User login
Botanical Briefs: Contact Dermatitis Induced by Western Poison Ivy (Toxicodendron rydbergii)
Clinical Importance
Western poison ivy (Toxicodendron rydbergii) is responsible for many of the cases of Toxicodendron contact dermatitis (TCD) reported in the western and northern United States. Toxicodendron plants cause more cases of allergic contact dermatitis (ACD) in North America than any other allergen1; 9 million Americans present to physician offices and 1.6 million present to emergency departments annually for ACD, emphasizing the notable medical burden of this condition.2,3 Exposure to urushiol, a plant resin containing potent allergens, precipitates this form of ACD.
An estimated 50% to 75% of adults in the United States demonstrate clinical sensitivity and exhibit ACD following contact with T rydbergii.4 Campers, hikers, firefighters, and forest workers often risk increased exposure through physical contact or aerosolized allergens in smoke. According to the Centers for Disease Control and Prevention, the incidence of visits to US emergency departments for TCD nearly doubled from 2002 to 2012,5 which may be explained by atmospheric CO2 levels that both promote increased growth of Toxicodendron species and augment their toxicity.6
Cutaneous Manifestations
The clinical presentation of T rydbergii contact dermatitis is similar to other allergenic members of the Toxicodendron genus. Patients sensitive to urushiol typically develop a pruritic erythematous rash within 1 to 2 days of exposure (range, 5 hours to 15 days).7 Erythematous and edematous streaks initially manifest on the extremities and often progress to bullae and oozing papulovesicles. In early disease, patients also may display black lesions on or near the rash8 (so-called black-dot dermatitis) caused by oxidized urushiol deposited on the skin—an uncommon yet classic presentation of TCD. Generally, symptoms resolve without complications and with few sequalae, though hyperpigmentation or a secondary infection can develop on or near affected areas.9,10
Taxonomy
The Toxicodendron genus belongs to the Anacardiaceae family, which includes pistachios, mangos, and cashews, and causes more cases of ACD than every other plant combined.4 (Shelled pistachios and cashews do not possess cross-reacting allergens and should not worry consumers; mango skin does contain urushiol.)
Toxicodendron (formerly part of the Rhus genus) includes several species of poison oak, poison ivy, and poison sumac and can be found in shrubs (T rydbergii and Toxicodendron diversilobum), vines (Toxicodendron radicans and Toxicodendron pubescens), and trees (Toxicodendron vernix). In addition, Toxicodendron taxa can hybridize with other taxa in close geographic proximity to form morphologic intermediates. Some individual plants have features of multiple species.11
Etymology
The common name of T rydbergii—western poison ivy—misleads the public; the plant contains no poison that can cause death and does not grow as ivy by wrapping around trees, as T radicans and English ivy (Hedera helix) do. Its formal genus, Toxicodendron, means “poison tree” in Greek and was given its generic name by the English botanist Phillip Miller in 1768,12 which caused the renaming of Rhus rydbergii as T rydbergii. The species name honors Per Axel Rydberg, a 19th and 20th century Swedish-American botanist.
Distribution
Toxicodendron rydbergii grows in California and other states in the western half of the United States as well as the states bordering Canada and Mexico. In Canada, it reigns as the most dominant form of poison ivy.13 Hikers and campers find T rydbergii in a variety of areas, including roadsides, river bottoms, sandy shores, talus slopes, precipices, and floodplains.11 This taxon grows under a variety of conditions and in distinct regions, and it thrives in both full sun or shade.
Identifying Features
Toxicodendron rydbergii turns red earlier than most plants; early red summer leaves should serve as a warning sign to hikers from a distance (Figure 1). It displays trifoliate ovate leaves (ie, each leaf contains 3 leaflets) on a dwarf nonclimbing shrub (Figure 2). Although the plant shares common features with its cousin T radicans (eastern poison ivy), T rydbergii is easily distinguished by its thicker stems, absence of aerial rootlets (abundant in T radicans), and short (approximately 1 meter) height.4

Curly hairs occupy the underside of T rydbergii leaflets and along the midrib; leaflet margins appear lobed or rounded. Lenticels appear as small holes in the bark that turn gray in the cold and become brighter come spring.13

The plant bears glabrous long petioles (leaf stems) and densely grouped clusters of yellow flowers. In autumn, the globose fruit—formed in clusters between each twig and leaf petiole (known as an axillary position)—change from yellow-green to tan (Figure 3). When urushiol exudes from damaged leaflets or other plant parts, it oxidizes on exposure to air and creates hardened black deposits on the plant. Even when grown in garden pots, T rydbergii maintains its distinguishing features.11

Dermatitis-Inducing Plant Parts
All parts of T rydbergii including leaves, stems, roots, and fruit contain the allergenic sap throughout the year.14 A person must damage or bruise the plant for urushiol to be released and produce its allergenic effects; softly brushing against undamaged plants typically does not induce dermatitis.4
Pathophysiology of Urushiol
Urushiol, a pale yellow, oily mixture of organic compounds conserved throughout all Toxicodendron species, contains highly allergenic alkyl catechols. These catechols possess hydroxyl groups at positions 1 and 2 on a benzene ring; the hydrocarbon side chain of poison ivies (typically 15–carbon atoms long) attaches at position 3.15 The catechols and the aliphatic side chain contribute to the plant’s antigenic and dermatitis-inducing properties.16
The high lipophilicity of urushiol allows for rapid and unforgiving absorption into the skin, notwithstanding attempts to wash it off. Upon direct contact, catechols of urushiol penetrate the epidermis and become oxidized to quinone intermediates that bind to antigen-presenting cells in the epidermis and dermis. Epidermal Langerhans cells and dermal macrophages internalize and present the antigen to CD4+ T cells in nearby lymph nodes. This sequence results in production of inflammatory mediators, clonal expansion of T-effector and T-memory cells specific to the allergenic catechols, and an ensuing cytotoxic response against epidermal cells and the dermal vasculature. Keratinocytes and monocytes mediate the inflammatory response by releasing other cytokines.4,17
Sensitization to urushiol generally occurs at 8 to 14 years of age; therefore, infants have lower susceptibility to dermatitis upon contact with T rydbergii.18 Most animals do not experience sensitization upon contact; in fact, birds and forest animals consume the urushiol-rich fruit of T rydbergii without harm.3
Prevention and Treatment
Toxicodendron dermatitis typically lasts 1 to 3 weeks but can remain for as long as 6 weeks without treatment.19 Recognition and physical avoidance of the plant provides the most promising preventive strategy. Immediate rinsing with soap and water can prevent TCD by breaking down urushiol and its allergenic components; however, this is an option for only a short time, as the skin absorbs 50% of urushiol within 10 minutes after contact.20 Nevertheless, patients must seize the earliest opportunity to wash off the affected area and remove any residual urushiol. Patients must be cautious when removing and washing clothing to prevent further contact.
Most health care providers treat TCD with a corticosteroid to reduce inflammation and intense pruritus. A high-potency topical corticosteroid (eg, clobetasol) may prove effective in providing early therapeutic relief in mild disease.21 A short course of a systemic steroid quickly and effectively quenches intense itching and should not be limited to what the clinician considers severe disease. Do not underestimate the patient’s symptoms with this eruption.
Prednisone dosing begins at 1 mg/kg daily and is then tapered slowly over 2 weeks (no shorter a time) for an optimal treatment course of 15 days.22 Prescribing an inadequate dosage and course of a corticosteroid leaves the patient susceptible to rebound dermatitis—and loss of trust in their provider.
Intramuscular injection of the long-acting corticosteroid triamcinolone acetonide with rapid-onset betamethasone provides rapid relief and fewer adverse effects than an oral corticosteroid.22 Despite the long-standing use of sedating oral antihistamines by clinicians, these drugs provide no benefit for pruritus or sleep because the histamine does not cause the itching of TCD, and antihistamines disrupt normal sleep architecture.23-25
Patients can consider several over-the-counter products that have varying degrees of efficacy.4,26 The few products for which prospective studies support their use include Tecnu (Tec Laboraties Inc), Zanfel (RhusTox), and the well-known soaps Dial (Henkel Corporation) and Goop (Critzas Industries, Inc).27,28
Aside from treating the direct effects of TCD, clinicians also must take note of any look for signs of secondary infection and occasionally should consider supplementing treatment with an antibiotic.
- Lofgran T, Mahabal GD. Toxicodendron toxicity. StatPearls [Internet]. Updated May 16, 2023. Accessed December 23, 2023. https://www.ncbi.nlm.nih.gov/books/NBK557866/
- The Lewin Group. The Burden of Skin Diseases 2005. Society for Investigative Dermatology and American Academy of Dermatology Association; 2005:37-40. Accessed December 26, 2023. https://www.lewin.com/content/dam/Lewin/Resources/Site_Sections/Publications/april2005skindisease.pdf
- Monroe J. Toxicodendron contact dermatitis: a case report and brief review. J Clin Aesthet Dermatol. 2020;13(9 Suppl 1):S29-S34.
- Gladman AC. Toxicodendron dermatitis: poison ivy, oak, and sumac. Wilderness Environ Med. 2006;17:120-128. doi:10.1580/pr31-05.1
- Fretwell S. Poison ivy cases on the rise. The State. Updated May 15,2017. Accessed December 26, 2023. https://www.thestate.com/news/local/article150403932.html
- Mohan JE, Ziska LH, Schlesinger WH, et al. Biomass and toxicity responses of poison ivy (Toxicodendron radicans) to elevated atmospheric CO2. Proc Natl Acad Sci U S A. 2006;103:9086-9089. doi:10.1073/pnas.0602392103
- Williams JV, Light J, Marks JG Jr. Individual variations in allergic contact dermatitis from urushiol. Arch Dermatol. 1999;135:1002-1003. doi:10.1001/archderm.135.8.1002
- Kurlan JG, Lucky AW. Black spot poison ivy: a report of 5 cases and a review of the literature. J Am Acad Dermatol. 2001;45:246-249. doi:10.1067/mjd.2001.114295
- Fisher AA. Poison ivy/oak/sumac. part II: specific features. Cutis. 1996;58:22-24.
- Brook I, Frazier EH, Yeager JK. Microbiology of infected poison ivy dermatitis. Br J Dermatol. 2000;142:943-946. doi:10.1046/j.1365-2133.2000.03475.x
- Gillis WT. The systematics and ecology of poison-ivy and the poison-oaks (Toxicodendron, Anacardiaceae). Rhodora. 1971;73:370-443.
- Reveal JL. Typification of six Philip Miller names of temperate North American Toxicodendron (Anacardiaceae) with proposals (999-1000) to reject T. crenatum and T. volubile. TAXON. 1991;40:333-335. doi:10.2307/1222994
- Guin JD, Gillis WT, Beaman JH. Recognizing the Toxicodendrons (poison ivy, poison oak, and poison sumac). J Am Acad Dermatol. 1981;4:99-114. doi:10.1016/s0190-9622(81)70014-8
- Lee NP, Arriola ER. Poison ivy, oak, and sumac dermatitis. West J Med. 1999;171:354-355.
- Marks JG Jr, Anderson BE, DeLeo VA, eds. Contact and Occupational Dermatology. Jaypee Brothers Medical Publishers Ltd; 2016.
- Dawson CR. The chemistry of poison ivy. Trans N Y Acad Sci. 1956;18:427-443. doi:10.1111/j.2164-0947.1956.tb00465.x
- Kalish RS. Recent developments in the pathogenesis of allergic contact dermatitis. Arch Dermatol. 1991;127:1558-1563.
- Fisher AA, Mitchell J. Toxicodendron plants and spices. In: Rietschel RL, Fowler JF Jr. Fisher’s Contact Dermatitis. 4th ed. Williams & Wilkins; 1995:461-523.
- Labib A, Yosipovitch G. Itchy Toxicodendron plant dermatitis. Allergies. 2022;2:16-22. doi:10.3390/allergies2010002
- Fisher AA. Poison ivy/oak dermatitis part I: prevention—soap and water, topical barriers, hyposensitization. Cutis. 1996;57:384-386.
- Kim Y, Flamm A, ElSohly MA, et al. Poison ivy, oak, and sumac dermatitis: what is known and what is new? 2019;30:183-190. doi:10.1097/DER.0000000000000472
- Prok L, McGovern T. Poison ivy (Toxicodendron) dermatitis. UpToDate. Updated October 16, 2023. Accessed December 26, 2023. https://www.uptodate.com/contents/poison-ivy-toxicodendron-dermatitis
- Klein PA, Clark RA. An evidence-based review of the efficacy of antihistamines in relieving pruritus in atopic dermatitis. Arch Dermatol. 1999;135:1522-1525. doi:10.1001/archderm.135.12.1522
- He A, Feldman SR, Fleischer AB Jr. An assessment of the use of antihistamines in the management of atopic dermatitis. J Am Acad Dermatol. 2018;79:92-96. doi:10.1016/j.jaad.2017.12.077
- van Zuuren EJ, Apfelbacher CJ, Fedorowicz Z, et al. No high level evidence to support the use of oral H1 antihistamines as monotherapy for eczema: a summary of a Cochrane systematic review. Syst Rev. 2014;3:25. doi:10.1186/2046-4053-3-25
- Neill BC, Neill JA, Brauker J, et al. Postexposure prevention of Toxicodendron dermatitis by early forceful unidirectional washing with liquid dishwashing soap. J Am Acad Dermatol. 2019;81:E25. doi:10.1016/j.jaad.2017.12.081
- Stibich AS, Yagan M, Sharma V, et al. Cost-effective post-exposure prevention of poison ivy dermatitis. Int J Dermatol. 2000;39:515-518. doi:10.1046/j.1365-4362.2000.00003.x
- Davila A, Laurora M, Fulton J, et al. A new topical agent, Zanfel, ameliorates urushiol-induced Toxicodendron allergic contact dermatitis [abstract]. Ann Emerg Med. 2003;42:S98.
Clinical Importance
Western poison ivy (Toxicodendron rydbergii) is responsible for many of the cases of Toxicodendron contact dermatitis (TCD) reported in the western and northern United States. Toxicodendron plants cause more cases of allergic contact dermatitis (ACD) in North America than any other allergen1; 9 million Americans present to physician offices and 1.6 million present to emergency departments annually for ACD, emphasizing the notable medical burden of this condition.2,3 Exposure to urushiol, a plant resin containing potent allergens, precipitates this form of ACD.
An estimated 50% to 75% of adults in the United States demonstrate clinical sensitivity and exhibit ACD following contact with T rydbergii.4 Campers, hikers, firefighters, and forest workers often risk increased exposure through physical contact or aerosolized allergens in smoke. According to the Centers for Disease Control and Prevention, the incidence of visits to US emergency departments for TCD nearly doubled from 2002 to 2012,5 which may be explained by atmospheric CO2 levels that both promote increased growth of Toxicodendron species and augment their toxicity.6
Cutaneous Manifestations
The clinical presentation of T rydbergii contact dermatitis is similar to other allergenic members of the Toxicodendron genus. Patients sensitive to urushiol typically develop a pruritic erythematous rash within 1 to 2 days of exposure (range, 5 hours to 15 days).7 Erythematous and edematous streaks initially manifest on the extremities and often progress to bullae and oozing papulovesicles. In early disease, patients also may display black lesions on or near the rash8 (so-called black-dot dermatitis) caused by oxidized urushiol deposited on the skin—an uncommon yet classic presentation of TCD. Generally, symptoms resolve without complications and with few sequalae, though hyperpigmentation or a secondary infection can develop on or near affected areas.9,10
Taxonomy
The Toxicodendron genus belongs to the Anacardiaceae family, which includes pistachios, mangos, and cashews, and causes more cases of ACD than every other plant combined.4 (Shelled pistachios and cashews do not possess cross-reacting allergens and should not worry consumers; mango skin does contain urushiol.)
Toxicodendron (formerly part of the Rhus genus) includes several species of poison oak, poison ivy, and poison sumac and can be found in shrubs (T rydbergii and Toxicodendron diversilobum), vines (Toxicodendron radicans and Toxicodendron pubescens), and trees (Toxicodendron vernix). In addition, Toxicodendron taxa can hybridize with other taxa in close geographic proximity to form morphologic intermediates. Some individual plants have features of multiple species.11
Etymology
The common name of T rydbergii—western poison ivy—misleads the public; the plant contains no poison that can cause death and does not grow as ivy by wrapping around trees, as T radicans and English ivy (Hedera helix) do. Its formal genus, Toxicodendron, means “poison tree” in Greek and was given its generic name by the English botanist Phillip Miller in 1768,12 which caused the renaming of Rhus rydbergii as T rydbergii. The species name honors Per Axel Rydberg, a 19th and 20th century Swedish-American botanist.
Distribution
Toxicodendron rydbergii grows in California and other states in the western half of the United States as well as the states bordering Canada and Mexico. In Canada, it reigns as the most dominant form of poison ivy.13 Hikers and campers find T rydbergii in a variety of areas, including roadsides, river bottoms, sandy shores, talus slopes, precipices, and floodplains.11 This taxon grows under a variety of conditions and in distinct regions, and it thrives in both full sun or shade.
Identifying Features
Toxicodendron rydbergii turns red earlier than most plants; early red summer leaves should serve as a warning sign to hikers from a distance (Figure 1). It displays trifoliate ovate leaves (ie, each leaf contains 3 leaflets) on a dwarf nonclimbing shrub (Figure 2). Although the plant shares common features with its cousin T radicans (eastern poison ivy), T rydbergii is easily distinguished by its thicker stems, absence of aerial rootlets (abundant in T radicans), and short (approximately 1 meter) height.4

Curly hairs occupy the underside of T rydbergii leaflets and along the midrib; leaflet margins appear lobed or rounded. Lenticels appear as small holes in the bark that turn gray in the cold and become brighter come spring.13

The plant bears glabrous long petioles (leaf stems) and densely grouped clusters of yellow flowers. In autumn, the globose fruit—formed in clusters between each twig and leaf petiole (known as an axillary position)—change from yellow-green to tan (Figure 3). When urushiol exudes from damaged leaflets or other plant parts, it oxidizes on exposure to air and creates hardened black deposits on the plant. Even when grown in garden pots, T rydbergii maintains its distinguishing features.11

Dermatitis-Inducing Plant Parts
All parts of T rydbergii including leaves, stems, roots, and fruit contain the allergenic sap throughout the year.14 A person must damage or bruise the plant for urushiol to be released and produce its allergenic effects; softly brushing against undamaged plants typically does not induce dermatitis.4
Pathophysiology of Urushiol
Urushiol, a pale yellow, oily mixture of organic compounds conserved throughout all Toxicodendron species, contains highly allergenic alkyl catechols. These catechols possess hydroxyl groups at positions 1 and 2 on a benzene ring; the hydrocarbon side chain of poison ivies (typically 15–carbon atoms long) attaches at position 3.15 The catechols and the aliphatic side chain contribute to the plant’s antigenic and dermatitis-inducing properties.16
The high lipophilicity of urushiol allows for rapid and unforgiving absorption into the skin, notwithstanding attempts to wash it off. Upon direct contact, catechols of urushiol penetrate the epidermis and become oxidized to quinone intermediates that bind to antigen-presenting cells in the epidermis and dermis. Epidermal Langerhans cells and dermal macrophages internalize and present the antigen to CD4+ T cells in nearby lymph nodes. This sequence results in production of inflammatory mediators, clonal expansion of T-effector and T-memory cells specific to the allergenic catechols, and an ensuing cytotoxic response against epidermal cells and the dermal vasculature. Keratinocytes and monocytes mediate the inflammatory response by releasing other cytokines.4,17
Sensitization to urushiol generally occurs at 8 to 14 years of age; therefore, infants have lower susceptibility to dermatitis upon contact with T rydbergii.18 Most animals do not experience sensitization upon contact; in fact, birds and forest animals consume the urushiol-rich fruit of T rydbergii without harm.3
Prevention and Treatment
Toxicodendron dermatitis typically lasts 1 to 3 weeks but can remain for as long as 6 weeks without treatment.19 Recognition and physical avoidance of the plant provides the most promising preventive strategy. Immediate rinsing with soap and water can prevent TCD by breaking down urushiol and its allergenic components; however, this is an option for only a short time, as the skin absorbs 50% of urushiol within 10 minutes after contact.20 Nevertheless, patients must seize the earliest opportunity to wash off the affected area and remove any residual urushiol. Patients must be cautious when removing and washing clothing to prevent further contact.
Most health care providers treat TCD with a corticosteroid to reduce inflammation and intense pruritus. A high-potency topical corticosteroid (eg, clobetasol) may prove effective in providing early therapeutic relief in mild disease.21 A short course of a systemic steroid quickly and effectively quenches intense itching and should not be limited to what the clinician considers severe disease. Do not underestimate the patient’s symptoms with this eruption.
Prednisone dosing begins at 1 mg/kg daily and is then tapered slowly over 2 weeks (no shorter a time) for an optimal treatment course of 15 days.22 Prescribing an inadequate dosage and course of a corticosteroid leaves the patient susceptible to rebound dermatitis—and loss of trust in their provider.
Intramuscular injection of the long-acting corticosteroid triamcinolone acetonide with rapid-onset betamethasone provides rapid relief and fewer adverse effects than an oral corticosteroid.22 Despite the long-standing use of sedating oral antihistamines by clinicians, these drugs provide no benefit for pruritus or sleep because the histamine does not cause the itching of TCD, and antihistamines disrupt normal sleep architecture.23-25
Patients can consider several over-the-counter products that have varying degrees of efficacy.4,26 The few products for which prospective studies support their use include Tecnu (Tec Laboraties Inc), Zanfel (RhusTox), and the well-known soaps Dial (Henkel Corporation) and Goop (Critzas Industries, Inc).27,28
Aside from treating the direct effects of TCD, clinicians also must take note of any look for signs of secondary infection and occasionally should consider supplementing treatment with an antibiotic.
Clinical Importance
Western poison ivy (Toxicodendron rydbergii) is responsible for many of the cases of Toxicodendron contact dermatitis (TCD) reported in the western and northern United States. Toxicodendron plants cause more cases of allergic contact dermatitis (ACD) in North America than any other allergen1; 9 million Americans present to physician offices and 1.6 million present to emergency departments annually for ACD, emphasizing the notable medical burden of this condition.2,3 Exposure to urushiol, a plant resin containing potent allergens, precipitates this form of ACD.
An estimated 50% to 75% of adults in the United States demonstrate clinical sensitivity and exhibit ACD following contact with T rydbergii.4 Campers, hikers, firefighters, and forest workers often risk increased exposure through physical contact or aerosolized allergens in smoke. According to the Centers for Disease Control and Prevention, the incidence of visits to US emergency departments for TCD nearly doubled from 2002 to 2012,5 which may be explained by atmospheric CO2 levels that both promote increased growth of Toxicodendron species and augment their toxicity.6
Cutaneous Manifestations
The clinical presentation of T rydbergii contact dermatitis is similar to other allergenic members of the Toxicodendron genus. Patients sensitive to urushiol typically develop a pruritic erythematous rash within 1 to 2 days of exposure (range, 5 hours to 15 days).7 Erythematous and edematous streaks initially manifest on the extremities and often progress to bullae and oozing papulovesicles. In early disease, patients also may display black lesions on or near the rash8 (so-called black-dot dermatitis) caused by oxidized urushiol deposited on the skin—an uncommon yet classic presentation of TCD. Generally, symptoms resolve without complications and with few sequalae, though hyperpigmentation or a secondary infection can develop on or near affected areas.9,10
Taxonomy
The Toxicodendron genus belongs to the Anacardiaceae family, which includes pistachios, mangos, and cashews, and causes more cases of ACD than every other plant combined.4 (Shelled pistachios and cashews do not possess cross-reacting allergens and should not worry consumers; mango skin does contain urushiol.)
Toxicodendron (formerly part of the Rhus genus) includes several species of poison oak, poison ivy, and poison sumac and can be found in shrubs (T rydbergii and Toxicodendron diversilobum), vines (Toxicodendron radicans and Toxicodendron pubescens), and trees (Toxicodendron vernix). In addition, Toxicodendron taxa can hybridize with other taxa in close geographic proximity to form morphologic intermediates. Some individual plants have features of multiple species.11
Etymology
The common name of T rydbergii—western poison ivy—misleads the public; the plant contains no poison that can cause death and does not grow as ivy by wrapping around trees, as T radicans and English ivy (Hedera helix) do. Its formal genus, Toxicodendron, means “poison tree” in Greek and was given its generic name by the English botanist Phillip Miller in 1768,12 which caused the renaming of Rhus rydbergii as T rydbergii. The species name honors Per Axel Rydberg, a 19th and 20th century Swedish-American botanist.
Distribution
Toxicodendron rydbergii grows in California and other states in the western half of the United States as well as the states bordering Canada and Mexico. In Canada, it reigns as the most dominant form of poison ivy.13 Hikers and campers find T rydbergii in a variety of areas, including roadsides, river bottoms, sandy shores, talus slopes, precipices, and floodplains.11 This taxon grows under a variety of conditions and in distinct regions, and it thrives in both full sun or shade.
Identifying Features
Toxicodendron rydbergii turns red earlier than most plants; early red summer leaves should serve as a warning sign to hikers from a distance (Figure 1). It displays trifoliate ovate leaves (ie, each leaf contains 3 leaflets) on a dwarf nonclimbing shrub (Figure 2). Although the plant shares common features with its cousin T radicans (eastern poison ivy), T rydbergii is easily distinguished by its thicker stems, absence of aerial rootlets (abundant in T radicans), and short (approximately 1 meter) height.4

Curly hairs occupy the underside of T rydbergii leaflets and along the midrib; leaflet margins appear lobed or rounded. Lenticels appear as small holes in the bark that turn gray in the cold and become brighter come spring.13

The plant bears glabrous long petioles (leaf stems) and densely grouped clusters of yellow flowers. In autumn, the globose fruit—formed in clusters between each twig and leaf petiole (known as an axillary position)—change from yellow-green to tan (Figure 3). When urushiol exudes from damaged leaflets or other plant parts, it oxidizes on exposure to air and creates hardened black deposits on the plant. Even when grown in garden pots, T rydbergii maintains its distinguishing features.11

Dermatitis-Inducing Plant Parts
All parts of T rydbergii including leaves, stems, roots, and fruit contain the allergenic sap throughout the year.14 A person must damage or bruise the plant for urushiol to be released and produce its allergenic effects; softly brushing against undamaged plants typically does not induce dermatitis.4
Pathophysiology of Urushiol
Urushiol, a pale yellow, oily mixture of organic compounds conserved throughout all Toxicodendron species, contains highly allergenic alkyl catechols. These catechols possess hydroxyl groups at positions 1 and 2 on a benzene ring; the hydrocarbon side chain of poison ivies (typically 15–carbon atoms long) attaches at position 3.15 The catechols and the aliphatic side chain contribute to the plant’s antigenic and dermatitis-inducing properties.16
The high lipophilicity of urushiol allows for rapid and unforgiving absorption into the skin, notwithstanding attempts to wash it off. Upon direct contact, catechols of urushiol penetrate the epidermis and become oxidized to quinone intermediates that bind to antigen-presenting cells in the epidermis and dermis. Epidermal Langerhans cells and dermal macrophages internalize and present the antigen to CD4+ T cells in nearby lymph nodes. This sequence results in production of inflammatory mediators, clonal expansion of T-effector and T-memory cells specific to the allergenic catechols, and an ensuing cytotoxic response against epidermal cells and the dermal vasculature. Keratinocytes and monocytes mediate the inflammatory response by releasing other cytokines.4,17
Sensitization to urushiol generally occurs at 8 to 14 years of age; therefore, infants have lower susceptibility to dermatitis upon contact with T rydbergii.18 Most animals do not experience sensitization upon contact; in fact, birds and forest animals consume the urushiol-rich fruit of T rydbergii without harm.3
Prevention and Treatment
Toxicodendron dermatitis typically lasts 1 to 3 weeks but can remain for as long as 6 weeks without treatment.19 Recognition and physical avoidance of the plant provides the most promising preventive strategy. Immediate rinsing with soap and water can prevent TCD by breaking down urushiol and its allergenic components; however, this is an option for only a short time, as the skin absorbs 50% of urushiol within 10 minutes after contact.20 Nevertheless, patients must seize the earliest opportunity to wash off the affected area and remove any residual urushiol. Patients must be cautious when removing and washing clothing to prevent further contact.
Most health care providers treat TCD with a corticosteroid to reduce inflammation and intense pruritus. A high-potency topical corticosteroid (eg, clobetasol) may prove effective in providing early therapeutic relief in mild disease.21 A short course of a systemic steroid quickly and effectively quenches intense itching and should not be limited to what the clinician considers severe disease. Do not underestimate the patient’s symptoms with this eruption.
Prednisone dosing begins at 1 mg/kg daily and is then tapered slowly over 2 weeks (no shorter a time) for an optimal treatment course of 15 days.22 Prescribing an inadequate dosage and course of a corticosteroid leaves the patient susceptible to rebound dermatitis—and loss of trust in their provider.
Intramuscular injection of the long-acting corticosteroid triamcinolone acetonide with rapid-onset betamethasone provides rapid relief and fewer adverse effects than an oral corticosteroid.22 Despite the long-standing use of sedating oral antihistamines by clinicians, these drugs provide no benefit for pruritus or sleep because the histamine does not cause the itching of TCD, and antihistamines disrupt normal sleep architecture.23-25
Patients can consider several over-the-counter products that have varying degrees of efficacy.4,26 The few products for which prospective studies support their use include Tecnu (Tec Laboraties Inc), Zanfel (RhusTox), and the well-known soaps Dial (Henkel Corporation) and Goop (Critzas Industries, Inc).27,28
Aside from treating the direct effects of TCD, clinicians also must take note of any look for signs of secondary infection and occasionally should consider supplementing treatment with an antibiotic.
- Lofgran T, Mahabal GD. Toxicodendron toxicity. StatPearls [Internet]. Updated May 16, 2023. Accessed December 23, 2023. https://www.ncbi.nlm.nih.gov/books/NBK557866/
- The Lewin Group. The Burden of Skin Diseases 2005. Society for Investigative Dermatology and American Academy of Dermatology Association; 2005:37-40. Accessed December 26, 2023. https://www.lewin.com/content/dam/Lewin/Resources/Site_Sections/Publications/april2005skindisease.pdf
- Monroe J. Toxicodendron contact dermatitis: a case report and brief review. J Clin Aesthet Dermatol. 2020;13(9 Suppl 1):S29-S34.
- Gladman AC. Toxicodendron dermatitis: poison ivy, oak, and sumac. Wilderness Environ Med. 2006;17:120-128. doi:10.1580/pr31-05.1
- Fretwell S. Poison ivy cases on the rise. The State. Updated May 15,2017. Accessed December 26, 2023. https://www.thestate.com/news/local/article150403932.html
- Mohan JE, Ziska LH, Schlesinger WH, et al. Biomass and toxicity responses of poison ivy (Toxicodendron radicans) to elevated atmospheric CO2. Proc Natl Acad Sci U S A. 2006;103:9086-9089. doi:10.1073/pnas.0602392103
- Williams JV, Light J, Marks JG Jr. Individual variations in allergic contact dermatitis from urushiol. Arch Dermatol. 1999;135:1002-1003. doi:10.1001/archderm.135.8.1002
- Kurlan JG, Lucky AW. Black spot poison ivy: a report of 5 cases and a review of the literature. J Am Acad Dermatol. 2001;45:246-249. doi:10.1067/mjd.2001.114295
- Fisher AA. Poison ivy/oak/sumac. part II: specific features. Cutis. 1996;58:22-24.
- Brook I, Frazier EH, Yeager JK. Microbiology of infected poison ivy dermatitis. Br J Dermatol. 2000;142:943-946. doi:10.1046/j.1365-2133.2000.03475.x
- Gillis WT. The systematics and ecology of poison-ivy and the poison-oaks (Toxicodendron, Anacardiaceae). Rhodora. 1971;73:370-443.
- Reveal JL. Typification of six Philip Miller names of temperate North American Toxicodendron (Anacardiaceae) with proposals (999-1000) to reject T. crenatum and T. volubile. TAXON. 1991;40:333-335. doi:10.2307/1222994
- Guin JD, Gillis WT, Beaman JH. Recognizing the Toxicodendrons (poison ivy, poison oak, and poison sumac). J Am Acad Dermatol. 1981;4:99-114. doi:10.1016/s0190-9622(81)70014-8
- Lee NP, Arriola ER. Poison ivy, oak, and sumac dermatitis. West J Med. 1999;171:354-355.
- Marks JG Jr, Anderson BE, DeLeo VA, eds. Contact and Occupational Dermatology. Jaypee Brothers Medical Publishers Ltd; 2016.
- Dawson CR. The chemistry of poison ivy. Trans N Y Acad Sci. 1956;18:427-443. doi:10.1111/j.2164-0947.1956.tb00465.x
- Kalish RS. Recent developments in the pathogenesis of allergic contact dermatitis. Arch Dermatol. 1991;127:1558-1563.
- Fisher AA, Mitchell J. Toxicodendron plants and spices. In: Rietschel RL, Fowler JF Jr. Fisher’s Contact Dermatitis. 4th ed. Williams & Wilkins; 1995:461-523.
- Labib A, Yosipovitch G. Itchy Toxicodendron plant dermatitis. Allergies. 2022;2:16-22. doi:10.3390/allergies2010002
- Fisher AA. Poison ivy/oak dermatitis part I: prevention—soap and water, topical barriers, hyposensitization. Cutis. 1996;57:384-386.
- Kim Y, Flamm A, ElSohly MA, et al. Poison ivy, oak, and sumac dermatitis: what is known and what is new? 2019;30:183-190. doi:10.1097/DER.0000000000000472
- Prok L, McGovern T. Poison ivy (Toxicodendron) dermatitis. UpToDate. Updated October 16, 2023. Accessed December 26, 2023. https://www.uptodate.com/contents/poison-ivy-toxicodendron-dermatitis
- Klein PA, Clark RA. An evidence-based review of the efficacy of antihistamines in relieving pruritus in atopic dermatitis. Arch Dermatol. 1999;135:1522-1525. doi:10.1001/archderm.135.12.1522
- He A, Feldman SR, Fleischer AB Jr. An assessment of the use of antihistamines in the management of atopic dermatitis. J Am Acad Dermatol. 2018;79:92-96. doi:10.1016/j.jaad.2017.12.077
- van Zuuren EJ, Apfelbacher CJ, Fedorowicz Z, et al. No high level evidence to support the use of oral H1 antihistamines as monotherapy for eczema: a summary of a Cochrane systematic review. Syst Rev. 2014;3:25. doi:10.1186/2046-4053-3-25
- Neill BC, Neill JA, Brauker J, et al. Postexposure prevention of Toxicodendron dermatitis by early forceful unidirectional washing with liquid dishwashing soap. J Am Acad Dermatol. 2019;81:E25. doi:10.1016/j.jaad.2017.12.081
- Stibich AS, Yagan M, Sharma V, et al. Cost-effective post-exposure prevention of poison ivy dermatitis. Int J Dermatol. 2000;39:515-518. doi:10.1046/j.1365-4362.2000.00003.x
- Davila A, Laurora M, Fulton J, et al. A new topical agent, Zanfel, ameliorates urushiol-induced Toxicodendron allergic contact dermatitis [abstract]. Ann Emerg Med. 2003;42:S98.
- Lofgran T, Mahabal GD. Toxicodendron toxicity. StatPearls [Internet]. Updated May 16, 2023. Accessed December 23, 2023. https://www.ncbi.nlm.nih.gov/books/NBK557866/
- The Lewin Group. The Burden of Skin Diseases 2005. Society for Investigative Dermatology and American Academy of Dermatology Association; 2005:37-40. Accessed December 26, 2023. https://www.lewin.com/content/dam/Lewin/Resources/Site_Sections/Publications/april2005skindisease.pdf
- Monroe J. Toxicodendron contact dermatitis: a case report and brief review. J Clin Aesthet Dermatol. 2020;13(9 Suppl 1):S29-S34.
- Gladman AC. Toxicodendron dermatitis: poison ivy, oak, and sumac. Wilderness Environ Med. 2006;17:120-128. doi:10.1580/pr31-05.1
- Fretwell S. Poison ivy cases on the rise. The State. Updated May 15,2017. Accessed December 26, 2023. https://www.thestate.com/news/local/article150403932.html
- Mohan JE, Ziska LH, Schlesinger WH, et al. Biomass and toxicity responses of poison ivy (Toxicodendron radicans) to elevated atmospheric CO2. Proc Natl Acad Sci U S A. 2006;103:9086-9089. doi:10.1073/pnas.0602392103
- Williams JV, Light J, Marks JG Jr. Individual variations in allergic contact dermatitis from urushiol. Arch Dermatol. 1999;135:1002-1003. doi:10.1001/archderm.135.8.1002
- Kurlan JG, Lucky AW. Black spot poison ivy: a report of 5 cases and a review of the literature. J Am Acad Dermatol. 2001;45:246-249. doi:10.1067/mjd.2001.114295
- Fisher AA. Poison ivy/oak/sumac. part II: specific features. Cutis. 1996;58:22-24.
- Brook I, Frazier EH, Yeager JK. Microbiology of infected poison ivy dermatitis. Br J Dermatol. 2000;142:943-946. doi:10.1046/j.1365-2133.2000.03475.x
- Gillis WT. The systematics and ecology of poison-ivy and the poison-oaks (Toxicodendron, Anacardiaceae). Rhodora. 1971;73:370-443.
- Reveal JL. Typification of six Philip Miller names of temperate North American Toxicodendron (Anacardiaceae) with proposals (999-1000) to reject T. crenatum and T. volubile. TAXON. 1991;40:333-335. doi:10.2307/1222994
- Guin JD, Gillis WT, Beaman JH. Recognizing the Toxicodendrons (poison ivy, poison oak, and poison sumac). J Am Acad Dermatol. 1981;4:99-114. doi:10.1016/s0190-9622(81)70014-8
- Lee NP, Arriola ER. Poison ivy, oak, and sumac dermatitis. West J Med. 1999;171:354-355.
- Marks JG Jr, Anderson BE, DeLeo VA, eds. Contact and Occupational Dermatology. Jaypee Brothers Medical Publishers Ltd; 2016.
- Dawson CR. The chemistry of poison ivy. Trans N Y Acad Sci. 1956;18:427-443. doi:10.1111/j.2164-0947.1956.tb00465.x
- Kalish RS. Recent developments in the pathogenesis of allergic contact dermatitis. Arch Dermatol. 1991;127:1558-1563.
- Fisher AA, Mitchell J. Toxicodendron plants and spices. In: Rietschel RL, Fowler JF Jr. Fisher’s Contact Dermatitis. 4th ed. Williams & Wilkins; 1995:461-523.
- Labib A, Yosipovitch G. Itchy Toxicodendron plant dermatitis. Allergies. 2022;2:16-22. doi:10.3390/allergies2010002
- Fisher AA. Poison ivy/oak dermatitis part I: prevention—soap and water, topical barriers, hyposensitization. Cutis. 1996;57:384-386.
- Kim Y, Flamm A, ElSohly MA, et al. Poison ivy, oak, and sumac dermatitis: what is known and what is new? 2019;30:183-190. doi:10.1097/DER.0000000000000472
- Prok L, McGovern T. Poison ivy (Toxicodendron) dermatitis. UpToDate. Updated October 16, 2023. Accessed December 26, 2023. https://www.uptodate.com/contents/poison-ivy-toxicodendron-dermatitis
- Klein PA, Clark RA. An evidence-based review of the efficacy of antihistamines in relieving pruritus in atopic dermatitis. Arch Dermatol. 1999;135:1522-1525. doi:10.1001/archderm.135.12.1522
- He A, Feldman SR, Fleischer AB Jr. An assessment of the use of antihistamines in the management of atopic dermatitis. J Am Acad Dermatol. 2018;79:92-96. doi:10.1016/j.jaad.2017.12.077
- van Zuuren EJ, Apfelbacher CJ, Fedorowicz Z, et al. No high level evidence to support the use of oral H1 antihistamines as monotherapy for eczema: a summary of a Cochrane systematic review. Syst Rev. 2014;3:25. doi:10.1186/2046-4053-3-25
- Neill BC, Neill JA, Brauker J, et al. Postexposure prevention of Toxicodendron dermatitis by early forceful unidirectional washing with liquid dishwashing soap. J Am Acad Dermatol. 2019;81:E25. doi:10.1016/j.jaad.2017.12.081
- Stibich AS, Yagan M, Sharma V, et al. Cost-effective post-exposure prevention of poison ivy dermatitis. Int J Dermatol. 2000;39:515-518. doi:10.1046/j.1365-4362.2000.00003.x
- Davila A, Laurora M, Fulton J, et al. A new topical agent, Zanfel, ameliorates urushiol-induced Toxicodendron allergic contact dermatitis [abstract]. Ann Emerg Med. 2003;42:S98.
PRACTICE POINTS
- Western poison ivy (Toxicodendron rydbergii) accounts for many of the cases of Toxicodendron contact dermatitis (TCD) in the western and northern United States. Individuals in these regions should be educated on how to identify T rydbergii to avoid TCD.
- Dermatologists should include TCD in the differential diagnosis when a patient presents with an erythematous pruritic rash in a linear pattern with sharp borders.
- Most patients who experience intense itching and pain from TCD benefit greatly from prompt treatment with an oral or intramuscular corticosteroid. Topical steroids rarely provide relief; oral antihistamines provide no benefit.
Multiple New-Onset Pyogenic Granulomas During Treatment With Paclitaxel and Ramucirumab
To the Editor:
Pyogenic granuloma (PG) is a benign vascular tumor that clinically is characterized as a small eruptive friable papule.1 Lesions typically are solitary and most commonly occur in children but also are associated with pregnancy; trauma to the skin or mucosa; and use of certain medications such as isotretinoin, capecitabine, vemurafenib, or indinavir.1 Numerous antineoplastic medications have been associated with the development of solitary PGs, including the taxane mitotic inhibitor paclitaxel (PTX) and the vascular endothelial growth factor receptor 2 (VEGFR2) monoclonal antibody ramucirumab.2 We report a case of multiple PGs in a patient undergoing treatment with PTX and ramucirumab.

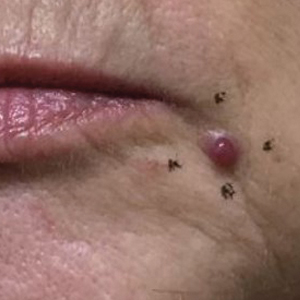
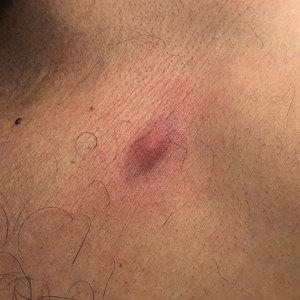
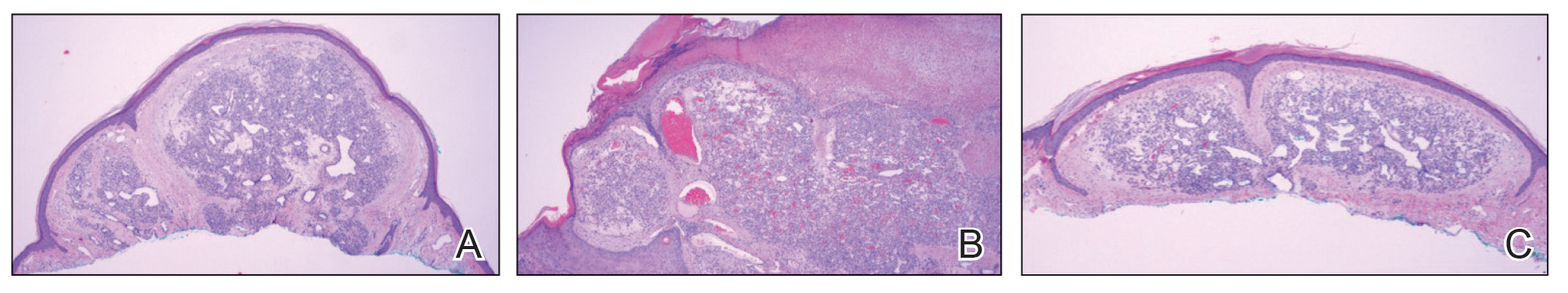
A 59-year-old woman presented to the dermatology clinic with red, itchy, bleeding skin lesions on the breast, superior chest, left cheek, and forearm of 1 month’s duration. She denied any preceding trauma to the areas. Her medical history was notable for gastroesophageal junction adenocarcinoma diagnosed more than 2 years prior to presentation. Her original treatment regimen included nivolumab, which was discontinued for unknown reasons 5 months prior to presentation, and she was started on combination therapy with PTX and ramucirumab at that time. She noted the formation of small red papules 2 months after the initiation of PTX-ramucirumab combination therapy, which grew larger over the course of the next month. Physical examination revealed 5 friable hemorrhagic papules and nodules ranging in size from 3 to 10 mm on the chest, cheek, and forearm consistent with PGs (Figure 1). Several scattered cherry angiomas were noted on the scalp and torso, but the patient reported these were not new. Biopsies of the PGs demonstrated lobular aggregates of small-caliber vessels set in an edematous inflamed stroma and partially enclosed by small collarettes of adnexal epithelium, confirming the clinical diagnosis of multiple PGs (Figure 2).
The first case of PTX-associated PG was reported in 2012.3 Based on a PubMed search of articles indexed for MEDLINE using the terms pyogenic granuloma, lobular capillary hemangioma, paclitaxel, taxane, and ramucirumab, there have been 9 cases of solitary PG development in the setting of PTX alone or in combination with ramucirumab since 2019 (Table).3-8 Pyogenic granulomas reported in patients who were treated exclusively with PTX were subungual, while the cases resulting from combined therapy were present on the scalp, face, oral mucosa, and surfaces of the hands sparing the nails. Ibe et al6 reported PG in a patient who received ramucirumab therapy without PTX but in combination with another taxane, docetaxel, which itself has been reported to cause subungual PG when used alone.9 Our case of the simultaneous development of multiple PGs in the setting of combined PTX and ramucirumab therapy added to the cutaneous distributions for which therapy-induced PGs have been observed (Table).

The development of PG, a vascular tumor, during treatment with the VEGFR2 inhibitor ramucirumab—whose mechanism of action is to inhibit angioneogenesis—is inherently paradoxical. In 2015, a rapidly expanding angioma with a mutation in the kinase domain receptor gene, KDR, that encodes VEGFR2 was identified in a patient undergoing ramucirumab therapy. The authors suggested that KDR mutation resulted in paradoxical activation of VEGFR2 in the setting of ramucirumab therapy.10 Since then, ramucirumab and PTX were suggested to have a synergistic effect in vascular proliferation,5 though an exact mechanism has not been proposed. Other authors have identified increased expression of VEGFR2 in biopsy specimens of PG during combined ramucirumab and taxane therapy.6 Although genetic studies have not been used to evaluate for the presence of KDR mutations specifically in our patient population, it is possible that patients who develop PG and other vascular tumors during combined taxane and ramucirumab therapy have a mutation that makes them more susceptible to VEGFR2 upregulation. UV exposure may have a role in the formation of PG in patients on combined ramucirumab and taxane therapy7; however, our patient’s lesions were distributed on both sun-exposed and unexposed areas. Although potential clinical implications have not yet been thoroughly investigated, following long-term outcomes for these patients may provide important information on the efficacy of the antineoplastic regimen in the subset of patients who develop cutaneous vascular tumors during antiangiogenic treatment.
Combination therapy with PTX and ramucirumab has been associated with the paradoxical development of cutaneous vascular tumors. We report a case of multiple new-onset PGs in a patient undergoing this treatment regimen.
- Elston D, Neuhaus I, James WD, et al. Andrews’ Diseases of the Skin: Clinical Dermatology. 13th ed. Elsevier; 2020.
- Pierson JC. Pyogenic granuloma (lobular capillary hemangioma) clinical presentation. Medscape. Updated February 21, 2020. Accessed December 26, 2023. https://emedicine.medscape.com/article/1084701-clinical#showall
- Paul LJ, Cohen PR. Paclitaxel-associated subungual pyogenic granuloma: report in a patient with breast cancer receiving paclitaxel and review of drug-induced pyogenic granulomas adjacent to and beneath the nail. J Drugs Dermatol. 2012;11:262-268.
- Alessandrini A, Starace M, Cerè G, et al. Management and outcome of taxane-induced nail side effects: experience of 79 patients from a single centre. Skin Appendage Disord. 2019;5:276-282.
- Watanabe R, Nakano E, Kawazoe A, et al. Four cases of paradoxical cephalocervical pyogenic granuloma during treatment with paclitaxel and ramucirumab. J Dermatol. 2019;46:E178-E180.
- Ibe T, Hamamoto Y, Takabatake M, et al. Development of pyogenic granuloma with strong vascular endothelial growth factor receptor-2 expression during ramucirumab treatment. BMJ Case Rep. 2019;12:E231464.
- Choi YH, Byun HJ, Lee JH, et al. Multiple cherry angiomas and pyogenic granuloma in a patient treated with ramucirumab and paclitaxel. Indian J Dermatol Venereol Leprol. 2020;86:199-202.
- Aragaki T, Tomomatsu N, Michi Y, et al. Ramucirumab-related oral pyogenic granuloma: a report of two cases [published online March 8, 2021]. Intern Med. 2021;60:2601-2605. doi:10.2169/internalmedicine.6650-20
- Devillers C, Vanhooteghem O, Henrijean A, et al. Subungual pyogenic granuloma secondary to docetaxel therapy. Clin Exp Dermatol. 2009;34:251-252.
- Lim YH, Odell ID, Ko CJ, et al. Somatic p.T771R KDR (VEGFR2) mutation arising in a sporadic angioma during ramucirumab therapy. JAMA Dermatol. 2015;151:1240-1243.
To the Editor:
Pyogenic granuloma (PG) is a benign vascular tumor that clinically is characterized as a small eruptive friable papule.1 Lesions typically are solitary and most commonly occur in children but also are associated with pregnancy; trauma to the skin or mucosa; and use of certain medications such as isotretinoin, capecitabine, vemurafenib, or indinavir.1 Numerous antineoplastic medications have been associated with the development of solitary PGs, including the taxane mitotic inhibitor paclitaxel (PTX) and the vascular endothelial growth factor receptor 2 (VEGFR2) monoclonal antibody ramucirumab.2 We report a case of multiple PGs in a patient undergoing treatment with PTX and ramucirumab.

A 59-year-old woman presented to the dermatology clinic with red, itchy, bleeding skin lesions on the breast, superior chest, left cheek, and forearm of 1 month’s duration. She denied any preceding trauma to the areas. Her medical history was notable for gastroesophageal junction adenocarcinoma diagnosed more than 2 years prior to presentation. Her original treatment regimen included nivolumab, which was discontinued for unknown reasons 5 months prior to presentation, and she was started on combination therapy with PTX and ramucirumab at that time. She noted the formation of small red papules 2 months after the initiation of PTX-ramucirumab combination therapy, which grew larger over the course of the next month. Physical examination revealed 5 friable hemorrhagic papules and nodules ranging in size from 3 to 10 mm on the chest, cheek, and forearm consistent with PGs (Figure 1). Several scattered cherry angiomas were noted on the scalp and torso, but the patient reported these were not new. Biopsies of the PGs demonstrated lobular aggregates of small-caliber vessels set in an edematous inflamed stroma and partially enclosed by small collarettes of adnexal epithelium, confirming the clinical diagnosis of multiple PGs (Figure 2).

The first case of PTX-associated PG was reported in 2012.3 Based on a PubMed search of articles indexed for MEDLINE using the terms pyogenic granuloma, lobular capillary hemangioma, paclitaxel, taxane, and ramucirumab, there have been 9 cases of solitary PG development in the setting of PTX alone or in combination with ramucirumab since 2019 (Table).3-8 Pyogenic granulomas reported in patients who were treated exclusively with PTX were subungual, while the cases resulting from combined therapy were present on the scalp, face, oral mucosa, and surfaces of the hands sparing the nails. Ibe et al6 reported PG in a patient who received ramucirumab therapy without PTX but in combination with another taxane, docetaxel, which itself has been reported to cause subungual PG when used alone.9 Our case of the simultaneous development of multiple PGs in the setting of combined PTX and ramucirumab therapy added to the cutaneous distributions for which therapy-induced PGs have been observed (Table).

The development of PG, a vascular tumor, during treatment with the VEGFR2 inhibitor ramucirumab—whose mechanism of action is to inhibit angioneogenesis—is inherently paradoxical. In 2015, a rapidly expanding angioma with a mutation in the kinase domain receptor gene, KDR, that encodes VEGFR2 was identified in a patient undergoing ramucirumab therapy. The authors suggested that KDR mutation resulted in paradoxical activation of VEGFR2 in the setting of ramucirumab therapy.10 Since then, ramucirumab and PTX were suggested to have a synergistic effect in vascular proliferation,5 though an exact mechanism has not been proposed. Other authors have identified increased expression of VEGFR2 in biopsy specimens of PG during combined ramucirumab and taxane therapy.6 Although genetic studies have not been used to evaluate for the presence of KDR mutations specifically in our patient population, it is possible that patients who develop PG and other vascular tumors during combined taxane and ramucirumab therapy have a mutation that makes them more susceptible to VEGFR2 upregulation. UV exposure may have a role in the formation of PG in patients on combined ramucirumab and taxane therapy7; however, our patient’s lesions were distributed on both sun-exposed and unexposed areas. Although potential clinical implications have not yet been thoroughly investigated, following long-term outcomes for these patients may provide important information on the efficacy of the antineoplastic regimen in the subset of patients who develop cutaneous vascular tumors during antiangiogenic treatment.
Combination therapy with PTX and ramucirumab has been associated with the paradoxical development of cutaneous vascular tumors. We report a case of multiple new-onset PGs in a patient undergoing this treatment regimen.
To the Editor:
Pyogenic granuloma (PG) is a benign vascular tumor that clinically is characterized as a small eruptive friable papule.1 Lesions typically are solitary and most commonly occur in children but also are associated with pregnancy; trauma to the skin or mucosa; and use of certain medications such as isotretinoin, capecitabine, vemurafenib, or indinavir.1 Numerous antineoplastic medications have been associated with the development of solitary PGs, including the taxane mitotic inhibitor paclitaxel (PTX) and the vascular endothelial growth factor receptor 2 (VEGFR2) monoclonal antibody ramucirumab.2 We report a case of multiple PGs in a patient undergoing treatment with PTX and ramucirumab.

A 59-year-old woman presented to the dermatology clinic with red, itchy, bleeding skin lesions on the breast, superior chest, left cheek, and forearm of 1 month’s duration. She denied any preceding trauma to the areas. Her medical history was notable for gastroesophageal junction adenocarcinoma diagnosed more than 2 years prior to presentation. Her original treatment regimen included nivolumab, which was discontinued for unknown reasons 5 months prior to presentation, and she was started on combination therapy with PTX and ramucirumab at that time. She noted the formation of small red papules 2 months after the initiation of PTX-ramucirumab combination therapy, which grew larger over the course of the next month. Physical examination revealed 5 friable hemorrhagic papules and nodules ranging in size from 3 to 10 mm on the chest, cheek, and forearm consistent with PGs (Figure 1). Several scattered cherry angiomas were noted on the scalp and torso, but the patient reported these were not new. Biopsies of the PGs demonstrated lobular aggregates of small-caliber vessels set in an edematous inflamed stroma and partially enclosed by small collarettes of adnexal epithelium, confirming the clinical diagnosis of multiple PGs (Figure 2).

The first case of PTX-associated PG was reported in 2012.3 Based on a PubMed search of articles indexed for MEDLINE using the terms pyogenic granuloma, lobular capillary hemangioma, paclitaxel, taxane, and ramucirumab, there have been 9 cases of solitary PG development in the setting of PTX alone or in combination with ramucirumab since 2019 (Table).3-8 Pyogenic granulomas reported in patients who were treated exclusively with PTX were subungual, while the cases resulting from combined therapy were present on the scalp, face, oral mucosa, and surfaces of the hands sparing the nails. Ibe et al6 reported PG in a patient who received ramucirumab therapy without PTX but in combination with another taxane, docetaxel, which itself has been reported to cause subungual PG when used alone.9 Our case of the simultaneous development of multiple PGs in the setting of combined PTX and ramucirumab therapy added to the cutaneous distributions for which therapy-induced PGs have been observed (Table).

The development of PG, a vascular tumor, during treatment with the VEGFR2 inhibitor ramucirumab—whose mechanism of action is to inhibit angioneogenesis—is inherently paradoxical. In 2015, a rapidly expanding angioma with a mutation in the kinase domain receptor gene, KDR, that encodes VEGFR2 was identified in a patient undergoing ramucirumab therapy. The authors suggested that KDR mutation resulted in paradoxical activation of VEGFR2 in the setting of ramucirumab therapy.10 Since then, ramucirumab and PTX were suggested to have a synergistic effect in vascular proliferation,5 though an exact mechanism has not been proposed. Other authors have identified increased expression of VEGFR2 in biopsy specimens of PG during combined ramucirumab and taxane therapy.6 Although genetic studies have not been used to evaluate for the presence of KDR mutations specifically in our patient population, it is possible that patients who develop PG and other vascular tumors during combined taxane and ramucirumab therapy have a mutation that makes them more susceptible to VEGFR2 upregulation. UV exposure may have a role in the formation of PG in patients on combined ramucirumab and taxane therapy7; however, our patient’s lesions were distributed on both sun-exposed and unexposed areas. Although potential clinical implications have not yet been thoroughly investigated, following long-term outcomes for these patients may provide important information on the efficacy of the antineoplastic regimen in the subset of patients who develop cutaneous vascular tumors during antiangiogenic treatment.
Combination therapy with PTX and ramucirumab has been associated with the paradoxical development of cutaneous vascular tumors. We report a case of multiple new-onset PGs in a patient undergoing this treatment regimen.
- Elston D, Neuhaus I, James WD, et al. Andrews’ Diseases of the Skin: Clinical Dermatology. 13th ed. Elsevier; 2020.
- Pierson JC. Pyogenic granuloma (lobular capillary hemangioma) clinical presentation. Medscape. Updated February 21, 2020. Accessed December 26, 2023. https://emedicine.medscape.com/article/1084701-clinical#showall
- Paul LJ, Cohen PR. Paclitaxel-associated subungual pyogenic granuloma: report in a patient with breast cancer receiving paclitaxel and review of drug-induced pyogenic granulomas adjacent to and beneath the nail. J Drugs Dermatol. 2012;11:262-268.
- Alessandrini A, Starace M, Cerè G, et al. Management and outcome of taxane-induced nail side effects: experience of 79 patients from a single centre. Skin Appendage Disord. 2019;5:276-282.
- Watanabe R, Nakano E, Kawazoe A, et al. Four cases of paradoxical cephalocervical pyogenic granuloma during treatment with paclitaxel and ramucirumab. J Dermatol. 2019;46:E178-E180.
- Ibe T, Hamamoto Y, Takabatake M, et al. Development of pyogenic granuloma with strong vascular endothelial growth factor receptor-2 expression during ramucirumab treatment. BMJ Case Rep. 2019;12:E231464.
- Choi YH, Byun HJ, Lee JH, et al. Multiple cherry angiomas and pyogenic granuloma in a patient treated with ramucirumab and paclitaxel. Indian J Dermatol Venereol Leprol. 2020;86:199-202.
- Aragaki T, Tomomatsu N, Michi Y, et al. Ramucirumab-related oral pyogenic granuloma: a report of two cases [published online March 8, 2021]. Intern Med. 2021;60:2601-2605. doi:10.2169/internalmedicine.6650-20
- Devillers C, Vanhooteghem O, Henrijean A, et al. Subungual pyogenic granuloma secondary to docetaxel therapy. Clin Exp Dermatol. 2009;34:251-252.
- Lim YH, Odell ID, Ko CJ, et al. Somatic p.T771R KDR (VEGFR2) mutation arising in a sporadic angioma during ramucirumab therapy. JAMA Dermatol. 2015;151:1240-1243.
- Elston D, Neuhaus I, James WD, et al. Andrews’ Diseases of the Skin: Clinical Dermatology. 13th ed. Elsevier; 2020.
- Pierson JC. Pyogenic granuloma (lobular capillary hemangioma) clinical presentation. Medscape. Updated February 21, 2020. Accessed December 26, 2023. https://emedicine.medscape.com/article/1084701-clinical#showall
- Paul LJ, Cohen PR. Paclitaxel-associated subungual pyogenic granuloma: report in a patient with breast cancer receiving paclitaxel and review of drug-induced pyogenic granulomas adjacent to and beneath the nail. J Drugs Dermatol. 2012;11:262-268.
- Alessandrini A, Starace M, Cerè G, et al. Management and outcome of taxane-induced nail side effects: experience of 79 patients from a single centre. Skin Appendage Disord. 2019;5:276-282.
- Watanabe R, Nakano E, Kawazoe A, et al. Four cases of paradoxical cephalocervical pyogenic granuloma during treatment with paclitaxel and ramucirumab. J Dermatol. 2019;46:E178-E180.
- Ibe T, Hamamoto Y, Takabatake M, et al. Development of pyogenic granuloma with strong vascular endothelial growth factor receptor-2 expression during ramucirumab treatment. BMJ Case Rep. 2019;12:E231464.
- Choi YH, Byun HJ, Lee JH, et al. Multiple cherry angiomas and pyogenic granuloma in a patient treated with ramucirumab and paclitaxel. Indian J Dermatol Venereol Leprol. 2020;86:199-202.
- Aragaki T, Tomomatsu N, Michi Y, et al. Ramucirumab-related oral pyogenic granuloma: a report of two cases [published online March 8, 2021]. Intern Med. 2021;60:2601-2605. doi:10.2169/internalmedicine.6650-20
- Devillers C, Vanhooteghem O, Henrijean A, et al. Subungual pyogenic granuloma secondary to docetaxel therapy. Clin Exp Dermatol. 2009;34:251-252.
- Lim YH, Odell ID, Ko CJ, et al. Somatic p.T771R KDR (VEGFR2) mutation arising in a sporadic angioma during ramucirumab therapy. JAMA Dermatol. 2015;151:1240-1243.
Practice Points
- Pyogenic granulomas (PGs) are benign vascular tumors that clinically are characterized as small, eruptive, friable papules.
- Ramucirumab is a monoclonal antibody against vascular endothelial growth factor receptor 2.
- Some patients experience paradoxical formation of vascular tumors such as PGs when treated with combination therapy with ramucirumab and a taxane such as paclitaxel.
Cemiplimab-Associated Eruption of Generalized Eruptive Keratoacanthoma of Grzybowski
To the Editor:
Treatment of cancer, including cutaneous malignancy, has been transformed by the use of immunotherapeutic agents such as immune checkpoint inhibitors (ICIs) that target cytotoxic T lymphocyte-associated antigen 4, programmed cell-death protein 1 (PD-1), or programmed cell-death ligand 1 (PD-L1). However, these drugs are associated with a distinct set of immune-related adverse events (IRAEs). We present a case of generalized eruptive keratoacanthoma of Grzybowski associated with the ICI cemiplimab.
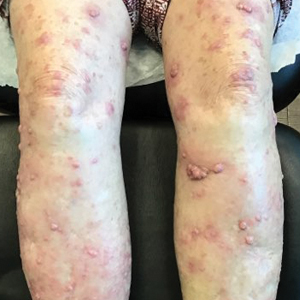
A 94-year-old White woman presented to the dermatology clinic with acute onset of extensive, locally advanced cutaneous squamous cell carcinoma (cSCC) of the upper right posterolateral calf as well as multiple noninvasive cSCCs of the arms and legs. Her medical history was remarkable for widespread actinic keratoses and numerous cSCCs. The patient had no personal or family history of melanoma. Various cSCCs had required treatment with electrodesiccation and curettage, topical or intralesional 5-fluorouracil, and Mohs micrographic surgery. Approximately 1 year prior to presentation, oral acitretin was initiated to help control the cSCC. Given the extent of locally advanced disease, which was considered unresectable, she was referred to oncology but continued to follow up with dermatology. Positron emission tomography was remarkable for hypermetabolic cutaneous thickening in the upper right posterolateral calf with no evidence of visceral disease.

The patient was started on cemiplimab, an anti-PD-1 monoclonal antibody ICI indicated for the treatment of both metastatic and advanced cSCC. After 4 cycles of intravenous cemiplimab, the patient developed widespread nodules covering the arms and legs (Figure 1) as well as associated tenderness and pruritus. Biopsies of nodules revealed superficially invasive, well-differentiated cSCC consistent with keratoacanthoma. Although a lymphocytic infiltrate was present, no other specific reaction pattern, such as a lichenoid infiltrate, was present (Figure 2).

Positron emission tomography was repeated, demonstrating resolution of the right calf lesion; however, new diffuse cutaneous lesions and inguinal lymph node involvement were present, again without evidence of visceral disease. Given the clinical and histologic findings, a diagnosis of generalized eruptive keratoacanthoma of Grzybowski was made. Cemiplimab was discontinued after the fifth cycle. The patient declined further systemic treatment, instead choosing a regimen of topical steroids and an emollient.
Immunotherapeutics have transformed cancer therapy, which includes ICIs that target cytotoxic T lymphocyte-associated antigen 4, PD-1, or PD-L1. Increased activity of these checkpoints allows tumor cells to downregulate T-cell activation, thereby evading immune destruction. When PD-1 on T cells binds PD-L1 on tumor cells, T lymphocytes are inhibited from cytotoxic-mediated killing. Therefore, anti-PD-1 ICIs such as cemiplimab permit T-lymphocyte activation and destruction of malignant cells. However, this unique mechanism of immunotherapy is associated with an array of IRAEs, which often manifest in a delayed and prolonged fashion.1 Immune-related adverse events most commonly affect the gastrointestinal tract as well as the endocrine and dermatologic systems.2 Notably, patients with certain tumors who experience these adverse effects might be more likely to have superior overall survival; therefore, IRAEs are sometimes used as an indicator of favorable treatment response.2,3
Dermatologic IRAEs associated with the use of a PD-1 inhibitor include lichenoid reactions, pruritus, morbilliform eruptions, vitiligo, and bullous pemphigoid.4,5 Eruptions of keratoacanthoma rarely have been reported following treatment with the PD-1 inhibitors nivolumab and pembrolizumab.3,6,7 In our patient, we believe the profound and generalized eruptive keratoacanthoma—a well-differentiated cSCC variant—was related to treatment of locally advanced cSCC with cemiplimab. The mechanism underlying the formation of anti-PD-1 eruptive keratoacanthoma is not well understood. In susceptible patients, it is plausible that the inflammatory environment permitted by ICIs paradoxically induces regression of tumors such as locally invasive cSCC and simultaneously promotes formation of keratoacanthoma.
The role of inflammation in the pathogenesis and progression of cSCC is complex and possibly involves contrasting roles of leukocyte subpopulations.8 The increased incidence of cSCC in the immunocompromised population,8 PD-L1 overexpression in cSCC,9,10 and successful treatment of cSCC with PD-1 inhibition10 all suggest that inhibition of specific inflammatory pathways is pivotal in tumor pathogenesis. However, increased inflammation, particularly inflammation driven by T lymphocytes and Langerhans cells, also is believed to play a key role in the formation of cSCCs, including the degeneration of actinic keratosis into cSCC. Moreover, because keratoacanthomas are believed to be a cSCC variant and also are associated with PD-L1 overexpression,9 it is perplexing that PD-1 blockade may result in eruptive keratoacanthoma in some patients while also treating locally advanced cSCC, as seen in our patient. Successful treatment of keratoacanthoma with anti-inflammatory intralesional or topical corticosteroids adds to this complicated picture.3
We hypothesize that the pathogenesis of invasive cSCC and keratoacanthoma shares certain immune-mediated mechanisms but also differs in distinct manners. To understand the relationship between systemic treatment of cSCC and eruptive keratoacanthoma, further research is required.
In addition, the RAS/BRAF/MEK oncogenic pathway may be involved in the development of cSCCs associated with anti-PD-1. It is hypothesized that BRAF and MEK inhibition increases T-cell infiltration and increases PD-L1 expression on tumor cells,11 thus increasing the susceptibility of those cells to PD-1 blockade. Further supporting a relationship between the RAS/BRAF/MEK and PD-1 pathways, BRAF inhibitors are associated with development of SCCs and verrucal keratosis by upregulation of the RAS pathway.12,13 Perhaps a common mechanism underlying these pathways results in their shared association for an increased risk for cSCC upon blockade. More research is needed to fully elucidate the underlying biochemical mechanism of immunotherapy and formation of SCCs, such as keratoacanthoma.
Treatment of solitary keratoacanthoma often involves surgical excision; however, the sheer number of lesions in eruptive keratoacanthoma presents a larger dilemma. Because oral systemic retinoids have been shown to be most effective for treating eruptive keratoacanthoma, they are considered first-line therapy as monotherapy or in combination with surgical excision.3 Other treatment options include intralesional or topical corticosteroids, cyclosporine, 5-fluorouracil, imiquimod, and cryotherapy.3,6
The development of ICIs has revolutionized the treatment of cutaneous malignancy, yet we have a great deal more to comprehend on the systemic effects of these medications. Although IRAEs may signal a better response to therapy, some of these effects regrettably can be dose limiting. In our patient, cemiplimab was successful in treating locally advanced cSCC, but treatment also resulted in devastating widespread eruptive keratoacanthoma. The mechanism of this kind of eruption has yet to be understood; we hypothesize that it likely involves T lymphocyte–driven inflammation and the interplay of molecular and immune-mediated pathways.
- Ramos-Casals M, Brahmer JR, Callahan MK, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers. 2020;6:38. doi:10.1038/s41572-020-0160-6
- Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019;7:306. doi:10.1186/s40425-019-0805-8
- Freites-Martinez A, Kwong BY, Rieger KE, et al. Eruptive keratoacanthomas associated with pembrolizumab therapy. JAMA Dermatol. 2017;153:694-697. doi:10.1001/jamadermatol.2017.0989
- Shen J, Chang J, Mendenhall M, et al. Diverse cutaneous adverse eruptions caused by anti-programmed cell death-1 (PD-1) and anti-programmed cell death ligand-1 (PD-L1) immunotherapies: clinicalfeatures and management. Ther Adv Med Oncol. 2018;10:1758834017751634. doi:10.1177/1758834017751634
- Bandino JP, Perry DM, Clarke CE, et al. Two cases of anti-programmed cell death 1-associated bullous pemphigoid-like disease and eruptive keratoacanthomas featuring combined histopathology. J Eur Acad Dermatol Venereol. 2017;31:E378-E380. doi:10.1111/jdv.14179
- Marsh RL, Kolodney JA, Iyengar S, et al. Formation of eruptive cutaneous squamous cell carcinomas after programmed cell death protein-1 blockade. JAAD Case Rep. 2020;6:390-393. doi:10.1016/j.jdcr.2020.02.024
- Antonov NK, Nair KG, Halasz CL. Transient eruptive keratoacanthomas associated with nivolumab. JAAD Case Rep. 2019;5:342-345. doi:10.1016/j.jdcr.2019.01.025
- Bottomley MJ, Thomson J, Harwood C, et al. The role of the immune system in cutaneous squamous cell carcinoma. Int J Mol Sci. 2019;20:2009. doi:10.3390/ijms20082009
- Gambichler T, Gnielka M, Rüddel I, et al. Expression of PD-L1 in keratoacanthoma and different stages of progression in cutaneous squamous cell carcinoma. Cancer Immunol Immunother. 2017;66:1199-1204. doi:10.1007/s00262-017-2015-x
- Patel R, Chang ALS. Immune checkpoint inhibitors for treating advanced cutaneous squamous cell carcinoma. Am J Clin Dermatol. 2019;20:477-482. doi:10.1007/s40257-019-00426-w
- Rozeman EA, Blank CU. Combining checkpoint inhibition and targeted therapy in melanoma. Nat Med. 2019;25:879-882. doi:10.1038/s41591-019-0482-7
- Dubauskas Z, Kunishige J, Prieto VG, Jonasch E, Hwu P, Tannir NM. Cutaneous squamous cell carcinoma and inflammation of actinic keratoses associated with sorafenib. Clin Genitourin Cancer. 2009;7:20-23. doi:10.3816/CGC.2009.n.003
- Chen P, Chen F, Zhou B. Systematic review and meta-analysis of prevalence of dermatological toxicities associated with vemurafenib treatment in patients with melanoma. Clin Exp Dermatol. 2019;44:243-251. doi:10.1111/ced.13751
To the Editor:
Treatment of cancer, including cutaneous malignancy, has been transformed by the use of immunotherapeutic agents such as immune checkpoint inhibitors (ICIs) that target cytotoxic T lymphocyte-associated antigen 4, programmed cell-death protein 1 (PD-1), or programmed cell-death ligand 1 (PD-L1). However, these drugs are associated with a distinct set of immune-related adverse events (IRAEs). We present a case of generalized eruptive keratoacanthoma of Grzybowski associated with the ICI cemiplimab.
A 94-year-old White woman presented to the dermatology clinic with acute onset of extensive, locally advanced cutaneous squamous cell carcinoma (cSCC) of the upper right posterolateral calf as well as multiple noninvasive cSCCs of the arms and legs. Her medical history was remarkable for widespread actinic keratoses and numerous cSCCs. The patient had no personal or family history of melanoma. Various cSCCs had required treatment with electrodesiccation and curettage, topical or intralesional 5-fluorouracil, and Mohs micrographic surgery. Approximately 1 year prior to presentation, oral acitretin was initiated to help control the cSCC. Given the extent of locally advanced disease, which was considered unresectable, she was referred to oncology but continued to follow up with dermatology. Positron emission tomography was remarkable for hypermetabolic cutaneous thickening in the upper right posterolateral calf with no evidence of visceral disease.

The patient was started on cemiplimab, an anti-PD-1 monoclonal antibody ICI indicated for the treatment of both metastatic and advanced cSCC. After 4 cycles of intravenous cemiplimab, the patient developed widespread nodules covering the arms and legs (Figure 1) as well as associated tenderness and pruritus. Biopsies of nodules revealed superficially invasive, well-differentiated cSCC consistent with keratoacanthoma. Although a lymphocytic infiltrate was present, no other specific reaction pattern, such as a lichenoid infiltrate, was present (Figure 2).

Positron emission tomography was repeated, demonstrating resolution of the right calf lesion; however, new diffuse cutaneous lesions and inguinal lymph node involvement were present, again without evidence of visceral disease. Given the clinical and histologic findings, a diagnosis of generalized eruptive keratoacanthoma of Grzybowski was made. Cemiplimab was discontinued after the fifth cycle. The patient declined further systemic treatment, instead choosing a regimen of topical steroids and an emollient.
Immunotherapeutics have transformed cancer therapy, which includes ICIs that target cytotoxic T lymphocyte-associated antigen 4, PD-1, or PD-L1. Increased activity of these checkpoints allows tumor cells to downregulate T-cell activation, thereby evading immune destruction. When PD-1 on T cells binds PD-L1 on tumor cells, T lymphocytes are inhibited from cytotoxic-mediated killing. Therefore, anti-PD-1 ICIs such as cemiplimab permit T-lymphocyte activation and destruction of malignant cells. However, this unique mechanism of immunotherapy is associated with an array of IRAEs, which often manifest in a delayed and prolonged fashion.1 Immune-related adverse events most commonly affect the gastrointestinal tract as well as the endocrine and dermatologic systems.2 Notably, patients with certain tumors who experience these adverse effects might be more likely to have superior overall survival; therefore, IRAEs are sometimes used as an indicator of favorable treatment response.2,3
Dermatologic IRAEs associated with the use of a PD-1 inhibitor include lichenoid reactions, pruritus, morbilliform eruptions, vitiligo, and bullous pemphigoid.4,5 Eruptions of keratoacanthoma rarely have been reported following treatment with the PD-1 inhibitors nivolumab and pembrolizumab.3,6,7 In our patient, we believe the profound and generalized eruptive keratoacanthoma—a well-differentiated cSCC variant—was related to treatment of locally advanced cSCC with cemiplimab. The mechanism underlying the formation of anti-PD-1 eruptive keratoacanthoma is not well understood. In susceptible patients, it is plausible that the inflammatory environment permitted by ICIs paradoxically induces regression of tumors such as locally invasive cSCC and simultaneously promotes formation of keratoacanthoma.
The role of inflammation in the pathogenesis and progression of cSCC is complex and possibly involves contrasting roles of leukocyte subpopulations.8 The increased incidence of cSCC in the immunocompromised population,8 PD-L1 overexpression in cSCC,9,10 and successful treatment of cSCC with PD-1 inhibition10 all suggest that inhibition of specific inflammatory pathways is pivotal in tumor pathogenesis. However, increased inflammation, particularly inflammation driven by T lymphocytes and Langerhans cells, also is believed to play a key role in the formation of cSCCs, including the degeneration of actinic keratosis into cSCC. Moreover, because keratoacanthomas are believed to be a cSCC variant and also are associated with PD-L1 overexpression,9 it is perplexing that PD-1 blockade may result in eruptive keratoacanthoma in some patients while also treating locally advanced cSCC, as seen in our patient. Successful treatment of keratoacanthoma with anti-inflammatory intralesional or topical corticosteroids adds to this complicated picture.3
We hypothesize that the pathogenesis of invasive cSCC and keratoacanthoma shares certain immune-mediated mechanisms but also differs in distinct manners. To understand the relationship between systemic treatment of cSCC and eruptive keratoacanthoma, further research is required.
In addition, the RAS/BRAF/MEK oncogenic pathway may be involved in the development of cSCCs associated with anti-PD-1. It is hypothesized that BRAF and MEK inhibition increases T-cell infiltration and increases PD-L1 expression on tumor cells,11 thus increasing the susceptibility of those cells to PD-1 blockade. Further supporting a relationship between the RAS/BRAF/MEK and PD-1 pathways, BRAF inhibitors are associated with development of SCCs and verrucal keratosis by upregulation of the RAS pathway.12,13 Perhaps a common mechanism underlying these pathways results in their shared association for an increased risk for cSCC upon blockade. More research is needed to fully elucidate the underlying biochemical mechanism of immunotherapy and formation of SCCs, such as keratoacanthoma.
Treatment of solitary keratoacanthoma often involves surgical excision; however, the sheer number of lesions in eruptive keratoacanthoma presents a larger dilemma. Because oral systemic retinoids have been shown to be most effective for treating eruptive keratoacanthoma, they are considered first-line therapy as monotherapy or in combination with surgical excision.3 Other treatment options include intralesional or topical corticosteroids, cyclosporine, 5-fluorouracil, imiquimod, and cryotherapy.3,6
The development of ICIs has revolutionized the treatment of cutaneous malignancy, yet we have a great deal more to comprehend on the systemic effects of these medications. Although IRAEs may signal a better response to therapy, some of these effects regrettably can be dose limiting. In our patient, cemiplimab was successful in treating locally advanced cSCC, but treatment also resulted in devastating widespread eruptive keratoacanthoma. The mechanism of this kind of eruption has yet to be understood; we hypothesize that it likely involves T lymphocyte–driven inflammation and the interplay of molecular and immune-mediated pathways.
To the Editor:
Treatment of cancer, including cutaneous malignancy, has been transformed by the use of immunotherapeutic agents such as immune checkpoint inhibitors (ICIs) that target cytotoxic T lymphocyte-associated antigen 4, programmed cell-death protein 1 (PD-1), or programmed cell-death ligand 1 (PD-L1). However, these drugs are associated with a distinct set of immune-related adverse events (IRAEs). We present a case of generalized eruptive keratoacanthoma of Grzybowski associated with the ICI cemiplimab.
A 94-year-old White woman presented to the dermatology clinic with acute onset of extensive, locally advanced cutaneous squamous cell carcinoma (cSCC) of the upper right posterolateral calf as well as multiple noninvasive cSCCs of the arms and legs. Her medical history was remarkable for widespread actinic keratoses and numerous cSCCs. The patient had no personal or family history of melanoma. Various cSCCs had required treatment with electrodesiccation and curettage, topical or intralesional 5-fluorouracil, and Mohs micrographic surgery. Approximately 1 year prior to presentation, oral acitretin was initiated to help control the cSCC. Given the extent of locally advanced disease, which was considered unresectable, she was referred to oncology but continued to follow up with dermatology. Positron emission tomography was remarkable for hypermetabolic cutaneous thickening in the upper right posterolateral calf with no evidence of visceral disease.

The patient was started on cemiplimab, an anti-PD-1 monoclonal antibody ICI indicated for the treatment of both metastatic and advanced cSCC. After 4 cycles of intravenous cemiplimab, the patient developed widespread nodules covering the arms and legs (Figure 1) as well as associated tenderness and pruritus. Biopsies of nodules revealed superficially invasive, well-differentiated cSCC consistent with keratoacanthoma. Although a lymphocytic infiltrate was present, no other specific reaction pattern, such as a lichenoid infiltrate, was present (Figure 2).

Positron emission tomography was repeated, demonstrating resolution of the right calf lesion; however, new diffuse cutaneous lesions and inguinal lymph node involvement were present, again without evidence of visceral disease. Given the clinical and histologic findings, a diagnosis of generalized eruptive keratoacanthoma of Grzybowski was made. Cemiplimab was discontinued after the fifth cycle. The patient declined further systemic treatment, instead choosing a regimen of topical steroids and an emollient.
Immunotherapeutics have transformed cancer therapy, which includes ICIs that target cytotoxic T lymphocyte-associated antigen 4, PD-1, or PD-L1. Increased activity of these checkpoints allows tumor cells to downregulate T-cell activation, thereby evading immune destruction. When PD-1 on T cells binds PD-L1 on tumor cells, T lymphocytes are inhibited from cytotoxic-mediated killing. Therefore, anti-PD-1 ICIs such as cemiplimab permit T-lymphocyte activation and destruction of malignant cells. However, this unique mechanism of immunotherapy is associated with an array of IRAEs, which often manifest in a delayed and prolonged fashion.1 Immune-related adverse events most commonly affect the gastrointestinal tract as well as the endocrine and dermatologic systems.2 Notably, patients with certain tumors who experience these adverse effects might be more likely to have superior overall survival; therefore, IRAEs are sometimes used as an indicator of favorable treatment response.2,3
Dermatologic IRAEs associated with the use of a PD-1 inhibitor include lichenoid reactions, pruritus, morbilliform eruptions, vitiligo, and bullous pemphigoid.4,5 Eruptions of keratoacanthoma rarely have been reported following treatment with the PD-1 inhibitors nivolumab and pembrolizumab.3,6,7 In our patient, we believe the profound and generalized eruptive keratoacanthoma—a well-differentiated cSCC variant—was related to treatment of locally advanced cSCC with cemiplimab. The mechanism underlying the formation of anti-PD-1 eruptive keratoacanthoma is not well understood. In susceptible patients, it is plausible that the inflammatory environment permitted by ICIs paradoxically induces regression of tumors such as locally invasive cSCC and simultaneously promotes formation of keratoacanthoma.
The role of inflammation in the pathogenesis and progression of cSCC is complex and possibly involves contrasting roles of leukocyte subpopulations.8 The increased incidence of cSCC in the immunocompromised population,8 PD-L1 overexpression in cSCC,9,10 and successful treatment of cSCC with PD-1 inhibition10 all suggest that inhibition of specific inflammatory pathways is pivotal in tumor pathogenesis. However, increased inflammation, particularly inflammation driven by T lymphocytes and Langerhans cells, also is believed to play a key role in the formation of cSCCs, including the degeneration of actinic keratosis into cSCC. Moreover, because keratoacanthomas are believed to be a cSCC variant and also are associated with PD-L1 overexpression,9 it is perplexing that PD-1 blockade may result in eruptive keratoacanthoma in some patients while also treating locally advanced cSCC, as seen in our patient. Successful treatment of keratoacanthoma with anti-inflammatory intralesional or topical corticosteroids adds to this complicated picture.3
We hypothesize that the pathogenesis of invasive cSCC and keratoacanthoma shares certain immune-mediated mechanisms but also differs in distinct manners. To understand the relationship between systemic treatment of cSCC and eruptive keratoacanthoma, further research is required.
In addition, the RAS/BRAF/MEK oncogenic pathway may be involved in the development of cSCCs associated with anti-PD-1. It is hypothesized that BRAF and MEK inhibition increases T-cell infiltration and increases PD-L1 expression on tumor cells,11 thus increasing the susceptibility of those cells to PD-1 blockade. Further supporting a relationship between the RAS/BRAF/MEK and PD-1 pathways, BRAF inhibitors are associated with development of SCCs and verrucal keratosis by upregulation of the RAS pathway.12,13 Perhaps a common mechanism underlying these pathways results in their shared association for an increased risk for cSCC upon blockade. More research is needed to fully elucidate the underlying biochemical mechanism of immunotherapy and formation of SCCs, such as keratoacanthoma.
Treatment of solitary keratoacanthoma often involves surgical excision; however, the sheer number of lesions in eruptive keratoacanthoma presents a larger dilemma. Because oral systemic retinoids have been shown to be most effective for treating eruptive keratoacanthoma, they are considered first-line therapy as monotherapy or in combination with surgical excision.3 Other treatment options include intralesional or topical corticosteroids, cyclosporine, 5-fluorouracil, imiquimod, and cryotherapy.3,6
The development of ICIs has revolutionized the treatment of cutaneous malignancy, yet we have a great deal more to comprehend on the systemic effects of these medications. Although IRAEs may signal a better response to therapy, some of these effects regrettably can be dose limiting. In our patient, cemiplimab was successful in treating locally advanced cSCC, but treatment also resulted in devastating widespread eruptive keratoacanthoma. The mechanism of this kind of eruption has yet to be understood; we hypothesize that it likely involves T lymphocyte–driven inflammation and the interplay of molecular and immune-mediated pathways.
- Ramos-Casals M, Brahmer JR, Callahan MK, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers. 2020;6:38. doi:10.1038/s41572-020-0160-6
- Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019;7:306. doi:10.1186/s40425-019-0805-8
- Freites-Martinez A, Kwong BY, Rieger KE, et al. Eruptive keratoacanthomas associated with pembrolizumab therapy. JAMA Dermatol. 2017;153:694-697. doi:10.1001/jamadermatol.2017.0989
- Shen J, Chang J, Mendenhall M, et al. Diverse cutaneous adverse eruptions caused by anti-programmed cell death-1 (PD-1) and anti-programmed cell death ligand-1 (PD-L1) immunotherapies: clinicalfeatures and management. Ther Adv Med Oncol. 2018;10:1758834017751634. doi:10.1177/1758834017751634
- Bandino JP, Perry DM, Clarke CE, et al. Two cases of anti-programmed cell death 1-associated bullous pemphigoid-like disease and eruptive keratoacanthomas featuring combined histopathology. J Eur Acad Dermatol Venereol. 2017;31:E378-E380. doi:10.1111/jdv.14179
- Marsh RL, Kolodney JA, Iyengar S, et al. Formation of eruptive cutaneous squamous cell carcinomas after programmed cell death protein-1 blockade. JAAD Case Rep. 2020;6:390-393. doi:10.1016/j.jdcr.2020.02.024
- Antonov NK, Nair KG, Halasz CL. Transient eruptive keratoacanthomas associated with nivolumab. JAAD Case Rep. 2019;5:342-345. doi:10.1016/j.jdcr.2019.01.025
- Bottomley MJ, Thomson J, Harwood C, et al. The role of the immune system in cutaneous squamous cell carcinoma. Int J Mol Sci. 2019;20:2009. doi:10.3390/ijms20082009
- Gambichler T, Gnielka M, Rüddel I, et al. Expression of PD-L1 in keratoacanthoma and different stages of progression in cutaneous squamous cell carcinoma. Cancer Immunol Immunother. 2017;66:1199-1204. doi:10.1007/s00262-017-2015-x
- Patel R, Chang ALS. Immune checkpoint inhibitors for treating advanced cutaneous squamous cell carcinoma. Am J Clin Dermatol. 2019;20:477-482. doi:10.1007/s40257-019-00426-w
- Rozeman EA, Blank CU. Combining checkpoint inhibition and targeted therapy in melanoma. Nat Med. 2019;25:879-882. doi:10.1038/s41591-019-0482-7
- Dubauskas Z, Kunishige J, Prieto VG, Jonasch E, Hwu P, Tannir NM. Cutaneous squamous cell carcinoma and inflammation of actinic keratoses associated with sorafenib. Clin Genitourin Cancer. 2009;7:20-23. doi:10.3816/CGC.2009.n.003
- Chen P, Chen F, Zhou B. Systematic review and meta-analysis of prevalence of dermatological toxicities associated with vemurafenib treatment in patients with melanoma. Clin Exp Dermatol. 2019;44:243-251. doi:10.1111/ced.13751
- Ramos-Casals M, Brahmer JR, Callahan MK, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers. 2020;6:38. doi:10.1038/s41572-020-0160-6
- Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019;7:306. doi:10.1186/s40425-019-0805-8
- Freites-Martinez A, Kwong BY, Rieger KE, et al. Eruptive keratoacanthomas associated with pembrolizumab therapy. JAMA Dermatol. 2017;153:694-697. doi:10.1001/jamadermatol.2017.0989
- Shen J, Chang J, Mendenhall M, et al. Diverse cutaneous adverse eruptions caused by anti-programmed cell death-1 (PD-1) and anti-programmed cell death ligand-1 (PD-L1) immunotherapies: clinicalfeatures and management. Ther Adv Med Oncol. 2018;10:1758834017751634. doi:10.1177/1758834017751634
- Bandino JP, Perry DM, Clarke CE, et al. Two cases of anti-programmed cell death 1-associated bullous pemphigoid-like disease and eruptive keratoacanthomas featuring combined histopathology. J Eur Acad Dermatol Venereol. 2017;31:E378-E380. doi:10.1111/jdv.14179
- Marsh RL, Kolodney JA, Iyengar S, et al. Formation of eruptive cutaneous squamous cell carcinomas after programmed cell death protein-1 blockade. JAAD Case Rep. 2020;6:390-393. doi:10.1016/j.jdcr.2020.02.024
- Antonov NK, Nair KG, Halasz CL. Transient eruptive keratoacanthomas associated with nivolumab. JAAD Case Rep. 2019;5:342-345. doi:10.1016/j.jdcr.2019.01.025
- Bottomley MJ, Thomson J, Harwood C, et al. The role of the immune system in cutaneous squamous cell carcinoma. Int J Mol Sci. 2019;20:2009. doi:10.3390/ijms20082009
- Gambichler T, Gnielka M, Rüddel I, et al. Expression of PD-L1 in keratoacanthoma and different stages of progression in cutaneous squamous cell carcinoma. Cancer Immunol Immunother. 2017;66:1199-1204. doi:10.1007/s00262-017-2015-x
- Patel R, Chang ALS. Immune checkpoint inhibitors for treating advanced cutaneous squamous cell carcinoma. Am J Clin Dermatol. 2019;20:477-482. doi:10.1007/s40257-019-00426-w
- Rozeman EA, Blank CU. Combining checkpoint inhibition and targeted therapy in melanoma. Nat Med. 2019;25:879-882. doi:10.1038/s41591-019-0482-7
- Dubauskas Z, Kunishige J, Prieto VG, Jonasch E, Hwu P, Tannir NM. Cutaneous squamous cell carcinoma and inflammation of actinic keratoses associated with sorafenib. Clin Genitourin Cancer. 2009;7:20-23. doi:10.3816/CGC.2009.n.003
- Chen P, Chen F, Zhou B. Systematic review and meta-analysis of prevalence of dermatological toxicities associated with vemurafenib treatment in patients with melanoma. Clin Exp Dermatol. 2019;44:243-251. doi:10.1111/ced.13751
Practice Points
- Immunotherapy, including immune checkpoint inhibitors such as programmed cell-death protein 1 (PD-1) inhibitors, is associated with an array of immune-related adverse events that often manifest in a delayed and prolonged manner. They most commonly affect the gastrointestinal tract as well as the endocrine and dermatologic systems.
- Dermatologic adverse effects associated with PD-1 inhibitors include lichenoid reactions, pruritus, morbilliform eruptions, vitiligo, and bullous pemphigoid.
- Eruptions of keratoacanthoma rarely have been reported following treatment with PD-1 inhibitors such as cemiplimab, nivolumab, and pembrolizumab.
Age-Friendly Health Systems and Meeting the Principles of High Reliability Organizations in the VHA
The Veterans Health Administration (VHA) is the largest integrated health care system in the US, providing care to more than 9 million enrolled veterans at 1298 facilities.1 In February 2019, the VHA identified key action steps to become a high reliability organization (HRO), transforming how employees think about patient safety and care quality.2 The VHA is also working toward becoming the largest age-friendly health system in the US to be recognized by the Institute for Healthcare Improvement (IHI) for its commitment to providing care guided by the 4Ms (what matters, medication, mentation, and mobility), causing no harm, and aligning care with what matters to older veterans.3 In this article, we describe how the Age-Friendly Health Systems (AFHS) movement supports the culture shift observed in HROs.
Age-Friendly Veteran Care
By 2060, the US population of adults aged ≥ 65 years is projected to increase to about 95 million.3 In the VHA, nearly half of veteran enrollees are aged ≥ 65 years, necessitating evidence-based models of care, such as the 4Ms, to meet their complex care needs.3 Historically, the VHA has been a leader in caring for older adults, recognizing the value of age-friendly care for veterans.4 In 1975, the VHA established the Geriatric Research, Education, and Clinical Centers (GRECCs) to serve as catalysts for developing, implementing, and refining enduring models of geriatric care.4 For 5 decades, GRECCs have driven innovations related to the 4Ms.
The VHA is well positioned to be a leader in the AFHS movement, building on decades of GRECC innovations and geriatric programs that align with the 4Ms and providing specialized geriatric training for health care professionals to expand age-friendly care to new settings and health systems.4 The AFHS movement organizes the 4Ms into a simple framework for frontline staff, and the VHA has recently begun tracking 4Ms care in the electronic health record (EHR) to facilitate evaluation and continuous improvement.
AFHS use the 4Ms as a framework to be implemented in every care setting, from the emergency department to inpatient units, outpatient settings, and postacute and long-term care. By assessing and acting on each M and practicing the 4Ms collectively, all members of the care team work to improve health outcomes and prevent avoidable harm.5
The 4Ms
What matters, is the driver of this person-centered approach. Any member of the care team may initiate a what matters conversation with the older adult to understand their personal values, health goals, and care preferences. When compared with usual care, care aligned with the older adult’s health priorities has been shown to decrease the use of high-risk medications and reduce treatment burden.6 The VHA has adopted Whole Health principles of care and the Patient Priorities Care approach to identify and support what matters to veterans.7,8
Addressing polypharmacy and identifying and deprescribing potentially inappropriate medications are essential in preventing adverse drug events, drug-drug interactions, and medication nonadherence.9 In the VHA, VIONE (Vital, Important, Optional, Not indicated, Every medication has an indication) is a rapidly expanding medication deprescribing program that exemplifies HRO principles.9 VIONE provides medication management that supports shared decision making, reducing risk and improving patient safety and quality of life.9 As of June 2023, > 600,000 unique veterans have benefited from VIONE, with an average of 2.2 medications deprescribed per patient with an annual cost avoidance of > $100 million.10
Assessing and acting on mentation includes preventing, identifying, and managing depression and dementia in outpatient settings and delirium in hospital and long-term care settings.5 There are many tools and clinical reminders available in the EHR so that interdisciplinary teams can document changes to mentation and identify opportunities for continuous improvement.
Closely aligned with mentation is mobility, with evidence suggesting that regular physical activity reduces the risk of falls (preventing associated complications), maintains physical functioning, and lowers the risk of cognitive impairment and depression.5 Ensuring early, frequent, and safe mobility helps patients achieve better health outcomes and prevent injury.5 Mobility programs within the VHA include the STRIDE program for the inpatient setting and Gerofit for outpatient settings.11,12
HRO Principles
An HRO is a complex environment of care that experiences fewer than anticipated accidents or adverse events by (1) establishing trust among leaders and staff by balancing individual accountability with systems thinking; (2) empowering staff to lead continuous process improvements; and (3) creating an environment where employees feel safe to report harm or near misses, focusing on the reasons errors occur.13 The work of AFHS incorporates HRO principles with an emphasis on 3 elements. First, it involves interactive systems and processes needed to support 4Ms care across care settings. Second, AFHS acknowledge the complexity of age-friendly work and deference to the expertise of interdisciplinary team members. Finally, AFHS are committed to resilience by overcoming failures and challenges to implementation and long-term sustainment as a standard of practice.
Case study
The names and details in this case have been modified to protect patient privacy. It is representative of many Community Living Centers (CLCs) involved in AFHS that work to create a safe, person-centered environment for veterans.
In a CLC team workroom, 2 nurses were discussing a long-term care resident. The nurses approached the attending physician and explained that they were worried about Sgt Johnson, who seemed depressed and sometimes combative. They had noticed a change in his behavior when they helped him clean up after an episode of incontinence and were concerned that he would try to get out of bed on his own and fall. The attending physician thanked them for sharing their concerns. Sgt Johnson was a retired Army veteran who had a long, decorated military career. His chronic health conditions had led to muscle weakness, and he fell and broke a hip before this admission. He had an uneventful hip replacement but was showing signs of depression due to his limited mobility, loss of independence, and inability to live at home without additional support.
The attending physician knocked on the door of his room, sat down next to the bed, and asked, “How are you feeling today?” Sgt Johnson tersely replied, “About the same.” The physician asked, “Sgt Johnson, what matters most to you related to your recovery? What is important to you?” Sgt Johnson responded, “Feeling like a man!” The doctor replied, “So what makes you feel ‘not like a man’?” The Sgt replied, “Having to be cleaned up by the nurses and not being able to use the toilet on my own.” The physician surmised that his decline in physical functioning had a connection to his worsening depression and combativeness and said to the Sgt, “Let’s get the team together and work out a plan to get you strong enough to use a bedside commode by yourself. Let’s make that the first goal in our plan to get you back to using the toilet independently. Can you work with us on that?” He smiled and said, “Sir, yes Sir!”
At the weekly interdisciplinary team meeting, the team discussed Sgt Johnson’s wishes and the nurses’ safety concerns. The physician reported to the team what mattered to the veteran. The nurses arranged for a bedside commode and supplies to be placed in his room, encouraged and assisted him, and provided a privacy screen. The physical therapist continued to support his mobility needs, concentrating on transfers, small steps like standing and turning with a walker to get in position to use the bedside commode, and later the bathroom toilet. The psychologist addressed what matters to Sgt Johnson and his mentation, health goals, and coping strategies. The social worker provided support and counseling for the veteran and his family. The pharmacist checked his medications to be sure that none were affecting his gastrointestinal tract and his ability to move safely and do what matters to him. Knowing what mattered to Sgt Johnson was the driver of the interdisciplinary care plan to provide 4Ms care.
The team worked collaboratively with the veteran to develop and set attainable goals around toileting and regaining his dignity. This improved his overall recovery. As Sgt Johnson became more independent, his mood gradually improved and he began to participate in other activities and interact with other residents on the unit, and he did not experience any falls. By addressing the 4Ms, the interdisciplinary team coordinated efforts to provide high-quality, person-centered care. They built trust with the veteran, shared accountability, and followed HRO principles to keep the veteran safe.
Becoming an Age-Friendly HRO
Becoming an HRO is a dynamic, ever-changing process to maintain high standards, improve care quality, and cause no harm. There are 3 pillars and 5 principles that guide an HRO. The pillars are critical areas of focus and include leadership commitment, culture of safety, and continuous process improvement.14 The first of 5 HRO principles is sensitivity to operations. This is defined as an awareness of how processes and systems impact the entire organization, the downstream impact.15 Focusing on the 4Ms helps develop the capability of frontline staff to provide high-quality care for older adults while ensuring that processes are in place to support the work. The 4Ms provide an efficient way to organize interdisciplinary team meetings, provide warm handoffs using Situation-Background-Assessment-Recommendation, and standardize documentation. Involvement in the AFHS movement improves communication, care quality, and patient and staff satisfaction to meet this HRO principle.15
The second HRO principle, reluctance to simplify, ensures that direct care staff and leaders delve further into issues to find solutions.15 AFHS use the Plan-Do-Study-Act cycle to put the 4Ms into practice; this cycle helps teams test small increments of change, study their performance, and act to ensure that all 4Ms are being practiced as a set. AFHS teams are encouraged to review at least 3 months of data after implementation of the 4Ms, working to find solutions if there are gaps or issues identified.
The third principle, preoccupation with failure, refers to shared attentiveness—being prepared for the unexpected and learning from mistakes.15 The entire AFHS team shares responsibility for providing 4Ms care, where staff are empowered to report any safety concerns or close calls. The fourth principle of deference to expertise includes listening to staff who have the most knowledge for the task at hand, which aligns with the collaborative interdisciplinary teamwork of age-friendly teams.15
The final HRO principle, commitment to resilience, includes continuous learning, interdisciplinary team training, and sharing of lessons learned.15 Although IHI offers 2 levels of AFHS recognition, teams are continuously learning to improve and sustain care beyond level 2, Committed to Care Excellence recognition.16
The Table shows the VHA’s AFHS implementation strategies and the HRO principles adapted from the Joint Commission’s High Reliability Health Care Maturity Model and the IHI’s Framework for Safe, Reliable, and Effective Care. The VHA is developing a national dashboard to capture age-friendly processes and health outcome measures that address patient safety and care quality.
Conclusions
AFHS empowers VHA teams to honor veterans’ care preferences and values, supporting their independence, dignity, and quality of life across care settings. The adoption of AFHS brings evidence-based practices to the point of care by addressing common pitfalls in the care of older adults, drawing attention to, and calling for action on inappropriate medication use, physical inactivity, and assessment of the vulnerable brain. The 4Ms also serve as a framework to continuously improve care and cause zero harm, reinforcing HRO pillars and principles across the VHA, and ensuring that older adults reliably receive the evidence-based, high-quality care they deserve.
1. Veterans Health Administration. Providing healthcare for veterans. Updated June 20, 2023. Accessed June 26, 2023. https://www.va.gov/health
2. Veazie S, Peterson K, Bourne D. Evidence brief: implementation of high reliability organization principles. Washington, DC: Evidence Synthesis Program, Health Services Research and Development Service, Office of Research and Development, Department of Veterans Affairs. VA ESP Project #09-199; 2019. Accessed November 30, 2023. https://www.hsrd.research.va.gov/publications/esp/high-reliability-org.cfm
3. Church K, Munro S, Shaughnessy M, Clancy C. Age-Friendly Health Systems: improving care for older adults in the Veterans Health Administration. Health Serv Res. 2023;58(suppl 1):5-8. doi:10.1111/1475-6773.14110
4. Farrell TW, Volden TA, Butler JM, et al. Age-friendly care in the Veterans Health Administration: past, present, and future. J Am Geriatr Soc. 2023;71(1):18-25. doi:10.1111/jgs.18070
5. Mate K, Fulmer T, Pelton L, et al. Evidence for the 4Ms: interactions and outcomes across the care continuum. J Aging Health. 2021;33(7-8):469-481. doi:10.1177/0898264321991658
6. Tinetti ME, Naik AD, Dindo L, et al. Association of patient priorities-aligned decision-making with patient outcomes and ambulatory health care burden among older adults with multiple chronic conditions: A nonrandomized clinical trial. JAMA Intern Med. 2019;179(12):1688-1697. doi:10.1001/jamainternmed.2019.4235
7. US Department of Veterans Affairs. What is whole health? Updated: October 31, 2023. November 30, 2023. https://www.va.gov/wholehealth
8. Patient Priorities Care. Updated 2019. Accessed November 30, 2023. https://patientprioritiescare.org
9. Battar S, Watson Dickerson KR, Sedgwick C, Cmelik T. Understanding principles of high reliability organizations through the eyes of VIONE: a clinical program to improve patient safety by deprescribing potentially inappropriate medications and reducing polypharmacy. Fed Pract. 2019;36(12):564-568.
10. VA Diffusion Marketplace. VIONE- medication optimization and polypharmacy reduction initiative. Accessed November 30, 2023. https://marketplace.va.gov/innovations/vione
11. US Department of Veterans Affairs, Office of Research and Development. STRIDE program to keep hospitalized veterans mobile. Updated November 6, 2018. Accessed November 30, 2023. https://www.research.va.gov/research_in_action/STRIDE-program-to-keep-hospitalized-Veterans-mobile.cfm
12. US Department of Veterans Affairs, VA Geriatrics and Extended Care. Gerofit: a program promoting exercise and health for older veterans. Updated August 2, 2023. Accessed November 30, 2023. https://www.va.gov/GERIATRICS/pages/gerofit_Home.asp
13. US Department of Veterans Affairs, Health Services Research and Development. VHA’s vision for a high reliability organization. Updated August 14, 2020. Accessed November 30, 2023. https://www.hsrd.research.va.gov/publications/forum/summer20/default.cfm?ForumMenu=summer20-1
14. US Department of Veterans Affairs, Health Services Research and Development. Three HRO evaluation priorities. Updated August 14, 2020. Accessed November 30, 2023. https://www.hsrd.research.va.gov/publications/forum/summer20/default.cfm?ForumMenu=summer20-2
15. Oster CA, Deakins S. Practical application of high-reliability principles in healthcare to optimize quality and safety outcomes. J Nurs Adm. 2018;48(1):50-55. doi:10.1097/NNA.0000000000000570
16. Institute for Healthcare Improvement. Age-Friendly Health Systems recognitions. Accessed November 30, 2023. https://www.ihi.org/Engage/Initiatives/Age-Friendly-Health-Systems/Pages/Recognition.aspx
The Veterans Health Administration (VHA) is the largest integrated health care system in the US, providing care to more than 9 million enrolled veterans at 1298 facilities.1 In February 2019, the VHA identified key action steps to become a high reliability organization (HRO), transforming how employees think about patient safety and care quality.2 The VHA is also working toward becoming the largest age-friendly health system in the US to be recognized by the Institute for Healthcare Improvement (IHI) for its commitment to providing care guided by the 4Ms (what matters, medication, mentation, and mobility), causing no harm, and aligning care with what matters to older veterans.3 In this article, we describe how the Age-Friendly Health Systems (AFHS) movement supports the culture shift observed in HROs.
Age-Friendly Veteran Care
By 2060, the US population of adults aged ≥ 65 years is projected to increase to about 95 million.3 In the VHA, nearly half of veteran enrollees are aged ≥ 65 years, necessitating evidence-based models of care, such as the 4Ms, to meet their complex care needs.3 Historically, the VHA has been a leader in caring for older adults, recognizing the value of age-friendly care for veterans.4 In 1975, the VHA established the Geriatric Research, Education, and Clinical Centers (GRECCs) to serve as catalysts for developing, implementing, and refining enduring models of geriatric care.4 For 5 decades, GRECCs have driven innovations related to the 4Ms.
The VHA is well positioned to be a leader in the AFHS movement, building on decades of GRECC innovations and geriatric programs that align with the 4Ms and providing specialized geriatric training for health care professionals to expand age-friendly care to new settings and health systems.4 The AFHS movement organizes the 4Ms into a simple framework for frontline staff, and the VHA has recently begun tracking 4Ms care in the electronic health record (EHR) to facilitate evaluation and continuous improvement.
AFHS use the 4Ms as a framework to be implemented in every care setting, from the emergency department to inpatient units, outpatient settings, and postacute and long-term care. By assessing and acting on each M and practicing the 4Ms collectively, all members of the care team work to improve health outcomes and prevent avoidable harm.5
The 4Ms
What matters, is the driver of this person-centered approach. Any member of the care team may initiate a what matters conversation with the older adult to understand their personal values, health goals, and care preferences. When compared with usual care, care aligned with the older adult’s health priorities has been shown to decrease the use of high-risk medications and reduce treatment burden.6 The VHA has adopted Whole Health principles of care and the Patient Priorities Care approach to identify and support what matters to veterans.7,8
Addressing polypharmacy and identifying and deprescribing potentially inappropriate medications are essential in preventing adverse drug events, drug-drug interactions, and medication nonadherence.9 In the VHA, VIONE (Vital, Important, Optional, Not indicated, Every medication has an indication) is a rapidly expanding medication deprescribing program that exemplifies HRO principles.9 VIONE provides medication management that supports shared decision making, reducing risk and improving patient safety and quality of life.9 As of June 2023, > 600,000 unique veterans have benefited from VIONE, with an average of 2.2 medications deprescribed per patient with an annual cost avoidance of > $100 million.10
Assessing and acting on mentation includes preventing, identifying, and managing depression and dementia in outpatient settings and delirium in hospital and long-term care settings.5 There are many tools and clinical reminders available in the EHR so that interdisciplinary teams can document changes to mentation and identify opportunities for continuous improvement.
Closely aligned with mentation is mobility, with evidence suggesting that regular physical activity reduces the risk of falls (preventing associated complications), maintains physical functioning, and lowers the risk of cognitive impairment and depression.5 Ensuring early, frequent, and safe mobility helps patients achieve better health outcomes and prevent injury.5 Mobility programs within the VHA include the STRIDE program for the inpatient setting and Gerofit for outpatient settings.11,12
HRO Principles
An HRO is a complex environment of care that experiences fewer than anticipated accidents or adverse events by (1) establishing trust among leaders and staff by balancing individual accountability with systems thinking; (2) empowering staff to lead continuous process improvements; and (3) creating an environment where employees feel safe to report harm or near misses, focusing on the reasons errors occur.13 The work of AFHS incorporates HRO principles with an emphasis on 3 elements. First, it involves interactive systems and processes needed to support 4Ms care across care settings. Second, AFHS acknowledge the complexity of age-friendly work and deference to the expertise of interdisciplinary team members. Finally, AFHS are committed to resilience by overcoming failures and challenges to implementation and long-term sustainment as a standard of practice.
Case study
The names and details in this case have been modified to protect patient privacy. It is representative of many Community Living Centers (CLCs) involved in AFHS that work to create a safe, person-centered environment for veterans.
In a CLC team workroom, 2 nurses were discussing a long-term care resident. The nurses approached the attending physician and explained that they were worried about Sgt Johnson, who seemed depressed and sometimes combative. They had noticed a change in his behavior when they helped him clean up after an episode of incontinence and were concerned that he would try to get out of bed on his own and fall. The attending physician thanked them for sharing their concerns. Sgt Johnson was a retired Army veteran who had a long, decorated military career. His chronic health conditions had led to muscle weakness, and he fell and broke a hip before this admission. He had an uneventful hip replacement but was showing signs of depression due to his limited mobility, loss of independence, and inability to live at home without additional support.
The attending physician knocked on the door of his room, sat down next to the bed, and asked, “How are you feeling today?” Sgt Johnson tersely replied, “About the same.” The physician asked, “Sgt Johnson, what matters most to you related to your recovery? What is important to you?” Sgt Johnson responded, “Feeling like a man!” The doctor replied, “So what makes you feel ‘not like a man’?” The Sgt replied, “Having to be cleaned up by the nurses and not being able to use the toilet on my own.” The physician surmised that his decline in physical functioning had a connection to his worsening depression and combativeness and said to the Sgt, “Let’s get the team together and work out a plan to get you strong enough to use a bedside commode by yourself. Let’s make that the first goal in our plan to get you back to using the toilet independently. Can you work with us on that?” He smiled and said, “Sir, yes Sir!”
At the weekly interdisciplinary team meeting, the team discussed Sgt Johnson’s wishes and the nurses’ safety concerns. The physician reported to the team what mattered to the veteran. The nurses arranged for a bedside commode and supplies to be placed in his room, encouraged and assisted him, and provided a privacy screen. The physical therapist continued to support his mobility needs, concentrating on transfers, small steps like standing and turning with a walker to get in position to use the bedside commode, and later the bathroom toilet. The psychologist addressed what matters to Sgt Johnson and his mentation, health goals, and coping strategies. The social worker provided support and counseling for the veteran and his family. The pharmacist checked his medications to be sure that none were affecting his gastrointestinal tract and his ability to move safely and do what matters to him. Knowing what mattered to Sgt Johnson was the driver of the interdisciplinary care plan to provide 4Ms care.
The team worked collaboratively with the veteran to develop and set attainable goals around toileting and regaining his dignity. This improved his overall recovery. As Sgt Johnson became more independent, his mood gradually improved and he began to participate in other activities and interact with other residents on the unit, and he did not experience any falls. By addressing the 4Ms, the interdisciplinary team coordinated efforts to provide high-quality, person-centered care. They built trust with the veteran, shared accountability, and followed HRO principles to keep the veteran safe.
Becoming an Age-Friendly HRO
Becoming an HRO is a dynamic, ever-changing process to maintain high standards, improve care quality, and cause no harm. There are 3 pillars and 5 principles that guide an HRO. The pillars are critical areas of focus and include leadership commitment, culture of safety, and continuous process improvement.14 The first of 5 HRO principles is sensitivity to operations. This is defined as an awareness of how processes and systems impact the entire organization, the downstream impact.15 Focusing on the 4Ms helps develop the capability of frontline staff to provide high-quality care for older adults while ensuring that processes are in place to support the work. The 4Ms provide an efficient way to organize interdisciplinary team meetings, provide warm handoffs using Situation-Background-Assessment-Recommendation, and standardize documentation. Involvement in the AFHS movement improves communication, care quality, and patient and staff satisfaction to meet this HRO principle.15
The second HRO principle, reluctance to simplify, ensures that direct care staff and leaders delve further into issues to find solutions.15 AFHS use the Plan-Do-Study-Act cycle to put the 4Ms into practice; this cycle helps teams test small increments of change, study their performance, and act to ensure that all 4Ms are being practiced as a set. AFHS teams are encouraged to review at least 3 months of data after implementation of the 4Ms, working to find solutions if there are gaps or issues identified.
The third principle, preoccupation with failure, refers to shared attentiveness—being prepared for the unexpected and learning from mistakes.15 The entire AFHS team shares responsibility for providing 4Ms care, where staff are empowered to report any safety concerns or close calls. The fourth principle of deference to expertise includes listening to staff who have the most knowledge for the task at hand, which aligns with the collaborative interdisciplinary teamwork of age-friendly teams.15
The final HRO principle, commitment to resilience, includes continuous learning, interdisciplinary team training, and sharing of lessons learned.15 Although IHI offers 2 levels of AFHS recognition, teams are continuously learning to improve and sustain care beyond level 2, Committed to Care Excellence recognition.16
The Table shows the VHA’s AFHS implementation strategies and the HRO principles adapted from the Joint Commission’s High Reliability Health Care Maturity Model and the IHI’s Framework for Safe, Reliable, and Effective Care. The VHA is developing a national dashboard to capture age-friendly processes and health outcome measures that address patient safety and care quality.
Conclusions
AFHS empowers VHA teams to honor veterans’ care preferences and values, supporting their independence, dignity, and quality of life across care settings. The adoption of AFHS brings evidence-based practices to the point of care by addressing common pitfalls in the care of older adults, drawing attention to, and calling for action on inappropriate medication use, physical inactivity, and assessment of the vulnerable brain. The 4Ms also serve as a framework to continuously improve care and cause zero harm, reinforcing HRO pillars and principles across the VHA, and ensuring that older adults reliably receive the evidence-based, high-quality care they deserve.
The Veterans Health Administration (VHA) is the largest integrated health care system in the US, providing care to more than 9 million enrolled veterans at 1298 facilities.1 In February 2019, the VHA identified key action steps to become a high reliability organization (HRO), transforming how employees think about patient safety and care quality.2 The VHA is also working toward becoming the largest age-friendly health system in the US to be recognized by the Institute for Healthcare Improvement (IHI) for its commitment to providing care guided by the 4Ms (what matters, medication, mentation, and mobility), causing no harm, and aligning care with what matters to older veterans.3 In this article, we describe how the Age-Friendly Health Systems (AFHS) movement supports the culture shift observed in HROs.
Age-Friendly Veteran Care
By 2060, the US population of adults aged ≥ 65 years is projected to increase to about 95 million.3 In the VHA, nearly half of veteran enrollees are aged ≥ 65 years, necessitating evidence-based models of care, such as the 4Ms, to meet their complex care needs.3 Historically, the VHA has been a leader in caring for older adults, recognizing the value of age-friendly care for veterans.4 In 1975, the VHA established the Geriatric Research, Education, and Clinical Centers (GRECCs) to serve as catalysts for developing, implementing, and refining enduring models of geriatric care.4 For 5 decades, GRECCs have driven innovations related to the 4Ms.
The VHA is well positioned to be a leader in the AFHS movement, building on decades of GRECC innovations and geriatric programs that align with the 4Ms and providing specialized geriatric training for health care professionals to expand age-friendly care to new settings and health systems.4 The AFHS movement organizes the 4Ms into a simple framework for frontline staff, and the VHA has recently begun tracking 4Ms care in the electronic health record (EHR) to facilitate evaluation and continuous improvement.
AFHS use the 4Ms as a framework to be implemented in every care setting, from the emergency department to inpatient units, outpatient settings, and postacute and long-term care. By assessing and acting on each M and practicing the 4Ms collectively, all members of the care team work to improve health outcomes and prevent avoidable harm.5
The 4Ms
What matters, is the driver of this person-centered approach. Any member of the care team may initiate a what matters conversation with the older adult to understand their personal values, health goals, and care preferences. When compared with usual care, care aligned with the older adult’s health priorities has been shown to decrease the use of high-risk medications and reduce treatment burden.6 The VHA has adopted Whole Health principles of care and the Patient Priorities Care approach to identify and support what matters to veterans.7,8
Addressing polypharmacy and identifying and deprescribing potentially inappropriate medications are essential in preventing adverse drug events, drug-drug interactions, and medication nonadherence.9 In the VHA, VIONE (Vital, Important, Optional, Not indicated, Every medication has an indication) is a rapidly expanding medication deprescribing program that exemplifies HRO principles.9 VIONE provides medication management that supports shared decision making, reducing risk and improving patient safety and quality of life.9 As of June 2023, > 600,000 unique veterans have benefited from VIONE, with an average of 2.2 medications deprescribed per patient with an annual cost avoidance of > $100 million.10
Assessing and acting on mentation includes preventing, identifying, and managing depression and dementia in outpatient settings and delirium in hospital and long-term care settings.5 There are many tools and clinical reminders available in the EHR so that interdisciplinary teams can document changes to mentation and identify opportunities for continuous improvement.
Closely aligned with mentation is mobility, with evidence suggesting that regular physical activity reduces the risk of falls (preventing associated complications), maintains physical functioning, and lowers the risk of cognitive impairment and depression.5 Ensuring early, frequent, and safe mobility helps patients achieve better health outcomes and prevent injury.5 Mobility programs within the VHA include the STRIDE program for the inpatient setting and Gerofit for outpatient settings.11,12
HRO Principles
An HRO is a complex environment of care that experiences fewer than anticipated accidents or adverse events by (1) establishing trust among leaders and staff by balancing individual accountability with systems thinking; (2) empowering staff to lead continuous process improvements; and (3) creating an environment where employees feel safe to report harm or near misses, focusing on the reasons errors occur.13 The work of AFHS incorporates HRO principles with an emphasis on 3 elements. First, it involves interactive systems and processes needed to support 4Ms care across care settings. Second, AFHS acknowledge the complexity of age-friendly work and deference to the expertise of interdisciplinary team members. Finally, AFHS are committed to resilience by overcoming failures and challenges to implementation and long-term sustainment as a standard of practice.
Case study
The names and details in this case have been modified to protect patient privacy. It is representative of many Community Living Centers (CLCs) involved in AFHS that work to create a safe, person-centered environment for veterans.
In a CLC team workroom, 2 nurses were discussing a long-term care resident. The nurses approached the attending physician and explained that they were worried about Sgt Johnson, who seemed depressed and sometimes combative. They had noticed a change in his behavior when they helped him clean up after an episode of incontinence and were concerned that he would try to get out of bed on his own and fall. The attending physician thanked them for sharing their concerns. Sgt Johnson was a retired Army veteran who had a long, decorated military career. His chronic health conditions had led to muscle weakness, and he fell and broke a hip before this admission. He had an uneventful hip replacement but was showing signs of depression due to his limited mobility, loss of independence, and inability to live at home without additional support.
The attending physician knocked on the door of his room, sat down next to the bed, and asked, “How are you feeling today?” Sgt Johnson tersely replied, “About the same.” The physician asked, “Sgt Johnson, what matters most to you related to your recovery? What is important to you?” Sgt Johnson responded, “Feeling like a man!” The doctor replied, “So what makes you feel ‘not like a man’?” The Sgt replied, “Having to be cleaned up by the nurses and not being able to use the toilet on my own.” The physician surmised that his decline in physical functioning had a connection to his worsening depression and combativeness and said to the Sgt, “Let’s get the team together and work out a plan to get you strong enough to use a bedside commode by yourself. Let’s make that the first goal in our plan to get you back to using the toilet independently. Can you work with us on that?” He smiled and said, “Sir, yes Sir!”
At the weekly interdisciplinary team meeting, the team discussed Sgt Johnson’s wishes and the nurses’ safety concerns. The physician reported to the team what mattered to the veteran. The nurses arranged for a bedside commode and supplies to be placed in his room, encouraged and assisted him, and provided a privacy screen. The physical therapist continued to support his mobility needs, concentrating on transfers, small steps like standing and turning with a walker to get in position to use the bedside commode, and later the bathroom toilet. The psychologist addressed what matters to Sgt Johnson and his mentation, health goals, and coping strategies. The social worker provided support and counseling for the veteran and his family. The pharmacist checked his medications to be sure that none were affecting his gastrointestinal tract and his ability to move safely and do what matters to him. Knowing what mattered to Sgt Johnson was the driver of the interdisciplinary care plan to provide 4Ms care.
The team worked collaboratively with the veteran to develop and set attainable goals around toileting and regaining his dignity. This improved his overall recovery. As Sgt Johnson became more independent, his mood gradually improved and he began to participate in other activities and interact with other residents on the unit, and he did not experience any falls. By addressing the 4Ms, the interdisciplinary team coordinated efforts to provide high-quality, person-centered care. They built trust with the veteran, shared accountability, and followed HRO principles to keep the veteran safe.
Becoming an Age-Friendly HRO
Becoming an HRO is a dynamic, ever-changing process to maintain high standards, improve care quality, and cause no harm. There are 3 pillars and 5 principles that guide an HRO. The pillars are critical areas of focus and include leadership commitment, culture of safety, and continuous process improvement.14 The first of 5 HRO principles is sensitivity to operations. This is defined as an awareness of how processes and systems impact the entire organization, the downstream impact.15 Focusing on the 4Ms helps develop the capability of frontline staff to provide high-quality care for older adults while ensuring that processes are in place to support the work. The 4Ms provide an efficient way to organize interdisciplinary team meetings, provide warm handoffs using Situation-Background-Assessment-Recommendation, and standardize documentation. Involvement in the AFHS movement improves communication, care quality, and patient and staff satisfaction to meet this HRO principle.15
The second HRO principle, reluctance to simplify, ensures that direct care staff and leaders delve further into issues to find solutions.15 AFHS use the Plan-Do-Study-Act cycle to put the 4Ms into practice; this cycle helps teams test small increments of change, study their performance, and act to ensure that all 4Ms are being practiced as a set. AFHS teams are encouraged to review at least 3 months of data after implementation of the 4Ms, working to find solutions if there are gaps or issues identified.
The third principle, preoccupation with failure, refers to shared attentiveness—being prepared for the unexpected and learning from mistakes.15 The entire AFHS team shares responsibility for providing 4Ms care, where staff are empowered to report any safety concerns or close calls. The fourth principle of deference to expertise includes listening to staff who have the most knowledge for the task at hand, which aligns with the collaborative interdisciplinary teamwork of age-friendly teams.15
The final HRO principle, commitment to resilience, includes continuous learning, interdisciplinary team training, and sharing of lessons learned.15 Although IHI offers 2 levels of AFHS recognition, teams are continuously learning to improve and sustain care beyond level 2, Committed to Care Excellence recognition.16
The Table shows the VHA’s AFHS implementation strategies and the HRO principles adapted from the Joint Commission’s High Reliability Health Care Maturity Model and the IHI’s Framework for Safe, Reliable, and Effective Care. The VHA is developing a national dashboard to capture age-friendly processes and health outcome measures that address patient safety and care quality.
Conclusions
AFHS empowers VHA teams to honor veterans’ care preferences and values, supporting their independence, dignity, and quality of life across care settings. The adoption of AFHS brings evidence-based practices to the point of care by addressing common pitfalls in the care of older adults, drawing attention to, and calling for action on inappropriate medication use, physical inactivity, and assessment of the vulnerable brain. The 4Ms also serve as a framework to continuously improve care and cause zero harm, reinforcing HRO pillars and principles across the VHA, and ensuring that older adults reliably receive the evidence-based, high-quality care they deserve.
1. Veterans Health Administration. Providing healthcare for veterans. Updated June 20, 2023. Accessed June 26, 2023. https://www.va.gov/health
2. Veazie S, Peterson K, Bourne D. Evidence brief: implementation of high reliability organization principles. Washington, DC: Evidence Synthesis Program, Health Services Research and Development Service, Office of Research and Development, Department of Veterans Affairs. VA ESP Project #09-199; 2019. Accessed November 30, 2023. https://www.hsrd.research.va.gov/publications/esp/high-reliability-org.cfm
3. Church K, Munro S, Shaughnessy M, Clancy C. Age-Friendly Health Systems: improving care for older adults in the Veterans Health Administration. Health Serv Res. 2023;58(suppl 1):5-8. doi:10.1111/1475-6773.14110
4. Farrell TW, Volden TA, Butler JM, et al. Age-friendly care in the Veterans Health Administration: past, present, and future. J Am Geriatr Soc. 2023;71(1):18-25. doi:10.1111/jgs.18070
5. Mate K, Fulmer T, Pelton L, et al. Evidence for the 4Ms: interactions and outcomes across the care continuum. J Aging Health. 2021;33(7-8):469-481. doi:10.1177/0898264321991658
6. Tinetti ME, Naik AD, Dindo L, et al. Association of patient priorities-aligned decision-making with patient outcomes and ambulatory health care burden among older adults with multiple chronic conditions: A nonrandomized clinical trial. JAMA Intern Med. 2019;179(12):1688-1697. doi:10.1001/jamainternmed.2019.4235
7. US Department of Veterans Affairs. What is whole health? Updated: October 31, 2023. November 30, 2023. https://www.va.gov/wholehealth
8. Patient Priorities Care. Updated 2019. Accessed November 30, 2023. https://patientprioritiescare.org
9. Battar S, Watson Dickerson KR, Sedgwick C, Cmelik T. Understanding principles of high reliability organizations through the eyes of VIONE: a clinical program to improve patient safety by deprescribing potentially inappropriate medications and reducing polypharmacy. Fed Pract. 2019;36(12):564-568.
10. VA Diffusion Marketplace. VIONE- medication optimization and polypharmacy reduction initiative. Accessed November 30, 2023. https://marketplace.va.gov/innovations/vione
11. US Department of Veterans Affairs, Office of Research and Development. STRIDE program to keep hospitalized veterans mobile. Updated November 6, 2018. Accessed November 30, 2023. https://www.research.va.gov/research_in_action/STRIDE-program-to-keep-hospitalized-Veterans-mobile.cfm
12. US Department of Veterans Affairs, VA Geriatrics and Extended Care. Gerofit: a program promoting exercise and health for older veterans. Updated August 2, 2023. Accessed November 30, 2023. https://www.va.gov/GERIATRICS/pages/gerofit_Home.asp
13. US Department of Veterans Affairs, Health Services Research and Development. VHA’s vision for a high reliability organization. Updated August 14, 2020. Accessed November 30, 2023. https://www.hsrd.research.va.gov/publications/forum/summer20/default.cfm?ForumMenu=summer20-1
14. US Department of Veterans Affairs, Health Services Research and Development. Three HRO evaluation priorities. Updated August 14, 2020. Accessed November 30, 2023. https://www.hsrd.research.va.gov/publications/forum/summer20/default.cfm?ForumMenu=summer20-2
15. Oster CA, Deakins S. Practical application of high-reliability principles in healthcare to optimize quality and safety outcomes. J Nurs Adm. 2018;48(1):50-55. doi:10.1097/NNA.0000000000000570
16. Institute for Healthcare Improvement. Age-Friendly Health Systems recognitions. Accessed November 30, 2023. https://www.ihi.org/Engage/Initiatives/Age-Friendly-Health-Systems/Pages/Recognition.aspx
1. Veterans Health Administration. Providing healthcare for veterans. Updated June 20, 2023. Accessed June 26, 2023. https://www.va.gov/health
2. Veazie S, Peterson K, Bourne D. Evidence brief: implementation of high reliability organization principles. Washington, DC: Evidence Synthesis Program, Health Services Research and Development Service, Office of Research and Development, Department of Veterans Affairs. VA ESP Project #09-199; 2019. Accessed November 30, 2023. https://www.hsrd.research.va.gov/publications/esp/high-reliability-org.cfm
3. Church K, Munro S, Shaughnessy M, Clancy C. Age-Friendly Health Systems: improving care for older adults in the Veterans Health Administration. Health Serv Res. 2023;58(suppl 1):5-8. doi:10.1111/1475-6773.14110
4. Farrell TW, Volden TA, Butler JM, et al. Age-friendly care in the Veterans Health Administration: past, present, and future. J Am Geriatr Soc. 2023;71(1):18-25. doi:10.1111/jgs.18070
5. Mate K, Fulmer T, Pelton L, et al. Evidence for the 4Ms: interactions and outcomes across the care continuum. J Aging Health. 2021;33(7-8):469-481. doi:10.1177/0898264321991658
6. Tinetti ME, Naik AD, Dindo L, et al. Association of patient priorities-aligned decision-making with patient outcomes and ambulatory health care burden among older adults with multiple chronic conditions: A nonrandomized clinical trial. JAMA Intern Med. 2019;179(12):1688-1697. doi:10.1001/jamainternmed.2019.4235
7. US Department of Veterans Affairs. What is whole health? Updated: October 31, 2023. November 30, 2023. https://www.va.gov/wholehealth
8. Patient Priorities Care. Updated 2019. Accessed November 30, 2023. https://patientprioritiescare.org
9. Battar S, Watson Dickerson KR, Sedgwick C, Cmelik T. Understanding principles of high reliability organizations through the eyes of VIONE: a clinical program to improve patient safety by deprescribing potentially inappropriate medications and reducing polypharmacy. Fed Pract. 2019;36(12):564-568.
10. VA Diffusion Marketplace. VIONE- medication optimization and polypharmacy reduction initiative. Accessed November 30, 2023. https://marketplace.va.gov/innovations/vione
11. US Department of Veterans Affairs, Office of Research and Development. STRIDE program to keep hospitalized veterans mobile. Updated November 6, 2018. Accessed November 30, 2023. https://www.research.va.gov/research_in_action/STRIDE-program-to-keep-hospitalized-Veterans-mobile.cfm
12. US Department of Veterans Affairs, VA Geriatrics and Extended Care. Gerofit: a program promoting exercise and health for older veterans. Updated August 2, 2023. Accessed November 30, 2023. https://www.va.gov/GERIATRICS/pages/gerofit_Home.asp
13. US Department of Veterans Affairs, Health Services Research and Development. VHA’s vision for a high reliability organization. Updated August 14, 2020. Accessed November 30, 2023. https://www.hsrd.research.va.gov/publications/forum/summer20/default.cfm?ForumMenu=summer20-1
14. US Department of Veterans Affairs, Health Services Research and Development. Three HRO evaluation priorities. Updated August 14, 2020. Accessed November 30, 2023. https://www.hsrd.research.va.gov/publications/forum/summer20/default.cfm?ForumMenu=summer20-2
15. Oster CA, Deakins S. Practical application of high-reliability principles in healthcare to optimize quality and safety outcomes. J Nurs Adm. 2018;48(1):50-55. doi:10.1097/NNA.0000000000000570
16. Institute for Healthcare Improvement. Age-Friendly Health Systems recognitions. Accessed November 30, 2023. https://www.ihi.org/Engage/Initiatives/Age-Friendly-Health-Systems/Pages/Recognition.aspx
Building Tailored Resource Guides to Address Social Risks and Advance Health Equity in the Veterans Health Administration
Social risk factors and social needs have significant, often cumulative, impacts on health outcomes and are closely tied to health inequities. Defined as the individual-level adverse social conditions associated with poor health, social risk factors broadly include experiences such as food insecurity and housing instability; whereas the term social needs incorporates a person’s perceptions of and priorities related to their health-related needs.1 One recent study examining data from the Veterans Health Administration (VHA) found a 27% higher odds of mortality with each additional identified social risk, underscoring the critical link between social risks and veteran health outcomes.2
Assessing Circumstances and Offering Resources for Needs (ACORN), a collaborative quality improvement initiative conducted in partnership with the VHA Office of Health Equity and VHA National Social Work Program, Care Management and Social Work Services, is a social risk screening and referral program that aims to systematically identify and address unmet social needs among veterans to improve health and advance health equity.3,4 ACORN consists of 2 components: (1) a veteran-tailored screener to identify social risks within 9 domains; and (2) provision of relevant VA and community resources and referrals to address identified needs.3,5 Veterans who screen positive for ≥ 1 need receive referrals to a social worker or other relevant services, such as nutrition and food services or mental health, support navigating resources, and/or geographically tailored resource guides. This article describes the development and use of resource guides as a cross-cutting intervention component to address unmet social needs in diverse clinical settings and shares lessons learned from implementation in VHA outpatient clinics.
BACKGROUND
Unequal distribution of resources combined with historical discriminatory policies and practices, often linked to institutionalized racism, create inequities that lead to health disparities and hinder advancements in population health.6,7 Although health care systems alone cannot eliminate all health inequities, they can implement programs to identify social risks and address individual-level needs as 1 component of the multilevel approach needed to achieve health equity.8
As a national health care system serving > 9 million veterans, the VHA is well positioned to address social needs as an essential part of health. The VHA routinely screens for certain social risks, including housing instability, food insecurity, and intimate partner violence, and has a robust system of supports to address these and other needs among veterans, such as supportive housing services, vocational rehabilitation, assistance for justice-involved veterans, technology access support, and peer-support services.9-11 However, the VHA lacks a systematic approach to broader screening for social risks.
To address this gap, ACORN was developed in 2018 by an advisory board of subject matter experts, including clinical leaders, clinical psychologists, social workers, and health services researchers with content expertise in social risks and social needs.3 This interprofessional team sought to develop a veteran-tailored screener and resource referral initiative that could be scaled efficiently across VHA clinical settings.
Although health care organizations are increasingly implementing screening and interventions for social risks within clinical care, best practices and evidence-based tools to support clinical staff in these efforts are limited.12 Resource guides—curated lists of supportive services and organizations—may serve as a scalable “low-touch” intervention to help clinical staff address needs either alone or with more intensive interventions, such as social worker case management or patient navigation services.13
RESOURCE GUIDES—A Cross-Cutting Tool
The VHA has a uniquely robust network of nearly 18,000 social workers with clinical expertise in identifying, comprehensively assessing, and addressing social risks and needs among veterans. Interprofessional patient aligned care teams (PACTs)—a patient-centered medical home initiative that includes embedding social workers into primary care teams—facilitate the VHA’s capacity to address both medical and social needs.14 Social workers in PACTs and other care settings provide in-depth assessment and case management services to veterans who have a range of complex social needs. However, despite these comprehensive social services, in the setting of universal screening with a tool such as ACORN, it may not be feasible or practical to refer all patients who screen positive to a social worker for immediate follow-up, particularly in settings with capacity or resource limitations. For example, rates of screening positive on ACORN for ≥ 1 social risk have ranged from 48% of veterans in primary care sites and 80% in social work sites to nearly 100% in a PACT clinic for veterans experiencing homelessness.15
Additionally, a key challenge in the design of social needs interventions is determining how to optimize intervention intensity based on individual patient needs, acuity, and preferences. A substantial proportion of individuals who screen positive for social risks decline offered assistance, such as referrals.16 Resource guides are a cross-cutting tool that can be offered to veterans across a variety of settings, including primary care, specialty clinics, or emergency departments, as either a standalone intervention or one provided in combination with other resources or services. For patients who may not be interested in or feel comfortable accepting assistance at the time of screening or for those who prefer to research and navigate resources on their own, tailored resource guides can serve as a lower touch intervention to ensure interprofessional clinical staff across a range of settings and specialties have accessible, reliable, and up-to-date information to give to patients at the point of clinical care.17
Resource guides also can be used with higher touch clinical social work interventions, such as crisis management, supportive counseling, and case management. For example, social workers can use resource guides to provide education on VHA or community resources during clinical encounters with veterans and/or provide the guides to veterans to reference for future needs. Resource guides can further be used as a tool to support community resource navigation provided by nonclinical staff, such as peer specialists or community health workers.
How to BUILD RESOURCE GUIDES
Our team created resource guides (Figure) to provide veterans with concise, geographically tailored lists of VHA and other federal, state, and community services for the social risk domains included on the ACORN screener. To inform and develop a framework for building and maintaining ACORN guides, we first reviewed existing models that use this approach, including Boston Medical Center's WE CARE (Well Child Care, Evaluation, Community Resources, Advocacy, Referral, Education) and Thrive programs. We provide an overview of our process, which can be applied to clinical settings both within and outside the VHA.18,19
Partnerships
Active collaboration with frontline clinical social workers and local social work leadership is a critical part of identifying and prioritizing quality resources. Equipped with the knowledge of the local resource landscape, social workers can provide recommendations pertaining to national or federal, state, and local programs that have a history of being responsive to patients’ expressed needs.20 VHA social workers have robust knowledge of the veteran-specific resources available at VHA medical centers and nationally, and their clinical training equips them with the expertise to provide guidance about which resources to prioritize for inclusion in the guides.20
After receiving initial guidance from clinical social workers, our team began outreach to compile detailed program information, gauge program serviceability, and build relationships with both VHA and community-based services. Aligning with programs that share a similar mission in addressing social needs has proved crucial when developing the resource guides. Beyond ensuring the accuracy of program information, regular contact provides an opportunity to address capacity and workflow concerns that may arise from increased referrals. Additionally, open lines of communication with various supportive services facilitate connections with additional organizations and resources within the area.
The value of these relationships was evident at the onset of the COVID-19 pandemic, when the ACORN resource guides in use by our clinical site partners required frequent modifications to reflect rapid changes in services (eg, closures, transition to fully virtual programs, social distancing and masking requirements). Having established connections with community organizations was essential to navigating the evolving landscape of available programs and supports.
Curating Quality Resources
ACORN currently screens for social risks in 9 domains: food, housing, utilities, transportation, education, employment, legal, social isolation/loneliness, and digital needs. Each resource guide pertains to a specific social risk domain and associated question(s) in the screener, allowing staff to quickly identify which guides a veteran may benefit from based on their screening responses. The guides are meant to be short and comprehensive but not exhaustive lists of programs and services. We limit the length of the guides to one single-sided page to provide high-yield, geographically tailored resources in an easy-to-use format. The guides should reflect the geographic area served by the VHA medical center or the community-based outpatient clinic (CBOC) where a veteran receives clinical care, but they also may include national- and state-level resources that provide services and programs to veterans.
Although there is local variation in the availability and accessibility of services across social risk domains, some domains have an abundance of resources and organizations at federal or national, state, and/or community levels. To narrow the list of resources to the highest yield programs, we developed a series of questions that serve as selection criteria to inform resource inclusion (Table).
Because the resource guides are intended to be broadly applicable to a large number of veterans, we prioritize generalizable resources over those with narrow eligibility criteria and/or services. When more intensive support is needed, social workers and other VHA clinical and nonclinical staff can supplement the resources on the guides with additional, more tailored resources that are based on individual factors, such as physical residence, income, transportation access, or household composition (eg, veteran families with children or older adults).
Formatting Resource Guides
Along with relevant information, such as the program name, location, and a specific point of contact, brief program descriptions provide information about services offered, eligibility criteria, application requirements, alternate contacts and locations, and website links. At the bottom of each guide, a section is included with the name and direct contact information for a social worker (often the individual assigned to the clinic where the veteran completed the ACORN screener) or another VHA staff member who can be reached for further assistance. These staff members are familiar with the content included on the guides and provide veterans with additional information or higher touch support as necessary. This contact information is useful for veterans who initially decline assistance or referrals but later want to follow up with staff for support or questions.
Visual consistency is a key feature of the ACORN resource guides, and layout and design elements are used to maximize space and enhance usability. Corresponding font colors for all program titles, contact information, and website links assist in visually separating and drawing attention to pertinent contact information. QR codes linked to program websites also are incorporated for veterans to easily access resource information from their smartphone or other electronic device.
Maintaining Resource Guides
To ensure continued accuracy, resource guides are updated about every 6 months to reflect changes in closures, transitions to virtual/in-person services, changes in location, new points of contact, and modifications to services or eligibility requirements. Notations also are made if any changes to services or eligibility are temporary or permanent. Recording these temporary adjustments was critical early in the COVID-19 pandemic as service offerings, eligibility requirements, and application processes changed often.
Updating the guides also facilitates continuous relationship building and connections with VHA and community-based services. Resource guides are living documents: they maintain lines of communication with designated contacts, allow for opportunities to improve the presentation of evolving program information in the guides, and offer the chance to learn about additional programs in the area that may meet veterans' needs.
Creating a Manual for ACORN Resource Guide Development
To facilitate dissemination of ACORN across VHA clinical settings and locations, our team developed a Resource Guide Manual to aid ACORN clinical sites in developing resource guides.21 The manual provides step-by-step guidance from recommendations for identifying resources to formatting and layout considerations. Supplemental materials include a checklist to ensure each program description includes the necessary information for veterans to successfully access the resource, as well as page templates and style suggestions to maximize usability. These templates standardize formatting across the social risk domain guides and include options for electronic and paper distribution.
RESOURCE GUIDE LIMITATIONS
The labor involved in building and maintaining multiple guides is considerable and requires a time investment both upfront and long term, which may not be feasible for clinical sites with limited staff. However, many VHA social workers maintain lists of resource and referral services for veterans as part of their routine clinical case management. These lists can serve as a valuable and timesaving starting point in curating high-yield resources for formal resource guides. To further reduce the time needed to develop guides, sites can use ACORN resource guide templates rather than designing and formatting guides from scratch. In addition to informing veterans of relevant services and programs, resource guides also can be provided to new staff, such as social workers or peer specialists, during onboarding to help familiarize them with available services to address veterans’ unmet needs.
Resources included on the guides also are geographically tailored, based on the physical location of the VHA medical center or CBOC. Some community-based services listed may not be as relevant, accessible, available, or convenient to veterans who live far from the clinic, which is relevant for nearly 25% of veterans who live in rural communities.22 This is a circumstance in which the expertise of VHA social workers should be used to recommend more appropriately tailored resources to a veteran. Use of free, publicly available electronic resource databases (eg, 211 Helpline Center) also can provide social workers and patients with an overview of all available resources within their community. There are paid referral platform services that health systems can contract with as well.23 However, the potential drawbacks to these alternative platforms include high startup costs or costly user-license fees for medical centers or clinics, inconsistent updates to resource information, and lack of compatibility with some electronic health record systems.23
Resource guides are not intended to take the place of a clinical social worker or other health professional but rather to serve in a supplemental capacity to clinical services. Certain circumstances necessitate a more comprehensive clinical assessment and/or a warm handoff to a social worker, including assistance with urgent food or housing needs, and ACORN workflows are created with urgent needs pathways in mind. Determining how to optimize intervention intensity based on individual patients’ expressed needs, preferences, and acuity remains a challenge for health care organizations conducting social risk screening.12 While distribution of geographically tailored resource guides can be a useful low touch intervention for some veterans, others will require more intensive case management to address or meet their needs. Some veterans also may fall in the middle of this spectrum, where a resource guide is not enough but intensive case management services facilitated by social workers are not needed or wanted by the patient. Integration of peer specialists, patient navigators, or community health workers who can work with veterans to support them in identifying, connecting with, and receiving support from relevant programs may help fill this gap. Given their knowledge and lived experience, these professionals also can promote patient-centered care as part of the health care team.
CONCLUSIONS
Whether used as a low-touch, standalone intervention or in combination with higher touch services (eg, case management or resource navigation), resource guides are a valuable tool for health care organizations working to address social needs as a component of efforts to advance health equity, reduce health disparities, and promote population health. We provide a pragmatic framework for developing and maintaining resource guides used in the ACORN initiative. However, additional work is needed to optimize the design, content, and format of resource guides for both usability and effectiveness as a social risk intervention across health care settings.
Acknowledgments
We express our gratitude for the Veterans Health Administration (VHA) Office of Health Equity and the VHA National Social Work Program, Care Management and Social Work Services for their support of the Assessing Circumstances and Offering Resources for Needs (ACORN) initiative. We also express our appreciation for those who supported the initial screener development as part of the ACORN Advisory Board, including Stacey Curran, BA; Charles Drebing, PhD; J. Stewart Evans, MD, MSc; Edward Federman, PhD; Maneesha Gulati, LICSW, ACSW; Nancy Kressin, PhD; Kenneth Link, LICSW; Monica Sharma, MD; and Jacqueline Spencer, MD, MPH. We also express our appreciation for those who supported the initial ACORN resource guide development, including Chuck Drebing, PhD, Ed Federman, PhD, and Ken Link, LICSW, and for the clinical care team members, especially the social workers and nurses, at our ACORN partner sites as well as the community-based partners who have helped us develop comprehensive resource guides for veterans. This work was supported by funding from the VHA Office of Health Equity and by resources and use of facilities at the VA Bedford Healthcare System, VA New England Healthcare System, and VA Providence Healthcare System. Alicia J. Cohen was additionally supported by a VA HSR&D Career Development Award (CDA 20-037).
1. Alderwick H, Gottlieb LM. Meanings and misunderstandings: a social determinants of health lexicon for health care systems. Milbank Q. 2019;97(2):407-419. doi:10.1111/1468-0009.12390
2. Blosnich JM, Montgomery AE, Taylor LD, Dichter ME. Adverse social factors and all-cause mortality among male and female patients receiving care in the Veterans Health Administration. Prev Med. 2020;141:106272. doi:10.1016/j.ypmed.2020.106272
3. Russell LE, Cohen AJ, Chrzas S, et al. Implementing a social needs screening and referral program among veterans: Assessing Circumstances & Offering Resources for Needs (ACORN). J Gen Intern Med. 2023;38(13):2906-2913. doi:10.1007/s11606-023-08181-9
4. Cohen AJ, Russell LE, Elwy AR, et al. Adaptation of a social risk screening and referral initiative across clinical populations, settings, and contexts in the Department of Veterans Affairs Health System. Front Health Serv. 2023;2. doi:10.3389/frhs.2022.958969
5. Cohen AJ, Kennedy MA, Mitchell KM, Russell LE. The Assessing Circumstances & Offering Resources for Needs (ACORN) initiative. Updated September 2022. Accessed December 4, 2023. https://www.va.gov/HEALTHEQUITY/docs/ACORN_Screening_Tool.pdf
6. Jones CP. Levels of racism: a theoretic framework and a gardener’s tale. Am J Public Health. 2000;90(8):1212-1215. doi:10.2105/ajph.90.8.1212
7. American Public Health Association. Creating the healthiest nation: advancing health equity. Accessed November 28, 2023. https://www.apha.org/-/media/files/pdf/factsheets/advancing_health_equity.ashx?la=en&hash=9144021FDA33B4E7E02447CB28CA3F9D4BE5EF18
8. Castrucci B, Auerbach J. Meeting individual social needs falls short of addressing social determinants of health. Health Aff. Published January 16, 2019. doi:10.1377/hblog20190115.234942
9. Montgomery AE, Fargo JD, Byrne TH, Kane VR, Culhane DP. Universal screening for homelessness and risk for homelessness in the Veterans Health Administration. Am J Public Health. 2013;103(suppl 2):S210-211. doi:10.2105/AJPH.2013.301398
10. Cohen AJ, Rudolph JL, Thomas KS, et al. Food insecurity among veterans: resources to screen and intervene. Fed Pract. 2020;37(1):16-23.
11. Iverson KM, Adjognon O, Grillo AR, et al. Intimate partner violence screening programs in the Veterans Health Administration: informing scale-up of successful practices. J Gen Intern Med. 2019;34(11):2435-2442. doi:10.1007/s11606-019-05240-y
12. National Academies of Sciences, Engineering, and Medicine. Integrating Social Care into the Delivery of Health Care: Moving Upstream to Improve the Nation’s Health. The National Academies Press; 2019. Accessed November 28, 2023. https://nap.nationalacademies.org/catalog/25467/integrating-social-care-into-the-delivery-of-health-care-moving
13. Gottlieb LM, Adler NE, Wing H, et al. Effects of in-person assistance vs personalized written resources about social services on household social risks and child and caregiver health: a randomized clinical trial. JAMA Netw Open. 2020;3(3):e200701. doi:10.1001/jamanetworkopen.2020.0701
14. Cornell PY, Halladay CW, Ader J, et al. Embedding social workers in Veterans Health Administration primary care teams reduces emergency department visits. Health Aff (Millwood). 2020;39(4):603-612. doi:10.1377/hlthaff.2019.01589
15. Cohen AJ, Bruton M, Hooshyar D. US Department of Veterans Affairs, Office of Health Services Research and Development. The WHO’s greatest ICD-10 hits for fiscal year 2022: social determinants of health. Published March 9, 2022. Updated November 6, 2023. Accessed December 4, 2023. https://www.hsrd.research.va.gov/for_researchers/cyber_seminars/archives/video_archive.cfm?SessionID=4125

16. De Marchis EH, Alderwick H, Gottlieb LM. Do patients want help addressing social risks? J Am Board Fam Med. 2020;33(2):170-175. doi:10.3122/jabfm.2020.02.190309
17. Cohen AJ, Isaacson N, Torby M, Smith A, Zhang G, Patel MR. Motivators, barriers, and preferences to engagement with offered social care assistance among people with diabetes: a mixed methods study. Am J Prev Med. 2022;63(3, suppl 2):S152-S163. doi:10.1016/j.amepre.2022.02.022
18. Buitron de la Vega P, Losi S, Sprague Martinez L, et al. Implementing an EHR-based screening and referral system to address social determinants of health in primary care. Med Care. 2019;57(suppl 6, suppl 2):S133-S139. doi:10.1097/MLR.0000000000001029
19. Boston Medical Center. The WE CARE Model. Accessed November 28, 2023. https://www.bmc.org/pediatrics-primary-care/we-care/we-care-model
20. US Department of Veterans Affairs, Office of Rural Health. VA social work. Updated July 11, 2023. Accessed December 4, 2023. https://www.socialwork.va.gov
21. Mitchell KM, Russell LE, Cohen AJ, Kennedy MA. Building ACORN resource guides for veterans. Accessed November 28, 2023. https://www.va.gov/HEALTHEQUITY/docs/ACORN_Resource_Guide_Manual.pdf
22. US Department of Veterans Affairs, Veterans Health Administration, Office of Rural Health. Rural Veterans. Accessed November 28, 2023. https://www.ruralhealth.va.gov/aboutus/ruralvets.asp
23. Cartier Y, Fichtenberg C, Gottlieb L. Community resource referral platforms: a guide for health care organizations. Published 2019. Accessed December 4, 2023. https://sirenetwork.ucsf.edu/tools-resources/resources/community-resource-referral-platforms-guide-health-care-organizations
Social risk factors and social needs have significant, often cumulative, impacts on health outcomes and are closely tied to health inequities. Defined as the individual-level adverse social conditions associated with poor health, social risk factors broadly include experiences such as food insecurity and housing instability; whereas the term social needs incorporates a person’s perceptions of and priorities related to their health-related needs.1 One recent study examining data from the Veterans Health Administration (VHA) found a 27% higher odds of mortality with each additional identified social risk, underscoring the critical link between social risks and veteran health outcomes.2
Assessing Circumstances and Offering Resources for Needs (ACORN), a collaborative quality improvement initiative conducted in partnership with the VHA Office of Health Equity and VHA National Social Work Program, Care Management and Social Work Services, is a social risk screening and referral program that aims to systematically identify and address unmet social needs among veterans to improve health and advance health equity.3,4 ACORN consists of 2 components: (1) a veteran-tailored screener to identify social risks within 9 domains; and (2) provision of relevant VA and community resources and referrals to address identified needs.3,5 Veterans who screen positive for ≥ 1 need receive referrals to a social worker or other relevant services, such as nutrition and food services or mental health, support navigating resources, and/or geographically tailored resource guides. This article describes the development and use of resource guides as a cross-cutting intervention component to address unmet social needs in diverse clinical settings and shares lessons learned from implementation in VHA outpatient clinics.
BACKGROUND
Unequal distribution of resources combined with historical discriminatory policies and practices, often linked to institutionalized racism, create inequities that lead to health disparities and hinder advancements in population health.6,7 Although health care systems alone cannot eliminate all health inequities, they can implement programs to identify social risks and address individual-level needs as 1 component of the multilevel approach needed to achieve health equity.8
As a national health care system serving > 9 million veterans, the VHA is well positioned to address social needs as an essential part of health. The VHA routinely screens for certain social risks, including housing instability, food insecurity, and intimate partner violence, and has a robust system of supports to address these and other needs among veterans, such as supportive housing services, vocational rehabilitation, assistance for justice-involved veterans, technology access support, and peer-support services.9-11 However, the VHA lacks a systematic approach to broader screening for social risks.
To address this gap, ACORN was developed in 2018 by an advisory board of subject matter experts, including clinical leaders, clinical psychologists, social workers, and health services researchers with content expertise in social risks and social needs.3 This interprofessional team sought to develop a veteran-tailored screener and resource referral initiative that could be scaled efficiently across VHA clinical settings.
Although health care organizations are increasingly implementing screening and interventions for social risks within clinical care, best practices and evidence-based tools to support clinical staff in these efforts are limited.12 Resource guides—curated lists of supportive services and organizations—may serve as a scalable “low-touch” intervention to help clinical staff address needs either alone or with more intensive interventions, such as social worker case management or patient navigation services.13
RESOURCE GUIDES—A Cross-Cutting Tool
The VHA has a uniquely robust network of nearly 18,000 social workers with clinical expertise in identifying, comprehensively assessing, and addressing social risks and needs among veterans. Interprofessional patient aligned care teams (PACTs)—a patient-centered medical home initiative that includes embedding social workers into primary care teams—facilitate the VHA’s capacity to address both medical and social needs.14 Social workers in PACTs and other care settings provide in-depth assessment and case management services to veterans who have a range of complex social needs. However, despite these comprehensive social services, in the setting of universal screening with a tool such as ACORN, it may not be feasible or practical to refer all patients who screen positive to a social worker for immediate follow-up, particularly in settings with capacity or resource limitations. For example, rates of screening positive on ACORN for ≥ 1 social risk have ranged from 48% of veterans in primary care sites and 80% in social work sites to nearly 100% in a PACT clinic for veterans experiencing homelessness.15
Additionally, a key challenge in the design of social needs interventions is determining how to optimize intervention intensity based on individual patient needs, acuity, and preferences. A substantial proportion of individuals who screen positive for social risks decline offered assistance, such as referrals.16 Resource guides are a cross-cutting tool that can be offered to veterans across a variety of settings, including primary care, specialty clinics, or emergency departments, as either a standalone intervention or one provided in combination with other resources or services. For patients who may not be interested in or feel comfortable accepting assistance at the time of screening or for those who prefer to research and navigate resources on their own, tailored resource guides can serve as a lower touch intervention to ensure interprofessional clinical staff across a range of settings and specialties have accessible, reliable, and up-to-date information to give to patients at the point of clinical care.17
Resource guides also can be used with higher touch clinical social work interventions, such as crisis management, supportive counseling, and case management. For example, social workers can use resource guides to provide education on VHA or community resources during clinical encounters with veterans and/or provide the guides to veterans to reference for future needs. Resource guides can further be used as a tool to support community resource navigation provided by nonclinical staff, such as peer specialists or community health workers.
How to BUILD RESOURCE GUIDES
Our team created resource guides (Figure) to provide veterans with concise, geographically tailored lists of VHA and other federal, state, and community services for the social risk domains included on the ACORN screener. To inform and develop a framework for building and maintaining ACORN guides, we first reviewed existing models that use this approach, including Boston Medical Center's WE CARE (Well Child Care, Evaluation, Community Resources, Advocacy, Referral, Education) and Thrive programs. We provide an overview of our process, which can be applied to clinical settings both within and outside the VHA.18,19
Partnerships
Active collaboration with frontline clinical social workers and local social work leadership is a critical part of identifying and prioritizing quality resources. Equipped with the knowledge of the local resource landscape, social workers can provide recommendations pertaining to national or federal, state, and local programs that have a history of being responsive to patients’ expressed needs.20 VHA social workers have robust knowledge of the veteran-specific resources available at VHA medical centers and nationally, and their clinical training equips them with the expertise to provide guidance about which resources to prioritize for inclusion in the guides.20
After receiving initial guidance from clinical social workers, our team began outreach to compile detailed program information, gauge program serviceability, and build relationships with both VHA and community-based services. Aligning with programs that share a similar mission in addressing social needs has proved crucial when developing the resource guides. Beyond ensuring the accuracy of program information, regular contact provides an opportunity to address capacity and workflow concerns that may arise from increased referrals. Additionally, open lines of communication with various supportive services facilitate connections with additional organizations and resources within the area.
The value of these relationships was evident at the onset of the COVID-19 pandemic, when the ACORN resource guides in use by our clinical site partners required frequent modifications to reflect rapid changes in services (eg, closures, transition to fully virtual programs, social distancing and masking requirements). Having established connections with community organizations was essential to navigating the evolving landscape of available programs and supports.
Curating Quality Resources
ACORN currently screens for social risks in 9 domains: food, housing, utilities, transportation, education, employment, legal, social isolation/loneliness, and digital needs. Each resource guide pertains to a specific social risk domain and associated question(s) in the screener, allowing staff to quickly identify which guides a veteran may benefit from based on their screening responses. The guides are meant to be short and comprehensive but not exhaustive lists of programs and services. We limit the length of the guides to one single-sided page to provide high-yield, geographically tailored resources in an easy-to-use format. The guides should reflect the geographic area served by the VHA medical center or the community-based outpatient clinic (CBOC) where a veteran receives clinical care, but they also may include national- and state-level resources that provide services and programs to veterans.
Although there is local variation in the availability and accessibility of services across social risk domains, some domains have an abundance of resources and organizations at federal or national, state, and/or community levels. To narrow the list of resources to the highest yield programs, we developed a series of questions that serve as selection criteria to inform resource inclusion (Table).
Because the resource guides are intended to be broadly applicable to a large number of veterans, we prioritize generalizable resources over those with narrow eligibility criteria and/or services. When more intensive support is needed, social workers and other VHA clinical and nonclinical staff can supplement the resources on the guides with additional, more tailored resources that are based on individual factors, such as physical residence, income, transportation access, or household composition (eg, veteran families with children or older adults).
Formatting Resource Guides
Along with relevant information, such as the program name, location, and a specific point of contact, brief program descriptions provide information about services offered, eligibility criteria, application requirements, alternate contacts and locations, and website links. At the bottom of each guide, a section is included with the name and direct contact information for a social worker (often the individual assigned to the clinic where the veteran completed the ACORN screener) or another VHA staff member who can be reached for further assistance. These staff members are familiar with the content included on the guides and provide veterans with additional information or higher touch support as necessary. This contact information is useful for veterans who initially decline assistance or referrals but later want to follow up with staff for support or questions.
Visual consistency is a key feature of the ACORN resource guides, and layout and design elements are used to maximize space and enhance usability. Corresponding font colors for all program titles, contact information, and website links assist in visually separating and drawing attention to pertinent contact information. QR codes linked to program websites also are incorporated for veterans to easily access resource information from their smartphone or other electronic device.
Maintaining Resource Guides
To ensure continued accuracy, resource guides are updated about every 6 months to reflect changes in closures, transitions to virtual/in-person services, changes in location, new points of contact, and modifications to services or eligibility requirements. Notations also are made if any changes to services or eligibility are temporary or permanent. Recording these temporary adjustments was critical early in the COVID-19 pandemic as service offerings, eligibility requirements, and application processes changed often.
Updating the guides also facilitates continuous relationship building and connections with VHA and community-based services. Resource guides are living documents: they maintain lines of communication with designated contacts, allow for opportunities to improve the presentation of evolving program information in the guides, and offer the chance to learn about additional programs in the area that may meet veterans' needs.
Creating a Manual for ACORN Resource Guide Development
To facilitate dissemination of ACORN across VHA clinical settings and locations, our team developed a Resource Guide Manual to aid ACORN clinical sites in developing resource guides.21 The manual provides step-by-step guidance from recommendations for identifying resources to formatting and layout considerations. Supplemental materials include a checklist to ensure each program description includes the necessary information for veterans to successfully access the resource, as well as page templates and style suggestions to maximize usability. These templates standardize formatting across the social risk domain guides and include options for electronic and paper distribution.
RESOURCE GUIDE LIMITATIONS
The labor involved in building and maintaining multiple guides is considerable and requires a time investment both upfront and long term, which may not be feasible for clinical sites with limited staff. However, many VHA social workers maintain lists of resource and referral services for veterans as part of their routine clinical case management. These lists can serve as a valuable and timesaving starting point in curating high-yield resources for formal resource guides. To further reduce the time needed to develop guides, sites can use ACORN resource guide templates rather than designing and formatting guides from scratch. In addition to informing veterans of relevant services and programs, resource guides also can be provided to new staff, such as social workers or peer specialists, during onboarding to help familiarize them with available services to address veterans’ unmet needs.
Resources included on the guides also are geographically tailored, based on the physical location of the VHA medical center or CBOC. Some community-based services listed may not be as relevant, accessible, available, or convenient to veterans who live far from the clinic, which is relevant for nearly 25% of veterans who live in rural communities.22 This is a circumstance in which the expertise of VHA social workers should be used to recommend more appropriately tailored resources to a veteran. Use of free, publicly available electronic resource databases (eg, 211 Helpline Center) also can provide social workers and patients with an overview of all available resources within their community. There are paid referral platform services that health systems can contract with as well.23 However, the potential drawbacks to these alternative platforms include high startup costs or costly user-license fees for medical centers or clinics, inconsistent updates to resource information, and lack of compatibility with some electronic health record systems.23
Resource guides are not intended to take the place of a clinical social worker or other health professional but rather to serve in a supplemental capacity to clinical services. Certain circumstances necessitate a more comprehensive clinical assessment and/or a warm handoff to a social worker, including assistance with urgent food or housing needs, and ACORN workflows are created with urgent needs pathways in mind. Determining how to optimize intervention intensity based on individual patients’ expressed needs, preferences, and acuity remains a challenge for health care organizations conducting social risk screening.12 While distribution of geographically tailored resource guides can be a useful low touch intervention for some veterans, others will require more intensive case management to address or meet their needs. Some veterans also may fall in the middle of this spectrum, where a resource guide is not enough but intensive case management services facilitated by social workers are not needed or wanted by the patient. Integration of peer specialists, patient navigators, or community health workers who can work with veterans to support them in identifying, connecting with, and receiving support from relevant programs may help fill this gap. Given their knowledge and lived experience, these professionals also can promote patient-centered care as part of the health care team.
CONCLUSIONS
Whether used as a low-touch, standalone intervention or in combination with higher touch services (eg, case management or resource navigation), resource guides are a valuable tool for health care organizations working to address social needs as a component of efforts to advance health equity, reduce health disparities, and promote population health. We provide a pragmatic framework for developing and maintaining resource guides used in the ACORN initiative. However, additional work is needed to optimize the design, content, and format of resource guides for both usability and effectiveness as a social risk intervention across health care settings.
Acknowledgments
We express our gratitude for the Veterans Health Administration (VHA) Office of Health Equity and the VHA National Social Work Program, Care Management and Social Work Services for their support of the Assessing Circumstances and Offering Resources for Needs (ACORN) initiative. We also express our appreciation for those who supported the initial screener development as part of the ACORN Advisory Board, including Stacey Curran, BA; Charles Drebing, PhD; J. Stewart Evans, MD, MSc; Edward Federman, PhD; Maneesha Gulati, LICSW, ACSW; Nancy Kressin, PhD; Kenneth Link, LICSW; Monica Sharma, MD; and Jacqueline Spencer, MD, MPH. We also express our appreciation for those who supported the initial ACORN resource guide development, including Chuck Drebing, PhD, Ed Federman, PhD, and Ken Link, LICSW, and for the clinical care team members, especially the social workers and nurses, at our ACORN partner sites as well as the community-based partners who have helped us develop comprehensive resource guides for veterans. This work was supported by funding from the VHA Office of Health Equity and by resources and use of facilities at the VA Bedford Healthcare System, VA New England Healthcare System, and VA Providence Healthcare System. Alicia J. Cohen was additionally supported by a VA HSR&D Career Development Award (CDA 20-037).
Social risk factors and social needs have significant, often cumulative, impacts on health outcomes and are closely tied to health inequities. Defined as the individual-level adverse social conditions associated with poor health, social risk factors broadly include experiences such as food insecurity and housing instability; whereas the term social needs incorporates a person’s perceptions of and priorities related to their health-related needs.1 One recent study examining data from the Veterans Health Administration (VHA) found a 27% higher odds of mortality with each additional identified social risk, underscoring the critical link between social risks and veteran health outcomes.2
Assessing Circumstances and Offering Resources for Needs (ACORN), a collaborative quality improvement initiative conducted in partnership with the VHA Office of Health Equity and VHA National Social Work Program, Care Management and Social Work Services, is a social risk screening and referral program that aims to systematically identify and address unmet social needs among veterans to improve health and advance health equity.3,4 ACORN consists of 2 components: (1) a veteran-tailored screener to identify social risks within 9 domains; and (2) provision of relevant VA and community resources and referrals to address identified needs.3,5 Veterans who screen positive for ≥ 1 need receive referrals to a social worker or other relevant services, such as nutrition and food services or mental health, support navigating resources, and/or geographically tailored resource guides. This article describes the development and use of resource guides as a cross-cutting intervention component to address unmet social needs in diverse clinical settings and shares lessons learned from implementation in VHA outpatient clinics.
BACKGROUND
Unequal distribution of resources combined with historical discriminatory policies and practices, often linked to institutionalized racism, create inequities that lead to health disparities and hinder advancements in population health.6,7 Although health care systems alone cannot eliminate all health inequities, they can implement programs to identify social risks and address individual-level needs as 1 component of the multilevel approach needed to achieve health equity.8
As a national health care system serving > 9 million veterans, the VHA is well positioned to address social needs as an essential part of health. The VHA routinely screens for certain social risks, including housing instability, food insecurity, and intimate partner violence, and has a robust system of supports to address these and other needs among veterans, such as supportive housing services, vocational rehabilitation, assistance for justice-involved veterans, technology access support, and peer-support services.9-11 However, the VHA lacks a systematic approach to broader screening for social risks.
To address this gap, ACORN was developed in 2018 by an advisory board of subject matter experts, including clinical leaders, clinical psychologists, social workers, and health services researchers with content expertise in social risks and social needs.3 This interprofessional team sought to develop a veteran-tailored screener and resource referral initiative that could be scaled efficiently across VHA clinical settings.
Although health care organizations are increasingly implementing screening and interventions for social risks within clinical care, best practices and evidence-based tools to support clinical staff in these efforts are limited.12 Resource guides—curated lists of supportive services and organizations—may serve as a scalable “low-touch” intervention to help clinical staff address needs either alone or with more intensive interventions, such as social worker case management or patient navigation services.13
RESOURCE GUIDES—A Cross-Cutting Tool
The VHA has a uniquely robust network of nearly 18,000 social workers with clinical expertise in identifying, comprehensively assessing, and addressing social risks and needs among veterans. Interprofessional patient aligned care teams (PACTs)—a patient-centered medical home initiative that includes embedding social workers into primary care teams—facilitate the VHA’s capacity to address both medical and social needs.14 Social workers in PACTs and other care settings provide in-depth assessment and case management services to veterans who have a range of complex social needs. However, despite these comprehensive social services, in the setting of universal screening with a tool such as ACORN, it may not be feasible or practical to refer all patients who screen positive to a social worker for immediate follow-up, particularly in settings with capacity or resource limitations. For example, rates of screening positive on ACORN for ≥ 1 social risk have ranged from 48% of veterans in primary care sites and 80% in social work sites to nearly 100% in a PACT clinic for veterans experiencing homelessness.15
Additionally, a key challenge in the design of social needs interventions is determining how to optimize intervention intensity based on individual patient needs, acuity, and preferences. A substantial proportion of individuals who screen positive for social risks decline offered assistance, such as referrals.16 Resource guides are a cross-cutting tool that can be offered to veterans across a variety of settings, including primary care, specialty clinics, or emergency departments, as either a standalone intervention or one provided in combination with other resources or services. For patients who may not be interested in or feel comfortable accepting assistance at the time of screening or for those who prefer to research and navigate resources on their own, tailored resource guides can serve as a lower touch intervention to ensure interprofessional clinical staff across a range of settings and specialties have accessible, reliable, and up-to-date information to give to patients at the point of clinical care.17
Resource guides also can be used with higher touch clinical social work interventions, such as crisis management, supportive counseling, and case management. For example, social workers can use resource guides to provide education on VHA or community resources during clinical encounters with veterans and/or provide the guides to veterans to reference for future needs. Resource guides can further be used as a tool to support community resource navigation provided by nonclinical staff, such as peer specialists or community health workers.
How to BUILD RESOURCE GUIDES
Our team created resource guides (Figure) to provide veterans with concise, geographically tailored lists of VHA and other federal, state, and community services for the social risk domains included on the ACORN screener. To inform and develop a framework for building and maintaining ACORN guides, we first reviewed existing models that use this approach, including Boston Medical Center's WE CARE (Well Child Care, Evaluation, Community Resources, Advocacy, Referral, Education) and Thrive programs. We provide an overview of our process, which can be applied to clinical settings both within and outside the VHA.18,19
Partnerships
Active collaboration with frontline clinical social workers and local social work leadership is a critical part of identifying and prioritizing quality resources. Equipped with the knowledge of the local resource landscape, social workers can provide recommendations pertaining to national or federal, state, and local programs that have a history of being responsive to patients’ expressed needs.20 VHA social workers have robust knowledge of the veteran-specific resources available at VHA medical centers and nationally, and their clinical training equips them with the expertise to provide guidance about which resources to prioritize for inclusion in the guides.20
After receiving initial guidance from clinical social workers, our team began outreach to compile detailed program information, gauge program serviceability, and build relationships with both VHA and community-based services. Aligning with programs that share a similar mission in addressing social needs has proved crucial when developing the resource guides. Beyond ensuring the accuracy of program information, regular contact provides an opportunity to address capacity and workflow concerns that may arise from increased referrals. Additionally, open lines of communication with various supportive services facilitate connections with additional organizations and resources within the area.
The value of these relationships was evident at the onset of the COVID-19 pandemic, when the ACORN resource guides in use by our clinical site partners required frequent modifications to reflect rapid changes in services (eg, closures, transition to fully virtual programs, social distancing and masking requirements). Having established connections with community organizations was essential to navigating the evolving landscape of available programs and supports.
Curating Quality Resources
ACORN currently screens for social risks in 9 domains: food, housing, utilities, transportation, education, employment, legal, social isolation/loneliness, and digital needs. Each resource guide pertains to a specific social risk domain and associated question(s) in the screener, allowing staff to quickly identify which guides a veteran may benefit from based on their screening responses. The guides are meant to be short and comprehensive but not exhaustive lists of programs and services. We limit the length of the guides to one single-sided page to provide high-yield, geographically tailored resources in an easy-to-use format. The guides should reflect the geographic area served by the VHA medical center or the community-based outpatient clinic (CBOC) where a veteran receives clinical care, but they also may include national- and state-level resources that provide services and programs to veterans.
Although there is local variation in the availability and accessibility of services across social risk domains, some domains have an abundance of resources and organizations at federal or national, state, and/or community levels. To narrow the list of resources to the highest yield programs, we developed a series of questions that serve as selection criteria to inform resource inclusion (Table).
Because the resource guides are intended to be broadly applicable to a large number of veterans, we prioritize generalizable resources over those with narrow eligibility criteria and/or services. When more intensive support is needed, social workers and other VHA clinical and nonclinical staff can supplement the resources on the guides with additional, more tailored resources that are based on individual factors, such as physical residence, income, transportation access, or household composition (eg, veteran families with children or older adults).
Formatting Resource Guides
Along with relevant information, such as the program name, location, and a specific point of contact, brief program descriptions provide information about services offered, eligibility criteria, application requirements, alternate contacts and locations, and website links. At the bottom of each guide, a section is included with the name and direct contact information for a social worker (often the individual assigned to the clinic where the veteran completed the ACORN screener) or another VHA staff member who can be reached for further assistance. These staff members are familiar with the content included on the guides and provide veterans with additional information or higher touch support as necessary. This contact information is useful for veterans who initially decline assistance or referrals but later want to follow up with staff for support or questions.
Visual consistency is a key feature of the ACORN resource guides, and layout and design elements are used to maximize space and enhance usability. Corresponding font colors for all program titles, contact information, and website links assist in visually separating and drawing attention to pertinent contact information. QR codes linked to program websites also are incorporated for veterans to easily access resource information from their smartphone or other electronic device.
Maintaining Resource Guides
To ensure continued accuracy, resource guides are updated about every 6 months to reflect changes in closures, transitions to virtual/in-person services, changes in location, new points of contact, and modifications to services or eligibility requirements. Notations also are made if any changes to services or eligibility are temporary or permanent. Recording these temporary adjustments was critical early in the COVID-19 pandemic as service offerings, eligibility requirements, and application processes changed often.
Updating the guides also facilitates continuous relationship building and connections with VHA and community-based services. Resource guides are living documents: they maintain lines of communication with designated contacts, allow for opportunities to improve the presentation of evolving program information in the guides, and offer the chance to learn about additional programs in the area that may meet veterans' needs.
Creating a Manual for ACORN Resource Guide Development
To facilitate dissemination of ACORN across VHA clinical settings and locations, our team developed a Resource Guide Manual to aid ACORN clinical sites in developing resource guides.21 The manual provides step-by-step guidance from recommendations for identifying resources to formatting and layout considerations. Supplemental materials include a checklist to ensure each program description includes the necessary information for veterans to successfully access the resource, as well as page templates and style suggestions to maximize usability. These templates standardize formatting across the social risk domain guides and include options for electronic and paper distribution.
RESOURCE GUIDE LIMITATIONS
The labor involved in building and maintaining multiple guides is considerable and requires a time investment both upfront and long term, which may not be feasible for clinical sites with limited staff. However, many VHA social workers maintain lists of resource and referral services for veterans as part of their routine clinical case management. These lists can serve as a valuable and timesaving starting point in curating high-yield resources for formal resource guides. To further reduce the time needed to develop guides, sites can use ACORN resource guide templates rather than designing and formatting guides from scratch. In addition to informing veterans of relevant services and programs, resource guides also can be provided to new staff, such as social workers or peer specialists, during onboarding to help familiarize them with available services to address veterans’ unmet needs.
Resources included on the guides also are geographically tailored, based on the physical location of the VHA medical center or CBOC. Some community-based services listed may not be as relevant, accessible, available, or convenient to veterans who live far from the clinic, which is relevant for nearly 25% of veterans who live in rural communities.22 This is a circumstance in which the expertise of VHA social workers should be used to recommend more appropriately tailored resources to a veteran. Use of free, publicly available electronic resource databases (eg, 211 Helpline Center) also can provide social workers and patients with an overview of all available resources within their community. There are paid referral platform services that health systems can contract with as well.23 However, the potential drawbacks to these alternative platforms include high startup costs or costly user-license fees for medical centers or clinics, inconsistent updates to resource information, and lack of compatibility with some electronic health record systems.23
Resource guides are not intended to take the place of a clinical social worker or other health professional but rather to serve in a supplemental capacity to clinical services. Certain circumstances necessitate a more comprehensive clinical assessment and/or a warm handoff to a social worker, including assistance with urgent food or housing needs, and ACORN workflows are created with urgent needs pathways in mind. Determining how to optimize intervention intensity based on individual patients’ expressed needs, preferences, and acuity remains a challenge for health care organizations conducting social risk screening.12 While distribution of geographically tailored resource guides can be a useful low touch intervention for some veterans, others will require more intensive case management to address or meet their needs. Some veterans also may fall in the middle of this spectrum, where a resource guide is not enough but intensive case management services facilitated by social workers are not needed or wanted by the patient. Integration of peer specialists, patient navigators, or community health workers who can work with veterans to support them in identifying, connecting with, and receiving support from relevant programs may help fill this gap. Given their knowledge and lived experience, these professionals also can promote patient-centered care as part of the health care team.
CONCLUSIONS
Whether used as a low-touch, standalone intervention or in combination with higher touch services (eg, case management or resource navigation), resource guides are a valuable tool for health care organizations working to address social needs as a component of efforts to advance health equity, reduce health disparities, and promote population health. We provide a pragmatic framework for developing and maintaining resource guides used in the ACORN initiative. However, additional work is needed to optimize the design, content, and format of resource guides for both usability and effectiveness as a social risk intervention across health care settings.
Acknowledgments
We express our gratitude for the Veterans Health Administration (VHA) Office of Health Equity and the VHA National Social Work Program, Care Management and Social Work Services for their support of the Assessing Circumstances and Offering Resources for Needs (ACORN) initiative. We also express our appreciation for those who supported the initial screener development as part of the ACORN Advisory Board, including Stacey Curran, BA; Charles Drebing, PhD; J. Stewart Evans, MD, MSc; Edward Federman, PhD; Maneesha Gulati, LICSW, ACSW; Nancy Kressin, PhD; Kenneth Link, LICSW; Monica Sharma, MD; and Jacqueline Spencer, MD, MPH. We also express our appreciation for those who supported the initial ACORN resource guide development, including Chuck Drebing, PhD, Ed Federman, PhD, and Ken Link, LICSW, and for the clinical care team members, especially the social workers and nurses, at our ACORN partner sites as well as the community-based partners who have helped us develop comprehensive resource guides for veterans. This work was supported by funding from the VHA Office of Health Equity and by resources and use of facilities at the VA Bedford Healthcare System, VA New England Healthcare System, and VA Providence Healthcare System. Alicia J. Cohen was additionally supported by a VA HSR&D Career Development Award (CDA 20-037).
1. Alderwick H, Gottlieb LM. Meanings and misunderstandings: a social determinants of health lexicon for health care systems. Milbank Q. 2019;97(2):407-419. doi:10.1111/1468-0009.12390
2. Blosnich JM, Montgomery AE, Taylor LD, Dichter ME. Adverse social factors and all-cause mortality among male and female patients receiving care in the Veterans Health Administration. Prev Med. 2020;141:106272. doi:10.1016/j.ypmed.2020.106272
3. Russell LE, Cohen AJ, Chrzas S, et al. Implementing a social needs screening and referral program among veterans: Assessing Circumstances & Offering Resources for Needs (ACORN). J Gen Intern Med. 2023;38(13):2906-2913. doi:10.1007/s11606-023-08181-9
4. Cohen AJ, Russell LE, Elwy AR, et al. Adaptation of a social risk screening and referral initiative across clinical populations, settings, and contexts in the Department of Veterans Affairs Health System. Front Health Serv. 2023;2. doi:10.3389/frhs.2022.958969
5. Cohen AJ, Kennedy MA, Mitchell KM, Russell LE. The Assessing Circumstances & Offering Resources for Needs (ACORN) initiative. Updated September 2022. Accessed December 4, 2023. https://www.va.gov/HEALTHEQUITY/docs/ACORN_Screening_Tool.pdf
6. Jones CP. Levels of racism: a theoretic framework and a gardener’s tale. Am J Public Health. 2000;90(8):1212-1215. doi:10.2105/ajph.90.8.1212
7. American Public Health Association. Creating the healthiest nation: advancing health equity. Accessed November 28, 2023. https://www.apha.org/-/media/files/pdf/factsheets/advancing_health_equity.ashx?la=en&hash=9144021FDA33B4E7E02447CB28CA3F9D4BE5EF18
8. Castrucci B, Auerbach J. Meeting individual social needs falls short of addressing social determinants of health. Health Aff. Published January 16, 2019. doi:10.1377/hblog20190115.234942
9. Montgomery AE, Fargo JD, Byrne TH, Kane VR, Culhane DP. Universal screening for homelessness and risk for homelessness in the Veterans Health Administration. Am J Public Health. 2013;103(suppl 2):S210-211. doi:10.2105/AJPH.2013.301398
10. Cohen AJ, Rudolph JL, Thomas KS, et al. Food insecurity among veterans: resources to screen and intervene. Fed Pract. 2020;37(1):16-23.
11. Iverson KM, Adjognon O, Grillo AR, et al. Intimate partner violence screening programs in the Veterans Health Administration: informing scale-up of successful practices. J Gen Intern Med. 2019;34(11):2435-2442. doi:10.1007/s11606-019-05240-y
12. National Academies of Sciences, Engineering, and Medicine. Integrating Social Care into the Delivery of Health Care: Moving Upstream to Improve the Nation’s Health. The National Academies Press; 2019. Accessed November 28, 2023. https://nap.nationalacademies.org/catalog/25467/integrating-social-care-into-the-delivery-of-health-care-moving
13. Gottlieb LM, Adler NE, Wing H, et al. Effects of in-person assistance vs personalized written resources about social services on household social risks and child and caregiver health: a randomized clinical trial. JAMA Netw Open. 2020;3(3):e200701. doi:10.1001/jamanetworkopen.2020.0701
14. Cornell PY, Halladay CW, Ader J, et al. Embedding social workers in Veterans Health Administration primary care teams reduces emergency department visits. Health Aff (Millwood). 2020;39(4):603-612. doi:10.1377/hlthaff.2019.01589
15. Cohen AJ, Bruton M, Hooshyar D. US Department of Veterans Affairs, Office of Health Services Research and Development. The WHO’s greatest ICD-10 hits for fiscal year 2022: social determinants of health. Published March 9, 2022. Updated November 6, 2023. Accessed December 4, 2023. https://www.hsrd.research.va.gov/for_researchers/cyber_seminars/archives/video_archive.cfm?SessionID=4125

16. De Marchis EH, Alderwick H, Gottlieb LM. Do patients want help addressing social risks? J Am Board Fam Med. 2020;33(2):170-175. doi:10.3122/jabfm.2020.02.190309
17. Cohen AJ, Isaacson N, Torby M, Smith A, Zhang G, Patel MR. Motivators, barriers, and preferences to engagement with offered social care assistance among people with diabetes: a mixed methods study. Am J Prev Med. 2022;63(3, suppl 2):S152-S163. doi:10.1016/j.amepre.2022.02.022
18. Buitron de la Vega P, Losi S, Sprague Martinez L, et al. Implementing an EHR-based screening and referral system to address social determinants of health in primary care. Med Care. 2019;57(suppl 6, suppl 2):S133-S139. doi:10.1097/MLR.0000000000001029
19. Boston Medical Center. The WE CARE Model. Accessed November 28, 2023. https://www.bmc.org/pediatrics-primary-care/we-care/we-care-model
20. US Department of Veterans Affairs, Office of Rural Health. VA social work. Updated July 11, 2023. Accessed December 4, 2023. https://www.socialwork.va.gov
21. Mitchell KM, Russell LE, Cohen AJ, Kennedy MA. Building ACORN resource guides for veterans. Accessed November 28, 2023. https://www.va.gov/HEALTHEQUITY/docs/ACORN_Resource_Guide_Manual.pdf
22. US Department of Veterans Affairs, Veterans Health Administration, Office of Rural Health. Rural Veterans. Accessed November 28, 2023. https://www.ruralhealth.va.gov/aboutus/ruralvets.asp
23. Cartier Y, Fichtenberg C, Gottlieb L. Community resource referral platforms: a guide for health care organizations. Published 2019. Accessed December 4, 2023. https://sirenetwork.ucsf.edu/tools-resources/resources/community-resource-referral-platforms-guide-health-care-organizations
1. Alderwick H, Gottlieb LM. Meanings and misunderstandings: a social determinants of health lexicon for health care systems. Milbank Q. 2019;97(2):407-419. doi:10.1111/1468-0009.12390
2. Blosnich JM, Montgomery AE, Taylor LD, Dichter ME. Adverse social factors and all-cause mortality among male and female patients receiving care in the Veterans Health Administration. Prev Med. 2020;141:106272. doi:10.1016/j.ypmed.2020.106272
3. Russell LE, Cohen AJ, Chrzas S, et al. Implementing a social needs screening and referral program among veterans: Assessing Circumstances & Offering Resources for Needs (ACORN). J Gen Intern Med. 2023;38(13):2906-2913. doi:10.1007/s11606-023-08181-9
4. Cohen AJ, Russell LE, Elwy AR, et al. Adaptation of a social risk screening and referral initiative across clinical populations, settings, and contexts in the Department of Veterans Affairs Health System. Front Health Serv. 2023;2. doi:10.3389/frhs.2022.958969
5. Cohen AJ, Kennedy MA, Mitchell KM, Russell LE. The Assessing Circumstances & Offering Resources for Needs (ACORN) initiative. Updated September 2022. Accessed December 4, 2023. https://www.va.gov/HEALTHEQUITY/docs/ACORN_Screening_Tool.pdf
6. Jones CP. Levels of racism: a theoretic framework and a gardener’s tale. Am J Public Health. 2000;90(8):1212-1215. doi:10.2105/ajph.90.8.1212
7. American Public Health Association. Creating the healthiest nation: advancing health equity. Accessed November 28, 2023. https://www.apha.org/-/media/files/pdf/factsheets/advancing_health_equity.ashx?la=en&hash=9144021FDA33B4E7E02447CB28CA3F9D4BE5EF18
8. Castrucci B, Auerbach J. Meeting individual social needs falls short of addressing social determinants of health. Health Aff. Published January 16, 2019. doi:10.1377/hblog20190115.234942
9. Montgomery AE, Fargo JD, Byrne TH, Kane VR, Culhane DP. Universal screening for homelessness and risk for homelessness in the Veterans Health Administration. Am J Public Health. 2013;103(suppl 2):S210-211. doi:10.2105/AJPH.2013.301398
10. Cohen AJ, Rudolph JL, Thomas KS, et al. Food insecurity among veterans: resources to screen and intervene. Fed Pract. 2020;37(1):16-23.
11. Iverson KM, Adjognon O, Grillo AR, et al. Intimate partner violence screening programs in the Veterans Health Administration: informing scale-up of successful practices. J Gen Intern Med. 2019;34(11):2435-2442. doi:10.1007/s11606-019-05240-y
12. National Academies of Sciences, Engineering, and Medicine. Integrating Social Care into the Delivery of Health Care: Moving Upstream to Improve the Nation’s Health. The National Academies Press; 2019. Accessed November 28, 2023. https://nap.nationalacademies.org/catalog/25467/integrating-social-care-into-the-delivery-of-health-care-moving
13. Gottlieb LM, Adler NE, Wing H, et al. Effects of in-person assistance vs personalized written resources about social services on household social risks and child and caregiver health: a randomized clinical trial. JAMA Netw Open. 2020;3(3):e200701. doi:10.1001/jamanetworkopen.2020.0701
14. Cornell PY, Halladay CW, Ader J, et al. Embedding social workers in Veterans Health Administration primary care teams reduces emergency department visits. Health Aff (Millwood). 2020;39(4):603-612. doi:10.1377/hlthaff.2019.01589
15. Cohen AJ, Bruton M, Hooshyar D. US Department of Veterans Affairs, Office of Health Services Research and Development. The WHO’s greatest ICD-10 hits for fiscal year 2022: social determinants of health. Published March 9, 2022. Updated November 6, 2023. Accessed December 4, 2023. https://www.hsrd.research.va.gov/for_researchers/cyber_seminars/archives/video_archive.cfm?SessionID=4125

16. De Marchis EH, Alderwick H, Gottlieb LM. Do patients want help addressing social risks? J Am Board Fam Med. 2020;33(2):170-175. doi:10.3122/jabfm.2020.02.190309
17. Cohen AJ, Isaacson N, Torby M, Smith A, Zhang G, Patel MR. Motivators, barriers, and preferences to engagement with offered social care assistance among people with diabetes: a mixed methods study. Am J Prev Med. 2022;63(3, suppl 2):S152-S163. doi:10.1016/j.amepre.2022.02.022
18. Buitron de la Vega P, Losi S, Sprague Martinez L, et al. Implementing an EHR-based screening and referral system to address social determinants of health in primary care. Med Care. 2019;57(suppl 6, suppl 2):S133-S139. doi:10.1097/MLR.0000000000001029
19. Boston Medical Center. The WE CARE Model. Accessed November 28, 2023. https://www.bmc.org/pediatrics-primary-care/we-care/we-care-model
20. US Department of Veterans Affairs, Office of Rural Health. VA social work. Updated July 11, 2023. Accessed December 4, 2023. https://www.socialwork.va.gov
21. Mitchell KM, Russell LE, Cohen AJ, Kennedy MA. Building ACORN resource guides for veterans. Accessed November 28, 2023. https://www.va.gov/HEALTHEQUITY/docs/ACORN_Resource_Guide_Manual.pdf
22. US Department of Veterans Affairs, Veterans Health Administration, Office of Rural Health. Rural Veterans. Accessed November 28, 2023. https://www.ruralhealth.va.gov/aboutus/ruralvets.asp
23. Cartier Y, Fichtenberg C, Gottlieb L. Community resource referral platforms: a guide for health care organizations. Published 2019. Accessed December 4, 2023. https://sirenetwork.ucsf.edu/tools-resources/resources/community-resource-referral-platforms-guide-health-care-organizations
Leader Rounding for High Reliability and Improved Patient Safety
The hospital is altogether the most complex human organization ever devised. Peter Drucker 1
The ever-changing landscape of today’s increasingly complex health care system depends on implementing multifaceted, team-based methods of care delivery to provide safe, effective patient care.2 Critical to establishing and sustaining exceptionally safe, effective patient care is open, transparent communication among members of interprofessional teams with senior leaders.3 However, current evidence shows that poor communication among interprofessional health care teams and leadership is commonplace and a significant contributing factor to inefficiencies, medical errors, and poor outcomes.4 One strategy for improving communication is through the implementation of
We describe the importance of leader rounding for high reliability as an approach to improving patient safety. Based on a review of the literature, our experiences, and lessons learned, we offer recommendations for how health care organizations on the journey to high reliability can improve patient safety.
Rounding in health care is not new. In fact, rounding has been a strong principal practice globally for more than 2 decades.6 During this time, varied rounding approaches have emerged, oftentimes focused on areas of interest, such as patient care, environmental services, facilities management, and discharge planning.4,7 Variations also might involve the location of the rounds, such as a patient’s bedside, unit hallways, and conference rooms as well as the naming of rounds, such as interdisciplinary/multidisciplinary, teaching, and walkrounds.7-10
A different type of rounding that is characteristic of high reliability organizations (HROs) is leader rounding for high reliability. The Veterans Health Administration (VHA) formally launched its journey to becoming an
Leader rounding for high reliability includes regularly scheduled, structured visits, with interdisciplinary teams to discuss high reliability, safety, and improvement efforts. The specific aim of these particular rounds is for senior leaders to be visible where teams are located and learn from staff (especially those on the frontlines of care) about day-to-day challenges that may contribute to patient harm.12,13 Leader rounding for high reliability is also an important approach to improving leadership visibility across the organization, demonstrating a commitment to high reliability, and building trust and relationships with staff through open and honest dialogue. It is also an important approach to increasing leadership understanding of operational, clinical, nonclinical, patient experience issues, and concern related to safety.11 This opportunity enables leaders to provide and receive real-time feedback from staff.9,11 This experience also gives leaders an opportunity to reinforce
In preparation for implementing a leader rounding for high reliability process at the VABHS, we conducted an extensive literature review for peer-reviewed publications published between January 2015 and September 2022 regarding how other organizations implemented leader rounding. This search found a dearth of evidence as it specifically relates to leader rounding for high reliability. This motivated us to create a process for developing and implementing leader rounding for high reliability in pursuit of improving patient safety. With this objective in mind, we created and piloted a process in the fall of 2023. The first 3 months were focused on the medical center director rounding with other members of the executive leadership team to assess the feasibility and acceptability of the process. In December 2023, members of the executive leadership team began conducting leader rounding for high reliability separately. The following steps are based on the lessons we have gleaned from evolving evidence, our experiences, and developing and implementing an approach to leader rounding for high reliability.
ESTABLISH A PROCESS
Leader rounding for high reliability is performed by health care organization executive leadership, directors, managers, and supervisors. When properly conducted, increased levels of teamwork and more effective bidirectional communication take place, resulting in a united team motivated to improve patient safety.16,17 Important early steps for implementing leader rounding for high reliability include establishing a process and getting leadership buy-in. Purposeful attention to planning is critical as is understanding the organizational factors that might deter success. Establishing a process should consider facilitators and barriers to implementation, which can include high vs low leadership turnover, structured vs unstructured rounding, and time for rounding vs competing demands.18,19 We have learned that effective planning is important for ensuring that leadership teams are well prepared and ambitious about leader rounding for high reliability.
Leader rounding for high reliability involves brief 10-to-15-minute interactions with interdisciplinary teams, including frontline staff. For health care organizations beginning to implement this approach, having scripts or checklists accessible might be of help. If possible, the rounds should be scheduled in advance. This helps to avoid rounding in areas at their busiest times. When possible, leader rounding for high reliability should occur as planned. Canceling rounds sends the message that leader rounding for high reliability and the valuable interactions they support are a low priority. When conflicts arise, another leader should be sent to participate. Developing a list of questions in advance helps to underscore key messages to be shared as well as reinforce principles, practices, behaviors, and attitudes related to high reliability (Appendix 1).11
Finally, closing the loop is critical to the leader rounding process and to improve bidirectional communication. Closed-loop communication, following up on and/or closing out an area of discussion, not only promotes a shared understanding of information but has been found to improve patient safety.19 Effective leader rounding for high reliability includes summarizing issues and opportunities, deciding on a date for resolution for open action items, and identifying who is responsible for taking action. Senior leaders are not responsible for resolving all issues. If a team or manager of a work area can solve any issues identified, this should be encouraged and supported so accountability is maintained at the most appropriate level of the organization.
Instrumental to leader rounding for high reliability is establishing a cadence for when leaders will visit work areas.14 The most critical strategy, especially in times of change, is consistency in rounding.11 At the start of implementation, we decided on a biweekly cadence. Initially leaders visited areas of the organization within their respective reporting structure. Once this was established, leaders periodically round in areas outside their scope of responsibility. This affords leaders the opportunity to observe other areas of the organization. As noted, it is important for leaders to be flexible with the rounding process especially in areas where direct patient care is being provided.
Tracking
Developing a tracking tool also is important for an effective leader rounding process. This tool is used to document issues and concerns identified during the rounding process, assign accountability, track the status of items, and close the loop when completed. One of the most commonly reported hurdles to staff sharing information to promote a culture of safety is the lack of feedback on what actions were taken to address the concern or issue raised with leadership. Closed-loop communication is critical for keeping staff continually engaged in efforts to promote a culture of safety.20 We have found that a tracking tool helps to ensure that closed-loop communication takes place.
Various platforms can be used for tracking items and providing follow-up, including paper worksheets, spreadsheets, databases, or third party software (eg, SharePoint, TruthPoint Rounds, GetWell Rounds). The tracking tool should have a standardized approach for prioritizing issues.
The stoplight classification system uses color coding (Figure 3).21 Green represents a safe space where there are no or low safety risks and are easily addressed at the local level by the area manager with or without assistance from the leadership team rounding, such as staffing.22,23 The unit manager has control of the situation and a plan is actively being implemented. Yellow signifies that areas are at risk, but with increased vigilance, issues do not escalate to a crisis state.22,23 Yellow-coded issues require further investigation by the leadership team. The senior leader on the team designates a process as well as a person responsible for closing the loop with the area manager regarding the status of problem resolution. For example, if the unit manager mentioned previously needing help to find staff, the area manager would suggest or take steps to help the unit manager. The area manager is then responsible for updating the frontline staff. Red-coded issues are urgent, identifying a state of crisis or high risk. Red issues need to be immediately addressed but cannot be resolved during rounds. Senior leaders must evaluate and make decisions to mitigate the threat. A member of the leadership team is tasked with following up with the area manager, typically within 24 hours. A staffing crisis that requires executive leadership help with identifying additional resources would be coded red.
The area manager is responsible for closing the loop with frontline staff. As frontline staff became more comfortable with the process, we observed an upward trend in the number of reported issues. We are now starting to see a downward trend in concerns shared during rounding as managers and frontline staff feel empowered to address issues at the lowest level.
Measuring Impact
Measuring the impact is a critical step to determine the overall effectiveness of leader rounding for high reliability. It can be as simple as requesting candid feedback from frontline staff, supervisors, managers, and service chiefs. For example, 4 months into the implementation process, the VABHS administered a brief staff survey on the overall process, perceived benefits, and challenges experienced (Appendix 2). Potential measures include the counts of leaders rounding, total rounds, rounds cancelled, and staff members actively participating in rounds. Outcomes that can be measured include issues identified, addressed, elevated, and remaining open; number of extended workdays due to rounds; staff staying overtime; and delays in patient care activities.23 Other measures to consider are the effects of rounding on staff as well as patient/family satisfaction, increase in the number of errors and near-miss events reported per month in a health care organizations’ patient safety reporting system, and increased engagement of staff members in continuous process improvement activities. Since the inception of leader rounding for high reliability, the VABHS has seen a slight increase in the number of events entered in the patient safety reporting system. Other factors that may have contributed to this change, including encouragement of reporting at safety forums, and tiered safety huddles.
DISCUSSION
This initiative involved the development and implementation of a leader rounding for high reliability process at the VABHS with the overarching goal of ensuring efficient communication exists among members of the health care team for delivering safe, quality patient care. The initiative was well received by staff from senior leadership to frontline personnel and promoted significant interest in efforts to improve safety across the health care system.
The pilot phase permitted us to examine the feasibility and acceptability of the process to leadership as well as frontline staff. The insight gained and lessons learned through the implementation process helped us make revisions where needed and develop the tools to ensure success. In the second phase of implementation, which commenced in December 2023, each executive leadership team member began leader rounding for high reliability with their respective department service chiefs. Throughout this phase, feedback will be sought on the overall process, perceived benefits, and challenges experienced to make improvements or changes as needed. We also will continue to monitor the number of events entered in the patient safety reporting system. Future efforts will focus on developing a robust program of evaluation to explore the impact of the program on patient/family satisfaction as well as safety outcomes.
Limitations
Developing and implementing a process for leader rounding for high reliability was undertaken to support the VABHS and VHA journey to high reliability. Other health care organizations and integrated systems might identify different processes for improving patient safety and to support their journey to becoming an HRO.
CONCLUSIONS
The importance of leader rounding for high reliability to improve patient safety cannot be emphasized enough in a time where health care systems have become increasingly complex. Health care is a complex adaptive system that requires effective, bidirectional communication and collaboration among all disciplines. One of the most useful, evidence-based strategies for promoting this communication and collaboration to improve a culture of safety is leader rounding for high reliability.
1. Drucker PF. They’re not employees, they’re people. Accessed November 15, 2023. https://hbr.org/2002/02/theyre-not-employees-theyre-people
2. Adams HA, Feudale RM. Implementation of a structured rounding tool for interprofessional care team rounds to improve communication and collaboration in patient care. Pediatr Nurs. 2018;44(5):229-233, 246.
3. Witz I, Lucchese S, Valenzano TJ, et al: Perceptions on implementation of a new standardized reporting tool to support structured morning rounds: recommendations for interprofessional teams and healthcare leaders. J Med Radiat Sci. 2022;53(4):S85-S92. doi:10.1016/j.jmir.2022.06.006
4. Blakeney EA, Chu F, White AA, et al. A scoping review of new implementations of interprofessional bedside rounding models to improve teamwork, care, and outcomes in hospitals. J Interprof Care. 2021;10:1-16 [Online ahead of print.] doi:10.1080/13561820.2021.1980379
5. Agency for Research and Healthcare Quality. High reliability. Accessed December 4, 2023. https://psnet.ahrq.gov/primer/high-reliability
6. Hedenstrom M, Harrilson A, Heath M, Dyass S. “What’s old is new again”: innovative health care leader rounding—a strategy to foster connection. Nurse Lead. 2022;20(4):366-370.
7. Walton V, Hogden A, Long JC, Johnson JK, Greenfield D. How do interprofessional healthcare teams perceive the benefits and challenges of interdisciplinary ward rounds. J Multidiscip Healthc. 2019;12:1023-1032. doi:10.2147/JMDH.S226330
8. Walton V, Hogden A, Johnson J, Greenfield D. Ward rounds, participants, roles and perceptions: literature review. Int J Health Care Qual Assur. 2016;29(4):364-379. doi:10.1108/IJHCQA-04-2015-0053
9. Sexton JB, Adair KC, Leonard MW, et al. Providing feedback following leadership walkrounds is associated with better patient safety culture, higher employee engagement and lower burnout. BMJ Qual Saf. 2018;27(4):261-270. doi:10.1136/bmjqs-2016-006399
10. Sexton JB, Adair KC, Profit J, et al. Safety culture and workforce well-being associations with positive leadership walkrounds. Jt Comm J Qual Patient Saf. 2021;47(7):403-411. doi:10.1016/j.jcjq.2021.04.001
11. US Department of Veterans Affairs, Veterans Health Administration. Leader’s guide to foundational high reliability organization (HRO) practices. Accessed December 5, 2023. https://dvagov.sharepoint.com/sites/OHT-PMO/high-reliability/Pages/default.aspx
12. Zajac S, Woods A, Tannenbaum S, Salas E, Hollada CL. Overcoming challenges to teamwork in healthcare: a team effectiveness framework and evidence-based guidance. Front Commun. 2021;6:1-20. doi:10.3389/fcomm.2021.606445
13. Department of Veterans Affairs, Veterans Health Administration. VHA’s Vision for a High Reliability Organization. Accessed December 5, 2023. https://www.hsrd.research.va.gov/publications/forum/summer20/default.cfm?ForumMenu=summer20-1
14. Merchant NB, O’Neal J, Dealino-Perez C, Xiang J, Montoya A Jr, Murray JS. A high-reliability organization mindset. Am J Med Qual. 2022;37(6):504-510. doi:10.1097/JMQ.0000000000000086
15. Verhaegh KJ, Seller-Boersma A, Simons R, et al. An exploratory study of healthcare professionals’ perceptions of interprofessional communication and collaboration. J Interprof Care. 2017;31(3):397-400. doi:10.1080/13561820.2017.1289158
16. Winter M, Tjiong L. HCAHPS Series Part 2: Does purposeful leader rounding make a difference? Nurs Manag. 2015;46(2):26-32. doi:10.1097/01.NUMA.0000460034.25697.06
17. Beaird G, Baernholdt M, White KR. Perceptions of interdisciplinary rounding practices. J Clin Nurs. 2020;29(7-8):1141-1150. doi:10.1111/jocn.15161
18. Hendricks S, LaMothe VJ, Kara A. Facilitators and barriers for interprofessional rounding: a qualitative study. Clin Nurse Spec. 2017;31(4):219-228. doi:10.1097/NUR.0000000000000310
19. Diaz MCG, Dawson K. Impact of simulation-based closed-loop communication training on medical errors in a pediatric emergency department. Am J Med Qual. 2020;35(6):474-478. doi:10.1177/1062860620912480
20. Williams S, Fiumara K, Kachalia A, Desai S. Closing the loop with ambulatory staff on safety reports. Jt Comm J Qual Saf. 2020;46(1):44-50. doi:10.1016/j.jcjq.2019.09.009
21. Parbhoo A, Batte J. Traffic lights: putting a stop to unsafe patient transfers. BMJ Qual Improv Rep. 2015;4(1):u204799.w2079. doi:10.1136/bmjquality.u204799.w2079
22. Prineas S, Culwick M, Endlich Y. A proposed system for standardization of colour-coding stages of escalating criticality in clinical incidents. Curr Opin Anaesthesiol. 2021;34(6):752-760. doi:10.1097/ACO.0000000000001071.
23. Merchant NB, O’Neal J, Montoya A, Cox GR, Murray JS. Creating a process for the implementation of tiered huddles in a Veterans Affairs medical center. Mil Med. 2023;188(5-6):901-906. doi:10.1093/milmed/usac073
The hospital is altogether the most complex human organization ever devised. Peter Drucker 1
The ever-changing landscape of today’s increasingly complex health care system depends on implementing multifaceted, team-based methods of care delivery to provide safe, effective patient care.2 Critical to establishing and sustaining exceptionally safe, effective patient care is open, transparent communication among members of interprofessional teams with senior leaders.3 However, current evidence shows that poor communication among interprofessional health care teams and leadership is commonplace and a significant contributing factor to inefficiencies, medical errors, and poor outcomes.4 One strategy for improving communication is through the implementation of
We describe the importance of leader rounding for high reliability as an approach to improving patient safety. Based on a review of the literature, our experiences, and lessons learned, we offer recommendations for how health care organizations on the journey to high reliability can improve patient safety.
Rounding in health care is not new. In fact, rounding has been a strong principal practice globally for more than 2 decades.6 During this time, varied rounding approaches have emerged, oftentimes focused on areas of interest, such as patient care, environmental services, facilities management, and discharge planning.4,7 Variations also might involve the location of the rounds, such as a patient’s bedside, unit hallways, and conference rooms as well as the naming of rounds, such as interdisciplinary/multidisciplinary, teaching, and walkrounds.7-10
A different type of rounding that is characteristic of high reliability organizations (HROs) is leader rounding for high reliability. The Veterans Health Administration (VHA) formally launched its journey to becoming an
Leader rounding for high reliability includes regularly scheduled, structured visits, with interdisciplinary teams to discuss high reliability, safety, and improvement efforts. The specific aim of these particular rounds is for senior leaders to be visible where teams are located and learn from staff (especially those on the frontlines of care) about day-to-day challenges that may contribute to patient harm.12,13 Leader rounding for high reliability is also an important approach to improving leadership visibility across the organization, demonstrating a commitment to high reliability, and building trust and relationships with staff through open and honest dialogue. It is also an important approach to increasing leadership understanding of operational, clinical, nonclinical, patient experience issues, and concern related to safety.11 This opportunity enables leaders to provide and receive real-time feedback from staff.9,11 This experience also gives leaders an opportunity to reinforce
In preparation for implementing a leader rounding for high reliability process at the VABHS, we conducted an extensive literature review for peer-reviewed publications published between January 2015 and September 2022 regarding how other organizations implemented leader rounding. This search found a dearth of evidence as it specifically relates to leader rounding for high reliability. This motivated us to create a process for developing and implementing leader rounding for high reliability in pursuit of improving patient safety. With this objective in mind, we created and piloted a process in the fall of 2023. The first 3 months were focused on the medical center director rounding with other members of the executive leadership team to assess the feasibility and acceptability of the process. In December 2023, members of the executive leadership team began conducting leader rounding for high reliability separately. The following steps are based on the lessons we have gleaned from evolving evidence, our experiences, and developing and implementing an approach to leader rounding for high reliability.
ESTABLISH A PROCESS
Leader rounding for high reliability is performed by health care organization executive leadership, directors, managers, and supervisors. When properly conducted, increased levels of teamwork and more effective bidirectional communication take place, resulting in a united team motivated to improve patient safety.16,17 Important early steps for implementing leader rounding for high reliability include establishing a process and getting leadership buy-in. Purposeful attention to planning is critical as is understanding the organizational factors that might deter success. Establishing a process should consider facilitators and barriers to implementation, which can include high vs low leadership turnover, structured vs unstructured rounding, and time for rounding vs competing demands.18,19 We have learned that effective planning is important for ensuring that leadership teams are well prepared and ambitious about leader rounding for high reliability.
Leader rounding for high reliability involves brief 10-to-15-minute interactions with interdisciplinary teams, including frontline staff. For health care organizations beginning to implement this approach, having scripts or checklists accessible might be of help. If possible, the rounds should be scheduled in advance. This helps to avoid rounding in areas at their busiest times. When possible, leader rounding for high reliability should occur as planned. Canceling rounds sends the message that leader rounding for high reliability and the valuable interactions they support are a low priority. When conflicts arise, another leader should be sent to participate. Developing a list of questions in advance helps to underscore key messages to be shared as well as reinforce principles, practices, behaviors, and attitudes related to high reliability (Appendix 1).11
Finally, closing the loop is critical to the leader rounding process and to improve bidirectional communication. Closed-loop communication, following up on and/or closing out an area of discussion, not only promotes a shared understanding of information but has been found to improve patient safety.19 Effective leader rounding for high reliability includes summarizing issues and opportunities, deciding on a date for resolution for open action items, and identifying who is responsible for taking action. Senior leaders are not responsible for resolving all issues. If a team or manager of a work area can solve any issues identified, this should be encouraged and supported so accountability is maintained at the most appropriate level of the organization.
Instrumental to leader rounding for high reliability is establishing a cadence for when leaders will visit work areas.14 The most critical strategy, especially in times of change, is consistency in rounding.11 At the start of implementation, we decided on a biweekly cadence. Initially leaders visited areas of the organization within their respective reporting structure. Once this was established, leaders periodically round in areas outside their scope of responsibility. This affords leaders the opportunity to observe other areas of the organization. As noted, it is important for leaders to be flexible with the rounding process especially in areas where direct patient care is being provided.
Tracking
Developing a tracking tool also is important for an effective leader rounding process. This tool is used to document issues and concerns identified during the rounding process, assign accountability, track the status of items, and close the loop when completed. One of the most commonly reported hurdles to staff sharing information to promote a culture of safety is the lack of feedback on what actions were taken to address the concern or issue raised with leadership. Closed-loop communication is critical for keeping staff continually engaged in efforts to promote a culture of safety.20 We have found that a tracking tool helps to ensure that closed-loop communication takes place.
Various platforms can be used for tracking items and providing follow-up, including paper worksheets, spreadsheets, databases, or third party software (eg, SharePoint, TruthPoint Rounds, GetWell Rounds). The tracking tool should have a standardized approach for prioritizing issues.
The stoplight classification system uses color coding (Figure 3).21 Green represents a safe space where there are no or low safety risks and are easily addressed at the local level by the area manager with or without assistance from the leadership team rounding, such as staffing.22,23 The unit manager has control of the situation and a plan is actively being implemented. Yellow signifies that areas are at risk, but with increased vigilance, issues do not escalate to a crisis state.22,23 Yellow-coded issues require further investigation by the leadership team. The senior leader on the team designates a process as well as a person responsible for closing the loop with the area manager regarding the status of problem resolution. For example, if the unit manager mentioned previously needing help to find staff, the area manager would suggest or take steps to help the unit manager. The area manager is then responsible for updating the frontline staff. Red-coded issues are urgent, identifying a state of crisis or high risk. Red issues need to be immediately addressed but cannot be resolved during rounds. Senior leaders must evaluate and make decisions to mitigate the threat. A member of the leadership team is tasked with following up with the area manager, typically within 24 hours. A staffing crisis that requires executive leadership help with identifying additional resources would be coded red.
The area manager is responsible for closing the loop with frontline staff. As frontline staff became more comfortable with the process, we observed an upward trend in the number of reported issues. We are now starting to see a downward trend in concerns shared during rounding as managers and frontline staff feel empowered to address issues at the lowest level.
Measuring Impact
Measuring the impact is a critical step to determine the overall effectiveness of leader rounding for high reliability. It can be as simple as requesting candid feedback from frontline staff, supervisors, managers, and service chiefs. For example, 4 months into the implementation process, the VABHS administered a brief staff survey on the overall process, perceived benefits, and challenges experienced (Appendix 2). Potential measures include the counts of leaders rounding, total rounds, rounds cancelled, and staff members actively participating in rounds. Outcomes that can be measured include issues identified, addressed, elevated, and remaining open; number of extended workdays due to rounds; staff staying overtime; and delays in patient care activities.23 Other measures to consider are the effects of rounding on staff as well as patient/family satisfaction, increase in the number of errors and near-miss events reported per month in a health care organizations’ patient safety reporting system, and increased engagement of staff members in continuous process improvement activities. Since the inception of leader rounding for high reliability, the VABHS has seen a slight increase in the number of events entered in the patient safety reporting system. Other factors that may have contributed to this change, including encouragement of reporting at safety forums, and tiered safety huddles.
DISCUSSION
This initiative involved the development and implementation of a leader rounding for high reliability process at the VABHS with the overarching goal of ensuring efficient communication exists among members of the health care team for delivering safe, quality patient care. The initiative was well received by staff from senior leadership to frontline personnel and promoted significant interest in efforts to improve safety across the health care system.
The pilot phase permitted us to examine the feasibility and acceptability of the process to leadership as well as frontline staff. The insight gained and lessons learned through the implementation process helped us make revisions where needed and develop the tools to ensure success. In the second phase of implementation, which commenced in December 2023, each executive leadership team member began leader rounding for high reliability with their respective department service chiefs. Throughout this phase, feedback will be sought on the overall process, perceived benefits, and challenges experienced to make improvements or changes as needed. We also will continue to monitor the number of events entered in the patient safety reporting system. Future efforts will focus on developing a robust program of evaluation to explore the impact of the program on patient/family satisfaction as well as safety outcomes.
Limitations
Developing and implementing a process for leader rounding for high reliability was undertaken to support the VABHS and VHA journey to high reliability. Other health care organizations and integrated systems might identify different processes for improving patient safety and to support their journey to becoming an HRO.
CONCLUSIONS
The importance of leader rounding for high reliability to improve patient safety cannot be emphasized enough in a time where health care systems have become increasingly complex. Health care is a complex adaptive system that requires effective, bidirectional communication and collaboration among all disciplines. One of the most useful, evidence-based strategies for promoting this communication and collaboration to improve a culture of safety is leader rounding for high reliability.
The hospital is altogether the most complex human organization ever devised. Peter Drucker 1
The ever-changing landscape of today’s increasingly complex health care system depends on implementing multifaceted, team-based methods of care delivery to provide safe, effective patient care.2 Critical to establishing and sustaining exceptionally safe, effective patient care is open, transparent communication among members of interprofessional teams with senior leaders.3 However, current evidence shows that poor communication among interprofessional health care teams and leadership is commonplace and a significant contributing factor to inefficiencies, medical errors, and poor outcomes.4 One strategy for improving communication is through the implementation of
We describe the importance of leader rounding for high reliability as an approach to improving patient safety. Based on a review of the literature, our experiences, and lessons learned, we offer recommendations for how health care organizations on the journey to high reliability can improve patient safety.
Rounding in health care is not new. In fact, rounding has been a strong principal practice globally for more than 2 decades.6 During this time, varied rounding approaches have emerged, oftentimes focused on areas of interest, such as patient care, environmental services, facilities management, and discharge planning.4,7 Variations also might involve the location of the rounds, such as a patient’s bedside, unit hallways, and conference rooms as well as the naming of rounds, such as interdisciplinary/multidisciplinary, teaching, and walkrounds.7-10
A different type of rounding that is characteristic of high reliability organizations (HROs) is leader rounding for high reliability. The Veterans Health Administration (VHA) formally launched its journey to becoming an
Leader rounding for high reliability includes regularly scheduled, structured visits, with interdisciplinary teams to discuss high reliability, safety, and improvement efforts. The specific aim of these particular rounds is for senior leaders to be visible where teams are located and learn from staff (especially those on the frontlines of care) about day-to-day challenges that may contribute to patient harm.12,13 Leader rounding for high reliability is also an important approach to improving leadership visibility across the organization, demonstrating a commitment to high reliability, and building trust and relationships with staff through open and honest dialogue. It is also an important approach to increasing leadership understanding of operational, clinical, nonclinical, patient experience issues, and concern related to safety.11 This opportunity enables leaders to provide and receive real-time feedback from staff.9,11 This experience also gives leaders an opportunity to reinforce
In preparation for implementing a leader rounding for high reliability process at the VABHS, we conducted an extensive literature review for peer-reviewed publications published between January 2015 and September 2022 regarding how other organizations implemented leader rounding. This search found a dearth of evidence as it specifically relates to leader rounding for high reliability. This motivated us to create a process for developing and implementing leader rounding for high reliability in pursuit of improving patient safety. With this objective in mind, we created and piloted a process in the fall of 2023. The first 3 months were focused on the medical center director rounding with other members of the executive leadership team to assess the feasibility and acceptability of the process. In December 2023, members of the executive leadership team began conducting leader rounding for high reliability separately. The following steps are based on the lessons we have gleaned from evolving evidence, our experiences, and developing and implementing an approach to leader rounding for high reliability.
ESTABLISH A PROCESS
Leader rounding for high reliability is performed by health care organization executive leadership, directors, managers, and supervisors. When properly conducted, increased levels of teamwork and more effective bidirectional communication take place, resulting in a united team motivated to improve patient safety.16,17 Important early steps for implementing leader rounding for high reliability include establishing a process and getting leadership buy-in. Purposeful attention to planning is critical as is understanding the organizational factors that might deter success. Establishing a process should consider facilitators and barriers to implementation, which can include high vs low leadership turnover, structured vs unstructured rounding, and time for rounding vs competing demands.18,19 We have learned that effective planning is important for ensuring that leadership teams are well prepared and ambitious about leader rounding for high reliability.
Leader rounding for high reliability involves brief 10-to-15-minute interactions with interdisciplinary teams, including frontline staff. For health care organizations beginning to implement this approach, having scripts or checklists accessible might be of help. If possible, the rounds should be scheduled in advance. This helps to avoid rounding in areas at their busiest times. When possible, leader rounding for high reliability should occur as planned. Canceling rounds sends the message that leader rounding for high reliability and the valuable interactions they support are a low priority. When conflicts arise, another leader should be sent to participate. Developing a list of questions in advance helps to underscore key messages to be shared as well as reinforce principles, practices, behaviors, and attitudes related to high reliability (Appendix 1).11
Finally, closing the loop is critical to the leader rounding process and to improve bidirectional communication. Closed-loop communication, following up on and/or closing out an area of discussion, not only promotes a shared understanding of information but has been found to improve patient safety.19 Effective leader rounding for high reliability includes summarizing issues and opportunities, deciding on a date for resolution for open action items, and identifying who is responsible for taking action. Senior leaders are not responsible for resolving all issues. If a team or manager of a work area can solve any issues identified, this should be encouraged and supported so accountability is maintained at the most appropriate level of the organization.
Instrumental to leader rounding for high reliability is establishing a cadence for when leaders will visit work areas.14 The most critical strategy, especially in times of change, is consistency in rounding.11 At the start of implementation, we decided on a biweekly cadence. Initially leaders visited areas of the organization within their respective reporting structure. Once this was established, leaders periodically round in areas outside their scope of responsibility. This affords leaders the opportunity to observe other areas of the organization. As noted, it is important for leaders to be flexible with the rounding process especially in areas where direct patient care is being provided.
Tracking
Developing a tracking tool also is important for an effective leader rounding process. This tool is used to document issues and concerns identified during the rounding process, assign accountability, track the status of items, and close the loop when completed. One of the most commonly reported hurdles to staff sharing information to promote a culture of safety is the lack of feedback on what actions were taken to address the concern or issue raised with leadership. Closed-loop communication is critical for keeping staff continually engaged in efforts to promote a culture of safety.20 We have found that a tracking tool helps to ensure that closed-loop communication takes place.
Various platforms can be used for tracking items and providing follow-up, including paper worksheets, spreadsheets, databases, or third party software (eg, SharePoint, TruthPoint Rounds, GetWell Rounds). The tracking tool should have a standardized approach for prioritizing issues.
The stoplight classification system uses color coding (Figure 3).21 Green represents a safe space where there are no or low safety risks and are easily addressed at the local level by the area manager with or without assistance from the leadership team rounding, such as staffing.22,23 The unit manager has control of the situation and a plan is actively being implemented. Yellow signifies that areas are at risk, but with increased vigilance, issues do not escalate to a crisis state.22,23 Yellow-coded issues require further investigation by the leadership team. The senior leader on the team designates a process as well as a person responsible for closing the loop with the area manager regarding the status of problem resolution. For example, if the unit manager mentioned previously needing help to find staff, the area manager would suggest or take steps to help the unit manager. The area manager is then responsible for updating the frontline staff. Red-coded issues are urgent, identifying a state of crisis or high risk. Red issues need to be immediately addressed but cannot be resolved during rounds. Senior leaders must evaluate and make decisions to mitigate the threat. A member of the leadership team is tasked with following up with the area manager, typically within 24 hours. A staffing crisis that requires executive leadership help with identifying additional resources would be coded red.
The area manager is responsible for closing the loop with frontline staff. As frontline staff became more comfortable with the process, we observed an upward trend in the number of reported issues. We are now starting to see a downward trend in concerns shared during rounding as managers and frontline staff feel empowered to address issues at the lowest level.
Measuring Impact
Measuring the impact is a critical step to determine the overall effectiveness of leader rounding for high reliability. It can be as simple as requesting candid feedback from frontline staff, supervisors, managers, and service chiefs. For example, 4 months into the implementation process, the VABHS administered a brief staff survey on the overall process, perceived benefits, and challenges experienced (Appendix 2). Potential measures include the counts of leaders rounding, total rounds, rounds cancelled, and staff members actively participating in rounds. Outcomes that can be measured include issues identified, addressed, elevated, and remaining open; number of extended workdays due to rounds; staff staying overtime; and delays in patient care activities.23 Other measures to consider are the effects of rounding on staff as well as patient/family satisfaction, increase in the number of errors and near-miss events reported per month in a health care organizations’ patient safety reporting system, and increased engagement of staff members in continuous process improvement activities. Since the inception of leader rounding for high reliability, the VABHS has seen a slight increase in the number of events entered in the patient safety reporting system. Other factors that may have contributed to this change, including encouragement of reporting at safety forums, and tiered safety huddles.
DISCUSSION
This initiative involved the development and implementation of a leader rounding for high reliability process at the VABHS with the overarching goal of ensuring efficient communication exists among members of the health care team for delivering safe, quality patient care. The initiative was well received by staff from senior leadership to frontline personnel and promoted significant interest in efforts to improve safety across the health care system.
The pilot phase permitted us to examine the feasibility and acceptability of the process to leadership as well as frontline staff. The insight gained and lessons learned through the implementation process helped us make revisions where needed and develop the tools to ensure success. In the second phase of implementation, which commenced in December 2023, each executive leadership team member began leader rounding for high reliability with their respective department service chiefs. Throughout this phase, feedback will be sought on the overall process, perceived benefits, and challenges experienced to make improvements or changes as needed. We also will continue to monitor the number of events entered in the patient safety reporting system. Future efforts will focus on developing a robust program of evaluation to explore the impact of the program on patient/family satisfaction as well as safety outcomes.
Limitations
Developing and implementing a process for leader rounding for high reliability was undertaken to support the VABHS and VHA journey to high reliability. Other health care organizations and integrated systems might identify different processes for improving patient safety and to support their journey to becoming an HRO.
CONCLUSIONS
The importance of leader rounding for high reliability to improve patient safety cannot be emphasized enough in a time where health care systems have become increasingly complex. Health care is a complex adaptive system that requires effective, bidirectional communication and collaboration among all disciplines. One of the most useful, evidence-based strategies for promoting this communication and collaboration to improve a culture of safety is leader rounding for high reliability.
1. Drucker PF. They’re not employees, they’re people. Accessed November 15, 2023. https://hbr.org/2002/02/theyre-not-employees-theyre-people
2. Adams HA, Feudale RM. Implementation of a structured rounding tool for interprofessional care team rounds to improve communication and collaboration in patient care. Pediatr Nurs. 2018;44(5):229-233, 246.
3. Witz I, Lucchese S, Valenzano TJ, et al: Perceptions on implementation of a new standardized reporting tool to support structured morning rounds: recommendations for interprofessional teams and healthcare leaders. J Med Radiat Sci. 2022;53(4):S85-S92. doi:10.1016/j.jmir.2022.06.006
4. Blakeney EA, Chu F, White AA, et al. A scoping review of new implementations of interprofessional bedside rounding models to improve teamwork, care, and outcomes in hospitals. J Interprof Care. 2021;10:1-16 [Online ahead of print.] doi:10.1080/13561820.2021.1980379
5. Agency for Research and Healthcare Quality. High reliability. Accessed December 4, 2023. https://psnet.ahrq.gov/primer/high-reliability
6. Hedenstrom M, Harrilson A, Heath M, Dyass S. “What’s old is new again”: innovative health care leader rounding—a strategy to foster connection. Nurse Lead. 2022;20(4):366-370.
7. Walton V, Hogden A, Long JC, Johnson JK, Greenfield D. How do interprofessional healthcare teams perceive the benefits and challenges of interdisciplinary ward rounds. J Multidiscip Healthc. 2019;12:1023-1032. doi:10.2147/JMDH.S226330
8. Walton V, Hogden A, Johnson J, Greenfield D. Ward rounds, participants, roles and perceptions: literature review. Int J Health Care Qual Assur. 2016;29(4):364-379. doi:10.1108/IJHCQA-04-2015-0053
9. Sexton JB, Adair KC, Leonard MW, et al. Providing feedback following leadership walkrounds is associated with better patient safety culture, higher employee engagement and lower burnout. BMJ Qual Saf. 2018;27(4):261-270. doi:10.1136/bmjqs-2016-006399
10. Sexton JB, Adair KC, Profit J, et al. Safety culture and workforce well-being associations with positive leadership walkrounds. Jt Comm J Qual Patient Saf. 2021;47(7):403-411. doi:10.1016/j.jcjq.2021.04.001
11. US Department of Veterans Affairs, Veterans Health Administration. Leader’s guide to foundational high reliability organization (HRO) practices. Accessed December 5, 2023. https://dvagov.sharepoint.com/sites/OHT-PMO/high-reliability/Pages/default.aspx
12. Zajac S, Woods A, Tannenbaum S, Salas E, Hollada CL. Overcoming challenges to teamwork in healthcare: a team effectiveness framework and evidence-based guidance. Front Commun. 2021;6:1-20. doi:10.3389/fcomm.2021.606445
13. Department of Veterans Affairs, Veterans Health Administration. VHA’s Vision for a High Reliability Organization. Accessed December 5, 2023. https://www.hsrd.research.va.gov/publications/forum/summer20/default.cfm?ForumMenu=summer20-1
14. Merchant NB, O’Neal J, Dealino-Perez C, Xiang J, Montoya A Jr, Murray JS. A high-reliability organization mindset. Am J Med Qual. 2022;37(6):504-510. doi:10.1097/JMQ.0000000000000086
15. Verhaegh KJ, Seller-Boersma A, Simons R, et al. An exploratory study of healthcare professionals’ perceptions of interprofessional communication and collaboration. J Interprof Care. 2017;31(3):397-400. doi:10.1080/13561820.2017.1289158
16. Winter M, Tjiong L. HCAHPS Series Part 2: Does purposeful leader rounding make a difference? Nurs Manag. 2015;46(2):26-32. doi:10.1097/01.NUMA.0000460034.25697.06
17. Beaird G, Baernholdt M, White KR. Perceptions of interdisciplinary rounding practices. J Clin Nurs. 2020;29(7-8):1141-1150. doi:10.1111/jocn.15161
18. Hendricks S, LaMothe VJ, Kara A. Facilitators and barriers for interprofessional rounding: a qualitative study. Clin Nurse Spec. 2017;31(4):219-228. doi:10.1097/NUR.0000000000000310
19. Diaz MCG, Dawson K. Impact of simulation-based closed-loop communication training on medical errors in a pediatric emergency department. Am J Med Qual. 2020;35(6):474-478. doi:10.1177/1062860620912480
20. Williams S, Fiumara K, Kachalia A, Desai S. Closing the loop with ambulatory staff on safety reports. Jt Comm J Qual Saf. 2020;46(1):44-50. doi:10.1016/j.jcjq.2019.09.009
21. Parbhoo A, Batte J. Traffic lights: putting a stop to unsafe patient transfers. BMJ Qual Improv Rep. 2015;4(1):u204799.w2079. doi:10.1136/bmjquality.u204799.w2079
22. Prineas S, Culwick M, Endlich Y. A proposed system for standardization of colour-coding stages of escalating criticality in clinical incidents. Curr Opin Anaesthesiol. 2021;34(6):752-760. doi:10.1097/ACO.0000000000001071.
23. Merchant NB, O’Neal J, Montoya A, Cox GR, Murray JS. Creating a process for the implementation of tiered huddles in a Veterans Affairs medical center. Mil Med. 2023;188(5-6):901-906. doi:10.1093/milmed/usac073
1. Drucker PF. They’re not employees, they’re people. Accessed November 15, 2023. https://hbr.org/2002/02/theyre-not-employees-theyre-people
2. Adams HA, Feudale RM. Implementation of a structured rounding tool for interprofessional care team rounds to improve communication and collaboration in patient care. Pediatr Nurs. 2018;44(5):229-233, 246.
3. Witz I, Lucchese S, Valenzano TJ, et al: Perceptions on implementation of a new standardized reporting tool to support structured morning rounds: recommendations for interprofessional teams and healthcare leaders. J Med Radiat Sci. 2022;53(4):S85-S92. doi:10.1016/j.jmir.2022.06.006
4. Blakeney EA, Chu F, White AA, et al. A scoping review of new implementations of interprofessional bedside rounding models to improve teamwork, care, and outcomes in hospitals. J Interprof Care. 2021;10:1-16 [Online ahead of print.] doi:10.1080/13561820.2021.1980379
5. Agency for Research and Healthcare Quality. High reliability. Accessed December 4, 2023. https://psnet.ahrq.gov/primer/high-reliability
6. Hedenstrom M, Harrilson A, Heath M, Dyass S. “What’s old is new again”: innovative health care leader rounding—a strategy to foster connection. Nurse Lead. 2022;20(4):366-370.
7. Walton V, Hogden A, Long JC, Johnson JK, Greenfield D. How do interprofessional healthcare teams perceive the benefits and challenges of interdisciplinary ward rounds. J Multidiscip Healthc. 2019;12:1023-1032. doi:10.2147/JMDH.S226330
8. Walton V, Hogden A, Johnson J, Greenfield D. Ward rounds, participants, roles and perceptions: literature review. Int J Health Care Qual Assur. 2016;29(4):364-379. doi:10.1108/IJHCQA-04-2015-0053
9. Sexton JB, Adair KC, Leonard MW, et al. Providing feedback following leadership walkrounds is associated with better patient safety culture, higher employee engagement and lower burnout. BMJ Qual Saf. 2018;27(4):261-270. doi:10.1136/bmjqs-2016-006399
10. Sexton JB, Adair KC, Profit J, et al. Safety culture and workforce well-being associations with positive leadership walkrounds. Jt Comm J Qual Patient Saf. 2021;47(7):403-411. doi:10.1016/j.jcjq.2021.04.001
11. US Department of Veterans Affairs, Veterans Health Administration. Leader’s guide to foundational high reliability organization (HRO) practices. Accessed December 5, 2023. https://dvagov.sharepoint.com/sites/OHT-PMO/high-reliability/Pages/default.aspx
12. Zajac S, Woods A, Tannenbaum S, Salas E, Hollada CL. Overcoming challenges to teamwork in healthcare: a team effectiveness framework and evidence-based guidance. Front Commun. 2021;6:1-20. doi:10.3389/fcomm.2021.606445
13. Department of Veterans Affairs, Veterans Health Administration. VHA’s Vision for a High Reliability Organization. Accessed December 5, 2023. https://www.hsrd.research.va.gov/publications/forum/summer20/default.cfm?ForumMenu=summer20-1
14. Merchant NB, O’Neal J, Dealino-Perez C, Xiang J, Montoya A Jr, Murray JS. A high-reliability organization mindset. Am J Med Qual. 2022;37(6):504-510. doi:10.1097/JMQ.0000000000000086
15. Verhaegh KJ, Seller-Boersma A, Simons R, et al. An exploratory study of healthcare professionals’ perceptions of interprofessional communication and collaboration. J Interprof Care. 2017;31(3):397-400. doi:10.1080/13561820.2017.1289158
16. Winter M, Tjiong L. HCAHPS Series Part 2: Does purposeful leader rounding make a difference? Nurs Manag. 2015;46(2):26-32. doi:10.1097/01.NUMA.0000460034.25697.06
17. Beaird G, Baernholdt M, White KR. Perceptions of interdisciplinary rounding practices. J Clin Nurs. 2020;29(7-8):1141-1150. doi:10.1111/jocn.15161
18. Hendricks S, LaMothe VJ, Kara A. Facilitators and barriers for interprofessional rounding: a qualitative study. Clin Nurse Spec. 2017;31(4):219-228. doi:10.1097/NUR.0000000000000310
19. Diaz MCG, Dawson K. Impact of simulation-based closed-loop communication training on medical errors in a pediatric emergency department. Am J Med Qual. 2020;35(6):474-478. doi:10.1177/1062860620912480
20. Williams S, Fiumara K, Kachalia A, Desai S. Closing the loop with ambulatory staff on safety reports. Jt Comm J Qual Saf. 2020;46(1):44-50. doi:10.1016/j.jcjq.2019.09.009
21. Parbhoo A, Batte J. Traffic lights: putting a stop to unsafe patient transfers. BMJ Qual Improv Rep. 2015;4(1):u204799.w2079. doi:10.1136/bmjquality.u204799.w2079
22. Prineas S, Culwick M, Endlich Y. A proposed system for standardization of colour-coding stages of escalating criticality in clinical incidents. Curr Opin Anaesthesiol. 2021;34(6):752-760. doi:10.1097/ACO.0000000000001071.
23. Merchant NB, O’Neal J, Montoya A, Cox GR, Murray JS. Creating a process for the implementation of tiered huddles in a Veterans Affairs medical center. Mil Med. 2023;188(5-6):901-906. doi:10.1093/milmed/usac073
Low-Carbohydrate and Ketogenic Dietary Patterns for Type 2 Diabetes Management
The prevalence of diabetes continues to increase despite advances in treatment options. In 2019, according to the Centers for Disease Control and Prevention (CDC), 37.1 million (14.7%) US adults had diabetes. Among adults aged ≥ 65 years, the prevalence is even higher at 29.2%.1 Research has also estimated that 45% of adults have evidence of prediabetes or diabetes.2 According to the Veterans Health Administration, almost 25% of enrolled veterans have diabetes.3
Background
Diabetes is associated with an increased risk of microvascular complications (eg, retinopathy, nephropathy, and neuropathy) and macrovascular complications (eg, atherosclerotic cardiovascular disease) and is one of the most common causes of morbidity and mortality in the US.4 In 2017, diabetes was estimated to cost $327 billion in the US, up from $261 billion in 2012.5 During this same period, the excess costs per person with diabetes increased from $8417 to $9601.5
Type 2 diabetes mellitus (T2DM) and its associated insulin resistance is typically considered a chronic disease with progressive loss of β-cell function. Controlling glycemia, delaying microvascular changes, and preventing macrovascular disease are major management goals. Lifestyle interventions are essential in the management and prevention of T2DM. Medication management for T2DM usually progresses through several medications, ending in insulin therapy.6 Within 10 years of diagnosis, almost half of all individuals with T2DM will require insulin to manage their glycemia.7
Bariatric surgery and nutrition approaches have been successful in reversing T2DM. Recently, there has been increased interest in nutritional approaches to place T2DM in remission, reverse the disease process, and improve insulin resistance. Contrary to popular belief, before the discovery of insulin in 1921, low-carbohydrate (LC) diets were the most common treatment for T2DM.8 With the discovery of insulin and the eventual development of low-fat dietary recommendations, LC diets were no longer favored by most clinicians.8 Low-fat diets are, by definition, also high-carbohydrate diets. By the early 1980s, low-fat diets had become the standard of care dietary recommendation, and the goal for clinicians became glycemic maintenance (with increased use of medications) rather than preventing hyperglycemia.8
With growing evidence regarding the use of LC diets for T2DM, the US Department of Veterans Affairs (VA) and US Department of Defense (DoD), the American Diabetes Association (ADA), the European Association for the Study of Diabetes (EASD), Diabetes Canada, and Diabetes Australia all include LC diets as a viable option for treating T2DM.4,9-12 This article will highlight a case using a reduced carbohydrate approach in lifestyle management and provide clinicians with practical guidance in its implementation. We will review the evidence that informs these guidelines, describe a practical approach to nutritional counseling, and review medication management and deprescribing approaches. Finally, barriers to implementation will be explored.
ILLUSTRATIVE CASE
A 64-year-old woman presented to the clinical pharmacist for the management of T2DM after her tenth hospitalization related to hyperglycemia in 10 years. She had previously been managed by primary care clinicians, clinical dietitians, endocrinologists, and certified diabetes care and education specialists. Pertinent history included diabetic ketoacidosis, coronary artery disease, hyperlipidemia, hypertension, obstructive sleep apnea, obesity, metabolic dysfunction-associated steatotic liver disease, and mild nonproliferative diabetic retinopathy with clinically significant macular edema. The patient expressed frustration with poor glycemic control during her many years of insulin therapy and an inability to lose weight due to insulin dose titrations. The patient reported prior education including but not limited to standardized sample menus, consistent carbohydrate intake, calorie reduction, general healthful nutrition, and the “move more, eat less” approach. The patient was unable to titrate insulin dosage and did not experience weight loss despite compliance with these methods.
Her medications included glargine insulin 45 units once daily, aspart insulin 5 units before meals 3 times daily, and metformin 1000 mg twice daily. Her hemoglobin A1c (HbA1c) level was 11.8%. A review of prior therapies for T2DM included glyburide 5 mg twice daily, metformin 1000 mg twice daily, 70/30 insulin (up to 340 units/d), glargine insulin (range, 10-140 units/d), regular insulin (range, 30-240 units/d), aspart insulin (range, 15-45 units/d), and U-500 regular insulin (range, 125-390 units/d). She took metoprolol 25 mg extended release daily and hydrochlorothiazide 25 mg daily, but both were discontinued after the most recent hospitalization. A review of HbA1c readings showed poor glycemic control for > 12 years (range, 10.3% to > 12.3%).
Education for lifestyle modifications, including an LC diet, was presented to the patient to assist with weight loss, improve glycemic control, and reduce insulin resistance. In addition, a glucagon-like peptide-1 agonist (liraglutide) was added to her pharmacotherapy. Continued dietary modifications with LC intake led to consistent reductions in glargine and aspart insulin therapy. The patient remained motivated throughout clinic visits due to improved glycemic control with sustainable dietary modifications, consistently reported feeling better overall, and deprescribed diabetes drug therapies. She remained off her blood pressure medications. After4 months of LC dietary modifications, all insulin therapy was discontinued. She continued with liraglutide 1.8 mg daily and metformin 1000 mg twice daily with an HbA1c of 6.3%. Two months later, her HbA1c level was 6.0%. She also lost 8 lb and her body mass index improved from 31 to 29.
Low-Carbohydrate T2DM DIET MANAGEMENT
LC diets are commonly defined as < 130 g of carbohydrates per day.13 Very LC ketogenic (VLCK) diets often contain ≤ 50 g of carbohydrates per day to induce nutritional ketosis.13 One of the first randomized controlled trials (RCTs) that compared a VLCK diet (< 30 g of carbohydrates per day) with a low-fat diet for obesity demonstrated greater weight loss at 6 months with the LC diet. In addition, patients with diabetes randomized to the LC group also showed improved insulin sensitivity. Notably, this study was done in a population of veterans enrolled at the VA Philadelphia Health Care System.14
A 2008 study comparing an LC diet with a calorie-restricted, low-glycemic diet for individuals with T2DM found that the LC diet group experienced a greater reduction in HbA1c and insulin levels and weight.15 Comparing these 2 diet groups after 24 weeks, 95% of individuals in the LC group reduced or discontinued T2DM medications vs 62% in the low-glycemic group.15 Another study of individuals with T2DM compared a VLCK diet with a low-fat diet. After 34 weeks, 55% of individuals in the LC diet group achieved an HbA1c level below the threshold for diabetes vs 0% in the low-fat diet group.16 A 2018 study of patients with T2DM investigated the impact of a very LC diet compared with the standard of care.17 After 1 year, the LC diet group experienced a mean HbA1c reduction of 1.3%, and 60% of individuals who completed the study achieved an HbA1c level < 6.5% without T2DM medications (not including metformin). This study also demonstrated that medications were significantly reduced, including 100% discontinuation of sulfonylureas and 94% reduction or elimination of insulin.
A recent study of an LC diet (< 20% energy from carbohydrates) demonstrated reduced HbA1c levels, weight, and waist circumference vs a control diet after 6 months. The control diet derived 50% to 60% of energy from carbohydrates.18 This study is typical of other LC interventions, which did not calorie restrict and instead allowed ad libitum intake.14,15
With mounting evidence, the VA/DoD guidelines on T2DM management included LC diets as dietary options for treating T2DM. The ADA also determined that LC diets had the most evidence in improving glycemia and included LC diets as an option for medical nutrition therapy (Table 1).10,19
A systematic review and meta-analysis looking at RCTs of LC diets found evidence for remission of T2DM without significant adverse effects (AEs).20 Another recent systematic review and network meta-analysis of 42 RCTs found that the ketogenic diet was superior for a reduction in HbA1c levels compared with 9 other dietary patterns, including low-fat, Mediterranean, and vegetarian/vegan diets. Overall, ketogenic, Mediterranean, moderate-carbohydrate, and low-glycemic index diets demonstrated improved glycemic control.21
Ideally, a comprehensive behavioral program, such as the VA Move! or Whole Health program, should incorporate patient aligned care teams (PACTs), behavioral health clinicians, clinical pharmacists, and dietitians to provide medical-nutrition therapy using LC diets. However, many facilities may not have adequate experience, expertise, or support. We provide practical approaches to provide LC nutrition counseling, medication management, and deprescribing for any primary care clinician applying LC diets for their patients. For simplicity and practicality, we define 3 types of LC dietary patterns: (1) VLCK (< 50 g); (2) LC (50-100 g); and (3) moderate LC (101-150 g).
Nutrition
All nutrition approaches, including LC diets, should be patient centered, individualized, and sensitive to the patient's culture. Typically, many patients have previously been instructed to consume low-fat (and subsequently) high-carbohydrate (> 150 g) meals. Most well-meaning clinicians have provided common-approach diet education from mainstream health organizations in the form of standardized handouts. For example, the Carbohydrate Counting for People with Diabetes patient education handout from the Academy of Nutrition and Dietetics provides a sample menu with 3 meals and 1 snack totaling 195 g of carbohydrates.22 In contrast, an example ADA diet has sample diets with 3 meals and 2 snacks with approximately 20 to 70 g of carbohydrates.23 In the VA, there are excellent resources to review and standardize handouts that emphasize an LC nutrition approach to T2DM, including ketogenic versions.24,25 Table 2 shows example meal plans based on different LC patterns—VLCK, LC, and moderate LC.
Starting an LC dietary pattern should maximize nutrient-dense and minimally processed proteins. Clinicians should begin with a baseline nutritional assessment through a 24-hour recall or food diary. After this has been completed, the patient’s baseline diet is assessed, and a gradual carbohydrate reduction plan is discussed. Generally, carbohydrate reduction is recommended at 1 meal per day per week. High-carbohydrate meals and snacks are restructured to favor satiating, minimally processed, high-protein food sources. Individual food preferences are considered and included in the recommended LC plan. For example, LC diets can be formulated for vegetarians and vegans as well as those who prefer meat and seafood. Prioritizing satiating and nutrient-dense foods can help increase the probability of diet acceptance and adherence.
A recent study showed that restricting carbohydrates at breakfast reduces 24-hour postprandial hyperglycemia and improves glycemic variability.26 Many patients consume upward of 50 g of carbohydrates at breakfast.27 For example, it is not uncommon for a patient to consume cereal with milk or oatmeal, orange juice, a banana, and toast at breakfast. Instead, the patient is advised to consume any combination of eggs, meat, no-sugar-added Greek yogurt, or berries.
To keep things simple for lunch and dinner, the patient is offered high-quality, minimally processed protein of their choosing with any nonstarchy vegetable. Should a patient desire additional carbohydrates with meals, they may reduce the baseline serving of carbohydrates by 50%. For example, if a patient normally fills 50% of their plate with spaghetti, they may reduce the pasta portion to 25% and add a meatball or increase the amount of vegetables consumed with the meal to satiety.
Snacks may include cheese, eggs, peanut butter, nuts, seeds, berries, no-sugar-added Greek yogurt, or guacamole. Oftentimes, when LC meals are adopted, the desire or need for snacking is diminished due to the satiating effect of high-quality protein sources and nonstarchy vegetables.
Adverse Effects
AEs have been reported with VLCK diets, including headache, diarrhea, constipation, muscle cramps, halitosis, light-headedness, and muscle weakness.28 These AEs may be mitigated with increased fluid intake, sodium intake, and magnesium supplementation.29 Increasing fluids to a minimum of 2 L/d and adding sodium (eg, bouillon supplementation) can minimize AEs.30 Milk of magnesia (5 mL) or slow-release magnesium chloride 200 mEq/d is suggested to reduce muscle cramps.30 There have been no studies looking at sodium intake and worsening hypertension or chronic heart failure in the setting of an LC diet, but fluid and electrolyte intake should be monitored closely, especially in patients with uncontrolled hypertension and heart failure. Other concerns of higher protein on worsening kidney function have generally not been founded.31 In some individuals, an LC and higher fat diet may increase low-density lipoprotein cholesterol (LDL-C).32 Therefore a baseline lipid panel is recommended and should be monitored along with HbA1c levels. An elevated LDL-C response may be managed by increasing protein and reducing saturated fat intake while maintaining the reduced carbohydrate content of the diet.
Medication Management
The adoption of an LC diet can cause a swift and profound reduction in blood sugar.33 Utilizing PACTs can help prevent adverse drug events by involving clinical pharmacists to provide recommendations and dose reductions as patients adopt an LC diet. Each approach must be individualized to the patient and can depend on several factors, including the number and strength of medications, the degree of carbohydrate reduction, baseline blood glucose, as well as assessing for medical literacy and ability to implement recommendations. Additionally, patients should monitor their blood sugar regularly and communicate with their primary care team (pharmacist, PACT registered nurse, primary care clinician, and registered dietician). Ultimately, the goal when adopting an LC diet while taking antihyperglycemics is safely avoiding hypoglycemia while reducing the number of medications the patient is taking. We summarize a practical approach to medication management that was recently published (Table 3).33,34
Medications to Reduce or Discontinue
Medications that can cause hypoglycemia should be the first to be reduced or discontinued upon starting an LC diet, including bolus insulin (although a small amount may be needed to correct for high blood sugar), sulfonylureas, and meglitinides. Combination insulin should be stopped and changed to basal insulin to avoid the risk of hypoglycemia (see Table 4 for insulin deprescribing recommendations). The mechanism of action in preventing the breakdown of carbohydrates in the gastrointestinal tract makes the use of α-glucosidase inhibitors superfluous, and they can be discontinued, reducing pill burden and polypharmacy risks. Sodium-glucose transport protein 2 inhibitors (SGLT2i) should be discontinued for patients on VLCK diets due to the risk of euglycemic diabetic ketoacidosis. However, with LC and moderate LC plans, the SGLT2i may be used with caution as long as patients are made aware of ketoacidosis symptoms. To help prevent the risk of hypoglycemia, basal/long-acting insulin can be continued, but at a 50% reduced dose. Patients should closely monitor blood sugar to assess for appropriateness of dose reductions. While thiazolidinediones are not contraindicated, clinicians can consider discontinuation given both their penchant for inducing weight gain and their limited outcomes data.
Medications to Continue
Medications that pose minimal risk for hypoglycemia can be continued, including metformin, dipeptidyl peptidase 4 inhibitors, and glucagon-like peptide-1 agonists. However, even though these may pose a low risk of hypoglycemia, patients should still closely monitor their blood glucose so medications can be deprescribed as soon as safely and reasonably possible.
Other Medications
The improvement in metabolic health with the reduction of carbohydrates can render other classes of medications unnecessary or require adjustment. Patients should be counseled to monitor their blood pressure as significant and rapid improvements can occur. In the event of a systolic blood pressure of 100 to 110 mm Hg or signs of hypotension, down titration or discontinuation of antihypertensives should be initiated. Limited evidence exists on the preferred order of discontinuation but should be informed by other comorbidities, such as coronary artery disease and chronic kidney disease. Given an LC diet’s diuretic effect, tapering and stopping diuretics may be an option. Other medications requiring closer monitoring include lithium (can be affected by fluid and electrolyte shifts), warfarin (may alter vitamin K intake), valproate (which may be reduced), and zonisamide and topiramate (kidney stone risk).
Remission of T2DM with LC Diets
As patients adopt LC diets and medications are deprescribed and glycemia improves, HbA1c and fasting glucose levels may drop below the diagnostic threshold for T2DM.20 As new evidence emerges surrounding the management of T2DM from a lifestyle perspective, major health care organizations have acknowledged that T2DM is not necessarily an incurable, progressive disease, but rather a disease that can be reversed or put in remission.35-37 In 2016, the World Health Organization (WHO) global report on diabetes acknowledged that T2DM reversal can be achieved via weight loss and calorie restriction.35
In 2021, a consensus statement from the ADA, the Endocrine Society, the EASD, and Diabetes UK defined T2DM remission as an HbA1c level < 6.5% for at least 3 months with no T2DM medications.36 Diabetes Australia also published a position statement in 2021 about T2DM remission.37 Like the WHO, Diabetes Australia acknowledged that remission of T2DM is possible following intensive dietary changes or bariatric surgery.37 Before the 2021 consensus statement, some experts argued that excluding metformin from the T2DM medication list may not be warranted since metformin has indications beyond T2DM. In this case, remission of T2DM could be defined as an HbA1c level < 6.5% for at least 3 months and on metformin or no T2DM medications.8
Emerging Strategies
Emerging strategies, such as continuous glucose monitors (CGMs) and the use of intermittent fasting/time-restricted eating (TRE), can be used with the LC diet to help improve the monitoring and management of T2DM. In the recently published VA/DoD guidelines for T2DM, the work group suggested real-time CGMs for qualified patients with T2DM.4 These include patients on daily insulin who are not achieving glycemic control or to reduce the risk for hypoglycemia. CGMs have shown evidence of improved glycemic control and decreased hypoglycemia in those with T2DM.38,39 It is currently unknown if CGMs improve long-term glycemic control, but they appear promising for managing and reducing medications for those on an LC diet.40
TRE can be supplemented with an LC plan that incorporates “eating windows.” Common patterns include 14 hours of fasting and a 10-hour eating window (14F:10E), or 16 hours of fasting and an 8-hour eating window (16F:8E). By eating only in the specified window, patients generally reduce caloric intake and minimize insulin and glucose excursions during the fasting window. No changes need to be made to the macronutrient composition of the diet, and LC approaches can be used with TRE. The mechanism of action is likely multifactorial, targeting hyperinsulinemia and insulin resistance as well as producing a caloric deficit to enable weight loss.41 Eating windows may improve insulin sensitivity, reduce insulin resistance, and enhance overall glycemic control. The recent VA/DoD guidelines recommended against intermittent fasting due to concerns over the risk of hypoglycemia despite larger weight loss in TRE groups.4 Recently, a study using CGMs and TRE demonstrated both improved glycemic control and no hypoglycemic episodes in patients with T2DM on insulin.42 Patients who would like to supplement TRE with an LC plan as a strategy for improved glycemic control should work closely with their PACT to help manage their TRE and LC plan and consider a CGM adjunct, especially if on insulin.
Barriers
Managing T2DM often requires comprehensive lifestyle modifications of nutrition, exercise, sleep, stress management, and other psychosocial issues, as well as an interdisciplinary team-based approach.43 The advantage of working within the VA includes a uniform system within a network of care. However, many patients continue to use both federal and private health care. This use of out-of-network care may result in fragmented, potentially disjointed, or even contradictory dietary advice.
The VA PACT, whole health for holistic health, and weight loss interventions such as the MOVE! program provide lifestyle interventions like nutrition, physical activity, and behavior change. However, these well-intentioned approaches may provide alternative and even diverging recommendations, which place additional barriers to effective patient management. In patients who are advised and accept a trial of an LC plan, each member of the team should embrace the self-management decision of the patient and support the plan.29 Any conflicts, questions, or concerns should be communicated directly with the team in an interdisciplinary approach to provide a unified message and counsel.
The long-term effects and sustainability of an LC diet have been questioned in the literature.44-46 Recently, the use of an app-based coaching plan has demonstrated short- and long-term sustainability on an LC diet.47 In just 5 months in a large VA system, 590 patients using a virtual coaching platform and a VLCK diet plan were found to have lower HbA1c levels, reduced diabetic medication fills, lower body mass index, fewer outpatient visits, and lower prescription drug costs.
A 5-year follow-up found nearly 50% of participants sustained a VLCK diet for T2DM. For patients who participated in the study after 2 years, 72% sustained the VLCK diet in years 2 to 5. Most required nearly 50% fewer medications and in those that started with insulin, half did not require it at 5 years.48 Further research, however, is necessary to determine the long-term effects on cardiometabolic markers and health with LC diets. There are no long-term RCTs on outcomes data looking at T2DM morbidity or mortality. While there are prospective cohort studies on LC diets in the general population on mortality, they demonstrate mixed results. These studies may be confounded by heterogeneous definitions of LC diets, diet quality, and other health factors.49-51
Conclusions
The effective use of LC diets within a PACT with close and intensive lifestyle counseling and a safe approach to medication management and deprescribing can improve glycemic control, reduce the overall need for insulin, reduce medication use, and provide sustained weight loss. Additionally, the use of therapeutic carbohydrate reduction and subsequent medication deprescription may lead to sustained remission of T2DM. The current efficacy and sustainment of therapeutic carbohydrate reduction for patients with T2DM appears promising. Further research on LC diets, emerging strategies, and long-term effects on cardiometabolic risk factors, morbidity, and mortality will continue to inform future practice in our health care system.
Acknowledgments
We thank Cecile Seth who has been instrumental in pushing us forward and the Metabolic Multiplier group who has helped encourage and provide input into this article.
1. Centers for Disease Control and Prevention. Prevalence of Both Diagnosed and Undiagnosed Diabetes. Updated September 30, 2022. Accessed October 6, 2023. https://www.cdc.gov/diabetes/data/statistics-report/diagnosed-undiagnosed-diabetes.html
2. Centers for Disease Control and Prevention. Diabetes and Prediabetes. Updated September 6, 2022. Accessed October 6, 2023. https://www.cdc.gov/chronicdisease/resources/publications/factsheets/diabetes-prediabetes.htm 3. US Department of Veterans Affairs. Diabetes information - Nutrition and food services. Updated May 4, 2023. Accessed October 6, 2023. https://www.nutrition.va.gov/diabetes.asp
4. US Department of Veterans Affairs. Management of Type 2 Diabetes Mellitus (2023) - VA/DoD Clinical Practice Guidelines. Updated September 1, 2023. Accessed October 6, 2023. https://www.healthquality.va.gov/guidelines/CD/diabetes/
5. American Diabetes Association. Economic Costs of Diabetes in the U.S. in 2017. Diabetes Care. 2018;41(5):917-928. doi:10.2337/dci18-0007
6. Home P, Riddle M, Cefalu WT, et al. Insulin therapy in people with type 2 diabetes: opportunities and challenges?. Diabetes Care. 2014;37(6):1499-1508. doi:10.2337/dc13-2743
7. Donath MY, Ehses JA, Maedler K, et al. Mechanisms of β-cell death in type 2 diabetes. Diabetes. 2005;54(suppl 2):S108-S113. doi:10.2337/DIABETES.54.SUPPL_2.S108
8. Hallberg SJ, Gershuni VM, Hazbun TL, Athinarayanan SJ. Reversing type 2 diabetes: a narrative review of the evidence. Nutrients. 2019;11(4):766. Published 2019 Apr 1. doi:10.3390/nu11040766
9. Davies MJ, D’Alessio DA, Fradkin J, et al. Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41(12):2669. doi:10.2337/DCI18-0033
10. Evert AB, Dennison M, Gardner CD, et al. Nutrition therapy for adults with diabetes or prediabetes: a consensus report. Diabetes Care. 2019;42(5):731-754. doi:10.2337/DCI19-0014
11. Diabetes Canada position statement on low-carbohydrate diets for adults with diabetes: a rapid review. Can J Diabetes. 2020;44(4):295-299. doi:10.1016/J.JCJD.2020.04.001
12. Diabetes Australia. Position statements. Accessed October 6, 2023. https://www.diabetesaustralia.com.au/research-advocacy/position-statements/
13. Feinman RD, Pogozelski WK, Astrup A, et al. Dietary carbohydrate restriction as the first approach in diabetes management: critical review and evidence base. Nutrition. 2014;31(1):1-13. doi:10.1016/j.nut.2014.06.011
14. Samaha FF, Iqbal N, Seshadri P, et al. A low-carbohydrate as compared with a low-fat diet in severe obesity. N Engl J Med. 2003;348(21):2074-2081. doi:10.1056/NEJMOA02263715. Westman EC, Yancy WS, Mavropoulos JC, Marquart M, McDuffie JR. The effect of a low-carbohydrate, ketogenic diet versus a low-glycemic index diet on glycemic control in type 2 diabetes mellitus. Nutr Metab (Lond). 2008;5(1):36. doi:10.1186/1743-7075-5-36
16. Saslow LR, Mason AE, Kim S, et al. An online intervention comparing a very low-carbohydrate ketogenic diet and lifestyle recommendations versus a plate method diet in overweight individuals with type 2 diabetes: a randomized controlled trial. J Med Internet Res. 2017;19(2). doi:10.2196/JMIR.5806
17. Hallberg SJ, McKenzie AL, Williams PT, et al. Effectiveness and safety of a novel care model for the management of type 2 diabetes at 1 year: an open-label, non-randomized, controlled study. Diabetes Ther. 2018;9(2):583-612. doi:10.1007/S13300-018-0373-9
18. Gram-Kampmann EM, Hansen CD, Hugger MB, et al. Effects of a 6-month, low-carbohydrate diet on glycaemic control, body composition, and cardiovascular risk factors in patients with type 2 diabetes: An open-label randomized controlled trial. Diabetes Obes Metab. 2022;24(4):693-703. doi:10.1111/DOM.14633
19. Committee ADAPP. 5. Facilitating behavior change and well-being to improve health outcomes: standards of medical care in diabetes—2022. Diabetes Care. 2022;45(suppl 1):S60-S82. doi:10.2337/DC22-S005
20. Goldenberg JZ, Johnston BC. Low and very low carbohydrate diets for diabetes remission. BMJ. 2021;373:m4743. doi:10.1136/BMJ.N262

21. Jing T, Zhang S, Bai M, et al. Effect of dietary approaches on glycemic control in patients with type 2 diabetes: a systematic review with network meta-analysis of randomized trials. Nutrients. 2023;15(14):3156. doi:10.3390/nu15143156
22. Academy of Nutrition and Dietetics. Nutrition care manual. Accessed October 6, 2023. https://www.nutritioncaremanual.org/
23. Low carbohydrate and very low carbohydrate eating patterns in adults with diabetes. ShopDiabetes.org. Accessed August 5, 2022. https://shopdiabetes.org/products/low-carbohydrate-and-very-low-carbohydrate-eating-patterns-in-adults-with-diabetes-a-guide-for-health-care-providers
24. US Department of Veterans Affairs. Diabetes education - nutrition and food services. Published July 31, 2022. http://vaww.nutrition.va.gov/docs/pted/ModifiedKetogenicDiet.pdf [Source not verified]
25. US Department of Veterans Affairs, My HealtheVet. Lowdown on low-carb diets. Updated June 1, 2021. Accessed October 6, 2023. https://www.myhealth.va.gov/mhv-portal-web/ss20190724-low-carb-diet
26. Chang CR, Francois ME, Little JP. Restricting carbohydrates at breakfast is sufficient to reduce 24-hour exposure to postprandial hyperglycemia and improve glycemic variability. Am J Clin Nutr. 2019;109(5):1302-1309. doi:10.1093/AJCN/NQY261
27. Hall KD, Ayuketah A, Brychta R, et al. Ultra-processed diets cause excess calorie intake and weight gain: an inpatient randomized controlled trial of ad libitum food intake. Cell Metab. 2019;30(1):226. doi:10.1016/j.cmet.2019.05.020
28. Harvey CJ d. C, Schofield GM, Zinn C, Thornley S. Effects of differing levels of carbohydrate restriction on mood achievement of nutritional ketosis, and symptoms of carbohydrate withdrawal in healthy adults: a randomized clinical trial. Nutrition. 2019;67-68:100005. doi:10.1016/J.NUTX.2019.100005
29. Griauzde DH, Standafer Lopez K, Saslow LR, Richardson CR. A pragmatic approach to translating low- and very low-carbohydrate diets into clinical practice for patients with obesity and type 2 diabetes. Front Nutr. 2021;8:416. doi:10.3389/FNUT.2021.682137/BIBTEX
30. Westman EC, Tondt J, Maguire E, Yancy WS. Implementing a low-carbohydrate, ketogenic diet to manage type 2 diabetes mellitus. Expert Rev Endocrinol Metab. 2018;13(5):263-272. doi:10.1080/17446651.2018.1523713
31. Suyoto PST. Effect of low-carbohydrate diet on markers of renal function in patients with type 2 diabetes: a meta-analysis. Diabetes Metab Res Rev. 2018;34(7). doi:10.1002/DMRR.3032
32. Norwitz NG, Feldman D, Soto-Mota A, Kalayjian T, Ludwig DS. Elevated LDL cholesterol with a carbohydrate-restricted diet: evidence for a “lean mass hyper-responder” phenotype. Curr Dev Nutr. 2021;6(1). doi:10.1093/CDN/NZAB144
33. Murdoch C, Unwin D, Cavan D, Cucuzzella M, Patel M. Adapting diabetes medication for low carbohydrate management of type 2 diabetes: a practical guide. Br J Gen Pract. 2019;69(684):360-361. doi:10.3399/bjgp19X704525
34. Cucuzzella M, Riley K, Isaacs D. Adapting medication for type 2 diabetes to a low carbohydrate diet. Front Nutr. 2021;8:486. doi:10.3389/FNUT.2021.688540/BIBTEX
35. World Health Organization. Global report on diabetes. 2016. Accessed October 6, 2023. https://iris.who.int/bitstream/handle/10665/204871/9789241565257_eng.pdf?sequence=1
36. Riddle MC, Cefalu WT, Evans PH, et al. Consensus report: definition and interpretation of remission in type 2 diabetes. Diabetes Care. 2021;44(10):2438-2444. doi:10.2337/DCI21-0034
37. Diabetes Australia. Type 2 Diabetes remission position statement. 2021. Accessed October 6, 2023. https://www.diabetesaustralia.com.au/wp-content/uploads/2021_Diabetes-Australia-Position-Statement_Type-2-diabetes-remission_2.pdf
38. Martens T, Beck RW, Bailey R, et al. Effect of continuous glucose monitoring on glycemic control in patients with type 2 diabetes treated with basal insulin: a randomized clinical trial. JAMA. 2021;325(22):2262-2272. doi:10.1001/JAMA.2021.7444
39. Jackson MA, Ahmann A, Shah VN. Type 2 diabetes and the use of real-time continuous glucose monitoring. Diabetes Technol Ther. 2021;23(S1):S27-S34. doi:10.1089/DIA.2021.0007
40. Oser TK, Cucuzzella M, Stasinopoulos M, Moncrief M, McCall A, Cox DJ. An innovative, paradigm-shifting lifestyle intervention to reduce glucose excursions with the use of continuous glucose monitoring to educate, motivate, and activate adults with newly diagnosed type 2 diabetes: pilot feasibility study. JMIR Diabetes. 2022;7(1). doi:10.2196/34465
41. Światkiewicz I, Woźniak A, Taub PR. Time-restricted eating and metabolic syndrome: current status and future perspectives. Nutrients. 2021;13(1):221. doi:10.3390/NU13010221
42. Obermayer A, Tripolt NJ, Pferschy PN, et al. Efficacy and safety of intermittent fasting in people with insulin-treated type 2 diabetes (INTERFAST-2)—a randomized controlled trial. Diabetes Care. 2023;46(2):463-468. doi:10.2337/dc22-1622
43. American Diabetes Association. 5. Lifestyle management: standards of medical care in diabetes—2019. Diabetes Care. 2019;42(suppl 1):S46-S60. doi:10.2337/DC19-S005
44. Li S, Ding L, Xiao X. Comparing the efficacy and safety of low-carbohydrate diets with low-fat diets for type 2 diabetes mellitus patients: a systematic review and meta-analysis of randomized clinical trials. Int J Endocrinol. 2021;2021:8521756. Published 2021 Dec 6. doi:10.1155/2021/8521756
45. Choi JH, Kang JH, Chon S. Comprehensive understanding for application in Korean patients with type 2 diabetes mellitus of the consensus statement on carbohydrate-restricted diets by Korean Diabetes Association, Korean Society for the Study of Obesity, and Korean Society of Hypertension. Diabetes Metab J. 2022;46(3):377. doi:10.4093/DMJ.2022.0051
46. Jayedi A, Zeraattalab-Motlagh S, Jabbarzadeh B, et al. Dose-dependent effect of carbohydrate restriction for type 2 diabetes management: a systematic review and dose-response meta-analysis of randomized controlled trials. Am J Clin Nutr. 2022;116(1). doi:10.1093/AJCN/NQAC066
47. Strombotne KL, Lum J, Ndugga NJ, et al. Effectiveness of a ketogenic diet and virtual coaching intervention for patients with diabetes: a difference-in-differences analysis. Diabetes Obes Metab. 2021;23(12):2643-2650. doi:10.1111/DOM.14515
48. Virta Health. Virta Health highlights lasting, transformative health improvements in 5-year diabetes reversal study. June 5, 2022. Accessed October 6, 2023. https://www.virtahealth.com/blog/virta-sustainable-health-improvements-5-year-diabetes-reversal-study
49. Wan Z, Shan Z, Geng T, et al. Associations of moderate low-carbohydrate diets with mortality among patients with type 2 diabetes: a prospective cohort study. J Clin Endocrinol Metab. 2022;107(7):E2702-E2709. doi:10.1210/CLINEM/DGAC235
50. Akter S, Mizoue T, Nanri A, et al. Low carbohydrate diet and all cause and cause-specific mortality. Clin Nutr. 2021;40(4):2016-2024. doi:10.1016/J.CLNU.2020.09.022
51. Shan Z, Guo Y, Hu FB, Liu L, Qi Q. Association of low-carbohydrate and low-fat diets with mortality among US adults. JAMA Intern Med. 2020;180(4):513-523. doi:10.1001/JAMAINTERNMED.2019.6980
The prevalence of diabetes continues to increase despite advances in treatment options. In 2019, according to the Centers for Disease Control and Prevention (CDC), 37.1 million (14.7%) US adults had diabetes. Among adults aged ≥ 65 years, the prevalence is even higher at 29.2%.1 Research has also estimated that 45% of adults have evidence of prediabetes or diabetes.2 According to the Veterans Health Administration, almost 25% of enrolled veterans have diabetes.3
Background
Diabetes is associated with an increased risk of microvascular complications (eg, retinopathy, nephropathy, and neuropathy) and macrovascular complications (eg, atherosclerotic cardiovascular disease) and is one of the most common causes of morbidity and mortality in the US.4 In 2017, diabetes was estimated to cost $327 billion in the US, up from $261 billion in 2012.5 During this same period, the excess costs per person with diabetes increased from $8417 to $9601.5
Type 2 diabetes mellitus (T2DM) and its associated insulin resistance is typically considered a chronic disease with progressive loss of β-cell function. Controlling glycemia, delaying microvascular changes, and preventing macrovascular disease are major management goals. Lifestyle interventions are essential in the management and prevention of T2DM. Medication management for T2DM usually progresses through several medications, ending in insulin therapy.6 Within 10 years of diagnosis, almost half of all individuals with T2DM will require insulin to manage their glycemia.7
Bariatric surgery and nutrition approaches have been successful in reversing T2DM. Recently, there has been increased interest in nutritional approaches to place T2DM in remission, reverse the disease process, and improve insulin resistance. Contrary to popular belief, before the discovery of insulin in 1921, low-carbohydrate (LC) diets were the most common treatment for T2DM.8 With the discovery of insulin and the eventual development of low-fat dietary recommendations, LC diets were no longer favored by most clinicians.8 Low-fat diets are, by definition, also high-carbohydrate diets. By the early 1980s, low-fat diets had become the standard of care dietary recommendation, and the goal for clinicians became glycemic maintenance (with increased use of medications) rather than preventing hyperglycemia.8
With growing evidence regarding the use of LC diets for T2DM, the US Department of Veterans Affairs (VA) and US Department of Defense (DoD), the American Diabetes Association (ADA), the European Association for the Study of Diabetes (EASD), Diabetes Canada, and Diabetes Australia all include LC diets as a viable option for treating T2DM.4,9-12 This article will highlight a case using a reduced carbohydrate approach in lifestyle management and provide clinicians with practical guidance in its implementation. We will review the evidence that informs these guidelines, describe a practical approach to nutritional counseling, and review medication management and deprescribing approaches. Finally, barriers to implementation will be explored.
ILLUSTRATIVE CASE
A 64-year-old woman presented to the clinical pharmacist for the management of T2DM after her tenth hospitalization related to hyperglycemia in 10 years. She had previously been managed by primary care clinicians, clinical dietitians, endocrinologists, and certified diabetes care and education specialists. Pertinent history included diabetic ketoacidosis, coronary artery disease, hyperlipidemia, hypertension, obstructive sleep apnea, obesity, metabolic dysfunction-associated steatotic liver disease, and mild nonproliferative diabetic retinopathy with clinically significant macular edema. The patient expressed frustration with poor glycemic control during her many years of insulin therapy and an inability to lose weight due to insulin dose titrations. The patient reported prior education including but not limited to standardized sample menus, consistent carbohydrate intake, calorie reduction, general healthful nutrition, and the “move more, eat less” approach. The patient was unable to titrate insulin dosage and did not experience weight loss despite compliance with these methods.
Her medications included glargine insulin 45 units once daily, aspart insulin 5 units before meals 3 times daily, and metformin 1000 mg twice daily. Her hemoglobin A1c (HbA1c) level was 11.8%. A review of prior therapies for T2DM included glyburide 5 mg twice daily, metformin 1000 mg twice daily, 70/30 insulin (up to 340 units/d), glargine insulin (range, 10-140 units/d), regular insulin (range, 30-240 units/d), aspart insulin (range, 15-45 units/d), and U-500 regular insulin (range, 125-390 units/d). She took metoprolol 25 mg extended release daily and hydrochlorothiazide 25 mg daily, but both were discontinued after the most recent hospitalization. A review of HbA1c readings showed poor glycemic control for > 12 years (range, 10.3% to > 12.3%).
Education for lifestyle modifications, including an LC diet, was presented to the patient to assist with weight loss, improve glycemic control, and reduce insulin resistance. In addition, a glucagon-like peptide-1 agonist (liraglutide) was added to her pharmacotherapy. Continued dietary modifications with LC intake led to consistent reductions in glargine and aspart insulin therapy. The patient remained motivated throughout clinic visits due to improved glycemic control with sustainable dietary modifications, consistently reported feeling better overall, and deprescribed diabetes drug therapies. She remained off her blood pressure medications. After4 months of LC dietary modifications, all insulin therapy was discontinued. She continued with liraglutide 1.8 mg daily and metformin 1000 mg twice daily with an HbA1c of 6.3%. Two months later, her HbA1c level was 6.0%. She also lost 8 lb and her body mass index improved from 31 to 29.
Low-Carbohydrate T2DM DIET MANAGEMENT
LC diets are commonly defined as < 130 g of carbohydrates per day.13 Very LC ketogenic (VLCK) diets often contain ≤ 50 g of carbohydrates per day to induce nutritional ketosis.13 One of the first randomized controlled trials (RCTs) that compared a VLCK diet (< 30 g of carbohydrates per day) with a low-fat diet for obesity demonstrated greater weight loss at 6 months with the LC diet. In addition, patients with diabetes randomized to the LC group also showed improved insulin sensitivity. Notably, this study was done in a population of veterans enrolled at the VA Philadelphia Health Care System.14
A 2008 study comparing an LC diet with a calorie-restricted, low-glycemic diet for individuals with T2DM found that the LC diet group experienced a greater reduction in HbA1c and insulin levels and weight.15 Comparing these 2 diet groups after 24 weeks, 95% of individuals in the LC group reduced or discontinued T2DM medications vs 62% in the low-glycemic group.15 Another study of individuals with T2DM compared a VLCK diet with a low-fat diet. After 34 weeks, 55% of individuals in the LC diet group achieved an HbA1c level below the threshold for diabetes vs 0% in the low-fat diet group.16 A 2018 study of patients with T2DM investigated the impact of a very LC diet compared with the standard of care.17 After 1 year, the LC diet group experienced a mean HbA1c reduction of 1.3%, and 60% of individuals who completed the study achieved an HbA1c level < 6.5% without T2DM medications (not including metformin). This study also demonstrated that medications were significantly reduced, including 100% discontinuation of sulfonylureas and 94% reduction or elimination of insulin.
A recent study of an LC diet (< 20% energy from carbohydrates) demonstrated reduced HbA1c levels, weight, and waist circumference vs a control diet after 6 months. The control diet derived 50% to 60% of energy from carbohydrates.18 This study is typical of other LC interventions, which did not calorie restrict and instead allowed ad libitum intake.14,15
With mounting evidence, the VA/DoD guidelines on T2DM management included LC diets as dietary options for treating T2DM. The ADA also determined that LC diets had the most evidence in improving glycemia and included LC diets as an option for medical nutrition therapy (Table 1).10,19
A systematic review and meta-analysis looking at RCTs of LC diets found evidence for remission of T2DM without significant adverse effects (AEs).20 Another recent systematic review and network meta-analysis of 42 RCTs found that the ketogenic diet was superior for a reduction in HbA1c levels compared with 9 other dietary patterns, including low-fat, Mediterranean, and vegetarian/vegan diets. Overall, ketogenic, Mediterranean, moderate-carbohydrate, and low-glycemic index diets demonstrated improved glycemic control.21
Ideally, a comprehensive behavioral program, such as the VA Move! or Whole Health program, should incorporate patient aligned care teams (PACTs), behavioral health clinicians, clinical pharmacists, and dietitians to provide medical-nutrition therapy using LC diets. However, many facilities may not have adequate experience, expertise, or support. We provide practical approaches to provide LC nutrition counseling, medication management, and deprescribing for any primary care clinician applying LC diets for their patients. For simplicity and practicality, we define 3 types of LC dietary patterns: (1) VLCK (< 50 g); (2) LC (50-100 g); and (3) moderate LC (101-150 g).
Nutrition
All nutrition approaches, including LC diets, should be patient centered, individualized, and sensitive to the patient's culture. Typically, many patients have previously been instructed to consume low-fat (and subsequently) high-carbohydrate (> 150 g) meals. Most well-meaning clinicians have provided common-approach diet education from mainstream health organizations in the form of standardized handouts. For example, the Carbohydrate Counting for People with Diabetes patient education handout from the Academy of Nutrition and Dietetics provides a sample menu with 3 meals and 1 snack totaling 195 g of carbohydrates.22 In contrast, an example ADA diet has sample diets with 3 meals and 2 snacks with approximately 20 to 70 g of carbohydrates.23 In the VA, there are excellent resources to review and standardize handouts that emphasize an LC nutrition approach to T2DM, including ketogenic versions.24,25 Table 2 shows example meal plans based on different LC patterns—VLCK, LC, and moderate LC.
Starting an LC dietary pattern should maximize nutrient-dense and minimally processed proteins. Clinicians should begin with a baseline nutritional assessment through a 24-hour recall or food diary. After this has been completed, the patient’s baseline diet is assessed, and a gradual carbohydrate reduction plan is discussed. Generally, carbohydrate reduction is recommended at 1 meal per day per week. High-carbohydrate meals and snacks are restructured to favor satiating, minimally processed, high-protein food sources. Individual food preferences are considered and included in the recommended LC plan. For example, LC diets can be formulated for vegetarians and vegans as well as those who prefer meat and seafood. Prioritizing satiating and nutrient-dense foods can help increase the probability of diet acceptance and adherence.
A recent study showed that restricting carbohydrates at breakfast reduces 24-hour postprandial hyperglycemia and improves glycemic variability.26 Many patients consume upward of 50 g of carbohydrates at breakfast.27 For example, it is not uncommon for a patient to consume cereal with milk or oatmeal, orange juice, a banana, and toast at breakfast. Instead, the patient is advised to consume any combination of eggs, meat, no-sugar-added Greek yogurt, or berries.
To keep things simple for lunch and dinner, the patient is offered high-quality, minimally processed protein of their choosing with any nonstarchy vegetable. Should a patient desire additional carbohydrates with meals, they may reduce the baseline serving of carbohydrates by 50%. For example, if a patient normally fills 50% of their plate with spaghetti, they may reduce the pasta portion to 25% and add a meatball or increase the amount of vegetables consumed with the meal to satiety.
Snacks may include cheese, eggs, peanut butter, nuts, seeds, berries, no-sugar-added Greek yogurt, or guacamole. Oftentimes, when LC meals are adopted, the desire or need for snacking is diminished due to the satiating effect of high-quality protein sources and nonstarchy vegetables.
Adverse Effects
AEs have been reported with VLCK diets, including headache, diarrhea, constipation, muscle cramps, halitosis, light-headedness, and muscle weakness.28 These AEs may be mitigated with increased fluid intake, sodium intake, and magnesium supplementation.29 Increasing fluids to a minimum of 2 L/d and adding sodium (eg, bouillon supplementation) can minimize AEs.30 Milk of magnesia (5 mL) or slow-release magnesium chloride 200 mEq/d is suggested to reduce muscle cramps.30 There have been no studies looking at sodium intake and worsening hypertension or chronic heart failure in the setting of an LC diet, but fluid and electrolyte intake should be monitored closely, especially in patients with uncontrolled hypertension and heart failure. Other concerns of higher protein on worsening kidney function have generally not been founded.31 In some individuals, an LC and higher fat diet may increase low-density lipoprotein cholesterol (LDL-C).32 Therefore a baseline lipid panel is recommended and should be monitored along with HbA1c levels. An elevated LDL-C response may be managed by increasing protein and reducing saturated fat intake while maintaining the reduced carbohydrate content of the diet.
Medication Management
The adoption of an LC diet can cause a swift and profound reduction in blood sugar.33 Utilizing PACTs can help prevent adverse drug events by involving clinical pharmacists to provide recommendations and dose reductions as patients adopt an LC diet. Each approach must be individualized to the patient and can depend on several factors, including the number and strength of medications, the degree of carbohydrate reduction, baseline blood glucose, as well as assessing for medical literacy and ability to implement recommendations. Additionally, patients should monitor their blood sugar regularly and communicate with their primary care team (pharmacist, PACT registered nurse, primary care clinician, and registered dietician). Ultimately, the goal when adopting an LC diet while taking antihyperglycemics is safely avoiding hypoglycemia while reducing the number of medications the patient is taking. We summarize a practical approach to medication management that was recently published (Table 3).33,34
Medications to Reduce or Discontinue
Medications that can cause hypoglycemia should be the first to be reduced or discontinued upon starting an LC diet, including bolus insulin (although a small amount may be needed to correct for high blood sugar), sulfonylureas, and meglitinides. Combination insulin should be stopped and changed to basal insulin to avoid the risk of hypoglycemia (see Table 4 for insulin deprescribing recommendations). The mechanism of action in preventing the breakdown of carbohydrates in the gastrointestinal tract makes the use of α-glucosidase inhibitors superfluous, and they can be discontinued, reducing pill burden and polypharmacy risks. Sodium-glucose transport protein 2 inhibitors (SGLT2i) should be discontinued for patients on VLCK diets due to the risk of euglycemic diabetic ketoacidosis. However, with LC and moderate LC plans, the SGLT2i may be used with caution as long as patients are made aware of ketoacidosis symptoms. To help prevent the risk of hypoglycemia, basal/long-acting insulin can be continued, but at a 50% reduced dose. Patients should closely monitor blood sugar to assess for appropriateness of dose reductions. While thiazolidinediones are not contraindicated, clinicians can consider discontinuation given both their penchant for inducing weight gain and their limited outcomes data.
Medications to Continue
Medications that pose minimal risk for hypoglycemia can be continued, including metformin, dipeptidyl peptidase 4 inhibitors, and glucagon-like peptide-1 agonists. However, even though these may pose a low risk of hypoglycemia, patients should still closely monitor their blood glucose so medications can be deprescribed as soon as safely and reasonably possible.
Other Medications
The improvement in metabolic health with the reduction of carbohydrates can render other classes of medications unnecessary or require adjustment. Patients should be counseled to monitor their blood pressure as significant and rapid improvements can occur. In the event of a systolic blood pressure of 100 to 110 mm Hg or signs of hypotension, down titration or discontinuation of antihypertensives should be initiated. Limited evidence exists on the preferred order of discontinuation but should be informed by other comorbidities, such as coronary artery disease and chronic kidney disease. Given an LC diet’s diuretic effect, tapering and stopping diuretics may be an option. Other medications requiring closer monitoring include lithium (can be affected by fluid and electrolyte shifts), warfarin (may alter vitamin K intake), valproate (which may be reduced), and zonisamide and topiramate (kidney stone risk).
Remission of T2DM with LC Diets
As patients adopt LC diets and medications are deprescribed and glycemia improves, HbA1c and fasting glucose levels may drop below the diagnostic threshold for T2DM.20 As new evidence emerges surrounding the management of T2DM from a lifestyle perspective, major health care organizations have acknowledged that T2DM is not necessarily an incurable, progressive disease, but rather a disease that can be reversed or put in remission.35-37 In 2016, the World Health Organization (WHO) global report on diabetes acknowledged that T2DM reversal can be achieved via weight loss and calorie restriction.35
In 2021, a consensus statement from the ADA, the Endocrine Society, the EASD, and Diabetes UK defined T2DM remission as an HbA1c level < 6.5% for at least 3 months with no T2DM medications.36 Diabetes Australia also published a position statement in 2021 about T2DM remission.37 Like the WHO, Diabetes Australia acknowledged that remission of T2DM is possible following intensive dietary changes or bariatric surgery.37 Before the 2021 consensus statement, some experts argued that excluding metformin from the T2DM medication list may not be warranted since metformin has indications beyond T2DM. In this case, remission of T2DM could be defined as an HbA1c level < 6.5% for at least 3 months and on metformin or no T2DM medications.8
Emerging Strategies
Emerging strategies, such as continuous glucose monitors (CGMs) and the use of intermittent fasting/time-restricted eating (TRE), can be used with the LC diet to help improve the monitoring and management of T2DM. In the recently published VA/DoD guidelines for T2DM, the work group suggested real-time CGMs for qualified patients with T2DM.4 These include patients on daily insulin who are not achieving glycemic control or to reduce the risk for hypoglycemia. CGMs have shown evidence of improved glycemic control and decreased hypoglycemia in those with T2DM.38,39 It is currently unknown if CGMs improve long-term glycemic control, but they appear promising for managing and reducing medications for those on an LC diet.40
TRE can be supplemented with an LC plan that incorporates “eating windows.” Common patterns include 14 hours of fasting and a 10-hour eating window (14F:10E), or 16 hours of fasting and an 8-hour eating window (16F:8E). By eating only in the specified window, patients generally reduce caloric intake and minimize insulin and glucose excursions during the fasting window. No changes need to be made to the macronutrient composition of the diet, and LC approaches can be used with TRE. The mechanism of action is likely multifactorial, targeting hyperinsulinemia and insulin resistance as well as producing a caloric deficit to enable weight loss.41 Eating windows may improve insulin sensitivity, reduce insulin resistance, and enhance overall glycemic control. The recent VA/DoD guidelines recommended against intermittent fasting due to concerns over the risk of hypoglycemia despite larger weight loss in TRE groups.4 Recently, a study using CGMs and TRE demonstrated both improved glycemic control and no hypoglycemic episodes in patients with T2DM on insulin.42 Patients who would like to supplement TRE with an LC plan as a strategy for improved glycemic control should work closely with their PACT to help manage their TRE and LC plan and consider a CGM adjunct, especially if on insulin.
Barriers
Managing T2DM often requires comprehensive lifestyle modifications of nutrition, exercise, sleep, stress management, and other psychosocial issues, as well as an interdisciplinary team-based approach.43 The advantage of working within the VA includes a uniform system within a network of care. However, many patients continue to use both federal and private health care. This use of out-of-network care may result in fragmented, potentially disjointed, or even contradictory dietary advice.
The VA PACT, whole health for holistic health, and weight loss interventions such as the MOVE! program provide lifestyle interventions like nutrition, physical activity, and behavior change. However, these well-intentioned approaches may provide alternative and even diverging recommendations, which place additional barriers to effective patient management. In patients who are advised and accept a trial of an LC plan, each member of the team should embrace the self-management decision of the patient and support the plan.29 Any conflicts, questions, or concerns should be communicated directly with the team in an interdisciplinary approach to provide a unified message and counsel.
The long-term effects and sustainability of an LC diet have been questioned in the literature.44-46 Recently, the use of an app-based coaching plan has demonstrated short- and long-term sustainability on an LC diet.47 In just 5 months in a large VA system, 590 patients using a virtual coaching platform and a VLCK diet plan were found to have lower HbA1c levels, reduced diabetic medication fills, lower body mass index, fewer outpatient visits, and lower prescription drug costs.
A 5-year follow-up found nearly 50% of participants sustained a VLCK diet for T2DM. For patients who participated in the study after 2 years, 72% sustained the VLCK diet in years 2 to 5. Most required nearly 50% fewer medications and in those that started with insulin, half did not require it at 5 years.48 Further research, however, is necessary to determine the long-term effects on cardiometabolic markers and health with LC diets. There are no long-term RCTs on outcomes data looking at T2DM morbidity or mortality. While there are prospective cohort studies on LC diets in the general population on mortality, they demonstrate mixed results. These studies may be confounded by heterogeneous definitions of LC diets, diet quality, and other health factors.49-51
Conclusions
The effective use of LC diets within a PACT with close and intensive lifestyle counseling and a safe approach to medication management and deprescribing can improve glycemic control, reduce the overall need for insulin, reduce medication use, and provide sustained weight loss. Additionally, the use of therapeutic carbohydrate reduction and subsequent medication deprescription may lead to sustained remission of T2DM. The current efficacy and sustainment of therapeutic carbohydrate reduction for patients with T2DM appears promising. Further research on LC diets, emerging strategies, and long-term effects on cardiometabolic risk factors, morbidity, and mortality will continue to inform future practice in our health care system.
Acknowledgments
We thank Cecile Seth who has been instrumental in pushing us forward and the Metabolic Multiplier group who has helped encourage and provide input into this article.
The prevalence of diabetes continues to increase despite advances in treatment options. In 2019, according to the Centers for Disease Control and Prevention (CDC), 37.1 million (14.7%) US adults had diabetes. Among adults aged ≥ 65 years, the prevalence is even higher at 29.2%.1 Research has also estimated that 45% of adults have evidence of prediabetes or diabetes.2 According to the Veterans Health Administration, almost 25% of enrolled veterans have diabetes.3
Background
Diabetes is associated with an increased risk of microvascular complications (eg, retinopathy, nephropathy, and neuropathy) and macrovascular complications (eg, atherosclerotic cardiovascular disease) and is one of the most common causes of morbidity and mortality in the US.4 In 2017, diabetes was estimated to cost $327 billion in the US, up from $261 billion in 2012.5 During this same period, the excess costs per person with diabetes increased from $8417 to $9601.5
Type 2 diabetes mellitus (T2DM) and its associated insulin resistance is typically considered a chronic disease with progressive loss of β-cell function. Controlling glycemia, delaying microvascular changes, and preventing macrovascular disease are major management goals. Lifestyle interventions are essential in the management and prevention of T2DM. Medication management for T2DM usually progresses through several medications, ending in insulin therapy.6 Within 10 years of diagnosis, almost half of all individuals with T2DM will require insulin to manage their glycemia.7
Bariatric surgery and nutrition approaches have been successful in reversing T2DM. Recently, there has been increased interest in nutritional approaches to place T2DM in remission, reverse the disease process, and improve insulin resistance. Contrary to popular belief, before the discovery of insulin in 1921, low-carbohydrate (LC) diets were the most common treatment for T2DM.8 With the discovery of insulin and the eventual development of low-fat dietary recommendations, LC diets were no longer favored by most clinicians.8 Low-fat diets are, by definition, also high-carbohydrate diets. By the early 1980s, low-fat diets had become the standard of care dietary recommendation, and the goal for clinicians became glycemic maintenance (with increased use of medications) rather than preventing hyperglycemia.8
With growing evidence regarding the use of LC diets for T2DM, the US Department of Veterans Affairs (VA) and US Department of Defense (DoD), the American Diabetes Association (ADA), the European Association for the Study of Diabetes (EASD), Diabetes Canada, and Diabetes Australia all include LC diets as a viable option for treating T2DM.4,9-12 This article will highlight a case using a reduced carbohydrate approach in lifestyle management and provide clinicians with practical guidance in its implementation. We will review the evidence that informs these guidelines, describe a practical approach to nutritional counseling, and review medication management and deprescribing approaches. Finally, barriers to implementation will be explored.
ILLUSTRATIVE CASE
A 64-year-old woman presented to the clinical pharmacist for the management of T2DM after her tenth hospitalization related to hyperglycemia in 10 years. She had previously been managed by primary care clinicians, clinical dietitians, endocrinologists, and certified diabetes care and education specialists. Pertinent history included diabetic ketoacidosis, coronary artery disease, hyperlipidemia, hypertension, obstructive sleep apnea, obesity, metabolic dysfunction-associated steatotic liver disease, and mild nonproliferative diabetic retinopathy with clinically significant macular edema. The patient expressed frustration with poor glycemic control during her many years of insulin therapy and an inability to lose weight due to insulin dose titrations. The patient reported prior education including but not limited to standardized sample menus, consistent carbohydrate intake, calorie reduction, general healthful nutrition, and the “move more, eat less” approach. The patient was unable to titrate insulin dosage and did not experience weight loss despite compliance with these methods.
Her medications included glargine insulin 45 units once daily, aspart insulin 5 units before meals 3 times daily, and metformin 1000 mg twice daily. Her hemoglobin A1c (HbA1c) level was 11.8%. A review of prior therapies for T2DM included glyburide 5 mg twice daily, metformin 1000 mg twice daily, 70/30 insulin (up to 340 units/d), glargine insulin (range, 10-140 units/d), regular insulin (range, 30-240 units/d), aspart insulin (range, 15-45 units/d), and U-500 regular insulin (range, 125-390 units/d). She took metoprolol 25 mg extended release daily and hydrochlorothiazide 25 mg daily, but both were discontinued after the most recent hospitalization. A review of HbA1c readings showed poor glycemic control for > 12 years (range, 10.3% to > 12.3%).
Education for lifestyle modifications, including an LC diet, was presented to the patient to assist with weight loss, improve glycemic control, and reduce insulin resistance. In addition, a glucagon-like peptide-1 agonist (liraglutide) was added to her pharmacotherapy. Continued dietary modifications with LC intake led to consistent reductions in glargine and aspart insulin therapy. The patient remained motivated throughout clinic visits due to improved glycemic control with sustainable dietary modifications, consistently reported feeling better overall, and deprescribed diabetes drug therapies. She remained off her blood pressure medications. After4 months of LC dietary modifications, all insulin therapy was discontinued. She continued with liraglutide 1.8 mg daily and metformin 1000 mg twice daily with an HbA1c of 6.3%. Two months later, her HbA1c level was 6.0%. She also lost 8 lb and her body mass index improved from 31 to 29.
Low-Carbohydrate T2DM DIET MANAGEMENT
LC diets are commonly defined as < 130 g of carbohydrates per day.13 Very LC ketogenic (VLCK) diets often contain ≤ 50 g of carbohydrates per day to induce nutritional ketosis.13 One of the first randomized controlled trials (RCTs) that compared a VLCK diet (< 30 g of carbohydrates per day) with a low-fat diet for obesity demonstrated greater weight loss at 6 months with the LC diet. In addition, patients with diabetes randomized to the LC group also showed improved insulin sensitivity. Notably, this study was done in a population of veterans enrolled at the VA Philadelphia Health Care System.14
A 2008 study comparing an LC diet with a calorie-restricted, low-glycemic diet for individuals with T2DM found that the LC diet group experienced a greater reduction in HbA1c and insulin levels and weight.15 Comparing these 2 diet groups after 24 weeks, 95% of individuals in the LC group reduced or discontinued T2DM medications vs 62% in the low-glycemic group.15 Another study of individuals with T2DM compared a VLCK diet with a low-fat diet. After 34 weeks, 55% of individuals in the LC diet group achieved an HbA1c level below the threshold for diabetes vs 0% in the low-fat diet group.16 A 2018 study of patients with T2DM investigated the impact of a very LC diet compared with the standard of care.17 After 1 year, the LC diet group experienced a mean HbA1c reduction of 1.3%, and 60% of individuals who completed the study achieved an HbA1c level < 6.5% without T2DM medications (not including metformin). This study also demonstrated that medications were significantly reduced, including 100% discontinuation of sulfonylureas and 94% reduction or elimination of insulin.
A recent study of an LC diet (< 20% energy from carbohydrates) demonstrated reduced HbA1c levels, weight, and waist circumference vs a control diet after 6 months. The control diet derived 50% to 60% of energy from carbohydrates.18 This study is typical of other LC interventions, which did not calorie restrict and instead allowed ad libitum intake.14,15
With mounting evidence, the VA/DoD guidelines on T2DM management included LC diets as dietary options for treating T2DM. The ADA also determined that LC diets had the most evidence in improving glycemia and included LC diets as an option for medical nutrition therapy (Table 1).10,19
A systematic review and meta-analysis looking at RCTs of LC diets found evidence for remission of T2DM without significant adverse effects (AEs).20 Another recent systematic review and network meta-analysis of 42 RCTs found that the ketogenic diet was superior for a reduction in HbA1c levels compared with 9 other dietary patterns, including low-fat, Mediterranean, and vegetarian/vegan diets. Overall, ketogenic, Mediterranean, moderate-carbohydrate, and low-glycemic index diets demonstrated improved glycemic control.21
Ideally, a comprehensive behavioral program, such as the VA Move! or Whole Health program, should incorporate patient aligned care teams (PACTs), behavioral health clinicians, clinical pharmacists, and dietitians to provide medical-nutrition therapy using LC diets. However, many facilities may not have adequate experience, expertise, or support. We provide practical approaches to provide LC nutrition counseling, medication management, and deprescribing for any primary care clinician applying LC diets for their patients. For simplicity and practicality, we define 3 types of LC dietary patterns: (1) VLCK (< 50 g); (2) LC (50-100 g); and (3) moderate LC (101-150 g).
Nutrition
All nutrition approaches, including LC diets, should be patient centered, individualized, and sensitive to the patient's culture. Typically, many patients have previously been instructed to consume low-fat (and subsequently) high-carbohydrate (> 150 g) meals. Most well-meaning clinicians have provided common-approach diet education from mainstream health organizations in the form of standardized handouts. For example, the Carbohydrate Counting for People with Diabetes patient education handout from the Academy of Nutrition and Dietetics provides a sample menu with 3 meals and 1 snack totaling 195 g of carbohydrates.22 In contrast, an example ADA diet has sample diets with 3 meals and 2 snacks with approximately 20 to 70 g of carbohydrates.23 In the VA, there are excellent resources to review and standardize handouts that emphasize an LC nutrition approach to T2DM, including ketogenic versions.24,25 Table 2 shows example meal plans based on different LC patterns—VLCK, LC, and moderate LC.
Starting an LC dietary pattern should maximize nutrient-dense and minimally processed proteins. Clinicians should begin with a baseline nutritional assessment through a 24-hour recall or food diary. After this has been completed, the patient’s baseline diet is assessed, and a gradual carbohydrate reduction plan is discussed. Generally, carbohydrate reduction is recommended at 1 meal per day per week. High-carbohydrate meals and snacks are restructured to favor satiating, minimally processed, high-protein food sources. Individual food preferences are considered and included in the recommended LC plan. For example, LC diets can be formulated for vegetarians and vegans as well as those who prefer meat and seafood. Prioritizing satiating and nutrient-dense foods can help increase the probability of diet acceptance and adherence.
A recent study showed that restricting carbohydrates at breakfast reduces 24-hour postprandial hyperglycemia and improves glycemic variability.26 Many patients consume upward of 50 g of carbohydrates at breakfast.27 For example, it is not uncommon for a patient to consume cereal with milk or oatmeal, orange juice, a banana, and toast at breakfast. Instead, the patient is advised to consume any combination of eggs, meat, no-sugar-added Greek yogurt, or berries.
To keep things simple for lunch and dinner, the patient is offered high-quality, minimally processed protein of their choosing with any nonstarchy vegetable. Should a patient desire additional carbohydrates with meals, they may reduce the baseline serving of carbohydrates by 50%. For example, if a patient normally fills 50% of their plate with spaghetti, they may reduce the pasta portion to 25% and add a meatball or increase the amount of vegetables consumed with the meal to satiety.
Snacks may include cheese, eggs, peanut butter, nuts, seeds, berries, no-sugar-added Greek yogurt, or guacamole. Oftentimes, when LC meals are adopted, the desire or need for snacking is diminished due to the satiating effect of high-quality protein sources and nonstarchy vegetables.
Adverse Effects
AEs have been reported with VLCK diets, including headache, diarrhea, constipation, muscle cramps, halitosis, light-headedness, and muscle weakness.28 These AEs may be mitigated with increased fluid intake, sodium intake, and magnesium supplementation.29 Increasing fluids to a minimum of 2 L/d and adding sodium (eg, bouillon supplementation) can minimize AEs.30 Milk of magnesia (5 mL) or slow-release magnesium chloride 200 mEq/d is suggested to reduce muscle cramps.30 There have been no studies looking at sodium intake and worsening hypertension or chronic heart failure in the setting of an LC diet, but fluid and electrolyte intake should be monitored closely, especially in patients with uncontrolled hypertension and heart failure. Other concerns of higher protein on worsening kidney function have generally not been founded.31 In some individuals, an LC and higher fat diet may increase low-density lipoprotein cholesterol (LDL-C).32 Therefore a baseline lipid panel is recommended and should be monitored along with HbA1c levels. An elevated LDL-C response may be managed by increasing protein and reducing saturated fat intake while maintaining the reduced carbohydrate content of the diet.
Medication Management
The adoption of an LC diet can cause a swift and profound reduction in blood sugar.33 Utilizing PACTs can help prevent adverse drug events by involving clinical pharmacists to provide recommendations and dose reductions as patients adopt an LC diet. Each approach must be individualized to the patient and can depend on several factors, including the number and strength of medications, the degree of carbohydrate reduction, baseline blood glucose, as well as assessing for medical literacy and ability to implement recommendations. Additionally, patients should monitor their blood sugar regularly and communicate with their primary care team (pharmacist, PACT registered nurse, primary care clinician, and registered dietician). Ultimately, the goal when adopting an LC diet while taking antihyperglycemics is safely avoiding hypoglycemia while reducing the number of medications the patient is taking. We summarize a practical approach to medication management that was recently published (Table 3).33,34
Medications to Reduce or Discontinue
Medications that can cause hypoglycemia should be the first to be reduced or discontinued upon starting an LC diet, including bolus insulin (although a small amount may be needed to correct for high blood sugar), sulfonylureas, and meglitinides. Combination insulin should be stopped and changed to basal insulin to avoid the risk of hypoglycemia (see Table 4 for insulin deprescribing recommendations). The mechanism of action in preventing the breakdown of carbohydrates in the gastrointestinal tract makes the use of α-glucosidase inhibitors superfluous, and they can be discontinued, reducing pill burden and polypharmacy risks. Sodium-glucose transport protein 2 inhibitors (SGLT2i) should be discontinued for patients on VLCK diets due to the risk of euglycemic diabetic ketoacidosis. However, with LC and moderate LC plans, the SGLT2i may be used with caution as long as patients are made aware of ketoacidosis symptoms. To help prevent the risk of hypoglycemia, basal/long-acting insulin can be continued, but at a 50% reduced dose. Patients should closely monitor blood sugar to assess for appropriateness of dose reductions. While thiazolidinediones are not contraindicated, clinicians can consider discontinuation given both their penchant for inducing weight gain and their limited outcomes data.
Medications to Continue
Medications that pose minimal risk for hypoglycemia can be continued, including metformin, dipeptidyl peptidase 4 inhibitors, and glucagon-like peptide-1 agonists. However, even though these may pose a low risk of hypoglycemia, patients should still closely monitor their blood glucose so medications can be deprescribed as soon as safely and reasonably possible.
Other Medications
The improvement in metabolic health with the reduction of carbohydrates can render other classes of medications unnecessary or require adjustment. Patients should be counseled to monitor their blood pressure as significant and rapid improvements can occur. In the event of a systolic blood pressure of 100 to 110 mm Hg or signs of hypotension, down titration or discontinuation of antihypertensives should be initiated. Limited evidence exists on the preferred order of discontinuation but should be informed by other comorbidities, such as coronary artery disease and chronic kidney disease. Given an LC diet’s diuretic effect, tapering and stopping diuretics may be an option. Other medications requiring closer monitoring include lithium (can be affected by fluid and electrolyte shifts), warfarin (may alter vitamin K intake), valproate (which may be reduced), and zonisamide and topiramate (kidney stone risk).
Remission of T2DM with LC Diets
As patients adopt LC diets and medications are deprescribed and glycemia improves, HbA1c and fasting glucose levels may drop below the diagnostic threshold for T2DM.20 As new evidence emerges surrounding the management of T2DM from a lifestyle perspective, major health care organizations have acknowledged that T2DM is not necessarily an incurable, progressive disease, but rather a disease that can be reversed or put in remission.35-37 In 2016, the World Health Organization (WHO) global report on diabetes acknowledged that T2DM reversal can be achieved via weight loss and calorie restriction.35
In 2021, a consensus statement from the ADA, the Endocrine Society, the EASD, and Diabetes UK defined T2DM remission as an HbA1c level < 6.5% for at least 3 months with no T2DM medications.36 Diabetes Australia also published a position statement in 2021 about T2DM remission.37 Like the WHO, Diabetes Australia acknowledged that remission of T2DM is possible following intensive dietary changes or bariatric surgery.37 Before the 2021 consensus statement, some experts argued that excluding metformin from the T2DM medication list may not be warranted since metformin has indications beyond T2DM. In this case, remission of T2DM could be defined as an HbA1c level < 6.5% for at least 3 months and on metformin or no T2DM medications.8
Emerging Strategies
Emerging strategies, such as continuous glucose monitors (CGMs) and the use of intermittent fasting/time-restricted eating (TRE), can be used with the LC diet to help improve the monitoring and management of T2DM. In the recently published VA/DoD guidelines for T2DM, the work group suggested real-time CGMs for qualified patients with T2DM.4 These include patients on daily insulin who are not achieving glycemic control or to reduce the risk for hypoglycemia. CGMs have shown evidence of improved glycemic control and decreased hypoglycemia in those with T2DM.38,39 It is currently unknown if CGMs improve long-term glycemic control, but they appear promising for managing and reducing medications for those on an LC diet.40
TRE can be supplemented with an LC plan that incorporates “eating windows.” Common patterns include 14 hours of fasting and a 10-hour eating window (14F:10E), or 16 hours of fasting and an 8-hour eating window (16F:8E). By eating only in the specified window, patients generally reduce caloric intake and minimize insulin and glucose excursions during the fasting window. No changes need to be made to the macronutrient composition of the diet, and LC approaches can be used with TRE. The mechanism of action is likely multifactorial, targeting hyperinsulinemia and insulin resistance as well as producing a caloric deficit to enable weight loss.41 Eating windows may improve insulin sensitivity, reduce insulin resistance, and enhance overall glycemic control. The recent VA/DoD guidelines recommended against intermittent fasting due to concerns over the risk of hypoglycemia despite larger weight loss in TRE groups.4 Recently, a study using CGMs and TRE demonstrated both improved glycemic control and no hypoglycemic episodes in patients with T2DM on insulin.42 Patients who would like to supplement TRE with an LC plan as a strategy for improved glycemic control should work closely with their PACT to help manage their TRE and LC plan and consider a CGM adjunct, especially if on insulin.
Barriers
Managing T2DM often requires comprehensive lifestyle modifications of nutrition, exercise, sleep, stress management, and other psychosocial issues, as well as an interdisciplinary team-based approach.43 The advantage of working within the VA includes a uniform system within a network of care. However, many patients continue to use both federal and private health care. This use of out-of-network care may result in fragmented, potentially disjointed, or even contradictory dietary advice.
The VA PACT, whole health for holistic health, and weight loss interventions such as the MOVE! program provide lifestyle interventions like nutrition, physical activity, and behavior change. However, these well-intentioned approaches may provide alternative and even diverging recommendations, which place additional barriers to effective patient management. In patients who are advised and accept a trial of an LC plan, each member of the team should embrace the self-management decision of the patient and support the plan.29 Any conflicts, questions, or concerns should be communicated directly with the team in an interdisciplinary approach to provide a unified message and counsel.
The long-term effects and sustainability of an LC diet have been questioned in the literature.44-46 Recently, the use of an app-based coaching plan has demonstrated short- and long-term sustainability on an LC diet.47 In just 5 months in a large VA system, 590 patients using a virtual coaching platform and a VLCK diet plan were found to have lower HbA1c levels, reduced diabetic medication fills, lower body mass index, fewer outpatient visits, and lower prescription drug costs.
A 5-year follow-up found nearly 50% of participants sustained a VLCK diet for T2DM. For patients who participated in the study after 2 years, 72% sustained the VLCK diet in years 2 to 5. Most required nearly 50% fewer medications and in those that started with insulin, half did not require it at 5 years.48 Further research, however, is necessary to determine the long-term effects on cardiometabolic markers and health with LC diets. There are no long-term RCTs on outcomes data looking at T2DM morbidity or mortality. While there are prospective cohort studies on LC diets in the general population on mortality, they demonstrate mixed results. These studies may be confounded by heterogeneous definitions of LC diets, diet quality, and other health factors.49-51
Conclusions
The effective use of LC diets within a PACT with close and intensive lifestyle counseling and a safe approach to medication management and deprescribing can improve glycemic control, reduce the overall need for insulin, reduce medication use, and provide sustained weight loss. Additionally, the use of therapeutic carbohydrate reduction and subsequent medication deprescription may lead to sustained remission of T2DM. The current efficacy and sustainment of therapeutic carbohydrate reduction for patients with T2DM appears promising. Further research on LC diets, emerging strategies, and long-term effects on cardiometabolic risk factors, morbidity, and mortality will continue to inform future practice in our health care system.
Acknowledgments
We thank Cecile Seth who has been instrumental in pushing us forward and the Metabolic Multiplier group who has helped encourage and provide input into this article.
1. Centers for Disease Control and Prevention. Prevalence of Both Diagnosed and Undiagnosed Diabetes. Updated September 30, 2022. Accessed October 6, 2023. https://www.cdc.gov/diabetes/data/statistics-report/diagnosed-undiagnosed-diabetes.html
2. Centers for Disease Control and Prevention. Diabetes and Prediabetes. Updated September 6, 2022. Accessed October 6, 2023. https://www.cdc.gov/chronicdisease/resources/publications/factsheets/diabetes-prediabetes.htm 3. US Department of Veterans Affairs. Diabetes information - Nutrition and food services. Updated May 4, 2023. Accessed October 6, 2023. https://www.nutrition.va.gov/diabetes.asp
4. US Department of Veterans Affairs. Management of Type 2 Diabetes Mellitus (2023) - VA/DoD Clinical Practice Guidelines. Updated September 1, 2023. Accessed October 6, 2023. https://www.healthquality.va.gov/guidelines/CD/diabetes/
5. American Diabetes Association. Economic Costs of Diabetes in the U.S. in 2017. Diabetes Care. 2018;41(5):917-928. doi:10.2337/dci18-0007
6. Home P, Riddle M, Cefalu WT, et al. Insulin therapy in people with type 2 diabetes: opportunities and challenges?. Diabetes Care. 2014;37(6):1499-1508. doi:10.2337/dc13-2743
7. Donath MY, Ehses JA, Maedler K, et al. Mechanisms of β-cell death in type 2 diabetes. Diabetes. 2005;54(suppl 2):S108-S113. doi:10.2337/DIABETES.54.SUPPL_2.S108
8. Hallberg SJ, Gershuni VM, Hazbun TL, Athinarayanan SJ. Reversing type 2 diabetes: a narrative review of the evidence. Nutrients. 2019;11(4):766. Published 2019 Apr 1. doi:10.3390/nu11040766
9. Davies MJ, D’Alessio DA, Fradkin J, et al. Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41(12):2669. doi:10.2337/DCI18-0033
10. Evert AB, Dennison M, Gardner CD, et al. Nutrition therapy for adults with diabetes or prediabetes: a consensus report. Diabetes Care. 2019;42(5):731-754. doi:10.2337/DCI19-0014
11. Diabetes Canada position statement on low-carbohydrate diets for adults with diabetes: a rapid review. Can J Diabetes. 2020;44(4):295-299. doi:10.1016/J.JCJD.2020.04.001
12. Diabetes Australia. Position statements. Accessed October 6, 2023. https://www.diabetesaustralia.com.au/research-advocacy/position-statements/
13. Feinman RD, Pogozelski WK, Astrup A, et al. Dietary carbohydrate restriction as the first approach in diabetes management: critical review and evidence base. Nutrition. 2014;31(1):1-13. doi:10.1016/j.nut.2014.06.011
14. Samaha FF, Iqbal N, Seshadri P, et al. A low-carbohydrate as compared with a low-fat diet in severe obesity. N Engl J Med. 2003;348(21):2074-2081. doi:10.1056/NEJMOA02263715. Westman EC, Yancy WS, Mavropoulos JC, Marquart M, McDuffie JR. The effect of a low-carbohydrate, ketogenic diet versus a low-glycemic index diet on glycemic control in type 2 diabetes mellitus. Nutr Metab (Lond). 2008;5(1):36. doi:10.1186/1743-7075-5-36
16. Saslow LR, Mason AE, Kim S, et al. An online intervention comparing a very low-carbohydrate ketogenic diet and lifestyle recommendations versus a plate method diet in overweight individuals with type 2 diabetes: a randomized controlled trial. J Med Internet Res. 2017;19(2). doi:10.2196/JMIR.5806
17. Hallberg SJ, McKenzie AL, Williams PT, et al. Effectiveness and safety of a novel care model for the management of type 2 diabetes at 1 year: an open-label, non-randomized, controlled study. Diabetes Ther. 2018;9(2):583-612. doi:10.1007/S13300-018-0373-9
18. Gram-Kampmann EM, Hansen CD, Hugger MB, et al. Effects of a 6-month, low-carbohydrate diet on glycaemic control, body composition, and cardiovascular risk factors in patients with type 2 diabetes: An open-label randomized controlled trial. Diabetes Obes Metab. 2022;24(4):693-703. doi:10.1111/DOM.14633
19. Committee ADAPP. 5. Facilitating behavior change and well-being to improve health outcomes: standards of medical care in diabetes—2022. Diabetes Care. 2022;45(suppl 1):S60-S82. doi:10.2337/DC22-S005
20. Goldenberg JZ, Johnston BC. Low and very low carbohydrate diets for diabetes remission. BMJ. 2021;373:m4743. doi:10.1136/BMJ.N262

21. Jing T, Zhang S, Bai M, et al. Effect of dietary approaches on glycemic control in patients with type 2 diabetes: a systematic review with network meta-analysis of randomized trials. Nutrients. 2023;15(14):3156. doi:10.3390/nu15143156
22. Academy of Nutrition and Dietetics. Nutrition care manual. Accessed October 6, 2023. https://www.nutritioncaremanual.org/
23. Low carbohydrate and very low carbohydrate eating patterns in adults with diabetes. ShopDiabetes.org. Accessed August 5, 2022. https://shopdiabetes.org/products/low-carbohydrate-and-very-low-carbohydrate-eating-patterns-in-adults-with-diabetes-a-guide-for-health-care-providers
24. US Department of Veterans Affairs. Diabetes education - nutrition and food services. Published July 31, 2022. http://vaww.nutrition.va.gov/docs/pted/ModifiedKetogenicDiet.pdf [Source not verified]
25. US Department of Veterans Affairs, My HealtheVet. Lowdown on low-carb diets. Updated June 1, 2021. Accessed October 6, 2023. https://www.myhealth.va.gov/mhv-portal-web/ss20190724-low-carb-diet
26. Chang CR, Francois ME, Little JP. Restricting carbohydrates at breakfast is sufficient to reduce 24-hour exposure to postprandial hyperglycemia and improve glycemic variability. Am J Clin Nutr. 2019;109(5):1302-1309. doi:10.1093/AJCN/NQY261
27. Hall KD, Ayuketah A, Brychta R, et al. Ultra-processed diets cause excess calorie intake and weight gain: an inpatient randomized controlled trial of ad libitum food intake. Cell Metab. 2019;30(1):226. doi:10.1016/j.cmet.2019.05.020
28. Harvey CJ d. C, Schofield GM, Zinn C, Thornley S. Effects of differing levels of carbohydrate restriction on mood achievement of nutritional ketosis, and symptoms of carbohydrate withdrawal in healthy adults: a randomized clinical trial. Nutrition. 2019;67-68:100005. doi:10.1016/J.NUTX.2019.100005
29. Griauzde DH, Standafer Lopez K, Saslow LR, Richardson CR. A pragmatic approach to translating low- and very low-carbohydrate diets into clinical practice for patients with obesity and type 2 diabetes. Front Nutr. 2021;8:416. doi:10.3389/FNUT.2021.682137/BIBTEX
30. Westman EC, Tondt J, Maguire E, Yancy WS. Implementing a low-carbohydrate, ketogenic diet to manage type 2 diabetes mellitus. Expert Rev Endocrinol Metab. 2018;13(5):263-272. doi:10.1080/17446651.2018.1523713
31. Suyoto PST. Effect of low-carbohydrate diet on markers of renal function in patients with type 2 diabetes: a meta-analysis. Diabetes Metab Res Rev. 2018;34(7). doi:10.1002/DMRR.3032
32. Norwitz NG, Feldman D, Soto-Mota A, Kalayjian T, Ludwig DS. Elevated LDL cholesterol with a carbohydrate-restricted diet: evidence for a “lean mass hyper-responder” phenotype. Curr Dev Nutr. 2021;6(1). doi:10.1093/CDN/NZAB144
33. Murdoch C, Unwin D, Cavan D, Cucuzzella M, Patel M. Adapting diabetes medication for low carbohydrate management of type 2 diabetes: a practical guide. Br J Gen Pract. 2019;69(684):360-361. doi:10.3399/bjgp19X704525
34. Cucuzzella M, Riley K, Isaacs D. Adapting medication for type 2 diabetes to a low carbohydrate diet. Front Nutr. 2021;8:486. doi:10.3389/FNUT.2021.688540/BIBTEX
35. World Health Organization. Global report on diabetes. 2016. Accessed October 6, 2023. https://iris.who.int/bitstream/handle/10665/204871/9789241565257_eng.pdf?sequence=1
36. Riddle MC, Cefalu WT, Evans PH, et al. Consensus report: definition and interpretation of remission in type 2 diabetes. Diabetes Care. 2021;44(10):2438-2444. doi:10.2337/DCI21-0034
37. Diabetes Australia. Type 2 Diabetes remission position statement. 2021. Accessed October 6, 2023. https://www.diabetesaustralia.com.au/wp-content/uploads/2021_Diabetes-Australia-Position-Statement_Type-2-diabetes-remission_2.pdf
38. Martens T, Beck RW, Bailey R, et al. Effect of continuous glucose monitoring on glycemic control in patients with type 2 diabetes treated with basal insulin: a randomized clinical trial. JAMA. 2021;325(22):2262-2272. doi:10.1001/JAMA.2021.7444
39. Jackson MA, Ahmann A, Shah VN. Type 2 diabetes and the use of real-time continuous glucose monitoring. Diabetes Technol Ther. 2021;23(S1):S27-S34. doi:10.1089/DIA.2021.0007
40. Oser TK, Cucuzzella M, Stasinopoulos M, Moncrief M, McCall A, Cox DJ. An innovative, paradigm-shifting lifestyle intervention to reduce glucose excursions with the use of continuous glucose monitoring to educate, motivate, and activate adults with newly diagnosed type 2 diabetes: pilot feasibility study. JMIR Diabetes. 2022;7(1). doi:10.2196/34465
41. Światkiewicz I, Woźniak A, Taub PR. Time-restricted eating and metabolic syndrome: current status and future perspectives. Nutrients. 2021;13(1):221. doi:10.3390/NU13010221
42. Obermayer A, Tripolt NJ, Pferschy PN, et al. Efficacy and safety of intermittent fasting in people with insulin-treated type 2 diabetes (INTERFAST-2)—a randomized controlled trial. Diabetes Care. 2023;46(2):463-468. doi:10.2337/dc22-1622
43. American Diabetes Association. 5. Lifestyle management: standards of medical care in diabetes—2019. Diabetes Care. 2019;42(suppl 1):S46-S60. doi:10.2337/DC19-S005
44. Li S, Ding L, Xiao X. Comparing the efficacy and safety of low-carbohydrate diets with low-fat diets for type 2 diabetes mellitus patients: a systematic review and meta-analysis of randomized clinical trials. Int J Endocrinol. 2021;2021:8521756. Published 2021 Dec 6. doi:10.1155/2021/8521756
45. Choi JH, Kang JH, Chon S. Comprehensive understanding for application in Korean patients with type 2 diabetes mellitus of the consensus statement on carbohydrate-restricted diets by Korean Diabetes Association, Korean Society for the Study of Obesity, and Korean Society of Hypertension. Diabetes Metab J. 2022;46(3):377. doi:10.4093/DMJ.2022.0051
46. Jayedi A, Zeraattalab-Motlagh S, Jabbarzadeh B, et al. Dose-dependent effect of carbohydrate restriction for type 2 diabetes management: a systematic review and dose-response meta-analysis of randomized controlled trials. Am J Clin Nutr. 2022;116(1). doi:10.1093/AJCN/NQAC066
47. Strombotne KL, Lum J, Ndugga NJ, et al. Effectiveness of a ketogenic diet and virtual coaching intervention for patients with diabetes: a difference-in-differences analysis. Diabetes Obes Metab. 2021;23(12):2643-2650. doi:10.1111/DOM.14515
48. Virta Health. Virta Health highlights lasting, transformative health improvements in 5-year diabetes reversal study. June 5, 2022. Accessed October 6, 2023. https://www.virtahealth.com/blog/virta-sustainable-health-improvements-5-year-diabetes-reversal-study
49. Wan Z, Shan Z, Geng T, et al. Associations of moderate low-carbohydrate diets with mortality among patients with type 2 diabetes: a prospective cohort study. J Clin Endocrinol Metab. 2022;107(7):E2702-E2709. doi:10.1210/CLINEM/DGAC235
50. Akter S, Mizoue T, Nanri A, et al. Low carbohydrate diet and all cause and cause-specific mortality. Clin Nutr. 2021;40(4):2016-2024. doi:10.1016/J.CLNU.2020.09.022
51. Shan Z, Guo Y, Hu FB, Liu L, Qi Q. Association of low-carbohydrate and low-fat diets with mortality among US adults. JAMA Intern Med. 2020;180(4):513-523. doi:10.1001/JAMAINTERNMED.2019.6980
1. Centers for Disease Control and Prevention. Prevalence of Both Diagnosed and Undiagnosed Diabetes. Updated September 30, 2022. Accessed October 6, 2023. https://www.cdc.gov/diabetes/data/statistics-report/diagnosed-undiagnosed-diabetes.html
2. Centers for Disease Control and Prevention. Diabetes and Prediabetes. Updated September 6, 2022. Accessed October 6, 2023. https://www.cdc.gov/chronicdisease/resources/publications/factsheets/diabetes-prediabetes.htm 3. US Department of Veterans Affairs. Diabetes information - Nutrition and food services. Updated May 4, 2023. Accessed October 6, 2023. https://www.nutrition.va.gov/diabetes.asp
4. US Department of Veterans Affairs. Management of Type 2 Diabetes Mellitus (2023) - VA/DoD Clinical Practice Guidelines. Updated September 1, 2023. Accessed October 6, 2023. https://www.healthquality.va.gov/guidelines/CD/diabetes/
5. American Diabetes Association. Economic Costs of Diabetes in the U.S. in 2017. Diabetes Care. 2018;41(5):917-928. doi:10.2337/dci18-0007
6. Home P, Riddle M, Cefalu WT, et al. Insulin therapy in people with type 2 diabetes: opportunities and challenges?. Diabetes Care. 2014;37(6):1499-1508. doi:10.2337/dc13-2743
7. Donath MY, Ehses JA, Maedler K, et al. Mechanisms of β-cell death in type 2 diabetes. Diabetes. 2005;54(suppl 2):S108-S113. doi:10.2337/DIABETES.54.SUPPL_2.S108
8. Hallberg SJ, Gershuni VM, Hazbun TL, Athinarayanan SJ. Reversing type 2 diabetes: a narrative review of the evidence. Nutrients. 2019;11(4):766. Published 2019 Apr 1. doi:10.3390/nu11040766
9. Davies MJ, D’Alessio DA, Fradkin J, et al. Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41(12):2669. doi:10.2337/DCI18-0033
10. Evert AB, Dennison M, Gardner CD, et al. Nutrition therapy for adults with diabetes or prediabetes: a consensus report. Diabetes Care. 2019;42(5):731-754. doi:10.2337/DCI19-0014
11. Diabetes Canada position statement on low-carbohydrate diets for adults with diabetes: a rapid review. Can J Diabetes. 2020;44(4):295-299. doi:10.1016/J.JCJD.2020.04.001
12. Diabetes Australia. Position statements. Accessed October 6, 2023. https://www.diabetesaustralia.com.au/research-advocacy/position-statements/
13. Feinman RD, Pogozelski WK, Astrup A, et al. Dietary carbohydrate restriction as the first approach in diabetes management: critical review and evidence base. Nutrition. 2014;31(1):1-13. doi:10.1016/j.nut.2014.06.011
14. Samaha FF, Iqbal N, Seshadri P, et al. A low-carbohydrate as compared with a low-fat diet in severe obesity. N Engl J Med. 2003;348(21):2074-2081. doi:10.1056/NEJMOA02263715. Westman EC, Yancy WS, Mavropoulos JC, Marquart M, McDuffie JR. The effect of a low-carbohydrate, ketogenic diet versus a low-glycemic index diet on glycemic control in type 2 diabetes mellitus. Nutr Metab (Lond). 2008;5(1):36. doi:10.1186/1743-7075-5-36
16. Saslow LR, Mason AE, Kim S, et al. An online intervention comparing a very low-carbohydrate ketogenic diet and lifestyle recommendations versus a plate method diet in overweight individuals with type 2 diabetes: a randomized controlled trial. J Med Internet Res. 2017;19(2). doi:10.2196/JMIR.5806
17. Hallberg SJ, McKenzie AL, Williams PT, et al. Effectiveness and safety of a novel care model for the management of type 2 diabetes at 1 year: an open-label, non-randomized, controlled study. Diabetes Ther. 2018;9(2):583-612. doi:10.1007/S13300-018-0373-9
18. Gram-Kampmann EM, Hansen CD, Hugger MB, et al. Effects of a 6-month, low-carbohydrate diet on glycaemic control, body composition, and cardiovascular risk factors in patients with type 2 diabetes: An open-label randomized controlled trial. Diabetes Obes Metab. 2022;24(4):693-703. doi:10.1111/DOM.14633
19. Committee ADAPP. 5. Facilitating behavior change and well-being to improve health outcomes: standards of medical care in diabetes—2022. Diabetes Care. 2022;45(suppl 1):S60-S82. doi:10.2337/DC22-S005
20. Goldenberg JZ, Johnston BC. Low and very low carbohydrate diets for diabetes remission. BMJ. 2021;373:m4743. doi:10.1136/BMJ.N262

21. Jing T, Zhang S, Bai M, et al. Effect of dietary approaches on glycemic control in patients with type 2 diabetes: a systematic review with network meta-analysis of randomized trials. Nutrients. 2023;15(14):3156. doi:10.3390/nu15143156
22. Academy of Nutrition and Dietetics. Nutrition care manual. Accessed October 6, 2023. https://www.nutritioncaremanual.org/
23. Low carbohydrate and very low carbohydrate eating patterns in adults with diabetes. ShopDiabetes.org. Accessed August 5, 2022. https://shopdiabetes.org/products/low-carbohydrate-and-very-low-carbohydrate-eating-patterns-in-adults-with-diabetes-a-guide-for-health-care-providers
24. US Department of Veterans Affairs. Diabetes education - nutrition and food services. Published July 31, 2022. http://vaww.nutrition.va.gov/docs/pted/ModifiedKetogenicDiet.pdf [Source not verified]
25. US Department of Veterans Affairs, My HealtheVet. Lowdown on low-carb diets. Updated June 1, 2021. Accessed October 6, 2023. https://www.myhealth.va.gov/mhv-portal-web/ss20190724-low-carb-diet
26. Chang CR, Francois ME, Little JP. Restricting carbohydrates at breakfast is sufficient to reduce 24-hour exposure to postprandial hyperglycemia and improve glycemic variability. Am J Clin Nutr. 2019;109(5):1302-1309. doi:10.1093/AJCN/NQY261
27. Hall KD, Ayuketah A, Brychta R, et al. Ultra-processed diets cause excess calorie intake and weight gain: an inpatient randomized controlled trial of ad libitum food intake. Cell Metab. 2019;30(1):226. doi:10.1016/j.cmet.2019.05.020
28. Harvey CJ d. C, Schofield GM, Zinn C, Thornley S. Effects of differing levels of carbohydrate restriction on mood achievement of nutritional ketosis, and symptoms of carbohydrate withdrawal in healthy adults: a randomized clinical trial. Nutrition. 2019;67-68:100005. doi:10.1016/J.NUTX.2019.100005
29. Griauzde DH, Standafer Lopez K, Saslow LR, Richardson CR. A pragmatic approach to translating low- and very low-carbohydrate diets into clinical practice for patients with obesity and type 2 diabetes. Front Nutr. 2021;8:416. doi:10.3389/FNUT.2021.682137/BIBTEX
30. Westman EC, Tondt J, Maguire E, Yancy WS. Implementing a low-carbohydrate, ketogenic diet to manage type 2 diabetes mellitus. Expert Rev Endocrinol Metab. 2018;13(5):263-272. doi:10.1080/17446651.2018.1523713
31. Suyoto PST. Effect of low-carbohydrate diet on markers of renal function in patients with type 2 diabetes: a meta-analysis. Diabetes Metab Res Rev. 2018;34(7). doi:10.1002/DMRR.3032
32. Norwitz NG, Feldman D, Soto-Mota A, Kalayjian T, Ludwig DS. Elevated LDL cholesterol with a carbohydrate-restricted diet: evidence for a “lean mass hyper-responder” phenotype. Curr Dev Nutr. 2021;6(1). doi:10.1093/CDN/NZAB144
33. Murdoch C, Unwin D, Cavan D, Cucuzzella M, Patel M. Adapting diabetes medication for low carbohydrate management of type 2 diabetes: a practical guide. Br J Gen Pract. 2019;69(684):360-361. doi:10.3399/bjgp19X704525
34. Cucuzzella M, Riley K, Isaacs D. Adapting medication for type 2 diabetes to a low carbohydrate diet. Front Nutr. 2021;8:486. doi:10.3389/FNUT.2021.688540/BIBTEX
35. World Health Organization. Global report on diabetes. 2016. Accessed October 6, 2023. https://iris.who.int/bitstream/handle/10665/204871/9789241565257_eng.pdf?sequence=1
36. Riddle MC, Cefalu WT, Evans PH, et al. Consensus report: definition and interpretation of remission in type 2 diabetes. Diabetes Care. 2021;44(10):2438-2444. doi:10.2337/DCI21-0034
37. Diabetes Australia. Type 2 Diabetes remission position statement. 2021. Accessed October 6, 2023. https://www.diabetesaustralia.com.au/wp-content/uploads/2021_Diabetes-Australia-Position-Statement_Type-2-diabetes-remission_2.pdf
38. Martens T, Beck RW, Bailey R, et al. Effect of continuous glucose monitoring on glycemic control in patients with type 2 diabetes treated with basal insulin: a randomized clinical trial. JAMA. 2021;325(22):2262-2272. doi:10.1001/JAMA.2021.7444
39. Jackson MA, Ahmann A, Shah VN. Type 2 diabetes and the use of real-time continuous glucose monitoring. Diabetes Technol Ther. 2021;23(S1):S27-S34. doi:10.1089/DIA.2021.0007
40. Oser TK, Cucuzzella M, Stasinopoulos M, Moncrief M, McCall A, Cox DJ. An innovative, paradigm-shifting lifestyle intervention to reduce glucose excursions with the use of continuous glucose monitoring to educate, motivate, and activate adults with newly diagnosed type 2 diabetes: pilot feasibility study. JMIR Diabetes. 2022;7(1). doi:10.2196/34465
41. Światkiewicz I, Woźniak A, Taub PR. Time-restricted eating and metabolic syndrome: current status and future perspectives. Nutrients. 2021;13(1):221. doi:10.3390/NU13010221
42. Obermayer A, Tripolt NJ, Pferschy PN, et al. Efficacy and safety of intermittent fasting in people with insulin-treated type 2 diabetes (INTERFAST-2)—a randomized controlled trial. Diabetes Care. 2023;46(2):463-468. doi:10.2337/dc22-1622
43. American Diabetes Association. 5. Lifestyle management: standards of medical care in diabetes—2019. Diabetes Care. 2019;42(suppl 1):S46-S60. doi:10.2337/DC19-S005
44. Li S, Ding L, Xiao X. Comparing the efficacy and safety of low-carbohydrate diets with low-fat diets for type 2 diabetes mellitus patients: a systematic review and meta-analysis of randomized clinical trials. Int J Endocrinol. 2021;2021:8521756. Published 2021 Dec 6. doi:10.1155/2021/8521756
45. Choi JH, Kang JH, Chon S. Comprehensive understanding for application in Korean patients with type 2 diabetes mellitus of the consensus statement on carbohydrate-restricted diets by Korean Diabetes Association, Korean Society for the Study of Obesity, and Korean Society of Hypertension. Diabetes Metab J. 2022;46(3):377. doi:10.4093/DMJ.2022.0051
46. Jayedi A, Zeraattalab-Motlagh S, Jabbarzadeh B, et al. Dose-dependent effect of carbohydrate restriction for type 2 diabetes management: a systematic review and dose-response meta-analysis of randomized controlled trials. Am J Clin Nutr. 2022;116(1). doi:10.1093/AJCN/NQAC066
47. Strombotne KL, Lum J, Ndugga NJ, et al. Effectiveness of a ketogenic diet and virtual coaching intervention for patients with diabetes: a difference-in-differences analysis. Diabetes Obes Metab. 2021;23(12):2643-2650. doi:10.1111/DOM.14515
48. Virta Health. Virta Health highlights lasting, transformative health improvements in 5-year diabetes reversal study. June 5, 2022. Accessed October 6, 2023. https://www.virtahealth.com/blog/virta-sustainable-health-improvements-5-year-diabetes-reversal-study
49. Wan Z, Shan Z, Geng T, et al. Associations of moderate low-carbohydrate diets with mortality among patients with type 2 diabetes: a prospective cohort study. J Clin Endocrinol Metab. 2022;107(7):E2702-E2709. doi:10.1210/CLINEM/DGAC235
50. Akter S, Mizoue T, Nanri A, et al. Low carbohydrate diet and all cause and cause-specific mortality. Clin Nutr. 2021;40(4):2016-2024. doi:10.1016/J.CLNU.2020.09.022
51. Shan Z, Guo Y, Hu FB, Liu L, Qi Q. Association of low-carbohydrate and low-fat diets with mortality among US adults. JAMA Intern Med. 2020;180(4):513-523. doi:10.1001/JAMAINTERNMED.2019.6980
Chronicling Health Care Transformation: Federal Practitioner Looks Back 40 Years
When VA Practitioner published its first issue in January 1984, federal health care was at the cusp of a dramatic transformation. VA Practitioner stepped in to serve “as a forum, as a bulletin, as an easy means of communication with colleagues who share your unique concerns,” founding editor James McCloskey noted in the first issue.
The need for this forum was most acute at the US Department of Veterans Affairs (VA). The agency of about 200,000 employees was decentralizing its management, developing the first electronic health record system, and caring for an aging population of World War II and Vietnam War era veterans with high comorbidity burdens. In the 1980s, the VA was at a nadir and under increasing pressure to change. At that moment of challenge, VA Practitioner offered columns suggesting a way forward and focused on clinical improvements with articles like, “The ghosts of budgets past,” “Psychoenvironment: a therapeutic redesign plan,” and “The VA’s geriatric goals.” Within a few years, the journal had enlisted an editorial advisory board to help guide the journal and provide the first peer review process for articles.
Peer Review and Expanded Focus
Ten years later, tremendous changes were underway for both VA Practitioner and the VA. Ken Kizer, MD, MPH, was named Under Secretary of Health in 1994 and almost immediately started the massive process of reforming and reorganizing the VA’s health care arm: Veterans Health Administration (VHA). The VHA would expand from 2.7 million enrolled veteran patients in 1993 to 8.9 million in 2014. In the process, the VA transformed from an oft derided institution to a major source of research and care that hosted most US physician residents while delivering the “best care anywhere.”
In 1994, VA Practitioner changed its name, becoming Federal Practitioner with an expanded mandate to address the needs of US Department of Defense (DoD) and US Public Health Service (PHS) clinicians working at the Indian Health Service (IHS), Bureau of Prisons, and US Coast Guard. In addition, the journal instituted a double-blind peer review process. Health care reform was clearly on the agenda for the new journal.
A new vision for VHA sought to redistribute resources, decentralize decision making, and make care more patient centered. The VHA began development of the Computerized Patient Record System (CPRS), which was fully implemented by 1999 as one of the earliest electronic health record systems and shared it with the IHS.
The DoD, on the other hand, was in a long-term period of reduction and consolidation. The active-duty service member population dropped from 2.1 million to 1.6 million between 1984 and 1994 and would continue to drop to 1.4 million in 2001, even with the onset of the first Gulf War. The DoD rolled out the Civilian Health and Medical Program of the United States (CHAMPUS), which would later become TRICARE, that reshaped the way the DoD delivered health care for active-duty service members, their families, and retirees.
From the outset, Federal Practitioner sought to play a role in those transformations. For PHS officers stationed across the Centers for Disease Control and Prevention, US Food and Drug Administration, IHS, and Bureau of Prisons, the journal provided a new way to share findings and best practices. With a growing group of dedicated peer reviewers, Federal Practitioner articles became more clinical and more patient centered. Frequent columns gave way to clinical reviews, continuing medical education, and best practice articles.
Addressing Post-9/11 Veteran Needs
All of these changes were well under way on the eve of September 11, 2001. After years of reductions, the size of the military stabilized, but the demographics were shifting in important ways. Women made up a larger proportion of the active-duty population, growing from 5% in 1975 to 10% in 1985 and 14% in 2005. The military was also becoming more diverse, with a growing number of service members indicating Hispanic, Asian, Pacific Islander, and other identities. More importantly, a new set of health care concerns emerged to challenge DoD and VHA clinicians. A growing number of service members and veterans of the Gulf Wars were seeking care for respiratory diseases, cancers, blast injuries, and prosthetics.
Federal Practitioner articles primarily focused on quality improvement but increasingly the journal published original research and case studies. Columns like Common Errors in Internal Medicine and Advances in Geriatrics focused on quality improvement and innovative therapies, respectively. To supplement its 12 regular issues, in 2011 Federal Practitioner began publishing special issues to provide even more depth of coverage in specific disease states, including hematology/oncology (in cooperation with the Association of VA Hematology/Oncology), mental health, neurology, infectious diseases, diabetes, among other topics.
The Last 10 Years and the Next 40
In 2013, the DoD formally reorganized its health care operations under the Defense Health Agency, starting an entirely new process that would dramatically reshape health care delivery for 8 million beneficiaries and 140,000 employees. This started a long process of consolidating separate systems and priorities for each branch into a single approach. Meanwhile, controversies around long wait times for VHA appointments (and veterans who died while waiting) put it under intense scrutiny. Legislation to privatize some or all of health care for veterans were discussed and considered, which finally resulted in the creation of the Veterans Choice Program, which greatly expanded the use of private health care services for covered conditions.
In 2018, Federal Practitioner was accepted by the national Library of Medicine’s PubMed Central, ensuring the widest possible access to journal articles. The journal saw a steady growth in submissions and published a combined 21 regular and special issues that year driven by increased submissions and more original research studies.
More and more through the work of its authors, Federal Practitioner has been in the middle of critical and ongoing federal health care concerns. Federal Practitioner authors have turned to the journal to address issues ranging from the deprescribing of opioid medications to measures taken to decrease the incidence of veteran suicide and the challenges presented by artificial intelligence and telehealth delivery. Whether it was the federal responses to Ebola outbreaks in Africa or the myriad ways that the PHS and VA responded to the COVID-19 pandemic in the US, Federal Practitioner has been at the center of federal health care.
Further reading
To learn more about the past 40 years of federal health care visit mdedge.com/fedprac or doi:10.12788/fp.0453.
When VA Practitioner published its first issue in January 1984, federal health care was at the cusp of a dramatic transformation. VA Practitioner stepped in to serve “as a forum, as a bulletin, as an easy means of communication with colleagues who share your unique concerns,” founding editor James McCloskey noted in the first issue.
The need for this forum was most acute at the US Department of Veterans Affairs (VA). The agency of about 200,000 employees was decentralizing its management, developing the first electronic health record system, and caring for an aging population of World War II and Vietnam War era veterans with high comorbidity burdens. In the 1980s, the VA was at a nadir and under increasing pressure to change. At that moment of challenge, VA Practitioner offered columns suggesting a way forward and focused on clinical improvements with articles like, “The ghosts of budgets past,” “Psychoenvironment: a therapeutic redesign plan,” and “The VA’s geriatric goals.” Within a few years, the journal had enlisted an editorial advisory board to help guide the journal and provide the first peer review process for articles.
Peer Review and Expanded Focus
Ten years later, tremendous changes were underway for both VA Practitioner and the VA. Ken Kizer, MD, MPH, was named Under Secretary of Health in 1994 and almost immediately started the massive process of reforming and reorganizing the VA’s health care arm: Veterans Health Administration (VHA). The VHA would expand from 2.7 million enrolled veteran patients in 1993 to 8.9 million in 2014. In the process, the VA transformed from an oft derided institution to a major source of research and care that hosted most US physician residents while delivering the “best care anywhere.”
In 1994, VA Practitioner changed its name, becoming Federal Practitioner with an expanded mandate to address the needs of US Department of Defense (DoD) and US Public Health Service (PHS) clinicians working at the Indian Health Service (IHS), Bureau of Prisons, and US Coast Guard. In addition, the journal instituted a double-blind peer review process. Health care reform was clearly on the agenda for the new journal.
A new vision for VHA sought to redistribute resources, decentralize decision making, and make care more patient centered. The VHA began development of the Computerized Patient Record System (CPRS), which was fully implemented by 1999 as one of the earliest electronic health record systems and shared it with the IHS.
The DoD, on the other hand, was in a long-term period of reduction and consolidation. The active-duty service member population dropped from 2.1 million to 1.6 million between 1984 and 1994 and would continue to drop to 1.4 million in 2001, even with the onset of the first Gulf War. The DoD rolled out the Civilian Health and Medical Program of the United States (CHAMPUS), which would later become TRICARE, that reshaped the way the DoD delivered health care for active-duty service members, their families, and retirees.
From the outset, Federal Practitioner sought to play a role in those transformations. For PHS officers stationed across the Centers for Disease Control and Prevention, US Food and Drug Administration, IHS, and Bureau of Prisons, the journal provided a new way to share findings and best practices. With a growing group of dedicated peer reviewers, Federal Practitioner articles became more clinical and more patient centered. Frequent columns gave way to clinical reviews, continuing medical education, and best practice articles.
Addressing Post-9/11 Veteran Needs
All of these changes were well under way on the eve of September 11, 2001. After years of reductions, the size of the military stabilized, but the demographics were shifting in important ways. Women made up a larger proportion of the active-duty population, growing from 5% in 1975 to 10% in 1985 and 14% in 2005. The military was also becoming more diverse, with a growing number of service members indicating Hispanic, Asian, Pacific Islander, and other identities. More importantly, a new set of health care concerns emerged to challenge DoD and VHA clinicians. A growing number of service members and veterans of the Gulf Wars were seeking care for respiratory diseases, cancers, blast injuries, and prosthetics.
Federal Practitioner articles primarily focused on quality improvement but increasingly the journal published original research and case studies. Columns like Common Errors in Internal Medicine and Advances in Geriatrics focused on quality improvement and innovative therapies, respectively. To supplement its 12 regular issues, in 2011 Federal Practitioner began publishing special issues to provide even more depth of coverage in specific disease states, including hematology/oncology (in cooperation with the Association of VA Hematology/Oncology), mental health, neurology, infectious diseases, diabetes, among other topics.
The Last 10 Years and the Next 40
In 2013, the DoD formally reorganized its health care operations under the Defense Health Agency, starting an entirely new process that would dramatically reshape health care delivery for 8 million beneficiaries and 140,000 employees. This started a long process of consolidating separate systems and priorities for each branch into a single approach. Meanwhile, controversies around long wait times for VHA appointments (and veterans who died while waiting) put it under intense scrutiny. Legislation to privatize some or all of health care for veterans were discussed and considered, which finally resulted in the creation of the Veterans Choice Program, which greatly expanded the use of private health care services for covered conditions.
In 2018, Federal Practitioner was accepted by the national Library of Medicine’s PubMed Central, ensuring the widest possible access to journal articles. The journal saw a steady growth in submissions and published a combined 21 regular and special issues that year driven by increased submissions and more original research studies.
More and more through the work of its authors, Federal Practitioner has been in the middle of critical and ongoing federal health care concerns. Federal Practitioner authors have turned to the journal to address issues ranging from the deprescribing of opioid medications to measures taken to decrease the incidence of veteran suicide and the challenges presented by artificial intelligence and telehealth delivery. Whether it was the federal responses to Ebola outbreaks in Africa or the myriad ways that the PHS and VA responded to the COVID-19 pandemic in the US, Federal Practitioner has been at the center of federal health care.
Further reading
To learn more about the past 40 years of federal health care visit mdedge.com/fedprac or doi:10.12788/fp.0453.
When VA Practitioner published its first issue in January 1984, federal health care was at the cusp of a dramatic transformation. VA Practitioner stepped in to serve “as a forum, as a bulletin, as an easy means of communication with colleagues who share your unique concerns,” founding editor James McCloskey noted in the first issue.
The need for this forum was most acute at the US Department of Veterans Affairs (VA). The agency of about 200,000 employees was decentralizing its management, developing the first electronic health record system, and caring for an aging population of World War II and Vietnam War era veterans with high comorbidity burdens. In the 1980s, the VA was at a nadir and under increasing pressure to change. At that moment of challenge, VA Practitioner offered columns suggesting a way forward and focused on clinical improvements with articles like, “The ghosts of budgets past,” “Psychoenvironment: a therapeutic redesign plan,” and “The VA’s geriatric goals.” Within a few years, the journal had enlisted an editorial advisory board to help guide the journal and provide the first peer review process for articles.
Peer Review and Expanded Focus
Ten years later, tremendous changes were underway for both VA Practitioner and the VA. Ken Kizer, MD, MPH, was named Under Secretary of Health in 1994 and almost immediately started the massive process of reforming and reorganizing the VA’s health care arm: Veterans Health Administration (VHA). The VHA would expand from 2.7 million enrolled veteran patients in 1993 to 8.9 million in 2014. In the process, the VA transformed from an oft derided institution to a major source of research and care that hosted most US physician residents while delivering the “best care anywhere.”
In 1994, VA Practitioner changed its name, becoming Federal Practitioner with an expanded mandate to address the needs of US Department of Defense (DoD) and US Public Health Service (PHS) clinicians working at the Indian Health Service (IHS), Bureau of Prisons, and US Coast Guard. In addition, the journal instituted a double-blind peer review process. Health care reform was clearly on the agenda for the new journal.
A new vision for VHA sought to redistribute resources, decentralize decision making, and make care more patient centered. The VHA began development of the Computerized Patient Record System (CPRS), which was fully implemented by 1999 as one of the earliest electronic health record systems and shared it with the IHS.
The DoD, on the other hand, was in a long-term period of reduction and consolidation. The active-duty service member population dropped from 2.1 million to 1.6 million between 1984 and 1994 and would continue to drop to 1.4 million in 2001, even with the onset of the first Gulf War. The DoD rolled out the Civilian Health and Medical Program of the United States (CHAMPUS), which would later become TRICARE, that reshaped the way the DoD delivered health care for active-duty service members, their families, and retirees.
From the outset, Federal Practitioner sought to play a role in those transformations. For PHS officers stationed across the Centers for Disease Control and Prevention, US Food and Drug Administration, IHS, and Bureau of Prisons, the journal provided a new way to share findings and best practices. With a growing group of dedicated peer reviewers, Federal Practitioner articles became more clinical and more patient centered. Frequent columns gave way to clinical reviews, continuing medical education, and best practice articles.
Addressing Post-9/11 Veteran Needs
All of these changes were well under way on the eve of September 11, 2001. After years of reductions, the size of the military stabilized, but the demographics were shifting in important ways. Women made up a larger proportion of the active-duty population, growing from 5% in 1975 to 10% in 1985 and 14% in 2005. The military was also becoming more diverse, with a growing number of service members indicating Hispanic, Asian, Pacific Islander, and other identities. More importantly, a new set of health care concerns emerged to challenge DoD and VHA clinicians. A growing number of service members and veterans of the Gulf Wars were seeking care for respiratory diseases, cancers, blast injuries, and prosthetics.
Federal Practitioner articles primarily focused on quality improvement but increasingly the journal published original research and case studies. Columns like Common Errors in Internal Medicine and Advances in Geriatrics focused on quality improvement and innovative therapies, respectively. To supplement its 12 regular issues, in 2011 Federal Practitioner began publishing special issues to provide even more depth of coverage in specific disease states, including hematology/oncology (in cooperation with the Association of VA Hematology/Oncology), mental health, neurology, infectious diseases, diabetes, among other topics.
The Last 10 Years and the Next 40
In 2013, the DoD formally reorganized its health care operations under the Defense Health Agency, starting an entirely new process that would dramatically reshape health care delivery for 8 million beneficiaries and 140,000 employees. This started a long process of consolidating separate systems and priorities for each branch into a single approach. Meanwhile, controversies around long wait times for VHA appointments (and veterans who died while waiting) put it under intense scrutiny. Legislation to privatize some or all of health care for veterans were discussed and considered, which finally resulted in the creation of the Veterans Choice Program, which greatly expanded the use of private health care services for covered conditions.
In 2018, Federal Practitioner was accepted by the national Library of Medicine’s PubMed Central, ensuring the widest possible access to journal articles. The journal saw a steady growth in submissions and published a combined 21 regular and special issues that year driven by increased submissions and more original research studies.
More and more through the work of its authors, Federal Practitioner has been in the middle of critical and ongoing federal health care concerns. Federal Practitioner authors have turned to the journal to address issues ranging from the deprescribing of opioid medications to measures taken to decrease the incidence of veteran suicide and the challenges presented by artificial intelligence and telehealth delivery. Whether it was the federal responses to Ebola outbreaks in Africa or the myriad ways that the PHS and VA responded to the COVID-19 pandemic in the US, Federal Practitioner has been at the center of federal health care.
Further reading
To learn more about the past 40 years of federal health care visit mdedge.com/fedprac or doi:10.12788/fp.0453.
Nasal Tanning Sprays: Illuminating the Risks of a Popular TikTok Trend
Nasal tanning spray is a recent phenomenon that has been gaining popularity among consumers on TikTok and other social media platforms. The active ingredient in the tanning spray is melanotan II—a synthetic analog of α‒melanocyte-stimulating hormone,1,2 a naturally occurring hormone responsible for skin pigmentation. α‒Melanocyte-stimulating hormone is a derivative of the precursor proopiomelanocortin, an agonist on the melanocortin-1 receptor that promotes formation of eumelanin.1,3 Eumelanin then provides pigmentation to the skin.3 Apart from its use for tanning, melanotan II has been reported to increase sexual function and aid in weight loss.1
Melanotan II is not approved by the US Food and Drug Administration; however, injectable formulations can be obtained illegally on the Internet as well as at some tanning salons and beauty parlors.4 Although injectable forms of melanotan II have been used for years to artificially increase skin pigmentation, the newly hyped nasal tanning sprays are drawing the attention of consumers. The synthetic chemical spray is inhaled into the nasal mucosae, where it is readily absorbed into the bloodstream to act on melanocortin receptors throughout the body, thus enhancing skin pigmentation.2 Because melanotan II is not approved, there is no guarantee that the product purchased from those sources is pure; therefore, consumers risk inhaling or injecting contaminated chemicals.5
In a 2017 study, Kirk and Greenfield6 cited self-image as a common concern among participants who expressed a preference for appearing tanned.6 Societal influence and standards to which young adults, particularly young women, often are accustomed drive some to take steps to achieve tanned skin, which they view as more attractive and healthier than untanned skin.7,8
Social media consumption is a significant risk factor for developing or exacerbating body dissatisfaction among impressionable teenagers and young adults, who may be less risk averse and therefore choose to embrace trends such as nasal tanning sprays to enhance their appearance, without considering possible consequences. Most young adults, and even teens, are aware of the risks associated with tanning beds, which may propel them to seek out what they perceive as a less-risky tanning alternative such as a tanner delivered via a nasal route, but it is unlikely that this group is fully informed about the possible dangers of nasal tanning sprays.
It is crucial for dermatologists and other clinicians to provide awareness and education about the potential harm of nasal tanning sprays. Along with the general risks of using an unregulated substance, common adverse effects include acne, facial flushing, gastrointestinal tract upset, and sensitivity to sunlight (Table).1,9,10 Several case reports have linked melanotan II to cutaneous changes, including dysplastic nevi and even melanoma.1 Less common complications, such as renal infarction and priapism, also have been observed with melanotan II use.9,10

Even with the known risks involving tanning beds and skin cancer, an analysis by Kream et al11 in 2020 showed that 90% (441/488) of tanning-related videos on TikTok promoted a positive view of tanning. Of these TikTok videos involving pro-tanning trends, 3% (12/441) were specifically about melanotan II nasal spray, injection, or both, which has only become more popular since this study was published.11
Dermatologists should be aware of the impact that tanning trends, such as nasal tanning spray, can have on all patients and initiate discussions regarding the risks of using these products with patients as appropriate. Alternatives to nasal tanning sprays such as spray-on tans and self-tanning lotions are safer ways for patients to achieve a tanned look without the health risks associated with melanotan II.
- Habbema L, Halk AB, Neumann M, et al. Risks of unregulated use of alpha-melanocyte-stimulating hormone analogues: a review. Int J Dermatol. 2017;56:975-980. doi:10.1111/ijd.13585
- Why you should never use nasal tanning spray. Cleveland Clinic Health Essentials [Internet]. November 1, 2022. Accessed December 18, 2023. https://health.clevelandclinic.org/nasal-tanning-spray
- Hjuler KF, Lorentzen HF. Melanoma associated with the use of melanotan-II. Dermatology. 2014;228:34-36. doi:10.1159/000356389
- Evans-Brown M, Dawson RT, Chandler M, et al. Use of melanotan I and II in the general population. BMJ. 2009;338:b566. doi:10.116/bmj.b566
- Callaghan DJ III. A glimpse into the underground market of melanotan. Dermatol Online J. 2018;24:1-5. doi:10.5070/D3245040036
- Kirk L, Greenfield S. Knowledge and attitudes of UK university students in relation to ultraviolet radiation (UVR) exposure and their sun-related behaviours: a qualitative study. BMJ Open. 2017;7:e014388. doi:10.1136/bmjopen-2016-014388
- Hay JL, Geller AC, Schoenhammer M, et al. Tanning and beauty: mother and teenage daughters in discussion. J Health Psychol. 2016;21:1261-1270. doi:10.1177/1359105314551621
- Gillen MM, Markey CN. The role of body image and depression in tanning behaviors and attitudes. Behav Med. 2017;38:74-82.
- Peters B, Hadimeri H, Wahlberg R, et al. Melanotan II: a possible cause of renal infarction: review of the literature and case report. CEN Case Rep. 2020;9:159-161. doi:10.1007/s13730-020-00447-z
- Mallory CW, Lopategui DM, Cordon BH. Melanotan tanning injection: a rare cause of priapism. Sex Med. 2021;9:100298. doi:10.1016/j.esxm.2020.100298
- Kream E, Watchmaker JD, Dover JS. TikTok sheds light on tanning: tanning is still popular and emerging trends pose new risks. Dermatol Surg. 2022;48:1018-1021. doi:10.1097/DSS.0000000000003549
Nasal tanning spray is a recent phenomenon that has been gaining popularity among consumers on TikTok and other social media platforms. The active ingredient in the tanning spray is melanotan II—a synthetic analog of α‒melanocyte-stimulating hormone,1,2 a naturally occurring hormone responsible for skin pigmentation. α‒Melanocyte-stimulating hormone is a derivative of the precursor proopiomelanocortin, an agonist on the melanocortin-1 receptor that promotes formation of eumelanin.1,3 Eumelanin then provides pigmentation to the skin.3 Apart from its use for tanning, melanotan II has been reported to increase sexual function and aid in weight loss.1
Melanotan II is not approved by the US Food and Drug Administration; however, injectable formulations can be obtained illegally on the Internet as well as at some tanning salons and beauty parlors.4 Although injectable forms of melanotan II have been used for years to artificially increase skin pigmentation, the newly hyped nasal tanning sprays are drawing the attention of consumers. The synthetic chemical spray is inhaled into the nasal mucosae, where it is readily absorbed into the bloodstream to act on melanocortin receptors throughout the body, thus enhancing skin pigmentation.2 Because melanotan II is not approved, there is no guarantee that the product purchased from those sources is pure; therefore, consumers risk inhaling or injecting contaminated chemicals.5
In a 2017 study, Kirk and Greenfield6 cited self-image as a common concern among participants who expressed a preference for appearing tanned.6 Societal influence and standards to which young adults, particularly young women, often are accustomed drive some to take steps to achieve tanned skin, which they view as more attractive and healthier than untanned skin.7,8
Social media consumption is a significant risk factor for developing or exacerbating body dissatisfaction among impressionable teenagers and young adults, who may be less risk averse and therefore choose to embrace trends such as nasal tanning sprays to enhance their appearance, without considering possible consequences. Most young adults, and even teens, are aware of the risks associated with tanning beds, which may propel them to seek out what they perceive as a less-risky tanning alternative such as a tanner delivered via a nasal route, but it is unlikely that this group is fully informed about the possible dangers of nasal tanning sprays.
It is crucial for dermatologists and other clinicians to provide awareness and education about the potential harm of nasal tanning sprays. Along with the general risks of using an unregulated substance, common adverse effects include acne, facial flushing, gastrointestinal tract upset, and sensitivity to sunlight (Table).1,9,10 Several case reports have linked melanotan II to cutaneous changes, including dysplastic nevi and even melanoma.1 Less common complications, such as renal infarction and priapism, also have been observed with melanotan II use.9,10

Even with the known risks involving tanning beds and skin cancer, an analysis by Kream et al11 in 2020 showed that 90% (441/488) of tanning-related videos on TikTok promoted a positive view of tanning. Of these TikTok videos involving pro-tanning trends, 3% (12/441) were specifically about melanotan II nasal spray, injection, or both, which has only become more popular since this study was published.11
Dermatologists should be aware of the impact that tanning trends, such as nasal tanning spray, can have on all patients and initiate discussions regarding the risks of using these products with patients as appropriate. Alternatives to nasal tanning sprays such as spray-on tans and self-tanning lotions are safer ways for patients to achieve a tanned look without the health risks associated with melanotan II.
Nasal tanning spray is a recent phenomenon that has been gaining popularity among consumers on TikTok and other social media platforms. The active ingredient in the tanning spray is melanotan II—a synthetic analog of α‒melanocyte-stimulating hormone,1,2 a naturally occurring hormone responsible for skin pigmentation. α‒Melanocyte-stimulating hormone is a derivative of the precursor proopiomelanocortin, an agonist on the melanocortin-1 receptor that promotes formation of eumelanin.1,3 Eumelanin then provides pigmentation to the skin.3 Apart from its use for tanning, melanotan II has been reported to increase sexual function and aid in weight loss.1
Melanotan II is not approved by the US Food and Drug Administration; however, injectable formulations can be obtained illegally on the Internet as well as at some tanning salons and beauty parlors.4 Although injectable forms of melanotan II have been used for years to artificially increase skin pigmentation, the newly hyped nasal tanning sprays are drawing the attention of consumers. The synthetic chemical spray is inhaled into the nasal mucosae, where it is readily absorbed into the bloodstream to act on melanocortin receptors throughout the body, thus enhancing skin pigmentation.2 Because melanotan II is not approved, there is no guarantee that the product purchased from those sources is pure; therefore, consumers risk inhaling or injecting contaminated chemicals.5
In a 2017 study, Kirk and Greenfield6 cited self-image as a common concern among participants who expressed a preference for appearing tanned.6 Societal influence and standards to which young adults, particularly young women, often are accustomed drive some to take steps to achieve tanned skin, which they view as more attractive and healthier than untanned skin.7,8
Social media consumption is a significant risk factor for developing or exacerbating body dissatisfaction among impressionable teenagers and young adults, who may be less risk averse and therefore choose to embrace trends such as nasal tanning sprays to enhance their appearance, without considering possible consequences. Most young adults, and even teens, are aware of the risks associated with tanning beds, which may propel them to seek out what they perceive as a less-risky tanning alternative such as a tanner delivered via a nasal route, but it is unlikely that this group is fully informed about the possible dangers of nasal tanning sprays.
It is crucial for dermatologists and other clinicians to provide awareness and education about the potential harm of nasal tanning sprays. Along with the general risks of using an unregulated substance, common adverse effects include acne, facial flushing, gastrointestinal tract upset, and sensitivity to sunlight (Table).1,9,10 Several case reports have linked melanotan II to cutaneous changes, including dysplastic nevi and even melanoma.1 Less common complications, such as renal infarction and priapism, also have been observed with melanotan II use.9,10

Even with the known risks involving tanning beds and skin cancer, an analysis by Kream et al11 in 2020 showed that 90% (441/488) of tanning-related videos on TikTok promoted a positive view of tanning. Of these TikTok videos involving pro-tanning trends, 3% (12/441) were specifically about melanotan II nasal spray, injection, or both, which has only become more popular since this study was published.11
Dermatologists should be aware of the impact that tanning trends, such as nasal tanning spray, can have on all patients and initiate discussions regarding the risks of using these products with patients as appropriate. Alternatives to nasal tanning sprays such as spray-on tans and self-tanning lotions are safer ways for patients to achieve a tanned look without the health risks associated with melanotan II.
- Habbema L, Halk AB, Neumann M, et al. Risks of unregulated use of alpha-melanocyte-stimulating hormone analogues: a review. Int J Dermatol. 2017;56:975-980. doi:10.1111/ijd.13585
- Why you should never use nasal tanning spray. Cleveland Clinic Health Essentials [Internet]. November 1, 2022. Accessed December 18, 2023. https://health.clevelandclinic.org/nasal-tanning-spray
- Hjuler KF, Lorentzen HF. Melanoma associated with the use of melanotan-II. Dermatology. 2014;228:34-36. doi:10.1159/000356389
- Evans-Brown M, Dawson RT, Chandler M, et al. Use of melanotan I and II in the general population. BMJ. 2009;338:b566. doi:10.116/bmj.b566
- Callaghan DJ III. A glimpse into the underground market of melanotan. Dermatol Online J. 2018;24:1-5. doi:10.5070/D3245040036
- Kirk L, Greenfield S. Knowledge and attitudes of UK university students in relation to ultraviolet radiation (UVR) exposure and their sun-related behaviours: a qualitative study. BMJ Open. 2017;7:e014388. doi:10.1136/bmjopen-2016-014388
- Hay JL, Geller AC, Schoenhammer M, et al. Tanning and beauty: mother and teenage daughters in discussion. J Health Psychol. 2016;21:1261-1270. doi:10.1177/1359105314551621
- Gillen MM, Markey CN. The role of body image and depression in tanning behaviors and attitudes. Behav Med. 2017;38:74-82.
- Peters B, Hadimeri H, Wahlberg R, et al. Melanotan II: a possible cause of renal infarction: review of the literature and case report. CEN Case Rep. 2020;9:159-161. doi:10.1007/s13730-020-00447-z
- Mallory CW, Lopategui DM, Cordon BH. Melanotan tanning injection: a rare cause of priapism. Sex Med. 2021;9:100298. doi:10.1016/j.esxm.2020.100298
- Kream E, Watchmaker JD, Dover JS. TikTok sheds light on tanning: tanning is still popular and emerging trends pose new risks. Dermatol Surg. 2022;48:1018-1021. doi:10.1097/DSS.0000000000003549
- Habbema L, Halk AB, Neumann M, et al. Risks of unregulated use of alpha-melanocyte-stimulating hormone analogues: a review. Int J Dermatol. 2017;56:975-980. doi:10.1111/ijd.13585
- Why you should never use nasal tanning spray. Cleveland Clinic Health Essentials [Internet]. November 1, 2022. Accessed December 18, 2023. https://health.clevelandclinic.org/nasal-tanning-spray
- Hjuler KF, Lorentzen HF. Melanoma associated with the use of melanotan-II. Dermatology. 2014;228:34-36. doi:10.1159/000356389
- Evans-Brown M, Dawson RT, Chandler M, et al. Use of melanotan I and II in the general population. BMJ. 2009;338:b566. doi:10.116/bmj.b566
- Callaghan DJ III. A glimpse into the underground market of melanotan. Dermatol Online J. 2018;24:1-5. doi:10.5070/D3245040036
- Kirk L, Greenfield S. Knowledge and attitudes of UK university students in relation to ultraviolet radiation (UVR) exposure and their sun-related behaviours: a qualitative study. BMJ Open. 2017;7:e014388. doi:10.1136/bmjopen-2016-014388
- Hay JL, Geller AC, Schoenhammer M, et al. Tanning and beauty: mother and teenage daughters in discussion. J Health Psychol. 2016;21:1261-1270. doi:10.1177/1359105314551621
- Gillen MM, Markey CN. The role of body image and depression in tanning behaviors and attitudes. Behav Med. 2017;38:74-82.
- Peters B, Hadimeri H, Wahlberg R, et al. Melanotan II: a possible cause of renal infarction: review of the literature and case report. CEN Case Rep. 2020;9:159-161. doi:10.1007/s13730-020-00447-z
- Mallory CW, Lopategui DM, Cordon BH. Melanotan tanning injection: a rare cause of priapism. Sex Med. 2021;9:100298. doi:10.1016/j.esxm.2020.100298
- Kream E, Watchmaker JD, Dover JS. TikTok sheds light on tanning: tanning is still popular and emerging trends pose new risks. Dermatol Surg. 2022;48:1018-1021. doi:10.1097/DSS.0000000000003549
PRACTICE POINTS
- Although tanning beds are arguably the most common and dangerous method used by patients to tan their skin, dermatologists should be aware of the other means by which patients may artificially increase skin pigmentation and the risks imposed by undertaking such practices.
- We challenge dermatologists to note the influence of social media on tanning trends and consider creating a platform on these mediums to combat misinformation and promote sun safety and skin health.
- We encourage dermatologists to diligently stay informed about the popular societal trends related to the skin such as the use of nasal tanning products (eg, melanotan I and II) and be proactive in discussing their risks with patients as deemed appropriate.
Migratory Nodules in a Traveler
The Diagnosis: Gnathostomiasis
The biopsy demonstrated a dense, eosinophilic, granulomatous infiltrate surrounding sections of a parasite with skeletal muscle bundles and intestines containing a brush border and luminal debris (Figure), which was consistent with a diagnosis of gnathostomiasis. Upon further questioning, he revealed that while in Peru he frequently consumed ceviche, which is a dish typically made from fresh raw fish cured in lemon or lime juice. He subsequently was treated with oral ivermectin 0.2 mg/kg once daily for 2 days with no evidence of recurrence 12 months later.
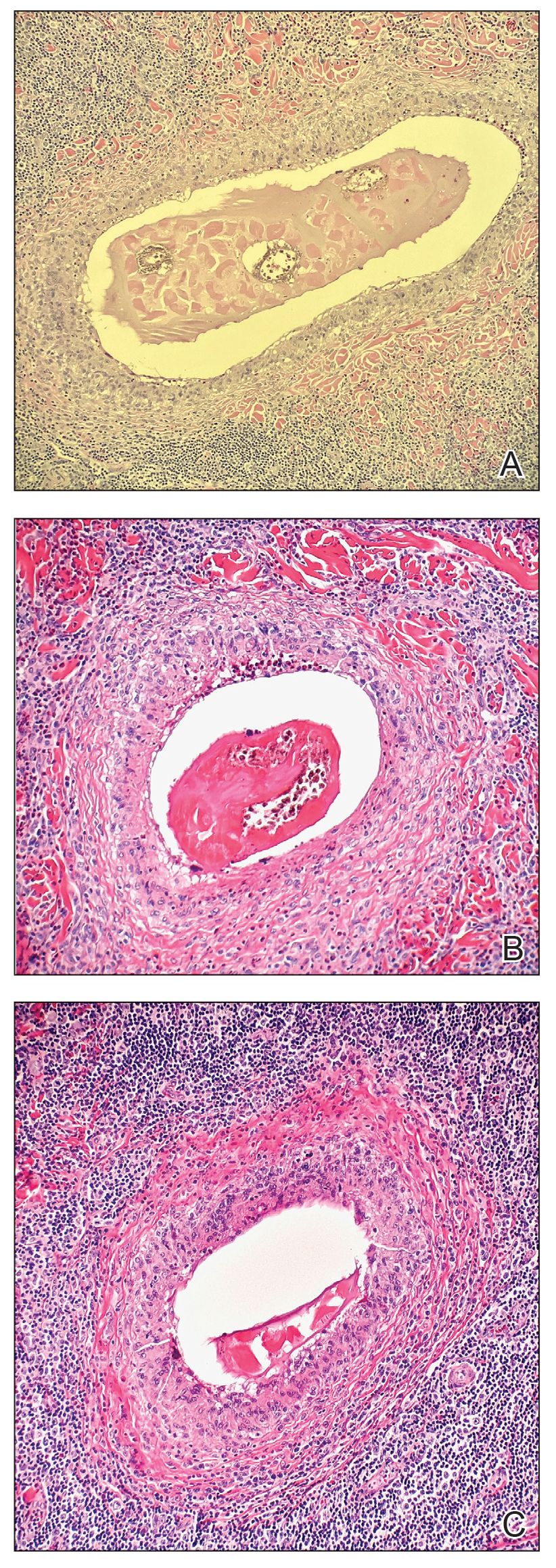
Cutaneous gnathostomiasis is the most common manifestation of infection caused by the third-stage larvae of the genus Gnathostoma. The nematode is endemic to tropical and subtropical regions of Japan and Southeast Asia, particularly Thailand. The disease has been increasingly observed in Central and South America. Humans can become infected through ingestion of undercooked meats, particularly freshwater fish but also poultry, snakes, or frogs. Few cases have been reported in North America and Europe presumably due to more stringent regulations governing the sourcing and storage of fish for consumption.1-3 Restaurants in endemic regions also may use cheaper local freshwater or brackish fish compared to restaurants in the West, which use more expensive saltwater fish that do not harbor Gnathostoma species.1 There is a false belief among restauranteurs and consumers that the larvae can be reliably killed by marinating meat in citrus juice or with concurrent consumption of alcohol or hot spices.2 Adequately cooking or freezing meat to 20 °C for 3 to 5 days are the only effective ways to ensure that the larvae are killed.1-3
The parasite requires its natural definitive hosts—fish-eating mammals such as pigs, cats, and dogs—to complete its life cycle and reproduce. Humans are accidental hosts in whom the parasite fails to reach sexual maturity.1-3 Consequently, symptoms commonly are due to the migration of only 1 larva, but occasionally infection with 2 or more has been observed.1,4
Human infection initially may result in malaise, fever, anorexia, abdominal pain, nausea, vomiting, and diarrhea as the parasite migrates through the stomach, intestines, and liver. After 2 to 4 weeks, larvae may reach the skin where they most commonly create ill-defined, erythematous, indurated, round or oval plaques or nodules described as nodular migratory panniculitis. These lesions tend to develop on the trunk or arms and correspond to the location of the migrating worm.1,3,5 The larvae have been observed to migrate at 1 cm/h.6 Symptoms often wax and wane, with individual nodules lasting approximately 1 to 2 weeks. Uniquely, larval migration can result in a trail of subcutaneous hemorrhage that is considered pathognomonic and helps to differentiate gnathostomiasis from other forms of parasitosis such as strongyloidiasis and sparganosis.1,3 Larvae are highly motile and invasive, and they are capable of producing a wide range of symptoms affecting virtually any part of the body.1,2 Depending on the anatomic location of the migrating worm, infection also may result in neurologic, gastrointestinal, pulmonary, or ocular symptoms.1-3,7 Eosinophilia is common but can subside in the chronic stage, as seen in our patient.1
The classic triad of intermittent migratory nodules, eosinophilia, and a history of travel to Southeast Asia or another endemic region should raise suspicion for gnathostomiasis.1-3,5,7 Unfortunately, confirmatory testing such as Gnathostoma serology is not readily available in the United States, and available serologic tests demonstrate frequent false positives and incomplete crossreactivity.1,2,8 Accordingly, the diagnosis most commonly is solidified by combining cardinal clinical features with histologic findings of a dense eosinophilic inflammatory infiltrate involving the dermis and hypodermis.2,5 In one study, the larva itself was only found in 12 of 66 (18%) skin biopsy specimens from patients with gnathostomiasis.5 If the larva is detected within the sections, it ranges from 2.5 to 12.5 mm in length and 0.4 to 1.2 mm in width and can exhibit cuticular spines, intestinal cells, and characteristic large lateral chords.1,5
The treatment of choice is surgical removal of the worm. Oral albendazole (400–800 mg/d for 21 days) also is considered a first-line treatment and results in clinical cure in approximately 90% of cases. Two doses of oral ivermectin (0.2 mg/kg) spaced 24 to 48 hours apart is an acceptable alternative with comparable efficacy.1-3 Care should be taken if involvement of the central nervous system is suspected, as antihelminthic treatment theoretically could be deleterious due to an inflammatory response to the dying larvae.1,2,9
In the differential diagnosis, loiasis can resemble gnathostomiasis, but the former is endemic to Africa.3 Cutaneous larva migrans most frequently is caused by hookworms from the genus Ancylostoma, which classically leads to superficial serpiginous linear plaques that migrate at a rate of several millimeters per day. However, the larvae are believed to lack the collagenase enzyme required to penetrate the epidermal basement membrane and thus are not capable of producing deep-seated nodules or visceral symptoms.3 Strongyloidiasis (larva currens) generally exhibits a more linear morphology, and infection would result in positive Strongyloides serology.7 Erythema nodosum is a septal panniculitis that can be triggered by infection, pregnancy, medications, connective tissue diseases, inflammatory conditions, and underlying malignancy.10
- Herman JS, Chiodini PL. Gnathostomiasis, another emerging imported disease. Clin Microbiol Rev. 2009;22:484-492.
- Liu GH, Sun MM, Elsheikha HM, et al. Human gnathostomiasis: a neglected food-borne zoonosis. Parasit Vectors. 2020;13:616.
- Tyring SK. Gnathostomiasis. In: Tyring SK, Lupi O, Hengge UR, eds. Tropical Dermatology. 2nd ed. Elsevier; 2017:77-78.
- Rusnak JM, Lucey DR. Clinical gnathostomiasis: case report and review of the English-language literature. Clin Infect Dis. 1993;16:33-50.
- Magaña M, Messina M, Bustamante F, et al. Gnathostomiasis: clinicopathologic study. Am J Dermatopathol. 2004;26:91-95.
- Chandenier J, Husson J, Canaple S, et al. Medullary gnathostomiasis in a white patient: use of immunodiagnosis and magnetic resonance imaging. Clin Infect Dis. 2001;32:E154-E157.
- Hamilton WL, Agranoff D. Imported gnathostomiasis manifesting as cutaneous larva migrans and Löffler’s syndrome. BMJ Case Rep. 2018;2018:bcr2017223132.
- Neumayr A, Ollague J, Bravo F, et al. Cross-reactivity pattern of Asian and American human gnathostomiasis in western blot assays using crude antigens prepared from Gnathostoma spinigerum and Gnathostoma binucleatum third-stage larvae. Am J Trop Med Hyg. 2016;95:413-416.
- Kraivichian K, Nuchprayoon S, Sitichalernchai P, et al. Treatment of cutaneous gnathostomiasis with ivermectin. Am J Trop Med Hyg. 2004;71:623-628.
- Pérez-Garza DM, Chavez-Alvarez S, Ocampo-Candiani J, et al. Erythema nodosum: a practical approach and diagnostic algorithm. Am J Clin Dermatol. 2021;22:367-378.
The Diagnosis: Gnathostomiasis
The biopsy demonstrated a dense, eosinophilic, granulomatous infiltrate surrounding sections of a parasite with skeletal muscle bundles and intestines containing a brush border and luminal debris (Figure), which was consistent with a diagnosis of gnathostomiasis. Upon further questioning, he revealed that while in Peru he frequently consumed ceviche, which is a dish typically made from fresh raw fish cured in lemon or lime juice. He subsequently was treated with oral ivermectin 0.2 mg/kg once daily for 2 days with no evidence of recurrence 12 months later.

Cutaneous gnathostomiasis is the most common manifestation of infection caused by the third-stage larvae of the genus Gnathostoma. The nematode is endemic to tropical and subtropical regions of Japan and Southeast Asia, particularly Thailand. The disease has been increasingly observed in Central and South America. Humans can become infected through ingestion of undercooked meats, particularly freshwater fish but also poultry, snakes, or frogs. Few cases have been reported in North America and Europe presumably due to more stringent regulations governing the sourcing and storage of fish for consumption.1-3 Restaurants in endemic regions also may use cheaper local freshwater or brackish fish compared to restaurants in the West, which use more expensive saltwater fish that do not harbor Gnathostoma species.1 There is a false belief among restauranteurs and consumers that the larvae can be reliably killed by marinating meat in citrus juice or with concurrent consumption of alcohol or hot spices.2 Adequately cooking or freezing meat to 20 °C for 3 to 5 days are the only effective ways to ensure that the larvae are killed.1-3
The parasite requires its natural definitive hosts—fish-eating mammals such as pigs, cats, and dogs—to complete its life cycle and reproduce. Humans are accidental hosts in whom the parasite fails to reach sexual maturity.1-3 Consequently, symptoms commonly are due to the migration of only 1 larva, but occasionally infection with 2 or more has been observed.1,4
Human infection initially may result in malaise, fever, anorexia, abdominal pain, nausea, vomiting, and diarrhea as the parasite migrates through the stomach, intestines, and liver. After 2 to 4 weeks, larvae may reach the skin where they most commonly create ill-defined, erythematous, indurated, round or oval plaques or nodules described as nodular migratory panniculitis. These lesions tend to develop on the trunk or arms and correspond to the location of the migrating worm.1,3,5 The larvae have been observed to migrate at 1 cm/h.6 Symptoms often wax and wane, with individual nodules lasting approximately 1 to 2 weeks. Uniquely, larval migration can result in a trail of subcutaneous hemorrhage that is considered pathognomonic and helps to differentiate gnathostomiasis from other forms of parasitosis such as strongyloidiasis and sparganosis.1,3 Larvae are highly motile and invasive, and they are capable of producing a wide range of symptoms affecting virtually any part of the body.1,2 Depending on the anatomic location of the migrating worm, infection also may result in neurologic, gastrointestinal, pulmonary, or ocular symptoms.1-3,7 Eosinophilia is common but can subside in the chronic stage, as seen in our patient.1
The classic triad of intermittent migratory nodules, eosinophilia, and a history of travel to Southeast Asia or another endemic region should raise suspicion for gnathostomiasis.1-3,5,7 Unfortunately, confirmatory testing such as Gnathostoma serology is not readily available in the United States, and available serologic tests demonstrate frequent false positives and incomplete crossreactivity.1,2,8 Accordingly, the diagnosis most commonly is solidified by combining cardinal clinical features with histologic findings of a dense eosinophilic inflammatory infiltrate involving the dermis and hypodermis.2,5 In one study, the larva itself was only found in 12 of 66 (18%) skin biopsy specimens from patients with gnathostomiasis.5 If the larva is detected within the sections, it ranges from 2.5 to 12.5 mm in length and 0.4 to 1.2 mm in width and can exhibit cuticular spines, intestinal cells, and characteristic large lateral chords.1,5
The treatment of choice is surgical removal of the worm. Oral albendazole (400–800 mg/d for 21 days) also is considered a first-line treatment and results in clinical cure in approximately 90% of cases. Two doses of oral ivermectin (0.2 mg/kg) spaced 24 to 48 hours apart is an acceptable alternative with comparable efficacy.1-3 Care should be taken if involvement of the central nervous system is suspected, as antihelminthic treatment theoretically could be deleterious due to an inflammatory response to the dying larvae.1,2,9
In the differential diagnosis, loiasis can resemble gnathostomiasis, but the former is endemic to Africa.3 Cutaneous larva migrans most frequently is caused by hookworms from the genus Ancylostoma, which classically leads to superficial serpiginous linear plaques that migrate at a rate of several millimeters per day. However, the larvae are believed to lack the collagenase enzyme required to penetrate the epidermal basement membrane and thus are not capable of producing deep-seated nodules or visceral symptoms.3 Strongyloidiasis (larva currens) generally exhibits a more linear morphology, and infection would result in positive Strongyloides serology.7 Erythema nodosum is a septal panniculitis that can be triggered by infection, pregnancy, medications, connective tissue diseases, inflammatory conditions, and underlying malignancy.10
The Diagnosis: Gnathostomiasis
The biopsy demonstrated a dense, eosinophilic, granulomatous infiltrate surrounding sections of a parasite with skeletal muscle bundles and intestines containing a brush border and luminal debris (Figure), which was consistent with a diagnosis of gnathostomiasis. Upon further questioning, he revealed that while in Peru he frequently consumed ceviche, which is a dish typically made from fresh raw fish cured in lemon or lime juice. He subsequently was treated with oral ivermectin 0.2 mg/kg once daily for 2 days with no evidence of recurrence 12 months later.

Cutaneous gnathostomiasis is the most common manifestation of infection caused by the third-stage larvae of the genus Gnathostoma. The nematode is endemic to tropical and subtropical regions of Japan and Southeast Asia, particularly Thailand. The disease has been increasingly observed in Central and South America. Humans can become infected through ingestion of undercooked meats, particularly freshwater fish but also poultry, snakes, or frogs. Few cases have been reported in North America and Europe presumably due to more stringent regulations governing the sourcing and storage of fish for consumption.1-3 Restaurants in endemic regions also may use cheaper local freshwater or brackish fish compared to restaurants in the West, which use more expensive saltwater fish that do not harbor Gnathostoma species.1 There is a false belief among restauranteurs and consumers that the larvae can be reliably killed by marinating meat in citrus juice or with concurrent consumption of alcohol or hot spices.2 Adequately cooking or freezing meat to 20 °C for 3 to 5 days are the only effective ways to ensure that the larvae are killed.1-3
The parasite requires its natural definitive hosts—fish-eating mammals such as pigs, cats, and dogs—to complete its life cycle and reproduce. Humans are accidental hosts in whom the parasite fails to reach sexual maturity.1-3 Consequently, symptoms commonly are due to the migration of only 1 larva, but occasionally infection with 2 or more has been observed.1,4
Human infection initially may result in malaise, fever, anorexia, abdominal pain, nausea, vomiting, and diarrhea as the parasite migrates through the stomach, intestines, and liver. After 2 to 4 weeks, larvae may reach the skin where they most commonly create ill-defined, erythematous, indurated, round or oval plaques or nodules described as nodular migratory panniculitis. These lesions tend to develop on the trunk or arms and correspond to the location of the migrating worm.1,3,5 The larvae have been observed to migrate at 1 cm/h.6 Symptoms often wax and wane, with individual nodules lasting approximately 1 to 2 weeks. Uniquely, larval migration can result in a trail of subcutaneous hemorrhage that is considered pathognomonic and helps to differentiate gnathostomiasis from other forms of parasitosis such as strongyloidiasis and sparganosis.1,3 Larvae are highly motile and invasive, and they are capable of producing a wide range of symptoms affecting virtually any part of the body.1,2 Depending on the anatomic location of the migrating worm, infection also may result in neurologic, gastrointestinal, pulmonary, or ocular symptoms.1-3,7 Eosinophilia is common but can subside in the chronic stage, as seen in our patient.1
The classic triad of intermittent migratory nodules, eosinophilia, and a history of travel to Southeast Asia or another endemic region should raise suspicion for gnathostomiasis.1-3,5,7 Unfortunately, confirmatory testing such as Gnathostoma serology is not readily available in the United States, and available serologic tests demonstrate frequent false positives and incomplete crossreactivity.1,2,8 Accordingly, the diagnosis most commonly is solidified by combining cardinal clinical features with histologic findings of a dense eosinophilic inflammatory infiltrate involving the dermis and hypodermis.2,5 In one study, the larva itself was only found in 12 of 66 (18%) skin biopsy specimens from patients with gnathostomiasis.5 If the larva is detected within the sections, it ranges from 2.5 to 12.5 mm in length and 0.4 to 1.2 mm in width and can exhibit cuticular spines, intestinal cells, and characteristic large lateral chords.1,5
The treatment of choice is surgical removal of the worm. Oral albendazole (400–800 mg/d for 21 days) also is considered a first-line treatment and results in clinical cure in approximately 90% of cases. Two doses of oral ivermectin (0.2 mg/kg) spaced 24 to 48 hours apart is an acceptable alternative with comparable efficacy.1-3 Care should be taken if involvement of the central nervous system is suspected, as antihelminthic treatment theoretically could be deleterious due to an inflammatory response to the dying larvae.1,2,9
In the differential diagnosis, loiasis can resemble gnathostomiasis, but the former is endemic to Africa.3 Cutaneous larva migrans most frequently is caused by hookworms from the genus Ancylostoma, which classically leads to superficial serpiginous linear plaques that migrate at a rate of several millimeters per day. However, the larvae are believed to lack the collagenase enzyme required to penetrate the epidermal basement membrane and thus are not capable of producing deep-seated nodules or visceral symptoms.3 Strongyloidiasis (larva currens) generally exhibits a more linear morphology, and infection would result in positive Strongyloides serology.7 Erythema nodosum is a septal panniculitis that can be triggered by infection, pregnancy, medications, connective tissue diseases, inflammatory conditions, and underlying malignancy.10
- Herman JS, Chiodini PL. Gnathostomiasis, another emerging imported disease. Clin Microbiol Rev. 2009;22:484-492.
- Liu GH, Sun MM, Elsheikha HM, et al. Human gnathostomiasis: a neglected food-borne zoonosis. Parasit Vectors. 2020;13:616.
- Tyring SK. Gnathostomiasis. In: Tyring SK, Lupi O, Hengge UR, eds. Tropical Dermatology. 2nd ed. Elsevier; 2017:77-78.
- Rusnak JM, Lucey DR. Clinical gnathostomiasis: case report and review of the English-language literature. Clin Infect Dis. 1993;16:33-50.
- Magaña M, Messina M, Bustamante F, et al. Gnathostomiasis: clinicopathologic study. Am J Dermatopathol. 2004;26:91-95.
- Chandenier J, Husson J, Canaple S, et al. Medullary gnathostomiasis in a white patient: use of immunodiagnosis and magnetic resonance imaging. Clin Infect Dis. 2001;32:E154-E157.
- Hamilton WL, Agranoff D. Imported gnathostomiasis manifesting as cutaneous larva migrans and Löffler’s syndrome. BMJ Case Rep. 2018;2018:bcr2017223132.
- Neumayr A, Ollague J, Bravo F, et al. Cross-reactivity pattern of Asian and American human gnathostomiasis in western blot assays using crude antigens prepared from Gnathostoma spinigerum and Gnathostoma binucleatum third-stage larvae. Am J Trop Med Hyg. 2016;95:413-416.
- Kraivichian K, Nuchprayoon S, Sitichalernchai P, et al. Treatment of cutaneous gnathostomiasis with ivermectin. Am J Trop Med Hyg. 2004;71:623-628.
- Pérez-Garza DM, Chavez-Alvarez S, Ocampo-Candiani J, et al. Erythema nodosum: a practical approach and diagnostic algorithm. Am J Clin Dermatol. 2021;22:367-378.
- Herman JS, Chiodini PL. Gnathostomiasis, another emerging imported disease. Clin Microbiol Rev. 2009;22:484-492.
- Liu GH, Sun MM, Elsheikha HM, et al. Human gnathostomiasis: a neglected food-borne zoonosis. Parasit Vectors. 2020;13:616.
- Tyring SK. Gnathostomiasis. In: Tyring SK, Lupi O, Hengge UR, eds. Tropical Dermatology. 2nd ed. Elsevier; 2017:77-78.
- Rusnak JM, Lucey DR. Clinical gnathostomiasis: case report and review of the English-language literature. Clin Infect Dis. 1993;16:33-50.
- Magaña M, Messina M, Bustamante F, et al. Gnathostomiasis: clinicopathologic study. Am J Dermatopathol. 2004;26:91-95.
- Chandenier J, Husson J, Canaple S, et al. Medullary gnathostomiasis in a white patient: use of immunodiagnosis and magnetic resonance imaging. Clin Infect Dis. 2001;32:E154-E157.
- Hamilton WL, Agranoff D. Imported gnathostomiasis manifesting as cutaneous larva migrans and Löffler’s syndrome. BMJ Case Rep. 2018;2018:bcr2017223132.
- Neumayr A, Ollague J, Bravo F, et al. Cross-reactivity pattern of Asian and American human gnathostomiasis in western blot assays using crude antigens prepared from Gnathostoma spinigerum and Gnathostoma binucleatum third-stage larvae. Am J Trop Med Hyg. 2016;95:413-416.
- Kraivichian K, Nuchprayoon S, Sitichalernchai P, et al. Treatment of cutaneous gnathostomiasis with ivermectin. Am J Trop Med Hyg. 2004;71:623-628.
- Pérez-Garza DM, Chavez-Alvarez S, Ocampo-Candiani J, et al. Erythema nodosum: a practical approach and diagnostic algorithm. Am J Clin Dermatol. 2021;22:367-378.
A 41-year-old man presented to a dermatology clinic in the United States with a migratory subcutaneous nodule overlying the left upper chest that initially developed 12 months prior and continued to migrate along the trunk and proximal aspect of the arms. The patient had spent the last 3 years residing in Peru. He never observed more than 1 nodule at a time and denied associated fever, headache, visual changes, chest pain, cough, abdominal pain, and diarrhea. Laboratory studies including a blood eosinophil count and serum Strongyloides immunoglobulins were within reference range. An excisional biopsy was performed.