User login
What infectious disease should parents be most worried about?
I think the question was intended as polite, dinner party chit chat ... maybe an attempt by a gracious hostess to make sure everyone was engaged in conversation.
“So what pediatric infectious disease should parents be most worried about?” she asked me.
I’ll admit that a couple of perfectly respectable and noncontroversial possibilities crossed my mind before I answered.
Acute flaccid myelitis? Measles?
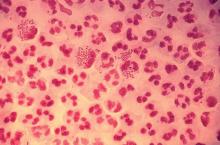
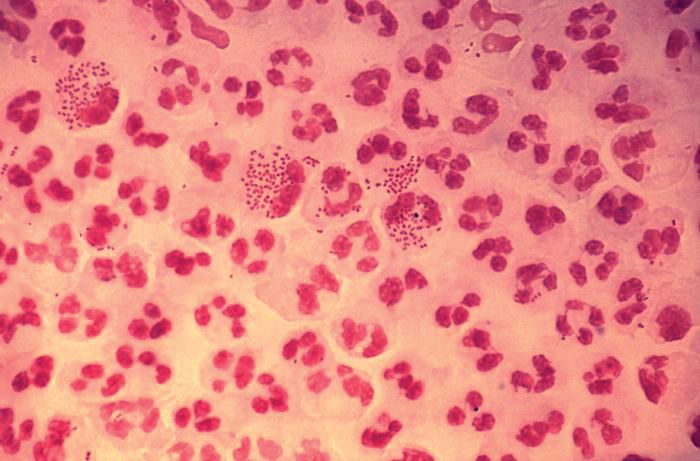
When I replied, “gonorrhea,” conversation at the table pretty much stopped.
Let me explain. Acute flaccid myelitis is a polio-like neurologic condition that has been grabbing headlines. Yes, it is concerning that most cases have occurred in children and some affected children are left with long-term deficits. Technically though, AFM is a neurologic rather than an infectious disease. When cases occur, we suspect a viral infection but according to the Centers for Disease Control and Prevention, no pathogen has been consistently identified from the spinal fluid of infected patients. From August 2014 to September 2018, the CDC received information on 368 confirmed cases, so AFM fortunately is still rare.
News reports describe measles outbreaks raging in Europe – more than 41,000 cases so far this year, and 40 deaths – and warn that the United States could be next. But let’s be honest: We have a safe and effective vaccine for measles and outbreaks like this don’t happen when individuals are appropriately immunized. Parents, immunize your children. If you are lucky enough to be traveling to Europe with your baby, remember that MMR vaccine is indicated for 6- to 11-month olds, but it doesn’t count in the 2-dose series.
But gonorrhea?
In 2017, the World Health Organization included Neisseria gonorrhoeae on its list of bacteria that pose the greatest threat to human health and for which new antibiotics are urgently needed. The popular media are calling N. gonorrhoeae one of the new “superbugs.” Globally, patients are being diagnosed with strains of gonorrhea that are resistant to all commonly used antibiotics. As reported during IDWeek 2018 this October, patients also are being diagnosed in the United States.
Sancta St. Cyr, MD, of the Centers for Disease Control and Prevention, and her colleagues reported data from the Gonococcal Isolate Surveillance Project (GISP) and trends in multidrug resistant (MDR) and extensively-drug resistant (XDR) gonorrhea in the United States. A gonococcal isolate with resistance or elevated minimum inhibitory concentrations (MIC) to greater than or equal to two classes of antimicrobials is classified as MDR and an isolate with elevated MICs to greater than or equal to three classes of antimicrobials is classified as XDR. The MIC is the lowest antimicrobial concentration that inhibits growth of bacteria in the laboratory and rising MICs – evidence that higher levels of an antibiotic are needed to stop bacterial growth – can be an early indicator that resistance is emerging.
More than 150,000 gonococcal isolates were tested between 1987 and 2016. The first isolates with elevated MICs to cephalosporins and macrolides were identified in 1998, and since 2011, MDR resistance rates have hovered around 1%. In 2016, the rate was 1.1%, down from 1.3% in 2011. A single XDR isolate with resistance to fluoroquinolones with elevated MICs to both cephalosporins and macrolides was identified in 2011.
One could look at these data and ask if this is a “glass half full or half empty” situation, but I propose that clinicians and public health officials should not look at these data and be reassured that rates of MDR-gonorrhea remained stable between 2010 and 2016. According to a recent surveillance report released by the CDC, the absolute number of cases of gonorrhea has continued to rise. In 2017, there were 555,608 cases reported in the United States, a 67% increase since 2013. If we assume that rates of resistance in 2017 were similar to those in 2016, that’s more than 5,000 cases of MDR-gonorrhea in a single year.
“That’s bad,” one of my dining companions agreed. “But is gonorrhea really a pediatric issue?”
To answer that question, we just have to look at the numbers. According to the 2017 Youth Risk Behavior Survey, the percentage of high school students who had ever had sex was approximately 40% and about 10% of students had four or more lifetime partners. More than 45% of sexually active students denied the use of a condom during the last sexual intercourse. Certainly, that puts many teenagers at risk for sexually transmitted infections (STIs). Perhaps it shouldn’t be surprising that public health authorities report that half of all new STIs occur in individuals aged 15-24 years. Moreover, 25% of sexually active adolescent girls contract at least one STI.
Gonorrhea is the second most commonly reported notifiable disease in the United States, and according to the CDC, rates of disease in 2017 were highest among adolescents and young adults. In females specifically, the highest rates of gonorrhea were observed among those aged 20-24 years (684.8 cases per 100,000 females) and 15-19 years (557.4 cases per 100,000 females).
It makes sense that pediatricians and parents advocate for making the reduction of gonorrhea transmission rates a public health priority. We also need to recognize that prompt diagnosis and appropriate treatment are critical. Since 2015, dual therapy with ceftriaxone and azithromycin is the only CDC-recommended treatment for gonorrhea.
At that dinner party, my closest friend, who also happens to be a pediatrician, rolled her eyes and shot me look that I’m sure meant, “Nobody really wants to talk about gonorrhea over dessert.” Still, because she is a good friend she said, “So basically you’re saying that and if this keeps up, we may see kids with untreatable infection. Now that is scary.”
I kept quiet after that but I wanted to mention that in 2017, less than 85% of patients diagnosed with gonorrhea at selected surveillance sites received the recommended treatment with two antibiotics. Patients with inadequately treated gonorrhea are at risk for a host of sequelae. Women can develop pelvic inflammatory disease, abscesses, chronic pelvic pain, and damage of the fallopian tubes that can lead to infertility. Men can develop epididymitis, which occasionally results in infertility. Rarely, N. gonorrhoeae can spread to the blood and cause life-threatening infection. Of course, patients who aren’t treated appropriately may continue to spread the bacteria. Scary? You bet.
For pediatricians who need a refresher course in the treatment of STIs, there are free resources available. The CDC’s 2015 STD Treatment Guidelines are available in a free app; the app contains a nice refresher on taking a sexual history. There also is a print version, wall chart, and pocket guide. Providers also may want to check out the National STD Curriculum offered by the University of Washington STD Prevention Training Center and the University of Washington. Visit https://www.std.uw.edu/.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
I think the question was intended as polite, dinner party chit chat ... maybe an attempt by a gracious hostess to make sure everyone was engaged in conversation.
“So what pediatric infectious disease should parents be most worried about?” she asked me.
I’ll admit that a couple of perfectly respectable and noncontroversial possibilities crossed my mind before I answered.
Acute flaccid myelitis? Measles?
When I replied, “gonorrhea,” conversation at the table pretty much stopped.
Let me explain. Acute flaccid myelitis is a polio-like neurologic condition that has been grabbing headlines. Yes, it is concerning that most cases have occurred in children and some affected children are left with long-term deficits. Technically though, AFM is a neurologic rather than an infectious disease. When cases occur, we suspect a viral infection but according to the Centers for Disease Control and Prevention, no pathogen has been consistently identified from the spinal fluid of infected patients. From August 2014 to September 2018, the CDC received information on 368 confirmed cases, so AFM fortunately is still rare.
News reports describe measles outbreaks raging in Europe – more than 41,000 cases so far this year, and 40 deaths – and warn that the United States could be next. But let’s be honest: We have a safe and effective vaccine for measles and outbreaks like this don’t happen when individuals are appropriately immunized. Parents, immunize your children. If you are lucky enough to be traveling to Europe with your baby, remember that MMR vaccine is indicated for 6- to 11-month olds, but it doesn’t count in the 2-dose series.
But gonorrhea?
In 2017, the World Health Organization included Neisseria gonorrhoeae on its list of bacteria that pose the greatest threat to human health and for which new antibiotics are urgently needed. The popular media are calling N. gonorrhoeae one of the new “superbugs.” Globally, patients are being diagnosed with strains of gonorrhea that are resistant to all commonly used antibiotics. As reported during IDWeek 2018 this October, patients also are being diagnosed in the United States.
Sancta St. Cyr, MD, of the Centers for Disease Control and Prevention, and her colleagues reported data from the Gonococcal Isolate Surveillance Project (GISP) and trends in multidrug resistant (MDR) and extensively-drug resistant (XDR) gonorrhea in the United States. A gonococcal isolate with resistance or elevated minimum inhibitory concentrations (MIC) to greater than or equal to two classes of antimicrobials is classified as MDR and an isolate with elevated MICs to greater than or equal to three classes of antimicrobials is classified as XDR. The MIC is the lowest antimicrobial concentration that inhibits growth of bacteria in the laboratory and rising MICs – evidence that higher levels of an antibiotic are needed to stop bacterial growth – can be an early indicator that resistance is emerging.
More than 150,000 gonococcal isolates were tested between 1987 and 2016. The first isolates with elevated MICs to cephalosporins and macrolides were identified in 1998, and since 2011, MDR resistance rates have hovered around 1%. In 2016, the rate was 1.1%, down from 1.3% in 2011. A single XDR isolate with resistance to fluoroquinolones with elevated MICs to both cephalosporins and macrolides was identified in 2011.
One could look at these data and ask if this is a “glass half full or half empty” situation, but I propose that clinicians and public health officials should not look at these data and be reassured that rates of MDR-gonorrhea remained stable between 2010 and 2016. According to a recent surveillance report released by the CDC, the absolute number of cases of gonorrhea has continued to rise. In 2017, there were 555,608 cases reported in the United States, a 67% increase since 2013. If we assume that rates of resistance in 2017 were similar to those in 2016, that’s more than 5,000 cases of MDR-gonorrhea in a single year.
“That’s bad,” one of my dining companions agreed. “But is gonorrhea really a pediatric issue?”
To answer that question, we just have to look at the numbers. According to the 2017 Youth Risk Behavior Survey, the percentage of high school students who had ever had sex was approximately 40% and about 10% of students had four or more lifetime partners. More than 45% of sexually active students denied the use of a condom during the last sexual intercourse. Certainly, that puts many teenagers at risk for sexually transmitted infections (STIs). Perhaps it shouldn’t be surprising that public health authorities report that half of all new STIs occur in individuals aged 15-24 years. Moreover, 25% of sexually active adolescent girls contract at least one STI.
Gonorrhea is the second most commonly reported notifiable disease in the United States, and according to the CDC, rates of disease in 2017 were highest among adolescents and young adults. In females specifically, the highest rates of gonorrhea were observed among those aged 20-24 years (684.8 cases per 100,000 females) and 15-19 years (557.4 cases per 100,000 females).
It makes sense that pediatricians and parents advocate for making the reduction of gonorrhea transmission rates a public health priority. We also need to recognize that prompt diagnosis and appropriate treatment are critical. Since 2015, dual therapy with ceftriaxone and azithromycin is the only CDC-recommended treatment for gonorrhea.
At that dinner party, my closest friend, who also happens to be a pediatrician, rolled her eyes and shot me look that I’m sure meant, “Nobody really wants to talk about gonorrhea over dessert.” Still, because she is a good friend she said, “So basically you’re saying that and if this keeps up, we may see kids with untreatable infection. Now that is scary.”
I kept quiet after that but I wanted to mention that in 2017, less than 85% of patients diagnosed with gonorrhea at selected surveillance sites received the recommended treatment with two antibiotics. Patients with inadequately treated gonorrhea are at risk for a host of sequelae. Women can develop pelvic inflammatory disease, abscesses, chronic pelvic pain, and damage of the fallopian tubes that can lead to infertility. Men can develop epididymitis, which occasionally results in infertility. Rarely, N. gonorrhoeae can spread to the blood and cause life-threatening infection. Of course, patients who aren’t treated appropriately may continue to spread the bacteria. Scary? You bet.
For pediatricians who need a refresher course in the treatment of STIs, there are free resources available. The CDC’s 2015 STD Treatment Guidelines are available in a free app; the app contains a nice refresher on taking a sexual history. There also is a print version, wall chart, and pocket guide. Providers also may want to check out the National STD Curriculum offered by the University of Washington STD Prevention Training Center and the University of Washington. Visit https://www.std.uw.edu/.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
I think the question was intended as polite, dinner party chit chat ... maybe an attempt by a gracious hostess to make sure everyone was engaged in conversation.
“So what pediatric infectious disease should parents be most worried about?” she asked me.
I’ll admit that a couple of perfectly respectable and noncontroversial possibilities crossed my mind before I answered.
Acute flaccid myelitis? Measles?
When I replied, “gonorrhea,” conversation at the table pretty much stopped.
Let me explain. Acute flaccid myelitis is a polio-like neurologic condition that has been grabbing headlines. Yes, it is concerning that most cases have occurred in children and some affected children are left with long-term deficits. Technically though, AFM is a neurologic rather than an infectious disease. When cases occur, we suspect a viral infection but according to the Centers for Disease Control and Prevention, no pathogen has been consistently identified from the spinal fluid of infected patients. From August 2014 to September 2018, the CDC received information on 368 confirmed cases, so AFM fortunately is still rare.
News reports describe measles outbreaks raging in Europe – more than 41,000 cases so far this year, and 40 deaths – and warn that the United States could be next. But let’s be honest: We have a safe and effective vaccine for measles and outbreaks like this don’t happen when individuals are appropriately immunized. Parents, immunize your children. If you are lucky enough to be traveling to Europe with your baby, remember that MMR vaccine is indicated for 6- to 11-month olds, but it doesn’t count in the 2-dose series.
But gonorrhea?
In 2017, the World Health Organization included Neisseria gonorrhoeae on its list of bacteria that pose the greatest threat to human health and for which new antibiotics are urgently needed. The popular media are calling N. gonorrhoeae one of the new “superbugs.” Globally, patients are being diagnosed with strains of gonorrhea that are resistant to all commonly used antibiotics. As reported during IDWeek 2018 this October, patients also are being diagnosed in the United States.
Sancta St. Cyr, MD, of the Centers for Disease Control and Prevention, and her colleagues reported data from the Gonococcal Isolate Surveillance Project (GISP) and trends in multidrug resistant (MDR) and extensively-drug resistant (XDR) gonorrhea in the United States. A gonococcal isolate with resistance or elevated minimum inhibitory concentrations (MIC) to greater than or equal to two classes of antimicrobials is classified as MDR and an isolate with elevated MICs to greater than or equal to three classes of antimicrobials is classified as XDR. The MIC is the lowest antimicrobial concentration that inhibits growth of bacteria in the laboratory and rising MICs – evidence that higher levels of an antibiotic are needed to stop bacterial growth – can be an early indicator that resistance is emerging.
More than 150,000 gonococcal isolates were tested between 1987 and 2016. The first isolates with elevated MICs to cephalosporins and macrolides were identified in 1998, and since 2011, MDR resistance rates have hovered around 1%. In 2016, the rate was 1.1%, down from 1.3% in 2011. A single XDR isolate with resistance to fluoroquinolones with elevated MICs to both cephalosporins and macrolides was identified in 2011.
One could look at these data and ask if this is a “glass half full or half empty” situation, but I propose that clinicians and public health officials should not look at these data and be reassured that rates of MDR-gonorrhea remained stable between 2010 and 2016. According to a recent surveillance report released by the CDC, the absolute number of cases of gonorrhea has continued to rise. In 2017, there were 555,608 cases reported in the United States, a 67% increase since 2013. If we assume that rates of resistance in 2017 were similar to those in 2016, that’s more than 5,000 cases of MDR-gonorrhea in a single year.
“That’s bad,” one of my dining companions agreed. “But is gonorrhea really a pediatric issue?”
To answer that question, we just have to look at the numbers. According to the 2017 Youth Risk Behavior Survey, the percentage of high school students who had ever had sex was approximately 40% and about 10% of students had four or more lifetime partners. More than 45% of sexually active students denied the use of a condom during the last sexual intercourse. Certainly, that puts many teenagers at risk for sexually transmitted infections (STIs). Perhaps it shouldn’t be surprising that public health authorities report that half of all new STIs occur in individuals aged 15-24 years. Moreover, 25% of sexually active adolescent girls contract at least one STI.
Gonorrhea is the second most commonly reported notifiable disease in the United States, and according to the CDC, rates of disease in 2017 were highest among adolescents and young adults. In females specifically, the highest rates of gonorrhea were observed among those aged 20-24 years (684.8 cases per 100,000 females) and 15-19 years (557.4 cases per 100,000 females).
It makes sense that pediatricians and parents advocate for making the reduction of gonorrhea transmission rates a public health priority. We also need to recognize that prompt diagnosis and appropriate treatment are critical. Since 2015, dual therapy with ceftriaxone and azithromycin is the only CDC-recommended treatment for gonorrhea.
At that dinner party, my closest friend, who also happens to be a pediatrician, rolled her eyes and shot me look that I’m sure meant, “Nobody really wants to talk about gonorrhea over dessert.” Still, because she is a good friend she said, “So basically you’re saying that and if this keeps up, we may see kids with untreatable infection. Now that is scary.”
I kept quiet after that but I wanted to mention that in 2017, less than 85% of patients diagnosed with gonorrhea at selected surveillance sites received the recommended treatment with two antibiotics. Patients with inadequately treated gonorrhea are at risk for a host of sequelae. Women can develop pelvic inflammatory disease, abscesses, chronic pelvic pain, and damage of the fallopian tubes that can lead to infertility. Men can develop epididymitis, which occasionally results in infertility. Rarely, N. gonorrhoeae can spread to the blood and cause life-threatening infection. Of course, patients who aren’t treated appropriately may continue to spread the bacteria. Scary? You bet.
For pediatricians who need a refresher course in the treatment of STIs, there are free resources available. The CDC’s 2015 STD Treatment Guidelines are available in a free app; the app contains a nice refresher on taking a sexual history. There also is a print version, wall chart, and pocket guide. Providers also may want to check out the National STD Curriculum offered by the University of Washington STD Prevention Training Center and the University of Washington. Visit https://www.std.uw.edu/.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
Low and high BMI tied to higher postpartum depression risk
Women with high and low body mass index in the first trimester of their first pregnancies are at an increased risk of developing postpartum depression, a population-based study of more than 600,000 new mothers shows.
“Our findings show a U-shaped association between BMI extremes and clinically significant depression after childbirth,” Michael E. Silverman, PhD, and his associates reported in the Journal of Affective Disorders. “Specifically, women in the lowest and highest groups were at a significantly increased risk for developing [postpartum depression].”
Dr. Silverman and his associates used the Swedish Medical Birth Register to identify women who delivered first live singleton infants from 1997 to 2008. They then calculated the risk of postpartum depression in relation to each woman’s BMI and history of depression. Postpartum depression was defined as a clinical depression diagnosis within 1 year after delivery, Dr. Silverman and his associates wrote.
The investigators found that women with low BMI (less than 18.5 kg/m2) were at an increased postpartum depression risk (relative risk [RR], 1.52; 95% confidence interval, 1.30-1.78), as were those with high BMI (greater than 35 kg/m2) (RR, 1.23; 95% CI, 1.04-1.45).
In addition, an important difference was found between women with low and high BMI.
“Women in the highest BMI group were only at an increased risk for [postpartum depression] if they had no history of depression, showing for the first time how [postpartum depression] risk factors associated with BMI are modified by maternal depression history,” said Dr. Silverman of the department of psychiatry at the Icahn School of Medicine at Mount Sinai, New York, and his associates.
The investigators cited several limitations. For example, only first births were analyzed, which suggests that the incidence of postpartum depression might have been underestimated. Another limitation is that the registry might not have captured women with mild depression. Nevertheless, they said, the study has important implications.
“ Because pregnant women represent a medically captured population,” they wrote, the findings support implementing preventive strategies for postpartum depression and health literacy for high-risk women.
Dr. Silverman and his associates reported having no conflicts of interest. The study was supported by a grant from the National Institutes of Health.
SOURCE: Silverman ME et al. J Affect Disord. 2018 Nov;240:193-8.
Women with high and low body mass index in the first trimester of their first pregnancies are at an increased risk of developing postpartum depression, a population-based study of more than 600,000 new mothers shows.
“Our findings show a U-shaped association between BMI extremes and clinically significant depression after childbirth,” Michael E. Silverman, PhD, and his associates reported in the Journal of Affective Disorders. “Specifically, women in the lowest and highest groups were at a significantly increased risk for developing [postpartum depression].”
Dr. Silverman and his associates used the Swedish Medical Birth Register to identify women who delivered first live singleton infants from 1997 to 2008. They then calculated the risk of postpartum depression in relation to each woman’s BMI and history of depression. Postpartum depression was defined as a clinical depression diagnosis within 1 year after delivery, Dr. Silverman and his associates wrote.
The investigators found that women with low BMI (less than 18.5 kg/m2) were at an increased postpartum depression risk (relative risk [RR], 1.52; 95% confidence interval, 1.30-1.78), as were those with high BMI (greater than 35 kg/m2) (RR, 1.23; 95% CI, 1.04-1.45).
In addition, an important difference was found between women with low and high BMI.
“Women in the highest BMI group were only at an increased risk for [postpartum depression] if they had no history of depression, showing for the first time how [postpartum depression] risk factors associated with BMI are modified by maternal depression history,” said Dr. Silverman of the department of psychiatry at the Icahn School of Medicine at Mount Sinai, New York, and his associates.
The investigators cited several limitations. For example, only first births were analyzed, which suggests that the incidence of postpartum depression might have been underestimated. Another limitation is that the registry might not have captured women with mild depression. Nevertheless, they said, the study has important implications.
“ Because pregnant women represent a medically captured population,” they wrote, the findings support implementing preventive strategies for postpartum depression and health literacy for high-risk women.
Dr. Silverman and his associates reported having no conflicts of interest. The study was supported by a grant from the National Institutes of Health.
SOURCE: Silverman ME et al. J Affect Disord. 2018 Nov;240:193-8.
Women with high and low body mass index in the first trimester of their first pregnancies are at an increased risk of developing postpartum depression, a population-based study of more than 600,000 new mothers shows.
“Our findings show a U-shaped association between BMI extremes and clinically significant depression after childbirth,” Michael E. Silverman, PhD, and his associates reported in the Journal of Affective Disorders. “Specifically, women in the lowest and highest groups were at a significantly increased risk for developing [postpartum depression].”
Dr. Silverman and his associates used the Swedish Medical Birth Register to identify women who delivered first live singleton infants from 1997 to 2008. They then calculated the risk of postpartum depression in relation to each woman’s BMI and history of depression. Postpartum depression was defined as a clinical depression diagnosis within 1 year after delivery, Dr. Silverman and his associates wrote.
The investigators found that women with low BMI (less than 18.5 kg/m2) were at an increased postpartum depression risk (relative risk [RR], 1.52; 95% confidence interval, 1.30-1.78), as were those with high BMI (greater than 35 kg/m2) (RR, 1.23; 95% CI, 1.04-1.45).
In addition, an important difference was found between women with low and high BMI.
“Women in the highest BMI group were only at an increased risk for [postpartum depression] if they had no history of depression, showing for the first time how [postpartum depression] risk factors associated with BMI are modified by maternal depression history,” said Dr. Silverman of the department of psychiatry at the Icahn School of Medicine at Mount Sinai, New York, and his associates.
The investigators cited several limitations. For example, only first births were analyzed, which suggests that the incidence of postpartum depression might have been underestimated. Another limitation is that the registry might not have captured women with mild depression. Nevertheless, they said, the study has important implications.
“ Because pregnant women represent a medically captured population,” they wrote, the findings support implementing preventive strategies for postpartum depression and health literacy for high-risk women.
Dr. Silverman and his associates reported having no conflicts of interest. The study was supported by a grant from the National Institutes of Health.
SOURCE: Silverman ME et al. J Affect Disord. 2018 Nov;240:193-8.
FROM THE JOURNAL OF AFFECTIVE DISORDERS
HIV testing low in U.S. women engaged in risky behavior
HIV testing rates were low among women whose sexual behaviors increased their risk of HIV infection, and they were especially low among women who reported having anal sex, according to a report published in the American Journal of Obstetrics & Gynecology.
Data from the 2011-2015 National Survey of Family Growth were analyzed to estimate the proportion of sexually active, nonpregnant U.S. women aged 15-44 years who had had an HIV test within the past year. The data was stratified by those who reported anal sex and other risk factors, including having more than two sexual partners, condomless sex with a new partner or multiple partners, gonorrhea in the past year, or any history of syphilis, according to Mary Evans, MD, of the National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention at the Centers for Disease Control and Prevention and her colleagues.
Among the 42.4 million sexually active women assessed, 9.0 million (20%) reported they had had anal sex in the past year. Of these, 19% reported that their providers asked about their types of intercourse, and 20% reported an HIV test within the past year. Overall, HIV testing was higher among women who reported anal sex and whose providers asked about types of sex engaged in than it was among those women whose provider did not ask (38% vs. 16%, respectively; P less than .001). However, HIV testing in the past year was higher for women with other forms of risky behaviors as compared with anal sex, ranging from 35.8% to 47.2%.
“Women who report sexual behaviors such as anal sex would benefit from an HIV test and an assessment for [prevention with preexposure prophylaxis] eligibility. Women’s health care providers are uniquely poised to provide HIV prevention for women who tend to have frequent encounters with the health care system,” the researchers concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Evans ME et al. Am J Obstet Gynecol. 2018 Oct;219(4):383.e1-7.
HIV testing rates were low among women whose sexual behaviors increased their risk of HIV infection, and they were especially low among women who reported having anal sex, according to a report published in the American Journal of Obstetrics & Gynecology.
Data from the 2011-2015 National Survey of Family Growth were analyzed to estimate the proportion of sexually active, nonpregnant U.S. women aged 15-44 years who had had an HIV test within the past year. The data was stratified by those who reported anal sex and other risk factors, including having more than two sexual partners, condomless sex with a new partner or multiple partners, gonorrhea in the past year, or any history of syphilis, according to Mary Evans, MD, of the National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention at the Centers for Disease Control and Prevention and her colleagues.
Among the 42.4 million sexually active women assessed, 9.0 million (20%) reported they had had anal sex in the past year. Of these, 19% reported that their providers asked about their types of intercourse, and 20% reported an HIV test within the past year. Overall, HIV testing was higher among women who reported anal sex and whose providers asked about types of sex engaged in than it was among those women whose provider did not ask (38% vs. 16%, respectively; P less than .001). However, HIV testing in the past year was higher for women with other forms of risky behaviors as compared with anal sex, ranging from 35.8% to 47.2%.
“Women who report sexual behaviors such as anal sex would benefit from an HIV test and an assessment for [prevention with preexposure prophylaxis] eligibility. Women’s health care providers are uniquely poised to provide HIV prevention for women who tend to have frequent encounters with the health care system,” the researchers concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Evans ME et al. Am J Obstet Gynecol. 2018 Oct;219(4):383.e1-7.
HIV testing rates were low among women whose sexual behaviors increased their risk of HIV infection, and they were especially low among women who reported having anal sex, according to a report published in the American Journal of Obstetrics & Gynecology.
Data from the 2011-2015 National Survey of Family Growth were analyzed to estimate the proportion of sexually active, nonpregnant U.S. women aged 15-44 years who had had an HIV test within the past year. The data was stratified by those who reported anal sex and other risk factors, including having more than two sexual partners, condomless sex with a new partner or multiple partners, gonorrhea in the past year, or any history of syphilis, according to Mary Evans, MD, of the National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention at the Centers for Disease Control and Prevention and her colleagues.
Among the 42.4 million sexually active women assessed, 9.0 million (20%) reported they had had anal sex in the past year. Of these, 19% reported that their providers asked about their types of intercourse, and 20% reported an HIV test within the past year. Overall, HIV testing was higher among women who reported anal sex and whose providers asked about types of sex engaged in than it was among those women whose provider did not ask (38% vs. 16%, respectively; P less than .001). However, HIV testing in the past year was higher for women with other forms of risky behaviors as compared with anal sex, ranging from 35.8% to 47.2%.
“Women who report sexual behaviors such as anal sex would benefit from an HIV test and an assessment for [prevention with preexposure prophylaxis] eligibility. Women’s health care providers are uniquely poised to provide HIV prevention for women who tend to have frequent encounters with the health care system,” the researchers concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Evans ME et al. Am J Obstet Gynecol. 2018 Oct;219(4):383.e1-7.
FROM THE AMERICAN JOURNAL OF OBSTETRICS & GYNECOLOGY
Key clinical point: Health care providers don’t ask sexually active women about risky behavior that would raise their risk of HIV infection.
Major finding: Of women who reported having anal sex, 19% reported that their providers asked about their types of intercourse.
Study details: Data from the 2011-2015 National Survey of Family Growth.
Disclosures: The authors reported that they had no conflicts of interest.
Source: Evans ME et al. Am J Obstet Gynecol. 2018 Oct;219(4):383.e1-7.
HF unlikely in pregnant cancer survivors without history of cardiotoxicity
The risk of adverse cardiac events in female cancer survivors during pregnancy is low unless there is a history of cardiotoxicity, according to Shiying Liu, MD, of the University of Toronto, and her associates.
In a research letter published in the Journal of the American College of Cardiology, Dr. Liu and her associates reported on a retrospective chart review of 78 women with 94 pregnancies who had previously received cancer therapy who were seen at Mount Sinai Hospital between 2005 and 2015. Of these, 15 pregnancies occurred in 13 women with a prior history of cardiotoxicity. The primary outcome was a composite of cardiac events including cardiac death, heart failure (HF), acute coronary syndrome, and sustained arrhythmia.
HF occurred during five pregnancies in four women; no other adverse cardiac events occurred during the study period. All four of the women who experienced HF had a history of cardiotoxicity. There was no difference in age at cancer diagnosis, age at pregnancy, cancer type, or exposure to anthracyclines between those who did and did not experience HF, but women who developed HF were more likely to have left ventricular systolic dysfunction at the first antenatal visit (75% vs. 8%; P = .004) and to be on cardiac medications (50% vs. 8%; P = .026).
“The risk of developing [HF] during pregnancy is rare in female cancer survivors without a history of cardiotoxicity. These women can be reassured that they are at a very low risk of developing [HF] during pregnancy. Women who have a history of cardiotoxicity have an approximately one in three chance of developing [HF] during pregnancy and should receive close cardiac surveillance during pregnancy at a center with expertise in cardiac disease in pregnancy,” the authors concluded.
Coauthor Paaladinesh Thavendiranathan, MD, reported support from the Canadian Institutes of Health Research New Investigator Award. None of the other authors had any relevant financial disclosures.
SOURCE: Liu S et al. J Am Coll Cardiol. 2018 Oct 15. doi: 10.1016/j.jacc.2018.07.085.
The risk of adverse cardiac events in female cancer survivors during pregnancy is low unless there is a history of cardiotoxicity, according to Shiying Liu, MD, of the University of Toronto, and her associates.
In a research letter published in the Journal of the American College of Cardiology, Dr. Liu and her associates reported on a retrospective chart review of 78 women with 94 pregnancies who had previously received cancer therapy who were seen at Mount Sinai Hospital between 2005 and 2015. Of these, 15 pregnancies occurred in 13 women with a prior history of cardiotoxicity. The primary outcome was a composite of cardiac events including cardiac death, heart failure (HF), acute coronary syndrome, and sustained arrhythmia.
HF occurred during five pregnancies in four women; no other adverse cardiac events occurred during the study period. All four of the women who experienced HF had a history of cardiotoxicity. There was no difference in age at cancer diagnosis, age at pregnancy, cancer type, or exposure to anthracyclines between those who did and did not experience HF, but women who developed HF were more likely to have left ventricular systolic dysfunction at the first antenatal visit (75% vs. 8%; P = .004) and to be on cardiac medications (50% vs. 8%; P = .026).
“The risk of developing [HF] during pregnancy is rare in female cancer survivors without a history of cardiotoxicity. These women can be reassured that they are at a very low risk of developing [HF] during pregnancy. Women who have a history of cardiotoxicity have an approximately one in three chance of developing [HF] during pregnancy and should receive close cardiac surveillance during pregnancy at a center with expertise in cardiac disease in pregnancy,” the authors concluded.
Coauthor Paaladinesh Thavendiranathan, MD, reported support from the Canadian Institutes of Health Research New Investigator Award. None of the other authors had any relevant financial disclosures.
SOURCE: Liu S et al. J Am Coll Cardiol. 2018 Oct 15. doi: 10.1016/j.jacc.2018.07.085.
The risk of adverse cardiac events in female cancer survivors during pregnancy is low unless there is a history of cardiotoxicity, according to Shiying Liu, MD, of the University of Toronto, and her associates.
In a research letter published in the Journal of the American College of Cardiology, Dr. Liu and her associates reported on a retrospective chart review of 78 women with 94 pregnancies who had previously received cancer therapy who were seen at Mount Sinai Hospital between 2005 and 2015. Of these, 15 pregnancies occurred in 13 women with a prior history of cardiotoxicity. The primary outcome was a composite of cardiac events including cardiac death, heart failure (HF), acute coronary syndrome, and sustained arrhythmia.
HF occurred during five pregnancies in four women; no other adverse cardiac events occurred during the study period. All four of the women who experienced HF had a history of cardiotoxicity. There was no difference in age at cancer diagnosis, age at pregnancy, cancer type, or exposure to anthracyclines between those who did and did not experience HF, but women who developed HF were more likely to have left ventricular systolic dysfunction at the first antenatal visit (75% vs. 8%; P = .004) and to be on cardiac medications (50% vs. 8%; P = .026).
“The risk of developing [HF] during pregnancy is rare in female cancer survivors without a history of cardiotoxicity. These women can be reassured that they are at a very low risk of developing [HF] during pregnancy. Women who have a history of cardiotoxicity have an approximately one in three chance of developing [HF] during pregnancy and should receive close cardiac surveillance during pregnancy at a center with expertise in cardiac disease in pregnancy,” the authors concluded.
Coauthor Paaladinesh Thavendiranathan, MD, reported support from the Canadian Institutes of Health Research New Investigator Award. None of the other authors had any relevant financial disclosures.
SOURCE: Liu S et al. J Am Coll Cardiol. 2018 Oct 15. doi: 10.1016/j.jacc.2018.07.085.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Can ultrasound screening improve survival in ovarian cancer?
Annual ultrasound screening of asymptomatic women at risk of epithelial ovarian cancer can lead to lower staging of cancer at detection and improved survival, compared with no screening, according to a prospective clinical trial that followed more than 46,000 women over 2 decades.
“The findings of this study support the concept that a major predictor of ovarian cancer survival is stage at detection,” said John R. van Nagell Jr., MD, of the University of Kentucky–Markey Cancer Center, Lexington, and his coauthors. “The 10-year survival of women whose ovarian cancer was detected at an early stage (I or II) was 35% higher than that of women diagnosed with stage III cancer.” Study results were published in the November issue of Obstetrics & Gynecology.
The study evaluated 46,101 women enrolled in the University of Kentucky Ovarian Cancer Screening Trial over 30.5 years. Trial participants, all of whom had annual ultrasound screening, were age 50 and older, or 25 and older with a family history of ovarian cancer. Overall, 23% and 44% of the women had a family history of either ovarian or breast cancer, respectively. Women in the study had an average of seven scans each. The unscreened comparator group was women with ovarian cancer referred to the UK Markey Cancer Center.
The study detected 71 cases of invasive epithelial ovarian cancers and 17 epithelial ovarian tumors of low malignant potential. None of the women with these tumors had a recurrence. Among the invasive cancers, the majority were either stage I (42%) or II (21%), and none were stage IV. The median age of these patients was 66 years. Of the low-malignancy tumors, 27% were stage I and 73% stage II, with none stage III or IV. A total of 699 women (1.5%) with persistent ovarian tumors had surgery.
Screened women also had improved survival compared to unscreened women: 86% vs. 45% at 5 years, 68% vs. 31% at 10 years (P less than .001).
However, the study also showed a high overall incidence rate for ovarian cancer, including false-positive and false-negative cases, compared with National Cancer Institute reports in the Kentucky state cancer profile: 271 per 100,000 vs. 10.4/100,000.
The study also looked at the economics of annual screening. “Ovarian screening reduced the 10-year mortality by 37% and produced 416 life years gained,” Dr. van Nagell and his coauthors said. Based on an estimated cost of $56 for each transvaginal ultrasound scan, that translates into a cost of $40,731 for each life year gained.
One concern of screening ultrasound is the high false-positive rate. “Although the sensitivity of transvaginal ultrasonography in detecting an ovarian abnormality is high, it has been unreliable in differentiating benign from malignant ovarian tumors,” they said. While they noted the accuracy of assessing malignancy has improved, the risk of complications in women who have surgery for benign tumors is an ongoing concern. “Additional research is necessary to identify high-risk populations who will benefit most from screening.”
Dr. van Nagell and his coauthors reported having no financial relationships. The study was supported by research grants from the Kentucky Department of Health and Human Services and the Telford Foundation.
SOURCE: Van Nagell JR Jr et al. Obstet Gynecol. 2018 Nov;132[5]:1091-100. doi: 10.1097/AOG.0000000000002921.
Use a measure of caution when interpreting the results of the study by van Nagell et al., Sharon E. Robertson, MD, MPH, and Jeffery E. Peipert, MD, PhD, said in an invited commentary. First, they noted the “surprisingly high” rate of ovarian cancer (271 per 100,000) – although it may improve the predictive value of ultrasound screening, when one applies the test sensitivity and specificity the trial reported to the general population, “the positive predictive value falls to an unacceptable 0.7%.”
They also questioned the rationale for comparing the study population to an unscreened cohort of women with ovarian cancer referred to the University of Kentucky. “However, are we really comparing apples to apples?” they asked, noting “important key baseline differences” between the two groups, including that the screened cohort “could have contained an unbalanced proportion of genetically related ovarian cancers.” They also noted the risk profile of the unscreened cohort is unknown.
Addressing the differences in survival rates between screened and unscreened patients, Dr. Robertson and Dr. Peipert noted the study population had a higher rate of type I tumors than that seen in National Cancer Institute data for the general population (27% vs. 11%), along with the absence of genetic analysis. “For these reasons, we cannot confidently agree with the authors’ conclusion that the ultrasound screening reduced ovarian cancer mortality,” they stated.
They commended the Kentucky group for a “landmark study,” but added, “the evidence that screening for ovarian cancer improves survival remains elusive.” They called for more evidence before widespread screening programs are implemented.
Dr. Robertson and Dr. Peipert of Indiana University, Indianapolis, commented on the article by van Nagell et al. in Obstetrics and Gynecology (2018 Nov;132[5]:1089-90. doi: 10.1097/AOG.0000000000002962). Dr. Peipert disclosed relationships with Cooper Surgical/Teva, Merck, and Bayer. Dr. Robertson had no relevant financial relationships.
Use a measure of caution when interpreting the results of the study by van Nagell et al., Sharon E. Robertson, MD, MPH, and Jeffery E. Peipert, MD, PhD, said in an invited commentary. First, they noted the “surprisingly high” rate of ovarian cancer (271 per 100,000) – although it may improve the predictive value of ultrasound screening, when one applies the test sensitivity and specificity the trial reported to the general population, “the positive predictive value falls to an unacceptable 0.7%.”
They also questioned the rationale for comparing the study population to an unscreened cohort of women with ovarian cancer referred to the University of Kentucky. “However, are we really comparing apples to apples?” they asked, noting “important key baseline differences” between the two groups, including that the screened cohort “could have contained an unbalanced proportion of genetically related ovarian cancers.” They also noted the risk profile of the unscreened cohort is unknown.
Addressing the differences in survival rates between screened and unscreened patients, Dr. Robertson and Dr. Peipert noted the study population had a higher rate of type I tumors than that seen in National Cancer Institute data for the general population (27% vs. 11%), along with the absence of genetic analysis. “For these reasons, we cannot confidently agree with the authors’ conclusion that the ultrasound screening reduced ovarian cancer mortality,” they stated.
They commended the Kentucky group for a “landmark study,” but added, “the evidence that screening for ovarian cancer improves survival remains elusive.” They called for more evidence before widespread screening programs are implemented.
Dr. Robertson and Dr. Peipert of Indiana University, Indianapolis, commented on the article by van Nagell et al. in Obstetrics and Gynecology (2018 Nov;132[5]:1089-90. doi: 10.1097/AOG.0000000000002962). Dr. Peipert disclosed relationships with Cooper Surgical/Teva, Merck, and Bayer. Dr. Robertson had no relevant financial relationships.
Use a measure of caution when interpreting the results of the study by van Nagell et al., Sharon E. Robertson, MD, MPH, and Jeffery E. Peipert, MD, PhD, said in an invited commentary. First, they noted the “surprisingly high” rate of ovarian cancer (271 per 100,000) – although it may improve the predictive value of ultrasound screening, when one applies the test sensitivity and specificity the trial reported to the general population, “the positive predictive value falls to an unacceptable 0.7%.”
They also questioned the rationale for comparing the study population to an unscreened cohort of women with ovarian cancer referred to the University of Kentucky. “However, are we really comparing apples to apples?” they asked, noting “important key baseline differences” between the two groups, including that the screened cohort “could have contained an unbalanced proportion of genetically related ovarian cancers.” They also noted the risk profile of the unscreened cohort is unknown.
Addressing the differences in survival rates between screened and unscreened patients, Dr. Robertson and Dr. Peipert noted the study population had a higher rate of type I tumors than that seen in National Cancer Institute data for the general population (27% vs. 11%), along with the absence of genetic analysis. “For these reasons, we cannot confidently agree with the authors’ conclusion that the ultrasound screening reduced ovarian cancer mortality,” they stated.
They commended the Kentucky group for a “landmark study,” but added, “the evidence that screening for ovarian cancer improves survival remains elusive.” They called for more evidence before widespread screening programs are implemented.
Dr. Robertson and Dr. Peipert of Indiana University, Indianapolis, commented on the article by van Nagell et al. in Obstetrics and Gynecology (2018 Nov;132[5]:1089-90. doi: 10.1097/AOG.0000000000002962). Dr. Peipert disclosed relationships with Cooper Surgical/Teva, Merck, and Bayer. Dr. Robertson had no relevant financial relationships.
Annual ultrasound screening of asymptomatic women at risk of epithelial ovarian cancer can lead to lower staging of cancer at detection and improved survival, compared with no screening, according to a prospective clinical trial that followed more than 46,000 women over 2 decades.
“The findings of this study support the concept that a major predictor of ovarian cancer survival is stage at detection,” said John R. van Nagell Jr., MD, of the University of Kentucky–Markey Cancer Center, Lexington, and his coauthors. “The 10-year survival of women whose ovarian cancer was detected at an early stage (I or II) was 35% higher than that of women diagnosed with stage III cancer.” Study results were published in the November issue of Obstetrics & Gynecology.
The study evaluated 46,101 women enrolled in the University of Kentucky Ovarian Cancer Screening Trial over 30.5 years. Trial participants, all of whom had annual ultrasound screening, were age 50 and older, or 25 and older with a family history of ovarian cancer. Overall, 23% and 44% of the women had a family history of either ovarian or breast cancer, respectively. Women in the study had an average of seven scans each. The unscreened comparator group was women with ovarian cancer referred to the UK Markey Cancer Center.
The study detected 71 cases of invasive epithelial ovarian cancers and 17 epithelial ovarian tumors of low malignant potential. None of the women with these tumors had a recurrence. Among the invasive cancers, the majority were either stage I (42%) or II (21%), and none were stage IV. The median age of these patients was 66 years. Of the low-malignancy tumors, 27% were stage I and 73% stage II, with none stage III or IV. A total of 699 women (1.5%) with persistent ovarian tumors had surgery.
Screened women also had improved survival compared to unscreened women: 86% vs. 45% at 5 years, 68% vs. 31% at 10 years (P less than .001).
However, the study also showed a high overall incidence rate for ovarian cancer, including false-positive and false-negative cases, compared with National Cancer Institute reports in the Kentucky state cancer profile: 271 per 100,000 vs. 10.4/100,000.
The study also looked at the economics of annual screening. “Ovarian screening reduced the 10-year mortality by 37% and produced 416 life years gained,” Dr. van Nagell and his coauthors said. Based on an estimated cost of $56 for each transvaginal ultrasound scan, that translates into a cost of $40,731 for each life year gained.
One concern of screening ultrasound is the high false-positive rate. “Although the sensitivity of transvaginal ultrasonography in detecting an ovarian abnormality is high, it has been unreliable in differentiating benign from malignant ovarian tumors,” they said. While they noted the accuracy of assessing malignancy has improved, the risk of complications in women who have surgery for benign tumors is an ongoing concern. “Additional research is necessary to identify high-risk populations who will benefit most from screening.”
Dr. van Nagell and his coauthors reported having no financial relationships. The study was supported by research grants from the Kentucky Department of Health and Human Services and the Telford Foundation.
SOURCE: Van Nagell JR Jr et al. Obstet Gynecol. 2018 Nov;132[5]:1091-100. doi: 10.1097/AOG.0000000000002921.
Annual ultrasound screening of asymptomatic women at risk of epithelial ovarian cancer can lead to lower staging of cancer at detection and improved survival, compared with no screening, according to a prospective clinical trial that followed more than 46,000 women over 2 decades.
“The findings of this study support the concept that a major predictor of ovarian cancer survival is stage at detection,” said John R. van Nagell Jr., MD, of the University of Kentucky–Markey Cancer Center, Lexington, and his coauthors. “The 10-year survival of women whose ovarian cancer was detected at an early stage (I or II) was 35% higher than that of women diagnosed with stage III cancer.” Study results were published in the November issue of Obstetrics & Gynecology.
The study evaluated 46,101 women enrolled in the University of Kentucky Ovarian Cancer Screening Trial over 30.5 years. Trial participants, all of whom had annual ultrasound screening, were age 50 and older, or 25 and older with a family history of ovarian cancer. Overall, 23% and 44% of the women had a family history of either ovarian or breast cancer, respectively. Women in the study had an average of seven scans each. The unscreened comparator group was women with ovarian cancer referred to the UK Markey Cancer Center.
The study detected 71 cases of invasive epithelial ovarian cancers and 17 epithelial ovarian tumors of low malignant potential. None of the women with these tumors had a recurrence. Among the invasive cancers, the majority were either stage I (42%) or II (21%), and none were stage IV. The median age of these patients was 66 years. Of the low-malignancy tumors, 27% were stage I and 73% stage II, with none stage III or IV. A total of 699 women (1.5%) with persistent ovarian tumors had surgery.
Screened women also had improved survival compared to unscreened women: 86% vs. 45% at 5 years, 68% vs. 31% at 10 years (P less than .001).
However, the study also showed a high overall incidence rate for ovarian cancer, including false-positive and false-negative cases, compared with National Cancer Institute reports in the Kentucky state cancer profile: 271 per 100,000 vs. 10.4/100,000.
The study also looked at the economics of annual screening. “Ovarian screening reduced the 10-year mortality by 37% and produced 416 life years gained,” Dr. van Nagell and his coauthors said. Based on an estimated cost of $56 for each transvaginal ultrasound scan, that translates into a cost of $40,731 for each life year gained.
One concern of screening ultrasound is the high false-positive rate. “Although the sensitivity of transvaginal ultrasonography in detecting an ovarian abnormality is high, it has been unreliable in differentiating benign from malignant ovarian tumors,” they said. While they noted the accuracy of assessing malignancy has improved, the risk of complications in women who have surgery for benign tumors is an ongoing concern. “Additional research is necessary to identify high-risk populations who will benefit most from screening.”
Dr. van Nagell and his coauthors reported having no financial relationships. The study was supported by research grants from the Kentucky Department of Health and Human Services and the Telford Foundation.
SOURCE: Van Nagell JR Jr et al. Obstet Gynecol. 2018 Nov;132[5]:1091-100. doi: 10.1097/AOG.0000000000002921.
FROM OBSTETRICS & GYNECOLOGY
Key clinical point: Annual ultrasound may improve outcomes for women at risk for epithelial ovarian cancer.
Major finding: Five-year survival was 86% for screened women vs. 45% for unscreened women (P less than .001).
Study details: Analysis of 46,101 at-risk women enrolled in the University of Kentucky Ovarian Cancer Screening Trial, a prospective cohort trial, from January 1987 to June 2017.
Disclosures: Dr. van Nagell and coauthors reported having no financial relationships. The study was supported by research grants from the Kentucky Department of Health and Human Services and the Telford Foundation.
Source: Van Nagell JR Jr et al. Obstet Gynecol. 2018 Nov;132[5]:1091-100. doi: 10.1097/AOG.0000000000002921.
Diagnosis is an ongoing concern in endometriosis
according to a new survey by Health Union, a family of online health communities.
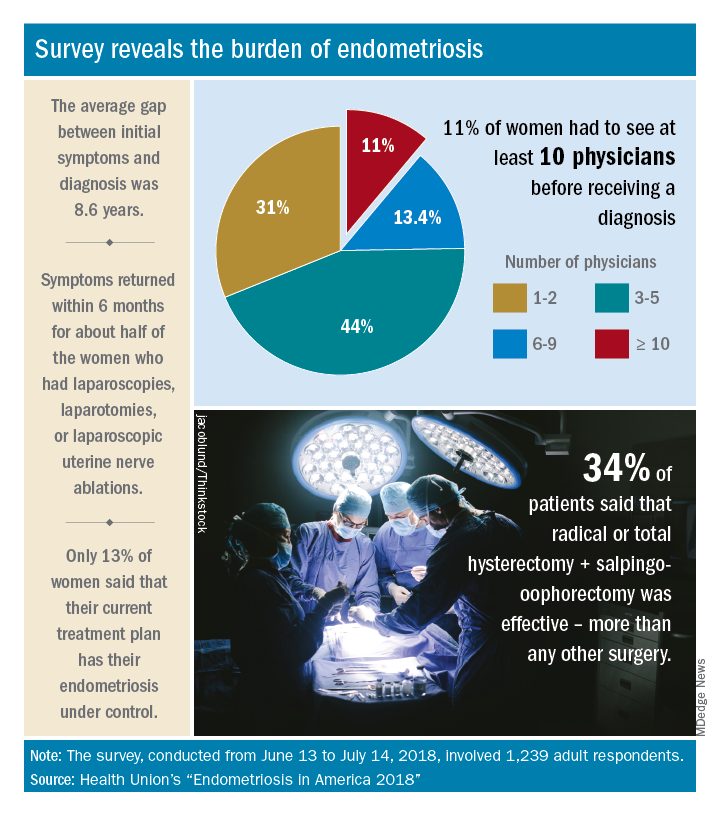
Advances in support and understanding have been made through research and dissemination of information via the Internet, but complete control of endometriosis remains elusive, as only 13% of the 1,239 women surveyed from June 13 to July 14, 2018, said that their condition was under control with their current treatment plan.
Before control, of course, comes diagnosis, and the average gap between onset of symptoms and diagnosis was 8.6 years. Such a gap “can lead to delayed treatment and a potentially negative impact on quality of life,” Health Union said in a written statement. Those years of delays often involved visits to multiple physicians: 44% of respondents saw 3-5 physicians before receiving a diagnosis and 11% had to see 10 or more physicians.
“When comparing differences between symptom onset-to-diagnosis groups, there are some significant findings that suggest a fair amount of progress has been made, for the better,” Health Union said, noting that women who received a diagnosis in less than 5 years “were significantly less likely to think their symptoms were related to their menstrual cycles than those with a longer symptoms-to-diagnosis gap.” Respondents who had a gap of less than 2 years “were more likely to seek medical care as soon as possible” and to have used hormone therapies than those with longer gaps, the group said.
The most common diagnostic tests were laparoscopy, reported by 85% of respondents, and pelvic/transvaginal ultrasound, reported by 46%. Of the women who did not have a laparoscopy, 43% were undergoing a surgical procedure for another condition when their endometriosis was discovered. Laparoscopy also was by far the most common surgery to treat endometriosis, with a 79% prevalence among respondents, compared with 16% for laparotomy and 12% for oophorectomy, Health Union reported in Endometriosis in America 2018.
Common nonsurgical tactics to improve symptoms included increased water intake (79%), use of a heating pad (75%), and increased fresh fruit (64%) or green vegetables (62%) in the diet. Three-quarters of respondents also tried alternative and complementary therapies such as vitamins, exercise, and acupuncture, the report showed.
“Living with endometriosis is much easier now than it was not even a decade ago, as the Internet and social media have definitely increased knowledge about the disease,” said Endometriosis.net (one of the Health Union online communities) patient advocate Laura Kiesel. “When I first suspected I had the disease, in the mid-90s, hardly anyone had heard about it, and those aware of it didn’t think it was very serious. All these years later, I get a lot more sympathy and support – both online and in person – and people understand how serious, painful, and life altering it could be.”
according to a new survey by Health Union, a family of online health communities.

Advances in support and understanding have been made through research and dissemination of information via the Internet, but complete control of endometriosis remains elusive, as only 13% of the 1,239 women surveyed from June 13 to July 14, 2018, said that their condition was under control with their current treatment plan.
Before control, of course, comes diagnosis, and the average gap between onset of symptoms and diagnosis was 8.6 years. Such a gap “can lead to delayed treatment and a potentially negative impact on quality of life,” Health Union said in a written statement. Those years of delays often involved visits to multiple physicians: 44% of respondents saw 3-5 physicians before receiving a diagnosis and 11% had to see 10 or more physicians.
“When comparing differences between symptom onset-to-diagnosis groups, there are some significant findings that suggest a fair amount of progress has been made, for the better,” Health Union said, noting that women who received a diagnosis in less than 5 years “were significantly less likely to think their symptoms were related to their menstrual cycles than those with a longer symptoms-to-diagnosis gap.” Respondents who had a gap of less than 2 years “were more likely to seek medical care as soon as possible” and to have used hormone therapies than those with longer gaps, the group said.
The most common diagnostic tests were laparoscopy, reported by 85% of respondents, and pelvic/transvaginal ultrasound, reported by 46%. Of the women who did not have a laparoscopy, 43% were undergoing a surgical procedure for another condition when their endometriosis was discovered. Laparoscopy also was by far the most common surgery to treat endometriosis, with a 79% prevalence among respondents, compared with 16% for laparotomy and 12% for oophorectomy, Health Union reported in Endometriosis in America 2018.
Common nonsurgical tactics to improve symptoms included increased water intake (79%), use of a heating pad (75%), and increased fresh fruit (64%) or green vegetables (62%) in the diet. Three-quarters of respondents also tried alternative and complementary therapies such as vitamins, exercise, and acupuncture, the report showed.
“Living with endometriosis is much easier now than it was not even a decade ago, as the Internet and social media have definitely increased knowledge about the disease,” said Endometriosis.net (one of the Health Union online communities) patient advocate Laura Kiesel. “When I first suspected I had the disease, in the mid-90s, hardly anyone had heard about it, and those aware of it didn’t think it was very serious. All these years later, I get a lot more sympathy and support – both online and in person – and people understand how serious, painful, and life altering it could be.”
according to a new survey by Health Union, a family of online health communities.

Advances in support and understanding have been made through research and dissemination of information via the Internet, but complete control of endometriosis remains elusive, as only 13% of the 1,239 women surveyed from June 13 to July 14, 2018, said that their condition was under control with their current treatment plan.
Before control, of course, comes diagnosis, and the average gap between onset of symptoms and diagnosis was 8.6 years. Such a gap “can lead to delayed treatment and a potentially negative impact on quality of life,” Health Union said in a written statement. Those years of delays often involved visits to multiple physicians: 44% of respondents saw 3-5 physicians before receiving a diagnosis and 11% had to see 10 or more physicians.
“When comparing differences between symptom onset-to-diagnosis groups, there are some significant findings that suggest a fair amount of progress has been made, for the better,” Health Union said, noting that women who received a diagnosis in less than 5 years “were significantly less likely to think their symptoms were related to their menstrual cycles than those with a longer symptoms-to-diagnosis gap.” Respondents who had a gap of less than 2 years “were more likely to seek medical care as soon as possible” and to have used hormone therapies than those with longer gaps, the group said.
The most common diagnostic tests were laparoscopy, reported by 85% of respondents, and pelvic/transvaginal ultrasound, reported by 46%. Of the women who did not have a laparoscopy, 43% were undergoing a surgical procedure for another condition when their endometriosis was discovered. Laparoscopy also was by far the most common surgery to treat endometriosis, with a 79% prevalence among respondents, compared with 16% for laparotomy and 12% for oophorectomy, Health Union reported in Endometriosis in America 2018.
Common nonsurgical tactics to improve symptoms included increased water intake (79%), use of a heating pad (75%), and increased fresh fruit (64%) or green vegetables (62%) in the diet. Three-quarters of respondents also tried alternative and complementary therapies such as vitamins, exercise, and acupuncture, the report showed.
“Living with endometriosis is much easier now than it was not even a decade ago, as the Internet and social media have definitely increased knowledge about the disease,” said Endometriosis.net (one of the Health Union online communities) patient advocate Laura Kiesel. “When I first suspected I had the disease, in the mid-90s, hardly anyone had heard about it, and those aware of it didn’t think it was very serious. All these years later, I get a lot more sympathy and support – both online and in person – and people understand how serious, painful, and life altering it could be.”
Aspirin cuts risk of ovarian and liver cancer
Regular long-term aspirin use may lower the risk of hepatocellular carcinoma (HCC) and ovarian cancer, adding to the growing evidence that aspirin may play a role as a chemopreventive agent, according to two new studies published in JAMA Oncology.
In the first study, led by Tracey G. Simon, MD, of Massachusetts General Hospital, Boston, the authors evaluated the associations between aspirin dose and duration of use and the risk of developing HCC. They conducted a population-based study, with a pooled analysis of two large prospective U.S. cohort studies: the Nurses’ Health Study and the Health Professionals Follow-up Study. The cohort included a total of 133,371 health care professionals who reported long-term data on aspirin use, frequency, dosage, and duration of use.
For the 87,507 female participants, reporting began in 1980, and for the 45,864 men, reporting began in 1986. The mean age for women was 62 years and was 64 years for men at the midpoint of follow-up (1996). Compared with nonaspirin users, those who used aspirin regularly tended to be older, former smokers, and regularly used statins and multivitamins. During the follow-up period, which was more than 26 years, there were 108 incident cases of HCC (65 women, 43 men; 47 with noncirrhotic HCC).
The investigators found that regular aspirin use was associated with a significantly lower HCC risk versus nonregular use (multivariable hazard ratio, 0.51; 95% confidence interval, 0.34-0.77), and estimates were similar for both sexes. Adjustments for regular NSAID use (for example, at least two tablets per week) did not change the data, and results were similar after further adjustment for coffee consumption and adherence to a healthy diet. The benefit also appeared to be dose related, as compared with nonuse, the multivariable-adjusted HR for HCC was 0.87 (95% CI, 0.51-1.48) for up to 1.5 tablets of standard-dose aspirin per week and 0.51 (95% CI, 0.30-0.86) for 1.5-5 tablets per week. The most benefit was for at least five tablets per week (HR, 0.49; 95% CI, 0.28-0.96; P = .006).
“Our findings add to the growing literature suggesting that the chemopreventive effects of aspirin may extend beyond colorectal cancer,” they wrote.
In the second study, Mollie E. Barnard, ScD, of the Harvard School of Public Health, Boston, and her colleagues looked at whether regular aspirin or NSAID use, as well as the patterns of use, were associated with a lower risk of ovarian cancer.
The data used were obtained from 93,664 women in the Nurses’ Health Study (NHS), who were followed up from 1980 to 2014, and 111,834 people in the Nurses’ Health Study II (NHSII), who were followed up from 1989 to 2015. For each type of agent, including aspirin, low-dose aspirin, nonaspirin NSAIDs, and acetaminophen, they evaluated the timing, duration, frequency, and number of tablets that were used. The mean age of participants in the NHS at baseline was 45.9 years and 34.2 years in the NHSII.
There were 1,054 incident cases of epithelial ovarian cancer identified during the study period. The authors did not detect any significant associations between aspirin and ovarian cancer risk when current users and nonusers were compared, regardless of dose (HR, 0.99; 95% CI, 0.83-1.19). But when low-dose (less than or equal to 100 mg) and standard-dose (325 mg) aspirin were analyzed separately, an inverse association for low-dose aspirin (HR, 0.77; 95% CI, 0.61-0.96) was observed. However, there was no association for standard-dose aspirin (HR, 1.17; 95% CI, 0.92-1.49).
In contrast, use of nonaspirin NSAIDs was positively associated with a higher risk of ovarian cancer when compared with nonuse (HR, 1.19; 95% CI, 1.00-1.41), and there were significant positive trends for duration of use (P = .02) and cumulative average tablets per week (P = .03). No clear associations were identified for acetaminophen use.
“Our results also suggest an increased risk of ovarian cancer among long-term, high-quantity users of nonaspirin analgesics, although this finding may reflect unmeasured confounding,” wrote Dr. Barnard and her coauthors. “Further exploration is warranted to evaluate the mechanisms by which heavy use of aspirin, nonaspirin NSAIDs, and acetaminophen may contribute to the development of ovarian cancer and to replicate our findings.”
The ovarian cancer study was supported by awards from the National Institutes of Health. Dr. Barnard was supported by awards from the National Cancer Institute, and her coauthors had no disclosures to report. The HCC study was funded by an infrastructure grant from the Nurses’ Health Study, an infrastructure grant from the Health Professionals Follow-up Study, and NIH grants to several of the authors. Dr. Chan has previously served as a consultant for Bayer on work unrelated to this article. No other disclosures were reported.
SOURCES: Barnard ME et al. JAMA Oncol. 2018 Oct 4. doi: 10.1001/jamaoncol.2018.4149; Simon TG et al. JAMA Oncol. 2018 Oct 4. doi: 10.1001/jamaoncol.2018.4154.
In an accompanying editorial published in JAMA Oncology, Victoria L. Seewaldt, MD, of the City of Hope Comprehensive Cancer Center in Duarte, Calif., asked if we “have arrived,” as these two studies are a critical step in realizing the potential of aspirin for cancer chemoprevention beyond colorectal cancer.
Aspirin use is very common in the United States, with almost half of adults aged between 45 and 75 years taking it regularly. Many regular users also believe that aspirin has potential to protect against cancer, and in a 2015 study – which was conducted prior to any formal cancer prevention guidelines – 18% of those taking aspirin on a regular basis reported doing so to prevent cancer.
Based on the strength of the association between aspirin use and colorectal cancer risk reduction, the U.S. Preventive Services Task Force recommended in 2015 that, among individuals aged between 50 and 69 years who have specific cardiovascular risk profiles, colorectal cancer prevention be included as part of the rationale for regular aspirin prophylaxis, Dr. Seewaldt noted. Aspirin became the first drug to be included in USPSTF recommendations for cancer chemoprevention in a “population not characterized as having a high risk of developing cancer.”
But it now appears aspirin may be able to go beyond colorectal cancer for chemoprevention. Ovarian cancer and hepatocellular carcinoma are in need of new prevention strategies and these findings provide important information that can help guide chemoprevention with aspirin.
These two studies “have the power to start to change clinical practice,” Dr. Seewaldt wrote, but more research is needed to better understand the underlying mechanism behind the appropriate dose and duration of use. Importantly, the authors of both studies cautioned that the potential benefits of aspirin must be weighed against the risk of bleeding, which is particularly important in patients with chronic liver disease.
“To reach the full promise of aspirin’s ability to prevent cancer, there needs to be better understanding of dose, duration, and mechanism,” she emphasized.
Dr. Seewaldt reported receiving grants from the National Institutes of Health/National Cancer Institute and is supported by the Prevent Cancer Foundation.
In an accompanying editorial published in JAMA Oncology, Victoria L. Seewaldt, MD, of the City of Hope Comprehensive Cancer Center in Duarte, Calif., asked if we “have arrived,” as these two studies are a critical step in realizing the potential of aspirin for cancer chemoprevention beyond colorectal cancer.
Aspirin use is very common in the United States, with almost half of adults aged between 45 and 75 years taking it regularly. Many regular users also believe that aspirin has potential to protect against cancer, and in a 2015 study – which was conducted prior to any formal cancer prevention guidelines – 18% of those taking aspirin on a regular basis reported doing so to prevent cancer.
Based on the strength of the association between aspirin use and colorectal cancer risk reduction, the U.S. Preventive Services Task Force recommended in 2015 that, among individuals aged between 50 and 69 years who have specific cardiovascular risk profiles, colorectal cancer prevention be included as part of the rationale for regular aspirin prophylaxis, Dr. Seewaldt noted. Aspirin became the first drug to be included in USPSTF recommendations for cancer chemoprevention in a “population not characterized as having a high risk of developing cancer.”
But it now appears aspirin may be able to go beyond colorectal cancer for chemoprevention. Ovarian cancer and hepatocellular carcinoma are in need of new prevention strategies and these findings provide important information that can help guide chemoprevention with aspirin.
These two studies “have the power to start to change clinical practice,” Dr. Seewaldt wrote, but more research is needed to better understand the underlying mechanism behind the appropriate dose and duration of use. Importantly, the authors of both studies cautioned that the potential benefits of aspirin must be weighed against the risk of bleeding, which is particularly important in patients with chronic liver disease.
“To reach the full promise of aspirin’s ability to prevent cancer, there needs to be better understanding of dose, duration, and mechanism,” she emphasized.
Dr. Seewaldt reported receiving grants from the National Institutes of Health/National Cancer Institute and is supported by the Prevent Cancer Foundation.
In an accompanying editorial published in JAMA Oncology, Victoria L. Seewaldt, MD, of the City of Hope Comprehensive Cancer Center in Duarte, Calif., asked if we “have arrived,” as these two studies are a critical step in realizing the potential of aspirin for cancer chemoprevention beyond colorectal cancer.
Aspirin use is very common in the United States, with almost half of adults aged between 45 and 75 years taking it regularly. Many regular users also believe that aspirin has potential to protect against cancer, and in a 2015 study – which was conducted prior to any formal cancer prevention guidelines – 18% of those taking aspirin on a regular basis reported doing so to prevent cancer.
Based on the strength of the association between aspirin use and colorectal cancer risk reduction, the U.S. Preventive Services Task Force recommended in 2015 that, among individuals aged between 50 and 69 years who have specific cardiovascular risk profiles, colorectal cancer prevention be included as part of the rationale for regular aspirin prophylaxis, Dr. Seewaldt noted. Aspirin became the first drug to be included in USPSTF recommendations for cancer chemoprevention in a “population not characterized as having a high risk of developing cancer.”
But it now appears aspirin may be able to go beyond colorectal cancer for chemoprevention. Ovarian cancer and hepatocellular carcinoma are in need of new prevention strategies and these findings provide important information that can help guide chemoprevention with aspirin.
These two studies “have the power to start to change clinical practice,” Dr. Seewaldt wrote, but more research is needed to better understand the underlying mechanism behind the appropriate dose and duration of use. Importantly, the authors of both studies cautioned that the potential benefits of aspirin must be weighed against the risk of bleeding, which is particularly important in patients with chronic liver disease.
“To reach the full promise of aspirin’s ability to prevent cancer, there needs to be better understanding of dose, duration, and mechanism,” she emphasized.
Dr. Seewaldt reported receiving grants from the National Institutes of Health/National Cancer Institute and is supported by the Prevent Cancer Foundation.
Regular long-term aspirin use may lower the risk of hepatocellular carcinoma (HCC) and ovarian cancer, adding to the growing evidence that aspirin may play a role as a chemopreventive agent, according to two new studies published in JAMA Oncology.
In the first study, led by Tracey G. Simon, MD, of Massachusetts General Hospital, Boston, the authors evaluated the associations between aspirin dose and duration of use and the risk of developing HCC. They conducted a population-based study, with a pooled analysis of two large prospective U.S. cohort studies: the Nurses’ Health Study and the Health Professionals Follow-up Study. The cohort included a total of 133,371 health care professionals who reported long-term data on aspirin use, frequency, dosage, and duration of use.
For the 87,507 female participants, reporting began in 1980, and for the 45,864 men, reporting began in 1986. The mean age for women was 62 years and was 64 years for men at the midpoint of follow-up (1996). Compared with nonaspirin users, those who used aspirin regularly tended to be older, former smokers, and regularly used statins and multivitamins. During the follow-up period, which was more than 26 years, there were 108 incident cases of HCC (65 women, 43 men; 47 with noncirrhotic HCC).
The investigators found that regular aspirin use was associated with a significantly lower HCC risk versus nonregular use (multivariable hazard ratio, 0.51; 95% confidence interval, 0.34-0.77), and estimates were similar for both sexes. Adjustments for regular NSAID use (for example, at least two tablets per week) did not change the data, and results were similar after further adjustment for coffee consumption and adherence to a healthy diet. The benefit also appeared to be dose related, as compared with nonuse, the multivariable-adjusted HR for HCC was 0.87 (95% CI, 0.51-1.48) for up to 1.5 tablets of standard-dose aspirin per week and 0.51 (95% CI, 0.30-0.86) for 1.5-5 tablets per week. The most benefit was for at least five tablets per week (HR, 0.49; 95% CI, 0.28-0.96; P = .006).
“Our findings add to the growing literature suggesting that the chemopreventive effects of aspirin may extend beyond colorectal cancer,” they wrote.
In the second study, Mollie E. Barnard, ScD, of the Harvard School of Public Health, Boston, and her colleagues looked at whether regular aspirin or NSAID use, as well as the patterns of use, were associated with a lower risk of ovarian cancer.
The data used were obtained from 93,664 women in the Nurses’ Health Study (NHS), who were followed up from 1980 to 2014, and 111,834 people in the Nurses’ Health Study II (NHSII), who were followed up from 1989 to 2015. For each type of agent, including aspirin, low-dose aspirin, nonaspirin NSAIDs, and acetaminophen, they evaluated the timing, duration, frequency, and number of tablets that were used. The mean age of participants in the NHS at baseline was 45.9 years and 34.2 years in the NHSII.
There were 1,054 incident cases of epithelial ovarian cancer identified during the study period. The authors did not detect any significant associations between aspirin and ovarian cancer risk when current users and nonusers were compared, regardless of dose (HR, 0.99; 95% CI, 0.83-1.19). But when low-dose (less than or equal to 100 mg) and standard-dose (325 mg) aspirin were analyzed separately, an inverse association for low-dose aspirin (HR, 0.77; 95% CI, 0.61-0.96) was observed. However, there was no association for standard-dose aspirin (HR, 1.17; 95% CI, 0.92-1.49).
In contrast, use of nonaspirin NSAIDs was positively associated with a higher risk of ovarian cancer when compared with nonuse (HR, 1.19; 95% CI, 1.00-1.41), and there were significant positive trends for duration of use (P = .02) and cumulative average tablets per week (P = .03). No clear associations were identified for acetaminophen use.
“Our results also suggest an increased risk of ovarian cancer among long-term, high-quantity users of nonaspirin analgesics, although this finding may reflect unmeasured confounding,” wrote Dr. Barnard and her coauthors. “Further exploration is warranted to evaluate the mechanisms by which heavy use of aspirin, nonaspirin NSAIDs, and acetaminophen may contribute to the development of ovarian cancer and to replicate our findings.”
The ovarian cancer study was supported by awards from the National Institutes of Health. Dr. Barnard was supported by awards from the National Cancer Institute, and her coauthors had no disclosures to report. The HCC study was funded by an infrastructure grant from the Nurses’ Health Study, an infrastructure grant from the Health Professionals Follow-up Study, and NIH grants to several of the authors. Dr. Chan has previously served as a consultant for Bayer on work unrelated to this article. No other disclosures were reported.
SOURCES: Barnard ME et al. JAMA Oncol. 2018 Oct 4. doi: 10.1001/jamaoncol.2018.4149; Simon TG et al. JAMA Oncol. 2018 Oct 4. doi: 10.1001/jamaoncol.2018.4154.
Regular long-term aspirin use may lower the risk of hepatocellular carcinoma (HCC) and ovarian cancer, adding to the growing evidence that aspirin may play a role as a chemopreventive agent, according to two new studies published in JAMA Oncology.
In the first study, led by Tracey G. Simon, MD, of Massachusetts General Hospital, Boston, the authors evaluated the associations between aspirin dose and duration of use and the risk of developing HCC. They conducted a population-based study, with a pooled analysis of two large prospective U.S. cohort studies: the Nurses’ Health Study and the Health Professionals Follow-up Study. The cohort included a total of 133,371 health care professionals who reported long-term data on aspirin use, frequency, dosage, and duration of use.
For the 87,507 female participants, reporting began in 1980, and for the 45,864 men, reporting began in 1986. The mean age for women was 62 years and was 64 years for men at the midpoint of follow-up (1996). Compared with nonaspirin users, those who used aspirin regularly tended to be older, former smokers, and regularly used statins and multivitamins. During the follow-up period, which was more than 26 years, there were 108 incident cases of HCC (65 women, 43 men; 47 with noncirrhotic HCC).
The investigators found that regular aspirin use was associated with a significantly lower HCC risk versus nonregular use (multivariable hazard ratio, 0.51; 95% confidence interval, 0.34-0.77), and estimates were similar for both sexes. Adjustments for regular NSAID use (for example, at least two tablets per week) did not change the data, and results were similar after further adjustment for coffee consumption and adherence to a healthy diet. The benefit also appeared to be dose related, as compared with nonuse, the multivariable-adjusted HR for HCC was 0.87 (95% CI, 0.51-1.48) for up to 1.5 tablets of standard-dose aspirin per week and 0.51 (95% CI, 0.30-0.86) for 1.5-5 tablets per week. The most benefit was for at least five tablets per week (HR, 0.49; 95% CI, 0.28-0.96; P = .006).
“Our findings add to the growing literature suggesting that the chemopreventive effects of aspirin may extend beyond colorectal cancer,” they wrote.
In the second study, Mollie E. Barnard, ScD, of the Harvard School of Public Health, Boston, and her colleagues looked at whether regular aspirin or NSAID use, as well as the patterns of use, were associated with a lower risk of ovarian cancer.
The data used were obtained from 93,664 women in the Nurses’ Health Study (NHS), who were followed up from 1980 to 2014, and 111,834 people in the Nurses’ Health Study II (NHSII), who were followed up from 1989 to 2015. For each type of agent, including aspirin, low-dose aspirin, nonaspirin NSAIDs, and acetaminophen, they evaluated the timing, duration, frequency, and number of tablets that were used. The mean age of participants in the NHS at baseline was 45.9 years and 34.2 years in the NHSII.
There were 1,054 incident cases of epithelial ovarian cancer identified during the study period. The authors did not detect any significant associations between aspirin and ovarian cancer risk when current users and nonusers were compared, regardless of dose (HR, 0.99; 95% CI, 0.83-1.19). But when low-dose (less than or equal to 100 mg) and standard-dose (325 mg) aspirin were analyzed separately, an inverse association for low-dose aspirin (HR, 0.77; 95% CI, 0.61-0.96) was observed. However, there was no association for standard-dose aspirin (HR, 1.17; 95% CI, 0.92-1.49).
In contrast, use of nonaspirin NSAIDs was positively associated with a higher risk of ovarian cancer when compared with nonuse (HR, 1.19; 95% CI, 1.00-1.41), and there were significant positive trends for duration of use (P = .02) and cumulative average tablets per week (P = .03). No clear associations were identified for acetaminophen use.
“Our results also suggest an increased risk of ovarian cancer among long-term, high-quantity users of nonaspirin analgesics, although this finding may reflect unmeasured confounding,” wrote Dr. Barnard and her coauthors. “Further exploration is warranted to evaluate the mechanisms by which heavy use of aspirin, nonaspirin NSAIDs, and acetaminophen may contribute to the development of ovarian cancer and to replicate our findings.”
The ovarian cancer study was supported by awards from the National Institutes of Health. Dr. Barnard was supported by awards from the National Cancer Institute, and her coauthors had no disclosures to report. The HCC study was funded by an infrastructure grant from the Nurses’ Health Study, an infrastructure grant from the Health Professionals Follow-up Study, and NIH grants to several of the authors. Dr. Chan has previously served as a consultant for Bayer on work unrelated to this article. No other disclosures were reported.
SOURCES: Barnard ME et al. JAMA Oncol. 2018 Oct 4. doi: 10.1001/jamaoncol.2018.4149; Simon TG et al. JAMA Oncol. 2018 Oct 4. doi: 10.1001/jamaoncol.2018.4154.
FROM JAMA ONCOLOGY
Key clinical point: Regular aspirin use was associated with a decreased risk of ovarian cancer and hepatocellular carcinoma.
Major finding: Low-dose aspirin was associated with a 23% lower risk of ovarian cancer and a 49% reduced risk of developing hepatocellular carcinoma.
Study details: The hepatocellular carcinoma study was a population-based study of two nationwide, prospective cohorts of 87,507 men and 45,864 women; the ovarian cancer study was a cohort study using data from two prospective cohorts, with 93,664 people in one and 111,834 in the other.
Disclosures: The ovarian cancer study was supported by awards from the National Institutes of Health. Dr. Barnard was supported by awards from the National Cancer Institute, and her coauthors had no disclosures to report. The hepatocellular carcinoma study was funded by an infrastructure grant from the Nurses’ Health Study, an infrastructure grant from the Health Professionals Follow-up Study, and NIH grants to several of the authors. Dr. Chan has previously served as a consultant for Bayer on work unrelated to this article. No other disclosures were reported.
Sources: Barnard ME et al. JAMA Oncol. 2018 Oct 4. doi: 10.1001/jamaoncol.2018.4149; Simon TG et al. JAMA Oncol. 2018 Oct 4. doi: 10.1001/jamaoncol.2018.4154.
Higher rate of loss seen in unplanned pregnancies for women with epilepsy
when compared against women with epilepsy who planned their pregnancy, according to recent results from a retrospective study published in JAMA Neurology.
“This analysis adds the finding that unplanned pregnancy may increase the risk of [spontaneous fetal loss] in women with epilepsy and identifies pregnancy planning, maternal age, and interpregnancy interval as significant modifiable variables,” Andrew G. Herzog, MD, of the Harvard Neuroendocrine Unit at Beth Israel Deaconess Medical Center in Wellesley, Mass., and colleagues wrote in their study.
The researchers examined results from a web-based survey completed by 1,144 women in the Epilepsy Birth Control Registry (EBCR) between 2010 and 2014 with data on contraception use, pregnancy history, and antiepileptic drug (AED) treatment. Patients were aged 18-47 years (mean 28.5 years) with 8.7% of the cohort consisting of minority women and 39.8% having household incomes of $25,000 or less.
Pregnancy history data included number of pregnancies, number of planned or unplanned pregnancies, AED type used during pregnancies, pregnancy outcomes such as live birth, induced abortion, and spontaneous fetal loss (SFL), while AED data included categorizing patients into no therapy, monotherapy, and polytherapy groups. AED use was further subdivided into no AED, enzyme-inducing AED, non–enzyme-inducing AED, enzyme-inhibiting AED, glucuronidated AED, and mixed category groups.
Of 794 pregnancies, 530 pregnancies (66.8%) were unplanned and 264 (33.2%) were planned, with 473 live births (59.6%), 141 induced abortions (17.8%), and 180 SFL (22.7%). Among patients who did not have an induced abortion, SFL risk was higher if the pregnancy was unplanned (137 patients, 35.0%), compared with those who planned (43 patients, 16.4%) their pregnancy (risk ratio = 2.14; 95% confidence interval, 1.59-2.90; P less than .001). According to a regression analysis, SFL risk was higher for patients where “planning was entered alone” in unplanned pregnancies (odds ratio = 2.75; 95% CI, 1.87-4.05; P less than .001) as well as when adjusted for AED category, maternal age, and interpregnancy interval (OR = 3.57; 95% CI, 1.54-8.78; P = .003).
There was an association between maternal age (OR = 0.957; 95% CI, 0.928-0.986; P = .02) and risk of SFL, with lower risk seen in the 18- to 27-year-old group (118 patients, 29.5%; RR = 0.57; 95% CI, 0.39-0.84; P less than .004) and 28- to 37-year-old group (44 patients, 20.8%; RR = 0.40; 95% CI, 0.26-0.62; P less than .001), compared with the under-18 group (15 patients, 51.7%). There was also a higher risk of SFL with regard to interpregnancy interval (OR = 2.878; 95% CI, 1.8094-4.5801; P = .008), with a greater risk seen if the interpregnancy interval was under 1 year (56 patients, 45.9%), compared with 1 year (56 patients, 22.8%) or higher (RR = 2.02; 95% CI, 1.49-2.72; P less than .001).
“In view of the finding of increased risk for SFL in unplanned pregnancies in women with epilepsy, and because a history of SFL in women with epilepsy may increase the risk that subsequent live-born offspring will develop epilepsy, the finding warrants prospective investigation with medical record verification of pregnancy outcomes,” Dr. Herzog and his colleagues wrote.
The Epilepsy Foundation and Lundbeck funded the study. Dr. Herzog reports support by grants, and two coauthors received salary support from grants, from the two organizations.
SOURCE: Herzog AG et al. JAMA Neurol. 2018 Oct 15. doi: 10.1001/jamaneurol.2018.3089.
when compared against women with epilepsy who planned their pregnancy, according to recent results from a retrospective study published in JAMA Neurology.
“This analysis adds the finding that unplanned pregnancy may increase the risk of [spontaneous fetal loss] in women with epilepsy and identifies pregnancy planning, maternal age, and interpregnancy interval as significant modifiable variables,” Andrew G. Herzog, MD, of the Harvard Neuroendocrine Unit at Beth Israel Deaconess Medical Center in Wellesley, Mass., and colleagues wrote in their study.
The researchers examined results from a web-based survey completed by 1,144 women in the Epilepsy Birth Control Registry (EBCR) between 2010 and 2014 with data on contraception use, pregnancy history, and antiepileptic drug (AED) treatment. Patients were aged 18-47 years (mean 28.5 years) with 8.7% of the cohort consisting of minority women and 39.8% having household incomes of $25,000 or less.
Pregnancy history data included number of pregnancies, number of planned or unplanned pregnancies, AED type used during pregnancies, pregnancy outcomes such as live birth, induced abortion, and spontaneous fetal loss (SFL), while AED data included categorizing patients into no therapy, monotherapy, and polytherapy groups. AED use was further subdivided into no AED, enzyme-inducing AED, non–enzyme-inducing AED, enzyme-inhibiting AED, glucuronidated AED, and mixed category groups.
Of 794 pregnancies, 530 pregnancies (66.8%) were unplanned and 264 (33.2%) were planned, with 473 live births (59.6%), 141 induced abortions (17.8%), and 180 SFL (22.7%). Among patients who did not have an induced abortion, SFL risk was higher if the pregnancy was unplanned (137 patients, 35.0%), compared with those who planned (43 patients, 16.4%) their pregnancy (risk ratio = 2.14; 95% confidence interval, 1.59-2.90; P less than .001). According to a regression analysis, SFL risk was higher for patients where “planning was entered alone” in unplanned pregnancies (odds ratio = 2.75; 95% CI, 1.87-4.05; P less than .001) as well as when adjusted for AED category, maternal age, and interpregnancy interval (OR = 3.57; 95% CI, 1.54-8.78; P = .003).
There was an association between maternal age (OR = 0.957; 95% CI, 0.928-0.986; P = .02) and risk of SFL, with lower risk seen in the 18- to 27-year-old group (118 patients, 29.5%; RR = 0.57; 95% CI, 0.39-0.84; P less than .004) and 28- to 37-year-old group (44 patients, 20.8%; RR = 0.40; 95% CI, 0.26-0.62; P less than .001), compared with the under-18 group (15 patients, 51.7%). There was also a higher risk of SFL with regard to interpregnancy interval (OR = 2.878; 95% CI, 1.8094-4.5801; P = .008), with a greater risk seen if the interpregnancy interval was under 1 year (56 patients, 45.9%), compared with 1 year (56 patients, 22.8%) or higher (RR = 2.02; 95% CI, 1.49-2.72; P less than .001).
“In view of the finding of increased risk for SFL in unplanned pregnancies in women with epilepsy, and because a history of SFL in women with epilepsy may increase the risk that subsequent live-born offspring will develop epilepsy, the finding warrants prospective investigation with medical record verification of pregnancy outcomes,” Dr. Herzog and his colleagues wrote.
The Epilepsy Foundation and Lundbeck funded the study. Dr. Herzog reports support by grants, and two coauthors received salary support from grants, from the two organizations.
SOURCE: Herzog AG et al. JAMA Neurol. 2018 Oct 15. doi: 10.1001/jamaneurol.2018.3089.
when compared against women with epilepsy who planned their pregnancy, according to recent results from a retrospective study published in JAMA Neurology.
“This analysis adds the finding that unplanned pregnancy may increase the risk of [spontaneous fetal loss] in women with epilepsy and identifies pregnancy planning, maternal age, and interpregnancy interval as significant modifiable variables,” Andrew G. Herzog, MD, of the Harvard Neuroendocrine Unit at Beth Israel Deaconess Medical Center in Wellesley, Mass., and colleagues wrote in their study.
The researchers examined results from a web-based survey completed by 1,144 women in the Epilepsy Birth Control Registry (EBCR) between 2010 and 2014 with data on contraception use, pregnancy history, and antiepileptic drug (AED) treatment. Patients were aged 18-47 years (mean 28.5 years) with 8.7% of the cohort consisting of minority women and 39.8% having household incomes of $25,000 or less.
Pregnancy history data included number of pregnancies, number of planned or unplanned pregnancies, AED type used during pregnancies, pregnancy outcomes such as live birth, induced abortion, and spontaneous fetal loss (SFL), while AED data included categorizing patients into no therapy, monotherapy, and polytherapy groups. AED use was further subdivided into no AED, enzyme-inducing AED, non–enzyme-inducing AED, enzyme-inhibiting AED, glucuronidated AED, and mixed category groups.
Of 794 pregnancies, 530 pregnancies (66.8%) were unplanned and 264 (33.2%) were planned, with 473 live births (59.6%), 141 induced abortions (17.8%), and 180 SFL (22.7%). Among patients who did not have an induced abortion, SFL risk was higher if the pregnancy was unplanned (137 patients, 35.0%), compared with those who planned (43 patients, 16.4%) their pregnancy (risk ratio = 2.14; 95% confidence interval, 1.59-2.90; P less than .001). According to a regression analysis, SFL risk was higher for patients where “planning was entered alone” in unplanned pregnancies (odds ratio = 2.75; 95% CI, 1.87-4.05; P less than .001) as well as when adjusted for AED category, maternal age, and interpregnancy interval (OR = 3.57; 95% CI, 1.54-8.78; P = .003).
There was an association between maternal age (OR = 0.957; 95% CI, 0.928-0.986; P = .02) and risk of SFL, with lower risk seen in the 18- to 27-year-old group (118 patients, 29.5%; RR = 0.57; 95% CI, 0.39-0.84; P less than .004) and 28- to 37-year-old group (44 patients, 20.8%; RR = 0.40; 95% CI, 0.26-0.62; P less than .001), compared with the under-18 group (15 patients, 51.7%). There was also a higher risk of SFL with regard to interpregnancy interval (OR = 2.878; 95% CI, 1.8094-4.5801; P = .008), with a greater risk seen if the interpregnancy interval was under 1 year (56 patients, 45.9%), compared with 1 year (56 patients, 22.8%) or higher (RR = 2.02; 95% CI, 1.49-2.72; P less than .001).
“In view of the finding of increased risk for SFL in unplanned pregnancies in women with epilepsy, and because a history of SFL in women with epilepsy may increase the risk that subsequent live-born offspring will develop epilepsy, the finding warrants prospective investigation with medical record verification of pregnancy outcomes,” Dr. Herzog and his colleagues wrote.
The Epilepsy Foundation and Lundbeck funded the study. Dr. Herzog reports support by grants, and two coauthors received salary support from grants, from the two organizations.
SOURCE: Herzog AG et al. JAMA Neurol. 2018 Oct 15. doi: 10.1001/jamaneurol.2018.3089.
FROM JAMA NEUROLOGY
Key clinical point: Women with epilepsy who experience unplanned pregnancies have a higher rate of spontaneous fetal loss, compared with those with epilepsy who plan their pregnancies.
Major finding: Thirty-five percent of women with unplanned pregnancies experienced spontaneous fetal loss, compared with 16.4% of women in the planned pregnancy group.
Study details: A retrospective analysis of results from a web-based survey of 1,144 women from the Epilepsy Birth Control Registry.
Disclosures: The Epilepsy Foundation and Lundbeck funded the study. Dr. Herzog reports support by grants, and two coauthors received salary support from grants, from the two organizations.
Source: Herzog AG et al. JAMA Neurol. 2018 Oct 15. doi: 10.1001/jamaneurol.2018.3089.
Brexanolone injection quickly improves postpartum depression
BARCELONA – Brexanolone injection provided rapid and durable improvement in postpartum depression in an integrated analysis of three pivotal randomized trials collectively known as the Hummingbird trials, Christine Clemson, PhD, reported at the annual congress of the European College of Neuropsychopharmacology.
This was accomplished with a favorable safety experience. The most common treatment-emergent adverse events – dizziness and sleepiness – were roughly twice as common as with placebo in the 247-patient Hummingbird safety analysis.
On Dec. 19, 2018, the agency is expected to respond to SAGE Therapeutics’s application for marketing approval of intravenous brexanolone given as a continuous 60-hour infusion at 90 mcg/kg per hour, according to Dr. Clemson, senior medical director at Cambridge, Mass.–based SAGE Therapeutics, which is developing the therapy.
Brexanolone is a proprietary IV formulation of allopregnanolone, a metabolite of progesterone. The drug’s mechanism of action involves modulation of the neurotransmitter gamma-aminobutyric acid (GABA). The drug binds to both synaptic and extra-synaptic GABA A receptors, thereby increasing receptor functionality.
The decision to target GABA as a novel therapeutic strategy in PPD was based upon translational studies demonstrating that GABA is the chief neuroinhibitory mechanism in the brain, and its actions are mediated mainly by GABA A receptors. Brexanolone’s efficacy is consistent with a theory that the pathogenesis of PPD involves triggers such as inflammation, hormonal fluctuations, or chronic stress, which in some women cause GABA hypofunction, both at the receptors and in terms of tissue GABA levels. This, in turn, leads to an overactive HPA axis and dysregulated neural networks, with resultant PPD, Dr. Clemson explained.
The three Hummingbird clinical trials were double blind, randomized, and placebo controlled. Two were restricted to women with severe PPD. The third and largest focused on moderately affected patients as defined by a baseline Hamilton Depression Scale for Depression (HAM-D) score of 20-25.
The efficacy analysis included 207 patients who received brexanolone at 90 mcg/kg per hour or placebo for 60 hours in an inpatient setting and were followed for 30 days. The primary endpoint was the change in HAM-D total score from baseline to 60 hours. The mean 17-point reduction in the active treatment arm was significantly better than the 12.8-point decrease with placebo. The between-group difference was significant within the first 24 hours and remained so at all time points out to the study’s end at day 30. There was no individual item on the HAM-D in which the drug performed worse than placebo, and there were many in which brexanolone performed significantly better, including depressed mood, anxiety, insomnia, and feelings of guilt.
In terms of the rigorous secondary endpoint of HAM-D remission as defined by a total score of 7 or less, the brexanolone injection significantly outperformed placebo at every time point except for day 30.
There was a 2% rate of serious adverse events in both study arms. These included suicidal ideation, an intentional overdose attempt post discharge, altered state of consciousness, and syncope.
A vastly more convenient once-daily oral formulation of brexanolone is now in phase 3 clinical trials for PPD and major depressive disorder, and in phase 2 for insomnia and bipolar depression.
Elsewhere at the ECNP congress, other investigators from Sage Therapeutics presented for the first time the outcomes of an 89-patient, randomized, double-blind, multicenter, placebo-controlled, phase 2 clinical trial of SAGE-217 for treatment of major depressive disorder.
Participants received a nightly 30-mg dose of the drug or placebo for 2 weeks, with a primary study endpoint being the change in HAM-D total score from baseline to day 15. Patients on the oral GABA A receptor positive modulator averaged a 17.4-point improvement, significantly better than the 10.3-point spread in placebo-treated controls. A statistically significant between-group difference was noted on days 2 through 28. HAM-D remission at day 15 was documented in 64% of the oral brexanolone group, compared with 26% of controls.
Those improvements in depression were accompanied by significant gains in numerous domains of health-related quality of life as assessed via the 36-Item Short Form Health Survey. Indeed, day 15 health-related quality of life scores in the oral brexanolone group approached normative values for the general population.
The studies were funded by SAGE Therapeutics.
BARCELONA – Brexanolone injection provided rapid and durable improvement in postpartum depression in an integrated analysis of three pivotal randomized trials collectively known as the Hummingbird trials, Christine Clemson, PhD, reported at the annual congress of the European College of Neuropsychopharmacology.
This was accomplished with a favorable safety experience. The most common treatment-emergent adverse events – dizziness and sleepiness – were roughly twice as common as with placebo in the 247-patient Hummingbird safety analysis.
On Dec. 19, 2018, the agency is expected to respond to SAGE Therapeutics’s application for marketing approval of intravenous brexanolone given as a continuous 60-hour infusion at 90 mcg/kg per hour, according to Dr. Clemson, senior medical director at Cambridge, Mass.–based SAGE Therapeutics, which is developing the therapy.
Brexanolone is a proprietary IV formulation of allopregnanolone, a metabolite of progesterone. The drug’s mechanism of action involves modulation of the neurotransmitter gamma-aminobutyric acid (GABA). The drug binds to both synaptic and extra-synaptic GABA A receptors, thereby increasing receptor functionality.
The decision to target GABA as a novel therapeutic strategy in PPD was based upon translational studies demonstrating that GABA is the chief neuroinhibitory mechanism in the brain, and its actions are mediated mainly by GABA A receptors. Brexanolone’s efficacy is consistent with a theory that the pathogenesis of PPD involves triggers such as inflammation, hormonal fluctuations, or chronic stress, which in some women cause GABA hypofunction, both at the receptors and in terms of tissue GABA levels. This, in turn, leads to an overactive HPA axis and dysregulated neural networks, with resultant PPD, Dr. Clemson explained.
The three Hummingbird clinical trials were double blind, randomized, and placebo controlled. Two were restricted to women with severe PPD. The third and largest focused on moderately affected patients as defined by a baseline Hamilton Depression Scale for Depression (HAM-D) score of 20-25.
The efficacy analysis included 207 patients who received brexanolone at 90 mcg/kg per hour or placebo for 60 hours in an inpatient setting and were followed for 30 days. The primary endpoint was the change in HAM-D total score from baseline to 60 hours. The mean 17-point reduction in the active treatment arm was significantly better than the 12.8-point decrease with placebo. The between-group difference was significant within the first 24 hours and remained so at all time points out to the study’s end at day 30. There was no individual item on the HAM-D in which the drug performed worse than placebo, and there were many in which brexanolone performed significantly better, including depressed mood, anxiety, insomnia, and feelings of guilt.
In terms of the rigorous secondary endpoint of HAM-D remission as defined by a total score of 7 or less, the brexanolone injection significantly outperformed placebo at every time point except for day 30.
There was a 2% rate of serious adverse events in both study arms. These included suicidal ideation, an intentional overdose attempt post discharge, altered state of consciousness, and syncope.
A vastly more convenient once-daily oral formulation of brexanolone is now in phase 3 clinical trials for PPD and major depressive disorder, and in phase 2 for insomnia and bipolar depression.
Elsewhere at the ECNP congress, other investigators from Sage Therapeutics presented for the first time the outcomes of an 89-patient, randomized, double-blind, multicenter, placebo-controlled, phase 2 clinical trial of SAGE-217 for treatment of major depressive disorder.
Participants received a nightly 30-mg dose of the drug or placebo for 2 weeks, with a primary study endpoint being the change in HAM-D total score from baseline to day 15. Patients on the oral GABA A receptor positive modulator averaged a 17.4-point improvement, significantly better than the 10.3-point spread in placebo-treated controls. A statistically significant between-group difference was noted on days 2 through 28. HAM-D remission at day 15 was documented in 64% of the oral brexanolone group, compared with 26% of controls.
Those improvements in depression were accompanied by significant gains in numerous domains of health-related quality of life as assessed via the 36-Item Short Form Health Survey. Indeed, day 15 health-related quality of life scores in the oral brexanolone group approached normative values for the general population.
The studies were funded by SAGE Therapeutics.
BARCELONA – Brexanolone injection provided rapid and durable improvement in postpartum depression in an integrated analysis of three pivotal randomized trials collectively known as the Hummingbird trials, Christine Clemson, PhD, reported at the annual congress of the European College of Neuropsychopharmacology.
This was accomplished with a favorable safety experience. The most common treatment-emergent adverse events – dizziness and sleepiness – were roughly twice as common as with placebo in the 247-patient Hummingbird safety analysis.
On Dec. 19, 2018, the agency is expected to respond to SAGE Therapeutics’s application for marketing approval of intravenous brexanolone given as a continuous 60-hour infusion at 90 mcg/kg per hour, according to Dr. Clemson, senior medical director at Cambridge, Mass.–based SAGE Therapeutics, which is developing the therapy.
Brexanolone is a proprietary IV formulation of allopregnanolone, a metabolite of progesterone. The drug’s mechanism of action involves modulation of the neurotransmitter gamma-aminobutyric acid (GABA). The drug binds to both synaptic and extra-synaptic GABA A receptors, thereby increasing receptor functionality.
The decision to target GABA as a novel therapeutic strategy in PPD was based upon translational studies demonstrating that GABA is the chief neuroinhibitory mechanism in the brain, and its actions are mediated mainly by GABA A receptors. Brexanolone’s efficacy is consistent with a theory that the pathogenesis of PPD involves triggers such as inflammation, hormonal fluctuations, or chronic stress, which in some women cause GABA hypofunction, both at the receptors and in terms of tissue GABA levels. This, in turn, leads to an overactive HPA axis and dysregulated neural networks, with resultant PPD, Dr. Clemson explained.
The three Hummingbird clinical trials were double blind, randomized, and placebo controlled. Two were restricted to women with severe PPD. The third and largest focused on moderately affected patients as defined by a baseline Hamilton Depression Scale for Depression (HAM-D) score of 20-25.
The efficacy analysis included 207 patients who received brexanolone at 90 mcg/kg per hour or placebo for 60 hours in an inpatient setting and were followed for 30 days. The primary endpoint was the change in HAM-D total score from baseline to 60 hours. The mean 17-point reduction in the active treatment arm was significantly better than the 12.8-point decrease with placebo. The between-group difference was significant within the first 24 hours and remained so at all time points out to the study’s end at day 30. There was no individual item on the HAM-D in which the drug performed worse than placebo, and there were many in which brexanolone performed significantly better, including depressed mood, anxiety, insomnia, and feelings of guilt.
In terms of the rigorous secondary endpoint of HAM-D remission as defined by a total score of 7 or less, the brexanolone injection significantly outperformed placebo at every time point except for day 30.
There was a 2% rate of serious adverse events in both study arms. These included suicidal ideation, an intentional overdose attempt post discharge, altered state of consciousness, and syncope.
A vastly more convenient once-daily oral formulation of brexanolone is now in phase 3 clinical trials for PPD and major depressive disorder, and in phase 2 for insomnia and bipolar depression.
Elsewhere at the ECNP congress, other investigators from Sage Therapeutics presented for the first time the outcomes of an 89-patient, randomized, double-blind, multicenter, placebo-controlled, phase 2 clinical trial of SAGE-217 for treatment of major depressive disorder.
Participants received a nightly 30-mg dose of the drug or placebo for 2 weeks, with a primary study endpoint being the change in HAM-D total score from baseline to day 15. Patients on the oral GABA A receptor positive modulator averaged a 17.4-point improvement, significantly better than the 10.3-point spread in placebo-treated controls. A statistically significant between-group difference was noted on days 2 through 28. HAM-D remission at day 15 was documented in 64% of the oral brexanolone group, compared with 26% of controls.
Those improvements in depression were accompanied by significant gains in numerous domains of health-related quality of life as assessed via the 36-Item Short Form Health Survey. Indeed, day 15 health-related quality of life scores in the oral brexanolone group approached normative values for the general population.
The studies were funded by SAGE Therapeutics.
REPORTING FROM THE ECNP CONGRESS
Key clinical point: A novel investigational GABA modulator is turning heads for treatment of postpartum depression.
Major finding: At 60 hours, mean HAM-D total scores had dropped by 17 points with brexanolone, versus 12.8 with placebo.
Study details: A prespecified integrated safety and efficacy analysis incorporating three pivotal clinical trials.
Disclosures: The studies were funded by SAGE Therapeutics and presented by a company executive.
Consider different etiologies in patients with vaginal pruritus
CHICAGO – Diagnosing the cause of vaginal itching, which can have a significant negative impact on a woman’s quality of life, can be particularly difficult for multiple reasons, according to Rachel Kornik, MD, of the departments of dermatology and obstetrics and gynecology at the University of Wisconsin, Madison.
“The anatomy is really challenging in this area, and there’s a broad differential. Often there’s more than one thing happening,” Dr. Kornik said during a session on diagnosing and managing genital pruritus in women at the American Academy of Dermatology summer meeting. Like hair loss, vaginal pruritus is also very emotionally distressing.
“Patients are very anxious when they have all this itching,” she said. “It has an impact on personal relationships. Some patients find it difficult to talk about because it’s a taboo subject, so we have to make them comfortable.”
Dr. Kornik showed a chart of the inflammatory, neoplastic, infections, infestations, environmental, neuropathic, and hormonal. But she focused her presentation primarily on the most common causes: contact dermatitis, lichen sclerosus, and lichen simplex chronicus.
Contact dermatitis
The most common factors that contribute to contact dermatitis are friction, hygiene practices, unique body exposures (such as body fluids and menstrual and personal care products), and occlusion/maceration, which facilitates penetration of external agents. Estrogen deficiency may also play a role.
Taking a thorough history from the patient is key to finding out possible causes. Dr. Kornik provided a list of common irritants to consider.
- Hygiene-related irritants, such as frequent washing and the use of soaps, wash cloths, loofahs, wipes, bath oil, bubbles, and water.
- Laundry products, such as fabric softeners or dryer sheets.
- Menstrual products, such as panty liners, pads, and scents or additives for retaining moisture.
- Over-the-counter itch products, such as those containing benzocaine.
- Medications, such as alcohol-based creams and gels, trichloroacetic acid, fluorouracil (Efudex), imiquimod, and topical antifungals.
- Heat-related irritants, such as use of hair dryers and heating pads.
- Body fluids, including urine, feces, menstrual blood, sweat, semen, and excessive discharge.
It’s also important to consider whether there is an allergic cause. “Contact dermatitis and allergic dermatitis can look very similar both clinically and histologically, and patients can even have them both at the same time,” Dr. Kornik said. “So really, patch testing is essential sometimes to identify a true allergic contact dermatitis.”
She cited a study that identified the top five most common allergens as fragrance mixes, balsam of Peru, benzocaine, terconazole, and quaternium-15 (a formaldehyde-releasing preservative) (Dermatitis. 2013 Mar-Apr;24(2):64-72).
“If somebody’s coming into your office and they have vulvar itching for any reason, the No. 1 thing is making sure that they eliminate and not use any products with fragrances,” Dr. Kornik said. “It’s also important to note that over time, industries’ use of preservatives does change, the concentrations change, and so we may see more emerging allergens or different ones over time.”
The causative allergens are rarely consumed orally, but they may be ectopic, such as shampoo or nail polish.
“What I’ve learned over the years in treating patients with vulvar itching is that they don’t always think to tell you about everything they are applying,” Dr. Kornik said. “You have to ask specific questions. Are you using any wipes or using any lubricants? What is the type and brand of menstrual pad you’re using?”
Patients might also think they can eliminate the cause of irritation by changing products, but “there are cross reactants in many preservatives and fragrances in many products, so they might not eliminate exposure, and intermittent exposures can lead to chronic dermatitis,” she pointed out.
One example is wipes: Some women may use them only periodically, such as after a yoga class, and not think of this as a possibility or realize that wipes could perpetuate chronic dermatitis.
Research has also found that it’s very common for patients with allergic contact dermatitis to have a concomitant vulvar diagnosis. In one study, more than half of patients had another condition, the most common of which was lichen sclerosus. Others included simplex chronicus, atopic dermatitis, condyloma acuminatum, psoriasis, and Paget disease.
Therefore, if patients are not responding as expected, it’s important to consider that the condition is multifactorial “and consider allergic contact dermatitis in addition to whatever other underlying dermatosis they have,” Dr. Kornik said.
Lichen sclerosus
Prevalence of the scarring disorder lichen sclerosus ranges from 1.7% to 3% in the research literature and pathogenesis is likely multifactorial.
“It’s a very frustrating condition for patients and for physicians because we don’t know exactly what causes it, but it definitely has a predilection for the vulva area, and it affects women of all ages,” she said. “I also think it’s more common than we think.”
Loss of normal anatomical structures are a key feature, so physicians need to know their anatomy well to look for what’s not there. Lichen sclerosus involves modified mucous membranes and the perianal area, and it may spread to the crural folds and upper thighs. Symptoms can include periclitoral edema, white patches, pale skin, textural changes (such as wrinkling, waxiness, or hyperkeratosis), fissures, melanosis, and sometimes ulcerations or erosions from scratching.
There is no standardized treatment for lichen sclerosus. Research suggests using a high potency topical steroid treatment daily until skin texture normalizes, which can take anywhere from 6 weeks to 5 months, depending on severity, Dr. Kornik said. Few data are available for management if topical steroids do not work, she added.*
If dealing with recalcitrant disease, she recommends first checking the patients’ compliance and then considering alternative diagnoses or secondary conditions. Do patch testing, rule out contact dermatitis, and rebiopsy if needed. Other options are to add tacrolimus ointment, offer intralesional triamcinolone, consider a systemic agent (acitretin, methotrexate, or possibly hydroxychloroquine), or try laser or photodynamic therapy. She emphasizes the importance of demonstrating to the patient where to apply ointment, since they may not be applying to the right areas.*
Lichen simplex chronicus
Lichen simplex chronicus is a clinical description of the result of chronic rubbing and scratching. It might be triggered by something that has now resolved or be linked to other itching conditions, but clinicians need to consider the possibility of neuropathic itch as well.
Features of lichen simplex chronicus can include bilateral or unilateral involvement of the labia majora, erythematous plaques with lichenification, hyper- or hypopigmentation, or angulated excoriations and hypertrophy of labia caused by thickened skin, though the signs may be subtle, she said.
Treatment requires management of the skin problem itself – the underlying cause of the itch – as well as the behavioral component. Topical steroids are first line, plus an antihistamine at night as needed to stop the scratching. If those are insufficient, the next treatments to consider are intralesional triamcinolone (Kenalog), tacrolimus ointment, topical or oral doxepin, mirtazapine, or even selective serotonin reuptake inhibitors.
Women using topical steroids should also be aware of the possible side effects, including atrophy, infections, and allergic contact dermatitis if the steroid itself or the cream it’s in is an allergen. If stinging or burning occurs, switch to a steroid without propylene glycol, she added.
If no changes occur in the skin, clinicians may have to consider the existence of neuropathic pruritus diagnosis, an injury or dysfunction along the afferent itch pathway. Burning is more common with this neuropathy, but itching can occur too.
Other issues include symptoms that worsen with sitting and pain that worsens throughout the day. Causes can include childbirth, surgery, pelvic trauma, infection, and chemoradiation, and diagnosis requires imaging to rule out other possible causes. Treatment involves pelvic floor physical therapy, pudendal nerve block, or gabapentin.
Dr. Kornik wrapped up with a reminder that vulvar itch is often multifactorial, so clinicians need to chip away at the potential causes – sometimes with cultures, scrapes, and biopsies as needed.
She reported no financial disclosures.
Correction, 10/26/18: Dr. Kornik's treatment recommendations for lichen sclerosus were misstated.
CHICAGO – Diagnosing the cause of vaginal itching, which can have a significant negative impact on a woman’s quality of life, can be particularly difficult for multiple reasons, according to Rachel Kornik, MD, of the departments of dermatology and obstetrics and gynecology at the University of Wisconsin, Madison.
“The anatomy is really challenging in this area, and there’s a broad differential. Often there’s more than one thing happening,” Dr. Kornik said during a session on diagnosing and managing genital pruritus in women at the American Academy of Dermatology summer meeting. Like hair loss, vaginal pruritus is also very emotionally distressing.
“Patients are very anxious when they have all this itching,” she said. “It has an impact on personal relationships. Some patients find it difficult to talk about because it’s a taboo subject, so we have to make them comfortable.”
Dr. Kornik showed a chart of the inflammatory, neoplastic, infections, infestations, environmental, neuropathic, and hormonal. But she focused her presentation primarily on the most common causes: contact dermatitis, lichen sclerosus, and lichen simplex chronicus.
Contact dermatitis
The most common factors that contribute to contact dermatitis are friction, hygiene practices, unique body exposures (such as body fluids and menstrual and personal care products), and occlusion/maceration, which facilitates penetration of external agents. Estrogen deficiency may also play a role.
Taking a thorough history from the patient is key to finding out possible causes. Dr. Kornik provided a list of common irritants to consider.
- Hygiene-related irritants, such as frequent washing and the use of soaps, wash cloths, loofahs, wipes, bath oil, bubbles, and water.
- Laundry products, such as fabric softeners or dryer sheets.
- Menstrual products, such as panty liners, pads, and scents or additives for retaining moisture.
- Over-the-counter itch products, such as those containing benzocaine.
- Medications, such as alcohol-based creams and gels, trichloroacetic acid, fluorouracil (Efudex), imiquimod, and topical antifungals.
- Heat-related irritants, such as use of hair dryers and heating pads.
- Body fluids, including urine, feces, menstrual blood, sweat, semen, and excessive discharge.
It’s also important to consider whether there is an allergic cause. “Contact dermatitis and allergic dermatitis can look very similar both clinically and histologically, and patients can even have them both at the same time,” Dr. Kornik said. “So really, patch testing is essential sometimes to identify a true allergic contact dermatitis.”
She cited a study that identified the top five most common allergens as fragrance mixes, balsam of Peru, benzocaine, terconazole, and quaternium-15 (a formaldehyde-releasing preservative) (Dermatitis. 2013 Mar-Apr;24(2):64-72).
“If somebody’s coming into your office and they have vulvar itching for any reason, the No. 1 thing is making sure that they eliminate and not use any products with fragrances,” Dr. Kornik said. “It’s also important to note that over time, industries’ use of preservatives does change, the concentrations change, and so we may see more emerging allergens or different ones over time.”
The causative allergens are rarely consumed orally, but they may be ectopic, such as shampoo or nail polish.
“What I’ve learned over the years in treating patients with vulvar itching is that they don’t always think to tell you about everything they are applying,” Dr. Kornik said. “You have to ask specific questions. Are you using any wipes or using any lubricants? What is the type and brand of menstrual pad you’re using?”
Patients might also think they can eliminate the cause of irritation by changing products, but “there are cross reactants in many preservatives and fragrances in many products, so they might not eliminate exposure, and intermittent exposures can lead to chronic dermatitis,” she pointed out.
One example is wipes: Some women may use them only periodically, such as after a yoga class, and not think of this as a possibility or realize that wipes could perpetuate chronic dermatitis.
Research has also found that it’s very common for patients with allergic contact dermatitis to have a concomitant vulvar diagnosis. In one study, more than half of patients had another condition, the most common of which was lichen sclerosus. Others included simplex chronicus, atopic dermatitis, condyloma acuminatum, psoriasis, and Paget disease.
Therefore, if patients are not responding as expected, it’s important to consider that the condition is multifactorial “and consider allergic contact dermatitis in addition to whatever other underlying dermatosis they have,” Dr. Kornik said.
Lichen sclerosus
Prevalence of the scarring disorder lichen sclerosus ranges from 1.7% to 3% in the research literature and pathogenesis is likely multifactorial.
“It’s a very frustrating condition for patients and for physicians because we don’t know exactly what causes it, but it definitely has a predilection for the vulva area, and it affects women of all ages,” she said. “I also think it’s more common than we think.”
Loss of normal anatomical structures are a key feature, so physicians need to know their anatomy well to look for what’s not there. Lichen sclerosus involves modified mucous membranes and the perianal area, and it may spread to the crural folds and upper thighs. Symptoms can include periclitoral edema, white patches, pale skin, textural changes (such as wrinkling, waxiness, or hyperkeratosis), fissures, melanosis, and sometimes ulcerations or erosions from scratching.
There is no standardized treatment for lichen sclerosus. Research suggests using a high potency topical steroid treatment daily until skin texture normalizes, which can take anywhere from 6 weeks to 5 months, depending on severity, Dr. Kornik said. Few data are available for management if topical steroids do not work, she added.*
If dealing with recalcitrant disease, she recommends first checking the patients’ compliance and then considering alternative diagnoses or secondary conditions. Do patch testing, rule out contact dermatitis, and rebiopsy if needed. Other options are to add tacrolimus ointment, offer intralesional triamcinolone, consider a systemic agent (acitretin, methotrexate, or possibly hydroxychloroquine), or try laser or photodynamic therapy. She emphasizes the importance of demonstrating to the patient where to apply ointment, since they may not be applying to the right areas.*
Lichen simplex chronicus
Lichen simplex chronicus is a clinical description of the result of chronic rubbing and scratching. It might be triggered by something that has now resolved or be linked to other itching conditions, but clinicians need to consider the possibility of neuropathic itch as well.
Features of lichen simplex chronicus can include bilateral or unilateral involvement of the labia majora, erythematous plaques with lichenification, hyper- or hypopigmentation, or angulated excoriations and hypertrophy of labia caused by thickened skin, though the signs may be subtle, she said.
Treatment requires management of the skin problem itself – the underlying cause of the itch – as well as the behavioral component. Topical steroids are first line, plus an antihistamine at night as needed to stop the scratching. If those are insufficient, the next treatments to consider are intralesional triamcinolone (Kenalog), tacrolimus ointment, topical or oral doxepin, mirtazapine, or even selective serotonin reuptake inhibitors.
Women using topical steroids should also be aware of the possible side effects, including atrophy, infections, and allergic contact dermatitis if the steroid itself or the cream it’s in is an allergen. If stinging or burning occurs, switch to a steroid without propylene glycol, she added.
If no changes occur in the skin, clinicians may have to consider the existence of neuropathic pruritus diagnosis, an injury or dysfunction along the afferent itch pathway. Burning is more common with this neuropathy, but itching can occur too.
Other issues include symptoms that worsen with sitting and pain that worsens throughout the day. Causes can include childbirth, surgery, pelvic trauma, infection, and chemoradiation, and diagnosis requires imaging to rule out other possible causes. Treatment involves pelvic floor physical therapy, pudendal nerve block, or gabapentin.
Dr. Kornik wrapped up with a reminder that vulvar itch is often multifactorial, so clinicians need to chip away at the potential causes – sometimes with cultures, scrapes, and biopsies as needed.
She reported no financial disclosures.
Correction, 10/26/18: Dr. Kornik's treatment recommendations for lichen sclerosus were misstated.
CHICAGO – Diagnosing the cause of vaginal itching, which can have a significant negative impact on a woman’s quality of life, can be particularly difficult for multiple reasons, according to Rachel Kornik, MD, of the departments of dermatology and obstetrics and gynecology at the University of Wisconsin, Madison.
“The anatomy is really challenging in this area, and there’s a broad differential. Often there’s more than one thing happening,” Dr. Kornik said during a session on diagnosing and managing genital pruritus in women at the American Academy of Dermatology summer meeting. Like hair loss, vaginal pruritus is also very emotionally distressing.
“Patients are very anxious when they have all this itching,” she said. “It has an impact on personal relationships. Some patients find it difficult to talk about because it’s a taboo subject, so we have to make them comfortable.”
Dr. Kornik showed a chart of the inflammatory, neoplastic, infections, infestations, environmental, neuropathic, and hormonal. But she focused her presentation primarily on the most common causes: contact dermatitis, lichen sclerosus, and lichen simplex chronicus.
Contact dermatitis
The most common factors that contribute to contact dermatitis are friction, hygiene practices, unique body exposures (such as body fluids and menstrual and personal care products), and occlusion/maceration, which facilitates penetration of external agents. Estrogen deficiency may also play a role.
Taking a thorough history from the patient is key to finding out possible causes. Dr. Kornik provided a list of common irritants to consider.
- Hygiene-related irritants, such as frequent washing and the use of soaps, wash cloths, loofahs, wipes, bath oil, bubbles, and water.
- Laundry products, such as fabric softeners or dryer sheets.
- Menstrual products, such as panty liners, pads, and scents or additives for retaining moisture.
- Over-the-counter itch products, such as those containing benzocaine.
- Medications, such as alcohol-based creams and gels, trichloroacetic acid, fluorouracil (Efudex), imiquimod, and topical antifungals.
- Heat-related irritants, such as use of hair dryers and heating pads.
- Body fluids, including urine, feces, menstrual blood, sweat, semen, and excessive discharge.
It’s also important to consider whether there is an allergic cause. “Contact dermatitis and allergic dermatitis can look very similar both clinically and histologically, and patients can even have them both at the same time,” Dr. Kornik said. “So really, patch testing is essential sometimes to identify a true allergic contact dermatitis.”
She cited a study that identified the top five most common allergens as fragrance mixes, balsam of Peru, benzocaine, terconazole, and quaternium-15 (a formaldehyde-releasing preservative) (Dermatitis. 2013 Mar-Apr;24(2):64-72).
“If somebody’s coming into your office and they have vulvar itching for any reason, the No. 1 thing is making sure that they eliminate and not use any products with fragrances,” Dr. Kornik said. “It’s also important to note that over time, industries’ use of preservatives does change, the concentrations change, and so we may see more emerging allergens or different ones over time.”
The causative allergens are rarely consumed orally, but they may be ectopic, such as shampoo or nail polish.
“What I’ve learned over the years in treating patients with vulvar itching is that they don’t always think to tell you about everything they are applying,” Dr. Kornik said. “You have to ask specific questions. Are you using any wipes or using any lubricants? What is the type and brand of menstrual pad you’re using?”
Patients might also think they can eliminate the cause of irritation by changing products, but “there are cross reactants in many preservatives and fragrances in many products, so they might not eliminate exposure, and intermittent exposures can lead to chronic dermatitis,” she pointed out.
One example is wipes: Some women may use them only periodically, such as after a yoga class, and not think of this as a possibility or realize that wipes could perpetuate chronic dermatitis.
Research has also found that it’s very common for patients with allergic contact dermatitis to have a concomitant vulvar diagnosis. In one study, more than half of patients had another condition, the most common of which was lichen sclerosus. Others included simplex chronicus, atopic dermatitis, condyloma acuminatum, psoriasis, and Paget disease.
Therefore, if patients are not responding as expected, it’s important to consider that the condition is multifactorial “and consider allergic contact dermatitis in addition to whatever other underlying dermatosis they have,” Dr. Kornik said.
Lichen sclerosus
Prevalence of the scarring disorder lichen sclerosus ranges from 1.7% to 3% in the research literature and pathogenesis is likely multifactorial.
“It’s a very frustrating condition for patients and for physicians because we don’t know exactly what causes it, but it definitely has a predilection for the vulva area, and it affects women of all ages,” she said. “I also think it’s more common than we think.”
Loss of normal anatomical structures are a key feature, so physicians need to know their anatomy well to look for what’s not there. Lichen sclerosus involves modified mucous membranes and the perianal area, and it may spread to the crural folds and upper thighs. Symptoms can include periclitoral edema, white patches, pale skin, textural changes (such as wrinkling, waxiness, or hyperkeratosis), fissures, melanosis, and sometimes ulcerations or erosions from scratching.
There is no standardized treatment for lichen sclerosus. Research suggests using a high potency topical steroid treatment daily until skin texture normalizes, which can take anywhere from 6 weeks to 5 months, depending on severity, Dr. Kornik said. Few data are available for management if topical steroids do not work, she added.*
If dealing with recalcitrant disease, she recommends first checking the patients’ compliance and then considering alternative diagnoses or secondary conditions. Do patch testing, rule out contact dermatitis, and rebiopsy if needed. Other options are to add tacrolimus ointment, offer intralesional triamcinolone, consider a systemic agent (acitretin, methotrexate, or possibly hydroxychloroquine), or try laser or photodynamic therapy. She emphasizes the importance of demonstrating to the patient where to apply ointment, since they may not be applying to the right areas.*
Lichen simplex chronicus
Lichen simplex chronicus is a clinical description of the result of chronic rubbing and scratching. It might be triggered by something that has now resolved or be linked to other itching conditions, but clinicians need to consider the possibility of neuropathic itch as well.
Features of lichen simplex chronicus can include bilateral or unilateral involvement of the labia majora, erythematous plaques with lichenification, hyper- or hypopigmentation, or angulated excoriations and hypertrophy of labia caused by thickened skin, though the signs may be subtle, she said.
Treatment requires management of the skin problem itself – the underlying cause of the itch – as well as the behavioral component. Topical steroids are first line, plus an antihistamine at night as needed to stop the scratching. If those are insufficient, the next treatments to consider are intralesional triamcinolone (Kenalog), tacrolimus ointment, topical or oral doxepin, mirtazapine, or even selective serotonin reuptake inhibitors.
Women using topical steroids should also be aware of the possible side effects, including atrophy, infections, and allergic contact dermatitis if the steroid itself or the cream it’s in is an allergen. If stinging or burning occurs, switch to a steroid without propylene glycol, she added.
If no changes occur in the skin, clinicians may have to consider the existence of neuropathic pruritus diagnosis, an injury or dysfunction along the afferent itch pathway. Burning is more common with this neuropathy, but itching can occur too.
Other issues include symptoms that worsen with sitting and pain that worsens throughout the day. Causes can include childbirth, surgery, pelvic trauma, infection, and chemoradiation, and diagnosis requires imaging to rule out other possible causes. Treatment involves pelvic floor physical therapy, pudendal nerve block, or gabapentin.
Dr. Kornik wrapped up with a reminder that vulvar itch is often multifactorial, so clinicians need to chip away at the potential causes – sometimes with cultures, scrapes, and biopsies as needed.
She reported no financial disclosures.
Correction, 10/26/18: Dr. Kornik's treatment recommendations for lichen sclerosus were misstated.
EXPERT ANALYSIS FROM SUMMER AAD 2018