User login
Preventing postpartum depression: Start with women at greatest risk
The last decade has brought appropriate attention to the high prevalence of postpartum mood and anxiety disorders, with postpartum depression (PPD) constituting the most common complication in modern obstetrics.
There have been very substantial efforts in more than 40 states in the United States to enhance screening for PPD and to increase support groups for women with postpartum depressive or anxiety symptoms. However, less focus has been paid to the outcomes of these screening initiatives.
A question that comes to mind is whether patients who are screened actually get referred for treatment, and if they do receive treatment, whether they recover and become well. One study referenced previously in this column noted that even in settings where women are screened for PPD, the vast majority of women are not referred, and of those who are referred, even fewer of those are treated or become well.1
It is noteworthy, then, that the U.S. Preventive Services Task Force has recommended screening for perinatal depression (just before and after birth) and issued draft recommendations regarding prevention of perinatal depression where it is suggested that patients at risk for perinatal depression be referred for appropriate “counseling interventions” – specifically, either cognitive-behavioral therapy (CBT) or interpersonal psychotherapy (IPT).2
The recommendation is a striking one because of the volume of patients who would be included. For example, the USPSTF recommends patients with histories of depression, depression during pregnancy, a history of child abuse, or even a family history of depression should receive preventive interventions with CBT or IPT. The recommendation is puzzling because of the data on risk for perinatal depression in those populations and the lack of available resources for patients who would be deemed “at risk.” Women with histories of depression are at a threefold increased risk for PPD (25%-30%). Depression during pregnancy is the strongest predictor of PPD and risk for PPD among these patients is as high as 75%.
So, there are a vast number of women who may be “at risk” for perinatal depression. But even with some data suggesting that IPT and CBT may be able to prevent perinatal depression, the suggestion that resources be made available to patients who are at risk is naive, because counseling interventions such as IPT or CBT, or even simply referrals to psychiatrists are not available even to patients who screen in for perinatal depression in real time during pregnancy and the postpartum period. I have previously written that the follow-up of women post partum who suffer from PPD is still far from meeting the needs who suffer from the disorder, and early detection and referrals to appropriate clinicians who are facile with both pharmacologic and nonpharmacologic interventions seem the most effective way to manage these patients and to see that they receive treatment.
The question then becomes: If the numbers or scale of the prevention initiative suggested in this draft recommendation from the USPSTF is an overreach, is there a group of patients for whom a preventive intervention could be pursued? The patients at highest risk for PPD include those with a history of PPD (50%), bipolar disorder (50%-60%), or postpartum psychosis (80%). And while there is not substantial literature for specifically using IPT, CBT, or other counseling interventions to mitigate risk for recurrence in women with histories of PPD, bipolar disorder, or postpartum psychosis, there are ways of identifying this population at risk and following them closely to mitigate the risk for recurrence.
To make this recommendation feasible, an infrastructure needs to be in place in both low resource settings and in all communities so that these patients can be referred and effectively treated. If we move to prevention, we ought to start with the populations that we already know are at greatest risk and that we can inquire about, and there are very easy-to-use screens that screen for bipolar disorder or that screen for past history of depression with which these women can be identified.
In committee opinion 757, the American College of Obstetricians and Gynecologists recommends women be screened at least once during the perinatal period for depression and anxiety symptoms and highlighted several validated tools, such as the Edinburgh Postnatal Depression Scale.3 We also need a better system of early detection and early intervention so that women at less-considerable risk for perinatal depression would have the opportunity for early identification, treatment, and referral, which we do not have at the current time.
An update of the ACOG committee opinion also states, “It is recommended that all obstetrician-gynecologists and other obstetric care providers complete a full assessment of mood and emotional well-being (including screening for PPD and anxiety with a validated instrument) during the comprehensive postpartum visit for each patient.” This is recommended in addition to any screening for depression and anxiety during the pregnancy.
It is exciting that after decades of failing to attend to such a common complication of modern obstetrics, particularly now that we understand the adverse effects of PPD as it affects child development, family functioning, and risk for later childhood psychopathology. But in addition to recognizing the problem, we must come up with methods to carefully identify a navigable route for the women suffering from PPD to get their needs met. The route includes publicly identifying the illness, understanding which treatments are most effective and can be scaled for delivery to large numbers of women, and then, most critically, configuring social systems to absorb, effectively manage, and monitor the women we identify as needing treatment.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email him at [email protected].
References
1. J Clin Psychiatry. 2016 Sep;77[9]:1189-200.
2. Draft Recommendation Statement: Perinatal Depression: Preventive Interventions. U.S. Preventive Services Task Force. Aug 2018.
The last decade has brought appropriate attention to the high prevalence of postpartum mood and anxiety disorders, with postpartum depression (PPD) constituting the most common complication in modern obstetrics.
There have been very substantial efforts in more than 40 states in the United States to enhance screening for PPD and to increase support groups for women with postpartum depressive or anxiety symptoms. However, less focus has been paid to the outcomes of these screening initiatives.
A question that comes to mind is whether patients who are screened actually get referred for treatment, and if they do receive treatment, whether they recover and become well. One study referenced previously in this column noted that even in settings where women are screened for PPD, the vast majority of women are not referred, and of those who are referred, even fewer of those are treated or become well.1
It is noteworthy, then, that the U.S. Preventive Services Task Force has recommended screening for perinatal depression (just before and after birth) and issued draft recommendations regarding prevention of perinatal depression where it is suggested that patients at risk for perinatal depression be referred for appropriate “counseling interventions” – specifically, either cognitive-behavioral therapy (CBT) or interpersonal psychotherapy (IPT).2
The recommendation is a striking one because of the volume of patients who would be included. For example, the USPSTF recommends patients with histories of depression, depression during pregnancy, a history of child abuse, or even a family history of depression should receive preventive interventions with CBT or IPT. The recommendation is puzzling because of the data on risk for perinatal depression in those populations and the lack of available resources for patients who would be deemed “at risk.” Women with histories of depression are at a threefold increased risk for PPD (25%-30%). Depression during pregnancy is the strongest predictor of PPD and risk for PPD among these patients is as high as 75%.
So, there are a vast number of women who may be “at risk” for perinatal depression. But even with some data suggesting that IPT and CBT may be able to prevent perinatal depression, the suggestion that resources be made available to patients who are at risk is naive, because counseling interventions such as IPT or CBT, or even simply referrals to psychiatrists are not available even to patients who screen in for perinatal depression in real time during pregnancy and the postpartum period. I have previously written that the follow-up of women post partum who suffer from PPD is still far from meeting the needs who suffer from the disorder, and early detection and referrals to appropriate clinicians who are facile with both pharmacologic and nonpharmacologic interventions seem the most effective way to manage these patients and to see that they receive treatment.
The question then becomes: If the numbers or scale of the prevention initiative suggested in this draft recommendation from the USPSTF is an overreach, is there a group of patients for whom a preventive intervention could be pursued? The patients at highest risk for PPD include those with a history of PPD (50%), bipolar disorder (50%-60%), or postpartum psychosis (80%). And while there is not substantial literature for specifically using IPT, CBT, or other counseling interventions to mitigate risk for recurrence in women with histories of PPD, bipolar disorder, or postpartum psychosis, there are ways of identifying this population at risk and following them closely to mitigate the risk for recurrence.
To make this recommendation feasible, an infrastructure needs to be in place in both low resource settings and in all communities so that these patients can be referred and effectively treated. If we move to prevention, we ought to start with the populations that we already know are at greatest risk and that we can inquire about, and there are very easy-to-use screens that screen for bipolar disorder or that screen for past history of depression with which these women can be identified.
In committee opinion 757, the American College of Obstetricians and Gynecologists recommends women be screened at least once during the perinatal period for depression and anxiety symptoms and highlighted several validated tools, such as the Edinburgh Postnatal Depression Scale.3 We also need a better system of early detection and early intervention so that women at less-considerable risk for perinatal depression would have the opportunity for early identification, treatment, and referral, which we do not have at the current time.
An update of the ACOG committee opinion also states, “It is recommended that all obstetrician-gynecologists and other obstetric care providers complete a full assessment of mood and emotional well-being (including screening for PPD and anxiety with a validated instrument) during the comprehensive postpartum visit for each patient.” This is recommended in addition to any screening for depression and anxiety during the pregnancy.
It is exciting that after decades of failing to attend to such a common complication of modern obstetrics, particularly now that we understand the adverse effects of PPD as it affects child development, family functioning, and risk for later childhood psychopathology. But in addition to recognizing the problem, we must come up with methods to carefully identify a navigable route for the women suffering from PPD to get their needs met. The route includes publicly identifying the illness, understanding which treatments are most effective and can be scaled for delivery to large numbers of women, and then, most critically, configuring social systems to absorb, effectively manage, and monitor the women we identify as needing treatment.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email him at [email protected].
References
1. J Clin Psychiatry. 2016 Sep;77[9]:1189-200.
2. Draft Recommendation Statement: Perinatal Depression: Preventive Interventions. U.S. Preventive Services Task Force. Aug 2018.
The last decade has brought appropriate attention to the high prevalence of postpartum mood and anxiety disorders, with postpartum depression (PPD) constituting the most common complication in modern obstetrics.
There have been very substantial efforts in more than 40 states in the United States to enhance screening for PPD and to increase support groups for women with postpartum depressive or anxiety symptoms. However, less focus has been paid to the outcomes of these screening initiatives.
A question that comes to mind is whether patients who are screened actually get referred for treatment, and if they do receive treatment, whether they recover and become well. One study referenced previously in this column noted that even in settings where women are screened for PPD, the vast majority of women are not referred, and of those who are referred, even fewer of those are treated or become well.1
It is noteworthy, then, that the U.S. Preventive Services Task Force has recommended screening for perinatal depression (just before and after birth) and issued draft recommendations regarding prevention of perinatal depression where it is suggested that patients at risk for perinatal depression be referred for appropriate “counseling interventions” – specifically, either cognitive-behavioral therapy (CBT) or interpersonal psychotherapy (IPT).2
The recommendation is a striking one because of the volume of patients who would be included. For example, the USPSTF recommends patients with histories of depression, depression during pregnancy, a history of child abuse, or even a family history of depression should receive preventive interventions with CBT or IPT. The recommendation is puzzling because of the data on risk for perinatal depression in those populations and the lack of available resources for patients who would be deemed “at risk.” Women with histories of depression are at a threefold increased risk for PPD (25%-30%). Depression during pregnancy is the strongest predictor of PPD and risk for PPD among these patients is as high as 75%.
So, there are a vast number of women who may be “at risk” for perinatal depression. But even with some data suggesting that IPT and CBT may be able to prevent perinatal depression, the suggestion that resources be made available to patients who are at risk is naive, because counseling interventions such as IPT or CBT, or even simply referrals to psychiatrists are not available even to patients who screen in for perinatal depression in real time during pregnancy and the postpartum period. I have previously written that the follow-up of women post partum who suffer from PPD is still far from meeting the needs who suffer from the disorder, and early detection and referrals to appropriate clinicians who are facile with both pharmacologic and nonpharmacologic interventions seem the most effective way to manage these patients and to see that they receive treatment.
The question then becomes: If the numbers or scale of the prevention initiative suggested in this draft recommendation from the USPSTF is an overreach, is there a group of patients for whom a preventive intervention could be pursued? The patients at highest risk for PPD include those with a history of PPD (50%), bipolar disorder (50%-60%), or postpartum psychosis (80%). And while there is not substantial literature for specifically using IPT, CBT, or other counseling interventions to mitigate risk for recurrence in women with histories of PPD, bipolar disorder, or postpartum psychosis, there are ways of identifying this population at risk and following them closely to mitigate the risk for recurrence.
To make this recommendation feasible, an infrastructure needs to be in place in both low resource settings and in all communities so that these patients can be referred and effectively treated. If we move to prevention, we ought to start with the populations that we already know are at greatest risk and that we can inquire about, and there are very easy-to-use screens that screen for bipolar disorder or that screen for past history of depression with which these women can be identified.
In committee opinion 757, the American College of Obstetricians and Gynecologists recommends women be screened at least once during the perinatal period for depression and anxiety symptoms and highlighted several validated tools, such as the Edinburgh Postnatal Depression Scale.3 We also need a better system of early detection and early intervention so that women at less-considerable risk for perinatal depression would have the opportunity for early identification, treatment, and referral, which we do not have at the current time.
An update of the ACOG committee opinion also states, “It is recommended that all obstetrician-gynecologists and other obstetric care providers complete a full assessment of mood and emotional well-being (including screening for PPD and anxiety with a validated instrument) during the comprehensive postpartum visit for each patient.” This is recommended in addition to any screening for depression and anxiety during the pregnancy.
It is exciting that after decades of failing to attend to such a common complication of modern obstetrics, particularly now that we understand the adverse effects of PPD as it affects child development, family functioning, and risk for later childhood psychopathology. But in addition to recognizing the problem, we must come up with methods to carefully identify a navigable route for the women suffering from PPD to get their needs met. The route includes publicly identifying the illness, understanding which treatments are most effective and can be scaled for delivery to large numbers of women, and then, most critically, configuring social systems to absorb, effectively manage, and monitor the women we identify as needing treatment.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email him at [email protected].
References
1. J Clin Psychiatry. 2016 Sep;77[9]:1189-200.
2. Draft Recommendation Statement: Perinatal Depression: Preventive Interventions. U.S. Preventive Services Task Force. Aug 2018.
Vulvar disease treatment tips: From lice to lichen sclerosus
LAS VEGAS – at the Pelvic Anatomy and Gynecologic Surgery Symposium.
Pubic lice
Treat with malathion 0.5% lotion (Ovide), permethrin 1%-5% (Nix), or lindane 1% (Kwell). Be aware that the U.S. Library of Medicine cautions that lindane can cause serious side effects, and patients should use it only “if there is some reason you cannot use the other medications or if you have tried the other medications and they have not worked.”
Pruritus (itchy skin)
Eliminate possible contact allergens such as soaps, detergents, and undergarments. Swabs with 2% acetic acid solution can assist with general hygiene. It’s important to address secondary infections, and control of diet and stress may be helpful.
Folliculitis (inflammation of hair follicles)
A salt water bath can be helpful. Try 2 cups of “Instant Ocean” – a sea salt product for aquariums – in a shallow bath twice daily.
It can be treated with silver sulfadiazine (Silvadene) cream (three times daily and at bedtime) or clindamycin (Cleocin) cream (three times daily and at bedtime).
Consider a systemic drug after culture results come back if needed.
Lichen sclerosus (a skin inflammation also known as white spot disease)
“I see a lot of lichen sclerosus,” Dr. Baggish said. “Every single practice day, I’m seeing two or three [cases].”
Topical treatments include testosterone cream (which has low efficacy) and topical corticosteroid creams and ointments (the standard treatment).
Other treatments provide better and more consistent results: Etretinate (Tegison), a retinoid that is expensive and can produce serious side effects, and injectable dexamethasone (Decadron), which can stop progression.
Be aware that 10% of patients with this condition may develop squamous cell carcinoma. Monitor for any changes in appearance and biopsy if needed.
Behçet’s disease (a blood vessel inflammation disorder also known as silk road disease)
This rare condition can cause mouth and genital ulcers and uveitis (eye inflammation). For treatment, start 40 mg prednisone for 2-3 days, then 20 mg for 2 days, then 10 mg for 4 days, then stop. Start treatment immediately if there are signs of an oral lesion.
Fox-Fordyce disease (an inflammatory response that blocks sweat ducts and causes intense itching)
Treatment includes estrogen (2.5 mg per day) and tretinoin (Retin-A, apply once daily), usually given together. Suggest that patients try the Instant Ocean salt water treatment in the bath once daily (see details above under folliculitis entry).
Genital warts
Vaporize the warts via laser. “If they look like they’re recurring, I put them on interferon for 3 months because otherwise they just keep recurring,” Dr. Baggish said. “You could put topical treatments on them, but they’ll recur.”
Dr. Baggish, of the University of California, San Francisco, had no relevant financial disclosures. The meeting was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
LAS VEGAS – at the Pelvic Anatomy and Gynecologic Surgery Symposium.
Pubic lice
Treat with malathion 0.5% lotion (Ovide), permethrin 1%-5% (Nix), or lindane 1% (Kwell). Be aware that the U.S. Library of Medicine cautions that lindane can cause serious side effects, and patients should use it only “if there is some reason you cannot use the other medications or if you have tried the other medications and they have not worked.”
Pruritus (itchy skin)
Eliminate possible contact allergens such as soaps, detergents, and undergarments. Swabs with 2% acetic acid solution can assist with general hygiene. It’s important to address secondary infections, and control of diet and stress may be helpful.
Folliculitis (inflammation of hair follicles)
A salt water bath can be helpful. Try 2 cups of “Instant Ocean” – a sea salt product for aquariums – in a shallow bath twice daily.
It can be treated with silver sulfadiazine (Silvadene) cream (three times daily and at bedtime) or clindamycin (Cleocin) cream (three times daily and at bedtime).
Consider a systemic drug after culture results come back if needed.
Lichen sclerosus (a skin inflammation also known as white spot disease)
“I see a lot of lichen sclerosus,” Dr. Baggish said. “Every single practice day, I’m seeing two or three [cases].”
Topical treatments include testosterone cream (which has low efficacy) and topical corticosteroid creams and ointments (the standard treatment).
Other treatments provide better and more consistent results: Etretinate (Tegison), a retinoid that is expensive and can produce serious side effects, and injectable dexamethasone (Decadron), which can stop progression.
Be aware that 10% of patients with this condition may develop squamous cell carcinoma. Monitor for any changes in appearance and biopsy if needed.
Behçet’s disease (a blood vessel inflammation disorder also known as silk road disease)
This rare condition can cause mouth and genital ulcers and uveitis (eye inflammation). For treatment, start 40 mg prednisone for 2-3 days, then 20 mg for 2 days, then 10 mg for 4 days, then stop. Start treatment immediately if there are signs of an oral lesion.
Fox-Fordyce disease (an inflammatory response that blocks sweat ducts and causes intense itching)
Treatment includes estrogen (2.5 mg per day) and tretinoin (Retin-A, apply once daily), usually given together. Suggest that patients try the Instant Ocean salt water treatment in the bath once daily (see details above under folliculitis entry).
Genital warts
Vaporize the warts via laser. “If they look like they’re recurring, I put them on interferon for 3 months because otherwise they just keep recurring,” Dr. Baggish said. “You could put topical treatments on them, but they’ll recur.”
Dr. Baggish, of the University of California, San Francisco, had no relevant financial disclosures. The meeting was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
LAS VEGAS – at the Pelvic Anatomy and Gynecologic Surgery Symposium.
Pubic lice
Treat with malathion 0.5% lotion (Ovide), permethrin 1%-5% (Nix), or lindane 1% (Kwell). Be aware that the U.S. Library of Medicine cautions that lindane can cause serious side effects, and patients should use it only “if there is some reason you cannot use the other medications or if you have tried the other medications and they have not worked.”
Pruritus (itchy skin)
Eliminate possible contact allergens such as soaps, detergents, and undergarments. Swabs with 2% acetic acid solution can assist with general hygiene. It’s important to address secondary infections, and control of diet and stress may be helpful.
Folliculitis (inflammation of hair follicles)
A salt water bath can be helpful. Try 2 cups of “Instant Ocean” – a sea salt product for aquariums – in a shallow bath twice daily.
It can be treated with silver sulfadiazine (Silvadene) cream (three times daily and at bedtime) or clindamycin (Cleocin) cream (three times daily and at bedtime).
Consider a systemic drug after culture results come back if needed.
Lichen sclerosus (a skin inflammation also known as white spot disease)
“I see a lot of lichen sclerosus,” Dr. Baggish said. “Every single practice day, I’m seeing two or three [cases].”
Topical treatments include testosterone cream (which has low efficacy) and topical corticosteroid creams and ointments (the standard treatment).
Other treatments provide better and more consistent results: Etretinate (Tegison), a retinoid that is expensive and can produce serious side effects, and injectable dexamethasone (Decadron), which can stop progression.
Be aware that 10% of patients with this condition may develop squamous cell carcinoma. Monitor for any changes in appearance and biopsy if needed.
Behçet’s disease (a blood vessel inflammation disorder also known as silk road disease)
This rare condition can cause mouth and genital ulcers and uveitis (eye inflammation). For treatment, start 40 mg prednisone for 2-3 days, then 20 mg for 2 days, then 10 mg for 4 days, then stop. Start treatment immediately if there are signs of an oral lesion.
Fox-Fordyce disease (an inflammatory response that blocks sweat ducts and causes intense itching)
Treatment includes estrogen (2.5 mg per day) and tretinoin (Retin-A, apply once daily), usually given together. Suggest that patients try the Instant Ocean salt water treatment in the bath once daily (see details above under folliculitis entry).
Genital warts
Vaporize the warts via laser. “If they look like they’re recurring, I put them on interferon for 3 months because otherwise they just keep recurring,” Dr. Baggish said. “You could put topical treatments on them, but they’ll recur.”
Dr. Baggish, of the University of California, San Francisco, had no relevant financial disclosures. The meeting was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
EXPERT ANALYSIS FROM PAGS
Insulin may be toxic to the placenta in early pregnancy
, according to findings from an experimental in vitro study published in Fertility and Sterility.
“Collectively these results demonstrate that insulin itself may be directly toxic to the early human placenta but that metformin can prevent these deleterious effects,” wrote Mario Vega, MD, of Columbia University Fertility Center, New York, and his colleagues. “If confirmed in animal and human studies, this would indicate that screening and treatment for insulin resistance should focus on hyperinsulinemia.”
Dr. Vega and his colleagues cultivated trophoblast cells from three healthy women scheduled for manual vacuum aspiration during the first trimester of pregnancy to study the effects of insulin exposure alone, while trophoblast cells were cultured from a different set of women for the insulin and metformin follow-up experiments. The researchers tested each experiment against a control group of cultivated lung fibroblast cells. Insulin was measured in doses of 0.2 nmol, 1 nmol, and 5 nmol, while metformin was measured at 10 micromol. The primary outcome measures examined were gamma-H2AX for DNA damage, cell proliferation assay for cell survival, and cleaved caspase-3 for apoptosis.
Within 48 hours, the cultures showed DNA damage and induction of apoptosis when exposed to 1 nmol of insulin, but researchers said pretreatment with metformin prevented these effects. Exposing cells to metformin after insulin reduced but did not eliminate the effects of insulin.
The researchers noted the study is limited because the effects of insulin and metformin have not been examined in vivo, and it is not known at what level insulin causes damage. In addition, they suggested downregulation of genes in trophoblasts caused by insulin could cause apoptosis and DNA damage to trophoblast cells.
“Although studies performed on kidney and colon cells suggest that one possible mechanism of action for insulin-mediated genotoxicity is through AKT activation of mitochondria and subsequent reactive oxygen species production, the exact mechanism is poorly understood,” Dr. Vega and colleagues said. “Future studies will be necessary to determine variability among subjects, as well as mechanisms of action through which insulin exerts its cytotoxicity and genotoxicity.”
This study was funded by a grant from the National Institutes of Health Human Placenta Project. The authors reported no relevant financial disclosures.
SOURCE: Vega M et al. Fertil Steril. 2019. doi: 10.1016/j.fertnstert.2018.11.032.
, according to findings from an experimental in vitro study published in Fertility and Sterility.
“Collectively these results demonstrate that insulin itself may be directly toxic to the early human placenta but that metformin can prevent these deleterious effects,” wrote Mario Vega, MD, of Columbia University Fertility Center, New York, and his colleagues. “If confirmed in animal and human studies, this would indicate that screening and treatment for insulin resistance should focus on hyperinsulinemia.”
Dr. Vega and his colleagues cultivated trophoblast cells from three healthy women scheduled for manual vacuum aspiration during the first trimester of pregnancy to study the effects of insulin exposure alone, while trophoblast cells were cultured from a different set of women for the insulin and metformin follow-up experiments. The researchers tested each experiment against a control group of cultivated lung fibroblast cells. Insulin was measured in doses of 0.2 nmol, 1 nmol, and 5 nmol, while metformin was measured at 10 micromol. The primary outcome measures examined were gamma-H2AX for DNA damage, cell proliferation assay for cell survival, and cleaved caspase-3 for apoptosis.
Within 48 hours, the cultures showed DNA damage and induction of apoptosis when exposed to 1 nmol of insulin, but researchers said pretreatment with metformin prevented these effects. Exposing cells to metformin after insulin reduced but did not eliminate the effects of insulin.
The researchers noted the study is limited because the effects of insulin and metformin have not been examined in vivo, and it is not known at what level insulin causes damage. In addition, they suggested downregulation of genes in trophoblasts caused by insulin could cause apoptosis and DNA damage to trophoblast cells.
“Although studies performed on kidney and colon cells suggest that one possible mechanism of action for insulin-mediated genotoxicity is through AKT activation of mitochondria and subsequent reactive oxygen species production, the exact mechanism is poorly understood,” Dr. Vega and colleagues said. “Future studies will be necessary to determine variability among subjects, as well as mechanisms of action through which insulin exerts its cytotoxicity and genotoxicity.”
This study was funded by a grant from the National Institutes of Health Human Placenta Project. The authors reported no relevant financial disclosures.
SOURCE: Vega M et al. Fertil Steril. 2019. doi: 10.1016/j.fertnstert.2018.11.032.
, according to findings from an experimental in vitro study published in Fertility and Sterility.
“Collectively these results demonstrate that insulin itself may be directly toxic to the early human placenta but that metformin can prevent these deleterious effects,” wrote Mario Vega, MD, of Columbia University Fertility Center, New York, and his colleagues. “If confirmed in animal and human studies, this would indicate that screening and treatment for insulin resistance should focus on hyperinsulinemia.”
Dr. Vega and his colleagues cultivated trophoblast cells from three healthy women scheduled for manual vacuum aspiration during the first trimester of pregnancy to study the effects of insulin exposure alone, while trophoblast cells were cultured from a different set of women for the insulin and metformin follow-up experiments. The researchers tested each experiment against a control group of cultivated lung fibroblast cells. Insulin was measured in doses of 0.2 nmol, 1 nmol, and 5 nmol, while metformin was measured at 10 micromol. The primary outcome measures examined were gamma-H2AX for DNA damage, cell proliferation assay for cell survival, and cleaved caspase-3 for apoptosis.
Within 48 hours, the cultures showed DNA damage and induction of apoptosis when exposed to 1 nmol of insulin, but researchers said pretreatment with metformin prevented these effects. Exposing cells to metformin after insulin reduced but did not eliminate the effects of insulin.
The researchers noted the study is limited because the effects of insulin and metformin have not been examined in vivo, and it is not known at what level insulin causes damage. In addition, they suggested downregulation of genes in trophoblasts caused by insulin could cause apoptosis and DNA damage to trophoblast cells.
“Although studies performed on kidney and colon cells suggest that one possible mechanism of action for insulin-mediated genotoxicity is through AKT activation of mitochondria and subsequent reactive oxygen species production, the exact mechanism is poorly understood,” Dr. Vega and colleagues said. “Future studies will be necessary to determine variability among subjects, as well as mechanisms of action through which insulin exerts its cytotoxicity and genotoxicity.”
This study was funded by a grant from the National Institutes of Health Human Placenta Project. The authors reported no relevant financial disclosures.
SOURCE: Vega M et al. Fertil Steril. 2019. doi: 10.1016/j.fertnstert.2018.11.032.
FROM FERTILITY & STERILITY
Key clinical point: Trophoblasts cultured during the first trimester of pregnancy exposed to insulin were more likely to have increased apoptosis, DNA damage, and decreased cell survival, while pretreatment with metformin prior to exposure with insulin prevented these effects.
Major finding: DNA damage and rate of apoptosis increased in trophoblast cells exposed to 1 nmol of insulin, and cell survival decreased, compared with primary lung fibroblast cells; treating the cells with metformin prior to exposure with insulin resulted in prevention of these effects.
Study details: An experimental in vitro study of first trimester trophoblast cells exposed to insulin and metformin.
Disclosures: This study was funded by a grant from the National Institutes of Health Human Placenta Project. The authors reported they had no relevant financial disclosures.
Source: Vega M et al. Fertil Steril. 2019. doi: 10.1016/j.fertnstert.2018.11.032.
Stopping TNF inhibitors before 20 weeks’ gestation not linked to worsening RA, JIA
In pregnant women with arthritis, discontinuing tumor necrosis factor inhibitors prior to gestational week 20 seems feasible without an increased risk of disease worsening, particularly in those with well-controlled disease, according to authors of a recent analysis of a prospective cohort study.
Stopping tumor necrosis factor inhibitor (TNFi) treatment at that point in the second trimester was not linked to any clinically important worsening of patient-reported outcomes later in pregnancy for women with rheumatoid arthritis (RA) or juvenile idiopathic arthritis (JIA), the researchers said.
However, continuing a TNFi past gestational week 20 may also be warranted for some patients, according to the researchers, led by Frauke Förger, MD, of the University of Bern (Switzerland).
“In case of active disease, the continuation of TNF inhibitors beyond gestational week 20 seems reasonable from the standpoint of improved disease activity in the third trimester, which may in turn lead to improved pregnancy outcomes,” Dr. Förger and her coinvestigators wrote in Arthritis & Rheumatology.
These findings stand in contrast to those of another recent study, in which stopping a TNF inhibitor after a positive pregnancy test was linked to disease flares in women with RA, according to the authors.
“The timing of drug discontinuation during pregnancy may be of importance,” they said in their report.
Beyond these studies, there are very limited data on the effects of discontinuing TNF inhibitors in pregnant women with rheumatoid arthritis, and “a lack of any data” in pregnant women with JIA, they said.
The current investigation by Dr. Förger and her colleagues included 490 pregnant women in the United States or Canada who were enrolled in the Organization of Teratology Information Specialists (OTIS) Autoimmune Diseases in Pregnancy Project, a prospective cohort study. Of those women, 397 had RA and 93 had JIA.
About one-quarter of the women (122, or 24.9%) discontinued TNF inhibitor therapy prior to gestational week 20, while 41% continued on TNF inhibitors beyond that point, and 34.1% did not use a TNF inhibitor in pregnancy.
For those women who discontinued TNF inhibitors before gestational week 20, scores on the Patient Activity Scale (PAS) were stable over time, Dr. Förger and her colleagues reported.
Women who continued TNF inhibitor treatment past gestational week 20 had improved PAS scores in the third trimester, according to results of a univariate analysis (P = .02). However, the improvement appeared to be attenuated after adjustment for factors including race, smoking, use of prednisone or disease-modifying antirheumatic drugs, and gestational age, the investigators said.
They were unable to analyze the effects of ongoing TNFi treatment or discontinuation on patients with JIA separately because of the limited number of such patients in each group.
Another limitation of the study is that a high proportion of women – nearly three-quarters – had low disease activity at the start of pregnancy, according to the investigators, who said that group of women might expect some degree of improvement in the third trimester with or without TNF inhibitor discontinuation.
“In this context, the ameliorating effect of pregnancy on RA and JIA, which is most pronounced in the third trimester, may play a role,” they explained.
A certain proportion of women choose to discontinue certain arthritis treatments during pregnancy because of concerns that the medication may lead to fetal harm, but that may be changing, the investigators noted in their report.
“In recent years, more patients requiring treatment have been continuing on effective TNF inhibitors beyond conception as the available data on the safety of TNF inhibitors during pregnancy has increased,” they wrote.
Dr. Förger and her coauthors reported no financial disclosures or conflicts of interest. Financial support for the OTIS Collaborative Research Group comes from industry sources including AbbVie, Bristol-Myers Squibb, Celgene, Hoffman La Roche-Genentech, Janssen, Pfizer, Regeneron, Sandoz, and UCB, among others.
SOURCE: Förger F et al. Arthritis Rheumatol. 2019 Jan 21. doi: 10.1002/art.40821
In pregnant women with arthritis, discontinuing tumor necrosis factor inhibitors prior to gestational week 20 seems feasible without an increased risk of disease worsening, particularly in those with well-controlled disease, according to authors of a recent analysis of a prospective cohort study.
Stopping tumor necrosis factor inhibitor (TNFi) treatment at that point in the second trimester was not linked to any clinically important worsening of patient-reported outcomes later in pregnancy for women with rheumatoid arthritis (RA) or juvenile idiopathic arthritis (JIA), the researchers said.
However, continuing a TNFi past gestational week 20 may also be warranted for some patients, according to the researchers, led by Frauke Förger, MD, of the University of Bern (Switzerland).
“In case of active disease, the continuation of TNF inhibitors beyond gestational week 20 seems reasonable from the standpoint of improved disease activity in the third trimester, which may in turn lead to improved pregnancy outcomes,” Dr. Förger and her coinvestigators wrote in Arthritis & Rheumatology.
These findings stand in contrast to those of another recent study, in which stopping a TNF inhibitor after a positive pregnancy test was linked to disease flares in women with RA, according to the authors.
“The timing of drug discontinuation during pregnancy may be of importance,” they said in their report.
Beyond these studies, there are very limited data on the effects of discontinuing TNF inhibitors in pregnant women with rheumatoid arthritis, and “a lack of any data” in pregnant women with JIA, they said.
The current investigation by Dr. Förger and her colleagues included 490 pregnant women in the United States or Canada who were enrolled in the Organization of Teratology Information Specialists (OTIS) Autoimmune Diseases in Pregnancy Project, a prospective cohort study. Of those women, 397 had RA and 93 had JIA.
About one-quarter of the women (122, or 24.9%) discontinued TNF inhibitor therapy prior to gestational week 20, while 41% continued on TNF inhibitors beyond that point, and 34.1% did not use a TNF inhibitor in pregnancy.
For those women who discontinued TNF inhibitors before gestational week 20, scores on the Patient Activity Scale (PAS) were stable over time, Dr. Förger and her colleagues reported.
Women who continued TNF inhibitor treatment past gestational week 20 had improved PAS scores in the third trimester, according to results of a univariate analysis (P = .02). However, the improvement appeared to be attenuated after adjustment for factors including race, smoking, use of prednisone or disease-modifying antirheumatic drugs, and gestational age, the investigators said.
They were unable to analyze the effects of ongoing TNFi treatment or discontinuation on patients with JIA separately because of the limited number of such patients in each group.
Another limitation of the study is that a high proportion of women – nearly three-quarters – had low disease activity at the start of pregnancy, according to the investigators, who said that group of women might expect some degree of improvement in the third trimester with or without TNF inhibitor discontinuation.
“In this context, the ameliorating effect of pregnancy on RA and JIA, which is most pronounced in the third trimester, may play a role,” they explained.
A certain proportion of women choose to discontinue certain arthritis treatments during pregnancy because of concerns that the medication may lead to fetal harm, but that may be changing, the investigators noted in their report.
“In recent years, more patients requiring treatment have been continuing on effective TNF inhibitors beyond conception as the available data on the safety of TNF inhibitors during pregnancy has increased,” they wrote.
Dr. Förger and her coauthors reported no financial disclosures or conflicts of interest. Financial support for the OTIS Collaborative Research Group comes from industry sources including AbbVie, Bristol-Myers Squibb, Celgene, Hoffman La Roche-Genentech, Janssen, Pfizer, Regeneron, Sandoz, and UCB, among others.
SOURCE: Förger F et al. Arthritis Rheumatol. 2019 Jan 21. doi: 10.1002/art.40821
In pregnant women with arthritis, discontinuing tumor necrosis factor inhibitors prior to gestational week 20 seems feasible without an increased risk of disease worsening, particularly in those with well-controlled disease, according to authors of a recent analysis of a prospective cohort study.
Stopping tumor necrosis factor inhibitor (TNFi) treatment at that point in the second trimester was not linked to any clinically important worsening of patient-reported outcomes later in pregnancy for women with rheumatoid arthritis (RA) or juvenile idiopathic arthritis (JIA), the researchers said.
However, continuing a TNFi past gestational week 20 may also be warranted for some patients, according to the researchers, led by Frauke Förger, MD, of the University of Bern (Switzerland).
“In case of active disease, the continuation of TNF inhibitors beyond gestational week 20 seems reasonable from the standpoint of improved disease activity in the third trimester, which may in turn lead to improved pregnancy outcomes,” Dr. Förger and her coinvestigators wrote in Arthritis & Rheumatology.
These findings stand in contrast to those of another recent study, in which stopping a TNF inhibitor after a positive pregnancy test was linked to disease flares in women with RA, according to the authors.
“The timing of drug discontinuation during pregnancy may be of importance,” they said in their report.
Beyond these studies, there are very limited data on the effects of discontinuing TNF inhibitors in pregnant women with rheumatoid arthritis, and “a lack of any data” in pregnant women with JIA, they said.
The current investigation by Dr. Förger and her colleagues included 490 pregnant women in the United States or Canada who were enrolled in the Organization of Teratology Information Specialists (OTIS) Autoimmune Diseases in Pregnancy Project, a prospective cohort study. Of those women, 397 had RA and 93 had JIA.
About one-quarter of the women (122, or 24.9%) discontinued TNF inhibitor therapy prior to gestational week 20, while 41% continued on TNF inhibitors beyond that point, and 34.1% did not use a TNF inhibitor in pregnancy.
For those women who discontinued TNF inhibitors before gestational week 20, scores on the Patient Activity Scale (PAS) were stable over time, Dr. Förger and her colleagues reported.
Women who continued TNF inhibitor treatment past gestational week 20 had improved PAS scores in the third trimester, according to results of a univariate analysis (P = .02). However, the improvement appeared to be attenuated after adjustment for factors including race, smoking, use of prednisone or disease-modifying antirheumatic drugs, and gestational age, the investigators said.
They were unable to analyze the effects of ongoing TNFi treatment or discontinuation on patients with JIA separately because of the limited number of such patients in each group.
Another limitation of the study is that a high proportion of women – nearly three-quarters – had low disease activity at the start of pregnancy, according to the investigators, who said that group of women might expect some degree of improvement in the third trimester with or without TNF inhibitor discontinuation.
“In this context, the ameliorating effect of pregnancy on RA and JIA, which is most pronounced in the third trimester, may play a role,” they explained.
A certain proportion of women choose to discontinue certain arthritis treatments during pregnancy because of concerns that the medication may lead to fetal harm, but that may be changing, the investigators noted in their report.
“In recent years, more patients requiring treatment have been continuing on effective TNF inhibitors beyond conception as the available data on the safety of TNF inhibitors during pregnancy has increased,” they wrote.
Dr. Förger and her coauthors reported no financial disclosures or conflicts of interest. Financial support for the OTIS Collaborative Research Group comes from industry sources including AbbVie, Bristol-Myers Squibb, Celgene, Hoffman La Roche-Genentech, Janssen, Pfizer, Regeneron, Sandoz, and UCB, among others.
SOURCE: Förger F et al. Arthritis Rheumatol. 2019 Jan 21. doi: 10.1002/art.40821
FROM ARTHRITIS & RHEUMATOLOGY
Key clinical point: In contrast to a previous report,
Major finding: Patient Activity Scale (PAS) scores were stable over time in women who discontinued TNF inhibitors before gestational week 20. Those who continued past week 20 had improved PAS scores in the third trimester (univariate analysis; P = .02).
Study details: Analysis including 490 pregnant women in the United States or Canada who enrolled in the Organization of Teratology Information Specialists (OTIS) Autoimmune Diseases in Pregnancy Project, a prospective cohort study.
Disclosures: Dr. Förger and her coauthors reported no financial disclosures or conflicts of interest. Financial support for the OTIS Collaborative Research Group comes from industry sources including AbbVie, Bristol-Myers Squibb, Celgene, Hoffman La Roche-Genentech, Janssen, Pfizer, Regeneron, Sandoz, and UCB, among others.
Source: Förger F et al. Arthritis Rheumatol. 2019 Jan 21. doi: 10.1002/art.40821.
When NOT to perform a Pap test
Pap tests have the reputation of being a simple, noninvasive, low-cost test to offer patients, and, therefore, it is understandable to believe there is no harm in offering it in all situations. However, if inappropriately applied in isolation, performing the Pap test may do more harm than good.
I recently saw a patient in consultation for cervical cancer. Her story was similar to one I’ve seen many times before. She was a 30-year-old non–English-speaking Hispanic woman who received regular care from the health department clinics.
In April of the prior year, she had noticed abnormal bleeding symptoms including intermenstrual and postcoital bleeding. She visited the health department and reported these symptoms to the provider who performed an examination. According to the provider’s notes, the cervix appeared “abnormal” and a Pap test was done. The result of this Pap test was high-grade dysplasia. The patient was promptly notified of the result and an appointment was arranged with the local ob.gyn. for a consultation, presumably for colposcopy and subsequent appropriate excisional procedure. Unfortunately, the patient did not attend that scheduled appointment. She later recounted to me that it was because she had not understood that it was important. She had a long history of abnormal Pap tests which, in the past, had only required repeat testing or minor interventions such as “freezing.”
Her bleeding symptoms became worse, and she developed abnormal discharge and pain. In November, she presented again for evaluation to the same provider. Now her cervix appeared very abnormal and was described as a “crater.” Again a Pap test was done. This time the Pap test showed “carcinoma,” and the patient was informed that she had cancer and was referred to gynecologic oncology. When I examined this unfortunate young woman, I discovered a 10 cm, stage IIB very locally advanced tumor. She is currently receiving primary chemotherapy/radiation with an approximately 60% probability of cure, and a high likelihood of lifelong sequelae of this toxic therapy.
This case highlights that, even when patients are engaged within our health care system, we can miss the opportunity to diagnose early-stage cancers if we are not utilizing screening versus diagnostic tests appropriately.
The purpose of a Pap test is as a screening test, which are designed to detect disease in asymptomatic individuals. The accuracy of these tests is determined in low-risk (asymptomatic) populations, which influences the pretest probability of disease. In asymptomatic patients with a normal screening test, it is safe to wait out the interval of time for the repeat screening test, because the combination of a low pretest probability and a high sensitivity of the test in finding disease means that there is a very low chance of missing disease.
Dysplasia rarely causes bleeding. However, invasive cervical cancer does. If a patient has a symptom such as abnormal bleeding, they no longer fit into the population with a low pretest probability for having cervical cancer. This same sensitivity of the Pap test in finding disease, combined with the now-higher pretest probability can raise the level of false-negative results to unacceptably high levels.
Patients with symptoms of cervical cancer should not receive screening tests exclusively; they should receive diagnostic tests. For example, Pap tests should not be used in isolation to diagnose pathology in patients with abnormal bleeding or discharge, just as screening mammograms should not be ordered in patients with symptomatic breast lumps, nipple discharge, retraction, etc. (these women should be referred for diagnostic mammography and ultrasound). It is not unusual for gynecologic oncologists to see patients with visible invasive cervical cancer who have only cervical intraepithelial neoplasia grade 3 on the preceding Pap test. There is a 34% positive predictive value that a cervical cancer will be found with a high-grade dysplastic Pap test.1 Cytology is an inferior diagnostic tool, compared with histology, in determining invasive cancer from preinvasive lesions. Cytology is an inferior diagnostic tool, compared with histology, in determining invasive cancer from preinvasive lesions. It analyzes individual cells rather than a piece of tissue with intercellular relationships.
The take-home message for this column is that, if a provider sees an abnormal lesion on a cervix, they should biopsy the visible lesion to obtain a histologic diagnosis. Simply performing a Pap test alone may result in false reassurance and in underestimating the severity of disease.
Some providers will tell me that they have concerns about performing a biopsy on a grossly abnormal cervix for fear that the subsequent bleeding will be difficult to manage in the outpatient setting. This is understandable, although it is unlikely that an office equipped with the ability to perform colposcopy or excisional procedures would not have the necessary equipment to manage this. Prolonged pressure applied to the cervix with topical hemostatic agents or – in extreme cases – vaginal packing with gauze always has been effective for me in these circumstances.
The additional benefit of establishing histologic confirmation prior to referral is expediting care, including additional imaging and referrals to treating providers. If the diagnosis is inadequately established prior to their appointment with a gynecologic oncologist, it can add further delays before definitive surgical or nonsurgical management can be initiated, which is particularly problematic if the patient is experiencing severe bleeding. If the provider feels uncomfortable with proceeding with biopsy, they should inform the patient very clearly that they suspect that there is a cancer of the cervix, and it needs attention from a cancer specialist to confirm the diagnosis. This clear communication will minimize the likelihood that the patient may not show up for the subsequent appointments before her diagnosis is definitively established.
Another common scenario in which Pap tests are inappropriately applied is in the surveillance of endometrial cancer. In 2013, the Society of Gynecologic Oncology released its five “Choosing Wisely” recommendations. This included the recommendation to not perform Pap tests in the surveillance of endometrial cancer. This recommendation was based on a body of evidence that demonstrates screening for endometrial cancer recurrence with Pap smears does not detect vaginal mucosal recurrences any sooner than visualization of lesions on speculum examination.2,3 These Pap-positive recurrences almost always are visible on exam. Additionally, false positives are common in this population, particularly among women who have had radiation or have atrophic tissues.
Using Pap tests for the surveillance of cervical cancer is somewhat more complicated. Similarly, they do not detect cervical cancer recurrence any sooner than comprehensive examination does. However, this population may suffer from chronic human papillomavirus (HPV) infection, and there remains a role of the Pap test in screening for future, new HPV-related preinvasive vaginal disease. Therefore, Pap tests, and/or HPV testing can be offered to cervical cancer survivors in accordance with the American Society for Colposcopy and Cervical Pathology guidelines for noncervical cancer patients, with the caveat that, if radiation has been given, false positives are more likely.2
Pap tests clearly have an important role as a screening test in asymptomatic individuals. However, when the patient has a symptom that might be cervical cancer or a visibly suspicious lesion, she should receive a diagnostic test, and Pap tests are not designed for that purpose.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no conflicts of interest. Email Dr. Rossi at [email protected].
References
1. Cytopathology. 2016 Jun;27(3):201-9.
2. Gynecol Oncol. 2017 Jul;146(1):3-10.
3. Gynecol Oncol. 2011 Nov;123(2):205-7.
Pap tests have the reputation of being a simple, noninvasive, low-cost test to offer patients, and, therefore, it is understandable to believe there is no harm in offering it in all situations. However, if inappropriately applied in isolation, performing the Pap test may do more harm than good.
I recently saw a patient in consultation for cervical cancer. Her story was similar to one I’ve seen many times before. She was a 30-year-old non–English-speaking Hispanic woman who received regular care from the health department clinics.
In April of the prior year, she had noticed abnormal bleeding symptoms including intermenstrual and postcoital bleeding. She visited the health department and reported these symptoms to the provider who performed an examination. According to the provider’s notes, the cervix appeared “abnormal” and a Pap test was done. The result of this Pap test was high-grade dysplasia. The patient was promptly notified of the result and an appointment was arranged with the local ob.gyn. for a consultation, presumably for colposcopy and subsequent appropriate excisional procedure. Unfortunately, the patient did not attend that scheduled appointment. She later recounted to me that it was because she had not understood that it was important. She had a long history of abnormal Pap tests which, in the past, had only required repeat testing or minor interventions such as “freezing.”
Her bleeding symptoms became worse, and she developed abnormal discharge and pain. In November, she presented again for evaluation to the same provider. Now her cervix appeared very abnormal and was described as a “crater.” Again a Pap test was done. This time the Pap test showed “carcinoma,” and the patient was informed that she had cancer and was referred to gynecologic oncology. When I examined this unfortunate young woman, I discovered a 10 cm, stage IIB very locally advanced tumor. She is currently receiving primary chemotherapy/radiation with an approximately 60% probability of cure, and a high likelihood of lifelong sequelae of this toxic therapy.
This case highlights that, even when patients are engaged within our health care system, we can miss the opportunity to diagnose early-stage cancers if we are not utilizing screening versus diagnostic tests appropriately.
The purpose of a Pap test is as a screening test, which are designed to detect disease in asymptomatic individuals. The accuracy of these tests is determined in low-risk (asymptomatic) populations, which influences the pretest probability of disease. In asymptomatic patients with a normal screening test, it is safe to wait out the interval of time for the repeat screening test, because the combination of a low pretest probability and a high sensitivity of the test in finding disease means that there is a very low chance of missing disease.
Dysplasia rarely causes bleeding. However, invasive cervical cancer does. If a patient has a symptom such as abnormal bleeding, they no longer fit into the population with a low pretest probability for having cervical cancer. This same sensitivity of the Pap test in finding disease, combined with the now-higher pretest probability can raise the level of false-negative results to unacceptably high levels.
Patients with symptoms of cervical cancer should not receive screening tests exclusively; they should receive diagnostic tests. For example, Pap tests should not be used in isolation to diagnose pathology in patients with abnormal bleeding or discharge, just as screening mammograms should not be ordered in patients with symptomatic breast lumps, nipple discharge, retraction, etc. (these women should be referred for diagnostic mammography and ultrasound). It is not unusual for gynecologic oncologists to see patients with visible invasive cervical cancer who have only cervical intraepithelial neoplasia grade 3 on the preceding Pap test. There is a 34% positive predictive value that a cervical cancer will be found with a high-grade dysplastic Pap test.1 Cytology is an inferior diagnostic tool, compared with histology, in determining invasive cancer from preinvasive lesions. Cytology is an inferior diagnostic tool, compared with histology, in determining invasive cancer from preinvasive lesions. It analyzes individual cells rather than a piece of tissue with intercellular relationships.
The take-home message for this column is that, if a provider sees an abnormal lesion on a cervix, they should biopsy the visible lesion to obtain a histologic diagnosis. Simply performing a Pap test alone may result in false reassurance and in underestimating the severity of disease.
Some providers will tell me that they have concerns about performing a biopsy on a grossly abnormal cervix for fear that the subsequent bleeding will be difficult to manage in the outpatient setting. This is understandable, although it is unlikely that an office equipped with the ability to perform colposcopy or excisional procedures would not have the necessary equipment to manage this. Prolonged pressure applied to the cervix with topical hemostatic agents or – in extreme cases – vaginal packing with gauze always has been effective for me in these circumstances.
The additional benefit of establishing histologic confirmation prior to referral is expediting care, including additional imaging and referrals to treating providers. If the diagnosis is inadequately established prior to their appointment with a gynecologic oncologist, it can add further delays before definitive surgical or nonsurgical management can be initiated, which is particularly problematic if the patient is experiencing severe bleeding. If the provider feels uncomfortable with proceeding with biopsy, they should inform the patient very clearly that they suspect that there is a cancer of the cervix, and it needs attention from a cancer specialist to confirm the diagnosis. This clear communication will minimize the likelihood that the patient may not show up for the subsequent appointments before her diagnosis is definitively established.
Another common scenario in which Pap tests are inappropriately applied is in the surveillance of endometrial cancer. In 2013, the Society of Gynecologic Oncology released its five “Choosing Wisely” recommendations. This included the recommendation to not perform Pap tests in the surveillance of endometrial cancer. This recommendation was based on a body of evidence that demonstrates screening for endometrial cancer recurrence with Pap smears does not detect vaginal mucosal recurrences any sooner than visualization of lesions on speculum examination.2,3 These Pap-positive recurrences almost always are visible on exam. Additionally, false positives are common in this population, particularly among women who have had radiation or have atrophic tissues.
Using Pap tests for the surveillance of cervical cancer is somewhat more complicated. Similarly, they do not detect cervical cancer recurrence any sooner than comprehensive examination does. However, this population may suffer from chronic human papillomavirus (HPV) infection, and there remains a role of the Pap test in screening for future, new HPV-related preinvasive vaginal disease. Therefore, Pap tests, and/or HPV testing can be offered to cervical cancer survivors in accordance with the American Society for Colposcopy and Cervical Pathology guidelines for noncervical cancer patients, with the caveat that, if radiation has been given, false positives are more likely.2
Pap tests clearly have an important role as a screening test in asymptomatic individuals. However, when the patient has a symptom that might be cervical cancer or a visibly suspicious lesion, she should receive a diagnostic test, and Pap tests are not designed for that purpose.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no conflicts of interest. Email Dr. Rossi at [email protected].
References
1. Cytopathology. 2016 Jun;27(3):201-9.
2. Gynecol Oncol. 2017 Jul;146(1):3-10.
3. Gynecol Oncol. 2011 Nov;123(2):205-7.
Pap tests have the reputation of being a simple, noninvasive, low-cost test to offer patients, and, therefore, it is understandable to believe there is no harm in offering it in all situations. However, if inappropriately applied in isolation, performing the Pap test may do more harm than good.
I recently saw a patient in consultation for cervical cancer. Her story was similar to one I’ve seen many times before. She was a 30-year-old non–English-speaking Hispanic woman who received regular care from the health department clinics.
In April of the prior year, she had noticed abnormal bleeding symptoms including intermenstrual and postcoital bleeding. She visited the health department and reported these symptoms to the provider who performed an examination. According to the provider’s notes, the cervix appeared “abnormal” and a Pap test was done. The result of this Pap test was high-grade dysplasia. The patient was promptly notified of the result and an appointment was arranged with the local ob.gyn. for a consultation, presumably for colposcopy and subsequent appropriate excisional procedure. Unfortunately, the patient did not attend that scheduled appointment. She later recounted to me that it was because she had not understood that it was important. She had a long history of abnormal Pap tests which, in the past, had only required repeat testing or minor interventions such as “freezing.”
Her bleeding symptoms became worse, and she developed abnormal discharge and pain. In November, she presented again for evaluation to the same provider. Now her cervix appeared very abnormal and was described as a “crater.” Again a Pap test was done. This time the Pap test showed “carcinoma,” and the patient was informed that she had cancer and was referred to gynecologic oncology. When I examined this unfortunate young woman, I discovered a 10 cm, stage IIB very locally advanced tumor. She is currently receiving primary chemotherapy/radiation with an approximately 60% probability of cure, and a high likelihood of lifelong sequelae of this toxic therapy.
This case highlights that, even when patients are engaged within our health care system, we can miss the opportunity to diagnose early-stage cancers if we are not utilizing screening versus diagnostic tests appropriately.
The purpose of a Pap test is as a screening test, which are designed to detect disease in asymptomatic individuals. The accuracy of these tests is determined in low-risk (asymptomatic) populations, which influences the pretest probability of disease. In asymptomatic patients with a normal screening test, it is safe to wait out the interval of time for the repeat screening test, because the combination of a low pretest probability and a high sensitivity of the test in finding disease means that there is a very low chance of missing disease.
Dysplasia rarely causes bleeding. However, invasive cervical cancer does. If a patient has a symptom such as abnormal bleeding, they no longer fit into the population with a low pretest probability for having cervical cancer. This same sensitivity of the Pap test in finding disease, combined with the now-higher pretest probability can raise the level of false-negative results to unacceptably high levels.
Patients with symptoms of cervical cancer should not receive screening tests exclusively; they should receive diagnostic tests. For example, Pap tests should not be used in isolation to diagnose pathology in patients with abnormal bleeding or discharge, just as screening mammograms should not be ordered in patients with symptomatic breast lumps, nipple discharge, retraction, etc. (these women should be referred for diagnostic mammography and ultrasound). It is not unusual for gynecologic oncologists to see patients with visible invasive cervical cancer who have only cervical intraepithelial neoplasia grade 3 on the preceding Pap test. There is a 34% positive predictive value that a cervical cancer will be found with a high-grade dysplastic Pap test.1 Cytology is an inferior diagnostic tool, compared with histology, in determining invasive cancer from preinvasive lesions. Cytology is an inferior diagnostic tool, compared with histology, in determining invasive cancer from preinvasive lesions. It analyzes individual cells rather than a piece of tissue with intercellular relationships.
The take-home message for this column is that, if a provider sees an abnormal lesion on a cervix, they should biopsy the visible lesion to obtain a histologic diagnosis. Simply performing a Pap test alone may result in false reassurance and in underestimating the severity of disease.
Some providers will tell me that they have concerns about performing a biopsy on a grossly abnormal cervix for fear that the subsequent bleeding will be difficult to manage in the outpatient setting. This is understandable, although it is unlikely that an office equipped with the ability to perform colposcopy or excisional procedures would not have the necessary equipment to manage this. Prolonged pressure applied to the cervix with topical hemostatic agents or – in extreme cases – vaginal packing with gauze always has been effective for me in these circumstances.
The additional benefit of establishing histologic confirmation prior to referral is expediting care, including additional imaging and referrals to treating providers. If the diagnosis is inadequately established prior to their appointment with a gynecologic oncologist, it can add further delays before definitive surgical or nonsurgical management can be initiated, which is particularly problematic if the patient is experiencing severe bleeding. If the provider feels uncomfortable with proceeding with biopsy, they should inform the patient very clearly that they suspect that there is a cancer of the cervix, and it needs attention from a cancer specialist to confirm the diagnosis. This clear communication will minimize the likelihood that the patient may not show up for the subsequent appointments before her diagnosis is definitively established.
Another common scenario in which Pap tests are inappropriately applied is in the surveillance of endometrial cancer. In 2013, the Society of Gynecologic Oncology released its five “Choosing Wisely” recommendations. This included the recommendation to not perform Pap tests in the surveillance of endometrial cancer. This recommendation was based on a body of evidence that demonstrates screening for endometrial cancer recurrence with Pap smears does not detect vaginal mucosal recurrences any sooner than visualization of lesions on speculum examination.2,3 These Pap-positive recurrences almost always are visible on exam. Additionally, false positives are common in this population, particularly among women who have had radiation or have atrophic tissues.
Using Pap tests for the surveillance of cervical cancer is somewhat more complicated. Similarly, they do not detect cervical cancer recurrence any sooner than comprehensive examination does. However, this population may suffer from chronic human papillomavirus (HPV) infection, and there remains a role of the Pap test in screening for future, new HPV-related preinvasive vaginal disease. Therefore, Pap tests, and/or HPV testing can be offered to cervical cancer survivors in accordance with the American Society for Colposcopy and Cervical Pathology guidelines for noncervical cancer patients, with the caveat that, if radiation has been given, false positives are more likely.2
Pap tests clearly have an important role as a screening test in asymptomatic individuals. However, when the patient has a symptom that might be cervical cancer or a visibly suspicious lesion, she should receive a diagnostic test, and Pap tests are not designed for that purpose.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no conflicts of interest. Email Dr. Rossi at [email protected].
References
1. Cytopathology. 2016 Jun;27(3):201-9.
2. Gynecol Oncol. 2017 Jul;146(1):3-10.
3. Gynecol Oncol. 2011 Nov;123(2):205-7.
Anxiety, depression, burnout higher in physician mothers caring for others at home
Physicians who are also mothers have a higher risk of burnout and mood and anxiety disorders if they are also caring for someone with a serious illness or disability outside of work, according to a cross-sectional survey reported in a letter in JAMA Internal Medicine.
“Our findings highlight the additional caregiving responsibilities of some women physicians and the potential consequences of these additional responsibilities for their behavioral health and careers,” wrote Veronica Yank, MD, of the department of medicine at the University of California, San Francisco, and her colleagues.
“To reduce burnout and improve workforce retention, health care systems should develop new approaches to identify and address the needs of these physician mothers,” they wrote.
The researchers used data from a June-July 2016 online survey of respondents from the Physicians Moms Group online community. Approximately 16,059 members saw the posting for the survey, and 5,613 United States–based mothers participated.
Among the questions was one on non–work related caregiving responsibilities that asked whether the respondent provided “regular care or assistance to a friend or family member with a serious health problem, long-term illness or disability” during the last year. Other questions assessed alcohol and drug use, history of a mood or anxiety disorder, career satisfaction and burnout.
Among the 16.4% of respondents who had additional caregiving responsibilities outside of work for someone chronically or seriously ill or disabled, nearly half (48.3%) said they cared for ill parents, 16.9% for children or infants, 7.7% for a partner, and 28.6% for another relative. In addition, 16.7% of respondents had such caregiving responsibilities for more than one person.
The women with these extra caregiving responsibilities were 21% more likely to have a mood or anxiety disorder (adjusted relative risk, 1.21; P = .02) and 25% more likely to report burnout (aRR, 1.25; P = .007), compared with those who did not have such extra responsibilities.
There were no significant differences, however, on rates of career satisfaction, risky drinking behaviors, or substance abuse between physician mothers who did have additional caregiving responsibilities and those who did not.
Among the study’s limitations were its cross-sectional nature, use of a convenience sample that may not be generalizable or representative, and lack of data on fathers or non-parent physicians for comparison.
SOURCE: Yank V et al. JAMA Intern Med. 2019 Jan 28. doi: 10.1001/jamainternmed.2018.6411.
Physicians who are also mothers have a higher risk of burnout and mood and anxiety disorders if they are also caring for someone with a serious illness or disability outside of work, according to a cross-sectional survey reported in a letter in JAMA Internal Medicine.
“Our findings highlight the additional caregiving responsibilities of some women physicians and the potential consequences of these additional responsibilities for their behavioral health and careers,” wrote Veronica Yank, MD, of the department of medicine at the University of California, San Francisco, and her colleagues.
“To reduce burnout and improve workforce retention, health care systems should develop new approaches to identify and address the needs of these physician mothers,” they wrote.
The researchers used data from a June-July 2016 online survey of respondents from the Physicians Moms Group online community. Approximately 16,059 members saw the posting for the survey, and 5,613 United States–based mothers participated.
Among the questions was one on non–work related caregiving responsibilities that asked whether the respondent provided “regular care or assistance to a friend or family member with a serious health problem, long-term illness or disability” during the last year. Other questions assessed alcohol and drug use, history of a mood or anxiety disorder, career satisfaction and burnout.
Among the 16.4% of respondents who had additional caregiving responsibilities outside of work for someone chronically or seriously ill or disabled, nearly half (48.3%) said they cared for ill parents, 16.9% for children or infants, 7.7% for a partner, and 28.6% for another relative. In addition, 16.7% of respondents had such caregiving responsibilities for more than one person.
The women with these extra caregiving responsibilities were 21% more likely to have a mood or anxiety disorder (adjusted relative risk, 1.21; P = .02) and 25% more likely to report burnout (aRR, 1.25; P = .007), compared with those who did not have such extra responsibilities.
There were no significant differences, however, on rates of career satisfaction, risky drinking behaviors, or substance abuse between physician mothers who did have additional caregiving responsibilities and those who did not.
Among the study’s limitations were its cross-sectional nature, use of a convenience sample that may not be generalizable or representative, and lack of data on fathers or non-parent physicians for comparison.
SOURCE: Yank V et al. JAMA Intern Med. 2019 Jan 28. doi: 10.1001/jamainternmed.2018.6411.
Physicians who are also mothers have a higher risk of burnout and mood and anxiety disorders if they are also caring for someone with a serious illness or disability outside of work, according to a cross-sectional survey reported in a letter in JAMA Internal Medicine.
“Our findings highlight the additional caregiving responsibilities of some women physicians and the potential consequences of these additional responsibilities for their behavioral health and careers,” wrote Veronica Yank, MD, of the department of medicine at the University of California, San Francisco, and her colleagues.
“To reduce burnout and improve workforce retention, health care systems should develop new approaches to identify and address the needs of these physician mothers,” they wrote.
The researchers used data from a June-July 2016 online survey of respondents from the Physicians Moms Group online community. Approximately 16,059 members saw the posting for the survey, and 5,613 United States–based mothers participated.
Among the questions was one on non–work related caregiving responsibilities that asked whether the respondent provided “regular care or assistance to a friend or family member with a serious health problem, long-term illness or disability” during the last year. Other questions assessed alcohol and drug use, history of a mood or anxiety disorder, career satisfaction and burnout.
Among the 16.4% of respondents who had additional caregiving responsibilities outside of work for someone chronically or seriously ill or disabled, nearly half (48.3%) said they cared for ill parents, 16.9% for children or infants, 7.7% for a partner, and 28.6% for another relative. In addition, 16.7% of respondents had such caregiving responsibilities for more than one person.
The women with these extra caregiving responsibilities were 21% more likely to have a mood or anxiety disorder (adjusted relative risk, 1.21; P = .02) and 25% more likely to report burnout (aRR, 1.25; P = .007), compared with those who did not have such extra responsibilities.
There were no significant differences, however, on rates of career satisfaction, risky drinking behaviors, or substance abuse between physician mothers who did have additional caregiving responsibilities and those who did not.
Among the study’s limitations were its cross-sectional nature, use of a convenience sample that may not be generalizable or representative, and lack of data on fathers or non-parent physicians for comparison.
SOURCE: Yank V et al. JAMA Intern Med. 2019 Jan 28. doi: 10.1001/jamainternmed.2018.6411.
FROM JAMA INTERNAL MEDICINE
Key clinical point:
Major finding: Risk of anxiety and mood disorders is 21% higher and burnout is 25% higher among physician mothers with extra caregiving at home.
Study details: The findings are based on an online cross-sectional survey of 5,613 United States–based physician mothers conducted from June to July 2016.
Disclosures: No single entity directly funded the study, but the authors were supported by a variety of grants from foundations and the National Institutes of Health at the time it was completed. One coauthor is founder of Equity Quotient, a company that provides gender equity culture analytics for institutions, and another has consulted for Amgen and Vizient and receives stock options as an Equity Quotient advisory board member.
Source: Yank V et al. JAMA Internal Medicine. 2018 Jan 28. doi: 10.1001/jamainternmed.2018.6411.
FDA permits marketing of first M. genitalium diagnostic test
the first test for the diagnoses of sexually transmitted infections (STIs) caused by the M. genitalium bacterium, the agency reported in a press release.
M. genitalium is associated with nongonococcal urethritis in men and cervicitis in women, causing 15%-30% of persistent or recurring urethritis cases and 10%-30% of cervicitis cases, according to the Centers for Disease Control and Prevention. It also can lead to pelvic inflammatory disease (PID) in women. The assay is a nucleic acid amplification test, which can detect the bacterium in urine, as well as urethral, penile meatal, endocervical, or vaginal swab samples.
In a clinical study of 11,774 samples, the Aptima assay correctly identified M. genitalium in about 90% of vaginal, male urethral, male urine, and penile samples. It also correctly identified the bacterium in female urine and endocervical samples 78% and 82% of the time, respectively. The test was even more accurate in identifying samples that did not have M. genitalium present, according to an FDA press release
“In the past, it has been hard to diagnose this organism. By being able to detect it more reliably, doctors may be able to more carefully tailor treatment and use medicines most likely to be effective,” FDA Commissioner Scott Gottlieb, MD, said in the press release. “Having accurate and reliable tests to identify the specific bacteria that’s causing an infection can assist doctors in choosing the right treatment for the right infection, which can reduce overuse of antibiotics and help in the fight against antimicrobial resistance.”
Find the full press release on the FDA website.
the first test for the diagnoses of sexually transmitted infections (STIs) caused by the M. genitalium bacterium, the agency reported in a press release.
M. genitalium is associated with nongonococcal urethritis in men and cervicitis in women, causing 15%-30% of persistent or recurring urethritis cases and 10%-30% of cervicitis cases, according to the Centers for Disease Control and Prevention. It also can lead to pelvic inflammatory disease (PID) in women. The assay is a nucleic acid amplification test, which can detect the bacterium in urine, as well as urethral, penile meatal, endocervical, or vaginal swab samples.
In a clinical study of 11,774 samples, the Aptima assay correctly identified M. genitalium in about 90% of vaginal, male urethral, male urine, and penile samples. It also correctly identified the bacterium in female urine and endocervical samples 78% and 82% of the time, respectively. The test was even more accurate in identifying samples that did not have M. genitalium present, according to an FDA press release
“In the past, it has been hard to diagnose this organism. By being able to detect it more reliably, doctors may be able to more carefully tailor treatment and use medicines most likely to be effective,” FDA Commissioner Scott Gottlieb, MD, said in the press release. “Having accurate and reliable tests to identify the specific bacteria that’s causing an infection can assist doctors in choosing the right treatment for the right infection, which can reduce overuse of antibiotics and help in the fight against antimicrobial resistance.”
Find the full press release on the FDA website.
the first test for the diagnoses of sexually transmitted infections (STIs) caused by the M. genitalium bacterium, the agency reported in a press release.
M. genitalium is associated with nongonococcal urethritis in men and cervicitis in women, causing 15%-30% of persistent or recurring urethritis cases and 10%-30% of cervicitis cases, according to the Centers for Disease Control and Prevention. It also can lead to pelvic inflammatory disease (PID) in women. The assay is a nucleic acid amplification test, which can detect the bacterium in urine, as well as urethral, penile meatal, endocervical, or vaginal swab samples.
In a clinical study of 11,774 samples, the Aptima assay correctly identified M. genitalium in about 90% of vaginal, male urethral, male urine, and penile samples. It also correctly identified the bacterium in female urine and endocervical samples 78% and 82% of the time, respectively. The test was even more accurate in identifying samples that did not have M. genitalium present, according to an FDA press release
“In the past, it has been hard to diagnose this organism. By being able to detect it more reliably, doctors may be able to more carefully tailor treatment and use medicines most likely to be effective,” FDA Commissioner Scott Gottlieb, MD, said in the press release. “Having accurate and reliable tests to identify the specific bacteria that’s causing an infection can assist doctors in choosing the right treatment for the right infection, which can reduce overuse of antibiotics and help in the fight against antimicrobial resistance.”
Find the full press release on the FDA website.
Migraine: Expanding our Tx arsenal
Migraine is a highly disabling primary headache disorder that affects more than 44 million Americans annually.1 The disorder causes pain, photophobia, phonophobia, and nausea that can last for hours, even days. Migraine headaches are 2 times more common in women than in men; although migraine is most common in people 30 to 39 years of age, all ages are affected.2,3 Frequency of migraine headache is variable; chronic migraineurs experience more than 15 headache days a month.
Recent estimates indicate that the cost of acute and chronic migraine headaches reaches approximately $78 million a year in the United States. 4 This high burden of disease has made effective migraine treatment options absolutely essential. Recent advances in our understanding of migraine pathophysiology have led to new therapeutic targets; there are now many novel treatment approaches on the horizon.
In this article, we review the diagnosis and management of migraine in detail. Our emphasis is on evidence-based approaches to acute and prophylactic treatment, including tried-and-true options and newly emerging therapies.
Neuronal dysfunction and a genetic predisposition
Although migraine was once thought to be caused by abnormalities of vasodilation, current research suggests that the disorder has its origins in primary neuronal dysfunction. There appears to be a genetic predisposition toward widespread neuronal hyperexcitability in migraineurs.5 In addition, hypothalamic neurons are thought to initiate migraine by responding to changes in brain homeostasis. Increased parasympathetic tone might activate meningeal pain receptors or lower the threshold for transmitting pain signals from the thalamus to the cortex.6
Prodromal symptoms and aura appear to originate from multiple areas across the brain, including the hypothalamus, cortex, limbic system, and brainstem. This widespread brain involvement might explain why some headache sufferers concurrently experience a variety of symptoms, including fatigue, depression, muscle pain, and an abnormal sensitivity to light, sound, and smell.6,7
Although the exact mechanisms behind each of these symptoms have yet to be defined precisely, waves of neuronal depolarization—known as cortical spreading depression—are suspected to cause migraine aura.8-10 Cortical spreading depression activates the trigeminal pain pathway and leads to the release of pro-inflammatory markers such as calcitonin gene-related protein (CGRP).6 A better understanding of these complex signaling pathways has helped provide potential therapeutic targets for new migraine drugs.
Diagnosis: Close patient inquiry is most helpful
The International Headache Society (IHS) criteria for primary headache disorders serve as the basis for the diagnosis of migraine and its subtypes, which include migraine without aura and migraine with aura. Due to variability of presentation, migraine with aura is further subdivided into migraine with typical aura (with and without headache), migraine with brainstem aura, hemiplegic migraine, and retinal migraine.11
Continue to: How is migraine defined?
How is migraine defined? Simply, migraine is classically defined as a unilateral, pulsating headache of moderate to severe intensity lasting 4 to 72 hours, associated with photophobia and phonophobia or nausea and vomiting, or both.11 Often visual in nature, aura is a set of neurologic symptoms that lasts for minutes and precedes the onset of the headache. The visual aura is often described as a scintillating scotoma that begins near the point of visual fixation and then spreads left or right. Other aura symptoms include tingling or numbness (second most common), speech disturbance (aphasia), motor changes and, in rare cases, a combination of these in succession. By definition, all of these symptoms fully resolve between attacks.11
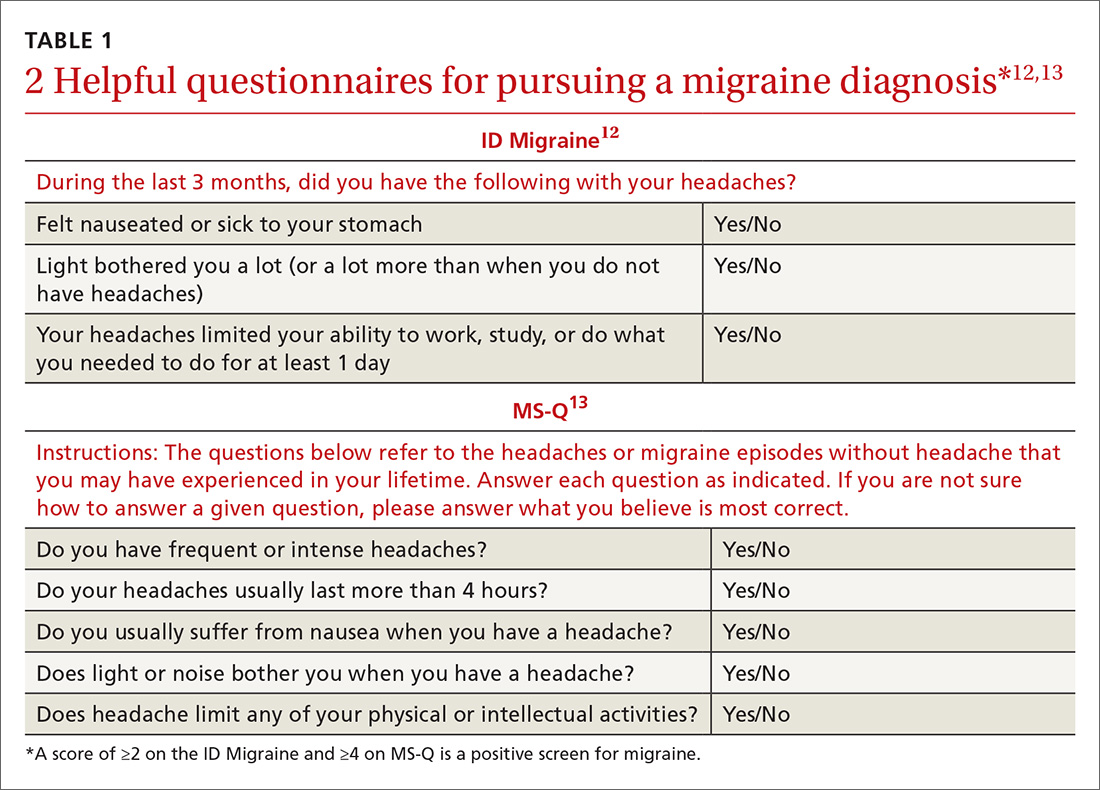
Validated valuable questionnaires. To help with accurate and timely diagnosis, researchers have developed and validated simplified questionnaires that can be completed independently by patients presenting to primary care (TABLE 112,13):
- ID Migraine is a set of 3 questions that scores positive when a patient endorses at least 2 of the 3 symptoms. 12
- MS-Q is similar to the ID Migraine but includes 5 items. A score of ≥4 is a positive screen. 13
The sensitivity and specificity of MS-Q (0.93 and 0.81, respectively) are slightly higher than those of ID Migraine (0.81 and 0.75).13
Remember POUND. This mnemonic device can also be used during history-taking to aid in diagnostic accuracy. Migraine is highly likely (92%) in patients who endorse 4 of the following 5 symptoms and unlikely (17%) in those who endorse ≤2 symptoms14: Pulsatile quality of headache 4 to 72 hOurs in duration, Unilateral location, Nausea or vomiting, and Disabling intensity.
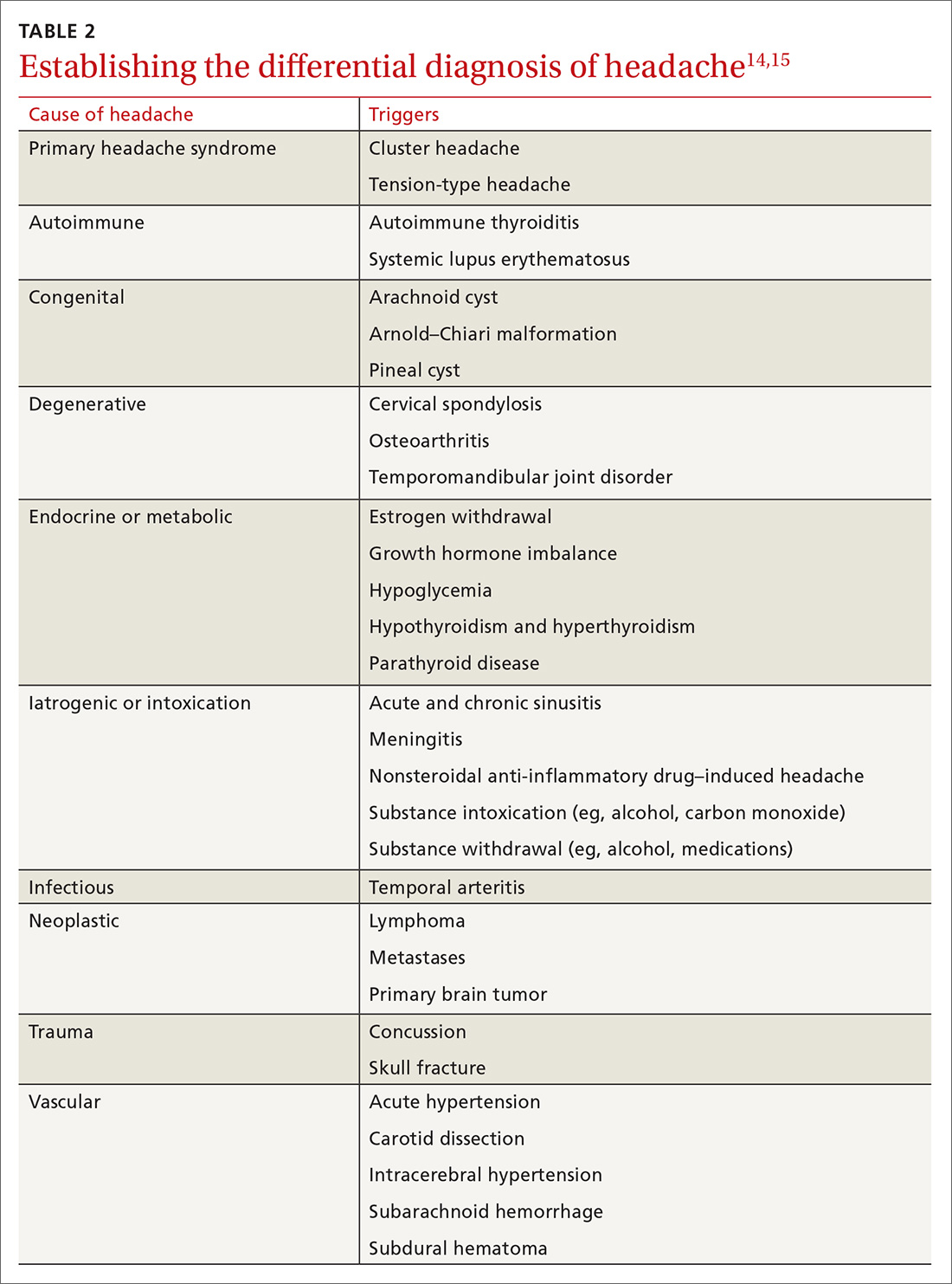
Differential Dx. Although the differential diagnosis of headache is broad (TABLE 214,15), the history alone can often guide clinicians towards the correct assessment. After taking the initial history (headache onset, location, duration, and associated symptoms), focus your attention on assessing the risk of intracranial pathology. This is best accomplished by assessing specific details of the history (TABLE 314) and findings on physical examination15:
- blood pressure measurement (seated, legs uncrossed, feet flat on the floor; having rested for 5 minutes; arm well supported)
- cranial nerve exam
- extremity strength testing
- eye exam (vision, extra-ocular muscles, visual fields, pupillary reactivity, and funduscopic exam)
- gait (tandem walk)
- reflexes.
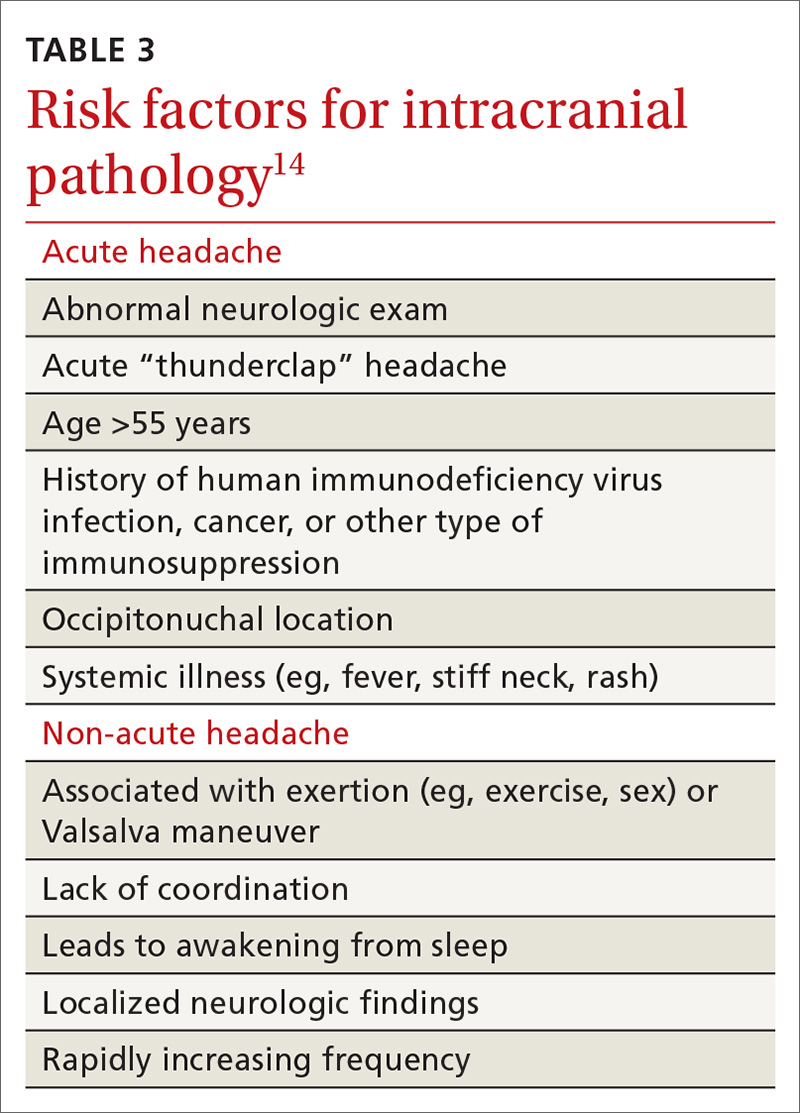
Continue to: Further testing needed?
Further testing needed? Neuroimaging should be considered only in patients with an abnormal neurologic exam, atypical headache features, or certain risk factors, such as an immune deficiency. There is no role for electroencephalography or other diagnostic testing in migraine.16
Take a multipronged approach to treatment
As with other complex, chronic conditions, the treatment of migraine should take a multifaceted approach, including management of acute symptoms as well as prevention of future headaches. In 2015, the American Headache Society published a systematic review that specified particular treatment goals for migraine sufferers. 17 These goals include:
- headache reduction
- headache relief
- decreased disability from headache
- elimination of nausea and vomiting
- elimination of photophobia and phonophobia.
Our review, which follows, of therapeutic options focuses on the management of migraine in adults. Approaches in special populations (older adults, pregnant women, and children) are discussed afterward.
Pharmacotherapy for acute migraine
Acute migraine should be treated with an abortive medication at the onset of headache. The immediate goal is to relieve pain within 2 hours and prevent its recurrence within the subsequent 48 hours (TABLE 412,18-20).
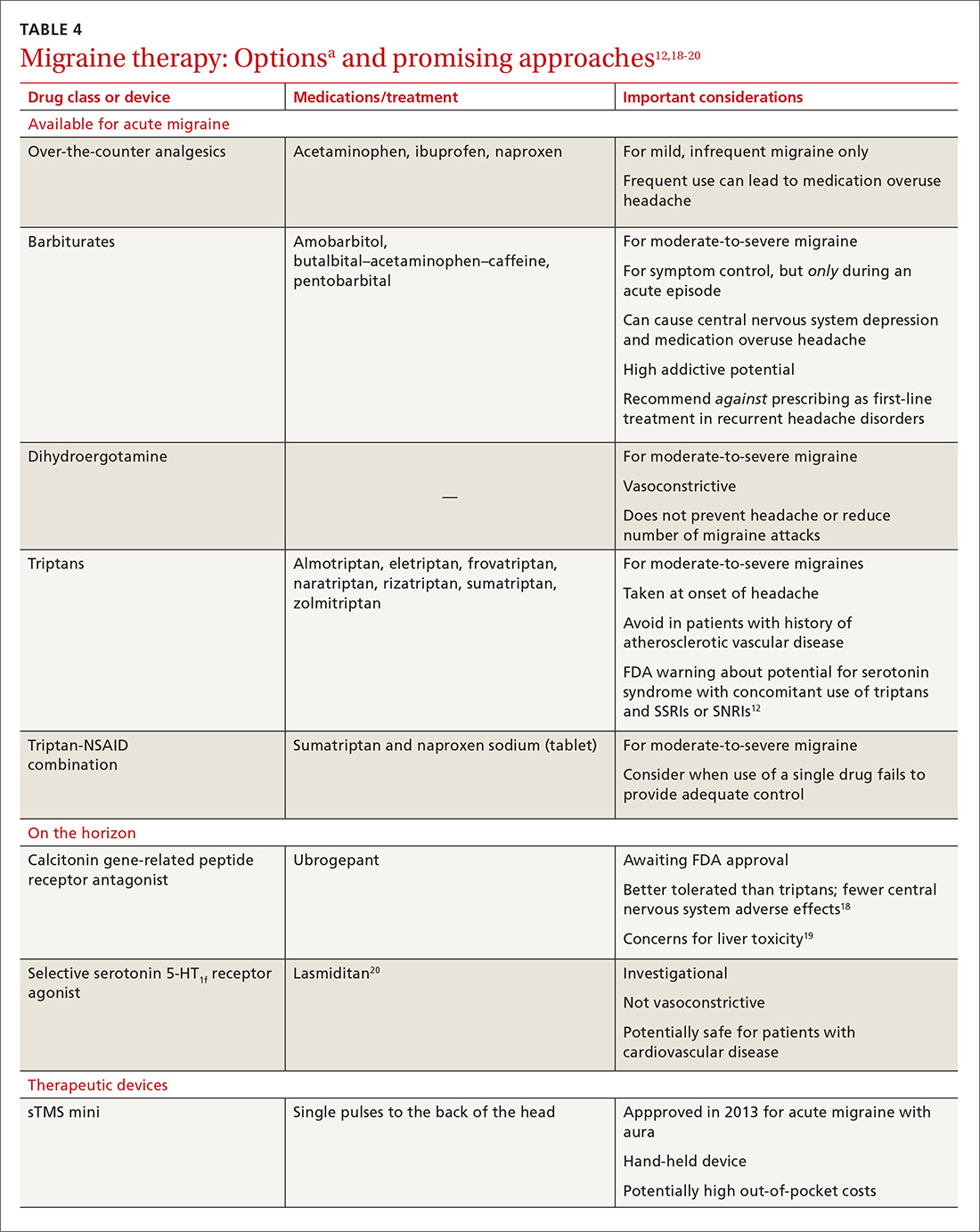
In the general population, mild, infrequent migraines can be managed with acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs).21
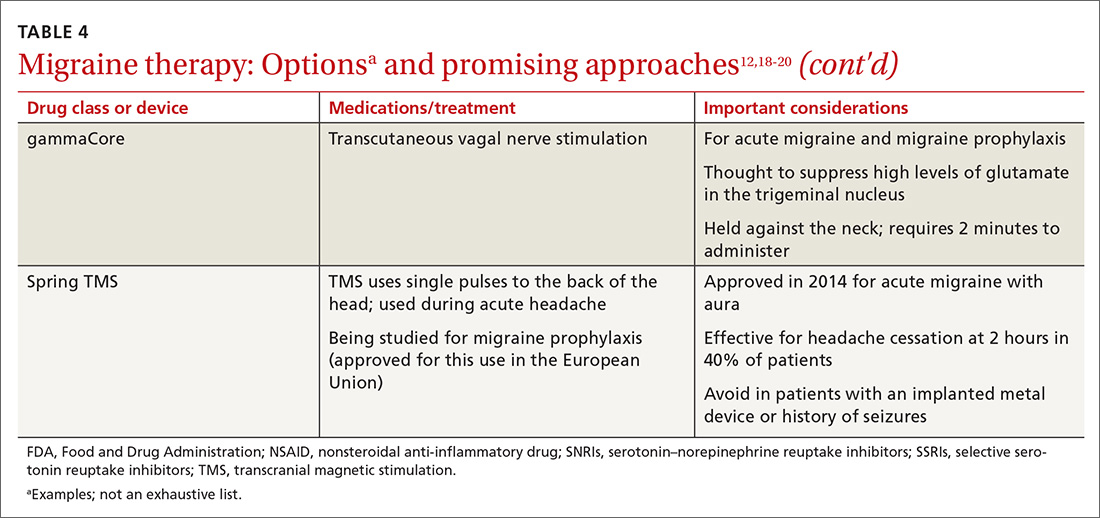
Continue to: For moderate-to-severe migraine...
For moderate-to-severe migraine, triptans, which target serotonin receptors, are the drug of choice for most patients.21 Triptans are superior to placebo in achieving a pain-free state at 2 and 24 hours after administration; eletriptan has the most desirable outcome, with 68% of patients pain free at 2 hours and 54% pain free at 24 hours.22 Triptans are available as sublingual tablets and nasal sprays, as well as subcutaneous injections for patients with significant associated nausea and vomiting. Avoid prescribing triptans for patients with known vascular disease (eg, history of stroke, myocardial infarction, peripheral vascular disease, uncontrolled hypertension, or signs and symptoms of these conditions), as well as for patients with severe hepatic impairment.
Importantly, although triptans all have a similar mechanism of action, patients might respond differently to different drugs within the class. If a patient does not get adequate headache relief from an appropriate dosage of a given triptan during a particular migraine episode, a different triptan can be tried during the next migraine.22 Additionally, if a patient experiences an adverse effect from one triptan, this does not necessarily mean that a trial of another triptan at a later time is contraindicated.
For patients who have an incomplete response to migraine treatment or for those with frequent recurrence, the combination formulation of sumatriptan, 85 mg, and naproxen, 500 mg, showed the highest rate of resolution of headache within 2 hours compared with either drug alone.23 A similar result might be found by combining a triptan known to be effective for a patient and an NSAID other than naproxen. If migraine persists despite initial treatment of an attack, a different class of medication should be tried during the course of that attack to attain relief of symptoms of that migraine.21
When a patient is seen in an acute care setting (eg, emergency department, urgent care center) while suffering a migraine, additional treatment options are available. Intravenous (IV) anti-emetics are useful for relieving the pain of migraine and nausea, and can be used in combination with an IV NSAID (eg, ketorolac).21 The most effective anti-emetics are dopamine receptor type-2 blockers, including chlorpromazine, droperidol, metoclopramide, and prochlorperazine, which has the highest level of efficacy.24 Note that these medications do present the risk of a dystonic reaction; diphenhydramine is therefore often used in tandem to mitigate such a response.
Looking ahead. Although triptans are the current first-line therapy for acute migraine, their effectiveness is limited. Only 20% of patients report sustained relief of pain in the 2 to 24 hours after treatment, and the response can vary from episode to episode.25
Continue to: With better understading of the pathophysiology of migraine...
With better understanding of the pathophysiology of migraine, a host of novel anti-migraine drugs are on the horizon.
CGRP receptor antagonists. The neuropeptide CGRP, which mediates central and peripheral nervous system pain signaling, has been noted to be elevated during acute migraine attacks26; clinical trials are therefore underway to evaluate the safety and efficacy of CGRP receptor antagonists.18 These agents appear to be better tolerated than triptans, have fewer vascular and central nervous system adverse effects, and present less of a risk of medication overuse headache.18 Liver toxicity has been seen with some medications in this class and remains an important concern in their development.19
Phase 3 clinical trials for 1 drug in this class, ubrogepant, were completed in late 2017; full analysis of the data is not yet available. Primary outcomes being evaluated include relief of pain at 2 hours and relief from the most bothersome symptoms again at 2 hours.27
Selective serotonin-HT1f receptor agonists, such as lasmiditan, offer another potential approach. Although the exact mechanism of action of these agents is not entirely clear, clinical trials have supported their efficacy and safety.20 Importantly, ongoing trials are specifically targeting patients with known cardiovascular risk factors because they are most likely to benefit from the nonvasoconstrictive mechanism of action.28,29 Adverse effects reported primarily include dizziness, fatigue, and vertigo.
Strategies for managing recurrent episodic migraine
Because of the risk of medication overuse headache with acute treatment, daily preventive therapy for migraine is indicated for any patient with 30 :
- ≥6 headache days a month
- ≥4 headache days a month with some impairment
- ≥3 headache days a month with severe impairment.
Continue to: Treatment begins by having patients identify...
Treatment begins by having patients identify, and then avoid, migraine triggers (TABLE 5). This can be accomplished by having patients keep a headache diary, in which they can enter notations about personal and environmental situations that precede a headache.
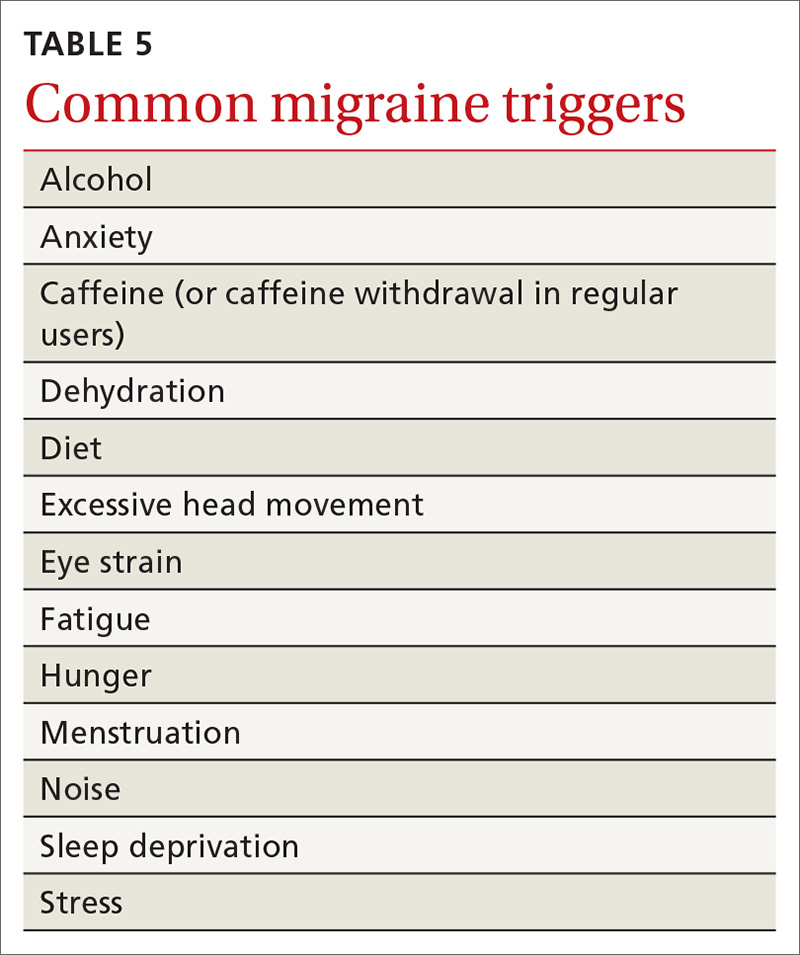
For the individual patient, some triggers are modifiable; others are not. Helping a patient develop strategies for coping with triggers, rather than aiming for complete avoidance, might help her (him) manage those that are inescapable (eg stress, menstruation, etc).31 For many patients, however, this is not an adequate intervention and other approaches must be explored. When considering which therapy might be best for a given patient, evaluate her (his) comorbidities and assess that particular treatment for potential secondary benefits and the possibility of adverse effects. Pay attention to the choice of preventive therapy in women who are considering pregnancy because many available treatments are potentially teratogenic.
Oral medications. Oral agents from several classes of drugs can be used for migraine prophylaxis, including anti-epileptics,antidepressants, and antihypertensives (TABLE 620,29,30,32-41). Selected anti-epileptics (divalproex sodium, sodium valproate, topiramate) and beta-blockers (metoprolol, propranolol, and timolol) have the strongest evidence to support their use.32 Overall, regular use of prophylactic medications can reduce headache frequency by 50% for approximately 40% to 45% of patients who take them.29 However, adherence may be limited by adverse effects or perceived lack of efficacy, thus reducing their potential for benefit.42
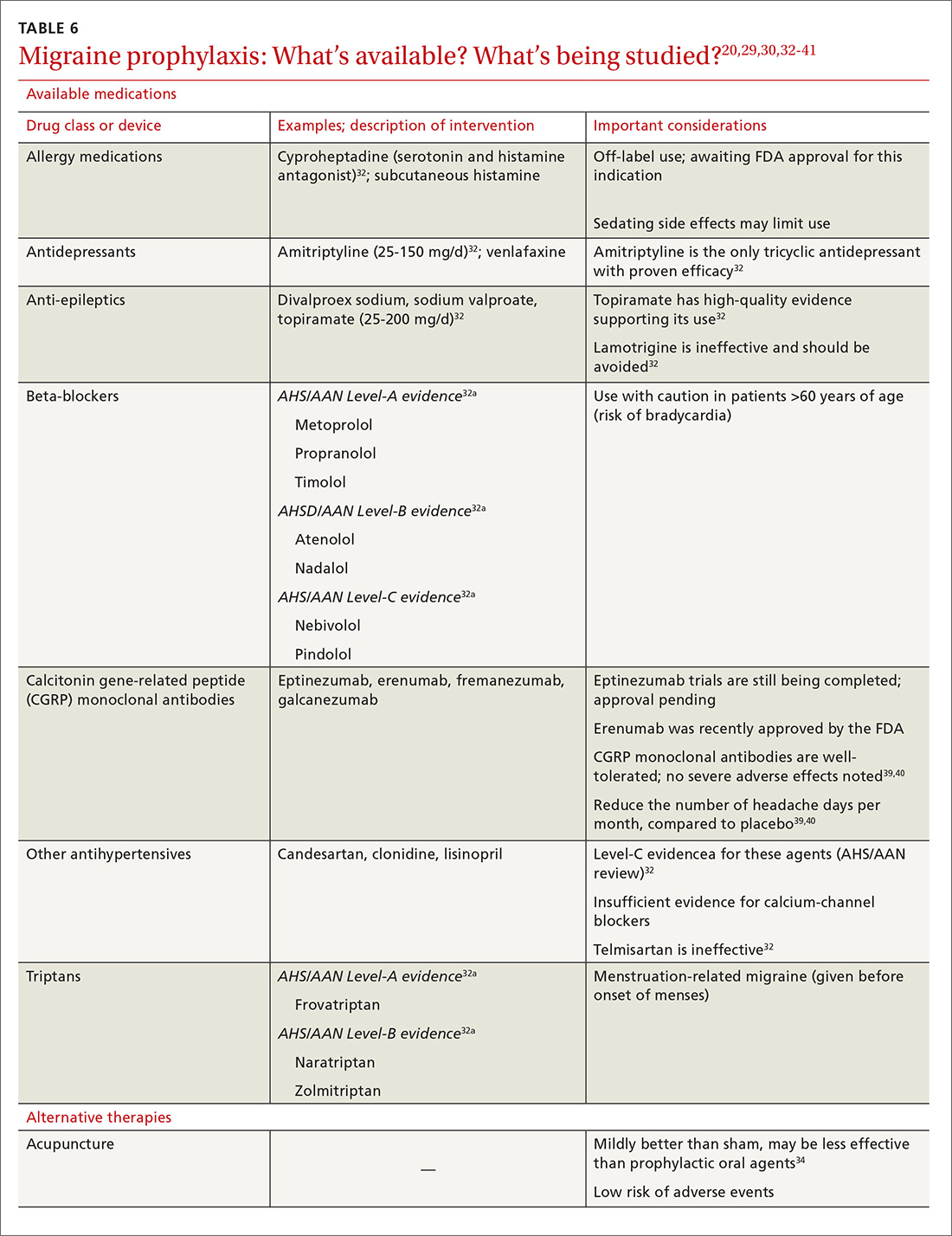
OnabotulinumtoxinA. In patients with chronic migraine (≥15 headache days a month for at least 3 months) who have failed oral medications, the American Academy of Neurology (AAN) recommends the use of onabotulinumtoxinA.30 The treatment regimen comprises 31 injections at various sites on the head, neck, and shoulders every 3 months.33
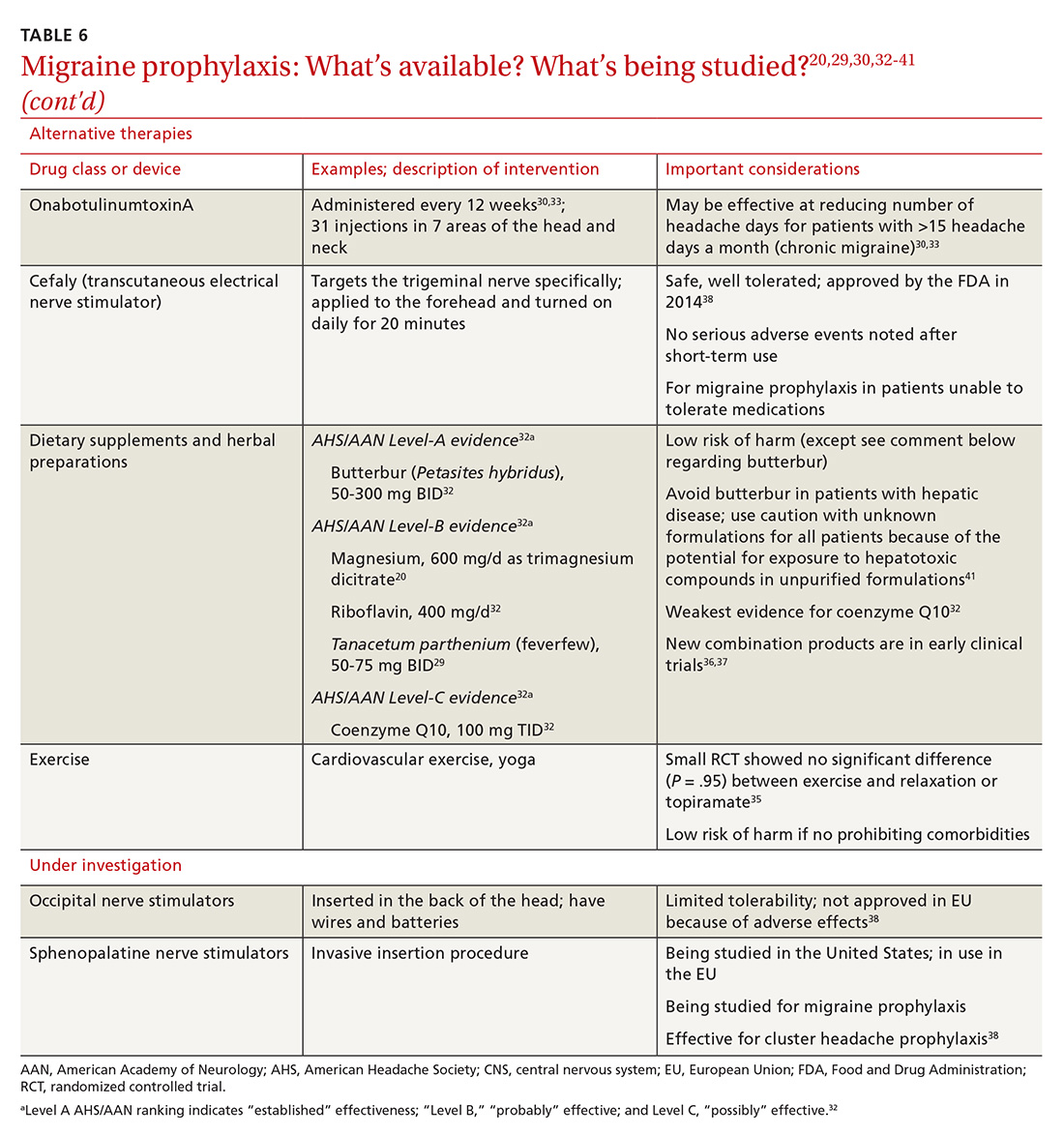
A 2010 large randomized controlled trial showed a decrease in the frequency of headache days for patients receiving onabotulinumtoxinA compared to placebo after a 24-week treatment period (7.8 fewer headache days a month, compared to 6.4 fewer in the placebo group).33 A recent systematic review also noted a reduction of 2 headache days a month compared with placebo; the authors cautioned, however, that data with which to evaluate onabotulinumtoxinA in comparison to other prophylactic agents are limited.43
Continue to: In both studies...
In both studies, the risk of adverse drug events due to onabotulinumtoxinA was high and led to a significant rate of discontinuation.33,43 Despite this, onabotulinumtoxinA remains the only Food and Drug Administration (FDA)–approved treatment for chronic migraine, making it reasonable to consider for appropriate patients.
Acupuncture. A 2016 Cochrane review found benefit for patients using acupuncture compared with sham acupuncture.34 When acupuncture was compared with prophylactic agents such as beta-blockers, calcium-channel blockers, and anti-epileptics, however, there was no significant difference between the procedure and pharmacotherapy. Patients willing and able to try acupuncture might see a reduction in the overall number of headaches. Acupuncture has few adverse effects; however, long-term data are lacking.34
Exercise is not supported by robust data for its role as a prophylactic treatment. It is generally considered safe in most populations, however, and can be pursued with little out-of-pocket cost.35
Cognitive behavioral therapy (CBT). The AAN recommends CBT, relaxation therapy, and biofeedback therapy. Accessibility of these services remains limited for many patients, and cost can be prohibitive.16
Supplements used to help prevent migraine include the root of Petasites hybridus (butterbur), magnesium, vitamin B2 (riboflavin), Tanacetum parthenium (feverfew), and coenzyme Q10.16 Although the strength of evidence for these therapies is limited by small trials, their overall risk of adverse effects is low, and they might be easier for patients to obtain than acupuncture or CBT.
Continue to: Butterbur, in particular...
Butterbur, in particular, has been found to be beneficial for migraine prevention in 2 small placebo-controlled trials. In a randomized controlled study of 245 patients P hybridus, (specifically, the German formulation, Petadolex), 75 mg BID, reduced the frequency of migraine attack by 48% at 4 months, compared to placebo (number needed to treat, 5.3).44 No difference was found at lower dosages. The most common reported adverse effect was burping.
Regrettably, unpurified butterbur extract contains pyrrolizidine alkaloids, potentially hepatotoxic and carcinogenic compounds. Because of variations in purification in production facilities in the United States, butterbur supplements might not have all of these compounds removed—and so should be used with caution.41
Magnesium. Studies evaluating the use of magnesium have demonstrated varied results; differences in methods and dosing have limited broad application of findings. As with most supplements considered for prophylactic treatment, magnesium dosing is poorly understood, and bioavailability varies in its different forms. Oral supplementation can be given as magnesium dicitrate, 600 mg/d.45
Recently, products containing various combinations of feverfew, coenzyme Q10, riboflavin, magnesium, and other supplements have shown benefit in early clinical trials.36,37
Neural stimulation. Over the past few years, a variety of transcutaneous nerve stimulator devices have gained FDA approval for use in migraine prophylaxis. The long-term safety and efficacy of these devices is not yet well understood, but they appear to provide headache relief in the short term and decrease the frequency of headache.38 Use of the noninvasive stimulators is limited today by high cost and poor coverage by US health care insurers.
Continue to: Newly available medical therapy
Newly available medical therapy. The FDA recently approved erenumab, a fully human monoclonal antibody for prevention of migraine in adults. This is the first drug in the CGRP antagonist class to be approved for this indication. Trials of this once-monthly, self-injectable drug show promising results for patients whose migraines have been refractory to other therapies.
A recent large trial evaluated 955 adults with migraine, randomizing them to receive erenumab, 70 mg; erenumab, 140 mg; or placebo over 28 weeks.39 The groups receiving erenumab had a nearly 2-fold higher odds of having their migraine reduced by 50%, compared with placebo (number needed to treat with the 140-mg dose, 4.27). Similar numbers of participants from all groups discontinued the study.39 Phase 3 trials that are not yet formally published have produced similarly beneficial results.40,46 The FDA has listed injection site reaction and constipation as the most reported adverse effects.40
Three other anti-CGRP antibodies are likely to be approved in the near future: fremanezumab, galcanezumab, and eptinezumab.
The approach to migraine in special populations
Management of acute and chronic migraine in children, pregnant women, and older adults requires special attention: Treatment approaches are different than they are for adults 19 to 65 years of age.
Pediatric patients. Migraine is the most common acute and recurrent headache syndrome in children. Headaches differ from those of adult migraine as a result of variations in brain maturation, plasticity, and cognitive development.47 Migraine attacks are often of shorter duration in children, lasting 1 to 2 hours, but can still be preceded by visual aura.48 Just as with adults, imaging, electroencephalography, lumbar puncture, and routine labs should be considered only if a child has an abnormal neurological exam or other concerning features (TABLE 214,15).
Continue to: The general approach to migraine treatment...
The general approach to migraine treatment in the pediatric population includes education of the child and family about symptom management. Acetaminophen, NSAIDs, and triptans are approved for abortive therapy in children and should be used for acute headache relief in the same way that they are used in adults. Oral rizatriptan, the most well studied triptan in the pediatric population, is approved for use in children as young as 6 years49; the pediatric dosage is 5 mg/d for patients weighing 20 to 39 kg and 10 mg/d for patients weighing more than 40 kg (same as the adult dosage).
Oral almotriptan and zolmitriptan are also approved for use in children 12 to 17 years of age. Usual dosages are: almotriptan, 12.5 mg at onset, can repeat in 2 hours as needed (maximum dosage, 25 mg/d); and zolmitriptan, 2.5 mg at onset, can repeat in 2 hours as needed (maximum dosage, 10 mg/d).50
For children who are unable to swallow pills or who are vomiting, a non-oral route of administration is preferable. Rizatriptan is available as an orally disintegrating tablet. Zolmitriptan is available in a nasal spray at a dose of 5 mg for children 12 years and older. Sumatriptan is not approved for use in patients younger than 18 years; however, recent studies have shown that it might have good efficacy and tolerability.50
Daily prophylactic treatment for recurrent migraine in the pediatric population is an evolving subject; published guidelines do not exist. It is reasonable to consider treatment using the same guidelines as those in place for adults.51 Topiramate, 1 to 2 mg/kg/d, is the only therapy approved by the FDA for episodic migraine preventive therapy in adolescents.50
Notably, a nonpharmacotherapeutic approach may be more effective for pediatric prevention. In 2017, a large double-blind, placebo-controlled trial investigated the use of amitriptyline, topiramate, and placebo for the treatment of recurrent migraine in children 8 to 17 years of age. An interim analysis of the 328 children enrolled found no significant differences in reduction of headache frequency with treatment compared with placebo over a 24-week period; the trial was stopped early due to futility.52
Continue to: The study did show...
The study did show, however, that reducing migraine triggers provided a high level of benefit to study participants. Stress is one of the most common migraine triggers in children; lack of sleep, exposure to a warm climate, and exposure to video games are also notable triggers.53 CBT may augment the efficacy of standard migraine medications in the pediatric population and may help prevent recurrence of episodes.54
Pregnancy. The treatment of migraine is different in pregnant women than it is in nonpregnant adults because of a concern over adverse effects on fetal development. For acute headache treatment, first-line therapies include trigger avoidance and acetaminophen, 1000 mg (maximum dosage, 4000 mg/d).55 If this is ineffective, a 10-mg dose of metoclopramide, as often as every 6 hours (not an FDA-approved indication), can be considered. During the second trimester, NSAIDs can be considered second-line therapy.
Triptans—specifically, sumatriptan and rizatriptan—can also be considered if first-line therapies fail.56 Triptan-exposed pregnant women with migraine have a rate of congenital malformations, spontaneous abortions, and prematurity that is similar to what is seen in pregnant women with migraine who have not been exposed to triptans. However, when triptan-exposed women are compared with healthy, non-migraine-suffering women, the rate of spontaneous abortion appears to be increased in the triptan-exposed population.57
Ergotamine is contraindicated during pregnancy because of its potential to induce uterine contractions and vasospasm, which can be detrimental to the fetus.56Nonpharmacotherapeutic interventions such as heat, ice, massage, rest, and avoidance of triggers are as successful in the pregnant population as in the nonpregnant population. For migraine prevention, coenzyme Q10, vitamins B2 and B6 (pyridoxine), and oral magnesium can be considered. Feverfew and butterbur should be avoided because of concerns about fetal malformation and preterm labor.58
Older adults. Choosing appropriate migraine therapy for older adults requires special consideration because of changes in drug metabolism and risks associated with drug adverse effects. Additionally, few studies of migraine drugs have included large populations of adults older than 65 years; medications should therefore be prescribed cautiously in this population, with particular attention to drug–drug interactions.
Continue to: Just as for younger adults...
Just as for younger adults, mild symptoms can be managed effectively with acetaminophen. NSAIDs may be used as well, but carry increased risks of gastric bleeding and elevation in blood pressure.59 The use of triptans is acceptable for the appropriate patient, but should be avoided in patients with known vascular disease.60 Antiemetics present an increased risk of extrapyramidal adverse effects in the elderly and should be used with caution at the lowest effective dosage.59 Novel mechanisms of action make some of the newer agents potentially safer for use in older adults when treating acute migraine.
For migraine prevention in older adults, particular attention should be paid to reducing triggers and minimizing polypharmacy.
More and more, successful treatment is within reach
With many clinical trials evaluating novel drugs underway, and additional studies contributing to our understanding of nonpharmacotherapeutic approaches to migraine treatment, improved headache control may become increasingly common over the next few years.
CORRESPONDENCE
Kathryn McGrath, MD, Department of Family and Community Medicine, Thomas Jefferson University, 1015 Walnut St, Philadelphia PA 19107; [email protected].
1. Stokes M, Becker WJ, Lipton RB, et al. Cost of health care among patients with chronic and episodic migraine in Canada and the USA: results from the International Burden of Migraine Study (IBMS). Headache. 2011;51:1058-1077.
2. Smitherman TA, Burch R, Sheikh H, et al. The prevalence, impact, and treatment of migraine and severe headaches in the United States: a review of statistics from national surveillance studies. Headache. 2013;53:427-436.
3. Burch RC, Loder S, Loder E, et al. The prevalence and burden of migraine and severe headache in the United States: updated statistics from government health surveillance studies. Headache. 2015;55:21-34.
4. Gooch CL, Pracht E, Borenstein AR. The burden of neurological disease in the United States: a summary report and call to action. Ann Neurol. 2017;81:479-484.
5. Ferrari MD, Klever RR, Terwindt GM, et al. Migraine pathophysiology: lessons from mouse models and human genetics. Lancet Neurol. 2015;14:65-80.
6. Burstein R, Noseda R, Borsook D. Migraine: multiple processes, complex pathophysiology. J Neurosc. 2015;35:6619-6629.
7. Maniyar FH, Sprenger T, Monteith T, et al. Brain activations in the premonitory phase of nitroglycerin-triggered migraine attacks. Brain. 2013;137(Pt 1):232-241.
8. Cutrer FM, Sorensen AG, Weisskoff RM, et al. Perfusion‐weighted imaging defects during spontaneous migrainous aura. Ann Neurol. 1998;43:25-31.
9. Hadjikhani N, Sanchez Del Rio MS, Wu O, et al. Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci U S A. 2001;98:4687-4692.
10. Pietrobon D, Moskowitz MA. Pathophysiology of migraine. Ann Rev Physiol. 2013;75:365-391.
11. Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, (beta version). Cephalalgia. 2013;33:629-808.
12. Lipton RB, Dodick D, Sadovsky RE, et al; ID Migraine validation study. A self-administered screener for migraine in primary care: The ID Migraine™ validation study. Neurology. 2003;61:375-382.
13. Láinez MJ, Domínguez M, Rejas J, et al. Development and validation of the Migraine Screen Questionnaire (MS‐Q). Headache. 2005;45:1328-1338.
14. Detsky ME, McDonald DR, Baerlocher MO, et al. Does this patient with headache have a migraine or need neuroimaging? JAMA. 2006;296:1274-1283.
15. Becker WJ, Findlay T, Moga C, et al. Guideline for primary care management of headache in adults. Can Fam Physician. 2015;61:670-679.
16. Silberstein SD. Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;55:754-762.
17. Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: the American Headache Society evidence assessment of migraine pharmacotherapies. Headache. 2015;55:3-20.
18. Voss T, Lipton RB, Dodick DW, et al. A phase IIb randomized, double-blind, placebo-controlled trial of ubrogepant for the acute treatment of migraine. Cephalalgia. 2016;36:887-898.
19. Russo AF. Calcitonin gene-related peptide (CGRP): a new target for migraine. Annu Rev Pharmacol Toxicol. 2015;55:533-552.
20. Färkkilä M, Diener HC, Géraud G, et al; COL MIG-202 study group. Efficacy and tolerability of lasmiditan, an oral 5-HT(1F) receptor agonist, for the acute treatment of migraine: a phase 2 randomised, placebo-controlled, parallel-group, dose-ranging study. Lancet Neurol. 2012;11:405-413.
21. Pringsheim T, Davenport WJ, Marmura MJ, et al. How to apply the AHS evidence assessment of the acute treatment of migraine in adults to your patient with migraine. Headache. 2016;56:1194-1200.
22. Thorlund K, Mills EJ, Wu P, et al. Comparative efficacy of triptans for the abortive treatment of migraine: a multiple treatment comparison meta-analysis. Cephalalgia. 2014;34:258-267.
23. Law S, Derry S, Moore RA. Sumatriptan plus naproxen for acute migraine attacks in adults. Cochrane Database Syst Rev. 2013;(10):CD008541.
24. Orr SL, Aubé M, Becker WJ, et al. Canadian Headache Society systematic review and recommendations on the treatment of migraine pain in emergency settings. Cephalalgia. 2015;35:271-284.
25. Ferrari MD, Goadsby PJ, Roon KI, et al. Triptans (serotonin, 5‐HT1B/1D agonists) in migraine: detailed results and methods of a meta‐analysis of 53 trials. Cephalalgia. 2002;22:633-658.
26. Goadsby PJ, Edvinsson L. The trigeminovascular system and migraine: studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann Neurol. 1993;33:48-56.
27. A phase 3, multicenter, randomized, double-blind, placebo-controlled single attack study to evaluate the efficacy, safety, and tolerability of oral ubrogepant in the acute treatment of migraine. https://clinicaltrials.gov/ct2/show/study/NCT02828020. Accessed November 16, 2018.
28. Rubio-Beltrán E, Labastida-Ramírez A, Villalón CM, et al. Is selective 5-HT1F receptor agonism an entity apart from that of the triptans in antimigraine therapy? Pharmacol Ther. 2018;186:88-97.
29. Diener HC, Charles A, Goadsby PJ, et al. New therapeutic approaches for the prevention and treatment of migraine. Lancet Neurol. 2015;14:1010-1022.
30. Lipton RB, Silberstein SD. Episodic and chronic migraine headache: breaking down barriers to optimal treatment and prevention. Headache. 2015;55 Suppl 2:103-122.
31. Martin PR. Behavioral management of migraine headache triggers: learning to cope with triggers. Curr Pain Headache Rep. 2010;14:221-227.
32. Loder E, Burch R, Rizzoli P. The 2012 AHS/AAN guidelines for prevention of episodic migraine: a summary and comparison with other recent clinical practice guidelines. Headache. 2012;52:930-945.
33. Dodick DW, Turkel CC, DeGryse RE, et al; PREEMPT Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: pooled results from the double‐blind, randomized, placebo‐controlled phases of the PREEMPT clinical program. Headache. 2010;50:921-936.
34. Linde K, Allais G, Brinkhaus B, et al. Acupuncture for the prevention of episodic migraine. Cochrane Database Syst Rev. 2016(6):CD001218.
35. Varkey E, Cider Å, Carlsson J, et al. Exercise as migraine prophylaxis: a randomized study using relaxation and topiramate as controls. Cephalalgia. 2011;31:1428-1438.
36. Guilbot A, Bangratz M, Abdellah SA, et al. A combination of coenzyme Q10, feverfew and magnesium for migraine prophylaxis: a prospective observational study. BMC Complement Altern Med. 2017;17:433.
37. Dalla Volta G, Zavarize P, Ngonga G, et al. Combination of Tanacethum partenium, 5-hydrossitriptophan (5-Http) and magnesium in the prophylaxis of episodic migraine without aura (AURASTOP®) an observational study. Int J Neuro Brain Dis. 2017;4:1-4.
38. Puledda F, Goadsby PJ. An update on non‐pharmacological neuromodulation for the acute and preventive treatment of migraine. Headache. 2017;57:685-691.
39. Goadsby PJ, Reuter U, Hallström Y, et al. A controlled trial of erenumab for episodic migraine. N Engl J Med. 2017;377:2123-2132.
40. Reuter U. Efficacy and safety of erenumab in episodic migraine patients with 2-4 prior preventive treatment failures: Results from the Phase 3b LIBERTY study. Abstract 009, AAN 2018 Annual Meeting; April 24, 2018.
41. Diener HC, Freitag FG, Danesch U. Safety profile of a special butterbur extract from Petasites hybridus in migraine prevention with emphasis on the liver. Cephalalgia Reports. https://journals.sagepub.com/doi/10.1177/2515816318759304. 2018 May 2. Accessed December 15, 2018.
42. Kingston WS, Halker R. Determinants of suboptimal migraine diagnosis and treatment in the primary care setting. J Clin Outcomes Manag. 2017;24:319-324.
43. Herd CP, Tomlinson CL, Rick C, et al. Botulinum toxins for the prevention of migraine in adults. Cochrane Database of Syst Rev. 2018;6:CD011616.
44. Lipton RB, Göbel H, Einhäupl KM, et al. Petasites hybridus root (butterbur) is an effective preventive treatment for migraine. Neurology. 2004;63:2240-2244.
45. Von Luckner A, Riederer F. Magnesium in migraine prophylaxis—is there an evidence‐based rationale? A systematic review. Headache. 2018;58:199-209.
46. Tepper S, Ashina M, Reuter U, et al. Safety and efficacy of erenumab for preventive treatment of chronic migraine: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2017;16:425-434.
47. Sonal Sekhar M, Sasidharan S, Joseph S, et al. Migraine management: How do the adult and paediatric migraines differ? Saudi Pharm J. 2012;20:1-7.
48. Lewis DW. Pediatric migraine. In: Lewis DW. Clinician’s Manual on Treatment of Pediatric Migraine. London, UK: Springer Healthcare Ltd; 2010:15-26.
49. Ho TW, Pearlman E, Lewis D, et al. Efficacy and tolerability of rizatriptan in pediatric migraineurs: results from a randomized double-blind, placebo controlled trial using a novel adaptive enrichment design. Cephalagia. 2012;32:750-765.
50. Khrizman M, Pakalnis A. Management of pediatric migraine: current therapies. Pediatr Ann. 2018;47:e55-e60.
51. Lipton RB, Bigal ME, Diamond M, et al; AMPP Advisory Group. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343-349.
52. Powers SW, Coffey CS, Chamberlin LA, et al; CHAMP Investigators. Trial of amitriptyline, topiramate, and placebo for pediatric migraine. N Engl J Med. 2017;376:115-124.
53. Neut D, Fily A, Cuvellier JC, et al. The prevalence of triggers in paediatric migraine: a questionnaire study in 102 children and adolescents. J Headache Pain. 2012;13:61-65.
54. Ng QX, Venkatanarayanan N, Kumar L. A systematic review and meta‐analysis of the efficacy of cognitive behavioral therapy for the management of pediatric migraine. Headache. s2017;57:349-362.
55. Lipton RB, Baggish JS, Stewart WF, et al. Efficacy and safety of acetaminophen in the treatment of migraine: results of a randomized, double-blind, placebo-controlled, population-based study. Arch Intern Med. 2000;160:3486-3492.
56. Lucas S. Medication use in the treatment of migraine during pregnancy and lactation. Curr Pain Headache Rep. 2009;13:392-398.
57. Marchenko A, Etwel F, Olutunfesse O, et al. Pregnancy outcome following prenatal exposure to triptan medications: a meta-analysis. Headache. 2015:55:490-501.
58. Wells RE, Turner DP, Lee M, et al. Managing migraine during pregnancy and lactation. Curr Neurol Neurosci Rep. 2016;16:40.
59. Haan J, Hollander J, Ferrari MD. Migraine in the elderly: a review. Cephalalgia. 2007;27:97-106.
60. Gladstone JP, Eross EJ, Dodick DW. Migraine in special populations. Treatment strategies for children and adolescents, pregnant women, and the elderly. Postgrad Med. 2004;115:39-44,47-50.
Migraine is a highly disabling primary headache disorder that affects more than 44 million Americans annually.1 The disorder causes pain, photophobia, phonophobia, and nausea that can last for hours, even days. Migraine headaches are 2 times more common in women than in men; although migraine is most common in people 30 to 39 years of age, all ages are affected.2,3 Frequency of migraine headache is variable; chronic migraineurs experience more than 15 headache days a month.

Recent estimates indicate that the cost of acute and chronic migraine headaches reaches approximately $78 million a year in the United States. 4 This high burden of disease has made effective migraine treatment options absolutely essential. Recent advances in our understanding of migraine pathophysiology have led to new therapeutic targets; there are now many novel treatment approaches on the horizon.
In this article, we review the diagnosis and management of migraine in detail. Our emphasis is on evidence-based approaches to acute and prophylactic treatment, including tried-and-true options and newly emerging therapies.
Neuronal dysfunction and a genetic predisposition
Although migraine was once thought to be caused by abnormalities of vasodilation, current research suggests that the disorder has its origins in primary neuronal dysfunction. There appears to be a genetic predisposition toward widespread neuronal hyperexcitability in migraineurs.5 In addition, hypothalamic neurons are thought to initiate migraine by responding to changes in brain homeostasis. Increased parasympathetic tone might activate meningeal pain receptors or lower the threshold for transmitting pain signals from the thalamus to the cortex.6
Prodromal symptoms and aura appear to originate from multiple areas across the brain, including the hypothalamus, cortex, limbic system, and brainstem. This widespread brain involvement might explain why some headache sufferers concurrently experience a variety of symptoms, including fatigue, depression, muscle pain, and an abnormal sensitivity to light, sound, and smell.6,7
Although the exact mechanisms behind each of these symptoms have yet to be defined precisely, waves of neuronal depolarization—known as cortical spreading depression—are suspected to cause migraine aura.8-10 Cortical spreading depression activates the trigeminal pain pathway and leads to the release of pro-inflammatory markers such as calcitonin gene-related protein (CGRP).6 A better understanding of these complex signaling pathways has helped provide potential therapeutic targets for new migraine drugs.
Diagnosis: Close patient inquiry is most helpful
The International Headache Society (IHS) criteria for primary headache disorders serve as the basis for the diagnosis of migraine and its subtypes, which include migraine without aura and migraine with aura. Due to variability of presentation, migraine with aura is further subdivided into migraine with typical aura (with and without headache), migraine with brainstem aura, hemiplegic migraine, and retinal migraine.11
Continue to: How is migraine defined?
How is migraine defined? Simply, migraine is classically defined as a unilateral, pulsating headache of moderate to severe intensity lasting 4 to 72 hours, associated with photophobia and phonophobia or nausea and vomiting, or both.11 Often visual in nature, aura is a set of neurologic symptoms that lasts for minutes and precedes the onset of the headache. The visual aura is often described as a scintillating scotoma that begins near the point of visual fixation and then spreads left or right. Other aura symptoms include tingling or numbness (second most common), speech disturbance (aphasia), motor changes and, in rare cases, a combination of these in succession. By definition, all of these symptoms fully resolve between attacks.11

Validated valuable questionnaires. To help with accurate and timely diagnosis, researchers have developed and validated simplified questionnaires that can be completed independently by patients presenting to primary care (TABLE 112,13):
- ID Migraine is a set of 3 questions that scores positive when a patient endorses at least 2 of the 3 symptoms. 12
- MS-Q is similar to the ID Migraine but includes 5 items. A score of ≥4 is a positive screen. 13
The sensitivity and specificity of MS-Q (0.93 and 0.81, respectively) are slightly higher than those of ID Migraine (0.81 and 0.75).13
Remember POUND. This mnemonic device can also be used during history-taking to aid in diagnostic accuracy. Migraine is highly likely (92%) in patients who endorse 4 of the following 5 symptoms and unlikely (17%) in those who endorse ≤2 symptoms14: Pulsatile quality of headache 4 to 72 hOurs in duration, Unilateral location, Nausea or vomiting, and Disabling intensity.

Differential Dx. Although the differential diagnosis of headache is broad (TABLE 214,15), the history alone can often guide clinicians towards the correct assessment. After taking the initial history (headache onset, location, duration, and associated symptoms), focus your attention on assessing the risk of intracranial pathology. This is best accomplished by assessing specific details of the history (TABLE 314) and findings on physical examination15:
- blood pressure measurement (seated, legs uncrossed, feet flat on the floor; having rested for 5 minutes; arm well supported)
- cranial nerve exam
- extremity strength testing
- eye exam (vision, extra-ocular muscles, visual fields, pupillary reactivity, and funduscopic exam)
- gait (tandem walk)
- reflexes.

Continue to: Further testing needed?
Further testing needed? Neuroimaging should be considered only in patients with an abnormal neurologic exam, atypical headache features, or certain risk factors, such as an immune deficiency. There is no role for electroencephalography or other diagnostic testing in migraine.16
Take a multipronged approach to treatment
As with other complex, chronic conditions, the treatment of migraine should take a multifaceted approach, including management of acute symptoms as well as prevention of future headaches. In 2015, the American Headache Society published a systematic review that specified particular treatment goals for migraine sufferers. 17 These goals include:
- headache reduction
- headache relief
- decreased disability from headache
- elimination of nausea and vomiting
- elimination of photophobia and phonophobia.
Our review, which follows, of therapeutic options focuses on the management of migraine in adults. Approaches in special populations (older adults, pregnant women, and children) are discussed afterward.
Pharmacotherapy for acute migraine
Acute migraine should be treated with an abortive medication at the onset of headache. The immediate goal is to relieve pain within 2 hours and prevent its recurrence within the subsequent 48 hours (TABLE 412,18-20).

In the general population, mild, infrequent migraines can be managed with acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs).21

Continue to: For moderate-to-severe migraine...
For moderate-to-severe migraine, triptans, which target serotonin receptors, are the drug of choice for most patients.21 Triptans are superior to placebo in achieving a pain-free state at 2 and 24 hours after administration; eletriptan has the most desirable outcome, with 68% of patients pain free at 2 hours and 54% pain free at 24 hours.22 Triptans are available as sublingual tablets and nasal sprays, as well as subcutaneous injections for patients with significant associated nausea and vomiting. Avoid prescribing triptans for patients with known vascular disease (eg, history of stroke, myocardial infarction, peripheral vascular disease, uncontrolled hypertension, or signs and symptoms of these conditions), as well as for patients with severe hepatic impairment.
Importantly, although triptans all have a similar mechanism of action, patients might respond differently to different drugs within the class. If a patient does not get adequate headache relief from an appropriate dosage of a given triptan during a particular migraine episode, a different triptan can be tried during the next migraine.22 Additionally, if a patient experiences an adverse effect from one triptan, this does not necessarily mean that a trial of another triptan at a later time is contraindicated.
For patients who have an incomplete response to migraine treatment or for those with frequent recurrence, the combination formulation of sumatriptan, 85 mg, and naproxen, 500 mg, showed the highest rate of resolution of headache within 2 hours compared with either drug alone.23 A similar result might be found by combining a triptan known to be effective for a patient and an NSAID other than naproxen. If migraine persists despite initial treatment of an attack, a different class of medication should be tried during the course of that attack to attain relief of symptoms of that migraine.21
When a patient is seen in an acute care setting (eg, emergency department, urgent care center) while suffering a migraine, additional treatment options are available. Intravenous (IV) anti-emetics are useful for relieving the pain of migraine and nausea, and can be used in combination with an IV NSAID (eg, ketorolac).21 The most effective anti-emetics are dopamine receptor type-2 blockers, including chlorpromazine, droperidol, metoclopramide, and prochlorperazine, which has the highest level of efficacy.24 Note that these medications do present the risk of a dystonic reaction; diphenhydramine is therefore often used in tandem to mitigate such a response.
Looking ahead. Although triptans are the current first-line therapy for acute migraine, their effectiveness is limited. Only 20% of patients report sustained relief of pain in the 2 to 24 hours after treatment, and the response can vary from episode to episode.25
Continue to: With better understading of the pathophysiology of migraine...
With better understanding of the pathophysiology of migraine, a host of novel anti-migraine drugs are on the horizon.
CGRP receptor antagonists. The neuropeptide CGRP, which mediates central and peripheral nervous system pain signaling, has been noted to be elevated during acute migraine attacks26; clinical trials are therefore underway to evaluate the safety and efficacy of CGRP receptor antagonists.18 These agents appear to be better tolerated than triptans, have fewer vascular and central nervous system adverse effects, and present less of a risk of medication overuse headache.18 Liver toxicity has been seen with some medications in this class and remains an important concern in their development.19
Phase 3 clinical trials for 1 drug in this class, ubrogepant, were completed in late 2017; full analysis of the data is not yet available. Primary outcomes being evaluated include relief of pain at 2 hours and relief from the most bothersome symptoms again at 2 hours.27
Selective serotonin-HT1f receptor agonists, such as lasmiditan, offer another potential approach. Although the exact mechanism of action of these agents is not entirely clear, clinical trials have supported their efficacy and safety.20 Importantly, ongoing trials are specifically targeting patients with known cardiovascular risk factors because they are most likely to benefit from the nonvasoconstrictive mechanism of action.28,29 Adverse effects reported primarily include dizziness, fatigue, and vertigo.
Strategies for managing recurrent episodic migraine
Because of the risk of medication overuse headache with acute treatment, daily preventive therapy for migraine is indicated for any patient with 30 :
- ≥6 headache days a month
- ≥4 headache days a month with some impairment
- ≥3 headache days a month with severe impairment.
Continue to: Treatment begins by having patients identify...
Treatment begins by having patients identify, and then avoid, migraine triggers (TABLE 5). This can be accomplished by having patients keep a headache diary, in which they can enter notations about personal and environmental situations that precede a headache.

For the individual patient, some triggers are modifiable; others are not. Helping a patient develop strategies for coping with triggers, rather than aiming for complete avoidance, might help her (him) manage those that are inescapable (eg stress, menstruation, etc).31 For many patients, however, this is not an adequate intervention and other approaches must be explored. When considering which therapy might be best for a given patient, evaluate her (his) comorbidities and assess that particular treatment for potential secondary benefits and the possibility of adverse effects. Pay attention to the choice of preventive therapy in women who are considering pregnancy because many available treatments are potentially teratogenic.
Oral medications. Oral agents from several classes of drugs can be used for migraine prophylaxis, including anti-epileptics,antidepressants, and antihypertensives (TABLE 620,29,30,32-41). Selected anti-epileptics (divalproex sodium, sodium valproate, topiramate) and beta-blockers (metoprolol, propranolol, and timolol) have the strongest evidence to support their use.32 Overall, regular use of prophylactic medications can reduce headache frequency by 50% for approximately 40% to 45% of patients who take them.29 However, adherence may be limited by adverse effects or perceived lack of efficacy, thus reducing their potential for benefit.42

OnabotulinumtoxinA. In patients with chronic migraine (≥15 headache days a month for at least 3 months) who have failed oral medications, the American Academy of Neurology (AAN) recommends the use of onabotulinumtoxinA.30 The treatment regimen comprises 31 injections at various sites on the head, neck, and shoulders every 3 months.33

A 2010 large randomized controlled trial showed a decrease in the frequency of headache days for patients receiving onabotulinumtoxinA compared to placebo after a 24-week treatment period (7.8 fewer headache days a month, compared to 6.4 fewer in the placebo group).33 A recent systematic review also noted a reduction of 2 headache days a month compared with placebo; the authors cautioned, however, that data with which to evaluate onabotulinumtoxinA in comparison to other prophylactic agents are limited.43
Continue to: In both studies...
In both studies, the risk of adverse drug events due to onabotulinumtoxinA was high and led to a significant rate of discontinuation.33,43 Despite this, onabotulinumtoxinA remains the only Food and Drug Administration (FDA)–approved treatment for chronic migraine, making it reasonable to consider for appropriate patients.
Acupuncture. A 2016 Cochrane review found benefit for patients using acupuncture compared with sham acupuncture.34 When acupuncture was compared with prophylactic agents such as beta-blockers, calcium-channel blockers, and anti-epileptics, however, there was no significant difference between the procedure and pharmacotherapy. Patients willing and able to try acupuncture might see a reduction in the overall number of headaches. Acupuncture has few adverse effects; however, long-term data are lacking.34
Exercise is not supported by robust data for its role as a prophylactic treatment. It is generally considered safe in most populations, however, and can be pursued with little out-of-pocket cost.35
Cognitive behavioral therapy (CBT). The AAN recommends CBT, relaxation therapy, and biofeedback therapy. Accessibility of these services remains limited for many patients, and cost can be prohibitive.16
Supplements used to help prevent migraine include the root of Petasites hybridus (butterbur), magnesium, vitamin B2 (riboflavin), Tanacetum parthenium (feverfew), and coenzyme Q10.16 Although the strength of evidence for these therapies is limited by small trials, their overall risk of adverse effects is low, and they might be easier for patients to obtain than acupuncture or CBT.
Continue to: Butterbur, in particular...
Butterbur, in particular, has been found to be beneficial for migraine prevention in 2 small placebo-controlled trials. In a randomized controlled study of 245 patients P hybridus, (specifically, the German formulation, Petadolex), 75 mg BID, reduced the frequency of migraine attack by 48% at 4 months, compared to placebo (number needed to treat, 5.3).44 No difference was found at lower dosages. The most common reported adverse effect was burping.
Regrettably, unpurified butterbur extract contains pyrrolizidine alkaloids, potentially hepatotoxic and carcinogenic compounds. Because of variations in purification in production facilities in the United States, butterbur supplements might not have all of these compounds removed—and so should be used with caution.41
Magnesium. Studies evaluating the use of magnesium have demonstrated varied results; differences in methods and dosing have limited broad application of findings. As with most supplements considered for prophylactic treatment, magnesium dosing is poorly understood, and bioavailability varies in its different forms. Oral supplementation can be given as magnesium dicitrate, 600 mg/d.45
Recently, products containing various combinations of feverfew, coenzyme Q10, riboflavin, magnesium, and other supplements have shown benefit in early clinical trials.36,37
Neural stimulation. Over the past few years, a variety of transcutaneous nerve stimulator devices have gained FDA approval for use in migraine prophylaxis. The long-term safety and efficacy of these devices is not yet well understood, but they appear to provide headache relief in the short term and decrease the frequency of headache.38 Use of the noninvasive stimulators is limited today by high cost and poor coverage by US health care insurers.
Continue to: Newly available medical therapy
Newly available medical therapy. The FDA recently approved erenumab, a fully human monoclonal antibody for prevention of migraine in adults. This is the first drug in the CGRP antagonist class to be approved for this indication. Trials of this once-monthly, self-injectable drug show promising results for patients whose migraines have been refractory to other therapies.
A recent large trial evaluated 955 adults with migraine, randomizing them to receive erenumab, 70 mg; erenumab, 140 mg; or placebo over 28 weeks.39 The groups receiving erenumab had a nearly 2-fold higher odds of having their migraine reduced by 50%, compared with placebo (number needed to treat with the 140-mg dose, 4.27). Similar numbers of participants from all groups discontinued the study.39 Phase 3 trials that are not yet formally published have produced similarly beneficial results.40,46 The FDA has listed injection site reaction and constipation as the most reported adverse effects.40
Three other anti-CGRP antibodies are likely to be approved in the near future: fremanezumab, galcanezumab, and eptinezumab.
The approach to migraine in special populations
Management of acute and chronic migraine in children, pregnant women, and older adults requires special attention: Treatment approaches are different than they are for adults 19 to 65 years of age.
Pediatric patients. Migraine is the most common acute and recurrent headache syndrome in children. Headaches differ from those of adult migraine as a result of variations in brain maturation, plasticity, and cognitive development.47 Migraine attacks are often of shorter duration in children, lasting 1 to 2 hours, but can still be preceded by visual aura.48 Just as with adults, imaging, electroencephalography, lumbar puncture, and routine labs should be considered only if a child has an abnormal neurological exam or other concerning features (TABLE 214,15).
Continue to: The general approach to migraine treatment...
The general approach to migraine treatment in the pediatric population includes education of the child and family about symptom management. Acetaminophen, NSAIDs, and triptans are approved for abortive therapy in children and should be used for acute headache relief in the same way that they are used in adults. Oral rizatriptan, the most well studied triptan in the pediatric population, is approved for use in children as young as 6 years49; the pediatric dosage is 5 mg/d for patients weighing 20 to 39 kg and 10 mg/d for patients weighing more than 40 kg (same as the adult dosage).
Oral almotriptan and zolmitriptan are also approved for use in children 12 to 17 years of age. Usual dosages are: almotriptan, 12.5 mg at onset, can repeat in 2 hours as needed (maximum dosage, 25 mg/d); and zolmitriptan, 2.5 mg at onset, can repeat in 2 hours as needed (maximum dosage, 10 mg/d).50
For children who are unable to swallow pills or who are vomiting, a non-oral route of administration is preferable. Rizatriptan is available as an orally disintegrating tablet. Zolmitriptan is available in a nasal spray at a dose of 5 mg for children 12 years and older. Sumatriptan is not approved for use in patients younger than 18 years; however, recent studies have shown that it might have good efficacy and tolerability.50
Daily prophylactic treatment for recurrent migraine in the pediatric population is an evolving subject; published guidelines do not exist. It is reasonable to consider treatment using the same guidelines as those in place for adults.51 Topiramate, 1 to 2 mg/kg/d, is the only therapy approved by the FDA for episodic migraine preventive therapy in adolescents.50
Notably, a nonpharmacotherapeutic approach may be more effective for pediatric prevention. In 2017, a large double-blind, placebo-controlled trial investigated the use of amitriptyline, topiramate, and placebo for the treatment of recurrent migraine in children 8 to 17 years of age. An interim analysis of the 328 children enrolled found no significant differences in reduction of headache frequency with treatment compared with placebo over a 24-week period; the trial was stopped early due to futility.52
Continue to: The study did show...
The study did show, however, that reducing migraine triggers provided a high level of benefit to study participants. Stress is one of the most common migraine triggers in children; lack of sleep, exposure to a warm climate, and exposure to video games are also notable triggers.53 CBT may augment the efficacy of standard migraine medications in the pediatric population and may help prevent recurrence of episodes.54
Pregnancy. The treatment of migraine is different in pregnant women than it is in nonpregnant adults because of a concern over adverse effects on fetal development. For acute headache treatment, first-line therapies include trigger avoidance and acetaminophen, 1000 mg (maximum dosage, 4000 mg/d).55 If this is ineffective, a 10-mg dose of metoclopramide, as often as every 6 hours (not an FDA-approved indication), can be considered. During the second trimester, NSAIDs can be considered second-line therapy.
Triptans—specifically, sumatriptan and rizatriptan—can also be considered if first-line therapies fail.56 Triptan-exposed pregnant women with migraine have a rate of congenital malformations, spontaneous abortions, and prematurity that is similar to what is seen in pregnant women with migraine who have not been exposed to triptans. However, when triptan-exposed women are compared with healthy, non-migraine-suffering women, the rate of spontaneous abortion appears to be increased in the triptan-exposed population.57
Ergotamine is contraindicated during pregnancy because of its potential to induce uterine contractions and vasospasm, which can be detrimental to the fetus.56Nonpharmacotherapeutic interventions such as heat, ice, massage, rest, and avoidance of triggers are as successful in the pregnant population as in the nonpregnant population. For migraine prevention, coenzyme Q10, vitamins B2 and B6 (pyridoxine), and oral magnesium can be considered. Feverfew and butterbur should be avoided because of concerns about fetal malformation and preterm labor.58
Older adults. Choosing appropriate migraine therapy for older adults requires special consideration because of changes in drug metabolism and risks associated with drug adverse effects. Additionally, few studies of migraine drugs have included large populations of adults older than 65 years; medications should therefore be prescribed cautiously in this population, with particular attention to drug–drug interactions.
Continue to: Just as for younger adults...
Just as for younger adults, mild symptoms can be managed effectively with acetaminophen. NSAIDs may be used as well, but carry increased risks of gastric bleeding and elevation in blood pressure.59 The use of triptans is acceptable for the appropriate patient, but should be avoided in patients with known vascular disease.60 Antiemetics present an increased risk of extrapyramidal adverse effects in the elderly and should be used with caution at the lowest effective dosage.59 Novel mechanisms of action make some of the newer agents potentially safer for use in older adults when treating acute migraine.
For migraine prevention in older adults, particular attention should be paid to reducing triggers and minimizing polypharmacy.
More and more, successful treatment is within reach
With many clinical trials evaluating novel drugs underway, and additional studies contributing to our understanding of nonpharmacotherapeutic approaches to migraine treatment, improved headache control may become increasingly common over the next few years.
CORRESPONDENCE
Kathryn McGrath, MD, Department of Family and Community Medicine, Thomas Jefferson University, 1015 Walnut St, Philadelphia PA 19107; [email protected].
Migraine is a highly disabling primary headache disorder that affects more than 44 million Americans annually.1 The disorder causes pain, photophobia, phonophobia, and nausea that can last for hours, even days. Migraine headaches are 2 times more common in women than in men; although migraine is most common in people 30 to 39 years of age, all ages are affected.2,3 Frequency of migraine headache is variable; chronic migraineurs experience more than 15 headache days a month.

Recent estimates indicate that the cost of acute and chronic migraine headaches reaches approximately $78 million a year in the United States. 4 This high burden of disease has made effective migraine treatment options absolutely essential. Recent advances in our understanding of migraine pathophysiology have led to new therapeutic targets; there are now many novel treatment approaches on the horizon.
In this article, we review the diagnosis and management of migraine in detail. Our emphasis is on evidence-based approaches to acute and prophylactic treatment, including tried-and-true options and newly emerging therapies.
Neuronal dysfunction and a genetic predisposition
Although migraine was once thought to be caused by abnormalities of vasodilation, current research suggests that the disorder has its origins in primary neuronal dysfunction. There appears to be a genetic predisposition toward widespread neuronal hyperexcitability in migraineurs.5 In addition, hypothalamic neurons are thought to initiate migraine by responding to changes in brain homeostasis. Increased parasympathetic tone might activate meningeal pain receptors or lower the threshold for transmitting pain signals from the thalamus to the cortex.6
Prodromal symptoms and aura appear to originate from multiple areas across the brain, including the hypothalamus, cortex, limbic system, and brainstem. This widespread brain involvement might explain why some headache sufferers concurrently experience a variety of symptoms, including fatigue, depression, muscle pain, and an abnormal sensitivity to light, sound, and smell.6,7
Although the exact mechanisms behind each of these symptoms have yet to be defined precisely, waves of neuronal depolarization—known as cortical spreading depression—are suspected to cause migraine aura.8-10 Cortical spreading depression activates the trigeminal pain pathway and leads to the release of pro-inflammatory markers such as calcitonin gene-related protein (CGRP).6 A better understanding of these complex signaling pathways has helped provide potential therapeutic targets for new migraine drugs.
Diagnosis: Close patient inquiry is most helpful
The International Headache Society (IHS) criteria for primary headache disorders serve as the basis for the diagnosis of migraine and its subtypes, which include migraine without aura and migraine with aura. Due to variability of presentation, migraine with aura is further subdivided into migraine with typical aura (with and without headache), migraine with brainstem aura, hemiplegic migraine, and retinal migraine.11
Continue to: How is migraine defined?
How is migraine defined? Simply, migraine is classically defined as a unilateral, pulsating headache of moderate to severe intensity lasting 4 to 72 hours, associated with photophobia and phonophobia or nausea and vomiting, or both.11 Often visual in nature, aura is a set of neurologic symptoms that lasts for minutes and precedes the onset of the headache. The visual aura is often described as a scintillating scotoma that begins near the point of visual fixation and then spreads left or right. Other aura symptoms include tingling or numbness (second most common), speech disturbance (aphasia), motor changes and, in rare cases, a combination of these in succession. By definition, all of these symptoms fully resolve between attacks.11

Validated valuable questionnaires. To help with accurate and timely diagnosis, researchers have developed and validated simplified questionnaires that can be completed independently by patients presenting to primary care (TABLE 112,13):
- ID Migraine is a set of 3 questions that scores positive when a patient endorses at least 2 of the 3 symptoms. 12
- MS-Q is similar to the ID Migraine but includes 5 items. A score of ≥4 is a positive screen. 13
The sensitivity and specificity of MS-Q (0.93 and 0.81, respectively) are slightly higher than those of ID Migraine (0.81 and 0.75).13
Remember POUND. This mnemonic device can also be used during history-taking to aid in diagnostic accuracy. Migraine is highly likely (92%) in patients who endorse 4 of the following 5 symptoms and unlikely (17%) in those who endorse ≤2 symptoms14: Pulsatile quality of headache 4 to 72 hOurs in duration, Unilateral location, Nausea or vomiting, and Disabling intensity.

Differential Dx. Although the differential diagnosis of headache is broad (TABLE 214,15), the history alone can often guide clinicians towards the correct assessment. After taking the initial history (headache onset, location, duration, and associated symptoms), focus your attention on assessing the risk of intracranial pathology. This is best accomplished by assessing specific details of the history (TABLE 314) and findings on physical examination15:
- blood pressure measurement (seated, legs uncrossed, feet flat on the floor; having rested for 5 minutes; arm well supported)
- cranial nerve exam
- extremity strength testing
- eye exam (vision, extra-ocular muscles, visual fields, pupillary reactivity, and funduscopic exam)
- gait (tandem walk)
- reflexes.

Continue to: Further testing needed?
Further testing needed? Neuroimaging should be considered only in patients with an abnormal neurologic exam, atypical headache features, or certain risk factors, such as an immune deficiency. There is no role for electroencephalography or other diagnostic testing in migraine.16
Take a multipronged approach to treatment
As with other complex, chronic conditions, the treatment of migraine should take a multifaceted approach, including management of acute symptoms as well as prevention of future headaches. In 2015, the American Headache Society published a systematic review that specified particular treatment goals for migraine sufferers. 17 These goals include:
- headache reduction
- headache relief
- decreased disability from headache
- elimination of nausea and vomiting
- elimination of photophobia and phonophobia.
Our review, which follows, of therapeutic options focuses on the management of migraine in adults. Approaches in special populations (older adults, pregnant women, and children) are discussed afterward.
Pharmacotherapy for acute migraine
Acute migraine should be treated with an abortive medication at the onset of headache. The immediate goal is to relieve pain within 2 hours and prevent its recurrence within the subsequent 48 hours (TABLE 412,18-20).

In the general population, mild, infrequent migraines can be managed with acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs).21

Continue to: For moderate-to-severe migraine...
For moderate-to-severe migraine, triptans, which target serotonin receptors, are the drug of choice for most patients.21 Triptans are superior to placebo in achieving a pain-free state at 2 and 24 hours after administration; eletriptan has the most desirable outcome, with 68% of patients pain free at 2 hours and 54% pain free at 24 hours.22 Triptans are available as sublingual tablets and nasal sprays, as well as subcutaneous injections for patients with significant associated nausea and vomiting. Avoid prescribing triptans for patients with known vascular disease (eg, history of stroke, myocardial infarction, peripheral vascular disease, uncontrolled hypertension, or signs and symptoms of these conditions), as well as for patients with severe hepatic impairment.
Importantly, although triptans all have a similar mechanism of action, patients might respond differently to different drugs within the class. If a patient does not get adequate headache relief from an appropriate dosage of a given triptan during a particular migraine episode, a different triptan can be tried during the next migraine.22 Additionally, if a patient experiences an adverse effect from one triptan, this does not necessarily mean that a trial of another triptan at a later time is contraindicated.
For patients who have an incomplete response to migraine treatment or for those with frequent recurrence, the combination formulation of sumatriptan, 85 mg, and naproxen, 500 mg, showed the highest rate of resolution of headache within 2 hours compared with either drug alone.23 A similar result might be found by combining a triptan known to be effective for a patient and an NSAID other than naproxen. If migraine persists despite initial treatment of an attack, a different class of medication should be tried during the course of that attack to attain relief of symptoms of that migraine.21
When a patient is seen in an acute care setting (eg, emergency department, urgent care center) while suffering a migraine, additional treatment options are available. Intravenous (IV) anti-emetics are useful for relieving the pain of migraine and nausea, and can be used in combination with an IV NSAID (eg, ketorolac).21 The most effective anti-emetics are dopamine receptor type-2 blockers, including chlorpromazine, droperidol, metoclopramide, and prochlorperazine, which has the highest level of efficacy.24 Note that these medications do present the risk of a dystonic reaction; diphenhydramine is therefore often used in tandem to mitigate such a response.
Looking ahead. Although triptans are the current first-line therapy for acute migraine, their effectiveness is limited. Only 20% of patients report sustained relief of pain in the 2 to 24 hours after treatment, and the response can vary from episode to episode.25
Continue to: With better understading of the pathophysiology of migraine...
With better understanding of the pathophysiology of migraine, a host of novel anti-migraine drugs are on the horizon.
CGRP receptor antagonists. The neuropeptide CGRP, which mediates central and peripheral nervous system pain signaling, has been noted to be elevated during acute migraine attacks26; clinical trials are therefore underway to evaluate the safety and efficacy of CGRP receptor antagonists.18 These agents appear to be better tolerated than triptans, have fewer vascular and central nervous system adverse effects, and present less of a risk of medication overuse headache.18 Liver toxicity has been seen with some medications in this class and remains an important concern in their development.19
Phase 3 clinical trials for 1 drug in this class, ubrogepant, were completed in late 2017; full analysis of the data is not yet available. Primary outcomes being evaluated include relief of pain at 2 hours and relief from the most bothersome symptoms again at 2 hours.27
Selective serotonin-HT1f receptor agonists, such as lasmiditan, offer another potential approach. Although the exact mechanism of action of these agents is not entirely clear, clinical trials have supported their efficacy and safety.20 Importantly, ongoing trials are specifically targeting patients with known cardiovascular risk factors because they are most likely to benefit from the nonvasoconstrictive mechanism of action.28,29 Adverse effects reported primarily include dizziness, fatigue, and vertigo.
Strategies for managing recurrent episodic migraine
Because of the risk of medication overuse headache with acute treatment, daily preventive therapy for migraine is indicated for any patient with 30 :
- ≥6 headache days a month
- ≥4 headache days a month with some impairment
- ≥3 headache days a month with severe impairment.
Continue to: Treatment begins by having patients identify...
Treatment begins by having patients identify, and then avoid, migraine triggers (TABLE 5). This can be accomplished by having patients keep a headache diary, in which they can enter notations about personal and environmental situations that precede a headache.

For the individual patient, some triggers are modifiable; others are not. Helping a patient develop strategies for coping with triggers, rather than aiming for complete avoidance, might help her (him) manage those that are inescapable (eg stress, menstruation, etc).31 For many patients, however, this is not an adequate intervention and other approaches must be explored. When considering which therapy might be best for a given patient, evaluate her (his) comorbidities and assess that particular treatment for potential secondary benefits and the possibility of adverse effects. Pay attention to the choice of preventive therapy in women who are considering pregnancy because many available treatments are potentially teratogenic.
Oral medications. Oral agents from several classes of drugs can be used for migraine prophylaxis, including anti-epileptics,antidepressants, and antihypertensives (TABLE 620,29,30,32-41). Selected anti-epileptics (divalproex sodium, sodium valproate, topiramate) and beta-blockers (metoprolol, propranolol, and timolol) have the strongest evidence to support their use.32 Overall, regular use of prophylactic medications can reduce headache frequency by 50% for approximately 40% to 45% of patients who take them.29 However, adherence may be limited by adverse effects or perceived lack of efficacy, thus reducing their potential for benefit.42

OnabotulinumtoxinA. In patients with chronic migraine (≥15 headache days a month for at least 3 months) who have failed oral medications, the American Academy of Neurology (AAN) recommends the use of onabotulinumtoxinA.30 The treatment regimen comprises 31 injections at various sites on the head, neck, and shoulders every 3 months.33

A 2010 large randomized controlled trial showed a decrease in the frequency of headache days for patients receiving onabotulinumtoxinA compared to placebo after a 24-week treatment period (7.8 fewer headache days a month, compared to 6.4 fewer in the placebo group).33 A recent systematic review also noted a reduction of 2 headache days a month compared with placebo; the authors cautioned, however, that data with which to evaluate onabotulinumtoxinA in comparison to other prophylactic agents are limited.43
Continue to: In both studies...
In both studies, the risk of adverse drug events due to onabotulinumtoxinA was high and led to a significant rate of discontinuation.33,43 Despite this, onabotulinumtoxinA remains the only Food and Drug Administration (FDA)–approved treatment for chronic migraine, making it reasonable to consider for appropriate patients.
Acupuncture. A 2016 Cochrane review found benefit for patients using acupuncture compared with sham acupuncture.34 When acupuncture was compared with prophylactic agents such as beta-blockers, calcium-channel blockers, and anti-epileptics, however, there was no significant difference between the procedure and pharmacotherapy. Patients willing and able to try acupuncture might see a reduction in the overall number of headaches. Acupuncture has few adverse effects; however, long-term data are lacking.34
Exercise is not supported by robust data for its role as a prophylactic treatment. It is generally considered safe in most populations, however, and can be pursued with little out-of-pocket cost.35
Cognitive behavioral therapy (CBT). The AAN recommends CBT, relaxation therapy, and biofeedback therapy. Accessibility of these services remains limited for many patients, and cost can be prohibitive.16
Supplements used to help prevent migraine include the root of Petasites hybridus (butterbur), magnesium, vitamin B2 (riboflavin), Tanacetum parthenium (feverfew), and coenzyme Q10.16 Although the strength of evidence for these therapies is limited by small trials, their overall risk of adverse effects is low, and they might be easier for patients to obtain than acupuncture or CBT.
Continue to: Butterbur, in particular...
Butterbur, in particular, has been found to be beneficial for migraine prevention in 2 small placebo-controlled trials. In a randomized controlled study of 245 patients P hybridus, (specifically, the German formulation, Petadolex), 75 mg BID, reduced the frequency of migraine attack by 48% at 4 months, compared to placebo (number needed to treat, 5.3).44 No difference was found at lower dosages. The most common reported adverse effect was burping.
Regrettably, unpurified butterbur extract contains pyrrolizidine alkaloids, potentially hepatotoxic and carcinogenic compounds. Because of variations in purification in production facilities in the United States, butterbur supplements might not have all of these compounds removed—and so should be used with caution.41
Magnesium. Studies evaluating the use of magnesium have demonstrated varied results; differences in methods and dosing have limited broad application of findings. As with most supplements considered for prophylactic treatment, magnesium dosing is poorly understood, and bioavailability varies in its different forms. Oral supplementation can be given as magnesium dicitrate, 600 mg/d.45
Recently, products containing various combinations of feverfew, coenzyme Q10, riboflavin, magnesium, and other supplements have shown benefit in early clinical trials.36,37
Neural stimulation. Over the past few years, a variety of transcutaneous nerve stimulator devices have gained FDA approval for use in migraine prophylaxis. The long-term safety and efficacy of these devices is not yet well understood, but they appear to provide headache relief in the short term and decrease the frequency of headache.38 Use of the noninvasive stimulators is limited today by high cost and poor coverage by US health care insurers.
Continue to: Newly available medical therapy
Newly available medical therapy. The FDA recently approved erenumab, a fully human monoclonal antibody for prevention of migraine in adults. This is the first drug in the CGRP antagonist class to be approved for this indication. Trials of this once-monthly, self-injectable drug show promising results for patients whose migraines have been refractory to other therapies.
A recent large trial evaluated 955 adults with migraine, randomizing them to receive erenumab, 70 mg; erenumab, 140 mg; or placebo over 28 weeks.39 The groups receiving erenumab had a nearly 2-fold higher odds of having their migraine reduced by 50%, compared with placebo (number needed to treat with the 140-mg dose, 4.27). Similar numbers of participants from all groups discontinued the study.39 Phase 3 trials that are not yet formally published have produced similarly beneficial results.40,46 The FDA has listed injection site reaction and constipation as the most reported adverse effects.40
Three other anti-CGRP antibodies are likely to be approved in the near future: fremanezumab, galcanezumab, and eptinezumab.
The approach to migraine in special populations
Management of acute and chronic migraine in children, pregnant women, and older adults requires special attention: Treatment approaches are different than they are for adults 19 to 65 years of age.
Pediatric patients. Migraine is the most common acute and recurrent headache syndrome in children. Headaches differ from those of adult migraine as a result of variations in brain maturation, plasticity, and cognitive development.47 Migraine attacks are often of shorter duration in children, lasting 1 to 2 hours, but can still be preceded by visual aura.48 Just as with adults, imaging, electroencephalography, lumbar puncture, and routine labs should be considered only if a child has an abnormal neurological exam or other concerning features (TABLE 214,15).
Continue to: The general approach to migraine treatment...
The general approach to migraine treatment in the pediatric population includes education of the child and family about symptom management. Acetaminophen, NSAIDs, and triptans are approved for abortive therapy in children and should be used for acute headache relief in the same way that they are used in adults. Oral rizatriptan, the most well studied triptan in the pediatric population, is approved for use in children as young as 6 years49; the pediatric dosage is 5 mg/d for patients weighing 20 to 39 kg and 10 mg/d for patients weighing more than 40 kg (same as the adult dosage).
Oral almotriptan and zolmitriptan are also approved for use in children 12 to 17 years of age. Usual dosages are: almotriptan, 12.5 mg at onset, can repeat in 2 hours as needed (maximum dosage, 25 mg/d); and zolmitriptan, 2.5 mg at onset, can repeat in 2 hours as needed (maximum dosage, 10 mg/d).50
For children who are unable to swallow pills or who are vomiting, a non-oral route of administration is preferable. Rizatriptan is available as an orally disintegrating tablet. Zolmitriptan is available in a nasal spray at a dose of 5 mg for children 12 years and older. Sumatriptan is not approved for use in patients younger than 18 years; however, recent studies have shown that it might have good efficacy and tolerability.50
Daily prophylactic treatment for recurrent migraine in the pediatric population is an evolving subject; published guidelines do not exist. It is reasonable to consider treatment using the same guidelines as those in place for adults.51 Topiramate, 1 to 2 mg/kg/d, is the only therapy approved by the FDA for episodic migraine preventive therapy in adolescents.50
Notably, a nonpharmacotherapeutic approach may be more effective for pediatric prevention. In 2017, a large double-blind, placebo-controlled trial investigated the use of amitriptyline, topiramate, and placebo for the treatment of recurrent migraine in children 8 to 17 years of age. An interim analysis of the 328 children enrolled found no significant differences in reduction of headache frequency with treatment compared with placebo over a 24-week period; the trial was stopped early due to futility.52
Continue to: The study did show...
The study did show, however, that reducing migraine triggers provided a high level of benefit to study participants. Stress is one of the most common migraine triggers in children; lack of sleep, exposure to a warm climate, and exposure to video games are also notable triggers.53 CBT may augment the efficacy of standard migraine medications in the pediatric population and may help prevent recurrence of episodes.54
Pregnancy. The treatment of migraine is different in pregnant women than it is in nonpregnant adults because of a concern over adverse effects on fetal development. For acute headache treatment, first-line therapies include trigger avoidance and acetaminophen, 1000 mg (maximum dosage, 4000 mg/d).55 If this is ineffective, a 10-mg dose of metoclopramide, as often as every 6 hours (not an FDA-approved indication), can be considered. During the second trimester, NSAIDs can be considered second-line therapy.
Triptans—specifically, sumatriptan and rizatriptan—can also be considered if first-line therapies fail.56 Triptan-exposed pregnant women with migraine have a rate of congenital malformations, spontaneous abortions, and prematurity that is similar to what is seen in pregnant women with migraine who have not been exposed to triptans. However, when triptan-exposed women are compared with healthy, non-migraine-suffering women, the rate of spontaneous abortion appears to be increased in the triptan-exposed population.57
Ergotamine is contraindicated during pregnancy because of its potential to induce uterine contractions and vasospasm, which can be detrimental to the fetus.56Nonpharmacotherapeutic interventions such as heat, ice, massage, rest, and avoidance of triggers are as successful in the pregnant population as in the nonpregnant population. For migraine prevention, coenzyme Q10, vitamins B2 and B6 (pyridoxine), and oral magnesium can be considered. Feverfew and butterbur should be avoided because of concerns about fetal malformation and preterm labor.58
Older adults. Choosing appropriate migraine therapy for older adults requires special consideration because of changes in drug metabolism and risks associated with drug adverse effects. Additionally, few studies of migraine drugs have included large populations of adults older than 65 years; medications should therefore be prescribed cautiously in this population, with particular attention to drug–drug interactions.
Continue to: Just as for younger adults...
Just as for younger adults, mild symptoms can be managed effectively with acetaminophen. NSAIDs may be used as well, but carry increased risks of gastric bleeding and elevation in blood pressure.59 The use of triptans is acceptable for the appropriate patient, but should be avoided in patients with known vascular disease.60 Antiemetics present an increased risk of extrapyramidal adverse effects in the elderly and should be used with caution at the lowest effective dosage.59 Novel mechanisms of action make some of the newer agents potentially safer for use in older adults when treating acute migraine.
For migraine prevention in older adults, particular attention should be paid to reducing triggers and minimizing polypharmacy.
More and more, successful treatment is within reach
With many clinical trials evaluating novel drugs underway, and additional studies contributing to our understanding of nonpharmacotherapeutic approaches to migraine treatment, improved headache control may become increasingly common over the next few years.
CORRESPONDENCE
Kathryn McGrath, MD, Department of Family and Community Medicine, Thomas Jefferson University, 1015 Walnut St, Philadelphia PA 19107; [email protected].
1. Stokes M, Becker WJ, Lipton RB, et al. Cost of health care among patients with chronic and episodic migraine in Canada and the USA: results from the International Burden of Migraine Study (IBMS). Headache. 2011;51:1058-1077.
2. Smitherman TA, Burch R, Sheikh H, et al. The prevalence, impact, and treatment of migraine and severe headaches in the United States: a review of statistics from national surveillance studies. Headache. 2013;53:427-436.
3. Burch RC, Loder S, Loder E, et al. The prevalence and burden of migraine and severe headache in the United States: updated statistics from government health surveillance studies. Headache. 2015;55:21-34.
4. Gooch CL, Pracht E, Borenstein AR. The burden of neurological disease in the United States: a summary report and call to action. Ann Neurol. 2017;81:479-484.
5. Ferrari MD, Klever RR, Terwindt GM, et al. Migraine pathophysiology: lessons from mouse models and human genetics. Lancet Neurol. 2015;14:65-80.
6. Burstein R, Noseda R, Borsook D. Migraine: multiple processes, complex pathophysiology. J Neurosc. 2015;35:6619-6629.
7. Maniyar FH, Sprenger T, Monteith T, et al. Brain activations in the premonitory phase of nitroglycerin-triggered migraine attacks. Brain. 2013;137(Pt 1):232-241.
8. Cutrer FM, Sorensen AG, Weisskoff RM, et al. Perfusion‐weighted imaging defects during spontaneous migrainous aura. Ann Neurol. 1998;43:25-31.
9. Hadjikhani N, Sanchez Del Rio MS, Wu O, et al. Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci U S A. 2001;98:4687-4692.
10. Pietrobon D, Moskowitz MA. Pathophysiology of migraine. Ann Rev Physiol. 2013;75:365-391.
11. Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, (beta version). Cephalalgia. 2013;33:629-808.
12. Lipton RB, Dodick D, Sadovsky RE, et al; ID Migraine validation study. A self-administered screener for migraine in primary care: The ID Migraine™ validation study. Neurology. 2003;61:375-382.
13. Láinez MJ, Domínguez M, Rejas J, et al. Development and validation of the Migraine Screen Questionnaire (MS‐Q). Headache. 2005;45:1328-1338.
14. Detsky ME, McDonald DR, Baerlocher MO, et al. Does this patient with headache have a migraine or need neuroimaging? JAMA. 2006;296:1274-1283.
15. Becker WJ, Findlay T, Moga C, et al. Guideline for primary care management of headache in adults. Can Fam Physician. 2015;61:670-679.
16. Silberstein SD. Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;55:754-762.
17. Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: the American Headache Society evidence assessment of migraine pharmacotherapies. Headache. 2015;55:3-20.
18. Voss T, Lipton RB, Dodick DW, et al. A phase IIb randomized, double-blind, placebo-controlled trial of ubrogepant for the acute treatment of migraine. Cephalalgia. 2016;36:887-898.
19. Russo AF. Calcitonin gene-related peptide (CGRP): a new target for migraine. Annu Rev Pharmacol Toxicol. 2015;55:533-552.
20. Färkkilä M, Diener HC, Géraud G, et al; COL MIG-202 study group. Efficacy and tolerability of lasmiditan, an oral 5-HT(1F) receptor agonist, for the acute treatment of migraine: a phase 2 randomised, placebo-controlled, parallel-group, dose-ranging study. Lancet Neurol. 2012;11:405-413.
21. Pringsheim T, Davenport WJ, Marmura MJ, et al. How to apply the AHS evidence assessment of the acute treatment of migraine in adults to your patient with migraine. Headache. 2016;56:1194-1200.
22. Thorlund K, Mills EJ, Wu P, et al. Comparative efficacy of triptans for the abortive treatment of migraine: a multiple treatment comparison meta-analysis. Cephalalgia. 2014;34:258-267.
23. Law S, Derry S, Moore RA. Sumatriptan plus naproxen for acute migraine attacks in adults. Cochrane Database Syst Rev. 2013;(10):CD008541.
24. Orr SL, Aubé M, Becker WJ, et al. Canadian Headache Society systematic review and recommendations on the treatment of migraine pain in emergency settings. Cephalalgia. 2015;35:271-284.
25. Ferrari MD, Goadsby PJ, Roon KI, et al. Triptans (serotonin, 5‐HT1B/1D agonists) in migraine: detailed results and methods of a meta‐analysis of 53 trials. Cephalalgia. 2002;22:633-658.
26. Goadsby PJ, Edvinsson L. The trigeminovascular system and migraine: studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann Neurol. 1993;33:48-56.
27. A phase 3, multicenter, randomized, double-blind, placebo-controlled single attack study to evaluate the efficacy, safety, and tolerability of oral ubrogepant in the acute treatment of migraine. https://clinicaltrials.gov/ct2/show/study/NCT02828020. Accessed November 16, 2018.
28. Rubio-Beltrán E, Labastida-Ramírez A, Villalón CM, et al. Is selective 5-HT1F receptor agonism an entity apart from that of the triptans in antimigraine therapy? Pharmacol Ther. 2018;186:88-97.
29. Diener HC, Charles A, Goadsby PJ, et al. New therapeutic approaches for the prevention and treatment of migraine. Lancet Neurol. 2015;14:1010-1022.
30. Lipton RB, Silberstein SD. Episodic and chronic migraine headache: breaking down barriers to optimal treatment and prevention. Headache. 2015;55 Suppl 2:103-122.
31. Martin PR. Behavioral management of migraine headache triggers: learning to cope with triggers. Curr Pain Headache Rep. 2010;14:221-227.
32. Loder E, Burch R, Rizzoli P. The 2012 AHS/AAN guidelines for prevention of episodic migraine: a summary and comparison with other recent clinical practice guidelines. Headache. 2012;52:930-945.
33. Dodick DW, Turkel CC, DeGryse RE, et al; PREEMPT Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: pooled results from the double‐blind, randomized, placebo‐controlled phases of the PREEMPT clinical program. Headache. 2010;50:921-936.
34. Linde K, Allais G, Brinkhaus B, et al. Acupuncture for the prevention of episodic migraine. Cochrane Database Syst Rev. 2016(6):CD001218.
35. Varkey E, Cider Å, Carlsson J, et al. Exercise as migraine prophylaxis: a randomized study using relaxation and topiramate as controls. Cephalalgia. 2011;31:1428-1438.
36. Guilbot A, Bangratz M, Abdellah SA, et al. A combination of coenzyme Q10, feverfew and magnesium for migraine prophylaxis: a prospective observational study. BMC Complement Altern Med. 2017;17:433.
37. Dalla Volta G, Zavarize P, Ngonga G, et al. Combination of Tanacethum partenium, 5-hydrossitriptophan (5-Http) and magnesium in the prophylaxis of episodic migraine without aura (AURASTOP®) an observational study. Int J Neuro Brain Dis. 2017;4:1-4.
38. Puledda F, Goadsby PJ. An update on non‐pharmacological neuromodulation for the acute and preventive treatment of migraine. Headache. 2017;57:685-691.
39. Goadsby PJ, Reuter U, Hallström Y, et al. A controlled trial of erenumab for episodic migraine. N Engl J Med. 2017;377:2123-2132.
40. Reuter U. Efficacy and safety of erenumab in episodic migraine patients with 2-4 prior preventive treatment failures: Results from the Phase 3b LIBERTY study. Abstract 009, AAN 2018 Annual Meeting; April 24, 2018.
41. Diener HC, Freitag FG, Danesch U. Safety profile of a special butterbur extract from Petasites hybridus in migraine prevention with emphasis on the liver. Cephalalgia Reports. https://journals.sagepub.com/doi/10.1177/2515816318759304. 2018 May 2. Accessed December 15, 2018.
42. Kingston WS, Halker R. Determinants of suboptimal migraine diagnosis and treatment in the primary care setting. J Clin Outcomes Manag. 2017;24:319-324.
43. Herd CP, Tomlinson CL, Rick C, et al. Botulinum toxins for the prevention of migraine in adults. Cochrane Database of Syst Rev. 2018;6:CD011616.
44. Lipton RB, Göbel H, Einhäupl KM, et al. Petasites hybridus root (butterbur) is an effective preventive treatment for migraine. Neurology. 2004;63:2240-2244.
45. Von Luckner A, Riederer F. Magnesium in migraine prophylaxis—is there an evidence‐based rationale? A systematic review. Headache. 2018;58:199-209.
46. Tepper S, Ashina M, Reuter U, et al. Safety and efficacy of erenumab for preventive treatment of chronic migraine: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2017;16:425-434.
47. Sonal Sekhar M, Sasidharan S, Joseph S, et al. Migraine management: How do the adult and paediatric migraines differ? Saudi Pharm J. 2012;20:1-7.
48. Lewis DW. Pediatric migraine. In: Lewis DW. Clinician’s Manual on Treatment of Pediatric Migraine. London, UK: Springer Healthcare Ltd; 2010:15-26.
49. Ho TW, Pearlman E, Lewis D, et al. Efficacy and tolerability of rizatriptan in pediatric migraineurs: results from a randomized double-blind, placebo controlled trial using a novel adaptive enrichment design. Cephalagia. 2012;32:750-765.
50. Khrizman M, Pakalnis A. Management of pediatric migraine: current therapies. Pediatr Ann. 2018;47:e55-e60.
51. Lipton RB, Bigal ME, Diamond M, et al; AMPP Advisory Group. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343-349.
52. Powers SW, Coffey CS, Chamberlin LA, et al; CHAMP Investigators. Trial of amitriptyline, topiramate, and placebo for pediatric migraine. N Engl J Med. 2017;376:115-124.
53. Neut D, Fily A, Cuvellier JC, et al. The prevalence of triggers in paediatric migraine: a questionnaire study in 102 children and adolescents. J Headache Pain. 2012;13:61-65.
54. Ng QX, Venkatanarayanan N, Kumar L. A systematic review and meta‐analysis of the efficacy of cognitive behavioral therapy for the management of pediatric migraine. Headache. s2017;57:349-362.
55. Lipton RB, Baggish JS, Stewart WF, et al. Efficacy and safety of acetaminophen in the treatment of migraine: results of a randomized, double-blind, placebo-controlled, population-based study. Arch Intern Med. 2000;160:3486-3492.
56. Lucas S. Medication use in the treatment of migraine during pregnancy and lactation. Curr Pain Headache Rep. 2009;13:392-398.
57. Marchenko A, Etwel F, Olutunfesse O, et al. Pregnancy outcome following prenatal exposure to triptan medications: a meta-analysis. Headache. 2015:55:490-501.
58. Wells RE, Turner DP, Lee M, et al. Managing migraine during pregnancy and lactation. Curr Neurol Neurosci Rep. 2016;16:40.
59. Haan J, Hollander J, Ferrari MD. Migraine in the elderly: a review. Cephalalgia. 2007;27:97-106.
60. Gladstone JP, Eross EJ, Dodick DW. Migraine in special populations. Treatment strategies for children and adolescents, pregnant women, and the elderly. Postgrad Med. 2004;115:39-44,47-50.
1. Stokes M, Becker WJ, Lipton RB, et al. Cost of health care among patients with chronic and episodic migraine in Canada and the USA: results from the International Burden of Migraine Study (IBMS). Headache. 2011;51:1058-1077.
2. Smitherman TA, Burch R, Sheikh H, et al. The prevalence, impact, and treatment of migraine and severe headaches in the United States: a review of statistics from national surveillance studies. Headache. 2013;53:427-436.
3. Burch RC, Loder S, Loder E, et al. The prevalence and burden of migraine and severe headache in the United States: updated statistics from government health surveillance studies. Headache. 2015;55:21-34.
4. Gooch CL, Pracht E, Borenstein AR. The burden of neurological disease in the United States: a summary report and call to action. Ann Neurol. 2017;81:479-484.
5. Ferrari MD, Klever RR, Terwindt GM, et al. Migraine pathophysiology: lessons from mouse models and human genetics. Lancet Neurol. 2015;14:65-80.
6. Burstein R, Noseda R, Borsook D. Migraine: multiple processes, complex pathophysiology. J Neurosc. 2015;35:6619-6629.
7. Maniyar FH, Sprenger T, Monteith T, et al. Brain activations in the premonitory phase of nitroglycerin-triggered migraine attacks. Brain. 2013;137(Pt 1):232-241.
8. Cutrer FM, Sorensen AG, Weisskoff RM, et al. Perfusion‐weighted imaging defects during spontaneous migrainous aura. Ann Neurol. 1998;43:25-31.
9. Hadjikhani N, Sanchez Del Rio MS, Wu O, et al. Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci U S A. 2001;98:4687-4692.
10. Pietrobon D, Moskowitz MA. Pathophysiology of migraine. Ann Rev Physiol. 2013;75:365-391.
11. Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, (beta version). Cephalalgia. 2013;33:629-808.
12. Lipton RB, Dodick D, Sadovsky RE, et al; ID Migraine validation study. A self-administered screener for migraine in primary care: The ID Migraine™ validation study. Neurology. 2003;61:375-382.
13. Láinez MJ, Domínguez M, Rejas J, et al. Development and validation of the Migraine Screen Questionnaire (MS‐Q). Headache. 2005;45:1328-1338.
14. Detsky ME, McDonald DR, Baerlocher MO, et al. Does this patient with headache have a migraine or need neuroimaging? JAMA. 2006;296:1274-1283.
15. Becker WJ, Findlay T, Moga C, et al. Guideline for primary care management of headache in adults. Can Fam Physician. 2015;61:670-679.
16. Silberstein SD. Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;55:754-762.
17. Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: the American Headache Society evidence assessment of migraine pharmacotherapies. Headache. 2015;55:3-20.
18. Voss T, Lipton RB, Dodick DW, et al. A phase IIb randomized, double-blind, placebo-controlled trial of ubrogepant for the acute treatment of migraine. Cephalalgia. 2016;36:887-898.
19. Russo AF. Calcitonin gene-related peptide (CGRP): a new target for migraine. Annu Rev Pharmacol Toxicol. 2015;55:533-552.
20. Färkkilä M, Diener HC, Géraud G, et al; COL MIG-202 study group. Efficacy and tolerability of lasmiditan, an oral 5-HT(1F) receptor agonist, for the acute treatment of migraine: a phase 2 randomised, placebo-controlled, parallel-group, dose-ranging study. Lancet Neurol. 2012;11:405-413.
21. Pringsheim T, Davenport WJ, Marmura MJ, et al. How to apply the AHS evidence assessment of the acute treatment of migraine in adults to your patient with migraine. Headache. 2016;56:1194-1200.
22. Thorlund K, Mills EJ, Wu P, et al. Comparative efficacy of triptans for the abortive treatment of migraine: a multiple treatment comparison meta-analysis. Cephalalgia. 2014;34:258-267.
23. Law S, Derry S, Moore RA. Sumatriptan plus naproxen for acute migraine attacks in adults. Cochrane Database Syst Rev. 2013;(10):CD008541.
24. Orr SL, Aubé M, Becker WJ, et al. Canadian Headache Society systematic review and recommendations on the treatment of migraine pain in emergency settings. Cephalalgia. 2015;35:271-284.
25. Ferrari MD, Goadsby PJ, Roon KI, et al. Triptans (serotonin, 5‐HT1B/1D agonists) in migraine: detailed results and methods of a meta‐analysis of 53 trials. Cephalalgia. 2002;22:633-658.
26. Goadsby PJ, Edvinsson L. The trigeminovascular system and migraine: studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann Neurol. 1993;33:48-56.
27. A phase 3, multicenter, randomized, double-blind, placebo-controlled single attack study to evaluate the efficacy, safety, and tolerability of oral ubrogepant in the acute treatment of migraine. https://clinicaltrials.gov/ct2/show/study/NCT02828020. Accessed November 16, 2018.
28. Rubio-Beltrán E, Labastida-Ramírez A, Villalón CM, et al. Is selective 5-HT1F receptor agonism an entity apart from that of the triptans in antimigraine therapy? Pharmacol Ther. 2018;186:88-97.
29. Diener HC, Charles A, Goadsby PJ, et al. New therapeutic approaches for the prevention and treatment of migraine. Lancet Neurol. 2015;14:1010-1022.
30. Lipton RB, Silberstein SD. Episodic and chronic migraine headache: breaking down barriers to optimal treatment and prevention. Headache. 2015;55 Suppl 2:103-122.
31. Martin PR. Behavioral management of migraine headache triggers: learning to cope with triggers. Curr Pain Headache Rep. 2010;14:221-227.
32. Loder E, Burch R, Rizzoli P. The 2012 AHS/AAN guidelines for prevention of episodic migraine: a summary and comparison with other recent clinical practice guidelines. Headache. 2012;52:930-945.
33. Dodick DW, Turkel CC, DeGryse RE, et al; PREEMPT Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: pooled results from the double‐blind, randomized, placebo‐controlled phases of the PREEMPT clinical program. Headache. 2010;50:921-936.
34. Linde K, Allais G, Brinkhaus B, et al. Acupuncture for the prevention of episodic migraine. Cochrane Database Syst Rev. 2016(6):CD001218.
35. Varkey E, Cider Å, Carlsson J, et al. Exercise as migraine prophylaxis: a randomized study using relaxation and topiramate as controls. Cephalalgia. 2011;31:1428-1438.
36. Guilbot A, Bangratz M, Abdellah SA, et al. A combination of coenzyme Q10, feverfew and magnesium for migraine prophylaxis: a prospective observational study. BMC Complement Altern Med. 2017;17:433.
37. Dalla Volta G, Zavarize P, Ngonga G, et al. Combination of Tanacethum partenium, 5-hydrossitriptophan (5-Http) and magnesium in the prophylaxis of episodic migraine without aura (AURASTOP®) an observational study. Int J Neuro Brain Dis. 2017;4:1-4.
38. Puledda F, Goadsby PJ. An update on non‐pharmacological neuromodulation for the acute and preventive treatment of migraine. Headache. 2017;57:685-691.
39. Goadsby PJ, Reuter U, Hallström Y, et al. A controlled trial of erenumab for episodic migraine. N Engl J Med. 2017;377:2123-2132.
40. Reuter U. Efficacy and safety of erenumab in episodic migraine patients with 2-4 prior preventive treatment failures: Results from the Phase 3b LIBERTY study. Abstract 009, AAN 2018 Annual Meeting; April 24, 2018.
41. Diener HC, Freitag FG, Danesch U. Safety profile of a special butterbur extract from Petasites hybridus in migraine prevention with emphasis on the liver. Cephalalgia Reports. https://journals.sagepub.com/doi/10.1177/2515816318759304. 2018 May 2. Accessed December 15, 2018.
42. Kingston WS, Halker R. Determinants of suboptimal migraine diagnosis and treatment in the primary care setting. J Clin Outcomes Manag. 2017;24:319-324.
43. Herd CP, Tomlinson CL, Rick C, et al. Botulinum toxins for the prevention of migraine in adults. Cochrane Database of Syst Rev. 2018;6:CD011616.
44. Lipton RB, Göbel H, Einhäupl KM, et al. Petasites hybridus root (butterbur) is an effective preventive treatment for migraine. Neurology. 2004;63:2240-2244.
45. Von Luckner A, Riederer F. Magnesium in migraine prophylaxis—is there an evidence‐based rationale? A systematic review. Headache. 2018;58:199-209.
46. Tepper S, Ashina M, Reuter U, et al. Safety and efficacy of erenumab for preventive treatment of chronic migraine: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2017;16:425-434.
47. Sonal Sekhar M, Sasidharan S, Joseph S, et al. Migraine management: How do the adult and paediatric migraines differ? Saudi Pharm J. 2012;20:1-7.
48. Lewis DW. Pediatric migraine. In: Lewis DW. Clinician’s Manual on Treatment of Pediatric Migraine. London, UK: Springer Healthcare Ltd; 2010:15-26.
49. Ho TW, Pearlman E, Lewis D, et al. Efficacy and tolerability of rizatriptan in pediatric migraineurs: results from a randomized double-blind, placebo controlled trial using a novel adaptive enrichment design. Cephalagia. 2012;32:750-765.
50. Khrizman M, Pakalnis A. Management of pediatric migraine: current therapies. Pediatr Ann. 2018;47:e55-e60.
51. Lipton RB, Bigal ME, Diamond M, et al; AMPP Advisory Group. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343-349.
52. Powers SW, Coffey CS, Chamberlin LA, et al; CHAMP Investigators. Trial of amitriptyline, topiramate, and placebo for pediatric migraine. N Engl J Med. 2017;376:115-124.
53. Neut D, Fily A, Cuvellier JC, et al. The prevalence of triggers in paediatric migraine: a questionnaire study in 102 children and adolescents. J Headache Pain. 2012;13:61-65.
54. Ng QX, Venkatanarayanan N, Kumar L. A systematic review and meta‐analysis of the efficacy of cognitive behavioral therapy for the management of pediatric migraine. Headache. s2017;57:349-362.
55. Lipton RB, Baggish JS, Stewart WF, et al. Efficacy and safety of acetaminophen in the treatment of migraine: results of a randomized, double-blind, placebo-controlled, population-based study. Arch Intern Med. 2000;160:3486-3492.
56. Lucas S. Medication use in the treatment of migraine during pregnancy and lactation. Curr Pain Headache Rep. 2009;13:392-398.
57. Marchenko A, Etwel F, Olutunfesse O, et al. Pregnancy outcome following prenatal exposure to triptan medications: a meta-analysis. Headache. 2015:55:490-501.
58. Wells RE, Turner DP, Lee M, et al. Managing migraine during pregnancy and lactation. Curr Neurol Neurosci Rep. 2016;16:40.
59. Haan J, Hollander J, Ferrari MD. Migraine in the elderly: a review. Cephalalgia. 2007;27:97-106.
60. Gladstone JP, Eross EJ, Dodick DW. Migraine in special populations. Treatment strategies for children and adolescents, pregnant women, and the elderly. Postgrad Med. 2004;115:39-44,47-50.
PRACTICE RECOMMENDATIONS
› Offer treatment with a triptan to adult patients with moderate-to-severe episodic migraine. A
› Consider prescribing topiramate, divalproex sodium, metoprolol, propranolol, or the herbal, Petasites hybridum, for the prevention of recurrent episodic migraine that has not responded to a reduction in headache triggers. A
› Add onabotulinumtoxinA injection to your therapeutic toolbox as an effective preventive treatment for chronic migraine (≥15 headache days a month for 3 months). B
› Recommend magnesium and feverfew as adjunctive preventive treatments for migraine. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Women with RA have reduced chance of live birth after assisted reproduction treatment
Women with rheumatoid arthritis who undergo assisted reproduction treatment have a decreased likelihood of live births versus women without rheumatoid arthritis, according to authors of a recent Denmark-wide cohort study.
The problem might be caused by an impaired chance of embryo implantation, authors of the study reported in the Annals of the Rheumatic Diseases.
Corticosteroids prescribed before embryo transfer might have improved the likelihood of live birth in these women with rheumatoid arthritis, according to the investigators, led by Professor Bente Mertz Nørgård of the center for clinical epidemiology at the Odense (Denmark) University Hospital.
However, findings with regard to that potential effect of corticosteroids were “not unambiguous,” said Prof. Nørgård and her coauthors said in their report.
The cohort study, based on Danish registry data from 1994-2017, included 1,149 embryo transfers in women and rheumatoid arthritis with 198,941 embryo transfers in women without rheumatoid arthritis.
Live births per embryo transfer were less likely in women with rheumatoid arthritis versus those they were in women with no rheumatoid arthritis, with an adjusted odds ratio of 0.78 (95% confidence interval, 0.65-0.92), according to investigators.
Chances of biochemical and clinical pregnancies were also lower in women with rheumatoid arthritis, with odds ratios of 0.81 (95% CI, 0.68-0.95) and 0.82 (95% CI, 0.59-1.15), respectively, the investigators found in an analysis of secondary outcomes in the study.
Corticosteroids prescribed before embryo transfer increased odds of live birth, with an adjusted odds ratio of 1.32 (95% CI, 0.85-2.05), though the underlying reason why corticosteroids were prescribed could not be established in this data set, investigators cautioned.
“The impact of corticosteroid prior to embryo transfer, found in our study, could be due to a suppression of ‘abnormalities in the immune system’ in women with RA, but we have to underline that this is speculative,” Prof. Nørgård and her colleagues said in a discussion of their results.
Future investigations are needed to clarify the role of corticosteroids in women with rheumatoid arthritis undergoing assisted reproduction treatment, they added.
Support for the study came from the Research Foundation of the Region of Southern Denmark and the Free Research Foundation at Odense University Hospital. Dr. Nørgård and her coauthors said they had no competing interests related to the research.
SOURCE: Nørgård BM et al. Ann Rheum Dis. 2019 Jan 12. doi: 10.1136/annrheumdis-2018-214619.
Women with rheumatoid arthritis who undergo assisted reproduction treatment have a decreased likelihood of live births versus women without rheumatoid arthritis, according to authors of a recent Denmark-wide cohort study.
The problem might be caused by an impaired chance of embryo implantation, authors of the study reported in the Annals of the Rheumatic Diseases.
Corticosteroids prescribed before embryo transfer might have improved the likelihood of live birth in these women with rheumatoid arthritis, according to the investigators, led by Professor Bente Mertz Nørgård of the center for clinical epidemiology at the Odense (Denmark) University Hospital.
However, findings with regard to that potential effect of corticosteroids were “not unambiguous,” said Prof. Nørgård and her coauthors said in their report.
The cohort study, based on Danish registry data from 1994-2017, included 1,149 embryo transfers in women and rheumatoid arthritis with 198,941 embryo transfers in women without rheumatoid arthritis.
Live births per embryo transfer were less likely in women with rheumatoid arthritis versus those they were in women with no rheumatoid arthritis, with an adjusted odds ratio of 0.78 (95% confidence interval, 0.65-0.92), according to investigators.
Chances of biochemical and clinical pregnancies were also lower in women with rheumatoid arthritis, with odds ratios of 0.81 (95% CI, 0.68-0.95) and 0.82 (95% CI, 0.59-1.15), respectively, the investigators found in an analysis of secondary outcomes in the study.
Corticosteroids prescribed before embryo transfer increased odds of live birth, with an adjusted odds ratio of 1.32 (95% CI, 0.85-2.05), though the underlying reason why corticosteroids were prescribed could not be established in this data set, investigators cautioned.
“The impact of corticosteroid prior to embryo transfer, found in our study, could be due to a suppression of ‘abnormalities in the immune system’ in women with RA, but we have to underline that this is speculative,” Prof. Nørgård and her colleagues said in a discussion of their results.
Future investigations are needed to clarify the role of corticosteroids in women with rheumatoid arthritis undergoing assisted reproduction treatment, they added.
Support for the study came from the Research Foundation of the Region of Southern Denmark and the Free Research Foundation at Odense University Hospital. Dr. Nørgård and her coauthors said they had no competing interests related to the research.
SOURCE: Nørgård BM et al. Ann Rheum Dis. 2019 Jan 12. doi: 10.1136/annrheumdis-2018-214619.
Women with rheumatoid arthritis who undergo assisted reproduction treatment have a decreased likelihood of live births versus women without rheumatoid arthritis, according to authors of a recent Denmark-wide cohort study.
The problem might be caused by an impaired chance of embryo implantation, authors of the study reported in the Annals of the Rheumatic Diseases.
Corticosteroids prescribed before embryo transfer might have improved the likelihood of live birth in these women with rheumatoid arthritis, according to the investigators, led by Professor Bente Mertz Nørgård of the center for clinical epidemiology at the Odense (Denmark) University Hospital.
However, findings with regard to that potential effect of corticosteroids were “not unambiguous,” said Prof. Nørgård and her coauthors said in their report.
The cohort study, based on Danish registry data from 1994-2017, included 1,149 embryo transfers in women and rheumatoid arthritis with 198,941 embryo transfers in women without rheumatoid arthritis.
Live births per embryo transfer were less likely in women with rheumatoid arthritis versus those they were in women with no rheumatoid arthritis, with an adjusted odds ratio of 0.78 (95% confidence interval, 0.65-0.92), according to investigators.
Chances of biochemical and clinical pregnancies were also lower in women with rheumatoid arthritis, with odds ratios of 0.81 (95% CI, 0.68-0.95) and 0.82 (95% CI, 0.59-1.15), respectively, the investigators found in an analysis of secondary outcomes in the study.
Corticosteroids prescribed before embryo transfer increased odds of live birth, with an adjusted odds ratio of 1.32 (95% CI, 0.85-2.05), though the underlying reason why corticosteroids were prescribed could not be established in this data set, investigators cautioned.
“The impact of corticosteroid prior to embryo transfer, found in our study, could be due to a suppression of ‘abnormalities in the immune system’ in women with RA, but we have to underline that this is speculative,” Prof. Nørgård and her colleagues said in a discussion of their results.
Future investigations are needed to clarify the role of corticosteroids in women with rheumatoid arthritis undergoing assisted reproduction treatment, they added.
Support for the study came from the Research Foundation of the Region of Southern Denmark and the Free Research Foundation at Odense University Hospital. Dr. Nørgård and her coauthors said they had no competing interests related to the research.
SOURCE: Nørgård BM et al. Ann Rheum Dis. 2019 Jan 12. doi: 10.1136/annrheumdis-2018-214619.
FROM ANNALS OF THE RHEUMATIC DISEASES
Key clinical point: Women with rheumatoid arthritis (RA) who undergo assisted reproduction treatment have decreased chances of live births, compared with women without RA.
Major finding: The odds ratio for live births per embryo transfer in women with RA, as compared to women without RA, was 0.78 (95% confidence interval, 0.65-0.92).
Study details: A nationwide cohort study including 1,149 embryo transfers in women with rheumatoid arthritis and 198,941 without rheumatoid arthritis
Disclosures: Study authors had no disclosures. Support for the study came from the Research Foundation of the Region of Southern Denmark and the Free Research Foundation at Odense (Denmark) University Hospital.
Source: Nørgård BM et al. Ann Rheum Dis. 2019 Jan 12. doi: 10.1136/annrheumdis-2018-214619.
Intrapartum molecular GBS screening reduced newborn early-onset disease, antibiotic use
Point-of-care intrapartum molecular screening of group B Streptococcus reduced the incidence of early-onset disease cases and antibiotic use, according to research published in Obstetrics & Gynecology.
Najoua El Helali, PharmD, from the Service de Microbiologie Clinique at Groupe Hospitalier Paris Saint-Joseph, and her colleagues measured the rate of early-onset disease group B Streptococcus (GBS) in a single-center study analyzing antenatal culture screening for 4 years prior to implementation (2006-2009) of polymerase chain reaction (PCR) screening (2010-2015). There were 11,226 deliveries (11,818 live births) during the antenatal screening period and 18,835 deliveries (18,980 live births) during the PCR screening period. Overall, 4% of deliveries during the antenatal period and 0.1% of deliveries during the intrapartum period were not screened for GBS (P less than .001).
During 2006-2015, the rate of early-onset disease of GBS decreased to 0.21/1,000 cases from 1.01/1,000 cases (risk ratio, 0.25; 95% confidence interval, 0.14-0.43; P = .026), while the rate of probable early-onset disease GBS decreased to 0.73/1,000 cases from 2.8/1,000 cases (RR, 0.25; (95% CI, 0.14-0.43; P less than .001).
For patients with early-onset GBS, length of stay in hospital decreased by 64%, and antibiotic therapy decreased by 60%, but there was no significant difference in average length of stay or duration of antibiotic therapy during the study period. There was a reduction in annual delivery- and treatment-associated costs of early-onset disease GBS from $41,875 to $11,945, while the estimated extra cost of PCR screening to avoid one additional case of early-onset disease GBS was $5,819 and a cost increase of $49 per newborn.
“The additional PCR costs were offset in part by the reduction in early-onset GBS disease treatment costs,” the investigators said.
“A randomized, controlled multicenter study is probably needed to evaluate the cost-effectiveness of this prevention strategy and demonstrate a better efficacy in populations where poorly followed women are of unknown GBS status at presentation for delivery,” the researchers said. “In term newborns, however, using infection rate as an endpoint is problematic given the sample size needed.”
The researchers said their study was potentially limited by lack of a control group and population selection, and described mothers in their center as “mostly well-informed and well-monitored during their pregnancy.”
The authors reported no relevant conflicts of interest.
SOURCE: El Helali N et al. Obstet Gynecol. 2019. doi: 10.1097/AOG.0000000000003057.
Point-of-care intrapartum molecular screening of group B Streptococcus reduced the incidence of early-onset disease cases and antibiotic use, according to research published in Obstetrics & Gynecology.
Najoua El Helali, PharmD, from the Service de Microbiologie Clinique at Groupe Hospitalier Paris Saint-Joseph, and her colleagues measured the rate of early-onset disease group B Streptococcus (GBS) in a single-center study analyzing antenatal culture screening for 4 years prior to implementation (2006-2009) of polymerase chain reaction (PCR) screening (2010-2015). There were 11,226 deliveries (11,818 live births) during the antenatal screening period and 18,835 deliveries (18,980 live births) during the PCR screening period. Overall, 4% of deliveries during the antenatal period and 0.1% of deliveries during the intrapartum period were not screened for GBS (P less than .001).
During 2006-2015, the rate of early-onset disease of GBS decreased to 0.21/1,000 cases from 1.01/1,000 cases (risk ratio, 0.25; 95% confidence interval, 0.14-0.43; P = .026), while the rate of probable early-onset disease GBS decreased to 0.73/1,000 cases from 2.8/1,000 cases (RR, 0.25; (95% CI, 0.14-0.43; P less than .001).
For patients with early-onset GBS, length of stay in hospital decreased by 64%, and antibiotic therapy decreased by 60%, but there was no significant difference in average length of stay or duration of antibiotic therapy during the study period. There was a reduction in annual delivery- and treatment-associated costs of early-onset disease GBS from $41,875 to $11,945, while the estimated extra cost of PCR screening to avoid one additional case of early-onset disease GBS was $5,819 and a cost increase of $49 per newborn.
“The additional PCR costs were offset in part by the reduction in early-onset GBS disease treatment costs,” the investigators said.
“A randomized, controlled multicenter study is probably needed to evaluate the cost-effectiveness of this prevention strategy and demonstrate a better efficacy in populations where poorly followed women are of unknown GBS status at presentation for delivery,” the researchers said. “In term newborns, however, using infection rate as an endpoint is problematic given the sample size needed.”
The researchers said their study was potentially limited by lack of a control group and population selection, and described mothers in their center as “mostly well-informed and well-monitored during their pregnancy.”
The authors reported no relevant conflicts of interest.
SOURCE: El Helali N et al. Obstet Gynecol. 2019. doi: 10.1097/AOG.0000000000003057.
Point-of-care intrapartum molecular screening of group B Streptococcus reduced the incidence of early-onset disease cases and antibiotic use, according to research published in Obstetrics & Gynecology.
Najoua El Helali, PharmD, from the Service de Microbiologie Clinique at Groupe Hospitalier Paris Saint-Joseph, and her colleagues measured the rate of early-onset disease group B Streptococcus (GBS) in a single-center study analyzing antenatal culture screening for 4 years prior to implementation (2006-2009) of polymerase chain reaction (PCR) screening (2010-2015). There were 11,226 deliveries (11,818 live births) during the antenatal screening period and 18,835 deliveries (18,980 live births) during the PCR screening period. Overall, 4% of deliveries during the antenatal period and 0.1% of deliveries during the intrapartum period were not screened for GBS (P less than .001).
During 2006-2015, the rate of early-onset disease of GBS decreased to 0.21/1,000 cases from 1.01/1,000 cases (risk ratio, 0.25; 95% confidence interval, 0.14-0.43; P = .026), while the rate of probable early-onset disease GBS decreased to 0.73/1,000 cases from 2.8/1,000 cases (RR, 0.25; (95% CI, 0.14-0.43; P less than .001).
For patients with early-onset GBS, length of stay in hospital decreased by 64%, and antibiotic therapy decreased by 60%, but there was no significant difference in average length of stay or duration of antibiotic therapy during the study period. There was a reduction in annual delivery- and treatment-associated costs of early-onset disease GBS from $41,875 to $11,945, while the estimated extra cost of PCR screening to avoid one additional case of early-onset disease GBS was $5,819 and a cost increase of $49 per newborn.
“The additional PCR costs were offset in part by the reduction in early-onset GBS disease treatment costs,” the investigators said.
“A randomized, controlled multicenter study is probably needed to evaluate the cost-effectiveness of this prevention strategy and demonstrate a better efficacy in populations where poorly followed women are of unknown GBS status at presentation for delivery,” the researchers said. “In term newborns, however, using infection rate as an endpoint is problematic given the sample size needed.”
The researchers said their study was potentially limited by lack of a control group and population selection, and described mothers in their center as “mostly well-informed and well-monitored during their pregnancy.”
The authors reported no relevant conflicts of interest.
SOURCE: El Helali N et al. Obstet Gynecol. 2019. doi: 10.1097/AOG.0000000000003057.
FROM OBSTETRICS & GYNECOLOGY
Key clinical point:
Major finding: The rate of early-onset disease group B Streptococcus decreased from 1.01/1,000 cases to 0.21/1,000 cases across the antenatal and intrapartum periods.
Study details: A single-center study of antenatal culture screening for 11,226 deliveries during 2006-2009 and intrapartum PCR screening for 18,835 deliveries during 2010-2015.
Disclosures: The authors reported no relevant conflicts of interest.
Source: El Helali N et al. Obstet Gynecol. 2019. doi: 10.1097/AOG.0000000000003057.