User login
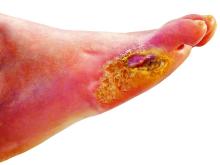
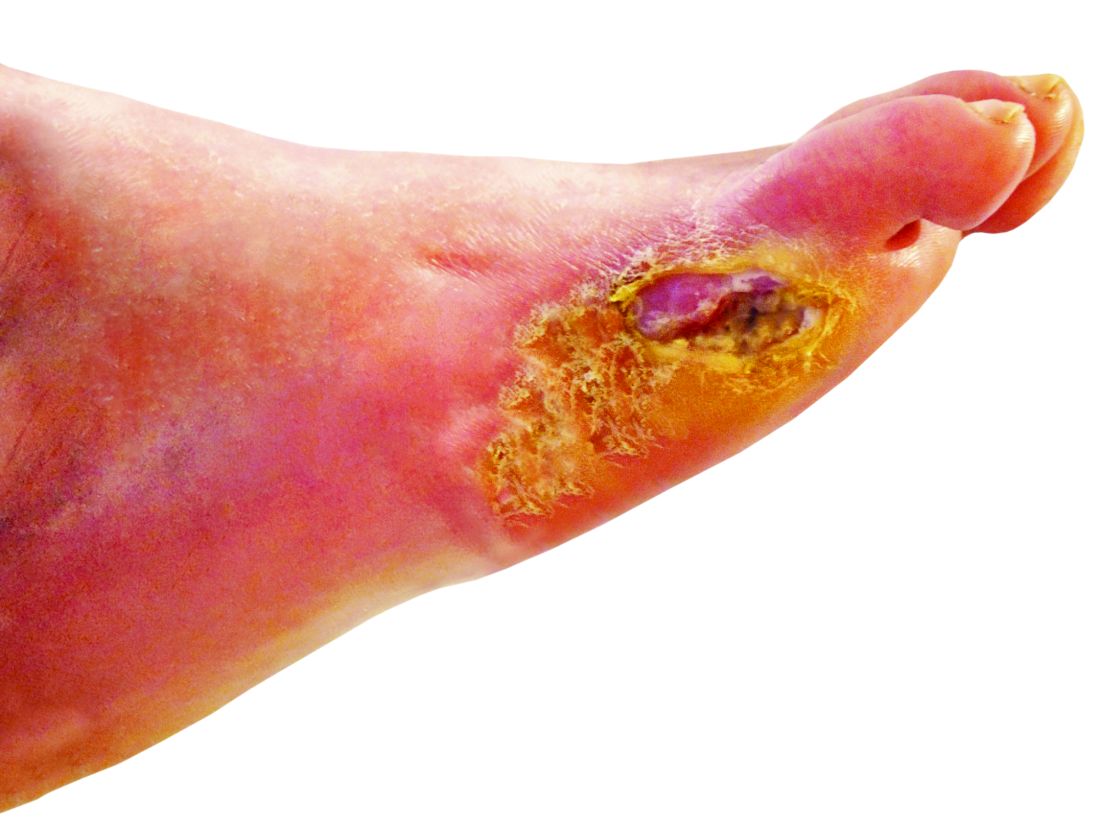
Nitric oxide–generating dressing holds promise for diabetic foot ulcers
Physicians and nurses turn to a wide variety of kinds of dressings to treat patients with diabetic foot ulcers (DFU).
The treatment is still in the research stage, and it’s not clear whether more studies will be conducted. For now, though, “we have a topical agent which specifically treats infection as well as increases perfusion of the ulcer,” study lead author Michael E. Edmonds, MD, a professor of diabetes and endocrinology at King’s College Hospital in London, said in an interview. “The study also showed that the agent not only improved healing but significantly reduced serious adverse events related to the ulcer, which included hospitalizations and amputations.”
The study appeared online April 4 in Wound Repair and Regeneration.
Researchers estimate that DFUs affect as many as 4% of patients with diabetes each year, with about a quarter developing the condition over their lifetimes.
A 2014 U.S. study found that 4%-5% percentage of patients with DFUs underwent lower limb amputations over a 12-month period. The same study also estimated that DFU-related care costs as much as $13 billion a year. (Diabetes Care. 2014 Mar;37[3]:651-8)
“There is no straightforward guideline to choose dressing,” said wound care specialist William H. Tettelbach, MD, the medical director of infection prevention, wound care, and antibiotic stewardship at Landmark Hospital in Salt Lake City, in an interview. Instead, he said, there are just some general tenets: Use an absorbing dressing for a wet ulcer, a moist dressing for a dry ulcer, and an antimicrobial dressing for a bacterial ulcer.
The new multi-center, randomized, controlled phase 2/3 study – funded by the biotech company Edixomed – examined the use of a nitric oxide–generating dressing known as EDX110. The dressing consists of a moist mesh and a second layer that keeps the first layer in place.
“The critical factors that delay the healing of diabetic foot ulcers are ischemia and infection,” Dr. Edmonds said. “Nitric oxide plays a crucial role in maintaining the microvascular supply and infection control in the skin, and its absence in diabetes contributes to poor ulcer healing. EDX110 generates a sustained release of nitric oxide which can treat both infection and ischemia simultaneously.”
The average age of patients in both groups was 59 years, and males made up 82%-87% of the total. Some had more than 1 ulcer.
All patients received standard DFU care for their institution with the exception of members of the treatment group, who were given the EDX110 dressing. Participants were treated for 12 weeks or until their ulcers healed followed by a 12-week follow-up period.
The institutes used a wide variety of dressings including absorbent pad, alginate, antimicrobial, foam, gauze, and other types. About a third were antimicrobial.
In the intent-to-treat population at 12 weeks, the median percentage area reduction of the ulcers was 89% in the treatment group, compared with 47% in the control group (P = .016).
The researchers reported significantly fewer serious adverse events in the treatment group, and none were reported to be linked to the various dressings used.
According to Dr. Edmonds, pricing information for the treatment is unavailable.
Dr. Tettelbach cautioned about the limitations of the study. For one, it doesn’t focus on chronic DFUs that can last well beyond a month and “are more problematic to heal and pose a greater relative risk of infection than acute DFUs.”
He added: “Surrogate end points such as 80% reduction in surface area at 12 weeks are difficult to extrapolate to expected closure. An open chronic ulcer is at risk for complicating infection no matter what size,” he said.
Overall, Dr. Tettelbach said, he doesn’t see the study as a “big deal,” but it’s “a welcomed addition to the wound dressing family that works using a novel mechanism of stimulating angiogenesis and antimicrobial properties.”
The biotech company Edixomed funded the study. The study authors report various disclosures or no disclosures; two disclose links to Edixomed.
SOURCE: Edmonds ME et al. Wound Repair Regen. 2018 April 4. doi: 10.1111/wrr.12630.
Physicians and nurses turn to a wide variety of kinds of dressings to treat patients with diabetic foot ulcers (DFU).
The treatment is still in the research stage, and it’s not clear whether more studies will be conducted. For now, though, “we have a topical agent which specifically treats infection as well as increases perfusion of the ulcer,” study lead author Michael E. Edmonds, MD, a professor of diabetes and endocrinology at King’s College Hospital in London, said in an interview. “The study also showed that the agent not only improved healing but significantly reduced serious adverse events related to the ulcer, which included hospitalizations and amputations.”
The study appeared online April 4 in Wound Repair and Regeneration.
Researchers estimate that DFUs affect as many as 4% of patients with diabetes each year, with about a quarter developing the condition over their lifetimes.
A 2014 U.S. study found that 4%-5% percentage of patients with DFUs underwent lower limb amputations over a 12-month period. The same study also estimated that DFU-related care costs as much as $13 billion a year. (Diabetes Care. 2014 Mar;37[3]:651-8)
“There is no straightforward guideline to choose dressing,” said wound care specialist William H. Tettelbach, MD, the medical director of infection prevention, wound care, and antibiotic stewardship at Landmark Hospital in Salt Lake City, in an interview. Instead, he said, there are just some general tenets: Use an absorbing dressing for a wet ulcer, a moist dressing for a dry ulcer, and an antimicrobial dressing for a bacterial ulcer.
The new multi-center, randomized, controlled phase 2/3 study – funded by the biotech company Edixomed – examined the use of a nitric oxide–generating dressing known as EDX110. The dressing consists of a moist mesh and a second layer that keeps the first layer in place.
“The critical factors that delay the healing of diabetic foot ulcers are ischemia and infection,” Dr. Edmonds said. “Nitric oxide plays a crucial role in maintaining the microvascular supply and infection control in the skin, and its absence in diabetes contributes to poor ulcer healing. EDX110 generates a sustained release of nitric oxide which can treat both infection and ischemia simultaneously.”
The average age of patients in both groups was 59 years, and males made up 82%-87% of the total. Some had more than 1 ulcer.
All patients received standard DFU care for their institution with the exception of members of the treatment group, who were given the EDX110 dressing. Participants were treated for 12 weeks or until their ulcers healed followed by a 12-week follow-up period.
The institutes used a wide variety of dressings including absorbent pad, alginate, antimicrobial, foam, gauze, and other types. About a third were antimicrobial.
In the intent-to-treat population at 12 weeks, the median percentage area reduction of the ulcers was 89% in the treatment group, compared with 47% in the control group (P = .016).
The researchers reported significantly fewer serious adverse events in the treatment group, and none were reported to be linked to the various dressings used.
According to Dr. Edmonds, pricing information for the treatment is unavailable.
Dr. Tettelbach cautioned about the limitations of the study. For one, it doesn’t focus on chronic DFUs that can last well beyond a month and “are more problematic to heal and pose a greater relative risk of infection than acute DFUs.”
He added: “Surrogate end points such as 80% reduction in surface area at 12 weeks are difficult to extrapolate to expected closure. An open chronic ulcer is at risk for complicating infection no matter what size,” he said.
Overall, Dr. Tettelbach said, he doesn’t see the study as a “big deal,” but it’s “a welcomed addition to the wound dressing family that works using a novel mechanism of stimulating angiogenesis and antimicrobial properties.”
The biotech company Edixomed funded the study. The study authors report various disclosures or no disclosures; two disclose links to Edixomed.
SOURCE: Edmonds ME et al. Wound Repair Regen. 2018 April 4. doi: 10.1111/wrr.12630.
Physicians and nurses turn to a wide variety of kinds of dressings to treat patients with diabetic foot ulcers (DFU).
The treatment is still in the research stage, and it’s not clear whether more studies will be conducted. For now, though, “we have a topical agent which specifically treats infection as well as increases perfusion of the ulcer,” study lead author Michael E. Edmonds, MD, a professor of diabetes and endocrinology at King’s College Hospital in London, said in an interview. “The study also showed that the agent not only improved healing but significantly reduced serious adverse events related to the ulcer, which included hospitalizations and amputations.”
The study appeared online April 4 in Wound Repair and Regeneration.
Researchers estimate that DFUs affect as many as 4% of patients with diabetes each year, with about a quarter developing the condition over their lifetimes.
A 2014 U.S. study found that 4%-5% percentage of patients with DFUs underwent lower limb amputations over a 12-month period. The same study also estimated that DFU-related care costs as much as $13 billion a year. (Diabetes Care. 2014 Mar;37[3]:651-8)
“There is no straightforward guideline to choose dressing,” said wound care specialist William H. Tettelbach, MD, the medical director of infection prevention, wound care, and antibiotic stewardship at Landmark Hospital in Salt Lake City, in an interview. Instead, he said, there are just some general tenets: Use an absorbing dressing for a wet ulcer, a moist dressing for a dry ulcer, and an antimicrobial dressing for a bacterial ulcer.
The new multi-center, randomized, controlled phase 2/3 study – funded by the biotech company Edixomed – examined the use of a nitric oxide–generating dressing known as EDX110. The dressing consists of a moist mesh and a second layer that keeps the first layer in place.
“The critical factors that delay the healing of diabetic foot ulcers are ischemia and infection,” Dr. Edmonds said. “Nitric oxide plays a crucial role in maintaining the microvascular supply and infection control in the skin, and its absence in diabetes contributes to poor ulcer healing. EDX110 generates a sustained release of nitric oxide which can treat both infection and ischemia simultaneously.”
The average age of patients in both groups was 59 years, and males made up 82%-87% of the total. Some had more than 1 ulcer.
All patients received standard DFU care for their institution with the exception of members of the treatment group, who were given the EDX110 dressing. Participants were treated for 12 weeks or until their ulcers healed followed by a 12-week follow-up period.
The institutes used a wide variety of dressings including absorbent pad, alginate, antimicrobial, foam, gauze, and other types. About a third were antimicrobial.
In the intent-to-treat population at 12 weeks, the median percentage area reduction of the ulcers was 89% in the treatment group, compared with 47% in the control group (P = .016).
The researchers reported significantly fewer serious adverse events in the treatment group, and none were reported to be linked to the various dressings used.
According to Dr. Edmonds, pricing information for the treatment is unavailable.
Dr. Tettelbach cautioned about the limitations of the study. For one, it doesn’t focus on chronic DFUs that can last well beyond a month and “are more problematic to heal and pose a greater relative risk of infection than acute DFUs.”
He added: “Surrogate end points such as 80% reduction in surface area at 12 weeks are difficult to extrapolate to expected closure. An open chronic ulcer is at risk for complicating infection no matter what size,” he said.
Overall, Dr. Tettelbach said, he doesn’t see the study as a “big deal,” but it’s “a welcomed addition to the wound dressing family that works using a novel mechanism of stimulating angiogenesis and antimicrobial properties.”
The biotech company Edixomed funded the study. The study authors report various disclosures or no disclosures; two disclose links to Edixomed.
SOURCE: Edmonds ME et al. Wound Repair Regen. 2018 April 4. doi: 10.1111/wrr.12630.
FROM WOUND REPAIR AND REGENERATION
Drug-induced Linear IgA Bullous Dermatosis in a Patient With a Vancomycin-impregnated Cement Spacer
Case Report
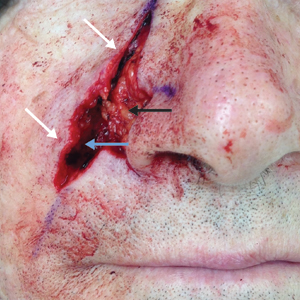
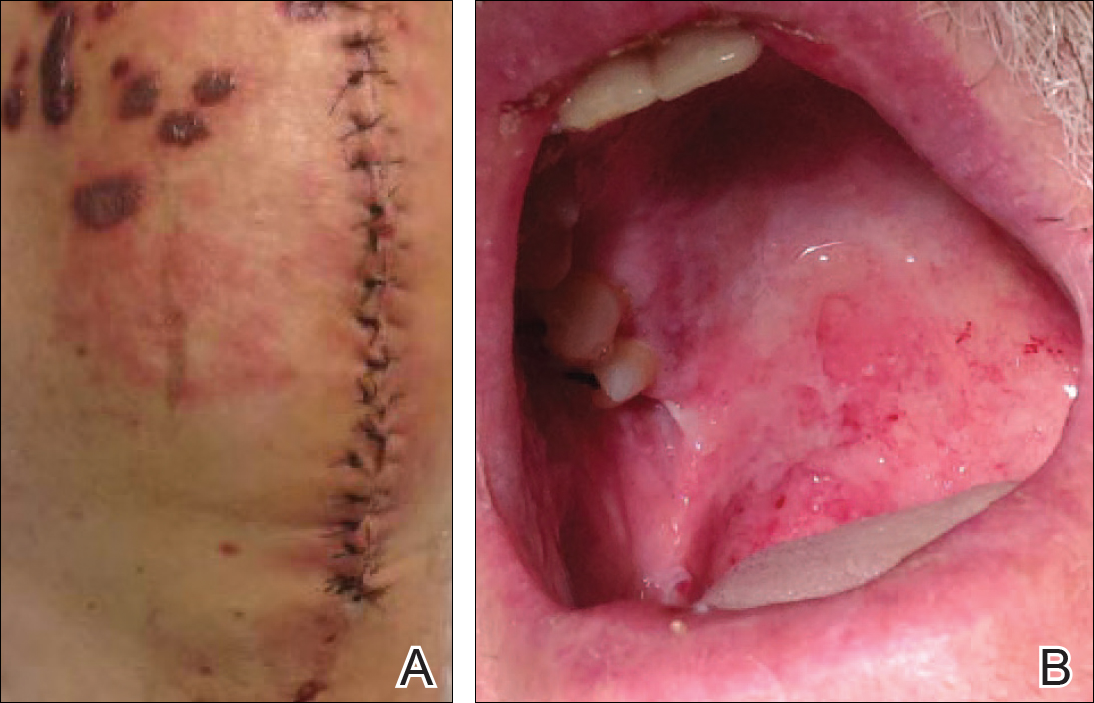
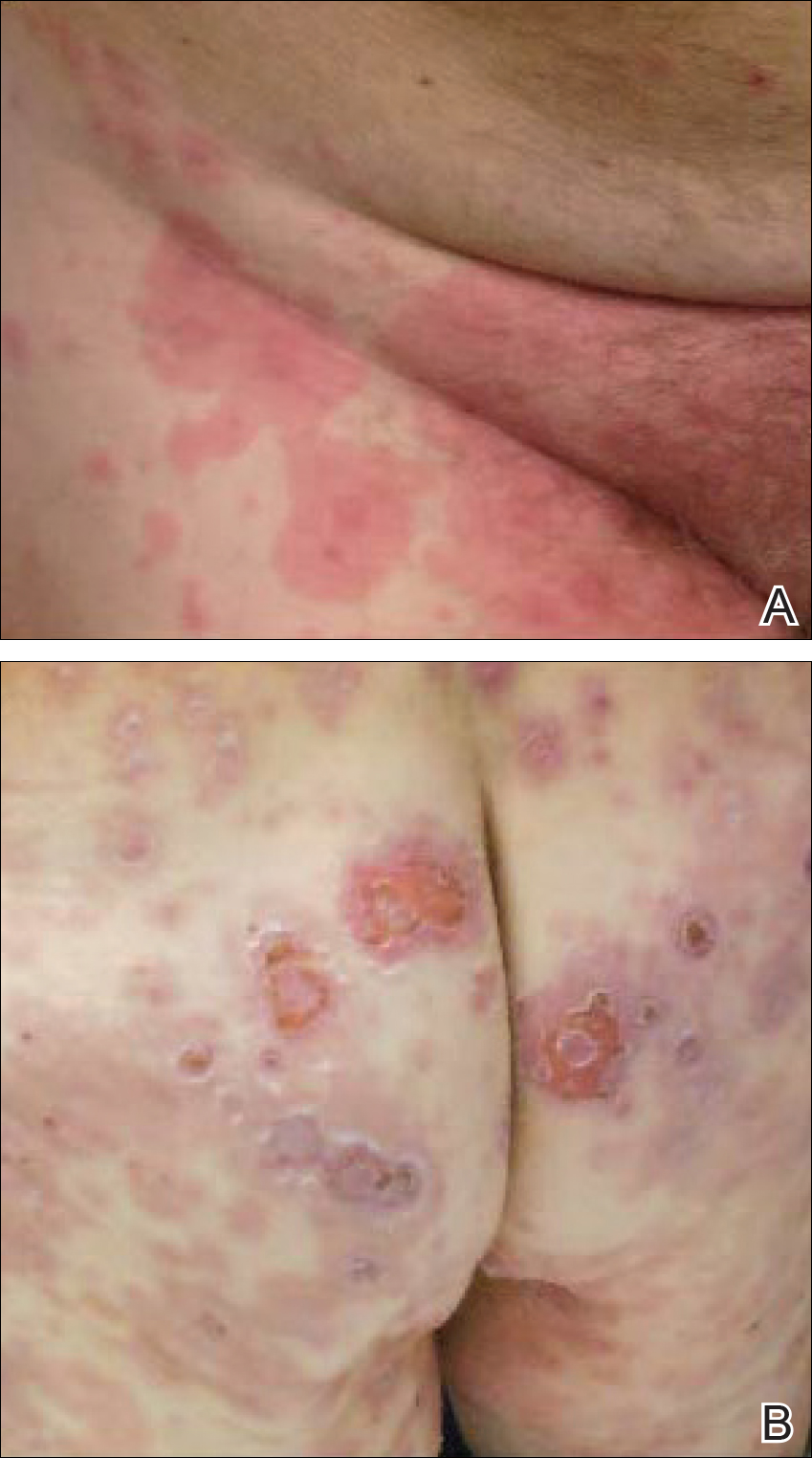
A 77-year-old man was admitted to the general medicine service at our institution for treatment of a diffuse macular eruption and hemorrhagic bullae 12 days after undergoing left-knee revision arthroplasty during which a cement spacer impregnated with vancomycin and tobramycin was placed. At the time of the surgery, the patient also received intravenous (IV) vancomycin and oral ciprofloxacin, which were continued postoperatively until his hospital presentation. The patient was recovering well until postoperative day 7, when he developed painful swelling and erythema surrounding the surgical wound on the left knee. Concerned that his symptoms indicated a flare of gout, he restarted a former allopurinol prescription from an outside physician after 2 years of nonuse. The skin changes progressed distally on the left leg over the next 48 hours. By postoperative day 10, he had developed serosanguinous blisters on the left knee (Figure 1A) and oral mucosa (Figure 1B), as well as erythematous nodules on the bilateral palms. He presented to our institution for emergent care on postoperative day 12 following progression of the eruption to the inguinal region (Figure 2A), buttocks (Figure 2B), and abdominal region.
Due to concerns about a potential drug reaction, the IV vancomycin, oral ciprofloxacin, and oral allopurinol were discontinued on hospital admission.
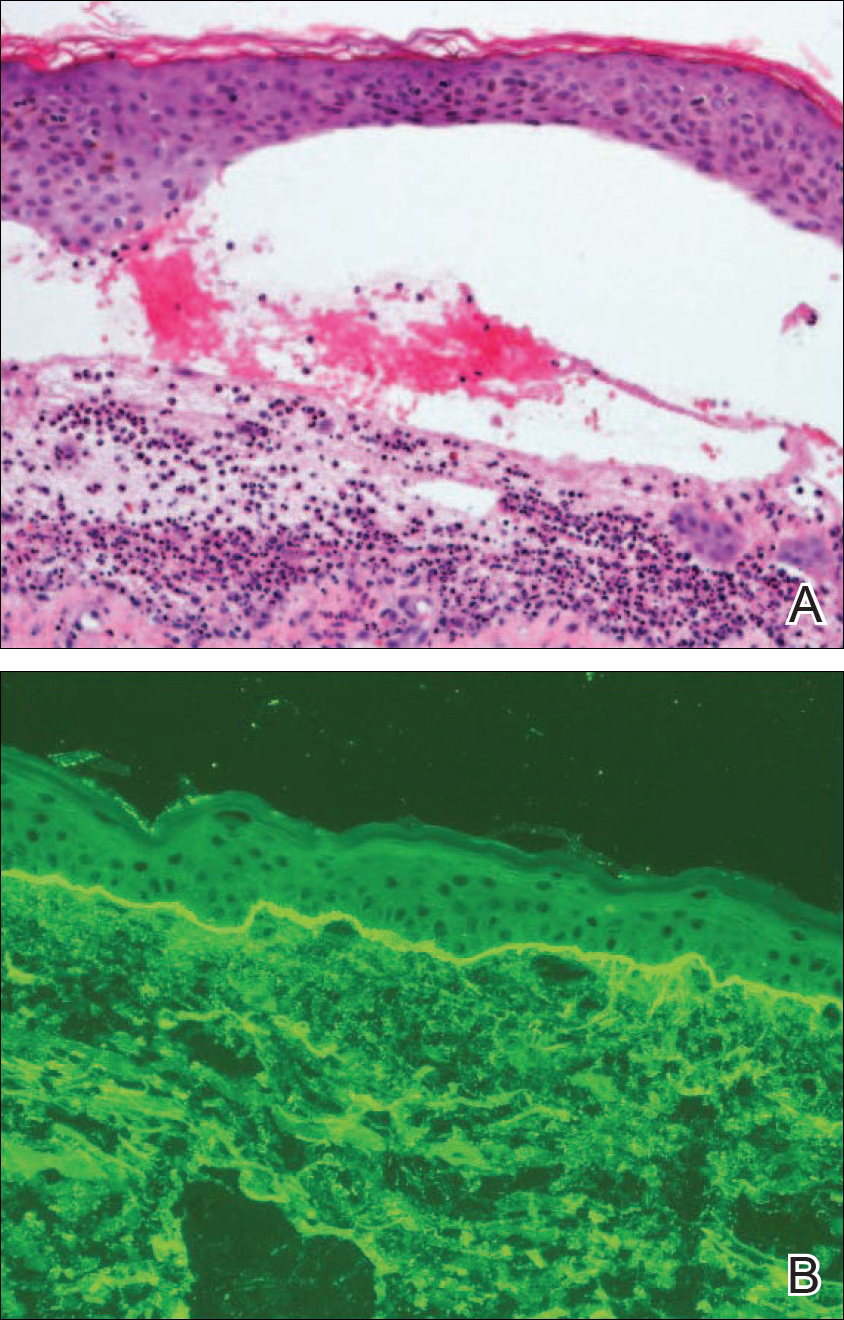
Oral prednisone 60 mg once daily and oral dapsone 25 mg once daily were initiated on hospital days 4 and 6 (postoperative days 15 and 17), respectively. A 6-week course of oral ciprofloxacin 750 mg twice daily and daptomycin 8 mg/kg once daily was initiated for bacterial coverage on hospital day 5 (postoperative day 16). Topical triamcinolone and an anesthetic mouthwash also were used to treat the mucosal involvement. The lesions stabilized on the third day of steroid therapy, and the patient was discharged 7 days after hospital admission (postoperative day 18). Dapsone was rapidly increased to 100 mg once daily over the next week for Pneumocystis jirovecii pneumonia prophylaxis. An increase in prednisone to 80 mg once daily was required 3 days after the patient was discharged due to worsening oral lesions. Five days after discharge, the patient was readmitted to the hospital for 3 days due to acute kidney injury (AKI) in which his baseline creatinine level tripled. The cause of renal impairment was unknown, resulting in empiric discontinuation of dapsone on postoperative day 27. Prophylaxis for P jirovecii pneumonia was replaced with once-monthly inhaled pentamidine. Prednisone was tapered 20 days after the original presentation (postoperative day 32) following gradual improvement of both the skin and oral lesions. At dermatology follow-up 2 weeks later, doxycycline 100 mg twice daily was added for residual inflammation of the left leg. A deep vein thrombosis was discovered in the left leg 10 days later, and 3 months of anticoagulation therapy was initiated with discontinuation of the doxycycline. The patient continued to have renal insufficiency several weeks after dapsone discontinuation and developed prominent peripheral motor neuropathy with bilateral thenar atrophy. He did not experience any skin eruptions or relapses in the weeks following prednisone cessation and underwent successful removal of the cement spacer with full left-knee reconstruction 4 months after his initial presentation to our institution. At 9-month dermatology follow-up, the LABD remained in remission.
Comment
Linear IgA bullous dermatosis is a well-documented autoimmune mucocutaneous disorder characterized by linear IgA deposits at the dermoepidermal junction. The development of autoantibodies to antigens within the basement membrane zone leads to both cellular and humoral immune responses that facilitate the subepidermal blistering rash in LABD.2,3 Linear IgA bullous dermatosis affects all ages and races with a bimodal epidemiology. The adult form typically appears after 60 years of age, whereas the childhood form (chronic bullous disease of childhood) appears between 6 months and 6 years of age.3 Medications—particularly vancomycin—are responsible for a substantial portion of cases.1-4 In one review, vancomycin was implicated in almost half (22/52 [42.3%]) of drug-related cases of LABD.4 Other associated medications include captopril, trimethoprim-sulfamethoxazole, phenytoin, and diclo-fenac.3,4 Vancomycin-associated LABD has a substantially shorter time to onset of symptoms, with a mean of 8.6 days compared to 63.8 days for other causative agents.4
The initial treatment of drug-induced LABD is immediate discontinuation of the suspected agent(s) and supportive care.9 Although future avoidance of vancomycin is recommended in patients with a history of LABD, there are reported cases of successful rechallenges.4,10 The early removal of our patient’s cement spacer was discouraged by both the orthopedics and infectious disease consultation services due to potential complications as well as the patient’s gradual improvement during his hospital course.
Dapsone is considered the standard systemic treatment for LABD. Sulfapyridine is an alternative to dapsone, or a combination of these 2 drugs may be used. Corticosteroids can be added to each of these regimens to achieve remission, as in our case.2 Although dapsone was discontinued in the setting of the patient’s AKI, the vancomycin in the dual-eluting spacer was more likely the culprit. A review of 544 postoperative outcomes following the use of an antibiotic-impregnated cement spacer (AICS) during 2-stage arthroplasty displayed an 8- to 10-fold increase in the development of AKIs compared to the rate of AKIs following primary joint arthroplasty.10 While our patient’s AKI was not attributed to dapsone, his prominent peripheral motor neuropathy with resultant bilateral thenar atrophy was a rare complication of dapsone use. While dapsone-associated neuropathy has been reported in daily dosages of as low as 75 mg, it typically is seen in doses of at least 300 mg per day and in larger cumulative dosages.11
Despite having a well-characterized vancomycin-induced LABD in the setting of known vancomycin exposure, our patient’s case was particularly challenging given the continued presence of the vancomycin-impregnated cement spacer (VICS) in the left knee, resulting in vancomycin levels at admission and during subsequent measurements over 2 weeks that were all several-fold higher than the renal clearance predicted.
Vancomycin-associated LABD does not appear to be dose dependent and has been reported at both subtherapeutic1-3 and supratherapeutic levels,5-9 whereas toxicity reactions are more common at supratherapeutic levels.9 The literature on AICS use suggests that drug elution occurs at relatively unpredictable rates based on a variety of factors, including the type of cement used and the initial antibiotic concentration.12,13 Furthermore, the addition of tobramycin to VICSs has been found to increase the rate of vancomycin delivery through a phenomenon known as passive opportunism.14
As AICS devices allow for the delivery of higher concentrations of antibiotics to a localized area, systemic complications are considered rare but have been reported.13 Our report describes a rare case of LABD in the setting of a VICS. One clinical aspect of our case that supports the implication of VICS as the cause of the patient’s LABD is the concentration of bullae overlying the incision site on the left knee. A case of a desquamating rash in a patient with an implanted VICS has been documented in which the early lesions were localized to the surgical leg, as in our case.15 Unlike our case, there was a history of Stevens-Johnson syndrome following previous vancomycin exposure. A case of a gentamicin-impregnated cement spacer causing allergic dermatitis that was most prominent in the surgical leg also has been reported.16 An isomorphic phenomenon (Köbner phenomenon) has been suggested in the setting of
- Plunkett RW, Chiarello SE, Beutner EH. Linear IgA bullous dermatosis in one of two piroxicam-induced eruptions: a distinct direct immunofluorescence trend revealed by the literature. J Am Acad Dermatol. 2001;45:691-696.
- Guide SV, Marinkovich MP. Linear IgA bullous dermatosis. Clin Dermatol. 2001;19:719-727.
- Fortuna G, Marinkovich MP. Linear immunoglobulin A bullous dermatosis. Clin Dermatol. 2012;30:38-50.
- Fortuna G, Salas-Alanis JC, Guidetti E, et al. A critical reappraisal of the current data on drug-induced linear immunoglobulin A bullous dermatosis: a real and separate nosological entity? J Am Acad Dermatol. 2012;66:988-994.
- Kuechle MK, Stegemeir E, Maynard B, et al. Drug-induced linear IgA bullous dermatosis: report of six cases and review of the literature. J Am Acad Dermatol. 1994;30(2, pt 1):187-192.
- Neughebauer BI, Negron G, Pelton S, et al. Bullous skin disease: an unusual allergic reaction to vancomycin. Am J Med Sci. 2002;323:273-278.
- Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-245.
- Wiadrowski TP, Reid CM. Drug-induced linear IgA bullous disease following antibiotics. Australas J Dermatol. 2001;42:196-199.
- Dang LV, Byrom L, Muir J, et al. Vancomycin-induced linear IgA with mucosal and ocular involvement: a case report. Infect Dis Clin Pract. 2014;22:e119-e121.
- Luu A, Syed F, Raman G, et al. Two-stage arthroplasty for prosthetic joint infection: a systematic review of acute kidney injury, systemic toxicity and infection control [published online April 8, 2013]. J Arthroplasty. 2013;28:1490.e1-1498.e1.
- Daneshmend TK. The neurotoxicity of dapsone. Adverse Drug React Acute Poisoning Rev. 1984;3:43-58.
- Jacobs C, Christensen CP, Berend ME. Static and mobile antibiotic-impregnated cement spacers for the management of prosthetic joint infection. J Am Acad Orthop Surg. 2009;17:356-368.
- Springer BD, Lee GC, Osmon D, et al. Systemic safety of high-dose antibiotic-loaded cement spacers after resection of an infected total knee arthroplasty. Clin Orthop Relat Res. 2004;427:47-51.
- Penner MJ, Masri BA, Duncan CP. Elution characteristics of vancomycin and tobramycin combined in acrylic bone-cement. J Arthroplasty. 1996;11:939-944.
- Williams B, Hanson A, Sha B. Diffuse desquamating rash following exposure to vancomycin-impregnated bone cement. Ann Pharmacother. 2014;48:1061-1065.
- Haeberle M, Wittner B. Is gentamicin-loaded bone cement a risk for developing systemic allergic dermatitis? Contact Dermatitis. 2009;60:176-177.
- McDonald HC, York NR, Pandya AG. Drug-induced linear IgA bullous dermatosis demonstrating the isomorphic phenomenon. J Am Acad Dermatol. 2010;62:897-898.
Case Report
A 77-year-old man was admitted to the general medicine service at our institution for treatment of a diffuse macular eruption and hemorrhagic bullae 12 days after undergoing left-knee revision arthroplasty during which a cement spacer impregnated with vancomycin and tobramycin was placed. At the time of the surgery, the patient also received intravenous (IV) vancomycin and oral ciprofloxacin, which were continued postoperatively until his hospital presentation. The patient was recovering well until postoperative day 7, when he developed painful swelling and erythema surrounding the surgical wound on the left knee. Concerned that his symptoms indicated a flare of gout, he restarted a former allopurinol prescription from an outside physician after 2 years of nonuse. The skin changes progressed distally on the left leg over the next 48 hours. By postoperative day 10, he had developed serosanguinous blisters on the left knee (Figure 1A) and oral mucosa (Figure 1B), as well as erythematous nodules on the bilateral palms. He presented to our institution for emergent care on postoperative day 12 following progression of the eruption to the inguinal region (Figure 2A), buttocks (Figure 2B), and abdominal region.


Due to concerns about a potential drug reaction, the IV vancomycin, oral ciprofloxacin, and oral allopurinol were discontinued on hospital admission.

Oral prednisone 60 mg once daily and oral dapsone 25 mg once daily were initiated on hospital days 4 and 6 (postoperative days 15 and 17), respectively. A 6-week course of oral ciprofloxacin 750 mg twice daily and daptomycin 8 mg/kg once daily was initiated for bacterial coverage on hospital day 5 (postoperative day 16). Topical triamcinolone and an anesthetic mouthwash also were used to treat the mucosal involvement. The lesions stabilized on the third day of steroid therapy, and the patient was discharged 7 days after hospital admission (postoperative day 18). Dapsone was rapidly increased to 100 mg once daily over the next week for Pneumocystis jirovecii pneumonia prophylaxis. An increase in prednisone to 80 mg once daily was required 3 days after the patient was discharged due to worsening oral lesions. Five days after discharge, the patient was readmitted to the hospital for 3 days due to acute kidney injury (AKI) in which his baseline creatinine level tripled. The cause of renal impairment was unknown, resulting in empiric discontinuation of dapsone on postoperative day 27. Prophylaxis for P jirovecii pneumonia was replaced with once-monthly inhaled pentamidine. Prednisone was tapered 20 days after the original presentation (postoperative day 32) following gradual improvement of both the skin and oral lesions. At dermatology follow-up 2 weeks later, doxycycline 100 mg twice daily was added for residual inflammation of the left leg. A deep vein thrombosis was discovered in the left leg 10 days later, and 3 months of anticoagulation therapy was initiated with discontinuation of the doxycycline. The patient continued to have renal insufficiency several weeks after dapsone discontinuation and developed prominent peripheral motor neuropathy with bilateral thenar atrophy. He did not experience any skin eruptions or relapses in the weeks following prednisone cessation and underwent successful removal of the cement spacer with full left-knee reconstruction 4 months after his initial presentation to our institution. At 9-month dermatology follow-up, the LABD remained in remission.
Comment
Linear IgA bullous dermatosis is a well-documented autoimmune mucocutaneous disorder characterized by linear IgA deposits at the dermoepidermal junction. The development of autoantibodies to antigens within the basement membrane zone leads to both cellular and humoral immune responses that facilitate the subepidermal blistering rash in LABD.2,3 Linear IgA bullous dermatosis affects all ages and races with a bimodal epidemiology. The adult form typically appears after 60 years of age, whereas the childhood form (chronic bullous disease of childhood) appears between 6 months and 6 years of age.3 Medications—particularly vancomycin—are responsible for a substantial portion of cases.1-4 In one review, vancomycin was implicated in almost half (22/52 [42.3%]) of drug-related cases of LABD.4 Other associated medications include captopril, trimethoprim-sulfamethoxazole, phenytoin, and diclo-fenac.3,4 Vancomycin-associated LABD has a substantially shorter time to onset of symptoms, with a mean of 8.6 days compared to 63.8 days for other causative agents.4
The initial treatment of drug-induced LABD is immediate discontinuation of the suspected agent(s) and supportive care.9 Although future avoidance of vancomycin is recommended in patients with a history of LABD, there are reported cases of successful rechallenges.4,10 The early removal of our patient’s cement spacer was discouraged by both the orthopedics and infectious disease consultation services due to potential complications as well as the patient’s gradual improvement during his hospital course.
Dapsone is considered the standard systemic treatment for LABD. Sulfapyridine is an alternative to dapsone, or a combination of these 2 drugs may be used. Corticosteroids can be added to each of these regimens to achieve remission, as in our case.2 Although dapsone was discontinued in the setting of the patient’s AKI, the vancomycin in the dual-eluting spacer was more likely the culprit. A review of 544 postoperative outcomes following the use of an antibiotic-impregnated cement spacer (AICS) during 2-stage arthroplasty displayed an 8- to 10-fold increase in the development of AKIs compared to the rate of AKIs following primary joint arthroplasty.10 While our patient’s AKI was not attributed to dapsone, his prominent peripheral motor neuropathy with resultant bilateral thenar atrophy was a rare complication of dapsone use. While dapsone-associated neuropathy has been reported in daily dosages of as low as 75 mg, it typically is seen in doses of at least 300 mg per day and in larger cumulative dosages.11
Despite having a well-characterized vancomycin-induced LABD in the setting of known vancomycin exposure, our patient’s case was particularly challenging given the continued presence of the vancomycin-impregnated cement spacer (VICS) in the left knee, resulting in vancomycin levels at admission and during subsequent measurements over 2 weeks that were all several-fold higher than the renal clearance predicted.
Vancomycin-associated LABD does not appear to be dose dependent and has been reported at both subtherapeutic1-3 and supratherapeutic levels,5-9 whereas toxicity reactions are more common at supratherapeutic levels.9 The literature on AICS use suggests that drug elution occurs at relatively unpredictable rates based on a variety of factors, including the type of cement used and the initial antibiotic concentration.12,13 Furthermore, the addition of tobramycin to VICSs has been found to increase the rate of vancomycin delivery through a phenomenon known as passive opportunism.14
As AICS devices allow for the delivery of higher concentrations of antibiotics to a localized area, systemic complications are considered rare but have been reported.13 Our report describes a rare case of LABD in the setting of a VICS. One clinical aspect of our case that supports the implication of VICS as the cause of the patient’s LABD is the concentration of bullae overlying the incision site on the left knee. A case of a desquamating rash in a patient with an implanted VICS has been documented in which the early lesions were localized to the surgical leg, as in our case.15 Unlike our case, there was a history of Stevens-Johnson syndrome following previous vancomycin exposure. A case of a gentamicin-impregnated cement spacer causing allergic dermatitis that was most prominent in the surgical leg also has been reported.16 An isomorphic phenomenon (Köbner phenomenon) has been suggested in the setting of
Case Report
A 77-year-old man was admitted to the general medicine service at our institution for treatment of a diffuse macular eruption and hemorrhagic bullae 12 days after undergoing left-knee revision arthroplasty during which a cement spacer impregnated with vancomycin and tobramycin was placed. At the time of the surgery, the patient also received intravenous (IV) vancomycin and oral ciprofloxacin, which were continued postoperatively until his hospital presentation. The patient was recovering well until postoperative day 7, when he developed painful swelling and erythema surrounding the surgical wound on the left knee. Concerned that his symptoms indicated a flare of gout, he restarted a former allopurinol prescription from an outside physician after 2 years of nonuse. The skin changes progressed distally on the left leg over the next 48 hours. By postoperative day 10, he had developed serosanguinous blisters on the left knee (Figure 1A) and oral mucosa (Figure 1B), as well as erythematous nodules on the bilateral palms. He presented to our institution for emergent care on postoperative day 12 following progression of the eruption to the inguinal region (Figure 2A), buttocks (Figure 2B), and abdominal region.


Due to concerns about a potential drug reaction, the IV vancomycin, oral ciprofloxacin, and oral allopurinol were discontinued on hospital admission.

Oral prednisone 60 mg once daily and oral dapsone 25 mg once daily were initiated on hospital days 4 and 6 (postoperative days 15 and 17), respectively. A 6-week course of oral ciprofloxacin 750 mg twice daily and daptomycin 8 mg/kg once daily was initiated for bacterial coverage on hospital day 5 (postoperative day 16). Topical triamcinolone and an anesthetic mouthwash also were used to treat the mucosal involvement. The lesions stabilized on the third day of steroid therapy, and the patient was discharged 7 days after hospital admission (postoperative day 18). Dapsone was rapidly increased to 100 mg once daily over the next week for Pneumocystis jirovecii pneumonia prophylaxis. An increase in prednisone to 80 mg once daily was required 3 days after the patient was discharged due to worsening oral lesions. Five days after discharge, the patient was readmitted to the hospital for 3 days due to acute kidney injury (AKI) in which his baseline creatinine level tripled. The cause of renal impairment was unknown, resulting in empiric discontinuation of dapsone on postoperative day 27. Prophylaxis for P jirovecii pneumonia was replaced with once-monthly inhaled pentamidine. Prednisone was tapered 20 days after the original presentation (postoperative day 32) following gradual improvement of both the skin and oral lesions. At dermatology follow-up 2 weeks later, doxycycline 100 mg twice daily was added for residual inflammation of the left leg. A deep vein thrombosis was discovered in the left leg 10 days later, and 3 months of anticoagulation therapy was initiated with discontinuation of the doxycycline. The patient continued to have renal insufficiency several weeks after dapsone discontinuation and developed prominent peripheral motor neuropathy with bilateral thenar atrophy. He did not experience any skin eruptions or relapses in the weeks following prednisone cessation and underwent successful removal of the cement spacer with full left-knee reconstruction 4 months after his initial presentation to our institution. At 9-month dermatology follow-up, the LABD remained in remission.
Comment
Linear IgA bullous dermatosis is a well-documented autoimmune mucocutaneous disorder characterized by linear IgA deposits at the dermoepidermal junction. The development of autoantibodies to antigens within the basement membrane zone leads to both cellular and humoral immune responses that facilitate the subepidermal blistering rash in LABD.2,3 Linear IgA bullous dermatosis affects all ages and races with a bimodal epidemiology. The adult form typically appears after 60 years of age, whereas the childhood form (chronic bullous disease of childhood) appears between 6 months and 6 years of age.3 Medications—particularly vancomycin—are responsible for a substantial portion of cases.1-4 In one review, vancomycin was implicated in almost half (22/52 [42.3%]) of drug-related cases of LABD.4 Other associated medications include captopril, trimethoprim-sulfamethoxazole, phenytoin, and diclo-fenac.3,4 Vancomycin-associated LABD has a substantially shorter time to onset of symptoms, with a mean of 8.6 days compared to 63.8 days for other causative agents.4
The initial treatment of drug-induced LABD is immediate discontinuation of the suspected agent(s) and supportive care.9 Although future avoidance of vancomycin is recommended in patients with a history of LABD, there are reported cases of successful rechallenges.4,10 The early removal of our patient’s cement spacer was discouraged by both the orthopedics and infectious disease consultation services due to potential complications as well as the patient’s gradual improvement during his hospital course.
Dapsone is considered the standard systemic treatment for LABD. Sulfapyridine is an alternative to dapsone, or a combination of these 2 drugs may be used. Corticosteroids can be added to each of these regimens to achieve remission, as in our case.2 Although dapsone was discontinued in the setting of the patient’s AKI, the vancomycin in the dual-eluting spacer was more likely the culprit. A review of 544 postoperative outcomes following the use of an antibiotic-impregnated cement spacer (AICS) during 2-stage arthroplasty displayed an 8- to 10-fold increase in the development of AKIs compared to the rate of AKIs following primary joint arthroplasty.10 While our patient’s AKI was not attributed to dapsone, his prominent peripheral motor neuropathy with resultant bilateral thenar atrophy was a rare complication of dapsone use. While dapsone-associated neuropathy has been reported in daily dosages of as low as 75 mg, it typically is seen in doses of at least 300 mg per day and in larger cumulative dosages.11
Despite having a well-characterized vancomycin-induced LABD in the setting of known vancomycin exposure, our patient’s case was particularly challenging given the continued presence of the vancomycin-impregnated cement spacer (VICS) in the left knee, resulting in vancomycin levels at admission and during subsequent measurements over 2 weeks that were all several-fold higher than the renal clearance predicted.
Vancomycin-associated LABD does not appear to be dose dependent and has been reported at both subtherapeutic1-3 and supratherapeutic levels,5-9 whereas toxicity reactions are more common at supratherapeutic levels.9 The literature on AICS use suggests that drug elution occurs at relatively unpredictable rates based on a variety of factors, including the type of cement used and the initial antibiotic concentration.12,13 Furthermore, the addition of tobramycin to VICSs has been found to increase the rate of vancomycin delivery through a phenomenon known as passive opportunism.14
As AICS devices allow for the delivery of higher concentrations of antibiotics to a localized area, systemic complications are considered rare but have been reported.13 Our report describes a rare case of LABD in the setting of a VICS. One clinical aspect of our case that supports the implication of VICS as the cause of the patient’s LABD is the concentration of bullae overlying the incision site on the left knee. A case of a desquamating rash in a patient with an implanted VICS has been documented in which the early lesions were localized to the surgical leg, as in our case.15 Unlike our case, there was a history of Stevens-Johnson syndrome following previous vancomycin exposure. A case of a gentamicin-impregnated cement spacer causing allergic dermatitis that was most prominent in the surgical leg also has been reported.16 An isomorphic phenomenon (Köbner phenomenon) has been suggested in the setting of
- Plunkett RW, Chiarello SE, Beutner EH. Linear IgA bullous dermatosis in one of two piroxicam-induced eruptions: a distinct direct immunofluorescence trend revealed by the literature. J Am Acad Dermatol. 2001;45:691-696.
- Guide SV, Marinkovich MP. Linear IgA bullous dermatosis. Clin Dermatol. 2001;19:719-727.
- Fortuna G, Marinkovich MP. Linear immunoglobulin A bullous dermatosis. Clin Dermatol. 2012;30:38-50.
- Fortuna G, Salas-Alanis JC, Guidetti E, et al. A critical reappraisal of the current data on drug-induced linear immunoglobulin A bullous dermatosis: a real and separate nosological entity? J Am Acad Dermatol. 2012;66:988-994.
- Kuechle MK, Stegemeir E, Maynard B, et al. Drug-induced linear IgA bullous dermatosis: report of six cases and review of the literature. J Am Acad Dermatol. 1994;30(2, pt 1):187-192.
- Neughebauer BI, Negron G, Pelton S, et al. Bullous skin disease: an unusual allergic reaction to vancomycin. Am J Med Sci. 2002;323:273-278.
- Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-245.
- Wiadrowski TP, Reid CM. Drug-induced linear IgA bullous disease following antibiotics. Australas J Dermatol. 2001;42:196-199.
- Dang LV, Byrom L, Muir J, et al. Vancomycin-induced linear IgA with mucosal and ocular involvement: a case report. Infect Dis Clin Pract. 2014;22:e119-e121.
- Luu A, Syed F, Raman G, et al. Two-stage arthroplasty for prosthetic joint infection: a systematic review of acute kidney injury, systemic toxicity and infection control [published online April 8, 2013]. J Arthroplasty. 2013;28:1490.e1-1498.e1.
- Daneshmend TK. The neurotoxicity of dapsone. Adverse Drug React Acute Poisoning Rev. 1984;3:43-58.
- Jacobs C, Christensen CP, Berend ME. Static and mobile antibiotic-impregnated cement spacers for the management of prosthetic joint infection. J Am Acad Orthop Surg. 2009;17:356-368.
- Springer BD, Lee GC, Osmon D, et al. Systemic safety of high-dose antibiotic-loaded cement spacers after resection of an infected total knee arthroplasty. Clin Orthop Relat Res. 2004;427:47-51.
- Penner MJ, Masri BA, Duncan CP. Elution characteristics of vancomycin and tobramycin combined in acrylic bone-cement. J Arthroplasty. 1996;11:939-944.
- Williams B, Hanson A, Sha B. Diffuse desquamating rash following exposure to vancomycin-impregnated bone cement. Ann Pharmacother. 2014;48:1061-1065.
- Haeberle M, Wittner B. Is gentamicin-loaded bone cement a risk for developing systemic allergic dermatitis? Contact Dermatitis. 2009;60:176-177.
- McDonald HC, York NR, Pandya AG. Drug-induced linear IgA bullous dermatosis demonstrating the isomorphic phenomenon. J Am Acad Dermatol. 2010;62:897-898.
- Plunkett RW, Chiarello SE, Beutner EH. Linear IgA bullous dermatosis in one of two piroxicam-induced eruptions: a distinct direct immunofluorescence trend revealed by the literature. J Am Acad Dermatol. 2001;45:691-696.
- Guide SV, Marinkovich MP. Linear IgA bullous dermatosis. Clin Dermatol. 2001;19:719-727.
- Fortuna G, Marinkovich MP. Linear immunoglobulin A bullous dermatosis. Clin Dermatol. 2012;30:38-50.
- Fortuna G, Salas-Alanis JC, Guidetti E, et al. A critical reappraisal of the current data on drug-induced linear immunoglobulin A bullous dermatosis: a real and separate nosological entity? J Am Acad Dermatol. 2012;66:988-994.
- Kuechle MK, Stegemeir E, Maynard B, et al. Drug-induced linear IgA bullous dermatosis: report of six cases and review of the literature. J Am Acad Dermatol. 1994;30(2, pt 1):187-192.
- Neughebauer BI, Negron G, Pelton S, et al. Bullous skin disease: an unusual allergic reaction to vancomycin. Am J Med Sci. 2002;323:273-278.
- Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-245.
- Wiadrowski TP, Reid CM. Drug-induced linear IgA bullous disease following antibiotics. Australas J Dermatol. 2001;42:196-199.
- Dang LV, Byrom L, Muir J, et al. Vancomycin-induced linear IgA with mucosal and ocular involvement: a case report. Infect Dis Clin Pract. 2014;22:e119-e121.
- Luu A, Syed F, Raman G, et al. Two-stage arthroplasty for prosthetic joint infection: a systematic review of acute kidney injury, systemic toxicity and infection control [published online April 8, 2013]. J Arthroplasty. 2013;28:1490.e1-1498.e1.
- Daneshmend TK. The neurotoxicity of dapsone. Adverse Drug React Acute Poisoning Rev. 1984;3:43-58.
- Jacobs C, Christensen CP, Berend ME. Static and mobile antibiotic-impregnated cement spacers for the management of prosthetic joint infection. J Am Acad Orthop Surg. 2009;17:356-368.
- Springer BD, Lee GC, Osmon D, et al. Systemic safety of high-dose antibiotic-loaded cement spacers after resection of an infected total knee arthroplasty. Clin Orthop Relat Res. 2004;427:47-51.
- Penner MJ, Masri BA, Duncan CP. Elution characteristics of vancomycin and tobramycin combined in acrylic bone-cement. J Arthroplasty. 1996;11:939-944.
- Williams B, Hanson A, Sha B. Diffuse desquamating rash following exposure to vancomycin-impregnated bone cement. Ann Pharmacother. 2014;48:1061-1065.
- Haeberle M, Wittner B. Is gentamicin-loaded bone cement a risk for developing systemic allergic dermatitis? Contact Dermatitis. 2009;60:176-177.
- McDonald HC, York NR, Pandya AG. Drug-induced linear IgA bullous dermatosis demonstrating the isomorphic phenomenon. J Am Acad Dermatol. 2010;62:897-898.
Practice Points
- Linear IgA bullous dermatosis (LABD) is an autoimmune mucocutaneous disorder characterized by linear IgA deposits at the dermoepidermal junction.
- A substantial number of cases of LABD are drug related, with vancomycin most commonly implicated.
- While antibiotic-impregnated cement spacers deliver high concentrations of local medications, systemic reactions are still possible.
- Dapsone is the first-line treatment for LABD.
Brown-Black Papulonodules on the Arm
The Diagnosis: Glochid Dermatitis
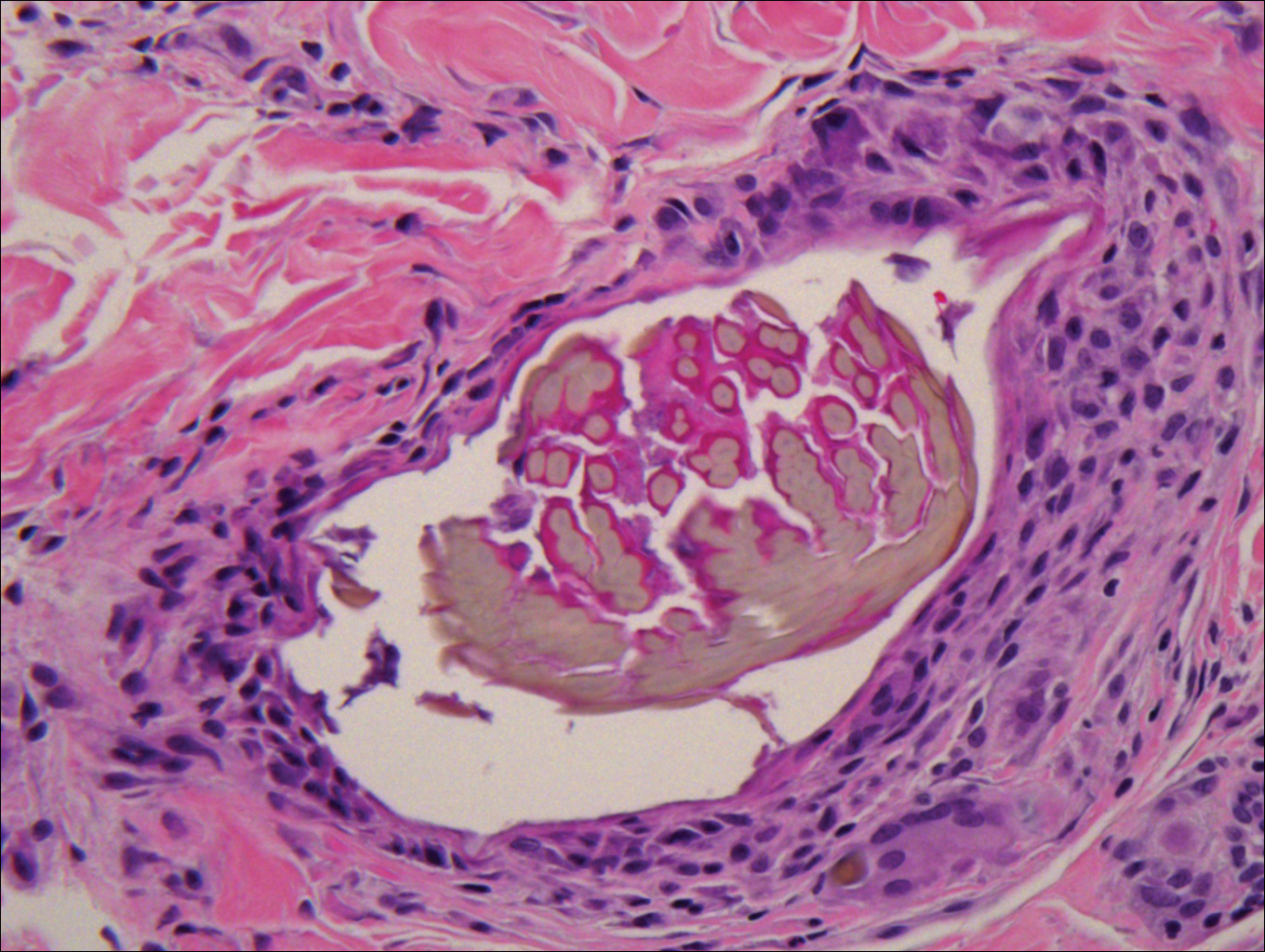
Biopsy of a nodule on the upper right arm showed chronic granulomatous inflammation and polarizable foreign material consistent with plant cellulose (Figure). A diagnosis of glochid dermatitis was made. The treatment plan included follow-up skin evaluation and punch excision of persistent papules 1 month after the initial presentation. The patient reported the rash began after he fell on a cactus plant while chasing his grandson. He was seen by various clinicians and was given hydrocortisone and clobetasol, which helped with pruritis but did not resolve the rash. His grandson developed a similar rash at the site of contact with the cactus plant. The patient and his grandson did not detect the presence of any cactus spines.
Injuries from cactus glochids most often occur due to accidental falls on cactus plants, but glochids also may be transferred from clothing to other individuals. The thin, hairlike glochids easily detach from the stem of the cactus and can become deeply embedded with virtually no pressure.1
Glochid implantation from the prickly pear cactus commonly presents as a pruritic papular eruption known as glochid dermatitis. These penetrating injuries can lead to inoculation of Clostridium tetani and Staphylococcus aureus. Additionally, unrecognized and unremoved cactus spines may be highly inflammatory and may cause chronic granulomatous inflammation.2
Initially, acute glochid dermatitis occurs due to mechanical damage caused by the detatched cactus spine and may not resolve for up to 4 months. Granuloma formation has been reported several weeks after exposure and may persist for more than 8 months.3 Although an immune mechanism has been suggested, the literature has indicated that delayed hypersensitivity reactions are a more probable cause of the granulomatous inflammation after glochid exposure.3 Madkan et al4 reported that relatively few patients developed granulomas after implantation of glochids in the skin, thus suggesting that granuloma formation is an allergic response.
With regard to the pathogenesis of glochid dermatitis, the initial response to foreign plant matter in the dermis involves a neutrophilic infiltrate, which later is replaced by histiocytes; however, the foreign material remains undegraded in the macrophage cytoplasm.5 Activated macrophages secrete cytokines that intensify the inflammatory response, resulting in formation of a granuloma around the foreign body. The granuloma acts as a wall to isolate the foreign matter from the rest of the body.5
Regarding treatment of chronic granulomas, Madkan et al4 reported a case that showed some improvement with clobetasol ointment; however, clinical lesions resolved only after punch biopsies were performed to confirm the diagnosis of cactus spine granuloma. In a controlled study in rabbits, glochids were successfully removed by first detaching the larger clumps with tweezers then applying glue and gauze to the affected area.6 After the glue dried, the gauze was peeled off, resulting in the removal of 95% of the implanted glochids. Overall, removal of embedded spines is difficult because the glochids typically radiate in several directions.7 Treatment of foreign body granulomas caused by cactus spines can be achieved by expulsion of plant matter remnants and symptomatic treatment using midpotency topical steroids twice daily.4 Uncovering and performing punch biopsies of papules also can result in rapid healing of the lesions. Without manual removal of the glochid, lesions can persist for 2 to 8 months until gradual resolution with possible postinflammatory hyperpigmentation.4
- Suzuki H, Baba S. Cactus granuloma of the skin. J Dermatol. 1993;20:424-427.
- Suárez A, Freeman S, Puls L, et al. Unusual presentation of cactus spines in the flank of an elderly man: a case report. J Med Case Rep. 2010;4:152.
- Spoerke DG, Spoerke SE. Granuloma formation induced by spines of the cactus, Opuntia acanthocarpa. Vet Hum Toxicol. 1991;33:342-344.
- Madkan VK, Abraham T, Lesher JL Jr. Cactus spine granuloma. Cutis. 2007;79:208-210.
- Molina-Ruiz AM, Requena L. Foreign body granulomas. Dermatol Clin. 2015;33:497-523.
- McGovern TW, Barkley TM. Botanical dermatology. Int J Dermatol. 1998;37:321-334.
- Lindsey D, Lindsey WE. Cactus spine injuries. Am J Emerg Med. 1988;6:362-369.
The Diagnosis: Glochid Dermatitis
Biopsy of a nodule on the upper right arm showed chronic granulomatous inflammation and polarizable foreign material consistent with plant cellulose (Figure). A diagnosis of glochid dermatitis was made. The treatment plan included follow-up skin evaluation and punch excision of persistent papules 1 month after the initial presentation. The patient reported the rash began after he fell on a cactus plant while chasing his grandson. He was seen by various clinicians and was given hydrocortisone and clobetasol, which helped with pruritis but did not resolve the rash. His grandson developed a similar rash at the site of contact with the cactus plant. The patient and his grandson did not detect the presence of any cactus spines.

Injuries from cactus glochids most often occur due to accidental falls on cactus plants, but glochids also may be transferred from clothing to other individuals. The thin, hairlike glochids easily detach from the stem of the cactus and can become deeply embedded with virtually no pressure.1
Glochid implantation from the prickly pear cactus commonly presents as a pruritic papular eruption known as glochid dermatitis. These penetrating injuries can lead to inoculation of Clostridium tetani and Staphylococcus aureus. Additionally, unrecognized and unremoved cactus spines may be highly inflammatory and may cause chronic granulomatous inflammation.2
Initially, acute glochid dermatitis occurs due to mechanical damage caused by the detatched cactus spine and may not resolve for up to 4 months. Granuloma formation has been reported several weeks after exposure and may persist for more than 8 months.3 Although an immune mechanism has been suggested, the literature has indicated that delayed hypersensitivity reactions are a more probable cause of the granulomatous inflammation after glochid exposure.3 Madkan et al4 reported that relatively few patients developed granulomas after implantation of glochids in the skin, thus suggesting that granuloma formation is an allergic response.
With regard to the pathogenesis of glochid dermatitis, the initial response to foreign plant matter in the dermis involves a neutrophilic infiltrate, which later is replaced by histiocytes; however, the foreign material remains undegraded in the macrophage cytoplasm.5 Activated macrophages secrete cytokines that intensify the inflammatory response, resulting in formation of a granuloma around the foreign body. The granuloma acts as a wall to isolate the foreign matter from the rest of the body.5
Regarding treatment of chronic granulomas, Madkan et al4 reported a case that showed some improvement with clobetasol ointment; however, clinical lesions resolved only after punch biopsies were performed to confirm the diagnosis of cactus spine granuloma. In a controlled study in rabbits, glochids were successfully removed by first detaching the larger clumps with tweezers then applying glue and gauze to the affected area.6 After the glue dried, the gauze was peeled off, resulting in the removal of 95% of the implanted glochids. Overall, removal of embedded spines is difficult because the glochids typically radiate in several directions.7 Treatment of foreign body granulomas caused by cactus spines can be achieved by expulsion of plant matter remnants and symptomatic treatment using midpotency topical steroids twice daily.4 Uncovering and performing punch biopsies of papules also can result in rapid healing of the lesions. Without manual removal of the glochid, lesions can persist for 2 to 8 months until gradual resolution with possible postinflammatory hyperpigmentation.4
The Diagnosis: Glochid Dermatitis
Biopsy of a nodule on the upper right arm showed chronic granulomatous inflammation and polarizable foreign material consistent with plant cellulose (Figure). A diagnosis of glochid dermatitis was made. The treatment plan included follow-up skin evaluation and punch excision of persistent papules 1 month after the initial presentation. The patient reported the rash began after he fell on a cactus plant while chasing his grandson. He was seen by various clinicians and was given hydrocortisone and clobetasol, which helped with pruritis but did not resolve the rash. His grandson developed a similar rash at the site of contact with the cactus plant. The patient and his grandson did not detect the presence of any cactus spines.

Injuries from cactus glochids most often occur due to accidental falls on cactus plants, but glochids also may be transferred from clothing to other individuals. The thin, hairlike glochids easily detach from the stem of the cactus and can become deeply embedded with virtually no pressure.1
Glochid implantation from the prickly pear cactus commonly presents as a pruritic papular eruption known as glochid dermatitis. These penetrating injuries can lead to inoculation of Clostridium tetani and Staphylococcus aureus. Additionally, unrecognized and unremoved cactus spines may be highly inflammatory and may cause chronic granulomatous inflammation.2
Initially, acute glochid dermatitis occurs due to mechanical damage caused by the detatched cactus spine and may not resolve for up to 4 months. Granuloma formation has been reported several weeks after exposure and may persist for more than 8 months.3 Although an immune mechanism has been suggested, the literature has indicated that delayed hypersensitivity reactions are a more probable cause of the granulomatous inflammation after glochid exposure.3 Madkan et al4 reported that relatively few patients developed granulomas after implantation of glochids in the skin, thus suggesting that granuloma formation is an allergic response.
With regard to the pathogenesis of glochid dermatitis, the initial response to foreign plant matter in the dermis involves a neutrophilic infiltrate, which later is replaced by histiocytes; however, the foreign material remains undegraded in the macrophage cytoplasm.5 Activated macrophages secrete cytokines that intensify the inflammatory response, resulting in formation of a granuloma around the foreign body. The granuloma acts as a wall to isolate the foreign matter from the rest of the body.5
Regarding treatment of chronic granulomas, Madkan et al4 reported a case that showed some improvement with clobetasol ointment; however, clinical lesions resolved only after punch biopsies were performed to confirm the diagnosis of cactus spine granuloma. In a controlled study in rabbits, glochids were successfully removed by first detaching the larger clumps with tweezers then applying glue and gauze to the affected area.6 After the glue dried, the gauze was peeled off, resulting in the removal of 95% of the implanted glochids. Overall, removal of embedded spines is difficult because the glochids typically radiate in several directions.7 Treatment of foreign body granulomas caused by cactus spines can be achieved by expulsion of plant matter remnants and symptomatic treatment using midpotency topical steroids twice daily.4 Uncovering and performing punch biopsies of papules also can result in rapid healing of the lesions. Without manual removal of the glochid, lesions can persist for 2 to 8 months until gradual resolution with possible postinflammatory hyperpigmentation.4
- Suzuki H, Baba S. Cactus granuloma of the skin. J Dermatol. 1993;20:424-427.
- Suárez A, Freeman S, Puls L, et al. Unusual presentation of cactus spines in the flank of an elderly man: a case report. J Med Case Rep. 2010;4:152.
- Spoerke DG, Spoerke SE. Granuloma formation induced by spines of the cactus, Opuntia acanthocarpa. Vet Hum Toxicol. 1991;33:342-344.
- Madkan VK, Abraham T, Lesher JL Jr. Cactus spine granuloma. Cutis. 2007;79:208-210.
- Molina-Ruiz AM, Requena L. Foreign body granulomas. Dermatol Clin. 2015;33:497-523.
- McGovern TW, Barkley TM. Botanical dermatology. Int J Dermatol. 1998;37:321-334.
- Lindsey D, Lindsey WE. Cactus spine injuries. Am J Emerg Med. 1988;6:362-369.
- Suzuki H, Baba S. Cactus granuloma of the skin. J Dermatol. 1993;20:424-427.
- Suárez A, Freeman S, Puls L, et al. Unusual presentation of cactus spines in the flank of an elderly man: a case report. J Med Case Rep. 2010;4:152.
- Spoerke DG, Spoerke SE. Granuloma formation induced by spines of the cactus, Opuntia acanthocarpa. Vet Hum Toxicol. 1991;33:342-344.
- Madkan VK, Abraham T, Lesher JL Jr. Cactus spine granuloma. Cutis. 2007;79:208-210.
- Molina-Ruiz AM, Requena L. Foreign body granulomas. Dermatol Clin. 2015;33:497-523.
- McGovern TW, Barkley TM. Botanical dermatology. Int J Dermatol. 1998;37:321-334.
- Lindsey D, Lindsey WE. Cactus spine injuries. Am J Emerg Med. 1988;6:362-369.
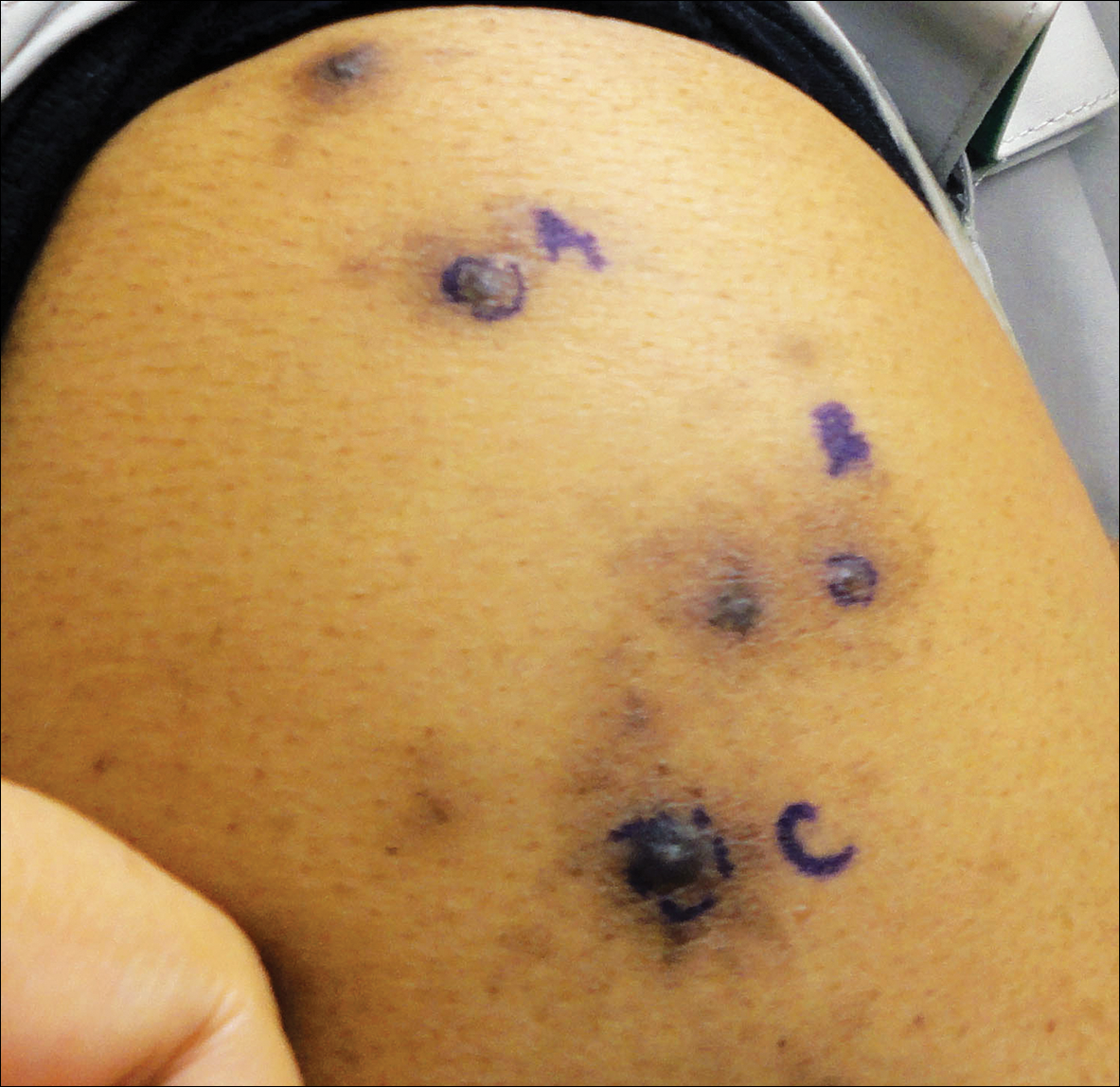
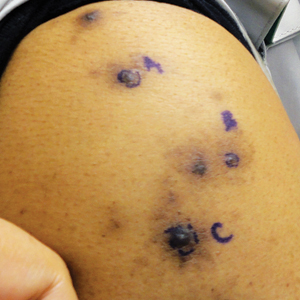
A 63-year-old man presented with a pruritic rash on the right arm of approximately 3 months' duration. On physical examination, several discrete, 4- to 5-mm, brown-black papulonodules with a central punctum were identified along the extensor aspects of the upper and lower right arm. No foreign bodies were appreciated. Biopsies of nodules on the right upper arm were performed (sites marked with letters).
ALT-70 score outperformed thermal imaging for cellulitis diagnosis
SAN DIEGO – A simple scoring system surpassed thermal imaging for diagnosing lower extremity cellulitis in a head-to-head, single-center comparison in 67 patients.
The ALT-70 score – which tallies points for asymmetry, leukocytosis, tachycardia, and age of at least 70 years – produced a positive predictive value for lower-extremity cellulitis (LEC) of 80.4% and a negative predictive value of 90.9%, compared with values of 75.5% and 57.1%, respectively, for thermal imaging when researchers applied both methods to 67 patients, said David G. Li, a clinical research fellow in the department of dermatology at Brigham and Women’s Hospital, Boston, where the study was conducted.
The senior author of Mr. Li’s report, Arash Mostaghimi, MD, director of the inpatient consultation service, department of dermatology at Brigham and Women’s, was also lead investigator for the team of dermatology researchers – from his center and from Massachusetts General Hospital in Boston – who recently devised the ALT-70 scoring system for diagnosing LEC (J Amer Acad Dermatol. 2017 April;76[4]:618-25.e2).
The four-item survey can generate a score of 0-7, with a score of 0-2 suggesting need for additional monitoring, a score of 3-4 initiating a dermatology consult, and a score of 5-7 triggering immediate treatment for cellulitis, Mr. Li said. The 2017 review of ALT-70 showed that among 259 patients, those with a score of 0-2 had an 83% likelihood of having pseudocellulitis, while patients with a score of 5-7 had an 82% likelihood of having true cellulitis.
The current study enrolled 67 patients who had a presumptive diagnosis of LEC while in the emergency department or inpatient wards during a 7-month period. In addition to undergoing blinded assessment by both thermal imaging and by ALT-70 scoring, all patients also underwent blinded assessment by a board-certified dermatologist, who provided the definitive diagnosis. The attending dermatologists determined that 46 of the patients had true LEC and 21 patients did not.
The calculated sensitivity of ALT-70 was 97.8%, compared with 87.0% for thermal imaging. Specificity was 47.6% for ALT-70 and 38.1% for thermal imaging, Mr. Li reported at the annual meeting of the American Academy of Dermatology.
He also presented an analysis of the results when he combined both methods, with a positive on both assessments required to produce a positive LEC diagnosis. This resulted in a positive predictive value of 86.7%, slightly higher than the 80.4% from ALT-70 alone, but the combination produced a negative predictive value of 68.2%, substantially less than the 90.9% rate with ALT-70 alone. This demonstrated the “marginal benefit” from combining the two methods, he said.
In a receiver operating characteristic curve analysis, in which the area under the curve (c-statistic) reflects a diagnostic test’s validity, ALT-70 produced a c-statistic of 0.85, thermal imaging had a c-statistic of 0.63, and when combined, the c-statistic was 0.88.
Mr. Li called for validation of the findings using larger and different patient populations.
He had no reported disclosures.
SOURCE: Li DG et al. AAD 18, Abstract 6744.
SAN DIEGO – A simple scoring system surpassed thermal imaging for diagnosing lower extremity cellulitis in a head-to-head, single-center comparison in 67 patients.
The ALT-70 score – which tallies points for asymmetry, leukocytosis, tachycardia, and age of at least 70 years – produced a positive predictive value for lower-extremity cellulitis (LEC) of 80.4% and a negative predictive value of 90.9%, compared with values of 75.5% and 57.1%, respectively, for thermal imaging when researchers applied both methods to 67 patients, said David G. Li, a clinical research fellow in the department of dermatology at Brigham and Women’s Hospital, Boston, where the study was conducted.
The senior author of Mr. Li’s report, Arash Mostaghimi, MD, director of the inpatient consultation service, department of dermatology at Brigham and Women’s, was also lead investigator for the team of dermatology researchers – from his center and from Massachusetts General Hospital in Boston – who recently devised the ALT-70 scoring system for diagnosing LEC (J Amer Acad Dermatol. 2017 April;76[4]:618-25.e2).
The four-item survey can generate a score of 0-7, with a score of 0-2 suggesting need for additional monitoring, a score of 3-4 initiating a dermatology consult, and a score of 5-7 triggering immediate treatment for cellulitis, Mr. Li said. The 2017 review of ALT-70 showed that among 259 patients, those with a score of 0-2 had an 83% likelihood of having pseudocellulitis, while patients with a score of 5-7 had an 82% likelihood of having true cellulitis.
The current study enrolled 67 patients who had a presumptive diagnosis of LEC while in the emergency department or inpatient wards during a 7-month period. In addition to undergoing blinded assessment by both thermal imaging and by ALT-70 scoring, all patients also underwent blinded assessment by a board-certified dermatologist, who provided the definitive diagnosis. The attending dermatologists determined that 46 of the patients had true LEC and 21 patients did not.
The calculated sensitivity of ALT-70 was 97.8%, compared with 87.0% for thermal imaging. Specificity was 47.6% for ALT-70 and 38.1% for thermal imaging, Mr. Li reported at the annual meeting of the American Academy of Dermatology.
He also presented an analysis of the results when he combined both methods, with a positive on both assessments required to produce a positive LEC diagnosis. This resulted in a positive predictive value of 86.7%, slightly higher than the 80.4% from ALT-70 alone, but the combination produced a negative predictive value of 68.2%, substantially less than the 90.9% rate with ALT-70 alone. This demonstrated the “marginal benefit” from combining the two methods, he said.
In a receiver operating characteristic curve analysis, in which the area under the curve (c-statistic) reflects a diagnostic test’s validity, ALT-70 produced a c-statistic of 0.85, thermal imaging had a c-statistic of 0.63, and when combined, the c-statistic was 0.88.
Mr. Li called for validation of the findings using larger and different patient populations.
He had no reported disclosures.
SOURCE: Li DG et al. AAD 18, Abstract 6744.
SAN DIEGO – A simple scoring system surpassed thermal imaging for diagnosing lower extremity cellulitis in a head-to-head, single-center comparison in 67 patients.
The ALT-70 score – which tallies points for asymmetry, leukocytosis, tachycardia, and age of at least 70 years – produced a positive predictive value for lower-extremity cellulitis (LEC) of 80.4% and a negative predictive value of 90.9%, compared with values of 75.5% and 57.1%, respectively, for thermal imaging when researchers applied both methods to 67 patients, said David G. Li, a clinical research fellow in the department of dermatology at Brigham and Women’s Hospital, Boston, where the study was conducted.
The senior author of Mr. Li’s report, Arash Mostaghimi, MD, director of the inpatient consultation service, department of dermatology at Brigham and Women’s, was also lead investigator for the team of dermatology researchers – from his center and from Massachusetts General Hospital in Boston – who recently devised the ALT-70 scoring system for diagnosing LEC (J Amer Acad Dermatol. 2017 April;76[4]:618-25.e2).
The four-item survey can generate a score of 0-7, with a score of 0-2 suggesting need for additional monitoring, a score of 3-4 initiating a dermatology consult, and a score of 5-7 triggering immediate treatment for cellulitis, Mr. Li said. The 2017 review of ALT-70 showed that among 259 patients, those with a score of 0-2 had an 83% likelihood of having pseudocellulitis, while patients with a score of 5-7 had an 82% likelihood of having true cellulitis.
The current study enrolled 67 patients who had a presumptive diagnosis of LEC while in the emergency department or inpatient wards during a 7-month period. In addition to undergoing blinded assessment by both thermal imaging and by ALT-70 scoring, all patients also underwent blinded assessment by a board-certified dermatologist, who provided the definitive diagnosis. The attending dermatologists determined that 46 of the patients had true LEC and 21 patients did not.
The calculated sensitivity of ALT-70 was 97.8%, compared with 87.0% for thermal imaging. Specificity was 47.6% for ALT-70 and 38.1% for thermal imaging, Mr. Li reported at the annual meeting of the American Academy of Dermatology.
He also presented an analysis of the results when he combined both methods, with a positive on both assessments required to produce a positive LEC diagnosis. This resulted in a positive predictive value of 86.7%, slightly higher than the 80.4% from ALT-70 alone, but the combination produced a negative predictive value of 68.2%, substantially less than the 90.9% rate with ALT-70 alone. This demonstrated the “marginal benefit” from combining the two methods, he said.
In a receiver operating characteristic curve analysis, in which the area under the curve (c-statistic) reflects a diagnostic test’s validity, ALT-70 produced a c-statistic of 0.85, thermal imaging had a c-statistic of 0.63, and when combined, the c-statistic was 0.88.
Mr. Li called for validation of the findings using larger and different patient populations.
He had no reported disclosures.
SOURCE: Li DG et al. AAD 18, Abstract 6744.
REPORTING FROM AAD 18
Key clinical point: The ALT-70 score surpassed thermal imaging for diagnosing lower-extremity cellulitis.
Major finding: Positive and negative predictive values were 80.4% and 90.9% for ALT-70 and 75.5% and 57.1% for thermal imaging.
Study details: A single-center study with 67 patients.
Disclosures: Mr. Li had no disclosures.
Source: Li DG et al. AAD 18, Abstract 6744.
Deepithelialized Flaps and Grafts: Applications in Dermatologic Surgery
Deepithelialized flaps and grafts have been widely used by reconstructive surgeons in a diverse range of medical specialties since the early 20th century. 1 These reconstructive modalities have more recently been applied to dermatologic surgery. Deepithelialized flaps and grafts involve removal of the epidermis from the dermis for a variety of surgical purposes. Although these techniques play an important role in dermatologic surgery, reports of application of deepithelialized flaps and grafts in the dermatology literature is limited. This article includes a presentation of the applications of deepithelialized flaps and grafts in procedural dermatology.
DEEPITHELIALIZATION TECHNIQUES
There are a variety of techniques for deepithelialization, although sharp deepithelialization generally is preferred by dermatologic surgeons. The scalpel technique can be accomplished by making an intradermal incision with a No. 15 blade. Traction is an essential component of the deepthelialization process and facilitates sharp removal of the epidermis and superficial dermis in an even plane. The peeling orange technique, which has been described in reduction mammoplasty, is a variant of the scalpel technique used for creating a large area of deepithelialized tissue.2 A No. 10 blade is used to make multiple partial-thickness intradermal incisions 1 to 2 cm apart along the pedicle. Traction facilitates rapid deepithelialization of the skin strips on the pedicle. A sharp curette is an alternative option for sharply removing the epithelium from a small area. Electric dermatome, laser, and electrocautery techniques for deepithelialization also can be considered.2,3
APPLICATION OF DEEPITHELIALIZED FLAPS
Deepithelialized flaps may be considered for single-stage reconstruction with tunneled interpolation flaps, reconstruction requiring contour preservation, and reconstruction involving free margins.4-17
Reconstruction With Single-Stage Tunneled Interpolated Flaps
Alar Base
A partially deepithelialized tunneled interpolated flap is an elegant reconstructive option for defects involving the upper cutaneous lip and alar base. The flap is elevated from the ipsilateral nasolabial fold, deepithelialized proximally, and tunneled under the intact portion of the cutaneous upper lip and ala. The flap is then deepithelialized superiorly to bolster the alar base and inset at the recipient site.4
Nasal Ala
The tunneled interpolated flap is useful for reconstruction of defects of the nasal ala. A flap with a superior deepithelialized pedicle and an anticipated inferior Burow triangle is designed along the axis of the nasolabial fold. The inferior Burow triangle and central flap are elevated at the level of the superficial subcutaneous fat and the pedicle is dissected. The donor and recipient sites are widely undermined, and the flap and pedicle pass through the tunnel. The donor site is closed primarily, the inferior Burow triangle is trimmed, and the flap is sutured into the defect.5 This flap allows for preservation of free margins and favorable placement of incision lines. Furthermore, pincushioning of the flap helps to recreate the rounded shape of the lateral ala.6
Nasal Tip
Nasal tip defects can be repaired with a retroangular flap, centered on the angular artery. The flap is elevated along the axis of the nasolabial fold, deepithelialized at its proximal base, and transferred through a subcutaneous tunnel to the nasal tip. The angular artery is ligated at the inferior aspect of the flap.7
Nasal Sidewall
A deepithelialized tunneled interpolated forehead flap, similar to the classic paramedian forehead flap, can be used to reconstruct nasal sidewall defects. A flap is elevated on the contralateral forehead and the proximal portion is deepithelialized. A tunnel is then bluntly dissected just above the periosteum, and the flap is introduced into the defect through the tunnel and inset. This flap has the advantages of being a single-stage procedure, restoring volume to the defect area, and maintaining excellent vascular supply.8
Eyelid
A tunneled interpolated forehead flap also can be used to repair medial canthal defects and for anterior lamellar repair of lower eyelid defects. In a study of 9 patients receiving a tunneled interpolated forehead flap in these anatomic locations, all flaps demonstrated viability, protection of the globe, and preservation of the concave architecture of the medial canthus.9
Earlobe
Earlobe defects may be repaired with a pull-through interpolated preauricular flap. A flap is elevated superiorly in the preauricular region and the proximal aspect of the flap is deepithelialized. The flap is pulled through a tunnel and inset at the anterior earlobe defect. The donor site is closed primarily.10,11
Concha
Reconstruction of anterior conchal defects with exposed cartilage can be accomplished with a pull-through interpolated postauricular flap based on the auriculomastoid fossa. The postauricular flap is elevated, the base is deepithelialized, an incision is made in the medial aspect of the defect, and the flap is moved through a tunnel between the posterior and anterior surfaces of the ear. The flap is secured to the anterior surface of the concha.12
Reconstruction Requiring Contour Preservation
Central Face
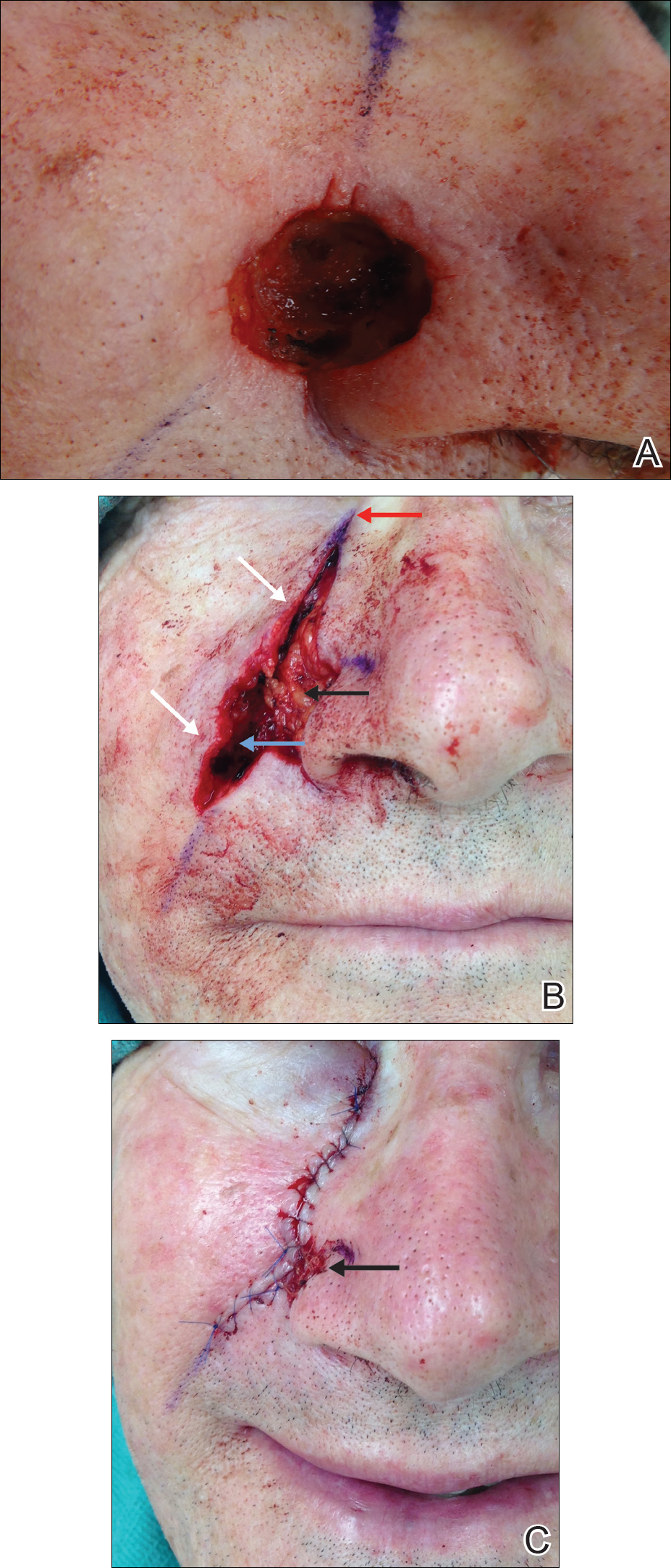
The hinge flap is optimal for reconstruction of deep central facial defects (Figure 1). The hinge flap is planned at a site contiguous with a margin of the defect and can include the dermis, subcutaneous tissue, muscle, or a combination of these. The desired tissue is folded over on the pedicle to fill the defect. Cutaneous coverage is accomplished through a primary closure, separate flap, or skin graft. In addition to restoring contour and therefore the cosmetic subunit, the hinge flap is performed in a single stage, resists wound contracture, and provides a well-vascularized wound bed resulting in a low incidence of graft failure.13,14 Muscular hinge flaps have been described for reconstruction of forehead defects with exposed bone based on the frontalis muscle.15
Lower Lip
A variant of a V-Y advancement flap has been described for reconstruction of defects greater than one-third the length of the lower lip. The top of the “V” is deepithelialized and the flap is advanced such that the top of the “V” abuts the inferior border of the defect. The “V” flap is inset at its advanced position, converting the “V”-shaped wound into a “Y.” An overlying buccal mucosal graft provides reconstruction of the lower red lip and labial mucosa.16
Helix of the Ear
Large defects of the scapha and helix of the ear can be reconstructed with the use of a staged interpolated postauricular flap. The postauricular flap is elevated into a subcutaneous plane. A full-thickness incision is made medial to the helical rim, and the flap is tunneled through and sutured into place. The pedicle is later divided, and the distal aspect of the flap is deepithelialized and inset into the helical rim for volume restoration.17
Reconstruction Involving Free Margins
Nasal Ala
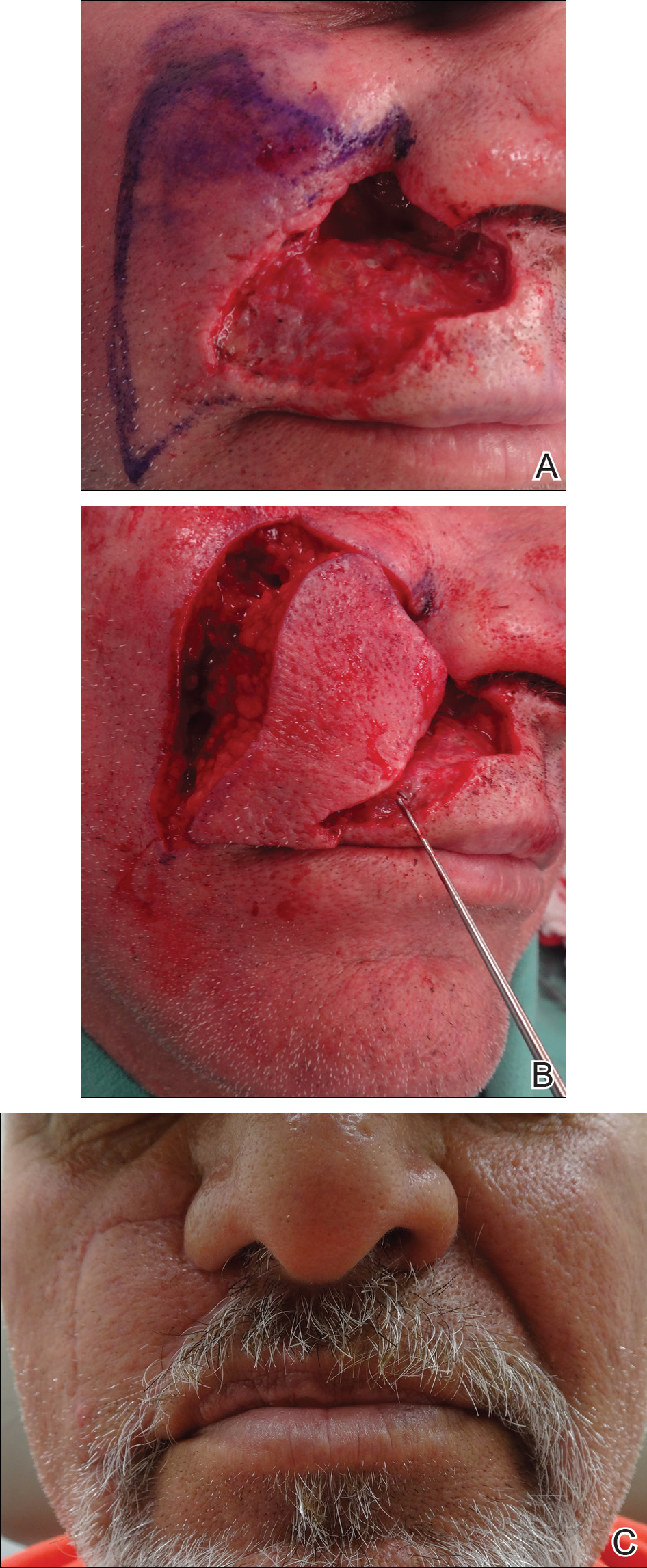
For large defects involving the upper cutaneous lip with adjacent alar base involvement, a partially deepithelialized V-Y flap is a useful reconstructive option (Figure 2).
Infraorbital Region
A deepithelialized variant of a V-Y advancement flap can be used for closure of infraorbital defects. The limbs of the V-Y flap are deepithelialized and anchored to the medial and lateral canthal tendons or periosteum. Ectropion prevention is the primary advantage of this flap.18
APPLICATION OF DEEPITHELIALIZED GRAFTS
Deepithelialized grafts may be considered for volume replacement, reconstruction requiring contour preservation, and restoration of mechanical integrity in areas of high mechanical tension.3,19-21
Reconstruction Requiring Contour Preservation
Deepithelialized grafts are used to improve depressed nasal scars and restore volume in deep nasal wounds. One method involves deepithelialization of 2 postauricular punch biopsies. An 18-gauge needle is used to make a small hole in the depressed nasal scar, the dermal grafts are inserted, and the defect is closed primarily.19 Dermal grafts may be harvested from excess full-thickness skin grafts (FTSGs) or dog-ear tissue. When used under flaps, the dermal graft is trimmed to the size of the defect. When used under FTSGs, thin dermal graft strips are placed in a gridlike pattern to allow for revascularization. A study of 15 patients with contour deformities reconstructed with dermal graft insertions demonstrated that 14 (94%) patients had no significant complications and improvement of scar depression was achieved.20
Reconstruction in Areas of High Mechanical Tension
Plantar Foot
A combined dermal and full-thickness sandwich graft has been described for reconstruction of plantar foot defects.3 The graft is created by obtaining a FTSG twice the size of the wound defect and deepithelializing half of the graft. The graft is then defatted and the deepithelialized portion is folded beneath the other half, allowing the papillary dermis to make contact with the wound surface.
Scalp
Dermal graft reconstruction for scalp defects may be accomplished with a split-thickness skin flap. The flap is harvested using an electronic dermatome that ensures the proximal aspect is still attached to adjacent skin. The dermis is removed from the area underneath the back-folded split-thickness skin flap. The dermal graft is meshed and sutured into the recipient site. The split-thickness skin flap is replaced over the donor site. Meshed reversed dermal grafts have excellent survival rates, even with direct placement on bone without periosteum. Querings et al21 reported graft survival with no complications in 19 of 21 (90.4%) patients undergoing scalp or plantar sole reconstruction.
CONCLUSION
With the widespread adoption of the fresh-tissue technique for Mohs micrographic surgery and the establishment of the American Society for Dermatologic Surgery in 1970, the depth and scope of techniques used by dermatologic surgeons has dramatically expanded. Although the use of dermal flaps and grafts is not as widespread in dermatology as other reconstructive techniques, their unique advantages should be considered. Deepithelialized flaps and grafts should be considered when the following reconstructive goals are desired: (1) conversion of a 2-stage interpolation flap to a single-stage tunneled flap, (2) contour and cosmetic subunit preservation of deep defects through volume augmentation, (3) reconstruction in areas of high mechanical tension, and (4) free margin preservation. The multiple applications of deepithelialized flaps and grafts as described in this review demonstrate their continued applicability in dermatologic surgery.
- Straatsma CR. Use of the dermal graft in the repairs of small saddle defects of the nose. Arch Otolaryngol. 1932;16:506-509.
- Cydeli A, Hunter J. Peeling orange: rapid deepithelialization in reduction mammoplasty. J Aesthet Surg. 2004;24:580-581.
- Bechara F, Sand M, Radenhausen M, et al. Erbium:YAG laser-assisted preparation of a combined dermal/full thickness sandwich skin graft. Dermatol Surg. 2006;32:353-358.
- Cook JL. Tunneled and transposed island flaps in facial reconstructive surgery. Dermatol Surg. 2014;40(suppl 9):S16-S29.
- Krishnan RS, Clark DP. Tunneled transposition flap for reconstruction of defects of the nasal ala. Dermatol Surg. 2007;33:1496-1501.
- Mahlberg M. Tunneled melolabial pedicle flap for small but deep lateral alar rim defect. Dermatol Surg. 2013;39:1527-1529.
- Ascari-Raccagni A, Balderi U. The retroangular flap used in the surgery of nasal tip defects. Dermatol Surg. 2004;30:1131-1137.
- Hollmig ST, Leach BC, Cook J. Single-staged interpolation flaps in facial reconstruction. Dermatol Surg. 2014;40(suppl 9):S62-S70.
- Mombaerts I, Gillis A. The tunneled forehead flap in medial canthal and eyelid reconstruction. Dermatol Surg. 2010:36:1118-1125.
- Wang SQ, Goldberg LH, Kimyah-Asadi A. Tunneled island pedicle flap for an earlobe defect. Dermatol Surg. 2007;33:835-838.
- Hatoko M, Kuwahara M, Shiba A, et al. Earlobe reconstruction using a subcutaneous island pedicle flap after resection of “earlobe keloid.” Dermatol Surg. 1998;24:257-261.
- Alder N, Ad-El D, Azaria R. Reconstruction of nonhelical auricular defects with local flaps. Dermatol Surg. 2008;34:501-507.
- Fader DJ, Wang TS, Johnson TM. Nasal reconstruction utilizing a muscle hinge flap with overlying FTSG. J Am Acad Dermatol. 2000;43:837-840.
- Braun MA, Cook J. Hinge flaps in facial reconstruction. Dermatol Surg. 2007;33:213-221.
- Salmon PL, Mortimer NL, Hill SE. Muscular hinge flaps: utility and technique in facial reconstructive surgery. Dermatol Surg. 2010;36:227-234.
- Seo Y, Song S, Choi Y, et al. A lower lip reconstruction. Dermatol Surg. 2015;41:505-507.
- Malone CH, Wagner RF. Partially de-epithelialized postauricular flap for ear reconstruction. J Am Acad Dermatol. 2015;73:E219-E220.
- Yildrim S, Akoz T, Akan M, et al. Nasolabial V-Y advancement for closure of the midface defects. Dermatol Surg. 2001;27:656-662.
- Jensen DJ, Cohen JL. Nasal tip revision using a dermal graft. Dermatol Surg. 2014;40:1140-1142.
- Meyers S, Rohrer T. Use of dermal grafts in reconstructing deep nasal defects and shaping the ala nasi. Dermatol Surg. 2001;27:300-305.
- Querings K, Bachter D, Balda B. Meshed reversed dermal graft in patients with surgical defects of sole and scalp: technique and long-term results. Dermatol Surg. 2002;28:122-126.
Deepithelialized flaps and grafts have been widely used by reconstructive surgeons in a diverse range of medical specialties since the early 20th century. 1 These reconstructive modalities have more recently been applied to dermatologic surgery. Deepithelialized flaps and grafts involve removal of the epidermis from the dermis for a variety of surgical purposes. Although these techniques play an important role in dermatologic surgery, reports of application of deepithelialized flaps and grafts in the dermatology literature is limited. This article includes a presentation of the applications of deepithelialized flaps and grafts in procedural dermatology.
DEEPITHELIALIZATION TECHNIQUES
There are a variety of techniques for deepithelialization, although sharp deepithelialization generally is preferred by dermatologic surgeons. The scalpel technique can be accomplished by making an intradermal incision with a No. 15 blade. Traction is an essential component of the deepthelialization process and facilitates sharp removal of the epidermis and superficial dermis in an even plane. The peeling orange technique, which has been described in reduction mammoplasty, is a variant of the scalpel technique used for creating a large area of deepithelialized tissue.2 A No. 10 blade is used to make multiple partial-thickness intradermal incisions 1 to 2 cm apart along the pedicle. Traction facilitates rapid deepithelialization of the skin strips on the pedicle. A sharp curette is an alternative option for sharply removing the epithelium from a small area. Electric dermatome, laser, and electrocautery techniques for deepithelialization also can be considered.2,3
APPLICATION OF DEEPITHELIALIZED FLAPS
Deepithelialized flaps may be considered for single-stage reconstruction with tunneled interpolation flaps, reconstruction requiring contour preservation, and reconstruction involving free margins.4-17
Reconstruction With Single-Stage Tunneled Interpolated Flaps
Alar Base
A partially deepithelialized tunneled interpolated flap is an elegant reconstructive option for defects involving the upper cutaneous lip and alar base. The flap is elevated from the ipsilateral nasolabial fold, deepithelialized proximally, and tunneled under the intact portion of the cutaneous upper lip and ala. The flap is then deepithelialized superiorly to bolster the alar base and inset at the recipient site.4
Nasal Ala
The tunneled interpolated flap is useful for reconstruction of defects of the nasal ala. A flap with a superior deepithelialized pedicle and an anticipated inferior Burow triangle is designed along the axis of the nasolabial fold. The inferior Burow triangle and central flap are elevated at the level of the superficial subcutaneous fat and the pedicle is dissected. The donor and recipient sites are widely undermined, and the flap and pedicle pass through the tunnel. The donor site is closed primarily, the inferior Burow triangle is trimmed, and the flap is sutured into the defect.5 This flap allows for preservation of free margins and favorable placement of incision lines. Furthermore, pincushioning of the flap helps to recreate the rounded shape of the lateral ala.6
Nasal Tip
Nasal tip defects can be repaired with a retroangular flap, centered on the angular artery. The flap is elevated along the axis of the nasolabial fold, deepithelialized at its proximal base, and transferred through a subcutaneous tunnel to the nasal tip. The angular artery is ligated at the inferior aspect of the flap.7
Nasal Sidewall
A deepithelialized tunneled interpolated forehead flap, similar to the classic paramedian forehead flap, can be used to reconstruct nasal sidewall defects. A flap is elevated on the contralateral forehead and the proximal portion is deepithelialized. A tunnel is then bluntly dissected just above the periosteum, and the flap is introduced into the defect through the tunnel and inset. This flap has the advantages of being a single-stage procedure, restoring volume to the defect area, and maintaining excellent vascular supply.8
Eyelid
A tunneled interpolated forehead flap also can be used to repair medial canthal defects and for anterior lamellar repair of lower eyelid defects. In a study of 9 patients receiving a tunneled interpolated forehead flap in these anatomic locations, all flaps demonstrated viability, protection of the globe, and preservation of the concave architecture of the medial canthus.9
Earlobe
Earlobe defects may be repaired with a pull-through interpolated preauricular flap. A flap is elevated superiorly in the preauricular region and the proximal aspect of the flap is deepithelialized. The flap is pulled through a tunnel and inset at the anterior earlobe defect. The donor site is closed primarily.10,11
Concha
Reconstruction of anterior conchal defects with exposed cartilage can be accomplished with a pull-through interpolated postauricular flap based on the auriculomastoid fossa. The postauricular flap is elevated, the base is deepithelialized, an incision is made in the medial aspect of the defect, and the flap is moved through a tunnel between the posterior and anterior surfaces of the ear. The flap is secured to the anterior surface of the concha.12
Reconstruction Requiring Contour Preservation
Central Face
The hinge flap is optimal for reconstruction of deep central facial defects (Figure 1). The hinge flap is planned at a site contiguous with a margin of the defect and can include the dermis, subcutaneous tissue, muscle, or a combination of these. The desired tissue is folded over on the pedicle to fill the defect. Cutaneous coverage is accomplished through a primary closure, separate flap, or skin graft. In addition to restoring contour and therefore the cosmetic subunit, the hinge flap is performed in a single stage, resists wound contracture, and provides a well-vascularized wound bed resulting in a low incidence of graft failure.13,14 Muscular hinge flaps have been described for reconstruction of forehead defects with exposed bone based on the frontalis muscle.15

Lower Lip
A variant of a V-Y advancement flap has been described for reconstruction of defects greater than one-third the length of the lower lip. The top of the “V” is deepithelialized and the flap is advanced such that the top of the “V” abuts the inferior border of the defect. The “V” flap is inset at its advanced position, converting the “V”-shaped wound into a “Y.” An overlying buccal mucosal graft provides reconstruction of the lower red lip and labial mucosa.16
Helix of the Ear
Large defects of the scapha and helix of the ear can be reconstructed with the use of a staged interpolated postauricular flap. The postauricular flap is elevated into a subcutaneous plane. A full-thickness incision is made medial to the helical rim, and the flap is tunneled through and sutured into place. The pedicle is later divided, and the distal aspect of the flap is deepithelialized and inset into the helical rim for volume restoration.17
Reconstruction Involving Free Margins
Nasal Ala
For large defects involving the upper cutaneous lip with adjacent alar base involvement, a partially deepithelialized V-Y flap is a useful reconstructive option (Figure 2).

Infraorbital Region
A deepithelialized variant of a V-Y advancement flap can be used for closure of infraorbital defects. The limbs of the V-Y flap are deepithelialized and anchored to the medial and lateral canthal tendons or periosteum. Ectropion prevention is the primary advantage of this flap.18
APPLICATION OF DEEPITHELIALIZED GRAFTS
Deepithelialized grafts may be considered for volume replacement, reconstruction requiring contour preservation, and restoration of mechanical integrity in areas of high mechanical tension.3,19-21
Reconstruction Requiring Contour Preservation
Deepithelialized grafts are used to improve depressed nasal scars and restore volume in deep nasal wounds. One method involves deepithelialization of 2 postauricular punch biopsies. An 18-gauge needle is used to make a small hole in the depressed nasal scar, the dermal grafts are inserted, and the defect is closed primarily.19 Dermal grafts may be harvested from excess full-thickness skin grafts (FTSGs) or dog-ear tissue. When used under flaps, the dermal graft is trimmed to the size of the defect. When used under FTSGs, thin dermal graft strips are placed in a gridlike pattern to allow for revascularization. A study of 15 patients with contour deformities reconstructed with dermal graft insertions demonstrated that 14 (94%) patients had no significant complications and improvement of scar depression was achieved.20
Reconstruction in Areas of High Mechanical Tension
Plantar Foot
A combined dermal and full-thickness sandwich graft has been described for reconstruction of plantar foot defects.3 The graft is created by obtaining a FTSG twice the size of the wound defect and deepithelializing half of the graft. The graft is then defatted and the deepithelialized portion is folded beneath the other half, allowing the papillary dermis to make contact with the wound surface.
Scalp
Dermal graft reconstruction for scalp defects may be accomplished with a split-thickness skin flap. The flap is harvested using an electronic dermatome that ensures the proximal aspect is still attached to adjacent skin. The dermis is removed from the area underneath the back-folded split-thickness skin flap. The dermal graft is meshed and sutured into the recipient site. The split-thickness skin flap is replaced over the donor site. Meshed reversed dermal grafts have excellent survival rates, even with direct placement on bone without periosteum. Querings et al21 reported graft survival with no complications in 19 of 21 (90.4%) patients undergoing scalp or plantar sole reconstruction.
CONCLUSION
With the widespread adoption of the fresh-tissue technique for Mohs micrographic surgery and the establishment of the American Society for Dermatologic Surgery in 1970, the depth and scope of techniques used by dermatologic surgeons has dramatically expanded. Although the use of dermal flaps and grafts is not as widespread in dermatology as other reconstructive techniques, their unique advantages should be considered. Deepithelialized flaps and grafts should be considered when the following reconstructive goals are desired: (1) conversion of a 2-stage interpolation flap to a single-stage tunneled flap, (2) contour and cosmetic subunit preservation of deep defects through volume augmentation, (3) reconstruction in areas of high mechanical tension, and (4) free margin preservation. The multiple applications of deepithelialized flaps and grafts as described in this review demonstrate their continued applicability in dermatologic surgery.
Deepithelialized flaps and grafts have been widely used by reconstructive surgeons in a diverse range of medical specialties since the early 20th century. 1 These reconstructive modalities have more recently been applied to dermatologic surgery. Deepithelialized flaps and grafts involve removal of the epidermis from the dermis for a variety of surgical purposes. Although these techniques play an important role in dermatologic surgery, reports of application of deepithelialized flaps and grafts in the dermatology literature is limited. This article includes a presentation of the applications of deepithelialized flaps and grafts in procedural dermatology.
DEEPITHELIALIZATION TECHNIQUES
There are a variety of techniques for deepithelialization, although sharp deepithelialization generally is preferred by dermatologic surgeons. The scalpel technique can be accomplished by making an intradermal incision with a No. 15 blade. Traction is an essential component of the deepthelialization process and facilitates sharp removal of the epidermis and superficial dermis in an even plane. The peeling orange technique, which has been described in reduction mammoplasty, is a variant of the scalpel technique used for creating a large area of deepithelialized tissue.2 A No. 10 blade is used to make multiple partial-thickness intradermal incisions 1 to 2 cm apart along the pedicle. Traction facilitates rapid deepithelialization of the skin strips on the pedicle. A sharp curette is an alternative option for sharply removing the epithelium from a small area. Electric dermatome, laser, and electrocautery techniques for deepithelialization also can be considered.2,3
APPLICATION OF DEEPITHELIALIZED FLAPS
Deepithelialized flaps may be considered for single-stage reconstruction with tunneled interpolation flaps, reconstruction requiring contour preservation, and reconstruction involving free margins.4-17
Reconstruction With Single-Stage Tunneled Interpolated Flaps
Alar Base
A partially deepithelialized tunneled interpolated flap is an elegant reconstructive option for defects involving the upper cutaneous lip and alar base. The flap is elevated from the ipsilateral nasolabial fold, deepithelialized proximally, and tunneled under the intact portion of the cutaneous upper lip and ala. The flap is then deepithelialized superiorly to bolster the alar base and inset at the recipient site.4
Nasal Ala
The tunneled interpolated flap is useful for reconstruction of defects of the nasal ala. A flap with a superior deepithelialized pedicle and an anticipated inferior Burow triangle is designed along the axis of the nasolabial fold. The inferior Burow triangle and central flap are elevated at the level of the superficial subcutaneous fat and the pedicle is dissected. The donor and recipient sites are widely undermined, and the flap and pedicle pass through the tunnel. The donor site is closed primarily, the inferior Burow triangle is trimmed, and the flap is sutured into the defect.5 This flap allows for preservation of free margins and favorable placement of incision lines. Furthermore, pincushioning of the flap helps to recreate the rounded shape of the lateral ala.6
Nasal Tip
Nasal tip defects can be repaired with a retroangular flap, centered on the angular artery. The flap is elevated along the axis of the nasolabial fold, deepithelialized at its proximal base, and transferred through a subcutaneous tunnel to the nasal tip. The angular artery is ligated at the inferior aspect of the flap.7
Nasal Sidewall
A deepithelialized tunneled interpolated forehead flap, similar to the classic paramedian forehead flap, can be used to reconstruct nasal sidewall defects. A flap is elevated on the contralateral forehead and the proximal portion is deepithelialized. A tunnel is then bluntly dissected just above the periosteum, and the flap is introduced into the defect through the tunnel and inset. This flap has the advantages of being a single-stage procedure, restoring volume to the defect area, and maintaining excellent vascular supply.8
Eyelid
A tunneled interpolated forehead flap also can be used to repair medial canthal defects and for anterior lamellar repair of lower eyelid defects. In a study of 9 patients receiving a tunneled interpolated forehead flap in these anatomic locations, all flaps demonstrated viability, protection of the globe, and preservation of the concave architecture of the medial canthus.9
Earlobe
Earlobe defects may be repaired with a pull-through interpolated preauricular flap. A flap is elevated superiorly in the preauricular region and the proximal aspect of the flap is deepithelialized. The flap is pulled through a tunnel and inset at the anterior earlobe defect. The donor site is closed primarily.10,11
Concha
Reconstruction of anterior conchal defects with exposed cartilage can be accomplished with a pull-through interpolated postauricular flap based on the auriculomastoid fossa. The postauricular flap is elevated, the base is deepithelialized, an incision is made in the medial aspect of the defect, and the flap is moved through a tunnel between the posterior and anterior surfaces of the ear. The flap is secured to the anterior surface of the concha.12
Reconstruction Requiring Contour Preservation
Central Face
The hinge flap is optimal for reconstruction of deep central facial defects (Figure 1). The hinge flap is planned at a site contiguous with a margin of the defect and can include the dermis, subcutaneous tissue, muscle, or a combination of these. The desired tissue is folded over on the pedicle to fill the defect. Cutaneous coverage is accomplished through a primary closure, separate flap, or skin graft. In addition to restoring contour and therefore the cosmetic subunit, the hinge flap is performed in a single stage, resists wound contracture, and provides a well-vascularized wound bed resulting in a low incidence of graft failure.13,14 Muscular hinge flaps have been described for reconstruction of forehead defects with exposed bone based on the frontalis muscle.15

Lower Lip
A variant of a V-Y advancement flap has been described for reconstruction of defects greater than one-third the length of the lower lip. The top of the “V” is deepithelialized and the flap is advanced such that the top of the “V” abuts the inferior border of the defect. The “V” flap is inset at its advanced position, converting the “V”-shaped wound into a “Y.” An overlying buccal mucosal graft provides reconstruction of the lower red lip and labial mucosa.16
Helix of the Ear
Large defects of the scapha and helix of the ear can be reconstructed with the use of a staged interpolated postauricular flap. The postauricular flap is elevated into a subcutaneous plane. A full-thickness incision is made medial to the helical rim, and the flap is tunneled through and sutured into place. The pedicle is later divided, and the distal aspect of the flap is deepithelialized and inset into the helical rim for volume restoration.17
Reconstruction Involving Free Margins
Nasal Ala
For large defects involving the upper cutaneous lip with adjacent alar base involvement, a partially deepithelialized V-Y flap is a useful reconstructive option (Figure 2).

Infraorbital Region
A deepithelialized variant of a V-Y advancement flap can be used for closure of infraorbital defects. The limbs of the V-Y flap are deepithelialized and anchored to the medial and lateral canthal tendons or periosteum. Ectropion prevention is the primary advantage of this flap.18
APPLICATION OF DEEPITHELIALIZED GRAFTS
Deepithelialized grafts may be considered for volume replacement, reconstruction requiring contour preservation, and restoration of mechanical integrity in areas of high mechanical tension.3,19-21
Reconstruction Requiring Contour Preservation
Deepithelialized grafts are used to improve depressed nasal scars and restore volume in deep nasal wounds. One method involves deepithelialization of 2 postauricular punch biopsies. An 18-gauge needle is used to make a small hole in the depressed nasal scar, the dermal grafts are inserted, and the defect is closed primarily.19 Dermal grafts may be harvested from excess full-thickness skin grafts (FTSGs) or dog-ear tissue. When used under flaps, the dermal graft is trimmed to the size of the defect. When used under FTSGs, thin dermal graft strips are placed in a gridlike pattern to allow for revascularization. A study of 15 patients with contour deformities reconstructed with dermal graft insertions demonstrated that 14 (94%) patients had no significant complications and improvement of scar depression was achieved.20
Reconstruction in Areas of High Mechanical Tension
Plantar Foot
A combined dermal and full-thickness sandwich graft has been described for reconstruction of plantar foot defects.3 The graft is created by obtaining a FTSG twice the size of the wound defect and deepithelializing half of the graft. The graft is then defatted and the deepithelialized portion is folded beneath the other half, allowing the papillary dermis to make contact with the wound surface.
Scalp
Dermal graft reconstruction for scalp defects may be accomplished with a split-thickness skin flap. The flap is harvested using an electronic dermatome that ensures the proximal aspect is still attached to adjacent skin. The dermis is removed from the area underneath the back-folded split-thickness skin flap. The dermal graft is meshed and sutured into the recipient site. The split-thickness skin flap is replaced over the donor site. Meshed reversed dermal grafts have excellent survival rates, even with direct placement on bone without periosteum. Querings et al21 reported graft survival with no complications in 19 of 21 (90.4%) patients undergoing scalp or plantar sole reconstruction.
CONCLUSION
With the widespread adoption of the fresh-tissue technique for Mohs micrographic surgery and the establishment of the American Society for Dermatologic Surgery in 1970, the depth and scope of techniques used by dermatologic surgeons has dramatically expanded. Although the use of dermal flaps and grafts is not as widespread in dermatology as other reconstructive techniques, their unique advantages should be considered. Deepithelialized flaps and grafts should be considered when the following reconstructive goals are desired: (1) conversion of a 2-stage interpolation flap to a single-stage tunneled flap, (2) contour and cosmetic subunit preservation of deep defects through volume augmentation, (3) reconstruction in areas of high mechanical tension, and (4) free margin preservation. The multiple applications of deepithelialized flaps and grafts as described in this review demonstrate their continued applicability in dermatologic surgery.
- Straatsma CR. Use of the dermal graft in the repairs of small saddle defects of the nose. Arch Otolaryngol. 1932;16:506-509.
- Cydeli A, Hunter J. Peeling orange: rapid deepithelialization in reduction mammoplasty. J Aesthet Surg. 2004;24:580-581.
- Bechara F, Sand M, Radenhausen M, et al. Erbium:YAG laser-assisted preparation of a combined dermal/full thickness sandwich skin graft. Dermatol Surg. 2006;32:353-358.
- Cook JL. Tunneled and transposed island flaps in facial reconstructive surgery. Dermatol Surg. 2014;40(suppl 9):S16-S29.
- Krishnan RS, Clark DP. Tunneled transposition flap for reconstruction of defects of the nasal ala. Dermatol Surg. 2007;33:1496-1501.
- Mahlberg M. Tunneled melolabial pedicle flap for small but deep lateral alar rim defect. Dermatol Surg. 2013;39:1527-1529.
- Ascari-Raccagni A, Balderi U. The retroangular flap used in the surgery of nasal tip defects. Dermatol Surg. 2004;30:1131-1137.
- Hollmig ST, Leach BC, Cook J. Single-staged interpolation flaps in facial reconstruction. Dermatol Surg. 2014;40(suppl 9):S62-S70.
- Mombaerts I, Gillis A. The tunneled forehead flap in medial canthal and eyelid reconstruction. Dermatol Surg. 2010:36:1118-1125.
- Wang SQ, Goldberg LH, Kimyah-Asadi A. Tunneled island pedicle flap for an earlobe defect. Dermatol Surg. 2007;33:835-838.
- Hatoko M, Kuwahara M, Shiba A, et al. Earlobe reconstruction using a subcutaneous island pedicle flap after resection of “earlobe keloid.” Dermatol Surg. 1998;24:257-261.
- Alder N, Ad-El D, Azaria R. Reconstruction of nonhelical auricular defects with local flaps. Dermatol Surg. 2008;34:501-507.
- Fader DJ, Wang TS, Johnson TM. Nasal reconstruction utilizing a muscle hinge flap with overlying FTSG. J Am Acad Dermatol. 2000;43:837-840.
- Braun MA, Cook J. Hinge flaps in facial reconstruction. Dermatol Surg. 2007;33:213-221.
- Salmon PL, Mortimer NL, Hill SE. Muscular hinge flaps: utility and technique in facial reconstructive surgery. Dermatol Surg. 2010;36:227-234.
- Seo Y, Song S, Choi Y, et al. A lower lip reconstruction. Dermatol Surg. 2015;41:505-507.
- Malone CH, Wagner RF. Partially de-epithelialized postauricular flap for ear reconstruction. J Am Acad Dermatol. 2015;73:E219-E220.
- Yildrim S, Akoz T, Akan M, et al. Nasolabial V-Y advancement for closure of the midface defects. Dermatol Surg. 2001;27:656-662.
- Jensen DJ, Cohen JL. Nasal tip revision using a dermal graft. Dermatol Surg. 2014;40:1140-1142.
- Meyers S, Rohrer T. Use of dermal grafts in reconstructing deep nasal defects and shaping the ala nasi. Dermatol Surg. 2001;27:300-305.
- Querings K, Bachter D, Balda B. Meshed reversed dermal graft in patients with surgical defects of sole and scalp: technique and long-term results. Dermatol Surg. 2002;28:122-126.
- Straatsma CR. Use of the dermal graft in the repairs of small saddle defects of the nose. Arch Otolaryngol. 1932;16:506-509.
- Cydeli A, Hunter J. Peeling orange: rapid deepithelialization in reduction mammoplasty. J Aesthet Surg. 2004;24:580-581.
- Bechara F, Sand M, Radenhausen M, et al. Erbium:YAG laser-assisted preparation of a combined dermal/full thickness sandwich skin graft. Dermatol Surg. 2006;32:353-358.
- Cook JL. Tunneled and transposed island flaps in facial reconstructive surgery. Dermatol Surg. 2014;40(suppl 9):S16-S29.
- Krishnan RS, Clark DP. Tunneled transposition flap for reconstruction of defects of the nasal ala. Dermatol Surg. 2007;33:1496-1501.
- Mahlberg M. Tunneled melolabial pedicle flap for small but deep lateral alar rim defect. Dermatol Surg. 2013;39:1527-1529.
- Ascari-Raccagni A, Balderi U. The retroangular flap used in the surgery of nasal tip defects. Dermatol Surg. 2004;30:1131-1137.
- Hollmig ST, Leach BC, Cook J. Single-staged interpolation flaps in facial reconstruction. Dermatol Surg. 2014;40(suppl 9):S62-S70.
- Mombaerts I, Gillis A. The tunneled forehead flap in medial canthal and eyelid reconstruction. Dermatol Surg. 2010:36:1118-1125.
- Wang SQ, Goldberg LH, Kimyah-Asadi A. Tunneled island pedicle flap for an earlobe defect. Dermatol Surg. 2007;33:835-838.
- Hatoko M, Kuwahara M, Shiba A, et al. Earlobe reconstruction using a subcutaneous island pedicle flap after resection of “earlobe keloid.” Dermatol Surg. 1998;24:257-261.
- Alder N, Ad-El D, Azaria R. Reconstruction of nonhelical auricular defects with local flaps. Dermatol Surg. 2008;34:501-507.
- Fader DJ, Wang TS, Johnson TM. Nasal reconstruction utilizing a muscle hinge flap with overlying FTSG. J Am Acad Dermatol. 2000;43:837-840.
- Braun MA, Cook J. Hinge flaps in facial reconstruction. Dermatol Surg. 2007;33:213-221.
- Salmon PL, Mortimer NL, Hill SE. Muscular hinge flaps: utility and technique in facial reconstructive surgery. Dermatol Surg. 2010;36:227-234.
- Seo Y, Song S, Choi Y, et al. A lower lip reconstruction. Dermatol Surg. 2015;41:505-507.
- Malone CH, Wagner RF. Partially de-epithelialized postauricular flap for ear reconstruction. J Am Acad Dermatol. 2015;73:E219-E220.
- Yildrim S, Akoz T, Akan M, et al. Nasolabial V-Y advancement for closure of the midface defects. Dermatol Surg. 2001;27:656-662.
- Jensen DJ, Cohen JL. Nasal tip revision using a dermal graft. Dermatol Surg. 2014;40:1140-1142.
- Meyers S, Rohrer T. Use of dermal grafts in reconstructing deep nasal defects and shaping the ala nasi. Dermatol Surg. 2001;27:300-305.
- Querings K, Bachter D, Balda B. Meshed reversed dermal graft in patients with surgical defects of sole and scalp: technique and long-term results. Dermatol Surg. 2002;28:122-126.
Practice Points
- Deepithelialized flaps should be considered for single-stage reconstruction with tunneled interpolation flaps, reconstruction requiring contour preservation, and reconstruction involving free margins.
- Deepithelialized grafts may be considered for volume replacement, reconstruction requiring contour preservation, and reconstruction in areas of high mechanical tension.
Dermatology practice gaps: Missed diagnoses
KAUAI, HAWAII – Up to 130,000 patients hospitalized for treatment of lower extremity cellulitis annually in the United States turn out to have been misdiagnosed – and therein lies an opportunity for dermatologists to make a difference, according to Erik J. Stratman, MD, chairman of the department of dermatology at the Marshfield (Wisc.) Clinic.
As a section editor for UptoDate, he monitors the medical literature to identify , which he defines as things he and, he suspects, many other dermatologists are “either doing or not doing in practice that we shouldn’t or should be doing,” he explained at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
At the Hawaii meeting, he zeroed in on two such practice gaps pertaining to missed diagnoses.
Lower extremity cellulitis
A 2017 American Academy of Dermatology report on the national burden of skin disease contained eye-popping figures on the heavy toll of cellulitis. Cellulitis is the most common form of skin and soft tissue infection (SSTI). To put that into perspective, the annual incidence of SSTIs is 10-fold greater than that of pneumonia. Indeed, SSTIs account for 10% of all infectious disease–related hospitalizations across the country. There are 2.3 million emergency department visits per year for cellulitis, 14%-17% of which result in hospitalization (J Am Acad Dermatol. 2017 May;76[5]:958-972.e2).
Dr. Stratman, who is on the board of directors of the American Board of Dermatology, was favorably impressed with the work of a multicenter group of investigators who scrutinized 259 consecutive patients admitted with a diagnosis of lower extremity cellulitis through the emergency department at Massachusetts General Hospital in Boston. Seventy-nine of them (30.5%), were found to be misdiagnosed. Fifty-two of the 79 misdiagnosed patients had been admitted primarily for treatment of their supposed cellulitis: 44 of these 52, or 85%, didn’t require hospitalization, and 48 of the 52, or 92%, received unnecessary antibiotics.
Extrapolating from this experience, with application of cost data provided by the U.S. Agency for Healthcare Research and Quality, the investigators estimated that misdiagnosis of cellulitis results in 50,000 to 130,000 unnecessary hospitalizations annually. These hospitalizations for what the investigators termed “pseudocellulitis,” the majority of which is stasis dermatitis, resulted in inpatient costs estimated at up to $515 million per year. The unnecessary hospitalizations also led to an estimated 9,000 nosocomial infections, up to 5,000 Clostridium difficile infections, and a projected two to six cases of anaphylaxis resulting from exposure to the unnecessary antibiotics (JAMA Dermatol. 2017;153[2]:141-6).
Dr. Stratman said that the large Massachusetts General Hospital study mirrors his own experience when called upon to do a hospital consultation, as well as that of other dermatologists he has spoken with: “The number-one reason we get consulted is for stuff that is wrongfully admitted, mainly cellulitis.”
The investigators then went on to develop a simple prediction model for lower extremity cellulitis based upon their data. It’s called the ALT-70 score, an acronym for Asymmetric, Leukocytosis, Tachycardia, and Age greater than 70. A patient gets 3 points if one leg is affected, zero if both are. Age 70 or more is worth 2 points. A heart rate of 90 beats per minute or higher gets 1 point, as does a WBC of at least 10,000 per uL. A score of 0-2 spells at least an 83% likelihood that the patient has pseudocellulitis, while a score of 5 or points indicates at least an 82% likelihood of true cellulitis (J Am Acad Dermatol. 2017 Apr;76[4]:618-625.e2).
“If you don’t reach a score of 3, you’d better think a little bit harder before you hang that bag of vancomycin,” Dr. Stratman observed.
He ascribed the huge problem of misdiagnosed lower extremity cellulitis to several causes: emergency medicine physicians, hospitalists, and primary care physicians receive minimal dermatology training. In addition, there are no reliable diagnostic studies for the infection, and dermatologists are seldom consulted on patients with red legs, either because there are no dermatologists in a particular community or they don’t want to be consulted.
“It’s not all the dermatologists’ fault. Have you tried to get credentialed at a hospital lately? It’s a 1½-inch stack of papers and 8½ hours of electronic medical record training, if you’re lucky. So there are definitely barriers to overcoming this gap,” Dr. Stratman pointed out.
The best solution, he continued, is for dermatologists to take the initiative in educating hospitalists, emergency medicine specialists, and primary care physicians on the common mimickers of cellulitis, especially stasis dermatitis and contact dermatitis. This can happen through grand rounds presentations and feedback to consulting physicians.
“I think dermatologists have to take the lead on this,” Dr. Stratman said.
Underscreening for autoimmune thyroid disease in vitiligo patients
The international Vitiligo Working Group, citing evidence that 19% of patients with vitiligo have concomitant autoimmune thyroid disease and that the risk of developing this endocrine disease doubles every 5 years that a patient has vitiligo, has issued a call to action for dermatologists to ensure that their patients with vitiligo undergo periodic screening (J Am Acad Dermatol. 2017 Jul;77[1]:1-13).
This recommendation was based upon insights provided by a French prospective, observational study of 626 patients with vitiligo. The French investigators found that the risk of autoimmune thyroid disease doubled every 5 years and was associated with female sex, younger age at vitiligo onset, vitiligo on the trunk, and a personal history of autoimmune disease. They recommended screening every 2 years for thyroid-stimulating hormone and free thyroxine levels, as well as checking for serum antithyroperoxidase antibodies (Br J Dermatol. 2013 Apr;168[4]:756-61).
Dr. Stratman noted that some dermatologists may feel that ordering thyroid screening tests is outside their scope of practice. In that case, it’s important to engage with their vitiligo patient’s primary care physician to make sure the screening gets done.
He reported having no financial conflicts of interest regarding his presentation.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – Up to 130,000 patients hospitalized for treatment of lower extremity cellulitis annually in the United States turn out to have been misdiagnosed – and therein lies an opportunity for dermatologists to make a difference, according to Erik J. Stratman, MD, chairman of the department of dermatology at the Marshfield (Wisc.) Clinic.
As a section editor for UptoDate, he monitors the medical literature to identify , which he defines as things he and, he suspects, many other dermatologists are “either doing or not doing in practice that we shouldn’t or should be doing,” he explained at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
At the Hawaii meeting, he zeroed in on two such practice gaps pertaining to missed diagnoses.
Lower extremity cellulitis
A 2017 American Academy of Dermatology report on the national burden of skin disease contained eye-popping figures on the heavy toll of cellulitis. Cellulitis is the most common form of skin and soft tissue infection (SSTI). To put that into perspective, the annual incidence of SSTIs is 10-fold greater than that of pneumonia. Indeed, SSTIs account for 10% of all infectious disease–related hospitalizations across the country. There are 2.3 million emergency department visits per year for cellulitis, 14%-17% of which result in hospitalization (J Am Acad Dermatol. 2017 May;76[5]:958-972.e2).
Dr. Stratman, who is on the board of directors of the American Board of Dermatology, was favorably impressed with the work of a multicenter group of investigators who scrutinized 259 consecutive patients admitted with a diagnosis of lower extremity cellulitis through the emergency department at Massachusetts General Hospital in Boston. Seventy-nine of them (30.5%), were found to be misdiagnosed. Fifty-two of the 79 misdiagnosed patients had been admitted primarily for treatment of their supposed cellulitis: 44 of these 52, or 85%, didn’t require hospitalization, and 48 of the 52, or 92%, received unnecessary antibiotics.
Extrapolating from this experience, with application of cost data provided by the U.S. Agency for Healthcare Research and Quality, the investigators estimated that misdiagnosis of cellulitis results in 50,000 to 130,000 unnecessary hospitalizations annually. These hospitalizations for what the investigators termed “pseudocellulitis,” the majority of which is stasis dermatitis, resulted in inpatient costs estimated at up to $515 million per year. The unnecessary hospitalizations also led to an estimated 9,000 nosocomial infections, up to 5,000 Clostridium difficile infections, and a projected two to six cases of anaphylaxis resulting from exposure to the unnecessary antibiotics (JAMA Dermatol. 2017;153[2]:141-6).
Dr. Stratman said that the large Massachusetts General Hospital study mirrors his own experience when called upon to do a hospital consultation, as well as that of other dermatologists he has spoken with: “The number-one reason we get consulted is for stuff that is wrongfully admitted, mainly cellulitis.”
The investigators then went on to develop a simple prediction model for lower extremity cellulitis based upon their data. It’s called the ALT-70 score, an acronym for Asymmetric, Leukocytosis, Tachycardia, and Age greater than 70. A patient gets 3 points if one leg is affected, zero if both are. Age 70 or more is worth 2 points. A heart rate of 90 beats per minute or higher gets 1 point, as does a WBC of at least 10,000 per uL. A score of 0-2 spells at least an 83% likelihood that the patient has pseudocellulitis, while a score of 5 or points indicates at least an 82% likelihood of true cellulitis (J Am Acad Dermatol. 2017 Apr;76[4]:618-625.e2).
“If you don’t reach a score of 3, you’d better think a little bit harder before you hang that bag of vancomycin,” Dr. Stratman observed.
He ascribed the huge problem of misdiagnosed lower extremity cellulitis to several causes: emergency medicine physicians, hospitalists, and primary care physicians receive minimal dermatology training. In addition, there are no reliable diagnostic studies for the infection, and dermatologists are seldom consulted on patients with red legs, either because there are no dermatologists in a particular community or they don’t want to be consulted.
“It’s not all the dermatologists’ fault. Have you tried to get credentialed at a hospital lately? It’s a 1½-inch stack of papers and 8½ hours of electronic medical record training, if you’re lucky. So there are definitely barriers to overcoming this gap,” Dr. Stratman pointed out.
The best solution, he continued, is for dermatologists to take the initiative in educating hospitalists, emergency medicine specialists, and primary care physicians on the common mimickers of cellulitis, especially stasis dermatitis and contact dermatitis. This can happen through grand rounds presentations and feedback to consulting physicians.
“I think dermatologists have to take the lead on this,” Dr. Stratman said.
Underscreening for autoimmune thyroid disease in vitiligo patients
The international Vitiligo Working Group, citing evidence that 19% of patients with vitiligo have concomitant autoimmune thyroid disease and that the risk of developing this endocrine disease doubles every 5 years that a patient has vitiligo, has issued a call to action for dermatologists to ensure that their patients with vitiligo undergo periodic screening (J Am Acad Dermatol. 2017 Jul;77[1]:1-13).
This recommendation was based upon insights provided by a French prospective, observational study of 626 patients with vitiligo. The French investigators found that the risk of autoimmune thyroid disease doubled every 5 years and was associated with female sex, younger age at vitiligo onset, vitiligo on the trunk, and a personal history of autoimmune disease. They recommended screening every 2 years for thyroid-stimulating hormone and free thyroxine levels, as well as checking for serum antithyroperoxidase antibodies (Br J Dermatol. 2013 Apr;168[4]:756-61).
Dr. Stratman noted that some dermatologists may feel that ordering thyroid screening tests is outside their scope of practice. In that case, it’s important to engage with their vitiligo patient’s primary care physician to make sure the screening gets done.
He reported having no financial conflicts of interest regarding his presentation.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – Up to 130,000 patients hospitalized for treatment of lower extremity cellulitis annually in the United States turn out to have been misdiagnosed – and therein lies an opportunity for dermatologists to make a difference, according to Erik J. Stratman, MD, chairman of the department of dermatology at the Marshfield (Wisc.) Clinic.
As a section editor for UptoDate, he monitors the medical literature to identify , which he defines as things he and, he suspects, many other dermatologists are “either doing or not doing in practice that we shouldn’t or should be doing,” he explained at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
At the Hawaii meeting, he zeroed in on two such practice gaps pertaining to missed diagnoses.
Lower extremity cellulitis
A 2017 American Academy of Dermatology report on the national burden of skin disease contained eye-popping figures on the heavy toll of cellulitis. Cellulitis is the most common form of skin and soft tissue infection (SSTI). To put that into perspective, the annual incidence of SSTIs is 10-fold greater than that of pneumonia. Indeed, SSTIs account for 10% of all infectious disease–related hospitalizations across the country. There are 2.3 million emergency department visits per year for cellulitis, 14%-17% of which result in hospitalization (J Am Acad Dermatol. 2017 May;76[5]:958-972.e2).
Dr. Stratman, who is on the board of directors of the American Board of Dermatology, was favorably impressed with the work of a multicenter group of investigators who scrutinized 259 consecutive patients admitted with a diagnosis of lower extremity cellulitis through the emergency department at Massachusetts General Hospital in Boston. Seventy-nine of them (30.5%), were found to be misdiagnosed. Fifty-two of the 79 misdiagnosed patients had been admitted primarily for treatment of their supposed cellulitis: 44 of these 52, or 85%, didn’t require hospitalization, and 48 of the 52, or 92%, received unnecessary antibiotics.
Extrapolating from this experience, with application of cost data provided by the U.S. Agency for Healthcare Research and Quality, the investigators estimated that misdiagnosis of cellulitis results in 50,000 to 130,000 unnecessary hospitalizations annually. These hospitalizations for what the investigators termed “pseudocellulitis,” the majority of which is stasis dermatitis, resulted in inpatient costs estimated at up to $515 million per year. The unnecessary hospitalizations also led to an estimated 9,000 nosocomial infections, up to 5,000 Clostridium difficile infections, and a projected two to six cases of anaphylaxis resulting from exposure to the unnecessary antibiotics (JAMA Dermatol. 2017;153[2]:141-6).
Dr. Stratman said that the large Massachusetts General Hospital study mirrors his own experience when called upon to do a hospital consultation, as well as that of other dermatologists he has spoken with: “The number-one reason we get consulted is for stuff that is wrongfully admitted, mainly cellulitis.”
The investigators then went on to develop a simple prediction model for lower extremity cellulitis based upon their data. It’s called the ALT-70 score, an acronym for Asymmetric, Leukocytosis, Tachycardia, and Age greater than 70. A patient gets 3 points if one leg is affected, zero if both are. Age 70 or more is worth 2 points. A heart rate of 90 beats per minute or higher gets 1 point, as does a WBC of at least 10,000 per uL. A score of 0-2 spells at least an 83% likelihood that the patient has pseudocellulitis, while a score of 5 or points indicates at least an 82% likelihood of true cellulitis (J Am Acad Dermatol. 2017 Apr;76[4]:618-625.e2).
“If you don’t reach a score of 3, you’d better think a little bit harder before you hang that bag of vancomycin,” Dr. Stratman observed.
He ascribed the huge problem of misdiagnosed lower extremity cellulitis to several causes: emergency medicine physicians, hospitalists, and primary care physicians receive minimal dermatology training. In addition, there are no reliable diagnostic studies for the infection, and dermatologists are seldom consulted on patients with red legs, either because there are no dermatologists in a particular community or they don’t want to be consulted.
“It’s not all the dermatologists’ fault. Have you tried to get credentialed at a hospital lately? It’s a 1½-inch stack of papers and 8½ hours of electronic medical record training, if you’re lucky. So there are definitely barriers to overcoming this gap,” Dr. Stratman pointed out.
The best solution, he continued, is for dermatologists to take the initiative in educating hospitalists, emergency medicine specialists, and primary care physicians on the common mimickers of cellulitis, especially stasis dermatitis and contact dermatitis. This can happen through grand rounds presentations and feedback to consulting physicians.
“I think dermatologists have to take the lead on this,” Dr. Stratman said.
Underscreening for autoimmune thyroid disease in vitiligo patients
The international Vitiligo Working Group, citing evidence that 19% of patients with vitiligo have concomitant autoimmune thyroid disease and that the risk of developing this endocrine disease doubles every 5 years that a patient has vitiligo, has issued a call to action for dermatologists to ensure that their patients with vitiligo undergo periodic screening (J Am Acad Dermatol. 2017 Jul;77[1]:1-13).
This recommendation was based upon insights provided by a French prospective, observational study of 626 patients with vitiligo. The French investigators found that the risk of autoimmune thyroid disease doubled every 5 years and was associated with female sex, younger age at vitiligo onset, vitiligo on the trunk, and a personal history of autoimmune disease. They recommended screening every 2 years for thyroid-stimulating hormone and free thyroxine levels, as well as checking for serum antithyroperoxidase antibodies (Br J Dermatol. 2013 Apr;168[4]:756-61).
Dr. Stratman noted that some dermatologists may feel that ordering thyroid screening tests is outside their scope of practice. In that case, it’s important to engage with their vitiligo patient’s primary care physician to make sure the screening gets done.
He reported having no financial conflicts of interest regarding his presentation.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Listen up: Acoustic device useful for diabetic foot ulcers
The Food & Drug Administration has approved the marketing of a device that uses acoustic shock waves to boost wound closure in patients with diabetic foot ulcers (DFUs), an especially stubborn and dangerous condition.
The treatment is experimental, and only limited research into its effectiveness has been published. Still, representatives of its manufacturer say the device, known as dermaPACE, has produced promising results as a secondary treatment in stubborn cases.
A wound care specialist said in an interview that the shock wave technology appears to hold promise.
“A shortcoming in the field of wound care is that providers are typically not trained in a standardized fashion on when and how to a perform meticulous excisional sharp debridement of a wound,” said Bill Tettelbach, MD, systems medical director of Wound Care & Hyperbaric Medicine Services at Intermountain Healthcare in Salt Lake City. “In the majority of cases, the better the debridement, the more rapidly the patient will obtain wound closure.”
This new therapy may provide a benefit as a secondary treatment, especially when the patient cannot tolerate extensive sharp debridement, he said. It also could potentially improve biofilm penetration of antimicrobial topical treatments, he said.
DFUs are believed to affect as many as 1 in 4 people with diabetes over the course of their lifetimes. A 2014 report estimated that care of these wounds costs insurers as much as $13 billion a year in the U.S. alone (Diabetes Care. 2014 Mar;37[3]:651-8).
Treatment options include debridement and, in more extreme cases, hyperbaric oxygen treatment. Amputation can be required if treatment is unsuccessful.
According to Mr. Stegagno, the shock wave device is about the size of a desktop computer from a decade ago. A high-voltage generator box is connected to a handheld therapy head and delivers an acoustic pulse to the patient. The system “is like a spark plug that you see in your automobile,” he said. “It’s pretty much the same technology as lithotripsy, just downsized significantly. The key part is a highly focused, high-energy pulse.”
In a news release, the FDA said it examined the results of two studies of patients with diabetes who received usual DFU care along with either the shock wave therapy or a sham therapy. A total of 336 patients took part in the multicenter, randomized, double-blind studies.
According to the FDA, the studies found a 44% wound closure rate at 24 weeks in patients who had undergone 1-7 shock wave treatments, compared with the 30% wound closure rate in those who received the sham treatment.
Side effects included pain while the device was applied, bruising and numbness, migraines, nausea, fainting, wound infection, fever, and infection beyond the wound such as cellulitis and osteomyelitis.
“There were no meaningful statistical differences in the adverse event rates between the dermaPACE-treated patients and the sham-control group,” Mr. Stegagno said. “There were no issues regarding the tolerability of the treatment, which suggests that a second course of treatment, if needed, is a clinically viable option.”
Mr. Stegagno said the FDA expressed concern about “increased incidences of osteomyelitis at later points in the trials, particularly at the 10-week mark and later.” In response to the agency’s concerns, warning statements were added to labeling, he said.
According to Mr. Stegagno, only one study into the shock wave treatment for DFU has been published, although research has been released through posters and abstracts. The small published study favorably compared shock wave therapy with hyperbaric oxygen therapy. (Diabetes Res Clin Pract. 2011 May;92[2]:187-93)
“Sanuwave will be sponsoring additional studies later this year in the [United States] as follow-on studies to the just-completed DFU trials,” Mr. Stegagno said.
The FDA says the device is intended to be used in adults aged 22 and up with certain types of chronic DFUs. The Sanuwave company says patients should be treated with 4-8 applications over 2-10 weeks.
The shock wave process appears to boost healing through a process that leads to inflammatory responses and oxygenation, Mr. Stegagno said, by first creating an initial compression phase that “squeezes the cell and creates a microtrauma.”
“The cell wakes up and says, ‘Something just punched me,’ ” he said. “This tissue and cellular disruption is believed to initiate the cellular signaling for growth factors and other proteins noted in studies.”
The effects of negative pressure also play a role in stimulation of the wound, he said.
The shock wave therapy will cost an estimated $3,000-$4,000 per protocol of 8 treatments, said Kevin A. Richardson II, the CEO and chairman of the board at Sanuwave, in an interview. The initial plan is for the company to place the devices with doctors while the firm still owns the machines, he said.
The FDA approved the marketing of the device as part of its de novo premarket review pathway, which allows certain new types of devices to be approved when approved similar devices don’t yet exist for the purposes of comparison.
Mr. Stegagno and Mr. Richardson work for Sanuwave. Dr. Tettelbach reported no relevant disclosures.
The Food & Drug Administration has approved the marketing of a device that uses acoustic shock waves to boost wound closure in patients with diabetic foot ulcers (DFUs), an especially stubborn and dangerous condition.
The treatment is experimental, and only limited research into its effectiveness has been published. Still, representatives of its manufacturer say the device, known as dermaPACE, has produced promising results as a secondary treatment in stubborn cases.
A wound care specialist said in an interview that the shock wave technology appears to hold promise.
“A shortcoming in the field of wound care is that providers are typically not trained in a standardized fashion on when and how to a perform meticulous excisional sharp debridement of a wound,” said Bill Tettelbach, MD, systems medical director of Wound Care & Hyperbaric Medicine Services at Intermountain Healthcare in Salt Lake City. “In the majority of cases, the better the debridement, the more rapidly the patient will obtain wound closure.”
This new therapy may provide a benefit as a secondary treatment, especially when the patient cannot tolerate extensive sharp debridement, he said. It also could potentially improve biofilm penetration of antimicrobial topical treatments, he said.
DFUs are believed to affect as many as 1 in 4 people with diabetes over the course of their lifetimes. A 2014 report estimated that care of these wounds costs insurers as much as $13 billion a year in the U.S. alone (Diabetes Care. 2014 Mar;37[3]:651-8).
Treatment options include debridement and, in more extreme cases, hyperbaric oxygen treatment. Amputation can be required if treatment is unsuccessful.
According to Mr. Stegagno, the shock wave device is about the size of a desktop computer from a decade ago. A high-voltage generator box is connected to a handheld therapy head and delivers an acoustic pulse to the patient. The system “is like a spark plug that you see in your automobile,” he said. “It’s pretty much the same technology as lithotripsy, just downsized significantly. The key part is a highly focused, high-energy pulse.”
In a news release, the FDA said it examined the results of two studies of patients with diabetes who received usual DFU care along with either the shock wave therapy or a sham therapy. A total of 336 patients took part in the multicenter, randomized, double-blind studies.
According to the FDA, the studies found a 44% wound closure rate at 24 weeks in patients who had undergone 1-7 shock wave treatments, compared with the 30% wound closure rate in those who received the sham treatment.
Side effects included pain while the device was applied, bruising and numbness, migraines, nausea, fainting, wound infection, fever, and infection beyond the wound such as cellulitis and osteomyelitis.
“There were no meaningful statistical differences in the adverse event rates between the dermaPACE-treated patients and the sham-control group,” Mr. Stegagno said. “There were no issues regarding the tolerability of the treatment, which suggests that a second course of treatment, if needed, is a clinically viable option.”
Mr. Stegagno said the FDA expressed concern about “increased incidences of osteomyelitis at later points in the trials, particularly at the 10-week mark and later.” In response to the agency’s concerns, warning statements were added to labeling, he said.
According to Mr. Stegagno, only one study into the shock wave treatment for DFU has been published, although research has been released through posters and abstracts. The small published study favorably compared shock wave therapy with hyperbaric oxygen therapy. (Diabetes Res Clin Pract. 2011 May;92[2]:187-93)
“Sanuwave will be sponsoring additional studies later this year in the [United States] as follow-on studies to the just-completed DFU trials,” Mr. Stegagno said.
The FDA says the device is intended to be used in adults aged 22 and up with certain types of chronic DFUs. The Sanuwave company says patients should be treated with 4-8 applications over 2-10 weeks.
The shock wave process appears to boost healing through a process that leads to inflammatory responses and oxygenation, Mr. Stegagno said, by first creating an initial compression phase that “squeezes the cell and creates a microtrauma.”
“The cell wakes up and says, ‘Something just punched me,’ ” he said. “This tissue and cellular disruption is believed to initiate the cellular signaling for growth factors and other proteins noted in studies.”
The effects of negative pressure also play a role in stimulation of the wound, he said.
The shock wave therapy will cost an estimated $3,000-$4,000 per protocol of 8 treatments, said Kevin A. Richardson II, the CEO and chairman of the board at Sanuwave, in an interview. The initial plan is for the company to place the devices with doctors while the firm still owns the machines, he said.
The FDA approved the marketing of the device as part of its de novo premarket review pathway, which allows certain new types of devices to be approved when approved similar devices don’t yet exist for the purposes of comparison.
Mr. Stegagno and Mr. Richardson work for Sanuwave. Dr. Tettelbach reported no relevant disclosures.
The Food & Drug Administration has approved the marketing of a device that uses acoustic shock waves to boost wound closure in patients with diabetic foot ulcers (DFUs), an especially stubborn and dangerous condition.
The treatment is experimental, and only limited research into its effectiveness has been published. Still, representatives of its manufacturer say the device, known as dermaPACE, has produced promising results as a secondary treatment in stubborn cases.
A wound care specialist said in an interview that the shock wave technology appears to hold promise.
“A shortcoming in the field of wound care is that providers are typically not trained in a standardized fashion on when and how to a perform meticulous excisional sharp debridement of a wound,” said Bill Tettelbach, MD, systems medical director of Wound Care & Hyperbaric Medicine Services at Intermountain Healthcare in Salt Lake City. “In the majority of cases, the better the debridement, the more rapidly the patient will obtain wound closure.”
This new therapy may provide a benefit as a secondary treatment, especially when the patient cannot tolerate extensive sharp debridement, he said. It also could potentially improve biofilm penetration of antimicrobial topical treatments, he said.
DFUs are believed to affect as many as 1 in 4 people with diabetes over the course of their lifetimes. A 2014 report estimated that care of these wounds costs insurers as much as $13 billion a year in the U.S. alone (Diabetes Care. 2014 Mar;37[3]:651-8).
Treatment options include debridement and, in more extreme cases, hyperbaric oxygen treatment. Amputation can be required if treatment is unsuccessful.
According to Mr. Stegagno, the shock wave device is about the size of a desktop computer from a decade ago. A high-voltage generator box is connected to a handheld therapy head and delivers an acoustic pulse to the patient. The system “is like a spark plug that you see in your automobile,” he said. “It’s pretty much the same technology as lithotripsy, just downsized significantly. The key part is a highly focused, high-energy pulse.”
In a news release, the FDA said it examined the results of two studies of patients with diabetes who received usual DFU care along with either the shock wave therapy or a sham therapy. A total of 336 patients took part in the multicenter, randomized, double-blind studies.
According to the FDA, the studies found a 44% wound closure rate at 24 weeks in patients who had undergone 1-7 shock wave treatments, compared with the 30% wound closure rate in those who received the sham treatment.
Side effects included pain while the device was applied, bruising and numbness, migraines, nausea, fainting, wound infection, fever, and infection beyond the wound such as cellulitis and osteomyelitis.
“There were no meaningful statistical differences in the adverse event rates between the dermaPACE-treated patients and the sham-control group,” Mr. Stegagno said. “There were no issues regarding the tolerability of the treatment, which suggests that a second course of treatment, if needed, is a clinically viable option.”
Mr. Stegagno said the FDA expressed concern about “increased incidences of osteomyelitis at later points in the trials, particularly at the 10-week mark and later.” In response to the agency’s concerns, warning statements were added to labeling, he said.
According to Mr. Stegagno, only one study into the shock wave treatment for DFU has been published, although research has been released through posters and abstracts. The small published study favorably compared shock wave therapy with hyperbaric oxygen therapy. (Diabetes Res Clin Pract. 2011 May;92[2]:187-93)
“Sanuwave will be sponsoring additional studies later this year in the [United States] as follow-on studies to the just-completed DFU trials,” Mr. Stegagno said.
The FDA says the device is intended to be used in adults aged 22 and up with certain types of chronic DFUs. The Sanuwave company says patients should be treated with 4-8 applications over 2-10 weeks.
The shock wave process appears to boost healing through a process that leads to inflammatory responses and oxygenation, Mr. Stegagno said, by first creating an initial compression phase that “squeezes the cell and creates a microtrauma.”
“The cell wakes up and says, ‘Something just punched me,’ ” he said. “This tissue and cellular disruption is believed to initiate the cellular signaling for growth factors and other proteins noted in studies.”
The effects of negative pressure also play a role in stimulation of the wound, he said.
The shock wave therapy will cost an estimated $3,000-$4,000 per protocol of 8 treatments, said Kevin A. Richardson II, the CEO and chairman of the board at Sanuwave, in an interview. The initial plan is for the company to place the devices with doctors while the firm still owns the machines, he said.
The FDA approved the marketing of the device as part of its de novo premarket review pathway, which allows certain new types of devices to be approved when approved similar devices don’t yet exist for the purposes of comparison.
Mr. Stegagno and Mr. Richardson work for Sanuwave. Dr. Tettelbach reported no relevant disclosures.
Five pearls target wound healing
MIAMI – Another reason not to prescribe opioids for postoperative pain – besides potentially adding to the epidemic the nation – comes from evidence showing these agents can impair wound healing.
In addition, epidermal sutures to close dermatologic surgery sites may be unnecessary if deep suturing is done proficiently. These and other pearls to optimize wound closure were suggested by Robert S. Kirsner, MD, PhD, professor and chair of the department of dermatology and cutaneous surgery at the University of Miami.
Avoid opioids for postoperative pain
“We know the opioid epidemic is a big problem. An estimated 5-8 million Americans use them for chronic pain,” Dr. Kirsner said at the Orlando Dermatology Aesthetic and Clinical Conference. “And there has been a steady increase in the use of illicit and prescription opioids.”
“The take-home message is that for the first time we have patient-oriented data that suggests that opioids impair healing,” Dr. Kirsner said. “So avoid opioids if at all possible.”
The precise mechanism remains unknown. The most likely explanation, he said, is that opioids inhibit substance P, a peptide that promotes healing in animal models. Interestingly, he added, adding the opioid antagonist naltrexone in animal studies improves healing.
Consider skipping epidermal sutures in some cases
Dermatologists who place really good deep sutures when closing a wound might be able to forgo traditional epidural suturing, Dr. Kirsner said. “If you believe the literature, you can actually forget epidermal sutures. That’s hard for us. We’re trained to put epidermal sutures in, and changing habits can be difficult.”
A prospective, randomized study demonstrated no difference in cosmesis at 6 months, for example, in a split scar study where half of each wound was closed with epidural suturing and half was not (Dermatol. Surg. 2015;41:1257-63). In another randomized study, researchers found something similar when comparing buried interrupted subcuticular suturing of wounds with and without adhesive strips to close the epidermis (JAMA Dermatol. 2015;15:862-7). “When they looked at the scars, complications, and cosmesis at 6 months, there was no difference,” Dr. Kirsner said.
“Forget epidermal sutures if you’re brave enough,” he said.
Dr. Kirsner acknowledged that some dermatologists might point out a requirement to evert wound edges with epidermal stitches. “It turns out you don’t need to, again, if you believe the literature.” He cited a randomized, controlled, split scar trial that revealed no difference in cosmetic outcomes according to blinded physician ratings or patient reports at 3 months (J Am Acad Dermatol. 2015;72;668-73). “So maybe the concept of wound eversion is not as important as we were originally taught.”
And speaking of wound edges …
When debriding a nonhealing wound ...
There may be something highly abnormal about a nonhealing wound edge, Dr. Kirsner said. In fact, they can be phenotypically and genotypically different from surrounding tissue, including characteristic overexpression of c-Myc and beta catenin. These two factors in higher amounts can inhibit the migration of keratinocytes into a wound to promote healing.
“Sometimes we debride the wound because it’s necrotic,” Dr. Kirsner said. But in the case of a nonhealing wound, it can be more effective to debride the edges to remove the abnormal tissue. “You can change the fortune of a wound by debriding the edge. You want to remove all the abnormal tissue, and give it a chance to heal.” Pathology supports the elevated presence of the c-Myc and beta catenin factors in the “healing incompetent” tissue around the edges of nonhealing wounds, he added.
If a patient is unusually anxious or stressed
Stress can impair wound healing by 40%, Dr. Kirsner said (Psychosom Med. 1998;60:362-5). Some anxiety before a dermatologic surgery procedure is normal for many patients, but there also are unusual circumstances. For example, “if a patient comes for cyst excision but learns while in the waiting room that his dog just died,” he said. It’s often better to reschedule the procedure than to proceed.
“What you can do on a daily basis is create a stress-free environment” as well, Dr. Kirsner said.
“From a practical standpoint, things that can impair healing include patient depression, negativism, isolation, and postoperative pain,” he added. The mechanism between elevated stress and impaired wound healing includes release of catecholamines that induce the action of endogenous steroids. This, in turn, can cause a cascade of events that reduce inflammatory cells and their pro-healing cytokines, thereby leading to poor healing.
“All of this is mediated through the love hormone, oxytocin. Maybe someday we will be able to give oxytocin to speed healing.”
Two technologies still look good for scarless donor sites
Epidermal grafting and technology based on fractional laser treatments continue to show promise for achieving a scarless donor site for patients who need grafting to promote wound healing, Dr. Kirsner said.
With epidermal grafting, dermatologists can apply a device to lift up on the epidermis from a donor site. The CelluTome Epidermal Harvesting System, for example, achieves this feat by applying both a little heat and some suction. “It creates little domes [of epidermis] in this Easy Bake oven looking device,” Dr. Kirsner said. Without any anesthetic, you place this device on the skin and you get these epidermal grafts in 30 minutes. Then you can transfer them to a sterile dressing and place them on the wound.”
As pointed out in a previous report in Dermatology News, avoiding the need for donor site anesthesia is one advantage of the epidermal grafting technique. In addition, the procedure is generally bloodless because the device does not go deep enough to reach the blood vessels, Dr. Kirsner said. In addition, healing of the donor site can be seen on histology in as little as 2 days.
Transferring the epidermis can promote healing because it also transfers keratinocytes and melanocytes to the wound.
“This technique is also excellent to add skin or cells to someone with pyoderma gangrenosum,” Dr. Kirsner said. “Because of the simplicity and the lack of trauma, you don’t get the pathergy you normally see on someone with pyoderma gangrenosum.”
An Autologous Regeneration of Tissue or ART device that transfers columns of healthy skin to a wound to help regenerate tissue and promote healing is a second technology with a lot of potential, Dr. Kirsner said. “With a fractional laser, you create a hole, and that hole heals without scarring. Instead of making holes, R. Rox Anderson, MD, professor of dermatology at Harvard University, Boston, created a device that picks out the microcolumns of skin.” When these full skin thickness columns of skin are transferred to a wound, Dr. Kirsner noted, “in 3 weeks you can pretty much have no visible or a much improved cosmetic scar. Histologically you don’t see a scar either.”
Dr. Kirsner said he had no relevant financial disclosures.
MIAMI – Another reason not to prescribe opioids for postoperative pain – besides potentially adding to the epidemic the nation – comes from evidence showing these agents can impair wound healing.
In addition, epidermal sutures to close dermatologic surgery sites may be unnecessary if deep suturing is done proficiently. These and other pearls to optimize wound closure were suggested by Robert S. Kirsner, MD, PhD, professor and chair of the department of dermatology and cutaneous surgery at the University of Miami.
Avoid opioids for postoperative pain
“We know the opioid epidemic is a big problem. An estimated 5-8 million Americans use them for chronic pain,” Dr. Kirsner said at the Orlando Dermatology Aesthetic and Clinical Conference. “And there has been a steady increase in the use of illicit and prescription opioids.”
“The take-home message is that for the first time we have patient-oriented data that suggests that opioids impair healing,” Dr. Kirsner said. “So avoid opioids if at all possible.”
The precise mechanism remains unknown. The most likely explanation, he said, is that opioids inhibit substance P, a peptide that promotes healing in animal models. Interestingly, he added, adding the opioid antagonist naltrexone in animal studies improves healing.
Consider skipping epidermal sutures in some cases
Dermatologists who place really good deep sutures when closing a wound might be able to forgo traditional epidural suturing, Dr. Kirsner said. “If you believe the literature, you can actually forget epidermal sutures. That’s hard for us. We’re trained to put epidermal sutures in, and changing habits can be difficult.”
A prospective, randomized study demonstrated no difference in cosmesis at 6 months, for example, in a split scar study where half of each wound was closed with epidural suturing and half was not (Dermatol. Surg. 2015;41:1257-63). In another randomized study, researchers found something similar when comparing buried interrupted subcuticular suturing of wounds with and without adhesive strips to close the epidermis (JAMA Dermatol. 2015;15:862-7). “When they looked at the scars, complications, and cosmesis at 6 months, there was no difference,” Dr. Kirsner said.
“Forget epidermal sutures if you’re brave enough,” he said.
Dr. Kirsner acknowledged that some dermatologists might point out a requirement to evert wound edges with epidermal stitches. “It turns out you don’t need to, again, if you believe the literature.” He cited a randomized, controlled, split scar trial that revealed no difference in cosmetic outcomes according to blinded physician ratings or patient reports at 3 months (J Am Acad Dermatol. 2015;72;668-73). “So maybe the concept of wound eversion is not as important as we were originally taught.”
And speaking of wound edges …
When debriding a nonhealing wound ...
There may be something highly abnormal about a nonhealing wound edge, Dr. Kirsner said. In fact, they can be phenotypically and genotypically different from surrounding tissue, including characteristic overexpression of c-Myc and beta catenin. These two factors in higher amounts can inhibit the migration of keratinocytes into a wound to promote healing.
“Sometimes we debride the wound because it’s necrotic,” Dr. Kirsner said. But in the case of a nonhealing wound, it can be more effective to debride the edges to remove the abnormal tissue. “You can change the fortune of a wound by debriding the edge. You want to remove all the abnormal tissue, and give it a chance to heal.” Pathology supports the elevated presence of the c-Myc and beta catenin factors in the “healing incompetent” tissue around the edges of nonhealing wounds, he added.
If a patient is unusually anxious or stressed
Stress can impair wound healing by 40%, Dr. Kirsner said (Psychosom Med. 1998;60:362-5). Some anxiety before a dermatologic surgery procedure is normal for many patients, but there also are unusual circumstances. For example, “if a patient comes for cyst excision but learns while in the waiting room that his dog just died,” he said. It’s often better to reschedule the procedure than to proceed.
“What you can do on a daily basis is create a stress-free environment” as well, Dr. Kirsner said.
“From a practical standpoint, things that can impair healing include patient depression, negativism, isolation, and postoperative pain,” he added. The mechanism between elevated stress and impaired wound healing includes release of catecholamines that induce the action of endogenous steroids. This, in turn, can cause a cascade of events that reduce inflammatory cells and their pro-healing cytokines, thereby leading to poor healing.
“All of this is mediated through the love hormone, oxytocin. Maybe someday we will be able to give oxytocin to speed healing.”
Two technologies still look good for scarless donor sites
Epidermal grafting and technology based on fractional laser treatments continue to show promise for achieving a scarless donor site for patients who need grafting to promote wound healing, Dr. Kirsner said.
With epidermal grafting, dermatologists can apply a device to lift up on the epidermis from a donor site. The CelluTome Epidermal Harvesting System, for example, achieves this feat by applying both a little heat and some suction. “It creates little domes [of epidermis] in this Easy Bake oven looking device,” Dr. Kirsner said. Without any anesthetic, you place this device on the skin and you get these epidermal grafts in 30 minutes. Then you can transfer them to a sterile dressing and place them on the wound.”
As pointed out in a previous report in Dermatology News, avoiding the need for donor site anesthesia is one advantage of the epidermal grafting technique. In addition, the procedure is generally bloodless because the device does not go deep enough to reach the blood vessels, Dr. Kirsner said. In addition, healing of the donor site can be seen on histology in as little as 2 days.
Transferring the epidermis can promote healing because it also transfers keratinocytes and melanocytes to the wound.
“This technique is also excellent to add skin or cells to someone with pyoderma gangrenosum,” Dr. Kirsner said. “Because of the simplicity and the lack of trauma, you don’t get the pathergy you normally see on someone with pyoderma gangrenosum.”
An Autologous Regeneration of Tissue or ART device that transfers columns of healthy skin to a wound to help regenerate tissue and promote healing is a second technology with a lot of potential, Dr. Kirsner said. “With a fractional laser, you create a hole, and that hole heals without scarring. Instead of making holes, R. Rox Anderson, MD, professor of dermatology at Harvard University, Boston, created a device that picks out the microcolumns of skin.” When these full skin thickness columns of skin are transferred to a wound, Dr. Kirsner noted, “in 3 weeks you can pretty much have no visible or a much improved cosmetic scar. Histologically you don’t see a scar either.”
Dr. Kirsner said he had no relevant financial disclosures.
MIAMI – Another reason not to prescribe opioids for postoperative pain – besides potentially adding to the epidemic the nation – comes from evidence showing these agents can impair wound healing.
In addition, epidermal sutures to close dermatologic surgery sites may be unnecessary if deep suturing is done proficiently. These and other pearls to optimize wound closure were suggested by Robert S. Kirsner, MD, PhD, professor and chair of the department of dermatology and cutaneous surgery at the University of Miami.
Avoid opioids for postoperative pain
“We know the opioid epidemic is a big problem. An estimated 5-8 million Americans use them for chronic pain,” Dr. Kirsner said at the Orlando Dermatology Aesthetic and Clinical Conference. “And there has been a steady increase in the use of illicit and prescription opioids.”
“The take-home message is that for the first time we have patient-oriented data that suggests that opioids impair healing,” Dr. Kirsner said. “So avoid opioids if at all possible.”
The precise mechanism remains unknown. The most likely explanation, he said, is that opioids inhibit substance P, a peptide that promotes healing in animal models. Interestingly, he added, adding the opioid antagonist naltrexone in animal studies improves healing.
Consider skipping epidermal sutures in some cases
Dermatologists who place really good deep sutures when closing a wound might be able to forgo traditional epidural suturing, Dr. Kirsner said. “If you believe the literature, you can actually forget epidermal sutures. That’s hard for us. We’re trained to put epidermal sutures in, and changing habits can be difficult.”
A prospective, randomized study demonstrated no difference in cosmesis at 6 months, for example, in a split scar study where half of each wound was closed with epidural suturing and half was not (Dermatol. Surg. 2015;41:1257-63). In another randomized study, researchers found something similar when comparing buried interrupted subcuticular suturing of wounds with and without adhesive strips to close the epidermis (JAMA Dermatol. 2015;15:862-7). “When they looked at the scars, complications, and cosmesis at 6 months, there was no difference,” Dr. Kirsner said.
“Forget epidermal sutures if you’re brave enough,” he said.
Dr. Kirsner acknowledged that some dermatologists might point out a requirement to evert wound edges with epidermal stitches. “It turns out you don’t need to, again, if you believe the literature.” He cited a randomized, controlled, split scar trial that revealed no difference in cosmetic outcomes according to blinded physician ratings or patient reports at 3 months (J Am Acad Dermatol. 2015;72;668-73). “So maybe the concept of wound eversion is not as important as we were originally taught.”
And speaking of wound edges …
When debriding a nonhealing wound ...
There may be something highly abnormal about a nonhealing wound edge, Dr. Kirsner said. In fact, they can be phenotypically and genotypically different from surrounding tissue, including characteristic overexpression of c-Myc and beta catenin. These two factors in higher amounts can inhibit the migration of keratinocytes into a wound to promote healing.
“Sometimes we debride the wound because it’s necrotic,” Dr. Kirsner said. But in the case of a nonhealing wound, it can be more effective to debride the edges to remove the abnormal tissue. “You can change the fortune of a wound by debriding the edge. You want to remove all the abnormal tissue, and give it a chance to heal.” Pathology supports the elevated presence of the c-Myc and beta catenin factors in the “healing incompetent” tissue around the edges of nonhealing wounds, he added.
If a patient is unusually anxious or stressed
Stress can impair wound healing by 40%, Dr. Kirsner said (Psychosom Med. 1998;60:362-5). Some anxiety before a dermatologic surgery procedure is normal for many patients, but there also are unusual circumstances. For example, “if a patient comes for cyst excision but learns while in the waiting room that his dog just died,” he said. It’s often better to reschedule the procedure than to proceed.
“What you can do on a daily basis is create a stress-free environment” as well, Dr. Kirsner said.
“From a practical standpoint, things that can impair healing include patient depression, negativism, isolation, and postoperative pain,” he added. The mechanism between elevated stress and impaired wound healing includes release of catecholamines that induce the action of endogenous steroids. This, in turn, can cause a cascade of events that reduce inflammatory cells and their pro-healing cytokines, thereby leading to poor healing.
“All of this is mediated through the love hormone, oxytocin. Maybe someday we will be able to give oxytocin to speed healing.”
Two technologies still look good for scarless donor sites
Epidermal grafting and technology based on fractional laser treatments continue to show promise for achieving a scarless donor site for patients who need grafting to promote wound healing, Dr. Kirsner said.
With epidermal grafting, dermatologists can apply a device to lift up on the epidermis from a donor site. The CelluTome Epidermal Harvesting System, for example, achieves this feat by applying both a little heat and some suction. “It creates little domes [of epidermis] in this Easy Bake oven looking device,” Dr. Kirsner said. Without any anesthetic, you place this device on the skin and you get these epidermal grafts in 30 minutes. Then you can transfer them to a sterile dressing and place them on the wound.”
As pointed out in a previous report in Dermatology News, avoiding the need for donor site anesthesia is one advantage of the epidermal grafting technique. In addition, the procedure is generally bloodless because the device does not go deep enough to reach the blood vessels, Dr. Kirsner said. In addition, healing of the donor site can be seen on histology in as little as 2 days.
Transferring the epidermis can promote healing because it also transfers keratinocytes and melanocytes to the wound.
“This technique is also excellent to add skin or cells to someone with pyoderma gangrenosum,” Dr. Kirsner said. “Because of the simplicity and the lack of trauma, you don’t get the pathergy you normally see on someone with pyoderma gangrenosum.”
An Autologous Regeneration of Tissue or ART device that transfers columns of healthy skin to a wound to help regenerate tissue and promote healing is a second technology with a lot of potential, Dr. Kirsner said. “With a fractional laser, you create a hole, and that hole heals without scarring. Instead of making holes, R. Rox Anderson, MD, professor of dermatology at Harvard University, Boston, created a device that picks out the microcolumns of skin.” When these full skin thickness columns of skin are transferred to a wound, Dr. Kirsner noted, “in 3 weeks you can pretty much have no visible or a much improved cosmetic scar. Histologically you don’t see a scar either.”
Dr. Kirsner said he had no relevant financial disclosures.
EXPERT ANALYSIS FROM ODAC 2018
Regenerative Medicine in Cosmetic Dermatology
Regenerative medicine encompasses innovative therapies that allow the body to repair or regenerate aging cells, tissues, and organs. The skin is a particularly attractive organ for the application of novel regenerative therapies due to its easy accessibility. Among these therapies, stem cells and platelet-rich plasma (PRP) have garnered interest based on their therapeutic potential in scar reduction, antiaging effects, and treatment of alopecia.
Stem cells possess the cardinal features of self-renewal and plasticity. Self-renewal refers to symmetric cell division generating daughter cells identical to the parent cell.1 Plasticity is the ability to generate cell types other than the germ line or tissue lineage from which stem cells derive.2 Stem cells can be categorized according to their differentiation potential. Totipotent stem cells may develop into any primary germ cell layer (ectoderm, mesoderm, endoderm) of the embryo, as well as extraembryonic tissue such as the trophoblast, which gives rise to the placenta. Pluripotent stem cells such as embryonic stem cells have the capacity to differentiate into any derivative of the 3 germ cell layers but have lost their ability to differentiate into the trophoblast.3 Adults lack totipotent or pluripotent cells; they have multipotent or unipotent cells. Multipotent stem cells are able to differentiate into multiple cell types from similar lineages; mesenchymal stem cells (MSCs), for example, can differentiate into adipogenic, osteogenic, chondrogenic, and myogenic cells.4 Unipotent stem cells have the lowest differentiation potential and can only self-regenerate. Herein, we review stem cell sources and their therapeutic potential in aesthetic dermatology.
Multipotent Stem Cells
Multipotent stem cells derived from the bone marrow, umbilical cord, adipose tissue, dermis, or hair follicle bulge have various clinical applications in dermatology. Stem cells from these sources are primarily utilized in an autologous manner in which they are processed outside the body and reintroduced into the donor. Autologous multipotent hematopoietic bone marrow cells were first successfully used for the treatment of chronic wounds and show promise for the treatment of atrophic scars.5,6 However, due to the invasive nature of extracting bone marrow stem cells and their declining number with age, other sources of multipotent stem cells have fallen into favor.
Umbilical cord blood is a source of multipotent hematopoietic stem cells for which surgical intervention is not necessary because they are retrieved after umbilical cord clamping.7 Advantages of sourcing stem cells from umbilical cord blood includes high regenerative power compared to a newborn’s skin and low immunogenicity given that the newborn is immunologically immature.8
Another popular source for autologous stem cells is adipose tissue due to its ease of accessibility and relative abundance. Given that adipose tissue–derived stem cells (ASCs) are capable of differentiating into adipocytes that help maintain volume over time, they are being used for midface contouring, lip augmentation, facial rejuvenation, facial scarring, lipodystrophy, penile girth enhancement, and vaginal augmentation. Adipose tissue–derived stem cells also are capable of differentiating into other types of tissue, including cartilage and bone. Thus, they have been successfully harnessed in the treatment of patients affected by systemic sclerosis and Parry-Romberg syndrome as well in the functional and aesthetic reconstruction of various military combat–related deformities.9,10
Adipose tissue–derived stem cells are commonly harvested from lipoaspirate of the abdomen and are combined with supportive mechanical scaffolds such as hydrogels. Lipoaspirate itself can serve as a scaffold for ASCs. Accordingly, ASCs also are being utilized as a scaffold for autologous fat transfer procedures in an effort to increase the viability of transplanted donor tissue, a process known as cell-assisted lipotransfer (CAL). In CAL, a fraction of the aspirated fat is processed for isolation of ASCs, which are then recombined with the remainder of the aspirated fat prior to grafting.11 However, there is conflicting evidence as to whether CAL leads to improved graft success relative to conventional autologous fat transfer.12,13
The skin also serves as an easily accessible and abundant autologous source of stem cells. A subtype of dermal fibroblasts has been proven to have multipotent potential.14,15 These dermal fibroblasts are harvested from one area of the skin using punch biopsy and are processed and reinjected into another desired area of the skin.16 Autologous human fibroblasts have proven to be effective for the treatment of wrinkles, rhytides, and acne scars.17 In June 2011, the US Food and Drug Administration approved azficel-T, an autologous cellular product created by harvesting fibroblasts from a patient’s own postauricular skin, culture-expanding them in vitro for 3 months, and reinjecting the cells into the desired area of dermis in a series of treatments. This product was the first personalized cell therapy approved by the US Food and Drug Administration for aesthetic uses, specifically for the improvement of nasolabial fold wrinkles.18
In adults, hair follicles contain an area known as the bulge, which is a site rich in epithelial and melanocytic stem cells. Bulge stem cells have the ability to reproduce the interfollicular epidermis, hair follicle structures, and sebaceous glands, and they have been used to construct entirely new hair follicles in an artificial in vivo system.19 Sugiyama-Nakagiri et al20 demonstrated that an entire hair follicle epithelium and interfollicular epidermis can be regenerated using cultured bulge stem cells. The cultured bulge stem cells were mixed with dermal papilla cells from neonatal rat vibrissae and engrafted into a silicone chamber implanted on the backs of severe combined immune deficient (SCID) mice. The grafts exhibited tufts of hair as well as a complete interfollicular epidermis at 4 weeks after transplantation.20 Thus, these bulge stem cells have the potential to treat male androgenic alopecia and female pattern hair loss. Bulge stem cells also have been shown to accelerate wound healing.21 Additionally, autologous melanocytic stem cells located at the hair follicle bulge are effective for treating vitiligo and are being investigated for the treatment of hair graying.22
Induced Pluripotent Stem Cells
Given the ethical concerns that surround the procurement and use of embryonic stem cells, efforts have been made to retrieve pluripotent stem cells from adults. A major breakthrough occurred in 2006 when researchers altered the genes of specialized adult mouse cells to cause dedifferentiation and the return to an embryoniclike stem cell state.23 Mouse somatic cells were reprogrammed through the activation of a combination of transcription factors. The resulting cells were termed induced pluripotent stem cells (iPSCs) and have since been recreated in human cell lines. The discovery of iPSCs precipitated a translational science revolution. Physician-scientists sought ways to apply the reprogrammed cells to the pathophysiology of obscure diseases, examination of drug targets, and regeneration of human tissue.24 Tissue regeneration via induced naïve somatic cells has shown promise as a future method to treat neurologic, cardiovascular, and ophthalmologic diseases.25
As the technology of cultivating and identifying optimal sources of iPSCs continues to advance, stem cell–based treatments have evolved as leading prospects in the field of biogerontology.26-29 Although much of the research in antiaging medicine has utilized iPSCs to reprogram cell senescence, the altering of iPSCs at a cellular level also allows for the stimulation of collagen synthesis. This potential for collagen generation may have direct applicability in dermatologic practice, particularly for aesthetic treatments.
Much of the research into iPSC-derived collagen has focused on genodermatoses. Itoh et al30 examined the creation of collagen through iPSCs to identify possible treatments for recessive dystrophic epidermolysis bullosa (DEB). Recessive DEB is characterized by mutations in the COL7A1 gene, which encodes type VII collagen, a basement membrane protein and component of the anchoring fibrils essential for skin integrity.31 Itoh et al30 began with source cells obtained from a skin biopsy. The cells were dedifferentiated to iPSCs and then induced into dermal fibroblasts according to the methods established in prior studies of embryonic stem cells, namely with the use of ascorbic acid and transforming growth factor b. The newly formed fibroblasts were determined to be functional based on their ability to synthesize mature type VII collagen.30 Once the viability of the iPSC-derived fibroblasts was confirmed in vitro, the cells were further tested through combination with human keratinocytes on SCID mice. The human keratinocytes grew together with the iPSC-derived fibroblasts, producing type VII collagen in the basement membrane zone and creating an epidermis with the normal markers.30 Similarly, Robbins et al32 utilized SCID mice to successfully demonstrate that the transfection of keratinocytes from patients with junctional epidermolysis bullosa into SCID mice produced phenotypically normal skin.
Sebastiano et al33 combined the concepts of iPSCs and genome editing in another study of recessive DEB. The investigators first cultured iPSCs from biopsies of affected patients. After deriving iPSCs and correcting their mutation via adenovirus-associated viral gene editing, the COL7A1 mutation-free cells were differentiated into keratinocytes. These iPSC-derived keratinocytes were subsequently grafted onto mice, which led to the production of wild-type collagen VII and a stratified epidermis. Despite this successful outcome, the grafts of iPSC-derived epidermis did not survive longer than 1 month.33
One of the many obstacles facing the practical use of stem cells is their successful incorporation into human tissue. A possible solution was uncovered by Zhang et al34 who examined iPSC-derived MSCs. Mesenchymal stem cells communicate via paracrine mechanisms, whereby exosomes containing RNA and proteins are released to potentiate a regenerative effect.35 Zhang et al34 found that injecting exosomes from human iPSC-derived MSCs into the wound sites of rats stimulated the production of type I collagen, type III collagen, and elastin. The wound sites demonstrated accelerated closure, narrower scar widths, and increased collagen maturity.
Understanding the role that local environment plays in stem cell differentiation, Xu et al36 aimed to create an extracellular scaffold to induce fibroblast behavior from iPSCs. The authors engineered a framework similar to the normal extracellular membrane using proteoglycans, glycosaminoglycans, fibrinogen, and connective tissue growth factor. The iPSCs were then applied to the scaffolding, which led to successful fibroblast differentiation and type I collagen synthesis.36 This use of local biosignaling cues holds important ramifications for controlling the fate of stem cells that have been introduced into a new environment.
Although the application of iPSCs in clinical dermatology has yet to be achieved, progress in the field is moving at a rapid pace. Several logistical elements require further mastery before therapeutics can be delivered. These areas include the optimal environment for iPSC differentiation, methods for maximization of graft survival, and different modes of transplanting iPSC-derived cells into patients. In cosmetic practice, success will depend on intradermal injections of collagen-producing iPSC-derived cells that possess long-term proliferative potential. Current research in mice models has demonstrated viability up to 16 weeks after intradermal injection of such cells.37
Plant Stem Cells
In discussing the dermatologic applications of stem cell technology, clinicians should be aware of the plant stem cell products that have become a popular cosmeceutical trend. Companies advertise plant cells as a natural source of regenerative cells that can induce rejuvenation in human skin; however, there are no significant data to indicate that plant stem cells encourage or activate cellular growth in humans. Indeed, for stem cells to differentiate and produce viable components, the cells must first be incorporated as living components in the host tissue. Because plant stem cells do not survive in human tissue and plant cell cytokines fail to interact with the receptors on human cells, their current value in cosmeceuticals may be overstated.
Platelet-Rich Plasma
Platelet-rich plasma also is commonly associated with stem cell therapy, as PRP is known to potentiate stem cell proliferation, migration, and differentiation. However, PRP does not contain stem cells and is instead autologous plasma concentrated with platelets. In fact, platelets cannot even be classified as cells given that they lack a nucleus; platelets are considered cell fragments. The regenerative potential of PRP can be attributed to the growth factors released from platelets, which play an important role in tissue regeneration and repair. Platelet-rich plasma currently is being used in dermatology for skin rejuvenation (reduction of wrinkles and furrows) and treatment of acne scars.38 There also is evidence supporting the effectiveness of PRP for alopecia and wound therapy, as growth factors play a vital role in hair growth and wound healing.38 Apart from the use of PRP on its own, it can be used as a supplement to enhance the effects of antiaging procedures such as microneedling.39
Future Directions
Multipotent stem cells and iPSCs discussed herein provide much promise in the field of regenerative dermatology. They are increasingly accessible and circumvent the use of ethically questionable embryonic stem cells. Although there is a general consensus on the great potential of stem cells for treating aesthetic skin conditions, high-quality randomized controlled trials remain scarce within the literature. Recognizing and optimizing these opportunities remains the next step in the future delivery of evidence-based care in regenerative dermatology.
- Thomas ED, Lochte HL, Lu WC, et al. Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. N Engl J Med. 1957;257:491-496.
- Ogliari KS, Marinowic D, Brum DE, et al. Stem cells in dermatology. An Bras Dermatol. 2014;89:286-291.
- Xu C, Inokuma MS, Denham J, et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001;19:971-974.
- Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211-228.
- Badiavas EV, Falanga V. Treatment of chronic wounds with bone marrow-derived cells. Arch Dermatol. 2003;139:510-516.
- Ibrahim ZA, Eltatawy RA, Ghaly NR, et al. Autologous bone marrow stem cells in atrophic acne scars: a pilot study. J Dermatolog Treat. 2015;26:260-265.
- Broxmeyer HE, Douglas GW, Hangoc G, et al. Human umbilical cord blood as a potential source of transplantable hematopoietic stem/progenitor cells. Proc Natl Acad Sci U S A. 1989;86:3828-3832.
- Gluckman E, Rocha V, Boyer-Chammard A, et al. Outcome of cord-blood transplantation from related and unrelated donors. Eurocord Transplant Group and the European Blood and Marrow Transplantation Group. N Engl J Med. 1997;337:373-381.
- Valerio IL, Sabino JM, Dearth CL. Plastic surgery challenges in war wounded II: regenerative medicine. Adv Wound Care (New Rochelle). 2016;5:412-419.
- Vescarelli E, D’Amici S, Onesti MG, et al. Adipose-derived stem cell: an innovative therapeutic approach in systemic sclerosis and Parry-Romberg syndrome. CellR4. 2014;2:E791-E797.
- Yoshimura K, Sato K, Aoi N, et al. Cell-assisted lipotransfer for cosmetic breast augmentation: supportive use of adipose-derived stem/stromal cells. Aesthetic Plast Surg. 2008;32:48-55.
- Grabin S, Antes G, Stark GB, et al. Cell-assisted lipotransfer: a critical appraisal of the evidence. Dtsch Arztebl Int. 2015;112:255.
- Zhou Y, Wang J, Li H, et al. Efficacy and safety of cell-assisted lipotransfer: a systematic review and meta-analysis. Plast Reconstr Surg. 2016;137:E44-E57.
- Toma JG, Akhavan M, Fernandes KJL, et al. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol. 2001;3:778-784.
- Toma JG, McKenzie IA, Bagli D, et al. Isolation and characterization of multipotent skin-derived precursors from human skin. Stem Cells. 2005;23:727-737.
- Homicz MR, Watson D. Review of injectable materials for soft tissue augmentation. Facial Plast Surg. 2004;20:21-29.
- Kumar S, Mahajan BB, Kaur S, et al. Autologous therapies in dermatology. J Clin Aesthet Dermatol. 2014;7:38-45.
- Schmidt C. FDA approves first cell therapy for wrinkle-free visage. Nat Biotech. 2011;29:674-675.
- Gentile P, Scioli MG, Bielli A, et al. Stem cells from human hair follicles: first mechanical isolation for immediate autologous clinical use in androgenetic alopecia and hair loss. Stem Cell Investig. 2017;4:58.
- Sugiyama-Nakagiri Y, Akiyama M, Shimizu H. Hair follicle stem cell-targeted gene transfer and reconstitution system. Gene Ther. 2006;13:732-737.
- Heidari F, Yari A, Rasoolijazi H, et al. Bulge hair follicle stem cells accelerate cutaneous wound healing in rats. Wounds. 2016;28:132-141.
- Lee JH, Fisher DE. Melanocyte stem cells as potential therapeutics in skin disorders. Expert Opin Biol Ther. 2014;14:1-11.
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663-676.
- Singh VK, Kalsan M, Kumar N, et al. Induced pluripotent stem cells: applications in regenerative medicine, disease modeling, and drug discovery. Front Cell Dev Biol. 2015;3:2.
- Aoi T. 10th anniversary of iPS cells: The challenges that lie ahead. J Biochem. 2016;160:121-129.
- Lowry WE, Plath K. The many ways to make an iPS cell. Nat Biotechnol. 2008;26:1246-1248.
- Kim K, Doi A, Wen B, et al. Epigenetic memory in induced pluripotent stem cells. Nature. 2010;467:285-290.
- Gafni O, Weinberger L, Mansour AA, et al. Derivation of novel human ground state naive pluripotent stem cells. Nature. 2013;504:282-286.
- Pareja-Galeano H, Sanchis-Gomar F, Pérez LM, et al. IPSCs-based anti-aging therapies: Recent discoveries and future challenges. Ageing Res Rev. 2016;27:37-41.
- Itoh M, Umegaki-Arao N, Guo Z, et al. Generation of 3D skin equivalents fully reconstituted from human induced pluripotent stem cells (iPSCs). PLoS One. 2013;8:e77673.
- Nyström A, Velati D, Mittapalli VR, et al. Collagen VII plays a dual role in wound healing. J Clin Invest. 2013;123:3498-3509.
- Robbins PB, Lin Q, Goodnough JB, et al. In vivo restoration of laminin 5 β3 expression and function in junctional epidermolysis bullosa. Proc Natl Acad Sci. 2001;98:5193-5198.
- Sebastiano V, Zhen HH, Haddad B, et al. Human COL7A1-corrected induced pluripotent stem cells for the treatment of recessive dystrophic epidermolysis bullosa. Sci Transl Med. 2014;6:264ra163.
- Zhang J, Guan J, Niu X, et al. Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J Transl Med. 2015;13:49.
- Pap E, Pállinger É, Pásztói M, et al. Highlights of a new type of intercellular communication: microvesicle-based information transfer. Inflamm Res. 2009;58:1-8.
- Xu R, Taskin MB, Rubert M, et al. hiPS-MSCs differentiation towards fibroblasts on a 3D ECM mimicking scaffold. Sci Rep. 2015;5:8480.
- Wenzel D, Bayerl J, Nyström A, et al. Genetically corrected iPSCs as cell therapy for recessive dystrophic epidermolysis bullosa. Sci Transl Med. 2014;6:264ra165.
- Bednarska K, Kieszek R, Domagała P, et al. The use of platelet-rich-plasma in aesthetic and regenerative medicine. MEDtube Science. 2015;2:8-15.
- Hashim PW, Levy Z, Cohen JL, et al. Microneedling therapy with and without platelet-rich plasma. Cutis. 2017;99:239-242.
Regenerative medicine encompasses innovative therapies that allow the body to repair or regenerate aging cells, tissues, and organs. The skin is a particularly attractive organ for the application of novel regenerative therapies due to its easy accessibility. Among these therapies, stem cells and platelet-rich plasma (PRP) have garnered interest based on their therapeutic potential in scar reduction, antiaging effects, and treatment of alopecia.
Stem cells possess the cardinal features of self-renewal and plasticity. Self-renewal refers to symmetric cell division generating daughter cells identical to the parent cell.1 Plasticity is the ability to generate cell types other than the germ line or tissue lineage from which stem cells derive.2 Stem cells can be categorized according to their differentiation potential. Totipotent stem cells may develop into any primary germ cell layer (ectoderm, mesoderm, endoderm) of the embryo, as well as extraembryonic tissue such as the trophoblast, which gives rise to the placenta. Pluripotent stem cells such as embryonic stem cells have the capacity to differentiate into any derivative of the 3 germ cell layers but have lost their ability to differentiate into the trophoblast.3 Adults lack totipotent or pluripotent cells; they have multipotent or unipotent cells. Multipotent stem cells are able to differentiate into multiple cell types from similar lineages; mesenchymal stem cells (MSCs), for example, can differentiate into adipogenic, osteogenic, chondrogenic, and myogenic cells.4 Unipotent stem cells have the lowest differentiation potential and can only self-regenerate. Herein, we review stem cell sources and their therapeutic potential in aesthetic dermatology.
Multipotent Stem Cells
Multipotent stem cells derived from the bone marrow, umbilical cord, adipose tissue, dermis, or hair follicle bulge have various clinical applications in dermatology. Stem cells from these sources are primarily utilized in an autologous manner in which they are processed outside the body and reintroduced into the donor. Autologous multipotent hematopoietic bone marrow cells were first successfully used for the treatment of chronic wounds and show promise for the treatment of atrophic scars.5,6 However, due to the invasive nature of extracting bone marrow stem cells and their declining number with age, other sources of multipotent stem cells have fallen into favor.
Umbilical cord blood is a source of multipotent hematopoietic stem cells for which surgical intervention is not necessary because they are retrieved after umbilical cord clamping.7 Advantages of sourcing stem cells from umbilical cord blood includes high regenerative power compared to a newborn’s skin and low immunogenicity given that the newborn is immunologically immature.8
Another popular source for autologous stem cells is adipose tissue due to its ease of accessibility and relative abundance. Given that adipose tissue–derived stem cells (ASCs) are capable of differentiating into adipocytes that help maintain volume over time, they are being used for midface contouring, lip augmentation, facial rejuvenation, facial scarring, lipodystrophy, penile girth enhancement, and vaginal augmentation. Adipose tissue–derived stem cells also are capable of differentiating into other types of tissue, including cartilage and bone. Thus, they have been successfully harnessed in the treatment of patients affected by systemic sclerosis and Parry-Romberg syndrome as well in the functional and aesthetic reconstruction of various military combat–related deformities.9,10
Adipose tissue–derived stem cells are commonly harvested from lipoaspirate of the abdomen and are combined with supportive mechanical scaffolds such as hydrogels. Lipoaspirate itself can serve as a scaffold for ASCs. Accordingly, ASCs also are being utilized as a scaffold for autologous fat transfer procedures in an effort to increase the viability of transplanted donor tissue, a process known as cell-assisted lipotransfer (CAL). In CAL, a fraction of the aspirated fat is processed for isolation of ASCs, which are then recombined with the remainder of the aspirated fat prior to grafting.11 However, there is conflicting evidence as to whether CAL leads to improved graft success relative to conventional autologous fat transfer.12,13
The skin also serves as an easily accessible and abundant autologous source of stem cells. A subtype of dermal fibroblasts has been proven to have multipotent potential.14,15 These dermal fibroblasts are harvested from one area of the skin using punch biopsy and are processed and reinjected into another desired area of the skin.16 Autologous human fibroblasts have proven to be effective for the treatment of wrinkles, rhytides, and acne scars.17 In June 2011, the US Food and Drug Administration approved azficel-T, an autologous cellular product created by harvesting fibroblasts from a patient’s own postauricular skin, culture-expanding them in vitro for 3 months, and reinjecting the cells into the desired area of dermis in a series of treatments. This product was the first personalized cell therapy approved by the US Food and Drug Administration for aesthetic uses, specifically for the improvement of nasolabial fold wrinkles.18
In adults, hair follicles contain an area known as the bulge, which is a site rich in epithelial and melanocytic stem cells. Bulge stem cells have the ability to reproduce the interfollicular epidermis, hair follicle structures, and sebaceous glands, and they have been used to construct entirely new hair follicles in an artificial in vivo system.19 Sugiyama-Nakagiri et al20 demonstrated that an entire hair follicle epithelium and interfollicular epidermis can be regenerated using cultured bulge stem cells. The cultured bulge stem cells were mixed with dermal papilla cells from neonatal rat vibrissae and engrafted into a silicone chamber implanted on the backs of severe combined immune deficient (SCID) mice. The grafts exhibited tufts of hair as well as a complete interfollicular epidermis at 4 weeks after transplantation.20 Thus, these bulge stem cells have the potential to treat male androgenic alopecia and female pattern hair loss. Bulge stem cells also have been shown to accelerate wound healing.21 Additionally, autologous melanocytic stem cells located at the hair follicle bulge are effective for treating vitiligo and are being investigated for the treatment of hair graying.22
Induced Pluripotent Stem Cells
Given the ethical concerns that surround the procurement and use of embryonic stem cells, efforts have been made to retrieve pluripotent stem cells from adults. A major breakthrough occurred in 2006 when researchers altered the genes of specialized adult mouse cells to cause dedifferentiation and the return to an embryoniclike stem cell state.23 Mouse somatic cells were reprogrammed through the activation of a combination of transcription factors. The resulting cells were termed induced pluripotent stem cells (iPSCs) and have since been recreated in human cell lines. The discovery of iPSCs precipitated a translational science revolution. Physician-scientists sought ways to apply the reprogrammed cells to the pathophysiology of obscure diseases, examination of drug targets, and regeneration of human tissue.24 Tissue regeneration via induced naïve somatic cells has shown promise as a future method to treat neurologic, cardiovascular, and ophthalmologic diseases.25
As the technology of cultivating and identifying optimal sources of iPSCs continues to advance, stem cell–based treatments have evolved as leading prospects in the field of biogerontology.26-29 Although much of the research in antiaging medicine has utilized iPSCs to reprogram cell senescence, the altering of iPSCs at a cellular level also allows for the stimulation of collagen synthesis. This potential for collagen generation may have direct applicability in dermatologic practice, particularly for aesthetic treatments.
Much of the research into iPSC-derived collagen has focused on genodermatoses. Itoh et al30 examined the creation of collagen through iPSCs to identify possible treatments for recessive dystrophic epidermolysis bullosa (DEB). Recessive DEB is characterized by mutations in the COL7A1 gene, which encodes type VII collagen, a basement membrane protein and component of the anchoring fibrils essential for skin integrity.31 Itoh et al30 began with source cells obtained from a skin biopsy. The cells were dedifferentiated to iPSCs and then induced into dermal fibroblasts according to the methods established in prior studies of embryonic stem cells, namely with the use of ascorbic acid and transforming growth factor b. The newly formed fibroblasts were determined to be functional based on their ability to synthesize mature type VII collagen.30 Once the viability of the iPSC-derived fibroblasts was confirmed in vitro, the cells were further tested through combination with human keratinocytes on SCID mice. The human keratinocytes grew together with the iPSC-derived fibroblasts, producing type VII collagen in the basement membrane zone and creating an epidermis with the normal markers.30 Similarly, Robbins et al32 utilized SCID mice to successfully demonstrate that the transfection of keratinocytes from patients with junctional epidermolysis bullosa into SCID mice produced phenotypically normal skin.
Sebastiano et al33 combined the concepts of iPSCs and genome editing in another study of recessive DEB. The investigators first cultured iPSCs from biopsies of affected patients. After deriving iPSCs and correcting their mutation via adenovirus-associated viral gene editing, the COL7A1 mutation-free cells were differentiated into keratinocytes. These iPSC-derived keratinocytes were subsequently grafted onto mice, which led to the production of wild-type collagen VII and a stratified epidermis. Despite this successful outcome, the grafts of iPSC-derived epidermis did not survive longer than 1 month.33
One of the many obstacles facing the practical use of stem cells is their successful incorporation into human tissue. A possible solution was uncovered by Zhang et al34 who examined iPSC-derived MSCs. Mesenchymal stem cells communicate via paracrine mechanisms, whereby exosomes containing RNA and proteins are released to potentiate a regenerative effect.35 Zhang et al34 found that injecting exosomes from human iPSC-derived MSCs into the wound sites of rats stimulated the production of type I collagen, type III collagen, and elastin. The wound sites demonstrated accelerated closure, narrower scar widths, and increased collagen maturity.
Understanding the role that local environment plays in stem cell differentiation, Xu et al36 aimed to create an extracellular scaffold to induce fibroblast behavior from iPSCs. The authors engineered a framework similar to the normal extracellular membrane using proteoglycans, glycosaminoglycans, fibrinogen, and connective tissue growth factor. The iPSCs were then applied to the scaffolding, which led to successful fibroblast differentiation and type I collagen synthesis.36 This use of local biosignaling cues holds important ramifications for controlling the fate of stem cells that have been introduced into a new environment.
Although the application of iPSCs in clinical dermatology has yet to be achieved, progress in the field is moving at a rapid pace. Several logistical elements require further mastery before therapeutics can be delivered. These areas include the optimal environment for iPSC differentiation, methods for maximization of graft survival, and different modes of transplanting iPSC-derived cells into patients. In cosmetic practice, success will depend on intradermal injections of collagen-producing iPSC-derived cells that possess long-term proliferative potential. Current research in mice models has demonstrated viability up to 16 weeks after intradermal injection of such cells.37
Plant Stem Cells
In discussing the dermatologic applications of stem cell technology, clinicians should be aware of the plant stem cell products that have become a popular cosmeceutical trend. Companies advertise plant cells as a natural source of regenerative cells that can induce rejuvenation in human skin; however, there are no significant data to indicate that plant stem cells encourage or activate cellular growth in humans. Indeed, for stem cells to differentiate and produce viable components, the cells must first be incorporated as living components in the host tissue. Because plant stem cells do not survive in human tissue and plant cell cytokines fail to interact with the receptors on human cells, their current value in cosmeceuticals may be overstated.
Platelet-Rich Plasma
Platelet-rich plasma also is commonly associated with stem cell therapy, as PRP is known to potentiate stem cell proliferation, migration, and differentiation. However, PRP does not contain stem cells and is instead autologous plasma concentrated with platelets. In fact, platelets cannot even be classified as cells given that they lack a nucleus; platelets are considered cell fragments. The regenerative potential of PRP can be attributed to the growth factors released from platelets, which play an important role in tissue regeneration and repair. Platelet-rich plasma currently is being used in dermatology for skin rejuvenation (reduction of wrinkles and furrows) and treatment of acne scars.38 There also is evidence supporting the effectiveness of PRP for alopecia and wound therapy, as growth factors play a vital role in hair growth and wound healing.38 Apart from the use of PRP on its own, it can be used as a supplement to enhance the effects of antiaging procedures such as microneedling.39
Future Directions
Multipotent stem cells and iPSCs discussed herein provide much promise in the field of regenerative dermatology. They are increasingly accessible and circumvent the use of ethically questionable embryonic stem cells. Although there is a general consensus on the great potential of stem cells for treating aesthetic skin conditions, high-quality randomized controlled trials remain scarce within the literature. Recognizing and optimizing these opportunities remains the next step in the future delivery of evidence-based care in regenerative dermatology.
Regenerative medicine encompasses innovative therapies that allow the body to repair or regenerate aging cells, tissues, and organs. The skin is a particularly attractive organ for the application of novel regenerative therapies due to its easy accessibility. Among these therapies, stem cells and platelet-rich plasma (PRP) have garnered interest based on their therapeutic potential in scar reduction, antiaging effects, and treatment of alopecia.
Stem cells possess the cardinal features of self-renewal and plasticity. Self-renewal refers to symmetric cell division generating daughter cells identical to the parent cell.1 Plasticity is the ability to generate cell types other than the germ line or tissue lineage from which stem cells derive.2 Stem cells can be categorized according to their differentiation potential. Totipotent stem cells may develop into any primary germ cell layer (ectoderm, mesoderm, endoderm) of the embryo, as well as extraembryonic tissue such as the trophoblast, which gives rise to the placenta. Pluripotent stem cells such as embryonic stem cells have the capacity to differentiate into any derivative of the 3 germ cell layers but have lost their ability to differentiate into the trophoblast.3 Adults lack totipotent or pluripotent cells; they have multipotent or unipotent cells. Multipotent stem cells are able to differentiate into multiple cell types from similar lineages; mesenchymal stem cells (MSCs), for example, can differentiate into adipogenic, osteogenic, chondrogenic, and myogenic cells.4 Unipotent stem cells have the lowest differentiation potential and can only self-regenerate. Herein, we review stem cell sources and their therapeutic potential in aesthetic dermatology.
Multipotent Stem Cells
Multipotent stem cells derived from the bone marrow, umbilical cord, adipose tissue, dermis, or hair follicle bulge have various clinical applications in dermatology. Stem cells from these sources are primarily utilized in an autologous manner in which they are processed outside the body and reintroduced into the donor. Autologous multipotent hematopoietic bone marrow cells were first successfully used for the treatment of chronic wounds and show promise for the treatment of atrophic scars.5,6 However, due to the invasive nature of extracting bone marrow stem cells and their declining number with age, other sources of multipotent stem cells have fallen into favor.
Umbilical cord blood is a source of multipotent hematopoietic stem cells for which surgical intervention is not necessary because they are retrieved after umbilical cord clamping.7 Advantages of sourcing stem cells from umbilical cord blood includes high regenerative power compared to a newborn’s skin and low immunogenicity given that the newborn is immunologically immature.8
Another popular source for autologous stem cells is adipose tissue due to its ease of accessibility and relative abundance. Given that adipose tissue–derived stem cells (ASCs) are capable of differentiating into adipocytes that help maintain volume over time, they are being used for midface contouring, lip augmentation, facial rejuvenation, facial scarring, lipodystrophy, penile girth enhancement, and vaginal augmentation. Adipose tissue–derived stem cells also are capable of differentiating into other types of tissue, including cartilage and bone. Thus, they have been successfully harnessed in the treatment of patients affected by systemic sclerosis and Parry-Romberg syndrome as well in the functional and aesthetic reconstruction of various military combat–related deformities.9,10
Adipose tissue–derived stem cells are commonly harvested from lipoaspirate of the abdomen and are combined with supportive mechanical scaffolds such as hydrogels. Lipoaspirate itself can serve as a scaffold for ASCs. Accordingly, ASCs also are being utilized as a scaffold for autologous fat transfer procedures in an effort to increase the viability of transplanted donor tissue, a process known as cell-assisted lipotransfer (CAL). In CAL, a fraction of the aspirated fat is processed for isolation of ASCs, which are then recombined with the remainder of the aspirated fat prior to grafting.11 However, there is conflicting evidence as to whether CAL leads to improved graft success relative to conventional autologous fat transfer.12,13
The skin also serves as an easily accessible and abundant autologous source of stem cells. A subtype of dermal fibroblasts has been proven to have multipotent potential.14,15 These dermal fibroblasts are harvested from one area of the skin using punch biopsy and are processed and reinjected into another desired area of the skin.16 Autologous human fibroblasts have proven to be effective for the treatment of wrinkles, rhytides, and acne scars.17 In June 2011, the US Food and Drug Administration approved azficel-T, an autologous cellular product created by harvesting fibroblasts from a patient’s own postauricular skin, culture-expanding them in vitro for 3 months, and reinjecting the cells into the desired area of dermis in a series of treatments. This product was the first personalized cell therapy approved by the US Food and Drug Administration for aesthetic uses, specifically for the improvement of nasolabial fold wrinkles.18
In adults, hair follicles contain an area known as the bulge, which is a site rich in epithelial and melanocytic stem cells. Bulge stem cells have the ability to reproduce the interfollicular epidermis, hair follicle structures, and sebaceous glands, and they have been used to construct entirely new hair follicles in an artificial in vivo system.19 Sugiyama-Nakagiri et al20 demonstrated that an entire hair follicle epithelium and interfollicular epidermis can be regenerated using cultured bulge stem cells. The cultured bulge stem cells were mixed with dermal papilla cells from neonatal rat vibrissae and engrafted into a silicone chamber implanted on the backs of severe combined immune deficient (SCID) mice. The grafts exhibited tufts of hair as well as a complete interfollicular epidermis at 4 weeks after transplantation.20 Thus, these bulge stem cells have the potential to treat male androgenic alopecia and female pattern hair loss. Bulge stem cells also have been shown to accelerate wound healing.21 Additionally, autologous melanocytic stem cells located at the hair follicle bulge are effective for treating vitiligo and are being investigated for the treatment of hair graying.22
Induced Pluripotent Stem Cells
Given the ethical concerns that surround the procurement and use of embryonic stem cells, efforts have been made to retrieve pluripotent stem cells from adults. A major breakthrough occurred in 2006 when researchers altered the genes of specialized adult mouse cells to cause dedifferentiation and the return to an embryoniclike stem cell state.23 Mouse somatic cells were reprogrammed through the activation of a combination of transcription factors. The resulting cells were termed induced pluripotent stem cells (iPSCs) and have since been recreated in human cell lines. The discovery of iPSCs precipitated a translational science revolution. Physician-scientists sought ways to apply the reprogrammed cells to the pathophysiology of obscure diseases, examination of drug targets, and regeneration of human tissue.24 Tissue regeneration via induced naïve somatic cells has shown promise as a future method to treat neurologic, cardiovascular, and ophthalmologic diseases.25
As the technology of cultivating and identifying optimal sources of iPSCs continues to advance, stem cell–based treatments have evolved as leading prospects in the field of biogerontology.26-29 Although much of the research in antiaging medicine has utilized iPSCs to reprogram cell senescence, the altering of iPSCs at a cellular level also allows for the stimulation of collagen synthesis. This potential for collagen generation may have direct applicability in dermatologic practice, particularly for aesthetic treatments.
Much of the research into iPSC-derived collagen has focused on genodermatoses. Itoh et al30 examined the creation of collagen through iPSCs to identify possible treatments for recessive dystrophic epidermolysis bullosa (DEB). Recessive DEB is characterized by mutations in the COL7A1 gene, which encodes type VII collagen, a basement membrane protein and component of the anchoring fibrils essential for skin integrity.31 Itoh et al30 began with source cells obtained from a skin biopsy. The cells were dedifferentiated to iPSCs and then induced into dermal fibroblasts according to the methods established in prior studies of embryonic stem cells, namely with the use of ascorbic acid and transforming growth factor b. The newly formed fibroblasts were determined to be functional based on their ability to synthesize mature type VII collagen.30 Once the viability of the iPSC-derived fibroblasts was confirmed in vitro, the cells were further tested through combination with human keratinocytes on SCID mice. The human keratinocytes grew together with the iPSC-derived fibroblasts, producing type VII collagen in the basement membrane zone and creating an epidermis with the normal markers.30 Similarly, Robbins et al32 utilized SCID mice to successfully demonstrate that the transfection of keratinocytes from patients with junctional epidermolysis bullosa into SCID mice produced phenotypically normal skin.
Sebastiano et al33 combined the concepts of iPSCs and genome editing in another study of recessive DEB. The investigators first cultured iPSCs from biopsies of affected patients. After deriving iPSCs and correcting their mutation via adenovirus-associated viral gene editing, the COL7A1 mutation-free cells were differentiated into keratinocytes. These iPSC-derived keratinocytes were subsequently grafted onto mice, which led to the production of wild-type collagen VII and a stratified epidermis. Despite this successful outcome, the grafts of iPSC-derived epidermis did not survive longer than 1 month.33
One of the many obstacles facing the practical use of stem cells is their successful incorporation into human tissue. A possible solution was uncovered by Zhang et al34 who examined iPSC-derived MSCs. Mesenchymal stem cells communicate via paracrine mechanisms, whereby exosomes containing RNA and proteins are released to potentiate a regenerative effect.35 Zhang et al34 found that injecting exosomes from human iPSC-derived MSCs into the wound sites of rats stimulated the production of type I collagen, type III collagen, and elastin. The wound sites demonstrated accelerated closure, narrower scar widths, and increased collagen maturity.
Understanding the role that local environment plays in stem cell differentiation, Xu et al36 aimed to create an extracellular scaffold to induce fibroblast behavior from iPSCs. The authors engineered a framework similar to the normal extracellular membrane using proteoglycans, glycosaminoglycans, fibrinogen, and connective tissue growth factor. The iPSCs were then applied to the scaffolding, which led to successful fibroblast differentiation and type I collagen synthesis.36 This use of local biosignaling cues holds important ramifications for controlling the fate of stem cells that have been introduced into a new environment.
Although the application of iPSCs in clinical dermatology has yet to be achieved, progress in the field is moving at a rapid pace. Several logistical elements require further mastery before therapeutics can be delivered. These areas include the optimal environment for iPSC differentiation, methods for maximization of graft survival, and different modes of transplanting iPSC-derived cells into patients. In cosmetic practice, success will depend on intradermal injections of collagen-producing iPSC-derived cells that possess long-term proliferative potential. Current research in mice models has demonstrated viability up to 16 weeks after intradermal injection of such cells.37
Plant Stem Cells
In discussing the dermatologic applications of stem cell technology, clinicians should be aware of the plant stem cell products that have become a popular cosmeceutical trend. Companies advertise plant cells as a natural source of regenerative cells that can induce rejuvenation in human skin; however, there are no significant data to indicate that plant stem cells encourage or activate cellular growth in humans. Indeed, for stem cells to differentiate and produce viable components, the cells must first be incorporated as living components in the host tissue. Because plant stem cells do not survive in human tissue and plant cell cytokines fail to interact with the receptors on human cells, their current value in cosmeceuticals may be overstated.
Platelet-Rich Plasma
Platelet-rich plasma also is commonly associated with stem cell therapy, as PRP is known to potentiate stem cell proliferation, migration, and differentiation. However, PRP does not contain stem cells and is instead autologous plasma concentrated with platelets. In fact, platelets cannot even be classified as cells given that they lack a nucleus; platelets are considered cell fragments. The regenerative potential of PRP can be attributed to the growth factors released from platelets, which play an important role in tissue regeneration and repair. Platelet-rich plasma currently is being used in dermatology for skin rejuvenation (reduction of wrinkles and furrows) and treatment of acne scars.38 There also is evidence supporting the effectiveness of PRP for alopecia and wound therapy, as growth factors play a vital role in hair growth and wound healing.38 Apart from the use of PRP on its own, it can be used as a supplement to enhance the effects of antiaging procedures such as microneedling.39
Future Directions
Multipotent stem cells and iPSCs discussed herein provide much promise in the field of regenerative dermatology. They are increasingly accessible and circumvent the use of ethically questionable embryonic stem cells. Although there is a general consensus on the great potential of stem cells for treating aesthetic skin conditions, high-quality randomized controlled trials remain scarce within the literature. Recognizing and optimizing these opportunities remains the next step in the future delivery of evidence-based care in regenerative dermatology.
- Thomas ED, Lochte HL, Lu WC, et al. Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. N Engl J Med. 1957;257:491-496.
- Ogliari KS, Marinowic D, Brum DE, et al. Stem cells in dermatology. An Bras Dermatol. 2014;89:286-291.
- Xu C, Inokuma MS, Denham J, et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001;19:971-974.
- Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211-228.
- Badiavas EV, Falanga V. Treatment of chronic wounds with bone marrow-derived cells. Arch Dermatol. 2003;139:510-516.
- Ibrahim ZA, Eltatawy RA, Ghaly NR, et al. Autologous bone marrow stem cells in atrophic acne scars: a pilot study. J Dermatolog Treat. 2015;26:260-265.
- Broxmeyer HE, Douglas GW, Hangoc G, et al. Human umbilical cord blood as a potential source of transplantable hematopoietic stem/progenitor cells. Proc Natl Acad Sci U S A. 1989;86:3828-3832.
- Gluckman E, Rocha V, Boyer-Chammard A, et al. Outcome of cord-blood transplantation from related and unrelated donors. Eurocord Transplant Group and the European Blood and Marrow Transplantation Group. N Engl J Med. 1997;337:373-381.
- Valerio IL, Sabino JM, Dearth CL. Plastic surgery challenges in war wounded II: regenerative medicine. Adv Wound Care (New Rochelle). 2016;5:412-419.
- Vescarelli E, D’Amici S, Onesti MG, et al. Adipose-derived stem cell: an innovative therapeutic approach in systemic sclerosis and Parry-Romberg syndrome. CellR4. 2014;2:E791-E797.
- Yoshimura K, Sato K, Aoi N, et al. Cell-assisted lipotransfer for cosmetic breast augmentation: supportive use of adipose-derived stem/stromal cells. Aesthetic Plast Surg. 2008;32:48-55.
- Grabin S, Antes G, Stark GB, et al. Cell-assisted lipotransfer: a critical appraisal of the evidence. Dtsch Arztebl Int. 2015;112:255.
- Zhou Y, Wang J, Li H, et al. Efficacy and safety of cell-assisted lipotransfer: a systematic review and meta-analysis. Plast Reconstr Surg. 2016;137:E44-E57.
- Toma JG, Akhavan M, Fernandes KJL, et al. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol. 2001;3:778-784.
- Toma JG, McKenzie IA, Bagli D, et al. Isolation and characterization of multipotent skin-derived precursors from human skin. Stem Cells. 2005;23:727-737.
- Homicz MR, Watson D. Review of injectable materials for soft tissue augmentation. Facial Plast Surg. 2004;20:21-29.
- Kumar S, Mahajan BB, Kaur S, et al. Autologous therapies in dermatology. J Clin Aesthet Dermatol. 2014;7:38-45.
- Schmidt C. FDA approves first cell therapy for wrinkle-free visage. Nat Biotech. 2011;29:674-675.
- Gentile P, Scioli MG, Bielli A, et al. Stem cells from human hair follicles: first mechanical isolation for immediate autologous clinical use in androgenetic alopecia and hair loss. Stem Cell Investig. 2017;4:58.
- Sugiyama-Nakagiri Y, Akiyama M, Shimizu H. Hair follicle stem cell-targeted gene transfer and reconstitution system. Gene Ther. 2006;13:732-737.
- Heidari F, Yari A, Rasoolijazi H, et al. Bulge hair follicle stem cells accelerate cutaneous wound healing in rats. Wounds. 2016;28:132-141.
- Lee JH, Fisher DE. Melanocyte stem cells as potential therapeutics in skin disorders. Expert Opin Biol Ther. 2014;14:1-11.
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663-676.
- Singh VK, Kalsan M, Kumar N, et al. Induced pluripotent stem cells: applications in regenerative medicine, disease modeling, and drug discovery. Front Cell Dev Biol. 2015;3:2.
- Aoi T. 10th anniversary of iPS cells: The challenges that lie ahead. J Biochem. 2016;160:121-129.
- Lowry WE, Plath K. The many ways to make an iPS cell. Nat Biotechnol. 2008;26:1246-1248.
- Kim K, Doi A, Wen B, et al. Epigenetic memory in induced pluripotent stem cells. Nature. 2010;467:285-290.
- Gafni O, Weinberger L, Mansour AA, et al. Derivation of novel human ground state naive pluripotent stem cells. Nature. 2013;504:282-286.
- Pareja-Galeano H, Sanchis-Gomar F, Pérez LM, et al. IPSCs-based anti-aging therapies: Recent discoveries and future challenges. Ageing Res Rev. 2016;27:37-41.
- Itoh M, Umegaki-Arao N, Guo Z, et al. Generation of 3D skin equivalents fully reconstituted from human induced pluripotent stem cells (iPSCs). PLoS One. 2013;8:e77673.
- Nyström A, Velati D, Mittapalli VR, et al. Collagen VII plays a dual role in wound healing. J Clin Invest. 2013;123:3498-3509.
- Robbins PB, Lin Q, Goodnough JB, et al. In vivo restoration of laminin 5 β3 expression and function in junctional epidermolysis bullosa. Proc Natl Acad Sci. 2001;98:5193-5198.
- Sebastiano V, Zhen HH, Haddad B, et al. Human COL7A1-corrected induced pluripotent stem cells for the treatment of recessive dystrophic epidermolysis bullosa. Sci Transl Med. 2014;6:264ra163.
- Zhang J, Guan J, Niu X, et al. Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J Transl Med. 2015;13:49.
- Pap E, Pállinger É, Pásztói M, et al. Highlights of a new type of intercellular communication: microvesicle-based information transfer. Inflamm Res. 2009;58:1-8.
- Xu R, Taskin MB, Rubert M, et al. hiPS-MSCs differentiation towards fibroblasts on a 3D ECM mimicking scaffold. Sci Rep. 2015;5:8480.
- Wenzel D, Bayerl J, Nyström A, et al. Genetically corrected iPSCs as cell therapy for recessive dystrophic epidermolysis bullosa. Sci Transl Med. 2014;6:264ra165.
- Bednarska K, Kieszek R, Domagała P, et al. The use of platelet-rich-plasma in aesthetic and regenerative medicine. MEDtube Science. 2015;2:8-15.
- Hashim PW, Levy Z, Cohen JL, et al. Microneedling therapy with and without platelet-rich plasma. Cutis. 2017;99:239-242.
- Thomas ED, Lochte HL, Lu WC, et al. Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. N Engl J Med. 1957;257:491-496.
- Ogliari KS, Marinowic D, Brum DE, et al. Stem cells in dermatology. An Bras Dermatol. 2014;89:286-291.
- Xu C, Inokuma MS, Denham J, et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001;19:971-974.
- Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211-228.
- Badiavas EV, Falanga V. Treatment of chronic wounds with bone marrow-derived cells. Arch Dermatol. 2003;139:510-516.
- Ibrahim ZA, Eltatawy RA, Ghaly NR, et al. Autologous bone marrow stem cells in atrophic acne scars: a pilot study. J Dermatolog Treat. 2015;26:260-265.
- Broxmeyer HE, Douglas GW, Hangoc G, et al. Human umbilical cord blood as a potential source of transplantable hematopoietic stem/progenitor cells. Proc Natl Acad Sci U S A. 1989;86:3828-3832.
- Gluckman E, Rocha V, Boyer-Chammard A, et al. Outcome of cord-blood transplantation from related and unrelated donors. Eurocord Transplant Group and the European Blood and Marrow Transplantation Group. N Engl J Med. 1997;337:373-381.
- Valerio IL, Sabino JM, Dearth CL. Plastic surgery challenges in war wounded II: regenerative medicine. Adv Wound Care (New Rochelle). 2016;5:412-419.
- Vescarelli E, D’Amici S, Onesti MG, et al. Adipose-derived stem cell: an innovative therapeutic approach in systemic sclerosis and Parry-Romberg syndrome. CellR4. 2014;2:E791-E797.
- Yoshimura K, Sato K, Aoi N, et al. Cell-assisted lipotransfer for cosmetic breast augmentation: supportive use of adipose-derived stem/stromal cells. Aesthetic Plast Surg. 2008;32:48-55.
- Grabin S, Antes G, Stark GB, et al. Cell-assisted lipotransfer: a critical appraisal of the evidence. Dtsch Arztebl Int. 2015;112:255.
- Zhou Y, Wang J, Li H, et al. Efficacy and safety of cell-assisted lipotransfer: a systematic review and meta-analysis. Plast Reconstr Surg. 2016;137:E44-E57.
- Toma JG, Akhavan M, Fernandes KJL, et al. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol. 2001;3:778-784.
- Toma JG, McKenzie IA, Bagli D, et al. Isolation and characterization of multipotent skin-derived precursors from human skin. Stem Cells. 2005;23:727-737.
- Homicz MR, Watson D. Review of injectable materials for soft tissue augmentation. Facial Plast Surg. 2004;20:21-29.
- Kumar S, Mahajan BB, Kaur S, et al. Autologous therapies in dermatology. J Clin Aesthet Dermatol. 2014;7:38-45.
- Schmidt C. FDA approves first cell therapy for wrinkle-free visage. Nat Biotech. 2011;29:674-675.
- Gentile P, Scioli MG, Bielli A, et al. Stem cells from human hair follicles: first mechanical isolation for immediate autologous clinical use in androgenetic alopecia and hair loss. Stem Cell Investig. 2017;4:58.
- Sugiyama-Nakagiri Y, Akiyama M, Shimizu H. Hair follicle stem cell-targeted gene transfer and reconstitution system. Gene Ther. 2006;13:732-737.
- Heidari F, Yari A, Rasoolijazi H, et al. Bulge hair follicle stem cells accelerate cutaneous wound healing in rats. Wounds. 2016;28:132-141.
- Lee JH, Fisher DE. Melanocyte stem cells as potential therapeutics in skin disorders. Expert Opin Biol Ther. 2014;14:1-11.
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663-676.
- Singh VK, Kalsan M, Kumar N, et al. Induced pluripotent stem cells: applications in regenerative medicine, disease modeling, and drug discovery. Front Cell Dev Biol. 2015;3:2.
- Aoi T. 10th anniversary of iPS cells: The challenges that lie ahead. J Biochem. 2016;160:121-129.
- Lowry WE, Plath K. The many ways to make an iPS cell. Nat Biotechnol. 2008;26:1246-1248.
- Kim K, Doi A, Wen B, et al. Epigenetic memory in induced pluripotent stem cells. Nature. 2010;467:285-290.
- Gafni O, Weinberger L, Mansour AA, et al. Derivation of novel human ground state naive pluripotent stem cells. Nature. 2013;504:282-286.
- Pareja-Galeano H, Sanchis-Gomar F, Pérez LM, et al. IPSCs-based anti-aging therapies: Recent discoveries and future challenges. Ageing Res Rev. 2016;27:37-41.
- Itoh M, Umegaki-Arao N, Guo Z, et al. Generation of 3D skin equivalents fully reconstituted from human induced pluripotent stem cells (iPSCs). PLoS One. 2013;8:e77673.
- Nyström A, Velati D, Mittapalli VR, et al. Collagen VII plays a dual role in wound healing. J Clin Invest. 2013;123:3498-3509.
- Robbins PB, Lin Q, Goodnough JB, et al. In vivo restoration of laminin 5 β3 expression and function in junctional epidermolysis bullosa. Proc Natl Acad Sci. 2001;98:5193-5198.
- Sebastiano V, Zhen HH, Haddad B, et al. Human COL7A1-corrected induced pluripotent stem cells for the treatment of recessive dystrophic epidermolysis bullosa. Sci Transl Med. 2014;6:264ra163.
- Zhang J, Guan J, Niu X, et al. Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J Transl Med. 2015;13:49.
- Pap E, Pállinger É, Pásztói M, et al. Highlights of a new type of intercellular communication: microvesicle-based information transfer. Inflamm Res. 2009;58:1-8.
- Xu R, Taskin MB, Rubert M, et al. hiPS-MSCs differentiation towards fibroblasts on a 3D ECM mimicking scaffold. Sci Rep. 2015;5:8480.
- Wenzel D, Bayerl J, Nyström A, et al. Genetically corrected iPSCs as cell therapy for recessive dystrophic epidermolysis bullosa. Sci Transl Med. 2014;6:264ra165.
- Bednarska K, Kieszek R, Domagała P, et al. The use of platelet-rich-plasma in aesthetic and regenerative medicine. MEDtube Science. 2015;2:8-15.
- Hashim PW, Levy Z, Cohen JL, et al. Microneedling therapy with and without platelet-rich plasma. Cutis. 2017;99:239-242.
Practice Points
- Multipotent stem cells derived from the bone marrow, umbilical cord, adipose tissue, dermis, and hair follicle bulge show promise in tissue regeneration for various dermatologic conditions and aesthetic applications.
- Induced pluripotent stem cells, progenitor cells that result from the dedifferentiation of specialized adult cells, have potential for collagen generation.
FDA approves topical antibiotic for impetigo infections
The Food and Drug Administration has approved in patients aged 2 months or older.
This is the first topical treatment for impetigo to be approved in more than 10 years, according to the press release from the manufacturer, Medimetriks Pharmaceuticals.
Ozenoxacin is a quinolone antimicrobial. The prescribing information is available on the FDA website.
The Food and Drug Administration has approved in patients aged 2 months or older.
This is the first topical treatment for impetigo to be approved in more than 10 years, according to the press release from the manufacturer, Medimetriks Pharmaceuticals.
Ozenoxacin is a quinolone antimicrobial. The prescribing information is available on the FDA website.
The Food and Drug Administration has approved in patients aged 2 months or older.
This is the first topical treatment for impetigo to be approved in more than 10 years, according to the press release from the manufacturer, Medimetriks Pharmaceuticals.
Ozenoxacin is a quinolone antimicrobial. The prescribing information is available on the FDA website.