User login
Cellular Versus Acellular Grafts for Diabetic Foot Ulcers: Altering the Protocol to Improve Recruitment to a Comparative Efficacy Trial
Chronic diabetic foot ulcers (DFUs) remain a serious therapeutic challenge worldwide.1-2 Patients with DFUs are at higher risk for infections, which may lead to limb loss.1-5 In fact, 1 in 6 patients with DFUs will undergo an amputation.6 The long-term consequences of DFUs are numerous and can severely affect patients’ quality of life, including loss of productivity.7 The current standard of care for DFUs consists of debridement of the necrotic tissue, application of a moist dressing, and use of an off-loading device that protects the wound from pressure or trauma related to ambulation and other acts of daily living.4-6,8 Unfortunately, studies have shown that the best standard of care (SOC) only heals 30% of DFUs after 20 weeks of therapy.9 With the estimated cost per episode of care approaching $40,000, DFUs remain a costly and important problem.10
The altered extracellular matrix (ECM) in DFUs has been a target for the development of new therapeutic devices that provide a new matrix that is either devoid of cells or can be enriched with fibroblasts.8,11 These bioengineered skin substitutes stimulate the growth of new vessels and generate cytokines essential for tissue repair. In 2013, Lev-Tov et al12 published this study protocol (Dermagraft Oasis Longitudinal Comparative Efficacy [DOLCE] trial) to compare the effectiveness of 2 advanced wound care devices, specifically to evaluate the clinical efficacy of a cellular matrix versus an acellular matrix, which we have amended. The cellular matrix used in the study is a dermal substitute composed of viable newborn foreskin fibroblasts seeded onto a bioabsorbable polyglactin mesh on which fibroblasts generate an ECM.13,14 It is supplied frozen and requires specific thawing steps prior to application. The recommended regimen for treatment of DFUs for this cellular matrix is 8 weekly applications.13,14 In 2016, the cost of the product was reported as $1411 per 5.0×7.5-cm sheet.15 The acellular matrix product used in the study is a bioabsorbable ECM that is derived from porcine small intestinal submucosa.16,17 It is stored at room temperature and has a long shelf life, with a current price of $112.6 for a 3.0×3.5-cm single-layer fenestrated sheet ($1126.60 per box of 10 sheets). The industry-supported randomized controlled trials for each of these devices have reported a 20% added benefit in the rate of wound closure at week 12 compared to SOC.14,17
This article provides the interim report of the trial (registered at www.clinicaltrials.gov with the identifier NCT01450943) described in the published protocol and initiated in 2011,12 focusing on elements that required modification during the trial’s duration.
Methods
Study Protocol
The clinical trial was approved by the Veterans’ Affairs Institutional Research and Development Committee and their institutional review board. This study was funded by the Veteran’s Administration Merit Award (#10554640), which was awarded to 2 of the investigators (S.E.D. and R.R.I.). Eligible veterans were recruited from all 7 sites of the VA Northern California Healthcare System. This trial is a randomized, single-blinded, 3-armed, controlled clinical equivalence trial comparing the effectiveness of an SOC treatment, cellular ECM, and acellular ECM.
Study Products
The SOC dressing applied in the clinical trial included a sterile antimicrobial gel, a nonadherent dressing, and gauze.12 The SOC dressing also was used as a secondary dressing for the active treatment arms. Bacitracin antibiotic ointment was used as an alternative for patients with allergy to iodine.12
Randomization
The inclusion and exclusion criteria were previously outlined.12 After a 2-week screening phase to exclude rapid healers, patients were randomized into a treatment arm and entered the active phase for 12 weeks.
Primary and Secondary Outcomes
The primary outcome was complete wound closure by week 12.12 Complete healing was defined as full reepithelialization with no drainage or dressing requirement. The secondary outcomes included healing at 28 weeks, rate of healing, ulcer recurrence at week 20, association of wound healing with ulcer characteristics or patients’ characteristic, incidence of adverse events, and cost-effectiveness of each treatment compared to the SOC arm.12
Statistical Analysis
To detect a 25% difference in the incidence of ulcer closure between the 2 study groups and the SOC group, the estimation of the sample size was based on 80% power with a significance level of 0.05. Specifically, it was expected that 50% of the cellular and acellular matrix groups and 25% of the SOC group would reach complete wound closure. The protocol indicated that 57 participants would be enrolled in each arm (total of 171 participants). Lev-Tov et al12 discussed the statistical analysis in more detail.
Results
Study Protocol Amendments
Given the number of diabetic patients in the US veteran population, we anticipated that there would be enough participants meeting the inclusion and exclusion criteria; however, because of the difficulty with recruitment, the initial study criteria were modified. The study was initially designed to incorporate DFUs with a minimum size of 1.0 cm2.12
Another limiting criterion was the percentage of total hemoglobin level for hemoglobin A1C (HbA1C). The study was originally established to include participants with an HbA1C level of 10% of total hemoglobin or below.12 Unfortunately, the majority of the potential participants had values substantially higher, and thus could not be enrolled in the trial, requiring another amendment to the study protocol in 2014, which was approved to include patients with an HbA1C level less than 12% of total hemoglobin. This change contributed considerably to the noted increase in enrollment rates in 2015, which almost doubled relative to enrollment under the original exclusion criteria (Figure).
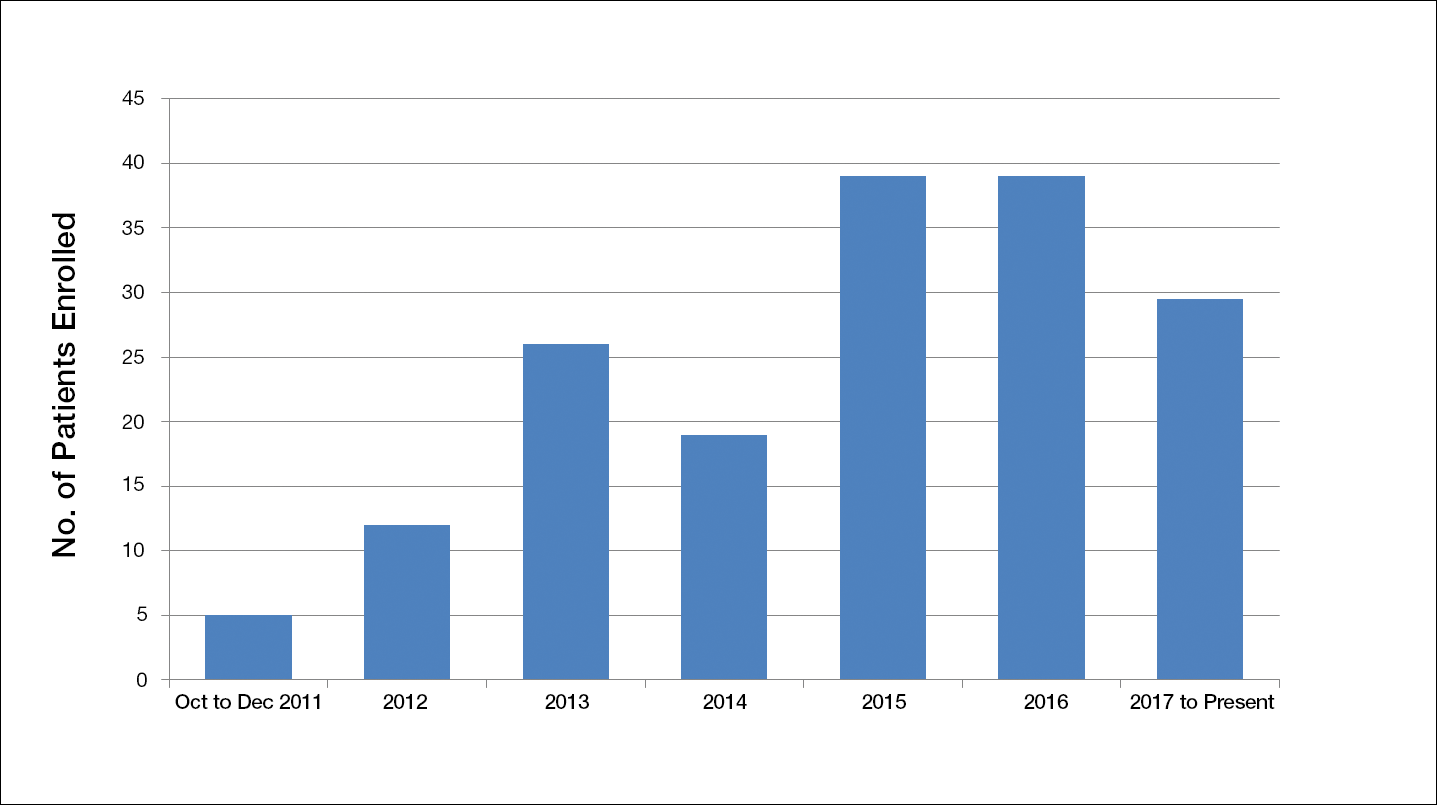
The study has screened more than 600 patients. Among them, 137 were assessed for eligibility; 71 were excluded for various reasons, including screen failure (eg, decrease in wound size by >40% during the 2-week screening phase), loss to follow-up, and adverse events. Sixty-six participants reached the primary outcome at week 12, while 55 participants completed the study (19 in the SOC group; 18 in the cellular matrix group; 18 in the acellular matrix group).
We have stopped enrolling patients from all sites and the community, as we have reached our target enrollment.
Comment
One of the challenges of clinical trials is the recruitment of an adequate number of participants within an appropriate time frame, which is explained by Lasagna’s Law,18 a well-described phenomenon whereby the investigator overestimates the number of potential participants available to meet the inclusion criteria. This so-called funnel-effect was partly encountered in our selection process. A review of the veteran population with DFUs seemed to be more than adequate to fulfill the sample size; however, some important participant-related factors also played a substantial role.
In addition, the Veterans’ Affairs network centralizes health information, making it readily available to all providers participating in their care. As a result, patients with diabetes mellitus typically are seen by a primary care physician along with an endocrinologist, a diabetic nurse, and/or a dietician. Despite the collaboration with an interdisciplinary team, the glycemic control of the participants remains an issue along with other psychosocial factors that are deterrents in patient compliance. As a result, patients with poorly controlled diabetes and an HbA1C level above 10% (and less than 12%) of total hemoglobin who were initially excluded from the study were reincluded after modifying the inclusion criteria. Some patients were interested in joining the study, but physical limitations (eg, impaired mobility) prompted their decision not to join the trial, even though they met all the inclusion criteria.
As far as research-related factors that could affect participation, it is notable that most of the patients were retired; thus, the interventions did not cause additional burden of taking time off from work or loss of productivity. Although randomization could be a deterrent in many clinical trials, the majority of patients were willing to participate without demanding to be assigned to a particular treatment group.
There are many factors that are intertwined and can lead to enrollment and/or attrition rates. It was critical for our team to make some adjustment without compromising the controlled nature of a randomized trial.
Acknowledgment
The authors wish to acknowledge Huong Le, DPM, MPH, who was the coauthor of the study protocol.
- Sen CK, Gordillo GM, Roy S, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17:763-771.
- Gurtner GC, Werner S, Barrandon Y, et al. Wound repair and regeneration. Nature. 2008;453:314-321.
- Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366:1736-1743.
- Boulton AJ. The diabetic foot: grand overview, epidemiology and pathogenesis. Diabetes Metab Res Rev. 2008;24(suppl 1):S3-S6.
- Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA. 2005;293:217-228.
- Vuorisalo S, Venermo M, Lepäntalo M. Treatment of diabetic foot ulcers. J Cardiovasc Surg (Torino). 2009;50:275-291.
- Meijer JW, Trip J, Jaegers SM, et al. Quality of life in patients with diabetic foot ulcers. Disabil Rehabil. 2001;23:336-340.
- Santema TB, Poyck PP, Ubbink DT. Skin grafting and tissue replacement for treating foot ulcers in people with diabetes. Cochrane Database Syst Rev. 2016;2:CD011255.
- Margolis DJ, Kantor J, Berlin JA. Healing of diabetic neuropathic foot ulcers receiving standard treatment. a meta-analysis. Diabetes Care. 1999;22:692-695.
- Cavanagh P, Attinger C, Abbas Z, et al. Cost of treating diabetic foot ulcers in five different countries. Diabetes Metab Res Rev. 2012;2(suppl 1):107-111.
- Panuncialman J, Falanga V. The science of wound bed preparation. Surg Clin North Am. 2009;89:611-626.
- Lev-Tov H, Li CS, Dahle S, et al. Cellular versus acellular matrix devices in treatment of diabetic foot ulcers: study protocol for a comparative efficacy randomized controlled trial. Trials. 2013;14:8.
- Gentzkow GD, Iwasaki SD, Hershon KS, et al. Use of dermagraft, a cultured human dermis, to treat diabetic foot ulcers. Diabetes Care. 1996;19:350-354.
- Marston WA, Hanft J, Norwood P, et al; Dermagraft Diabetic Foot Ulcer Study Group. The efficacy and safety of Dermagraft in improving the healing of chronic diabetic foot ulcers: results of a prospective randomized trial. Diabetes Care. 2003;26:1701-1705.
- 2016 Dermagraft® Medicare Product and Related Procedure Payment. http://www.dermagraft.com/wp-content/uploads/sites/1/Dermagraft_Hotsheet%202016%20Q1%20HOSPITAL_FINAL.pdf. Accessed November 23, 2017.
- Oasis® Wound Matrix. http://www.oasiswoundmatrix.com/aboutowm. Accessed November 23, 2017.
- Niezgoda JA, Van Gils CC, Frykberg RG, et al. Randomized clinical trial comparing OASIS Wound Matrix to Regranex Gel for diabetic ulcers. Adv Skin Wound Care. 2005;18(5, pt 1):258-266.
- Torgerson JS, Arlinger K, Käppi M, et al. Principles for enhanced recruitment of subjects in a large clinical trial. the XENDOS (XENical in the prevention of Diabetes in Obese Subjects) study experience. Controlled Clin Trials. 2001;22:515-525.
Chronic diabetic foot ulcers (DFUs) remain a serious therapeutic challenge worldwide.1-2 Patients with DFUs are at higher risk for infections, which may lead to limb loss.1-5 In fact, 1 in 6 patients with DFUs will undergo an amputation.6 The long-term consequences of DFUs are numerous and can severely affect patients’ quality of life, including loss of productivity.7 The current standard of care for DFUs consists of debridement of the necrotic tissue, application of a moist dressing, and use of an off-loading device that protects the wound from pressure or trauma related to ambulation and other acts of daily living.4-6,8 Unfortunately, studies have shown that the best standard of care (SOC) only heals 30% of DFUs after 20 weeks of therapy.9 With the estimated cost per episode of care approaching $40,000, DFUs remain a costly and important problem.10
The altered extracellular matrix (ECM) in DFUs has been a target for the development of new therapeutic devices that provide a new matrix that is either devoid of cells or can be enriched with fibroblasts.8,11 These bioengineered skin substitutes stimulate the growth of new vessels and generate cytokines essential for tissue repair. In 2013, Lev-Tov et al12 published this study protocol (Dermagraft Oasis Longitudinal Comparative Efficacy [DOLCE] trial) to compare the effectiveness of 2 advanced wound care devices, specifically to evaluate the clinical efficacy of a cellular matrix versus an acellular matrix, which we have amended. The cellular matrix used in the study is a dermal substitute composed of viable newborn foreskin fibroblasts seeded onto a bioabsorbable polyglactin mesh on which fibroblasts generate an ECM.13,14 It is supplied frozen and requires specific thawing steps prior to application. The recommended regimen for treatment of DFUs for this cellular matrix is 8 weekly applications.13,14 In 2016, the cost of the product was reported as $1411 per 5.0×7.5-cm sheet.15 The acellular matrix product used in the study is a bioabsorbable ECM that is derived from porcine small intestinal submucosa.16,17 It is stored at room temperature and has a long shelf life, with a current price of $112.6 for a 3.0×3.5-cm single-layer fenestrated sheet ($1126.60 per box of 10 sheets). The industry-supported randomized controlled trials for each of these devices have reported a 20% added benefit in the rate of wound closure at week 12 compared to SOC.14,17
This article provides the interim report of the trial (registered at www.clinicaltrials.gov with the identifier NCT01450943) described in the published protocol and initiated in 2011,12 focusing on elements that required modification during the trial’s duration.
Methods
Study Protocol
The clinical trial was approved by the Veterans’ Affairs Institutional Research and Development Committee and their institutional review board. This study was funded by the Veteran’s Administration Merit Award (#10554640), which was awarded to 2 of the investigators (S.E.D. and R.R.I.). Eligible veterans were recruited from all 7 sites of the VA Northern California Healthcare System. This trial is a randomized, single-blinded, 3-armed, controlled clinical equivalence trial comparing the effectiveness of an SOC treatment, cellular ECM, and acellular ECM.
Study Products
The SOC dressing applied in the clinical trial included a sterile antimicrobial gel, a nonadherent dressing, and gauze.12 The SOC dressing also was used as a secondary dressing for the active treatment arms. Bacitracin antibiotic ointment was used as an alternative for patients with allergy to iodine.12
Randomization
The inclusion and exclusion criteria were previously outlined.12 After a 2-week screening phase to exclude rapid healers, patients were randomized into a treatment arm and entered the active phase for 12 weeks.
Primary and Secondary Outcomes
The primary outcome was complete wound closure by week 12.12 Complete healing was defined as full reepithelialization with no drainage or dressing requirement. The secondary outcomes included healing at 28 weeks, rate of healing, ulcer recurrence at week 20, association of wound healing with ulcer characteristics or patients’ characteristic, incidence of adverse events, and cost-effectiveness of each treatment compared to the SOC arm.12
Statistical Analysis
To detect a 25% difference in the incidence of ulcer closure between the 2 study groups and the SOC group, the estimation of the sample size was based on 80% power with a significance level of 0.05. Specifically, it was expected that 50% of the cellular and acellular matrix groups and 25% of the SOC group would reach complete wound closure. The protocol indicated that 57 participants would be enrolled in each arm (total of 171 participants). Lev-Tov et al12 discussed the statistical analysis in more detail.
Results
Study Protocol Amendments
Given the number of diabetic patients in the US veteran population, we anticipated that there would be enough participants meeting the inclusion and exclusion criteria; however, because of the difficulty with recruitment, the initial study criteria were modified. The study was initially designed to incorporate DFUs with a minimum size of 1.0 cm2.12
Another limiting criterion was the percentage of total hemoglobin level for hemoglobin A1C (HbA1C). The study was originally established to include participants with an HbA1C level of 10% of total hemoglobin or below.12 Unfortunately, the majority of the potential participants had values substantially higher, and thus could not be enrolled in the trial, requiring another amendment to the study protocol in 2014, which was approved to include patients with an HbA1C level less than 12% of total hemoglobin. This change contributed considerably to the noted increase in enrollment rates in 2015, which almost doubled relative to enrollment under the original exclusion criteria (Figure).

The study has screened more than 600 patients. Among them, 137 were assessed for eligibility; 71 were excluded for various reasons, including screen failure (eg, decrease in wound size by >40% during the 2-week screening phase), loss to follow-up, and adverse events. Sixty-six participants reached the primary outcome at week 12, while 55 participants completed the study (19 in the SOC group; 18 in the cellular matrix group; 18 in the acellular matrix group).
We have stopped enrolling patients from all sites and the community, as we have reached our target enrollment.
Comment
One of the challenges of clinical trials is the recruitment of an adequate number of participants within an appropriate time frame, which is explained by Lasagna’s Law,18 a well-described phenomenon whereby the investigator overestimates the number of potential participants available to meet the inclusion criteria. This so-called funnel-effect was partly encountered in our selection process. A review of the veteran population with DFUs seemed to be more than adequate to fulfill the sample size; however, some important participant-related factors also played a substantial role.
In addition, the Veterans’ Affairs network centralizes health information, making it readily available to all providers participating in their care. As a result, patients with diabetes mellitus typically are seen by a primary care physician along with an endocrinologist, a diabetic nurse, and/or a dietician. Despite the collaboration with an interdisciplinary team, the glycemic control of the participants remains an issue along with other psychosocial factors that are deterrents in patient compliance. As a result, patients with poorly controlled diabetes and an HbA1C level above 10% (and less than 12%) of total hemoglobin who were initially excluded from the study were reincluded after modifying the inclusion criteria. Some patients were interested in joining the study, but physical limitations (eg, impaired mobility) prompted their decision not to join the trial, even though they met all the inclusion criteria.
As far as research-related factors that could affect participation, it is notable that most of the patients were retired; thus, the interventions did not cause additional burden of taking time off from work or loss of productivity. Although randomization could be a deterrent in many clinical trials, the majority of patients were willing to participate without demanding to be assigned to a particular treatment group.
There are many factors that are intertwined and can lead to enrollment and/or attrition rates. It was critical for our team to make some adjustment without compromising the controlled nature of a randomized trial.
Acknowledgment
The authors wish to acknowledge Huong Le, DPM, MPH, who was the coauthor of the study protocol.
Chronic diabetic foot ulcers (DFUs) remain a serious therapeutic challenge worldwide.1-2 Patients with DFUs are at higher risk for infections, which may lead to limb loss.1-5 In fact, 1 in 6 patients with DFUs will undergo an amputation.6 The long-term consequences of DFUs are numerous and can severely affect patients’ quality of life, including loss of productivity.7 The current standard of care for DFUs consists of debridement of the necrotic tissue, application of a moist dressing, and use of an off-loading device that protects the wound from pressure or trauma related to ambulation and other acts of daily living.4-6,8 Unfortunately, studies have shown that the best standard of care (SOC) only heals 30% of DFUs after 20 weeks of therapy.9 With the estimated cost per episode of care approaching $40,000, DFUs remain a costly and important problem.10
The altered extracellular matrix (ECM) in DFUs has been a target for the development of new therapeutic devices that provide a new matrix that is either devoid of cells or can be enriched with fibroblasts.8,11 These bioengineered skin substitutes stimulate the growth of new vessels and generate cytokines essential for tissue repair. In 2013, Lev-Tov et al12 published this study protocol (Dermagraft Oasis Longitudinal Comparative Efficacy [DOLCE] trial) to compare the effectiveness of 2 advanced wound care devices, specifically to evaluate the clinical efficacy of a cellular matrix versus an acellular matrix, which we have amended. The cellular matrix used in the study is a dermal substitute composed of viable newborn foreskin fibroblasts seeded onto a bioabsorbable polyglactin mesh on which fibroblasts generate an ECM.13,14 It is supplied frozen and requires specific thawing steps prior to application. The recommended regimen for treatment of DFUs for this cellular matrix is 8 weekly applications.13,14 In 2016, the cost of the product was reported as $1411 per 5.0×7.5-cm sheet.15 The acellular matrix product used in the study is a bioabsorbable ECM that is derived from porcine small intestinal submucosa.16,17 It is stored at room temperature and has a long shelf life, with a current price of $112.6 for a 3.0×3.5-cm single-layer fenestrated sheet ($1126.60 per box of 10 sheets). The industry-supported randomized controlled trials for each of these devices have reported a 20% added benefit in the rate of wound closure at week 12 compared to SOC.14,17
This article provides the interim report of the trial (registered at www.clinicaltrials.gov with the identifier NCT01450943) described in the published protocol and initiated in 2011,12 focusing on elements that required modification during the trial’s duration.
Methods
Study Protocol
The clinical trial was approved by the Veterans’ Affairs Institutional Research and Development Committee and their institutional review board. This study was funded by the Veteran’s Administration Merit Award (#10554640), which was awarded to 2 of the investigators (S.E.D. and R.R.I.). Eligible veterans were recruited from all 7 sites of the VA Northern California Healthcare System. This trial is a randomized, single-blinded, 3-armed, controlled clinical equivalence trial comparing the effectiveness of an SOC treatment, cellular ECM, and acellular ECM.
Study Products
The SOC dressing applied in the clinical trial included a sterile antimicrobial gel, a nonadherent dressing, and gauze.12 The SOC dressing also was used as a secondary dressing for the active treatment arms. Bacitracin antibiotic ointment was used as an alternative for patients with allergy to iodine.12
Randomization
The inclusion and exclusion criteria were previously outlined.12 After a 2-week screening phase to exclude rapid healers, patients were randomized into a treatment arm and entered the active phase for 12 weeks.
Primary and Secondary Outcomes
The primary outcome was complete wound closure by week 12.12 Complete healing was defined as full reepithelialization with no drainage or dressing requirement. The secondary outcomes included healing at 28 weeks, rate of healing, ulcer recurrence at week 20, association of wound healing with ulcer characteristics or patients’ characteristic, incidence of adverse events, and cost-effectiveness of each treatment compared to the SOC arm.12
Statistical Analysis
To detect a 25% difference in the incidence of ulcer closure between the 2 study groups and the SOC group, the estimation of the sample size was based on 80% power with a significance level of 0.05. Specifically, it was expected that 50% of the cellular and acellular matrix groups and 25% of the SOC group would reach complete wound closure. The protocol indicated that 57 participants would be enrolled in each arm (total of 171 participants). Lev-Tov et al12 discussed the statistical analysis in more detail.
Results
Study Protocol Amendments
Given the number of diabetic patients in the US veteran population, we anticipated that there would be enough participants meeting the inclusion and exclusion criteria; however, because of the difficulty with recruitment, the initial study criteria were modified. The study was initially designed to incorporate DFUs with a minimum size of 1.0 cm2.12
Another limiting criterion was the percentage of total hemoglobin level for hemoglobin A1C (HbA1C). The study was originally established to include participants with an HbA1C level of 10% of total hemoglobin or below.12 Unfortunately, the majority of the potential participants had values substantially higher, and thus could not be enrolled in the trial, requiring another amendment to the study protocol in 2014, which was approved to include patients with an HbA1C level less than 12% of total hemoglobin. This change contributed considerably to the noted increase in enrollment rates in 2015, which almost doubled relative to enrollment under the original exclusion criteria (Figure).

The study has screened more than 600 patients. Among them, 137 were assessed for eligibility; 71 were excluded for various reasons, including screen failure (eg, decrease in wound size by >40% during the 2-week screening phase), loss to follow-up, and adverse events. Sixty-six participants reached the primary outcome at week 12, while 55 participants completed the study (19 in the SOC group; 18 in the cellular matrix group; 18 in the acellular matrix group).
We have stopped enrolling patients from all sites and the community, as we have reached our target enrollment.
Comment
One of the challenges of clinical trials is the recruitment of an adequate number of participants within an appropriate time frame, which is explained by Lasagna’s Law,18 a well-described phenomenon whereby the investigator overestimates the number of potential participants available to meet the inclusion criteria. This so-called funnel-effect was partly encountered in our selection process. A review of the veteran population with DFUs seemed to be more than adequate to fulfill the sample size; however, some important participant-related factors also played a substantial role.
In addition, the Veterans’ Affairs network centralizes health information, making it readily available to all providers participating in their care. As a result, patients with diabetes mellitus typically are seen by a primary care physician along with an endocrinologist, a diabetic nurse, and/or a dietician. Despite the collaboration with an interdisciplinary team, the glycemic control of the participants remains an issue along with other psychosocial factors that are deterrents in patient compliance. As a result, patients with poorly controlled diabetes and an HbA1C level above 10% (and less than 12%) of total hemoglobin who were initially excluded from the study were reincluded after modifying the inclusion criteria. Some patients were interested in joining the study, but physical limitations (eg, impaired mobility) prompted their decision not to join the trial, even though they met all the inclusion criteria.
As far as research-related factors that could affect participation, it is notable that most of the patients were retired; thus, the interventions did not cause additional burden of taking time off from work or loss of productivity. Although randomization could be a deterrent in many clinical trials, the majority of patients were willing to participate without demanding to be assigned to a particular treatment group.
There are many factors that are intertwined and can lead to enrollment and/or attrition rates. It was critical for our team to make some adjustment without compromising the controlled nature of a randomized trial.
Acknowledgment
The authors wish to acknowledge Huong Le, DPM, MPH, who was the coauthor of the study protocol.
- Sen CK, Gordillo GM, Roy S, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17:763-771.
- Gurtner GC, Werner S, Barrandon Y, et al. Wound repair and regeneration. Nature. 2008;453:314-321.
- Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366:1736-1743.
- Boulton AJ. The diabetic foot: grand overview, epidemiology and pathogenesis. Diabetes Metab Res Rev. 2008;24(suppl 1):S3-S6.
- Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA. 2005;293:217-228.
- Vuorisalo S, Venermo M, Lepäntalo M. Treatment of diabetic foot ulcers. J Cardiovasc Surg (Torino). 2009;50:275-291.
- Meijer JW, Trip J, Jaegers SM, et al. Quality of life in patients with diabetic foot ulcers. Disabil Rehabil. 2001;23:336-340.
- Santema TB, Poyck PP, Ubbink DT. Skin grafting and tissue replacement for treating foot ulcers in people with diabetes. Cochrane Database Syst Rev. 2016;2:CD011255.
- Margolis DJ, Kantor J, Berlin JA. Healing of diabetic neuropathic foot ulcers receiving standard treatment. a meta-analysis. Diabetes Care. 1999;22:692-695.
- Cavanagh P, Attinger C, Abbas Z, et al. Cost of treating diabetic foot ulcers in five different countries. Diabetes Metab Res Rev. 2012;2(suppl 1):107-111.
- Panuncialman J, Falanga V. The science of wound bed preparation. Surg Clin North Am. 2009;89:611-626.
- Lev-Tov H, Li CS, Dahle S, et al. Cellular versus acellular matrix devices in treatment of diabetic foot ulcers: study protocol for a comparative efficacy randomized controlled trial. Trials. 2013;14:8.
- Gentzkow GD, Iwasaki SD, Hershon KS, et al. Use of dermagraft, a cultured human dermis, to treat diabetic foot ulcers. Diabetes Care. 1996;19:350-354.
- Marston WA, Hanft J, Norwood P, et al; Dermagraft Diabetic Foot Ulcer Study Group. The efficacy and safety of Dermagraft in improving the healing of chronic diabetic foot ulcers: results of a prospective randomized trial. Diabetes Care. 2003;26:1701-1705.
- 2016 Dermagraft® Medicare Product and Related Procedure Payment. http://www.dermagraft.com/wp-content/uploads/sites/1/Dermagraft_Hotsheet%202016%20Q1%20HOSPITAL_FINAL.pdf. Accessed November 23, 2017.
- Oasis® Wound Matrix. http://www.oasiswoundmatrix.com/aboutowm. Accessed November 23, 2017.
- Niezgoda JA, Van Gils CC, Frykberg RG, et al. Randomized clinical trial comparing OASIS Wound Matrix to Regranex Gel for diabetic ulcers. Adv Skin Wound Care. 2005;18(5, pt 1):258-266.
- Torgerson JS, Arlinger K, Käppi M, et al. Principles for enhanced recruitment of subjects in a large clinical trial. the XENDOS (XENical in the prevention of Diabetes in Obese Subjects) study experience. Controlled Clin Trials. 2001;22:515-525.
- Sen CK, Gordillo GM, Roy S, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17:763-771.
- Gurtner GC, Werner S, Barrandon Y, et al. Wound repair and regeneration. Nature. 2008;453:314-321.
- Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366:1736-1743.
- Boulton AJ. The diabetic foot: grand overview, epidemiology and pathogenesis. Diabetes Metab Res Rev. 2008;24(suppl 1):S3-S6.
- Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA. 2005;293:217-228.
- Vuorisalo S, Venermo M, Lepäntalo M. Treatment of diabetic foot ulcers. J Cardiovasc Surg (Torino). 2009;50:275-291.
- Meijer JW, Trip J, Jaegers SM, et al. Quality of life in patients with diabetic foot ulcers. Disabil Rehabil. 2001;23:336-340.
- Santema TB, Poyck PP, Ubbink DT. Skin grafting and tissue replacement for treating foot ulcers in people with diabetes. Cochrane Database Syst Rev. 2016;2:CD011255.
- Margolis DJ, Kantor J, Berlin JA. Healing of diabetic neuropathic foot ulcers receiving standard treatment. a meta-analysis. Diabetes Care. 1999;22:692-695.
- Cavanagh P, Attinger C, Abbas Z, et al. Cost of treating diabetic foot ulcers in five different countries. Diabetes Metab Res Rev. 2012;2(suppl 1):107-111.
- Panuncialman J, Falanga V. The science of wound bed preparation. Surg Clin North Am. 2009;89:611-626.
- Lev-Tov H, Li CS, Dahle S, et al. Cellular versus acellular matrix devices in treatment of diabetic foot ulcers: study protocol for a comparative efficacy randomized controlled trial. Trials. 2013;14:8.
- Gentzkow GD, Iwasaki SD, Hershon KS, et al. Use of dermagraft, a cultured human dermis, to treat diabetic foot ulcers. Diabetes Care. 1996;19:350-354.
- Marston WA, Hanft J, Norwood P, et al; Dermagraft Diabetic Foot Ulcer Study Group. The efficacy and safety of Dermagraft in improving the healing of chronic diabetic foot ulcers: results of a prospective randomized trial. Diabetes Care. 2003;26:1701-1705.
- 2016 Dermagraft® Medicare Product and Related Procedure Payment. http://www.dermagraft.com/wp-content/uploads/sites/1/Dermagraft_Hotsheet%202016%20Q1%20HOSPITAL_FINAL.pdf. Accessed November 23, 2017.
- Oasis® Wound Matrix. http://www.oasiswoundmatrix.com/aboutowm. Accessed November 23, 2017.
- Niezgoda JA, Van Gils CC, Frykberg RG, et al. Randomized clinical trial comparing OASIS Wound Matrix to Regranex Gel for diabetic ulcers. Adv Skin Wound Care. 2005;18(5, pt 1):258-266.
- Torgerson JS, Arlinger K, Käppi M, et al. Principles for enhanced recruitment of subjects in a large clinical trial. the XENDOS (XENical in the prevention of Diabetes in Obese Subjects) study experience. Controlled Clin Trials. 2001;22:515-525.
Resident Pearl
- Deciding on the appropriate wound care regimen for diabetic foot ulcers is difficult given the vast amount of wound products on the market. This head-to-head clinical trial compared the use of an expensive cellular matrix and an inexpensive acellular matrix relative to the standard of care. We hope that this study will help to guide therapy based on cost-effectiveness of wound adjuncts without compromising patient care.
Exercise program speeds healing of venous leg ulcers
A supervised exercise program for patients with venous leg ulcers has shown improved healing times over compression therapy alone, according to a paper published online on Oct. 27 in the British Journal of Dermatology.
In a parallel group feasibility trial, researchers randomized 39 patients with venous ulcers either to a 12-week program of supervised exercise three times a week plus compression therapy (18 patients), or compression therapy alone (21 patients). The exercise program combined aerobic, resistance, and flexibility exercises.
This group showed a median ulcer healing time of 13 weeks (3.9-52 weeks), compared with 34.7 weeks (4.3-52 weeks) for the compression therapy–only group, although the median ulcer size was similar between the two groups at 12 months. At last follow-up of 12 months, 83% of the ulcers in the exercise group had healed, compared with 60% in the control group (Br J Dermatol. 2017 Oct 27. doi: 10.1111/bjd.16089).
The intervention group had a slightly higher quality of life at baseline, as measured by the EQ-5D utility score, and this difference was maintained throughout the study.
Nearly three-quarters (72%) of the exercise group participants went to all the scheduled exercise sessions, with an overall attendance rate of 79%, which the authors noted was high considering many were old, frail, and had no previous exercise experience.
“This was achieved without employing any specific adherence-enhancing components or provision of behavioral change support, which could have potentially improved attendance rates and the effect of the intervention even further,” wrote Markos Klonizakis, DPhil, from the Centre for Sport and Exercise Science at Sheffield (England) Hallam University, and his coinvestigators.
There were no serious adverse events, and only two exercise-related adverse events in the intervention group – both excessive discharge from the ulcer – which resulted in postponement of the exercise sessions for those two individuals.
The exercise regimen was associated with modest reductions in weight, while those in the control group showed an overall increase in weight.
Researchers also assessed the relative costs of the two interventions by getting participants to keep a diary of their use of National Health Service resources, health care visits, prescriptions, and other out-of-pocket expenses.
They calculated that the total mean National Health Service cost per participant for the exercise intervention was £813.27, and £2,298.57 for the control group who received compression therapy only.
The investigators noted that their initial plan had been met with some skepticism from clinicians and patients, some of whom felt that exercise would have a detrimental rather than positive effect on venous ulcer healing.
“Our results suggest that there may be significant potential benefit in healing rates and that, if this were confirmed in a full trial, the introduction of supervised exercise for venous leg ulcers may well also be cost-saving for the National Health Service.”
The study was funded by the National Institute for Health Research. No conflicts of interest were declared.
A supervised exercise program for patients with venous leg ulcers has shown improved healing times over compression therapy alone, according to a paper published online on Oct. 27 in the British Journal of Dermatology.
In a parallel group feasibility trial, researchers randomized 39 patients with venous ulcers either to a 12-week program of supervised exercise three times a week plus compression therapy (18 patients), or compression therapy alone (21 patients). The exercise program combined aerobic, resistance, and flexibility exercises.
This group showed a median ulcer healing time of 13 weeks (3.9-52 weeks), compared with 34.7 weeks (4.3-52 weeks) for the compression therapy–only group, although the median ulcer size was similar between the two groups at 12 months. At last follow-up of 12 months, 83% of the ulcers in the exercise group had healed, compared with 60% in the control group (Br J Dermatol. 2017 Oct 27. doi: 10.1111/bjd.16089).
The intervention group had a slightly higher quality of life at baseline, as measured by the EQ-5D utility score, and this difference was maintained throughout the study.
Nearly three-quarters (72%) of the exercise group participants went to all the scheduled exercise sessions, with an overall attendance rate of 79%, which the authors noted was high considering many were old, frail, and had no previous exercise experience.
“This was achieved without employing any specific adherence-enhancing components or provision of behavioral change support, which could have potentially improved attendance rates and the effect of the intervention even further,” wrote Markos Klonizakis, DPhil, from the Centre for Sport and Exercise Science at Sheffield (England) Hallam University, and his coinvestigators.
There were no serious adverse events, and only two exercise-related adverse events in the intervention group – both excessive discharge from the ulcer – which resulted in postponement of the exercise sessions for those two individuals.
The exercise regimen was associated with modest reductions in weight, while those in the control group showed an overall increase in weight.
Researchers also assessed the relative costs of the two interventions by getting participants to keep a diary of their use of National Health Service resources, health care visits, prescriptions, and other out-of-pocket expenses.
They calculated that the total mean National Health Service cost per participant for the exercise intervention was £813.27, and £2,298.57 for the control group who received compression therapy only.
The investigators noted that their initial plan had been met with some skepticism from clinicians and patients, some of whom felt that exercise would have a detrimental rather than positive effect on venous ulcer healing.
“Our results suggest that there may be significant potential benefit in healing rates and that, if this were confirmed in a full trial, the introduction of supervised exercise for venous leg ulcers may well also be cost-saving for the National Health Service.”
The study was funded by the National Institute for Health Research. No conflicts of interest were declared.
A supervised exercise program for patients with venous leg ulcers has shown improved healing times over compression therapy alone, according to a paper published online on Oct. 27 in the British Journal of Dermatology.
In a parallel group feasibility trial, researchers randomized 39 patients with venous ulcers either to a 12-week program of supervised exercise three times a week plus compression therapy (18 patients), or compression therapy alone (21 patients). The exercise program combined aerobic, resistance, and flexibility exercises.
This group showed a median ulcer healing time of 13 weeks (3.9-52 weeks), compared with 34.7 weeks (4.3-52 weeks) for the compression therapy–only group, although the median ulcer size was similar between the two groups at 12 months. At last follow-up of 12 months, 83% of the ulcers in the exercise group had healed, compared with 60% in the control group (Br J Dermatol. 2017 Oct 27. doi: 10.1111/bjd.16089).
The intervention group had a slightly higher quality of life at baseline, as measured by the EQ-5D utility score, and this difference was maintained throughout the study.
Nearly three-quarters (72%) of the exercise group participants went to all the scheduled exercise sessions, with an overall attendance rate of 79%, which the authors noted was high considering many were old, frail, and had no previous exercise experience.
“This was achieved without employing any specific adherence-enhancing components or provision of behavioral change support, which could have potentially improved attendance rates and the effect of the intervention even further,” wrote Markos Klonizakis, DPhil, from the Centre for Sport and Exercise Science at Sheffield (England) Hallam University, and his coinvestigators.
There were no serious adverse events, and only two exercise-related adverse events in the intervention group – both excessive discharge from the ulcer – which resulted in postponement of the exercise sessions for those two individuals.
The exercise regimen was associated with modest reductions in weight, while those in the control group showed an overall increase in weight.
Researchers also assessed the relative costs of the two interventions by getting participants to keep a diary of their use of National Health Service resources, health care visits, prescriptions, and other out-of-pocket expenses.
They calculated that the total mean National Health Service cost per participant for the exercise intervention was £813.27, and £2,298.57 for the control group who received compression therapy only.
The investigators noted that their initial plan had been met with some skepticism from clinicians and patients, some of whom felt that exercise would have a detrimental rather than positive effect on venous ulcer healing.
“Our results suggest that there may be significant potential benefit in healing rates and that, if this were confirmed in a full trial, the introduction of supervised exercise for venous leg ulcers may well also be cost-saving for the National Health Service.”
The study was funded by the National Institute for Health Research. No conflicts of interest were declared.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
Key clinical point: A supervised exercise program for patients with venous leg ulcers has shown significantly improved healing times over compression therapy alone.
Major finding: Patients who underwent a program of supervised exercise in addition to compression therapy showed a median ulcer healing time of 13 weeks, compared with 35 weeks for patients who received compression therapy alone.
Data source: A randomized, parallel group feasibility trial in 39 patients with venous ulcers.
Disclosures: The study was funded by the National Institute for Health Research. No conflicts of interest were declared.
Topical timolol improved chronic leg ulcer healing
The use of topical timolol maleate as a treatment for chronic diabetic and chronic venous ulcers showed increased wound healing compared with controls, according to the results of a prospective observational study of 60 patients.
In the treatment group, 30 patients with chronic leg ulcer (15 diabetic ulcers; 15 venous) received topical application of 0.5% timolol maleate (a beta-blocker) plus conventional antibiotic and wound dressing therapy. In the control group, 30 patients (identical split between diabetic and venous ulcers) were treated with conventional therapy alone, according to a report published in the November issue of the Journal of Vascular Surgery: Venous and Lymphatic Disorders.
The researchers found no significant difference in healing rates due to sex, between smokers and nonsmokers, or alcohol consumers vs. nonconsumers and they saw no major adverse effects due to timolol application (J Vasc Surg: Venous and Lym Dis 2017;5:844-50).
They reported that the limitations of their study included the lack of randomization and a formal power assessment.
“Topical application of timolol maleate is an effective therapeutic option for the treatment of chronic diabetic ulcer and chronic venous ulcer patients to improve ulcer healing by promoting keratinocyte migration,” the researchers concluded.
They reported having no relevant conflicts.
The use of topical timolol maleate as a treatment for chronic diabetic and chronic venous ulcers showed increased wound healing compared with controls, according to the results of a prospective observational study of 60 patients.
In the treatment group, 30 patients with chronic leg ulcer (15 diabetic ulcers; 15 venous) received topical application of 0.5% timolol maleate (a beta-blocker) plus conventional antibiotic and wound dressing therapy. In the control group, 30 patients (identical split between diabetic and venous ulcers) were treated with conventional therapy alone, according to a report published in the November issue of the Journal of Vascular Surgery: Venous and Lymphatic Disorders.
The researchers found no significant difference in healing rates due to sex, between smokers and nonsmokers, or alcohol consumers vs. nonconsumers and they saw no major adverse effects due to timolol application (J Vasc Surg: Venous and Lym Dis 2017;5:844-50).
They reported that the limitations of their study included the lack of randomization and a formal power assessment.
“Topical application of timolol maleate is an effective therapeutic option for the treatment of chronic diabetic ulcer and chronic venous ulcer patients to improve ulcer healing by promoting keratinocyte migration,” the researchers concluded.
They reported having no relevant conflicts.
The use of topical timolol maleate as a treatment for chronic diabetic and chronic venous ulcers showed increased wound healing compared with controls, according to the results of a prospective observational study of 60 patients.
In the treatment group, 30 patients with chronic leg ulcer (15 diabetic ulcers; 15 venous) received topical application of 0.5% timolol maleate (a beta-blocker) plus conventional antibiotic and wound dressing therapy. In the control group, 30 patients (identical split between diabetic and venous ulcers) were treated with conventional therapy alone, according to a report published in the November issue of the Journal of Vascular Surgery: Venous and Lymphatic Disorders.
The researchers found no significant difference in healing rates due to sex, between smokers and nonsmokers, or alcohol consumers vs. nonconsumers and they saw no major adverse effects due to timolol application (J Vasc Surg: Venous and Lym Dis 2017;5:844-50).
They reported that the limitations of their study included the lack of randomization and a formal power assessment.
“Topical application of timolol maleate is an effective therapeutic option for the treatment of chronic diabetic ulcer and chronic venous ulcer patients to improve ulcer healing by promoting keratinocyte migration,” the researchers concluded.
They reported having no relevant conflicts.
FROM THE JOURNAL OF VASCULAR SURGERY: VENOUS AND LYMPHATIC DISORDERS
Clinical Pearl: A Simple and Effective Technique for Improving Surgical Closures for the Early-Learning Resident
Practice Gap
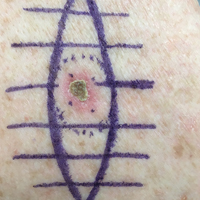
For first-year dermatology residents, dermatologic surgeries can present many challenges. Although approximation of wound edges following excision may be intuitive for the experienced surgeon, an early trainee may need some guidance. Infusion of anesthetics can distort the normal skin field or it may be difficult for the patient to remain in the same position for the required period of time; for example, an elderly patient who requires an excision on the posterior aspect of the neck may be unable to assume the same position for the full duration of the procedure. We offer a simple and effective technique for early-learning dermatology residents to improve surgical closures.
The Technique
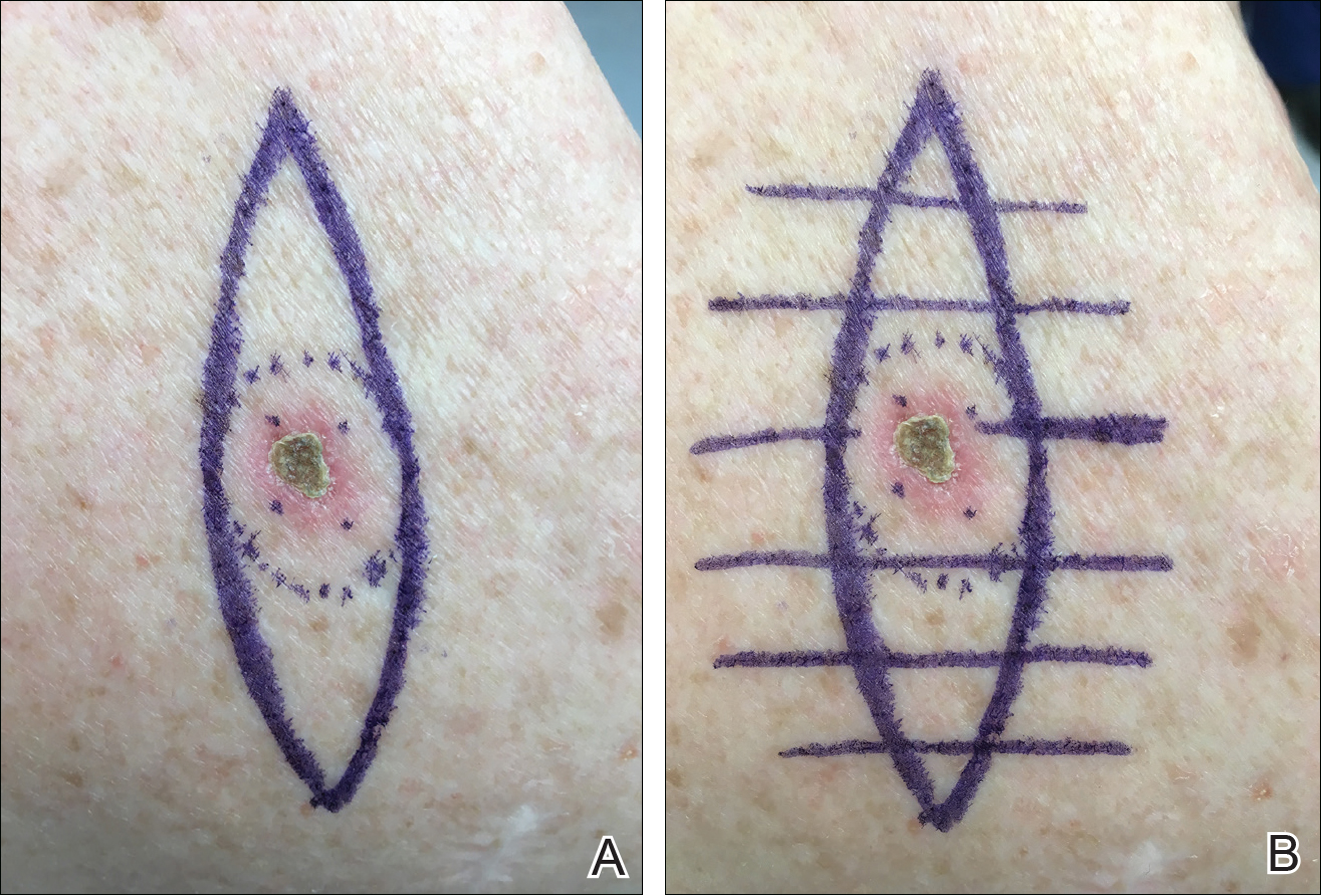
We propose drawing straight lines using a sterile marking pen perpendicular to the fusiform plane laid out for any simple, intermediate, or complex linear closure (Figure 1). These lines can then be used as scaffolding for the surgical closure (Figure 2). We recommend drawing the lines at the time of initial planning when the site of excision is in the normal anatomic position.
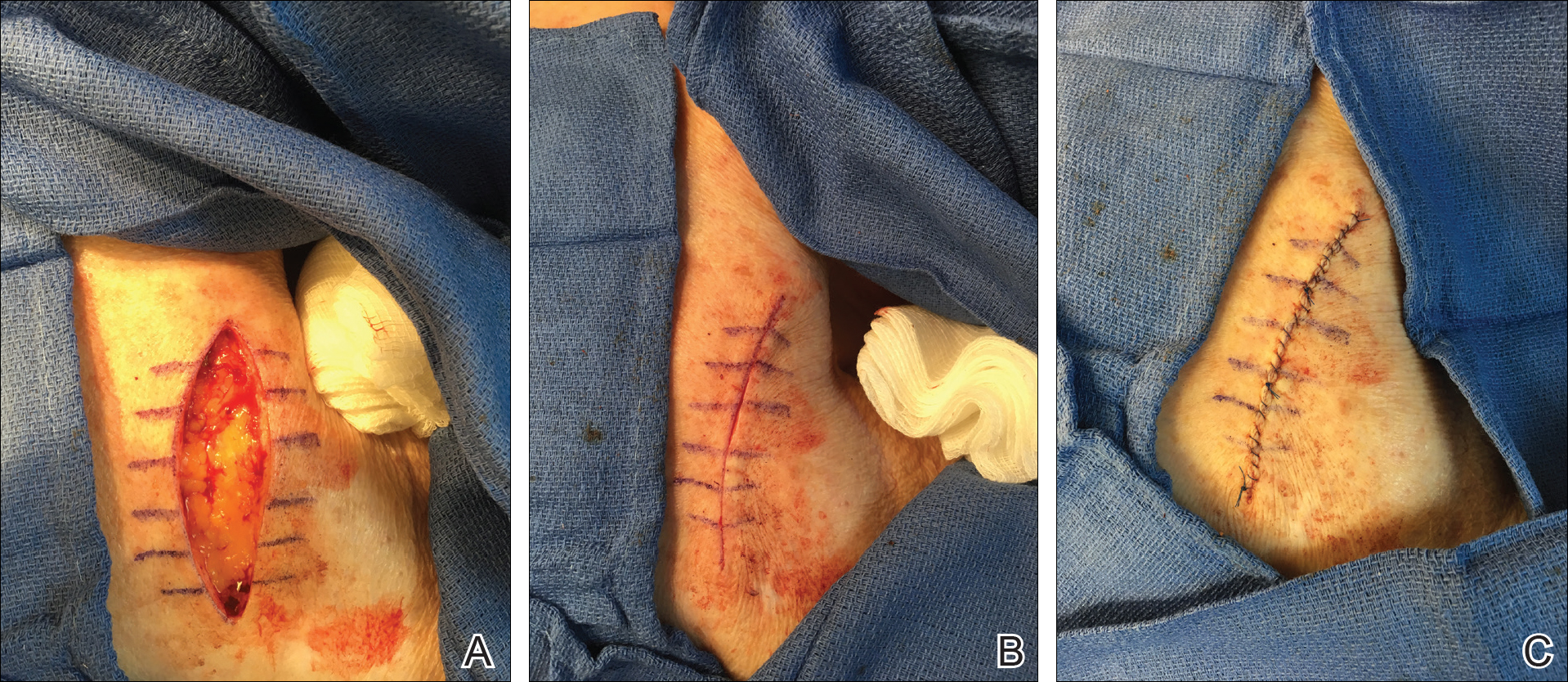
Practice Implications
By creating a sketch with perpendicular lines, approximation of skin edges and surgical closures may become easier for the learning resident. Patients also can rest more comfortably during the procedure, and the overall cosmesis, healing, and outcome of the procedure may improve. The addition of a sterile marking pen to the surgical tray may aide in highlighting faded pen markings for easier visualization after cleansing of the surgical site.
Practice Gap
For first-year dermatology residents, dermatologic surgeries can present many challenges. Although approximation of wound edges following excision may be intuitive for the experienced surgeon, an early trainee may need some guidance. Infusion of anesthetics can distort the normal skin field or it may be difficult for the patient to remain in the same position for the required period of time; for example, an elderly patient who requires an excision on the posterior aspect of the neck may be unable to assume the same position for the full duration of the procedure. We offer a simple and effective technique for early-learning dermatology residents to improve surgical closures.
The Technique
We propose drawing straight lines using a sterile marking pen perpendicular to the fusiform plane laid out for any simple, intermediate, or complex linear closure (Figure 1). These lines can then be used as scaffolding for the surgical closure (Figure 2). We recommend drawing the lines at the time of initial planning when the site of excision is in the normal anatomic position.


Practice Implications
By creating a sketch with perpendicular lines, approximation of skin edges and surgical closures may become easier for the learning resident. Patients also can rest more comfortably during the procedure, and the overall cosmesis, healing, and outcome of the procedure may improve. The addition of a sterile marking pen to the surgical tray may aide in highlighting faded pen markings for easier visualization after cleansing of the surgical site.
Practice Gap
For first-year dermatology residents, dermatologic surgeries can present many challenges. Although approximation of wound edges following excision may be intuitive for the experienced surgeon, an early trainee may need some guidance. Infusion of anesthetics can distort the normal skin field or it may be difficult for the patient to remain in the same position for the required period of time; for example, an elderly patient who requires an excision on the posterior aspect of the neck may be unable to assume the same position for the full duration of the procedure. We offer a simple and effective technique for early-learning dermatology residents to improve surgical closures.
The Technique
We propose drawing straight lines using a sterile marking pen perpendicular to the fusiform plane laid out for any simple, intermediate, or complex linear closure (Figure 1). These lines can then be used as scaffolding for the surgical closure (Figure 2). We recommend drawing the lines at the time of initial planning when the site of excision is in the normal anatomic position.


Practice Implications
By creating a sketch with perpendicular lines, approximation of skin edges and surgical closures may become easier for the learning resident. Patients also can rest more comfortably during the procedure, and the overall cosmesis, healing, and outcome of the procedure may improve. The addition of a sterile marking pen to the surgical tray may aide in highlighting faded pen markings for easier visualization after cleansing of the surgical site.
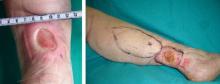
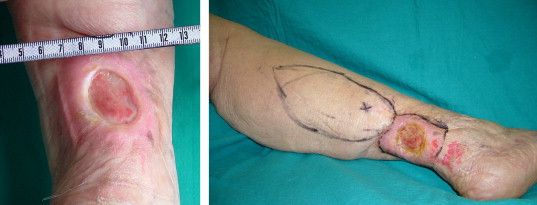
Recalcitrant Ulcer on the Lower Leg
The Diagnosis: Nonuremic Calciphylaxis
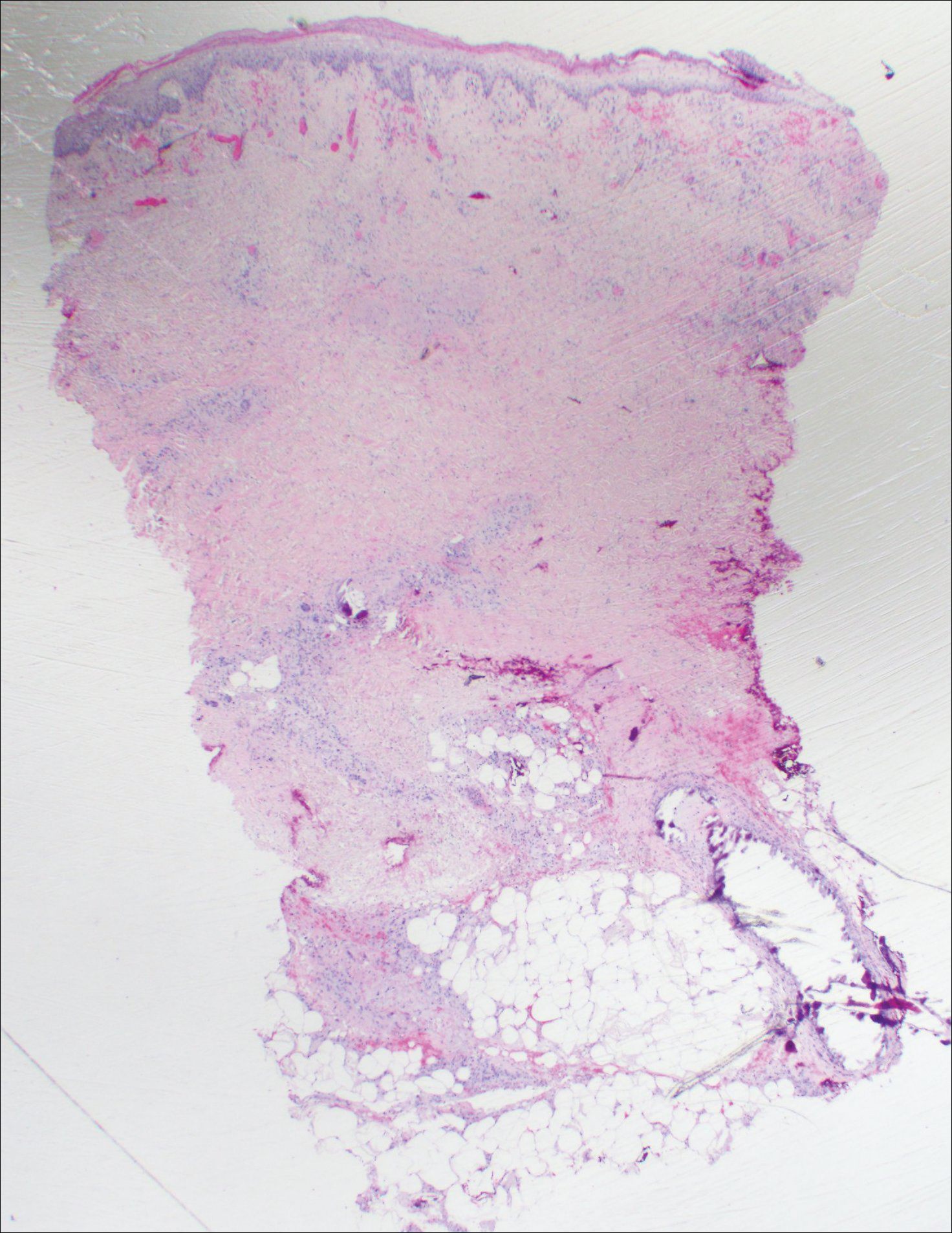
Histopathologic findings revealed ischemic necrosis and a subepidermal blister (Figure 1) with arteriosclerotic changes and fat necrosis. Foci of calcification were noted within the fat lobules. Arterioles within the deeper dermis and subcutis showed thickened hyalinized walls, narrowed lumina, and medial calcification (Figure 2). Multiple sections did not reveal any granulomatous inflammation. Periodic acid-Schiff and Gram stains were negative for fungal and bacterial elements, respectively. No dense neutrophilic infiltrate was seen. Multifocal calcific deposits within fat lobules and vessel walls (endothelium highlighted by the CD31 stain) suggested calciphylaxis.
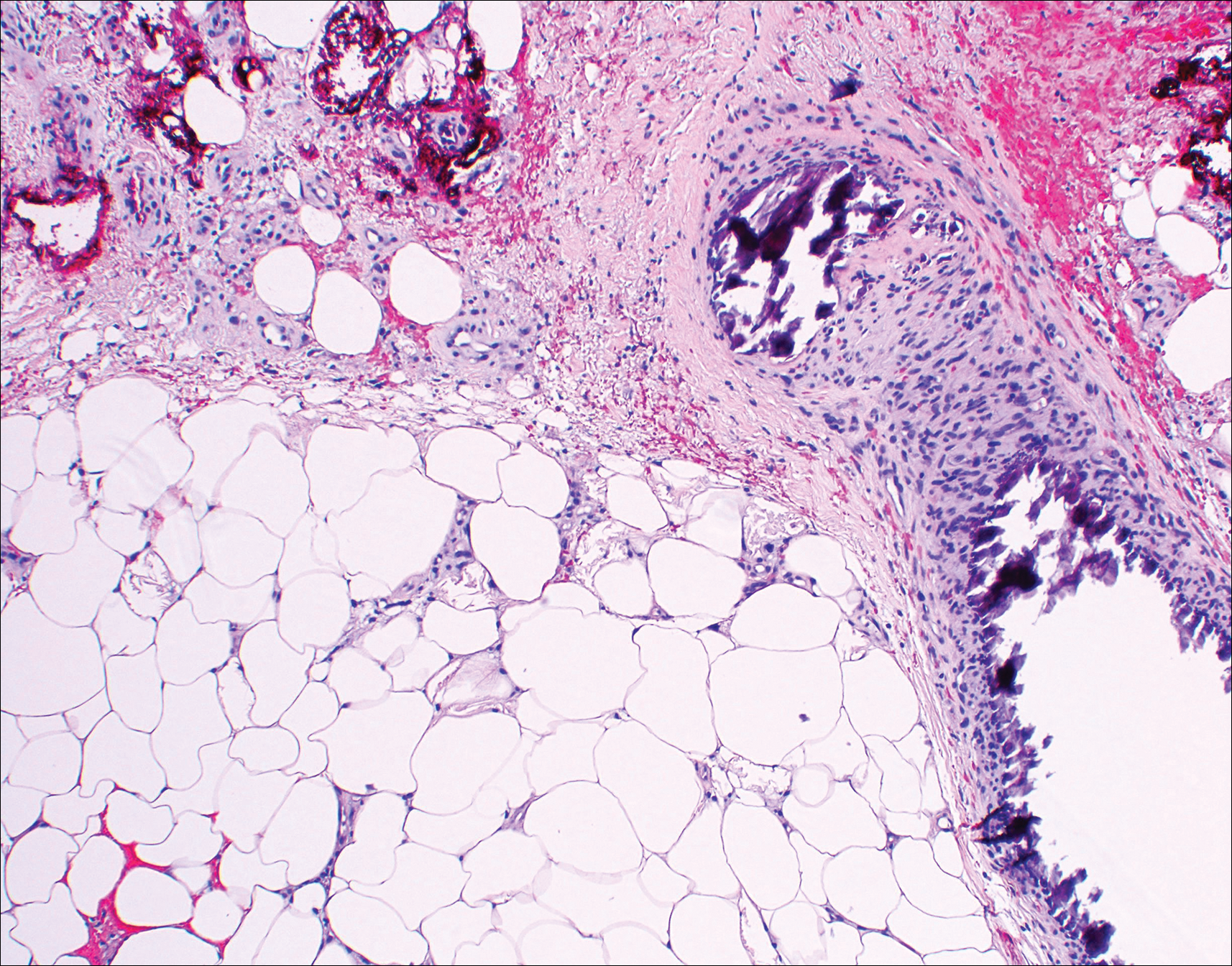
Laboratory test results revealed a normal white blood cell count, international normalized ratio level of 4 (on warfarin), and an elevated sedimentation rate at 72 mm/h (reference range, 0-20 mm/h). Serum creatinine was 1.1 mg/dL (reference range, 0.6-1.2 mg/dL) and the calcium-phosphorous product was 40.8 mg2/dL (reference range, <55 mg2/dL). Hemoglobin A1C (glycated hemoglobin) was 8.2% (reference range, 4%-7%). Wound cultures grew Proteus mirabilis sensitive to cefazolin. Acid-fast bacilli and fungal cultures were negative. Computed tomography of the left lower leg without contrast showed no evidence of osteomyelitis. Of note, the popliteal arteries and distal vessels showed moderate vascular calcification.
Histopathology findings as well as a clinical picture of painful ulceration on the distal extremities and uncontrolled diabetes with normal renal function favored a diagnosis of nonuremic calciphylaxis (NUC). The patient was treated with intravenous infusions of sodium thiosulfate 25 mg 3 times weekly and oral cefazolin for superadded bacterial infection. Local wound care included collagenase dressings with light compression. Warfarin was discontinued, as it can worsen calciphylaxis. Complete reepithelialization of the ulcer along with substantial reduction in pain was noted within 4 weeks.
Ulceration of the lower legs is a relatively common condition in the Western world, the prevalence of which increases up to 5% in patients older than 65 years.1 Of the myriad of causes that lead to ulceration of the distal aspect of the leg, NUC is a rare but known phenomenon. The pathogenesis of NUC is complicated based on theories of derangement of receptor activator of nuclear factor κβ, receptor activator of nuclear factor κβ ligand, and osteoprotegerin, leading to calcium deposits in the media of the arteries.2 This deposition precipitates vascular occlusion coupled with ischemic necrosis of the subcutaneous tissue and skin.3 Some of the more common causes of NUC are primary hyperparathyroidism, malignancy, and rheumatoid arthritis. Type 2 diabetes mellitus is a less common cause but often is found in association with NUC, as noted by Nigwekar et al.2 According to their study, the laboratory parameters commonly found in NUC included a calcium-phosphorous product greater than 50 mg2/dL and serum creatinine of 1.2 mg/dL or less.2
Our patient displayed these laboratory findings. However, distinguishing NUC from other atypical lower extremity ulcers such as Martorell hypertensive ischemic ulcer, pyoderma gangrenosum, and warfarin necrosis can pose a challenge to the dermatologist. Martorell hypertensive ischemic ulcer is excruciatingly painful and occurs more frequently near the Achilles tendon, responding well to surgical debridement. Histopathologically, medial calcinosis and arteriosclerosis are seen.4
Pyoderma gangrenosum is a neutrophilic dermatosis wherein the classical ulcerative variant is painful. It occurs mostly on the pretibial area and worsens after debridement.5 Clinically and histopathologically, it is a diagnosis of exclusion in which a dense neutrophilic to mixed lymphocytic infiltrate is seen with necrosis of dermal vessels.6
Warfarin necrosis is extremely rare, affecting 0.01% to 0.1% of patients on warfarin-derived anticoagulant therapy.7 Necrosis occurs mostly on fat-bearing areas such as the breasts, abdomen, and thighs 3 to 5 days after initiating treatment. Histologically, fibrin deposits occlude dermal vessels without perivascular inflammation.8
Necrobiosis lipoidica is a rare cutaneous entity seen in 0.3% of diabetic patients.9 The exact pathogenesis is unknown; however, microangiopathy in collaboration with cross-linking of abnormal collagen fibers play a role. These lesions appear as erythematous plaques with a slightly depressed to atrophic center, ultimately taking on a waxy porcelain appearance. Although most of these lesions either resolve or become chronically persistent, approximately 15% undergo ulceration, which can be painful. Histologically, with hematoxylin and eosin staining, areas of necrobiosis are seen surrounded by an inflammatory infiltrate comprised mainly of histiocytes along with lymphocytes and plasma cells.9
Nonuremic calciphylaxis can mimic the aforementioned conditions to a greater extent in female patients with obesity, diabetes mellitus, and hypertension. However, microscopic calcium deposition in the media of dermal arterioles, extravascular calcification within fat lobules, and cutaneous necrosis, along with remarkable response to intravenous sodium thiosulfate, confirmed a diagnosis of NUC in our patient. Sodium thiosulfate scavenges reactive oxygen species and promotes nitric oxygen generation, thereby reducing endothelial damage.10 Although there are no randomized controlled trials to support its use, sodium thiosulfate has been successfully used to treat established cases of NUC.11
- Spentzouris G, Labropoulos N. The evaluation of lower-extremity ulcers. Semin Intervent Radiol. 2009;26:286-295.
- Nigwekar SU, Wolf M, Sterns RH, et al. Calciphylaxis from nonuremic causes: a systematic review. Clin J Am Soc Nephrol. 2008;3:1139-1143.
- Bardin T. Musculoskeletal manifestations of chronic renal failure. Curr Opin Rheumatol. 2003;15:48-54.
- Hafner J, Nobbe S, Partsch H, et al. Martorell hypertensive ischemic leg ulcer: a model of ischemic subcutaneous arteriolosclerosis. Arch Dermatol. 2010;146:961-968.
- Sedda S, Caruso R, Marafini I, et al. Pyoderma gangrenosum in refractory celiac disease: a case report. BMC Gastroenterol. 2013;13:162.
- Su WP, Davis MD, Weenig RH, et al. Pyoderma gangrenosum: clinicopathologic correlation and proposed diagnostic criteria. Int J Dermatol. 2004;43:790-800.
- Breakey W, Hall C, Vann Jones S, et al. Warfarin-induced skin necrosis progressing to calciphylaxis. J Plast Reconstr Aesthet Surg. 2014;67:244-246.
- Kakagia DD, Papanas N, Karadimas E, et al. Warfarin-induced skin necrosis. Ann Dermatol. 2014;26:96-98.
- Kota SK, Jammula S, Kota SK, et al. Necrobiosis lipoidica diabeticorum: a case-based review of literature. Indian J Endocrinol Metab. 2012;16:614-620.
- Hayden MR, Goldsmith DJ. Sodium thiosulfate: new hope for the treatment of calciphylaxis. Semin Dial. 2010;23:258-262.
- Ning MS, Dahir KM, Castellanos EH, et al. Sodium thiosulfate in the treatment of non-uremic calciphylaxis. J Dermatol. 2013;40:649-652.
The Diagnosis: Nonuremic Calciphylaxis
Histopathologic findings revealed ischemic necrosis and a subepidermal blister (Figure 1) with arteriosclerotic changes and fat necrosis. Foci of calcification were noted within the fat lobules. Arterioles within the deeper dermis and subcutis showed thickened hyalinized walls, narrowed lumina, and medial calcification (Figure 2). Multiple sections did not reveal any granulomatous inflammation. Periodic acid-Schiff and Gram stains were negative for fungal and bacterial elements, respectively. No dense neutrophilic infiltrate was seen. Multifocal calcific deposits within fat lobules and vessel walls (endothelium highlighted by the CD31 stain) suggested calciphylaxis.


Laboratory test results revealed a normal white blood cell count, international normalized ratio level of 4 (on warfarin), and an elevated sedimentation rate at 72 mm/h (reference range, 0-20 mm/h). Serum creatinine was 1.1 mg/dL (reference range, 0.6-1.2 mg/dL) and the calcium-phosphorous product was 40.8 mg2/dL (reference range, <55 mg2/dL). Hemoglobin A1C (glycated hemoglobin) was 8.2% (reference range, 4%-7%). Wound cultures grew Proteus mirabilis sensitive to cefazolin. Acid-fast bacilli and fungal cultures were negative. Computed tomography of the left lower leg without contrast showed no evidence of osteomyelitis. Of note, the popliteal arteries and distal vessels showed moderate vascular calcification.
Histopathology findings as well as a clinical picture of painful ulceration on the distal extremities and uncontrolled diabetes with normal renal function favored a diagnosis of nonuremic calciphylaxis (NUC). The patient was treated with intravenous infusions of sodium thiosulfate 25 mg 3 times weekly and oral cefazolin for superadded bacterial infection. Local wound care included collagenase dressings with light compression. Warfarin was discontinued, as it can worsen calciphylaxis. Complete reepithelialization of the ulcer along with substantial reduction in pain was noted within 4 weeks.
Ulceration of the lower legs is a relatively common condition in the Western world, the prevalence of which increases up to 5% in patients older than 65 years.1 Of the myriad of causes that lead to ulceration of the distal aspect of the leg, NUC is a rare but known phenomenon. The pathogenesis of NUC is complicated based on theories of derangement of receptor activator of nuclear factor κβ, receptor activator of nuclear factor κβ ligand, and osteoprotegerin, leading to calcium deposits in the media of the arteries.2 This deposition precipitates vascular occlusion coupled with ischemic necrosis of the subcutaneous tissue and skin.3 Some of the more common causes of NUC are primary hyperparathyroidism, malignancy, and rheumatoid arthritis. Type 2 diabetes mellitus is a less common cause but often is found in association with NUC, as noted by Nigwekar et al.2 According to their study, the laboratory parameters commonly found in NUC included a calcium-phosphorous product greater than 50 mg2/dL and serum creatinine of 1.2 mg/dL or less.2
Our patient displayed these laboratory findings. However, distinguishing NUC from other atypical lower extremity ulcers such as Martorell hypertensive ischemic ulcer, pyoderma gangrenosum, and warfarin necrosis can pose a challenge to the dermatologist. Martorell hypertensive ischemic ulcer is excruciatingly painful and occurs more frequently near the Achilles tendon, responding well to surgical debridement. Histopathologically, medial calcinosis and arteriosclerosis are seen.4
Pyoderma gangrenosum is a neutrophilic dermatosis wherein the classical ulcerative variant is painful. It occurs mostly on the pretibial area and worsens after debridement.5 Clinically and histopathologically, it is a diagnosis of exclusion in which a dense neutrophilic to mixed lymphocytic infiltrate is seen with necrosis of dermal vessels.6
Warfarin necrosis is extremely rare, affecting 0.01% to 0.1% of patients on warfarin-derived anticoagulant therapy.7 Necrosis occurs mostly on fat-bearing areas such as the breasts, abdomen, and thighs 3 to 5 days after initiating treatment. Histologically, fibrin deposits occlude dermal vessels without perivascular inflammation.8
Necrobiosis lipoidica is a rare cutaneous entity seen in 0.3% of diabetic patients.9 The exact pathogenesis is unknown; however, microangiopathy in collaboration with cross-linking of abnormal collagen fibers play a role. These lesions appear as erythematous plaques with a slightly depressed to atrophic center, ultimately taking on a waxy porcelain appearance. Although most of these lesions either resolve or become chronically persistent, approximately 15% undergo ulceration, which can be painful. Histologically, with hematoxylin and eosin staining, areas of necrobiosis are seen surrounded by an inflammatory infiltrate comprised mainly of histiocytes along with lymphocytes and plasma cells.9
Nonuremic calciphylaxis can mimic the aforementioned conditions to a greater extent in female patients with obesity, diabetes mellitus, and hypertension. However, microscopic calcium deposition in the media of dermal arterioles, extravascular calcification within fat lobules, and cutaneous necrosis, along with remarkable response to intravenous sodium thiosulfate, confirmed a diagnosis of NUC in our patient. Sodium thiosulfate scavenges reactive oxygen species and promotes nitric oxygen generation, thereby reducing endothelial damage.10 Although there are no randomized controlled trials to support its use, sodium thiosulfate has been successfully used to treat established cases of NUC.11
The Diagnosis: Nonuremic Calciphylaxis
Histopathologic findings revealed ischemic necrosis and a subepidermal blister (Figure 1) with arteriosclerotic changes and fat necrosis. Foci of calcification were noted within the fat lobules. Arterioles within the deeper dermis and subcutis showed thickened hyalinized walls, narrowed lumina, and medial calcification (Figure 2). Multiple sections did not reveal any granulomatous inflammation. Periodic acid-Schiff and Gram stains were negative for fungal and bacterial elements, respectively. No dense neutrophilic infiltrate was seen. Multifocal calcific deposits within fat lobules and vessel walls (endothelium highlighted by the CD31 stain) suggested calciphylaxis.


Laboratory test results revealed a normal white blood cell count, international normalized ratio level of 4 (on warfarin), and an elevated sedimentation rate at 72 mm/h (reference range, 0-20 mm/h). Serum creatinine was 1.1 mg/dL (reference range, 0.6-1.2 mg/dL) and the calcium-phosphorous product was 40.8 mg2/dL (reference range, <55 mg2/dL). Hemoglobin A1C (glycated hemoglobin) was 8.2% (reference range, 4%-7%). Wound cultures grew Proteus mirabilis sensitive to cefazolin. Acid-fast bacilli and fungal cultures were negative. Computed tomography of the left lower leg without contrast showed no evidence of osteomyelitis. Of note, the popliteal arteries and distal vessels showed moderate vascular calcification.
Histopathology findings as well as a clinical picture of painful ulceration on the distal extremities and uncontrolled diabetes with normal renal function favored a diagnosis of nonuremic calciphylaxis (NUC). The patient was treated with intravenous infusions of sodium thiosulfate 25 mg 3 times weekly and oral cefazolin for superadded bacterial infection. Local wound care included collagenase dressings with light compression. Warfarin was discontinued, as it can worsen calciphylaxis. Complete reepithelialization of the ulcer along with substantial reduction in pain was noted within 4 weeks.
Ulceration of the lower legs is a relatively common condition in the Western world, the prevalence of which increases up to 5% in patients older than 65 years.1 Of the myriad of causes that lead to ulceration of the distal aspect of the leg, NUC is a rare but known phenomenon. The pathogenesis of NUC is complicated based on theories of derangement of receptor activator of nuclear factor κβ, receptor activator of nuclear factor κβ ligand, and osteoprotegerin, leading to calcium deposits in the media of the arteries.2 This deposition precipitates vascular occlusion coupled with ischemic necrosis of the subcutaneous tissue and skin.3 Some of the more common causes of NUC are primary hyperparathyroidism, malignancy, and rheumatoid arthritis. Type 2 diabetes mellitus is a less common cause but often is found in association with NUC, as noted by Nigwekar et al.2 According to their study, the laboratory parameters commonly found in NUC included a calcium-phosphorous product greater than 50 mg2/dL and serum creatinine of 1.2 mg/dL or less.2
Our patient displayed these laboratory findings. However, distinguishing NUC from other atypical lower extremity ulcers such as Martorell hypertensive ischemic ulcer, pyoderma gangrenosum, and warfarin necrosis can pose a challenge to the dermatologist. Martorell hypertensive ischemic ulcer is excruciatingly painful and occurs more frequently near the Achilles tendon, responding well to surgical debridement. Histopathologically, medial calcinosis and arteriosclerosis are seen.4
Pyoderma gangrenosum is a neutrophilic dermatosis wherein the classical ulcerative variant is painful. It occurs mostly on the pretibial area and worsens after debridement.5 Clinically and histopathologically, it is a diagnosis of exclusion in which a dense neutrophilic to mixed lymphocytic infiltrate is seen with necrosis of dermal vessels.6
Warfarin necrosis is extremely rare, affecting 0.01% to 0.1% of patients on warfarin-derived anticoagulant therapy.7 Necrosis occurs mostly on fat-bearing areas such as the breasts, abdomen, and thighs 3 to 5 days after initiating treatment. Histologically, fibrin deposits occlude dermal vessels without perivascular inflammation.8
Necrobiosis lipoidica is a rare cutaneous entity seen in 0.3% of diabetic patients.9 The exact pathogenesis is unknown; however, microangiopathy in collaboration with cross-linking of abnormal collagen fibers play a role. These lesions appear as erythematous plaques with a slightly depressed to atrophic center, ultimately taking on a waxy porcelain appearance. Although most of these lesions either resolve or become chronically persistent, approximately 15% undergo ulceration, which can be painful. Histologically, with hematoxylin and eosin staining, areas of necrobiosis are seen surrounded by an inflammatory infiltrate comprised mainly of histiocytes along with lymphocytes and plasma cells.9
Nonuremic calciphylaxis can mimic the aforementioned conditions to a greater extent in female patients with obesity, diabetes mellitus, and hypertension. However, microscopic calcium deposition in the media of dermal arterioles, extravascular calcification within fat lobules, and cutaneous necrosis, along with remarkable response to intravenous sodium thiosulfate, confirmed a diagnosis of NUC in our patient. Sodium thiosulfate scavenges reactive oxygen species and promotes nitric oxygen generation, thereby reducing endothelial damage.10 Although there are no randomized controlled trials to support its use, sodium thiosulfate has been successfully used to treat established cases of NUC.11
- Spentzouris G, Labropoulos N. The evaluation of lower-extremity ulcers. Semin Intervent Radiol. 2009;26:286-295.
- Nigwekar SU, Wolf M, Sterns RH, et al. Calciphylaxis from nonuremic causes: a systematic review. Clin J Am Soc Nephrol. 2008;3:1139-1143.
- Bardin T. Musculoskeletal manifestations of chronic renal failure. Curr Opin Rheumatol. 2003;15:48-54.
- Hafner J, Nobbe S, Partsch H, et al. Martorell hypertensive ischemic leg ulcer: a model of ischemic subcutaneous arteriolosclerosis. Arch Dermatol. 2010;146:961-968.
- Sedda S, Caruso R, Marafini I, et al. Pyoderma gangrenosum in refractory celiac disease: a case report. BMC Gastroenterol. 2013;13:162.
- Su WP, Davis MD, Weenig RH, et al. Pyoderma gangrenosum: clinicopathologic correlation and proposed diagnostic criteria. Int J Dermatol. 2004;43:790-800.
- Breakey W, Hall C, Vann Jones S, et al. Warfarin-induced skin necrosis progressing to calciphylaxis. J Plast Reconstr Aesthet Surg. 2014;67:244-246.
- Kakagia DD, Papanas N, Karadimas E, et al. Warfarin-induced skin necrosis. Ann Dermatol. 2014;26:96-98.
- Kota SK, Jammula S, Kota SK, et al. Necrobiosis lipoidica diabeticorum: a case-based review of literature. Indian J Endocrinol Metab. 2012;16:614-620.
- Hayden MR, Goldsmith DJ. Sodium thiosulfate: new hope for the treatment of calciphylaxis. Semin Dial. 2010;23:258-262.
- Ning MS, Dahir KM, Castellanos EH, et al. Sodium thiosulfate in the treatment of non-uremic calciphylaxis. J Dermatol. 2013;40:649-652.
- Spentzouris G, Labropoulos N. The evaluation of lower-extremity ulcers. Semin Intervent Radiol. 2009;26:286-295.
- Nigwekar SU, Wolf M, Sterns RH, et al. Calciphylaxis from nonuremic causes: a systematic review. Clin J Am Soc Nephrol. 2008;3:1139-1143.
- Bardin T. Musculoskeletal manifestations of chronic renal failure. Curr Opin Rheumatol. 2003;15:48-54.
- Hafner J, Nobbe S, Partsch H, et al. Martorell hypertensive ischemic leg ulcer: a model of ischemic subcutaneous arteriolosclerosis. Arch Dermatol. 2010;146:961-968.
- Sedda S, Caruso R, Marafini I, et al. Pyoderma gangrenosum in refractory celiac disease: a case report. BMC Gastroenterol. 2013;13:162.
- Su WP, Davis MD, Weenig RH, et al. Pyoderma gangrenosum: clinicopathologic correlation and proposed diagnostic criteria. Int J Dermatol. 2004;43:790-800.
- Breakey W, Hall C, Vann Jones S, et al. Warfarin-induced skin necrosis progressing to calciphylaxis. J Plast Reconstr Aesthet Surg. 2014;67:244-246.
- Kakagia DD, Papanas N, Karadimas E, et al. Warfarin-induced skin necrosis. Ann Dermatol. 2014;26:96-98.
- Kota SK, Jammula S, Kota SK, et al. Necrobiosis lipoidica diabeticorum: a case-based review of literature. Indian J Endocrinol Metab. 2012;16:614-620.
- Hayden MR, Goldsmith DJ. Sodium thiosulfate: new hope for the treatment of calciphylaxis. Semin Dial. 2010;23:258-262.
- Ning MS, Dahir KM, Castellanos EH, et al. Sodium thiosulfate in the treatment of non-uremic calciphylaxis. J Dermatol. 2013;40:649-652.
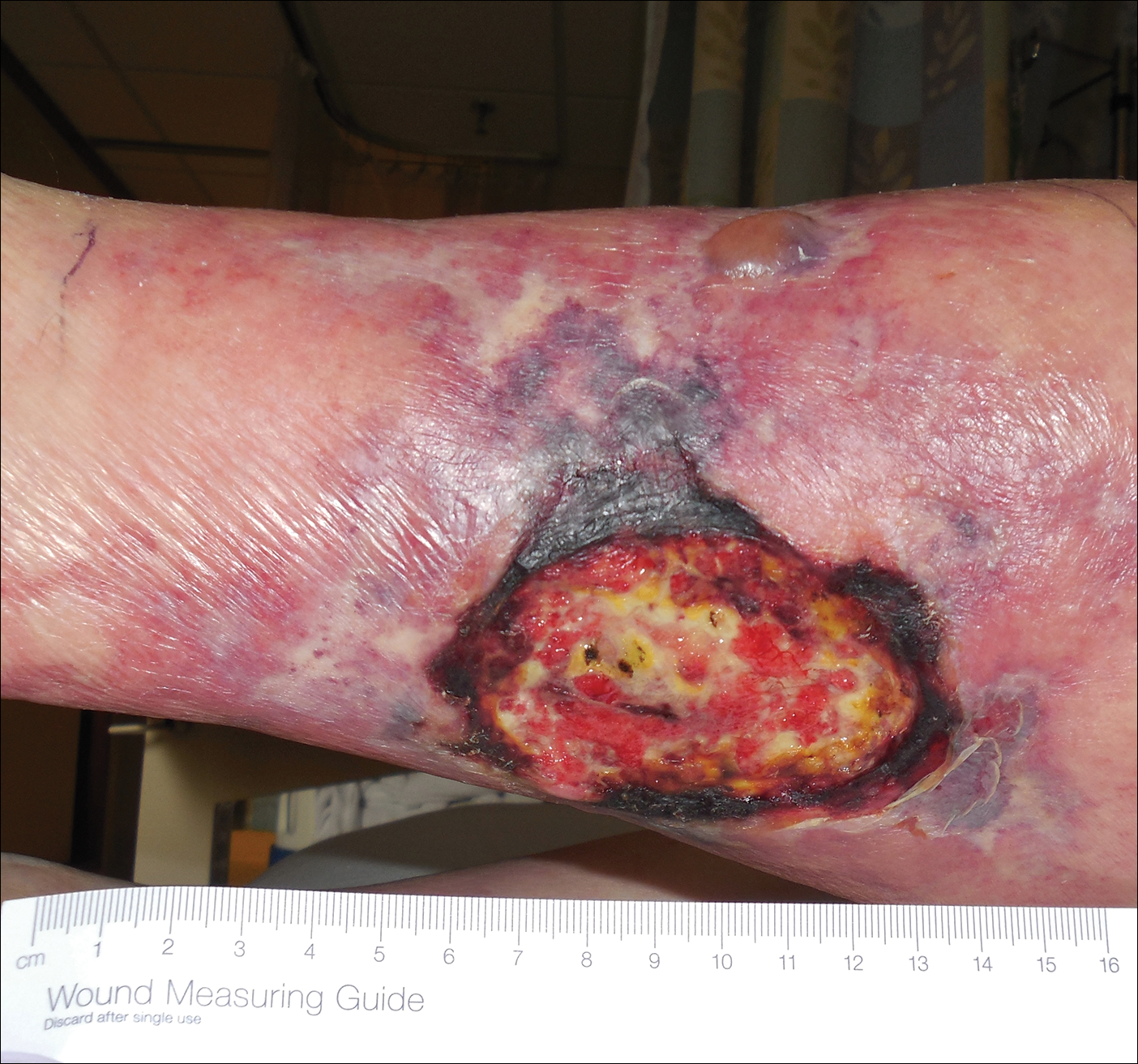
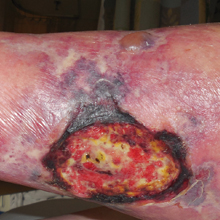
An 80-year-old woman with a medical history notable for obesity (body mass index, 31.2), type 2 diabetes mellitus, hypertension, and chronic atrial fibrillation treated with warfarin presented with a chronic painful wound on the left lower calf of 1 month's duration. A 7×7-cm ulcer on the posterior aspect of the left calf with necrotic debris was seen surrounded by skin of mottled purple discoloration. The edge of the ulcer was not undermined. There were tense nonhemorrhagic bullae on the medial aspect of the left leg and on bilateral anterior tibial areas. Two punch biopsy specimens were obtained from the anterior tibial bulla and the edge of the ulcer.
Wound expert: Consider hyperbaric oxygen therapy for diabetic foot ulcers
SAN DIEGO – Hyperbaric oxygen therapy, a mainstay of wound care, has a long and controversial history as a treatment for diabetic foot ulcers. Conflicting studies have spawned plenty of debate, and the most recent Cochrane Library review of existing research didn’t shed much light on the value of the treatment because the evidence was weak (Cochrane Database Syst Rev. 2015 Jun 24;[6]:CD004123).
But William H. Tettelbach, MD, a wound care specialist, told an audience at the annual scientific sessions of the American Diabetic Association that hyperbaric treatments are worth a try in certain cases. And he brought evidence to prove it – a 2015 report he coauthored that reviewed studies and offered clinical practice guidelines for hyperbaric oxygen therapy for the treatment of diabetic foot ulcers (DFUs) (Undersea Hyperb Med. 2015 May-Jun;42[3]:205-47).
In an interview, Dr. Tettelbach discussed ideal candidates for the treatment and offered clinical advice to endocrinologists.
Question: What did your review of research tell you about the value of hyperbaric oxygen treatment for DFUs?
Answer: We came to the same conclusion that most of the papers have indicated over the years: Hyperbaric oxygen is effective and attains goals such as reducing rates of amputation in a select population of diabetic ulcer patients.
Patients who have Wagner grade 3 or greater ulcers or admitted for surgery due to a septic diabetic foot benefit from an evaluation by a hyperbaric medicine–trained physician and treatment when indicated. There is evidence and years of clinical experience indicating that these patients benefit and have improved outcomes when evaluated and treated appropriately with hyperbaric oxygen therapy.
In the United States, hyperbaric oxygen therapy is not indicated in Wagner grade 2, 1 or 0 diabetic foot ulcers, the ulcers that involve soft tissue but not deep structure like bone.
Q: Why has there been so much controversy over the value of this treatment?
A: In the past, there have been problems with commercial outpatient wound centers that are heavily driven by profits. Financial margins in wound care clinics can be tight, and the need to remain profitable has at times resulted in patients being treated inappropriately with hyperbaric oxygen therapy (Adv Skin Wound Care. 2017 Apr;30[4]:181-90).
Q: Why does hyperbaric oxygen treatment work in some cases?
A: When you place a patient in a hyperbaric chamber where they breathe 100% oxygen under pressure, you increase the percentage of oxygen in the blood. At such a high percentage, oxygen saturates the plasma versus just being carried by red blood cells, thereby allowing the oxygen to penetrate farther into hypoxic tissues. By increasing the oxygen, you have the ability to make the environment unfavorable for rapid proliferation of anaerobic or microaerophilic bacteria that do not survive a highly oxygen-rich environment. Increasing tissue oxygen tension to 30 mm Hg or greater increases the macrophages’ ability to have an oxidative burst needed to kill bacteria. Furthermore, there are antibiotics that require certain levels of oxygen for transport across the bacterial cell wall.
Q: What should physicians understand about hyperbaric oxygen therapy for DFUs?
A: Overall, hyperbaric practitioners need to be more selective in identifying and treating patients according to what the evidence supports. Poorly designed trials with misleading results should not drive medical decisions. We should revisit diabetic foot ulcers through well-thought-out studies that target those who would benefit as suggested by current evidence. Prior trials have been heavily weighted with Wagner grade 1 and 2 candidates or ischemic diabetic ulcers that are not revascularized. These are biased toward poor outcomes since the current evidence does not strongly support treating these types of individuals with adjunctive hyperbaric oxygen therapy (Ont Health Technol Assess Ser. 2017 May 12;17[5]:1-142. eCollection 2017).
Q: What conditions should trigger endocrinologists to think about hyperbaric oxygen therapy for their DFU patients?
A: Candidates for the therapy include diabetic ulcers that have persisted for longer than 30 days, since these ulcers are at a significantly higher risk of a complicating infection, along with those that have failed treatment or are becoming more symptomatic over time (Undersea Hyperb Med. 2017 Mar-Apr;44[2]:157-60).
At that point, it might make sense to refer those patients to a wound and hyperbaric specialist for further evaluation and management, especially to a wound center that offers hyperbaric oxygen therapy.
These wound centers can be found in smaller towns. But some folks will have to travel, perhaps to a wound center at a hospital that has room and board like they do for cancer patients.
Q: What about treatment after surgery?
A: Using hyperbariatric oxygen therapy to treat inpatients with septic diabetic foot ulcers – Wagner grade 3 or higher – immediately after surgery may reduce length of stay as well as lower the risk of requiring multiple surgical debridements.
Q: What are the best-case scenarios for treatment?
A: A significant portion of what we do is limb preservation. Hyperbaric oxygen therapy often can help save a digit, forefoot, or even an extremity.
But it’s not something that just happens overnight. It’s a long-term process. Underlying complicating osteomyelitis may require up to 40-60 adjunctive hyperbaric oxygen treatments, 5 days a week with weekends off, along with concurrent antibiotics, wound care, and vascular interventions when indicated.
Q: Is insurance ever an issue for this treatment?
A: Typically, not if one follows the indications set by the Centers for Medicare & Medicaid Services and the Undersea and Hyperbaric Medical Society.
Medicare lists 15 medical indications that it will cover, and a majority of commercial insurers will cover the same 15 indications and possibly more. But commercial insurers may require prior authorization of medical necessity before preceding with hyperbaric oxygen therapy (Diving Hyperb Med. 2016 Sep;46[3]:133-4).
SAN DIEGO – Hyperbaric oxygen therapy, a mainstay of wound care, has a long and controversial history as a treatment for diabetic foot ulcers. Conflicting studies have spawned plenty of debate, and the most recent Cochrane Library review of existing research didn’t shed much light on the value of the treatment because the evidence was weak (Cochrane Database Syst Rev. 2015 Jun 24;[6]:CD004123).
But William H. Tettelbach, MD, a wound care specialist, told an audience at the annual scientific sessions of the American Diabetic Association that hyperbaric treatments are worth a try in certain cases. And he brought evidence to prove it – a 2015 report he coauthored that reviewed studies and offered clinical practice guidelines for hyperbaric oxygen therapy for the treatment of diabetic foot ulcers (DFUs) (Undersea Hyperb Med. 2015 May-Jun;42[3]:205-47).
In an interview, Dr. Tettelbach discussed ideal candidates for the treatment and offered clinical advice to endocrinologists.
Question: What did your review of research tell you about the value of hyperbaric oxygen treatment for DFUs?
Answer: We came to the same conclusion that most of the papers have indicated over the years: Hyperbaric oxygen is effective and attains goals such as reducing rates of amputation in a select population of diabetic ulcer patients.
Patients who have Wagner grade 3 or greater ulcers or admitted for surgery due to a septic diabetic foot benefit from an evaluation by a hyperbaric medicine–trained physician and treatment when indicated. There is evidence and years of clinical experience indicating that these patients benefit and have improved outcomes when evaluated and treated appropriately with hyperbaric oxygen therapy.
In the United States, hyperbaric oxygen therapy is not indicated in Wagner grade 2, 1 or 0 diabetic foot ulcers, the ulcers that involve soft tissue but not deep structure like bone.
Q: Why has there been so much controversy over the value of this treatment?
A: In the past, there have been problems with commercial outpatient wound centers that are heavily driven by profits. Financial margins in wound care clinics can be tight, and the need to remain profitable has at times resulted in patients being treated inappropriately with hyperbaric oxygen therapy (Adv Skin Wound Care. 2017 Apr;30[4]:181-90).
Q: Why does hyperbaric oxygen treatment work in some cases?
A: When you place a patient in a hyperbaric chamber where they breathe 100% oxygen under pressure, you increase the percentage of oxygen in the blood. At such a high percentage, oxygen saturates the plasma versus just being carried by red blood cells, thereby allowing the oxygen to penetrate farther into hypoxic tissues. By increasing the oxygen, you have the ability to make the environment unfavorable for rapid proliferation of anaerobic or microaerophilic bacteria that do not survive a highly oxygen-rich environment. Increasing tissue oxygen tension to 30 mm Hg or greater increases the macrophages’ ability to have an oxidative burst needed to kill bacteria. Furthermore, there are antibiotics that require certain levels of oxygen for transport across the bacterial cell wall.
Q: What should physicians understand about hyperbaric oxygen therapy for DFUs?
A: Overall, hyperbaric practitioners need to be more selective in identifying and treating patients according to what the evidence supports. Poorly designed trials with misleading results should not drive medical decisions. We should revisit diabetic foot ulcers through well-thought-out studies that target those who would benefit as suggested by current evidence. Prior trials have been heavily weighted with Wagner grade 1 and 2 candidates or ischemic diabetic ulcers that are not revascularized. These are biased toward poor outcomes since the current evidence does not strongly support treating these types of individuals with adjunctive hyperbaric oxygen therapy (Ont Health Technol Assess Ser. 2017 May 12;17[5]:1-142. eCollection 2017).
Q: What conditions should trigger endocrinologists to think about hyperbaric oxygen therapy for their DFU patients?
A: Candidates for the therapy include diabetic ulcers that have persisted for longer than 30 days, since these ulcers are at a significantly higher risk of a complicating infection, along with those that have failed treatment or are becoming more symptomatic over time (Undersea Hyperb Med. 2017 Mar-Apr;44[2]:157-60).
At that point, it might make sense to refer those patients to a wound and hyperbaric specialist for further evaluation and management, especially to a wound center that offers hyperbaric oxygen therapy.
These wound centers can be found in smaller towns. But some folks will have to travel, perhaps to a wound center at a hospital that has room and board like they do for cancer patients.
Q: What about treatment after surgery?
A: Using hyperbariatric oxygen therapy to treat inpatients with septic diabetic foot ulcers – Wagner grade 3 or higher – immediately after surgery may reduce length of stay as well as lower the risk of requiring multiple surgical debridements.
Q: What are the best-case scenarios for treatment?
A: A significant portion of what we do is limb preservation. Hyperbaric oxygen therapy often can help save a digit, forefoot, or even an extremity.
But it’s not something that just happens overnight. It’s a long-term process. Underlying complicating osteomyelitis may require up to 40-60 adjunctive hyperbaric oxygen treatments, 5 days a week with weekends off, along with concurrent antibiotics, wound care, and vascular interventions when indicated.
Q: Is insurance ever an issue for this treatment?
A: Typically, not if one follows the indications set by the Centers for Medicare & Medicaid Services and the Undersea and Hyperbaric Medical Society.
Medicare lists 15 medical indications that it will cover, and a majority of commercial insurers will cover the same 15 indications and possibly more. But commercial insurers may require prior authorization of medical necessity before preceding with hyperbaric oxygen therapy (Diving Hyperb Med. 2016 Sep;46[3]:133-4).
SAN DIEGO – Hyperbaric oxygen therapy, a mainstay of wound care, has a long and controversial history as a treatment for diabetic foot ulcers. Conflicting studies have spawned plenty of debate, and the most recent Cochrane Library review of existing research didn’t shed much light on the value of the treatment because the evidence was weak (Cochrane Database Syst Rev. 2015 Jun 24;[6]:CD004123).
But William H. Tettelbach, MD, a wound care specialist, told an audience at the annual scientific sessions of the American Diabetic Association that hyperbaric treatments are worth a try in certain cases. And he brought evidence to prove it – a 2015 report he coauthored that reviewed studies and offered clinical practice guidelines for hyperbaric oxygen therapy for the treatment of diabetic foot ulcers (DFUs) (Undersea Hyperb Med. 2015 May-Jun;42[3]:205-47).
In an interview, Dr. Tettelbach discussed ideal candidates for the treatment and offered clinical advice to endocrinologists.
Question: What did your review of research tell you about the value of hyperbaric oxygen treatment for DFUs?
Answer: We came to the same conclusion that most of the papers have indicated over the years: Hyperbaric oxygen is effective and attains goals such as reducing rates of amputation in a select population of diabetic ulcer patients.
Patients who have Wagner grade 3 or greater ulcers or admitted for surgery due to a septic diabetic foot benefit from an evaluation by a hyperbaric medicine–trained physician and treatment when indicated. There is evidence and years of clinical experience indicating that these patients benefit and have improved outcomes when evaluated and treated appropriately with hyperbaric oxygen therapy.
In the United States, hyperbaric oxygen therapy is not indicated in Wagner grade 2, 1 or 0 diabetic foot ulcers, the ulcers that involve soft tissue but not deep structure like bone.
Q: Why has there been so much controversy over the value of this treatment?
A: In the past, there have been problems with commercial outpatient wound centers that are heavily driven by profits. Financial margins in wound care clinics can be tight, and the need to remain profitable has at times resulted in patients being treated inappropriately with hyperbaric oxygen therapy (Adv Skin Wound Care. 2017 Apr;30[4]:181-90).
Q: Why does hyperbaric oxygen treatment work in some cases?
A: When you place a patient in a hyperbaric chamber where they breathe 100% oxygen under pressure, you increase the percentage of oxygen in the blood. At such a high percentage, oxygen saturates the plasma versus just being carried by red blood cells, thereby allowing the oxygen to penetrate farther into hypoxic tissues. By increasing the oxygen, you have the ability to make the environment unfavorable for rapid proliferation of anaerobic or microaerophilic bacteria that do not survive a highly oxygen-rich environment. Increasing tissue oxygen tension to 30 mm Hg or greater increases the macrophages’ ability to have an oxidative burst needed to kill bacteria. Furthermore, there are antibiotics that require certain levels of oxygen for transport across the bacterial cell wall.
Q: What should physicians understand about hyperbaric oxygen therapy for DFUs?
A: Overall, hyperbaric practitioners need to be more selective in identifying and treating patients according to what the evidence supports. Poorly designed trials with misleading results should not drive medical decisions. We should revisit diabetic foot ulcers through well-thought-out studies that target those who would benefit as suggested by current evidence. Prior trials have been heavily weighted with Wagner grade 1 and 2 candidates or ischemic diabetic ulcers that are not revascularized. These are biased toward poor outcomes since the current evidence does not strongly support treating these types of individuals with adjunctive hyperbaric oxygen therapy (Ont Health Technol Assess Ser. 2017 May 12;17[5]:1-142. eCollection 2017).
Q: What conditions should trigger endocrinologists to think about hyperbaric oxygen therapy for their DFU patients?
A: Candidates for the therapy include diabetic ulcers that have persisted for longer than 30 days, since these ulcers are at a significantly higher risk of a complicating infection, along with those that have failed treatment or are becoming more symptomatic over time (Undersea Hyperb Med. 2017 Mar-Apr;44[2]:157-60).
At that point, it might make sense to refer those patients to a wound and hyperbaric specialist for further evaluation and management, especially to a wound center that offers hyperbaric oxygen therapy.
These wound centers can be found in smaller towns. But some folks will have to travel, perhaps to a wound center at a hospital that has room and board like they do for cancer patients.
Q: What about treatment after surgery?
A: Using hyperbariatric oxygen therapy to treat inpatients with septic diabetic foot ulcers – Wagner grade 3 or higher – immediately after surgery may reduce length of stay as well as lower the risk of requiring multiple surgical debridements.
Q: What are the best-case scenarios for treatment?
A: A significant portion of what we do is limb preservation. Hyperbaric oxygen therapy often can help save a digit, forefoot, or even an extremity.
But it’s not something that just happens overnight. It’s a long-term process. Underlying complicating osteomyelitis may require up to 40-60 adjunctive hyperbaric oxygen treatments, 5 days a week with weekends off, along with concurrent antibiotics, wound care, and vascular interventions when indicated.
Q: Is insurance ever an issue for this treatment?
A: Typically, not if one follows the indications set by the Centers for Medicare & Medicaid Services and the Undersea and Hyperbaric Medical Society.
Medicare lists 15 medical indications that it will cover, and a majority of commercial insurers will cover the same 15 indications and possibly more. But commercial insurers may require prior authorization of medical necessity before preceding with hyperbaric oxygen therapy (Diving Hyperb Med. 2016 Sep;46[3]:133-4).
EXPERT ANALYSIS AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Clinical Pearl: Mastering the Flexible Scalpel Blade With the Banana Practice Model
The flexible scalpel blade (FSB) is a 2-sided handheld razor blade that serves as a pivotal instrument in certain dermatologic procedures. Its unrivaled sharpness1 permits pinpoint precision for shave biopsies, excisions of superficial lesions,2 scar contouring, and harvesting of split-thickness skin grafts.3 Given its flexibility and long edge, considerable manual dexterity and skill are required to maximize its full potential.
Practice Gap
Prior to practicing on live patients, students on clinical rotation would benefit from in vitro skin simulators to practice correct hand position, FSB control for concave and convex surface cutting, and safety. Prior practice models have included mannequins, tomatoes, and eggplants.4,5 Here, the authors recommend the use of a banana (genus Musa). In addition to its year-round availability, economic feasibility, simplicity, and portability, the banana has colored skin that well represents the epidermis, dermis, and subcutaneous tissue, allowing for visual feedback. Furthermore, its contour irregularities simulate convexities and concavities for various anatomic locations. Although the firmness of a yellow-green banana provides immediate tissue feedback, the softness and pliability of a ripe banana simulates the consistency of older skin and the use of appropriate traction.
Tools
To begin, one simply requires a marking pen, banana, and razor blade. Various shapes, including a circle, ellipse, rectangle, trapezoid, triangle, and multilobed lesion are demarcated by students or attendings (Figure 1).

The Technique
To handle the FSB, one can hold the lateral edges of the blade between the thumb and index finger or between the thumb and middle finger. The thumb and index finger position allows for additional flexible working space and visualization, increased traction by the remaining 3 fingers, and greater ease of removal of lesions with considerable height. The thumb and middle finger hold allows for versatile use of the index finger of the same hand for stabilizing the center of the blade, fixing the tissue on the FSB while it is removed, and sliding the specimen off the FSB. It is important to maintain a fixed distance from the blade to the metacarpals at all times to ensure smooth advancement of the blade and visualization. Beginners can lift the pinky finger of the hand holding the FSB and move the finger up and down to control the angle of the blade.
Practice Implications
Generally, we utilize various techniques of shaving using the FSB. We approach the target lesion 2 to 3 mm from the marked location and slide parallel to the skin surface and perpendicular to the lesion until the epidermis is penetrated. Second, we advance the blade toward the lesion with careful attention paid to the perimeter of the lesion and the points of contact of the FSB. For lesions with hardier consistencies, a sawing motion of the blade is employed, which also requires controlled tilting of the wrist to maintain an even depth and smooth bevel. To cut deeper, flexing the FSB with lateral pressure is helpful. More shallow lesions require the instrument to be flatter and less bowed. When finishing the shave, it is important to start angling the blade upward early, either at the center of the targeted lesion or 2 to 3 mm before the demarcated edge of the skin graft, while applying traction away from the lesion and slight downward pressure with the nondominant hand.
For larger lesions, the perimeter may be more difficult to remove precisely and can be achieved by rotating the blade around the lesion with focus on one point of contact of the FSB to cut and glide through the tissue’s perimeter. To achieve a more exact wound edge and to preclude jagged borders, a No. 15 blade can be used to score the perimeter very superficially to the papillary dermis prior to shave removal. The main disadvantage, however, is that the beveled edge is removed.
In summary, the FSB is an exceptional tool for biopsies, tumor removal, scar contouring, and split-thickness skin grafts. Through the banana practice model, one can attain fine control and reap the benefits of the FSB after meticulous and dedicated training.
- Awadalla B, Hexsel C, Goldberg LH. The sharpness of blades used in dermatologic surgery. Dermatol Surg. 2016;42:105-107.
- Vergilis-Kalner IJ, Goldberg LH, Firoz B, et al. Horizontal excision of in situ epidermal tumors using a flexible blade. Dermatol Surg. 2011;37:234-236.
- Hexsel CL, Loosemore M, Goldberg LH, et al. Postauricular skin: an excellent donor site for split-thickness skin grafts for the head, neck, and upper chest. Dermatol Surg. 2015;41:48-52.
- Chen TM, Mellette JR. Surgical pearl: tomato—an alternative model for shave biopsy training. J Am Acad Dermatol. 2006;54:517-518.
- Wang X, Albahrani Y, Pan M, et al. Skin simulators for dermatological procedures. Dermatol Online J. 2015;21. pii:13030/qt33j6x4nx.
The flexible scalpel blade (FSB) is a 2-sided handheld razor blade that serves as a pivotal instrument in certain dermatologic procedures. Its unrivaled sharpness1 permits pinpoint precision for shave biopsies, excisions of superficial lesions,2 scar contouring, and harvesting of split-thickness skin grafts.3 Given its flexibility and long edge, considerable manual dexterity and skill are required to maximize its full potential.
Practice Gap
Prior to practicing on live patients, students on clinical rotation would benefit from in vitro skin simulators to practice correct hand position, FSB control for concave and convex surface cutting, and safety. Prior practice models have included mannequins, tomatoes, and eggplants.4,5 Here, the authors recommend the use of a banana (genus Musa). In addition to its year-round availability, economic feasibility, simplicity, and portability, the banana has colored skin that well represents the epidermis, dermis, and subcutaneous tissue, allowing for visual feedback. Furthermore, its contour irregularities simulate convexities and concavities for various anatomic locations. Although the firmness of a yellow-green banana provides immediate tissue feedback, the softness and pliability of a ripe banana simulates the consistency of older skin and the use of appropriate traction.
Tools
To begin, one simply requires a marking pen, banana, and razor blade. Various shapes, including a circle, ellipse, rectangle, trapezoid, triangle, and multilobed lesion are demarcated by students or attendings (Figure 1).


The Technique
To handle the FSB, one can hold the lateral edges of the blade between the thumb and index finger or between the thumb and middle finger. The thumb and index finger position allows for additional flexible working space and visualization, increased traction by the remaining 3 fingers, and greater ease of removal of lesions with considerable height. The thumb and middle finger hold allows for versatile use of the index finger of the same hand for stabilizing the center of the blade, fixing the tissue on the FSB while it is removed, and sliding the specimen off the FSB. It is important to maintain a fixed distance from the blade to the metacarpals at all times to ensure smooth advancement of the blade and visualization. Beginners can lift the pinky finger of the hand holding the FSB and move the finger up and down to control the angle of the blade.
Practice Implications
Generally, we utilize various techniques of shaving using the FSB. We approach the target lesion 2 to 3 mm from the marked location and slide parallel to the skin surface and perpendicular to the lesion until the epidermis is penetrated. Second, we advance the blade toward the lesion with careful attention paid to the perimeter of the lesion and the points of contact of the FSB. For lesions with hardier consistencies, a sawing motion of the blade is employed, which also requires controlled tilting of the wrist to maintain an even depth and smooth bevel. To cut deeper, flexing the FSB with lateral pressure is helpful. More shallow lesions require the instrument to be flatter and less bowed. When finishing the shave, it is important to start angling the blade upward early, either at the center of the targeted lesion or 2 to 3 mm before the demarcated edge of the skin graft, while applying traction away from the lesion and slight downward pressure with the nondominant hand.
For larger lesions, the perimeter may be more difficult to remove precisely and can be achieved by rotating the blade around the lesion with focus on one point of contact of the FSB to cut and glide through the tissue’s perimeter. To achieve a more exact wound edge and to preclude jagged borders, a No. 15 blade can be used to score the perimeter very superficially to the papillary dermis prior to shave removal. The main disadvantage, however, is that the beveled edge is removed.
In summary, the FSB is an exceptional tool for biopsies, tumor removal, scar contouring, and split-thickness skin grafts. Through the banana practice model, one can attain fine control and reap the benefits of the FSB after meticulous and dedicated training.
The flexible scalpel blade (FSB) is a 2-sided handheld razor blade that serves as a pivotal instrument in certain dermatologic procedures. Its unrivaled sharpness1 permits pinpoint precision for shave biopsies, excisions of superficial lesions,2 scar contouring, and harvesting of split-thickness skin grafts.3 Given its flexibility and long edge, considerable manual dexterity and skill are required to maximize its full potential.
Practice Gap
Prior to practicing on live patients, students on clinical rotation would benefit from in vitro skin simulators to practice correct hand position, FSB control for concave and convex surface cutting, and safety. Prior practice models have included mannequins, tomatoes, and eggplants.4,5 Here, the authors recommend the use of a banana (genus Musa). In addition to its year-round availability, economic feasibility, simplicity, and portability, the banana has colored skin that well represents the epidermis, dermis, and subcutaneous tissue, allowing for visual feedback. Furthermore, its contour irregularities simulate convexities and concavities for various anatomic locations. Although the firmness of a yellow-green banana provides immediate tissue feedback, the softness and pliability of a ripe banana simulates the consistency of older skin and the use of appropriate traction.
Tools
To begin, one simply requires a marking pen, banana, and razor blade. Various shapes, including a circle, ellipse, rectangle, trapezoid, triangle, and multilobed lesion are demarcated by students or attendings (Figure 1).


The Technique
To handle the FSB, one can hold the lateral edges of the blade between the thumb and index finger or between the thumb and middle finger. The thumb and index finger position allows for additional flexible working space and visualization, increased traction by the remaining 3 fingers, and greater ease of removal of lesions with considerable height. The thumb and middle finger hold allows for versatile use of the index finger of the same hand for stabilizing the center of the blade, fixing the tissue on the FSB while it is removed, and sliding the specimen off the FSB. It is important to maintain a fixed distance from the blade to the metacarpals at all times to ensure smooth advancement of the blade and visualization. Beginners can lift the pinky finger of the hand holding the FSB and move the finger up and down to control the angle of the blade.
Practice Implications
Generally, we utilize various techniques of shaving using the FSB. We approach the target lesion 2 to 3 mm from the marked location and slide parallel to the skin surface and perpendicular to the lesion until the epidermis is penetrated. Second, we advance the blade toward the lesion with careful attention paid to the perimeter of the lesion and the points of contact of the FSB. For lesions with hardier consistencies, a sawing motion of the blade is employed, which also requires controlled tilting of the wrist to maintain an even depth and smooth bevel. To cut deeper, flexing the FSB with lateral pressure is helpful. More shallow lesions require the instrument to be flatter and less bowed. When finishing the shave, it is important to start angling the blade upward early, either at the center of the targeted lesion or 2 to 3 mm before the demarcated edge of the skin graft, while applying traction away from the lesion and slight downward pressure with the nondominant hand.
For larger lesions, the perimeter may be more difficult to remove precisely and can be achieved by rotating the blade around the lesion with focus on one point of contact of the FSB to cut and glide through the tissue’s perimeter. To achieve a more exact wound edge and to preclude jagged borders, a No. 15 blade can be used to score the perimeter very superficially to the papillary dermis prior to shave removal. The main disadvantage, however, is that the beveled edge is removed.
In summary, the FSB is an exceptional tool for biopsies, tumor removal, scar contouring, and split-thickness skin grafts. Through the banana practice model, one can attain fine control and reap the benefits of the FSB after meticulous and dedicated training.
- Awadalla B, Hexsel C, Goldberg LH. The sharpness of blades used in dermatologic surgery. Dermatol Surg. 2016;42:105-107.
- Vergilis-Kalner IJ, Goldberg LH, Firoz B, et al. Horizontal excision of in situ epidermal tumors using a flexible blade. Dermatol Surg. 2011;37:234-236.
- Hexsel CL, Loosemore M, Goldberg LH, et al. Postauricular skin: an excellent donor site for split-thickness skin grafts for the head, neck, and upper chest. Dermatol Surg. 2015;41:48-52.
- Chen TM, Mellette JR. Surgical pearl: tomato—an alternative model for shave biopsy training. J Am Acad Dermatol. 2006;54:517-518.
- Wang X, Albahrani Y, Pan M, et al. Skin simulators for dermatological procedures. Dermatol Online J. 2015;21. pii:13030/qt33j6x4nx.
- Awadalla B, Hexsel C, Goldberg LH. The sharpness of blades used in dermatologic surgery. Dermatol Surg. 2016;42:105-107.
- Vergilis-Kalner IJ, Goldberg LH, Firoz B, et al. Horizontal excision of in situ epidermal tumors using a flexible blade. Dermatol Surg. 2011;37:234-236.
- Hexsel CL, Loosemore M, Goldberg LH, et al. Postauricular skin: an excellent donor site for split-thickness skin grafts for the head, neck, and upper chest. Dermatol Surg. 2015;41:48-52.
- Chen TM, Mellette JR. Surgical pearl: tomato—an alternative model for shave biopsy training. J Am Acad Dermatol. 2006;54:517-518.
- Wang X, Albahrani Y, Pan M, et al. Skin simulators for dermatological procedures. Dermatol Online J. 2015;21. pii:13030/qt33j6x4nx.
Sodium fusidate noninferior to linezolid for acute skin infections
NEW ORLEANS – An oral antibiotic in development in the United States, fusidic acid (oral formulation, sodium fusidate) was noninferior to linezolid based on early clinical response in a randomized, double-blind, multicenter trial of 716 people with acute bacterial skin and skin structure infections (ABSSSI), including cellulitis, wound infection, and major cutaneous abscesses.
Early clinical response was defined as a 20% or greater reduction from baseline in the surface area of redness, edema, or induration at 48-72 hours after starting treatment with the study drugs. In an intent-to-treat analysis, 87.2% of patients randomized to fusidic acid and 86.6% of the linezolid group met this primary endpoint of the phase 3 study.
“Fusidic acid showed similar efficacy and comparable safety” that persisted through treatment, said Andy Strayer, PharmD, vice president of clinical programs at Cempra Pharmaceuticals, which is developing sodium fusidate as an oral agent to treat ABSSSI patients in the United States. Leo Pharmaceuticals has marketed sodium fusidate outside the United States in various formulations for decades.
Fusidic acid has potent activity against gram-positive aerobic organisms, including methicillin-resistant Staphylococcus aureus (MRSA). “Strikingly, fusidic acid showed 100% success in patients with MRSA in the microbiologically evaluable population at the end of treatment and posttherapy evaluation time points,” Dr. Strayer said at the annual meeting of the American Society for Microbiology. “Fusidic acid may offer an important oral therapy alternative for MRSA infection.”
“Fusidic acid, a drug long used in other parts of the world, has been demonstrated in this first phase 3 trial, to be a potential new option for the treatment of MRSA skin and skin structure infections in the U.S.,” said Carrie Cardenas, MD, lead study author and a principal investigator at eStudySite, San Diego, and an internist in private practice in La Mesa, California.
There was a microbiological diagnosis established in 75% of patients. S. aureus was the most commonly detected pathogen (422 patients; 59%), and the study included 235 patients diagnosed with MRSA infection.
About two-thirds, 65%, of participants were men. Mean age was 45 years. Infections were classified as wounds in 61%, cellulitis in 26%, and abscess in 13%. Notably, 68% of the recruited participants had ABSSSI associated with intravenous drug use, a “sometimes overlooked consequence of the ongoing epidemic of IV drug use in the U.S.,” Dr. Strayer said.
In terms of safety, treatment-emergent adverse event rates were comparable between the two groups (37.9% with fusidic acid versus 36.1% with linezolid). Gastrointestinal events were the most common adverse events, 22.8% versus 18.2%, respectively.
“Considering complicated skin infections are one of the most rapidly growing reasons for hospitalizations and emergency department visits each year, we anticipate that fusidic acid, if approved, may help clinicians decrease the length of inpatient stay or avoid hospitalization altogether,” Dr. Strayer said.
Cempra sponsored the study. Dr. Strayer is a Cempra employee and shareholder. Dr. Carrie Cardenas is a principal investigator at eStudySite, San Diego, and performs research for Cempra, Paratek, Debiopharm, Motif, Durata, MicuRx, Bristol-Myers Squibb, and Bayer.
NEW ORLEANS – An oral antibiotic in development in the United States, fusidic acid (oral formulation, sodium fusidate) was noninferior to linezolid based on early clinical response in a randomized, double-blind, multicenter trial of 716 people with acute bacterial skin and skin structure infections (ABSSSI), including cellulitis, wound infection, and major cutaneous abscesses.
Early clinical response was defined as a 20% or greater reduction from baseline in the surface area of redness, edema, or induration at 48-72 hours after starting treatment with the study drugs. In an intent-to-treat analysis, 87.2% of patients randomized to fusidic acid and 86.6% of the linezolid group met this primary endpoint of the phase 3 study.
“Fusidic acid showed similar efficacy and comparable safety” that persisted through treatment, said Andy Strayer, PharmD, vice president of clinical programs at Cempra Pharmaceuticals, which is developing sodium fusidate as an oral agent to treat ABSSSI patients in the United States. Leo Pharmaceuticals has marketed sodium fusidate outside the United States in various formulations for decades.
Fusidic acid has potent activity against gram-positive aerobic organisms, including methicillin-resistant Staphylococcus aureus (MRSA). “Strikingly, fusidic acid showed 100% success in patients with MRSA in the microbiologically evaluable population at the end of treatment and posttherapy evaluation time points,” Dr. Strayer said at the annual meeting of the American Society for Microbiology. “Fusidic acid may offer an important oral therapy alternative for MRSA infection.”
“Fusidic acid, a drug long used in other parts of the world, has been demonstrated in this first phase 3 trial, to be a potential new option for the treatment of MRSA skin and skin structure infections in the U.S.,” said Carrie Cardenas, MD, lead study author and a principal investigator at eStudySite, San Diego, and an internist in private practice in La Mesa, California.
There was a microbiological diagnosis established in 75% of patients. S. aureus was the most commonly detected pathogen (422 patients; 59%), and the study included 235 patients diagnosed with MRSA infection.
About two-thirds, 65%, of participants were men. Mean age was 45 years. Infections were classified as wounds in 61%, cellulitis in 26%, and abscess in 13%. Notably, 68% of the recruited participants had ABSSSI associated with intravenous drug use, a “sometimes overlooked consequence of the ongoing epidemic of IV drug use in the U.S.,” Dr. Strayer said.
In terms of safety, treatment-emergent adverse event rates were comparable between the two groups (37.9% with fusidic acid versus 36.1% with linezolid). Gastrointestinal events were the most common adverse events, 22.8% versus 18.2%, respectively.
“Considering complicated skin infections are one of the most rapidly growing reasons for hospitalizations and emergency department visits each year, we anticipate that fusidic acid, if approved, may help clinicians decrease the length of inpatient stay or avoid hospitalization altogether,” Dr. Strayer said.
Cempra sponsored the study. Dr. Strayer is a Cempra employee and shareholder. Dr. Carrie Cardenas is a principal investigator at eStudySite, San Diego, and performs research for Cempra, Paratek, Debiopharm, Motif, Durata, MicuRx, Bristol-Myers Squibb, and Bayer.
NEW ORLEANS – An oral antibiotic in development in the United States, fusidic acid (oral formulation, sodium fusidate) was noninferior to linezolid based on early clinical response in a randomized, double-blind, multicenter trial of 716 people with acute bacterial skin and skin structure infections (ABSSSI), including cellulitis, wound infection, and major cutaneous abscesses.
Early clinical response was defined as a 20% or greater reduction from baseline in the surface area of redness, edema, or induration at 48-72 hours after starting treatment with the study drugs. In an intent-to-treat analysis, 87.2% of patients randomized to fusidic acid and 86.6% of the linezolid group met this primary endpoint of the phase 3 study.
“Fusidic acid showed similar efficacy and comparable safety” that persisted through treatment, said Andy Strayer, PharmD, vice president of clinical programs at Cempra Pharmaceuticals, which is developing sodium fusidate as an oral agent to treat ABSSSI patients in the United States. Leo Pharmaceuticals has marketed sodium fusidate outside the United States in various formulations for decades.
Fusidic acid has potent activity against gram-positive aerobic organisms, including methicillin-resistant Staphylococcus aureus (MRSA). “Strikingly, fusidic acid showed 100% success in patients with MRSA in the microbiologically evaluable population at the end of treatment and posttherapy evaluation time points,” Dr. Strayer said at the annual meeting of the American Society for Microbiology. “Fusidic acid may offer an important oral therapy alternative for MRSA infection.”
“Fusidic acid, a drug long used in other parts of the world, has been demonstrated in this first phase 3 trial, to be a potential new option for the treatment of MRSA skin and skin structure infections in the U.S.,” said Carrie Cardenas, MD, lead study author and a principal investigator at eStudySite, San Diego, and an internist in private practice in La Mesa, California.
There was a microbiological diagnosis established in 75% of patients. S. aureus was the most commonly detected pathogen (422 patients; 59%), and the study included 235 patients diagnosed with MRSA infection.
About two-thirds, 65%, of participants were men. Mean age was 45 years. Infections were classified as wounds in 61%, cellulitis in 26%, and abscess in 13%. Notably, 68% of the recruited participants had ABSSSI associated with intravenous drug use, a “sometimes overlooked consequence of the ongoing epidemic of IV drug use in the U.S.,” Dr. Strayer said.
In terms of safety, treatment-emergent adverse event rates were comparable between the two groups (37.9% with fusidic acid versus 36.1% with linezolid). Gastrointestinal events were the most common adverse events, 22.8% versus 18.2%, respectively.
“Considering complicated skin infections are one of the most rapidly growing reasons for hospitalizations and emergency department visits each year, we anticipate that fusidic acid, if approved, may help clinicians decrease the length of inpatient stay or avoid hospitalization altogether,” Dr. Strayer said.
Cempra sponsored the study. Dr. Strayer is a Cempra employee and shareholder. Dr. Carrie Cardenas is a principal investigator at eStudySite, San Diego, and performs research for Cempra, Paratek, Debiopharm, Motif, Durata, MicuRx, Bristol-Myers Squibb, and Bayer.
AT ASM MICROBE 2017
Key clinical point: Sodium fusidate, active as fusidic acid, showed noninferiority to linezolid for early clinical response in ABSSI patients.
Major finding: 87.2% of patients given sodium fusidate and 86.6% of those receiving linezolid achieved an early clinical response.
Data source: Randomized, controlled, double-blind, phase 3 study with 716 participants.
Disclosures: Cempra sponsored the study. Dr. Carrier Cardenas is a researcher for Cempra, Paratek, Debiopharm, Motif, Durata, MicuRx, Bristol-Myers Squibb, and Bayer. Dr. Strayer is a Cempra employee and shareholder.
Topical Timolol May Improve Overall Scar Cosmesis in Acute Surgical Wounds
Timolol is a nonselective β-adrenergic receptor antagonist indicated for treating glaucoma, heart attacks, hypertension, and migraine headaches. It is made in both an oral and ophthalmic form. In dermatology, the beta-blocker propranolol is approved for the treatment of infantile hemangiomas (IHs). The exact mechanism of action of beta-blockers for the treatment of IHs is not yet completely understood, but it is postulated that they inhibit growth by at least 4 distinct mechanisms: (1) vasoconstriction, (2) inhibition of angiogenesis or vasculogenesis, (3) induction of apoptosis, and (4) recruitment of endothelial progenitor cells to the site of the hemangioma.1
Scar cosmesis can be calculated using the visual analog scale (VAS), which is a subjective scar assessment scored from poor to excellent. The multidimensional VAS is a photograph-based scale derived from evaluating standardized digital photographs in 4 dimensions—pigmentation, vascularity, acceptability, and observer comfort—plus contour. It uses the sum of the individual scores to obtain a single overall score ranging from excellent to poor.2 In this study, we sought to determine if the use of topical timolol after excision or Mohs micrographic surgery (MMS) treatment of nonmelanoma skin cancers improved the overall cosmesis of the scar.
Methods
The study protocol was approved by the institutional review board at Roger Williams Medical Center (Providence, Rhode Island). Eligibility criteria included patients who required excision or MMS for their nonmelanoma skin cancer located below the patella and those who agreed to allow their wounds to heal by secondary intention when given options for closure of their wounds. Patients were randomized to either the timolol (study medication) group or the saline (placebo) group. The initial defects were measured and photographed. Patients were educated on how to apply the study medication. All patients were prescribed 40 mm Hg compression stockings to wear following application of the study medication. Patients were asked to return at 1 and 5 weeks postsurgery and then every 1 to 2 weeks for wound assessment and measurement until their wounds had healed or at 13 weeks, depending on which came first. A healed wound was defined as having no exudate, exhibiting complete reepithelialization, and being stable for 1 week.
Healed wounds were assessed by a blinded outside dermatologist who examined photographs of the wounds and then completed the VAS for each participant’s scar.
Results
A total of 9 participants were enrolled in the study. Three participants were lost to follow-up; 6 completed the study (4 females, 2 males). The mean age was 70 years (age range, 46–89 years). The average wound size was 2×2 cm with a depth of 1 mm. Three participants were in the active medication group and 3 were in the control group.
A VAS was completed for each participant’s scar by an outside blinded dermatologist. Based on the VAS, wounds treated with timolol resulted in more cosmetically favorable scars (scored higher on the VAS) compared to control (mean [SD]: 6.5±0.9 vs 2.5±0.7; P<0.05). See Figures 1 and 2 for representative results.
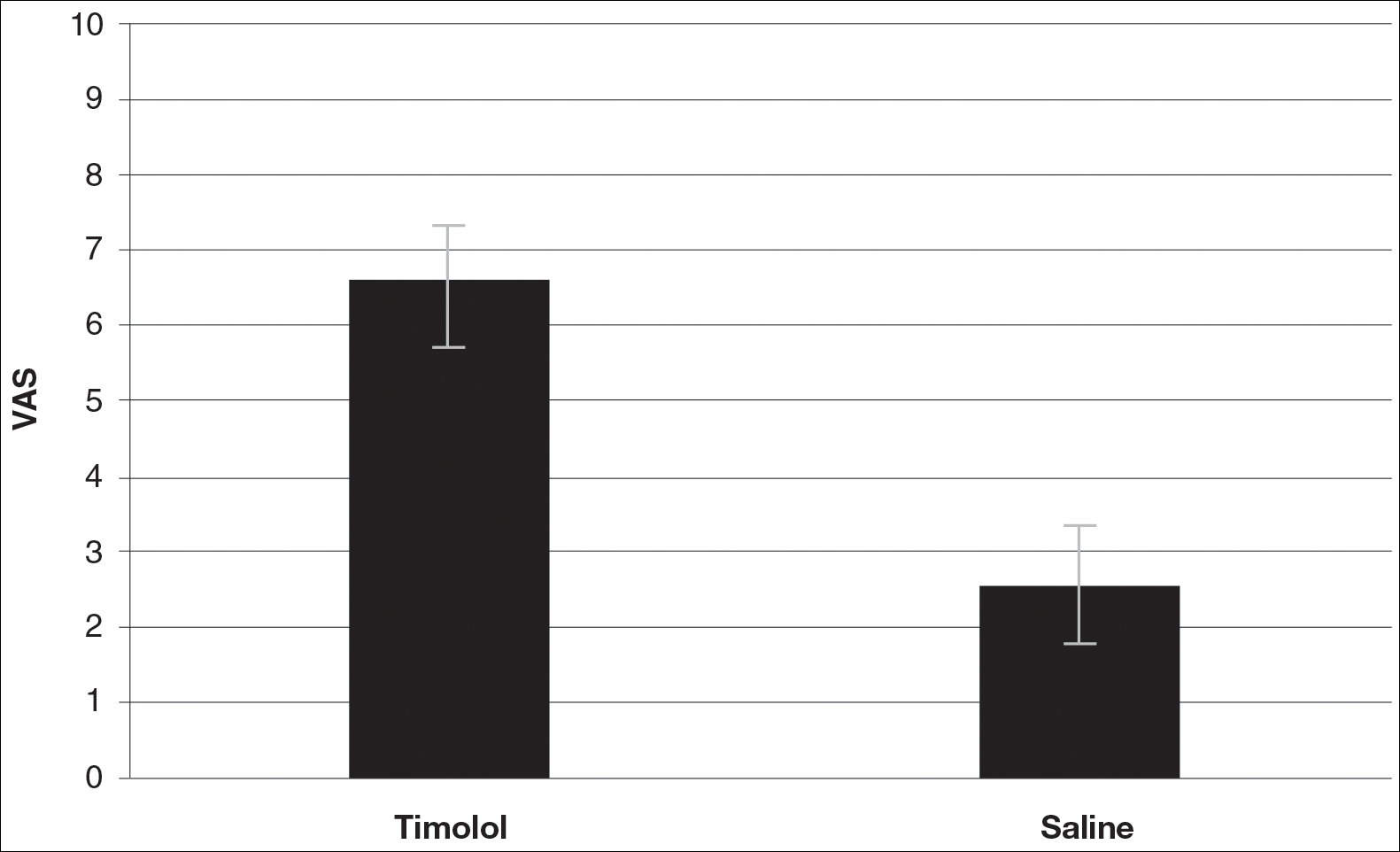
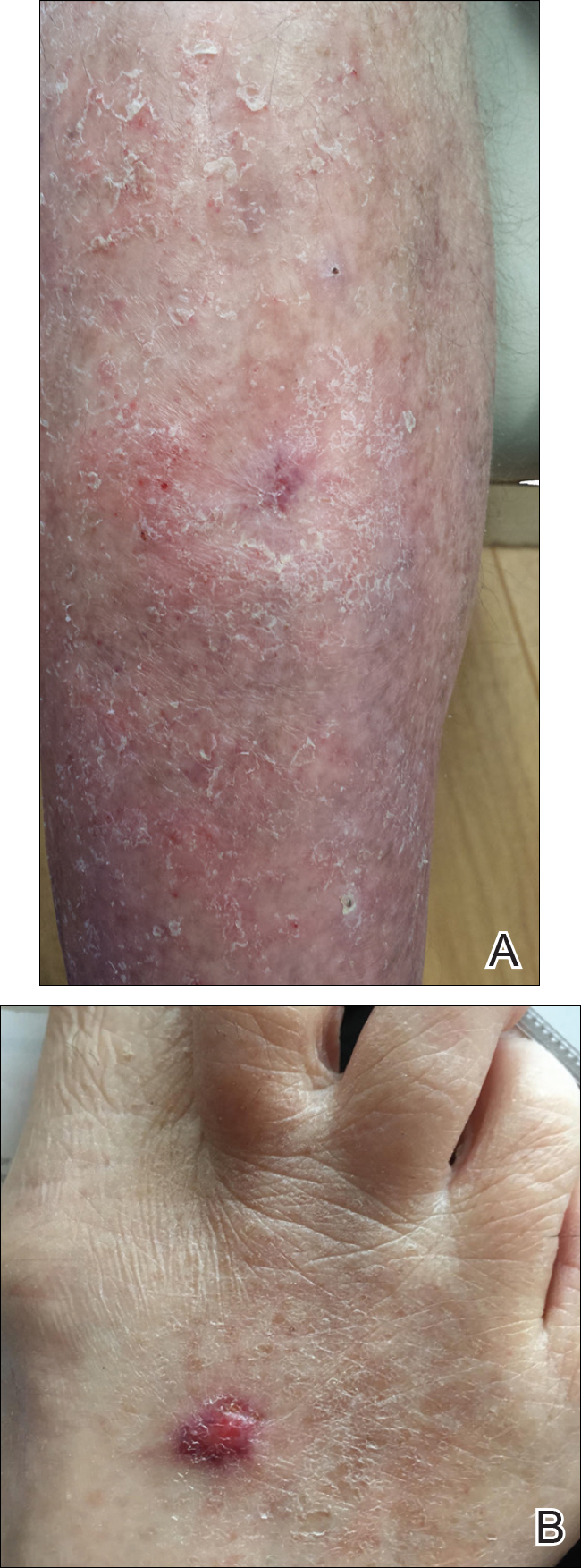
Comment
Dermatologists create acute wounds in patients on a daily basis. Ensuring that patients achieve the most desirable cosmetic outcome is a primary goal for dermatologists and an important component of patient satisfaction. A number of studies have examined patient satisfaction following MMS.3,4 Patient satisfaction is an especially important outcome measure in dermatology, as dermatologic diseases affect cosmetic appearance and are related to quality of life.3,4
Timolol is a nonselective β-adrenergic receptor antagonist that is used in dermatology to treat IHs. In this preliminary study, the authors sought to determine if topical timolol applied to acute wounds following surgical removal of nonmelanoma skin cancers could improve the overall cosmetic outcome of acute surgical scars. The results showed that compared to control, topical timolol resulted in a more cosmetically favorable scar. The results are preliminary, and it would be of future interest to further study the effects of topical timolol on acute surgical wounds from a wound-healing standpoint as well as to further test its effects on the cosmesis of these wounds.
- Chisholm KM, Chang KW, Truong MT, et al. β-Adrenergic receptor expression in vascular tumors [published online June 29, 2012]. Mod Pathol. 2012;25:1446-1451.
- Fearmonti R, Bond J, Erdmann D, et al. A review of scar scales and scar measuring devices. Eplasty. 2010;10:e43.
- Asgari MM, Warton EM, Neugebauer R, et al. Predictors of patient satisfaction with Mohs surgery: analysis of preoperative, intraoperative, and postoperative factors in a prospective cohort. Arch Dermatol. 2011;147:1387-1394.
- Asgari MM, Bertenthal D, Sen S, et al. Patient satisfaction after treatment of nonmelanoma skin cancer. Dermatol Surg. 2009;35:1041-1049.
Timolol is a nonselective β-adrenergic receptor antagonist indicated for treating glaucoma, heart attacks, hypertension, and migraine headaches. It is made in both an oral and ophthalmic form. In dermatology, the beta-blocker propranolol is approved for the treatment of infantile hemangiomas (IHs). The exact mechanism of action of beta-blockers for the treatment of IHs is not yet completely understood, but it is postulated that they inhibit growth by at least 4 distinct mechanisms: (1) vasoconstriction, (2) inhibition of angiogenesis or vasculogenesis, (3) induction of apoptosis, and (4) recruitment of endothelial progenitor cells to the site of the hemangioma.1
Scar cosmesis can be calculated using the visual analog scale (VAS), which is a subjective scar assessment scored from poor to excellent. The multidimensional VAS is a photograph-based scale derived from evaluating standardized digital photographs in 4 dimensions—pigmentation, vascularity, acceptability, and observer comfort—plus contour. It uses the sum of the individual scores to obtain a single overall score ranging from excellent to poor.2 In this study, we sought to determine if the use of topical timolol after excision or Mohs micrographic surgery (MMS) treatment of nonmelanoma skin cancers improved the overall cosmesis of the scar.
Methods
The study protocol was approved by the institutional review board at Roger Williams Medical Center (Providence, Rhode Island). Eligibility criteria included patients who required excision or MMS for their nonmelanoma skin cancer located below the patella and those who agreed to allow their wounds to heal by secondary intention when given options for closure of their wounds. Patients were randomized to either the timolol (study medication) group or the saline (placebo) group. The initial defects were measured and photographed. Patients were educated on how to apply the study medication. All patients were prescribed 40 mm Hg compression stockings to wear following application of the study medication. Patients were asked to return at 1 and 5 weeks postsurgery and then every 1 to 2 weeks for wound assessment and measurement until their wounds had healed or at 13 weeks, depending on which came first. A healed wound was defined as having no exudate, exhibiting complete reepithelialization, and being stable for 1 week.
Healed wounds were assessed by a blinded outside dermatologist who examined photographs of the wounds and then completed the VAS for each participant’s scar.
Results
A total of 9 participants were enrolled in the study. Three participants were lost to follow-up; 6 completed the study (4 females, 2 males). The mean age was 70 years (age range, 46–89 years). The average wound size was 2×2 cm with a depth of 1 mm. Three participants were in the active medication group and 3 were in the control group.
A VAS was completed for each participant’s scar by an outside blinded dermatologist. Based on the VAS, wounds treated with timolol resulted in more cosmetically favorable scars (scored higher on the VAS) compared to control (mean [SD]: 6.5±0.9 vs 2.5±0.7; P<0.05). See Figures 1 and 2 for representative results.


Comment
Dermatologists create acute wounds in patients on a daily basis. Ensuring that patients achieve the most desirable cosmetic outcome is a primary goal for dermatologists and an important component of patient satisfaction. A number of studies have examined patient satisfaction following MMS.3,4 Patient satisfaction is an especially important outcome measure in dermatology, as dermatologic diseases affect cosmetic appearance and are related to quality of life.3,4
Timolol is a nonselective β-adrenergic receptor antagonist that is used in dermatology to treat IHs. In this preliminary study, the authors sought to determine if topical timolol applied to acute wounds following surgical removal of nonmelanoma skin cancers could improve the overall cosmetic outcome of acute surgical scars. The results showed that compared to control, topical timolol resulted in a more cosmetically favorable scar. The results are preliminary, and it would be of future interest to further study the effects of topical timolol on acute surgical wounds from a wound-healing standpoint as well as to further test its effects on the cosmesis of these wounds.
Timolol is a nonselective β-adrenergic receptor antagonist indicated for treating glaucoma, heart attacks, hypertension, and migraine headaches. It is made in both an oral and ophthalmic form. In dermatology, the beta-blocker propranolol is approved for the treatment of infantile hemangiomas (IHs). The exact mechanism of action of beta-blockers for the treatment of IHs is not yet completely understood, but it is postulated that they inhibit growth by at least 4 distinct mechanisms: (1) vasoconstriction, (2) inhibition of angiogenesis or vasculogenesis, (3) induction of apoptosis, and (4) recruitment of endothelial progenitor cells to the site of the hemangioma.1
Scar cosmesis can be calculated using the visual analog scale (VAS), which is a subjective scar assessment scored from poor to excellent. The multidimensional VAS is a photograph-based scale derived from evaluating standardized digital photographs in 4 dimensions—pigmentation, vascularity, acceptability, and observer comfort—plus contour. It uses the sum of the individual scores to obtain a single overall score ranging from excellent to poor.2 In this study, we sought to determine if the use of topical timolol after excision or Mohs micrographic surgery (MMS) treatment of nonmelanoma skin cancers improved the overall cosmesis of the scar.
Methods
The study protocol was approved by the institutional review board at Roger Williams Medical Center (Providence, Rhode Island). Eligibility criteria included patients who required excision or MMS for their nonmelanoma skin cancer located below the patella and those who agreed to allow their wounds to heal by secondary intention when given options for closure of their wounds. Patients were randomized to either the timolol (study medication) group or the saline (placebo) group. The initial defects were measured and photographed. Patients were educated on how to apply the study medication. All patients were prescribed 40 mm Hg compression stockings to wear following application of the study medication. Patients were asked to return at 1 and 5 weeks postsurgery and then every 1 to 2 weeks for wound assessment and measurement until their wounds had healed or at 13 weeks, depending on which came first. A healed wound was defined as having no exudate, exhibiting complete reepithelialization, and being stable for 1 week.
Healed wounds were assessed by a blinded outside dermatologist who examined photographs of the wounds and then completed the VAS for each participant’s scar.
Results
A total of 9 participants were enrolled in the study. Three participants were lost to follow-up; 6 completed the study (4 females, 2 males). The mean age was 70 years (age range, 46–89 years). The average wound size was 2×2 cm with a depth of 1 mm. Three participants were in the active medication group and 3 were in the control group.
A VAS was completed for each participant’s scar by an outside blinded dermatologist. Based on the VAS, wounds treated with timolol resulted in more cosmetically favorable scars (scored higher on the VAS) compared to control (mean [SD]: 6.5±0.9 vs 2.5±0.7; P<0.05). See Figures 1 and 2 for representative results.


Comment
Dermatologists create acute wounds in patients on a daily basis. Ensuring that patients achieve the most desirable cosmetic outcome is a primary goal for dermatologists and an important component of patient satisfaction. A number of studies have examined patient satisfaction following MMS.3,4 Patient satisfaction is an especially important outcome measure in dermatology, as dermatologic diseases affect cosmetic appearance and are related to quality of life.3,4
Timolol is a nonselective β-adrenergic receptor antagonist that is used in dermatology to treat IHs. In this preliminary study, the authors sought to determine if topical timolol applied to acute wounds following surgical removal of nonmelanoma skin cancers could improve the overall cosmetic outcome of acute surgical scars. The results showed that compared to control, topical timolol resulted in a more cosmetically favorable scar. The results are preliminary, and it would be of future interest to further study the effects of topical timolol on acute surgical wounds from a wound-healing standpoint as well as to further test its effects on the cosmesis of these wounds.
- Chisholm KM, Chang KW, Truong MT, et al. β-Adrenergic receptor expression in vascular tumors [published online June 29, 2012]. Mod Pathol. 2012;25:1446-1451.
- Fearmonti R, Bond J, Erdmann D, et al. A review of scar scales and scar measuring devices. Eplasty. 2010;10:e43.
- Asgari MM, Warton EM, Neugebauer R, et al. Predictors of patient satisfaction with Mohs surgery: analysis of preoperative, intraoperative, and postoperative factors in a prospective cohort. Arch Dermatol. 2011;147:1387-1394.
- Asgari MM, Bertenthal D, Sen S, et al. Patient satisfaction after treatment of nonmelanoma skin cancer. Dermatol Surg. 2009;35:1041-1049.
- Chisholm KM, Chang KW, Truong MT, et al. β-Adrenergic receptor expression in vascular tumors [published online June 29, 2012]. Mod Pathol. 2012;25:1446-1451.
- Fearmonti R, Bond J, Erdmann D, et al. A review of scar scales and scar measuring devices. Eplasty. 2010;10:e43.
- Asgari MM, Warton EM, Neugebauer R, et al. Predictors of patient satisfaction with Mohs surgery: analysis of preoperative, intraoperative, and postoperative factors in a prospective cohort. Arch Dermatol. 2011;147:1387-1394.
- Asgari MM, Bertenthal D, Sen S, et al. Patient satisfaction after treatment of nonmelanoma skin cancer. Dermatol Surg. 2009;35:1041-1049.
Resident Pearl
- Dermatologists create acute surgical wounds on a daily basis. We should strive for excellent patient outcomes as well as the most desirable cosmetic result. This research article points to a possible new application of a longstanding medication to improve the cosmetic outcome in acute surgical wounds.
Many aspects to caring for epidermolysis bullosa patients
CHICAGO – Ask patients with recessive dystrophic epidermolysis bullosa (EB) to name their most bothersome symptom, and they’re likely to say itch, followed closely by pain, according to Jemima Mellerio, MD.
“We don’t really understand a lot about the mechanism of itch in patients with this disease, which is one of the reasons why we don’t have good treatments,” Prof. Mellerio said at the World Congress of Pediatric Dermatology.
A key resource for patients with EB and clinicians who care for them is Debra International, a network of national groups working on behalf of people with EB, which is undertaking a longterm initiative to develop clinical practice guidelines for the disorder. “This has been going on for about five years and is gathering momentum,” Prof. Mellerio said. “In the EB literature, there is very little that is good quality, evidence-based medicine.” Links to existing guidelines can be accessed DEBRA website.
She shared clinical tips for managing various aspects of EB, including pain, which was the subject of a recent 23-page clinical practice guideline (BMC Med. 2014;12:178). “It’s important to take a proper history: What kind of pain is it and when do they get it?” she commented. “Is there anything that is triggering it?” Basic treatment principles are to start with simple options like acetaminophen/NSAIDs and add in weak opiates as appropriate. Go a bit stronger if necessary, titrating to get the desired effect. “If you have specialist pain services, that can be extremely useful in some of the more complex cases,” she said.
Many EB patients are plagued by neuropathic pain that burns and stings. “For these cases, you might try tricyclic antidepressants or anticonvulsants like gabapentin and pregabalin,” she noted. Anxiolytics such as midazolam can be used to reduce anxiety during procedures, bathing, and dressings. A wide range of pain formulations exist to meet patient needs or preferences, including oral tablets or suspensions, lozenges, intranasal preparations, transdermal patches and intramuscular and intravenous injections.
Topical measures for isolated, painful wounds include ibuprofen-impregnated dressings such as Biatain Ibu and topical morphine gel. “You can get this made up by using morphine sulfate and mixing it in a hydrogel,” Prof. Mellerio said. “You apply that when you have a limited number of painful wounds, so you don’t get the systemic effects from having morphine but you get the local beneficial effects.” [This approach was described in Arch Dis Child. 2004;89:679-81.] Adding salt to a bath can also ameliorate pain for patients. She recommends adding 90g of salt to 10L bath water for a 0.9% solution, which translates into about 800g salt for a half-full tub of water.
Basic skin care is another challenge for EB patients. For those with extremely fragile skin, Prof. Mellerio recommends applying a primary layer of a soft silicone or lipidocolloid dressing under a secondary dressing layer. “There’s a whole range of soft silicone foam dressings or polymeric membrane, which is a nice soft dressing that can go over a primary dressing or directly on the skin if fragility is not a problem,” she said. “Really, it comes down to patient and carer choice as to what to use. It depends on many factors including site, exudate, pain, and dressing size. The frequency of wound changes will also vary. So, if you’ve got an infected, more heavily exuding wound, the dressing changes will be more frequent.”
Critical colonization and infection are significant problems for EB patients and are ideally managed with topical antimicrobials such as hydrogen peroxide cream, enzymatic antimicrobials, polyhexamethylene biguanide, and medical grade honey. “Topical antibiotics such as mupirocin can also be used, but there are problems with resistance if you’re using it long-term and potential for sensitization,” Prof. Mellerio said. “Other options include antimicrobial dressings such as polymeric membrane, polyhexamethylene biguanide, and silver. With silver dressings, there is the potential to absorb silver, so, if you’re a child and you have a lot of wounds on your skin, you can absorb silver at significant levels, which could be a problem.”
If patients don’t respond to topical measures, consider using oral antibiotics for 10-14 days, she said. Swab first for sensitivity and to look for Streptococcal carriage “because you can get a lot of problems like renal damage,” and use IV antibiotics only for severe infections, she said. “Best Practice Guidelines for Skin and Wound Care in Epidermolysis Bullosa,” supported by an award from the Urgo Foundation and produced by Wounds International/Wounds UK, are available.
Prof. Mellerio noted that EB can also adversely affect oral health and lead to the formation of painful blisters, scarring, microstomia, and ankyloglossia, which “can contribute to difficulties eating and speaking and make it hard to keep the teeth clean.” Analgesics can be helpful, as can an NSAID mouthwash or spray or mucoprotectants like Episil that coat the surface of lesions. “Alcohol-free chlorhexidine washes and fluoride mouth washes can help, as can high fluoride toothpaste and trying to limit the consumption of sugary foods and snacks,” she said. “You can adapt things like toothbrushes with a grip, which means that it’s a bit easier for somebody with EB to be able to keep their teeth clean.”
Keeping bones strong is also a concern, since osteopenia and osteoporosis are common in EB. “We’ve seen vertebral crush fractures in children as young as five years old,” Prof. Mellerio said. “Optimize calcium and vitamin D and mobility, which is important in helping people accrue their bone mineral density throughout life. Sometimes we have to use bisphosphonate therapies, but there isn’t a great deal in the literature to support what the best way of doing this is.”
Prof. Mellerio reported having no financial disclosures.
CHICAGO – Ask patients with recessive dystrophic epidermolysis bullosa (EB) to name their most bothersome symptom, and they’re likely to say itch, followed closely by pain, according to Jemima Mellerio, MD.
“We don’t really understand a lot about the mechanism of itch in patients with this disease, which is one of the reasons why we don’t have good treatments,” Prof. Mellerio said at the World Congress of Pediatric Dermatology.
A key resource for patients with EB and clinicians who care for them is Debra International, a network of national groups working on behalf of people with EB, which is undertaking a longterm initiative to develop clinical practice guidelines for the disorder. “This has been going on for about five years and is gathering momentum,” Prof. Mellerio said. “In the EB literature, there is very little that is good quality, evidence-based medicine.” Links to existing guidelines can be accessed DEBRA website.
She shared clinical tips for managing various aspects of EB, including pain, which was the subject of a recent 23-page clinical practice guideline (BMC Med. 2014;12:178). “It’s important to take a proper history: What kind of pain is it and when do they get it?” she commented. “Is there anything that is triggering it?” Basic treatment principles are to start with simple options like acetaminophen/NSAIDs and add in weak opiates as appropriate. Go a bit stronger if necessary, titrating to get the desired effect. “If you have specialist pain services, that can be extremely useful in some of the more complex cases,” she said.
Many EB patients are plagued by neuropathic pain that burns and stings. “For these cases, you might try tricyclic antidepressants or anticonvulsants like gabapentin and pregabalin,” she noted. Anxiolytics such as midazolam can be used to reduce anxiety during procedures, bathing, and dressings. A wide range of pain formulations exist to meet patient needs or preferences, including oral tablets or suspensions, lozenges, intranasal preparations, transdermal patches and intramuscular and intravenous injections.
Topical measures for isolated, painful wounds include ibuprofen-impregnated dressings such as Biatain Ibu and topical morphine gel. “You can get this made up by using morphine sulfate and mixing it in a hydrogel,” Prof. Mellerio said. “You apply that when you have a limited number of painful wounds, so you don’t get the systemic effects from having morphine but you get the local beneficial effects.” [This approach was described in Arch Dis Child. 2004;89:679-81.] Adding salt to a bath can also ameliorate pain for patients. She recommends adding 90g of salt to 10L bath water for a 0.9% solution, which translates into about 800g salt for a half-full tub of water.
Basic skin care is another challenge for EB patients. For those with extremely fragile skin, Prof. Mellerio recommends applying a primary layer of a soft silicone or lipidocolloid dressing under a secondary dressing layer. “There’s a whole range of soft silicone foam dressings or polymeric membrane, which is a nice soft dressing that can go over a primary dressing or directly on the skin if fragility is not a problem,” she said. “Really, it comes down to patient and carer choice as to what to use. It depends on many factors including site, exudate, pain, and dressing size. The frequency of wound changes will also vary. So, if you’ve got an infected, more heavily exuding wound, the dressing changes will be more frequent.”
Critical colonization and infection are significant problems for EB patients and are ideally managed with topical antimicrobials such as hydrogen peroxide cream, enzymatic antimicrobials, polyhexamethylene biguanide, and medical grade honey. “Topical antibiotics such as mupirocin can also be used, but there are problems with resistance if you’re using it long-term and potential for sensitization,” Prof. Mellerio said. “Other options include antimicrobial dressings such as polymeric membrane, polyhexamethylene biguanide, and silver. With silver dressings, there is the potential to absorb silver, so, if you’re a child and you have a lot of wounds on your skin, you can absorb silver at significant levels, which could be a problem.”
If patients don’t respond to topical measures, consider using oral antibiotics for 10-14 days, she said. Swab first for sensitivity and to look for Streptococcal carriage “because you can get a lot of problems like renal damage,” and use IV antibiotics only for severe infections, she said. “Best Practice Guidelines for Skin and Wound Care in Epidermolysis Bullosa,” supported by an award from the Urgo Foundation and produced by Wounds International/Wounds UK, are available.
Prof. Mellerio noted that EB can also adversely affect oral health and lead to the formation of painful blisters, scarring, microstomia, and ankyloglossia, which “can contribute to difficulties eating and speaking and make it hard to keep the teeth clean.” Analgesics can be helpful, as can an NSAID mouthwash or spray or mucoprotectants like Episil that coat the surface of lesions. “Alcohol-free chlorhexidine washes and fluoride mouth washes can help, as can high fluoride toothpaste and trying to limit the consumption of sugary foods and snacks,” she said. “You can adapt things like toothbrushes with a grip, which means that it’s a bit easier for somebody with EB to be able to keep their teeth clean.”
Keeping bones strong is also a concern, since osteopenia and osteoporosis are common in EB. “We’ve seen vertebral crush fractures in children as young as five years old,” Prof. Mellerio said. “Optimize calcium and vitamin D and mobility, which is important in helping people accrue their bone mineral density throughout life. Sometimes we have to use bisphosphonate therapies, but there isn’t a great deal in the literature to support what the best way of doing this is.”
Prof. Mellerio reported having no financial disclosures.
CHICAGO – Ask patients with recessive dystrophic epidermolysis bullosa (EB) to name their most bothersome symptom, and they’re likely to say itch, followed closely by pain, according to Jemima Mellerio, MD.
“We don’t really understand a lot about the mechanism of itch in patients with this disease, which is one of the reasons why we don’t have good treatments,” Prof. Mellerio said at the World Congress of Pediatric Dermatology.
A key resource for patients with EB and clinicians who care for them is Debra International, a network of national groups working on behalf of people with EB, which is undertaking a longterm initiative to develop clinical practice guidelines for the disorder. “This has been going on for about five years and is gathering momentum,” Prof. Mellerio said. “In the EB literature, there is very little that is good quality, evidence-based medicine.” Links to existing guidelines can be accessed DEBRA website.
She shared clinical tips for managing various aspects of EB, including pain, which was the subject of a recent 23-page clinical practice guideline (BMC Med. 2014;12:178). “It’s important to take a proper history: What kind of pain is it and when do they get it?” she commented. “Is there anything that is triggering it?” Basic treatment principles are to start with simple options like acetaminophen/NSAIDs and add in weak opiates as appropriate. Go a bit stronger if necessary, titrating to get the desired effect. “If you have specialist pain services, that can be extremely useful in some of the more complex cases,” she said.
Many EB patients are plagued by neuropathic pain that burns and stings. “For these cases, you might try tricyclic antidepressants or anticonvulsants like gabapentin and pregabalin,” she noted. Anxiolytics such as midazolam can be used to reduce anxiety during procedures, bathing, and dressings. A wide range of pain formulations exist to meet patient needs or preferences, including oral tablets or suspensions, lozenges, intranasal preparations, transdermal patches and intramuscular and intravenous injections.
Topical measures for isolated, painful wounds include ibuprofen-impregnated dressings such as Biatain Ibu and topical morphine gel. “You can get this made up by using morphine sulfate and mixing it in a hydrogel,” Prof. Mellerio said. “You apply that when you have a limited number of painful wounds, so you don’t get the systemic effects from having morphine but you get the local beneficial effects.” [This approach was described in Arch Dis Child. 2004;89:679-81.] Adding salt to a bath can also ameliorate pain for patients. She recommends adding 90g of salt to 10L bath water for a 0.9% solution, which translates into about 800g salt for a half-full tub of water.
Basic skin care is another challenge for EB patients. For those with extremely fragile skin, Prof. Mellerio recommends applying a primary layer of a soft silicone or lipidocolloid dressing under a secondary dressing layer. “There’s a whole range of soft silicone foam dressings or polymeric membrane, which is a nice soft dressing that can go over a primary dressing or directly on the skin if fragility is not a problem,” she said. “Really, it comes down to patient and carer choice as to what to use. It depends on many factors including site, exudate, pain, and dressing size. The frequency of wound changes will also vary. So, if you’ve got an infected, more heavily exuding wound, the dressing changes will be more frequent.”
Critical colonization and infection are significant problems for EB patients and are ideally managed with topical antimicrobials such as hydrogen peroxide cream, enzymatic antimicrobials, polyhexamethylene biguanide, and medical grade honey. “Topical antibiotics such as mupirocin can also be used, but there are problems with resistance if you’re using it long-term and potential for sensitization,” Prof. Mellerio said. “Other options include antimicrobial dressings such as polymeric membrane, polyhexamethylene biguanide, and silver. With silver dressings, there is the potential to absorb silver, so, if you’re a child and you have a lot of wounds on your skin, you can absorb silver at significant levels, which could be a problem.”
If patients don’t respond to topical measures, consider using oral antibiotics for 10-14 days, she said. Swab first for sensitivity and to look for Streptococcal carriage “because you can get a lot of problems like renal damage,” and use IV antibiotics only for severe infections, she said. “Best Practice Guidelines for Skin and Wound Care in Epidermolysis Bullosa,” supported by an award from the Urgo Foundation and produced by Wounds International/Wounds UK, are available.
Prof. Mellerio noted that EB can also adversely affect oral health and lead to the formation of painful blisters, scarring, microstomia, and ankyloglossia, which “can contribute to difficulties eating and speaking and make it hard to keep the teeth clean.” Analgesics can be helpful, as can an NSAID mouthwash or spray or mucoprotectants like Episil that coat the surface of lesions. “Alcohol-free chlorhexidine washes and fluoride mouth washes can help, as can high fluoride toothpaste and trying to limit the consumption of sugary foods and snacks,” she said. “You can adapt things like toothbrushes with a grip, which means that it’s a bit easier for somebody with EB to be able to keep their teeth clean.”
Keeping bones strong is also a concern, since osteopenia and osteoporosis are common in EB. “We’ve seen vertebral crush fractures in children as young as five years old,” Prof. Mellerio said. “Optimize calcium and vitamin D and mobility, which is important in helping people accrue their bone mineral density throughout life. Sometimes we have to use bisphosphonate therapies, but there isn’t a great deal in the literature to support what the best way of doing this is.”
Prof. Mellerio reported having no financial disclosures.
AT WCPD 2017