User login
Pairing vascular reconstruction, pancreatic cancer resection
CHICAGO – More than 53,000 people will develop pancreatic ductal adenocarcinoma in the United States this year, and upwards of 41,000 will die from the disease, many of them with tumors considered unresectable because they involve adjacent vessels. However, researchers at the University of California, Irvine, have found that careful removal of the tumor around involved veins and arteries, even in borderline cases, can improve outcomes for these patients.
Roy M. Fujitani, MD, updated previously published data on a single-center study he coauthored in 2015 of 270 patients who had undergone a Whipple operation, 183 for pancreatic adenocarcinoma (J Vasc Surg. 2015;61:475-80) at a symposium on vascular surgery sponsored by Northwestern University.
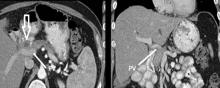
Resection of pancreatic tumors without vascular involvement is fairly straightforward for surgical oncologists to perform, Dr. Fujitani said, but pancreatic tumors enter the borderline resectable category when preoperative CT scan shows portal vein abutment, for which vascular surgery should provide counsel and assist. However, even in some cases when preoperative CT scan shows unresectable, locally advanced pancreatic tumor with celiac artery encasement, neoadjuvant therapy may downstage the disease into the borderline category, he said.
“Patients with borderline resectable or stage II disease are those one should consider for reconstruction,” Dr. Fujitani said. Resectable findings of borderline disease include encasement of the portal vein, superior mesenteric vein and the confluence of the portal venous system (with suitable proximal and distal targets for reconstruction); and less-than-circumferential involvement of the common hepatic artery or right hepatic artery – but without involvement of the superior mesenteric artery or the celiac axis and “certainly not” the aorta. “This would account for about one-fourth of patients in high-volume centers as being able to receive concomitant vascular reconstruction,” Dr. Fujitani said.
In the UCI series, 60 patients with borderline lesions underwent vascular reconstruction. “As it turned out, there was no significant difference in survival between the reconstruction group and the nonreconstruction group,” Dr. Fujitani said, “but it’s important to note that these patients who had the reconstruction would never have been operated on if we were not able to do the reconstruction.” Thirty-day mortality was around 5% and 1-year survival around 70% in both groups, he said. However, at about 1.5 years the Kaplan-Meier survival curves between the two groups diverged, which Dr. Fujitani attributed to more advanced disease in the reconstruction group.
“We found lymph node status and tumor margins were most important in determining survival of these patients,” he said. “Gaining an R0 resection is the most important thing that determines favorable survivability.”
Dr. Fujitani also reviewed different techniques for vascular reconstruction, and while differences in complication rates or 1-, 2-, or 3-year survival were not statistically significant, he did note that mean survival with lateral venorrhaphy exceeded that of primary anastomosis and interposition graft – 21 months vs. 13 months vs. 4 months, suggesting the merits of a more aggressive approach to vascular resection and reconstruction.
“Improvement of survival outcomes may be achieved with concomitant advanced vascular reconstruction in carefully selected patients,” Dr. Fujitani said. “There are multiple options for vascular reconstruction for mesenteric portal venous and visceral arterial involvement using standard vascular surgical techniques.” He added that a dedicated team of experienced surgical oncologists and vascular surgeons for these reconstructions “is essential for successful outcomes.”
Dr. Fujitani had no relevant financial relationships to disclose.
CHICAGO – More than 53,000 people will develop pancreatic ductal adenocarcinoma in the United States this year, and upwards of 41,000 will die from the disease, many of them with tumors considered unresectable because they involve adjacent vessels. However, researchers at the University of California, Irvine, have found that careful removal of the tumor around involved veins and arteries, even in borderline cases, can improve outcomes for these patients.
Roy M. Fujitani, MD, updated previously published data on a single-center study he coauthored in 2015 of 270 patients who had undergone a Whipple operation, 183 for pancreatic adenocarcinoma (J Vasc Surg. 2015;61:475-80) at a symposium on vascular surgery sponsored by Northwestern University.
Resection of pancreatic tumors without vascular involvement is fairly straightforward for surgical oncologists to perform, Dr. Fujitani said, but pancreatic tumors enter the borderline resectable category when preoperative CT scan shows portal vein abutment, for which vascular surgery should provide counsel and assist. However, even in some cases when preoperative CT scan shows unresectable, locally advanced pancreatic tumor with celiac artery encasement, neoadjuvant therapy may downstage the disease into the borderline category, he said.
“Patients with borderline resectable or stage II disease are those one should consider for reconstruction,” Dr. Fujitani said. Resectable findings of borderline disease include encasement of the portal vein, superior mesenteric vein and the confluence of the portal venous system (with suitable proximal and distal targets for reconstruction); and less-than-circumferential involvement of the common hepatic artery or right hepatic artery – but without involvement of the superior mesenteric artery or the celiac axis and “certainly not” the aorta. “This would account for about one-fourth of patients in high-volume centers as being able to receive concomitant vascular reconstruction,” Dr. Fujitani said.
In the UCI series, 60 patients with borderline lesions underwent vascular reconstruction. “As it turned out, there was no significant difference in survival between the reconstruction group and the nonreconstruction group,” Dr. Fujitani said, “but it’s important to note that these patients who had the reconstruction would never have been operated on if we were not able to do the reconstruction.” Thirty-day mortality was around 5% and 1-year survival around 70% in both groups, he said. However, at about 1.5 years the Kaplan-Meier survival curves between the two groups diverged, which Dr. Fujitani attributed to more advanced disease in the reconstruction group.
“We found lymph node status and tumor margins were most important in determining survival of these patients,” he said. “Gaining an R0 resection is the most important thing that determines favorable survivability.”
Dr. Fujitani also reviewed different techniques for vascular reconstruction, and while differences in complication rates or 1-, 2-, or 3-year survival were not statistically significant, he did note that mean survival with lateral venorrhaphy exceeded that of primary anastomosis and interposition graft – 21 months vs. 13 months vs. 4 months, suggesting the merits of a more aggressive approach to vascular resection and reconstruction.
“Improvement of survival outcomes may be achieved with concomitant advanced vascular reconstruction in carefully selected patients,” Dr. Fujitani said. “There are multiple options for vascular reconstruction for mesenteric portal venous and visceral arterial involvement using standard vascular surgical techniques.” He added that a dedicated team of experienced surgical oncologists and vascular surgeons for these reconstructions “is essential for successful outcomes.”
Dr. Fujitani had no relevant financial relationships to disclose.
CHICAGO – More than 53,000 people will develop pancreatic ductal adenocarcinoma in the United States this year, and upwards of 41,000 will die from the disease, many of them with tumors considered unresectable because they involve adjacent vessels. However, researchers at the University of California, Irvine, have found that careful removal of the tumor around involved veins and arteries, even in borderline cases, can improve outcomes for these patients.
Roy M. Fujitani, MD, updated previously published data on a single-center study he coauthored in 2015 of 270 patients who had undergone a Whipple operation, 183 for pancreatic adenocarcinoma (J Vasc Surg. 2015;61:475-80) at a symposium on vascular surgery sponsored by Northwestern University.
Resection of pancreatic tumors without vascular involvement is fairly straightforward for surgical oncologists to perform, Dr. Fujitani said, but pancreatic tumors enter the borderline resectable category when preoperative CT scan shows portal vein abutment, for which vascular surgery should provide counsel and assist. However, even in some cases when preoperative CT scan shows unresectable, locally advanced pancreatic tumor with celiac artery encasement, neoadjuvant therapy may downstage the disease into the borderline category, he said.
“Patients with borderline resectable or stage II disease are those one should consider for reconstruction,” Dr. Fujitani said. Resectable findings of borderline disease include encasement of the portal vein, superior mesenteric vein and the confluence of the portal venous system (with suitable proximal and distal targets for reconstruction); and less-than-circumferential involvement of the common hepatic artery or right hepatic artery – but without involvement of the superior mesenteric artery or the celiac axis and “certainly not” the aorta. “This would account for about one-fourth of patients in high-volume centers as being able to receive concomitant vascular reconstruction,” Dr. Fujitani said.
In the UCI series, 60 patients with borderline lesions underwent vascular reconstruction. “As it turned out, there was no significant difference in survival between the reconstruction group and the nonreconstruction group,” Dr. Fujitani said, “but it’s important to note that these patients who had the reconstruction would never have been operated on if we were not able to do the reconstruction.” Thirty-day mortality was around 5% and 1-year survival around 70% in both groups, he said. However, at about 1.5 years the Kaplan-Meier survival curves between the two groups diverged, which Dr. Fujitani attributed to more advanced disease in the reconstruction group.
“We found lymph node status and tumor margins were most important in determining survival of these patients,” he said. “Gaining an R0 resection is the most important thing that determines favorable survivability.”
Dr. Fujitani also reviewed different techniques for vascular reconstruction, and while differences in complication rates or 1-, 2-, or 3-year survival were not statistically significant, he did note that mean survival with lateral venorrhaphy exceeded that of primary anastomosis and interposition graft – 21 months vs. 13 months vs. 4 months, suggesting the merits of a more aggressive approach to vascular resection and reconstruction.
“Improvement of survival outcomes may be achieved with concomitant advanced vascular reconstruction in carefully selected patients,” Dr. Fujitani said. “There are multiple options for vascular reconstruction for mesenteric portal venous and visceral arterial involvement using standard vascular surgical techniques.” He added that a dedicated team of experienced surgical oncologists and vascular surgeons for these reconstructions “is essential for successful outcomes.”
Dr. Fujitani had no relevant financial relationships to disclose.
AT THE NORTHWESTERN VASCULAR SYMPOSIUM
Key clinical point: A more aggressive vascular resection and reconstruction in pancreatic cancer may improve outcomes and palliation in these patients.
Major finding: Mean survival with lateral venorrhaphy exceeded primary anastomosis and interposition graft (21 months vs. 13 months vs. 4 months).
Data source: Updated data of previously published single-center retrospective review of 183 patients who had Whipple procedure for pancreatic adenocarcinoma.
Disclosures: Dr. Fujitani reported having no financial disclosures.
Vascular anomalies often misdiagnosed amidst confusion
CHICAGO – Thanks to convoluted terminology, not to mention confusion in the literature, physicians have been known to frequently misdiagnose vascular malformations as hemangiomas, but an evolving understanding of their differences may lead to more precise diagnoses, according to a report at a symposium on vascular surgery sponsored by Northwestern University.
“Historically there has been a great deal of confusion in the literature when it comes to the nomenclature used to describe vascular anomalies,” said Naiem Nassiri, MD, of Robert Wood Johnson Medical School, New Brunswick, N.J. He pointed out that the term hemangioma “or derivatives thereof” – cavernous hemangioma, cavernous angioma, lymphangioma and cystic hygroma – are “absolute misnomers and continue to be misused and applied almost haphazardly to any anomalous vascular lesion.”
He cited reports that 71% of vascular anomalies have been improperly called hemangiomas, 69% have initially been diagnosed incorrectly, and 21% received the wrong treatment (Pediatr Dermatol. 2008;25[1]:7-12; Plast Reconstr Surg. 2011:127[1]:347-51). “Erroneous terminology has prognostic as well as diagnostic and therapeutic implications, and these can actually be quite devastating for the patient, not only clinically and physically but psychologically as well,” Dr. Nassiri said.
Using the International Society for the Study of Vascular Anomalies classification for hemangiomas and vascular malformations can help physicians make the differential diagnosis, Dr. Nassiri said. Hemangiomas are neoplastic lesions of infancy, though not always congenital, with a finite growth phase, whereas vascular malformations (VMs) are nonneoplastic, congenital lesions that can appear at any age and do not regress spontaneously, he said.
Infantile hemangiomas typically appear as the classic strawberry birthmark in children, whereas VMs tend to appear later in life. “They require some environmental trigger, such as trauma, activity, or changes in the hormonal milieu to manifest onset,” he said of VMs.
Simply put, VMs fall into three broad categories: slow-flow malformations, which include lymphatic and venous malformations; high-flow arteriovenous malformations (AVMs) and fistulas; and congenital mixed syndromes, which can include combinations thereof.
Dr. Nassiri noted that contrast-enhanced MRI is the standard imaging modality for diagnosis of VMs, and can differentiate between slow-flow and high-flow lesions. However, vascular specialists must be vigilant in ordering imaging for slow-flow lesions. “Orders can be changed to MR venography, and I’ve had patients who’ve gone decades with multiple MR venograms and no one can figure out what’s going on as no identifiable lesion is readily detected,” he said. “MR venograms are fantastic for detecting truncular blood flow where there typically are no anomalies in the vast majority of patients with isolated venous malformations, but on contrast-enhanced MRI these convoluted cluster of anomalous veins light up like Christmas trees.”
Lymphatic malformations affect the head and neck more so than the extremities, trunk or viscera, and are prone to infection and bleeding. “You can think of these as fluid-filled balloons, and the goal of treatment is fairly simple: You want to puncture the balloon and drain the fluid inside so as to obtain maximum wall collapse,” Dr. Nassiri said. Infusion of a sclerosant causes an inflammatory reaction leading to fibrosis, which then prevents balloon re-expansion. Surgical excision is best used as a secondary adjunct.
Venous malformations, comprising about 80% of all VMs, typically present as soft, spongy blue or purple compressible masses with associated pain that worsens with exertion, Dr. Nassiri said. “The most dangerous thing that is often overlooked, even by some of the physicians that treat these on a regular basis, is localized intravascular coagulopathy, which if left untreated can progress to fulminant disseminated intravascular coagulopathy,” he said. This tends to occur more in the more widespread varieties of venous malformations.
A common misnomer associated with venous malformations in adults is “liver hemangioma,” owing to the confusing nomenclature, Dr. Nassiri said. “When interrogated angiographically,” he said, “what is often labeled as a hepatic hemangioma is in fact a venous malformation. Natural history of the two entities is completely different.”
Dr. Nassiri described congenital high-flow AVMs as “convoluted networks of blood vessels with poorly differentiated endothelial cells that have neither a venous nor an arterial designation; this entity, otherwise known as a nidus, sits between the feeding arteries and the draining veins.” Treatment aims to eliminate the flow within that nidus.
Super-selective microcatheterization is the best option for nidus access and embolization using liquid embolic agents, preferably those that polymerize when infused. “This is probably the most potent angiogenic entity I’ve ever seen,” Dr. Nassiri said of the nidus.
“It’s like a low-pressure sump and it will recruit collaterals vigorously, so you have to eliminate that nidus.” A variety of different embolic agents, some off label, may be used for high flow AVMs.
For congenital mixed syndromes, the same diagnostic and therapeutic concepts hold true depending on the type of VM involved. Dr. Nassiri advised a multidisciplinary approach, and noted that early trials have investigated the use of sirolimus in severe, life-threatening cases (Br J Clin Pharmacol. 2016;82[5]:1171-9. doi: 10.1111/bcp.13022).
Dr. Nassiri disclosed serving on the speakers bureaus for Boston Scientific, Penumbra, and Merritt Medical, and is a consultant to Merritt Medical.
CHICAGO – Thanks to convoluted terminology, not to mention confusion in the literature, physicians have been known to frequently misdiagnose vascular malformations as hemangiomas, but an evolving understanding of their differences may lead to more precise diagnoses, according to a report at a symposium on vascular surgery sponsored by Northwestern University.
“Historically there has been a great deal of confusion in the literature when it comes to the nomenclature used to describe vascular anomalies,” said Naiem Nassiri, MD, of Robert Wood Johnson Medical School, New Brunswick, N.J. He pointed out that the term hemangioma “or derivatives thereof” – cavernous hemangioma, cavernous angioma, lymphangioma and cystic hygroma – are “absolute misnomers and continue to be misused and applied almost haphazardly to any anomalous vascular lesion.”
He cited reports that 71% of vascular anomalies have been improperly called hemangiomas, 69% have initially been diagnosed incorrectly, and 21% received the wrong treatment (Pediatr Dermatol. 2008;25[1]:7-12; Plast Reconstr Surg. 2011:127[1]:347-51). “Erroneous terminology has prognostic as well as diagnostic and therapeutic implications, and these can actually be quite devastating for the patient, not only clinically and physically but psychologically as well,” Dr. Nassiri said.
Using the International Society for the Study of Vascular Anomalies classification for hemangiomas and vascular malformations can help physicians make the differential diagnosis, Dr. Nassiri said. Hemangiomas are neoplastic lesions of infancy, though not always congenital, with a finite growth phase, whereas vascular malformations (VMs) are nonneoplastic, congenital lesions that can appear at any age and do not regress spontaneously, he said.
Infantile hemangiomas typically appear as the classic strawberry birthmark in children, whereas VMs tend to appear later in life. “They require some environmental trigger, such as trauma, activity, or changes in the hormonal milieu to manifest onset,” he said of VMs.
Simply put, VMs fall into three broad categories: slow-flow malformations, which include lymphatic and venous malformations; high-flow arteriovenous malformations (AVMs) and fistulas; and congenital mixed syndromes, which can include combinations thereof.
Dr. Nassiri noted that contrast-enhanced MRI is the standard imaging modality for diagnosis of VMs, and can differentiate between slow-flow and high-flow lesions. However, vascular specialists must be vigilant in ordering imaging for slow-flow lesions. “Orders can be changed to MR venography, and I’ve had patients who’ve gone decades with multiple MR venograms and no one can figure out what’s going on as no identifiable lesion is readily detected,” he said. “MR venograms are fantastic for detecting truncular blood flow where there typically are no anomalies in the vast majority of patients with isolated venous malformations, but on contrast-enhanced MRI these convoluted cluster of anomalous veins light up like Christmas trees.”
Lymphatic malformations affect the head and neck more so than the extremities, trunk or viscera, and are prone to infection and bleeding. “You can think of these as fluid-filled balloons, and the goal of treatment is fairly simple: You want to puncture the balloon and drain the fluid inside so as to obtain maximum wall collapse,” Dr. Nassiri said. Infusion of a sclerosant causes an inflammatory reaction leading to fibrosis, which then prevents balloon re-expansion. Surgical excision is best used as a secondary adjunct.
Venous malformations, comprising about 80% of all VMs, typically present as soft, spongy blue or purple compressible masses with associated pain that worsens with exertion, Dr. Nassiri said. “The most dangerous thing that is often overlooked, even by some of the physicians that treat these on a regular basis, is localized intravascular coagulopathy, which if left untreated can progress to fulminant disseminated intravascular coagulopathy,” he said. This tends to occur more in the more widespread varieties of venous malformations.
A common misnomer associated with venous malformations in adults is “liver hemangioma,” owing to the confusing nomenclature, Dr. Nassiri said. “When interrogated angiographically,” he said, “what is often labeled as a hepatic hemangioma is in fact a venous malformation. Natural history of the two entities is completely different.”
Dr. Nassiri described congenital high-flow AVMs as “convoluted networks of blood vessels with poorly differentiated endothelial cells that have neither a venous nor an arterial designation; this entity, otherwise known as a nidus, sits between the feeding arteries and the draining veins.” Treatment aims to eliminate the flow within that nidus.
Super-selective microcatheterization is the best option for nidus access and embolization using liquid embolic agents, preferably those that polymerize when infused. “This is probably the most potent angiogenic entity I’ve ever seen,” Dr. Nassiri said of the nidus.
“It’s like a low-pressure sump and it will recruit collaterals vigorously, so you have to eliminate that nidus.” A variety of different embolic agents, some off label, may be used for high flow AVMs.
For congenital mixed syndromes, the same diagnostic and therapeutic concepts hold true depending on the type of VM involved. Dr. Nassiri advised a multidisciplinary approach, and noted that early trials have investigated the use of sirolimus in severe, life-threatening cases (Br J Clin Pharmacol. 2016;82[5]:1171-9. doi: 10.1111/bcp.13022).
Dr. Nassiri disclosed serving on the speakers bureaus for Boston Scientific, Penumbra, and Merritt Medical, and is a consultant to Merritt Medical.
CHICAGO – Thanks to convoluted terminology, not to mention confusion in the literature, physicians have been known to frequently misdiagnose vascular malformations as hemangiomas, but an evolving understanding of their differences may lead to more precise diagnoses, according to a report at a symposium on vascular surgery sponsored by Northwestern University.
“Historically there has been a great deal of confusion in the literature when it comes to the nomenclature used to describe vascular anomalies,” said Naiem Nassiri, MD, of Robert Wood Johnson Medical School, New Brunswick, N.J. He pointed out that the term hemangioma “or derivatives thereof” – cavernous hemangioma, cavernous angioma, lymphangioma and cystic hygroma – are “absolute misnomers and continue to be misused and applied almost haphazardly to any anomalous vascular lesion.”
He cited reports that 71% of vascular anomalies have been improperly called hemangiomas, 69% have initially been diagnosed incorrectly, and 21% received the wrong treatment (Pediatr Dermatol. 2008;25[1]:7-12; Plast Reconstr Surg. 2011:127[1]:347-51). “Erroneous terminology has prognostic as well as diagnostic and therapeutic implications, and these can actually be quite devastating for the patient, not only clinically and physically but psychologically as well,” Dr. Nassiri said.
Using the International Society for the Study of Vascular Anomalies classification for hemangiomas and vascular malformations can help physicians make the differential diagnosis, Dr. Nassiri said. Hemangiomas are neoplastic lesions of infancy, though not always congenital, with a finite growth phase, whereas vascular malformations (VMs) are nonneoplastic, congenital lesions that can appear at any age and do not regress spontaneously, he said.
Infantile hemangiomas typically appear as the classic strawberry birthmark in children, whereas VMs tend to appear later in life. “They require some environmental trigger, such as trauma, activity, or changes in the hormonal milieu to manifest onset,” he said of VMs.
Simply put, VMs fall into three broad categories: slow-flow malformations, which include lymphatic and venous malformations; high-flow arteriovenous malformations (AVMs) and fistulas; and congenital mixed syndromes, which can include combinations thereof.
Dr. Nassiri noted that contrast-enhanced MRI is the standard imaging modality for diagnosis of VMs, and can differentiate between slow-flow and high-flow lesions. However, vascular specialists must be vigilant in ordering imaging for slow-flow lesions. “Orders can be changed to MR venography, and I’ve had patients who’ve gone decades with multiple MR venograms and no one can figure out what’s going on as no identifiable lesion is readily detected,” he said. “MR venograms are fantastic for detecting truncular blood flow where there typically are no anomalies in the vast majority of patients with isolated venous malformations, but on contrast-enhanced MRI these convoluted cluster of anomalous veins light up like Christmas trees.”
Lymphatic malformations affect the head and neck more so than the extremities, trunk or viscera, and are prone to infection and bleeding. “You can think of these as fluid-filled balloons, and the goal of treatment is fairly simple: You want to puncture the balloon and drain the fluid inside so as to obtain maximum wall collapse,” Dr. Nassiri said. Infusion of a sclerosant causes an inflammatory reaction leading to fibrosis, which then prevents balloon re-expansion. Surgical excision is best used as a secondary adjunct.
Venous malformations, comprising about 80% of all VMs, typically present as soft, spongy blue or purple compressible masses with associated pain that worsens with exertion, Dr. Nassiri said. “The most dangerous thing that is often overlooked, even by some of the physicians that treat these on a regular basis, is localized intravascular coagulopathy, which if left untreated can progress to fulminant disseminated intravascular coagulopathy,” he said. This tends to occur more in the more widespread varieties of venous malformations.
A common misnomer associated with venous malformations in adults is “liver hemangioma,” owing to the confusing nomenclature, Dr. Nassiri said. “When interrogated angiographically,” he said, “what is often labeled as a hepatic hemangioma is in fact a venous malformation. Natural history of the two entities is completely different.”
Dr. Nassiri described congenital high-flow AVMs as “convoluted networks of blood vessels with poorly differentiated endothelial cells that have neither a venous nor an arterial designation; this entity, otherwise known as a nidus, sits between the feeding arteries and the draining veins.” Treatment aims to eliminate the flow within that nidus.
Super-selective microcatheterization is the best option for nidus access and embolization using liquid embolic agents, preferably those that polymerize when infused. “This is probably the most potent angiogenic entity I’ve ever seen,” Dr. Nassiri said of the nidus.
“It’s like a low-pressure sump and it will recruit collaterals vigorously, so you have to eliminate that nidus.” A variety of different embolic agents, some off label, may be used for high flow AVMs.
For congenital mixed syndromes, the same diagnostic and therapeutic concepts hold true depending on the type of VM involved. Dr. Nassiri advised a multidisciplinary approach, and noted that early trials have investigated the use of sirolimus in severe, life-threatening cases (Br J Clin Pharmacol. 2016;82[5]:1171-9. doi: 10.1111/bcp.13022).
Dr. Nassiri disclosed serving on the speakers bureaus for Boston Scientific, Penumbra, and Merritt Medical, and is a consultant to Merritt Medical.
AT THE NORTHWESTERN VASCULAR SYMPOSIUM
Key clinical point:
Major finding: Use of imaging and a clearer understanding of the lack of neoplastic activity are key to more precisely diagnosing vascular malformations.
Data source: Review of literature and center experience.
Disclosure: Dr. Nassiri disclosed serving on the speakers bureaus for Boston Scientific, Penumbra, and Merritt Medical, and is a consultant to Merritt Medical.
An alternative device for ESRD patients with central venous obstruction
CHICAGO – Catheter dependence is often the final option available for hemodialysis patients who have exhausted upper extremity access because of central venous obstruction. But an alternative device that combines a standard expanded polytetrafluoroethylene (ePTFE) arterial graft component with an entirely internalized central venous catheter component may provide an additional option that can help avoid catheters in selected patients, according to pooled results reported at a symposium on vascular surgery sponsored by Northwestern University.
Virginia L. Wong, MD, of University Hospitals Cleveland Medical Center, reported on her group’s and others’ experience using the Hemodialysis Reliable Outflow (HeRO) graft (Merit Medical) to gain access to the superior vena cava (SVC), thus allowing for further upper extremity access options. The device has its limitations in patients with CVO, Dr. Wong noted, “but it can be an important tool for the dedicated access surgeon who is likely to be referred the most complicated patients who have run out of just about every other option.”
The Food and Drug Administration approved the HeRO graft for CVO in 2008, but a recent pooled analysis (Eur J Vasc Endovasc Surg. 2015;50[1]:108-13), which showed a 1-year primary patency rate of 22% and a secondary patency rate of 60%, may provide clarity on how the device can be used to treat CVO in end-stage renal disease (ESRD) patients when the care team desires an alternative to femoral arteriovenous graft, Dr. Wong said. “The 1-year primary patency rate overall was not very good, but with aggressive thrombectomy programs the 1-year patency rate was decent,” she said.
The pooled analysis involved eight series from 2009 to 2015, but the largest series, which involved 164 patients, reported primary and secondary patency rates of 48.8% and 90.8%, respectively (Eur J Vasc Endovasc Surg. 2012;44[1]:93-9). “Patency for these alternative accesses may not be quite what we can achieve with standard upper-extremity access,” Dr. Wong said, “but these patients do not have the standard access as an option.”
Dr. Wong explained where the HeRO fits into the existing vascular practice. “The current data suggest that we should try to exhaust all traditional upper extremity access options before considering anything else, but the HeRO could be considered as an acceptable option for suitable patients,” she said. However, to achieve those outcomes, “you need to have an aggressive thrombectomy program.”
HeRO may be an option for salvage of an existing arm access, plagued by recalcitrant CVO, while still preserving the femoral sites and for future hemodialysis access and/or renal transplantation, Dr. Wong said.
The HeRO also has been used in alternative configurations, taking advantage of axillary or subclavian routes to the SVC when both internal jugular veins are occluded. Dr. Wong has used the femoral route to the inferior vena cava (IVC) for salvaging the femoral AV graft in which iliofemoral venous outflow has been compromised.
Anatomically, the patient must be able to accept a large-bore (19-Fr) access catheter into the central vein. Physiologically, the patient must be able to maintain patency of the long, low-resistance HeRO circuit, which can be up to 50 cm in length, she said. The protocol at Dr. Wong’s institution recommends an inflow arterial diameter of at least 3 mm, along with a left ventricular ejection fraction of 20% or greater and a minimum systolic blood pressure of 100 mm Hg for HeRO on the right side, and possibly higher when coming from the left.
Chronic hypotension is a frequent disqualifier, although some of these patients may benefit from midodrine hydrochloride, she said. In any event, a review of medications and consultation with nephrology and the dialysis unit are mandatory elements of patient screening. “I usually request hemodialysis run sheets from the last three sessions to see what systolic blood pressure excursion is like over the course of treatment,” she said.
The basic principles of hemo-access care are important when considering the HeRO for CVO patients, Dr. Wong said. These include site/side preservation, catheter avoidance and “not to burn any bridges” for future access. “Individualization of care and careful patient selection are probably the best bets if you’re just starting out,” she said. “Choose good patients before resorting to HeRO as the last option for a fairly marginal candidate.”
Dr. Wong had no relevant financial relationships to disclose.
CHICAGO – Catheter dependence is often the final option available for hemodialysis patients who have exhausted upper extremity access because of central venous obstruction. But an alternative device that combines a standard expanded polytetrafluoroethylene (ePTFE) arterial graft component with an entirely internalized central venous catheter component may provide an additional option that can help avoid catheters in selected patients, according to pooled results reported at a symposium on vascular surgery sponsored by Northwestern University.
Virginia L. Wong, MD, of University Hospitals Cleveland Medical Center, reported on her group’s and others’ experience using the Hemodialysis Reliable Outflow (HeRO) graft (Merit Medical) to gain access to the superior vena cava (SVC), thus allowing for further upper extremity access options. The device has its limitations in patients with CVO, Dr. Wong noted, “but it can be an important tool for the dedicated access surgeon who is likely to be referred the most complicated patients who have run out of just about every other option.”
The Food and Drug Administration approved the HeRO graft for CVO in 2008, but a recent pooled analysis (Eur J Vasc Endovasc Surg. 2015;50[1]:108-13), which showed a 1-year primary patency rate of 22% and a secondary patency rate of 60%, may provide clarity on how the device can be used to treat CVO in end-stage renal disease (ESRD) patients when the care team desires an alternative to femoral arteriovenous graft, Dr. Wong said. “The 1-year primary patency rate overall was not very good, but with aggressive thrombectomy programs the 1-year patency rate was decent,” she said.
The pooled analysis involved eight series from 2009 to 2015, but the largest series, which involved 164 patients, reported primary and secondary patency rates of 48.8% and 90.8%, respectively (Eur J Vasc Endovasc Surg. 2012;44[1]:93-9). “Patency for these alternative accesses may not be quite what we can achieve with standard upper-extremity access,” Dr. Wong said, “but these patients do not have the standard access as an option.”
Dr. Wong explained where the HeRO fits into the existing vascular practice. “The current data suggest that we should try to exhaust all traditional upper extremity access options before considering anything else, but the HeRO could be considered as an acceptable option for suitable patients,” she said. However, to achieve those outcomes, “you need to have an aggressive thrombectomy program.”
HeRO may be an option for salvage of an existing arm access, plagued by recalcitrant CVO, while still preserving the femoral sites and for future hemodialysis access and/or renal transplantation, Dr. Wong said.
The HeRO also has been used in alternative configurations, taking advantage of axillary or subclavian routes to the SVC when both internal jugular veins are occluded. Dr. Wong has used the femoral route to the inferior vena cava (IVC) for salvaging the femoral AV graft in which iliofemoral venous outflow has been compromised.
Anatomically, the patient must be able to accept a large-bore (19-Fr) access catheter into the central vein. Physiologically, the patient must be able to maintain patency of the long, low-resistance HeRO circuit, which can be up to 50 cm in length, she said. The protocol at Dr. Wong’s institution recommends an inflow arterial diameter of at least 3 mm, along with a left ventricular ejection fraction of 20% or greater and a minimum systolic blood pressure of 100 mm Hg for HeRO on the right side, and possibly higher when coming from the left.
Chronic hypotension is a frequent disqualifier, although some of these patients may benefit from midodrine hydrochloride, she said. In any event, a review of medications and consultation with nephrology and the dialysis unit are mandatory elements of patient screening. “I usually request hemodialysis run sheets from the last three sessions to see what systolic blood pressure excursion is like over the course of treatment,” she said.
The basic principles of hemo-access care are important when considering the HeRO for CVO patients, Dr. Wong said. These include site/side preservation, catheter avoidance and “not to burn any bridges” for future access. “Individualization of care and careful patient selection are probably the best bets if you’re just starting out,” she said. “Choose good patients before resorting to HeRO as the last option for a fairly marginal candidate.”
Dr. Wong had no relevant financial relationships to disclose.
CHICAGO – Catheter dependence is often the final option available for hemodialysis patients who have exhausted upper extremity access because of central venous obstruction. But an alternative device that combines a standard expanded polytetrafluoroethylene (ePTFE) arterial graft component with an entirely internalized central venous catheter component may provide an additional option that can help avoid catheters in selected patients, according to pooled results reported at a symposium on vascular surgery sponsored by Northwestern University.
Virginia L. Wong, MD, of University Hospitals Cleveland Medical Center, reported on her group’s and others’ experience using the Hemodialysis Reliable Outflow (HeRO) graft (Merit Medical) to gain access to the superior vena cava (SVC), thus allowing for further upper extremity access options. The device has its limitations in patients with CVO, Dr. Wong noted, “but it can be an important tool for the dedicated access surgeon who is likely to be referred the most complicated patients who have run out of just about every other option.”
The Food and Drug Administration approved the HeRO graft for CVO in 2008, but a recent pooled analysis (Eur J Vasc Endovasc Surg. 2015;50[1]:108-13), which showed a 1-year primary patency rate of 22% and a secondary patency rate of 60%, may provide clarity on how the device can be used to treat CVO in end-stage renal disease (ESRD) patients when the care team desires an alternative to femoral arteriovenous graft, Dr. Wong said. “The 1-year primary patency rate overall was not very good, but with aggressive thrombectomy programs the 1-year patency rate was decent,” she said.
The pooled analysis involved eight series from 2009 to 2015, but the largest series, which involved 164 patients, reported primary and secondary patency rates of 48.8% and 90.8%, respectively (Eur J Vasc Endovasc Surg. 2012;44[1]:93-9). “Patency for these alternative accesses may not be quite what we can achieve with standard upper-extremity access,” Dr. Wong said, “but these patients do not have the standard access as an option.”
Dr. Wong explained where the HeRO fits into the existing vascular practice. “The current data suggest that we should try to exhaust all traditional upper extremity access options before considering anything else, but the HeRO could be considered as an acceptable option for suitable patients,” she said. However, to achieve those outcomes, “you need to have an aggressive thrombectomy program.”
HeRO may be an option for salvage of an existing arm access, plagued by recalcitrant CVO, while still preserving the femoral sites and for future hemodialysis access and/or renal transplantation, Dr. Wong said.
The HeRO also has been used in alternative configurations, taking advantage of axillary or subclavian routes to the SVC when both internal jugular veins are occluded. Dr. Wong has used the femoral route to the inferior vena cava (IVC) for salvaging the femoral AV graft in which iliofemoral venous outflow has been compromised.
Anatomically, the patient must be able to accept a large-bore (19-Fr) access catheter into the central vein. Physiologically, the patient must be able to maintain patency of the long, low-resistance HeRO circuit, which can be up to 50 cm in length, she said. The protocol at Dr. Wong’s institution recommends an inflow arterial diameter of at least 3 mm, along with a left ventricular ejection fraction of 20% or greater and a minimum systolic blood pressure of 100 mm Hg for HeRO on the right side, and possibly higher when coming from the left.
Chronic hypotension is a frequent disqualifier, although some of these patients may benefit from midodrine hydrochloride, she said. In any event, a review of medications and consultation with nephrology and the dialysis unit are mandatory elements of patient screening. “I usually request hemodialysis run sheets from the last three sessions to see what systolic blood pressure excursion is like over the course of treatment,” she said.
The basic principles of hemo-access care are important when considering the HeRO for CVO patients, Dr. Wong said. These include site/side preservation, catheter avoidance and “not to burn any bridges” for future access. “Individualization of care and careful patient selection are probably the best bets if you’re just starting out,” she said. “Choose good patients before resorting to HeRO as the last option for a fairly marginal candidate.”
Dr. Wong had no relevant financial relationships to disclose.
Key clinical point: Combined graft-catheter device may preserve femoral access for hemodialysis for patients with central venous obstruction.
Major finding: One-year primary potency rate was 22% and secondary patency rate 60% for device recipients.
Data source: Literature review, including pooled results from eight studies involving 408 subjects.
Disclosures: Dr. Wong reported having no financial disclosures.
Idle intravenous catheters are associated with preventable complications
Intravenous catheters (ICs) are common and necessary for inpatient care. However, peripheral and especially central venous catheters (CVCs) are associated with increased risk for local and systemic complications, including bloodstream infections and endocarditis.
Prevention of these complications is important and should be a major focus of infection control and patient safety practices. There are three main points of focus on infection prevention with regard to ICs – proper insertion techniques, proper care of the catheter, and prompt removal when it is no longer necessary.
We focused our review, published in the American Journal of Infection Control (2016 Oct. doi: 10.1016/j.ajic.2016.03.073), on the final point – determining the prevalence, risk factors, and outcomes related to idle intravenous catheters. To accomplish this, we conducted an integrative review of published studies related to idle catheters, excluding reviews, abstracts, and commentaries. Thirteen studies met the inclusion criteria and four of these focused on CVCs.
Generally, an idle catheter is one that remains in place even though it is not being used for patient care. However, the definition of an “idle” catheter varied amongst the reviewed studies, as did the unit of measure, especially for peripheral catheters. Central venous catheter-focused studies were more consistent in using “idle catheter days” and “catheter days.”
Studies of peripheral catheters revealed that 16%-50% of patients had an idle catheter of some type. For the studies focused on CVCs, the percentage of patients with idle catheters ranged from 2.7% in one intensive care unit to 26.2% in a different study. Interestingly, in the study with 2.7% idle CVCs in the ICU, there was a higher percentage of idle CVCs outside of the ICU in the same hospital.
The major reasons for leaving catheters in place in studies where reasons were noted were convenience, future intention to use intravenous medication, and inappropriate use of intravenous medications when oral could be used.
Although data are scarce, complications in the reviewed studies were relatively common with idle peripheral catheters, where 9%-12% suffered thrombophlebitis. Obviously, the risk for catheter-related bloodstream infection increases as the number of catheter days increases – this is especially important with regard to idle CVCs.
Decreasing the prevalence of idle catheters is likely to decrease the risk for infection and improve patient safety. Based on our review of the data, a standardized definition of an “idle catheter” is needed. At the very least, a standard definition should be developed at each institution. This would allow an individual hospital the ability to identify and track the presence of these lines, and implement targeted interventions to decrease the proportion of idle lines. Ideally, a common definition would be created and validated so that data and interventions could be comparable across institutions and guidelines could be developed.
The goal of targeted interventions should be zero idle lines. Prevention of idle peripheral catheters should also be pursued, but because CVC-related complications are often more serious, these lines are often the focus of efforts. Use of peripherally inserted central catheters (PICCs) has increased and while these catheters in some settings may have decreased complication risk, compared with femoral/internal jugular/subclavian CVCs, prevention of idle catheter days is paramount for these catheters as well.
Many ICUs, including at our own institution, have instituted programs to closely monitor for ongoing need for CVCs. This increased focus on the CVC likely explains the lower rates of idle catheters in ICUs noted in the reviewed studies. This close surveillance can be done outside of the ICU as well, and could include peripheral catheters.
At our own institution, the need for catheters is reviewed on some units as part of formalized patient safety rounds. Another potential group of interventions could focus on electronic medical record (EMR)-based changes such as limits on the duration of the order, requirement for renewal of the order, or on-screen reminders of the presence of a catheter. This sort of intervention could possibly be expanded as EMR use becomes more common and robust. For instance, if intravenous medications have not been ordered or given in a certain amount of time, an alert might be triggered. Another EMR-based mechanism could be to require an indication for ongoing catheter use.
Education about the potential adverse outcomes of idle catheters is important. Promoting a team-based approach to interventions, where all involved team members can discuss patient safety issues on equal ground is paramount to successfully decreasing idle catheters and improving patient care and safety in general. As with other hospital-wide initiatives, engagement of hospital administration is important to decrease barriers to implementation.
Intravenous catheter use will remain an integral part of patient care, but efforts should be made to create standardization around the definition of an idle catheter, standardize units of measure, and institute programs to prevent idle catheters.
Daniel Shirley, MD, MS, is assistant professor in the division of infectious disease at the University of Wisconsin–Madison School of Medicine and Public Health and the William S. Middleton Memorial Veterans Hospital. Nasia Safdar, MD, PhD, is associate professor in the division of infectious disease at the University of Wisconsin–Madison School of Medicine and Public Health and the William S. Middleton Memorial Veterans Hospital.
Intravenous catheters (ICs) are common and necessary for inpatient care. However, peripheral and especially central venous catheters (CVCs) are associated with increased risk for local and systemic complications, including bloodstream infections and endocarditis.
Prevention of these complications is important and should be a major focus of infection control and patient safety practices. There are three main points of focus on infection prevention with regard to ICs – proper insertion techniques, proper care of the catheter, and prompt removal when it is no longer necessary.
We focused our review, published in the American Journal of Infection Control (2016 Oct. doi: 10.1016/j.ajic.2016.03.073), on the final point – determining the prevalence, risk factors, and outcomes related to idle intravenous catheters. To accomplish this, we conducted an integrative review of published studies related to idle catheters, excluding reviews, abstracts, and commentaries. Thirteen studies met the inclusion criteria and four of these focused on CVCs.
Generally, an idle catheter is one that remains in place even though it is not being used for patient care. However, the definition of an “idle” catheter varied amongst the reviewed studies, as did the unit of measure, especially for peripheral catheters. Central venous catheter-focused studies were more consistent in using “idle catheter days” and “catheter days.”
Studies of peripheral catheters revealed that 16%-50% of patients had an idle catheter of some type. For the studies focused on CVCs, the percentage of patients with idle catheters ranged from 2.7% in one intensive care unit to 26.2% in a different study. Interestingly, in the study with 2.7% idle CVCs in the ICU, there was a higher percentage of idle CVCs outside of the ICU in the same hospital.
The major reasons for leaving catheters in place in studies where reasons were noted were convenience, future intention to use intravenous medication, and inappropriate use of intravenous medications when oral could be used.
Although data are scarce, complications in the reviewed studies were relatively common with idle peripheral catheters, where 9%-12% suffered thrombophlebitis. Obviously, the risk for catheter-related bloodstream infection increases as the number of catheter days increases – this is especially important with regard to idle CVCs.
Decreasing the prevalence of idle catheters is likely to decrease the risk for infection and improve patient safety. Based on our review of the data, a standardized definition of an “idle catheter” is needed. At the very least, a standard definition should be developed at each institution. This would allow an individual hospital the ability to identify and track the presence of these lines, and implement targeted interventions to decrease the proportion of idle lines. Ideally, a common definition would be created and validated so that data and interventions could be comparable across institutions and guidelines could be developed.
The goal of targeted interventions should be zero idle lines. Prevention of idle peripheral catheters should also be pursued, but because CVC-related complications are often more serious, these lines are often the focus of efforts. Use of peripherally inserted central catheters (PICCs) has increased and while these catheters in some settings may have decreased complication risk, compared with femoral/internal jugular/subclavian CVCs, prevention of idle catheter days is paramount for these catheters as well.
Many ICUs, including at our own institution, have instituted programs to closely monitor for ongoing need for CVCs. This increased focus on the CVC likely explains the lower rates of idle catheters in ICUs noted in the reviewed studies. This close surveillance can be done outside of the ICU as well, and could include peripheral catheters.
At our own institution, the need for catheters is reviewed on some units as part of formalized patient safety rounds. Another potential group of interventions could focus on electronic medical record (EMR)-based changes such as limits on the duration of the order, requirement for renewal of the order, or on-screen reminders of the presence of a catheter. This sort of intervention could possibly be expanded as EMR use becomes more common and robust. For instance, if intravenous medications have not been ordered or given in a certain amount of time, an alert might be triggered. Another EMR-based mechanism could be to require an indication for ongoing catheter use.
Education about the potential adverse outcomes of idle catheters is important. Promoting a team-based approach to interventions, where all involved team members can discuss patient safety issues on equal ground is paramount to successfully decreasing idle catheters and improving patient care and safety in general. As with other hospital-wide initiatives, engagement of hospital administration is important to decrease barriers to implementation.
Intravenous catheter use will remain an integral part of patient care, but efforts should be made to create standardization around the definition of an idle catheter, standardize units of measure, and institute programs to prevent idle catheters.
Daniel Shirley, MD, MS, is assistant professor in the division of infectious disease at the University of Wisconsin–Madison School of Medicine and Public Health and the William S. Middleton Memorial Veterans Hospital. Nasia Safdar, MD, PhD, is associate professor in the division of infectious disease at the University of Wisconsin–Madison School of Medicine and Public Health and the William S. Middleton Memorial Veterans Hospital.
Intravenous catheters (ICs) are common and necessary for inpatient care. However, peripheral and especially central venous catheters (CVCs) are associated with increased risk for local and systemic complications, including bloodstream infections and endocarditis.
Prevention of these complications is important and should be a major focus of infection control and patient safety practices. There are three main points of focus on infection prevention with regard to ICs – proper insertion techniques, proper care of the catheter, and prompt removal when it is no longer necessary.
We focused our review, published in the American Journal of Infection Control (2016 Oct. doi: 10.1016/j.ajic.2016.03.073), on the final point – determining the prevalence, risk factors, and outcomes related to idle intravenous catheters. To accomplish this, we conducted an integrative review of published studies related to idle catheters, excluding reviews, abstracts, and commentaries. Thirteen studies met the inclusion criteria and four of these focused on CVCs.
Generally, an idle catheter is one that remains in place even though it is not being used for patient care. However, the definition of an “idle” catheter varied amongst the reviewed studies, as did the unit of measure, especially for peripheral catheters. Central venous catheter-focused studies were more consistent in using “idle catheter days” and “catheter days.”
Studies of peripheral catheters revealed that 16%-50% of patients had an idle catheter of some type. For the studies focused on CVCs, the percentage of patients with idle catheters ranged from 2.7% in one intensive care unit to 26.2% in a different study. Interestingly, in the study with 2.7% idle CVCs in the ICU, there was a higher percentage of idle CVCs outside of the ICU in the same hospital.
The major reasons for leaving catheters in place in studies where reasons were noted were convenience, future intention to use intravenous medication, and inappropriate use of intravenous medications when oral could be used.
Although data are scarce, complications in the reviewed studies were relatively common with idle peripheral catheters, where 9%-12% suffered thrombophlebitis. Obviously, the risk for catheter-related bloodstream infection increases as the number of catheter days increases – this is especially important with regard to idle CVCs.
Decreasing the prevalence of idle catheters is likely to decrease the risk for infection and improve patient safety. Based on our review of the data, a standardized definition of an “idle catheter” is needed. At the very least, a standard definition should be developed at each institution. This would allow an individual hospital the ability to identify and track the presence of these lines, and implement targeted interventions to decrease the proportion of idle lines. Ideally, a common definition would be created and validated so that data and interventions could be comparable across institutions and guidelines could be developed.
The goal of targeted interventions should be zero idle lines. Prevention of idle peripheral catheters should also be pursued, but because CVC-related complications are often more serious, these lines are often the focus of efforts. Use of peripherally inserted central catheters (PICCs) has increased and while these catheters in some settings may have decreased complication risk, compared with femoral/internal jugular/subclavian CVCs, prevention of idle catheter days is paramount for these catheters as well.
Many ICUs, including at our own institution, have instituted programs to closely monitor for ongoing need for CVCs. This increased focus on the CVC likely explains the lower rates of idle catheters in ICUs noted in the reviewed studies. This close surveillance can be done outside of the ICU as well, and could include peripheral catheters.
At our own institution, the need for catheters is reviewed on some units as part of formalized patient safety rounds. Another potential group of interventions could focus on electronic medical record (EMR)-based changes such as limits on the duration of the order, requirement for renewal of the order, or on-screen reminders of the presence of a catheter. This sort of intervention could possibly be expanded as EMR use becomes more common and robust. For instance, if intravenous medications have not been ordered or given in a certain amount of time, an alert might be triggered. Another EMR-based mechanism could be to require an indication for ongoing catheter use.
Education about the potential adverse outcomes of idle catheters is important. Promoting a team-based approach to interventions, where all involved team members can discuss patient safety issues on equal ground is paramount to successfully decreasing idle catheters and improving patient care and safety in general. As with other hospital-wide initiatives, engagement of hospital administration is important to decrease barriers to implementation.
Intravenous catheter use will remain an integral part of patient care, but efforts should be made to create standardization around the definition of an idle catheter, standardize units of measure, and institute programs to prevent idle catheters.
Daniel Shirley, MD, MS, is assistant professor in the division of infectious disease at the University of Wisconsin–Madison School of Medicine and Public Health and the William S. Middleton Memorial Veterans Hospital. Nasia Safdar, MD, PhD, is associate professor in the division of infectious disease at the University of Wisconsin–Madison School of Medicine and Public Health and the William S. Middleton Memorial Veterans Hospital.
Sofosbuvir, daclatasvir combo best treatment for HCV cryoglobulinemia vasculitis
WASHINGTON – A combined regimen of sofosbuvir and daclatasvir is the best option to treat patients with hepatitis C virus infections experiencing cryoglobulinemia vasculitis, according to the findings of a new study presented at the annual meeting of the American College of Rheumatology.
“The HCV cryoglobulinemia vasculitis is a very important vasculitis because it represents 5% of chronically infected HCV patients in the world,” explained David Saadoun, MD, of Sorbonne Universities, Paris. “It’s sometimes a life-threatening vasculitis because patients may develop inflammation [so] there’s a need for very active and well-tolerated treatment.”
The primary endpoint – complete response to treatment at the end of the regimen – was achieved in 91% of subjects by the end of 24 weeks. Furthermore, 50% of patients experienced complete immunological response, defined as the complete clearance of cryoglobulin, within 24 weeks. At 12 weeks, average cryoglobulin levels decreased from 0.36 ± 0.12 to 0.10 ± 0.08 g/L, (P = .019), while average aminotransferase levels decreased from 57.6 ± 7.1 to 20.4 ± 2.0 IU/mL, (P less than .01).
But perhaps most significant, according to Dr. Saadoun, is that less than 5% of subjects required any additional treatment via immunosuppressants, such as steroids or rituximab. Average HCV viral loads dropped from 5.6 to 1.18 IU/mL at week 4 (P less than .01), with similarly sustained results through to week 12, indicating good virological responses. No serious adverse events were reported by any subjects throughout the trial period.
“The limitation is that there are quite a few patients, because it is only 35 patients this time, [and] that it’s a prospective, open-label study with no comparators,” Dr. Saadoun explained, adding that, in terms of further research, “[any] new study would focus on the way to avoid rituximab and steroid use in these patients, and to also have more patients treated with this regimen.”
No funding source was disclosed for this study. Dr. Saadoun did not report any relevant financial disclosures.
WASHINGTON – A combined regimen of sofosbuvir and daclatasvir is the best option to treat patients with hepatitis C virus infections experiencing cryoglobulinemia vasculitis, according to the findings of a new study presented at the annual meeting of the American College of Rheumatology.
“The HCV cryoglobulinemia vasculitis is a very important vasculitis because it represents 5% of chronically infected HCV patients in the world,” explained David Saadoun, MD, of Sorbonne Universities, Paris. “It’s sometimes a life-threatening vasculitis because patients may develop inflammation [so] there’s a need for very active and well-tolerated treatment.”
The primary endpoint – complete response to treatment at the end of the regimen – was achieved in 91% of subjects by the end of 24 weeks. Furthermore, 50% of patients experienced complete immunological response, defined as the complete clearance of cryoglobulin, within 24 weeks. At 12 weeks, average cryoglobulin levels decreased from 0.36 ± 0.12 to 0.10 ± 0.08 g/L, (P = .019), while average aminotransferase levels decreased from 57.6 ± 7.1 to 20.4 ± 2.0 IU/mL, (P less than .01).
But perhaps most significant, according to Dr. Saadoun, is that less than 5% of subjects required any additional treatment via immunosuppressants, such as steroids or rituximab. Average HCV viral loads dropped from 5.6 to 1.18 IU/mL at week 4 (P less than .01), with similarly sustained results through to week 12, indicating good virological responses. No serious adverse events were reported by any subjects throughout the trial period.
“The limitation is that there are quite a few patients, because it is only 35 patients this time, [and] that it’s a prospective, open-label study with no comparators,” Dr. Saadoun explained, adding that, in terms of further research, “[any] new study would focus on the way to avoid rituximab and steroid use in these patients, and to also have more patients treated with this regimen.”
No funding source was disclosed for this study. Dr. Saadoun did not report any relevant financial disclosures.
WASHINGTON – A combined regimen of sofosbuvir and daclatasvir is the best option to treat patients with hepatitis C virus infections experiencing cryoglobulinemia vasculitis, according to the findings of a new study presented at the annual meeting of the American College of Rheumatology.
“The HCV cryoglobulinemia vasculitis is a very important vasculitis because it represents 5% of chronically infected HCV patients in the world,” explained David Saadoun, MD, of Sorbonne Universities, Paris. “It’s sometimes a life-threatening vasculitis because patients may develop inflammation [so] there’s a need for very active and well-tolerated treatment.”
The primary endpoint – complete response to treatment at the end of the regimen – was achieved in 91% of subjects by the end of 24 weeks. Furthermore, 50% of patients experienced complete immunological response, defined as the complete clearance of cryoglobulin, within 24 weeks. At 12 weeks, average cryoglobulin levels decreased from 0.36 ± 0.12 to 0.10 ± 0.08 g/L, (P = .019), while average aminotransferase levels decreased from 57.6 ± 7.1 to 20.4 ± 2.0 IU/mL, (P less than .01).
But perhaps most significant, according to Dr. Saadoun, is that less than 5% of subjects required any additional treatment via immunosuppressants, such as steroids or rituximab. Average HCV viral loads dropped from 5.6 to 1.18 IU/mL at week 4 (P less than .01), with similarly sustained results through to week 12, indicating good virological responses. No serious adverse events were reported by any subjects throughout the trial period.
“The limitation is that there are quite a few patients, because it is only 35 patients this time, [and] that it’s a prospective, open-label study with no comparators,” Dr. Saadoun explained, adding that, in terms of further research, “[any] new study would focus on the way to avoid rituximab and steroid use in these patients, and to also have more patients treated with this regimen.”
No funding source was disclosed for this study. Dr. Saadoun did not report any relevant financial disclosures.
AT THE ACR ANNUAL MEETING
Key clinical point:
Major finding: Of 35 patients, 32 (91%) achieved complete clinical response in 6 months, with less than 5% requiring the use of additional immunosuppressants and none experiencing serious adverse events.
Data source: A prospective, open-label study of 35 patients with cryoglobulinemia vasculitis brought on by HCV infection.
Disclosures: Dr. Saadoun did not report any relevant financial disclosures.
Bone marrow cells prove limb-saving for some
NEW ORLEANS – Treatment with autologous bone marrow cells can avert amputation in selected patients who have critical limb ischemia and are not candidates for revascularization surgery, based on results from the randomized phase III MOBILE trial.
Critical limb ischemia, the end stage of peripheral arterial disease, accounts for more than 53,000 major limb amputations in the United States annually, noted principal investigator Dr. Michael P. Murphy, director of the Vascular and Cardiac Center for Adult Stem Cell Therapy at Indiana University, Indianapolis.
“About 30% of our patient population with critical limb ischemia have no options for the standard of care, such as surgical bypass, due to absence of a surgical target or chronic total occlusion, which mitigates an endovascular approach,” he said at the American Heart Association scientific sessions. “Thus, these no-option critical limb ischemia patients represent an unmet medical need for a novel agent that may promote limb salvage and prevent amputation and its associated disabilities.”
In the trial, investigators randomized 155 affected limbs (in 152 patients) 3:1 to receive double-blinded treatment with injections of concentrated autologous bone marrow aspirate or placebo.
Amputation-free survival 1 year after treatment, the trial’s primary endpoint, was not significantly better in the aspirate group than in the placebo group, according to the researchers. However, the aspirate reduced the risk of major amputation by 73% in the subgroup with less severe (Rutherford class 4) disease and by 67% in the subgroup of patients who did not have diabetes.
“Personally, I would recommend cell therapy for my Rutherford 4 patients and my nondiabetic Rutherford 5 patients,” Dr. Murphy said. “And one would say even for the Rutherford 5 diabetics, there were no safety concerns and there is hope for improvement rather than amputation – it might be worth the roll of the dice.”
The investigators are preparing a manuscript based on the full data and working with Biomet, the trial sponsor, to apply for Food and Drug Administration approval of the product for the treatment of critical limb ischemia, he said.
Lessons learned
A session attendee asked whether stronger demonstration of benefit in a larger trial is needed to justify the use and cost of such new cellular therapies.
“If we could go back and do it all over again, we would do things differently knowing what we know now,” Dr. Murphy replied, noting that the investigators had to stick to a trial design created more than a decade ago.
“We discovered that the event rate in patients with critical limb ischemia in the control group is actually 30% and not 40% because medical management has changed,” he said. “Secondly, we would do a 1:1 randomization and most likely would increase our sample size to 200, and we probably would have seen a difference overall. That difference overall would have been provided by, of course, increasing the Rutherford 4 and Rutherford 5 nondiabetics, which would overshadow the increased amputation rate in the diabetic group.”
“I think from this [experience], we can launch a much larger study, if there were funding for it, and look at autologous versus allogeneic cells in this domain,” Dr. Murphy concluded.
Identifying patients who benefit
Session panelist Roberto Bolli, MD, chief of the division of cardiovascular medicine at the University of Louisville (Ky.), commended the MOBILE trial for its study design and prespecified subgroup analyses.
“The whole problem we are coping with, not just in this trial, but in other studies, is that patients are different and some patients may respond better than others, or some patients may not respond at all. And really, we still don’t understand why some patients respond or not – it may have to do with their immune system, their regenerative capacity, as well as the type of cells that are being used,” he said.
“Even though it was in its entirety a negative study, it’s very important because it identified a possible target for future trials, which is patients without diabetes in Rutherford class 4, which may benefit from therapy,” Dr. Bolli concluded.
Trial details
Patients were enrolled in MOBILE from 24 U.S. centers. All had critical limb ischemia, were ineligible for revascularization, had an ankle-brachial index of less than 0.60 or a toe-brachial index of less than 0.40, and had Rutherford 4 disease (rest pain) or Rutherford 5 disease (tissue loss).
They received concentrated autologous bone marrow aspirate or placebo by intramuscular injection at 35-40 sites in the affected limb.
Results showed that the groups did not differ significantly with respect to the rates of adverse events or serious adverse events overall, Dr. Murphy reported. The aspirate group had lower rates of respiratory failure and fever; they also had a higher rate of anemia (68.9% vs. 36.1%, P less than .001) as expected, from the aspiration procedure, but with no associated complications.
The 1-year rate of amputation-free survival events, reflecting both major amputations and death, was lower with the aspirate, at 20.2%, than with placebo, at 30.5%, but not significantly so (P = .224). Findings were similar for major amputation in the entire trial population (16.0% vs. 22.2%, P = .392). However, this outcome was less common with the aspirate among patients with Rutherford 4 disease (7.7% vs. 26.3%, P = .041) and nondiabetic patients (10.0% vs. 27.7%, P = .046).
“Looking at these data, it became apparent to us that the Rutherford 5 diabetic was the outlying group,” said Dr. Murphy, who disclosed that he had no relevant conflicts of interest.
Considering all other patients – Rutherford 4 regardless of diabetes status and nondiabetic Rutherford 5 – the aspirate was associated with a dramatically lower rate of amputation compared with the placebo (9.6% vs. 26.7%, P = .021; hazard ratio, 0.33). In this subset, the number needed to treat with bone marrow aspirate to prevent a single amputation was just 6.
The aspirate and placebo groups did not differ with respect to ankle-brachial index, toe-brachial index, or 6-minute walk test distance in the entire trial population. Transcutaneous oxygen pressure (TcP02), an indicator of microvascular perfusion in the subcutaneous tissue of the ischemic limb, increased by 59% in the aspirate group but by only 7% in the placebo group.
NEW ORLEANS – Treatment with autologous bone marrow cells can avert amputation in selected patients who have critical limb ischemia and are not candidates for revascularization surgery, based on results from the randomized phase III MOBILE trial.
Critical limb ischemia, the end stage of peripheral arterial disease, accounts for more than 53,000 major limb amputations in the United States annually, noted principal investigator Dr. Michael P. Murphy, director of the Vascular and Cardiac Center for Adult Stem Cell Therapy at Indiana University, Indianapolis.
“About 30% of our patient population with critical limb ischemia have no options for the standard of care, such as surgical bypass, due to absence of a surgical target or chronic total occlusion, which mitigates an endovascular approach,” he said at the American Heart Association scientific sessions. “Thus, these no-option critical limb ischemia patients represent an unmet medical need for a novel agent that may promote limb salvage and prevent amputation and its associated disabilities.”
In the trial, investigators randomized 155 affected limbs (in 152 patients) 3:1 to receive double-blinded treatment with injections of concentrated autologous bone marrow aspirate or placebo.
Amputation-free survival 1 year after treatment, the trial’s primary endpoint, was not significantly better in the aspirate group than in the placebo group, according to the researchers. However, the aspirate reduced the risk of major amputation by 73% in the subgroup with less severe (Rutherford class 4) disease and by 67% in the subgroup of patients who did not have diabetes.
“Personally, I would recommend cell therapy for my Rutherford 4 patients and my nondiabetic Rutherford 5 patients,” Dr. Murphy said. “And one would say even for the Rutherford 5 diabetics, there were no safety concerns and there is hope for improvement rather than amputation – it might be worth the roll of the dice.”
The investigators are preparing a manuscript based on the full data and working with Biomet, the trial sponsor, to apply for Food and Drug Administration approval of the product for the treatment of critical limb ischemia, he said.
Lessons learned
A session attendee asked whether stronger demonstration of benefit in a larger trial is needed to justify the use and cost of such new cellular therapies.
“If we could go back and do it all over again, we would do things differently knowing what we know now,” Dr. Murphy replied, noting that the investigators had to stick to a trial design created more than a decade ago.
“We discovered that the event rate in patients with critical limb ischemia in the control group is actually 30% and not 40% because medical management has changed,” he said. “Secondly, we would do a 1:1 randomization and most likely would increase our sample size to 200, and we probably would have seen a difference overall. That difference overall would have been provided by, of course, increasing the Rutherford 4 and Rutherford 5 nondiabetics, which would overshadow the increased amputation rate in the diabetic group.”
“I think from this [experience], we can launch a much larger study, if there were funding for it, and look at autologous versus allogeneic cells in this domain,” Dr. Murphy concluded.
Identifying patients who benefit
Session panelist Roberto Bolli, MD, chief of the division of cardiovascular medicine at the University of Louisville (Ky.), commended the MOBILE trial for its study design and prespecified subgroup analyses.
“The whole problem we are coping with, not just in this trial, but in other studies, is that patients are different and some patients may respond better than others, or some patients may not respond at all. And really, we still don’t understand why some patients respond or not – it may have to do with their immune system, their regenerative capacity, as well as the type of cells that are being used,” he said.
“Even though it was in its entirety a negative study, it’s very important because it identified a possible target for future trials, which is patients without diabetes in Rutherford class 4, which may benefit from therapy,” Dr. Bolli concluded.
Trial details
Patients were enrolled in MOBILE from 24 U.S. centers. All had critical limb ischemia, were ineligible for revascularization, had an ankle-brachial index of less than 0.60 or a toe-brachial index of less than 0.40, and had Rutherford 4 disease (rest pain) or Rutherford 5 disease (tissue loss).
They received concentrated autologous bone marrow aspirate or placebo by intramuscular injection at 35-40 sites in the affected limb.
Results showed that the groups did not differ significantly with respect to the rates of adverse events or serious adverse events overall, Dr. Murphy reported. The aspirate group had lower rates of respiratory failure and fever; they also had a higher rate of anemia (68.9% vs. 36.1%, P less than .001) as expected, from the aspiration procedure, but with no associated complications.
The 1-year rate of amputation-free survival events, reflecting both major amputations and death, was lower with the aspirate, at 20.2%, than with placebo, at 30.5%, but not significantly so (P = .224). Findings were similar for major amputation in the entire trial population (16.0% vs. 22.2%, P = .392). However, this outcome was less common with the aspirate among patients with Rutherford 4 disease (7.7% vs. 26.3%, P = .041) and nondiabetic patients (10.0% vs. 27.7%, P = .046).
“Looking at these data, it became apparent to us that the Rutherford 5 diabetic was the outlying group,” said Dr. Murphy, who disclosed that he had no relevant conflicts of interest.
Considering all other patients – Rutherford 4 regardless of diabetes status and nondiabetic Rutherford 5 – the aspirate was associated with a dramatically lower rate of amputation compared with the placebo (9.6% vs. 26.7%, P = .021; hazard ratio, 0.33). In this subset, the number needed to treat with bone marrow aspirate to prevent a single amputation was just 6.
The aspirate and placebo groups did not differ with respect to ankle-brachial index, toe-brachial index, or 6-minute walk test distance in the entire trial population. Transcutaneous oxygen pressure (TcP02), an indicator of microvascular perfusion in the subcutaneous tissue of the ischemic limb, increased by 59% in the aspirate group but by only 7% in the placebo group.
NEW ORLEANS – Treatment with autologous bone marrow cells can avert amputation in selected patients who have critical limb ischemia and are not candidates for revascularization surgery, based on results from the randomized phase III MOBILE trial.
Critical limb ischemia, the end stage of peripheral arterial disease, accounts for more than 53,000 major limb amputations in the United States annually, noted principal investigator Dr. Michael P. Murphy, director of the Vascular and Cardiac Center for Adult Stem Cell Therapy at Indiana University, Indianapolis.
“About 30% of our patient population with critical limb ischemia have no options for the standard of care, such as surgical bypass, due to absence of a surgical target or chronic total occlusion, which mitigates an endovascular approach,” he said at the American Heart Association scientific sessions. “Thus, these no-option critical limb ischemia patients represent an unmet medical need for a novel agent that may promote limb salvage and prevent amputation and its associated disabilities.”
In the trial, investigators randomized 155 affected limbs (in 152 patients) 3:1 to receive double-blinded treatment with injections of concentrated autologous bone marrow aspirate or placebo.
Amputation-free survival 1 year after treatment, the trial’s primary endpoint, was not significantly better in the aspirate group than in the placebo group, according to the researchers. However, the aspirate reduced the risk of major amputation by 73% in the subgroup with less severe (Rutherford class 4) disease and by 67% in the subgroup of patients who did not have diabetes.
“Personally, I would recommend cell therapy for my Rutherford 4 patients and my nondiabetic Rutherford 5 patients,” Dr. Murphy said. “And one would say even for the Rutherford 5 diabetics, there were no safety concerns and there is hope for improvement rather than amputation – it might be worth the roll of the dice.”
The investigators are preparing a manuscript based on the full data and working with Biomet, the trial sponsor, to apply for Food and Drug Administration approval of the product for the treatment of critical limb ischemia, he said.
Lessons learned
A session attendee asked whether stronger demonstration of benefit in a larger trial is needed to justify the use and cost of such new cellular therapies.
“If we could go back and do it all over again, we would do things differently knowing what we know now,” Dr. Murphy replied, noting that the investigators had to stick to a trial design created more than a decade ago.
“We discovered that the event rate in patients with critical limb ischemia in the control group is actually 30% and not 40% because medical management has changed,” he said. “Secondly, we would do a 1:1 randomization and most likely would increase our sample size to 200, and we probably would have seen a difference overall. That difference overall would have been provided by, of course, increasing the Rutherford 4 and Rutherford 5 nondiabetics, which would overshadow the increased amputation rate in the diabetic group.”
“I think from this [experience], we can launch a much larger study, if there were funding for it, and look at autologous versus allogeneic cells in this domain,” Dr. Murphy concluded.
Identifying patients who benefit
Session panelist Roberto Bolli, MD, chief of the division of cardiovascular medicine at the University of Louisville (Ky.), commended the MOBILE trial for its study design and prespecified subgroup analyses.
“The whole problem we are coping with, not just in this trial, but in other studies, is that patients are different and some patients may respond better than others, or some patients may not respond at all. And really, we still don’t understand why some patients respond or not – it may have to do with their immune system, their regenerative capacity, as well as the type of cells that are being used,” he said.
“Even though it was in its entirety a negative study, it’s very important because it identified a possible target for future trials, which is patients without diabetes in Rutherford class 4, which may benefit from therapy,” Dr. Bolli concluded.
Trial details
Patients were enrolled in MOBILE from 24 U.S. centers. All had critical limb ischemia, were ineligible for revascularization, had an ankle-brachial index of less than 0.60 or a toe-brachial index of less than 0.40, and had Rutherford 4 disease (rest pain) or Rutherford 5 disease (tissue loss).
They received concentrated autologous bone marrow aspirate or placebo by intramuscular injection at 35-40 sites in the affected limb.
Results showed that the groups did not differ significantly with respect to the rates of adverse events or serious adverse events overall, Dr. Murphy reported. The aspirate group had lower rates of respiratory failure and fever; they also had a higher rate of anemia (68.9% vs. 36.1%, P less than .001) as expected, from the aspiration procedure, but with no associated complications.
The 1-year rate of amputation-free survival events, reflecting both major amputations and death, was lower with the aspirate, at 20.2%, than with placebo, at 30.5%, but not significantly so (P = .224). Findings were similar for major amputation in the entire trial population (16.0% vs. 22.2%, P = .392). However, this outcome was less common with the aspirate among patients with Rutherford 4 disease (7.7% vs. 26.3%, P = .041) and nondiabetic patients (10.0% vs. 27.7%, P = .046).
“Looking at these data, it became apparent to us that the Rutherford 5 diabetic was the outlying group,” said Dr. Murphy, who disclosed that he had no relevant conflicts of interest.
Considering all other patients – Rutherford 4 regardless of diabetes status and nondiabetic Rutherford 5 – the aspirate was associated with a dramatically lower rate of amputation compared with the placebo (9.6% vs. 26.7%, P = .021; hazard ratio, 0.33). In this subset, the number needed to treat with bone marrow aspirate to prevent a single amputation was just 6.
The aspirate and placebo groups did not differ with respect to ankle-brachial index, toe-brachial index, or 6-minute walk test distance in the entire trial population. Transcutaneous oxygen pressure (TcP02), an indicator of microvascular perfusion in the subcutaneous tissue of the ischemic limb, increased by 59% in the aspirate group but by only 7% in the placebo group.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: The 52-week rate of amputation-free survival events was 20.2% with the aspirate and 30.5% with placebo (P = .224).
Data source: A randomized phase III trial among 152 patients with critical limb ischemia who were not candidates for revascularization surgery (MOBILE trial).
Disclosures: Dr. Murphy disclosed that he had no relevant conflicts of interest. The trial was sponsored by Biomet Biologics, LLC.
Long-term remission maintenance in ANCA-associated vasculitis leans toward rituximab over azathioprine
WASHINGTON – Rituximab was superior to azathioprine as maintenance therapy for antineutrophil cytoplasmic antibody–associated vasculitis over long-term follow-up of the MAINRITSAN trial. At 60 months, rituximab significantly improved overall survival and relapse-free survival, compared with azathioprine.
At 60 months, overall survival (OS) rates were 100% for rituximab (Rituxan) versus 93% for azathioprine (P = .045), and relapse-free survival (RFS) rates were 57.9% versus 37.3%, respectively (P = .012).
These long-term maintenance results build on the primary results of MAINRITSAN that were previously published in 2014 (N Engl J Med. 2014;Nov 6;371[19]:1771-80). The primary results showed the superiority of rituximab maintenance therapy versus the then-gold standard azathioprine in maintaining antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis (AAV) remission at 28 months following induction therapy with a cyclophosphamide/glucocorticoid regimen.
“Following publication of the primary results, some uncertainties remained as to the duration of remission on rituximab. There is a need for therapy that can prevent relapse over the longer term,” lead author Benjamin Terrier, MD, of the National Referral Center for Rare Systemic Autoimmune Diseases at Cochin Hospital and Paris Descartes University in France, said in his presentation of the long-term follow-up data at the annual meeting of the American College of Rheumatology.
The follow-up results indicate that “over the long term, despite late relapses after the 28-month initial follow-up period, maintenance therapy with rituximab remained significantly superior to azathioprine to maintain remission at 60 months and was associated with better survival,” Dr. Terrier said.
The study included 115 newly diagnosed or relapsing patients with AAV (granulomatosis with polyangiitis, microscopic polyangiitis, or eosinophilic granulomatosis with polyangiitis). Of these, 80% were newly diagnosed. After achieving remission on induction therapy, patients were randomized to rituximab infusion 500 mg on day 1, day 15, and 5.5 months later, then every 6 months for 18 months or to azathioprine for 22 months.
The investigators collected data prospectively on major and minor relapses and adverse events, using a Q-TWIST (Quality Adjusted Time Without Symptoms and Toxicity) analysis to show the trade-off between toxicity and disease activity.
For all relapses, major and minor, rituximab maintained superiority over azathioprine at 60 months. There were 24 events in the rituximab arm: 11 minor relapses and 13 major relapses. There were 36 events in the azathioprine arm: 10 minor relapses, 25 major relapses, and 1 death. Major RFS survival rates were 71.9% versus 49.4%, respectively (P = .003).
“There was an absolute difference of 12 months favoring rituximab for RFS,” Dr. Terrier said.
Serious infections were numerically increased in the rituximab-treated group: 30 compared with 20 in the azathioprine group. Cardiovascular event rates were similar for both groups.
Q-TWIST analysis found significantly longer quality-adjusted time without progression or toxicity in the rituximab arm (P less than .001). The cumulative dose of steroids was comparable between treatment groups.
Six cancers were found in the azathioprine arm (including four skin cancers), compared with two in the rituximab arm.
In the rituximab group, PR3 ANCA positivity or ANCA persistence 12 months after starting rituximab maintenance therapy were associated with higher major relapse rates.
“Combining these two factors allows discerning low relapse rate. Patients negative for ANCA and for PR3 ANCA were at low risk,” Dr. Terrier told the audience. “ANCA monitoring seems to be relevant to guide treatment duration.”
Best rituximab regimen?
A separate study presented at a poster session compared the systemic regimen used in MAINRITSAN (as a control group) versus an experimental regimen of fixed 500-mg rituximab infusions on day 0 post randomization and then every 3 months until month 18, based on ANCA status/titer and/or circulating CD19 B-cell reappearance. The open-label, randomized study included 163 patients with granulomatosis with polyangiitis or microscopic polyangiitis in complete remission after induction therapy with glucocorticoids and cyclophosphamide or rituximab or methotrexate.
At 28 months, the relapse rate was 8% in the control arm and 14% in the experimental arm, a difference that was not statistically significant.
“We found no difference in the primary endpoint of relapse between the two regimens, but the experimental arm received fewer infusions. From this study, we cannot make a strong recommendation for the experimental regimen, but we think it is better, because there is less cumulative exposure to rituximab,” stated lead author Pierre Charles, MD, also of Cochin Hospital.
Both studies were funded by Hoffman-LaRoche. Dr. Terrier and Dr. Charles disclosed financial support from Hoffman-LaRoche.
Over the long term, patients initially randomized to rituximab maintenance therapy in the initial phase of the MAINRITSAN trial continued to be more likely to remain in remission than were those who had been randomized to azathioprine. The maintenance therapy data for rituximab look better than for azathioprine.
In clinical practice, individualizing the timing of repeat rituximab may be favorable for remission maintenance rather than having the same fixed dose for all comers, as was used in the MAINRITSAN trial.
By tailoring therapy, it may be possible to use less medication. If patients’ B cells remain depleted and ANCA is stable, flare is unlikely in the next 3 months, but if B cells are reconstituting, particularly in concert with a rising ANCA, I would generally administer a “remission maintenance” dose of rituximab, particularly in an individual who has had relapsing disease in the past. Perhaps patients could use less cumulative rituximab if these parameters are used to make treatment decisions. The poster presentation by Pierre Charles, MD, suggested that this approach indeed is feasible.
Robert F. Spiera, MD, is director of the Scleroderma, Vasculitis, & Myositis Center at the Hospital for Special Surgery, New York. He is also professor of clinical medicine at Cornell University, New York. He made these comments in an interview. Dr. Spiera has received research funding and consulting fees from Roche/Genentech, which markets rituximab.
Over the long term, patients initially randomized to rituximab maintenance therapy in the initial phase of the MAINRITSAN trial continued to be more likely to remain in remission than were those who had been randomized to azathioprine. The maintenance therapy data for rituximab look better than for azathioprine.
In clinical practice, individualizing the timing of repeat rituximab may be favorable for remission maintenance rather than having the same fixed dose for all comers, as was used in the MAINRITSAN trial.
By tailoring therapy, it may be possible to use less medication. If patients’ B cells remain depleted and ANCA is stable, flare is unlikely in the next 3 months, but if B cells are reconstituting, particularly in concert with a rising ANCA, I would generally administer a “remission maintenance” dose of rituximab, particularly in an individual who has had relapsing disease in the past. Perhaps patients could use less cumulative rituximab if these parameters are used to make treatment decisions. The poster presentation by Pierre Charles, MD, suggested that this approach indeed is feasible.
Robert F. Spiera, MD, is director of the Scleroderma, Vasculitis, & Myositis Center at the Hospital for Special Surgery, New York. He is also professor of clinical medicine at Cornell University, New York. He made these comments in an interview. Dr. Spiera has received research funding and consulting fees from Roche/Genentech, which markets rituximab.
Over the long term, patients initially randomized to rituximab maintenance therapy in the initial phase of the MAINRITSAN trial continued to be more likely to remain in remission than were those who had been randomized to azathioprine. The maintenance therapy data for rituximab look better than for azathioprine.
In clinical practice, individualizing the timing of repeat rituximab may be favorable for remission maintenance rather than having the same fixed dose for all comers, as was used in the MAINRITSAN trial.
By tailoring therapy, it may be possible to use less medication. If patients’ B cells remain depleted and ANCA is stable, flare is unlikely in the next 3 months, but if B cells are reconstituting, particularly in concert with a rising ANCA, I would generally administer a “remission maintenance” dose of rituximab, particularly in an individual who has had relapsing disease in the past. Perhaps patients could use less cumulative rituximab if these parameters are used to make treatment decisions. The poster presentation by Pierre Charles, MD, suggested that this approach indeed is feasible.
Robert F. Spiera, MD, is director of the Scleroderma, Vasculitis, & Myositis Center at the Hospital for Special Surgery, New York. He is also professor of clinical medicine at Cornell University, New York. He made these comments in an interview. Dr. Spiera has received research funding and consulting fees from Roche/Genentech, which markets rituximab.
WASHINGTON – Rituximab was superior to azathioprine as maintenance therapy for antineutrophil cytoplasmic antibody–associated vasculitis over long-term follow-up of the MAINRITSAN trial. At 60 months, rituximab significantly improved overall survival and relapse-free survival, compared with azathioprine.
At 60 months, overall survival (OS) rates were 100% for rituximab (Rituxan) versus 93% for azathioprine (P = .045), and relapse-free survival (RFS) rates were 57.9% versus 37.3%, respectively (P = .012).
These long-term maintenance results build on the primary results of MAINRITSAN that were previously published in 2014 (N Engl J Med. 2014;Nov 6;371[19]:1771-80). The primary results showed the superiority of rituximab maintenance therapy versus the then-gold standard azathioprine in maintaining antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis (AAV) remission at 28 months following induction therapy with a cyclophosphamide/glucocorticoid regimen.
“Following publication of the primary results, some uncertainties remained as to the duration of remission on rituximab. There is a need for therapy that can prevent relapse over the longer term,” lead author Benjamin Terrier, MD, of the National Referral Center for Rare Systemic Autoimmune Diseases at Cochin Hospital and Paris Descartes University in France, said in his presentation of the long-term follow-up data at the annual meeting of the American College of Rheumatology.
The follow-up results indicate that “over the long term, despite late relapses after the 28-month initial follow-up period, maintenance therapy with rituximab remained significantly superior to azathioprine to maintain remission at 60 months and was associated with better survival,” Dr. Terrier said.
The study included 115 newly diagnosed or relapsing patients with AAV (granulomatosis with polyangiitis, microscopic polyangiitis, or eosinophilic granulomatosis with polyangiitis). Of these, 80% were newly diagnosed. After achieving remission on induction therapy, patients were randomized to rituximab infusion 500 mg on day 1, day 15, and 5.5 months later, then every 6 months for 18 months or to azathioprine for 22 months.
The investigators collected data prospectively on major and minor relapses and adverse events, using a Q-TWIST (Quality Adjusted Time Without Symptoms and Toxicity) analysis to show the trade-off between toxicity and disease activity.
For all relapses, major and minor, rituximab maintained superiority over azathioprine at 60 months. There were 24 events in the rituximab arm: 11 minor relapses and 13 major relapses. There were 36 events in the azathioprine arm: 10 minor relapses, 25 major relapses, and 1 death. Major RFS survival rates were 71.9% versus 49.4%, respectively (P = .003).
“There was an absolute difference of 12 months favoring rituximab for RFS,” Dr. Terrier said.
Serious infections were numerically increased in the rituximab-treated group: 30 compared with 20 in the azathioprine group. Cardiovascular event rates were similar for both groups.
Q-TWIST analysis found significantly longer quality-adjusted time without progression or toxicity in the rituximab arm (P less than .001). The cumulative dose of steroids was comparable between treatment groups.
Six cancers were found in the azathioprine arm (including four skin cancers), compared with two in the rituximab arm.
In the rituximab group, PR3 ANCA positivity or ANCA persistence 12 months after starting rituximab maintenance therapy were associated with higher major relapse rates.
“Combining these two factors allows discerning low relapse rate. Patients negative for ANCA and for PR3 ANCA were at low risk,” Dr. Terrier told the audience. “ANCA monitoring seems to be relevant to guide treatment duration.”
Best rituximab regimen?
A separate study presented at a poster session compared the systemic regimen used in MAINRITSAN (as a control group) versus an experimental regimen of fixed 500-mg rituximab infusions on day 0 post randomization and then every 3 months until month 18, based on ANCA status/titer and/or circulating CD19 B-cell reappearance. The open-label, randomized study included 163 patients with granulomatosis with polyangiitis or microscopic polyangiitis in complete remission after induction therapy with glucocorticoids and cyclophosphamide or rituximab or methotrexate.
At 28 months, the relapse rate was 8% in the control arm and 14% in the experimental arm, a difference that was not statistically significant.
“We found no difference in the primary endpoint of relapse between the two regimens, but the experimental arm received fewer infusions. From this study, we cannot make a strong recommendation for the experimental regimen, but we think it is better, because there is less cumulative exposure to rituximab,” stated lead author Pierre Charles, MD, also of Cochin Hospital.
Both studies were funded by Hoffman-LaRoche. Dr. Terrier and Dr. Charles disclosed financial support from Hoffman-LaRoche.
WASHINGTON – Rituximab was superior to azathioprine as maintenance therapy for antineutrophil cytoplasmic antibody–associated vasculitis over long-term follow-up of the MAINRITSAN trial. At 60 months, rituximab significantly improved overall survival and relapse-free survival, compared with azathioprine.
At 60 months, overall survival (OS) rates were 100% for rituximab (Rituxan) versus 93% for azathioprine (P = .045), and relapse-free survival (RFS) rates were 57.9% versus 37.3%, respectively (P = .012).
These long-term maintenance results build on the primary results of MAINRITSAN that were previously published in 2014 (N Engl J Med. 2014;Nov 6;371[19]:1771-80). The primary results showed the superiority of rituximab maintenance therapy versus the then-gold standard azathioprine in maintaining antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis (AAV) remission at 28 months following induction therapy with a cyclophosphamide/glucocorticoid regimen.
“Following publication of the primary results, some uncertainties remained as to the duration of remission on rituximab. There is a need for therapy that can prevent relapse over the longer term,” lead author Benjamin Terrier, MD, of the National Referral Center for Rare Systemic Autoimmune Diseases at Cochin Hospital and Paris Descartes University in France, said in his presentation of the long-term follow-up data at the annual meeting of the American College of Rheumatology.
The follow-up results indicate that “over the long term, despite late relapses after the 28-month initial follow-up period, maintenance therapy with rituximab remained significantly superior to azathioprine to maintain remission at 60 months and was associated with better survival,” Dr. Terrier said.
The study included 115 newly diagnosed or relapsing patients with AAV (granulomatosis with polyangiitis, microscopic polyangiitis, or eosinophilic granulomatosis with polyangiitis). Of these, 80% were newly diagnosed. After achieving remission on induction therapy, patients were randomized to rituximab infusion 500 mg on day 1, day 15, and 5.5 months later, then every 6 months for 18 months or to azathioprine for 22 months.
The investigators collected data prospectively on major and minor relapses and adverse events, using a Q-TWIST (Quality Adjusted Time Without Symptoms and Toxicity) analysis to show the trade-off between toxicity and disease activity.
For all relapses, major and minor, rituximab maintained superiority over azathioprine at 60 months. There were 24 events in the rituximab arm: 11 minor relapses and 13 major relapses. There were 36 events in the azathioprine arm: 10 minor relapses, 25 major relapses, and 1 death. Major RFS survival rates were 71.9% versus 49.4%, respectively (P = .003).
“There was an absolute difference of 12 months favoring rituximab for RFS,” Dr. Terrier said.
Serious infections were numerically increased in the rituximab-treated group: 30 compared with 20 in the azathioprine group. Cardiovascular event rates were similar for both groups.
Q-TWIST analysis found significantly longer quality-adjusted time without progression or toxicity in the rituximab arm (P less than .001). The cumulative dose of steroids was comparable between treatment groups.
Six cancers were found in the azathioprine arm (including four skin cancers), compared with two in the rituximab arm.
In the rituximab group, PR3 ANCA positivity or ANCA persistence 12 months after starting rituximab maintenance therapy were associated with higher major relapse rates.
“Combining these two factors allows discerning low relapse rate. Patients negative for ANCA and for PR3 ANCA were at low risk,” Dr. Terrier told the audience. “ANCA monitoring seems to be relevant to guide treatment duration.”
Best rituximab regimen?
A separate study presented at a poster session compared the systemic regimen used in MAINRITSAN (as a control group) versus an experimental regimen of fixed 500-mg rituximab infusions on day 0 post randomization and then every 3 months until month 18, based on ANCA status/titer and/or circulating CD19 B-cell reappearance. The open-label, randomized study included 163 patients with granulomatosis with polyangiitis or microscopic polyangiitis in complete remission after induction therapy with glucocorticoids and cyclophosphamide or rituximab or methotrexate.
At 28 months, the relapse rate was 8% in the control arm and 14% in the experimental arm, a difference that was not statistically significant.
“We found no difference in the primary endpoint of relapse between the two regimens, but the experimental arm received fewer infusions. From this study, we cannot make a strong recommendation for the experimental regimen, but we think it is better, because there is less cumulative exposure to rituximab,” stated lead author Pierre Charles, MD, also of Cochin Hospital.
Both studies were funded by Hoffman-LaRoche. Dr. Terrier and Dr. Charles disclosed financial support from Hoffman-LaRoche.
AT THE ACR ANNUAL MEETING
Key clinical point:
Major finding: At 60 months, overall survival rates were 100% for rituximab versus 93% azathioprine (P = .045) and relapse-free survival rates were 57.9% versus 37.3%, respectively (P = .012).
Data source: 60-month follow-up of a randomized, controlled trial of 115 patients.
Disclosures: Both studies were funded by Hoffman-LaRoche. Dr. Terrier and Dr. Charles each disclosed financial support from Hoffman-LaRoche.
Endovascular construction of arteriovenous fistulas shows promise
LAS VEGAS – Patients who had an arteriovenous fistula (AVF) created endovascularly using a new magnetic catheter system required fewer interventions and had fewer health care costs than patients whose AVF was created surgically, according to a late-breaking trial presented by Charmaine E. Lok, MD, at the 2016 Vascular Interventional Advances meeting.
The study compared AVF postcreation interventions between patients undergoing surgical (sAVF) creation and those whose fistula was created using a new endovascular AVF (endoAVF) system.
Medicare Standard Analytical Files were used to determine patient demographic and clinical characteristics and to identify and determine rates of sAVF postcreation interventions in patients with sAVF created from 2011 to 2013, according to Dr. Lok, a professor of medicine at the University of Toronto and senior scientist at the Toronto General Research Institute.
The rates of postcreation interventions per patient-year were determined based on patients’ outpatient and physician claims. Demographics and clinical information for patients with endoAVF were obtained from the single-arm Novel Endovascular Access Trial (NEAT) performed in Canada, Australia, and New Zealand.
The researchers determined the rates of postcreation interventions per patient-year from the trial based on patients’ outpatient and physician claims during specified follow-up.
Propensity score matching based on clinical and demographic factors was successful for comparing 60 Medicare patients who had surgical AVFs to NEAT patients. The matched surgical cohort had a significantly higher number of interventions than the endovascular cohort (3.4 vs. 0.6 per patient-year, respectively; P less than .0001). The associated average annual costs were $11,240 less for the endovascular patients, compared with the surgical patients.
In a breakdown of procedures, the endovascular cohort had lower event rates for angioplasty (0.04 vs. 0.93),respectively; thrombectomy (0.04 vs. 0.20); embolization/ligation of vein (0.13 vs. 0.1); revision (0.04 vs. 0.17); new AVF or transposition (0.11 vs. 0.30); catheter placement (0.11 vs. 0.43); vascular access–related infection (0.02 vs. 1.23), and arteriovenous graft placement (0.02 vs. 0.07), according to Dr. Lok.
The NEAT study assessed the FLEX system, which percutaneously creates a fistula in chronic kidney disease patients who require hemodialysis vascular access.
The FLEX system uses two catheters delivered percutaneously to an artery and a vein in proximity to each other in the arm. The catheters use magnets for alignment and a radio frequency system as an energy source. The catheters are magnetically aligned and an RF pulse creates an arteriovenous fistula endovascularly between the artery and vein and the catheters are then removed.
The technology used is not commercially available in the United States and is pending Food and Drug Administration review, according to Dr. Lok
The study was sponsored by TVA Medical. Dr. Lok has received honoraria from Maquet and W.L. Gore, and is a consultant for TVA Medical and W. L. Gore, and has received research funding from Maquet, Proteon, and TVA Medical.
LAS VEGAS – Patients who had an arteriovenous fistula (AVF) created endovascularly using a new magnetic catheter system required fewer interventions and had fewer health care costs than patients whose AVF was created surgically, according to a late-breaking trial presented by Charmaine E. Lok, MD, at the 2016 Vascular Interventional Advances meeting.
The study compared AVF postcreation interventions between patients undergoing surgical (sAVF) creation and those whose fistula was created using a new endovascular AVF (endoAVF) system.
Medicare Standard Analytical Files were used to determine patient demographic and clinical characteristics and to identify and determine rates of sAVF postcreation interventions in patients with sAVF created from 2011 to 2013, according to Dr. Lok, a professor of medicine at the University of Toronto and senior scientist at the Toronto General Research Institute.
The rates of postcreation interventions per patient-year were determined based on patients’ outpatient and physician claims. Demographics and clinical information for patients with endoAVF were obtained from the single-arm Novel Endovascular Access Trial (NEAT) performed in Canada, Australia, and New Zealand.
The researchers determined the rates of postcreation interventions per patient-year from the trial based on patients’ outpatient and physician claims during specified follow-up.
Propensity score matching based on clinical and demographic factors was successful for comparing 60 Medicare patients who had surgical AVFs to NEAT patients. The matched surgical cohort had a significantly higher number of interventions than the endovascular cohort (3.4 vs. 0.6 per patient-year, respectively; P less than .0001). The associated average annual costs were $11,240 less for the endovascular patients, compared with the surgical patients.
In a breakdown of procedures, the endovascular cohort had lower event rates for angioplasty (0.04 vs. 0.93),respectively; thrombectomy (0.04 vs. 0.20); embolization/ligation of vein (0.13 vs. 0.1); revision (0.04 vs. 0.17); new AVF or transposition (0.11 vs. 0.30); catheter placement (0.11 vs. 0.43); vascular access–related infection (0.02 vs. 1.23), and arteriovenous graft placement (0.02 vs. 0.07), according to Dr. Lok.
The NEAT study assessed the FLEX system, which percutaneously creates a fistula in chronic kidney disease patients who require hemodialysis vascular access.
The FLEX system uses two catheters delivered percutaneously to an artery and a vein in proximity to each other in the arm. The catheters use magnets for alignment and a radio frequency system as an energy source. The catheters are magnetically aligned and an RF pulse creates an arteriovenous fistula endovascularly between the artery and vein and the catheters are then removed.
The technology used is not commercially available in the United States and is pending Food and Drug Administration review, according to Dr. Lok
The study was sponsored by TVA Medical. Dr. Lok has received honoraria from Maquet and W.L. Gore, and is a consultant for TVA Medical and W. L. Gore, and has received research funding from Maquet, Proteon, and TVA Medical.
LAS VEGAS – Patients who had an arteriovenous fistula (AVF) created endovascularly using a new magnetic catheter system required fewer interventions and had fewer health care costs than patients whose AVF was created surgically, according to a late-breaking trial presented by Charmaine E. Lok, MD, at the 2016 Vascular Interventional Advances meeting.
The study compared AVF postcreation interventions between patients undergoing surgical (sAVF) creation and those whose fistula was created using a new endovascular AVF (endoAVF) system.
Medicare Standard Analytical Files were used to determine patient demographic and clinical characteristics and to identify and determine rates of sAVF postcreation interventions in patients with sAVF created from 2011 to 2013, according to Dr. Lok, a professor of medicine at the University of Toronto and senior scientist at the Toronto General Research Institute.
The rates of postcreation interventions per patient-year were determined based on patients’ outpatient and physician claims. Demographics and clinical information for patients with endoAVF were obtained from the single-arm Novel Endovascular Access Trial (NEAT) performed in Canada, Australia, and New Zealand.
The researchers determined the rates of postcreation interventions per patient-year from the trial based on patients’ outpatient and physician claims during specified follow-up.
Propensity score matching based on clinical and demographic factors was successful for comparing 60 Medicare patients who had surgical AVFs to NEAT patients. The matched surgical cohort had a significantly higher number of interventions than the endovascular cohort (3.4 vs. 0.6 per patient-year, respectively; P less than .0001). The associated average annual costs were $11,240 less for the endovascular patients, compared with the surgical patients.
In a breakdown of procedures, the endovascular cohort had lower event rates for angioplasty (0.04 vs. 0.93),respectively; thrombectomy (0.04 vs. 0.20); embolization/ligation of vein (0.13 vs. 0.1); revision (0.04 vs. 0.17); new AVF or transposition (0.11 vs. 0.30); catheter placement (0.11 vs. 0.43); vascular access–related infection (0.02 vs. 1.23), and arteriovenous graft placement (0.02 vs. 0.07), according to Dr. Lok.
The NEAT study assessed the FLEX system, which percutaneously creates a fistula in chronic kidney disease patients who require hemodialysis vascular access.
The FLEX system uses two catheters delivered percutaneously to an artery and a vein in proximity to each other in the arm. The catheters use magnets for alignment and a radio frequency system as an energy source. The catheters are magnetically aligned and an RF pulse creates an arteriovenous fistula endovascularly between the artery and vein and the catheters are then removed.
The technology used is not commercially available in the United States and is pending Food and Drug Administration review, according to Dr. Lok
The study was sponsored by TVA Medical. Dr. Lok has received honoraria from Maquet and W.L. Gore, and is a consultant for TVA Medical and W. L. Gore, and has received research funding from Maquet, Proteon, and TVA Medical.
Key clinical point: than patients whose AVF was created surgically
Major finding: A matched surgical cohort of patients with surgically constructed AVFs had a significantly higher number of interventions than an endovascular AVF cohort (3.4 vs. 0.6 per patient-year).
Data source: Researchers performed a propensity-matching analysis of Medicare patients to those patient who received an endovascularly created AVF in the NEAT trial.
Disclosures: The study was sponsored by TVA Medical. Dr. Lok has received honoraria from Maquet and W.L. Gore, and is a consultant for TVA Medical and W. L. Gore, and has received research funding from Maquet, Proteon, and TVA Medical.
EADV: Venous leg ulcers herald increased cancer risk
COPENHAGEN – Patients with venous leg ulcers have an increased risk of occult cancer – especially hematologic and immune-related malignancies, according to a Danish nationwide cohort study.
The risk of newly detected cancer was greatest during the first 89 days after diagnosis of a venous leg ulcer. Indeed, during that initial period the risk of being diagnosed with a hematologic cancer was 3.48-fold greater than expected based upon Danish Cancer Registry data, Dr. Sigrun Alba Johannesdottir Schmidt reported at the annual congress of the European Academy of Dermatology and Venereology.
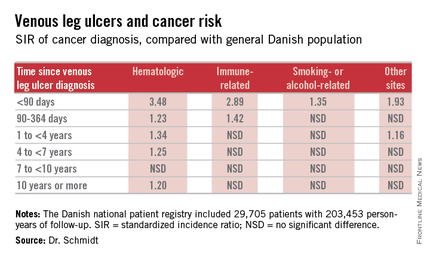
It’s reasonable to assume that the vast majority of cancers identified within a year following diagnosis of a venous leg ulcer were probably present at the time when the ulcer was first diagnosed, meaning venous leg ulcers can serve as a red flag for occult cancer.
However, it’s also worth noting that the increased risk of hematologic malignancies persisted, albeit to a lesser degree, for up to 10 years. This suggests that venous ulceration could also have a carcinogenic effect. It’s biologically plausible that a venous leg ulcer could promote development of cancer through a variety of mechanisms, including inflammation, alteration of plasma viscosity and the adhesive properties of blood cells, and disruptions of venous pressure that encourage direct neoplastic invasion, according to Dr. Schmidt of Aarhus (Denmark) University.
She presented a Danish national patient registry study, which included all 29,705 patients with a first-time inpatient, outpatient, or emergency department diagnosis of a venous leg ulcer during 1982-2010. Fifty-five percent of them were age 70 years or older at the time of ulcer diagnosis. And 42% had moderate to very severe comorbid conditions based upon their Charlson Comorbidity Index score. During a median of 5.1 years of follow-up, or a total of 203,453 person-years, their overall risk of a first-time cancer diagnosis was significantly increased by 11%,compared with the general Danish population.
The malignancy risk was strongly time-dependent . However, the absolute risk of cancer was relatively low: less than 1% within the first 90 days after diagnosis of a venous leg ulcer. The number of patients who would need to be examined for a possible malignancy at the time of diagnosis of a venous leg ulcer in order to diagnosis one excess cancer was 146.
Dr. Schmidt indicated she would defer to experts in cost-benefit analysis as to whether an extensive work-up for occult malignancy is worthwhile in patients with a newly diagnosed venous leg ulcer, given the low absolute risk of cancer.
She reported having no financial conflicts of interest regarding her study, which was conducted with Danish institutional research funds.
COPENHAGEN – Patients with venous leg ulcers have an increased risk of occult cancer – especially hematologic and immune-related malignancies, according to a Danish nationwide cohort study.
The risk of newly detected cancer was greatest during the first 89 days after diagnosis of a venous leg ulcer. Indeed, during that initial period the risk of being diagnosed with a hematologic cancer was 3.48-fold greater than expected based upon Danish Cancer Registry data, Dr. Sigrun Alba Johannesdottir Schmidt reported at the annual congress of the European Academy of Dermatology and Venereology.

It’s reasonable to assume that the vast majority of cancers identified within a year following diagnosis of a venous leg ulcer were probably present at the time when the ulcer was first diagnosed, meaning venous leg ulcers can serve as a red flag for occult cancer.
However, it’s also worth noting that the increased risk of hematologic malignancies persisted, albeit to a lesser degree, for up to 10 years. This suggests that venous ulceration could also have a carcinogenic effect. It’s biologically plausible that a venous leg ulcer could promote development of cancer through a variety of mechanisms, including inflammation, alteration of plasma viscosity and the adhesive properties of blood cells, and disruptions of venous pressure that encourage direct neoplastic invasion, according to Dr. Schmidt of Aarhus (Denmark) University.
She presented a Danish national patient registry study, which included all 29,705 patients with a first-time inpatient, outpatient, or emergency department diagnosis of a venous leg ulcer during 1982-2010. Fifty-five percent of them were age 70 years or older at the time of ulcer diagnosis. And 42% had moderate to very severe comorbid conditions based upon their Charlson Comorbidity Index score. During a median of 5.1 years of follow-up, or a total of 203,453 person-years, their overall risk of a first-time cancer diagnosis was significantly increased by 11%,compared with the general Danish population.
The malignancy risk was strongly time-dependent . However, the absolute risk of cancer was relatively low: less than 1% within the first 90 days after diagnosis of a venous leg ulcer. The number of patients who would need to be examined for a possible malignancy at the time of diagnosis of a venous leg ulcer in order to diagnosis one excess cancer was 146.
Dr. Schmidt indicated she would defer to experts in cost-benefit analysis as to whether an extensive work-up for occult malignancy is worthwhile in patients with a newly diagnosed venous leg ulcer, given the low absolute risk of cancer.
She reported having no financial conflicts of interest regarding her study, which was conducted with Danish institutional research funds.
COPENHAGEN – Patients with venous leg ulcers have an increased risk of occult cancer – especially hematologic and immune-related malignancies, according to a Danish nationwide cohort study.
The risk of newly detected cancer was greatest during the first 89 days after diagnosis of a venous leg ulcer. Indeed, during that initial period the risk of being diagnosed with a hematologic cancer was 3.48-fold greater than expected based upon Danish Cancer Registry data, Dr. Sigrun Alba Johannesdottir Schmidt reported at the annual congress of the European Academy of Dermatology and Venereology.

It’s reasonable to assume that the vast majority of cancers identified within a year following diagnosis of a venous leg ulcer were probably present at the time when the ulcer was first diagnosed, meaning venous leg ulcers can serve as a red flag for occult cancer.
However, it’s also worth noting that the increased risk of hematologic malignancies persisted, albeit to a lesser degree, for up to 10 years. This suggests that venous ulceration could also have a carcinogenic effect. It’s biologically plausible that a venous leg ulcer could promote development of cancer through a variety of mechanisms, including inflammation, alteration of plasma viscosity and the adhesive properties of blood cells, and disruptions of venous pressure that encourage direct neoplastic invasion, according to Dr. Schmidt of Aarhus (Denmark) University.
She presented a Danish national patient registry study, which included all 29,705 patients with a first-time inpatient, outpatient, or emergency department diagnosis of a venous leg ulcer during 1982-2010. Fifty-five percent of them were age 70 years or older at the time of ulcer diagnosis. And 42% had moderate to very severe comorbid conditions based upon their Charlson Comorbidity Index score. During a median of 5.1 years of follow-up, or a total of 203,453 person-years, their overall risk of a first-time cancer diagnosis was significantly increased by 11%,compared with the general Danish population.
The malignancy risk was strongly time-dependent . However, the absolute risk of cancer was relatively low: less than 1% within the first 90 days after diagnosis of a venous leg ulcer. The number of patients who would need to be examined for a possible malignancy at the time of diagnosis of a venous leg ulcer in order to diagnosis one excess cancer was 146.
Dr. Schmidt indicated she would defer to experts in cost-benefit analysis as to whether an extensive work-up for occult malignancy is worthwhile in patients with a newly diagnosed venous leg ulcer, given the low absolute risk of cancer.
She reported having no financial conflicts of interest regarding her study, which was conducted with Danish institutional research funds.
AT THE EADV CONGRESS
Key clinical point: Venous leg ulceration appears to be a red flag for occult malignancy.
Major finding: During the first 89 days following diagnosis of a venous leg ulcer, affected patients are at a 3.48-fold increased risk of being diagnosed with a hematologic malignancy and a 2.89-fold greater risk of immune-related cancer compared with the general population.
Data source: This Danish nationwide cohort study compared standardized incidence ratios for various types of cancer during more than 200,000 person-years of follow-up in 29,705 patients with a venous leg ulcer vs. the general population.
Disclosures: The presenter reported having no financial conflicts of interest regarding her study, which was supported by Danish institutional funds.
EADV: Best treatments for great saphenous vein reflux
COPENHAGEN – Superior 5-year outcomes for great saphenous vein reflux were achieved with conventional surgery and endovenous laser ablation as compared with ultrasound-guided foam sclerotherapy in a randomized trial, Dr. Simone van der Velden reported at the annual congress of the European Academy of Dermatology and Venereology.
The multicenter study included 224 randomized legs belonging to symptomatic patients with a target great saphenous vein diameter of at least 5 mm. If deemed necessary, patients could undergo one re-treatment at 3 or 12 months after their initial procedure. At 5 years of follow-up, 86% of the treated legs were available for long-term evaluation, noted Dr. van der Velden of Erasmus University Medical Center in Rotterdam, the Netherlands.
The primary endpoint was obliteration or absence of the treated great saphenous vein segment. This was achieved with conventional surgery in 85% of treated cases, in 77% of legs treated with endovenous laser ablation (EVLA), and in 23% with ultrasound-guided foam sclerotherapy (UGFS).
Absence of above-the-knee greater saphenous vein reflux – a secondary endpoint – was achieved in 85% of the conventional surgery group and in 82% of the EVLA group, both of which were significantly better results than the 41% response with UGFS.
Another secondary endpoint was grade II neovascularization. Here again, both conventional surgery and EVLA outperformed UGFS, with rates of 17%, 13%, and 4%, respectively. In contrast, there was no significant difference between the three treatment groups in terms of the presence of refluxing tributaries above or below knee level, she continued.
Scores on the disease-specific Chronic Venous Insufficiency quality of life Questionnaire (CIVIQ) deteriorated over time in the UGFS group, improved in the EVLA-treated patients, and remained stable in the conventional surgery group.
Conventional surgery was performed under general anesthesia and entailed high ligation of the saphenofemoral junction and phlebectomy of tributaries. In contrast, EVLA was done under local tumescent anesthesia using a 940-nm laser. The laser fiber was introduced at knee level, positioned 1-2 cm below the saphenofemoral junction, and delivered an energy of roughly 60 Joules/cm2.
For UGFS, operators utilized a foam comprising 1 mL of sodium tetradecyl sulfate per 3 mL of air. A maximum of 10 mL of foam could be injected per treatment session, depending upon the diameter of the great saphenous vein and length of the refluxing trunk. Phlebectomies in this group were performed only in the event of patient complaints.
Of note, patients in the minimally invasive UGFS group required re-treatment three times more often than did those in the other two study arms.
Dr. van der Velden said she has heard from some UGFS partisans that she and her coinvestigators may have undertreated patients in that study arm because they didn’t routinely perform phlebectomies of the tributaries, and the average amount of foam they injected, about 4.5 mL, was on the low side.
The study was sponsored by Erasmus University. Dr. van der Velden reported having no financial conflicts of interest.
COPENHAGEN – Superior 5-year outcomes for great saphenous vein reflux were achieved with conventional surgery and endovenous laser ablation as compared with ultrasound-guided foam sclerotherapy in a randomized trial, Dr. Simone van der Velden reported at the annual congress of the European Academy of Dermatology and Venereology.
The multicenter study included 224 randomized legs belonging to symptomatic patients with a target great saphenous vein diameter of at least 5 mm. If deemed necessary, patients could undergo one re-treatment at 3 or 12 months after their initial procedure. At 5 years of follow-up, 86% of the treated legs were available for long-term evaluation, noted Dr. van der Velden of Erasmus University Medical Center in Rotterdam, the Netherlands.
The primary endpoint was obliteration or absence of the treated great saphenous vein segment. This was achieved with conventional surgery in 85% of treated cases, in 77% of legs treated with endovenous laser ablation (EVLA), and in 23% with ultrasound-guided foam sclerotherapy (UGFS).
Absence of above-the-knee greater saphenous vein reflux – a secondary endpoint – was achieved in 85% of the conventional surgery group and in 82% of the EVLA group, both of which were significantly better results than the 41% response with UGFS.
Another secondary endpoint was grade II neovascularization. Here again, both conventional surgery and EVLA outperformed UGFS, with rates of 17%, 13%, and 4%, respectively. In contrast, there was no significant difference between the three treatment groups in terms of the presence of refluxing tributaries above or below knee level, she continued.
Scores on the disease-specific Chronic Venous Insufficiency quality of life Questionnaire (CIVIQ) deteriorated over time in the UGFS group, improved in the EVLA-treated patients, and remained stable in the conventional surgery group.
Conventional surgery was performed under general anesthesia and entailed high ligation of the saphenofemoral junction and phlebectomy of tributaries. In contrast, EVLA was done under local tumescent anesthesia using a 940-nm laser. The laser fiber was introduced at knee level, positioned 1-2 cm below the saphenofemoral junction, and delivered an energy of roughly 60 Joules/cm2.
For UGFS, operators utilized a foam comprising 1 mL of sodium tetradecyl sulfate per 3 mL of air. A maximum of 10 mL of foam could be injected per treatment session, depending upon the diameter of the great saphenous vein and length of the refluxing trunk. Phlebectomies in this group were performed only in the event of patient complaints.
Of note, patients in the minimally invasive UGFS group required re-treatment three times more often than did those in the other two study arms.
Dr. van der Velden said she has heard from some UGFS partisans that she and her coinvestigators may have undertreated patients in that study arm because they didn’t routinely perform phlebectomies of the tributaries, and the average amount of foam they injected, about 4.5 mL, was on the low side.
The study was sponsored by Erasmus University. Dr. van der Velden reported having no financial conflicts of interest.
COPENHAGEN – Superior 5-year outcomes for great saphenous vein reflux were achieved with conventional surgery and endovenous laser ablation as compared with ultrasound-guided foam sclerotherapy in a randomized trial, Dr. Simone van der Velden reported at the annual congress of the European Academy of Dermatology and Venereology.
The multicenter study included 224 randomized legs belonging to symptomatic patients with a target great saphenous vein diameter of at least 5 mm. If deemed necessary, patients could undergo one re-treatment at 3 or 12 months after their initial procedure. At 5 years of follow-up, 86% of the treated legs were available for long-term evaluation, noted Dr. van der Velden of Erasmus University Medical Center in Rotterdam, the Netherlands.
The primary endpoint was obliteration or absence of the treated great saphenous vein segment. This was achieved with conventional surgery in 85% of treated cases, in 77% of legs treated with endovenous laser ablation (EVLA), and in 23% with ultrasound-guided foam sclerotherapy (UGFS).
Absence of above-the-knee greater saphenous vein reflux – a secondary endpoint – was achieved in 85% of the conventional surgery group and in 82% of the EVLA group, both of which were significantly better results than the 41% response with UGFS.
Another secondary endpoint was grade II neovascularization. Here again, both conventional surgery and EVLA outperformed UGFS, with rates of 17%, 13%, and 4%, respectively. In contrast, there was no significant difference between the three treatment groups in terms of the presence of refluxing tributaries above or below knee level, she continued.
Scores on the disease-specific Chronic Venous Insufficiency quality of life Questionnaire (CIVIQ) deteriorated over time in the UGFS group, improved in the EVLA-treated patients, and remained stable in the conventional surgery group.
Conventional surgery was performed under general anesthesia and entailed high ligation of the saphenofemoral junction and phlebectomy of tributaries. In contrast, EVLA was done under local tumescent anesthesia using a 940-nm laser. The laser fiber was introduced at knee level, positioned 1-2 cm below the saphenofemoral junction, and delivered an energy of roughly 60 Joules/cm2.
For UGFS, operators utilized a foam comprising 1 mL of sodium tetradecyl sulfate per 3 mL of air. A maximum of 10 mL of foam could be injected per treatment session, depending upon the diameter of the great saphenous vein and length of the refluxing trunk. Phlebectomies in this group were performed only in the event of patient complaints.
Of note, patients in the minimally invasive UGFS group required re-treatment three times more often than did those in the other two study arms.
Dr. van der Velden said she has heard from some UGFS partisans that she and her coinvestigators may have undertreated patients in that study arm because they didn’t routinely perform phlebectomies of the tributaries, and the average amount of foam they injected, about 4.5 mL, was on the low side.
The study was sponsored by Erasmus University. Dr. van der Velden reported having no financial conflicts of interest.
AT THE EADV CONGRESS
Key clinical point: Long-term outcomes for treatment of great saphenous vein reflux were significantly better with conventional surgery or endovenous laser ablation than with ultrasound-guided foam sclerotherapy.
Major finding: Obliteration or absence of the treated great saphenous vein segment was achieved with conventional surgery in 85% of treated cases, with endovenous laser ablation (EVLA) in 77% of legs treated, and with ultrasound-guided foam sclerotherapy (UGFS) in 23%.
Data source: This multicenter clinical trial with 5-year follow-up included 224 legs randomized to one of three popular treatments for great saphenous varicose veins.
Disclosures: The study was sponsored by Erasmus University. The presenter reported having no financial conflicts of interest.