User login
Commentary: Genetics, Juvenile PsA, and Weight Loss in PsA, September 2023
Although the usual age at onset of PsA is in the fourth or fifth decade of life, children may develop juvenile-onset PsA (JPsA). Less attention has been paid to this form of juvenile idiopathic arthritis (JIA), and the impact of JPsA vis-à-vis other forms of JIA is not well known. In addition, only about half of the patients with JPsA have cutaneous psoriasis. The impact of psoriasis on children with JPsA is not known. In order to evaluate differences in disease outcomes in patients with JPsA, Low and colleagues evaluated 1653 children and young people with JIA who were recruited to the Childhood Arthritis Prospective Study, of whom 111 had JPsA at diagnosis. They demonstrated that there were no significant differences in patient-reported outcomes between children with JPsA and other JIA categories. However, children with JPsA and psoriasis at JPsA diagnosis had more depressive symptoms compared with those without psoriasis. Moreover, children with JPsA vs other JIA categories had 2.35 times higher odds of having persistently poor well-being scores despite improvements in joint counts and physician global scores. Thus, children with JPsA have poorer well-being scores and a higher prevalence of depression, which requires multidisciplinary care.
Apart from immunomodulatory therapies, weight loss leads to improvement in disease activity in patients with obesity and PsA. However, the mechanisms by which weight loss improves PsA is currently not known, but is likely to be due to changes in adipokines and inflammation-related cytokines. In a recent study1that included patients with PsA and obesity, it was demonstrated that weight loss through a Very Low Energy Diet (VLED) resulted in significant improvements in PsA disease activity. Landgren and colleagues now aimed to determine the effects of VLED on cytokines and adipokines. They obtained blood samples from patients with PsA and obesity (n = 41) and matched control individuals without rheumatic disease or psoriasis (n = 39) who were on VLED. At month 6, along with significant weight loss, serum levels of interleukin-23 and leptin decreased significantly, while those of total adiponectin and high-molecular-weight adiponectin increased significantly in patients with PsA and control individuals. The change in body mass index correlated positively with a reduction in serum interleukin-23 (rS = 0.671, P < .001) and improvement in PsA disease activity (P = .003). This study highlights the anti-inflammatory effect of weight loss in patients with PsA. Weight loss can complement immunomodulatory therapy in PsA patients with obesity.
Additional Reference
- Klingberg E, Bilberg A, Björkman S, et al. Weight loss improves disease activity in patients with psoriatic arthritis and obesity: an interventional study. Arthritis Res Ther. 2019;21:17. doi: 10.1186/s13075-019-1810-5
Although the usual age at onset of PsA is in the fourth or fifth decade of life, children may develop juvenile-onset PsA (JPsA). Less attention has been paid to this form of juvenile idiopathic arthritis (JIA), and the impact of JPsA vis-à-vis other forms of JIA is not well known. In addition, only about half of the patients with JPsA have cutaneous psoriasis. The impact of psoriasis on children with JPsA is not known. In order to evaluate differences in disease outcomes in patients with JPsA, Low and colleagues evaluated 1653 children and young people with JIA who were recruited to the Childhood Arthritis Prospective Study, of whom 111 had JPsA at diagnosis. They demonstrated that there were no significant differences in patient-reported outcomes between children with JPsA and other JIA categories. However, children with JPsA and psoriasis at JPsA diagnosis had more depressive symptoms compared with those without psoriasis. Moreover, children with JPsA vs other JIA categories had 2.35 times higher odds of having persistently poor well-being scores despite improvements in joint counts and physician global scores. Thus, children with JPsA have poorer well-being scores and a higher prevalence of depression, which requires multidisciplinary care.
Apart from immunomodulatory therapies, weight loss leads to improvement in disease activity in patients with obesity and PsA. However, the mechanisms by which weight loss improves PsA is currently not known, but is likely to be due to changes in adipokines and inflammation-related cytokines. In a recent study1that included patients with PsA and obesity, it was demonstrated that weight loss through a Very Low Energy Diet (VLED) resulted in significant improvements in PsA disease activity. Landgren and colleagues now aimed to determine the effects of VLED on cytokines and adipokines. They obtained blood samples from patients with PsA and obesity (n = 41) and matched control individuals without rheumatic disease or psoriasis (n = 39) who were on VLED. At month 6, along with significant weight loss, serum levels of interleukin-23 and leptin decreased significantly, while those of total adiponectin and high-molecular-weight adiponectin increased significantly in patients with PsA and control individuals. The change in body mass index correlated positively with a reduction in serum interleukin-23 (rS = 0.671, P < .001) and improvement in PsA disease activity (P = .003). This study highlights the anti-inflammatory effect of weight loss in patients with PsA. Weight loss can complement immunomodulatory therapy in PsA patients with obesity.
Additional Reference
- Klingberg E, Bilberg A, Björkman S, et al. Weight loss improves disease activity in patients with psoriatic arthritis and obesity: an interventional study. Arthritis Res Ther. 2019;21:17. doi: 10.1186/s13075-019-1810-5
Although the usual age at onset of PsA is in the fourth or fifth decade of life, children may develop juvenile-onset PsA (JPsA). Less attention has been paid to this form of juvenile idiopathic arthritis (JIA), and the impact of JPsA vis-à-vis other forms of JIA is not well known. In addition, only about half of the patients with JPsA have cutaneous psoriasis. The impact of psoriasis on children with JPsA is not known. In order to evaluate differences in disease outcomes in patients with JPsA, Low and colleagues evaluated 1653 children and young people with JIA who were recruited to the Childhood Arthritis Prospective Study, of whom 111 had JPsA at diagnosis. They demonstrated that there were no significant differences in patient-reported outcomes between children with JPsA and other JIA categories. However, children with JPsA and psoriasis at JPsA diagnosis had more depressive symptoms compared with those without psoriasis. Moreover, children with JPsA vs other JIA categories had 2.35 times higher odds of having persistently poor well-being scores despite improvements in joint counts and physician global scores. Thus, children with JPsA have poorer well-being scores and a higher prevalence of depression, which requires multidisciplinary care.
Apart from immunomodulatory therapies, weight loss leads to improvement in disease activity in patients with obesity and PsA. However, the mechanisms by which weight loss improves PsA is currently not known, but is likely to be due to changes in adipokines and inflammation-related cytokines. In a recent study1that included patients with PsA and obesity, it was demonstrated that weight loss through a Very Low Energy Diet (VLED) resulted in significant improvements in PsA disease activity. Landgren and colleagues now aimed to determine the effects of VLED on cytokines and adipokines. They obtained blood samples from patients with PsA and obesity (n = 41) and matched control individuals without rheumatic disease or psoriasis (n = 39) who were on VLED. At month 6, along with significant weight loss, serum levels of interleukin-23 and leptin decreased significantly, while those of total adiponectin and high-molecular-weight adiponectin increased significantly in patients with PsA and control individuals. The change in body mass index correlated positively with a reduction in serum interleukin-23 (rS = 0.671, P < .001) and improvement in PsA disease activity (P = .003). This study highlights the anti-inflammatory effect of weight loss in patients with PsA. Weight loss can complement immunomodulatory therapy in PsA patients with obesity.
Additional Reference
- Klingberg E, Bilberg A, Björkman S, et al. Weight loss improves disease activity in patients with psoriatic arthritis and obesity: an interventional study. Arthritis Res Ther. 2019;21:17. doi: 10.1186/s13075-019-1810-5
What’s New in Diffuse Large B-cell Lymphoma?
Diffuse large B-cell lymphoma (DLBCL) is the most diagnosed non-Hodgkin lymphoma (NHL), accounting for up to one-third of cases. For many decades, R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) has been the standard first-line treatment approach for eligible patients in the first-line setting, resulting in long-term remissions in about two-thirds of patients. However, as our understanding of the biologic heterogeneity of this disease has advanced with the ability to perform more sophisticated molecular testing at diagnosis, researchers have been able to identify high-risk patient subtypes with suboptimal outcomes. While survival outcomes among low-risk patient subgroups are favorable with first-line immunochemotherapy, the majority of high-risk patients will experience relapse and often succumb to their disease.
Given the poor outcomes among patients with relapsed or refractory (R/R) DLBCL, there has been a massive research effort over the last decade to improve survival in this setting. Many experts agree that the approval of chimeric antigen receptor (CAR) T-cell therapy was the first major victory in this uphill battle. First approved in October of 2017, axicabtagene ciloleucel was the first of the 3 currently available commercial CAR T-cell therapy constructs to be approved in the third-line setting for DLBCL. Compared to historical controls, CAR T-cell therapy is associated with significant improvement in patient survival with complete response (CR) rates of 40%-50% compared to <20% with standard salvage immunochemotherapy.
Following approval in the third-line setting, these agents were quickly expedited to second-line therapy with pivotal trials demonstrating superiority with CAR T-cell therapy in the second line compared to salvage immunochemotherapy followed by autologous stem cell transplant. In 2022 the ZUMA-7 study reported a 24-month event-free survival (EFS) of 41% with axicabtagene ciloleucel compared to 16% with standard of care, and the TRANSFORM study documented a median EFS not yet reached with lisocabtagene ciloleucel compared to 2.3 months with standard of care. Despite these drastic improvements in patient outcomes, more than half of patients will still fail CAR T-cell therapy and require further systemic therapy.
Thankfully, this year has seen even more advancement in the treatment landscape of R/R DLBCL with two new commercially approved agents in yet another novel therapeutic category: bispecific antibodies. The following is a description of the newest data leading to the latest approvals by the US Food and Drug Administration.
Bispecific antibodies (BsAbs) are an off-the-shelf product that activate endogenous immune cells by cotargeting both tumor antigens as well as host T cells or natural killer cells. Several different experimental agents with varying constructs are under active observation in a wide variety of both hematologic and solid malignancies. Specifically within the realm of B-cell NHL, however, this class of agents is extremely promising and possibly represents the next significant milestone in the treatment of lymphoma.
The toxicity profile of these agents has been reliably predictable in most early phase clinical studies and is related predominantly to T-cell overactivation. The most commonly reported adverse events consist of cytokine release syndrome (CRS) as well as neutropenia, anemia, and hypophosphatemia. While neurologic toxicity has been reported, the incidence is low, and the mechanism is thought to be different than that reported with CAR T-cell therapy given that BsAbs are not likely to cross the blood–brain barrier.
Epcoritamab is a subcutaneously administered bispecific antibody that targets CD3 and CD20 in a 1:1 ratio and activates T cells to destroy CD20-expressing malignant cells. The recent EPCORE NHL-1 clinical trial investigated epcoritamab monotherapy in R/R mature B-cell lymphomas. This agent is administered with a step-up dosing strategy seen consistently across the BsAb drug class. Patients receive a first priming dose of 0.16 mg on cycle 1 day 1, followed by an intermediate dose of 0.8 mg on cycle 1 day 8, followed by the first full dose of 48 mg on cycle 1 day 15. Subsequent doses are administered once weekly for cycles 1-3 followed by every 2 weeks for cycles 4-9, and every 4 weeks starting with cycle 10.
The study enrolled 157 patients globally with median age of 64 and 3 median prior lines of antilymphoma therapy. Nearly 40% of patients had received at least 4 prior lines of therapy, and 83% of patients were refractory to last systemic therapy. Thirty-nine percent of patients had received prior CAR T-cell therapy; 75% of these patients developed progressive disease within 6 months of CAR T-cell therapy.
Among patients treated in the study, the results were as follows:
CR rate 39% with an overall response rate (ORR) of 63%
Duration of response 12 months; duration of objective response not reached in patients with CR
Duration of CR 12 months
Median PFS 4.4 months; median OS not reached
Time to CR of 2.7 months
Toxicity profile was notable for the following:
Any grade CRS in 50%, grade ≥3 in 2.5%
Most CRS occurs with first full dose on cycle 1 day 15 with median time to onset of 20 hours and median time to resolution of 48 hours
Any grade neutropenia in 22%, grade ≥3 in 15%, febrile neutropenia in 2.5%
Any grade anemia in 18%, grade ≥3 in 10%
Injection site reaction, any grade, in 20%
Any grade neurotoxicity in 6%, grade ≥3 in 1 patient (0.6%)
Epcoritamab was granted accelerated approval on May 19, 2023, for use in patients with R/R DLBCL who have received at least 2 prior lines of systemic therapy.
Glofitamab is the more recently approved BsAb for DLBCL. This agent is distinguished by its 2:1 binding configuration that confers bivalency for the CD20 binding site. Glofitamab is delivered intravenously and requires pretreatment with obinutuzumab 1000 mg 7 days before the first dose. With a similar step-up dosing strategy, patients receive a priming dose of 2.5mg on cycle 1 day 8, an intermediate dose of 10mg on cycle 1 day 15, and a first full dose of 30mg on cycle 2 day 1. Subsequent treatments are administered every 21 days for up to 12 cycles.
The open-label phase 1-2 clinical trial of glofitamab monotherapy enrolled 155 patients with a median age of 66 and 3 median prior lines of therapy. Thirty-three percent of patients had received prior CAR T-cell therapy, and 86% were refractory to last line of therapy with 30% refractory to CAR T-cell therapy.
Results were as follows:
CR rate of 39%, ORR 52%
Median duration of CR not reached, median duration of objective response 18.4 months
Median PFS 4.9 months, median OS not reached
Toxicity profile demonstrated the following:
Any grade CRS 66%, grade ≥ 2 in 18%
Median time to onset 13.5 hours from cycle 1 day 8, median duration 30.5 hours
Any grade neutropenia in 38%, grade ≥ 3 in 27%
Grade ≥ 2 neurologic event in 15%
Glofitamab received accelerated approval from the FDA on June 15, 2023, with an identical indication to epcoritamab.
The introduction of BsAbs in DLBCL has highlighted some important issues. Will BsAbs supplant CAR T-cell therapy in DLBCL? Experts can be found on both sides of this debate. BsAbs circumvent the logistics surrounding the production of CAR T-cell therapy products and can, for the large part, be administered in the outpatient setting. However, CAR T-cell therapy has significantly longer follow-up times, which speaks to the curative potential of these agents even in the third-line setting. BsAbs, some may argue, seem to carry a more favorable toxicity profile with the CRS mitigation strategies. However, we still have much to learn about the downstream side effects with prolonged T-cell activation and the potential for T-cell exhaustion.
Finally, with the continued development of new agents in this arena, the art of sequencing therapies will become ever more important. What is the efficacy of CAR T-cell therapy after BsAb exposure? Can BsAbs be used as bridging therapy to a curative option with CAR T-cell therapy? With longer-term follow-up in several years, will we see late relapses after CR with BsAbs? Ongoing clinical trials investigating combination strategies and CAR T-cell therapy consolidation with BsAbs will hopefully eventually clarify some of these questions.
Diffuse large B-cell lymphoma (DLBCL) is the most diagnosed non-Hodgkin lymphoma (NHL), accounting for up to one-third of cases. For many decades, R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) has been the standard first-line treatment approach for eligible patients in the first-line setting, resulting in long-term remissions in about two-thirds of patients. However, as our understanding of the biologic heterogeneity of this disease has advanced with the ability to perform more sophisticated molecular testing at diagnosis, researchers have been able to identify high-risk patient subtypes with suboptimal outcomes. While survival outcomes among low-risk patient subgroups are favorable with first-line immunochemotherapy, the majority of high-risk patients will experience relapse and often succumb to their disease.
Given the poor outcomes among patients with relapsed or refractory (R/R) DLBCL, there has been a massive research effort over the last decade to improve survival in this setting. Many experts agree that the approval of chimeric antigen receptor (CAR) T-cell therapy was the first major victory in this uphill battle. First approved in October of 2017, axicabtagene ciloleucel was the first of the 3 currently available commercial CAR T-cell therapy constructs to be approved in the third-line setting for DLBCL. Compared to historical controls, CAR T-cell therapy is associated with significant improvement in patient survival with complete response (CR) rates of 40%-50% compared to <20% with standard salvage immunochemotherapy.
Following approval in the third-line setting, these agents were quickly expedited to second-line therapy with pivotal trials demonstrating superiority with CAR T-cell therapy in the second line compared to salvage immunochemotherapy followed by autologous stem cell transplant. In 2022 the ZUMA-7 study reported a 24-month event-free survival (EFS) of 41% with axicabtagene ciloleucel compared to 16% with standard of care, and the TRANSFORM study documented a median EFS not yet reached with lisocabtagene ciloleucel compared to 2.3 months with standard of care. Despite these drastic improvements in patient outcomes, more than half of patients will still fail CAR T-cell therapy and require further systemic therapy.
Thankfully, this year has seen even more advancement in the treatment landscape of R/R DLBCL with two new commercially approved agents in yet another novel therapeutic category: bispecific antibodies. The following is a description of the newest data leading to the latest approvals by the US Food and Drug Administration.
Bispecific antibodies (BsAbs) are an off-the-shelf product that activate endogenous immune cells by cotargeting both tumor antigens as well as host T cells or natural killer cells. Several different experimental agents with varying constructs are under active observation in a wide variety of both hematologic and solid malignancies. Specifically within the realm of B-cell NHL, however, this class of agents is extremely promising and possibly represents the next significant milestone in the treatment of lymphoma.
The toxicity profile of these agents has been reliably predictable in most early phase clinical studies and is related predominantly to T-cell overactivation. The most commonly reported adverse events consist of cytokine release syndrome (CRS) as well as neutropenia, anemia, and hypophosphatemia. While neurologic toxicity has been reported, the incidence is low, and the mechanism is thought to be different than that reported with CAR T-cell therapy given that BsAbs are not likely to cross the blood–brain barrier.
Epcoritamab is a subcutaneously administered bispecific antibody that targets CD3 and CD20 in a 1:1 ratio and activates T cells to destroy CD20-expressing malignant cells. The recent EPCORE NHL-1 clinical trial investigated epcoritamab monotherapy in R/R mature B-cell lymphomas. This agent is administered with a step-up dosing strategy seen consistently across the BsAb drug class. Patients receive a first priming dose of 0.16 mg on cycle 1 day 1, followed by an intermediate dose of 0.8 mg on cycle 1 day 8, followed by the first full dose of 48 mg on cycle 1 day 15. Subsequent doses are administered once weekly for cycles 1-3 followed by every 2 weeks for cycles 4-9, and every 4 weeks starting with cycle 10.
The study enrolled 157 patients globally with median age of 64 and 3 median prior lines of antilymphoma therapy. Nearly 40% of patients had received at least 4 prior lines of therapy, and 83% of patients were refractory to last systemic therapy. Thirty-nine percent of patients had received prior CAR T-cell therapy; 75% of these patients developed progressive disease within 6 months of CAR T-cell therapy.
Among patients treated in the study, the results were as follows:
CR rate 39% with an overall response rate (ORR) of 63%
Duration of response 12 months; duration of objective response not reached in patients with CR
Duration of CR 12 months
Median PFS 4.4 months; median OS not reached
Time to CR of 2.7 months
Toxicity profile was notable for the following:
Any grade CRS in 50%, grade ≥3 in 2.5%
Most CRS occurs with first full dose on cycle 1 day 15 with median time to onset of 20 hours and median time to resolution of 48 hours
Any grade neutropenia in 22%, grade ≥3 in 15%, febrile neutropenia in 2.5%
Any grade anemia in 18%, grade ≥3 in 10%
Injection site reaction, any grade, in 20%
Any grade neurotoxicity in 6%, grade ≥3 in 1 patient (0.6%)
Epcoritamab was granted accelerated approval on May 19, 2023, for use in patients with R/R DLBCL who have received at least 2 prior lines of systemic therapy.
Glofitamab is the more recently approved BsAb for DLBCL. This agent is distinguished by its 2:1 binding configuration that confers bivalency for the CD20 binding site. Glofitamab is delivered intravenously and requires pretreatment with obinutuzumab 1000 mg 7 days before the first dose. With a similar step-up dosing strategy, patients receive a priming dose of 2.5mg on cycle 1 day 8, an intermediate dose of 10mg on cycle 1 day 15, and a first full dose of 30mg on cycle 2 day 1. Subsequent treatments are administered every 21 days for up to 12 cycles.
The open-label phase 1-2 clinical trial of glofitamab monotherapy enrolled 155 patients with a median age of 66 and 3 median prior lines of therapy. Thirty-three percent of patients had received prior CAR T-cell therapy, and 86% were refractory to last line of therapy with 30% refractory to CAR T-cell therapy.
Results were as follows:
CR rate of 39%, ORR 52%
Median duration of CR not reached, median duration of objective response 18.4 months
Median PFS 4.9 months, median OS not reached
Toxicity profile demonstrated the following:
Any grade CRS 66%, grade ≥ 2 in 18%
Median time to onset 13.5 hours from cycle 1 day 8, median duration 30.5 hours
Any grade neutropenia in 38%, grade ≥ 3 in 27%
Grade ≥ 2 neurologic event in 15%
Glofitamab received accelerated approval from the FDA on June 15, 2023, with an identical indication to epcoritamab.
The introduction of BsAbs in DLBCL has highlighted some important issues. Will BsAbs supplant CAR T-cell therapy in DLBCL? Experts can be found on both sides of this debate. BsAbs circumvent the logistics surrounding the production of CAR T-cell therapy products and can, for the large part, be administered in the outpatient setting. However, CAR T-cell therapy has significantly longer follow-up times, which speaks to the curative potential of these agents even in the third-line setting. BsAbs, some may argue, seem to carry a more favorable toxicity profile with the CRS mitigation strategies. However, we still have much to learn about the downstream side effects with prolonged T-cell activation and the potential for T-cell exhaustion.
Finally, with the continued development of new agents in this arena, the art of sequencing therapies will become ever more important. What is the efficacy of CAR T-cell therapy after BsAb exposure? Can BsAbs be used as bridging therapy to a curative option with CAR T-cell therapy? With longer-term follow-up in several years, will we see late relapses after CR with BsAbs? Ongoing clinical trials investigating combination strategies and CAR T-cell therapy consolidation with BsAbs will hopefully eventually clarify some of these questions.
Diffuse large B-cell lymphoma (DLBCL) is the most diagnosed non-Hodgkin lymphoma (NHL), accounting for up to one-third of cases. For many decades, R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) has been the standard first-line treatment approach for eligible patients in the first-line setting, resulting in long-term remissions in about two-thirds of patients. However, as our understanding of the biologic heterogeneity of this disease has advanced with the ability to perform more sophisticated molecular testing at diagnosis, researchers have been able to identify high-risk patient subtypes with suboptimal outcomes. While survival outcomes among low-risk patient subgroups are favorable with first-line immunochemotherapy, the majority of high-risk patients will experience relapse and often succumb to their disease.
Given the poor outcomes among patients with relapsed or refractory (R/R) DLBCL, there has been a massive research effort over the last decade to improve survival in this setting. Many experts agree that the approval of chimeric antigen receptor (CAR) T-cell therapy was the first major victory in this uphill battle. First approved in October of 2017, axicabtagene ciloleucel was the first of the 3 currently available commercial CAR T-cell therapy constructs to be approved in the third-line setting for DLBCL. Compared to historical controls, CAR T-cell therapy is associated with significant improvement in patient survival with complete response (CR) rates of 40%-50% compared to <20% with standard salvage immunochemotherapy.
Following approval in the third-line setting, these agents were quickly expedited to second-line therapy with pivotal trials demonstrating superiority with CAR T-cell therapy in the second line compared to salvage immunochemotherapy followed by autologous stem cell transplant. In 2022 the ZUMA-7 study reported a 24-month event-free survival (EFS) of 41% with axicabtagene ciloleucel compared to 16% with standard of care, and the TRANSFORM study documented a median EFS not yet reached with lisocabtagene ciloleucel compared to 2.3 months with standard of care. Despite these drastic improvements in patient outcomes, more than half of patients will still fail CAR T-cell therapy and require further systemic therapy.
Thankfully, this year has seen even more advancement in the treatment landscape of R/R DLBCL with two new commercially approved agents in yet another novel therapeutic category: bispecific antibodies. The following is a description of the newest data leading to the latest approvals by the US Food and Drug Administration.
Bispecific antibodies (BsAbs) are an off-the-shelf product that activate endogenous immune cells by cotargeting both tumor antigens as well as host T cells or natural killer cells. Several different experimental agents with varying constructs are under active observation in a wide variety of both hematologic and solid malignancies. Specifically within the realm of B-cell NHL, however, this class of agents is extremely promising and possibly represents the next significant milestone in the treatment of lymphoma.
The toxicity profile of these agents has been reliably predictable in most early phase clinical studies and is related predominantly to T-cell overactivation. The most commonly reported adverse events consist of cytokine release syndrome (CRS) as well as neutropenia, anemia, and hypophosphatemia. While neurologic toxicity has been reported, the incidence is low, and the mechanism is thought to be different than that reported with CAR T-cell therapy given that BsAbs are not likely to cross the blood–brain barrier.
Epcoritamab is a subcutaneously administered bispecific antibody that targets CD3 and CD20 in a 1:1 ratio and activates T cells to destroy CD20-expressing malignant cells. The recent EPCORE NHL-1 clinical trial investigated epcoritamab monotherapy in R/R mature B-cell lymphomas. This agent is administered with a step-up dosing strategy seen consistently across the BsAb drug class. Patients receive a first priming dose of 0.16 mg on cycle 1 day 1, followed by an intermediate dose of 0.8 mg on cycle 1 day 8, followed by the first full dose of 48 mg on cycle 1 day 15. Subsequent doses are administered once weekly for cycles 1-3 followed by every 2 weeks for cycles 4-9, and every 4 weeks starting with cycle 10.
The study enrolled 157 patients globally with median age of 64 and 3 median prior lines of antilymphoma therapy. Nearly 40% of patients had received at least 4 prior lines of therapy, and 83% of patients were refractory to last systemic therapy. Thirty-nine percent of patients had received prior CAR T-cell therapy; 75% of these patients developed progressive disease within 6 months of CAR T-cell therapy.
Among patients treated in the study, the results were as follows:
CR rate 39% with an overall response rate (ORR) of 63%
Duration of response 12 months; duration of objective response not reached in patients with CR
Duration of CR 12 months
Median PFS 4.4 months; median OS not reached
Time to CR of 2.7 months
Toxicity profile was notable for the following:
Any grade CRS in 50%, grade ≥3 in 2.5%
Most CRS occurs with first full dose on cycle 1 day 15 with median time to onset of 20 hours and median time to resolution of 48 hours
Any grade neutropenia in 22%, grade ≥3 in 15%, febrile neutropenia in 2.5%
Any grade anemia in 18%, grade ≥3 in 10%
Injection site reaction, any grade, in 20%
Any grade neurotoxicity in 6%, grade ≥3 in 1 patient (0.6%)
Epcoritamab was granted accelerated approval on May 19, 2023, for use in patients with R/R DLBCL who have received at least 2 prior lines of systemic therapy.
Glofitamab is the more recently approved BsAb for DLBCL. This agent is distinguished by its 2:1 binding configuration that confers bivalency for the CD20 binding site. Glofitamab is delivered intravenously and requires pretreatment with obinutuzumab 1000 mg 7 days before the first dose. With a similar step-up dosing strategy, patients receive a priming dose of 2.5mg on cycle 1 day 8, an intermediate dose of 10mg on cycle 1 day 15, and a first full dose of 30mg on cycle 2 day 1. Subsequent treatments are administered every 21 days for up to 12 cycles.
The open-label phase 1-2 clinical trial of glofitamab monotherapy enrolled 155 patients with a median age of 66 and 3 median prior lines of therapy. Thirty-three percent of patients had received prior CAR T-cell therapy, and 86% were refractory to last line of therapy with 30% refractory to CAR T-cell therapy.
Results were as follows:
CR rate of 39%, ORR 52%
Median duration of CR not reached, median duration of objective response 18.4 months
Median PFS 4.9 months, median OS not reached
Toxicity profile demonstrated the following:
Any grade CRS 66%, grade ≥ 2 in 18%
Median time to onset 13.5 hours from cycle 1 day 8, median duration 30.5 hours
Any grade neutropenia in 38%, grade ≥ 3 in 27%
Grade ≥ 2 neurologic event in 15%
Glofitamab received accelerated approval from the FDA on June 15, 2023, with an identical indication to epcoritamab.
The introduction of BsAbs in DLBCL has highlighted some important issues. Will BsAbs supplant CAR T-cell therapy in DLBCL? Experts can be found on both sides of this debate. BsAbs circumvent the logistics surrounding the production of CAR T-cell therapy products and can, for the large part, be administered in the outpatient setting. However, CAR T-cell therapy has significantly longer follow-up times, which speaks to the curative potential of these agents even in the third-line setting. BsAbs, some may argue, seem to carry a more favorable toxicity profile with the CRS mitigation strategies. However, we still have much to learn about the downstream side effects with prolonged T-cell activation and the potential for T-cell exhaustion.
Finally, with the continued development of new agents in this arena, the art of sequencing therapies will become ever more important. What is the efficacy of CAR T-cell therapy after BsAb exposure? Can BsAbs be used as bridging therapy to a curative option with CAR T-cell therapy? With longer-term follow-up in several years, will we see late relapses after CR with BsAbs? Ongoing clinical trials investigating combination strategies and CAR T-cell therapy consolidation with BsAbs will hopefully eventually clarify some of these questions.
Commentary: Newer Drugs for AD Plus Dupilumab and Other Issues, September 2023
Amlitelimab is a monoclonal antibody that targets the OX40 ligand (Weidinger et al). It is predicted to have broad potential therapeutic application for multiple immune diseases, including atopic dermatitis. I'm not looking for that. I've been spoiled by drugs that have narrow therapeutic application (like IL-23 blockade and IL-4/IL-13 blockade) that target a specific disease very effectively with very little in the way of side effects.
The OX40 ligand/receptor interaction may be too important. When I Google "OX40 deficiency," the first thing that pops up is a combined T- and B-cell immunodeficiency associated with possible aggressive, childhood-onset, disseminated, cutaneous, and systemic Kaposi sarcoma. That doesn't mean that such a horrible outcome will come with the level of pharmacologic OX40 blockade that we would try to achieve in our patients. Clinical trials don't show horrible adverse events — so far. I'm in no hurry to find out in my patients whether real-life efficacy in large numbers of people treated for long periods of time matches up with the short-term safety profiles seen in relatively small clinical trial populations.
It might be nice to give patients upadacitinib only as needed, for example for a flare of their atopic dermatitis, then cut down the dose or stop altogether until the next flare. The study by Guttman-Yassky and colleagues found that atopic dermatitis came back quickly when upadacitinib was stopped. However, their study looked at patients with chronically bad atopic dermatitis. If we have a patient who tends to flare only intermittently, it may be that we could use upadacitinib or other systemic treatments on an intermittent basis. I know when it came to my son's mild atopic dermatitis, intermittent use of a little triamcinolone ointment was all that was needed. Yes, I know that's a "reactive," roller-coaster approach. Yes, I know that a "proactive" keep-the-disease-away approach sounds better. But I'm realistic when it comes to patients' adherence behaviors. I think there's a lot to be said for minimizing drug exposure and just using treatments as needed. Guttman-Yassky's work makes me believe that a lot of patients will need continuous treatment to keep their severe disease under control. I'm not convinced that everyone will need continuous treatment to be happy with their treatment.
O'Connor and colleagues found that emollient bathing is associated with later development of atopic dermatitis. They defined emollient bathing as baths with oil or emulsifier-based additives. This study illustrates the importance of randomization in a controlled trial. Because their study was not randomized, we don't know whether the emollient bathing caused atopic dermatitis or whether families that had more dry skin or more family history of atopic dermatitis were more likely to use emollient bathing.
When dupilumab was first approved, I prescribed it to my patients to take every 2 weeks as recommended on the label. I'm not so sure how many patients actually used it that way. I suspect that a lot of them took the medicine less often than recommended, especially when they were doing well. This report by Sánchez-García and colleagues suggests that patients who are doing very well on dupilumab may be able to take the drug less often. That's probably not news to my patients who are already taking the drug less often than I told them to.
I think less frequent dosing may become even more common over time, particularly for drugs that may have more safety risks than dupilumab. Many patients with atopic dermatitis probably don't need to be taking drugs all the time. Patients who tend to have flare-ups but who do very well for a long period of time between flares may only need drugs intermittently. It will be interesting to see if our patients can use oral treatments for atopic dermatitis that way.
Siegfried and colleagues assessed how well dupilumab worked in children with atopic dermatitis in different areas of the body: head and neck, trunk, upper extremities, lower extremities. Dupilumab worked well in all these areas, as expected.
Xu and colleagues did a meta-analysis of studies of dupilumab for atopic dermatitis and concluded, not shockingly, that dupilumab is safe and effective for atopic dermatitis. Okay, I believe that. They further concluded: "More long-term, high-quality, controlled studies in different regions are needed for further verification." I don't think so. I think the evidence is clear already.
Studies that measure the levels of things are generally not particularly helpful. The study by García-Reyes and colleagues studied the levels of serum thymic stromal lymphopoietin (TSLP) in patients with atopic dermatitis. TSLP levels were higher in patients with atopic dermatitis compared with patients without atopic dermatitis. This basically tells us nothing about the role of TSLP in atopic dermatitis. The elevated levels could be causing atopic dermatitis or they could be the body's response to having atopic dermatitis.
To tell whether something is causal we have to look at either genetic studies or studies with specific inhibitors. A specific inhibitor study was done by atopic dermatitis expert Eric Simpson and colleagues.1 This was a randomized, placebo-controlled study in which an anti-TSLP antibody was given to patients with atopic dermatitis. Both the anti-TSLP antibody and placebo groups were permitted to use topical steroids. While the anti-TSLP antibody–treated patients did better than placebo-treated patients, the difference did not achieve statistical significance, probably, I believe, because the placebo-treated patients used more topical steroids. When you want to assess whether a drug for atopic dermatitis is better than placebo, you must be careful about how much topical steroid you let patients in the study use!
Additional Reference
- Simpson EL, Parnes JR, She D, et al. Tezepelumab, an anti-thymic stromal lymphopoietin monoclonal antibody, in the treatment of moderate to severe atopic dermatitis: A randomized phase 2a clinical trial. J Am Acad Dermatol. 2019;80(4):1013-1021. doi: 10.1016/j.jaad.2018.11.059
Amlitelimab is a monoclonal antibody that targets the OX40 ligand (Weidinger et al). It is predicted to have broad potential therapeutic application for multiple immune diseases, including atopic dermatitis. I'm not looking for that. I've been spoiled by drugs that have narrow therapeutic application (like IL-23 blockade and IL-4/IL-13 blockade) that target a specific disease very effectively with very little in the way of side effects.
The OX40 ligand/receptor interaction may be too important. When I Google "OX40 deficiency," the first thing that pops up is a combined T- and B-cell immunodeficiency associated with possible aggressive, childhood-onset, disseminated, cutaneous, and systemic Kaposi sarcoma. That doesn't mean that such a horrible outcome will come with the level of pharmacologic OX40 blockade that we would try to achieve in our patients. Clinical trials don't show horrible adverse events — so far. I'm in no hurry to find out in my patients whether real-life efficacy in large numbers of people treated for long periods of time matches up with the short-term safety profiles seen in relatively small clinical trial populations.
It might be nice to give patients upadacitinib only as needed, for example for a flare of their atopic dermatitis, then cut down the dose or stop altogether until the next flare. The study by Guttman-Yassky and colleagues found that atopic dermatitis came back quickly when upadacitinib was stopped. However, their study looked at patients with chronically bad atopic dermatitis. If we have a patient who tends to flare only intermittently, it may be that we could use upadacitinib or other systemic treatments on an intermittent basis. I know when it came to my son's mild atopic dermatitis, intermittent use of a little triamcinolone ointment was all that was needed. Yes, I know that's a "reactive," roller-coaster approach. Yes, I know that a "proactive" keep-the-disease-away approach sounds better. But I'm realistic when it comes to patients' adherence behaviors. I think there's a lot to be said for minimizing drug exposure and just using treatments as needed. Guttman-Yassky's work makes me believe that a lot of patients will need continuous treatment to keep their severe disease under control. I'm not convinced that everyone will need continuous treatment to be happy with their treatment.
O'Connor and colleagues found that emollient bathing is associated with later development of atopic dermatitis. They defined emollient bathing as baths with oil or emulsifier-based additives. This study illustrates the importance of randomization in a controlled trial. Because their study was not randomized, we don't know whether the emollient bathing caused atopic dermatitis or whether families that had more dry skin or more family history of atopic dermatitis were more likely to use emollient bathing.
When dupilumab was first approved, I prescribed it to my patients to take every 2 weeks as recommended on the label. I'm not so sure how many patients actually used it that way. I suspect that a lot of them took the medicine less often than recommended, especially when they were doing well. This report by Sánchez-García and colleagues suggests that patients who are doing very well on dupilumab may be able to take the drug less often. That's probably not news to my patients who are already taking the drug less often than I told them to.
I think less frequent dosing may become even more common over time, particularly for drugs that may have more safety risks than dupilumab. Many patients with atopic dermatitis probably don't need to be taking drugs all the time. Patients who tend to have flare-ups but who do very well for a long period of time between flares may only need drugs intermittently. It will be interesting to see if our patients can use oral treatments for atopic dermatitis that way.
Siegfried and colleagues assessed how well dupilumab worked in children with atopic dermatitis in different areas of the body: head and neck, trunk, upper extremities, lower extremities. Dupilumab worked well in all these areas, as expected.
Xu and colleagues did a meta-analysis of studies of dupilumab for atopic dermatitis and concluded, not shockingly, that dupilumab is safe and effective for atopic dermatitis. Okay, I believe that. They further concluded: "More long-term, high-quality, controlled studies in different regions are needed for further verification." I don't think so. I think the evidence is clear already.
Studies that measure the levels of things are generally not particularly helpful. The study by García-Reyes and colleagues studied the levels of serum thymic stromal lymphopoietin (TSLP) in patients with atopic dermatitis. TSLP levels were higher in patients with atopic dermatitis compared with patients without atopic dermatitis. This basically tells us nothing about the role of TSLP in atopic dermatitis. The elevated levels could be causing atopic dermatitis or they could be the body's response to having atopic dermatitis.
To tell whether something is causal we have to look at either genetic studies or studies with specific inhibitors. A specific inhibitor study was done by atopic dermatitis expert Eric Simpson and colleagues.1 This was a randomized, placebo-controlled study in which an anti-TSLP antibody was given to patients with atopic dermatitis. Both the anti-TSLP antibody and placebo groups were permitted to use topical steroids. While the anti-TSLP antibody–treated patients did better than placebo-treated patients, the difference did not achieve statistical significance, probably, I believe, because the placebo-treated patients used more topical steroids. When you want to assess whether a drug for atopic dermatitis is better than placebo, you must be careful about how much topical steroid you let patients in the study use!
Additional Reference
- Simpson EL, Parnes JR, She D, et al. Tezepelumab, an anti-thymic stromal lymphopoietin monoclonal antibody, in the treatment of moderate to severe atopic dermatitis: A randomized phase 2a clinical trial. J Am Acad Dermatol. 2019;80(4):1013-1021. doi: 10.1016/j.jaad.2018.11.059
Amlitelimab is a monoclonal antibody that targets the OX40 ligand (Weidinger et al). It is predicted to have broad potential therapeutic application for multiple immune diseases, including atopic dermatitis. I'm not looking for that. I've been spoiled by drugs that have narrow therapeutic application (like IL-23 blockade and IL-4/IL-13 blockade) that target a specific disease very effectively with very little in the way of side effects.
The OX40 ligand/receptor interaction may be too important. When I Google "OX40 deficiency," the first thing that pops up is a combined T- and B-cell immunodeficiency associated with possible aggressive, childhood-onset, disseminated, cutaneous, and systemic Kaposi sarcoma. That doesn't mean that such a horrible outcome will come with the level of pharmacologic OX40 blockade that we would try to achieve in our patients. Clinical trials don't show horrible adverse events — so far. I'm in no hurry to find out in my patients whether real-life efficacy in large numbers of people treated for long periods of time matches up with the short-term safety profiles seen in relatively small clinical trial populations.
It might be nice to give patients upadacitinib only as needed, for example for a flare of their atopic dermatitis, then cut down the dose or stop altogether until the next flare. The study by Guttman-Yassky and colleagues found that atopic dermatitis came back quickly when upadacitinib was stopped. However, their study looked at patients with chronically bad atopic dermatitis. If we have a patient who tends to flare only intermittently, it may be that we could use upadacitinib or other systemic treatments on an intermittent basis. I know when it came to my son's mild atopic dermatitis, intermittent use of a little triamcinolone ointment was all that was needed. Yes, I know that's a "reactive," roller-coaster approach. Yes, I know that a "proactive" keep-the-disease-away approach sounds better. But I'm realistic when it comes to patients' adherence behaviors. I think there's a lot to be said for minimizing drug exposure and just using treatments as needed. Guttman-Yassky's work makes me believe that a lot of patients will need continuous treatment to keep their severe disease under control. I'm not convinced that everyone will need continuous treatment to be happy with their treatment.
O'Connor and colleagues found that emollient bathing is associated with later development of atopic dermatitis. They defined emollient bathing as baths with oil or emulsifier-based additives. This study illustrates the importance of randomization in a controlled trial. Because their study was not randomized, we don't know whether the emollient bathing caused atopic dermatitis or whether families that had more dry skin or more family history of atopic dermatitis were more likely to use emollient bathing.
When dupilumab was first approved, I prescribed it to my patients to take every 2 weeks as recommended on the label. I'm not so sure how many patients actually used it that way. I suspect that a lot of them took the medicine less often than recommended, especially when they were doing well. This report by Sánchez-García and colleagues suggests that patients who are doing very well on dupilumab may be able to take the drug less often. That's probably not news to my patients who are already taking the drug less often than I told them to.
I think less frequent dosing may become even more common over time, particularly for drugs that may have more safety risks than dupilumab. Many patients with atopic dermatitis probably don't need to be taking drugs all the time. Patients who tend to have flare-ups but who do very well for a long period of time between flares may only need drugs intermittently. It will be interesting to see if our patients can use oral treatments for atopic dermatitis that way.
Siegfried and colleagues assessed how well dupilumab worked in children with atopic dermatitis in different areas of the body: head and neck, trunk, upper extremities, lower extremities. Dupilumab worked well in all these areas, as expected.
Xu and colleagues did a meta-analysis of studies of dupilumab for atopic dermatitis and concluded, not shockingly, that dupilumab is safe and effective for atopic dermatitis. Okay, I believe that. They further concluded: "More long-term, high-quality, controlled studies in different regions are needed for further verification." I don't think so. I think the evidence is clear already.
Studies that measure the levels of things are generally not particularly helpful. The study by García-Reyes and colleagues studied the levels of serum thymic stromal lymphopoietin (TSLP) in patients with atopic dermatitis. TSLP levels were higher in patients with atopic dermatitis compared with patients without atopic dermatitis. This basically tells us nothing about the role of TSLP in atopic dermatitis. The elevated levels could be causing atopic dermatitis or they could be the body's response to having atopic dermatitis.
To tell whether something is causal we have to look at either genetic studies or studies with specific inhibitors. A specific inhibitor study was done by atopic dermatitis expert Eric Simpson and colleagues.1 This was a randomized, placebo-controlled study in which an anti-TSLP antibody was given to patients with atopic dermatitis. Both the anti-TSLP antibody and placebo groups were permitted to use topical steroids. While the anti-TSLP antibody–treated patients did better than placebo-treated patients, the difference did not achieve statistical significance, probably, I believe, because the placebo-treated patients used more topical steroids. When you want to assess whether a drug for atopic dermatitis is better than placebo, you must be careful about how much topical steroid you let patients in the study use!
Additional Reference
- Simpson EL, Parnes JR, She D, et al. Tezepelumab, an anti-thymic stromal lymphopoietin monoclonal antibody, in the treatment of moderate to severe atopic dermatitis: A randomized phase 2a clinical trial. J Am Acad Dermatol. 2019;80(4):1013-1021. doi: 10.1016/j.jaad.2018.11.059
Financial Insecurity Among US Adults With Psoriasis
To the Editor:
Approximately 3% of the US population, or 6.9 million adults, is affected by psoriasis.1 Psoriasis has a substantial impact on quality of life and is associated with increased health care expenses and medication costs. In 2013, it was reported that the estimated US annual cost—direct, indirect, intangible, and comorbidity costs—of psoriasis for adults was $112 billion.2 We investigated the prevalence and sociodemographic characteristics of adult psoriasis patients (aged ≥20 years) with financial insecurity utilizing the 2009–2014 National Health and Nutrition Examination Survey (NHANES) data.3
We conducted a population-based, cross-sectional study focused on patients 20 years and older with psoriasis from the 2009-2014 NHANES database to evaluate financial insecurity. Financial insecurity was evaluated by 2 outcome variables. The primary outcome variable was assessed by the question “Are you covered by health insurance or some other kind of health care plan (including health insurance obtained through employment or purchased directly as well as government programs like Medicare and Medicaid that provide medical care or help pay medical bills)?”3 Our secondary outcome variable was evaluated by a reported annual household income of less than $20,000. P values in Table 1 were calculated using Pearson χ2 tests. In Table 2, multivariate logistic regressions were performed using Stata/MP 17 (StataCorp LLC) to analyze associations between outcome variables and sociodemographic characteristics. Additionally, we controlled for age, race/ethnicity, sex, education, marital status, US citizenship status, and tobacco use. Subsequently, relationships with P<.05 were considered statistically significant.
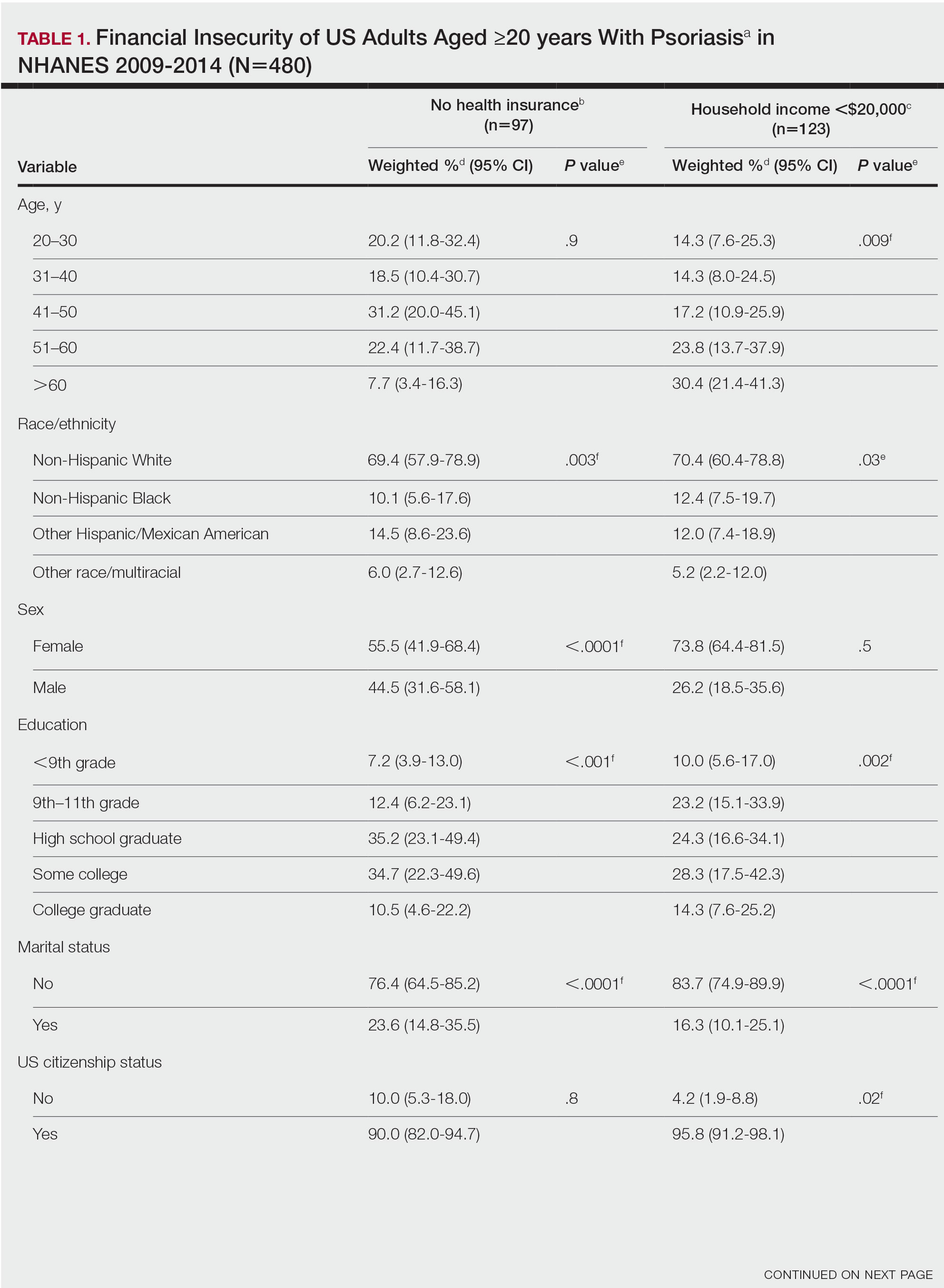

Our analysis comprised 480 individuals with psoriasis; 40 individuals were excluded from our analysis because they did not report annual household income and health insurance status (Table 1). Among the 480 individuals with psoriasis, approximately 16% (weighted) reported a lack of health insurance, and approximately 17% (weighted) reported an annual household income of less than $20,000. Among those who reported an annual household income of less than $20,000, approximately 38% (weighted) of them reported that they did not have health insurance.
Multivariate logistic regression analyses revealed that elderly individuals (aged >60 years), college graduates, married individuals, and US citizens had decreased odds of lacking health insurance (Table 2). Additionally, those with a history of tobacco use (adjusted odds ratio [AOR] 2.02; 95% CI, 1.00-4.05) were associated with lacking health insurance. Non-Hispanic Black individuals (AOR 2.26; 95% CI, 1.09-4.71) and US citizens (AOR 5.01; 95% CI, 1.28-19.63) had a significant association with an annual household income of less than $20,000 (P<.05). Lastly, males, those with education beyond ninth grade, and married individuals had a significantly decreased odds of having an annual household income of less than $20,000 (P<.05)(Table 2).
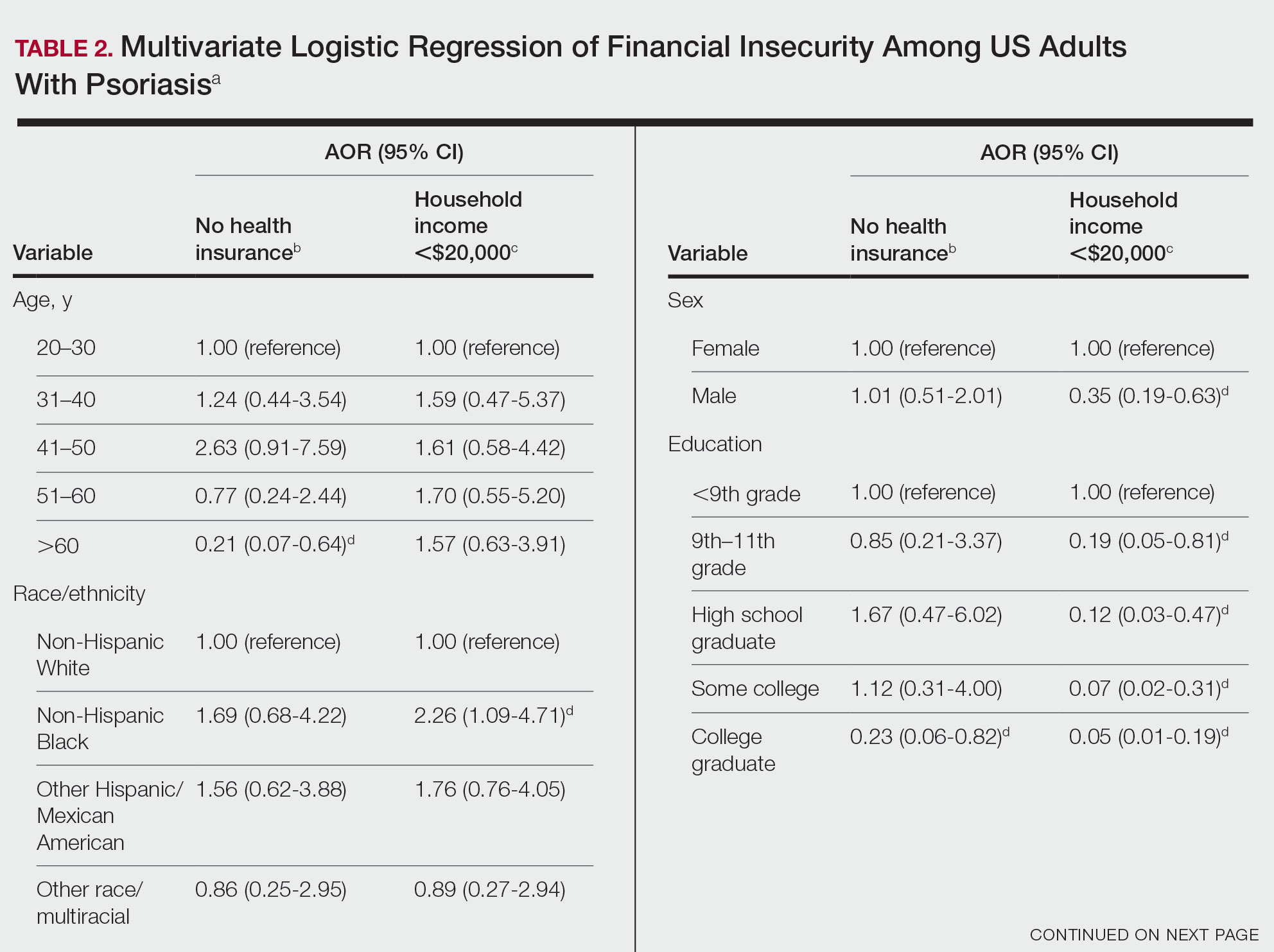
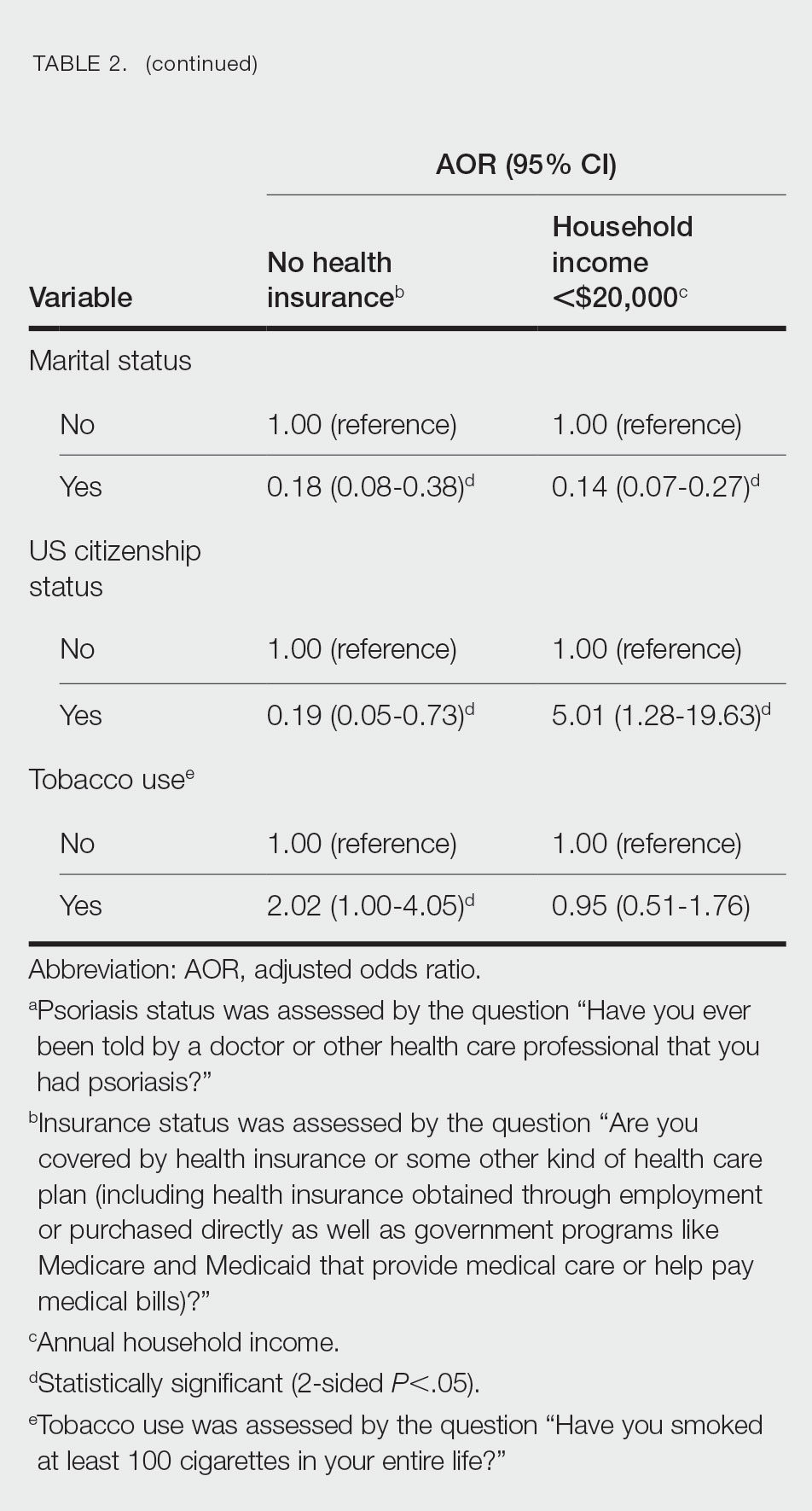
Our findings indicate that certain sociodemographic groups of psoriasis patients have an increased risk for being financially insecure. It is important to evaluate the cost of treatment, number of necessary visits to the office, and cost of transportation, as these factors can serve as a major economic burden to patients being managed for psoriasis.4 Additionally, the cost of biologics has been increasing over time.5 Taking all of this into account when caring for psoriasis patients is crucial, as understanding the financial status of patients can assist with determining appropriate individualized treatment regimens.
- Liu J, Thatiparthi A, Martin A, et al. Prevalence of psoriasis among adults in the US 2009-2010 and 2013-2014 National Health and Nutrition Examination Surveys. J Am Acad Dermatol. 2021;84:767-769. doi:10.1016/j.jaad.2020.10.035
- Brezinski EA, Dhillon JS, Armstrong AW. Economic burden of psoriasis in the United States: a systematic review. JAMA Dermatol. 2015;151:651-658. doi:10.1001/jamadermatol.2014.3593
- National Center for Health Statistics. NHANES questionnaires, datasets, and related documentation. Centers for Disease Control and Prevention website. Accessed June 22, 2023. https://wwwn.cdc.govnchs/nhanes/Default.aspx
- Maya-Rico AM, Londoño-García Á, Palacios-Barahona AU, et al. Out-of-pocket costs for patients with psoriasis in an outpatient dermatology referral service. An Bras Dermatol. 2021;96:295-300. doi:10.1016/j.abd.2020.09.004
- Cheng J, Feldman SR. The cost of biologics for psoriasis is increasing. Drugs Context. 2014;3:212266. doi:10.7573/dic.212266
To the Editor:
Approximately 3% of the US population, or 6.9 million adults, is affected by psoriasis.1 Psoriasis has a substantial impact on quality of life and is associated with increased health care expenses and medication costs. In 2013, it was reported that the estimated US annual cost—direct, indirect, intangible, and comorbidity costs—of psoriasis for adults was $112 billion.2 We investigated the prevalence and sociodemographic characteristics of adult psoriasis patients (aged ≥20 years) with financial insecurity utilizing the 2009–2014 National Health and Nutrition Examination Survey (NHANES) data.3
We conducted a population-based, cross-sectional study focused on patients 20 years and older with psoriasis from the 2009-2014 NHANES database to evaluate financial insecurity. Financial insecurity was evaluated by 2 outcome variables. The primary outcome variable was assessed by the question “Are you covered by health insurance or some other kind of health care plan (including health insurance obtained through employment or purchased directly as well as government programs like Medicare and Medicaid that provide medical care or help pay medical bills)?”3 Our secondary outcome variable was evaluated by a reported annual household income of less than $20,000. P values in Table 1 were calculated using Pearson χ2 tests. In Table 2, multivariate logistic regressions were performed using Stata/MP 17 (StataCorp LLC) to analyze associations between outcome variables and sociodemographic characteristics. Additionally, we controlled for age, race/ethnicity, sex, education, marital status, US citizenship status, and tobacco use. Subsequently, relationships with P<.05 were considered statistically significant.


Our analysis comprised 480 individuals with psoriasis; 40 individuals were excluded from our analysis because they did not report annual household income and health insurance status (Table 1). Among the 480 individuals with psoriasis, approximately 16% (weighted) reported a lack of health insurance, and approximately 17% (weighted) reported an annual household income of less than $20,000. Among those who reported an annual household income of less than $20,000, approximately 38% (weighted) of them reported that they did not have health insurance.
Multivariate logistic regression analyses revealed that elderly individuals (aged >60 years), college graduates, married individuals, and US citizens had decreased odds of lacking health insurance (Table 2). Additionally, those with a history of tobacco use (adjusted odds ratio [AOR] 2.02; 95% CI, 1.00-4.05) were associated with lacking health insurance. Non-Hispanic Black individuals (AOR 2.26; 95% CI, 1.09-4.71) and US citizens (AOR 5.01; 95% CI, 1.28-19.63) had a significant association with an annual household income of less than $20,000 (P<.05). Lastly, males, those with education beyond ninth grade, and married individuals had a significantly decreased odds of having an annual household income of less than $20,000 (P<.05)(Table 2).


Our findings indicate that certain sociodemographic groups of psoriasis patients have an increased risk for being financially insecure. It is important to evaluate the cost of treatment, number of necessary visits to the office, and cost of transportation, as these factors can serve as a major economic burden to patients being managed for psoriasis.4 Additionally, the cost of biologics has been increasing over time.5 Taking all of this into account when caring for psoriasis patients is crucial, as understanding the financial status of patients can assist with determining appropriate individualized treatment regimens.
To the Editor:
Approximately 3% of the US population, or 6.9 million adults, is affected by psoriasis.1 Psoriasis has a substantial impact on quality of life and is associated with increased health care expenses and medication costs. In 2013, it was reported that the estimated US annual cost—direct, indirect, intangible, and comorbidity costs—of psoriasis for adults was $112 billion.2 We investigated the prevalence and sociodemographic characteristics of adult psoriasis patients (aged ≥20 years) with financial insecurity utilizing the 2009–2014 National Health and Nutrition Examination Survey (NHANES) data.3
We conducted a population-based, cross-sectional study focused on patients 20 years and older with psoriasis from the 2009-2014 NHANES database to evaluate financial insecurity. Financial insecurity was evaluated by 2 outcome variables. The primary outcome variable was assessed by the question “Are you covered by health insurance or some other kind of health care plan (including health insurance obtained through employment or purchased directly as well as government programs like Medicare and Medicaid that provide medical care or help pay medical bills)?”3 Our secondary outcome variable was evaluated by a reported annual household income of less than $20,000. P values in Table 1 were calculated using Pearson χ2 tests. In Table 2, multivariate logistic regressions were performed using Stata/MP 17 (StataCorp LLC) to analyze associations between outcome variables and sociodemographic characteristics. Additionally, we controlled for age, race/ethnicity, sex, education, marital status, US citizenship status, and tobacco use. Subsequently, relationships with P<.05 were considered statistically significant.


Our analysis comprised 480 individuals with psoriasis; 40 individuals were excluded from our analysis because they did not report annual household income and health insurance status (Table 1). Among the 480 individuals with psoriasis, approximately 16% (weighted) reported a lack of health insurance, and approximately 17% (weighted) reported an annual household income of less than $20,000. Among those who reported an annual household income of less than $20,000, approximately 38% (weighted) of them reported that they did not have health insurance.
Multivariate logistic regression analyses revealed that elderly individuals (aged >60 years), college graduates, married individuals, and US citizens had decreased odds of lacking health insurance (Table 2). Additionally, those with a history of tobacco use (adjusted odds ratio [AOR] 2.02; 95% CI, 1.00-4.05) were associated with lacking health insurance. Non-Hispanic Black individuals (AOR 2.26; 95% CI, 1.09-4.71) and US citizens (AOR 5.01; 95% CI, 1.28-19.63) had a significant association with an annual household income of less than $20,000 (P<.05). Lastly, males, those with education beyond ninth grade, and married individuals had a significantly decreased odds of having an annual household income of less than $20,000 (P<.05)(Table 2).


Our findings indicate that certain sociodemographic groups of psoriasis patients have an increased risk for being financially insecure. It is important to evaluate the cost of treatment, number of necessary visits to the office, and cost of transportation, as these factors can serve as a major economic burden to patients being managed for psoriasis.4 Additionally, the cost of biologics has been increasing over time.5 Taking all of this into account when caring for psoriasis patients is crucial, as understanding the financial status of patients can assist with determining appropriate individualized treatment regimens.
- Liu J, Thatiparthi A, Martin A, et al. Prevalence of psoriasis among adults in the US 2009-2010 and 2013-2014 National Health and Nutrition Examination Surveys. J Am Acad Dermatol. 2021;84:767-769. doi:10.1016/j.jaad.2020.10.035
- Brezinski EA, Dhillon JS, Armstrong AW. Economic burden of psoriasis in the United States: a systematic review. JAMA Dermatol. 2015;151:651-658. doi:10.1001/jamadermatol.2014.3593
- National Center for Health Statistics. NHANES questionnaires, datasets, and related documentation. Centers for Disease Control and Prevention website. Accessed June 22, 2023. https://wwwn.cdc.govnchs/nhanes/Default.aspx
- Maya-Rico AM, Londoño-García Á, Palacios-Barahona AU, et al. Out-of-pocket costs for patients with psoriasis in an outpatient dermatology referral service. An Bras Dermatol. 2021;96:295-300. doi:10.1016/j.abd.2020.09.004
- Cheng J, Feldman SR. The cost of biologics for psoriasis is increasing. Drugs Context. 2014;3:212266. doi:10.7573/dic.212266
- Liu J, Thatiparthi A, Martin A, et al. Prevalence of psoriasis among adults in the US 2009-2010 and 2013-2014 National Health and Nutrition Examination Surveys. J Am Acad Dermatol. 2021;84:767-769. doi:10.1016/j.jaad.2020.10.035
- Brezinski EA, Dhillon JS, Armstrong AW. Economic burden of psoriasis in the United States: a systematic review. JAMA Dermatol. 2015;151:651-658. doi:10.1001/jamadermatol.2014.3593
- National Center for Health Statistics. NHANES questionnaires, datasets, and related documentation. Centers for Disease Control and Prevention website. Accessed June 22, 2023. https://wwwn.cdc.govnchs/nhanes/Default.aspx
- Maya-Rico AM, Londoño-García Á, Palacios-Barahona AU, et al. Out-of-pocket costs for patients with psoriasis in an outpatient dermatology referral service. An Bras Dermatol. 2021;96:295-300. doi:10.1016/j.abd.2020.09.004
- Cheng J, Feldman SR. The cost of biologics for psoriasis is increasing. Drugs Context. 2014;3:212266. doi:10.7573/dic.212266
Practice Points
- The economic burden on patients with psoriasis has been rising over time, as the disease impacts many aspects of patients’ lives.
- Various sociodemographic groups among patients with psoriasis are financially insecure. Knowing which groups are at higher risk for poor outcomes due to financial insecurity can assist with appropriate treatment regimens.
Commentary: Age and breast cancer, and cardiometabolic comorbidities, September 2023
Studies have shown that breast cancer survivors have increased rates of age-related conditions, including cardiovascular disease and osteoporosis among others, therefore postulating that the biological aging process may be accelerated in this population.2 Among 417 women enrolled in the prospective Sister Study cohort, paired blood samples collected an average of 7.7 years apart compared three epigenetic metrics of biological aging (calculated on the basis of DNA methylation data) between women who were diagnosed and treated for breast cancer (n = 190) vs those who remained breast cancer–free (n = 227) (Kresovich et al). Women diagnosed and treated for breast cancer had higher biological aging metrics than women who were cancer-free at the time of follow-up: PhenoAgeAccel3 (standardized mean difference [β] = 0.13; P = .04), GrimAgeAccel4 (β = 0.14; P = .01), and DunedinPACE5 (β = 0.37; P < .001). Regarding breast cancer therapies received, the increases in biological aging were most striking for those women who underwent radiation. The effect of cancer treatments, specifically chemotherapy and radiation, on DNA methylation profiles and accelerating the aging process has been demonstrated in prior studies as well.6 Future research should strive to improve our understanding of the specific mechanisms underlying these age-related changes, identify ways to affect those which are modifiable, and positively influence long-term cognitive and functional consequences.
The association between cardiometabolic abnormalities, including obesity, hyperinsulinemia, diabetes, hypertension, and dyslipidemia, and an elevated breast cancer risk has been demonstrated in various studies.7 Furthermore, dysregulation of obesity-related proteins plays a role in breast cancer development and progression. A study by Xu and colleagues evaluated the temporal relationships and longitudinal associations of body mass index (BMI), cardiometabolic risk score (CRS), and obesity-related protein score (OPS) among 444 healthy women in a breast cancer screening cohort. After adjustment for demographics, lifestyle, and reproductive factors, a 1-kg/m2 increase in BMI per year increased CRS in both premenopausal (0.057 unit; P = .025) and postmenopausal women (0.054 unit; P = .033) and increased OPS by 0.588 unit (P = .001) in postmenopausal women. A significant association was also observed between CRS and OPS in postmenopausal women (β = 0.281; P = .034). These results support the importance of weight management and its effect on cardiometabolic and obesity-related parameters in breast cancer prevention. Research focused on lifestyle interventions to modify risk factors and effective implementation of these techniques will contribute to further reducing breast cancer risk.
Additional References
- García-Albéniz X, Hernán MA, Logan RW, et al. Continuation of annual screening mammography and breast cancer mortality in women older than 70 years. Ann Intern Med. 2020;172(6):381-389. doi: 10.7326/M18-1199
- Greenlee H, Iribarren C, Rana JS, et al. Risk of cardiovascular disease in women with and without breast cancer: The Pathways Heart Study. J Clin Oncol. 2022;40(15):1647-1658. doi: 10.1200/JCO.21.01736
- Levine ME, Lu AT, Quach A, et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging (Albany NY). 2018;10(4):573-591. doi: 10.18632/aging.101414
- Lu AT, Quach A, Wilson JG, et al. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging (Albany NY). 2019;11(2):303-327. doi: 10.18632/aging.101684
- Belsky DW, Caspi A, Corcoran DL, et al. DunedinPACE, a DNA methylation biomarker of the pace of aging. eLife. 2022:11:e73420. doi: 10.7554/eLife.73420
- Sehl ME, Carroll JE, Horvath S, Bower JE. The acute effects of adjuvant radiation and chemotherapy on peripheral blood epigenetic age in early stage breast cancer patients. NPJ Breast Cancer. 2020;6:23. doi: 10.1038/s41523-020-0161-3
- Nouri M, Mohsenpour MA, Katsiki N, et al. Effect of serum lipid profile on the risk of breast cancer: Systematic review and meta-analysis of 1,628,871 women. J Clin Med. 2022;11(15):4503. doi: 10.3390/jcm11154503
Studies have shown that breast cancer survivors have increased rates of age-related conditions, including cardiovascular disease and osteoporosis among others, therefore postulating that the biological aging process may be accelerated in this population.2 Among 417 women enrolled in the prospective Sister Study cohort, paired blood samples collected an average of 7.7 years apart compared three epigenetic metrics of biological aging (calculated on the basis of DNA methylation data) between women who were diagnosed and treated for breast cancer (n = 190) vs those who remained breast cancer–free (n = 227) (Kresovich et al). Women diagnosed and treated for breast cancer had higher biological aging metrics than women who were cancer-free at the time of follow-up: PhenoAgeAccel3 (standardized mean difference [β] = 0.13; P = .04), GrimAgeAccel4 (β = 0.14; P = .01), and DunedinPACE5 (β = 0.37; P < .001). Regarding breast cancer therapies received, the increases in biological aging were most striking for those women who underwent radiation. The effect of cancer treatments, specifically chemotherapy and radiation, on DNA methylation profiles and accelerating the aging process has been demonstrated in prior studies as well.6 Future research should strive to improve our understanding of the specific mechanisms underlying these age-related changes, identify ways to affect those which are modifiable, and positively influence long-term cognitive and functional consequences.
The association between cardiometabolic abnormalities, including obesity, hyperinsulinemia, diabetes, hypertension, and dyslipidemia, and an elevated breast cancer risk has been demonstrated in various studies.7 Furthermore, dysregulation of obesity-related proteins plays a role in breast cancer development and progression. A study by Xu and colleagues evaluated the temporal relationships and longitudinal associations of body mass index (BMI), cardiometabolic risk score (CRS), and obesity-related protein score (OPS) among 444 healthy women in a breast cancer screening cohort. After adjustment for demographics, lifestyle, and reproductive factors, a 1-kg/m2 increase in BMI per year increased CRS in both premenopausal (0.057 unit; P = .025) and postmenopausal women (0.054 unit; P = .033) and increased OPS by 0.588 unit (P = .001) in postmenopausal women. A significant association was also observed between CRS and OPS in postmenopausal women (β = 0.281; P = .034). These results support the importance of weight management and its effect on cardiometabolic and obesity-related parameters in breast cancer prevention. Research focused on lifestyle interventions to modify risk factors and effective implementation of these techniques will contribute to further reducing breast cancer risk.
Additional References
- García-Albéniz X, Hernán MA, Logan RW, et al. Continuation of annual screening mammography and breast cancer mortality in women older than 70 years. Ann Intern Med. 2020;172(6):381-389. doi: 10.7326/M18-1199
- Greenlee H, Iribarren C, Rana JS, et al. Risk of cardiovascular disease in women with and without breast cancer: The Pathways Heart Study. J Clin Oncol. 2022;40(15):1647-1658. doi: 10.1200/JCO.21.01736
- Levine ME, Lu AT, Quach A, et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging (Albany NY). 2018;10(4):573-591. doi: 10.18632/aging.101414
- Lu AT, Quach A, Wilson JG, et al. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging (Albany NY). 2019;11(2):303-327. doi: 10.18632/aging.101684
- Belsky DW, Caspi A, Corcoran DL, et al. DunedinPACE, a DNA methylation biomarker of the pace of aging. eLife. 2022:11:e73420. doi: 10.7554/eLife.73420
- Sehl ME, Carroll JE, Horvath S, Bower JE. The acute effects of adjuvant radiation and chemotherapy on peripheral blood epigenetic age in early stage breast cancer patients. NPJ Breast Cancer. 2020;6:23. doi: 10.1038/s41523-020-0161-3
- Nouri M, Mohsenpour MA, Katsiki N, et al. Effect of serum lipid profile on the risk of breast cancer: Systematic review and meta-analysis of 1,628,871 women. J Clin Med. 2022;11(15):4503. doi: 10.3390/jcm11154503
Studies have shown that breast cancer survivors have increased rates of age-related conditions, including cardiovascular disease and osteoporosis among others, therefore postulating that the biological aging process may be accelerated in this population.2 Among 417 women enrolled in the prospective Sister Study cohort, paired blood samples collected an average of 7.7 years apart compared three epigenetic metrics of biological aging (calculated on the basis of DNA methylation data) between women who were diagnosed and treated for breast cancer (n = 190) vs those who remained breast cancer–free (n = 227) (Kresovich et al). Women diagnosed and treated for breast cancer had higher biological aging metrics than women who were cancer-free at the time of follow-up: PhenoAgeAccel3 (standardized mean difference [β] = 0.13; P = .04), GrimAgeAccel4 (β = 0.14; P = .01), and DunedinPACE5 (β = 0.37; P < .001). Regarding breast cancer therapies received, the increases in biological aging were most striking for those women who underwent radiation. The effect of cancer treatments, specifically chemotherapy and radiation, on DNA methylation profiles and accelerating the aging process has been demonstrated in prior studies as well.6 Future research should strive to improve our understanding of the specific mechanisms underlying these age-related changes, identify ways to affect those which are modifiable, and positively influence long-term cognitive and functional consequences.
The association between cardiometabolic abnormalities, including obesity, hyperinsulinemia, diabetes, hypertension, and dyslipidemia, and an elevated breast cancer risk has been demonstrated in various studies.7 Furthermore, dysregulation of obesity-related proteins plays a role in breast cancer development and progression. A study by Xu and colleagues evaluated the temporal relationships and longitudinal associations of body mass index (BMI), cardiometabolic risk score (CRS), and obesity-related protein score (OPS) among 444 healthy women in a breast cancer screening cohort. After adjustment for demographics, lifestyle, and reproductive factors, a 1-kg/m2 increase in BMI per year increased CRS in both premenopausal (0.057 unit; P = .025) and postmenopausal women (0.054 unit; P = .033) and increased OPS by 0.588 unit (P = .001) in postmenopausal women. A significant association was also observed between CRS and OPS in postmenopausal women (β = 0.281; P = .034). These results support the importance of weight management and its effect on cardiometabolic and obesity-related parameters in breast cancer prevention. Research focused on lifestyle interventions to modify risk factors and effective implementation of these techniques will contribute to further reducing breast cancer risk.
Additional References
- García-Albéniz X, Hernán MA, Logan RW, et al. Continuation of annual screening mammography and breast cancer mortality in women older than 70 years. Ann Intern Med. 2020;172(6):381-389. doi: 10.7326/M18-1199
- Greenlee H, Iribarren C, Rana JS, et al. Risk of cardiovascular disease in women with and without breast cancer: The Pathways Heart Study. J Clin Oncol. 2022;40(15):1647-1658. doi: 10.1200/JCO.21.01736
- Levine ME, Lu AT, Quach A, et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging (Albany NY). 2018;10(4):573-591. doi: 10.18632/aging.101414
- Lu AT, Quach A, Wilson JG, et al. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging (Albany NY). 2019;11(2):303-327. doi: 10.18632/aging.101684
- Belsky DW, Caspi A, Corcoran DL, et al. DunedinPACE, a DNA methylation biomarker of the pace of aging. eLife. 2022:11:e73420. doi: 10.7554/eLife.73420
- Sehl ME, Carroll JE, Horvath S, Bower JE. The acute effects of adjuvant radiation and chemotherapy on peripheral blood epigenetic age in early stage breast cancer patients. NPJ Breast Cancer. 2020;6:23. doi: 10.1038/s41523-020-0161-3
- Nouri M, Mohsenpour MA, Katsiki N, et al. Effect of serum lipid profile on the risk of breast cancer: Systematic review and meta-analysis of 1,628,871 women. J Clin Med. 2022;11(15):4503. doi: 10.3390/jcm11154503
Cystic Presentation of High-Grade Ductal Carcinoma In Situ in an Inframammary Accessory Nipple
To the Editor:
The term ectopic breast tissue serves as an umbrella term that encompasses breast tissue positioned in anatomically incorrect locations, including the subtypes of supernumerary and aberrant breasts.1 However, the more frequently used term is accessory breast tissue (ABT).1 Supernumerary breasts have diverse variations of a nipple, areola, and/or ductal tissue and can span in size from a small mole to a fully functioning breast. This breast type maintains structured ductal systems connected to the overlying skin and experiences regular changes during the reproductive cycle. In contrast, an aberrant breast is isolated breast tissue that does not contain organized ductal systems.1 Accessory breast tissue is prevalent in up to 6.0% of the world population, with Japanese individuals being the most affected and White individuals being the least affected.1
Accessory breasts typically are located along the milk line—the embryologic precursor to mammary glands and nipples, which extend from the axillae to the groin and regress from the caudal end spanning to the groin.2 For this reason, incomplete regression of the mammary ridge results in ABT, most commonly in the axillary region.3 Accessory breast tissue usually is benign and is considered an anatomical variant; however, because the histomorphology is similar to mammary gland tissue, accessory breasts have the same proliferative potential as anatomically correct breasts and therefore can form fibroadenomas, cysts, abscesses, mastitis, or breast cancer.4 Accessory breast carcinomas comprise 0.3% to 0.6% of all breast malignancies.5 Certain genodermatoses (ie, Cowden syndrome) also may predispose patients to benign or malignant pathology in ABT.6 We present a rare case of accessory breast cancer in the inframammary region masquerading as a cyst. These findings were further supported by ultrasonography and mammography.
A 45-year-old White woman presented to our clinic for removal of a dermal mass underlying a supernumerary nipple at the left inframammary fold. Her medical history was noncontributory and was only remarkable for uterine fibroids. She developed pain and swelling in the left breast 1 year prior, which prompted her to seek medical attention from her primary care physician. Diagnostic mammography was negative for any concerning malignant nodules, and subsequent BRCA genetic testing also was negative. Six months after the diagnostic mammography, she continued to experience pain and swelling in the left breast and was then referred for diagnostic ultrasonography; 2 masses in the left breast suspected as infected cysts with rupture were identified (Figure 1). She was then referred to our dermatology clinic for evaluation and surgical extirpation of the suspected cyst underlying the accessory breast. The area subsequently was excised under local anesthesia, and a second similar but smaller mass also was identified adjacent to the initial growth. Dermatopathologic examination revealed an estrogen receptor– (Figure 2A) and progesterone receptor–positive (Figure 2B), ERBB2 (HER2/neu)–negative, nuclear grade III ductal carcinoma in situ (Figure 3).
Various ABT classification methods have been proposed with Brightmore7 categorizing polymastia into 8 subtypes: (1) complete breast; (2) glandular tissue and nipple; (3) glandular tissue and areola; (4) glandular tissue only; (5) nipple, areola, and fat; (6) nipple only; (7) areola only; and (8) patch of hair only. De Cholnokey8 focused on axillary polymastia, dividing it into 4 classes: (1) axillary tumor in milk line without nipple or areola; (2) axillary tumor with areola with or without pigmentation; (3) nipple or areola without underlying breast tissue; and (4) complete breast with nipple, areola, and glandular tissue. Fenench’s9 method is preferred and simply describes ABT as 2 subtypes: supernumerary and aberrant.1,2,10 One study observed 6% of ABT cancers were the supernumerary type and 94% were the aberrant type.1 Ductal lumen stagnation increases the risk for accessory breast carcinoma development.10 Men have a higher prevalence of cancer in ABT compared to anatomically correct breast tissue.11
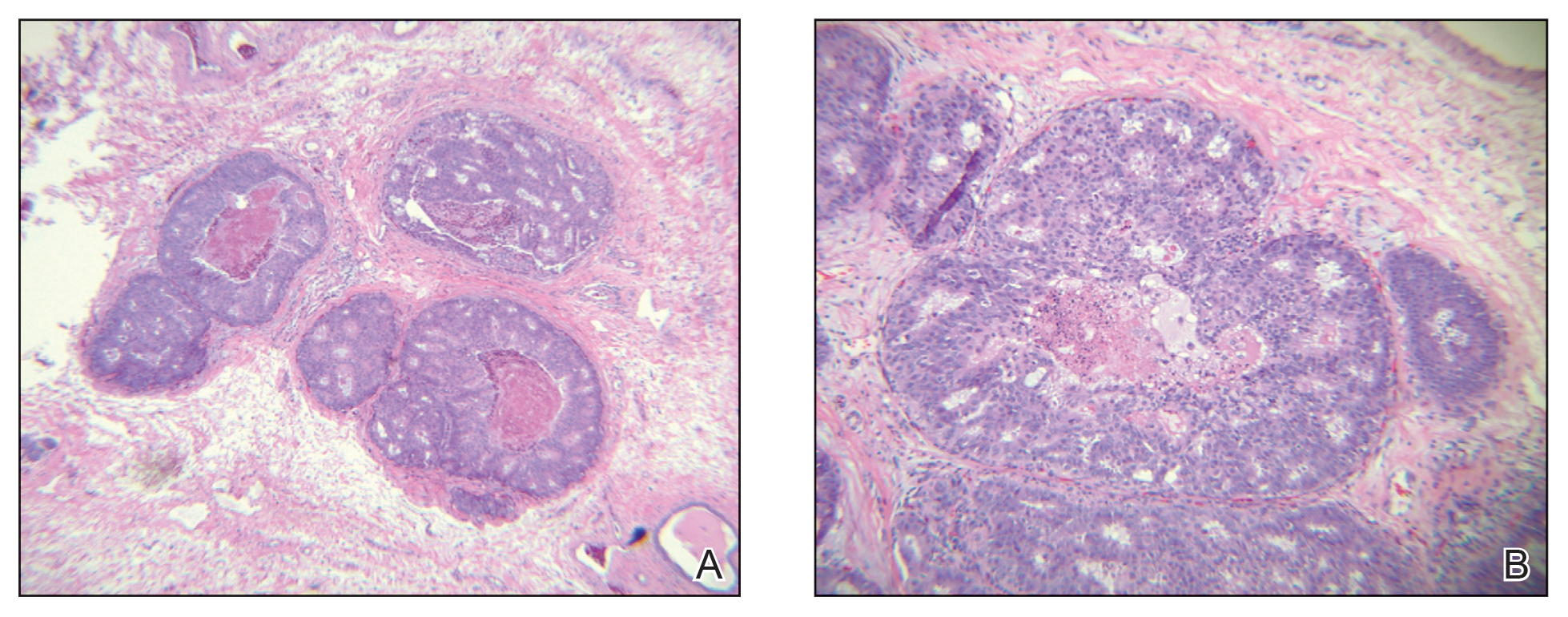
There currently is no standardized guideline for ABT cancer treatment. The initial clinical impression of cancer of ABT may be misdiagnosed as lymphadenopathy, abscesses, or lipomas.12 The risk for misdiagnosis is higher for cancer of ABT compared to normal breast tissue and is associated with a poorer prognosis.1 Despite multiple screening modalities, our patient’s initial breast cancer screenings proved unreliable. A mammogram failed to detect malignancy, likely secondary to the area of concern being out of the standard imaging field. Ultrasonography also was unreliable and led to misdiagnosis as an infected sebaceous cyst with rupture in our patient. Upon review of the ultrasound, concerns were raised by dermatology that the mass was more likely an epidermal inclusion cyst with rupture given the more superficial and sac-free nature of sebaceous cysts, which commonly are associated with steatocystoma multiplex.13 Definitive diagnosis of ductal carcinoma in situ was made with dermatopathologic examination.
Prophylactic surgical excision of ABT has been recommended, suggesting that excisional biopsy and histopathologic examination is the more appropriate method to rule out malignancy. Surgical treatment of ABT may omit any risk for malignant transformation and may provide psychological relief to patients for aesthetic reasons.10,12,14 The risk and benefits of prophylactic excision of ABT has been compared to prophylactic mastectomy of anatomically correct breasts,15 with some clinicians considering this definitive procedure unnecessary except in high-risk patients with a strong genetic predisposition.16,17
Accessory breast tissue should be viewed as an anatomical variant with the option of surgical removal for symptomatic concerns, such as firm nodules, discharge, and pain. Although ABT is rare and cancer in ABT is even more uncommon (<1% of all breast cancers),5,11 clinicians should be suspicious of benign diagnostic reports when the clinical situation does not fit the proposed narrative.
- Marshall MB, Moynihan JJ, Frost A, et al. Ectopic breast cancer: case report and literature review. Surg Oncol. 1994;3:295-304. doi:10.1016/0960-7404(94)90032-9
- DeFilippis EM, Arleo EK. The ABCs of accessory breast tissue: basic information every radiologist should know. Am J Roentgenol. 2014;202:1157-1162. doi:10.2214/AJR.13.10930
- Famá F, Cicciú M, Sindoni A, et al. Prevalence of ectopic breast tissue and tumor: a 20-year single center experience. Clin Breast Cancer. 2016;16:E107-E112. doi:10.1016/j.clbc.2016.03.004
- Brown J, Schwartz RA. Supernumerary nipples: an overview. Cutis. 2003;71:344-346.
- Nihon-Yanagi Y, Ueda T, Kameda N, et al. A case of ectopic breast cancer with a literature review. Surg Oncol. 2011;20:35-42. doi:10.1016/j.suronc.2009.09.005
- Hedayat AA, Pettus JR, Marotti JD, et al. Proliferative lesion of anogenital mammary-like glands in the setting of Cowden syndrome: case report and review of the literature. J Cutan Pathol. 2016;43:707-710. doi:10.1111/cup.12721
- Brightmore T. Bilateral double nipples. Br J Surg. 1972;59:55-57. https://doi.org/10.1002/bjs.1800590114
- De Cholnoky T. Accessory breast tissue in the axilla. N Y State J Med. 1951;51:2245-2248.
- Fenech HB. Aberrant breast tissue; case report. Harper Hosp Bull. 1949;7:268-271.
- Francone E, Nathan MJ, Murelli F, et al. Ectopic breast cancer: case report and review of the literature. Aesthetic Plast Surg. 2013;37:746-749. doi:10.1007/s00266-013-0125-1
- Yamamura J, Masuda N, Kodama Y, et al. Male breast cancer originating in an accessory mammary gland in the axilla: a case report. Case Rep Med. 2012;2012:286210. doi:10.1155/2012/286210.
- Ghosn SH, Khatri KA, Bhawan J. Bilateral aberrant axillary breast tissue mimicking lipomas: report of a case and review of the literature. J Cutan Pathol. 2007;34(suppl 1):9-13. doi:10.1111/j.1600-0560.2006.00713.x
- Arceu M, Martinez G, Alfaro D, et al. Ultrasound morphologic features of steatocystoma multiplex with clinical correlation. J Ultrasound Med. 2020;39:2255-2260. doi:10.1002/jum.15320
- Lesavoy MA, Gomez-Garcia A, Nejdl R, et al. Axillary breast tissue: clinical presentation and surgical treatment. Ann Plast Surg. 1995;35:356-360. doi:10.1097/00000637-199510000-00004
- Bank J. Management of ectopic breast tissue. Aesthetic Plast Surg. 2013;37:750-751. doi:10.1007/s00266-013-0143-z
- Morrow M. Prophylactic mastectomy of the contralateral breast. Breast. 2011;20(suppl 3):S108-S110. doi:10.1016/S0960-9776(11)70306-X
- Teoh V, Tasoulis M-K, Gui G. Contralateral prophylactic mastectomy in women with unilateral breast cancer who are genetic carriers, have a strong family history or are just young at presentation. Cancers (Basel). 2020;12:140. doi:10.3390/cancers12010140
To the Editor:
The term ectopic breast tissue serves as an umbrella term that encompasses breast tissue positioned in anatomically incorrect locations, including the subtypes of supernumerary and aberrant breasts.1 However, the more frequently used term is accessory breast tissue (ABT).1 Supernumerary breasts have diverse variations of a nipple, areola, and/or ductal tissue and can span in size from a small mole to a fully functioning breast. This breast type maintains structured ductal systems connected to the overlying skin and experiences regular changes during the reproductive cycle. In contrast, an aberrant breast is isolated breast tissue that does not contain organized ductal systems.1 Accessory breast tissue is prevalent in up to 6.0% of the world population, with Japanese individuals being the most affected and White individuals being the least affected.1
Accessory breasts typically are located along the milk line—the embryologic precursor to mammary glands and nipples, which extend from the axillae to the groin and regress from the caudal end spanning to the groin.2 For this reason, incomplete regression of the mammary ridge results in ABT, most commonly in the axillary region.3 Accessory breast tissue usually is benign and is considered an anatomical variant; however, because the histomorphology is similar to mammary gland tissue, accessory breasts have the same proliferative potential as anatomically correct breasts and therefore can form fibroadenomas, cysts, abscesses, mastitis, or breast cancer.4 Accessory breast carcinomas comprise 0.3% to 0.6% of all breast malignancies.5 Certain genodermatoses (ie, Cowden syndrome) also may predispose patients to benign or malignant pathology in ABT.6 We present a rare case of accessory breast cancer in the inframammary region masquerading as a cyst. These findings were further supported by ultrasonography and mammography.

A 45-year-old White woman presented to our clinic for removal of a dermal mass underlying a supernumerary nipple at the left inframammary fold. Her medical history was noncontributory and was only remarkable for uterine fibroids. She developed pain and swelling in the left breast 1 year prior, which prompted her to seek medical attention from her primary care physician. Diagnostic mammography was negative for any concerning malignant nodules, and subsequent BRCA genetic testing also was negative. Six months after the diagnostic mammography, she continued to experience pain and swelling in the left breast and was then referred for diagnostic ultrasonography; 2 masses in the left breast suspected as infected cysts with rupture were identified (Figure 1). She was then referred to our dermatology clinic for evaluation and surgical extirpation of the suspected cyst underlying the accessory breast. The area subsequently was excised under local anesthesia, and a second similar but smaller mass also was identified adjacent to the initial growth. Dermatopathologic examination revealed an estrogen receptor– (Figure 2A) and progesterone receptor–positive (Figure 2B), ERBB2 (HER2/neu)–negative, nuclear grade III ductal carcinoma in situ (Figure 3).

Various ABT classification methods have been proposed with Brightmore7 categorizing polymastia into 8 subtypes: (1) complete breast; (2) glandular tissue and nipple; (3) glandular tissue and areola; (4) glandular tissue only; (5) nipple, areola, and fat; (6) nipple only; (7) areola only; and (8) patch of hair only. De Cholnokey8 focused on axillary polymastia, dividing it into 4 classes: (1) axillary tumor in milk line without nipple or areola; (2) axillary tumor with areola with or without pigmentation; (3) nipple or areola without underlying breast tissue; and (4) complete breast with nipple, areola, and glandular tissue. Fenench’s9 method is preferred and simply describes ABT as 2 subtypes: supernumerary and aberrant.1,2,10 One study observed 6% of ABT cancers were the supernumerary type and 94% were the aberrant type.1 Ductal lumen stagnation increases the risk for accessory breast carcinoma development.10 Men have a higher prevalence of cancer in ABT compared to anatomically correct breast tissue.11

There currently is no standardized guideline for ABT cancer treatment. The initial clinical impression of cancer of ABT may be misdiagnosed as lymphadenopathy, abscesses, or lipomas.12 The risk for misdiagnosis is higher for cancer of ABT compared to normal breast tissue and is associated with a poorer prognosis.1 Despite multiple screening modalities, our patient’s initial breast cancer screenings proved unreliable. A mammogram failed to detect malignancy, likely secondary to the area of concern being out of the standard imaging field. Ultrasonography also was unreliable and led to misdiagnosis as an infected sebaceous cyst with rupture in our patient. Upon review of the ultrasound, concerns were raised by dermatology that the mass was more likely an epidermal inclusion cyst with rupture given the more superficial and sac-free nature of sebaceous cysts, which commonly are associated with steatocystoma multiplex.13 Definitive diagnosis of ductal carcinoma in situ was made with dermatopathologic examination.
Prophylactic surgical excision of ABT has been recommended, suggesting that excisional biopsy and histopathologic examination is the more appropriate method to rule out malignancy. Surgical treatment of ABT may omit any risk for malignant transformation and may provide psychological relief to patients for aesthetic reasons.10,12,14 The risk and benefits of prophylactic excision of ABT has been compared to prophylactic mastectomy of anatomically correct breasts,15 with some clinicians considering this definitive procedure unnecessary except in high-risk patients with a strong genetic predisposition.16,17
Accessory breast tissue should be viewed as an anatomical variant with the option of surgical removal for symptomatic concerns, such as firm nodules, discharge, and pain. Although ABT is rare and cancer in ABT is even more uncommon (<1% of all breast cancers),5,11 clinicians should be suspicious of benign diagnostic reports when the clinical situation does not fit the proposed narrative.
To the Editor:
The term ectopic breast tissue serves as an umbrella term that encompasses breast tissue positioned in anatomically incorrect locations, including the subtypes of supernumerary and aberrant breasts.1 However, the more frequently used term is accessory breast tissue (ABT).1 Supernumerary breasts have diverse variations of a nipple, areola, and/or ductal tissue and can span in size from a small mole to a fully functioning breast. This breast type maintains structured ductal systems connected to the overlying skin and experiences regular changes during the reproductive cycle. In contrast, an aberrant breast is isolated breast tissue that does not contain organized ductal systems.1 Accessory breast tissue is prevalent in up to 6.0% of the world population, with Japanese individuals being the most affected and White individuals being the least affected.1
Accessory breasts typically are located along the milk line—the embryologic precursor to mammary glands and nipples, which extend from the axillae to the groin and regress from the caudal end spanning to the groin.2 For this reason, incomplete regression of the mammary ridge results in ABT, most commonly in the axillary region.3 Accessory breast tissue usually is benign and is considered an anatomical variant; however, because the histomorphology is similar to mammary gland tissue, accessory breasts have the same proliferative potential as anatomically correct breasts and therefore can form fibroadenomas, cysts, abscesses, mastitis, or breast cancer.4 Accessory breast carcinomas comprise 0.3% to 0.6% of all breast malignancies.5 Certain genodermatoses (ie, Cowden syndrome) also may predispose patients to benign or malignant pathology in ABT.6 We present a rare case of accessory breast cancer in the inframammary region masquerading as a cyst. These findings were further supported by ultrasonography and mammography.

A 45-year-old White woman presented to our clinic for removal of a dermal mass underlying a supernumerary nipple at the left inframammary fold. Her medical history was noncontributory and was only remarkable for uterine fibroids. She developed pain and swelling in the left breast 1 year prior, which prompted her to seek medical attention from her primary care physician. Diagnostic mammography was negative for any concerning malignant nodules, and subsequent BRCA genetic testing also was negative. Six months after the diagnostic mammography, she continued to experience pain and swelling in the left breast and was then referred for diagnostic ultrasonography; 2 masses in the left breast suspected as infected cysts with rupture were identified (Figure 1). She was then referred to our dermatology clinic for evaluation and surgical extirpation of the suspected cyst underlying the accessory breast. The area subsequently was excised under local anesthesia, and a second similar but smaller mass also was identified adjacent to the initial growth. Dermatopathologic examination revealed an estrogen receptor– (Figure 2A) and progesterone receptor–positive (Figure 2B), ERBB2 (HER2/neu)–negative, nuclear grade III ductal carcinoma in situ (Figure 3).

Various ABT classification methods have been proposed with Brightmore7 categorizing polymastia into 8 subtypes: (1) complete breast; (2) glandular tissue and nipple; (3) glandular tissue and areola; (4) glandular tissue only; (5) nipple, areola, and fat; (6) nipple only; (7) areola only; and (8) patch of hair only. De Cholnokey8 focused on axillary polymastia, dividing it into 4 classes: (1) axillary tumor in milk line without nipple or areola; (2) axillary tumor with areola with or without pigmentation; (3) nipple or areola without underlying breast tissue; and (4) complete breast with nipple, areola, and glandular tissue. Fenench’s9 method is preferred and simply describes ABT as 2 subtypes: supernumerary and aberrant.1,2,10 One study observed 6% of ABT cancers were the supernumerary type and 94% were the aberrant type.1 Ductal lumen stagnation increases the risk for accessory breast carcinoma development.10 Men have a higher prevalence of cancer in ABT compared to anatomically correct breast tissue.11

There currently is no standardized guideline for ABT cancer treatment. The initial clinical impression of cancer of ABT may be misdiagnosed as lymphadenopathy, abscesses, or lipomas.12 The risk for misdiagnosis is higher for cancer of ABT compared to normal breast tissue and is associated with a poorer prognosis.1 Despite multiple screening modalities, our patient’s initial breast cancer screenings proved unreliable. A mammogram failed to detect malignancy, likely secondary to the area of concern being out of the standard imaging field. Ultrasonography also was unreliable and led to misdiagnosis as an infected sebaceous cyst with rupture in our patient. Upon review of the ultrasound, concerns were raised by dermatology that the mass was more likely an epidermal inclusion cyst with rupture given the more superficial and sac-free nature of sebaceous cysts, which commonly are associated with steatocystoma multiplex.13 Definitive diagnosis of ductal carcinoma in situ was made with dermatopathologic examination.
Prophylactic surgical excision of ABT has been recommended, suggesting that excisional biopsy and histopathologic examination is the more appropriate method to rule out malignancy. Surgical treatment of ABT may omit any risk for malignant transformation and may provide psychological relief to patients for aesthetic reasons.10,12,14 The risk and benefits of prophylactic excision of ABT has been compared to prophylactic mastectomy of anatomically correct breasts,15 with some clinicians considering this definitive procedure unnecessary except in high-risk patients with a strong genetic predisposition.16,17
Accessory breast tissue should be viewed as an anatomical variant with the option of surgical removal for symptomatic concerns, such as firm nodules, discharge, and pain. Although ABT is rare and cancer in ABT is even more uncommon (<1% of all breast cancers),5,11 clinicians should be suspicious of benign diagnostic reports when the clinical situation does not fit the proposed narrative.
- Marshall MB, Moynihan JJ, Frost A, et al. Ectopic breast cancer: case report and literature review. Surg Oncol. 1994;3:295-304. doi:10.1016/0960-7404(94)90032-9
- DeFilippis EM, Arleo EK. The ABCs of accessory breast tissue: basic information every radiologist should know. Am J Roentgenol. 2014;202:1157-1162. doi:10.2214/AJR.13.10930
- Famá F, Cicciú M, Sindoni A, et al. Prevalence of ectopic breast tissue and tumor: a 20-year single center experience. Clin Breast Cancer. 2016;16:E107-E112. doi:10.1016/j.clbc.2016.03.004
- Brown J, Schwartz RA. Supernumerary nipples: an overview. Cutis. 2003;71:344-346.
- Nihon-Yanagi Y, Ueda T, Kameda N, et al. A case of ectopic breast cancer with a literature review. Surg Oncol. 2011;20:35-42. doi:10.1016/j.suronc.2009.09.005
- Hedayat AA, Pettus JR, Marotti JD, et al. Proliferative lesion of anogenital mammary-like glands in the setting of Cowden syndrome: case report and review of the literature. J Cutan Pathol. 2016;43:707-710. doi:10.1111/cup.12721
- Brightmore T. Bilateral double nipples. Br J Surg. 1972;59:55-57. https://doi.org/10.1002/bjs.1800590114
- De Cholnoky T. Accessory breast tissue in the axilla. N Y State J Med. 1951;51:2245-2248.
- Fenech HB. Aberrant breast tissue; case report. Harper Hosp Bull. 1949;7:268-271.
- Francone E, Nathan MJ, Murelli F, et al. Ectopic breast cancer: case report and review of the literature. Aesthetic Plast Surg. 2013;37:746-749. doi:10.1007/s00266-013-0125-1
- Yamamura J, Masuda N, Kodama Y, et al. Male breast cancer originating in an accessory mammary gland in the axilla: a case report. Case Rep Med. 2012;2012:286210. doi:10.1155/2012/286210.
- Ghosn SH, Khatri KA, Bhawan J. Bilateral aberrant axillary breast tissue mimicking lipomas: report of a case and review of the literature. J Cutan Pathol. 2007;34(suppl 1):9-13. doi:10.1111/j.1600-0560.2006.00713.x
- Arceu M, Martinez G, Alfaro D, et al. Ultrasound morphologic features of steatocystoma multiplex with clinical correlation. J Ultrasound Med. 2020;39:2255-2260. doi:10.1002/jum.15320
- Lesavoy MA, Gomez-Garcia A, Nejdl R, et al. Axillary breast tissue: clinical presentation and surgical treatment. Ann Plast Surg. 1995;35:356-360. doi:10.1097/00000637-199510000-00004
- Bank J. Management of ectopic breast tissue. Aesthetic Plast Surg. 2013;37:750-751. doi:10.1007/s00266-013-0143-z
- Morrow M. Prophylactic mastectomy of the contralateral breast. Breast. 2011;20(suppl 3):S108-S110. doi:10.1016/S0960-9776(11)70306-X
- Teoh V, Tasoulis M-K, Gui G. Contralateral prophylactic mastectomy in women with unilateral breast cancer who are genetic carriers, have a strong family history or are just young at presentation. Cancers (Basel). 2020;12:140. doi:10.3390/cancers12010140
- Marshall MB, Moynihan JJ, Frost A, et al. Ectopic breast cancer: case report and literature review. Surg Oncol. 1994;3:295-304. doi:10.1016/0960-7404(94)90032-9
- DeFilippis EM, Arleo EK. The ABCs of accessory breast tissue: basic information every radiologist should know. Am J Roentgenol. 2014;202:1157-1162. doi:10.2214/AJR.13.10930
- Famá F, Cicciú M, Sindoni A, et al. Prevalence of ectopic breast tissue and tumor: a 20-year single center experience. Clin Breast Cancer. 2016;16:E107-E112. doi:10.1016/j.clbc.2016.03.004
- Brown J, Schwartz RA. Supernumerary nipples: an overview. Cutis. 2003;71:344-346.
- Nihon-Yanagi Y, Ueda T, Kameda N, et al. A case of ectopic breast cancer with a literature review. Surg Oncol. 2011;20:35-42. doi:10.1016/j.suronc.2009.09.005
- Hedayat AA, Pettus JR, Marotti JD, et al. Proliferative lesion of anogenital mammary-like glands in the setting of Cowden syndrome: case report and review of the literature. J Cutan Pathol. 2016;43:707-710. doi:10.1111/cup.12721
- Brightmore T. Bilateral double nipples. Br J Surg. 1972;59:55-57. https://doi.org/10.1002/bjs.1800590114
- De Cholnoky T. Accessory breast tissue in the axilla. N Y State J Med. 1951;51:2245-2248.
- Fenech HB. Aberrant breast tissue; case report. Harper Hosp Bull. 1949;7:268-271.
- Francone E, Nathan MJ, Murelli F, et al. Ectopic breast cancer: case report and review of the literature. Aesthetic Plast Surg. 2013;37:746-749. doi:10.1007/s00266-013-0125-1
- Yamamura J, Masuda N, Kodama Y, et al. Male breast cancer originating in an accessory mammary gland in the axilla: a case report. Case Rep Med. 2012;2012:286210. doi:10.1155/2012/286210.
- Ghosn SH, Khatri KA, Bhawan J. Bilateral aberrant axillary breast tissue mimicking lipomas: report of a case and review of the literature. J Cutan Pathol. 2007;34(suppl 1):9-13. doi:10.1111/j.1600-0560.2006.00713.x
- Arceu M, Martinez G, Alfaro D, et al. Ultrasound morphologic features of steatocystoma multiplex with clinical correlation. J Ultrasound Med. 2020;39:2255-2260. doi:10.1002/jum.15320
- Lesavoy MA, Gomez-Garcia A, Nejdl R, et al. Axillary breast tissue: clinical presentation and surgical treatment. Ann Plast Surg. 1995;35:356-360. doi:10.1097/00000637-199510000-00004
- Bank J. Management of ectopic breast tissue. Aesthetic Plast Surg. 2013;37:750-751. doi:10.1007/s00266-013-0143-z
- Morrow M. Prophylactic mastectomy of the contralateral breast. Breast. 2011;20(suppl 3):S108-S110. doi:10.1016/S0960-9776(11)70306-X
- Teoh V, Tasoulis M-K, Gui G. Contralateral prophylactic mastectomy in women with unilateral breast cancer who are genetic carriers, have a strong family history or are just young at presentation. Cancers (Basel). 2020;12:140. doi:10.3390/cancers12010140
Practice Points
- Accessory breasts (also referred to as ectopic breast tissue) develop when breast tissue is retained along the mammary ridge outside of the usual pectoral regions.
- Because accessory breasts may contain the same structures as anatomically correct breasts, they can be subject to the same benign or malignant changes.
- Clinical and pathologic correlation is prudent when interpreting ectopic mammary tissue, as various benign or malignant neoplasms may arise in this setting, especially if there are underlying genetic aberrancies or genodermatoses.
ChatGPT in Dermatology Clinical Practice: Potential Uses and Pitfalls
Artificial intelligence (AI) technology has increasingly been incorporated in medicine. In dermatology, AI has been used to detect and diagnose skin lesions, including skin cancer.1 ChatGPT (OpenAI) is a novel, highly popular development in generative AI technology. A large language model released in 2022, ChatGPT is a chatbot designed to mimic human conversation and generate specific detailed information when prompted. Free and publicly available, it has been used by millions of people. ChatGPT’s application in the medical field currently is being evaluated across several specialties, including plastic surgery, radiology, and urology.2-4 ChatGPT has the potential to assist health care professionals, including dermatologists, though its use raises important ethical considerations. Herein, we focus on the potential benefits as well as the pitfalls of using ChatGPT in dermatology clinical practice.
Potential Uses of ChatGPT in Practice
A major benefit of ChatGPT is its ability to improve clinical efficiency. First, ChatGPT can provide quick access to general medical information, similar to a search engine but with more natural language processing and contextual understanding to synthesize information.5 This function is useful for rapid concise answers to specific and directed questions. ChatGPT also can interact with its user by asking follow-up questions to produce more precise and relevant responses; this feature may help dermatologists form more accurate differential diagnoses. Additionally, ChatGPT can increase efficiency in clinical practice by drafting generic medical documents,2 including templates for after-visit summaries, postprocedure instructions, referrals, prior authorization appeal letters, and educational handouts. Importantly, increased efficiency can reduce provider burnout and lead to improved patient care. Another useful feature of ChatGPT is its ability to output information modeling human conversation. Because of this feature, ChatGPT also could be employed in clinical practice to serve as an interpreter for patients during clinic visits. Currently, the use of virtual translators can be cumbersome and subject to technical constraints. ChatGPT can provide accurate and conversational translations for patients and dermatologists, improving the patient-provider relationship.
ChatGPT also can contribute to major advancements in the field of dermatology beyond the clinical setting. Because of its ability to draw from extensive data that have already been uploaded, there are some uses of ChatGPT in a research context: to assist in finding resources for research and reviews, formulating hypotheses, drafting study protocols, and collecting large amounts of data within seconds.6
ChatGPT also has potential in advancing medical education. It could be used by medical schools to model interactive patient encounters to help students practice taking a patient’s history and creating differential diagnoses.6 This application of ChatGPT may help medical students hone their clinical skills in a low-stress environment without the restrictions that can come with hiring and training standardized patients, especially when mimicking dermatologic clinical encounters.
Other possibilities for ChatGPT in dermatologic practice include survey administration, clinical trial recruitment, and even automatic high-risk medication monitoring. Despite the many potential applications of ChatGPT in clinical practice, the question raised in each scenario is the quality, accuracy, and safety of what it produces.
Potential Pitfalls of ChatGPT in Practice and Possible Mitigation Strategies
A main concern in using ChatGPT in clinical practice is its potential to produce inaccurate or biased information. When prompted to create a research abstract based on previously published research, ChatGPT drafted abstracts that were clear and digestible but supplemented with incorrect data.7 A group of medical researchers who reviewed these ChatGPT-generated abstracts mistook 32% of the abstracts as having been written by human researchers. The implications of this finding are worrisome. If inaccurate or false information is used by ChatGPT in documents sent to insurance companies or patients, the patient’s safety as well as the dermatologist’s license and credibility are at stake. Thus, dermatologists looking to use ChatGPT to draft generic medical documents should actively review the output to ensure that the information is accurate. Importantly, ChatGPT also is only currently programmed with information up to 2021, limiting its access to recently published research articles and updated International Classification of Diseases, Tenth Revision codes.5 The continued development of ChatGPT will lead to regular updates by OpenAI that resolve this shortcoming in the future. Further, AI models may encode and perpetuate harmful stereotypes and social biases that are present in training data.8
When considering its potential in clinical practice, ChatGPT itself states that it can aid in clinical decision-making by processing patient information, including history, current symptoms, and biopsy and test results. This is uncharted territory, and providers who use ChatGPT at this stage to aid in decision-making should regard it similarly to information retrieved from a search engine. The information produced by ChatGPT should be used to substantiate but not definitively confirm a diagnosis or dictate management. Clinical suspicion by the dermatologist should always trump ChatGPT output. If physicians recommend ChatGPT output over their own advice, it can become a liability, as the technology is not regulated to any degree. Patients also should be cautious when seeking and following medical advice from ChatGPT, as it could be misleading or incorrect and could undermine the patient-physician relationship.6
Additionally, the specific use of ChatGPT to evaluate patient health records raises concerns related to patient privacy and confidentiality. Providers should never enter patient information protected by the Health Insurance Portability and Accountability Act into ChatGPT or anywhere else on the internet. Mitigation of this risk is not currently possible; ChatGPT requires more development and further understanding to even consider its integration into current electronic health records without compromising patient confidentiality.
The Bottom Line
Overall, ChatGPT has the potential to improve efficiency in clinical practice by assisting with informational searches and document drafting. Importantly, increased efficiency can reduce provider burnout and lead to improved patient care. However, dermatologists should carefully weigh the ethical issues of nonmaleficence and justice in evaluating ChatGPT for accuracy, biases, and the protection of patient confidentiality.
Finally, we asked ChatGPT on February 28, 2023, what it thinks:
User: Should dermatologists use ChatGPT in clinical practice?
ChatGPT: As an AI language model, ChatGPT can be a useful tool in dermatology clinical practice, but it should be used with caution in conjunction with other sources of information and clinical judgement.
Dermatologists should be aware of the potential benefits and risks of using ChatGPT and make informed decisions about how to integrate it into their clinical practice. It’s important to remember that ChatGPT is not a substitute for clinical expertise or human interaction with patients, and it should only be used as a supplement to enhance the quality of patient care.
Dermatologists who choose to use ChatGPT should also be aware of its limitations and potential risks, such as limited accuracy, lack of context, and liability issues if it provides incorrect information or recommendations that lead to patient harm. Dermatologists should use their professional judgement and consult with other health care professionals when making important decisions regarding patient care.
- Thomsen K, Iversen L, Titlestad TL, et al. Systematic review of machine learning for diagnosis and prognosis in dermatology. J Dermatol Treat. 2020;31:496-510. doi:10.1080/09546634.2019.1682500
- Shen Y, Heacock L, Elias J, et al. ChatGPT and other large language models are double-edged swords. Radiology. 2023;307:E230163. doi:10.1148/radiol.230163
- Gupta R, Pande P, Herzog I, et al. Application of ChatGPT in cosmetic plastic surgery: ally or antagonist? Aesthet Surg J. 2023;43:NP587-NP590. doi: 10.1093/asj/sjad042
- Gabrielson AT, Odisho AY, Canes D. Harnessing generative artificial intelligence to improve efficiency among urologists: welcome ChatGPT. J Urol. 2023;209:827-829. doi:10.1097/JU.0000000000003383
- What is ChatGPT? OpenAI. Accessed August 10, 2023. https://help.openai.com/en/articles/6783457-chatgpt-general-faq
- Haupt CE, Marks M. AI-generated medical advice—GPT and beyond. JAMA. 2023;329:1349-1350. doi:10.1001/jama.2023.5321
- Gao CA, Howard FM, Markov NS, et al. Comparing Scientific Abstracts Generated by ChatGPT to Original Abstracts Using an Artificial Intelligence Output Detector, Plagiarism Detector, and Blinded Human Reviewers. Scientific Communication and Education; 2022. doi:10.1101/2022.12.23.521610
- Weidinger L, Mellor J, Rauh M, et al. Ethical and social risks of harm from language models. arXiv. Preprint posted online December 8, 2021. https://doi.org/10.48550/arXiv.2112.04359
Artificial intelligence (AI) technology has increasingly been incorporated in medicine. In dermatology, AI has been used to detect and diagnose skin lesions, including skin cancer.1 ChatGPT (OpenAI) is a novel, highly popular development in generative AI technology. A large language model released in 2022, ChatGPT is a chatbot designed to mimic human conversation and generate specific detailed information when prompted. Free and publicly available, it has been used by millions of people. ChatGPT’s application in the medical field currently is being evaluated across several specialties, including plastic surgery, radiology, and urology.2-4 ChatGPT has the potential to assist health care professionals, including dermatologists, though its use raises important ethical considerations. Herein, we focus on the potential benefits as well as the pitfalls of using ChatGPT in dermatology clinical practice.
Potential Uses of ChatGPT in Practice
A major benefit of ChatGPT is its ability to improve clinical efficiency. First, ChatGPT can provide quick access to general medical information, similar to a search engine but with more natural language processing and contextual understanding to synthesize information.5 This function is useful for rapid concise answers to specific and directed questions. ChatGPT also can interact with its user by asking follow-up questions to produce more precise and relevant responses; this feature may help dermatologists form more accurate differential diagnoses. Additionally, ChatGPT can increase efficiency in clinical practice by drafting generic medical documents,2 including templates for after-visit summaries, postprocedure instructions, referrals, prior authorization appeal letters, and educational handouts. Importantly, increased efficiency can reduce provider burnout and lead to improved patient care. Another useful feature of ChatGPT is its ability to output information modeling human conversation. Because of this feature, ChatGPT also could be employed in clinical practice to serve as an interpreter for patients during clinic visits. Currently, the use of virtual translators can be cumbersome and subject to technical constraints. ChatGPT can provide accurate and conversational translations for patients and dermatologists, improving the patient-provider relationship.
ChatGPT also can contribute to major advancements in the field of dermatology beyond the clinical setting. Because of its ability to draw from extensive data that have already been uploaded, there are some uses of ChatGPT in a research context: to assist in finding resources for research and reviews, formulating hypotheses, drafting study protocols, and collecting large amounts of data within seconds.6
ChatGPT also has potential in advancing medical education. It could be used by medical schools to model interactive patient encounters to help students practice taking a patient’s history and creating differential diagnoses.6 This application of ChatGPT may help medical students hone their clinical skills in a low-stress environment without the restrictions that can come with hiring and training standardized patients, especially when mimicking dermatologic clinical encounters.
Other possibilities for ChatGPT in dermatologic practice include survey administration, clinical trial recruitment, and even automatic high-risk medication monitoring. Despite the many potential applications of ChatGPT in clinical practice, the question raised in each scenario is the quality, accuracy, and safety of what it produces.
Potential Pitfalls of ChatGPT in Practice and Possible Mitigation Strategies
A main concern in using ChatGPT in clinical practice is its potential to produce inaccurate or biased information. When prompted to create a research abstract based on previously published research, ChatGPT drafted abstracts that were clear and digestible but supplemented with incorrect data.7 A group of medical researchers who reviewed these ChatGPT-generated abstracts mistook 32% of the abstracts as having been written by human researchers. The implications of this finding are worrisome. If inaccurate or false information is used by ChatGPT in documents sent to insurance companies or patients, the patient’s safety as well as the dermatologist’s license and credibility are at stake. Thus, dermatologists looking to use ChatGPT to draft generic medical documents should actively review the output to ensure that the information is accurate. Importantly, ChatGPT also is only currently programmed with information up to 2021, limiting its access to recently published research articles and updated International Classification of Diseases, Tenth Revision codes.5 The continued development of ChatGPT will lead to regular updates by OpenAI that resolve this shortcoming in the future. Further, AI models may encode and perpetuate harmful stereotypes and social biases that are present in training data.8
When considering its potential in clinical practice, ChatGPT itself states that it can aid in clinical decision-making by processing patient information, including history, current symptoms, and biopsy and test results. This is uncharted territory, and providers who use ChatGPT at this stage to aid in decision-making should regard it similarly to information retrieved from a search engine. The information produced by ChatGPT should be used to substantiate but not definitively confirm a diagnosis or dictate management. Clinical suspicion by the dermatologist should always trump ChatGPT output. If physicians recommend ChatGPT output over their own advice, it can become a liability, as the technology is not regulated to any degree. Patients also should be cautious when seeking and following medical advice from ChatGPT, as it could be misleading or incorrect and could undermine the patient-physician relationship.6
Additionally, the specific use of ChatGPT to evaluate patient health records raises concerns related to patient privacy and confidentiality. Providers should never enter patient information protected by the Health Insurance Portability and Accountability Act into ChatGPT or anywhere else on the internet. Mitigation of this risk is not currently possible; ChatGPT requires more development and further understanding to even consider its integration into current electronic health records without compromising patient confidentiality.
The Bottom Line
Overall, ChatGPT has the potential to improve efficiency in clinical practice by assisting with informational searches and document drafting. Importantly, increased efficiency can reduce provider burnout and lead to improved patient care. However, dermatologists should carefully weigh the ethical issues of nonmaleficence and justice in evaluating ChatGPT for accuracy, biases, and the protection of patient confidentiality.
Finally, we asked ChatGPT on February 28, 2023, what it thinks:
User: Should dermatologists use ChatGPT in clinical practice?
ChatGPT: As an AI language model, ChatGPT can be a useful tool in dermatology clinical practice, but it should be used with caution in conjunction with other sources of information and clinical judgement.
Dermatologists should be aware of the potential benefits and risks of using ChatGPT and make informed decisions about how to integrate it into their clinical practice. It’s important to remember that ChatGPT is not a substitute for clinical expertise or human interaction with patients, and it should only be used as a supplement to enhance the quality of patient care.
Dermatologists who choose to use ChatGPT should also be aware of its limitations and potential risks, such as limited accuracy, lack of context, and liability issues if it provides incorrect information or recommendations that lead to patient harm. Dermatologists should use their professional judgement and consult with other health care professionals when making important decisions regarding patient care.
Artificial intelligence (AI) technology has increasingly been incorporated in medicine. In dermatology, AI has been used to detect and diagnose skin lesions, including skin cancer.1 ChatGPT (OpenAI) is a novel, highly popular development in generative AI technology. A large language model released in 2022, ChatGPT is a chatbot designed to mimic human conversation and generate specific detailed information when prompted. Free and publicly available, it has been used by millions of people. ChatGPT’s application in the medical field currently is being evaluated across several specialties, including plastic surgery, radiology, and urology.2-4 ChatGPT has the potential to assist health care professionals, including dermatologists, though its use raises important ethical considerations. Herein, we focus on the potential benefits as well as the pitfalls of using ChatGPT in dermatology clinical practice.
Potential Uses of ChatGPT in Practice
A major benefit of ChatGPT is its ability to improve clinical efficiency. First, ChatGPT can provide quick access to general medical information, similar to a search engine but with more natural language processing and contextual understanding to synthesize information.5 This function is useful for rapid concise answers to specific and directed questions. ChatGPT also can interact with its user by asking follow-up questions to produce more precise and relevant responses; this feature may help dermatologists form more accurate differential diagnoses. Additionally, ChatGPT can increase efficiency in clinical practice by drafting generic medical documents,2 including templates for after-visit summaries, postprocedure instructions, referrals, prior authorization appeal letters, and educational handouts. Importantly, increased efficiency can reduce provider burnout and lead to improved patient care. Another useful feature of ChatGPT is its ability to output information modeling human conversation. Because of this feature, ChatGPT also could be employed in clinical practice to serve as an interpreter for patients during clinic visits. Currently, the use of virtual translators can be cumbersome and subject to technical constraints. ChatGPT can provide accurate and conversational translations for patients and dermatologists, improving the patient-provider relationship.
ChatGPT also can contribute to major advancements in the field of dermatology beyond the clinical setting. Because of its ability to draw from extensive data that have already been uploaded, there are some uses of ChatGPT in a research context: to assist in finding resources for research and reviews, formulating hypotheses, drafting study protocols, and collecting large amounts of data within seconds.6
ChatGPT also has potential in advancing medical education. It could be used by medical schools to model interactive patient encounters to help students practice taking a patient’s history and creating differential diagnoses.6 This application of ChatGPT may help medical students hone their clinical skills in a low-stress environment without the restrictions that can come with hiring and training standardized patients, especially when mimicking dermatologic clinical encounters.
Other possibilities for ChatGPT in dermatologic practice include survey administration, clinical trial recruitment, and even automatic high-risk medication monitoring. Despite the many potential applications of ChatGPT in clinical practice, the question raised in each scenario is the quality, accuracy, and safety of what it produces.
Potential Pitfalls of ChatGPT in Practice and Possible Mitigation Strategies
A main concern in using ChatGPT in clinical practice is its potential to produce inaccurate or biased information. When prompted to create a research abstract based on previously published research, ChatGPT drafted abstracts that were clear and digestible but supplemented with incorrect data.7 A group of medical researchers who reviewed these ChatGPT-generated abstracts mistook 32% of the abstracts as having been written by human researchers. The implications of this finding are worrisome. If inaccurate or false information is used by ChatGPT in documents sent to insurance companies or patients, the patient’s safety as well as the dermatologist’s license and credibility are at stake. Thus, dermatologists looking to use ChatGPT to draft generic medical documents should actively review the output to ensure that the information is accurate. Importantly, ChatGPT also is only currently programmed with information up to 2021, limiting its access to recently published research articles and updated International Classification of Diseases, Tenth Revision codes.5 The continued development of ChatGPT will lead to regular updates by OpenAI that resolve this shortcoming in the future. Further, AI models may encode and perpetuate harmful stereotypes and social biases that are present in training data.8
When considering its potential in clinical practice, ChatGPT itself states that it can aid in clinical decision-making by processing patient information, including history, current symptoms, and biopsy and test results. This is uncharted territory, and providers who use ChatGPT at this stage to aid in decision-making should regard it similarly to information retrieved from a search engine. The information produced by ChatGPT should be used to substantiate but not definitively confirm a diagnosis or dictate management. Clinical suspicion by the dermatologist should always trump ChatGPT output. If physicians recommend ChatGPT output over their own advice, it can become a liability, as the technology is not regulated to any degree. Patients also should be cautious when seeking and following medical advice from ChatGPT, as it could be misleading or incorrect and could undermine the patient-physician relationship.6
Additionally, the specific use of ChatGPT to evaluate patient health records raises concerns related to patient privacy and confidentiality. Providers should never enter patient information protected by the Health Insurance Portability and Accountability Act into ChatGPT or anywhere else on the internet. Mitigation of this risk is not currently possible; ChatGPT requires more development and further understanding to even consider its integration into current electronic health records without compromising patient confidentiality.
The Bottom Line
Overall, ChatGPT has the potential to improve efficiency in clinical practice by assisting with informational searches and document drafting. Importantly, increased efficiency can reduce provider burnout and lead to improved patient care. However, dermatologists should carefully weigh the ethical issues of nonmaleficence and justice in evaluating ChatGPT for accuracy, biases, and the protection of patient confidentiality.
Finally, we asked ChatGPT on February 28, 2023, what it thinks:
User: Should dermatologists use ChatGPT in clinical practice?
ChatGPT: As an AI language model, ChatGPT can be a useful tool in dermatology clinical practice, but it should be used with caution in conjunction with other sources of information and clinical judgement.
Dermatologists should be aware of the potential benefits and risks of using ChatGPT and make informed decisions about how to integrate it into their clinical practice. It’s important to remember that ChatGPT is not a substitute for clinical expertise or human interaction with patients, and it should only be used as a supplement to enhance the quality of patient care.
Dermatologists who choose to use ChatGPT should also be aware of its limitations and potential risks, such as limited accuracy, lack of context, and liability issues if it provides incorrect information or recommendations that lead to patient harm. Dermatologists should use their professional judgement and consult with other health care professionals when making important decisions regarding patient care.
- Thomsen K, Iversen L, Titlestad TL, et al. Systematic review of machine learning for diagnosis and prognosis in dermatology. J Dermatol Treat. 2020;31:496-510. doi:10.1080/09546634.2019.1682500
- Shen Y, Heacock L, Elias J, et al. ChatGPT and other large language models are double-edged swords. Radiology. 2023;307:E230163. doi:10.1148/radiol.230163
- Gupta R, Pande P, Herzog I, et al. Application of ChatGPT in cosmetic plastic surgery: ally or antagonist? Aesthet Surg J. 2023;43:NP587-NP590. doi: 10.1093/asj/sjad042
- Gabrielson AT, Odisho AY, Canes D. Harnessing generative artificial intelligence to improve efficiency among urologists: welcome ChatGPT. J Urol. 2023;209:827-829. doi:10.1097/JU.0000000000003383
- What is ChatGPT? OpenAI. Accessed August 10, 2023. https://help.openai.com/en/articles/6783457-chatgpt-general-faq
- Haupt CE, Marks M. AI-generated medical advice—GPT and beyond. JAMA. 2023;329:1349-1350. doi:10.1001/jama.2023.5321
- Gao CA, Howard FM, Markov NS, et al. Comparing Scientific Abstracts Generated by ChatGPT to Original Abstracts Using an Artificial Intelligence Output Detector, Plagiarism Detector, and Blinded Human Reviewers. Scientific Communication and Education; 2022. doi:10.1101/2022.12.23.521610
- Weidinger L, Mellor J, Rauh M, et al. Ethical and social risks of harm from language models. arXiv. Preprint posted online December 8, 2021. https://doi.org/10.48550/arXiv.2112.04359
- Thomsen K, Iversen L, Titlestad TL, et al. Systematic review of machine learning for diagnosis and prognosis in dermatology. J Dermatol Treat. 2020;31:496-510. doi:10.1080/09546634.2019.1682500
- Shen Y, Heacock L, Elias J, et al. ChatGPT and other large language models are double-edged swords. Radiology. 2023;307:E230163. doi:10.1148/radiol.230163
- Gupta R, Pande P, Herzog I, et al. Application of ChatGPT in cosmetic plastic surgery: ally or antagonist? Aesthet Surg J. 2023;43:NP587-NP590. doi: 10.1093/asj/sjad042
- Gabrielson AT, Odisho AY, Canes D. Harnessing generative artificial intelligence to improve efficiency among urologists: welcome ChatGPT. J Urol. 2023;209:827-829. doi:10.1097/JU.0000000000003383
- What is ChatGPT? OpenAI. Accessed August 10, 2023. https://help.openai.com/en/articles/6783457-chatgpt-general-faq
- Haupt CE, Marks M. AI-generated medical advice—GPT and beyond. JAMA. 2023;329:1349-1350. doi:10.1001/jama.2023.5321
- Gao CA, Howard FM, Markov NS, et al. Comparing Scientific Abstracts Generated by ChatGPT to Original Abstracts Using an Artificial Intelligence Output Detector, Plagiarism Detector, and Blinded Human Reviewers. Scientific Communication and Education; 2022. doi:10.1101/2022.12.23.521610
- Weidinger L, Mellor J, Rauh M, et al. Ethical and social risks of harm from language models. arXiv. Preprint posted online December 8, 2021. https://doi.org/10.48550/arXiv.2112.04359
Practice Points
- ChatGPT potentially can play a beneficial role in dermatologic practice by quickly accessing and synthesizing information, drafting generic medical documents, interpreting visits, advancing medical education, and more.
- Dermatologists using ChatGPT should be extremely cautious, as it can produce false or biased information, perpetuate harmful stereotypes, and present information that is not up-to-date.
Applications for the CUTIS 2024 Resident Corner Column
The Cutis Editorial Board is now accepting applications for the 2024 Resident Corner column. The Editorial Board will select 2 to 3 residents to serve as the Resident Corner columnists for 1 year. Articles are posted online only at www.mdedge.com/dermatology but will be referenced in Index Medicus. All applicants must be current residents and will be in residency throughout 2024.
For consideration, send your curriculum vitae along with a brief (not to exceed 500 words) statement of why you enjoy Cutis and what you can offer your fellow residents in contributing a monthly column.
A signed letter of recommendation from the Director of the dermatology residency program also should be supplied.
All materials should be submitted via email to Melissa Sears ([email protected]) by November 1. The residents who are selected to write the column for the upcoming year will be notified by November 15.
We look forward to continuing to educate dermatology residents on topics that are most important to them!
The Cutis Editorial Board is now accepting applications for the 2024 Resident Corner column. The Editorial Board will select 2 to 3 residents to serve as the Resident Corner columnists for 1 year. Articles are posted online only at www.mdedge.com/dermatology but will be referenced in Index Medicus. All applicants must be current residents and will be in residency throughout 2024.
For consideration, send your curriculum vitae along with a brief (not to exceed 500 words) statement of why you enjoy Cutis and what you can offer your fellow residents in contributing a monthly column.
A signed letter of recommendation from the Director of the dermatology residency program also should be supplied.
All materials should be submitted via email to Melissa Sears ([email protected]) by November 1. The residents who are selected to write the column for the upcoming year will be notified by November 15.
We look forward to continuing to educate dermatology residents on topics that are most important to them!
The Cutis Editorial Board is now accepting applications for the 2024 Resident Corner column. The Editorial Board will select 2 to 3 residents to serve as the Resident Corner columnists for 1 year. Articles are posted online only at www.mdedge.com/dermatology but will be referenced in Index Medicus. All applicants must be current residents and will be in residency throughout 2024.
For consideration, send your curriculum vitae along with a brief (not to exceed 500 words) statement of why you enjoy Cutis and what you can offer your fellow residents in contributing a monthly column.
A signed letter of recommendation from the Director of the dermatology residency program also should be supplied.
All materials should be submitted via email to Melissa Sears ([email protected]) by November 1. The residents who are selected to write the column for the upcoming year will be notified by November 15.
We look forward to continuing to educate dermatology residents on topics that are most important to them!
Diffuse Annular Plaques in an Infant
The Diagnosis: Neonatal Lupus Erythematosus
A review of the medical records of the patient’s mother from her first pregnancy revealed positive anti-Ro/SSA (Sjögren syndrome A) (>8.0 U [reference range <1.0 U]) and anti-La/SSB (Sjögren syndrome B) antibodies (>8.0 U [reference range <1.0 U]), which were reconfirmed during her pregnancy with our patient (the second child). The patient’s older brother was diagnosed with neonatal lupus erythematosus (NLE) 2 years prior at 1 month of age; therefore, the mother took hydroxychloroquine during the pregnancy with the second child to help prevent heart block if the child was diagnosed with NLE. Given the family history, positive antibodies in the mother, and clinical presentation, our patient was diagnosed with NLE. He was referred to a pediatric cardiologist and pediatrician to continue the workup of systemic manifestations of NLE and to rule out the presence of congenital heart block. The rash resolved 6 months after the initial presentation, and he did not develop any systemic manifestations of NLE.
Neonatal lupus erythematosus is a rare acquired autoimmune disorder caused by the placental transfer of anti-Ro/SSA and anti-La/SSB antibodies and less commonly anti-U1 ribonucleoprotein antinuclear autoantibodies.1,2 Approximately 1% to 2% of mothers with these positive antibodies will have infants affected with NLE.2 The annual prevalence of NLE in the United States is approximately 1 in 20,000 live births. Mothers of children with NLE most commonly have clinical Sjögren syndrome; however, anti-Ro/SSA and anti-LA/SSB antibodies may be present in 0.1% to 1.5% of healthy women, and 25% to 60% of women with autoimmune disease may be asymptomatic.1 As demonstrated in our case, when there is a family history of NLE in an infant from an earlier pregnancy, the risk for NLE increases to 17% to 20% in subsequent pregnancies1,3 and up to 25% in subsequent pregnancies if the initial child was diagnosed with a congenital heart block in the setting of NLE.1
Neonatal lupus erythematosus classically presents as annular erythematous macules and plaques with central scaling, telangictasia, atrophy, and pigmentary changes. It may start on the scalp and face and spread caudally.1,2 Patients may develop these lesions after UV exposure, and 80% of infants may not have dermatologic findings at birth. Importantly, 40% to 60% of mothers may be asymptomatic at the time of presentation of their child’s NLE.1 The diagnosis can be confirmed via antibody testing in the mother and/or infant. If performed, a punch biopsy shows interface dermatitis, vacuolar degeneration, and possible periadnexal lymphocytic infiltrates on histopathology.1,2
Management of cutaneous NLE includes sun protection (eg, application of sunscreen) and topical corticosteroids. Most dermatologic manifestations of NLE are transient, resolving after clearance of maternal IgG antibodies in 6 to 9 months; however, some telangiectasia, dyspigmentation, and atrophic scarring may persist.1-3
Neonatal lupus erythematosus also may have hepatobiliary, cardiac, hematologic, and less commonly neurologic manifestations. Hepatobiliary manifestations usually present as hepatomegaly or asymptomatic elevated transaminases or γ-glutamyl transferase.1,3 Approximately 10% to 20% of infants with NLE may present with transient anemia and thrombocytopenia.1 Cardiac manifestations are permanent and may require pacemaker implantation.1,3 The incidence of a congenital heart block in infants with NLE is 15% to 30%.3 Cardiac NLE most commonly injures the conductive tissue, leading to a congenital atrioventricular block. The development of a congenital heart block develops in the 18th to 24th week of gestation. Manifestations of a more advanced condition can include dilation of the ascending aorta and dilated cardiomyopathy.1 As such, patients need to be followed by a pediatric cardiologist for monitoring and treatment of any cardiac manifestations.
The overall prognosis of infants affected with NLE varies. Cardiac involvement is associated with a poor prognosis, while isolated cutaneous involvement requires little treatment and portends a favorable prognosis. It is critical for dermatologists to recognize NLE to refer patients to appropriate specialists to investigate and further monitor possible extracutaneous manifestations. With an understanding of the increased risk for a congenital heart block and NLE in subsequent pregnancies, mothers with positive anti-Ro/La antibodies should receive timely counseling and screening. In expectant mothers with suspected autoimmune disease, testing for antinuclear antibodies and SSA and SSB antibodies can be considered, as administration of hydroxychloroquine or prenatal systemic corticosteroids has proven to be effective in preventing a congenital heart block.1 Our patient was followed by pediatric cardiology and was not found to have a congenital heart block.
The differential diagnosis includes other causes of annular erythema in infants, as NLE can mimic several conditions. Tinea corporis may present as scaly annular plaques with central clearing; however, it rarely is encountered fulminantly in neonates.4 Erythema multiforme is a mucocutaneous hypersensitivy reaction distinguished by targetoid morphology.5 It is an exceedingly rare diagnosis in neonates; the average pediatric age of onset is 5.6 years.6 Erythema multiforme often is associated with an infection, most commonly herpes simplex virus,5 and mucosal involvement is common.6 Urticaria multiforme (also known as acute annular urticaria) is a benign disease that appears between 2 months to 3 years of age with blanchable urticarial plaques that likely are triggered by viral or bacterial infections, antibiotics, or vaccines.6 Specific lesions usually will resolve within 24 hours. Annular erythema of infancy is a benign and asymptomatic gyrate erythema that presents as annular plaques with palpable borders that spread centrifugally in patients younger than 1 year. Notably, lesions should periodically fade and may reappear cyclically for months to years. Evaluation for underlying disease usually is negative.6
- Derdulska JM, Rudnicka L, Szykut-Badaczewska A, et al. Neonatal lupus erythematosus—practical guidelines. J Perinat Med. 2021;49:529-538. doi:10.1515/jpm-2020-0543
- Wu J, Berk-Krauss J, Glick SA. Neonatal lupus erythematosus. JAMA Dermatol. 2021;157:590. doi:10.1001/jamadermatol.2021.0041
- Hon KL, Leung AK. Neonatal lupus erythematosus. Autoimmune Dis. 2012;2012:301274. doi:10.1155/2012/301274
- Khare AK, Gupta LK, Mittal A, et al. Neonatal tinea corporis. Indian J Dermatol. 2010;55:201. doi:10.4103/0019-5154.6274
- Ang-Tiu CU, Nicolas ME. Erythema multiforme in a 25-day old neonate. Pediatr Dermatol. 2013;30:E118-E120. doi:10.1111 /j.1525-1470.2012.01873.x
- Agnihotri G, Tsoukas MM. Annular skin lesions in infancy [published online February 3, 2022]. Clin Dermatol. 2022;40:505-512. doi:10.1016/j.clindermatol.2021.12.011
The Diagnosis: Neonatal Lupus Erythematosus
A review of the medical records of the patient’s mother from her first pregnancy revealed positive anti-Ro/SSA (Sjögren syndrome A) (>8.0 U [reference range <1.0 U]) and anti-La/SSB (Sjögren syndrome B) antibodies (>8.0 U [reference range <1.0 U]), which were reconfirmed during her pregnancy with our patient (the second child). The patient’s older brother was diagnosed with neonatal lupus erythematosus (NLE) 2 years prior at 1 month of age; therefore, the mother took hydroxychloroquine during the pregnancy with the second child to help prevent heart block if the child was diagnosed with NLE. Given the family history, positive antibodies in the mother, and clinical presentation, our patient was diagnosed with NLE. He was referred to a pediatric cardiologist and pediatrician to continue the workup of systemic manifestations of NLE and to rule out the presence of congenital heart block. The rash resolved 6 months after the initial presentation, and he did not develop any systemic manifestations of NLE.
Neonatal lupus erythematosus is a rare acquired autoimmune disorder caused by the placental transfer of anti-Ro/SSA and anti-La/SSB antibodies and less commonly anti-U1 ribonucleoprotein antinuclear autoantibodies.1,2 Approximately 1% to 2% of mothers with these positive antibodies will have infants affected with NLE.2 The annual prevalence of NLE in the United States is approximately 1 in 20,000 live births. Mothers of children with NLE most commonly have clinical Sjögren syndrome; however, anti-Ro/SSA and anti-LA/SSB antibodies may be present in 0.1% to 1.5% of healthy women, and 25% to 60% of women with autoimmune disease may be asymptomatic.1 As demonstrated in our case, when there is a family history of NLE in an infant from an earlier pregnancy, the risk for NLE increases to 17% to 20% in subsequent pregnancies1,3 and up to 25% in subsequent pregnancies if the initial child was diagnosed with a congenital heart block in the setting of NLE.1
Neonatal lupus erythematosus classically presents as annular erythematous macules and plaques with central scaling, telangictasia, atrophy, and pigmentary changes. It may start on the scalp and face and spread caudally.1,2 Patients may develop these lesions after UV exposure, and 80% of infants may not have dermatologic findings at birth. Importantly, 40% to 60% of mothers may be asymptomatic at the time of presentation of their child’s NLE.1 The diagnosis can be confirmed via antibody testing in the mother and/or infant. If performed, a punch biopsy shows interface dermatitis, vacuolar degeneration, and possible periadnexal lymphocytic infiltrates on histopathology.1,2
Management of cutaneous NLE includes sun protection (eg, application of sunscreen) and topical corticosteroids. Most dermatologic manifestations of NLE are transient, resolving after clearance of maternal IgG antibodies in 6 to 9 months; however, some telangiectasia, dyspigmentation, and atrophic scarring may persist.1-3
Neonatal lupus erythematosus also may have hepatobiliary, cardiac, hematologic, and less commonly neurologic manifestations. Hepatobiliary manifestations usually present as hepatomegaly or asymptomatic elevated transaminases or γ-glutamyl transferase.1,3 Approximately 10% to 20% of infants with NLE may present with transient anemia and thrombocytopenia.1 Cardiac manifestations are permanent and may require pacemaker implantation.1,3 The incidence of a congenital heart block in infants with NLE is 15% to 30%.3 Cardiac NLE most commonly injures the conductive tissue, leading to a congenital atrioventricular block. The development of a congenital heart block develops in the 18th to 24th week of gestation. Manifestations of a more advanced condition can include dilation of the ascending aorta and dilated cardiomyopathy.1 As such, patients need to be followed by a pediatric cardiologist for monitoring and treatment of any cardiac manifestations.
The overall prognosis of infants affected with NLE varies. Cardiac involvement is associated with a poor prognosis, while isolated cutaneous involvement requires little treatment and portends a favorable prognosis. It is critical for dermatologists to recognize NLE to refer patients to appropriate specialists to investigate and further monitor possible extracutaneous manifestations. With an understanding of the increased risk for a congenital heart block and NLE in subsequent pregnancies, mothers with positive anti-Ro/La antibodies should receive timely counseling and screening. In expectant mothers with suspected autoimmune disease, testing for antinuclear antibodies and SSA and SSB antibodies can be considered, as administration of hydroxychloroquine or prenatal systemic corticosteroids has proven to be effective in preventing a congenital heart block.1 Our patient was followed by pediatric cardiology and was not found to have a congenital heart block.
The differential diagnosis includes other causes of annular erythema in infants, as NLE can mimic several conditions. Tinea corporis may present as scaly annular plaques with central clearing; however, it rarely is encountered fulminantly in neonates.4 Erythema multiforme is a mucocutaneous hypersensitivy reaction distinguished by targetoid morphology.5 It is an exceedingly rare diagnosis in neonates; the average pediatric age of onset is 5.6 years.6 Erythema multiforme often is associated with an infection, most commonly herpes simplex virus,5 and mucosal involvement is common.6 Urticaria multiforme (also known as acute annular urticaria) is a benign disease that appears between 2 months to 3 years of age with blanchable urticarial plaques that likely are triggered by viral or bacterial infections, antibiotics, or vaccines.6 Specific lesions usually will resolve within 24 hours. Annular erythema of infancy is a benign and asymptomatic gyrate erythema that presents as annular plaques with palpable borders that spread centrifugally in patients younger than 1 year. Notably, lesions should periodically fade and may reappear cyclically for months to years. Evaluation for underlying disease usually is negative.6
The Diagnosis: Neonatal Lupus Erythematosus
A review of the medical records of the patient’s mother from her first pregnancy revealed positive anti-Ro/SSA (Sjögren syndrome A) (>8.0 U [reference range <1.0 U]) and anti-La/SSB (Sjögren syndrome B) antibodies (>8.0 U [reference range <1.0 U]), which were reconfirmed during her pregnancy with our patient (the second child). The patient’s older brother was diagnosed with neonatal lupus erythematosus (NLE) 2 years prior at 1 month of age; therefore, the mother took hydroxychloroquine during the pregnancy with the second child to help prevent heart block if the child was diagnosed with NLE. Given the family history, positive antibodies in the mother, and clinical presentation, our patient was diagnosed with NLE. He was referred to a pediatric cardiologist and pediatrician to continue the workup of systemic manifestations of NLE and to rule out the presence of congenital heart block. The rash resolved 6 months after the initial presentation, and he did not develop any systemic manifestations of NLE.
Neonatal lupus erythematosus is a rare acquired autoimmune disorder caused by the placental transfer of anti-Ro/SSA and anti-La/SSB antibodies and less commonly anti-U1 ribonucleoprotein antinuclear autoantibodies.1,2 Approximately 1% to 2% of mothers with these positive antibodies will have infants affected with NLE.2 The annual prevalence of NLE in the United States is approximately 1 in 20,000 live births. Mothers of children with NLE most commonly have clinical Sjögren syndrome; however, anti-Ro/SSA and anti-LA/SSB antibodies may be present in 0.1% to 1.5% of healthy women, and 25% to 60% of women with autoimmune disease may be asymptomatic.1 As demonstrated in our case, when there is a family history of NLE in an infant from an earlier pregnancy, the risk for NLE increases to 17% to 20% in subsequent pregnancies1,3 and up to 25% in subsequent pregnancies if the initial child was diagnosed with a congenital heart block in the setting of NLE.1
Neonatal lupus erythematosus classically presents as annular erythematous macules and plaques with central scaling, telangictasia, atrophy, and pigmentary changes. It may start on the scalp and face and spread caudally.1,2 Patients may develop these lesions after UV exposure, and 80% of infants may not have dermatologic findings at birth. Importantly, 40% to 60% of mothers may be asymptomatic at the time of presentation of their child’s NLE.1 The diagnosis can be confirmed via antibody testing in the mother and/or infant. If performed, a punch biopsy shows interface dermatitis, vacuolar degeneration, and possible periadnexal lymphocytic infiltrates on histopathology.1,2
Management of cutaneous NLE includes sun protection (eg, application of sunscreen) and topical corticosteroids. Most dermatologic manifestations of NLE are transient, resolving after clearance of maternal IgG antibodies in 6 to 9 months; however, some telangiectasia, dyspigmentation, and atrophic scarring may persist.1-3
Neonatal lupus erythematosus also may have hepatobiliary, cardiac, hematologic, and less commonly neurologic manifestations. Hepatobiliary manifestations usually present as hepatomegaly or asymptomatic elevated transaminases or γ-glutamyl transferase.1,3 Approximately 10% to 20% of infants with NLE may present with transient anemia and thrombocytopenia.1 Cardiac manifestations are permanent and may require pacemaker implantation.1,3 The incidence of a congenital heart block in infants with NLE is 15% to 30%.3 Cardiac NLE most commonly injures the conductive tissue, leading to a congenital atrioventricular block. The development of a congenital heart block develops in the 18th to 24th week of gestation. Manifestations of a more advanced condition can include dilation of the ascending aorta and dilated cardiomyopathy.1 As such, patients need to be followed by a pediatric cardiologist for monitoring and treatment of any cardiac manifestations.
The overall prognosis of infants affected with NLE varies. Cardiac involvement is associated with a poor prognosis, while isolated cutaneous involvement requires little treatment and portends a favorable prognosis. It is critical for dermatologists to recognize NLE to refer patients to appropriate specialists to investigate and further monitor possible extracutaneous manifestations. With an understanding of the increased risk for a congenital heart block and NLE in subsequent pregnancies, mothers with positive anti-Ro/La antibodies should receive timely counseling and screening. In expectant mothers with suspected autoimmune disease, testing for antinuclear antibodies and SSA and SSB antibodies can be considered, as administration of hydroxychloroquine or prenatal systemic corticosteroids has proven to be effective in preventing a congenital heart block.1 Our patient was followed by pediatric cardiology and was not found to have a congenital heart block.
The differential diagnosis includes other causes of annular erythema in infants, as NLE can mimic several conditions. Tinea corporis may present as scaly annular plaques with central clearing; however, it rarely is encountered fulminantly in neonates.4 Erythema multiforme is a mucocutaneous hypersensitivy reaction distinguished by targetoid morphology.5 It is an exceedingly rare diagnosis in neonates; the average pediatric age of onset is 5.6 years.6 Erythema multiforme often is associated with an infection, most commonly herpes simplex virus,5 and mucosal involvement is common.6 Urticaria multiforme (also known as acute annular urticaria) is a benign disease that appears between 2 months to 3 years of age with blanchable urticarial plaques that likely are triggered by viral or bacterial infections, antibiotics, or vaccines.6 Specific lesions usually will resolve within 24 hours. Annular erythema of infancy is a benign and asymptomatic gyrate erythema that presents as annular plaques with palpable borders that spread centrifugally in patients younger than 1 year. Notably, lesions should periodically fade and may reappear cyclically for months to years. Evaluation for underlying disease usually is negative.6
- Derdulska JM, Rudnicka L, Szykut-Badaczewska A, et al. Neonatal lupus erythematosus—practical guidelines. J Perinat Med. 2021;49:529-538. doi:10.1515/jpm-2020-0543
- Wu J, Berk-Krauss J, Glick SA. Neonatal lupus erythematosus. JAMA Dermatol. 2021;157:590. doi:10.1001/jamadermatol.2021.0041
- Hon KL, Leung AK. Neonatal lupus erythematosus. Autoimmune Dis. 2012;2012:301274. doi:10.1155/2012/301274
- Khare AK, Gupta LK, Mittal A, et al. Neonatal tinea corporis. Indian J Dermatol. 2010;55:201. doi:10.4103/0019-5154.6274
- Ang-Tiu CU, Nicolas ME. Erythema multiforme in a 25-day old neonate. Pediatr Dermatol. 2013;30:E118-E120. doi:10.1111 /j.1525-1470.2012.01873.x
- Agnihotri G, Tsoukas MM. Annular skin lesions in infancy [published online February 3, 2022]. Clin Dermatol. 2022;40:505-512. doi:10.1016/j.clindermatol.2021.12.011
- Derdulska JM, Rudnicka L, Szykut-Badaczewska A, et al. Neonatal lupus erythematosus—practical guidelines. J Perinat Med. 2021;49:529-538. doi:10.1515/jpm-2020-0543
- Wu J, Berk-Krauss J, Glick SA. Neonatal lupus erythematosus. JAMA Dermatol. 2021;157:590. doi:10.1001/jamadermatol.2021.0041
- Hon KL, Leung AK. Neonatal lupus erythematosus. Autoimmune Dis. 2012;2012:301274. doi:10.1155/2012/301274
- Khare AK, Gupta LK, Mittal A, et al. Neonatal tinea corporis. Indian J Dermatol. 2010;55:201. doi:10.4103/0019-5154.6274
- Ang-Tiu CU, Nicolas ME. Erythema multiforme in a 25-day old neonate. Pediatr Dermatol. 2013;30:E118-E120. doi:10.1111 /j.1525-1470.2012.01873.x
- Agnihotri G, Tsoukas MM. Annular skin lesions in infancy [published online February 3, 2022]. Clin Dermatol. 2022;40:505-512. doi:10.1016/j.clindermatol.2021.12.011
A 5-week-old infant boy presented with a rash at birth (left). The pregnancy was full term without complications, and he was otherwise healthy. A family history revealed that his older brother developed a similar rash 2 weeks after birth (right). Physical examination revealed polycyclic annular patches with an erythematous border and central clearing diffusely located on the trunk, extremities, scalp, and face with periorbital edema.
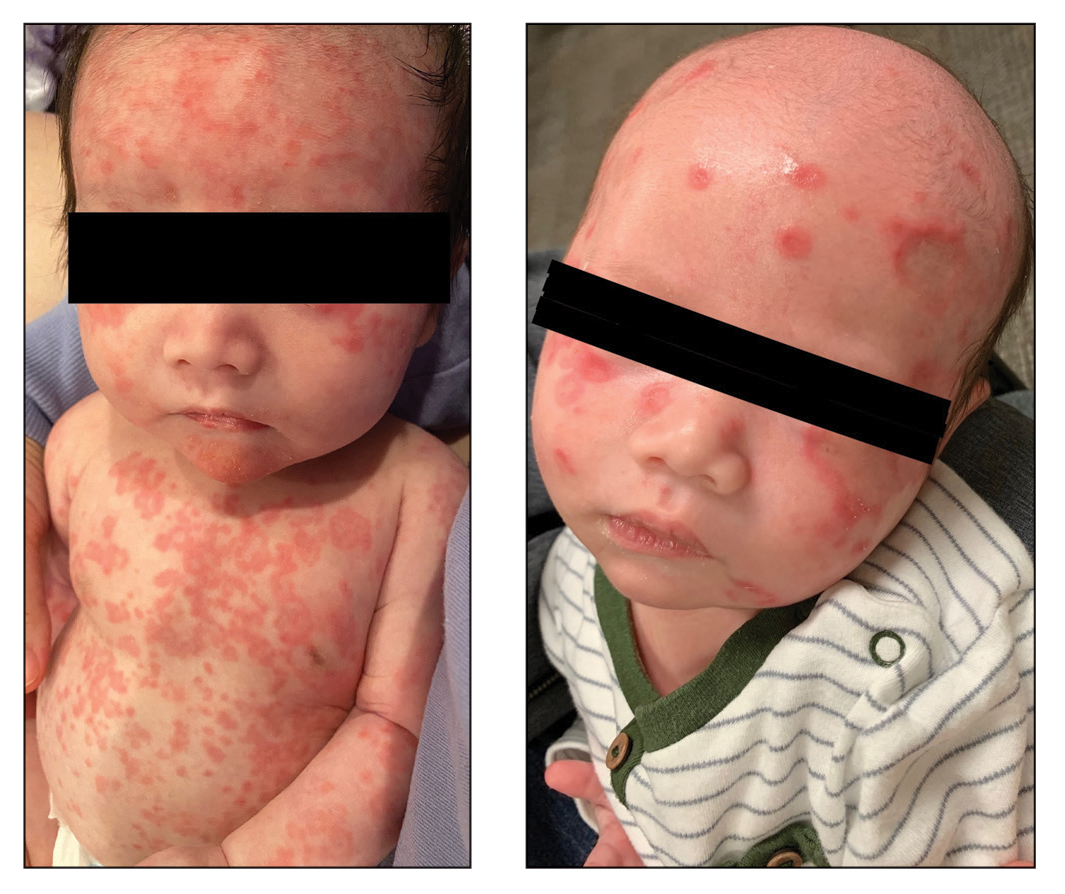
Evaluating Pharmacists’ Time Collecting Self-Monitoring Blood Glucose Data
The American Diabetes Association recommends that patients on intensive insulin regimens self-monitor blood glucose (SMBG) to assist in therapy optimization.1 To be useful, SMBG data must be captured by patients, shared with care teams, and used and interpreted by patients and practitioners.2,3 Communication of SMBG data from the patient to practitioner can be challenging. Although technology can help in this process, limitations exist, such as manual data entry into systems, patient and/or practitioner technological challenges (eg, accessing interface), and compatibility and integration between SMBG devices and electronic health record (EHR) systems.4
The Boise Veterans Affairs Medical Center (BVAMC) in Idaho serves more than 100,000 veterans. It includes a main site, community-based outpatient clinics, and a clinical resource hub that provides telehealth services to veterans residing in rural neighboring states. The BVAMC pharmacy department provides both inpatient and outpatient services. At the BVAMC, clinical pharmacist practitioners (CPPs) are independent practitioners who support their care teams in comprehensive medication management and have the ability to initiate, modify, and discontinue drug therapy for referred patients.5 A prominent role of CPPs in primary care teams is to manage patients with uncontrolled diabetes and intensive insulin regimens, in which SMBG data are vital to therapy optimization. As collecting SMBG data from patients is seen anecdotally as time intensive, we determined the mean time spent by CPPs collecting patient SMBG data and its potential implications.
Methods
Pharmacists at BVAMC were asked to estimate and record the following: SMBG data collection method, time spent collecting data, extra time spent documenting or formatting SMBG readings, total patient visit time, and visit type. Time was collected in minutes. Extra time spent documenting or formatting SMBG readings included any additional time formatting or entering data in the clinical note after talking to the patient; if this was done while multitasking and talking to the patient, it was not considered extra time. For total patient visit time, pharmacists were asked to estimate only time spent discussing diabetes care and collecting SMBG data. Visit types were categorized as in-person/face-to-face, telephone, and telehealth using clinical video telehealth (CVT)/VA Video Connect (VVC). Data were collected using a standardized spreadsheet. The spreadsheet was pilot tested by a CPP before distribution to all pharmacists.
CPPs were educated about the project in March 2021 and were asked to record data for a 1-week period between April 5, 2021, and April 30, 2021. One CPP also provided delayed data collected from May 17 to 21, 2021, and these data were included in our analysis.
Descriptive statistics were used to determine the mean time spent by CPPs collecting SMBG data. Unpaired t tests were used to compare time spent collecting SMBG data by different collection methods and patient visit types. A P value of ≤ .05 was considered statistically significant. Data were organized in Microsoft Excel, and statistics were completed with JMP Pro v15.
Results
Eight CPPs provided data from 120 patient encounters. For all pa
When compared by the SMBG collection method, the longest time spent collecting SMBG data was with patient report (3.7 minutes), and the longest time spent documenting/formatting time was with meter download/home telehealth (2 minutes). There was no statistically significant difference in the time to collect SMBG data between patient report and other methods (3.7 minutes vs 2.8 minutes; P = .07).
When compared by visit type, there was not a statistically significant difference between time spent collecting SMBG data (3.8 minutes vs 3.2 minutes; P = .39) (Table 2).
Discussion
We found that the mean amount of time spent collecting and documenting/formatting SMBG data was only 4.6 minutes; however, this still represented a substantial portion of visit time. For telephone and CVT/VVC appointments, this represented > 25% of total visit time. While CPPs make important contributions to interprofessional team management of patients with diabetes, their cost is not trivial.6-8 It is worth exploring the most effective and efficient ways to use CPPs. Our results indicate that streamlining SMBG data collection may be beneficial.
Pharmacy technicians, licensed practical nurses/clinical associates, registered nurses/nurse care managers, or other team members could help improve SMBG data collection. Using other team members is also an opportunity for comanagement, for team collaboration, and for more patients to be seen. For example, if a CPP currently has 12 patient encounters that last 20 minutes each, this results in about 240 minutes of direct patient care. If patient encounters were 16 minutes, CPPS could have 15 patient encounters in 240 minutes. Saved time could be used for other clinical tasks involved in disease management or clinical reminder reviews. While there are benefits to CPPs collecting SMBG data, such as further inquiry about patient-reported values, other team members could also be trained to ask appropriate follow-up questions for abnormal blood glucose readings. In addition, leveraging current team members and optimizing their roles could prevent the need to acquire additional full-time equivalent employees.
Another opportunity to increase efficiency in SMBG data collection is with SMBG devices and EHR integration.4,9 However, integration can be difficult with different types of SMBG devices and EHR platforms. Education for patients and practitioners could help to ensure accurate and reliable data uploads; patient internet availability; data protection, privacy, and sharing; workflow management; and clear patient-practitioner expectations.10 For example, if patient SMBG data are automatically uploaded to practitioners, patients’ expectations for practitioner review of data and follow-up need to be determined.
We found a subset of patient encounters (n = 23) where data collection and documenting/formatting represented more than half of the total visit time. In this subset, 13 SMBG reports were pulled from a log or meter, 8 were patient reported, and 3 were meter download or home telehealth.
Limitations
A potential reason for the lack of statistically significant differences in SMBG collection method or visit type in this study includes the small sample size. Participation in this work was voluntary, and all participating CPPs had ≥ 3 years of practice in their current setting, which includes a heavy workload of diabetes management. These pharmacists noted self-established procedures/systems for SMBG data collection, including the use of Excel spreadsheets with pregenerated formulas. For less experienced CPPs, SMBG data collection time may be even longer. Pharmacists also noted that they may limit time spent collecting SMBG data depending on the patient encounter and whether they have gathered sufficient data to guide clinical care. Other limitations of this work include data collection from a single institution and that the time documented represented estimates; there was no external monitor.
Conclusions
In this analysis, we found that CPPs spend about 3 minutes collecting SMBG data from patients, and about an additional 1 minute documenting and formatting data. While 4 to 5 minutes may not represent a substantial amount of time for one patient, it can be when multiplied by several patient encounters. The time spent collecting SMBG data did not significantly differ by collection method or visit type. Opportunities to increase efficiency in SMBG data collection, such as the use of nonpharmacist team members are worth exploring.
Acknowledgments
Thank you to the pharmacists at the Boise Veterans Affairs Medical Center for their time and support of this work: Danielle Ahlstrom, Paul Black, Robyn Cruz, Sarah Naidoo, Anthony Nelson, Laura Spoutz, Eileen Twomey, Donovan Victorine, and Michelle Wilkin.
1. American Diabetes Association. 7. Diabetes Technology: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44(suppl 1):S85-S99. doi:10.2337/dc21-S007
2. Austin MM. The two skill sets of self-monitoring of blood glucose education: the operational and the interpretive. Diabetes Spectr. 2013;26(2):83-90. doi:10.2337/diaspect.26.2.83
3. Gallichan M. Self monitoring of glucose by people with diabetes: evidence based practice. BMJ. 1997;314(7085):964-967. doi:10.1136/bmj.314.7085.964
4. Lewinski AA, Drake C, Shaw RJ, et al. Bridging the integration gap between patient-generated blood glucose data and electronic health records. J Am Med Inform Assoc. 2019;26(7):667-672. doi:10.1093/jamia/ocz039
5. McFarland MS, Groppi J, Jorgenson T, et al. Role of the US Veterans Health Administration clinical pharmacy specialist provider: shaping the future of comprehensive medication management. Can J Hosp Pharm. 2020;73(2):152-158. doi:10.4212/cjhp.v73i2.2982
6. Schmidt K, Caudill J. Hamilton T. Impact of clinical pharmacy specialists on glycemic control in veterans with type 2 diabetes. Am J Health Syst Pharm. 2019;76(suppl 1):S9-S14. doi:10.1093/ajhp/zxy015
7. Sullivan J, Jett BP, Cradick M, Zuber J. Effect of clinical pharmacist intervention on hemoglobin A1c reduction in veteran patients with type 2 diabetes in a rural setting. Ann Pharmacother. 2016;50(12):1023-1027. doi:10.1177/1060028016663564
8. Bloom CI, Ku M, Williams M. Clinical pharmacy specialists’ impact in patient aligned care teams for type 2 diabetes management. J Am Pharm Assoc (2003). 2019;59(5):717-721. doi:10.1016/j.japh.2019.05.002
9. Kumar RB, Goren ND, Stark DE, Wall DP, Longhurst CA. Automated integration of continuous glucose monitor data in the electronic health record using consumer technology. J Am Med Inform Assoc. 2016;23(3):532-537. doi:10.1093/jamia/ocv206
10. Reading MJ, Merrill JA. Converging and diverging needs between patients and providers who are collecting and using patient-generated health data: an integrative review. J Am Med Inform Assoc. 2018;25(6):759-771. doi:10.1093/jamia/ocy006
The American Diabetes Association recommends that patients on intensive insulin regimens self-monitor blood glucose (SMBG) to assist in therapy optimization.1 To be useful, SMBG data must be captured by patients, shared with care teams, and used and interpreted by patients and practitioners.2,3 Communication of SMBG data from the patient to practitioner can be challenging. Although technology can help in this process, limitations exist, such as manual data entry into systems, patient and/or practitioner technological challenges (eg, accessing interface), and compatibility and integration between SMBG devices and electronic health record (EHR) systems.4
The Boise Veterans Affairs Medical Center (BVAMC) in Idaho serves more than 100,000 veterans. It includes a main site, community-based outpatient clinics, and a clinical resource hub that provides telehealth services to veterans residing in rural neighboring states. The BVAMC pharmacy department provides both inpatient and outpatient services. At the BVAMC, clinical pharmacist practitioners (CPPs) are independent practitioners who support their care teams in comprehensive medication management and have the ability to initiate, modify, and discontinue drug therapy for referred patients.5 A prominent role of CPPs in primary care teams is to manage patients with uncontrolled diabetes and intensive insulin regimens, in which SMBG data are vital to therapy optimization. As collecting SMBG data from patients is seen anecdotally as time intensive, we determined the mean time spent by CPPs collecting patient SMBG data and its potential implications.
Methods
Pharmacists at BVAMC were asked to estimate and record the following: SMBG data collection method, time spent collecting data, extra time spent documenting or formatting SMBG readings, total patient visit time, and visit type. Time was collected in minutes. Extra time spent documenting or formatting SMBG readings included any additional time formatting or entering data in the clinical note after talking to the patient; if this was done while multitasking and talking to the patient, it was not considered extra time. For total patient visit time, pharmacists were asked to estimate only time spent discussing diabetes care and collecting SMBG data. Visit types were categorized as in-person/face-to-face, telephone, and telehealth using clinical video telehealth (CVT)/VA Video Connect (VVC). Data were collected using a standardized spreadsheet. The spreadsheet was pilot tested by a CPP before distribution to all pharmacists.
CPPs were educated about the project in March 2021 and were asked to record data for a 1-week period between April 5, 2021, and April 30, 2021. One CPP also provided delayed data collected from May 17 to 21, 2021, and these data were included in our analysis.
Descriptive statistics were used to determine the mean time spent by CPPs collecting SMBG data. Unpaired t tests were used to compare time spent collecting SMBG data by different collection methods and patient visit types. A P value of ≤ .05 was considered statistically significant. Data were organized in Microsoft Excel, and statistics were completed with JMP Pro v15.
Results
Eight CPPs provided data from 120 patient encounters. For all pa
When compared by the SMBG collection method, the longest time spent collecting SMBG data was with patient report (3.7 minutes), and the longest time spent documenting/formatting time was with meter download/home telehealth (2 minutes). There was no statistically significant difference in the time to collect SMBG data between patient report and other methods (3.7 minutes vs 2.8 minutes; P = .07).
When compared by visit type, there was not a statistically significant difference between time spent collecting SMBG data (3.8 minutes vs 3.2 minutes; P = .39) (Table 2).
Discussion
We found that the mean amount of time spent collecting and documenting/formatting SMBG data was only 4.6 minutes; however, this still represented a substantial portion of visit time. For telephone and CVT/VVC appointments, this represented > 25% of total visit time. While CPPs make important contributions to interprofessional team management of patients with diabetes, their cost is not trivial.6-8 It is worth exploring the most effective and efficient ways to use CPPs. Our results indicate that streamlining SMBG data collection may be beneficial.
Pharmacy technicians, licensed practical nurses/clinical associates, registered nurses/nurse care managers, or other team members could help improve SMBG data collection. Using other team members is also an opportunity for comanagement, for team collaboration, and for more patients to be seen. For example, if a CPP currently has 12 patient encounters that last 20 minutes each, this results in about 240 minutes of direct patient care. If patient encounters were 16 minutes, CPPS could have 15 patient encounters in 240 minutes. Saved time could be used for other clinical tasks involved in disease management or clinical reminder reviews. While there are benefits to CPPs collecting SMBG data, such as further inquiry about patient-reported values, other team members could also be trained to ask appropriate follow-up questions for abnormal blood glucose readings. In addition, leveraging current team members and optimizing their roles could prevent the need to acquire additional full-time equivalent employees.
Another opportunity to increase efficiency in SMBG data collection is with SMBG devices and EHR integration.4,9 However, integration can be difficult with different types of SMBG devices and EHR platforms. Education for patients and practitioners could help to ensure accurate and reliable data uploads; patient internet availability; data protection, privacy, and sharing; workflow management; and clear patient-practitioner expectations.10 For example, if patient SMBG data are automatically uploaded to practitioners, patients’ expectations for practitioner review of data and follow-up need to be determined.
We found a subset of patient encounters (n = 23) where data collection and documenting/formatting represented more than half of the total visit time. In this subset, 13 SMBG reports were pulled from a log or meter, 8 were patient reported, and 3 were meter download or home telehealth.
Limitations
A potential reason for the lack of statistically significant differences in SMBG collection method or visit type in this study includes the small sample size. Participation in this work was voluntary, and all participating CPPs had ≥ 3 years of practice in their current setting, which includes a heavy workload of diabetes management. These pharmacists noted self-established procedures/systems for SMBG data collection, including the use of Excel spreadsheets with pregenerated formulas. For less experienced CPPs, SMBG data collection time may be even longer. Pharmacists also noted that they may limit time spent collecting SMBG data depending on the patient encounter and whether they have gathered sufficient data to guide clinical care. Other limitations of this work include data collection from a single institution and that the time documented represented estimates; there was no external monitor.
Conclusions
In this analysis, we found that CPPs spend about 3 minutes collecting SMBG data from patients, and about an additional 1 minute documenting and formatting data. While 4 to 5 minutes may not represent a substantial amount of time for one patient, it can be when multiplied by several patient encounters. The time spent collecting SMBG data did not significantly differ by collection method or visit type. Opportunities to increase efficiency in SMBG data collection, such as the use of nonpharmacist team members are worth exploring.
Acknowledgments
Thank you to the pharmacists at the Boise Veterans Affairs Medical Center for their time and support of this work: Danielle Ahlstrom, Paul Black, Robyn Cruz, Sarah Naidoo, Anthony Nelson, Laura Spoutz, Eileen Twomey, Donovan Victorine, and Michelle Wilkin.
The American Diabetes Association recommends that patients on intensive insulin regimens self-monitor blood glucose (SMBG) to assist in therapy optimization.1 To be useful, SMBG data must be captured by patients, shared with care teams, and used and interpreted by patients and practitioners.2,3 Communication of SMBG data from the patient to practitioner can be challenging. Although technology can help in this process, limitations exist, such as manual data entry into systems, patient and/or practitioner technological challenges (eg, accessing interface), and compatibility and integration between SMBG devices and electronic health record (EHR) systems.4
The Boise Veterans Affairs Medical Center (BVAMC) in Idaho serves more than 100,000 veterans. It includes a main site, community-based outpatient clinics, and a clinical resource hub that provides telehealth services to veterans residing in rural neighboring states. The BVAMC pharmacy department provides both inpatient and outpatient services. At the BVAMC, clinical pharmacist practitioners (CPPs) are independent practitioners who support their care teams in comprehensive medication management and have the ability to initiate, modify, and discontinue drug therapy for referred patients.5 A prominent role of CPPs in primary care teams is to manage patients with uncontrolled diabetes and intensive insulin regimens, in which SMBG data are vital to therapy optimization. As collecting SMBG data from patients is seen anecdotally as time intensive, we determined the mean time spent by CPPs collecting patient SMBG data and its potential implications.
Methods
Pharmacists at BVAMC were asked to estimate and record the following: SMBG data collection method, time spent collecting data, extra time spent documenting or formatting SMBG readings, total patient visit time, and visit type. Time was collected in minutes. Extra time spent documenting or formatting SMBG readings included any additional time formatting or entering data in the clinical note after talking to the patient; if this was done while multitasking and talking to the patient, it was not considered extra time. For total patient visit time, pharmacists were asked to estimate only time spent discussing diabetes care and collecting SMBG data. Visit types were categorized as in-person/face-to-face, telephone, and telehealth using clinical video telehealth (CVT)/VA Video Connect (VVC). Data were collected using a standardized spreadsheet. The spreadsheet was pilot tested by a CPP before distribution to all pharmacists.
CPPs were educated about the project in March 2021 and were asked to record data for a 1-week period between April 5, 2021, and April 30, 2021. One CPP also provided delayed data collected from May 17 to 21, 2021, and these data were included in our analysis.
Descriptive statistics were used to determine the mean time spent by CPPs collecting SMBG data. Unpaired t tests were used to compare time spent collecting SMBG data by different collection methods and patient visit types. A P value of ≤ .05 was considered statistically significant. Data were organized in Microsoft Excel, and statistics were completed with JMP Pro v15.
Results
Eight CPPs provided data from 120 patient encounters. For all pa
When compared by the SMBG collection method, the longest time spent collecting SMBG data was with patient report (3.7 minutes), and the longest time spent documenting/formatting time was with meter download/home telehealth (2 minutes). There was no statistically significant difference in the time to collect SMBG data between patient report and other methods (3.7 minutes vs 2.8 minutes; P = .07).
When compared by visit type, there was not a statistically significant difference between time spent collecting SMBG data (3.8 minutes vs 3.2 minutes; P = .39) (Table 2).
Discussion
We found that the mean amount of time spent collecting and documenting/formatting SMBG data was only 4.6 minutes; however, this still represented a substantial portion of visit time. For telephone and CVT/VVC appointments, this represented > 25% of total visit time. While CPPs make important contributions to interprofessional team management of patients with diabetes, their cost is not trivial.6-8 It is worth exploring the most effective and efficient ways to use CPPs. Our results indicate that streamlining SMBG data collection may be beneficial.
Pharmacy technicians, licensed practical nurses/clinical associates, registered nurses/nurse care managers, or other team members could help improve SMBG data collection. Using other team members is also an opportunity for comanagement, for team collaboration, and for more patients to be seen. For example, if a CPP currently has 12 patient encounters that last 20 minutes each, this results in about 240 minutes of direct patient care. If patient encounters were 16 minutes, CPPS could have 15 patient encounters in 240 minutes. Saved time could be used for other clinical tasks involved in disease management or clinical reminder reviews. While there are benefits to CPPs collecting SMBG data, such as further inquiry about patient-reported values, other team members could also be trained to ask appropriate follow-up questions for abnormal blood glucose readings. In addition, leveraging current team members and optimizing their roles could prevent the need to acquire additional full-time equivalent employees.
Another opportunity to increase efficiency in SMBG data collection is with SMBG devices and EHR integration.4,9 However, integration can be difficult with different types of SMBG devices and EHR platforms. Education for patients and practitioners could help to ensure accurate and reliable data uploads; patient internet availability; data protection, privacy, and sharing; workflow management; and clear patient-practitioner expectations.10 For example, if patient SMBG data are automatically uploaded to practitioners, patients’ expectations for practitioner review of data and follow-up need to be determined.
We found a subset of patient encounters (n = 23) where data collection and documenting/formatting represented more than half of the total visit time. In this subset, 13 SMBG reports were pulled from a log or meter, 8 were patient reported, and 3 were meter download or home telehealth.
Limitations
A potential reason for the lack of statistically significant differences in SMBG collection method or visit type in this study includes the small sample size. Participation in this work was voluntary, and all participating CPPs had ≥ 3 years of practice in their current setting, which includes a heavy workload of diabetes management. These pharmacists noted self-established procedures/systems for SMBG data collection, including the use of Excel spreadsheets with pregenerated formulas. For less experienced CPPs, SMBG data collection time may be even longer. Pharmacists also noted that they may limit time spent collecting SMBG data depending on the patient encounter and whether they have gathered sufficient data to guide clinical care. Other limitations of this work include data collection from a single institution and that the time documented represented estimates; there was no external monitor.
Conclusions
In this analysis, we found that CPPs spend about 3 minutes collecting SMBG data from patients, and about an additional 1 minute documenting and formatting data. While 4 to 5 minutes may not represent a substantial amount of time for one patient, it can be when multiplied by several patient encounters. The time spent collecting SMBG data did not significantly differ by collection method or visit type. Opportunities to increase efficiency in SMBG data collection, such as the use of nonpharmacist team members are worth exploring.
Acknowledgments
Thank you to the pharmacists at the Boise Veterans Affairs Medical Center for their time and support of this work: Danielle Ahlstrom, Paul Black, Robyn Cruz, Sarah Naidoo, Anthony Nelson, Laura Spoutz, Eileen Twomey, Donovan Victorine, and Michelle Wilkin.
1. American Diabetes Association. 7. Diabetes Technology: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44(suppl 1):S85-S99. doi:10.2337/dc21-S007
2. Austin MM. The two skill sets of self-monitoring of blood glucose education: the operational and the interpretive. Diabetes Spectr. 2013;26(2):83-90. doi:10.2337/diaspect.26.2.83
3. Gallichan M. Self monitoring of glucose by people with diabetes: evidence based practice. BMJ. 1997;314(7085):964-967. doi:10.1136/bmj.314.7085.964
4. Lewinski AA, Drake C, Shaw RJ, et al. Bridging the integration gap between patient-generated blood glucose data and electronic health records. J Am Med Inform Assoc. 2019;26(7):667-672. doi:10.1093/jamia/ocz039
5. McFarland MS, Groppi J, Jorgenson T, et al. Role of the US Veterans Health Administration clinical pharmacy specialist provider: shaping the future of comprehensive medication management. Can J Hosp Pharm. 2020;73(2):152-158. doi:10.4212/cjhp.v73i2.2982
6. Schmidt K, Caudill J. Hamilton T. Impact of clinical pharmacy specialists on glycemic control in veterans with type 2 diabetes. Am J Health Syst Pharm. 2019;76(suppl 1):S9-S14. doi:10.1093/ajhp/zxy015
7. Sullivan J, Jett BP, Cradick M, Zuber J. Effect of clinical pharmacist intervention on hemoglobin A1c reduction in veteran patients with type 2 diabetes in a rural setting. Ann Pharmacother. 2016;50(12):1023-1027. doi:10.1177/1060028016663564
8. Bloom CI, Ku M, Williams M. Clinical pharmacy specialists’ impact in patient aligned care teams for type 2 diabetes management. J Am Pharm Assoc (2003). 2019;59(5):717-721. doi:10.1016/j.japh.2019.05.002
9. Kumar RB, Goren ND, Stark DE, Wall DP, Longhurst CA. Automated integration of continuous glucose monitor data in the electronic health record using consumer technology. J Am Med Inform Assoc. 2016;23(3):532-537. doi:10.1093/jamia/ocv206
10. Reading MJ, Merrill JA. Converging and diverging needs between patients and providers who are collecting and using patient-generated health data: an integrative review. J Am Med Inform Assoc. 2018;25(6):759-771. doi:10.1093/jamia/ocy006
1. American Diabetes Association. 7. Diabetes Technology: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44(suppl 1):S85-S99. doi:10.2337/dc21-S007
2. Austin MM. The two skill sets of self-monitoring of blood glucose education: the operational and the interpretive. Diabetes Spectr. 2013;26(2):83-90. doi:10.2337/diaspect.26.2.83
3. Gallichan M. Self monitoring of glucose by people with diabetes: evidence based practice. BMJ. 1997;314(7085):964-967. doi:10.1136/bmj.314.7085.964
4. Lewinski AA, Drake C, Shaw RJ, et al. Bridging the integration gap between patient-generated blood glucose data and electronic health records. J Am Med Inform Assoc. 2019;26(7):667-672. doi:10.1093/jamia/ocz039
5. McFarland MS, Groppi J, Jorgenson T, et al. Role of the US Veterans Health Administration clinical pharmacy specialist provider: shaping the future of comprehensive medication management. Can J Hosp Pharm. 2020;73(2):152-158. doi:10.4212/cjhp.v73i2.2982
6. Schmidt K, Caudill J. Hamilton T. Impact of clinical pharmacy specialists on glycemic control in veterans with type 2 diabetes. Am J Health Syst Pharm. 2019;76(suppl 1):S9-S14. doi:10.1093/ajhp/zxy015
7. Sullivan J, Jett BP, Cradick M, Zuber J. Effect of clinical pharmacist intervention on hemoglobin A1c reduction in veteran patients with type 2 diabetes in a rural setting. Ann Pharmacother. 2016;50(12):1023-1027. doi:10.1177/1060028016663564
8. Bloom CI, Ku M, Williams M. Clinical pharmacy specialists’ impact in patient aligned care teams for type 2 diabetes management. J Am Pharm Assoc (2003). 2019;59(5):717-721. doi:10.1016/j.japh.2019.05.002
9. Kumar RB, Goren ND, Stark DE, Wall DP, Longhurst CA. Automated integration of continuous glucose monitor data in the electronic health record using consumer technology. J Am Med Inform Assoc. 2016;23(3):532-537. doi:10.1093/jamia/ocv206
10. Reading MJ, Merrill JA. Converging and diverging needs between patients and providers who are collecting and using patient-generated health data: an integrative review. J Am Med Inform Assoc. 2018;25(6):759-771. doi:10.1093/jamia/ocy006