User login
Tax Questions Frequently Asked by Physicians
Physicians spend years of their lives in education and training. There are countless hours devoted to studying, researching, and clinical training, not to mention residency and possible fellowships. Then literally overnight, they transition out of a resident salary into a full-time attending pay with little to no education around what to do with this significant increase in salary.
Every job position is unique in terms of benefits, how compensation is earned, job expectations, etc. But they all share one thing in common — taxes. Increased income comes with increased taxes.
FAQ 1. What is the difference between W2 income and 1099 income?
A: If you are a W2 employee, your employer is responsible for paying half of your Social Security and Medicare taxes. You, as the employee, are then responsible only for the remaining half of your Social Security and Medicare taxes. Additionally, your employer will withhold these taxes, along with federal income taxes, from your paycheck each pay period. You are not responsible for remitting any taxes to the IRS or state agencies, as your employer will do this for you. As a W2 employee, you are not able to deduct any employee expenses against your income.
As a 1099 contractor, you are considered self-employed and are responsible for the employer and employee portion of the Social Security and Medicare taxes. You are also responsible for remitting these taxes, as well as quarterly estimated federal withholding, to the IRS and state agencies. You can deduct work-related expenses against your 1099 income.
Both types of income have pros and cons. Either of these can be more beneficial to a specific situation.
FAQ 2. How do I know if I am withholding enough taxes?
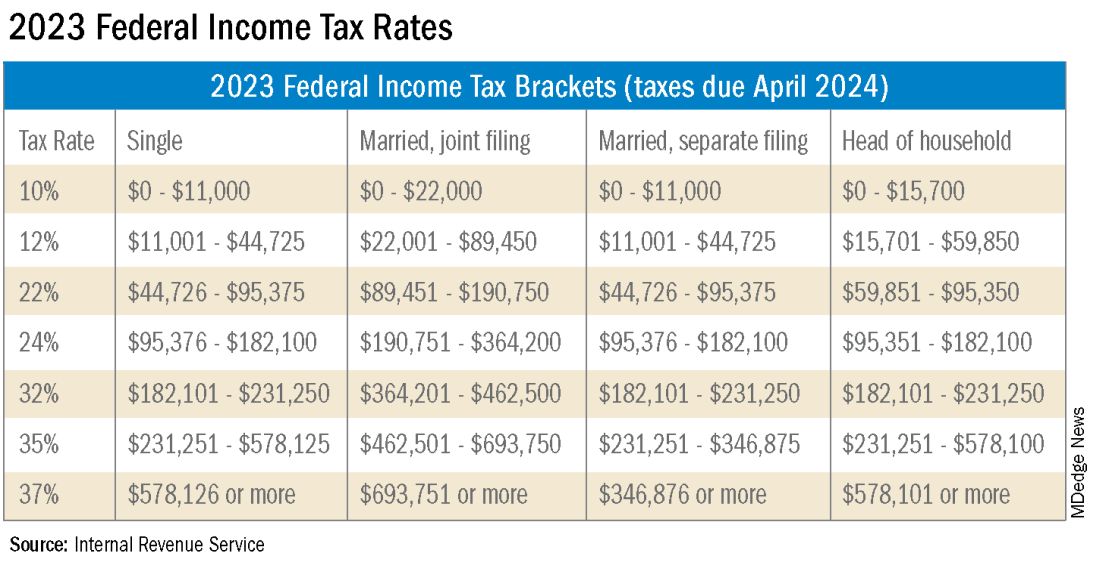
A: This is a very common issue I see, especially with physicians who are transitioning out of training into their full-time attending salary. Because this transition happens mid-year, often the first half of the year you are withholding at a rate much lower than what you will be earning as an attending and end up with a tax surprise at filing. One way to remedy this is to look at how much taxes are being withheld from your paycheck and compare this to what tax bracket you anticipate to be in, depending on filing status (Figure 1). If you do this and realize you are not withholding enough taxes, you can submit an amended form W4 to your employer to have additional withholding taken out each pay period.
FAQ 3. I am a 1099 contractor; do I need a PLLC, and should I file as an S-Corporation?
A: The term “S-Corp” gets mentioned often related to 1099 contractors and can be extremely beneficial from a tax savings perspective. Often physicians may moonlight — in addition to working in their W2 positions — and would receive this compensation as a 1099 contractor rather than an employee. This is an example of when a Professional Limited Liability Company (PLLC) might be advisable. A PLLC is created at a state level and helps shield owners from potential litigation. The owner of a PLLC pays Social Security and Medicare taxes on all income earned from the entity, and the PLLC is included in the owner’s individual income tax return.
A Small-Corporation (S-Corporation) is a tax classification that passes income through to the owners. The PLLC is now taxed as an S-Corporation, rather than a disregarded entity. The shareholders of the S-Corporation are required to pay a reasonable salary (W2 income). The remaining income passes through to the owner and is not subject to Social Security and Medicare taxes, only federal income tax. This taxation status requires an additional tax return and payroll service. Because there are additional expenses with being taxed as an S-Corporation, a cost-benefit analysis should be done before changing the tax classification to confirm that the tax savings are greater than the additional costs.
FAQ 4. What is the ‘backdoor Roth’ strategy? Should I implement it?
A: A Roth IRA is a specific type of Individual Retirement Account (IRA) that is funded with after-tax dollars. The contributions and growth in a Roth IRA can be withdrawn at retirement, tax free. As physicians who are typically high earners, you are not able to contribute directly to a Roth IRA because of income limitations. This is where the Roth conversion strategy — the backdoor Roth — comes into play. This strategy allows you to make a nondeductible traditional IRA contribution and then convert those dollars into a Roth IRA. In 2023, you can contribute up to $6,500 into this type of account. There are many additional considerations that must be made before implementing this strategy. Discussion with a financial advisor or CPA is recommended.
FAQ 5. I’ve always done my own taxes. Do I need to hire a CPA?
A: For many physicians, especially during training, your tax situation may not warrant the need for a Certified Public Accountant (CPA). However, as your income and tax complexity increase, working with a CPA not only decreases your risk for error, but also helps ensure you are not overpaying in taxes. There are many different types of services that a CPA can offer, the most basic being tax preparation. This is simply compiling your tax return based on the circumstances that occurred in the prior year. Tax planning is an additional level of service that may not be included in tax preparation cost. Tax planning is a proactive approach to taxes and helps maximize tax savings opportunities before return preparation. When interviewing a potential CPA, you can ask what level of services are included in the fees quoted.
These are just a few of the questions I regularly answer related to physicians’ taxation. The tax code is complex and ever changing. Recommendations that are made today might not be applicable or advisable in the future to any given situation. Working with a professional can ensure you have the most up-to-date and accurate information related to your taxes.
Ms. Anderson is with Physician’s Resource Services and is on Instagram @physiciansrs . Dr. Anderson is a CA-1 Resident in Anesthesia at Baylor Scott and White Health. The authors have no conflicts of interest.
Physicians spend years of their lives in education and training. There are countless hours devoted to studying, researching, and clinical training, not to mention residency and possible fellowships. Then literally overnight, they transition out of a resident salary into a full-time attending pay with little to no education around what to do with this significant increase in salary.
Every job position is unique in terms of benefits, how compensation is earned, job expectations, etc. But they all share one thing in common — taxes. Increased income comes with increased taxes.
FAQ 1. What is the difference between W2 income and 1099 income?
A: If you are a W2 employee, your employer is responsible for paying half of your Social Security and Medicare taxes. You, as the employee, are then responsible only for the remaining half of your Social Security and Medicare taxes. Additionally, your employer will withhold these taxes, along with federal income taxes, from your paycheck each pay period. You are not responsible for remitting any taxes to the IRS or state agencies, as your employer will do this for you. As a W2 employee, you are not able to deduct any employee expenses against your income.
As a 1099 contractor, you are considered self-employed and are responsible for the employer and employee portion of the Social Security and Medicare taxes. You are also responsible for remitting these taxes, as well as quarterly estimated federal withholding, to the IRS and state agencies. You can deduct work-related expenses against your 1099 income.
Both types of income have pros and cons. Either of these can be more beneficial to a specific situation.
FAQ 2. How do I know if I am withholding enough taxes?
A: This is a very common issue I see, especially with physicians who are transitioning out of training into their full-time attending salary. Because this transition happens mid-year, often the first half of the year you are withholding at a rate much lower than what you will be earning as an attending and end up with a tax surprise at filing. One way to remedy this is to look at how much taxes are being withheld from your paycheck and compare this to what tax bracket you anticipate to be in, depending on filing status (Figure 1). If you do this and realize you are not withholding enough taxes, you can submit an amended form W4 to your employer to have additional withholding taken out each pay period.

FAQ 3. I am a 1099 contractor; do I need a PLLC, and should I file as an S-Corporation?
A: The term “S-Corp” gets mentioned often related to 1099 contractors and can be extremely beneficial from a tax savings perspective. Often physicians may moonlight — in addition to working in their W2 positions — and would receive this compensation as a 1099 contractor rather than an employee. This is an example of when a Professional Limited Liability Company (PLLC) might be advisable. A PLLC is created at a state level and helps shield owners from potential litigation. The owner of a PLLC pays Social Security and Medicare taxes on all income earned from the entity, and the PLLC is included in the owner’s individual income tax return.
A Small-Corporation (S-Corporation) is a tax classification that passes income through to the owners. The PLLC is now taxed as an S-Corporation, rather than a disregarded entity. The shareholders of the S-Corporation are required to pay a reasonable salary (W2 income). The remaining income passes through to the owner and is not subject to Social Security and Medicare taxes, only federal income tax. This taxation status requires an additional tax return and payroll service. Because there are additional expenses with being taxed as an S-Corporation, a cost-benefit analysis should be done before changing the tax classification to confirm that the tax savings are greater than the additional costs.
FAQ 4. What is the ‘backdoor Roth’ strategy? Should I implement it?
A: A Roth IRA is a specific type of Individual Retirement Account (IRA) that is funded with after-tax dollars. The contributions and growth in a Roth IRA can be withdrawn at retirement, tax free. As physicians who are typically high earners, you are not able to contribute directly to a Roth IRA because of income limitations. This is where the Roth conversion strategy — the backdoor Roth — comes into play. This strategy allows you to make a nondeductible traditional IRA contribution and then convert those dollars into a Roth IRA. In 2023, you can contribute up to $6,500 into this type of account. There are many additional considerations that must be made before implementing this strategy. Discussion with a financial advisor or CPA is recommended.
FAQ 5. I’ve always done my own taxes. Do I need to hire a CPA?
A: For many physicians, especially during training, your tax situation may not warrant the need for a Certified Public Accountant (CPA). However, as your income and tax complexity increase, working with a CPA not only decreases your risk for error, but also helps ensure you are not overpaying in taxes. There are many different types of services that a CPA can offer, the most basic being tax preparation. This is simply compiling your tax return based on the circumstances that occurred in the prior year. Tax planning is an additional level of service that may not be included in tax preparation cost. Tax planning is a proactive approach to taxes and helps maximize tax savings opportunities before return preparation. When interviewing a potential CPA, you can ask what level of services are included in the fees quoted.
These are just a few of the questions I regularly answer related to physicians’ taxation. The tax code is complex and ever changing. Recommendations that are made today might not be applicable or advisable in the future to any given situation. Working with a professional can ensure you have the most up-to-date and accurate information related to your taxes.
Ms. Anderson is with Physician’s Resource Services and is on Instagram @physiciansrs . Dr. Anderson is a CA-1 Resident in Anesthesia at Baylor Scott and White Health. The authors have no conflicts of interest.
Physicians spend years of their lives in education and training. There are countless hours devoted to studying, researching, and clinical training, not to mention residency and possible fellowships. Then literally overnight, they transition out of a resident salary into a full-time attending pay with little to no education around what to do with this significant increase in salary.
Every job position is unique in terms of benefits, how compensation is earned, job expectations, etc. But they all share one thing in common — taxes. Increased income comes with increased taxes.
FAQ 1. What is the difference between W2 income and 1099 income?
A: If you are a W2 employee, your employer is responsible for paying half of your Social Security and Medicare taxes. You, as the employee, are then responsible only for the remaining half of your Social Security and Medicare taxes. Additionally, your employer will withhold these taxes, along with federal income taxes, from your paycheck each pay period. You are not responsible for remitting any taxes to the IRS or state agencies, as your employer will do this for you. As a W2 employee, you are not able to deduct any employee expenses against your income.
As a 1099 contractor, you are considered self-employed and are responsible for the employer and employee portion of the Social Security and Medicare taxes. You are also responsible for remitting these taxes, as well as quarterly estimated federal withholding, to the IRS and state agencies. You can deduct work-related expenses against your 1099 income.
Both types of income have pros and cons. Either of these can be more beneficial to a specific situation.
FAQ 2. How do I know if I am withholding enough taxes?
A: This is a very common issue I see, especially with physicians who are transitioning out of training into their full-time attending salary. Because this transition happens mid-year, often the first half of the year you are withholding at a rate much lower than what you will be earning as an attending and end up with a tax surprise at filing. One way to remedy this is to look at how much taxes are being withheld from your paycheck and compare this to what tax bracket you anticipate to be in, depending on filing status (Figure 1). If you do this and realize you are not withholding enough taxes, you can submit an amended form W4 to your employer to have additional withholding taken out each pay period.

FAQ 3. I am a 1099 contractor; do I need a PLLC, and should I file as an S-Corporation?
A: The term “S-Corp” gets mentioned often related to 1099 contractors and can be extremely beneficial from a tax savings perspective. Often physicians may moonlight — in addition to working in their W2 positions — and would receive this compensation as a 1099 contractor rather than an employee. This is an example of when a Professional Limited Liability Company (PLLC) might be advisable. A PLLC is created at a state level and helps shield owners from potential litigation. The owner of a PLLC pays Social Security and Medicare taxes on all income earned from the entity, and the PLLC is included in the owner’s individual income tax return.
A Small-Corporation (S-Corporation) is a tax classification that passes income through to the owners. The PLLC is now taxed as an S-Corporation, rather than a disregarded entity. The shareholders of the S-Corporation are required to pay a reasonable salary (W2 income). The remaining income passes through to the owner and is not subject to Social Security and Medicare taxes, only federal income tax. This taxation status requires an additional tax return and payroll service. Because there are additional expenses with being taxed as an S-Corporation, a cost-benefit analysis should be done before changing the tax classification to confirm that the tax savings are greater than the additional costs.
FAQ 4. What is the ‘backdoor Roth’ strategy? Should I implement it?
A: A Roth IRA is a specific type of Individual Retirement Account (IRA) that is funded with after-tax dollars. The contributions and growth in a Roth IRA can be withdrawn at retirement, tax free. As physicians who are typically high earners, you are not able to contribute directly to a Roth IRA because of income limitations. This is where the Roth conversion strategy — the backdoor Roth — comes into play. This strategy allows you to make a nondeductible traditional IRA contribution and then convert those dollars into a Roth IRA. In 2023, you can contribute up to $6,500 into this type of account. There are many additional considerations that must be made before implementing this strategy. Discussion with a financial advisor or CPA is recommended.
FAQ 5. I’ve always done my own taxes. Do I need to hire a CPA?
A: For many physicians, especially during training, your tax situation may not warrant the need for a Certified Public Accountant (CPA). However, as your income and tax complexity increase, working with a CPA not only decreases your risk for error, but also helps ensure you are not overpaying in taxes. There are many different types of services that a CPA can offer, the most basic being tax preparation. This is simply compiling your tax return based on the circumstances that occurred in the prior year. Tax planning is an additional level of service that may not be included in tax preparation cost. Tax planning is a proactive approach to taxes and helps maximize tax savings opportunities before return preparation. When interviewing a potential CPA, you can ask what level of services are included in the fees quoted.
These are just a few of the questions I regularly answer related to physicians’ taxation. The tax code is complex and ever changing. Recommendations that are made today might not be applicable or advisable in the future to any given situation. Working with a professional can ensure you have the most up-to-date and accurate information related to your taxes.
Ms. Anderson is with Physician’s Resource Services and is on Instagram @physiciansrs . Dr. Anderson is a CA-1 Resident in Anesthesia at Baylor Scott and White Health. The authors have no conflicts of interest.
Deciphering the usefulness of probiotics
The idea of the use of probiotics has a history going back more than a century when Russian scientist, Elie Metchnikoff, theorized that lactic acid bacteria may offer health benefits as well as promote longevity. In the early 1900s, intestinal disorders were frequently treated with nonpathogenic bacteria to replace gut microbes.
Today, the market is flooded with products from foods to prescription medications containing probiotics that extol their health benefits. It has been estimated that the global market for probiotics is more than $32 billion dollars annually and is expected to increase 8% per year.
As family doctors, patients come to us with many questions about the use of probiotics. Look online or on store shelves — there are so many types, doses, and brands of probiotics it is hard to decipher which are worth using. We older doctors never received much education about them.
Earlier this year, the World Gastroenterology Organization (WGO) developed recommendations around the use of probiotics and defined them as “live microbes that have been shown in controlled human studies to impart a health benefit.” Their recommendation is to use the strains that have been shown to be beneficial for the condition they claim to help and have been shown to do so in controlled studies. The dosage advised should be that shown to be useful in studies.
While this is an easy statement to make, it is much less so in clinical practice. The guidelines do a good job breaking down the conditions they help and the strains that have shown to be beneficial for specific conditions.
There have been claims that probiotics have been shown to be beneficial in colorectal cancer. While there have been some studies to show that they can improve markers associated with colorectal cancer, there are no data that probiotics actually do much in terms of prevention. Eating a healthy diet is more helpful here.
One area where probiotics have been shown to be beneficial is in the prevention of antibiotic-associated diarrhea. This makes sense since we know that antibiotics can kill the “good bacteria” lining the gut wall and probiotics work to replace them. Other conditions where these agents have been shown to be beneficial include radiation-induced diarrhea, acute diarrhea, irritable bowel syndrome, and colic in breast-fed infants.
The guideline contains good evidence of where and which types of probiotics are useful and it is good to look at the charts in the paper to see the specific strains recommended. It also contains an extensive reference section, and as primary care physicians, it is imperative that we educate ourselves on these agents.
While probiotics are typically sold as supplements, we should not dismiss them summarily. It is easy to do that when supplemental products are marketed and sold unethically with no clinical evidence of benefit. We need to remember that just because something is a supplement doesn’t necessarily mean that it was not studied.
Family physicians need to be able to educate their patients and answer their questions. When we don’t have the answers, we need to find them. Any time our patient doesn’t get good information from us, they will probably go to the Internet and get bad advice from someone else.
There is much ongoing research about the gut microbiome and the bacteria that can be found in the gut. Researchers are looking into the “gut-brain” axis but there is not much good evidence of this link yet. There is no evidence that probiotics can cure Alzheimer’s disease or Parkinsonism. The future may reveal different stories, but for now, we need to follow the evidence we have available.
There are many outlandish claims about what the gut microbiome is responsible for and can do for health. It is easy to have a knee-jerk reaction when anyone brings it up in conversation. We need to arm ourselves with the evidence. We are stewards of the health and safety of our patients.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, N.J. She was paid by Pfizer as a consultant on Paxlovid and is the editor in chief of Physician’s Weekly.
The idea of the use of probiotics has a history going back more than a century when Russian scientist, Elie Metchnikoff, theorized that lactic acid bacteria may offer health benefits as well as promote longevity. In the early 1900s, intestinal disorders were frequently treated with nonpathogenic bacteria to replace gut microbes.
Today, the market is flooded with products from foods to prescription medications containing probiotics that extol their health benefits. It has been estimated that the global market for probiotics is more than $32 billion dollars annually and is expected to increase 8% per year.
As family doctors, patients come to us with many questions about the use of probiotics. Look online or on store shelves — there are so many types, doses, and brands of probiotics it is hard to decipher which are worth using. We older doctors never received much education about them.
Earlier this year, the World Gastroenterology Organization (WGO) developed recommendations around the use of probiotics and defined them as “live microbes that have been shown in controlled human studies to impart a health benefit.” Their recommendation is to use the strains that have been shown to be beneficial for the condition they claim to help and have been shown to do so in controlled studies. The dosage advised should be that shown to be useful in studies.
While this is an easy statement to make, it is much less so in clinical practice. The guidelines do a good job breaking down the conditions they help and the strains that have shown to be beneficial for specific conditions.
There have been claims that probiotics have been shown to be beneficial in colorectal cancer. While there have been some studies to show that they can improve markers associated with colorectal cancer, there are no data that probiotics actually do much in terms of prevention. Eating a healthy diet is more helpful here.
One area where probiotics have been shown to be beneficial is in the prevention of antibiotic-associated diarrhea. This makes sense since we know that antibiotics can kill the “good bacteria” lining the gut wall and probiotics work to replace them. Other conditions where these agents have been shown to be beneficial include radiation-induced diarrhea, acute diarrhea, irritable bowel syndrome, and colic in breast-fed infants.
The guideline contains good evidence of where and which types of probiotics are useful and it is good to look at the charts in the paper to see the specific strains recommended. It also contains an extensive reference section, and as primary care physicians, it is imperative that we educate ourselves on these agents.
While probiotics are typically sold as supplements, we should not dismiss them summarily. It is easy to do that when supplemental products are marketed and sold unethically with no clinical evidence of benefit. We need to remember that just because something is a supplement doesn’t necessarily mean that it was not studied.
Family physicians need to be able to educate their patients and answer their questions. When we don’t have the answers, we need to find them. Any time our patient doesn’t get good information from us, they will probably go to the Internet and get bad advice from someone else.
There is much ongoing research about the gut microbiome and the bacteria that can be found in the gut. Researchers are looking into the “gut-brain” axis but there is not much good evidence of this link yet. There is no evidence that probiotics can cure Alzheimer’s disease or Parkinsonism. The future may reveal different stories, but for now, we need to follow the evidence we have available.
There are many outlandish claims about what the gut microbiome is responsible for and can do for health. It is easy to have a knee-jerk reaction when anyone brings it up in conversation. We need to arm ourselves with the evidence. We are stewards of the health and safety of our patients.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, N.J. She was paid by Pfizer as a consultant on Paxlovid and is the editor in chief of Physician’s Weekly.
The idea of the use of probiotics has a history going back more than a century when Russian scientist, Elie Metchnikoff, theorized that lactic acid bacteria may offer health benefits as well as promote longevity. In the early 1900s, intestinal disorders were frequently treated with nonpathogenic bacteria to replace gut microbes.
Today, the market is flooded with products from foods to prescription medications containing probiotics that extol their health benefits. It has been estimated that the global market for probiotics is more than $32 billion dollars annually and is expected to increase 8% per year.
As family doctors, patients come to us with many questions about the use of probiotics. Look online or on store shelves — there are so many types, doses, and brands of probiotics it is hard to decipher which are worth using. We older doctors never received much education about them.
Earlier this year, the World Gastroenterology Organization (WGO) developed recommendations around the use of probiotics and defined them as “live microbes that have been shown in controlled human studies to impart a health benefit.” Their recommendation is to use the strains that have been shown to be beneficial for the condition they claim to help and have been shown to do so in controlled studies. The dosage advised should be that shown to be useful in studies.
While this is an easy statement to make, it is much less so in clinical practice. The guidelines do a good job breaking down the conditions they help and the strains that have shown to be beneficial for specific conditions.
There have been claims that probiotics have been shown to be beneficial in colorectal cancer. While there have been some studies to show that they can improve markers associated with colorectal cancer, there are no data that probiotics actually do much in terms of prevention. Eating a healthy diet is more helpful here.
One area where probiotics have been shown to be beneficial is in the prevention of antibiotic-associated diarrhea. This makes sense since we know that antibiotics can kill the “good bacteria” lining the gut wall and probiotics work to replace them. Other conditions where these agents have been shown to be beneficial include radiation-induced diarrhea, acute diarrhea, irritable bowel syndrome, and colic in breast-fed infants.
The guideline contains good evidence of where and which types of probiotics are useful and it is good to look at the charts in the paper to see the specific strains recommended. It also contains an extensive reference section, and as primary care physicians, it is imperative that we educate ourselves on these agents.
While probiotics are typically sold as supplements, we should not dismiss them summarily. It is easy to do that when supplemental products are marketed and sold unethically with no clinical evidence of benefit. We need to remember that just because something is a supplement doesn’t necessarily mean that it was not studied.
Family physicians need to be able to educate their patients and answer their questions. When we don’t have the answers, we need to find them. Any time our patient doesn’t get good information from us, they will probably go to the Internet and get bad advice from someone else.
There is much ongoing research about the gut microbiome and the bacteria that can be found in the gut. Researchers are looking into the “gut-brain” axis but there is not much good evidence of this link yet. There is no evidence that probiotics can cure Alzheimer’s disease or Parkinsonism. The future may reveal different stories, but for now, we need to follow the evidence we have available.
There are many outlandish claims about what the gut microbiome is responsible for and can do for health. It is easy to have a knee-jerk reaction when anyone brings it up in conversation. We need to arm ourselves with the evidence. We are stewards of the health and safety of our patients.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, N.J. She was paid by Pfizer as a consultant on Paxlovid and is the editor in chief of Physician’s Weekly.
Sometimes well-intended mental health treatment hurts
We love psychiatry. We love the idea that someone can come to receive care from a physician to alleviate psychological suffering.
Some people experience such severe anguish that they are unable to relate to others. Some are so despondent that they are unable to make decisions. Some are so distressed that their thoughts become inconsistent with reality. We want all those people, and many more, to have access to effective psychiatric care. However, there are reasonable expectations that one should be able to have that a treatment will help, and that appropriate informed consent is given.
One recent article reminded us of this in a particularly poignant way.
The study in question is a recent publication looking at the universal use of psychotherapy for teenagers.1 At face value, we would have certainly considered this to be a benevolent and well-meaning intervention. Anyone who has been a teenager or has talked to one, is aware of the emotional instability punctuated by episodes of intense anxiety or irritability. It is age appropriate for a teenager to question and explore their identity. Teenagers are notoriously impulsive with a deep desire for validating interpersonal relationships. One could continue to list the symptoms of borderline personality disorder (BPD) and find a lot of similarity with the condition of transitioning from a child to an adult.
It is thus common sense to consider applying the most established therapy for BPD, dialectical behavioral therapy (DBT), to teenagers. The basics of DBT would seem to be helpful to anyone but appear particularly appropriate to this population. Mindfulness, the practice of paying attention to your present experience, allows one to realize that they are trapped in past or hypothetical future moments. Emotional regulation provides the tools that offer a frame for our feelings and involves recognizing feelings and understanding what they mean. Interpersonal work allows one to recognize and adapt to the feelings of others, while learning how to have a healthy voice with others. Distress tolerance is the exercise of learning to experience and contain our feelings.
The study looked at about 1,000 young adolescents, around 13 years old across high schools in Sydney, Australia: 598 adolescents were allocated to the intervention, and 566 to the control. The intervention consisted of eight weekly sessions of DBT lasting about 50 minutes. The results were “contrary to predictions.” Participants who received DBT “reported significantly increased total difficulties,” and “significant increases in depression and anxiety.” The effects were worse in males yet significant in both genders. The study concludes with “a reminder that present enthusiasm for universal dissemination of short-term DBT-based group skills training within schools, specifically in early adolescence, is ahead of the research evidence.”
We can’t help but wonder why the outcomes of the study were this way; here are some ideas:
• Society has natural ways of developing interpersonal skills, emotional regulation, and the ability to appreciate the present. Interpersonal skills are consistently fostered and tested in schools. Navigating high school parties, the process of organizing them, and getting invited to them requires significant social dexterity. Rejection from romantic interest, alienation from peers, rewards for accomplishment, and acceptance by other peers are some of the daily emotional obstacles that teenagers face. Being constantly taught by older individuals and scolded by parents is its own course in mindfulness. Those are few of the many natural processes of interpersonal growth that formalized therapy may impede.
• The universal discussion of psychological terms and psychiatric symptoms may not only destigmatize mental illness, but also normalize and possibly even promote it. While punishing or stigmatizing a child for having mental illness is obviously unacceptable and cruel, we do wonder if the compulsory psychotherapy may provide negative effects. Psychotherapies, especially manualized ones, were developed to alleviate mental suffering. It seems possible that this format normalizes pathology.
In 1961, Erving Goffman described the concept of sane people appearing insane in an asylum as “mortification.” In 2023, we have much improved, but have we done something to internalize patterns of suffering and alienation rather than dispel them? They are given forms that explain what the feeling of depression is when they may have never considered it. They are given tools to handle distress, when distress may not be present.
• Many human beings live on a fairly tight rope of suppression and the less adaptive repression. Suppression is the defense mechanism by which individuals make an effort to put distressing thoughts out of conscious awareness. After a difficult breakup a teenager may ask some friends to go out and watch a movie, making efforts to put negative feelings out of conscious awareness until there is an opportunity to cope adaptively with those stressors.
Repression is the defense mechanism by which individuals make an effort to prevent distressing thoughts from entering conscious awareness in the first place. After a difficult breakup a teenager acts like nothing happened. While not particularly adaptive, many people live with significant repression and without particular anguish. It is possible that uncovering all of those repressed and suppressed feelings through the exploratory work of therapy may destabilize individuals from their tight rope.
• A less problematic explanation could also be what was previously referred to as therapeutic regression. In psychoanalytic theory, patients are generally thought to have a compromise formation, a psychological strategy used to reconcile conflicting drives. The compromise formation is the way a patient balances their desires against moral expectations and the realities of the external world. In therapy, that compromise formation can be challenged, leading to therapeutic regression.
By uncovering and confronting deeply rooted feelings, a patient may find that their symptoms temporarily intensify. This may not be a problem, but a necessary step to growth in some patients. It is possible that a program longer than 8 weeks would have overcome a temporary worsening in outcome measures.
While it’s easy to highlight the darker moments in psychiatric history, psychiatry has grown into a field which offers well-accepted and uncontroversially promoted forms of treatment. This is evolution, exemplified by the mere consideration of the universal use of psychotherapy for teenagers. But this raises important questions about the potential unintended consequences of normalizing and formalizing therapy. It prompted us to reflect on whether psychiatric treatment is always the best solution and if it might, at times, impede natural processes of growth and coping.
In this context, the study on universal DBT-based group skills training for teenagers challenged our assumptions. The unexpected outcomes suggest that societal and educational systems may naturally foster many of the skills that formalized therapy seeks to provide, and may do so with greater efficacy than that which prescriptive psychiatric treatments have to offer. Moreover, the universal discussion of psychiatric symptoms may not only destigmatize mental illness but also normalize it, potentially leading to unnecessary pathology.
Finally, the study prompted us to consider the fine balance that people find themselves in, questioning whether we should be so certain that our interventions can always provide a better outcome than an individual’s current coping mechanisms. These findings serve as a valuable reminder that our enthusiasm for widespread psychiatric interventions should be tempered by rigorous research and a nuanced understanding of human psychology and development.
This study could be an example of the grandiose stance psychiatry has at times taken of late, suggesting the field has an intervention for all that ails you and can serve as a corrective to society’s maladaptive deviations. Rising rates of mental illness in the community are not interpreted as a failing of the field of psychiatry, but as evidence that we need more psychiatrists. Acts of gun violence, ever increasing rates suicides, and even political disagreements are met with the idea that if only we had more mental health capacity, this could be avoided.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. He has no conflicts of interest. Dr. ZoBell is a fourth-year senior resident at UCSD Psychiatry Residency Program. She is currently serving as the program’s Chief Resident at the VA San Diego on the inpatient psychiatric unit. Dr. ZoBell is interested in outpatient and emergency psychiatry as well as psychotherapy. Dr. Lehman is a professor of psychiatry at the University of California, San Diego. He is codirector of all acute and intensive psychiatric treatment at the Veterans Affairs Medical Center in San Diego, where he practices clinical psychiatry. He has no conflicts of interest.
Reference
1. Harvey, LJ, et al. Investigating the efficacy of a Dialectical behaviour therapy-based universal intervention on adolescent social and emotional well-being outcomes. Behav Res Ther. 2023 Oct. doi: 10.1016/j.brat.2023.104408.
We love psychiatry. We love the idea that someone can come to receive care from a physician to alleviate psychological suffering.
Some people experience such severe anguish that they are unable to relate to others. Some are so despondent that they are unable to make decisions. Some are so distressed that their thoughts become inconsistent with reality. We want all those people, and many more, to have access to effective psychiatric care. However, there are reasonable expectations that one should be able to have that a treatment will help, and that appropriate informed consent is given.
One recent article reminded us of this in a particularly poignant way.
The study in question is a recent publication looking at the universal use of psychotherapy for teenagers.1 At face value, we would have certainly considered this to be a benevolent and well-meaning intervention. Anyone who has been a teenager or has talked to one, is aware of the emotional instability punctuated by episodes of intense anxiety or irritability. It is age appropriate for a teenager to question and explore their identity. Teenagers are notoriously impulsive with a deep desire for validating interpersonal relationships. One could continue to list the symptoms of borderline personality disorder (BPD) and find a lot of similarity with the condition of transitioning from a child to an adult.
It is thus common sense to consider applying the most established therapy for BPD, dialectical behavioral therapy (DBT), to teenagers. The basics of DBT would seem to be helpful to anyone but appear particularly appropriate to this population. Mindfulness, the practice of paying attention to your present experience, allows one to realize that they are trapped in past or hypothetical future moments. Emotional regulation provides the tools that offer a frame for our feelings and involves recognizing feelings and understanding what they mean. Interpersonal work allows one to recognize and adapt to the feelings of others, while learning how to have a healthy voice with others. Distress tolerance is the exercise of learning to experience and contain our feelings.
The study looked at about 1,000 young adolescents, around 13 years old across high schools in Sydney, Australia: 598 adolescents were allocated to the intervention, and 566 to the control. The intervention consisted of eight weekly sessions of DBT lasting about 50 minutes. The results were “contrary to predictions.” Participants who received DBT “reported significantly increased total difficulties,” and “significant increases in depression and anxiety.” The effects were worse in males yet significant in both genders. The study concludes with “a reminder that present enthusiasm for universal dissemination of short-term DBT-based group skills training within schools, specifically in early adolescence, is ahead of the research evidence.”
We can’t help but wonder why the outcomes of the study were this way; here are some ideas:
• Society has natural ways of developing interpersonal skills, emotional regulation, and the ability to appreciate the present. Interpersonal skills are consistently fostered and tested in schools. Navigating high school parties, the process of organizing them, and getting invited to them requires significant social dexterity. Rejection from romantic interest, alienation from peers, rewards for accomplishment, and acceptance by other peers are some of the daily emotional obstacles that teenagers face. Being constantly taught by older individuals and scolded by parents is its own course in mindfulness. Those are few of the many natural processes of interpersonal growth that formalized therapy may impede.
• The universal discussion of psychological terms and psychiatric symptoms may not only destigmatize mental illness, but also normalize and possibly even promote it. While punishing or stigmatizing a child for having mental illness is obviously unacceptable and cruel, we do wonder if the compulsory psychotherapy may provide negative effects. Psychotherapies, especially manualized ones, were developed to alleviate mental suffering. It seems possible that this format normalizes pathology.
In 1961, Erving Goffman described the concept of sane people appearing insane in an asylum as “mortification.” In 2023, we have much improved, but have we done something to internalize patterns of suffering and alienation rather than dispel them? They are given forms that explain what the feeling of depression is when they may have never considered it. They are given tools to handle distress, when distress may not be present.
• Many human beings live on a fairly tight rope of suppression and the less adaptive repression. Suppression is the defense mechanism by which individuals make an effort to put distressing thoughts out of conscious awareness. After a difficult breakup a teenager may ask some friends to go out and watch a movie, making efforts to put negative feelings out of conscious awareness until there is an opportunity to cope adaptively with those stressors.
Repression is the defense mechanism by which individuals make an effort to prevent distressing thoughts from entering conscious awareness in the first place. After a difficult breakup a teenager acts like nothing happened. While not particularly adaptive, many people live with significant repression and without particular anguish. It is possible that uncovering all of those repressed and suppressed feelings through the exploratory work of therapy may destabilize individuals from their tight rope.
• A less problematic explanation could also be what was previously referred to as therapeutic regression. In psychoanalytic theory, patients are generally thought to have a compromise formation, a psychological strategy used to reconcile conflicting drives. The compromise formation is the way a patient balances their desires against moral expectations and the realities of the external world. In therapy, that compromise formation can be challenged, leading to therapeutic regression.
By uncovering and confronting deeply rooted feelings, a patient may find that their symptoms temporarily intensify. This may not be a problem, but a necessary step to growth in some patients. It is possible that a program longer than 8 weeks would have overcome a temporary worsening in outcome measures.
While it’s easy to highlight the darker moments in psychiatric history, psychiatry has grown into a field which offers well-accepted and uncontroversially promoted forms of treatment. This is evolution, exemplified by the mere consideration of the universal use of psychotherapy for teenagers. But this raises important questions about the potential unintended consequences of normalizing and formalizing therapy. It prompted us to reflect on whether psychiatric treatment is always the best solution and if it might, at times, impede natural processes of growth and coping.
In this context, the study on universal DBT-based group skills training for teenagers challenged our assumptions. The unexpected outcomes suggest that societal and educational systems may naturally foster many of the skills that formalized therapy seeks to provide, and may do so with greater efficacy than that which prescriptive psychiatric treatments have to offer. Moreover, the universal discussion of psychiatric symptoms may not only destigmatize mental illness but also normalize it, potentially leading to unnecessary pathology.
Finally, the study prompted us to consider the fine balance that people find themselves in, questioning whether we should be so certain that our interventions can always provide a better outcome than an individual’s current coping mechanisms. These findings serve as a valuable reminder that our enthusiasm for widespread psychiatric interventions should be tempered by rigorous research and a nuanced understanding of human psychology and development.
This study could be an example of the grandiose stance psychiatry has at times taken of late, suggesting the field has an intervention for all that ails you and can serve as a corrective to society’s maladaptive deviations. Rising rates of mental illness in the community are not interpreted as a failing of the field of psychiatry, but as evidence that we need more psychiatrists. Acts of gun violence, ever increasing rates suicides, and even political disagreements are met with the idea that if only we had more mental health capacity, this could be avoided.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. He has no conflicts of interest. Dr. ZoBell is a fourth-year senior resident at UCSD Psychiatry Residency Program. She is currently serving as the program’s Chief Resident at the VA San Diego on the inpatient psychiatric unit. Dr. ZoBell is interested in outpatient and emergency psychiatry as well as psychotherapy. Dr. Lehman is a professor of psychiatry at the University of California, San Diego. He is codirector of all acute and intensive psychiatric treatment at the Veterans Affairs Medical Center in San Diego, where he practices clinical psychiatry. He has no conflicts of interest.
Reference
1. Harvey, LJ, et al. Investigating the efficacy of a Dialectical behaviour therapy-based universal intervention on adolescent social and emotional well-being outcomes. Behav Res Ther. 2023 Oct. doi: 10.1016/j.brat.2023.104408.
We love psychiatry. We love the idea that someone can come to receive care from a physician to alleviate psychological suffering.
Some people experience such severe anguish that they are unable to relate to others. Some are so despondent that they are unable to make decisions. Some are so distressed that their thoughts become inconsistent with reality. We want all those people, and many more, to have access to effective psychiatric care. However, there are reasonable expectations that one should be able to have that a treatment will help, and that appropriate informed consent is given.
One recent article reminded us of this in a particularly poignant way.
The study in question is a recent publication looking at the universal use of psychotherapy for teenagers.1 At face value, we would have certainly considered this to be a benevolent and well-meaning intervention. Anyone who has been a teenager or has talked to one, is aware of the emotional instability punctuated by episodes of intense anxiety or irritability. It is age appropriate for a teenager to question and explore their identity. Teenagers are notoriously impulsive with a deep desire for validating interpersonal relationships. One could continue to list the symptoms of borderline personality disorder (BPD) and find a lot of similarity with the condition of transitioning from a child to an adult.
It is thus common sense to consider applying the most established therapy for BPD, dialectical behavioral therapy (DBT), to teenagers. The basics of DBT would seem to be helpful to anyone but appear particularly appropriate to this population. Mindfulness, the practice of paying attention to your present experience, allows one to realize that they are trapped in past or hypothetical future moments. Emotional regulation provides the tools that offer a frame for our feelings and involves recognizing feelings and understanding what they mean. Interpersonal work allows one to recognize and adapt to the feelings of others, while learning how to have a healthy voice with others. Distress tolerance is the exercise of learning to experience and contain our feelings.
The study looked at about 1,000 young adolescents, around 13 years old across high schools in Sydney, Australia: 598 adolescents were allocated to the intervention, and 566 to the control. The intervention consisted of eight weekly sessions of DBT lasting about 50 minutes. The results were “contrary to predictions.” Participants who received DBT “reported significantly increased total difficulties,” and “significant increases in depression and anxiety.” The effects were worse in males yet significant in both genders. The study concludes with “a reminder that present enthusiasm for universal dissemination of short-term DBT-based group skills training within schools, specifically in early adolescence, is ahead of the research evidence.”
We can’t help but wonder why the outcomes of the study were this way; here are some ideas:
• Society has natural ways of developing interpersonal skills, emotional regulation, and the ability to appreciate the present. Interpersonal skills are consistently fostered and tested in schools. Navigating high school parties, the process of organizing them, and getting invited to them requires significant social dexterity. Rejection from romantic interest, alienation from peers, rewards for accomplishment, and acceptance by other peers are some of the daily emotional obstacles that teenagers face. Being constantly taught by older individuals and scolded by parents is its own course in mindfulness. Those are few of the many natural processes of interpersonal growth that formalized therapy may impede.
• The universal discussion of psychological terms and psychiatric symptoms may not only destigmatize mental illness, but also normalize and possibly even promote it. While punishing or stigmatizing a child for having mental illness is obviously unacceptable and cruel, we do wonder if the compulsory psychotherapy may provide negative effects. Psychotherapies, especially manualized ones, were developed to alleviate mental suffering. It seems possible that this format normalizes pathology.
In 1961, Erving Goffman described the concept of sane people appearing insane in an asylum as “mortification.” In 2023, we have much improved, but have we done something to internalize patterns of suffering and alienation rather than dispel them? They are given forms that explain what the feeling of depression is when they may have never considered it. They are given tools to handle distress, when distress may not be present.
• Many human beings live on a fairly tight rope of suppression and the less adaptive repression. Suppression is the defense mechanism by which individuals make an effort to put distressing thoughts out of conscious awareness. After a difficult breakup a teenager may ask some friends to go out and watch a movie, making efforts to put negative feelings out of conscious awareness until there is an opportunity to cope adaptively with those stressors.
Repression is the defense mechanism by which individuals make an effort to prevent distressing thoughts from entering conscious awareness in the first place. After a difficult breakup a teenager acts like nothing happened. While not particularly adaptive, many people live with significant repression and without particular anguish. It is possible that uncovering all of those repressed and suppressed feelings through the exploratory work of therapy may destabilize individuals from their tight rope.
• A less problematic explanation could also be what was previously referred to as therapeutic regression. In psychoanalytic theory, patients are generally thought to have a compromise formation, a psychological strategy used to reconcile conflicting drives. The compromise formation is the way a patient balances their desires against moral expectations and the realities of the external world. In therapy, that compromise formation can be challenged, leading to therapeutic regression.
By uncovering and confronting deeply rooted feelings, a patient may find that their symptoms temporarily intensify. This may not be a problem, but a necessary step to growth in some patients. It is possible that a program longer than 8 weeks would have overcome a temporary worsening in outcome measures.
While it’s easy to highlight the darker moments in psychiatric history, psychiatry has grown into a field which offers well-accepted and uncontroversially promoted forms of treatment. This is evolution, exemplified by the mere consideration of the universal use of psychotherapy for teenagers. But this raises important questions about the potential unintended consequences of normalizing and formalizing therapy. It prompted us to reflect on whether psychiatric treatment is always the best solution and if it might, at times, impede natural processes of growth and coping.
In this context, the study on universal DBT-based group skills training for teenagers challenged our assumptions. The unexpected outcomes suggest that societal and educational systems may naturally foster many of the skills that formalized therapy seeks to provide, and may do so with greater efficacy than that which prescriptive psychiatric treatments have to offer. Moreover, the universal discussion of psychiatric symptoms may not only destigmatize mental illness but also normalize it, potentially leading to unnecessary pathology.
Finally, the study prompted us to consider the fine balance that people find themselves in, questioning whether we should be so certain that our interventions can always provide a better outcome than an individual’s current coping mechanisms. These findings serve as a valuable reminder that our enthusiasm for widespread psychiatric interventions should be tempered by rigorous research and a nuanced understanding of human psychology and development.
This study could be an example of the grandiose stance psychiatry has at times taken of late, suggesting the field has an intervention for all that ails you and can serve as a corrective to society’s maladaptive deviations. Rising rates of mental illness in the community are not interpreted as a failing of the field of psychiatry, but as evidence that we need more psychiatrists. Acts of gun violence, ever increasing rates suicides, and even political disagreements are met with the idea that if only we had more mental health capacity, this could be avoided.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. He has no conflicts of interest. Dr. ZoBell is a fourth-year senior resident at UCSD Psychiatry Residency Program. She is currently serving as the program’s Chief Resident at the VA San Diego on the inpatient psychiatric unit. Dr. ZoBell is interested in outpatient and emergency psychiatry as well as psychotherapy. Dr. Lehman is a professor of psychiatry at the University of California, San Diego. He is codirector of all acute and intensive psychiatric treatment at the Veterans Affairs Medical Center in San Diego, where he practices clinical psychiatry. He has no conflicts of interest.
Reference
1. Harvey, LJ, et al. Investigating the efficacy of a Dialectical behaviour therapy-based universal intervention on adolescent social and emotional well-being outcomes. Behav Res Ther. 2023 Oct. doi: 10.1016/j.brat.2023.104408.
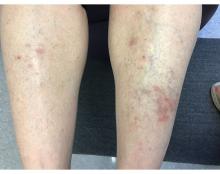
A 55-year-old female presented a with few years' history of pruritic plaques on her shins and wrists
. Lesions may have a covering of scale. HLP commonly affects middle aged men and women. Lesions are most commonly located bilaterally on the shins and ankles and can be painful or pruritic. The differential diagnosis for the condition includes lichen simplex chronicus, connective tissue disease, and other skin disorders that cause hyperkeratosis. This wide differential makes histopathological analysis a useful tool in confirming the diagnosis of HLP.
A definitive diagnosis can be made via skin biopsy. Histopathology reveals hyperkeratosis, acanthosis, and a band-like lymphocytic infiltrate in the dermis. An eosinophilic infiltrate may be present. Other common features include saw tooth rete ridges and Civatte bodies, which are apoptotic keratinocytes. The lymphocytic infiltrate may indicate an autoimmune etiology in which the body’s immune system erroneously attacks itself. However, the exact cause is not known and genetic and environmental factors may play a role.
The treatment of HLP includes symptomatic management and control of inflammation. Topical steroids can be prescribed to manage the inflammation and associated pruritus, and emollient creams and moisturizers are helpful in controlling the dryness. Oral steroids, immunosuppressant medications, or retinoids may be necessary in more severe cases. In addition, psoralen plus ultraviolet A (PUVA) light therapy has been found to be beneficial in some cases. Squamous cell carcinoma may arise in lesions.
This case and photo were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Florida, and Donna Bilu Martin, MD; Premier Dermatology, MD, Aventura, Florida. The column was edited by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Arnold DL, Krishnamurthy K. Lichen Planus. [Updated 2023 Jun 1]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK526126/
Jaime TJ et al. An Bras Dermatol. 2011 Jul-Aug;86(4 Suppl 1):S96-9.
Mirchandani S et al. Med Pharm Rep. 2020 Apr;93(2):210-2. .
Whittington CP et al. Arch Pathol Lab Med. 2023 Jun 19. doi: 10.5858/arpa.2022-0515-RA.
. Lesions may have a covering of scale. HLP commonly affects middle aged men and women. Lesions are most commonly located bilaterally on the shins and ankles and can be painful or pruritic. The differential diagnosis for the condition includes lichen simplex chronicus, connective tissue disease, and other skin disorders that cause hyperkeratosis. This wide differential makes histopathological analysis a useful tool in confirming the diagnosis of HLP.
A definitive diagnosis can be made via skin biopsy. Histopathology reveals hyperkeratosis, acanthosis, and a band-like lymphocytic infiltrate in the dermis. An eosinophilic infiltrate may be present. Other common features include saw tooth rete ridges and Civatte bodies, which are apoptotic keratinocytes. The lymphocytic infiltrate may indicate an autoimmune etiology in which the body’s immune system erroneously attacks itself. However, the exact cause is not known and genetic and environmental factors may play a role.
The treatment of HLP includes symptomatic management and control of inflammation. Topical steroids can be prescribed to manage the inflammation and associated pruritus, and emollient creams and moisturizers are helpful in controlling the dryness. Oral steroids, immunosuppressant medications, or retinoids may be necessary in more severe cases. In addition, psoralen plus ultraviolet A (PUVA) light therapy has been found to be beneficial in some cases. Squamous cell carcinoma may arise in lesions.
This case and photo were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Florida, and Donna Bilu Martin, MD; Premier Dermatology, MD, Aventura, Florida. The column was edited by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Arnold DL, Krishnamurthy K. Lichen Planus. [Updated 2023 Jun 1]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK526126/
Jaime TJ et al. An Bras Dermatol. 2011 Jul-Aug;86(4 Suppl 1):S96-9.
Mirchandani S et al. Med Pharm Rep. 2020 Apr;93(2):210-2. .
Whittington CP et al. Arch Pathol Lab Med. 2023 Jun 19. doi: 10.5858/arpa.2022-0515-RA.
. Lesions may have a covering of scale. HLP commonly affects middle aged men and women. Lesions are most commonly located bilaterally on the shins and ankles and can be painful or pruritic. The differential diagnosis for the condition includes lichen simplex chronicus, connective tissue disease, and other skin disorders that cause hyperkeratosis. This wide differential makes histopathological analysis a useful tool in confirming the diagnosis of HLP.
A definitive diagnosis can be made via skin biopsy. Histopathology reveals hyperkeratosis, acanthosis, and a band-like lymphocytic infiltrate in the dermis. An eosinophilic infiltrate may be present. Other common features include saw tooth rete ridges and Civatte bodies, which are apoptotic keratinocytes. The lymphocytic infiltrate may indicate an autoimmune etiology in which the body’s immune system erroneously attacks itself. However, the exact cause is not known and genetic and environmental factors may play a role.
The treatment of HLP includes symptomatic management and control of inflammation. Topical steroids can be prescribed to manage the inflammation and associated pruritus, and emollient creams and moisturizers are helpful in controlling the dryness. Oral steroids, immunosuppressant medications, or retinoids may be necessary in more severe cases. In addition, psoralen plus ultraviolet A (PUVA) light therapy has been found to be beneficial in some cases. Squamous cell carcinoma may arise in lesions.
This case and photo were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Florida, and Donna Bilu Martin, MD; Premier Dermatology, MD, Aventura, Florida. The column was edited by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Arnold DL, Krishnamurthy K. Lichen Planus. [Updated 2023 Jun 1]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK526126/
Jaime TJ et al. An Bras Dermatol. 2011 Jul-Aug;86(4 Suppl 1):S96-9.
Mirchandani S et al. Med Pharm Rep. 2020 Apr;93(2):210-2. .
Whittington CP et al. Arch Pathol Lab Med. 2023 Jun 19. doi: 10.5858/arpa.2022-0515-RA.
Erectile Dysfunction Rx: Give It a Shot
This transcript has been edited for clarity.
I’m Dr Rachel Rubin. I am a urologist with fellowship training in sexual medicine. Today I’m going to explain why I may recommend that your patients put a needle directly into their penises for help with erectile dysfunction (ED).
I know that sounds crazy, but in a recent video when I talked about erection hardness, I acknowledged that it may not be easy to talk with patients about their penises, but it’s important.
ED can be a marker for cardiovascular disease, with 50% of our 50-year-old patients having ED. As physicians, we must do a better job of talking to our patients about ED and letting them know that it’s a marker for overall health.
How do we treat ED? Primary care doctors can do a great deal for patients with ED, and there are other things that urologists can do when you run out of options in your own toolbox.
What’s important for a healthy erection? You need three things: healthy muscle, healthy nerves, and healthy arteries. If anything goes wrong with muscles, nerves, or arteries, this is what leads to ED. Think through the algorithm of your patient’s medical history: Do they have diabetes, which can affect their nerves? Do they have high blood pressure, which can affect their arteries? Do they have problems with testosterone, which can affect the smooth muscles of the penis? Understanding your patient’s history can be really helpful when you figure out what is the best treatment strategy for your patient.
For the penis to work, those smooth muscles have to relax; therefore, your brain has to be relaxed, along with your pelvic floor muscles. The smooth muscle of the penis has to be relaxed so it can fill with blood, increase in girth and size, and hold that erection in place.
To treat ED, we have a biopsychosocial toolbox. Biology refers to the muscles, arteries, and nerves. The psychosocial component is stress: If your brain is stressed, you have a lot of adrenaline around that can tighten those smooth muscles and cause you to lose an erection.
So, what are these treatments? I’ll start with lifestyle. A healthy heart means a healthy penis, so, all of the things you already recommend for lifestyle changes can really help with ED. Sleep is important. Does your patient need a sleep study? Do they have sleep apnea? Are they exercising? Recent data show that exercise may be just as effective, if not more effective, than Viagra. How about a good diet? The Mediterranean diet seems to be the most helpful. So, encourage your patients to make dietary, exercise, sleep, and other lifestyle changes if they want to improve erectile function.
What about sex education? Most physicians didn’t get great education about sex in medical school, but it’s very important to our patients who likewise have had inadequate sex education. Ask questions, talk to them, explain what is normal.
I can’t stress enough how important mental health is to a great sex life. Everyone would benefit from sex therapy and becoming better at sex. We need to get better at communicating and educating patients and their partners to maximize their quality of life. If you need to refer to a specialist, we recommend going to psychologytoday.com or aasect.org to find a local sex therapist. Call them and use them in your referral networks.
In the “bio” component of the biopsychosocial approach, we can do a lot to treat ED with medications and hormones. Testosterone has been shown to help with low libido and erectile function. Checking the patient’s testosterone level can be very helpful. Pills — we are familiar with Viagra, Cialis, Levitra, and Stendra. The oral PDE-5 inhibitors have been around since the late 1990s and they work quite well for many people with ED. Viagra and Cialis are generic now and patients can get them fairly inexpensively with discount coupons from GoodRx or Cost Plus Drugs. They may not even have to worry about insurance coverage.
Pills relax the smooth muscle of the penis so that it fills with blood and becomes erect, but they don’t work for everybody. If pills stop working, we often talk about synergistic treatments — combining pills and devices. Devices for ED should be discussed more often, and clinicians should consider prescribing them. We commonly discuss eyeglasses and wheelchairs, but we don’t talk about the sexual health devices that could help patients have more success and fun in the bedroom.
What are the various types of devices for ED? One common device is a vacuum pump, which can be very effective. This is how they work: The penis is lubricated and placed into the pump. A button on the pump creates suction that brings blood into the penis. The patient then applies a constriction band around the base of the penis to hold that erection in place.
“Sex tech” has really expanded to help patients with ED with devices that vibrate and hold the erection in place. Vibrating devices allow for a better orgasm. We even have devices that monitor erectile fitness (like a Fitbit for the penis), gathering data to help patients understand the firmness of their erections.
Devices are helpful adjuncts, but they don’t always do enough to achieve an erect penis that’s hard enough for penetration. In those cases, we can recommend injections that increase smooth muscle relaxation of the penis. I know it sounds crazy. If the muscles, arteries, and nerves of the penis aren’t functioning well, additional smooth muscle relaxation can be achieved by injecting alprostadil (prostaglandin E1) directly into the penis. It’s a tiny needle. It doesn’t hurt. These injections can be quite helpful for our patients, and we often recommend them.
But what happens when your patient doesn’t even respond to injections or any of the synergistic treatments? They’ve tried everything. Urologists may suggest a surgical option, the penile implant. Penile implants contain a pump inside the scrotum that fills with fluid, allowing a rigid erection. Penile implants are wonderful for patients who can no longer get erections. Talking to a urologist about the pros and the cons and the risks and benefits of surgically placed implants is very important.
Finally, ED is a marker for cardiovascular disease. These patients may need a cardiology workup. They need to improve their general health. We have to ask our patients about their goals and what they care about, and find a toolbox that makes sense for each patient and couple to maximize their sexual health and quality of life. Don’t give up. If you have questions, let us know.
Rachel S. Rubin, MD, is Assistant Clinical Professor, Department of Urology, Georgetown University, Washington, DC; Private practice, Rachel Rubin MD PLLC, North Bethesda, Maryland. She disclosed ties with Sprout, Maternal Medical, Absorption Pharmaceuticals, GSK, and Endo.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I’m Dr Rachel Rubin. I am a urologist with fellowship training in sexual medicine. Today I’m going to explain why I may recommend that your patients put a needle directly into their penises for help with erectile dysfunction (ED).
I know that sounds crazy, but in a recent video when I talked about erection hardness, I acknowledged that it may not be easy to talk with patients about their penises, but it’s important.
ED can be a marker for cardiovascular disease, with 50% of our 50-year-old patients having ED. As physicians, we must do a better job of talking to our patients about ED and letting them know that it’s a marker for overall health.
How do we treat ED? Primary care doctors can do a great deal for patients with ED, and there are other things that urologists can do when you run out of options in your own toolbox.
What’s important for a healthy erection? You need three things: healthy muscle, healthy nerves, and healthy arteries. If anything goes wrong with muscles, nerves, or arteries, this is what leads to ED. Think through the algorithm of your patient’s medical history: Do they have diabetes, which can affect their nerves? Do they have high blood pressure, which can affect their arteries? Do they have problems with testosterone, which can affect the smooth muscles of the penis? Understanding your patient’s history can be really helpful when you figure out what is the best treatment strategy for your patient.
For the penis to work, those smooth muscles have to relax; therefore, your brain has to be relaxed, along with your pelvic floor muscles. The smooth muscle of the penis has to be relaxed so it can fill with blood, increase in girth and size, and hold that erection in place.
To treat ED, we have a biopsychosocial toolbox. Biology refers to the muscles, arteries, and nerves. The psychosocial component is stress: If your brain is stressed, you have a lot of adrenaline around that can tighten those smooth muscles and cause you to lose an erection.
So, what are these treatments? I’ll start with lifestyle. A healthy heart means a healthy penis, so, all of the things you already recommend for lifestyle changes can really help with ED. Sleep is important. Does your patient need a sleep study? Do they have sleep apnea? Are they exercising? Recent data show that exercise may be just as effective, if not more effective, than Viagra. How about a good diet? The Mediterranean diet seems to be the most helpful. So, encourage your patients to make dietary, exercise, sleep, and other lifestyle changes if they want to improve erectile function.
What about sex education? Most physicians didn’t get great education about sex in medical school, but it’s very important to our patients who likewise have had inadequate sex education. Ask questions, talk to them, explain what is normal.
I can’t stress enough how important mental health is to a great sex life. Everyone would benefit from sex therapy and becoming better at sex. We need to get better at communicating and educating patients and their partners to maximize their quality of life. If you need to refer to a specialist, we recommend going to psychologytoday.com or aasect.org to find a local sex therapist. Call them and use them in your referral networks.
In the “bio” component of the biopsychosocial approach, we can do a lot to treat ED with medications and hormones. Testosterone has been shown to help with low libido and erectile function. Checking the patient’s testosterone level can be very helpful. Pills — we are familiar with Viagra, Cialis, Levitra, and Stendra. The oral PDE-5 inhibitors have been around since the late 1990s and they work quite well for many people with ED. Viagra and Cialis are generic now and patients can get them fairly inexpensively with discount coupons from GoodRx or Cost Plus Drugs. They may not even have to worry about insurance coverage.
Pills relax the smooth muscle of the penis so that it fills with blood and becomes erect, but they don’t work for everybody. If pills stop working, we often talk about synergistic treatments — combining pills and devices. Devices for ED should be discussed more often, and clinicians should consider prescribing them. We commonly discuss eyeglasses and wheelchairs, but we don’t talk about the sexual health devices that could help patients have more success and fun in the bedroom.
What are the various types of devices for ED? One common device is a vacuum pump, which can be very effective. This is how they work: The penis is lubricated and placed into the pump. A button on the pump creates suction that brings blood into the penis. The patient then applies a constriction band around the base of the penis to hold that erection in place.
“Sex tech” has really expanded to help patients with ED with devices that vibrate and hold the erection in place. Vibrating devices allow for a better orgasm. We even have devices that monitor erectile fitness (like a Fitbit for the penis), gathering data to help patients understand the firmness of their erections.
Devices are helpful adjuncts, but they don’t always do enough to achieve an erect penis that’s hard enough for penetration. In those cases, we can recommend injections that increase smooth muscle relaxation of the penis. I know it sounds crazy. If the muscles, arteries, and nerves of the penis aren’t functioning well, additional smooth muscle relaxation can be achieved by injecting alprostadil (prostaglandin E1) directly into the penis. It’s a tiny needle. It doesn’t hurt. These injections can be quite helpful for our patients, and we often recommend them.
But what happens when your patient doesn’t even respond to injections or any of the synergistic treatments? They’ve tried everything. Urologists may suggest a surgical option, the penile implant. Penile implants contain a pump inside the scrotum that fills with fluid, allowing a rigid erection. Penile implants are wonderful for patients who can no longer get erections. Talking to a urologist about the pros and the cons and the risks and benefits of surgically placed implants is very important.
Finally, ED is a marker for cardiovascular disease. These patients may need a cardiology workup. They need to improve their general health. We have to ask our patients about their goals and what they care about, and find a toolbox that makes sense for each patient and couple to maximize their sexual health and quality of life. Don’t give up. If you have questions, let us know.
Rachel S. Rubin, MD, is Assistant Clinical Professor, Department of Urology, Georgetown University, Washington, DC; Private practice, Rachel Rubin MD PLLC, North Bethesda, Maryland. She disclosed ties with Sprout, Maternal Medical, Absorption Pharmaceuticals, GSK, and Endo.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I’m Dr Rachel Rubin. I am a urologist with fellowship training in sexual medicine. Today I’m going to explain why I may recommend that your patients put a needle directly into their penises for help with erectile dysfunction (ED).
I know that sounds crazy, but in a recent video when I talked about erection hardness, I acknowledged that it may not be easy to talk with patients about their penises, but it’s important.
ED can be a marker for cardiovascular disease, with 50% of our 50-year-old patients having ED. As physicians, we must do a better job of talking to our patients about ED and letting them know that it’s a marker for overall health.
How do we treat ED? Primary care doctors can do a great deal for patients with ED, and there are other things that urologists can do when you run out of options in your own toolbox.
What’s important for a healthy erection? You need three things: healthy muscle, healthy nerves, and healthy arteries. If anything goes wrong with muscles, nerves, or arteries, this is what leads to ED. Think through the algorithm of your patient’s medical history: Do they have diabetes, which can affect their nerves? Do they have high blood pressure, which can affect their arteries? Do they have problems with testosterone, which can affect the smooth muscles of the penis? Understanding your patient’s history can be really helpful when you figure out what is the best treatment strategy for your patient.
For the penis to work, those smooth muscles have to relax; therefore, your brain has to be relaxed, along with your pelvic floor muscles. The smooth muscle of the penis has to be relaxed so it can fill with blood, increase in girth and size, and hold that erection in place.
To treat ED, we have a biopsychosocial toolbox. Biology refers to the muscles, arteries, and nerves. The psychosocial component is stress: If your brain is stressed, you have a lot of adrenaline around that can tighten those smooth muscles and cause you to lose an erection.
So, what are these treatments? I’ll start with lifestyle. A healthy heart means a healthy penis, so, all of the things you already recommend for lifestyle changes can really help with ED. Sleep is important. Does your patient need a sleep study? Do they have sleep apnea? Are they exercising? Recent data show that exercise may be just as effective, if not more effective, than Viagra. How about a good diet? The Mediterranean diet seems to be the most helpful. So, encourage your patients to make dietary, exercise, sleep, and other lifestyle changes if they want to improve erectile function.
What about sex education? Most physicians didn’t get great education about sex in medical school, but it’s very important to our patients who likewise have had inadequate sex education. Ask questions, talk to them, explain what is normal.
I can’t stress enough how important mental health is to a great sex life. Everyone would benefit from sex therapy and becoming better at sex. We need to get better at communicating and educating patients and their partners to maximize their quality of life. If you need to refer to a specialist, we recommend going to psychologytoday.com or aasect.org to find a local sex therapist. Call them and use them in your referral networks.
In the “bio” component of the biopsychosocial approach, we can do a lot to treat ED with medications and hormones. Testosterone has been shown to help with low libido and erectile function. Checking the patient’s testosterone level can be very helpful. Pills — we are familiar with Viagra, Cialis, Levitra, and Stendra. The oral PDE-5 inhibitors have been around since the late 1990s and they work quite well for many people with ED. Viagra and Cialis are generic now and patients can get them fairly inexpensively with discount coupons from GoodRx or Cost Plus Drugs. They may not even have to worry about insurance coverage.
Pills relax the smooth muscle of the penis so that it fills with blood and becomes erect, but they don’t work for everybody. If pills stop working, we often talk about synergistic treatments — combining pills and devices. Devices for ED should be discussed more often, and clinicians should consider prescribing them. We commonly discuss eyeglasses and wheelchairs, but we don’t talk about the sexual health devices that could help patients have more success and fun in the bedroom.
What are the various types of devices for ED? One common device is a vacuum pump, which can be very effective. This is how they work: The penis is lubricated and placed into the pump. A button on the pump creates suction that brings blood into the penis. The patient then applies a constriction band around the base of the penis to hold that erection in place.
“Sex tech” has really expanded to help patients with ED with devices that vibrate and hold the erection in place. Vibrating devices allow for a better orgasm. We even have devices that monitor erectile fitness (like a Fitbit for the penis), gathering data to help patients understand the firmness of their erections.
Devices are helpful adjuncts, but they don’t always do enough to achieve an erect penis that’s hard enough for penetration. In those cases, we can recommend injections that increase smooth muscle relaxation of the penis. I know it sounds crazy. If the muscles, arteries, and nerves of the penis aren’t functioning well, additional smooth muscle relaxation can be achieved by injecting alprostadil (prostaglandin E1) directly into the penis. It’s a tiny needle. It doesn’t hurt. These injections can be quite helpful for our patients, and we often recommend them.
But what happens when your patient doesn’t even respond to injections or any of the synergistic treatments? They’ve tried everything. Urologists may suggest a surgical option, the penile implant. Penile implants contain a pump inside the scrotum that fills with fluid, allowing a rigid erection. Penile implants are wonderful for patients who can no longer get erections. Talking to a urologist about the pros and the cons and the risks and benefits of surgically placed implants is very important.
Finally, ED is a marker for cardiovascular disease. These patients may need a cardiology workup. They need to improve their general health. We have to ask our patients about their goals and what they care about, and find a toolbox that makes sense for each patient and couple to maximize their sexual health and quality of life. Don’t give up. If you have questions, let us know.
Rachel S. Rubin, MD, is Assistant Clinical Professor, Department of Urology, Georgetown University, Washington, DC; Private practice, Rachel Rubin MD PLLC, North Bethesda, Maryland. She disclosed ties with Sprout, Maternal Medical, Absorption Pharmaceuticals, GSK, and Endo.
A version of this article appeared on Medscape.com.
Supercharge your medical practice with ChatGPT: Here’s why you should upgrade
Artificial intelligence (AI) has already demonstrated its potential in various areas of healthcare, from early disease detection and drug discovery to genomics and personalized care. OpenAI’s ChatGPT, a large language model, is one AI tool that has been transforming practices across the globe, including mine.
Let me walk you through it.
ChatGPT is essentially an AI-fueled assistant, capable of interpreting and generating human-like text in response to user inputs. Imagine a well-informed and competent trainee working with you, ready to tackle tasks from handling patient inquiries to summarizing intricate medical literature.
Currently, ChatGPT works on the “freemium” pricing model; there is a free version built upon GPT-3.5 as well as a subscription “ChatGPT Plus” version based on GPT-4 which offers additional features such as the use of third-party plug-ins.
Now, you may ask, “Isn’t the free version enough?” The free version is indeed impressive, but upgrading to the paid version for $20 per month unlocks the full potential of this tool, particularly if we add plug-ins.
Here are some of the best ways to incorporate ChatGPT Plus into your practice.
Time saver and efficiency multiplier. The paid version of ChatGPT is an extraordinary time-saving tool. It can help you sort through vast amounts of medical literature in a fraction of the time it would normally take. Imagine having to sift through hundreds of articles to find the latest research relevant to a patient’s case. With the paid version of ChatGPT, you can simply ask it to provide summaries of the most recent and relevant studies, all in seconds.
Did you forget about that PowerPoint you need to make but know the potential papers you would use? No problem. ChatGPT can create slides in a few minutes. It becomes your on-demand research assistant.
Of course, you need to provide the source you find most relevant to you. Using plug-ins such as ScholarAI and Link Reader are great.
Improved patient communication. Explaining complex medical terminology and procedures to patients can sometimes be a challenge. ChatGPT can generate simplified and personalized explanations for your patients, fostering their understanding and involvement in their care process.
Epic is currently collaborating with Nuance Communications, Microsoft’s speech recognition subsidiary, to use generative AI tools for medical note-taking in the electronic health record. However, you do not need to wait for it; it just takes a prompt in ChatGPT and then copying/pasting the results into the chart.
Smoother administrative management. The premium version of ChatGPT can automate administrative tasks such as creating letters of medical necessity, clearance to other physicians for services, or even communications to staff on specific topics. This frees you to focus more on your core work: providing patient care.
Precision medicine aid. ChatGPT can be a powerful ally in the field of precision medicine. Its capabilities for analyzing large datasets and unearthing valuable insights can help deliver more personalized and potentially effective treatment plans. For example, one can prompt ChatGPT to query the reported frequency of certain genomic variants and their implications; with the upgraded version and plug-ins, the results will have fewer hallucinations — inaccurate results — and key data references.
Unlimited accessibility. Uninterrupted access is a compelling reason to upgrade. While the free version may have usage limitations, the premium version provides unrestricted, round-the-clock access. Be it a late-night research quest or an early-morning patient query, your AI assistant will always be available.
Strengthened privacy and security. The premium version of ChatGPT includes heightened privacy and security measures. Just make sure to follow HIPAA and not include identifiers when making queries.
Embracing AI tools like ChatGPT in your practice can help you stay at the cutting edge of medical care, saving you time, enhancing patient communication, and supporting you in providing personalized care.
While the free version can serve as a good starting point (there are apps for both iOS and Android), upgrading to the paid version opens up a world of possibilities that can truly supercharge your practice.
I would love to hear your comments on this column or on future topics. Contact me at [email protected].
Arturo Loaiza-Bonilla, MD, MSEd, is the cofounder and chief medical officer at Massive Bio, a company connecting patients to clinical trials using artificial intelligence. His research and professional interests focus on precision medicine, clinical trial design, digital health, entrepreneurship, and patient advocacy. Dr. Loaiza-Bonilla is Assistant Professor of Medicine, Drexel University School of Medicine, Philadelphia, Pennsylvania, and serves as medical director of oncology research at Capital Health in New Jersey, where he maintains a connection to patient care by attending to patients 2 days a week. He has financial relationships with Verify, PSI CRO, Bayer, AstraZeneca, Cardinal Health, BrightInsight, The Lynx Group, Fresenius, Pfizer, Ipsen, Guardant, Amgen, Eisai, Natera, Merck, and Bristol Myers Squibb.
A version of this article appeared on Medscape.com.
Artificial intelligence (AI) has already demonstrated its potential in various areas of healthcare, from early disease detection and drug discovery to genomics and personalized care. OpenAI’s ChatGPT, a large language model, is one AI tool that has been transforming practices across the globe, including mine.
Let me walk you through it.
ChatGPT is essentially an AI-fueled assistant, capable of interpreting and generating human-like text in response to user inputs. Imagine a well-informed and competent trainee working with you, ready to tackle tasks from handling patient inquiries to summarizing intricate medical literature.
Currently, ChatGPT works on the “freemium” pricing model; there is a free version built upon GPT-3.5 as well as a subscription “ChatGPT Plus” version based on GPT-4 which offers additional features such as the use of third-party plug-ins.
Now, you may ask, “Isn’t the free version enough?” The free version is indeed impressive, but upgrading to the paid version for $20 per month unlocks the full potential of this tool, particularly if we add plug-ins.
Here are some of the best ways to incorporate ChatGPT Plus into your practice.
Time saver and efficiency multiplier. The paid version of ChatGPT is an extraordinary time-saving tool. It can help you sort through vast amounts of medical literature in a fraction of the time it would normally take. Imagine having to sift through hundreds of articles to find the latest research relevant to a patient’s case. With the paid version of ChatGPT, you can simply ask it to provide summaries of the most recent and relevant studies, all in seconds.
Did you forget about that PowerPoint you need to make but know the potential papers you would use? No problem. ChatGPT can create slides in a few minutes. It becomes your on-demand research assistant.
Of course, you need to provide the source you find most relevant to you. Using plug-ins such as ScholarAI and Link Reader are great.
Improved patient communication. Explaining complex medical terminology and procedures to patients can sometimes be a challenge. ChatGPT can generate simplified and personalized explanations for your patients, fostering their understanding and involvement in their care process.
Epic is currently collaborating with Nuance Communications, Microsoft’s speech recognition subsidiary, to use generative AI tools for medical note-taking in the electronic health record. However, you do not need to wait for it; it just takes a prompt in ChatGPT and then copying/pasting the results into the chart.
Smoother administrative management. The premium version of ChatGPT can automate administrative tasks such as creating letters of medical necessity, clearance to other physicians for services, or even communications to staff on specific topics. This frees you to focus more on your core work: providing patient care.
Precision medicine aid. ChatGPT can be a powerful ally in the field of precision medicine. Its capabilities for analyzing large datasets and unearthing valuable insights can help deliver more personalized and potentially effective treatment plans. For example, one can prompt ChatGPT to query the reported frequency of certain genomic variants and their implications; with the upgraded version and plug-ins, the results will have fewer hallucinations — inaccurate results — and key data references.
Unlimited accessibility. Uninterrupted access is a compelling reason to upgrade. While the free version may have usage limitations, the premium version provides unrestricted, round-the-clock access. Be it a late-night research quest or an early-morning patient query, your AI assistant will always be available.
Strengthened privacy and security. The premium version of ChatGPT includes heightened privacy and security measures. Just make sure to follow HIPAA and not include identifiers when making queries.
Embracing AI tools like ChatGPT in your practice can help you stay at the cutting edge of medical care, saving you time, enhancing patient communication, and supporting you in providing personalized care.
While the free version can serve as a good starting point (there are apps for both iOS and Android), upgrading to the paid version opens up a world of possibilities that can truly supercharge your practice.
I would love to hear your comments on this column or on future topics. Contact me at [email protected].
Arturo Loaiza-Bonilla, MD, MSEd, is the cofounder and chief medical officer at Massive Bio, a company connecting patients to clinical trials using artificial intelligence. His research and professional interests focus on precision medicine, clinical trial design, digital health, entrepreneurship, and patient advocacy. Dr. Loaiza-Bonilla is Assistant Professor of Medicine, Drexel University School of Medicine, Philadelphia, Pennsylvania, and serves as medical director of oncology research at Capital Health in New Jersey, where he maintains a connection to patient care by attending to patients 2 days a week. He has financial relationships with Verify, PSI CRO, Bayer, AstraZeneca, Cardinal Health, BrightInsight, The Lynx Group, Fresenius, Pfizer, Ipsen, Guardant, Amgen, Eisai, Natera, Merck, and Bristol Myers Squibb.
A version of this article appeared on Medscape.com.
Artificial intelligence (AI) has already demonstrated its potential in various areas of healthcare, from early disease detection and drug discovery to genomics and personalized care. OpenAI’s ChatGPT, a large language model, is one AI tool that has been transforming practices across the globe, including mine.
Let me walk you through it.
ChatGPT is essentially an AI-fueled assistant, capable of interpreting and generating human-like text in response to user inputs. Imagine a well-informed and competent trainee working with you, ready to tackle tasks from handling patient inquiries to summarizing intricate medical literature.
Currently, ChatGPT works on the “freemium” pricing model; there is a free version built upon GPT-3.5 as well as a subscription “ChatGPT Plus” version based on GPT-4 which offers additional features such as the use of third-party plug-ins.
Now, you may ask, “Isn’t the free version enough?” The free version is indeed impressive, but upgrading to the paid version for $20 per month unlocks the full potential of this tool, particularly if we add plug-ins.
Here are some of the best ways to incorporate ChatGPT Plus into your practice.
Time saver and efficiency multiplier. The paid version of ChatGPT is an extraordinary time-saving tool. It can help you sort through vast amounts of medical literature in a fraction of the time it would normally take. Imagine having to sift through hundreds of articles to find the latest research relevant to a patient’s case. With the paid version of ChatGPT, you can simply ask it to provide summaries of the most recent and relevant studies, all in seconds.
Did you forget about that PowerPoint you need to make but know the potential papers you would use? No problem. ChatGPT can create slides in a few minutes. It becomes your on-demand research assistant.
Of course, you need to provide the source you find most relevant to you. Using plug-ins such as ScholarAI and Link Reader are great.
Improved patient communication. Explaining complex medical terminology and procedures to patients can sometimes be a challenge. ChatGPT can generate simplified and personalized explanations for your patients, fostering their understanding and involvement in their care process.
Epic is currently collaborating with Nuance Communications, Microsoft’s speech recognition subsidiary, to use generative AI tools for medical note-taking in the electronic health record. However, you do not need to wait for it; it just takes a prompt in ChatGPT and then copying/pasting the results into the chart.
Smoother administrative management. The premium version of ChatGPT can automate administrative tasks such as creating letters of medical necessity, clearance to other physicians for services, or even communications to staff on specific topics. This frees you to focus more on your core work: providing patient care.
Precision medicine aid. ChatGPT can be a powerful ally in the field of precision medicine. Its capabilities for analyzing large datasets and unearthing valuable insights can help deliver more personalized and potentially effective treatment plans. For example, one can prompt ChatGPT to query the reported frequency of certain genomic variants and their implications; with the upgraded version and plug-ins, the results will have fewer hallucinations — inaccurate results — and key data references.
Unlimited accessibility. Uninterrupted access is a compelling reason to upgrade. While the free version may have usage limitations, the premium version provides unrestricted, round-the-clock access. Be it a late-night research quest or an early-morning patient query, your AI assistant will always be available.
Strengthened privacy and security. The premium version of ChatGPT includes heightened privacy and security measures. Just make sure to follow HIPAA and not include identifiers when making queries.
Embracing AI tools like ChatGPT in your practice can help you stay at the cutting edge of medical care, saving you time, enhancing patient communication, and supporting you in providing personalized care.
While the free version can serve as a good starting point (there are apps for both iOS and Android), upgrading to the paid version opens up a world of possibilities that can truly supercharge your practice.
I would love to hear your comments on this column or on future topics. Contact me at [email protected].
Arturo Loaiza-Bonilla, MD, MSEd, is the cofounder and chief medical officer at Massive Bio, a company connecting patients to clinical trials using artificial intelligence. His research and professional interests focus on precision medicine, clinical trial design, digital health, entrepreneurship, and patient advocacy. Dr. Loaiza-Bonilla is Assistant Professor of Medicine, Drexel University School of Medicine, Philadelphia, Pennsylvania, and serves as medical director of oncology research at Capital Health in New Jersey, where he maintains a connection to patient care by attending to patients 2 days a week. He has financial relationships with Verify, PSI CRO, Bayer, AstraZeneca, Cardinal Health, BrightInsight, The Lynx Group, Fresenius, Pfizer, Ipsen, Guardant, Amgen, Eisai, Natera, Merck, and Bristol Myers Squibb.
A version of this article appeared on Medscape.com.
Electronic Health Records — Recent Survey Results
I have been writing about electronic health records since the mid-1990s. While the basic concept has always been sound, I have always been (and continue to be) a critic of its implementation, which I have compared to the work of the Underpants Gnomes from the television show South Park.
You may recall that Phase One of the Gnomes’ grand scheme was to collect underpants, and Phase Three was to reap enormous profits. Unfortunately, they never quite figured out Phase Two.
EHR’s problems have run a similar course, ever since George W. Bush introduced the EHR Incentive Program (later renamed the Promoting Interoperability Program) in 2000. “By computerizing health records,” the president said, “we can avoid dangerous medical mistakes, reduce costs, and improve care.” That was the ultimate goal — Phase Three, if you will — but nearly a quarter-century later, we are still struggling with Phase Two.
According to the results of a recent survey by this news organization, progress has been made, but issues with usability, reliability, and patient privacy remain.
surveys, respectively. But 56% of them continue to worry about harmful effects from incorrect or misdirected information as a result of inputs from multiple sources, and the rapid turnover of staff that is doing the inputting. Many doctors worry about the potential for incorrect medications and “rule out” diagnoses getting embedded in some patients’ records and undermining future care.
The lack of information sharing among different EHR systems has been the technology’s greatest unmet promise, according to the survey. A lack of interoperability was cited as the most common reason for switching EHR systems. Other reasons included difficulties in clinical documentation and extracting data for quality reporting, as well as the inability to merge inpatient and outpatient records.
A clear majority (72%) felt EHR systems are getting easier to use. The recent decrease in government mandates has freed vendors to work on improving ease of documentation and information retrieval. The incorporation of virtual assistants and other artificial intelligence–based features (as I discussed in two recent columns) have also contributed to improved overall usability. Some newer applications even allow users to build workarounds to compensate for inherent deficiencies in the system.
Physicians tended to be most praiseworthy of functions related to electronic prescribing and retrieval of individual patient data. They felt that much more improvement was needed in helpful prompt features, internal messaging, and communications from patients.
The survey found that 38% of physicians “always” or “often” copy and paste information in patient charts, with another 37% doing so “occasionally.” Noting some of the problems inherent in copy and paste, such as note bloat, internal inconsistencies, error propagation, and documentation in the wrong patient chart, the survey authors suggest that EHR developers could help by shifting away from timelines that appear as one long note. They could also add functionality to allow new information to be displayed as updates on a digital chart.
Improvement is also needed in the way the EHR affects patient interactions, according to the survey results. Physicians are still often forced to click to a different screen to find lab results, another for current medications, and still another for past notes, all while trying to communicate with the patient. Such issues are likely to decrease in the next few years as doctors gain the ability to give voice commands to AI-based system add-ons to obtain this information.
Security concerns seem to be decreasing. In this year’s survey, nearly half of all physicians voiced no EHR privacy problems or concerns, even though a recent review of medical literature concluded that security risks remain meaningful. Those who did have privacy concerns were mostly worried about hackers and other unauthorized access to patient information.
The survey found that around 40% of EHR systems are not using patient portals to post lab results, diagnoses and procedure notes, or prescriptions. However, other physicians complained that their systems were too prompt in posting results, so that patients often received them before the doctor did. This is certainly another area where improvement at both extremes is necessary.
Other areas in which physicians saw a need for improvement were in system reliability, user training, and ongoing customer service. And among the dwindling ranks of physicians with no EHR experience, the most common reasons given for refusing to invest in an EHR system were affordability and interference with the doctor-patient relationship.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
I have been writing about electronic health records since the mid-1990s. While the basic concept has always been sound, I have always been (and continue to be) a critic of its implementation, which I have compared to the work of the Underpants Gnomes from the television show South Park.
You may recall that Phase One of the Gnomes’ grand scheme was to collect underpants, and Phase Three was to reap enormous profits. Unfortunately, they never quite figured out Phase Two.
EHR’s problems have run a similar course, ever since George W. Bush introduced the EHR Incentive Program (later renamed the Promoting Interoperability Program) in 2000. “By computerizing health records,” the president said, “we can avoid dangerous medical mistakes, reduce costs, and improve care.” That was the ultimate goal — Phase Three, if you will — but nearly a quarter-century later, we are still struggling with Phase Two.
According to the results of a recent survey by this news organization, progress has been made, but issues with usability, reliability, and patient privacy remain.
surveys, respectively. But 56% of them continue to worry about harmful effects from incorrect or misdirected information as a result of inputs from multiple sources, and the rapid turnover of staff that is doing the inputting. Many doctors worry about the potential for incorrect medications and “rule out” diagnoses getting embedded in some patients’ records and undermining future care.
The lack of information sharing among different EHR systems has been the technology’s greatest unmet promise, according to the survey. A lack of interoperability was cited as the most common reason for switching EHR systems. Other reasons included difficulties in clinical documentation and extracting data for quality reporting, as well as the inability to merge inpatient and outpatient records.
A clear majority (72%) felt EHR systems are getting easier to use. The recent decrease in government mandates has freed vendors to work on improving ease of documentation and information retrieval. The incorporation of virtual assistants and other artificial intelligence–based features (as I discussed in two recent columns) have also contributed to improved overall usability. Some newer applications even allow users to build workarounds to compensate for inherent deficiencies in the system.
Physicians tended to be most praiseworthy of functions related to electronic prescribing and retrieval of individual patient data. They felt that much more improvement was needed in helpful prompt features, internal messaging, and communications from patients.
The survey found that 38% of physicians “always” or “often” copy and paste information in patient charts, with another 37% doing so “occasionally.” Noting some of the problems inherent in copy and paste, such as note bloat, internal inconsistencies, error propagation, and documentation in the wrong patient chart, the survey authors suggest that EHR developers could help by shifting away from timelines that appear as one long note. They could also add functionality to allow new information to be displayed as updates on a digital chart.
Improvement is also needed in the way the EHR affects patient interactions, according to the survey results. Physicians are still often forced to click to a different screen to find lab results, another for current medications, and still another for past notes, all while trying to communicate with the patient. Such issues are likely to decrease in the next few years as doctors gain the ability to give voice commands to AI-based system add-ons to obtain this information.
Security concerns seem to be decreasing. In this year’s survey, nearly half of all physicians voiced no EHR privacy problems or concerns, even though a recent review of medical literature concluded that security risks remain meaningful. Those who did have privacy concerns were mostly worried about hackers and other unauthorized access to patient information.
The survey found that around 40% of EHR systems are not using patient portals to post lab results, diagnoses and procedure notes, or prescriptions. However, other physicians complained that their systems were too prompt in posting results, so that patients often received them before the doctor did. This is certainly another area where improvement at both extremes is necessary.
Other areas in which physicians saw a need for improvement were in system reliability, user training, and ongoing customer service. And among the dwindling ranks of physicians with no EHR experience, the most common reasons given for refusing to invest in an EHR system were affordability and interference with the doctor-patient relationship.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
I have been writing about electronic health records since the mid-1990s. While the basic concept has always been sound, I have always been (and continue to be) a critic of its implementation, which I have compared to the work of the Underpants Gnomes from the television show South Park.
You may recall that Phase One of the Gnomes’ grand scheme was to collect underpants, and Phase Three was to reap enormous profits. Unfortunately, they never quite figured out Phase Two.
EHR’s problems have run a similar course, ever since George W. Bush introduced the EHR Incentive Program (later renamed the Promoting Interoperability Program) in 2000. “By computerizing health records,” the president said, “we can avoid dangerous medical mistakes, reduce costs, and improve care.” That was the ultimate goal — Phase Three, if you will — but nearly a quarter-century later, we are still struggling with Phase Two.
According to the results of a recent survey by this news organization, progress has been made, but issues with usability, reliability, and patient privacy remain.
surveys, respectively. But 56% of them continue to worry about harmful effects from incorrect or misdirected information as a result of inputs from multiple sources, and the rapid turnover of staff that is doing the inputting. Many doctors worry about the potential for incorrect medications and “rule out” diagnoses getting embedded in some patients’ records and undermining future care.
The lack of information sharing among different EHR systems has been the technology’s greatest unmet promise, according to the survey. A lack of interoperability was cited as the most common reason for switching EHR systems. Other reasons included difficulties in clinical documentation and extracting data for quality reporting, as well as the inability to merge inpatient and outpatient records.
A clear majority (72%) felt EHR systems are getting easier to use. The recent decrease in government mandates has freed vendors to work on improving ease of documentation and information retrieval. The incorporation of virtual assistants and other artificial intelligence–based features (as I discussed in two recent columns) have also contributed to improved overall usability. Some newer applications even allow users to build workarounds to compensate for inherent deficiencies in the system.
Physicians tended to be most praiseworthy of functions related to electronic prescribing and retrieval of individual patient data. They felt that much more improvement was needed in helpful prompt features, internal messaging, and communications from patients.
The survey found that 38% of physicians “always” or “often” copy and paste information in patient charts, with another 37% doing so “occasionally.” Noting some of the problems inherent in copy and paste, such as note bloat, internal inconsistencies, error propagation, and documentation in the wrong patient chart, the survey authors suggest that EHR developers could help by shifting away from timelines that appear as one long note. They could also add functionality to allow new information to be displayed as updates on a digital chart.
Improvement is also needed in the way the EHR affects patient interactions, according to the survey results. Physicians are still often forced to click to a different screen to find lab results, another for current medications, and still another for past notes, all while trying to communicate with the patient. Such issues are likely to decrease in the next few years as doctors gain the ability to give voice commands to AI-based system add-ons to obtain this information.
Security concerns seem to be decreasing. In this year’s survey, nearly half of all physicians voiced no EHR privacy problems or concerns, even though a recent review of medical literature concluded that security risks remain meaningful. Those who did have privacy concerns were mostly worried about hackers and other unauthorized access to patient information.
The survey found that around 40% of EHR systems are not using patient portals to post lab results, diagnoses and procedure notes, or prescriptions. However, other physicians complained that their systems were too prompt in posting results, so that patients often received them before the doctor did. This is certainly another area where improvement at both extremes is necessary.
Other areas in which physicians saw a need for improvement were in system reliability, user training, and ongoing customer service. And among the dwindling ranks of physicians with no EHR experience, the most common reasons given for refusing to invest in an EHR system were affordability and interference with the doctor-patient relationship.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Toward a better framework for postmarketing reproductive safety surveillance of medications
For the last 30 years, the Center for Women’s Mental Health at Massachusetts General Hospital (MGH) has had as part of its mission, the conveying of accurate information about the reproductive safety of psychiatric medications. There has been a spectrum of medicines developed across psychiatric indications over the last several decades, and many studies over those decades have attempted to delineate the reproductive safety of these agents.
With the development of new antidepressants and second-generation antipsychotics has come an appreciation of the utility of these agents across a wide range of psychiatric disease states and psychiatric symptoms. More and more data demonstrate the efficacy of these medicines for mood and anxiety disorders; these agents are also used for a broad array of symptoms from insomnia, irritability, and symptoms of posttraumatic stress disorder (PTSD) just as examples — even absent formal approval by the US Food and Drug Administration (FDA) for these specific indications. With the growing use of medicines, including new antidepressants like selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors, and second-generation atypical antipsychotics, there has been a greater interest and appreciation of the need to provide women with the best information about reproductive safety of these medicines as well.
When I began working in reproductive psychiatry, the FDA was using the pregnancy labeling categories introduced in 1979. The categories were simple, but also oversimplified in terms of incompletely conveying information about reproductive safety. For instance, category labels of B and C under the old labeling system could be nebulous, containing sparse information (in the case of category B) or animal data and some conflicting human data (in the case of category C) that may not have translated into relevant or easily interpretable safety information for patients and clinicians.
It was on that basis the current Pregnancy and Lactation Labeling (PLLR) Final Rule was published in 2014, which was a shift from categorical labeling to more descriptive labeling, including updated actual information on the package insert about available reproductive safety data, animal data, and data on lactation.
Even following the publication of the PLLR, there has still been an acknowledgment in the field that our assessment tools for postmarketing reproductive safety surveillance are incomplete. A recent 2-day FDA workshop hosted by the Duke-Margolis Center for Health Policy on optimizing the use of postapproval pregnancy safety studies sought to discuss the many questions that still surround this issue. Based on presentations at this workshop, a framework emerged for the future of assessing the reproductive safety of medications, which included an effort to develop the most effective model using tools such as pregnancy registries and harnessing “big data,” whether through electronic health records or large administrative databases from public and private insurers. Together, these various sources of information can provide signals of potential concern, prompting the need for a more rigorous look at the reproductive safety of a medication, or provide reassurance if data fail to indicate the absence of a signal of risk.
FDA’s new commitments under the latest reauthorization of the Prescription Drug User Fee Act (PDUFA VII) include pregnancy-specific postmarketing safety requirements as well as the creation of a framework for how data from pregnancy-specific postmarketing studies can be used. The agency is also conducting demonstration projects, including one for assessing the performance of pregnancy registries for the potential to detect safety signals for medications early in pregnancy. FDA is expanding its Sentinel Initiative to help accomplish these aims, and is implementing an Active Risk Identification and Analysis (ARIA) system to conduct active safety surveillance of medications used during pregnancy.
Pregnancy registries have now been available for decades, and some have been more successful than others across different classes of medicines, with the most rigorous registries including prospective follow-up of women across pregnancies and careful documentation of malformations (at best with original source data and with a blinded dysmorphologist). Still, with all of its rigor, even the best-intentioned efforts with respect to pregnancy registries have limitations. As I mentioned in my testimony during the public comment portion of the workshop, the sheer volume of pregnancy data from administrative databases we now have access to is attractive, but the quality of these data needs to be good enough to ascertain a signal of risk if they are to be used as a basis for reproductive safety determination.
The flip side of using data from large administrative databases is using carefully collected data from pregnancy registries. With a pregnancy registry, accrual of a substantial number of participants can also take a considerable period of time, and initial risk estimates of outcomes can have typically large confidence intervals, which can make it difficult to discern whether a drug is safe for women of reproductive age.
Another key issue is a lack of participation from manufacturers with respect to commitment to collection of high-quality reproductive safety data. History has shown that many medication manufacturers, unless required to have a dedicated registry as part of a postmarketing requirement or commitment, will invest sparse resources to track data on safety of fetal drug exposure. Participation is typically voluntary and varies from company to company unless, as noted previously, there is a postmarketing requirement or commitment tied to the approval of a medication. Just as a recent concrete example, the manufacturer of a new medication recently approved by the FDA for the treatment of postpartum depression (which will include presumably sexually active women well into the first postpartum year) has no plan to support the collection of reproductive safety data on this new medication because it is not required to, based on current FDA guidelines and the absence of a postmarketing requirement to do so.
Looking ahead
While the PLLR was a huge step forward in the field from the old pregnancy category system that could misinform women contemplating pregnancy, it also sets the stage for the next iteration of a system that allows us to generate information more quickly about the reproductive safety of medications. In psychiatry, as many as 10% of women use SSRIs during pregnancy. With drugs like atypical antipsychotics being used across disease states — in schizophrenia, bipolar disorder, depression, anxiety, insomnia, and PTSD — and where new classes of medicine are becoming available, like with ketamine or steroids, we need to have a system by which we can more quickly ascertain reproductive safety information. This information informs treatment decisions during a critical life event of deciding to try to become pregnant or during an actual pregnancy.
In my mind, it is reassuring when a registry has even as few as 50-60 cases of fetal exposure without an increase in the risk for malformation, because it can mean we are not seeing a repeat of the past with medications like thalidomide and sodium valproate. However, patients and clinicians are starved for better data. Risk assessment is also different from clinician to clinician and patient to patient. We want to empower patients to make decisions that work for them based on more rapidly accumulating information and help inform their decisions.
To come out on the “other side” of the PLLR, , which can be confusing when study results frequently conflict. I believe we have an obligation today to do this better, because the areas of reproductive toxicology and pharmacovigilance are growing incredibly quickly, and clinicians and patients are seeing these volumes of data being published without the ability to integrate that information in a systematic way.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Full disclosure information for Dr. Cohen is available at womensmentalhealth.org. Email Dr. Cohen at [email protected].
For the last 30 years, the Center for Women’s Mental Health at Massachusetts General Hospital (MGH) has had as part of its mission, the conveying of accurate information about the reproductive safety of psychiatric medications. There has been a spectrum of medicines developed across psychiatric indications over the last several decades, and many studies over those decades have attempted to delineate the reproductive safety of these agents.
With the development of new antidepressants and second-generation antipsychotics has come an appreciation of the utility of these agents across a wide range of psychiatric disease states and psychiatric symptoms. More and more data demonstrate the efficacy of these medicines for mood and anxiety disorders; these agents are also used for a broad array of symptoms from insomnia, irritability, and symptoms of posttraumatic stress disorder (PTSD) just as examples — even absent formal approval by the US Food and Drug Administration (FDA) for these specific indications. With the growing use of medicines, including new antidepressants like selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors, and second-generation atypical antipsychotics, there has been a greater interest and appreciation of the need to provide women with the best information about reproductive safety of these medicines as well.
When I began working in reproductive psychiatry, the FDA was using the pregnancy labeling categories introduced in 1979. The categories were simple, but also oversimplified in terms of incompletely conveying information about reproductive safety. For instance, category labels of B and C under the old labeling system could be nebulous, containing sparse information (in the case of category B) or animal data and some conflicting human data (in the case of category C) that may not have translated into relevant or easily interpretable safety information for patients and clinicians.
It was on that basis the current Pregnancy and Lactation Labeling (PLLR) Final Rule was published in 2014, which was a shift from categorical labeling to more descriptive labeling, including updated actual information on the package insert about available reproductive safety data, animal data, and data on lactation.
Even following the publication of the PLLR, there has still been an acknowledgment in the field that our assessment tools for postmarketing reproductive safety surveillance are incomplete. A recent 2-day FDA workshop hosted by the Duke-Margolis Center for Health Policy on optimizing the use of postapproval pregnancy safety studies sought to discuss the many questions that still surround this issue. Based on presentations at this workshop, a framework emerged for the future of assessing the reproductive safety of medications, which included an effort to develop the most effective model using tools such as pregnancy registries and harnessing “big data,” whether through electronic health records or large administrative databases from public and private insurers. Together, these various sources of information can provide signals of potential concern, prompting the need for a more rigorous look at the reproductive safety of a medication, or provide reassurance if data fail to indicate the absence of a signal of risk.
FDA’s new commitments under the latest reauthorization of the Prescription Drug User Fee Act (PDUFA VII) include pregnancy-specific postmarketing safety requirements as well as the creation of a framework for how data from pregnancy-specific postmarketing studies can be used. The agency is also conducting demonstration projects, including one for assessing the performance of pregnancy registries for the potential to detect safety signals for medications early in pregnancy. FDA is expanding its Sentinel Initiative to help accomplish these aims, and is implementing an Active Risk Identification and Analysis (ARIA) system to conduct active safety surveillance of medications used during pregnancy.
Pregnancy registries have now been available for decades, and some have been more successful than others across different classes of medicines, with the most rigorous registries including prospective follow-up of women across pregnancies and careful documentation of malformations (at best with original source data and with a blinded dysmorphologist). Still, with all of its rigor, even the best-intentioned efforts with respect to pregnancy registries have limitations. As I mentioned in my testimony during the public comment portion of the workshop, the sheer volume of pregnancy data from administrative databases we now have access to is attractive, but the quality of these data needs to be good enough to ascertain a signal of risk if they are to be used as a basis for reproductive safety determination.
The flip side of using data from large administrative databases is using carefully collected data from pregnancy registries. With a pregnancy registry, accrual of a substantial number of participants can also take a considerable period of time, and initial risk estimates of outcomes can have typically large confidence intervals, which can make it difficult to discern whether a drug is safe for women of reproductive age.
Another key issue is a lack of participation from manufacturers with respect to commitment to collection of high-quality reproductive safety data. History has shown that many medication manufacturers, unless required to have a dedicated registry as part of a postmarketing requirement or commitment, will invest sparse resources to track data on safety of fetal drug exposure. Participation is typically voluntary and varies from company to company unless, as noted previously, there is a postmarketing requirement or commitment tied to the approval of a medication. Just as a recent concrete example, the manufacturer of a new medication recently approved by the FDA for the treatment of postpartum depression (which will include presumably sexually active women well into the first postpartum year) has no plan to support the collection of reproductive safety data on this new medication because it is not required to, based on current FDA guidelines and the absence of a postmarketing requirement to do so.
Looking ahead
While the PLLR was a huge step forward in the field from the old pregnancy category system that could misinform women contemplating pregnancy, it also sets the stage for the next iteration of a system that allows us to generate information more quickly about the reproductive safety of medications. In psychiatry, as many as 10% of women use SSRIs during pregnancy. With drugs like atypical antipsychotics being used across disease states — in schizophrenia, bipolar disorder, depression, anxiety, insomnia, and PTSD — and where new classes of medicine are becoming available, like with ketamine or steroids, we need to have a system by which we can more quickly ascertain reproductive safety information. This information informs treatment decisions during a critical life event of deciding to try to become pregnant or during an actual pregnancy.
In my mind, it is reassuring when a registry has even as few as 50-60 cases of fetal exposure without an increase in the risk for malformation, because it can mean we are not seeing a repeat of the past with medications like thalidomide and sodium valproate. However, patients and clinicians are starved for better data. Risk assessment is also different from clinician to clinician and patient to patient. We want to empower patients to make decisions that work for them based on more rapidly accumulating information and help inform their decisions.
To come out on the “other side” of the PLLR, , which can be confusing when study results frequently conflict. I believe we have an obligation today to do this better, because the areas of reproductive toxicology and pharmacovigilance are growing incredibly quickly, and clinicians and patients are seeing these volumes of data being published without the ability to integrate that information in a systematic way.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Full disclosure information for Dr. Cohen is available at womensmentalhealth.org. Email Dr. Cohen at [email protected].
For the last 30 years, the Center for Women’s Mental Health at Massachusetts General Hospital (MGH) has had as part of its mission, the conveying of accurate information about the reproductive safety of psychiatric medications. There has been a spectrum of medicines developed across psychiatric indications over the last several decades, and many studies over those decades have attempted to delineate the reproductive safety of these agents.
With the development of new antidepressants and second-generation antipsychotics has come an appreciation of the utility of these agents across a wide range of psychiatric disease states and psychiatric symptoms. More and more data demonstrate the efficacy of these medicines for mood and anxiety disorders; these agents are also used for a broad array of symptoms from insomnia, irritability, and symptoms of posttraumatic stress disorder (PTSD) just as examples — even absent formal approval by the US Food and Drug Administration (FDA) for these specific indications. With the growing use of medicines, including new antidepressants like selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors, and second-generation atypical antipsychotics, there has been a greater interest and appreciation of the need to provide women with the best information about reproductive safety of these medicines as well.
When I began working in reproductive psychiatry, the FDA was using the pregnancy labeling categories introduced in 1979. The categories were simple, but also oversimplified in terms of incompletely conveying information about reproductive safety. For instance, category labels of B and C under the old labeling system could be nebulous, containing sparse information (in the case of category B) or animal data and some conflicting human data (in the case of category C) that may not have translated into relevant or easily interpretable safety information for patients and clinicians.
It was on that basis the current Pregnancy and Lactation Labeling (PLLR) Final Rule was published in 2014, which was a shift from categorical labeling to more descriptive labeling, including updated actual information on the package insert about available reproductive safety data, animal data, and data on lactation.
Even following the publication of the PLLR, there has still been an acknowledgment in the field that our assessment tools for postmarketing reproductive safety surveillance are incomplete. A recent 2-day FDA workshop hosted by the Duke-Margolis Center for Health Policy on optimizing the use of postapproval pregnancy safety studies sought to discuss the many questions that still surround this issue. Based on presentations at this workshop, a framework emerged for the future of assessing the reproductive safety of medications, which included an effort to develop the most effective model using tools such as pregnancy registries and harnessing “big data,” whether through electronic health records or large administrative databases from public and private insurers. Together, these various sources of information can provide signals of potential concern, prompting the need for a more rigorous look at the reproductive safety of a medication, or provide reassurance if data fail to indicate the absence of a signal of risk.
FDA’s new commitments under the latest reauthorization of the Prescription Drug User Fee Act (PDUFA VII) include pregnancy-specific postmarketing safety requirements as well as the creation of a framework for how data from pregnancy-specific postmarketing studies can be used. The agency is also conducting demonstration projects, including one for assessing the performance of pregnancy registries for the potential to detect safety signals for medications early in pregnancy. FDA is expanding its Sentinel Initiative to help accomplish these aims, and is implementing an Active Risk Identification and Analysis (ARIA) system to conduct active safety surveillance of medications used during pregnancy.
Pregnancy registries have now been available for decades, and some have been more successful than others across different classes of medicines, with the most rigorous registries including prospective follow-up of women across pregnancies and careful documentation of malformations (at best with original source data and with a blinded dysmorphologist). Still, with all of its rigor, even the best-intentioned efforts with respect to pregnancy registries have limitations. As I mentioned in my testimony during the public comment portion of the workshop, the sheer volume of pregnancy data from administrative databases we now have access to is attractive, but the quality of these data needs to be good enough to ascertain a signal of risk if they are to be used as a basis for reproductive safety determination.
The flip side of using data from large administrative databases is using carefully collected data from pregnancy registries. With a pregnancy registry, accrual of a substantial number of participants can also take a considerable period of time, and initial risk estimates of outcomes can have typically large confidence intervals, which can make it difficult to discern whether a drug is safe for women of reproductive age.
Another key issue is a lack of participation from manufacturers with respect to commitment to collection of high-quality reproductive safety data. History has shown that many medication manufacturers, unless required to have a dedicated registry as part of a postmarketing requirement or commitment, will invest sparse resources to track data on safety of fetal drug exposure. Participation is typically voluntary and varies from company to company unless, as noted previously, there is a postmarketing requirement or commitment tied to the approval of a medication. Just as a recent concrete example, the manufacturer of a new medication recently approved by the FDA for the treatment of postpartum depression (which will include presumably sexually active women well into the first postpartum year) has no plan to support the collection of reproductive safety data on this new medication because it is not required to, based on current FDA guidelines and the absence of a postmarketing requirement to do so.
Looking ahead
While the PLLR was a huge step forward in the field from the old pregnancy category system that could misinform women contemplating pregnancy, it also sets the stage for the next iteration of a system that allows us to generate information more quickly about the reproductive safety of medications. In psychiatry, as many as 10% of women use SSRIs during pregnancy. With drugs like atypical antipsychotics being used across disease states — in schizophrenia, bipolar disorder, depression, anxiety, insomnia, and PTSD — and where new classes of medicine are becoming available, like with ketamine or steroids, we need to have a system by which we can more quickly ascertain reproductive safety information. This information informs treatment decisions during a critical life event of deciding to try to become pregnant or during an actual pregnancy.
In my mind, it is reassuring when a registry has even as few as 50-60 cases of fetal exposure without an increase in the risk for malformation, because it can mean we are not seeing a repeat of the past with medications like thalidomide and sodium valproate. However, patients and clinicians are starved for better data. Risk assessment is also different from clinician to clinician and patient to patient. We want to empower patients to make decisions that work for them based on more rapidly accumulating information and help inform their decisions.
To come out on the “other side” of the PLLR, , which can be confusing when study results frequently conflict. I believe we have an obligation today to do this better, because the areas of reproductive toxicology and pharmacovigilance are growing incredibly quickly, and clinicians and patients are seeing these volumes of data being published without the ability to integrate that information in a systematic way.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Full disclosure information for Dr. Cohen is available at womensmentalhealth.org. Email Dr. Cohen at [email protected].
How to prescribe Zepbound
December marks the advent of the approval of tirzepatide (Zepbound) for on-label treatment of obesity. In November 2023, the US Food and Drug Administration (FDA) approved it for the treatment of obesity in adults.
In May 2022, the FDA approved Mounjaro, which is tirzepatide, for type 2 diabetes. Since then, many physicians, including myself, have prescribed it off-label for obesity. As an endocrinologist treating both obesity and diabetes, 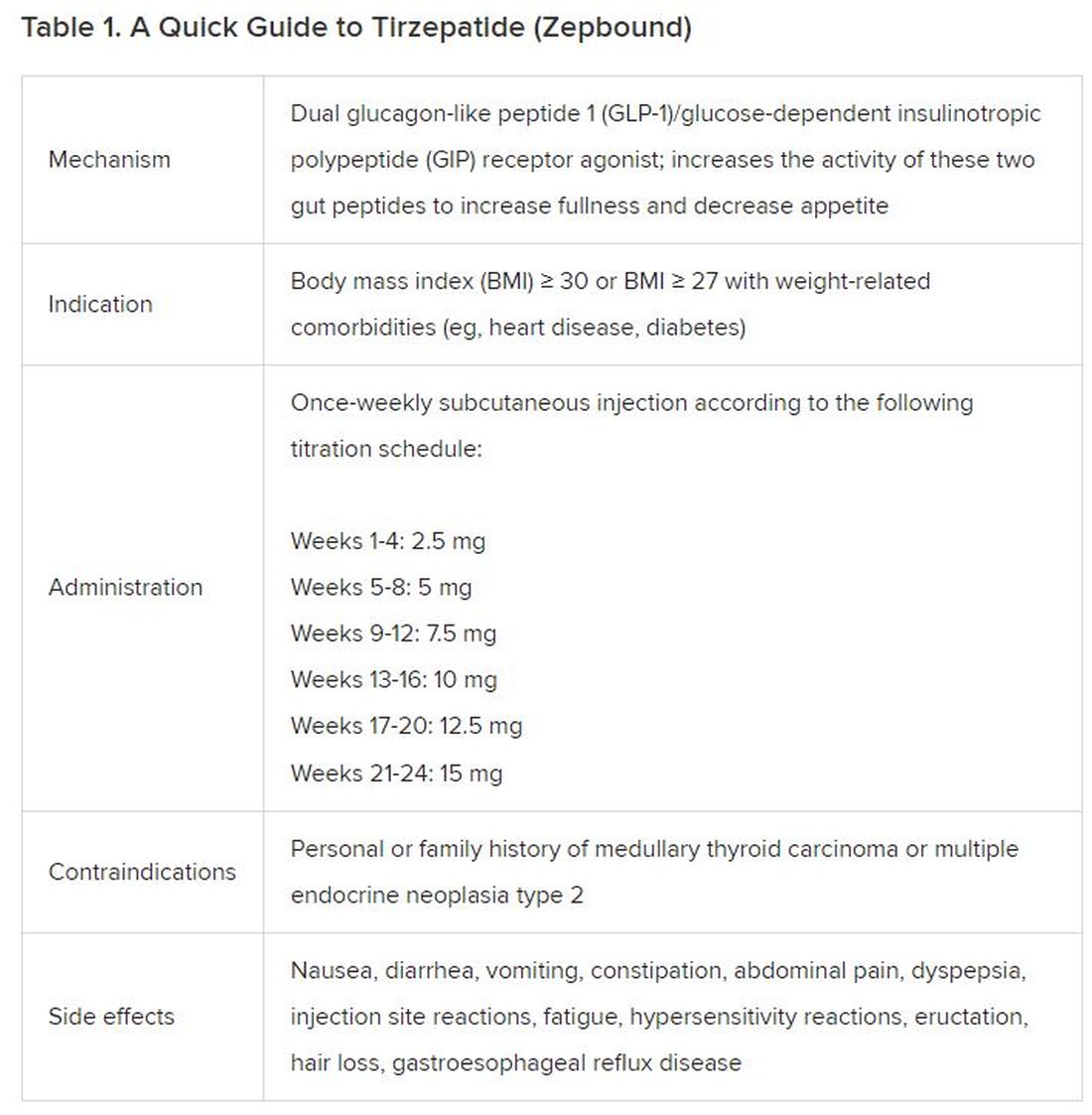
The Expertise
Because GLP-1 receptor agonists have been around since 2005, we’ve had over a decade of clinical experience with these medications. Table 2 provides more nuanced information on tirzepatide (as Zepbound, for obesity) based on our experiences with dulaglutide, liraglutide, semaglutide, and tirzepatide (as Mounjaro).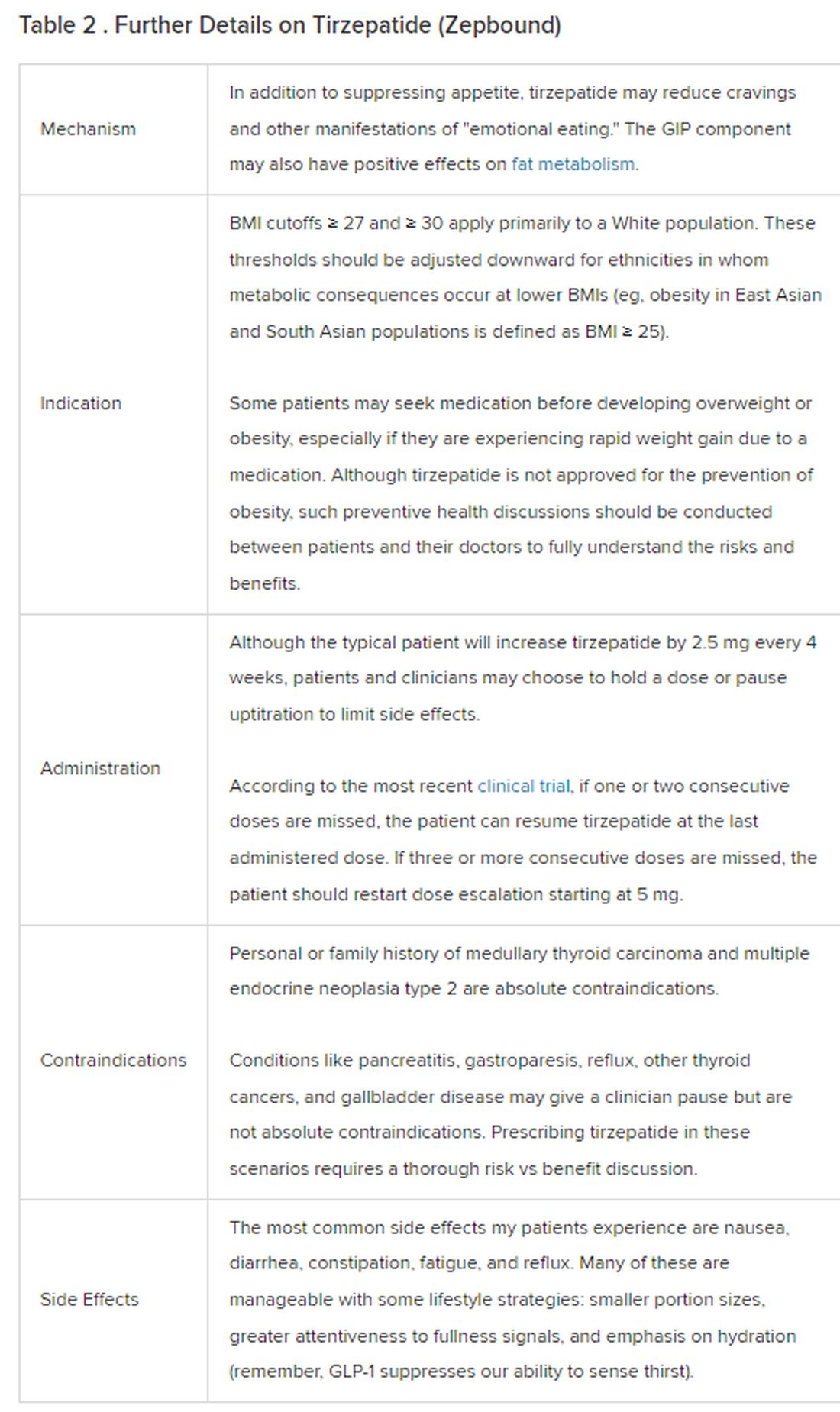
The Reality
In today’s increasingly complex healthcare system, the reality of providing high-quality obesity care is challenging. When discussing tirzepatide with patients, I use a 4 Cs schematic — comorbidities, cautions, costs, choices — to cover the most frequently asked questions.
Comorbidities
In trials, tirzepatide reduced A1c by about 2%. In one diabetes trial, tirzepatide reduced liver fat content significantly more than the comparator (insulin), and trials of tirzepatide in nonalcoholic steatohepatitis are ongoing. A prespecified meta-analysis of tirzepatide and cardiovascular disease estimated a 20% reduction in the risk for cardiovascular death, myocardial infarction, stroke, and hospitalized unstable angina. Tirzepatide as well as other GLP-1 agonists may be beneficial in alcohol use disorder. Prescribing tirzepatide to patients who have or are at risk of developing such comorbidities is an ideal way to target multiple metabolic diseases with one agent.
Cautions
The first principle of medicine is “do no harm.” Tirzepatide may be a poor option for individuals with a history of pancreatitis, gastroparesis, or severe gastroesophageal reflux disease. Because tirzepatide may interfere with the efficacy of estrogen-containing contraceptives during its uptitration phase, women should speak with their doctors about appropriate birth control options (eg, progestin-only, barrier methods). In clinical trials of tirzepatide, male participants were also advised to use reliable contraception. If patients are family-planning, tirzepatide should be discontinued 2 months (for women) and 4 months (for men) before conception, because its effects on fertility or pregnancy are currently unknown.
Costs
At a retail price of $1279 per month, Zepbound is only slightly more affordable than its main competitor, Wegovy (semaglutide 2.4 mg). Complex pharmacy negotiations may reduce this cost, but even with rebates, coupons, and commercial insurance, these costs still place tirzepatide out of reach for many patients. For patients who cannot access tirzepatide, clinicians should discuss more cost-feasible, evidence-based alternatives: for example, phentermine, phentermine-topiramate, naltrexone-bupropion, metformin, bupropion, or topiramate.
Choices
Patient preference drives much of today’s clinical decision-making. Some patients may be switching from semaglutide to tirzepatide, whether by choice or on the basis of physician recommendation. Although no head-to-head obesity trial exists, data from SURPASS-2 and SUSTAIN-FORTE can inform therapeutic equivalence:
- Semaglutide 1.0 mg to tirzepatide 2.5 mg will be a step-down; 5 mg will be a step-up
- Semaglutide 2.0 or 2.4 mg to tirzepatide 5 mg is probably equivalent
The decision to switch therapeutics may depend on weight loss goals, side effect tolerability, or insurance coverage. As with all medications, the use of tirzepatide should progress with shared decision-making, thorough discussions of risks vs benefits, and individualized regimens tailored to each patient’s needs.
The newly approved Zepbound is a valuable addition to our toolbox of obesity treatments. Patients and providers alike are excited for its potential as a highly effective antiobesity medication that can cause a degree of weight loss necessary to reverse comorbidities. The medical management of obesity with agents like tirzepatide holds great promise in addressing today’s obesity epidemic.
Dr. Tchang is Assistant Professor, Clinical Medicine, Division of Endocrinology, Diabetes, and Metabolism, Weill Cornell Medicine; Physician, Department of Medicine, Iris Cantor Women’s Health Center, Comprehensive Weight Control Center, New York, NY. She disclosed ties to Gelesis and Novo Nordisk.
A version of this article appeared on Medscape.com.
December marks the advent of the approval of tirzepatide (Zepbound) for on-label treatment of obesity. In November 2023, the US Food and Drug Administration (FDA) approved it for the treatment of obesity in adults.
In May 2022, the FDA approved Mounjaro, which is tirzepatide, for type 2 diabetes. Since then, many physicians, including myself, have prescribed it off-label for obesity. As an endocrinologist treating both obesity and diabetes, 
The Expertise
Because GLP-1 receptor agonists have been around since 2005, we’ve had over a decade of clinical experience with these medications. Table 2 provides more nuanced information on tirzepatide (as Zepbound, for obesity) based on our experiences with dulaglutide, liraglutide, semaglutide, and tirzepatide (as Mounjaro).
The Reality
In today’s increasingly complex healthcare system, the reality of providing high-quality obesity care is challenging. When discussing tirzepatide with patients, I use a 4 Cs schematic — comorbidities, cautions, costs, choices — to cover the most frequently asked questions.
Comorbidities
In trials, tirzepatide reduced A1c by about 2%. In one diabetes trial, tirzepatide reduced liver fat content significantly more than the comparator (insulin), and trials of tirzepatide in nonalcoholic steatohepatitis are ongoing. A prespecified meta-analysis of tirzepatide and cardiovascular disease estimated a 20% reduction in the risk for cardiovascular death, myocardial infarction, stroke, and hospitalized unstable angina. Tirzepatide as well as other GLP-1 agonists may be beneficial in alcohol use disorder. Prescribing tirzepatide to patients who have or are at risk of developing such comorbidities is an ideal way to target multiple metabolic diseases with one agent.
Cautions
The first principle of medicine is “do no harm.” Tirzepatide may be a poor option for individuals with a history of pancreatitis, gastroparesis, or severe gastroesophageal reflux disease. Because tirzepatide may interfere with the efficacy of estrogen-containing contraceptives during its uptitration phase, women should speak with their doctors about appropriate birth control options (eg, progestin-only, barrier methods). In clinical trials of tirzepatide, male participants were also advised to use reliable contraception. If patients are family-planning, tirzepatide should be discontinued 2 months (for women) and 4 months (for men) before conception, because its effects on fertility or pregnancy are currently unknown.
Costs
At a retail price of $1279 per month, Zepbound is only slightly more affordable than its main competitor, Wegovy (semaglutide 2.4 mg). Complex pharmacy negotiations may reduce this cost, but even with rebates, coupons, and commercial insurance, these costs still place tirzepatide out of reach for many patients. For patients who cannot access tirzepatide, clinicians should discuss more cost-feasible, evidence-based alternatives: for example, phentermine, phentermine-topiramate, naltrexone-bupropion, metformin, bupropion, or topiramate.
Choices
Patient preference drives much of today’s clinical decision-making. Some patients may be switching from semaglutide to tirzepatide, whether by choice or on the basis of physician recommendation. Although no head-to-head obesity trial exists, data from SURPASS-2 and SUSTAIN-FORTE can inform therapeutic equivalence:
- Semaglutide 1.0 mg to tirzepatide 2.5 mg will be a step-down; 5 mg will be a step-up
- Semaglutide 2.0 or 2.4 mg to tirzepatide 5 mg is probably equivalent
The decision to switch therapeutics may depend on weight loss goals, side effect tolerability, or insurance coverage. As with all medications, the use of tirzepatide should progress with shared decision-making, thorough discussions of risks vs benefits, and individualized regimens tailored to each patient’s needs.
The newly approved Zepbound is a valuable addition to our toolbox of obesity treatments. Patients and providers alike are excited for its potential as a highly effective antiobesity medication that can cause a degree of weight loss necessary to reverse comorbidities. The medical management of obesity with agents like tirzepatide holds great promise in addressing today’s obesity epidemic.
Dr. Tchang is Assistant Professor, Clinical Medicine, Division of Endocrinology, Diabetes, and Metabolism, Weill Cornell Medicine; Physician, Department of Medicine, Iris Cantor Women’s Health Center, Comprehensive Weight Control Center, New York, NY. She disclosed ties to Gelesis and Novo Nordisk.
A version of this article appeared on Medscape.com.
December marks the advent of the approval of tirzepatide (Zepbound) for on-label treatment of obesity. In November 2023, the US Food and Drug Administration (FDA) approved it for the treatment of obesity in adults.
In May 2022, the FDA approved Mounjaro, which is tirzepatide, for type 2 diabetes. Since then, many physicians, including myself, have prescribed it off-label for obesity. As an endocrinologist treating both obesity and diabetes, 
The Expertise
Because GLP-1 receptor agonists have been around since 2005, we’ve had over a decade of clinical experience with these medications. Table 2 provides more nuanced information on tirzepatide (as Zepbound, for obesity) based on our experiences with dulaglutide, liraglutide, semaglutide, and tirzepatide (as Mounjaro).
The Reality
In today’s increasingly complex healthcare system, the reality of providing high-quality obesity care is challenging. When discussing tirzepatide with patients, I use a 4 Cs schematic — comorbidities, cautions, costs, choices — to cover the most frequently asked questions.
Comorbidities
In trials, tirzepatide reduced A1c by about 2%. In one diabetes trial, tirzepatide reduced liver fat content significantly more than the comparator (insulin), and trials of tirzepatide in nonalcoholic steatohepatitis are ongoing. A prespecified meta-analysis of tirzepatide and cardiovascular disease estimated a 20% reduction in the risk for cardiovascular death, myocardial infarction, stroke, and hospitalized unstable angina. Tirzepatide as well as other GLP-1 agonists may be beneficial in alcohol use disorder. Prescribing tirzepatide to patients who have or are at risk of developing such comorbidities is an ideal way to target multiple metabolic diseases with one agent.
Cautions
The first principle of medicine is “do no harm.” Tirzepatide may be a poor option for individuals with a history of pancreatitis, gastroparesis, or severe gastroesophageal reflux disease. Because tirzepatide may interfere with the efficacy of estrogen-containing contraceptives during its uptitration phase, women should speak with their doctors about appropriate birth control options (eg, progestin-only, barrier methods). In clinical trials of tirzepatide, male participants were also advised to use reliable contraception. If patients are family-planning, tirzepatide should be discontinued 2 months (for women) and 4 months (for men) before conception, because its effects on fertility or pregnancy are currently unknown.
Costs
At a retail price of $1279 per month, Zepbound is only slightly more affordable than its main competitor, Wegovy (semaglutide 2.4 mg). Complex pharmacy negotiations may reduce this cost, but even with rebates, coupons, and commercial insurance, these costs still place tirzepatide out of reach for many patients. For patients who cannot access tirzepatide, clinicians should discuss more cost-feasible, evidence-based alternatives: for example, phentermine, phentermine-topiramate, naltrexone-bupropion, metformin, bupropion, or topiramate.
Choices
Patient preference drives much of today’s clinical decision-making. Some patients may be switching from semaglutide to tirzepatide, whether by choice or on the basis of physician recommendation. Although no head-to-head obesity trial exists, data from SURPASS-2 and SUSTAIN-FORTE can inform therapeutic equivalence:
- Semaglutide 1.0 mg to tirzepatide 2.5 mg will be a step-down; 5 mg will be a step-up
- Semaglutide 2.0 or 2.4 mg to tirzepatide 5 mg is probably equivalent
The decision to switch therapeutics may depend on weight loss goals, side effect tolerability, or insurance coverage. As with all medications, the use of tirzepatide should progress with shared decision-making, thorough discussions of risks vs benefits, and individualized regimens tailored to each patient’s needs.
The newly approved Zepbound is a valuable addition to our toolbox of obesity treatments. Patients and providers alike are excited for its potential as a highly effective antiobesity medication that can cause a degree of weight loss necessary to reverse comorbidities. The medical management of obesity with agents like tirzepatide holds great promise in addressing today’s obesity epidemic.
Dr. Tchang is Assistant Professor, Clinical Medicine, Division of Endocrinology, Diabetes, and Metabolism, Weill Cornell Medicine; Physician, Department of Medicine, Iris Cantor Women’s Health Center, Comprehensive Weight Control Center, New York, NY. She disclosed ties to Gelesis and Novo Nordisk.
A version of this article appeared on Medscape.com.
Technology for primary care — terrific, terrifying, or both?
We have all been using technology in our primary care practices for a long time but newer formats have been emerging so fast that our minds, much less our staff’s minds, may be spinning.
Our old friend the telephone, a time-soaking nemesis for scheduling, checking coverage, questions calls, prescribing, quick consults, and follow-up is being replaced by EHR portals and SMS for messaging (e.g. DoctorConnect, SimplePractice), drop-in televisits and patient education links on our websites (e.g. Schmitt Pediatric Care, Remedy Connect), and chatbots for scheduling (e.g. CHEC-UP). While time is saved, what is lost may be hearing the subtext of anxiety or misperceptions in parents’ voices that would change our advice and the empathetic human connection in conversations with our patients. A hybrid approach may be better.
The paper appointment book has been replaced by scheduling systems sometimes lacking in flexibility for double booking, sibling visits, and variable length or extremely valuable multi-professional visits. Allowing patients to book their own visits may place complex problems in inappropriate slots, so only allowing online requests for visits is safer. On the other hand, many of us can now squeeze in “same day” televisits (e.g. Blueberry Pediatrics), sometimes from outside our practice (e.g., zocdoc), to increase payments and even entice new patients to enroll.
Amazing advances in technology are being made in specialty care such as genetic modifications (CRISPR), immunotherapies (mRNA vaccines and AI drug design), robot-assisted surgery, and 3-D printing of body parts and prosthetics. Technology as treatment such as transcranial magnetic stimulation and vagal stimulation are finding value in psychiatry.
But beside being aware of and able to order such specialty technologies, innovations are now extending our senses in primary care such as amplified or visual stethoscopes, bedside ultrasound (e.g. Butterfly), remote visualization (oto-, endo-)scopes, photographic vision screens (e.g. iScreen) for skin lesion (VisualDx) and genetic syndrome facial recognition. We need to be sure that technologies are tested and calibrated for children and different racial groups and genders to provide safe and equitable care. Early adoption may not always be the best approach. Costs of technology, as usual, may limit access to these advanced care aids especially, as usual, in practices serving low income and rural communities.
Patients, especially younger parents and youth, now expect to participate and can directly benefit from technology as part of their health care. Validated parent or self-report screens (e.g. EHRs, Phreesia) can detect important issues early for more effective intervention. Such questionnaires typically provide a pass/fail result or score, but other delivery systems (e.g. CHADIS) include interpretation, assist patients/parents in setting visit priorities and health goals, and even chain results of one questionnaire to secondary screens to hone in on problems, sometimes obviating a time-consuming second visit. Patient-completed comprehensive questionnaires (e.g. Well Visit Planner, CHADIS) allow us time to use our skills to focus on concerns, education, and management rather than asking myriad routine questions. Some (e.g. CHADIS) even create visit documentation reducing our “pajama time” write ups (and burnout); automate repeated online measures to track progress; and use questionnaire results to trigger related patient-specific education and resources rather than the often-ignored generic EHR handouts.
Digital therapeutics such as apps for anxiety (e.g. Calm), depression (e.g. SparkRx, Cass), weight control (e.g. Noom, Lose it), fitness, or sleep tracking (e.g. Whoop) help educate and, in some cases, provide real-time feedback to personalize discovery of contributing factors in order to maintain motivation for positive health behavior change. Some video games improve ADHD symptoms (e.g. EndeavorRX). Virtual reality scenarios have been shown to desensitize those with PTSD and social anxiety or teach social skills to children with autism.
Systems that trigger resource listings (including apps) from screen results can help, but now with over 10,000 apps for mental health, knowing what to recommend for what conditions is a challenge for which ratings (e.g. MINDapps.org) can help. With few product reps visiting to tell us what’s new, we need to read critically about innovations, search the web, subscribe to the AAP SOAPM LISTSERV, visit exhibitors at professional meetings, and talk with peers.
All the digital data collected from health care technology, if assembled with privacy constraints and analyzed with advanced statistical methods, have the possibility, with or without inclusion of genomic data, to allow for more accurate diagnostic and treatment decision support. While AI can search widely for patterns, it needs to be “trained” on appropriate data to make correct conclusions. We are all aware that the history determines 85% of both diagnosis and treatment decisions, particularly in primary care where x-rays or lab tests are not often needed.
But history in EHR notes is often idiosyncratic, entered hours after the visit by the clinician, and does not include the information needed to define diagnostic or guideline criteria, even if the clinician knows and considered those criteria. EHR templates are presented blank and are onerous and time consuming for clinicians. In addition, individual patient barriers to care, preferences, and environmental or subjective concerns are infrequently documented even though they may make the biggest difference to adherence and/or outcomes.
Notes made from voice to text digital AI translation of the encounter (e.g. Nuance DAX) are even less likely to include diagnostic criteria as it would be inappropriate to speak these. To use EHR history data to train AI and to test efficacy of care using variations of guidelines, guideline-related data is needed from online patient entries in questionnaires that are transformed to fill in templates along with some structured choices for clinician entries forming visit notes (e.g. CHADIS). New apps to facilitate clinician documentation of guidelines (e.g. AvoMD) could streamline visits as well as help document guideline criteria. The resulting combination of guideline-relevant patient histories and objective data to test and iteratively refine guidelines will allow a process known as a “Learning Health System.”
Technology to collect this kind of data can allow for the aspirational American Academy of Pediatrics CHILD Registry to approach this goal. Population-level data can provide surveillance for illness, toxins, effects of climate change, social drivers of health, and even effects of technologies themselves such as social media and remote learning so that we can attempt to make the best choices for the future.
Clinicians, staff, and patients will need to develop trust in technology as it infiltrates all aspects of health care. Professionals need both evidence and experience to trust a technology, which takes time and effort. Disinformation in the media may reduce trust or evoke unwarranted trust, as we have all seen regarding vaccines. Clear and coherent public health messaging can help but is no longer a panacea for developing trust in health care. Our nonjudgmental listening and informed opinions are needed more than ever.
The biggest issues for new technology are likely to be the need for workflow adjustments, changing our habit patterns, training, and cost/benefit analyses. With today’s high staff churn, confusion and even chaos can ensue when adopting new technology.
Staff need to be part of the selection process, if at all possible, and discuss how roles and flow will need to change. Having one staff member be a champion and expert for new tech can move adoption to a shared process rather than imposing “one more thing.” It is crucial to discuss the benefits for patients and staff even if the change is required. Sometimes cost savings can include a bonus for staff or free group lunches. Providing a certificate of achievement or title promotion for mastering new tech may be appropriate. Giving some time off from other tasks to learn new workflows can reduce resistance rather than just adding it on to a regular workload. Office “huddles” going forward can include examples of benefits staff have observed or heard about from the adoption. There are quality improvement processes that engage the team — some that earn MOC-4 or CEU credits — that apply to making workflow changes and measuring them iteratively.
If technology takes over important aspects of the work of medical professionals, even if it is faster and/or more accurate, it may degrade clinical observational, interactional, and decision-making skills through lack of use. It may also remove the sense of self-efficacy that motivates professionals to endure onerous training and desire to enter the field. Using technology may reduce empathetic interactions that are basic to humanistic motivation, work satisfaction, and even community respect. Moral injury is already rampant in medicine from restrictions on freedom to do what we see as important for our patients. Technology has great potential and already is enhancing our ability to provide the best care for patients but the risks need to be watched for and ameliorated.
When technology automates comprehensive visit documentation that highlights priority and risk areas from patient input and individualizes decision support, it can facilitate the personalized care that we and our patients want to experience. We must not be so awed, intrigued, or wary of new technology to miss its benefits nor give up our good clinical judgment about the technology or about our patients.
Dr. Howard is assistant professor of pediatrics at The Johns Hopkins University School of Medicine, Baltimore, and creator of CHADIS. She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
We have all been using technology in our primary care practices for a long time but newer formats have been emerging so fast that our minds, much less our staff’s minds, may be spinning.
Our old friend the telephone, a time-soaking nemesis for scheduling, checking coverage, questions calls, prescribing, quick consults, and follow-up is being replaced by EHR portals and SMS for messaging (e.g. DoctorConnect, SimplePractice), drop-in televisits and patient education links on our websites (e.g. Schmitt Pediatric Care, Remedy Connect), and chatbots for scheduling (e.g. CHEC-UP). While time is saved, what is lost may be hearing the subtext of anxiety or misperceptions in parents’ voices that would change our advice and the empathetic human connection in conversations with our patients. A hybrid approach may be better.
The paper appointment book has been replaced by scheduling systems sometimes lacking in flexibility for double booking, sibling visits, and variable length or extremely valuable multi-professional visits. Allowing patients to book their own visits may place complex problems in inappropriate slots, so only allowing online requests for visits is safer. On the other hand, many of us can now squeeze in “same day” televisits (e.g. Blueberry Pediatrics), sometimes from outside our practice (e.g., zocdoc), to increase payments and even entice new patients to enroll.
Amazing advances in technology are being made in specialty care such as genetic modifications (CRISPR), immunotherapies (mRNA vaccines and AI drug design), robot-assisted surgery, and 3-D printing of body parts and prosthetics. Technology as treatment such as transcranial magnetic stimulation and vagal stimulation are finding value in psychiatry.
But beside being aware of and able to order such specialty technologies, innovations are now extending our senses in primary care such as amplified or visual stethoscopes, bedside ultrasound (e.g. Butterfly), remote visualization (oto-, endo-)scopes, photographic vision screens (e.g. iScreen) for skin lesion (VisualDx) and genetic syndrome facial recognition. We need to be sure that technologies are tested and calibrated for children and different racial groups and genders to provide safe and equitable care. Early adoption may not always be the best approach. Costs of technology, as usual, may limit access to these advanced care aids especially, as usual, in practices serving low income and rural communities.
Patients, especially younger parents and youth, now expect to participate and can directly benefit from technology as part of their health care. Validated parent or self-report screens (e.g. EHRs, Phreesia) can detect important issues early for more effective intervention. Such questionnaires typically provide a pass/fail result or score, but other delivery systems (e.g. CHADIS) include interpretation, assist patients/parents in setting visit priorities and health goals, and even chain results of one questionnaire to secondary screens to hone in on problems, sometimes obviating a time-consuming second visit. Patient-completed comprehensive questionnaires (e.g. Well Visit Planner, CHADIS) allow us time to use our skills to focus on concerns, education, and management rather than asking myriad routine questions. Some (e.g. CHADIS) even create visit documentation reducing our “pajama time” write ups (and burnout); automate repeated online measures to track progress; and use questionnaire results to trigger related patient-specific education and resources rather than the often-ignored generic EHR handouts.
Digital therapeutics such as apps for anxiety (e.g. Calm), depression (e.g. SparkRx, Cass), weight control (e.g. Noom, Lose it), fitness, or sleep tracking (e.g. Whoop) help educate and, in some cases, provide real-time feedback to personalize discovery of contributing factors in order to maintain motivation for positive health behavior change. Some video games improve ADHD symptoms (e.g. EndeavorRX). Virtual reality scenarios have been shown to desensitize those with PTSD and social anxiety or teach social skills to children with autism.
Systems that trigger resource listings (including apps) from screen results can help, but now with over 10,000 apps for mental health, knowing what to recommend for what conditions is a challenge for which ratings (e.g. MINDapps.org) can help. With few product reps visiting to tell us what’s new, we need to read critically about innovations, search the web, subscribe to the AAP SOAPM LISTSERV, visit exhibitors at professional meetings, and talk with peers.
All the digital data collected from health care technology, if assembled with privacy constraints and analyzed with advanced statistical methods, have the possibility, with or without inclusion of genomic data, to allow for more accurate diagnostic and treatment decision support. While AI can search widely for patterns, it needs to be “trained” on appropriate data to make correct conclusions. We are all aware that the history determines 85% of both diagnosis and treatment decisions, particularly in primary care where x-rays or lab tests are not often needed.
But history in EHR notes is often idiosyncratic, entered hours after the visit by the clinician, and does not include the information needed to define diagnostic or guideline criteria, even if the clinician knows and considered those criteria. EHR templates are presented blank and are onerous and time consuming for clinicians. In addition, individual patient barriers to care, preferences, and environmental or subjective concerns are infrequently documented even though they may make the biggest difference to adherence and/or outcomes.
Notes made from voice to text digital AI translation of the encounter (e.g. Nuance DAX) are even less likely to include diagnostic criteria as it would be inappropriate to speak these. To use EHR history data to train AI and to test efficacy of care using variations of guidelines, guideline-related data is needed from online patient entries in questionnaires that are transformed to fill in templates along with some structured choices for clinician entries forming visit notes (e.g. CHADIS). New apps to facilitate clinician documentation of guidelines (e.g. AvoMD) could streamline visits as well as help document guideline criteria. The resulting combination of guideline-relevant patient histories and objective data to test and iteratively refine guidelines will allow a process known as a “Learning Health System.”
Technology to collect this kind of data can allow for the aspirational American Academy of Pediatrics CHILD Registry to approach this goal. Population-level data can provide surveillance for illness, toxins, effects of climate change, social drivers of health, and even effects of technologies themselves such as social media and remote learning so that we can attempt to make the best choices for the future.
Clinicians, staff, and patients will need to develop trust in technology as it infiltrates all aspects of health care. Professionals need both evidence and experience to trust a technology, which takes time and effort. Disinformation in the media may reduce trust or evoke unwarranted trust, as we have all seen regarding vaccines. Clear and coherent public health messaging can help but is no longer a panacea for developing trust in health care. Our nonjudgmental listening and informed opinions are needed more than ever.
The biggest issues for new technology are likely to be the need for workflow adjustments, changing our habit patterns, training, and cost/benefit analyses. With today’s high staff churn, confusion and even chaos can ensue when adopting new technology.
Staff need to be part of the selection process, if at all possible, and discuss how roles and flow will need to change. Having one staff member be a champion and expert for new tech can move adoption to a shared process rather than imposing “one more thing.” It is crucial to discuss the benefits for patients and staff even if the change is required. Sometimes cost savings can include a bonus for staff or free group lunches. Providing a certificate of achievement or title promotion for mastering new tech may be appropriate. Giving some time off from other tasks to learn new workflows can reduce resistance rather than just adding it on to a regular workload. Office “huddles” going forward can include examples of benefits staff have observed or heard about from the adoption. There are quality improvement processes that engage the team — some that earn MOC-4 or CEU credits — that apply to making workflow changes and measuring them iteratively.
If technology takes over important aspects of the work of medical professionals, even if it is faster and/or more accurate, it may degrade clinical observational, interactional, and decision-making skills through lack of use. It may also remove the sense of self-efficacy that motivates professionals to endure onerous training and desire to enter the field. Using technology may reduce empathetic interactions that are basic to humanistic motivation, work satisfaction, and even community respect. Moral injury is already rampant in medicine from restrictions on freedom to do what we see as important for our patients. Technology has great potential and already is enhancing our ability to provide the best care for patients but the risks need to be watched for and ameliorated.
When technology automates comprehensive visit documentation that highlights priority and risk areas from patient input and individualizes decision support, it can facilitate the personalized care that we and our patients want to experience. We must not be so awed, intrigued, or wary of new technology to miss its benefits nor give up our good clinical judgment about the technology or about our patients.
Dr. Howard is assistant professor of pediatrics at The Johns Hopkins University School of Medicine, Baltimore, and creator of CHADIS. She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
We have all been using technology in our primary care practices for a long time but newer formats have been emerging so fast that our minds, much less our staff’s minds, may be spinning.
Our old friend the telephone, a time-soaking nemesis for scheduling, checking coverage, questions calls, prescribing, quick consults, and follow-up is being replaced by EHR portals and SMS for messaging (e.g. DoctorConnect, SimplePractice), drop-in televisits and patient education links on our websites (e.g. Schmitt Pediatric Care, Remedy Connect), and chatbots for scheduling (e.g. CHEC-UP). While time is saved, what is lost may be hearing the subtext of anxiety or misperceptions in parents’ voices that would change our advice and the empathetic human connection in conversations with our patients. A hybrid approach may be better.
The paper appointment book has been replaced by scheduling systems sometimes lacking in flexibility for double booking, sibling visits, and variable length or extremely valuable multi-professional visits. Allowing patients to book their own visits may place complex problems in inappropriate slots, so only allowing online requests for visits is safer. On the other hand, many of us can now squeeze in “same day” televisits (e.g. Blueberry Pediatrics), sometimes from outside our practice (e.g., zocdoc), to increase payments and even entice new patients to enroll.
Amazing advances in technology are being made in specialty care such as genetic modifications (CRISPR), immunotherapies (mRNA vaccines and AI drug design), robot-assisted surgery, and 3-D printing of body parts and prosthetics. Technology as treatment such as transcranial magnetic stimulation and vagal stimulation are finding value in psychiatry.
But beside being aware of and able to order such specialty technologies, innovations are now extending our senses in primary care such as amplified or visual stethoscopes, bedside ultrasound (e.g. Butterfly), remote visualization (oto-, endo-)scopes, photographic vision screens (e.g. iScreen) for skin lesion (VisualDx) and genetic syndrome facial recognition. We need to be sure that technologies are tested and calibrated for children and different racial groups and genders to provide safe and equitable care. Early adoption may not always be the best approach. Costs of technology, as usual, may limit access to these advanced care aids especially, as usual, in practices serving low income and rural communities.
Patients, especially younger parents and youth, now expect to participate and can directly benefit from technology as part of their health care. Validated parent or self-report screens (e.g. EHRs, Phreesia) can detect important issues early for more effective intervention. Such questionnaires typically provide a pass/fail result or score, but other delivery systems (e.g. CHADIS) include interpretation, assist patients/parents in setting visit priorities and health goals, and even chain results of one questionnaire to secondary screens to hone in on problems, sometimes obviating a time-consuming second visit. Patient-completed comprehensive questionnaires (e.g. Well Visit Planner, CHADIS) allow us time to use our skills to focus on concerns, education, and management rather than asking myriad routine questions. Some (e.g. CHADIS) even create visit documentation reducing our “pajama time” write ups (and burnout); automate repeated online measures to track progress; and use questionnaire results to trigger related patient-specific education and resources rather than the often-ignored generic EHR handouts.
Digital therapeutics such as apps for anxiety (e.g. Calm), depression (e.g. SparkRx, Cass), weight control (e.g. Noom, Lose it), fitness, or sleep tracking (e.g. Whoop) help educate and, in some cases, provide real-time feedback to personalize discovery of contributing factors in order to maintain motivation for positive health behavior change. Some video games improve ADHD symptoms (e.g. EndeavorRX). Virtual reality scenarios have been shown to desensitize those with PTSD and social anxiety or teach social skills to children with autism.
Systems that trigger resource listings (including apps) from screen results can help, but now with over 10,000 apps for mental health, knowing what to recommend for what conditions is a challenge for which ratings (e.g. MINDapps.org) can help. With few product reps visiting to tell us what’s new, we need to read critically about innovations, search the web, subscribe to the AAP SOAPM LISTSERV, visit exhibitors at professional meetings, and talk with peers.
All the digital data collected from health care technology, if assembled with privacy constraints and analyzed with advanced statistical methods, have the possibility, with or without inclusion of genomic data, to allow for more accurate diagnostic and treatment decision support. While AI can search widely for patterns, it needs to be “trained” on appropriate data to make correct conclusions. We are all aware that the history determines 85% of both diagnosis and treatment decisions, particularly in primary care where x-rays or lab tests are not often needed.
But history in EHR notes is often idiosyncratic, entered hours after the visit by the clinician, and does not include the information needed to define diagnostic or guideline criteria, even if the clinician knows and considered those criteria. EHR templates are presented blank and are onerous and time consuming for clinicians. In addition, individual patient barriers to care, preferences, and environmental or subjective concerns are infrequently documented even though they may make the biggest difference to adherence and/or outcomes.
Notes made from voice to text digital AI translation of the encounter (e.g. Nuance DAX) are even less likely to include diagnostic criteria as it would be inappropriate to speak these. To use EHR history data to train AI and to test efficacy of care using variations of guidelines, guideline-related data is needed from online patient entries in questionnaires that are transformed to fill in templates along with some structured choices for clinician entries forming visit notes (e.g. CHADIS). New apps to facilitate clinician documentation of guidelines (e.g. AvoMD) could streamline visits as well as help document guideline criteria. The resulting combination of guideline-relevant patient histories and objective data to test and iteratively refine guidelines will allow a process known as a “Learning Health System.”
Technology to collect this kind of data can allow for the aspirational American Academy of Pediatrics CHILD Registry to approach this goal. Population-level data can provide surveillance for illness, toxins, effects of climate change, social drivers of health, and even effects of technologies themselves such as social media and remote learning so that we can attempt to make the best choices for the future.
Clinicians, staff, and patients will need to develop trust in technology as it infiltrates all aspects of health care. Professionals need both evidence and experience to trust a technology, which takes time and effort. Disinformation in the media may reduce trust or evoke unwarranted trust, as we have all seen regarding vaccines. Clear and coherent public health messaging can help but is no longer a panacea for developing trust in health care. Our nonjudgmental listening and informed opinions are needed more than ever.
The biggest issues for new technology are likely to be the need for workflow adjustments, changing our habit patterns, training, and cost/benefit analyses. With today’s high staff churn, confusion and even chaos can ensue when adopting new technology.
Staff need to be part of the selection process, if at all possible, and discuss how roles and flow will need to change. Having one staff member be a champion and expert for new tech can move adoption to a shared process rather than imposing “one more thing.” It is crucial to discuss the benefits for patients and staff even if the change is required. Sometimes cost savings can include a bonus for staff or free group lunches. Providing a certificate of achievement or title promotion for mastering new tech may be appropriate. Giving some time off from other tasks to learn new workflows can reduce resistance rather than just adding it on to a regular workload. Office “huddles” going forward can include examples of benefits staff have observed or heard about from the adoption. There are quality improvement processes that engage the team — some that earn MOC-4 or CEU credits — that apply to making workflow changes and measuring them iteratively.
If technology takes over important aspects of the work of medical professionals, even if it is faster and/or more accurate, it may degrade clinical observational, interactional, and decision-making skills through lack of use. It may also remove the sense of self-efficacy that motivates professionals to endure onerous training and desire to enter the field. Using technology may reduce empathetic interactions that are basic to humanistic motivation, work satisfaction, and even community respect. Moral injury is already rampant in medicine from restrictions on freedom to do what we see as important for our patients. Technology has great potential and already is enhancing our ability to provide the best care for patients but the risks need to be watched for and ameliorated.
When technology automates comprehensive visit documentation that highlights priority and risk areas from patient input and individualizes decision support, it can facilitate the personalized care that we and our patients want to experience. We must not be so awed, intrigued, or wary of new technology to miss its benefits nor give up our good clinical judgment about the technology or about our patients.
Dr. Howard is assistant professor of pediatrics at The Johns Hopkins University School of Medicine, Baltimore, and creator of CHADIS. She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].