User login
More on prescribing controlled substances
I was disheartened with the June 2023 issue of
The benzodiazepine pharmacology discussed in this article is interesting, but it would be helpful if it had been integrated within a much more extensive discussion of careful prescribing practices. In 2020, the FDA updated the boxed warning to alert prescribers to the serious risks of abuse, addiction, physical dependence, and withdrawal reactions associated with benzodiazepines.2 I would hope that an article on benzodiazepines would provide more discussion and guidance surrounding these important issues.
The June 2023 issue also included “High-dose stimulants for adult ADHD” (p. 34-39, doi:10.12788/cp.0366). This article provided esoteric advice on managing stimulant therapy in the setting of Roux-en-Y gastric bypass surgery, yet I would regard stimulant misuse as a far more common and pressing issue.3,4 The recent Drug Enforcement Administration investigation of telehealth stimulant prescribing is a notable example of this problem.5
The patient discussed in this article was receiving large doses of stimulants for a purported case of refractory attention-deficit/hyperactivity disorder (ADHD). The article provided a sparse differential diagnosis for the patient’s intractable symptoms. While rapid metabolism may be an explanation, I would also like to know how the authors ruled out physiological dependence and/or addiction to a controlled substance. How was misuse excluded? Was urine drug testing (UDS) performed? UDS is highly irregular among prescribers,6 which suggests that practices for detecting covert substance abuse and stimulant misuse are inadequate. Wouldn’t such investigations be fundamental to ethical stimulant prescribing?
Jeff Sanders, MD, PhD
Atlanta, Georgia
References
1. Centers for Disease Control and Prevention. Trends in nonfatal and fatal overdoses involving benzodiazepines—38 states and the District of Columbia, 2019-2020. Accessed August 9, 2023. https://www.cdc.gov/mmwr/volumes/70/wr/mm7034a2.htm
2. US Food & Drug Administration. FDA requiring boxed warning updated to improve safe use of benzodiazepine drug class. Accessed August 14, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requiring-boxed-warning-updated-improve-safe-use-benzodiazepine-drug-class
3. McCabe SE, Schulenberg JE, Wilens TE, et al. Prescription stimulant medical and nonmedical use among US secondary school students, 2005 to 2020. JAMA Netw Open. 2023;6(4):e238707. doi:10.1001/jamanetworkopen.2023.8707
4. US Food & Drug Administration. FDA updating warnings to improve safe use of prescription stimulants used to treat ADHD and other conditions. Accessed August 14, 2023. https://www.fda.gov/safety/medical-product-safety-information/fda-updating-warnings-improve-safe-use-prescription-stimulants-used-treat-adhd-and-other-conditions
5. Vaidya A. Report: telehealth company’s prescribing practices come under DEA scrutiny. September 16, 2022. Accessed August 9, 2023. https://mhealthintelligence.com/news/report-telehealth-company-dones-prescribing-practices-come-under-dea-scrutiny
6. Zionts A. Some ADHD patients are drug-tested often, while others are never asked. Kaiser Health News. March 25, 2023. Accessed August 9, 2023. https://www.nbcnews.com/news/amp/rcna76330
Continue to: Drs. Stimpfl and Strawn respond
Drs. Stimpfl and Strawn respond
We thank Dr. Sanders for highlighting the need for clinical equipoise in considering the risks and benefits of medications—something that is true for benzodiazepines, antipsychotics, antidepressants, and in fact all medications. He reminds us that the risks of misuse, dependence, and withdrawal associated with benzodiazepines led to a boxed warning in September 2020 and highlights recent trends of fatal and nonfatal benzodiazepine overdose, especially when combined with opiates.
Our article, which aimed to educate clinicians on benzodiazepine pharmacology and patient-specific factors influencing benzodiazepine selection and dosing, did not focus significantly on the risks associated with benzodiazepines. We do encourage careful and individualized benzodiazepine prescribing. However, we wish to remind our colleagues that benzodiazepines, while associated with risks, continue to have utility in acute and periprocedural settings, and remain an important treatment option for patients with panic disorder, generalized anxiety disorder (especially while waiting for other medications to take effect), catatonia, seizure disorders, and alcohol withdrawal.
We agree that patient-specific risk assessment is essential, as some patients benefit from benzodiazepines despite the risks. However, we also acknowledge that some individuals are at higher risk for adverse outcomes, including those with concurrent opiate use or who are prescribed other sedative-hypnotics; older adults and those with neurocognitive disorders; and patients susceptible to respiratory depression due to other medical reasons (eg, myasthenia gravis, sleep apnea, and chronic obstructive pulmonary disease). Further, we agree that benzodiazepine use during pregnancy is generally not advised due to the risks of neonatal hypotonia and neonatal withdrawal syndrome1 as well as a possible risk of cleft palate that has been reported in some studies.2 Finally, paradoxical reactions may be more common at the extremes of age and in patients with intellectual disability or personality disorders.3,4
Patient characteristics that have been associated with a higher risk of benzodiazepine use disorder include lower education/income, unemployment, having another substance use disorder, and severe psychopathology.5 In some studies, using benzodiazepines for prolonged periods at high doses as well as using those with a rapid onset of action was associated with an increased risk of benzodiazepine use disorder.5-7
Ultimately, we concur with Dr. Sanders on the perils of the “irresponsible use” of medication and emphasize the need for discernment when choosing treatments to avoid rashly discarding an effective remedy while attempting to mitigate all conceivable risks.
Julia Stimpfl, MD
Jeffrey R. Strawn, MD
Cincinnati, Ohio
References
1. McElhatton PR. The effects of benzodiazepine use during pregnancy and lactation. Reprod Toxicol. 1994;8(6):461-475. doi:10.1016/0890-6238(94)90029-9
2. Enato E, Moretti M, Koren G. The fetal safety of benzodiazepines: an updated meta-analysis. J Obstet Gynaecol Can. 2011;33(1):46-48. doi:10.1016/S1701-2163(16)34772-7 Erratum in: J Obstet Gynaecol Can. 2011;33(4):319.
3. Hakimi Y, Petitpain N, Pinzani V, et al. Paradoxical adverse drug reactions: descriptive analysis of French reports. Eur J Clin Pharmacol. 2020;76(8):1169-1174. doi:10.1007/s00228-020-02892-2
4. Paton C. Benzodiazepines and disinhibition: a review. Psychiatric Bulletin. 2002;26(12):460-462. doi:10.1192/pb.26.12.460
5. Fride Tvete I, Bjørner T, Skomedal T. Risk factors for excessive benzodiazepine use in a working age population: a nationwide 5-year survey in Norway. Scand J Prim Health Care. 2015;33(4):252-259. doi:10.3109/02813432.2015.1117282
6. Griffiths RR, Johnson MW. Relative abuse liability of hypnotic drugs: a conceptual framework and algorithm for differentiating among compounds. J Clin Psychiatry. 2005;66 Suppl 9:31-41.
7. Kan CC, Hilberink SR, Breteler MH. Determination of the main risk factors for benzodiazepine dependence using a multivariate and multidimensional approach. Compr Psychiatry. 2004;45(2):88-94. doi:10.1016/j.comppsych.2003.12.007
Continue to: Drs. Sarma and Grady respond
Drs. Sarma and Grady respond
Dr. Sanders’ letter highlights the potential caveats associated with prescribing controlled substances. We agree that our short case summary includes numerous interesting elements, each of which would be worthy of further exploration and discussion. Our choice was to highlight the patient history of bariatric surgery and use this as a springboard into a review of stimulants, including the newest formulations for ADHD. For more than 1 year, many generic stimulants have been in short supply, and patients and clinicians have been seeking other therapeutic options. Given this background and with newer, branded stimulant use becoming more commonplace, we believe our article was useful and timely.
Our original intent had been to include an example of a controlled substance agreement. Regrettably, there was simply not enough space for this document or the additional discussion that its inclusion would deem necessary. Nevertheless, had the May 2023 FDA requirement for manufacturers to update the labeling of prescription stimulants1 to clarify misuse and abuse been published before our article’s final revision, we would have mentioned it and provided the appropriate link.
Subbu J. Sarma, MD, FAPA
Kansas City, Missouri
Sarah E. Grady, PharmD, BCPS, BCPP
Des Moines, Iowa
References
1. US Food & Drug Administration. FDA requires updates to clarify labeling of prescription stimulants used to treat ADHD and other conditions. Accessed August 9, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-updates-clarify-labeling-prescription-stimulants-used-treat-adhd-and-other-conditions
I was disheartened with the June 2023 issue of
The benzodiazepine pharmacology discussed in this article is interesting, but it would be helpful if it had been integrated within a much more extensive discussion of careful prescribing practices. In 2020, the FDA updated the boxed warning to alert prescribers to the serious risks of abuse, addiction, physical dependence, and withdrawal reactions associated with benzodiazepines.2 I would hope that an article on benzodiazepines would provide more discussion and guidance surrounding these important issues.
The June 2023 issue also included “High-dose stimulants for adult ADHD” (p. 34-39, doi:10.12788/cp.0366). This article provided esoteric advice on managing stimulant therapy in the setting of Roux-en-Y gastric bypass surgery, yet I would regard stimulant misuse as a far more common and pressing issue.3,4 The recent Drug Enforcement Administration investigation of telehealth stimulant prescribing is a notable example of this problem.5
The patient discussed in this article was receiving large doses of stimulants for a purported case of refractory attention-deficit/hyperactivity disorder (ADHD). The article provided a sparse differential diagnosis for the patient’s intractable symptoms. While rapid metabolism may be an explanation, I would also like to know how the authors ruled out physiological dependence and/or addiction to a controlled substance. How was misuse excluded? Was urine drug testing (UDS) performed? UDS is highly irregular among prescribers,6 which suggests that practices for detecting covert substance abuse and stimulant misuse are inadequate. Wouldn’t such investigations be fundamental to ethical stimulant prescribing?
Jeff Sanders, MD, PhD
Atlanta, Georgia
References
1. Centers for Disease Control and Prevention. Trends in nonfatal and fatal overdoses involving benzodiazepines—38 states and the District of Columbia, 2019-2020. Accessed August 9, 2023. https://www.cdc.gov/mmwr/volumes/70/wr/mm7034a2.htm
2. US Food & Drug Administration. FDA requiring boxed warning updated to improve safe use of benzodiazepine drug class. Accessed August 14, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requiring-boxed-warning-updated-improve-safe-use-benzodiazepine-drug-class
3. McCabe SE, Schulenberg JE, Wilens TE, et al. Prescription stimulant medical and nonmedical use among US secondary school students, 2005 to 2020. JAMA Netw Open. 2023;6(4):e238707. doi:10.1001/jamanetworkopen.2023.8707
4. US Food & Drug Administration. FDA updating warnings to improve safe use of prescription stimulants used to treat ADHD and other conditions. Accessed August 14, 2023. https://www.fda.gov/safety/medical-product-safety-information/fda-updating-warnings-improve-safe-use-prescription-stimulants-used-treat-adhd-and-other-conditions
5. Vaidya A. Report: telehealth company’s prescribing practices come under DEA scrutiny. September 16, 2022. Accessed August 9, 2023. https://mhealthintelligence.com/news/report-telehealth-company-dones-prescribing-practices-come-under-dea-scrutiny
6. Zionts A. Some ADHD patients are drug-tested often, while others are never asked. Kaiser Health News. March 25, 2023. Accessed August 9, 2023. https://www.nbcnews.com/news/amp/rcna76330
Continue to: Drs. Stimpfl and Strawn respond
Drs. Stimpfl and Strawn respond
We thank Dr. Sanders for highlighting the need for clinical equipoise in considering the risks and benefits of medications—something that is true for benzodiazepines, antipsychotics, antidepressants, and in fact all medications. He reminds us that the risks of misuse, dependence, and withdrawal associated with benzodiazepines led to a boxed warning in September 2020 and highlights recent trends of fatal and nonfatal benzodiazepine overdose, especially when combined with opiates.
Our article, which aimed to educate clinicians on benzodiazepine pharmacology and patient-specific factors influencing benzodiazepine selection and dosing, did not focus significantly on the risks associated with benzodiazepines. We do encourage careful and individualized benzodiazepine prescribing. However, we wish to remind our colleagues that benzodiazepines, while associated with risks, continue to have utility in acute and periprocedural settings, and remain an important treatment option for patients with panic disorder, generalized anxiety disorder (especially while waiting for other medications to take effect), catatonia, seizure disorders, and alcohol withdrawal.
We agree that patient-specific risk assessment is essential, as some patients benefit from benzodiazepines despite the risks. However, we also acknowledge that some individuals are at higher risk for adverse outcomes, including those with concurrent opiate use or who are prescribed other sedative-hypnotics; older adults and those with neurocognitive disorders; and patients susceptible to respiratory depression due to other medical reasons (eg, myasthenia gravis, sleep apnea, and chronic obstructive pulmonary disease). Further, we agree that benzodiazepine use during pregnancy is generally not advised due to the risks of neonatal hypotonia and neonatal withdrawal syndrome1 as well as a possible risk of cleft palate that has been reported in some studies.2 Finally, paradoxical reactions may be more common at the extremes of age and in patients with intellectual disability or personality disorders.3,4
Patient characteristics that have been associated with a higher risk of benzodiazepine use disorder include lower education/income, unemployment, having another substance use disorder, and severe psychopathology.5 In some studies, using benzodiazepines for prolonged periods at high doses as well as using those with a rapid onset of action was associated with an increased risk of benzodiazepine use disorder.5-7
Ultimately, we concur with Dr. Sanders on the perils of the “irresponsible use” of medication and emphasize the need for discernment when choosing treatments to avoid rashly discarding an effective remedy while attempting to mitigate all conceivable risks.
Julia Stimpfl, MD
Jeffrey R. Strawn, MD
Cincinnati, Ohio
References
1. McElhatton PR. The effects of benzodiazepine use during pregnancy and lactation. Reprod Toxicol. 1994;8(6):461-475. doi:10.1016/0890-6238(94)90029-9
2. Enato E, Moretti M, Koren G. The fetal safety of benzodiazepines: an updated meta-analysis. J Obstet Gynaecol Can. 2011;33(1):46-48. doi:10.1016/S1701-2163(16)34772-7 Erratum in: J Obstet Gynaecol Can. 2011;33(4):319.
3. Hakimi Y, Petitpain N, Pinzani V, et al. Paradoxical adverse drug reactions: descriptive analysis of French reports. Eur J Clin Pharmacol. 2020;76(8):1169-1174. doi:10.1007/s00228-020-02892-2
4. Paton C. Benzodiazepines and disinhibition: a review. Psychiatric Bulletin. 2002;26(12):460-462. doi:10.1192/pb.26.12.460
5. Fride Tvete I, Bjørner T, Skomedal T. Risk factors for excessive benzodiazepine use in a working age population: a nationwide 5-year survey in Norway. Scand J Prim Health Care. 2015;33(4):252-259. doi:10.3109/02813432.2015.1117282
6. Griffiths RR, Johnson MW. Relative abuse liability of hypnotic drugs: a conceptual framework and algorithm for differentiating among compounds. J Clin Psychiatry. 2005;66 Suppl 9:31-41.
7. Kan CC, Hilberink SR, Breteler MH. Determination of the main risk factors for benzodiazepine dependence using a multivariate and multidimensional approach. Compr Psychiatry. 2004;45(2):88-94. doi:10.1016/j.comppsych.2003.12.007
Continue to: Drs. Sarma and Grady respond
Drs. Sarma and Grady respond
Dr. Sanders’ letter highlights the potential caveats associated with prescribing controlled substances. We agree that our short case summary includes numerous interesting elements, each of which would be worthy of further exploration and discussion. Our choice was to highlight the patient history of bariatric surgery and use this as a springboard into a review of stimulants, including the newest formulations for ADHD. For more than 1 year, many generic stimulants have been in short supply, and patients and clinicians have been seeking other therapeutic options. Given this background and with newer, branded stimulant use becoming more commonplace, we believe our article was useful and timely.
Our original intent had been to include an example of a controlled substance agreement. Regrettably, there was simply not enough space for this document or the additional discussion that its inclusion would deem necessary. Nevertheless, had the May 2023 FDA requirement for manufacturers to update the labeling of prescription stimulants1 to clarify misuse and abuse been published before our article’s final revision, we would have mentioned it and provided the appropriate link.
Subbu J. Sarma, MD, FAPA
Kansas City, Missouri
Sarah E. Grady, PharmD, BCPS, BCPP
Des Moines, Iowa
References
1. US Food & Drug Administration. FDA requires updates to clarify labeling of prescription stimulants used to treat ADHD and other conditions. Accessed August 9, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-updates-clarify-labeling-prescription-stimulants-used-treat-adhd-and-other-conditions
I was disheartened with the June 2023 issue of
The benzodiazepine pharmacology discussed in this article is interesting, but it would be helpful if it had been integrated within a much more extensive discussion of careful prescribing practices. In 2020, the FDA updated the boxed warning to alert prescribers to the serious risks of abuse, addiction, physical dependence, and withdrawal reactions associated with benzodiazepines.2 I would hope that an article on benzodiazepines would provide more discussion and guidance surrounding these important issues.
The June 2023 issue also included “High-dose stimulants for adult ADHD” (p. 34-39, doi:10.12788/cp.0366). This article provided esoteric advice on managing stimulant therapy in the setting of Roux-en-Y gastric bypass surgery, yet I would regard stimulant misuse as a far more common and pressing issue.3,4 The recent Drug Enforcement Administration investigation of telehealth stimulant prescribing is a notable example of this problem.5
The patient discussed in this article was receiving large doses of stimulants for a purported case of refractory attention-deficit/hyperactivity disorder (ADHD). The article provided a sparse differential diagnosis for the patient’s intractable symptoms. While rapid metabolism may be an explanation, I would also like to know how the authors ruled out physiological dependence and/or addiction to a controlled substance. How was misuse excluded? Was urine drug testing (UDS) performed? UDS is highly irregular among prescribers,6 which suggests that practices for detecting covert substance abuse and stimulant misuse are inadequate. Wouldn’t such investigations be fundamental to ethical stimulant prescribing?
Jeff Sanders, MD, PhD
Atlanta, Georgia
References
1. Centers for Disease Control and Prevention. Trends in nonfatal and fatal overdoses involving benzodiazepines—38 states and the District of Columbia, 2019-2020. Accessed August 9, 2023. https://www.cdc.gov/mmwr/volumes/70/wr/mm7034a2.htm
2. US Food & Drug Administration. FDA requiring boxed warning updated to improve safe use of benzodiazepine drug class. Accessed August 14, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requiring-boxed-warning-updated-improve-safe-use-benzodiazepine-drug-class
3. McCabe SE, Schulenberg JE, Wilens TE, et al. Prescription stimulant medical and nonmedical use among US secondary school students, 2005 to 2020. JAMA Netw Open. 2023;6(4):e238707. doi:10.1001/jamanetworkopen.2023.8707
4. US Food & Drug Administration. FDA updating warnings to improve safe use of prescription stimulants used to treat ADHD and other conditions. Accessed August 14, 2023. https://www.fda.gov/safety/medical-product-safety-information/fda-updating-warnings-improve-safe-use-prescription-stimulants-used-treat-adhd-and-other-conditions
5. Vaidya A. Report: telehealth company’s prescribing practices come under DEA scrutiny. September 16, 2022. Accessed August 9, 2023. https://mhealthintelligence.com/news/report-telehealth-company-dones-prescribing-practices-come-under-dea-scrutiny
6. Zionts A. Some ADHD patients are drug-tested often, while others are never asked. Kaiser Health News. March 25, 2023. Accessed August 9, 2023. https://www.nbcnews.com/news/amp/rcna76330
Continue to: Drs. Stimpfl and Strawn respond
Drs. Stimpfl and Strawn respond
We thank Dr. Sanders for highlighting the need for clinical equipoise in considering the risks and benefits of medications—something that is true for benzodiazepines, antipsychotics, antidepressants, and in fact all medications. He reminds us that the risks of misuse, dependence, and withdrawal associated with benzodiazepines led to a boxed warning in September 2020 and highlights recent trends of fatal and nonfatal benzodiazepine overdose, especially when combined with opiates.
Our article, which aimed to educate clinicians on benzodiazepine pharmacology and patient-specific factors influencing benzodiazepine selection and dosing, did not focus significantly on the risks associated with benzodiazepines. We do encourage careful and individualized benzodiazepine prescribing. However, we wish to remind our colleagues that benzodiazepines, while associated with risks, continue to have utility in acute and periprocedural settings, and remain an important treatment option for patients with panic disorder, generalized anxiety disorder (especially while waiting for other medications to take effect), catatonia, seizure disorders, and alcohol withdrawal.
We agree that patient-specific risk assessment is essential, as some patients benefit from benzodiazepines despite the risks. However, we also acknowledge that some individuals are at higher risk for adverse outcomes, including those with concurrent opiate use or who are prescribed other sedative-hypnotics; older adults and those with neurocognitive disorders; and patients susceptible to respiratory depression due to other medical reasons (eg, myasthenia gravis, sleep apnea, and chronic obstructive pulmonary disease). Further, we agree that benzodiazepine use during pregnancy is generally not advised due to the risks of neonatal hypotonia and neonatal withdrawal syndrome1 as well as a possible risk of cleft palate that has been reported in some studies.2 Finally, paradoxical reactions may be more common at the extremes of age and in patients with intellectual disability or personality disorders.3,4
Patient characteristics that have been associated with a higher risk of benzodiazepine use disorder include lower education/income, unemployment, having another substance use disorder, and severe psychopathology.5 In some studies, using benzodiazepines for prolonged periods at high doses as well as using those with a rapid onset of action was associated with an increased risk of benzodiazepine use disorder.5-7
Ultimately, we concur with Dr. Sanders on the perils of the “irresponsible use” of medication and emphasize the need for discernment when choosing treatments to avoid rashly discarding an effective remedy while attempting to mitigate all conceivable risks.
Julia Stimpfl, MD
Jeffrey R. Strawn, MD
Cincinnati, Ohio
References
1. McElhatton PR. The effects of benzodiazepine use during pregnancy and lactation. Reprod Toxicol. 1994;8(6):461-475. doi:10.1016/0890-6238(94)90029-9
2. Enato E, Moretti M, Koren G. The fetal safety of benzodiazepines: an updated meta-analysis. J Obstet Gynaecol Can. 2011;33(1):46-48. doi:10.1016/S1701-2163(16)34772-7 Erratum in: J Obstet Gynaecol Can. 2011;33(4):319.
3. Hakimi Y, Petitpain N, Pinzani V, et al. Paradoxical adverse drug reactions: descriptive analysis of French reports. Eur J Clin Pharmacol. 2020;76(8):1169-1174. doi:10.1007/s00228-020-02892-2
4. Paton C. Benzodiazepines and disinhibition: a review. Psychiatric Bulletin. 2002;26(12):460-462. doi:10.1192/pb.26.12.460
5. Fride Tvete I, Bjørner T, Skomedal T. Risk factors for excessive benzodiazepine use in a working age population: a nationwide 5-year survey in Norway. Scand J Prim Health Care. 2015;33(4):252-259. doi:10.3109/02813432.2015.1117282
6. Griffiths RR, Johnson MW. Relative abuse liability of hypnotic drugs: a conceptual framework and algorithm for differentiating among compounds. J Clin Psychiatry. 2005;66 Suppl 9:31-41.
7. Kan CC, Hilberink SR, Breteler MH. Determination of the main risk factors for benzodiazepine dependence using a multivariate and multidimensional approach. Compr Psychiatry. 2004;45(2):88-94. doi:10.1016/j.comppsych.2003.12.007
Continue to: Drs. Sarma and Grady respond
Drs. Sarma and Grady respond
Dr. Sanders’ letter highlights the potential caveats associated with prescribing controlled substances. We agree that our short case summary includes numerous interesting elements, each of which would be worthy of further exploration and discussion. Our choice was to highlight the patient history of bariatric surgery and use this as a springboard into a review of stimulants, including the newest formulations for ADHD. For more than 1 year, many generic stimulants have been in short supply, and patients and clinicians have been seeking other therapeutic options. Given this background and with newer, branded stimulant use becoming more commonplace, we believe our article was useful and timely.
Our original intent had been to include an example of a controlled substance agreement. Regrettably, there was simply not enough space for this document or the additional discussion that its inclusion would deem necessary. Nevertheless, had the May 2023 FDA requirement for manufacturers to update the labeling of prescription stimulants1 to clarify misuse and abuse been published before our article’s final revision, we would have mentioned it and provided the appropriate link.
Subbu J. Sarma, MD, FAPA
Kansas City, Missouri
Sarah E. Grady, PharmD, BCPS, BCPP
Des Moines, Iowa
References
1. US Food & Drug Administration. FDA requires updates to clarify labeling of prescription stimulants used to treat ADHD and other conditions. Accessed August 9, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-updates-clarify-labeling-prescription-stimulants-used-treat-adhd-and-other-conditions
Diversity in Multiple Sclerosis Care: How the Field of Underrepresented Minorities Has Evolved, and Where We Still See Areas for Improvement
The persistent notion that multiple sclerosis (MS) is predominantly a White patient’s disease has been challenged by scientific data and our clinical experience in the field. Recent research has shown a higher risk of MS in non-White populations than originally thought. This may be surprising, but new data are influencing the way we now approach MS in under-represented minorities, bringing this topic to the forefront of scientific interest.
The early conviction that “there is no MS in minorities” led to underdiagnosis and misdiagnosis of MS in those patients, which in turn deepened these patients’ distrust of physicians and reluctance to seek further medical care, very often delivered by non-minority providers. Inequities in social determinants of health, low health literacy, and lack of private insurance, along with structural racism in healthcare, has further hindered active engagement with an already marginalized patient population in their MS care. This lack of engagement and lack of minorities in scientific research has proved to be unfavorable for MS research as well, creating large and persistent knowledge gaps in understanding MS course, severity, and response to treatment specific to this group. A 2014 PubMed search found 52,000 publications on MS in English, but in only 136 of those were minority patients with MS (Black or Hispanic/Latino) the primary research focus. In 2019, the same search indicated that the subsequent 5 years produced only 30 more articles focusing solely on minority patients.
Research participation of underrepresented minorities is another area where we, as a field, continue to fail these patients. A review of participant enrollment in MS clinical trials that took place between 1993 and 2006 showed a significant decrease in the percentage of enrolled Black patients (from 7% to about 4%). This trend did not improve by the DEFINE treatment trial (2012), in which only 2% of enrolled patients were Black. Of the 1246 participants in the 2019 SUNBEAM MS study, only 2 were Black. Low numbers of minority patients in trials prevent us from drawing any reasonable conclusion as to the efficacy of disease-modifying agents in those patients and make the goal of personalized medicine for this group impossible.
The results of the research conducted on these groups are compelling and should be prompting further work. Not only do Black patients have a higher risk of MS, but there is also now convincing evidence that MS in minorities is more severe overall, causing early progression of disability and necessitating assistive gait devices such as a cane or wheelchair. Minority patients tend to have more extensive involvement of spinal cord and infra-tentorial brain structures during the disease, which could explain the increased likelihood of more severe disease and earlier disability. Minority patients were admitted to nursing homes at a younger age, with greater physical and cognitive impairment than nonminority patients. A study looking at MS mortality between 1999 and 2015 found that Black males with MS had the highest mortality rate before age 45, and Black females before age 53. MS mortality increased with age but peaked at age 55 to 64 for Black patients and 65 to 74 for White patients. Underrepresented minorities are also less likely to use community resources, case management, medical equipment, and home nursing services. When looking at other measures of disease impact on these patients, studies evaluating magnetic resonance imaging (MRI) data showed higher lesion volume in Black patients with MS, as well as a higher degree of brain demyelination and atrophy when compared with White patients.
Treatment strategies currently used for underrepresented minority patients, as well as estimations of medication efficacy, treatment responses, and adverse-event profiles are largely driven by data from clinical trials with only minimal representation of those patients. How can we propose a patient-tailored and individualized treatment plan without these crucial data? Given that, to this day, not a single trial has focused solely on underrepresented minorities, we are left with either post hoc exploratory subgroup analyses of existing trials or pragmatic, observational, and very often retrospective studies using chart analysis. Notwithstanding the methodological flaws of either approach, prior studies did suggest worse response to platform therapies in Black patients, but equal response to high-efficacy therapies when compared with White patients.
Definitive biological underpinnings of disparities in disease severity have not been identified. In recent years, the field of health outcomes research has suggested we move away from considering racial categories as biologically distinct and instead focus on long-overlooked sociodemographic and modifiable lifestyle
factors. The role of diet, exercise, body mass index, smoking, and vascular comorbidities as risk factors associated with worse MS outcomes has been previously shown; however, these factors have not been rigorously assessed in underrepresented populations with MS. Recent studies focused on uncovering what drives the differences in MS severity in underrepresented populations disagree on the role biological differences, socioeconomic disparities, and structural racism in both healthcare settings and society play in answering this question. While it is plausible that a combination of these factors might explain our observations, more research on larger, underserved patient populations and better-defined measures of socioeconomic differences are needed to answer this complex question.
The path of recognizing and correcting our mistakes is not simple but must be done, and our underrepresented minority patients depend on our swift action. There are many places where we as a field of experts can and must do better—in communities, healthcare systems, and society in general.
Increasing community health literacy around MS, rebuilding trust, and addressing structural racism on every level is important. Outreach and educational programs that include in-person meetings and leverage social media platforms can help empower patients and their families—and hopefully increase trust in healthcare providers. Devising targeted interventions focusing on modifiable factors of a healthy lifestyle such as diet and exercise can increase community engagement and strengthen the support system for our patients. Increasing diversity in our own field of physicians, nurses, and other healthcare providers can also aid in strengthening mutual relationships.
Improving access to comprehensive MS care for underrepresented minorities who very often also lack robust insurance coverage is paramount. Recipients of comprehensive care are more likely to participate in research, as these patients receive more well-rounded care and have a lower risk of mismanaged comorbidities. Their involvement in the treatment plan is higher, which also improves compliance with treatment. Patients in comprehensive care centers are more likely to receive newer treatment agents with better efficacy without hindrance of monitoring barriers, and they are likely to benefit from treatment strategies using newly approved agents soon after US Food and Drug Administration approval.
Increasing research participation and, ideally, conducting a clinical trial devoted solely to studying MS in underrepresented minorities is something for which we should actively strive. Identifying the main factors prohibiting enrollment and retention of a high number of minority participants in trials is critical to success. Multiple deterrents in day-to-day life, very often directly connected to economic hardship and racism, pose a very real threat to equitable trial participation. To even consider a successful trial for underrepresented minorities, we must do better in devising strategies and accommodations to help overcome those barriers.
The underserved minorities with MS deserve and need our attention and focus. These patients have largely been neglected and forgotten, but now are emerging at the forefront of our attention—where they belong.
The persistent notion that multiple sclerosis (MS) is predominantly a White patient’s disease has been challenged by scientific data and our clinical experience in the field. Recent research has shown a higher risk of MS in non-White populations than originally thought. This may be surprising, but new data are influencing the way we now approach MS in under-represented minorities, bringing this topic to the forefront of scientific interest.
The early conviction that “there is no MS in minorities” led to underdiagnosis and misdiagnosis of MS in those patients, which in turn deepened these patients’ distrust of physicians and reluctance to seek further medical care, very often delivered by non-minority providers. Inequities in social determinants of health, low health literacy, and lack of private insurance, along with structural racism in healthcare, has further hindered active engagement with an already marginalized patient population in their MS care. This lack of engagement and lack of minorities in scientific research has proved to be unfavorable for MS research as well, creating large and persistent knowledge gaps in understanding MS course, severity, and response to treatment specific to this group. A 2014 PubMed search found 52,000 publications on MS in English, but in only 136 of those were minority patients with MS (Black or Hispanic/Latino) the primary research focus. In 2019, the same search indicated that the subsequent 5 years produced only 30 more articles focusing solely on minority patients.
Research participation of underrepresented minorities is another area where we, as a field, continue to fail these patients. A review of participant enrollment in MS clinical trials that took place between 1993 and 2006 showed a significant decrease in the percentage of enrolled Black patients (from 7% to about 4%). This trend did not improve by the DEFINE treatment trial (2012), in which only 2% of enrolled patients were Black. Of the 1246 participants in the 2019 SUNBEAM MS study, only 2 were Black. Low numbers of minority patients in trials prevent us from drawing any reasonable conclusion as to the efficacy of disease-modifying agents in those patients and make the goal of personalized medicine for this group impossible.
The results of the research conducted on these groups are compelling and should be prompting further work. Not only do Black patients have a higher risk of MS, but there is also now convincing evidence that MS in minorities is more severe overall, causing early progression of disability and necessitating assistive gait devices such as a cane or wheelchair. Minority patients tend to have more extensive involvement of spinal cord and infra-tentorial brain structures during the disease, which could explain the increased likelihood of more severe disease and earlier disability. Minority patients were admitted to nursing homes at a younger age, with greater physical and cognitive impairment than nonminority patients. A study looking at MS mortality between 1999 and 2015 found that Black males with MS had the highest mortality rate before age 45, and Black females before age 53. MS mortality increased with age but peaked at age 55 to 64 for Black patients and 65 to 74 for White patients. Underrepresented minorities are also less likely to use community resources, case management, medical equipment, and home nursing services. When looking at other measures of disease impact on these patients, studies evaluating magnetic resonance imaging (MRI) data showed higher lesion volume in Black patients with MS, as well as a higher degree of brain demyelination and atrophy when compared with White patients.
Treatment strategies currently used for underrepresented minority patients, as well as estimations of medication efficacy, treatment responses, and adverse-event profiles are largely driven by data from clinical trials with only minimal representation of those patients. How can we propose a patient-tailored and individualized treatment plan without these crucial data? Given that, to this day, not a single trial has focused solely on underrepresented minorities, we are left with either post hoc exploratory subgroup analyses of existing trials or pragmatic, observational, and very often retrospective studies using chart analysis. Notwithstanding the methodological flaws of either approach, prior studies did suggest worse response to platform therapies in Black patients, but equal response to high-efficacy therapies when compared with White patients.
Definitive biological underpinnings of disparities in disease severity have not been identified. In recent years, the field of health outcomes research has suggested we move away from considering racial categories as biologically distinct and instead focus on long-overlooked sociodemographic and modifiable lifestyle
factors. The role of diet, exercise, body mass index, smoking, and vascular comorbidities as risk factors associated with worse MS outcomes has been previously shown; however, these factors have not been rigorously assessed in underrepresented populations with MS. Recent studies focused on uncovering what drives the differences in MS severity in underrepresented populations disagree on the role biological differences, socioeconomic disparities, and structural racism in both healthcare settings and society play in answering this question. While it is plausible that a combination of these factors might explain our observations, more research on larger, underserved patient populations and better-defined measures of socioeconomic differences are needed to answer this complex question.
The path of recognizing and correcting our mistakes is not simple but must be done, and our underrepresented minority patients depend on our swift action. There are many places where we as a field of experts can and must do better—in communities, healthcare systems, and society in general.
Increasing community health literacy around MS, rebuilding trust, and addressing structural racism on every level is important. Outreach and educational programs that include in-person meetings and leverage social media platforms can help empower patients and their families—and hopefully increase trust in healthcare providers. Devising targeted interventions focusing on modifiable factors of a healthy lifestyle such as diet and exercise can increase community engagement and strengthen the support system for our patients. Increasing diversity in our own field of physicians, nurses, and other healthcare providers can also aid in strengthening mutual relationships.
Improving access to comprehensive MS care for underrepresented minorities who very often also lack robust insurance coverage is paramount. Recipients of comprehensive care are more likely to participate in research, as these patients receive more well-rounded care and have a lower risk of mismanaged comorbidities. Their involvement in the treatment plan is higher, which also improves compliance with treatment. Patients in comprehensive care centers are more likely to receive newer treatment agents with better efficacy without hindrance of monitoring barriers, and they are likely to benefit from treatment strategies using newly approved agents soon after US Food and Drug Administration approval.
Increasing research participation and, ideally, conducting a clinical trial devoted solely to studying MS in underrepresented minorities is something for which we should actively strive. Identifying the main factors prohibiting enrollment and retention of a high number of minority participants in trials is critical to success. Multiple deterrents in day-to-day life, very often directly connected to economic hardship and racism, pose a very real threat to equitable trial participation. To even consider a successful trial for underrepresented minorities, we must do better in devising strategies and accommodations to help overcome those barriers.
The underserved minorities with MS deserve and need our attention and focus. These patients have largely been neglected and forgotten, but now are emerging at the forefront of our attention—where they belong.
The persistent notion that multiple sclerosis (MS) is predominantly a White patient’s disease has been challenged by scientific data and our clinical experience in the field. Recent research has shown a higher risk of MS in non-White populations than originally thought. This may be surprising, but new data are influencing the way we now approach MS in under-represented minorities, bringing this topic to the forefront of scientific interest.
The early conviction that “there is no MS in minorities” led to underdiagnosis and misdiagnosis of MS in those patients, which in turn deepened these patients’ distrust of physicians and reluctance to seek further medical care, very often delivered by non-minority providers. Inequities in social determinants of health, low health literacy, and lack of private insurance, along with structural racism in healthcare, has further hindered active engagement with an already marginalized patient population in their MS care. This lack of engagement and lack of minorities in scientific research has proved to be unfavorable for MS research as well, creating large and persistent knowledge gaps in understanding MS course, severity, and response to treatment specific to this group. A 2014 PubMed search found 52,000 publications on MS in English, but in only 136 of those were minority patients with MS (Black or Hispanic/Latino) the primary research focus. In 2019, the same search indicated that the subsequent 5 years produced only 30 more articles focusing solely on minority patients.
Research participation of underrepresented minorities is another area where we, as a field, continue to fail these patients. A review of participant enrollment in MS clinical trials that took place between 1993 and 2006 showed a significant decrease in the percentage of enrolled Black patients (from 7% to about 4%). This trend did not improve by the DEFINE treatment trial (2012), in which only 2% of enrolled patients were Black. Of the 1246 participants in the 2019 SUNBEAM MS study, only 2 were Black. Low numbers of minority patients in trials prevent us from drawing any reasonable conclusion as to the efficacy of disease-modifying agents in those patients and make the goal of personalized medicine for this group impossible.
The results of the research conducted on these groups are compelling and should be prompting further work. Not only do Black patients have a higher risk of MS, but there is also now convincing evidence that MS in minorities is more severe overall, causing early progression of disability and necessitating assistive gait devices such as a cane or wheelchair. Minority patients tend to have more extensive involvement of spinal cord and infra-tentorial brain structures during the disease, which could explain the increased likelihood of more severe disease and earlier disability. Minority patients were admitted to nursing homes at a younger age, with greater physical and cognitive impairment than nonminority patients. A study looking at MS mortality between 1999 and 2015 found that Black males with MS had the highest mortality rate before age 45, and Black females before age 53. MS mortality increased with age but peaked at age 55 to 64 for Black patients and 65 to 74 for White patients. Underrepresented minorities are also less likely to use community resources, case management, medical equipment, and home nursing services. When looking at other measures of disease impact on these patients, studies evaluating magnetic resonance imaging (MRI) data showed higher lesion volume in Black patients with MS, as well as a higher degree of brain demyelination and atrophy when compared with White patients.
Treatment strategies currently used for underrepresented minority patients, as well as estimations of medication efficacy, treatment responses, and adverse-event profiles are largely driven by data from clinical trials with only minimal representation of those patients. How can we propose a patient-tailored and individualized treatment plan without these crucial data? Given that, to this day, not a single trial has focused solely on underrepresented minorities, we are left with either post hoc exploratory subgroup analyses of existing trials or pragmatic, observational, and very often retrospective studies using chart analysis. Notwithstanding the methodological flaws of either approach, prior studies did suggest worse response to platform therapies in Black patients, but equal response to high-efficacy therapies when compared with White patients.
Definitive biological underpinnings of disparities in disease severity have not been identified. In recent years, the field of health outcomes research has suggested we move away from considering racial categories as biologically distinct and instead focus on long-overlooked sociodemographic and modifiable lifestyle
factors. The role of diet, exercise, body mass index, smoking, and vascular comorbidities as risk factors associated with worse MS outcomes has been previously shown; however, these factors have not been rigorously assessed in underrepresented populations with MS. Recent studies focused on uncovering what drives the differences in MS severity in underrepresented populations disagree on the role biological differences, socioeconomic disparities, and structural racism in both healthcare settings and society play in answering this question. While it is plausible that a combination of these factors might explain our observations, more research on larger, underserved patient populations and better-defined measures of socioeconomic differences are needed to answer this complex question.
The path of recognizing and correcting our mistakes is not simple but must be done, and our underrepresented minority patients depend on our swift action. There are many places where we as a field of experts can and must do better—in communities, healthcare systems, and society in general.
Increasing community health literacy around MS, rebuilding trust, and addressing structural racism on every level is important. Outreach and educational programs that include in-person meetings and leverage social media platforms can help empower patients and their families—and hopefully increase trust in healthcare providers. Devising targeted interventions focusing on modifiable factors of a healthy lifestyle such as diet and exercise can increase community engagement and strengthen the support system for our patients. Increasing diversity in our own field of physicians, nurses, and other healthcare providers can also aid in strengthening mutual relationships.
Improving access to comprehensive MS care for underrepresented minorities who very often also lack robust insurance coverage is paramount. Recipients of comprehensive care are more likely to participate in research, as these patients receive more well-rounded care and have a lower risk of mismanaged comorbidities. Their involvement in the treatment plan is higher, which also improves compliance with treatment. Patients in comprehensive care centers are more likely to receive newer treatment agents with better efficacy without hindrance of monitoring barriers, and they are likely to benefit from treatment strategies using newly approved agents soon after US Food and Drug Administration approval.
Increasing research participation and, ideally, conducting a clinical trial devoted solely to studying MS in underrepresented minorities is something for which we should actively strive. Identifying the main factors prohibiting enrollment and retention of a high number of minority participants in trials is critical to success. Multiple deterrents in day-to-day life, very often directly connected to economic hardship and racism, pose a very real threat to equitable trial participation. To even consider a successful trial for underrepresented minorities, we must do better in devising strategies and accommodations to help overcome those barriers.
The underserved minorities with MS deserve and need our attention and focus. These patients have largely been neglected and forgotten, but now are emerging at the forefront of our attention—where they belong.
Commentary: Updates in mantle cell lymphoma, September 2023
Mantle cell lymphoma (MCL) is a rare subtype of non-Hodgkin lymphoma that is characterized by t(11;14) and cyclin D1 overexpression. It is also known to be clinically heterogenous, with disease presentations ranging from indolent to aggressive. Baseline risk can be determined on the basis of a combination of clinical and pathologic features. A key prognostic tool, for example, is the Mantle Cell Lymphoma International Prognostic Index-Combined (MIPI-c), which integrates the standard MIPI clinical factors (age, performance status, lactate dehydrogenase, and leukocyte count) with estimates of proliferation (Ki-67).1 Other features, including the presence of TP53 alterations, have also been associated with poor outcomes, even with intensive therapy.2
Recently, a study aimed to further refine prognostication in MCL in order to identify high-risk patients that may be more likely to benefit from novel treatment strategies (Scheubeck et al). This retrospective study included 684 patients with MCL from the MCL-Younger and MCL-Elderly trials with evaluable data for Ki-67 or p53 expression (a surrogate for TP53 alterations). Patients were classified as having high-risk disease on the basis of a high-risk MIPI-c or p53 expression > 50% or as having low-risk disease on the basis of low, low-intermediate, or high-intermediate MIPI-c and p53 expression ≤ 50%. Patients with high-risk disease had significantly shorter median failure-free survival (1.1 vs 5.6 years; P < .0001) and overall survival (2.2 vs 13.2 years; P < .0001) compared with those with low-risk disease. The differences were confirmed in two validation cohorts from the Italian MCL0208 and Nordic-MCL4 trials. These data highlight the poor outcomes of conventional therapy in patients with high-risk MCL. Evaluation of novel approaches should be considered in these patients.
Bruton tyrosine kinase (BTK) inhibitors have been promising options for patients with MCL, including those with high-risk features. Acalabrutinib is a second-generation covalent BTK inhibitor that is approved by the US Food and Drug Administration for patients who have received at least one prior line of therapy. The final results of the single-arm, phase 2 ACE-LY-004 study recently demonstrated long-term safety and efficacy in patients with relapsed/refractory MCL (Le Gouill et al). The overall and complete response rates were 81.5% (95% CI 73.5%-87.9%) and 47.6% (95% CI 38.5%-56.7%), respectively. After a 38.1-month median follow-up, the median duration of response and progression-free survival were 28.6 months (95% CI 17.5-39.1) and 22.0 months (95% CI 16.6-33.3), respectively. Responses were also seen in patients with high-risk features, including blastoid morphology, high-risk MIPI score, and high Ki-67. No new safety signals were observed. This study confirms the role of BTK inhibitors in MCL and providers longer-term estimates of response. Evaluation of BTK inhibitors in earlier lines of therapy and in combination with other agents are ongoing.
Although the majority of patients with MCL will have favorable responses to initial therapy, those with high-risk features, particularly TP53 aberrations, have poor outcomes with standard approaches. Despite a growing number of treatment options in the relapsed setting, such as targeted therapies and chimeric antigen receptor (CAR) T-cell therapy, relapses remain common. Allogenic stem cell transplantation can be associated with prolonged response for patients with relapsed MCL, though it has the potential for significant treatment-associated toxicity.
Recently, prolonged follow-up of a retrospective cohort of patients with MCL, including a subset with TP53 aberrations, was reported (Lew et al). Thirty-six patients with MCL were included, including 13 with TP53-mutated disease. A subset of patients (61%) received an allogeneic transplant in first remission. The estimated overall survival rates after allogenic transplant were 56% (95% CI 36%-72%) at 10 years for the overall cohort and 59% (95% CI 21%-75%) at 4 years for patients with TP53-mutated disease at median follow-ups of 10.8 and 4.2 years, respectively. No relapses were observed in the TP53-mutated subset beyond 6 months after transplantation. These data suggest a potentially curative option for patients with high-risk MCL. Given the availability of CAR T-cell therapy, the optimal timing of allogenic stem cell transplant has become less clear for patients with TP53-mutant disease. Although this study was small and retrospective, these data are encouraging for patients with high-risk disease.
Additional References
1. Hoster E, Rosenwald A, Berger F, et al. Prognostic value of Ki-67 index, cytology, and growth pattern in mantle-cell lymphoma: Results from randomized trials of the European Mantle Cell Lymphoma Network. J Clin Oncol. 2016;34:1386-1394. doi: 10.1200/JCO.2015.63.8387
2. Eskelund CW, Dahl C, Hansen JW, et al. TP53 mutations identify younger mantle cell lymphoma patients who do not benefit from intensive chemoimmunotherapy. Blood. 2017;130:1903-1910. doi: 10.1182/blood-2017-04-77973
Mantle cell lymphoma (MCL) is a rare subtype of non-Hodgkin lymphoma that is characterized by t(11;14) and cyclin D1 overexpression. It is also known to be clinically heterogenous, with disease presentations ranging from indolent to aggressive. Baseline risk can be determined on the basis of a combination of clinical and pathologic features. A key prognostic tool, for example, is the Mantle Cell Lymphoma International Prognostic Index-Combined (MIPI-c), which integrates the standard MIPI clinical factors (age, performance status, lactate dehydrogenase, and leukocyte count) with estimates of proliferation (Ki-67).1 Other features, including the presence of TP53 alterations, have also been associated with poor outcomes, even with intensive therapy.2
Recently, a study aimed to further refine prognostication in MCL in order to identify high-risk patients that may be more likely to benefit from novel treatment strategies (Scheubeck et al). This retrospective study included 684 patients with MCL from the MCL-Younger and MCL-Elderly trials with evaluable data for Ki-67 or p53 expression (a surrogate for TP53 alterations). Patients were classified as having high-risk disease on the basis of a high-risk MIPI-c or p53 expression > 50% or as having low-risk disease on the basis of low, low-intermediate, or high-intermediate MIPI-c and p53 expression ≤ 50%. Patients with high-risk disease had significantly shorter median failure-free survival (1.1 vs 5.6 years; P < .0001) and overall survival (2.2 vs 13.2 years; P < .0001) compared with those with low-risk disease. The differences were confirmed in two validation cohorts from the Italian MCL0208 and Nordic-MCL4 trials. These data highlight the poor outcomes of conventional therapy in patients with high-risk MCL. Evaluation of novel approaches should be considered in these patients.
Bruton tyrosine kinase (BTK) inhibitors have been promising options for patients with MCL, including those with high-risk features. Acalabrutinib is a second-generation covalent BTK inhibitor that is approved by the US Food and Drug Administration for patients who have received at least one prior line of therapy. The final results of the single-arm, phase 2 ACE-LY-004 study recently demonstrated long-term safety and efficacy in patients with relapsed/refractory MCL (Le Gouill et al). The overall and complete response rates were 81.5% (95% CI 73.5%-87.9%) and 47.6% (95% CI 38.5%-56.7%), respectively. After a 38.1-month median follow-up, the median duration of response and progression-free survival were 28.6 months (95% CI 17.5-39.1) and 22.0 months (95% CI 16.6-33.3), respectively. Responses were also seen in patients with high-risk features, including blastoid morphology, high-risk MIPI score, and high Ki-67. No new safety signals were observed. This study confirms the role of BTK inhibitors in MCL and providers longer-term estimates of response. Evaluation of BTK inhibitors in earlier lines of therapy and in combination with other agents are ongoing.
Although the majority of patients with MCL will have favorable responses to initial therapy, those with high-risk features, particularly TP53 aberrations, have poor outcomes with standard approaches. Despite a growing number of treatment options in the relapsed setting, such as targeted therapies and chimeric antigen receptor (CAR) T-cell therapy, relapses remain common. Allogenic stem cell transplantation can be associated with prolonged response for patients with relapsed MCL, though it has the potential for significant treatment-associated toxicity.
Recently, prolonged follow-up of a retrospective cohort of patients with MCL, including a subset with TP53 aberrations, was reported (Lew et al). Thirty-six patients with MCL were included, including 13 with TP53-mutated disease. A subset of patients (61%) received an allogeneic transplant in first remission. The estimated overall survival rates after allogenic transplant were 56% (95% CI 36%-72%) at 10 years for the overall cohort and 59% (95% CI 21%-75%) at 4 years for patients with TP53-mutated disease at median follow-ups of 10.8 and 4.2 years, respectively. No relapses were observed in the TP53-mutated subset beyond 6 months after transplantation. These data suggest a potentially curative option for patients with high-risk MCL. Given the availability of CAR T-cell therapy, the optimal timing of allogenic stem cell transplant has become less clear for patients with TP53-mutant disease. Although this study was small and retrospective, these data are encouraging for patients with high-risk disease.
Additional References
1. Hoster E, Rosenwald A, Berger F, et al. Prognostic value of Ki-67 index, cytology, and growth pattern in mantle-cell lymphoma: Results from randomized trials of the European Mantle Cell Lymphoma Network. J Clin Oncol. 2016;34:1386-1394. doi: 10.1200/JCO.2015.63.8387
2. Eskelund CW, Dahl C, Hansen JW, et al. TP53 mutations identify younger mantle cell lymphoma patients who do not benefit from intensive chemoimmunotherapy. Blood. 2017;130:1903-1910. doi: 10.1182/blood-2017-04-77973
Mantle cell lymphoma (MCL) is a rare subtype of non-Hodgkin lymphoma that is characterized by t(11;14) and cyclin D1 overexpression. It is also known to be clinically heterogenous, with disease presentations ranging from indolent to aggressive. Baseline risk can be determined on the basis of a combination of clinical and pathologic features. A key prognostic tool, for example, is the Mantle Cell Lymphoma International Prognostic Index-Combined (MIPI-c), which integrates the standard MIPI clinical factors (age, performance status, lactate dehydrogenase, and leukocyte count) with estimates of proliferation (Ki-67).1 Other features, including the presence of TP53 alterations, have also been associated with poor outcomes, even with intensive therapy.2
Recently, a study aimed to further refine prognostication in MCL in order to identify high-risk patients that may be more likely to benefit from novel treatment strategies (Scheubeck et al). This retrospective study included 684 patients with MCL from the MCL-Younger and MCL-Elderly trials with evaluable data for Ki-67 or p53 expression (a surrogate for TP53 alterations). Patients were classified as having high-risk disease on the basis of a high-risk MIPI-c or p53 expression > 50% or as having low-risk disease on the basis of low, low-intermediate, or high-intermediate MIPI-c and p53 expression ≤ 50%. Patients with high-risk disease had significantly shorter median failure-free survival (1.1 vs 5.6 years; P < .0001) and overall survival (2.2 vs 13.2 years; P < .0001) compared with those with low-risk disease. The differences were confirmed in two validation cohorts from the Italian MCL0208 and Nordic-MCL4 trials. These data highlight the poor outcomes of conventional therapy in patients with high-risk MCL. Evaluation of novel approaches should be considered in these patients.
Bruton tyrosine kinase (BTK) inhibitors have been promising options for patients with MCL, including those with high-risk features. Acalabrutinib is a second-generation covalent BTK inhibitor that is approved by the US Food and Drug Administration for patients who have received at least one prior line of therapy. The final results of the single-arm, phase 2 ACE-LY-004 study recently demonstrated long-term safety and efficacy in patients with relapsed/refractory MCL (Le Gouill et al). The overall and complete response rates were 81.5% (95% CI 73.5%-87.9%) and 47.6% (95% CI 38.5%-56.7%), respectively. After a 38.1-month median follow-up, the median duration of response and progression-free survival were 28.6 months (95% CI 17.5-39.1) and 22.0 months (95% CI 16.6-33.3), respectively. Responses were also seen in patients with high-risk features, including blastoid morphology, high-risk MIPI score, and high Ki-67. No new safety signals were observed. This study confirms the role of BTK inhibitors in MCL and providers longer-term estimates of response. Evaluation of BTK inhibitors in earlier lines of therapy and in combination with other agents are ongoing.
Although the majority of patients with MCL will have favorable responses to initial therapy, those with high-risk features, particularly TP53 aberrations, have poor outcomes with standard approaches. Despite a growing number of treatment options in the relapsed setting, such as targeted therapies and chimeric antigen receptor (CAR) T-cell therapy, relapses remain common. Allogenic stem cell transplantation can be associated with prolonged response for patients with relapsed MCL, though it has the potential for significant treatment-associated toxicity.
Recently, prolonged follow-up of a retrospective cohort of patients with MCL, including a subset with TP53 aberrations, was reported (Lew et al). Thirty-six patients with MCL were included, including 13 with TP53-mutated disease. A subset of patients (61%) received an allogeneic transplant in first remission. The estimated overall survival rates after allogenic transplant were 56% (95% CI 36%-72%) at 10 years for the overall cohort and 59% (95% CI 21%-75%) at 4 years for patients with TP53-mutated disease at median follow-ups of 10.8 and 4.2 years, respectively. No relapses were observed in the TP53-mutated subset beyond 6 months after transplantation. These data suggest a potentially curative option for patients with high-risk MCL. Given the availability of CAR T-cell therapy, the optimal timing of allogenic stem cell transplant has become less clear for patients with TP53-mutant disease. Although this study was small and retrospective, these data are encouraging for patients with high-risk disease.
Additional References
1. Hoster E, Rosenwald A, Berger F, et al. Prognostic value of Ki-67 index, cytology, and growth pattern in mantle-cell lymphoma: Results from randomized trials of the European Mantle Cell Lymphoma Network. J Clin Oncol. 2016;34:1386-1394. doi: 10.1200/JCO.2015.63.8387
2. Eskelund CW, Dahl C, Hansen JW, et al. TP53 mutations identify younger mantle cell lymphoma patients who do not benefit from intensive chemoimmunotherapy. Blood. 2017;130:1903-1910. doi: 10.1182/blood-2017-04-77973
Commentary: Cardiovascular risk, anti-drug antibodies, and prednisolone in RA, September 2023
Anti-drug antibody (ADA) testing for biologics, particularly anti–tumor necrosis factor (TNF) agents, has been commercially available for several years, though its clinical use in rheumatoid arthritis (RA) is not known owing to lack of prospective data. Bitoun and colleagues analyzed data from the European ABI-RA registry to evaluate the association between ADA and the anti–TNF monoclonal antibodies (mAb) etanercept, tocilizumab, and rituximab, and clinical response (as measured by disease activity scores, inflammatory markers, and European Alliance of Associations for Rheumatology [EULAR] response rate). Higher rates of ADA positivity were seen in patients treated with rituximab (50%), anti-TNF mAb (38%), and tocilizumab (20%) compared with etanercept (6%). Patients who had a positive ADA test were less likely to have a EULAR response. In addition, patients treated with methotrexate were less likely to have persistent ADA. Though the study was not powered enough to detect differences between the drug classes, the evidence presented is compelling and suggests a role for measuring ADA in patients with RA who do not respond to treatment.
RA is well-known to be associated with cardiovascular disease, particularly atherosclerotic disease and heart failure, but its association with valvular heart disease and its progression has not been well-explored in the literature. Johnson and colleagues performed a cohort study of over 73,000 patients with RA in the Veterans Health Administration (VHA) system compared with 640,000 patients without RA to evaluate the incidence of aortic stenosis, need for intervention, and risk for death. Though the overall incidence rate was low (about 3%), patients with RA had a higher risk for aortic stenosis, with a hazard ratio of 1.48 compared with those without RA, as well as a higher risk for aortic valve replacement and aortic stenosis–related death. The risk for aortic stenosis was associated with hypertension, stroke, and other cardiovascular disease, as well as a body mass index > 30 kg/m2, although not with a history of smoking or diabetes. Because the study was performed using data from the VHA — that is, from predominantly male patients — this finding may not be generalizable. In addition, the diagnosis of aortic stenosis is generally reliant on echocardiography and may be detected while searching for other conditions not evaluated here (such as pericarditis). As such, these findings would not support routine screening in patients with RA without other reasons for suspicion of valvular heart disease.
In particular, the increase in cardiovascular risk associated with glucocorticoid therapy in patients with RA has received increased scrutiny, along with other side effects of systemic glucocorticoids. In a recent retrospective study, So and colleagues examined the clinical data of over 12,000 patients with RA treated in public hospitals in Hong Kong with a mean of 9 years of follow-up. Consistent with prior studies, systemic glucocorticoid use (prednisolone equivalent > 5 mg daily) was associated with an increased risk for adverse cardiovascular events, whereas lower doses did not increase cardiovascular risk. Because the data on some disease activity measures and traditional cardiovascular risk factors (such as smoking or obesity) were not available in the database, the study supports, but does not expand on, prior evidence regarding cardiovascular risk.
Almayali and colleagues also looked at glucocorticoid therapy in RA in a follow-up study to the previously published pragmatic randomized double-blinded placebo-controlled GLORIA study, which evaluated the effects of 5 mg/d prednisolone added to standard care for 2 years in patients with active RA who were age 65 years or older. In the current study, 191 patients out of the initial 451 were followed for 3 months and prednisolone tapered off. Patients who tapered off prednisolone had, as expected, an increased risk for flare but no evidence of adrenal insufficiency. Although, again, this is not likely to change practice, it does suggest that glucocorticoid tapering is a reasonable goal in RA therapeutic trials.
Anti-drug antibody (ADA) testing for biologics, particularly anti–tumor necrosis factor (TNF) agents, has been commercially available for several years, though its clinical use in rheumatoid arthritis (RA) is not known owing to lack of prospective data. Bitoun and colleagues analyzed data from the European ABI-RA registry to evaluate the association between ADA and the anti–TNF monoclonal antibodies (mAb) etanercept, tocilizumab, and rituximab, and clinical response (as measured by disease activity scores, inflammatory markers, and European Alliance of Associations for Rheumatology [EULAR] response rate). Higher rates of ADA positivity were seen in patients treated with rituximab (50%), anti-TNF mAb (38%), and tocilizumab (20%) compared with etanercept (6%). Patients who had a positive ADA test were less likely to have a EULAR response. In addition, patients treated with methotrexate were less likely to have persistent ADA. Though the study was not powered enough to detect differences between the drug classes, the evidence presented is compelling and suggests a role for measuring ADA in patients with RA who do not respond to treatment.
RA is well-known to be associated with cardiovascular disease, particularly atherosclerotic disease and heart failure, but its association with valvular heart disease and its progression has not been well-explored in the literature. Johnson and colleagues performed a cohort study of over 73,000 patients with RA in the Veterans Health Administration (VHA) system compared with 640,000 patients without RA to evaluate the incidence of aortic stenosis, need for intervention, and risk for death. Though the overall incidence rate was low (about 3%), patients with RA had a higher risk for aortic stenosis, with a hazard ratio of 1.48 compared with those without RA, as well as a higher risk for aortic valve replacement and aortic stenosis–related death. The risk for aortic stenosis was associated with hypertension, stroke, and other cardiovascular disease, as well as a body mass index > 30 kg/m2, although not with a history of smoking or diabetes. Because the study was performed using data from the VHA — that is, from predominantly male patients — this finding may not be generalizable. In addition, the diagnosis of aortic stenosis is generally reliant on echocardiography and may be detected while searching for other conditions not evaluated here (such as pericarditis). As such, these findings would not support routine screening in patients with RA without other reasons for suspicion of valvular heart disease.
In particular, the increase in cardiovascular risk associated with glucocorticoid therapy in patients with RA has received increased scrutiny, along with other side effects of systemic glucocorticoids. In a recent retrospective study, So and colleagues examined the clinical data of over 12,000 patients with RA treated in public hospitals in Hong Kong with a mean of 9 years of follow-up. Consistent with prior studies, systemic glucocorticoid use (prednisolone equivalent > 5 mg daily) was associated with an increased risk for adverse cardiovascular events, whereas lower doses did not increase cardiovascular risk. Because the data on some disease activity measures and traditional cardiovascular risk factors (such as smoking or obesity) were not available in the database, the study supports, but does not expand on, prior evidence regarding cardiovascular risk.
Almayali and colleagues also looked at glucocorticoid therapy in RA in a follow-up study to the previously published pragmatic randomized double-blinded placebo-controlled GLORIA study, which evaluated the effects of 5 mg/d prednisolone added to standard care for 2 years in patients with active RA who were age 65 years or older. In the current study, 191 patients out of the initial 451 were followed for 3 months and prednisolone tapered off. Patients who tapered off prednisolone had, as expected, an increased risk for flare but no evidence of adrenal insufficiency. Although, again, this is not likely to change practice, it does suggest that glucocorticoid tapering is a reasonable goal in RA therapeutic trials.
Anti-drug antibody (ADA) testing for biologics, particularly anti–tumor necrosis factor (TNF) agents, has been commercially available for several years, though its clinical use in rheumatoid arthritis (RA) is not known owing to lack of prospective data. Bitoun and colleagues analyzed data from the European ABI-RA registry to evaluate the association between ADA and the anti–TNF monoclonal antibodies (mAb) etanercept, tocilizumab, and rituximab, and clinical response (as measured by disease activity scores, inflammatory markers, and European Alliance of Associations for Rheumatology [EULAR] response rate). Higher rates of ADA positivity were seen in patients treated with rituximab (50%), anti-TNF mAb (38%), and tocilizumab (20%) compared with etanercept (6%). Patients who had a positive ADA test were less likely to have a EULAR response. In addition, patients treated with methotrexate were less likely to have persistent ADA. Though the study was not powered enough to detect differences between the drug classes, the evidence presented is compelling and suggests a role for measuring ADA in patients with RA who do not respond to treatment.
RA is well-known to be associated with cardiovascular disease, particularly atherosclerotic disease and heart failure, but its association with valvular heart disease and its progression has not been well-explored in the literature. Johnson and colleagues performed a cohort study of over 73,000 patients with RA in the Veterans Health Administration (VHA) system compared with 640,000 patients without RA to evaluate the incidence of aortic stenosis, need for intervention, and risk for death. Though the overall incidence rate was low (about 3%), patients with RA had a higher risk for aortic stenosis, with a hazard ratio of 1.48 compared with those without RA, as well as a higher risk for aortic valve replacement and aortic stenosis–related death. The risk for aortic stenosis was associated with hypertension, stroke, and other cardiovascular disease, as well as a body mass index > 30 kg/m2, although not with a history of smoking or diabetes. Because the study was performed using data from the VHA — that is, from predominantly male patients — this finding may not be generalizable. In addition, the diagnosis of aortic stenosis is generally reliant on echocardiography and may be detected while searching for other conditions not evaluated here (such as pericarditis). As such, these findings would not support routine screening in patients with RA without other reasons for suspicion of valvular heart disease.
In particular, the increase in cardiovascular risk associated with glucocorticoid therapy in patients with RA has received increased scrutiny, along with other side effects of systemic glucocorticoids. In a recent retrospective study, So and colleagues examined the clinical data of over 12,000 patients with RA treated in public hospitals in Hong Kong with a mean of 9 years of follow-up. Consistent with prior studies, systemic glucocorticoid use (prednisolone equivalent > 5 mg daily) was associated with an increased risk for adverse cardiovascular events, whereas lower doses did not increase cardiovascular risk. Because the data on some disease activity measures and traditional cardiovascular risk factors (such as smoking or obesity) were not available in the database, the study supports, but does not expand on, prior evidence regarding cardiovascular risk.
Almayali and colleagues also looked at glucocorticoid therapy in RA in a follow-up study to the previously published pragmatic randomized double-blinded placebo-controlled GLORIA study, which evaluated the effects of 5 mg/d prednisolone added to standard care for 2 years in patients with active RA who were age 65 years or older. In the current study, 191 patients out of the initial 451 were followed for 3 months and prednisolone tapered off. Patients who tapered off prednisolone had, as expected, an increased risk for flare but no evidence of adrenal insufficiency. Although, again, this is not likely to change practice, it does suggest that glucocorticoid tapering is a reasonable goal in RA therapeutic trials.
Commentary: Alcohol, PPI use, BMI, and lymph node dissection in BC, September 2023
The use of proton pump inhibitors (PPI) can affect the bioavailability and effectiveness of concomitant medications, including cancer therapies. A retrospective study by Lee and colleagues aimed to identify the clinical outcomes of patients with hormone receptor–positive (HR+) and human epidermal growth factor receptor 2–negative advanced or metastatic BC who were concomitantly using PPI and palbociclib. The study included 1310 patients, of which 344 received concomitant PPI plus palbociclib and 966 patients received palbociclib alone. Results showed that patients who received concomitant PPI plus palbociclib had significantly shorter progression-free survival (hazard ratio 1.76; 95% CI 1.46-2.13) and overall survival (hazard ratio 2.72; 95% CI 2.07-3.53) rates compared with those who received palbociclib alone. These results suggest that the concomitant use of PPI with palbociclib may alter the therapeutic efficacy of the drug. More research studies are needed to confirm these findings.
Pfeiler and colleagues examined the association of BMI with side effects, treatment discontinuation, and efficacy of palbociclib. This study looked at 5698 patients with early-stage HR+ BC who received palbociclib plus endocrine therapy as part of a preplanned analysis of the PALLAS trial. Results showed that in women who received adjuvant palbociclib, higher BMI was associated with a significantly lower rate of neutropenia (odds ratio for a 1-unit change in BMI 0.93; 95% CI 0.92-0.95) and a lower rate of treatment discontinuation (adjusted hazard ratio for a 10-unit change in BMI 0.75; 95% CI 0.67-0.83) compared with normal-weight patients. No effect of BMI on palbociclib efficacy was observed at 31 months of follow-up. Further studies are needed to validate these findings in different cohorts.
In cases of early-stage breast cancer (clinical T1, T2) where patients undergo upfront breast-conserving therapy and sentinel lymph node biopsy (SLNB), completion of axillary lymph node dissection (CLND) is often omitted if only one or two positive sentinel lymph nodes are detected. A study by Zaveri and colleagues looked at outcomes among 548 patients with cT1-2 N0 BC who were treated with upfront mastectomy and had one or two positive lymph nodes on SLNB. The 5-year cumulative incidence rate of overall locoregional recurrence was comparable between patients who underwent vs those who did not undergo CLND (1.8% vs 1.3%; P = .93); receipt of post-mastectomy radiation therapy did not affect the locoregional recurrence rate in both categories of patients who underwent SLNB alone and SLNB with CLND (P = .1638). These results suggest that CLND may not necessarily improve outcomes in this patient population. Larger prospective studies are needed to confirm these findings.
The use of proton pump inhibitors (PPI) can affect the bioavailability and effectiveness of concomitant medications, including cancer therapies. A retrospective study by Lee and colleagues aimed to identify the clinical outcomes of patients with hormone receptor–positive (HR+) and human epidermal growth factor receptor 2–negative advanced or metastatic BC who were concomitantly using PPI and palbociclib. The study included 1310 patients, of which 344 received concomitant PPI plus palbociclib and 966 patients received palbociclib alone. Results showed that patients who received concomitant PPI plus palbociclib had significantly shorter progression-free survival (hazard ratio 1.76; 95% CI 1.46-2.13) and overall survival (hazard ratio 2.72; 95% CI 2.07-3.53) rates compared with those who received palbociclib alone. These results suggest that the concomitant use of PPI with palbociclib may alter the therapeutic efficacy of the drug. More research studies are needed to confirm these findings.
Pfeiler and colleagues examined the association of BMI with side effects, treatment discontinuation, and efficacy of palbociclib. This study looked at 5698 patients with early-stage HR+ BC who received palbociclib plus endocrine therapy as part of a preplanned analysis of the PALLAS trial. Results showed that in women who received adjuvant palbociclib, higher BMI was associated with a significantly lower rate of neutropenia (odds ratio for a 1-unit change in BMI 0.93; 95% CI 0.92-0.95) and a lower rate of treatment discontinuation (adjusted hazard ratio for a 10-unit change in BMI 0.75; 95% CI 0.67-0.83) compared with normal-weight patients. No effect of BMI on palbociclib efficacy was observed at 31 months of follow-up. Further studies are needed to validate these findings in different cohorts.
In cases of early-stage breast cancer (clinical T1, T2) where patients undergo upfront breast-conserving therapy and sentinel lymph node biopsy (SLNB), completion of axillary lymph node dissection (CLND) is often omitted if only one or two positive sentinel lymph nodes are detected. A study by Zaveri and colleagues looked at outcomes among 548 patients with cT1-2 N0 BC who were treated with upfront mastectomy and had one or two positive lymph nodes on SLNB. The 5-year cumulative incidence rate of overall locoregional recurrence was comparable between patients who underwent vs those who did not undergo CLND (1.8% vs 1.3%; P = .93); receipt of post-mastectomy radiation therapy did not affect the locoregional recurrence rate in both categories of patients who underwent SLNB alone and SLNB with CLND (P = .1638). These results suggest that CLND may not necessarily improve outcomes in this patient population. Larger prospective studies are needed to confirm these findings.
The use of proton pump inhibitors (PPI) can affect the bioavailability and effectiveness of concomitant medications, including cancer therapies. A retrospective study by Lee and colleagues aimed to identify the clinical outcomes of patients with hormone receptor–positive (HR+) and human epidermal growth factor receptor 2–negative advanced or metastatic BC who were concomitantly using PPI and palbociclib. The study included 1310 patients, of which 344 received concomitant PPI plus palbociclib and 966 patients received palbociclib alone. Results showed that patients who received concomitant PPI plus palbociclib had significantly shorter progression-free survival (hazard ratio 1.76; 95% CI 1.46-2.13) and overall survival (hazard ratio 2.72; 95% CI 2.07-3.53) rates compared with those who received palbociclib alone. These results suggest that the concomitant use of PPI with palbociclib may alter the therapeutic efficacy of the drug. More research studies are needed to confirm these findings.
Pfeiler and colleagues examined the association of BMI with side effects, treatment discontinuation, and efficacy of palbociclib. This study looked at 5698 patients with early-stage HR+ BC who received palbociclib plus endocrine therapy as part of a preplanned analysis of the PALLAS trial. Results showed that in women who received adjuvant palbociclib, higher BMI was associated with a significantly lower rate of neutropenia (odds ratio for a 1-unit change in BMI 0.93; 95% CI 0.92-0.95) and a lower rate of treatment discontinuation (adjusted hazard ratio for a 10-unit change in BMI 0.75; 95% CI 0.67-0.83) compared with normal-weight patients. No effect of BMI on palbociclib efficacy was observed at 31 months of follow-up. Further studies are needed to validate these findings in different cohorts.
In cases of early-stage breast cancer (clinical T1, T2) where patients undergo upfront breast-conserving therapy and sentinel lymph node biopsy (SLNB), completion of axillary lymph node dissection (CLND) is often omitted if only one or two positive sentinel lymph nodes are detected. A study by Zaveri and colleagues looked at outcomes among 548 patients with cT1-2 N0 BC who were treated with upfront mastectomy and had one or two positive lymph nodes on SLNB. The 5-year cumulative incidence rate of overall locoregional recurrence was comparable between patients who underwent vs those who did not undergo CLND (1.8% vs 1.3%; P = .93); receipt of post-mastectomy radiation therapy did not affect the locoregional recurrence rate in both categories of patients who underwent SLNB alone and SLNB with CLND (P = .1638). These results suggest that CLND may not necessarily improve outcomes in this patient population. Larger prospective studies are needed to confirm these findings.
Raised Linear Plaques on the Back
The Diagnosis: Flagellate Dermatitis
Upon further questioning by dermatology, the patient noted recent ingestion of shiitake mushrooms, which were not a part of his typical diet. Based on the appearance of the rash in the context of ingesting shiitake mushrooms, our patient was diagnosed with flagellate dermatitis. At 6-week followup, the patient’s rash had resolved spontaneously without further intervention.
Flagellate dermatitis usually appears on the torso as linear whiplike streaks.1 The eruption often is pruritic and may be preceded by severe pruritus. Flagellate dermatitis also is a well-documented complication of bleomycin sulfate therapy with an incidence rate of 8% to 66%.2
Other chemotherapeutic causes include peplomycin, bendamustine, docetaxel, cisplatin, and trastuzumab.3 Flagellate dermatitis also is seen in some patients with dermatomyositis.4 A thorough patient history, including medications and dietary habits, is necessary to differentiate flagellate dermatitis from dermatomyositis.
Flagellate dermatitis, also known as shiitake dermatitis, is observed as erythematous flagellate eruptions involving the trunk or extremities that present within 2 hours to 5 days of handling or consuming undercooked or raw shiitake mushrooms (Lentinula edodes),5,6 as was observed in our patient. Lentinan is the polysaccharide component of the shiitake species and is destabilized by heat.6 Ingestion of polysaccharide is associated with dermatitis, particularly in Japan, China, and Korea; however, the consumption of shiitake mushrooms has increased worldwide, and cases increasingly are reported outside of these typical regions. The rash typically resolves spontaneously; therefore, treatment is supportive. However, more severe symptomatic cases may require courses of topical corticosteroids and antihistamines.6
In our case, the differential diagnosis consisted of acute urticaria, cutaneous dermatomyositis, dermatographism, and maculopapular cutaneous mastocytosis. Acute urticaria displays well-circumscribed edematous papules or plaques, and individual lesions last less than 24 hours. Cutaneous dermatomyositis includes additional systemic manifestations such as fatigue, malaise, and myalgia, as well as involvement of the gastrointestinal, respiratory, or cardiac organs. Dermatographism is evoked by stroking or rubbing of the skin, which results in asymptomatic lesions that persist for 15 to 30 minutes. Cases of maculopapular cutaneous mastocytosis more often are seen in children, and the histamine release most often causes gastrointestinal tract symptoms such as nausea, vomiting, and diarrhea, as well as flushing, blushing, pruritus, respiratory difficulty, and malaise.
- Biswas A, Chaudhari PB, Sharma P, et al. Bleomycin induced flagellate erythema: revisiting a unique complication. J Cancer Res Ther. 2013;9:500-503.
- Yagoda A, Mukherji B, Young C, et al. Bleomycin, an anti-tumor antibiotic: clinical experience in 274 patients. Ann Intern Med. 1972;77:861-870.
- Cohen PR. Trastuzumab-associated flagellate erythema: report in a woman with metastatic breast cancer and review of antineoplastic therapy-induced flagellate dermatoses. Dermatol Ther (Heidelb). 2015;5:253-264. doi:10.1007/s13555-015-0085-2
- Grynszpan R, Niemeyer-Corbellini JP, Lopes MS, et al. Bleomycininduced flagellate dermatitis. BMJ Case Rep. 2013;2013:bcr2013009764. doi:10.1136/bcr-2013-009764
- Stephany MP, Chung S, Handler MZ, et al. Shiitake mushroom dermatitis: a review. Am J Clin Dermatol. 2016;17:485-489.
- Boels D, Landreau A, Bruneau C, et al. Shiitake dermatitis recorded by French Poison Control Centers—new case series with clinical observations. Clin Toxicol (Phila). 2014;52:625-628.
The Diagnosis: Flagellate Dermatitis
Upon further questioning by dermatology, the patient noted recent ingestion of shiitake mushrooms, which were not a part of his typical diet. Based on the appearance of the rash in the context of ingesting shiitake mushrooms, our patient was diagnosed with flagellate dermatitis. At 6-week followup, the patient’s rash had resolved spontaneously without further intervention.
Flagellate dermatitis usually appears on the torso as linear whiplike streaks.1 The eruption often is pruritic and may be preceded by severe pruritus. Flagellate dermatitis also is a well-documented complication of bleomycin sulfate therapy with an incidence rate of 8% to 66%.2
Other chemotherapeutic causes include peplomycin, bendamustine, docetaxel, cisplatin, and trastuzumab.3 Flagellate dermatitis also is seen in some patients with dermatomyositis.4 A thorough patient history, including medications and dietary habits, is necessary to differentiate flagellate dermatitis from dermatomyositis.
Flagellate dermatitis, also known as shiitake dermatitis, is observed as erythematous flagellate eruptions involving the trunk or extremities that present within 2 hours to 5 days of handling or consuming undercooked or raw shiitake mushrooms (Lentinula edodes),5,6 as was observed in our patient. Lentinan is the polysaccharide component of the shiitake species and is destabilized by heat.6 Ingestion of polysaccharide is associated with dermatitis, particularly in Japan, China, and Korea; however, the consumption of shiitake mushrooms has increased worldwide, and cases increasingly are reported outside of these typical regions. The rash typically resolves spontaneously; therefore, treatment is supportive. However, more severe symptomatic cases may require courses of topical corticosteroids and antihistamines.6
In our case, the differential diagnosis consisted of acute urticaria, cutaneous dermatomyositis, dermatographism, and maculopapular cutaneous mastocytosis. Acute urticaria displays well-circumscribed edematous papules or plaques, and individual lesions last less than 24 hours. Cutaneous dermatomyositis includes additional systemic manifestations such as fatigue, malaise, and myalgia, as well as involvement of the gastrointestinal, respiratory, or cardiac organs. Dermatographism is evoked by stroking or rubbing of the skin, which results in asymptomatic lesions that persist for 15 to 30 minutes. Cases of maculopapular cutaneous mastocytosis more often are seen in children, and the histamine release most often causes gastrointestinal tract symptoms such as nausea, vomiting, and diarrhea, as well as flushing, blushing, pruritus, respiratory difficulty, and malaise.
The Diagnosis: Flagellate Dermatitis
Upon further questioning by dermatology, the patient noted recent ingestion of shiitake mushrooms, which were not a part of his typical diet. Based on the appearance of the rash in the context of ingesting shiitake mushrooms, our patient was diagnosed with flagellate dermatitis. At 6-week followup, the patient’s rash had resolved spontaneously without further intervention.
Flagellate dermatitis usually appears on the torso as linear whiplike streaks.1 The eruption often is pruritic and may be preceded by severe pruritus. Flagellate dermatitis also is a well-documented complication of bleomycin sulfate therapy with an incidence rate of 8% to 66%.2
Other chemotherapeutic causes include peplomycin, bendamustine, docetaxel, cisplatin, and trastuzumab.3 Flagellate dermatitis also is seen in some patients with dermatomyositis.4 A thorough patient history, including medications and dietary habits, is necessary to differentiate flagellate dermatitis from dermatomyositis.
Flagellate dermatitis, also known as shiitake dermatitis, is observed as erythematous flagellate eruptions involving the trunk or extremities that present within 2 hours to 5 days of handling or consuming undercooked or raw shiitake mushrooms (Lentinula edodes),5,6 as was observed in our patient. Lentinan is the polysaccharide component of the shiitake species and is destabilized by heat.6 Ingestion of polysaccharide is associated with dermatitis, particularly in Japan, China, and Korea; however, the consumption of shiitake mushrooms has increased worldwide, and cases increasingly are reported outside of these typical regions. The rash typically resolves spontaneously; therefore, treatment is supportive. However, more severe symptomatic cases may require courses of topical corticosteroids and antihistamines.6
In our case, the differential diagnosis consisted of acute urticaria, cutaneous dermatomyositis, dermatographism, and maculopapular cutaneous mastocytosis. Acute urticaria displays well-circumscribed edematous papules or plaques, and individual lesions last less than 24 hours. Cutaneous dermatomyositis includes additional systemic manifestations such as fatigue, malaise, and myalgia, as well as involvement of the gastrointestinal, respiratory, or cardiac organs. Dermatographism is evoked by stroking or rubbing of the skin, which results in asymptomatic lesions that persist for 15 to 30 minutes. Cases of maculopapular cutaneous mastocytosis more often are seen in children, and the histamine release most often causes gastrointestinal tract symptoms such as nausea, vomiting, and diarrhea, as well as flushing, blushing, pruritus, respiratory difficulty, and malaise.
- Biswas A, Chaudhari PB, Sharma P, et al. Bleomycin induced flagellate erythema: revisiting a unique complication. J Cancer Res Ther. 2013;9:500-503.
- Yagoda A, Mukherji B, Young C, et al. Bleomycin, an anti-tumor antibiotic: clinical experience in 274 patients. Ann Intern Med. 1972;77:861-870.
- Cohen PR. Trastuzumab-associated flagellate erythema: report in a woman with metastatic breast cancer and review of antineoplastic therapy-induced flagellate dermatoses. Dermatol Ther (Heidelb). 2015;5:253-264. doi:10.1007/s13555-015-0085-2
- Grynszpan R, Niemeyer-Corbellini JP, Lopes MS, et al. Bleomycininduced flagellate dermatitis. BMJ Case Rep. 2013;2013:bcr2013009764. doi:10.1136/bcr-2013-009764
- Stephany MP, Chung S, Handler MZ, et al. Shiitake mushroom dermatitis: a review. Am J Clin Dermatol. 2016;17:485-489.
- Boels D, Landreau A, Bruneau C, et al. Shiitake dermatitis recorded by French Poison Control Centers—new case series with clinical observations. Clin Toxicol (Phila). 2014;52:625-628.
- Biswas A, Chaudhari PB, Sharma P, et al. Bleomycin induced flagellate erythema: revisiting a unique complication. J Cancer Res Ther. 2013;9:500-503.
- Yagoda A, Mukherji B, Young C, et al. Bleomycin, an anti-tumor antibiotic: clinical experience in 274 patients. Ann Intern Med. 1972;77:861-870.
- Cohen PR. Trastuzumab-associated flagellate erythema: report in a woman with metastatic breast cancer and review of antineoplastic therapy-induced flagellate dermatoses. Dermatol Ther (Heidelb). 2015;5:253-264. doi:10.1007/s13555-015-0085-2
- Grynszpan R, Niemeyer-Corbellini JP, Lopes MS, et al. Bleomycininduced flagellate dermatitis. BMJ Case Rep. 2013;2013:bcr2013009764. doi:10.1136/bcr-2013-009764
- Stephany MP, Chung S, Handler MZ, et al. Shiitake mushroom dermatitis: a review. Am J Clin Dermatol. 2016;17:485-489.
- Boels D, Landreau A, Bruneau C, et al. Shiitake dermatitis recorded by French Poison Control Centers—new case series with clinical observations. Clin Toxicol (Phila). 2014;52:625-628.
A 77-year-old man with a history of hypertension, hyperlipidemia, and nonmelanoma skin cancer presented to the dermatology clinic for evaluation of a new rash of 2 days’ duration. He trialed a previously prescribed triamcinolone cream 0.1% without improvement. The patient denied any recent travel, as well as fever, nausea, vomiting, or changes in bowel habits. Physical examination revealed diffuse, erythematous, raised, linear plaques on the mid to lower back.
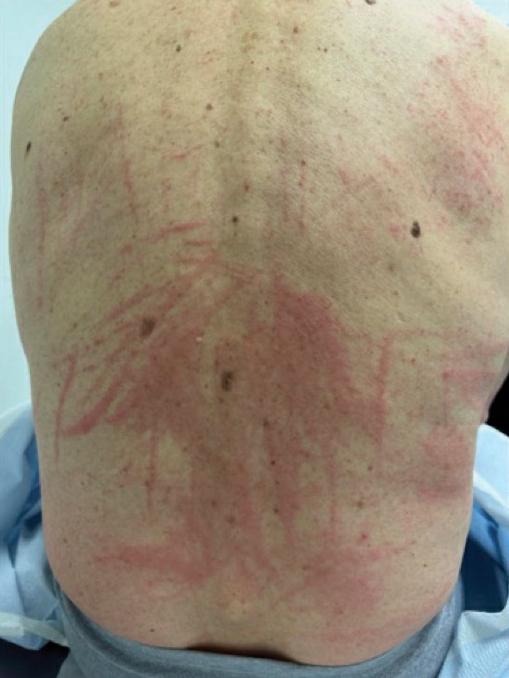
Inner lip erosions
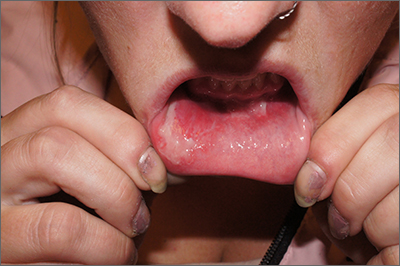
The patient was having a flare of pemphigus vulgaris (PV), a rare and sometimes life-threatening acquired autoimmune blistering disease that affects the skin and/or mucosa. Ashkenazi Jewish patients and patients from Mediterranean and Middle Eastern countries are more likely to be affected.
In PV, acquired autoantibodies target the desmosomes that connect epithelial cells together, weakening the intercellular adhesion. It can affect skin, mucosa, or both. Patients present with fragile bullae or ulcers. The connections between the cells are often so damaged that rubbing on the skin creates a new blister called “Nikolsky sign.” In the mouth, bullae erode rapidly. Look for disease affecting the ocular conjunctiva or sclera, as well. PV can also occasionally affect the nasopharynx and esophagus, usually manifesting as hemoptysis, dysphagia, and nosebleeds with ulcer seen on endoscopy or otolaryngoscopy.
Although PV is often severe (and can warrant hospitalization when significant body surface area is involved), some patients may have few active lesions and can be managed safely as outpatients.
The diagnosis requires 2 biopsies and serum for indirect immunofluorescence. One biopsy (either by punch or shave to the upper dermis) is taken from the edge of a bulla or ulcer. Another biopsy (by punch or shave) is taken from nearby normal-looking skin or mucosa for testing the direct immunofluorescence pattern. In the mucosa, a punch biopsy may be left open or closed with absorbable sutures. A serum sample is taken for indirect immunofluorescence to differentiate pemphigus vulgaris from other forms of pemphigus.1
PV is treated by suppressing the immune system. Focal disease may be treated with super-potent topical steroids, including clobetasol 0.05% ointment. Even in the mouth, topical clobetasol 0.05% may be used off-label twice daily until control is achieved. When topical treatment is used in the mouth, advise patients to apply the clobetasol ointment to a piece of gauze and place the gauze (ointment side down) over affected areas for 20 to 30 minutes twice daily.
Patients with widespread or severe disease should be hospitalized. In severe cases, supportive wound care is provided, and treatment is aimed at immunosuppression. Systemic options include high-dose prednisone 0.5 to 1 mg/kg daily until clear, a steroid-sparing immunosuppressant such as mycophenolate mofetil up to 1000 mg bid, or rituximab in 1 of several regimens.
Three years prior to this patient’s visit, she had been successfully treated for PV with a course of rituximab. To treat the current flare, she was started on prednisone 60 mg/d. In addition, the plan was for her to complete 2 infusions of 1000 mg rituximab 2 weeks apart.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, Maine.
1. Didona, D, Schmidt, MF, Maglie, R, et al. Pemphigus and pemphigoids: clinical presentation, diagnosis and therapy. J Dtsch Dermatol Ges. 2023;1-20. doi: 10.1111/ddg.15174

The patient was having a flare of pemphigus vulgaris (PV), a rare and sometimes life-threatening acquired autoimmune blistering disease that affects the skin and/or mucosa. Ashkenazi Jewish patients and patients from Mediterranean and Middle Eastern countries are more likely to be affected.
In PV, acquired autoantibodies target the desmosomes that connect epithelial cells together, weakening the intercellular adhesion. It can affect skin, mucosa, or both. Patients present with fragile bullae or ulcers. The connections between the cells are often so damaged that rubbing on the skin creates a new blister called “Nikolsky sign.” In the mouth, bullae erode rapidly. Look for disease affecting the ocular conjunctiva or sclera, as well. PV can also occasionally affect the nasopharynx and esophagus, usually manifesting as hemoptysis, dysphagia, and nosebleeds with ulcer seen on endoscopy or otolaryngoscopy.
Although PV is often severe (and can warrant hospitalization when significant body surface area is involved), some patients may have few active lesions and can be managed safely as outpatients.
The diagnosis requires 2 biopsies and serum for indirect immunofluorescence. One biopsy (either by punch or shave to the upper dermis) is taken from the edge of a bulla or ulcer. Another biopsy (by punch or shave) is taken from nearby normal-looking skin or mucosa for testing the direct immunofluorescence pattern. In the mucosa, a punch biopsy may be left open or closed with absorbable sutures. A serum sample is taken for indirect immunofluorescence to differentiate pemphigus vulgaris from other forms of pemphigus.1
PV is treated by suppressing the immune system. Focal disease may be treated with super-potent topical steroids, including clobetasol 0.05% ointment. Even in the mouth, topical clobetasol 0.05% may be used off-label twice daily until control is achieved. When topical treatment is used in the mouth, advise patients to apply the clobetasol ointment to a piece of gauze and place the gauze (ointment side down) over affected areas for 20 to 30 minutes twice daily.
Patients with widespread or severe disease should be hospitalized. In severe cases, supportive wound care is provided, and treatment is aimed at immunosuppression. Systemic options include high-dose prednisone 0.5 to 1 mg/kg daily until clear, a steroid-sparing immunosuppressant such as mycophenolate mofetil up to 1000 mg bid, or rituximab in 1 of several regimens.
Three years prior to this patient’s visit, she had been successfully treated for PV with a course of rituximab. To treat the current flare, she was started on prednisone 60 mg/d. In addition, the plan was for her to complete 2 infusions of 1000 mg rituximab 2 weeks apart.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, Maine.

The patient was having a flare of pemphigus vulgaris (PV), a rare and sometimes life-threatening acquired autoimmune blistering disease that affects the skin and/or mucosa. Ashkenazi Jewish patients and patients from Mediterranean and Middle Eastern countries are more likely to be affected.
In PV, acquired autoantibodies target the desmosomes that connect epithelial cells together, weakening the intercellular adhesion. It can affect skin, mucosa, or both. Patients present with fragile bullae or ulcers. The connections between the cells are often so damaged that rubbing on the skin creates a new blister called “Nikolsky sign.” In the mouth, bullae erode rapidly. Look for disease affecting the ocular conjunctiva or sclera, as well. PV can also occasionally affect the nasopharynx and esophagus, usually manifesting as hemoptysis, dysphagia, and nosebleeds with ulcer seen on endoscopy or otolaryngoscopy.
Although PV is often severe (and can warrant hospitalization when significant body surface area is involved), some patients may have few active lesions and can be managed safely as outpatients.
The diagnosis requires 2 biopsies and serum for indirect immunofluorescence. One biopsy (either by punch or shave to the upper dermis) is taken from the edge of a bulla or ulcer. Another biopsy (by punch or shave) is taken from nearby normal-looking skin or mucosa for testing the direct immunofluorescence pattern. In the mucosa, a punch biopsy may be left open or closed with absorbable sutures. A serum sample is taken for indirect immunofluorescence to differentiate pemphigus vulgaris from other forms of pemphigus.1
PV is treated by suppressing the immune system. Focal disease may be treated with super-potent topical steroids, including clobetasol 0.05% ointment. Even in the mouth, topical clobetasol 0.05% may be used off-label twice daily until control is achieved. When topical treatment is used in the mouth, advise patients to apply the clobetasol ointment to a piece of gauze and place the gauze (ointment side down) over affected areas for 20 to 30 minutes twice daily.
Patients with widespread or severe disease should be hospitalized. In severe cases, supportive wound care is provided, and treatment is aimed at immunosuppression. Systemic options include high-dose prednisone 0.5 to 1 mg/kg daily until clear, a steroid-sparing immunosuppressant such as mycophenolate mofetil up to 1000 mg bid, or rituximab in 1 of several regimens.
Three years prior to this patient’s visit, she had been successfully treated for PV with a course of rituximab. To treat the current flare, she was started on prednisone 60 mg/d. In addition, the plan was for her to complete 2 infusions of 1000 mg rituximab 2 weeks apart.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, Maine.
1. Didona, D, Schmidt, MF, Maglie, R, et al. Pemphigus and pemphigoids: clinical presentation, diagnosis and therapy. J Dtsch Dermatol Ges. 2023;1-20. doi: 10.1111/ddg.15174
1. Didona, D, Schmidt, MF, Maglie, R, et al. Pemphigus and pemphigoids: clinical presentation, diagnosis and therapy. J Dtsch Dermatol Ges. 2023;1-20. doi: 10.1111/ddg.15174
Commentary: Genetics, Juvenile PsA, and Weight Loss in PsA, September 2023
Although the usual age at onset of PsA is in the fourth or fifth decade of life, children may develop juvenile-onset PsA (JPsA). Less attention has been paid to this form of juvenile idiopathic arthritis (JIA), and the impact of JPsA vis-à-vis other forms of JIA is not well known. In addition, only about half of the patients with JPsA have cutaneous psoriasis. The impact of psoriasis on children with JPsA is not known. In order to evaluate differences in disease outcomes in patients with JPsA, Low and colleagues evaluated 1653 children and young people with JIA who were recruited to the Childhood Arthritis Prospective Study, of whom 111 had JPsA at diagnosis. They demonstrated that there were no significant differences in patient-reported outcomes between children with JPsA and other JIA categories. However, children with JPsA and psoriasis at JPsA diagnosis had more depressive symptoms compared with those without psoriasis. Moreover, children with JPsA vs other JIA categories had 2.35 times higher odds of having persistently poor well-being scores despite improvements in joint counts and physician global scores. Thus, children with JPsA have poorer well-being scores and a higher prevalence of depression, which requires multidisciplinary care.
Apart from immunomodulatory therapies, weight loss leads to improvement in disease activity in patients with obesity and PsA. However, the mechanisms by which weight loss improves PsA is currently not known, but is likely to be due to changes in adipokines and inflammation-related cytokines. In a recent study1that included patients with PsA and obesity, it was demonstrated that weight loss through a Very Low Energy Diet (VLED) resulted in significant improvements in PsA disease activity. Landgren and colleagues now aimed to determine the effects of VLED on cytokines and adipokines. They obtained blood samples from patients with PsA and obesity (n = 41) and matched control individuals without rheumatic disease or psoriasis (n = 39) who were on VLED. At month 6, along with significant weight loss, serum levels of interleukin-23 and leptin decreased significantly, while those of total adiponectin and high-molecular-weight adiponectin increased significantly in patients with PsA and control individuals. The change in body mass index correlated positively with a reduction in serum interleukin-23 (rS = 0.671, P < .001) and improvement in PsA disease activity (P = .003). This study highlights the anti-inflammatory effect of weight loss in patients with PsA. Weight loss can complement immunomodulatory therapy in PsA patients with obesity.
Additional Reference
- Klingberg E, Bilberg A, Björkman S, et al. Weight loss improves disease activity in patients with psoriatic arthritis and obesity: an interventional study. Arthritis Res Ther. 2019;21:17. doi: 10.1186/s13075-019-1810-5
Although the usual age at onset of PsA is in the fourth or fifth decade of life, children may develop juvenile-onset PsA (JPsA). Less attention has been paid to this form of juvenile idiopathic arthritis (JIA), and the impact of JPsA vis-à-vis other forms of JIA is not well known. In addition, only about half of the patients with JPsA have cutaneous psoriasis. The impact of psoriasis on children with JPsA is not known. In order to evaluate differences in disease outcomes in patients with JPsA, Low and colleagues evaluated 1653 children and young people with JIA who were recruited to the Childhood Arthritis Prospective Study, of whom 111 had JPsA at diagnosis. They demonstrated that there were no significant differences in patient-reported outcomes between children with JPsA and other JIA categories. However, children with JPsA and psoriasis at JPsA diagnosis had more depressive symptoms compared with those without psoriasis. Moreover, children with JPsA vs other JIA categories had 2.35 times higher odds of having persistently poor well-being scores despite improvements in joint counts and physician global scores. Thus, children with JPsA have poorer well-being scores and a higher prevalence of depression, which requires multidisciplinary care.
Apart from immunomodulatory therapies, weight loss leads to improvement in disease activity in patients with obesity and PsA. However, the mechanisms by which weight loss improves PsA is currently not known, but is likely to be due to changes in adipokines and inflammation-related cytokines. In a recent study1that included patients with PsA and obesity, it was demonstrated that weight loss through a Very Low Energy Diet (VLED) resulted in significant improvements in PsA disease activity. Landgren and colleagues now aimed to determine the effects of VLED on cytokines and adipokines. They obtained blood samples from patients with PsA and obesity (n = 41) and matched control individuals without rheumatic disease or psoriasis (n = 39) who were on VLED. At month 6, along with significant weight loss, serum levels of interleukin-23 and leptin decreased significantly, while those of total adiponectin and high-molecular-weight adiponectin increased significantly in patients with PsA and control individuals. The change in body mass index correlated positively with a reduction in serum interleukin-23 (rS = 0.671, P < .001) and improvement in PsA disease activity (P = .003). This study highlights the anti-inflammatory effect of weight loss in patients with PsA. Weight loss can complement immunomodulatory therapy in PsA patients with obesity.
Additional Reference
- Klingberg E, Bilberg A, Björkman S, et al. Weight loss improves disease activity in patients with psoriatic arthritis and obesity: an interventional study. Arthritis Res Ther. 2019;21:17. doi: 10.1186/s13075-019-1810-5
Although the usual age at onset of PsA is in the fourth or fifth decade of life, children may develop juvenile-onset PsA (JPsA). Less attention has been paid to this form of juvenile idiopathic arthritis (JIA), and the impact of JPsA vis-à-vis other forms of JIA is not well known. In addition, only about half of the patients with JPsA have cutaneous psoriasis. The impact of psoriasis on children with JPsA is not known. In order to evaluate differences in disease outcomes in patients with JPsA, Low and colleagues evaluated 1653 children and young people with JIA who were recruited to the Childhood Arthritis Prospective Study, of whom 111 had JPsA at diagnosis. They demonstrated that there were no significant differences in patient-reported outcomes between children with JPsA and other JIA categories. However, children with JPsA and psoriasis at JPsA diagnosis had more depressive symptoms compared with those without psoriasis. Moreover, children with JPsA vs other JIA categories had 2.35 times higher odds of having persistently poor well-being scores despite improvements in joint counts and physician global scores. Thus, children with JPsA have poorer well-being scores and a higher prevalence of depression, which requires multidisciplinary care.
Apart from immunomodulatory therapies, weight loss leads to improvement in disease activity in patients with obesity and PsA. However, the mechanisms by which weight loss improves PsA is currently not known, but is likely to be due to changes in adipokines and inflammation-related cytokines. In a recent study1that included patients with PsA and obesity, it was demonstrated that weight loss through a Very Low Energy Diet (VLED) resulted in significant improvements in PsA disease activity. Landgren and colleagues now aimed to determine the effects of VLED on cytokines and adipokines. They obtained blood samples from patients with PsA and obesity (n = 41) and matched control individuals without rheumatic disease or psoriasis (n = 39) who were on VLED. At month 6, along with significant weight loss, serum levels of interleukin-23 and leptin decreased significantly, while those of total adiponectin and high-molecular-weight adiponectin increased significantly in patients with PsA and control individuals. The change in body mass index correlated positively with a reduction in serum interleukin-23 (rS = 0.671, P < .001) and improvement in PsA disease activity (P = .003). This study highlights the anti-inflammatory effect of weight loss in patients with PsA. Weight loss can complement immunomodulatory therapy in PsA patients with obesity.
Additional Reference
- Klingberg E, Bilberg A, Björkman S, et al. Weight loss improves disease activity in patients with psoriatic arthritis and obesity: an interventional study. Arthritis Res Ther. 2019;21:17. doi: 10.1186/s13075-019-1810-5
What’s New in Diffuse Large B-cell Lymphoma?
Diffuse large B-cell lymphoma (DLBCL) is the most diagnosed non-Hodgkin lymphoma (NHL), accounting for up to one-third of cases. For many decades, R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) has been the standard first-line treatment approach for eligible patients in the first-line setting, resulting in long-term remissions in about two-thirds of patients. However, as our understanding of the biologic heterogeneity of this disease has advanced with the ability to perform more sophisticated molecular testing at diagnosis, researchers have been able to identify high-risk patient subtypes with suboptimal outcomes. While survival outcomes among low-risk patient subgroups are favorable with first-line immunochemotherapy, the majority of high-risk patients will experience relapse and often succumb to their disease.
Given the poor outcomes among patients with relapsed or refractory (R/R) DLBCL, there has been a massive research effort over the last decade to improve survival in this setting. Many experts agree that the approval of chimeric antigen receptor (CAR) T-cell therapy was the first major victory in this uphill battle. First approved in October of 2017, axicabtagene ciloleucel was the first of the 3 currently available commercial CAR T-cell therapy constructs to be approved in the third-line setting for DLBCL. Compared to historical controls, CAR T-cell therapy is associated with significant improvement in patient survival with complete response (CR) rates of 40%-50% compared to <20% with standard salvage immunochemotherapy.
Following approval in the third-line setting, these agents were quickly expedited to second-line therapy with pivotal trials demonstrating superiority with CAR T-cell therapy in the second line compared to salvage immunochemotherapy followed by autologous stem cell transplant. In 2022 the ZUMA-7 study reported a 24-month event-free survival (EFS) of 41% with axicabtagene ciloleucel compared to 16% with standard of care, and the TRANSFORM study documented a median EFS not yet reached with lisocabtagene ciloleucel compared to 2.3 months with standard of care. Despite these drastic improvements in patient outcomes, more than half of patients will still fail CAR T-cell therapy and require further systemic therapy.
Thankfully, this year has seen even more advancement in the treatment landscape of R/R DLBCL with two new commercially approved agents in yet another novel therapeutic category: bispecific antibodies. The following is a description of the newest data leading to the latest approvals by the US Food and Drug Administration.
Bispecific antibodies (BsAbs) are an off-the-shelf product that activate endogenous immune cells by cotargeting both tumor antigens as well as host T cells or natural killer cells. Several different experimental agents with varying constructs are under active observation in a wide variety of both hematologic and solid malignancies. Specifically within the realm of B-cell NHL, however, this class of agents is extremely promising and possibly represents the next significant milestone in the treatment of lymphoma.
The toxicity profile of these agents has been reliably predictable in most early phase clinical studies and is related predominantly to T-cell overactivation. The most commonly reported adverse events consist of cytokine release syndrome (CRS) as well as neutropenia, anemia, and hypophosphatemia. While neurologic toxicity has been reported, the incidence is low, and the mechanism is thought to be different than that reported with CAR T-cell therapy given that BsAbs are not likely to cross the blood–brain barrier.
Epcoritamab is a subcutaneously administered bispecific antibody that targets CD3 and CD20 in a 1:1 ratio and activates T cells to destroy CD20-expressing malignant cells. The recent EPCORE NHL-1 clinical trial investigated epcoritamab monotherapy in R/R mature B-cell lymphomas. This agent is administered with a step-up dosing strategy seen consistently across the BsAb drug class. Patients receive a first priming dose of 0.16 mg on cycle 1 day 1, followed by an intermediate dose of 0.8 mg on cycle 1 day 8, followed by the first full dose of 48 mg on cycle 1 day 15. Subsequent doses are administered once weekly for cycles 1-3 followed by every 2 weeks for cycles 4-9, and every 4 weeks starting with cycle 10.
The study enrolled 157 patients globally with median age of 64 and 3 median prior lines of antilymphoma therapy. Nearly 40% of patients had received at least 4 prior lines of therapy, and 83% of patients were refractory to last systemic therapy. Thirty-nine percent of patients had received prior CAR T-cell therapy; 75% of these patients developed progressive disease within 6 months of CAR T-cell therapy.
Among patients treated in the study, the results were as follows:
CR rate 39% with an overall response rate (ORR) of 63%
Duration of response 12 months; duration of objective response not reached in patients with CR
Duration of CR 12 months
Median PFS 4.4 months; median OS not reached
Time to CR of 2.7 months
Toxicity profile was notable for the following:
Any grade CRS in 50%, grade ≥3 in 2.5%
Most CRS occurs with first full dose on cycle 1 day 15 with median time to onset of 20 hours and median time to resolution of 48 hours
Any grade neutropenia in 22%, grade ≥3 in 15%, febrile neutropenia in 2.5%
Any grade anemia in 18%, grade ≥3 in 10%
Injection site reaction, any grade, in 20%
Any grade neurotoxicity in 6%, grade ≥3 in 1 patient (0.6%)
Epcoritamab was granted accelerated approval on May 19, 2023, for use in patients with R/R DLBCL who have received at least 2 prior lines of systemic therapy.
Glofitamab is the more recently approved BsAb for DLBCL. This agent is distinguished by its 2:1 binding configuration that confers bivalency for the CD20 binding site. Glofitamab is delivered intravenously and requires pretreatment with obinutuzumab 1000 mg 7 days before the first dose. With a similar step-up dosing strategy, patients receive a priming dose of 2.5mg on cycle 1 day 8, an intermediate dose of 10mg on cycle 1 day 15, and a first full dose of 30mg on cycle 2 day 1. Subsequent treatments are administered every 21 days for up to 12 cycles.
The open-label phase 1-2 clinical trial of glofitamab monotherapy enrolled 155 patients with a median age of 66 and 3 median prior lines of therapy. Thirty-three percent of patients had received prior CAR T-cell therapy, and 86% were refractory to last line of therapy with 30% refractory to CAR T-cell therapy.
Results were as follows:
CR rate of 39%, ORR 52%
Median duration of CR not reached, median duration of objective response 18.4 months
Median PFS 4.9 months, median OS not reached
Toxicity profile demonstrated the following:
Any grade CRS 66%, grade ≥ 2 in 18%
Median time to onset 13.5 hours from cycle 1 day 8, median duration 30.5 hours
Any grade neutropenia in 38%, grade ≥ 3 in 27%
Grade ≥ 2 neurologic event in 15%
Glofitamab received accelerated approval from the FDA on June 15, 2023, with an identical indication to epcoritamab.
The introduction of BsAbs in DLBCL has highlighted some important issues. Will BsAbs supplant CAR T-cell therapy in DLBCL? Experts can be found on both sides of this debate. BsAbs circumvent the logistics surrounding the production of CAR T-cell therapy products and can, for the large part, be administered in the outpatient setting. However, CAR T-cell therapy has significantly longer follow-up times, which speaks to the curative potential of these agents even in the third-line setting. BsAbs, some may argue, seem to carry a more favorable toxicity profile with the CRS mitigation strategies. However, we still have much to learn about the downstream side effects with prolonged T-cell activation and the potential for T-cell exhaustion.
Finally, with the continued development of new agents in this arena, the art of sequencing therapies will become ever more important. What is the efficacy of CAR T-cell therapy after BsAb exposure? Can BsAbs be used as bridging therapy to a curative option with CAR T-cell therapy? With longer-term follow-up in several years, will we see late relapses after CR with BsAbs? Ongoing clinical trials investigating combination strategies and CAR T-cell therapy consolidation with BsAbs will hopefully eventually clarify some of these questions.
Diffuse large B-cell lymphoma (DLBCL) is the most diagnosed non-Hodgkin lymphoma (NHL), accounting for up to one-third of cases. For many decades, R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) has been the standard first-line treatment approach for eligible patients in the first-line setting, resulting in long-term remissions in about two-thirds of patients. However, as our understanding of the biologic heterogeneity of this disease has advanced with the ability to perform more sophisticated molecular testing at diagnosis, researchers have been able to identify high-risk patient subtypes with suboptimal outcomes. While survival outcomes among low-risk patient subgroups are favorable with first-line immunochemotherapy, the majority of high-risk patients will experience relapse and often succumb to their disease.
Given the poor outcomes among patients with relapsed or refractory (R/R) DLBCL, there has been a massive research effort over the last decade to improve survival in this setting. Many experts agree that the approval of chimeric antigen receptor (CAR) T-cell therapy was the first major victory in this uphill battle. First approved in October of 2017, axicabtagene ciloleucel was the first of the 3 currently available commercial CAR T-cell therapy constructs to be approved in the third-line setting for DLBCL. Compared to historical controls, CAR T-cell therapy is associated with significant improvement in patient survival with complete response (CR) rates of 40%-50% compared to <20% with standard salvage immunochemotherapy.
Following approval in the third-line setting, these agents were quickly expedited to second-line therapy with pivotal trials demonstrating superiority with CAR T-cell therapy in the second line compared to salvage immunochemotherapy followed by autologous stem cell transplant. In 2022 the ZUMA-7 study reported a 24-month event-free survival (EFS) of 41% with axicabtagene ciloleucel compared to 16% with standard of care, and the TRANSFORM study documented a median EFS not yet reached with lisocabtagene ciloleucel compared to 2.3 months with standard of care. Despite these drastic improvements in patient outcomes, more than half of patients will still fail CAR T-cell therapy and require further systemic therapy.
Thankfully, this year has seen even more advancement in the treatment landscape of R/R DLBCL with two new commercially approved agents in yet another novel therapeutic category: bispecific antibodies. The following is a description of the newest data leading to the latest approvals by the US Food and Drug Administration.
Bispecific antibodies (BsAbs) are an off-the-shelf product that activate endogenous immune cells by cotargeting both tumor antigens as well as host T cells or natural killer cells. Several different experimental agents with varying constructs are under active observation in a wide variety of both hematologic and solid malignancies. Specifically within the realm of B-cell NHL, however, this class of agents is extremely promising and possibly represents the next significant milestone in the treatment of lymphoma.
The toxicity profile of these agents has been reliably predictable in most early phase clinical studies and is related predominantly to T-cell overactivation. The most commonly reported adverse events consist of cytokine release syndrome (CRS) as well as neutropenia, anemia, and hypophosphatemia. While neurologic toxicity has been reported, the incidence is low, and the mechanism is thought to be different than that reported with CAR T-cell therapy given that BsAbs are not likely to cross the blood–brain barrier.
Epcoritamab is a subcutaneously administered bispecific antibody that targets CD3 and CD20 in a 1:1 ratio and activates T cells to destroy CD20-expressing malignant cells. The recent EPCORE NHL-1 clinical trial investigated epcoritamab monotherapy in R/R mature B-cell lymphomas. This agent is administered with a step-up dosing strategy seen consistently across the BsAb drug class. Patients receive a first priming dose of 0.16 mg on cycle 1 day 1, followed by an intermediate dose of 0.8 mg on cycle 1 day 8, followed by the first full dose of 48 mg on cycle 1 day 15. Subsequent doses are administered once weekly for cycles 1-3 followed by every 2 weeks for cycles 4-9, and every 4 weeks starting with cycle 10.
The study enrolled 157 patients globally with median age of 64 and 3 median prior lines of antilymphoma therapy. Nearly 40% of patients had received at least 4 prior lines of therapy, and 83% of patients were refractory to last systemic therapy. Thirty-nine percent of patients had received prior CAR T-cell therapy; 75% of these patients developed progressive disease within 6 months of CAR T-cell therapy.
Among patients treated in the study, the results were as follows:
CR rate 39% with an overall response rate (ORR) of 63%
Duration of response 12 months; duration of objective response not reached in patients with CR
Duration of CR 12 months
Median PFS 4.4 months; median OS not reached
Time to CR of 2.7 months
Toxicity profile was notable for the following:
Any grade CRS in 50%, grade ≥3 in 2.5%
Most CRS occurs with first full dose on cycle 1 day 15 with median time to onset of 20 hours and median time to resolution of 48 hours
Any grade neutropenia in 22%, grade ≥3 in 15%, febrile neutropenia in 2.5%
Any grade anemia in 18%, grade ≥3 in 10%
Injection site reaction, any grade, in 20%
Any grade neurotoxicity in 6%, grade ≥3 in 1 patient (0.6%)
Epcoritamab was granted accelerated approval on May 19, 2023, for use in patients with R/R DLBCL who have received at least 2 prior lines of systemic therapy.
Glofitamab is the more recently approved BsAb for DLBCL. This agent is distinguished by its 2:1 binding configuration that confers bivalency for the CD20 binding site. Glofitamab is delivered intravenously and requires pretreatment with obinutuzumab 1000 mg 7 days before the first dose. With a similar step-up dosing strategy, patients receive a priming dose of 2.5mg on cycle 1 day 8, an intermediate dose of 10mg on cycle 1 day 15, and a first full dose of 30mg on cycle 2 day 1. Subsequent treatments are administered every 21 days for up to 12 cycles.
The open-label phase 1-2 clinical trial of glofitamab monotherapy enrolled 155 patients with a median age of 66 and 3 median prior lines of therapy. Thirty-three percent of patients had received prior CAR T-cell therapy, and 86% were refractory to last line of therapy with 30% refractory to CAR T-cell therapy.
Results were as follows:
CR rate of 39%, ORR 52%
Median duration of CR not reached, median duration of objective response 18.4 months
Median PFS 4.9 months, median OS not reached
Toxicity profile demonstrated the following:
Any grade CRS 66%, grade ≥ 2 in 18%
Median time to onset 13.5 hours from cycle 1 day 8, median duration 30.5 hours
Any grade neutropenia in 38%, grade ≥ 3 in 27%
Grade ≥ 2 neurologic event in 15%
Glofitamab received accelerated approval from the FDA on June 15, 2023, with an identical indication to epcoritamab.
The introduction of BsAbs in DLBCL has highlighted some important issues. Will BsAbs supplant CAR T-cell therapy in DLBCL? Experts can be found on both sides of this debate. BsAbs circumvent the logistics surrounding the production of CAR T-cell therapy products and can, for the large part, be administered in the outpatient setting. However, CAR T-cell therapy has significantly longer follow-up times, which speaks to the curative potential of these agents even in the third-line setting. BsAbs, some may argue, seem to carry a more favorable toxicity profile with the CRS mitigation strategies. However, we still have much to learn about the downstream side effects with prolonged T-cell activation and the potential for T-cell exhaustion.
Finally, with the continued development of new agents in this arena, the art of sequencing therapies will become ever more important. What is the efficacy of CAR T-cell therapy after BsAb exposure? Can BsAbs be used as bridging therapy to a curative option with CAR T-cell therapy? With longer-term follow-up in several years, will we see late relapses after CR with BsAbs? Ongoing clinical trials investigating combination strategies and CAR T-cell therapy consolidation with BsAbs will hopefully eventually clarify some of these questions.
Diffuse large B-cell lymphoma (DLBCL) is the most diagnosed non-Hodgkin lymphoma (NHL), accounting for up to one-third of cases. For many decades, R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) has been the standard first-line treatment approach for eligible patients in the first-line setting, resulting in long-term remissions in about two-thirds of patients. However, as our understanding of the biologic heterogeneity of this disease has advanced with the ability to perform more sophisticated molecular testing at diagnosis, researchers have been able to identify high-risk patient subtypes with suboptimal outcomes. While survival outcomes among low-risk patient subgroups are favorable with first-line immunochemotherapy, the majority of high-risk patients will experience relapse and often succumb to their disease.
Given the poor outcomes among patients with relapsed or refractory (R/R) DLBCL, there has been a massive research effort over the last decade to improve survival in this setting. Many experts agree that the approval of chimeric antigen receptor (CAR) T-cell therapy was the first major victory in this uphill battle. First approved in October of 2017, axicabtagene ciloleucel was the first of the 3 currently available commercial CAR T-cell therapy constructs to be approved in the third-line setting for DLBCL. Compared to historical controls, CAR T-cell therapy is associated with significant improvement in patient survival with complete response (CR) rates of 40%-50% compared to <20% with standard salvage immunochemotherapy.
Following approval in the third-line setting, these agents were quickly expedited to second-line therapy with pivotal trials demonstrating superiority with CAR T-cell therapy in the second line compared to salvage immunochemotherapy followed by autologous stem cell transplant. In 2022 the ZUMA-7 study reported a 24-month event-free survival (EFS) of 41% with axicabtagene ciloleucel compared to 16% with standard of care, and the TRANSFORM study documented a median EFS not yet reached with lisocabtagene ciloleucel compared to 2.3 months with standard of care. Despite these drastic improvements in patient outcomes, more than half of patients will still fail CAR T-cell therapy and require further systemic therapy.
Thankfully, this year has seen even more advancement in the treatment landscape of R/R DLBCL with two new commercially approved agents in yet another novel therapeutic category: bispecific antibodies. The following is a description of the newest data leading to the latest approvals by the US Food and Drug Administration.
Bispecific antibodies (BsAbs) are an off-the-shelf product that activate endogenous immune cells by cotargeting both tumor antigens as well as host T cells or natural killer cells. Several different experimental agents with varying constructs are under active observation in a wide variety of both hematologic and solid malignancies. Specifically within the realm of B-cell NHL, however, this class of agents is extremely promising and possibly represents the next significant milestone in the treatment of lymphoma.
The toxicity profile of these agents has been reliably predictable in most early phase clinical studies and is related predominantly to T-cell overactivation. The most commonly reported adverse events consist of cytokine release syndrome (CRS) as well as neutropenia, anemia, and hypophosphatemia. While neurologic toxicity has been reported, the incidence is low, and the mechanism is thought to be different than that reported with CAR T-cell therapy given that BsAbs are not likely to cross the blood–brain barrier.
Epcoritamab is a subcutaneously administered bispecific antibody that targets CD3 and CD20 in a 1:1 ratio and activates T cells to destroy CD20-expressing malignant cells. The recent EPCORE NHL-1 clinical trial investigated epcoritamab monotherapy in R/R mature B-cell lymphomas. This agent is administered with a step-up dosing strategy seen consistently across the BsAb drug class. Patients receive a first priming dose of 0.16 mg on cycle 1 day 1, followed by an intermediate dose of 0.8 mg on cycle 1 day 8, followed by the first full dose of 48 mg on cycle 1 day 15. Subsequent doses are administered once weekly for cycles 1-3 followed by every 2 weeks for cycles 4-9, and every 4 weeks starting with cycle 10.
The study enrolled 157 patients globally with median age of 64 and 3 median prior lines of antilymphoma therapy. Nearly 40% of patients had received at least 4 prior lines of therapy, and 83% of patients were refractory to last systemic therapy. Thirty-nine percent of patients had received prior CAR T-cell therapy; 75% of these patients developed progressive disease within 6 months of CAR T-cell therapy.
Among patients treated in the study, the results were as follows:
CR rate 39% with an overall response rate (ORR) of 63%
Duration of response 12 months; duration of objective response not reached in patients with CR
Duration of CR 12 months
Median PFS 4.4 months; median OS not reached
Time to CR of 2.7 months
Toxicity profile was notable for the following:
Any grade CRS in 50%, grade ≥3 in 2.5%
Most CRS occurs with first full dose on cycle 1 day 15 with median time to onset of 20 hours and median time to resolution of 48 hours
Any grade neutropenia in 22%, grade ≥3 in 15%, febrile neutropenia in 2.5%
Any grade anemia in 18%, grade ≥3 in 10%
Injection site reaction, any grade, in 20%
Any grade neurotoxicity in 6%, grade ≥3 in 1 patient (0.6%)
Epcoritamab was granted accelerated approval on May 19, 2023, for use in patients with R/R DLBCL who have received at least 2 prior lines of systemic therapy.
Glofitamab is the more recently approved BsAb for DLBCL. This agent is distinguished by its 2:1 binding configuration that confers bivalency for the CD20 binding site. Glofitamab is delivered intravenously and requires pretreatment with obinutuzumab 1000 mg 7 days before the first dose. With a similar step-up dosing strategy, patients receive a priming dose of 2.5mg on cycle 1 day 8, an intermediate dose of 10mg on cycle 1 day 15, and a first full dose of 30mg on cycle 2 day 1. Subsequent treatments are administered every 21 days for up to 12 cycles.
The open-label phase 1-2 clinical trial of glofitamab monotherapy enrolled 155 patients with a median age of 66 and 3 median prior lines of therapy. Thirty-three percent of patients had received prior CAR T-cell therapy, and 86% were refractory to last line of therapy with 30% refractory to CAR T-cell therapy.
Results were as follows:
CR rate of 39%, ORR 52%
Median duration of CR not reached, median duration of objective response 18.4 months
Median PFS 4.9 months, median OS not reached
Toxicity profile demonstrated the following:
Any grade CRS 66%, grade ≥ 2 in 18%
Median time to onset 13.5 hours from cycle 1 day 8, median duration 30.5 hours
Any grade neutropenia in 38%, grade ≥ 3 in 27%
Grade ≥ 2 neurologic event in 15%
Glofitamab received accelerated approval from the FDA on June 15, 2023, with an identical indication to epcoritamab.
The introduction of BsAbs in DLBCL has highlighted some important issues. Will BsAbs supplant CAR T-cell therapy in DLBCL? Experts can be found on both sides of this debate. BsAbs circumvent the logistics surrounding the production of CAR T-cell therapy products and can, for the large part, be administered in the outpatient setting. However, CAR T-cell therapy has significantly longer follow-up times, which speaks to the curative potential of these agents even in the third-line setting. BsAbs, some may argue, seem to carry a more favorable toxicity profile with the CRS mitigation strategies. However, we still have much to learn about the downstream side effects with prolonged T-cell activation and the potential for T-cell exhaustion.
Finally, with the continued development of new agents in this arena, the art of sequencing therapies will become ever more important. What is the efficacy of CAR T-cell therapy after BsAb exposure? Can BsAbs be used as bridging therapy to a curative option with CAR T-cell therapy? With longer-term follow-up in several years, will we see late relapses after CR with BsAbs? Ongoing clinical trials investigating combination strategies and CAR T-cell therapy consolidation with BsAbs will hopefully eventually clarify some of these questions.