User login
Clinical Takeaways in Thrombocytopenia From ASH 2023
The clinical takeaways in immune thrombocytopenia (ITP) from the 2023 American Society of Hematology (ASH) Annual Meeting and Exposition include the relative merits of advanced therapies, efficacy of novel therapies, and infection risk for patients with chronic ITP.
Dr Howard Liebman, of the Keck School of Medicine in Los Angeles, California, opens with a database analysis of patients with primary ITP who were first-time users of advanced therapies, such as rituximab and thrombopoietin (TPO) receptor agonists. The analysis found that despite their relative merits, all the drugs carried a significant risk for adverse events.
Next, he reports on a study examining whether newly diagnosed patients or patients with chronic ITP had better results from the use of avatrombopag, a TPO receptor agonist. Reassuringly, there were no differences in outcomes.
Dr Liebman then discusses the updated results of an ongoing study of rilzabrutinib, a Bruton kinase inhibitor. This analysis showed that the drug achieved rapid, stable, and durable platelet responses.
He next turns to a Danish registry study on infection in patients with chronic ITP, which revealed an ongoing, cumulative risk for infection over 10 years.
Finally, Dr Liebman reports on a study that showed women who develop ITP during pregnancy require more interventions than do women with chronic ITP who become pregnant, and many develop chronic disease after delivery.
--
Howard A. Liebman, MD, Professor of Medicine and Pathology, University of Southern California, Keck School of Medicine; Attending Physician, Department of Medicine, Hematology, Norris Comprehensive Cancer Center, Keck Medicine at University of Southern California, Los Angeles, California
Howard A. Liebman, MD, has disclosed the following relevant financial relationships:
Serve(d) as a consultant for: Novartis; Sanofi; Sobi
Received research grant from: Janssen Pharmaceuticals; Sanofi
The clinical takeaways in immune thrombocytopenia (ITP) from the 2023 American Society of Hematology (ASH) Annual Meeting and Exposition include the relative merits of advanced therapies, efficacy of novel therapies, and infection risk for patients with chronic ITP.
Dr Howard Liebman, of the Keck School of Medicine in Los Angeles, California, opens with a database analysis of patients with primary ITP who were first-time users of advanced therapies, such as rituximab and thrombopoietin (TPO) receptor agonists. The analysis found that despite their relative merits, all the drugs carried a significant risk for adverse events.
Next, he reports on a study examining whether newly diagnosed patients or patients with chronic ITP had better results from the use of avatrombopag, a TPO receptor agonist. Reassuringly, there were no differences in outcomes.
Dr Liebman then discusses the updated results of an ongoing study of rilzabrutinib, a Bruton kinase inhibitor. This analysis showed that the drug achieved rapid, stable, and durable platelet responses.
He next turns to a Danish registry study on infection in patients with chronic ITP, which revealed an ongoing, cumulative risk for infection over 10 years.
Finally, Dr Liebman reports on a study that showed women who develop ITP during pregnancy require more interventions than do women with chronic ITP who become pregnant, and many develop chronic disease after delivery.
--
Howard A. Liebman, MD, Professor of Medicine and Pathology, University of Southern California, Keck School of Medicine; Attending Physician, Department of Medicine, Hematology, Norris Comprehensive Cancer Center, Keck Medicine at University of Southern California, Los Angeles, California
Howard A. Liebman, MD, has disclosed the following relevant financial relationships:
Serve(d) as a consultant for: Novartis; Sanofi; Sobi
Received research grant from: Janssen Pharmaceuticals; Sanofi
The clinical takeaways in immune thrombocytopenia (ITP) from the 2023 American Society of Hematology (ASH) Annual Meeting and Exposition include the relative merits of advanced therapies, efficacy of novel therapies, and infection risk for patients with chronic ITP.
Dr Howard Liebman, of the Keck School of Medicine in Los Angeles, California, opens with a database analysis of patients with primary ITP who were first-time users of advanced therapies, such as rituximab and thrombopoietin (TPO) receptor agonists. The analysis found that despite their relative merits, all the drugs carried a significant risk for adverse events.
Next, he reports on a study examining whether newly diagnosed patients or patients with chronic ITP had better results from the use of avatrombopag, a TPO receptor agonist. Reassuringly, there were no differences in outcomes.
Dr Liebman then discusses the updated results of an ongoing study of rilzabrutinib, a Bruton kinase inhibitor. This analysis showed that the drug achieved rapid, stable, and durable platelet responses.
He next turns to a Danish registry study on infection in patients with chronic ITP, which revealed an ongoing, cumulative risk for infection over 10 years.
Finally, Dr Liebman reports on a study that showed women who develop ITP during pregnancy require more interventions than do women with chronic ITP who become pregnant, and many develop chronic disease after delivery.
--
Howard A. Liebman, MD, Professor of Medicine and Pathology, University of Southern California, Keck School of Medicine; Attending Physician, Department of Medicine, Hematology, Norris Comprehensive Cancer Center, Keck Medicine at University of Southern California, Los Angeles, California
Howard A. Liebman, MD, has disclosed the following relevant financial relationships:
Serve(d) as a consultant for: Novartis; Sanofi; Sobi
Received research grant from: Janssen Pharmaceuticals; Sanofi

Commentary: Risks for Eosinophilic Esophagitis: IBD, Eczema, Diet, and Acid Suppressants, January 2024
Although there exists a recognized association between IBD and secondary EoE diagnoses, studies focusing on the primary diagnosis of EoE alongside IBD have yielded conflicting results. Dr Amiko Uchida, from the University of Utah Department of Medicine, working with colleagues from the Department of Medical Epidemiology and Biostatistics at Karolinska Institutet in Stockholm, Sweden, led by Dr Jonas F. Ludvigsson, conducted a comprehensive study spanning 1990-2019 to explore the relationship between these two diseases.
Dr Uchida and colleagues assessed the association among Swedish patients diagnosed with biopsy-verified EoE (n = 1587) between 1990 and 2017. These patients were age- and sex-matched with up to five reference individuals from the general population (n = 7808). The primary focus was to discern the relationship between the primary diagnosis of EoE and the subsequent diagnosis of IBD.
The study's findings underscore the importance of heightened awareness among healthcare professionals regarding the potential association between EoE and the development of subsequent IBD. Collaborative efforts between physicians, gastroenterologists, and specialists in allergic diseases are crucial to ensure comprehensive care and timely identification of gastrointestinal complications in patients with EoE.
These results indicate a potential interplay between EoE and the pathogenesis of IBD, particularly Crohn's disease. In patients with EoE, careful consideration of gastrointestinal symptoms suggestive of IBD, such as abdominal pain, diarrhea, and rectal bleeding, is pivotal, necessitating further evaluation and appropriate monitoring for early detection and management of IBD.
Upon diagnosis, clinicians must develop a management plan for this chronic and critical disease. This study aids in planning future screenings for these patients, because one third of those diagnosed with EoE are at risk of developing IBD within a year. Therefore, primary physicians must remain vigilant for the development of gastrointestinal symptoms leading to a diagnosis of Crohn's disease or ulcerative colitis. Additionally, family awareness is crucial owing to observed associations between siblings, suggesting a potential role of genetics or early environmental factors in EoE development and future IBD diagnoses.
Further research is necessary to elucidate the shared pathophysiologic mechanisms connecting EoE and IBD. It is important to consider certain details, however; for instance, 31% of all subsequent IBD cases were diagnosed within the first year after an EoE diagnosis, potentially indicating a role of detection bias in these findings. Using a validated nationwide cohort and comparing study individuals with their siblings helped control for potential intrafamilial confounders as well as some environmental confounders, minimizing such biases as selection bias due to socioeconomic status. This strengthens the observed association in this study.
These insights into the increased risk for IBD, notably Crohn's disease, among patients with EoE underscore the need for thorough clinical evaluation and vigilant monitoring for gastrointestinal complications in this population.
A retrospective study conducted in pediatric patients presenting with an aerodigestive manifestation aimed to assess the factors associated with EoE and the diagnostic role of triple endoscopy. The results suggested a potential association between a family history of eczema and a diet lacking allergenic foods with a future diagnosis of EoE.
This study by Sheila Moran and colleagues, led by Dr Christina J. Yang from the Department of Otorhinolaryngology, Head and Neck Surgery at the Albert Einstein College of Medicine, Bronx, New York, aimed to identify preoperative risk factors linked to an EoE diagnosis in children undergoing triple endoscopy. They evaluated 119 pediatric patients aged 0-21 years who underwent triple endoscopy (including flexible bronchoscopy, rigid direct laryngoscopy and bronchoscopy, and esophagoscopy with biopsy) at the Children's Hospital at Montefiore between January 1, 2015, and December 31, 2019.
The study underscored the significance of both genetic predisposition and dietary influences in EoE development. Understanding the interplay between familial atopic conditions and dietary choices among pediatric patients with aerodigestive dysfunction is crucial for early identification and implementing preventive strategies against EoE.
As clinicians, it's essential to consider rare diseases like EoE when patients present with mixed symptoms, including aerodigestive symptoms, a family history of eczema, and a history of environmental allergies, given the association between these conditions. The potential link between a family history of eczema and increased EoE risk suggests a shared genetic susceptibility among allergic conditions. Therefore, clinicians evaluating children with aerodigestive dysfunction, particularly those with a familial history of eczema, should maintain a high index of suspicion for EoE, prompting vigilant monitoring and appropriate diagnostic assessments.
When contemplating advanced procedures, such as triple endoscopy or biopsy sampling, considering the patient's previous medical history and the effect of dietary modifications, such as incorporating or excluding dairy from the diet, warrants further investigation in the context of EoE prevention. Clinicians should consider providing dietary counseling and personalized nutritional plans based on evidence-based approaches to potentially mitigate EoE risk in susceptible pediatric populations.
Additionally, it's crucial to consider EoE in minority racial groups and underserved communities and encourage the use of diagnostic tests, such as triple endoscopy, to facilitate early diagnosis. Healthcare providers should contemplate integrating family history assessments, particularly regarding eczema, into the evaluation of children with aerodigestive dysfunction. This information can assist in risk assessment and early identification of individuals at a higher risk of developing EoE, enabling prompt intervention and management.
The increasing incidence of EoE across different nations, including the United States, has underscored the need for a deeper understanding of its causes, early diagnosis, and treatment. A novel population-based study conducted in Denmark aimed to explore the relationship between maternal and infant use of antibiotics and acid suppressants in the development of EoE. The study yielded significant results based on a population of 392 cases. Dr Elizabeth T. Jensen, MPH, PhD, from the Department of Epidemiology & Prevention at Wake Forest University School of Medicine, Winston-Salem, North Carolina, conducted a comprehensive study spanning the last 20 years to decipher potential causes contributing to the rising incidence of EoE.
Dr Jensen and her colleagues evaluated the association between maternal and infant use of antibiotics and acid suppressants in Denmark. They used pathology, prescription, birth, inpatient, and outpatient health registry data, ensuring complete ascertainment of all EoE cases among Danish residents born between 1997 and 2018. The research obtained a census of cases from a registered sample of approximately 1.4 million children, matching EoE cases to controls using a 1:10 ratio through incidence density sampling. A total of 392 patients with EoE and 3637 control patients were enrolled. The primary outcome of the study focused on the development of EoE, revealing a dose-response association between maternal and infant antibiotic and acid suppressant use and increased EoE risk.
This study demonstrated a robust correlation between the dosage of antibiotics and acid suppressants and the development of EoE in offspring during childhood. These findings hold significance because these medications represent some of the most common prescriptions in clinical practice. Pregnancy triggers significant physiologic changes in women, including increased hormonal effects and abdominal pressure on the lower esophageal sphincter, making pregnant individuals more prone to esophageal reflux and necessitating the use of gastric acid suppressants. As clinicians, it's crucial to consider lifestyle modifications and dietary adjustments before resorting to acid suppressants, reserving their use for only when absolutely necessary.
Postpregnancy, emphasizing exclusive breastfeeding for the first 6 months and proper feeding techniques can aid in reducing the likelihood of reflux disease in newborns. Acid suppressants have been linked to alterations in infant microbiome colonization, potentially increasing the susceptibility to immunoreactive diseases, such as asthma, atopic dermatitis, and allergic rhinitis. Given that exclusive breastfeeding in the initial 6 months has demonstrated preventive benefits against such diseases, primary physicians play a crucial role in advocating its importance. Although gastric acid suppressants and antibiotics are essential for managing various health conditions, including infections and gastroesophageal reflux disease (GERD), their potential impact on EoE development should not be overlooked.
Though this study had a relatively small sample size, the strong population registry of Denmark significantly reduced recall bias. However, cultural differences and over-the-counter access to drugs, such as acid suppressants, in other countries, including the United States, warrant further research to ascertain their effect on EoE development.
In light of these findings, clinicians should carefully weigh the risks and benefits of prescribing antibiotics and acid suppressants during pregnancy and infancy. Adherence to evidence-based guidelines and considering alternative treatment options, such as lifestyle modifications, should be prioritized. Prescribing antibiotics only when medically necessary and using nonpharmacologic strategies for managing GERD in infants should be considered to mitigate potential risks associated with these medications.
Although there exists a recognized association between IBD and secondary EoE diagnoses, studies focusing on the primary diagnosis of EoE alongside IBD have yielded conflicting results. Dr Amiko Uchida, from the University of Utah Department of Medicine, working with colleagues from the Department of Medical Epidemiology and Biostatistics at Karolinska Institutet in Stockholm, Sweden, led by Dr Jonas F. Ludvigsson, conducted a comprehensive study spanning 1990-2019 to explore the relationship between these two diseases.
Dr Uchida and colleagues assessed the association among Swedish patients diagnosed with biopsy-verified EoE (n = 1587) between 1990 and 2017. These patients were age- and sex-matched with up to five reference individuals from the general population (n = 7808). The primary focus was to discern the relationship between the primary diagnosis of EoE and the subsequent diagnosis of IBD.
The study's findings underscore the importance of heightened awareness among healthcare professionals regarding the potential association between EoE and the development of subsequent IBD. Collaborative efforts between physicians, gastroenterologists, and specialists in allergic diseases are crucial to ensure comprehensive care and timely identification of gastrointestinal complications in patients with EoE.
These results indicate a potential interplay between EoE and the pathogenesis of IBD, particularly Crohn's disease. In patients with EoE, careful consideration of gastrointestinal symptoms suggestive of IBD, such as abdominal pain, diarrhea, and rectal bleeding, is pivotal, necessitating further evaluation and appropriate monitoring for early detection and management of IBD.
Upon diagnosis, clinicians must develop a management plan for this chronic and critical disease. This study aids in planning future screenings for these patients, because one third of those diagnosed with EoE are at risk of developing IBD within a year. Therefore, primary physicians must remain vigilant for the development of gastrointestinal symptoms leading to a diagnosis of Crohn's disease or ulcerative colitis. Additionally, family awareness is crucial owing to observed associations between siblings, suggesting a potential role of genetics or early environmental factors in EoE development and future IBD diagnoses.
Further research is necessary to elucidate the shared pathophysiologic mechanisms connecting EoE and IBD. It is important to consider certain details, however; for instance, 31% of all subsequent IBD cases were diagnosed within the first year after an EoE diagnosis, potentially indicating a role of detection bias in these findings. Using a validated nationwide cohort and comparing study individuals with their siblings helped control for potential intrafamilial confounders as well as some environmental confounders, minimizing such biases as selection bias due to socioeconomic status. This strengthens the observed association in this study.
These insights into the increased risk for IBD, notably Crohn's disease, among patients with EoE underscore the need for thorough clinical evaluation and vigilant monitoring for gastrointestinal complications in this population.
A retrospective study conducted in pediatric patients presenting with an aerodigestive manifestation aimed to assess the factors associated with EoE and the diagnostic role of triple endoscopy. The results suggested a potential association between a family history of eczema and a diet lacking allergenic foods with a future diagnosis of EoE.
This study by Sheila Moran and colleagues, led by Dr Christina J. Yang from the Department of Otorhinolaryngology, Head and Neck Surgery at the Albert Einstein College of Medicine, Bronx, New York, aimed to identify preoperative risk factors linked to an EoE diagnosis in children undergoing triple endoscopy. They evaluated 119 pediatric patients aged 0-21 years who underwent triple endoscopy (including flexible bronchoscopy, rigid direct laryngoscopy and bronchoscopy, and esophagoscopy with biopsy) at the Children's Hospital at Montefiore between January 1, 2015, and December 31, 2019.
The study underscored the significance of both genetic predisposition and dietary influences in EoE development. Understanding the interplay between familial atopic conditions and dietary choices among pediatric patients with aerodigestive dysfunction is crucial for early identification and implementing preventive strategies against EoE.
As clinicians, it's essential to consider rare diseases like EoE when patients present with mixed symptoms, including aerodigestive symptoms, a family history of eczema, and a history of environmental allergies, given the association between these conditions. The potential link between a family history of eczema and increased EoE risk suggests a shared genetic susceptibility among allergic conditions. Therefore, clinicians evaluating children with aerodigestive dysfunction, particularly those with a familial history of eczema, should maintain a high index of suspicion for EoE, prompting vigilant monitoring and appropriate diagnostic assessments.
When contemplating advanced procedures, such as triple endoscopy or biopsy sampling, considering the patient's previous medical history and the effect of dietary modifications, such as incorporating or excluding dairy from the diet, warrants further investigation in the context of EoE prevention. Clinicians should consider providing dietary counseling and personalized nutritional plans based on evidence-based approaches to potentially mitigate EoE risk in susceptible pediatric populations.
Additionally, it's crucial to consider EoE in minority racial groups and underserved communities and encourage the use of diagnostic tests, such as triple endoscopy, to facilitate early diagnosis. Healthcare providers should contemplate integrating family history assessments, particularly regarding eczema, into the evaluation of children with aerodigestive dysfunction. This information can assist in risk assessment and early identification of individuals at a higher risk of developing EoE, enabling prompt intervention and management.
The increasing incidence of EoE across different nations, including the United States, has underscored the need for a deeper understanding of its causes, early diagnosis, and treatment. A novel population-based study conducted in Denmark aimed to explore the relationship between maternal and infant use of antibiotics and acid suppressants in the development of EoE. The study yielded significant results based on a population of 392 cases. Dr Elizabeth T. Jensen, MPH, PhD, from the Department of Epidemiology & Prevention at Wake Forest University School of Medicine, Winston-Salem, North Carolina, conducted a comprehensive study spanning the last 20 years to decipher potential causes contributing to the rising incidence of EoE.
Dr Jensen and her colleagues evaluated the association between maternal and infant use of antibiotics and acid suppressants in Denmark. They used pathology, prescription, birth, inpatient, and outpatient health registry data, ensuring complete ascertainment of all EoE cases among Danish residents born between 1997 and 2018. The research obtained a census of cases from a registered sample of approximately 1.4 million children, matching EoE cases to controls using a 1:10 ratio through incidence density sampling. A total of 392 patients with EoE and 3637 control patients were enrolled. The primary outcome of the study focused on the development of EoE, revealing a dose-response association between maternal and infant antibiotic and acid suppressant use and increased EoE risk.
This study demonstrated a robust correlation between the dosage of antibiotics and acid suppressants and the development of EoE in offspring during childhood. These findings hold significance because these medications represent some of the most common prescriptions in clinical practice. Pregnancy triggers significant physiologic changes in women, including increased hormonal effects and abdominal pressure on the lower esophageal sphincter, making pregnant individuals more prone to esophageal reflux and necessitating the use of gastric acid suppressants. As clinicians, it's crucial to consider lifestyle modifications and dietary adjustments before resorting to acid suppressants, reserving their use for only when absolutely necessary.
Postpregnancy, emphasizing exclusive breastfeeding for the first 6 months and proper feeding techniques can aid in reducing the likelihood of reflux disease in newborns. Acid suppressants have been linked to alterations in infant microbiome colonization, potentially increasing the susceptibility to immunoreactive diseases, such as asthma, atopic dermatitis, and allergic rhinitis. Given that exclusive breastfeeding in the initial 6 months has demonstrated preventive benefits against such diseases, primary physicians play a crucial role in advocating its importance. Although gastric acid suppressants and antibiotics are essential for managing various health conditions, including infections and gastroesophageal reflux disease (GERD), their potential impact on EoE development should not be overlooked.
Though this study had a relatively small sample size, the strong population registry of Denmark significantly reduced recall bias. However, cultural differences and over-the-counter access to drugs, such as acid suppressants, in other countries, including the United States, warrant further research to ascertain their effect on EoE development.
In light of these findings, clinicians should carefully weigh the risks and benefits of prescribing antibiotics and acid suppressants during pregnancy and infancy. Adherence to evidence-based guidelines and considering alternative treatment options, such as lifestyle modifications, should be prioritized. Prescribing antibiotics only when medically necessary and using nonpharmacologic strategies for managing GERD in infants should be considered to mitigate potential risks associated with these medications.
Although there exists a recognized association between IBD and secondary EoE diagnoses, studies focusing on the primary diagnosis of EoE alongside IBD have yielded conflicting results. Dr Amiko Uchida, from the University of Utah Department of Medicine, working with colleagues from the Department of Medical Epidemiology and Biostatistics at Karolinska Institutet in Stockholm, Sweden, led by Dr Jonas F. Ludvigsson, conducted a comprehensive study spanning 1990-2019 to explore the relationship between these two diseases.
Dr Uchida and colleagues assessed the association among Swedish patients diagnosed with biopsy-verified EoE (n = 1587) between 1990 and 2017. These patients were age- and sex-matched with up to five reference individuals from the general population (n = 7808). The primary focus was to discern the relationship between the primary diagnosis of EoE and the subsequent diagnosis of IBD.
The study's findings underscore the importance of heightened awareness among healthcare professionals regarding the potential association between EoE and the development of subsequent IBD. Collaborative efforts between physicians, gastroenterologists, and specialists in allergic diseases are crucial to ensure comprehensive care and timely identification of gastrointestinal complications in patients with EoE.
These results indicate a potential interplay between EoE and the pathogenesis of IBD, particularly Crohn's disease. In patients with EoE, careful consideration of gastrointestinal symptoms suggestive of IBD, such as abdominal pain, diarrhea, and rectal bleeding, is pivotal, necessitating further evaluation and appropriate monitoring for early detection and management of IBD.
Upon diagnosis, clinicians must develop a management plan for this chronic and critical disease. This study aids in planning future screenings for these patients, because one third of those diagnosed with EoE are at risk of developing IBD within a year. Therefore, primary physicians must remain vigilant for the development of gastrointestinal symptoms leading to a diagnosis of Crohn's disease or ulcerative colitis. Additionally, family awareness is crucial owing to observed associations between siblings, suggesting a potential role of genetics or early environmental factors in EoE development and future IBD diagnoses.
Further research is necessary to elucidate the shared pathophysiologic mechanisms connecting EoE and IBD. It is important to consider certain details, however; for instance, 31% of all subsequent IBD cases were diagnosed within the first year after an EoE diagnosis, potentially indicating a role of detection bias in these findings. Using a validated nationwide cohort and comparing study individuals with their siblings helped control for potential intrafamilial confounders as well as some environmental confounders, minimizing such biases as selection bias due to socioeconomic status. This strengthens the observed association in this study.
These insights into the increased risk for IBD, notably Crohn's disease, among patients with EoE underscore the need for thorough clinical evaluation and vigilant monitoring for gastrointestinal complications in this population.
A retrospective study conducted in pediatric patients presenting with an aerodigestive manifestation aimed to assess the factors associated with EoE and the diagnostic role of triple endoscopy. The results suggested a potential association between a family history of eczema and a diet lacking allergenic foods with a future diagnosis of EoE.
This study by Sheila Moran and colleagues, led by Dr Christina J. Yang from the Department of Otorhinolaryngology, Head and Neck Surgery at the Albert Einstein College of Medicine, Bronx, New York, aimed to identify preoperative risk factors linked to an EoE diagnosis in children undergoing triple endoscopy. They evaluated 119 pediatric patients aged 0-21 years who underwent triple endoscopy (including flexible bronchoscopy, rigid direct laryngoscopy and bronchoscopy, and esophagoscopy with biopsy) at the Children's Hospital at Montefiore between January 1, 2015, and December 31, 2019.
The study underscored the significance of both genetic predisposition and dietary influences in EoE development. Understanding the interplay between familial atopic conditions and dietary choices among pediatric patients with aerodigestive dysfunction is crucial for early identification and implementing preventive strategies against EoE.
As clinicians, it's essential to consider rare diseases like EoE when patients present with mixed symptoms, including aerodigestive symptoms, a family history of eczema, and a history of environmental allergies, given the association between these conditions. The potential link between a family history of eczema and increased EoE risk suggests a shared genetic susceptibility among allergic conditions. Therefore, clinicians evaluating children with aerodigestive dysfunction, particularly those with a familial history of eczema, should maintain a high index of suspicion for EoE, prompting vigilant monitoring and appropriate diagnostic assessments.
When contemplating advanced procedures, such as triple endoscopy or biopsy sampling, considering the patient's previous medical history and the effect of dietary modifications, such as incorporating or excluding dairy from the diet, warrants further investigation in the context of EoE prevention. Clinicians should consider providing dietary counseling and personalized nutritional plans based on evidence-based approaches to potentially mitigate EoE risk in susceptible pediatric populations.
Additionally, it's crucial to consider EoE in minority racial groups and underserved communities and encourage the use of diagnostic tests, such as triple endoscopy, to facilitate early diagnosis. Healthcare providers should contemplate integrating family history assessments, particularly regarding eczema, into the evaluation of children with aerodigestive dysfunction. This information can assist in risk assessment and early identification of individuals at a higher risk of developing EoE, enabling prompt intervention and management.
The increasing incidence of EoE across different nations, including the United States, has underscored the need for a deeper understanding of its causes, early diagnosis, and treatment. A novel population-based study conducted in Denmark aimed to explore the relationship between maternal and infant use of antibiotics and acid suppressants in the development of EoE. The study yielded significant results based on a population of 392 cases. Dr Elizabeth T. Jensen, MPH, PhD, from the Department of Epidemiology & Prevention at Wake Forest University School of Medicine, Winston-Salem, North Carolina, conducted a comprehensive study spanning the last 20 years to decipher potential causes contributing to the rising incidence of EoE.
Dr Jensen and her colleagues evaluated the association between maternal and infant use of antibiotics and acid suppressants in Denmark. They used pathology, prescription, birth, inpatient, and outpatient health registry data, ensuring complete ascertainment of all EoE cases among Danish residents born between 1997 and 2018. The research obtained a census of cases from a registered sample of approximately 1.4 million children, matching EoE cases to controls using a 1:10 ratio through incidence density sampling. A total of 392 patients with EoE and 3637 control patients were enrolled. The primary outcome of the study focused on the development of EoE, revealing a dose-response association between maternal and infant antibiotic and acid suppressant use and increased EoE risk.
This study demonstrated a robust correlation between the dosage of antibiotics and acid suppressants and the development of EoE in offspring during childhood. These findings hold significance because these medications represent some of the most common prescriptions in clinical practice. Pregnancy triggers significant physiologic changes in women, including increased hormonal effects and abdominal pressure on the lower esophageal sphincter, making pregnant individuals more prone to esophageal reflux and necessitating the use of gastric acid suppressants. As clinicians, it's crucial to consider lifestyle modifications and dietary adjustments before resorting to acid suppressants, reserving their use for only when absolutely necessary.
Postpregnancy, emphasizing exclusive breastfeeding for the first 6 months and proper feeding techniques can aid in reducing the likelihood of reflux disease in newborns. Acid suppressants have been linked to alterations in infant microbiome colonization, potentially increasing the susceptibility to immunoreactive diseases, such as asthma, atopic dermatitis, and allergic rhinitis. Given that exclusive breastfeeding in the initial 6 months has demonstrated preventive benefits against such diseases, primary physicians play a crucial role in advocating its importance. Although gastric acid suppressants and antibiotics are essential for managing various health conditions, including infections and gastroesophageal reflux disease (GERD), their potential impact on EoE development should not be overlooked.
Though this study had a relatively small sample size, the strong population registry of Denmark significantly reduced recall bias. However, cultural differences and over-the-counter access to drugs, such as acid suppressants, in other countries, including the United States, warrant further research to ascertain their effect on EoE development.
In light of these findings, clinicians should carefully weigh the risks and benefits of prescribing antibiotics and acid suppressants during pregnancy and infancy. Adherence to evidence-based guidelines and considering alternative treatment options, such as lifestyle modifications, should be prioritized. Prescribing antibiotics only when medically necessary and using nonpharmacologic strategies for managing GERD in infants should be considered to mitigate potential risks associated with these medications.
Commentary: Fertility Concerns and Treatment-Related QOL After Breast Cancer, January 2024
Young women diagnosed with breast cancer have been shown to experience higher rates of symptoms that may adversely affect quality of life (QOL), including depression, weight gain, vasomotor symptoms, and sexual dysfunction; they may also have a harder time managing these issues.3 Chemotherapy-related amenorrhea (CRA) is one of the side effects of breast cancer treatment that can affect premenopausal women, and is associated with both patient- (age, body mass index) and treatment-related (regimen, duration) factors.4 A study analyzing data derived from the prospective, longitudinal Cancer Toxicities Study included 1636 premenopausal women ≤ 50 years of age with stage I-III breast cancer treated with chemotherapy but not receiving ovarian suppression (Kabirian et al). A total of 83.0% of women reported CRA at year 1, 72.5% at year 2, and 66.1% at year 4. A higher likelihood of CRA was observed for women of older age vs those age 18-34 years (adjusted odds ratio [aOR] for 35-39 years 1.84; 40-44 years 5.90; and ≥ 45 years 21.29; P < .001 for all), those who received adjuvant tamoxifen (aOR 1.97; P < .001), and those who had hot flashes at baseline (aOR 1.83; P = .01). In the QOL analysis, 57.1% reported no recovery of menses. Persistent CRA was associated with worse insomnia, more systemic therapy–related adverse effects, and worse sexual functioning. These findings highlight the importance of identifying and discussing CRA with our patients, as this can have both physical and psychological effects in the survivorship setting.
The phase 3 KEYNOTE-522 trial has established immunotherapy plus an anthracycline-based chemotherapy backbone for the treatment of stage II-III triple-negative breast cancer (TNBC), with improvements in pathologic complete response (pCR) rates and survival outcomes.5 This regimen can present tolerance issues in clinical practice, and rare risks for cardiotoxicity and secondary hematologic malignancies are also relevant to consider. Furthermore, some patients may not be candidates for anthracycline-based treatment due to prior receipt of a drug in this class or cardiac comorbidities. De-escalation strategies are desired to lessen toxicity and maintain (or improve) outcomes. An open-label phase 2 trial (NeoPACT) investigated the efficacy of neoadjuvant carboplatin (AUC 6), docetaxel (75 mg/m2), and pembrolizumab (200 mg) every 21 days for six cycles among 115 patients with stage I-III TNBC (Sharma et al). The overall pCR and residual cancer burden (RCB 0+1) rates were 58% (95% CI 48%-67%) and 69% (95% CI 60%-78%), respectively. Estimated 3-year event-free survival was 86% (95% CI 77%-95%) in all patients, 98% in those with a pCR, and 68% in those with residual disease. This study also demonstrated a positive association of immune biomarkers and pathologic response. The most common grade ≥ 3 treatment-related adverse events were diarrhea (4.3%), anemia (3.5%), and peripheral sensory neuropathy (2.6%). The phase 3 SCARLET (Shorter Anthracycline-Free Chemoimmunotherapy Adapted to Pathologic Response in Early TNBC) trial is comparing the NeoPACT regimen with the standard KEYNOTE-522 regimen in early-stage TNBC and will be critical to further defining this treatment space.6 Presently, considering the described efficacy outcomes with the NeoPACT regimen, this regimen would be very reasonable to consider in patients who are not candidates for an anthracycline. Future prospective evaluation of immune biomarkers and additional predictors of response will also be valuable to further individualize treatment for our patients.
Additional References
- Lambertini M, Blondeaux E, Bruzzone M, et al. Pregnancy after breast cancer: A systematic review and meta-analysis. J Clin Oncol. 2021;39:3293-3305. doi: 10.1200/JCO.21.00535
- Partridge AH, Niman SM, Ruggeri M, et al, for the International Breast Cancer Study Group and POSITIVE Trial Collaborators. Interrupting endocrine therapy to attempt pregnancy after breast cancer. N Engl J Med. 2023;388:1645-1656. doi: 10.1056/NEJMoa2212856
- Howard-Anderson J, Ganz PA, Bower JE, Stanton AL. Quality of life, fertility concerns, and behavioral health outcomes in younger breast cancer survivors: A systematic review. J Natl Cancer Inst. 2012;104:386-405. doi: 10.1093/jnci/djr541
- Turnbull AK, Patel S, Martinez-Perez C, et al. Risk of chemotherapy-related amenorrhoea (CRA) in premenopausal women undergoing chemotherapy for early stage breast cancer. Breast Cancer Res Treat. 2021;186:237-245. doi: 10.1007/s10549-020-05951-5
- Schmid P, Cortes J, Dent R, et al; KEYNOTE-522 Investigators. Event-free survival with pembrolizumab in early triple-negative breast cancer. N Engl J Med. 2022;386:556-567. doi: 10.1056/NEJMoa2112651
- US National Cancer Institute, Cancer Therapy Evaluation Program. Shorter anthracycline-free chemoimmunotherapy adapted to pathological response in early TNBC (SCARLET); SWOG S2212. Source
Young women diagnosed with breast cancer have been shown to experience higher rates of symptoms that may adversely affect quality of life (QOL), including depression, weight gain, vasomotor symptoms, and sexual dysfunction; they may also have a harder time managing these issues.3 Chemotherapy-related amenorrhea (CRA) is one of the side effects of breast cancer treatment that can affect premenopausal women, and is associated with both patient- (age, body mass index) and treatment-related (regimen, duration) factors.4 A study analyzing data derived from the prospective, longitudinal Cancer Toxicities Study included 1636 premenopausal women ≤ 50 years of age with stage I-III breast cancer treated with chemotherapy but not receiving ovarian suppression (Kabirian et al). A total of 83.0% of women reported CRA at year 1, 72.5% at year 2, and 66.1% at year 4. A higher likelihood of CRA was observed for women of older age vs those age 18-34 years (adjusted odds ratio [aOR] for 35-39 years 1.84; 40-44 years 5.90; and ≥ 45 years 21.29; P < .001 for all), those who received adjuvant tamoxifen (aOR 1.97; P < .001), and those who had hot flashes at baseline (aOR 1.83; P = .01). In the QOL analysis, 57.1% reported no recovery of menses. Persistent CRA was associated with worse insomnia, more systemic therapy–related adverse effects, and worse sexual functioning. These findings highlight the importance of identifying and discussing CRA with our patients, as this can have both physical and psychological effects in the survivorship setting.
The phase 3 KEYNOTE-522 trial has established immunotherapy plus an anthracycline-based chemotherapy backbone for the treatment of stage II-III triple-negative breast cancer (TNBC), with improvements in pathologic complete response (pCR) rates and survival outcomes.5 This regimen can present tolerance issues in clinical practice, and rare risks for cardiotoxicity and secondary hematologic malignancies are also relevant to consider. Furthermore, some patients may not be candidates for anthracycline-based treatment due to prior receipt of a drug in this class or cardiac comorbidities. De-escalation strategies are desired to lessen toxicity and maintain (or improve) outcomes. An open-label phase 2 trial (NeoPACT) investigated the efficacy of neoadjuvant carboplatin (AUC 6), docetaxel (75 mg/m2), and pembrolizumab (200 mg) every 21 days for six cycles among 115 patients with stage I-III TNBC (Sharma et al). The overall pCR and residual cancer burden (RCB 0+1) rates were 58% (95% CI 48%-67%) and 69% (95% CI 60%-78%), respectively. Estimated 3-year event-free survival was 86% (95% CI 77%-95%) in all patients, 98% in those with a pCR, and 68% in those with residual disease. This study also demonstrated a positive association of immune biomarkers and pathologic response. The most common grade ≥ 3 treatment-related adverse events were diarrhea (4.3%), anemia (3.5%), and peripheral sensory neuropathy (2.6%). The phase 3 SCARLET (Shorter Anthracycline-Free Chemoimmunotherapy Adapted to Pathologic Response in Early TNBC) trial is comparing the NeoPACT regimen with the standard KEYNOTE-522 regimen in early-stage TNBC and will be critical to further defining this treatment space.6 Presently, considering the described efficacy outcomes with the NeoPACT regimen, this regimen would be very reasonable to consider in patients who are not candidates for an anthracycline. Future prospective evaluation of immune biomarkers and additional predictors of response will also be valuable to further individualize treatment for our patients.
Additional References
- Lambertini M, Blondeaux E, Bruzzone M, et al. Pregnancy after breast cancer: A systematic review and meta-analysis. J Clin Oncol. 2021;39:3293-3305. doi: 10.1200/JCO.21.00535
- Partridge AH, Niman SM, Ruggeri M, et al, for the International Breast Cancer Study Group and POSITIVE Trial Collaborators. Interrupting endocrine therapy to attempt pregnancy after breast cancer. N Engl J Med. 2023;388:1645-1656. doi: 10.1056/NEJMoa2212856
- Howard-Anderson J, Ganz PA, Bower JE, Stanton AL. Quality of life, fertility concerns, and behavioral health outcomes in younger breast cancer survivors: A systematic review. J Natl Cancer Inst. 2012;104:386-405. doi: 10.1093/jnci/djr541
- Turnbull AK, Patel S, Martinez-Perez C, et al. Risk of chemotherapy-related amenorrhoea (CRA) in premenopausal women undergoing chemotherapy for early stage breast cancer. Breast Cancer Res Treat. 2021;186:237-245. doi: 10.1007/s10549-020-05951-5
- Schmid P, Cortes J, Dent R, et al; KEYNOTE-522 Investigators. Event-free survival with pembrolizumab in early triple-negative breast cancer. N Engl J Med. 2022;386:556-567. doi: 10.1056/NEJMoa2112651
- US National Cancer Institute, Cancer Therapy Evaluation Program. Shorter anthracycline-free chemoimmunotherapy adapted to pathological response in early TNBC (SCARLET); SWOG S2212. Source
Young women diagnosed with breast cancer have been shown to experience higher rates of symptoms that may adversely affect quality of life (QOL), including depression, weight gain, vasomotor symptoms, and sexual dysfunction; they may also have a harder time managing these issues.3 Chemotherapy-related amenorrhea (CRA) is one of the side effects of breast cancer treatment that can affect premenopausal women, and is associated with both patient- (age, body mass index) and treatment-related (regimen, duration) factors.4 A study analyzing data derived from the prospective, longitudinal Cancer Toxicities Study included 1636 premenopausal women ≤ 50 years of age with stage I-III breast cancer treated with chemotherapy but not receiving ovarian suppression (Kabirian et al). A total of 83.0% of women reported CRA at year 1, 72.5% at year 2, and 66.1% at year 4. A higher likelihood of CRA was observed for women of older age vs those age 18-34 years (adjusted odds ratio [aOR] for 35-39 years 1.84; 40-44 years 5.90; and ≥ 45 years 21.29; P < .001 for all), those who received adjuvant tamoxifen (aOR 1.97; P < .001), and those who had hot flashes at baseline (aOR 1.83; P = .01). In the QOL analysis, 57.1% reported no recovery of menses. Persistent CRA was associated with worse insomnia, more systemic therapy–related adverse effects, and worse sexual functioning. These findings highlight the importance of identifying and discussing CRA with our patients, as this can have both physical and psychological effects in the survivorship setting.
The phase 3 KEYNOTE-522 trial has established immunotherapy plus an anthracycline-based chemotherapy backbone for the treatment of stage II-III triple-negative breast cancer (TNBC), with improvements in pathologic complete response (pCR) rates and survival outcomes.5 This regimen can present tolerance issues in clinical practice, and rare risks for cardiotoxicity and secondary hematologic malignancies are also relevant to consider. Furthermore, some patients may not be candidates for anthracycline-based treatment due to prior receipt of a drug in this class or cardiac comorbidities. De-escalation strategies are desired to lessen toxicity and maintain (or improve) outcomes. An open-label phase 2 trial (NeoPACT) investigated the efficacy of neoadjuvant carboplatin (AUC 6), docetaxel (75 mg/m2), and pembrolizumab (200 mg) every 21 days for six cycles among 115 patients with stage I-III TNBC (Sharma et al). The overall pCR and residual cancer burden (RCB 0+1) rates were 58% (95% CI 48%-67%) and 69% (95% CI 60%-78%), respectively. Estimated 3-year event-free survival was 86% (95% CI 77%-95%) in all patients, 98% in those with a pCR, and 68% in those with residual disease. This study also demonstrated a positive association of immune biomarkers and pathologic response. The most common grade ≥ 3 treatment-related adverse events were diarrhea (4.3%), anemia (3.5%), and peripheral sensory neuropathy (2.6%). The phase 3 SCARLET (Shorter Anthracycline-Free Chemoimmunotherapy Adapted to Pathologic Response in Early TNBC) trial is comparing the NeoPACT regimen with the standard KEYNOTE-522 regimen in early-stage TNBC and will be critical to further defining this treatment space.6 Presently, considering the described efficacy outcomes with the NeoPACT regimen, this regimen would be very reasonable to consider in patients who are not candidates for an anthracycline. Future prospective evaluation of immune biomarkers and additional predictors of response will also be valuable to further individualize treatment for our patients.
Additional References
- Lambertini M, Blondeaux E, Bruzzone M, et al. Pregnancy after breast cancer: A systematic review and meta-analysis. J Clin Oncol. 2021;39:3293-3305. doi: 10.1200/JCO.21.00535
- Partridge AH, Niman SM, Ruggeri M, et al, for the International Breast Cancer Study Group and POSITIVE Trial Collaborators. Interrupting endocrine therapy to attempt pregnancy after breast cancer. N Engl J Med. 2023;388:1645-1656. doi: 10.1056/NEJMoa2212856
- Howard-Anderson J, Ganz PA, Bower JE, Stanton AL. Quality of life, fertility concerns, and behavioral health outcomes in younger breast cancer survivors: A systematic review. J Natl Cancer Inst. 2012;104:386-405. doi: 10.1093/jnci/djr541
- Turnbull AK, Patel S, Martinez-Perez C, et al. Risk of chemotherapy-related amenorrhoea (CRA) in premenopausal women undergoing chemotherapy for early stage breast cancer. Breast Cancer Res Treat. 2021;186:237-245. doi: 10.1007/s10549-020-05951-5
- Schmid P, Cortes J, Dent R, et al; KEYNOTE-522 Investigators. Event-free survival with pembrolizumab in early triple-negative breast cancer. N Engl J Med. 2022;386:556-567. doi: 10.1056/NEJMoa2112651
- US National Cancer Institute, Cancer Therapy Evaluation Program. Shorter anthracycline-free chemoimmunotherapy adapted to pathological response in early TNBC (SCARLET); SWOG S2212. Source
Commentary: Variations in DMARD Effectiveness and Enthesitis Treatment in PsA, January 2024
Treatment of enthesitis can be challenging. Head-to-head clinical trials using clinical enthesitis indices have indicated that TNF inhibitors and IL-17 inhibitors have similar efficacy in treating enthesitis. However, clinically determined enthesitis may not be true inflammatory enthesitis. Ultrasonography-confirmed enthesitis probably reflects true enthesitis. Therefore, Elliot and colleagues conducted an observational study that compared the change in MAdrid Sonographic Enthesitis Index (MASEI) at 16 weeks of treatment with either TNF inhibitors or secukinumab. They observed that the mean reduction in MASEI that assesses both active and chronic entheseal disease was not significantly different with TNF inhibitors vs secukinumab treatment. However, TNF inhibitors were significantly more effective than secukinumab when only active entheseal lesions were considered. Thus, TNF inhibitors may be more effective for active enthesitis; randomized trials using ultrasonographic enthesitis indices comparing the two treatments are required.
Serum drug levels have previously been shown to be associated with response to bDMARD therapy, but use of drug-level measurement is not routine in rheumatology practice. Moreover, trough levels are emphasized and may not often be feasible to obtain. Curry and colleagues investigated the relationship between serum non-trough drug levels (SDL) and treatment response at 3 months in patients with PsA who initiated treatment with adalimumab (n = 104) or etanercept (n = 97). They demonstrated that patients with higher etanercept SDL or higher adalimumab SDL were significantly more likely to be responders. A non-trough etanercept SDL of 2.0 µg/mL and adalimumab SDL of 3.6 µg/mL could differentiate between responders and nonresponders with ~50% specificity and > 60% sensitivity. However, the area under the receiver operating characteristic curves were only about 65%; thus, the ability of SDL to discriminate between responders and nonresponders is low.
Treatment of enthesitis can be challenging. Head-to-head clinical trials using clinical enthesitis indices have indicated that TNF inhibitors and IL-17 inhibitors have similar efficacy in treating enthesitis. However, clinically determined enthesitis may not be true inflammatory enthesitis. Ultrasonography-confirmed enthesitis probably reflects true enthesitis. Therefore, Elliot and colleagues conducted an observational study that compared the change in MAdrid Sonographic Enthesitis Index (MASEI) at 16 weeks of treatment with either TNF inhibitors or secukinumab. They observed that the mean reduction in MASEI that assesses both active and chronic entheseal disease was not significantly different with TNF inhibitors vs secukinumab treatment. However, TNF inhibitors were significantly more effective than secukinumab when only active entheseal lesions were considered. Thus, TNF inhibitors may be more effective for active enthesitis; randomized trials using ultrasonographic enthesitis indices comparing the two treatments are required.
Serum drug levels have previously been shown to be associated with response to bDMARD therapy, but use of drug-level measurement is not routine in rheumatology practice. Moreover, trough levels are emphasized and may not often be feasible to obtain. Curry and colleagues investigated the relationship between serum non-trough drug levels (SDL) and treatment response at 3 months in patients with PsA who initiated treatment with adalimumab (n = 104) or etanercept (n = 97). They demonstrated that patients with higher etanercept SDL or higher adalimumab SDL were significantly more likely to be responders. A non-trough etanercept SDL of 2.0 µg/mL and adalimumab SDL of 3.6 µg/mL could differentiate between responders and nonresponders with ~50% specificity and > 60% sensitivity. However, the area under the receiver operating characteristic curves were only about 65%; thus, the ability of SDL to discriminate between responders and nonresponders is low.
Treatment of enthesitis can be challenging. Head-to-head clinical trials using clinical enthesitis indices have indicated that TNF inhibitors and IL-17 inhibitors have similar efficacy in treating enthesitis. However, clinically determined enthesitis may not be true inflammatory enthesitis. Ultrasonography-confirmed enthesitis probably reflects true enthesitis. Therefore, Elliot and colleagues conducted an observational study that compared the change in MAdrid Sonographic Enthesitis Index (MASEI) at 16 weeks of treatment with either TNF inhibitors or secukinumab. They observed that the mean reduction in MASEI that assesses both active and chronic entheseal disease was not significantly different with TNF inhibitors vs secukinumab treatment. However, TNF inhibitors were significantly more effective than secukinumab when only active entheseal lesions were considered. Thus, TNF inhibitors may be more effective for active enthesitis; randomized trials using ultrasonographic enthesitis indices comparing the two treatments are required.
Serum drug levels have previously been shown to be associated with response to bDMARD therapy, but use of drug-level measurement is not routine in rheumatology practice. Moreover, trough levels are emphasized and may not often be feasible to obtain. Curry and colleagues investigated the relationship between serum non-trough drug levels (SDL) and treatment response at 3 months in patients with PsA who initiated treatment with adalimumab (n = 104) or etanercept (n = 97). They demonstrated that patients with higher etanercept SDL or higher adalimumab SDL were significantly more likely to be responders. A non-trough etanercept SDL of 2.0 µg/mL and adalimumab SDL of 3.6 µg/mL could differentiate between responders and nonresponders with ~50% specificity and > 60% sensitivity. However, the area under the receiver operating characteristic curves were only about 65%; thus, the ability of SDL to discriminate between responders and nonresponders is low.
Commentary: Examining CGRP Antagonists for Migraine Relief, January 2024
The recent 3-month double-blind study by Schwedt and colleagues included 580 participants and compared the effects of galcanezumab (Emgality) with those of rimegepant (Nurtec ODT). These medications are administered by different methods when used for migraine prevention; galcanezumab is given subcutaneously (SC) every month, whereas rimegepant is taken by mouth every other day. To blind the study, participants were randomly assigned to receive either 120 mg galcanezumab SC per month (after a 240 mg loading dose) and a placebo oral disintegrating tablet every other day or every-other-day 75 mg rimegepant as oral disintegrating tablets and a monthly SC placebo. According to the study authors, 62% of the patients receiving galcanezumab vs 61% of those receiving rimegepant achieved ≥ 50% reduction in monthly migraine headache days after 3 months, with no statistically significant difference between the groups. Comparisons between CGRP receptor antagonists are scarce. The studies tend to be of a short duration and to include small sample sizes — and most are retrospective. To date, physicians who treat patients with these drugs do not have information about the distinguishing characteristics between these treatments that could be used to guide drug selection for subtypes of migraine or different patient populations. As further research emerges, we may see distinctions between these therapies, or we might continue to see that their effects are similar in terms of benefits, duration of action, and patient characteristics.
Many patients who are prescribed these new medications have already been treated with a variety of other previously available migraine therapies, with varying degrees of improvement. Physicians who prescribe treatments for migraine patients often move on to new therapeutic options when patients only experience partial relief, but recent research suggests that even these incomplete responses could be beneficial for patients.
Researchers at the Headache Centre — Neurology Clinic at the Spedali Civili di Brescia in Brescia, Italy, conducted a retrospective study to examine whether previous treatment with onabotulinumtoxinA affected patent response to anti-CGRP mAb (Ceccardi et al). These treatments have differing mechanisms. OnabotulinumtoxinA is an exotoxin produced by Clostridium botulinum that blocks the acetylcholine release from nerve endings temporarily disabling postsynaptic action. Anti-CGRP mAb work by inhibiting the inflammatory receptor, thereby inhibiting the pain sensation.2
Several studies have examined the effects of combining onabotulinumtoxinA (Botox) with anti-CGRP mAb, with varying results. For example, researchers of a study designed to compare the two treatments concluded, "In patients with chronic migraine who have only had a partial response to Botox, adjunctive preventative therapy with a CGRP-mAb drug is safe and effective."2 A review examining several small studies that evaluated the response of dual therapy included a few studies that found no significant differences between an anti-CGRP mAb monotherapy and dual therapy with onabotulinumtoxinA, as well as some studies that noted improvement with dual therapy over either therapy alone. The review authors concluded that a real-life application is not yet determined and that "Further sufficiently powered, placebo-controlled studies are warranted to shed light on potential additive or synergistic effects of combining onabotulinumtoxin A with a CGRP antagonist."3
The Brescia study was designed to examine the effect of previous onabotulinumtoxinA treatment on subsequent anti-CGRP mAb response. The researchers enrolled 128 patients, of whom 51 (39.9%) had previously been treated with onabotulinumtoxinA, with the last dose 3 months before preventive treatment with an anti-CGRP mAb was started. The study was conducted between November 2018 and May 2023. The outcomes noted included monthly headache days, monthly migraine days, mean analgesic consumption, and clinical disability according to the Migraine Disability Assessment test (MIDAS). Participants received 3 months of treatment with an anti-CGRP mAb.
In addition to comparing patients who had previously received onabotulinumtoxinA with those who did not, the researchers also "aimed to evaluate whether the clinical response to anti-CGRP mAb was affected by the number of previous Onabotulinumtoxin-A administrations.
The documented baseline prior to treatment with an anti-CGRP mAb was as follows: mean monthly headache days 23.7 (SD 5.7), monthly migraine days 13.9 (SD 8.0); mean MIDAS score 108.9 (SD 76.1); and mean analgesic consumption 24.8 (SD 18.8). After 3 months of treatment with an anti-CGRP mAb, both groups experienced significant improvement in all these parameters. Furthermore, after 3 months of treatment with an anti-CGRP mAb, the patients who received at least three onabotulinumtoxinA administrations prior to the study experienced lower MMD compared with those who had received fewer cycles.
For physicians and patients, this outcome provides validation that patients can potentially gain long-term benefits from migraine treatment, even if such interventions do not provide sufficient migraine relief. The conclusion cannot be generalized to other migraine treatment sequences, and the authors did not suggest deliberately postponing any treatment or using any treatment as "priming" for another treatment. Yet physicians may be able to give patients some reassurance that an incomplete response in migraine therapy is not futile.
Migraine treatment can be very effective, but sometimes it is not clear whether patients should take their medication before or during a migraine episode, or whether the signal to take medication should be based on specific symptoms. Many patients wait to take their migraine treatment until they are sure that they will have a migraine, especially if they frequently have prodromal symptoms that do not consistently lead to a migraine. Additionally, some of the new CGRP receptor antagonists are expensive, and many payers only approve a limited amount per month. Patients might not want to waste their CGRP receptor antagonist supply in case they run out before their next refill authorization.
AbbVie, the makers of ubrogepant (Ubrelvy), a CGRP receptor antagonist approved for acute treatment of migraine, conducted a phase 3, multicenter, randomized, double-blind, placebo-controlled, crossover trial of ubrogepant at 75 research centers and headache clinics in the US (Dodick et al). According to the manufacturer, the aim of the trial was to evaluate the efficacy, safety, and tolerability of 100 mg ubrogepant for the acute treatment of migraine when administered during the prodrome of a migraine attack. The study included 518 participants age 18-75 years who had at least a 1-year history of migraine and had had two to eight migraine attacks per month that included symptoms of a moderate to severe headache in each of the 3 months before the study. Because this was a crossover trial, the participants were randomly assigned to either receive placebo for treatment of the first qualifying prodrome event and 100 mg ubrogepant for treatment of the second qualifying prodrome event or to receive 100 mg ubrogepant to treat the first qualifying prodrome event and placebo to treat the second qualifying prodrome event.
According to AbbVie's news release following publication of the study, "Absence of moderate or severe intensity headache within 24 hours was achieved following 46% of qualifying prodrome events when treated with UBRELVY vs 29% of placebo-treated events" and "absence of moderate or severe intensity headache within 48 hours was achieved following 41% of qualifying prodrome events when treated with UBRELVY vs 25% of placebo-treated events" (both P < .0001).4 Safety and tolerability of treatment during the prodromal period were also established.
In clinical practice, these results hold promise because patients can gain some assurance in knowing that taking their migraine treatment during their early prodromal symptoms is safe and could potentially improve the outcome of the event, preventing migraine symptoms for 48 hours. Even for patients who do not have an ample supply of ubrogepant or another CGRP antagonist, taking a treatment that is approved by their doctor at the onset of prodromal symptoms can provide relief compared with waiting until symptoms worsen.
Additional References
1. Waliszewska-Prosół M, Vuralli D, Martelletti P. What to do with non-responders to CGRP(r) monoclonal antibodies: Switch to another or move to gepants? J Headache Pain. 2023;24:163. doi: 10.1186/s10194-023-01698-8
2. Pallapothu MR, Quintana Mariñez MG, Chakkera M, et al. Long-term management of migraine with OnabotulinumtoxinA (Botox) vs calcitonin gene-related peptide antibodies (Anti-CGRP). Cureus. 2023;15:e46696. doi: 10.7759/cureus.46696
3. Pellesi L. Combining onabotulinumtoxin A with a CGRP antagonist for chronic migraine prophylaxis: Where do we stand? Front Pain Res (Lausanne). 2023;4:1292994. doi: 10.3389/fpain.2023.1292994
4. AbbVie. Results published in The Lancet show UBRELVY® (ubrogepant) reduces the headache phase of a migraine attack when dosed during the prodrome of migraine. November 16, 2023. Source
The recent 3-month double-blind study by Schwedt and colleagues included 580 participants and compared the effects of galcanezumab (Emgality) with those of rimegepant (Nurtec ODT). These medications are administered by different methods when used for migraine prevention; galcanezumab is given subcutaneously (SC) every month, whereas rimegepant is taken by mouth every other day. To blind the study, participants were randomly assigned to receive either 120 mg galcanezumab SC per month (after a 240 mg loading dose) and a placebo oral disintegrating tablet every other day or every-other-day 75 mg rimegepant as oral disintegrating tablets and a monthly SC placebo. According to the study authors, 62% of the patients receiving galcanezumab vs 61% of those receiving rimegepant achieved ≥ 50% reduction in monthly migraine headache days after 3 months, with no statistically significant difference between the groups. Comparisons between CGRP receptor antagonists are scarce. The studies tend to be of a short duration and to include small sample sizes — and most are retrospective. To date, physicians who treat patients with these drugs do not have information about the distinguishing characteristics between these treatments that could be used to guide drug selection for subtypes of migraine or different patient populations. As further research emerges, we may see distinctions between these therapies, or we might continue to see that their effects are similar in terms of benefits, duration of action, and patient characteristics.
Many patients who are prescribed these new medications have already been treated with a variety of other previously available migraine therapies, with varying degrees of improvement. Physicians who prescribe treatments for migraine patients often move on to new therapeutic options when patients only experience partial relief, but recent research suggests that even these incomplete responses could be beneficial for patients.
Researchers at the Headache Centre — Neurology Clinic at the Spedali Civili di Brescia in Brescia, Italy, conducted a retrospective study to examine whether previous treatment with onabotulinumtoxinA affected patent response to anti-CGRP mAb (Ceccardi et al). These treatments have differing mechanisms. OnabotulinumtoxinA is an exotoxin produced by Clostridium botulinum that blocks the acetylcholine release from nerve endings temporarily disabling postsynaptic action. Anti-CGRP mAb work by inhibiting the inflammatory receptor, thereby inhibiting the pain sensation.2
Several studies have examined the effects of combining onabotulinumtoxinA (Botox) with anti-CGRP mAb, with varying results. For example, researchers of a study designed to compare the two treatments concluded, "In patients with chronic migraine who have only had a partial response to Botox, adjunctive preventative therapy with a CGRP-mAb drug is safe and effective."2 A review examining several small studies that evaluated the response of dual therapy included a few studies that found no significant differences between an anti-CGRP mAb monotherapy and dual therapy with onabotulinumtoxinA, as well as some studies that noted improvement with dual therapy over either therapy alone. The review authors concluded that a real-life application is not yet determined and that "Further sufficiently powered, placebo-controlled studies are warranted to shed light on potential additive or synergistic effects of combining onabotulinumtoxin A with a CGRP antagonist."3
The Brescia study was designed to examine the effect of previous onabotulinumtoxinA treatment on subsequent anti-CGRP mAb response. The researchers enrolled 128 patients, of whom 51 (39.9%) had previously been treated with onabotulinumtoxinA, with the last dose 3 months before preventive treatment with an anti-CGRP mAb was started. The study was conducted between November 2018 and May 2023. The outcomes noted included monthly headache days, monthly migraine days, mean analgesic consumption, and clinical disability according to the Migraine Disability Assessment test (MIDAS). Participants received 3 months of treatment with an anti-CGRP mAb.
In addition to comparing patients who had previously received onabotulinumtoxinA with those who did not, the researchers also "aimed to evaluate whether the clinical response to anti-CGRP mAb was affected by the number of previous Onabotulinumtoxin-A administrations.
The documented baseline prior to treatment with an anti-CGRP mAb was as follows: mean monthly headache days 23.7 (SD 5.7), monthly migraine days 13.9 (SD 8.0); mean MIDAS score 108.9 (SD 76.1); and mean analgesic consumption 24.8 (SD 18.8). After 3 months of treatment with an anti-CGRP mAb, both groups experienced significant improvement in all these parameters. Furthermore, after 3 months of treatment with an anti-CGRP mAb, the patients who received at least three onabotulinumtoxinA administrations prior to the study experienced lower MMD compared with those who had received fewer cycles.
For physicians and patients, this outcome provides validation that patients can potentially gain long-term benefits from migraine treatment, even if such interventions do not provide sufficient migraine relief. The conclusion cannot be generalized to other migraine treatment sequences, and the authors did not suggest deliberately postponing any treatment or using any treatment as "priming" for another treatment. Yet physicians may be able to give patients some reassurance that an incomplete response in migraine therapy is not futile.
Migraine treatment can be very effective, but sometimes it is not clear whether patients should take their medication before or during a migraine episode, or whether the signal to take medication should be based on specific symptoms. Many patients wait to take their migraine treatment until they are sure that they will have a migraine, especially if they frequently have prodromal symptoms that do not consistently lead to a migraine. Additionally, some of the new CGRP receptor antagonists are expensive, and many payers only approve a limited amount per month. Patients might not want to waste their CGRP receptor antagonist supply in case they run out before their next refill authorization.
AbbVie, the makers of ubrogepant (Ubrelvy), a CGRP receptor antagonist approved for acute treatment of migraine, conducted a phase 3, multicenter, randomized, double-blind, placebo-controlled, crossover trial of ubrogepant at 75 research centers and headache clinics in the US (Dodick et al). According to the manufacturer, the aim of the trial was to evaluate the efficacy, safety, and tolerability of 100 mg ubrogepant for the acute treatment of migraine when administered during the prodrome of a migraine attack. The study included 518 participants age 18-75 years who had at least a 1-year history of migraine and had had two to eight migraine attacks per month that included symptoms of a moderate to severe headache in each of the 3 months before the study. Because this was a crossover trial, the participants were randomly assigned to either receive placebo for treatment of the first qualifying prodrome event and 100 mg ubrogepant for treatment of the second qualifying prodrome event or to receive 100 mg ubrogepant to treat the first qualifying prodrome event and placebo to treat the second qualifying prodrome event.
According to AbbVie's news release following publication of the study, "Absence of moderate or severe intensity headache within 24 hours was achieved following 46% of qualifying prodrome events when treated with UBRELVY vs 29% of placebo-treated events" and "absence of moderate or severe intensity headache within 48 hours was achieved following 41% of qualifying prodrome events when treated with UBRELVY vs 25% of placebo-treated events" (both P < .0001).4 Safety and tolerability of treatment during the prodromal period were also established.
In clinical practice, these results hold promise because patients can gain some assurance in knowing that taking their migraine treatment during their early prodromal symptoms is safe and could potentially improve the outcome of the event, preventing migraine symptoms for 48 hours. Even for patients who do not have an ample supply of ubrogepant or another CGRP antagonist, taking a treatment that is approved by their doctor at the onset of prodromal symptoms can provide relief compared with waiting until symptoms worsen.
Additional References
1. Waliszewska-Prosół M, Vuralli D, Martelletti P. What to do with non-responders to CGRP(r) monoclonal antibodies: Switch to another or move to gepants? J Headache Pain. 2023;24:163. doi: 10.1186/s10194-023-01698-8
2. Pallapothu MR, Quintana Mariñez MG, Chakkera M, et al. Long-term management of migraine with OnabotulinumtoxinA (Botox) vs calcitonin gene-related peptide antibodies (Anti-CGRP). Cureus. 2023;15:e46696. doi: 10.7759/cureus.46696
3. Pellesi L. Combining onabotulinumtoxin A with a CGRP antagonist for chronic migraine prophylaxis: Where do we stand? Front Pain Res (Lausanne). 2023;4:1292994. doi: 10.3389/fpain.2023.1292994
4. AbbVie. Results published in The Lancet show UBRELVY® (ubrogepant) reduces the headache phase of a migraine attack when dosed during the prodrome of migraine. November 16, 2023. Source
The recent 3-month double-blind study by Schwedt and colleagues included 580 participants and compared the effects of galcanezumab (Emgality) with those of rimegepant (Nurtec ODT). These medications are administered by different methods when used for migraine prevention; galcanezumab is given subcutaneously (SC) every month, whereas rimegepant is taken by mouth every other day. To blind the study, participants were randomly assigned to receive either 120 mg galcanezumab SC per month (after a 240 mg loading dose) and a placebo oral disintegrating tablet every other day or every-other-day 75 mg rimegepant as oral disintegrating tablets and a monthly SC placebo. According to the study authors, 62% of the patients receiving galcanezumab vs 61% of those receiving rimegepant achieved ≥ 50% reduction in monthly migraine headache days after 3 months, with no statistically significant difference between the groups. Comparisons between CGRP receptor antagonists are scarce. The studies tend to be of a short duration and to include small sample sizes — and most are retrospective. To date, physicians who treat patients with these drugs do not have information about the distinguishing characteristics between these treatments that could be used to guide drug selection for subtypes of migraine or different patient populations. As further research emerges, we may see distinctions between these therapies, or we might continue to see that their effects are similar in terms of benefits, duration of action, and patient characteristics.
Many patients who are prescribed these new medications have already been treated with a variety of other previously available migraine therapies, with varying degrees of improvement. Physicians who prescribe treatments for migraine patients often move on to new therapeutic options when patients only experience partial relief, but recent research suggests that even these incomplete responses could be beneficial for patients.
Researchers at the Headache Centre — Neurology Clinic at the Spedali Civili di Brescia in Brescia, Italy, conducted a retrospective study to examine whether previous treatment with onabotulinumtoxinA affected patent response to anti-CGRP mAb (Ceccardi et al). These treatments have differing mechanisms. OnabotulinumtoxinA is an exotoxin produced by Clostridium botulinum that blocks the acetylcholine release from nerve endings temporarily disabling postsynaptic action. Anti-CGRP mAb work by inhibiting the inflammatory receptor, thereby inhibiting the pain sensation.2
Several studies have examined the effects of combining onabotulinumtoxinA (Botox) with anti-CGRP mAb, with varying results. For example, researchers of a study designed to compare the two treatments concluded, "In patients with chronic migraine who have only had a partial response to Botox, adjunctive preventative therapy with a CGRP-mAb drug is safe and effective."2 A review examining several small studies that evaluated the response of dual therapy included a few studies that found no significant differences between an anti-CGRP mAb monotherapy and dual therapy with onabotulinumtoxinA, as well as some studies that noted improvement with dual therapy over either therapy alone. The review authors concluded that a real-life application is not yet determined and that "Further sufficiently powered, placebo-controlled studies are warranted to shed light on potential additive or synergistic effects of combining onabotulinumtoxin A with a CGRP antagonist."3
The Brescia study was designed to examine the effect of previous onabotulinumtoxinA treatment on subsequent anti-CGRP mAb response. The researchers enrolled 128 patients, of whom 51 (39.9%) had previously been treated with onabotulinumtoxinA, with the last dose 3 months before preventive treatment with an anti-CGRP mAb was started. The study was conducted between November 2018 and May 2023. The outcomes noted included monthly headache days, monthly migraine days, mean analgesic consumption, and clinical disability according to the Migraine Disability Assessment test (MIDAS). Participants received 3 months of treatment with an anti-CGRP mAb.
In addition to comparing patients who had previously received onabotulinumtoxinA with those who did not, the researchers also "aimed to evaluate whether the clinical response to anti-CGRP mAb was affected by the number of previous Onabotulinumtoxin-A administrations.
The documented baseline prior to treatment with an anti-CGRP mAb was as follows: mean monthly headache days 23.7 (SD 5.7), monthly migraine days 13.9 (SD 8.0); mean MIDAS score 108.9 (SD 76.1); and mean analgesic consumption 24.8 (SD 18.8). After 3 months of treatment with an anti-CGRP mAb, both groups experienced significant improvement in all these parameters. Furthermore, after 3 months of treatment with an anti-CGRP mAb, the patients who received at least three onabotulinumtoxinA administrations prior to the study experienced lower MMD compared with those who had received fewer cycles.
For physicians and patients, this outcome provides validation that patients can potentially gain long-term benefits from migraine treatment, even if such interventions do not provide sufficient migraine relief. The conclusion cannot be generalized to other migraine treatment sequences, and the authors did not suggest deliberately postponing any treatment or using any treatment as "priming" for another treatment. Yet physicians may be able to give patients some reassurance that an incomplete response in migraine therapy is not futile.
Migraine treatment can be very effective, but sometimes it is not clear whether patients should take their medication before or during a migraine episode, or whether the signal to take medication should be based on specific symptoms. Many patients wait to take their migraine treatment until they are sure that they will have a migraine, especially if they frequently have prodromal symptoms that do not consistently lead to a migraine. Additionally, some of the new CGRP receptor antagonists are expensive, and many payers only approve a limited amount per month. Patients might not want to waste their CGRP receptor antagonist supply in case they run out before their next refill authorization.
AbbVie, the makers of ubrogepant (Ubrelvy), a CGRP receptor antagonist approved for acute treatment of migraine, conducted a phase 3, multicenter, randomized, double-blind, placebo-controlled, crossover trial of ubrogepant at 75 research centers and headache clinics in the US (Dodick et al). According to the manufacturer, the aim of the trial was to evaluate the efficacy, safety, and tolerability of 100 mg ubrogepant for the acute treatment of migraine when administered during the prodrome of a migraine attack. The study included 518 participants age 18-75 years who had at least a 1-year history of migraine and had had two to eight migraine attacks per month that included symptoms of a moderate to severe headache in each of the 3 months before the study. Because this was a crossover trial, the participants were randomly assigned to either receive placebo for treatment of the first qualifying prodrome event and 100 mg ubrogepant for treatment of the second qualifying prodrome event or to receive 100 mg ubrogepant to treat the first qualifying prodrome event and placebo to treat the second qualifying prodrome event.
According to AbbVie's news release following publication of the study, "Absence of moderate or severe intensity headache within 24 hours was achieved following 46% of qualifying prodrome events when treated with UBRELVY vs 29% of placebo-treated events" and "absence of moderate or severe intensity headache within 48 hours was achieved following 41% of qualifying prodrome events when treated with UBRELVY vs 25% of placebo-treated events" (both P < .0001).4 Safety and tolerability of treatment during the prodromal period were also established.
In clinical practice, these results hold promise because patients can gain some assurance in knowing that taking their migraine treatment during their early prodromal symptoms is safe and could potentially improve the outcome of the event, preventing migraine symptoms for 48 hours. Even for patients who do not have an ample supply of ubrogepant or another CGRP antagonist, taking a treatment that is approved by their doctor at the onset of prodromal symptoms can provide relief compared with waiting until symptoms worsen.
Additional References
1. Waliszewska-Prosół M, Vuralli D, Martelletti P. What to do with non-responders to CGRP(r) monoclonal antibodies: Switch to another or move to gepants? J Headache Pain. 2023;24:163. doi: 10.1186/s10194-023-01698-8
2. Pallapothu MR, Quintana Mariñez MG, Chakkera M, et al. Long-term management of migraine with OnabotulinumtoxinA (Botox) vs calcitonin gene-related peptide antibodies (Anti-CGRP). Cureus. 2023;15:e46696. doi: 10.7759/cureus.46696
3. Pellesi L. Combining onabotulinumtoxin A with a CGRP antagonist for chronic migraine prophylaxis: Where do we stand? Front Pain Res (Lausanne). 2023;4:1292994. doi: 10.3389/fpain.2023.1292994
4. AbbVie. Results published in The Lancet show UBRELVY® (ubrogepant) reduces the headache phase of a migraine attack when dosed during the prodrome of migraine. November 16, 2023. Source
SCLC Imaging
PsA Imaging
Depression Medications
Navigating Hair Loss in Medical School: Experiences of 2 Young Black Women
As medical students, we often assume we are exempt from the diagnoses we learn about. During the first 2 years of medical school, we learn about alopecia as a condition that may be associated with stress, hormonal imbalances, nutrient deficiencies, and aging. However, our curricula do not explore the subtypes, psychosocial impact, or even the overwhelming number of Black women who are disproportionately affected by alopecia. For Black women, hair is a colossal part of their cultural identity, learning from a young age how to nurture and style natural coils. It becomes devastating when women begin to lose them.
The diagnosis of alopecia subtypes in Black women has been explored in the literature; however, understanding the unique experiences of young Black women is an important part of patient care, as alopecia often is destructive to the patient’s self-image. Therefore, it is important to shed light on these experiences so others feel empowered and supported in their journeys. Herein, we share the experiences of 2 authors (J.D. and C.A.V.O.)—both young Black women—who navigated unexpected hair loss in medical school.
Jewell’s Story
During my first year of medical school, I noticed my hair was shedding more than usual, and my ponytail was not as thick as it once was. I also had an area in my crown that was abnormally thin. My parents suggested that it was a consequence of stress, but I knew something was not right. With only 1 Black dermatologist within 2 hours of Nashville, Tennessee, I remember worrying about seeing a dermatologist who did not understand Black hair. I still scheduled an appointment, but I remember debating if I should straighten my hair or wear my naturally curly Afro. The first dermatologist I saw diagnosed me with seborrheic dermatitis—without even examining my scalp. She told me that I had a “full head of hair” and that I had nothing to worry about. I was unconvinced. Weeks later, I met with another dermatologist who took the time to listen to my concerns. After a scalp biopsy and laboratory work, she diagnosed me with telogen effluvium and androgenetic alopecia. Months later, I had the opportunity to visit the Black dermatologist, and she diagnosed me with central centrifugal cicatricial alopecia. I am grateful for the earlier dermatologists I saw, but I finally feel at ease with my diagnosis and treatment plan after being seen by the latter.
Chidubem’s Story
From a young age, I was conditioned to think my hair was thick, unmanageable, and a nuisance. I grew accustomed to people yanking on my hair, and my gentle whispers of “this hurts” and “the braid is too tight” being ignored. That continued into adulthood. While studying for the US Medical Licensing Examination, I noticed a burning sensation on my scalp. I decided to ignore it. However, as the days progressed, the slight burning sensation turned into intense burning and itching. I still ignored it. Not only did I lack the funds for a dermatology appointment, but my licensing examination was approaching, and it was more important than anything related to my hair. After the examination, I eventually made an appointment with my primary care physician, who attributed my symptoms to the stressors of medical school. “I think you are having migraines,” she told me. So, I continued to ignore my symptoms. A year passed, and a hair braider pointed out that I had 2 well-defined bald patches on my scalp. I remember feeling angry and confused as to how I missed those findings. I could no longer ignore it—it bothered me less when no one else knew about it. I quickly made a dermatology appointment. Although I opted out of a biopsy, we decided to treat my hair loss empirically, and I have experienced drastic improvement.
Final Thoughts
We are 2 Black women living more than 500 miles away from each other at different medical institutions, yet we share the same experience, which many other women unfortunately face alone. It is not uncommon for us to feel unheard, dismissed, or misdiagnosed. We write this for the Black woman sorting through the feelings of confusion and shock as she traces the hairless spot on her scalp. We write this for the medical student ignoring their symptoms until after their examination. We even write this for any nondermatologists uncomfortable with diagnosing and treating textured hair. To improve patient satisfaction and overall health outcomes, physicians must approach patients with both knowledge and cultural competency. Most importantly, dermatologists (and other physicians) should be appropriately trained in not only the structural differences of textured hair but also the unique practices and beliefs among Black women in relation to their hair.
Acknowledgments—Jewell Dinkins is the inaugural recipient of the Janssen–Skin of Color Research Fellowship at Howard University (Washington, DC), and Chidubem A.V. Okeke is the inaugural recipient of the Women’s Dermatologic Society–La Roche-Posay dermatology fellowship at Howard University.
As medical students, we often assume we are exempt from the diagnoses we learn about. During the first 2 years of medical school, we learn about alopecia as a condition that may be associated with stress, hormonal imbalances, nutrient deficiencies, and aging. However, our curricula do not explore the subtypes, psychosocial impact, or even the overwhelming number of Black women who are disproportionately affected by alopecia. For Black women, hair is a colossal part of their cultural identity, learning from a young age how to nurture and style natural coils. It becomes devastating when women begin to lose them.
The diagnosis of alopecia subtypes in Black women has been explored in the literature; however, understanding the unique experiences of young Black women is an important part of patient care, as alopecia often is destructive to the patient’s self-image. Therefore, it is important to shed light on these experiences so others feel empowered and supported in their journeys. Herein, we share the experiences of 2 authors (J.D. and C.A.V.O.)—both young Black women—who navigated unexpected hair loss in medical school.
Jewell’s Story
During my first year of medical school, I noticed my hair was shedding more than usual, and my ponytail was not as thick as it once was. I also had an area in my crown that was abnormally thin. My parents suggested that it was a consequence of stress, but I knew something was not right. With only 1 Black dermatologist within 2 hours of Nashville, Tennessee, I remember worrying about seeing a dermatologist who did not understand Black hair. I still scheduled an appointment, but I remember debating if I should straighten my hair or wear my naturally curly Afro. The first dermatologist I saw diagnosed me with seborrheic dermatitis—without even examining my scalp. She told me that I had a “full head of hair” and that I had nothing to worry about. I was unconvinced. Weeks later, I met with another dermatologist who took the time to listen to my concerns. After a scalp biopsy and laboratory work, she diagnosed me with telogen effluvium and androgenetic alopecia. Months later, I had the opportunity to visit the Black dermatologist, and she diagnosed me with central centrifugal cicatricial alopecia. I am grateful for the earlier dermatologists I saw, but I finally feel at ease with my diagnosis and treatment plan after being seen by the latter.
Chidubem’s Story
From a young age, I was conditioned to think my hair was thick, unmanageable, and a nuisance. I grew accustomed to people yanking on my hair, and my gentle whispers of “this hurts” and “the braid is too tight” being ignored. That continued into adulthood. While studying for the US Medical Licensing Examination, I noticed a burning sensation on my scalp. I decided to ignore it. However, as the days progressed, the slight burning sensation turned into intense burning and itching. I still ignored it. Not only did I lack the funds for a dermatology appointment, but my licensing examination was approaching, and it was more important than anything related to my hair. After the examination, I eventually made an appointment with my primary care physician, who attributed my symptoms to the stressors of medical school. “I think you are having migraines,” she told me. So, I continued to ignore my symptoms. A year passed, and a hair braider pointed out that I had 2 well-defined bald patches on my scalp. I remember feeling angry and confused as to how I missed those findings. I could no longer ignore it—it bothered me less when no one else knew about it. I quickly made a dermatology appointment. Although I opted out of a biopsy, we decided to treat my hair loss empirically, and I have experienced drastic improvement.
Final Thoughts
We are 2 Black women living more than 500 miles away from each other at different medical institutions, yet we share the same experience, which many other women unfortunately face alone. It is not uncommon for us to feel unheard, dismissed, or misdiagnosed. We write this for the Black woman sorting through the feelings of confusion and shock as she traces the hairless spot on her scalp. We write this for the medical student ignoring their symptoms until after their examination. We even write this for any nondermatologists uncomfortable with diagnosing and treating textured hair. To improve patient satisfaction and overall health outcomes, physicians must approach patients with both knowledge and cultural competency. Most importantly, dermatologists (and other physicians) should be appropriately trained in not only the structural differences of textured hair but also the unique practices and beliefs among Black women in relation to their hair.
Acknowledgments—Jewell Dinkins is the inaugural recipient of the Janssen–Skin of Color Research Fellowship at Howard University (Washington, DC), and Chidubem A.V. Okeke is the inaugural recipient of the Women’s Dermatologic Society–La Roche-Posay dermatology fellowship at Howard University.
As medical students, we often assume we are exempt from the diagnoses we learn about. During the first 2 years of medical school, we learn about alopecia as a condition that may be associated with stress, hormonal imbalances, nutrient deficiencies, and aging. However, our curricula do not explore the subtypes, psychosocial impact, or even the overwhelming number of Black women who are disproportionately affected by alopecia. For Black women, hair is a colossal part of their cultural identity, learning from a young age how to nurture and style natural coils. It becomes devastating when women begin to lose them.
The diagnosis of alopecia subtypes in Black women has been explored in the literature; however, understanding the unique experiences of young Black women is an important part of patient care, as alopecia often is destructive to the patient’s self-image. Therefore, it is important to shed light on these experiences so others feel empowered and supported in their journeys. Herein, we share the experiences of 2 authors (J.D. and C.A.V.O.)—both young Black women—who navigated unexpected hair loss in medical school.
Jewell’s Story
During my first year of medical school, I noticed my hair was shedding more than usual, and my ponytail was not as thick as it once was. I also had an area in my crown that was abnormally thin. My parents suggested that it was a consequence of stress, but I knew something was not right. With only 1 Black dermatologist within 2 hours of Nashville, Tennessee, I remember worrying about seeing a dermatologist who did not understand Black hair. I still scheduled an appointment, but I remember debating if I should straighten my hair or wear my naturally curly Afro. The first dermatologist I saw diagnosed me with seborrheic dermatitis—without even examining my scalp. She told me that I had a “full head of hair” and that I had nothing to worry about. I was unconvinced. Weeks later, I met with another dermatologist who took the time to listen to my concerns. After a scalp biopsy and laboratory work, she diagnosed me with telogen effluvium and androgenetic alopecia. Months later, I had the opportunity to visit the Black dermatologist, and she diagnosed me with central centrifugal cicatricial alopecia. I am grateful for the earlier dermatologists I saw, but I finally feel at ease with my diagnosis and treatment plan after being seen by the latter.
Chidubem’s Story
From a young age, I was conditioned to think my hair was thick, unmanageable, and a nuisance. I grew accustomed to people yanking on my hair, and my gentle whispers of “this hurts” and “the braid is too tight” being ignored. That continued into adulthood. While studying for the US Medical Licensing Examination, I noticed a burning sensation on my scalp. I decided to ignore it. However, as the days progressed, the slight burning sensation turned into intense burning and itching. I still ignored it. Not only did I lack the funds for a dermatology appointment, but my licensing examination was approaching, and it was more important than anything related to my hair. After the examination, I eventually made an appointment with my primary care physician, who attributed my symptoms to the stressors of medical school. “I think you are having migraines,” she told me. So, I continued to ignore my symptoms. A year passed, and a hair braider pointed out that I had 2 well-defined bald patches on my scalp. I remember feeling angry and confused as to how I missed those findings. I could no longer ignore it—it bothered me less when no one else knew about it. I quickly made a dermatology appointment. Although I opted out of a biopsy, we decided to treat my hair loss empirically, and I have experienced drastic improvement.
Final Thoughts
We are 2 Black women living more than 500 miles away from each other at different medical institutions, yet we share the same experience, which many other women unfortunately face alone. It is not uncommon for us to feel unheard, dismissed, or misdiagnosed. We write this for the Black woman sorting through the feelings of confusion and shock as she traces the hairless spot on her scalp. We write this for the medical student ignoring their symptoms until after their examination. We even write this for any nondermatologists uncomfortable with diagnosing and treating textured hair. To improve patient satisfaction and overall health outcomes, physicians must approach patients with both knowledge and cultural competency. Most importantly, dermatologists (and other physicians) should be appropriately trained in not only the structural differences of textured hair but also the unique practices and beliefs among Black women in relation to their hair.
Acknowledgments—Jewell Dinkins is the inaugural recipient of the Janssen–Skin of Color Research Fellowship at Howard University (Washington, DC), and Chidubem A.V. Okeke is the inaugural recipient of the Women’s Dermatologic Society–La Roche-Posay dermatology fellowship at Howard University.
Practice Points
- Hair loss is a common dermatologic concern among Black women and can represent a diagnostic challenge to dermatologists who may not be familiar with textured hair.
- Dermatologists should practice cultural sensitivity and provide relevant recommendations to Black patients dealing with hair loss.
Thalidomide Analogue Drug Eruption Along the Lines of Blaschko
To the Editor:
Lenalidomide is a thalidomide analogue used to treat various hematologic malignancies, including non-Hodgkin lymphoma, myelodysplastic syndrome, and multiple myeloma (MM).1 Lenalidomide is referred to as a degrader therapeutic because it induces targeted protein degradation of disease-relevant proteins (eg, Ikaros family zinc finger protein 1 [IKZF1], Ikaros family zinc finger protein 3 [IKZF3], and casein kinase I isoform-α [CK1α]) as its primary mechanism of action.1,2 Although cutaneous adverse events are relatively common among thalidomide analogues, the morphologic and histopathologic descriptions of these drug eruptions have not been fully elucidated.3,4 We report a novel pityriasiform drug eruption followed by a clinical eruption suggestive of blaschkitis in a patient with MM who was being treated with lenalidomide.
A 76-year-old man presented to the dermatology clinic with a progressive, mildly pruritic eruption on the chest and axillae of 1 year’s duration. He had a medical history of chronic hepatitis B, malignant carcinoid tumor of the colon, prostate cancer, and MM. The eruption emerged 1 to 2 weeks after the patient started oral lenalidomide 10 mg/d and oral dexamethasone40 mg/wk following autologous stem cell transplantation for MM. The patient had not received any other therapy for MM.
Physical examination revealed multiple erythematous, hyperpigmented, scaly papules and plaques on the lateral chest and within the axillae (Figure 1). A skin biopsy from the left axilla demonstrated a mild lichenoid and perivascular lymphocytic infiltrate with scattered eosinophils, neutrophils, and extravasated erythrocytes. The overlying epidermis showed spongiosis with parakeratosis in addition to lymphocytic exocytosis (Figure 2). No fungal organisms were highlighted on periodic acid–Schiff staining. After this evaluation, we recommended that the patient discontinue lenalidomide and start taking a topical over-the-counter corticosteroid for 2 weeks. Over time, he noted marked improvement in the eruption and associated pruritus.
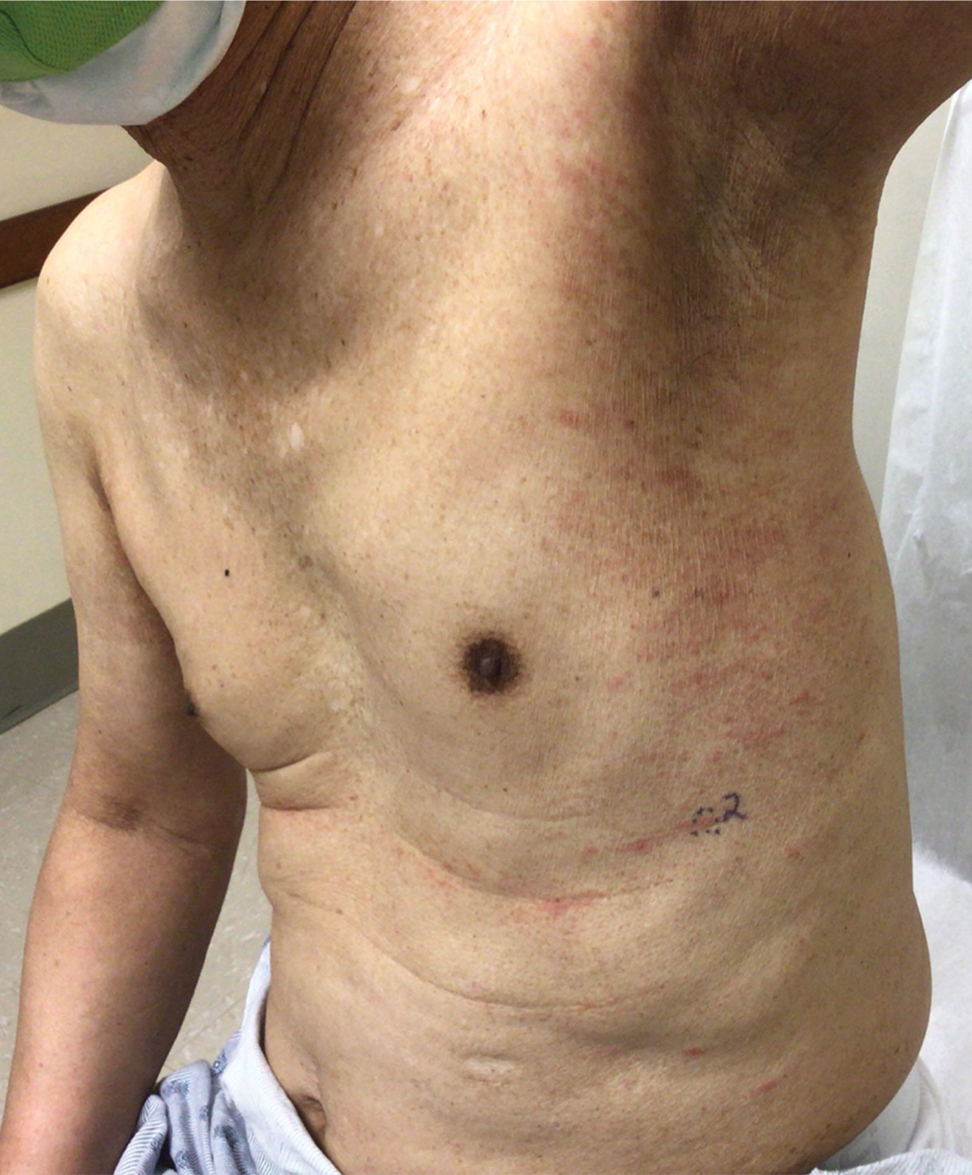
After a drug holiday of 2 months, the patient resumed a maintenance dosage of oral lenalidomide 10 mg/d. Four or 5 days after restarting lenalidomide, a pruritic eruption appeared that involved the axillae and the left lower abdomen, circling around to the left lower back. The axillary eruption resolved with a topical over-the-counter corticosteroid; the abdominal eruption persisted.
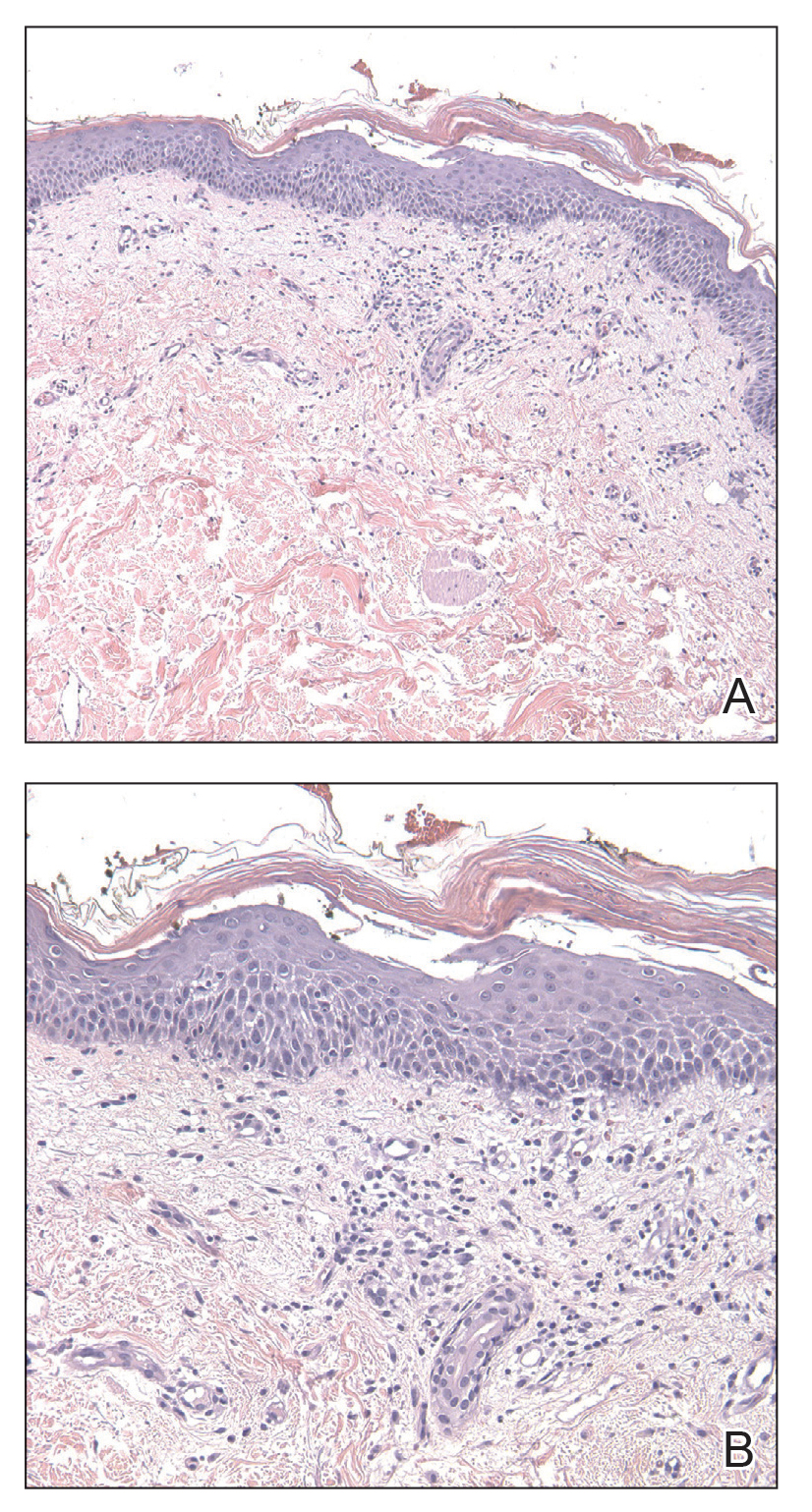
At the 3-month follow-up visit, physical examination revealed erythematous macules and papules that coalesced over a salmon-colored base along the lines of Blaschko extending from the left lower abdominal quadrant, crossing the left flank, and continuing to the left lower back without crossing the midline (Figure 3).
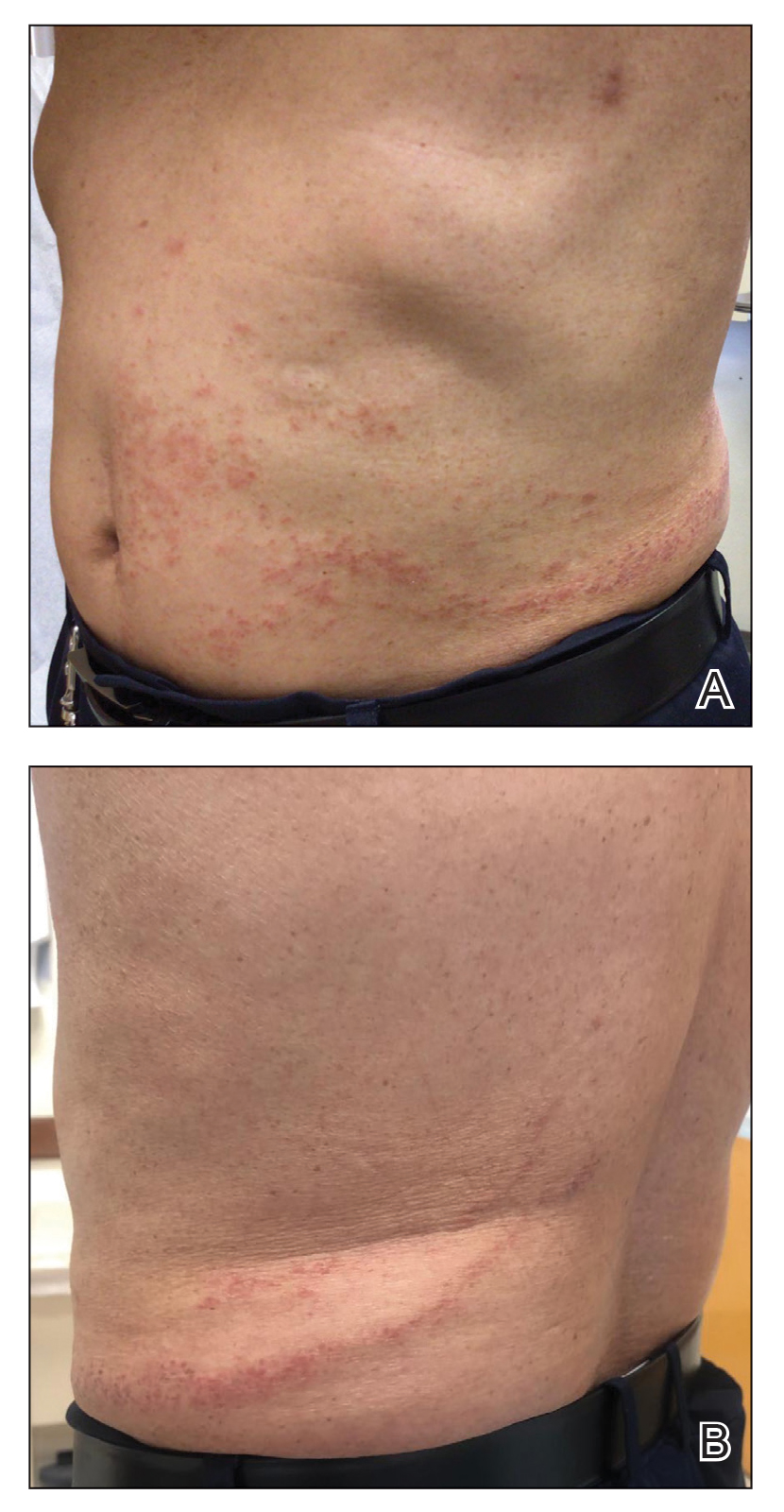
We recommended that the patient continue treatment through this eruption; he was instructed to apply a corticosteroid cream and resume lenalidomide at the maintenance dosage. A month later, he reported that the eruption and associated pruritus resolved with the corticosteroid cream and resumption of the maintenance dose of lenalidomide. The patient noted no further spread of the eruption.
Cutaneous adverse events are common following lenalidomide. In prior trials, the overall incidence of any-grade rash following lenalidomide exposure was 22% to 33%.5 A meta-analysis of 10 trials determined the overall incidence of all-grade and high-grade cutaneous adverse events after exposure to lenalidomide was 27.2% and 3.6%, respectively.6 Our case represents a pityriasiform eruption due to lenalidomide followed by a secondary eruption suggestive of blaschkitis.
The rash due to lenalidomide has been described as morbilliform, urticarial, dermatitic, acneform, and undefined.7 Lenalidomide-induced rash typically develops during the first month of therapy, similar to our patient’s presentation. It has even been observed in the first week of therapy.8 Severe reactions such as Stevens-Johnson syndrome and toxic epidermal necrolysis have been reported.5,6 Risk factors associated with rash secondary to lenalidomide include advanced age (≥70 years), presence of Bence-Jones protein-type MM in urine, and no prior chemotherapy.8 Our patient had 2 of these risk factors: advanced age and no prior chemotherapy for MM. The exact pathogenesis by which lenalidomide leads to a pityriasiform eruption, as in our patient, or to a rash in general is unclear. Studies have hypothesized that a lenalidomide-induced rash could be attributable to a delayed hypersensitivity type IV reaction or to a reaction related to the molecular mechanism of action of the drug.9
At the molecular level, the antimyeloma effects of lenalidomide include promoting degradation of transcription factors IKZF1 and IKZF3, which subsequently increases production of IL-2.1,2,9 Recombinant IL-2 has been associated with an increased incidence of rash in other cancers.9 Overexpression of programmed death 1(PD-1) and its ligand (PD-L1) has been demonstrated in MM; lenalidomide has been shown to downregulate both PD-1 and PD-L1. Patients receiving PD-1 and PD-L1 inhibitors commonly have developed rash.9 However, the association between lenalidomide and its downregulation of PD-1 and PD-L1 leading to rash has not been fully elucidated. Given the multiple malignancies in our patient—MM, prostate cancer, malignant carcinoid tumor—an underlying paraneoplastic phenomenon may be possible. Additionally, because our patient initially received dexamethasone along with lenalidomide, the manifestation of the initial pityriasiform rash may have been less severe due to the steroid use. Although our patient underwent a 2-month drug holiday following the initial pityriasiform eruption, most lenalidomide-induced rashes do not necessitate discontinuation of the drug.5,7
Our patient’s secondary drug eruption was clinically suggestive of lenalidomide-induced blaschkitis. A report of a German patient with plasmacytoma described a unilateral papular exanthem that developed 4 months after lenalidomide was initiated.10 The papular exanthem following the lines of Blaschko lines extended from that patient’s posterior left foot to the calf and on to the thigh and flank,10 which was more extensive than our patient’s eruption. Blaschkitis in this patient resolved with a corticosteroid cream and UV light therapy10; lenalidomide was not discontinued, similar to our patient.
The pathogenesis of our patient’s secondary eruption that preferentially involved the lines of Blaschko is unclear. After the initial pityriasiform eruption, the secondary eruption was blaschkitis. Distinguishing dermatomes from the lines of Blaschko, which are thought to represent pathways of epidermal cell migration and proliferation during embryologic development, is important. Genodermatoses such as incontinentia pigmenti and hypomelanosis of Ito involve the lines of Blaschko11; other disorders in the differential diagnosis of linear configurations include linear lichen planus, linear cutaneous lupus erythematosus, linear morphea, and lichen striatus.11 Notably, drug-induced blaschkitis is rare.
Cutaneous adverse reactions from thalidomide analogues are relatively common. Our case of lenalidomide-associated blaschkitis that developed following an initial pityriasiform drug eruption in a patient with MM highlights that dermatologists need to collaborate with the oncologist regarding the severity of drug eruptions to determine if the patient should continue treatment through the cutaneous eruptions or discontinue a vital medication.
- Jan M, Sperling AS, Ebert BL. Cancer therapies based on targeted protein degradation—lessons learned with lenalidomide. Nat Rev Clin Oncol. 2021;18:401-417. doi:10.1038/s41571-021-00479-z
- Shah UA, Mailankody S. Emerging immunotherapies in multiple myeloma. BMJ. 2020;370:3176. doi:10.1136/BMJ.M3176
- Richardson PG, Blood E, Mitsiades CS, et al. A randomized phase 2 study of lenalidomide therapy for patients with relapsed or relapsed and refractory multiple myeloma. Blood. 2006;108:3458-3464. doi:10.1182/BLOOD-2006-04-015909
- Benboubker L, Dimopoulos MA, Dispenzieri A, et al. Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. N Engl J Med. 2014;371:906-917. doi:10.1056/NEJMOA1402551
- Tinsley SM, Kurtin SE, Ridgeway JA. Practical management of lenalidomide-related rash. Clin Lymphoma Myeloma Leuk. 2015;15(suppl):S64-S69. doi:10.1016/J.CLML.2015.02.008
- Nardone B, Wu S, Garden BC, et al. Risk of rash associated with lenalidomide in cancer patients: a systematic review of the literature and meta-analysis. Clin Lymphoma Myeloma Leuk. 2013;13:424-429. doi:10.1016/J.CLML.2013.03.006
- Sviggum HP, Davis MDP, Rajkumar SV, et al. Dermatologic adverse effects of lenalidomide therapy for amyloidosis and multiple myeloma. Arch Dermatol. 2006;142:1298-1302. doi:10.1001/ARCHDERM.142.10.1298
- Sugi T, Nishigami Y, Saigo H, et al. Analysis of risk factors for lenalidomide-associated skin rash in patients with multiple myeloma. Leuk Lymphoma. 2021;62:1405-1410. doi:10.1080/10428194.2021.1876867
- Barley K, He W, Agarwal S, et al. Outcomes and management of lenalidomide-associated rash in patients with multiple myeloma. Leuk Lymphoma. 2016;57:2510-2515. doi:10.3109/10428194.2016.1151507
- Grape J, Frosch P. Papular drug eruption along the lines of Blaschko caused by lenalidomide [in German]. Hautarzt. 2011;62:618-620. doi:10.1007/S00105-010-2121-6
- Bolognia JL, Orlow SJ, Glick SA. Lines of Blaschko. J Am Acad Dermatol. 1994;31(2 pt 1):157-190. doi:10.1016/S0190-9622(94)70143-1
To the Editor:
Lenalidomide is a thalidomide analogue used to treat various hematologic malignancies, including non-Hodgkin lymphoma, myelodysplastic syndrome, and multiple myeloma (MM).1 Lenalidomide is referred to as a degrader therapeutic because it induces targeted protein degradation of disease-relevant proteins (eg, Ikaros family zinc finger protein 1 [IKZF1], Ikaros family zinc finger protein 3 [IKZF3], and casein kinase I isoform-α [CK1α]) as its primary mechanism of action.1,2 Although cutaneous adverse events are relatively common among thalidomide analogues, the morphologic and histopathologic descriptions of these drug eruptions have not been fully elucidated.3,4 We report a novel pityriasiform drug eruption followed by a clinical eruption suggestive of blaschkitis in a patient with MM who was being treated with lenalidomide.
A 76-year-old man presented to the dermatology clinic with a progressive, mildly pruritic eruption on the chest and axillae of 1 year’s duration. He had a medical history of chronic hepatitis B, malignant carcinoid tumor of the colon, prostate cancer, and MM. The eruption emerged 1 to 2 weeks after the patient started oral lenalidomide 10 mg/d and oral dexamethasone40 mg/wk following autologous stem cell transplantation for MM. The patient had not received any other therapy for MM.
Physical examination revealed multiple erythematous, hyperpigmented, scaly papules and plaques on the lateral chest and within the axillae (Figure 1). A skin biopsy from the left axilla demonstrated a mild lichenoid and perivascular lymphocytic infiltrate with scattered eosinophils, neutrophils, and extravasated erythrocytes. The overlying epidermis showed spongiosis with parakeratosis in addition to lymphocytic exocytosis (Figure 2). No fungal organisms were highlighted on periodic acid–Schiff staining. After this evaluation, we recommended that the patient discontinue lenalidomide and start taking a topical over-the-counter corticosteroid for 2 weeks. Over time, he noted marked improvement in the eruption and associated pruritus.

After a drug holiday of 2 months, the patient resumed a maintenance dosage of oral lenalidomide 10 mg/d. Four or 5 days after restarting lenalidomide, a pruritic eruption appeared that involved the axillae and the left lower abdomen, circling around to the left lower back. The axillary eruption resolved with a topical over-the-counter corticosteroid; the abdominal eruption persisted.

At the 3-month follow-up visit, physical examination revealed erythematous macules and papules that coalesced over a salmon-colored base along the lines of Blaschko extending from the left lower abdominal quadrant, crossing the left flank, and continuing to the left lower back without crossing the midline (Figure 3).

We recommended that the patient continue treatment through this eruption; he was instructed to apply a corticosteroid cream and resume lenalidomide at the maintenance dosage. A month later, he reported that the eruption and associated pruritus resolved with the corticosteroid cream and resumption of the maintenance dose of lenalidomide. The patient noted no further spread of the eruption.
Cutaneous adverse events are common following lenalidomide. In prior trials, the overall incidence of any-grade rash following lenalidomide exposure was 22% to 33%.5 A meta-analysis of 10 trials determined the overall incidence of all-grade and high-grade cutaneous adverse events after exposure to lenalidomide was 27.2% and 3.6%, respectively.6 Our case represents a pityriasiform eruption due to lenalidomide followed by a secondary eruption suggestive of blaschkitis.
The rash due to lenalidomide has been described as morbilliform, urticarial, dermatitic, acneform, and undefined.7 Lenalidomide-induced rash typically develops during the first month of therapy, similar to our patient’s presentation. It has even been observed in the first week of therapy.8 Severe reactions such as Stevens-Johnson syndrome and toxic epidermal necrolysis have been reported.5,6 Risk factors associated with rash secondary to lenalidomide include advanced age (≥70 years), presence of Bence-Jones protein-type MM in urine, and no prior chemotherapy.8 Our patient had 2 of these risk factors: advanced age and no prior chemotherapy for MM. The exact pathogenesis by which lenalidomide leads to a pityriasiform eruption, as in our patient, or to a rash in general is unclear. Studies have hypothesized that a lenalidomide-induced rash could be attributable to a delayed hypersensitivity type IV reaction or to a reaction related to the molecular mechanism of action of the drug.9
At the molecular level, the antimyeloma effects of lenalidomide include promoting degradation of transcription factors IKZF1 and IKZF3, which subsequently increases production of IL-2.1,2,9 Recombinant IL-2 has been associated with an increased incidence of rash in other cancers.9 Overexpression of programmed death 1(PD-1) and its ligand (PD-L1) has been demonstrated in MM; lenalidomide has been shown to downregulate both PD-1 and PD-L1. Patients receiving PD-1 and PD-L1 inhibitors commonly have developed rash.9 However, the association between lenalidomide and its downregulation of PD-1 and PD-L1 leading to rash has not been fully elucidated. Given the multiple malignancies in our patient—MM, prostate cancer, malignant carcinoid tumor—an underlying paraneoplastic phenomenon may be possible. Additionally, because our patient initially received dexamethasone along with lenalidomide, the manifestation of the initial pityriasiform rash may have been less severe due to the steroid use. Although our patient underwent a 2-month drug holiday following the initial pityriasiform eruption, most lenalidomide-induced rashes do not necessitate discontinuation of the drug.5,7
Our patient’s secondary drug eruption was clinically suggestive of lenalidomide-induced blaschkitis. A report of a German patient with plasmacytoma described a unilateral papular exanthem that developed 4 months after lenalidomide was initiated.10 The papular exanthem following the lines of Blaschko lines extended from that patient’s posterior left foot to the calf and on to the thigh and flank,10 which was more extensive than our patient’s eruption. Blaschkitis in this patient resolved with a corticosteroid cream and UV light therapy10; lenalidomide was not discontinued, similar to our patient.
The pathogenesis of our patient’s secondary eruption that preferentially involved the lines of Blaschko is unclear. After the initial pityriasiform eruption, the secondary eruption was blaschkitis. Distinguishing dermatomes from the lines of Blaschko, which are thought to represent pathways of epidermal cell migration and proliferation during embryologic development, is important. Genodermatoses such as incontinentia pigmenti and hypomelanosis of Ito involve the lines of Blaschko11; other disorders in the differential diagnosis of linear configurations include linear lichen planus, linear cutaneous lupus erythematosus, linear morphea, and lichen striatus.11 Notably, drug-induced blaschkitis is rare.
Cutaneous adverse reactions from thalidomide analogues are relatively common. Our case of lenalidomide-associated blaschkitis that developed following an initial pityriasiform drug eruption in a patient with MM highlights that dermatologists need to collaborate with the oncologist regarding the severity of drug eruptions to determine if the patient should continue treatment through the cutaneous eruptions or discontinue a vital medication.
To the Editor:
Lenalidomide is a thalidomide analogue used to treat various hematologic malignancies, including non-Hodgkin lymphoma, myelodysplastic syndrome, and multiple myeloma (MM).1 Lenalidomide is referred to as a degrader therapeutic because it induces targeted protein degradation of disease-relevant proteins (eg, Ikaros family zinc finger protein 1 [IKZF1], Ikaros family zinc finger protein 3 [IKZF3], and casein kinase I isoform-α [CK1α]) as its primary mechanism of action.1,2 Although cutaneous adverse events are relatively common among thalidomide analogues, the morphologic and histopathologic descriptions of these drug eruptions have not been fully elucidated.3,4 We report a novel pityriasiform drug eruption followed by a clinical eruption suggestive of blaschkitis in a patient with MM who was being treated with lenalidomide.
A 76-year-old man presented to the dermatology clinic with a progressive, mildly pruritic eruption on the chest and axillae of 1 year’s duration. He had a medical history of chronic hepatitis B, malignant carcinoid tumor of the colon, prostate cancer, and MM. The eruption emerged 1 to 2 weeks after the patient started oral lenalidomide 10 mg/d and oral dexamethasone40 mg/wk following autologous stem cell transplantation for MM. The patient had not received any other therapy for MM.
Physical examination revealed multiple erythematous, hyperpigmented, scaly papules and plaques on the lateral chest and within the axillae (Figure 1). A skin biopsy from the left axilla demonstrated a mild lichenoid and perivascular lymphocytic infiltrate with scattered eosinophils, neutrophils, and extravasated erythrocytes. The overlying epidermis showed spongiosis with parakeratosis in addition to lymphocytic exocytosis (Figure 2). No fungal organisms were highlighted on periodic acid–Schiff staining. After this evaluation, we recommended that the patient discontinue lenalidomide and start taking a topical over-the-counter corticosteroid for 2 weeks. Over time, he noted marked improvement in the eruption and associated pruritus.

After a drug holiday of 2 months, the patient resumed a maintenance dosage of oral lenalidomide 10 mg/d. Four or 5 days after restarting lenalidomide, a pruritic eruption appeared that involved the axillae and the left lower abdomen, circling around to the left lower back. The axillary eruption resolved with a topical over-the-counter corticosteroid; the abdominal eruption persisted.

At the 3-month follow-up visit, physical examination revealed erythematous macules and papules that coalesced over a salmon-colored base along the lines of Blaschko extending from the left lower abdominal quadrant, crossing the left flank, and continuing to the left lower back without crossing the midline (Figure 3).

We recommended that the patient continue treatment through this eruption; he was instructed to apply a corticosteroid cream and resume lenalidomide at the maintenance dosage. A month later, he reported that the eruption and associated pruritus resolved with the corticosteroid cream and resumption of the maintenance dose of lenalidomide. The patient noted no further spread of the eruption.
Cutaneous adverse events are common following lenalidomide. In prior trials, the overall incidence of any-grade rash following lenalidomide exposure was 22% to 33%.5 A meta-analysis of 10 trials determined the overall incidence of all-grade and high-grade cutaneous adverse events after exposure to lenalidomide was 27.2% and 3.6%, respectively.6 Our case represents a pityriasiform eruption due to lenalidomide followed by a secondary eruption suggestive of blaschkitis.
The rash due to lenalidomide has been described as morbilliform, urticarial, dermatitic, acneform, and undefined.7 Lenalidomide-induced rash typically develops during the first month of therapy, similar to our patient’s presentation. It has even been observed in the first week of therapy.8 Severe reactions such as Stevens-Johnson syndrome and toxic epidermal necrolysis have been reported.5,6 Risk factors associated with rash secondary to lenalidomide include advanced age (≥70 years), presence of Bence-Jones protein-type MM in urine, and no prior chemotherapy.8 Our patient had 2 of these risk factors: advanced age and no prior chemotherapy for MM. The exact pathogenesis by which lenalidomide leads to a pityriasiform eruption, as in our patient, or to a rash in general is unclear. Studies have hypothesized that a lenalidomide-induced rash could be attributable to a delayed hypersensitivity type IV reaction or to a reaction related to the molecular mechanism of action of the drug.9
At the molecular level, the antimyeloma effects of lenalidomide include promoting degradation of transcription factors IKZF1 and IKZF3, which subsequently increases production of IL-2.1,2,9 Recombinant IL-2 has been associated with an increased incidence of rash in other cancers.9 Overexpression of programmed death 1(PD-1) and its ligand (PD-L1) has been demonstrated in MM; lenalidomide has been shown to downregulate both PD-1 and PD-L1. Patients receiving PD-1 and PD-L1 inhibitors commonly have developed rash.9 However, the association between lenalidomide and its downregulation of PD-1 and PD-L1 leading to rash has not been fully elucidated. Given the multiple malignancies in our patient—MM, prostate cancer, malignant carcinoid tumor—an underlying paraneoplastic phenomenon may be possible. Additionally, because our patient initially received dexamethasone along with lenalidomide, the manifestation of the initial pityriasiform rash may have been less severe due to the steroid use. Although our patient underwent a 2-month drug holiday following the initial pityriasiform eruption, most lenalidomide-induced rashes do not necessitate discontinuation of the drug.5,7
Our patient’s secondary drug eruption was clinically suggestive of lenalidomide-induced blaschkitis. A report of a German patient with plasmacytoma described a unilateral papular exanthem that developed 4 months after lenalidomide was initiated.10 The papular exanthem following the lines of Blaschko lines extended from that patient’s posterior left foot to the calf and on to the thigh and flank,10 which was more extensive than our patient’s eruption. Blaschkitis in this patient resolved with a corticosteroid cream and UV light therapy10; lenalidomide was not discontinued, similar to our patient.
The pathogenesis of our patient’s secondary eruption that preferentially involved the lines of Blaschko is unclear. After the initial pityriasiform eruption, the secondary eruption was blaschkitis. Distinguishing dermatomes from the lines of Blaschko, which are thought to represent pathways of epidermal cell migration and proliferation during embryologic development, is important. Genodermatoses such as incontinentia pigmenti and hypomelanosis of Ito involve the lines of Blaschko11; other disorders in the differential diagnosis of linear configurations include linear lichen planus, linear cutaneous lupus erythematosus, linear morphea, and lichen striatus.11 Notably, drug-induced blaschkitis is rare.
Cutaneous adverse reactions from thalidomide analogues are relatively common. Our case of lenalidomide-associated blaschkitis that developed following an initial pityriasiform drug eruption in a patient with MM highlights that dermatologists need to collaborate with the oncologist regarding the severity of drug eruptions to determine if the patient should continue treatment through the cutaneous eruptions or discontinue a vital medication.
- Jan M, Sperling AS, Ebert BL. Cancer therapies based on targeted protein degradation—lessons learned with lenalidomide. Nat Rev Clin Oncol. 2021;18:401-417. doi:10.1038/s41571-021-00479-z
- Shah UA, Mailankody S. Emerging immunotherapies in multiple myeloma. BMJ. 2020;370:3176. doi:10.1136/BMJ.M3176
- Richardson PG, Blood E, Mitsiades CS, et al. A randomized phase 2 study of lenalidomide therapy for patients with relapsed or relapsed and refractory multiple myeloma. Blood. 2006;108:3458-3464. doi:10.1182/BLOOD-2006-04-015909
- Benboubker L, Dimopoulos MA, Dispenzieri A, et al. Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. N Engl J Med. 2014;371:906-917. doi:10.1056/NEJMOA1402551
- Tinsley SM, Kurtin SE, Ridgeway JA. Practical management of lenalidomide-related rash. Clin Lymphoma Myeloma Leuk. 2015;15(suppl):S64-S69. doi:10.1016/J.CLML.2015.02.008
- Nardone B, Wu S, Garden BC, et al. Risk of rash associated with lenalidomide in cancer patients: a systematic review of the literature and meta-analysis. Clin Lymphoma Myeloma Leuk. 2013;13:424-429. doi:10.1016/J.CLML.2013.03.006
- Sviggum HP, Davis MDP, Rajkumar SV, et al. Dermatologic adverse effects of lenalidomide therapy for amyloidosis and multiple myeloma. Arch Dermatol. 2006;142:1298-1302. doi:10.1001/ARCHDERM.142.10.1298
- Sugi T, Nishigami Y, Saigo H, et al. Analysis of risk factors for lenalidomide-associated skin rash in patients with multiple myeloma. Leuk Lymphoma. 2021;62:1405-1410. doi:10.1080/10428194.2021.1876867
- Barley K, He W, Agarwal S, et al. Outcomes and management of lenalidomide-associated rash in patients with multiple myeloma. Leuk Lymphoma. 2016;57:2510-2515. doi:10.3109/10428194.2016.1151507
- Grape J, Frosch P. Papular drug eruption along the lines of Blaschko caused by lenalidomide [in German]. Hautarzt. 2011;62:618-620. doi:10.1007/S00105-010-2121-6
- Bolognia JL, Orlow SJ, Glick SA. Lines of Blaschko. J Am Acad Dermatol. 1994;31(2 pt 1):157-190. doi:10.1016/S0190-9622(94)70143-1
- Jan M, Sperling AS, Ebert BL. Cancer therapies based on targeted protein degradation—lessons learned with lenalidomide. Nat Rev Clin Oncol. 2021;18:401-417. doi:10.1038/s41571-021-00479-z
- Shah UA, Mailankody S. Emerging immunotherapies in multiple myeloma. BMJ. 2020;370:3176. doi:10.1136/BMJ.M3176
- Richardson PG, Blood E, Mitsiades CS, et al. A randomized phase 2 study of lenalidomide therapy for patients with relapsed or relapsed and refractory multiple myeloma. Blood. 2006;108:3458-3464. doi:10.1182/BLOOD-2006-04-015909
- Benboubker L, Dimopoulos MA, Dispenzieri A, et al. Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. N Engl J Med. 2014;371:906-917. doi:10.1056/NEJMOA1402551
- Tinsley SM, Kurtin SE, Ridgeway JA. Practical management of lenalidomide-related rash. Clin Lymphoma Myeloma Leuk. 2015;15(suppl):S64-S69. doi:10.1016/J.CLML.2015.02.008
- Nardone B, Wu S, Garden BC, et al. Risk of rash associated with lenalidomide in cancer patients: a systematic review of the literature and meta-analysis. Clin Lymphoma Myeloma Leuk. 2013;13:424-429. doi:10.1016/J.CLML.2013.03.006
- Sviggum HP, Davis MDP, Rajkumar SV, et al. Dermatologic adverse effects of lenalidomide therapy for amyloidosis and multiple myeloma. Arch Dermatol. 2006;142:1298-1302. doi:10.1001/ARCHDERM.142.10.1298
- Sugi T, Nishigami Y, Saigo H, et al. Analysis of risk factors for lenalidomide-associated skin rash in patients with multiple myeloma. Leuk Lymphoma. 2021;62:1405-1410. doi:10.1080/10428194.2021.1876867
- Barley K, He W, Agarwal S, et al. Outcomes and management of lenalidomide-associated rash in patients with multiple myeloma. Leuk Lymphoma. 2016;57:2510-2515. doi:10.3109/10428194.2016.1151507
- Grape J, Frosch P. Papular drug eruption along the lines of Blaschko caused by lenalidomide [in German]. Hautarzt. 2011;62:618-620. doi:10.1007/S00105-010-2121-6
- Bolognia JL, Orlow SJ, Glick SA. Lines of Blaschko. J Am Acad Dermatol. 1994;31(2 pt 1):157-190. doi:10.1016/S0190-9622(94)70143-1
Practice Points
- Dermatologists should be aware of the variety of cutaneous adverse events that can arise from the use of immunotherapeutic agents for hematologic malignancies.
- Some cutaneous reactions to immunotherapeutic medications, such as pityriasiform eruption and blaschkitis, generally are benign and may not necessitate halting an important therapy.