User login
Improved Communication Center Stage in Multiple Sclerosis
This transcript has been edited for clarity.
Joseph R. Berger, MD: Hi. I'm Dr Joseph Berger, and I'm joined for this Care Cues conversation with my patient, Michelle Biloon, who has had multiple sclerosis (MS) for the past 6 years. Hello, Michelle. Welcome.
Michelle Biloon: Thank you, Dr Berger.
Berger: Can you tell us a little bit about yourself, how you came to understand you had MS, and how you've done since the diagnosis was rendered?
Biloon: Yeah. It was a very short diagnosis period for me. In the winter of 2017, I started experiencing dizzy spells, and I didn't really know why. I eventually went to my primary care clinic where my doctor is, and they did blood work. Then, they did a CT and didn't see anything, and I just kind of kept feeling worse.
Then, finally, I went to an ENT just to see if it was maybe related to my ears. The ENT actually said, "You need to go to the ER and get an MRI." And while I was in the MRI, I could feel the dizzy spells. And I thought, Well, something is happening. I don't know what it is. And then a resident came in and said that they saw lesions on my brain, and they knew that it was going to be MS or something like it.
Berger: How did you feel about that?
Biloon: At the time, I was kind of glad to hear it was something. And I just asked her if, like, you die from it. That was the first thing I asked. It was like falling off a cliff.
It was making it hard for me to function in what I was doing, which was stand-up comedy, because of the cognitive issues I was having, the cognitive fog. That was how I ended up with you. Right away, you talked to me and were actually able to introduce to me some new medications that are out and are phenomenally better for MS plus were not pills or shots every day. It's made my MS over the years a lot more manageable.
Berger: I'd like to pick up on a couple of things you said.
Biloon: Sure.
Berger: One is, because most people envision MS as this terrible, crippling illness that's going to leave them wheelchair-bound, deprived of their profession, finding it difficult to stay in a marriage it's vested with what has been termed "lamentable results." And one of the first things that we as physicians have to do is to calm people down and say, "You know what. You have MS. You're going to be just fine. Trust me. We have wonderful medications for what you have, and we'll take care of it." In fact, I've made a habit of telling people quit worrying. You hired me to worry for you.
Biloon: Yep.
Berger: And I think that's helpful.
Biloon: I've been just so appreciative of that. There's a balance of being condescended to — do you know what I mean — and also being given information. I'm very sensitive to that balance because I consider myself an intelligent person. And you're being put in a position where someone knows more than you, and you have to listen.
Berger: One of the other challenges we face is getting somebody on a treatment. And we elected to put you on an intravenous therapy every 6 months.
Biloon: Especially because as a stand-up comedian, I was traveling a lot, doing these every-6-months infusion, especially with the high efficacy rate that it had been reported from what we had read and the low amount of side effects. I mean, just those things together was just something that seemed the easiest for me.
Berger: So did you encounter any challenges when we first got you started on the infusion therapy?
Biloon: The first infusion I got was at the hospital. But then after that, I had to go to the suburbs, to a center out there for the infusion. That was difficult because to get a ride out there and a ride back — it was a long trip for someone to wait with me. Taking an Uber is expensive, so was it for me to drive. You don't feel good for a couple of days after. So that was how it was, and I complained about it. Probably at every appointment we had, I complained about it.
Berger: Yeah. So some of the challenges you talked about are very, very common. As a physician on medications myself, I can tell you that I am not particularly compliant. And what I love about infusion therapies is that I know that the patient is getting their medicine. Because when they don't show up for a scheduled appointment, I'm called, and I know.
Biloon: I do have a bit of an allergic reaction to the drug. But that's been easily managed over time. Now, the drug infusions are actually being done at my home, which makes the whole process twice-a-year–world's better.
Berger: But there are other barriers that people confront other than the initiation of drugs. Had you encountered any?
Biloon: I think the problem that I had more so was finding the drugs that would manage some of my symptoms. It took a couple of years to sort of figure out what that would be, both with figuring them out and both dealing with insurance on certain medications.
Berger: That's one sort of problem that we confront. The other, of course, are those individuals who, for a variety of reasons, have difficulty with the diagnosis because of their backgrounds. And they may be sociocultural in nature. Every time you go to the physical therapist, it's some degree of money.
Now for some people, it's trivial. But for others, it's a considerable amount of money, relative to what it is that they earn. And you simply have to work within those confines as best you can.
We do have various programs that help people. So we try to employ them. There are, in addition to the sociocultural barriers, language barriers that we often confront. We, in our situation here in a large city, have a very large migrant population.
Fortunately, most of the people speak languages that either you speak as well, or there's somebody in the next room that speaks pretty well. But that's not always the case. So we do have an interpreter service that has to be employed.
Biloon: I cannot imagine the nuance in speaking to people from different ages and different backgrounds, who have different types of lifestyles, for them to understand.
Berger: I don't write at a computer. I think that really degrades the patient-physician relationship. What I do is I obtain a history. I do it on a piece of paper with a pen or a pencil.
I recapitulate them to the patient in paraphrasing it, to make sure that I have gotten it right and that they understand what I think I heard. That, I think, has been enormously helpful in helping people understand what may happen in the absence of treatment and why the treatment is important. That you can do, regardless of what the person's background is. So that's how I approach it.
Biloon: How do you deal with patients when they're not on the same page with you?
Berger: One important thing is that you have to be patient. That is something that it took me 50 years in medicine to learn. And then accepting the patient's opinion and saying, "All right, go home and think about it," because you often don't convince them when they're in the office with you.
Biloon: I did have a little bit of a cushion between my diagnosis and when we actually saw each other, where I was able to really sit in my thoughts on the different treatments and stuff. By the time that we were able to talk, it reassured me on that was the right plan.
Berger: I'm curious what your experience has been with our MS center.
Biloon: Through the portal, every time I need something, I'm usually reaching out, keeping you up-to-date on my primary care or whether it's trying to get a refill on one of my medications that I have to reach out. I really do feel that having that team there, being able to reach out, that's been extremely helpful to have and keeps me very secure because that's all I really need, especially during the pandemic, right? Because then I was very isolated and dealing with going through MS. So it was great to at least — and I did — shoot off emails or texts in the portal, and that's usually primarily how I communicated.
Berger: I will tell you, in my opinion, maybe nine out of 10 messages in the portal or calls that we get simply require reassurance.
Biloon: Yes.
Berger: You just either pick up the phone or shoot back a note, say, "This is not your MS. Don't worry about it." I mean, the most important thing for me is to keep people from worrying because that doesn't solve any problem.
Biloon: No, and it causes stress, which causes fatigue. I mean, it's a bad cycle.
Berger: In the past year, you've actually felt better, and you've gone back to performing. It sounds like the volume of performances has gotten back to what it was pre-illness. What do you see for the future?
Biloon: What I see is traveling more for stand-up and doing the sort of clubs and cities that I had kind of stopped doing from before I was diagnosed, so 2017 and prior to that. And then also even working on other things, writing and maybe even doing sort of books or one-person shows that even talk about sort of my struggles with MS and kind of coming back to where I am. I'm looking forward to the future, and I hope that that's the track I can keep going on.
Berger: I see no reason why you shouldn't.
Biloon: Thank you.
Berger: Michelle, thank you very much for joining me today in this conversation.
Biloon: Thank you so much for having me. It's been really wonderful to be able to sit down here with you.
Joseph R. Berger, MD, has disclosed the following relevant financial relationships: Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Celgene/Bristol-Myers Squibb; Cellevolve; EMD Serono/Merck/Genentech; Genzyme; Janssen/Johnson & Johnson; Morphic; Novartis; Roche; Sanofi; Takeda; TG Therapeutics; MAPI; Excision Bio
Received research grant from: Genentech/Roche
Michelle Biloon has disclosed no relevant financial relationships
This transcript has been edited for clarity.
Joseph R. Berger, MD: Hi. I'm Dr Joseph Berger, and I'm joined for this Care Cues conversation with my patient, Michelle Biloon, who has had multiple sclerosis (MS) for the past 6 years. Hello, Michelle. Welcome.
Michelle Biloon: Thank you, Dr Berger.
Berger: Can you tell us a little bit about yourself, how you came to understand you had MS, and how you've done since the diagnosis was rendered?
Biloon: Yeah. It was a very short diagnosis period for me. In the winter of 2017, I started experiencing dizzy spells, and I didn't really know why. I eventually went to my primary care clinic where my doctor is, and they did blood work. Then, they did a CT and didn't see anything, and I just kind of kept feeling worse.
Then, finally, I went to an ENT just to see if it was maybe related to my ears. The ENT actually said, "You need to go to the ER and get an MRI." And while I was in the MRI, I could feel the dizzy spells. And I thought, Well, something is happening. I don't know what it is. And then a resident came in and said that they saw lesions on my brain, and they knew that it was going to be MS or something like it.
Berger: How did you feel about that?
Biloon: At the time, I was kind of glad to hear it was something. And I just asked her if, like, you die from it. That was the first thing I asked. It was like falling off a cliff.
It was making it hard for me to function in what I was doing, which was stand-up comedy, because of the cognitive issues I was having, the cognitive fog. That was how I ended up with you. Right away, you talked to me and were actually able to introduce to me some new medications that are out and are phenomenally better for MS plus were not pills or shots every day. It's made my MS over the years a lot more manageable.
Berger: I'd like to pick up on a couple of things you said.
Biloon: Sure.
Berger: One is, because most people envision MS as this terrible, crippling illness that's going to leave them wheelchair-bound, deprived of their profession, finding it difficult to stay in a marriage it's vested with what has been termed "lamentable results." And one of the first things that we as physicians have to do is to calm people down and say, "You know what. You have MS. You're going to be just fine. Trust me. We have wonderful medications for what you have, and we'll take care of it." In fact, I've made a habit of telling people quit worrying. You hired me to worry for you.
Biloon: Yep.
Berger: And I think that's helpful.
Biloon: I've been just so appreciative of that. There's a balance of being condescended to — do you know what I mean — and also being given information. I'm very sensitive to that balance because I consider myself an intelligent person. And you're being put in a position where someone knows more than you, and you have to listen.
Berger: One of the other challenges we face is getting somebody on a treatment. And we elected to put you on an intravenous therapy every 6 months.
Biloon: Especially because as a stand-up comedian, I was traveling a lot, doing these every-6-months infusion, especially with the high efficacy rate that it had been reported from what we had read and the low amount of side effects. I mean, just those things together was just something that seemed the easiest for me.
Berger: So did you encounter any challenges when we first got you started on the infusion therapy?
Biloon: The first infusion I got was at the hospital. But then after that, I had to go to the suburbs, to a center out there for the infusion. That was difficult because to get a ride out there and a ride back — it was a long trip for someone to wait with me. Taking an Uber is expensive, so was it for me to drive. You don't feel good for a couple of days after. So that was how it was, and I complained about it. Probably at every appointment we had, I complained about it.
Berger: Yeah. So some of the challenges you talked about are very, very common. As a physician on medications myself, I can tell you that I am not particularly compliant. And what I love about infusion therapies is that I know that the patient is getting their medicine. Because when they don't show up for a scheduled appointment, I'm called, and I know.
Biloon: I do have a bit of an allergic reaction to the drug. But that's been easily managed over time. Now, the drug infusions are actually being done at my home, which makes the whole process twice-a-year–world's better.
Berger: But there are other barriers that people confront other than the initiation of drugs. Had you encountered any?
Biloon: I think the problem that I had more so was finding the drugs that would manage some of my symptoms. It took a couple of years to sort of figure out what that would be, both with figuring them out and both dealing with insurance on certain medications.
Berger: That's one sort of problem that we confront. The other, of course, are those individuals who, for a variety of reasons, have difficulty with the diagnosis because of their backgrounds. And they may be sociocultural in nature. Every time you go to the physical therapist, it's some degree of money.
Now for some people, it's trivial. But for others, it's a considerable amount of money, relative to what it is that they earn. And you simply have to work within those confines as best you can.
We do have various programs that help people. So we try to employ them. There are, in addition to the sociocultural barriers, language barriers that we often confront. We, in our situation here in a large city, have a very large migrant population.
Fortunately, most of the people speak languages that either you speak as well, or there's somebody in the next room that speaks pretty well. But that's not always the case. So we do have an interpreter service that has to be employed.
Biloon: I cannot imagine the nuance in speaking to people from different ages and different backgrounds, who have different types of lifestyles, for them to understand.
Berger: I don't write at a computer. I think that really degrades the patient-physician relationship. What I do is I obtain a history. I do it on a piece of paper with a pen or a pencil.
I recapitulate them to the patient in paraphrasing it, to make sure that I have gotten it right and that they understand what I think I heard. That, I think, has been enormously helpful in helping people understand what may happen in the absence of treatment and why the treatment is important. That you can do, regardless of what the person's background is. So that's how I approach it.
Biloon: How do you deal with patients when they're not on the same page with you?
Berger: One important thing is that you have to be patient. That is something that it took me 50 years in medicine to learn. And then accepting the patient's opinion and saying, "All right, go home and think about it," because you often don't convince them when they're in the office with you.
Biloon: I did have a little bit of a cushion between my diagnosis and when we actually saw each other, where I was able to really sit in my thoughts on the different treatments and stuff. By the time that we were able to talk, it reassured me on that was the right plan.
Berger: I'm curious what your experience has been with our MS center.
Biloon: Through the portal, every time I need something, I'm usually reaching out, keeping you up-to-date on my primary care or whether it's trying to get a refill on one of my medications that I have to reach out. I really do feel that having that team there, being able to reach out, that's been extremely helpful to have and keeps me very secure because that's all I really need, especially during the pandemic, right? Because then I was very isolated and dealing with going through MS. So it was great to at least — and I did — shoot off emails or texts in the portal, and that's usually primarily how I communicated.
Berger: I will tell you, in my opinion, maybe nine out of 10 messages in the portal or calls that we get simply require reassurance.
Biloon: Yes.
Berger: You just either pick up the phone or shoot back a note, say, "This is not your MS. Don't worry about it." I mean, the most important thing for me is to keep people from worrying because that doesn't solve any problem.
Biloon: No, and it causes stress, which causes fatigue. I mean, it's a bad cycle.
Berger: In the past year, you've actually felt better, and you've gone back to performing. It sounds like the volume of performances has gotten back to what it was pre-illness. What do you see for the future?
Biloon: What I see is traveling more for stand-up and doing the sort of clubs and cities that I had kind of stopped doing from before I was diagnosed, so 2017 and prior to that. And then also even working on other things, writing and maybe even doing sort of books or one-person shows that even talk about sort of my struggles with MS and kind of coming back to where I am. I'm looking forward to the future, and I hope that that's the track I can keep going on.
Berger: I see no reason why you shouldn't.
Biloon: Thank you.
Berger: Michelle, thank you very much for joining me today in this conversation.
Biloon: Thank you so much for having me. It's been really wonderful to be able to sit down here with you.
Joseph R. Berger, MD, has disclosed the following relevant financial relationships: Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Celgene/Bristol-Myers Squibb; Cellevolve; EMD Serono/Merck/Genentech; Genzyme; Janssen/Johnson & Johnson; Morphic; Novartis; Roche; Sanofi; Takeda; TG Therapeutics; MAPI; Excision Bio
Received research grant from: Genentech/Roche
Michelle Biloon has disclosed no relevant financial relationships
This transcript has been edited for clarity.
Joseph R. Berger, MD: Hi. I'm Dr Joseph Berger, and I'm joined for this Care Cues conversation with my patient, Michelle Biloon, who has had multiple sclerosis (MS) for the past 6 years. Hello, Michelle. Welcome.
Michelle Biloon: Thank you, Dr Berger.
Berger: Can you tell us a little bit about yourself, how you came to understand you had MS, and how you've done since the diagnosis was rendered?
Biloon: Yeah. It was a very short diagnosis period for me. In the winter of 2017, I started experiencing dizzy spells, and I didn't really know why. I eventually went to my primary care clinic where my doctor is, and they did blood work. Then, they did a CT and didn't see anything, and I just kind of kept feeling worse.
Then, finally, I went to an ENT just to see if it was maybe related to my ears. The ENT actually said, "You need to go to the ER and get an MRI." And while I was in the MRI, I could feel the dizzy spells. And I thought, Well, something is happening. I don't know what it is. And then a resident came in and said that they saw lesions on my brain, and they knew that it was going to be MS or something like it.
Berger: How did you feel about that?
Biloon: At the time, I was kind of glad to hear it was something. And I just asked her if, like, you die from it. That was the first thing I asked. It was like falling off a cliff.
It was making it hard for me to function in what I was doing, which was stand-up comedy, because of the cognitive issues I was having, the cognitive fog. That was how I ended up with you. Right away, you talked to me and were actually able to introduce to me some new medications that are out and are phenomenally better for MS plus were not pills or shots every day. It's made my MS over the years a lot more manageable.
Berger: I'd like to pick up on a couple of things you said.
Biloon: Sure.
Berger: One is, because most people envision MS as this terrible, crippling illness that's going to leave them wheelchair-bound, deprived of their profession, finding it difficult to stay in a marriage it's vested with what has been termed "lamentable results." And one of the first things that we as physicians have to do is to calm people down and say, "You know what. You have MS. You're going to be just fine. Trust me. We have wonderful medications for what you have, and we'll take care of it." In fact, I've made a habit of telling people quit worrying. You hired me to worry for you.
Biloon: Yep.
Berger: And I think that's helpful.
Biloon: I've been just so appreciative of that. There's a balance of being condescended to — do you know what I mean — and also being given information. I'm very sensitive to that balance because I consider myself an intelligent person. And you're being put in a position where someone knows more than you, and you have to listen.
Berger: One of the other challenges we face is getting somebody on a treatment. And we elected to put you on an intravenous therapy every 6 months.
Biloon: Especially because as a stand-up comedian, I was traveling a lot, doing these every-6-months infusion, especially with the high efficacy rate that it had been reported from what we had read and the low amount of side effects. I mean, just those things together was just something that seemed the easiest for me.
Berger: So did you encounter any challenges when we first got you started on the infusion therapy?
Biloon: The first infusion I got was at the hospital. But then after that, I had to go to the suburbs, to a center out there for the infusion. That was difficult because to get a ride out there and a ride back — it was a long trip for someone to wait with me. Taking an Uber is expensive, so was it for me to drive. You don't feel good for a couple of days after. So that was how it was, and I complained about it. Probably at every appointment we had, I complained about it.
Berger: Yeah. So some of the challenges you talked about are very, very common. As a physician on medications myself, I can tell you that I am not particularly compliant. And what I love about infusion therapies is that I know that the patient is getting their medicine. Because when they don't show up for a scheduled appointment, I'm called, and I know.
Biloon: I do have a bit of an allergic reaction to the drug. But that's been easily managed over time. Now, the drug infusions are actually being done at my home, which makes the whole process twice-a-year–world's better.
Berger: But there are other barriers that people confront other than the initiation of drugs. Had you encountered any?
Biloon: I think the problem that I had more so was finding the drugs that would manage some of my symptoms. It took a couple of years to sort of figure out what that would be, both with figuring them out and both dealing with insurance on certain medications.
Berger: That's one sort of problem that we confront. The other, of course, are those individuals who, for a variety of reasons, have difficulty with the diagnosis because of their backgrounds. And they may be sociocultural in nature. Every time you go to the physical therapist, it's some degree of money.
Now for some people, it's trivial. But for others, it's a considerable amount of money, relative to what it is that they earn. And you simply have to work within those confines as best you can.
We do have various programs that help people. So we try to employ them. There are, in addition to the sociocultural barriers, language barriers that we often confront. We, in our situation here in a large city, have a very large migrant population.
Fortunately, most of the people speak languages that either you speak as well, or there's somebody in the next room that speaks pretty well. But that's not always the case. So we do have an interpreter service that has to be employed.
Biloon: I cannot imagine the nuance in speaking to people from different ages and different backgrounds, who have different types of lifestyles, for them to understand.
Berger: I don't write at a computer. I think that really degrades the patient-physician relationship. What I do is I obtain a history. I do it on a piece of paper with a pen or a pencil.
I recapitulate them to the patient in paraphrasing it, to make sure that I have gotten it right and that they understand what I think I heard. That, I think, has been enormously helpful in helping people understand what may happen in the absence of treatment and why the treatment is important. That you can do, regardless of what the person's background is. So that's how I approach it.
Biloon: How do you deal with patients when they're not on the same page with you?
Berger: One important thing is that you have to be patient. That is something that it took me 50 years in medicine to learn. And then accepting the patient's opinion and saying, "All right, go home and think about it," because you often don't convince them when they're in the office with you.
Biloon: I did have a little bit of a cushion between my diagnosis and when we actually saw each other, where I was able to really sit in my thoughts on the different treatments and stuff. By the time that we were able to talk, it reassured me on that was the right plan.
Berger: I'm curious what your experience has been with our MS center.
Biloon: Through the portal, every time I need something, I'm usually reaching out, keeping you up-to-date on my primary care or whether it's trying to get a refill on one of my medications that I have to reach out. I really do feel that having that team there, being able to reach out, that's been extremely helpful to have and keeps me very secure because that's all I really need, especially during the pandemic, right? Because then I was very isolated and dealing with going through MS. So it was great to at least — and I did — shoot off emails or texts in the portal, and that's usually primarily how I communicated.
Berger: I will tell you, in my opinion, maybe nine out of 10 messages in the portal or calls that we get simply require reassurance.
Biloon: Yes.
Berger: You just either pick up the phone or shoot back a note, say, "This is not your MS. Don't worry about it." I mean, the most important thing for me is to keep people from worrying because that doesn't solve any problem.
Biloon: No, and it causes stress, which causes fatigue. I mean, it's a bad cycle.
Berger: In the past year, you've actually felt better, and you've gone back to performing. It sounds like the volume of performances has gotten back to what it was pre-illness. What do you see for the future?
Biloon: What I see is traveling more for stand-up and doing the sort of clubs and cities that I had kind of stopped doing from before I was diagnosed, so 2017 and prior to that. And then also even working on other things, writing and maybe even doing sort of books or one-person shows that even talk about sort of my struggles with MS and kind of coming back to where I am. I'm looking forward to the future, and I hope that that's the track I can keep going on.
Berger: I see no reason why you shouldn't.
Biloon: Thank you.
Berger: Michelle, thank you very much for joining me today in this conversation.
Biloon: Thank you so much for having me. It's been really wonderful to be able to sit down here with you.
Joseph R. Berger, MD, has disclosed the following relevant financial relationships: Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Celgene/Bristol-Myers Squibb; Cellevolve; EMD Serono/Merck/Genentech; Genzyme; Janssen/Johnson & Johnson; Morphic; Novartis; Roche; Sanofi; Takeda; TG Therapeutics; MAPI; Excision Bio
Received research grant from: Genentech/Roche
Michelle Biloon has disclosed no relevant financial relationships
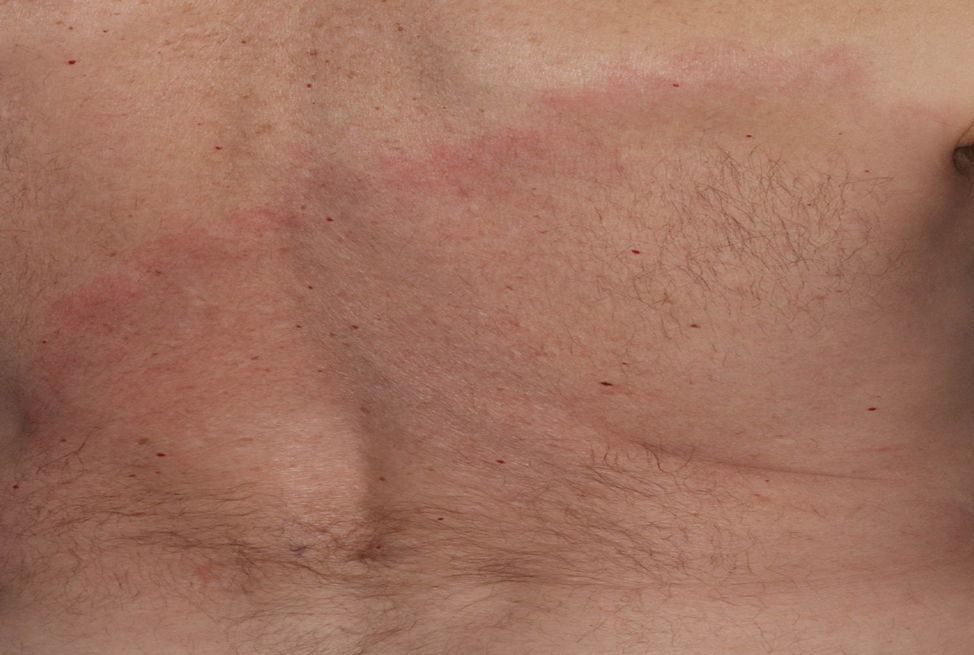
Asymptomatic Erythematous Plaque in an Outdoorsman
The Diagnosis: Erythema Migrans
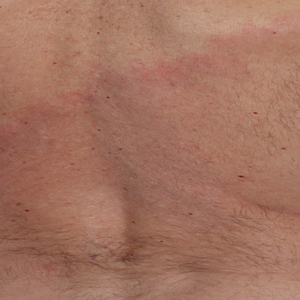
The patient was clinically diagnosed with erythema migrans. He did not recall a tick bite but spent a lot of time outdoors. He was treated with 10 days of doxycycline 100 mg twice daily with complete resolution of the rash.
Lyme disease is a spirochete infection caused by the Borrelia burgdorferi sensu lato species complex and transmitted by the Ixodidae tick family. It is the most common tick-borne disease in the United States and mostly is reported in the northeastern and upper midwestern states during the warmer seasons, but it is prevalent worldwide. In geographic areas where Lyme disease is common, the incidence is approximately 40 cases per 100,000 individuals.1 Our patient resided in coastal South Carolina. Lyme disease is more commonly reported in White individuals. The skin lesions may be more difficult to discern and diagnose in patients with darker skin types, leading to delayed diagnosis and treatment.2,3
Patients may be diagnosed with early localized, early disseminated, or late Lyme disease. Erythema migrans is the early localized form of the disease and is classically described as an erythematous targetlike plaque with raised borders arising at the site of the tick bite 1 to 2 weeks later.4 However, many patients simply have a homogeneous erythematous plaque with raised advancing borders ranging in size from 5 to 68 cm.5 In a 2022 study of 69 patients with suspected Lyme disease, only 35 (50.7%) were determined to truly have acute Lyme disease.6 Of them, only 2 (5.7%) had the classic ringwithin- a-ring pattern. Most plaques were uniform, pink, oval-shaped lesions with well-demarcated borders.6
The rash may present with a burning sensation, or patients may experience no symptoms at all, which can lead to delayed diagnosis and progression to late disease. Patients may develop malaise, fever, headache, body aches, or joint pain. Early disseminated disease manifests similarly. Patients with disseminated disease also may develop more serious complications, including lymphadenopathy; cranial nerve palsies; ocular involvement; meningitis; or cardiac abnormalities such as myocarditis, pericarditis, or arrhythmia. Late disease most often causes arthritis of the large joints, though it also can have cardiac or neurologic manifestations. Some patients with chronic disease—the majority of whom were diagnosed in Europe—may develop acrodermatitis chronica atrophicans with edematous blue-red plaques that become atrophic and hyperpigmented fibrotic plaques over the course of years.
Allergic contact dermatitis to a plant more likely would cause itchy or painful, oozy, weepy, vesicular lesions arranged in a linear pattern. A dermatophyte infection likely would cause a scaly eruption. Although our patient presented with a sharply demarcated, raised, erythematous lesion, the distribution did not follow normal clothing lines and would be unusual for a photosensitive drug eruption. Cellulitis likely would be associated with tenderness or warmth to the touch. Finally, southern tick-associated rash illness, which is associated with Amblyomma americanum (lone star tick) bites, may appear with a similar rash but few systemic symptoms. It also can be treated with tetracycline antibiotics.7
Our case in South Carolina demonstrates the importance of keeping Lyme disease in the differential. Clinicians should remember to ask patients about their travel history. In endemic areas, patients with erythema migrans can be started on treatment without waiting for serology. Patients with early Lyme disease may or may not have positive serologies at the time of presentation.6 Guidelines for the treatment of Lyme disease have been revised in recent years to decrease patient antibiotic exposure by reducing the number of days of antibiotic therapy.8 A recent randomized controlled trial found no significant difference in recurrence for patients treated with 7 days of doxycycline compared with 14 days.9 We typically prescribe a 10-day course of doxycycline, which also is adequate for concurrent rickettsial disease. Patients who develop malarialike symptoms should be evaluated for babesiosis, which is treated with clindamycin.
- Skar GL, Simonsen KA. Lyme disease. StatPearls [Internet]. Updated February 4, 2024. Accessed March 20, 2024. https://www.ncbi.nlm.nih.gov/books/NBK431066/
- Dennison R, Novak C, Rebman A, et al. Lyme disease with erythema migrans and seventh nerve palsy in an African-American man. Cureus. 2019;11:E6509.
- Bax CE, Clark AK, Oboite M, et al. A case of disseminated Lyme disease in a child with skin of color. Pediatr Dermatol. 2021;38 (suppl 2):140-141.
- Shah AS, Varatharaj Palraj BR. Multiple erythema migrans rashes characteristic of early disseminated lyme disease, before and after therapy. Mayo Clin Proc. 2019;94:172-173.
- Feder HM Jr, Abeles M, Bernstein M, et al. Diagnosis, treatment, and prognosis of erythema migrans and Lyme arthritis. Clin Dermatol. 2006;24:509-520.
- Schotthoefer AM, Green CB, Dempsey G, et al. The spectrum of erythema migrans in early Lyme disease: can we improve its recognition? Cureus. 2022;14:E30673.
- Strle F, Wormser GP. Early Lyme disease (erythema migrans) and its mimics (southern tick-associated rash illness and tick-associated rash illness). Infect Dis Clin North Am. 2022;36:523-539.
- Torbahn G, Hofmann H, Rücker G, et al. Efficacy and safety of antibiotic therapy in early cutaneous Lyme borreliosis: a network meta-analysis. JAMA Dermatol. 2018;154:1292-1303.
- Stupica D, Collinet-Adler S, Blagus R, et al. Treatment of erythema migrans with doxycycline for 7 days versus 14 days in Slovenia: a randomised open-label non-inferiority trial. Lancet Infect Dis. 2023;23:371-379.
The Diagnosis: Erythema Migrans
The patient was clinically diagnosed with erythema migrans. He did not recall a tick bite but spent a lot of time outdoors. He was treated with 10 days of doxycycline 100 mg twice daily with complete resolution of the rash.
Lyme disease is a spirochete infection caused by the Borrelia burgdorferi sensu lato species complex and transmitted by the Ixodidae tick family. It is the most common tick-borne disease in the United States and mostly is reported in the northeastern and upper midwestern states during the warmer seasons, but it is prevalent worldwide. In geographic areas where Lyme disease is common, the incidence is approximately 40 cases per 100,000 individuals.1 Our patient resided in coastal South Carolina. Lyme disease is more commonly reported in White individuals. The skin lesions may be more difficult to discern and diagnose in patients with darker skin types, leading to delayed diagnosis and treatment.2,3
Patients may be diagnosed with early localized, early disseminated, or late Lyme disease. Erythema migrans is the early localized form of the disease and is classically described as an erythematous targetlike plaque with raised borders arising at the site of the tick bite 1 to 2 weeks later.4 However, many patients simply have a homogeneous erythematous plaque with raised advancing borders ranging in size from 5 to 68 cm.5 In a 2022 study of 69 patients with suspected Lyme disease, only 35 (50.7%) were determined to truly have acute Lyme disease.6 Of them, only 2 (5.7%) had the classic ringwithin- a-ring pattern. Most plaques were uniform, pink, oval-shaped lesions with well-demarcated borders.6
The rash may present with a burning sensation, or patients may experience no symptoms at all, which can lead to delayed diagnosis and progression to late disease. Patients may develop malaise, fever, headache, body aches, or joint pain. Early disseminated disease manifests similarly. Patients with disseminated disease also may develop more serious complications, including lymphadenopathy; cranial nerve palsies; ocular involvement; meningitis; or cardiac abnormalities such as myocarditis, pericarditis, or arrhythmia. Late disease most often causes arthritis of the large joints, though it also can have cardiac or neurologic manifestations. Some patients with chronic disease—the majority of whom were diagnosed in Europe—may develop acrodermatitis chronica atrophicans with edematous blue-red plaques that become atrophic and hyperpigmented fibrotic plaques over the course of years.
Allergic contact dermatitis to a plant more likely would cause itchy or painful, oozy, weepy, vesicular lesions arranged in a linear pattern. A dermatophyte infection likely would cause a scaly eruption. Although our patient presented with a sharply demarcated, raised, erythematous lesion, the distribution did not follow normal clothing lines and would be unusual for a photosensitive drug eruption. Cellulitis likely would be associated with tenderness or warmth to the touch. Finally, southern tick-associated rash illness, which is associated with Amblyomma americanum (lone star tick) bites, may appear with a similar rash but few systemic symptoms. It also can be treated with tetracycline antibiotics.7
Our case in South Carolina demonstrates the importance of keeping Lyme disease in the differential. Clinicians should remember to ask patients about their travel history. In endemic areas, patients with erythema migrans can be started on treatment without waiting for serology. Patients with early Lyme disease may or may not have positive serologies at the time of presentation.6 Guidelines for the treatment of Lyme disease have been revised in recent years to decrease patient antibiotic exposure by reducing the number of days of antibiotic therapy.8 A recent randomized controlled trial found no significant difference in recurrence for patients treated with 7 days of doxycycline compared with 14 days.9 We typically prescribe a 10-day course of doxycycline, which also is adequate for concurrent rickettsial disease. Patients who develop malarialike symptoms should be evaluated for babesiosis, which is treated with clindamycin.
The Diagnosis: Erythema Migrans
The patient was clinically diagnosed with erythema migrans. He did not recall a tick bite but spent a lot of time outdoors. He was treated with 10 days of doxycycline 100 mg twice daily with complete resolution of the rash.
Lyme disease is a spirochete infection caused by the Borrelia burgdorferi sensu lato species complex and transmitted by the Ixodidae tick family. It is the most common tick-borne disease in the United States and mostly is reported in the northeastern and upper midwestern states during the warmer seasons, but it is prevalent worldwide. In geographic areas where Lyme disease is common, the incidence is approximately 40 cases per 100,000 individuals.1 Our patient resided in coastal South Carolina. Lyme disease is more commonly reported in White individuals. The skin lesions may be more difficult to discern and diagnose in patients with darker skin types, leading to delayed diagnosis and treatment.2,3
Patients may be diagnosed with early localized, early disseminated, or late Lyme disease. Erythema migrans is the early localized form of the disease and is classically described as an erythematous targetlike plaque with raised borders arising at the site of the tick bite 1 to 2 weeks later.4 However, many patients simply have a homogeneous erythematous plaque with raised advancing borders ranging in size from 5 to 68 cm.5 In a 2022 study of 69 patients with suspected Lyme disease, only 35 (50.7%) were determined to truly have acute Lyme disease.6 Of them, only 2 (5.7%) had the classic ringwithin- a-ring pattern. Most plaques were uniform, pink, oval-shaped lesions with well-demarcated borders.6
The rash may present with a burning sensation, or patients may experience no symptoms at all, which can lead to delayed diagnosis and progression to late disease. Patients may develop malaise, fever, headache, body aches, or joint pain. Early disseminated disease manifests similarly. Patients with disseminated disease also may develop more serious complications, including lymphadenopathy; cranial nerve palsies; ocular involvement; meningitis; or cardiac abnormalities such as myocarditis, pericarditis, or arrhythmia. Late disease most often causes arthritis of the large joints, though it also can have cardiac or neurologic manifestations. Some patients with chronic disease—the majority of whom were diagnosed in Europe—may develop acrodermatitis chronica atrophicans with edematous blue-red plaques that become atrophic and hyperpigmented fibrotic plaques over the course of years.
Allergic contact dermatitis to a plant more likely would cause itchy or painful, oozy, weepy, vesicular lesions arranged in a linear pattern. A dermatophyte infection likely would cause a scaly eruption. Although our patient presented with a sharply demarcated, raised, erythematous lesion, the distribution did not follow normal clothing lines and would be unusual for a photosensitive drug eruption. Cellulitis likely would be associated with tenderness or warmth to the touch. Finally, southern tick-associated rash illness, which is associated with Amblyomma americanum (lone star tick) bites, may appear with a similar rash but few systemic symptoms. It also can be treated with tetracycline antibiotics.7
Our case in South Carolina demonstrates the importance of keeping Lyme disease in the differential. Clinicians should remember to ask patients about their travel history. In endemic areas, patients with erythema migrans can be started on treatment without waiting for serology. Patients with early Lyme disease may or may not have positive serologies at the time of presentation.6 Guidelines for the treatment of Lyme disease have been revised in recent years to decrease patient antibiotic exposure by reducing the number of days of antibiotic therapy.8 A recent randomized controlled trial found no significant difference in recurrence for patients treated with 7 days of doxycycline compared with 14 days.9 We typically prescribe a 10-day course of doxycycline, which also is adequate for concurrent rickettsial disease. Patients who develop malarialike symptoms should be evaluated for babesiosis, which is treated with clindamycin.
- Skar GL, Simonsen KA. Lyme disease. StatPearls [Internet]. Updated February 4, 2024. Accessed March 20, 2024. https://www.ncbi.nlm.nih.gov/books/NBK431066/
- Dennison R, Novak C, Rebman A, et al. Lyme disease with erythema migrans and seventh nerve palsy in an African-American man. Cureus. 2019;11:E6509.
- Bax CE, Clark AK, Oboite M, et al. A case of disseminated Lyme disease in a child with skin of color. Pediatr Dermatol. 2021;38 (suppl 2):140-141.
- Shah AS, Varatharaj Palraj BR. Multiple erythema migrans rashes characteristic of early disseminated lyme disease, before and after therapy. Mayo Clin Proc. 2019;94:172-173.
- Feder HM Jr, Abeles M, Bernstein M, et al. Diagnosis, treatment, and prognosis of erythema migrans and Lyme arthritis. Clin Dermatol. 2006;24:509-520.
- Schotthoefer AM, Green CB, Dempsey G, et al. The spectrum of erythema migrans in early Lyme disease: can we improve its recognition? Cureus. 2022;14:E30673.
- Strle F, Wormser GP. Early Lyme disease (erythema migrans) and its mimics (southern tick-associated rash illness and tick-associated rash illness). Infect Dis Clin North Am. 2022;36:523-539.
- Torbahn G, Hofmann H, Rücker G, et al. Efficacy and safety of antibiotic therapy in early cutaneous Lyme borreliosis: a network meta-analysis. JAMA Dermatol. 2018;154:1292-1303.
- Stupica D, Collinet-Adler S, Blagus R, et al. Treatment of erythema migrans with doxycycline for 7 days versus 14 days in Slovenia: a randomised open-label non-inferiority trial. Lancet Infect Dis. 2023;23:371-379.
- Skar GL, Simonsen KA. Lyme disease. StatPearls [Internet]. Updated February 4, 2024. Accessed March 20, 2024. https://www.ncbi.nlm.nih.gov/books/NBK431066/
- Dennison R, Novak C, Rebman A, et al. Lyme disease with erythema migrans and seventh nerve palsy in an African-American man. Cureus. 2019;11:E6509.
- Bax CE, Clark AK, Oboite M, et al. A case of disseminated Lyme disease in a child with skin of color. Pediatr Dermatol. 2021;38 (suppl 2):140-141.
- Shah AS, Varatharaj Palraj BR. Multiple erythema migrans rashes characteristic of early disseminated lyme disease, before and after therapy. Mayo Clin Proc. 2019;94:172-173.
- Feder HM Jr, Abeles M, Bernstein M, et al. Diagnosis, treatment, and prognosis of erythema migrans and Lyme arthritis. Clin Dermatol. 2006;24:509-520.
- Schotthoefer AM, Green CB, Dempsey G, et al. The spectrum of erythema migrans in early Lyme disease: can we improve its recognition? Cureus. 2022;14:E30673.
- Strle F, Wormser GP. Early Lyme disease (erythema migrans) and its mimics (southern tick-associated rash illness and tick-associated rash illness). Infect Dis Clin North Am. 2022;36:523-539.
- Torbahn G, Hofmann H, Rücker G, et al. Efficacy and safety of antibiotic therapy in early cutaneous Lyme borreliosis: a network meta-analysis. JAMA Dermatol. 2018;154:1292-1303.
- Stupica D, Collinet-Adler S, Blagus R, et al. Treatment of erythema migrans with doxycycline for 7 days versus 14 days in Slovenia: a randomised open-label non-inferiority trial. Lancet Infect Dis. 2023;23:371-379.
A middle-aged man presented with a well-demarcated, hyperpigmented, erythematous patch with an annular erythematous border that extended from the mid-back to the lower back. The patient was otherwise asymptomatic. He was an avid gardener who resided in South Carolina and had recently adopted 2 puppies.
Evaluation of Anti-Agitation Medication Prescribing Patterns by Age in the Emergency Department
Each year, about 2.6% of emergency department (ED) visits involve agitation.1 ED clinicians are especially prone to workplace violence and assault, facing the challenge of caring for patients while maintaining safety. A 2013 prospective study found an average of 4.15 violent events per employee in 9 months; nurses and patient care assistants were most frequently affected.2 A 2022 survey from the American College of Emergency Physicians found 55% of respondents reported being physically assaulted in the ED and 79% of respondents reported witnessing another assault. Most of these assaults (98%) were committed by the patients.3 Appropriate management of patients experiencing acute agitation is critical for the safety of all parties involved.
The initial approach to acute agitation management involves nonpharmacologic measures in an attempt to avoid coercive actions, such as physical restraints. Reducing environmental stimulation and verbal de-escalation are effective and help the patients with agitation regain control over their behavior.4
When these measures fail, however, pharmacologic therapy is often administered to ensure safety. The goal of pharmacologic therapy is to calm the patient without causing sedation.5 This allows the patient to continue participating in their care and allows the care team to accurately assess them, which is critical in determining the underlying etiology of agitation. Historically, haloperidol has commonly been used to manage acute agitation. It is frequently administered with lorazepam and diphenhydramine to reduce the incidence of haloperidol’s extrapyramidal adverse effects. However, there are several potential concerns with this method, including oversedation, QTc prolongation, potential drug interactions, and polypharmacy.5,6
The American Association of Emergency Psychiatry Project BETA Psychopharmacology Workgroup published a Consensus Statement in 2012 regarding the psychopharmacology of agitation.5 When considering medication for agitation management, clinicians must first determine a provisional diagnosis outlining the most probable etiology of the patient’s behavior, such as delirium, intoxication, or a psychiatric disorder. Apart from alcohol intoxication, benzodiazepines (BZDs) or second-generation antipsychotics as monotherapy are generally preferred over haloperidol for acute agitation.5 Second-generation antipsychotics have demonstrated to be as effective as haloperidol but are thought to be safer options. Quetiapine is not recommended for use in the ED due to the risk of orthostatic hypotension, as patients are often volume depleted.5The Veterans Affairs Southern Nevada Healthcare System (VASNHS) serves veterans in the Las Vegas area. Among the nearly 220,000 veterans in Nevada, about 100,000 veterans are aged ≥ 65 years.7 The 2012 consensus statement on psychopharmacology for agitation offers no specific age-related guidance. However, there are safety concerns in older adults both with antipsychotics and BZDs, even with acute use. The US Food and Drug Administration (FDA) issued a boxed warning for all antipsychotics due to increased mortality in older adult patients with dementia-related psychosis.8 The 2023 American Geriatrics Society Beers Criteria provides guidance on pharmacological therapy for adults aged ≥ 65 years and recommends avoiding antipsychotics and BZDs.9 In addition to the FDA boxed warning, data suggest increased mortality with antipsychotic use independent of dementia. With BZDs, changes in pharmacodynamics make older adults more prone to adverse effects, including cognitive impairment, delirium, falls, and fractures. A retrospective chart review evaluated risperidone use in the ED and found that adults aged ≥ 65 years experienced higher rates of hypotension, even though this age group received about half the dose of risperidone compared with younger patients.10 For this patient population, the general approach in treating acute agitation has been to avoid the use of medications, but prescribe lower doses when necessary.11
With limited research on acute agitation management in older adults, the purpose of this study was to compare current prescribing practices of anti-agitation medications between adults aged 18 to 64 years and adults aged ≥ 65 years in the VASNHS ED. This study was also conducted to better understand the anti-agitation prescribing practices at VASNHS, as no order sets or protocols existed at the time of the study to guide medication selection in agitation management. To our knowledge, this is the first observational study evaluating pharmacologic acute agitation management in the ED based on age.
Methods
This study was a retrospective chart review of patients aged ≥ 18 years who presented to the VASNHS ED and received medication for acute agitation. Patients were identified through active orders for a formulary agitation medication from August 1, 2019, to July 31, 2022. Formulary medication options included intravenous, oral, and intramuscular routes for haloperidol, droperidol, lorazepam, olanzapine, or ziprasidone. Veterans were excluded if they presented with alcohol intoxication, alcohol or BZD withdrawal, if the medication administration was unrelated to agitation, or whether the medication was not administered. While alcohol and/or BZDs can contribute to acute agitation, these patients were excluded due to a clear indication for BZD therapy and the challenge in a retrospective chart review to determine whether patients received medication for agitation vs other withdrawal-related symptoms.
Endpoints
The primary endpoint was the medication selection between 2 age groups: 18 to 64 years and ≥ 65 years. The secondary endpoints included ordered medication dose by regimen, additional anti-agitation medication use within 3 hours of initial medication administration, and disposition. Safety outcomes included incidence of newly occurring oxygen desaturation < 95%, supplemental oxygen requirement, intubation, QTc prolongation, and hypotension with systolic blood pressure < 90 mm Hg within 1 hour of medication administration. Data collected included patient demographics, substance use, conditions contributing to altered mental status, active psychotropic medication prescriptions, medication adherence, agitation medication prescriber, and doses. Adherence to psychotropic medication in the past 6 months was defined as ≥ 80% of days covered with medication and based on fill history. This was only calculated for applicable patients and did not include patients with only as-needed medications, such as hydroxyzine for anxiety.
Statistical Analysis
Statistical analyses were performed using IBM SPSS. Baseline characteristics were analyzed using descriptive statistics. χ2 and Fisher exact tests were used to analyze categorical data. A student t test was used for continuous variables and a 2-sided P value of < .05 was considered statistically significant.
Results
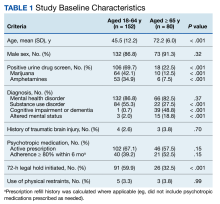
During the study period, 2342 unique patient encounters with active anti-agitation medication orders in the ED were identified and 232 encounters met the inclusion criteria. Of those excluded, 605 encounters had alcohol involvement. The study included 152 patient encounters for 128 patients aged 18 to 64 years of whom 16 patients had > 1 encounter with a mean (SD) 2.5 (1.1) visits. The study included 80 patient encounters for 72 patients aged ≥ 65 years of whom 7 patients had > 1 encounter with a mean (SD) 2.1 (0.3) visits. The mean age was 45.5 years in the younger cohort and 72.2 years in the older cohort. For data analysis and characterization of the ED population, each patient encounter was treated as a unique patient.
Baseline characteristics significantly differed between the 2 groups (Table 1). When comparing patients aged 18 to 64 years and those aged ≥ 65 years, the younger cohort had higher rates of substance use disorder diagnosis (55.3% vs 27.5%, P < .001), positive urine drug screen (69.7% vs 22.5%, P < .001), and 72-hour legal hold (59.9% vs 32.5%, P < .001) and lower rates of cognitive impairment or dementia (0.7% vs 48.8%, P < .001), and altered mental status-related diagnosis (2.0% vs 18.8%, P < .001). Diagnoses in the younger cohort included 1 each for hyperglycemia, urinary tract infection, and hyponatremia. Diagnoses in the older cohort included 4 for urinary tract infections, 4 for sepsis, 2 for encephalopathy, 2, for hyperglycemia, 1 gastrointestinal bleed, 1 thyrotoxicosis, and 1 respiratory failure.
Endpoints
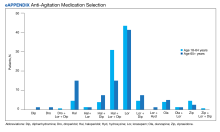
The primary outcome of anti-agitation medication selection significantly differed between the younger cohort and older cohort (P = .02). All medication combinations ordered are shown in the eAppendix based on patient age and the percentage of patients in the age cohort that received that medication combination. Lorazepam monotherapy was the most common anti-agitation medication regimen ordered: 43.4% in patients aged 18 to 64 years and 41.3% in patients aged ≥ 65 years. Second-generation antipsychotic use was low.
Only 10.5% of patients aged 18 to 64 years and 8.8% of patients aged ≥ 65 years received a medication combination including a second-generation antipsychotic. Intramuscular administration (41.4%) was most common followed by intravenous (37.5%), oral (19.8%), and oral disintegrating tablets (1.3%). The median (IQR) number of anti-agitation medications ordered by a prescriber was 6 (3-11) and 18 of 28 prescribers did not prescribe second-generation antipsychotics.
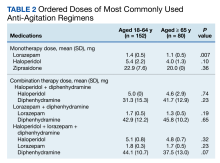
Medication doses ordered did not significantly differ except lorazepam monotherapy, as patients aged ≥ 65 received a lower dose (P = .007) (Table 2). Given the limited data within 1 hour, the first set of vital signs available after medication administration was used for analysis of safety outcomes. Vital signs were documented within 1 hour after medication administration for only 28.3% of patients aged 18 to 64 years and 42.5% of patients aged ≥ 65 years. The median (IQR) time to documentation for vital signs after medication administration was 96 minutes (56-177) for patients aged 18 to 64 years and 64 minutes (25-121) for patients aged ≥ 65 years. Electrocardiogram measurement after medication administration only occurred in 7.9% of patients aged 18 to 64 years and 5% of patients aged ≥ 65 years.
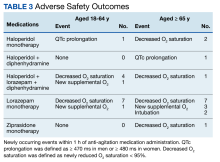
Fourteen patients (7.9%) aged 18 to 64 years and 17 patients (15.0%) aged ≥ 65 years experienced an adverse outcome (P = .09) (Table 3). Most patients who had an adverse safety outcome experienced new oxygen desaturation < 95%. Of those patients, only a small proportion required new supplemental oxygen or intubation. The 2 patients intubated had ongoing medical issues complicating their course in the ED. New QTc prolongation was only documented in haloperidol-containing regimens.
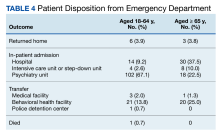
The proportion of patients requiring additional anti-agitation medication doses within 3 hours following initial administration was similar between the 2 groups. The mean (SD) amount of time to administration of subsequent dose was 55 minutes (30) in the younger cohort and 64 minutes (36) in the older cohort. Patient disposition from the ED, significantly differed based on age (P < .001) (Table 4). Patients aged 18 to 64 years were more frequently admitted to the psychiatry unit, while patients aged ≥ 65 years were primarily admitted to the hospital. One patient in the younger cohort died due to hyponatremia.
Discussion
The most likely causes of acute agitation significantly differed between patients aged 18 to 64 years and patients aged ≥ 65 years. Patients in the younger cohort were more likely to present with a history of substance use disorder or a positive urine drug screen for illicit substances. They were also more likely to have a 72-hour legal hold initiated, suggesting higher rates of suicidal and/or homicidal ideations. Patients in the older cohort were likely to present with a history of cognitive impairment or be diagnosed with a condition contributing to an altered mental status. To our knowledge, this is the first study that has assessed characteristics of patients experiencing acute agitation in the ED based on age and demonstrated significant differences in potential contributing factors to acute agitation. These findings may have important implications in helping guide the selection of empiric regimens, especially when the cause of agitation cannot immediately be elucidated.
Lorazepam monotherapy, haloperidol monotherapy, and a combination of haloperidol, lorazepam, and diphenhydramine were the 3 most frequently prescribed regimens for acute agitation. There was low second-generation antipsychotic use. Outside of the VASNHS formulary, there were no policies or restrictions that would have prevented clinicians from ordering a particular anti-agitation medication during the study period.
Since the end of the period assessed in this study, VASNHS clinicians have been educated on the guidelines for anti-agitation medication regimens to encourage higher use of second-generation antipsychotics when appropriate. Training has been developed to prevent unnecessary delays when using these products. Barriers to second-generation antipsychotic use at VASNHS have also been identified and addressed. Previously, second-generation antipsychotics and the sterile water required for medication reconstitution were not overridable in Pyxis machines, often resulting in delays in administering these medications to acutely agitated patients. As of February 2023, olanzapine, ziprasidone, and sterile water are overridable, making them more accessible in situations when medication is urgently needed. Clinicians also expressed concern regarding a lack of familiarity with reconstituting and administering intramuscular second-generation antipsychotics.
While the general guidance has been to use lower doses of anti-agitation medications in patients aged ≥ 65 years, no significant differences were seen in doses ordered other than for lorazepam. In our study, however, there were no significant differences in adverse safety outcomes, though a higher proportion of patients in the older cohort experienced new respiratory-related outcomes after medication administration. Given the retrospective nature of this study and limited documentation of vital signs after medication administration, we cannot conclude the adverse safety outcomes were directly related to the anti-agitation medications. Most patients in both groups did not require additional doses of anti-agitation medications. The results of this study have been used to guide the development of an order set for anti-agitation medications.
Limitations
As a retrospective chart review, this study is unable to prove any differences in prescribing patterns for anti-agitation medications based on age. As a single-center study, the prescribing patterns and baseline characteristics are unique to the facility and not generalizable to all patients with acute agitation in the ED. Future, higher-quality studies with adequate power in diverse patient populations are needed to further elucidate differences in acute agitation etiology and anti-agitation medications based on patient age.
The anti-agitation medication used may have been skewed for patients with multiple and/or previous ED encounters. If information was available on previous causes of agitation and/or previous efficacy of regimens, this may have influenced selection. Additionally, clinical pharmacy specialists began providing daytime coverage in the ED in April 2022. As a part of their role, these pharmacists provide recommendations for medication selection in the management of acute agitation and can order anti-agitation medications. While no pharmacist prescriptions were identified in the study, their recommendations may have influenced medication selection toward the end of the study period.
Given the retrospective nature of the study, it is unclear whether medication selection may have been guided by the patient’s presentation or comorbidities to avoid adverse effects. This may have influenced the safety outcomes observed. Another limitation to this data is vital signs documentation. Vital signs were rarely documented in the ED within 1 hour of medication administration, meaning the vital signs captured may not be related to the agitation medication. Among the patients with documented vital signs, 20 patients were documented within 10 minutes, likely prior to when the medication had taken full effect. This time variability further limits the ability to link safety outcomes to medications and demonstrates a need for additional research. Very few patients had electrocardiogram data after medication administration. If patients did have an electrocardiogram measured in the ED, this more commonly occurred prior to any medication administration, which may have also guided clinicians in initial medication selection.
This study may have also overlooked risperidone use. Though risperidone is on the VASNHS formulary, it was not expected to be commonly used in the ED setting due to it only being available by mouth. However, oral medication use was higher than expected, and there were instances where clinicians initially ordered 1 of the included anti-agitation medications but patients ultimately received risperidone. Based on these findings, the current study may have overlooked this as an anti-agitation medication regimen. In addition, by excluding alcohol intoxication, alcohol withdrawal, and BZD withdrawal, this study did not fully capture the agitated population in our ED.
Conclusions
Anti-agitation medication prescribing patterns may differ between adults aged 18 to 64 years and those aged ≥ 65 years. The findings of this study also suggest that the most common agitation etiologies may differ based on patient age. Future studies should further explore anti-agitation medication use and agitation etiologies among older adults to guide medication prescribing.
Acknowledgments
We acknowledge Ted Turner, PharmD, BCPP, and Phong Ly, PharmD, BCPS, for their support and assistance on this project.
1. Miner JR, Klein LR, Cole JB, Driver BE, Moore JC, Ho JD. The characteristics and prevalence of agitation in an urban county emergency department. Ann Emerg Med. 2018;72(4):361-370. doi:10.1016/j.annemergmed.2018.06.001
2. Kowalenko T, Gates D, Gillespie GL, Succop P, Mentzel TK. Prospective study of violence against ED workers. Am J Emerg Med. 2013;31(1):197-205. doi:10.1016/j.ajem.2012.07.010
3. Marketing General Incorporated. ACEP emergency department violence poll results. American College of Emergency Physicians. August 2022. Accessed January 10, 2024. https://www.emergencyphysicians.org/siteassets/emphysicians/all-pdfs/acep-emergency-department-violence-report-2022-abridged.pdf
4. Richmond JS, Berlin JS, Fishkind AB, et al. Verbal de-escalation of the agitated patient: consensus statement of the American Association for Emergency Psychiatry Project BETA De-escalation Workgroup. West J Emerg Med. 2012;13(1):17-25. doi:10.5811/westjem.2011.9.6864
5. Wilson MP, Pepper D, Currier GW, Holloman GH Jr, Feifel D. The psychopharmacology of agitation: consensus statement of the American Association for Emergency Psychiatry Project BETA Psychopharmacology Workgroup. West J Emerg Med. 2012;13(1):26-34. doi:10.5811/westjem.2011.9.6866
6. Pierre JM. Time to retire haloperidol? Current Psychiatry. 2020;19(5):18-28.
7. US Department of Veteran Affairs. National Center for Veterans Analysis and Statistics. Updated September 7, 2022. Accessed January 10, 2024. https://www.va.gov/vetdata/Veteran_Population.asp
8. Yan J. FDA extends black-box warning to all antipsychotics. Psychiatric News. 2008;43(14):1-27. doi:10.1176/pn.43.14.0001
9. 2023 American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2023 updated AGS Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2023;71(7):2052-2081. doi:10.1111/jgs.18372
10. Wilson MP, Nordstrom K, Hopper A, Porter A, Castillo EM, Vilke GM. Risperidone in the emergency setting is associated with more hypotension in elderly patients. J Emerg Med. 2017;53(5):735-739. doi:10.1016/j.jemermed.2017.06.026
11. Gottlieb M, Long B, Koyfman A. Approach to the agitated emergency department patient. J Emerg Med. 2018;54(4):447-457. doi:10.1016/j.jemermed.2017.12.049
Each year, about 2.6% of emergency department (ED) visits involve agitation.1 ED clinicians are especially prone to workplace violence and assault, facing the challenge of caring for patients while maintaining safety. A 2013 prospective study found an average of 4.15 violent events per employee in 9 months; nurses and patient care assistants were most frequently affected.2 A 2022 survey from the American College of Emergency Physicians found 55% of respondents reported being physically assaulted in the ED and 79% of respondents reported witnessing another assault. Most of these assaults (98%) were committed by the patients.3 Appropriate management of patients experiencing acute agitation is critical for the safety of all parties involved.
The initial approach to acute agitation management involves nonpharmacologic measures in an attempt to avoid coercive actions, such as physical restraints. Reducing environmental stimulation and verbal de-escalation are effective and help the patients with agitation regain control over their behavior.4
When these measures fail, however, pharmacologic therapy is often administered to ensure safety. The goal of pharmacologic therapy is to calm the patient without causing sedation.5 This allows the patient to continue participating in their care and allows the care team to accurately assess them, which is critical in determining the underlying etiology of agitation. Historically, haloperidol has commonly been used to manage acute agitation. It is frequently administered with lorazepam and diphenhydramine to reduce the incidence of haloperidol’s extrapyramidal adverse effects. However, there are several potential concerns with this method, including oversedation, QTc prolongation, potential drug interactions, and polypharmacy.5,6
The American Association of Emergency Psychiatry Project BETA Psychopharmacology Workgroup published a Consensus Statement in 2012 regarding the psychopharmacology of agitation.5 When considering medication for agitation management, clinicians must first determine a provisional diagnosis outlining the most probable etiology of the patient’s behavior, such as delirium, intoxication, or a psychiatric disorder. Apart from alcohol intoxication, benzodiazepines (BZDs) or second-generation antipsychotics as monotherapy are generally preferred over haloperidol for acute agitation.5 Second-generation antipsychotics have demonstrated to be as effective as haloperidol but are thought to be safer options. Quetiapine is not recommended for use in the ED due to the risk of orthostatic hypotension, as patients are often volume depleted.5The Veterans Affairs Southern Nevada Healthcare System (VASNHS) serves veterans in the Las Vegas area. Among the nearly 220,000 veterans in Nevada, about 100,000 veterans are aged ≥ 65 years.7 The 2012 consensus statement on psychopharmacology for agitation offers no specific age-related guidance. However, there are safety concerns in older adults both with antipsychotics and BZDs, even with acute use. The US Food and Drug Administration (FDA) issued a boxed warning for all antipsychotics due to increased mortality in older adult patients with dementia-related psychosis.8 The 2023 American Geriatrics Society Beers Criteria provides guidance on pharmacological therapy for adults aged ≥ 65 years and recommends avoiding antipsychotics and BZDs.9 In addition to the FDA boxed warning, data suggest increased mortality with antipsychotic use independent of dementia. With BZDs, changes in pharmacodynamics make older adults more prone to adverse effects, including cognitive impairment, delirium, falls, and fractures. A retrospective chart review evaluated risperidone use in the ED and found that adults aged ≥ 65 years experienced higher rates of hypotension, even though this age group received about half the dose of risperidone compared with younger patients.10 For this patient population, the general approach in treating acute agitation has been to avoid the use of medications, but prescribe lower doses when necessary.11
With limited research on acute agitation management in older adults, the purpose of this study was to compare current prescribing practices of anti-agitation medications between adults aged 18 to 64 years and adults aged ≥ 65 years in the VASNHS ED. This study was also conducted to better understand the anti-agitation prescribing practices at VASNHS, as no order sets or protocols existed at the time of the study to guide medication selection in agitation management. To our knowledge, this is the first observational study evaluating pharmacologic acute agitation management in the ED based on age.
Methods
This study was a retrospective chart review of patients aged ≥ 18 years who presented to the VASNHS ED and received medication for acute agitation. Patients were identified through active orders for a formulary agitation medication from August 1, 2019, to July 31, 2022. Formulary medication options included intravenous, oral, and intramuscular routes for haloperidol, droperidol, lorazepam, olanzapine, or ziprasidone. Veterans were excluded if they presented with alcohol intoxication, alcohol or BZD withdrawal, if the medication administration was unrelated to agitation, or whether the medication was not administered. While alcohol and/or BZDs can contribute to acute agitation, these patients were excluded due to a clear indication for BZD therapy and the challenge in a retrospective chart review to determine whether patients received medication for agitation vs other withdrawal-related symptoms.
Endpoints
The primary endpoint was the medication selection between 2 age groups: 18 to 64 years and ≥ 65 years. The secondary endpoints included ordered medication dose by regimen, additional anti-agitation medication use within 3 hours of initial medication administration, and disposition. Safety outcomes included incidence of newly occurring oxygen desaturation < 95%, supplemental oxygen requirement, intubation, QTc prolongation, and hypotension with systolic blood pressure < 90 mm Hg within 1 hour of medication administration. Data collected included patient demographics, substance use, conditions contributing to altered mental status, active psychotropic medication prescriptions, medication adherence, agitation medication prescriber, and doses. Adherence to psychotropic medication in the past 6 months was defined as ≥ 80% of days covered with medication and based on fill history. This was only calculated for applicable patients and did not include patients with only as-needed medications, such as hydroxyzine for anxiety.
Statistical Analysis
Statistical analyses were performed using IBM SPSS. Baseline characteristics were analyzed using descriptive statistics. χ2 and Fisher exact tests were used to analyze categorical data. A student t test was used for continuous variables and a 2-sided P value of < .05 was considered statistically significant.
Results
During the study period, 2342 unique patient encounters with active anti-agitation medication orders in the ED were identified and 232 encounters met the inclusion criteria. Of those excluded, 605 encounters had alcohol involvement. The study included 152 patient encounters for 128 patients aged 18 to 64 years of whom 16 patients had > 1 encounter with a mean (SD) 2.5 (1.1) visits. The study included 80 patient encounters for 72 patients aged ≥ 65 years of whom 7 patients had > 1 encounter with a mean (SD) 2.1 (0.3) visits. The mean age was 45.5 years in the younger cohort and 72.2 years in the older cohort. For data analysis and characterization of the ED population, each patient encounter was treated as a unique patient.
Baseline characteristics significantly differed between the 2 groups (Table 1). When comparing patients aged 18 to 64 years and those aged ≥ 65 years, the younger cohort had higher rates of substance use disorder diagnosis (55.3% vs 27.5%, P < .001), positive urine drug screen (69.7% vs 22.5%, P < .001), and 72-hour legal hold (59.9% vs 32.5%, P < .001) and lower rates of cognitive impairment or dementia (0.7% vs 48.8%, P < .001), and altered mental status-related diagnosis (2.0% vs 18.8%, P < .001). Diagnoses in the younger cohort included 1 each for hyperglycemia, urinary tract infection, and hyponatremia. Diagnoses in the older cohort included 4 for urinary tract infections, 4 for sepsis, 2 for encephalopathy, 2, for hyperglycemia, 1 gastrointestinal bleed, 1 thyrotoxicosis, and 1 respiratory failure.
Endpoints
The primary outcome of anti-agitation medication selection significantly differed between the younger cohort and older cohort (P = .02). All medication combinations ordered are shown in the eAppendix based on patient age and the percentage of patients in the age cohort that received that medication combination. Lorazepam monotherapy was the most common anti-agitation medication regimen ordered: 43.4% in patients aged 18 to 64 years and 41.3% in patients aged ≥ 65 years. Second-generation antipsychotic use was low.
Only 10.5% of patients aged 18 to 64 years and 8.8% of patients aged ≥ 65 years received a medication combination including a second-generation antipsychotic. Intramuscular administration (41.4%) was most common followed by intravenous (37.5%), oral (19.8%), and oral disintegrating tablets (1.3%). The median (IQR) number of anti-agitation medications ordered by a prescriber was 6 (3-11) and 18 of 28 prescribers did not prescribe second-generation antipsychotics.
Medication doses ordered did not significantly differ except lorazepam monotherapy, as patients aged ≥ 65 received a lower dose (P = .007) (Table 2). Given the limited data within 1 hour, the first set of vital signs available after medication administration was used for analysis of safety outcomes. Vital signs were documented within 1 hour after medication administration for only 28.3% of patients aged 18 to 64 years and 42.5% of patients aged ≥ 65 years. The median (IQR) time to documentation for vital signs after medication administration was 96 minutes (56-177) for patients aged 18 to 64 years and 64 minutes (25-121) for patients aged ≥ 65 years. Electrocardiogram measurement after medication administration only occurred in 7.9% of patients aged 18 to 64 years and 5% of patients aged ≥ 65 years.
Fourteen patients (7.9%) aged 18 to 64 years and 17 patients (15.0%) aged ≥ 65 years experienced an adverse outcome (P = .09) (Table 3). Most patients who had an adverse safety outcome experienced new oxygen desaturation < 95%. Of those patients, only a small proportion required new supplemental oxygen or intubation. The 2 patients intubated had ongoing medical issues complicating their course in the ED. New QTc prolongation was only documented in haloperidol-containing regimens.
The proportion of patients requiring additional anti-agitation medication doses within 3 hours following initial administration was similar between the 2 groups. The mean (SD) amount of time to administration of subsequent dose was 55 minutes (30) in the younger cohort and 64 minutes (36) in the older cohort. Patient disposition from the ED, significantly differed based on age (P < .001) (Table 4). Patients aged 18 to 64 years were more frequently admitted to the psychiatry unit, while patients aged ≥ 65 years were primarily admitted to the hospital. One patient in the younger cohort died due to hyponatremia.
Discussion
The most likely causes of acute agitation significantly differed between patients aged 18 to 64 years and patients aged ≥ 65 years. Patients in the younger cohort were more likely to present with a history of substance use disorder or a positive urine drug screen for illicit substances. They were also more likely to have a 72-hour legal hold initiated, suggesting higher rates of suicidal and/or homicidal ideations. Patients in the older cohort were likely to present with a history of cognitive impairment or be diagnosed with a condition contributing to an altered mental status. To our knowledge, this is the first study that has assessed characteristics of patients experiencing acute agitation in the ED based on age and demonstrated significant differences in potential contributing factors to acute agitation. These findings may have important implications in helping guide the selection of empiric regimens, especially when the cause of agitation cannot immediately be elucidated.
Lorazepam monotherapy, haloperidol monotherapy, and a combination of haloperidol, lorazepam, and diphenhydramine were the 3 most frequently prescribed regimens for acute agitation. There was low second-generation antipsychotic use. Outside of the VASNHS formulary, there were no policies or restrictions that would have prevented clinicians from ordering a particular anti-agitation medication during the study period.
Since the end of the period assessed in this study, VASNHS clinicians have been educated on the guidelines for anti-agitation medication regimens to encourage higher use of second-generation antipsychotics when appropriate. Training has been developed to prevent unnecessary delays when using these products. Barriers to second-generation antipsychotic use at VASNHS have also been identified and addressed. Previously, second-generation antipsychotics and the sterile water required for medication reconstitution were not overridable in Pyxis machines, often resulting in delays in administering these medications to acutely agitated patients. As of February 2023, olanzapine, ziprasidone, and sterile water are overridable, making them more accessible in situations when medication is urgently needed. Clinicians also expressed concern regarding a lack of familiarity with reconstituting and administering intramuscular second-generation antipsychotics.
While the general guidance has been to use lower doses of anti-agitation medications in patients aged ≥ 65 years, no significant differences were seen in doses ordered other than for lorazepam. In our study, however, there were no significant differences in adverse safety outcomes, though a higher proportion of patients in the older cohort experienced new respiratory-related outcomes after medication administration. Given the retrospective nature of this study and limited documentation of vital signs after medication administration, we cannot conclude the adverse safety outcomes were directly related to the anti-agitation medications. Most patients in both groups did not require additional doses of anti-agitation medications. The results of this study have been used to guide the development of an order set for anti-agitation medications.
Limitations
As a retrospective chart review, this study is unable to prove any differences in prescribing patterns for anti-agitation medications based on age. As a single-center study, the prescribing patterns and baseline characteristics are unique to the facility and not generalizable to all patients with acute agitation in the ED. Future, higher-quality studies with adequate power in diverse patient populations are needed to further elucidate differences in acute agitation etiology and anti-agitation medications based on patient age.
The anti-agitation medication used may have been skewed for patients with multiple and/or previous ED encounters. If information was available on previous causes of agitation and/or previous efficacy of regimens, this may have influenced selection. Additionally, clinical pharmacy specialists began providing daytime coverage in the ED in April 2022. As a part of their role, these pharmacists provide recommendations for medication selection in the management of acute agitation and can order anti-agitation medications. While no pharmacist prescriptions were identified in the study, their recommendations may have influenced medication selection toward the end of the study period.
Given the retrospective nature of the study, it is unclear whether medication selection may have been guided by the patient’s presentation or comorbidities to avoid adverse effects. This may have influenced the safety outcomes observed. Another limitation to this data is vital signs documentation. Vital signs were rarely documented in the ED within 1 hour of medication administration, meaning the vital signs captured may not be related to the agitation medication. Among the patients with documented vital signs, 20 patients were documented within 10 minutes, likely prior to when the medication had taken full effect. This time variability further limits the ability to link safety outcomes to medications and demonstrates a need for additional research. Very few patients had electrocardiogram data after medication administration. If patients did have an electrocardiogram measured in the ED, this more commonly occurred prior to any medication administration, which may have also guided clinicians in initial medication selection.
This study may have also overlooked risperidone use. Though risperidone is on the VASNHS formulary, it was not expected to be commonly used in the ED setting due to it only being available by mouth. However, oral medication use was higher than expected, and there were instances where clinicians initially ordered 1 of the included anti-agitation medications but patients ultimately received risperidone. Based on these findings, the current study may have overlooked this as an anti-agitation medication regimen. In addition, by excluding alcohol intoxication, alcohol withdrawal, and BZD withdrawal, this study did not fully capture the agitated population in our ED.
Conclusions
Anti-agitation medication prescribing patterns may differ between adults aged 18 to 64 years and those aged ≥ 65 years. The findings of this study also suggest that the most common agitation etiologies may differ based on patient age. Future studies should further explore anti-agitation medication use and agitation etiologies among older adults to guide medication prescribing.
Acknowledgments
We acknowledge Ted Turner, PharmD, BCPP, and Phong Ly, PharmD, BCPS, for their support and assistance on this project.
Each year, about 2.6% of emergency department (ED) visits involve agitation.1 ED clinicians are especially prone to workplace violence and assault, facing the challenge of caring for patients while maintaining safety. A 2013 prospective study found an average of 4.15 violent events per employee in 9 months; nurses and patient care assistants were most frequently affected.2 A 2022 survey from the American College of Emergency Physicians found 55% of respondents reported being physically assaulted in the ED and 79% of respondents reported witnessing another assault. Most of these assaults (98%) were committed by the patients.3 Appropriate management of patients experiencing acute agitation is critical for the safety of all parties involved.
The initial approach to acute agitation management involves nonpharmacologic measures in an attempt to avoid coercive actions, such as physical restraints. Reducing environmental stimulation and verbal de-escalation are effective and help the patients with agitation regain control over their behavior.4
When these measures fail, however, pharmacologic therapy is often administered to ensure safety. The goal of pharmacologic therapy is to calm the patient without causing sedation.5 This allows the patient to continue participating in their care and allows the care team to accurately assess them, which is critical in determining the underlying etiology of agitation. Historically, haloperidol has commonly been used to manage acute agitation. It is frequently administered with lorazepam and diphenhydramine to reduce the incidence of haloperidol’s extrapyramidal adverse effects. However, there are several potential concerns with this method, including oversedation, QTc prolongation, potential drug interactions, and polypharmacy.5,6
The American Association of Emergency Psychiatry Project BETA Psychopharmacology Workgroup published a Consensus Statement in 2012 regarding the psychopharmacology of agitation.5 When considering medication for agitation management, clinicians must first determine a provisional diagnosis outlining the most probable etiology of the patient’s behavior, such as delirium, intoxication, or a psychiatric disorder. Apart from alcohol intoxication, benzodiazepines (BZDs) or second-generation antipsychotics as monotherapy are generally preferred over haloperidol for acute agitation.5 Second-generation antipsychotics have demonstrated to be as effective as haloperidol but are thought to be safer options. Quetiapine is not recommended for use in the ED due to the risk of orthostatic hypotension, as patients are often volume depleted.5The Veterans Affairs Southern Nevada Healthcare System (VASNHS) serves veterans in the Las Vegas area. Among the nearly 220,000 veterans in Nevada, about 100,000 veterans are aged ≥ 65 years.7 The 2012 consensus statement on psychopharmacology for agitation offers no specific age-related guidance. However, there are safety concerns in older adults both with antipsychotics and BZDs, even with acute use. The US Food and Drug Administration (FDA) issued a boxed warning for all antipsychotics due to increased mortality in older adult patients with dementia-related psychosis.8 The 2023 American Geriatrics Society Beers Criteria provides guidance on pharmacological therapy for adults aged ≥ 65 years and recommends avoiding antipsychotics and BZDs.9 In addition to the FDA boxed warning, data suggest increased mortality with antipsychotic use independent of dementia. With BZDs, changes in pharmacodynamics make older adults more prone to adverse effects, including cognitive impairment, delirium, falls, and fractures. A retrospective chart review evaluated risperidone use in the ED and found that adults aged ≥ 65 years experienced higher rates of hypotension, even though this age group received about half the dose of risperidone compared with younger patients.10 For this patient population, the general approach in treating acute agitation has been to avoid the use of medications, but prescribe lower doses when necessary.11
With limited research on acute agitation management in older adults, the purpose of this study was to compare current prescribing practices of anti-agitation medications between adults aged 18 to 64 years and adults aged ≥ 65 years in the VASNHS ED. This study was also conducted to better understand the anti-agitation prescribing practices at VASNHS, as no order sets or protocols existed at the time of the study to guide medication selection in agitation management. To our knowledge, this is the first observational study evaluating pharmacologic acute agitation management in the ED based on age.
Methods
This study was a retrospective chart review of patients aged ≥ 18 years who presented to the VASNHS ED and received medication for acute agitation. Patients were identified through active orders for a formulary agitation medication from August 1, 2019, to July 31, 2022. Formulary medication options included intravenous, oral, and intramuscular routes for haloperidol, droperidol, lorazepam, olanzapine, or ziprasidone. Veterans were excluded if they presented with alcohol intoxication, alcohol or BZD withdrawal, if the medication administration was unrelated to agitation, or whether the medication was not administered. While alcohol and/or BZDs can contribute to acute agitation, these patients were excluded due to a clear indication for BZD therapy and the challenge in a retrospective chart review to determine whether patients received medication for agitation vs other withdrawal-related symptoms.
Endpoints
The primary endpoint was the medication selection between 2 age groups: 18 to 64 years and ≥ 65 years. The secondary endpoints included ordered medication dose by regimen, additional anti-agitation medication use within 3 hours of initial medication administration, and disposition. Safety outcomes included incidence of newly occurring oxygen desaturation < 95%, supplemental oxygen requirement, intubation, QTc prolongation, and hypotension with systolic blood pressure < 90 mm Hg within 1 hour of medication administration. Data collected included patient demographics, substance use, conditions contributing to altered mental status, active psychotropic medication prescriptions, medication adherence, agitation medication prescriber, and doses. Adherence to psychotropic medication in the past 6 months was defined as ≥ 80% of days covered with medication and based on fill history. This was only calculated for applicable patients and did not include patients with only as-needed medications, such as hydroxyzine for anxiety.
Statistical Analysis
Statistical analyses were performed using IBM SPSS. Baseline characteristics were analyzed using descriptive statistics. χ2 and Fisher exact tests were used to analyze categorical data. A student t test was used for continuous variables and a 2-sided P value of < .05 was considered statistically significant.
Results
During the study period, 2342 unique patient encounters with active anti-agitation medication orders in the ED were identified and 232 encounters met the inclusion criteria. Of those excluded, 605 encounters had alcohol involvement. The study included 152 patient encounters for 128 patients aged 18 to 64 years of whom 16 patients had > 1 encounter with a mean (SD) 2.5 (1.1) visits. The study included 80 patient encounters for 72 patients aged ≥ 65 years of whom 7 patients had > 1 encounter with a mean (SD) 2.1 (0.3) visits. The mean age was 45.5 years in the younger cohort and 72.2 years in the older cohort. For data analysis and characterization of the ED population, each patient encounter was treated as a unique patient.
Baseline characteristics significantly differed between the 2 groups (Table 1). When comparing patients aged 18 to 64 years and those aged ≥ 65 years, the younger cohort had higher rates of substance use disorder diagnosis (55.3% vs 27.5%, P < .001), positive urine drug screen (69.7% vs 22.5%, P < .001), and 72-hour legal hold (59.9% vs 32.5%, P < .001) and lower rates of cognitive impairment or dementia (0.7% vs 48.8%, P < .001), and altered mental status-related diagnosis (2.0% vs 18.8%, P < .001). Diagnoses in the younger cohort included 1 each for hyperglycemia, urinary tract infection, and hyponatremia. Diagnoses in the older cohort included 4 for urinary tract infections, 4 for sepsis, 2 for encephalopathy, 2, for hyperglycemia, 1 gastrointestinal bleed, 1 thyrotoxicosis, and 1 respiratory failure.
Endpoints
The primary outcome of anti-agitation medication selection significantly differed between the younger cohort and older cohort (P = .02). All medication combinations ordered are shown in the eAppendix based on patient age and the percentage of patients in the age cohort that received that medication combination. Lorazepam monotherapy was the most common anti-agitation medication regimen ordered: 43.4% in patients aged 18 to 64 years and 41.3% in patients aged ≥ 65 years. Second-generation antipsychotic use was low.
Only 10.5% of patients aged 18 to 64 years and 8.8% of patients aged ≥ 65 years received a medication combination including a second-generation antipsychotic. Intramuscular administration (41.4%) was most common followed by intravenous (37.5%), oral (19.8%), and oral disintegrating tablets (1.3%). The median (IQR) number of anti-agitation medications ordered by a prescriber was 6 (3-11) and 18 of 28 prescribers did not prescribe second-generation antipsychotics.
Medication doses ordered did not significantly differ except lorazepam monotherapy, as patients aged ≥ 65 received a lower dose (P = .007) (Table 2). Given the limited data within 1 hour, the first set of vital signs available after medication administration was used for analysis of safety outcomes. Vital signs were documented within 1 hour after medication administration for only 28.3% of patients aged 18 to 64 years and 42.5% of patients aged ≥ 65 years. The median (IQR) time to documentation for vital signs after medication administration was 96 minutes (56-177) for patients aged 18 to 64 years and 64 minutes (25-121) for patients aged ≥ 65 years. Electrocardiogram measurement after medication administration only occurred in 7.9% of patients aged 18 to 64 years and 5% of patients aged ≥ 65 years.
Fourteen patients (7.9%) aged 18 to 64 years and 17 patients (15.0%) aged ≥ 65 years experienced an adverse outcome (P = .09) (Table 3). Most patients who had an adverse safety outcome experienced new oxygen desaturation < 95%. Of those patients, only a small proportion required new supplemental oxygen or intubation. The 2 patients intubated had ongoing medical issues complicating their course in the ED. New QTc prolongation was only documented in haloperidol-containing regimens.
The proportion of patients requiring additional anti-agitation medication doses within 3 hours following initial administration was similar between the 2 groups. The mean (SD) amount of time to administration of subsequent dose was 55 minutes (30) in the younger cohort and 64 minutes (36) in the older cohort. Patient disposition from the ED, significantly differed based on age (P < .001) (Table 4). Patients aged 18 to 64 years were more frequently admitted to the psychiatry unit, while patients aged ≥ 65 years were primarily admitted to the hospital. One patient in the younger cohort died due to hyponatremia.
Discussion
The most likely causes of acute agitation significantly differed between patients aged 18 to 64 years and patients aged ≥ 65 years. Patients in the younger cohort were more likely to present with a history of substance use disorder or a positive urine drug screen for illicit substances. They were also more likely to have a 72-hour legal hold initiated, suggesting higher rates of suicidal and/or homicidal ideations. Patients in the older cohort were likely to present with a history of cognitive impairment or be diagnosed with a condition contributing to an altered mental status. To our knowledge, this is the first study that has assessed characteristics of patients experiencing acute agitation in the ED based on age and demonstrated significant differences in potential contributing factors to acute agitation. These findings may have important implications in helping guide the selection of empiric regimens, especially when the cause of agitation cannot immediately be elucidated.
Lorazepam monotherapy, haloperidol monotherapy, and a combination of haloperidol, lorazepam, and diphenhydramine were the 3 most frequently prescribed regimens for acute agitation. There was low second-generation antipsychotic use. Outside of the VASNHS formulary, there were no policies or restrictions that would have prevented clinicians from ordering a particular anti-agitation medication during the study period.
Since the end of the period assessed in this study, VASNHS clinicians have been educated on the guidelines for anti-agitation medication regimens to encourage higher use of second-generation antipsychotics when appropriate. Training has been developed to prevent unnecessary delays when using these products. Barriers to second-generation antipsychotic use at VASNHS have also been identified and addressed. Previously, second-generation antipsychotics and the sterile water required for medication reconstitution were not overridable in Pyxis machines, often resulting in delays in administering these medications to acutely agitated patients. As of February 2023, olanzapine, ziprasidone, and sterile water are overridable, making them more accessible in situations when medication is urgently needed. Clinicians also expressed concern regarding a lack of familiarity with reconstituting and administering intramuscular second-generation antipsychotics.
While the general guidance has been to use lower doses of anti-agitation medications in patients aged ≥ 65 years, no significant differences were seen in doses ordered other than for lorazepam. In our study, however, there were no significant differences in adverse safety outcomes, though a higher proportion of patients in the older cohort experienced new respiratory-related outcomes after medication administration. Given the retrospective nature of this study and limited documentation of vital signs after medication administration, we cannot conclude the adverse safety outcomes were directly related to the anti-agitation medications. Most patients in both groups did not require additional doses of anti-agitation medications. The results of this study have been used to guide the development of an order set for anti-agitation medications.
Limitations
As a retrospective chart review, this study is unable to prove any differences in prescribing patterns for anti-agitation medications based on age. As a single-center study, the prescribing patterns and baseline characteristics are unique to the facility and not generalizable to all patients with acute agitation in the ED. Future, higher-quality studies with adequate power in diverse patient populations are needed to further elucidate differences in acute agitation etiology and anti-agitation medications based on patient age.
The anti-agitation medication used may have been skewed for patients with multiple and/or previous ED encounters. If information was available on previous causes of agitation and/or previous efficacy of regimens, this may have influenced selection. Additionally, clinical pharmacy specialists began providing daytime coverage in the ED in April 2022. As a part of their role, these pharmacists provide recommendations for medication selection in the management of acute agitation and can order anti-agitation medications. While no pharmacist prescriptions were identified in the study, their recommendations may have influenced medication selection toward the end of the study period.
Given the retrospective nature of the study, it is unclear whether medication selection may have been guided by the patient’s presentation or comorbidities to avoid adverse effects. This may have influenced the safety outcomes observed. Another limitation to this data is vital signs documentation. Vital signs were rarely documented in the ED within 1 hour of medication administration, meaning the vital signs captured may not be related to the agitation medication. Among the patients with documented vital signs, 20 patients were documented within 10 minutes, likely prior to when the medication had taken full effect. This time variability further limits the ability to link safety outcomes to medications and demonstrates a need for additional research. Very few patients had electrocardiogram data after medication administration. If patients did have an electrocardiogram measured in the ED, this more commonly occurred prior to any medication administration, which may have also guided clinicians in initial medication selection.
This study may have also overlooked risperidone use. Though risperidone is on the VASNHS formulary, it was not expected to be commonly used in the ED setting due to it only being available by mouth. However, oral medication use was higher than expected, and there were instances where clinicians initially ordered 1 of the included anti-agitation medications but patients ultimately received risperidone. Based on these findings, the current study may have overlooked this as an anti-agitation medication regimen. In addition, by excluding alcohol intoxication, alcohol withdrawal, and BZD withdrawal, this study did not fully capture the agitated population in our ED.
Conclusions
Anti-agitation medication prescribing patterns may differ between adults aged 18 to 64 years and those aged ≥ 65 years. The findings of this study also suggest that the most common agitation etiologies may differ based on patient age. Future studies should further explore anti-agitation medication use and agitation etiologies among older adults to guide medication prescribing.
Acknowledgments
We acknowledge Ted Turner, PharmD, BCPP, and Phong Ly, PharmD, BCPS, for their support and assistance on this project.
1. Miner JR, Klein LR, Cole JB, Driver BE, Moore JC, Ho JD. The characteristics and prevalence of agitation in an urban county emergency department. Ann Emerg Med. 2018;72(4):361-370. doi:10.1016/j.annemergmed.2018.06.001
2. Kowalenko T, Gates D, Gillespie GL, Succop P, Mentzel TK. Prospective study of violence against ED workers. Am J Emerg Med. 2013;31(1):197-205. doi:10.1016/j.ajem.2012.07.010
3. Marketing General Incorporated. ACEP emergency department violence poll results. American College of Emergency Physicians. August 2022. Accessed January 10, 2024. https://www.emergencyphysicians.org/siteassets/emphysicians/all-pdfs/acep-emergency-department-violence-report-2022-abridged.pdf
4. Richmond JS, Berlin JS, Fishkind AB, et al. Verbal de-escalation of the agitated patient: consensus statement of the American Association for Emergency Psychiatry Project BETA De-escalation Workgroup. West J Emerg Med. 2012;13(1):17-25. doi:10.5811/westjem.2011.9.6864
5. Wilson MP, Pepper D, Currier GW, Holloman GH Jr, Feifel D. The psychopharmacology of agitation: consensus statement of the American Association for Emergency Psychiatry Project BETA Psychopharmacology Workgroup. West J Emerg Med. 2012;13(1):26-34. doi:10.5811/westjem.2011.9.6866
6. Pierre JM. Time to retire haloperidol? Current Psychiatry. 2020;19(5):18-28.
7. US Department of Veteran Affairs. National Center for Veterans Analysis and Statistics. Updated September 7, 2022. Accessed January 10, 2024. https://www.va.gov/vetdata/Veteran_Population.asp
8. Yan J. FDA extends black-box warning to all antipsychotics. Psychiatric News. 2008;43(14):1-27. doi:10.1176/pn.43.14.0001
9. 2023 American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2023 updated AGS Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2023;71(7):2052-2081. doi:10.1111/jgs.18372
10. Wilson MP, Nordstrom K, Hopper A, Porter A, Castillo EM, Vilke GM. Risperidone in the emergency setting is associated with more hypotension in elderly patients. J Emerg Med. 2017;53(5):735-739. doi:10.1016/j.jemermed.2017.06.026
11. Gottlieb M, Long B, Koyfman A. Approach to the agitated emergency department patient. J Emerg Med. 2018;54(4):447-457. doi:10.1016/j.jemermed.2017.12.049
1. Miner JR, Klein LR, Cole JB, Driver BE, Moore JC, Ho JD. The characteristics and prevalence of agitation in an urban county emergency department. Ann Emerg Med. 2018;72(4):361-370. doi:10.1016/j.annemergmed.2018.06.001
2. Kowalenko T, Gates D, Gillespie GL, Succop P, Mentzel TK. Prospective study of violence against ED workers. Am J Emerg Med. 2013;31(1):197-205. doi:10.1016/j.ajem.2012.07.010
3. Marketing General Incorporated. ACEP emergency department violence poll results. American College of Emergency Physicians. August 2022. Accessed January 10, 2024. https://www.emergencyphysicians.org/siteassets/emphysicians/all-pdfs/acep-emergency-department-violence-report-2022-abridged.pdf
4. Richmond JS, Berlin JS, Fishkind AB, et al. Verbal de-escalation of the agitated patient: consensus statement of the American Association for Emergency Psychiatry Project BETA De-escalation Workgroup. West J Emerg Med. 2012;13(1):17-25. doi:10.5811/westjem.2011.9.6864
5. Wilson MP, Pepper D, Currier GW, Holloman GH Jr, Feifel D. The psychopharmacology of agitation: consensus statement of the American Association for Emergency Psychiatry Project BETA Psychopharmacology Workgroup. West J Emerg Med. 2012;13(1):26-34. doi:10.5811/westjem.2011.9.6866
6. Pierre JM. Time to retire haloperidol? Current Psychiatry. 2020;19(5):18-28.
7. US Department of Veteran Affairs. National Center for Veterans Analysis and Statistics. Updated September 7, 2022. Accessed January 10, 2024. https://www.va.gov/vetdata/Veteran_Population.asp
8. Yan J. FDA extends black-box warning to all antipsychotics. Psychiatric News. 2008;43(14):1-27. doi:10.1176/pn.43.14.0001
9. 2023 American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2023 updated AGS Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2023;71(7):2052-2081. doi:10.1111/jgs.18372
10. Wilson MP, Nordstrom K, Hopper A, Porter A, Castillo EM, Vilke GM. Risperidone in the emergency setting is associated with more hypotension in elderly patients. J Emerg Med. 2017;53(5):735-739. doi:10.1016/j.jemermed.2017.06.026
11. Gottlieb M, Long B, Koyfman A. Approach to the agitated emergency department patient. J Emerg Med. 2018;54(4):447-457. doi:10.1016/j.jemermed.2017.12.049
Preparing Veterans Health Administration Psychologists to Meet the Complex Needs of Aging Veterans
The Veterans Health Administration (VHA) is understaffed for clinical psychologists who have specialty training in geriatrics (ie, geropsychologists) to meet the needs of aging veterans. Though only 16.8% of US adults are aged ≥ 65 years,1 this age group comprises 45.9% of patients within the VHA.2 The needs of older adults are complex and warrant specialized services from mental health clinicians trained to understand lifespan developmental processes, biological changes associated with aging, and changes in psychosocial functioning.
Older veterans (aged ≥ 65 years) present with higher rates of combined medical and mental health diagnoses compared to both younger veterans and older adults who are not veterans.3 Nearly 1 of 5 (18.1%) older veterans who use VHA services have confirmed mental health diagnoses, and an additional 25.5% have documented mental health concerns without a formal diagnosis in their health record.4 The clinical presentations of older veterans frequently differ from younger adults and include greater complexity. For example, older veterans face an increased risk of cognitive impairment compared to the general population, due in part to higher prevalence of posttraumatic stress, which doubles their risk of developing dementia.5 Additional examples of multicomplexity among older veterans may include co-occurring medical and psychiatric diagnoses, the presence of delirium, social isolation/loneliness, and concerns related to polypharmacy. These complex presentations result in significant challenges for mental health clinicians in areas like assessment (eg, accuracy of case conceptualization), intervention (eg, selection and prioritization), and consultation (eg, coordination among multiple medical and mental health specialists).
Older veterans also present with substantial resilience. Research has found that aging veterans exposed to trauma during their military service often review their memories and past experiences, which is known as later-adulthood trauma reengagement.6 Through this normative life review process, veterans engage with memories and experiences from their past that they previously avoided, which could lead to posttraumatic growth for some. Unfortunately, others may experience an increase in psychological distress. Mental health clinicians with specialty expertise and training in aging and lifespan development can facilitate positive outcomes to reduce distress.7
The United States in general, and the VHA specifically, face a growing shortage of geriatric mental health clinicians.
The Geriatric Scholars Program (GSP) was developed in 2008 to address the training gap and provide education in geriatrics to VHA clinicians that treat older veterans, particularly in rural areas.11,12 The GSP initially focused on primary care physicians, nurse practitioners, physician assistants, and pharmacists. It was later expanded to include other disciplines (ie, social work, rehabilitation therapists, and psychiatrists). In 2013, the GSP – Psychology Track (GSP-P) was developed with funding from the VHA Offices of Rural Health and Geriatrics and Extended Care specifically for psychologists.
This article describes the multicomponent longitudinal GSP-P, which has evolved to meet the target audience’s ongoing needs for knowledge, skills, and opportunities to refine practice behaviors. GSP-P received the 2020 Award for Excellence in Geropsychology Training from the Council of Professional Geropsychology Training Programs. GSP-P has grown within the context of the larger GSP and aligns with the other existing elective learning opportunities (Figure 1).
Program description
Introductory Course
Psychologist subject matter experts (SMEs) developed an intensive course in geropsychology in the absence of a similar course in the geriatric medicine board review curriculum. SMEs reviewed the guidelines for practice by professional organizations like the Pikes Peak Geropsychology Competencies, which outline knowledge and skills in various domains.13 SMEs integrated this review with findings from a needs assessment for postlicensed VHA psychology staff in 4 health care systems, drafted a syllabus, and circulated it to geropsychology experts for feedback. The resulting multiday course covered general mental health as well as topics particularly salient for mental health clinicians treating older veterans including suicide prevention and posttraumatic stress disorder (PTSD).14 This Geropsychology Competencies Review Course was piloted in 1 region initially before being offered nationally in 2014.
Quality Improvement
Introductory course attendees also participate in an intensive day-long interactive workshop in quality improvement (QI). After completing these trainings, they apply what they have learned at their home facility by embarking on a QI project related to geriatrics. The QI projects reinforce learning and initiate practice changes not only for attendees but at times the larger health care system. Topics are selected by scholars in response to the needs they observe in their clinics. Recent GSP projects include efforts to increase screenings for depression and anxiety, improve adherence to VHA dementia policy, increase access to virtual care, and increase referrals to programs such as whole health or cognitive behavioral therapy for insomnia, a first-line treatment for insomnia in older adults.17 Another project targeted the improvement of referrals to the Compassionate Contact Corps in an effort to reduce social isolation and loneliness among older veterans.18 Evaluations demonstrate significant improvement in scholars’ confidence in related program development and management from precourse to 3 months postcourse.15
Webinars
The Addressing Geriatric Mental Health webinar series was created to introduce learners to topics that could not be covered in the introductory course. Topics were suggested by the expert reviewers of the curriculum or identified by the scholars themselves (eg, chronic pain, sexuality, or serious mental illness). A secondary function of the webinars was to reach a broader audience. Over time, scholars and webinar attendees requested opportunities to explore topics in greater depth (eg, PTSD later in life). These requests led the webinars to focus on annual themes.
The series is open to all disciplines of geriatric scholars, VHA staff, and non-VHA staff through the Veterans Affairs Talent Management System and the TRAIN Learning Network (train.org). Attendance for the 37 webinars was captured from logins to the virtual learning platform and may underestimate attendance if a group attended on a single screen. Average attendance increased from 157 attendees/webinar in 2015 to 418 attendees/webinar in 2023 (Figure 2). This may have been related to the increase in virtual learning during the COVID-19 pandemic, but represents a 166% increase in audience from the inaugural year of the series.
Advanced Learning Opportunities
To invest in the ongoing growth and development of introductory course graduates, GSP-P developed and offered an advanced workshop in 2019. This multiday workshop focused on further enhancement of geropsychology competencies, with an emphasis on treating older veterans with mental and physical comorbidities. Didactics and experiential learning exercises led by SMEs covered topics such as adjusting to chronic illness, capacity assessment, PTSD, insomnia and sleep changes, chronic pain, and psychological interventions in palliative care and hospice settings. Evaluation findings demonstrated significant improvements from precourse to 6 months postcourse in confidence and knowledge as
To facilitate ongoing and individually tailored learning following the advanced workshop, scholars also developed and executed independent learning plans (ILPs) during a 6-month window with consultation from an experienced geropsychologist. Fifteen of 19 scholars (78.9%) completed ILPs with an average of 3 learning goals listed. After completing the ILPs, scholars endorsed their clinical and/or personal usefulness, citing increased confidence, enhanced skills for use with patients with complex needs, personal fulfillment, and career advancement. Most scholars noted ILPs were feasible and learning resources were accessible. Overall, the evaluation found ILPs to be a valuable way to enhance psychologists’ learning and effectiveness in treating older veterans with complex health needs.20
Clinical Practica
All geriatric scholars who completed the program have additional opportunities for professional development through practicum experiences focused on specific clinical approaches to the care of older veterans, such as dementia care, pain management, geriatric assessment, and palliative care. These practica provide scholars with individualized learning experiences in an individualized or small group setting and may be conducted either in-person or virtually.
In response to an expressed need from those who completed the program, the GSP-P planning committee collaborated with an SME to develop a virtual practicum to assess patients’ decision making capacity. Evaluating capacities among older adults is a common request, yet clinicians report little to no formal training in how to conceptualize and approach these complex questions.21,22 Utilizing an evidence-informed and structured approach promotes the balancing of an older adult’s autonomy and professional ethics. Learning capacity evaluation skills could better position psychologists to not only navigate complex ethical, legal, and clinical situations, but also serve as expert consultants to interdisciplinary teams. This virtual practicum was initiated in 2022 and to date has included 10 scholars. The practicum includes multiple modalities of learning: (1) self-directed review of core concepts; (2) attendance at 4 capacity didactics focused on introduction to evaluating capacities, medical consent and decision making, financial decision making and management, and independent living; and (3) participation in 5 group consultations on capacity evaluations conducted at their home sites. During these group consultations, additional case examples were shared to reinforce capacity concepts.
Discussion
The objective of GSP-P is to enhance geropsychology practice competencies among VHA psychologists given the outsized representation of older adults within the VHA system and their complex care needs. The curricula have significantly evolved to accomplish this, expanding the reach and investing in the continuing growth and development of scholars.
There are several elements that set GSP apart from other geriatric and geropsychology continuing medical education programs. The first is that the training is veteran focused, allowing us to discuss the unique impact military service has on aging. Similarly, because all scholars work within the integrated health care system, we can introduce and review key resources and programs that benefit all veterans and their families/care partners across the system. Through the GSP, the VA invests in ongoing professional development. Scholars can participate in additional experiential practica, webinars, and advanced workshops tailored to their individual learning needs. Lastly, the GSP works to create a community among its scholars where they can not only continue to consult with presenters/instructors, but also one another. A planned future direction for the GSP-P is to incorporate quarterly office hours and discussions for alumni to develop an increased sense of community. This may strengthen commitment to the overall VA mission, leading to increased retainment of talent who now have the knowledge, skills, and confidence to care for aging veterans.
Limitations
GSP is limited by its available funding. Additionally, the number of participants who can enroll each year in GSP-P (not including webinars) is capped by policy. Another limitation is the number of QI coaches available to mentor scholars on their projects.
Conclusions
Outcomes of GSP-P have been extremely favorable. Following participation in the program, we have found a significant increase in confidence in geropsychology practice among clinicians, as well as enhanced knowledge and skills across competency domains.15,19 We have observed rising attendance in our annual webinar series and graduates of our introductory courses participate in subsequent trainings (eg, advanced workshop or virtual practicum). Several graduates of GSP-P have become board certified in geropsychology by the American Board of Geropsychology and many proceed to supervise geropsychology-focused clinical rotations for psychology practicum students, predoctoral interns, and postdoctoral fellows. This suggests that the reach of GSP-P programming may extend farther than reported in this article.
The needs of aging veterans have also changed based on cohort differences, as the population of World War II and Korean War era veterans has declined and the number of older Vietnam era veterans has grown. We expect different challenges with older Gulf War and post-9/11 era veterans. For instance, 17% of troops deployed to Iraq or Afghanistan following 9/11 experienced mild traumatic brain injury (TBI), and 59% of those experienced > 1 mild TBI.23 Research indicates that younger post-9/11 veterans have a 3-fold risk of developing early onset dementia after experiencing a TBI.24 Therefore, even though post-9/11 veterans are not older in terms of chronological age, some may experience symptoms and conditions more often occurring in older veterans. As a result, it would be beneficial for clinicians to learn about the presentation and treatment of geriatric conditions such as dementia.
Moving forward, the GSP-P should identify potential opportunities to collaborate with the non-VHA mental health community–which also faces a shortage of geriatric mental health clinicians–to extend educational opportunities to improve care for veterans in all settings (eg, cosponsor training opportunities open to both VHA and non-VHA clinicians).8,25 Many aging veterans may receive portions of their health care outside the VHA, particularly those who reside in rural areas. Additionally, as veterans age, so do their support systems (eg, family members, friends, spouses, caregivers, and even clinicians), most of whom will receive care outside of the VHA. Community education collaborations will not only improve the care of older veterans, but also the care of older adults in the general population.
Promising directions include the adoption of the GSP model in other health care settings. Recently, Indian Health Service has adapted the model, beginning with primary care clinicians and pharmacists and is beginning to expand to other disciplines. Additional investments in VHA workforce training include the availability of geropsychology internship and fellowship training opportunities through the Office of Academic Affiliations, which provide earlier opportunities to specialize in geropsychology. Continued investment in both prelicensure and postpsychology licensure training efforts are needed within the VHA to meet the geriatric mental health needs of veterans.
Acknowledgments
The authors wish to acknowledge Terri Huh, PhD, for her contributions to the development and initiation of the GSP-P. The authors also appreciate the collaboration and quality initiative training led by Carol Callaway-Lane, DNP, ACNP-BC, and her team.
1. Caplan Z, Rabe M; US Department of Commerce, US Census Bureau. The Older Population: 2020 (Census Brief No. C2020BR-07). May 2023. Accessed February 27, 2024. https://www2.census.gov/library/publications/decennial/2020/census-briefs/c2020br-07.pdf
2. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. VA benefits & health care utilization. Updated February 2023. Accessed February 27, 2024. https://www.va.gov/vetdata/docs/pocketcards/fy2023q2.PDF
3. O’Malley KA, Vinson L, Pless Kaiser A, Sager Z, Hinrichs K. Mental health and aging veterans: How the Veterans Health Administration meets the needs of aging veterans. Public Policy Aging Rep. 2020;30(1):19-23. doi:10.1093/ppar/prz027
4. Greenberg G, Hoff R. FY 2021 Older Adult (65+ on October 1st) Veteran Data Sheet: National, VISN, and Healthcare System Tables. West Haven, CT: U.S. Department of Veterans Affairs, Northeast Program Evaluation Center. 2022.
5. Yaffe K, Vittinghoff E, Lindquist K, et al. Posttraumatic stress disorder and risk of dementia among US veterans. Arch Gen Psychiatry. 2010;67(6):608-613. doi:10.1001/archgenpsychiatry.2010.61
6. Davison EH, Kaiser AP, Spiro A 3rd, Moye J, King LA, King DW. From late-onset stress symptomatology to later-adulthood trauma reengagement in aging combat veterans: Taking a broader view. Gerontologist. 2016;56(1):14-21. doi:10.1093/geront/gnv097
7. Kaiser AP, Boyle JT, Bamonti PM, O’Malley K, Moye J. Development, adaptation, and clinical implementation of the Later-Adulthood Trauma Reengagement (LATR) group intervention for older veterans. Psychol Serv. 2023;20(4):863-875. doi:10.1037/ser0000736
8. Moye J, Karel MJ, Stamm KE, et al. Workforce analysis of psychological practice with older adults: Growing crisis requires urgent action. Train Educ Prof Psychol. 2019;13(1):46-55. doi:10.1037/tep0000206
9. Stamm K, Lin L, Conroy J. Critical needs in geropsychology. Monitor on Psychology. 2021;52(4):21.
10. American Board of Geropsychology. Specialists. 2024. Accessed February 6, 2024. https://abgero.org/board-members/specialists/
11. Kramer BJ. The VA Geriatric Scholars Program. Fed Pract. 2015;32(5):46-48.
12. Kramer BJ, Creekmur B, Howe JL, et al. Veterans Affairs Geriatric Scholars Program: Enhancing existing primary care clinician skills in caring for older veterans. J Am Geriatr Soc. 2016;64(11):2343-2348. doi:10.1111/jgs.14382
13. Knight BG, Karel MJ, Hinrichsen GA, Qualls SH, Duffy M. Pikes Peak model for training in professional geropsychology. Am Psychol. 2009;64(3):205-14. doi:10.1037/a0015059
14. Huh JWT, Rodriguez R, Gould CE, R Brunskill S, Melendez L, Kramer BJ. Developing a program to increase geropsychology competencies of Veterans Health Administration (VHA) psychologists. Gerontol Geriatr Educ. 2020;41(4):463-479. doi:10.1080/02701960.2018.1491402
15. Huh JWT, Rodriguez RL, Gregg JJ, Scales AN, Kramer BJ, Gould CE. Improving geropsychology competencies of Veterans Affairs psychologists. J Am Geriatr Soc. 2021;69(3):798-805. doi:10.1111/jgs.17029
16. Karel MJ, Emery EE, Molinari V; CoPGTP Task Force on the Assessment of Geropsychology Competencies. Development of a tool to evaluate geropsychology knowledge and skill competencies. Int Psychogeriatr. 2010;22(6):886-896. doi:10.1017/S1041610209991736
17. Morgenthaler T, Kramer M, Alessi C, et al. Practice parameters for the psychological and behavioral treatment of insomnia: an update. An American Academy of Sleep Medicine report. Sleep. 2006;29(11):1415-1419.
18. Sullivan J, Gualtieri L, Campbell M, Davila H, Pendergast J, Taylor P. VA Compassionate Contact Corps: a phone-based intervention for veterans interested in speaking with peers. Innov Aging. 2021;5(Suppl 1):204. doi:10.1093/geroni/igab046.788
19. Gregg JJ, Rodriguez RL, Mehta PS, Kramer BJ, Gould CE. Enhancing specialty training in geropsychology competencies: an evaluation of a VA Geriatric Scholars Program advanced topics workshop. Gerontol Geriatr Educ. 2023;44(3):329-338. doi:10.1080/02701960.2022.2069764
20. Gould CE, Rodriguez RL, Gregg J, Mehta PS, Kramer J. Mentored independent learning plans among psychologists: a mixed methods investigation. J Amer Geriatr Soc. 2023;71(S1):S53.
21. Mullaly E, Kinsella G, Berberovic N, et al. Assessment of decision-making capacity: exploration of common practices among neuropsychologists. Aust Psychol. 2007;42:178-186. doi:10.1080/00050060601187142
22. Seyfried L, Ryan KA, Kim SYH. Assessment of decision-making capacity: Views and experiences of consultation psychiatrists. Psychosomatics. 2013;54(2):115-123. doi:10.1016/j.psym.2012.08.001
23. Wilk JE, Herrell RK, Wynn GH, Riviere LA, Hoge CW. Mild traumatic brain injury (concussion), posttraumatic stress disorder, and depression in U.S. soldiers involved in combat deployments: association with postdeployment symptoms. Psychosom Med. 2012;74(3):249-257. doi:10.1097/PSY.0b013e318244c604
24. Kennedy E, Panahi S, Stewart IJ, et al. Traumatic brain injury and early onset dementia in post 9-11 veterans. Brain Inj. 2022;36(5):620-627.doi:10.1080/02699052.2022.2033846
25. Merz CC, Koh D, Sakai EY, et al. The big shortage: Geropsychologists discuss facilitators and barriers to working in the field of aging. Transl Issues Psychol Sci. 2017;3(4):388-399. doi:10.1037/tps0000137
The Veterans Health Administration (VHA) is understaffed for clinical psychologists who have specialty training in geriatrics (ie, geropsychologists) to meet the needs of aging veterans. Though only 16.8% of US adults are aged ≥ 65 years,1 this age group comprises 45.9% of patients within the VHA.2 The needs of older adults are complex and warrant specialized services from mental health clinicians trained to understand lifespan developmental processes, biological changes associated with aging, and changes in psychosocial functioning.
Older veterans (aged ≥ 65 years) present with higher rates of combined medical and mental health diagnoses compared to both younger veterans and older adults who are not veterans.3 Nearly 1 of 5 (18.1%) older veterans who use VHA services have confirmed mental health diagnoses, and an additional 25.5% have documented mental health concerns without a formal diagnosis in their health record.4 The clinical presentations of older veterans frequently differ from younger adults and include greater complexity. For example, older veterans face an increased risk of cognitive impairment compared to the general population, due in part to higher prevalence of posttraumatic stress, which doubles their risk of developing dementia.5 Additional examples of multicomplexity among older veterans may include co-occurring medical and psychiatric diagnoses, the presence of delirium, social isolation/loneliness, and concerns related to polypharmacy. These complex presentations result in significant challenges for mental health clinicians in areas like assessment (eg, accuracy of case conceptualization), intervention (eg, selection and prioritization), and consultation (eg, coordination among multiple medical and mental health specialists).
Older veterans also present with substantial resilience. Research has found that aging veterans exposed to trauma during their military service often review their memories and past experiences, which is known as later-adulthood trauma reengagement.6 Through this normative life review process, veterans engage with memories and experiences from their past that they previously avoided, which could lead to posttraumatic growth for some. Unfortunately, others may experience an increase in psychological distress. Mental health clinicians with specialty expertise and training in aging and lifespan development can facilitate positive outcomes to reduce distress.7
The United States in general, and the VHA specifically, face a growing shortage of geriatric mental health clinicians.
The Geriatric Scholars Program (GSP) was developed in 2008 to address the training gap and provide education in geriatrics to VHA clinicians that treat older veterans, particularly in rural areas.11,12 The GSP initially focused on primary care physicians, nurse practitioners, physician assistants, and pharmacists. It was later expanded to include other disciplines (ie, social work, rehabilitation therapists, and psychiatrists). In 2013, the GSP – Psychology Track (GSP-P) was developed with funding from the VHA Offices of Rural Health and Geriatrics and Extended Care specifically for psychologists.
This article describes the multicomponent longitudinal GSP-P, which has evolved to meet the target audience’s ongoing needs for knowledge, skills, and opportunities to refine practice behaviors. GSP-P received the 2020 Award for Excellence in Geropsychology Training from the Council of Professional Geropsychology Training Programs. GSP-P has grown within the context of the larger GSP and aligns with the other existing elective learning opportunities (Figure 1).
Program description
Introductory Course
Psychologist subject matter experts (SMEs) developed an intensive course in geropsychology in the absence of a similar course in the geriatric medicine board review curriculum. SMEs reviewed the guidelines for practice by professional organizations like the Pikes Peak Geropsychology Competencies, which outline knowledge and skills in various domains.13 SMEs integrated this review with findings from a needs assessment for postlicensed VHA psychology staff in 4 health care systems, drafted a syllabus, and circulated it to geropsychology experts for feedback. The resulting multiday course covered general mental health as well as topics particularly salient for mental health clinicians treating older veterans including suicide prevention and posttraumatic stress disorder (PTSD).14 This Geropsychology Competencies Review Course was piloted in 1 region initially before being offered nationally in 2014.
Quality Improvement
Introductory course attendees also participate in an intensive day-long interactive workshop in quality improvement (QI). After completing these trainings, they apply what they have learned at their home facility by embarking on a QI project related to geriatrics. The QI projects reinforce learning and initiate practice changes not only for attendees but at times the larger health care system. Topics are selected by scholars in response to the needs they observe in their clinics. Recent GSP projects include efforts to increase screenings for depression and anxiety, improve adherence to VHA dementia policy, increase access to virtual care, and increase referrals to programs such as whole health or cognitive behavioral therapy for insomnia, a first-line treatment for insomnia in older adults.17 Another project targeted the improvement of referrals to the Compassionate Contact Corps in an effort to reduce social isolation and loneliness among older veterans.18 Evaluations demonstrate significant improvement in scholars’ confidence in related program development and management from precourse to 3 months postcourse.15
Webinars
The Addressing Geriatric Mental Health webinar series was created to introduce learners to topics that could not be covered in the introductory course. Topics were suggested by the expert reviewers of the curriculum or identified by the scholars themselves (eg, chronic pain, sexuality, or serious mental illness). A secondary function of the webinars was to reach a broader audience. Over time, scholars and webinar attendees requested opportunities to explore topics in greater depth (eg, PTSD later in life). These requests led the webinars to focus on annual themes.
The series is open to all disciplines of geriatric scholars, VHA staff, and non-VHA staff through the Veterans Affairs Talent Management System and the TRAIN Learning Network (train.org). Attendance for the 37 webinars was captured from logins to the virtual learning platform and may underestimate attendance if a group attended on a single screen. Average attendance increased from 157 attendees/webinar in 2015 to 418 attendees/webinar in 2023 (Figure 2). This may have been related to the increase in virtual learning during the COVID-19 pandemic, but represents a 166% increase in audience from the inaugural year of the series.
Advanced Learning Opportunities
To invest in the ongoing growth and development of introductory course graduates, GSP-P developed and offered an advanced workshop in 2019. This multiday workshop focused on further enhancement of geropsychology competencies, with an emphasis on treating older veterans with mental and physical comorbidities. Didactics and experiential learning exercises led by SMEs covered topics such as adjusting to chronic illness, capacity assessment, PTSD, insomnia and sleep changes, chronic pain, and psychological interventions in palliative care and hospice settings. Evaluation findings demonstrated significant improvements from precourse to 6 months postcourse in confidence and knowledge as
To facilitate ongoing and individually tailored learning following the advanced workshop, scholars also developed and executed independent learning plans (ILPs) during a 6-month window with consultation from an experienced geropsychologist. Fifteen of 19 scholars (78.9%) completed ILPs with an average of 3 learning goals listed. After completing the ILPs, scholars endorsed their clinical and/or personal usefulness, citing increased confidence, enhanced skills for use with patients with complex needs, personal fulfillment, and career advancement. Most scholars noted ILPs were feasible and learning resources were accessible. Overall, the evaluation found ILPs to be a valuable way to enhance psychologists’ learning and effectiveness in treating older veterans with complex health needs.20
Clinical Practica
All geriatric scholars who completed the program have additional opportunities for professional development through practicum experiences focused on specific clinical approaches to the care of older veterans, such as dementia care, pain management, geriatric assessment, and palliative care. These practica provide scholars with individualized learning experiences in an individualized or small group setting and may be conducted either in-person or virtually.
In response to an expressed need from those who completed the program, the GSP-P planning committee collaborated with an SME to develop a virtual practicum to assess patients’ decision making capacity. Evaluating capacities among older adults is a common request, yet clinicians report little to no formal training in how to conceptualize and approach these complex questions.21,22 Utilizing an evidence-informed and structured approach promotes the balancing of an older adult’s autonomy and professional ethics. Learning capacity evaluation skills could better position psychologists to not only navigate complex ethical, legal, and clinical situations, but also serve as expert consultants to interdisciplinary teams. This virtual practicum was initiated in 2022 and to date has included 10 scholars. The practicum includes multiple modalities of learning: (1) self-directed review of core concepts; (2) attendance at 4 capacity didactics focused on introduction to evaluating capacities, medical consent and decision making, financial decision making and management, and independent living; and (3) participation in 5 group consultations on capacity evaluations conducted at their home sites. During these group consultations, additional case examples were shared to reinforce capacity concepts.
Discussion
The objective of GSP-P is to enhance geropsychology practice competencies among VHA psychologists given the outsized representation of older adults within the VHA system and their complex care needs. The curricula have significantly evolved to accomplish this, expanding the reach and investing in the continuing growth and development of scholars.
There are several elements that set GSP apart from other geriatric and geropsychology continuing medical education programs. The first is that the training is veteran focused, allowing us to discuss the unique impact military service has on aging. Similarly, because all scholars work within the integrated health care system, we can introduce and review key resources and programs that benefit all veterans and their families/care partners across the system. Through the GSP, the VA invests in ongoing professional development. Scholars can participate in additional experiential practica, webinars, and advanced workshops tailored to their individual learning needs. Lastly, the GSP works to create a community among its scholars where they can not only continue to consult with presenters/instructors, but also one another. A planned future direction for the GSP-P is to incorporate quarterly office hours and discussions for alumni to develop an increased sense of community. This may strengthen commitment to the overall VA mission, leading to increased retainment of talent who now have the knowledge, skills, and confidence to care for aging veterans.
Limitations
GSP is limited by its available funding. Additionally, the number of participants who can enroll each year in GSP-P (not including webinars) is capped by policy. Another limitation is the number of QI coaches available to mentor scholars on their projects.
Conclusions
Outcomes of GSP-P have been extremely favorable. Following participation in the program, we have found a significant increase in confidence in geropsychology practice among clinicians, as well as enhanced knowledge and skills across competency domains.15,19 We have observed rising attendance in our annual webinar series and graduates of our introductory courses participate in subsequent trainings (eg, advanced workshop or virtual practicum). Several graduates of GSP-P have become board certified in geropsychology by the American Board of Geropsychology and many proceed to supervise geropsychology-focused clinical rotations for psychology practicum students, predoctoral interns, and postdoctoral fellows. This suggests that the reach of GSP-P programming may extend farther than reported in this article.
The needs of aging veterans have also changed based on cohort differences, as the population of World War II and Korean War era veterans has declined and the number of older Vietnam era veterans has grown. We expect different challenges with older Gulf War and post-9/11 era veterans. For instance, 17% of troops deployed to Iraq or Afghanistan following 9/11 experienced mild traumatic brain injury (TBI), and 59% of those experienced > 1 mild TBI.23 Research indicates that younger post-9/11 veterans have a 3-fold risk of developing early onset dementia after experiencing a TBI.24 Therefore, even though post-9/11 veterans are not older in terms of chronological age, some may experience symptoms and conditions more often occurring in older veterans. As a result, it would be beneficial for clinicians to learn about the presentation and treatment of geriatric conditions such as dementia.
Moving forward, the GSP-P should identify potential opportunities to collaborate with the non-VHA mental health community–which also faces a shortage of geriatric mental health clinicians–to extend educational opportunities to improve care for veterans in all settings (eg, cosponsor training opportunities open to both VHA and non-VHA clinicians).8,25 Many aging veterans may receive portions of their health care outside the VHA, particularly those who reside in rural areas. Additionally, as veterans age, so do their support systems (eg, family members, friends, spouses, caregivers, and even clinicians), most of whom will receive care outside of the VHA. Community education collaborations will not only improve the care of older veterans, but also the care of older adults in the general population.
Promising directions include the adoption of the GSP model in other health care settings. Recently, Indian Health Service has adapted the model, beginning with primary care clinicians and pharmacists and is beginning to expand to other disciplines. Additional investments in VHA workforce training include the availability of geropsychology internship and fellowship training opportunities through the Office of Academic Affiliations, which provide earlier opportunities to specialize in geropsychology. Continued investment in both prelicensure and postpsychology licensure training efforts are needed within the VHA to meet the geriatric mental health needs of veterans.
Acknowledgments
The authors wish to acknowledge Terri Huh, PhD, for her contributions to the development and initiation of the GSP-P. The authors also appreciate the collaboration and quality initiative training led by Carol Callaway-Lane, DNP, ACNP-BC, and her team.
The Veterans Health Administration (VHA) is understaffed for clinical psychologists who have specialty training in geriatrics (ie, geropsychologists) to meet the needs of aging veterans. Though only 16.8% of US adults are aged ≥ 65 years,1 this age group comprises 45.9% of patients within the VHA.2 The needs of older adults are complex and warrant specialized services from mental health clinicians trained to understand lifespan developmental processes, biological changes associated with aging, and changes in psychosocial functioning.
Older veterans (aged ≥ 65 years) present with higher rates of combined medical and mental health diagnoses compared to both younger veterans and older adults who are not veterans.3 Nearly 1 of 5 (18.1%) older veterans who use VHA services have confirmed mental health diagnoses, and an additional 25.5% have documented mental health concerns without a formal diagnosis in their health record.4 The clinical presentations of older veterans frequently differ from younger adults and include greater complexity. For example, older veterans face an increased risk of cognitive impairment compared to the general population, due in part to higher prevalence of posttraumatic stress, which doubles their risk of developing dementia.5 Additional examples of multicomplexity among older veterans may include co-occurring medical and psychiatric diagnoses, the presence of delirium, social isolation/loneliness, and concerns related to polypharmacy. These complex presentations result in significant challenges for mental health clinicians in areas like assessment (eg, accuracy of case conceptualization), intervention (eg, selection and prioritization), and consultation (eg, coordination among multiple medical and mental health specialists).
Older veterans also present with substantial resilience. Research has found that aging veterans exposed to trauma during their military service often review their memories and past experiences, which is known as later-adulthood trauma reengagement.6 Through this normative life review process, veterans engage with memories and experiences from their past that they previously avoided, which could lead to posttraumatic growth for some. Unfortunately, others may experience an increase in psychological distress. Mental health clinicians with specialty expertise and training in aging and lifespan development can facilitate positive outcomes to reduce distress.7
The United States in general, and the VHA specifically, face a growing shortage of geriatric mental health clinicians.
The Geriatric Scholars Program (GSP) was developed in 2008 to address the training gap and provide education in geriatrics to VHA clinicians that treat older veterans, particularly in rural areas.11,12 The GSP initially focused on primary care physicians, nurse practitioners, physician assistants, and pharmacists. It was later expanded to include other disciplines (ie, social work, rehabilitation therapists, and psychiatrists). In 2013, the GSP – Psychology Track (GSP-P) was developed with funding from the VHA Offices of Rural Health and Geriatrics and Extended Care specifically for psychologists.
This article describes the multicomponent longitudinal GSP-P, which has evolved to meet the target audience’s ongoing needs for knowledge, skills, and opportunities to refine practice behaviors. GSP-P received the 2020 Award for Excellence in Geropsychology Training from the Council of Professional Geropsychology Training Programs. GSP-P has grown within the context of the larger GSP and aligns with the other existing elective learning opportunities (Figure 1).
Program description
Introductory Course
Psychologist subject matter experts (SMEs) developed an intensive course in geropsychology in the absence of a similar course in the geriatric medicine board review curriculum. SMEs reviewed the guidelines for practice by professional organizations like the Pikes Peak Geropsychology Competencies, which outline knowledge and skills in various domains.13 SMEs integrated this review with findings from a needs assessment for postlicensed VHA psychology staff in 4 health care systems, drafted a syllabus, and circulated it to geropsychology experts for feedback. The resulting multiday course covered general mental health as well as topics particularly salient for mental health clinicians treating older veterans including suicide prevention and posttraumatic stress disorder (PTSD).14 This Geropsychology Competencies Review Course was piloted in 1 region initially before being offered nationally in 2014.
Quality Improvement
Introductory course attendees also participate in an intensive day-long interactive workshop in quality improvement (QI). After completing these trainings, they apply what they have learned at their home facility by embarking on a QI project related to geriatrics. The QI projects reinforce learning and initiate practice changes not only for attendees but at times the larger health care system. Topics are selected by scholars in response to the needs they observe in their clinics. Recent GSP projects include efforts to increase screenings for depression and anxiety, improve adherence to VHA dementia policy, increase access to virtual care, and increase referrals to programs such as whole health or cognitive behavioral therapy for insomnia, a first-line treatment for insomnia in older adults.17 Another project targeted the improvement of referrals to the Compassionate Contact Corps in an effort to reduce social isolation and loneliness among older veterans.18 Evaluations demonstrate significant improvement in scholars’ confidence in related program development and management from precourse to 3 months postcourse.15
Webinars
The Addressing Geriatric Mental Health webinar series was created to introduce learners to topics that could not be covered in the introductory course. Topics were suggested by the expert reviewers of the curriculum or identified by the scholars themselves (eg, chronic pain, sexuality, or serious mental illness). A secondary function of the webinars was to reach a broader audience. Over time, scholars and webinar attendees requested opportunities to explore topics in greater depth (eg, PTSD later in life). These requests led the webinars to focus on annual themes.
The series is open to all disciplines of geriatric scholars, VHA staff, and non-VHA staff through the Veterans Affairs Talent Management System and the TRAIN Learning Network (train.org). Attendance for the 37 webinars was captured from logins to the virtual learning platform and may underestimate attendance if a group attended on a single screen. Average attendance increased from 157 attendees/webinar in 2015 to 418 attendees/webinar in 2023 (Figure 2). This may have been related to the increase in virtual learning during the COVID-19 pandemic, but represents a 166% increase in audience from the inaugural year of the series.
Advanced Learning Opportunities
To invest in the ongoing growth and development of introductory course graduates, GSP-P developed and offered an advanced workshop in 2019. This multiday workshop focused on further enhancement of geropsychology competencies, with an emphasis on treating older veterans with mental and physical comorbidities. Didactics and experiential learning exercises led by SMEs covered topics such as adjusting to chronic illness, capacity assessment, PTSD, insomnia and sleep changes, chronic pain, and psychological interventions in palliative care and hospice settings. Evaluation findings demonstrated significant improvements from precourse to 6 months postcourse in confidence and knowledge as
To facilitate ongoing and individually tailored learning following the advanced workshop, scholars also developed and executed independent learning plans (ILPs) during a 6-month window with consultation from an experienced geropsychologist. Fifteen of 19 scholars (78.9%) completed ILPs with an average of 3 learning goals listed. After completing the ILPs, scholars endorsed their clinical and/or personal usefulness, citing increased confidence, enhanced skills for use with patients with complex needs, personal fulfillment, and career advancement. Most scholars noted ILPs were feasible and learning resources were accessible. Overall, the evaluation found ILPs to be a valuable way to enhance psychologists’ learning and effectiveness in treating older veterans with complex health needs.20
Clinical Practica
All geriatric scholars who completed the program have additional opportunities for professional development through practicum experiences focused on specific clinical approaches to the care of older veterans, such as dementia care, pain management, geriatric assessment, and palliative care. These practica provide scholars with individualized learning experiences in an individualized or small group setting and may be conducted either in-person or virtually.
In response to an expressed need from those who completed the program, the GSP-P planning committee collaborated with an SME to develop a virtual practicum to assess patients’ decision making capacity. Evaluating capacities among older adults is a common request, yet clinicians report little to no formal training in how to conceptualize and approach these complex questions.21,22 Utilizing an evidence-informed and structured approach promotes the balancing of an older adult’s autonomy and professional ethics. Learning capacity evaluation skills could better position psychologists to not only navigate complex ethical, legal, and clinical situations, but also serve as expert consultants to interdisciplinary teams. This virtual practicum was initiated in 2022 and to date has included 10 scholars. The practicum includes multiple modalities of learning: (1) self-directed review of core concepts; (2) attendance at 4 capacity didactics focused on introduction to evaluating capacities, medical consent and decision making, financial decision making and management, and independent living; and (3) participation in 5 group consultations on capacity evaluations conducted at their home sites. During these group consultations, additional case examples were shared to reinforce capacity concepts.
Discussion
The objective of GSP-P is to enhance geropsychology practice competencies among VHA psychologists given the outsized representation of older adults within the VHA system and their complex care needs. The curricula have significantly evolved to accomplish this, expanding the reach and investing in the continuing growth and development of scholars.
There are several elements that set GSP apart from other geriatric and geropsychology continuing medical education programs. The first is that the training is veteran focused, allowing us to discuss the unique impact military service has on aging. Similarly, because all scholars work within the integrated health care system, we can introduce and review key resources and programs that benefit all veterans and their families/care partners across the system. Through the GSP, the VA invests in ongoing professional development. Scholars can participate in additional experiential practica, webinars, and advanced workshops tailored to their individual learning needs. Lastly, the GSP works to create a community among its scholars where they can not only continue to consult with presenters/instructors, but also one another. A planned future direction for the GSP-P is to incorporate quarterly office hours and discussions for alumni to develop an increased sense of community. This may strengthen commitment to the overall VA mission, leading to increased retainment of talent who now have the knowledge, skills, and confidence to care for aging veterans.
Limitations
GSP is limited by its available funding. Additionally, the number of participants who can enroll each year in GSP-P (not including webinars) is capped by policy. Another limitation is the number of QI coaches available to mentor scholars on their projects.
Conclusions
Outcomes of GSP-P have been extremely favorable. Following participation in the program, we have found a significant increase in confidence in geropsychology practice among clinicians, as well as enhanced knowledge and skills across competency domains.15,19 We have observed rising attendance in our annual webinar series and graduates of our introductory courses participate in subsequent trainings (eg, advanced workshop or virtual practicum). Several graduates of GSP-P have become board certified in geropsychology by the American Board of Geropsychology and many proceed to supervise geropsychology-focused clinical rotations for psychology practicum students, predoctoral interns, and postdoctoral fellows. This suggests that the reach of GSP-P programming may extend farther than reported in this article.
The needs of aging veterans have also changed based on cohort differences, as the population of World War II and Korean War era veterans has declined and the number of older Vietnam era veterans has grown. We expect different challenges with older Gulf War and post-9/11 era veterans. For instance, 17% of troops deployed to Iraq or Afghanistan following 9/11 experienced mild traumatic brain injury (TBI), and 59% of those experienced > 1 mild TBI.23 Research indicates that younger post-9/11 veterans have a 3-fold risk of developing early onset dementia after experiencing a TBI.24 Therefore, even though post-9/11 veterans are not older in terms of chronological age, some may experience symptoms and conditions more often occurring in older veterans. As a result, it would be beneficial for clinicians to learn about the presentation and treatment of geriatric conditions such as dementia.
Moving forward, the GSP-P should identify potential opportunities to collaborate with the non-VHA mental health community–which also faces a shortage of geriatric mental health clinicians–to extend educational opportunities to improve care for veterans in all settings (eg, cosponsor training opportunities open to both VHA and non-VHA clinicians).8,25 Many aging veterans may receive portions of their health care outside the VHA, particularly those who reside in rural areas. Additionally, as veterans age, so do their support systems (eg, family members, friends, spouses, caregivers, and even clinicians), most of whom will receive care outside of the VHA. Community education collaborations will not only improve the care of older veterans, but also the care of older adults in the general population.
Promising directions include the adoption of the GSP model in other health care settings. Recently, Indian Health Service has adapted the model, beginning with primary care clinicians and pharmacists and is beginning to expand to other disciplines. Additional investments in VHA workforce training include the availability of geropsychology internship and fellowship training opportunities through the Office of Academic Affiliations, which provide earlier opportunities to specialize in geropsychology. Continued investment in both prelicensure and postpsychology licensure training efforts are needed within the VHA to meet the geriatric mental health needs of veterans.
Acknowledgments
The authors wish to acknowledge Terri Huh, PhD, for her contributions to the development and initiation of the GSP-P. The authors also appreciate the collaboration and quality initiative training led by Carol Callaway-Lane, DNP, ACNP-BC, and her team.
1. Caplan Z, Rabe M; US Department of Commerce, US Census Bureau. The Older Population: 2020 (Census Brief No. C2020BR-07). May 2023. Accessed February 27, 2024. https://www2.census.gov/library/publications/decennial/2020/census-briefs/c2020br-07.pdf
2. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. VA benefits & health care utilization. Updated February 2023. Accessed February 27, 2024. https://www.va.gov/vetdata/docs/pocketcards/fy2023q2.PDF
3. O’Malley KA, Vinson L, Pless Kaiser A, Sager Z, Hinrichs K. Mental health and aging veterans: How the Veterans Health Administration meets the needs of aging veterans. Public Policy Aging Rep. 2020;30(1):19-23. doi:10.1093/ppar/prz027
4. Greenberg G, Hoff R. FY 2021 Older Adult (65+ on October 1st) Veteran Data Sheet: National, VISN, and Healthcare System Tables. West Haven, CT: U.S. Department of Veterans Affairs, Northeast Program Evaluation Center. 2022.
5. Yaffe K, Vittinghoff E, Lindquist K, et al. Posttraumatic stress disorder and risk of dementia among US veterans. Arch Gen Psychiatry. 2010;67(6):608-613. doi:10.1001/archgenpsychiatry.2010.61
6. Davison EH, Kaiser AP, Spiro A 3rd, Moye J, King LA, King DW. From late-onset stress symptomatology to later-adulthood trauma reengagement in aging combat veterans: Taking a broader view. Gerontologist. 2016;56(1):14-21. doi:10.1093/geront/gnv097
7. Kaiser AP, Boyle JT, Bamonti PM, O’Malley K, Moye J. Development, adaptation, and clinical implementation of the Later-Adulthood Trauma Reengagement (LATR) group intervention for older veterans. Psychol Serv. 2023;20(4):863-875. doi:10.1037/ser0000736
8. Moye J, Karel MJ, Stamm KE, et al. Workforce analysis of psychological practice with older adults: Growing crisis requires urgent action. Train Educ Prof Psychol. 2019;13(1):46-55. doi:10.1037/tep0000206
9. Stamm K, Lin L, Conroy J. Critical needs in geropsychology. Monitor on Psychology. 2021;52(4):21.
10. American Board of Geropsychology. Specialists. 2024. Accessed February 6, 2024. https://abgero.org/board-members/specialists/
11. Kramer BJ. The VA Geriatric Scholars Program. Fed Pract. 2015;32(5):46-48.
12. Kramer BJ, Creekmur B, Howe JL, et al. Veterans Affairs Geriatric Scholars Program: Enhancing existing primary care clinician skills in caring for older veterans. J Am Geriatr Soc. 2016;64(11):2343-2348. doi:10.1111/jgs.14382
13. Knight BG, Karel MJ, Hinrichsen GA, Qualls SH, Duffy M. Pikes Peak model for training in professional geropsychology. Am Psychol. 2009;64(3):205-14. doi:10.1037/a0015059
14. Huh JWT, Rodriguez R, Gould CE, R Brunskill S, Melendez L, Kramer BJ. Developing a program to increase geropsychology competencies of Veterans Health Administration (VHA) psychologists. Gerontol Geriatr Educ. 2020;41(4):463-479. doi:10.1080/02701960.2018.1491402
15. Huh JWT, Rodriguez RL, Gregg JJ, Scales AN, Kramer BJ, Gould CE. Improving geropsychology competencies of Veterans Affairs psychologists. J Am Geriatr Soc. 2021;69(3):798-805. doi:10.1111/jgs.17029
16. Karel MJ, Emery EE, Molinari V; CoPGTP Task Force on the Assessment of Geropsychology Competencies. Development of a tool to evaluate geropsychology knowledge and skill competencies. Int Psychogeriatr. 2010;22(6):886-896. doi:10.1017/S1041610209991736
17. Morgenthaler T, Kramer M, Alessi C, et al. Practice parameters for the psychological and behavioral treatment of insomnia: an update. An American Academy of Sleep Medicine report. Sleep. 2006;29(11):1415-1419.
18. Sullivan J, Gualtieri L, Campbell M, Davila H, Pendergast J, Taylor P. VA Compassionate Contact Corps: a phone-based intervention for veterans interested in speaking with peers. Innov Aging. 2021;5(Suppl 1):204. doi:10.1093/geroni/igab046.788
19. Gregg JJ, Rodriguez RL, Mehta PS, Kramer BJ, Gould CE. Enhancing specialty training in geropsychology competencies: an evaluation of a VA Geriatric Scholars Program advanced topics workshop. Gerontol Geriatr Educ. 2023;44(3):329-338. doi:10.1080/02701960.2022.2069764
20. Gould CE, Rodriguez RL, Gregg J, Mehta PS, Kramer J. Mentored independent learning plans among psychologists: a mixed methods investigation. J Amer Geriatr Soc. 2023;71(S1):S53.
21. Mullaly E, Kinsella G, Berberovic N, et al. Assessment of decision-making capacity: exploration of common practices among neuropsychologists. Aust Psychol. 2007;42:178-186. doi:10.1080/00050060601187142
22. Seyfried L, Ryan KA, Kim SYH. Assessment of decision-making capacity: Views and experiences of consultation psychiatrists. Psychosomatics. 2013;54(2):115-123. doi:10.1016/j.psym.2012.08.001
23. Wilk JE, Herrell RK, Wynn GH, Riviere LA, Hoge CW. Mild traumatic brain injury (concussion), posttraumatic stress disorder, and depression in U.S. soldiers involved in combat deployments: association with postdeployment symptoms. Psychosom Med. 2012;74(3):249-257. doi:10.1097/PSY.0b013e318244c604
24. Kennedy E, Panahi S, Stewart IJ, et al. Traumatic brain injury and early onset dementia in post 9-11 veterans. Brain Inj. 2022;36(5):620-627.doi:10.1080/02699052.2022.2033846
25. Merz CC, Koh D, Sakai EY, et al. The big shortage: Geropsychologists discuss facilitators and barriers to working in the field of aging. Transl Issues Psychol Sci. 2017;3(4):388-399. doi:10.1037/tps0000137
1. Caplan Z, Rabe M; US Department of Commerce, US Census Bureau. The Older Population: 2020 (Census Brief No. C2020BR-07). May 2023. Accessed February 27, 2024. https://www2.census.gov/library/publications/decennial/2020/census-briefs/c2020br-07.pdf
2. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. VA benefits & health care utilization. Updated February 2023. Accessed February 27, 2024. https://www.va.gov/vetdata/docs/pocketcards/fy2023q2.PDF
3. O’Malley KA, Vinson L, Pless Kaiser A, Sager Z, Hinrichs K. Mental health and aging veterans: How the Veterans Health Administration meets the needs of aging veterans. Public Policy Aging Rep. 2020;30(1):19-23. doi:10.1093/ppar/prz027
4. Greenberg G, Hoff R. FY 2021 Older Adult (65+ on October 1st) Veteran Data Sheet: National, VISN, and Healthcare System Tables. West Haven, CT: U.S. Department of Veterans Affairs, Northeast Program Evaluation Center. 2022.
5. Yaffe K, Vittinghoff E, Lindquist K, et al. Posttraumatic stress disorder and risk of dementia among US veterans. Arch Gen Psychiatry. 2010;67(6):608-613. doi:10.1001/archgenpsychiatry.2010.61
6. Davison EH, Kaiser AP, Spiro A 3rd, Moye J, King LA, King DW. From late-onset stress symptomatology to later-adulthood trauma reengagement in aging combat veterans: Taking a broader view. Gerontologist. 2016;56(1):14-21. doi:10.1093/geront/gnv097
7. Kaiser AP, Boyle JT, Bamonti PM, O’Malley K, Moye J. Development, adaptation, and clinical implementation of the Later-Adulthood Trauma Reengagement (LATR) group intervention for older veterans. Psychol Serv. 2023;20(4):863-875. doi:10.1037/ser0000736
8. Moye J, Karel MJ, Stamm KE, et al. Workforce analysis of psychological practice with older adults: Growing crisis requires urgent action. Train Educ Prof Psychol. 2019;13(1):46-55. doi:10.1037/tep0000206
9. Stamm K, Lin L, Conroy J. Critical needs in geropsychology. Monitor on Psychology. 2021;52(4):21.
10. American Board of Geropsychology. Specialists. 2024. Accessed February 6, 2024. https://abgero.org/board-members/specialists/
11. Kramer BJ. The VA Geriatric Scholars Program. Fed Pract. 2015;32(5):46-48.
12. Kramer BJ, Creekmur B, Howe JL, et al. Veterans Affairs Geriatric Scholars Program: Enhancing existing primary care clinician skills in caring for older veterans. J Am Geriatr Soc. 2016;64(11):2343-2348. doi:10.1111/jgs.14382
13. Knight BG, Karel MJ, Hinrichsen GA, Qualls SH, Duffy M. Pikes Peak model for training in professional geropsychology. Am Psychol. 2009;64(3):205-14. doi:10.1037/a0015059
14. Huh JWT, Rodriguez R, Gould CE, R Brunskill S, Melendez L, Kramer BJ. Developing a program to increase geropsychology competencies of Veterans Health Administration (VHA) psychologists. Gerontol Geriatr Educ. 2020;41(4):463-479. doi:10.1080/02701960.2018.1491402
15. Huh JWT, Rodriguez RL, Gregg JJ, Scales AN, Kramer BJ, Gould CE. Improving geropsychology competencies of Veterans Affairs psychologists. J Am Geriatr Soc. 2021;69(3):798-805. doi:10.1111/jgs.17029
16. Karel MJ, Emery EE, Molinari V; CoPGTP Task Force on the Assessment of Geropsychology Competencies. Development of a tool to evaluate geropsychology knowledge and skill competencies. Int Psychogeriatr. 2010;22(6):886-896. doi:10.1017/S1041610209991736
17. Morgenthaler T, Kramer M, Alessi C, et al. Practice parameters for the psychological and behavioral treatment of insomnia: an update. An American Academy of Sleep Medicine report. Sleep. 2006;29(11):1415-1419.
18. Sullivan J, Gualtieri L, Campbell M, Davila H, Pendergast J, Taylor P. VA Compassionate Contact Corps: a phone-based intervention for veterans interested in speaking with peers. Innov Aging. 2021;5(Suppl 1):204. doi:10.1093/geroni/igab046.788
19. Gregg JJ, Rodriguez RL, Mehta PS, Kramer BJ, Gould CE. Enhancing specialty training in geropsychology competencies: an evaluation of a VA Geriatric Scholars Program advanced topics workshop. Gerontol Geriatr Educ. 2023;44(3):329-338. doi:10.1080/02701960.2022.2069764
20. Gould CE, Rodriguez RL, Gregg J, Mehta PS, Kramer J. Mentored independent learning plans among psychologists: a mixed methods investigation. J Amer Geriatr Soc. 2023;71(S1):S53.
21. Mullaly E, Kinsella G, Berberovic N, et al. Assessment of decision-making capacity: exploration of common practices among neuropsychologists. Aust Psychol. 2007;42:178-186. doi:10.1080/00050060601187142
22. Seyfried L, Ryan KA, Kim SYH. Assessment of decision-making capacity: Views and experiences of consultation psychiatrists. Psychosomatics. 2013;54(2):115-123. doi:10.1016/j.psym.2012.08.001
23. Wilk JE, Herrell RK, Wynn GH, Riviere LA, Hoge CW. Mild traumatic brain injury (concussion), posttraumatic stress disorder, and depression in U.S. soldiers involved in combat deployments: association with postdeployment symptoms. Psychosom Med. 2012;74(3):249-257. doi:10.1097/PSY.0b013e318244c604
24. Kennedy E, Panahi S, Stewart IJ, et al. Traumatic brain injury and early onset dementia in post 9-11 veterans. Brain Inj. 2022;36(5):620-627.doi:10.1080/02699052.2022.2033846
25. Merz CC, Koh D, Sakai EY, et al. The big shortage: Geropsychologists discuss facilitators and barriers to working in the field of aging. Transl Issues Psychol Sci. 2017;3(4):388-399. doi:10.1037/tps0000137
Underlying Mental Illness and Risk of Severe Outcomes Associated With COVID-19
The Centers for Disease Control and Prevention (CDC) has identified factors that put patients at a higher risk of severe COVID-19 infection, which include advanced age, obesity, cardiovascular disease, diabetes, chronic kidney disease, lung disease, and immunocompromising conditions. The CDC also acknowledges that mood disorders, including depression and schizophrenia, contribute to the progression to severe COVID-19.1 Antiviral therapies, such as nirmatrelvir and ritonavir combination, remdesivir, and molnupiravir, and monoclonal antibody (mAb) therapies, have been used to prevent hospitalization and mortality from COVID-19 infection for individuals with mild-to-moderate COVID-19 who are at high risk of progressing to severe infection.2 Although antiviral and mAb therapies likely have mitigated many infections, poor prognoses are prevalent. It is important to identify all patients at risk of progressing to severe COVID-19 infection.
Although the CDC considers depression and schizophrenia to be risk factors for severe COVID-19 infection, the Captain James A. Lovell Federal Health Care Center (FHCC) in North Chicago, Illinois, does not, making these patients ineligible for antiviral or mAb therapies unless they have another risk factor. As a result, these patients could be at risk of severe COVID-19 infection, but might not be treated appropriately. Psychiatric diagnoses are common among veterans, with 19.7% experiencing a mental illness in 2020.3 It is imperative to determine whether depression or schizophrenia play a role in the progression of COVID-19 to expand access to individuals who are eligible for antiviral or mAb therapies.
Because COVID-19 is a novel virus, there are few studies of psychiatric disorders and COVID-19 prognosis. A 2020 case control study determined that those with a recent mental illness diagnosis were at higher risk of COVID-19 infection with worse outcomes compared with those without psychiatric diagnoses. This effect was most prevalent among individuals with depression and schizophrenia.4 However, these individuals also were found to have additional comorbidities that could have contributed to poorer outcomes. A meta-analysis determined that psychiatric disorders were associated with increased COVID-19-related mortality.5 A 2022 cohort study that included vaccinated US Department of Veterans Affairs (VA) patients determined that having a psychiatric diagnosis was associated with increased incidence of breakthrough infections.6 Individuals with psychiatric conditions are thought to be at higher risk of severe COVID-19 outcomes because of poor access to care and higher incidence of untreated underlying health conditions.7 Lifestyle factors also could play a role. Because there is minimal data on COVID-19 prognosis and mental illness, further research is warranted to determine whether psychiatric diagnoses could contribute to more severe COVID-19 infections.
Methods
This was a retrospective cohort chart review study at FHCC that compared COVID-19 outcomes in individuals with depression or schizophrenia with those without these diagnoses. FHCC patients with the International Classification of Diseases code for COVID-19 (U07.1) from fiscal years 2020 to 2022 were included. We then selected patients with a depression or schizophrenia diagnosis noted in the electronic health record (EHR). These 2 patient lists were consolidated to identify every individual with a COVID-19 diagnosis and a diagnosis of depression or schizophrenia.
Patients were included if they were aged ≥ 18 years with a positive COVID-19 infection confirmed via polymerase chain reaction or blood test. Patients also had to have mild-to-moderate COVID-19 with ≥ 1 symptom such as fever, cough, sore throat, malaise, headache, muscle pain, loss of taste and smell, or shortness of breath. Patients were excluded if they had an asymptomatic infection, presented with severe COVID-19 infection, or were an FHCC employee. Severe COVID-19 was defined as having oxygen saturation < 94%, a respiratory rate > 30 breaths per minute, or supplemental oxygen requirement.
Patient EHRs were reviewed and analyzed using the VA Computerized Patient Record System and Joint Legacy Viewer. Collected data included age, medical history, use of antiviral or mAb therapy, and admission or death within 30 days of a positive COVID-19 test. The primary outcome of this study was severe COVID-19 outcomes defined as hospitalization, admission to the intensive care unit, intubation or mechanical ventilation, or death within 30 days of infection. The primary outcome was analyzed with a student t test; P < .05 was considered statistically significant.
Results
More than 5000 individuals had a COVID-19 diagnosis during the study period. Among these patients, 4530 had no depression or schizophrenia diagnosis; 1021 individuals had COVID-19 and a preexisting diagnosis of depression or schizophrenia. Among these 1021 patients, 279 charts were reviewed due to time constraints; 128 patients met exclusion criteria and 151 patients were included in the study. Of the 151 patients with COVID-19, 78 had no depression or schizophrenia and 73 patients with COVID-19 had a preexisting depression or schizophrenia diagnosis (Figure).
The 2 groups were similar at baseline. The most common risk factors for severe COVID-19 included age > 60 years, obesity, and cardiovascular disease. However, more than half of the individuals analyzed had no risk factors (Table 1). Some patients with risk factors received antiviral or mAb therapy to prevent severe COVID-19 infection; combination nirmatrelvir and ritonavir was the most common agent (Table 2). Of the 73 individuals with a psychiatric diagnosis, 67 had depression (91.8%), and 6 had schizophrenia (8.2%).
Hospitalization or death within 30 days of COVID-19 infection between patients with depression or schizophrenia and patients without these psychiatric diagnoses was not statistically significant (P = .36). Sixteen individuals were hospitalized, 8 in each group. Three individuals died within 30 days; death only occurred in patients who had depression or schizophrenia (Table 3).
Discussion
This study found that hospitalization or death within 30 days of COVID-19 infection occurred more frequently among individuals with depression or schizophrenia compared with those without these psychiatric comorbidities. However, this difference was not statistically significant.
This study had several limitations. It was a retrospective, chart review study, which relied on accurate documentation. In addition, we reviewed COVID-19 cases from fiscal years 2020 to 2022 and as a result, several viral variants were analyzed. This made it difficult to draw conclusions, especially because the omicron variant is thought to be less deadly, which may have skewed the data. Vaccinations and COVID-19 treatments became available in late 2020, which likely affected the progression to severe disease. Our study did not assess vaccination status, therefore it is unclear whether COVID-19 vaccination played a role in mitigating infection. When the pandemic began, many individuals were afraid to come to the hospital and did not receive care until they progressed to severe COVID-19, which would have excluded them from the study. Many individuals had additional comorbidities that likely impacted their COVID-19 outcomes. It is not possible to conclude if the depression or schizophrenia diagnoses were responsible for hospitalization or death within 30 days of infection or if it was because of other known risk factors. Future research is needed to address these limitations.
Conclusions
More COVID-19 hospitalizations and deaths occurred within 30 days of infection among those with depression and schizophrenia compared with individuals without these comorbidities. However, this effect was not statistically significant. Many limitations could have contributed to this finding, which should be addressed in future studies. Because the sample size was small, further research with a larger patient population is warranted to explore the association between psychiatric comorbidities such as depression and schizophrenia and COVID-19 disease progression. Future studies also could include assessment of vaccination status and exclude individuals with other high-risk comorbidities for severe COVID-19 outcomes. These studies could determine if depression and schizophrenia are correlated with worse COVID-19 outcomes and ensure that all high-risk patients are identified and treated appropriately to prevent morbidity and mortality.
Acknowledgements
Thank you to the research committee at the Captain James A. Lovell Federal Health Care Center who assisted in the completion of this project, including Shaiza Khan, PharmD, BCPS; Yinka Alaka, PharmD; and Hong-Yen Vi, PharmD, BCPS, BCCCP.
1. Centers for Disease Control and Prevention. Underlying medical conditions associated with higher risk for severe COVID-19: information for healthcare professionals. Updated February 9, 2023. Accessed February 27, 2024. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html
2. National Institutes of Health. Therapeutic management of nonhospitalized adults with COVID-19. Updated November 2, 2023. Accessed February 27, 2024. https://www.covid19treatmentguidelines.nih.gov/management/clinical-management-of-adults/nonhospitalized-adults-therapeutic-management
3. National Alliance on Mental Illness. Mental health by the numbers. Updated April 2023. Accessed February 27, 2024. https://www.nami.org/mhstats
4. Wang Q, Xu R, Volkow ND. Increased risk of COVID-19 infection and mortality in people with mental disorders: analysis from electronic health records in the United States. World Psychiatry . 2021;20(1):124-130. doi:10.1002/wps.20806
5. Fond G, Nemani K, Etchecopar-Etchart D, et al. Association Between Mental Health Disorders and Mortality Among Patients With COVID-19 in 7 Countries: A Systematic Review and Meta-analysis. JAMA Psychiatry . 2021;78(11):1208-1217. doi:10.1001/jamapsychiatry.2021.2274
6. Nishimi K, Neylan TC, Bertenthal D, Seal KH, O’Donovan A. Association of Psychiatric Disorders With Incidence of SARS-CoV-2 Breakthrough Infection Among Vaccinated Adults. JAMA Netw Open . 2022;5(4):e227287. Published 2022 Apr 1. doi:10.1001/jamanetworkopen.2022.7287
7. Koyama AK, Koumans EH, Sircar K, et al. Mental Health Conditions and Severe COVID-19 Outcomes after Hospitalization, United States. Emerg Infect Dis . 2022;28(7):1533-1536. doi:10.3201/eid2807.212208
The Centers for Disease Control and Prevention (CDC) has identified factors that put patients at a higher risk of severe COVID-19 infection, which include advanced age, obesity, cardiovascular disease, diabetes, chronic kidney disease, lung disease, and immunocompromising conditions. The CDC also acknowledges that mood disorders, including depression and schizophrenia, contribute to the progression to severe COVID-19.1 Antiviral therapies, such as nirmatrelvir and ritonavir combination, remdesivir, and molnupiravir, and monoclonal antibody (mAb) therapies, have been used to prevent hospitalization and mortality from COVID-19 infection for individuals with mild-to-moderate COVID-19 who are at high risk of progressing to severe infection.2 Although antiviral and mAb therapies likely have mitigated many infections, poor prognoses are prevalent. It is important to identify all patients at risk of progressing to severe COVID-19 infection.
Although the CDC considers depression and schizophrenia to be risk factors for severe COVID-19 infection, the Captain James A. Lovell Federal Health Care Center (FHCC) in North Chicago, Illinois, does not, making these patients ineligible for antiviral or mAb therapies unless they have another risk factor. As a result, these patients could be at risk of severe COVID-19 infection, but might not be treated appropriately. Psychiatric diagnoses are common among veterans, with 19.7% experiencing a mental illness in 2020.3 It is imperative to determine whether depression or schizophrenia play a role in the progression of COVID-19 to expand access to individuals who are eligible for antiviral or mAb therapies.
Because COVID-19 is a novel virus, there are few studies of psychiatric disorders and COVID-19 prognosis. A 2020 case control study determined that those with a recent mental illness diagnosis were at higher risk of COVID-19 infection with worse outcomes compared with those without psychiatric diagnoses. This effect was most prevalent among individuals with depression and schizophrenia.4 However, these individuals also were found to have additional comorbidities that could have contributed to poorer outcomes. A meta-analysis determined that psychiatric disorders were associated with increased COVID-19-related mortality.5 A 2022 cohort study that included vaccinated US Department of Veterans Affairs (VA) patients determined that having a psychiatric diagnosis was associated with increased incidence of breakthrough infections.6 Individuals with psychiatric conditions are thought to be at higher risk of severe COVID-19 outcomes because of poor access to care and higher incidence of untreated underlying health conditions.7 Lifestyle factors also could play a role. Because there is minimal data on COVID-19 prognosis and mental illness, further research is warranted to determine whether psychiatric diagnoses could contribute to more severe COVID-19 infections.
Methods
This was a retrospective cohort chart review study at FHCC that compared COVID-19 outcomes in individuals with depression or schizophrenia with those without these diagnoses. FHCC patients with the International Classification of Diseases code for COVID-19 (U07.1) from fiscal years 2020 to 2022 were included. We then selected patients with a depression or schizophrenia diagnosis noted in the electronic health record (EHR). These 2 patient lists were consolidated to identify every individual with a COVID-19 diagnosis and a diagnosis of depression or schizophrenia.
Patients were included if they were aged ≥ 18 years with a positive COVID-19 infection confirmed via polymerase chain reaction or blood test. Patients also had to have mild-to-moderate COVID-19 with ≥ 1 symptom such as fever, cough, sore throat, malaise, headache, muscle pain, loss of taste and smell, or shortness of breath. Patients were excluded if they had an asymptomatic infection, presented with severe COVID-19 infection, or were an FHCC employee. Severe COVID-19 was defined as having oxygen saturation < 94%, a respiratory rate > 30 breaths per minute, or supplemental oxygen requirement.
Patient EHRs were reviewed and analyzed using the VA Computerized Patient Record System and Joint Legacy Viewer. Collected data included age, medical history, use of antiviral or mAb therapy, and admission or death within 30 days of a positive COVID-19 test. The primary outcome of this study was severe COVID-19 outcomes defined as hospitalization, admission to the intensive care unit, intubation or mechanical ventilation, or death within 30 days of infection. The primary outcome was analyzed with a student t test; P < .05 was considered statistically significant.
Results
More than 5000 individuals had a COVID-19 diagnosis during the study period. Among these patients, 4530 had no depression or schizophrenia diagnosis; 1021 individuals had COVID-19 and a preexisting diagnosis of depression or schizophrenia. Among these 1021 patients, 279 charts were reviewed due to time constraints; 128 patients met exclusion criteria and 151 patients were included in the study. Of the 151 patients with COVID-19, 78 had no depression or schizophrenia and 73 patients with COVID-19 had a preexisting depression or schizophrenia diagnosis (Figure).
The 2 groups were similar at baseline. The most common risk factors for severe COVID-19 included age > 60 years, obesity, and cardiovascular disease. However, more than half of the individuals analyzed had no risk factors (Table 1). Some patients with risk factors received antiviral or mAb therapy to prevent severe COVID-19 infection; combination nirmatrelvir and ritonavir was the most common agent (Table 2). Of the 73 individuals with a psychiatric diagnosis, 67 had depression (91.8%), and 6 had schizophrenia (8.2%).
Hospitalization or death within 30 days of COVID-19 infection between patients with depression or schizophrenia and patients without these psychiatric diagnoses was not statistically significant (P = .36). Sixteen individuals were hospitalized, 8 in each group. Three individuals died within 30 days; death only occurred in patients who had depression or schizophrenia (Table 3).
Discussion
This study found that hospitalization or death within 30 days of COVID-19 infection occurred more frequently among individuals with depression or schizophrenia compared with those without these psychiatric comorbidities. However, this difference was not statistically significant.
This study had several limitations. It was a retrospective, chart review study, which relied on accurate documentation. In addition, we reviewed COVID-19 cases from fiscal years 2020 to 2022 and as a result, several viral variants were analyzed. This made it difficult to draw conclusions, especially because the omicron variant is thought to be less deadly, which may have skewed the data. Vaccinations and COVID-19 treatments became available in late 2020, which likely affected the progression to severe disease. Our study did not assess vaccination status, therefore it is unclear whether COVID-19 vaccination played a role in mitigating infection. When the pandemic began, many individuals were afraid to come to the hospital and did not receive care until they progressed to severe COVID-19, which would have excluded them from the study. Many individuals had additional comorbidities that likely impacted their COVID-19 outcomes. It is not possible to conclude if the depression or schizophrenia diagnoses were responsible for hospitalization or death within 30 days of infection or if it was because of other known risk factors. Future research is needed to address these limitations.
Conclusions
More COVID-19 hospitalizations and deaths occurred within 30 days of infection among those with depression and schizophrenia compared with individuals without these comorbidities. However, this effect was not statistically significant. Many limitations could have contributed to this finding, which should be addressed in future studies. Because the sample size was small, further research with a larger patient population is warranted to explore the association between psychiatric comorbidities such as depression and schizophrenia and COVID-19 disease progression. Future studies also could include assessment of vaccination status and exclude individuals with other high-risk comorbidities for severe COVID-19 outcomes. These studies could determine if depression and schizophrenia are correlated with worse COVID-19 outcomes and ensure that all high-risk patients are identified and treated appropriately to prevent morbidity and mortality.
Acknowledgements
Thank you to the research committee at the Captain James A. Lovell Federal Health Care Center who assisted in the completion of this project, including Shaiza Khan, PharmD, BCPS; Yinka Alaka, PharmD; and Hong-Yen Vi, PharmD, BCPS, BCCCP.
The Centers for Disease Control and Prevention (CDC) has identified factors that put patients at a higher risk of severe COVID-19 infection, which include advanced age, obesity, cardiovascular disease, diabetes, chronic kidney disease, lung disease, and immunocompromising conditions. The CDC also acknowledges that mood disorders, including depression and schizophrenia, contribute to the progression to severe COVID-19.1 Antiviral therapies, such as nirmatrelvir and ritonavir combination, remdesivir, and molnupiravir, and monoclonal antibody (mAb) therapies, have been used to prevent hospitalization and mortality from COVID-19 infection for individuals with mild-to-moderate COVID-19 who are at high risk of progressing to severe infection.2 Although antiviral and mAb therapies likely have mitigated many infections, poor prognoses are prevalent. It is important to identify all patients at risk of progressing to severe COVID-19 infection.
Although the CDC considers depression and schizophrenia to be risk factors for severe COVID-19 infection, the Captain James A. Lovell Federal Health Care Center (FHCC) in North Chicago, Illinois, does not, making these patients ineligible for antiviral or mAb therapies unless they have another risk factor. As a result, these patients could be at risk of severe COVID-19 infection, but might not be treated appropriately. Psychiatric diagnoses are common among veterans, with 19.7% experiencing a mental illness in 2020.3 It is imperative to determine whether depression or schizophrenia play a role in the progression of COVID-19 to expand access to individuals who are eligible for antiviral or mAb therapies.
Because COVID-19 is a novel virus, there are few studies of psychiatric disorders and COVID-19 prognosis. A 2020 case control study determined that those with a recent mental illness diagnosis were at higher risk of COVID-19 infection with worse outcomes compared with those without psychiatric diagnoses. This effect was most prevalent among individuals with depression and schizophrenia.4 However, these individuals also were found to have additional comorbidities that could have contributed to poorer outcomes. A meta-analysis determined that psychiatric disorders were associated with increased COVID-19-related mortality.5 A 2022 cohort study that included vaccinated US Department of Veterans Affairs (VA) patients determined that having a psychiatric diagnosis was associated with increased incidence of breakthrough infections.6 Individuals with psychiatric conditions are thought to be at higher risk of severe COVID-19 outcomes because of poor access to care and higher incidence of untreated underlying health conditions.7 Lifestyle factors also could play a role. Because there is minimal data on COVID-19 prognosis and mental illness, further research is warranted to determine whether psychiatric diagnoses could contribute to more severe COVID-19 infections.
Methods
This was a retrospective cohort chart review study at FHCC that compared COVID-19 outcomes in individuals with depression or schizophrenia with those without these diagnoses. FHCC patients with the International Classification of Diseases code for COVID-19 (U07.1) from fiscal years 2020 to 2022 were included. We then selected patients with a depression or schizophrenia diagnosis noted in the electronic health record (EHR). These 2 patient lists were consolidated to identify every individual with a COVID-19 diagnosis and a diagnosis of depression or schizophrenia.
Patients were included if they were aged ≥ 18 years with a positive COVID-19 infection confirmed via polymerase chain reaction or blood test. Patients also had to have mild-to-moderate COVID-19 with ≥ 1 symptom such as fever, cough, sore throat, malaise, headache, muscle pain, loss of taste and smell, or shortness of breath. Patients were excluded if they had an asymptomatic infection, presented with severe COVID-19 infection, or were an FHCC employee. Severe COVID-19 was defined as having oxygen saturation < 94%, a respiratory rate > 30 breaths per minute, or supplemental oxygen requirement.
Patient EHRs were reviewed and analyzed using the VA Computerized Patient Record System and Joint Legacy Viewer. Collected data included age, medical history, use of antiviral or mAb therapy, and admission or death within 30 days of a positive COVID-19 test. The primary outcome of this study was severe COVID-19 outcomes defined as hospitalization, admission to the intensive care unit, intubation or mechanical ventilation, or death within 30 days of infection. The primary outcome was analyzed with a student t test; P < .05 was considered statistically significant.
Results
More than 5000 individuals had a COVID-19 diagnosis during the study period. Among these patients, 4530 had no depression or schizophrenia diagnosis; 1021 individuals had COVID-19 and a preexisting diagnosis of depression or schizophrenia. Among these 1021 patients, 279 charts were reviewed due to time constraints; 128 patients met exclusion criteria and 151 patients were included in the study. Of the 151 patients with COVID-19, 78 had no depression or schizophrenia and 73 patients with COVID-19 had a preexisting depression or schizophrenia diagnosis (Figure).
The 2 groups were similar at baseline. The most common risk factors for severe COVID-19 included age > 60 years, obesity, and cardiovascular disease. However, more than half of the individuals analyzed had no risk factors (Table 1). Some patients with risk factors received antiviral or mAb therapy to prevent severe COVID-19 infection; combination nirmatrelvir and ritonavir was the most common agent (Table 2). Of the 73 individuals with a psychiatric diagnosis, 67 had depression (91.8%), and 6 had schizophrenia (8.2%).
Hospitalization or death within 30 days of COVID-19 infection between patients with depression or schizophrenia and patients without these psychiatric diagnoses was not statistically significant (P = .36). Sixteen individuals were hospitalized, 8 in each group. Three individuals died within 30 days; death only occurred in patients who had depression or schizophrenia (Table 3).
Discussion
This study found that hospitalization or death within 30 days of COVID-19 infection occurred more frequently among individuals with depression or schizophrenia compared with those without these psychiatric comorbidities. However, this difference was not statistically significant.
This study had several limitations. It was a retrospective, chart review study, which relied on accurate documentation. In addition, we reviewed COVID-19 cases from fiscal years 2020 to 2022 and as a result, several viral variants were analyzed. This made it difficult to draw conclusions, especially because the omicron variant is thought to be less deadly, which may have skewed the data. Vaccinations and COVID-19 treatments became available in late 2020, which likely affected the progression to severe disease. Our study did not assess vaccination status, therefore it is unclear whether COVID-19 vaccination played a role in mitigating infection. When the pandemic began, many individuals were afraid to come to the hospital and did not receive care until they progressed to severe COVID-19, which would have excluded them from the study. Many individuals had additional comorbidities that likely impacted their COVID-19 outcomes. It is not possible to conclude if the depression or schizophrenia diagnoses were responsible for hospitalization or death within 30 days of infection or if it was because of other known risk factors. Future research is needed to address these limitations.
Conclusions
More COVID-19 hospitalizations and deaths occurred within 30 days of infection among those with depression and schizophrenia compared with individuals without these comorbidities. However, this effect was not statistically significant. Many limitations could have contributed to this finding, which should be addressed in future studies. Because the sample size was small, further research with a larger patient population is warranted to explore the association between psychiatric comorbidities such as depression and schizophrenia and COVID-19 disease progression. Future studies also could include assessment of vaccination status and exclude individuals with other high-risk comorbidities for severe COVID-19 outcomes. These studies could determine if depression and schizophrenia are correlated with worse COVID-19 outcomes and ensure that all high-risk patients are identified and treated appropriately to prevent morbidity and mortality.
Acknowledgements
Thank you to the research committee at the Captain James A. Lovell Federal Health Care Center who assisted in the completion of this project, including Shaiza Khan, PharmD, BCPS; Yinka Alaka, PharmD; and Hong-Yen Vi, PharmD, BCPS, BCCCP.
1. Centers for Disease Control and Prevention. Underlying medical conditions associated with higher risk for severe COVID-19: information for healthcare professionals. Updated February 9, 2023. Accessed February 27, 2024. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html
2. National Institutes of Health. Therapeutic management of nonhospitalized adults with COVID-19. Updated November 2, 2023. Accessed February 27, 2024. https://www.covid19treatmentguidelines.nih.gov/management/clinical-management-of-adults/nonhospitalized-adults-therapeutic-management
3. National Alliance on Mental Illness. Mental health by the numbers. Updated April 2023. Accessed February 27, 2024. https://www.nami.org/mhstats
4. Wang Q, Xu R, Volkow ND. Increased risk of COVID-19 infection and mortality in people with mental disorders: analysis from electronic health records in the United States. World Psychiatry . 2021;20(1):124-130. doi:10.1002/wps.20806
5. Fond G, Nemani K, Etchecopar-Etchart D, et al. Association Between Mental Health Disorders and Mortality Among Patients With COVID-19 in 7 Countries: A Systematic Review and Meta-analysis. JAMA Psychiatry . 2021;78(11):1208-1217. doi:10.1001/jamapsychiatry.2021.2274
6. Nishimi K, Neylan TC, Bertenthal D, Seal KH, O’Donovan A. Association of Psychiatric Disorders With Incidence of SARS-CoV-2 Breakthrough Infection Among Vaccinated Adults. JAMA Netw Open . 2022;5(4):e227287. Published 2022 Apr 1. doi:10.1001/jamanetworkopen.2022.7287
7. Koyama AK, Koumans EH, Sircar K, et al. Mental Health Conditions and Severe COVID-19 Outcomes after Hospitalization, United States. Emerg Infect Dis . 2022;28(7):1533-1536. doi:10.3201/eid2807.212208
1. Centers for Disease Control and Prevention. Underlying medical conditions associated with higher risk for severe COVID-19: information for healthcare professionals. Updated February 9, 2023. Accessed February 27, 2024. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html
2. National Institutes of Health. Therapeutic management of nonhospitalized adults with COVID-19. Updated November 2, 2023. Accessed February 27, 2024. https://www.covid19treatmentguidelines.nih.gov/management/clinical-management-of-adults/nonhospitalized-adults-therapeutic-management
3. National Alliance on Mental Illness. Mental health by the numbers. Updated April 2023. Accessed February 27, 2024. https://www.nami.org/mhstats
4. Wang Q, Xu R, Volkow ND. Increased risk of COVID-19 infection and mortality in people with mental disorders: analysis from electronic health records in the United States. World Psychiatry . 2021;20(1):124-130. doi:10.1002/wps.20806
5. Fond G, Nemani K, Etchecopar-Etchart D, et al. Association Between Mental Health Disorders and Mortality Among Patients With COVID-19 in 7 Countries: A Systematic Review and Meta-analysis. JAMA Psychiatry . 2021;78(11):1208-1217. doi:10.1001/jamapsychiatry.2021.2274
6. Nishimi K, Neylan TC, Bertenthal D, Seal KH, O’Donovan A. Association of Psychiatric Disorders With Incidence of SARS-CoV-2 Breakthrough Infection Among Vaccinated Adults. JAMA Netw Open . 2022;5(4):e227287. Published 2022 Apr 1. doi:10.1001/jamanetworkopen.2022.7287
7. Koyama AK, Koumans EH, Sircar K, et al. Mental Health Conditions and Severe COVID-19 Outcomes after Hospitalization, United States. Emerg Infect Dis . 2022;28(7):1533-1536. doi:10.3201/eid2807.212208
Commentary: Migraine and Cardiovascular Risk, April 2024
A recent study, published in the March 2024 issue of Sleep Medicine, identified shift work as one of the risk factors for headache and migraine. The researchers conducted a meta-analysis, including seven studies and involving 422,869 participants. The authors defined shift work as characterized by individuals or teams working consecutively to exceed the standard 8-hour day. They reported that the pooled analysis revealed a significant association between shift work and an increased risk for headache. Specifically, they determined that "individuals working night shifts had a 44% higher risk of developing headaches and a higher incidence of migraines." The authors stated that this association did not establish any causal relationship, and they suggested that future research should investigate the impact of genetics or health behaviors, which could be considered shared risk factors.
An article that had been published in 2019 in Headache included two case reports detailing the effects of shift work on patients with migraine. The authors of the case reports stated that "in the two cases presented, shift work appeared to be associated with chronification of migraine and higher headache-related disability, despite optimal headache management and good patient adherence."[1] They observed that "a switch to only day shifts promoted transition to an episodic, less disabling pattern of migraine."[1] These publications both support the idea that, while patients may have an underlying predisposition to migraine, certain lifestyle factors can play a role in exacerbating symptoms.
Erenumab, one of the relatively new therapies for migraine, was found to have a potential link to worsening hypertension. According to an article published in February in Headache: The Journal of Head and Face Pain, there has not been evidence of hypertension in preclinical models or clinical trials, yet postmarketing data suggest that erenumab may be associated with hypertension. The authors conducted an observational retrospective cohort study that included 335 patients who had been seen at a tertiary headache or neurology department. At baseline, 20.9% (70/335) of patients had a prior diagnosis of hypertension. The researchers observed that 23.3% (78/335) of the patients were found to have worsening hypertension, and 13 patients of the 225 who continued on erenumab experienced an improvement in their blood pressure. The authors noted that there was no association between worsening hypertension and preexisting hypertension, sex, body mass index, or age, but patients with atrial fibrillation were more likely to develop worsening hypertension (odds ratio 4.9; 95% CI 1.12-21.4; P = .035).
Consideration of a relationship between hypertension and anti-calcitonin gene–related peptide migraine (CGRP) therapies has been found in other studies as well. Results of a retrospective study conducted at the University Hospital of Modena, to explore the rate of hypertension among patients treated with anti-CGRP monoclonal antibodies, were published in April 2024 in Neurological Sciences (published online November 6, 2023). Those authors reported that no significant increase in blood pressure was detected overall, yet 5.7% of the patients developed a significant increase in their blood pressure.[2] Specifically, the researchers reported that patients with preexisting hypertension were more likely to have a significant increase in blood pressure.[2] The study authors of the Neurological Sciences publication suggested that patients with preexisting hypertension should be cautiously monitored for signs of hypertension. A more recent publication noted that "CGRP is involved in the regulation of vasomotor tone under physiologic and pathologic conditions, including hypertension," which could explain these findings. As the two studies noted different underlying risk factors for hypertension for patients taking anti-CGRP migraine therapies, it is important to monitor patients for signs of hypertension regardless of their underlying cardiovascular status.
Migraine was also noted to potentially be associated with an increased risk for cerebrovascular disease and stroke among women who have underlying cardiovascular disease risk factors. According to a cross-sectional analysis whose results were published in Mayo Clinic Proceedings in May 2023, women with migraine were significantly more likely to have severe hot flashes compared with women without migraine.[3] Additionally, the authors stated that migraine was associated with a diagnosis of hypertension.[3]
Results of a secondary data analysis of a subset of 1954 women in the Coronary Artery Risk Development in Young Adults (CARDIA) study were published in the April 2024 issue of Menopause. After adjustment for age, race, estrogen use, oophorectomy, and hysterectomy, women with histories of migraine and persistent vasomotor symptoms were found to have a greater risk for cerebrovascular disease (hazard ratio [HR] 2.25; 95% CI 1.15-4.38), and stroke (HR 3.15; 95% CI 1.35-7.34), compared with women without migraine histories and with minimal vasomotor symptoms. After adjustment for cerebrovascular disease risk factors, the associations between migraine/vasomotor symptoms and cerebrovascular disease were attenuated (HR 1.51; 95% CI 0.73-3.10), and associations between migraine/vasomotor symptoms and stroke were similarly attenuated (HR 1.70; 95% CI 0.66-4.38). The authors of this research article concluded that migraine and persistent vasomotor symptoms are jointly associated with greater risk for cerebrovascular disease and stroke, particularly for women who already have traditional risk factors for cerebrovascular disease.
This new research brings the importance of managing migraine risk factors and symptoms to the forefront. Patients who experience migraine may have a higher risk for cerebrovascular disease. Minimizing migraine risk factors could potentially help reduce this risk for cerebrovascular disease for some patients, and effectively treating migraines may also play a role in reducing the risk for cerebrovascular disease. Some migraine therapies could worsen cardiovascular disease for some patients, however — particularly patients who already have underlying risk factors. Therefore, it is crucial for physicians to approach migraine care with a comprehensive strategy to reduce risk factors, assess underlying disease, and monitor for comorbidities.
Additional References
1. Sandoe CH, Sasikumar S, Lay C, Lawler V. The impact of shift work on migraine: A case series and narrative review. Headache. 2019;59:1631-1640. doi: 10.1111/head.13622 Source
2. Guerzoni S, Castro FL, Brovia D, Baraldi C, Pani L. Evaluation of the risk of hypertension in patients treated with anti-CGRP monoclonal antibodies in a real-life study. Neurol Sci. 2024;45:1661-1668. doi: 10.1007/s10072-023-07167-z Source
3. Faubion SS, Smith T, Thielen J, et al. Association of migraine and vasomotor symptoms. Mayo Clin Proc. 2023;98:701-712. doi: 10.1016/j.mayocp.2023.01.010 Source
A recent study, published in the March 2024 issue of Sleep Medicine, identified shift work as one of the risk factors for headache and migraine. The researchers conducted a meta-analysis, including seven studies and involving 422,869 participants. The authors defined shift work as characterized by individuals or teams working consecutively to exceed the standard 8-hour day. They reported that the pooled analysis revealed a significant association between shift work and an increased risk for headache. Specifically, they determined that "individuals working night shifts had a 44% higher risk of developing headaches and a higher incidence of migraines." The authors stated that this association did not establish any causal relationship, and they suggested that future research should investigate the impact of genetics or health behaviors, which could be considered shared risk factors.
An article that had been published in 2019 in Headache included two case reports detailing the effects of shift work on patients with migraine. The authors of the case reports stated that "in the two cases presented, shift work appeared to be associated with chronification of migraine and higher headache-related disability, despite optimal headache management and good patient adherence."[1] They observed that "a switch to only day shifts promoted transition to an episodic, less disabling pattern of migraine."[1] These publications both support the idea that, while patients may have an underlying predisposition to migraine, certain lifestyle factors can play a role in exacerbating symptoms.
Erenumab, one of the relatively new therapies for migraine, was found to have a potential link to worsening hypertension. According to an article published in February in Headache: The Journal of Head and Face Pain, there has not been evidence of hypertension in preclinical models or clinical trials, yet postmarketing data suggest that erenumab may be associated with hypertension. The authors conducted an observational retrospective cohort study that included 335 patients who had been seen at a tertiary headache or neurology department. At baseline, 20.9% (70/335) of patients had a prior diagnosis of hypertension. The researchers observed that 23.3% (78/335) of the patients were found to have worsening hypertension, and 13 patients of the 225 who continued on erenumab experienced an improvement in their blood pressure. The authors noted that there was no association between worsening hypertension and preexisting hypertension, sex, body mass index, or age, but patients with atrial fibrillation were more likely to develop worsening hypertension (odds ratio 4.9; 95% CI 1.12-21.4; P = .035).
Consideration of a relationship between hypertension and anti-calcitonin gene–related peptide migraine (CGRP) therapies has been found in other studies as well. Results of a retrospective study conducted at the University Hospital of Modena, to explore the rate of hypertension among patients treated with anti-CGRP monoclonal antibodies, were published in April 2024 in Neurological Sciences (published online November 6, 2023). Those authors reported that no significant increase in blood pressure was detected overall, yet 5.7% of the patients developed a significant increase in their blood pressure.[2] Specifically, the researchers reported that patients with preexisting hypertension were more likely to have a significant increase in blood pressure.[2] The study authors of the Neurological Sciences publication suggested that patients with preexisting hypertension should be cautiously monitored for signs of hypertension. A more recent publication noted that "CGRP is involved in the regulation of vasomotor tone under physiologic and pathologic conditions, including hypertension," which could explain these findings. As the two studies noted different underlying risk factors for hypertension for patients taking anti-CGRP migraine therapies, it is important to monitor patients for signs of hypertension regardless of their underlying cardiovascular status.
Migraine was also noted to potentially be associated with an increased risk for cerebrovascular disease and stroke among women who have underlying cardiovascular disease risk factors. According to a cross-sectional analysis whose results were published in Mayo Clinic Proceedings in May 2023, women with migraine were significantly more likely to have severe hot flashes compared with women without migraine.[3] Additionally, the authors stated that migraine was associated with a diagnosis of hypertension.[3]
Results of a secondary data analysis of a subset of 1954 women in the Coronary Artery Risk Development in Young Adults (CARDIA) study were published in the April 2024 issue of Menopause. After adjustment for age, race, estrogen use, oophorectomy, and hysterectomy, women with histories of migraine and persistent vasomotor symptoms were found to have a greater risk for cerebrovascular disease (hazard ratio [HR] 2.25; 95% CI 1.15-4.38), and stroke (HR 3.15; 95% CI 1.35-7.34), compared with women without migraine histories and with minimal vasomotor symptoms. After adjustment for cerebrovascular disease risk factors, the associations between migraine/vasomotor symptoms and cerebrovascular disease were attenuated (HR 1.51; 95% CI 0.73-3.10), and associations between migraine/vasomotor symptoms and stroke were similarly attenuated (HR 1.70; 95% CI 0.66-4.38). The authors of this research article concluded that migraine and persistent vasomotor symptoms are jointly associated with greater risk for cerebrovascular disease and stroke, particularly for women who already have traditional risk factors for cerebrovascular disease.
This new research brings the importance of managing migraine risk factors and symptoms to the forefront. Patients who experience migraine may have a higher risk for cerebrovascular disease. Minimizing migraine risk factors could potentially help reduce this risk for cerebrovascular disease for some patients, and effectively treating migraines may also play a role in reducing the risk for cerebrovascular disease. Some migraine therapies could worsen cardiovascular disease for some patients, however — particularly patients who already have underlying risk factors. Therefore, it is crucial for physicians to approach migraine care with a comprehensive strategy to reduce risk factors, assess underlying disease, and monitor for comorbidities.
Additional References
1. Sandoe CH, Sasikumar S, Lay C, Lawler V. The impact of shift work on migraine: A case series and narrative review. Headache. 2019;59:1631-1640. doi: 10.1111/head.13622 Source
2. Guerzoni S, Castro FL, Brovia D, Baraldi C, Pani L. Evaluation of the risk of hypertension in patients treated with anti-CGRP monoclonal antibodies in a real-life study. Neurol Sci. 2024;45:1661-1668. doi: 10.1007/s10072-023-07167-z Source
3. Faubion SS, Smith T, Thielen J, et al. Association of migraine and vasomotor symptoms. Mayo Clin Proc. 2023;98:701-712. doi: 10.1016/j.mayocp.2023.01.010 Source
A recent study, published in the March 2024 issue of Sleep Medicine, identified shift work as one of the risk factors for headache and migraine. The researchers conducted a meta-analysis, including seven studies and involving 422,869 participants. The authors defined shift work as characterized by individuals or teams working consecutively to exceed the standard 8-hour day. They reported that the pooled analysis revealed a significant association between shift work and an increased risk for headache. Specifically, they determined that "individuals working night shifts had a 44% higher risk of developing headaches and a higher incidence of migraines." The authors stated that this association did not establish any causal relationship, and they suggested that future research should investigate the impact of genetics or health behaviors, which could be considered shared risk factors.
An article that had been published in 2019 in Headache included two case reports detailing the effects of shift work on patients with migraine. The authors of the case reports stated that "in the two cases presented, shift work appeared to be associated with chronification of migraine and higher headache-related disability, despite optimal headache management and good patient adherence."[1] They observed that "a switch to only day shifts promoted transition to an episodic, less disabling pattern of migraine."[1] These publications both support the idea that, while patients may have an underlying predisposition to migraine, certain lifestyle factors can play a role in exacerbating symptoms.
Erenumab, one of the relatively new therapies for migraine, was found to have a potential link to worsening hypertension. According to an article published in February in Headache: The Journal of Head and Face Pain, there has not been evidence of hypertension in preclinical models or clinical trials, yet postmarketing data suggest that erenumab may be associated with hypertension. The authors conducted an observational retrospective cohort study that included 335 patients who had been seen at a tertiary headache or neurology department. At baseline, 20.9% (70/335) of patients had a prior diagnosis of hypertension. The researchers observed that 23.3% (78/335) of the patients were found to have worsening hypertension, and 13 patients of the 225 who continued on erenumab experienced an improvement in their blood pressure. The authors noted that there was no association between worsening hypertension and preexisting hypertension, sex, body mass index, or age, but patients with atrial fibrillation were more likely to develop worsening hypertension (odds ratio 4.9; 95% CI 1.12-21.4; P = .035).
Consideration of a relationship between hypertension and anti-calcitonin gene–related peptide migraine (CGRP) therapies has been found in other studies as well. Results of a retrospective study conducted at the University Hospital of Modena, to explore the rate of hypertension among patients treated with anti-CGRP monoclonal antibodies, were published in April 2024 in Neurological Sciences (published online November 6, 2023). Those authors reported that no significant increase in blood pressure was detected overall, yet 5.7% of the patients developed a significant increase in their blood pressure.[2] Specifically, the researchers reported that patients with preexisting hypertension were more likely to have a significant increase in blood pressure.[2] The study authors of the Neurological Sciences publication suggested that patients with preexisting hypertension should be cautiously monitored for signs of hypertension. A more recent publication noted that "CGRP is involved in the regulation of vasomotor tone under physiologic and pathologic conditions, including hypertension," which could explain these findings. As the two studies noted different underlying risk factors for hypertension for patients taking anti-CGRP migraine therapies, it is important to monitor patients for signs of hypertension regardless of their underlying cardiovascular status.
Migraine was also noted to potentially be associated with an increased risk for cerebrovascular disease and stroke among women who have underlying cardiovascular disease risk factors. According to a cross-sectional analysis whose results were published in Mayo Clinic Proceedings in May 2023, women with migraine were significantly more likely to have severe hot flashes compared with women without migraine.[3] Additionally, the authors stated that migraine was associated with a diagnosis of hypertension.[3]
Results of a secondary data analysis of a subset of 1954 women in the Coronary Artery Risk Development in Young Adults (CARDIA) study were published in the April 2024 issue of Menopause. After adjustment for age, race, estrogen use, oophorectomy, and hysterectomy, women with histories of migraine and persistent vasomotor symptoms were found to have a greater risk for cerebrovascular disease (hazard ratio [HR] 2.25; 95% CI 1.15-4.38), and stroke (HR 3.15; 95% CI 1.35-7.34), compared with women without migraine histories and with minimal vasomotor symptoms. After adjustment for cerebrovascular disease risk factors, the associations between migraine/vasomotor symptoms and cerebrovascular disease were attenuated (HR 1.51; 95% CI 0.73-3.10), and associations between migraine/vasomotor symptoms and stroke were similarly attenuated (HR 1.70; 95% CI 0.66-4.38). The authors of this research article concluded that migraine and persistent vasomotor symptoms are jointly associated with greater risk for cerebrovascular disease and stroke, particularly for women who already have traditional risk factors for cerebrovascular disease.
This new research brings the importance of managing migraine risk factors and symptoms to the forefront. Patients who experience migraine may have a higher risk for cerebrovascular disease. Minimizing migraine risk factors could potentially help reduce this risk for cerebrovascular disease for some patients, and effectively treating migraines may also play a role in reducing the risk for cerebrovascular disease. Some migraine therapies could worsen cardiovascular disease for some patients, however — particularly patients who already have underlying risk factors. Therefore, it is crucial for physicians to approach migraine care with a comprehensive strategy to reduce risk factors, assess underlying disease, and monitor for comorbidities.
Additional References
1. Sandoe CH, Sasikumar S, Lay C, Lawler V. The impact of shift work on migraine: A case series and narrative review. Headache. 2019;59:1631-1640. doi: 10.1111/head.13622 Source
2. Guerzoni S, Castro FL, Brovia D, Baraldi C, Pani L. Evaluation of the risk of hypertension in patients treated with anti-CGRP monoclonal antibodies in a real-life study. Neurol Sci. 2024;45:1661-1668. doi: 10.1007/s10072-023-07167-z Source
3. Faubion SS, Smith T, Thielen J, et al. Association of migraine and vasomotor symptoms. Mayo Clin Proc. 2023;98:701-712. doi: 10.1016/j.mayocp.2023.01.010 Source
DermGPT Can Help Improve Your Office Productivity
For anyone (physicians included) concerned about whether generative artificial intelligence (AI) tools will take your job, the likely answer is no—but those who use generative AI will have an advantage, according to Faranak (Fara) Kamangar, MD, Department Chair, Palo Alto Medical Foundation, who presented on AI at the 2024 Annual Meeting of the American Academy of Dermatology, San Diego, California.
Dr. Kamangar is a dermatologist and inventor of DermGPT, an AI tool created specifically to help health care providers with clinical tasks to increase productivity. According to Dr. Kamangar, “For every 8 hours of scheduled patient time, ambulatory physicians spend more than 5 hours on the [electronic health record].” Her advice is to use AI when you can to complete clinical tasks and move on.
DermGPT utilizes a learned language model that is based on dermatology knowledge acquired from more than 3000 peer-reviewed articles and texts (eg, systematic literature reviews, other published sources in the field of dermatology). Search output includes citations so that users can confirm that the answers and sources are accurate. “Still, with all of these safeguards, all AI models can create inaccuracies and it is important to proofread all content,” says Dr. Kamangar.
During her presentation, Dr. Kamangar gave the following examples of potentially useful DermGPT prompts for dermatologists:
- Can you help me write a response to a denial letter to an insurance company for upadacitinib in a patient with atopic dermatitis?
- I am seeing a patient with blisters. What is the differential diagnosis?
- I am prescribing bimekizumab. What labs do I need to check?
Other potential time-saving uses for a dermatologist include:
- prior authorization letters
- coding support
- responses to common patient messages
- letters of recommendation
- information on new treatments.
In Dr. Kamangar’s practice, they have been able to save at least 30 to 60 minutes at the end of the day that is usually spent updating the electronic health record. “This allows us to complete the clinic day much earlier and does not leave work that will spill over to the next day,” she shares. “During my workday, I have [DermGPT] open on my computer, and as clinical tasks arise, if they require more information or assistance, I turn to DermGPT to help de-escalate the task from a moderate to difficult level to an easy task that can be easily managed.”
The next stage of health technology—AI—is here, and physicians are understandably cautious. Board-certified dermatologists, dermatology residents, fellows, and medical students can try DermGPT for free at https://www.dermgpt.com/.
Dr. Kamangar is the founder of DermGPT.
Melissa Sears is the Director, Editorial, of Cutis.
For anyone (physicians included) concerned about whether generative artificial intelligence (AI) tools will take your job, the likely answer is no—but those who use generative AI will have an advantage, according to Faranak (Fara) Kamangar, MD, Department Chair, Palo Alto Medical Foundation, who presented on AI at the 2024 Annual Meeting of the American Academy of Dermatology, San Diego, California.
Dr. Kamangar is a dermatologist and inventor of DermGPT, an AI tool created specifically to help health care providers with clinical tasks to increase productivity. According to Dr. Kamangar, “For every 8 hours of scheduled patient time, ambulatory physicians spend more than 5 hours on the [electronic health record].” Her advice is to use AI when you can to complete clinical tasks and move on.
DermGPT utilizes a learned language model that is based on dermatology knowledge acquired from more than 3000 peer-reviewed articles and texts (eg, systematic literature reviews, other published sources in the field of dermatology). Search output includes citations so that users can confirm that the answers and sources are accurate. “Still, with all of these safeguards, all AI models can create inaccuracies and it is important to proofread all content,” says Dr. Kamangar.
During her presentation, Dr. Kamangar gave the following examples of potentially useful DermGPT prompts for dermatologists:
- Can you help me write a response to a denial letter to an insurance company for upadacitinib in a patient with atopic dermatitis?
- I am seeing a patient with blisters. What is the differential diagnosis?
- I am prescribing bimekizumab. What labs do I need to check?
Other potential time-saving uses for a dermatologist include:
- prior authorization letters
- coding support
- responses to common patient messages
- letters of recommendation
- information on new treatments.
In Dr. Kamangar’s practice, they have been able to save at least 30 to 60 minutes at the end of the day that is usually spent updating the electronic health record. “This allows us to complete the clinic day much earlier and does not leave work that will spill over to the next day,” she shares. “During my workday, I have [DermGPT] open on my computer, and as clinical tasks arise, if they require more information or assistance, I turn to DermGPT to help de-escalate the task from a moderate to difficult level to an easy task that can be easily managed.”
The next stage of health technology—AI—is here, and physicians are understandably cautious. Board-certified dermatologists, dermatology residents, fellows, and medical students can try DermGPT for free at https://www.dermgpt.com/.
Dr. Kamangar is the founder of DermGPT.
Melissa Sears is the Director, Editorial, of Cutis.
For anyone (physicians included) concerned about whether generative artificial intelligence (AI) tools will take your job, the likely answer is no—but those who use generative AI will have an advantage, according to Faranak (Fara) Kamangar, MD, Department Chair, Palo Alto Medical Foundation, who presented on AI at the 2024 Annual Meeting of the American Academy of Dermatology, San Diego, California.
Dr. Kamangar is a dermatologist and inventor of DermGPT, an AI tool created specifically to help health care providers with clinical tasks to increase productivity. According to Dr. Kamangar, “For every 8 hours of scheduled patient time, ambulatory physicians spend more than 5 hours on the [electronic health record].” Her advice is to use AI when you can to complete clinical tasks and move on.
DermGPT utilizes a learned language model that is based on dermatology knowledge acquired from more than 3000 peer-reviewed articles and texts (eg, systematic literature reviews, other published sources in the field of dermatology). Search output includes citations so that users can confirm that the answers and sources are accurate. “Still, with all of these safeguards, all AI models can create inaccuracies and it is important to proofread all content,” says Dr. Kamangar.
During her presentation, Dr. Kamangar gave the following examples of potentially useful DermGPT prompts for dermatologists:
- Can you help me write a response to a denial letter to an insurance company for upadacitinib in a patient with atopic dermatitis?
- I am seeing a patient with blisters. What is the differential diagnosis?
- I am prescribing bimekizumab. What labs do I need to check?
Other potential time-saving uses for a dermatologist include:
- prior authorization letters
- coding support
- responses to common patient messages
- letters of recommendation
- information on new treatments.
In Dr. Kamangar’s practice, they have been able to save at least 30 to 60 minutes at the end of the day that is usually spent updating the electronic health record. “This allows us to complete the clinic day much earlier and does not leave work that will spill over to the next day,” she shares. “During my workday, I have [DermGPT] open on my computer, and as clinical tasks arise, if they require more information or assistance, I turn to DermGPT to help de-escalate the task from a moderate to difficult level to an easy task that can be easily managed.”
The next stage of health technology—AI—is here, and physicians are understandably cautious. Board-certified dermatologists, dermatology residents, fellows, and medical students can try DermGPT for free at https://www.dermgpt.com/.
Dr. Kamangar is the founder of DermGPT.
Melissa Sears is the Director, Editorial, of Cutis.
Dry Eye: A Hands-On Guide for Diagnosis and Treatment in Primary Care
Read More
Read More
Read More
Commentary: Gut Dysbiosis, DMARD, Joint Involvement, and MACE in PsA, April 2024
After PsA onset, early diagnosis and management leads to better long-term outcomes. These prior observations were confirmed in a study by Snoeck Henkemans and colleagues that included 708 newly diagnosed patients with PsA naive to disease-modifying antirheumatic drugs (DMARD) who were followed up for 3 years or more. Patients with a short (<12 weeks) vs long delay (>1 year) in PsA diagnosis after symptom onset were more likely to achieve minimum disease activity (OR 2.55; 95% CI 1.37-4.76). Thus, longer delay in diagnosing PsA is associated with worse clinical outcomes.
Bimekizumab is a novel biologic therapy that inhibits interleukins (IL)-17A and -17F and is efficacious in the treatment of psoriasis, PsA, and axial spondyloarthritis. However, the effectiveness in PsA vis-à-vis other IL-17A inhibitors is not known. In the absence of a formal head-to-head study, matching-adjusted indirect comparisons is a method to evaluate comparative effectiveness. Such a study by Mease and colleagues included the data of patients with PsA who were biological DMARD–naive or who had an inadequate response to tumor necrosis factor inhibitors (TNFi-IR), and who received bimekizumab from the BE OPTIMAL (n = 236) and BE COMPLETE (n = 146) trials and secukinumab from the FUTURE 2 trial (n = 200). They demonstrated that, in the biological DMARD–naive subgroup, the probability of achieving at least 70% improvement in American College of Rheumatology (ACR) response was two times higher with bimekizumab (160 mg every 4 weeks) vs secukinumab (150 mg or 300 mg every 4 weeks) at week 52. In the TNFi-IR subgroup, bimekizumab had a greater likelihood of response compared with 150 mg secukinumab for ACR20, ACR70, and minimal disease activity outcomes and a greater likelihood of response compared with 300 mg secukinumab for ACR50 and minimal disease activity. Thus, bimekizumab is at least as effective as secukinumab in PsA. Formal head-to-head studies comparing bimekizumab with other IL-17A inhibitors are required.
Distal interphalangeal (DIP) joint involvement is an important manifestation of PsA and is closely related to nail dystrophy in the adjacent nail. Ixekizumab is another biologic that targets IL-17A. In a post hoc analysis of the SPIRIT-H2H study, McGonagle and colleagues confirmed that over 96% of patients with PsA and simultaneous DIP joint involvement reported adjacent nail psoriasis. When compared with adalimumab, ixekizumab led to greater improvements in DIP involvement and adjacent nail psoriasis as early as week 12 (38.8% vs 28.4%; P < .0001), with improvements sustained up to week 52 (64.9% vs 57.5%; P = .0055). This probably reflects a greater effectiveness of IL-17A inhibition in treating skin and nail psoriasis compared with TNFi.
Finally, in a population-based retrospective cohort study that included 13,905 patients with PsA (n = 1672) or rheumatoid arthritis (n = 12,233) who did not have any previous history of major adverse cardiovascular events (MACE), Meng and colleagues showed that the incidence rates of MACE were similar in patients with PsA and rheumatoid arthritis. Thus, cardiovascular risk management should be similarly aggressive in patients with PsA and rheumatoid arthritis.
After PsA onset, early diagnosis and management leads to better long-term outcomes. These prior observations were confirmed in a study by Snoeck Henkemans and colleagues that included 708 newly diagnosed patients with PsA naive to disease-modifying antirheumatic drugs (DMARD) who were followed up for 3 years or more. Patients with a short (<12 weeks) vs long delay (>1 year) in PsA diagnosis after symptom onset were more likely to achieve minimum disease activity (OR 2.55; 95% CI 1.37-4.76). Thus, longer delay in diagnosing PsA is associated with worse clinical outcomes.
Bimekizumab is a novel biologic therapy that inhibits interleukins (IL)-17A and -17F and is efficacious in the treatment of psoriasis, PsA, and axial spondyloarthritis. However, the effectiveness in PsA vis-à-vis other IL-17A inhibitors is not known. In the absence of a formal head-to-head study, matching-adjusted indirect comparisons is a method to evaluate comparative effectiveness. Such a study by Mease and colleagues included the data of patients with PsA who were biological DMARD–naive or who had an inadequate response to tumor necrosis factor inhibitors (TNFi-IR), and who received bimekizumab from the BE OPTIMAL (n = 236) and BE COMPLETE (n = 146) trials and secukinumab from the FUTURE 2 trial (n = 200). They demonstrated that, in the biological DMARD–naive subgroup, the probability of achieving at least 70% improvement in American College of Rheumatology (ACR) response was two times higher with bimekizumab (160 mg every 4 weeks) vs secukinumab (150 mg or 300 mg every 4 weeks) at week 52. In the TNFi-IR subgroup, bimekizumab had a greater likelihood of response compared with 150 mg secukinumab for ACR20, ACR70, and minimal disease activity outcomes and a greater likelihood of response compared with 300 mg secukinumab for ACR50 and minimal disease activity. Thus, bimekizumab is at least as effective as secukinumab in PsA. Formal head-to-head studies comparing bimekizumab with other IL-17A inhibitors are required.
Distal interphalangeal (DIP) joint involvement is an important manifestation of PsA and is closely related to nail dystrophy in the adjacent nail. Ixekizumab is another biologic that targets IL-17A. In a post hoc analysis of the SPIRIT-H2H study, McGonagle and colleagues confirmed that over 96% of patients with PsA and simultaneous DIP joint involvement reported adjacent nail psoriasis. When compared with adalimumab, ixekizumab led to greater improvements in DIP involvement and adjacent nail psoriasis as early as week 12 (38.8% vs 28.4%; P < .0001), with improvements sustained up to week 52 (64.9% vs 57.5%; P = .0055). This probably reflects a greater effectiveness of IL-17A inhibition in treating skin and nail psoriasis compared with TNFi.
Finally, in a population-based retrospective cohort study that included 13,905 patients with PsA (n = 1672) or rheumatoid arthritis (n = 12,233) who did not have any previous history of major adverse cardiovascular events (MACE), Meng and colleagues showed that the incidence rates of MACE were similar in patients with PsA and rheumatoid arthritis. Thus, cardiovascular risk management should be similarly aggressive in patients with PsA and rheumatoid arthritis.
After PsA onset, early diagnosis and management leads to better long-term outcomes. These prior observations were confirmed in a study by Snoeck Henkemans and colleagues that included 708 newly diagnosed patients with PsA naive to disease-modifying antirheumatic drugs (DMARD) who were followed up for 3 years or more. Patients with a short (<12 weeks) vs long delay (>1 year) in PsA diagnosis after symptom onset were more likely to achieve minimum disease activity (OR 2.55; 95% CI 1.37-4.76). Thus, longer delay in diagnosing PsA is associated with worse clinical outcomes.
Bimekizumab is a novel biologic therapy that inhibits interleukins (IL)-17A and -17F and is efficacious in the treatment of psoriasis, PsA, and axial spondyloarthritis. However, the effectiveness in PsA vis-à-vis other IL-17A inhibitors is not known. In the absence of a formal head-to-head study, matching-adjusted indirect comparisons is a method to evaluate comparative effectiveness. Such a study by Mease and colleagues included the data of patients with PsA who were biological DMARD–naive or who had an inadequate response to tumor necrosis factor inhibitors (TNFi-IR), and who received bimekizumab from the BE OPTIMAL (n = 236) and BE COMPLETE (n = 146) trials and secukinumab from the FUTURE 2 trial (n = 200). They demonstrated that, in the biological DMARD–naive subgroup, the probability of achieving at least 70% improvement in American College of Rheumatology (ACR) response was two times higher with bimekizumab (160 mg every 4 weeks) vs secukinumab (150 mg or 300 mg every 4 weeks) at week 52. In the TNFi-IR subgroup, bimekizumab had a greater likelihood of response compared with 150 mg secukinumab for ACR20, ACR70, and minimal disease activity outcomes and a greater likelihood of response compared with 300 mg secukinumab for ACR50 and minimal disease activity. Thus, bimekizumab is at least as effective as secukinumab in PsA. Formal head-to-head studies comparing bimekizumab with other IL-17A inhibitors are required.
Distal interphalangeal (DIP) joint involvement is an important manifestation of PsA and is closely related to nail dystrophy in the adjacent nail. Ixekizumab is another biologic that targets IL-17A. In a post hoc analysis of the SPIRIT-H2H study, McGonagle and colleagues confirmed that over 96% of patients with PsA and simultaneous DIP joint involvement reported adjacent nail psoriasis. When compared with adalimumab, ixekizumab led to greater improvements in DIP involvement and adjacent nail psoriasis as early as week 12 (38.8% vs 28.4%; P < .0001), with improvements sustained up to week 52 (64.9% vs 57.5%; P = .0055). This probably reflects a greater effectiveness of IL-17A inhibition in treating skin and nail psoriasis compared with TNFi.
Finally, in a population-based retrospective cohort study that included 13,905 patients with PsA (n = 1672) or rheumatoid arthritis (n = 12,233) who did not have any previous history of major adverse cardiovascular events (MACE), Meng and colleagues showed that the incidence rates of MACE were similar in patients with PsA and rheumatoid arthritis. Thus, cardiovascular risk management should be similarly aggressive in patients with PsA and rheumatoid arthritis.
Commentary: MRI Surveillance and Risk Factors in Breast Cancer, April 2024
A positive family history of cancer and obesity are established risk factors for development of breast cancer among women.[4,5] A population-based cohort study that included 15,055 Chinese women evaluated the association and interaction between body mass index (BMI) and family history of cancer on the risk for breast cancer (Cao et al). The incidence risk for breast cancer was highest in the group with obesity vs the group with normal weight (adjusted HR 2.09; 95% CI 1.42-3.07), and those with a family history of cancer also had an increased risk vs those without a family history of cancer (adjusted HR 1.63; 95% CI 1.22-2.49). Furthermore, women with a BMI ≥ 24 and family history of cancer had a higher risk for breast cancer development compared with women with a BMI < 24 and no family history of cancer (adjusted HR 2.06; 95% CI 1.39-3.06). This study indicates a heightened breast cancer risk when cancer family history and obesity coexist, suggesting the importance of addressing modifiable risk factors and targeting lifestyle interventions in this population.
Triple-negative breast cancer (TNBC), although exhibiting its own heterogeneity, has various features that differentiate this subtype from luminal breast cancers. For example, TNBC generally has a more aggressive course, increased responsiveness to chemotherapy, and earlier pattern of recurrence compared with hormone receptor–positive disease. Prior studies have also shown that established breast cancer risk factors reflect those for the luminal A subtype, whereas those for TNBC are less consistent.[6] A meta-analysis that included 33 studies evaluated the association between traditional breast cancer risk factors and TNBC incidence (Kumar et al). Family history (odds ratio [OR] 1.55; 95% CI 1.34-1.81; P < .001), longer duration of oral contraceptive use (OR 1.29; 95% CI 1.08-1.55; P < .001), and higher breast density (OR 2.19; 95% CI 1.67-2.88; P < .001) were significantly associated with an increased risk for TNBC. Factors including later age at menarche, later age at first birth, and breastfeeding were associated with reduced risk for TNBC. Furthermore, there was no significant association with parity, menopausal hormone therapy, alcohol, smoking, and BMI. This study highlights distinct risk factors that may contribute to a higher risk for TNBC, and future research will be valuable to better elucidate the mechanisms at play and to further understand the differences within this subtype itself.
Additional References
- National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology. Genetic/familial high-risk assessment: breast, ovarian, and pancreatic. Version 3.2024. Source
- Saadatmand S, Geuzinge HA, Rutgers EJT, et al; on behalf of the FaMRIsc study group. MRI versus mammography for breast cancer screening in women with familial risk (FaMRIsc): A multicentre, randomised, controlled trial. Lancet Oncol. 2019;20:1136-1147. doi: 10.1016/S1470-2045(19)30275-X Source
- Warner E, Zhu S, Plewes DB, et al. Breast cancer mortality among women with a BRCA1 or BRCA2 mutation in a magnetic resonance imaging plus mammography screening program. Cancers (Basel). 2020;12:3479. doi: 10.3390/cancers12113479 Source
- Picon-Ruiz M, Morata-Tarifa C, Valle-Goffin JJ, et al. Obesity and adverse breast cancer risk and outcome: Mechanistic insights and strategies for intervention. CA Cancer J Clin. 2017;67:378-397. doi: 10.3322/caac.21405 Source
- Engmann NJ, Golmakani MK, Miglioretti DL, et al; for the Breast Cancer Surveillance Consortium. Population-attributable risk proportion of clinical risk factors for breast cancer. JAMA Oncol. 2017;3:1228-1236. doi: 10.1001/jamaoncol.2016.6326 Source
- Barnard ME, Boeke CE, Tamimi RM. Established breast cancer risk factors and risk of intrinsic tumor subtypes. Biochim Biophys Acta Rev Cancer. 2015;1856:73-85. doi: 10.1016/j.bbcan.2015.0002 Source
A positive family history of cancer and obesity are established risk factors for development of breast cancer among women.[4,5] A population-based cohort study that included 15,055 Chinese women evaluated the association and interaction between body mass index (BMI) and family history of cancer on the risk for breast cancer (Cao et al). The incidence risk for breast cancer was highest in the group with obesity vs the group with normal weight (adjusted HR 2.09; 95% CI 1.42-3.07), and those with a family history of cancer also had an increased risk vs those without a family history of cancer (adjusted HR 1.63; 95% CI 1.22-2.49). Furthermore, women with a BMI ≥ 24 and family history of cancer had a higher risk for breast cancer development compared with women with a BMI < 24 and no family history of cancer (adjusted HR 2.06; 95% CI 1.39-3.06). This study indicates a heightened breast cancer risk when cancer family history and obesity coexist, suggesting the importance of addressing modifiable risk factors and targeting lifestyle interventions in this population.
Triple-negative breast cancer (TNBC), although exhibiting its own heterogeneity, has various features that differentiate this subtype from luminal breast cancers. For example, TNBC generally has a more aggressive course, increased responsiveness to chemotherapy, and earlier pattern of recurrence compared with hormone receptor–positive disease. Prior studies have also shown that established breast cancer risk factors reflect those for the luminal A subtype, whereas those for TNBC are less consistent.[6] A meta-analysis that included 33 studies evaluated the association between traditional breast cancer risk factors and TNBC incidence (Kumar et al). Family history (odds ratio [OR] 1.55; 95% CI 1.34-1.81; P < .001), longer duration of oral contraceptive use (OR 1.29; 95% CI 1.08-1.55; P < .001), and higher breast density (OR 2.19; 95% CI 1.67-2.88; P < .001) were significantly associated with an increased risk for TNBC. Factors including later age at menarche, later age at first birth, and breastfeeding were associated with reduced risk for TNBC. Furthermore, there was no significant association with parity, menopausal hormone therapy, alcohol, smoking, and BMI. This study highlights distinct risk factors that may contribute to a higher risk for TNBC, and future research will be valuable to better elucidate the mechanisms at play and to further understand the differences within this subtype itself.
Additional References
- National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology. Genetic/familial high-risk assessment: breast, ovarian, and pancreatic. Version 3.2024. Source
- Saadatmand S, Geuzinge HA, Rutgers EJT, et al; on behalf of the FaMRIsc study group. MRI versus mammography for breast cancer screening in women with familial risk (FaMRIsc): A multicentre, randomised, controlled trial. Lancet Oncol. 2019;20:1136-1147. doi: 10.1016/S1470-2045(19)30275-X Source
- Warner E, Zhu S, Plewes DB, et al. Breast cancer mortality among women with a BRCA1 or BRCA2 mutation in a magnetic resonance imaging plus mammography screening program. Cancers (Basel). 2020;12:3479. doi: 10.3390/cancers12113479 Source
- Picon-Ruiz M, Morata-Tarifa C, Valle-Goffin JJ, et al. Obesity and adverse breast cancer risk and outcome: Mechanistic insights and strategies for intervention. CA Cancer J Clin. 2017;67:378-397. doi: 10.3322/caac.21405 Source
- Engmann NJ, Golmakani MK, Miglioretti DL, et al; for the Breast Cancer Surveillance Consortium. Population-attributable risk proportion of clinical risk factors for breast cancer. JAMA Oncol. 2017;3:1228-1236. doi: 10.1001/jamaoncol.2016.6326 Source
- Barnard ME, Boeke CE, Tamimi RM. Established breast cancer risk factors and risk of intrinsic tumor subtypes. Biochim Biophys Acta Rev Cancer. 2015;1856:73-85. doi: 10.1016/j.bbcan.2015.0002 Source
A positive family history of cancer and obesity are established risk factors for development of breast cancer among women.[4,5] A population-based cohort study that included 15,055 Chinese women evaluated the association and interaction between body mass index (BMI) and family history of cancer on the risk for breast cancer (Cao et al). The incidence risk for breast cancer was highest in the group with obesity vs the group with normal weight (adjusted HR 2.09; 95% CI 1.42-3.07), and those with a family history of cancer also had an increased risk vs those without a family history of cancer (adjusted HR 1.63; 95% CI 1.22-2.49). Furthermore, women with a BMI ≥ 24 and family history of cancer had a higher risk for breast cancer development compared with women with a BMI < 24 and no family history of cancer (adjusted HR 2.06; 95% CI 1.39-3.06). This study indicates a heightened breast cancer risk when cancer family history and obesity coexist, suggesting the importance of addressing modifiable risk factors and targeting lifestyle interventions in this population.
Triple-negative breast cancer (TNBC), although exhibiting its own heterogeneity, has various features that differentiate this subtype from luminal breast cancers. For example, TNBC generally has a more aggressive course, increased responsiveness to chemotherapy, and earlier pattern of recurrence compared with hormone receptor–positive disease. Prior studies have also shown that established breast cancer risk factors reflect those for the luminal A subtype, whereas those for TNBC are less consistent.[6] A meta-analysis that included 33 studies evaluated the association between traditional breast cancer risk factors and TNBC incidence (Kumar et al). Family history (odds ratio [OR] 1.55; 95% CI 1.34-1.81; P < .001), longer duration of oral contraceptive use (OR 1.29; 95% CI 1.08-1.55; P < .001), and higher breast density (OR 2.19; 95% CI 1.67-2.88; P < .001) were significantly associated with an increased risk for TNBC. Factors including later age at menarche, later age at first birth, and breastfeeding were associated with reduced risk for TNBC. Furthermore, there was no significant association with parity, menopausal hormone therapy, alcohol, smoking, and BMI. This study highlights distinct risk factors that may contribute to a higher risk for TNBC, and future research will be valuable to better elucidate the mechanisms at play and to further understand the differences within this subtype itself.
Additional References
- National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology. Genetic/familial high-risk assessment: breast, ovarian, and pancreatic. Version 3.2024. Source
- Saadatmand S, Geuzinge HA, Rutgers EJT, et al; on behalf of the FaMRIsc study group. MRI versus mammography for breast cancer screening in women with familial risk (FaMRIsc): A multicentre, randomised, controlled trial. Lancet Oncol. 2019;20:1136-1147. doi: 10.1016/S1470-2045(19)30275-X Source
- Warner E, Zhu S, Plewes DB, et al. Breast cancer mortality among women with a BRCA1 or BRCA2 mutation in a magnetic resonance imaging plus mammography screening program. Cancers (Basel). 2020;12:3479. doi: 10.3390/cancers12113479 Source
- Picon-Ruiz M, Morata-Tarifa C, Valle-Goffin JJ, et al. Obesity and adverse breast cancer risk and outcome: Mechanistic insights and strategies for intervention. CA Cancer J Clin. 2017;67:378-397. doi: 10.3322/caac.21405 Source
- Engmann NJ, Golmakani MK, Miglioretti DL, et al; for the Breast Cancer Surveillance Consortium. Population-attributable risk proportion of clinical risk factors for breast cancer. JAMA Oncol. 2017;3:1228-1236. doi: 10.1001/jamaoncol.2016.6326 Source
- Barnard ME, Boeke CE, Tamimi RM. Established breast cancer risk factors and risk of intrinsic tumor subtypes. Biochim Biophys Acta Rev Cancer. 2015;1856:73-85. doi: 10.1016/j.bbcan.2015.0002 Source