User login
Why Are Prion Diseases on the Rise?
This transcript has been edited for clarity.
In 1986, in Britain, cattle started dying.
The condition, quickly nicknamed “mad cow disease,” was clearly infectious, but the particular pathogen was difficult to identify. By 1993, 120,000 cattle in Britain were identified as being infected. As yet, no human cases had occurred and the UK government insisted that cattle were a dead-end host for the pathogen. By the mid-1990s, however, multiple human cases, attributable to ingestion of meat and organs from infected cattle, were discovered. In humans, variant Creutzfeldt-Jakob disease (CJD) was a media sensation — a nearly uniformly fatal, untreatable condition with a rapid onset of dementia, mobility issues characterized by jerky movements, and autopsy reports finding that the brain itself had turned into a spongy mess.
The United States banned UK beef imports in 1996 and only lifted the ban in 2020.
The disease was made all the more mysterious because the pathogen involved was not a bacterium, parasite, or virus, but a protein — or a proteinaceous infectious particle, shortened to “prion.”
Prions are misfolded proteins that aggregate in cells — in this case, in nerve cells. But what makes prions different from other misfolded proteins is that the misfolded protein catalyzes the conversion of its non-misfolded counterpart into the misfolded configuration. It creates a chain reaction, leading to rapid accumulation of misfolded proteins and cell death.
And, like a time bomb, we all have prion protein inside us. In its normally folded state, the function of prion protein remains unclear — knockout mice do okay without it — but it is also highly conserved across mammalian species, so it probably does something worthwhile, perhaps protecting nerve fibers.
Far more common than humans contracting mad cow disease is the condition known as sporadic CJD, responsible for 85% of all cases of prion-induced brain disease. The cause of sporadic CJD is unknown.
But one thing is known: Cases are increasing.
I don’t want you to freak out; we are not in the midst of a CJD epidemic. But it’s been a while since I’ve seen people discussing the condition — which remains as horrible as it was in the 1990s — and a new research letter appearing in JAMA Neurology brought it back to the top of my mind.
Researchers, led by Matthew Crane at Hopkins, used the CDC’s WONDER cause-of-death database, which pulls diagnoses from death certificates. Normally, I’m not a fan of using death certificates for cause-of-death analyses, but in this case I’ll give it a pass. Assuming that the diagnosis of CJD is made, it would be really unlikely for it not to appear on a death certificate.
The main findings are seen here. 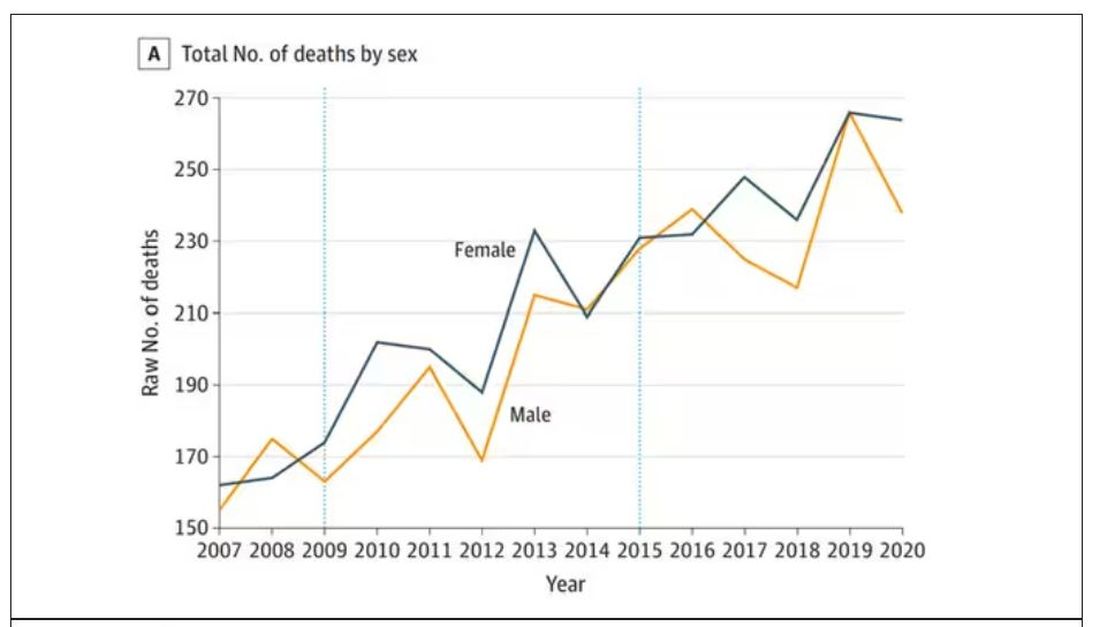
Note that we can’t tell whether these are sporadic CJD cases or variant CJD cases or even familial CJD cases; however, unless there has been a dramatic change in epidemiology, the vast majority of these will be sporadic.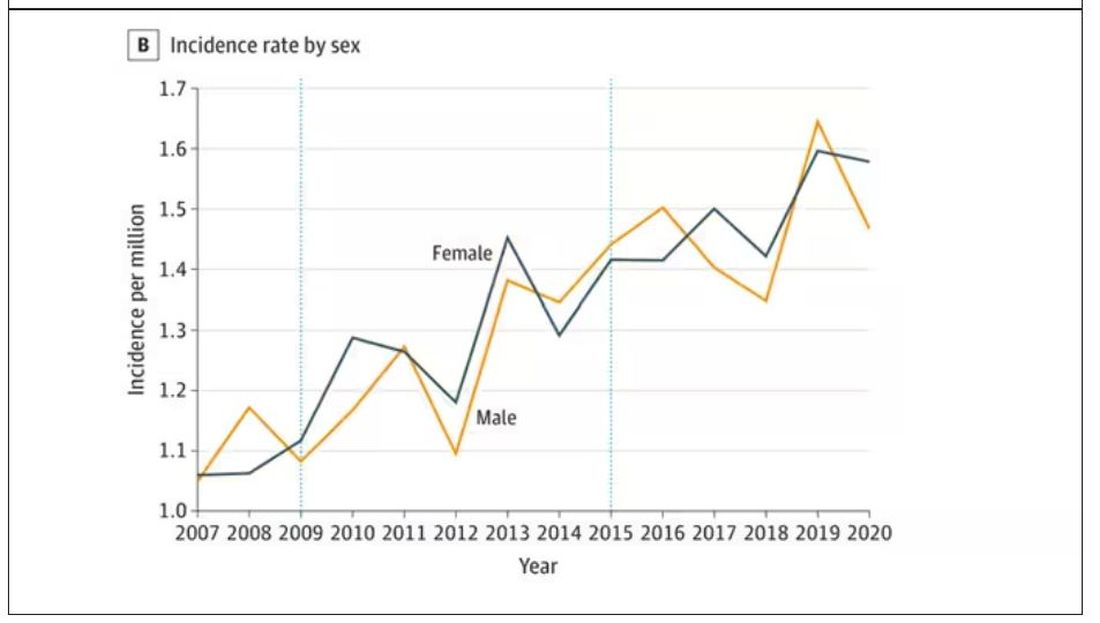
The question is, why are there more cases?
Whenever this type of question comes up with any disease, there are basically three possibilities:
First, there may be an increase in the susceptible, or at-risk, population. In this case, we know that older people are at higher risk of developing sporadic CJD, and over time, the population has aged. To be fair, the authors adjusted for this and still saw an increase, though it was attenuated.
Second, we might be better at diagnosing the condition. A lot has happened since the mid-1990s, when the diagnosis was based more or less on symptoms. The advent of more sophisticated MRI protocols as well as a new diagnostic test called “real-time quaking-induced conversion testing” may mean we are just better at detecting people with this disease.
Third (and most concerning), a new exposure has occurred. What that exposure might be, where it might come from, is anyone’s guess. It’s hard to do broad-scale epidemiology on very rare diseases.
But given these findings, it seems that a bit more surveillance for this rare but devastating condition is well merited.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here on Medscape. He tweets @fperrywilson and his new book, How Medicine Works and When It Doesn’t, is available now.
F. Perry Wilson, MD, MSCE, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
In 1986, in Britain, cattle started dying.
The condition, quickly nicknamed “mad cow disease,” was clearly infectious, but the particular pathogen was difficult to identify. By 1993, 120,000 cattle in Britain were identified as being infected. As yet, no human cases had occurred and the UK government insisted that cattle were a dead-end host for the pathogen. By the mid-1990s, however, multiple human cases, attributable to ingestion of meat and organs from infected cattle, were discovered. In humans, variant Creutzfeldt-Jakob disease (CJD) was a media sensation — a nearly uniformly fatal, untreatable condition with a rapid onset of dementia, mobility issues characterized by jerky movements, and autopsy reports finding that the brain itself had turned into a spongy mess.
The United States banned UK beef imports in 1996 and only lifted the ban in 2020.
The disease was made all the more mysterious because the pathogen involved was not a bacterium, parasite, or virus, but a protein — or a proteinaceous infectious particle, shortened to “prion.”
Prions are misfolded proteins that aggregate in cells — in this case, in nerve cells. But what makes prions different from other misfolded proteins is that the misfolded protein catalyzes the conversion of its non-misfolded counterpart into the misfolded configuration. It creates a chain reaction, leading to rapid accumulation of misfolded proteins and cell death.
And, like a time bomb, we all have prion protein inside us. In its normally folded state, the function of prion protein remains unclear — knockout mice do okay without it — but it is also highly conserved across mammalian species, so it probably does something worthwhile, perhaps protecting nerve fibers.
Far more common than humans contracting mad cow disease is the condition known as sporadic CJD, responsible for 85% of all cases of prion-induced brain disease. The cause of sporadic CJD is unknown.
But one thing is known: Cases are increasing.
I don’t want you to freak out; we are not in the midst of a CJD epidemic. But it’s been a while since I’ve seen people discussing the condition — which remains as horrible as it was in the 1990s — and a new research letter appearing in JAMA Neurology brought it back to the top of my mind.
Researchers, led by Matthew Crane at Hopkins, used the CDC’s WONDER cause-of-death database, which pulls diagnoses from death certificates. Normally, I’m not a fan of using death certificates for cause-of-death analyses, but in this case I’ll give it a pass. Assuming that the diagnosis of CJD is made, it would be really unlikely for it not to appear on a death certificate.
The main findings are seen here. 
Note that we can’t tell whether these are sporadic CJD cases or variant CJD cases or even familial CJD cases; however, unless there has been a dramatic change in epidemiology, the vast majority of these will be sporadic.
The question is, why are there more cases?
Whenever this type of question comes up with any disease, there are basically three possibilities:
First, there may be an increase in the susceptible, or at-risk, population. In this case, we know that older people are at higher risk of developing sporadic CJD, and over time, the population has aged. To be fair, the authors adjusted for this and still saw an increase, though it was attenuated.
Second, we might be better at diagnosing the condition. A lot has happened since the mid-1990s, when the diagnosis was based more or less on symptoms. The advent of more sophisticated MRI protocols as well as a new diagnostic test called “real-time quaking-induced conversion testing” may mean we are just better at detecting people with this disease.
Third (and most concerning), a new exposure has occurred. What that exposure might be, where it might come from, is anyone’s guess. It’s hard to do broad-scale epidemiology on very rare diseases.
But given these findings, it seems that a bit more surveillance for this rare but devastating condition is well merited.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here on Medscape. He tweets @fperrywilson and his new book, How Medicine Works and When It Doesn’t, is available now.
F. Perry Wilson, MD, MSCE, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
In 1986, in Britain, cattle started dying.
The condition, quickly nicknamed “mad cow disease,” was clearly infectious, but the particular pathogen was difficult to identify. By 1993, 120,000 cattle in Britain were identified as being infected. As yet, no human cases had occurred and the UK government insisted that cattle were a dead-end host for the pathogen. By the mid-1990s, however, multiple human cases, attributable to ingestion of meat and organs from infected cattle, were discovered. In humans, variant Creutzfeldt-Jakob disease (CJD) was a media sensation — a nearly uniformly fatal, untreatable condition with a rapid onset of dementia, mobility issues characterized by jerky movements, and autopsy reports finding that the brain itself had turned into a spongy mess.
The United States banned UK beef imports in 1996 and only lifted the ban in 2020.
The disease was made all the more mysterious because the pathogen involved was not a bacterium, parasite, or virus, but a protein — or a proteinaceous infectious particle, shortened to “prion.”
Prions are misfolded proteins that aggregate in cells — in this case, in nerve cells. But what makes prions different from other misfolded proteins is that the misfolded protein catalyzes the conversion of its non-misfolded counterpart into the misfolded configuration. It creates a chain reaction, leading to rapid accumulation of misfolded proteins and cell death.
And, like a time bomb, we all have prion protein inside us. In its normally folded state, the function of prion protein remains unclear — knockout mice do okay without it — but it is also highly conserved across mammalian species, so it probably does something worthwhile, perhaps protecting nerve fibers.
Far more common than humans contracting mad cow disease is the condition known as sporadic CJD, responsible for 85% of all cases of prion-induced brain disease. The cause of sporadic CJD is unknown.
But one thing is known: Cases are increasing.
I don’t want you to freak out; we are not in the midst of a CJD epidemic. But it’s been a while since I’ve seen people discussing the condition — which remains as horrible as it was in the 1990s — and a new research letter appearing in JAMA Neurology brought it back to the top of my mind.
Researchers, led by Matthew Crane at Hopkins, used the CDC’s WONDER cause-of-death database, which pulls diagnoses from death certificates. Normally, I’m not a fan of using death certificates for cause-of-death analyses, but in this case I’ll give it a pass. Assuming that the diagnosis of CJD is made, it would be really unlikely for it not to appear on a death certificate.
The main findings are seen here. 
Note that we can’t tell whether these are sporadic CJD cases or variant CJD cases or even familial CJD cases; however, unless there has been a dramatic change in epidemiology, the vast majority of these will be sporadic.
The question is, why are there more cases?
Whenever this type of question comes up with any disease, there are basically three possibilities:
First, there may be an increase in the susceptible, or at-risk, population. In this case, we know that older people are at higher risk of developing sporadic CJD, and over time, the population has aged. To be fair, the authors adjusted for this and still saw an increase, though it was attenuated.
Second, we might be better at diagnosing the condition. A lot has happened since the mid-1990s, when the diagnosis was based more or less on symptoms. The advent of more sophisticated MRI protocols as well as a new diagnostic test called “real-time quaking-induced conversion testing” may mean we are just better at detecting people with this disease.
Third (and most concerning), a new exposure has occurred. What that exposure might be, where it might come from, is anyone’s guess. It’s hard to do broad-scale epidemiology on very rare diseases.
But given these findings, it seems that a bit more surveillance for this rare but devastating condition is well merited.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here on Medscape. He tweets @fperrywilson and his new book, How Medicine Works and When It Doesn’t, is available now.
F. Perry Wilson, MD, MSCE, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
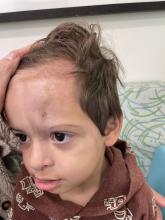
An 18-month-old male presents with a red mark on the forehead and nose
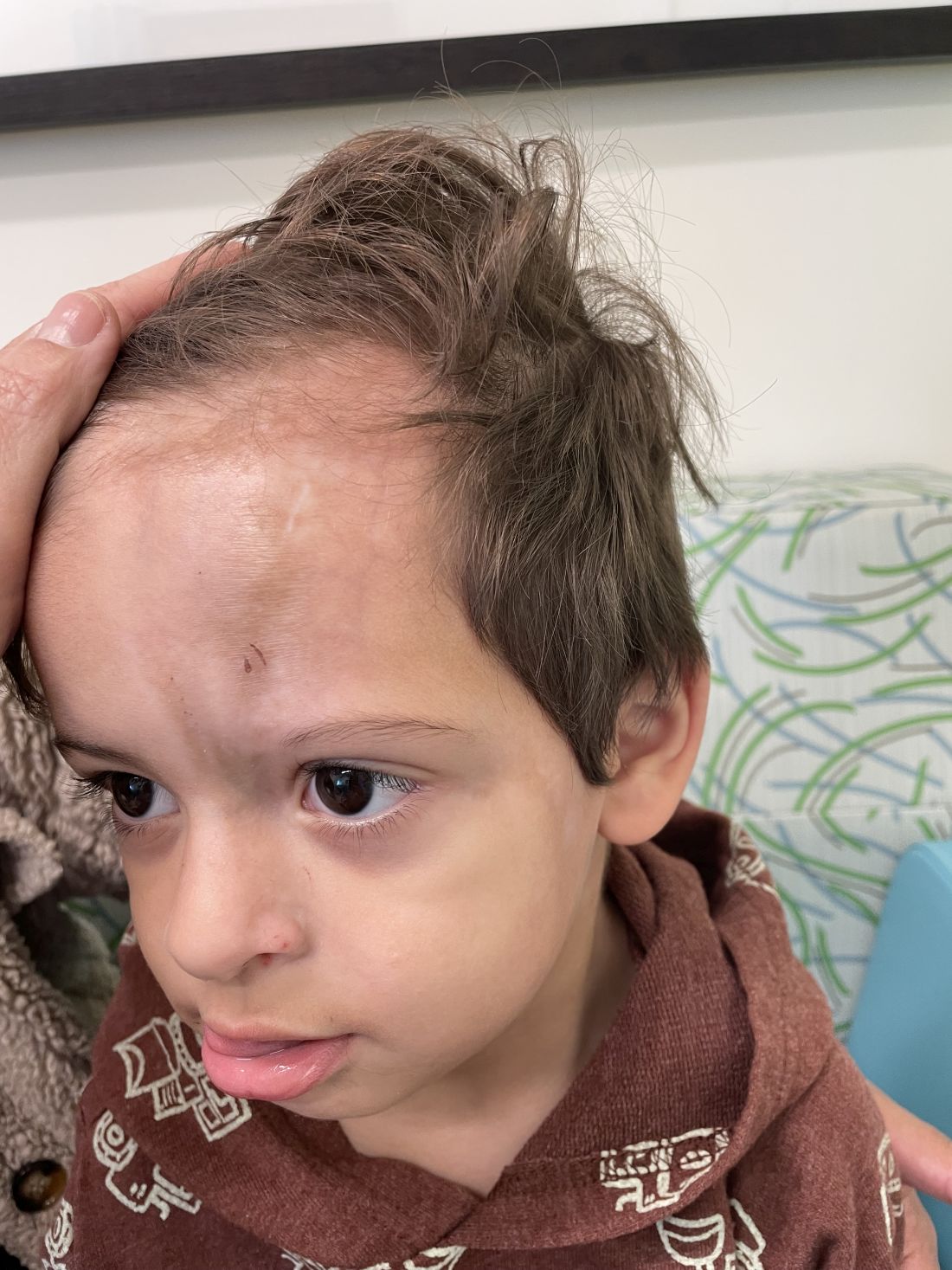
Following the initial presentation, the lesion was initially considered an acquired port wine stain and the child was referred for laser treatment. Upon reassessment during laser treatment a few months later, the lesion had progressed to hyper- and hypopigmented plaques with associated tissue sclerosis and bone atrophy on the mid forehead, nose, and scalp. Patches of alopecia and atrophy were observed on the frontal scalp. The diagnosis was revised to linear morphea en coup de sabre and the child was referred to pediatric rheumatology and commenced treatment with methotrexate and oral corticosteroids.
Linear morphea, a rare connective tissue disorder, primarily affects girls in the first 2 decades of life. Lesions can initially present in many ways. Usually, they present as hypo- or hyperpigmented patches, but may also present as lichenoid uncolored or pink plaques resembling lichen striatus. There may also be erythematous patches mimicking a capillary malformation, as seen in our patient. A recent article reviewing the progression of the lesions from erythematous patches to sclerosis suggests it occurs between 3 and 7 months of age. Subsequent stages manifest as significant atrophy, hypo- and hyperpigmentation, and in severe cases, bone atrophy and deformity, often causing substantial cosmetic disfigurement and functional impairment.
Pathophysiologically, linear morphea involves a complex interplay of immunologic, vascular, and fibrotic processes. While the initial triggers remain elusive, dysregulated immune responses leading to endothelial injury, subsequent activation of fibroblasts and myofibroblasts, and excessive collagen deposition are implicated. Angiogenic disturbances exacerbate tissue ischemia, perpetuating the fibrotic cascade. Alterations in cytokine signaling pathways, particularly TGF-beta and interleukin-6, play pivotal roles in promoting fibrosis and modulating the inflammatory milieu.
Diagnosis of linear morphea en coup de sabre relies on clinical examination, imaging (ultrasonography, MRI, CT scan), and skin biopsy for histopathological analysis. Imaging helps evaluate tissue involvement, while histology reveals characteristic dermal sclerosis, collagen deposition, and inflammation. Early-stage histology may show telangiectatic changes, complicating its differentiation from capillary malformation.
Treatment aims to mitigate symptoms, halt disease progression, and improve cosmesis and functionality. This involves a multidisciplinary approach with systemic medications, phototherapy, physical therapy, and surgical interventions in severe cases. Early identification is crucial for systemic treatments such as methotrexate and systemic corticosteroids to arrest disease progression. Other adjunctive therapies include topical corticosteroids, calcineurin inhibitors, and phototherapy. Surgical procedures like tissue expansion or autologous fat grafting may address tissue atrophy and deformities.
Linear morphea en coup de sabre presents diagnostic and therapeutic challenges because of its rarity and variable clinical course. Collaborative efforts among dermatologists, rheumatologists, radiologists, and surgeons are essential for accurate diagnosis, evaluation, and tailored management. Continued research into pathogenesis and novel therapeutic agents is pivotal to enhance understanding and improve outcomes for those affected by this enigmatic dermatologic condition.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
Gomez-Garcia LA et al. Pediatr Dermatol. 2022 Mar;39(2):275-80.
Ng SS, Tay YK. J Cosmet Laser Ther. 2015;17(5):277-80.
Nijhawan RI et al. J Am Acad Dermatol. 2011 Apr;64(4):779-82.
Following the initial presentation, the lesion was initially considered an acquired port wine stain and the child was referred for laser treatment. Upon reassessment during laser treatment a few months later, the lesion had progressed to hyper- and hypopigmented plaques with associated tissue sclerosis and bone atrophy on the mid forehead, nose, and scalp. Patches of alopecia and atrophy were observed on the frontal scalp. The diagnosis was revised to linear morphea en coup de sabre and the child was referred to pediatric rheumatology and commenced treatment with methotrexate and oral corticosteroids.
Linear morphea, a rare connective tissue disorder, primarily affects girls in the first 2 decades of life. Lesions can initially present in many ways. Usually, they present as hypo- or hyperpigmented patches, but may also present as lichenoid uncolored or pink plaques resembling lichen striatus. There may also be erythematous patches mimicking a capillary malformation, as seen in our patient. A recent article reviewing the progression of the lesions from erythematous patches to sclerosis suggests it occurs between 3 and 7 months of age. Subsequent stages manifest as significant atrophy, hypo- and hyperpigmentation, and in severe cases, bone atrophy and deformity, often causing substantial cosmetic disfigurement and functional impairment.
Pathophysiologically, linear morphea involves a complex interplay of immunologic, vascular, and fibrotic processes. While the initial triggers remain elusive, dysregulated immune responses leading to endothelial injury, subsequent activation of fibroblasts and myofibroblasts, and excessive collagen deposition are implicated. Angiogenic disturbances exacerbate tissue ischemia, perpetuating the fibrotic cascade. Alterations in cytokine signaling pathways, particularly TGF-beta and interleukin-6, play pivotal roles in promoting fibrosis and modulating the inflammatory milieu.
Diagnosis of linear morphea en coup de sabre relies on clinical examination, imaging (ultrasonography, MRI, CT scan), and skin biopsy for histopathological analysis. Imaging helps evaluate tissue involvement, while histology reveals characteristic dermal sclerosis, collagen deposition, and inflammation. Early-stage histology may show telangiectatic changes, complicating its differentiation from capillary malformation.
Treatment aims to mitigate symptoms, halt disease progression, and improve cosmesis and functionality. This involves a multidisciplinary approach with systemic medications, phototherapy, physical therapy, and surgical interventions in severe cases. Early identification is crucial for systemic treatments such as methotrexate and systemic corticosteroids to arrest disease progression. Other adjunctive therapies include topical corticosteroids, calcineurin inhibitors, and phototherapy. Surgical procedures like tissue expansion or autologous fat grafting may address tissue atrophy and deformities.
Linear morphea en coup de sabre presents diagnostic and therapeutic challenges because of its rarity and variable clinical course. Collaborative efforts among dermatologists, rheumatologists, radiologists, and surgeons are essential for accurate diagnosis, evaluation, and tailored management. Continued research into pathogenesis and novel therapeutic agents is pivotal to enhance understanding and improve outcomes for those affected by this enigmatic dermatologic condition.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
Gomez-Garcia LA et al. Pediatr Dermatol. 2022 Mar;39(2):275-80.
Ng SS, Tay YK. J Cosmet Laser Ther. 2015;17(5):277-80.
Nijhawan RI et al. J Am Acad Dermatol. 2011 Apr;64(4):779-82.
Following the initial presentation, the lesion was initially considered an acquired port wine stain and the child was referred for laser treatment. Upon reassessment during laser treatment a few months later, the lesion had progressed to hyper- and hypopigmented plaques with associated tissue sclerosis and bone atrophy on the mid forehead, nose, and scalp. Patches of alopecia and atrophy were observed on the frontal scalp. The diagnosis was revised to linear morphea en coup de sabre and the child was referred to pediatric rheumatology and commenced treatment with methotrexate and oral corticosteroids.
Linear morphea, a rare connective tissue disorder, primarily affects girls in the first 2 decades of life. Lesions can initially present in many ways. Usually, they present as hypo- or hyperpigmented patches, but may also present as lichenoid uncolored or pink plaques resembling lichen striatus. There may also be erythematous patches mimicking a capillary malformation, as seen in our patient. A recent article reviewing the progression of the lesions from erythematous patches to sclerosis suggests it occurs between 3 and 7 months of age. Subsequent stages manifest as significant atrophy, hypo- and hyperpigmentation, and in severe cases, bone atrophy and deformity, often causing substantial cosmetic disfigurement and functional impairment.
Pathophysiologically, linear morphea involves a complex interplay of immunologic, vascular, and fibrotic processes. While the initial triggers remain elusive, dysregulated immune responses leading to endothelial injury, subsequent activation of fibroblasts and myofibroblasts, and excessive collagen deposition are implicated. Angiogenic disturbances exacerbate tissue ischemia, perpetuating the fibrotic cascade. Alterations in cytokine signaling pathways, particularly TGF-beta and interleukin-6, play pivotal roles in promoting fibrosis and modulating the inflammatory milieu.
Diagnosis of linear morphea en coup de sabre relies on clinical examination, imaging (ultrasonography, MRI, CT scan), and skin biopsy for histopathological analysis. Imaging helps evaluate tissue involvement, while histology reveals characteristic dermal sclerosis, collagen deposition, and inflammation. Early-stage histology may show telangiectatic changes, complicating its differentiation from capillary malformation.
Treatment aims to mitigate symptoms, halt disease progression, and improve cosmesis and functionality. This involves a multidisciplinary approach with systemic medications, phototherapy, physical therapy, and surgical interventions in severe cases. Early identification is crucial for systemic treatments such as methotrexate and systemic corticosteroids to arrest disease progression. Other adjunctive therapies include topical corticosteroids, calcineurin inhibitors, and phototherapy. Surgical procedures like tissue expansion or autologous fat grafting may address tissue atrophy and deformities.
Linear morphea en coup de sabre presents diagnostic and therapeutic challenges because of its rarity and variable clinical course. Collaborative efforts among dermatologists, rheumatologists, radiologists, and surgeons are essential for accurate diagnosis, evaluation, and tailored management. Continued research into pathogenesis and novel therapeutic agents is pivotal to enhance understanding and improve outcomes for those affected by this enigmatic dermatologic condition.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
Gomez-Garcia LA et al. Pediatr Dermatol. 2022 Mar;39(2):275-80.
Ng SS, Tay YK. J Cosmet Laser Ther. 2015;17(5):277-80.
Nijhawan RI et al. J Am Acad Dermatol. 2011 Apr;64(4):779-82.
On examination, a faint pink patch was observed on the right forehead, frontal scalp, and nose. The lesion paled under pressure, with small areas of hair loss on the scalp. No atrophy was noted.
Can AI enhance mental health treatment?
Three questions for clinicians
Artificial intelligence (AI) is already impacting the mental health care space, with several new tools available to both clinicians and patients. While this technology could be a game-changer amid a mental health crisis and clinician shortage, there are important ethical and efficacy concerns clinicians should be aware of.
Current use cases illustrate both the potential and risks of AI. On one hand, AI has the potential to improve patient care with tools that can support diagnoses and inform treatment decisions at scale. The UK’s National Health Service is using an AI-powered diagnostic tool to help clinicians diagnose mental health disorders and determine the severity of a patient’s needs. Other tools leverage AI to analyze a patient’s voice for signs of depression or anxiety.
On the other hand, there are serious potential risks involving privacy, bias, and misinformation. One chatbot tool designed to counsel patients through disordered eating was shut down after giving problematic weight-loss advice.
The number of AI tools in the healthcare space is expected to increase fivefold by 2035. Keeping up with these advances is just as important for clinicians as keeping up with the latest medication and treatment options. That means being aware of both the limitations and the potential of AI. Here are three questions clinicians can ask as they explore ways to integrate these tools into their practice while navigating the risks.
• How can AI augment, not replace, the work of my staff?
For example, documentation and the use of electronic health records have consistently been linked to clinician burnout. Using AI to cut down on documentation would leave clinicians with more time and energy to focus on patient care.
One study from the National Library of Medicine found that physicians who did not have enough time to complete documentation were nearly three times more likely to report burnout. In some cases, clinic schedules were deliberately shortened to allow time for documentation.
New tools are emerging that use audio recording, transcription services, and large language models to generate clinical summaries and other documentation support. Amazon and 3M have partnered to solve documentation challenges using AI. This is an area I’ll definitely be keeping an eye on as it develops.
• Do I have patient consent to use this tool?
Since most AI tools remain relatively new, there is a gap in the legal and regulatory framework needed to ensure patient privacy and data protection. Clinicians should draw on existing guardrails and best practices to protect patient privacy and prioritize informed consent. The bottom line: Patients need to know how their data will be used and agree to it.
In the example above regarding documentation, a clinician should obtain patient consent before using technology that records or transcribes sessions. This extends to disclosing the use of AI chat tools and other touch points that occur between sessions. One mental health nonprofit has come under fire for using ChatGPT to provide mental health counseling to thousands of patients who weren’t aware the responses were generated by AI.
Beyond disclosing the use of these tools, clinicians should sufficiently explain how they work to ensure patients understand what they’re consenting to. Some technology companies offer guidance on how informed consent applies to their products and even offer template consent forms to support clinicians. Ultimately, accountability for maintaining patient privacy rests with the clinician, not the company behind the AI tool.
• Where is there a risk of bias?
There has been much discussion around the issue of bias within large language models in particular, since these programs will inherit any bias from the data points or text used to train them. However, there is often little to no visibility into how these models are trained, the algorithms they rely on, and how efficacy is measured.
This is especially concerning within the mental health care space, where bias can contribute to lower-quality care based on a patient’s race, gender or other characteristics. One systemic review published in JAMA Network Open found that most of the AI models used for psychiatric diagnoses that have been studied had a high overall risk of bias — which can lead to outputs that are misleading or incorrect, which can be dangerous in the healthcare field.
It’s important to keep the risk of bias top-of-mind when exploring AI tools and consider whether a tool would pose any direct harm to patients. Clinicians should have active oversight with any use of AI and, ultimately, consider an AI tool’s outputs alongside their own insights, expertise, and instincts.
Clinicians have the power to shape AI’s impact
While there is plenty to be excited about as these new tools develop, clinicians should explore AI with an eye toward the risks as well as the rewards. Practitioners have a significant opportunity to help shape how this technology develops by making informed decisions about which products to invest in and holding tech companies accountable. By educating patients, prioritizing informed consent, and seeking ways to augment their work that ultimately improve quality and scale of care, clinicians can help ensure positive outcomes while minimizing unintended consequences.
Dr. Patel-Dunn is a psychiatrist and chief medical officer at Lifestance Health, Scottsdale, Ariz.
Three questions for clinicians
Three questions for clinicians
Artificial intelligence (AI) is already impacting the mental health care space, with several new tools available to both clinicians and patients. While this technology could be a game-changer amid a mental health crisis and clinician shortage, there are important ethical and efficacy concerns clinicians should be aware of.
Current use cases illustrate both the potential and risks of AI. On one hand, AI has the potential to improve patient care with tools that can support diagnoses and inform treatment decisions at scale. The UK’s National Health Service is using an AI-powered diagnostic tool to help clinicians diagnose mental health disorders and determine the severity of a patient’s needs. Other tools leverage AI to analyze a patient’s voice for signs of depression or anxiety.
On the other hand, there are serious potential risks involving privacy, bias, and misinformation. One chatbot tool designed to counsel patients through disordered eating was shut down after giving problematic weight-loss advice.
The number of AI tools in the healthcare space is expected to increase fivefold by 2035. Keeping up with these advances is just as important for clinicians as keeping up with the latest medication and treatment options. That means being aware of both the limitations and the potential of AI. Here are three questions clinicians can ask as they explore ways to integrate these tools into their practice while navigating the risks.
• How can AI augment, not replace, the work of my staff?
For example, documentation and the use of electronic health records have consistently been linked to clinician burnout. Using AI to cut down on documentation would leave clinicians with more time and energy to focus on patient care.
One study from the National Library of Medicine found that physicians who did not have enough time to complete documentation were nearly three times more likely to report burnout. In some cases, clinic schedules were deliberately shortened to allow time for documentation.
New tools are emerging that use audio recording, transcription services, and large language models to generate clinical summaries and other documentation support. Amazon and 3M have partnered to solve documentation challenges using AI. This is an area I’ll definitely be keeping an eye on as it develops.
• Do I have patient consent to use this tool?
Since most AI tools remain relatively new, there is a gap in the legal and regulatory framework needed to ensure patient privacy and data protection. Clinicians should draw on existing guardrails and best practices to protect patient privacy and prioritize informed consent. The bottom line: Patients need to know how their data will be used and agree to it.
In the example above regarding documentation, a clinician should obtain patient consent before using technology that records or transcribes sessions. This extends to disclosing the use of AI chat tools and other touch points that occur between sessions. One mental health nonprofit has come under fire for using ChatGPT to provide mental health counseling to thousands of patients who weren’t aware the responses were generated by AI.
Beyond disclosing the use of these tools, clinicians should sufficiently explain how they work to ensure patients understand what they’re consenting to. Some technology companies offer guidance on how informed consent applies to their products and even offer template consent forms to support clinicians. Ultimately, accountability for maintaining patient privacy rests with the clinician, not the company behind the AI tool.
• Where is there a risk of bias?
There has been much discussion around the issue of bias within large language models in particular, since these programs will inherit any bias from the data points or text used to train them. However, there is often little to no visibility into how these models are trained, the algorithms they rely on, and how efficacy is measured.
This is especially concerning within the mental health care space, where bias can contribute to lower-quality care based on a patient’s race, gender or other characteristics. One systemic review published in JAMA Network Open found that most of the AI models used for psychiatric diagnoses that have been studied had a high overall risk of bias — which can lead to outputs that are misleading or incorrect, which can be dangerous in the healthcare field.
It’s important to keep the risk of bias top-of-mind when exploring AI tools and consider whether a tool would pose any direct harm to patients. Clinicians should have active oversight with any use of AI and, ultimately, consider an AI tool’s outputs alongside their own insights, expertise, and instincts.
Clinicians have the power to shape AI’s impact
While there is plenty to be excited about as these new tools develop, clinicians should explore AI with an eye toward the risks as well as the rewards. Practitioners have a significant opportunity to help shape how this technology develops by making informed decisions about which products to invest in and holding tech companies accountable. By educating patients, prioritizing informed consent, and seeking ways to augment their work that ultimately improve quality and scale of care, clinicians can help ensure positive outcomes while minimizing unintended consequences.
Dr. Patel-Dunn is a psychiatrist and chief medical officer at Lifestance Health, Scottsdale, Ariz.
Artificial intelligence (AI) is already impacting the mental health care space, with several new tools available to both clinicians and patients. While this technology could be a game-changer amid a mental health crisis and clinician shortage, there are important ethical and efficacy concerns clinicians should be aware of.
Current use cases illustrate both the potential and risks of AI. On one hand, AI has the potential to improve patient care with tools that can support diagnoses and inform treatment decisions at scale. The UK’s National Health Service is using an AI-powered diagnostic tool to help clinicians diagnose mental health disorders and determine the severity of a patient’s needs. Other tools leverage AI to analyze a patient’s voice for signs of depression or anxiety.
On the other hand, there are serious potential risks involving privacy, bias, and misinformation. One chatbot tool designed to counsel patients through disordered eating was shut down after giving problematic weight-loss advice.
The number of AI tools in the healthcare space is expected to increase fivefold by 2035. Keeping up with these advances is just as important for clinicians as keeping up with the latest medication and treatment options. That means being aware of both the limitations and the potential of AI. Here are three questions clinicians can ask as they explore ways to integrate these tools into their practice while navigating the risks.
• How can AI augment, not replace, the work of my staff?
For example, documentation and the use of electronic health records have consistently been linked to clinician burnout. Using AI to cut down on documentation would leave clinicians with more time and energy to focus on patient care.
One study from the National Library of Medicine found that physicians who did not have enough time to complete documentation were nearly three times more likely to report burnout. In some cases, clinic schedules were deliberately shortened to allow time for documentation.
New tools are emerging that use audio recording, transcription services, and large language models to generate clinical summaries and other documentation support. Amazon and 3M have partnered to solve documentation challenges using AI. This is an area I’ll definitely be keeping an eye on as it develops.
• Do I have patient consent to use this tool?
Since most AI tools remain relatively new, there is a gap in the legal and regulatory framework needed to ensure patient privacy and data protection. Clinicians should draw on existing guardrails and best practices to protect patient privacy and prioritize informed consent. The bottom line: Patients need to know how their data will be used and agree to it.
In the example above regarding documentation, a clinician should obtain patient consent before using technology that records or transcribes sessions. This extends to disclosing the use of AI chat tools and other touch points that occur between sessions. One mental health nonprofit has come under fire for using ChatGPT to provide mental health counseling to thousands of patients who weren’t aware the responses were generated by AI.
Beyond disclosing the use of these tools, clinicians should sufficiently explain how they work to ensure patients understand what they’re consenting to. Some technology companies offer guidance on how informed consent applies to their products and even offer template consent forms to support clinicians. Ultimately, accountability for maintaining patient privacy rests with the clinician, not the company behind the AI tool.
• Where is there a risk of bias?
There has been much discussion around the issue of bias within large language models in particular, since these programs will inherit any bias from the data points or text used to train them. However, there is often little to no visibility into how these models are trained, the algorithms they rely on, and how efficacy is measured.
This is especially concerning within the mental health care space, where bias can contribute to lower-quality care based on a patient’s race, gender or other characteristics. One systemic review published in JAMA Network Open found that most of the AI models used for psychiatric diagnoses that have been studied had a high overall risk of bias — which can lead to outputs that are misleading or incorrect, which can be dangerous in the healthcare field.
It’s important to keep the risk of bias top-of-mind when exploring AI tools and consider whether a tool would pose any direct harm to patients. Clinicians should have active oversight with any use of AI and, ultimately, consider an AI tool’s outputs alongside their own insights, expertise, and instincts.
Clinicians have the power to shape AI’s impact
While there is plenty to be excited about as these new tools develop, clinicians should explore AI with an eye toward the risks as well as the rewards. Practitioners have a significant opportunity to help shape how this technology develops by making informed decisions about which products to invest in and holding tech companies accountable. By educating patients, prioritizing informed consent, and seeking ways to augment their work that ultimately improve quality and scale of care, clinicians can help ensure positive outcomes while minimizing unintended consequences.
Dr. Patel-Dunn is a psychiatrist and chief medical officer at Lifestance Health, Scottsdale, Ariz.
Clinician responsibilities during times of geopolitical conflict
In the realm of clinical psychology and psychiatry, our primary duty and commitment is (and should be) to the well-being of our patients. Yet, as we find ourselves in an era marked by escalating geopolitical conflict, such as the Israel-Hamas war, probably more aptly titled the Israeli-Hamas-Hezbollah-Houthi war (a clarification that elucidates a later point), clinicians are increasingly confronted with ethical dilemmas that extend far beyond what is outlined in our code of ethics.
These challenges are not only impacting us on a personal level but are also spilling over into our professional lives, creating a divisive and non-collegial environment within the healthcare community. We commit to “do no harm” when delivering care and yet we are doing harm to one another as colleagues.
We are no strangers to the complexities of human behavior and the intricate tapestry of emotions that are involved with our professional work. However, the current geopolitical landscape has added an extra layer of difficulty to our already taxing professional lives. We are, after all, human first with unconscious drives that govern how we negotiate cognitive dissonance and our need for the illusion of absolute justice as Yuval Noah Harari explains in a recent podcast.
Humans are notoriously bad at holding the multiplicity of experience in mind and various (often competing narratives) that impede the capacity for nuanced thinking. We would like to believe we are better and more capable than the average person in doing so, but divisiveness in our profession has become disturbingly pronounced, making it essential for us to carve out reflective space, more than ever.
The personal and professional divide
Geopolitical conflicts like the current war have a unique capacity to ignite strong emotions and deeply held convictions. It’s not hard to quickly become embroiled in passionate and engaged debate.
While discussion and discourse are healthy, these are bleeding into professional spheres, creating rifts within our clinical communities and contributing to a culture where not everyone feels safe. Look at any professional listserv in medicine or psychology and you will find the evidence. It should be an immediate call to action that we need to be fostering a different type of environment.
The impact of divisiveness is profound, hindering opportunities for collaboration, mentorship, and the free exchange of ideas among clinicians. It may lead to misunderstandings, mistrust, and an erosion of the support systems we rely on, ultimately diverting energy away from the pursuit of providing quality patient-care.
Balancing obligations and limits
Because of the inherent power differential that accompanies being in a provider role (physician and psychologist alike), we have a social and moral responsibility to be mindful of what we share – for the sake of humanity. There is an implicit assumption that a provider’s guidance should be adhered to and respected. In other words, words carry tremendous weight and deeply matter, and people in the general public ascribe significant meaning to messages put out by professionals.
When providers steer from their lanes of professional expertise to provide the general public with opinions or recommendations on nonmedical topics, problematic precedents can be set. We may be doing people a disservice.
Unfortunately, I have heard several anecdotes about clinicians who spend their patient’s time in session pushing their own ideological agendas. The patient-provider relationship is founded on principles of trust, empathy, and collaboration, with the primary goal of improving overall well-being and addressing a specific presenting problem. Of course, issues emerge that need to be addressed outside of the initial scope of treatment, an inherent part of the process. However, a grave concern emerges when clinicians initiate dialogue that is not meaningful to a patient, disclose and discuss their personal ideologies, or put pressure on patients to explain their beliefs in an attempt to change the patients’ minds.
Clinicians pushing their own agenda during patient sessions is antithetical to the objectives of psychotherapy and compromises the therapeutic alliance by diverting the focus of care in a way that serves the clinician rather than the client. It is quite the opposite of the patient-centered care that we strive for in training and practice.
Even within one’s theoretical professional scope of competence, I have seen the impact of emotions running high during this conflict, and have witnessed trained professionals making light of, or even mocking, hostages and their behavior upon release. These are care providers who could elucidate the complexities of captor-captive dynamics and the impact of trauma for the general public, yet they are contributing to dangerous perceptions and divisiveness.
I have also seen providers justify sexual violence, diminishing survivor and witness testimony due to ideological differences and strong personal beliefs. This is harmful to those impacted and does a disservice to our profession at large. In a helping profession we should strive to support and advocate for anyone who has been maltreated or experienced any form of victimization, violence, or abuse. This should be a professional standard.
As clinicians, we have an ethical obligation to uphold the well-being, autonomy, and dignity of our patients — and humanity. It is crucial to recognize the limits of our expertise and the ethical concerns that can arise in light of geopolitical conflict. How can we balance our duty to provide psychological support while also being cautious about delving into the realms of political analysis, foreign policy, or international relations?
The pitfalls of well-intentioned speaking out
In the age of social media and instant communication, a critical aspect to consider is the role of speaking out. The point I made above, in naming all partaking in the current conflict, speaks to this issue.
As providers and programs, we must be mindful of the inadvertent harm that can arise from making brief, underdeveloped, uninformed, or emotionally charged statements. Expressing opinions without a solid understanding of the historical, cultural, and political nuances of a conflict can contribute to misinformation and further polarization.
Anecdotally, there appears to be some significant degree of bias emerging within professional fields (e.g., psychology, medicine) and an innate calling for providers to “weigh in” as the war continues. Obviously, physicians and psychologists are trained to provide care and to be humanistic and empathic, but the majority do not have expertise in geopolitics or a nuanced awareness of the complexities of the conflict in the Middle East.
While hearts may be in the right place, issuing statements on complicated humanitarian/political situations can inadvertently have unintended and harmful consequences (in terms of antisemitism and islamophobia, increased incidence of hate crimes, and colleagues not feeling safe within professional societies or member organizations).
Unsophisticated, overly simplistic, and reductionistic statements that do not adequately convey nuance will not reflect the range of experience reflected by providers in the field (or the patients we treat). It is essential for clinicians and institutions putting out public statements to engage in deep reflection and utilize discernment. We must recognize that our words carry weight, given our position of influence as treatment providers. To minimize harm, we should seek to provide information that is fair, vetted, and balanced, and encourage open, respectful dialogue rather than asserting definitive positions.
Ultimately, as providers we must strive to seek unity and inclusivity amidst the current challenges. It is important for us to embody a spirit of collaboration during a time demarcated by deep fragmentation.
By acknowledging our limitations, promoting informed discussion, and avoiding the pitfalls of uninformed advocacy, we can contribute to a more compassionate and understanding world, even in the face of the most divisive geopolitical conflicts. We have an obligation to uphold when it comes to ourselves as professionals, and we need to foster healthy, respectful dialogue while maintaining an awareness of our blind spots.
Dr. Feldman is a licensed clinical psychologist in private practice in Miami. She is an adjunct professor in the College of Psychology at Nova Southeastern University, Fort Lauderdale, Fla., where she teaches clinical psychology doctoral students. She is an affiliate of Baptist West Kendall Hospital/FIU Family Medicine Residency Program and serves as president on the board of directors of The Southeast Florida Association for Psychoanalytic Psychology. The opinions expressed by Dr. Feldman are her own and do not represent the institutions with which she is affiliated. She has no disclosures.
In the realm of clinical psychology and psychiatry, our primary duty and commitment is (and should be) to the well-being of our patients. Yet, as we find ourselves in an era marked by escalating geopolitical conflict, such as the Israel-Hamas war, probably more aptly titled the Israeli-Hamas-Hezbollah-Houthi war (a clarification that elucidates a later point), clinicians are increasingly confronted with ethical dilemmas that extend far beyond what is outlined in our code of ethics.
These challenges are not only impacting us on a personal level but are also spilling over into our professional lives, creating a divisive and non-collegial environment within the healthcare community. We commit to “do no harm” when delivering care and yet we are doing harm to one another as colleagues.
We are no strangers to the complexities of human behavior and the intricate tapestry of emotions that are involved with our professional work. However, the current geopolitical landscape has added an extra layer of difficulty to our already taxing professional lives. We are, after all, human first with unconscious drives that govern how we negotiate cognitive dissonance and our need for the illusion of absolute justice as Yuval Noah Harari explains in a recent podcast.
Humans are notoriously bad at holding the multiplicity of experience in mind and various (often competing narratives) that impede the capacity for nuanced thinking. We would like to believe we are better and more capable than the average person in doing so, but divisiveness in our profession has become disturbingly pronounced, making it essential for us to carve out reflective space, more than ever.
The personal and professional divide
Geopolitical conflicts like the current war have a unique capacity to ignite strong emotions and deeply held convictions. It’s not hard to quickly become embroiled in passionate and engaged debate.
While discussion and discourse are healthy, these are bleeding into professional spheres, creating rifts within our clinical communities and contributing to a culture where not everyone feels safe. Look at any professional listserv in medicine or psychology and you will find the evidence. It should be an immediate call to action that we need to be fostering a different type of environment.
The impact of divisiveness is profound, hindering opportunities for collaboration, mentorship, and the free exchange of ideas among clinicians. It may lead to misunderstandings, mistrust, and an erosion of the support systems we rely on, ultimately diverting energy away from the pursuit of providing quality patient-care.
Balancing obligations and limits
Because of the inherent power differential that accompanies being in a provider role (physician and psychologist alike), we have a social and moral responsibility to be mindful of what we share – for the sake of humanity. There is an implicit assumption that a provider’s guidance should be adhered to and respected. In other words, words carry tremendous weight and deeply matter, and people in the general public ascribe significant meaning to messages put out by professionals.
When providers steer from their lanes of professional expertise to provide the general public with opinions or recommendations on nonmedical topics, problematic precedents can be set. We may be doing people a disservice.
Unfortunately, I have heard several anecdotes about clinicians who spend their patient’s time in session pushing their own ideological agendas. The patient-provider relationship is founded on principles of trust, empathy, and collaboration, with the primary goal of improving overall well-being and addressing a specific presenting problem. Of course, issues emerge that need to be addressed outside of the initial scope of treatment, an inherent part of the process. However, a grave concern emerges when clinicians initiate dialogue that is not meaningful to a patient, disclose and discuss their personal ideologies, or put pressure on patients to explain their beliefs in an attempt to change the patients’ minds.
Clinicians pushing their own agenda during patient sessions is antithetical to the objectives of psychotherapy and compromises the therapeutic alliance by diverting the focus of care in a way that serves the clinician rather than the client. It is quite the opposite of the patient-centered care that we strive for in training and practice.
Even within one’s theoretical professional scope of competence, I have seen the impact of emotions running high during this conflict, and have witnessed trained professionals making light of, or even mocking, hostages and their behavior upon release. These are care providers who could elucidate the complexities of captor-captive dynamics and the impact of trauma for the general public, yet they are contributing to dangerous perceptions and divisiveness.
I have also seen providers justify sexual violence, diminishing survivor and witness testimony due to ideological differences and strong personal beliefs. This is harmful to those impacted and does a disservice to our profession at large. In a helping profession we should strive to support and advocate for anyone who has been maltreated or experienced any form of victimization, violence, or abuse. This should be a professional standard.
As clinicians, we have an ethical obligation to uphold the well-being, autonomy, and dignity of our patients — and humanity. It is crucial to recognize the limits of our expertise and the ethical concerns that can arise in light of geopolitical conflict. How can we balance our duty to provide psychological support while also being cautious about delving into the realms of political analysis, foreign policy, or international relations?
The pitfalls of well-intentioned speaking out
In the age of social media and instant communication, a critical aspect to consider is the role of speaking out. The point I made above, in naming all partaking in the current conflict, speaks to this issue.
As providers and programs, we must be mindful of the inadvertent harm that can arise from making brief, underdeveloped, uninformed, or emotionally charged statements. Expressing opinions without a solid understanding of the historical, cultural, and political nuances of a conflict can contribute to misinformation and further polarization.
Anecdotally, there appears to be some significant degree of bias emerging within professional fields (e.g., psychology, medicine) and an innate calling for providers to “weigh in” as the war continues. Obviously, physicians and psychologists are trained to provide care and to be humanistic and empathic, but the majority do not have expertise in geopolitics or a nuanced awareness of the complexities of the conflict in the Middle East.
While hearts may be in the right place, issuing statements on complicated humanitarian/political situations can inadvertently have unintended and harmful consequences (in terms of antisemitism and islamophobia, increased incidence of hate crimes, and colleagues not feeling safe within professional societies or member organizations).
Unsophisticated, overly simplistic, and reductionistic statements that do not adequately convey nuance will not reflect the range of experience reflected by providers in the field (or the patients we treat). It is essential for clinicians and institutions putting out public statements to engage in deep reflection and utilize discernment. We must recognize that our words carry weight, given our position of influence as treatment providers. To minimize harm, we should seek to provide information that is fair, vetted, and balanced, and encourage open, respectful dialogue rather than asserting definitive positions.
Ultimately, as providers we must strive to seek unity and inclusivity amidst the current challenges. It is important for us to embody a spirit of collaboration during a time demarcated by deep fragmentation.
By acknowledging our limitations, promoting informed discussion, and avoiding the pitfalls of uninformed advocacy, we can contribute to a more compassionate and understanding world, even in the face of the most divisive geopolitical conflicts. We have an obligation to uphold when it comes to ourselves as professionals, and we need to foster healthy, respectful dialogue while maintaining an awareness of our blind spots.
Dr. Feldman is a licensed clinical psychologist in private practice in Miami. She is an adjunct professor in the College of Psychology at Nova Southeastern University, Fort Lauderdale, Fla., where she teaches clinical psychology doctoral students. She is an affiliate of Baptist West Kendall Hospital/FIU Family Medicine Residency Program and serves as president on the board of directors of The Southeast Florida Association for Psychoanalytic Psychology. The opinions expressed by Dr. Feldman are her own and do not represent the institutions with which she is affiliated. She has no disclosures.
In the realm of clinical psychology and psychiatry, our primary duty and commitment is (and should be) to the well-being of our patients. Yet, as we find ourselves in an era marked by escalating geopolitical conflict, such as the Israel-Hamas war, probably more aptly titled the Israeli-Hamas-Hezbollah-Houthi war (a clarification that elucidates a later point), clinicians are increasingly confronted with ethical dilemmas that extend far beyond what is outlined in our code of ethics.
These challenges are not only impacting us on a personal level but are also spilling over into our professional lives, creating a divisive and non-collegial environment within the healthcare community. We commit to “do no harm” when delivering care and yet we are doing harm to one another as colleagues.
We are no strangers to the complexities of human behavior and the intricate tapestry of emotions that are involved with our professional work. However, the current geopolitical landscape has added an extra layer of difficulty to our already taxing professional lives. We are, after all, human first with unconscious drives that govern how we negotiate cognitive dissonance and our need for the illusion of absolute justice as Yuval Noah Harari explains in a recent podcast.
Humans are notoriously bad at holding the multiplicity of experience in mind and various (often competing narratives) that impede the capacity for nuanced thinking. We would like to believe we are better and more capable than the average person in doing so, but divisiveness in our profession has become disturbingly pronounced, making it essential for us to carve out reflective space, more than ever.
The personal and professional divide
Geopolitical conflicts like the current war have a unique capacity to ignite strong emotions and deeply held convictions. It’s not hard to quickly become embroiled in passionate and engaged debate.
While discussion and discourse are healthy, these are bleeding into professional spheres, creating rifts within our clinical communities and contributing to a culture where not everyone feels safe. Look at any professional listserv in medicine or psychology and you will find the evidence. It should be an immediate call to action that we need to be fostering a different type of environment.
The impact of divisiveness is profound, hindering opportunities for collaboration, mentorship, and the free exchange of ideas among clinicians. It may lead to misunderstandings, mistrust, and an erosion of the support systems we rely on, ultimately diverting energy away from the pursuit of providing quality patient-care.
Balancing obligations and limits
Because of the inherent power differential that accompanies being in a provider role (physician and psychologist alike), we have a social and moral responsibility to be mindful of what we share – for the sake of humanity. There is an implicit assumption that a provider’s guidance should be adhered to and respected. In other words, words carry tremendous weight and deeply matter, and people in the general public ascribe significant meaning to messages put out by professionals.
When providers steer from their lanes of professional expertise to provide the general public with opinions or recommendations on nonmedical topics, problematic precedents can be set. We may be doing people a disservice.
Unfortunately, I have heard several anecdotes about clinicians who spend their patient’s time in session pushing their own ideological agendas. The patient-provider relationship is founded on principles of trust, empathy, and collaboration, with the primary goal of improving overall well-being and addressing a specific presenting problem. Of course, issues emerge that need to be addressed outside of the initial scope of treatment, an inherent part of the process. However, a grave concern emerges when clinicians initiate dialogue that is not meaningful to a patient, disclose and discuss their personal ideologies, or put pressure on patients to explain their beliefs in an attempt to change the patients’ minds.
Clinicians pushing their own agenda during patient sessions is antithetical to the objectives of psychotherapy and compromises the therapeutic alliance by diverting the focus of care in a way that serves the clinician rather than the client. It is quite the opposite of the patient-centered care that we strive for in training and practice.
Even within one’s theoretical professional scope of competence, I have seen the impact of emotions running high during this conflict, and have witnessed trained professionals making light of, or even mocking, hostages and their behavior upon release. These are care providers who could elucidate the complexities of captor-captive dynamics and the impact of trauma for the general public, yet they are contributing to dangerous perceptions and divisiveness.
I have also seen providers justify sexual violence, diminishing survivor and witness testimony due to ideological differences and strong personal beliefs. This is harmful to those impacted and does a disservice to our profession at large. In a helping profession we should strive to support and advocate for anyone who has been maltreated or experienced any form of victimization, violence, or abuse. This should be a professional standard.
As clinicians, we have an ethical obligation to uphold the well-being, autonomy, and dignity of our patients — and humanity. It is crucial to recognize the limits of our expertise and the ethical concerns that can arise in light of geopolitical conflict. How can we balance our duty to provide psychological support while also being cautious about delving into the realms of political analysis, foreign policy, or international relations?
The pitfalls of well-intentioned speaking out
In the age of social media and instant communication, a critical aspect to consider is the role of speaking out. The point I made above, in naming all partaking in the current conflict, speaks to this issue.
As providers and programs, we must be mindful of the inadvertent harm that can arise from making brief, underdeveloped, uninformed, or emotionally charged statements. Expressing opinions without a solid understanding of the historical, cultural, and political nuances of a conflict can contribute to misinformation and further polarization.
Anecdotally, there appears to be some significant degree of bias emerging within professional fields (e.g., psychology, medicine) and an innate calling for providers to “weigh in” as the war continues. Obviously, physicians and psychologists are trained to provide care and to be humanistic and empathic, but the majority do not have expertise in geopolitics or a nuanced awareness of the complexities of the conflict in the Middle East.
While hearts may be in the right place, issuing statements on complicated humanitarian/political situations can inadvertently have unintended and harmful consequences (in terms of antisemitism and islamophobia, increased incidence of hate crimes, and colleagues not feeling safe within professional societies or member organizations).
Unsophisticated, overly simplistic, and reductionistic statements that do not adequately convey nuance will not reflect the range of experience reflected by providers in the field (or the patients we treat). It is essential for clinicians and institutions putting out public statements to engage in deep reflection and utilize discernment. We must recognize that our words carry weight, given our position of influence as treatment providers. To minimize harm, we should seek to provide information that is fair, vetted, and balanced, and encourage open, respectful dialogue rather than asserting definitive positions.
Ultimately, as providers we must strive to seek unity and inclusivity amidst the current challenges. It is important for us to embody a spirit of collaboration during a time demarcated by deep fragmentation.
By acknowledging our limitations, promoting informed discussion, and avoiding the pitfalls of uninformed advocacy, we can contribute to a more compassionate and understanding world, even in the face of the most divisive geopolitical conflicts. We have an obligation to uphold when it comes to ourselves as professionals, and we need to foster healthy, respectful dialogue while maintaining an awareness of our blind spots.
Dr. Feldman is a licensed clinical psychologist in private practice in Miami. She is an adjunct professor in the College of Psychology at Nova Southeastern University, Fort Lauderdale, Fla., where she teaches clinical psychology doctoral students. She is an affiliate of Baptist West Kendall Hospital/FIU Family Medicine Residency Program and serves as president on the board of directors of The Southeast Florida Association for Psychoanalytic Psychology. The opinions expressed by Dr. Feldman are her own and do not represent the institutions with which she is affiliated. She has no disclosures.
EMRs: gumming up the works
I don’t like EMR systems, with all their requirements, click boxes, endless cut & paste abuse, and 20-page notes that say nothing.
But I am a fan of what computers have brought to medical charts.
When I started out in 2000, I had no patients, hence no charts. I had the advantage of being able to start from scratch — there was nothing to convert to digital. So, from the beginning, that’s how I went. Back then, of course, everything came to the office as paper. It had to be scanned in, then named, then placed in the right computer file.
But it was still easier than amassing paper records. At that time I subleased from a doc who’d been in practice for 15 years. His charts were all paper. Charts were neatly filed on shelves, everything was initialed, hole-punched, and put in the right section (which involved pulling out other stuff and putting it back). A few times a year, his staff would comb through the charts in front, and anyone who hadn’t been seen in 2 years would have their chart moved to a storage room in the back. Once a year they’d pull the charts of anyone not seen in 7 years and a company would come in and shred those records.
After 23 years, I still have it all. The whole thing takes up a little over 50 gigabytes on a hard drive, which realistically is nothing these days. Electrons don’t take up much space.
The majority of the charts — those that are more than 7 years old — I’ll probably never need to access, but it still happens sometimes. People call in and say they’ve moved back to Phoenix, or need to see a neurologist again, or need the records for insurance reasons, or whatever. My staff is also spared from moving charts to a storage room, then to shredding. Since they don’t take up any physical space, it’s no effort to keep everything.
And they aren’t just at my office. They’re at home, on my phone, wherever I am. If I get called from an ER, I can pull them up quickly. If I travel, they’re with me. My memory is good, but not that good, and I’d rather be able to look things up than guess.
This, at least to me, is the advantage of computers. Their data storage and retrieval advantages far exceed that of paper. In my opinion EMRs, while well-intentioned, have taken these benefits and twisted them into something cumbersome, geared more to meet nonmedical requirements and billing purposes.
In the process they’ve lost sight of our age-old job of caring for patients.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I don’t like EMR systems, with all their requirements, click boxes, endless cut & paste abuse, and 20-page notes that say nothing.
But I am a fan of what computers have brought to medical charts.
When I started out in 2000, I had no patients, hence no charts. I had the advantage of being able to start from scratch — there was nothing to convert to digital. So, from the beginning, that’s how I went. Back then, of course, everything came to the office as paper. It had to be scanned in, then named, then placed in the right computer file.
But it was still easier than amassing paper records. At that time I subleased from a doc who’d been in practice for 15 years. His charts were all paper. Charts were neatly filed on shelves, everything was initialed, hole-punched, and put in the right section (which involved pulling out other stuff and putting it back). A few times a year, his staff would comb through the charts in front, and anyone who hadn’t been seen in 2 years would have their chart moved to a storage room in the back. Once a year they’d pull the charts of anyone not seen in 7 years and a company would come in and shred those records.
After 23 years, I still have it all. The whole thing takes up a little over 50 gigabytes on a hard drive, which realistically is nothing these days. Electrons don’t take up much space.
The majority of the charts — those that are more than 7 years old — I’ll probably never need to access, but it still happens sometimes. People call in and say they’ve moved back to Phoenix, or need to see a neurologist again, or need the records for insurance reasons, or whatever. My staff is also spared from moving charts to a storage room, then to shredding. Since they don’t take up any physical space, it’s no effort to keep everything.
And they aren’t just at my office. They’re at home, on my phone, wherever I am. If I get called from an ER, I can pull them up quickly. If I travel, they’re with me. My memory is good, but not that good, and I’d rather be able to look things up than guess.
This, at least to me, is the advantage of computers. Their data storage and retrieval advantages far exceed that of paper. In my opinion EMRs, while well-intentioned, have taken these benefits and twisted them into something cumbersome, geared more to meet nonmedical requirements and billing purposes.
In the process they’ve lost sight of our age-old job of caring for patients.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I don’t like EMR systems, with all their requirements, click boxes, endless cut & paste abuse, and 20-page notes that say nothing.
But I am a fan of what computers have brought to medical charts.
When I started out in 2000, I had no patients, hence no charts. I had the advantage of being able to start from scratch — there was nothing to convert to digital. So, from the beginning, that’s how I went. Back then, of course, everything came to the office as paper. It had to be scanned in, then named, then placed in the right computer file.
But it was still easier than amassing paper records. At that time I subleased from a doc who’d been in practice for 15 years. His charts were all paper. Charts were neatly filed on shelves, everything was initialed, hole-punched, and put in the right section (which involved pulling out other stuff and putting it back). A few times a year, his staff would comb through the charts in front, and anyone who hadn’t been seen in 2 years would have their chart moved to a storage room in the back. Once a year they’d pull the charts of anyone not seen in 7 years and a company would come in and shred those records.
After 23 years, I still have it all. The whole thing takes up a little over 50 gigabytes on a hard drive, which realistically is nothing these days. Electrons don’t take up much space.
The majority of the charts — those that are more than 7 years old — I’ll probably never need to access, but it still happens sometimes. People call in and say they’ve moved back to Phoenix, or need to see a neurologist again, or need the records for insurance reasons, or whatever. My staff is also spared from moving charts to a storage room, then to shredding. Since they don’t take up any physical space, it’s no effort to keep everything.
And they aren’t just at my office. They’re at home, on my phone, wherever I am. If I get called from an ER, I can pull them up quickly. If I travel, they’re with me. My memory is good, but not that good, and I’d rather be able to look things up than guess.
This, at least to me, is the advantage of computers. Their data storage and retrieval advantages far exceed that of paper. In my opinion EMRs, while well-intentioned, have taken these benefits and twisted them into something cumbersome, geared more to meet nonmedical requirements and billing purposes.
In the process they’ve lost sight of our age-old job of caring for patients.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
An alternative to walking out
Organized labor seems to be experiencing a rebirth of sorts. In October 2022 a strike by railroad workers was averted when a tentative agreement about wages, working conditions, health insurance, and medical leave was hammered out. This past fall, strikes by auto workers that threatened to paralyze the big three manufacturers have now been resolved with agreements that meet many of the workers’ demands. The President even made an appearance on a picket line. Baristas at coffee shops, screenwriters, and actors have all been involved in work actions around the country.
While the health care industry has been relatively immune to threatened work stoppages, there are a growing number of hospitals and clinics where nurses and physicians are exploring the possibility of organizing to give themselves a stronger voice in how health care is being delivered. The realities that come when you transition from owner to employee are finally beginning to sink in for physicians, whether they are specialists or primary care providers.
One of the most significant efforts toward unionization recently occurred in Minnesota and Wisconsin. About 400 physicians and 150 physician’s assistants and nurse practitioners employed at Allina Health System voted to unionize and join the Doctors Council.
In an interview with Jacobin, a publication that offers a socialist perspective, three of the providers involved in the process that led to the vote shared their observations. The physicians claim that the first steps toward unionization came after multiple efforts to work with the Allina’s administration were rebuffed. As primary care physicians, their initial demands focused on getting help with hiring staffing and getting support with paperwork and administrative obligations.
The organizers complained that while Medicare hoped to bolster primary care by paying the providers more, the funds went to the companies, who then distributed them in a way that often did little to help the overworked providers. In addition to achieving a more equitable distribution of the monies, one of the organizers sees unionization as a way to provide a layer of protection when providers feel they must speak out about situations which clearly put quality of care at risk.
The organizers say the idea of unionization has been particularly appealing to the younger providers who are feeling threatened by burnout. When these new physicians look to their older coworkers for advice, they often find that the seasoned employees are as stressed as they are. Realizing that things aren’t going to improve with time, acting now to strengthen their voices sounds appealing.
With the vote for unionization behind them, the organizers are now ready to formulate a prioritized list of demands. Those of you who are regular readers of Letters from Maine know that I have been urging primary care physicians to find their voices. Unfortunately, unionization seems to be becoming a more common fall-back strategy when other avenues have failed to reach a sympathetic ear in the corporate boardrooms.
As more unions form, it will be interesting to see how the organizers structure their demands and job actions. While walkouts and strikes can certainly be effective in gaining attention, that attention can carry a risk of counter productivity sometimes by alienating patients, who should become allies.
Since an unsustainable burden of paperwork and administrative demands seems to be at the top of everyone’s priority list, it might make sense to adopt this message as a scaffolding on which to built a work action. Instead of walking off the job or marching on a picket line, why not stay in the hospital and continue to see patients but only for part of the work day. The remainder of the day would be spent doing all the clerical work that has become so onerous.
Providers would agree to see patients in the mornings, saving up the clerical work and administrative obligations for the afternoon. The definition of “morning” could vary depending on local conditions.
The important message to the public and the patients would be that the providers were not abandoning them by walking out. The patients’ access to face-to-face care was being limited not because the doctors didn’t want to see them but because the providers were being forced to accept other responsibilities by the administration. The physicians would always be on site in case of a crisis, but until reasonable demands for support from the company were met, a certain portion of the providers’ day would be spent doing things not directly related to face-to-face patient care. This burden of meaningless work is the reality as it stands already. Why not organize it in a way that makes it startlingly visible to the patients and the public.
There would be no video clips of physicians walking the picket lines carrying signs. Any images released to the media would be of empty waiting rooms while providers sat hunched over their computers or talking on the phone to insurance companies.
The strategy needs a catchy phrase like “a paperwork-in” but I’m still struggling with a name. Let me know if you have a better one or even a better strategy.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Organized labor seems to be experiencing a rebirth of sorts. In October 2022 a strike by railroad workers was averted when a tentative agreement about wages, working conditions, health insurance, and medical leave was hammered out. This past fall, strikes by auto workers that threatened to paralyze the big three manufacturers have now been resolved with agreements that meet many of the workers’ demands. The President even made an appearance on a picket line. Baristas at coffee shops, screenwriters, and actors have all been involved in work actions around the country.
While the health care industry has been relatively immune to threatened work stoppages, there are a growing number of hospitals and clinics where nurses and physicians are exploring the possibility of organizing to give themselves a stronger voice in how health care is being delivered. The realities that come when you transition from owner to employee are finally beginning to sink in for physicians, whether they are specialists or primary care providers.
One of the most significant efforts toward unionization recently occurred in Minnesota and Wisconsin. About 400 physicians and 150 physician’s assistants and nurse practitioners employed at Allina Health System voted to unionize and join the Doctors Council.
In an interview with Jacobin, a publication that offers a socialist perspective, three of the providers involved in the process that led to the vote shared their observations. The physicians claim that the first steps toward unionization came after multiple efforts to work with the Allina’s administration were rebuffed. As primary care physicians, their initial demands focused on getting help with hiring staffing and getting support with paperwork and administrative obligations.
The organizers complained that while Medicare hoped to bolster primary care by paying the providers more, the funds went to the companies, who then distributed them in a way that often did little to help the overworked providers. In addition to achieving a more equitable distribution of the monies, one of the organizers sees unionization as a way to provide a layer of protection when providers feel they must speak out about situations which clearly put quality of care at risk.
The organizers say the idea of unionization has been particularly appealing to the younger providers who are feeling threatened by burnout. When these new physicians look to their older coworkers for advice, they often find that the seasoned employees are as stressed as they are. Realizing that things aren’t going to improve with time, acting now to strengthen their voices sounds appealing.
With the vote for unionization behind them, the organizers are now ready to formulate a prioritized list of demands. Those of you who are regular readers of Letters from Maine know that I have been urging primary care physicians to find their voices. Unfortunately, unionization seems to be becoming a more common fall-back strategy when other avenues have failed to reach a sympathetic ear in the corporate boardrooms.
As more unions form, it will be interesting to see how the organizers structure their demands and job actions. While walkouts and strikes can certainly be effective in gaining attention, that attention can carry a risk of counter productivity sometimes by alienating patients, who should become allies.
Since an unsustainable burden of paperwork and administrative demands seems to be at the top of everyone’s priority list, it might make sense to adopt this message as a scaffolding on which to built a work action. Instead of walking off the job or marching on a picket line, why not stay in the hospital and continue to see patients but only for part of the work day. The remainder of the day would be spent doing all the clerical work that has become so onerous.
Providers would agree to see patients in the mornings, saving up the clerical work and administrative obligations for the afternoon. The definition of “morning” could vary depending on local conditions.
The important message to the public and the patients would be that the providers were not abandoning them by walking out. The patients’ access to face-to-face care was being limited not because the doctors didn’t want to see them but because the providers were being forced to accept other responsibilities by the administration. The physicians would always be on site in case of a crisis, but until reasonable demands for support from the company were met, a certain portion of the providers’ day would be spent doing things not directly related to face-to-face patient care. This burden of meaningless work is the reality as it stands already. Why not organize it in a way that makes it startlingly visible to the patients and the public.
There would be no video clips of physicians walking the picket lines carrying signs. Any images released to the media would be of empty waiting rooms while providers sat hunched over their computers or talking on the phone to insurance companies.
The strategy needs a catchy phrase like “a paperwork-in” but I’m still struggling with a name. Let me know if you have a better one or even a better strategy.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Organized labor seems to be experiencing a rebirth of sorts. In October 2022 a strike by railroad workers was averted when a tentative agreement about wages, working conditions, health insurance, and medical leave was hammered out. This past fall, strikes by auto workers that threatened to paralyze the big three manufacturers have now been resolved with agreements that meet many of the workers’ demands. The President even made an appearance on a picket line. Baristas at coffee shops, screenwriters, and actors have all been involved in work actions around the country.
While the health care industry has been relatively immune to threatened work stoppages, there are a growing number of hospitals and clinics where nurses and physicians are exploring the possibility of organizing to give themselves a stronger voice in how health care is being delivered. The realities that come when you transition from owner to employee are finally beginning to sink in for physicians, whether they are specialists or primary care providers.
One of the most significant efforts toward unionization recently occurred in Minnesota and Wisconsin. About 400 physicians and 150 physician’s assistants and nurse practitioners employed at Allina Health System voted to unionize and join the Doctors Council.
In an interview with Jacobin, a publication that offers a socialist perspective, three of the providers involved in the process that led to the vote shared their observations. The physicians claim that the first steps toward unionization came after multiple efforts to work with the Allina’s administration were rebuffed. As primary care physicians, their initial demands focused on getting help with hiring staffing and getting support with paperwork and administrative obligations.
The organizers complained that while Medicare hoped to bolster primary care by paying the providers more, the funds went to the companies, who then distributed them in a way that often did little to help the overworked providers. In addition to achieving a more equitable distribution of the monies, one of the organizers sees unionization as a way to provide a layer of protection when providers feel they must speak out about situations which clearly put quality of care at risk.
The organizers say the idea of unionization has been particularly appealing to the younger providers who are feeling threatened by burnout. When these new physicians look to their older coworkers for advice, they often find that the seasoned employees are as stressed as they are. Realizing that things aren’t going to improve with time, acting now to strengthen their voices sounds appealing.
With the vote for unionization behind them, the organizers are now ready to formulate a prioritized list of demands. Those of you who are regular readers of Letters from Maine know that I have been urging primary care physicians to find their voices. Unfortunately, unionization seems to be becoming a more common fall-back strategy when other avenues have failed to reach a sympathetic ear in the corporate boardrooms.
As more unions form, it will be interesting to see how the organizers structure their demands and job actions. While walkouts and strikes can certainly be effective in gaining attention, that attention can carry a risk of counter productivity sometimes by alienating patients, who should become allies.
Since an unsustainable burden of paperwork and administrative demands seems to be at the top of everyone’s priority list, it might make sense to adopt this message as a scaffolding on which to built a work action. Instead of walking off the job or marching on a picket line, why not stay in the hospital and continue to see patients but only for part of the work day. The remainder of the day would be spent doing all the clerical work that has become so onerous.
Providers would agree to see patients in the mornings, saving up the clerical work and administrative obligations for the afternoon. The definition of “morning” could vary depending on local conditions.
The important message to the public and the patients would be that the providers were not abandoning them by walking out. The patients’ access to face-to-face care was being limited not because the doctors didn’t want to see them but because the providers were being forced to accept other responsibilities by the administration. The physicians would always be on site in case of a crisis, but until reasonable demands for support from the company were met, a certain portion of the providers’ day would be spent doing things not directly related to face-to-face patient care. This burden of meaningless work is the reality as it stands already. Why not organize it in a way that makes it startlingly visible to the patients and the public.
There would be no video clips of physicians walking the picket lines carrying signs. Any images released to the media would be of empty waiting rooms while providers sat hunched over their computers or talking on the phone to insurance companies.
The strategy needs a catchy phrase like “a paperwork-in” but I’m still struggling with a name. Let me know if you have a better one or even a better strategy.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Are you sure your patient is alive?
This transcript has been edited for clarity.
Much of my research focuses on what is known as clinical decision support — prompts and messages to providers to help them make good decisions for their patients. I know that these things can be annoying, which is exactly why I study them — to figure out which ones actually help.
When I got started on this about 10 years ago, we were learning a lot about how best to message providers about their patients. My team had developed a simple alert for acute kidney injury (AKI). We knew that providers often missed the diagnosis, so maybe letting them know would improve patient outcomes.
As we tested the alert, we got feedback, and I have kept an email from an ICU doctor from those early days. It read:
Dear Dr. Wilson: Thank you for the automated alert informing me that my patient had AKI. Regrettably, the alert fired about an hour after the patient had died. I feel that the information is less than actionable at this time.
Our early system had neglected to add a conditional flag ensuring that the patient was still alive at the time it sent the alert message. A small oversight, but one that had very large implications. Future studies would show that “false positive” alerts like this seriously degrade physician confidence in the system. And why wouldn’t they?
Not knowing the vital status of a patient can have major consequences.
Health systems send messages to their patients all the time: reminders of appointments, reminders for preventive care, reminders for vaccinations, and so on.
But what if the patient being reminded has died? It’s a waste of resources, of course, but more than that, it can be painful for their families and reflects poorly on the health care system. Of all the people who should know whether someone is alive or dead, shouldn’t their doctor be at the top of the list?
A new study in JAMA Internal Medicine quantifies this very phenomenon.
Researchers examined 11,658 primary care patients in their health system who met the criteria of being “seriously ill” and followed them for 2 years. During that period of time, 25% were recorded as deceased in the electronic health record. But 30.8% had died. That left 676 patients who had died, but were not known to have died, left in the system.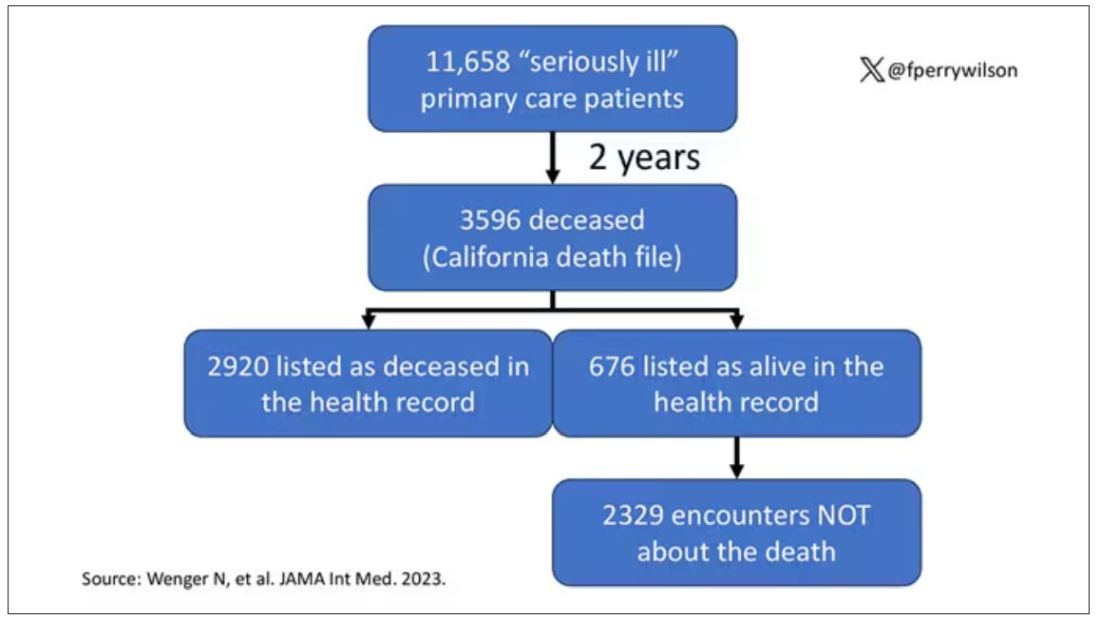
And those 676 were not left to rest in peace. They received 221 telephone and 338 health portal messages not related to death, and 920 letters reminding them about unmet primary care metrics like flu shots and cancer screening. Orders were entered into the health record for things like vaccines and routine screenings for 158 patients, and 310 future appointments — destined to be no-shows — were still on the books. One can only imagine the frustration of families checking their mail and finding yet another letter reminding their deceased loved one to get a mammogram.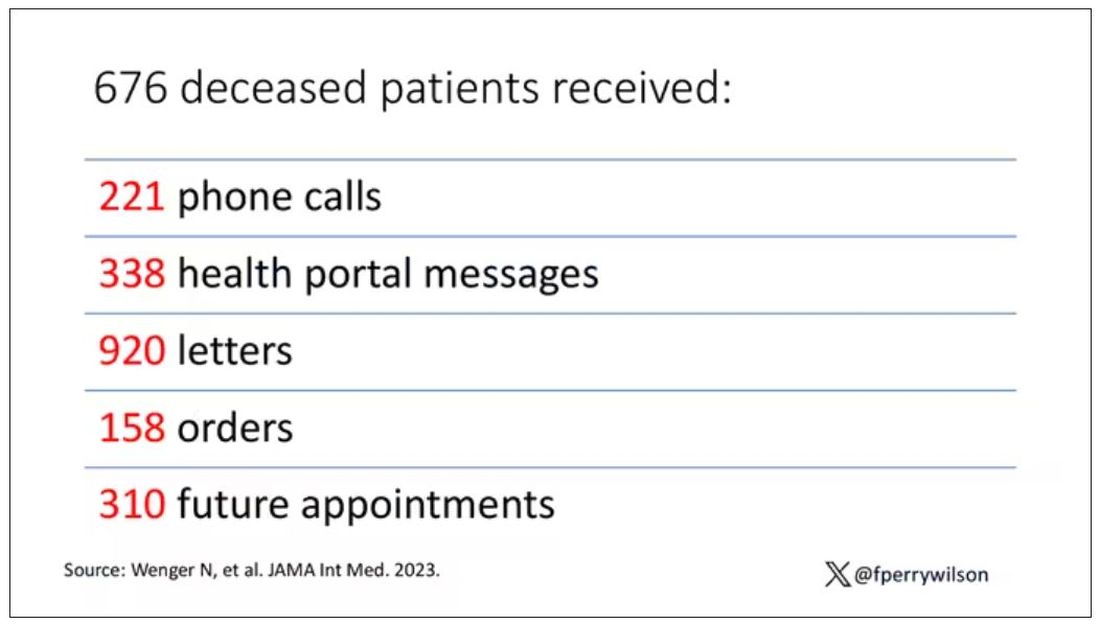
How did the researchers figure out who had died? It turns out it’s not that hard. California keeps a record of all deaths in the state; they simply had to search it. Like all state death records, they tend to lag a bit so it’s not clinically terribly useful, but it works. California and most other states also have a very accurate and up-to-date death file which can only be used by law enforcement to investigate criminal activity and fraud; health care is left in the lurch.
Nationwide, there is the real-time fact of death service, supported by the National Association for Public Health Statistics and Information Systems. This allows employers to verify, in real time, whether the person applying for a job is alive. Healthcare systems are not allowed to use it.
Let’s also remember that very few people die in this country without some health care agency knowing about it and recording it. But sharing of medical information is so poor in the United States that your patient could die in a hospital one city away from you and you might not find out until you’re calling them to see why they missed a scheduled follow-up appointment.
These events — the embarrassing lack of knowledge about the very vital status of our patients — highlight a huge problem with health care in our country. The fragmented health care system is terrible at data sharing, in part because of poor protocols, in part because of unfounded concerns about patient privacy, and in part because of a tendency to hoard data that might be valuable in the future. It has to stop. We need to know how our patients are doing even when they are not sitting in front of us. When it comes to life and death, the knowledge is out there; we just can’t access it. Seems like a pretty easy fix.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com .
This transcript has been edited for clarity.
Much of my research focuses on what is known as clinical decision support — prompts and messages to providers to help them make good decisions for their patients. I know that these things can be annoying, which is exactly why I study them — to figure out which ones actually help.
When I got started on this about 10 years ago, we were learning a lot about how best to message providers about their patients. My team had developed a simple alert for acute kidney injury (AKI). We knew that providers often missed the diagnosis, so maybe letting them know would improve patient outcomes.
As we tested the alert, we got feedback, and I have kept an email from an ICU doctor from those early days. It read:
Dear Dr. Wilson: Thank you for the automated alert informing me that my patient had AKI. Regrettably, the alert fired about an hour after the patient had died. I feel that the information is less than actionable at this time.
Our early system had neglected to add a conditional flag ensuring that the patient was still alive at the time it sent the alert message. A small oversight, but one that had very large implications. Future studies would show that “false positive” alerts like this seriously degrade physician confidence in the system. And why wouldn’t they?
Not knowing the vital status of a patient can have major consequences.
Health systems send messages to their patients all the time: reminders of appointments, reminders for preventive care, reminders for vaccinations, and so on.
But what if the patient being reminded has died? It’s a waste of resources, of course, but more than that, it can be painful for their families and reflects poorly on the health care system. Of all the people who should know whether someone is alive or dead, shouldn’t their doctor be at the top of the list?
A new study in JAMA Internal Medicine quantifies this very phenomenon.
Researchers examined 11,658 primary care patients in their health system who met the criteria of being “seriously ill” and followed them for 2 years. During that period of time, 25% were recorded as deceased in the electronic health record. But 30.8% had died. That left 676 patients who had died, but were not known to have died, left in the system.
And those 676 were not left to rest in peace. They received 221 telephone and 338 health portal messages not related to death, and 920 letters reminding them about unmet primary care metrics like flu shots and cancer screening. Orders were entered into the health record for things like vaccines and routine screenings for 158 patients, and 310 future appointments — destined to be no-shows — were still on the books. One can only imagine the frustration of families checking their mail and finding yet another letter reminding their deceased loved one to get a mammogram.
How did the researchers figure out who had died? It turns out it’s not that hard. California keeps a record of all deaths in the state; they simply had to search it. Like all state death records, they tend to lag a bit so it’s not clinically terribly useful, but it works. California and most other states also have a very accurate and up-to-date death file which can only be used by law enforcement to investigate criminal activity and fraud; health care is left in the lurch.
Nationwide, there is the real-time fact of death service, supported by the National Association for Public Health Statistics and Information Systems. This allows employers to verify, in real time, whether the person applying for a job is alive. Healthcare systems are not allowed to use it.
Let’s also remember that very few people die in this country without some health care agency knowing about it and recording it. But sharing of medical information is so poor in the United States that your patient could die in a hospital one city away from you and you might not find out until you’re calling them to see why they missed a scheduled follow-up appointment.
These events — the embarrassing lack of knowledge about the very vital status of our patients — highlight a huge problem with health care in our country. The fragmented health care system is terrible at data sharing, in part because of poor protocols, in part because of unfounded concerns about patient privacy, and in part because of a tendency to hoard data that might be valuable in the future. It has to stop. We need to know how our patients are doing even when they are not sitting in front of us. When it comes to life and death, the knowledge is out there; we just can’t access it. Seems like a pretty easy fix.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com .
This transcript has been edited for clarity.
Much of my research focuses on what is known as clinical decision support — prompts and messages to providers to help them make good decisions for their patients. I know that these things can be annoying, which is exactly why I study them — to figure out which ones actually help.
When I got started on this about 10 years ago, we were learning a lot about how best to message providers about their patients. My team had developed a simple alert for acute kidney injury (AKI). We knew that providers often missed the diagnosis, so maybe letting them know would improve patient outcomes.
As we tested the alert, we got feedback, and I have kept an email from an ICU doctor from those early days. It read:
Dear Dr. Wilson: Thank you for the automated alert informing me that my patient had AKI. Regrettably, the alert fired about an hour after the patient had died. I feel that the information is less than actionable at this time.
Our early system had neglected to add a conditional flag ensuring that the patient was still alive at the time it sent the alert message. A small oversight, but one that had very large implications. Future studies would show that “false positive” alerts like this seriously degrade physician confidence in the system. And why wouldn’t they?
Not knowing the vital status of a patient can have major consequences.
Health systems send messages to their patients all the time: reminders of appointments, reminders for preventive care, reminders for vaccinations, and so on.
But what if the patient being reminded has died? It’s a waste of resources, of course, but more than that, it can be painful for their families and reflects poorly on the health care system. Of all the people who should know whether someone is alive or dead, shouldn’t their doctor be at the top of the list?
A new study in JAMA Internal Medicine quantifies this very phenomenon.
Researchers examined 11,658 primary care patients in their health system who met the criteria of being “seriously ill” and followed them for 2 years. During that period of time, 25% were recorded as deceased in the electronic health record. But 30.8% had died. That left 676 patients who had died, but were not known to have died, left in the system.
And those 676 were not left to rest in peace. They received 221 telephone and 338 health portal messages not related to death, and 920 letters reminding them about unmet primary care metrics like flu shots and cancer screening. Orders were entered into the health record for things like vaccines and routine screenings for 158 patients, and 310 future appointments — destined to be no-shows — were still on the books. One can only imagine the frustration of families checking their mail and finding yet another letter reminding their deceased loved one to get a mammogram.
How did the researchers figure out who had died? It turns out it’s not that hard. California keeps a record of all deaths in the state; they simply had to search it. Like all state death records, they tend to lag a bit so it’s not clinically terribly useful, but it works. California and most other states also have a very accurate and up-to-date death file which can only be used by law enforcement to investigate criminal activity and fraud; health care is left in the lurch.
Nationwide, there is the real-time fact of death service, supported by the National Association for Public Health Statistics and Information Systems. This allows employers to verify, in real time, whether the person applying for a job is alive. Healthcare systems are not allowed to use it.
Let’s also remember that very few people die in this country without some health care agency knowing about it and recording it. But sharing of medical information is so poor in the United States that your patient could die in a hospital one city away from you and you might not find out until you’re calling them to see why they missed a scheduled follow-up appointment.
These events — the embarrassing lack of knowledge about the very vital status of our patients — highlight a huge problem with health care in our country. The fragmented health care system is terrible at data sharing, in part because of poor protocols, in part because of unfounded concerns about patient privacy, and in part because of a tendency to hoard data that might be valuable in the future. It has to stop. We need to know how our patients are doing even when they are not sitting in front of us. When it comes to life and death, the knowledge is out there; we just can’t access it. Seems like a pretty easy fix.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com .
Statins and the liver: Not harmful and perhaps beneficial
, including glucose intolerance and hyperlipidemia. A lingering concern about potential hepatotoxicity has resulted in widespread reluctance to prescribe statins to treat hyperlipidemia in patients with liver disease; however, their safety in this setting has been documented in the literature as well as in clinical practice. Therefore, statins should not be withheld in patients with liver disease when indicated — with a few caveats. Baseline liver chemistries should be obtained. After initiation of statin therapy, a modest rise in serum aminotransferase levels may occur but is not an indication to discontinue the drug. In fact, monitoring of liver biochemical tests more frequently than is appropriate for any patient with chronic liver disease is unnecessary. The role of statins in cirrhosis may even expand, as recent reports suggest that statin use in patients with cirrhosis may slow the progression of liver disease and reduce the frequency of complications, such as hepatocellular carcinoma. These observations, however, require confirmation before statins can be suggested for any indication other than treating hyperlipidemia in patients with chronic liver disease, and statins are generally not appropriate in patients with decompensated cirrhosis.
Pearls from the Pros was published in Gastro Hep Advances .
Dr. Friedman is the Anton R. Fried, MD, Chair of the Department of Medicine at Newton-Wellesley Hospital in Newton, Mass., and assistant chief of medicine at Massachusetts General Hospital, and a professor of medicine at Harvard Medical School and Tufts University, Boston. Dr. Martin is chief of the division of digestive health and liver diseases at the University of Miami, where he is the Mandel Chair of Gastroenterology. The authors disclose no conflicts.
, including glucose intolerance and hyperlipidemia. A lingering concern about potential hepatotoxicity has resulted in widespread reluctance to prescribe statins to treat hyperlipidemia in patients with liver disease; however, their safety in this setting has been documented in the literature as well as in clinical practice. Therefore, statins should not be withheld in patients with liver disease when indicated — with a few caveats. Baseline liver chemistries should be obtained. After initiation of statin therapy, a modest rise in serum aminotransferase levels may occur but is not an indication to discontinue the drug. In fact, monitoring of liver biochemical tests more frequently than is appropriate for any patient with chronic liver disease is unnecessary. The role of statins in cirrhosis may even expand, as recent reports suggest that statin use in patients with cirrhosis may slow the progression of liver disease and reduce the frequency of complications, such as hepatocellular carcinoma. These observations, however, require confirmation before statins can be suggested for any indication other than treating hyperlipidemia in patients with chronic liver disease, and statins are generally not appropriate in patients with decompensated cirrhosis.
Pearls from the Pros was published in Gastro Hep Advances .
Dr. Friedman is the Anton R. Fried, MD, Chair of the Department of Medicine at Newton-Wellesley Hospital in Newton, Mass., and assistant chief of medicine at Massachusetts General Hospital, and a professor of medicine at Harvard Medical School and Tufts University, Boston. Dr. Martin is chief of the division of digestive health and liver diseases at the University of Miami, where he is the Mandel Chair of Gastroenterology. The authors disclose no conflicts.
, including glucose intolerance and hyperlipidemia. A lingering concern about potential hepatotoxicity has resulted in widespread reluctance to prescribe statins to treat hyperlipidemia in patients with liver disease; however, their safety in this setting has been documented in the literature as well as in clinical practice. Therefore, statins should not be withheld in patients with liver disease when indicated — with a few caveats. Baseline liver chemistries should be obtained. After initiation of statin therapy, a modest rise in serum aminotransferase levels may occur but is not an indication to discontinue the drug. In fact, monitoring of liver biochemical tests more frequently than is appropriate for any patient with chronic liver disease is unnecessary. The role of statins in cirrhosis may even expand, as recent reports suggest that statin use in patients with cirrhosis may slow the progression of liver disease and reduce the frequency of complications, such as hepatocellular carcinoma. These observations, however, require confirmation before statins can be suggested for any indication other than treating hyperlipidemia in patients with chronic liver disease, and statins are generally not appropriate in patients with decompensated cirrhosis.
Pearls from the Pros was published in Gastro Hep Advances .
Dr. Friedman is the Anton R. Fried, MD, Chair of the Department of Medicine at Newton-Wellesley Hospital in Newton, Mass., and assistant chief of medicine at Massachusetts General Hospital, and a professor of medicine at Harvard Medical School and Tufts University, Boston. Dr. Martin is chief of the division of digestive health and liver diseases at the University of Miami, where he is the Mandel Chair of Gastroenterology. The authors disclose no conflicts.
Mass shooters and mental illness: Reexamining the connection
Our psychiatric research, which found a high incidence of undiagnosed mental illness in mass shooters, was recently awarded the esteemed Psychodynamic Psychiatry Journal Prize for best paper published in the last 2 years (2022-2023). The editors noted our integrity in using quantitative data to argue against the common, careless assumption that mass shooters are not mentally ill.
Some of the mass shooters we studied were motivated by religious or political ideologies that were considered forms of terrorism. Given the current tragically violent landscape both at home and in Israel/Palestine, the “desire for destruction” is vital to understand.
Although there have been a limited number of psychiatric studies of perpetrators of mass shootings, our team took the first step to lay the groundwork by conducting a systematic, quantitative study. Our psychiatric research team’s research findings were published in the Journal of Clinical Psychopharmacology and then in greater detail in Psychodynamic Psychiatry,1,2 which provided important context to the complicated backgrounds of these mass shooters who suffer from abuse, marginalization, and severe undiagnosed brain illness.3
The Mother Jones database of 115 mass shootings from 1982 to 2019 was used to study retrospectively 55 shooters in the United States. We developed a uniform, comprehensive, 62-item questionnaire to compile the data collection from multiple sources and record our psychiatric assessments of the assailants, using DSM-5 criteria. After developing this detailed psychiatric assessment questionnaire, psychiatric researchers evaluated the weight and quality of clinical evidence by (1) interviewing forensic psychiatrists who had assessed the assailant following the crime, and/or (2) reviewing court records of psychiatric evaluations conducted during the postcrime judicial proceedings to determine the prevalence of psychiatric illness. Rather than accepting diagnoses from forensic psychiatrists and/or court records, our team independently reviewed the clinical data gathered from multiple sources to apply the DSM-5 criteria to diagnose mental illness.
In most incidents in the database, the perpetrator died either during or shortly after the crime. We examined every case (n=35) in which the assailant survived, and criminal proceedings were instituted.
Of the 35 cases in which the assailant survived and criminal proceedings were instituted, there was insufficient information to make a diagnosis in 3 cases. Of the remaining 32 cases in which we had sufficient information, we determined that 87.5% had the following psychiatric diagnosis: 18 assailants (56%) had schizophrenia, while 10 assailants (31%) had other psychiatric diagnoses: 3 had bipolar I disorder, 2 had delusional disorders (persecutory), 2 had personality disorders (1 paranoid, 1 borderline), 2 had substance-related disorders without other psychiatric diagnosis, and 1 had post-traumatic stress disorder (PTSD).
Out of the 32 surviving assailants for whom we have sufficient evidence, 87.5% of perpetrators of mass shootings were diagnosed with major psychiatric illness, and none were treated appropriately with medication at the time of the crime. Four assailants (12.5%) had no psychiatric diagnosis that we could discern. Of the 18 surviving assailants with schizophrenia, no assailant was on antipsychotic medication for the treatment of schizophrenia prior to the crime. Of the 10 surviving assailants with other psychiatric illnesses, no assailant was on antipsychotic and/or appropriate medication.
In addition, we found that the clinical misdiagnosis of early-onset schizophrenia was associated with the worsening of many of these assailants’ psychotic symptoms. Many of our adolescent shooters prior to the massacre had been misdiagnosed with attention-deficit disorder (ADD), major depression disorder (MDD), or autism spectrum disorder.
Though the vast majority of those suffering from psychiatric illnesses who are appropriately treated are not violent, .4,5,6 This research demonstrates that such untreated illness combined with access to firearms poses a lethal threat to society.
Most of the assailants also experienced profound estrangement, not only from families and friends, but most importantly from themselves. Being marginalized rendered them more vulnerable to their untreated psychiatric illness and to radicalization online, which fostered their violence. While there are complex reasons that a person is not diagnosed, there remains a vital need to decrease the stigma of mental illness to enable those with psychiatric illness to be more respected, less marginalized, and encouraged to receive effective psychiatric treatments.
Dr. Cerfolio is author of “Psychoanalytic and Spiritual Perspectives on Terrorism: Desire for Destruction.” She is clinical assistant professor at the Icahn School of Medicine at Mount Sinai, New York. Dr. Glick is Professor Emeritus, Department of Psychiatry and Behavioral Sciences at Stanford University School of Medicine, Stanford, Calif.
References
1. Glick ID, et al. Domestic Mass Shooters: The Association With Unmedicated and Untreated Psychiatric Illness. J Clin Psychopharmacol. 2021 Jul-Aug;41(4):366-369. doi: 10.1097/JCP.0000000000001417.
2. Cerfolio NE, et al. A Retrospective Observational Study of Psychosocial Determinants and Psychiatric Diagnoses of Mass Shooters in the United States. Psychodyn Psychiatry. 2022 Fall;50(3):1-16. doi: 10.1521/pdps.2022.50.5.001.
3. Cerfolio NE. The Parkland gunman, a horrific crime, and mental illness. The New York Times. 2022 Oct 14. www.nytimes.com/2022/10/14/opinion/letters/jan-6-panel-trump.html#link-5e2ccc1.
4. Corner E, et al. Mental Health Disorders and the Terrorist: A Research Note Probing Selection Effects and Disorder Prevalence. Stud Confl Terror. 2016 Jan;39(6):560–568. doi: 10.1080/1057610X.2015.1120099.
5. Gruenewald J, et al. Distinguishing “Loner” Attacks from Other Domestic Extremist Violence. Criminol Public Policy. 2013 Feb;12(1):65–91. doi: 10.1111/1745-9133.12008.
6. Lankford A. Detecting mental health problems and suicidal motives among terrorists and mass shooters. Crim Behav Ment Health. 2016 Dec;26(5):315-321. doi: 10.1002/cbm.2020.
Our psychiatric research, which found a high incidence of undiagnosed mental illness in mass shooters, was recently awarded the esteemed Psychodynamic Psychiatry Journal Prize for best paper published in the last 2 years (2022-2023). The editors noted our integrity in using quantitative data to argue against the common, careless assumption that mass shooters are not mentally ill.
Some of the mass shooters we studied were motivated by religious or political ideologies that were considered forms of terrorism. Given the current tragically violent landscape both at home and in Israel/Palestine, the “desire for destruction” is vital to understand.
Although there have been a limited number of psychiatric studies of perpetrators of mass shootings, our team took the first step to lay the groundwork by conducting a systematic, quantitative study. Our psychiatric research team’s research findings were published in the Journal of Clinical Psychopharmacology and then in greater detail in Psychodynamic Psychiatry,1,2 which provided important context to the complicated backgrounds of these mass shooters who suffer from abuse, marginalization, and severe undiagnosed brain illness.3
The Mother Jones database of 115 mass shootings from 1982 to 2019 was used to study retrospectively 55 shooters in the United States. We developed a uniform, comprehensive, 62-item questionnaire to compile the data collection from multiple sources and record our psychiatric assessments of the assailants, using DSM-5 criteria. After developing this detailed psychiatric assessment questionnaire, psychiatric researchers evaluated the weight and quality of clinical evidence by (1) interviewing forensic psychiatrists who had assessed the assailant following the crime, and/or (2) reviewing court records of psychiatric evaluations conducted during the postcrime judicial proceedings to determine the prevalence of psychiatric illness. Rather than accepting diagnoses from forensic psychiatrists and/or court records, our team independently reviewed the clinical data gathered from multiple sources to apply the DSM-5 criteria to diagnose mental illness.
In most incidents in the database, the perpetrator died either during or shortly after the crime. We examined every case (n=35) in which the assailant survived, and criminal proceedings were instituted.
Of the 35 cases in which the assailant survived and criminal proceedings were instituted, there was insufficient information to make a diagnosis in 3 cases. Of the remaining 32 cases in which we had sufficient information, we determined that 87.5% had the following psychiatric diagnosis: 18 assailants (56%) had schizophrenia, while 10 assailants (31%) had other psychiatric diagnoses: 3 had bipolar I disorder, 2 had delusional disorders (persecutory), 2 had personality disorders (1 paranoid, 1 borderline), 2 had substance-related disorders without other psychiatric diagnosis, and 1 had post-traumatic stress disorder (PTSD).
Out of the 32 surviving assailants for whom we have sufficient evidence, 87.5% of perpetrators of mass shootings were diagnosed with major psychiatric illness, and none were treated appropriately with medication at the time of the crime. Four assailants (12.5%) had no psychiatric diagnosis that we could discern. Of the 18 surviving assailants with schizophrenia, no assailant was on antipsychotic medication for the treatment of schizophrenia prior to the crime. Of the 10 surviving assailants with other psychiatric illnesses, no assailant was on antipsychotic and/or appropriate medication.
In addition, we found that the clinical misdiagnosis of early-onset schizophrenia was associated with the worsening of many of these assailants’ psychotic symptoms. Many of our adolescent shooters prior to the massacre had been misdiagnosed with attention-deficit disorder (ADD), major depression disorder (MDD), or autism spectrum disorder.
Though the vast majority of those suffering from psychiatric illnesses who are appropriately treated are not violent, .4,5,6 This research demonstrates that such untreated illness combined with access to firearms poses a lethal threat to society.
Most of the assailants also experienced profound estrangement, not only from families and friends, but most importantly from themselves. Being marginalized rendered them more vulnerable to their untreated psychiatric illness and to radicalization online, which fostered their violence. While there are complex reasons that a person is not diagnosed, there remains a vital need to decrease the stigma of mental illness to enable those with psychiatric illness to be more respected, less marginalized, and encouraged to receive effective psychiatric treatments.
Dr. Cerfolio is author of “Psychoanalytic and Spiritual Perspectives on Terrorism: Desire for Destruction.” She is clinical assistant professor at the Icahn School of Medicine at Mount Sinai, New York. Dr. Glick is Professor Emeritus, Department of Psychiatry and Behavioral Sciences at Stanford University School of Medicine, Stanford, Calif.
References
1. Glick ID, et al. Domestic Mass Shooters: The Association With Unmedicated and Untreated Psychiatric Illness. J Clin Psychopharmacol. 2021 Jul-Aug;41(4):366-369. doi: 10.1097/JCP.0000000000001417.
2. Cerfolio NE, et al. A Retrospective Observational Study of Psychosocial Determinants and Psychiatric Diagnoses of Mass Shooters in the United States. Psychodyn Psychiatry. 2022 Fall;50(3):1-16. doi: 10.1521/pdps.2022.50.5.001.
3. Cerfolio NE. The Parkland gunman, a horrific crime, and mental illness. The New York Times. 2022 Oct 14. www.nytimes.com/2022/10/14/opinion/letters/jan-6-panel-trump.html#link-5e2ccc1.
4. Corner E, et al. Mental Health Disorders and the Terrorist: A Research Note Probing Selection Effects and Disorder Prevalence. Stud Confl Terror. 2016 Jan;39(6):560–568. doi: 10.1080/1057610X.2015.1120099.
5. Gruenewald J, et al. Distinguishing “Loner” Attacks from Other Domestic Extremist Violence. Criminol Public Policy. 2013 Feb;12(1):65–91. doi: 10.1111/1745-9133.12008.
6. Lankford A. Detecting mental health problems and suicidal motives among terrorists and mass shooters. Crim Behav Ment Health. 2016 Dec;26(5):315-321. doi: 10.1002/cbm.2020.
Our psychiatric research, which found a high incidence of undiagnosed mental illness in mass shooters, was recently awarded the esteemed Psychodynamic Psychiatry Journal Prize for best paper published in the last 2 years (2022-2023). The editors noted our integrity in using quantitative data to argue against the common, careless assumption that mass shooters are not mentally ill.
Some of the mass shooters we studied were motivated by religious or political ideologies that were considered forms of terrorism. Given the current tragically violent landscape both at home and in Israel/Palestine, the “desire for destruction” is vital to understand.
Although there have been a limited number of psychiatric studies of perpetrators of mass shootings, our team took the first step to lay the groundwork by conducting a systematic, quantitative study. Our psychiatric research team’s research findings were published in the Journal of Clinical Psychopharmacology and then in greater detail in Psychodynamic Psychiatry,1,2 which provided important context to the complicated backgrounds of these mass shooters who suffer from abuse, marginalization, and severe undiagnosed brain illness.3
The Mother Jones database of 115 mass shootings from 1982 to 2019 was used to study retrospectively 55 shooters in the United States. We developed a uniform, comprehensive, 62-item questionnaire to compile the data collection from multiple sources and record our psychiatric assessments of the assailants, using DSM-5 criteria. After developing this detailed psychiatric assessment questionnaire, psychiatric researchers evaluated the weight and quality of clinical evidence by (1) interviewing forensic psychiatrists who had assessed the assailant following the crime, and/or (2) reviewing court records of psychiatric evaluations conducted during the postcrime judicial proceedings to determine the prevalence of psychiatric illness. Rather than accepting diagnoses from forensic psychiatrists and/or court records, our team independently reviewed the clinical data gathered from multiple sources to apply the DSM-5 criteria to diagnose mental illness.
In most incidents in the database, the perpetrator died either during or shortly after the crime. We examined every case (n=35) in which the assailant survived, and criminal proceedings were instituted.
Of the 35 cases in which the assailant survived and criminal proceedings were instituted, there was insufficient information to make a diagnosis in 3 cases. Of the remaining 32 cases in which we had sufficient information, we determined that 87.5% had the following psychiatric diagnosis: 18 assailants (56%) had schizophrenia, while 10 assailants (31%) had other psychiatric diagnoses: 3 had bipolar I disorder, 2 had delusional disorders (persecutory), 2 had personality disorders (1 paranoid, 1 borderline), 2 had substance-related disorders without other psychiatric diagnosis, and 1 had post-traumatic stress disorder (PTSD).
Out of the 32 surviving assailants for whom we have sufficient evidence, 87.5% of perpetrators of mass shootings were diagnosed with major psychiatric illness, and none were treated appropriately with medication at the time of the crime. Four assailants (12.5%) had no psychiatric diagnosis that we could discern. Of the 18 surviving assailants with schizophrenia, no assailant was on antipsychotic medication for the treatment of schizophrenia prior to the crime. Of the 10 surviving assailants with other psychiatric illnesses, no assailant was on antipsychotic and/or appropriate medication.
In addition, we found that the clinical misdiagnosis of early-onset schizophrenia was associated with the worsening of many of these assailants’ psychotic symptoms. Many of our adolescent shooters prior to the massacre had been misdiagnosed with attention-deficit disorder (ADD), major depression disorder (MDD), or autism spectrum disorder.
Though the vast majority of those suffering from psychiatric illnesses who are appropriately treated are not violent, .4,5,6 This research demonstrates that such untreated illness combined with access to firearms poses a lethal threat to society.
Most of the assailants also experienced profound estrangement, not only from families and friends, but most importantly from themselves. Being marginalized rendered them more vulnerable to their untreated psychiatric illness and to radicalization online, which fostered their violence. While there are complex reasons that a person is not diagnosed, there remains a vital need to decrease the stigma of mental illness to enable those with psychiatric illness to be more respected, less marginalized, and encouraged to receive effective psychiatric treatments.
Dr. Cerfolio is author of “Psychoanalytic and Spiritual Perspectives on Terrorism: Desire for Destruction.” She is clinical assistant professor at the Icahn School of Medicine at Mount Sinai, New York. Dr. Glick is Professor Emeritus, Department of Psychiatry and Behavioral Sciences at Stanford University School of Medicine, Stanford, Calif.
References
1. Glick ID, et al. Domestic Mass Shooters: The Association With Unmedicated and Untreated Psychiatric Illness. J Clin Psychopharmacol. 2021 Jul-Aug;41(4):366-369. doi: 10.1097/JCP.0000000000001417.
2. Cerfolio NE, et al. A Retrospective Observational Study of Psychosocial Determinants and Psychiatric Diagnoses of Mass Shooters in the United States. Psychodyn Psychiatry. 2022 Fall;50(3):1-16. doi: 10.1521/pdps.2022.50.5.001.
3. Cerfolio NE. The Parkland gunman, a horrific crime, and mental illness. The New York Times. 2022 Oct 14. www.nytimes.com/2022/10/14/opinion/letters/jan-6-panel-trump.html#link-5e2ccc1.
4. Corner E, et al. Mental Health Disorders and the Terrorist: A Research Note Probing Selection Effects and Disorder Prevalence. Stud Confl Terror. 2016 Jan;39(6):560–568. doi: 10.1080/1057610X.2015.1120099.
5. Gruenewald J, et al. Distinguishing “Loner” Attacks from Other Domestic Extremist Violence. Criminol Public Policy. 2013 Feb;12(1):65–91. doi: 10.1111/1745-9133.12008.
6. Lankford A. Detecting mental health problems and suicidal motives among terrorists and mass shooters. Crim Behav Ment Health. 2016 Dec;26(5):315-321. doi: 10.1002/cbm.2020.
What gastroenterologists need to know about the 2024 Medicare payment rules
Medicare Physician Fee Schedule (MPFS) Final Rule
Cuts to physician payments continue: The final calendar year (CY) 2024 MPFS conversion factor will be $32.7442, a cut of approximately 3.4% from CY 2023, unless Congress acts. The reduction is the result of several factors, including the statutory base payment update of 0 percent, the reduction in assistance provided by the Consolidated Appropriations Act, 2023 (from 2.5% for 2023 to 1.25% for 2024), and budget neutrality adjustments of –2.18 percent resulting from CMS’ finalized policies.
New add-on code for complex care: CMS is finalizing complexity add-on code, G2211 (Visit complexity inherent to evaluation and management associated with medical care services that serve as the continuing focal point for all needed health care services and/or with medical care services that are part of ongoing care related to a patient’s single, serious condition or a complex condition), that it originally proposed in 2018 rulemaking. CMS noted that G2211 cannot be used with an office and outpatient E/M procedure reported with modifier –25. CMS further clarified that the add-on code “is not intended for use by a professional whose relationship with the patient is of a discrete, routine, or time-limited nature ...” CMS further stated, “The inherent complexity that this code (G2211) captures is not in the clinical condition itself ... but rather the cognitive load of the continued responsibility of being the focal point for all needed services for this patient.” For gastroenterologists, it is reasonable to assume G2211 could be reported for care of patients with complex, chronic conditions such as inflammatory bowel disease (IBD), celiac disease, and/or chronic liver disease.
CMS to align split (or shared) visit policy with CPT rules: Originally, CMS proposed to again delay “through at least December 31, 2024” its planned implementation of defining the “substantive portion” of a split/shared visit as more than half of the total time. However, after the American Medical Association’s CPT Editorial Panel, the body responsible for maintaining the CPT code set, issued new guidelines for split (or shared) services CMS decided to finalize the following policy to align with those guidelines: “Substantive portion means more than half of the total time spent by the physician and nonphysician practitioner performing the split (or shared) visit, or a substantive part of the medical decision making except as otherwise provided in this paragraph. For critical care visits, substantive portion means more than half of the total time spent by the physician and nonphysician practitioner performing the split (or shared) visit.”
While the CPT guidance states, “If code selection is based on total time on the date of the encounter, the service is reported by the professional who spent the majority of the face-to-face or non-face-to-face time performing the service,” this direction does not appear in the finalized CMS language.
CMS has extended Telehealth flexibility provisions through Dec. 31, 2024:
- Reporting of Home Address — CMS will continue to permit distant site practitioners to use their currently enrolled practice location instead of their home address when providing telehealth services from their home through CY 2024.
- Place of Service (POS) for Medicare Telehealth Services — Beginning in CY 2024, claims billed with POS 10 (Telehealth Provided in Patient’s Home) will be paid at the non-facility rate, and claims billed with POS 02 (Telehealth Provided Other than in Patient’s Home) will be paid at the facility rate. CMS also clarified that modifier –95 should be used when the clinician is in the hospital and the patient is at home.
- Direct Supervision with Virtual Presence — CMS will continue to define direct supervision to permit the presence and “immediate availability” of the supervising practitioner through real-time audio and visual interactive telecommunications through CY 2024.
- Supervision of Residents in Teaching Settings — CMS will allow teaching physicians to have a virtual presence (to continue to include real-time audio and video observation by the teaching physician) in all teaching settings, but only in clinical instances when the service is furnished virtually, through CY 2024.
- Telephone E/M Services — CMS will continue to pay for CPT codes for telephone assessment and management services (99441-99443) through CY 2024.
Hospital Outpatient Prospective Payment System (OPPS) and Ambulatory Surgery Center (ASC) Final Rule
Hospital and ASC payments will increase: Conversion factors will increase 3.1% to $87.38 for hospitals and $53.51 for ASCs that meet applicable quality reporting requirements.
Hospital payments for Peroral Endoscopic Myotomy (POEM) increase: The GI societies successfully advocated for a 67% increase to the facility payment for POEM. To better align with the procedure’s cost, CMS will place CPT code 43497 for POEM into a higher-level Ambulatory Payment Classification (APC) (5331 — Complex GI procedures) with a facility payment of $5,435.83.
Cuts to hospital payments for some Level 3 upper GI procedures: CMS has finalized moving the following GI CPT codes that had previously been assigned to APC 5303 (Level 3 Upper GI Procedures — $3,260.69) to APC 5302 (Level 2 Upper GI Procedures — $1,814.88) without explanation and against advice from AGA and the GI societies. This will result in payment cuts of 44% to hospitals.
- 43252 (EGD, flexible transoral with optical microscopy)
- 43263 (ERCP with pressure measurement, sphincter of Oddi)
- 43275 (ERCP, remove foreign body/stent biliary/pancreatic duct)
GI Comprehensive APC complexity adjustments: Based on a cost and volume threshold, CMS sometimes makes payment adjustments for Comprehensive APCs when two procedures are performed together. In response to comments received, CMS is adding the following procedures to the list of code combinations eligible for an increased payment via the Complexity Adjustment.
- CPT 43270 (EGD, ablate tumor polyp/lesion with dilation and wire)
- CPT 43252 (EGD, flexible transoral with optical microscopy)
For more information, see 2024 the payment rules summary and payment tables at https://gastro.org/practice-resources/reimbursement.
The Coverage and Reimbursement Subcommittee members have no conflicts of interest.
Medicare Physician Fee Schedule (MPFS) Final Rule
Cuts to physician payments continue: The final calendar year (CY) 2024 MPFS conversion factor will be $32.7442, a cut of approximately 3.4% from CY 2023, unless Congress acts. The reduction is the result of several factors, including the statutory base payment update of 0 percent, the reduction in assistance provided by the Consolidated Appropriations Act, 2023 (from 2.5% for 2023 to 1.25% for 2024), and budget neutrality adjustments of –2.18 percent resulting from CMS’ finalized policies.
New add-on code for complex care: CMS is finalizing complexity add-on code, G2211 (Visit complexity inherent to evaluation and management associated with medical care services that serve as the continuing focal point for all needed health care services and/or with medical care services that are part of ongoing care related to a patient’s single, serious condition or a complex condition), that it originally proposed in 2018 rulemaking. CMS noted that G2211 cannot be used with an office and outpatient E/M procedure reported with modifier –25. CMS further clarified that the add-on code “is not intended for use by a professional whose relationship with the patient is of a discrete, routine, or time-limited nature ...” CMS further stated, “The inherent complexity that this code (G2211) captures is not in the clinical condition itself ... but rather the cognitive load of the continued responsibility of being the focal point for all needed services for this patient.” For gastroenterologists, it is reasonable to assume G2211 could be reported for care of patients with complex, chronic conditions such as inflammatory bowel disease (IBD), celiac disease, and/or chronic liver disease.
CMS to align split (or shared) visit policy with CPT rules: Originally, CMS proposed to again delay “through at least December 31, 2024” its planned implementation of defining the “substantive portion” of a split/shared visit as more than half of the total time. However, after the American Medical Association’s CPT Editorial Panel, the body responsible for maintaining the CPT code set, issued new guidelines for split (or shared) services CMS decided to finalize the following policy to align with those guidelines: “Substantive portion means more than half of the total time spent by the physician and nonphysician practitioner performing the split (or shared) visit, or a substantive part of the medical decision making except as otherwise provided in this paragraph. For critical care visits, substantive portion means more than half of the total time spent by the physician and nonphysician practitioner performing the split (or shared) visit.”
While the CPT guidance states, “If code selection is based on total time on the date of the encounter, the service is reported by the professional who spent the majority of the face-to-face or non-face-to-face time performing the service,” this direction does not appear in the finalized CMS language.
CMS has extended Telehealth flexibility provisions through Dec. 31, 2024:
- Reporting of Home Address — CMS will continue to permit distant site practitioners to use their currently enrolled practice location instead of their home address when providing telehealth services from their home through CY 2024.
- Place of Service (POS) for Medicare Telehealth Services — Beginning in CY 2024, claims billed with POS 10 (Telehealth Provided in Patient’s Home) will be paid at the non-facility rate, and claims billed with POS 02 (Telehealth Provided Other than in Patient’s Home) will be paid at the facility rate. CMS also clarified that modifier –95 should be used when the clinician is in the hospital and the patient is at home.
- Direct Supervision with Virtual Presence — CMS will continue to define direct supervision to permit the presence and “immediate availability” of the supervising practitioner through real-time audio and visual interactive telecommunications through CY 2024.
- Supervision of Residents in Teaching Settings — CMS will allow teaching physicians to have a virtual presence (to continue to include real-time audio and video observation by the teaching physician) in all teaching settings, but only in clinical instances when the service is furnished virtually, through CY 2024.
- Telephone E/M Services — CMS will continue to pay for CPT codes for telephone assessment and management services (99441-99443) through CY 2024.
Hospital Outpatient Prospective Payment System (OPPS) and Ambulatory Surgery Center (ASC) Final Rule
Hospital and ASC payments will increase: Conversion factors will increase 3.1% to $87.38 for hospitals and $53.51 for ASCs that meet applicable quality reporting requirements.
Hospital payments for Peroral Endoscopic Myotomy (POEM) increase: The GI societies successfully advocated for a 67% increase to the facility payment for POEM. To better align with the procedure’s cost, CMS will place CPT code 43497 for POEM into a higher-level Ambulatory Payment Classification (APC) (5331 — Complex GI procedures) with a facility payment of $5,435.83.
Cuts to hospital payments for some Level 3 upper GI procedures: CMS has finalized moving the following GI CPT codes that had previously been assigned to APC 5303 (Level 3 Upper GI Procedures — $3,260.69) to APC 5302 (Level 2 Upper GI Procedures — $1,814.88) without explanation and against advice from AGA and the GI societies. This will result in payment cuts of 44% to hospitals.
- 43252 (EGD, flexible transoral with optical microscopy)
- 43263 (ERCP with pressure measurement, sphincter of Oddi)
- 43275 (ERCP, remove foreign body/stent biliary/pancreatic duct)
GI Comprehensive APC complexity adjustments: Based on a cost and volume threshold, CMS sometimes makes payment adjustments for Comprehensive APCs when two procedures are performed together. In response to comments received, CMS is adding the following procedures to the list of code combinations eligible for an increased payment via the Complexity Adjustment.
- CPT 43270 (EGD, ablate tumor polyp/lesion with dilation and wire)
- CPT 43252 (EGD, flexible transoral with optical microscopy)
For more information, see 2024 the payment rules summary and payment tables at https://gastro.org/practice-resources/reimbursement.
The Coverage and Reimbursement Subcommittee members have no conflicts of interest.
Medicare Physician Fee Schedule (MPFS) Final Rule
Cuts to physician payments continue: The final calendar year (CY) 2024 MPFS conversion factor will be $32.7442, a cut of approximately 3.4% from CY 2023, unless Congress acts. The reduction is the result of several factors, including the statutory base payment update of 0 percent, the reduction in assistance provided by the Consolidated Appropriations Act, 2023 (from 2.5% for 2023 to 1.25% for 2024), and budget neutrality adjustments of –2.18 percent resulting from CMS’ finalized policies.
New add-on code for complex care: CMS is finalizing complexity add-on code, G2211 (Visit complexity inherent to evaluation and management associated with medical care services that serve as the continuing focal point for all needed health care services and/or with medical care services that are part of ongoing care related to a patient’s single, serious condition or a complex condition), that it originally proposed in 2018 rulemaking. CMS noted that G2211 cannot be used with an office and outpatient E/M procedure reported with modifier –25. CMS further clarified that the add-on code “is not intended for use by a professional whose relationship with the patient is of a discrete, routine, or time-limited nature ...” CMS further stated, “The inherent complexity that this code (G2211) captures is not in the clinical condition itself ... but rather the cognitive load of the continued responsibility of being the focal point for all needed services for this patient.” For gastroenterologists, it is reasonable to assume G2211 could be reported for care of patients with complex, chronic conditions such as inflammatory bowel disease (IBD), celiac disease, and/or chronic liver disease.
CMS to align split (or shared) visit policy with CPT rules: Originally, CMS proposed to again delay “through at least December 31, 2024” its planned implementation of defining the “substantive portion” of a split/shared visit as more than half of the total time. However, after the American Medical Association’s CPT Editorial Panel, the body responsible for maintaining the CPT code set, issued new guidelines for split (or shared) services CMS decided to finalize the following policy to align with those guidelines: “Substantive portion means more than half of the total time spent by the physician and nonphysician practitioner performing the split (or shared) visit, or a substantive part of the medical decision making except as otherwise provided in this paragraph. For critical care visits, substantive portion means more than half of the total time spent by the physician and nonphysician practitioner performing the split (or shared) visit.”
While the CPT guidance states, “If code selection is based on total time on the date of the encounter, the service is reported by the professional who spent the majority of the face-to-face or non-face-to-face time performing the service,” this direction does not appear in the finalized CMS language.
CMS has extended Telehealth flexibility provisions through Dec. 31, 2024:
- Reporting of Home Address — CMS will continue to permit distant site practitioners to use their currently enrolled practice location instead of their home address when providing telehealth services from their home through CY 2024.
- Place of Service (POS) for Medicare Telehealth Services — Beginning in CY 2024, claims billed with POS 10 (Telehealth Provided in Patient’s Home) will be paid at the non-facility rate, and claims billed with POS 02 (Telehealth Provided Other than in Patient’s Home) will be paid at the facility rate. CMS also clarified that modifier –95 should be used when the clinician is in the hospital and the patient is at home.
- Direct Supervision with Virtual Presence — CMS will continue to define direct supervision to permit the presence and “immediate availability” of the supervising practitioner through real-time audio and visual interactive telecommunications through CY 2024.
- Supervision of Residents in Teaching Settings — CMS will allow teaching physicians to have a virtual presence (to continue to include real-time audio and video observation by the teaching physician) in all teaching settings, but only in clinical instances when the service is furnished virtually, through CY 2024.
- Telephone E/M Services — CMS will continue to pay for CPT codes for telephone assessment and management services (99441-99443) through CY 2024.
Hospital Outpatient Prospective Payment System (OPPS) and Ambulatory Surgery Center (ASC) Final Rule
Hospital and ASC payments will increase: Conversion factors will increase 3.1% to $87.38 for hospitals and $53.51 for ASCs that meet applicable quality reporting requirements.
Hospital payments for Peroral Endoscopic Myotomy (POEM) increase: The GI societies successfully advocated for a 67% increase to the facility payment for POEM. To better align with the procedure’s cost, CMS will place CPT code 43497 for POEM into a higher-level Ambulatory Payment Classification (APC) (5331 — Complex GI procedures) with a facility payment of $5,435.83.
Cuts to hospital payments for some Level 3 upper GI procedures: CMS has finalized moving the following GI CPT codes that had previously been assigned to APC 5303 (Level 3 Upper GI Procedures — $3,260.69) to APC 5302 (Level 2 Upper GI Procedures — $1,814.88) without explanation and against advice from AGA and the GI societies. This will result in payment cuts of 44% to hospitals.
- 43252 (EGD, flexible transoral with optical microscopy)
- 43263 (ERCP with pressure measurement, sphincter of Oddi)
- 43275 (ERCP, remove foreign body/stent biliary/pancreatic duct)
GI Comprehensive APC complexity adjustments: Based on a cost and volume threshold, CMS sometimes makes payment adjustments for Comprehensive APCs when two procedures are performed together. In response to comments received, CMS is adding the following procedures to the list of code combinations eligible for an increased payment via the Complexity Adjustment.
- CPT 43270 (EGD, ablate tumor polyp/lesion with dilation and wire)
- CPT 43252 (EGD, flexible transoral with optical microscopy)
For more information, see 2024 the payment rules summary and payment tables at https://gastro.org/practice-resources/reimbursement.
The Coverage and Reimbursement Subcommittee members have no conflicts of interest.