User login
Lp(a) molar concentration flags CVD, diabetes risk
Lipoprotein(a) molar concentration, rather than apolipoprotein(a) size, appears to be the factor that drives lipoprotein(a)-based cardiovascular disease, according to research published in the Journal of the American College of Cardiology.
The causal association between lipoprotein(a), or Lp(a), and cardiovascular disease has been previously established, but exactly what attribute of Lp(a) is related to cardiovascular risk is not known, Daniel F. Gudbjartsson, PhD, of deCODE genetics and the University of Iceland in Reykjavik, and colleagues wrote in their study. The researchers set out to determine whether Lp(a) molar concentration or apolipoprotein(a), or apo(a), size affects cardiovascular risk. In addition, Dr. Gudbjartsson and colleagues examined the relationship between Lp(a) and type 2 diabetes. While low levels of Lp(a) have been linked to type 2 diabetes, the researchers sought to examine whether low Lp(a) molar concentration levels were also associated with type 2 diabetes risk.
“With Lp(a)-lowering drugs being developed, it is important to understand which attributes of Lp(a) best capture the cardiovascular risk and the consequences of Lp(a) lowering,” noted Dr. Gudbjartsson and colleagues.
Using Mendelian randomization, the researchers assessed Lp(a) molar concentration and kringle IV type 2 (KIV-2) repeat sequence variants to determine a causal relationship between both variants and disease risk. Lp(a) molar concentration serum samples were measured using particle-enhanced turbidimetric immunoassay, while KIV-2 repeats were genotyped with real-time polymerase chain reaction.
Overall, 143,087 participants from Iceland had their genetic information analyzed; of these, 17,715 participants had coronary artery disease, and 8,734 had type 2 diabetes. Lp(a) molecular concentration was analyzed in 12,137 participants and genetically imputed into 130,950 Icelanders, and KIV-2 repeats were estimated in 22,771 Icelanders and genetically imputed into 120,316 Icelanders.
Dr. Gudbjartsson and colleagues found there was a dose-dependent association between Lp(a) molar concentration and risk of coronary artery disease (CAD), peripheral artery disease, aortic valve stenosis, heart failure, and lifespan. In participants in whom Lp(a) molar concentration was at the 79th percentile (50 units of molarity [nM]), the odds ratio was 1.11, and for those in the 99th percentile (250 nM), there was an odds ratio of 2.01 when compared with participants with a median Lp(a) molar concentration of 14 nM. “Lp(a) molar concentration fully explained the Lp(a) association with CAD, and there was no residual association with apo(a) size,” the researchers said.
Participants who were not at increased risk for CAD included those with few KIV-2 repeats and participants with the splice variant G4925A. “This suggested that risk prediction based on Lp(a) should only depend on molar concentration and that treatment of Lp(a) should focus on lowering the molar concentration in subjects with high Lp(a) levels, regardless of the apo(a) size distribution,” Dr. Gudbjartsson and colleagues wrote.
Among participants with type 2 diabetes examined, the 10% of participants with Lp(a) molar concentrations of less than 3.5 nM were at the highest risk of developing type 2 diabetes.
In an accompanying editorial, Benoit J. Arsenault, PhD, of the Quebec Heart and Lung Institute, said that the findings of an association between Lp(a) concentration and atherosclerotic cardiovascular diseases (ASCVD) from Gudbjartsson et al. are important, particularly if they can be replicated in more diverse populations (doi: 10.1016/j.jacc.2019.06.083). “Investigating the association between Lp(a) levels, apo(a) isoform size, and ASCVD risk in different populations is important because the distribution of Lp(a) levels appears to be different across ethnic groups,” he said.
Despite the link between absolute Lp(a) concentrations and cardiovascular disease, cardiovascular outcome trials will need be conducted, Dr. Arsenault noted.
“In the post-statin and post-genomic era, finding much needed therapeutic targets for residual cardiovascular risk can be compared to a gold-digging expedition. Like a map to the location of the gold, GWAS [genome-wide association studies] and Mendelian randomization studies are consistently pointing us in the direction of Lp(a),” he said. “It is time to coordinate our efforts to dig where the map told us, to see once and for all if we will find the golden target of residual cardiovascular risk that we are hoping for and to give hope to high-risk patients with elevated Lp(a) levels.”
Dr. Gudbjartsson and 19 other authors reported being employees of deCODE genetics, owned by Amgen, which is developing Lp(a)-lowering drugs related to the study findings. The other authors reported no relevant conflict of interest. Dr. Arsenault reported being supported by the Fonds de recherche du Québec: Santé and the Canadian Institutes of Health Research; has received research funding from Pfizer, Merck, and Ionis; and was a former consultant for Pfizer and Novartis.
SOURCE: Gudbjartsson DF et al. J Am Coll Cardiol. 2019. doi: 10.1016/j.jacc.2019.10.019.
Lipoprotein(a) molar concentration, rather than apolipoprotein(a) size, appears to be the factor that drives lipoprotein(a)-based cardiovascular disease, according to research published in the Journal of the American College of Cardiology.
The causal association between lipoprotein(a), or Lp(a), and cardiovascular disease has been previously established, but exactly what attribute of Lp(a) is related to cardiovascular risk is not known, Daniel F. Gudbjartsson, PhD, of deCODE genetics and the University of Iceland in Reykjavik, and colleagues wrote in their study. The researchers set out to determine whether Lp(a) molar concentration or apolipoprotein(a), or apo(a), size affects cardiovascular risk. In addition, Dr. Gudbjartsson and colleagues examined the relationship between Lp(a) and type 2 diabetes. While low levels of Lp(a) have been linked to type 2 diabetes, the researchers sought to examine whether low Lp(a) molar concentration levels were also associated with type 2 diabetes risk.
“With Lp(a)-lowering drugs being developed, it is important to understand which attributes of Lp(a) best capture the cardiovascular risk and the consequences of Lp(a) lowering,” noted Dr. Gudbjartsson and colleagues.
Using Mendelian randomization, the researchers assessed Lp(a) molar concentration and kringle IV type 2 (KIV-2) repeat sequence variants to determine a causal relationship between both variants and disease risk. Lp(a) molar concentration serum samples were measured using particle-enhanced turbidimetric immunoassay, while KIV-2 repeats were genotyped with real-time polymerase chain reaction.
Overall, 143,087 participants from Iceland had their genetic information analyzed; of these, 17,715 participants had coronary artery disease, and 8,734 had type 2 diabetes. Lp(a) molecular concentration was analyzed in 12,137 participants and genetically imputed into 130,950 Icelanders, and KIV-2 repeats were estimated in 22,771 Icelanders and genetically imputed into 120,316 Icelanders.
Dr. Gudbjartsson and colleagues found there was a dose-dependent association between Lp(a) molar concentration and risk of coronary artery disease (CAD), peripheral artery disease, aortic valve stenosis, heart failure, and lifespan. In participants in whom Lp(a) molar concentration was at the 79th percentile (50 units of molarity [nM]), the odds ratio was 1.11, and for those in the 99th percentile (250 nM), there was an odds ratio of 2.01 when compared with participants with a median Lp(a) molar concentration of 14 nM. “Lp(a) molar concentration fully explained the Lp(a) association with CAD, and there was no residual association with apo(a) size,” the researchers said.
Participants who were not at increased risk for CAD included those with few KIV-2 repeats and participants with the splice variant G4925A. “This suggested that risk prediction based on Lp(a) should only depend on molar concentration and that treatment of Lp(a) should focus on lowering the molar concentration in subjects with high Lp(a) levels, regardless of the apo(a) size distribution,” Dr. Gudbjartsson and colleagues wrote.
Among participants with type 2 diabetes examined, the 10% of participants with Lp(a) molar concentrations of less than 3.5 nM were at the highest risk of developing type 2 diabetes.
In an accompanying editorial, Benoit J. Arsenault, PhD, of the Quebec Heart and Lung Institute, said that the findings of an association between Lp(a) concentration and atherosclerotic cardiovascular diseases (ASCVD) from Gudbjartsson et al. are important, particularly if they can be replicated in more diverse populations (doi: 10.1016/j.jacc.2019.06.083). “Investigating the association between Lp(a) levels, apo(a) isoform size, and ASCVD risk in different populations is important because the distribution of Lp(a) levels appears to be different across ethnic groups,” he said.
Despite the link between absolute Lp(a) concentrations and cardiovascular disease, cardiovascular outcome trials will need be conducted, Dr. Arsenault noted.
“In the post-statin and post-genomic era, finding much needed therapeutic targets for residual cardiovascular risk can be compared to a gold-digging expedition. Like a map to the location of the gold, GWAS [genome-wide association studies] and Mendelian randomization studies are consistently pointing us in the direction of Lp(a),” he said. “It is time to coordinate our efforts to dig where the map told us, to see once and for all if we will find the golden target of residual cardiovascular risk that we are hoping for and to give hope to high-risk patients with elevated Lp(a) levels.”
Dr. Gudbjartsson and 19 other authors reported being employees of deCODE genetics, owned by Amgen, which is developing Lp(a)-lowering drugs related to the study findings. The other authors reported no relevant conflict of interest. Dr. Arsenault reported being supported by the Fonds de recherche du Québec: Santé and the Canadian Institutes of Health Research; has received research funding from Pfizer, Merck, and Ionis; and was a former consultant for Pfizer and Novartis.
SOURCE: Gudbjartsson DF et al. J Am Coll Cardiol. 2019. doi: 10.1016/j.jacc.2019.10.019.
Lipoprotein(a) molar concentration, rather than apolipoprotein(a) size, appears to be the factor that drives lipoprotein(a)-based cardiovascular disease, according to research published in the Journal of the American College of Cardiology.
The causal association between lipoprotein(a), or Lp(a), and cardiovascular disease has been previously established, but exactly what attribute of Lp(a) is related to cardiovascular risk is not known, Daniel F. Gudbjartsson, PhD, of deCODE genetics and the University of Iceland in Reykjavik, and colleagues wrote in their study. The researchers set out to determine whether Lp(a) molar concentration or apolipoprotein(a), or apo(a), size affects cardiovascular risk. In addition, Dr. Gudbjartsson and colleagues examined the relationship between Lp(a) and type 2 diabetes. While low levels of Lp(a) have been linked to type 2 diabetes, the researchers sought to examine whether low Lp(a) molar concentration levels were also associated with type 2 diabetes risk.
“With Lp(a)-lowering drugs being developed, it is important to understand which attributes of Lp(a) best capture the cardiovascular risk and the consequences of Lp(a) lowering,” noted Dr. Gudbjartsson and colleagues.
Using Mendelian randomization, the researchers assessed Lp(a) molar concentration and kringle IV type 2 (KIV-2) repeat sequence variants to determine a causal relationship between both variants and disease risk. Lp(a) molar concentration serum samples were measured using particle-enhanced turbidimetric immunoassay, while KIV-2 repeats were genotyped with real-time polymerase chain reaction.
Overall, 143,087 participants from Iceland had their genetic information analyzed; of these, 17,715 participants had coronary artery disease, and 8,734 had type 2 diabetes. Lp(a) molecular concentration was analyzed in 12,137 participants and genetically imputed into 130,950 Icelanders, and KIV-2 repeats were estimated in 22,771 Icelanders and genetically imputed into 120,316 Icelanders.
Dr. Gudbjartsson and colleagues found there was a dose-dependent association between Lp(a) molar concentration and risk of coronary artery disease (CAD), peripheral artery disease, aortic valve stenosis, heart failure, and lifespan. In participants in whom Lp(a) molar concentration was at the 79th percentile (50 units of molarity [nM]), the odds ratio was 1.11, and for those in the 99th percentile (250 nM), there was an odds ratio of 2.01 when compared with participants with a median Lp(a) molar concentration of 14 nM. “Lp(a) molar concentration fully explained the Lp(a) association with CAD, and there was no residual association with apo(a) size,” the researchers said.
Participants who were not at increased risk for CAD included those with few KIV-2 repeats and participants with the splice variant G4925A. “This suggested that risk prediction based on Lp(a) should only depend on molar concentration and that treatment of Lp(a) should focus on lowering the molar concentration in subjects with high Lp(a) levels, regardless of the apo(a) size distribution,” Dr. Gudbjartsson and colleagues wrote.
Among participants with type 2 diabetes examined, the 10% of participants with Lp(a) molar concentrations of less than 3.5 nM were at the highest risk of developing type 2 diabetes.
In an accompanying editorial, Benoit J. Arsenault, PhD, of the Quebec Heart and Lung Institute, said that the findings of an association between Lp(a) concentration and atherosclerotic cardiovascular diseases (ASCVD) from Gudbjartsson et al. are important, particularly if they can be replicated in more diverse populations (doi: 10.1016/j.jacc.2019.06.083). “Investigating the association between Lp(a) levels, apo(a) isoform size, and ASCVD risk in different populations is important because the distribution of Lp(a) levels appears to be different across ethnic groups,” he said.
Despite the link between absolute Lp(a) concentrations and cardiovascular disease, cardiovascular outcome trials will need be conducted, Dr. Arsenault noted.
“In the post-statin and post-genomic era, finding much needed therapeutic targets for residual cardiovascular risk can be compared to a gold-digging expedition. Like a map to the location of the gold, GWAS [genome-wide association studies] and Mendelian randomization studies are consistently pointing us in the direction of Lp(a),” he said. “It is time to coordinate our efforts to dig where the map told us, to see once and for all if we will find the golden target of residual cardiovascular risk that we are hoping for and to give hope to high-risk patients with elevated Lp(a) levels.”
Dr. Gudbjartsson and 19 other authors reported being employees of deCODE genetics, owned by Amgen, which is developing Lp(a)-lowering drugs related to the study findings. The other authors reported no relevant conflict of interest. Dr. Arsenault reported being supported by the Fonds de recherche du Québec: Santé and the Canadian Institutes of Health Research; has received research funding from Pfizer, Merck, and Ionis; and was a former consultant for Pfizer and Novartis.
SOURCE: Gudbjartsson DF et al. J Am Coll Cardiol. 2019. doi: 10.1016/j.jacc.2019.10.019.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Key clinical point:
Major finding: There was a dose-dependent association between Lp(a) molar concentration and risk of coronary artery disease (CAD), peripheral artery disease, aortic valve stenosis, heart failure, and lifespan.
Study details: A case-control study of genetic information from 143,087 Icelandic participants.
Disclosures: Dr. Gudbjartsson and 19 other authors reported being employees of deCODE genetics, owned by Amgen, which is developing Lp(a)-lowering drugs related to the study findings. The other authors reported no relevant conflict of interest. Dr. Arsenault reported being supported by the Fonds de recherche du Québec: Santé and the Canadian Institutes of Health Research; has received research funding from Pfizer, Merck, and Ionis; and was a former consultant for Pfizer and Novartis.
Source: Gudbjartsson DF et al. J Am Coll Cardiol. 2019. doi: 10.1016/j.jacc.2019.10.019.
Perioperative antirheumatic drug use does not impact postsurgery infection rate in RA patients
ATLANTA – Patients with rheumatoid arthritis were more at risk of postoperative infection because of a high Charlson Comorbidity Index or longer surgery time than because of perioperative use of antirheumatic medications, according to a presentation at the annual meeting of the American College of Rheumatology.
Anna Shmagel, MD, of the University of Minnesota in Minneapolis and colleagues performed a retrospective cohort study of 154 patients with seropositive RA who were in the Fairview Health System between Jan. 2010 and Dec. 2017 and underwent either orthopedic or major organ surgery. The patients were classified based on their use of disease-modifying antirheumatic drugs (DMARDs) and biologics alone or in combination, with patients divided into “no DMARD or biologic,” “DMARD but no biologic” and “biologic with or without DMARD” groups.
The question of whether to discontinue antirheumatic medications before surgery is still controversial, with conflicting evidence across studies, Dr. Shmagel said in her presentation. A study by Giles and colleagues found 10 of 91 patients (11%) RA who underwent an orthopedic surgical procedure developed a postoperative infection, with patients receiving tumor necrosis factor (TNF) inhibitors more likely to develop an infection, compared with patients who were not receiving TNF inhibitors (Arthritis Care Res. 2006. doi: 10.1002/art.21841).
However, other studies have challenged that idea, and a 2018 study from Goodman and colleagues raised the issue of whether patients stopping biologics prior to surgery are at increased risk of flares. Of 120 RA patients in their study who underwent total hip or total knee arthroplasty, 75% of patients flared at 6 weeks after surgery. While patients who halted biologics before surgery were more likely to flare, stopping biologics did not predict flaring after surgery (J Rheumatol. 2018. doi: 10.3899/jrheum.170366).
“It’s not entirely clear whether these theories are related to what we do with antirheumatic medications, but we felt that it was pertinent to further study this question.” Dr. Shmagel said.
Dr. Shmagel and colleagues examined the 30-day infection rate of RA patients postoperatively, with 30-day readmission and 30-day mortality rates as secondary outcomes. Patient-associated factors such as age, gender, race, body mass index, smoking status, Charlson Comorbidity Index, income, and use of corticosteroids were analyzed as covariates in addition to factors involving surgery such as expected surgery time, perioperative antibiotic use, and whether the procedure was elective or emergency surgery.
A majority of the patients in the study across all groups were white women about 63 years old with a body mass index above 30 kg/m2 and almost all undergoing electing surgery compared with emergency surgery. While patients in each group were similar with regard to Charlson Comorbidity Index, expected length of surgery, and percentage of patients undergoing elective surgery, patients in the biologic with or without DMARD group had a significantly lower median income level compared with those in the other two groups (P = .01).
Overall, there were 244 surgeries in 154 patients, with 117 surgeries in the group not receiving biologics or DMARDs, 95 surgeries in the group receiving DMARDs but no biologics, and 32 surgeries in the biologics with or without DMARD group. In the DMARD but no biologics group, most patients were receiving methotrexate (45%) or hydroxychloroquine (44%), while the most common biologics in the biologics with or without DMARD group were infliximab (25%), tocilizumab (19%), abatacept (16%), etanercept (13%), rituximab (9%), and tofacitinib (9%).
There was an 11% overall rate of infection, with a similar rate of infection across all groups (P = .09). While there was a higher rate of surgical site infections among patients in the biologics with or without DMARD group (9%) and a higher percentage of urinary tract infections in the no DMARD and no biologics group (4%), the results were not statistically significant. When the rate of infections was examined by type of surgery, there were no significant differences between infections from musculoskeletal surgery (P = .7) and major organ surgery (P = .8).
The overall 30-day readmission rate was 12%, but there were no statistically significant differences between groups. Although there were five deaths in the study, four deaths were in the group not receiving DMARDs or biologics, and one death was in the biologic with or without DMARD group.
Higher Charlson Comorbidity Index did predict infection risk, with an odds ratio of 1.37 per 1-point increase in the index (95% confidence interval, 1.10-1.70). Length of surgery also increased the risk of infection, with an OR of 1.16 per 15-minute increase in surgery time (95% CI, 1.09-1.23).
Dr. Shmagel noted that the retrospective nature of the study and the midwestern cohort may mean the results are not generalizable to other populations and that larger randomized trials should be considered. “Certainly, a larger study with more events would be needed,” she said.
This study was funded by the University of Minnesota. Dr. Shmagel reported no relevant conflicts of interest.
SOURCE: Kerski M et al. Arthritis Rheumatol. 2019;71 (suppl 10), Abstract 1805.
ATLANTA – Patients with rheumatoid arthritis were more at risk of postoperative infection because of a high Charlson Comorbidity Index or longer surgery time than because of perioperative use of antirheumatic medications, according to a presentation at the annual meeting of the American College of Rheumatology.
Anna Shmagel, MD, of the University of Minnesota in Minneapolis and colleagues performed a retrospective cohort study of 154 patients with seropositive RA who were in the Fairview Health System between Jan. 2010 and Dec. 2017 and underwent either orthopedic or major organ surgery. The patients were classified based on their use of disease-modifying antirheumatic drugs (DMARDs) and biologics alone or in combination, with patients divided into “no DMARD or biologic,” “DMARD but no biologic” and “biologic with or without DMARD” groups.
The question of whether to discontinue antirheumatic medications before surgery is still controversial, with conflicting evidence across studies, Dr. Shmagel said in her presentation. A study by Giles and colleagues found 10 of 91 patients (11%) RA who underwent an orthopedic surgical procedure developed a postoperative infection, with patients receiving tumor necrosis factor (TNF) inhibitors more likely to develop an infection, compared with patients who were not receiving TNF inhibitors (Arthritis Care Res. 2006. doi: 10.1002/art.21841).
However, other studies have challenged that idea, and a 2018 study from Goodman and colleagues raised the issue of whether patients stopping biologics prior to surgery are at increased risk of flares. Of 120 RA patients in their study who underwent total hip or total knee arthroplasty, 75% of patients flared at 6 weeks after surgery. While patients who halted biologics before surgery were more likely to flare, stopping biologics did not predict flaring after surgery (J Rheumatol. 2018. doi: 10.3899/jrheum.170366).
“It’s not entirely clear whether these theories are related to what we do with antirheumatic medications, but we felt that it was pertinent to further study this question.” Dr. Shmagel said.
Dr. Shmagel and colleagues examined the 30-day infection rate of RA patients postoperatively, with 30-day readmission and 30-day mortality rates as secondary outcomes. Patient-associated factors such as age, gender, race, body mass index, smoking status, Charlson Comorbidity Index, income, and use of corticosteroids were analyzed as covariates in addition to factors involving surgery such as expected surgery time, perioperative antibiotic use, and whether the procedure was elective or emergency surgery.
A majority of the patients in the study across all groups were white women about 63 years old with a body mass index above 30 kg/m2 and almost all undergoing electing surgery compared with emergency surgery. While patients in each group were similar with regard to Charlson Comorbidity Index, expected length of surgery, and percentage of patients undergoing elective surgery, patients in the biologic with or without DMARD group had a significantly lower median income level compared with those in the other two groups (P = .01).
Overall, there were 244 surgeries in 154 patients, with 117 surgeries in the group not receiving biologics or DMARDs, 95 surgeries in the group receiving DMARDs but no biologics, and 32 surgeries in the biologics with or without DMARD group. In the DMARD but no biologics group, most patients were receiving methotrexate (45%) or hydroxychloroquine (44%), while the most common biologics in the biologics with or without DMARD group were infliximab (25%), tocilizumab (19%), abatacept (16%), etanercept (13%), rituximab (9%), and tofacitinib (9%).
There was an 11% overall rate of infection, with a similar rate of infection across all groups (P = .09). While there was a higher rate of surgical site infections among patients in the biologics with or without DMARD group (9%) and a higher percentage of urinary tract infections in the no DMARD and no biologics group (4%), the results were not statistically significant. When the rate of infections was examined by type of surgery, there were no significant differences between infections from musculoskeletal surgery (P = .7) and major organ surgery (P = .8).
The overall 30-day readmission rate was 12%, but there were no statistically significant differences between groups. Although there were five deaths in the study, four deaths were in the group not receiving DMARDs or biologics, and one death was in the biologic with or without DMARD group.
Higher Charlson Comorbidity Index did predict infection risk, with an odds ratio of 1.37 per 1-point increase in the index (95% confidence interval, 1.10-1.70). Length of surgery also increased the risk of infection, with an OR of 1.16 per 15-minute increase in surgery time (95% CI, 1.09-1.23).
Dr. Shmagel noted that the retrospective nature of the study and the midwestern cohort may mean the results are not generalizable to other populations and that larger randomized trials should be considered. “Certainly, a larger study with more events would be needed,” she said.
This study was funded by the University of Minnesota. Dr. Shmagel reported no relevant conflicts of interest.
SOURCE: Kerski M et al. Arthritis Rheumatol. 2019;71 (suppl 10), Abstract 1805.
ATLANTA – Patients with rheumatoid arthritis were more at risk of postoperative infection because of a high Charlson Comorbidity Index or longer surgery time than because of perioperative use of antirheumatic medications, according to a presentation at the annual meeting of the American College of Rheumatology.
Anna Shmagel, MD, of the University of Minnesota in Minneapolis and colleagues performed a retrospective cohort study of 154 patients with seropositive RA who were in the Fairview Health System between Jan. 2010 and Dec. 2017 and underwent either orthopedic or major organ surgery. The patients were classified based on their use of disease-modifying antirheumatic drugs (DMARDs) and biologics alone or in combination, with patients divided into “no DMARD or biologic,” “DMARD but no biologic” and “biologic with or without DMARD” groups.
The question of whether to discontinue antirheumatic medications before surgery is still controversial, with conflicting evidence across studies, Dr. Shmagel said in her presentation. A study by Giles and colleagues found 10 of 91 patients (11%) RA who underwent an orthopedic surgical procedure developed a postoperative infection, with patients receiving tumor necrosis factor (TNF) inhibitors more likely to develop an infection, compared with patients who were not receiving TNF inhibitors (Arthritis Care Res. 2006. doi: 10.1002/art.21841).
However, other studies have challenged that idea, and a 2018 study from Goodman and colleagues raised the issue of whether patients stopping biologics prior to surgery are at increased risk of flares. Of 120 RA patients in their study who underwent total hip or total knee arthroplasty, 75% of patients flared at 6 weeks after surgery. While patients who halted biologics before surgery were more likely to flare, stopping biologics did not predict flaring after surgery (J Rheumatol. 2018. doi: 10.3899/jrheum.170366).
“It’s not entirely clear whether these theories are related to what we do with antirheumatic medications, but we felt that it was pertinent to further study this question.” Dr. Shmagel said.
Dr. Shmagel and colleagues examined the 30-day infection rate of RA patients postoperatively, with 30-day readmission and 30-day mortality rates as secondary outcomes. Patient-associated factors such as age, gender, race, body mass index, smoking status, Charlson Comorbidity Index, income, and use of corticosteroids were analyzed as covariates in addition to factors involving surgery such as expected surgery time, perioperative antibiotic use, and whether the procedure was elective or emergency surgery.
A majority of the patients in the study across all groups were white women about 63 years old with a body mass index above 30 kg/m2 and almost all undergoing electing surgery compared with emergency surgery. While patients in each group were similar with regard to Charlson Comorbidity Index, expected length of surgery, and percentage of patients undergoing elective surgery, patients in the biologic with or without DMARD group had a significantly lower median income level compared with those in the other two groups (P = .01).
Overall, there were 244 surgeries in 154 patients, with 117 surgeries in the group not receiving biologics or DMARDs, 95 surgeries in the group receiving DMARDs but no biologics, and 32 surgeries in the biologics with or without DMARD group. In the DMARD but no biologics group, most patients were receiving methotrexate (45%) or hydroxychloroquine (44%), while the most common biologics in the biologics with or without DMARD group were infliximab (25%), tocilizumab (19%), abatacept (16%), etanercept (13%), rituximab (9%), and tofacitinib (9%).
There was an 11% overall rate of infection, with a similar rate of infection across all groups (P = .09). While there was a higher rate of surgical site infections among patients in the biologics with or without DMARD group (9%) and a higher percentage of urinary tract infections in the no DMARD and no biologics group (4%), the results were not statistically significant. When the rate of infections was examined by type of surgery, there were no significant differences between infections from musculoskeletal surgery (P = .7) and major organ surgery (P = .8).
The overall 30-day readmission rate was 12%, but there were no statistically significant differences between groups. Although there were five deaths in the study, four deaths were in the group not receiving DMARDs or biologics, and one death was in the biologic with or without DMARD group.
Higher Charlson Comorbidity Index did predict infection risk, with an odds ratio of 1.37 per 1-point increase in the index (95% confidence interval, 1.10-1.70). Length of surgery also increased the risk of infection, with an OR of 1.16 per 15-minute increase in surgery time (95% CI, 1.09-1.23).
Dr. Shmagel noted that the retrospective nature of the study and the midwestern cohort may mean the results are not generalizable to other populations and that larger randomized trials should be considered. “Certainly, a larger study with more events would be needed,” she said.
This study was funded by the University of Minnesota. Dr. Shmagel reported no relevant conflicts of interest.
SOURCE: Kerski M et al. Arthritis Rheumatol. 2019;71 (suppl 10), Abstract 1805.
REPORTING FROM ACR 2019
Be Part of VAM Scholarship Program; Apply for VAM Travel Scholarship Today
Are you interested in vascular surgery? Consider applying for a travel scholarship that will help defray costs of attending the 2020 Vascular Annual Meeting June 17 to 20 in Toronto, Canada. The Society for Vascular Surgery offers travel scholarships for medical students or general surgery residents. The Vascular Annual Meeting – June 17 to 20 – includes a dedicated resident/student educational and networking program that will include a mock interview session, mentor program, residency fair and more. Learn more.
Are you interested in vascular surgery? Consider applying for a travel scholarship that will help defray costs of attending the 2020 Vascular Annual Meeting June 17 to 20 in Toronto, Canada. The Society for Vascular Surgery offers travel scholarships for medical students or general surgery residents. The Vascular Annual Meeting – June 17 to 20 – includes a dedicated resident/student educational and networking program that will include a mock interview session, mentor program, residency fair and more. Learn more.
Are you interested in vascular surgery? Consider applying for a travel scholarship that will help defray costs of attending the 2020 Vascular Annual Meeting June 17 to 20 in Toronto, Canada. The Society for Vascular Surgery offers travel scholarships for medical students or general surgery residents. The Vascular Annual Meeting – June 17 to 20 – includes a dedicated resident/student educational and networking program that will include a mock interview session, mentor program, residency fair and more. Learn more.
SVS Members: You Have Options When It Comes to Disability Insurance
The Affinity Program of expanded benefits gives SVS members more options for disability insurance. Many members are part of a group plan in which the employer or group pays the premium. These plans may meet the needs of members, but sometimes they may not. With the options offered through the Affinity Program, members could avoid offsets that reduce tax-free benefits or ones that limit the time period in which they are covered. The program can connect interested members with three companies: Principal Life Insurance Company, Securian and Lloyd’s of London. These group plans are unlike many others, providing tax-free benefits that could potentially protect hundreds of thousands of dollars. Click here for details.
The Affinity Program of expanded benefits gives SVS members more options for disability insurance. Many members are part of a group plan in which the employer or group pays the premium. These plans may meet the needs of members, but sometimes they may not. With the options offered through the Affinity Program, members could avoid offsets that reduce tax-free benefits or ones that limit the time period in which they are covered. The program can connect interested members with three companies: Principal Life Insurance Company, Securian and Lloyd’s of London. These group plans are unlike many others, providing tax-free benefits that could potentially protect hundreds of thousands of dollars. Click here for details.
The Affinity Program of expanded benefits gives SVS members more options for disability insurance. Many members are part of a group plan in which the employer or group pays the premium. These plans may meet the needs of members, but sometimes they may not. With the options offered through the Affinity Program, members could avoid offsets that reduce tax-free benefits or ones that limit the time period in which they are covered. The program can connect interested members with three companies: Principal Life Insurance Company, Securian and Lloyd’s of London. These group plans are unlike many others, providing tax-free benefits that could potentially protect hundreds of thousands of dollars. Click here for details.
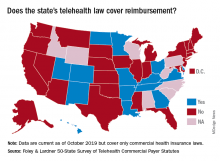
More states pushing plans to pay for telehealth care
More states are enacting laws that require private plans to cover telehealth services, but fair payment remains a challenge for providers, a new analysis finds.
In 2019, 42 states and the District of Columbia had commercial payer telehealth laws, according to a December report by Foley & Lardner LLP, an international law firm. In contrast, about 30 states had such laws in 2015, according to a 2015 report by the National Conference of State Legislatures. Telehealth coverage laws generally require private plans to cover services provided via telehealth to the extent they cover in-person services of the same nature. The measures also frequently protect patients from cost-shifting, in which an insurer imposes higher deductibles or copays for telehealth services.
Private coverage for asynchronous telehealth and remote patient monitoring (RPM) is also growing. Twenty-four states mandate coverage for store and forward asynchronous telehealth, while 13 states require commercial health plans to cover RPM services, the analysis found. In addition, most telehealth coverage laws do not limit where a patient can receive telehealth services. However, some states, such as Arizona, Tennessee, and Washington, still require that patients be located in a particular clinical setting at the time of the telehealth consultation.
Overall, the landscape for reimbursement of telehealth services by commercial payers has improved, said Jacqueline Acosta, a health care attorney with Foley & Lardner and a coauthor of the report.
“[Foley& Lardner’s] 2017 report really noted that implementation [of telehealth] had really picked up both from providers and patients asking for telemedicine, but reimbursement still lagged behind,” Ms. Acosta said in an interview. “This one shows real progress on that front.”
However, the survey notes that payment parity for telehealth services remains lacking. Payment parity refers to insurers paying for telehealth services at the same or an equivalent rate as those delivered in-person. In 2019, 16 states had laws that specifically addressed reimbursement of telehealth services, but only 10 offer true payment parity, according to the Foley analysis. The 10 states with payment parity laws are Arkansas, Delaware, Georgia, Hawaii, Kentucky, Minnesota, Missouri, New Mexico, Utah, and Virginia. Other telehealth reimbursement measures often include ambiguity or allow room for payment negotiation, Ms. Acosta said.
She predicts that more payment parity laws and improved telehealth coverage laws are on the horizon for 2020 and beyond. California, for example, recently revised its telehealth law to require both coverage and payment parity for telehealth services. Mississippi meanwhile, recently expanded its law to include RPM coverage.
That states are revising existing laws and expanding their statutes shows an optimistic trend toward telehealth acceptance and coverage growth, Ms. Acosta said.
More states are enacting laws that require private plans to cover telehealth services, but fair payment remains a challenge for providers, a new analysis finds.
In 2019, 42 states and the District of Columbia had commercial payer telehealth laws, according to a December report by Foley & Lardner LLP, an international law firm. In contrast, about 30 states had such laws in 2015, according to a 2015 report by the National Conference of State Legislatures. Telehealth coverage laws generally require private plans to cover services provided via telehealth to the extent they cover in-person services of the same nature. The measures also frequently protect patients from cost-shifting, in which an insurer imposes higher deductibles or copays for telehealth services.
Private coverage for asynchronous telehealth and remote patient monitoring (RPM) is also growing. Twenty-four states mandate coverage for store and forward asynchronous telehealth, while 13 states require commercial health plans to cover RPM services, the analysis found. In addition, most telehealth coverage laws do not limit where a patient can receive telehealth services. However, some states, such as Arizona, Tennessee, and Washington, still require that patients be located in a particular clinical setting at the time of the telehealth consultation.
Overall, the landscape for reimbursement of telehealth services by commercial payers has improved, said Jacqueline Acosta, a health care attorney with Foley & Lardner and a coauthor of the report.
“[Foley& Lardner’s] 2017 report really noted that implementation [of telehealth] had really picked up both from providers and patients asking for telemedicine, but reimbursement still lagged behind,” Ms. Acosta said in an interview. “This one shows real progress on that front.”
However, the survey notes that payment parity for telehealth services remains lacking. Payment parity refers to insurers paying for telehealth services at the same or an equivalent rate as those delivered in-person. In 2019, 16 states had laws that specifically addressed reimbursement of telehealth services, but only 10 offer true payment parity, according to the Foley analysis. The 10 states with payment parity laws are Arkansas, Delaware, Georgia, Hawaii, Kentucky, Minnesota, Missouri, New Mexico, Utah, and Virginia. Other telehealth reimbursement measures often include ambiguity or allow room for payment negotiation, Ms. Acosta said.
She predicts that more payment parity laws and improved telehealth coverage laws are on the horizon for 2020 and beyond. California, for example, recently revised its telehealth law to require both coverage and payment parity for telehealth services. Mississippi meanwhile, recently expanded its law to include RPM coverage.
That states are revising existing laws and expanding their statutes shows an optimistic trend toward telehealth acceptance and coverage growth, Ms. Acosta said.
More states are enacting laws that require private plans to cover telehealth services, but fair payment remains a challenge for providers, a new analysis finds.
In 2019, 42 states and the District of Columbia had commercial payer telehealth laws, according to a December report by Foley & Lardner LLP, an international law firm. In contrast, about 30 states had such laws in 2015, according to a 2015 report by the National Conference of State Legislatures. Telehealth coverage laws generally require private plans to cover services provided via telehealth to the extent they cover in-person services of the same nature. The measures also frequently protect patients from cost-shifting, in which an insurer imposes higher deductibles or copays for telehealth services.
Private coverage for asynchronous telehealth and remote patient monitoring (RPM) is also growing. Twenty-four states mandate coverage for store and forward asynchronous telehealth, while 13 states require commercial health plans to cover RPM services, the analysis found. In addition, most telehealth coverage laws do not limit where a patient can receive telehealth services. However, some states, such as Arizona, Tennessee, and Washington, still require that patients be located in a particular clinical setting at the time of the telehealth consultation.
Overall, the landscape for reimbursement of telehealth services by commercial payers has improved, said Jacqueline Acosta, a health care attorney with Foley & Lardner and a coauthor of the report.
“[Foley& Lardner’s] 2017 report really noted that implementation [of telehealth] had really picked up both from providers and patients asking for telemedicine, but reimbursement still lagged behind,” Ms. Acosta said in an interview. “This one shows real progress on that front.”
However, the survey notes that payment parity for telehealth services remains lacking. Payment parity refers to insurers paying for telehealth services at the same or an equivalent rate as those delivered in-person. In 2019, 16 states had laws that specifically addressed reimbursement of telehealth services, but only 10 offer true payment parity, according to the Foley analysis. The 10 states with payment parity laws are Arkansas, Delaware, Georgia, Hawaii, Kentucky, Minnesota, Missouri, New Mexico, Utah, and Virginia. Other telehealth reimbursement measures often include ambiguity or allow room for payment negotiation, Ms. Acosta said.
She predicts that more payment parity laws and improved telehealth coverage laws are on the horizon for 2020 and beyond. California, for example, recently revised its telehealth law to require both coverage and payment parity for telehealth services. Mississippi meanwhile, recently expanded its law to include RPM coverage.
That states are revising existing laws and expanding their statutes shows an optimistic trend toward telehealth acceptance and coverage growth, Ms. Acosta said.
Health care: More uninsured as insurance costs grow faster
WASHINGTON – The number of uninsured grew in 2018 as the rate of health care spending grew, according to data from the Centers for Medicare & Medicaid Services.
A total of 30.7 million people in the United States were uninsured in 2018 – up 1 million from 2017. It was the second year in a row that the number of uninsured grew by that amount.
The newly uninsured came from the private insurance sector, which saw the number of insured decrease to 200.5 million in 2018 from 202.1 million in the previous year, partially offset by increases in Americans covered by Medicare and Medicaid.
The increase in uninsured people comes as the growth rate in health care spending rose to 4.6% in 2018 from 4.2% in 2017, though much of that growth in the rate of spending was attributed to the application of a health insurance tax in 2018 that Congress put a moratorium on in the previous year. The tax was part of the Affordable Care Act and was enacted in 2014.
“We see that health care spending reached $3.6 trillion, or $11,172 per person, and spending was faster,” Micah Hartman, statistician in the National Health Statistics Group in the CMS Office of the Actuary, said during a press conference to review the national health expenditure results. “The main reason for the acceleration was faster growth in the net cost of insurance, and that was particularly the case for private health insurance and also for Medicare.”
The net cost of insurance includes nonmedical expenses such as administration, taxes, and fees, as well as gains or losses for private health insurers. The ACA’s health insurance tax generated $14.3 billion in spending, according to Internal Revenue Service data.
Also contributing to the rise in the rate of growth was faster growth in medical prices, “and that was due to underlying economy-wide inflation, as well as the impacts of the tax,” Mr. Hartman said.
Despite this growth in the rate of spending, health care spending as a percentage of GDP fell slightly from 17.9% in 2017 in 17.7%, as the GDP grew faster than health care spending in 2018.
The faster growth in prices more than offset the slightly slower growth in the use and intensity of medical services, CMS reported.
The growth rate on spending on physician and clinical services slowed to 4.1% in 2018 from 4.7% in 2017. Overall spending on physician and clinical services in 2018 reached $725.6 billion and accounted for 20% of overall health care spending.
Spending on hospital services also slowed, but only slightly, dropping to a growth rate of 4.5% from 4.7% during this period. Hospital spending in 2018, at $1.2 trillion, accounted for 33% of overall health care spending.
On a personal level, overall growth in personal health care spending held steady with growth rate of 4.1% in 2018, the same as 2017, though individual components that feed into the figure varied. For example, growth rate in the spending on retail pharmaceuticals rose to 2.5% from 1.4% during this period. Spending on retail pharmaceuticals reached $335 billion and accounted for 9% of overall health care spending.
Another factor in the rising growth rate in spending came from employer-sponsored insurance.
“Growth in health spending by private business was due to faster growth in employer contributions to private health insurance premiums,” Anne B. Martin, economist in the National Health Statistics Group, said during the press conference. There also was faster growth in spending by the federal government, “driven mainly by faster growth in the federally funded portions of Medicare and Medicaid.”
Spending by private health insurance grew at a rate of 5.8% and reached $1.2 trillion in 2018. Medicare spending grew by 6.4% and reached $750.2 billion, while Medicaid spending grew 3.0%, reaching $597.4 billion.
SOURCE: Hartman M et al. Health Affairs. 2019. doi: 10.1377/hlthaff.2019.00451
WASHINGTON – The number of uninsured grew in 2018 as the rate of health care spending grew, according to data from the Centers for Medicare & Medicaid Services.
A total of 30.7 million people in the United States were uninsured in 2018 – up 1 million from 2017. It was the second year in a row that the number of uninsured grew by that amount.
The newly uninsured came from the private insurance sector, which saw the number of insured decrease to 200.5 million in 2018 from 202.1 million in the previous year, partially offset by increases in Americans covered by Medicare and Medicaid.
The increase in uninsured people comes as the growth rate in health care spending rose to 4.6% in 2018 from 4.2% in 2017, though much of that growth in the rate of spending was attributed to the application of a health insurance tax in 2018 that Congress put a moratorium on in the previous year. The tax was part of the Affordable Care Act and was enacted in 2014.
“We see that health care spending reached $3.6 trillion, or $11,172 per person, and spending was faster,” Micah Hartman, statistician in the National Health Statistics Group in the CMS Office of the Actuary, said during a press conference to review the national health expenditure results. “The main reason for the acceleration was faster growth in the net cost of insurance, and that was particularly the case for private health insurance and also for Medicare.”
The net cost of insurance includes nonmedical expenses such as administration, taxes, and fees, as well as gains or losses for private health insurers. The ACA’s health insurance tax generated $14.3 billion in spending, according to Internal Revenue Service data.
Also contributing to the rise in the rate of growth was faster growth in medical prices, “and that was due to underlying economy-wide inflation, as well as the impacts of the tax,” Mr. Hartman said.
Despite this growth in the rate of spending, health care spending as a percentage of GDP fell slightly from 17.9% in 2017 in 17.7%, as the GDP grew faster than health care spending in 2018.
The faster growth in prices more than offset the slightly slower growth in the use and intensity of medical services, CMS reported.
The growth rate on spending on physician and clinical services slowed to 4.1% in 2018 from 4.7% in 2017. Overall spending on physician and clinical services in 2018 reached $725.6 billion and accounted for 20% of overall health care spending.
Spending on hospital services also slowed, but only slightly, dropping to a growth rate of 4.5% from 4.7% during this period. Hospital spending in 2018, at $1.2 trillion, accounted for 33% of overall health care spending.
On a personal level, overall growth in personal health care spending held steady with growth rate of 4.1% in 2018, the same as 2017, though individual components that feed into the figure varied. For example, growth rate in the spending on retail pharmaceuticals rose to 2.5% from 1.4% during this period. Spending on retail pharmaceuticals reached $335 billion and accounted for 9% of overall health care spending.
Another factor in the rising growth rate in spending came from employer-sponsored insurance.
“Growth in health spending by private business was due to faster growth in employer contributions to private health insurance premiums,” Anne B. Martin, economist in the National Health Statistics Group, said during the press conference. There also was faster growth in spending by the federal government, “driven mainly by faster growth in the federally funded portions of Medicare and Medicaid.”
Spending by private health insurance grew at a rate of 5.8% and reached $1.2 trillion in 2018. Medicare spending grew by 6.4% and reached $750.2 billion, while Medicaid spending grew 3.0%, reaching $597.4 billion.
SOURCE: Hartman M et al. Health Affairs. 2019. doi: 10.1377/hlthaff.2019.00451
WASHINGTON – The number of uninsured grew in 2018 as the rate of health care spending grew, according to data from the Centers for Medicare & Medicaid Services.
A total of 30.7 million people in the United States were uninsured in 2018 – up 1 million from 2017. It was the second year in a row that the number of uninsured grew by that amount.
The newly uninsured came from the private insurance sector, which saw the number of insured decrease to 200.5 million in 2018 from 202.1 million in the previous year, partially offset by increases in Americans covered by Medicare and Medicaid.
The increase in uninsured people comes as the growth rate in health care spending rose to 4.6% in 2018 from 4.2% in 2017, though much of that growth in the rate of spending was attributed to the application of a health insurance tax in 2018 that Congress put a moratorium on in the previous year. The tax was part of the Affordable Care Act and was enacted in 2014.
“We see that health care spending reached $3.6 trillion, or $11,172 per person, and spending was faster,” Micah Hartman, statistician in the National Health Statistics Group in the CMS Office of the Actuary, said during a press conference to review the national health expenditure results. “The main reason for the acceleration was faster growth in the net cost of insurance, and that was particularly the case for private health insurance and also for Medicare.”
The net cost of insurance includes nonmedical expenses such as administration, taxes, and fees, as well as gains or losses for private health insurers. The ACA’s health insurance tax generated $14.3 billion in spending, according to Internal Revenue Service data.
Also contributing to the rise in the rate of growth was faster growth in medical prices, “and that was due to underlying economy-wide inflation, as well as the impacts of the tax,” Mr. Hartman said.
Despite this growth in the rate of spending, health care spending as a percentage of GDP fell slightly from 17.9% in 2017 in 17.7%, as the GDP grew faster than health care spending in 2018.
The faster growth in prices more than offset the slightly slower growth in the use and intensity of medical services, CMS reported.
The growth rate on spending on physician and clinical services slowed to 4.1% in 2018 from 4.7% in 2017. Overall spending on physician and clinical services in 2018 reached $725.6 billion and accounted for 20% of overall health care spending.
Spending on hospital services also slowed, but only slightly, dropping to a growth rate of 4.5% from 4.7% during this period. Hospital spending in 2018, at $1.2 trillion, accounted for 33% of overall health care spending.
On a personal level, overall growth in personal health care spending held steady with growth rate of 4.1% in 2018, the same as 2017, though individual components that feed into the figure varied. For example, growth rate in the spending on retail pharmaceuticals rose to 2.5% from 1.4% during this period. Spending on retail pharmaceuticals reached $335 billion and accounted for 9% of overall health care spending.
Another factor in the rising growth rate in spending came from employer-sponsored insurance.
“Growth in health spending by private business was due to faster growth in employer contributions to private health insurance premiums,” Anne B. Martin, economist in the National Health Statistics Group, said during the press conference. There also was faster growth in spending by the federal government, “driven mainly by faster growth in the federally funded portions of Medicare and Medicaid.”
Spending by private health insurance grew at a rate of 5.8% and reached $1.2 trillion in 2018. Medicare spending grew by 6.4% and reached $750.2 billion, while Medicaid spending grew 3.0%, reaching $597.4 billion.
SOURCE: Hartman M et al. Health Affairs. 2019. doi: 10.1377/hlthaff.2019.00451
Extent of insulin rationing in the U.S. is ‘shameful,’ say experts
BUSAN, SOUTH KOREA – The practice of insulin rationing because of cost by people with type 1 diabetes is considerably more common in the United States than in other high-income countries, and is even higher than in some low- and middle-income countries, new data suggest.
Findings from the latest survey conducted by the nonprofit advocacy organization T1International were presented at the International Diabetes Federation Congress 2019 by organization trustee James Elliott, MMSc, of Toronto.
The data were also simultaneously posted on the organization’s website.
The 2018 online survey is an update of T1International’s 2016 survey. It was disseminated through the organization’s website, partner organizations, and social media. The survey questions were developed by people living with type 1 diabetes to ensure they made sense to patients.
A total of 1,478 respondents from 90 countries completed the online survey in 2018.
Overall, 18% reported rationing insulin in the previous year because of cost. About 26% of 627 respondents from the United States reported the practice, compared with 6.5% of 525 respondents from other high-income countries, and 10.9% of 256 respondents from low- and middle-income countries. Rates of rationing suplies for blood glucose testing were even higher.
“The take-home point is that insulin rationing and blood glucose testing rationing is a reality for far more people with diabetes than I think is acknowledged,” said Mr. Elliott.
“One of the key findings is that many people are actually better off living in lower- and middle-income countries than in the United States, which is quite shameful,” Mr. Elliott told Medscape Medical News in an interview.
He advised clinicians to ask patients if they’re insulin rationing, but to be mindful that “not everyone is going to be upfront. There’re a lot of associated stigmas.”
Endocrinologist Irl B. Hirsch, MD, noted that the rationing rate reported for the United States in the survey is similar to that found in a recently published study from Yale University, New Haven, Conn., as reported by Medscape Medical News.
Dr. Hirsch, who is chair of Diabetes Treatment and Teaching at the University of Washington, Seattle, agreed wholeheartedly with Mr. Elliott.
“It is shameful and embarrassing sitting here with colleagues from around the world at IDF. It is time for our elected officials [in the United States] to do something instead of simply talking about it,” Dr. Hirsch said.
Many have no coverage, blood glucose test rationing also common
Overall, 66.2% of survey respondents reported having no financial coverage for diabetes expenses, many instead relying on support from family and friends, charities and nonprofit organizations, donations including online programs such as GoFundMe, and/or assistance from government or pharmaceutical company programs.
By region, the proportions reporting no coverage for diabetes supplies were 79.2% in the United States, 54.0% in other high-income countries, and 59.8% in low- and middle-income countries.
“Many countries still lack any kind of support system to help people with type 1 diabetes survive,” Mr. Elliott noted.
Also asked to comment, Edward W. Gregg, PhD, a professor in the department of epidemiology and biostatistics at Imperial College, London, said: “It’s pretty astounding to me that two thirds of people with type 1 diabetes have no coverage whatsoever for out-of-pocket costs.”
“For as much concern as we have [for the US], it’s really staggering to think about how it must be in the low- and middle-income countries where having to pay for insulin takes away a large proportion of income,” he added.
Rationing of blood glucose testing was considerably more common than insulin rationing, with 33.5% overall reporting having done so in the last year.
The proportion was higher in the United States and in low- and middle-income countries, at 38.6% and 55.5%, respectively, compared with just 17.2% of high-income countries other than the United States.
Mr. Elliott told Medscape Medical News that the recent World Health Organization’s launch of its first-ever insulin prequalification program to expand access to treatment is a “start” and that T1International is pushing to expand that beyond human insulins to also include analogues.
“It’s a tough disease to survive in lower- and middle-income countries. Oftentimes, it’s a death sentence,” Mr. Elliott said.
This story first appeared on Medscape.com.
BUSAN, SOUTH KOREA – The practice of insulin rationing because of cost by people with type 1 diabetes is considerably more common in the United States than in other high-income countries, and is even higher than in some low- and middle-income countries, new data suggest.
Findings from the latest survey conducted by the nonprofit advocacy organization T1International were presented at the International Diabetes Federation Congress 2019 by organization trustee James Elliott, MMSc, of Toronto.
The data were also simultaneously posted on the organization’s website.
The 2018 online survey is an update of T1International’s 2016 survey. It was disseminated through the organization’s website, partner organizations, and social media. The survey questions were developed by people living with type 1 diabetes to ensure they made sense to patients.
A total of 1,478 respondents from 90 countries completed the online survey in 2018.
Overall, 18% reported rationing insulin in the previous year because of cost. About 26% of 627 respondents from the United States reported the practice, compared with 6.5% of 525 respondents from other high-income countries, and 10.9% of 256 respondents from low- and middle-income countries. Rates of rationing suplies for blood glucose testing were even higher.
“The take-home point is that insulin rationing and blood glucose testing rationing is a reality for far more people with diabetes than I think is acknowledged,” said Mr. Elliott.
“One of the key findings is that many people are actually better off living in lower- and middle-income countries than in the United States, which is quite shameful,” Mr. Elliott told Medscape Medical News in an interview.
He advised clinicians to ask patients if they’re insulin rationing, but to be mindful that “not everyone is going to be upfront. There’re a lot of associated stigmas.”
Endocrinologist Irl B. Hirsch, MD, noted that the rationing rate reported for the United States in the survey is similar to that found in a recently published study from Yale University, New Haven, Conn., as reported by Medscape Medical News.
Dr. Hirsch, who is chair of Diabetes Treatment and Teaching at the University of Washington, Seattle, agreed wholeheartedly with Mr. Elliott.
“It is shameful and embarrassing sitting here with colleagues from around the world at IDF. It is time for our elected officials [in the United States] to do something instead of simply talking about it,” Dr. Hirsch said.
Many have no coverage, blood glucose test rationing also common
Overall, 66.2% of survey respondents reported having no financial coverage for diabetes expenses, many instead relying on support from family and friends, charities and nonprofit organizations, donations including online programs such as GoFundMe, and/or assistance from government or pharmaceutical company programs.
By region, the proportions reporting no coverage for diabetes supplies were 79.2% in the United States, 54.0% in other high-income countries, and 59.8% in low- and middle-income countries.
“Many countries still lack any kind of support system to help people with type 1 diabetes survive,” Mr. Elliott noted.
Also asked to comment, Edward W. Gregg, PhD, a professor in the department of epidemiology and biostatistics at Imperial College, London, said: “It’s pretty astounding to me that two thirds of people with type 1 diabetes have no coverage whatsoever for out-of-pocket costs.”
“For as much concern as we have [for the US], it’s really staggering to think about how it must be in the low- and middle-income countries where having to pay for insulin takes away a large proportion of income,” he added.
Rationing of blood glucose testing was considerably more common than insulin rationing, with 33.5% overall reporting having done so in the last year.
The proportion was higher in the United States and in low- and middle-income countries, at 38.6% and 55.5%, respectively, compared with just 17.2% of high-income countries other than the United States.
Mr. Elliott told Medscape Medical News that the recent World Health Organization’s launch of its first-ever insulin prequalification program to expand access to treatment is a “start” and that T1International is pushing to expand that beyond human insulins to also include analogues.
“It’s a tough disease to survive in lower- and middle-income countries. Oftentimes, it’s a death sentence,” Mr. Elliott said.
This story first appeared on Medscape.com.
BUSAN, SOUTH KOREA – The practice of insulin rationing because of cost by people with type 1 diabetes is considerably more common in the United States than in other high-income countries, and is even higher than in some low- and middle-income countries, new data suggest.
Findings from the latest survey conducted by the nonprofit advocacy organization T1International were presented at the International Diabetes Federation Congress 2019 by organization trustee James Elliott, MMSc, of Toronto.
The data were also simultaneously posted on the organization’s website.
The 2018 online survey is an update of T1International’s 2016 survey. It was disseminated through the organization’s website, partner organizations, and social media. The survey questions were developed by people living with type 1 diabetes to ensure they made sense to patients.
A total of 1,478 respondents from 90 countries completed the online survey in 2018.
Overall, 18% reported rationing insulin in the previous year because of cost. About 26% of 627 respondents from the United States reported the practice, compared with 6.5% of 525 respondents from other high-income countries, and 10.9% of 256 respondents from low- and middle-income countries. Rates of rationing suplies for blood glucose testing were even higher.
“The take-home point is that insulin rationing and blood glucose testing rationing is a reality for far more people with diabetes than I think is acknowledged,” said Mr. Elliott.
“One of the key findings is that many people are actually better off living in lower- and middle-income countries than in the United States, which is quite shameful,” Mr. Elliott told Medscape Medical News in an interview.
He advised clinicians to ask patients if they’re insulin rationing, but to be mindful that “not everyone is going to be upfront. There’re a lot of associated stigmas.”
Endocrinologist Irl B. Hirsch, MD, noted that the rationing rate reported for the United States in the survey is similar to that found in a recently published study from Yale University, New Haven, Conn., as reported by Medscape Medical News.
Dr. Hirsch, who is chair of Diabetes Treatment and Teaching at the University of Washington, Seattle, agreed wholeheartedly with Mr. Elliott.
“It is shameful and embarrassing sitting here with colleagues from around the world at IDF. It is time for our elected officials [in the United States] to do something instead of simply talking about it,” Dr. Hirsch said.
Many have no coverage, blood glucose test rationing also common
Overall, 66.2% of survey respondents reported having no financial coverage for diabetes expenses, many instead relying on support from family and friends, charities and nonprofit organizations, donations including online programs such as GoFundMe, and/or assistance from government or pharmaceutical company programs.
By region, the proportions reporting no coverage for diabetes supplies were 79.2% in the United States, 54.0% in other high-income countries, and 59.8% in low- and middle-income countries.
“Many countries still lack any kind of support system to help people with type 1 diabetes survive,” Mr. Elliott noted.
Also asked to comment, Edward W. Gregg, PhD, a professor in the department of epidemiology and biostatistics at Imperial College, London, said: “It’s pretty astounding to me that two thirds of people with type 1 diabetes have no coverage whatsoever for out-of-pocket costs.”
“For as much concern as we have [for the US], it’s really staggering to think about how it must be in the low- and middle-income countries where having to pay for insulin takes away a large proportion of income,” he added.
Rationing of blood glucose testing was considerably more common than insulin rationing, with 33.5% overall reporting having done so in the last year.
The proportion was higher in the United States and in low- and middle-income countries, at 38.6% and 55.5%, respectively, compared with just 17.2% of high-income countries other than the United States.
Mr. Elliott told Medscape Medical News that the recent World Health Organization’s launch of its first-ever insulin prequalification program to expand access to treatment is a “start” and that T1International is pushing to expand that beyond human insulins to also include analogues.
“It’s a tough disease to survive in lower- and middle-income countries. Oftentimes, it’s a death sentence,” Mr. Elliott said.
This story first appeared on Medscape.com.
Icosapent ethyl cost effective in REDUCE-IT analysis
PHILADELPHIA – The overall costs of icosapent ethyl were less than placebo, and the medication reduced cardiovascular events by 30% at a cost that fits well within acceptable quality-adjusted life-year (QALY) parameters, according to a cost-effectiveness analysis of the REDUCE-IT trial.
Days before the presentation of the analysis at the American Heart Association scientific sessions, a Food and Drug Administration advisory panel unanimously recommended approval of icosapent ethyl (Vascepa) for a new indication for reducing CV event risk. Icosapent ethyl, a highly purified form of the ethyl ester of eicosapentaenoic acid derived from fish oil, received FDA approval in 2012 for treatment of triglyceride levels of at least 500 mg/dL.
“What we found here is that icosapent ethyl is a dominant strategy,” said William S. Weintraub, MD, director of outcomes research at MedStar Heart & Vascular Institute in Washington, in reporting preliminary cost-analysis findings from REDUCE-IT (Reduction of Cardiovascular Events With Icosapent Ethyl – Intervention Trial). “It’s offering better outcomes at a lower cost.”
The dominant strategy was demonstrated by cost savings in 70% of simulations the cost-effectiveness analysis ran, Dr. Weintraub said.
“These are very impressive results,” said session moderator Seth S. Martin, MD, an internist and cardiologist at Johns Hopkins University, Baltimore. “We don’t often see dominant strategies for new drugs. This is very exciting.”
“Almost never,” Dr. Weintraub responded.
REDUCE-IT randomized 8,179 patients with a diagnosis of CVD or with diabetes and other risk factors who had been on statins and had triglycerides of 135-499 mg/dL to either 4 g of icosapent ethyl daily or placebo (N Engl J Med. 2019;380:11-22). Trial results showed the treatment group had an absolute risk reduction of 4.8% and a relative risk reduction of 25% of first CV events and a 30% relative risk reduction for total events, Dr. Weintraub said.
The analysis determined that the QALYs for icosapent ethyl versus those for placebo were 3.34 and 3.27, respectively, during the trial period and 11.61 and 11.35 over a lifetime. The mean costs for the two treatments were $27,576 and $28,205 during the trial period and $235,352 and $236,636 lifetime, respectively, Dr. Weintraub said.
An analysis of cost effectiveness showed that almost all of the estimates fell below the willingness-to-pay (WTP) threshold of $50,000 per QALY gained, Dr. Weintraub said. “In fact, some 70% plus are in what’s called quadrant two; that is, decreased cost and increased efficacy.”
The analysis also calculated the value of icosapent ethyl at three different WTP thresholds: up to $6 a day at a WTP of $50,000, up to $12 a day at $100,000, and up to $18 a day at $150,000. The analysis used the actual net pricing of $4.16 a day, Dr. Weintraub said. “That’s why we showed we have the dominant strategy,” he said.
Further cost-effectiveness analyses of the REDUCE-IT data will focus on subgroups, such as U.S. and non–U.S. patients and people with diabetes. He also emphasized the data he reported were preliminary. “We have a lot more work to do,” Dr. Weintraub said.
Dr. Weintraub reported having financial relationships with Amarin Pharma, which markets Vascepa, and AstraZeneca.
SOURCE: Weintraub WS. AHA 2019, Session FS.AOS.F1.
PHILADELPHIA – The overall costs of icosapent ethyl were less than placebo, and the medication reduced cardiovascular events by 30% at a cost that fits well within acceptable quality-adjusted life-year (QALY) parameters, according to a cost-effectiveness analysis of the REDUCE-IT trial.
Days before the presentation of the analysis at the American Heart Association scientific sessions, a Food and Drug Administration advisory panel unanimously recommended approval of icosapent ethyl (Vascepa) for a new indication for reducing CV event risk. Icosapent ethyl, a highly purified form of the ethyl ester of eicosapentaenoic acid derived from fish oil, received FDA approval in 2012 for treatment of triglyceride levels of at least 500 mg/dL.
“What we found here is that icosapent ethyl is a dominant strategy,” said William S. Weintraub, MD, director of outcomes research at MedStar Heart & Vascular Institute in Washington, in reporting preliminary cost-analysis findings from REDUCE-IT (Reduction of Cardiovascular Events With Icosapent Ethyl – Intervention Trial). “It’s offering better outcomes at a lower cost.”
The dominant strategy was demonstrated by cost savings in 70% of simulations the cost-effectiveness analysis ran, Dr. Weintraub said.
“These are very impressive results,” said session moderator Seth S. Martin, MD, an internist and cardiologist at Johns Hopkins University, Baltimore. “We don’t often see dominant strategies for new drugs. This is very exciting.”
“Almost never,” Dr. Weintraub responded.
REDUCE-IT randomized 8,179 patients with a diagnosis of CVD or with diabetes and other risk factors who had been on statins and had triglycerides of 135-499 mg/dL to either 4 g of icosapent ethyl daily or placebo (N Engl J Med. 2019;380:11-22). Trial results showed the treatment group had an absolute risk reduction of 4.8% and a relative risk reduction of 25% of first CV events and a 30% relative risk reduction for total events, Dr. Weintraub said.
The analysis determined that the QALYs for icosapent ethyl versus those for placebo were 3.34 and 3.27, respectively, during the trial period and 11.61 and 11.35 over a lifetime. The mean costs for the two treatments were $27,576 and $28,205 during the trial period and $235,352 and $236,636 lifetime, respectively, Dr. Weintraub said.
An analysis of cost effectiveness showed that almost all of the estimates fell below the willingness-to-pay (WTP) threshold of $50,000 per QALY gained, Dr. Weintraub said. “In fact, some 70% plus are in what’s called quadrant two; that is, decreased cost and increased efficacy.”
The analysis also calculated the value of icosapent ethyl at three different WTP thresholds: up to $6 a day at a WTP of $50,000, up to $12 a day at $100,000, and up to $18 a day at $150,000. The analysis used the actual net pricing of $4.16 a day, Dr. Weintraub said. “That’s why we showed we have the dominant strategy,” he said.
Further cost-effectiveness analyses of the REDUCE-IT data will focus on subgroups, such as U.S. and non–U.S. patients and people with diabetes. He also emphasized the data he reported were preliminary. “We have a lot more work to do,” Dr. Weintraub said.
Dr. Weintraub reported having financial relationships with Amarin Pharma, which markets Vascepa, and AstraZeneca.
SOURCE: Weintraub WS. AHA 2019, Session FS.AOS.F1.
PHILADELPHIA – The overall costs of icosapent ethyl were less than placebo, and the medication reduced cardiovascular events by 30% at a cost that fits well within acceptable quality-adjusted life-year (QALY) parameters, according to a cost-effectiveness analysis of the REDUCE-IT trial.
Days before the presentation of the analysis at the American Heart Association scientific sessions, a Food and Drug Administration advisory panel unanimously recommended approval of icosapent ethyl (Vascepa) for a new indication for reducing CV event risk. Icosapent ethyl, a highly purified form of the ethyl ester of eicosapentaenoic acid derived from fish oil, received FDA approval in 2012 for treatment of triglyceride levels of at least 500 mg/dL.
“What we found here is that icosapent ethyl is a dominant strategy,” said William S. Weintraub, MD, director of outcomes research at MedStar Heart & Vascular Institute in Washington, in reporting preliminary cost-analysis findings from REDUCE-IT (Reduction of Cardiovascular Events With Icosapent Ethyl – Intervention Trial). “It’s offering better outcomes at a lower cost.”
The dominant strategy was demonstrated by cost savings in 70% of simulations the cost-effectiveness analysis ran, Dr. Weintraub said.
“These are very impressive results,” said session moderator Seth S. Martin, MD, an internist and cardiologist at Johns Hopkins University, Baltimore. “We don’t often see dominant strategies for new drugs. This is very exciting.”
“Almost never,” Dr. Weintraub responded.
REDUCE-IT randomized 8,179 patients with a diagnosis of CVD or with diabetes and other risk factors who had been on statins and had triglycerides of 135-499 mg/dL to either 4 g of icosapent ethyl daily or placebo (N Engl J Med. 2019;380:11-22). Trial results showed the treatment group had an absolute risk reduction of 4.8% and a relative risk reduction of 25% of first CV events and a 30% relative risk reduction for total events, Dr. Weintraub said.
The analysis determined that the QALYs for icosapent ethyl versus those for placebo were 3.34 and 3.27, respectively, during the trial period and 11.61 and 11.35 over a lifetime. The mean costs for the two treatments were $27,576 and $28,205 during the trial period and $235,352 and $236,636 lifetime, respectively, Dr. Weintraub said.
An analysis of cost effectiveness showed that almost all of the estimates fell below the willingness-to-pay (WTP) threshold of $50,000 per QALY gained, Dr. Weintraub said. “In fact, some 70% plus are in what’s called quadrant two; that is, decreased cost and increased efficacy.”
The analysis also calculated the value of icosapent ethyl at three different WTP thresholds: up to $6 a day at a WTP of $50,000, up to $12 a day at $100,000, and up to $18 a day at $150,000. The analysis used the actual net pricing of $4.16 a day, Dr. Weintraub said. “That’s why we showed we have the dominant strategy,” he said.
Further cost-effectiveness analyses of the REDUCE-IT data will focus on subgroups, such as U.S. and non–U.S. patients and people with diabetes. He also emphasized the data he reported were preliminary. “We have a lot more work to do,” Dr. Weintraub said.
Dr. Weintraub reported having financial relationships with Amarin Pharma, which markets Vascepa, and AstraZeneca.
SOURCE: Weintraub WS. AHA 2019, Session FS.AOS.F1.
REPORTING FROM AHA 2019
ACGME deepening its commitment to physician well-being, leader says
NEW ORLEANS – When Timothy P. Brigham, MDiv, PhD, thinks about the impact of burnout and stress on the ability of physicians to practice medicine, Lewin’s equation comes to mind.
Developed by psychologist Kurt Lewin in 1936, the equation holds that behavior stems from a person’s personality and the environment that person inhabits.
Dr. Brigham, chief of staff and chief education and organizational development officer at the Chicago-based Accreditation Council for Graduate Medical Education (ACGME), said at the annual meeting of the American Academy of Pediatrics.
“It’s a toxic mine, in some ways. What we tend to do is when we detect that physicians in general are, or a particular residency program is, too stressed out or burned out, we give them resilience training. Not that that’s unimportant, but it’s like putting a canary in a toxic mine full of poison and saying, ‘We’re going to teach you to hold your breath a little bit longer.’ Our job is to detoxify the mine.”
Troubled by the rise of suicides among physicians in recent years as well as mounting evidence about the adverse impact of burnout and stress on the practice of medicine, Dr. Brigham said that the ACGME is deepening its commitment to the well-being of faculty, residents, patients, and all members of the health care team. Since launching a “call to arms” on the topic at its annual educational conference in 2015, the ACGME has added courses on well-being to its annual meeting and remolded its Clinical Learning Environmental Review program to include all clinicians, “because everybody is affected by this: nurses, coordinators, et cetera,” he said. The ACGME also has revised Common Program Requirements, disseminated tools and resources to promote well-being and new knowledge on the topic, and partnered with the National Academy of Medicine Action Collaborative on Clinician Well-Being and Resilience – all in an effort to bring about culture change.
“But we’re well aware that the ACGME can’t do this alone,” Dr. Brigham said. “We can’t ‘requirement’ our way out of this problem. It’s going to take a culture shift. Only you physicians, in collaboration with everyone in your community of learning, can create the systemic change required to improve our culture. We have a good handle on the problem at this point, but the solutions are a little bit more difficult to get a hold of. As Martin Luther King Jr. once said, ‘You don’t have to see the whole staircase, just take the first step.’ ”
The ACGME wants to work with physicians “to collect data and do joint research, to share insights, and to share tools and resources to create a better world for practicing physicians, for other members of the health care team, and for patients. After all, clinicians who care for themselves provide better care for others. They’re less likely to make errors or leave the profession,” Dr. Brigham told attendees.
He added that clinicians can gauge their risk for burnout by asking themselves three simple questions about their work environment: Does it support self-care? Does it increase and support connection with colleagues? Does it connect people to purpose and meaningful work?
“One of the problems with our resident clinical work hours is not terrible program directors saying, ‘work longer.’ It’s residents who want to take care of families for 1 more hour,” Dr. Brigham continued. “It’s residents who want to take care of patients who are going through a difficult time. You represent the top 2% in the world in terms of your intelligence and achievement, yet that’s not what makes you special. What makes you special is that the level of self-doubt in this room exceeds that of the general population by about 10 times. You also tend to run toward what everyone else runs away from: disease, despair, people who are injured and suffering. That takes a toll.”
He emphasized that positive social relationships with others are crucial to joy and well-being in the practice of medicine. “Burnout isn’t just about exhaustion; it’s about loneliness,” Dr. Brigham said. “There’s a surprising power in just asking people how they’re doing, and really wanting to know the answer.”
Negative social connections are highly correlated with burnout and depression, such as harassment, bullying, mistreatment, discrimination, “and using the power gradient to squash somebody who’s trying their best to be a physician,” he said.
Dr. Brigham acknowledged the tall task of bringing a spotlight to well-being as physicians continue to engage in tasks such as the burden and lack of standardization of prior authorization requirements, the burden of clinical documentation requirements, electronic health records and related work flow, and quality payment programs. “This is what we need to shift; this is what we need to take away so you can get back in touch with why you became a physician in the first place.”
Dr. Brigham reported having no financial disclosures.
NEW ORLEANS – When Timothy P. Brigham, MDiv, PhD, thinks about the impact of burnout and stress on the ability of physicians to practice medicine, Lewin’s equation comes to mind.
Developed by psychologist Kurt Lewin in 1936, the equation holds that behavior stems from a person’s personality and the environment that person inhabits.
Dr. Brigham, chief of staff and chief education and organizational development officer at the Chicago-based Accreditation Council for Graduate Medical Education (ACGME), said at the annual meeting of the American Academy of Pediatrics.
“It’s a toxic mine, in some ways. What we tend to do is when we detect that physicians in general are, or a particular residency program is, too stressed out or burned out, we give them resilience training. Not that that’s unimportant, but it’s like putting a canary in a toxic mine full of poison and saying, ‘We’re going to teach you to hold your breath a little bit longer.’ Our job is to detoxify the mine.”
Troubled by the rise of suicides among physicians in recent years as well as mounting evidence about the adverse impact of burnout and stress on the practice of medicine, Dr. Brigham said that the ACGME is deepening its commitment to the well-being of faculty, residents, patients, and all members of the health care team. Since launching a “call to arms” on the topic at its annual educational conference in 2015, the ACGME has added courses on well-being to its annual meeting and remolded its Clinical Learning Environmental Review program to include all clinicians, “because everybody is affected by this: nurses, coordinators, et cetera,” he said. The ACGME also has revised Common Program Requirements, disseminated tools and resources to promote well-being and new knowledge on the topic, and partnered with the National Academy of Medicine Action Collaborative on Clinician Well-Being and Resilience – all in an effort to bring about culture change.
“But we’re well aware that the ACGME can’t do this alone,” Dr. Brigham said. “We can’t ‘requirement’ our way out of this problem. It’s going to take a culture shift. Only you physicians, in collaboration with everyone in your community of learning, can create the systemic change required to improve our culture. We have a good handle on the problem at this point, but the solutions are a little bit more difficult to get a hold of. As Martin Luther King Jr. once said, ‘You don’t have to see the whole staircase, just take the first step.’ ”
The ACGME wants to work with physicians “to collect data and do joint research, to share insights, and to share tools and resources to create a better world for practicing physicians, for other members of the health care team, and for patients. After all, clinicians who care for themselves provide better care for others. They’re less likely to make errors or leave the profession,” Dr. Brigham told attendees.
He added that clinicians can gauge their risk for burnout by asking themselves three simple questions about their work environment: Does it support self-care? Does it increase and support connection with colleagues? Does it connect people to purpose and meaningful work?
“One of the problems with our resident clinical work hours is not terrible program directors saying, ‘work longer.’ It’s residents who want to take care of families for 1 more hour,” Dr. Brigham continued. “It’s residents who want to take care of patients who are going through a difficult time. You represent the top 2% in the world in terms of your intelligence and achievement, yet that’s not what makes you special. What makes you special is that the level of self-doubt in this room exceeds that of the general population by about 10 times. You also tend to run toward what everyone else runs away from: disease, despair, people who are injured and suffering. That takes a toll.”
He emphasized that positive social relationships with others are crucial to joy and well-being in the practice of medicine. “Burnout isn’t just about exhaustion; it’s about loneliness,” Dr. Brigham said. “There’s a surprising power in just asking people how they’re doing, and really wanting to know the answer.”
Negative social connections are highly correlated with burnout and depression, such as harassment, bullying, mistreatment, discrimination, “and using the power gradient to squash somebody who’s trying their best to be a physician,” he said.
Dr. Brigham acknowledged the tall task of bringing a spotlight to well-being as physicians continue to engage in tasks such as the burden and lack of standardization of prior authorization requirements, the burden of clinical documentation requirements, electronic health records and related work flow, and quality payment programs. “This is what we need to shift; this is what we need to take away so you can get back in touch with why you became a physician in the first place.”
Dr. Brigham reported having no financial disclosures.
NEW ORLEANS – When Timothy P. Brigham, MDiv, PhD, thinks about the impact of burnout and stress on the ability of physicians to practice medicine, Lewin’s equation comes to mind.
Developed by psychologist Kurt Lewin in 1936, the equation holds that behavior stems from a person’s personality and the environment that person inhabits.
Dr. Brigham, chief of staff and chief education and organizational development officer at the Chicago-based Accreditation Council for Graduate Medical Education (ACGME), said at the annual meeting of the American Academy of Pediatrics.
“It’s a toxic mine, in some ways. What we tend to do is when we detect that physicians in general are, or a particular residency program is, too stressed out or burned out, we give them resilience training. Not that that’s unimportant, but it’s like putting a canary in a toxic mine full of poison and saying, ‘We’re going to teach you to hold your breath a little bit longer.’ Our job is to detoxify the mine.”
Troubled by the rise of suicides among physicians in recent years as well as mounting evidence about the adverse impact of burnout and stress on the practice of medicine, Dr. Brigham said that the ACGME is deepening its commitment to the well-being of faculty, residents, patients, and all members of the health care team. Since launching a “call to arms” on the topic at its annual educational conference in 2015, the ACGME has added courses on well-being to its annual meeting and remolded its Clinical Learning Environmental Review program to include all clinicians, “because everybody is affected by this: nurses, coordinators, et cetera,” he said. The ACGME also has revised Common Program Requirements, disseminated tools and resources to promote well-being and new knowledge on the topic, and partnered with the National Academy of Medicine Action Collaborative on Clinician Well-Being and Resilience – all in an effort to bring about culture change.
“But we’re well aware that the ACGME can’t do this alone,” Dr. Brigham said. “We can’t ‘requirement’ our way out of this problem. It’s going to take a culture shift. Only you physicians, in collaboration with everyone in your community of learning, can create the systemic change required to improve our culture. We have a good handle on the problem at this point, but the solutions are a little bit more difficult to get a hold of. As Martin Luther King Jr. once said, ‘You don’t have to see the whole staircase, just take the first step.’ ”
The ACGME wants to work with physicians “to collect data and do joint research, to share insights, and to share tools and resources to create a better world for practicing physicians, for other members of the health care team, and for patients. After all, clinicians who care for themselves provide better care for others. They’re less likely to make errors or leave the profession,” Dr. Brigham told attendees.
He added that clinicians can gauge their risk for burnout by asking themselves three simple questions about their work environment: Does it support self-care? Does it increase and support connection with colleagues? Does it connect people to purpose and meaningful work?
“One of the problems with our resident clinical work hours is not terrible program directors saying, ‘work longer.’ It’s residents who want to take care of families for 1 more hour,” Dr. Brigham continued. “It’s residents who want to take care of patients who are going through a difficult time. You represent the top 2% in the world in terms of your intelligence and achievement, yet that’s not what makes you special. What makes you special is that the level of self-doubt in this room exceeds that of the general population by about 10 times. You also tend to run toward what everyone else runs away from: disease, despair, people who are injured and suffering. That takes a toll.”
He emphasized that positive social relationships with others are crucial to joy and well-being in the practice of medicine. “Burnout isn’t just about exhaustion; it’s about loneliness,” Dr. Brigham said. “There’s a surprising power in just asking people how they’re doing, and really wanting to know the answer.”
Negative social connections are highly correlated with burnout and depression, such as harassment, bullying, mistreatment, discrimination, “and using the power gradient to squash somebody who’s trying their best to be a physician,” he said.
Dr. Brigham acknowledged the tall task of bringing a spotlight to well-being as physicians continue to engage in tasks such as the burden and lack of standardization of prior authorization requirements, the burden of clinical documentation requirements, electronic health records and related work flow, and quality payment programs. “This is what we need to shift; this is what we need to take away so you can get back in touch with why you became a physician in the first place.”
Dr. Brigham reported having no financial disclosures.
EXPERT ANALYSIS AT AAP 2019
Upcoming Ask Us Anything Will Focus on Outpatient Vascular Surgery Through OBLs
More patients are receiving vascular surgery procedures in outpatient-based labs (OBLs). This trend is expected to increase in years to come. Right now, there are many questions surrounding OBLs , including questions about safety, equipment required for emergencies, types of procedures that can be safely performed, how an OBL starts and more. Join Drs. Deepak Nair and Cliff Sales on Monday, Dec. 2 from 7-8pm for an Ask Us Anything on SVSConnect. For this one-hour time-frame, both doctors will be available in the community to answer any and all questions related to this topic. Make sure you’re a user on SVSConnect by signing in here.
More patients are receiving vascular surgery procedures in outpatient-based labs (OBLs). This trend is expected to increase in years to come. Right now, there are many questions surrounding OBLs , including questions about safety, equipment required for emergencies, types of procedures that can be safely performed, how an OBL starts and more. Join Drs. Deepak Nair and Cliff Sales on Monday, Dec. 2 from 7-8pm for an Ask Us Anything on SVSConnect. For this one-hour time-frame, both doctors will be available in the community to answer any and all questions related to this topic. Make sure you’re a user on SVSConnect by signing in here.
More patients are receiving vascular surgery procedures in outpatient-based labs (OBLs). This trend is expected to increase in years to come. Right now, there are many questions surrounding OBLs , including questions about safety, equipment required for emergencies, types of procedures that can be safely performed, how an OBL starts and more. Join Drs. Deepak Nair and Cliff Sales on Monday, Dec. 2 from 7-8pm for an Ask Us Anything on SVSConnect. For this one-hour time-frame, both doctors will be available in the community to answer any and all questions related to this topic. Make sure you’re a user on SVSConnect by signing in here.