User login
FDA panels back brexanolone infusion for postpartum depression
A joint panel of the Food and Drug Administration voted Nov. 2 in support of brexanolone infusion as a treatment for postpartum depression.
The 17-1 vote by members of the Psychopharmacologic Drugs Advisory Committee and the Drug Safety and Risk Management Advisory Committee was based primarily on data from three studies, including 247 women aged 18-44 years with postpartum depression; 140 received brexanolone and 107 received placebo. Effectiveness was assessed based on the Hamilton Depression Scale (HAM-D) at the end of the infusion (hour 60).
In all three studies, patients given brexanolone showed significantly improved HAM-D scores, compared with placebo. The patients experienced significant differences at hour 24, which illustrated the rapid response. “The individual item scores of the HAM-D consistently favored brexanolone IV over placebo, confirming an overall antidepressant effect of the drug,” according to the briefing document of Sage Therapeutics, developer of the drug. In addition, more than 80% of the patients in the treatment and placebo groups sustained their improvement in symptoms at 30 days after the end of the infusion.
“[Postpartum depression] is symptomatically indistinguishable from an episode of major depression,” the FDA briefing document said. “However, the timing of its onset has led to its recognition as a potentially unique illness. There are no drugs specifically approved to treat [postpartum depression].”
Some clinicians use drugs approved for major depression to treat postpartum depression, but the effectiveness of these drugs is limited, the agency said. Other interventions, such as electroconvulsive therapy, repetitive transcranial magnetic stimulation, and psychotherapy also are used, but they can take several weeks to show results.
Recent estimates of postpartum depression in the United States range from about 8% to 20%, according to the FDA document. and both the FDA and Sage Therapeutics agreed on the need for additional treatment options for women with postpartum depression. , according to Sage.
The treatment protocol for brexanolone involves a single 60-hour continuous infusion with a recommended maximum dose of 90 µg/kg/h, referred to as a “90 dose regimen.” The patient receives a single infusion per episode of postpartum depression. The infusion includes three dosing phases: titration at 30 μg/kg/h for 4 hours followed by 60 μg/kg/h for 20 hours (hour 0-24), maintenance at 90 μg/kg/h for 28 hours (hour 24-52), and taper at 60 μg/kg/h for 4 hours – followed by 30 μg/kg/h for 4 hours (hour 52-60).
Brexanolone is an allosteric modulator of GABAA receptors and “a new molecular entity not currently marketed anywhere in the world for any indication,” according to the FDA document. The drug originally was studied as a treatment for seizure patients before its antidepressant properties were discovered.
Adverse reactions observed in 3% or more of the brexanolone patients during the 60-hour treatment and 4-week follow-up included dry mouth, infusion site pain, fatigue, headache, sedation/somnolence, dizziness/vertigo, and loss of consciousness.
Of those reactions, loss of consciousness was the issue of greatest concern to the committee members and informed their discussion of the strict Risk Evaluation and Mitigation Strategy protocol that would be needed to accompany approval of the drug. The details of the REMS will be determined, but the basics of the FDA’s proposed REMS to mitigate the risk of loss of consciousness include administration of the drug only in medically supervised settings by an authorized representative.
In addition, the proposed REMS states that the authorized representative must “establish policies and procedures to ensure that 1) all staff are trained on the risks and 2) the product is not dispensed for use outside the health care setting.”
The proposed REMS also stated that, “Patients must be continuously monitored for the duration of the infusion and 12 hours after, by health care provider who can intervene if the patient experiences excessive sedation or loss of consciousness.”
Despite those concerns, which most committee members thought could be addressed by the REMS, the overall impression of the committees’ members was that brexanolone could have a significant impact on postpartum depression. According to one member, brexanolone is mechanistically “groundbreaking” and “could be a tremendous help in changing the trajectory of postpartum depression.”
The FDA usually follows its panels’ recommendations, which are not binding.
A joint panel of the Food and Drug Administration voted Nov. 2 in support of brexanolone infusion as a treatment for postpartum depression.
The 17-1 vote by members of the Psychopharmacologic Drugs Advisory Committee and the Drug Safety and Risk Management Advisory Committee was based primarily on data from three studies, including 247 women aged 18-44 years with postpartum depression; 140 received brexanolone and 107 received placebo. Effectiveness was assessed based on the Hamilton Depression Scale (HAM-D) at the end of the infusion (hour 60).
In all three studies, patients given brexanolone showed significantly improved HAM-D scores, compared with placebo. The patients experienced significant differences at hour 24, which illustrated the rapid response. “The individual item scores of the HAM-D consistently favored brexanolone IV over placebo, confirming an overall antidepressant effect of the drug,” according to the briefing document of Sage Therapeutics, developer of the drug. In addition, more than 80% of the patients in the treatment and placebo groups sustained their improvement in symptoms at 30 days after the end of the infusion.
“[Postpartum depression] is symptomatically indistinguishable from an episode of major depression,” the FDA briefing document said. “However, the timing of its onset has led to its recognition as a potentially unique illness. There are no drugs specifically approved to treat [postpartum depression].”
Some clinicians use drugs approved for major depression to treat postpartum depression, but the effectiveness of these drugs is limited, the agency said. Other interventions, such as electroconvulsive therapy, repetitive transcranial magnetic stimulation, and psychotherapy also are used, but they can take several weeks to show results.
Recent estimates of postpartum depression in the United States range from about 8% to 20%, according to the FDA document. and both the FDA and Sage Therapeutics agreed on the need for additional treatment options for women with postpartum depression. , according to Sage.
The treatment protocol for brexanolone involves a single 60-hour continuous infusion with a recommended maximum dose of 90 µg/kg/h, referred to as a “90 dose regimen.” The patient receives a single infusion per episode of postpartum depression. The infusion includes three dosing phases: titration at 30 μg/kg/h for 4 hours followed by 60 μg/kg/h for 20 hours (hour 0-24), maintenance at 90 μg/kg/h for 28 hours (hour 24-52), and taper at 60 μg/kg/h for 4 hours – followed by 30 μg/kg/h for 4 hours (hour 52-60).
Brexanolone is an allosteric modulator of GABAA receptors and “a new molecular entity not currently marketed anywhere in the world for any indication,” according to the FDA document. The drug originally was studied as a treatment for seizure patients before its antidepressant properties were discovered.
Adverse reactions observed in 3% or more of the brexanolone patients during the 60-hour treatment and 4-week follow-up included dry mouth, infusion site pain, fatigue, headache, sedation/somnolence, dizziness/vertigo, and loss of consciousness.
Of those reactions, loss of consciousness was the issue of greatest concern to the committee members and informed their discussion of the strict Risk Evaluation and Mitigation Strategy protocol that would be needed to accompany approval of the drug. The details of the REMS will be determined, but the basics of the FDA’s proposed REMS to mitigate the risk of loss of consciousness include administration of the drug only in medically supervised settings by an authorized representative.
In addition, the proposed REMS states that the authorized representative must “establish policies and procedures to ensure that 1) all staff are trained on the risks and 2) the product is not dispensed for use outside the health care setting.”
The proposed REMS also stated that, “Patients must be continuously monitored for the duration of the infusion and 12 hours after, by health care provider who can intervene if the patient experiences excessive sedation or loss of consciousness.”
Despite those concerns, which most committee members thought could be addressed by the REMS, the overall impression of the committees’ members was that brexanolone could have a significant impact on postpartum depression. According to one member, brexanolone is mechanistically “groundbreaking” and “could be a tremendous help in changing the trajectory of postpartum depression.”
The FDA usually follows its panels’ recommendations, which are not binding.
A joint panel of the Food and Drug Administration voted Nov. 2 in support of brexanolone infusion as a treatment for postpartum depression.
The 17-1 vote by members of the Psychopharmacologic Drugs Advisory Committee and the Drug Safety and Risk Management Advisory Committee was based primarily on data from three studies, including 247 women aged 18-44 years with postpartum depression; 140 received brexanolone and 107 received placebo. Effectiveness was assessed based on the Hamilton Depression Scale (HAM-D) at the end of the infusion (hour 60).
In all three studies, patients given brexanolone showed significantly improved HAM-D scores, compared with placebo. The patients experienced significant differences at hour 24, which illustrated the rapid response. “The individual item scores of the HAM-D consistently favored brexanolone IV over placebo, confirming an overall antidepressant effect of the drug,” according to the briefing document of Sage Therapeutics, developer of the drug. In addition, more than 80% of the patients in the treatment and placebo groups sustained their improvement in symptoms at 30 days after the end of the infusion.
“[Postpartum depression] is symptomatically indistinguishable from an episode of major depression,” the FDA briefing document said. “However, the timing of its onset has led to its recognition as a potentially unique illness. There are no drugs specifically approved to treat [postpartum depression].”
Some clinicians use drugs approved for major depression to treat postpartum depression, but the effectiveness of these drugs is limited, the agency said. Other interventions, such as electroconvulsive therapy, repetitive transcranial magnetic stimulation, and psychotherapy also are used, but they can take several weeks to show results.
Recent estimates of postpartum depression in the United States range from about 8% to 20%, according to the FDA document. and both the FDA and Sage Therapeutics agreed on the need for additional treatment options for women with postpartum depression. , according to Sage.
The treatment protocol for brexanolone involves a single 60-hour continuous infusion with a recommended maximum dose of 90 µg/kg/h, referred to as a “90 dose regimen.” The patient receives a single infusion per episode of postpartum depression. The infusion includes three dosing phases: titration at 30 μg/kg/h for 4 hours followed by 60 μg/kg/h for 20 hours (hour 0-24), maintenance at 90 μg/kg/h for 28 hours (hour 24-52), and taper at 60 μg/kg/h for 4 hours – followed by 30 μg/kg/h for 4 hours (hour 52-60).
Brexanolone is an allosteric modulator of GABAA receptors and “a new molecular entity not currently marketed anywhere in the world for any indication,” according to the FDA document. The drug originally was studied as a treatment for seizure patients before its antidepressant properties were discovered.
Adverse reactions observed in 3% or more of the brexanolone patients during the 60-hour treatment and 4-week follow-up included dry mouth, infusion site pain, fatigue, headache, sedation/somnolence, dizziness/vertigo, and loss of consciousness.
Of those reactions, loss of consciousness was the issue of greatest concern to the committee members and informed their discussion of the strict Risk Evaluation and Mitigation Strategy protocol that would be needed to accompany approval of the drug. The details of the REMS will be determined, but the basics of the FDA’s proposed REMS to mitigate the risk of loss of consciousness include administration of the drug only in medically supervised settings by an authorized representative.
In addition, the proposed REMS states that the authorized representative must “establish policies and procedures to ensure that 1) all staff are trained on the risks and 2) the product is not dispensed for use outside the health care setting.”
The proposed REMS also stated that, “Patients must be continuously monitored for the duration of the infusion and 12 hours after, by health care provider who can intervene if the patient experiences excessive sedation or loss of consciousness.”
Despite those concerns, which most committee members thought could be addressed by the REMS, the overall impression of the committees’ members was that brexanolone could have a significant impact on postpartum depression. According to one member, brexanolone is mechanistically “groundbreaking” and “could be a tremendous help in changing the trajectory of postpartum depression.”
The FDA usually follows its panels’ recommendations, which are not binding.
Hospitals could reduce maternal mortality with four achievable steps
rate in the United States, according to a new perspective from leading obstetricians published in the New England Journal of Medicine.
The authors, including Kimberlee McKay, MD, president of the American College of Obstetricians and Gynecologists (ACOG), also call for collaboration with family physicians to increase access to obstetric care in rural areas.
The president of the American Academy of Family Physicians (AAFP), John S. Cullen, MD, in a separate statement, welcomed the opportunity for collaboration in addressing the maternal mortality crisis. However, some distance still lies between the finer points of how the two organizations see family physicians helping curb the crisis.
“Women in the United States are more likely to die from childbirth- or pregnancy-related causes than women in any other high-income country, and black women die at a rate three to four times that of white women,” noted Susan Mann, MD, along with her coauthors of the obstetric perspective, calling increasing maternal mortality a “tragedy.”
In an interview, Dr. Cullen concurred, calling the current situation “unconscionable.” One of the primary reasons he sought AAFP leadership, he said, was to bring experiences learned during his 25 years of obstetric practice in rural Alaska to bear on the current crisis.
A set of maternal care bundles created by the Alliance for Innovation on Maternal Health (AIM) provides the framework for the first action recommended by Dr. Mann and her coauthors. The AIM bundles focus on protocols that improve readiness, recognition, response, and reporting in maternal care. The protocols are institution specific. For example, the authors noted, antihypertensive medications should be readily available around the clock, because not all facilities will have a pharmacist in-house at all times, and hypertensive emergencies are among the gravest obstetric complications.
“Although management may vary from institution to institution, each unit can be required to demonstrate readiness to deal with emergencies 24/7,” said Dr. Mann, a physician in private practice in Boston, and her coauthors.
The second recommended action revolves around multidisciplinary staff meetings to perform individual assessments for each woman’s obstetric risk factors. These huddles should include assessing hemorrhage risk by using the California Maternal Quality Care Collaborative guidelines, and briefings with the full care team to “develop shared understandings of the patient and the procedure” before elective or nonurgent cesarean deliveries, said Dr. Mann and her colleagues. Patients should be informed of any safety concerns, and caregivers should use shared decision-making moving forward.
“Approximately 50% of U.S. hospitals provide care for three or fewer deliveries per day, but the need to identify women at risk is equally important for these small obstetrics services,” Dr. Mann and her coauthors noted.
Third, simulation of obstetric emergencies lets all members of the team understand the speed at which decisions must be made and actions taken when minutes, or even seconds, count. Dr. Mann and her coauthors pointed out that logistics problems come out during a well-run simulation, giving such examples as a lag in receiving blood products or a poorly placed hemorrhage cart.
Drawing an analogy to the extensive time pilots spend in flight simulators, Dr. Mann and her coauthors noted that “because severe maternity-related events are rare and often unpredictable, and because members of the care team may not know each other, it is important to train for low-probability but high-risk events.”
Using the Maternal Health Compact will help ensure that lower-resource hospitals have a relationship in place with a facility that can provide a higher level of maternal care when needed, they said.
This fourth action means that there’s a connection that “can be activated by lower-resource hospitals to get immediate consultation in the event of an unexpected obstetrical emergency whose care demands exceed their resources,” said Dr. Mann and her coauthors. When transport is necessary, it too can be facilitated if a compact is already in place.
The final, broader, recommendation from the obstetricians is that ACOG and AAFP collaborate “on an additional year of comprehensive training for family medicine physicians who are considering practicing obstetrics in rural areas.” The fourth year of training for these family physicians could help address widespread shortages of obstetricians and midwives in rural communities, Dr. Mann and her coauthors wrote. They observed that “pregnant women who live in rural areas may need to travel hundreds of miles to obtain routine prenatal care or consultation with an obstetrical specialist.”
Dr. Cullen, whose practice is in Valdez, Alaska, said he happily reciprocates the desire for collaboration. In his inaugural blog post after assuming the AAFP presidency in October, 2018, he extended warm thanks to his obstetrician and perinatologist colleagues. “The relationship between family physicians and obstetricians is vital in rural communities like mine. The training I received has saved lives and prevented catastrophe,” he wrote.
At the same time, Dr. Cullen said that he believes family physicians are the best hope for quality obstetrical care in rural communities. Dr. Cullen’s own training, he said, was structured to involve significant obstetric experience with obstetrician mentorship. “A 3-year residency left me very comfortable with fairly sophisticated obstetric management at graduation,” he added.
In his practice, Dr. Cullen said, a family physician hired a decade ago after a 3-year residency came with excellent obstetric skills, which have been further honed during the succeeding years of rural practice. During a 3-year residency, “family physicians receive training and demonstrate the skills and competencies required to deliver high-quality maternity care in any community, including those in rural settings,” he said in his statement.
“The bigger issue is really the closure of obstetric units in rural hospitals,” said Dr. Cullen. He pointed out that although obstetric services may have left a community, that rural hospital will still have miscarrying patients with hemorrhage, patients in preterm labor, and other obstetric emergencies arriving at the doorstep. These and other instances often “represent true emergencies without possibility of transfer, requiring immediate and effective response,” he wrote.
“We would encourage the authors to ensure an equal focus on improving care at all levels and in all hospitals, as well as relying on transfer as appropriate,” Dr. Cullen said in his statement.
Such mutual support can be bolstered by new technology, wrote Dr. Mann and her coauthors, because “telehealth and collaborations and consultation with clinics and regional hospitals can help increase the availability of maternity care in the United States.”
For Dr. Cullen also, the way forward is together. In his blog, he wrote: “As family physicians, we can only deliver obstetrical care to our patients with the cooperation of obstetricians and perinatologists. Conversely, obstetricians can only improve maternal and infant mortality rates, especially in rural areas, with the help of family physicians.”
SOURCE: Mann S et al. N Engl J Med 2018;379:1689-91.
rate in the United States, according to a new perspective from leading obstetricians published in the New England Journal of Medicine.
The authors, including Kimberlee McKay, MD, president of the American College of Obstetricians and Gynecologists (ACOG), also call for collaboration with family physicians to increase access to obstetric care in rural areas.
The president of the American Academy of Family Physicians (AAFP), John S. Cullen, MD, in a separate statement, welcomed the opportunity for collaboration in addressing the maternal mortality crisis. However, some distance still lies between the finer points of how the two organizations see family physicians helping curb the crisis.
“Women in the United States are more likely to die from childbirth- or pregnancy-related causes than women in any other high-income country, and black women die at a rate three to four times that of white women,” noted Susan Mann, MD, along with her coauthors of the obstetric perspective, calling increasing maternal mortality a “tragedy.”
In an interview, Dr. Cullen concurred, calling the current situation “unconscionable.” One of the primary reasons he sought AAFP leadership, he said, was to bring experiences learned during his 25 years of obstetric practice in rural Alaska to bear on the current crisis.
A set of maternal care bundles created by the Alliance for Innovation on Maternal Health (AIM) provides the framework for the first action recommended by Dr. Mann and her coauthors. The AIM bundles focus on protocols that improve readiness, recognition, response, and reporting in maternal care. The protocols are institution specific. For example, the authors noted, antihypertensive medications should be readily available around the clock, because not all facilities will have a pharmacist in-house at all times, and hypertensive emergencies are among the gravest obstetric complications.
“Although management may vary from institution to institution, each unit can be required to demonstrate readiness to deal with emergencies 24/7,” said Dr. Mann, a physician in private practice in Boston, and her coauthors.
The second recommended action revolves around multidisciplinary staff meetings to perform individual assessments for each woman’s obstetric risk factors. These huddles should include assessing hemorrhage risk by using the California Maternal Quality Care Collaborative guidelines, and briefings with the full care team to “develop shared understandings of the patient and the procedure” before elective or nonurgent cesarean deliveries, said Dr. Mann and her colleagues. Patients should be informed of any safety concerns, and caregivers should use shared decision-making moving forward.
“Approximately 50% of U.S. hospitals provide care for three or fewer deliveries per day, but the need to identify women at risk is equally important for these small obstetrics services,” Dr. Mann and her coauthors noted.
Third, simulation of obstetric emergencies lets all members of the team understand the speed at which decisions must be made and actions taken when minutes, or even seconds, count. Dr. Mann and her coauthors pointed out that logistics problems come out during a well-run simulation, giving such examples as a lag in receiving blood products or a poorly placed hemorrhage cart.
Drawing an analogy to the extensive time pilots spend in flight simulators, Dr. Mann and her coauthors noted that “because severe maternity-related events are rare and often unpredictable, and because members of the care team may not know each other, it is important to train for low-probability but high-risk events.”
Using the Maternal Health Compact will help ensure that lower-resource hospitals have a relationship in place with a facility that can provide a higher level of maternal care when needed, they said.
This fourth action means that there’s a connection that “can be activated by lower-resource hospitals to get immediate consultation in the event of an unexpected obstetrical emergency whose care demands exceed their resources,” said Dr. Mann and her coauthors. When transport is necessary, it too can be facilitated if a compact is already in place.
The final, broader, recommendation from the obstetricians is that ACOG and AAFP collaborate “on an additional year of comprehensive training for family medicine physicians who are considering practicing obstetrics in rural areas.” The fourth year of training for these family physicians could help address widespread shortages of obstetricians and midwives in rural communities, Dr. Mann and her coauthors wrote. They observed that “pregnant women who live in rural areas may need to travel hundreds of miles to obtain routine prenatal care or consultation with an obstetrical specialist.”
Dr. Cullen, whose practice is in Valdez, Alaska, said he happily reciprocates the desire for collaboration. In his inaugural blog post after assuming the AAFP presidency in October, 2018, he extended warm thanks to his obstetrician and perinatologist colleagues. “The relationship between family physicians and obstetricians is vital in rural communities like mine. The training I received has saved lives and prevented catastrophe,” he wrote.
At the same time, Dr. Cullen said that he believes family physicians are the best hope for quality obstetrical care in rural communities. Dr. Cullen’s own training, he said, was structured to involve significant obstetric experience with obstetrician mentorship. “A 3-year residency left me very comfortable with fairly sophisticated obstetric management at graduation,” he added.
In his practice, Dr. Cullen said, a family physician hired a decade ago after a 3-year residency came with excellent obstetric skills, which have been further honed during the succeeding years of rural practice. During a 3-year residency, “family physicians receive training and demonstrate the skills and competencies required to deliver high-quality maternity care in any community, including those in rural settings,” he said in his statement.
“The bigger issue is really the closure of obstetric units in rural hospitals,” said Dr. Cullen. He pointed out that although obstetric services may have left a community, that rural hospital will still have miscarrying patients with hemorrhage, patients in preterm labor, and other obstetric emergencies arriving at the doorstep. These and other instances often “represent true emergencies without possibility of transfer, requiring immediate and effective response,” he wrote.
“We would encourage the authors to ensure an equal focus on improving care at all levels and in all hospitals, as well as relying on transfer as appropriate,” Dr. Cullen said in his statement.
Such mutual support can be bolstered by new technology, wrote Dr. Mann and her coauthors, because “telehealth and collaborations and consultation with clinics and regional hospitals can help increase the availability of maternity care in the United States.”
For Dr. Cullen also, the way forward is together. In his blog, he wrote: “As family physicians, we can only deliver obstetrical care to our patients with the cooperation of obstetricians and perinatologists. Conversely, obstetricians can only improve maternal and infant mortality rates, especially in rural areas, with the help of family physicians.”
SOURCE: Mann S et al. N Engl J Med 2018;379:1689-91.
rate in the United States, according to a new perspective from leading obstetricians published in the New England Journal of Medicine.
The authors, including Kimberlee McKay, MD, president of the American College of Obstetricians and Gynecologists (ACOG), also call for collaboration with family physicians to increase access to obstetric care in rural areas.
The president of the American Academy of Family Physicians (AAFP), John S. Cullen, MD, in a separate statement, welcomed the opportunity for collaboration in addressing the maternal mortality crisis. However, some distance still lies between the finer points of how the two organizations see family physicians helping curb the crisis.
“Women in the United States are more likely to die from childbirth- or pregnancy-related causes than women in any other high-income country, and black women die at a rate three to four times that of white women,” noted Susan Mann, MD, along with her coauthors of the obstetric perspective, calling increasing maternal mortality a “tragedy.”
In an interview, Dr. Cullen concurred, calling the current situation “unconscionable.” One of the primary reasons he sought AAFP leadership, he said, was to bring experiences learned during his 25 years of obstetric practice in rural Alaska to bear on the current crisis.
A set of maternal care bundles created by the Alliance for Innovation on Maternal Health (AIM) provides the framework for the first action recommended by Dr. Mann and her coauthors. The AIM bundles focus on protocols that improve readiness, recognition, response, and reporting in maternal care. The protocols are institution specific. For example, the authors noted, antihypertensive medications should be readily available around the clock, because not all facilities will have a pharmacist in-house at all times, and hypertensive emergencies are among the gravest obstetric complications.
“Although management may vary from institution to institution, each unit can be required to demonstrate readiness to deal with emergencies 24/7,” said Dr. Mann, a physician in private practice in Boston, and her coauthors.
The second recommended action revolves around multidisciplinary staff meetings to perform individual assessments for each woman’s obstetric risk factors. These huddles should include assessing hemorrhage risk by using the California Maternal Quality Care Collaborative guidelines, and briefings with the full care team to “develop shared understandings of the patient and the procedure” before elective or nonurgent cesarean deliveries, said Dr. Mann and her colleagues. Patients should be informed of any safety concerns, and caregivers should use shared decision-making moving forward.
“Approximately 50% of U.S. hospitals provide care for three or fewer deliveries per day, but the need to identify women at risk is equally important for these small obstetrics services,” Dr. Mann and her coauthors noted.
Third, simulation of obstetric emergencies lets all members of the team understand the speed at which decisions must be made and actions taken when minutes, or even seconds, count. Dr. Mann and her coauthors pointed out that logistics problems come out during a well-run simulation, giving such examples as a lag in receiving blood products or a poorly placed hemorrhage cart.
Drawing an analogy to the extensive time pilots spend in flight simulators, Dr. Mann and her coauthors noted that “because severe maternity-related events are rare and often unpredictable, and because members of the care team may not know each other, it is important to train for low-probability but high-risk events.”
Using the Maternal Health Compact will help ensure that lower-resource hospitals have a relationship in place with a facility that can provide a higher level of maternal care when needed, they said.
This fourth action means that there’s a connection that “can be activated by lower-resource hospitals to get immediate consultation in the event of an unexpected obstetrical emergency whose care demands exceed their resources,” said Dr. Mann and her coauthors. When transport is necessary, it too can be facilitated if a compact is already in place.
The final, broader, recommendation from the obstetricians is that ACOG and AAFP collaborate “on an additional year of comprehensive training for family medicine physicians who are considering practicing obstetrics in rural areas.” The fourth year of training for these family physicians could help address widespread shortages of obstetricians and midwives in rural communities, Dr. Mann and her coauthors wrote. They observed that “pregnant women who live in rural areas may need to travel hundreds of miles to obtain routine prenatal care or consultation with an obstetrical specialist.”
Dr. Cullen, whose practice is in Valdez, Alaska, said he happily reciprocates the desire for collaboration. In his inaugural blog post after assuming the AAFP presidency in October, 2018, he extended warm thanks to his obstetrician and perinatologist colleagues. “The relationship between family physicians and obstetricians is vital in rural communities like mine. The training I received has saved lives and prevented catastrophe,” he wrote.
At the same time, Dr. Cullen said that he believes family physicians are the best hope for quality obstetrical care in rural communities. Dr. Cullen’s own training, he said, was structured to involve significant obstetric experience with obstetrician mentorship. “A 3-year residency left me very comfortable with fairly sophisticated obstetric management at graduation,” he added.
In his practice, Dr. Cullen said, a family physician hired a decade ago after a 3-year residency came with excellent obstetric skills, which have been further honed during the succeeding years of rural practice. During a 3-year residency, “family physicians receive training and demonstrate the skills and competencies required to deliver high-quality maternity care in any community, including those in rural settings,” he said in his statement.
“The bigger issue is really the closure of obstetric units in rural hospitals,” said Dr. Cullen. He pointed out that although obstetric services may have left a community, that rural hospital will still have miscarrying patients with hemorrhage, patients in preterm labor, and other obstetric emergencies arriving at the doorstep. These and other instances often “represent true emergencies without possibility of transfer, requiring immediate and effective response,” he wrote.
“We would encourage the authors to ensure an equal focus on improving care at all levels and in all hospitals, as well as relying on transfer as appropriate,” Dr. Cullen said in his statement.
Such mutual support can be bolstered by new technology, wrote Dr. Mann and her coauthors, because “telehealth and collaborations and consultation with clinics and regional hospitals can help increase the availability of maternity care in the United States.”
For Dr. Cullen also, the way forward is together. In his blog, he wrote: “As family physicians, we can only deliver obstetrical care to our patients with the cooperation of obstetricians and perinatologists. Conversely, obstetricians can only improve maternal and infant mortality rates, especially in rural areas, with the help of family physicians.”
SOURCE: Mann S et al. N Engl J Med 2018;379:1689-91.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Yoga feasible, provides modest benefits for women with urinary incontinence
according to Alison J. Huang, MD, of the University of California, San Francisco, and her associates.
In a small study published in the American Journal of Obstetrics and Gynecology, 28 women enrolled in a 3-month yoga intervention program and 28 were enrolled in a control program consisting of nonspecific muscle stretching and strengthening. The mean age was 65 years, baseline urinary incontinence was 3.5 episodes/day, and 37 women had urgency-predominant incontinence.
Of those who completed the study, 89% of 27 patients in the yoga group attended at least 80% of classes, compared with 87% in the control group; over 90% of women in the yoga group completed at least 9 home practice hours.
Overall incontinence frequency was reduced by 76% in the yoga group and by 56% in the control group. Incontinence caused by stress was significantly reduced in the yoga group, compared with the control group (61% vs. 35%; P = .045), but the rate of incontinence caused by urgency did not noticeably differ. A total of 48 nonserious adverse events were reported over the 3-month period (23 in the yoga and 25 in the control group), but none were associated with either yoga or control treatment.
“Yoga may be useful for incontinent women in the community who lack access to incontinence specialists, are unable to use clinical therapies, or wish to enhance conventional care. Since yoga can be practiced in a group setting without continuous supervision by health care specialists, it offers a potentially cost-effective, community-based self-management strategy for incontinence, provided that it can be taught with appropriate attention to safety and to patients’ clinical needs,” the authors noted.
The study was supported with grants from the National Center for Complementary and Integrative Medicine and the UCSF Osher Center for Integrative Medicine’s Bradley fund. One of the authors received a grant from the National Institute of Diabetes and Digestive and Kidney Disorders. Two study authors reported receiving funding from Pfizer and Astellas to conduct research unrelated to the current study.
SOURCE: Huang AJ et al. Am J Obstet Gynecol. 2018 Oct 26. doi: 10.1016/j.ajog.2018.10.031.
according to Alison J. Huang, MD, of the University of California, San Francisco, and her associates.
In a small study published in the American Journal of Obstetrics and Gynecology, 28 women enrolled in a 3-month yoga intervention program and 28 were enrolled in a control program consisting of nonspecific muscle stretching and strengthening. The mean age was 65 years, baseline urinary incontinence was 3.5 episodes/day, and 37 women had urgency-predominant incontinence.
Of those who completed the study, 89% of 27 patients in the yoga group attended at least 80% of classes, compared with 87% in the control group; over 90% of women in the yoga group completed at least 9 home practice hours.
Overall incontinence frequency was reduced by 76% in the yoga group and by 56% in the control group. Incontinence caused by stress was significantly reduced in the yoga group, compared with the control group (61% vs. 35%; P = .045), but the rate of incontinence caused by urgency did not noticeably differ. A total of 48 nonserious adverse events were reported over the 3-month period (23 in the yoga and 25 in the control group), but none were associated with either yoga or control treatment.
“Yoga may be useful for incontinent women in the community who lack access to incontinence specialists, are unable to use clinical therapies, or wish to enhance conventional care. Since yoga can be practiced in a group setting without continuous supervision by health care specialists, it offers a potentially cost-effective, community-based self-management strategy for incontinence, provided that it can be taught with appropriate attention to safety and to patients’ clinical needs,” the authors noted.
The study was supported with grants from the National Center for Complementary and Integrative Medicine and the UCSF Osher Center for Integrative Medicine’s Bradley fund. One of the authors received a grant from the National Institute of Diabetes and Digestive and Kidney Disorders. Two study authors reported receiving funding from Pfizer and Astellas to conduct research unrelated to the current study.
SOURCE: Huang AJ et al. Am J Obstet Gynecol. 2018 Oct 26. doi: 10.1016/j.ajog.2018.10.031.
according to Alison J. Huang, MD, of the University of California, San Francisco, and her associates.
In a small study published in the American Journal of Obstetrics and Gynecology, 28 women enrolled in a 3-month yoga intervention program and 28 were enrolled in a control program consisting of nonspecific muscle stretching and strengthening. The mean age was 65 years, baseline urinary incontinence was 3.5 episodes/day, and 37 women had urgency-predominant incontinence.
Of those who completed the study, 89% of 27 patients in the yoga group attended at least 80% of classes, compared with 87% in the control group; over 90% of women in the yoga group completed at least 9 home practice hours.
Overall incontinence frequency was reduced by 76% in the yoga group and by 56% in the control group. Incontinence caused by stress was significantly reduced in the yoga group, compared with the control group (61% vs. 35%; P = .045), but the rate of incontinence caused by urgency did not noticeably differ. A total of 48 nonserious adverse events were reported over the 3-month period (23 in the yoga and 25 in the control group), but none were associated with either yoga or control treatment.
“Yoga may be useful for incontinent women in the community who lack access to incontinence specialists, are unable to use clinical therapies, or wish to enhance conventional care. Since yoga can be practiced in a group setting without continuous supervision by health care specialists, it offers a potentially cost-effective, community-based self-management strategy for incontinence, provided that it can be taught with appropriate attention to safety and to patients’ clinical needs,” the authors noted.
The study was supported with grants from the National Center for Complementary and Integrative Medicine and the UCSF Osher Center for Integrative Medicine’s Bradley fund. One of the authors received a grant from the National Institute of Diabetes and Digestive and Kidney Disorders. Two study authors reported receiving funding from Pfizer and Astellas to conduct research unrelated to the current study.
SOURCE: Huang AJ et al. Am J Obstet Gynecol. 2018 Oct 26. doi: 10.1016/j.ajog.2018.10.031.
FROM THE AMERICAN JOURNAL OF OBSTETRICS AND GYNECOLOGY
Estetrol safely limited menopause symptoms in a phase 2b study
Estetrol (Donesta) relieved vasomotor menopausal symptoms without stimulating breast tenderness or raising triglyceride levels in a dose-finding study of the investigational drug presented at the annual meeting of the North American Menopause Society.
Estetrol (E4), an estrogen produced by the fetal liver, is the first native estrogen acting selectively in tissues. Since it crosses the placenta, estetrol is present in maternal urine at about 9 weeks’ gestation. In fetal plasma, it circulates at concentrations about 12 times higher than maternal estetrol levels. The hormone has a half-life of 28-32 hours, longer than the half-life of most other estrogens.
E4 “has been shown to have a remarkably safe profile and I like to describe it as the first natural oral estrogen with the safety profile of a transdermal estrogen,” said Wulf H. Utian, MD, the Arthur H. Bill Professor of Obstetrics & Gynecology at Case Western Reserve University, Cleveland. Estetrol uniquely activates nuclear estrogen receptor–alpha, while antagonizing membrane estrogen receptor-alpha. These properties give E4 selective tissue action, with low breast stimulation meaning less breast tenderness and “low carcinogenic impact.”
Additionally, triglyceride levels are minimally affected, and serum markers for venous thromboembolism generally remain unchanged with E4 exposure, he said.
In a phase 2b dose-finding study, a variety of estetrol doses were compared with placebo to treat vasomotor symptoms in postmenopausal women aged 40-65 years with at least 7 moderate to severe hot flashes daily, or at least 50 moderate to severe hot flashes in the week before randomization. The multicenter, double-blind randomized controlled trial took place in five European countries, with 200 women overall completing the study. The study design excluded women with personal histories of malignancy, thromboembolism, or coagulopathy, and women with diabetes and poor glycemic control. Women with a uterus who had current or past endometrial hyperplasia, polyps, or an abnormal cervical smear were also excluded.
Women who had an intact uterus were included if transvaginal ultrasound showed endometrial thickness of 5 mm or less. Participants were randomized 1:1:1:1 to receive four different E4 doses: 2.5 mg, 5 mg, 10 mg, or 15 mg.
At the highest dose of 15 mg, E4 significantly reduced the frequency of vasomotor symptoms compared with placebo by study week 4 and throughout the 12-week study period (P less than .05).
This high dose also resulted in a 28% reduction in vasomotor symptom severity, compared with placebo by study week 12, with a significant separation from placebo by week 12.
In terms of the number of women who experienced at least a 50% drop in severe vasomotor symptoms, the 15-mg E4 dose also bested placebo (P less than .01). Among the group taking the 15 mg dose, significantly more also saw at least a 75% drop in frequency of severe vasomotor symptom (P less than .001).
Vaginal cytology showed that by week 12 all doses of E4 significantly increased the vaginal maturation index from baseline, a finding that corresponds with less thinning of vaginal tissues (P less than .001, compared with placebo for all doses).
The safety profile of E4 was good, Dr. Utian said. Coagulation markers were unaffected, and most lipid and blood glucose markers were also unchanged. There were “small but potentially beneficial changes in HDL-C [HDL cholesterol levels] and HbA1c values [hemoglobin A1c]” in groups taking the two highest doses of E4.
Also, C-telopeptide of type 1 collagen and osteocalcin values were reduced, “suggesting reduction in bone resorption,” he said.
According to Dr. Utian, those taking the two highest doses also saw a “slight though significant increase” from baseline in sex hormone–binding globulin levels, “indicating that the E4 estrogenic effect was mild and dose dependent.”
Endometrial biopsies showed no hyperplasia. In those taking the 15-mg E4 dose, mean endometrial thickness did increase from a mean 2 mm at baseline to 6 mm at 12 weeks. However, endometrial thickness returned to baseline after progestin therapy, said Dr. Utian. There were no unexpected adverse events during the study.
Dr. Utian reported consultant relationships with Mithra, the maker of estetrol; AMAG; Pharmavite; and Endoceutics. The study was funded by Mithra.
SOURCE: Utian W. NAMS 2018, Friday concurrent session 1.
Estetrol (Donesta) relieved vasomotor menopausal symptoms without stimulating breast tenderness or raising triglyceride levels in a dose-finding study of the investigational drug presented at the annual meeting of the North American Menopause Society.
Estetrol (E4), an estrogen produced by the fetal liver, is the first native estrogen acting selectively in tissues. Since it crosses the placenta, estetrol is present in maternal urine at about 9 weeks’ gestation. In fetal plasma, it circulates at concentrations about 12 times higher than maternal estetrol levels. The hormone has a half-life of 28-32 hours, longer than the half-life of most other estrogens.
E4 “has been shown to have a remarkably safe profile and I like to describe it as the first natural oral estrogen with the safety profile of a transdermal estrogen,” said Wulf H. Utian, MD, the Arthur H. Bill Professor of Obstetrics & Gynecology at Case Western Reserve University, Cleveland. Estetrol uniquely activates nuclear estrogen receptor–alpha, while antagonizing membrane estrogen receptor-alpha. These properties give E4 selective tissue action, with low breast stimulation meaning less breast tenderness and “low carcinogenic impact.”
Additionally, triglyceride levels are minimally affected, and serum markers for venous thromboembolism generally remain unchanged with E4 exposure, he said.
In a phase 2b dose-finding study, a variety of estetrol doses were compared with placebo to treat vasomotor symptoms in postmenopausal women aged 40-65 years with at least 7 moderate to severe hot flashes daily, or at least 50 moderate to severe hot flashes in the week before randomization. The multicenter, double-blind randomized controlled trial took place in five European countries, with 200 women overall completing the study. The study design excluded women with personal histories of malignancy, thromboembolism, or coagulopathy, and women with diabetes and poor glycemic control. Women with a uterus who had current or past endometrial hyperplasia, polyps, or an abnormal cervical smear were also excluded.
Women who had an intact uterus were included if transvaginal ultrasound showed endometrial thickness of 5 mm or less. Participants were randomized 1:1:1:1 to receive four different E4 doses: 2.5 mg, 5 mg, 10 mg, or 15 mg.
At the highest dose of 15 mg, E4 significantly reduced the frequency of vasomotor symptoms compared with placebo by study week 4 and throughout the 12-week study period (P less than .05).
This high dose also resulted in a 28% reduction in vasomotor symptom severity, compared with placebo by study week 12, with a significant separation from placebo by week 12.
In terms of the number of women who experienced at least a 50% drop in severe vasomotor symptoms, the 15-mg E4 dose also bested placebo (P less than .01). Among the group taking the 15 mg dose, significantly more also saw at least a 75% drop in frequency of severe vasomotor symptom (P less than .001).
Vaginal cytology showed that by week 12 all doses of E4 significantly increased the vaginal maturation index from baseline, a finding that corresponds with less thinning of vaginal tissues (P less than .001, compared with placebo for all doses).
The safety profile of E4 was good, Dr. Utian said. Coagulation markers were unaffected, and most lipid and blood glucose markers were also unchanged. There were “small but potentially beneficial changes in HDL-C [HDL cholesterol levels] and HbA1c values [hemoglobin A1c]” in groups taking the two highest doses of E4.
Also, C-telopeptide of type 1 collagen and osteocalcin values were reduced, “suggesting reduction in bone resorption,” he said.
According to Dr. Utian, those taking the two highest doses also saw a “slight though significant increase” from baseline in sex hormone–binding globulin levels, “indicating that the E4 estrogenic effect was mild and dose dependent.”
Endometrial biopsies showed no hyperplasia. In those taking the 15-mg E4 dose, mean endometrial thickness did increase from a mean 2 mm at baseline to 6 mm at 12 weeks. However, endometrial thickness returned to baseline after progestin therapy, said Dr. Utian. There were no unexpected adverse events during the study.
Dr. Utian reported consultant relationships with Mithra, the maker of estetrol; AMAG; Pharmavite; and Endoceutics. The study was funded by Mithra.
SOURCE: Utian W. NAMS 2018, Friday concurrent session 1.
Estetrol (Donesta) relieved vasomotor menopausal symptoms without stimulating breast tenderness or raising triglyceride levels in a dose-finding study of the investigational drug presented at the annual meeting of the North American Menopause Society.
Estetrol (E4), an estrogen produced by the fetal liver, is the first native estrogen acting selectively in tissues. Since it crosses the placenta, estetrol is present in maternal urine at about 9 weeks’ gestation. In fetal plasma, it circulates at concentrations about 12 times higher than maternal estetrol levels. The hormone has a half-life of 28-32 hours, longer than the half-life of most other estrogens.
E4 “has been shown to have a remarkably safe profile and I like to describe it as the first natural oral estrogen with the safety profile of a transdermal estrogen,” said Wulf H. Utian, MD, the Arthur H. Bill Professor of Obstetrics & Gynecology at Case Western Reserve University, Cleveland. Estetrol uniquely activates nuclear estrogen receptor–alpha, while antagonizing membrane estrogen receptor-alpha. These properties give E4 selective tissue action, with low breast stimulation meaning less breast tenderness and “low carcinogenic impact.”
Additionally, triglyceride levels are minimally affected, and serum markers for venous thromboembolism generally remain unchanged with E4 exposure, he said.
In a phase 2b dose-finding study, a variety of estetrol doses were compared with placebo to treat vasomotor symptoms in postmenopausal women aged 40-65 years with at least 7 moderate to severe hot flashes daily, or at least 50 moderate to severe hot flashes in the week before randomization. The multicenter, double-blind randomized controlled trial took place in five European countries, with 200 women overall completing the study. The study design excluded women with personal histories of malignancy, thromboembolism, or coagulopathy, and women with diabetes and poor glycemic control. Women with a uterus who had current or past endometrial hyperplasia, polyps, or an abnormal cervical smear were also excluded.
Women who had an intact uterus were included if transvaginal ultrasound showed endometrial thickness of 5 mm or less. Participants were randomized 1:1:1:1 to receive four different E4 doses: 2.5 mg, 5 mg, 10 mg, or 15 mg.
At the highest dose of 15 mg, E4 significantly reduced the frequency of vasomotor symptoms compared with placebo by study week 4 and throughout the 12-week study period (P less than .05).
This high dose also resulted in a 28% reduction in vasomotor symptom severity, compared with placebo by study week 12, with a significant separation from placebo by week 12.
In terms of the number of women who experienced at least a 50% drop in severe vasomotor symptoms, the 15-mg E4 dose also bested placebo (P less than .01). Among the group taking the 15 mg dose, significantly more also saw at least a 75% drop in frequency of severe vasomotor symptom (P less than .001).
Vaginal cytology showed that by week 12 all doses of E4 significantly increased the vaginal maturation index from baseline, a finding that corresponds with less thinning of vaginal tissues (P less than .001, compared with placebo for all doses).
The safety profile of E4 was good, Dr. Utian said. Coagulation markers were unaffected, and most lipid and blood glucose markers were also unchanged. There were “small but potentially beneficial changes in HDL-C [HDL cholesterol levels] and HbA1c values [hemoglobin A1c]” in groups taking the two highest doses of E4.
Also, C-telopeptide of type 1 collagen and osteocalcin values were reduced, “suggesting reduction in bone resorption,” he said.
According to Dr. Utian, those taking the two highest doses also saw a “slight though significant increase” from baseline in sex hormone–binding globulin levels, “indicating that the E4 estrogenic effect was mild and dose dependent.”
Endometrial biopsies showed no hyperplasia. In those taking the 15-mg E4 dose, mean endometrial thickness did increase from a mean 2 mm at baseline to 6 mm at 12 weeks. However, endometrial thickness returned to baseline after progestin therapy, said Dr. Utian. There were no unexpected adverse events during the study.
Dr. Utian reported consultant relationships with Mithra, the maker of estetrol; AMAG; Pharmavite; and Endoceutics. The study was funded by Mithra.
SOURCE: Utian W. NAMS 2018, Friday concurrent session 1.
FROM NAMS 2018
Key clinical point: Estetrol relieved hot flashes without adversely affecting lipid markers.
Major finding: Estetrol 15 mg reduced vasomotor symptom severity by 28%.
Study details: Randomized, double-blind, placebo-controlled phase 2b dose-finding study of 200 women.
Disclosures: Dr. Utian reported financial relationships with several pharmaceutical companies, including Mithra, the sponsor of the study.
Source: Utian WH. NAMS 2018, Friday concurrent session 1.
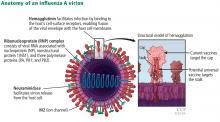
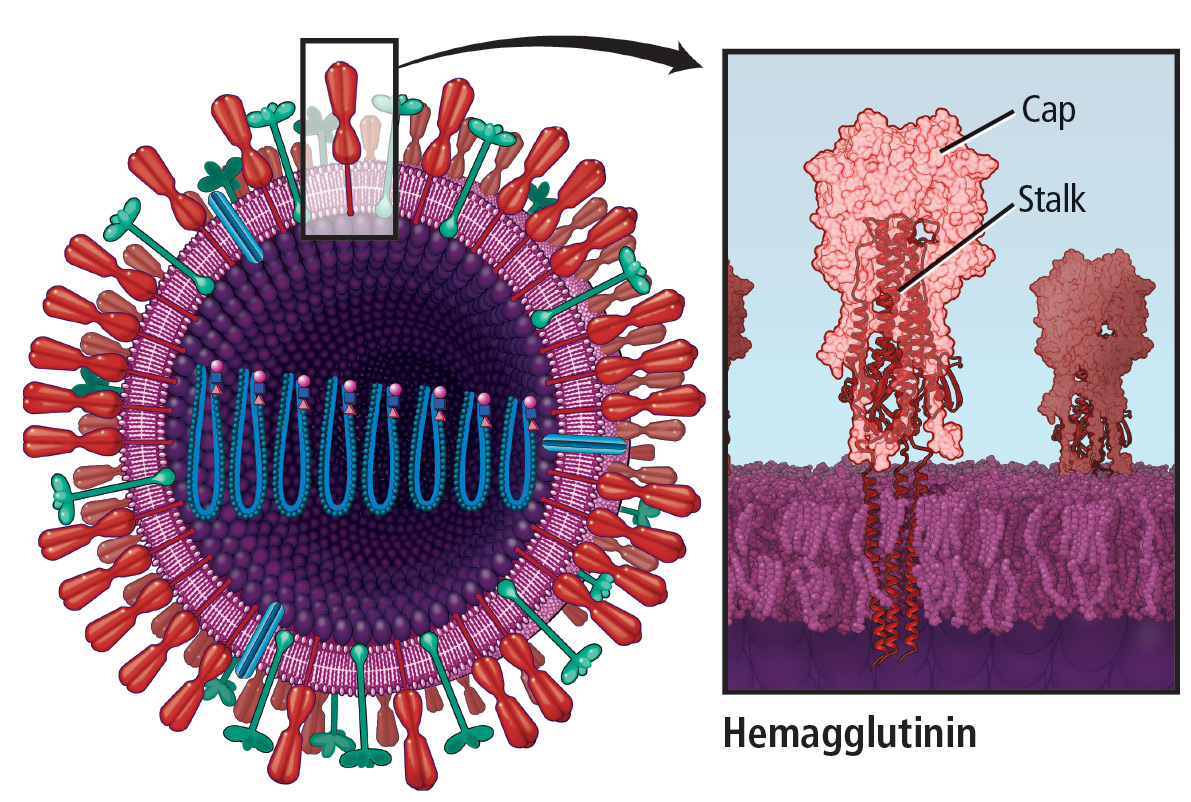
Influenza update 2018–2019: 100 years after the great pandemic
This centennial year update focuses primarily on immunization, but also reviews epidemiology, transmission, and treatment.
EPIDEMIOLOGY
2017–2018 was a bad season
The 2017–2018 influenza epidemic was memorable, dominated by influenza A(H3N2) viruses with morbidity and mortality rates approaching pandemic numbers. It lasted 19 weeks, killed more people than any other epidemic since 2010, particularly children, and was associated with 30,453 hospitalizations—almost twice the previous season high in some parts of the United States.2
Regrettably, 171 unvaccinated children died during 2017–2018, accounting for almost 80% of deaths.2 The mean age of the children who died was 7.1 years; 51% had at least 1 underlying medical condition placing them at risk for influenza-related complications, and 57% died after hospitalization.2
Recent estimates of the incidence of symptomatic influenza among all ages ranged from 3% to 11%, which is slightly lower than historical estimates. The rates were higher for children under age 18 than for adults.3 Interestingly, influenza A(H3N2) accounted for 50% of cases of non-mumps viral parotitis during the 2014–2015 influenza season in the United States.4
Influenza C exists but is rare
Influenza A and B account for almost all influenza-related outpatient visits and hospitalizations. Surveillance data from May 2013 through December 2016 showed that influenza C accounts for 0.5% of influenza-related outpatient visits and hospitalizations, particularly affecting children ages 6 to 24 months. Medical comorbidities and copathogens were seen in all patients requiring intensive care and in most hospitalizations.5 Diagnostic tests for influenza C are not widely available.
Dogs and cats: Factories for new flu strains?
While pigs and birds are the major reservoirs of influenza viral genetic diversity from which infection is transmitted to humans, dogs and cats have recently emerged as possible sources of novel reassortant influenza A.6 With their frequent close contact with humans, our pets may prove to pose a significant threat.
Obesity a risk factor for influenza
Obesity emerged as a risk factor for severe influenza in the 2009 pandemic. Recent data also showed that obesity increases the duration of influenza A virus shedding, thus increasing duration of contagiousness.7
Influenza a cardiovascular risk factor
Previous data showed that influenza was a risk factor for cardiovascular events. Two recent epidemiologic studies from the United Kingdom showed that laboratory-confirmed influenza was associated with higher rates of myocardial infarction and stroke for up to 4 weeks.8,9
Which strain is the biggest threat?
Predicting which emerging influenza serotype may cause the next pandemic is difficult, but influenza A(H7N9), which had not infected humans until 2013 but has since infected about 1,600 people in China and killed 37% of them, appears to have the greatest potential.10
National influenza surveillance programs and influenza-related social media applications have been developed and may get a boost from technology. A smartphone equipped with a temperature sensor can instantly detect one’s temperature with great precision. A 2018 study suggested that a smartphone-driven thermometry application correlated well with national influenza-like illness activity and improved its forecast in real time and up to 3 weeks in advance.11
TRANSMISSION
Humidity may not block transmission
Animal studies have suggested that humidity in the air interferes with transmission of airborne influenza virus, partially from biologic inactivation. But when a recent study used humidity-controlled chambers to investigate the stability of the 2009 influenza A(H1N1) virus in suspended aerosols and stationary droplets, the virus remained infectious in aerosols across a wide range of relative humidities, challenging the common belief that humidity destabilizes respiratory viruses in aerosols.12
One sick passenger may not infect the whole plane
Transmission of respiratory viruses on airplane flights has long been considered a potential avenue for spreading influenza. However, a recent study that monitored movements of individuals on 10 transcontinental US flights and simulated inflight transmission based on these data showed a low probability of direct transmission, except for passengers seated in close proximity to an infectious passenger.13
WHAT’S IN THE NEW FLU SHOT?
The 2018–2019 quadrivalent vaccine for the Northern Hemisphere14 contains the following strains:
- A/Michigan/45/2015 A(H1N1)pdm09-like virus
- A/Singapore/INFIMH-16-0019/2016 (H3N2)-like virus
- B/Colorado/06/2017-like virus (Victoria lineage)
- B/Phuket/3073/2013-like virus (Yamagata lineage).
The A(H3N2) (Singapore) and B/Victoria lineage components are new this year. The A(H3N2) strain was the main cause of the 2018 influenza epidemic in the Southern Hemisphere.
The quadrivalent live-attenuated vaccine, which was not recommended during the 2016–2017 and 2017–2018 influenza seasons, has made a comeback and is recommended for the 2018–2019 season in people for whom it is appropriate based on age and comorbidities.15 Although it was effective against influenza B and A(H3N2) viruses, it was less effective against the influenza A(H1N1)pdm09-like viruses during the 2013–2014 and 2015–2016 seasons.
A/Slovenia/2903/2015, the new A(H1N1)pdm09-like virus included in the 2018–2019 quadrivalent live-attenuated vaccine, is significantly more immunogenic than its predecessor, A/Bolivia/559/2013, but its clinical effectiveness remains to be seen.
PROMOTING VACCINATION
How effective is it?
Influenza vaccine effectiveness in the 2017–2018 influenza season was 36% overall, 67% against A(H1N1), 42% against influenza B, and 25% against A(H3N2).16 It is estimated that influenza vaccine prevents 300 to 4,000 deaths annually in the United States alone.17
A 2018 Cochrane review17 concluded that vaccination reduced the incidence of influenza by about half, with 2.3% of the population contracting the flu without vaccination compared with 0.9% with vaccination (risk ratio 0.41, 95% confidence interval 0.36–0.47). The same review found that 71 healthy adults need to be vaccinated to prevent 1 from experiencing influenza, and 29 to prevent 1 influenza-like illness.
Several recent studies showed that influenza vaccine effectiveness varied based on age and influenza serotype, with higher effectiveness in people ages 5 to 17 and ages 18 to 64 than in those age 65 and older.18–20 A mathematical model of influenza transmission and vaccination in the United States determined that even relatively low-efficacy influenza vaccines can be very useful if optimally distributed across age groups.21
Vaccination rates are low, and ‘antivaxxers’ are on the rise
Although the influenza vaccine is recommended in the United States for all people age 6 months and older regardless of the state of their health, vaccination rates remain low. In 2016, only 37% of employed adults were vaccinated. The highest rate was for government employees (45%), followed by private employees (36%), followed by the self-employed (30%).22
A national goal is to immunize 80% of all Americans and 90% of at-risk populations (which include children and the elderly).23 The number of US hospitals that require their employees to be vaccinated increased from 37.1% in 2013 to 61.4% in 2017.24 Regrettably, as of March 2018, 14 lawsuits addressing religious objections to hospital influenza vaccination mandates have been filed.25
Despite hundreds of studies demonstrating the efficacy, safety, and cost savings of influenza vaccination, the antivaccine movement has been growing in the United States and worldwide.26 All US states except West Virginia, Mississippi, and California allow nonmedical exemptions from vaccination based on religious or personal belief.27 Several US metropolitan areas represent “hot spots” for these exemptions.28 This may render such areas vulnerable to vaccine-preventable diseases, including influenza.
Herd immunity: We’re all in this together
Some argue that the potential adverse effects and the cost of vaccination outweigh the benefits, but the protective benefits of herd immunity are significant for those with comorbidities or compromised immunity.
Educating the public about herd immunity and local influenza vaccination uptake increases people’s willingness to be vaccinated.29 A key educational point is that at least 70% of a community needs to be vaccinated to prevent community outbreaks; this protects everyone, including those who do not mount a protective antibody response to influenza vaccination and those who are not vaccinated.
DOES ANNUAL VACCINATION BLUNT ITS EFFECTIVENESS?
Some studies from the 1970s and 1980s raised concern over a possible negative effect of annual influenza vaccination on vaccine effectiveness. The “antigenic distance hypothesis” holds that vaccine effectiveness is influenced by antigenic similarity between the previous season’s vaccine serotypes and the epidemic serotypes, as well as the antigenic similarity between the serotypes of the current and previous seasons.
A meta-analysis of studies from 2010 through 2015 showed significant inconsistencies in repeat vaccination effects within and between seasons and serotypes. It also showed that vaccine effectiveness may be influenced by more than 1 previous season, particularly for influenza A(H3N2), in which repeated vaccination can blunt the hemagglutinin antibody response.30
A study from Japan showed that people who needed medical attention for influenza in the previous season were at lower risk of a similar event in the current season.31 Prior-season influenza vaccination reduced current-season vaccine effectiveness only in those who did not have medically attended influenza in the prior season. This suggests that infection is more immunogenic than vaccination, but only against the serotype causing the infection and not the other serotypes included in the vaccine.
An Australian study showed that annual influenza vaccination did not decrease vaccine effectiveness against influenza-associated hospitalization. Rather, effectiveness increased by about 15% in those vaccinated in both current and previous seasons compared with those vaccinated in either season alone.32
European investigators showed that repeated seasonal influenza vaccination in the elderly prevented the need for hospitalization due to influenza A(H3N2) and B, but not A(H1N1)pdm09.33
VACCINATION IN SPECIAL POPULATIONS
High-dose vaccine for older adults
The high-dose influenza vaccine has been licensed since 2009 for use in the United States for people ages 65 and older.
Recent studies confirmed that high-dose vaccine is more effective than standard-dose vaccine in veterans34 and US Medicare beneficiaries.35
The high-dose vaccine is rapidly becoming the primary vaccine given to people ages 65 and older in retail pharmacies, where vaccination begins earlier in the season than in providers’ offices.36 Some studies have shown that the standard-dose vaccine wanes in effectiveness toward the end of the influenza season (particularly if the season is long) if it is given very early. It remains to be seen whether the same applies to the high-dose influenza vaccine.
Some advocate twice-annual influenza vaccination, particularly for older adults living in tropical and subtropical areas, where influenza seasons are more prolonged. However, a recently published study observed reductions in influenza-specific hemagglutination inhibition and cell-mediated immunity after twice-annual vaccination.37
Vaccination is beneficial during pregnancy
Many studies have shown the value of influenza vaccination during pregnancy for both mothers and their infants.
One recently published study showed that 18% of infants who developed influenza required hospitalization.38 In that study, prenatal and postpartum maternal influenza vaccination decreased the odds of influenza in infants by 61% and 53%, respectively.
Another study showed that vaccine effectiveness did not vary by gestational age at vaccination.39
Some studies have shown that influenza virus infection can increase susceptibility to certain bacterial infections. A post hoc analysis of an influenza vaccination study in pregnant women suggested that the vaccine was also associated with decreased rates of pertussis in these women.40
Factors that make vaccination less effective
Several factors including age-related frailty and iatrogenic and disease-related immunosuppression can affect vaccine effectiveness.
Frailty. A recent study showed that vaccine effectiveness was 77.6% in nonfrail older adults but only 58.7% in frail older adults.41
Immunosuppression. Temporary discontinuation of methotrexate for 2 weeks after influenza vaccination in patients with rheumatoid arthritis improves vaccine immunogenicity without precipitating disease flare.42 Solid-organ and hematopoietic stem cell transplant recipients who received influenza vaccine were less likely to develop pneumonia and require intensive care unit admission.43
The high-dose influenza vaccine is more immunogenic than the standard-dose vaccine in solid-organ transplant recipients.44
Statins are widely prescribed and have recently been associated with reduced influenza vaccine effectiveness against medically attended acute respiratory illness, but their benefits in preventing cardiovascular events outweigh this risk.45
FUTURE VACCINE CONSIDERATIONS
Moving away from eggs
During the annual egg-based production process, which takes several months, the influenza vaccine acquires antigenic changes that allow replication in eggs, particularly in the hemagglutinin protein, which mediates receptor binding. This process of egg adaptation may cause antigenic changes that decrease vaccine effectiveness against circulating viruses.
The cell-based baculovirus influenza vaccine grown in dog kidney cells has higher antigenic content and is not subject to the limitations of egg-based vaccine, although it still requires annual updates. A recombinant influenza vaccine reduces the probability of influenza-like illness by 30% compared with the egg-based influenza vaccine, but also still requires annual updates.46 The market share of these non-egg-based vaccines is small, and thus their effectiveness has yet to be demonstrated.
The US Department of Defense administered the cell-based influenza vaccine to about one-third of Armed Forces personnel, their families, and retirees in the 2017–2018 influenza seasons, and data on its effectiveness are expected in the near future.47
A universal vaccine would be ideal
The quest continues for a universal influenza vaccine, one that remains protective for several years and does not require annual updates.48 Such a vaccine would protect against seasonal epidemic influenza drift variants and pandemic strains. More people could likely be persuaded to be vaccinated once rather than every year.
An ideal universal vaccine would be suitable for all age groups, at least 75% effective against symptomatic influenza virus infection, protective against all influenza A viruses (influenza A, not B, causes pandemics and seasonal epidemics), and durable through multiple influenza seasons.51
Research and production of such a vaccine are expected to require funding of about $1 billion over the next 5 years.
Boosting effectiveness
Estimates of influenza vaccine effectiveness range from 40% to 60% in years when the vaccine viruses closely match the circulating viruses, and variably lower when they do not match. The efficacy of most other vaccines given to prevent other infections is much higher.
New technologies to improve influenza vaccine effectiveness are needed, particularly for influenza A(H3N2) viruses, which are rapidly evolving and are highly susceptible to egg-adaptive mutations in the manufacturing process.
In one study, a nanoparticle vaccine formulated with a saponin-based adjuvant induced hemagglutination inhibition responses that were even greater than those induced by the high-dose vaccine.52
Immunoglobulin A (IgA) may be a more effective vaccine target than traditional influenza vaccines that target IgG, since different parts of IgA may engage the influenza virus simultaneously.53
Vaccines can be developed more quickly than in the past. The timeline from viral sequencing to human studies with deoxyribonucleic acid plasmid vaccines decreased from 20 months in 2003 for the severe acquired respiratory syndrome coronavirus to 11 months in 2006 for influenza A/Indonesia/2006 (H5), to 4 months in 2009 for influenza A/California/2009 (H1), to 3.5 months in 2016 for Zika virus.54 This is because it is possible today to sequence a virus and insert the genetic material into a vaccine platform without ever having to grow the virus.
TREATMENT
Numerous studies have found anti-influenza medications to be effective. Nevertheless, in an analysis of the 2011–2016 influenza seasons, only 15% of high-risk patients were prescribed anti-influenza medications within 2 days of symptom onset, including 37% in those with laboratory-confirmed influenza.55 Fever was associated with an increased rate of antiviral treatment, but 25% of high-risk outpatients were afebrile. Empiric treatment of 4 high-risk outpatients with acute respiratory illness was needed to treat 1 patient with influenza.55
Treatment with a neuraminidase inhibitor within 2 days of illness has recently been shown to improve survival and shorten duration of viral shedding in patients with avian influenza A(H7N9) infection.56 Antiviral treatment within 2 days of illness is associated with improved outcomes in transplant recipients57 and with a lower risk of otitis media in children.58
Appropriate anti-influenza treatment is as important as avoiding unnecessary antibiotics. Regrettably, as many as one-third of patients with laboratory-confirmed influenza are prescribed antibiotics.59
The US Food and Drug Administration warns against fraudulent unapproved over-the-counter influenza products.60
Baloxavir marboxil
Baloxavir marboxil is a new anti-influenza medication approved in Japan in February 2018 and anticipated to be available in the United States sometime in 2019.
This prodrug is hydrolyzed in vivo to the active metabolite, which selectively inhibits cap-dependent endonuclease enzyme, a key enzyme in initiation of messenger ribonucleic acid synthesis required for influenza viral replication.61
In a double-blind phase 3 trial, the median time to alleviation of influenza symptoms is 26.5 hours shorter with baloxavir marboxil than with placebo. One tablet was as effective as 5 days of the neuraminidase inhibitor oseltamivir and was associated with greater reduction in viral load 1 day after initiation, and similar side effects.62 Of concern is the emergence of nucleic acid substitutions conferring resistance to baloxavir; this occurred in 2.2% and 9.7% of baloxavir recipients in the phase 2 and 3 trials, respectively.
CLOSING THE GAPS
Several gaps in the management of influenza persist since the 1918 pandemic.1 These include gaps in epidemiology, prevention, diagnosis, treatment, and prognosis.
- Global networks wider than current ones are needed to address this global disease and to prioritize coordination efforts.
- Establishing and strengthening clinical capacity is needed in limited resource settings. New technologies are needed to expedite vaccine development and to achieve progress toward a universal vaccine.
- Current diagnostic tests do not distinguish between seasonal and novel influenza A viruses of zoonotic origin, which are expected to cause the next pandemic.
- Current antivirals have been shown to shorten duration of illness in outpatients with uncomplicated influenza, but the benefit in hospitalized patients has been less well established.
- In 2007, resistance of seasonal influenza A(H1N1) to oseltamivir became widespread. In 2009, pandemic influenza A(H1N1), which is highly susceptible to oseltamivir, replaced the seasonal virus and remains the predominantly circulating A(H1N1) strain.
- A small-molecule fragment, N-cyclohexyaltaurine, binds to the conserved hemagglutinin receptor-binding site in a manner that mimics the binding mode of the natural receptor sialic acid. This can serve as a template to guide the development of novel broad-spectrum small-molecule anti-influenza drugs.63
- Biomarkers that can accurately predict development of severe disease in patients with influenza are needed.
- Uyeki TM, Fowler RA, Fischer WA. Gaps in the clinical management of influenza: a century since the 1918 pandemic. JAMA 2018; 320(8):755–756. doi:10.1001/jama.2018.8113
- Garten R, Blanton L, Elal AI, et al. Update: influenza activity in the United States during the 2017–18 season and composition of the 2018–19 influenza vaccine. MMWR Morb Mortal Wkly Rep 2018; 67(22):634–642. doi:10.15585/mmwr.mm6722a4
- Tokars JI, Olsen SJ, Reed C. Seasonal incidence of symptomatic influenza in the United States. Clin Infect Dis 2018; 66(10):1511–1518. doi:10.1093/cid/cix1060
- Elbadawi LI, Talley P, Rolfes MA, et al. Non-mumps viral parotitis during the 2014–2015 influenza season in the United States. Clin Infect Dis 2018. Epub ahead of print. doi:10.1093/cid/ciy137
- Thielen BK, Friedlander H, Bistodeau S, et al. Detection of influenza C viruses among outpatients and patients hospitalized for severe acute respiratory infection, Minnesota, 2013–2016. Clin Infect Dis 2018; 66(7):1092–1098. doi:10.1093/cid/cix931
- Chena Y, Trovãob NS, Wang G, et al. Emergence and evolution of novel reassortant influenza A viruses in canines in southern China. MBio 2018; 9(3):e00909–e00918. doi:10.1128/mBio.00909-18
- Maier HE, Lopez R, Sanchez N, et al. Obesity increases the duration of influenza A virus shedding in adults. J Infect Dis 2018. Epub ahead of print. doi:10.1093/infdis/jiy370
- Warren-Gash C, Blackburn R, Whitaker H, McMenamin J, Hayward AC. Laboratory-confirmed respiratory infections as triggers for acute myocardial infarction and stroke: a self-controlled case series analysis of national linked datasets from Scotland. Eur Respir J 2018; 51(3):1701794. doi:10.1183/13993003.01794-2017
- Blackburn R, Zhao H, Pebody R, Hayward A, Warren-Gash C. Laboratory-confirmed respiratory infections as predictors of hospital admission for myocardial infarction and stroke: time-series analysis of English data for 2004–2015. Clin Infect Dis 2018; 67(1):8–17. doi:10.1093/cid/cix1144
- Newsweek; Andrew S. What is disease X? Deadly bird flu virus could be next pandemic. www.newsweek.com/disease-x-bird-flu-deaths-pandemic-what-h7n9-979723. Accessed October 3, 2018.
- Miller AC, Singh I, Koehler E, Polgreen PM. A smartphone-driven thermometer application for real-time population- and individual-level influenza surveillance. Clin Infect Dis 2018; 67(3):388–397. doi:10.1093/cid/ciy073
- Kormuth KA, Lin K, Prussin AJ 2nd, et al. Influenza virus infectivity is retained in aerosols and droplets independent of relative humidity, J Infect Dis 2018; 218(5):739–747. doi:10.1093/infdis/jiy221
- Hertzberg VS, Weiss H, Elon L, et. al. Behaviors, movements, and transmission of droplet-mediated respiratory diseases during transcontinental airline flights. Proc Natl Acad Sci U S A 2018; 115(14):3623–3627. doi:10.1073/pnas.1711611115
- Grohskopf LA, Sokolow LZ, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2018–19 influenza season. MMWR Recomm Rep 2018; 67(3):1–20. doi:10.15585/mmwr.rr6703a1
- Grohskopf LA, Sokolow LZ, Fry AM, Walter EB, Jernigan DB. Update: ACIP recommendations for the use of quadrivalent live attenuated influenza vaccine (LAIV4)—United States, 2018–19 influenza season. MMWR Morb Mortal Wkly Rep 2018; 67(22):643–645. doi:10.15585/mmwr.mm6722a5
- Flannery B, Chung JR, Belongia EA, et al. Interim estimates of 2017–18 seasonal influenza vaccine effectiveness—United States, February 2018. MMWR Morb Mortal Wkly Rep 2018; 67(6):180–185. doi:10.15585/mmwr.mm6706a2
- Demicheli V, Jefferson T, Ferroni E, Rivetti A, Di Pietrantonj C. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev 2018; 2:CD001269. doi:10.1002/14651858.CD001269.pub6
- Flannery B, Smith C, Garten RJ, et al. Influence of birth cohort on effectiveness of 2015–2016 influenza vaccine against medically attended illness due to 2009 pandemic influenza A(H1N1) virus in the United States. J Infect Dis 2018; 218(2):189–196. doi:10.1093/infdis/jix634
- Rondy M, El Omeiri N, Thompson MG, Leveque A, Moren A, Sullivan SG. Effectiveness of influenza vaccines in preventing severe influenza illness among adults: a systematic review and meta-analysis of test-negative design case-control studies. J Infect 2017; 75(5):381–394. doi:10.1016/j.jinf.2017.09.010
- Stein Y, Mandelboim M, Sefty H, et al; Israeli Influenza Surveillance Network (IISN). Seasonal influenza vaccine effectiveness in preventing laboratory-confirmed influenza in primary care in Israel, 2016–2017 season: insights into novel age-specific analysis. Clin Infect Dis 2018; 66(9):1383–1391. doi:10.1093/cid/cix1013
- Sah P, Medlock J, Fitzpatrick MC, Singer BH, Galvani AP. Optimizing the impact of low-efficacy influenza vaccines. Proc Natl Acad Sci U S A 2018; 115(20):5151–5156. doi:10.1073/pnas.1802479115
- QuickStats: percentage of currently employed adults aged ≥ 18 years who received influenza vaccine in the past 12 months, by employment category—national health interview survey, United States, 2012 and 2016. MMWR Morb Mortal Wkly Rep 2018; 67(16):480. doi:10.15585/mmwr.mm6716a8
- Healthy People.gov. Immunization and infectious diseases. IID-12. Increase the percentage of children and adults who are vaccinated annually against seasonal influenza. www.healthypeople.gov/2020/topics-objectives/topic/immunization-and-infectious-diseases/objectives. Accessed October 3, 2018.
- Greene MT, Fowler KE, Ratz D, Krein SL, Bradley SF, Saint S. Changes in influenza vaccination requirements for health care personnel in US hospitals. JAMA Network Open 2018; 1(2):e180143. doi:10.1001/jamanetworkopen.2018.0143
- Opel DJ, Sonne JA, Mello MM. Vaccination without litigation—addressing religious objections to hospital influenza-vaccination mandates. N Engl J Med 2018; 378(9):785–788. doi:10.1056/NEJMp1716147
- Horowitz J. Italy loosens vaccine law just as children return to school. New York Times Sept. 20, 2018. www.nytimes.com/2018/09/20/world/europe/italy-vaccines-five-star-movement.html.
- National Conference of State Legislature. States with religious and philosophical exemptions from school immunization requirements. www.ncsl.org/research/health/school-immunization-exemption-state-laws.aspx. Accessed October 3, 2018.
- Olive JK, Hotez PJ, Damania A, Nolan MS. The state of the antivaccine movement in the United States: a focused examination of nonmedical exemptions in states and counties. PLoS Med 2018; 15(6):e1002578. doi:10.1371/journal.pmed.1002578
- Logan J, Nederhoff D, Koch B, et al. ‘What have you HEARD about the HERD?’ Does education about local influenza vaccination coverage and herd immunity affect willingness to vaccinate? Vaccine 2018; 36(28):4118–4125. doi:10.1016/j.vaccine.2018.05.037
- Belongia EA, Skowronski DM, McLean HQ, Chambers C, Sundaram ME, De Serres G. Repeated annual influenza vaccination and vaccine effectiveness: review of evidence. Expert Rev Vaccines 2017; 16(7):1–14. doi:10.1080/14760584.2017.1334554
- Saito N, Komori K, Suzuki M, et al. Negative impact of prior influenza vaccination on current influenza vaccination among people infected and not infected in prior season: a test-negative case-control study in Japan. Vaccine 2017; 35(4):687–693. doi:10.1016/j.vaccine.2016.11.024
- Cheng AC, Macartney KK, Waterer GW, Kotsimbos T, Kelly PM, Blyth CC; Influenza Complications Alert Network (FluCAN) Investigators. Repeated vaccination does not appear to impact upon influenza vaccine effectiveness against hospitalization with confirmed influenza. Clin Infect Dis 2017; 64(11):1564–1572. doi:10.1093/cid/cix209
- Rondy M, Launay O, Castilla J, et al; InNHOVE/I-MOVE+working group. Repeated seasonal influenza vaccination among elderly in Europe: effects on laboratory confirmed hospitalised influenza. Vaccine 2017; 35(34):4298–4306. doi:10.1016/j.vaccine.2017.06.088
- Young-Xu Y, van Aalst R, Mahmud SM, et al. Relative vaccine effectiveness of high-dose versus standard-dose influenza vaccines among Veterans Health Administration patients. J Infect Dis 2018; 217(11):1718–1727. doi:10.1093/infdis/jiy088
- Shay DK, Chillarige Y, Kelman J, et al. Comparative effectiveness of high-dose versus standard-dose influenza vaccines among US Medicare beneficiaries in preventing postinfluenza deaths during 2012–2013 and 2013–2014. J Infect Dis 2017; 215(4):510–517. doi:10.1093/infdis/jiw641
- Madaras-Kelly K, Remington R, Hruza H, Xu D. Comparative effectiveness of high-dose versus standard-dose influenza vaccines in preventing postinfluenza deaths. J Infect Dis 2018; 218(2):336–337. doi:10.1093/infdis/jix645
- Tam YH, Valkenburg SA, Perera RAPM, et al. Immune responses to twice-annual influenza vaccination in older adults in Hong Kong. Clin Infect Dis 2018; 66(6):904–912. doi:10.1093/cid/cix900
- Ohfuji S, Deguchi M, Tachibana D, et al; Osaka Pregnant Women Influenza Study Group. Protective effect of maternal influenza vaccination on influenza in their infants: a prospective cohort study. J Infect Dis 2018; 217(6):878–886. doi:10.1093/infdis/jix629
- Katz J, Englund JA, Steinhoff MC, et al. Impact of timing of influenza vaccination in pregnancy on transplacental antibody transfer, influenza incidence, and birth outcomes: a randomized trial in rural Nepal. Clin Infect Dis 2018; 67(3):334–340. doi:10.1093/cid/ciy090
- Nunes MC, Cutland CL, Madhi SA. Influenza vaccination during pregnancy and protection against pertussis. N Engl J Med 2018; 378(13):1257–1258. doi:10.1056/NEJMc1705208
- Andrew MK, Shinde V, Ye L, et al; Serious Outcomes Surveillance Network of the Public Health Agency of Canada/Canadian Institutes of Health Research Influenza Research Network (PCIRN) and the Toronto Invasive Bacterial Diseases Network (TIBDN). The importance of frailty in the assessment of influenza vaccine effectiveness against influenza-related hospitalization in elderly people. J Infect Dis 2017; 216(4):405–414. doi:10.1093/infdis/jix282
- Park JK, Lee YJ, Shin K, et al. Impact of temporary methotrexate discontinuation for 2 weeks on immunogenicity of seasonal influenza vaccination in patients with rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis 2018; 77(6):898–904. doi:10.1136/annrheumdis-2018-213222
- Kumar D, Ferreira VH, Blumberg E, et al. A five-year prospective multi-center evaluation of influenza infection in transplant recipients. Clin Infect Dis 2018. Epub ahead of print. doi:10.1093/cid/ciy294
- Natori Y, Shiotsuka M, Slomovic J, et al. A double-blind, randomized trial of high-dose vs standard-dose influenza vaccine in adult solid-organ transplant recipients. Clin Infect Dis 2018; 66(11):1698–1704. doi:10.1093/cid/cix1082
- Omer SB, Phadke VK, Bednarczyk BA, Chamberlain AT, Brosseau JL, Orenstein WA. Impact of statins on influenza vaccine effectiveness against medically attended acute respiratory illness. J Infect Dis 2016; 213(8):1216–1223. doi:10.1093/infdis/jiv457
- Dunkle LM, Izikson R, Patriarca P, et al. Efficacy of recombinant influenza vaccine in adults 50 years of age or older. N Engl J Med 2017; 376(25):2427–2436. doi:10.1056/NEJMoa1608862
- STAT; Branswell H. How the US military might help answer a critical question about the flu vaccine. www.statnews.com/2018/03/02/flu-vaccine-egg-production-data. Accessed October 3, 2018.
- Paules CI, Sullivan SG, Subbarao K, Fauci AS. Chasing seasonal influenza—the need for a universal influenza vaccine. N Engl J Med 2018; 378(1):7–9. doi:10.1056/NEJMp1714916
- Jin XW, Mossad SB. Avian influenza: an emerging pandemic threat. Cleve Clin J Med 2005; 72:1129-1134. pmid:16392727
- Wei WI, Brunger AT, Skehel JJ, Wiley DC. Refinement of the influenza virus hemagglutinin by simulated annealing. J Mol Biol 1990; 212(4):737–761. doi:10.1016/0022-2836(90)90234-D
- Erbelding EJ, Post DJ, Stemmy EJ, et al. A universal influenza vaccine: the strategic plan for the National Institute of Allergy and Infectious Diseases, J Infect Dis 2018; 218(3):347–354. doi:10.1093/infdis/jiy103
- Shinde V, Fries L, Wu Y, et al. Improved titers against influenza drift variants with a nanoparticle vaccine. N Engl J Med 2018; 378(24):2346–2348. doi:10.1056/NEJMc1803554
- Maurer MA, Meyer L, Bianchi M, et al. Glycosylation of human IgA directly inhibits influenza A and other sialic-acid-binding viruses. Cell Rep 2018; 23(1):90–99. doi:10.1016/j.celrep.2018.03.027
- Graham BS, Mascola JR, Fauci AS. Novel vaccine technologies: essential components of an adequate response to emerging viral diseases. JAMA 2018; 319(14):1431–1432. doi:10.1001/jama.2018.0345
- Stewart RJ, Flannery B, Chung JR, et al. Influenza antiviral prescribing for outpatients with an acute respiratory illness and at high risk for influenza-associated complications during 5 influenza seasons—United States, 2011–2016. Clin Infect Dis 2018; 66(7):1035–1041. doi:10.1093/cid/cix922
- Zheng S, Tang L, Gao H, et al. Benefit of early initiation of neuraminidase inhibitor treatment to hospitalized patients with avian influenza A(H7N9) virus. Clin Infect Dis 2018; 66(7):1054–1060. doi:10.1093/cid/cix930
- Kumar D, Ferreira VH, Blumberg E, et al. A five-year prospective multi-center evaluation of influenza infection in transplant recipients. Clin Infect Dis 2018. Epub ahead of print. doi:10.1093/cid/ciy294
- Malosh RE, Martin ET, Heikkinen T, Brooks WA, Whitley RJ, Monto AS. Efficacy and safety of oseltamivir in children: systematic review and individual patient data meta-analysis of randomized controlled trials. Clin Infect Dis 2018; 66(10):1492–1500. doi:10.1093/cid/cix1040
- Havers FP, Hicks LA, Chung JR, et al. Outpatient antibiotic prescribing for acute respiratory infections during influenza seasons. JAMA Network Open 2018; 1(2):e180243. doi:10.1001/jamanetworkopen.2018.0243
- US Food and Drug Administration. FDA warns of fraudulent and unapproved flu products. www.fda.gov/newsevents/newsroom/pressannouncements/ucm599223.htm. Accessed October 3, 2018.
- Portsmouth S, Kawaguchi K, Arai M, Tsuchiya K, Uehara T. Cap-dependent endonuclease inhibitor S-033188 for the treatment of influenza: results from a phase 3, randomized, double-blind, placebo- and active-controlled study in otherwise healthy adolescents and adults with seasonal influenza. Open Forum Infect Dis 2017; 4(suppl 1):S734. doi:10.1093/ofid/ofx180.001
- Hayden FG, Sugaya N, Hirotsu N, et al; Baloxavir Marboxil Investigators Group. Baloxavir Marboxil for uncomplicated influenza in adults and adolescents. N Engl J Med 2018; 379(10):913–923. doi:10.1056/NEJMoa1716197
- Kadam RU, Wilson IA. A small-molecule fragment that emulates binding of receptor and broadly neutralizing antibodies to influenza A hemagglutinin. Proc Natl Acad Sci U S A 2018; 115(16):4240–4245. doi:10.1073/pnas.1801999115
This centennial year update focuses primarily on immunization, but also reviews epidemiology, transmission, and treatment.
EPIDEMIOLOGY
2017–2018 was a bad season
The 2017–2018 influenza epidemic was memorable, dominated by influenza A(H3N2) viruses with morbidity and mortality rates approaching pandemic numbers. It lasted 19 weeks, killed more people than any other epidemic since 2010, particularly children, and was associated with 30,453 hospitalizations—almost twice the previous season high in some parts of the United States.2
Regrettably, 171 unvaccinated children died during 2017–2018, accounting for almost 80% of deaths.2 The mean age of the children who died was 7.1 years; 51% had at least 1 underlying medical condition placing them at risk for influenza-related complications, and 57% died after hospitalization.2
Recent estimates of the incidence of symptomatic influenza among all ages ranged from 3% to 11%, which is slightly lower than historical estimates. The rates were higher for children under age 18 than for adults.3 Interestingly, influenza A(H3N2) accounted for 50% of cases of non-mumps viral parotitis during the 2014–2015 influenza season in the United States.4
Influenza C exists but is rare
Influenza A and B account for almost all influenza-related outpatient visits and hospitalizations. Surveillance data from May 2013 through December 2016 showed that influenza C accounts for 0.5% of influenza-related outpatient visits and hospitalizations, particularly affecting children ages 6 to 24 months. Medical comorbidities and copathogens were seen in all patients requiring intensive care and in most hospitalizations.5 Diagnostic tests for influenza C are not widely available.
Dogs and cats: Factories for new flu strains?
While pigs and birds are the major reservoirs of influenza viral genetic diversity from which infection is transmitted to humans, dogs and cats have recently emerged as possible sources of novel reassortant influenza A.6 With their frequent close contact with humans, our pets may prove to pose a significant threat.
Obesity a risk factor for influenza
Obesity emerged as a risk factor for severe influenza in the 2009 pandemic. Recent data also showed that obesity increases the duration of influenza A virus shedding, thus increasing duration of contagiousness.7
Influenza a cardiovascular risk factor
Previous data showed that influenza was a risk factor for cardiovascular events. Two recent epidemiologic studies from the United Kingdom showed that laboratory-confirmed influenza was associated with higher rates of myocardial infarction and stroke for up to 4 weeks.8,9
Which strain is the biggest threat?
Predicting which emerging influenza serotype may cause the next pandemic is difficult, but influenza A(H7N9), which had not infected humans until 2013 but has since infected about 1,600 people in China and killed 37% of them, appears to have the greatest potential.10
National influenza surveillance programs and influenza-related social media applications have been developed and may get a boost from technology. A smartphone equipped with a temperature sensor can instantly detect one’s temperature with great precision. A 2018 study suggested that a smartphone-driven thermometry application correlated well with national influenza-like illness activity and improved its forecast in real time and up to 3 weeks in advance.11
TRANSMISSION
Humidity may not block transmission
Animal studies have suggested that humidity in the air interferes with transmission of airborne influenza virus, partially from biologic inactivation. But when a recent study used humidity-controlled chambers to investigate the stability of the 2009 influenza A(H1N1) virus in suspended aerosols and stationary droplets, the virus remained infectious in aerosols across a wide range of relative humidities, challenging the common belief that humidity destabilizes respiratory viruses in aerosols.12
One sick passenger may not infect the whole plane
Transmission of respiratory viruses on airplane flights has long been considered a potential avenue for spreading influenza. However, a recent study that monitored movements of individuals on 10 transcontinental US flights and simulated inflight transmission based on these data showed a low probability of direct transmission, except for passengers seated in close proximity to an infectious passenger.13
WHAT’S IN THE NEW FLU SHOT?
The 2018–2019 quadrivalent vaccine for the Northern Hemisphere14 contains the following strains:
- A/Michigan/45/2015 A(H1N1)pdm09-like virus
- A/Singapore/INFIMH-16-0019/2016 (H3N2)-like virus
- B/Colorado/06/2017-like virus (Victoria lineage)
- B/Phuket/3073/2013-like virus (Yamagata lineage).
The A(H3N2) (Singapore) and B/Victoria lineage components are new this year. The A(H3N2) strain was the main cause of the 2018 influenza epidemic in the Southern Hemisphere.
The quadrivalent live-attenuated vaccine, which was not recommended during the 2016–2017 and 2017–2018 influenza seasons, has made a comeback and is recommended for the 2018–2019 season in people for whom it is appropriate based on age and comorbidities.15 Although it was effective against influenza B and A(H3N2) viruses, it was less effective against the influenza A(H1N1)pdm09-like viruses during the 2013–2014 and 2015–2016 seasons.
A/Slovenia/2903/2015, the new A(H1N1)pdm09-like virus included in the 2018–2019 quadrivalent live-attenuated vaccine, is significantly more immunogenic than its predecessor, A/Bolivia/559/2013, but its clinical effectiveness remains to be seen.
PROMOTING VACCINATION
How effective is it?
Influenza vaccine effectiveness in the 2017–2018 influenza season was 36% overall, 67% against A(H1N1), 42% against influenza B, and 25% against A(H3N2).16 It is estimated that influenza vaccine prevents 300 to 4,000 deaths annually in the United States alone.17
A 2018 Cochrane review17 concluded that vaccination reduced the incidence of influenza by about half, with 2.3% of the population contracting the flu without vaccination compared with 0.9% with vaccination (risk ratio 0.41, 95% confidence interval 0.36–0.47). The same review found that 71 healthy adults need to be vaccinated to prevent 1 from experiencing influenza, and 29 to prevent 1 influenza-like illness.
Several recent studies showed that influenza vaccine effectiveness varied based on age and influenza serotype, with higher effectiveness in people ages 5 to 17 and ages 18 to 64 than in those age 65 and older.18–20 A mathematical model of influenza transmission and vaccination in the United States determined that even relatively low-efficacy influenza vaccines can be very useful if optimally distributed across age groups.21
Vaccination rates are low, and ‘antivaxxers’ are on the rise
Although the influenza vaccine is recommended in the United States for all people age 6 months and older regardless of the state of their health, vaccination rates remain low. In 2016, only 37% of employed adults were vaccinated. The highest rate was for government employees (45%), followed by private employees (36%), followed by the self-employed (30%).22
A national goal is to immunize 80% of all Americans and 90% of at-risk populations (which include children and the elderly).23 The number of US hospitals that require their employees to be vaccinated increased from 37.1% in 2013 to 61.4% in 2017.24 Regrettably, as of March 2018, 14 lawsuits addressing religious objections to hospital influenza vaccination mandates have been filed.25
Despite hundreds of studies demonstrating the efficacy, safety, and cost savings of influenza vaccination, the antivaccine movement has been growing in the United States and worldwide.26 All US states except West Virginia, Mississippi, and California allow nonmedical exemptions from vaccination based on religious or personal belief.27 Several US metropolitan areas represent “hot spots” for these exemptions.28 This may render such areas vulnerable to vaccine-preventable diseases, including influenza.
Herd immunity: We’re all in this together
Some argue that the potential adverse effects and the cost of vaccination outweigh the benefits, but the protective benefits of herd immunity are significant for those with comorbidities or compromised immunity.
Educating the public about herd immunity and local influenza vaccination uptake increases people’s willingness to be vaccinated.29 A key educational point is that at least 70% of a community needs to be vaccinated to prevent community outbreaks; this protects everyone, including those who do not mount a protective antibody response to influenza vaccination and those who are not vaccinated.
DOES ANNUAL VACCINATION BLUNT ITS EFFECTIVENESS?
Some studies from the 1970s and 1980s raised concern over a possible negative effect of annual influenza vaccination on vaccine effectiveness. The “antigenic distance hypothesis” holds that vaccine effectiveness is influenced by antigenic similarity between the previous season’s vaccine serotypes and the epidemic serotypes, as well as the antigenic similarity between the serotypes of the current and previous seasons.
A meta-analysis of studies from 2010 through 2015 showed significant inconsistencies in repeat vaccination effects within and between seasons and serotypes. It also showed that vaccine effectiveness may be influenced by more than 1 previous season, particularly for influenza A(H3N2), in which repeated vaccination can blunt the hemagglutinin antibody response.30
A study from Japan showed that people who needed medical attention for influenza in the previous season were at lower risk of a similar event in the current season.31 Prior-season influenza vaccination reduced current-season vaccine effectiveness only in those who did not have medically attended influenza in the prior season. This suggests that infection is more immunogenic than vaccination, but only against the serotype causing the infection and not the other serotypes included in the vaccine.
An Australian study showed that annual influenza vaccination did not decrease vaccine effectiveness against influenza-associated hospitalization. Rather, effectiveness increased by about 15% in those vaccinated in both current and previous seasons compared with those vaccinated in either season alone.32
European investigators showed that repeated seasonal influenza vaccination in the elderly prevented the need for hospitalization due to influenza A(H3N2) and B, but not A(H1N1)pdm09.33
VACCINATION IN SPECIAL POPULATIONS
High-dose vaccine for older adults
The high-dose influenza vaccine has been licensed since 2009 for use in the United States for people ages 65 and older.
Recent studies confirmed that high-dose vaccine is more effective than standard-dose vaccine in veterans34 and US Medicare beneficiaries.35
The high-dose vaccine is rapidly becoming the primary vaccine given to people ages 65 and older in retail pharmacies, where vaccination begins earlier in the season than in providers’ offices.36 Some studies have shown that the standard-dose vaccine wanes in effectiveness toward the end of the influenza season (particularly if the season is long) if it is given very early. It remains to be seen whether the same applies to the high-dose influenza vaccine.
Some advocate twice-annual influenza vaccination, particularly for older adults living in tropical and subtropical areas, where influenza seasons are more prolonged. However, a recently published study observed reductions in influenza-specific hemagglutination inhibition and cell-mediated immunity after twice-annual vaccination.37
Vaccination is beneficial during pregnancy
Many studies have shown the value of influenza vaccination during pregnancy for both mothers and their infants.
One recently published study showed that 18% of infants who developed influenza required hospitalization.38 In that study, prenatal and postpartum maternal influenza vaccination decreased the odds of influenza in infants by 61% and 53%, respectively.
Another study showed that vaccine effectiveness did not vary by gestational age at vaccination.39
Some studies have shown that influenza virus infection can increase susceptibility to certain bacterial infections. A post hoc analysis of an influenza vaccination study in pregnant women suggested that the vaccine was also associated with decreased rates of pertussis in these women.40
Factors that make vaccination less effective
Several factors including age-related frailty and iatrogenic and disease-related immunosuppression can affect vaccine effectiveness.
Frailty. A recent study showed that vaccine effectiveness was 77.6% in nonfrail older adults but only 58.7% in frail older adults.41
Immunosuppression. Temporary discontinuation of methotrexate for 2 weeks after influenza vaccination in patients with rheumatoid arthritis improves vaccine immunogenicity without precipitating disease flare.42 Solid-organ and hematopoietic stem cell transplant recipients who received influenza vaccine were less likely to develop pneumonia and require intensive care unit admission.43
The high-dose influenza vaccine is more immunogenic than the standard-dose vaccine in solid-organ transplant recipients.44
Statins are widely prescribed and have recently been associated with reduced influenza vaccine effectiveness against medically attended acute respiratory illness, but their benefits in preventing cardiovascular events outweigh this risk.45
FUTURE VACCINE CONSIDERATIONS
Moving away from eggs
During the annual egg-based production process, which takes several months, the influenza vaccine acquires antigenic changes that allow replication in eggs, particularly in the hemagglutinin protein, which mediates receptor binding. This process of egg adaptation may cause antigenic changes that decrease vaccine effectiveness against circulating viruses.
The cell-based baculovirus influenza vaccine grown in dog kidney cells has higher antigenic content and is not subject to the limitations of egg-based vaccine, although it still requires annual updates. A recombinant influenza vaccine reduces the probability of influenza-like illness by 30% compared with the egg-based influenza vaccine, but also still requires annual updates.46 The market share of these non-egg-based vaccines is small, and thus their effectiveness has yet to be demonstrated.
The US Department of Defense administered the cell-based influenza vaccine to about one-third of Armed Forces personnel, their families, and retirees in the 2017–2018 influenza seasons, and data on its effectiveness are expected in the near future.47
A universal vaccine would be ideal
The quest continues for a universal influenza vaccine, one that remains protective for several years and does not require annual updates.48 Such a vaccine would protect against seasonal epidemic influenza drift variants and pandemic strains. More people could likely be persuaded to be vaccinated once rather than every year.
An ideal universal vaccine would be suitable for all age groups, at least 75% effective against symptomatic influenza virus infection, protective against all influenza A viruses (influenza A, not B, causes pandemics and seasonal epidemics), and durable through multiple influenza seasons.51
Research and production of such a vaccine are expected to require funding of about $1 billion over the next 5 years.
Boosting effectiveness
Estimates of influenza vaccine effectiveness range from 40% to 60% in years when the vaccine viruses closely match the circulating viruses, and variably lower when they do not match. The efficacy of most other vaccines given to prevent other infections is much higher.
New technologies to improve influenza vaccine effectiveness are needed, particularly for influenza A(H3N2) viruses, which are rapidly evolving and are highly susceptible to egg-adaptive mutations in the manufacturing process.
In one study, a nanoparticle vaccine formulated with a saponin-based adjuvant induced hemagglutination inhibition responses that were even greater than those induced by the high-dose vaccine.52
Immunoglobulin A (IgA) may be a more effective vaccine target than traditional influenza vaccines that target IgG, since different parts of IgA may engage the influenza virus simultaneously.53
Vaccines can be developed more quickly than in the past. The timeline from viral sequencing to human studies with deoxyribonucleic acid plasmid vaccines decreased from 20 months in 2003 for the severe acquired respiratory syndrome coronavirus to 11 months in 2006 for influenza A/Indonesia/2006 (H5), to 4 months in 2009 for influenza A/California/2009 (H1), to 3.5 months in 2016 for Zika virus.54 This is because it is possible today to sequence a virus and insert the genetic material into a vaccine platform without ever having to grow the virus.
TREATMENT
Numerous studies have found anti-influenza medications to be effective. Nevertheless, in an analysis of the 2011–2016 influenza seasons, only 15% of high-risk patients were prescribed anti-influenza medications within 2 days of symptom onset, including 37% in those with laboratory-confirmed influenza.55 Fever was associated with an increased rate of antiviral treatment, but 25% of high-risk outpatients were afebrile. Empiric treatment of 4 high-risk outpatients with acute respiratory illness was needed to treat 1 patient with influenza.55
Treatment with a neuraminidase inhibitor within 2 days of illness has recently been shown to improve survival and shorten duration of viral shedding in patients with avian influenza A(H7N9) infection.56 Antiviral treatment within 2 days of illness is associated with improved outcomes in transplant recipients57 and with a lower risk of otitis media in children.58
Appropriate anti-influenza treatment is as important as avoiding unnecessary antibiotics. Regrettably, as many as one-third of patients with laboratory-confirmed influenza are prescribed antibiotics.59
The US Food and Drug Administration warns against fraudulent unapproved over-the-counter influenza products.60
Baloxavir marboxil
Baloxavir marboxil is a new anti-influenza medication approved in Japan in February 2018 and anticipated to be available in the United States sometime in 2019.
This prodrug is hydrolyzed in vivo to the active metabolite, which selectively inhibits cap-dependent endonuclease enzyme, a key enzyme in initiation of messenger ribonucleic acid synthesis required for influenza viral replication.61
In a double-blind phase 3 trial, the median time to alleviation of influenza symptoms is 26.5 hours shorter with baloxavir marboxil than with placebo. One tablet was as effective as 5 days of the neuraminidase inhibitor oseltamivir and was associated with greater reduction in viral load 1 day after initiation, and similar side effects.62 Of concern is the emergence of nucleic acid substitutions conferring resistance to baloxavir; this occurred in 2.2% and 9.7% of baloxavir recipients in the phase 2 and 3 trials, respectively.
CLOSING THE GAPS
Several gaps in the management of influenza persist since the 1918 pandemic.1 These include gaps in epidemiology, prevention, diagnosis, treatment, and prognosis.
- Global networks wider than current ones are needed to address this global disease and to prioritize coordination efforts.
- Establishing and strengthening clinical capacity is needed in limited resource settings. New technologies are needed to expedite vaccine development and to achieve progress toward a universal vaccine.
- Current diagnostic tests do not distinguish between seasonal and novel influenza A viruses of zoonotic origin, which are expected to cause the next pandemic.
- Current antivirals have been shown to shorten duration of illness in outpatients with uncomplicated influenza, but the benefit in hospitalized patients has been less well established.
- In 2007, resistance of seasonal influenza A(H1N1) to oseltamivir became widespread. In 2009, pandemic influenza A(H1N1), which is highly susceptible to oseltamivir, replaced the seasonal virus and remains the predominantly circulating A(H1N1) strain.
- A small-molecule fragment, N-cyclohexyaltaurine, binds to the conserved hemagglutinin receptor-binding site in a manner that mimics the binding mode of the natural receptor sialic acid. This can serve as a template to guide the development of novel broad-spectrum small-molecule anti-influenza drugs.63
- Biomarkers that can accurately predict development of severe disease in patients with influenza are needed.
This centennial year update focuses primarily on immunization, but also reviews epidemiology, transmission, and treatment.
EPIDEMIOLOGY
2017–2018 was a bad season
The 2017–2018 influenza epidemic was memorable, dominated by influenza A(H3N2) viruses with morbidity and mortality rates approaching pandemic numbers. It lasted 19 weeks, killed more people than any other epidemic since 2010, particularly children, and was associated with 30,453 hospitalizations—almost twice the previous season high in some parts of the United States.2
Regrettably, 171 unvaccinated children died during 2017–2018, accounting for almost 80% of deaths.2 The mean age of the children who died was 7.1 years; 51% had at least 1 underlying medical condition placing them at risk for influenza-related complications, and 57% died after hospitalization.2
Recent estimates of the incidence of symptomatic influenza among all ages ranged from 3% to 11%, which is slightly lower than historical estimates. The rates were higher for children under age 18 than for adults.3 Interestingly, influenza A(H3N2) accounted for 50% of cases of non-mumps viral parotitis during the 2014–2015 influenza season in the United States.4
Influenza C exists but is rare
Influenza A and B account for almost all influenza-related outpatient visits and hospitalizations. Surveillance data from May 2013 through December 2016 showed that influenza C accounts for 0.5% of influenza-related outpatient visits and hospitalizations, particularly affecting children ages 6 to 24 months. Medical comorbidities and copathogens were seen in all patients requiring intensive care and in most hospitalizations.5 Diagnostic tests for influenza C are not widely available.
Dogs and cats: Factories for new flu strains?
While pigs and birds are the major reservoirs of influenza viral genetic diversity from which infection is transmitted to humans, dogs and cats have recently emerged as possible sources of novel reassortant influenza A.6 With their frequent close contact with humans, our pets may prove to pose a significant threat.
Obesity a risk factor for influenza
Obesity emerged as a risk factor for severe influenza in the 2009 pandemic. Recent data also showed that obesity increases the duration of influenza A virus shedding, thus increasing duration of contagiousness.7
Influenza a cardiovascular risk factor
Previous data showed that influenza was a risk factor for cardiovascular events. Two recent epidemiologic studies from the United Kingdom showed that laboratory-confirmed influenza was associated with higher rates of myocardial infarction and stroke for up to 4 weeks.8,9
Which strain is the biggest threat?
Predicting which emerging influenza serotype may cause the next pandemic is difficult, but influenza A(H7N9), which had not infected humans until 2013 but has since infected about 1,600 people in China and killed 37% of them, appears to have the greatest potential.10
National influenza surveillance programs and influenza-related social media applications have been developed and may get a boost from technology. A smartphone equipped with a temperature sensor can instantly detect one’s temperature with great precision. A 2018 study suggested that a smartphone-driven thermometry application correlated well with national influenza-like illness activity and improved its forecast in real time and up to 3 weeks in advance.11
TRANSMISSION
Humidity may not block transmission
Animal studies have suggested that humidity in the air interferes with transmission of airborne influenza virus, partially from biologic inactivation. But when a recent study used humidity-controlled chambers to investigate the stability of the 2009 influenza A(H1N1) virus in suspended aerosols and stationary droplets, the virus remained infectious in aerosols across a wide range of relative humidities, challenging the common belief that humidity destabilizes respiratory viruses in aerosols.12
One sick passenger may not infect the whole plane
Transmission of respiratory viruses on airplane flights has long been considered a potential avenue for spreading influenza. However, a recent study that monitored movements of individuals on 10 transcontinental US flights and simulated inflight transmission based on these data showed a low probability of direct transmission, except for passengers seated in close proximity to an infectious passenger.13
WHAT’S IN THE NEW FLU SHOT?
The 2018–2019 quadrivalent vaccine for the Northern Hemisphere14 contains the following strains:
- A/Michigan/45/2015 A(H1N1)pdm09-like virus
- A/Singapore/INFIMH-16-0019/2016 (H3N2)-like virus
- B/Colorado/06/2017-like virus (Victoria lineage)
- B/Phuket/3073/2013-like virus (Yamagata lineage).
The A(H3N2) (Singapore) and B/Victoria lineage components are new this year. The A(H3N2) strain was the main cause of the 2018 influenza epidemic in the Southern Hemisphere.
The quadrivalent live-attenuated vaccine, which was not recommended during the 2016–2017 and 2017–2018 influenza seasons, has made a comeback and is recommended for the 2018–2019 season in people for whom it is appropriate based on age and comorbidities.15 Although it was effective against influenza B and A(H3N2) viruses, it was less effective against the influenza A(H1N1)pdm09-like viruses during the 2013–2014 and 2015–2016 seasons.
A/Slovenia/2903/2015, the new A(H1N1)pdm09-like virus included in the 2018–2019 quadrivalent live-attenuated vaccine, is significantly more immunogenic than its predecessor, A/Bolivia/559/2013, but its clinical effectiveness remains to be seen.
PROMOTING VACCINATION
How effective is it?
Influenza vaccine effectiveness in the 2017–2018 influenza season was 36% overall, 67% against A(H1N1), 42% against influenza B, and 25% against A(H3N2).16 It is estimated that influenza vaccine prevents 300 to 4,000 deaths annually in the United States alone.17
A 2018 Cochrane review17 concluded that vaccination reduced the incidence of influenza by about half, with 2.3% of the population contracting the flu without vaccination compared with 0.9% with vaccination (risk ratio 0.41, 95% confidence interval 0.36–0.47). The same review found that 71 healthy adults need to be vaccinated to prevent 1 from experiencing influenza, and 29 to prevent 1 influenza-like illness.
Several recent studies showed that influenza vaccine effectiveness varied based on age and influenza serotype, with higher effectiveness in people ages 5 to 17 and ages 18 to 64 than in those age 65 and older.18–20 A mathematical model of influenza transmission and vaccination in the United States determined that even relatively low-efficacy influenza vaccines can be very useful if optimally distributed across age groups.21
Vaccination rates are low, and ‘antivaxxers’ are on the rise
Although the influenza vaccine is recommended in the United States for all people age 6 months and older regardless of the state of their health, vaccination rates remain low. In 2016, only 37% of employed adults were vaccinated. The highest rate was for government employees (45%), followed by private employees (36%), followed by the self-employed (30%).22
A national goal is to immunize 80% of all Americans and 90% of at-risk populations (which include children and the elderly).23 The number of US hospitals that require their employees to be vaccinated increased from 37.1% in 2013 to 61.4% in 2017.24 Regrettably, as of March 2018, 14 lawsuits addressing religious objections to hospital influenza vaccination mandates have been filed.25
Despite hundreds of studies demonstrating the efficacy, safety, and cost savings of influenza vaccination, the antivaccine movement has been growing in the United States and worldwide.26 All US states except West Virginia, Mississippi, and California allow nonmedical exemptions from vaccination based on religious or personal belief.27 Several US metropolitan areas represent “hot spots” for these exemptions.28 This may render such areas vulnerable to vaccine-preventable diseases, including influenza.
Herd immunity: We’re all in this together
Some argue that the potential adverse effects and the cost of vaccination outweigh the benefits, but the protective benefits of herd immunity are significant for those with comorbidities or compromised immunity.
Educating the public about herd immunity and local influenza vaccination uptake increases people’s willingness to be vaccinated.29 A key educational point is that at least 70% of a community needs to be vaccinated to prevent community outbreaks; this protects everyone, including those who do not mount a protective antibody response to influenza vaccination and those who are not vaccinated.
DOES ANNUAL VACCINATION BLUNT ITS EFFECTIVENESS?
Some studies from the 1970s and 1980s raised concern over a possible negative effect of annual influenza vaccination on vaccine effectiveness. The “antigenic distance hypothesis” holds that vaccine effectiveness is influenced by antigenic similarity between the previous season’s vaccine serotypes and the epidemic serotypes, as well as the antigenic similarity between the serotypes of the current and previous seasons.
A meta-analysis of studies from 2010 through 2015 showed significant inconsistencies in repeat vaccination effects within and between seasons and serotypes. It also showed that vaccine effectiveness may be influenced by more than 1 previous season, particularly for influenza A(H3N2), in which repeated vaccination can blunt the hemagglutinin antibody response.30
A study from Japan showed that people who needed medical attention for influenza in the previous season were at lower risk of a similar event in the current season.31 Prior-season influenza vaccination reduced current-season vaccine effectiveness only in those who did not have medically attended influenza in the prior season. This suggests that infection is more immunogenic than vaccination, but only against the serotype causing the infection and not the other serotypes included in the vaccine.
An Australian study showed that annual influenza vaccination did not decrease vaccine effectiveness against influenza-associated hospitalization. Rather, effectiveness increased by about 15% in those vaccinated in both current and previous seasons compared with those vaccinated in either season alone.32
European investigators showed that repeated seasonal influenza vaccination in the elderly prevented the need for hospitalization due to influenza A(H3N2) and B, but not A(H1N1)pdm09.33
VACCINATION IN SPECIAL POPULATIONS
High-dose vaccine for older adults
The high-dose influenza vaccine has been licensed since 2009 for use in the United States for people ages 65 and older.
Recent studies confirmed that high-dose vaccine is more effective than standard-dose vaccine in veterans34 and US Medicare beneficiaries.35
The high-dose vaccine is rapidly becoming the primary vaccine given to people ages 65 and older in retail pharmacies, where vaccination begins earlier in the season than in providers’ offices.36 Some studies have shown that the standard-dose vaccine wanes in effectiveness toward the end of the influenza season (particularly if the season is long) if it is given very early. It remains to be seen whether the same applies to the high-dose influenza vaccine.
Some advocate twice-annual influenza vaccination, particularly for older adults living in tropical and subtropical areas, where influenza seasons are more prolonged. However, a recently published study observed reductions in influenza-specific hemagglutination inhibition and cell-mediated immunity after twice-annual vaccination.37
Vaccination is beneficial during pregnancy
Many studies have shown the value of influenza vaccination during pregnancy for both mothers and their infants.
One recently published study showed that 18% of infants who developed influenza required hospitalization.38 In that study, prenatal and postpartum maternal influenza vaccination decreased the odds of influenza in infants by 61% and 53%, respectively.
Another study showed that vaccine effectiveness did not vary by gestational age at vaccination.39
Some studies have shown that influenza virus infection can increase susceptibility to certain bacterial infections. A post hoc analysis of an influenza vaccination study in pregnant women suggested that the vaccine was also associated with decreased rates of pertussis in these women.40
Factors that make vaccination less effective
Several factors including age-related frailty and iatrogenic and disease-related immunosuppression can affect vaccine effectiveness.
Frailty. A recent study showed that vaccine effectiveness was 77.6% in nonfrail older adults but only 58.7% in frail older adults.41
Immunosuppression. Temporary discontinuation of methotrexate for 2 weeks after influenza vaccination in patients with rheumatoid arthritis improves vaccine immunogenicity without precipitating disease flare.42 Solid-organ and hematopoietic stem cell transplant recipients who received influenza vaccine were less likely to develop pneumonia and require intensive care unit admission.43
The high-dose influenza vaccine is more immunogenic than the standard-dose vaccine in solid-organ transplant recipients.44
Statins are widely prescribed and have recently been associated with reduced influenza vaccine effectiveness against medically attended acute respiratory illness, but their benefits in preventing cardiovascular events outweigh this risk.45
FUTURE VACCINE CONSIDERATIONS
Moving away from eggs
During the annual egg-based production process, which takes several months, the influenza vaccine acquires antigenic changes that allow replication in eggs, particularly in the hemagglutinin protein, which mediates receptor binding. This process of egg adaptation may cause antigenic changes that decrease vaccine effectiveness against circulating viruses.
The cell-based baculovirus influenza vaccine grown in dog kidney cells has higher antigenic content and is not subject to the limitations of egg-based vaccine, although it still requires annual updates. A recombinant influenza vaccine reduces the probability of influenza-like illness by 30% compared with the egg-based influenza vaccine, but also still requires annual updates.46 The market share of these non-egg-based vaccines is small, and thus their effectiveness has yet to be demonstrated.
The US Department of Defense administered the cell-based influenza vaccine to about one-third of Armed Forces personnel, their families, and retirees in the 2017–2018 influenza seasons, and data on its effectiveness are expected in the near future.47
A universal vaccine would be ideal
The quest continues for a universal influenza vaccine, one that remains protective for several years and does not require annual updates.48 Such a vaccine would protect against seasonal epidemic influenza drift variants and pandemic strains. More people could likely be persuaded to be vaccinated once rather than every year.
An ideal universal vaccine would be suitable for all age groups, at least 75% effective against symptomatic influenza virus infection, protective against all influenza A viruses (influenza A, not B, causes pandemics and seasonal epidemics), and durable through multiple influenza seasons.51
Research and production of such a vaccine are expected to require funding of about $1 billion over the next 5 years.
Boosting effectiveness
Estimates of influenza vaccine effectiveness range from 40% to 60% in years when the vaccine viruses closely match the circulating viruses, and variably lower when they do not match. The efficacy of most other vaccines given to prevent other infections is much higher.
New technologies to improve influenza vaccine effectiveness are needed, particularly for influenza A(H3N2) viruses, which are rapidly evolving and are highly susceptible to egg-adaptive mutations in the manufacturing process.
In one study, a nanoparticle vaccine formulated with a saponin-based adjuvant induced hemagglutination inhibition responses that were even greater than those induced by the high-dose vaccine.52
Immunoglobulin A (IgA) may be a more effective vaccine target than traditional influenza vaccines that target IgG, since different parts of IgA may engage the influenza virus simultaneously.53
Vaccines can be developed more quickly than in the past. The timeline from viral sequencing to human studies with deoxyribonucleic acid plasmid vaccines decreased from 20 months in 2003 for the severe acquired respiratory syndrome coronavirus to 11 months in 2006 for influenza A/Indonesia/2006 (H5), to 4 months in 2009 for influenza A/California/2009 (H1), to 3.5 months in 2016 for Zika virus.54 This is because it is possible today to sequence a virus and insert the genetic material into a vaccine platform without ever having to grow the virus.
TREATMENT
Numerous studies have found anti-influenza medications to be effective. Nevertheless, in an analysis of the 2011–2016 influenza seasons, only 15% of high-risk patients were prescribed anti-influenza medications within 2 days of symptom onset, including 37% in those with laboratory-confirmed influenza.55 Fever was associated with an increased rate of antiviral treatment, but 25% of high-risk outpatients were afebrile. Empiric treatment of 4 high-risk outpatients with acute respiratory illness was needed to treat 1 patient with influenza.55
Treatment with a neuraminidase inhibitor within 2 days of illness has recently been shown to improve survival and shorten duration of viral shedding in patients with avian influenza A(H7N9) infection.56 Antiviral treatment within 2 days of illness is associated with improved outcomes in transplant recipients57 and with a lower risk of otitis media in children.58
Appropriate anti-influenza treatment is as important as avoiding unnecessary antibiotics. Regrettably, as many as one-third of patients with laboratory-confirmed influenza are prescribed antibiotics.59
The US Food and Drug Administration warns against fraudulent unapproved over-the-counter influenza products.60
Baloxavir marboxil
Baloxavir marboxil is a new anti-influenza medication approved in Japan in February 2018 and anticipated to be available in the United States sometime in 2019.
This prodrug is hydrolyzed in vivo to the active metabolite, which selectively inhibits cap-dependent endonuclease enzyme, a key enzyme in initiation of messenger ribonucleic acid synthesis required for influenza viral replication.61
In a double-blind phase 3 trial, the median time to alleviation of influenza symptoms is 26.5 hours shorter with baloxavir marboxil than with placebo. One tablet was as effective as 5 days of the neuraminidase inhibitor oseltamivir and was associated with greater reduction in viral load 1 day after initiation, and similar side effects.62 Of concern is the emergence of nucleic acid substitutions conferring resistance to baloxavir; this occurred in 2.2% and 9.7% of baloxavir recipients in the phase 2 and 3 trials, respectively.
CLOSING THE GAPS
Several gaps in the management of influenza persist since the 1918 pandemic.1 These include gaps in epidemiology, prevention, diagnosis, treatment, and prognosis.
- Global networks wider than current ones are needed to address this global disease and to prioritize coordination efforts.
- Establishing and strengthening clinical capacity is needed in limited resource settings. New technologies are needed to expedite vaccine development and to achieve progress toward a universal vaccine.
- Current diagnostic tests do not distinguish between seasonal and novel influenza A viruses of zoonotic origin, which are expected to cause the next pandemic.
- Current antivirals have been shown to shorten duration of illness in outpatients with uncomplicated influenza, but the benefit in hospitalized patients has been less well established.
- In 2007, resistance of seasonal influenza A(H1N1) to oseltamivir became widespread. In 2009, pandemic influenza A(H1N1), which is highly susceptible to oseltamivir, replaced the seasonal virus and remains the predominantly circulating A(H1N1) strain.
- A small-molecule fragment, N-cyclohexyaltaurine, binds to the conserved hemagglutinin receptor-binding site in a manner that mimics the binding mode of the natural receptor sialic acid. This can serve as a template to guide the development of novel broad-spectrum small-molecule anti-influenza drugs.63
- Biomarkers that can accurately predict development of severe disease in patients with influenza are needed.
- Uyeki TM, Fowler RA, Fischer WA. Gaps in the clinical management of influenza: a century since the 1918 pandemic. JAMA 2018; 320(8):755–756. doi:10.1001/jama.2018.8113
- Garten R, Blanton L, Elal AI, et al. Update: influenza activity in the United States during the 2017–18 season and composition of the 2018–19 influenza vaccine. MMWR Morb Mortal Wkly Rep 2018; 67(22):634–642. doi:10.15585/mmwr.mm6722a4
- Tokars JI, Olsen SJ, Reed C. Seasonal incidence of symptomatic influenza in the United States. Clin Infect Dis 2018; 66(10):1511–1518. doi:10.1093/cid/cix1060
- Elbadawi LI, Talley P, Rolfes MA, et al. Non-mumps viral parotitis during the 2014–2015 influenza season in the United States. Clin Infect Dis 2018. Epub ahead of print. doi:10.1093/cid/ciy137
- Thielen BK, Friedlander H, Bistodeau S, et al. Detection of influenza C viruses among outpatients and patients hospitalized for severe acute respiratory infection, Minnesota, 2013–2016. Clin Infect Dis 2018; 66(7):1092–1098. doi:10.1093/cid/cix931
- Chena Y, Trovãob NS, Wang G, et al. Emergence and evolution of novel reassortant influenza A viruses in canines in southern China. MBio 2018; 9(3):e00909–e00918. doi:10.1128/mBio.00909-18
- Maier HE, Lopez R, Sanchez N, et al. Obesity increases the duration of influenza A virus shedding in adults. J Infect Dis 2018. Epub ahead of print. doi:10.1093/infdis/jiy370
- Warren-Gash C, Blackburn R, Whitaker H, McMenamin J, Hayward AC. Laboratory-confirmed respiratory infections as triggers for acute myocardial infarction and stroke: a self-controlled case series analysis of national linked datasets from Scotland. Eur Respir J 2018; 51(3):1701794. doi:10.1183/13993003.01794-2017
- Blackburn R, Zhao H, Pebody R, Hayward A, Warren-Gash C. Laboratory-confirmed respiratory infections as predictors of hospital admission for myocardial infarction and stroke: time-series analysis of English data for 2004–2015. Clin Infect Dis 2018; 67(1):8–17. doi:10.1093/cid/cix1144
- Newsweek; Andrew S. What is disease X? Deadly bird flu virus could be next pandemic. www.newsweek.com/disease-x-bird-flu-deaths-pandemic-what-h7n9-979723. Accessed October 3, 2018.
- Miller AC, Singh I, Koehler E, Polgreen PM. A smartphone-driven thermometer application for real-time population- and individual-level influenza surveillance. Clin Infect Dis 2018; 67(3):388–397. doi:10.1093/cid/ciy073
- Kormuth KA, Lin K, Prussin AJ 2nd, et al. Influenza virus infectivity is retained in aerosols and droplets independent of relative humidity, J Infect Dis 2018; 218(5):739–747. doi:10.1093/infdis/jiy221
- Hertzberg VS, Weiss H, Elon L, et. al. Behaviors, movements, and transmission of droplet-mediated respiratory diseases during transcontinental airline flights. Proc Natl Acad Sci U S A 2018; 115(14):3623–3627. doi:10.1073/pnas.1711611115
- Grohskopf LA, Sokolow LZ, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2018–19 influenza season. MMWR Recomm Rep 2018; 67(3):1–20. doi:10.15585/mmwr.rr6703a1
- Grohskopf LA, Sokolow LZ, Fry AM, Walter EB, Jernigan DB. Update: ACIP recommendations for the use of quadrivalent live attenuated influenza vaccine (LAIV4)—United States, 2018–19 influenza season. MMWR Morb Mortal Wkly Rep 2018; 67(22):643–645. doi:10.15585/mmwr.mm6722a5
- Flannery B, Chung JR, Belongia EA, et al. Interim estimates of 2017–18 seasonal influenza vaccine effectiveness—United States, February 2018. MMWR Morb Mortal Wkly Rep 2018; 67(6):180–185. doi:10.15585/mmwr.mm6706a2
- Demicheli V, Jefferson T, Ferroni E, Rivetti A, Di Pietrantonj C. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev 2018; 2:CD001269. doi:10.1002/14651858.CD001269.pub6
- Flannery B, Smith C, Garten RJ, et al. Influence of birth cohort on effectiveness of 2015–2016 influenza vaccine against medically attended illness due to 2009 pandemic influenza A(H1N1) virus in the United States. J Infect Dis 2018; 218(2):189–196. doi:10.1093/infdis/jix634
- Rondy M, El Omeiri N, Thompson MG, Leveque A, Moren A, Sullivan SG. Effectiveness of influenza vaccines in preventing severe influenza illness among adults: a systematic review and meta-analysis of test-negative design case-control studies. J Infect 2017; 75(5):381–394. doi:10.1016/j.jinf.2017.09.010
- Stein Y, Mandelboim M, Sefty H, et al; Israeli Influenza Surveillance Network (IISN). Seasonal influenza vaccine effectiveness in preventing laboratory-confirmed influenza in primary care in Israel, 2016–2017 season: insights into novel age-specific analysis. Clin Infect Dis 2018; 66(9):1383–1391. doi:10.1093/cid/cix1013
- Sah P, Medlock J, Fitzpatrick MC, Singer BH, Galvani AP. Optimizing the impact of low-efficacy influenza vaccines. Proc Natl Acad Sci U S A 2018; 115(20):5151–5156. doi:10.1073/pnas.1802479115
- QuickStats: percentage of currently employed adults aged ≥ 18 years who received influenza vaccine in the past 12 months, by employment category—national health interview survey, United States, 2012 and 2016. MMWR Morb Mortal Wkly Rep 2018; 67(16):480. doi:10.15585/mmwr.mm6716a8
- Healthy People.gov. Immunization and infectious diseases. IID-12. Increase the percentage of children and adults who are vaccinated annually against seasonal influenza. www.healthypeople.gov/2020/topics-objectives/topic/immunization-and-infectious-diseases/objectives. Accessed October 3, 2018.
- Greene MT, Fowler KE, Ratz D, Krein SL, Bradley SF, Saint S. Changes in influenza vaccination requirements for health care personnel in US hospitals. JAMA Network Open 2018; 1(2):e180143. doi:10.1001/jamanetworkopen.2018.0143
- Opel DJ, Sonne JA, Mello MM. Vaccination without litigation—addressing religious objections to hospital influenza-vaccination mandates. N Engl J Med 2018; 378(9):785–788. doi:10.1056/NEJMp1716147
- Horowitz J. Italy loosens vaccine law just as children return to school. New York Times Sept. 20, 2018. www.nytimes.com/2018/09/20/world/europe/italy-vaccines-five-star-movement.html.
- National Conference of State Legislature. States with religious and philosophical exemptions from school immunization requirements. www.ncsl.org/research/health/school-immunization-exemption-state-laws.aspx. Accessed October 3, 2018.
- Olive JK, Hotez PJ, Damania A, Nolan MS. The state of the antivaccine movement in the United States: a focused examination of nonmedical exemptions in states and counties. PLoS Med 2018; 15(6):e1002578. doi:10.1371/journal.pmed.1002578
- Logan J, Nederhoff D, Koch B, et al. ‘What have you HEARD about the HERD?’ Does education about local influenza vaccination coverage and herd immunity affect willingness to vaccinate? Vaccine 2018; 36(28):4118–4125. doi:10.1016/j.vaccine.2018.05.037
- Belongia EA, Skowronski DM, McLean HQ, Chambers C, Sundaram ME, De Serres G. Repeated annual influenza vaccination and vaccine effectiveness: review of evidence. Expert Rev Vaccines 2017; 16(7):1–14. doi:10.1080/14760584.2017.1334554
- Saito N, Komori K, Suzuki M, et al. Negative impact of prior influenza vaccination on current influenza vaccination among people infected and not infected in prior season: a test-negative case-control study in Japan. Vaccine 2017; 35(4):687–693. doi:10.1016/j.vaccine.2016.11.024
- Cheng AC, Macartney KK, Waterer GW, Kotsimbos T, Kelly PM, Blyth CC; Influenza Complications Alert Network (FluCAN) Investigators. Repeated vaccination does not appear to impact upon influenza vaccine effectiveness against hospitalization with confirmed influenza. Clin Infect Dis 2017; 64(11):1564–1572. doi:10.1093/cid/cix209
- Rondy M, Launay O, Castilla J, et al; InNHOVE/I-MOVE+working group. Repeated seasonal influenza vaccination among elderly in Europe: effects on laboratory confirmed hospitalised influenza. Vaccine 2017; 35(34):4298–4306. doi:10.1016/j.vaccine.2017.06.088
- Young-Xu Y, van Aalst R, Mahmud SM, et al. Relative vaccine effectiveness of high-dose versus standard-dose influenza vaccines among Veterans Health Administration patients. J Infect Dis 2018; 217(11):1718–1727. doi:10.1093/infdis/jiy088
- Shay DK, Chillarige Y, Kelman J, et al. Comparative effectiveness of high-dose versus standard-dose influenza vaccines among US Medicare beneficiaries in preventing postinfluenza deaths during 2012–2013 and 2013–2014. J Infect Dis 2017; 215(4):510–517. doi:10.1093/infdis/jiw641
- Madaras-Kelly K, Remington R, Hruza H, Xu D. Comparative effectiveness of high-dose versus standard-dose influenza vaccines in preventing postinfluenza deaths. J Infect Dis 2018; 218(2):336–337. doi:10.1093/infdis/jix645
- Tam YH, Valkenburg SA, Perera RAPM, et al. Immune responses to twice-annual influenza vaccination in older adults in Hong Kong. Clin Infect Dis 2018; 66(6):904–912. doi:10.1093/cid/cix900
- Ohfuji S, Deguchi M, Tachibana D, et al; Osaka Pregnant Women Influenza Study Group. Protective effect of maternal influenza vaccination on influenza in their infants: a prospective cohort study. J Infect Dis 2018; 217(6):878–886. doi:10.1093/infdis/jix629
- Katz J, Englund JA, Steinhoff MC, et al. Impact of timing of influenza vaccination in pregnancy on transplacental antibody transfer, influenza incidence, and birth outcomes: a randomized trial in rural Nepal. Clin Infect Dis 2018; 67(3):334–340. doi:10.1093/cid/ciy090
- Nunes MC, Cutland CL, Madhi SA. Influenza vaccination during pregnancy and protection against pertussis. N Engl J Med 2018; 378(13):1257–1258. doi:10.1056/NEJMc1705208
- Andrew MK, Shinde V, Ye L, et al; Serious Outcomes Surveillance Network of the Public Health Agency of Canada/Canadian Institutes of Health Research Influenza Research Network (PCIRN) and the Toronto Invasive Bacterial Diseases Network (TIBDN). The importance of frailty in the assessment of influenza vaccine effectiveness against influenza-related hospitalization in elderly people. J Infect Dis 2017; 216(4):405–414. doi:10.1093/infdis/jix282
- Park JK, Lee YJ, Shin K, et al. Impact of temporary methotrexate discontinuation for 2 weeks on immunogenicity of seasonal influenza vaccination in patients with rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis 2018; 77(6):898–904. doi:10.1136/annrheumdis-2018-213222
- Kumar D, Ferreira VH, Blumberg E, et al. A five-year prospective multi-center evaluation of influenza infection in transplant recipients. Clin Infect Dis 2018. Epub ahead of print. doi:10.1093/cid/ciy294
- Natori Y, Shiotsuka M, Slomovic J, et al. A double-blind, randomized trial of high-dose vs standard-dose influenza vaccine in adult solid-organ transplant recipients. Clin Infect Dis 2018; 66(11):1698–1704. doi:10.1093/cid/cix1082
- Omer SB, Phadke VK, Bednarczyk BA, Chamberlain AT, Brosseau JL, Orenstein WA. Impact of statins on influenza vaccine effectiveness against medically attended acute respiratory illness. J Infect Dis 2016; 213(8):1216–1223. doi:10.1093/infdis/jiv457
- Dunkle LM, Izikson R, Patriarca P, et al. Efficacy of recombinant influenza vaccine in adults 50 years of age or older. N Engl J Med 2017; 376(25):2427–2436. doi:10.1056/NEJMoa1608862
- STAT; Branswell H. How the US military might help answer a critical question about the flu vaccine. www.statnews.com/2018/03/02/flu-vaccine-egg-production-data. Accessed October 3, 2018.
- Paules CI, Sullivan SG, Subbarao K, Fauci AS. Chasing seasonal influenza—the need for a universal influenza vaccine. N Engl J Med 2018; 378(1):7–9. doi:10.1056/NEJMp1714916
- Jin XW, Mossad SB. Avian influenza: an emerging pandemic threat. Cleve Clin J Med 2005; 72:1129-1134. pmid:16392727
- Wei WI, Brunger AT, Skehel JJ, Wiley DC. Refinement of the influenza virus hemagglutinin by simulated annealing. J Mol Biol 1990; 212(4):737–761. doi:10.1016/0022-2836(90)90234-D
- Erbelding EJ, Post DJ, Stemmy EJ, et al. A universal influenza vaccine: the strategic plan for the National Institute of Allergy and Infectious Diseases, J Infect Dis 2018; 218(3):347–354. doi:10.1093/infdis/jiy103
- Shinde V, Fries L, Wu Y, et al. Improved titers against influenza drift variants with a nanoparticle vaccine. N Engl J Med 2018; 378(24):2346–2348. doi:10.1056/NEJMc1803554
- Maurer MA, Meyer L, Bianchi M, et al. Glycosylation of human IgA directly inhibits influenza A and other sialic-acid-binding viruses. Cell Rep 2018; 23(1):90–99. doi:10.1016/j.celrep.2018.03.027
- Graham BS, Mascola JR, Fauci AS. Novel vaccine technologies: essential components of an adequate response to emerging viral diseases. JAMA 2018; 319(14):1431–1432. doi:10.1001/jama.2018.0345
- Stewart RJ, Flannery B, Chung JR, et al. Influenza antiviral prescribing for outpatients with an acute respiratory illness and at high risk for influenza-associated complications during 5 influenza seasons—United States, 2011–2016. Clin Infect Dis 2018; 66(7):1035–1041. doi:10.1093/cid/cix922
- Zheng S, Tang L, Gao H, et al. Benefit of early initiation of neuraminidase inhibitor treatment to hospitalized patients with avian influenza A(H7N9) virus. Clin Infect Dis 2018; 66(7):1054–1060. doi:10.1093/cid/cix930
- Kumar D, Ferreira VH, Blumberg E, et al. A five-year prospective multi-center evaluation of influenza infection in transplant recipients. Clin Infect Dis 2018. Epub ahead of print. doi:10.1093/cid/ciy294
- Malosh RE, Martin ET, Heikkinen T, Brooks WA, Whitley RJ, Monto AS. Efficacy and safety of oseltamivir in children: systematic review and individual patient data meta-analysis of randomized controlled trials. Clin Infect Dis 2018; 66(10):1492–1500. doi:10.1093/cid/cix1040
- Havers FP, Hicks LA, Chung JR, et al. Outpatient antibiotic prescribing for acute respiratory infections during influenza seasons. JAMA Network Open 2018; 1(2):e180243. doi:10.1001/jamanetworkopen.2018.0243
- US Food and Drug Administration. FDA warns of fraudulent and unapproved flu products. www.fda.gov/newsevents/newsroom/pressannouncements/ucm599223.htm. Accessed October 3, 2018.
- Portsmouth S, Kawaguchi K, Arai M, Tsuchiya K, Uehara T. Cap-dependent endonuclease inhibitor S-033188 for the treatment of influenza: results from a phase 3, randomized, double-blind, placebo- and active-controlled study in otherwise healthy adolescents and adults with seasonal influenza. Open Forum Infect Dis 2017; 4(suppl 1):S734. doi:10.1093/ofid/ofx180.001
- Hayden FG, Sugaya N, Hirotsu N, et al; Baloxavir Marboxil Investigators Group. Baloxavir Marboxil for uncomplicated influenza in adults and adolescents. N Engl J Med 2018; 379(10):913–923. doi:10.1056/NEJMoa1716197
- Kadam RU, Wilson IA. A small-molecule fragment that emulates binding of receptor and broadly neutralizing antibodies to influenza A hemagglutinin. Proc Natl Acad Sci U S A 2018; 115(16):4240–4245. doi:10.1073/pnas.1801999115
- Uyeki TM, Fowler RA, Fischer WA. Gaps in the clinical management of influenza: a century since the 1918 pandemic. JAMA 2018; 320(8):755–756. doi:10.1001/jama.2018.8113
- Garten R, Blanton L, Elal AI, et al. Update: influenza activity in the United States during the 2017–18 season and composition of the 2018–19 influenza vaccine. MMWR Morb Mortal Wkly Rep 2018; 67(22):634–642. doi:10.15585/mmwr.mm6722a4
- Tokars JI, Olsen SJ, Reed C. Seasonal incidence of symptomatic influenza in the United States. Clin Infect Dis 2018; 66(10):1511–1518. doi:10.1093/cid/cix1060
- Elbadawi LI, Talley P, Rolfes MA, et al. Non-mumps viral parotitis during the 2014–2015 influenza season in the United States. Clin Infect Dis 2018. Epub ahead of print. doi:10.1093/cid/ciy137
- Thielen BK, Friedlander H, Bistodeau S, et al. Detection of influenza C viruses among outpatients and patients hospitalized for severe acute respiratory infection, Minnesota, 2013–2016. Clin Infect Dis 2018; 66(7):1092–1098. doi:10.1093/cid/cix931
- Chena Y, Trovãob NS, Wang G, et al. Emergence and evolution of novel reassortant influenza A viruses in canines in southern China. MBio 2018; 9(3):e00909–e00918. doi:10.1128/mBio.00909-18
- Maier HE, Lopez R, Sanchez N, et al. Obesity increases the duration of influenza A virus shedding in adults. J Infect Dis 2018. Epub ahead of print. doi:10.1093/infdis/jiy370
- Warren-Gash C, Blackburn R, Whitaker H, McMenamin J, Hayward AC. Laboratory-confirmed respiratory infections as triggers for acute myocardial infarction and stroke: a self-controlled case series analysis of national linked datasets from Scotland. Eur Respir J 2018; 51(3):1701794. doi:10.1183/13993003.01794-2017
- Blackburn R, Zhao H, Pebody R, Hayward A, Warren-Gash C. Laboratory-confirmed respiratory infections as predictors of hospital admission for myocardial infarction and stroke: time-series analysis of English data for 2004–2015. Clin Infect Dis 2018; 67(1):8–17. doi:10.1093/cid/cix1144
- Newsweek; Andrew S. What is disease X? Deadly bird flu virus could be next pandemic. www.newsweek.com/disease-x-bird-flu-deaths-pandemic-what-h7n9-979723. Accessed October 3, 2018.
- Miller AC, Singh I, Koehler E, Polgreen PM. A smartphone-driven thermometer application for real-time population- and individual-level influenza surveillance. Clin Infect Dis 2018; 67(3):388–397. doi:10.1093/cid/ciy073
- Kormuth KA, Lin K, Prussin AJ 2nd, et al. Influenza virus infectivity is retained in aerosols and droplets independent of relative humidity, J Infect Dis 2018; 218(5):739–747. doi:10.1093/infdis/jiy221
- Hertzberg VS, Weiss H, Elon L, et. al. Behaviors, movements, and transmission of droplet-mediated respiratory diseases during transcontinental airline flights. Proc Natl Acad Sci U S A 2018; 115(14):3623–3627. doi:10.1073/pnas.1711611115
- Grohskopf LA, Sokolow LZ, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2018–19 influenza season. MMWR Recomm Rep 2018; 67(3):1–20. doi:10.15585/mmwr.rr6703a1
- Grohskopf LA, Sokolow LZ, Fry AM, Walter EB, Jernigan DB. Update: ACIP recommendations for the use of quadrivalent live attenuated influenza vaccine (LAIV4)—United States, 2018–19 influenza season. MMWR Morb Mortal Wkly Rep 2018; 67(22):643–645. doi:10.15585/mmwr.mm6722a5
- Flannery B, Chung JR, Belongia EA, et al. Interim estimates of 2017–18 seasonal influenza vaccine effectiveness—United States, February 2018. MMWR Morb Mortal Wkly Rep 2018; 67(6):180–185. doi:10.15585/mmwr.mm6706a2
- Demicheli V, Jefferson T, Ferroni E, Rivetti A, Di Pietrantonj C. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev 2018; 2:CD001269. doi:10.1002/14651858.CD001269.pub6
- Flannery B, Smith C, Garten RJ, et al. Influence of birth cohort on effectiveness of 2015–2016 influenza vaccine against medically attended illness due to 2009 pandemic influenza A(H1N1) virus in the United States. J Infect Dis 2018; 218(2):189–196. doi:10.1093/infdis/jix634
- Rondy M, El Omeiri N, Thompson MG, Leveque A, Moren A, Sullivan SG. Effectiveness of influenza vaccines in preventing severe influenza illness among adults: a systematic review and meta-analysis of test-negative design case-control studies. J Infect 2017; 75(5):381–394. doi:10.1016/j.jinf.2017.09.010
- Stein Y, Mandelboim M, Sefty H, et al; Israeli Influenza Surveillance Network (IISN). Seasonal influenza vaccine effectiveness in preventing laboratory-confirmed influenza in primary care in Israel, 2016–2017 season: insights into novel age-specific analysis. Clin Infect Dis 2018; 66(9):1383–1391. doi:10.1093/cid/cix1013
- Sah P, Medlock J, Fitzpatrick MC, Singer BH, Galvani AP. Optimizing the impact of low-efficacy influenza vaccines. Proc Natl Acad Sci U S A 2018; 115(20):5151–5156. doi:10.1073/pnas.1802479115
- QuickStats: percentage of currently employed adults aged ≥ 18 years who received influenza vaccine in the past 12 months, by employment category—national health interview survey, United States, 2012 and 2016. MMWR Morb Mortal Wkly Rep 2018; 67(16):480. doi:10.15585/mmwr.mm6716a8
- Healthy People.gov. Immunization and infectious diseases. IID-12. Increase the percentage of children and adults who are vaccinated annually against seasonal influenza. www.healthypeople.gov/2020/topics-objectives/topic/immunization-and-infectious-diseases/objectives. Accessed October 3, 2018.
- Greene MT, Fowler KE, Ratz D, Krein SL, Bradley SF, Saint S. Changes in influenza vaccination requirements for health care personnel in US hospitals. JAMA Network Open 2018; 1(2):e180143. doi:10.1001/jamanetworkopen.2018.0143
- Opel DJ, Sonne JA, Mello MM. Vaccination without litigation—addressing religious objections to hospital influenza-vaccination mandates. N Engl J Med 2018; 378(9):785–788. doi:10.1056/NEJMp1716147
- Horowitz J. Italy loosens vaccine law just as children return to school. New York Times Sept. 20, 2018. www.nytimes.com/2018/09/20/world/europe/italy-vaccines-five-star-movement.html.
- National Conference of State Legislature. States with religious and philosophical exemptions from school immunization requirements. www.ncsl.org/research/health/school-immunization-exemption-state-laws.aspx. Accessed October 3, 2018.
- Olive JK, Hotez PJ, Damania A, Nolan MS. The state of the antivaccine movement in the United States: a focused examination of nonmedical exemptions in states and counties. PLoS Med 2018; 15(6):e1002578. doi:10.1371/journal.pmed.1002578
- Logan J, Nederhoff D, Koch B, et al. ‘What have you HEARD about the HERD?’ Does education about local influenza vaccination coverage and herd immunity affect willingness to vaccinate? Vaccine 2018; 36(28):4118–4125. doi:10.1016/j.vaccine.2018.05.037
- Belongia EA, Skowronski DM, McLean HQ, Chambers C, Sundaram ME, De Serres G. Repeated annual influenza vaccination and vaccine effectiveness: review of evidence. Expert Rev Vaccines 2017; 16(7):1–14. doi:10.1080/14760584.2017.1334554
- Saito N, Komori K, Suzuki M, et al. Negative impact of prior influenza vaccination on current influenza vaccination among people infected and not infected in prior season: a test-negative case-control study in Japan. Vaccine 2017; 35(4):687–693. doi:10.1016/j.vaccine.2016.11.024
- Cheng AC, Macartney KK, Waterer GW, Kotsimbos T, Kelly PM, Blyth CC; Influenza Complications Alert Network (FluCAN) Investigators. Repeated vaccination does not appear to impact upon influenza vaccine effectiveness against hospitalization with confirmed influenza. Clin Infect Dis 2017; 64(11):1564–1572. doi:10.1093/cid/cix209
- Rondy M, Launay O, Castilla J, et al; InNHOVE/I-MOVE+working group. Repeated seasonal influenza vaccination among elderly in Europe: effects on laboratory confirmed hospitalised influenza. Vaccine 2017; 35(34):4298–4306. doi:10.1016/j.vaccine.2017.06.088
- Young-Xu Y, van Aalst R, Mahmud SM, et al. Relative vaccine effectiveness of high-dose versus standard-dose influenza vaccines among Veterans Health Administration patients. J Infect Dis 2018; 217(11):1718–1727. doi:10.1093/infdis/jiy088
- Shay DK, Chillarige Y, Kelman J, et al. Comparative effectiveness of high-dose versus standard-dose influenza vaccines among US Medicare beneficiaries in preventing postinfluenza deaths during 2012–2013 and 2013–2014. J Infect Dis 2017; 215(4):510–517. doi:10.1093/infdis/jiw641
- Madaras-Kelly K, Remington R, Hruza H, Xu D. Comparative effectiveness of high-dose versus standard-dose influenza vaccines in preventing postinfluenza deaths. J Infect Dis 2018; 218(2):336–337. doi:10.1093/infdis/jix645
- Tam YH, Valkenburg SA, Perera RAPM, et al. Immune responses to twice-annual influenza vaccination in older adults in Hong Kong. Clin Infect Dis 2018; 66(6):904–912. doi:10.1093/cid/cix900
- Ohfuji S, Deguchi M, Tachibana D, et al; Osaka Pregnant Women Influenza Study Group. Protective effect of maternal influenza vaccination on influenza in their infants: a prospective cohort study. J Infect Dis 2018; 217(6):878–886. doi:10.1093/infdis/jix629
- Katz J, Englund JA, Steinhoff MC, et al. Impact of timing of influenza vaccination in pregnancy on transplacental antibody transfer, influenza incidence, and birth outcomes: a randomized trial in rural Nepal. Clin Infect Dis 2018; 67(3):334–340. doi:10.1093/cid/ciy090
- Nunes MC, Cutland CL, Madhi SA. Influenza vaccination during pregnancy and protection against pertussis. N Engl J Med 2018; 378(13):1257–1258. doi:10.1056/NEJMc1705208
- Andrew MK, Shinde V, Ye L, et al; Serious Outcomes Surveillance Network of the Public Health Agency of Canada/Canadian Institutes of Health Research Influenza Research Network (PCIRN) and the Toronto Invasive Bacterial Diseases Network (TIBDN). The importance of frailty in the assessment of influenza vaccine effectiveness against influenza-related hospitalization in elderly people. J Infect Dis 2017; 216(4):405–414. doi:10.1093/infdis/jix282
- Park JK, Lee YJ, Shin K, et al. Impact of temporary methotrexate discontinuation for 2 weeks on immunogenicity of seasonal influenza vaccination in patients with rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis 2018; 77(6):898–904. doi:10.1136/annrheumdis-2018-213222
- Kumar D, Ferreira VH, Blumberg E, et al. A five-year prospective multi-center evaluation of influenza infection in transplant recipients. Clin Infect Dis 2018. Epub ahead of print. doi:10.1093/cid/ciy294
- Natori Y, Shiotsuka M, Slomovic J, et al. A double-blind, randomized trial of high-dose vs standard-dose influenza vaccine in adult solid-organ transplant recipients. Clin Infect Dis 2018; 66(11):1698–1704. doi:10.1093/cid/cix1082
- Omer SB, Phadke VK, Bednarczyk BA, Chamberlain AT, Brosseau JL, Orenstein WA. Impact of statins on influenza vaccine effectiveness against medically attended acute respiratory illness. J Infect Dis 2016; 213(8):1216–1223. doi:10.1093/infdis/jiv457
- Dunkle LM, Izikson R, Patriarca P, et al. Efficacy of recombinant influenza vaccine in adults 50 years of age or older. N Engl J Med 2017; 376(25):2427–2436. doi:10.1056/NEJMoa1608862
- STAT; Branswell H. How the US military might help answer a critical question about the flu vaccine. www.statnews.com/2018/03/02/flu-vaccine-egg-production-data. Accessed October 3, 2018.
- Paules CI, Sullivan SG, Subbarao K, Fauci AS. Chasing seasonal influenza—the need for a universal influenza vaccine. N Engl J Med 2018; 378(1):7–9. doi:10.1056/NEJMp1714916
- Jin XW, Mossad SB. Avian influenza: an emerging pandemic threat. Cleve Clin J Med 2005; 72:1129-1134. pmid:16392727
- Wei WI, Brunger AT, Skehel JJ, Wiley DC. Refinement of the influenza virus hemagglutinin by simulated annealing. J Mol Biol 1990; 212(4):737–761. doi:10.1016/0022-2836(90)90234-D
- Erbelding EJ, Post DJ, Stemmy EJ, et al. A universal influenza vaccine: the strategic plan for the National Institute of Allergy and Infectious Diseases, J Infect Dis 2018; 218(3):347–354. doi:10.1093/infdis/jiy103
- Shinde V, Fries L, Wu Y, et al. Improved titers against influenza drift variants with a nanoparticle vaccine. N Engl J Med 2018; 378(24):2346–2348. doi:10.1056/NEJMc1803554
- Maurer MA, Meyer L, Bianchi M, et al. Glycosylation of human IgA directly inhibits influenza A and other sialic-acid-binding viruses. Cell Rep 2018; 23(1):90–99. doi:10.1016/j.celrep.2018.03.027
- Graham BS, Mascola JR, Fauci AS. Novel vaccine technologies: essential components of an adequate response to emerging viral diseases. JAMA 2018; 319(14):1431–1432. doi:10.1001/jama.2018.0345
- Stewart RJ, Flannery B, Chung JR, et al. Influenza antiviral prescribing for outpatients with an acute respiratory illness and at high risk for influenza-associated complications during 5 influenza seasons—United States, 2011–2016. Clin Infect Dis 2018; 66(7):1035–1041. doi:10.1093/cid/cix922
- Zheng S, Tang L, Gao H, et al. Benefit of early initiation of neuraminidase inhibitor treatment to hospitalized patients with avian influenza A(H7N9) virus. Clin Infect Dis 2018; 66(7):1054–1060. doi:10.1093/cid/cix930
- Kumar D, Ferreira VH, Blumberg E, et al. A five-year prospective multi-center evaluation of influenza infection in transplant recipients. Clin Infect Dis 2018. Epub ahead of print. doi:10.1093/cid/ciy294
- Malosh RE, Martin ET, Heikkinen T, Brooks WA, Whitley RJ, Monto AS. Efficacy and safety of oseltamivir in children: systematic review and individual patient data meta-analysis of randomized controlled trials. Clin Infect Dis 2018; 66(10):1492–1500. doi:10.1093/cid/cix1040
- Havers FP, Hicks LA, Chung JR, et al. Outpatient antibiotic prescribing for acute respiratory infections during influenza seasons. JAMA Network Open 2018; 1(2):e180243. doi:10.1001/jamanetworkopen.2018.0243
- US Food and Drug Administration. FDA warns of fraudulent and unapproved flu products. www.fda.gov/newsevents/newsroom/pressannouncements/ucm599223.htm. Accessed October 3, 2018.
- Portsmouth S, Kawaguchi K, Arai M, Tsuchiya K, Uehara T. Cap-dependent endonuclease inhibitor S-033188 for the treatment of influenza: results from a phase 3, randomized, double-blind, placebo- and active-controlled study in otherwise healthy adolescents and adults with seasonal influenza. Open Forum Infect Dis 2017; 4(suppl 1):S734. doi:10.1093/ofid/ofx180.001
- Hayden FG, Sugaya N, Hirotsu N, et al; Baloxavir Marboxil Investigators Group. Baloxavir Marboxil for uncomplicated influenza in adults and adolescents. N Engl J Med 2018; 379(10):913–923. doi:10.1056/NEJMoa1716197
- Kadam RU, Wilson IA. A small-molecule fragment that emulates binding of receptor and broadly neutralizing antibodies to influenza A hemagglutinin. Proc Natl Acad Sci U S A 2018; 115(16):4240–4245. doi:10.1073/pnas.1801999115
KEY POINTS
- Influenza A(H7N9) is a prime candidate to cause the next influenza pandemic.
- Influenza vaccine prevents 300 to 4,000 deaths in the United States every year.
- The 2018–2019 quadrivalent influenza vaccine contains updated A(H3N2) and B/Victoria lineage components different from those in the 2017–2018 Northern Hemisphere vaccine.
- The live-attenuated influenza vaccine, which was not recommended during the 2016–2017 and 2017–2018 influenza seasons, is recommended for the 2018–2019 influenza season.
- Influenza vaccine is recommended any time during pregnancy and is associated with lower infant mortality rates.
- Overall influenza vaccination rates remain below the 80% target for all Americans and 90% for at-risk populations.
Bisphosphonate-related atypical femoral fracture: Managing a rare but serious complication
Bisphosphonate therapy minimizes bone loss and reduces fracture risk by up to 50% in patients with osteoporosis,1 but it is also associated with increased risks of osteonecrosis of the jaw and atypical femoral fracture. Although atypical femoral fractures are rare, they can have a devastating effect. Patient concern about this complication has contributed to a decrease in bisphosphonate use by about half in the last decade or so,2,3 and we fear this could result in an increase in hip fracture rates.
In this article, we examine the evidence on bisphosphonate-associated atypical femoral fractures, including risks, pathogenesis, treatment, and prevention.
ATYPICAL FRACTURES INVOLVE THE FEMORAL SHAFT, NOT THE HEAD
An atypical femoral fracture is a transverse fracture of the femoral shaft (diaphysis), defined by both clinical criteria and radiographic appearance.
To be defined as atypical, a femoral fracture must meet 4 of the following 5 criteria4:
- Occurs with minimal or no trauma
- Has a predominantly transverse fracture line, originating at the lateral cortex and sometimes becoming oblique as it progresses medially across the femur
- Extends through both cortices and may be associated with a medial spike (complete fractures); or involves only the lateral cortex (incomplete fractures)
- Is noncomminuted or minimally comminuted
- Shows localized periosteal or endosteal thickening (termed “beaking” or “flaring”) of the lateral cortex at the fracture site.
Several minor features are also important but are not required, eg:
- Cortical thickening of the femoral shaft
- Unilateral or bilateral prodromal pain preceding the fracture
- Bilateral incomplete or complete femoral diaphysis fractures
- Delayed fracture healing.
Atypical femoral fracture can occur anywhere along the shaft, from just distal to the lesser trochanter to just proximal to the supracondylar flare. However, most occur in 2 areas, with 1 cluster centered at about 41 mm from the lesser trochanter (more common in relatively younger patients) and the other at 187 mm.5
ABSOLUTE RISK IS LOW BUT INCREASES WITH LONGER USE
Atypical femoral fractures are rare. Schilcher et al6 reviewed radiographs of 1,234 women who had a subtrochanteric or shaft fracture and found 59 (4.6%) of fractures were atypical. In a systematic review of 14 studies,7 the incidence ranged from 3.0 to 9.8 cases per 100,000 patient-years.
Furthermore, not all atypical femoral fractures are in bisphosphonate users: 7.4% were in nonusers in 1 series8 and 22% in another.9
Nevertheless, most studies show that bisphosphonate use increases the incidence of atypical femoral fracture, and the incidence increases with duration of use, especially after 3 years.7
An international task force of the American Society for Bone and Mineral Research listed the absolute risk as between 3.2 and 50 cases per 100,000 patient-years, with longer use (> 5 years) increasing the risk to about 100 per 100,000 patient-years.4 After stopping bisphosphonate therapy, the risk diminished by 70% per year.9
In another study, for 0.1 to 1.9 years of therapy, the age-adjusted atypical fracture rates were 1.78 per 100,000 per year (95% confidence interval [CI] 1.5–2.0), increasing to 113.1 per 100,000 per year (95% CI 69.3–156.8) with exposure from 8 to 9.9 years.10
A case-control study found that more than 5 years of bisphosphonate use increased the fracture risk by an odds ratio of 2.74 (95% CI 1.25–6.02).11
The incidence of typical femoral fracture was higher in those who adhered better to their oral bisphosphonate regimen in some studies,12 but the opposite was true in others.13
The benefits of bisphosphonate therapy in reducing fracture risk, however, outweigh the risk of atypical fracture.4
We do not know whether the rate of atypical femoral fracture is increasing. A review of Kaiser Permanente Northwest records found that the rates of atypical femoral shaft fracture had remained stable from 1996 to 2009. However, 61.9% of patients who met the strict radiographic criteria had taken oral bisphosphonates.14 These data suggest that bisphosphonate use has not increased the overall population-based risk for subtrochanteric and femoral shaft fractures, but that bisphosphonates and other risk factors may have increased the likelihood that such fractures will exhibit atypical radiographic features.
A population-based study in Denmark13 found that alendronate use longer than 10 years was associated with an adjusted 30% lower risk of hip fracture and no increase in the risk of subtrochanteric and femoral shaft fracture. In addition, the risk of subtrochanteric and femoral shaft fracture was lower with high adherence to alendronate treatment (based on medication possession ratio > 80%) compared with low adherence (ratio < 50%) (odds ratio 0.88, 95% CI 0.77–0.99). The risk was not increased in current vs past users.
The Danish study13 used the coding of the 10th revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10) to identify subtrochanteric and femoral shaft fractures without radiologic review for atypical radiographic features. The lack of specific ICD-10 coding for subtrochanteric and femoral shaft fractures with atypical radiographic features has limited our knowledge of their incidence.
Contralateral fracture in more than one-fourth of cases
After an atypical femoral fracture, patients have a significant risk of fracture on the contralateral side. In a case-control study, 28% of patients with atypical femoral fracture suffered a contralateral fracture, compared with 0.9% of patients presenting with a typical fracture pattern (odds ratio 42.6, 95% CI 12.8–142.4).15
Contralateral fracture occurs from 1 month to 4 years after the index atypical femoral fracture.16
There are reports of bisphosphonate-related low-impact fractures in other sites such as the tibia17 and forearm.18 However, they may be too rare to warrant screening.
Mortality rates
A Swedish database study found that patients with atypical femoral fractures, whether bisphosphonate users or nonusers, do not have higher mortality rates than patients with ordinary subtrochanteric or femoral shaft fractures.19 Furthermore, the mortality rates for those with atypical femoral fracture were similar to rates in the general population. In contrast, patients with an ordinary femoral fracture had a higher mortality risk than the general population.19
Other studies suggest that atypical femoral fracture may be associated with a less favorable prognosis in older patients,20 but this could be due to differences in demographics, treatment adherence, or postfracture care.21
In addition, functional outcomes as measured by independent mobility at discharge and at 3 months were comparable between patients with atypical fracture and those with typical fracture.22
IMAGING STUDIES
If a long-term bisphosphonate user presents with hip, thigh, or groin pain, imaging studies are recommended.
Plain radiography
Radiography is usually the first step and should include a frontal view of the pelvis (Figure 1) and 2 views of the full length of each femur. If radiography is not conclusive, bone scan or magnetic resonance imaging (MRI) should be considered.
A linear cortex transverse fracture pattern and focal lateral cortical thickening are the most sensitive and specific radiographic features.23,24 Because of the risk of fracture on the contralateral side, radiographic study of that side is recommended as well.
Computed tomography
Computed tomography (CT) is not sensitive for early stress fractures and, given the radiation burden, is not recommended in the workup of atypical fracture.
Bone scanning
Bone scanning using technetium 99m-labeled methylene diphosphonate with a gamma camera shows active bone turnover. Stress fractures and atypical femoral fractures are most easily identified in the third (delayed) phase of the bone scan. Although bone scanning is highly sensitive, the specificity is limited by lack of spatial resolution. Atypical femoral fracture appears as increased activity in the subtrochanteric region with a predilection for the lateral cortex.
Dual-energy x-ray absorptiometry
Conventional dual-energy x-ray absorptiometry (DXA) extends only to 1 to 2 cm below the lesser trochanter and can therefore miss atypical fractures, which usually occur farther down. The overall detection rate for DXA was 61% in a sample of 33 patients.25
Newer scanners can look at the entire femoral shaft.26 In addition, newer software can quantify focal thickening (beaking) of the lateral cortex and screen patients who have no symptoms. The results of serial measurements can be graphed so that the practitioner can view trends to help assess or rule out potential asymptomatic atypical femoral fracture.
A localized reaction (periosteal thickening of the lateral cortex or beaking) often precedes atypical femoral fracture. A 2017 study reported that patients with high localized reaction (mean height 3.3 mm) that was of the pointed type and was accompanied by prodromal pain had an increased risk of complete or incomplete atypical femoral fracture at that site.27 This finding is used by the newer DXA software. The predictive value of beaking on extended femoral DXA may be as high as 83%.26
Magnetic resonance imaging
The MRI characteristics of atypical femoral fracture are similar to those of other stress fractures except that there is a lateral-to-medial pattern rather than a medial pattern. The earliest findings include periosteal reaction about the lateral cortex with a normal marrow signal.
MRI may be of particular benefit in patients with known atypical femoral fracture to screen the contralateral leg. It should image the entire length of both femurs. Contrast enhancement is not needed.
Regardless of whether initial findings were discovered on conventional radiographs or DXA, MRI confirmation is needed. Radionuclide bone scanning is currently not recommended because it lacks specificity. Combination imaging is recommended, with either radiography plus MRI or DXA plus MRI.
DIFFERENTIAL DIAGNOSIS
The differential diagnosis of atypical femoral fracture includes stress fracture, pathologic fracture, hypophosphatasia, and osteogenesis imperfecta.28 Hypophosphatemic osteomalacia can cause Looser zones, which can be confused with atypical femoral fractures but usually occur on the medial side.4 Stress fracture of the femur can occur below the lesser trochanter but usually begins in the medial, not the lateral, cortex.
Pathologic fractures from underlying osseous lesions can mimic the cortical beaking of bisphosphonate-related fracture, but they usually show the associated underlying lucent lesion and poorly defined margins. A sinus tract along the region of a chronic osteomyelitis may also appear similar.
Hypophosphatasia is an inborn error of metabolism caused by a loss-of-function mutation in the gene encoding alkaline phosphatase, resulting in pyrophosphate accumulation and causing osteomalacia from impaired mineralization. This can result in femoral pseudofracture that is often bilateral and occurs in the subtrochanteric region.29
ADDITIONAL RISK FACTORS
Patients with atypical femoral fracture are generally a heterogeneous group, but there are risk factors to note other than bisphosphonate exposure.
Asian women had a risk 8 times higher than white women in 1 study.30
Bone geometry. Mahjoub et al8 reported that compared with controls, patients with atypical femoral fracture had greater offset of the femoral shaft from the center of rotation of the femoral head, a more acute angle between the femoral neck and shaft, and greater proximal cortical thickness.
Medications. In addition to bisphosphonates, other drugs associated with atypical femoral fracture include RANK-ligand inhibitors such as denosumab (another drug for osteoporosis),31 glucocorticoids,32,33 and proton pump inhibitors.32,33
Genetics. Three sisters with atypical femoral fracture were found to have 37 rare mutations in 34 genes, including one in the GGPS1 gene, which codes for geranylgeranyl pyrophosphate synthase—an enzyme that bisphosphonates inhibit.34
Medical conditions other than osteoporosis include collagen diseases, chronic pulmonary disease, asthma, rheumatoid arthritis, and diabetes.35
Clinical recommendations
Current recommendations are to reevaluate bisphosphonate use in patients with osteoporosis after 5 or more years of therapy.36
Given that patients with osteoporosis are at increased risk of typical fracture, those at higher risk should be considered for continued bisphosphonate therapy. Factors for high risk include the following:
- History of fracture on therapy
- Hip T score –2.5 or lower
- Older age (≥ 70)
- Other strong risk factors for fracture such as smoking, alcohol use, corticosteroid use, rheumatoid arthritis, and family history
- World Health Organization FRAX fracture risk score above the country-specific threshold.
Those at lower risk should be considered for a 2- to 3-year bisphosphonate holiday with periodic reevaluation of bone density and, possibly, bone markers.36
WHAT IS THE UNDERLYING PATHOPHYSIOLOGY?
The mechanism by which bisphosphonates increase the risk of atypical femoral fracture is not clear. These drugs work by suppressing bone turnover; however, in theory, prolonged use could suppress it too much and increase bone fragility.
One hypothesis is that bisphosphonates impair the toughening of cortical bone, an important barrier to clinical fracture. This is supported by a study that found bisphosphonate users with atypical femoral fracture had deficits in intrinsic and extrinsic bone toughness, perhaps due to treatment-related increases in matrix mineralization.37 Although this study and others showed an increase in matrix mineralization and reduced mineralization heterogeneity with bisphosphonate use,38,39 it is unclear whether such changes contributed to reduced toughness or to atypical femoral fracture.
Changes in the skeletal geometry of the lower limb such as femoral neck-shaft angle and femoral curvature alter the stresses and strains experienced by the femoral diaphysis with loading. Because the incidence of incomplete atypical femoral fracture is much greater than that of complete fracture, most incomplete atypical femoral fractures heal before the fracture progresses.
Ultimately, all fractures, including atypical femoral fractures, occur when mechanical stress and strain exceed bone strength.
Antiresorptive drugs such as bisphosphonates, estrogen, calcitonin, and RANK ligand inhibitors prevent hip fracture by increasing the strength of the proximal femur—perhaps at the expense of the strength (or toughness) of the subtrochanteric shaft. It is also possible that treatment-related increases in hip strength (and reduced hip fracture rates) promote or sustain the transfer of stress and strain to femoral regions that experience lesser or no increases in strength from treatment, which likely includes the shaft.40,41
CT studies in Japanese women with osteoporosis have shown that 2 years of zoledronate therapy had greater effects in the hip than in the femoral shaft, with significant increases in cortical thickness and volumetric bone mineral density at the femoral neck and intertrochanteric region compared with baseline.42 But zoledronate did not increase femoral shaft cortical thickness and caused only a minor increase in femoral shaft volumetric bone mineral density. Fracture patterns may have depended on damage and effects of bone turnover on mass and structure.
This hypothetical scenario portrays a possible “hip survival bias” mechanism for atypical femoral fracture, with the association with antiresorptive drugs arising from greater stress and strain in cortical regions where these fractures occur rather than from treatment-related reductions in cortical bone strength or toughness.
PRODROMAL PAIN IS COMMON
From 32% to 76% of patients who have incomplete or developing atypical femoral fracture present with a prodrome of groin or hip pain.4,43 Prodromal pain occurs any time from 2 weeks to several years before the fracture, presenting as pain in the anterior or lateral thigh or in the groin.
Prodromal pain in a patient on antiresorptive therapy should be a signal for the clinician to obtain a radiograph of the hip and to look for contralateral symptoms and fractures. The most common mechanism of injury appears to be a ground-level fall or even a nontraumatic activity such as walking or stepping off a curb.
MEDICAL MANAGEMENT
In bisphosphonate users with radiographic evidence of atypical femoral fracture, the bisphosphonate should be discontinued and the patient assessed for calcium and vitamin D deficiency, with supplements prescribed if needed.4
For patients with incomplete fracture and persistent pain after 3 months of medical management, prophylactic surgical nail fixation is recommended to prevent complete fracture.
Teriparatide, which has been associated with enhanced bone fracture healing, is a possible treatment to promote healing of atypical femoral fracture, either alone or as an adjunct to surgical fixation. A systematic review published in 2015 supported the use of teriparatide for enhancing fracture healing in atypical femoral fracture.44 In addition, a 10-patient series45 showed that incomplete fractures without radiolucent lines responded to teriparatide alone, whereas those with radiolucent lines needed intramedullary nailing.
These results suggest that teriparatide works best when the fracture site is stable, either inherently or with surgical fixation.
ORTHOPEDIC CARE
Orthopedic care for atypical femoral fracture differs depending on whether the patient experiences pain and whether the fracture is incomplete or complete. Figure 2 shows a treatment algorithm for atypical femoral fracture.
These are difficult fractures to manage, complicated by delayed healing in the elderly, complex displacement patterns, altered bone geometry, and risk of fracture in the opposite limb, all of which raise questions about recommending protected weight-bearing exercise.
Furthermore, atypical femoral fracture is often associated with increased anterolateral bowing of the femur, making it difficult to insert an intramedullary nail: the radius of curvature of the bone is shorter than that of a standard femoral nail. This mismatch can lead to intraoperative complications such as iatrogenic fracture during prophylactic nailing, malunion from excess straightening of the femur (which can itself lead to leg length discrepancy), and gapping of the fracture site, particularly on the medial side.
Intramedullary nailing for complete fracture
Intramedullary nailing is the first-line treatment for complete atypical femoral fracture, although the risk of delayed healing and revision surgery may be somewhat higher than with typical femoral fracture.46 Prophylactic intramedullary nailing should be considered for a patient with intractable pain.2
A radiograph of the opposite leg should be obtained routinely, looking for an asymptomatic fracture. Bisphosphonates should be discontinued and calcium and vitamin D continued. Teriparatide therapy can be considered as an alternative treatment.
Conservative management for incomplete fracture without pain
Incomplete atypical femoral fracture unaccompanied by pain can be followed conservatively.47 In addition to stopping antiresorptive therapy, patients need to avoid high-impact and repetitive-impact activities such as jumping or running. If pain occurs, patients should begin protected weight-bearing exercise.
Treatment is uncertain for incomplete fracture with pain
For patients with incomplete atypical femoral fracture and pain, treatment is controversial. Regimens that include 2 to 3 months of protected weight-bearing exercise, a full metabolic bone workup, calcium and vitamin D supplementation, and anabolic bone agents have produced some success. Some authors have reported poor results from conservative care, with few patients achieving pain relief or signs of complete healing.48,49 Additionally, if an incomplete fracture is found in the opposite femur, protected weight-bearing of both legs may not be possible.
Patients with incomplete fracture should be monitored regularly with radiography and physical examination. If there is progression of the fracture, escalation of pain, or failure to heal within 2 to 3 months, then surgical treatment is necessary.
Prophylactic placement of an intramedullary nail to prevent completion of the fracture and allow a return to full weight-bearing is generally advised.50 A long locking plate can be used if bone deformities make it difficult to place an intramedullary nail; however, nails are preferred because they allow formation of endochondral callus, which can be helpful in these difficult-to-heal fractures.
Results from retrospective reviews have shown that surgically treated patients with bisphosphonate-associated incomplete atypical femoral fracture were more likely than those treated nonsurgically to be pain-free (81% vs 64%) and have radiographic healing (100% vs 18% at final follow-up).46 Results have also been positive for those with complete atypical femoral fracture. At 6 months, 64% of surgically treated patients were pain-free and 98% were radiographically healed.51
The unusual geometry of the femur in patients with atypical femoral fracture and the presence of intramedullary cortical callus makes the placement of an intramedullary femoral rod more complex than in typical femoral fracture.8
Intramedullary nailing of atypical femoral fracture is a challenge for even the most experienced surgeon, and vigilance is imperative to avoid iatrogenic fracture and malunion.
MANY QUESTIONS REMAIN
We need more studies on the pathophysiology of bisphosphonate-associated atypical femoral fracture, the value of periodic screening with DXA, and which factors predict high risk (eg, Asian ethnicity, use of certain medications, femoral geometry). In addition, we need more data on the success of conservative management of incomplete fracture, including use of teriparatide.
- Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 1996; 348(9041):1535–1541. pmid:8950879
- Jha S, Wang Z, Laucis N, Bhattacharyya T. Trends in media reports, oral bisphosphonate prescriptions, and hip fractures 1996–2012: an ecological analysis. J Bone Miner Res 2015; 30(12):2179–2187. doi:10.1002/jbmr.2565
- Solomon DH, Johnston SS, Boytsov NN, McMorrow D, Lane JM, Krohn KD. Osteoporosis medication use after hip fracture in US patients between 2002 and 2011. J Bone Miner Res 2014; 29(9):1929–1937. doi:10.1002/jbmr.2202
- Shane E, Burr D, Abrahamsen B, et al. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 2014; 29(1):1–23. doi:10.1002/jbmr.1998
- Koeppen VA, Schilcher J, Aspenberg P. Dichotomous location of 160 atypical femoral fractures. Acta Orthop 2013; 84(6):561–564. doi:10.3109/17453674.2013.866193
- Schilcher J, Koeppen V, Aspenberg P, Michäelsson K. Risk of atypical femoral fracture during and after bisphosphonate use. Acta Orthop 2015; 86(1):100–107. doi:10.3109/17453674.2015.1004149
- Khow KS, Shibu P, Yu SC, Chehade MJ, Visvanathan R. Epidemiology and postoperative outcomes of atypical femoral fractures in older adults: a systematic review. J Nutr Health Aging 2017; 21(1):83–91. doi:10.1007/s12603-015-0652-3
- Mahjoub Z, Jean S, Leclerc JT, et al. Incidence and characteristics of atypical femoral fractures: clinical and geometrical data. J Bone Miner Res 2016; 31(4):767–776. doi:10.1002/jbmr.2748
- Schilcher J, Michaelsson K, Aspenberg P. Bisphosphonate use and atypical fractures of the femoral shaft. N Engl J Med 2011; 364(18):1728–1737. doi:10.1056/NEJMoa1010650
- Dell RM, Adams AL, Greene DF, et al. Incidence of atypical nontraumatic diaphyseal fractures of the femur. J Bone Miner Res 2012; 27(12):2544–2550. doi:10.1002/jbmr.1719
- Park-Wyllie LY, Mamdani MM, Juurlink DN, et al. Bisphosphonate use and the risk of subtrochanteric or femoral shaft fractures in older women. JAMA 2011; 305(8):783–789. doi:10.1001/jama.2011.190
- Wang Z, Ward MM, Chan L, Bhattacharyya T. Adherence to oral bisphosphonates and the risk of subtrochanteric and femoral shaft fractures among female Medicare beneficiaries. Osteoporos Int 2014; 25(8):2109–2116. doi:10.1007/s00198-014-2738-x
- Abrahamsen B, Eiken P, Prieto-Alhambra D, Eastell R. Risk of hip, subtrochanteric, and femoral shaft fractures among mid and long term users of alendronate: nationwide cohort and nested case-control study. BMJ 2016; 353:i3365. doi:10.1136/bmj.i3365
- Feldstein AC, Black D, Perrin N, et al. Incidence and demography of femur fractures with and without atypical features. J Bone Miner Res 2012; 27(5):977–986. doi:10.1002/jbmr.1550
- Meier RP, Perneger TV, Stern R, Rizzoli R, Peter RE. Increasing occurrence of atypical femoral fractures associated with bisphosphonate use. Arch Intern Med 2012; 172(12):930–936. doi:10.1001/archinternmed.2012.1796
- La Rocca Vieira R, Rosenberg ZS, Allison MB, Im SA, Babb J, Peck V. Frequency of incomplete atypical femoral fractures in asymptomatic patients on long term bisphosphonate therapy. AJR Am J Roentgenol 2012; 198(5):1144–1151. doi:10.2214/AJR.11.7442
- Bissonnette L, April PM, Dumais R, Boire G, Roux S. Atypical fracture of the tibial diaphysis associated with bisphosphonate therapy: a case report. Bone 2013; 56(2):406–409. doi:10.1016/j.bone.2013.07.012
- Moon J, Bither N, Lee T. Atypical forearm fractures associated with long-term use of bisphosphonate. Arch Orthop Trauma Surg 2013; 133(7):889–892. doi:10.1007/s00402-013-1760-3
- Kharazmi M, Hallberg P, Schilcher J, Aspenberg P, Michaëlsson K. Mortality after atypical femoral fractures: a cohort study. J Bone Miner Res 2016; 31(3):491–497. doi:10.1002/jbmr.2767
- Medin E, Goude F, Melberg HO, Tediosi F, Belicza E, Peltola M; EuroHOPE Study Group. European regional differences in all-cause mortality and length of stay for patients with hip fracture. Health Econ 2015; 24(suppl 2):53–64. doi:10.1002/hec.3278
- Abrahamsen B, Prieto-Alhambra D. Patients with atypical femur fractures have the same mortality as the background population-drug channeling bias, bisphosphonate effects and public health implications. J Bone Miner Res 2016; 31(3):488–490. doi:10.1002/jbmr.2801
- Khow KS, Paterson F, Shibu P, Yu SC, Chehade MJ, Visvanathan R. Outcomes between older adults with atypical and typical femoral fractures are comparable. Injury 2017; 48(2):394–398. doi:10.1016/j.injury.2016.10.035
- Adams AL, Xue F, Chantra JQ, et al. Sensitivity and specificity of radiographic characteristics in atypical femoral fractures. Osteoporos Int 2017; 28(1):413–417. doi:10.1007/s00198-016-3809-y
- Rosenberg ZS, La Rocca Vieira R, Chan SS, et al. Bisphosphonate-related complete atypical subtrochanteric femoral fractures: diagnostic utility of radiography. AJR Am J Roentgenol 2011; 197(4):954–960. doi:10.2214/AJR.10.6262
- Kim S, Yang KH, Lim H, et al. Detection of prefracture hip lesions in atypical subtrochanteric fracture with dual-energy x-ray absorptiometry images. Radiology 2014; 270(2):487–495. doi:10.1148/radiol.13122691
- van de Laarschot DM, Smits AA, Buitendijk SK, Stegenga MT, Zillikens MC. Screening for atypical femur fractures using extended femur scans by DXA. J Bone Miner Res 2017; 32(8):1632–1639. doi:10.1002/jbmr.3164
- Sato H, Kondo N, Nakatsue T, et al. High and pointed type of femoral localized reaction frequently extends to complete an incomplete atypical femoral fracture in patients with autoimmune diseases on long-term glucocorticoids and bisphosphonates. Osteoporos Int 2017; 28(8):2367–2376. doi:10.1007/s00198-017-4038-8
- Giaconi JC, Watterson CT. Bisphosphonate-related atypical femur fractures and the radiographic features. In: Silverman SL, Abrahamsen B, eds. The Duration and Safety of Osteoporosis Treatment. Switzerland: Springer International Publishing; 2016:107–124. doi:10.1007/978-3-319-23639-1
- Whyte MP. Atypical femoral fractures, bisphosphonates, and adult hypophosphatasia. J Bone Miner Res 2009; 24(6):1132–1134. doi:10.1359/jbmr.081253
- Lo JC, Hui RL, Grimsrud CD, et al. The association of race/ethnicity and risk of atypical femoral fracture among older women receiving oral bisphosphonate therapy. Bone 2016; 85:142–147. doi:10.1016/j.bone.2016.01.002
- Bone HG, Wagman RB, Brandi ML, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol 2017; 5(7):513–523. doi:10.1016/S2213-8587(17)30138-9
- Koh JH, Myong JP, Yoo J, et al. Predisposing factors associated with atypical femur fracture among postmenopausal Korean women receiving bisphosphonate therapy: 8 years' experience in a single center. Osteoporos Int 2017; 28(11):3251–3259. doi:10.1007/s00198-017-4169-y
- Kim D, Sung YK, Cho SK, Han M, Kim YS. Factors associated with atypical femoral fracture. Rheumatol Int 2016; 36(1):65–71. doi:10.1007/s00296-015-3323-0
- Roca-Ayats N, Balcells S, Garcia-Giralt N, et al. GGPS1 mutation and atypical femoral fractures with bisphosphonates. N Engl J Med 2017; 376(18):1794–1795. doi:10.1056/NEJMc1612804
- Giusti A, Hamdy NA, Dekkers OM, Ramautar SR, Dijkstra S, Papapoulos SE. Atypical fractures and bisphosphonate therapy: a cohort study of patients with femoral fracture with radiographic adjudication of fracture site and features. Bone 2011; 48(5):966–971. doi:10.1016/j.bone.2010.12.033
- Adler RA, El-Hajj Fuleihan G, Bauer DC, et al. Managing osteoporosis in patients on long-term bisphosphonate treatment: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 2016; 31(1):16–35. doi:10.1002/jbmr.2708
- Lloyd AA, Gludovatz B, Riedel C, et al. Atypical fracture with long-term bisphosphonate therapy is associated with altered cortical composition and reduced fracture resistance. Proc Natl Acad Sci USA 2017; 114(33):8722–8727. doi:10.1073/pnas.1704460114
- Ettinger B, Burr DB, Ritchie RO. Proposed pathogenesis for atypical femoral fractures; lessons from materials research. Bone 2013; 55(2):495–500. doi:10.1016/j.bone.2013.02.004
- Burr DB, Liu Z, Allen MR. Duration-dependent effects of clinically relevant oral alendronate doses on cortical bone toughness in beagle dogs. Bone 2015; 71:58–62. doi:10.1016/j.bone.2014.10.010
- Sasaki S, Miyakoshi N, Hongo M, Kasukawa Y, Shimada Y. Low-energy diaphyseal femoral fractures associated with bisphosphonate use and severe curved femur: a case series. J Bone Miner Metab 2012; 30(5):561–567. doi:10.1007/s00774-012-0358-0
- Pulkkinen P, Gluer C, Jamsa T. Investigation of differences between hip fracture types: a worthy strategy of improved risk assessment and fracture prevention. Bone 2011; 49(4):600–604. doi:10.1016/j.bone.2011.07.022
- Ito M, Sone T, Shiraki M, et al. The effect of once-yearly zoledronic acid on hip structural and biomechanical properties derived using computed tomography (CT) in Japanese women with osteoporosis. Bone 2018; 106:179–186. doi:10.1016/j.bone.2017.10.013
- Bogdan Y, Einhorn TA. Clinical presentation of atypical femur fractures. In: Silverman SL, Abrahamsen B, eds. The Duration and Safety of Osteoporosis Treatment. Switzerland: Springer International Publishing; 2016:137–140. doi:10.1007/978-3-319-23639-1
- Im GI, Lee SH. Effect of teriparatide on healing of atypical femoral fractures: a systemic review. J Bone Metab 2015; 22(4):183–189. doi:10.11005/jbm.2015.22.4.183
- Saleh A, Hegde VV, Potty AG, Schneider R, Cornell CN, Lane JM. Management strategy for symptomatic bisphosphonate-associated incomplete atypical femoral fractures. HSS J 2012; 8(2):103–110. doi:10.1007/s11420-012-9275-y
- Egol KA, Park JH, Prensky C, Rosenberg ZS, Peck V, Tejwani NC. Surgical treatment improves clinical and functional outcomes for patients who sustain incomplete bisphosphonate-related femur fractures. J Orthop Trauma 2013; 27(6):331–335. doi:10.1097/BOT.0b013e31827240ae
- Koh A, Guerado E, Giannoudis PV. Atypical femoral fractures related to bisphosphonate treatment: issues and controversies related to their surgical management. Bone Joint J 2017; 99-B(3):295–302. doi:10.1302/0301-620X.99B3.BJJ-2016-0276.R2
- Oh CW, Oh JK, Park KC, Kim JW, Yoon YC. Prophylactic nailing of incomplete atypical femoral fractures. ScientificWorldJournal 2013; 2013:450148. doi:10.1155/2013/450148
- Ha YC, Cho MR, Park KH, Kim SY, Koo KH. Is surgery necessary for femoral insufficiency fractures after long-term bisphosphonate therapy? Clin Orthop Relat Res 2010; 468(12):3393–3398. doi:10.1007/s11999-010-1583-2
- Tosounidis TH, Lampropoulou-Adamidou, Kanakaris NK. Intramedullary nailing of sequential bilateral atypical subtrochanteric fractures and the management of distal femoral intraoperative fracture. J Orthop Trauma 2015 Jun 11. Epub ahead of print. doi:10.1097/BOT.0000000000000370
- Egol KA, Park JH, Rosenberg ZS, Peck V, Tejwani NC. Healing delayed but generally reliable after bisphosphonate-associated complete femur fractures treated with IM nails. Clin Orthop Relat Res 2014; 472(9):2728–2734. doi:10.1007/s11999-013-2963-1
Bisphosphonate therapy minimizes bone loss and reduces fracture risk by up to 50% in patients with osteoporosis,1 but it is also associated with increased risks of osteonecrosis of the jaw and atypical femoral fracture. Although atypical femoral fractures are rare, they can have a devastating effect. Patient concern about this complication has contributed to a decrease in bisphosphonate use by about half in the last decade or so,2,3 and we fear this could result in an increase in hip fracture rates.
In this article, we examine the evidence on bisphosphonate-associated atypical femoral fractures, including risks, pathogenesis, treatment, and prevention.
ATYPICAL FRACTURES INVOLVE THE FEMORAL SHAFT, NOT THE HEAD
An atypical femoral fracture is a transverse fracture of the femoral shaft (diaphysis), defined by both clinical criteria and radiographic appearance.
To be defined as atypical, a femoral fracture must meet 4 of the following 5 criteria4:
- Occurs with minimal or no trauma
- Has a predominantly transverse fracture line, originating at the lateral cortex and sometimes becoming oblique as it progresses medially across the femur
- Extends through both cortices and may be associated with a medial spike (complete fractures); or involves only the lateral cortex (incomplete fractures)
- Is noncomminuted or minimally comminuted
- Shows localized periosteal or endosteal thickening (termed “beaking” or “flaring”) of the lateral cortex at the fracture site.
Several minor features are also important but are not required, eg:
- Cortical thickening of the femoral shaft
- Unilateral or bilateral prodromal pain preceding the fracture
- Bilateral incomplete or complete femoral diaphysis fractures
- Delayed fracture healing.
Atypical femoral fracture can occur anywhere along the shaft, from just distal to the lesser trochanter to just proximal to the supracondylar flare. However, most occur in 2 areas, with 1 cluster centered at about 41 mm from the lesser trochanter (more common in relatively younger patients) and the other at 187 mm.5
ABSOLUTE RISK IS LOW BUT INCREASES WITH LONGER USE
Atypical femoral fractures are rare. Schilcher et al6 reviewed radiographs of 1,234 women who had a subtrochanteric or shaft fracture and found 59 (4.6%) of fractures were atypical. In a systematic review of 14 studies,7 the incidence ranged from 3.0 to 9.8 cases per 100,000 patient-years.
Furthermore, not all atypical femoral fractures are in bisphosphonate users: 7.4% were in nonusers in 1 series8 and 22% in another.9
Nevertheless, most studies show that bisphosphonate use increases the incidence of atypical femoral fracture, and the incidence increases with duration of use, especially after 3 years.7
An international task force of the American Society for Bone and Mineral Research listed the absolute risk as between 3.2 and 50 cases per 100,000 patient-years, with longer use (> 5 years) increasing the risk to about 100 per 100,000 patient-years.4 After stopping bisphosphonate therapy, the risk diminished by 70% per year.9
In another study, for 0.1 to 1.9 years of therapy, the age-adjusted atypical fracture rates were 1.78 per 100,000 per year (95% confidence interval [CI] 1.5–2.0), increasing to 113.1 per 100,000 per year (95% CI 69.3–156.8) with exposure from 8 to 9.9 years.10
A case-control study found that more than 5 years of bisphosphonate use increased the fracture risk by an odds ratio of 2.74 (95% CI 1.25–6.02).11
The incidence of typical femoral fracture was higher in those who adhered better to their oral bisphosphonate regimen in some studies,12 but the opposite was true in others.13
The benefits of bisphosphonate therapy in reducing fracture risk, however, outweigh the risk of atypical fracture.4
We do not know whether the rate of atypical femoral fracture is increasing. A review of Kaiser Permanente Northwest records found that the rates of atypical femoral shaft fracture had remained stable from 1996 to 2009. However, 61.9% of patients who met the strict radiographic criteria had taken oral bisphosphonates.14 These data suggest that bisphosphonate use has not increased the overall population-based risk for subtrochanteric and femoral shaft fractures, but that bisphosphonates and other risk factors may have increased the likelihood that such fractures will exhibit atypical radiographic features.
A population-based study in Denmark13 found that alendronate use longer than 10 years was associated with an adjusted 30% lower risk of hip fracture and no increase in the risk of subtrochanteric and femoral shaft fracture. In addition, the risk of subtrochanteric and femoral shaft fracture was lower with high adherence to alendronate treatment (based on medication possession ratio > 80%) compared with low adherence (ratio < 50%) (odds ratio 0.88, 95% CI 0.77–0.99). The risk was not increased in current vs past users.
The Danish study13 used the coding of the 10th revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10) to identify subtrochanteric and femoral shaft fractures without radiologic review for atypical radiographic features. The lack of specific ICD-10 coding for subtrochanteric and femoral shaft fractures with atypical radiographic features has limited our knowledge of their incidence.
Contralateral fracture in more than one-fourth of cases
After an atypical femoral fracture, patients have a significant risk of fracture on the contralateral side. In a case-control study, 28% of patients with atypical femoral fracture suffered a contralateral fracture, compared with 0.9% of patients presenting with a typical fracture pattern (odds ratio 42.6, 95% CI 12.8–142.4).15
Contralateral fracture occurs from 1 month to 4 years after the index atypical femoral fracture.16
There are reports of bisphosphonate-related low-impact fractures in other sites such as the tibia17 and forearm.18 However, they may be too rare to warrant screening.
Mortality rates
A Swedish database study found that patients with atypical femoral fractures, whether bisphosphonate users or nonusers, do not have higher mortality rates than patients with ordinary subtrochanteric or femoral shaft fractures.19 Furthermore, the mortality rates for those with atypical femoral fracture were similar to rates in the general population. In contrast, patients with an ordinary femoral fracture had a higher mortality risk than the general population.19
Other studies suggest that atypical femoral fracture may be associated with a less favorable prognosis in older patients,20 but this could be due to differences in demographics, treatment adherence, or postfracture care.21
In addition, functional outcomes as measured by independent mobility at discharge and at 3 months were comparable between patients with atypical fracture and those with typical fracture.22
IMAGING STUDIES
If a long-term bisphosphonate user presents with hip, thigh, or groin pain, imaging studies are recommended.
Plain radiography
Radiography is usually the first step and should include a frontal view of the pelvis (Figure 1) and 2 views of the full length of each femur. If radiography is not conclusive, bone scan or magnetic resonance imaging (MRI) should be considered.
A linear cortex transverse fracture pattern and focal lateral cortical thickening are the most sensitive and specific radiographic features.23,24 Because of the risk of fracture on the contralateral side, radiographic study of that side is recommended as well.
Computed tomography
Computed tomography (CT) is not sensitive for early stress fractures and, given the radiation burden, is not recommended in the workup of atypical fracture.
Bone scanning
Bone scanning using technetium 99m-labeled methylene diphosphonate with a gamma camera shows active bone turnover. Stress fractures and atypical femoral fractures are most easily identified in the third (delayed) phase of the bone scan. Although bone scanning is highly sensitive, the specificity is limited by lack of spatial resolution. Atypical femoral fracture appears as increased activity in the subtrochanteric region with a predilection for the lateral cortex.
Dual-energy x-ray absorptiometry
Conventional dual-energy x-ray absorptiometry (DXA) extends only to 1 to 2 cm below the lesser trochanter and can therefore miss atypical fractures, which usually occur farther down. The overall detection rate for DXA was 61% in a sample of 33 patients.25
Newer scanners can look at the entire femoral shaft.26 In addition, newer software can quantify focal thickening (beaking) of the lateral cortex and screen patients who have no symptoms. The results of serial measurements can be graphed so that the practitioner can view trends to help assess or rule out potential asymptomatic atypical femoral fracture.
A localized reaction (periosteal thickening of the lateral cortex or beaking) often precedes atypical femoral fracture. A 2017 study reported that patients with high localized reaction (mean height 3.3 mm) that was of the pointed type and was accompanied by prodromal pain had an increased risk of complete or incomplete atypical femoral fracture at that site.27 This finding is used by the newer DXA software. The predictive value of beaking on extended femoral DXA may be as high as 83%.26
Magnetic resonance imaging
The MRI characteristics of atypical femoral fracture are similar to those of other stress fractures except that there is a lateral-to-medial pattern rather than a medial pattern. The earliest findings include periosteal reaction about the lateral cortex with a normal marrow signal.
MRI may be of particular benefit in patients with known atypical femoral fracture to screen the contralateral leg. It should image the entire length of both femurs. Contrast enhancement is not needed.
Regardless of whether initial findings were discovered on conventional radiographs or DXA, MRI confirmation is needed. Radionuclide bone scanning is currently not recommended because it lacks specificity. Combination imaging is recommended, with either radiography plus MRI or DXA plus MRI.
DIFFERENTIAL DIAGNOSIS
The differential diagnosis of atypical femoral fracture includes stress fracture, pathologic fracture, hypophosphatasia, and osteogenesis imperfecta.28 Hypophosphatemic osteomalacia can cause Looser zones, which can be confused with atypical femoral fractures but usually occur on the medial side.4 Stress fracture of the femur can occur below the lesser trochanter but usually begins in the medial, not the lateral, cortex.
Pathologic fractures from underlying osseous lesions can mimic the cortical beaking of bisphosphonate-related fracture, but they usually show the associated underlying lucent lesion and poorly defined margins. A sinus tract along the region of a chronic osteomyelitis may also appear similar.
Hypophosphatasia is an inborn error of metabolism caused by a loss-of-function mutation in the gene encoding alkaline phosphatase, resulting in pyrophosphate accumulation and causing osteomalacia from impaired mineralization. This can result in femoral pseudofracture that is often bilateral and occurs in the subtrochanteric region.29
ADDITIONAL RISK FACTORS
Patients with atypical femoral fracture are generally a heterogeneous group, but there are risk factors to note other than bisphosphonate exposure.
Asian women had a risk 8 times higher than white women in 1 study.30
Bone geometry. Mahjoub et al8 reported that compared with controls, patients with atypical femoral fracture had greater offset of the femoral shaft from the center of rotation of the femoral head, a more acute angle between the femoral neck and shaft, and greater proximal cortical thickness.
Medications. In addition to bisphosphonates, other drugs associated with atypical femoral fracture include RANK-ligand inhibitors such as denosumab (another drug for osteoporosis),31 glucocorticoids,32,33 and proton pump inhibitors.32,33
Genetics. Three sisters with atypical femoral fracture were found to have 37 rare mutations in 34 genes, including one in the GGPS1 gene, which codes for geranylgeranyl pyrophosphate synthase—an enzyme that bisphosphonates inhibit.34
Medical conditions other than osteoporosis include collagen diseases, chronic pulmonary disease, asthma, rheumatoid arthritis, and diabetes.35
Clinical recommendations
Current recommendations are to reevaluate bisphosphonate use in patients with osteoporosis after 5 or more years of therapy.36
Given that patients with osteoporosis are at increased risk of typical fracture, those at higher risk should be considered for continued bisphosphonate therapy. Factors for high risk include the following:
- History of fracture on therapy
- Hip T score –2.5 or lower
- Older age (≥ 70)
- Other strong risk factors for fracture such as smoking, alcohol use, corticosteroid use, rheumatoid arthritis, and family history
- World Health Organization FRAX fracture risk score above the country-specific threshold.
Those at lower risk should be considered for a 2- to 3-year bisphosphonate holiday with periodic reevaluation of bone density and, possibly, bone markers.36
WHAT IS THE UNDERLYING PATHOPHYSIOLOGY?
The mechanism by which bisphosphonates increase the risk of atypical femoral fracture is not clear. These drugs work by suppressing bone turnover; however, in theory, prolonged use could suppress it too much and increase bone fragility.
One hypothesis is that bisphosphonates impair the toughening of cortical bone, an important barrier to clinical fracture. This is supported by a study that found bisphosphonate users with atypical femoral fracture had deficits in intrinsic and extrinsic bone toughness, perhaps due to treatment-related increases in matrix mineralization.37 Although this study and others showed an increase in matrix mineralization and reduced mineralization heterogeneity with bisphosphonate use,38,39 it is unclear whether such changes contributed to reduced toughness or to atypical femoral fracture.
Changes in the skeletal geometry of the lower limb such as femoral neck-shaft angle and femoral curvature alter the stresses and strains experienced by the femoral diaphysis with loading. Because the incidence of incomplete atypical femoral fracture is much greater than that of complete fracture, most incomplete atypical femoral fractures heal before the fracture progresses.
Ultimately, all fractures, including atypical femoral fractures, occur when mechanical stress and strain exceed bone strength.
Antiresorptive drugs such as bisphosphonates, estrogen, calcitonin, and RANK ligand inhibitors prevent hip fracture by increasing the strength of the proximal femur—perhaps at the expense of the strength (or toughness) of the subtrochanteric shaft. It is also possible that treatment-related increases in hip strength (and reduced hip fracture rates) promote or sustain the transfer of stress and strain to femoral regions that experience lesser or no increases in strength from treatment, which likely includes the shaft.40,41
CT studies in Japanese women with osteoporosis have shown that 2 years of zoledronate therapy had greater effects in the hip than in the femoral shaft, with significant increases in cortical thickness and volumetric bone mineral density at the femoral neck and intertrochanteric region compared with baseline.42 But zoledronate did not increase femoral shaft cortical thickness and caused only a minor increase in femoral shaft volumetric bone mineral density. Fracture patterns may have depended on damage and effects of bone turnover on mass and structure.
This hypothetical scenario portrays a possible “hip survival bias” mechanism for atypical femoral fracture, with the association with antiresorptive drugs arising from greater stress and strain in cortical regions where these fractures occur rather than from treatment-related reductions in cortical bone strength or toughness.
PRODROMAL PAIN IS COMMON
From 32% to 76% of patients who have incomplete or developing atypical femoral fracture present with a prodrome of groin or hip pain.4,43 Prodromal pain occurs any time from 2 weeks to several years before the fracture, presenting as pain in the anterior or lateral thigh or in the groin.
Prodromal pain in a patient on antiresorptive therapy should be a signal for the clinician to obtain a radiograph of the hip and to look for contralateral symptoms and fractures. The most common mechanism of injury appears to be a ground-level fall or even a nontraumatic activity such as walking or stepping off a curb.
MEDICAL MANAGEMENT
In bisphosphonate users with radiographic evidence of atypical femoral fracture, the bisphosphonate should be discontinued and the patient assessed for calcium and vitamin D deficiency, with supplements prescribed if needed.4
For patients with incomplete fracture and persistent pain after 3 months of medical management, prophylactic surgical nail fixation is recommended to prevent complete fracture.
Teriparatide, which has been associated with enhanced bone fracture healing, is a possible treatment to promote healing of atypical femoral fracture, either alone or as an adjunct to surgical fixation. A systematic review published in 2015 supported the use of teriparatide for enhancing fracture healing in atypical femoral fracture.44 In addition, a 10-patient series45 showed that incomplete fractures without radiolucent lines responded to teriparatide alone, whereas those with radiolucent lines needed intramedullary nailing.
These results suggest that teriparatide works best when the fracture site is stable, either inherently or with surgical fixation.
ORTHOPEDIC CARE
Orthopedic care for atypical femoral fracture differs depending on whether the patient experiences pain and whether the fracture is incomplete or complete. Figure 2 shows a treatment algorithm for atypical femoral fracture.
These are difficult fractures to manage, complicated by delayed healing in the elderly, complex displacement patterns, altered bone geometry, and risk of fracture in the opposite limb, all of which raise questions about recommending protected weight-bearing exercise.
Furthermore, atypical femoral fracture is often associated with increased anterolateral bowing of the femur, making it difficult to insert an intramedullary nail: the radius of curvature of the bone is shorter than that of a standard femoral nail. This mismatch can lead to intraoperative complications such as iatrogenic fracture during prophylactic nailing, malunion from excess straightening of the femur (which can itself lead to leg length discrepancy), and gapping of the fracture site, particularly on the medial side.
Intramedullary nailing for complete fracture
Intramedullary nailing is the first-line treatment for complete atypical femoral fracture, although the risk of delayed healing and revision surgery may be somewhat higher than with typical femoral fracture.46 Prophylactic intramedullary nailing should be considered for a patient with intractable pain.2
A radiograph of the opposite leg should be obtained routinely, looking for an asymptomatic fracture. Bisphosphonates should be discontinued and calcium and vitamin D continued. Teriparatide therapy can be considered as an alternative treatment.
Conservative management for incomplete fracture without pain
Incomplete atypical femoral fracture unaccompanied by pain can be followed conservatively.47 In addition to stopping antiresorptive therapy, patients need to avoid high-impact and repetitive-impact activities such as jumping or running. If pain occurs, patients should begin protected weight-bearing exercise.
Treatment is uncertain for incomplete fracture with pain
For patients with incomplete atypical femoral fracture and pain, treatment is controversial. Regimens that include 2 to 3 months of protected weight-bearing exercise, a full metabolic bone workup, calcium and vitamin D supplementation, and anabolic bone agents have produced some success. Some authors have reported poor results from conservative care, with few patients achieving pain relief or signs of complete healing.48,49 Additionally, if an incomplete fracture is found in the opposite femur, protected weight-bearing of both legs may not be possible.
Patients with incomplete fracture should be monitored regularly with radiography and physical examination. If there is progression of the fracture, escalation of pain, or failure to heal within 2 to 3 months, then surgical treatment is necessary.
Prophylactic placement of an intramedullary nail to prevent completion of the fracture and allow a return to full weight-bearing is generally advised.50 A long locking plate can be used if bone deformities make it difficult to place an intramedullary nail; however, nails are preferred because they allow formation of endochondral callus, which can be helpful in these difficult-to-heal fractures.
Results from retrospective reviews have shown that surgically treated patients with bisphosphonate-associated incomplete atypical femoral fracture were more likely than those treated nonsurgically to be pain-free (81% vs 64%) and have radiographic healing (100% vs 18% at final follow-up).46 Results have also been positive for those with complete atypical femoral fracture. At 6 months, 64% of surgically treated patients were pain-free and 98% were radiographically healed.51
The unusual geometry of the femur in patients with atypical femoral fracture and the presence of intramedullary cortical callus makes the placement of an intramedullary femoral rod more complex than in typical femoral fracture.8
Intramedullary nailing of atypical femoral fracture is a challenge for even the most experienced surgeon, and vigilance is imperative to avoid iatrogenic fracture and malunion.
MANY QUESTIONS REMAIN
We need more studies on the pathophysiology of bisphosphonate-associated atypical femoral fracture, the value of periodic screening with DXA, and which factors predict high risk (eg, Asian ethnicity, use of certain medications, femoral geometry). In addition, we need more data on the success of conservative management of incomplete fracture, including use of teriparatide.
Bisphosphonate therapy minimizes bone loss and reduces fracture risk by up to 50% in patients with osteoporosis,1 but it is also associated with increased risks of osteonecrosis of the jaw and atypical femoral fracture. Although atypical femoral fractures are rare, they can have a devastating effect. Patient concern about this complication has contributed to a decrease in bisphosphonate use by about half in the last decade or so,2,3 and we fear this could result in an increase in hip fracture rates.
In this article, we examine the evidence on bisphosphonate-associated atypical femoral fractures, including risks, pathogenesis, treatment, and prevention.
ATYPICAL FRACTURES INVOLVE THE FEMORAL SHAFT, NOT THE HEAD
An atypical femoral fracture is a transverse fracture of the femoral shaft (diaphysis), defined by both clinical criteria and radiographic appearance.
To be defined as atypical, a femoral fracture must meet 4 of the following 5 criteria4:
- Occurs with minimal or no trauma
- Has a predominantly transverse fracture line, originating at the lateral cortex and sometimes becoming oblique as it progresses medially across the femur
- Extends through both cortices and may be associated with a medial spike (complete fractures); or involves only the lateral cortex (incomplete fractures)
- Is noncomminuted or minimally comminuted
- Shows localized periosteal or endosteal thickening (termed “beaking” or “flaring”) of the lateral cortex at the fracture site.
Several minor features are also important but are not required, eg:
- Cortical thickening of the femoral shaft
- Unilateral or bilateral prodromal pain preceding the fracture
- Bilateral incomplete or complete femoral diaphysis fractures
- Delayed fracture healing.
Atypical femoral fracture can occur anywhere along the shaft, from just distal to the lesser trochanter to just proximal to the supracondylar flare. However, most occur in 2 areas, with 1 cluster centered at about 41 mm from the lesser trochanter (more common in relatively younger patients) and the other at 187 mm.5
ABSOLUTE RISK IS LOW BUT INCREASES WITH LONGER USE
Atypical femoral fractures are rare. Schilcher et al6 reviewed radiographs of 1,234 women who had a subtrochanteric or shaft fracture and found 59 (4.6%) of fractures were atypical. In a systematic review of 14 studies,7 the incidence ranged from 3.0 to 9.8 cases per 100,000 patient-years.
Furthermore, not all atypical femoral fractures are in bisphosphonate users: 7.4% were in nonusers in 1 series8 and 22% in another.9
Nevertheless, most studies show that bisphosphonate use increases the incidence of atypical femoral fracture, and the incidence increases with duration of use, especially after 3 years.7
An international task force of the American Society for Bone and Mineral Research listed the absolute risk as between 3.2 and 50 cases per 100,000 patient-years, with longer use (> 5 years) increasing the risk to about 100 per 100,000 patient-years.4 After stopping bisphosphonate therapy, the risk diminished by 70% per year.9
In another study, for 0.1 to 1.9 years of therapy, the age-adjusted atypical fracture rates were 1.78 per 100,000 per year (95% confidence interval [CI] 1.5–2.0), increasing to 113.1 per 100,000 per year (95% CI 69.3–156.8) with exposure from 8 to 9.9 years.10
A case-control study found that more than 5 years of bisphosphonate use increased the fracture risk by an odds ratio of 2.74 (95% CI 1.25–6.02).11
The incidence of typical femoral fracture was higher in those who adhered better to their oral bisphosphonate regimen in some studies,12 but the opposite was true in others.13
The benefits of bisphosphonate therapy in reducing fracture risk, however, outweigh the risk of atypical fracture.4
We do not know whether the rate of atypical femoral fracture is increasing. A review of Kaiser Permanente Northwest records found that the rates of atypical femoral shaft fracture had remained stable from 1996 to 2009. However, 61.9% of patients who met the strict radiographic criteria had taken oral bisphosphonates.14 These data suggest that bisphosphonate use has not increased the overall population-based risk for subtrochanteric and femoral shaft fractures, but that bisphosphonates and other risk factors may have increased the likelihood that such fractures will exhibit atypical radiographic features.
A population-based study in Denmark13 found that alendronate use longer than 10 years was associated with an adjusted 30% lower risk of hip fracture and no increase in the risk of subtrochanteric and femoral shaft fracture. In addition, the risk of subtrochanteric and femoral shaft fracture was lower with high adherence to alendronate treatment (based on medication possession ratio > 80%) compared with low adherence (ratio < 50%) (odds ratio 0.88, 95% CI 0.77–0.99). The risk was not increased in current vs past users.
The Danish study13 used the coding of the 10th revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10) to identify subtrochanteric and femoral shaft fractures without radiologic review for atypical radiographic features. The lack of specific ICD-10 coding for subtrochanteric and femoral shaft fractures with atypical radiographic features has limited our knowledge of their incidence.
Contralateral fracture in more than one-fourth of cases
After an atypical femoral fracture, patients have a significant risk of fracture on the contralateral side. In a case-control study, 28% of patients with atypical femoral fracture suffered a contralateral fracture, compared with 0.9% of patients presenting with a typical fracture pattern (odds ratio 42.6, 95% CI 12.8–142.4).15
Contralateral fracture occurs from 1 month to 4 years after the index atypical femoral fracture.16
There are reports of bisphosphonate-related low-impact fractures in other sites such as the tibia17 and forearm.18 However, they may be too rare to warrant screening.
Mortality rates
A Swedish database study found that patients with atypical femoral fractures, whether bisphosphonate users or nonusers, do not have higher mortality rates than patients with ordinary subtrochanteric or femoral shaft fractures.19 Furthermore, the mortality rates for those with atypical femoral fracture were similar to rates in the general population. In contrast, patients with an ordinary femoral fracture had a higher mortality risk than the general population.19
Other studies suggest that atypical femoral fracture may be associated with a less favorable prognosis in older patients,20 but this could be due to differences in demographics, treatment adherence, or postfracture care.21
In addition, functional outcomes as measured by independent mobility at discharge and at 3 months were comparable between patients with atypical fracture and those with typical fracture.22
IMAGING STUDIES
If a long-term bisphosphonate user presents with hip, thigh, or groin pain, imaging studies are recommended.
Plain radiography
Radiography is usually the first step and should include a frontal view of the pelvis (Figure 1) and 2 views of the full length of each femur. If radiography is not conclusive, bone scan or magnetic resonance imaging (MRI) should be considered.
A linear cortex transverse fracture pattern and focal lateral cortical thickening are the most sensitive and specific radiographic features.23,24 Because of the risk of fracture on the contralateral side, radiographic study of that side is recommended as well.
Computed tomography
Computed tomography (CT) is not sensitive for early stress fractures and, given the radiation burden, is not recommended in the workup of atypical fracture.
Bone scanning
Bone scanning using technetium 99m-labeled methylene diphosphonate with a gamma camera shows active bone turnover. Stress fractures and atypical femoral fractures are most easily identified in the third (delayed) phase of the bone scan. Although bone scanning is highly sensitive, the specificity is limited by lack of spatial resolution. Atypical femoral fracture appears as increased activity in the subtrochanteric region with a predilection for the lateral cortex.
Dual-energy x-ray absorptiometry
Conventional dual-energy x-ray absorptiometry (DXA) extends only to 1 to 2 cm below the lesser trochanter and can therefore miss atypical fractures, which usually occur farther down. The overall detection rate for DXA was 61% in a sample of 33 patients.25
Newer scanners can look at the entire femoral shaft.26 In addition, newer software can quantify focal thickening (beaking) of the lateral cortex and screen patients who have no symptoms. The results of serial measurements can be graphed so that the practitioner can view trends to help assess or rule out potential asymptomatic atypical femoral fracture.
A localized reaction (periosteal thickening of the lateral cortex or beaking) often precedes atypical femoral fracture. A 2017 study reported that patients with high localized reaction (mean height 3.3 mm) that was of the pointed type and was accompanied by prodromal pain had an increased risk of complete or incomplete atypical femoral fracture at that site.27 This finding is used by the newer DXA software. The predictive value of beaking on extended femoral DXA may be as high as 83%.26
Magnetic resonance imaging
The MRI characteristics of atypical femoral fracture are similar to those of other stress fractures except that there is a lateral-to-medial pattern rather than a medial pattern. The earliest findings include periosteal reaction about the lateral cortex with a normal marrow signal.
MRI may be of particular benefit in patients with known atypical femoral fracture to screen the contralateral leg. It should image the entire length of both femurs. Contrast enhancement is not needed.
Regardless of whether initial findings were discovered on conventional radiographs or DXA, MRI confirmation is needed. Radionuclide bone scanning is currently not recommended because it lacks specificity. Combination imaging is recommended, with either radiography plus MRI or DXA plus MRI.
DIFFERENTIAL DIAGNOSIS
The differential diagnosis of atypical femoral fracture includes stress fracture, pathologic fracture, hypophosphatasia, and osteogenesis imperfecta.28 Hypophosphatemic osteomalacia can cause Looser zones, which can be confused with atypical femoral fractures but usually occur on the medial side.4 Stress fracture of the femur can occur below the lesser trochanter but usually begins in the medial, not the lateral, cortex.
Pathologic fractures from underlying osseous lesions can mimic the cortical beaking of bisphosphonate-related fracture, but they usually show the associated underlying lucent lesion and poorly defined margins. A sinus tract along the region of a chronic osteomyelitis may also appear similar.
Hypophosphatasia is an inborn error of metabolism caused by a loss-of-function mutation in the gene encoding alkaline phosphatase, resulting in pyrophosphate accumulation and causing osteomalacia from impaired mineralization. This can result in femoral pseudofracture that is often bilateral and occurs in the subtrochanteric region.29
ADDITIONAL RISK FACTORS
Patients with atypical femoral fracture are generally a heterogeneous group, but there are risk factors to note other than bisphosphonate exposure.
Asian women had a risk 8 times higher than white women in 1 study.30
Bone geometry. Mahjoub et al8 reported that compared with controls, patients with atypical femoral fracture had greater offset of the femoral shaft from the center of rotation of the femoral head, a more acute angle between the femoral neck and shaft, and greater proximal cortical thickness.
Medications. In addition to bisphosphonates, other drugs associated with atypical femoral fracture include RANK-ligand inhibitors such as denosumab (another drug for osteoporosis),31 glucocorticoids,32,33 and proton pump inhibitors.32,33
Genetics. Three sisters with atypical femoral fracture were found to have 37 rare mutations in 34 genes, including one in the GGPS1 gene, which codes for geranylgeranyl pyrophosphate synthase—an enzyme that bisphosphonates inhibit.34
Medical conditions other than osteoporosis include collagen diseases, chronic pulmonary disease, asthma, rheumatoid arthritis, and diabetes.35
Clinical recommendations
Current recommendations are to reevaluate bisphosphonate use in patients with osteoporosis after 5 or more years of therapy.36
Given that patients with osteoporosis are at increased risk of typical fracture, those at higher risk should be considered for continued bisphosphonate therapy. Factors for high risk include the following:
- History of fracture on therapy
- Hip T score –2.5 or lower
- Older age (≥ 70)
- Other strong risk factors for fracture such as smoking, alcohol use, corticosteroid use, rheumatoid arthritis, and family history
- World Health Organization FRAX fracture risk score above the country-specific threshold.
Those at lower risk should be considered for a 2- to 3-year bisphosphonate holiday with periodic reevaluation of bone density and, possibly, bone markers.36
WHAT IS THE UNDERLYING PATHOPHYSIOLOGY?
The mechanism by which bisphosphonates increase the risk of atypical femoral fracture is not clear. These drugs work by suppressing bone turnover; however, in theory, prolonged use could suppress it too much and increase bone fragility.
One hypothesis is that bisphosphonates impair the toughening of cortical bone, an important barrier to clinical fracture. This is supported by a study that found bisphosphonate users with atypical femoral fracture had deficits in intrinsic and extrinsic bone toughness, perhaps due to treatment-related increases in matrix mineralization.37 Although this study and others showed an increase in matrix mineralization and reduced mineralization heterogeneity with bisphosphonate use,38,39 it is unclear whether such changes contributed to reduced toughness or to atypical femoral fracture.
Changes in the skeletal geometry of the lower limb such as femoral neck-shaft angle and femoral curvature alter the stresses and strains experienced by the femoral diaphysis with loading. Because the incidence of incomplete atypical femoral fracture is much greater than that of complete fracture, most incomplete atypical femoral fractures heal before the fracture progresses.
Ultimately, all fractures, including atypical femoral fractures, occur when mechanical stress and strain exceed bone strength.
Antiresorptive drugs such as bisphosphonates, estrogen, calcitonin, and RANK ligand inhibitors prevent hip fracture by increasing the strength of the proximal femur—perhaps at the expense of the strength (or toughness) of the subtrochanteric shaft. It is also possible that treatment-related increases in hip strength (and reduced hip fracture rates) promote or sustain the transfer of stress and strain to femoral regions that experience lesser or no increases in strength from treatment, which likely includes the shaft.40,41
CT studies in Japanese women with osteoporosis have shown that 2 years of zoledronate therapy had greater effects in the hip than in the femoral shaft, with significant increases in cortical thickness and volumetric bone mineral density at the femoral neck and intertrochanteric region compared with baseline.42 But zoledronate did not increase femoral shaft cortical thickness and caused only a minor increase in femoral shaft volumetric bone mineral density. Fracture patterns may have depended on damage and effects of bone turnover on mass and structure.
This hypothetical scenario portrays a possible “hip survival bias” mechanism for atypical femoral fracture, with the association with antiresorptive drugs arising from greater stress and strain in cortical regions where these fractures occur rather than from treatment-related reductions in cortical bone strength or toughness.
PRODROMAL PAIN IS COMMON
From 32% to 76% of patients who have incomplete or developing atypical femoral fracture present with a prodrome of groin or hip pain.4,43 Prodromal pain occurs any time from 2 weeks to several years before the fracture, presenting as pain in the anterior or lateral thigh or in the groin.
Prodromal pain in a patient on antiresorptive therapy should be a signal for the clinician to obtain a radiograph of the hip and to look for contralateral symptoms and fractures. The most common mechanism of injury appears to be a ground-level fall or even a nontraumatic activity such as walking or stepping off a curb.
MEDICAL MANAGEMENT
In bisphosphonate users with radiographic evidence of atypical femoral fracture, the bisphosphonate should be discontinued and the patient assessed for calcium and vitamin D deficiency, with supplements prescribed if needed.4
For patients with incomplete fracture and persistent pain after 3 months of medical management, prophylactic surgical nail fixation is recommended to prevent complete fracture.
Teriparatide, which has been associated with enhanced bone fracture healing, is a possible treatment to promote healing of atypical femoral fracture, either alone or as an adjunct to surgical fixation. A systematic review published in 2015 supported the use of teriparatide for enhancing fracture healing in atypical femoral fracture.44 In addition, a 10-patient series45 showed that incomplete fractures without radiolucent lines responded to teriparatide alone, whereas those with radiolucent lines needed intramedullary nailing.
These results suggest that teriparatide works best when the fracture site is stable, either inherently or with surgical fixation.
ORTHOPEDIC CARE
Orthopedic care for atypical femoral fracture differs depending on whether the patient experiences pain and whether the fracture is incomplete or complete. Figure 2 shows a treatment algorithm for atypical femoral fracture.
These are difficult fractures to manage, complicated by delayed healing in the elderly, complex displacement patterns, altered bone geometry, and risk of fracture in the opposite limb, all of which raise questions about recommending protected weight-bearing exercise.
Furthermore, atypical femoral fracture is often associated with increased anterolateral bowing of the femur, making it difficult to insert an intramedullary nail: the radius of curvature of the bone is shorter than that of a standard femoral nail. This mismatch can lead to intraoperative complications such as iatrogenic fracture during prophylactic nailing, malunion from excess straightening of the femur (which can itself lead to leg length discrepancy), and gapping of the fracture site, particularly on the medial side.
Intramedullary nailing for complete fracture
Intramedullary nailing is the first-line treatment for complete atypical femoral fracture, although the risk of delayed healing and revision surgery may be somewhat higher than with typical femoral fracture.46 Prophylactic intramedullary nailing should be considered for a patient with intractable pain.2
A radiograph of the opposite leg should be obtained routinely, looking for an asymptomatic fracture. Bisphosphonates should be discontinued and calcium and vitamin D continued. Teriparatide therapy can be considered as an alternative treatment.
Conservative management for incomplete fracture without pain
Incomplete atypical femoral fracture unaccompanied by pain can be followed conservatively.47 In addition to stopping antiresorptive therapy, patients need to avoid high-impact and repetitive-impact activities such as jumping or running. If pain occurs, patients should begin protected weight-bearing exercise.
Treatment is uncertain for incomplete fracture with pain
For patients with incomplete atypical femoral fracture and pain, treatment is controversial. Regimens that include 2 to 3 months of protected weight-bearing exercise, a full metabolic bone workup, calcium and vitamin D supplementation, and anabolic bone agents have produced some success. Some authors have reported poor results from conservative care, with few patients achieving pain relief or signs of complete healing.48,49 Additionally, if an incomplete fracture is found in the opposite femur, protected weight-bearing of both legs may not be possible.
Patients with incomplete fracture should be monitored regularly with radiography and physical examination. If there is progression of the fracture, escalation of pain, or failure to heal within 2 to 3 months, then surgical treatment is necessary.
Prophylactic placement of an intramedullary nail to prevent completion of the fracture and allow a return to full weight-bearing is generally advised.50 A long locking plate can be used if bone deformities make it difficult to place an intramedullary nail; however, nails are preferred because they allow formation of endochondral callus, which can be helpful in these difficult-to-heal fractures.
Results from retrospective reviews have shown that surgically treated patients with bisphosphonate-associated incomplete atypical femoral fracture were more likely than those treated nonsurgically to be pain-free (81% vs 64%) and have radiographic healing (100% vs 18% at final follow-up).46 Results have also been positive for those with complete atypical femoral fracture. At 6 months, 64% of surgically treated patients were pain-free and 98% were radiographically healed.51
The unusual geometry of the femur in patients with atypical femoral fracture and the presence of intramedullary cortical callus makes the placement of an intramedullary femoral rod more complex than in typical femoral fracture.8
Intramedullary nailing of atypical femoral fracture is a challenge for even the most experienced surgeon, and vigilance is imperative to avoid iatrogenic fracture and malunion.
MANY QUESTIONS REMAIN
We need more studies on the pathophysiology of bisphosphonate-associated atypical femoral fracture, the value of periodic screening with DXA, and which factors predict high risk (eg, Asian ethnicity, use of certain medications, femoral geometry). In addition, we need more data on the success of conservative management of incomplete fracture, including use of teriparatide.
- Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 1996; 348(9041):1535–1541. pmid:8950879
- Jha S, Wang Z, Laucis N, Bhattacharyya T. Trends in media reports, oral bisphosphonate prescriptions, and hip fractures 1996–2012: an ecological analysis. J Bone Miner Res 2015; 30(12):2179–2187. doi:10.1002/jbmr.2565
- Solomon DH, Johnston SS, Boytsov NN, McMorrow D, Lane JM, Krohn KD. Osteoporosis medication use after hip fracture in US patients between 2002 and 2011. J Bone Miner Res 2014; 29(9):1929–1937. doi:10.1002/jbmr.2202
- Shane E, Burr D, Abrahamsen B, et al. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 2014; 29(1):1–23. doi:10.1002/jbmr.1998
- Koeppen VA, Schilcher J, Aspenberg P. Dichotomous location of 160 atypical femoral fractures. Acta Orthop 2013; 84(6):561–564. doi:10.3109/17453674.2013.866193
- Schilcher J, Koeppen V, Aspenberg P, Michäelsson K. Risk of atypical femoral fracture during and after bisphosphonate use. Acta Orthop 2015; 86(1):100–107. doi:10.3109/17453674.2015.1004149
- Khow KS, Shibu P, Yu SC, Chehade MJ, Visvanathan R. Epidemiology and postoperative outcomes of atypical femoral fractures in older adults: a systematic review. J Nutr Health Aging 2017; 21(1):83–91. doi:10.1007/s12603-015-0652-3
- Mahjoub Z, Jean S, Leclerc JT, et al. Incidence and characteristics of atypical femoral fractures: clinical and geometrical data. J Bone Miner Res 2016; 31(4):767–776. doi:10.1002/jbmr.2748
- Schilcher J, Michaelsson K, Aspenberg P. Bisphosphonate use and atypical fractures of the femoral shaft. N Engl J Med 2011; 364(18):1728–1737. doi:10.1056/NEJMoa1010650
- Dell RM, Adams AL, Greene DF, et al. Incidence of atypical nontraumatic diaphyseal fractures of the femur. J Bone Miner Res 2012; 27(12):2544–2550. doi:10.1002/jbmr.1719
- Park-Wyllie LY, Mamdani MM, Juurlink DN, et al. Bisphosphonate use and the risk of subtrochanteric or femoral shaft fractures in older women. JAMA 2011; 305(8):783–789. doi:10.1001/jama.2011.190
- Wang Z, Ward MM, Chan L, Bhattacharyya T. Adherence to oral bisphosphonates and the risk of subtrochanteric and femoral shaft fractures among female Medicare beneficiaries. Osteoporos Int 2014; 25(8):2109–2116. doi:10.1007/s00198-014-2738-x
- Abrahamsen B, Eiken P, Prieto-Alhambra D, Eastell R. Risk of hip, subtrochanteric, and femoral shaft fractures among mid and long term users of alendronate: nationwide cohort and nested case-control study. BMJ 2016; 353:i3365. doi:10.1136/bmj.i3365
- Feldstein AC, Black D, Perrin N, et al. Incidence and demography of femur fractures with and without atypical features. J Bone Miner Res 2012; 27(5):977–986. doi:10.1002/jbmr.1550
- Meier RP, Perneger TV, Stern R, Rizzoli R, Peter RE. Increasing occurrence of atypical femoral fractures associated with bisphosphonate use. Arch Intern Med 2012; 172(12):930–936. doi:10.1001/archinternmed.2012.1796
- La Rocca Vieira R, Rosenberg ZS, Allison MB, Im SA, Babb J, Peck V. Frequency of incomplete atypical femoral fractures in asymptomatic patients on long term bisphosphonate therapy. AJR Am J Roentgenol 2012; 198(5):1144–1151. doi:10.2214/AJR.11.7442
- Bissonnette L, April PM, Dumais R, Boire G, Roux S. Atypical fracture of the tibial diaphysis associated with bisphosphonate therapy: a case report. Bone 2013; 56(2):406–409. doi:10.1016/j.bone.2013.07.012
- Moon J, Bither N, Lee T. Atypical forearm fractures associated with long-term use of bisphosphonate. Arch Orthop Trauma Surg 2013; 133(7):889–892. doi:10.1007/s00402-013-1760-3
- Kharazmi M, Hallberg P, Schilcher J, Aspenberg P, Michaëlsson K. Mortality after atypical femoral fractures: a cohort study. J Bone Miner Res 2016; 31(3):491–497. doi:10.1002/jbmr.2767
- Medin E, Goude F, Melberg HO, Tediosi F, Belicza E, Peltola M; EuroHOPE Study Group. European regional differences in all-cause mortality and length of stay for patients with hip fracture. Health Econ 2015; 24(suppl 2):53–64. doi:10.1002/hec.3278
- Abrahamsen B, Prieto-Alhambra D. Patients with atypical femur fractures have the same mortality as the background population-drug channeling bias, bisphosphonate effects and public health implications. J Bone Miner Res 2016; 31(3):488–490. doi:10.1002/jbmr.2801
- Khow KS, Paterson F, Shibu P, Yu SC, Chehade MJ, Visvanathan R. Outcomes between older adults with atypical and typical femoral fractures are comparable. Injury 2017; 48(2):394–398. doi:10.1016/j.injury.2016.10.035
- Adams AL, Xue F, Chantra JQ, et al. Sensitivity and specificity of radiographic characteristics in atypical femoral fractures. Osteoporos Int 2017; 28(1):413–417. doi:10.1007/s00198-016-3809-y
- Rosenberg ZS, La Rocca Vieira R, Chan SS, et al. Bisphosphonate-related complete atypical subtrochanteric femoral fractures: diagnostic utility of radiography. AJR Am J Roentgenol 2011; 197(4):954–960. doi:10.2214/AJR.10.6262
- Kim S, Yang KH, Lim H, et al. Detection of prefracture hip lesions in atypical subtrochanteric fracture with dual-energy x-ray absorptiometry images. Radiology 2014; 270(2):487–495. doi:10.1148/radiol.13122691
- van de Laarschot DM, Smits AA, Buitendijk SK, Stegenga MT, Zillikens MC. Screening for atypical femur fractures using extended femur scans by DXA. J Bone Miner Res 2017; 32(8):1632–1639. doi:10.1002/jbmr.3164
- Sato H, Kondo N, Nakatsue T, et al. High and pointed type of femoral localized reaction frequently extends to complete an incomplete atypical femoral fracture in patients with autoimmune diseases on long-term glucocorticoids and bisphosphonates. Osteoporos Int 2017; 28(8):2367–2376. doi:10.1007/s00198-017-4038-8
- Giaconi JC, Watterson CT. Bisphosphonate-related atypical femur fractures and the radiographic features. In: Silverman SL, Abrahamsen B, eds. The Duration and Safety of Osteoporosis Treatment. Switzerland: Springer International Publishing; 2016:107–124. doi:10.1007/978-3-319-23639-1
- Whyte MP. Atypical femoral fractures, bisphosphonates, and adult hypophosphatasia. J Bone Miner Res 2009; 24(6):1132–1134. doi:10.1359/jbmr.081253
- Lo JC, Hui RL, Grimsrud CD, et al. The association of race/ethnicity and risk of atypical femoral fracture among older women receiving oral bisphosphonate therapy. Bone 2016; 85:142–147. doi:10.1016/j.bone.2016.01.002
- Bone HG, Wagman RB, Brandi ML, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol 2017; 5(7):513–523. doi:10.1016/S2213-8587(17)30138-9
- Koh JH, Myong JP, Yoo J, et al. Predisposing factors associated with atypical femur fracture among postmenopausal Korean women receiving bisphosphonate therapy: 8 years' experience in a single center. Osteoporos Int 2017; 28(11):3251–3259. doi:10.1007/s00198-017-4169-y
- Kim D, Sung YK, Cho SK, Han M, Kim YS. Factors associated with atypical femoral fracture. Rheumatol Int 2016; 36(1):65–71. doi:10.1007/s00296-015-3323-0
- Roca-Ayats N, Balcells S, Garcia-Giralt N, et al. GGPS1 mutation and atypical femoral fractures with bisphosphonates. N Engl J Med 2017; 376(18):1794–1795. doi:10.1056/NEJMc1612804
- Giusti A, Hamdy NA, Dekkers OM, Ramautar SR, Dijkstra S, Papapoulos SE. Atypical fractures and bisphosphonate therapy: a cohort study of patients with femoral fracture with radiographic adjudication of fracture site and features. Bone 2011; 48(5):966–971. doi:10.1016/j.bone.2010.12.033
- Adler RA, El-Hajj Fuleihan G, Bauer DC, et al. Managing osteoporosis in patients on long-term bisphosphonate treatment: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 2016; 31(1):16–35. doi:10.1002/jbmr.2708
- Lloyd AA, Gludovatz B, Riedel C, et al. Atypical fracture with long-term bisphosphonate therapy is associated with altered cortical composition and reduced fracture resistance. Proc Natl Acad Sci USA 2017; 114(33):8722–8727. doi:10.1073/pnas.1704460114
- Ettinger B, Burr DB, Ritchie RO. Proposed pathogenesis for atypical femoral fractures; lessons from materials research. Bone 2013; 55(2):495–500. doi:10.1016/j.bone.2013.02.004
- Burr DB, Liu Z, Allen MR. Duration-dependent effects of clinically relevant oral alendronate doses on cortical bone toughness in beagle dogs. Bone 2015; 71:58–62. doi:10.1016/j.bone.2014.10.010
- Sasaki S, Miyakoshi N, Hongo M, Kasukawa Y, Shimada Y. Low-energy diaphyseal femoral fractures associated with bisphosphonate use and severe curved femur: a case series. J Bone Miner Metab 2012; 30(5):561–567. doi:10.1007/s00774-012-0358-0
- Pulkkinen P, Gluer C, Jamsa T. Investigation of differences between hip fracture types: a worthy strategy of improved risk assessment and fracture prevention. Bone 2011; 49(4):600–604. doi:10.1016/j.bone.2011.07.022
- Ito M, Sone T, Shiraki M, et al. The effect of once-yearly zoledronic acid on hip structural and biomechanical properties derived using computed tomography (CT) in Japanese women with osteoporosis. Bone 2018; 106:179–186. doi:10.1016/j.bone.2017.10.013
- Bogdan Y, Einhorn TA. Clinical presentation of atypical femur fractures. In: Silverman SL, Abrahamsen B, eds. The Duration and Safety of Osteoporosis Treatment. Switzerland: Springer International Publishing; 2016:137–140. doi:10.1007/978-3-319-23639-1
- Im GI, Lee SH. Effect of teriparatide on healing of atypical femoral fractures: a systemic review. J Bone Metab 2015; 22(4):183–189. doi:10.11005/jbm.2015.22.4.183
- Saleh A, Hegde VV, Potty AG, Schneider R, Cornell CN, Lane JM. Management strategy for symptomatic bisphosphonate-associated incomplete atypical femoral fractures. HSS J 2012; 8(2):103–110. doi:10.1007/s11420-012-9275-y
- Egol KA, Park JH, Prensky C, Rosenberg ZS, Peck V, Tejwani NC. Surgical treatment improves clinical and functional outcomes for patients who sustain incomplete bisphosphonate-related femur fractures. J Orthop Trauma 2013; 27(6):331–335. doi:10.1097/BOT.0b013e31827240ae
- Koh A, Guerado E, Giannoudis PV. Atypical femoral fractures related to bisphosphonate treatment: issues and controversies related to their surgical management. Bone Joint J 2017; 99-B(3):295–302. doi:10.1302/0301-620X.99B3.BJJ-2016-0276.R2
- Oh CW, Oh JK, Park KC, Kim JW, Yoon YC. Prophylactic nailing of incomplete atypical femoral fractures. ScientificWorldJournal 2013; 2013:450148. doi:10.1155/2013/450148
- Ha YC, Cho MR, Park KH, Kim SY, Koo KH. Is surgery necessary for femoral insufficiency fractures after long-term bisphosphonate therapy? Clin Orthop Relat Res 2010; 468(12):3393–3398. doi:10.1007/s11999-010-1583-2
- Tosounidis TH, Lampropoulou-Adamidou, Kanakaris NK. Intramedullary nailing of sequential bilateral atypical subtrochanteric fractures and the management of distal femoral intraoperative fracture. J Orthop Trauma 2015 Jun 11. Epub ahead of print. doi:10.1097/BOT.0000000000000370
- Egol KA, Park JH, Rosenberg ZS, Peck V, Tejwani NC. Healing delayed but generally reliable after bisphosphonate-associated complete femur fractures treated with IM nails. Clin Orthop Relat Res 2014; 472(9):2728–2734. doi:10.1007/s11999-013-2963-1
- Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 1996; 348(9041):1535–1541. pmid:8950879
- Jha S, Wang Z, Laucis N, Bhattacharyya T. Trends in media reports, oral bisphosphonate prescriptions, and hip fractures 1996–2012: an ecological analysis. J Bone Miner Res 2015; 30(12):2179–2187. doi:10.1002/jbmr.2565
- Solomon DH, Johnston SS, Boytsov NN, McMorrow D, Lane JM, Krohn KD. Osteoporosis medication use after hip fracture in US patients between 2002 and 2011. J Bone Miner Res 2014; 29(9):1929–1937. doi:10.1002/jbmr.2202
- Shane E, Burr D, Abrahamsen B, et al. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 2014; 29(1):1–23. doi:10.1002/jbmr.1998
- Koeppen VA, Schilcher J, Aspenberg P. Dichotomous location of 160 atypical femoral fractures. Acta Orthop 2013; 84(6):561–564. doi:10.3109/17453674.2013.866193
- Schilcher J, Koeppen V, Aspenberg P, Michäelsson K. Risk of atypical femoral fracture during and after bisphosphonate use. Acta Orthop 2015; 86(1):100–107. doi:10.3109/17453674.2015.1004149
- Khow KS, Shibu P, Yu SC, Chehade MJ, Visvanathan R. Epidemiology and postoperative outcomes of atypical femoral fractures in older adults: a systematic review. J Nutr Health Aging 2017; 21(1):83–91. doi:10.1007/s12603-015-0652-3
- Mahjoub Z, Jean S, Leclerc JT, et al. Incidence and characteristics of atypical femoral fractures: clinical and geometrical data. J Bone Miner Res 2016; 31(4):767–776. doi:10.1002/jbmr.2748
- Schilcher J, Michaelsson K, Aspenberg P. Bisphosphonate use and atypical fractures of the femoral shaft. N Engl J Med 2011; 364(18):1728–1737. doi:10.1056/NEJMoa1010650
- Dell RM, Adams AL, Greene DF, et al. Incidence of atypical nontraumatic diaphyseal fractures of the femur. J Bone Miner Res 2012; 27(12):2544–2550. doi:10.1002/jbmr.1719
- Park-Wyllie LY, Mamdani MM, Juurlink DN, et al. Bisphosphonate use and the risk of subtrochanteric or femoral shaft fractures in older women. JAMA 2011; 305(8):783–789. doi:10.1001/jama.2011.190
- Wang Z, Ward MM, Chan L, Bhattacharyya T. Adherence to oral bisphosphonates and the risk of subtrochanteric and femoral shaft fractures among female Medicare beneficiaries. Osteoporos Int 2014; 25(8):2109–2116. doi:10.1007/s00198-014-2738-x
- Abrahamsen B, Eiken P, Prieto-Alhambra D, Eastell R. Risk of hip, subtrochanteric, and femoral shaft fractures among mid and long term users of alendronate: nationwide cohort and nested case-control study. BMJ 2016; 353:i3365. doi:10.1136/bmj.i3365
- Feldstein AC, Black D, Perrin N, et al. Incidence and demography of femur fractures with and without atypical features. J Bone Miner Res 2012; 27(5):977–986. doi:10.1002/jbmr.1550
- Meier RP, Perneger TV, Stern R, Rizzoli R, Peter RE. Increasing occurrence of atypical femoral fractures associated with bisphosphonate use. Arch Intern Med 2012; 172(12):930–936. doi:10.1001/archinternmed.2012.1796
- La Rocca Vieira R, Rosenberg ZS, Allison MB, Im SA, Babb J, Peck V. Frequency of incomplete atypical femoral fractures in asymptomatic patients on long term bisphosphonate therapy. AJR Am J Roentgenol 2012; 198(5):1144–1151. doi:10.2214/AJR.11.7442
- Bissonnette L, April PM, Dumais R, Boire G, Roux S. Atypical fracture of the tibial diaphysis associated with bisphosphonate therapy: a case report. Bone 2013; 56(2):406–409. doi:10.1016/j.bone.2013.07.012
- Moon J, Bither N, Lee T. Atypical forearm fractures associated with long-term use of bisphosphonate. Arch Orthop Trauma Surg 2013; 133(7):889–892. doi:10.1007/s00402-013-1760-3
- Kharazmi M, Hallberg P, Schilcher J, Aspenberg P, Michaëlsson K. Mortality after atypical femoral fractures: a cohort study. J Bone Miner Res 2016; 31(3):491–497. doi:10.1002/jbmr.2767
- Medin E, Goude F, Melberg HO, Tediosi F, Belicza E, Peltola M; EuroHOPE Study Group. European regional differences in all-cause mortality and length of stay for patients with hip fracture. Health Econ 2015; 24(suppl 2):53–64. doi:10.1002/hec.3278
- Abrahamsen B, Prieto-Alhambra D. Patients with atypical femur fractures have the same mortality as the background population-drug channeling bias, bisphosphonate effects and public health implications. J Bone Miner Res 2016; 31(3):488–490. doi:10.1002/jbmr.2801
- Khow KS, Paterson F, Shibu P, Yu SC, Chehade MJ, Visvanathan R. Outcomes between older adults with atypical and typical femoral fractures are comparable. Injury 2017; 48(2):394–398. doi:10.1016/j.injury.2016.10.035
- Adams AL, Xue F, Chantra JQ, et al. Sensitivity and specificity of radiographic characteristics in atypical femoral fractures. Osteoporos Int 2017; 28(1):413–417. doi:10.1007/s00198-016-3809-y
- Rosenberg ZS, La Rocca Vieira R, Chan SS, et al. Bisphosphonate-related complete atypical subtrochanteric femoral fractures: diagnostic utility of radiography. AJR Am J Roentgenol 2011; 197(4):954–960. doi:10.2214/AJR.10.6262
- Kim S, Yang KH, Lim H, et al. Detection of prefracture hip lesions in atypical subtrochanteric fracture with dual-energy x-ray absorptiometry images. Radiology 2014; 270(2):487–495. doi:10.1148/radiol.13122691
- van de Laarschot DM, Smits AA, Buitendijk SK, Stegenga MT, Zillikens MC. Screening for atypical femur fractures using extended femur scans by DXA. J Bone Miner Res 2017; 32(8):1632–1639. doi:10.1002/jbmr.3164
- Sato H, Kondo N, Nakatsue T, et al. High and pointed type of femoral localized reaction frequently extends to complete an incomplete atypical femoral fracture in patients with autoimmune diseases on long-term glucocorticoids and bisphosphonates. Osteoporos Int 2017; 28(8):2367–2376. doi:10.1007/s00198-017-4038-8
- Giaconi JC, Watterson CT. Bisphosphonate-related atypical femur fractures and the radiographic features. In: Silverman SL, Abrahamsen B, eds. The Duration and Safety of Osteoporosis Treatment. Switzerland: Springer International Publishing; 2016:107–124. doi:10.1007/978-3-319-23639-1
- Whyte MP. Atypical femoral fractures, bisphosphonates, and adult hypophosphatasia. J Bone Miner Res 2009; 24(6):1132–1134. doi:10.1359/jbmr.081253
- Lo JC, Hui RL, Grimsrud CD, et al. The association of race/ethnicity and risk of atypical femoral fracture among older women receiving oral bisphosphonate therapy. Bone 2016; 85:142–147. doi:10.1016/j.bone.2016.01.002
- Bone HG, Wagman RB, Brandi ML, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol 2017; 5(7):513–523. doi:10.1016/S2213-8587(17)30138-9
- Koh JH, Myong JP, Yoo J, et al. Predisposing factors associated with atypical femur fracture among postmenopausal Korean women receiving bisphosphonate therapy: 8 years' experience in a single center. Osteoporos Int 2017; 28(11):3251–3259. doi:10.1007/s00198-017-4169-y
- Kim D, Sung YK, Cho SK, Han M, Kim YS. Factors associated with atypical femoral fracture. Rheumatol Int 2016; 36(1):65–71. doi:10.1007/s00296-015-3323-0
- Roca-Ayats N, Balcells S, Garcia-Giralt N, et al. GGPS1 mutation and atypical femoral fractures with bisphosphonates. N Engl J Med 2017; 376(18):1794–1795. doi:10.1056/NEJMc1612804
- Giusti A, Hamdy NA, Dekkers OM, Ramautar SR, Dijkstra S, Papapoulos SE. Atypical fractures and bisphosphonate therapy: a cohort study of patients with femoral fracture with radiographic adjudication of fracture site and features. Bone 2011; 48(5):966–971. doi:10.1016/j.bone.2010.12.033
- Adler RA, El-Hajj Fuleihan G, Bauer DC, et al. Managing osteoporosis in patients on long-term bisphosphonate treatment: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 2016; 31(1):16–35. doi:10.1002/jbmr.2708
- Lloyd AA, Gludovatz B, Riedel C, et al. Atypical fracture with long-term bisphosphonate therapy is associated with altered cortical composition and reduced fracture resistance. Proc Natl Acad Sci USA 2017; 114(33):8722–8727. doi:10.1073/pnas.1704460114
- Ettinger B, Burr DB, Ritchie RO. Proposed pathogenesis for atypical femoral fractures; lessons from materials research. Bone 2013; 55(2):495–500. doi:10.1016/j.bone.2013.02.004
- Burr DB, Liu Z, Allen MR. Duration-dependent effects of clinically relevant oral alendronate doses on cortical bone toughness in beagle dogs. Bone 2015; 71:58–62. doi:10.1016/j.bone.2014.10.010
- Sasaki S, Miyakoshi N, Hongo M, Kasukawa Y, Shimada Y. Low-energy diaphyseal femoral fractures associated with bisphosphonate use and severe curved femur: a case series. J Bone Miner Metab 2012; 30(5):561–567. doi:10.1007/s00774-012-0358-0
- Pulkkinen P, Gluer C, Jamsa T. Investigation of differences between hip fracture types: a worthy strategy of improved risk assessment and fracture prevention. Bone 2011; 49(4):600–604. doi:10.1016/j.bone.2011.07.022
- Ito M, Sone T, Shiraki M, et al. The effect of once-yearly zoledronic acid on hip structural and biomechanical properties derived using computed tomography (CT) in Japanese women with osteoporosis. Bone 2018; 106:179–186. doi:10.1016/j.bone.2017.10.013
- Bogdan Y, Einhorn TA. Clinical presentation of atypical femur fractures. In: Silverman SL, Abrahamsen B, eds. The Duration and Safety of Osteoporosis Treatment. Switzerland: Springer International Publishing; 2016:137–140. doi:10.1007/978-3-319-23639-1
- Im GI, Lee SH. Effect of teriparatide on healing of atypical femoral fractures: a systemic review. J Bone Metab 2015; 22(4):183–189. doi:10.11005/jbm.2015.22.4.183
- Saleh A, Hegde VV, Potty AG, Schneider R, Cornell CN, Lane JM. Management strategy for symptomatic bisphosphonate-associated incomplete atypical femoral fractures. HSS J 2012; 8(2):103–110. doi:10.1007/s11420-012-9275-y
- Egol KA, Park JH, Prensky C, Rosenberg ZS, Peck V, Tejwani NC. Surgical treatment improves clinical and functional outcomes for patients who sustain incomplete bisphosphonate-related femur fractures. J Orthop Trauma 2013; 27(6):331–335. doi:10.1097/BOT.0b013e31827240ae
- Koh A, Guerado E, Giannoudis PV. Atypical femoral fractures related to bisphosphonate treatment: issues and controversies related to their surgical management. Bone Joint J 2017; 99-B(3):295–302. doi:10.1302/0301-620X.99B3.BJJ-2016-0276.R2
- Oh CW, Oh JK, Park KC, Kim JW, Yoon YC. Prophylactic nailing of incomplete atypical femoral fractures. ScientificWorldJournal 2013; 2013:450148. doi:10.1155/2013/450148
- Ha YC, Cho MR, Park KH, Kim SY, Koo KH. Is surgery necessary for femoral insufficiency fractures after long-term bisphosphonate therapy? Clin Orthop Relat Res 2010; 468(12):3393–3398. doi:10.1007/s11999-010-1583-2
- Tosounidis TH, Lampropoulou-Adamidou, Kanakaris NK. Intramedullary nailing of sequential bilateral atypical subtrochanteric fractures and the management of distal femoral intraoperative fracture. J Orthop Trauma 2015 Jun 11. Epub ahead of print. doi:10.1097/BOT.0000000000000370
- Egol KA, Park JH, Rosenberg ZS, Peck V, Tejwani NC. Healing delayed but generally reliable after bisphosphonate-associated complete femur fractures treated with IM nails. Clin Orthop Relat Res 2014; 472(9):2728–2734. doi:10.1007/s11999-013-2963-1
KEY POINTS
- The benefits of bisphosphonate therapy in reducing fracture risk outweigh the risk of atypical fracture.
- Bisphosphonate use for longer than 5 years greatly increases the risk of atypical femoral fracture.
- Treatment of atypical femoral fracture varies depending on whether the patient has pain and whether the fracture is complete or incomplete.
How acute pain leads to chronic opioid use
Mary, age 38, was hospitalized for acute cholecystitis requiring laparoscopic surgery. Her hospital course was uneventful. At the time of discharge, I, her inpatient doctor, prescribed 15 hydrocodone tablets for postoperative pain. I never saw her again. Did she struggle to stop taking the hydrocodone I prescribed?
Heather is a 50-year-old patient in my addiction medicine clinic who developed opioid use disorder while being treated for chronic pain. After much hardship and to her credit, she is now in long-term remission. Did her opioid use disorder start with an opioid prescription for an accepted indication?
The issues Mary and Heather face seem unrelated, but these 2 patients may be at different time points in the progression of the same disease. As a hospitalist, I want to optimize the chances that patients taking opioids for acute pain will be able to stop taking them.
CHRONIC USE VS OPIOID USE DISORDER
There is a distinction between chronic use of opioids and opioid use disorder. The latter is also known as addiction.
Patients who take opioids daily do not necessarily have opioid use disorder, even if they have physiologic dependence on them. Physiologic opioid dependence is commonly confused with opioid use disorder, but it is the expected result of regularly taking these drugs.
Opioid use disorder is a chronic disease of the brain characterized by loss of control over opioid use, resulting in harm. The Diagnostic and Statistical Manual, fifth edition, excludes physiologic dependence on opioids (tolerance and withdrawal) from its criteria for opioid use disorder if the patient is taking opioids solely under medical supervision.1 To be diagnosed with opioid use disorder, patients need to do only 2 of the following within 12 months:
- Take more of the drug than intended
- Want or try to cut down without success
- Spend a lot of time in getting, using, or recovering from the drug
- Crave the drug
- Fail to meet commitments due to the drug
- Continue to use the drug, even though it causes social or relationship problems
- Give up or reduce other activities because of the drug
- Use the drug even when it isn’t safe
- Continue to use even when it causes physical or psychological problems
- Develop tolerance (but, as noted, not if taking the drug as directed under a doctor’s supervision)
- Experience withdrawal (again, but not if taking the drug under medical supervision).
WHY DO SOME PATIENTS STRUGGLE TO STOP TAKING OPIOIDS?
Studying opioid use disorder as an outcome in large groups of patients is complicated by imperfect medical documentation. However, using pharmacy claims data, researchers can accurately describe opioid prescription patterns in large groups of patients over time. This means we can count how many patients keep taking prescribed opioids but not how many become addicted.
In a country where nearly 40% of adults are prescribed an opioid annually, the question is not why people start taking opioids, but why some have to struggle to stop.2 Several recent studies used pharmacy claims data to identify factors that may predict chronic opioid use in patients prescribed opioids for acute pain. The findings suggest that we can better treat acute pain to prevent chronic opioid use.
We don’t yet know how to protect patients like Mary from opioid use disorder, but the following 3 studies have already changed my practice.
HIGHER TOTAL DOSE MEANS HIGHER RISK
[Shah A, Hayes CJ, Martin BC. Characteristics of initial prescription episodes and likelihood of long-term opioid use—United States, 2006–2015. MMWR Morb Mortal Wkly Rep 2017; 66(10):265–269.]
Shah et al3 reported a study of nearly 1.3 million opioid-naive patients who received opioid prescriptions. Of those prescribed at least 1 day of opioids, 6% were still taking them 1 year later, and 2.9% were still taking them 3 years later.
Opioid exposure in acute pain was measured in total “morphine milligram equivalents” (MME), ie, the cumulative amount of opioids prescribed in the treatment episode, standardized across different types of opioids. We usually think of exposure in terms of how many milligrams a patient takes per day, which correlates with mortality in chronic opioid use.4 But this study showed a linear relationship between total MME prescribed for acute pain and ongoing opioid use in opioid-naive patients. By itself, the difference between daily and total MME made the article revelatory.
But the study went further, asking how much is too much: ie, What is the cutoff MME above which the patient is at risk of chronic opioid use? The relationship between acute opioid dose and chronic use is linear and starts early. Shah et al suggested that a total threshold of 700 MME predicts chronic opioid use—140 hydrocodone tablets, or 1 month of regular use.3
Many doctors worry that specific opioids such as oxycodone, hydromorphone, and fentanyl may be more habit-forming. Surprisingly, this study showed that these drugs were associated with rates of chronic use similar to those of other opioids when they controlled for potency.
Bottom line. Total opioid use in acute pain was the best predictor of chronic opioid use, and it showed that chronicity begins earlier than thought.
DON’T BE A ‘HIGH-INTENSITY’ PRESCRIBER
[Barnett ML, Olenski AR, Jena AB. Opioid-prescribing patterns of emergency physicians and risk of long-term use. N Engl J Med 2017; 376(7):663–673.]
Barnett et al5 analyzed opioid prescribing for acute pain in the emergency department, using Medicare pharmacy data from 377,629 previously opioid-naive patients. They categorized the emergency providers into quartiles based on the frequency of opioid prescribing.
The relative risk of ongoing opioid use 1 year after being treated by a “high-intensity” prescriber (ie, one in the top quartile) was 30% greater than in similar patients seen by a low-intensity prescriber (ie, one in the bottom quartile). In addition, those who were treated by high-intensity prescribers were more likely to have a serious fall.
In designing the study, the authors assumed that patients visiting an emergency department had their doctor assigned randomly. They controlled for many patient variables that might have confounded the results, such as age, sex, race, depression, medical comorbidities, and geographic region. Were the higher rates of ongoing opioid use in the high-intensity-prescriber group due to the higher prescribing rates of their emergency providers, or did the providers counsel patients differently? This is not known.
Bottom line. Different doctors manage similar patients differently when it comes to pain, and those who prescribe more opioids for acute pain put their patients at risk of chronic opioid use and falls. I don’t want to be a high-intensity opioid prescriber.
SURGERY AND CHRONIC OPIOID USE
[Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg 2017; 152(6):e170504.]
Brummett et al6 examined ongoing opioid use after surgery in 36,177 opioid-naive patients and in a nonsurgical control group. After 3 months, 6% of the patients who underwent surgery remained on opioids, compared with only 0.4% of the nonsurgical controls. Whether the surgery was major or minor did not affect the rate of postoperative opioid use.
Risk factors for ongoing opioid use were preexisting addiction to anything (including tobacco), mood disorders, and preoperative pain disorders. These risk factors have previously been reported in nonsurgical patients.7
Brummett et al speculated that patients are counseled about postoperative opioids in a way that leads them to overestimate the safety and efficacy of these drugs for treating other common pain conditions.6
Bottom line. Patients with mental health comorbidities have a hard time stopping opioids. The remarkable finding in this study was the similarity between major and minor surgery in terms of chronic opioid use. If postoperative opioids treat only the pain caused by the surgery, major surgery should be associated with greater opioid use. The similarity suggests that a mechanism other than postoperative pain confers risk of chronic opioid use.
THINKING ABOUT OPIOIDS
Collectively, these articles describe elements of acute pain treatment that correlate with chronic ongoing opioid use: a higher cumulative dose,3 being seen by a physician who prescribes a lot of opioids,5 undergoing surgery,6 and psychiatric comorbidity.6 They made me wonder if opioid use for acute pain acts as an inoculation, analogous to inoculating a Petri dish with bacteria. The likelihood of chronic opioid use arises from the inoculum dose, the host response, and the context of inoculation.
These articles do not show how patients taking opioids chronically for pain become addicted. Stumbo et al8 interviewed 283 opioid-dependent patients and identified 5 pathways to opioid use disorder, 3 of which were related to pain control: inadequately controlled chronic pain, exposure to opioids during acute pain episodes, and chronic pain in patients who already had substance use disorders. Brat et al9 recently estimated the risk of opioid use disorder after receiving opioids postoperatively to be less than 1%, but it increased dramatically with duration of opioid treatment.
A patient who fills an opioid prescription does not necessarily have chronic pain. Nor do all patients with chronic pain require an opioid prescription. These studies did not establish whether the patients had a pain syndrome. In practice, we call our patients who chronically take opioids our “chronic pain patients.” But 40% of Americans have chronic pain, while only 5% take opioids daily for pain.11,12
We assume that those taking opioids have the most severe pain. But Brummett et al suggested that continued opioid use is predicted less by pain and more by psychiatric comorbidity.6 More than half of the opioid prescriptions in the United States are written for patients with serious mental illness, who represent one-sixth of that population.11 Maybe chronic opioid use for pain has more to do with vulnerability to opioids and less to do with a pain syndrome.
I now think about daily opioid use in much the same way as I think about daily prednisone use. Patients on daily prednisone have a characteristic set of medical risks from the prednisone itself, regardless of its indication. Yet we do not consider these patients addicted to prednisone. Opioid use may be similar.
Like most doctors, I am troubled by the continued rise in the opioid overdose rate.13 Yet addiction and death from overdose are not the only risks that patients on chronic opioids face; they also have higher rates of falls, cardiovascular death, pneumonia, death from chronic obstructive pulmonary disease, and motor vehicle crashes.14–17 Patients on chronic opioids for pain have greater mental health comorbidity and worse function.18
Most concerning, chronic opioid treatment for pain lacks proof of benefit. In fact, a recent study disproved the benefit of opioids for chronic pain compared with nonopioid options.19 When I meet with patients who are taking chronic opioids for pain, I often can’t identify why the drugs were started or ought to be continued, and I anticipate a bad outcome. Yet the patient is afraid to stop the drug. For these reasons, chronic opioid use for pain strikes me as worth considering separately from opioid use disorder.
HOW THIS CHANGED MY PRACTICE
The studies described above have had a powerful effect on my clinical care as a hospitalist.
I now talk to all patients starting opioids about how hard it can be to stop. Some patients are defensive at first, believing this does not apply to them. But I politely continue.
People with depression and anxiety can have a harder time stopping opioids. Addiction is both a risk with ongoing opioid use and a possible outcome of acute opioid use.8 But one can struggle to stop opioids without being addicted or depressed. Even the healthiest person may wish to continue opioids past the point of benefit.
I am careful not to invalidate the patient’s experience of pain. It is challenging for patients to find the balance between current discomfort and a possible future adverse effect. In these conversations, I imagine how I would want a loved one counseled on their pain control. This centers me as I choose my words and my tone.
I now monitor the total amount of opioid I prescribe for acute pain in addition to the daily dose. I give my patients as few opioids as reasonable, and advise them to take the minimum dose required for tolerable comfort. I offer nonopioid options as the preferred choice, presenting them as effective and safe. I do this irrespective of the indication for opioids.
I limit opioids in all patients, not just those with comorbidities. I include in my shared decision-making process the risk of chronic opioid use when I prescribe opioids for acute pain, carefully distinguishing it from opioid use disorder. Instead of excess opioids, I give patients my office phone number to call in case they struggle. I rarely get calls. But I find patients would rather have access to a doctor than extra pills. And offering them my contact information lets me limit opioids while letting them know that I am committed to their comfort and health.
As an addiction medicine doctor, I consult on patients not taking their opioids as prescribed. Caring for these patients is intellectually and emotionally draining; they suffer daily, and the opioids they take provide a modicum of relief at a high cost. The publications I have discussed here provide insight into how a troubled relationship with opioids begins. I remind myself that these patients have an iatrogenic condition. Their behaviors that we label “aberrant” may reflect an adverse reaction to medications prescribed to them for acute pain.
Mary, my patient with postoperative pain after cholecystectomy, may over time develop opioid use disorder as Heather did. That progression may have begun with the hydrocodone I prescribed and the counseling I gave her, and it may proceed to chronic opioid use and then opioid use disorder.
I am looking closely at the care I give for acute pain in light of these innovative studies. But even more so, they have increased the compassion with which I care for patients like Heather, those harmed by prescribed opioids.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association Publishing; 2013:541–546.
- Han B, Compton WM, Blanco C, Crane E, Lee J, Jones CM. Prescription opioid use, misuse, and use disorders in US adults: 2015 national survey on drug use and health. Ann Intern Med 2017; 167(5):293–301. doi:10.7326/M17-0865
- Shah A, Hayes CJ, Martin BC. Characteristics of initial prescription episodes and likelihood of long-term opioid use—United States, 2006–2015. MMWR Morb Mortal Wkly Rep 2017; 66(10):265–269. doi:10.15585/mmwr.mm6610a1
- Dasgupta N, Funk MJ, Proescholdbell S, Hirsch A, Ribisl KM, Marshall S. Cohort study of the impact of high-dose opioid analgesics on overdose mortality. Pain Med 2016; 17(1):85–98. doi:10.1111/pme.12907
- Barnett ML, Olenski AR, Jena AB. Opioid-prescribing patterns of emergency physicians and risk of long-term use. N Engl J Med 2017; 376(7):663–673. doi:10.1056/NEJMsa1610524
- Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg 2017; 152(6):e170504. doi:10.1001/jamasurg.2017.0504
- Volkow ND, McLellan AT. Opioid abuse in chronic pain—misconceptions and mitigation strategies. N Engl J Med 2016; 374(13):1253–1263. doi:10.1056/NEJMra1507771
- Stumbo SP, Yarborough BJ, McCarty D, Weisner C, Green CA. Patient-reported pathways to opioid use disorders and pain-related barriers to treatment engagement. J Subst Abuse Treat 2017; 73:47–54. doi:10.1016/j.jsat.2016.11.003
- Brat GA, Agniel D, Beam A, et al. Postsurgical prescriptions for opioid naive patients and association with overdose and misuse: retrospective cohort study. BMJ 2018; 360:j5790. doi:10.1136/bmj.j5790
- Vowles KE, McEntee ML, Julnes PS, Frohe T, Ney JP, van der Goes DN. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain 2015; 156(4):569–576. doi:10.1097/01.j.pain.0000460357.01998.f1
- Davis MA, Lin LA, Liu H, Sites BD. Prescription opioid use among adults with mental health disorders in the United States. J Am Board Fam Med 2017; 30(4):407–417. doi:10.3122/jabfm.2017.04.170112
- Tsang A, Von Korff M, Lee S, et al. Common chronic pain conditions in developed and developing countries: gender and age differences and comorbidity with depression-anxiety disorders. J Pain 2008; 9(10):883–891. doi:10.1016/j.jpain.2008.05.005
- QuickStats: age-adjusted death rates for drug overdose, by race/ethnicity—national vital statistics system, United States, 2015–2016. MMWR Morb Mortal Wkly Rep 2018; 67(12):374. doi:10.15585/mmwr.mm6712a9
- Solomon DH, Rassen JA, Glynn RJ, Lee J, Levin R, Schneeweiss S. The comparative safety of analgesics in older adults with arthritis. Arch Intern Med 2010; 170(22):1968–1976. doi:10.1001/archinternmed.2010.391
- Vozoris NT, Wang X, Fischer HD, et al. Incident opioid drug use and adverse respiratory outcomes among older adults with COPD. Eur Respir J 2016; 48(3):683–693. doi:10.1183/13993003.01967-2015
- Wiese AD, Griffin MR, Schaffner W, et al. Opioid analgesic use and risk for invasive pneumococcal diseases: a nested case-control study. Ann Intern Med 2018; 168(6):396–404. doi:10.7326/M17-1907
- Chihuri S, Li G. Use of prescription opioids and motor vehicle crashes: a meta analysis. Accid Anal Prev 2017; 109:123–131. doi:10.1016/j.aap.2017.10.004
- Morasco BJ, Yarborough BJ, Smith NX, et al. Higher prescription opioid dose is associated with worse patient-reported pain outcomes and more health care utilization. J Pain 2017; 18(4):437–445. doi:10.1016/j.jpain.2016.12.004
- Krebs EE, Gravely A, Nugent S, et al. Effect of opioid vs nonopioid medications on pain-related function in patients with chronic back pain or hip or knee osteoarthritis pain: the SPACE randomized clinical trial. JAMA 2018; 319(9):872–882. doi:10.1001/jama.2018.0899
Mary, age 38, was hospitalized for acute cholecystitis requiring laparoscopic surgery. Her hospital course was uneventful. At the time of discharge, I, her inpatient doctor, prescribed 15 hydrocodone tablets for postoperative pain. I never saw her again. Did she struggle to stop taking the hydrocodone I prescribed?
Heather is a 50-year-old patient in my addiction medicine clinic who developed opioid use disorder while being treated for chronic pain. After much hardship and to her credit, she is now in long-term remission. Did her opioid use disorder start with an opioid prescription for an accepted indication?
The issues Mary and Heather face seem unrelated, but these 2 patients may be at different time points in the progression of the same disease. As a hospitalist, I want to optimize the chances that patients taking opioids for acute pain will be able to stop taking them.
CHRONIC USE VS OPIOID USE DISORDER
There is a distinction between chronic use of opioids and opioid use disorder. The latter is also known as addiction.
Patients who take opioids daily do not necessarily have opioid use disorder, even if they have physiologic dependence on them. Physiologic opioid dependence is commonly confused with opioid use disorder, but it is the expected result of regularly taking these drugs.
Opioid use disorder is a chronic disease of the brain characterized by loss of control over opioid use, resulting in harm. The Diagnostic and Statistical Manual, fifth edition, excludes physiologic dependence on opioids (tolerance and withdrawal) from its criteria for opioid use disorder if the patient is taking opioids solely under medical supervision.1 To be diagnosed with opioid use disorder, patients need to do only 2 of the following within 12 months:
- Take more of the drug than intended
- Want or try to cut down without success
- Spend a lot of time in getting, using, or recovering from the drug
- Crave the drug
- Fail to meet commitments due to the drug
- Continue to use the drug, even though it causes social or relationship problems
- Give up or reduce other activities because of the drug
- Use the drug even when it isn’t safe
- Continue to use even when it causes physical or psychological problems
- Develop tolerance (but, as noted, not if taking the drug as directed under a doctor’s supervision)
- Experience withdrawal (again, but not if taking the drug under medical supervision).
WHY DO SOME PATIENTS STRUGGLE TO STOP TAKING OPIOIDS?
Studying opioid use disorder as an outcome in large groups of patients is complicated by imperfect medical documentation. However, using pharmacy claims data, researchers can accurately describe opioid prescription patterns in large groups of patients over time. This means we can count how many patients keep taking prescribed opioids but not how many become addicted.
In a country where nearly 40% of adults are prescribed an opioid annually, the question is not why people start taking opioids, but why some have to struggle to stop.2 Several recent studies used pharmacy claims data to identify factors that may predict chronic opioid use in patients prescribed opioids for acute pain. The findings suggest that we can better treat acute pain to prevent chronic opioid use.
We don’t yet know how to protect patients like Mary from opioid use disorder, but the following 3 studies have already changed my practice.
HIGHER TOTAL DOSE MEANS HIGHER RISK
[Shah A, Hayes CJ, Martin BC. Characteristics of initial prescription episodes and likelihood of long-term opioid use—United States, 2006–2015. MMWR Morb Mortal Wkly Rep 2017; 66(10):265–269.]
Shah et al3 reported a study of nearly 1.3 million opioid-naive patients who received opioid prescriptions. Of those prescribed at least 1 day of opioids, 6% were still taking them 1 year later, and 2.9% were still taking them 3 years later.
Opioid exposure in acute pain was measured in total “morphine milligram equivalents” (MME), ie, the cumulative amount of opioids prescribed in the treatment episode, standardized across different types of opioids. We usually think of exposure in terms of how many milligrams a patient takes per day, which correlates with mortality in chronic opioid use.4 But this study showed a linear relationship between total MME prescribed for acute pain and ongoing opioid use in opioid-naive patients. By itself, the difference between daily and total MME made the article revelatory.
But the study went further, asking how much is too much: ie, What is the cutoff MME above which the patient is at risk of chronic opioid use? The relationship between acute opioid dose and chronic use is linear and starts early. Shah et al suggested that a total threshold of 700 MME predicts chronic opioid use—140 hydrocodone tablets, or 1 month of regular use.3
Many doctors worry that specific opioids such as oxycodone, hydromorphone, and fentanyl may be more habit-forming. Surprisingly, this study showed that these drugs were associated with rates of chronic use similar to those of other opioids when they controlled for potency.
Bottom line. Total opioid use in acute pain was the best predictor of chronic opioid use, and it showed that chronicity begins earlier than thought.
DON’T BE A ‘HIGH-INTENSITY’ PRESCRIBER
[Barnett ML, Olenski AR, Jena AB. Opioid-prescribing patterns of emergency physicians and risk of long-term use. N Engl J Med 2017; 376(7):663–673.]
Barnett et al5 analyzed opioid prescribing for acute pain in the emergency department, using Medicare pharmacy data from 377,629 previously opioid-naive patients. They categorized the emergency providers into quartiles based on the frequency of opioid prescribing.
The relative risk of ongoing opioid use 1 year after being treated by a “high-intensity” prescriber (ie, one in the top quartile) was 30% greater than in similar patients seen by a low-intensity prescriber (ie, one in the bottom quartile). In addition, those who were treated by high-intensity prescribers were more likely to have a serious fall.
In designing the study, the authors assumed that patients visiting an emergency department had their doctor assigned randomly. They controlled for many patient variables that might have confounded the results, such as age, sex, race, depression, medical comorbidities, and geographic region. Were the higher rates of ongoing opioid use in the high-intensity-prescriber group due to the higher prescribing rates of their emergency providers, or did the providers counsel patients differently? This is not known.
Bottom line. Different doctors manage similar patients differently when it comes to pain, and those who prescribe more opioids for acute pain put their patients at risk of chronic opioid use and falls. I don’t want to be a high-intensity opioid prescriber.
SURGERY AND CHRONIC OPIOID USE
[Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg 2017; 152(6):e170504.]
Brummett et al6 examined ongoing opioid use after surgery in 36,177 opioid-naive patients and in a nonsurgical control group. After 3 months, 6% of the patients who underwent surgery remained on opioids, compared with only 0.4% of the nonsurgical controls. Whether the surgery was major or minor did not affect the rate of postoperative opioid use.
Risk factors for ongoing opioid use were preexisting addiction to anything (including tobacco), mood disorders, and preoperative pain disorders. These risk factors have previously been reported in nonsurgical patients.7
Brummett et al speculated that patients are counseled about postoperative opioids in a way that leads them to overestimate the safety and efficacy of these drugs for treating other common pain conditions.6
Bottom line. Patients with mental health comorbidities have a hard time stopping opioids. The remarkable finding in this study was the similarity between major and minor surgery in terms of chronic opioid use. If postoperative opioids treat only the pain caused by the surgery, major surgery should be associated with greater opioid use. The similarity suggests that a mechanism other than postoperative pain confers risk of chronic opioid use.
THINKING ABOUT OPIOIDS
Collectively, these articles describe elements of acute pain treatment that correlate with chronic ongoing opioid use: a higher cumulative dose,3 being seen by a physician who prescribes a lot of opioids,5 undergoing surgery,6 and psychiatric comorbidity.6 They made me wonder if opioid use for acute pain acts as an inoculation, analogous to inoculating a Petri dish with bacteria. The likelihood of chronic opioid use arises from the inoculum dose, the host response, and the context of inoculation.
These articles do not show how patients taking opioids chronically for pain become addicted. Stumbo et al8 interviewed 283 opioid-dependent patients and identified 5 pathways to opioid use disorder, 3 of which were related to pain control: inadequately controlled chronic pain, exposure to opioids during acute pain episodes, and chronic pain in patients who already had substance use disorders. Brat et al9 recently estimated the risk of opioid use disorder after receiving opioids postoperatively to be less than 1%, but it increased dramatically with duration of opioid treatment.
A patient who fills an opioid prescription does not necessarily have chronic pain. Nor do all patients with chronic pain require an opioid prescription. These studies did not establish whether the patients had a pain syndrome. In practice, we call our patients who chronically take opioids our “chronic pain patients.” But 40% of Americans have chronic pain, while only 5% take opioids daily for pain.11,12
We assume that those taking opioids have the most severe pain. But Brummett et al suggested that continued opioid use is predicted less by pain and more by psychiatric comorbidity.6 More than half of the opioid prescriptions in the United States are written for patients with serious mental illness, who represent one-sixth of that population.11 Maybe chronic opioid use for pain has more to do with vulnerability to opioids and less to do with a pain syndrome.
I now think about daily opioid use in much the same way as I think about daily prednisone use. Patients on daily prednisone have a characteristic set of medical risks from the prednisone itself, regardless of its indication. Yet we do not consider these patients addicted to prednisone. Opioid use may be similar.
Like most doctors, I am troubled by the continued rise in the opioid overdose rate.13 Yet addiction and death from overdose are not the only risks that patients on chronic opioids face; they also have higher rates of falls, cardiovascular death, pneumonia, death from chronic obstructive pulmonary disease, and motor vehicle crashes.14–17 Patients on chronic opioids for pain have greater mental health comorbidity and worse function.18
Most concerning, chronic opioid treatment for pain lacks proof of benefit. In fact, a recent study disproved the benefit of opioids for chronic pain compared with nonopioid options.19 When I meet with patients who are taking chronic opioids for pain, I often can’t identify why the drugs were started or ought to be continued, and I anticipate a bad outcome. Yet the patient is afraid to stop the drug. For these reasons, chronic opioid use for pain strikes me as worth considering separately from opioid use disorder.
HOW THIS CHANGED MY PRACTICE
The studies described above have had a powerful effect on my clinical care as a hospitalist.
I now talk to all patients starting opioids about how hard it can be to stop. Some patients are defensive at first, believing this does not apply to them. But I politely continue.
People with depression and anxiety can have a harder time stopping opioids. Addiction is both a risk with ongoing opioid use and a possible outcome of acute opioid use.8 But one can struggle to stop opioids without being addicted or depressed. Even the healthiest person may wish to continue opioids past the point of benefit.
I am careful not to invalidate the patient’s experience of pain. It is challenging for patients to find the balance between current discomfort and a possible future adverse effect. In these conversations, I imagine how I would want a loved one counseled on their pain control. This centers me as I choose my words and my tone.
I now monitor the total amount of opioid I prescribe for acute pain in addition to the daily dose. I give my patients as few opioids as reasonable, and advise them to take the minimum dose required for tolerable comfort. I offer nonopioid options as the preferred choice, presenting them as effective and safe. I do this irrespective of the indication for opioids.
I limit opioids in all patients, not just those with comorbidities. I include in my shared decision-making process the risk of chronic opioid use when I prescribe opioids for acute pain, carefully distinguishing it from opioid use disorder. Instead of excess opioids, I give patients my office phone number to call in case they struggle. I rarely get calls. But I find patients would rather have access to a doctor than extra pills. And offering them my contact information lets me limit opioids while letting them know that I am committed to their comfort and health.
As an addiction medicine doctor, I consult on patients not taking their opioids as prescribed. Caring for these patients is intellectually and emotionally draining; they suffer daily, and the opioids they take provide a modicum of relief at a high cost. The publications I have discussed here provide insight into how a troubled relationship with opioids begins. I remind myself that these patients have an iatrogenic condition. Their behaviors that we label “aberrant” may reflect an adverse reaction to medications prescribed to them for acute pain.
Mary, my patient with postoperative pain after cholecystectomy, may over time develop opioid use disorder as Heather did. That progression may have begun with the hydrocodone I prescribed and the counseling I gave her, and it may proceed to chronic opioid use and then opioid use disorder.
I am looking closely at the care I give for acute pain in light of these innovative studies. But even more so, they have increased the compassion with which I care for patients like Heather, those harmed by prescribed opioids.
Mary, age 38, was hospitalized for acute cholecystitis requiring laparoscopic surgery. Her hospital course was uneventful. At the time of discharge, I, her inpatient doctor, prescribed 15 hydrocodone tablets for postoperative pain. I never saw her again. Did she struggle to stop taking the hydrocodone I prescribed?
Heather is a 50-year-old patient in my addiction medicine clinic who developed opioid use disorder while being treated for chronic pain. After much hardship and to her credit, she is now in long-term remission. Did her opioid use disorder start with an opioid prescription for an accepted indication?
The issues Mary and Heather face seem unrelated, but these 2 patients may be at different time points in the progression of the same disease. As a hospitalist, I want to optimize the chances that patients taking opioids for acute pain will be able to stop taking them.
CHRONIC USE VS OPIOID USE DISORDER
There is a distinction between chronic use of opioids and opioid use disorder. The latter is also known as addiction.
Patients who take opioids daily do not necessarily have opioid use disorder, even if they have physiologic dependence on them. Physiologic opioid dependence is commonly confused with opioid use disorder, but it is the expected result of regularly taking these drugs.
Opioid use disorder is a chronic disease of the brain characterized by loss of control over opioid use, resulting in harm. The Diagnostic and Statistical Manual, fifth edition, excludes physiologic dependence on opioids (tolerance and withdrawal) from its criteria for opioid use disorder if the patient is taking opioids solely under medical supervision.1 To be diagnosed with opioid use disorder, patients need to do only 2 of the following within 12 months:
- Take more of the drug than intended
- Want or try to cut down without success
- Spend a lot of time in getting, using, or recovering from the drug
- Crave the drug
- Fail to meet commitments due to the drug
- Continue to use the drug, even though it causes social or relationship problems
- Give up or reduce other activities because of the drug
- Use the drug even when it isn’t safe
- Continue to use even when it causes physical or psychological problems
- Develop tolerance (but, as noted, not if taking the drug as directed under a doctor’s supervision)
- Experience withdrawal (again, but not if taking the drug under medical supervision).
WHY DO SOME PATIENTS STRUGGLE TO STOP TAKING OPIOIDS?
Studying opioid use disorder as an outcome in large groups of patients is complicated by imperfect medical documentation. However, using pharmacy claims data, researchers can accurately describe opioid prescription patterns in large groups of patients over time. This means we can count how many patients keep taking prescribed opioids but not how many become addicted.
In a country where nearly 40% of adults are prescribed an opioid annually, the question is not why people start taking opioids, but why some have to struggle to stop.2 Several recent studies used pharmacy claims data to identify factors that may predict chronic opioid use in patients prescribed opioids for acute pain. The findings suggest that we can better treat acute pain to prevent chronic opioid use.
We don’t yet know how to protect patients like Mary from opioid use disorder, but the following 3 studies have already changed my practice.
HIGHER TOTAL DOSE MEANS HIGHER RISK
[Shah A, Hayes CJ, Martin BC. Characteristics of initial prescription episodes and likelihood of long-term opioid use—United States, 2006–2015. MMWR Morb Mortal Wkly Rep 2017; 66(10):265–269.]
Shah et al3 reported a study of nearly 1.3 million opioid-naive patients who received opioid prescriptions. Of those prescribed at least 1 day of opioids, 6% were still taking them 1 year later, and 2.9% were still taking them 3 years later.
Opioid exposure in acute pain was measured in total “morphine milligram equivalents” (MME), ie, the cumulative amount of opioids prescribed in the treatment episode, standardized across different types of opioids. We usually think of exposure in terms of how many milligrams a patient takes per day, which correlates with mortality in chronic opioid use.4 But this study showed a linear relationship between total MME prescribed for acute pain and ongoing opioid use in opioid-naive patients. By itself, the difference between daily and total MME made the article revelatory.
But the study went further, asking how much is too much: ie, What is the cutoff MME above which the patient is at risk of chronic opioid use? The relationship between acute opioid dose and chronic use is linear and starts early. Shah et al suggested that a total threshold of 700 MME predicts chronic opioid use—140 hydrocodone tablets, or 1 month of regular use.3
Many doctors worry that specific opioids such as oxycodone, hydromorphone, and fentanyl may be more habit-forming. Surprisingly, this study showed that these drugs were associated with rates of chronic use similar to those of other opioids when they controlled for potency.
Bottom line. Total opioid use in acute pain was the best predictor of chronic opioid use, and it showed that chronicity begins earlier than thought.
DON’T BE A ‘HIGH-INTENSITY’ PRESCRIBER
[Barnett ML, Olenski AR, Jena AB. Opioid-prescribing patterns of emergency physicians and risk of long-term use. N Engl J Med 2017; 376(7):663–673.]
Barnett et al5 analyzed opioid prescribing for acute pain in the emergency department, using Medicare pharmacy data from 377,629 previously opioid-naive patients. They categorized the emergency providers into quartiles based on the frequency of opioid prescribing.
The relative risk of ongoing opioid use 1 year after being treated by a “high-intensity” prescriber (ie, one in the top quartile) was 30% greater than in similar patients seen by a low-intensity prescriber (ie, one in the bottom quartile). In addition, those who were treated by high-intensity prescribers were more likely to have a serious fall.
In designing the study, the authors assumed that patients visiting an emergency department had their doctor assigned randomly. They controlled for many patient variables that might have confounded the results, such as age, sex, race, depression, medical comorbidities, and geographic region. Were the higher rates of ongoing opioid use in the high-intensity-prescriber group due to the higher prescribing rates of their emergency providers, or did the providers counsel patients differently? This is not known.
Bottom line. Different doctors manage similar patients differently when it comes to pain, and those who prescribe more opioids for acute pain put their patients at risk of chronic opioid use and falls. I don’t want to be a high-intensity opioid prescriber.
SURGERY AND CHRONIC OPIOID USE
[Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg 2017; 152(6):e170504.]
Brummett et al6 examined ongoing opioid use after surgery in 36,177 opioid-naive patients and in a nonsurgical control group. After 3 months, 6% of the patients who underwent surgery remained on opioids, compared with only 0.4% of the nonsurgical controls. Whether the surgery was major or minor did not affect the rate of postoperative opioid use.
Risk factors for ongoing opioid use were preexisting addiction to anything (including tobacco), mood disorders, and preoperative pain disorders. These risk factors have previously been reported in nonsurgical patients.7
Brummett et al speculated that patients are counseled about postoperative opioids in a way that leads them to overestimate the safety and efficacy of these drugs for treating other common pain conditions.6
Bottom line. Patients with mental health comorbidities have a hard time stopping opioids. The remarkable finding in this study was the similarity between major and minor surgery in terms of chronic opioid use. If postoperative opioids treat only the pain caused by the surgery, major surgery should be associated with greater opioid use. The similarity suggests that a mechanism other than postoperative pain confers risk of chronic opioid use.
THINKING ABOUT OPIOIDS
Collectively, these articles describe elements of acute pain treatment that correlate with chronic ongoing opioid use: a higher cumulative dose,3 being seen by a physician who prescribes a lot of opioids,5 undergoing surgery,6 and psychiatric comorbidity.6 They made me wonder if opioid use for acute pain acts as an inoculation, analogous to inoculating a Petri dish with bacteria. The likelihood of chronic opioid use arises from the inoculum dose, the host response, and the context of inoculation.
These articles do not show how patients taking opioids chronically for pain become addicted. Stumbo et al8 interviewed 283 opioid-dependent patients and identified 5 pathways to opioid use disorder, 3 of which were related to pain control: inadequately controlled chronic pain, exposure to opioids during acute pain episodes, and chronic pain in patients who already had substance use disorders. Brat et al9 recently estimated the risk of opioid use disorder after receiving opioids postoperatively to be less than 1%, but it increased dramatically with duration of opioid treatment.
A patient who fills an opioid prescription does not necessarily have chronic pain. Nor do all patients with chronic pain require an opioid prescription. These studies did not establish whether the patients had a pain syndrome. In practice, we call our patients who chronically take opioids our “chronic pain patients.” But 40% of Americans have chronic pain, while only 5% take opioids daily for pain.11,12
We assume that those taking opioids have the most severe pain. But Brummett et al suggested that continued opioid use is predicted less by pain and more by psychiatric comorbidity.6 More than half of the opioid prescriptions in the United States are written for patients with serious mental illness, who represent one-sixth of that population.11 Maybe chronic opioid use for pain has more to do with vulnerability to opioids and less to do with a pain syndrome.
I now think about daily opioid use in much the same way as I think about daily prednisone use. Patients on daily prednisone have a characteristic set of medical risks from the prednisone itself, regardless of its indication. Yet we do not consider these patients addicted to prednisone. Opioid use may be similar.
Like most doctors, I am troubled by the continued rise in the opioid overdose rate.13 Yet addiction and death from overdose are not the only risks that patients on chronic opioids face; they also have higher rates of falls, cardiovascular death, pneumonia, death from chronic obstructive pulmonary disease, and motor vehicle crashes.14–17 Patients on chronic opioids for pain have greater mental health comorbidity and worse function.18
Most concerning, chronic opioid treatment for pain lacks proof of benefit. In fact, a recent study disproved the benefit of opioids for chronic pain compared with nonopioid options.19 When I meet with patients who are taking chronic opioids for pain, I often can’t identify why the drugs were started or ought to be continued, and I anticipate a bad outcome. Yet the patient is afraid to stop the drug. For these reasons, chronic opioid use for pain strikes me as worth considering separately from opioid use disorder.
HOW THIS CHANGED MY PRACTICE
The studies described above have had a powerful effect on my clinical care as a hospitalist.
I now talk to all patients starting opioids about how hard it can be to stop. Some patients are defensive at first, believing this does not apply to them. But I politely continue.
People with depression and anxiety can have a harder time stopping opioids. Addiction is both a risk with ongoing opioid use and a possible outcome of acute opioid use.8 But one can struggle to stop opioids without being addicted or depressed. Even the healthiest person may wish to continue opioids past the point of benefit.
I am careful not to invalidate the patient’s experience of pain. It is challenging for patients to find the balance between current discomfort and a possible future adverse effect. In these conversations, I imagine how I would want a loved one counseled on their pain control. This centers me as I choose my words and my tone.
I now monitor the total amount of opioid I prescribe for acute pain in addition to the daily dose. I give my patients as few opioids as reasonable, and advise them to take the minimum dose required for tolerable comfort. I offer nonopioid options as the preferred choice, presenting them as effective and safe. I do this irrespective of the indication for opioids.
I limit opioids in all patients, not just those with comorbidities. I include in my shared decision-making process the risk of chronic opioid use when I prescribe opioids for acute pain, carefully distinguishing it from opioid use disorder. Instead of excess opioids, I give patients my office phone number to call in case they struggle. I rarely get calls. But I find patients would rather have access to a doctor than extra pills. And offering them my contact information lets me limit opioids while letting them know that I am committed to their comfort and health.
As an addiction medicine doctor, I consult on patients not taking their opioids as prescribed. Caring for these patients is intellectually and emotionally draining; they suffer daily, and the opioids they take provide a modicum of relief at a high cost. The publications I have discussed here provide insight into how a troubled relationship with opioids begins. I remind myself that these patients have an iatrogenic condition. Their behaviors that we label “aberrant” may reflect an adverse reaction to medications prescribed to them for acute pain.
Mary, my patient with postoperative pain after cholecystectomy, may over time develop opioid use disorder as Heather did. That progression may have begun with the hydrocodone I prescribed and the counseling I gave her, and it may proceed to chronic opioid use and then opioid use disorder.
I am looking closely at the care I give for acute pain in light of these innovative studies. But even more so, they have increased the compassion with which I care for patients like Heather, those harmed by prescribed opioids.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association Publishing; 2013:541–546.
- Han B, Compton WM, Blanco C, Crane E, Lee J, Jones CM. Prescription opioid use, misuse, and use disorders in US adults: 2015 national survey on drug use and health. Ann Intern Med 2017; 167(5):293–301. doi:10.7326/M17-0865
- Shah A, Hayes CJ, Martin BC. Characteristics of initial prescription episodes and likelihood of long-term opioid use—United States, 2006–2015. MMWR Morb Mortal Wkly Rep 2017; 66(10):265–269. doi:10.15585/mmwr.mm6610a1
- Dasgupta N, Funk MJ, Proescholdbell S, Hirsch A, Ribisl KM, Marshall S. Cohort study of the impact of high-dose opioid analgesics on overdose mortality. Pain Med 2016; 17(1):85–98. doi:10.1111/pme.12907
- Barnett ML, Olenski AR, Jena AB. Opioid-prescribing patterns of emergency physicians and risk of long-term use. N Engl J Med 2017; 376(7):663–673. doi:10.1056/NEJMsa1610524
- Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg 2017; 152(6):e170504. doi:10.1001/jamasurg.2017.0504
- Volkow ND, McLellan AT. Opioid abuse in chronic pain—misconceptions and mitigation strategies. N Engl J Med 2016; 374(13):1253–1263. doi:10.1056/NEJMra1507771
- Stumbo SP, Yarborough BJ, McCarty D, Weisner C, Green CA. Patient-reported pathways to opioid use disorders and pain-related barriers to treatment engagement. J Subst Abuse Treat 2017; 73:47–54. doi:10.1016/j.jsat.2016.11.003
- Brat GA, Agniel D, Beam A, et al. Postsurgical prescriptions for opioid naive patients and association with overdose and misuse: retrospective cohort study. BMJ 2018; 360:j5790. doi:10.1136/bmj.j5790
- Vowles KE, McEntee ML, Julnes PS, Frohe T, Ney JP, van der Goes DN. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain 2015; 156(4):569–576. doi:10.1097/01.j.pain.0000460357.01998.f1
- Davis MA, Lin LA, Liu H, Sites BD. Prescription opioid use among adults with mental health disorders in the United States. J Am Board Fam Med 2017; 30(4):407–417. doi:10.3122/jabfm.2017.04.170112
- Tsang A, Von Korff M, Lee S, et al. Common chronic pain conditions in developed and developing countries: gender and age differences and comorbidity with depression-anxiety disorders. J Pain 2008; 9(10):883–891. doi:10.1016/j.jpain.2008.05.005
- QuickStats: age-adjusted death rates for drug overdose, by race/ethnicity—national vital statistics system, United States, 2015–2016. MMWR Morb Mortal Wkly Rep 2018; 67(12):374. doi:10.15585/mmwr.mm6712a9
- Solomon DH, Rassen JA, Glynn RJ, Lee J, Levin R, Schneeweiss S. The comparative safety of analgesics in older adults with arthritis. Arch Intern Med 2010; 170(22):1968–1976. doi:10.1001/archinternmed.2010.391
- Vozoris NT, Wang X, Fischer HD, et al. Incident opioid drug use and adverse respiratory outcomes among older adults with COPD. Eur Respir J 2016; 48(3):683–693. doi:10.1183/13993003.01967-2015
- Wiese AD, Griffin MR, Schaffner W, et al. Opioid analgesic use and risk for invasive pneumococcal diseases: a nested case-control study. Ann Intern Med 2018; 168(6):396–404. doi:10.7326/M17-1907
- Chihuri S, Li G. Use of prescription opioids and motor vehicle crashes: a meta analysis. Accid Anal Prev 2017; 109:123–131. doi:10.1016/j.aap.2017.10.004
- Morasco BJ, Yarborough BJ, Smith NX, et al. Higher prescription opioid dose is associated with worse patient-reported pain outcomes and more health care utilization. J Pain 2017; 18(4):437–445. doi:10.1016/j.jpain.2016.12.004
- Krebs EE, Gravely A, Nugent S, et al. Effect of opioid vs nonopioid medications on pain-related function in patients with chronic back pain or hip or knee osteoarthritis pain: the SPACE randomized clinical trial. JAMA 2018; 319(9):872–882. doi:10.1001/jama.2018.0899
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association Publishing; 2013:541–546.
- Han B, Compton WM, Blanco C, Crane E, Lee J, Jones CM. Prescription opioid use, misuse, and use disorders in US adults: 2015 national survey on drug use and health. Ann Intern Med 2017; 167(5):293–301. doi:10.7326/M17-0865
- Shah A, Hayes CJ, Martin BC. Characteristics of initial prescription episodes and likelihood of long-term opioid use—United States, 2006–2015. MMWR Morb Mortal Wkly Rep 2017; 66(10):265–269. doi:10.15585/mmwr.mm6610a1
- Dasgupta N, Funk MJ, Proescholdbell S, Hirsch A, Ribisl KM, Marshall S. Cohort study of the impact of high-dose opioid analgesics on overdose mortality. Pain Med 2016; 17(1):85–98. doi:10.1111/pme.12907
- Barnett ML, Olenski AR, Jena AB. Opioid-prescribing patterns of emergency physicians and risk of long-term use. N Engl J Med 2017; 376(7):663–673. doi:10.1056/NEJMsa1610524
- Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg 2017; 152(6):e170504. doi:10.1001/jamasurg.2017.0504
- Volkow ND, McLellan AT. Opioid abuse in chronic pain—misconceptions and mitigation strategies. N Engl J Med 2016; 374(13):1253–1263. doi:10.1056/NEJMra1507771
- Stumbo SP, Yarborough BJ, McCarty D, Weisner C, Green CA. Patient-reported pathways to opioid use disorders and pain-related barriers to treatment engagement. J Subst Abuse Treat 2017; 73:47–54. doi:10.1016/j.jsat.2016.11.003
- Brat GA, Agniel D, Beam A, et al. Postsurgical prescriptions for opioid naive patients and association with overdose and misuse: retrospective cohort study. BMJ 2018; 360:j5790. doi:10.1136/bmj.j5790
- Vowles KE, McEntee ML, Julnes PS, Frohe T, Ney JP, van der Goes DN. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain 2015; 156(4):569–576. doi:10.1097/01.j.pain.0000460357.01998.f1
- Davis MA, Lin LA, Liu H, Sites BD. Prescription opioid use among adults with mental health disorders in the United States. J Am Board Fam Med 2017; 30(4):407–417. doi:10.3122/jabfm.2017.04.170112
- Tsang A, Von Korff M, Lee S, et al. Common chronic pain conditions in developed and developing countries: gender and age differences and comorbidity with depression-anxiety disorders. J Pain 2008; 9(10):883–891. doi:10.1016/j.jpain.2008.05.005
- QuickStats: age-adjusted death rates for drug overdose, by race/ethnicity—national vital statistics system, United States, 2015–2016. MMWR Morb Mortal Wkly Rep 2018; 67(12):374. doi:10.15585/mmwr.mm6712a9
- Solomon DH, Rassen JA, Glynn RJ, Lee J, Levin R, Schneeweiss S. The comparative safety of analgesics in older adults with arthritis. Arch Intern Med 2010; 170(22):1968–1976. doi:10.1001/archinternmed.2010.391
- Vozoris NT, Wang X, Fischer HD, et al. Incident opioid drug use and adverse respiratory outcomes among older adults with COPD. Eur Respir J 2016; 48(3):683–693. doi:10.1183/13993003.01967-2015
- Wiese AD, Griffin MR, Schaffner W, et al. Opioid analgesic use and risk for invasive pneumococcal diseases: a nested case-control study. Ann Intern Med 2018; 168(6):396–404. doi:10.7326/M17-1907
- Chihuri S, Li G. Use of prescription opioids and motor vehicle crashes: a meta analysis. Accid Anal Prev 2017; 109:123–131. doi:10.1016/j.aap.2017.10.004
- Morasco BJ, Yarborough BJ, Smith NX, et al. Higher prescription opioid dose is associated with worse patient-reported pain outcomes and more health care utilization. J Pain 2017; 18(4):437–445. doi:10.1016/j.jpain.2016.12.004
- Krebs EE, Gravely A, Nugent S, et al. Effect of opioid vs nonopioid medications on pain-related function in patients with chronic back pain or hip or knee osteoarthritis pain: the SPACE randomized clinical trial. JAMA 2018; 319(9):872–882. doi:10.1001/jama.2018.0899
Correction: Genitourinary syndrome of menopause
Persistent erythematous papulonodular rash
An 80-year-old white woman presented to our dermatology clinic with a rash across her abdomen that had been there for more than a year. While not itchy or painful, the rash was slowly expanding. The patient had tried treatments including topical antifungals and topical corticosteroids, but none had helped.
Her medical history was significant for dementia and stage III triple-negative breast cancer in the left breast (diagnosed 8 years prior), which was treated with a simple left mastectomy, chemotherapy, and radiation. She reported no history of skin cancer. She was not taking any medications and had no known drug allergies. A physical examination revealed an erythematous, papulonodular rash with diffuse induration in a band-like pattern across her entire upper abdomen and left flank (FIGURE).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Cutaneous metastasis of primary breast cancer
Based on our patient’s history, we gave a presumptive diagnosis of cutaneous breast cancer metastasis. A punch biopsy was performed. The pathology report showed nests of neoplastic cells within the dermis, which was consistent with this diagnosis. Immunohistochemical stains and fluorescence in-situ hybridization confirmed triple-negative breast markers for estrogen receptor, progesterone receptor, and human epidermal growth factor receptor-2.
An uncommon phenomenonseen mostly with breast cancer
Cutaneous metastatic carcinoma is relatively uncommon; one meta-analysis reported the overall incidence to be 5.3%.1 While it is unusual, any internal malignancy can metastasize to the skin. In women, the most common malignancy to do so is breast cancer. One study found breast cancer to be associated with 26.5% of cutaneous metastatic cases.2 These metastases often occur well after the patient has been treated for the primary malignancy.
Identifying features. Most cutaneous metastases occur near the site of the primary tumor, initially in the form of a firm, mobile, nonpainful nodule.3 This nodule is typically skin-colored or red, but in the case of cutaneous metastases of melanomas, it can appear blue or black. In the case of breast cancer, the lesions most often arise on the chest and abdomen.4 Occasionally, metastases can ulcerate through the skin.
Some forms of cutaneous metastasis, such as carcinoma erysipeloides, can appear in specific patterns. Carcinoma erysipeloides has a similar appearance to cellulitis; it manifests as a sharply demarcated, red, inflammatory patch in the skin adjacent to the primary tumor.
Consider the clinical picture
Cutaneous metastatic lesions have a wide range of differential diagnoses due to their varied appearances. It is important to view the overall clinical picture when distinguishing such lesions. Although cutaneous metastasis is uncommon, it should always be considered when asymptomatic skin lesions resist treatment—even in someone without a known history of malignancy.
Perform a biopsy. The diagnosis can be confirmed with a skin biopsy. A punch biopsy is preferable, as visualization of the dermis is crucial, and histology often reveals nests of pleomorphic cells. Further cellular cytology can elicit the primary malignancy of origin.
Making our diagnosis
We ruled out several possibilities before arriving at our diagnosis. An infectious etiology (eg, cutaneous candidiasis) was considered, as was a cutaneous change due to radiation therapy. We also considered shingles, the early stages of which would have been similar in appearance to our patient’s lesions, and urticaria, which can manifest as erythematous papules and wheals across various parts of the body. A lack of specific symptoms (eg, pruritis, pain, fever) made these alternative diagnoses less likely. The fact that our patient’s lesions persisted for more than a year without any response to treatment—and that they continued to grow—alerted us of a more sinister etiology.
Continue to: Treating the tumor is often not possible
Treating the tumor is often not possible
Treatment first involves treating the underlying tumor. For cases in which cutaneous lesions are the first manifestation of an internal malignancy, investigation as to the source should be performed. The lesions can then be treated with a combination of chemotherapy, radiation, and surgery.5,6
Unfortunately, in most cases of cutaneous metastases, the primary malignancy is already widespread and possibly untreatable. In such instances, palliative care is offered. Lesions are managed symptomatically, and prevention of skin irritation becomes the primary focus. Keeping the skin clean and dry helps to prevent ulceration and secondary infection.
In cases where the lesions ulcerate or crust, debridement can help. Excision of lesions, as well as pairing laser therapy with electrochemotherapy, may be helpful to improve the patient’s quality of life when lesions cause discomfort.
The prognosis for cutaneous metastasis due to breast cancer is often hard to predict because it is determined by other factors, such as the presence of internal metastases, which indicates a worse prognosis (on the scale of months). Some case reports have demonstrated that patients with metastases limited to the skin may have prolonged survival (on the scale of years).7
Our patient was offered an initial trial of radiation therapy, but she refused all treatment because the lesions did not cause discomfort, and she preferred to not go through further aggressive cancer treatment that could potentially cause complications and pain. We respected the patient’s wishes and counseled her on follow-up if the lesions became symptomatic or she decided she wanted to try treatment.
CORRESPONDENCE
Araya Zaesim, 1550 College St, Macon, GA, 31207; [email protected]
1. Krathen RA, Orengo IF, Rosen T. Cutaneous metastasis: a meta-analysis of data. South Med J. 2003;96:164-167.
2. Brownstein MH, Helwig EB. Patterns of cutaneous metastasis. Arch Dermatol. 1972;105:862-868.
3. De Giorgi V, Grazzini M, Alfaioli B, et al. Cutaneous manifestations of breast carcinoma. Dermatol Ther. 2010;23:581-589.
4. Wong CYB, Helm MA, Kalb RE, et al. The presentation, pathology, and current management strategies of cutaneous metastasis. N Am J Med Sci. 2013;5:499-504.
5. Moore S. Cutaneous metastatic breast cancer. Clin J Oncol Nurs. 2002;6:255-260.
6. Ahmed M. Cutaneous metastases from breast carcinoma. BMJ Case Rep. 2011;2011: bcr0620114398.
7. Cho J, Park Y, Lee JC, et al. Case series of different onset of skin metastasis according to the breast cancer subtypes. Cancer Res Treat. 2014;46:194-199.
An 80-year-old white woman presented to our dermatology clinic with a rash across her abdomen that had been there for more than a year. While not itchy or painful, the rash was slowly expanding. The patient had tried treatments including topical antifungals and topical corticosteroids, but none had helped.
Her medical history was significant for dementia and stage III triple-negative breast cancer in the left breast (diagnosed 8 years prior), which was treated with a simple left mastectomy, chemotherapy, and radiation. She reported no history of skin cancer. She was not taking any medications and had no known drug allergies. A physical examination revealed an erythematous, papulonodular rash with diffuse induration in a band-like pattern across her entire upper abdomen and left flank (FIGURE).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Cutaneous metastasis of primary breast cancer
Based on our patient’s history, we gave a presumptive diagnosis of cutaneous breast cancer metastasis. A punch biopsy was performed. The pathology report showed nests of neoplastic cells within the dermis, which was consistent with this diagnosis. Immunohistochemical stains and fluorescence in-situ hybridization confirmed triple-negative breast markers for estrogen receptor, progesterone receptor, and human epidermal growth factor receptor-2.
An uncommon phenomenonseen mostly with breast cancer
Cutaneous metastatic carcinoma is relatively uncommon; one meta-analysis reported the overall incidence to be 5.3%.1 While it is unusual, any internal malignancy can metastasize to the skin. In women, the most common malignancy to do so is breast cancer. One study found breast cancer to be associated with 26.5% of cutaneous metastatic cases.2 These metastases often occur well after the patient has been treated for the primary malignancy.
Identifying features. Most cutaneous metastases occur near the site of the primary tumor, initially in the form of a firm, mobile, nonpainful nodule.3 This nodule is typically skin-colored or red, but in the case of cutaneous metastases of melanomas, it can appear blue or black. In the case of breast cancer, the lesions most often arise on the chest and abdomen.4 Occasionally, metastases can ulcerate through the skin.
Some forms of cutaneous metastasis, such as carcinoma erysipeloides, can appear in specific patterns. Carcinoma erysipeloides has a similar appearance to cellulitis; it manifests as a sharply demarcated, red, inflammatory patch in the skin adjacent to the primary tumor.
Consider the clinical picture
Cutaneous metastatic lesions have a wide range of differential diagnoses due to their varied appearances. It is important to view the overall clinical picture when distinguishing such lesions. Although cutaneous metastasis is uncommon, it should always be considered when asymptomatic skin lesions resist treatment—even in someone without a known history of malignancy.
Perform a biopsy. The diagnosis can be confirmed with a skin biopsy. A punch biopsy is preferable, as visualization of the dermis is crucial, and histology often reveals nests of pleomorphic cells. Further cellular cytology can elicit the primary malignancy of origin.
Making our diagnosis
We ruled out several possibilities before arriving at our diagnosis. An infectious etiology (eg, cutaneous candidiasis) was considered, as was a cutaneous change due to radiation therapy. We also considered shingles, the early stages of which would have been similar in appearance to our patient’s lesions, and urticaria, which can manifest as erythematous papules and wheals across various parts of the body. A lack of specific symptoms (eg, pruritis, pain, fever) made these alternative diagnoses less likely. The fact that our patient’s lesions persisted for more than a year without any response to treatment—and that they continued to grow—alerted us of a more sinister etiology.
Continue to: Treating the tumor is often not possible
Treating the tumor is often not possible
Treatment first involves treating the underlying tumor. For cases in which cutaneous lesions are the first manifestation of an internal malignancy, investigation as to the source should be performed. The lesions can then be treated with a combination of chemotherapy, radiation, and surgery.5,6
Unfortunately, in most cases of cutaneous metastases, the primary malignancy is already widespread and possibly untreatable. In such instances, palliative care is offered. Lesions are managed symptomatically, and prevention of skin irritation becomes the primary focus. Keeping the skin clean and dry helps to prevent ulceration and secondary infection.
In cases where the lesions ulcerate or crust, debridement can help. Excision of lesions, as well as pairing laser therapy with electrochemotherapy, may be helpful to improve the patient’s quality of life when lesions cause discomfort.
The prognosis for cutaneous metastasis due to breast cancer is often hard to predict because it is determined by other factors, such as the presence of internal metastases, which indicates a worse prognosis (on the scale of months). Some case reports have demonstrated that patients with metastases limited to the skin may have prolonged survival (on the scale of years).7
Our patient was offered an initial trial of radiation therapy, but she refused all treatment because the lesions did not cause discomfort, and she preferred to not go through further aggressive cancer treatment that could potentially cause complications and pain. We respected the patient’s wishes and counseled her on follow-up if the lesions became symptomatic or she decided she wanted to try treatment.
CORRESPONDENCE
Araya Zaesim, 1550 College St, Macon, GA, 31207; [email protected]
An 80-year-old white woman presented to our dermatology clinic with a rash across her abdomen that had been there for more than a year. While not itchy or painful, the rash was slowly expanding. The patient had tried treatments including topical antifungals and topical corticosteroids, but none had helped.
Her medical history was significant for dementia and stage III triple-negative breast cancer in the left breast (diagnosed 8 years prior), which was treated with a simple left mastectomy, chemotherapy, and radiation. She reported no history of skin cancer. She was not taking any medications and had no known drug allergies. A physical examination revealed an erythematous, papulonodular rash with diffuse induration in a band-like pattern across her entire upper abdomen and left flank (FIGURE).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Cutaneous metastasis of primary breast cancer
Based on our patient’s history, we gave a presumptive diagnosis of cutaneous breast cancer metastasis. A punch biopsy was performed. The pathology report showed nests of neoplastic cells within the dermis, which was consistent with this diagnosis. Immunohistochemical stains and fluorescence in-situ hybridization confirmed triple-negative breast markers for estrogen receptor, progesterone receptor, and human epidermal growth factor receptor-2.
An uncommon phenomenonseen mostly with breast cancer
Cutaneous metastatic carcinoma is relatively uncommon; one meta-analysis reported the overall incidence to be 5.3%.1 While it is unusual, any internal malignancy can metastasize to the skin. In women, the most common malignancy to do so is breast cancer. One study found breast cancer to be associated with 26.5% of cutaneous metastatic cases.2 These metastases often occur well after the patient has been treated for the primary malignancy.
Identifying features. Most cutaneous metastases occur near the site of the primary tumor, initially in the form of a firm, mobile, nonpainful nodule.3 This nodule is typically skin-colored or red, but in the case of cutaneous metastases of melanomas, it can appear blue or black. In the case of breast cancer, the lesions most often arise on the chest and abdomen.4 Occasionally, metastases can ulcerate through the skin.
Some forms of cutaneous metastasis, such as carcinoma erysipeloides, can appear in specific patterns. Carcinoma erysipeloides has a similar appearance to cellulitis; it manifests as a sharply demarcated, red, inflammatory patch in the skin adjacent to the primary tumor.
Consider the clinical picture
Cutaneous metastatic lesions have a wide range of differential diagnoses due to their varied appearances. It is important to view the overall clinical picture when distinguishing such lesions. Although cutaneous metastasis is uncommon, it should always be considered when asymptomatic skin lesions resist treatment—even in someone without a known history of malignancy.
Perform a biopsy. The diagnosis can be confirmed with a skin biopsy. A punch biopsy is preferable, as visualization of the dermis is crucial, and histology often reveals nests of pleomorphic cells. Further cellular cytology can elicit the primary malignancy of origin.
Making our diagnosis
We ruled out several possibilities before arriving at our diagnosis. An infectious etiology (eg, cutaneous candidiasis) was considered, as was a cutaneous change due to radiation therapy. We also considered shingles, the early stages of which would have been similar in appearance to our patient’s lesions, and urticaria, which can manifest as erythematous papules and wheals across various parts of the body. A lack of specific symptoms (eg, pruritis, pain, fever) made these alternative diagnoses less likely. The fact that our patient’s lesions persisted for more than a year without any response to treatment—and that they continued to grow—alerted us of a more sinister etiology.
Continue to: Treating the tumor is often not possible
Treating the tumor is often not possible
Treatment first involves treating the underlying tumor. For cases in which cutaneous lesions are the first manifestation of an internal malignancy, investigation as to the source should be performed. The lesions can then be treated with a combination of chemotherapy, radiation, and surgery.5,6
Unfortunately, in most cases of cutaneous metastases, the primary malignancy is already widespread and possibly untreatable. In such instances, palliative care is offered. Lesions are managed symptomatically, and prevention of skin irritation becomes the primary focus. Keeping the skin clean and dry helps to prevent ulceration and secondary infection.
In cases where the lesions ulcerate or crust, debridement can help. Excision of lesions, as well as pairing laser therapy with electrochemotherapy, may be helpful to improve the patient’s quality of life when lesions cause discomfort.
The prognosis for cutaneous metastasis due to breast cancer is often hard to predict because it is determined by other factors, such as the presence of internal metastases, which indicates a worse prognosis (on the scale of months). Some case reports have demonstrated that patients with metastases limited to the skin may have prolonged survival (on the scale of years).7
Our patient was offered an initial trial of radiation therapy, but she refused all treatment because the lesions did not cause discomfort, and she preferred to not go through further aggressive cancer treatment that could potentially cause complications and pain. We respected the patient’s wishes and counseled her on follow-up if the lesions became symptomatic or she decided she wanted to try treatment.
CORRESPONDENCE
Araya Zaesim, 1550 College St, Macon, GA, 31207; [email protected]
1. Krathen RA, Orengo IF, Rosen T. Cutaneous metastasis: a meta-analysis of data. South Med J. 2003;96:164-167.
2. Brownstein MH, Helwig EB. Patterns of cutaneous metastasis. Arch Dermatol. 1972;105:862-868.
3. De Giorgi V, Grazzini M, Alfaioli B, et al. Cutaneous manifestations of breast carcinoma. Dermatol Ther. 2010;23:581-589.
4. Wong CYB, Helm MA, Kalb RE, et al. The presentation, pathology, and current management strategies of cutaneous metastasis. N Am J Med Sci. 2013;5:499-504.
5. Moore S. Cutaneous metastatic breast cancer. Clin J Oncol Nurs. 2002;6:255-260.
6. Ahmed M. Cutaneous metastases from breast carcinoma. BMJ Case Rep. 2011;2011: bcr0620114398.
7. Cho J, Park Y, Lee JC, et al. Case series of different onset of skin metastasis according to the breast cancer subtypes. Cancer Res Treat. 2014;46:194-199.
1. Krathen RA, Orengo IF, Rosen T. Cutaneous metastasis: a meta-analysis of data. South Med J. 2003;96:164-167.
2. Brownstein MH, Helwig EB. Patterns of cutaneous metastasis. Arch Dermatol. 1972;105:862-868.
3. De Giorgi V, Grazzini M, Alfaioli B, et al. Cutaneous manifestations of breast carcinoma. Dermatol Ther. 2010;23:581-589.
4. Wong CYB, Helm MA, Kalb RE, et al. The presentation, pathology, and current management strategies of cutaneous metastasis. N Am J Med Sci. 2013;5:499-504.
5. Moore S. Cutaneous metastatic breast cancer. Clin J Oncol Nurs. 2002;6:255-260.
6. Ahmed M. Cutaneous metastases from breast carcinoma. BMJ Case Rep. 2011;2011: bcr0620114398.
7. Cho J, Park Y, Lee JC, et al. Case series of different onset of skin metastasis according to the breast cancer subtypes. Cancer Res Treat. 2014;46:194-199.