User login
Official Newspaper of the American College of Surgeons
Doctors, payers collaborate to simplify and align quality measures
The Centers for Medicare & Medicaid Services and America’s Health Insurance Plans on Feb 16 announced a set of core quality measures across seven areas that will serve as the foundation for a more uniform set of quality metrics that will be used by both public and private payers.
The CMS and AHIP are working with the National Quality Forum, medical specialty societies, employer groups, and consumer groups under an umbrella organization called the Core Quality Measures Collaborative. Together, they derived a set of measures that are “meaningful to patients, consumers, and physicians, while reducing variability in measure selection, collection burden, and cost,” the CMS noted in a fact sheet.
Involved medical specialty societies include the American Academy of Family Physicians, American College of Cardiology, American Heart Association, American College of Physicians, American Gastroenterological Association, HIV Medicine Association, Infectious Diseases Society of America, American Academy of Pediatrics, American Society of Clinical Oncology, and the American Medical Association.
The collaborative announced core measures in seven areas: accountable care organizations/patient centered medical homes/primary care, cardiology, gastroenterology, HIV and hepatitis C, medical oncology, obstetrics and gynecology, and orthopedics. Measures in additional areas will be released in the future and the core sets will be reviewed and updated on a regular basis.
“I think [this collaboration] strikes the best balance between what is feasible and what is desirable,” Dr. Ziad Gellad, chair of the AGA Quality Measures Committee, said in an interview.
For Medicare, Medicaid, and other public programs, the measure sets will be implemented and updated through the physician fee schedule in the coming Merit-Based Incentive Payment System (MIPS) and alternative payment models (APMs). Private payers are expected to begin implementing these quality measures as physician contracts come up for renewal.
“I think one of the biggest challenges will be how to collect these measures, because the decision about what measures to include was only driven in part by the feasibility,” Dr. Gellad said. “A large part was driven by what are the best metrics and what are most important outcomes and processes to evaluate. I think in terms of feasibility, that is going to be the next important question. How do you improve the feasibility of these measurements in practice?”
Electronic health records will play an integral part, as a FAQ released by AHIP notes that several of the measures “require that clinical data be extracted from EHRs or registries or be self-reported by clinicians.” It adds that clinicians and payers “will need to work together to create a reporting infrastructure for such measures” in areas where it is currently lacking.
The Centers for Medicare & Medicaid Services and America’s Health Insurance Plans on Feb 16 announced a set of core quality measures across seven areas that will serve as the foundation for a more uniform set of quality metrics that will be used by both public and private payers.
The CMS and AHIP are working with the National Quality Forum, medical specialty societies, employer groups, and consumer groups under an umbrella organization called the Core Quality Measures Collaborative. Together, they derived a set of measures that are “meaningful to patients, consumers, and physicians, while reducing variability in measure selection, collection burden, and cost,” the CMS noted in a fact sheet.
Involved medical specialty societies include the American Academy of Family Physicians, American College of Cardiology, American Heart Association, American College of Physicians, American Gastroenterological Association, HIV Medicine Association, Infectious Diseases Society of America, American Academy of Pediatrics, American Society of Clinical Oncology, and the American Medical Association.
The collaborative announced core measures in seven areas: accountable care organizations/patient centered medical homes/primary care, cardiology, gastroenterology, HIV and hepatitis C, medical oncology, obstetrics and gynecology, and orthopedics. Measures in additional areas will be released in the future and the core sets will be reviewed and updated on a regular basis.
“I think [this collaboration] strikes the best balance between what is feasible and what is desirable,” Dr. Ziad Gellad, chair of the AGA Quality Measures Committee, said in an interview.
For Medicare, Medicaid, and other public programs, the measure sets will be implemented and updated through the physician fee schedule in the coming Merit-Based Incentive Payment System (MIPS) and alternative payment models (APMs). Private payers are expected to begin implementing these quality measures as physician contracts come up for renewal.
“I think one of the biggest challenges will be how to collect these measures, because the decision about what measures to include was only driven in part by the feasibility,” Dr. Gellad said. “A large part was driven by what are the best metrics and what are most important outcomes and processes to evaluate. I think in terms of feasibility, that is going to be the next important question. How do you improve the feasibility of these measurements in practice?”
Electronic health records will play an integral part, as a FAQ released by AHIP notes that several of the measures “require that clinical data be extracted from EHRs or registries or be self-reported by clinicians.” It adds that clinicians and payers “will need to work together to create a reporting infrastructure for such measures” in areas where it is currently lacking.
The Centers for Medicare & Medicaid Services and America’s Health Insurance Plans on Feb 16 announced a set of core quality measures across seven areas that will serve as the foundation for a more uniform set of quality metrics that will be used by both public and private payers.
The CMS and AHIP are working with the National Quality Forum, medical specialty societies, employer groups, and consumer groups under an umbrella organization called the Core Quality Measures Collaborative. Together, they derived a set of measures that are “meaningful to patients, consumers, and physicians, while reducing variability in measure selection, collection burden, and cost,” the CMS noted in a fact sheet.
Involved medical specialty societies include the American Academy of Family Physicians, American College of Cardiology, American Heart Association, American College of Physicians, American Gastroenterological Association, HIV Medicine Association, Infectious Diseases Society of America, American Academy of Pediatrics, American Society of Clinical Oncology, and the American Medical Association.
The collaborative announced core measures in seven areas: accountable care organizations/patient centered medical homes/primary care, cardiology, gastroenterology, HIV and hepatitis C, medical oncology, obstetrics and gynecology, and orthopedics. Measures in additional areas will be released in the future and the core sets will be reviewed and updated on a regular basis.
“I think [this collaboration] strikes the best balance between what is feasible and what is desirable,” Dr. Ziad Gellad, chair of the AGA Quality Measures Committee, said in an interview.
For Medicare, Medicaid, and other public programs, the measure sets will be implemented and updated through the physician fee schedule in the coming Merit-Based Incentive Payment System (MIPS) and alternative payment models (APMs). Private payers are expected to begin implementing these quality measures as physician contracts come up for renewal.
“I think one of the biggest challenges will be how to collect these measures, because the decision about what measures to include was only driven in part by the feasibility,” Dr. Gellad said. “A large part was driven by what are the best metrics and what are most important outcomes and processes to evaluate. I think in terms of feasibility, that is going to be the next important question. How do you improve the feasibility of these measurements in practice?”
Electronic health records will play an integral part, as a FAQ released by AHIP notes that several of the measures “require that clinical data be extracted from EHRs or registries or be self-reported by clinicians.” It adds that clinicians and payers “will need to work together to create a reporting infrastructure for such measures” in areas where it is currently lacking.
STS: Valved conduit shows right ventricular outflow durability
PHOENIX – A prosthetic conduit that contains a porcine valve showed excellent intermediate-term durability for repairing the right ventricular outflow tract in 100 teenagers and young adults at a single U.S. center.
“The Carpentier-Edwards xenograft for right ventricular outflow tract [RVOT] reconstruction provides excellent freedom from reoperation and valve dysfunction, as well as sustained improvement in right-ventricular chamber size at intermediate-term follow-up,” Dr. Heidi B. Schubmehl said at the Society of Thoracic Surgeons annual meeting.
Dr. Schubmehl reported a 92% rate of freedom from valve dysfunction with follow-up out to about 10 years, and significant reductions in right ventricular size at follow-up, compared with baseline, as measured by both echocardiography and by MRI.
The Carpentier-Edwards porcine valve and conduit “seemed to hold up better than a lot of other [prosthetic] valves,” said Dr. George M. Alfieris, director of pediatric cardiac surgery at the University of Rochester (N.Y.), and senior author for the study. In addition to the valve’s durability over approximately the first 10 years following placement, the results also showed the positive impact the valve had on right ventricular size, an important result of the repair’s efficacy, Dr. Alfieris said.
“It’s a mistake to allow the right ventricle to be under high pressure or to reach a large volume. We now focus on preserving the right ventricle,” he said in an interview. “I’ve become very concerned about preventing right ventricular dilation and preserving right ventricular function.”
Dr. Alfieris noted that his prior experience using other types of valves in the pulmonary valve and RVOT position showed those valves “did great for the first 10 years and then failed. What’s different in this series is that after 10 years, we have not seen the same dysfunction as with the prior generation of valves. I will be very interested to see what happens to them” as follow-up continues beyond 10 years. He also expressed dismay that recently the company that had been marketing the valve and conduit used in the current study, the Carpentier-Edwards, stopped selling them. He expects that as his supply of conduits runs out he’ll have to start using a different commercial valve and conduit that he believes will not perform as well or create his own conduits with a porcine valve from a different supplier.
The series of 100 patients comprised individuals aged 17 or older who received a pulmonary artery and had RVOT reconstruction at the University of Rochester during 2000-2010, Dr. Schubmehl reported. The series included 78 patients with a history of tetralogy of Fallot, 8 patients born with transposition of their great arteries, 8 patients with truncus arteriosus, and 6 patients with other congenital heart diseases. Their median age at the time they received the RVOT conduit was 24 years, 59% were men, and 99 had undergone a prior sternotomy. At the time they received the conduit, 55 had pulmonary valve insufficiency, 30 had valve stenosis, and 15 had both. Follow-up occurred an average of 7 years after conduit placement.
Two recipients died: One death occurred perioperatively in a 41-year old who had a massive cerebrovascular event, and the second death was in a 39-year old who died 2.6 years after conduit placement from respiratory failure. Two additional patients required a reintervention during follow-up, said Dr. Schubmehl, a general surgeon at the University of Rochester. One reintervention occurred after 11 years to treat endocarditis, and the second after 11 years to perform balloon valvuloplasty because of valve stenosis.
The results reported by Dr. Schubmehl for echocardiography examinations showed that the patients had a statistically significant reduction in their RVOT pressure gradient from baseline to 1-year follow-up that was sustained through their intermediate-term follow-up. Seventy-seven patients had pulmonary valve insufficiency at baseline that resolved in all patients at 1-year follow-up and remained resolved in all but one patient at extended follow-up. Nineteen patients underwent additional imaging with MRI at an average follow-up of 7 years, and these findings confirmed the echo results.
On Twitter @mitchelzoler
The intermediate-term results reported by Dr. Schubmehl using a Carpentier-Edwards conduit in the right-ventricular outflow tract are clearly better than what we have seen using other types of valves and conduits in this position. If the valve and conduit they used persists with similar performance beyond 10 years, it would be a very good option. However, what typically happens is that replacement valves look good for about 10 years and then start to fail, often with a steep failure curve. I suspect that during the next 10 years of follow-up many more of the valves they placed will start to fail. The 10- to 20-year follow-up period is critical for demonstrating long-term durability of this valve and conduit.

|
Dr. James Jaggers |
One additional potential advantage of the Carpentier-Edwards prosthesis is that the valve it contains is larger than the usual valve placed in the right ventricular outflow tract (RVOT). Failed valves increasingly are replaced by a transcatheter approach that puts a new valve inside the old, failed valve. As patients who received these replacement valves continue to survive we anticipate their need over time for a series of valve-in-valve procedures. The larger the valve at the outset, the more feasible it will be to have multiple episodes of valve-in-valve replacement.
At one time, we regarded early surgical repair of a tetralogy of Fallot defect as curative. We now know that as children with a repaired tetralogy of Fallot grow into teens and adults they require additional repairs, most often replacement of their RVOTs. This has made pulmonary valve replacement the most common surgery for adult survivors of congenital heart disease. The numbers of teen or adult patients who require a new RVOT will steadily increase as more of these children survive.
Dr. James Jaggers, professor of surgery at the University of Colorado and chief of cardiothoracic surgery at Children’s Hospital Colorado in Denver, made these comments in an interview. He had no disclosures.
The intermediate-term results reported by Dr. Schubmehl using a Carpentier-Edwards conduit in the right-ventricular outflow tract are clearly better than what we have seen using other types of valves and conduits in this position. If the valve and conduit they used persists with similar performance beyond 10 years, it would be a very good option. However, what typically happens is that replacement valves look good for about 10 years and then start to fail, often with a steep failure curve. I suspect that during the next 10 years of follow-up many more of the valves they placed will start to fail. The 10- to 20-year follow-up period is critical for demonstrating long-term durability of this valve and conduit.

|
Dr. James Jaggers |
One additional potential advantage of the Carpentier-Edwards prosthesis is that the valve it contains is larger than the usual valve placed in the right ventricular outflow tract (RVOT). Failed valves increasingly are replaced by a transcatheter approach that puts a new valve inside the old, failed valve. As patients who received these replacement valves continue to survive we anticipate their need over time for a series of valve-in-valve procedures. The larger the valve at the outset, the more feasible it will be to have multiple episodes of valve-in-valve replacement.
At one time, we regarded early surgical repair of a tetralogy of Fallot defect as curative. We now know that as children with a repaired tetralogy of Fallot grow into teens and adults they require additional repairs, most often replacement of their RVOTs. This has made pulmonary valve replacement the most common surgery for adult survivors of congenital heart disease. The numbers of teen or adult patients who require a new RVOT will steadily increase as more of these children survive.
Dr. James Jaggers, professor of surgery at the University of Colorado and chief of cardiothoracic surgery at Children’s Hospital Colorado in Denver, made these comments in an interview. He had no disclosures.
The intermediate-term results reported by Dr. Schubmehl using a Carpentier-Edwards conduit in the right-ventricular outflow tract are clearly better than what we have seen using other types of valves and conduits in this position. If the valve and conduit they used persists with similar performance beyond 10 years, it would be a very good option. However, what typically happens is that replacement valves look good for about 10 years and then start to fail, often with a steep failure curve. I suspect that during the next 10 years of follow-up many more of the valves they placed will start to fail. The 10- to 20-year follow-up period is critical for demonstrating long-term durability of this valve and conduit.

|
Dr. James Jaggers |
One additional potential advantage of the Carpentier-Edwards prosthesis is that the valve it contains is larger than the usual valve placed in the right ventricular outflow tract (RVOT). Failed valves increasingly are replaced by a transcatheter approach that puts a new valve inside the old, failed valve. As patients who received these replacement valves continue to survive we anticipate their need over time for a series of valve-in-valve procedures. The larger the valve at the outset, the more feasible it will be to have multiple episodes of valve-in-valve replacement.
At one time, we regarded early surgical repair of a tetralogy of Fallot defect as curative. We now know that as children with a repaired tetralogy of Fallot grow into teens and adults they require additional repairs, most often replacement of their RVOTs. This has made pulmonary valve replacement the most common surgery for adult survivors of congenital heart disease. The numbers of teen or adult patients who require a new RVOT will steadily increase as more of these children survive.
Dr. James Jaggers, professor of surgery at the University of Colorado and chief of cardiothoracic surgery at Children’s Hospital Colorado in Denver, made these comments in an interview. He had no disclosures.
PHOENIX – A prosthetic conduit that contains a porcine valve showed excellent intermediate-term durability for repairing the right ventricular outflow tract in 100 teenagers and young adults at a single U.S. center.
“The Carpentier-Edwards xenograft for right ventricular outflow tract [RVOT] reconstruction provides excellent freedom from reoperation and valve dysfunction, as well as sustained improvement in right-ventricular chamber size at intermediate-term follow-up,” Dr. Heidi B. Schubmehl said at the Society of Thoracic Surgeons annual meeting.
Dr. Schubmehl reported a 92% rate of freedom from valve dysfunction with follow-up out to about 10 years, and significant reductions in right ventricular size at follow-up, compared with baseline, as measured by both echocardiography and by MRI.
The Carpentier-Edwards porcine valve and conduit “seemed to hold up better than a lot of other [prosthetic] valves,” said Dr. George M. Alfieris, director of pediatric cardiac surgery at the University of Rochester (N.Y.), and senior author for the study. In addition to the valve’s durability over approximately the first 10 years following placement, the results also showed the positive impact the valve had on right ventricular size, an important result of the repair’s efficacy, Dr. Alfieris said.
“It’s a mistake to allow the right ventricle to be under high pressure or to reach a large volume. We now focus on preserving the right ventricle,” he said in an interview. “I’ve become very concerned about preventing right ventricular dilation and preserving right ventricular function.”
Dr. Alfieris noted that his prior experience using other types of valves in the pulmonary valve and RVOT position showed those valves “did great for the first 10 years and then failed. What’s different in this series is that after 10 years, we have not seen the same dysfunction as with the prior generation of valves. I will be very interested to see what happens to them” as follow-up continues beyond 10 years. He also expressed dismay that recently the company that had been marketing the valve and conduit used in the current study, the Carpentier-Edwards, stopped selling them. He expects that as his supply of conduits runs out he’ll have to start using a different commercial valve and conduit that he believes will not perform as well or create his own conduits with a porcine valve from a different supplier.
The series of 100 patients comprised individuals aged 17 or older who received a pulmonary artery and had RVOT reconstruction at the University of Rochester during 2000-2010, Dr. Schubmehl reported. The series included 78 patients with a history of tetralogy of Fallot, 8 patients born with transposition of their great arteries, 8 patients with truncus arteriosus, and 6 patients with other congenital heart diseases. Their median age at the time they received the RVOT conduit was 24 years, 59% were men, and 99 had undergone a prior sternotomy. At the time they received the conduit, 55 had pulmonary valve insufficiency, 30 had valve stenosis, and 15 had both. Follow-up occurred an average of 7 years after conduit placement.
Two recipients died: One death occurred perioperatively in a 41-year old who had a massive cerebrovascular event, and the second death was in a 39-year old who died 2.6 years after conduit placement from respiratory failure. Two additional patients required a reintervention during follow-up, said Dr. Schubmehl, a general surgeon at the University of Rochester. One reintervention occurred after 11 years to treat endocarditis, and the second after 11 years to perform balloon valvuloplasty because of valve stenosis.
The results reported by Dr. Schubmehl for echocardiography examinations showed that the patients had a statistically significant reduction in their RVOT pressure gradient from baseline to 1-year follow-up that was sustained through their intermediate-term follow-up. Seventy-seven patients had pulmonary valve insufficiency at baseline that resolved in all patients at 1-year follow-up and remained resolved in all but one patient at extended follow-up. Nineteen patients underwent additional imaging with MRI at an average follow-up of 7 years, and these findings confirmed the echo results.
On Twitter @mitchelzoler
PHOENIX – A prosthetic conduit that contains a porcine valve showed excellent intermediate-term durability for repairing the right ventricular outflow tract in 100 teenagers and young adults at a single U.S. center.
“The Carpentier-Edwards xenograft for right ventricular outflow tract [RVOT] reconstruction provides excellent freedom from reoperation and valve dysfunction, as well as sustained improvement in right-ventricular chamber size at intermediate-term follow-up,” Dr. Heidi B. Schubmehl said at the Society of Thoracic Surgeons annual meeting.
Dr. Schubmehl reported a 92% rate of freedom from valve dysfunction with follow-up out to about 10 years, and significant reductions in right ventricular size at follow-up, compared with baseline, as measured by both echocardiography and by MRI.
The Carpentier-Edwards porcine valve and conduit “seemed to hold up better than a lot of other [prosthetic] valves,” said Dr. George M. Alfieris, director of pediatric cardiac surgery at the University of Rochester (N.Y.), and senior author for the study. In addition to the valve’s durability over approximately the first 10 years following placement, the results also showed the positive impact the valve had on right ventricular size, an important result of the repair’s efficacy, Dr. Alfieris said.
“It’s a mistake to allow the right ventricle to be under high pressure or to reach a large volume. We now focus on preserving the right ventricle,” he said in an interview. “I’ve become very concerned about preventing right ventricular dilation and preserving right ventricular function.”
Dr. Alfieris noted that his prior experience using other types of valves in the pulmonary valve and RVOT position showed those valves “did great for the first 10 years and then failed. What’s different in this series is that after 10 years, we have not seen the same dysfunction as with the prior generation of valves. I will be very interested to see what happens to them” as follow-up continues beyond 10 years. He also expressed dismay that recently the company that had been marketing the valve and conduit used in the current study, the Carpentier-Edwards, stopped selling them. He expects that as his supply of conduits runs out he’ll have to start using a different commercial valve and conduit that he believes will not perform as well or create his own conduits with a porcine valve from a different supplier.
The series of 100 patients comprised individuals aged 17 or older who received a pulmonary artery and had RVOT reconstruction at the University of Rochester during 2000-2010, Dr. Schubmehl reported. The series included 78 patients with a history of tetralogy of Fallot, 8 patients born with transposition of their great arteries, 8 patients with truncus arteriosus, and 6 patients with other congenital heart diseases. Their median age at the time they received the RVOT conduit was 24 years, 59% were men, and 99 had undergone a prior sternotomy. At the time they received the conduit, 55 had pulmonary valve insufficiency, 30 had valve stenosis, and 15 had both. Follow-up occurred an average of 7 years after conduit placement.
Two recipients died: One death occurred perioperatively in a 41-year old who had a massive cerebrovascular event, and the second death was in a 39-year old who died 2.6 years after conduit placement from respiratory failure. Two additional patients required a reintervention during follow-up, said Dr. Schubmehl, a general surgeon at the University of Rochester. One reintervention occurred after 11 years to treat endocarditis, and the second after 11 years to perform balloon valvuloplasty because of valve stenosis.
The results reported by Dr. Schubmehl for echocardiography examinations showed that the patients had a statistically significant reduction in their RVOT pressure gradient from baseline to 1-year follow-up that was sustained through their intermediate-term follow-up. Seventy-seven patients had pulmonary valve insufficiency at baseline that resolved in all patients at 1-year follow-up and remained resolved in all but one patient at extended follow-up. Nineteen patients underwent additional imaging with MRI at an average follow-up of 7 years, and these findings confirmed the echo results.
On Twitter @mitchelzoler
AT THE STS ANNUAL MEETING
Key clinical point: A prosthetic conduit with a porcine valve showed excellent durability for congenital heart defect repairs at intermediate-term follow-up.
Major finding: After an average 7-year follow-up, the replacement valve and conduit had a 92% rate of freedom from valve dysfunction.
Data source: Single-center series of 100 patients.
Disclosures: Dr. Schubmehl and Dr. Alfieris had no disclosures.
TAVR forges ahead in PARTNER III for low-risk patients
SNOWMASS, COLO. – The Food and Drug Administration has approved the first-ever U.S. randomized clinical trial of transcatheter aortic valve replacement versus open surgical replacement in low–surgical risk patients with symptomatic severe aortic stenosis.
The PARTNER III trial will enroll roughly 1,200 patients age 65 or older, all with a Society of Thoracic Surgeons risk score of less than 4%, at 50 sites beginning this spring, Dr. Vinod H. Thourani said at the Annual Cardiovascular Conference at Snowmass.
This is a noninferiority trial with a primary endpoint comprising a 1-year composite of death, stroke, or rehospitalization. The study is sponsored by Edwards Lifesciences, and patients randomized to transcatheter aortic valve replacement (TAVR) will receive the company’s low-profile Sapien 3 valve.
Coprincipal investigators are Dr. Michael J. Mack of the Baylor Health Care System in Plano, Tex., and Dr. Martin B. Leon of Columbia University, New York. Dr. Thourani is a member of the PARTNER III executive committee.
This is a study that could upend clinical practice, he observed.
“Are we going to have within the next 5 years 80%-90% of all patients who present with severe symptomatic aortic stenosis treated with transcatheter valves? We’re really at a major crossroads here, I believe,” said Dr. Thourani, professor of surgery and medicine and codirector of the structural heart and valve center at Emory University in Atlanta.
He ran down the numbers: Today, roughly 80% of all surgical aortic valve replacements (SAVR) in the United States are performed in low–surgical risk patients. These low-risk patients comprise roughly 65% of the total operable population with severe aortic stenosis. If PARTNER III and other data show that TAVR provides results comparable to SAVR in this group, Dr. Thourani predicted that it’s likely most low–surgical risk patients will opt for the less invasive approach. The appeal is no surgical incision, less pain, a shorter or no ICU stay, and faster return to normal activity.
Right now, U.S. and European guidelines state that TAVR is the preferred or alternative strategy to SAVR only in the relatively small group comprised of inoperable or high–surgical risk patients. In clinical practice, TAVR has already supplanted SAVR in the 10% of operable patients with high surgical risk. And TAVR is poised to do so in the roughly 25% of patients who fall into the intermediate–surgical risk category, according to the cardiothoracic surgeon.
He predicted that the 1-year outcomes of TAVR in more than 1,000 intermediate-risk participants in the PARTNER II trial will create a stir when presented this year, as a late-breaker at the annual meeting of the American College of Cardiology in Chicago. Although he stressed that he doesn’t know the results, the 30-day outcomes presented at last year’s Transcatheter Cardiovascular Therapeutics conference are extremely promising: a 1.1% all-cause mortality rate in patients with an average Society of Thoracic Surgeons risk score of 5.3%, for a stunning observed-to-expected ratio of just 0.21. Plus, a 1.0% rate of disabling stroke in this large multicenter randomized experience.
“That becomes really compelling data for us to think we’re ready now to go to the next step,” Dr. Thourani said. “My belief is at the rate we’re going, we’ll see most intermediate-risk patients going to TAVR.”
To date there has been only one randomized trial of TAVR versus SAVR in low–surgical risk patients: the Nordic Aortic Valve Intervention Trial (NOTION), which included 280 randomized patients with an average Society of Thoracic Surgeons risk score of 3%.
In the 2-year results presented by Dr. Lars Søndergaard of the University of Copenhagen at TCT 2015, all-cause mortality was 2.1% with TAVR and 3.7% with SAVR at 30 days, 4.9% with TAVR and 7.5% with SAVR at 12 months, and 8.0% versus 9.8% at 24 months. The 30-day rates of major bleeding, cardiogenic shock, atrial fibrillation, and acute kidney injury were all substantially lower in the TAVR group. All very impressive. However, Dr. Thourani found the TAVR patients’ pacemaker-requirement rate troubling. At 30 days post TAVR, 34% of patients had a pacemaker, compared with 1.6% of the SAVR group. By 24 months, 41% of the TAVR group had received a pacemaker, compared with just 4% of the SAVR group.
“What’s the acceptable pacemaker rate for someone utilizing TAVR – 5%, 10%, 40%? That’s something we as a community have to look at,” the surgeon observed. He noted that his purchase price for a TAVR valve is roughly $32,500, whereas a SAVR valve costs him $4,500. And at Emory, putting in a pacemaker costs an added $10,000-$15,000 for the device.
“If I’m putting a pacemaker in 40% of my TAVR patients at a cost of $40,000-$45,000 per patient for the valve and pacemaker, that becomes an issue,” Dr. Thourani said.
Other concerns surrounding TAVR, in addition to reimbursement, include the uncertain long-term impact of residual minimal paravalvular leak, which is common.
“We’re not done talking about paravalvular leak rates. As cardiologists you’re not okay with me giving your patient a minimal paravalvular leak post-SAVR. Are we going to change the bar a little bit for TAVR?” he mused.
Another issue is thrombosis of TAVR valve leaflets, Dr. Thourani continued. In a large patient series reported last year, this event occurred in 0.6% of patients, with an average of 181 days from TAVR to confirmatory abnormal imaging (Circ Cardiovasc Interv. 2015 Apr;8[4]. pii: e001779). Two clinical trials are gearing up to examine various anticoagulant strategies to address the problem.
Despite the various concerns, however, Dr. Thourani is extremely optimistic about TAVR’s future. It’s a booming field, with 396 U.S. TAVR centers as of 2015. The indications appear to be on the verge of expansion. Technical progress continues, with half a dozen TAVR valves in development in addition to the two now FDA approved.
“We have just scratched the surface of what we’re going to do in the management of severe aortic stenosis,” the surgeon promised.
The latest results of minimalist TAVR provide another reason for optimism regarding TAVR’s future.
Emory University surgeons and interventional cardiologists have been pacesetters in the minimalist TAVR approach. The key elements of minimalist TAVR are that the procedure is performed in the cardiac catheterization laboratory via transfemoral access, under conscious sedation, with transthoracic echocardiographic guidance, no Swan-Ganz catheter, and no ICU stay for most patients.
Dr. Thourani presented as-yet unpublished data on a recent series of 111 high–surgical risk patients who underwent minimalist TAVR with implantation of a Sapien 3 valve at Emory. Although their Society of Thoracic Surgeons risk score was 8%, there was zero 30-day mortality in this group. One patient had a major stroke, two had major vascular complications, and the 30-day readmission rate was just 3.8%.
“Can we get to these results universally? We think we can. This is the bar we need to start thinking about,” Dr. Thourani said.
Dr. Thourani reported serving as a consultant to Edwards Lifesciences and St. Jude Medical and receiving research grants from Abbott, Boston Scientific, Medtronic, and Sorin.
SNOWMASS, COLO. – The Food and Drug Administration has approved the first-ever U.S. randomized clinical trial of transcatheter aortic valve replacement versus open surgical replacement in low–surgical risk patients with symptomatic severe aortic stenosis.
The PARTNER III trial will enroll roughly 1,200 patients age 65 or older, all with a Society of Thoracic Surgeons risk score of less than 4%, at 50 sites beginning this spring, Dr. Vinod H. Thourani said at the Annual Cardiovascular Conference at Snowmass.
This is a noninferiority trial with a primary endpoint comprising a 1-year composite of death, stroke, or rehospitalization. The study is sponsored by Edwards Lifesciences, and patients randomized to transcatheter aortic valve replacement (TAVR) will receive the company’s low-profile Sapien 3 valve.
Coprincipal investigators are Dr. Michael J. Mack of the Baylor Health Care System in Plano, Tex., and Dr. Martin B. Leon of Columbia University, New York. Dr. Thourani is a member of the PARTNER III executive committee.
This is a study that could upend clinical practice, he observed.
“Are we going to have within the next 5 years 80%-90% of all patients who present with severe symptomatic aortic stenosis treated with transcatheter valves? We’re really at a major crossroads here, I believe,” said Dr. Thourani, professor of surgery and medicine and codirector of the structural heart and valve center at Emory University in Atlanta.
He ran down the numbers: Today, roughly 80% of all surgical aortic valve replacements (SAVR) in the United States are performed in low–surgical risk patients. These low-risk patients comprise roughly 65% of the total operable population with severe aortic stenosis. If PARTNER III and other data show that TAVR provides results comparable to SAVR in this group, Dr. Thourani predicted that it’s likely most low–surgical risk patients will opt for the less invasive approach. The appeal is no surgical incision, less pain, a shorter or no ICU stay, and faster return to normal activity.
Right now, U.S. and European guidelines state that TAVR is the preferred or alternative strategy to SAVR only in the relatively small group comprised of inoperable or high–surgical risk patients. In clinical practice, TAVR has already supplanted SAVR in the 10% of operable patients with high surgical risk. And TAVR is poised to do so in the roughly 25% of patients who fall into the intermediate–surgical risk category, according to the cardiothoracic surgeon.
He predicted that the 1-year outcomes of TAVR in more than 1,000 intermediate-risk participants in the PARTNER II trial will create a stir when presented this year, as a late-breaker at the annual meeting of the American College of Cardiology in Chicago. Although he stressed that he doesn’t know the results, the 30-day outcomes presented at last year’s Transcatheter Cardiovascular Therapeutics conference are extremely promising: a 1.1% all-cause mortality rate in patients with an average Society of Thoracic Surgeons risk score of 5.3%, for a stunning observed-to-expected ratio of just 0.21. Plus, a 1.0% rate of disabling stroke in this large multicenter randomized experience.
“That becomes really compelling data for us to think we’re ready now to go to the next step,” Dr. Thourani said. “My belief is at the rate we’re going, we’ll see most intermediate-risk patients going to TAVR.”
To date there has been only one randomized trial of TAVR versus SAVR in low–surgical risk patients: the Nordic Aortic Valve Intervention Trial (NOTION), which included 280 randomized patients with an average Society of Thoracic Surgeons risk score of 3%.
In the 2-year results presented by Dr. Lars Søndergaard of the University of Copenhagen at TCT 2015, all-cause mortality was 2.1% with TAVR and 3.7% with SAVR at 30 days, 4.9% with TAVR and 7.5% with SAVR at 12 months, and 8.0% versus 9.8% at 24 months. The 30-day rates of major bleeding, cardiogenic shock, atrial fibrillation, and acute kidney injury were all substantially lower in the TAVR group. All very impressive. However, Dr. Thourani found the TAVR patients’ pacemaker-requirement rate troubling. At 30 days post TAVR, 34% of patients had a pacemaker, compared with 1.6% of the SAVR group. By 24 months, 41% of the TAVR group had received a pacemaker, compared with just 4% of the SAVR group.
“What’s the acceptable pacemaker rate for someone utilizing TAVR – 5%, 10%, 40%? That’s something we as a community have to look at,” the surgeon observed. He noted that his purchase price for a TAVR valve is roughly $32,500, whereas a SAVR valve costs him $4,500. And at Emory, putting in a pacemaker costs an added $10,000-$15,000 for the device.
“If I’m putting a pacemaker in 40% of my TAVR patients at a cost of $40,000-$45,000 per patient for the valve and pacemaker, that becomes an issue,” Dr. Thourani said.
Other concerns surrounding TAVR, in addition to reimbursement, include the uncertain long-term impact of residual minimal paravalvular leak, which is common.
“We’re not done talking about paravalvular leak rates. As cardiologists you’re not okay with me giving your patient a minimal paravalvular leak post-SAVR. Are we going to change the bar a little bit for TAVR?” he mused.
Another issue is thrombosis of TAVR valve leaflets, Dr. Thourani continued. In a large patient series reported last year, this event occurred in 0.6% of patients, with an average of 181 days from TAVR to confirmatory abnormal imaging (Circ Cardiovasc Interv. 2015 Apr;8[4]. pii: e001779). Two clinical trials are gearing up to examine various anticoagulant strategies to address the problem.
Despite the various concerns, however, Dr. Thourani is extremely optimistic about TAVR’s future. It’s a booming field, with 396 U.S. TAVR centers as of 2015. The indications appear to be on the verge of expansion. Technical progress continues, with half a dozen TAVR valves in development in addition to the two now FDA approved.
“We have just scratched the surface of what we’re going to do in the management of severe aortic stenosis,” the surgeon promised.
The latest results of minimalist TAVR provide another reason for optimism regarding TAVR’s future.
Emory University surgeons and interventional cardiologists have been pacesetters in the minimalist TAVR approach. The key elements of minimalist TAVR are that the procedure is performed in the cardiac catheterization laboratory via transfemoral access, under conscious sedation, with transthoracic echocardiographic guidance, no Swan-Ganz catheter, and no ICU stay for most patients.
Dr. Thourani presented as-yet unpublished data on a recent series of 111 high–surgical risk patients who underwent minimalist TAVR with implantation of a Sapien 3 valve at Emory. Although their Society of Thoracic Surgeons risk score was 8%, there was zero 30-day mortality in this group. One patient had a major stroke, two had major vascular complications, and the 30-day readmission rate was just 3.8%.
“Can we get to these results universally? We think we can. This is the bar we need to start thinking about,” Dr. Thourani said.
Dr. Thourani reported serving as a consultant to Edwards Lifesciences and St. Jude Medical and receiving research grants from Abbott, Boston Scientific, Medtronic, and Sorin.
SNOWMASS, COLO. – The Food and Drug Administration has approved the first-ever U.S. randomized clinical trial of transcatheter aortic valve replacement versus open surgical replacement in low–surgical risk patients with symptomatic severe aortic stenosis.
The PARTNER III trial will enroll roughly 1,200 patients age 65 or older, all with a Society of Thoracic Surgeons risk score of less than 4%, at 50 sites beginning this spring, Dr. Vinod H. Thourani said at the Annual Cardiovascular Conference at Snowmass.
This is a noninferiority trial with a primary endpoint comprising a 1-year composite of death, stroke, or rehospitalization. The study is sponsored by Edwards Lifesciences, and patients randomized to transcatheter aortic valve replacement (TAVR) will receive the company’s low-profile Sapien 3 valve.
Coprincipal investigators are Dr. Michael J. Mack of the Baylor Health Care System in Plano, Tex., and Dr. Martin B. Leon of Columbia University, New York. Dr. Thourani is a member of the PARTNER III executive committee.
This is a study that could upend clinical practice, he observed.
“Are we going to have within the next 5 years 80%-90% of all patients who present with severe symptomatic aortic stenosis treated with transcatheter valves? We’re really at a major crossroads here, I believe,” said Dr. Thourani, professor of surgery and medicine and codirector of the structural heart and valve center at Emory University in Atlanta.
He ran down the numbers: Today, roughly 80% of all surgical aortic valve replacements (SAVR) in the United States are performed in low–surgical risk patients. These low-risk patients comprise roughly 65% of the total operable population with severe aortic stenosis. If PARTNER III and other data show that TAVR provides results comparable to SAVR in this group, Dr. Thourani predicted that it’s likely most low–surgical risk patients will opt for the less invasive approach. The appeal is no surgical incision, less pain, a shorter or no ICU stay, and faster return to normal activity.
Right now, U.S. and European guidelines state that TAVR is the preferred or alternative strategy to SAVR only in the relatively small group comprised of inoperable or high–surgical risk patients. In clinical practice, TAVR has already supplanted SAVR in the 10% of operable patients with high surgical risk. And TAVR is poised to do so in the roughly 25% of patients who fall into the intermediate–surgical risk category, according to the cardiothoracic surgeon.
He predicted that the 1-year outcomes of TAVR in more than 1,000 intermediate-risk participants in the PARTNER II trial will create a stir when presented this year, as a late-breaker at the annual meeting of the American College of Cardiology in Chicago. Although he stressed that he doesn’t know the results, the 30-day outcomes presented at last year’s Transcatheter Cardiovascular Therapeutics conference are extremely promising: a 1.1% all-cause mortality rate in patients with an average Society of Thoracic Surgeons risk score of 5.3%, for a stunning observed-to-expected ratio of just 0.21. Plus, a 1.0% rate of disabling stroke in this large multicenter randomized experience.
“That becomes really compelling data for us to think we’re ready now to go to the next step,” Dr. Thourani said. “My belief is at the rate we’re going, we’ll see most intermediate-risk patients going to TAVR.”
To date there has been only one randomized trial of TAVR versus SAVR in low–surgical risk patients: the Nordic Aortic Valve Intervention Trial (NOTION), which included 280 randomized patients with an average Society of Thoracic Surgeons risk score of 3%.
In the 2-year results presented by Dr. Lars Søndergaard of the University of Copenhagen at TCT 2015, all-cause mortality was 2.1% with TAVR and 3.7% with SAVR at 30 days, 4.9% with TAVR and 7.5% with SAVR at 12 months, and 8.0% versus 9.8% at 24 months. The 30-day rates of major bleeding, cardiogenic shock, atrial fibrillation, and acute kidney injury were all substantially lower in the TAVR group. All very impressive. However, Dr. Thourani found the TAVR patients’ pacemaker-requirement rate troubling. At 30 days post TAVR, 34% of patients had a pacemaker, compared with 1.6% of the SAVR group. By 24 months, 41% of the TAVR group had received a pacemaker, compared with just 4% of the SAVR group.
“What’s the acceptable pacemaker rate for someone utilizing TAVR – 5%, 10%, 40%? That’s something we as a community have to look at,” the surgeon observed. He noted that his purchase price for a TAVR valve is roughly $32,500, whereas a SAVR valve costs him $4,500. And at Emory, putting in a pacemaker costs an added $10,000-$15,000 for the device.
“If I’m putting a pacemaker in 40% of my TAVR patients at a cost of $40,000-$45,000 per patient for the valve and pacemaker, that becomes an issue,” Dr. Thourani said.
Other concerns surrounding TAVR, in addition to reimbursement, include the uncertain long-term impact of residual minimal paravalvular leak, which is common.
“We’re not done talking about paravalvular leak rates. As cardiologists you’re not okay with me giving your patient a minimal paravalvular leak post-SAVR. Are we going to change the bar a little bit for TAVR?” he mused.
Another issue is thrombosis of TAVR valve leaflets, Dr. Thourani continued. In a large patient series reported last year, this event occurred in 0.6% of patients, with an average of 181 days from TAVR to confirmatory abnormal imaging (Circ Cardiovasc Interv. 2015 Apr;8[4]. pii: e001779). Two clinical trials are gearing up to examine various anticoagulant strategies to address the problem.
Despite the various concerns, however, Dr. Thourani is extremely optimistic about TAVR’s future. It’s a booming field, with 396 U.S. TAVR centers as of 2015. The indications appear to be on the verge of expansion. Technical progress continues, with half a dozen TAVR valves in development in addition to the two now FDA approved.
“We have just scratched the surface of what we’re going to do in the management of severe aortic stenosis,” the surgeon promised.
The latest results of minimalist TAVR provide another reason for optimism regarding TAVR’s future.
Emory University surgeons and interventional cardiologists have been pacesetters in the minimalist TAVR approach. The key elements of minimalist TAVR are that the procedure is performed in the cardiac catheterization laboratory via transfemoral access, under conscious sedation, with transthoracic echocardiographic guidance, no Swan-Ganz catheter, and no ICU stay for most patients.
Dr. Thourani presented as-yet unpublished data on a recent series of 111 high–surgical risk patients who underwent minimalist TAVR with implantation of a Sapien 3 valve at Emory. Although their Society of Thoracic Surgeons risk score was 8%, there was zero 30-day mortality in this group. One patient had a major stroke, two had major vascular complications, and the 30-day readmission rate was just 3.8%.
“Can we get to these results universally? We think we can. This is the bar we need to start thinking about,” Dr. Thourani said.
Dr. Thourani reported serving as a consultant to Edwards Lifesciences and St. Jude Medical and receiving research grants from Abbott, Boston Scientific, Medtronic, and Sorin.
EXPERT ANALYSIS FROM THE CARDIOVASCULAR CONFERENCE AT SNOWMASS
CMS clarifies how to report Medicare overpayments
There is finally some clarity about how to report and return Medicare overpayments, under a final rule released by the Centers for Medicare & Medicaid Services Feb. 11.
The final regulation clarifies that health providers have identified an overpayment when they “have or should have, through the exercise of reasonable diligence, determined [they have] received an overpayment and quantified the amount of the overpayment.”
Overpayments must be reported and returned only if identified within 6 years of the date the payment was received – down from the 10 years included in the proposed rule released in 2012. Physician organizations and other health care stakeholders had criticized the proposal, calling the 10-year time frame unreasonable and burdensome.
The revised definition of identification makes more sense for physicians, particularly that identification exists when providers have quantified the amount of the overpayment, said Scot T. Hasselman, a Washington health law attorney. In many cases, it takes time to decipher how much money is owed after discovering a potential overpayment, he said in an interview.
“This all goes to: When does the clock begin ticking for the 60 days?” he said. “The language in the final rule provides for a standard that is easier to apply.
The 6-year time frame is also more reasonable and will save practices money by limiting their audit obligations, Mr. Hasselman noted.
The final rule also allows the 60-day deadline for returning overpayments to be suspended if a provider requests an extended repayment schedule. In the past, “people could be in a real pickle if they didn’t have the money to return,” Mr. Hasselman said. “This [provision] is important, especially for smaller [practices] and physicians who may not have big credit lines or the cash flow of an institutional provider.”
The final rule also clarifies how to report overpayments. Providers and suppliers must use an applicable claims adjustment, credit balance, self-reported refund, or another appropriate process to satisfy the obligation to report and return overpayments, the rule states. If a provider has reported a self-identified overpayment using the self-referral disclosure protocol managed by CMS or the self-disclosure protocol managed by the HHS Office of Inspector General (OIG), the provider is considered to be in compliance with the rule.
But the final rule is not entirely positive, according to Houston-based health law attorney Michael E. Clark. Many health providers had requested clarification about the level of resources small providers are expected to devote to investigating potential overpayments. Commenters suggested CMS allow for more defined overpayment responses based on provider size and resources. The agency did not do so, saying that providers, “large and small have a duty to ensure claims are accurate and appropriate and to report and return overpayments they have received.”
Refusing to allow scalable responses is unfortunate for practices that do not have the ability to react to overpayments as robustly as larger chains, Mr. Clark said.
“The agency was unwilling to go that far,” Mr. Clark said. “They’re not going to give a lesser standard for smaller providers. They’re going to look at the facts and circumstances. It gives [CMS] subjectivity, whereas doctors would rather have more clarification and objectivity.”
On Twitter @legal_med
There is finally some clarity about how to report and return Medicare overpayments, under a final rule released by the Centers for Medicare & Medicaid Services Feb. 11.
The final regulation clarifies that health providers have identified an overpayment when they “have or should have, through the exercise of reasonable diligence, determined [they have] received an overpayment and quantified the amount of the overpayment.”
Overpayments must be reported and returned only if identified within 6 years of the date the payment was received – down from the 10 years included in the proposed rule released in 2012. Physician organizations and other health care stakeholders had criticized the proposal, calling the 10-year time frame unreasonable and burdensome.
The revised definition of identification makes more sense for physicians, particularly that identification exists when providers have quantified the amount of the overpayment, said Scot T. Hasselman, a Washington health law attorney. In many cases, it takes time to decipher how much money is owed after discovering a potential overpayment, he said in an interview.
“This all goes to: When does the clock begin ticking for the 60 days?” he said. “The language in the final rule provides for a standard that is easier to apply.
The 6-year time frame is also more reasonable and will save practices money by limiting their audit obligations, Mr. Hasselman noted.
The final rule also allows the 60-day deadline for returning overpayments to be suspended if a provider requests an extended repayment schedule. In the past, “people could be in a real pickle if they didn’t have the money to return,” Mr. Hasselman said. “This [provision] is important, especially for smaller [practices] and physicians who may not have big credit lines or the cash flow of an institutional provider.”
The final rule also clarifies how to report overpayments. Providers and suppliers must use an applicable claims adjustment, credit balance, self-reported refund, or another appropriate process to satisfy the obligation to report and return overpayments, the rule states. If a provider has reported a self-identified overpayment using the self-referral disclosure protocol managed by CMS or the self-disclosure protocol managed by the HHS Office of Inspector General (OIG), the provider is considered to be in compliance with the rule.
But the final rule is not entirely positive, according to Houston-based health law attorney Michael E. Clark. Many health providers had requested clarification about the level of resources small providers are expected to devote to investigating potential overpayments. Commenters suggested CMS allow for more defined overpayment responses based on provider size and resources. The agency did not do so, saying that providers, “large and small have a duty to ensure claims are accurate and appropriate and to report and return overpayments they have received.”
Refusing to allow scalable responses is unfortunate for practices that do not have the ability to react to overpayments as robustly as larger chains, Mr. Clark said.
“The agency was unwilling to go that far,” Mr. Clark said. “They’re not going to give a lesser standard for smaller providers. They’re going to look at the facts and circumstances. It gives [CMS] subjectivity, whereas doctors would rather have more clarification and objectivity.”
On Twitter @legal_med
There is finally some clarity about how to report and return Medicare overpayments, under a final rule released by the Centers for Medicare & Medicaid Services Feb. 11.
The final regulation clarifies that health providers have identified an overpayment when they “have or should have, through the exercise of reasonable diligence, determined [they have] received an overpayment and quantified the amount of the overpayment.”
Overpayments must be reported and returned only if identified within 6 years of the date the payment was received – down from the 10 years included in the proposed rule released in 2012. Physician organizations and other health care stakeholders had criticized the proposal, calling the 10-year time frame unreasonable and burdensome.
The revised definition of identification makes more sense for physicians, particularly that identification exists when providers have quantified the amount of the overpayment, said Scot T. Hasselman, a Washington health law attorney. In many cases, it takes time to decipher how much money is owed after discovering a potential overpayment, he said in an interview.
“This all goes to: When does the clock begin ticking for the 60 days?” he said. “The language in the final rule provides for a standard that is easier to apply.
The 6-year time frame is also more reasonable and will save practices money by limiting their audit obligations, Mr. Hasselman noted.
The final rule also allows the 60-day deadline for returning overpayments to be suspended if a provider requests an extended repayment schedule. In the past, “people could be in a real pickle if they didn’t have the money to return,” Mr. Hasselman said. “This [provision] is important, especially for smaller [practices] and physicians who may not have big credit lines or the cash flow of an institutional provider.”
The final rule also clarifies how to report overpayments. Providers and suppliers must use an applicable claims adjustment, credit balance, self-reported refund, or another appropriate process to satisfy the obligation to report and return overpayments, the rule states. If a provider has reported a self-identified overpayment using the self-referral disclosure protocol managed by CMS or the self-disclosure protocol managed by the HHS Office of Inspector General (OIG), the provider is considered to be in compliance with the rule.
But the final rule is not entirely positive, according to Houston-based health law attorney Michael E. Clark. Many health providers had requested clarification about the level of resources small providers are expected to devote to investigating potential overpayments. Commenters suggested CMS allow for more defined overpayment responses based on provider size and resources. The agency did not do so, saying that providers, “large and small have a duty to ensure claims are accurate and appropriate and to report and return overpayments they have received.”
Refusing to allow scalable responses is unfortunate for practices that do not have the ability to react to overpayments as robustly as larger chains, Mr. Clark said.
“The agency was unwilling to go that far,” Mr. Clark said. “They’re not going to give a lesser standard for smaller providers. They’re going to look at the facts and circumstances. It gives [CMS] subjectivity, whereas doctors would rather have more clarification and objectivity.”
On Twitter @legal_med
CMS extends deadline for 2015 EHR attestation
Physicians and hospitals will have an extra 2 weeks to attest to meaningful use of electronic health records in 2015.
The deadline for attesting to 90 days of continuous use during the calendar year for physicians or from Oct. 1, 2014, through the end of 2015 for hospitals is now March 11. Previously, attestations were due Feb. 29 to avoid penalties under the EHR Incentive Program for not meeting meaningful use requirements in 2015.
The deadline was extended to align EHR attestation with the Physician Quality Reporting System, according to CMS officials.
Attestations can be made on the CMS website.
Physicians and hospitals will have an extra 2 weeks to attest to meaningful use of electronic health records in 2015.
The deadline for attesting to 90 days of continuous use during the calendar year for physicians or from Oct. 1, 2014, through the end of 2015 for hospitals is now March 11. Previously, attestations were due Feb. 29 to avoid penalties under the EHR Incentive Program for not meeting meaningful use requirements in 2015.
The deadline was extended to align EHR attestation with the Physician Quality Reporting System, according to CMS officials.
Attestations can be made on the CMS website.
Physicians and hospitals will have an extra 2 weeks to attest to meaningful use of electronic health records in 2015.
The deadline for attesting to 90 days of continuous use during the calendar year for physicians or from Oct. 1, 2014, through the end of 2015 for hospitals is now March 11. Previously, attestations were due Feb. 29 to avoid penalties under the EHR Incentive Program for not meeting meaningful use requirements in 2015.
The deadline was extended to align EHR attestation with the Physician Quality Reporting System, according to CMS officials.
Attestations can be made on the CMS website.
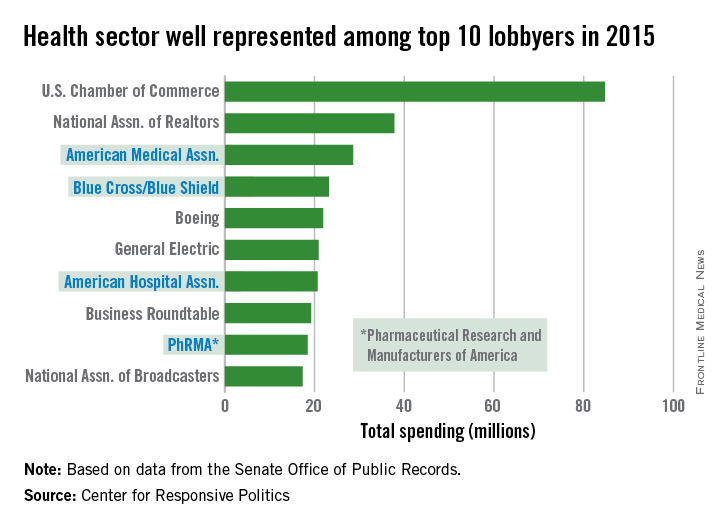
AMA spending tops health-sector lobbying
The health sector let itself be heard in Washington in 2015, as the American Medical Association and three other organizations spent their way into the lobbying top 10, according to the Center for Responsive Politics.
The AMA spent $28.6 million on lobbying last year – tops among the health sector and third among all lobbyers. The other health sector entities among the top 10 in spending were Blue Cross/Blue Shield at $23.2 million (fourth overall), the American Hospital Association at $20.7 million (seventh), and the Pharmaceutical Research and Manufacturers of America at $18.4 million (ninth), the center reported.
That’s definitely nothing to sneeze at, but it would have taken the combined spending of all four, about $90.9 million, to surpass the U.S. Chamber of Commerce, which led all lobbyers at $84.7 million.
Total spending on lobbying for the health sector was $507 million in 2015, which was second to “miscellaneous business” (including the Chamber of Commerce) among the center’s 13 ranked sectors. Total spending on lobbying for all sectors was $3.2 billion for the year, according to the center’s calculations, which were based on data from the Senate Office of Public Records.
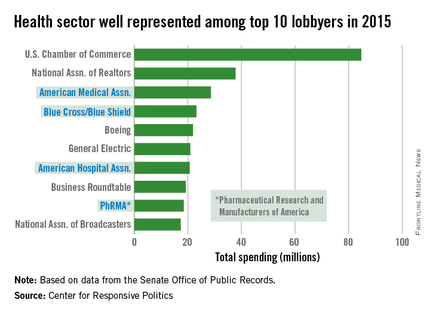
The health sector let itself be heard in Washington in 2015, as the American Medical Association and three other organizations spent their way into the lobbying top 10, according to the Center for Responsive Politics.
The AMA spent $28.6 million on lobbying last year – tops among the health sector and third among all lobbyers. The other health sector entities among the top 10 in spending were Blue Cross/Blue Shield at $23.2 million (fourth overall), the American Hospital Association at $20.7 million (seventh), and the Pharmaceutical Research and Manufacturers of America at $18.4 million (ninth), the center reported.
That’s definitely nothing to sneeze at, but it would have taken the combined spending of all four, about $90.9 million, to surpass the U.S. Chamber of Commerce, which led all lobbyers at $84.7 million.
Total spending on lobbying for the health sector was $507 million in 2015, which was second to “miscellaneous business” (including the Chamber of Commerce) among the center’s 13 ranked sectors. Total spending on lobbying for all sectors was $3.2 billion for the year, according to the center’s calculations, which were based on data from the Senate Office of Public Records.

The health sector let itself be heard in Washington in 2015, as the American Medical Association and three other organizations spent their way into the lobbying top 10, according to the Center for Responsive Politics.
The AMA spent $28.6 million on lobbying last year – tops among the health sector and third among all lobbyers. The other health sector entities among the top 10 in spending were Blue Cross/Blue Shield at $23.2 million (fourth overall), the American Hospital Association at $20.7 million (seventh), and the Pharmaceutical Research and Manufacturers of America at $18.4 million (ninth), the center reported.
That’s definitely nothing to sneeze at, but it would have taken the combined spending of all four, about $90.9 million, to surpass the U.S. Chamber of Commerce, which led all lobbyers at $84.7 million.
Total spending on lobbying for the health sector was $507 million in 2015, which was second to “miscellaneous business” (including the Chamber of Commerce) among the center’s 13 ranked sectors. Total spending on lobbying for all sectors was $3.2 billion for the year, according to the center’s calculations, which were based on data from the Senate Office of Public Records.

What’s next for Watchman stroke prevention device
SNOWMASS, COLO. – The goal is finally in sight following an odyssey to develop the Watchman left atrial appendage closure device as a safe and effective alternative to oral anticoagulation for stroke prevention in patients with nonvalvular atrial fibrillation, Dr. David R. Holmes Jr. said at the Annual Cardiovascular Conference at Snowmass.
It’s all coming together: The Watchman, a small percutaneously delivered parachute-like device, has received FDA marketing approval as the sole authorized left atrial appendage closure device in this country on the strength of two compellingly positive randomized controlled trials. A recent meta-analysis of those trials showed significantly fewer hemorrhagic strokes, fewer cardiovascular or unexplained deaths, and fewer nonprocedural bleeding events in Watchman recipients than in patients randomized to warfarin. And a cost-effectiveness analysis has concluded that after 8 years, the Watchman becomes “the dominant strategy” – meaning more effective and less costly for stroke prevention in patients with atrial fibrillation (AF) having a contraindication to warfarin – compared with the novel oral anticoagulant apixaban (J Am Coll Cardiol. 2015 Dec 22;66[24]:2728-39).
Moreover, the final pieces required for the Watchman to become a mainstream reimbursable therapy are falling into place. The Society for Cardiovascular Angiography and Interventions (SCAI), the American College of Cardiology, and the Heart Rhythm Society (HRS) have jointly issued institutional and operator requirements for left atrial appendage occlusion programs (J Am Coll Cardiol. 2015 Dec 8. pii: S0735-1097[15]07550-6). The ACC’s National Cardiovascular Data Registry has set up a new left atrial occlusion registry. And most important of all from a reimbursement standpoint, the Centers for Medicare and Medicaid Services has released a preliminary National Coverage Determination for left atrial appendage occlusion.
“This will affect your patients and your lives,” noted Dr. Holmes of the Mayo Clinic in Rochester, Minn. He and the Mayo Clinic share a financial interest in the Watchman technology, which has been licensed to Boston Scientific.
The CMS will cover the Watchman only when the catheter procedure is performed by an experienced interventional cardiologist or electrophysiologist in an experienced center as defined by the SCAI/ACC/HRS standards, and only in patients enrolled in the national prospective registry. The registry will monitor operator and device-related complications, stroke and systemic embolism rates, deaths, and major bleeding rates for 5 years post-procedure.
“If you want to be in this field, you will be in that registry because reimbursement will depend on that,” Dr. Holmes explained. “This registry will be incredibly important. It will tell us how we’re doing, what we should be doing, and what we could potentially be doing in the future.”
One hitch is that the preliminary National Coverage Determination states that coverage will be limited to AF patients with high stroke-risk and HAS-BLED scores as well as a contraindication to warfarin, whereas the FDA-approved indication says patients must be deemed by their physician to be suitable for warfarin.
“In this particular case, CMS was not talking with FDA. We don’t know how that will get sorted out,” according to the cardiologist. “But as soon as CMS comes through with their final regulatory coverage determination, I think we will finally be there. We’ll then be able to offer this as a treatment strategy for stroke prevention in selected patients with atrial fibrillation, realizing that with this device there’s a 40% reduction in the composite endpoint of cardiovascular or unexplained death, stroke, and systemic embolism compared to warfarin.”
That figure of a 40% relative risk reduction comes from the 46-month follow-up data in the randomized PROTECT AF trial (JAMA. 2014 Nov 19;312[19]:1988-98).
More recently, Dr. Holmes and his coinvestigators published a patient-level meta-analysis of data from PROTECT AF and PREVAIL, the other randomized trial of the Watchman versus warfarin. They reported that Watchman recipients had a 78% reduction in hemorrhagic strokes, a 52% reduction in cardiovascular or unexplained deaths, and a 49% lower rate of nonprocedural bleeding, compared with patients assigned to warfarin (J Am Coll Cardiol. 2015 Jun 23;65[24]:2614-23).
There have been no randomized trials comparing the Watchman to novel oral anticoagulants.
Dr. Holmes said the worldwide experience to date has been that roughly 95% of AF patients are able to safely go off warfarin or a novel oral anticoagulant 12 months after Watchman placement.
“So instead of taking eight drugs when you’re 75 years old, you can take seven. Most patients think that’s a pretty good deal,” the cardiologist observed.
Session moderator Dr. Samuel J. Asirvatham posed a question: Since recurrence of AF following catheter ablation is common and it’s now thought that up to 20% of AF arises from foci located in the left atrial appendage, what about combining standard catheter ablation of AF via pulmonary vein isolation with placement of the Watchman in a single procedure?
If such a combined procedure can be done efficiently, it should enable recipients of AF catheter ablation to safely go off oral anticoagulation, noted Dr. Asirvatham, an electrophysiologist who is professor of medicine and pediatrics at the Mayo Clinic, Rochester, Minn.
Dr. Holmes said several small studies of patients who have received combined AF ablation and Watchman implantation have been published.
“It’s uncertain whether left atrial appendage closure will affect AF recurrence rates post ablation, but it should reduce stroke risk. It’s a terribly important field of exploration that will be pursued in registries both in Europe and the United States,” he said.
SNOWMASS, COLO. – The goal is finally in sight following an odyssey to develop the Watchman left atrial appendage closure device as a safe and effective alternative to oral anticoagulation for stroke prevention in patients with nonvalvular atrial fibrillation, Dr. David R. Holmes Jr. said at the Annual Cardiovascular Conference at Snowmass.
It’s all coming together: The Watchman, a small percutaneously delivered parachute-like device, has received FDA marketing approval as the sole authorized left atrial appendage closure device in this country on the strength of two compellingly positive randomized controlled trials. A recent meta-analysis of those trials showed significantly fewer hemorrhagic strokes, fewer cardiovascular or unexplained deaths, and fewer nonprocedural bleeding events in Watchman recipients than in patients randomized to warfarin. And a cost-effectiveness analysis has concluded that after 8 years, the Watchman becomes “the dominant strategy” – meaning more effective and less costly for stroke prevention in patients with atrial fibrillation (AF) having a contraindication to warfarin – compared with the novel oral anticoagulant apixaban (J Am Coll Cardiol. 2015 Dec 22;66[24]:2728-39).
Moreover, the final pieces required for the Watchman to become a mainstream reimbursable therapy are falling into place. The Society for Cardiovascular Angiography and Interventions (SCAI), the American College of Cardiology, and the Heart Rhythm Society (HRS) have jointly issued institutional and operator requirements for left atrial appendage occlusion programs (J Am Coll Cardiol. 2015 Dec 8. pii: S0735-1097[15]07550-6). The ACC’s National Cardiovascular Data Registry has set up a new left atrial occlusion registry. And most important of all from a reimbursement standpoint, the Centers for Medicare and Medicaid Services has released a preliminary National Coverage Determination for left atrial appendage occlusion.
“This will affect your patients and your lives,” noted Dr. Holmes of the Mayo Clinic in Rochester, Minn. He and the Mayo Clinic share a financial interest in the Watchman technology, which has been licensed to Boston Scientific.
The CMS will cover the Watchman only when the catheter procedure is performed by an experienced interventional cardiologist or electrophysiologist in an experienced center as defined by the SCAI/ACC/HRS standards, and only in patients enrolled in the national prospective registry. The registry will monitor operator and device-related complications, stroke and systemic embolism rates, deaths, and major bleeding rates for 5 years post-procedure.
“If you want to be in this field, you will be in that registry because reimbursement will depend on that,” Dr. Holmes explained. “This registry will be incredibly important. It will tell us how we’re doing, what we should be doing, and what we could potentially be doing in the future.”
One hitch is that the preliminary National Coverage Determination states that coverage will be limited to AF patients with high stroke-risk and HAS-BLED scores as well as a contraindication to warfarin, whereas the FDA-approved indication says patients must be deemed by their physician to be suitable for warfarin.
“In this particular case, CMS was not talking with FDA. We don’t know how that will get sorted out,” according to the cardiologist. “But as soon as CMS comes through with their final regulatory coverage determination, I think we will finally be there. We’ll then be able to offer this as a treatment strategy for stroke prevention in selected patients with atrial fibrillation, realizing that with this device there’s a 40% reduction in the composite endpoint of cardiovascular or unexplained death, stroke, and systemic embolism compared to warfarin.”
That figure of a 40% relative risk reduction comes from the 46-month follow-up data in the randomized PROTECT AF trial (JAMA. 2014 Nov 19;312[19]:1988-98).
More recently, Dr. Holmes and his coinvestigators published a patient-level meta-analysis of data from PROTECT AF and PREVAIL, the other randomized trial of the Watchman versus warfarin. They reported that Watchman recipients had a 78% reduction in hemorrhagic strokes, a 52% reduction in cardiovascular or unexplained deaths, and a 49% lower rate of nonprocedural bleeding, compared with patients assigned to warfarin (J Am Coll Cardiol. 2015 Jun 23;65[24]:2614-23).
There have been no randomized trials comparing the Watchman to novel oral anticoagulants.
Dr. Holmes said the worldwide experience to date has been that roughly 95% of AF patients are able to safely go off warfarin or a novel oral anticoagulant 12 months after Watchman placement.
“So instead of taking eight drugs when you’re 75 years old, you can take seven. Most patients think that’s a pretty good deal,” the cardiologist observed.
Session moderator Dr. Samuel J. Asirvatham posed a question: Since recurrence of AF following catheter ablation is common and it’s now thought that up to 20% of AF arises from foci located in the left atrial appendage, what about combining standard catheter ablation of AF via pulmonary vein isolation with placement of the Watchman in a single procedure?
If such a combined procedure can be done efficiently, it should enable recipients of AF catheter ablation to safely go off oral anticoagulation, noted Dr. Asirvatham, an electrophysiologist who is professor of medicine and pediatrics at the Mayo Clinic, Rochester, Minn.
Dr. Holmes said several small studies of patients who have received combined AF ablation and Watchman implantation have been published.
“It’s uncertain whether left atrial appendage closure will affect AF recurrence rates post ablation, but it should reduce stroke risk. It’s a terribly important field of exploration that will be pursued in registries both in Europe and the United States,” he said.
SNOWMASS, COLO. – The goal is finally in sight following an odyssey to develop the Watchman left atrial appendage closure device as a safe and effective alternative to oral anticoagulation for stroke prevention in patients with nonvalvular atrial fibrillation, Dr. David R. Holmes Jr. said at the Annual Cardiovascular Conference at Snowmass.
It’s all coming together: The Watchman, a small percutaneously delivered parachute-like device, has received FDA marketing approval as the sole authorized left atrial appendage closure device in this country on the strength of two compellingly positive randomized controlled trials. A recent meta-analysis of those trials showed significantly fewer hemorrhagic strokes, fewer cardiovascular or unexplained deaths, and fewer nonprocedural bleeding events in Watchman recipients than in patients randomized to warfarin. And a cost-effectiveness analysis has concluded that after 8 years, the Watchman becomes “the dominant strategy” – meaning more effective and less costly for stroke prevention in patients with atrial fibrillation (AF) having a contraindication to warfarin – compared with the novel oral anticoagulant apixaban (J Am Coll Cardiol. 2015 Dec 22;66[24]:2728-39).
Moreover, the final pieces required for the Watchman to become a mainstream reimbursable therapy are falling into place. The Society for Cardiovascular Angiography and Interventions (SCAI), the American College of Cardiology, and the Heart Rhythm Society (HRS) have jointly issued institutional and operator requirements for left atrial appendage occlusion programs (J Am Coll Cardiol. 2015 Dec 8. pii: S0735-1097[15]07550-6). The ACC’s National Cardiovascular Data Registry has set up a new left atrial occlusion registry. And most important of all from a reimbursement standpoint, the Centers for Medicare and Medicaid Services has released a preliminary National Coverage Determination for left atrial appendage occlusion.
“This will affect your patients and your lives,” noted Dr. Holmes of the Mayo Clinic in Rochester, Minn. He and the Mayo Clinic share a financial interest in the Watchman technology, which has been licensed to Boston Scientific.
The CMS will cover the Watchman only when the catheter procedure is performed by an experienced interventional cardiologist or electrophysiologist in an experienced center as defined by the SCAI/ACC/HRS standards, and only in patients enrolled in the national prospective registry. The registry will monitor operator and device-related complications, stroke and systemic embolism rates, deaths, and major bleeding rates for 5 years post-procedure.
“If you want to be in this field, you will be in that registry because reimbursement will depend on that,” Dr. Holmes explained. “This registry will be incredibly important. It will tell us how we’re doing, what we should be doing, and what we could potentially be doing in the future.”
One hitch is that the preliminary National Coverage Determination states that coverage will be limited to AF patients with high stroke-risk and HAS-BLED scores as well as a contraindication to warfarin, whereas the FDA-approved indication says patients must be deemed by their physician to be suitable for warfarin.
“In this particular case, CMS was not talking with FDA. We don’t know how that will get sorted out,” according to the cardiologist. “But as soon as CMS comes through with their final regulatory coverage determination, I think we will finally be there. We’ll then be able to offer this as a treatment strategy for stroke prevention in selected patients with atrial fibrillation, realizing that with this device there’s a 40% reduction in the composite endpoint of cardiovascular or unexplained death, stroke, and systemic embolism compared to warfarin.”
That figure of a 40% relative risk reduction comes from the 46-month follow-up data in the randomized PROTECT AF trial (JAMA. 2014 Nov 19;312[19]:1988-98).
More recently, Dr. Holmes and his coinvestigators published a patient-level meta-analysis of data from PROTECT AF and PREVAIL, the other randomized trial of the Watchman versus warfarin. They reported that Watchman recipients had a 78% reduction in hemorrhagic strokes, a 52% reduction in cardiovascular or unexplained deaths, and a 49% lower rate of nonprocedural bleeding, compared with patients assigned to warfarin (J Am Coll Cardiol. 2015 Jun 23;65[24]:2614-23).
There have been no randomized trials comparing the Watchman to novel oral anticoagulants.
Dr. Holmes said the worldwide experience to date has been that roughly 95% of AF patients are able to safely go off warfarin or a novel oral anticoagulant 12 months after Watchman placement.
“So instead of taking eight drugs when you’re 75 years old, you can take seven. Most patients think that’s a pretty good deal,” the cardiologist observed.
Session moderator Dr. Samuel J. Asirvatham posed a question: Since recurrence of AF following catheter ablation is common and it’s now thought that up to 20% of AF arises from foci located in the left atrial appendage, what about combining standard catheter ablation of AF via pulmonary vein isolation with placement of the Watchman in a single procedure?
If such a combined procedure can be done efficiently, it should enable recipients of AF catheter ablation to safely go off oral anticoagulation, noted Dr. Asirvatham, an electrophysiologist who is professor of medicine and pediatrics at the Mayo Clinic, Rochester, Minn.
Dr. Holmes said several small studies of patients who have received combined AF ablation and Watchman implantation have been published.
“It’s uncertain whether left atrial appendage closure will affect AF recurrence rates post ablation, but it should reduce stroke risk. It’s a terribly important field of exploration that will be pursued in registries both in Europe and the United States,” he said.
EXPERT ANALYSIS FROM THE CARDIOVASCULAR CONFERENCE AT SNOWMASS
Cancer death rates show wide geographic variation
Over 595,000 cancer deaths – an average of about 1,600 each day – are expected in the United States in 2016, but those deaths are not evenly distributed among the states, according to investigators from the American Cancer Society.
Estimates from the ACS show that, in 2016, Utah will have a cancer death rate of 99 per 100,000 population, the lowest in the country. Those same estimates predict that West Virginia will have a highest-in-the-country death rate of 258.2 per 100,000 – 2.6 times higher than Utah’s. Other states with high estimated death rates include Maine, Kentucky, Arkansas, and Pennsylvania, noted Rebecca L. Siegel and her associates at the ACS (CA Cancer J Clin. 2016 Jan;66[1]:7-30).
Besides Utah, the states with the lowest estimated cancer death rates in 2016 are Colorado, Texas, and Alaska, along with the District of Columbia. The national death rate for cancer has dropped 23% since 1991, the investigators said, but cancer is now the leading cause of death in 21 states. This good news/bad news situation comes about mainly as a result of “exceptionally large reductions in death from heart disease,” they added.
Ms. Siegel and her associates calculated the expected number of cancer deaths “based on the annual percent change in reported numbers of cancer deaths from 1998 through 2012 at the state and national levels as reported to the [National Center for Health Statistics].” Death rates were calculated here using estimated populations for 2015 from the U.S. Census Bureau.
Over 595,000 cancer deaths – an average of about 1,600 each day – are expected in the United States in 2016, but those deaths are not evenly distributed among the states, according to investigators from the American Cancer Society.
Estimates from the ACS show that, in 2016, Utah will have a cancer death rate of 99 per 100,000 population, the lowest in the country. Those same estimates predict that West Virginia will have a highest-in-the-country death rate of 258.2 per 100,000 – 2.6 times higher than Utah’s. Other states with high estimated death rates include Maine, Kentucky, Arkansas, and Pennsylvania, noted Rebecca L. Siegel and her associates at the ACS (CA Cancer J Clin. 2016 Jan;66[1]:7-30).

Besides Utah, the states with the lowest estimated cancer death rates in 2016 are Colorado, Texas, and Alaska, along with the District of Columbia. The national death rate for cancer has dropped 23% since 1991, the investigators said, but cancer is now the leading cause of death in 21 states. This good news/bad news situation comes about mainly as a result of “exceptionally large reductions in death from heart disease,” they added.
Ms. Siegel and her associates calculated the expected number of cancer deaths “based on the annual percent change in reported numbers of cancer deaths from 1998 through 2012 at the state and national levels as reported to the [National Center for Health Statistics].” Death rates were calculated here using estimated populations for 2015 from the U.S. Census Bureau.
Over 595,000 cancer deaths – an average of about 1,600 each day – are expected in the United States in 2016, but those deaths are not evenly distributed among the states, according to investigators from the American Cancer Society.
Estimates from the ACS show that, in 2016, Utah will have a cancer death rate of 99 per 100,000 population, the lowest in the country. Those same estimates predict that West Virginia will have a highest-in-the-country death rate of 258.2 per 100,000 – 2.6 times higher than Utah’s. Other states with high estimated death rates include Maine, Kentucky, Arkansas, and Pennsylvania, noted Rebecca L. Siegel and her associates at the ACS (CA Cancer J Clin. 2016 Jan;66[1]:7-30).

Besides Utah, the states with the lowest estimated cancer death rates in 2016 are Colorado, Texas, and Alaska, along with the District of Columbia. The national death rate for cancer has dropped 23% since 1991, the investigators said, but cancer is now the leading cause of death in 21 states. This good news/bad news situation comes about mainly as a result of “exceptionally large reductions in death from heart disease,” they added.
Ms. Siegel and her associates calculated the expected number of cancer deaths “based on the annual percent change in reported numbers of cancer deaths from 1998 through 2012 at the state and national levels as reported to the [National Center for Health Statistics].” Death rates were calculated here using estimated populations for 2015 from the U.S. Census Bureau.
Early elective colon resection common in diverticulitis
A significant number of elective colon resections for uncomplicated diverticulitis are done in individuals who have experienced fewer than three episodes.
Researchers analyzed nationwide data from 87,461 immunocompetent patients with at least one claim for diverticulitis, of whom 5,604 (6.4%) underwent a resection.
According to a paper published online Feb. 10 in JAMA Surgery, 94.9% of resections, in a final cohort of 3,054 patients, occurred in individuals with fewer than three episodes of diverticulitis, if only inpatient claims were counted (doi:10.1001/jamasurg.2015.5478).
If both inpatient and outpatient claims for diverticulitis were counted, 80.5% of patients who underwent resection had experienced fewer than three episodes, and if all types of claims (including antibiotic prescription claims for diverticulitis) were counted, that figure dropped to 56.3%.
Individuals who underwent early resection were slightly more likely to be male (risk ratio [RR], 1.07; 95% confidence interval [CI], 1.02-1.13; P = .004) but were of a similar age to those who underwent resection after three or more episodes of diverticulitis.
The mean time between the last two episodes of diverticulitis was longer in individuals who underwent early surgery compared to those who delayed surgery (157 days vs. 96 days; P less than .001).
Patients residing in the South were also significantly more likely to undergo early surgery than were those residing in an other regions, with 60.5% of policy holders there undergoing early surgery compared to 50.7% in the West.
Insurance status also influenced the likelihood of early surgery, as patients with HMO or capitated insurance plans were less likely to undergo early surgery than were patients with other plan types.
In the last decade, professional guidelines have moved toward recommending elective surgery for diverticulitis after three or more episodes, but at the same time, the incidence of elective resection has more than doubled, reported Dr. Vlad V. Simianu of the University of Washington, Seattle, and coauthors.
This study covered a period of data in which guidelines on elective resection have remained in a relatively steady state, offering an opportunity to assess guideline adherence.
“Within this context, the suspected drivers of early elective surgery (younger age, laparoscopy, more frequent episodes, and personal financial risk) were not found to be associated with earlier operations for diverticulitis,” the authors wrote.
The analysis found laparoscopy was not associated with early surgery, which the authors said challenged the hypothesis that the threshold for early surgical resection might be lowered by the availability of laparoscopy.
The lack of an age difference between those undergoing early resection also challenged the notion that younger patients may experience more severe diverticulitis and suffer a greater impact on their quality of life and that this may drive physicians to operate earlier.
The authors noted that patient factors such as quality of life and anxiety about future episodes of diverticulitis, and surgeon-related factors such as training, local practice, and referral patterns were not tested, and that these may account for some of decisions about early surgery.
The National Institute of Diabetes and Digestive and Kidney Diseases and the University of Washington supported the study. No conflicts of interest were declared.
A significant number of elective colon resections for uncomplicated diverticulitis are done in individuals who have experienced fewer than three episodes.
Researchers analyzed nationwide data from 87,461 immunocompetent patients with at least one claim for diverticulitis, of whom 5,604 (6.4%) underwent a resection.
According to a paper published online Feb. 10 in JAMA Surgery, 94.9% of resections, in a final cohort of 3,054 patients, occurred in individuals with fewer than three episodes of diverticulitis, if only inpatient claims were counted (doi:10.1001/jamasurg.2015.5478).
If both inpatient and outpatient claims for diverticulitis were counted, 80.5% of patients who underwent resection had experienced fewer than three episodes, and if all types of claims (including antibiotic prescription claims for diverticulitis) were counted, that figure dropped to 56.3%.
Individuals who underwent early resection were slightly more likely to be male (risk ratio [RR], 1.07; 95% confidence interval [CI], 1.02-1.13; P = .004) but were of a similar age to those who underwent resection after three or more episodes of diverticulitis.
The mean time between the last two episodes of diverticulitis was longer in individuals who underwent early surgery compared to those who delayed surgery (157 days vs. 96 days; P less than .001).
Patients residing in the South were also significantly more likely to undergo early surgery than were those residing in an other regions, with 60.5% of policy holders there undergoing early surgery compared to 50.7% in the West.
Insurance status also influenced the likelihood of early surgery, as patients with HMO or capitated insurance plans were less likely to undergo early surgery than were patients with other plan types.
In the last decade, professional guidelines have moved toward recommending elective surgery for diverticulitis after three or more episodes, but at the same time, the incidence of elective resection has more than doubled, reported Dr. Vlad V. Simianu of the University of Washington, Seattle, and coauthors.
This study covered a period of data in which guidelines on elective resection have remained in a relatively steady state, offering an opportunity to assess guideline adherence.
“Within this context, the suspected drivers of early elective surgery (younger age, laparoscopy, more frequent episodes, and personal financial risk) were not found to be associated with earlier operations for diverticulitis,” the authors wrote.
The analysis found laparoscopy was not associated with early surgery, which the authors said challenged the hypothesis that the threshold for early surgical resection might be lowered by the availability of laparoscopy.
The lack of an age difference between those undergoing early resection also challenged the notion that younger patients may experience more severe diverticulitis and suffer a greater impact on their quality of life and that this may drive physicians to operate earlier.
The authors noted that patient factors such as quality of life and anxiety about future episodes of diverticulitis, and surgeon-related factors such as training, local practice, and referral patterns were not tested, and that these may account for some of decisions about early surgery.
The National Institute of Diabetes and Digestive and Kidney Diseases and the University of Washington supported the study. No conflicts of interest were declared.
A significant number of elective colon resections for uncomplicated diverticulitis are done in individuals who have experienced fewer than three episodes.
Researchers analyzed nationwide data from 87,461 immunocompetent patients with at least one claim for diverticulitis, of whom 5,604 (6.4%) underwent a resection.
According to a paper published online Feb. 10 in JAMA Surgery, 94.9% of resections, in a final cohort of 3,054 patients, occurred in individuals with fewer than three episodes of diverticulitis, if only inpatient claims were counted (doi:10.1001/jamasurg.2015.5478).
If both inpatient and outpatient claims for diverticulitis were counted, 80.5% of patients who underwent resection had experienced fewer than three episodes, and if all types of claims (including antibiotic prescription claims for diverticulitis) were counted, that figure dropped to 56.3%.
Individuals who underwent early resection were slightly more likely to be male (risk ratio [RR], 1.07; 95% confidence interval [CI], 1.02-1.13; P = .004) but were of a similar age to those who underwent resection after three or more episodes of diverticulitis.
The mean time between the last two episodes of diverticulitis was longer in individuals who underwent early surgery compared to those who delayed surgery (157 days vs. 96 days; P less than .001).
Patients residing in the South were also significantly more likely to undergo early surgery than were those residing in an other regions, with 60.5% of policy holders there undergoing early surgery compared to 50.7% in the West.
Insurance status also influenced the likelihood of early surgery, as patients with HMO or capitated insurance plans were less likely to undergo early surgery than were patients with other plan types.
In the last decade, professional guidelines have moved toward recommending elective surgery for diverticulitis after three or more episodes, but at the same time, the incidence of elective resection has more than doubled, reported Dr. Vlad V. Simianu of the University of Washington, Seattle, and coauthors.
This study covered a period of data in which guidelines on elective resection have remained in a relatively steady state, offering an opportunity to assess guideline adherence.
“Within this context, the suspected drivers of early elective surgery (younger age, laparoscopy, more frequent episodes, and personal financial risk) were not found to be associated with earlier operations for diverticulitis,” the authors wrote.
The analysis found laparoscopy was not associated with early surgery, which the authors said challenged the hypothesis that the threshold for early surgical resection might be lowered by the availability of laparoscopy.
The lack of an age difference between those undergoing early resection also challenged the notion that younger patients may experience more severe diverticulitis and suffer a greater impact on their quality of life and that this may drive physicians to operate earlier.
The authors noted that patient factors such as quality of life and anxiety about future episodes of diverticulitis, and surgeon-related factors such as training, local practice, and referral patterns were not tested, and that these may account for some of decisions about early surgery.
The National Institute of Diabetes and Digestive and Kidney Diseases and the University of Washington supported the study. No conflicts of interest were declared.
FROM JAMA SURGERY
Key clinical point: A majority of elective colon resections for uncomplicated diverticulitis are done in individuals who have experienced fewer than three episodes.
Major finding: More than 90% of elective resections occur in patients who have experienced fewer than three inpatient-managed episodes of diverticulitis.
Data source: Retrospective cohort study of 87,461 patients who underwent surgical resection.
Disclosures: The National Institute of Diabetes and Digestive and Kidney Diseases and the University of Washington supported the study. No conflicts of interest were declared.
Aortic aneurysms pose unique challenges in transplant recipients
CHICAGO – Surgeons can expect to see more abdominal organ transplant recipients presenting with aortic aneurysms, as transplant survival rates increase along with the age of organ donors and recipients.
“The consensus is that abdominal aortic aneurysms (AAAs) have a more aggressive course post-transplant and within that context, probably need to be managed more aggressively,” Dr. Michael J. Englesbe of the University of Michigan, Ann Arbor said at the annual Northwestern Vascular Symposium.
Some 270,000 Americans are living with a functioning liver or kidney graft, and their average age has risen from 47 years to 57 years over the last decade.
Though the data isn’t great, it’s hypothesized that the immunosuppression prerequisite for successful organ transplantation promotes the progression of atherosclerosis and aneurysm growth in transplant patients, he said.
New-onset diabetes, hyperlipidemia, and hypertension are all common post-transplant due to immunosuppression therapy. Aortic aneurysms are also reported to rupture at smaller sizes in transplant recipients.
Intriguingly, the opposite effect has been observed in experimental animal models, where immunosuppression with calcineurin inhibitors and mammalian target of rapamycin (mTOR) inhibitors has been shown to stabilize atherosclerotic lesions and inhibit aneurysm expansion.
The reason for this disparity is unclear, but immunosuppressants likely augment other cardiovascular comorbidities such as hypertension and atherosclerosis and this may trump their anti-inflammatory effects and lead to worse aneurysm disease and faster expansion in humans, Dr. Englesbe speculated in an interview.
As for when aneurysms should be fixed, kidney transplant candidates should undergo AAA repair prior to transplantation since the risk of renal complications after aneurysm repair puts the allograft at risk, Dr. Englesbe advised. Either an open or endovascular approach can be used.
In liver transplant candidates, elective AAA repair should be avoided if possible and is contraindicated if any signs of hepatic decompensation are present such as muscle wasting, ascites, platelet count less than 50 x 109/L, or encephalopathy. For well-compensated cirrhotic patients, endovascular repair is best.
One of the most important considerations for any solid-organ transplant patient undergoing aneurysm repair is perioperative management of immunosuppression, Dr. Englesbe stressed.
Transplant patients are maintained on oral calcineurin inhibitors such as cyclosporine and tacrolimus (Prograf) throughout the perioperative period to prevent organ rejection, but these drugs have nephrotoxic effects. About 10% of recipients, typically the sicker patients, will be switched to mTOR inhibitors such as everolimus (Afinitor) and sirolumus (Rapamune) as a kidney-sparing alternative.
“Part of the mechanism of these [mTOR] drugs is that they really affect fibroblast functioning, so patients that are on these medications, their wound will fall apart and they will invariably get a hernia,” Dr. Englesbe said. “You have to stop them upwards of about 6 weeks before surgical intervention, and I think this is also true for many endografts.”
He highlighted a case in which an mTOR inhibitor was started three months after liver transplant due to renal dysfunction in a patient who was fully healed, but within three weeks, “her wound fell apart, completely fell apart.” She developed several seromas underneath her incision, one of which became infected and took months to close.
“The transplant professionals – your nephrologists, your cardiologists – aren’t going to know this fact, but as a transplant surgeon it’s usually the first question we’re going to ask with respect to any post-transplant patient we’re going to operate on, so it’s something to keep in mind,” Dr. Englesbe said.
Another take-home message was the importance of maintaining kidney function in kidney recipients presenting with aortic aneurysm, as mortality in these patients is about 10-fold higher once the kidney fails, he said. A recent study reported that AAAs are significantly more common in kidney than liver transplant recipients (29.6% vs. 11.4%; P = .02), despite a similar prevalence for any aneurysm (4%) in both groups (J Vasc Surg. 2014 Mar;59;594-8).
When kidney recipients present, preoperative imaging of the aorta from the aneurysm to the kidney allograft is mandatory, he said. Endovascular repair is preferred, whenever possible.
The renal graft is typically sewn to the external iliac artery 3 cm to 10 cm from the bifurcation of the external and internal iliac arteries. Because of this, repair is challenging when aneurysmal disease involves the iliac artery, Dr. Englesbe observed. Aneurysmal dilation is less common in the external iliac, but stenting an iliac aneurysm can still compromise inflow to the transplanted kidney.
Several surgical techniques including axillofemoral bypass, aortofemoral shunt, or extracorporeal circuit have been reported to preserve renal function during open AAA repair in renal transplant recipients. These techniques are not without their own risk of complications and should be avoided in patients with low creatinine, but are appropriate in patients with marginal or impaired renal function, according to Dr. Englesbe, who reported having no relevant disclosures.
CHICAGO – Surgeons can expect to see more abdominal organ transplant recipients presenting with aortic aneurysms, as transplant survival rates increase along with the age of organ donors and recipients.
“The consensus is that abdominal aortic aneurysms (AAAs) have a more aggressive course post-transplant and within that context, probably need to be managed more aggressively,” Dr. Michael J. Englesbe of the University of Michigan, Ann Arbor said at the annual Northwestern Vascular Symposium.
Some 270,000 Americans are living with a functioning liver or kidney graft, and their average age has risen from 47 years to 57 years over the last decade.
Though the data isn’t great, it’s hypothesized that the immunosuppression prerequisite for successful organ transplantation promotes the progression of atherosclerosis and aneurysm growth in transplant patients, he said.
New-onset diabetes, hyperlipidemia, and hypertension are all common post-transplant due to immunosuppression therapy. Aortic aneurysms are also reported to rupture at smaller sizes in transplant recipients.
Intriguingly, the opposite effect has been observed in experimental animal models, where immunosuppression with calcineurin inhibitors and mammalian target of rapamycin (mTOR) inhibitors has been shown to stabilize atherosclerotic lesions and inhibit aneurysm expansion.
The reason for this disparity is unclear, but immunosuppressants likely augment other cardiovascular comorbidities such as hypertension and atherosclerosis and this may trump their anti-inflammatory effects and lead to worse aneurysm disease and faster expansion in humans, Dr. Englesbe speculated in an interview.
As for when aneurysms should be fixed, kidney transplant candidates should undergo AAA repair prior to transplantation since the risk of renal complications after aneurysm repair puts the allograft at risk, Dr. Englesbe advised. Either an open or endovascular approach can be used.
In liver transplant candidates, elective AAA repair should be avoided if possible and is contraindicated if any signs of hepatic decompensation are present such as muscle wasting, ascites, platelet count less than 50 x 109/L, or encephalopathy. For well-compensated cirrhotic patients, endovascular repair is best.
One of the most important considerations for any solid-organ transplant patient undergoing aneurysm repair is perioperative management of immunosuppression, Dr. Englesbe stressed.
Transplant patients are maintained on oral calcineurin inhibitors such as cyclosporine and tacrolimus (Prograf) throughout the perioperative period to prevent organ rejection, but these drugs have nephrotoxic effects. About 10% of recipients, typically the sicker patients, will be switched to mTOR inhibitors such as everolimus (Afinitor) and sirolumus (Rapamune) as a kidney-sparing alternative.
“Part of the mechanism of these [mTOR] drugs is that they really affect fibroblast functioning, so patients that are on these medications, their wound will fall apart and they will invariably get a hernia,” Dr. Englesbe said. “You have to stop them upwards of about 6 weeks before surgical intervention, and I think this is also true for many endografts.”
He highlighted a case in which an mTOR inhibitor was started three months after liver transplant due to renal dysfunction in a patient who was fully healed, but within three weeks, “her wound fell apart, completely fell apart.” She developed several seromas underneath her incision, one of which became infected and took months to close.
“The transplant professionals – your nephrologists, your cardiologists – aren’t going to know this fact, but as a transplant surgeon it’s usually the first question we’re going to ask with respect to any post-transplant patient we’re going to operate on, so it’s something to keep in mind,” Dr. Englesbe said.
Another take-home message was the importance of maintaining kidney function in kidney recipients presenting with aortic aneurysm, as mortality in these patients is about 10-fold higher once the kidney fails, he said. A recent study reported that AAAs are significantly more common in kidney than liver transplant recipients (29.6% vs. 11.4%; P = .02), despite a similar prevalence for any aneurysm (4%) in both groups (J Vasc Surg. 2014 Mar;59;594-8).
When kidney recipients present, preoperative imaging of the aorta from the aneurysm to the kidney allograft is mandatory, he said. Endovascular repair is preferred, whenever possible.
The renal graft is typically sewn to the external iliac artery 3 cm to 10 cm from the bifurcation of the external and internal iliac arteries. Because of this, repair is challenging when aneurysmal disease involves the iliac artery, Dr. Englesbe observed. Aneurysmal dilation is less common in the external iliac, but stenting an iliac aneurysm can still compromise inflow to the transplanted kidney.
Several surgical techniques including axillofemoral bypass, aortofemoral shunt, or extracorporeal circuit have been reported to preserve renal function during open AAA repair in renal transplant recipients. These techniques are not without their own risk of complications and should be avoided in patients with low creatinine, but are appropriate in patients with marginal or impaired renal function, according to Dr. Englesbe, who reported having no relevant disclosures.
CHICAGO – Surgeons can expect to see more abdominal organ transplant recipients presenting with aortic aneurysms, as transplant survival rates increase along with the age of organ donors and recipients.
“The consensus is that abdominal aortic aneurysms (AAAs) have a more aggressive course post-transplant and within that context, probably need to be managed more aggressively,” Dr. Michael J. Englesbe of the University of Michigan, Ann Arbor said at the annual Northwestern Vascular Symposium.
Some 270,000 Americans are living with a functioning liver or kidney graft, and their average age has risen from 47 years to 57 years over the last decade.
Though the data isn’t great, it’s hypothesized that the immunosuppression prerequisite for successful organ transplantation promotes the progression of atherosclerosis and aneurysm growth in transplant patients, he said.
New-onset diabetes, hyperlipidemia, and hypertension are all common post-transplant due to immunosuppression therapy. Aortic aneurysms are also reported to rupture at smaller sizes in transplant recipients.
Intriguingly, the opposite effect has been observed in experimental animal models, where immunosuppression with calcineurin inhibitors and mammalian target of rapamycin (mTOR) inhibitors has been shown to stabilize atherosclerotic lesions and inhibit aneurysm expansion.
The reason for this disparity is unclear, but immunosuppressants likely augment other cardiovascular comorbidities such as hypertension and atherosclerosis and this may trump their anti-inflammatory effects and lead to worse aneurysm disease and faster expansion in humans, Dr. Englesbe speculated in an interview.
As for when aneurysms should be fixed, kidney transplant candidates should undergo AAA repair prior to transplantation since the risk of renal complications after aneurysm repair puts the allograft at risk, Dr. Englesbe advised. Either an open or endovascular approach can be used.
In liver transplant candidates, elective AAA repair should be avoided if possible and is contraindicated if any signs of hepatic decompensation are present such as muscle wasting, ascites, platelet count less than 50 x 109/L, or encephalopathy. For well-compensated cirrhotic patients, endovascular repair is best.
One of the most important considerations for any solid-organ transplant patient undergoing aneurysm repair is perioperative management of immunosuppression, Dr. Englesbe stressed.
Transplant patients are maintained on oral calcineurin inhibitors such as cyclosporine and tacrolimus (Prograf) throughout the perioperative period to prevent organ rejection, but these drugs have nephrotoxic effects. About 10% of recipients, typically the sicker patients, will be switched to mTOR inhibitors such as everolimus (Afinitor) and sirolumus (Rapamune) as a kidney-sparing alternative.
“Part of the mechanism of these [mTOR] drugs is that they really affect fibroblast functioning, so patients that are on these medications, their wound will fall apart and they will invariably get a hernia,” Dr. Englesbe said. “You have to stop them upwards of about 6 weeks before surgical intervention, and I think this is also true for many endografts.”
He highlighted a case in which an mTOR inhibitor was started three months after liver transplant due to renal dysfunction in a patient who was fully healed, but within three weeks, “her wound fell apart, completely fell apart.” She developed several seromas underneath her incision, one of which became infected and took months to close.
“The transplant professionals – your nephrologists, your cardiologists – aren’t going to know this fact, but as a transplant surgeon it’s usually the first question we’re going to ask with respect to any post-transplant patient we’re going to operate on, so it’s something to keep in mind,” Dr. Englesbe said.
Another take-home message was the importance of maintaining kidney function in kidney recipients presenting with aortic aneurysm, as mortality in these patients is about 10-fold higher once the kidney fails, he said. A recent study reported that AAAs are significantly more common in kidney than liver transplant recipients (29.6% vs. 11.4%; P = .02), despite a similar prevalence for any aneurysm (4%) in both groups (J Vasc Surg. 2014 Mar;59;594-8).
When kidney recipients present, preoperative imaging of the aorta from the aneurysm to the kidney allograft is mandatory, he said. Endovascular repair is preferred, whenever possible.
The renal graft is typically sewn to the external iliac artery 3 cm to 10 cm from the bifurcation of the external and internal iliac arteries. Because of this, repair is challenging when aneurysmal disease involves the iliac artery, Dr. Englesbe observed. Aneurysmal dilation is less common in the external iliac, but stenting an iliac aneurysm can still compromise inflow to the transplanted kidney.
Several surgical techniques including axillofemoral bypass, aortofemoral shunt, or extracorporeal circuit have been reported to preserve renal function during open AAA repair in renal transplant recipients. These techniques are not without their own risk of complications and should be avoided in patients with low creatinine, but are appropriate in patients with marginal or impaired renal function, according to Dr. Englesbe, who reported having no relevant disclosures.
EXPERT ANALYSIS FROM THE NORTHWESTERN VASCULAR SYMPOSIUM