User login
Heard of ApoB Testing? New Guidelines
This transcript has been edited for clarity.
I've been hearing a lot about apolipoprotein B (apoB) lately. It keeps popping up, but I've not been sure where it fits in or what I should do about it. The new Expert Clinical Consensus from the National Lipid Association now finally gives us clear guidance.
ApoB is the main protein that is found on all atherogenic lipoproteins. It is found on low-density lipoprotein (LDL) but also on other atherogenic lipoprotein particles. Because it is a part of all atherogenic particles, it predicts cardiovascular (CV) risk more accurately than does LDL cholesterol (LDL-C).
ApoB and LDL-C tend to run together, but not always. While they are correlated fairly well on a population level, for a given individual they can diverge; and when they do, apoB is the better predictor of future CV outcomes. This divergence occurs frequently, and it can occur even more frequently after treatment with statins. When LDL decreases to reach the LDL threshold for treatment, but apoB remains elevated, there is the potential for misclassification of CV risk and essentially the risk for undertreatment of someone whose CV risk is actually higher than it appears to be if we only look at their LDL-C. The consensus statement says, "Where there is discordance between apoB and LDL-C, risk follows apoB."
This understanding leads to the places where measurement of apoB may be helpful:
In patients with borderline atherosclerotic cardiovascular disease risk in whom a shared decision about statin therapy is being determined and the patient prefers not to start a statin, apoB can be useful for further risk stratification. If apoB suggests low risk, then statin therapy could be withheld, and if apoB is high, that would favor starting statin therapy. Certain common conditions, such as obesity and insulin resistance, can lead to smaller cholesterol-depleted LDL particles that result in lower LDL-C, but elevated apoB levels in this circumstance may drive the decision to treat with a statin.
In patients already treated with statins, but a decision must be made about whether treatment intensification is warranted. If the LDL-C is to goal and apoB is above threshold, treatment intensification may be considered. In patients who are not yet to goal, based on an elevated apoB, the first step is intensification of statin therapy. After that, intensification would be the same as has already been addressed in my review of the 2022 ACC Expert Consensus Decision Pathway on the Role of Nonstatin Therapies for LDL-Cholesterol Lowering.
After clarifying the importance of apoB in providing additional discrimination of CV risk, the consensus statement clarifies the treatment thresholds, or goals for treatment, for apoB that correlate with established LDL-C thresholds, as shown in this table:
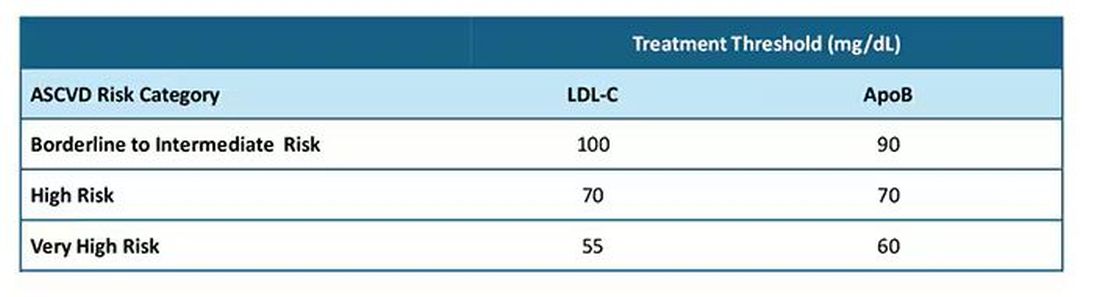
Let me be really clear: The consensus statement does not say that we need to measure apoB in all patients or that such measurement is the standard of care. It is not. It says, and I'll quote, "At present, the use of apoB to assess the effectiveness of lipid-lowering therapies remains a matter of clinical judgment." This guideline is helpful in pointing out the patients most likely to benefit from this additional measurement, including those with hypertriglyceridemia, diabetes, visceral adiposity, insulin resistance/metabolic syndrome, low HDL-C, or very low LDL-C levels.
In summary, measurement of apoB can be helpful for further risk stratification in patients with borderline or intermediate LDL-C levels, and for deciding whether further intensification of lipid-lowering therapy may be warranted when the LDL threshold has been reached.
Lipid management is something that we do every day in the office. This is new information, or at least clarifying information, for most of us. Hopefully it is helpful. I'm interested in your thoughts on this topic, including whether and how you plan to use apoB measurements.
Dr. Skolnik, Professor, Department of Family Medicine, Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia; Associate Director, Department of Family Medicine, Abington Jefferson Health, Abington, Pennsylvania, disclosed ties with AstraZeneca, Teva, Eli Lilly, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck, and Bayer.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I've been hearing a lot about apolipoprotein B (apoB) lately. It keeps popping up, but I've not been sure where it fits in or what I should do about it. The new Expert Clinical Consensus from the National Lipid Association now finally gives us clear guidance.
ApoB is the main protein that is found on all atherogenic lipoproteins. It is found on low-density lipoprotein (LDL) but also on other atherogenic lipoprotein particles. Because it is a part of all atherogenic particles, it predicts cardiovascular (CV) risk more accurately than does LDL cholesterol (LDL-C).
ApoB and LDL-C tend to run together, but not always. While they are correlated fairly well on a population level, for a given individual they can diverge; and when they do, apoB is the better predictor of future CV outcomes. This divergence occurs frequently, and it can occur even more frequently after treatment with statins. When LDL decreases to reach the LDL threshold for treatment, but apoB remains elevated, there is the potential for misclassification of CV risk and essentially the risk for undertreatment of someone whose CV risk is actually higher than it appears to be if we only look at their LDL-C. The consensus statement says, "Where there is discordance between apoB and LDL-C, risk follows apoB."
This understanding leads to the places where measurement of apoB may be helpful:
In patients with borderline atherosclerotic cardiovascular disease risk in whom a shared decision about statin therapy is being determined and the patient prefers not to start a statin, apoB can be useful for further risk stratification. If apoB suggests low risk, then statin therapy could be withheld, and if apoB is high, that would favor starting statin therapy. Certain common conditions, such as obesity and insulin resistance, can lead to smaller cholesterol-depleted LDL particles that result in lower LDL-C, but elevated apoB levels in this circumstance may drive the decision to treat with a statin.
In patients already treated with statins, but a decision must be made about whether treatment intensification is warranted. If the LDL-C is to goal and apoB is above threshold, treatment intensification may be considered. In patients who are not yet to goal, based on an elevated apoB, the first step is intensification of statin therapy. After that, intensification would be the same as has already been addressed in my review of the 2022 ACC Expert Consensus Decision Pathway on the Role of Nonstatin Therapies for LDL-Cholesterol Lowering.
After clarifying the importance of apoB in providing additional discrimination of CV risk, the consensus statement clarifies the treatment thresholds, or goals for treatment, for apoB that correlate with established LDL-C thresholds, as shown in this table:

Let me be really clear: The consensus statement does not say that we need to measure apoB in all patients or that such measurement is the standard of care. It is not. It says, and I'll quote, "At present, the use of apoB to assess the effectiveness of lipid-lowering therapies remains a matter of clinical judgment." This guideline is helpful in pointing out the patients most likely to benefit from this additional measurement, including those with hypertriglyceridemia, diabetes, visceral adiposity, insulin resistance/metabolic syndrome, low HDL-C, or very low LDL-C levels.
In summary, measurement of apoB can be helpful for further risk stratification in patients with borderline or intermediate LDL-C levels, and for deciding whether further intensification of lipid-lowering therapy may be warranted when the LDL threshold has been reached.
Lipid management is something that we do every day in the office. This is new information, or at least clarifying information, for most of us. Hopefully it is helpful. I'm interested in your thoughts on this topic, including whether and how you plan to use apoB measurements.
Dr. Skolnik, Professor, Department of Family Medicine, Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia; Associate Director, Department of Family Medicine, Abington Jefferson Health, Abington, Pennsylvania, disclosed ties with AstraZeneca, Teva, Eli Lilly, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck, and Bayer.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I've been hearing a lot about apolipoprotein B (apoB) lately. It keeps popping up, but I've not been sure where it fits in or what I should do about it. The new Expert Clinical Consensus from the National Lipid Association now finally gives us clear guidance.
ApoB is the main protein that is found on all atherogenic lipoproteins. It is found on low-density lipoprotein (LDL) but also on other atherogenic lipoprotein particles. Because it is a part of all atherogenic particles, it predicts cardiovascular (CV) risk more accurately than does LDL cholesterol (LDL-C).
ApoB and LDL-C tend to run together, but not always. While they are correlated fairly well on a population level, for a given individual they can diverge; and when they do, apoB is the better predictor of future CV outcomes. This divergence occurs frequently, and it can occur even more frequently after treatment with statins. When LDL decreases to reach the LDL threshold for treatment, but apoB remains elevated, there is the potential for misclassification of CV risk and essentially the risk for undertreatment of someone whose CV risk is actually higher than it appears to be if we only look at their LDL-C. The consensus statement says, "Where there is discordance between apoB and LDL-C, risk follows apoB."
This understanding leads to the places where measurement of apoB may be helpful:
In patients with borderline atherosclerotic cardiovascular disease risk in whom a shared decision about statin therapy is being determined and the patient prefers not to start a statin, apoB can be useful for further risk stratification. If apoB suggests low risk, then statin therapy could be withheld, and if apoB is high, that would favor starting statin therapy. Certain common conditions, such as obesity and insulin resistance, can lead to smaller cholesterol-depleted LDL particles that result in lower LDL-C, but elevated apoB levels in this circumstance may drive the decision to treat with a statin.
In patients already treated with statins, but a decision must be made about whether treatment intensification is warranted. If the LDL-C is to goal and apoB is above threshold, treatment intensification may be considered. In patients who are not yet to goal, based on an elevated apoB, the first step is intensification of statin therapy. After that, intensification would be the same as has already been addressed in my review of the 2022 ACC Expert Consensus Decision Pathway on the Role of Nonstatin Therapies for LDL-Cholesterol Lowering.
After clarifying the importance of apoB in providing additional discrimination of CV risk, the consensus statement clarifies the treatment thresholds, or goals for treatment, for apoB that correlate with established LDL-C thresholds, as shown in this table:

Let me be really clear: The consensus statement does not say that we need to measure apoB in all patients or that such measurement is the standard of care. It is not. It says, and I'll quote, "At present, the use of apoB to assess the effectiveness of lipid-lowering therapies remains a matter of clinical judgment." This guideline is helpful in pointing out the patients most likely to benefit from this additional measurement, including those with hypertriglyceridemia, diabetes, visceral adiposity, insulin resistance/metabolic syndrome, low HDL-C, or very low LDL-C levels.
In summary, measurement of apoB can be helpful for further risk stratification in patients with borderline or intermediate LDL-C levels, and for deciding whether further intensification of lipid-lowering therapy may be warranted when the LDL threshold has been reached.
Lipid management is something that we do every day in the office. This is new information, or at least clarifying information, for most of us. Hopefully it is helpful. I'm interested in your thoughts on this topic, including whether and how you plan to use apoB measurements.
Dr. Skolnik, Professor, Department of Family Medicine, Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia; Associate Director, Department of Family Medicine, Abington Jefferson Health, Abington, Pennsylvania, disclosed ties with AstraZeneca, Teva, Eli Lilly, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck, and Bayer.
A version of this article first appeared on Medscape.com.
Mechanism of Action
MOA — Mechanism of action — gets bandied about a lot.
Drug reps love it. Saying your product is a “first-in-class MOA” sounds great as they hand you a glossy brochure. It also features prominently in print ads, usually with pics of smiling people.
It’s a good thing to know, too, both medically and in a cool-science-geeky way. We want to understand what we’re prescribing will do to patients. We want to explain it to them, too.
It certainly helps to know that what we’re doing when treating a disorder using rational polypharmacy.
But at the same time we face the realization that it may not mean as much as we think it should. I don’t have to go back very far in my career to find Food and Drug Administration–approved medications that worked, but we didn’t have a clear reason why. I mean, we had a vague idea on a scientific basis, but we’re still guessing.
This didn’t stop us from using them, which is nothing new. The ancients had learned certain plants reduced pain and fever long before they understood what aspirin (and its MOA) was.
At the same time we’re now using drugs, such as the anti-amyloid treatments for Alzheimer’s disease, that should be more effective than one would think. Pulling the damaged molecules out of the brain should, on paper, make a dramatic difference ... but it doesn’t. I’m not saying they don’t have some benefit, but certainly not as much as you’d think. Of course, that’s based on our understanding of the disease mechanism being correct. We find there’s a lot more going on than we know.
Like so much in science (and this aspect of medicine is a science) the answers often lead to more questions.
Observation takes the lead over understanding in most things. Our ancestors knew what fire was, and how to use it, without any idea of what rapid exothermic oxidation was. (Admittedly, I have a degree in chemistry and can’t explain it myself anymore.)
The glossy ads and scientific data about MOA doesn’t mean much in my world if they don’t work. My patients would say the same.
Clinical medicine, after all, is both an art and a science.
Dr. Block has a solo neurology practice in Scottsdale, Arizona.
MOA — Mechanism of action — gets bandied about a lot.
Drug reps love it. Saying your product is a “first-in-class MOA” sounds great as they hand you a glossy brochure. It also features prominently in print ads, usually with pics of smiling people.
It’s a good thing to know, too, both medically and in a cool-science-geeky way. We want to understand what we’re prescribing will do to patients. We want to explain it to them, too.
It certainly helps to know that what we’re doing when treating a disorder using rational polypharmacy.
But at the same time we face the realization that it may not mean as much as we think it should. I don’t have to go back very far in my career to find Food and Drug Administration–approved medications that worked, but we didn’t have a clear reason why. I mean, we had a vague idea on a scientific basis, but we’re still guessing.
This didn’t stop us from using them, which is nothing new. The ancients had learned certain plants reduced pain and fever long before they understood what aspirin (and its MOA) was.
At the same time we’re now using drugs, such as the anti-amyloid treatments for Alzheimer’s disease, that should be more effective than one would think. Pulling the damaged molecules out of the brain should, on paper, make a dramatic difference ... but it doesn’t. I’m not saying they don’t have some benefit, but certainly not as much as you’d think. Of course, that’s based on our understanding of the disease mechanism being correct. We find there’s a lot more going on than we know.
Like so much in science (and this aspect of medicine is a science) the answers often lead to more questions.
Observation takes the lead over understanding in most things. Our ancestors knew what fire was, and how to use it, without any idea of what rapid exothermic oxidation was. (Admittedly, I have a degree in chemistry and can’t explain it myself anymore.)
The glossy ads and scientific data about MOA doesn’t mean much in my world if they don’t work. My patients would say the same.
Clinical medicine, after all, is both an art and a science.
Dr. Block has a solo neurology practice in Scottsdale, Arizona.
MOA — Mechanism of action — gets bandied about a lot.
Drug reps love it. Saying your product is a “first-in-class MOA” sounds great as they hand you a glossy brochure. It also features prominently in print ads, usually with pics of smiling people.
It’s a good thing to know, too, both medically and in a cool-science-geeky way. We want to understand what we’re prescribing will do to patients. We want to explain it to them, too.
It certainly helps to know that what we’re doing when treating a disorder using rational polypharmacy.
But at the same time we face the realization that it may not mean as much as we think it should. I don’t have to go back very far in my career to find Food and Drug Administration–approved medications that worked, but we didn’t have a clear reason why. I mean, we had a vague idea on a scientific basis, but we’re still guessing.
This didn’t stop us from using them, which is nothing new. The ancients had learned certain plants reduced pain and fever long before they understood what aspirin (and its MOA) was.
At the same time we’re now using drugs, such as the anti-amyloid treatments for Alzheimer’s disease, that should be more effective than one would think. Pulling the damaged molecules out of the brain should, on paper, make a dramatic difference ... but it doesn’t. I’m not saying they don’t have some benefit, but certainly not as much as you’d think. Of course, that’s based on our understanding of the disease mechanism being correct. We find there’s a lot more going on than we know.
Like so much in science (and this aspect of medicine is a science) the answers often lead to more questions.
Observation takes the lead over understanding in most things. Our ancestors knew what fire was, and how to use it, without any idea of what rapid exothermic oxidation was. (Admittedly, I have a degree in chemistry and can’t explain it myself anymore.)
The glossy ads and scientific data about MOA doesn’t mean much in my world if they don’t work. My patients would say the same.
Clinical medicine, after all, is both an art and a science.
Dr. Block has a solo neurology practice in Scottsdale, Arizona.
What Should You Do When a Patient Asks for a PSA Test?
Many patients ask us to request a prostate-specific antigen (PSA) test. According to the Brazilian Ministry of Health, prostate cancer is the second most common type of cancer in the male population in all regions of our country. It is the second-leading cause of cancer death in the male population, reaffirming its epidemiologic importance in Brazil. On the other hand, a Ministry of Health technical paper recommends against population-based screening for prostate cancer. So, what should we do?
First, it is important to distinguish early diagnosis from screening. Early diagnosis is the identification of cancer in early stages in people with signs and symptoms. Screening is characterized by the systematic application of exams — digital rectal exam and PSA test — in asymptomatic people, with the aim of identifying cancer in an early stage.
A recent European epidemiologic study reinforced this thesis and helps guide us.
The study included men aged 35-84 years from 26 European countries. Data on cancer incidence and mortality were collected between 1980 and 2017. The data suggested overdiagnosis of prostate cancer, which varied over time and among populations. The findings supported previous recommendations that any implementation of prostate cancer screening should be carefully designed, with an emphasis on minimizing the harms of overdiagnosis.
The clinical evolution of prostate cancer is still not well understood. Increasing age is associated with increased mortality. Many men with less aggressive disease tend to die with cancer rather than die of cancer. However, it is not always possible at the time of diagnosis to determine which tumors will be aggressive and which will grow slowly.
On the other hand, with screening, many of these indolent cancers are unnecessarily detected, generating excessive exams and treatments with negative repercussions (eg, pain, bleeding, infections, stress, and urinary and sexual dysfunction).
So, how should we as clinicians proceed regarding screening?
We should request the PSA test and emphasize the importance of digital rectal exam by a urologist for those at high risk for prostatic neoplasia (ie, those with family history) or those with urinary symptoms that may be associated with prostate cancer.
In general, we should draw attention to the possible risks and benefits of testing and adopt a shared decision-making approach with asymptomatic men or those at low risk who wish to have the screening exam. But achieving a shared decision is not a simple task.
I always have a thorough conversation with patients, but I confess that I request the exam in most cases.
Dr. Wajngarten is a professor of cardiology, Faculty of Medicine, at the University of São Paulo in Brazil. Dr. Wajngarten reported no conflicts of interest.
This story was translated from the Medscape Portuguese edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Many patients ask us to request a prostate-specific antigen (PSA) test. According to the Brazilian Ministry of Health, prostate cancer is the second most common type of cancer in the male population in all regions of our country. It is the second-leading cause of cancer death in the male population, reaffirming its epidemiologic importance in Brazil. On the other hand, a Ministry of Health technical paper recommends against population-based screening for prostate cancer. So, what should we do?
First, it is important to distinguish early diagnosis from screening. Early diagnosis is the identification of cancer in early stages in people with signs and symptoms. Screening is characterized by the systematic application of exams — digital rectal exam and PSA test — in asymptomatic people, with the aim of identifying cancer in an early stage.
A recent European epidemiologic study reinforced this thesis and helps guide us.
The study included men aged 35-84 years from 26 European countries. Data on cancer incidence and mortality were collected between 1980 and 2017. The data suggested overdiagnosis of prostate cancer, which varied over time and among populations. The findings supported previous recommendations that any implementation of prostate cancer screening should be carefully designed, with an emphasis on minimizing the harms of overdiagnosis.
The clinical evolution of prostate cancer is still not well understood. Increasing age is associated with increased mortality. Many men with less aggressive disease tend to die with cancer rather than die of cancer. However, it is not always possible at the time of diagnosis to determine which tumors will be aggressive and which will grow slowly.
On the other hand, with screening, many of these indolent cancers are unnecessarily detected, generating excessive exams and treatments with negative repercussions (eg, pain, bleeding, infections, stress, and urinary and sexual dysfunction).
So, how should we as clinicians proceed regarding screening?
We should request the PSA test and emphasize the importance of digital rectal exam by a urologist for those at high risk for prostatic neoplasia (ie, those with family history) or those with urinary symptoms that may be associated with prostate cancer.
In general, we should draw attention to the possible risks and benefits of testing and adopt a shared decision-making approach with asymptomatic men or those at low risk who wish to have the screening exam. But achieving a shared decision is not a simple task.
I always have a thorough conversation with patients, but I confess that I request the exam in most cases.
Dr. Wajngarten is a professor of cardiology, Faculty of Medicine, at the University of São Paulo in Brazil. Dr. Wajngarten reported no conflicts of interest.
This story was translated from the Medscape Portuguese edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Many patients ask us to request a prostate-specific antigen (PSA) test. According to the Brazilian Ministry of Health, prostate cancer is the second most common type of cancer in the male population in all regions of our country. It is the second-leading cause of cancer death in the male population, reaffirming its epidemiologic importance in Brazil. On the other hand, a Ministry of Health technical paper recommends against population-based screening for prostate cancer. So, what should we do?
First, it is important to distinguish early diagnosis from screening. Early diagnosis is the identification of cancer in early stages in people with signs and symptoms. Screening is characterized by the systematic application of exams — digital rectal exam and PSA test — in asymptomatic people, with the aim of identifying cancer in an early stage.
A recent European epidemiologic study reinforced this thesis and helps guide us.
The study included men aged 35-84 years from 26 European countries. Data on cancer incidence and mortality were collected between 1980 and 2017. The data suggested overdiagnosis of prostate cancer, which varied over time and among populations. The findings supported previous recommendations that any implementation of prostate cancer screening should be carefully designed, with an emphasis on minimizing the harms of overdiagnosis.
The clinical evolution of prostate cancer is still not well understood. Increasing age is associated with increased mortality. Many men with less aggressive disease tend to die with cancer rather than die of cancer. However, it is not always possible at the time of diagnosis to determine which tumors will be aggressive and which will grow slowly.
On the other hand, with screening, many of these indolent cancers are unnecessarily detected, generating excessive exams and treatments with negative repercussions (eg, pain, bleeding, infections, stress, and urinary and sexual dysfunction).
So, how should we as clinicians proceed regarding screening?
We should request the PSA test and emphasize the importance of digital rectal exam by a urologist for those at high risk for prostatic neoplasia (ie, those with family history) or those with urinary symptoms that may be associated with prostate cancer.
In general, we should draw attention to the possible risks and benefits of testing and adopt a shared decision-making approach with asymptomatic men or those at low risk who wish to have the screening exam. But achieving a shared decision is not a simple task.
I always have a thorough conversation with patients, but I confess that I request the exam in most cases.
Dr. Wajngarten is a professor of cardiology, Faculty of Medicine, at the University of São Paulo in Brazil. Dr. Wajngarten reported no conflicts of interest.
This story was translated from the Medscape Portuguese edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Maternal Immunization to Prevent Serious Respiratory Illness
Editor’s Note: Sadly, this is the last column in the Master Class Obstetrics series. This award-winning column has been part of Ob.Gyn. News for 20 years. The deep discussion of cutting-edge topics in obstetrics by specialists and researchers will be missed as will the leadership and curation of topics by Dr. E. Albert Reece.
Introduction: The Need for Increased Vigilance About Maternal Immunization
Viruses are becoming increasingly prevalent in our world and the consequences of viral infections are implicated in a growing number of disease states. It is well established that certain cancers are caused by viruses and it is increasingly evident that viral infections can trigger the development of chronic illness. In pregnant women, viruses such as cytomegalovirus can cause infection in utero and lead to long-term impairments for the baby.
Likewise, it appears that the virulence of viruses is increasing, whether it be the respiratory syncytial virus (RSV) in children or the severe acute respiratory syndrome (SARS) coronaviruses in adults. Clearly, our environment is changing, with increases in population growth and urbanization, for instance, and an intensification of climate change and its effects. Viruses are part of this changing background.
Vaccines are our most powerful tool to protect people of all ages against viral threats, and fortunately, we benefit from increasing expertise in vaccinology. Since 1974, the University of Maryland School of Medicine has a Center for Vaccine Development and Global Health that has conducted research on vaccines to defend against the Zika virus, H1N1, Ebola, and SARS-CoV-2.
We’re not alone. Other vaccinology centers across the country — as well as the National Institutes of Health at the national level, through its National Institute of Allergy and Infectious Diseases — are doing research and developing vaccines to combat viral diseases.
In this column, we are focused on viral diseases in pregnancy and the role that vaccines can play in preventing serious respiratory illness in mothers and their newborns. I have invited Laura E. Riley, MD, the Given Foundation Professor and Chair of Obstetrics and Gynecology at Weill Cornell Medicine, to address the importance of maternal immunization and how we can best counsel our patients and improve immunization rates.
As Dr. Riley explains, we are in a new era, and it behooves us all to be more vigilant about recommending vaccines, combating misperceptions, addressing patients’ knowledge gaps, and administering vaccines whenever possible.
Dr. Reece is the former Dean of Medicine & University Executive VP, and The Distinguished University and Endowed Professor & Director of the Center for Advanced Research Training and Innovation (CARTI) at the University of Maryland School of Medicine, as well as senior scientist at the Center for Birth Defects Research.
The alarming decline in maternal immunization rates that occurred in the wake of the COVID-19 pandemic means that, now more than ever, we must fully embrace our responsibility to recommend immunizations in pregnancy and to communicate what is known about their efficacy and safety. Data show that vaccination rates drop when we do not offer vaccines in our offices, so whenever possible, we should administer them as well.
The ob.gyn. is the patient’s most trusted person in pregnancy. When patients decline or express hesitancy about vaccines, it is incumbent upon us to ask why. Oftentimes, we can identify areas in which patients lack knowledge or have misperceptions and we can successfully educate the patient or change their perspective or misunderstanding concerning the importance of vaccination for themselves and their babies. (See Table 1.) We can also successfully address concerns about safety.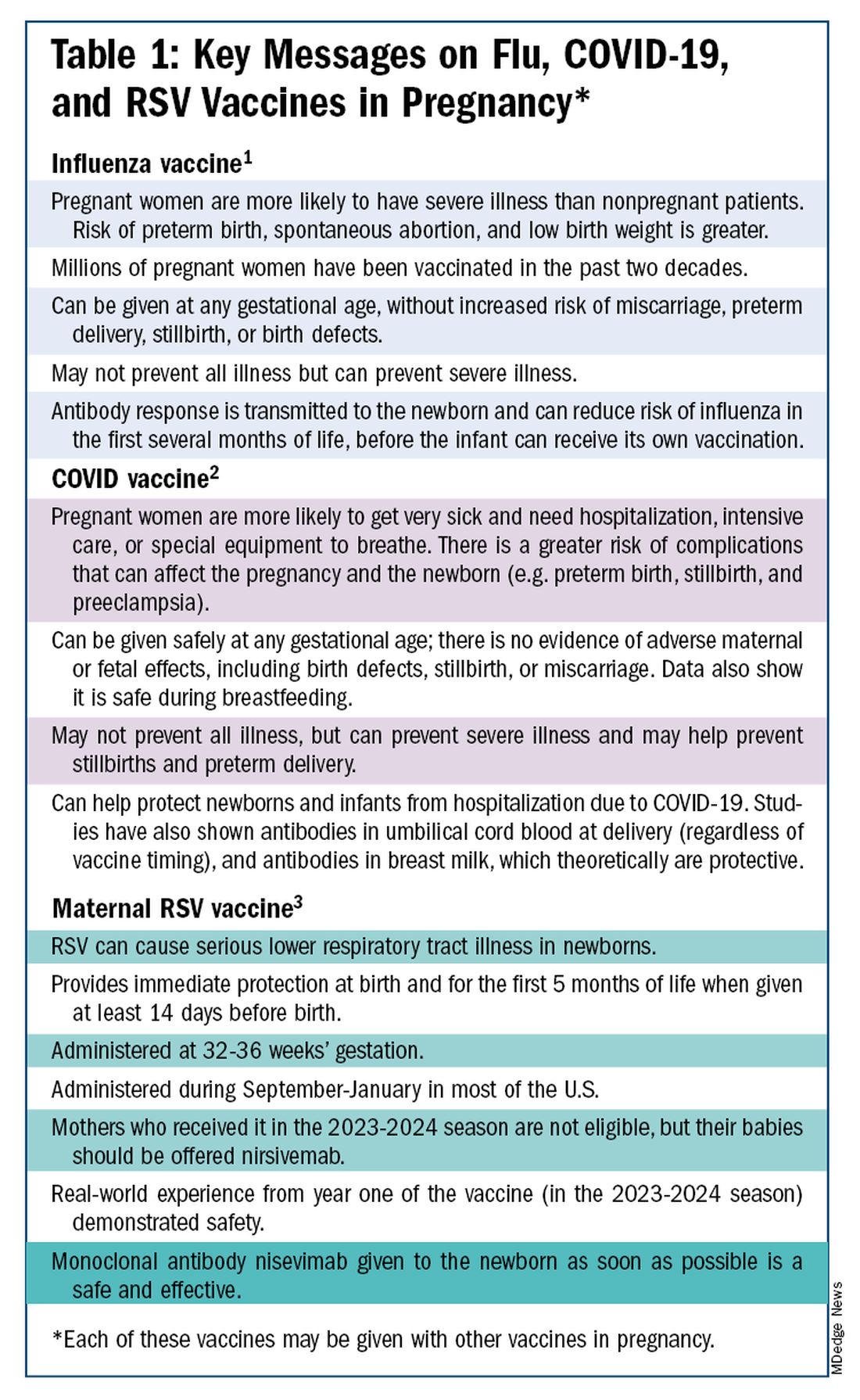
The safety of COVID-19 vaccinations in pregnancy is now backed by several years of data from multiple studies showing no increase in birth defects, preterm delivery, miscarriage, or stillbirth.
Data also show that pregnant patients are more likely than patients who are not pregnant to need hospitalization and intensive care when infected with SARS-CoV-2 and are at risk of having complications that can affect pregnancy and the newborn, including preterm birth and stillbirth. Vaccination has been shown to reduce the risk of severe illness and the risk of such adverse obstetrical outcomes, in addition to providing protection for the infant early on.
Similarly, influenza has long been more likely to be severe in pregnant patients, with an increased risk of poor obstetrical outcomes. Vaccines similarly provide “two for one protection,” protecting both mother and baby, and are, of course, backed by many years of safety and efficacy data.
With the new maternal respiratory syncytial virus (RSV) vaccine, now in its second year of availability, the goal is to protect the baby from RSV-caused serious lower respiratory tract illness. The illness has contributed to tens of thousands of annual hospitalizations and up to several hundred deaths every year in children younger than 5 years — particularly in those under age 6 months.
The RSV monoclonal antibody nirsevimab is available for the newborn as an alternative to maternal immunization but the maternal vaccine is optimal in that it will provide immediate rather than delayed protection for the newborn. The maternal vaccine is recommended during weeks 32-36 of pregnancy in mothers who were not vaccinated during last year’s RSV season. With real-world experience from year one, the available safety data are reassuring.
Counseling About Influenza and COVID-19 Vaccination
The COVID-19 pandemic took a toll on vaccination interest/receptivity broadly in pregnant and nonpregnant people. Among pregnant individuals, influenza vaccination coverage declined from 71% in the 2019-2020 influenza season to 56% in the 2021-2022 season, according to data from the Centers for Disease Control and Prevention’s Vaccine Safety Datalink.4 Coverage for the 2022-2023 and 2023-2024 influenza seasons was even worse: well under 50%.5
Fewer pregnant women have received updated COVID-19 vaccines. Only 13% of pregnant persons overall received the updated 2023-2024 COVID-19 booster vaccine (through March 30, 2024), according to the CDC.6
Maternal immunization for influenza has been recommended in the United States since 2004 (part of the recommendation that everyone over the age of 6 months receive an annual flu vaccine), and flu vaccines have been given to millions of pregnant women, but the H1N1 pandemic of 2009 reinforced its value as a priority for prenatal care. Most of the women who became severely ill from the H1N1 virus were young and healthy, without co-existing conditions known to increase risk.7
It became clearer during the H1N1 pandemic that pregnancy itself — which is associated with physiologic changes such as decreased lung capacity, increased nasal congestion and changes in the immune system – is its own significant risk factor for severe illness from the influenza virus. This increased risk applies to COVID-19 as well.
As COVID-19 has become endemic, with hospitalizations and deaths not reaching the levels of previous surges — and with mask-wearing and other preventive measures having declined — patients understandably have become more complacent. Some patients are vaccine deniers, but in my practice, these patients are a much smaller group than those who believe COVID-19 “is no big deal,” especially if they have had infections recently.
This is why it’s important to actively listen to concerns and to ask patients who decline a vaccination why they are hesitant. Blanket messages about vaccine efficacy and safety are the first step, but individualized, more pointed conversations based on the patient’s personal experiences and beliefs have become increasingly important.
I routinely tell pregnant patients about the risks of COVID-19 and I explain that it has been difficult to predict who will develop severe illness. Sometimes more conversation is needed. For those who are still hesitant or who tell me they feel protected by a recent infection, for instance, I provide more detail on the unique risks of pregnancy — the fact that “pregnancy is different” — and that natural immunity wanes while the protection afforded by immunization is believed to last longer. Many women are also concerned about the safety of the COVID-19 vaccine, so having safety data at your fingertips is helpful. (See Table 2.)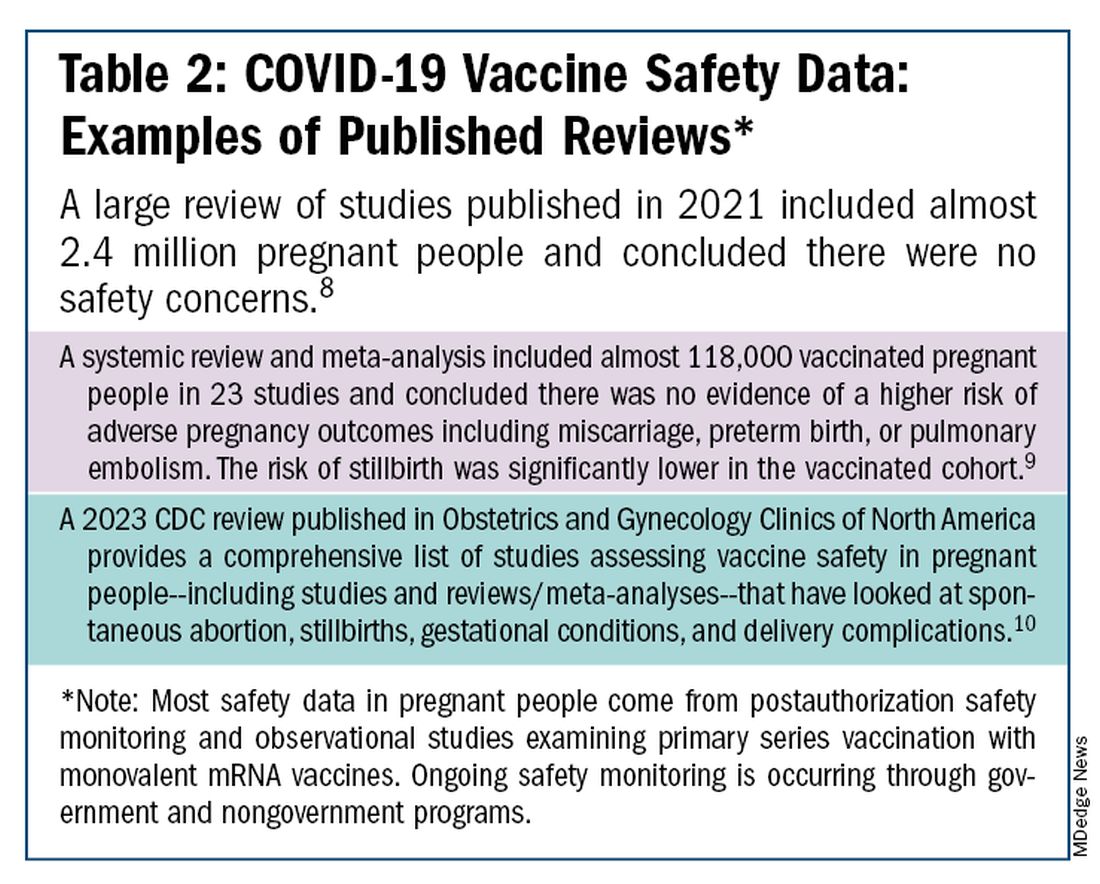
The fact that influenza and COVID-19 vaccination protect the newborn as well as the mother is something that I find is underappreciated by many patients. Explaining that infants likely benefit from the passage of antibodies across the placenta should be part of patient counseling.
Counseling About RSV Vaccination
Importantly, for the 2024-2025 RSV season, the maternal RSV vaccine (Abrysvo, Pfizer) is recommended only for pregnant women who did not receive the vaccine during the 2023-2024 season. When more research is done and more data are obtained showing how long the immune response persists post vaccination, it may be that the US Food and Drug Administration (FDA) will approve the maternal RSV vaccine for use in every pregnancy.
The later timing of the vaccination recommendation — 32-36 weeks’ gestation — reflects a conservative approach taken by the FDA in response to data from one of the pivotal trials showing a numerical trend toward more preterm deliveries among vaccinated compared with unvaccinated patients. This imbalance in the original trial, which administered the vaccine during 24-36 weeks of gestation, was seen only in low-income countries with no temporal association, however.
In our experience at two Weill Cornell Medical College–associated hospitals we did not see this trend. Our cohort study of almost 3000 pregnant patients who delivered at 32 weeks’ gestation or later found no increased risk of preterm birth among the 35% of patients who received the RSV vaccine during the 2023-2024 RSV season. We also did not see any difference in preeclampsia, in contrast with original trial data that showed a signal for increased risk.11
When fewer than 2 weeks have elapsed between maternal vaccination and delivery, the monoclonal antibody nirsevimab is recommended for the newborn — ideally before the newborn leaves the hospital. Nirsevimab is also recommended for newborns of mothers who decline vaccination or were not candidates (e.g. vaccinated in a previous pregnancy), or when there is concern about the adequacy of the maternal immune response to the vaccine (e.g. in cases of immunosuppression).
While there was a limited supply of the monoclonal antibody last year, limitations are not expected this year, especially after October.
The ultimate goal is that patients choose the vaccine or the immunoglobulin, given the severity of RSV disease. Patient preferences should be considered. However, given that it takes 2 weeks after vaccination for protection to build up, I stress to patients that if they’ve vaccinated themselves, their newborn will leave the hospital with protection. If nirsevimab is relied upon, I explain, their newborn may not be protected for some period of time.
Take-home Messages
- When patients decline or are hesitant about vaccines, ask why. Listen actively, and work to correct misperceptions and knowledge gaps.
- Whenever possible, offer vaccines in your practice. Vaccination rates drop when this does not occur.
- COVID-vaccine safety is backed by many studies showing no increase in birth defects, preterm delivery, miscarriage, or stillbirth.
- Pregnant women are more likely to have severe illness from the influenza and SARS-CoV-2 viruses. Vaccines can prevent severe illness and can protect the newborn as well as the mother.
- Recommend/administer the maternal RSV vaccine at 32-36 weeks’ gestation in women who did not receive the vaccine in the 2023-2024 season. If mothers aren’t eligible their babies should be offered nirsevimab.
Dr. Riley is the Given Foundation Professor and Chair of Obstetrics and Gynecology at Weill Cornell Medicine and the obstetrician and gynecologist-in-chief at New York Presbyterian Hospital. She disclosed that she has provided one-time consultations to Pfizer (Abrysvo RSV vaccine) and GSK (cytomegalovirus vaccine), and is providing consultant education on CMV for Moderna. She is chair of ACOG’s task force on immunization and emerging infectious diseases, serves on the medical advisory board for MAVEN, and serves as an editor or editorial board member for several medical publications.
References
1. ACOG Committee Opinion No. 741: Maternal Immunization. Obstet Gynecol. 2018;131(6):e214-e217.
2. Centers for Disease Control and Prevention. COVID-19 Vaccination for People Who are Pregnant or Breastfeeding. https://www.cdc.gov/covid/vaccines/pregnant-or-breastfeeding.html.
3. ACOG Practice Advisory on Maternal Respiratory Syncytial Virus Vaccination, September 2023. (Updated August 2024).4. Irving S et al. Open Forum Infect Dis. 2023;10(Suppl 2):ofad500.1002.
5. Flu Vaccination Dashboard, CDC, National Center for Immunization and Respiratory Diseases.
6. Weekly COVID-19 Vaccination Dashboard, CDC. https://www.cdc.gov/covidvaxview/weekly-dashboard/index.html
7. Louie JK et al. N Engl J Med. 2010;362:27-35. 8. Ciapponi A et al. Vaccine. 2021;39(40):5891-908.
9. Prasad S et al. Nature Communications. 2022;13:2414. 10. Fleming-Dutra KE et al. Obstet Gynecol Clin North Am 2023;50(2):279-97. 11. Mouen S et al. JAMA Network Open 2024;7(7):e2419268.
Editor’s Note: Sadly, this is the last column in the Master Class Obstetrics series. This award-winning column has been part of Ob.Gyn. News for 20 years. The deep discussion of cutting-edge topics in obstetrics by specialists and researchers will be missed as will the leadership and curation of topics by Dr. E. Albert Reece.
Introduction: The Need for Increased Vigilance About Maternal Immunization
Viruses are becoming increasingly prevalent in our world and the consequences of viral infections are implicated in a growing number of disease states. It is well established that certain cancers are caused by viruses and it is increasingly evident that viral infections can trigger the development of chronic illness. In pregnant women, viruses such as cytomegalovirus can cause infection in utero and lead to long-term impairments for the baby.
Likewise, it appears that the virulence of viruses is increasing, whether it be the respiratory syncytial virus (RSV) in children or the severe acute respiratory syndrome (SARS) coronaviruses in adults. Clearly, our environment is changing, with increases in population growth and urbanization, for instance, and an intensification of climate change and its effects. Viruses are part of this changing background.
Vaccines are our most powerful tool to protect people of all ages against viral threats, and fortunately, we benefit from increasing expertise in vaccinology. Since 1974, the University of Maryland School of Medicine has a Center for Vaccine Development and Global Health that has conducted research on vaccines to defend against the Zika virus, H1N1, Ebola, and SARS-CoV-2.
We’re not alone. Other vaccinology centers across the country — as well as the National Institutes of Health at the national level, through its National Institute of Allergy and Infectious Diseases — are doing research and developing vaccines to combat viral diseases.
In this column, we are focused on viral diseases in pregnancy and the role that vaccines can play in preventing serious respiratory illness in mothers and their newborns. I have invited Laura E. Riley, MD, the Given Foundation Professor and Chair of Obstetrics and Gynecology at Weill Cornell Medicine, to address the importance of maternal immunization and how we can best counsel our patients and improve immunization rates.
As Dr. Riley explains, we are in a new era, and it behooves us all to be more vigilant about recommending vaccines, combating misperceptions, addressing patients’ knowledge gaps, and administering vaccines whenever possible.
Dr. Reece is the former Dean of Medicine & University Executive VP, and The Distinguished University and Endowed Professor & Director of the Center for Advanced Research Training and Innovation (CARTI) at the University of Maryland School of Medicine, as well as senior scientist at the Center for Birth Defects Research.
The alarming decline in maternal immunization rates that occurred in the wake of the COVID-19 pandemic means that, now more than ever, we must fully embrace our responsibility to recommend immunizations in pregnancy and to communicate what is known about their efficacy and safety. Data show that vaccination rates drop when we do not offer vaccines in our offices, so whenever possible, we should administer them as well.
The ob.gyn. is the patient’s most trusted person in pregnancy. When patients decline or express hesitancy about vaccines, it is incumbent upon us to ask why. Oftentimes, we can identify areas in which patients lack knowledge or have misperceptions and we can successfully educate the patient or change their perspective or misunderstanding concerning the importance of vaccination for themselves and their babies. (See Table 1.) We can also successfully address concerns about safety.
The safety of COVID-19 vaccinations in pregnancy is now backed by several years of data from multiple studies showing no increase in birth defects, preterm delivery, miscarriage, or stillbirth.
Data also show that pregnant patients are more likely than patients who are not pregnant to need hospitalization and intensive care when infected with SARS-CoV-2 and are at risk of having complications that can affect pregnancy and the newborn, including preterm birth and stillbirth. Vaccination has been shown to reduce the risk of severe illness and the risk of such adverse obstetrical outcomes, in addition to providing protection for the infant early on.
Similarly, influenza has long been more likely to be severe in pregnant patients, with an increased risk of poor obstetrical outcomes. Vaccines similarly provide “two for one protection,” protecting both mother and baby, and are, of course, backed by many years of safety and efficacy data.
With the new maternal respiratory syncytial virus (RSV) vaccine, now in its second year of availability, the goal is to protect the baby from RSV-caused serious lower respiratory tract illness. The illness has contributed to tens of thousands of annual hospitalizations and up to several hundred deaths every year in children younger than 5 years — particularly in those under age 6 months.
The RSV monoclonal antibody nirsevimab is available for the newborn as an alternative to maternal immunization but the maternal vaccine is optimal in that it will provide immediate rather than delayed protection for the newborn. The maternal vaccine is recommended during weeks 32-36 of pregnancy in mothers who were not vaccinated during last year’s RSV season. With real-world experience from year one, the available safety data are reassuring.
Counseling About Influenza and COVID-19 Vaccination
The COVID-19 pandemic took a toll on vaccination interest/receptivity broadly in pregnant and nonpregnant people. Among pregnant individuals, influenza vaccination coverage declined from 71% in the 2019-2020 influenza season to 56% in the 2021-2022 season, according to data from the Centers for Disease Control and Prevention’s Vaccine Safety Datalink.4 Coverage for the 2022-2023 and 2023-2024 influenza seasons was even worse: well under 50%.5
Fewer pregnant women have received updated COVID-19 vaccines. Only 13% of pregnant persons overall received the updated 2023-2024 COVID-19 booster vaccine (through March 30, 2024), according to the CDC.6
Maternal immunization for influenza has been recommended in the United States since 2004 (part of the recommendation that everyone over the age of 6 months receive an annual flu vaccine), and flu vaccines have been given to millions of pregnant women, but the H1N1 pandemic of 2009 reinforced its value as a priority for prenatal care. Most of the women who became severely ill from the H1N1 virus were young and healthy, without co-existing conditions known to increase risk.7
It became clearer during the H1N1 pandemic that pregnancy itself — which is associated with physiologic changes such as decreased lung capacity, increased nasal congestion and changes in the immune system – is its own significant risk factor for severe illness from the influenza virus. This increased risk applies to COVID-19 as well.
As COVID-19 has become endemic, with hospitalizations and deaths not reaching the levels of previous surges — and with mask-wearing and other preventive measures having declined — patients understandably have become more complacent. Some patients are vaccine deniers, but in my practice, these patients are a much smaller group than those who believe COVID-19 “is no big deal,” especially if they have had infections recently.
This is why it’s important to actively listen to concerns and to ask patients who decline a vaccination why they are hesitant. Blanket messages about vaccine efficacy and safety are the first step, but individualized, more pointed conversations based on the patient’s personal experiences and beliefs have become increasingly important.
I routinely tell pregnant patients about the risks of COVID-19 and I explain that it has been difficult to predict who will develop severe illness. Sometimes more conversation is needed. For those who are still hesitant or who tell me they feel protected by a recent infection, for instance, I provide more detail on the unique risks of pregnancy — the fact that “pregnancy is different” — and that natural immunity wanes while the protection afforded by immunization is believed to last longer. Many women are also concerned about the safety of the COVID-19 vaccine, so having safety data at your fingertips is helpful. (See Table 2.)
The fact that influenza and COVID-19 vaccination protect the newborn as well as the mother is something that I find is underappreciated by many patients. Explaining that infants likely benefit from the passage of antibodies across the placenta should be part of patient counseling.
Counseling About RSV Vaccination
Importantly, for the 2024-2025 RSV season, the maternal RSV vaccine (Abrysvo, Pfizer) is recommended only for pregnant women who did not receive the vaccine during the 2023-2024 season. When more research is done and more data are obtained showing how long the immune response persists post vaccination, it may be that the US Food and Drug Administration (FDA) will approve the maternal RSV vaccine for use in every pregnancy.
The later timing of the vaccination recommendation — 32-36 weeks’ gestation — reflects a conservative approach taken by the FDA in response to data from one of the pivotal trials showing a numerical trend toward more preterm deliveries among vaccinated compared with unvaccinated patients. This imbalance in the original trial, which administered the vaccine during 24-36 weeks of gestation, was seen only in low-income countries with no temporal association, however.
In our experience at two Weill Cornell Medical College–associated hospitals we did not see this trend. Our cohort study of almost 3000 pregnant patients who delivered at 32 weeks’ gestation or later found no increased risk of preterm birth among the 35% of patients who received the RSV vaccine during the 2023-2024 RSV season. We also did not see any difference in preeclampsia, in contrast with original trial data that showed a signal for increased risk.11
When fewer than 2 weeks have elapsed between maternal vaccination and delivery, the monoclonal antibody nirsevimab is recommended for the newborn — ideally before the newborn leaves the hospital. Nirsevimab is also recommended for newborns of mothers who decline vaccination or were not candidates (e.g. vaccinated in a previous pregnancy), or when there is concern about the adequacy of the maternal immune response to the vaccine (e.g. in cases of immunosuppression).
While there was a limited supply of the monoclonal antibody last year, limitations are not expected this year, especially after October.
The ultimate goal is that patients choose the vaccine or the immunoglobulin, given the severity of RSV disease. Patient preferences should be considered. However, given that it takes 2 weeks after vaccination for protection to build up, I stress to patients that if they’ve vaccinated themselves, their newborn will leave the hospital with protection. If nirsevimab is relied upon, I explain, their newborn may not be protected for some period of time.
Take-home Messages
- When patients decline or are hesitant about vaccines, ask why. Listen actively, and work to correct misperceptions and knowledge gaps.
- Whenever possible, offer vaccines in your practice. Vaccination rates drop when this does not occur.
- COVID-vaccine safety is backed by many studies showing no increase in birth defects, preterm delivery, miscarriage, or stillbirth.
- Pregnant women are more likely to have severe illness from the influenza and SARS-CoV-2 viruses. Vaccines can prevent severe illness and can protect the newborn as well as the mother.
- Recommend/administer the maternal RSV vaccine at 32-36 weeks’ gestation in women who did not receive the vaccine in the 2023-2024 season. If mothers aren’t eligible their babies should be offered nirsevimab.
Dr. Riley is the Given Foundation Professor and Chair of Obstetrics and Gynecology at Weill Cornell Medicine and the obstetrician and gynecologist-in-chief at New York Presbyterian Hospital. She disclosed that she has provided one-time consultations to Pfizer (Abrysvo RSV vaccine) and GSK (cytomegalovirus vaccine), and is providing consultant education on CMV for Moderna. She is chair of ACOG’s task force on immunization and emerging infectious diseases, serves on the medical advisory board for MAVEN, and serves as an editor or editorial board member for several medical publications.
References
1. ACOG Committee Opinion No. 741: Maternal Immunization. Obstet Gynecol. 2018;131(6):e214-e217.
2. Centers for Disease Control and Prevention. COVID-19 Vaccination for People Who are Pregnant or Breastfeeding. https://www.cdc.gov/covid/vaccines/pregnant-or-breastfeeding.html.
3. ACOG Practice Advisory on Maternal Respiratory Syncytial Virus Vaccination, September 2023. (Updated August 2024).4. Irving S et al. Open Forum Infect Dis. 2023;10(Suppl 2):ofad500.1002.
5. Flu Vaccination Dashboard, CDC, National Center for Immunization and Respiratory Diseases.
6. Weekly COVID-19 Vaccination Dashboard, CDC. https://www.cdc.gov/covidvaxview/weekly-dashboard/index.html
7. Louie JK et al. N Engl J Med. 2010;362:27-35. 8. Ciapponi A et al. Vaccine. 2021;39(40):5891-908.
9. Prasad S et al. Nature Communications. 2022;13:2414. 10. Fleming-Dutra KE et al. Obstet Gynecol Clin North Am 2023;50(2):279-97. 11. Mouen S et al. JAMA Network Open 2024;7(7):e2419268.
Editor’s Note: Sadly, this is the last column in the Master Class Obstetrics series. This award-winning column has been part of Ob.Gyn. News for 20 years. The deep discussion of cutting-edge topics in obstetrics by specialists and researchers will be missed as will the leadership and curation of topics by Dr. E. Albert Reece.
Introduction: The Need for Increased Vigilance About Maternal Immunization
Viruses are becoming increasingly prevalent in our world and the consequences of viral infections are implicated in a growing number of disease states. It is well established that certain cancers are caused by viruses and it is increasingly evident that viral infections can trigger the development of chronic illness. In pregnant women, viruses such as cytomegalovirus can cause infection in utero and lead to long-term impairments for the baby.
Likewise, it appears that the virulence of viruses is increasing, whether it be the respiratory syncytial virus (RSV) in children or the severe acute respiratory syndrome (SARS) coronaviruses in adults. Clearly, our environment is changing, with increases in population growth and urbanization, for instance, and an intensification of climate change and its effects. Viruses are part of this changing background.
Vaccines are our most powerful tool to protect people of all ages against viral threats, and fortunately, we benefit from increasing expertise in vaccinology. Since 1974, the University of Maryland School of Medicine has a Center for Vaccine Development and Global Health that has conducted research on vaccines to defend against the Zika virus, H1N1, Ebola, and SARS-CoV-2.
We’re not alone. Other vaccinology centers across the country — as well as the National Institutes of Health at the national level, through its National Institute of Allergy and Infectious Diseases — are doing research and developing vaccines to combat viral diseases.
In this column, we are focused on viral diseases in pregnancy and the role that vaccines can play in preventing serious respiratory illness in mothers and their newborns. I have invited Laura E. Riley, MD, the Given Foundation Professor and Chair of Obstetrics and Gynecology at Weill Cornell Medicine, to address the importance of maternal immunization and how we can best counsel our patients and improve immunization rates.
As Dr. Riley explains, we are in a new era, and it behooves us all to be more vigilant about recommending vaccines, combating misperceptions, addressing patients’ knowledge gaps, and administering vaccines whenever possible.
Dr. Reece is the former Dean of Medicine & University Executive VP, and The Distinguished University and Endowed Professor & Director of the Center for Advanced Research Training and Innovation (CARTI) at the University of Maryland School of Medicine, as well as senior scientist at the Center for Birth Defects Research.
The alarming decline in maternal immunization rates that occurred in the wake of the COVID-19 pandemic means that, now more than ever, we must fully embrace our responsibility to recommend immunizations in pregnancy and to communicate what is known about their efficacy and safety. Data show that vaccination rates drop when we do not offer vaccines in our offices, so whenever possible, we should administer them as well.
The ob.gyn. is the patient’s most trusted person in pregnancy. When patients decline or express hesitancy about vaccines, it is incumbent upon us to ask why. Oftentimes, we can identify areas in which patients lack knowledge or have misperceptions and we can successfully educate the patient or change their perspective or misunderstanding concerning the importance of vaccination for themselves and their babies. (See Table 1.) We can also successfully address concerns about safety.
The safety of COVID-19 vaccinations in pregnancy is now backed by several years of data from multiple studies showing no increase in birth defects, preterm delivery, miscarriage, or stillbirth.
Data also show that pregnant patients are more likely than patients who are not pregnant to need hospitalization and intensive care when infected with SARS-CoV-2 and are at risk of having complications that can affect pregnancy and the newborn, including preterm birth and stillbirth. Vaccination has been shown to reduce the risk of severe illness and the risk of such adverse obstetrical outcomes, in addition to providing protection for the infant early on.
Similarly, influenza has long been more likely to be severe in pregnant patients, with an increased risk of poor obstetrical outcomes. Vaccines similarly provide “two for one protection,” protecting both mother and baby, and are, of course, backed by many years of safety and efficacy data.
With the new maternal respiratory syncytial virus (RSV) vaccine, now in its second year of availability, the goal is to protect the baby from RSV-caused serious lower respiratory tract illness. The illness has contributed to tens of thousands of annual hospitalizations and up to several hundred deaths every year in children younger than 5 years — particularly in those under age 6 months.
The RSV monoclonal antibody nirsevimab is available for the newborn as an alternative to maternal immunization but the maternal vaccine is optimal in that it will provide immediate rather than delayed protection for the newborn. The maternal vaccine is recommended during weeks 32-36 of pregnancy in mothers who were not vaccinated during last year’s RSV season. With real-world experience from year one, the available safety data are reassuring.
Counseling About Influenza and COVID-19 Vaccination
The COVID-19 pandemic took a toll on vaccination interest/receptivity broadly in pregnant and nonpregnant people. Among pregnant individuals, influenza vaccination coverage declined from 71% in the 2019-2020 influenza season to 56% in the 2021-2022 season, according to data from the Centers for Disease Control and Prevention’s Vaccine Safety Datalink.4 Coverage for the 2022-2023 and 2023-2024 influenza seasons was even worse: well under 50%.5
Fewer pregnant women have received updated COVID-19 vaccines. Only 13% of pregnant persons overall received the updated 2023-2024 COVID-19 booster vaccine (through March 30, 2024), according to the CDC.6
Maternal immunization for influenza has been recommended in the United States since 2004 (part of the recommendation that everyone over the age of 6 months receive an annual flu vaccine), and flu vaccines have been given to millions of pregnant women, but the H1N1 pandemic of 2009 reinforced its value as a priority for prenatal care. Most of the women who became severely ill from the H1N1 virus were young and healthy, without co-existing conditions known to increase risk.7
It became clearer during the H1N1 pandemic that pregnancy itself — which is associated with physiologic changes such as decreased lung capacity, increased nasal congestion and changes in the immune system – is its own significant risk factor for severe illness from the influenza virus. This increased risk applies to COVID-19 as well.
As COVID-19 has become endemic, with hospitalizations and deaths not reaching the levels of previous surges — and with mask-wearing and other preventive measures having declined — patients understandably have become more complacent. Some patients are vaccine deniers, but in my practice, these patients are a much smaller group than those who believe COVID-19 “is no big deal,” especially if they have had infections recently.
This is why it’s important to actively listen to concerns and to ask patients who decline a vaccination why they are hesitant. Blanket messages about vaccine efficacy and safety are the first step, but individualized, more pointed conversations based on the patient’s personal experiences and beliefs have become increasingly important.
I routinely tell pregnant patients about the risks of COVID-19 and I explain that it has been difficult to predict who will develop severe illness. Sometimes more conversation is needed. For those who are still hesitant or who tell me they feel protected by a recent infection, for instance, I provide more detail on the unique risks of pregnancy — the fact that “pregnancy is different” — and that natural immunity wanes while the protection afforded by immunization is believed to last longer. Many women are also concerned about the safety of the COVID-19 vaccine, so having safety data at your fingertips is helpful. (See Table 2.)
The fact that influenza and COVID-19 vaccination protect the newborn as well as the mother is something that I find is underappreciated by many patients. Explaining that infants likely benefit from the passage of antibodies across the placenta should be part of patient counseling.
Counseling About RSV Vaccination
Importantly, for the 2024-2025 RSV season, the maternal RSV vaccine (Abrysvo, Pfizer) is recommended only for pregnant women who did not receive the vaccine during the 2023-2024 season. When more research is done and more data are obtained showing how long the immune response persists post vaccination, it may be that the US Food and Drug Administration (FDA) will approve the maternal RSV vaccine for use in every pregnancy.
The later timing of the vaccination recommendation — 32-36 weeks’ gestation — reflects a conservative approach taken by the FDA in response to data from one of the pivotal trials showing a numerical trend toward more preterm deliveries among vaccinated compared with unvaccinated patients. This imbalance in the original trial, which administered the vaccine during 24-36 weeks of gestation, was seen only in low-income countries with no temporal association, however.
In our experience at two Weill Cornell Medical College–associated hospitals we did not see this trend. Our cohort study of almost 3000 pregnant patients who delivered at 32 weeks’ gestation or later found no increased risk of preterm birth among the 35% of patients who received the RSV vaccine during the 2023-2024 RSV season. We also did not see any difference in preeclampsia, in contrast with original trial data that showed a signal for increased risk.11
When fewer than 2 weeks have elapsed between maternal vaccination and delivery, the monoclonal antibody nirsevimab is recommended for the newborn — ideally before the newborn leaves the hospital. Nirsevimab is also recommended for newborns of mothers who decline vaccination or were not candidates (e.g. vaccinated in a previous pregnancy), or when there is concern about the adequacy of the maternal immune response to the vaccine (e.g. in cases of immunosuppression).
While there was a limited supply of the monoclonal antibody last year, limitations are not expected this year, especially after October.
The ultimate goal is that patients choose the vaccine or the immunoglobulin, given the severity of RSV disease. Patient preferences should be considered. However, given that it takes 2 weeks after vaccination for protection to build up, I stress to patients that if they’ve vaccinated themselves, their newborn will leave the hospital with protection. If nirsevimab is relied upon, I explain, their newborn may not be protected for some period of time.
Take-home Messages
- When patients decline or are hesitant about vaccines, ask why. Listen actively, and work to correct misperceptions and knowledge gaps.
- Whenever possible, offer vaccines in your practice. Vaccination rates drop when this does not occur.
- COVID-vaccine safety is backed by many studies showing no increase in birth defects, preterm delivery, miscarriage, or stillbirth.
- Pregnant women are more likely to have severe illness from the influenza and SARS-CoV-2 viruses. Vaccines can prevent severe illness and can protect the newborn as well as the mother.
- Recommend/administer the maternal RSV vaccine at 32-36 weeks’ gestation in women who did not receive the vaccine in the 2023-2024 season. If mothers aren’t eligible their babies should be offered nirsevimab.
Dr. Riley is the Given Foundation Professor and Chair of Obstetrics and Gynecology at Weill Cornell Medicine and the obstetrician and gynecologist-in-chief at New York Presbyterian Hospital. She disclosed that she has provided one-time consultations to Pfizer (Abrysvo RSV vaccine) and GSK (cytomegalovirus vaccine), and is providing consultant education on CMV for Moderna. She is chair of ACOG’s task force on immunization and emerging infectious diseases, serves on the medical advisory board for MAVEN, and serves as an editor or editorial board member for several medical publications.
References
1. ACOG Committee Opinion No. 741: Maternal Immunization. Obstet Gynecol. 2018;131(6):e214-e217.
2. Centers for Disease Control and Prevention. COVID-19 Vaccination for People Who are Pregnant or Breastfeeding. https://www.cdc.gov/covid/vaccines/pregnant-or-breastfeeding.html.
3. ACOG Practice Advisory on Maternal Respiratory Syncytial Virus Vaccination, September 2023. (Updated August 2024).4. Irving S et al. Open Forum Infect Dis. 2023;10(Suppl 2):ofad500.1002.
5. Flu Vaccination Dashboard, CDC, National Center for Immunization and Respiratory Diseases.
6. Weekly COVID-19 Vaccination Dashboard, CDC. https://www.cdc.gov/covidvaxview/weekly-dashboard/index.html
7. Louie JK et al. N Engl J Med. 2010;362:27-35. 8. Ciapponi A et al. Vaccine. 2021;39(40):5891-908.
9. Prasad S et al. Nature Communications. 2022;13:2414. 10. Fleming-Dutra KE et al. Obstet Gynecol Clin North Am 2023;50(2):279-97. 11. Mouen S et al. JAMA Network Open 2024;7(7):e2419268.
SAFE: Ensuring Access for Children With Neurodevelopmental Disabilities
We pediatricians consider ourselves as compassionate professionals, optimistic about the potential of all children. This is reflected in the American Academy of Pediatrics’ equity statement of “its mission to ensure the health and well-being of all children. This includes promoting nurturing, inclusive environments and actively opposing intolerance, bigotry, bias, and discrimination.”
A committee of the Developmental Behavioral Pediatric Network developed and published a consensus statement specifically about problems in the care of individuals with neurodevelopmental disabilities (NDD) called the Supporting Access for Everyone (SAFE) initiative. All of us care for children with NDD as one in six are affected with these conditions that impact cognition, communication, motor, social, and/or behavior skills such as autism, ADHD, intellectual disabilities (ID), learning disorders, hearing or vision impairment, and motor disabilities such as cerebral palsy. Children with NDD are overrepresented in our daily practice schedule due to their multiple medical, behavioral, and social needs. NDD are also more common among marginalized children with racial, ethnic, sexual, or gender identity minority status compounding their difficulties in accessing quality care.
NDD present similar challenges to care as other chronic conditions that also require longer visits, more documentation, long-term monitoring, team-based care, care coordination, and often referrals. But most chronic medical conditions we care for such as asthma, diabetes, cancer, hypertension, and renal disease have clear national guidelines and appropriate billing codes and are not stigmatizing. Most also do not intrinsically affect the nervous system or cause disability as for NDD that alter the behavioral presentation of the individual in a way that changes their care.
Discrimination against individuals with NDD and other disabilities, called “ableism,” can take many forms: assuming a child with communication difficulty or ID is unable to understand explanations about their care; the presence of one NDD condition ending the clinician’s search for other issues; complicated problems or difficult behaviors in the medical setting truncating care, etc.
Adjustments Needed for Special Needs
As pediatricians we already adjust our interactions, starting instinctively, to the development level of the child we perceive before us. We approach infants slowly and softly, we speak in shorter sentences to toddlers, we joke around with school-aged children, and we take extra care about privacy with teens. This serves the relationships well for neurotypical children. But our (and our staff’s) perceptions of children with autism, ID, genetic syndromes that include NDD, or motor disabilities based on their behavioral presentation may not accurately recognize or accommodate their abilities or needs. Communication and environmental adjustments may need to be much more individualized to provide respectful care, comfort and even safety.
As an example, at this time 1 in 36 children have autism with or without ID. Defining features of autism include differences in social communication, repetitive or restrictive interests or behaviors, and hypersensitivity to the environment plus any coexisting conditions such as anxiety and hyperactivity. But most children with autism have completely age appropriate and typical physical appearance and their underlying condition may not even be known. The office setting, without special attention to the needs of a child with autism, may be frightening, loud, too bright, too crowded, fast paced, and confusing. The result of their sensitivities and difficulty communicating may lead to increased agitation, repetitive behaviors (sometimes called “stimming”), shrieking, attempts to escape the room, refusal to allow for vital signs or undressing, even aggression. Strategies for calming a neurotypical child such as talking or touching may make matters worse instead of better. We need help from the child and family and a plan to optimize their medical encounters.
If not adequately accommodated, children with many varieties of NDD end up not getting all the routine healthcare they need (eg vaccinations, blood tests, vital signs, even complete physical exams including dental) as well as having more adverse events during health care, including traumatizing seclusion, not allowing a support person to be present, restraint, injuries, and accidents. When more complex procedures are needed, eg x-ray, MRI, EEG, lab studies, or surgery, successful outcomes may be lower. Children with NDD have higher rates of often avoidable morbidity and mortality than those without, in part due to these barriers to complete care. While environmental accommodations to wheelchair users for accessibility has greatly improved in recent years, access to other kinds of individualized accommodations have lagged behind.
Accommodation Planning
There are a variety of factors that need to be taken into consideration in accommodating an individual with NDD. The family becomes the expert, along with the child, in knowing the child’s triggers, preferences, abilities, and level of understanding to accept and consent for care. An accommodation plan should be created using shared and supported decision making with the family and child and allowing for child preferences, regardless of their ability level, whenever possible. Development of an accommodation plan may benefit from multidisciplinary input, eg psychology, physical therapy, speech pathology, depending on the child’s needs and the practice’s ability to adapt.
The SAFE initiative is in the process of creating a checklist aiming to facilitate a description being created for each individual to help plan for a successful medical encounter while optimizing the child’s comfort, participation, and safety. While the checklist is not yet ready, we can start now by asking families and children in preparation for or at the start of a visit about their needs and writing a shared document that can also be placed in the electronic health record for the entire care team for informing care going forward.
It is especially important for the family to keep a copy of the care plan and for it to be sent as part of referrals for procedures or specialty visits so that the professionals can prepare and adapt the encounter. An excellent example is a how some hospitals schedule a practice visit for the child to experience the sights and sounds and people the child will encounter, for example, before an EEG, when nothing is required of the child. Scheduling the actual procedure at times of day when clinics are less crowded and wait times are shorter can improve the chances of success.
Some categories and details that might be included in an accommodation plan are listed below:
You might start the plan with the child’s preferred name/nickname, family member or support person names, and diagnoses along with a brief overview of the child’s level of functioning. Then list categories of needs and preferences along with suggestions or requests.
- Motor: Does the child have or need assistance entering the building, visit room, bathroom, or transferring to the exam table? What kind of assistance, if any, and by whom?
- Sensory: Is the child disturbed by noise, lights, or being touched? Does the child want to use equipment to be comfortable such as headphones, earplugs, or sunglasses or need a quiet room, care without perfumes, or dimmed lighting? Does the child typically refuse aspects of the physical examination?
- Behavioral regulation: What helps the child to stay calm? Are there certain triggers to becoming upset? Are there early cues that an upset is coming? What and who can help in the case of an upset?
- Habits/preferences: Are there certain comfort objects or habits your child needs? Are there habits your child needs to do, such as a certain order of events, or use of social stories or pictures, to cooperate or feel comfortable?
- Communication: How does the child make his/her needs known? Does the child/family speak English or another language? Does he/she use sign language or an augmentative communication device? What level of understanding does your child have; for example, similar to what age for a typical child? Is there a care plan with accommodations already available that needs review or needs revision with the child’s development or is a new one needed? Was the care plan developed including the child’s participation and assent or is more collaboration needed?
- History: Has your child had any very upsetting experiences in healthcare settings? What happened? Has the trauma been addressed? Are there reminders of the trauma that should be avoided?
- Other: Are there other things we should know about your child as an individual to provide the best care?
There are many actions needed to do better at ensuring equitable care for individuals with NDD. We should educate our office and medical staff about NDD in children and the importance of accommodating their needs, and ways to do it. The morning huddle can be used to remind staff of upcoming visits of children who may need accommodations. We then need to use quality improvement methods to check in periodically on how the changes are working for the children, families, and practice in order to continually improve.
The overall healthcare system also needs to change. Billing codes should reflect the time, complexity of accommodations, and documentation that were required for care. Episodes of the visit may need to be broken up within the day or over several days to allow the child to practice, calm down, and cooperate and this should be accounted for in billing. Given that NDD are generally lifelong conditions, payment systems that require measures of progress such as value-based payment based on improved outcomes will need to be adjusted to measure quality of care rather than significant progress.
We need to advocate for both individual children and for system changes to work toward equity of care for those with disabilities to make their lives more comfortable as well as ours.
Dr. Howard is assistant professor of pediatrics at The Johns Hopkins University School of Medicine, Baltimore, and creator of CHADIS. She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
We pediatricians consider ourselves as compassionate professionals, optimistic about the potential of all children. This is reflected in the American Academy of Pediatrics’ equity statement of “its mission to ensure the health and well-being of all children. This includes promoting nurturing, inclusive environments and actively opposing intolerance, bigotry, bias, and discrimination.”
A committee of the Developmental Behavioral Pediatric Network developed and published a consensus statement specifically about problems in the care of individuals with neurodevelopmental disabilities (NDD) called the Supporting Access for Everyone (SAFE) initiative. All of us care for children with NDD as one in six are affected with these conditions that impact cognition, communication, motor, social, and/or behavior skills such as autism, ADHD, intellectual disabilities (ID), learning disorders, hearing or vision impairment, and motor disabilities such as cerebral palsy. Children with NDD are overrepresented in our daily practice schedule due to their multiple medical, behavioral, and social needs. NDD are also more common among marginalized children with racial, ethnic, sexual, or gender identity minority status compounding their difficulties in accessing quality care.
NDD present similar challenges to care as other chronic conditions that also require longer visits, more documentation, long-term monitoring, team-based care, care coordination, and often referrals. But most chronic medical conditions we care for such as asthma, diabetes, cancer, hypertension, and renal disease have clear national guidelines and appropriate billing codes and are not stigmatizing. Most also do not intrinsically affect the nervous system or cause disability as for NDD that alter the behavioral presentation of the individual in a way that changes their care.
Discrimination against individuals with NDD and other disabilities, called “ableism,” can take many forms: assuming a child with communication difficulty or ID is unable to understand explanations about their care; the presence of one NDD condition ending the clinician’s search for other issues; complicated problems or difficult behaviors in the medical setting truncating care, etc.
Adjustments Needed for Special Needs
As pediatricians we already adjust our interactions, starting instinctively, to the development level of the child we perceive before us. We approach infants slowly and softly, we speak in shorter sentences to toddlers, we joke around with school-aged children, and we take extra care about privacy with teens. This serves the relationships well for neurotypical children. But our (and our staff’s) perceptions of children with autism, ID, genetic syndromes that include NDD, or motor disabilities based on their behavioral presentation may not accurately recognize or accommodate their abilities or needs. Communication and environmental adjustments may need to be much more individualized to provide respectful care, comfort and even safety.
As an example, at this time 1 in 36 children have autism with or without ID. Defining features of autism include differences in social communication, repetitive or restrictive interests or behaviors, and hypersensitivity to the environment plus any coexisting conditions such as anxiety and hyperactivity. But most children with autism have completely age appropriate and typical physical appearance and their underlying condition may not even be known. The office setting, without special attention to the needs of a child with autism, may be frightening, loud, too bright, too crowded, fast paced, and confusing. The result of their sensitivities and difficulty communicating may lead to increased agitation, repetitive behaviors (sometimes called “stimming”), shrieking, attempts to escape the room, refusal to allow for vital signs or undressing, even aggression. Strategies for calming a neurotypical child such as talking or touching may make matters worse instead of better. We need help from the child and family and a plan to optimize their medical encounters.
If not adequately accommodated, children with many varieties of NDD end up not getting all the routine healthcare they need (eg vaccinations, blood tests, vital signs, even complete physical exams including dental) as well as having more adverse events during health care, including traumatizing seclusion, not allowing a support person to be present, restraint, injuries, and accidents. When more complex procedures are needed, eg x-ray, MRI, EEG, lab studies, or surgery, successful outcomes may be lower. Children with NDD have higher rates of often avoidable morbidity and mortality than those without, in part due to these barriers to complete care. While environmental accommodations to wheelchair users for accessibility has greatly improved in recent years, access to other kinds of individualized accommodations have lagged behind.
Accommodation Planning
There are a variety of factors that need to be taken into consideration in accommodating an individual with NDD. The family becomes the expert, along with the child, in knowing the child’s triggers, preferences, abilities, and level of understanding to accept and consent for care. An accommodation plan should be created using shared and supported decision making with the family and child and allowing for child preferences, regardless of their ability level, whenever possible. Development of an accommodation plan may benefit from multidisciplinary input, eg psychology, physical therapy, speech pathology, depending on the child’s needs and the practice’s ability to adapt.
The SAFE initiative is in the process of creating a checklist aiming to facilitate a description being created for each individual to help plan for a successful medical encounter while optimizing the child’s comfort, participation, and safety. While the checklist is not yet ready, we can start now by asking families and children in preparation for or at the start of a visit about their needs and writing a shared document that can also be placed in the electronic health record for the entire care team for informing care going forward.
It is especially important for the family to keep a copy of the care plan and for it to be sent as part of referrals for procedures or specialty visits so that the professionals can prepare and adapt the encounter. An excellent example is a how some hospitals schedule a practice visit for the child to experience the sights and sounds and people the child will encounter, for example, before an EEG, when nothing is required of the child. Scheduling the actual procedure at times of day when clinics are less crowded and wait times are shorter can improve the chances of success.
Some categories and details that might be included in an accommodation plan are listed below:
You might start the plan with the child’s preferred name/nickname, family member or support person names, and diagnoses along with a brief overview of the child’s level of functioning. Then list categories of needs and preferences along with suggestions or requests.
- Motor: Does the child have or need assistance entering the building, visit room, bathroom, or transferring to the exam table? What kind of assistance, if any, and by whom?
- Sensory: Is the child disturbed by noise, lights, or being touched? Does the child want to use equipment to be comfortable such as headphones, earplugs, or sunglasses or need a quiet room, care without perfumes, or dimmed lighting? Does the child typically refuse aspects of the physical examination?
- Behavioral regulation: What helps the child to stay calm? Are there certain triggers to becoming upset? Are there early cues that an upset is coming? What and who can help in the case of an upset?
- Habits/preferences: Are there certain comfort objects or habits your child needs? Are there habits your child needs to do, such as a certain order of events, or use of social stories or pictures, to cooperate or feel comfortable?
- Communication: How does the child make his/her needs known? Does the child/family speak English or another language? Does he/she use sign language or an augmentative communication device? What level of understanding does your child have; for example, similar to what age for a typical child? Is there a care plan with accommodations already available that needs review or needs revision with the child’s development or is a new one needed? Was the care plan developed including the child’s participation and assent or is more collaboration needed?
- History: Has your child had any very upsetting experiences in healthcare settings? What happened? Has the trauma been addressed? Are there reminders of the trauma that should be avoided?
- Other: Are there other things we should know about your child as an individual to provide the best care?
There are many actions needed to do better at ensuring equitable care for individuals with NDD. We should educate our office and medical staff about NDD in children and the importance of accommodating their needs, and ways to do it. The morning huddle can be used to remind staff of upcoming visits of children who may need accommodations. We then need to use quality improvement methods to check in periodically on how the changes are working for the children, families, and practice in order to continually improve.
The overall healthcare system also needs to change. Billing codes should reflect the time, complexity of accommodations, and documentation that were required for care. Episodes of the visit may need to be broken up within the day or over several days to allow the child to practice, calm down, and cooperate and this should be accounted for in billing. Given that NDD are generally lifelong conditions, payment systems that require measures of progress such as value-based payment based on improved outcomes will need to be adjusted to measure quality of care rather than significant progress.
We need to advocate for both individual children and for system changes to work toward equity of care for those with disabilities to make their lives more comfortable as well as ours.
Dr. Howard is assistant professor of pediatrics at The Johns Hopkins University School of Medicine, Baltimore, and creator of CHADIS. She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
We pediatricians consider ourselves as compassionate professionals, optimistic about the potential of all children. This is reflected in the American Academy of Pediatrics’ equity statement of “its mission to ensure the health and well-being of all children. This includes promoting nurturing, inclusive environments and actively opposing intolerance, bigotry, bias, and discrimination.”
A committee of the Developmental Behavioral Pediatric Network developed and published a consensus statement specifically about problems in the care of individuals with neurodevelopmental disabilities (NDD) called the Supporting Access for Everyone (SAFE) initiative. All of us care for children with NDD as one in six are affected with these conditions that impact cognition, communication, motor, social, and/or behavior skills such as autism, ADHD, intellectual disabilities (ID), learning disorders, hearing or vision impairment, and motor disabilities such as cerebral palsy. Children with NDD are overrepresented in our daily practice schedule due to their multiple medical, behavioral, and social needs. NDD are also more common among marginalized children with racial, ethnic, sexual, or gender identity minority status compounding their difficulties in accessing quality care.
NDD present similar challenges to care as other chronic conditions that also require longer visits, more documentation, long-term monitoring, team-based care, care coordination, and often referrals. But most chronic medical conditions we care for such as asthma, diabetes, cancer, hypertension, and renal disease have clear national guidelines and appropriate billing codes and are not stigmatizing. Most also do not intrinsically affect the nervous system or cause disability as for NDD that alter the behavioral presentation of the individual in a way that changes their care.
Discrimination against individuals with NDD and other disabilities, called “ableism,” can take many forms: assuming a child with communication difficulty or ID is unable to understand explanations about their care; the presence of one NDD condition ending the clinician’s search for other issues; complicated problems or difficult behaviors in the medical setting truncating care, etc.
Adjustments Needed for Special Needs
As pediatricians we already adjust our interactions, starting instinctively, to the development level of the child we perceive before us. We approach infants slowly and softly, we speak in shorter sentences to toddlers, we joke around with school-aged children, and we take extra care about privacy with teens. This serves the relationships well for neurotypical children. But our (and our staff’s) perceptions of children with autism, ID, genetic syndromes that include NDD, or motor disabilities based on their behavioral presentation may not accurately recognize or accommodate their abilities or needs. Communication and environmental adjustments may need to be much more individualized to provide respectful care, comfort and even safety.
As an example, at this time 1 in 36 children have autism with or without ID. Defining features of autism include differences in social communication, repetitive or restrictive interests or behaviors, and hypersensitivity to the environment plus any coexisting conditions such as anxiety and hyperactivity. But most children with autism have completely age appropriate and typical physical appearance and their underlying condition may not even be known. The office setting, without special attention to the needs of a child with autism, may be frightening, loud, too bright, too crowded, fast paced, and confusing. The result of their sensitivities and difficulty communicating may lead to increased agitation, repetitive behaviors (sometimes called “stimming”), shrieking, attempts to escape the room, refusal to allow for vital signs or undressing, even aggression. Strategies for calming a neurotypical child such as talking or touching may make matters worse instead of better. We need help from the child and family and a plan to optimize their medical encounters.
If not adequately accommodated, children with many varieties of NDD end up not getting all the routine healthcare they need (eg vaccinations, blood tests, vital signs, even complete physical exams including dental) as well as having more adverse events during health care, including traumatizing seclusion, not allowing a support person to be present, restraint, injuries, and accidents. When more complex procedures are needed, eg x-ray, MRI, EEG, lab studies, or surgery, successful outcomes may be lower. Children with NDD have higher rates of often avoidable morbidity and mortality than those without, in part due to these barriers to complete care. While environmental accommodations to wheelchair users for accessibility has greatly improved in recent years, access to other kinds of individualized accommodations have lagged behind.
Accommodation Planning
There are a variety of factors that need to be taken into consideration in accommodating an individual with NDD. The family becomes the expert, along with the child, in knowing the child’s triggers, preferences, abilities, and level of understanding to accept and consent for care. An accommodation plan should be created using shared and supported decision making with the family and child and allowing for child preferences, regardless of their ability level, whenever possible. Development of an accommodation plan may benefit from multidisciplinary input, eg psychology, physical therapy, speech pathology, depending on the child’s needs and the practice’s ability to adapt.
The SAFE initiative is in the process of creating a checklist aiming to facilitate a description being created for each individual to help plan for a successful medical encounter while optimizing the child’s comfort, participation, and safety. While the checklist is not yet ready, we can start now by asking families and children in preparation for or at the start of a visit about their needs and writing a shared document that can also be placed in the electronic health record for the entire care team for informing care going forward.
It is especially important for the family to keep a copy of the care plan and for it to be sent as part of referrals for procedures or specialty visits so that the professionals can prepare and adapt the encounter. An excellent example is a how some hospitals schedule a practice visit for the child to experience the sights and sounds and people the child will encounter, for example, before an EEG, when nothing is required of the child. Scheduling the actual procedure at times of day when clinics are less crowded and wait times are shorter can improve the chances of success.
Some categories and details that might be included in an accommodation plan are listed below:
You might start the plan with the child’s preferred name/nickname, family member or support person names, and diagnoses along with a brief overview of the child’s level of functioning. Then list categories of needs and preferences along with suggestions or requests.
- Motor: Does the child have or need assistance entering the building, visit room, bathroom, or transferring to the exam table? What kind of assistance, if any, and by whom?
- Sensory: Is the child disturbed by noise, lights, or being touched? Does the child want to use equipment to be comfortable such as headphones, earplugs, or sunglasses or need a quiet room, care without perfumes, or dimmed lighting? Does the child typically refuse aspects of the physical examination?
- Behavioral regulation: What helps the child to stay calm? Are there certain triggers to becoming upset? Are there early cues that an upset is coming? What and who can help in the case of an upset?
- Habits/preferences: Are there certain comfort objects or habits your child needs? Are there habits your child needs to do, such as a certain order of events, or use of social stories or pictures, to cooperate or feel comfortable?
- Communication: How does the child make his/her needs known? Does the child/family speak English or another language? Does he/she use sign language or an augmentative communication device? What level of understanding does your child have; for example, similar to what age for a typical child? Is there a care plan with accommodations already available that needs review or needs revision with the child’s development or is a new one needed? Was the care plan developed including the child’s participation and assent or is more collaboration needed?
- History: Has your child had any very upsetting experiences in healthcare settings? What happened? Has the trauma been addressed? Are there reminders of the trauma that should be avoided?
- Other: Are there other things we should know about your child as an individual to provide the best care?
There are many actions needed to do better at ensuring equitable care for individuals with NDD. We should educate our office and medical staff about NDD in children and the importance of accommodating their needs, and ways to do it. The morning huddle can be used to remind staff of upcoming visits of children who may need accommodations. We then need to use quality improvement methods to check in periodically on how the changes are working for the children, families, and practice in order to continually improve.
The overall healthcare system also needs to change. Billing codes should reflect the time, complexity of accommodations, and documentation that were required for care. Episodes of the visit may need to be broken up within the day or over several days to allow the child to practice, calm down, and cooperate and this should be accounted for in billing. Given that NDD are generally lifelong conditions, payment systems that require measures of progress such as value-based payment based on improved outcomes will need to be adjusted to measure quality of care rather than significant progress.
We need to advocate for both individual children and for system changes to work toward equity of care for those with disabilities to make their lives more comfortable as well as ours.
Dr. Howard is assistant professor of pediatrics at The Johns Hopkins University School of Medicine, Baltimore, and creator of CHADIS. She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
Are Targeted Drugs the Future in Colorectal Cancer?
This transcript has been edited for clarity.
Welcome back, everybody, from the European Society for Medical Oncology (ESMO) Congress in the wonderful city of Barcelona in Spain. I was coming from ESMO drenched in huge amounts of new data.
They looked at molecularly targeted drugs, some early-stage and a later-stage study in which there’s some evidence of promise.
She talked a little about the preliminary results from three trials suggesting some benefits, pretty marginal, of cetuximab plus irinotecan in patients who’d already had epidermal growth factor receptor (EGFR) receptor inhibitory treatment.
Amivantamab plus FOLFOX or FOLFIRI was also discussed. This is a bispecific antibody against EGFR and MET. Again, very early, but there are some potential marginal benefits coming through. She also discussed the results of a larger phase 3 randomized trial with an old friend, ramucirumab, the anti-angiogenic agent, in which the ramucirumab in combination with trifluridine-tipiracil failed to meet its primary endpoint of improving overall survival.
There were some interesting post hoc subgroup analyses showing potential benefits for women, left-sided tumors, and so on. She made an excellent presentation, which she summarized by saying that the future of colorectal cancer treatment lies in further defining molecularly targeted treatment.
Nobody would disagree with that. What is interesting, though, is that, if I were to use the analogy of mining, the more deeply we mine, perhaps the lower marginal the benefits are becoming. There’s no doubt that we’re understanding better the exquisite machinery of cell signaling. We understand that there’s redundancy, there’s repeatability, and the possibility of emergence of resistance can come quite quickly.
Although we can develop ever more precise molecularly targeted drugs, it does seem as if the clinical benefits of these, in some cases, are marginally small. I’d like to suggest that, in addition to Sara’s call for more molecularly targeted drugs, we should think about cellular targets.
We did a large amount of work (as have many others, of course) looking at the immune tumor microenvironment and trying to, in a way, separate and understand the contribution of the individual component cells — of which there are many, including cancer-associated fibroblasts, natural killer (NK) cells, whole hosts of different types of T-cell subsets, B cells, tumor-associated neutrophils, and so on — and how these interact together and of interact with the epithelial colorectal cancer cells.
We are collaborating with Patrick Soon-Shiong, a clever chap, who believes in combination immunotherapy, dissecting and understanding the individual role of these different cells, and coming up with cellular therapies or targeted therapies that either inhibit or stimulate some of the different cell components to be the way ahead for an immunologically cold tumor such as microsatellite-stable colorectal cancer.
For example, we’re looking at combinations of our histone deacetylase (HDAC) inhibitor, which switches on the machinery of antigen presentation, up-regulating major histocompatibility complex (MHC) class 1 and class 2, and some other of the molecules involved in antigen chopping and presentation; it’s like turning a microsatellite-stable immunologically cold tumor hot; an interleukin-15 superagonist that stimulates NK cells; and we’ve found a way to manipulate and reduce the number of Treg cells.
We have various approaches to reducing the microenvironment transforming growth factor beta and some of the downstream elements from that. We can look at combinatorial immunotherapy, but thinking at a cellular level and developing anticancer agents that either activate or inhibit these different cell components. I’d bring the two together.
Of course, the future has got to be better molecularly targeted drugs, but let’s think at a macro level as to how we can look at the different cellular interactions within the tumor microenvironment, and perhaps through that, come up with synergistic immunotherapeutic combinations.
Dr. Kerr is Professor, Nuffield Department of Clinical Laboratory Science, University of Oxford, and Professor of Cancer Medicine, Oxford Cancer Centre, both in England. He reported conflicts of interest with Celleron Therapeutics, Oxford Cancer Biomarkers, Afrox, GlaxoSmithKline, Genomic Health, and Merck Serono.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome back, everybody, from the European Society for Medical Oncology (ESMO) Congress in the wonderful city of Barcelona in Spain. I was coming from ESMO drenched in huge amounts of new data.
They looked at molecularly targeted drugs, some early-stage and a later-stage study in which there’s some evidence of promise.
She talked a little about the preliminary results from three trials suggesting some benefits, pretty marginal, of cetuximab plus irinotecan in patients who’d already had epidermal growth factor receptor (EGFR) receptor inhibitory treatment.
Amivantamab plus FOLFOX or FOLFIRI was also discussed. This is a bispecific antibody against EGFR and MET. Again, very early, but there are some potential marginal benefits coming through. She also discussed the results of a larger phase 3 randomized trial with an old friend, ramucirumab, the anti-angiogenic agent, in which the ramucirumab in combination with trifluridine-tipiracil failed to meet its primary endpoint of improving overall survival.
There were some interesting post hoc subgroup analyses showing potential benefits for women, left-sided tumors, and so on. She made an excellent presentation, which she summarized by saying that the future of colorectal cancer treatment lies in further defining molecularly targeted treatment.
Nobody would disagree with that. What is interesting, though, is that, if I were to use the analogy of mining, the more deeply we mine, perhaps the lower marginal the benefits are becoming. There’s no doubt that we’re understanding better the exquisite machinery of cell signaling. We understand that there’s redundancy, there’s repeatability, and the possibility of emergence of resistance can come quite quickly.
Although we can develop ever more precise molecularly targeted drugs, it does seem as if the clinical benefits of these, in some cases, are marginally small. I’d like to suggest that, in addition to Sara’s call for more molecularly targeted drugs, we should think about cellular targets.
We did a large amount of work (as have many others, of course) looking at the immune tumor microenvironment and trying to, in a way, separate and understand the contribution of the individual component cells — of which there are many, including cancer-associated fibroblasts, natural killer (NK) cells, whole hosts of different types of T-cell subsets, B cells, tumor-associated neutrophils, and so on — and how these interact together and of interact with the epithelial colorectal cancer cells.
We are collaborating with Patrick Soon-Shiong, a clever chap, who believes in combination immunotherapy, dissecting and understanding the individual role of these different cells, and coming up with cellular therapies or targeted therapies that either inhibit or stimulate some of the different cell components to be the way ahead for an immunologically cold tumor such as microsatellite-stable colorectal cancer.
For example, we’re looking at combinations of our histone deacetylase (HDAC) inhibitor, which switches on the machinery of antigen presentation, up-regulating major histocompatibility complex (MHC) class 1 and class 2, and some other of the molecules involved in antigen chopping and presentation; it’s like turning a microsatellite-stable immunologically cold tumor hot; an interleukin-15 superagonist that stimulates NK cells; and we’ve found a way to manipulate and reduce the number of Treg cells.
We have various approaches to reducing the microenvironment transforming growth factor beta and some of the downstream elements from that. We can look at combinatorial immunotherapy, but thinking at a cellular level and developing anticancer agents that either activate or inhibit these different cell components. I’d bring the two together.
Of course, the future has got to be better molecularly targeted drugs, but let’s think at a macro level as to how we can look at the different cellular interactions within the tumor microenvironment, and perhaps through that, come up with synergistic immunotherapeutic combinations.
Dr. Kerr is Professor, Nuffield Department of Clinical Laboratory Science, University of Oxford, and Professor of Cancer Medicine, Oxford Cancer Centre, both in England. He reported conflicts of interest with Celleron Therapeutics, Oxford Cancer Biomarkers, Afrox, GlaxoSmithKline, Genomic Health, and Merck Serono.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome back, everybody, from the European Society for Medical Oncology (ESMO) Congress in the wonderful city of Barcelona in Spain. I was coming from ESMO drenched in huge amounts of new data.
They looked at molecularly targeted drugs, some early-stage and a later-stage study in which there’s some evidence of promise.
She talked a little about the preliminary results from three trials suggesting some benefits, pretty marginal, of cetuximab plus irinotecan in patients who’d already had epidermal growth factor receptor (EGFR) receptor inhibitory treatment.
Amivantamab plus FOLFOX or FOLFIRI was also discussed. This is a bispecific antibody against EGFR and MET. Again, very early, but there are some potential marginal benefits coming through. She also discussed the results of a larger phase 3 randomized trial with an old friend, ramucirumab, the anti-angiogenic agent, in which the ramucirumab in combination with trifluridine-tipiracil failed to meet its primary endpoint of improving overall survival.
There were some interesting post hoc subgroup analyses showing potential benefits for women, left-sided tumors, and so on. She made an excellent presentation, which she summarized by saying that the future of colorectal cancer treatment lies in further defining molecularly targeted treatment.
Nobody would disagree with that. What is interesting, though, is that, if I were to use the analogy of mining, the more deeply we mine, perhaps the lower marginal the benefits are becoming. There’s no doubt that we’re understanding better the exquisite machinery of cell signaling. We understand that there’s redundancy, there’s repeatability, and the possibility of emergence of resistance can come quite quickly.
Although we can develop ever more precise molecularly targeted drugs, it does seem as if the clinical benefits of these, in some cases, are marginally small. I’d like to suggest that, in addition to Sara’s call for more molecularly targeted drugs, we should think about cellular targets.
We did a large amount of work (as have many others, of course) looking at the immune tumor microenvironment and trying to, in a way, separate and understand the contribution of the individual component cells — of which there are many, including cancer-associated fibroblasts, natural killer (NK) cells, whole hosts of different types of T-cell subsets, B cells, tumor-associated neutrophils, and so on — and how these interact together and of interact with the epithelial colorectal cancer cells.
We are collaborating with Patrick Soon-Shiong, a clever chap, who believes in combination immunotherapy, dissecting and understanding the individual role of these different cells, and coming up with cellular therapies or targeted therapies that either inhibit or stimulate some of the different cell components to be the way ahead for an immunologically cold tumor such as microsatellite-stable colorectal cancer.
For example, we’re looking at combinations of our histone deacetylase (HDAC) inhibitor, which switches on the machinery of antigen presentation, up-regulating major histocompatibility complex (MHC) class 1 and class 2, and some other of the molecules involved in antigen chopping and presentation; it’s like turning a microsatellite-stable immunologically cold tumor hot; an interleukin-15 superagonist that stimulates NK cells; and we’ve found a way to manipulate and reduce the number of Treg cells.
We have various approaches to reducing the microenvironment transforming growth factor beta and some of the downstream elements from that. We can look at combinatorial immunotherapy, but thinking at a cellular level and developing anticancer agents that either activate or inhibit these different cell components. I’d bring the two together.
Of course, the future has got to be better molecularly targeted drugs, but let’s think at a macro level as to how we can look at the different cellular interactions within the tumor microenvironment, and perhaps through that, come up with synergistic immunotherapeutic combinations.
Dr. Kerr is Professor, Nuffield Department of Clinical Laboratory Science, University of Oxford, and Professor of Cancer Medicine, Oxford Cancer Centre, both in England. He reported conflicts of interest with Celleron Therapeutics, Oxford Cancer Biomarkers, Afrox, GlaxoSmithKline, Genomic Health, and Merck Serono.
A version of this article first appeared on Medscape.com.
Ghost Fat: The Unseen Consequences of Weight Loss
Many people who lose weight, whether through diet and lifestyle changes, medication, or bariatric surgery, recognize their body has changed. While they also experience improvements in quality of life and psychosocial areas, that’s not true for everyone. Some patients don’t “see” they’ve lost weight — a phenomenon referred to as “phantom fat,” “ghost fat,” or “vestigial body image.”
“Most people are happy with their appearance, or at least their body shape, after weight loss — although some are unhappy with the loose, sagging skin that can follow weight loss and seek plastic surgery to remedy that,” David B. Sarwer, PhD, director of the Center for Obesity Research and Education and professor of social and behavioral sciences, Temple University College of Public Health, Philadelphia, told this news organization. “There’s a subset of people who remain dissatisfied with their body image, including their shape.”
This body dissatisfaction of people who lose weight may be long-standing, predating the weight loss, or may be new because weight loss has catalyzed a host of previously unaddressed psychosocial issues. Some may show up at assessments on treatment onset, while others may be detected by monitoring changes during or after weight loss. “Mental health counseling after bariatric surgery is greatly underutilized,” Dr. Sarwer observed.
Ghost Fat
Research has corroborated the lingering self-perception of being “obese” vs “ex-obese.” In one study, patients who had undergone bariatric surgery reported being unable to see the difference in their size and shape 18-30 months following their procedure, despite substantial weight loss.
Some research suggests that rapid weight loss (eg, through bariatric surgery) is more likely to generate the perception of “phantom fat,” but additional research is needed to investigate whether the mode and speed of weight loss affect subsequent body image.
Being habituated to one’s former appearance may play a role, Dr. Sarwer suggested. “We see this not only with weight loss but with other body-altering procedures. It takes the brain time to catch up to the new appearance. In rhinoplasty, for example, it may take patients a while before they become accustomed to looking at their new face in the mirror after decades of looking at a more prominent nose.”
Years of Social Stigma
It may also take time for people to overcome years of enduring the stigma of obesity.
There are “pervasive” negative attitudes implying that individuals who are overweight and/or obese are “lazy, weak-willed, lacking in self-discipline and willpower” — a problem compounded by social media and media in general, which present unrealistic, glorified body images and disparaging messages about those with weight problems.
“Body image is a construct, rather than what you see in the mirror,” Sheethal Reddy, PhD, a psychologist at the Emory Bariatric Center, Emory University Hospital Midtown, Atlanta, told this news organization. “It’s the mental construct of our physical selves.”
According to Dr. Reddy, body image develops “within a broader societal context and is influenced by the person’s ethnic, racial, and cultural heritage.”
Adolescents are particularly vulnerable to body dissatisfaction. This is compounded in those with obesity, who often experience weight-based victimization and internalized weight-based stigma, compared with adolescents with lower weights. Weight stigma often takes the form of teasing and bullying.
“Appearance-related bullying and teasing during childhood and adolescence can reverberate into adulthood and persist throughout the lifespan,” Dr. Sarwer said. “When we see these patients and ask if they’ve ever been teased or bullied, not only do many say yes but it takes them back to those moments, to that origin story, and they remember someone saying something mean, cruel, and hurtful.”
Stigmatizing experiences can affect subjective body image, even after the weight has been lost and the person’s body is objectively thinner. Research comparing individuals who were overweight and lost weight to individuals who are currently overweight and haven’t lost weight and individuals who were never overweight suggests that “vestigial” body disparagement may persist following weight loss — especially in those with early-onset obesity.
The Role of Genetics
Genetics may contribute to people’s self-perception and body dissatisfaction, both before and after weight loss. A study of 827 community-based adolescents examined the association between polygenic risk scores (PRS) for body mass index (BMI) and type 2 diabetes and symptoms of body dissatisfaction and depression.
“Given the significant genetic role in BMI, we wanted to explore whether genetic risk for BMI might also predict body dissatisfaction,” lead author Krista Ekberg, MS, a doctoral candidate in clinical psychology, Rosalind Franklin University of Medicine and Science, North Chicago, Illinois, told this news organization.
Genetic influences on BMI, as measured by PRS, were significantly associated with both phenotypic BMI and body dissatisfaction. “The association between PRS and body dissatisfaction was largely explained by BMI, suggesting that BMI itself accounts for much of the link between genetic risk and body dissatisfaction.”
Psychiatric History and Trauma
Adverse experiences, particularly sexual or physical abuse, may also account for body dissatisfaction after weight loss. “When some people with a history of this type of abuse lose a large amount of weight — typically after bariatric surgery — they often go through a period of emotional turbulence,” Dr. Sarwer said.
Childhood maltreatment can also be associated with body image disturbances in adulthood, according to a meta-analysis of 12 studies, encompassing 15,481 participants. Sexual abuse is “surprisingly common” among patients with obesity, according to Dr. Sarwer. A chart review of 131 patients revealed that 60% of those who reported a history of rape or sexual molestation were ≥ 50 pounds overweight vs only 28% of age- and sex-matched controls without a history of abuse. Other studies have corroborated these findings.
Excess weight can serve an “adaptive function,” Dr. Sarwer noted. It can be a self-protective mechanism that “insulates” them from sexual advances by potential romantic partners or abusers. Some may find that, after weight loss, repressed memories of a sexual assault surface as a result of the newer, more “attractive” appearance. Feeling vulnerable in their thinner bodies, they may need to regard themselves as overweight to maintain that feeling of “protection.” Weight loss may also trigger memories, flashbacks, or nightmares, as people return to a weight at which they were abused.
Dissociation is another mechanism linking trauma with post–weight loss body dysmorphia, Supatra Tovar, PsyD, RD, a clinical psychologist and registered dietitian with a practice in California, told this news organization. Dissociation from the body is often a coping mechanism for dealing with an overwhelming traumatic experience.
Individuals with a history of depression, anxiety, or posttraumatic stress disorder have higher levels of body dysmorphia, both before and after weight loss. One study found that patients undergoing bariatric surgery who had some type of psychopathology and other psychological risk factors were significantly more likely to report body image concerns 3 months after the surgery. Body image concerns were also more common in patients with preoperative depression, current psychotropic medication use, and a history of outpatient therapy or psychotropic medication use.
“Depression, anxiety, and trauma play a role in how you see yourself and how you carry yourself,” Dr. Reddy said. “This is wrapped up in any type of psychopathology. Being depressed is like looking at yourself through a cloud. It’s the opposite of ‘rose-colored glasses’ and instead, looking at yourself through a negative lens.”
Diagnosis and Interventions
Some helpful tools to assess the presence and extent of weight dissatisfaction and body dysmorphia include the Eating Disorder Inventory — Body Dissatisfaction Subscale and the Body Shape Questionnaire. It’s also important to take into account “the extent to which people are invested in their appearance psychologically,” Dr. Sarwer advised. The AO subscale of the Multidimensional Body-Self Relations Questionnaire generally assesses this. The Body Image Quality of Life Inventory assesses how and to what extent the perceived body image affects the person’s quality of life.
Experts recommend cognitive behavioral therapy (CBT) as an evidence-based intervention for body image issues, including those following weight loss.
“There’s an extensive CBT body image therapy program specifically tailored to the needs of overweight and obese individuals,” Dr. Sarwer said. “We don’t ignore historical variables that may have contributed to the problem, like early bullying, but we encourage people to think about what’s going on in their day-to-day life today. We drill down not only into the maladaptive behaviors but also the cognition and beliefs that may be erroneous but underlie these behaviors.”
The aim of CBT is to “modify irrational and dysfunctional thoughts, emotions, and behaviors through techniques such as self-monitoring, cognitive structuring, psychoeducation, desensitization, and exposure and response prevention.” The program laid out in Cash’s body image workbook includes eight steps. (Figure).
Weight Loss Doesn’t Automatically Equate With Happiness
Another realistic expectation runs counter to a common misperception that becoming thin will automatically translate into becoming happier. That’s not always the case, according to Dr. Tovar.
“If you haven’t worked deeply on addressing self-compassion and understanding that who you are at the core has nothing to do with your physical appearance, you can have an empty feeling once you’ve reached this point,” she said. “You still don’t know who you are and what you’re contributing to the world [because] you’ve been so focused on losing weight.”
Weight loss can also “unmask” questions about self-worth, even when receiving compliments about one’s “improved” appearance. “Praise and compliments after weight loss can be a double-edged sword,” Dr. Tovar observed. “You might think, ‘I wasn’t accepted or praised when I was overweight. The only way to be acceptable or validated is by losing weight, so I have to continue losing weight.’ ” This fuels fear of regaining the weight and can lead to continuing to see oneself as overweight, perhaps as a way to stay motivated to continue with weight loss. “Feeling that one’s value depends on remaining thin hampers body satisfaction,” she said.
Dr. Tovar, author of the book Deprogram Diet Culture: Rethink Your Relationship with Food, Heal Your Mind, and Live a Diet-Free Life, encourages people to shift the emphasis from weight loss to a holistic focus on self-worth and to explore obstacles to those feelings both before and after weight loss.
Endocrinologists and other medical professionals can help by not engaging in “weight and body shaming,” Dr. Tovar said.
She recommends physicians “encourage patients to tune in to their own bodies, helping them become more aware of how different foods affect their physical and emotional well-being.”
Set realistic expectations through “open, nonjudgmental conversations about the complexities of metabolism, weight, and health.”
Dr. Tovar advises rather than focusing on weight loss as the primary goal, physicians should focus on health markers such as blood glucose, energy levels, mental well-being, and physical fitness.
Prioritize “listening over lecturing.” Begin with empathy, asking questions such as “How do you feel about your health right now? What changes have you noticed in your body lately?” Doing this “creates space for the patient to express their concerns without feeling judged or shamed.”
Refer patients to a mental health professional when a patient exhibits signs of disordered eating or poor body image or when emotional factors are playing a significant role in the relationship with food and weight. “If a patient is caught in a cycle of dieting and weight gain, struggles with binge eating, or displays symptoms of depression or anxiety related to body, then psychological help is crucial.”
Ultimately, the goal of treatment “should be to provide a safe, supportive environment where patients can heal — not just physically but also emotionally and mentally,” Dr. Tovar added.
Dr. Tovar, Ms. Ekberg, and Dr. Reddy reported no relevant financial relationships. Dr. Sarwer received grant funding from the National Institute of Dental and Craniofacial Research and National Institute of Diabetes and Digestive and Kidney Diseases. He has consulting relationships with Novo Nordisk and Twenty30 Health. He is an associate editor for Obesity Surgery and editor in chief of Obesity Science & Practice.
A version of this article first appeared on Medscape.com.
Many people who lose weight, whether through diet and lifestyle changes, medication, or bariatric surgery, recognize their body has changed. While they also experience improvements in quality of life and psychosocial areas, that’s not true for everyone. Some patients don’t “see” they’ve lost weight — a phenomenon referred to as “phantom fat,” “ghost fat,” or “vestigial body image.”
“Most people are happy with their appearance, or at least their body shape, after weight loss — although some are unhappy with the loose, sagging skin that can follow weight loss and seek plastic surgery to remedy that,” David B. Sarwer, PhD, director of the Center for Obesity Research and Education and professor of social and behavioral sciences, Temple University College of Public Health, Philadelphia, told this news organization. “There’s a subset of people who remain dissatisfied with their body image, including their shape.”
This body dissatisfaction of people who lose weight may be long-standing, predating the weight loss, or may be new because weight loss has catalyzed a host of previously unaddressed psychosocial issues. Some may show up at assessments on treatment onset, while others may be detected by monitoring changes during or after weight loss. “Mental health counseling after bariatric surgery is greatly underutilized,” Dr. Sarwer observed.
Ghost Fat
Research has corroborated the lingering self-perception of being “obese” vs “ex-obese.” In one study, patients who had undergone bariatric surgery reported being unable to see the difference in their size and shape 18-30 months following their procedure, despite substantial weight loss.
Some research suggests that rapid weight loss (eg, through bariatric surgery) is more likely to generate the perception of “phantom fat,” but additional research is needed to investigate whether the mode and speed of weight loss affect subsequent body image.
Being habituated to one’s former appearance may play a role, Dr. Sarwer suggested. “We see this not only with weight loss but with other body-altering procedures. It takes the brain time to catch up to the new appearance. In rhinoplasty, for example, it may take patients a while before they become accustomed to looking at their new face in the mirror after decades of looking at a more prominent nose.”
Years of Social Stigma
It may also take time for people to overcome years of enduring the stigma of obesity.
There are “pervasive” negative attitudes implying that individuals who are overweight and/or obese are “lazy, weak-willed, lacking in self-discipline and willpower” — a problem compounded by social media and media in general, which present unrealistic, glorified body images and disparaging messages about those with weight problems.
“Body image is a construct, rather than what you see in the mirror,” Sheethal Reddy, PhD, a psychologist at the Emory Bariatric Center, Emory University Hospital Midtown, Atlanta, told this news organization. “It’s the mental construct of our physical selves.”
According to Dr. Reddy, body image develops “within a broader societal context and is influenced by the person’s ethnic, racial, and cultural heritage.”
Adolescents are particularly vulnerable to body dissatisfaction. This is compounded in those with obesity, who often experience weight-based victimization and internalized weight-based stigma, compared with adolescents with lower weights. Weight stigma often takes the form of teasing and bullying.
“Appearance-related bullying and teasing during childhood and adolescence can reverberate into adulthood and persist throughout the lifespan,” Dr. Sarwer said. “When we see these patients and ask if they’ve ever been teased or bullied, not only do many say yes but it takes them back to those moments, to that origin story, and they remember someone saying something mean, cruel, and hurtful.”
Stigmatizing experiences can affect subjective body image, even after the weight has been lost and the person’s body is objectively thinner. Research comparing individuals who were overweight and lost weight to individuals who are currently overweight and haven’t lost weight and individuals who were never overweight suggests that “vestigial” body disparagement may persist following weight loss — especially in those with early-onset obesity.
The Role of Genetics
Genetics may contribute to people’s self-perception and body dissatisfaction, both before and after weight loss. A study of 827 community-based adolescents examined the association between polygenic risk scores (PRS) for body mass index (BMI) and type 2 diabetes and symptoms of body dissatisfaction and depression.
“Given the significant genetic role in BMI, we wanted to explore whether genetic risk for BMI might also predict body dissatisfaction,” lead author Krista Ekberg, MS, a doctoral candidate in clinical psychology, Rosalind Franklin University of Medicine and Science, North Chicago, Illinois, told this news organization.
Genetic influences on BMI, as measured by PRS, were significantly associated with both phenotypic BMI and body dissatisfaction. “The association between PRS and body dissatisfaction was largely explained by BMI, suggesting that BMI itself accounts for much of the link between genetic risk and body dissatisfaction.”
Psychiatric History and Trauma
Adverse experiences, particularly sexual or physical abuse, may also account for body dissatisfaction after weight loss. “When some people with a history of this type of abuse lose a large amount of weight — typically after bariatric surgery — they often go through a period of emotional turbulence,” Dr. Sarwer said.
Childhood maltreatment can also be associated with body image disturbances in adulthood, according to a meta-analysis of 12 studies, encompassing 15,481 participants. Sexual abuse is “surprisingly common” among patients with obesity, according to Dr. Sarwer. A chart review of 131 patients revealed that 60% of those who reported a history of rape or sexual molestation were ≥ 50 pounds overweight vs only 28% of age- and sex-matched controls without a history of abuse. Other studies have corroborated these findings.
Excess weight can serve an “adaptive function,” Dr. Sarwer noted. It can be a self-protective mechanism that “insulates” them from sexual advances by potential romantic partners or abusers. Some may find that, after weight loss, repressed memories of a sexual assault surface as a result of the newer, more “attractive” appearance. Feeling vulnerable in their thinner bodies, they may need to regard themselves as overweight to maintain that feeling of “protection.” Weight loss may also trigger memories, flashbacks, or nightmares, as people return to a weight at which they were abused.
Dissociation is another mechanism linking trauma with post–weight loss body dysmorphia, Supatra Tovar, PsyD, RD, a clinical psychologist and registered dietitian with a practice in California, told this news organization. Dissociation from the body is often a coping mechanism for dealing with an overwhelming traumatic experience.
Individuals with a history of depression, anxiety, or posttraumatic stress disorder have higher levels of body dysmorphia, both before and after weight loss. One study found that patients undergoing bariatric surgery who had some type of psychopathology and other psychological risk factors were significantly more likely to report body image concerns 3 months after the surgery. Body image concerns were also more common in patients with preoperative depression, current psychotropic medication use, and a history of outpatient therapy or psychotropic medication use.
“Depression, anxiety, and trauma play a role in how you see yourself and how you carry yourself,” Dr. Reddy said. “This is wrapped up in any type of psychopathology. Being depressed is like looking at yourself through a cloud. It’s the opposite of ‘rose-colored glasses’ and instead, looking at yourself through a negative lens.”
Diagnosis and Interventions
Some helpful tools to assess the presence and extent of weight dissatisfaction and body dysmorphia include the Eating Disorder Inventory — Body Dissatisfaction Subscale and the Body Shape Questionnaire. It’s also important to take into account “the extent to which people are invested in their appearance psychologically,” Dr. Sarwer advised. The AO subscale of the Multidimensional Body-Self Relations Questionnaire generally assesses this. The Body Image Quality of Life Inventory assesses how and to what extent the perceived body image affects the person’s quality of life.
Experts recommend cognitive behavioral therapy (CBT) as an evidence-based intervention for body image issues, including those following weight loss.
“There’s an extensive CBT body image therapy program specifically tailored to the needs of overweight and obese individuals,” Dr. Sarwer said. “We don’t ignore historical variables that may have contributed to the problem, like early bullying, but we encourage people to think about what’s going on in their day-to-day life today. We drill down not only into the maladaptive behaviors but also the cognition and beliefs that may be erroneous but underlie these behaviors.”
The aim of CBT is to “modify irrational and dysfunctional thoughts, emotions, and behaviors through techniques such as self-monitoring, cognitive structuring, psychoeducation, desensitization, and exposure and response prevention.” The program laid out in Cash’s body image workbook includes eight steps. (Figure).
Weight Loss Doesn’t Automatically Equate With Happiness
Another realistic expectation runs counter to a common misperception that becoming thin will automatically translate into becoming happier. That’s not always the case, according to Dr. Tovar.
“If you haven’t worked deeply on addressing self-compassion and understanding that who you are at the core has nothing to do with your physical appearance, you can have an empty feeling once you’ve reached this point,” she said. “You still don’t know who you are and what you’re contributing to the world [because] you’ve been so focused on losing weight.”
Weight loss can also “unmask” questions about self-worth, even when receiving compliments about one’s “improved” appearance. “Praise and compliments after weight loss can be a double-edged sword,” Dr. Tovar observed. “You might think, ‘I wasn’t accepted or praised when I was overweight. The only way to be acceptable or validated is by losing weight, so I have to continue losing weight.’ ” This fuels fear of regaining the weight and can lead to continuing to see oneself as overweight, perhaps as a way to stay motivated to continue with weight loss. “Feeling that one’s value depends on remaining thin hampers body satisfaction,” she said.
Dr. Tovar, author of the book Deprogram Diet Culture: Rethink Your Relationship with Food, Heal Your Mind, and Live a Diet-Free Life, encourages people to shift the emphasis from weight loss to a holistic focus on self-worth and to explore obstacles to those feelings both before and after weight loss.
Endocrinologists and other medical professionals can help by not engaging in “weight and body shaming,” Dr. Tovar said.
She recommends physicians “encourage patients to tune in to their own bodies, helping them become more aware of how different foods affect their physical and emotional well-being.”
Set realistic expectations through “open, nonjudgmental conversations about the complexities of metabolism, weight, and health.”
Dr. Tovar advises rather than focusing on weight loss as the primary goal, physicians should focus on health markers such as blood glucose, energy levels, mental well-being, and physical fitness.
Prioritize “listening over lecturing.” Begin with empathy, asking questions such as “How do you feel about your health right now? What changes have you noticed in your body lately?” Doing this “creates space for the patient to express their concerns without feeling judged or shamed.”
Refer patients to a mental health professional when a patient exhibits signs of disordered eating or poor body image or when emotional factors are playing a significant role in the relationship with food and weight. “If a patient is caught in a cycle of dieting and weight gain, struggles with binge eating, or displays symptoms of depression or anxiety related to body, then psychological help is crucial.”
Ultimately, the goal of treatment “should be to provide a safe, supportive environment where patients can heal — not just physically but also emotionally and mentally,” Dr. Tovar added.
Dr. Tovar, Ms. Ekberg, and Dr. Reddy reported no relevant financial relationships. Dr. Sarwer received grant funding from the National Institute of Dental and Craniofacial Research and National Institute of Diabetes and Digestive and Kidney Diseases. He has consulting relationships with Novo Nordisk and Twenty30 Health. He is an associate editor for Obesity Surgery and editor in chief of Obesity Science & Practice.
A version of this article first appeared on Medscape.com.
Many people who lose weight, whether through diet and lifestyle changes, medication, or bariatric surgery, recognize their body has changed. While they also experience improvements in quality of life and psychosocial areas, that’s not true for everyone. Some patients don’t “see” they’ve lost weight — a phenomenon referred to as “phantom fat,” “ghost fat,” or “vestigial body image.”
“Most people are happy with their appearance, or at least their body shape, after weight loss — although some are unhappy with the loose, sagging skin that can follow weight loss and seek plastic surgery to remedy that,” David B. Sarwer, PhD, director of the Center for Obesity Research and Education and professor of social and behavioral sciences, Temple University College of Public Health, Philadelphia, told this news organization. “There’s a subset of people who remain dissatisfied with their body image, including their shape.”
This body dissatisfaction of people who lose weight may be long-standing, predating the weight loss, or may be new because weight loss has catalyzed a host of previously unaddressed psychosocial issues. Some may show up at assessments on treatment onset, while others may be detected by monitoring changes during or after weight loss. “Mental health counseling after bariatric surgery is greatly underutilized,” Dr. Sarwer observed.
Ghost Fat
Research has corroborated the lingering self-perception of being “obese” vs “ex-obese.” In one study, patients who had undergone bariatric surgery reported being unable to see the difference in their size and shape 18-30 months following their procedure, despite substantial weight loss.
Some research suggests that rapid weight loss (eg, through bariatric surgery) is more likely to generate the perception of “phantom fat,” but additional research is needed to investigate whether the mode and speed of weight loss affect subsequent body image.
Being habituated to one’s former appearance may play a role, Dr. Sarwer suggested. “We see this not only with weight loss but with other body-altering procedures. It takes the brain time to catch up to the new appearance. In rhinoplasty, for example, it may take patients a while before they become accustomed to looking at their new face in the mirror after decades of looking at a more prominent nose.”
Years of Social Stigma
It may also take time for people to overcome years of enduring the stigma of obesity.
There are “pervasive” negative attitudes implying that individuals who are overweight and/or obese are “lazy, weak-willed, lacking in self-discipline and willpower” — a problem compounded by social media and media in general, which present unrealistic, glorified body images and disparaging messages about those with weight problems.
“Body image is a construct, rather than what you see in the mirror,” Sheethal Reddy, PhD, a psychologist at the Emory Bariatric Center, Emory University Hospital Midtown, Atlanta, told this news organization. “It’s the mental construct of our physical selves.”
According to Dr. Reddy, body image develops “within a broader societal context and is influenced by the person’s ethnic, racial, and cultural heritage.”
Adolescents are particularly vulnerable to body dissatisfaction. This is compounded in those with obesity, who often experience weight-based victimization and internalized weight-based stigma, compared with adolescents with lower weights. Weight stigma often takes the form of teasing and bullying.
“Appearance-related bullying and teasing during childhood and adolescence can reverberate into adulthood and persist throughout the lifespan,” Dr. Sarwer said. “When we see these patients and ask if they’ve ever been teased or bullied, not only do many say yes but it takes them back to those moments, to that origin story, and they remember someone saying something mean, cruel, and hurtful.”
Stigmatizing experiences can affect subjective body image, even after the weight has been lost and the person’s body is objectively thinner. Research comparing individuals who were overweight and lost weight to individuals who are currently overweight and haven’t lost weight and individuals who were never overweight suggests that “vestigial” body disparagement may persist following weight loss — especially in those with early-onset obesity.
The Role of Genetics
Genetics may contribute to people’s self-perception and body dissatisfaction, both before and after weight loss. A study of 827 community-based adolescents examined the association between polygenic risk scores (PRS) for body mass index (BMI) and type 2 diabetes and symptoms of body dissatisfaction and depression.
“Given the significant genetic role in BMI, we wanted to explore whether genetic risk for BMI might also predict body dissatisfaction,” lead author Krista Ekberg, MS, a doctoral candidate in clinical psychology, Rosalind Franklin University of Medicine and Science, North Chicago, Illinois, told this news organization.
Genetic influences on BMI, as measured by PRS, were significantly associated with both phenotypic BMI and body dissatisfaction. “The association between PRS and body dissatisfaction was largely explained by BMI, suggesting that BMI itself accounts for much of the link between genetic risk and body dissatisfaction.”
Psychiatric History and Trauma
Adverse experiences, particularly sexual or physical abuse, may also account for body dissatisfaction after weight loss. “When some people with a history of this type of abuse lose a large amount of weight — typically after bariatric surgery — they often go through a period of emotional turbulence,” Dr. Sarwer said.
Childhood maltreatment can also be associated with body image disturbances in adulthood, according to a meta-analysis of 12 studies, encompassing 15,481 participants. Sexual abuse is “surprisingly common” among patients with obesity, according to Dr. Sarwer. A chart review of 131 patients revealed that 60% of those who reported a history of rape or sexual molestation were ≥ 50 pounds overweight vs only 28% of age- and sex-matched controls without a history of abuse. Other studies have corroborated these findings.
Excess weight can serve an “adaptive function,” Dr. Sarwer noted. It can be a self-protective mechanism that “insulates” them from sexual advances by potential romantic partners or abusers. Some may find that, after weight loss, repressed memories of a sexual assault surface as a result of the newer, more “attractive” appearance. Feeling vulnerable in their thinner bodies, they may need to regard themselves as overweight to maintain that feeling of “protection.” Weight loss may also trigger memories, flashbacks, or nightmares, as people return to a weight at which they were abused.
Dissociation is another mechanism linking trauma with post–weight loss body dysmorphia, Supatra Tovar, PsyD, RD, a clinical psychologist and registered dietitian with a practice in California, told this news organization. Dissociation from the body is often a coping mechanism for dealing with an overwhelming traumatic experience.
Individuals with a history of depression, anxiety, or posttraumatic stress disorder have higher levels of body dysmorphia, both before and after weight loss. One study found that patients undergoing bariatric surgery who had some type of psychopathology and other psychological risk factors were significantly more likely to report body image concerns 3 months after the surgery. Body image concerns were also more common in patients with preoperative depression, current psychotropic medication use, and a history of outpatient therapy or psychotropic medication use.
“Depression, anxiety, and trauma play a role in how you see yourself and how you carry yourself,” Dr. Reddy said. “This is wrapped up in any type of psychopathology. Being depressed is like looking at yourself through a cloud. It’s the opposite of ‘rose-colored glasses’ and instead, looking at yourself through a negative lens.”
Diagnosis and Interventions
Some helpful tools to assess the presence and extent of weight dissatisfaction and body dysmorphia include the Eating Disorder Inventory — Body Dissatisfaction Subscale and the Body Shape Questionnaire. It’s also important to take into account “the extent to which people are invested in their appearance psychologically,” Dr. Sarwer advised. The AO subscale of the Multidimensional Body-Self Relations Questionnaire generally assesses this. The Body Image Quality of Life Inventory assesses how and to what extent the perceived body image affects the person’s quality of life.
Experts recommend cognitive behavioral therapy (CBT) as an evidence-based intervention for body image issues, including those following weight loss.
“There’s an extensive CBT body image therapy program specifically tailored to the needs of overweight and obese individuals,” Dr. Sarwer said. “We don’t ignore historical variables that may have contributed to the problem, like early bullying, but we encourage people to think about what’s going on in their day-to-day life today. We drill down not only into the maladaptive behaviors but also the cognition and beliefs that may be erroneous but underlie these behaviors.”
The aim of CBT is to “modify irrational and dysfunctional thoughts, emotions, and behaviors through techniques such as self-monitoring, cognitive structuring, psychoeducation, desensitization, and exposure and response prevention.” The program laid out in Cash’s body image workbook includes eight steps. (Figure).
Weight Loss Doesn’t Automatically Equate With Happiness
Another realistic expectation runs counter to a common misperception that becoming thin will automatically translate into becoming happier. That’s not always the case, according to Dr. Tovar.
“If you haven’t worked deeply on addressing self-compassion and understanding that who you are at the core has nothing to do with your physical appearance, you can have an empty feeling once you’ve reached this point,” she said. “You still don’t know who you are and what you’re contributing to the world [because] you’ve been so focused on losing weight.”
Weight loss can also “unmask” questions about self-worth, even when receiving compliments about one’s “improved” appearance. “Praise and compliments after weight loss can be a double-edged sword,” Dr. Tovar observed. “You might think, ‘I wasn’t accepted or praised when I was overweight. The only way to be acceptable or validated is by losing weight, so I have to continue losing weight.’ ” This fuels fear of regaining the weight and can lead to continuing to see oneself as overweight, perhaps as a way to stay motivated to continue with weight loss. “Feeling that one’s value depends on remaining thin hampers body satisfaction,” she said.
Dr. Tovar, author of the book Deprogram Diet Culture: Rethink Your Relationship with Food, Heal Your Mind, and Live a Diet-Free Life, encourages people to shift the emphasis from weight loss to a holistic focus on self-worth and to explore obstacles to those feelings both before and after weight loss.
Endocrinologists and other medical professionals can help by not engaging in “weight and body shaming,” Dr. Tovar said.
She recommends physicians “encourage patients to tune in to their own bodies, helping them become more aware of how different foods affect their physical and emotional well-being.”
Set realistic expectations through “open, nonjudgmental conversations about the complexities of metabolism, weight, and health.”
Dr. Tovar advises rather than focusing on weight loss as the primary goal, physicians should focus on health markers such as blood glucose, energy levels, mental well-being, and physical fitness.
Prioritize “listening over lecturing.” Begin with empathy, asking questions such as “How do you feel about your health right now? What changes have you noticed in your body lately?” Doing this “creates space for the patient to express their concerns without feeling judged or shamed.”
Refer patients to a mental health professional when a patient exhibits signs of disordered eating or poor body image or when emotional factors are playing a significant role in the relationship with food and weight. “If a patient is caught in a cycle of dieting and weight gain, struggles with binge eating, or displays symptoms of depression or anxiety related to body, then psychological help is crucial.”
Ultimately, the goal of treatment “should be to provide a safe, supportive environment where patients can heal — not just physically but also emotionally and mentally,” Dr. Tovar added.
Dr. Tovar, Ms. Ekberg, and Dr. Reddy reported no relevant financial relationships. Dr. Sarwer received grant funding from the National Institute of Dental and Craniofacial Research and National Institute of Diabetes and Digestive and Kidney Diseases. He has consulting relationships with Novo Nordisk and Twenty30 Health. He is an associate editor for Obesity Surgery and editor in chief of Obesity Science & Practice.
A version of this article first appeared on Medscape.com.
Nonalcoholic Beer and Underage Drinking
Several months ago in a letter about healthcare providers and the decision to use alcohol and other mind-altering substances on the job, I waxed enthusiastically about the new wave of no alcohol (NA) and zero (00) alcohol beers that have come on the market. In the last 2 years our local grocery store’s cooler space for nonalcoholic beer has grown from less than 24 inches to something approaching the height of the average sixth grader.
In a bold act of chivalry at the beginning of the pandemic I accepted the mantle of designated grocery shopper and over the last 3 years have become uncommonly proud of my ability to bring home the groceries efficiently and cost effectively, without catching COVID in the process. I have developed a sixth sense of choosing which human checker/bagger combination is fastest or whether the self-checkout is the way to go.
For obvious reasons the human checkers don’t ask for my ID when I am buying adult beverages. However, the self-check register freezes up instantly when I scan my 12-pack of Run Wild nonalcoholic. This necessitates a search for the MIA store person assigned to patrol the self-check corral, ever on the lookout for shoplifters, underage drinkers, and other generally shifty looking characters.
When I find one of the grocery store detectives (who is likely to have been a former patient), I say: “You know, this doesn’t have any alcohol in it.” They invariably reply with a shrug. “I know. But, the rules are the rules.” Occasionally, they may add: “It doesn’t make sense, does it?”
At first blush checking IDs for a nonalcoholic beverage may sound dumb, certainly to someone who is just a few years on either side of the legal drinking age. Why are we trying to protect some crazy teenager from the futility of getting high on a six-pack of something that at worst will make him spend most of the next couple of hours peeing?
But, there is concern in some corners that nonalcoholic drinks pose a significant threat to teenagers. Two PhDs at Stanford University have recently published a paper in which they worry that the dramatic rise in US sales of nonalcoholic drinks from 15% to 30% since 2018 may be socializing “users of alcohol drinking experiences by exposing them to the taste, look, and even brands of alcoholic beverages”.
Is there evidence to support their concern? I could only find one brief report in the Japanese literature that states that among young people “who experienced the nonalcoholic beverage intake, interest in or motivation for drinking alcoholic beverages, and/or smoking is higher than [among] those who did not.” The study didn’t appear to clearly separate the exposure in a family setting from the actual intake.
Beer is an acquired taste. If someone offered you your first taste of beer after a hot-weather set of tennis most of you would reject it and ask for water or lemonade. I can recall my first taste of beer. For some reason my father thought at age 11 or 12 I might like to try some from his glass. I’m not sure of his motivation, but he tried the same thing with oysters. I didn’t drink beer again until I was 16, motivated at that time by a group dynamic. The oyster trial, however, backfired on him and from then on he had to share his coveted dozen with me. Alcohol, unless heavily disguised by a mixer, is also not a taste that most young people find appealing.
It is unlikely that the average thrill-seeking teenager is going to ask his older-appearing buddy with a fake ID to buy him some nonalcoholic beer. Nor would he go to the effort or risk of acquiring his own fake ID just to see how it tastes. It just doesn’t compute, especially to a self-check corral patroller.
I guess one could envision a scenario in which a teenager wanting to fit in with the fast crowd would ask a trusted adult (or clueless parent) to buy him some nonalcoholic beer to bring to a party. He is running a serious risk of being laughed at by his friends if they find he’s drinking the fake stuff. It also seems unlikely that a parent would buy nonalcoholic beer to introduce his teenager to the taste of beer.
So,
Although it runs counter to my usual commitment to evidence-based decisions, making it difficult for adolescents to buy nonalcoholic beverages feels like the right think to do. As long as alcoholic and nonalcoholic beverages share the same display space and are packaged in nearly identical containers, there is ample opportunity for confusion. Recent evidence suggesting that even small amounts of alcohol increases some health risks should strengthen our resolve to minimize that confusion.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Several months ago in a letter about healthcare providers and the decision to use alcohol and other mind-altering substances on the job, I waxed enthusiastically about the new wave of no alcohol (NA) and zero (00) alcohol beers that have come on the market. In the last 2 years our local grocery store’s cooler space for nonalcoholic beer has grown from less than 24 inches to something approaching the height of the average sixth grader.
In a bold act of chivalry at the beginning of the pandemic I accepted the mantle of designated grocery shopper and over the last 3 years have become uncommonly proud of my ability to bring home the groceries efficiently and cost effectively, without catching COVID in the process. I have developed a sixth sense of choosing which human checker/bagger combination is fastest or whether the self-checkout is the way to go.
For obvious reasons the human checkers don’t ask for my ID when I am buying adult beverages. However, the self-check register freezes up instantly when I scan my 12-pack of Run Wild nonalcoholic. This necessitates a search for the MIA store person assigned to patrol the self-check corral, ever on the lookout for shoplifters, underage drinkers, and other generally shifty looking characters.
When I find one of the grocery store detectives (who is likely to have been a former patient), I say: “You know, this doesn’t have any alcohol in it.” They invariably reply with a shrug. “I know. But, the rules are the rules.” Occasionally, they may add: “It doesn’t make sense, does it?”
At first blush checking IDs for a nonalcoholic beverage may sound dumb, certainly to someone who is just a few years on either side of the legal drinking age. Why are we trying to protect some crazy teenager from the futility of getting high on a six-pack of something that at worst will make him spend most of the next couple of hours peeing?
But, there is concern in some corners that nonalcoholic drinks pose a significant threat to teenagers. Two PhDs at Stanford University have recently published a paper in which they worry that the dramatic rise in US sales of nonalcoholic drinks from 15% to 30% since 2018 may be socializing “users of alcohol drinking experiences by exposing them to the taste, look, and even brands of alcoholic beverages”.
Is there evidence to support their concern? I could only find one brief report in the Japanese literature that states that among young people “who experienced the nonalcoholic beverage intake, interest in or motivation for drinking alcoholic beverages, and/or smoking is higher than [among] those who did not.” The study didn’t appear to clearly separate the exposure in a family setting from the actual intake.
Beer is an acquired taste. If someone offered you your first taste of beer after a hot-weather set of tennis most of you would reject it and ask for water or lemonade. I can recall my first taste of beer. For some reason my father thought at age 11 or 12 I might like to try some from his glass. I’m not sure of his motivation, but he tried the same thing with oysters. I didn’t drink beer again until I was 16, motivated at that time by a group dynamic. The oyster trial, however, backfired on him and from then on he had to share his coveted dozen with me. Alcohol, unless heavily disguised by a mixer, is also not a taste that most young people find appealing.
It is unlikely that the average thrill-seeking teenager is going to ask his older-appearing buddy with a fake ID to buy him some nonalcoholic beer. Nor would he go to the effort or risk of acquiring his own fake ID just to see how it tastes. It just doesn’t compute, especially to a self-check corral patroller.
I guess one could envision a scenario in which a teenager wanting to fit in with the fast crowd would ask a trusted adult (or clueless parent) to buy him some nonalcoholic beer to bring to a party. He is running a serious risk of being laughed at by his friends if they find he’s drinking the fake stuff. It also seems unlikely that a parent would buy nonalcoholic beer to introduce his teenager to the taste of beer.
So,
Although it runs counter to my usual commitment to evidence-based decisions, making it difficult for adolescents to buy nonalcoholic beverages feels like the right think to do. As long as alcoholic and nonalcoholic beverages share the same display space and are packaged in nearly identical containers, there is ample opportunity for confusion. Recent evidence suggesting that even small amounts of alcohol increases some health risks should strengthen our resolve to minimize that confusion.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Several months ago in a letter about healthcare providers and the decision to use alcohol and other mind-altering substances on the job, I waxed enthusiastically about the new wave of no alcohol (NA) and zero (00) alcohol beers that have come on the market. In the last 2 years our local grocery store’s cooler space for nonalcoholic beer has grown from less than 24 inches to something approaching the height of the average sixth grader.
In a bold act of chivalry at the beginning of the pandemic I accepted the mantle of designated grocery shopper and over the last 3 years have become uncommonly proud of my ability to bring home the groceries efficiently and cost effectively, without catching COVID in the process. I have developed a sixth sense of choosing which human checker/bagger combination is fastest or whether the self-checkout is the way to go.
For obvious reasons the human checkers don’t ask for my ID when I am buying adult beverages. However, the self-check register freezes up instantly when I scan my 12-pack of Run Wild nonalcoholic. This necessitates a search for the MIA store person assigned to patrol the self-check corral, ever on the lookout for shoplifters, underage drinkers, and other generally shifty looking characters.
When I find one of the grocery store detectives (who is likely to have been a former patient), I say: “You know, this doesn’t have any alcohol in it.” They invariably reply with a shrug. “I know. But, the rules are the rules.” Occasionally, they may add: “It doesn’t make sense, does it?”
At first blush checking IDs for a nonalcoholic beverage may sound dumb, certainly to someone who is just a few years on either side of the legal drinking age. Why are we trying to protect some crazy teenager from the futility of getting high on a six-pack of something that at worst will make him spend most of the next couple of hours peeing?
But, there is concern in some corners that nonalcoholic drinks pose a significant threat to teenagers. Two PhDs at Stanford University have recently published a paper in which they worry that the dramatic rise in US sales of nonalcoholic drinks from 15% to 30% since 2018 may be socializing “users of alcohol drinking experiences by exposing them to the taste, look, and even brands of alcoholic beverages”.
Is there evidence to support their concern? I could only find one brief report in the Japanese literature that states that among young people “who experienced the nonalcoholic beverage intake, interest in or motivation for drinking alcoholic beverages, and/or smoking is higher than [among] those who did not.” The study didn’t appear to clearly separate the exposure in a family setting from the actual intake.
Beer is an acquired taste. If someone offered you your first taste of beer after a hot-weather set of tennis most of you would reject it and ask for water or lemonade. I can recall my first taste of beer. For some reason my father thought at age 11 or 12 I might like to try some from his glass. I’m not sure of his motivation, but he tried the same thing with oysters. I didn’t drink beer again until I was 16, motivated at that time by a group dynamic. The oyster trial, however, backfired on him and from then on he had to share his coveted dozen with me. Alcohol, unless heavily disguised by a mixer, is also not a taste that most young people find appealing.
It is unlikely that the average thrill-seeking teenager is going to ask his older-appearing buddy with a fake ID to buy him some nonalcoholic beer. Nor would he go to the effort or risk of acquiring his own fake ID just to see how it tastes. It just doesn’t compute, especially to a self-check corral patroller.
I guess one could envision a scenario in which a teenager wanting to fit in with the fast crowd would ask a trusted adult (or clueless parent) to buy him some nonalcoholic beer to bring to a party. He is running a serious risk of being laughed at by his friends if they find he’s drinking the fake stuff. It also seems unlikely that a parent would buy nonalcoholic beer to introduce his teenager to the taste of beer.
So,
Although it runs counter to my usual commitment to evidence-based decisions, making it difficult for adolescents to buy nonalcoholic beverages feels like the right think to do. As long as alcoholic and nonalcoholic beverages share the same display space and are packaged in nearly identical containers, there is ample opportunity for confusion. Recent evidence suggesting that even small amounts of alcohol increases some health risks should strengthen our resolve to minimize that confusion.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Statins for MS (Not)
Hidden behind all of the new drugs and breakthroughs reported at the 2024 ECTRIMS meetings was one paper that caught my attention.
It was that, after several years of study, simvastatin had no benefit for multiple sclerosis.
Statins for MS (and for Alzheimer’s disease) have been bandied about for some time, with arguments based on theoretical ideas, and small studies, that they’d have a beneficial effect on the disease – maybe from anti-inflammatory and other properties. In addition, they offered the benefit of being widely available and comparatively inexpensive.
Because of those studies, 15-20 years ago I used them off label for MS in a handful of patients – sometimes as an adjunct to their current treatment (limited at that point to interferons and Copaxone), or in patients who couldn’t afford the FDA-approved drugs. Although not without their drawbacks, the statins are relatively well understood and tolerated.
At some point, for reasons I’ve long forgotten, they all came off of them (at least for MS purposes). Maybe for side effects, or lack of benefit, or because new medications, with much clearer efficacies, were rolling out.
Now it seems pretty clear that statins don’t work for MS.
So was it a bad idea to try? No. Without asking questions we don’t find answers. If they’d worked out it would have been great, another tool on the neurology workbench to reach for in the right situation. It might also have led us to new avenues in MS treatment.
But it didn’t, and that’s fine. Although they don’t get the attention, we learn as much (sometimes more) from negative studies as we do from positive ones. If we put people on every drug that initially showed promise for their conditions, my patients would have a pretty huge medication list. For Alzheimer’s disease alone I remember studies that once suggested ibuprofen, statins, estrogen, nicotine, and several vitamins might be effective (“might” being the key word). Today we’re looking at the PDE5 inhibitors and semaglutide. The jury is still out on them, but whichever way it goes we’ll still learn something.
The statins are good drugs. Their benefits in cardiac and cerebrovascular disease can’t be disputed (I’m sure someone would, but that’s not the point of this piece). But, like all drugs, they don’t work for everything.
We learn from both and keep moving forward.
Dr. Block has a solo neurology practice in Scottsdale, Arizona.
Hidden behind all of the new drugs and breakthroughs reported at the 2024 ECTRIMS meetings was one paper that caught my attention.
It was that, after several years of study, simvastatin had no benefit for multiple sclerosis.
Statins for MS (and for Alzheimer’s disease) have been bandied about for some time, with arguments based on theoretical ideas, and small studies, that they’d have a beneficial effect on the disease – maybe from anti-inflammatory and other properties. In addition, they offered the benefit of being widely available and comparatively inexpensive.
Because of those studies, 15-20 years ago I used them off label for MS in a handful of patients – sometimes as an adjunct to their current treatment (limited at that point to interferons and Copaxone), or in patients who couldn’t afford the FDA-approved drugs. Although not without their drawbacks, the statins are relatively well understood and tolerated.
At some point, for reasons I’ve long forgotten, they all came off of them (at least for MS purposes). Maybe for side effects, or lack of benefit, or because new medications, with much clearer efficacies, were rolling out.
Now it seems pretty clear that statins don’t work for MS.
So was it a bad idea to try? No. Without asking questions we don’t find answers. If they’d worked out it would have been great, another tool on the neurology workbench to reach for in the right situation. It might also have led us to new avenues in MS treatment.
But it didn’t, and that’s fine. Although they don’t get the attention, we learn as much (sometimes more) from negative studies as we do from positive ones. If we put people on every drug that initially showed promise for their conditions, my patients would have a pretty huge medication list. For Alzheimer’s disease alone I remember studies that once suggested ibuprofen, statins, estrogen, nicotine, and several vitamins might be effective (“might” being the key word). Today we’re looking at the PDE5 inhibitors and semaglutide. The jury is still out on them, but whichever way it goes we’ll still learn something.
The statins are good drugs. Their benefits in cardiac and cerebrovascular disease can’t be disputed (I’m sure someone would, but that’s not the point of this piece). But, like all drugs, they don’t work for everything.
We learn from both and keep moving forward.
Dr. Block has a solo neurology practice in Scottsdale, Arizona.
Hidden behind all of the new drugs and breakthroughs reported at the 2024 ECTRIMS meetings was one paper that caught my attention.
It was that, after several years of study, simvastatin had no benefit for multiple sclerosis.
Statins for MS (and for Alzheimer’s disease) have been bandied about for some time, with arguments based on theoretical ideas, and small studies, that they’d have a beneficial effect on the disease – maybe from anti-inflammatory and other properties. In addition, they offered the benefit of being widely available and comparatively inexpensive.
Because of those studies, 15-20 years ago I used them off label for MS in a handful of patients – sometimes as an adjunct to their current treatment (limited at that point to interferons and Copaxone), or in patients who couldn’t afford the FDA-approved drugs. Although not without their drawbacks, the statins are relatively well understood and tolerated.
At some point, for reasons I’ve long forgotten, they all came off of them (at least for MS purposes). Maybe for side effects, or lack of benefit, or because new medications, with much clearer efficacies, were rolling out.
Now it seems pretty clear that statins don’t work for MS.
So was it a bad idea to try? No. Without asking questions we don’t find answers. If they’d worked out it would have been great, another tool on the neurology workbench to reach for in the right situation. It might also have led us to new avenues in MS treatment.
But it didn’t, and that’s fine. Although they don’t get the attention, we learn as much (sometimes more) from negative studies as we do from positive ones. If we put people on every drug that initially showed promise for their conditions, my patients would have a pretty huge medication list. For Alzheimer’s disease alone I remember studies that once suggested ibuprofen, statins, estrogen, nicotine, and several vitamins might be effective (“might” being the key word). Today we’re looking at the PDE5 inhibitors and semaglutide. The jury is still out on them, but whichever way it goes we’ll still learn something.
The statins are good drugs. Their benefits in cardiac and cerebrovascular disease can’t be disputed (I’m sure someone would, but that’s not the point of this piece). But, like all drugs, they don’t work for everything.
We learn from both and keep moving forward.
Dr. Block has a solo neurology practice in Scottsdale, Arizona.
Time-Restricted Eating Is Not a Metabolic Magic Bullet
This transcript has been edited for clarity.
One out of three American adults — about 100 million people in this country — have the metabolic syndrome. I’m showing you the official criteria here, but essentially this is a syndrome of insulin resistance and visceral adiposity that predisposes us to a host of chronic diseases such as diabetes, heart disease, and even dementia.
The metabolic syndrome is, fundamentally, a lifestyle disease. There is a direct line between our dietary habits and the wide availability of carbohydrate-rich, highly processed foods, and the rise in the syndrome in the population.
A saying I learned from one of my epidemiology teachers comes to mind: “Lifestyle diseases require lifestyle reinterventions.” But you know what? I’m not so sure anymore.
I’ve been around long enough to see multiple dietary fads come and go with varying efficacy. I grew up in the low-fat era, probably the most detrimental time to our national health as food manufacturers started replacing fats with carbohydrates, driving much of the problem we’re faced with today.
But I was also around for the Atkins diet and the low-carb craze — a healthier approach, all things being equal. And I’ve seen variants of these: the paleo diet (essentially a low-carb, high-protein diet based on minimally processed foods) and the Mediterranean diet, which sought to replace some percentage of fats with healthier fats.
And, of course, there is time-restricted eating.
Time-restricted eating, a variant of intermittent fasting, has the advantage of being very simple. No cookbooks, no recipes. Eat what you want — but limit it to certain hours in the day, ideally a window of less than 10 hours, such as 8 a.m. to 6 p.m.
When it comes to weight loss, the diets that work tend to work because they reduce calorie intake. I know, people will get angry about this, but thermodynamics is not just a good idea, it’s the law.
But weight loss is not the only reason we need to eat healthier. What we eat can impact our health in multiple ways; certain foods lead to more atherosclerosis, more inflammation, increased strain on the kidney and liver, and can affect our glucose homeostasis.
So I was really interested when I saw this article, “Time-Restricted Eating in Adults With Metabolic Syndrome,” appearing in Annals of Internal Medicine October 1, which examined the effect of time-restricted eating on the metabolic syndrome itself. Could this lifestyle intervention cure this lifestyle disease?
In the study, 108 individuals, all of whom had the metabolic syndrome but not full-blown diabetes, were randomized to usual care — basically, nutrition education — vs time-restricted eating. In that group, participants were instructed to reduce their window of eating by at least 4 hours to achieve an 8- to 10-hour eating window. The groups were followed for 3 months.
Now, before we get to the results, it’s important to remember that the success of a lifestyle intervention trial is quite dependent on how well people adhere to the lifestyle intervention. Time-restricted eating is not as easy as taking a pill once a day.
The researchers had participants log their consumption using a smartphone app to confirm whether they were adhering to that restricted eating window.
Broadly speaking, they did. At baseline, both groups had an eating window of about 14 hours a day — think 7 a.m. to 9 p.m. The intervention group reduced that to just under 10 hours, with 10% of days falling outside of the target window.
Lifestyle change achieved, the primary outcome was the change in hemoglobin A1c at 3 months. A1c integrates the serum glucose over time and is thus a good indicator of the success of the intervention in terms of insulin resistance. But the effect was, honestly, disappointing.
Technically, the time-restricted-eating group had a greater A1c change than the control group — by 0.1 percentage points. On average, they went from a baseline A1c of 5.87 to a 3-month A1c of 5.75.
Other metabolic syndrome markers were equally lackluster: no difference in fasting glucose, mean glucose, or fasting insulin.
There was some weight change. The control group, which got that dietary education, lost 1.5% of body weight over the 3 months. The time-restricted-eating group lost 3.3% — about 7 pounds, which is reasonable.
With that weight loss came statistically significant, albeit modest improvements in BMI, body fat percentage, and LDL cholesterol.
Of interest, despite the larger weight loss in the intermittent-fasting group, there was no difference in muscle mass loss, which is encouraging.
Taken together, we can say that, yes, it seems like time-restricted eating can help people lose some weight. This is essentially due to the fact that people eat fewer calories when they do time-restricted eating, as you can see here.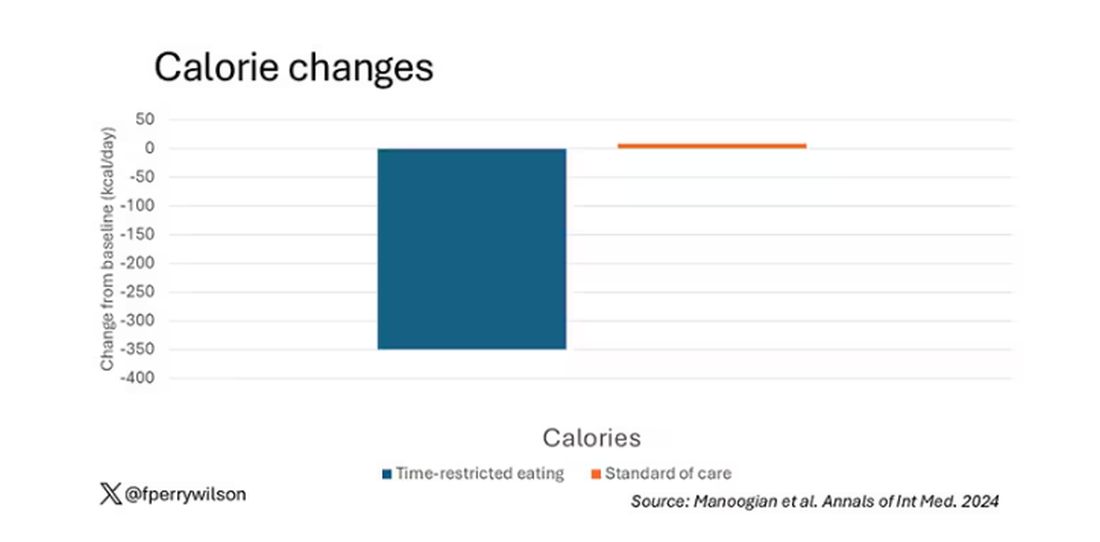
But, in the end, this trial examined whether this relatively straightforward lifestyle intervention would move the needle in terms of metabolic syndrome, and the data are not very compelling for that.
This graph shows how many of those five factors for metabolic syndrome the individuals in this trial had from the start to the end. You see that, over the 3 months, seven people in the time-restricted-eating group moved from having three criteria to two or one — being “cured” of metabolic syndrome, if you will. Nine people in the standard group were cured by that definition. Remember, they had to have at least three to have the syndrome and thus be eligible for the trial. 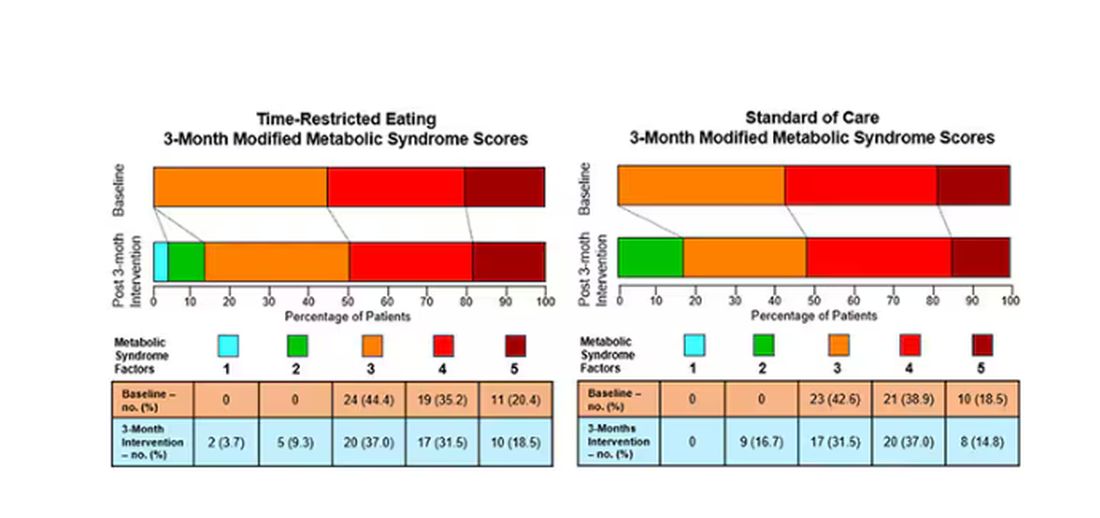
So If it just leads to weight loss by forcing people to consume less calories, then we need to acknowledge that we probably have better methods to achieve this same end. Ten years ago, I would have said that lifestyle change is the only way to end the epidemic of the metabolic syndrome in this country. Today, well, we live in a world of GLP-1 weight loss drugs. It is simply a different world now. Yes, they are expensive. Yes, they have side effects. But we need to evaluate them against the comparison. And so far, lifestyle changes alone are really no comparison.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
One out of three American adults — about 100 million people in this country — have the metabolic syndrome. I’m showing you the official criteria here, but essentially this is a syndrome of insulin resistance and visceral adiposity that predisposes us to a host of chronic diseases such as diabetes, heart disease, and even dementia.
The metabolic syndrome is, fundamentally, a lifestyle disease. There is a direct line between our dietary habits and the wide availability of carbohydrate-rich, highly processed foods, and the rise in the syndrome in the population.
A saying I learned from one of my epidemiology teachers comes to mind: “Lifestyle diseases require lifestyle reinterventions.” But you know what? I’m not so sure anymore.
I’ve been around long enough to see multiple dietary fads come and go with varying efficacy. I grew up in the low-fat era, probably the most detrimental time to our national health as food manufacturers started replacing fats with carbohydrates, driving much of the problem we’re faced with today.
But I was also around for the Atkins diet and the low-carb craze — a healthier approach, all things being equal. And I’ve seen variants of these: the paleo diet (essentially a low-carb, high-protein diet based on minimally processed foods) and the Mediterranean diet, which sought to replace some percentage of fats with healthier fats.
And, of course, there is time-restricted eating.
Time-restricted eating, a variant of intermittent fasting, has the advantage of being very simple. No cookbooks, no recipes. Eat what you want — but limit it to certain hours in the day, ideally a window of less than 10 hours, such as 8 a.m. to 6 p.m.
When it comes to weight loss, the diets that work tend to work because they reduce calorie intake. I know, people will get angry about this, but thermodynamics is not just a good idea, it’s the law.
But weight loss is not the only reason we need to eat healthier. What we eat can impact our health in multiple ways; certain foods lead to more atherosclerosis, more inflammation, increased strain on the kidney and liver, and can affect our glucose homeostasis.
So I was really interested when I saw this article, “Time-Restricted Eating in Adults With Metabolic Syndrome,” appearing in Annals of Internal Medicine October 1, which examined the effect of time-restricted eating on the metabolic syndrome itself. Could this lifestyle intervention cure this lifestyle disease?
In the study, 108 individuals, all of whom had the metabolic syndrome but not full-blown diabetes, were randomized to usual care — basically, nutrition education — vs time-restricted eating. In that group, participants were instructed to reduce their window of eating by at least 4 hours to achieve an 8- to 10-hour eating window. The groups were followed for 3 months.
Now, before we get to the results, it’s important to remember that the success of a lifestyle intervention trial is quite dependent on how well people adhere to the lifestyle intervention. Time-restricted eating is not as easy as taking a pill once a day.
The researchers had participants log their consumption using a smartphone app to confirm whether they were adhering to that restricted eating window.
Broadly speaking, they did. At baseline, both groups had an eating window of about 14 hours a day — think 7 a.m. to 9 p.m. The intervention group reduced that to just under 10 hours, with 10% of days falling outside of the target window.
Lifestyle change achieved, the primary outcome was the change in hemoglobin A1c at 3 months. A1c integrates the serum glucose over time and is thus a good indicator of the success of the intervention in terms of insulin resistance. But the effect was, honestly, disappointing.
Technically, the time-restricted-eating group had a greater A1c change than the control group — by 0.1 percentage points. On average, they went from a baseline A1c of 5.87 to a 3-month A1c of 5.75.
Other metabolic syndrome markers were equally lackluster: no difference in fasting glucose, mean glucose, or fasting insulin.
There was some weight change. The control group, which got that dietary education, lost 1.5% of body weight over the 3 months. The time-restricted-eating group lost 3.3% — about 7 pounds, which is reasonable.
With that weight loss came statistically significant, albeit modest improvements in BMI, body fat percentage, and LDL cholesterol.
Of interest, despite the larger weight loss in the intermittent-fasting group, there was no difference in muscle mass loss, which is encouraging.
Taken together, we can say that, yes, it seems like time-restricted eating can help people lose some weight. This is essentially due to the fact that people eat fewer calories when they do time-restricted eating, as you can see here.
But, in the end, this trial examined whether this relatively straightforward lifestyle intervention would move the needle in terms of metabolic syndrome, and the data are not very compelling for that.
This graph shows how many of those five factors for metabolic syndrome the individuals in this trial had from the start to the end. You see that, over the 3 months, seven people in the time-restricted-eating group moved from having three criteria to two or one — being “cured” of metabolic syndrome, if you will. Nine people in the standard group were cured by that definition. Remember, they had to have at least three to have the syndrome and thus be eligible for the trial. 
So If it just leads to weight loss by forcing people to consume less calories, then we need to acknowledge that we probably have better methods to achieve this same end. Ten years ago, I would have said that lifestyle change is the only way to end the epidemic of the metabolic syndrome in this country. Today, well, we live in a world of GLP-1 weight loss drugs. It is simply a different world now. Yes, they are expensive. Yes, they have side effects. But we need to evaluate them against the comparison. And so far, lifestyle changes alone are really no comparison.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
One out of three American adults — about 100 million people in this country — have the metabolic syndrome. I’m showing you the official criteria here, but essentially this is a syndrome of insulin resistance and visceral adiposity that predisposes us to a host of chronic diseases such as diabetes, heart disease, and even dementia.
The metabolic syndrome is, fundamentally, a lifestyle disease. There is a direct line between our dietary habits and the wide availability of carbohydrate-rich, highly processed foods, and the rise in the syndrome in the population.
A saying I learned from one of my epidemiology teachers comes to mind: “Lifestyle diseases require lifestyle reinterventions.” But you know what? I’m not so sure anymore.
I’ve been around long enough to see multiple dietary fads come and go with varying efficacy. I grew up in the low-fat era, probably the most detrimental time to our national health as food manufacturers started replacing fats with carbohydrates, driving much of the problem we’re faced with today.
But I was also around for the Atkins diet and the low-carb craze — a healthier approach, all things being equal. And I’ve seen variants of these: the paleo diet (essentially a low-carb, high-protein diet based on minimally processed foods) and the Mediterranean diet, which sought to replace some percentage of fats with healthier fats.
And, of course, there is time-restricted eating.
Time-restricted eating, a variant of intermittent fasting, has the advantage of being very simple. No cookbooks, no recipes. Eat what you want — but limit it to certain hours in the day, ideally a window of less than 10 hours, such as 8 a.m. to 6 p.m.
When it comes to weight loss, the diets that work tend to work because they reduce calorie intake. I know, people will get angry about this, but thermodynamics is not just a good idea, it’s the law.
But weight loss is not the only reason we need to eat healthier. What we eat can impact our health in multiple ways; certain foods lead to more atherosclerosis, more inflammation, increased strain on the kidney and liver, and can affect our glucose homeostasis.
So I was really interested when I saw this article, “Time-Restricted Eating in Adults With Metabolic Syndrome,” appearing in Annals of Internal Medicine October 1, which examined the effect of time-restricted eating on the metabolic syndrome itself. Could this lifestyle intervention cure this lifestyle disease?
In the study, 108 individuals, all of whom had the metabolic syndrome but not full-blown diabetes, were randomized to usual care — basically, nutrition education — vs time-restricted eating. In that group, participants were instructed to reduce their window of eating by at least 4 hours to achieve an 8- to 10-hour eating window. The groups were followed for 3 months.
Now, before we get to the results, it’s important to remember that the success of a lifestyle intervention trial is quite dependent on how well people adhere to the lifestyle intervention. Time-restricted eating is not as easy as taking a pill once a day.
The researchers had participants log their consumption using a smartphone app to confirm whether they were adhering to that restricted eating window.
Broadly speaking, they did. At baseline, both groups had an eating window of about 14 hours a day — think 7 a.m. to 9 p.m. The intervention group reduced that to just under 10 hours, with 10% of days falling outside of the target window.
Lifestyle change achieved, the primary outcome was the change in hemoglobin A1c at 3 months. A1c integrates the serum glucose over time and is thus a good indicator of the success of the intervention in terms of insulin resistance. But the effect was, honestly, disappointing.
Technically, the time-restricted-eating group had a greater A1c change than the control group — by 0.1 percentage points. On average, they went from a baseline A1c of 5.87 to a 3-month A1c of 5.75.
Other metabolic syndrome markers were equally lackluster: no difference in fasting glucose, mean glucose, or fasting insulin.
There was some weight change. The control group, which got that dietary education, lost 1.5% of body weight over the 3 months. The time-restricted-eating group lost 3.3% — about 7 pounds, which is reasonable.
With that weight loss came statistically significant, albeit modest improvements in BMI, body fat percentage, and LDL cholesterol.
Of interest, despite the larger weight loss in the intermittent-fasting group, there was no difference in muscle mass loss, which is encouraging.
Taken together, we can say that, yes, it seems like time-restricted eating can help people lose some weight. This is essentially due to the fact that people eat fewer calories when they do time-restricted eating, as you can see here.
But, in the end, this trial examined whether this relatively straightforward lifestyle intervention would move the needle in terms of metabolic syndrome, and the data are not very compelling for that.
This graph shows how many of those five factors for metabolic syndrome the individuals in this trial had from the start to the end. You see that, over the 3 months, seven people in the time-restricted-eating group moved from having three criteria to two or one — being “cured” of metabolic syndrome, if you will. Nine people in the standard group were cured by that definition. Remember, they had to have at least three to have the syndrome and thus be eligible for the trial. 
So If it just leads to weight loss by forcing people to consume less calories, then we need to acknowledge that we probably have better methods to achieve this same end. Ten years ago, I would have said that lifestyle change is the only way to end the epidemic of the metabolic syndrome in this country. Today, well, we live in a world of GLP-1 weight loss drugs. It is simply a different world now. Yes, they are expensive. Yes, they have side effects. But we need to evaluate them against the comparison. And so far, lifestyle changes alone are really no comparison.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.












