of advanced practice providers. J Am Coll Cardiol. 2015;65:2118-2136.
8. Centers for Disease Control and Prevention. Best practices for cardiovascular disease prevention programs: a guide to effective health care system interventions and community programs linked to clinical services, promoting team-based care to improve high blood pressure control. www.cdc.gov/dhdsp/pubs/guides/best-practices/team-based-care.htm. Accessed April 30, 2020.
9. Centers for Disease Control and Prevention. Task Force recommends team-based care for improving blood pressure [press release]. May 15, 2012. www.cdc.gov/media/releases/2012/p0515_bp_control.html
10. Mitchell P, Wynia M, Golden R, et al. Core principles & values of effective team-based health care. 2012. Institute of Medicine, Washington, DC.
11. Campbell SM, Hann M, Hacker J, et al. Identifying predictors of high-quality care in English general practice: observational study. BMJ. 2001;323:784-787.
12. Shojania KG, Ranji SR, McDonald KM, et al. Effects of quality improvement strategies for type 2 diabetes on glycemic control: a meta-regression analysis. JAMA. 2006;296:427-440.
13. Walsh JM, McDonald KM, Shojania KG, et al. Quality improvement strategies for hypertension management: a systematic review. Med Care. 2006;44:646-657.
14. Wagner E. The role of patient care teams in chronic disease management. BMJ. 2000;320:560-572.
15. Coleman K, Reid R. Continuous and team-based healing relationships: improving patient care through teams. In: Phillips KE, Weir V, eds. Safety Net Medical Home Initiative Implementation Guide Series. 2nd ed. Seattle, WA: Qualis Health and The MacColl Center for Health Care Innovation at the Group Health Research Institute; 2013.
16. Sinsky CA, Willard-Grace R, Schutzbank AM, et al. In search of joy in practice: a report of 23 high-functioning primary care practices. Ann Fam Med. 2013;11:272-278
17. Howard J, Etz RS, Crocker JB, et al. Maximizing the patient-centered medical home (PCMH) by choosing words wisely. J Am Board Fam Med. 2016;29:248-253.
18. Solberg LI, Crain AL, Tillema JO, et al. Challenges of medical home transformation reported by 118 patient-centered medical home (PCMH) leaders. J Am Board Fam Med. 2014;27:449-457.
19. Crabtree BF, Chase SM, Wise CG, et al. Evaluation of patient centered medical home practice transformation initiatives. Med Care. 2011;49:10-16.
20. Carter BL. Blood pressure control—implementing a team approach. US Cardiol. 2011;8:108-113.
21. Carter BL, Rogers M, Daly J, et al. The potency of team-based care interventions for hypertension: a meta-analysis. Arch Intern Med. 2009;169:1748-1755.
22. Proia KK, Thota AB, Njie GJ, et al. Team-based care and improved blood pressure control: a community guide systematic review. Am J Prev Med. 2014;47:86-99.
23. Santschi V, Chiolero A, Colosimo AL, et al. Improving blood pressure control through pharmacist interventions: a meta-analysis of randomized controlled trials. J Am Heart Assoc. 2014;3:e000718.
24. Shaw RJ, McDuffie JR, Hendrix CC, et al. Effects of nurse-managed protocols in the outpatient management of adults with chronic conditions: a systematic review and meta-analysis. Ann Intern Med. 2014;161:113-121.
25. Carter BL, Coffey CS, Ardery G, et al. Cluster-randomized trial of a physician/pharmacist collaborative model to improve blood pressure control. Circ Cardiovasc Qual Outcomes. 2015;8:235-243
26. Carter BL, Bosworth HB, Green BB. The hypertension team: the role of the pharmacist, nurse and teamwork in hypertension therapy. J Clin Hypertens. 2012;14:51-65.
27. Kennelty KA, Polgreen LA, Carter BL. Team-based care with pharmacists to improve blood pressure: a review of recent literature. Curr Hypertens Rep. 2018;20:1.
28. Brownstein JN, Chowdhury FM, Norris SL, et al. Effectiveness of community health workers in the care of people with hypertension. Am J Prev Med. 2007;32:435-447.
29. Derington CG, King JB, Bryant KB, et al. Cost-effectiveness and challenges of implementing intensive blood pressure goals and team-based care. Curr Hypertens Rep. 2019;21:91.
30. Mills KT, Obst KM, Shen W, et al. Comparative effectiveness of implementation strategies for blood pressure control in hypertensive patients: a systematic review and meta-analysis. Ann Intern Med. 2018;168:110-120.
31. Clark CE, Smith LFP, Taylor RS, et al. Nurse led interventions to improve control of blood pressure in people with hypertension: systematic review and meta-analysis. BMJ. 2010;341:c3995.
32. Santschi V, Chiolero A, Burnand B, et al. Impact of pharmacist care in the management of cardiovascular disease risk factors: a systematic review and meta-analysis of randomized trials. Arch Intern Med. 2011;171:1441-1453.
33. Dennison Himmelfarb CR, Commodore-Mensah Y, Hill MN. Expanding the role of nurses to improve hypertension care and control globally. Ann Glob Health. 2016;82:243-253.
34. Clark CE, Smith LFP, Taylor RS, Campbell JL. Nurse-led interventions used to improve control of high blood pressure in people with diabetes: a systematic review and meta-analysis. DiabetMed. 2011;28:250-261.
35. Zhu X, Wong FKY, Wu CLH. Development and evaluation of a nurse-led hypertension management model: A randomized controlled trial. Int J Nurs Stud. 2018;77:171-178.
36. Spruill TM, Williams O, Teresi JA, et al. Comparative effectiveness of home blood pressure telemonitoring (HBPTM) plus nurse case management versus HBPTM alone among Black and Hispanic stroke survivors: study protocol for a randomized controlled trial. Trials. 2015;16:97.
37. Ogedegbe G. Comparative effectiveness of home BP telemonitoring plus nurse case management (HBPTM+NCM) versus HBPTM alone on systolic BP (SBP) reduction among minority stroke survivors. International Stroke Conference 2020; February 19-21, 2020; Los Angeles, CA. Abstract LB19.
38. Dunn SP, Birtcher KK, Beavers CJ, et al. The role of the clinical pharmacist in the care of patients with cardiovascular disease. J Am Coll Cardiol. 2015;66:2129-2139.
39. Santschi V, Chiolero A, Paradis G et al. Pharmacist interventions to improve cardiovascular disease risk factors in diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabetes Care. 2012;35:2706-2717.
40. Anderegg MD, Gums TH, Uribe L, et al. Pharmacist intervention for blood pressure control in patients with diabetes and/or chronic kidney disease. Pharmacotherapy. 2018;38:309-318.
41. Anderegg MD, Gums TH, Uribe L et al. Physician-pharmacist collaborative management: narrowing the socioeconomic blood pressure gap. Hypertension. 2016;68:1314-1320.
42. Sisson EM, Dixon DL, Kildow DC, et al. Effectiveness of a pharmacist-physician team-based collaboration to improve long-term blood pressure control at an inner-city safety-net clinic. Pharmacotherapy. 2016;36:342-347.
43. Dixon DL, Sisson EM, Parod ED, et al. Pharmacist-physician collaborative care model and time to goal blood pressure in the uninsured population. J Clin Hypertens (Greenwich). 2018;20:88-95.
44. Dixon DL, Parod ED, Sisson EM et al. Impact of a pharmacist-physician collaborative care model on time-in-therapeutic blood pressure range in patients with hypertension. J Am Coll Clin Pharm. 2020;3:404-409.
45. Tsuyuki RT, Houle SK, Charrois TL, et al. Randomized trial of the effect of pharmacist prescribing on improving blood pressure in the community: the Alberta Clinical Trial in Optimizing Hypertension (RxACTION). Circulation. 2015;132:93-100.
46. Tsuyuki RT, Al Hamarneh YN, Jones CA, et al. The effectiveness of pharmacist interventions on cardiovascular risk: The Multicenter Randomized Controlled RxEACH trial. J Am Coll Cardiol. 2016;67:2846-2854.
47. Victor RG, Lynch K, Li N, et al. A cluster-randomized trial of blood-pressure reduction in black barbershops. N Engl J Med. 2018;378:1291-1301.
48. Victor RG, Blyler CA, Li N et al. Sustainability of blood pressure reduction in black barbershops. Circulation. 2019;139:10-19.
49. Panattoni L, Hurlimann L, Wilson C, et al. Workflow standardization of a novel team care model to improve chronic care: a quasi-experimental study. BMC Health Serv Res. 2017;17:286.
50. Chang AR, Bonaparte H, Yule C. Randomized controlled trial comparing a self-guided vs. dietitian-led approach using web-based tools to lower blood pressure: study design and rationale. International Stroke Conference 2020; February 19-21, 2020; Los Angeles, CA. Abstract P169.
51. Stephen C, Halcomb E, Mcinnes S, et al. Improving blood pressure control in primary care: The ImPress study. Int J Nurs Stud. 2019;95:28-33.
52. He J, Shi X, Lin M. Comparative effectiveness of implementation strategies on cardiovascular risk factor control in patients with diabetes: The D4C cluster randomized trial. International Stroke Conference 2020; February 19-21, 2020; Los Angeles, CA. Abstract 17.
53. Jafar TH, Gandhi M, de Silva HA, et al. A community-based intervention for managing hypertension in rural South Asia. N Engl J Med. 2020;382:717-726.
54. Centers for Disease Control and Prevention. Promoting team-based care to improve high blood pressure control. www.cdc.gov/dhdsp/pubs/guides/best-practices/team-based-care.htm. Accessed April 30, 2020.
55. Jacob V, Chattopadhyay SK, Thota AB, et al. Economics of team-based care in controlling blood pressure: a community guide systematic review. Am J Prev Med. 2015;49:772-783.
56. Dehmer SP, Baker-Goering MM, Maciosek MV, et al. Modeled health and economic impact of team-based care for hypertension. Am J Prev Med. 2016;50(5 suppl 1):S34-S44.
57. Zhang D, Wang G, Joo H. A systematic review of economic evidence on community hypertension interventions. Am J Prev Med. 2017;53:S121-S130.
58. Community Preventive Services Task Force. Cardiovascular disease: team-based care to improve blood pressure control. 2011. www.thecommunityguide.org/findings/cardiovascular-disease-team-based-care-improve-blood-pressure-control. Accessed April 30, 2020.
59. Kulchaitanaroaj P, Brooks JM, Ardery G et al. Incremental costs associated with physician and pharmacist collaboration to improve blood pressure control. Pharmacotherapy. 2012;32:772-780.
60. Mason JM, Freemantle N, Gibson JM, New JP. Specialist nurse-led clinics to improve control of hypertension and hyperlipidemia in diabetes. Diabetes Care. 2005;28:40-46.
61. Kulchaitanaroaj P, Brooks JM, Chaiyakunapruk N et al. Cost-utility analysis of physician-pharmacist collaborative intervention for treating hypertension compared with usual care. J Hypertens. 2017;35:178-187.
62. Lall D, Engel N, Devadasan N, et al. Models of care for chronic conditions in low/middle-income countries: a ‘best fit’ framework synthesis. BMJ Glob Health. 2018;3:e001077.
63. Bodenheimer T, Chen E, Bennett HD. Confronting the growing burden of chronic disease: can the U.S. health care workforce do the job? Health Aff (Millwood). 2009;28:64-74.
64. Smith M, Bates DW, Bodenheimer T, Cleary PD. Why pharmacists belong in the medical home. Health Aff (Millwood). 2010;29:906-913.
65. Wright JT, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
66. Phillips LS, Branch WT, Cook CB, et al. Clinical inertia. Ann Intern Med. 2001;135:825-834.
67. McManus RJ, Mant J, Franssen M, et al. Efficacy of self-monitored blood pressure, with or without telemonitoring, for titration of antihypertensive medication (TASMINH4): an unmasked randomised controlled trial. Lancet. 2018;391:949-959.
68. Tucker KL, Sheppard JP, Stevens R, et al. Self-monitoring of blood pressure in hypertension: a systematic review and individual patient data meta-analysis. PLoS Med. 2017;14:e1002389.
69. Casey DE, Thomas RJ, Bhalla V, et al. 2019 AHA/ACC clinical performance and quality measures for adults with high blood pressure: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures. J Am Coll Cardiol. 2019;74:2661-2706.
From Western University of Health Sciences College of Pharmacy, Department of Pharmacy Practice and Administration, Pomona, CA.
Abstract
- Objective: To review the current literature regarding the clinical effectiveness and cost-effectiveness of implementing hypertension team-based care (TBC) interventions in the outpatient setting, and discuss challenges to implementation.
- Methods: A literature review was conducted of meta-analyses, systematic reviews, and randomized controlled trials comparing TBC models to usual care for hypertension management.
- Results: Compared to usual care, TBC models have demonstrated greater blood pressure reductions and improved blood pressure control rates. Evidence was strongest for models involving nurses and pharmacists whose roles included medication management, patient education and counseling, coordination of care and follow-up, population health management, and performance measurement with quality improvement. Although TBC results in an increase in health care costs, the overall long-term benefits support the cost-effectiveness of these models over usual care. The most common barriers to TBC implementation include underutilization of technology, stakeholder engagement, and reimbursement issues.
- Conclusion: Hypertension TBC models have been shown to be clinically effective and cost-effective, but continued research comparing different models is warranted to determine which combination of health professionals and interventions is most impactful and cost-effective in practice. An implementation science approach, in which TBC models unique to each organization’s situation are created, will be useful to identify and overcome challenges and provide a solid foundation for sustainment.
Keywords: blood pressure; pharmacist; nurse; nurse practitioner; cost-effectiveness; team-based care.
Approximately 1 in 3 US adults—or about 100 million people—have high blood pressure, and only about half (48%) have their blood pressure under control.1 Effective blood pressure management has been shown to decrease the incidence of stroke, heart attack, and heart failure.2-4 The American College of Cardiology/American Heart Association (ACC/AHA) 2017 blood pressure guidelines recommended lower thresholds for diagnosing hypertension and initiating antihypertensive medication, and intensified the blood pressure goal to less than 130/80 mm Hg.5 Changing practice standards to more intensive blood pressure goals requires significant adjustments by clinicians and health care systems. In fact, new guideline uptake is often delayed, ignored, or sparsely applied.6 Due to this dramatic change in hypertension practice standards, the ACC/AHA guidelines support interdisciplinary team-based care (TBC) for hypertension management.5,7 Additionally, the Centers for Disease Control and Prevention (CDC) and the Community Preventive Services Task Force (CPSTF) promote TBC to improve blood pressure control in their initiatives to prevent heart disease and stroke.8,9
The National Academy of Medicine defines TBC as “the provision of health services to individuals, families, and/or their communities by at least 2 healthcare providers who work collaboratively with patients and their caregivers—to the extent preferred by each patient—to accomplish shared goals within and across settings to achieve coordinated, high-quality care.”10 Specific goals for TBC in hypertension treatment are listed in Table 1, and a checklist of key elements of TBC to consider before implementation are presented in Table 2.

TBC has been shown to have many advantages, including increased access to care due to expanded hours of operation and shorter wait times.11 Team-based models also provide effective and efficient delivery of patient education, behavioral health care, and care coordination.12-14 Patients are more likely to receive high-quality care when multiple providers, each with varied expertise, are on the health care team.11,15 Furthermore, clinicians report improved professional job satisfaction related to their ability to practice in environments where they are encouraged to work at the top of their licenses.16 Consequently, TBC has been accepted as a vital part of the patient-centered medical home (PCMH) model.17-19 Standards set by the National Committee for Quality Assurance (NCQA) include TBC as a requirement health systems must meet in order to achieve the highest level of PCMH recognition. While a team-based approach offers substantial benefits and is recognized as a marker of quality, implementation has presented various challenges, and the sustainability of these models in care settings has been questioned.20

In this article, we review the current literature regarding the clinical effectiveness and cost-effectiveness of implementing hypertension TBC interventions in the outpatient setting. We also discuss the challenges and opportunities of implementing this strategy in health systems and community settings in the United States.
Evidence of Impact and Effectiveness
Various models of hypertension TBC have been shown to increase the proportion of individuals with controlled blood pressure and to lead to a reduction in both systolic (SBP) and diastolic blood pressure (DBP), resulting in a strong recommendation for TBC approaches by the 2017 ACC/AHA blood pressure guidelines.5,21-25 There is great diversity in the types of hypertension treatment models studied, with few utilizing physician specialists and most utilizing nonphysician providers, such as community health workers, physician assistants, nurses, nurse practitioners, dietitians, social workers, and pharmacists.22,26-29 These professionals share duties of hypertension management with primary care physicians to reduce the burden of responsibility for care on any single provider type. TBC is patient-centered, and typically includes interprofessional collaboration, treatment algorithms, adherence counseling, frequent follow-up, home blood pressure monitoring, and patient self-management education.
Numerous studies have supported implementation of TBC in recent years. A systematic review and meta-analysis of 100 trials of hypertension TBC involving 55,920 patients concluded that the most effective blood pressure–lowering strategies use multilevel, multicomponent approaches to address barriers to hypertension control. Nonphysician providers are often involved in measuring blood pressure, ordering and assessing laboratory tests, and titrating medications.30 Compared with usual care, TBC with physician medication titration resulted in reductions in mean SBP and DBP (6.2 mm Hg and 2.7 mm Hg, respectively), while TBC with nonphysician medication titration also resulted in reductions in mean SBP and DBP (7.1 mm Hg and 3.1 mm Hg, respectively). Nurses and pharmacists are specifically mentioned by the 2017 ACC/AHA blood pressure guidelines as essential members of the hypertension treatment team.5 Randomized controlled trials (RCTs) and meta-analyses of TBC involving nurse or pharmacist interventions demonstrated greater reductions in SBP and/or greater attainment of blood pressure goals compared to usual care.21,26,31,32 The literature supports the roles of nurses and pharmacists in hypertension management in all aspects of care, including medication management, patient education and counseling, coordination of care and follow-up, population health management, and performance measurement with quality improvement.33
Nurses
Nurses are commonly part of TBC hypertension management programs. One meta-analysis and systematic review of international RCTs compared nurse, nurse prescriber (United Kingdom), and nurse practitioner interventions for hypertension with usual care. Interventions that included a stepped treatment algorithm and nurse prescribing showed greater reductions in SBP (8.2 mm Hg and 8.9 mm Hg, respectively) compared to usual care.31 Similarly, models that utilized telephone monitoring demonstrated greater achievement of blood pressure targets, while those that involved home monitoring showed significant reductions in blood pressure. Another international meta-analysis and systematic review of 11 nurse-led interventions in hypertensive patients with diabetes demonstrated a 5.8 mm Hg mean decrease in SBP compared to physician-led care. However, nurse-led care was not superior in achievement of study targets.34
A recent meta-analysis and systematic review, performed by Shaw and colleagues, sought to determine whether nurse-led protocols are effective for outpatient management of adults with diabetes, hypertension, and hyperlipidemia. All of the included studies involved a registered nurse who titrated medications by following a protocol, and most were RCTs comparing the nurse protocols to usual care. Overall, mean SBP and DBP decreased by 3.86 mm Hg and 1.56 mm Hg, respectively, while blood glucose and lipid levels were also reduced compared to usual care.24
Limited RCT data have been published since the Shaw et al meta-analysis. A single-blind RCT was performed in an urban community health care center in China among patients with uncontrolled blood pressure (SBP ≥ 140 mm Hg and/or DBP ≥ 90 mm Hg).35 The study group received care via a nurse-led model, which included a delivery design system, decision support, clinical information system, and self-management support, and the control group received usual care. At 12 weeks, patients in the study group had significantly lower blood pressure than control patients, with mean SBP/DBP reduction of 14.37/7.43 mm Hg and 5.10/2.69 mm Hg, respectively (P < 0.01). Improved medication adherence and increased patient satisfaction were other benefits of the nurse-led model.
Nurse case managers (NCM) also play a critical role in hypertension management, coordinating health care services to meet patient health needs. Ogedegbe sought to evaluate the comparative effectiveness of home blood pressure telemonitoring (HBPTM)+NCM versus HBPTM alone on SBP reduction in black and Hispanic stroke survivors.36,37 NCMs evaluated patient profiles, counseled patients on target lifestyle behaviors, and reviewed home blood pressure data. At 6 months, SBP declined by 13.63 mm Hg from baseline in the HBPTM+NCM group and 6.31 mm Hg in the HBPTM alone group (P < 0.0001). At 12 months, SBP in the HBPTM+NCM group declined by 14.76 mm Hg, while blood pressure in the HBPTM alone group declined by 5.53 mm Hg (P < 0.0001).
Pharmacists
Clinical pharmacists are also widely utilized in TBC models for hypertension management. Typical models involve pharmacists entering into collaborative practice agreements with physicians, leading to optimization of medications, avoidance of adverse drug events, and transitional care activities focusing on medication reconciliation and patient education in outpatient settings.30,38 The largest and most recent meta-analysis of pharmacist interventions, conducted in 2014 by Santschi et al,23 combined 2 previous systematic reviews to include a total of 39 RCTs with 14,224 patients.32,39 Pharmacist interventions included patient education, recommendations to physicians, and medication management. Compared with usual care, pharmacist interventions showed greater reductions in SBP (7.6 mm Hg) and DBP (3.9 mm Hg).23
Numerous studies substantiating the impact of pharmacist interventions on clinical outcomes have heavily influenced clinical practice and guideline development. Carter et al conducted a prospective, multi-state, cluster-randomized trial in 32 primary care clinics to evaluate whether clinics randomized to receive the pharmacist-physician collaborative care model (PPCCM) achieved better blood pressure outcomes versus clinics randomized to usual care.25 Investigators enrolled 625 patients with uncontrolled hypertension, 50% of whom had a prior diagnosis of diabetes mellitus or chronic kidney disease. The primary outcome of blood pressure control at 9 months in the intervention clinics compared to the control clinics was 43% and 34%, respectively (P = 0.059). The difference in mean SBP/DBP between the intervention and control clinics for all patients at 9 months was −6.1/−2.9 mm Hg. In a post-hoc analysis of patients with chronic kidney disease and diabetes, the pharmacist-intervention group had a significantly greater mean SBP reduction and higher blood pressure control rates compared to usual care at 9 months.40
A pre-specified secondary analysis from the Carter et al study determined that, in patients from racial minority groups, the mean SBP was 7.3 mm Hg lower in those who received the intervention compared to those in the control group (P = 0.0042).41 In patients with less than 12 years of education, those in the intervention group had a mean SBP 8.1 mm Hg lower than the SBP of those in the control group (P = 0.0001). Similar reductions in blood pressure occurred in patients with low income, Medicaid beneficiaries, or those without insurance. This study demonstrated that pharmacist interventions reduced racial and socioeconomic disparities in blood pressure treatment.
Other studies of pharmacist interventions in underserved populations have yielded positive results. In a retrospective review of uninsured patients, blood pressure control rates in a pharmacist-driven primary care clinic ranked in the 90th percentile of NCQA benchmarks, and was superior to the 2013 reported mean for commercial insurers.42 Similarly, another retrospective cohort study of a PPCCM on time to goal blood pressure in uninsured patients with hypertension showed the median time to blood pressure goal was 36 days in the PPCCM cohort versus 259 days in usual care cohorts (P < 0.001).43 A post-hoc analysis revealed the mean time-in-therapeutic blood pressure range was 46.2% ± 24.3% in the PPCCM group and 24.8% ± 27.4% in the usual care group (P < 0.0001). The blood pressure control rates at 12 months were 89% in the PPCCM group compared with 50% in the usual care group (P < 0.0001).44
Tsuyuki et al conducted the RxACTION study, a multicenter RCT evaluating the effectiveness of enhanced pharmacist care versus usual care in 23 Canadian community pharmacies and outpatient clinics following a 6-month intervention.45 Enhanced pharmacy services included pharmacist assessment of and counseling about cardiovascular disease risk and blood pressure control, review of current antihypertensive medications, and prescribing/titrating drug therapy, as needed, through independent prescriptive authority. Compared to the usual care group (n = 67), the intervention group had a reduction in SBP of 6.6 mm Hg (P = 0.006) and in DBP of 3.2 mm Hg (P = 0.01). This study expanded the pharmacists’ scope of practice, showing evidence for enhancing pharmacist roles on the hypertension care team. Tsuyuki et al also conducted the RxEACH randomized trial, which evaluated community pharmacist cardiovascular risk reduction interventions and showed an improvement in SBP and DBP, with reported results comparable to RxACTION.46
Victor et al conducted the landmark Black Barbershop Study, a cluster RCT involving 319 non-Hispanic black male patients with hypertension from 52 black-owned barbershops.47,48 Barbershops were assigned to 1 of 2 groups. The control group consisted of barbers who encouraged lifestyle modifications and made referrals to primary care providers. The intervention group had pharmacists who met regularly with participants at the barbershops and measured blood pressure, encouraged lifestyle changes, and prescribed drug therapy under collaborative practice agreements with physicians. Both groups demonstrated improvements in blood pressure outcomes, but the intervention group showed greater improvement in SBP and achievement of blood pressure goals compared to the control group. The results in the intervention group proved sustainable over the course of a year, even after the frequency of pharmacists’ visits was reduced. At 6 months, the mean SBP fell by 27.0 mm Hg (to 125.8 mm Hg) in the intervention group, as compared to a 9.3 mm Hg (to 145.4 mm Hg) reduction in the control group (P < 0.001), and blood pressure less than 130/80 mm Hg was achieved among 63.6% of the participants in the intervention group versus 11.7% in the control group (P < 0.001).
This community-level trial brought pharmacists to the barbershop and made them an essential part of the health care team through the endorsement of the barber, who the participants trusted and with whom they had a relationship. Long-standing issues related to distrust of the medical profession by this population were addressed, and trusted community barbershops were utilized as safe spaces for health care delivery. Health care professionals should consider utilizing community locations that other minority populations perceive as social centers and safe places, to reduce health disparities and barriers to care. However, models that bring care to patients need further economic and feasibility evaluations.
Other Health Care Professionals and Future Studies
In addition to models led by nurses and pharmacists, studies have also assessed models of TBC incorporating other health care professionals, including registered dietitians, medical assistants, community health workers, and health coaches (NCT02674464).49,50 Ongoing studies are also looking at the impact of TBC on underserved communities (NCT02674464, NCT03504124). Involving a variety of health care professionals with different communities and populations in TBC studies is warranted to determine the optimal settings in which to utilize different skill sets.
The Impress Study involves nurses who are assessing lifestyle risk and developing an action plan according to a standardized procedure, which may be advantageous given the degree of heterogeneity found in other TBC models.51 There are also studies underway or recently published that compare different components of TBC in order to determine which combination of TBC elements is preferred. Some of these have shown the benefits of using clinical decision-support systems (through a guideline-based treatment protocol) or training programs with ongoing support.52,53 Continued research comparing different TBC models is needed to determine which combination of health professionals and interventions is most impactful in practice.
Cost-Effectiveness
According to the CDC, TBC in hypertension management has proven to be cost-effective.54 Systematic reviews and meta-analyses assessing the cost-effectiveness of TBC in hypertension management have been conducted.26,27,29,55-58 While the general consensus supports this approach as being cost-effective, these determinations are based on studies that are widely heterogeneous. In each of these studies, different types of costs are taken into account when determining cost-effectiveness. The range of costs can be quite wide, depending on how they are calculated, making it difficult to determine the true cost-effectiveness of different TBC models.
Intervention cost is represented by the amount of money spent to implement and maintain the intervention beyond the cost of usual care or the cost without the intervention. For TBC, intervention cost consists of personnel resources such as provider time, patient time, and non-personnel resources, including rent and utilities. Studies show that intervention costs for TBC can range from $35 to $1350 per person per year (mean, $618; median, $428).27,56 One analysis, based on 20 studies comparing TBC to usual care, calculated an intervention cost of $284 per person per year,55 while another study showed an intervention cost of $525 per enrollee per year.56 Intervention cost can vary by the type of provider that is used, the amount of time spent per patient, and the setting where services are provided. Overall, the intervention cost of implementing TBC for hypertension management is consistently higher than the cost of usual care.
Health care cost is another factor to consider. It is the difference in the cost of health care products and services that are utilized in the process of TBC, as compared to care that is provided in the absence of TBC. Health care costs include the costs associated with hospitalizations, outpatient visits, emergency room visits, and medications. One study estimated a median health care cost of hypertension TBC of $65 per person per year.55 Overall, studies evaluating the impact of TBC for hypertension management on health care costs were mixed, with some showing that TBC resulted in an increase in health care cost, and others showing a savings compared to usual care.58 The variability in health care costs was due to the different number of health care components and comorbidities of the patients included in the studies. Also, study duration affected the estimated health care costs of TBC. Most studies did not assess long-term health care cost savings that could be achieved from prolonged blood pressure control.58 When considering both intervention and health care cost, Jacob et al estimated that TBC increased overall net cost by a median value of $329 per person per year.55 While some studies did attribute an overall reduction in health care costs to TBC for hypertension management, on average, team-based models increased health care costs compared to usual care.27,29,55,58,59
However, health care costs do not take into account the long-term reductions in morbidity and mortality or increased quality-adjusted life years (QALY) that result from improved blood pressure control attributed to TBC. In most cost-effectiveness studies, an intervention is considered to be cost-effective if the cost per QALY gained is less than the accepted threshold of $50,000.55 One study estimated that the cost per QALY of TBC in hypertension management is $4763,55,60 while another study estimated a median cost per QALY of $9716 to $13,992.55 A systematic review of 34 international studies estimated the median cost per QALY to be $13,986, ranging from $6683 to $58,610.57 The wide range in cost can be attributed to the variability in interventions, health outcomes used to measure effectiveness, and the settings and countries where the studies were conducted. In another study, a TBC intervention involving pharmacists resulted in a cost per QALY of $26,800.61 The intervention was found to be cost-effective for higher-risk patients, defined as those having diabetes, a smoking history, dyslipidemia, or obesity. For patients who did not have these risk factors, the cost per QALY increased to $43,330.61 Thus, the patient population should be considered before implementing a TBC model. Furthermore, the increased use of technology, allowing for more efficient provision of services and communication between providers, could reduce intervention costs and lead to increased cost efficacy in these models.
The variation in the models used for TBC makes it difficult to draw conclusions on the cost-effectiveness of these interventions. Although it is apparent that TBC in general is cost-effective, more studies are needed comparing different team-based models to determine which specific ones are most cost-effective.
Challenges to Implementation of Team-Based Care
Recognizing and addressing the challenges inherent to a TBC approach is important to the sustainability of such a model within various settings and institutions. Numerous studies conducted on team-based models have identified common challenges that appear to be consistent across multiple settings. These challenges can be categorized as financial, provider-specific, and technology.
Financial Barriers
Although studies have demonstrated the cost-effectiveness of controlling hypertension and preventing serious complications, health systems are still confronted with the challenge of covering the cost for TBC implementation and maintenance.29 The 2 main financial barriers for TBC services are stakeholder engagement and reimbursement for services. According to Kennelty et al, stakeholder engagement is key to the sustainability of the service.27 However, decisions by stakeholders on cost are influenced by many factors, which include available funds, perceived value, and estimates for return on investment. Additionally, interventions must align with the organization’s mission and vision and be feasible to implement, and organizations must have the capacity for administrative support.29 These various financial decisions may greatly influence the sustainability of a TBC model.
The reimbursement challenges for individual providers are an additional barrier to the sustainability of the service. In the United States, most providers are reimbursed via fee-for-service payment plans, but these plans do not reimburse all clinical providers because they are not all recognized as licensed providers.62,63 For example, pharmacists are not recognized by the Centers for Medicare & Medicaid Services as licensed health care providers, which limits their ability to be reimbursed for clinical services provided outside of a traditional dispensing role. Furthermore, state laws determine the services nonphysician providers can offer and how they are recognized for reimbursement by tertiary payers. For instance, pharmacist roles, such as ordering labs and modifying or prescribing medication regimens, vary greatly between states.7,63,64
Financial barriers are a major challenge facing the sustainability of a TBC hypertension service, so including all stakeholders in the decision-making process may improve the organization’s ability to sustain the service.
Provider-Specific Barriers
Notable barriers that are attributed to providers include lack of knowledge, lack of time, lack of initiative to change blood pressure medications, and inability to reach intensive blood pressure goals set in guidelines.29 Studies such as the SPRINT trial have significantly impacted clinical guideline cut-offs for blood pressure, but reaching the intensive blood pressure goals from clinical trials is difficult to emulate in clinical practice.65 In a typical clinical setting, providers may lack the confidence to make adjustments in therapy based on a single blood pressure measurement, and clinical inertia, defined as failure of health care providers to modify therapy when indicated,66 may contribute to the inability to achieve blood pressure goals. Many factors contribute to clinical inertia, including lack of knowledge, time, or clinical protocols on how to modify therapy, causing providers to delay clinical decisions. Implementing site-specific protocols and utilizing hypertension specialist health care professionals in TBC can address the barriers contributing to clinical inertia.
Technology Barriers
A common barrier in a variety of services, but especially prevalent in a TBC service, is access to an electronic health record (EHR) for all providers treating the patient. Some providers who are not directly tied to the same clinical site as the patient’s primary care provider may not have adequate access to the full EHR. For example, pharmacists who are managing hypertension in a TBC model in a community pharmacy may have access only to health information from prescription records. Patient interviews may not provide the pharmacist with adequate information about laboratory results, vitals, and other medical information and history for the patient, making it difficult for the pharmacist to make a proper recommendation for treatment.27 Depending on the setting, communication between providers may be a barrier in achieving optimal outcomes, especially when providers do not have access to a shared medical record.
In addition, patients often lack access to technology used to manage hypertension. Many new technologies exist that aid patients in managing their blood pressure, such as smart phone applications to track blood pressure readings and alarms to remind patients to take their medications. Studies have shown that telemonitoring of blood pressure measurements and management of hypertension, especially in combination with TBC, is effective and reduces costs compared to usual care.67 However, the lack of equal access to the various technologies available may inhibit the success of a TBC hypertension program. Patients may lack access, knowledge, or financial means to utilize the various methods available for managing their hypertension electronically.29
Conclusion
Incorporating nonphysician providers into the health care team for the treatment of hypertension has proven to be more effective than usual care and has been recognized by recent guidelines as a best practice approach to achieving blood pressure goals. Multiple studies have demonstrated that TBC utilizing nurses and pharmacists can improve blood pressure management. While adding members to the team increases health care costs, the long-term benefits of achieving optimal blood pressure goals contribute to the overall cost-effectiveness of TBC strategies over usual care. However, comparisons between different TBC models are warranted to determine which combination of health care professionals and/or interventions is most effective. Cost-analysis estimates are difficult to compare due to widely varied methodology and variance in the models that have been employed. Studies must consider pathways to overcoming reimbursement issues, provider-specific challenges, and technology barriers. Follow-up and monitoring after initiation of drug therapy for hypertension control should include systematic strategies to help improve blood pressure, including use of home blood pressure monitoring, TBC, and telehealth strategies. Future implementation science approaches to hypertension TBC models within specific clinic settings will be useful to identify and overcome challenges and will help to determine the populations who will benefit most, allowing for greater success in sustaining TBC models.
Corresponding author: Shawn R. Smith, PharmD, 309 E. 2nd Street, Pomona, CA 91766; [email protected].
Financial disclosures: None.
From Western University of Health Sciences College of Pharmacy, Department of Pharmacy Practice and Administration, Pomona, CA.
Abstract
- Objective: To review the current literature regarding the clinical effectiveness and cost-effectiveness of implementing hypertension team-based care (TBC) interventions in the outpatient setting, and discuss challenges to implementation.
- Methods: A literature review was conducted of meta-analyses, systematic reviews, and randomized controlled trials comparing TBC models to usual care for hypertension management.
- Results: Compared to usual care, TBC models have demonstrated greater blood pressure reductions and improved blood pressure control rates. Evidence was strongest for models involving nurses and pharmacists whose roles included medication management, patient education and counseling, coordination of care and follow-up, population health management, and performance measurement with quality improvement. Although TBC results in an increase in health care costs, the overall long-term benefits support the cost-effectiveness of these models over usual care. The most common barriers to TBC implementation include underutilization of technology, stakeholder engagement, and reimbursement issues.
- Conclusion: Hypertension TBC models have been shown to be clinically effective and cost-effective, but continued research comparing different models is warranted to determine which combination of health professionals and interventions is most impactful and cost-effective in practice. An implementation science approach, in which TBC models unique to each organization’s situation are created, will be useful to identify and overcome challenges and provide a solid foundation for sustainment.
Keywords: blood pressure; pharmacist; nurse; nurse practitioner; cost-effectiveness; team-based care.
Approximately 1 in 3 US adults—or about 100 million people—have high blood pressure, and only about half (48%) have their blood pressure under control.1 Effective blood pressure management has been shown to decrease the incidence of stroke, heart attack, and heart failure.2-4 The American College of Cardiology/American Heart Association (ACC/AHA) 2017 blood pressure guidelines recommended lower thresholds for diagnosing hypertension and initiating antihypertensive medication, and intensified the blood pressure goal to less than 130/80 mm Hg.5 Changing practice standards to more intensive blood pressure goals requires significant adjustments by clinicians and health care systems. In fact, new guideline uptake is often delayed, ignored, or sparsely applied.6 Due to this dramatic change in hypertension practice standards, the ACC/AHA guidelines support interdisciplinary team-based care (TBC) for hypertension management.5,7 Additionally, the Centers for Disease Control and Prevention (CDC) and the Community Preventive Services Task Force (CPSTF) promote TBC to improve blood pressure control in their initiatives to prevent heart disease and stroke.8,9
The National Academy of Medicine defines TBC as “the provision of health services to individuals, families, and/or their communities by at least 2 healthcare providers who work collaboratively with patients and their caregivers—to the extent preferred by each patient—to accomplish shared goals within and across settings to achieve coordinated, high-quality care.”10 Specific goals for TBC in hypertension treatment are listed in Table 1, and a checklist of key elements of TBC to consider before implementation are presented in Table 2.

TBC has been shown to have many advantages, including increased access to care due to expanded hours of operation and shorter wait times.11 Team-based models also provide effective and efficient delivery of patient education, behavioral health care, and care coordination.12-14 Patients are more likely to receive high-quality care when multiple providers, each with varied expertise, are on the health care team.11,15 Furthermore, clinicians report improved professional job satisfaction related to their ability to practice in environments where they are encouraged to work at the top of their licenses.16 Consequently, TBC has been accepted as a vital part of the patient-centered medical home (PCMH) model.17-19 Standards set by the National Committee for Quality Assurance (NCQA) include TBC as a requirement health systems must meet in order to achieve the highest level of PCMH recognition. While a team-based approach offers substantial benefits and is recognized as a marker of quality, implementation has presented various challenges, and the sustainability of these models in care settings has been questioned.20

In this article, we review the current literature regarding the clinical effectiveness and cost-effectiveness of implementing hypertension TBC interventions in the outpatient setting. We also discuss the challenges and opportunities of implementing this strategy in health systems and community settings in the United States.
Evidence of Impact and Effectiveness
Various models of hypertension TBC have been shown to increase the proportion of individuals with controlled blood pressure and to lead to a reduction in both systolic (SBP) and diastolic blood pressure (DBP), resulting in a strong recommendation for TBC approaches by the 2017 ACC/AHA blood pressure guidelines.5,21-25 There is great diversity in the types of hypertension treatment models studied, with few utilizing physician specialists and most utilizing nonphysician providers, such as community health workers, physician assistants, nurses, nurse practitioners, dietitians, social workers, and pharmacists.22,26-29 These professionals share duties of hypertension management with primary care physicians to reduce the burden of responsibility for care on any single provider type. TBC is patient-centered, and typically includes interprofessional collaboration, treatment algorithms, adherence counseling, frequent follow-up, home blood pressure monitoring, and patient self-management education.
Numerous studies have supported implementation of TBC in recent years. A systematic review and meta-analysis of 100 trials of hypertension TBC involving 55,920 patients concluded that the most effective blood pressure–lowering strategies use multilevel, multicomponent approaches to address barriers to hypertension control. Nonphysician providers are often involved in measuring blood pressure, ordering and assessing laboratory tests, and titrating medications.30 Compared with usual care, TBC with physician medication titration resulted in reductions in mean SBP and DBP (6.2 mm Hg and 2.7 mm Hg, respectively), while TBC with nonphysician medication titration also resulted in reductions in mean SBP and DBP (7.1 mm Hg and 3.1 mm Hg, respectively). Nurses and pharmacists are specifically mentioned by the 2017 ACC/AHA blood pressure guidelines as essential members of the hypertension treatment team.5 Randomized controlled trials (RCTs) and meta-analyses of TBC involving nurse or pharmacist interventions demonstrated greater reductions in SBP and/or greater attainment of blood pressure goals compared to usual care.21,26,31,32 The literature supports the roles of nurses and pharmacists in hypertension management in all aspects of care, including medication management, patient education and counseling, coordination of care and follow-up, population health management, and performance measurement with quality improvement.33
Nurses
Nurses are commonly part of TBC hypertension management programs. One meta-analysis and systematic review of international RCTs compared nurse, nurse prescriber (United Kingdom), and nurse practitioner interventions for hypertension with usual care. Interventions that included a stepped treatment algorithm and nurse prescribing showed greater reductions in SBP (8.2 mm Hg and 8.9 mm Hg, respectively) compared to usual care.31 Similarly, models that utilized telephone monitoring demonstrated greater achievement of blood pressure targets, while those that involved home monitoring showed significant reductions in blood pressure. Another international meta-analysis and systematic review of 11 nurse-led interventions in hypertensive patients with diabetes demonstrated a 5.8 mm Hg mean decrease in SBP compared to physician-led care. However, nurse-led care was not superior in achievement of study targets.34
A recent meta-analysis and systematic review, performed by Shaw and colleagues, sought to determine whether nurse-led protocols are effective for outpatient management of adults with diabetes, hypertension, and hyperlipidemia. All of the included studies involved a registered nurse who titrated medications by following a protocol, and most were RCTs comparing the nurse protocols to usual care. Overall, mean SBP and DBP decreased by 3.86 mm Hg and 1.56 mm Hg, respectively, while blood glucose and lipid levels were also reduced compared to usual care.24
Limited RCT data have been published since the Shaw et al meta-analysis. A single-blind RCT was performed in an urban community health care center in China among patients with uncontrolled blood pressure (SBP ≥ 140 mm Hg and/or DBP ≥ 90 mm Hg).35 The study group received care via a nurse-led model, which included a delivery design system, decision support, clinical information system, and self-management support, and the control group received usual care. At 12 weeks, patients in the study group had significantly lower blood pressure than control patients, with mean SBP/DBP reduction of 14.37/7.43 mm Hg and 5.10/2.69 mm Hg, respectively (P < 0.01). Improved medication adherence and increased patient satisfaction were other benefits of the nurse-led model.
Nurse case managers (NCM) also play a critical role in hypertension management, coordinating health care services to meet patient health needs. Ogedegbe sought to evaluate the comparative effectiveness of home blood pressure telemonitoring (HBPTM)+NCM versus HBPTM alone on SBP reduction in black and Hispanic stroke survivors.36,37 NCMs evaluated patient profiles, counseled patients on target lifestyle behaviors, and reviewed home blood pressure data. At 6 months, SBP declined by 13.63 mm Hg from baseline in the HBPTM+NCM group and 6.31 mm Hg in the HBPTM alone group (P < 0.0001). At 12 months, SBP in the HBPTM+NCM group declined by 14.76 mm Hg, while blood pressure in the HBPTM alone group declined by 5.53 mm Hg (P < 0.0001).
Pharmacists
Clinical pharmacists are also widely utilized in TBC models for hypertension management. Typical models involve pharmacists entering into collaborative practice agreements with physicians, leading to optimization of medications, avoidance of adverse drug events, and transitional care activities focusing on medication reconciliation and patient education in outpatient settings.30,38 The largest and most recent meta-analysis of pharmacist interventions, conducted in 2014 by Santschi et al,23 combined 2 previous systematic reviews to include a total of 39 RCTs with 14,224 patients.32,39 Pharmacist interventions included patient education, recommendations to physicians, and medication management. Compared with usual care, pharmacist interventions showed greater reductions in SBP (7.6 mm Hg) and DBP (3.9 mm Hg).23
Numerous studies substantiating the impact of pharmacist interventions on clinical outcomes have heavily influenced clinical practice and guideline development. Carter et al conducted a prospective, multi-state, cluster-randomized trial in 32 primary care clinics to evaluate whether clinics randomized to receive the pharmacist-physician collaborative care model (PPCCM) achieved better blood pressure outcomes versus clinics randomized to usual care.25 Investigators enrolled 625 patients with uncontrolled hypertension, 50% of whom had a prior diagnosis of diabetes mellitus or chronic kidney disease. The primary outcome of blood pressure control at 9 months in the intervention clinics compared to the control clinics was 43% and 34%, respectively (P = 0.059). The difference in mean SBP/DBP between the intervention and control clinics for all patients at 9 months was −6.1/−2.9 mm Hg. In a post-hoc analysis of patients with chronic kidney disease and diabetes, the pharmacist-intervention group had a significantly greater mean SBP reduction and higher blood pressure control rates compared to usual care at 9 months.40
A pre-specified secondary analysis from the Carter et al study determined that, in patients from racial minority groups, the mean SBP was 7.3 mm Hg lower in those who received the intervention compared to those in the control group (P = 0.0042).41 In patients with less than 12 years of education, those in the intervention group had a mean SBP 8.1 mm Hg lower than the SBP of those in the control group (P = 0.0001). Similar reductions in blood pressure occurred in patients with low income, Medicaid beneficiaries, or those without insurance. This study demonstrated that pharmacist interventions reduced racial and socioeconomic disparities in blood pressure treatment.
Other studies of pharmacist interventions in underserved populations have yielded positive results. In a retrospective review of uninsured patients, blood pressure control rates in a pharmacist-driven primary care clinic ranked in the 90th percentile of NCQA benchmarks, and was superior to the 2013 reported mean for commercial insurers.42 Similarly, another retrospective cohort study of a PPCCM on time to goal blood pressure in uninsured patients with hypertension showed the median time to blood pressure goal was 36 days in the PPCCM cohort versus 259 days in usual care cohorts (P < 0.001).43 A post-hoc analysis revealed the mean time-in-therapeutic blood pressure range was 46.2% ± 24.3% in the PPCCM group and 24.8% ± 27.4% in the usual care group (P < 0.0001). The blood pressure control rates at 12 months were 89% in the PPCCM group compared with 50% in the usual care group (P < 0.0001).44
Tsuyuki et al conducted the RxACTION study, a multicenter RCT evaluating the effectiveness of enhanced pharmacist care versus usual care in 23 Canadian community pharmacies and outpatient clinics following a 6-month intervention.45 Enhanced pharmacy services included pharmacist assessment of and counseling about cardiovascular disease risk and blood pressure control, review of current antihypertensive medications, and prescribing/titrating drug therapy, as needed, through independent prescriptive authority. Compared to the usual care group (n = 67), the intervention group had a reduction in SBP of 6.6 mm Hg (P = 0.006) and in DBP of 3.2 mm Hg (P = 0.01). This study expanded the pharmacists’ scope of practice, showing evidence for enhancing pharmacist roles on the hypertension care team. Tsuyuki et al also conducted the RxEACH randomized trial, which evaluated community pharmacist cardiovascular risk reduction interventions and showed an improvement in SBP and DBP, with reported results comparable to RxACTION.46
Victor et al conducted the landmark Black Barbershop Study, a cluster RCT involving 319 non-Hispanic black male patients with hypertension from 52 black-owned barbershops.47,48 Barbershops were assigned to 1 of 2 groups. The control group consisted of barbers who encouraged lifestyle modifications and made referrals to primary care providers. The intervention group had pharmacists who met regularly with participants at the barbershops and measured blood pressure, encouraged lifestyle changes, and prescribed drug therapy under collaborative practice agreements with physicians. Both groups demonstrated improvements in blood pressure outcomes, but the intervention group showed greater improvement in SBP and achievement of blood pressure goals compared to the control group. The results in the intervention group proved sustainable over the course of a year, even after the frequency of pharmacists’ visits was reduced. At 6 months, the mean SBP fell by 27.0 mm Hg (to 125.8 mm Hg) in the intervention group, as compared to a 9.3 mm Hg (to 145.4 mm Hg) reduction in the control group (P < 0.001), and blood pressure less than 130/80 mm Hg was achieved among 63.6% of the participants in the intervention group versus 11.7% in the control group (P < 0.001).
This community-level trial brought pharmacists to the barbershop and made them an essential part of the health care team through the endorsement of the barber, who the participants trusted and with whom they had a relationship. Long-standing issues related to distrust of the medical profession by this population were addressed, and trusted community barbershops were utilized as safe spaces for health care delivery. Health care professionals should consider utilizing community locations that other minority populations perceive as social centers and safe places, to reduce health disparities and barriers to care. However, models that bring care to patients need further economic and feasibility evaluations.
Other Health Care Professionals and Future Studies
In addition to models led by nurses and pharmacists, studies have also assessed models of TBC incorporating other health care professionals, including registered dietitians, medical assistants, community health workers, and health coaches (NCT02674464).49,50 Ongoing studies are also looking at the impact of TBC on underserved communities (NCT02674464, NCT03504124). Involving a variety of health care professionals with different communities and populations in TBC studies is warranted to determine the optimal settings in which to utilize different skill sets.
The Impress Study involves nurses who are assessing lifestyle risk and developing an action plan according to a standardized procedure, which may be advantageous given the degree of heterogeneity found in other TBC models.51 There are also studies underway or recently published that compare different components of TBC in order to determine which combination of TBC elements is preferred. Some of these have shown the benefits of using clinical decision-support systems (through a guideline-based treatment protocol) or training programs with ongoing support.52,53 Continued research comparing different TBC models is needed to determine which combination of health professionals and interventions is most impactful in practice.
Cost-Effectiveness
According to the CDC, TBC in hypertension management has proven to be cost-effective.54 Systematic reviews and meta-analyses assessing the cost-effectiveness of TBC in hypertension management have been conducted.26,27,29,55-58 While the general consensus supports this approach as being cost-effective, these determinations are based on studies that are widely heterogeneous. In each of these studies, different types of costs are taken into account when determining cost-effectiveness. The range of costs can be quite wide, depending on how they are calculated, making it difficult to determine the true cost-effectiveness of different TBC models.
Intervention cost is represented by the amount of money spent to implement and maintain the intervention beyond the cost of usual care or the cost without the intervention. For TBC, intervention cost consists of personnel resources such as provider time, patient time, and non-personnel resources, including rent and utilities. Studies show that intervention costs for TBC can range from $35 to $1350 per person per year (mean, $618; median, $428).27,56 One analysis, based on 20 studies comparing TBC to usual care, calculated an intervention cost of $284 per person per year,55 while another study showed an intervention cost of $525 per enrollee per year.56 Intervention cost can vary by the type of provider that is used, the amount of time spent per patient, and the setting where services are provided. Overall, the intervention cost of implementing TBC for hypertension management is consistently higher than the cost of usual care.
Health care cost is another factor to consider. It is the difference in the cost of health care products and services that are utilized in the process of TBC, as compared to care that is provided in the absence of TBC. Health care costs include the costs associated with hospitalizations, outpatient visits, emergency room visits, and medications. One study estimated a median health care cost of hypertension TBC of $65 per person per year.55 Overall, studies evaluating the impact of TBC for hypertension management on health care costs were mixed, with some showing that TBC resulted in an increase in health care cost, and others showing a savings compared to usual care.58 The variability in health care costs was due to the different number of health care components and comorbidities of the patients included in the studies. Also, study duration affected the estimated health care costs of TBC. Most studies did not assess long-term health care cost savings that could be achieved from prolonged blood pressure control.58 When considering both intervention and health care cost, Jacob et al estimated that TBC increased overall net cost by a median value of $329 per person per year.55 While some studies did attribute an overall reduction in health care costs to TBC for hypertension management, on average, team-based models increased health care costs compared to usual care.27,29,55,58,59
However, health care costs do not take into account the long-term reductions in morbidity and mortality or increased quality-adjusted life years (QALY) that result from improved blood pressure control attributed to TBC. In most cost-effectiveness studies, an intervention is considered to be cost-effective if the cost per QALY gained is less than the accepted threshold of $50,000.55 One study estimated that the cost per QALY of TBC in hypertension management is $4763,55,60 while another study estimated a median cost per QALY of $9716 to $13,992.55 A systematic review of 34 international studies estimated the median cost per QALY to be $13,986, ranging from $6683 to $58,610.57 The wide range in cost can be attributed to the variability in interventions, health outcomes used to measure effectiveness, and the settings and countries where the studies were conducted. In another study, a TBC intervention involving pharmacists resulted in a cost per QALY of $26,800.61 The intervention was found to be cost-effective for higher-risk patients, defined as those having diabetes, a smoking history, dyslipidemia, or obesity. For patients who did not have these risk factors, the cost per QALY increased to $43,330.61 Thus, the patient population should be considered before implementing a TBC model. Furthermore, the increased use of technology, allowing for more efficient provision of services and communication between providers, could reduce intervention costs and lead to increased cost efficacy in these models.
The variation in the models used for TBC makes it difficult to draw conclusions on the cost-effectiveness of these interventions. Although it is apparent that TBC in general is cost-effective, more studies are needed comparing different team-based models to determine which specific ones are most cost-effective.
Challenges to Implementation of Team-Based Care
Recognizing and addressing the challenges inherent to a TBC approach is important to the sustainability of such a model within various settings and institutions. Numerous studies conducted on team-based models have identified common challenges that appear to be consistent across multiple settings. These challenges can be categorized as financial, provider-specific, and technology.
Financial Barriers
Although studies have demonstrated the cost-effectiveness of controlling hypertension and preventing serious complications, health systems are still confronted with the challenge of covering the cost for TBC implementation and maintenance.29 The 2 main financial barriers for TBC services are stakeholder engagement and reimbursement for services. According to Kennelty et al, stakeholder engagement is key to the sustainability of the service.27 However, decisions by stakeholders on cost are influenced by many factors, which include available funds, perceived value, and estimates for return on investment. Additionally, interventions must align with the organization’s mission and vision and be feasible to implement, and organizations must have the capacity for administrative support.29 These various financial decisions may greatly influence the sustainability of a TBC model.
The reimbursement challenges for individual providers are an additional barrier to the sustainability of the service. In the United States, most providers are reimbursed via fee-for-service payment plans, but these plans do not reimburse all clinical providers because they are not all recognized as licensed providers.62,63 For example, pharmacists are not recognized by the Centers for Medicare & Medicaid Services as licensed health care providers, which limits their ability to be reimbursed for clinical services provided outside of a traditional dispensing role. Furthermore, state laws determine the services nonphysician providers can offer and how they are recognized for reimbursement by tertiary payers. For instance, pharmacist roles, such as ordering labs and modifying or prescribing medication regimens, vary greatly between states.7,63,64
Financial barriers are a major challenge facing the sustainability of a TBC hypertension service, so including all stakeholders in the decision-making process may improve the organization’s ability to sustain the service.
Provider-Specific Barriers
Notable barriers that are attributed to providers include lack of knowledge, lack of time, lack of initiative to change blood pressure medications, and inability to reach intensive blood pressure goals set in guidelines.29 Studies such as the SPRINT trial have significantly impacted clinical guideline cut-offs for blood pressure, but reaching the intensive blood pressure goals from clinical trials is difficult to emulate in clinical practice.65 In a typical clinical setting, providers may lack the confidence to make adjustments in therapy based on a single blood pressure measurement, and clinical inertia, defined as failure of health care providers to modify therapy when indicated,66 may contribute to the inability to achieve blood pressure goals. Many factors contribute to clinical inertia, including lack of knowledge, time, or clinical protocols on how to modify therapy, causing providers to delay clinical decisions. Implementing site-specific protocols and utilizing hypertension specialist health care professionals in TBC can address the barriers contributing to clinical inertia.
Technology Barriers
A common barrier in a variety of services, but especially prevalent in a TBC service, is access to an electronic health record (EHR) for all providers treating the patient. Some providers who are not directly tied to the same clinical site as the patient’s primary care provider may not have adequate access to the full EHR. For example, pharmacists who are managing hypertension in a TBC model in a community pharmacy may have access only to health information from prescription records. Patient interviews may not provide the pharmacist with adequate information about laboratory results, vitals, and other medical information and history for the patient, making it difficult for the pharmacist to make a proper recommendation for treatment.27 Depending on the setting, communication between providers may be a barrier in achieving optimal outcomes, especially when providers do not have access to a shared medical record.
In addition, patients often lack access to technology used to manage hypertension. Many new technologies exist that aid patients in managing their blood pressure, such as smart phone applications to track blood pressure readings and alarms to remind patients to take their medications. Studies have shown that telemonitoring of blood pressure measurements and management of hypertension, especially in combination with TBC, is effective and reduces costs compared to usual care.67 However, the lack of equal access to the various technologies available may inhibit the success of a TBC hypertension program. Patients may lack access, knowledge, or financial means to utilize the various methods available for managing their hypertension electronically.29
Conclusion
Incorporating nonphysician providers into the health care team for the treatment of hypertension has proven to be more effective than usual care and has been recognized by recent guidelines as a best practice approach to achieving blood pressure goals. Multiple studies have demonstrated that TBC utilizing nurses and pharmacists can improve blood pressure management. While adding members to the team increases health care costs, the long-term benefits of achieving optimal blood pressure goals contribute to the overall cost-effectiveness of TBC strategies over usual care. However, comparisons between different TBC models are warranted to determine which combination of health care professionals and/or interventions is most effective. Cost-analysis estimates are difficult to compare due to widely varied methodology and variance in the models that have been employed. Studies must consider pathways to overcoming reimbursement issues, provider-specific challenges, and technology barriers. Follow-up and monitoring after initiation of drug therapy for hypertension control should include systematic strategies to help improve blood pressure, including use of home blood pressure monitoring, TBC, and telehealth strategies. Future implementation science approaches to hypertension TBC models within specific clinic settings will be useful to identify and overcome challenges and will help to determine the populations who will benefit most, allowing for greater success in sustaining TBC models.
Corresponding author: Shawn R. Smith, PharmD, 309 E. 2nd Street, Pomona, CA 91766; [email protected].
Financial disclosures: None.
1. Fryar CD, Ostchega Y, Hales CM, et al. Hypertension prevalence and control among adults: United States, 2015–2016. NCHS Data Brief. 2017(289):1-8.
2. Ambrosius WT, Sink KM, Foy CG, et al. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: The Systolic Blood Pressure Intervention Trial (SPRINT). Clin Trials. 2014;11:532-546.
3. Lawes CM, Bennett DA, Feigin VL, Rodgers A. Blood pressure and stroke: an overview of published reviews. Stroke. 2017;35:776-785.
4. Zanchetti A, Thomopoulos C, Parati G. Randomized controlled trials of blood pressure lowering in hypertension: A critical reappraisal. Circ Res. 2015;116:1058-1073.
5. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/ AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127-e248.
6. Grol R. Successes and failures in the implementation of evidence-based guidelines for clinical practice. Med Care. 2001;39:II46-II54.
7. Brush JE, Handberg EM, Biga C, et al. 2015 ACC health policy statement on cardiovascular team-based care and the role of advanced practice providers. J Am Coll Cardiol. 2015;65:2118-2136.
8. Centers for Disease Control and Prevention. Best practices for cardiovascular disease prevention programs: a guide to effective health care system interventions and community programs linked to clinical services, promoting team-based care to improve high blood pressure control. www.cdc.gov/dhdsp/pubs/guides/best-practices/team-based-care.htm. Accessed April 30, 2020.
9. Centers for Disease Control and Prevention. Task Force recommends team-based care for improving blood pressure [press release]. May 15, 2012. www.cdc.gov/media/releases/2012/p0515_bp_control.html
10. Mitchell P, Wynia M, Golden R, et al. Core principles & values of effective team-based health care. 2012. Institute of Medicine, Washington, DC.
11. Campbell SM, Hann M, Hacker J, et al. Identifying predictors of high-quality care in English general practice: observational study. BMJ. 2001;323:784-787.
12. Shojania KG, Ranji SR, McDonald KM, et al. Effects of quality improvement strategies for type 2 diabetes on glycemic control: a meta-regression analysis. JAMA. 2006;296:427-440.
13. Walsh JM, McDonald KM, Shojania KG, et al. Quality improvement strategies for hypertension management: a systematic review. Med Care. 2006;44:646-657.
14. Wagner E. The role of patient care teams in chronic disease management. BMJ. 2000;320:560-572.
15. Coleman K, Reid R. Continuous and team-based healing relationships: improving patient care through teams. In: Phillips KE, Weir V, eds. Safety Net Medical Home Initiative Implementation Guide Series. 2nd ed. Seattle, WA: Qualis Health and The MacColl Center for Health Care Innovation at the Group Health Research Institute; 2013.
16. Sinsky CA, Willard-Grace R, Schutzbank AM, et al. In search of joy in practice: a report of 23 high-functioning primary care practices. Ann Fam Med. 2013;11:272-278
17. Howard J, Etz RS, Crocker JB, et al. Maximizing the patient-centered medical home (PCMH) by choosing words wisely. J Am Board Fam Med. 2016;29:248-253.
18. Solberg LI, Crain AL, Tillema JO, et al. Challenges of medical home transformation reported by 118 patient-centered medical home (PCMH) leaders. J Am Board Fam Med. 2014;27:449-457.
19. Crabtree BF, Chase SM, Wise CG, et al. Evaluation of patient centered medical home practice transformation initiatives. Med Care. 2011;49:10-16.
20. Carter BL. Blood pressure control—implementing a team approach. US Cardiol. 2011;8:108-113.
21. Carter BL, Rogers M, Daly J, et al. The potency of team-based care interventions for hypertension: a meta-analysis. Arch Intern Med. 2009;169:1748-1755.
22. Proia KK, Thota AB, Njie GJ, et al. Team-based care and improved blood pressure control: a community guide systematic review. Am J Prev Med. 2014;47:86-99.
23. Santschi V, Chiolero A, Colosimo AL, et al. Improving blood pressure control through pharmacist interventions: a meta-analysis of randomized controlled trials. J Am Heart Assoc. 2014;3:e000718.
24. Shaw RJ, McDuffie JR, Hendrix CC, et al. Effects of nurse-managed protocols in the outpatient management of adults with chronic conditions: a systematic review and meta-analysis. Ann Intern Med. 2014;161:113-121.
25. Carter BL, Coffey CS, Ardery G, et al. Cluster-randomized trial of a physician/pharmacist collaborative model to improve blood pressure control. Circ Cardiovasc Qual Outcomes. 2015;8:235-243
26. Carter BL, Bosworth HB, Green BB. The hypertension team: the role of the pharmacist, nurse and teamwork in hypertension therapy. J Clin Hypertens. 2012;14:51-65.
27. Kennelty KA, Polgreen LA, Carter BL. Team-based care with pharmacists to improve blood pressure: a review of recent literature. Curr Hypertens Rep. 2018;20:1.
28. Brownstein JN, Chowdhury FM, Norris SL, et al. Effectiveness of community health workers in the care of people with hypertension. Am J Prev Med. 2007;32:435-447.
29. Derington CG, King JB, Bryant KB, et al. Cost-effectiveness and challenges of implementing intensive blood pressure goals and team-based care. Curr Hypertens Rep. 2019;21:91.
30. Mills KT, Obst KM, Shen W, et al. Comparative effectiveness of implementation strategies for blood pressure control in hypertensive patients: a systematic review and meta-analysis. Ann Intern Med. 2018;168:110-120.
31. Clark CE, Smith LFP, Taylor RS, et al. Nurse led interventions to improve control of blood pressure in people with hypertension: systematic review and meta-analysis. BMJ. 2010;341:c3995.
32. Santschi V, Chiolero A, Burnand B, et al. Impact of pharmacist care in the management of cardiovascular disease risk factors: a systematic review and meta-analysis of randomized trials. Arch Intern Med. 2011;171:1441-1453.
33. Dennison Himmelfarb CR, Commodore-Mensah Y, Hill MN. Expanding the role of nurses to improve hypertension care and control globally. Ann Glob Health. 2016;82:243-253.
34. Clark CE, Smith LFP, Taylor RS, Campbell JL. Nurse-led interventions used to improve control of high blood pressure in people with diabetes: a systematic review and meta-analysis. DiabetMed. 2011;28:250-261.
35. Zhu X, Wong FKY, Wu CLH. Development and evaluation of a nurse-led hypertension management model: A randomized controlled trial. Int J Nurs Stud. 2018;77:171-178.
36. Spruill TM, Williams O, Teresi JA, et al. Comparative effectiveness of home blood pressure telemonitoring (HBPTM) plus nurse case management versus HBPTM alone among Black and Hispanic stroke survivors: study protocol for a randomized controlled trial. Trials. 2015;16:97.
37. Ogedegbe G. Comparative effectiveness of home BP telemonitoring plus nurse case management (HBPTM+NCM) versus HBPTM alone on systolic BP (SBP) reduction among minority stroke survivors. International Stroke Conference 2020; February 19-21, 2020; Los Angeles, CA. Abstract LB19.
38. Dunn SP, Birtcher KK, Beavers CJ, et al. The role of the clinical pharmacist in the care of patients with cardiovascular disease. J Am Coll Cardiol. 2015;66:2129-2139.
39. Santschi V, Chiolero A, Paradis G et al. Pharmacist interventions to improve cardiovascular disease risk factors in diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabetes Care. 2012;35:2706-2717.
40. Anderegg MD, Gums TH, Uribe L, et al. Pharmacist intervention for blood pressure control in patients with diabetes and/or chronic kidney disease. Pharmacotherapy. 2018;38:309-318.
41. Anderegg MD, Gums TH, Uribe L et al. Physician-pharmacist collaborative management: narrowing the socioeconomic blood pressure gap. Hypertension. 2016;68:1314-1320.
42. Sisson EM, Dixon DL, Kildow DC, et al. Effectiveness of a pharmacist-physician team-based collaboration to improve long-term blood pressure control at an inner-city safety-net clinic. Pharmacotherapy. 2016;36:342-347.
43. Dixon DL, Sisson EM, Parod ED, et al. Pharmacist-physician collaborative care model and time to goal blood pressure in the uninsured population. J Clin Hypertens (Greenwich). 2018;20:88-95.
44. Dixon DL, Parod ED, Sisson EM et al. Impact of a pharmacist-physician collaborative care model on time-in-therapeutic blood pressure range in patients with hypertension. J Am Coll Clin Pharm. 2020;3:404-409.
45. Tsuyuki RT, Houle SK, Charrois TL, et al. Randomized trial of the effect of pharmacist prescribing on improving blood pressure in the community: the Alberta Clinical Trial in Optimizing Hypertension (RxACTION). Circulation. 2015;132:93-100.
46. Tsuyuki RT, Al Hamarneh YN, Jones CA, et al. The effectiveness of pharmacist interventions on cardiovascular risk: The Multicenter Randomized Controlled RxEACH trial. J Am Coll Cardiol. 2016;67:2846-2854.
47. Victor RG, Lynch K, Li N, et al. A cluster-randomized trial of blood-pressure reduction in black barbershops. N Engl J Med. 2018;378:1291-1301.
48. Victor RG, Blyler CA, Li N et al. Sustainability of blood pressure reduction in black barbershops. Circulation. 2019;139:10-19.
49. Panattoni L, Hurlimann L, Wilson C, et al. Workflow standardization of a novel team care model to improve chronic care: a quasi-experimental study. BMC Health Serv Res. 2017;17:286.
50. Chang AR, Bonaparte H, Yule C. Randomized controlled trial comparing a self-guided vs. dietitian-led approach using web-based tools to lower blood pressure: study design and rationale. International Stroke Conference 2020; February 19-21, 2020; Los Angeles, CA. Abstract P169.
51. Stephen C, Halcomb E, Mcinnes S, et al. Improving blood pressure control in primary care: The ImPress study. Int J Nurs Stud. 2019;95:28-33.
52. He J, Shi X, Lin M. Comparative effectiveness of implementation strategies on cardiovascular risk factor control in patients with diabetes: The D4C cluster randomized trial. International Stroke Conference 2020; February 19-21, 2020; Los Angeles, CA. Abstract 17.
53. Jafar TH, Gandhi M, de Silva HA, et al. A community-based intervention for managing hypertension in rural South Asia. N Engl J Med. 2020;382:717-726.
54. Centers for Disease Control and Prevention. Promoting team-based care to improve high blood pressure control. www.cdc.gov/dhdsp/pubs/guides/best-practices/team-based-care.htm. Accessed April 30, 2020.
55. Jacob V, Chattopadhyay SK, Thota AB, et al. Economics of team-based care in controlling blood pressure: a community guide systematic review. Am J Prev Med. 2015;49:772-783.
56. Dehmer SP, Baker-Goering MM, Maciosek MV, et al. Modeled health and economic impact of team-based care for hypertension. Am J Prev Med. 2016;50(5 suppl 1):S34-S44.
57. Zhang D, Wang G, Joo H. A systematic review of economic evidence on community hypertension interventions. Am J Prev Med. 2017;53:S121-S130.
58. Community Preventive Services Task Force. Cardiovascular disease: team-based care to improve blood pressure control. 2011. www.thecommunityguide.org/findings/cardiovascular-disease-team-based-care-improve-blood-pressure-control. Accessed April 30, 2020.
59. Kulchaitanaroaj P, Brooks JM, Ardery G et al. Incremental costs associated with physician and pharmacist collaboration to improve blood pressure control. Pharmacotherapy. 2012;32:772-780.
60. Mason JM, Freemantle N, Gibson JM, New JP. Specialist nurse-led clinics to improve control of hypertension and hyperlipidemia in diabetes. Diabetes Care. 2005;28:40-46.
61. Kulchaitanaroaj P, Brooks JM, Chaiyakunapruk N et al. Cost-utility analysis of physician-pharmacist collaborative intervention for treating hypertension compared with usual care. J Hypertens. 2017;35:178-187.
62. Lall D, Engel N, Devadasan N, et al. Models of care for chronic conditions in low/middle-income countries: a ‘best fit’ framework synthesis. BMJ Glob Health. 2018;3:e001077.
63. Bodenheimer T, Chen E, Bennett HD. Confronting the growing burden of chronic disease: can the U.S. health care workforce do the job? Health Aff (Millwood). 2009;28:64-74.
64. Smith M, Bates DW, Bodenheimer T, Cleary PD. Why pharmacists belong in the medical home. Health Aff (Millwood). 2010;29:906-913.
65. Wright JT, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
66. Phillips LS, Branch WT, Cook CB, et al. Clinical inertia. Ann Intern Med. 2001;135:825-834.
67. McManus RJ, Mant J, Franssen M, et al. Efficacy of self-monitored blood pressure, with or without telemonitoring, for titration of antihypertensive medication (TASMINH4): an unmasked randomised controlled trial. Lancet. 2018;391:949-959.
68. Tucker KL, Sheppard JP, Stevens R, et al. Self-monitoring of blood pressure in hypertension: a systematic review and individual patient data meta-analysis. PLoS Med. 2017;14:e1002389.
69. Casey DE, Thomas RJ, Bhalla V, et al. 2019 AHA/ACC clinical performance and quality measures for adults with high blood pressure: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures. J Am Coll Cardiol. 2019;74:2661-2706.
1. Fryar CD, Ostchega Y, Hales CM, et al. Hypertension prevalence and control among adults: United States, 2015–2016. NCHS Data Brief. 2017(289):1-8.
2. Ambrosius WT, Sink KM, Foy CG, et al. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: The Systolic Blood Pressure Intervention Trial (SPRINT). Clin Trials. 2014;11:532-546.
3. Lawes CM, Bennett DA, Feigin VL, Rodgers A. Blood pressure and stroke: an overview of published reviews. Stroke. 2017;35:776-785.
4. Zanchetti A, Thomopoulos C, Parati G. Randomized controlled trials of blood pressure lowering in hypertension: A critical reappraisal. Circ Res. 2015;116:1058-1073.
5. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/ AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127-e248.
6. Grol R. Successes and failures in the implementation of evidence-based guidelines for clinical practice. Med Care. 2001;39:II46-II54.
7. Brush JE, Handberg EM, Biga C, et al. 2015 ACC health policy statement on cardiovascular team-based care and the role of advanced practice providers. J Am Coll Cardiol. 2015;65:2118-2136.
8. Centers for Disease Control and Prevention. Best practices for cardiovascular disease prevention programs: a guide to effective health care system interventions and community programs linked to clinical services, promoting team-based care to improve high blood pressure control. www.cdc.gov/dhdsp/pubs/guides/best-practices/team-based-care.htm. Accessed April 30, 2020.
9. Centers for Disease Control and Prevention. Task Force recommends team-based care for improving blood pressure [press release]. May 15, 2012. www.cdc.gov/media/releases/2012/p0515_bp_control.html
10. Mitchell P, Wynia M, Golden R, et al. Core principles & values of effective team-based health care. 2012. Institute of Medicine, Washington, DC.
11. Campbell SM, Hann M, Hacker J, et al. Identifying predictors of high-quality care in English general practice: observational study. BMJ. 2001;323:784-787.
12. Shojania KG, Ranji SR, McDonald KM, et al. Effects of quality improvement strategies for type 2 diabetes on glycemic control: a meta-regression analysis. JAMA. 2006;296:427-440.
13. Walsh JM, McDonald KM, Shojania KG, et al. Quality improvement strategies for hypertension management: a systematic review. Med Care. 2006;44:646-657.
14. Wagner E. The role of patient care teams in chronic disease management. BMJ. 2000;320:560-572.
15. Coleman K, Reid R. Continuous and team-based healing relationships: improving patient care through teams. In: Phillips KE, Weir V, eds. Safety Net Medical Home Initiative Implementation Guide Series. 2nd ed. Seattle, WA: Qualis Health and The MacColl Center for Health Care Innovation at the Group Health Research Institute; 2013.
16. Sinsky CA, Willard-Grace R, Schutzbank AM, et al. In search of joy in practice: a report of 23 high-functioning primary care practices. Ann Fam Med. 2013;11:272-278
17. Howard J, Etz RS, Crocker JB, et al. Maximizing the patient-centered medical home (PCMH) by choosing words wisely. J Am Board Fam Med. 2016;29:248-253.
18. Solberg LI, Crain AL, Tillema JO, et al. Challenges of medical home transformation reported by 118 patient-centered medical home (PCMH) leaders. J Am Board Fam Med. 2014;27:449-457.
19. Crabtree BF, Chase SM, Wise CG, et al. Evaluation of patient centered medical home practice transformation initiatives. Med Care. 2011;49:10-16.
20. Carter BL. Blood pressure control—implementing a team approach. US Cardiol. 2011;8:108-113.
21. Carter BL, Rogers M, Daly J, et al. The potency of team-based care interventions for hypertension: a meta-analysis. Arch Intern Med. 2009;169:1748-1755.
22. Proia KK, Thota AB, Njie GJ, et al. Team-based care and improved blood pressure control: a community guide systematic review. Am J Prev Med. 2014;47:86-99.
23. Santschi V, Chiolero A, Colosimo AL, et al. Improving blood pressure control through pharmacist interventions: a meta-analysis of randomized controlled trials. J Am Heart Assoc. 2014;3:e000718.
24. Shaw RJ, McDuffie JR, Hendrix CC, et al. Effects of nurse-managed protocols in the outpatient management of adults with chronic conditions: a systematic review and meta-analysis. Ann Intern Med. 2014;161:113-121.
25. Carter BL, Coffey CS, Ardery G, et al. Cluster-randomized trial of a physician/pharmacist collaborative model to improve blood pressure control. Circ Cardiovasc Qual Outcomes. 2015;8:235-243
26. Carter BL, Bosworth HB, Green BB. The hypertension team: the role of the pharmacist, nurse and teamwork in hypertension therapy. J Clin Hypertens. 2012;14:51-65.
27. Kennelty KA, Polgreen LA, Carter BL. Team-based care with pharmacists to improve blood pressure: a review of recent literature. Curr Hypertens Rep. 2018;20:1.
28. Brownstein JN, Chowdhury FM, Norris SL, et al. Effectiveness of community health workers in the care of people with hypertension. Am J Prev Med. 2007;32:435-447.
29. Derington CG, King JB, Bryant KB, et al. Cost-effectiveness and challenges of implementing intensive blood pressure goals and team-based care. Curr Hypertens Rep. 2019;21:91.
30. Mills KT, Obst KM, Shen W, et al. Comparative effectiveness of implementation strategies for blood pressure control in hypertensive patients: a systematic review and meta-analysis. Ann Intern Med. 2018;168:110-120.
31. Clark CE, Smith LFP, Taylor RS, et al. Nurse led interventions to improve control of blood pressure in people with hypertension: systematic review and meta-analysis. BMJ. 2010;341:c3995.
32. Santschi V, Chiolero A, Burnand B, et al. Impact of pharmacist care in the management of cardiovascular disease risk factors: a systematic review and meta-analysis of randomized trials. Arch Intern Med. 2011;171:1441-1453.
33. Dennison Himmelfarb CR, Commodore-Mensah Y, Hill MN. Expanding the role of nurses to improve hypertension care and control globally. Ann Glob Health. 2016;82:243-253.
34. Clark CE, Smith LFP, Taylor RS, Campbell JL. Nurse-led interventions used to improve control of high blood pressure in people with diabetes: a systematic review and meta-analysis. DiabetMed. 2011;28:250-261.
35. Zhu X, Wong FKY, Wu CLH. Development and evaluation of a nurse-led hypertension management model: A randomized controlled trial. Int J Nurs Stud. 2018;77:171-178.
36. Spruill TM, Williams O, Teresi JA, et al. Comparative effectiveness of home blood pressure telemonitoring (HBPTM) plus nurse case management versus HBPTM alone among Black and Hispanic stroke survivors: study protocol for a randomized controlled trial. Trials. 2015;16:97.
37. Ogedegbe G. Comparative effectiveness of home BP telemonitoring plus nurse case management (HBPTM+NCM) versus HBPTM alone on systolic BP (SBP) reduction among minority stroke survivors. International Stroke Conference 2020; February 19-21, 2020; Los Angeles, CA. Abstract LB19.
38. Dunn SP, Birtcher KK, Beavers CJ, et al. The role of the clinical pharmacist in the care of patients with cardiovascular disease. J Am Coll Cardiol. 2015;66:2129-2139.
39. Santschi V, Chiolero A, Paradis G et al. Pharmacist interventions to improve cardiovascular disease risk factors in diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabetes Care. 2012;35:2706-2717.
40. Anderegg MD, Gums TH, Uribe L, et al. Pharmacist intervention for blood pressure control in patients with diabetes and/or chronic kidney disease. Pharmacotherapy. 2018;38:309-318.
41. Anderegg MD, Gums TH, Uribe L et al. Physician-pharmacist collaborative management: narrowing the socioeconomic blood pressure gap. Hypertension. 2016;68:1314-1320.
42. Sisson EM, Dixon DL, Kildow DC, et al. Effectiveness of a pharmacist-physician team-based collaboration to improve long-term blood pressure control at an inner-city safety-net clinic. Pharmacotherapy. 2016;36:342-347.
43. Dixon DL, Sisson EM, Parod ED, et al. Pharmacist-physician collaborative care model and time to goal blood pressure in the uninsured population. J Clin Hypertens (Greenwich). 2018;20:88-95.
44. Dixon DL, Parod ED, Sisson EM et al. Impact of a pharmacist-physician collaborative care model on time-in-therapeutic blood pressure range in patients with hypertension. J Am Coll Clin Pharm. 2020;3:404-409.
45. Tsuyuki RT, Houle SK, Charrois TL, et al. Randomized trial of the effect of pharmacist prescribing on improving blood pressure in the community: the Alberta Clinical Trial in Optimizing Hypertension (RxACTION). Circulation. 2015;132:93-100.
46. Tsuyuki RT, Al Hamarneh YN, Jones CA, et al. The effectiveness of pharmacist interventions on cardiovascular risk: The Multicenter Randomized Controlled RxEACH trial. J Am Coll Cardiol. 2016;67:2846-2854.
47. Victor RG, Lynch K, Li N, et al. A cluster-randomized trial of blood-pressure reduction in black barbershops. N Engl J Med. 2018;378:1291-1301.
48. Victor RG, Blyler CA, Li N et al. Sustainability of blood pressure reduction in black barbershops. Circulation. 2019;139:10-19.
49. Panattoni L, Hurlimann L, Wilson C, et al. Workflow standardization of a novel team care model to improve chronic care: a quasi-experimental study. BMC Health Serv Res. 2017;17:286.
50. Chang AR, Bonaparte H, Yule C. Randomized controlled trial comparing a self-guided vs. dietitian-led approach using web-based tools to lower blood pressure: study design and rationale. International Stroke Conference 2020; February 19-21, 2020; Los Angeles, CA. Abstract P169.
51. Stephen C, Halcomb E, Mcinnes S, et al. Improving blood pressure control in primary care: The ImPress study. Int J Nurs Stud. 2019;95:28-33.
52. He J, Shi X, Lin M. Comparative effectiveness of implementation strategies on cardiovascular risk factor control in patients with diabetes: The D4C cluster randomized trial. International Stroke Conference 2020; February 19-21, 2020; Los Angeles, CA. Abstract 17.
53. Jafar TH, Gandhi M, de Silva HA, et al. A community-based intervention for managing hypertension in rural South Asia. N Engl J Med. 2020;382:717-726.
54. Centers for Disease Control and Prevention. Promoting team-based care to improve high blood pressure control. www.cdc.gov/dhdsp/pubs/guides/best-practices/team-based-care.htm. Accessed April 30, 2020.
55. Jacob V, Chattopadhyay SK, Thota AB, et al. Economics of team-based care in controlling blood pressure: a community guide systematic review. Am J Prev Med. 2015;49:772-783.
56. Dehmer SP, Baker-Goering MM, Maciosek MV, et al. Modeled health and economic impact of team-based care for hypertension. Am J Prev Med. 2016;50(5 suppl 1):S34-S44.
57. Zhang D, Wang G, Joo H. A systematic review of economic evidence on community hypertension interventions. Am J Prev Med. 2017;53:S121-S130.
58. Community Preventive Services Task Force. Cardiovascular disease: team-based care to improve blood pressure control. 2011. www.thecommunityguide.org/findings/cardiovascular-disease-team-based-care-improve-blood-pressure-control. Accessed April 30, 2020.
59. Kulchaitanaroaj P, Brooks JM, Ardery G et al. Incremental costs associated with physician and pharmacist collaboration to improve blood pressure control. Pharmacotherapy. 2012;32:772-780.
60. Mason JM, Freemantle N, Gibson JM, New JP. Specialist nurse-led clinics to improve control of hypertension and hyperlipidemia in diabetes. Diabetes Care. 2005;28:40-46.
61. Kulchaitanaroaj P, Brooks JM, Chaiyakunapruk N et al. Cost-utility analysis of physician-pharmacist collaborative intervention for treating hypertension compared with usual care. J Hypertens. 2017;35:178-187.
62. Lall D, Engel N, Devadasan N, et al. Models of care for chronic conditions in low/middle-income countries: a ‘best fit’ framework synthesis. BMJ Glob Health. 2018;3:e001077.
63. Bodenheimer T, Chen E, Bennett HD. Confronting the growing burden of chronic disease: can the U.S. health care workforce do the job? Health Aff (Millwood). 2009;28:64-74.
64. Smith M, Bates DW, Bodenheimer T, Cleary PD. Why pharmacists belong in the medical home. Health Aff (Millwood). 2010;29:906-913.
65. Wright JT, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
66. Phillips LS, Branch WT, Cook CB, et al. Clinical inertia. Ann Intern Med. 2001;135:825-834.
67. McManus RJ, Mant J, Franssen M, et al. Efficacy of self-monitored blood pressure, with or without telemonitoring, for titration of antihypertensive medication (TASMINH4): an unmasked randomised controlled trial. Lancet. 2018;391:949-959.
68. Tucker KL, Sheppard JP, Stevens R, et al. Self-monitoring of blood pressure in hypertension: a systematic review and individual patient data meta-analysis. PLoS Med. 2017;14:e1002389.
69. Casey DE, Thomas RJ, Bhalla V, et al. 2019 AHA/ACC clinical performance and quality measures for adults with high blood pressure: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures. J Am Coll Cardiol. 2019;74:2661-2706.
Rivaroxaban plus aspirin safely benefits PAD patients after limb revascularization
A combined antithrombotic regimen of rivaroxaban plus aspirin was safe and effective for reducing ischemic events in patients with symptomatic peripheral artery disease who had just undergone peripheral artery revascularization in VOYAGER PAD, a multicenter randomized trial with nearly 6,600 patients.
The study and its results were a groundbreaking advance for this patient population, who until now have had no evidence-based treatment available, Mark P. Bonaca, MD, said on March 28 at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
The study design excluded a small percentage of patients (about 2%) because of their very high bleeding-risk history. Among the treated patients, in those who received a combination of 2.5 mg rivaroxaban twice daily plus 100 mg of aspirin daily, bleeding events were more common, compared with control patients who received aspirin alone. But the patients who received both drugs showed no excess of fatal bleeds or intracranial hemorrhages, and the rate of ischemic events prevented by rivaroxaban plus aspirin exceeded the excess rate of bleeds by three- to sixfold, depending on how bleeding episodes were defined, noted Dr. Bonaca, executive director of CPC Clinical Research and CPC Community Health, an academic research organization affiliated with the University of Colorado at Denver in Aurora.
“This was a much anticipated and important trial. Those of us who treat patients with lower-limb peripheral artery disease have not had much evidence on how to treat these patients, particularly those who have just undergone revascularization. This trial gives us the evidence,” commented Mark A. Creager, MD, professor of medicine and director of the Heart and Vascular Center at Dartmouth-Hitchcock Medical Center in Lebanon, N.H. “The bleeding risk [from adding rivaroxaban treatment] was substantially less than the benefit from preventing major adverse limb events and major adverse cardiovascular events,” producing a “favorable balance” of benefit, compared with risk, Dr. Creager said in an interview. “In the right patients, the benefit greatly outweighed the risk.”
“This was an incredible trial that will advance care,” commented Joshua A. Beckman, MD, professor of medicine and director of Vascular Medicine at Vanderbilt University in Nashville, Tenn. “The treatment was beneficial for patients across a range of symptom severity, from claudication to critical limb ischemia,” and the results expand the range of patients proven to benefit from the rivaroxaban plus aspirin combination from the types of patients with peripheral artery disease (PAD) enrolled in the COMPASS trial. That pivotal trial showed similar benefit from the dual-antithrombotic regimen, but in patients who had both coronary artery disease as well as atherosclerotic disease in at least one additional vascular bed, such as lower-limb arteries (N Engl J Med. 2017 Oct 5;377[14]:1319-30). In addition to “bringing acute limb ischemia to the cardiovascular community,” the results also identified a very useful time point in the clinical presentation of these patients for starting a combined rivaroxaban plus aspirin regimen: when patients are hospitalized for their revascularization procedure, said Dr. Beckman, a designated discussant for the report.
Among the 6,564 patients randomized in the study, about two-thirds underwent endovascular revascularization within 10 days before starting their study treatment, and the remaining third had undergone surgical revascularization. The study focused on patients “with symptomatic PAD but without known coronary artery disease,” noted Dr. Bonaca.
VOYAGER PAD trial
The VOYAGER PAD (Vascular Outcomes Study of Acetylsalicylic Acid Along With Rivaroxaban in Endovascular Or Surgical Limb Revascularization for Peripheral Artery Disease) trial enrolled patients during 2015-2018 at 534 sites in 34 countries. The study’s primary endpoint was a composite of acute limb ischemia, major amputation for vascular causes, myocardial infarction, ischemic stroke, or death from cardiovascular causes, and was reduced during a median follow-up of 28 months from 19.9% with aspirin alone to 17.3% on the combined regimen, a 2.6% absolute difference and a 15% relative risk reduction that was statistically significant, an endpoint primarily driven by a reduction in acute limb ischemia. The primary safety endpoint was the rate of TIMI (Thrombolysis in Myocardial Infarction) major bleeds, which was 0.8% higher in the patients who received the anticoagulant, a 43% relative increase that just missed statistical significance. But that result demonstrated the small but important increased risk for bleeding events that the dual regimen produced in these patients, Dr. Bonaca said. Simultaneously with his report the findings also appeared in an article published online (N Engl J Med. 2020 Mar 28. doi: 10.1056/NEJMoa2000052).
Dr. Bonaca cautioned that one limitation of his report on the primary outcome of VOYAGER PAD is that the results of an important subgroup analysis won’t be known until a second report during the ACC online sessions on March 29, which will examine the impact that treatment with the antiplatelet drug clopidogrel had on both the efficacy and safety outcomes. Half of the enrolled patients received clopidogrel at the discretion of their treating physicians; addition or exclusion of concurrent clopidogrel treatment was outside of the study’s design. “Is efficacy the same with or without clopidogrel, and what is the bleeding cost,” especially in patients who receive three antithrombotic drugs? “It will be very important to understand,” Dr. Bonaca said.
“Until now, we had no idea of what was the best antithrombotic strategy for patients after a successful peripheral vascular intervention.” VOYAGER PAD was “an unprecedented vascular study that addressed an unmet patient need,” commented Roxana Mehran, MD, a designated discussant for the study and professor of medicine and director of Interventional Cardiovascular Research at Mount Sinai Medical Center in New York.
VOYAGER PAD was sponsored by Bayer and Janssen, the companies that market rivaroxaban (Xarelto). The institution that Dr. Bonaca directs has received research funding from Bayer and Janssen, and also from Amgen, Aralez, AstraZeneca, Merck, Novo Nordisk, Pfizer, and Sanofi. Dr. Creager had no disclosures. Dr. Beckman has served as a data safety monitor for Bayer and for Novartis, and has been a consultant to Amgen, AstraZeneca, JanOne and Sanofi. Dr. Mehran has received research funding from Bayer and has been a consultant to Janssen, and she has also received research funding or been a consultant to several other companies.
SOURCE: Bonaca MP et al. ACC 20, Abstract 402-10.
A combined antithrombotic regimen of rivaroxaban plus aspirin was safe and effective for reducing ischemic events in patients with symptomatic peripheral artery disease who had just undergone peripheral artery revascularization in VOYAGER PAD, a multicenter randomized trial with nearly 6,600 patients.
The study and its results were a groundbreaking advance for this patient population, who until now have had no evidence-based treatment available, Mark P. Bonaca, MD, said on March 28 at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
The study design excluded a small percentage of patients (about 2%) because of their very high bleeding-risk history. Among the treated patients, in those who received a combination of 2.5 mg rivaroxaban twice daily plus 100 mg of aspirin daily, bleeding events were more common, compared with control patients who received aspirin alone. But the patients who received both drugs showed no excess of fatal bleeds or intracranial hemorrhages, and the rate of ischemic events prevented by rivaroxaban plus aspirin exceeded the excess rate of bleeds by three- to sixfold, depending on how bleeding episodes were defined, noted Dr. Bonaca, executive director of CPC Clinical Research and CPC Community Health, an academic research organization affiliated with the University of Colorado at Denver in Aurora.
“This was a much anticipated and important trial. Those of us who treat patients with lower-limb peripheral artery disease have not had much evidence on how to treat these patients, particularly those who have just undergone revascularization. This trial gives us the evidence,” commented Mark A. Creager, MD, professor of medicine and director of the Heart and Vascular Center at Dartmouth-Hitchcock Medical Center in Lebanon, N.H. “The bleeding risk [from adding rivaroxaban treatment] was substantially less than the benefit from preventing major adverse limb events and major adverse cardiovascular events,” producing a “favorable balance” of benefit, compared with risk, Dr. Creager said in an interview. “In the right patients, the benefit greatly outweighed the risk.”
“This was an incredible trial that will advance care,” commented Joshua A. Beckman, MD, professor of medicine and director of Vascular Medicine at Vanderbilt University in Nashville, Tenn. “The treatment was beneficial for patients across a range of symptom severity, from claudication to critical limb ischemia,” and the results expand the range of patients proven to benefit from the rivaroxaban plus aspirin combination from the types of patients with peripheral artery disease (PAD) enrolled in the COMPASS trial. That pivotal trial showed similar benefit from the dual-antithrombotic regimen, but in patients who had both coronary artery disease as well as atherosclerotic disease in at least one additional vascular bed, such as lower-limb arteries (N Engl J Med. 2017 Oct 5;377[14]:1319-30). In addition to “bringing acute limb ischemia to the cardiovascular community,” the results also identified a very useful time point in the clinical presentation of these patients for starting a combined rivaroxaban plus aspirin regimen: when patients are hospitalized for their revascularization procedure, said Dr. Beckman, a designated discussant for the report.
Among the 6,564 patients randomized in the study, about two-thirds underwent endovascular revascularization within 10 days before starting their study treatment, and the remaining third had undergone surgical revascularization. The study focused on patients “with symptomatic PAD but without known coronary artery disease,” noted Dr. Bonaca.
VOYAGER PAD trial
The VOYAGER PAD (Vascular Outcomes Study of Acetylsalicylic Acid Along With Rivaroxaban in Endovascular Or Surgical Limb Revascularization for Peripheral Artery Disease) trial enrolled patients during 2015-2018 at 534 sites in 34 countries. The study’s primary endpoint was a composite of acute limb ischemia, major amputation for vascular causes, myocardial infarction, ischemic stroke, or death from cardiovascular causes, and was reduced during a median follow-up of 28 months from 19.9% with aspirin alone to 17.3% on the combined regimen, a 2.6% absolute difference and a 15% relative risk reduction that was statistically significant, an endpoint primarily driven by a reduction in acute limb ischemia. The primary safety endpoint was the rate of TIMI (Thrombolysis in Myocardial Infarction) major bleeds, which was 0.8% higher in the patients who received the anticoagulant, a 43% relative increase that just missed statistical significance. But that result demonstrated the small but important increased risk for bleeding events that the dual regimen produced in these patients, Dr. Bonaca said. Simultaneously with his report the findings also appeared in an article published online (N Engl J Med. 2020 Mar 28. doi: 10.1056/NEJMoa2000052).
Dr. Bonaca cautioned that one limitation of his report on the primary outcome of VOYAGER PAD is that the results of an important subgroup analysis won’t be known until a second report during the ACC online sessions on March 29, which will examine the impact that treatment with the antiplatelet drug clopidogrel had on both the efficacy and safety outcomes. Half of the enrolled patients received clopidogrel at the discretion of their treating physicians; addition or exclusion of concurrent clopidogrel treatment was outside of the study’s design. “Is efficacy the same with or without clopidogrel, and what is the bleeding cost,” especially in patients who receive three antithrombotic drugs? “It will be very important to understand,” Dr. Bonaca said.
“Until now, we had no idea of what was the best antithrombotic strategy for patients after a successful peripheral vascular intervention.” VOYAGER PAD was “an unprecedented vascular study that addressed an unmet patient need,” commented Roxana Mehran, MD, a designated discussant for the study and professor of medicine and director of Interventional Cardiovascular Research at Mount Sinai Medical Center in New York.
VOYAGER PAD was sponsored by Bayer and Janssen, the companies that market rivaroxaban (Xarelto). The institution that Dr. Bonaca directs has received research funding from Bayer and Janssen, and also from Amgen, Aralez, AstraZeneca, Merck, Novo Nordisk, Pfizer, and Sanofi. Dr. Creager had no disclosures. Dr. Beckman has served as a data safety monitor for Bayer and for Novartis, and has been a consultant to Amgen, AstraZeneca, JanOne and Sanofi. Dr. Mehran has received research funding from Bayer and has been a consultant to Janssen, and she has also received research funding or been a consultant to several other companies.
SOURCE: Bonaca MP et al. ACC 20, Abstract 402-10.
A combined antithrombotic regimen of rivaroxaban plus aspirin was safe and effective for reducing ischemic events in patients with symptomatic peripheral artery disease who had just undergone peripheral artery revascularization in VOYAGER PAD, a multicenter randomized trial with nearly 6,600 patients.
The study and its results were a groundbreaking advance for this patient population, who until now have had no evidence-based treatment available, Mark P. Bonaca, MD, said on March 28 at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
The study design excluded a small percentage of patients (about 2%) because of their very high bleeding-risk history. Among the treated patients, in those who received a combination of 2.5 mg rivaroxaban twice daily plus 100 mg of aspirin daily, bleeding events were more common, compared with control patients who received aspirin alone. But the patients who received both drugs showed no excess of fatal bleeds or intracranial hemorrhages, and the rate of ischemic events prevented by rivaroxaban plus aspirin exceeded the excess rate of bleeds by three- to sixfold, depending on how bleeding episodes were defined, noted Dr. Bonaca, executive director of CPC Clinical Research and CPC Community Health, an academic research organization affiliated with the University of Colorado at Denver in Aurora.
“This was a much anticipated and important trial. Those of us who treat patients with lower-limb peripheral artery disease have not had much evidence on how to treat these patients, particularly those who have just undergone revascularization. This trial gives us the evidence,” commented Mark A. Creager, MD, professor of medicine and director of the Heart and Vascular Center at Dartmouth-Hitchcock Medical Center in Lebanon, N.H. “The bleeding risk [from adding rivaroxaban treatment] was substantially less than the benefit from preventing major adverse limb events and major adverse cardiovascular events,” producing a “favorable balance” of benefit, compared with risk, Dr. Creager said in an interview. “In the right patients, the benefit greatly outweighed the risk.”
“This was an incredible trial that will advance care,” commented Joshua A. Beckman, MD, professor of medicine and director of Vascular Medicine at Vanderbilt University in Nashville, Tenn. “The treatment was beneficial for patients across a range of symptom severity, from claudication to critical limb ischemia,” and the results expand the range of patients proven to benefit from the rivaroxaban plus aspirin combination from the types of patients with peripheral artery disease (PAD) enrolled in the COMPASS trial. That pivotal trial showed similar benefit from the dual-antithrombotic regimen, but in patients who had both coronary artery disease as well as atherosclerotic disease in at least one additional vascular bed, such as lower-limb arteries (N Engl J Med. 2017 Oct 5;377[14]:1319-30). In addition to “bringing acute limb ischemia to the cardiovascular community,” the results also identified a very useful time point in the clinical presentation of these patients for starting a combined rivaroxaban plus aspirin regimen: when patients are hospitalized for their revascularization procedure, said Dr. Beckman, a designated discussant for the report.
Among the 6,564 patients randomized in the study, about two-thirds underwent endovascular revascularization within 10 days before starting their study treatment, and the remaining third had undergone surgical revascularization. The study focused on patients “with symptomatic PAD but without known coronary artery disease,” noted Dr. Bonaca.
VOYAGER PAD trial
The VOYAGER PAD (Vascular Outcomes Study of Acetylsalicylic Acid Along With Rivaroxaban in Endovascular Or Surgical Limb Revascularization for Peripheral Artery Disease) trial enrolled patients during 2015-2018 at 534 sites in 34 countries. The study’s primary endpoint was a composite of acute limb ischemia, major amputation for vascular causes, myocardial infarction, ischemic stroke, or death from cardiovascular causes, and was reduced during a median follow-up of 28 months from 19.9% with aspirin alone to 17.3% on the combined regimen, a 2.6% absolute difference and a 15% relative risk reduction that was statistically significant, an endpoint primarily driven by a reduction in acute limb ischemia. The primary safety endpoint was the rate of TIMI (Thrombolysis in Myocardial Infarction) major bleeds, which was 0.8% higher in the patients who received the anticoagulant, a 43% relative increase that just missed statistical significance. But that result demonstrated the small but important increased risk for bleeding events that the dual regimen produced in these patients, Dr. Bonaca said. Simultaneously with his report the findings also appeared in an article published online (N Engl J Med. 2020 Mar 28. doi: 10.1056/NEJMoa2000052).
Dr. Bonaca cautioned that one limitation of his report on the primary outcome of VOYAGER PAD is that the results of an important subgroup analysis won’t be known until a second report during the ACC online sessions on March 29, which will examine the impact that treatment with the antiplatelet drug clopidogrel had on both the efficacy and safety outcomes. Half of the enrolled patients received clopidogrel at the discretion of their treating physicians; addition or exclusion of concurrent clopidogrel treatment was outside of the study’s design. “Is efficacy the same with or without clopidogrel, and what is the bleeding cost,” especially in patients who receive three antithrombotic drugs? “It will be very important to understand,” Dr. Bonaca said.
“Until now, we had no idea of what was the best antithrombotic strategy for patients after a successful peripheral vascular intervention.” VOYAGER PAD was “an unprecedented vascular study that addressed an unmet patient need,” commented Roxana Mehran, MD, a designated discussant for the study and professor of medicine and director of Interventional Cardiovascular Research at Mount Sinai Medical Center in New York.
VOYAGER PAD was sponsored by Bayer and Janssen, the companies that market rivaroxaban (Xarelto). The institution that Dr. Bonaca directs has received research funding from Bayer and Janssen, and also from Amgen, Aralez, AstraZeneca, Merck, Novo Nordisk, Pfizer, and Sanofi. Dr. Creager had no disclosures. Dr. Beckman has served as a data safety monitor for Bayer and for Novartis, and has been a consultant to Amgen, AstraZeneca, JanOne and Sanofi. Dr. Mehran has received research funding from Bayer and has been a consultant to Janssen, and she has also received research funding or been a consultant to several other companies.
SOURCE: Bonaca MP et al. ACC 20, Abstract 402-10.
REPORTING FROM ACC 20
Key clinical point: Combined treatment with rivaroxaban plus aspirin safely reduced a composite measure of adverse ischemic events in PAD patients following lower-limb revascularization.
Major finding: The primary event outcome occurred in 17.3% of patients on rivaroxaban plus aspirin, and in 19.9% on aspirin alone.
Study details: VOYAGER PAD, a multicenter, international randomized trial with 6,564 patients.
Disclosures: VOYAGER PAD was sponsored by Bayer and Janssen, the companies that market rivaroxaban (Xarelto). The institution that Dr. Bonaca directs has received research funding from Bayer and Janssen, and also from Amgen, Aralez, AstraZeneca, Merck, Novo Nordisk, Pfizer, and Sanofi. Dr. Creager had no disclosures. Dr. Beckman has served as a data safety monitor for Bayer and for Novartis, and has been a consultant to Amgen, AstraZeneca, JanOne and Sanofi. Dr. Mehran has received research funding from Bayer and has been a consultant to Janssen, and she has also received research funding or been a consultant to several other companies.
Source: Bonaca MP. ACC 20, Abstract 402-10.
Carotid endarterectomy surpasses stenting in elderly, asymptomatic patients
LOS ANGELES – Carotid artery stenting in older, asymptomatic patients with severe carotid artery stenosis is, in general, as bad an idea as it has already proven to be in symptomatic patients, with a multifold increase in adverse short- and mid-term outcomes, compared with similar older, asymptomatic patients who underwent endarterectomy, according to a combined-study analysis with more than 2,500 patients.
The risk for poor outcomes in patients with severe but asymptomatic carotid artery disease who underwent carotid artery stenting (CAS), compared with patients who instead underwent carotid endarterectomy (CEA) “abruptly increased around age 75,” in an analysis that combined data from the two major, published, randomized trials that compared these two interventions in this patient population, Jenifer H. Voeks, PhD said at the International Stroke Conference sponsored by the American Heart Association.
These results “largely mirror” the findings from a similar combined analysis of data from four major, randomized trials that compared CEA and CAS in patients with symptomatic carotid disease, she noted (Lancet. 2016 Mar 26;387[10025]:1305-11). The new findings in an expanded population of asymptomatic patients derived from two separate studies showed that, in patients aged 70 years or less, “CAS appears to be a reasonable alternative to CEA, but above age 70, and certainly above age 75, age-related risk factors such as cerebrovascular anatomy and underlying cerebral pathology should be carefully considered before selecting patients for CAS,” said Dr. Voeks, a neurology researcher at the Medical University of South Carolina, Charleston. Many experts also believe that, for asymptomatic patients, intensive medical management may have returned as an alternative to either of these invasive approaches for treating severe carotid stenosis and has achieved a level of equipoise that led to the launch of CREST 2 (Carotid Revascularization and Medical Management for Asymptomatic Carotid Stenosis Trial). CREST 2 is comparing CEA and CAS with medical management, and is scheduled to report results in 2021.
The data for this analysis in asymptomatic patients came from the first CREST (Carotid Revascularization Endarterectomy Versus Stenting Trial; N Engl J Med. 2010 Jul 1;363[1]:11-23), which included 1,181 asymptomatic patients (nearly half the total enrollment, with symptomatic patients making up the balance) and had no age ceiling, as well as all 1,453 patients from the ACT 1 trial, which enrolled exclusively asymptomatic patients and limited enrollment to patients aged 79 years or less (N Engl J Med. 2016 Mar 17;374[11]: 1011-20). Because the maximum age of patients in ACT 1 was 79 years, for this analysis Dr. Voeks and associates only included the 1,091 asymptomatic CREST patients who also were within the same age ceiling. The resulting cohort of 2,544 included 1,637 patients who underwent CAS and 907 who underwent CEA (because of a 3:1 randomization ratio in ACT 1), creating the largest data set to compare CAS and CEA by age in asymptomatic patients, Dr. Voeks noted. When subdivided by age, 30% of the cohort was younger that 65 years, 54% were 65-74, and 16% were 75-79.
The primary outcome the researchers used for their analysis was the combined incidence of periprocedural stroke, MI, or death, plus the incidence of ipsilateral stroke during 4 years of follow-up post procedure. Among patients who underwent CAS, this outcome occurred in roughly 9% of patients aged 75-79 years and in about 3% of those younger than 65 years, a hazard ratio of 2.9 that was statistically significant. In contrast, the incidence of the primary outcome among patients aged 65-74 years was just 30% higher, compared with patients aged less than 65 years, a difference that was not statistically significant.
Patients who underwent CEA showed no similar relationship between age and outcome. The incidence of the primary outcome among the CEA patients was roughly the same, about 3.5%, regardless of their age.
A second analysis that considered age as a continuous variable showed a sharply spiked increase in the risk for CAS patients, compared with CEA patients once they reached about age 73-75 years. Until about age 72, the rate of the primary outcome was nearly the same regardless of whether patients underwent CAS or CEA, but the risk for adverse outcomes rose “steeply” starting at about age 75 so that by age 79 the rate of the primary outcome approached 300% higher among the CAS patients compared with CEA patients, Dr. Voeks said.
She cautioned that the analysis included just 115 total primary-outcome events, which makes the incidence rate estimates somewhat imprecise, and that the data reflect outcomes in patients who were treated more than a decade ago, but these data remain the only reported results from large randomized trials that compared CAS and CEA in asymptomatic patients.
Dr. Voeks reported no disclosures.
SOURCE: Voeks JH al. Stroke. 2020 Feb 12;51[suppl 1], Abstract 70.
The role for carotid intervention in asymptomatic patients with severe carotid stenosis, usually defined as a stenosis that obstructs at least 70% of the carotid lumen, is controversial right now because intensive medical management has not been compared with invasive treatments, such as carotid endarterectomy and carotid stenting, for well over a decade. New drugs and new regimens have become treatment options for patients with advanced atherosclerotic carotid artery disease, and this has returned us to a state of equipoise for medical versus interventional management. That’s the premise behind CREST 2 (Carotid Revascularization and Medical Management for Asymptomatic Carotid Stenosis Trial), which is comparing medical treatment against endarterectomy and against carotid stenting in a randomized study. The results may be available in 2021.
The new findings are very important for helping patients and their families make informed decisions. CAS is often perceived as the safer option for older patients because it is less traumatic and invasive than CEA. The data that Dr. Voeks reported show once again that this intuitive impression about CAS in the elderly is belied by the evidence. But the findings also require cautious interpretation because they came from a post hoc, subgroup analysis.
Mai N. Nguyen-Huynh, MD , is a vascular neurologist with Kaiser Permanente Northern California in Oakland. She had no relevant disclosures. She made these comments in an interview.
The role for carotid intervention in asymptomatic patients with severe carotid stenosis, usually defined as a stenosis that obstructs at least 70% of the carotid lumen, is controversial right now because intensive medical management has not been compared with invasive treatments, such as carotid endarterectomy and carotid stenting, for well over a decade. New drugs and new regimens have become treatment options for patients with advanced atherosclerotic carotid artery disease, and this has returned us to a state of equipoise for medical versus interventional management. That’s the premise behind CREST 2 (Carotid Revascularization and Medical Management for Asymptomatic Carotid Stenosis Trial), which is comparing medical treatment against endarterectomy and against carotid stenting in a randomized study. The results may be available in 2021.
The new findings are very important for helping patients and their families make informed decisions. CAS is often perceived as the safer option for older patients because it is less traumatic and invasive than CEA. The data that Dr. Voeks reported show once again that this intuitive impression about CAS in the elderly is belied by the evidence. But the findings also require cautious interpretation because they came from a post hoc, subgroup analysis.
Mai N. Nguyen-Huynh, MD , is a vascular neurologist with Kaiser Permanente Northern California in Oakland. She had no relevant disclosures. She made these comments in an interview.
The role for carotid intervention in asymptomatic patients with severe carotid stenosis, usually defined as a stenosis that obstructs at least 70% of the carotid lumen, is controversial right now because intensive medical management has not been compared with invasive treatments, such as carotid endarterectomy and carotid stenting, for well over a decade. New drugs and new regimens have become treatment options for patients with advanced atherosclerotic carotid artery disease, and this has returned us to a state of equipoise for medical versus interventional management. That’s the premise behind CREST 2 (Carotid Revascularization and Medical Management for Asymptomatic Carotid Stenosis Trial), which is comparing medical treatment against endarterectomy and against carotid stenting in a randomized study. The results may be available in 2021.
The new findings are very important for helping patients and their families make informed decisions. CAS is often perceived as the safer option for older patients because it is less traumatic and invasive than CEA. The data that Dr. Voeks reported show once again that this intuitive impression about CAS in the elderly is belied by the evidence. But the findings also require cautious interpretation because they came from a post hoc, subgroup analysis.
Mai N. Nguyen-Huynh, MD , is a vascular neurologist with Kaiser Permanente Northern California in Oakland. She had no relevant disclosures. She made these comments in an interview.
LOS ANGELES – Carotid artery stenting in older, asymptomatic patients with severe carotid artery stenosis is, in general, as bad an idea as it has already proven to be in symptomatic patients, with a multifold increase in adverse short- and mid-term outcomes, compared with similar older, asymptomatic patients who underwent endarterectomy, according to a combined-study analysis with more than 2,500 patients.
The risk for poor outcomes in patients with severe but asymptomatic carotid artery disease who underwent carotid artery stenting (CAS), compared with patients who instead underwent carotid endarterectomy (CEA) “abruptly increased around age 75,” in an analysis that combined data from the two major, published, randomized trials that compared these two interventions in this patient population, Jenifer H. Voeks, PhD said at the International Stroke Conference sponsored by the American Heart Association.
These results “largely mirror” the findings from a similar combined analysis of data from four major, randomized trials that compared CEA and CAS in patients with symptomatic carotid disease, she noted (Lancet. 2016 Mar 26;387[10025]:1305-11). The new findings in an expanded population of asymptomatic patients derived from two separate studies showed that, in patients aged 70 years or less, “CAS appears to be a reasonable alternative to CEA, but above age 70, and certainly above age 75, age-related risk factors such as cerebrovascular anatomy and underlying cerebral pathology should be carefully considered before selecting patients for CAS,” said Dr. Voeks, a neurology researcher at the Medical University of South Carolina, Charleston. Many experts also believe that, for asymptomatic patients, intensive medical management may have returned as an alternative to either of these invasive approaches for treating severe carotid stenosis and has achieved a level of equipoise that led to the launch of CREST 2 (Carotid Revascularization and Medical Management for Asymptomatic Carotid Stenosis Trial). CREST 2 is comparing CEA and CAS with medical management, and is scheduled to report results in 2021.
The data for this analysis in asymptomatic patients came from the first CREST (Carotid Revascularization Endarterectomy Versus Stenting Trial; N Engl J Med. 2010 Jul 1;363[1]:11-23), which included 1,181 asymptomatic patients (nearly half the total enrollment, with symptomatic patients making up the balance) and had no age ceiling, as well as all 1,453 patients from the ACT 1 trial, which enrolled exclusively asymptomatic patients and limited enrollment to patients aged 79 years or less (N Engl J Med. 2016 Mar 17;374[11]: 1011-20). Because the maximum age of patients in ACT 1 was 79 years, for this analysis Dr. Voeks and associates only included the 1,091 asymptomatic CREST patients who also were within the same age ceiling. The resulting cohort of 2,544 included 1,637 patients who underwent CAS and 907 who underwent CEA (because of a 3:1 randomization ratio in ACT 1), creating the largest data set to compare CAS and CEA by age in asymptomatic patients, Dr. Voeks noted. When subdivided by age, 30% of the cohort was younger that 65 years, 54% were 65-74, and 16% were 75-79.
The primary outcome the researchers used for their analysis was the combined incidence of periprocedural stroke, MI, or death, plus the incidence of ipsilateral stroke during 4 years of follow-up post procedure. Among patients who underwent CAS, this outcome occurred in roughly 9% of patients aged 75-79 years and in about 3% of those younger than 65 years, a hazard ratio of 2.9 that was statistically significant. In contrast, the incidence of the primary outcome among patients aged 65-74 years was just 30% higher, compared with patients aged less than 65 years, a difference that was not statistically significant.
Patients who underwent CEA showed no similar relationship between age and outcome. The incidence of the primary outcome among the CEA patients was roughly the same, about 3.5%, regardless of their age.
A second analysis that considered age as a continuous variable showed a sharply spiked increase in the risk for CAS patients, compared with CEA patients once they reached about age 73-75 years. Until about age 72, the rate of the primary outcome was nearly the same regardless of whether patients underwent CAS or CEA, but the risk for adverse outcomes rose “steeply” starting at about age 75 so that by age 79 the rate of the primary outcome approached 300% higher among the CAS patients compared with CEA patients, Dr. Voeks said.
She cautioned that the analysis included just 115 total primary-outcome events, which makes the incidence rate estimates somewhat imprecise, and that the data reflect outcomes in patients who were treated more than a decade ago, but these data remain the only reported results from large randomized trials that compared CAS and CEA in asymptomatic patients.
Dr. Voeks reported no disclosures.
SOURCE: Voeks JH al. Stroke. 2020 Feb 12;51[suppl 1], Abstract 70.
LOS ANGELES – Carotid artery stenting in older, asymptomatic patients with severe carotid artery stenosis is, in general, as bad an idea as it has already proven to be in symptomatic patients, with a multifold increase in adverse short- and mid-term outcomes, compared with similar older, asymptomatic patients who underwent endarterectomy, according to a combined-study analysis with more than 2,500 patients.
The risk for poor outcomes in patients with severe but asymptomatic carotid artery disease who underwent carotid artery stenting (CAS), compared with patients who instead underwent carotid endarterectomy (CEA) “abruptly increased around age 75,” in an analysis that combined data from the two major, published, randomized trials that compared these two interventions in this patient population, Jenifer H. Voeks, PhD said at the International Stroke Conference sponsored by the American Heart Association.
These results “largely mirror” the findings from a similar combined analysis of data from four major, randomized trials that compared CEA and CAS in patients with symptomatic carotid disease, she noted (Lancet. 2016 Mar 26;387[10025]:1305-11). The new findings in an expanded population of asymptomatic patients derived from two separate studies showed that, in patients aged 70 years or less, “CAS appears to be a reasonable alternative to CEA, but above age 70, and certainly above age 75, age-related risk factors such as cerebrovascular anatomy and underlying cerebral pathology should be carefully considered before selecting patients for CAS,” said Dr. Voeks, a neurology researcher at the Medical University of South Carolina, Charleston. Many experts also believe that, for asymptomatic patients, intensive medical management may have returned as an alternative to either of these invasive approaches for treating severe carotid stenosis and has achieved a level of equipoise that led to the launch of CREST 2 (Carotid Revascularization and Medical Management for Asymptomatic Carotid Stenosis Trial). CREST 2 is comparing CEA and CAS with medical management, and is scheduled to report results in 2021.
The data for this analysis in asymptomatic patients came from the first CREST (Carotid Revascularization Endarterectomy Versus Stenting Trial; N Engl J Med. 2010 Jul 1;363[1]:11-23), which included 1,181 asymptomatic patients (nearly half the total enrollment, with symptomatic patients making up the balance) and had no age ceiling, as well as all 1,453 patients from the ACT 1 trial, which enrolled exclusively asymptomatic patients and limited enrollment to patients aged 79 years or less (N Engl J Med. 2016 Mar 17;374[11]: 1011-20). Because the maximum age of patients in ACT 1 was 79 years, for this analysis Dr. Voeks and associates only included the 1,091 asymptomatic CREST patients who also were within the same age ceiling. The resulting cohort of 2,544 included 1,637 patients who underwent CAS and 907 who underwent CEA (because of a 3:1 randomization ratio in ACT 1), creating the largest data set to compare CAS and CEA by age in asymptomatic patients, Dr. Voeks noted. When subdivided by age, 30% of the cohort was younger that 65 years, 54% were 65-74, and 16% were 75-79.
The primary outcome the researchers used for their analysis was the combined incidence of periprocedural stroke, MI, or death, plus the incidence of ipsilateral stroke during 4 years of follow-up post procedure. Among patients who underwent CAS, this outcome occurred in roughly 9% of patients aged 75-79 years and in about 3% of those younger than 65 years, a hazard ratio of 2.9 that was statistically significant. In contrast, the incidence of the primary outcome among patients aged 65-74 years was just 30% higher, compared with patients aged less than 65 years, a difference that was not statistically significant.
Patients who underwent CEA showed no similar relationship between age and outcome. The incidence of the primary outcome among the CEA patients was roughly the same, about 3.5%, regardless of their age.
A second analysis that considered age as a continuous variable showed a sharply spiked increase in the risk for CAS patients, compared with CEA patients once they reached about age 73-75 years. Until about age 72, the rate of the primary outcome was nearly the same regardless of whether patients underwent CAS or CEA, but the risk for adverse outcomes rose “steeply” starting at about age 75 so that by age 79 the rate of the primary outcome approached 300% higher among the CAS patients compared with CEA patients, Dr. Voeks said.
She cautioned that the analysis included just 115 total primary-outcome events, which makes the incidence rate estimates somewhat imprecise, and that the data reflect outcomes in patients who were treated more than a decade ago, but these data remain the only reported results from large randomized trials that compared CAS and CEA in asymptomatic patients.
Dr. Voeks reported no disclosures.
SOURCE: Voeks JH al. Stroke. 2020 Feb 12;51[suppl 1], Abstract 70.
REPORTING FROM ISC 2020
Nonculprit Lesion PCI Strategies in Patients With STEMI Without Cardiogenic Shock
Study Overview
Objective. To determine whether percutaneous coronary intervention (PCI) of a nonculprit lesion in patients with ST-segment elevation myocardial infarction (STEMI) reduces the risk of cardiovascular death or myocardial infarction.
Design. International, multicenter, randomized controlled trial blinded to outcome.
Setting and participants. Patients with STEMI who had multivessel coronary disease and had undergone successful PCI to the culprit lesion.
Intervention. A total of 4041 patients were randomly assigned to either PCI of angiographically significant nonculprit lesions or optimal medical therapy without further revascularization. Randomization was stratified according to intended timing of nonculprit lesion PCI (either during or after the index hospitalization).
Main outcome measures. The first co-primary endpoint was the composite of cardiovascular death or myocardial infarction (MI). The second co-primary endpoint was the composite of cardiovascular death, MI or ischemia-driven revascularization.
Main results. At a median follow-up of 3 years, the composite of cardiovascular death or MI occurred in 158 of the 2016 patients (7.8%) in the nonculprit PCI group and in 213 of the 2025 patients (10.5%) in the culprit-lesion-only group (hazard ratio, 0.73; 95% confidence interval [CI], 0.60-0.91; P = 0.004). The second co-primary endpoint occurred in 179 patients (8.9%) in the nonculprit PCI group and in 339 patients (16.7%) in the culprit-lesion-only group (hazard ratio, 0.51; 95% CI, 0.43-0.61; P < 0.001).
Conclusion. Among patients with STEMI and multivessel disease, those who underwent complete revascularization with nonculprit lesion PCI had lower rates of cardiovascular death or MI compared to patients with culprit-lesion-only revascularization.
Commentary
Patients presenting with STEMI often have multivessel disease.1 Although it is known that mortality can be reduced by early revascularization of the culprit vessel,2 whether the nonculprit vessel should be revascularized at the time of presentation with STEMI remains controversial.
Recently, multiple studies have reported the benefit of nonculprit vessel revascularization in patients presenting with hemodynamically stable STEMI. Four trials (PRAMI, CvPRIT, DANAMI-PRIMULTI, and COMPARE ACUTE) investigated this clinical question with different designs, and all reported benefit of nonculprit vessel revascularization compared to a culprit-only strategy.3-6 However, the differences in the composite endpoints were mainly driven by the softer endpoints used in these trials, such as refractory angina and ischemia-driven revascularization, and none of these previous trials had adequate power to evaluate differences in hard outcomes, such as death or MI.
In this context, Mehta et al investigated whether achieving complete revascularization by performing PCI on nonculprit vessels would improve the composite of cardiovascular death or MI compared to the culprit-only strategy by conducting a well-designed randomized controlled study. At median follow-up of 3 years, patients who underwent nonculprit vessel PCI had a lower incidence of death or MI compared to those who received the culprit-only strategy (7.8% versus 10.5%). The second co-primary endpoint (composite of death, MI, or ischemia-driven revascularization) also occurred significantly less frequently in the nonculprit PCI group than in the culprit-only PCI group (8.9% versus 16.7%).
The current study has a number of strengths. First, this was a multicenter, international study, and a large number of patients were enrolled (> 4000), achieving adequate power to evaluate for the composite of death and MI. Second, the treatments the patients received reflect contemporary medical therapy and interventional practice: the third-generation thienopyridine ticagrelor, high-dose statins, and ACE inhibitors were prescribed at high rates, and radial access (> 80%) and current-generation drug-eluting stents were used at high rates as well. Third, all angiograms were reviewed by the core lab to evaluate for completeness of revascularization. Fourth, the trial mandated use of fractional flow reserve to assess lesion stenosis 50% to 69% before considering revascularization, ensuring that only ischemic or very-high-grade lesions were revascularized. Fifth, the crossover rate in each group was low compared to the previous studies (4.7% into the complete revascularization group, 3.9% into the lesion-only group). Finally, this study evaluated the timing of the nonculprit PCI. Randomization to each group was stratified according to the intended timing of the nonculprit PCI during the index hospitalization or after hospital discharge (within 45 days). They found that benefit was consistent regardless of when the nonculprit PCI was performed.
Although the COMPLETE study’s design has a number of strengths, it is important to note that patients enrolled in this trial represent a lower-risk STEMI population. Patients with complex anatomy likely were not included, as evidenced by a lower SYNTAX score (mean, 16). Furthermore, no patients who presented with STEMI complicated by cardiogenic shock were enrolled. In the recent CULPRIT SHOCK trial, which focused on patients who had multivessel disease, acute MI, and cardiogenic shock, patients who underwent the culprit-only strategy had a lower rate of death or renal replacement therapy, as compared to patients who underwent immediate complete revascularization.7 Therefore, whether the findings from the COMPLETE study can be extended to a sicker population requires further study.
In 2015, the results from the previous trials, such as PRAMI and CvPRIT, led to a focused update of US PCI guidelines.8 Recommendations for noninfarct-related artery PCI in hemodynamically stable patients presenting with acute MI were upgraded from class III to class IIb. The results from the COMPLETE trial will likely influence the future guidelines, with stronger recommendations toward complete revascularization in patients presenting with hemodynamically stable STEMI.
Applications for Clinical Practice
In patients presenting with hemodynamically stable STEMI, staged complete revascularization, including the nonculprit vessel, should be considered.
– Taishi Hirai, MD, University of Missouri, Columbia, MO, and John EA Blair, MD, University of Chicago Medical Center, Chicago, IL
1. Park DW, Clare RM, Schulte PJ, et al. Extent, location, and clinical significance of non-infarct-related coronary artery disease among patients with ST-elevation myocardial infarction. JAMA. 2014;312:2019-2027.
2. Hochman JS, Sleeper LA, Webb JG, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock. N Engl J Med. 1999;341:625-634.
3. Wald DS, Morris JK, Wald NJ, et al. Randomized trial of preventive angioplasty in myocardial infarction. N Engl J Med. 2013;369:1115-1123.
4. Gershlick AH, Khan JN, Kelly DJ, et al. Randomized trial of complete versus lesion-only revascularization in patients undergoing primary percutaneous coronary intervention for STEMI and multivessel disease: the CvLPRIT trial. J Am Coll Cardiol. 2015;65:963-972.
5. Engstrom T, Kelbaek H, Helqvist S, et al. Complete revascularisation versus treatment of the culprit lesion only in patients with ST-segment elevation myocardial infarction and multivessel disease (DANAMI-3-PRIMULTI): an open-label, randomised controlled trial. Lancet. 2015;386:665-671.
6. Smits PC, Abdel-Wahab M, Neumann FJ, et al. Fractional flow reserve-guided multivessel angioplasty in myocardial infarction. N Engl J Med. 2017;376:1234-1244.
7. Thiele H, Akin I, Sandri M, et al. PCI strategies in patients with acute myocardial infarction and cardiogenic shock. N Engl J Med. 2017;377:2419-2432.
8. Levine GN, Bates ER, Blankenship JC, et al. 2015 ACC/AHA/SCAI focused update on primary percutaneous coronary intervention for patients with ST-elevation myocardial infarction: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention and the 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction. J Am Coll Cardiol. 2016;67:1235-1250.
Study Overview
Objective. To determine whether percutaneous coronary intervention (PCI) of a nonculprit lesion in patients with ST-segment elevation myocardial infarction (STEMI) reduces the risk of cardiovascular death or myocardial infarction.
Design. International, multicenter, randomized controlled trial blinded to outcome.
Setting and participants. Patients with STEMI who had multivessel coronary disease and had undergone successful PCI to the culprit lesion.
Intervention. A total of 4041 patients were randomly assigned to either PCI of angiographically significant nonculprit lesions or optimal medical therapy without further revascularization. Randomization was stratified according to intended timing of nonculprit lesion PCI (either during or after the index hospitalization).
Main outcome measures. The first co-primary endpoint was the composite of cardiovascular death or myocardial infarction (MI). The second co-primary endpoint was the composite of cardiovascular death, MI or ischemia-driven revascularization.
Main results. At a median follow-up of 3 years, the composite of cardiovascular death or MI occurred in 158 of the 2016 patients (7.8%) in the nonculprit PCI group and in 213 of the 2025 patients (10.5%) in the culprit-lesion-only group (hazard ratio, 0.73; 95% confidence interval [CI], 0.60-0.91; P = 0.004). The second co-primary endpoint occurred in 179 patients (8.9%) in the nonculprit PCI group and in 339 patients (16.7%) in the culprit-lesion-only group (hazard ratio, 0.51; 95% CI, 0.43-0.61; P < 0.001).
Conclusion. Among patients with STEMI and multivessel disease, those who underwent complete revascularization with nonculprit lesion PCI had lower rates of cardiovascular death or MI compared to patients with culprit-lesion-only revascularization.
Commentary
Patients presenting with STEMI often have multivessel disease.1 Although it is known that mortality can be reduced by early revascularization of the culprit vessel,2 whether the nonculprit vessel should be revascularized at the time of presentation with STEMI remains controversial.
Recently, multiple studies have reported the benefit of nonculprit vessel revascularization in patients presenting with hemodynamically stable STEMI. Four trials (PRAMI, CvPRIT, DANAMI-PRIMULTI, and COMPARE ACUTE) investigated this clinical question with different designs, and all reported benefit of nonculprit vessel revascularization compared to a culprit-only strategy.3-6 However, the differences in the composite endpoints were mainly driven by the softer endpoints used in these trials, such as refractory angina and ischemia-driven revascularization, and none of these previous trials had adequate power to evaluate differences in hard outcomes, such as death or MI.
In this context, Mehta et al investigated whether achieving complete revascularization by performing PCI on nonculprit vessels would improve the composite of cardiovascular death or MI compared to the culprit-only strategy by conducting a well-designed randomized controlled study. At median follow-up of 3 years, patients who underwent nonculprit vessel PCI had a lower incidence of death or MI compared to those who received the culprit-only strategy (7.8% versus 10.5%). The second co-primary endpoint (composite of death, MI, or ischemia-driven revascularization) also occurred significantly less frequently in the nonculprit PCI group than in the culprit-only PCI group (8.9% versus 16.7%).
The current study has a number of strengths. First, this was a multicenter, international study, and a large number of patients were enrolled (> 4000), achieving adequate power to evaluate for the composite of death and MI. Second, the treatments the patients received reflect contemporary medical therapy and interventional practice: the third-generation thienopyridine ticagrelor, high-dose statins, and ACE inhibitors were prescribed at high rates, and radial access (> 80%) and current-generation drug-eluting stents were used at high rates as well. Third, all angiograms were reviewed by the core lab to evaluate for completeness of revascularization. Fourth, the trial mandated use of fractional flow reserve to assess lesion stenosis 50% to 69% before considering revascularization, ensuring that only ischemic or very-high-grade lesions were revascularized. Fifth, the crossover rate in each group was low compared to the previous studies (4.7% into the complete revascularization group, 3.9% into the lesion-only group). Finally, this study evaluated the timing of the nonculprit PCI. Randomization to each group was stratified according to the intended timing of the nonculprit PCI during the index hospitalization or after hospital discharge (within 45 days). They found that benefit was consistent regardless of when the nonculprit PCI was performed.
Although the COMPLETE study’s design has a number of strengths, it is important to note that patients enrolled in this trial represent a lower-risk STEMI population. Patients with complex anatomy likely were not included, as evidenced by a lower SYNTAX score (mean, 16). Furthermore, no patients who presented with STEMI complicated by cardiogenic shock were enrolled. In the recent CULPRIT SHOCK trial, which focused on patients who had multivessel disease, acute MI, and cardiogenic shock, patients who underwent the culprit-only strategy had a lower rate of death or renal replacement therapy, as compared to patients who underwent immediate complete revascularization.7 Therefore, whether the findings from the COMPLETE study can be extended to a sicker population requires further study.
In 2015, the results from the previous trials, such as PRAMI and CvPRIT, led to a focused update of US PCI guidelines.8 Recommendations for noninfarct-related artery PCI in hemodynamically stable patients presenting with acute MI were upgraded from class III to class IIb. The results from the COMPLETE trial will likely influence the future guidelines, with stronger recommendations toward complete revascularization in patients presenting with hemodynamically stable STEMI.
Applications for Clinical Practice
In patients presenting with hemodynamically stable STEMI, staged complete revascularization, including the nonculprit vessel, should be considered.
– Taishi Hirai, MD, University of Missouri, Columbia, MO, and John EA Blair, MD, University of Chicago Medical Center, Chicago, IL
Study Overview
Objective. To determine whether percutaneous coronary intervention (PCI) of a nonculprit lesion in patients with ST-segment elevation myocardial infarction (STEMI) reduces the risk of cardiovascular death or myocardial infarction.
Design. International, multicenter, randomized controlled trial blinded to outcome.
Setting and participants. Patients with STEMI who had multivessel coronary disease and had undergone successful PCI to the culprit lesion.
Intervention. A total of 4041 patients were randomly assigned to either PCI of angiographically significant nonculprit lesions or optimal medical therapy without further revascularization. Randomization was stratified according to intended timing of nonculprit lesion PCI (either during or after the index hospitalization).
Main outcome measures. The first co-primary endpoint was the composite of cardiovascular death or myocardial infarction (MI). The second co-primary endpoint was the composite of cardiovascular death, MI or ischemia-driven revascularization.
Main results. At a median follow-up of 3 years, the composite of cardiovascular death or MI occurred in 158 of the 2016 patients (7.8%) in the nonculprit PCI group and in 213 of the 2025 patients (10.5%) in the culprit-lesion-only group (hazard ratio, 0.73; 95% confidence interval [CI], 0.60-0.91; P = 0.004). The second co-primary endpoint occurred in 179 patients (8.9%) in the nonculprit PCI group and in 339 patients (16.7%) in the culprit-lesion-only group (hazard ratio, 0.51; 95% CI, 0.43-0.61; P < 0.001).
Conclusion. Among patients with STEMI and multivessel disease, those who underwent complete revascularization with nonculprit lesion PCI had lower rates of cardiovascular death or MI compared to patients with culprit-lesion-only revascularization.
Commentary
Patients presenting with STEMI often have multivessel disease.1 Although it is known that mortality can be reduced by early revascularization of the culprit vessel,2 whether the nonculprit vessel should be revascularized at the time of presentation with STEMI remains controversial.
Recently, multiple studies have reported the benefit of nonculprit vessel revascularization in patients presenting with hemodynamically stable STEMI. Four trials (PRAMI, CvPRIT, DANAMI-PRIMULTI, and COMPARE ACUTE) investigated this clinical question with different designs, and all reported benefit of nonculprit vessel revascularization compared to a culprit-only strategy.3-6 However, the differences in the composite endpoints were mainly driven by the softer endpoints used in these trials, such as refractory angina and ischemia-driven revascularization, and none of these previous trials had adequate power to evaluate differences in hard outcomes, such as death or MI.
In this context, Mehta et al investigated whether achieving complete revascularization by performing PCI on nonculprit vessels would improve the composite of cardiovascular death or MI compared to the culprit-only strategy by conducting a well-designed randomized controlled study. At median follow-up of 3 years, patients who underwent nonculprit vessel PCI had a lower incidence of death or MI compared to those who received the culprit-only strategy (7.8% versus 10.5%). The second co-primary endpoint (composite of death, MI, or ischemia-driven revascularization) also occurred significantly less frequently in the nonculprit PCI group than in the culprit-only PCI group (8.9% versus 16.7%).
The current study has a number of strengths. First, this was a multicenter, international study, and a large number of patients were enrolled (> 4000), achieving adequate power to evaluate for the composite of death and MI. Second, the treatments the patients received reflect contemporary medical therapy and interventional practice: the third-generation thienopyridine ticagrelor, high-dose statins, and ACE inhibitors were prescribed at high rates, and radial access (> 80%) and current-generation drug-eluting stents were used at high rates as well. Third, all angiograms were reviewed by the core lab to evaluate for completeness of revascularization. Fourth, the trial mandated use of fractional flow reserve to assess lesion stenosis 50% to 69% before considering revascularization, ensuring that only ischemic or very-high-grade lesions were revascularized. Fifth, the crossover rate in each group was low compared to the previous studies (4.7% into the complete revascularization group, 3.9% into the lesion-only group). Finally, this study evaluated the timing of the nonculprit PCI. Randomization to each group was stratified according to the intended timing of the nonculprit PCI during the index hospitalization or after hospital discharge (within 45 days). They found that benefit was consistent regardless of when the nonculprit PCI was performed.
Although the COMPLETE study’s design has a number of strengths, it is important to note that patients enrolled in this trial represent a lower-risk STEMI population. Patients with complex anatomy likely were not included, as evidenced by a lower SYNTAX score (mean, 16). Furthermore, no patients who presented with STEMI complicated by cardiogenic shock were enrolled. In the recent CULPRIT SHOCK trial, which focused on patients who had multivessel disease, acute MI, and cardiogenic shock, patients who underwent the culprit-only strategy had a lower rate of death or renal replacement therapy, as compared to patients who underwent immediate complete revascularization.7 Therefore, whether the findings from the COMPLETE study can be extended to a sicker population requires further study.
In 2015, the results from the previous trials, such as PRAMI and CvPRIT, led to a focused update of US PCI guidelines.8 Recommendations for noninfarct-related artery PCI in hemodynamically stable patients presenting with acute MI were upgraded from class III to class IIb. The results from the COMPLETE trial will likely influence the future guidelines, with stronger recommendations toward complete revascularization in patients presenting with hemodynamically stable STEMI.
Applications for Clinical Practice
In patients presenting with hemodynamically stable STEMI, staged complete revascularization, including the nonculprit vessel, should be considered.
– Taishi Hirai, MD, University of Missouri, Columbia, MO, and John EA Blair, MD, University of Chicago Medical Center, Chicago, IL
1. Park DW, Clare RM, Schulte PJ, et al. Extent, location, and clinical significance of non-infarct-related coronary artery disease among patients with ST-elevation myocardial infarction. JAMA. 2014;312:2019-2027.
2. Hochman JS, Sleeper LA, Webb JG, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock. N Engl J Med. 1999;341:625-634.
3. Wald DS, Morris JK, Wald NJ, et al. Randomized trial of preventive angioplasty in myocardial infarction. N Engl J Med. 2013;369:1115-1123.
4. Gershlick AH, Khan JN, Kelly DJ, et al. Randomized trial of complete versus lesion-only revascularization in patients undergoing primary percutaneous coronary intervention for STEMI and multivessel disease: the CvLPRIT trial. J Am Coll Cardiol. 2015;65:963-972.
5. Engstrom T, Kelbaek H, Helqvist S, et al. Complete revascularisation versus treatment of the culprit lesion only in patients with ST-segment elevation myocardial infarction and multivessel disease (DANAMI-3-PRIMULTI): an open-label, randomised controlled trial. Lancet. 2015;386:665-671.
6. Smits PC, Abdel-Wahab M, Neumann FJ, et al. Fractional flow reserve-guided multivessel angioplasty in myocardial infarction. N Engl J Med. 2017;376:1234-1244.
7. Thiele H, Akin I, Sandri M, et al. PCI strategies in patients with acute myocardial infarction and cardiogenic shock. N Engl J Med. 2017;377:2419-2432.
8. Levine GN, Bates ER, Blankenship JC, et al. 2015 ACC/AHA/SCAI focused update on primary percutaneous coronary intervention for patients with ST-elevation myocardial infarction: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention and the 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction. J Am Coll Cardiol. 2016;67:1235-1250.
1. Park DW, Clare RM, Schulte PJ, et al. Extent, location, and clinical significance of non-infarct-related coronary artery disease among patients with ST-elevation myocardial infarction. JAMA. 2014;312:2019-2027.
2. Hochman JS, Sleeper LA, Webb JG, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock. N Engl J Med. 1999;341:625-634.
3. Wald DS, Morris JK, Wald NJ, et al. Randomized trial of preventive angioplasty in myocardial infarction. N Engl J Med. 2013;369:1115-1123.
4. Gershlick AH, Khan JN, Kelly DJ, et al. Randomized trial of complete versus lesion-only revascularization in patients undergoing primary percutaneous coronary intervention for STEMI and multivessel disease: the CvLPRIT trial. J Am Coll Cardiol. 2015;65:963-972.
5. Engstrom T, Kelbaek H, Helqvist S, et al. Complete revascularisation versus treatment of the culprit lesion only in patients with ST-segment elevation myocardial infarction and multivessel disease (DANAMI-3-PRIMULTI): an open-label, randomised controlled trial. Lancet. 2015;386:665-671.
6. Smits PC, Abdel-Wahab M, Neumann FJ, et al. Fractional flow reserve-guided multivessel angioplasty in myocardial infarction. N Engl J Med. 2017;376:1234-1244.
7. Thiele H, Akin I, Sandri M, et al. PCI strategies in patients with acute myocardial infarction and cardiogenic shock. N Engl J Med. 2017;377:2419-2432.
8. Levine GN, Bates ER, Blankenship JC, et al. 2015 ACC/AHA/SCAI focused update on primary percutaneous coronary intervention for patients with ST-elevation myocardial infarction: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention and the 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction. J Am Coll Cardiol. 2016;67:1235-1250.
Draft ACR Takayasu’s guidelines: Surgery is the last resort
ATLANTA – One of the goals in soon-to-be-published Takayasu’s arteritis guidelines from the American College of Rheumatology is to wean patients off high-dose steroids once they are in remission.
This recommendation is in opposition to another option – namely, switching these patients to low-dose glucocorticoids. The idea is to prevent long-term side effects, particularly in children. The guidelines also recommend against escalating immunotherapy for asymptomatic increases in inflammatory markers and generally recommend against surgery – stenting in most cases – unless there is threat to life, limb, or organ and also if limb pain is so severe it cramps quality of life and dose escalation doesn’t get the job done. If surgery is planned, patients should be on perioperative steroids if there’s active disease.
It’s draft guidance for now, but it’s probably what the final document will say when it’s published in 2020, according to a presentation at the annual meeting of the American College of Rheumatology by one of the authors, Anisha Dua, MD, an associate professor of rheumatology at Northwestern University, Chicago. She gave a sneak preview at the meeting.
In general, severe, active Takayasu’s calls for high-dose oral steroids in conjunction with a nonsteroid immunosuppressive, such as methotrexate, azathioprine, leflunomide, or mycophenolate mofetil. There’s evidence that dual therapy gives a more durable remission and also reduces the need for steroids.
When that approach doesn’t do the trick, the next step is a tumor necrosis factor (TNF) inhibitor. There’s evidence for infliximab, adalimumab, certolizumab, and etanercept. Dr. Dua noted, “We still can consider” tocilizumab, but it failed to meet its primary endpoint in a randomized trial, and evidence for other biologics is sparse or nonexistent. “TNF inhibitors are the first line” for refractory disease, Dr. Dua said.
The steroid taper comes after 6-12 months of remission. Given their toxicity, “our goal for steroids is zero,” especially in pediatric populations. Even in remission, patients should have a clinical assessment, including inflammatory markers, every 3-12 months.
A rise in C-reactive protein or erythrocyte sedimentation rate, with no new symptoms, might be a reason for more frequent monitoring, but it’s not a reason to escalate immunosuppression. That should be kept in reserve for new vascular lesions, rapid progression on an old one, or worsening of organ or limb ischemia.
“We recommend [escalation] over surgical intervention” because patients often develop collateral circulation that solves the problem; it also gives the disease time to quiet down should the patient eventually go into surgery. Immediate surgery is reserved for organ or life-threatening disease, Dr. Dua said.
“Takayasu’s is different from other vasculitides in the sense that patients often present with certain nonspecific constitutional symptoms,” and there’s not a lot of pathology or histology to work with, “so we do tend to rely on imaging a lot,” Dr. Dua said.
Angiography has fallen out of favor because it’s invasive and exposes patients to radiation, among other problems. The field has moved to noninvasive imaging such as color Doppler ultrasound, CT angiography, magnetic resonance angiography, and PET CT.
“We do recommend regularly scheduled, noninvasive imaging every 6-12 months, in addition to the routine clinical assessment,” except in children with inactive disease; the risk of sedation outweighs the imaging benefit, Dr. Dua said.
In patients with single-vessel cranial or cervical stenosis, without symptoms, “we recommend medical over surgical management because of the risk of surgery. Surgery can be considered for multivessel involvement,” she said.
She and her colleagues also recommend medical management for renal artery stenosis, including antihypertensives and immunotherapy escalation for active disease. Surgery is considered for refractory hypertension or worsening kidney function
Dr. Dua is a primary investigator and adviser for Chemocentryx and an adviser for Novartis and AbbVie.
ATLANTA – One of the goals in soon-to-be-published Takayasu’s arteritis guidelines from the American College of Rheumatology is to wean patients off high-dose steroids once they are in remission.
This recommendation is in opposition to another option – namely, switching these patients to low-dose glucocorticoids. The idea is to prevent long-term side effects, particularly in children. The guidelines also recommend against escalating immunotherapy for asymptomatic increases in inflammatory markers and generally recommend against surgery – stenting in most cases – unless there is threat to life, limb, or organ and also if limb pain is so severe it cramps quality of life and dose escalation doesn’t get the job done. If surgery is planned, patients should be on perioperative steroids if there’s active disease.
It’s draft guidance for now, but it’s probably what the final document will say when it’s published in 2020, according to a presentation at the annual meeting of the American College of Rheumatology by one of the authors, Anisha Dua, MD, an associate professor of rheumatology at Northwestern University, Chicago. She gave a sneak preview at the meeting.
In general, severe, active Takayasu’s calls for high-dose oral steroids in conjunction with a nonsteroid immunosuppressive, such as methotrexate, azathioprine, leflunomide, or mycophenolate mofetil. There’s evidence that dual therapy gives a more durable remission and also reduces the need for steroids.
When that approach doesn’t do the trick, the next step is a tumor necrosis factor (TNF) inhibitor. There’s evidence for infliximab, adalimumab, certolizumab, and etanercept. Dr. Dua noted, “We still can consider” tocilizumab, but it failed to meet its primary endpoint in a randomized trial, and evidence for other biologics is sparse or nonexistent. “TNF inhibitors are the first line” for refractory disease, Dr. Dua said.
The steroid taper comes after 6-12 months of remission. Given their toxicity, “our goal for steroids is zero,” especially in pediatric populations. Even in remission, patients should have a clinical assessment, including inflammatory markers, every 3-12 months.
A rise in C-reactive protein or erythrocyte sedimentation rate, with no new symptoms, might be a reason for more frequent monitoring, but it’s not a reason to escalate immunosuppression. That should be kept in reserve for new vascular lesions, rapid progression on an old one, or worsening of organ or limb ischemia.
“We recommend [escalation] over surgical intervention” because patients often develop collateral circulation that solves the problem; it also gives the disease time to quiet down should the patient eventually go into surgery. Immediate surgery is reserved for organ or life-threatening disease, Dr. Dua said.
“Takayasu’s is different from other vasculitides in the sense that patients often present with certain nonspecific constitutional symptoms,” and there’s not a lot of pathology or histology to work with, “so we do tend to rely on imaging a lot,” Dr. Dua said.
Angiography has fallen out of favor because it’s invasive and exposes patients to radiation, among other problems. The field has moved to noninvasive imaging such as color Doppler ultrasound, CT angiography, magnetic resonance angiography, and PET CT.
“We do recommend regularly scheduled, noninvasive imaging every 6-12 months, in addition to the routine clinical assessment,” except in children with inactive disease; the risk of sedation outweighs the imaging benefit, Dr. Dua said.
In patients with single-vessel cranial or cervical stenosis, without symptoms, “we recommend medical over surgical management because of the risk of surgery. Surgery can be considered for multivessel involvement,” she said.
She and her colleagues also recommend medical management for renal artery stenosis, including antihypertensives and immunotherapy escalation for active disease. Surgery is considered for refractory hypertension or worsening kidney function
Dr. Dua is a primary investigator and adviser for Chemocentryx and an adviser for Novartis and AbbVie.
ATLANTA – One of the goals in soon-to-be-published Takayasu’s arteritis guidelines from the American College of Rheumatology is to wean patients off high-dose steroids once they are in remission.
This recommendation is in opposition to another option – namely, switching these patients to low-dose glucocorticoids. The idea is to prevent long-term side effects, particularly in children. The guidelines also recommend against escalating immunotherapy for asymptomatic increases in inflammatory markers and generally recommend against surgery – stenting in most cases – unless there is threat to life, limb, or organ and also if limb pain is so severe it cramps quality of life and dose escalation doesn’t get the job done. If surgery is planned, patients should be on perioperative steroids if there’s active disease.
It’s draft guidance for now, but it’s probably what the final document will say when it’s published in 2020, according to a presentation at the annual meeting of the American College of Rheumatology by one of the authors, Anisha Dua, MD, an associate professor of rheumatology at Northwestern University, Chicago. She gave a sneak preview at the meeting.
In general, severe, active Takayasu’s calls for high-dose oral steroids in conjunction with a nonsteroid immunosuppressive, such as methotrexate, azathioprine, leflunomide, or mycophenolate mofetil. There’s evidence that dual therapy gives a more durable remission and also reduces the need for steroids.
When that approach doesn’t do the trick, the next step is a tumor necrosis factor (TNF) inhibitor. There’s evidence for infliximab, adalimumab, certolizumab, and etanercept. Dr. Dua noted, “We still can consider” tocilizumab, but it failed to meet its primary endpoint in a randomized trial, and evidence for other biologics is sparse or nonexistent. “TNF inhibitors are the first line” for refractory disease, Dr. Dua said.
The steroid taper comes after 6-12 months of remission. Given their toxicity, “our goal for steroids is zero,” especially in pediatric populations. Even in remission, patients should have a clinical assessment, including inflammatory markers, every 3-12 months.
A rise in C-reactive protein or erythrocyte sedimentation rate, with no new symptoms, might be a reason for more frequent monitoring, but it’s not a reason to escalate immunosuppression. That should be kept in reserve for new vascular lesions, rapid progression on an old one, or worsening of organ or limb ischemia.
“We recommend [escalation] over surgical intervention” because patients often develop collateral circulation that solves the problem; it also gives the disease time to quiet down should the patient eventually go into surgery. Immediate surgery is reserved for organ or life-threatening disease, Dr. Dua said.
“Takayasu’s is different from other vasculitides in the sense that patients often present with certain nonspecific constitutional symptoms,” and there’s not a lot of pathology or histology to work with, “so we do tend to rely on imaging a lot,” Dr. Dua said.
Angiography has fallen out of favor because it’s invasive and exposes patients to radiation, among other problems. The field has moved to noninvasive imaging such as color Doppler ultrasound, CT angiography, magnetic resonance angiography, and PET CT.
“We do recommend regularly scheduled, noninvasive imaging every 6-12 months, in addition to the routine clinical assessment,” except in children with inactive disease; the risk of sedation outweighs the imaging benefit, Dr. Dua said.
In patients with single-vessel cranial or cervical stenosis, without symptoms, “we recommend medical over surgical management because of the risk of surgery. Surgery can be considered for multivessel involvement,” she said.
She and her colleagues also recommend medical management for renal artery stenosis, including antihypertensives and immunotherapy escalation for active disease. Surgery is considered for refractory hypertension or worsening kidney function
Dr. Dua is a primary investigator and adviser for Chemocentryx and an adviser for Novartis and AbbVie.
REPORTING FROM ACR 2019
ODYSSEY Outcomes: Alirocumab cut stroke, PAD, VTE
PHILADELPHIA – Treatment with the PCSK9 inhibitor alirocumab linked with a significant cut in the rates of peripheral artery disease events and ischemic strokes without increasing the rate of hemorrhagic strokes, and alirocumab treatment also showed a trend toward an association with a reduced rate of venous thromboembolic events in prespecified, ancillary analyses of data collected from more than 18,000 patients in the ODYSSEY Outcomes trial.
The analyses that looked at peripheral artery disease (PAD) events and venous thromboembolism (VTE) events also suggested that the apparent ability of alirocumab to reduce their incidence may have been largely mediated through a reduction in Lp(a) lipoprotein, with less of a contribution from the drug’s primary action of reducing LDL cholesterol, Gregory G. Schwartz, MD, said at the American Heart Association scientific sessions.
When used on top of intensive statin treatment, as in the ODYSSEY Outcomes trial, treatment with the PCSK9 inhibitor alirocumab “may be useful to prevent PAD events, particularly in patients with high levels of Lp(a),” said Dr. Schwartz, professor of medicine at the University of Colorado Denver in Aurora. In the analysis he reported, patients treated with alirocumab for a median of 2.8 years had a statistically significant 31% reduced rate of PAD or VTE event and a significant 31% reduced rate of PAD events alone, compared with control patients who received placebo, he reported. Alirocumab treatment was also associated with a 33% lower rate of VTE events only, but the overall rate of these events was low, and this difference just missed statistical significance with a P value of .06.
“Levels of Lp(a), but not LDL cholesterol, predicted the risk of PAD events,” and in patients on alirocumab treatment “the magnitude of Lp(a) reduction, but not LDL-cholesterol reduction, was associated with a reduction in PAD events and VTE.” The reduction in PAD events linked with alirocumab treatment “may be related to Lp(a) lowering,” Dr. Schwartz suggested.
The link between alirocumab treatment and a reduction in ischemic stroke with no increase in hemorrhagic strokes appeared in a separate prespecified analysis from ODYSSEY Outcomes that looked at the rates of ischemic stroke, hemorrhagic stroke, and their combined incidence during the median 2.8 year of study follow-up. Patients treated with alirocumab had a statistically significant 27% reduction in their rate of ischemic strokes compared with patients on placebo, and a statistically significant 28% relative reduction in the rate of any stroke with alirocumab treatment, J. Wouter Jukema, MD, said in a separate report at the meeting. The rate of hemorrhagic strokes was small, and showed a nominal 17% reduction in patients treated with alirocumab, compared with controls, a difference that was not statistically significant.
Further analysis of the stroke outcomes also showed that these reductions in total strokes occurred with alirocumab treatment at roughly similar rates regardless of baseline level of LDL cholesterol or history of a prior cerebrovascular event. Analysis also showed that the rate of hemorrhagic strokes was consistently low regardless of the on-treatment level of LDL cholesterol. Even among patients whose LDL cholesterol level fell below 25 mg/dL on alirocumab treatment, the incidence of hemorrhagic strokes during follow-up was 0.1%, “a very reassuring finding,” said Dr. Jukema, professor of cardiology at Leiden (The Netherlands) University. The stroke analyses did not examine possible linkages of these effects with changes in level of Lp(a).
ODYSSEY Outcomes (Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab) included 18,924 patients who had experienced an acute coronary syndrome event within the prior 12 months and had an LDL cholesterol level of at least 70 mg/dL despite maximally tolerated statin treatment and randomized them to treatment with alirocumab or placebo. The primary endpoint was the combination of coronary heart disease death, nonfatal MI, ischemic stroke, and hospitalization for unstable angina, which alirocumab effectively reduced compared with placebo (N Engl J Med. 2018 Nov 29;379[22]:2097-107).
The PAD analysis tallied the combined rate of acute limb ischemia, revascularization, or amputation related to PAD, and the VTE cases included patients who developed deep vein thrombosis or pulmonary embolism. All cases were nonadjudicated reports from participating investigators. Because Lp(a) makes up a portion of LDL cholesterol, Dr. Schwartz and associates calculated adjusted values for LDL cholesterol that were independent of Lp(a).
In a multivariable analysis that adjusted for demographic and clinical characteristics as well as baseline Lp(a) and the calculated level of LDL cholesterol, every 1 mg/dL decrease in Lp(a) linked with a statistically significant, nearly 1% decrease in the rate of either a PAD or VTE event, while the change in LDL cholesterol had no significant relationship with this endpoint, said Dr. Schwartz.
The impact of Lp(a) lowering was most dramatic among the subgroups of patients who entered the study with the highest levels of Lp(a). “In the lowest quartile [for baseline level of Lp(a)] the effect of treatment [with alirocumab] was inconsequential; all of the action was in the upper two quartiles,” he said. Dr. Schwartz highlighted that 90% of patients in the study were on an “intense” statin dosage, and 97% received some statin treatment. Against that treatment background, the findings showed that patients still had residual cardiovascular disease risk that did not appear to respond to changes in LDL cholesterol but which did appear to respond to a reduction in Lp(a) produced by alirocumab. Dr. Schwartz further suggested that alirocumab’s reduction of Lp(a) might also mediate the drug’s apparent effect on reducing VTE incidence, possibly because Lp(a) is structurally similar to plasminogen and hence can have prothrombotic effects.
ODYSSEY Outcomes was sponsored by Sanofi and Regeneron, the companies that market alirocumab (Praluent). Dr. Schwartz has received research support from Sanofi and from Resverlogix, Roche, and The Medicines Company. Dr. Jukema has been a speaker for and received research support from Sanofi Regeneron, and has also been a speaker for Amgen, MSD, and Roche and has also received research support from Biotronik
SOURCE: Schwartz GG et al. AHA 2019, Abstract 309; Jukema JW et al. AHA 2019, Abstract 334.
PHILADELPHIA – Treatment with the PCSK9 inhibitor alirocumab linked with a significant cut in the rates of peripheral artery disease events and ischemic strokes without increasing the rate of hemorrhagic strokes, and alirocumab treatment also showed a trend toward an association with a reduced rate of venous thromboembolic events in prespecified, ancillary analyses of data collected from more than 18,000 patients in the ODYSSEY Outcomes trial.
The analyses that looked at peripheral artery disease (PAD) events and venous thromboembolism (VTE) events also suggested that the apparent ability of alirocumab to reduce their incidence may have been largely mediated through a reduction in Lp(a) lipoprotein, with less of a contribution from the drug’s primary action of reducing LDL cholesterol, Gregory G. Schwartz, MD, said at the American Heart Association scientific sessions.
When used on top of intensive statin treatment, as in the ODYSSEY Outcomes trial, treatment with the PCSK9 inhibitor alirocumab “may be useful to prevent PAD events, particularly in patients with high levels of Lp(a),” said Dr. Schwartz, professor of medicine at the University of Colorado Denver in Aurora. In the analysis he reported, patients treated with alirocumab for a median of 2.8 years had a statistically significant 31% reduced rate of PAD or VTE event and a significant 31% reduced rate of PAD events alone, compared with control patients who received placebo, he reported. Alirocumab treatment was also associated with a 33% lower rate of VTE events only, but the overall rate of these events was low, and this difference just missed statistical significance with a P value of .06.
“Levels of Lp(a), but not LDL cholesterol, predicted the risk of PAD events,” and in patients on alirocumab treatment “the magnitude of Lp(a) reduction, but not LDL-cholesterol reduction, was associated with a reduction in PAD events and VTE.” The reduction in PAD events linked with alirocumab treatment “may be related to Lp(a) lowering,” Dr. Schwartz suggested.
The link between alirocumab treatment and a reduction in ischemic stroke with no increase in hemorrhagic strokes appeared in a separate prespecified analysis from ODYSSEY Outcomes that looked at the rates of ischemic stroke, hemorrhagic stroke, and their combined incidence during the median 2.8 year of study follow-up. Patients treated with alirocumab had a statistically significant 27% reduction in their rate of ischemic strokes compared with patients on placebo, and a statistically significant 28% relative reduction in the rate of any stroke with alirocumab treatment, J. Wouter Jukema, MD, said in a separate report at the meeting. The rate of hemorrhagic strokes was small, and showed a nominal 17% reduction in patients treated with alirocumab, compared with controls, a difference that was not statistically significant.
Further analysis of the stroke outcomes also showed that these reductions in total strokes occurred with alirocumab treatment at roughly similar rates regardless of baseline level of LDL cholesterol or history of a prior cerebrovascular event. Analysis also showed that the rate of hemorrhagic strokes was consistently low regardless of the on-treatment level of LDL cholesterol. Even among patients whose LDL cholesterol level fell below 25 mg/dL on alirocumab treatment, the incidence of hemorrhagic strokes during follow-up was 0.1%, “a very reassuring finding,” said Dr. Jukema, professor of cardiology at Leiden (The Netherlands) University. The stroke analyses did not examine possible linkages of these effects with changes in level of Lp(a).
ODYSSEY Outcomes (Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab) included 18,924 patients who had experienced an acute coronary syndrome event within the prior 12 months and had an LDL cholesterol level of at least 70 mg/dL despite maximally tolerated statin treatment and randomized them to treatment with alirocumab or placebo. The primary endpoint was the combination of coronary heart disease death, nonfatal MI, ischemic stroke, and hospitalization for unstable angina, which alirocumab effectively reduced compared with placebo (N Engl J Med. 2018 Nov 29;379[22]:2097-107).
The PAD analysis tallied the combined rate of acute limb ischemia, revascularization, or amputation related to PAD, and the VTE cases included patients who developed deep vein thrombosis or pulmonary embolism. All cases were nonadjudicated reports from participating investigators. Because Lp(a) makes up a portion of LDL cholesterol, Dr. Schwartz and associates calculated adjusted values for LDL cholesterol that were independent of Lp(a).
In a multivariable analysis that adjusted for demographic and clinical characteristics as well as baseline Lp(a) and the calculated level of LDL cholesterol, every 1 mg/dL decrease in Lp(a) linked with a statistically significant, nearly 1% decrease in the rate of either a PAD or VTE event, while the change in LDL cholesterol had no significant relationship with this endpoint, said Dr. Schwartz.
The impact of Lp(a) lowering was most dramatic among the subgroups of patients who entered the study with the highest levels of Lp(a). “In the lowest quartile [for baseline level of Lp(a)] the effect of treatment [with alirocumab] was inconsequential; all of the action was in the upper two quartiles,” he said. Dr. Schwartz highlighted that 90% of patients in the study were on an “intense” statin dosage, and 97% received some statin treatment. Against that treatment background, the findings showed that patients still had residual cardiovascular disease risk that did not appear to respond to changes in LDL cholesterol but which did appear to respond to a reduction in Lp(a) produced by alirocumab. Dr. Schwartz further suggested that alirocumab’s reduction of Lp(a) might also mediate the drug’s apparent effect on reducing VTE incidence, possibly because Lp(a) is structurally similar to plasminogen and hence can have prothrombotic effects.
ODYSSEY Outcomes was sponsored by Sanofi and Regeneron, the companies that market alirocumab (Praluent). Dr. Schwartz has received research support from Sanofi and from Resverlogix, Roche, and The Medicines Company. Dr. Jukema has been a speaker for and received research support from Sanofi Regeneron, and has also been a speaker for Amgen, MSD, and Roche and has also received research support from Biotronik
SOURCE: Schwartz GG et al. AHA 2019, Abstract 309; Jukema JW et al. AHA 2019, Abstract 334.
PHILADELPHIA – Treatment with the PCSK9 inhibitor alirocumab linked with a significant cut in the rates of peripheral artery disease events and ischemic strokes without increasing the rate of hemorrhagic strokes, and alirocumab treatment also showed a trend toward an association with a reduced rate of venous thromboembolic events in prespecified, ancillary analyses of data collected from more than 18,000 patients in the ODYSSEY Outcomes trial.
The analyses that looked at peripheral artery disease (PAD) events and venous thromboembolism (VTE) events also suggested that the apparent ability of alirocumab to reduce their incidence may have been largely mediated through a reduction in Lp(a) lipoprotein, with less of a contribution from the drug’s primary action of reducing LDL cholesterol, Gregory G. Schwartz, MD, said at the American Heart Association scientific sessions.
When used on top of intensive statin treatment, as in the ODYSSEY Outcomes trial, treatment with the PCSK9 inhibitor alirocumab “may be useful to prevent PAD events, particularly in patients with high levels of Lp(a),” said Dr. Schwartz, professor of medicine at the University of Colorado Denver in Aurora. In the analysis he reported, patients treated with alirocumab for a median of 2.8 years had a statistically significant 31% reduced rate of PAD or VTE event and a significant 31% reduced rate of PAD events alone, compared with control patients who received placebo, he reported. Alirocumab treatment was also associated with a 33% lower rate of VTE events only, but the overall rate of these events was low, and this difference just missed statistical significance with a P value of .06.
“Levels of Lp(a), but not LDL cholesterol, predicted the risk of PAD events,” and in patients on alirocumab treatment “the magnitude of Lp(a) reduction, but not LDL-cholesterol reduction, was associated with a reduction in PAD events and VTE.” The reduction in PAD events linked with alirocumab treatment “may be related to Lp(a) lowering,” Dr. Schwartz suggested.
The link between alirocumab treatment and a reduction in ischemic stroke with no increase in hemorrhagic strokes appeared in a separate prespecified analysis from ODYSSEY Outcomes that looked at the rates of ischemic stroke, hemorrhagic stroke, and their combined incidence during the median 2.8 year of study follow-up. Patients treated with alirocumab had a statistically significant 27% reduction in their rate of ischemic strokes compared with patients on placebo, and a statistically significant 28% relative reduction in the rate of any stroke with alirocumab treatment, J. Wouter Jukema, MD, said in a separate report at the meeting. The rate of hemorrhagic strokes was small, and showed a nominal 17% reduction in patients treated with alirocumab, compared with controls, a difference that was not statistically significant.
Further analysis of the stroke outcomes also showed that these reductions in total strokes occurred with alirocumab treatment at roughly similar rates regardless of baseline level of LDL cholesterol or history of a prior cerebrovascular event. Analysis also showed that the rate of hemorrhagic strokes was consistently low regardless of the on-treatment level of LDL cholesterol. Even among patients whose LDL cholesterol level fell below 25 mg/dL on alirocumab treatment, the incidence of hemorrhagic strokes during follow-up was 0.1%, “a very reassuring finding,” said Dr. Jukema, professor of cardiology at Leiden (The Netherlands) University. The stroke analyses did not examine possible linkages of these effects with changes in level of Lp(a).
ODYSSEY Outcomes (Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab) included 18,924 patients who had experienced an acute coronary syndrome event within the prior 12 months and had an LDL cholesterol level of at least 70 mg/dL despite maximally tolerated statin treatment and randomized them to treatment with alirocumab or placebo. The primary endpoint was the combination of coronary heart disease death, nonfatal MI, ischemic stroke, and hospitalization for unstable angina, which alirocumab effectively reduced compared with placebo (N Engl J Med. 2018 Nov 29;379[22]:2097-107).
The PAD analysis tallied the combined rate of acute limb ischemia, revascularization, or amputation related to PAD, and the VTE cases included patients who developed deep vein thrombosis or pulmonary embolism. All cases were nonadjudicated reports from participating investigators. Because Lp(a) makes up a portion of LDL cholesterol, Dr. Schwartz and associates calculated adjusted values for LDL cholesterol that were independent of Lp(a).
In a multivariable analysis that adjusted for demographic and clinical characteristics as well as baseline Lp(a) and the calculated level of LDL cholesterol, every 1 mg/dL decrease in Lp(a) linked with a statistically significant, nearly 1% decrease in the rate of either a PAD or VTE event, while the change in LDL cholesterol had no significant relationship with this endpoint, said Dr. Schwartz.
The impact of Lp(a) lowering was most dramatic among the subgroups of patients who entered the study with the highest levels of Lp(a). “In the lowest quartile [for baseline level of Lp(a)] the effect of treatment [with alirocumab] was inconsequential; all of the action was in the upper two quartiles,” he said. Dr. Schwartz highlighted that 90% of patients in the study were on an “intense” statin dosage, and 97% received some statin treatment. Against that treatment background, the findings showed that patients still had residual cardiovascular disease risk that did not appear to respond to changes in LDL cholesterol but which did appear to respond to a reduction in Lp(a) produced by alirocumab. Dr. Schwartz further suggested that alirocumab’s reduction of Lp(a) might also mediate the drug’s apparent effect on reducing VTE incidence, possibly because Lp(a) is structurally similar to plasminogen and hence can have prothrombotic effects.
ODYSSEY Outcomes was sponsored by Sanofi and Regeneron, the companies that market alirocumab (Praluent). Dr. Schwartz has received research support from Sanofi and from Resverlogix, Roche, and The Medicines Company. Dr. Jukema has been a speaker for and received research support from Sanofi Regeneron, and has also been a speaker for Amgen, MSD, and Roche and has also received research support from Biotronik
SOURCE: Schwartz GG et al. AHA 2019, Abstract 309; Jukema JW et al. AHA 2019, Abstract 334.
REPORTING FROM THE AHA 2019
Differences in U.S. and European aneurysm guidelines called unavoidable
NEW YORK – Published 12 months apart, guidelines on management of abdominal aortic aneurysm (AAA) from the European Society for Vascular Surgery are similar but diverged in instructive ways from those of the Society for Vascular Surgery, according to a critical review at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation. “Some of the differences were almost unavoidable in the sense that the ESVS guidelines represent multiple idiosyncratic health care systems across Europe,” reported Ronald L. Dalman, MD, chief of vascular surgery, Stanford (Calif.) University.
As a result, the ESVS guidelines provide very little specificity about pharmacologic options because of the differences in availability of these treatments within specific health systems. In addition, both open and endovascular aneurysm repair (EVAR) are given similar emphasis because of the limited availability of EVAR in some parts of Europe.
“The ESVS guidelines specifically recommend repair of an aneurysm within 8 weeks when repair is indicated, but there are not many aneurysms that go 8 weeks in the U.S. without being fixed by a fee-for-service surgeon,” Dr. Dalman observed.
The SVS AAA guidelines were published in January 2018 (J Vasc Surg 2018;67:2-77) and the ESVS guidelines followed 1 year later (Eur J Vasc Surg 2019;57:8-93).
The differences in the guidelines, although modest, are interesting because each set of guidelines was based largely on the same set of trials and published studies, according to Dr. Dalman, who was a coauthor of the SVS guidelines and an external reviewer for the ESVS guidelines.
In the lag between completion of the two guidelines, new information led to three ESVS additions not found in the SVS guidelines, according to Dr. Dalman. They involved the importance of considering aneurysm diameter as a prognostic factor, new understanding of the limitations on endovascular aneurysm sealing (EVAS), and new information about how aneurysm size should affect frequency of surveillance.
Overall, the U.S. guidelines contain 111 recommendations based on 177 references, while the ESVS guidelines contain 125 guidelines based on 189 references. In retrospect, Dr. Dalman believes both sets of guidelines omitted some clinically meaningful information, such as the risk of large-diameter devices for causing endoleaks.
The authors of the ESVS guidelines did have an opportunity to review of a draft of the SVS guidelines, so differences can be interpreted as intentional. For example, the SVS guidelines recommend risk calculators, but Dr. Dalman suggested that the authors of the ESVS guidelines were less convinced that their utility was established.
The decision not to recommend a door-to-treatment time for ruptured aneurysms, as in the SVS recommendations, might have been in deference to disparate practice across European countries, Dr. Dalman suggested.
Ultimately, the guidelines are “substantially similar,” according to Dr. Dalman, but he expressed concerned that neither guideline is accompanied by a specific mechanism or recommended strategy to ensure implementation.
Many of the SVS recommendations are likely to be translated into quality metrics at U.S. institutions, but “there are implementation issues” for ensuring that each guideline is applied, Dr. Dalman said.
Given the agreement on the vast majority of the recommendations, Dr. Dalman suggested that “it might be time to consider global guidelines” for management of AAA and other vascular diseases. Some type of language might be required to accommodate divergent resources or practices across borders, but Dr. Dalman questioned the need to review the same literature to arrive at mostly the same conclusions.
NEW YORK – Published 12 months apart, guidelines on management of abdominal aortic aneurysm (AAA) from the European Society for Vascular Surgery are similar but diverged in instructive ways from those of the Society for Vascular Surgery, according to a critical review at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation. “Some of the differences were almost unavoidable in the sense that the ESVS guidelines represent multiple idiosyncratic health care systems across Europe,” reported Ronald L. Dalman, MD, chief of vascular surgery, Stanford (Calif.) University.
As a result, the ESVS guidelines provide very little specificity about pharmacologic options because of the differences in availability of these treatments within specific health systems. In addition, both open and endovascular aneurysm repair (EVAR) are given similar emphasis because of the limited availability of EVAR in some parts of Europe.
“The ESVS guidelines specifically recommend repair of an aneurysm within 8 weeks when repair is indicated, but there are not many aneurysms that go 8 weeks in the U.S. without being fixed by a fee-for-service surgeon,” Dr. Dalman observed.
The SVS AAA guidelines were published in January 2018 (J Vasc Surg 2018;67:2-77) and the ESVS guidelines followed 1 year later (Eur J Vasc Surg 2019;57:8-93).
The differences in the guidelines, although modest, are interesting because each set of guidelines was based largely on the same set of trials and published studies, according to Dr. Dalman, who was a coauthor of the SVS guidelines and an external reviewer for the ESVS guidelines.
In the lag between completion of the two guidelines, new information led to three ESVS additions not found in the SVS guidelines, according to Dr. Dalman. They involved the importance of considering aneurysm diameter as a prognostic factor, new understanding of the limitations on endovascular aneurysm sealing (EVAS), and new information about how aneurysm size should affect frequency of surveillance.
Overall, the U.S. guidelines contain 111 recommendations based on 177 references, while the ESVS guidelines contain 125 guidelines based on 189 references. In retrospect, Dr. Dalman believes both sets of guidelines omitted some clinically meaningful information, such as the risk of large-diameter devices for causing endoleaks.
The authors of the ESVS guidelines did have an opportunity to review of a draft of the SVS guidelines, so differences can be interpreted as intentional. For example, the SVS guidelines recommend risk calculators, but Dr. Dalman suggested that the authors of the ESVS guidelines were less convinced that their utility was established.
The decision not to recommend a door-to-treatment time for ruptured aneurysms, as in the SVS recommendations, might have been in deference to disparate practice across European countries, Dr. Dalman suggested.
Ultimately, the guidelines are “substantially similar,” according to Dr. Dalman, but he expressed concerned that neither guideline is accompanied by a specific mechanism or recommended strategy to ensure implementation.
Many of the SVS recommendations are likely to be translated into quality metrics at U.S. institutions, but “there are implementation issues” for ensuring that each guideline is applied, Dr. Dalman said.
Given the agreement on the vast majority of the recommendations, Dr. Dalman suggested that “it might be time to consider global guidelines” for management of AAA and other vascular diseases. Some type of language might be required to accommodate divergent resources or practices across borders, but Dr. Dalman questioned the need to review the same literature to arrive at mostly the same conclusions.
NEW YORK – Published 12 months apart, guidelines on management of abdominal aortic aneurysm (AAA) from the European Society for Vascular Surgery are similar but diverged in instructive ways from those of the Society for Vascular Surgery, according to a critical review at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation. “Some of the differences were almost unavoidable in the sense that the ESVS guidelines represent multiple idiosyncratic health care systems across Europe,” reported Ronald L. Dalman, MD, chief of vascular surgery, Stanford (Calif.) University.
As a result, the ESVS guidelines provide very little specificity about pharmacologic options because of the differences in availability of these treatments within specific health systems. In addition, both open and endovascular aneurysm repair (EVAR) are given similar emphasis because of the limited availability of EVAR in some parts of Europe.
“The ESVS guidelines specifically recommend repair of an aneurysm within 8 weeks when repair is indicated, but there are not many aneurysms that go 8 weeks in the U.S. without being fixed by a fee-for-service surgeon,” Dr. Dalman observed.
The SVS AAA guidelines were published in January 2018 (J Vasc Surg 2018;67:2-77) and the ESVS guidelines followed 1 year later (Eur J Vasc Surg 2019;57:8-93).
The differences in the guidelines, although modest, are interesting because each set of guidelines was based largely on the same set of trials and published studies, according to Dr. Dalman, who was a coauthor of the SVS guidelines and an external reviewer for the ESVS guidelines.
In the lag between completion of the two guidelines, new information led to three ESVS additions not found in the SVS guidelines, according to Dr. Dalman. They involved the importance of considering aneurysm diameter as a prognostic factor, new understanding of the limitations on endovascular aneurysm sealing (EVAS), and new information about how aneurysm size should affect frequency of surveillance.
Overall, the U.S. guidelines contain 111 recommendations based on 177 references, while the ESVS guidelines contain 125 guidelines based on 189 references. In retrospect, Dr. Dalman believes both sets of guidelines omitted some clinically meaningful information, such as the risk of large-diameter devices for causing endoleaks.
The authors of the ESVS guidelines did have an opportunity to review of a draft of the SVS guidelines, so differences can be interpreted as intentional. For example, the SVS guidelines recommend risk calculators, but Dr. Dalman suggested that the authors of the ESVS guidelines were less convinced that their utility was established.
The decision not to recommend a door-to-treatment time for ruptured aneurysms, as in the SVS recommendations, might have been in deference to disparate practice across European countries, Dr. Dalman suggested.
Ultimately, the guidelines are “substantially similar,” according to Dr. Dalman, but he expressed concerned that neither guideline is accompanied by a specific mechanism or recommended strategy to ensure implementation.
Many of the SVS recommendations are likely to be translated into quality metrics at U.S. institutions, but “there are implementation issues” for ensuring that each guideline is applied, Dr. Dalman said.
Given the agreement on the vast majority of the recommendations, Dr. Dalman suggested that “it might be time to consider global guidelines” for management of AAA and other vascular diseases. Some type of language might be required to accommodate divergent resources or practices across borders, but Dr. Dalman questioned the need to review the same literature to arrive at mostly the same conclusions.
REPORTING FROM VEITHsymposium
Key clinical point:
Major finding: Less emphasis on endovascular repair and specific drugs in Europe reflects accommodation of nationalized health systems.
Study details: Expert review.
Disclosures: Dr. Dalman reports no potential financial conflicts of interest relevant to this topic.
Source: Dalman RL et al. 46th VEITHsymposium.
Challenges outlined for teaching open surgery to Gen Z in endovascular era
NEW YORK – The dual challenges of teaching open vascular surgery techniques when few are performed and reaching a generation that has a different attitude to absorbing information requires new and innovative approaches, according to an academic surgeon speaking at symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation.
“The see one, do one, teach one approach to surgical training is no longer possible,” explained R. Clement Darling III, MD, chief of the division of vascular surgery at Albany (N.Y.) Medical Center Hospital.
For open procedures, the problem is a rapidly declining number of cases in the era of endovascular surgery, but Dr. Darling also recommended adjusting training to the outlook and expectations of a new generation. First observed in the millennial generation, an attitude of firm work-related boundaries is also being seen in generation Z. Generation Z, characterized by birth after 1995, is just now beginning to reach residency programs.
“The approach to work-life balance is extremely different for these individuals than it was for my generation,” Dr. Darling said. While previous generations were often motivated by fear and pressure, newer generations appear to respond less well to anxiety.
“People learn differently. Some from their tactile sense, some intellectually, and some from fear or pressure, but mostly, particularly those who are younger, now learn from positive reinforcement,” Dr. Darling said.
For teaching open procedures at his own institution, Dr. Darling has switched from the traditional model of one-on-one instruction undertaken in the surgical suite to a group approach. The limited number of open cases was the impetus, but group instruction now extends beyond the operating room.
“We have a meeting before the case, when we go over the technical aspects,” Dr. Darling explained. Fellows are asked to envision and describe potential problems and potential solutions.
“We try to make them visualize as well as verbalize exactly what will be done in the operating room,” Dr. Darling said. The plans are outlined carefully “so no one does any thinking in the OR. All the thinking is done in advance.”
Videos and simulators are teaching aids, but a great deal of learning can be accomplished independent of doing, according to Dr. Darling. Moreover, understanding the anatomy, which comes before developing surgical skills, is the same for open and endovascular procedures, so each is relevant to the other.
After witnessing an open case, all of the trainees along with the nurses and attending physicians go through a debriefing to consider the potential lessons. At Dr. Darling’s center, open procedures increasingly involve sicker and older patients, conferring case analysis with a particularly vital learning function in the curriculum.
Because of the diminishing number of open cases and the diminishing open skills, even among experienced vascular surgeons, residents in an increasing number of training programs “graduate without any open experience, which is a little shocking,” Dr. Darling said.
Importantly, group instruction, although valuable and necessary for exposing residents and fellows to open vascular surgery, has its own lessons to impart even if it was born out of necessity.
“We always emphasize that it is not the sewing that counts, it is the setup that counts,” said Dr. Darling, indicating that this is a clear message when the group is assembled for case planning. The group planning also emphasizes that surgery is a team sport.
“All of us is smarter than one of us,” said Dr. Darling, articulating the implicit message of group training.
Although this is a departure from a bygone era where infallible surgeons ruled the OR, it is fits nicely with changing attitudes about the best attributes of a competent surgeon.
NEW YORK – The dual challenges of teaching open vascular surgery techniques when few are performed and reaching a generation that has a different attitude to absorbing information requires new and innovative approaches, according to an academic surgeon speaking at symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation.
“The see one, do one, teach one approach to surgical training is no longer possible,” explained R. Clement Darling III, MD, chief of the division of vascular surgery at Albany (N.Y.) Medical Center Hospital.
For open procedures, the problem is a rapidly declining number of cases in the era of endovascular surgery, but Dr. Darling also recommended adjusting training to the outlook and expectations of a new generation. First observed in the millennial generation, an attitude of firm work-related boundaries is also being seen in generation Z. Generation Z, characterized by birth after 1995, is just now beginning to reach residency programs.
“The approach to work-life balance is extremely different for these individuals than it was for my generation,” Dr. Darling said. While previous generations were often motivated by fear and pressure, newer generations appear to respond less well to anxiety.
“People learn differently. Some from their tactile sense, some intellectually, and some from fear or pressure, but mostly, particularly those who are younger, now learn from positive reinforcement,” Dr. Darling said.
For teaching open procedures at his own institution, Dr. Darling has switched from the traditional model of one-on-one instruction undertaken in the surgical suite to a group approach. The limited number of open cases was the impetus, but group instruction now extends beyond the operating room.
“We have a meeting before the case, when we go over the technical aspects,” Dr. Darling explained. Fellows are asked to envision and describe potential problems and potential solutions.
“We try to make them visualize as well as verbalize exactly what will be done in the operating room,” Dr. Darling said. The plans are outlined carefully “so no one does any thinking in the OR. All the thinking is done in advance.”
Videos and simulators are teaching aids, but a great deal of learning can be accomplished independent of doing, according to Dr. Darling. Moreover, understanding the anatomy, which comes before developing surgical skills, is the same for open and endovascular procedures, so each is relevant to the other.
After witnessing an open case, all of the trainees along with the nurses and attending physicians go through a debriefing to consider the potential lessons. At Dr. Darling’s center, open procedures increasingly involve sicker and older patients, conferring case analysis with a particularly vital learning function in the curriculum.
Because of the diminishing number of open cases and the diminishing open skills, even among experienced vascular surgeons, residents in an increasing number of training programs “graduate without any open experience, which is a little shocking,” Dr. Darling said.
Importantly, group instruction, although valuable and necessary for exposing residents and fellows to open vascular surgery, has its own lessons to impart even if it was born out of necessity.
“We always emphasize that it is not the sewing that counts, it is the setup that counts,” said Dr. Darling, indicating that this is a clear message when the group is assembled for case planning. The group planning also emphasizes that surgery is a team sport.
“All of us is smarter than one of us,” said Dr. Darling, articulating the implicit message of group training.
Although this is a departure from a bygone era where infallible surgeons ruled the OR, it is fits nicely with changing attitudes about the best attributes of a competent surgeon.
NEW YORK – The dual challenges of teaching open vascular surgery techniques when few are performed and reaching a generation that has a different attitude to absorbing information requires new and innovative approaches, according to an academic surgeon speaking at symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation.
“The see one, do one, teach one approach to surgical training is no longer possible,” explained R. Clement Darling III, MD, chief of the division of vascular surgery at Albany (N.Y.) Medical Center Hospital.
For open procedures, the problem is a rapidly declining number of cases in the era of endovascular surgery, but Dr. Darling also recommended adjusting training to the outlook and expectations of a new generation. First observed in the millennial generation, an attitude of firm work-related boundaries is also being seen in generation Z. Generation Z, characterized by birth after 1995, is just now beginning to reach residency programs.
“The approach to work-life balance is extremely different for these individuals than it was for my generation,” Dr. Darling said. While previous generations were often motivated by fear and pressure, newer generations appear to respond less well to anxiety.
“People learn differently. Some from their tactile sense, some intellectually, and some from fear or pressure, but mostly, particularly those who are younger, now learn from positive reinforcement,” Dr. Darling said.
For teaching open procedures at his own institution, Dr. Darling has switched from the traditional model of one-on-one instruction undertaken in the surgical suite to a group approach. The limited number of open cases was the impetus, but group instruction now extends beyond the operating room.
“We have a meeting before the case, when we go over the technical aspects,” Dr. Darling explained. Fellows are asked to envision and describe potential problems and potential solutions.
“We try to make them visualize as well as verbalize exactly what will be done in the operating room,” Dr. Darling said. The plans are outlined carefully “so no one does any thinking in the OR. All the thinking is done in advance.”
Videos and simulators are teaching aids, but a great deal of learning can be accomplished independent of doing, according to Dr. Darling. Moreover, understanding the anatomy, which comes before developing surgical skills, is the same for open and endovascular procedures, so each is relevant to the other.
After witnessing an open case, all of the trainees along with the nurses and attending physicians go through a debriefing to consider the potential lessons. At Dr. Darling’s center, open procedures increasingly involve sicker and older patients, conferring case analysis with a particularly vital learning function in the curriculum.
Because of the diminishing number of open cases and the diminishing open skills, even among experienced vascular surgeons, residents in an increasing number of training programs “graduate without any open experience, which is a little shocking,” Dr. Darling said.
Importantly, group instruction, although valuable and necessary for exposing residents and fellows to open vascular surgery, has its own lessons to impart even if it was born out of necessity.
“We always emphasize that it is not the sewing that counts, it is the setup that counts,” said Dr. Darling, indicating that this is a clear message when the group is assembled for case planning. The group planning also emphasizes that surgery is a team sport.
“All of us is smarter than one of us,” said Dr. Darling, articulating the implicit message of group training.
Although this is a departure from a bygone era where infallible surgeons ruled the OR, it is fits nicely with changing attitudes about the best attributes of a competent surgeon.
REPORTING FROM VEITHsymposium
















