User login
Diversity, equity, and inclusion in reproductive health care
These barriers represent inequality in access to reproductive medical services.
These challenges are also seen in other reproductive disorders such as polycystic ovary syndrome (PCOS), fibroids, and endometriosis. It is estimated that < 25% of individuals with infertility in the United States access the resources required to have their treatment needs met (Fertil Steril. 2015 Nov;104(5):1104-10. doi: 10.1016/j.fertnstert.2015.07.113)
In 2020, the American Society for Reproductive Medicine (ASRM) created a task force on Diversity, Equity, and Inclusion (DEI) chaired by Board Member Michael A. Thomas, MD. Two years later, the ASRM elevated this task force to a committee that is now chaired by Gloria Richard-Davis, MD. As health care systems and societies increasingly recognize these obstacles to care, I invited Dr. Thomas, the current president of the ASRM, to address this vital concern. Dr. Thomas is professor and chair, department of obstetrics and gynecology, at the University of Cincinnati.
While not limited to reproductive health care, how prevalent is the lack of DEI and what factors contribute to this problem?
When we established the initial ASRM DEI task force, we wanted to look at DEI issues within our profession and as an access-to-care initiative. We found that ASRM and ABOG (American Board of Obstetrics and Gynecology) were not asking questions about the makeup of our REI (Reproductive Endocrinology & Infertility) providers, nursing staff, and lab personnel. We had some older data from 2018 about the REI fellowships. Since that time, there appears to be an upward trend of people of color in REI fellowships.
We still need more data about academic, hybrid, and private REI practices when it comes to all employees. The goal would be to increase the number of people of color in all aspects of our field.
As far as access to care, we know that people of color do not have the ability to undergo ART (assisted reproductive technology) procedures at the same rate. This could be due to affordability, slower and/or later referral patterns, and personal stigma issues. Even in mandated states, people of color are seen by IVF providers in lower numbers. There is a need for a better understanding of the access-to-care issues, especially when affordability is not a problem, and the barriers to our LGBTQ+ patients.
Can you provide information about actions by the ASRM DEI task force and any plans for the future?
The DEI task force is now an ASRM committee. This committee is chaired by Dr. Gloria Richard-Davis and continues to work on increasing people of color in the REI workforce and understanding and decreasing access to care issues faced by people of color and members of the LGBTQ+ community.
What can physicians do at the local, state, and national level to support DEI?
All REI and ob.gyn. physicians can work with insurance companies to work on the current barriers that stand in the way of patients who want to have a family. For example, physicians can work with insurance companies to remove their definition of infertility as exposure to sperm for 1 year before fertility coverage can take effect. Also, mandated insurance coverage in all 50 states would allow even smaller companies to require this benefit to patients.
Many people of color work in smaller companies that, unfortunately, are not required to offer IVF coverage in states where mandated insurance coverage is available. As potential encouraging news, ASRM, RESOLVE (The National Infertility Association) and other patient advocacy groups are working with each state to help enact fertility mandates.
Which group, if any, has been most negatively affected by a lack of DEI?
People of color, LGBTQ+ communities, people with disabilities, single individuals, and those with income challenges are the most likely to be affected by adverse DEI policies.
While it is long overdue, why do you believe DEI has become such a touchstone and pervasive movement at this time?
This is the million-dollar question. After the George Floyd death, there was a global re-examination of how people of color were treated in every aspect of society. ASRM was the first to start this DEI initiative in women’s health.
ASRM and its patient advocacy partners are working with every nonmandated state toward the goal of passing infertility legislation to dramatically reduce the financial burden on all patients. We are starting to see more states either coming on board with mandates or at least discussing the possibilities. ASRM and RESOLVE have seen some recent positive outcomes with improved insurance for military families and government workers.
We can all agree that access to infertility treatment, particularly IVF, is not equivalent among different racial/ethnic populations. Part of the ASRM DEI task force is to evaluate research on IVF outcomes and race/ethnicity. Can you share why pregnancy outcomes would be included to potentially improve DEI?
More research needs to be done on pregnancy outcomes in women of color. We know that women of color have a decreased pregnancy rate in ART cycles even when controlling for age and other factors. We also know that birth outcomes are worse in these women. More understanding of this problem for women of color, especially African American women needs to be done.
Estimates are that more than one in eight LGBTQ+ patients live in states where physicians can refuse to treat them. Consequently, how can we improve DEI in these regions?
As someone with a number of family members in the LGBTQ+ community, this is a problem that is close to my heart. There appear to be many barriers that are being built to disenfranchise our LGBTQ+ community members. It is up to ASRM and patient advocacy groups to work with legislators to pass more inclusive laws and for insurance companies to update their definitions of infertility to be more inclusive for all.
Any final comments?
Everyone should have the right to become a parent whether they want to now or in the future!
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
These barriers represent inequality in access to reproductive medical services.
These challenges are also seen in other reproductive disorders such as polycystic ovary syndrome (PCOS), fibroids, and endometriosis. It is estimated that < 25% of individuals with infertility in the United States access the resources required to have their treatment needs met (Fertil Steril. 2015 Nov;104(5):1104-10. doi: 10.1016/j.fertnstert.2015.07.113)
In 2020, the American Society for Reproductive Medicine (ASRM) created a task force on Diversity, Equity, and Inclusion (DEI) chaired by Board Member Michael A. Thomas, MD. Two years later, the ASRM elevated this task force to a committee that is now chaired by Gloria Richard-Davis, MD. As health care systems and societies increasingly recognize these obstacles to care, I invited Dr. Thomas, the current president of the ASRM, to address this vital concern. Dr. Thomas is professor and chair, department of obstetrics and gynecology, at the University of Cincinnati.
While not limited to reproductive health care, how prevalent is the lack of DEI and what factors contribute to this problem?
When we established the initial ASRM DEI task force, we wanted to look at DEI issues within our profession and as an access-to-care initiative. We found that ASRM and ABOG (American Board of Obstetrics and Gynecology) were not asking questions about the makeup of our REI (Reproductive Endocrinology & Infertility) providers, nursing staff, and lab personnel. We had some older data from 2018 about the REI fellowships. Since that time, there appears to be an upward trend of people of color in REI fellowships.
We still need more data about academic, hybrid, and private REI practices when it comes to all employees. The goal would be to increase the number of people of color in all aspects of our field.
As far as access to care, we know that people of color do not have the ability to undergo ART (assisted reproductive technology) procedures at the same rate. This could be due to affordability, slower and/or later referral patterns, and personal stigma issues. Even in mandated states, people of color are seen by IVF providers in lower numbers. There is a need for a better understanding of the access-to-care issues, especially when affordability is not a problem, and the barriers to our LGBTQ+ patients.
Can you provide information about actions by the ASRM DEI task force and any plans for the future?
The DEI task force is now an ASRM committee. This committee is chaired by Dr. Gloria Richard-Davis and continues to work on increasing people of color in the REI workforce and understanding and decreasing access to care issues faced by people of color and members of the LGBTQ+ community.
What can physicians do at the local, state, and national level to support DEI?
All REI and ob.gyn. physicians can work with insurance companies to work on the current barriers that stand in the way of patients who want to have a family. For example, physicians can work with insurance companies to remove their definition of infertility as exposure to sperm for 1 year before fertility coverage can take effect. Also, mandated insurance coverage in all 50 states would allow even smaller companies to require this benefit to patients.
Many people of color work in smaller companies that, unfortunately, are not required to offer IVF coverage in states where mandated insurance coverage is available. As potential encouraging news, ASRM, RESOLVE (The National Infertility Association) and other patient advocacy groups are working with each state to help enact fertility mandates.
Which group, if any, has been most negatively affected by a lack of DEI?
People of color, LGBTQ+ communities, people with disabilities, single individuals, and those with income challenges are the most likely to be affected by adverse DEI policies.
While it is long overdue, why do you believe DEI has become such a touchstone and pervasive movement at this time?
This is the million-dollar question. After the George Floyd death, there was a global re-examination of how people of color were treated in every aspect of society. ASRM was the first to start this DEI initiative in women’s health.
ASRM and its patient advocacy partners are working with every nonmandated state toward the goal of passing infertility legislation to dramatically reduce the financial burden on all patients. We are starting to see more states either coming on board with mandates or at least discussing the possibilities. ASRM and RESOLVE have seen some recent positive outcomes with improved insurance for military families and government workers.
We can all agree that access to infertility treatment, particularly IVF, is not equivalent among different racial/ethnic populations. Part of the ASRM DEI task force is to evaluate research on IVF outcomes and race/ethnicity. Can you share why pregnancy outcomes would be included to potentially improve DEI?
More research needs to be done on pregnancy outcomes in women of color. We know that women of color have a decreased pregnancy rate in ART cycles even when controlling for age and other factors. We also know that birth outcomes are worse in these women. More understanding of this problem for women of color, especially African American women needs to be done.
Estimates are that more than one in eight LGBTQ+ patients live in states where physicians can refuse to treat them. Consequently, how can we improve DEI in these regions?
As someone with a number of family members in the LGBTQ+ community, this is a problem that is close to my heart. There appear to be many barriers that are being built to disenfranchise our LGBTQ+ community members. It is up to ASRM and patient advocacy groups to work with legislators to pass more inclusive laws and for insurance companies to update their definitions of infertility to be more inclusive for all.
Any final comments?
Everyone should have the right to become a parent whether they want to now or in the future!
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
These barriers represent inequality in access to reproductive medical services.
These challenges are also seen in other reproductive disorders such as polycystic ovary syndrome (PCOS), fibroids, and endometriosis. It is estimated that < 25% of individuals with infertility in the United States access the resources required to have their treatment needs met (Fertil Steril. 2015 Nov;104(5):1104-10. doi: 10.1016/j.fertnstert.2015.07.113)
In 2020, the American Society for Reproductive Medicine (ASRM) created a task force on Diversity, Equity, and Inclusion (DEI) chaired by Board Member Michael A. Thomas, MD. Two years later, the ASRM elevated this task force to a committee that is now chaired by Gloria Richard-Davis, MD. As health care systems and societies increasingly recognize these obstacles to care, I invited Dr. Thomas, the current president of the ASRM, to address this vital concern. Dr. Thomas is professor and chair, department of obstetrics and gynecology, at the University of Cincinnati.
While not limited to reproductive health care, how prevalent is the lack of DEI and what factors contribute to this problem?
When we established the initial ASRM DEI task force, we wanted to look at DEI issues within our profession and as an access-to-care initiative. We found that ASRM and ABOG (American Board of Obstetrics and Gynecology) were not asking questions about the makeup of our REI (Reproductive Endocrinology & Infertility) providers, nursing staff, and lab personnel. We had some older data from 2018 about the REI fellowships. Since that time, there appears to be an upward trend of people of color in REI fellowships.
We still need more data about academic, hybrid, and private REI practices when it comes to all employees. The goal would be to increase the number of people of color in all aspects of our field.
As far as access to care, we know that people of color do not have the ability to undergo ART (assisted reproductive technology) procedures at the same rate. This could be due to affordability, slower and/or later referral patterns, and personal stigma issues. Even in mandated states, people of color are seen by IVF providers in lower numbers. There is a need for a better understanding of the access-to-care issues, especially when affordability is not a problem, and the barriers to our LGBTQ+ patients.
Can you provide information about actions by the ASRM DEI task force and any plans for the future?
The DEI task force is now an ASRM committee. This committee is chaired by Dr. Gloria Richard-Davis and continues to work on increasing people of color in the REI workforce and understanding and decreasing access to care issues faced by people of color and members of the LGBTQ+ community.
What can physicians do at the local, state, and national level to support DEI?
All REI and ob.gyn. physicians can work with insurance companies to work on the current barriers that stand in the way of patients who want to have a family. For example, physicians can work with insurance companies to remove their definition of infertility as exposure to sperm for 1 year before fertility coverage can take effect. Also, mandated insurance coverage in all 50 states would allow even smaller companies to require this benefit to patients.
Many people of color work in smaller companies that, unfortunately, are not required to offer IVF coverage in states where mandated insurance coverage is available. As potential encouraging news, ASRM, RESOLVE (The National Infertility Association) and other patient advocacy groups are working with each state to help enact fertility mandates.
Which group, if any, has been most negatively affected by a lack of DEI?
People of color, LGBTQ+ communities, people with disabilities, single individuals, and those with income challenges are the most likely to be affected by adverse DEI policies.
While it is long overdue, why do you believe DEI has become such a touchstone and pervasive movement at this time?
This is the million-dollar question. After the George Floyd death, there was a global re-examination of how people of color were treated in every aspect of society. ASRM was the first to start this DEI initiative in women’s health.
ASRM and its patient advocacy partners are working with every nonmandated state toward the goal of passing infertility legislation to dramatically reduce the financial burden on all patients. We are starting to see more states either coming on board with mandates or at least discussing the possibilities. ASRM and RESOLVE have seen some recent positive outcomes with improved insurance for military families and government workers.
We can all agree that access to infertility treatment, particularly IVF, is not equivalent among different racial/ethnic populations. Part of the ASRM DEI task force is to evaluate research on IVF outcomes and race/ethnicity. Can you share why pregnancy outcomes would be included to potentially improve DEI?
More research needs to be done on pregnancy outcomes in women of color. We know that women of color have a decreased pregnancy rate in ART cycles even when controlling for age and other factors. We also know that birth outcomes are worse in these women. More understanding of this problem for women of color, especially African American women needs to be done.
Estimates are that more than one in eight LGBTQ+ patients live in states where physicians can refuse to treat them. Consequently, how can we improve DEI in these regions?
As someone with a number of family members in the LGBTQ+ community, this is a problem that is close to my heart. There appear to be many barriers that are being built to disenfranchise our LGBTQ+ community members. It is up to ASRM and patient advocacy groups to work with legislators to pass more inclusive laws and for insurance companies to update their definitions of infertility to be more inclusive for all.
Any final comments?
Everyone should have the right to become a parent whether they want to now or in the future!
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
Does an elevated TSH value always require therapy?
Thyroxine and L-thyroxine are two of the 10 most frequently prescribed medicinal products. “One large health insurance company ranks thyroid hormone at fourth place in the list of most-sold medications in the United States. It is possibly the second most commonly prescribed preparation,” said Joachim Feldkamp, MD, PhD, director of the University Clinic for General Internal Medicine, Endocrinology, Diabetology, and Infectious Diseases at Central Hospital, Bielefeld, Germany, at the online press conference for the German Society of Endocrinology’s hormone week.
The preparation is prescribed when the thyroid gland produces too little thyroid hormone. The messenger substance thyroid-stimulating hormone (TSH) is used as a screening value to assess thyroid function. An increase in TSH can indicate that too little thyroid hormone is being produced.
“But this does not mean that an underactive thyroid gland is hiding behind every elevated TSH value,” said Dr. Feldkamp. Normally, the TSH value lies between 0.3 and 4.2 mU/L. “Hypothyroidism, as it’s known, is formally present if the TSH value lies above the upper limit of 4.2 mU/L,” said Dr. Feldkamp.
Check again
However, not every elevated TSH value needs to be treated immediately. “From large-scale investigations, we know that TSH values are subject to fluctuations,” said Dr. Feldkamp. Individual measurements must therefore be taken with a grain of salt and almost never justify a therapeutic decision. Therefore, a slightly elevated TSH value should be checked again 2-6 months later, and the patient should be asked if they are experiencing any symptoms. “In 50%-60% of cases, the TSH value normalized at the second checkup without requiring any treatment,” Dr. Feldkamp explained.
The TSH value could be elevated for several reasons:
- Fluctuations depending on the time of day. At night and early in the morning, the TSH value is much higher than in the afternoon. An acute lack of sleep can lead to higher TSH values in the morning.
- Fluctuations depending on the time of year. In winter, TSH values are slightly higher than in the summer owing to adaptation to cooler temperatures. Researchers in the Arctic, for example, have significantly higher TSH values than people who live in warmer regions.
- Age-dependent differences. Children and adolescents have higher TSH values than adults do. The TSH values of adolescents cannot be based on those of adults because this would lead to incorrect treatment. In addition, TSH values increase with age, and slightly elevated values are initially no cause for treatment in people aged 70-80 years. Caution is advised during treatment, because overtreatment can lead to cardiac arrhythmias and a decrease in bone density.
- Sex-specific differences. The TSH values of women are generally a little higher than those in men.
- Obesity. In obesity, TSH increases and often exceeds the normal values usually recorded in persons of normal weight. The elevated values do not reflect a state of hypofunction but rather the body’s adjustment mechanism. If these patients lose weight, the TSH values will drop spontaneously. Slightly elevated TSH values in obese people should not be treated with thyroid hormones.
The nutritional supplement biotin (vitamin H or vitamin B7), which is often taken for skin, hair, and nail growth disorders, can distort measured values. In many of the laboratory methods used, the biotin competes with the test substances used. As a result, it can lead to falsely high and falsely low TSH values. At high doses of biotin (for example, 10 mg), there should be at least a 3-day pause (and ideally a pause of 1 week) before measuring TSH.
Hasty prescriptions
“Sometimes, because of the assumption that every high TSH value is due to sickness-related hypothyroidism, thyroid hormones can be prescribed too quickly,” said Dr. Feldkamp. This is also true for patients with thyroid nodules due to iodine deficiency, who are often still treated with thyroid hormones.
“These days, because we are generally an iodine-deficient nation, iodine would potentially be given in combination with thyroid hormones but not with thyroid hormones alone. There are lots of patients who have been taking thyroid hormones for 30 or 40 years due to thyroid nodules. That should definitely be reviewed,” said Dr. Feldkamp.
When to treat?
Dr. Feldkamp does not believe that standard determination of the TSH value is sensible and advises that clinicians examine patients with newly occurring symptoms, such as excess weight, impaired weight regulation despite reduced appetite, depression, or a high need for sleep.
If there are symptoms, the thyroid function must be clarified further. “This includes determination of free thyroid hormones T3 and T4; detection of antibodies against autologous thyroid tissue such as TPO-Ab [antibody against thyroid peroxidase], TG-Ab [antibody against thyroglobulin], and TRAb [antibody against TSH receptor]; and ultrasound examination of the metabolic organ,” said Dr. Feldkamp. Autoimmune-related hypothyroidism (Hashimoto’s thyroiditis) is the most common cause of an overly high TSH level.
Treatment should take place in the following situations:
- In young patients with TSH values greater than 10 mU/L;
- In young (< 65 years) symptomatic patients with TSH values of 4 to less than 10 mU/L;
- With elevated TSH values that result from thyroid surgery or radioactive iodine therapy;
- In patients with a diffuse enlarged or severely nodular thyroid gland
- In pregnant women with elevated TSH values.
This article was translated from Medscape’s German Edition and a version appeared on Medscape.com.
Thyroxine and L-thyroxine are two of the 10 most frequently prescribed medicinal products. “One large health insurance company ranks thyroid hormone at fourth place in the list of most-sold medications in the United States. It is possibly the second most commonly prescribed preparation,” said Joachim Feldkamp, MD, PhD, director of the University Clinic for General Internal Medicine, Endocrinology, Diabetology, and Infectious Diseases at Central Hospital, Bielefeld, Germany, at the online press conference for the German Society of Endocrinology’s hormone week.
The preparation is prescribed when the thyroid gland produces too little thyroid hormone. The messenger substance thyroid-stimulating hormone (TSH) is used as a screening value to assess thyroid function. An increase in TSH can indicate that too little thyroid hormone is being produced.
“But this does not mean that an underactive thyroid gland is hiding behind every elevated TSH value,” said Dr. Feldkamp. Normally, the TSH value lies between 0.3 and 4.2 mU/L. “Hypothyroidism, as it’s known, is formally present if the TSH value lies above the upper limit of 4.2 mU/L,” said Dr. Feldkamp.
Check again
However, not every elevated TSH value needs to be treated immediately. “From large-scale investigations, we know that TSH values are subject to fluctuations,” said Dr. Feldkamp. Individual measurements must therefore be taken with a grain of salt and almost never justify a therapeutic decision. Therefore, a slightly elevated TSH value should be checked again 2-6 months later, and the patient should be asked if they are experiencing any symptoms. “In 50%-60% of cases, the TSH value normalized at the second checkup without requiring any treatment,” Dr. Feldkamp explained.
The TSH value could be elevated for several reasons:
- Fluctuations depending on the time of day. At night and early in the morning, the TSH value is much higher than in the afternoon. An acute lack of sleep can lead to higher TSH values in the morning.
- Fluctuations depending on the time of year. In winter, TSH values are slightly higher than in the summer owing to adaptation to cooler temperatures. Researchers in the Arctic, for example, have significantly higher TSH values than people who live in warmer regions.
- Age-dependent differences. Children and adolescents have higher TSH values than adults do. The TSH values of adolescents cannot be based on those of adults because this would lead to incorrect treatment. In addition, TSH values increase with age, and slightly elevated values are initially no cause for treatment in people aged 70-80 years. Caution is advised during treatment, because overtreatment can lead to cardiac arrhythmias and a decrease in bone density.
- Sex-specific differences. The TSH values of women are generally a little higher than those in men.
- Obesity. In obesity, TSH increases and often exceeds the normal values usually recorded in persons of normal weight. The elevated values do not reflect a state of hypofunction but rather the body’s adjustment mechanism. If these patients lose weight, the TSH values will drop spontaneously. Slightly elevated TSH values in obese people should not be treated with thyroid hormones.
The nutritional supplement biotin (vitamin H or vitamin B7), which is often taken for skin, hair, and nail growth disorders, can distort measured values. In many of the laboratory methods used, the biotin competes with the test substances used. As a result, it can lead to falsely high and falsely low TSH values. At high doses of biotin (for example, 10 mg), there should be at least a 3-day pause (and ideally a pause of 1 week) before measuring TSH.
Hasty prescriptions
“Sometimes, because of the assumption that every high TSH value is due to sickness-related hypothyroidism, thyroid hormones can be prescribed too quickly,” said Dr. Feldkamp. This is also true for patients with thyroid nodules due to iodine deficiency, who are often still treated with thyroid hormones.
“These days, because we are generally an iodine-deficient nation, iodine would potentially be given in combination with thyroid hormones but not with thyroid hormones alone. There are lots of patients who have been taking thyroid hormones for 30 or 40 years due to thyroid nodules. That should definitely be reviewed,” said Dr. Feldkamp.
When to treat?
Dr. Feldkamp does not believe that standard determination of the TSH value is sensible and advises that clinicians examine patients with newly occurring symptoms, such as excess weight, impaired weight regulation despite reduced appetite, depression, or a high need for sleep.
If there are symptoms, the thyroid function must be clarified further. “This includes determination of free thyroid hormones T3 and T4; detection of antibodies against autologous thyroid tissue such as TPO-Ab [antibody against thyroid peroxidase], TG-Ab [antibody against thyroglobulin], and TRAb [antibody against TSH receptor]; and ultrasound examination of the metabolic organ,” said Dr. Feldkamp. Autoimmune-related hypothyroidism (Hashimoto’s thyroiditis) is the most common cause of an overly high TSH level.
Treatment should take place in the following situations:
- In young patients with TSH values greater than 10 mU/L;
- In young (< 65 years) symptomatic patients with TSH values of 4 to less than 10 mU/L;
- With elevated TSH values that result from thyroid surgery or radioactive iodine therapy;
- In patients with a diffuse enlarged or severely nodular thyroid gland
- In pregnant women with elevated TSH values.
This article was translated from Medscape’s German Edition and a version appeared on Medscape.com.
Thyroxine and L-thyroxine are two of the 10 most frequently prescribed medicinal products. “One large health insurance company ranks thyroid hormone at fourth place in the list of most-sold medications in the United States. It is possibly the second most commonly prescribed preparation,” said Joachim Feldkamp, MD, PhD, director of the University Clinic for General Internal Medicine, Endocrinology, Diabetology, and Infectious Diseases at Central Hospital, Bielefeld, Germany, at the online press conference for the German Society of Endocrinology’s hormone week.
The preparation is prescribed when the thyroid gland produces too little thyroid hormone. The messenger substance thyroid-stimulating hormone (TSH) is used as a screening value to assess thyroid function. An increase in TSH can indicate that too little thyroid hormone is being produced.
“But this does not mean that an underactive thyroid gland is hiding behind every elevated TSH value,” said Dr. Feldkamp. Normally, the TSH value lies between 0.3 and 4.2 mU/L. “Hypothyroidism, as it’s known, is formally present if the TSH value lies above the upper limit of 4.2 mU/L,” said Dr. Feldkamp.
Check again
However, not every elevated TSH value needs to be treated immediately. “From large-scale investigations, we know that TSH values are subject to fluctuations,” said Dr. Feldkamp. Individual measurements must therefore be taken with a grain of salt and almost never justify a therapeutic decision. Therefore, a slightly elevated TSH value should be checked again 2-6 months later, and the patient should be asked if they are experiencing any symptoms. “In 50%-60% of cases, the TSH value normalized at the second checkup without requiring any treatment,” Dr. Feldkamp explained.
The TSH value could be elevated for several reasons:
- Fluctuations depending on the time of day. At night and early in the morning, the TSH value is much higher than in the afternoon. An acute lack of sleep can lead to higher TSH values in the morning.
- Fluctuations depending on the time of year. In winter, TSH values are slightly higher than in the summer owing to adaptation to cooler temperatures. Researchers in the Arctic, for example, have significantly higher TSH values than people who live in warmer regions.
- Age-dependent differences. Children and adolescents have higher TSH values than adults do. The TSH values of adolescents cannot be based on those of adults because this would lead to incorrect treatment. In addition, TSH values increase with age, and slightly elevated values are initially no cause for treatment in people aged 70-80 years. Caution is advised during treatment, because overtreatment can lead to cardiac arrhythmias and a decrease in bone density.
- Sex-specific differences. The TSH values of women are generally a little higher than those in men.
- Obesity. In obesity, TSH increases and often exceeds the normal values usually recorded in persons of normal weight. The elevated values do not reflect a state of hypofunction but rather the body’s adjustment mechanism. If these patients lose weight, the TSH values will drop spontaneously. Slightly elevated TSH values in obese people should not be treated with thyroid hormones.
The nutritional supplement biotin (vitamin H or vitamin B7), which is often taken for skin, hair, and nail growth disorders, can distort measured values. In many of the laboratory methods used, the biotin competes with the test substances used. As a result, it can lead to falsely high and falsely low TSH values. At high doses of biotin (for example, 10 mg), there should be at least a 3-day pause (and ideally a pause of 1 week) before measuring TSH.
Hasty prescriptions
“Sometimes, because of the assumption that every high TSH value is due to sickness-related hypothyroidism, thyroid hormones can be prescribed too quickly,” said Dr. Feldkamp. This is also true for patients with thyroid nodules due to iodine deficiency, who are often still treated with thyroid hormones.
“These days, because we are generally an iodine-deficient nation, iodine would potentially be given in combination with thyroid hormones but not with thyroid hormones alone. There are lots of patients who have been taking thyroid hormones for 30 or 40 years due to thyroid nodules. That should definitely be reviewed,” said Dr. Feldkamp.
When to treat?
Dr. Feldkamp does not believe that standard determination of the TSH value is sensible and advises that clinicians examine patients with newly occurring symptoms, such as excess weight, impaired weight regulation despite reduced appetite, depression, or a high need for sleep.
If there are symptoms, the thyroid function must be clarified further. “This includes determination of free thyroid hormones T3 and T4; detection of antibodies against autologous thyroid tissue such as TPO-Ab [antibody against thyroid peroxidase], TG-Ab [antibody against thyroglobulin], and TRAb [antibody against TSH receptor]; and ultrasound examination of the metabolic organ,” said Dr. Feldkamp. Autoimmune-related hypothyroidism (Hashimoto’s thyroiditis) is the most common cause of an overly high TSH level.
Treatment should take place in the following situations:
- In young patients with TSH values greater than 10 mU/L;
- In young (< 65 years) symptomatic patients with TSH values of 4 to less than 10 mU/L;
- With elevated TSH values that result from thyroid surgery or radioactive iodine therapy;
- In patients with a diffuse enlarged or severely nodular thyroid gland
- In pregnant women with elevated TSH values.
This article was translated from Medscape’s German Edition and a version appeared on Medscape.com.
Do new Alzheimer’s drugs get us closer to solving the Alzheimer’s disease riddle?
Two antiamyloid drugs were recently approved by the Food and Drug Administration for treating early-stage Alzheimer’s disease (AD). In trials of both lecanemab (Leqembi) and donanemab, a long-held neuropharmacologic dream was realized: Most amyloid plaques – the primary pathologic marker for AD – were eliminated from the brains of patients with late pre-AD or early AD.
Implications for the amyloid hypothesis
The reduction of amyloid plaques has been argued by many scientists and clinical authorities to be the likely pharmacologic solution for AD. These trials are appropriately viewed as a test of the hypothesis that amyloid bodies are a primary cause of the neurobehavioral symptoms we call AD.
In parallel with that striking reduction in amyloid bodies, drug-treated patients had an initially slower progression of neurobehavioral decline than did placebo-treated control patients. That slowing in symptom progression was accompanied by a modest but statistically significant difference in neurobehavioral ability. After several months in treatment, the rate of decline again paralleled that recorded in the control group. The sustained difference of about a half point on cognitive assessment scores separating treatment and control participants was well short of the 1.5-point difference typically considered clinically significant.
A small number of unexpected and unexplained deaths occurred in the treatment groups. Brain swelling and/or micro-hemorrhages were seen in 20%-30% of treated individuals. Significant brain shrinkage was recorded. These adverse findings are indicative of drug-induced trauma in the target organ for these drugs (i.e., the brain) and were the basis for a boxed warning label for drug usage. Antiamyloid drug treatment was not effective in patients who had higher initial numbers of amyloid plaques, indicating that these drugs would not measurably help the majority of AD patients, who are at more advanced disease stages.
These drugs do not appear to be an “answer” for AD. A modest delay in progression does not mean that we’re on a path to a “cure.” Treatment cost estimates are high – more than $80,000 per year. With requisite PET exams and high copays, patient accessibility issues will be daunting.
Of note, To the contrary, they add strong support for the counterargument that the emergence of amyloid plaques is an effect and not a fundamental cause of that progressive loss of neurologic function that we ultimately define as “Alzheimer’s disease.”
Time to switch gears
The more obvious path to winning the battle against this human scourge is prevention. A recent analysis published in The Lancet argued that about 40% of AD and other dementias are potentially preventable. I disagree. I believe that 80%-90% of prospective cases can be substantially delayed or prevented. Studies have shown that progression to AD or other dementias is driven primarily by the progressive deterioration of organic brain health, expressed by the loss of what psychologists have termed “cognitive reserve.” Cognitive reserve is resilience arising from active brain usage, akin to physical resilience attributable to a physically active life. Scientific studies have shown us that an individual’s cognitive resilience (reserve) is a greater predictor of risk for dementia than are amyloid plaques – indeed, greater than any combination of pathologic markers in dementia patients.
Building up cognitive reserve
It’s increasingly clear to this observer that cognitive reserve is synonymous with organic brain health. The primary factors that underlie cognitive reserve are processing speed in the brain, executive control, response withholding, memory acquisition, reasoning, and attention abilities. Faster, more accurate brains are necessarily more physically optimized. They necessarily sustain brain system connectivity. They are necessarily healthier. Such brains bear a relatively low risk of developing AD or other dementias, just as physically healthier bodies bear a lower risk of being prematurely banished to semi-permanent residence in an easy chair or a bed.
Brain health can be sustained by deploying inexpensive, self-administered, app-based assessments of neurologic performance limits, which inform patients and their medical teams about general brain health status. These assessments can help doctors guide their patients to adopt more intelligent brain-healthy lifestyles, or direct them to the “brain gym” to progressively exercise their brains in ways that contribute to rapid, potentially large-scale, rejuvenating improvements in physical and functional brain health.
Randomized controlled trials incorporating different combinations of physical exercise, diet, and cognitive training have recorded significant improvements in physical and functional neurologic status, indicating substantially advanced brain health. Consistent moderate-to-intense physical exercise, brain- and heart-healthy eating habits, and, particularly, computerized brain training have repeatedly been shown to improve cognitive function and physically rejuvenate the brain. With cognitive training in the right forms, improvements in processing speed and other measures manifest improving brain health and greater safety.
In the National Institutes of Health–funded ACTIVE study with more than 2,800 older adults, just 10-18 hours of a specific speed of processing training (now part of BrainHQ, a program that I was involved in developing) reduced the probability of a progression to dementia over the following 10 years by 29%, and by 48% in those who did the most training.
This approach is several orders of magnitude less expensive than the pricey new AD drugs. It presents less serious issues of accessibility and has no side effects. It delivers far more powerful therapeutic benefits in older normal and at-risk populations.
Sustained wellness supporting prevention is the far more sensible medical way forward to save people from AD and other dementias – at a far lower medical and societal cost.
Dr. Merzenich is professor emeritus, department of neuroscience, University of California, San Francisco. He reported conflicts of interest with Posit Science, Stronger Brains, and the National Institutes of Health.
A version of this article first appeared on Medscape.com.
Two antiamyloid drugs were recently approved by the Food and Drug Administration for treating early-stage Alzheimer’s disease (AD). In trials of both lecanemab (Leqembi) and donanemab, a long-held neuropharmacologic dream was realized: Most amyloid plaques – the primary pathologic marker for AD – were eliminated from the brains of patients with late pre-AD or early AD.
Implications for the amyloid hypothesis
The reduction of amyloid plaques has been argued by many scientists and clinical authorities to be the likely pharmacologic solution for AD. These trials are appropriately viewed as a test of the hypothesis that amyloid bodies are a primary cause of the neurobehavioral symptoms we call AD.
In parallel with that striking reduction in amyloid bodies, drug-treated patients had an initially slower progression of neurobehavioral decline than did placebo-treated control patients. That slowing in symptom progression was accompanied by a modest but statistically significant difference in neurobehavioral ability. After several months in treatment, the rate of decline again paralleled that recorded in the control group. The sustained difference of about a half point on cognitive assessment scores separating treatment and control participants was well short of the 1.5-point difference typically considered clinically significant.
A small number of unexpected and unexplained deaths occurred in the treatment groups. Brain swelling and/or micro-hemorrhages were seen in 20%-30% of treated individuals. Significant brain shrinkage was recorded. These adverse findings are indicative of drug-induced trauma in the target organ for these drugs (i.e., the brain) and were the basis for a boxed warning label for drug usage. Antiamyloid drug treatment was not effective in patients who had higher initial numbers of amyloid plaques, indicating that these drugs would not measurably help the majority of AD patients, who are at more advanced disease stages.
These drugs do not appear to be an “answer” for AD. A modest delay in progression does not mean that we’re on a path to a “cure.” Treatment cost estimates are high – more than $80,000 per year. With requisite PET exams and high copays, patient accessibility issues will be daunting.
Of note, To the contrary, they add strong support for the counterargument that the emergence of amyloid plaques is an effect and not a fundamental cause of that progressive loss of neurologic function that we ultimately define as “Alzheimer’s disease.”
Time to switch gears
The more obvious path to winning the battle against this human scourge is prevention. A recent analysis published in The Lancet argued that about 40% of AD and other dementias are potentially preventable. I disagree. I believe that 80%-90% of prospective cases can be substantially delayed or prevented. Studies have shown that progression to AD or other dementias is driven primarily by the progressive deterioration of organic brain health, expressed by the loss of what psychologists have termed “cognitive reserve.” Cognitive reserve is resilience arising from active brain usage, akin to physical resilience attributable to a physically active life. Scientific studies have shown us that an individual’s cognitive resilience (reserve) is a greater predictor of risk for dementia than are amyloid plaques – indeed, greater than any combination of pathologic markers in dementia patients.
Building up cognitive reserve
It’s increasingly clear to this observer that cognitive reserve is synonymous with organic brain health. The primary factors that underlie cognitive reserve are processing speed in the brain, executive control, response withholding, memory acquisition, reasoning, and attention abilities. Faster, more accurate brains are necessarily more physically optimized. They necessarily sustain brain system connectivity. They are necessarily healthier. Such brains bear a relatively low risk of developing AD or other dementias, just as physically healthier bodies bear a lower risk of being prematurely banished to semi-permanent residence in an easy chair or a bed.
Brain health can be sustained by deploying inexpensive, self-administered, app-based assessments of neurologic performance limits, which inform patients and their medical teams about general brain health status. These assessments can help doctors guide their patients to adopt more intelligent brain-healthy lifestyles, or direct them to the “brain gym” to progressively exercise their brains in ways that contribute to rapid, potentially large-scale, rejuvenating improvements in physical and functional brain health.
Randomized controlled trials incorporating different combinations of physical exercise, diet, and cognitive training have recorded significant improvements in physical and functional neurologic status, indicating substantially advanced brain health. Consistent moderate-to-intense physical exercise, brain- and heart-healthy eating habits, and, particularly, computerized brain training have repeatedly been shown to improve cognitive function and physically rejuvenate the brain. With cognitive training in the right forms, improvements in processing speed and other measures manifest improving brain health and greater safety.
In the National Institutes of Health–funded ACTIVE study with more than 2,800 older adults, just 10-18 hours of a specific speed of processing training (now part of BrainHQ, a program that I was involved in developing) reduced the probability of a progression to dementia over the following 10 years by 29%, and by 48% in those who did the most training.
This approach is several orders of magnitude less expensive than the pricey new AD drugs. It presents less serious issues of accessibility and has no side effects. It delivers far more powerful therapeutic benefits in older normal and at-risk populations.
Sustained wellness supporting prevention is the far more sensible medical way forward to save people from AD and other dementias – at a far lower medical and societal cost.
Dr. Merzenich is professor emeritus, department of neuroscience, University of California, San Francisco. He reported conflicts of interest with Posit Science, Stronger Brains, and the National Institutes of Health.
A version of this article first appeared on Medscape.com.
Two antiamyloid drugs were recently approved by the Food and Drug Administration for treating early-stage Alzheimer’s disease (AD). In trials of both lecanemab (Leqembi) and donanemab, a long-held neuropharmacologic dream was realized: Most amyloid plaques – the primary pathologic marker for AD – were eliminated from the brains of patients with late pre-AD or early AD.
Implications for the amyloid hypothesis
The reduction of amyloid plaques has been argued by many scientists and clinical authorities to be the likely pharmacologic solution for AD. These trials are appropriately viewed as a test of the hypothesis that amyloid bodies are a primary cause of the neurobehavioral symptoms we call AD.
In parallel with that striking reduction in amyloid bodies, drug-treated patients had an initially slower progression of neurobehavioral decline than did placebo-treated control patients. That slowing in symptom progression was accompanied by a modest but statistically significant difference in neurobehavioral ability. After several months in treatment, the rate of decline again paralleled that recorded in the control group. The sustained difference of about a half point on cognitive assessment scores separating treatment and control participants was well short of the 1.5-point difference typically considered clinically significant.
A small number of unexpected and unexplained deaths occurred in the treatment groups. Brain swelling and/or micro-hemorrhages were seen in 20%-30% of treated individuals. Significant brain shrinkage was recorded. These adverse findings are indicative of drug-induced trauma in the target organ for these drugs (i.e., the brain) and were the basis for a boxed warning label for drug usage. Antiamyloid drug treatment was not effective in patients who had higher initial numbers of amyloid plaques, indicating that these drugs would not measurably help the majority of AD patients, who are at more advanced disease stages.
These drugs do not appear to be an “answer” for AD. A modest delay in progression does not mean that we’re on a path to a “cure.” Treatment cost estimates are high – more than $80,000 per year. With requisite PET exams and high copays, patient accessibility issues will be daunting.
Of note, To the contrary, they add strong support for the counterargument that the emergence of amyloid plaques is an effect and not a fundamental cause of that progressive loss of neurologic function that we ultimately define as “Alzheimer’s disease.”
Time to switch gears
The more obvious path to winning the battle against this human scourge is prevention. A recent analysis published in The Lancet argued that about 40% of AD and other dementias are potentially preventable. I disagree. I believe that 80%-90% of prospective cases can be substantially delayed or prevented. Studies have shown that progression to AD or other dementias is driven primarily by the progressive deterioration of organic brain health, expressed by the loss of what psychologists have termed “cognitive reserve.” Cognitive reserve is resilience arising from active brain usage, akin to physical resilience attributable to a physically active life. Scientific studies have shown us that an individual’s cognitive resilience (reserve) is a greater predictor of risk for dementia than are amyloid plaques – indeed, greater than any combination of pathologic markers in dementia patients.
Building up cognitive reserve
It’s increasingly clear to this observer that cognitive reserve is synonymous with organic brain health. The primary factors that underlie cognitive reserve are processing speed in the brain, executive control, response withholding, memory acquisition, reasoning, and attention abilities. Faster, more accurate brains are necessarily more physically optimized. They necessarily sustain brain system connectivity. They are necessarily healthier. Such brains bear a relatively low risk of developing AD or other dementias, just as physically healthier bodies bear a lower risk of being prematurely banished to semi-permanent residence in an easy chair or a bed.
Brain health can be sustained by deploying inexpensive, self-administered, app-based assessments of neurologic performance limits, which inform patients and their medical teams about general brain health status. These assessments can help doctors guide their patients to adopt more intelligent brain-healthy lifestyles, or direct them to the “brain gym” to progressively exercise their brains in ways that contribute to rapid, potentially large-scale, rejuvenating improvements in physical and functional brain health.
Randomized controlled trials incorporating different combinations of physical exercise, diet, and cognitive training have recorded significant improvements in physical and functional neurologic status, indicating substantially advanced brain health. Consistent moderate-to-intense physical exercise, brain- and heart-healthy eating habits, and, particularly, computerized brain training have repeatedly been shown to improve cognitive function and physically rejuvenate the brain. With cognitive training in the right forms, improvements in processing speed and other measures manifest improving brain health and greater safety.
In the National Institutes of Health–funded ACTIVE study with more than 2,800 older adults, just 10-18 hours of a specific speed of processing training (now part of BrainHQ, a program that I was involved in developing) reduced the probability of a progression to dementia over the following 10 years by 29%, and by 48% in those who did the most training.
This approach is several orders of magnitude less expensive than the pricey new AD drugs. It presents less serious issues of accessibility and has no side effects. It delivers far more powerful therapeutic benefits in older normal and at-risk populations.
Sustained wellness supporting prevention is the far more sensible medical way forward to save people from AD and other dementias – at a far lower medical and societal cost.
Dr. Merzenich is professor emeritus, department of neuroscience, University of California, San Francisco. He reported conflicts of interest with Posit Science, Stronger Brains, and the National Institutes of Health.
A version of this article first appeared on Medscape.com.
Social media makes kids with type 1 diabetes feel less alone
After being diagnosed with type 1 diabetes in 2021, British teenager Johnny Bailey felt isolated. That’s when he turned to social media, where he found others living with type 1 diabetes. He began to share his experience and now has more than 329,000 followers on his TikTok account, where he regularly posts videos.
These include short clips of him demonstrating how he changes his FreeStyle Libre sensor for his flash glucose monitor. In the videos, Johnny appropriately places his sensor on the back of his arm with background music, makes facial expressions, and transforms a dreaded diabetes-related task into an experience that appears fun and entertaining. In the limited videos I was able to review, he follows all the appropriate steps for sensor placement.
Many youths living with type 1 diabetes struggle with living with a chronic medical condition. Because type 1 diabetes is a rare condition, affecting about 1 in 500 children in the United States, many youth may not meet anyone else their age with type 1 diabetes through school, social events, or extracurricular activities.
For adolescents with intensively managed conditions like type 1 diabetes, this can present numerous psychosocial challenges – specifically, many youth experience shame or stigma associated with managing type 1 diabetes.
Diabetes-specific tasks may include wearing an insulin pump, monitoring blood glucose with finger pricks or a continuous glucose monitor (CGM), giving injections of insulin before meals and snacks, adjusting times for meals and snacks based on metabolic needs, waking up in the middle of the night to treat high or low blood glucose – the list goes on and on.
One study estimated that the average time it takes a child with type 1 diabetes to perform diabetes-specific tasks is over 5 hours per day.
Although much of this diabetes management time is spent by parents, as children get older and become teenagers, they are gradually transitioning to taking on more of this responsibility themselves. Wearing diabetes technology (insulin pumps and CGMs) can draw unwanted attention, leading to diabetes-specific body image concerns. Kids may also have to excuse themselves from an activity to treat a low or high blood glucose, creating uncomfortable situations when others inquire about why the activity was interrupted. As a result, many youths will avoid managing their diabetes properly to avoid drawing unwanted attention, consequently put their health at risk.
Those who are afraid of placing their glucose sensor owing to fear of pain may be reassured by seeing Johnny placing his sensor with a smile on his face. Some of his content also highlights other stigmatizing situations that teens may face, for example someone with a judgmental look questioning why he needed to give an insulin injection here.
This highlights an important concept – that people with type 1 diabetes may face criticism when dosing insulin in public, but it doesn’t mean they should feel forced to manage diabetes in private unless they choose to. Johnny is an inspirational individual who has bravely taken his type 1 diabetes experiences and used his creative skills to make these seemingly boring health-related tasks fun, interesting, and accessible.
Social media has become an outlet for people with type 1 diabetes to connect with others who can relate to their experiences.
However, there’s another side to consider. Although social media may provide a great source of support for youth, it may also adversely affect mental health. Just as quickly as social media outlets have grown, so has concern over excessive social media use and its impact on adolescents’ mental health. There’s a growing body of literature that describes the negative mental health aspects related to social media use.
Some adolescents struggling to manage type 1 diabetes may feel worse when seeing others thrive on social media, which has the potential to worsen stigma and shame. Youth may wonder how someone else is able to manage their type 1 diabetes so well when they are facing so many challenges.
Short videos on social media provide an incomplete picture of living with type 1 diabetes – just a glimpse into others’ lives, and only the parts that they want others to see. Managing a chronic condition can’t be fully represented in 10-second videos. And if youths choose to post their type 1 diabetes experiences on social media, they also risk receiving backlash or criticism, which can negatively their impact mental health in return.
Furthermore, the content being posted may not always be accurate or educational, leading to the potential for some youth to misunderstand type 1 diabetes.
Although I wouldn’t discourage youth with type 1 diabetes from engaging on social media and viewing diabetes-related content, they need to know that social media is flooded with misinformation. Creating an open space for youth to ask their clinicians questions about type 1 diabetes–related topics they view on social media is vital to ensuring they are viewing accurate information, so they are able to continue to manage their diabetes safely.
As a pediatric endocrinologist, I sometimes share resources on social media with patients if I believe it will help them cope with their type 1 diabetes diagnosis and management. I have had numerous patients – many of whom have struggled to accept their diagnosis – mention with joy and excitement that they were following an organization addressing type 1 diabetes on social media.
When making suggestions, I may refer them to The Diabetes Link, an organization with resources for young adults with type 1 diabetes that creates a space to connect with other young adults with type 1 diabetes. diaTribe is another organization created and led by people with diabetes that has a plethora of resources and provides evidence-based education for patients. I have also shared Project 50-in-50, which highlights two individuals with type 1 diabetes hiking the highest peak in each state in less than 50 days. Being able to see type 1 diabetes in a positive light is a huge step toward a more positive outlook on diabetes management.
Dr. Nally is an assistant professor, department of pediatrics, and a pediatric endocrinologist, division of pediatric endocrinology, at Yale University, New Haven, Conn. She reported conflicts of interest with Medtronic and the National Institutes of Health.
A version of this article first appeared on Medscape.com.
After being diagnosed with type 1 diabetes in 2021, British teenager Johnny Bailey felt isolated. That’s when he turned to social media, where he found others living with type 1 diabetes. He began to share his experience and now has more than 329,000 followers on his TikTok account, where he regularly posts videos.
These include short clips of him demonstrating how he changes his FreeStyle Libre sensor for his flash glucose monitor. In the videos, Johnny appropriately places his sensor on the back of his arm with background music, makes facial expressions, and transforms a dreaded diabetes-related task into an experience that appears fun and entertaining. In the limited videos I was able to review, he follows all the appropriate steps for sensor placement.
Many youths living with type 1 diabetes struggle with living with a chronic medical condition. Because type 1 diabetes is a rare condition, affecting about 1 in 500 children in the United States, many youth may not meet anyone else their age with type 1 diabetes through school, social events, or extracurricular activities.
For adolescents with intensively managed conditions like type 1 diabetes, this can present numerous psychosocial challenges – specifically, many youth experience shame or stigma associated with managing type 1 diabetes.
Diabetes-specific tasks may include wearing an insulin pump, monitoring blood glucose with finger pricks or a continuous glucose monitor (CGM), giving injections of insulin before meals and snacks, adjusting times for meals and snacks based on metabolic needs, waking up in the middle of the night to treat high or low blood glucose – the list goes on and on.
One study estimated that the average time it takes a child with type 1 diabetes to perform diabetes-specific tasks is over 5 hours per day.
Although much of this diabetes management time is spent by parents, as children get older and become teenagers, they are gradually transitioning to taking on more of this responsibility themselves. Wearing diabetes technology (insulin pumps and CGMs) can draw unwanted attention, leading to diabetes-specific body image concerns. Kids may also have to excuse themselves from an activity to treat a low or high blood glucose, creating uncomfortable situations when others inquire about why the activity was interrupted. As a result, many youths will avoid managing their diabetes properly to avoid drawing unwanted attention, consequently put their health at risk.
Those who are afraid of placing their glucose sensor owing to fear of pain may be reassured by seeing Johnny placing his sensor with a smile on his face. Some of his content also highlights other stigmatizing situations that teens may face, for example someone with a judgmental look questioning why he needed to give an insulin injection here.
This highlights an important concept – that people with type 1 diabetes may face criticism when dosing insulin in public, but it doesn’t mean they should feel forced to manage diabetes in private unless they choose to. Johnny is an inspirational individual who has bravely taken his type 1 diabetes experiences and used his creative skills to make these seemingly boring health-related tasks fun, interesting, and accessible.
Social media has become an outlet for people with type 1 diabetes to connect with others who can relate to their experiences.
However, there’s another side to consider. Although social media may provide a great source of support for youth, it may also adversely affect mental health. Just as quickly as social media outlets have grown, so has concern over excessive social media use and its impact on adolescents’ mental health. There’s a growing body of literature that describes the negative mental health aspects related to social media use.
Some adolescents struggling to manage type 1 diabetes may feel worse when seeing others thrive on social media, which has the potential to worsen stigma and shame. Youth may wonder how someone else is able to manage their type 1 diabetes so well when they are facing so many challenges.
Short videos on social media provide an incomplete picture of living with type 1 diabetes – just a glimpse into others’ lives, and only the parts that they want others to see. Managing a chronic condition can’t be fully represented in 10-second videos. And if youths choose to post their type 1 diabetes experiences on social media, they also risk receiving backlash or criticism, which can negatively their impact mental health in return.
Furthermore, the content being posted may not always be accurate or educational, leading to the potential for some youth to misunderstand type 1 diabetes.
Although I wouldn’t discourage youth with type 1 diabetes from engaging on social media and viewing diabetes-related content, they need to know that social media is flooded with misinformation. Creating an open space for youth to ask their clinicians questions about type 1 diabetes–related topics they view on social media is vital to ensuring they are viewing accurate information, so they are able to continue to manage their diabetes safely.
As a pediatric endocrinologist, I sometimes share resources on social media with patients if I believe it will help them cope with their type 1 diabetes diagnosis and management. I have had numerous patients – many of whom have struggled to accept their diagnosis – mention with joy and excitement that they were following an organization addressing type 1 diabetes on social media.
When making suggestions, I may refer them to The Diabetes Link, an organization with resources for young adults with type 1 diabetes that creates a space to connect with other young adults with type 1 diabetes. diaTribe is another organization created and led by people with diabetes that has a plethora of resources and provides evidence-based education for patients. I have also shared Project 50-in-50, which highlights two individuals with type 1 diabetes hiking the highest peak in each state in less than 50 days. Being able to see type 1 diabetes in a positive light is a huge step toward a more positive outlook on diabetes management.
Dr. Nally is an assistant professor, department of pediatrics, and a pediatric endocrinologist, division of pediatric endocrinology, at Yale University, New Haven, Conn. She reported conflicts of interest with Medtronic and the National Institutes of Health.
A version of this article first appeared on Medscape.com.
After being diagnosed with type 1 diabetes in 2021, British teenager Johnny Bailey felt isolated. That’s when he turned to social media, where he found others living with type 1 diabetes. He began to share his experience and now has more than 329,000 followers on his TikTok account, where he regularly posts videos.
These include short clips of him demonstrating how he changes his FreeStyle Libre sensor for his flash glucose monitor. In the videos, Johnny appropriately places his sensor on the back of his arm with background music, makes facial expressions, and transforms a dreaded diabetes-related task into an experience that appears fun and entertaining. In the limited videos I was able to review, he follows all the appropriate steps for sensor placement.
Many youths living with type 1 diabetes struggle with living with a chronic medical condition. Because type 1 diabetes is a rare condition, affecting about 1 in 500 children in the United States, many youth may not meet anyone else their age with type 1 diabetes through school, social events, or extracurricular activities.
For adolescents with intensively managed conditions like type 1 diabetes, this can present numerous psychosocial challenges – specifically, many youth experience shame or stigma associated with managing type 1 diabetes.
Diabetes-specific tasks may include wearing an insulin pump, monitoring blood glucose with finger pricks or a continuous glucose monitor (CGM), giving injections of insulin before meals and snacks, adjusting times for meals and snacks based on metabolic needs, waking up in the middle of the night to treat high or low blood glucose – the list goes on and on.
One study estimated that the average time it takes a child with type 1 diabetes to perform diabetes-specific tasks is over 5 hours per day.
Although much of this diabetes management time is spent by parents, as children get older and become teenagers, they are gradually transitioning to taking on more of this responsibility themselves. Wearing diabetes technology (insulin pumps and CGMs) can draw unwanted attention, leading to diabetes-specific body image concerns. Kids may also have to excuse themselves from an activity to treat a low or high blood glucose, creating uncomfortable situations when others inquire about why the activity was interrupted. As a result, many youths will avoid managing their diabetes properly to avoid drawing unwanted attention, consequently put their health at risk.
Those who are afraid of placing their glucose sensor owing to fear of pain may be reassured by seeing Johnny placing his sensor with a smile on his face. Some of his content also highlights other stigmatizing situations that teens may face, for example someone with a judgmental look questioning why he needed to give an insulin injection here.
This highlights an important concept – that people with type 1 diabetes may face criticism when dosing insulin in public, but it doesn’t mean they should feel forced to manage diabetes in private unless they choose to. Johnny is an inspirational individual who has bravely taken his type 1 diabetes experiences and used his creative skills to make these seemingly boring health-related tasks fun, interesting, and accessible.
Social media has become an outlet for people with type 1 diabetes to connect with others who can relate to their experiences.
However, there’s another side to consider. Although social media may provide a great source of support for youth, it may also adversely affect mental health. Just as quickly as social media outlets have grown, so has concern over excessive social media use and its impact on adolescents’ mental health. There’s a growing body of literature that describes the negative mental health aspects related to social media use.
Some adolescents struggling to manage type 1 diabetes may feel worse when seeing others thrive on social media, which has the potential to worsen stigma and shame. Youth may wonder how someone else is able to manage their type 1 diabetes so well when they are facing so many challenges.
Short videos on social media provide an incomplete picture of living with type 1 diabetes – just a glimpse into others’ lives, and only the parts that they want others to see. Managing a chronic condition can’t be fully represented in 10-second videos. And if youths choose to post their type 1 diabetes experiences on social media, they also risk receiving backlash or criticism, which can negatively their impact mental health in return.
Furthermore, the content being posted may not always be accurate or educational, leading to the potential for some youth to misunderstand type 1 diabetes.
Although I wouldn’t discourage youth with type 1 diabetes from engaging on social media and viewing diabetes-related content, they need to know that social media is flooded with misinformation. Creating an open space for youth to ask their clinicians questions about type 1 diabetes–related topics they view on social media is vital to ensuring they are viewing accurate information, so they are able to continue to manage their diabetes safely.
As a pediatric endocrinologist, I sometimes share resources on social media with patients if I believe it will help them cope with their type 1 diabetes diagnosis and management. I have had numerous patients – many of whom have struggled to accept their diagnosis – mention with joy and excitement that they were following an organization addressing type 1 diabetes on social media.
When making suggestions, I may refer them to The Diabetes Link, an organization with resources for young adults with type 1 diabetes that creates a space to connect with other young adults with type 1 diabetes. diaTribe is another organization created and led by people with diabetes that has a plethora of resources and provides evidence-based education for patients. I have also shared Project 50-in-50, which highlights two individuals with type 1 diabetes hiking the highest peak in each state in less than 50 days. Being able to see type 1 diabetes in a positive light is a huge step toward a more positive outlook on diabetes management.
Dr. Nally is an assistant professor, department of pediatrics, and a pediatric endocrinologist, division of pediatric endocrinology, at Yale University, New Haven, Conn. She reported conflicts of interest with Medtronic and the National Institutes of Health.
A version of this article first appeared on Medscape.com.
Hyperbaric oxygen therapy for traumatic brain injury: Promising or wishful thinking?
A recent review by Hadanny and colleagues recommends hyperbaric oxygen therapy (HBOT) for acute moderate to severe traumatic brain injury (TBI) and selected patients with prolonged postconcussive syndrome.
This article piqued my curiosity because I trained in HBOT more than 20 years ago. As a passionate scuba diver, my motivation was to master treatment for air embolism and decompression illness. Thankfully, these diving accidents are rare. However, I used HBOT for nonhealing wounds, and its efficacy was sometimes remarkable.
Paradoxical results with oxygen therapy
Although it may seem self-evident that “more oxygen is better” for medical illness, this is not necessarily true. I recently interviewed Ola Didrik Saugstad, MD, who demonstrated that the traditional practice of resuscitating newborns with 100% oxygen was more toxic than resuscitation with air (which contains 21% oxygen). His counterintuitive discovery led to a lifesaving change in the international newborn resuscitation guidelines.
The Food and Drug Administration has approved HBOT for a wide variety of conditions, but some practitioners enthusiastically promote it for off-label indications. These include antiaging, autism, multiple sclerosis, and the aforementioned TBI.
More than 50 years ago, HBOT was proposed for stroke, another disorder where the brain has been deprived of oxygen. Despite obvious logic, clinical trials have been unconvincing. The FDA has not approved HBOT for stroke.
HBOT in practice
During HBOT, the patient breathes 100% oxygen while the whole body is pressurized within a hyperbaric chamber. The chamber’s construction allows pressures above normal sea level of 1.0 atmosphere absolute (ATA). For example, The U.S. Navy Treatment Table for decompression sickness recommends 100% oxygen at 2.8 ATA. Chambers may hold one or more patients at a time.
The frequency of therapy varies but often consists of 20-60 sessions lasting 90-120 minutes. For off-label use like TBI, patients usually pay out of pocket. Given the multiple treatments, costs can add up.
Inconsistent evidence and sham controls
The unwieldy 33-page evidence review by Hadanny and colleagues cites multiple studies supporting HBOT for TBI. However, many, if not all, suffer from methodological flaws. These include vague inclusion criteria, lack of a control group, small patient numbers, treatment at different times since injury, poorly defined or varying HBOT protocols, varying outcome measures, and superficial results analysis.
A sham or control arm is essential for HBOT research trials, given the potential placebo effect of placing a human being inside a large, high-tech, sealed tube for an hour or more. In some sham-controlled studies, which consisted of low-pressure oxygen (that is, 1.3 ATA as sham vs. 2.4 ATA as treatment), all groups experienced symptom improvement. The review authors argue that the low-dose HBOT sham arms were biologically active and that the improvements seen mean that both high- and low-dose HBOT is therapeutic. The alternative explanation is that the placebo effect accounted for improvement in both groups.
The late Michael Bennett, a world authority on hyperbaric and underwater medicine, doubted that conventional HBOT sham controls could genuinely have a therapeutic effect, and I agree. The upcoming HOT-POCS trial (discussed below) should answer the question more definitively.
Mechanisms of action and safety
Mechanisms of benefit for HBOT include increased oxygen availability and angiogenesis. Animal research suggests that it may reduce secondary cell death from TBI, through stabilization of the blood-brain barrier and inflammation reduction.
HBOT is generally safe and well tolerated. A retrospective analysis of 1.5 million outpatient hyperbaric treatments revealed that less than 1% were associated with adverse events. The most common were ear and sinus barotrauma. Because HBOT uses increased air pressure, patients must equalize their ears and sinuses. Those who cannot because of altered consciousness, anatomical defects, or congestion must undergo myringotomy or terminate therapy. Claustrophobia was the second most common adverse effect. Convulsions and tension pneumocephalus were rare.
Perhaps the most concerning risk of HBOT for patients with TBI is the potential waste of human and financial resources.
Desperate physicians and patients
As a neurologist who regularly treats patients with TBI, I share the review authors’ frustration regarding the limited efficacy of available treatments. However, the suboptimal efficacy of currently available therapy is insufficient justification to recommend HBOT.
With respect to chronic TBI, it is difficult to imagine how HBOT could reverse brain injury that has been present for months or years. No other therapy exists that reliably encourages neuronal regeneration or prevents the development of posttraumatic epilepsy.
Frank Conidi, MD, a board-certified sports neurologist and headache specialist, shared his thoughts via email. He agrees that HBOT may have a role in TBI, but after reviewing Hadanny and colleagues’ paper, he concluded that there is insufficient evidence for the use of HBOT in all forms of TBI. He would like to see large multicenter, well-designed studies with standardized pressures and duration and a standard definition of the various types of head injury.
Ongoing research
There are at least five ongoing trials on HBOT for TBI or postconcussive syndrome, including the well-designed placebo-controlled HOT-POCS study. The latter has a novel placebo gas system that addresses Hadanny and colleagues’ contention that even low-dose HBOT might be effective.
The placebo arm in HOT-POCS mimics the HBO environment but provides only 0.21 ATA of oxygen, the same as room air. The active arm provides 100% oxygen at 2.0 ATA. If patients in both arms improve, the benefit will be caused by a placebo response, not HBOT.
Conflict of interest
Another concern with the review is that all three authors are affiliated with Aviv Scientific. This company has an exclusive partnership with the world’s largest hyperbaric medicine and research facility, the Sagol Center at Shamir Medical Center in Be’er Ya’akov, Israel.
This conflict of interest does not a priori invalidate their conclusions. However, official HBOT guidelines from a leading organization like the Undersea and Hyperbaric Medicine Society or the American Academy of Neurology would be preferable.
Conclusion
There is an urgent unmet need for more effective treatments for postconcussive syndrome and chronic TBI.
The review authors’ recommendations for HBOT seem premature. They are arguably a disservice to the many desperate patients and their families who will be tempted to expend valuable resources of time and money for an appealing but unproven therapy. Appropriately designed placebo-controlled studies such as HOT-POCS will help separate fact from wishful thinking.
Dr. Wilner is associate professor of neurology at University of Tennessee Health Science Center, Memphis. He reported a conflict of interest with Accordant Health Services.
A version of this article first appeared on Medscape.com.
A recent review by Hadanny and colleagues recommends hyperbaric oxygen therapy (HBOT) for acute moderate to severe traumatic brain injury (TBI) and selected patients with prolonged postconcussive syndrome.
This article piqued my curiosity because I trained in HBOT more than 20 years ago. As a passionate scuba diver, my motivation was to master treatment for air embolism and decompression illness. Thankfully, these diving accidents are rare. However, I used HBOT for nonhealing wounds, and its efficacy was sometimes remarkable.
Paradoxical results with oxygen therapy
Although it may seem self-evident that “more oxygen is better” for medical illness, this is not necessarily true. I recently interviewed Ola Didrik Saugstad, MD, who demonstrated that the traditional practice of resuscitating newborns with 100% oxygen was more toxic than resuscitation with air (which contains 21% oxygen). His counterintuitive discovery led to a lifesaving change in the international newborn resuscitation guidelines.
The Food and Drug Administration has approved HBOT for a wide variety of conditions, but some practitioners enthusiastically promote it for off-label indications. These include antiaging, autism, multiple sclerosis, and the aforementioned TBI.
More than 50 years ago, HBOT was proposed for stroke, another disorder where the brain has been deprived of oxygen. Despite obvious logic, clinical trials have been unconvincing. The FDA has not approved HBOT for stroke.
HBOT in practice
During HBOT, the patient breathes 100% oxygen while the whole body is pressurized within a hyperbaric chamber. The chamber’s construction allows pressures above normal sea level of 1.0 atmosphere absolute (ATA). For example, The U.S. Navy Treatment Table for decompression sickness recommends 100% oxygen at 2.8 ATA. Chambers may hold one or more patients at a time.
The frequency of therapy varies but often consists of 20-60 sessions lasting 90-120 minutes. For off-label use like TBI, patients usually pay out of pocket. Given the multiple treatments, costs can add up.
Inconsistent evidence and sham controls
The unwieldy 33-page evidence review by Hadanny and colleagues cites multiple studies supporting HBOT for TBI. However, many, if not all, suffer from methodological flaws. These include vague inclusion criteria, lack of a control group, small patient numbers, treatment at different times since injury, poorly defined or varying HBOT protocols, varying outcome measures, and superficial results analysis.
A sham or control arm is essential for HBOT research trials, given the potential placebo effect of placing a human being inside a large, high-tech, sealed tube for an hour or more. In some sham-controlled studies, which consisted of low-pressure oxygen (that is, 1.3 ATA as sham vs. 2.4 ATA as treatment), all groups experienced symptom improvement. The review authors argue that the low-dose HBOT sham arms were biologically active and that the improvements seen mean that both high- and low-dose HBOT is therapeutic. The alternative explanation is that the placebo effect accounted for improvement in both groups.
The late Michael Bennett, a world authority on hyperbaric and underwater medicine, doubted that conventional HBOT sham controls could genuinely have a therapeutic effect, and I agree. The upcoming HOT-POCS trial (discussed below) should answer the question more definitively.
Mechanisms of action and safety
Mechanisms of benefit for HBOT include increased oxygen availability and angiogenesis. Animal research suggests that it may reduce secondary cell death from TBI, through stabilization of the blood-brain barrier and inflammation reduction.
HBOT is generally safe and well tolerated. A retrospective analysis of 1.5 million outpatient hyperbaric treatments revealed that less than 1% were associated with adverse events. The most common were ear and sinus barotrauma. Because HBOT uses increased air pressure, patients must equalize their ears and sinuses. Those who cannot because of altered consciousness, anatomical defects, or congestion must undergo myringotomy or terminate therapy. Claustrophobia was the second most common adverse effect. Convulsions and tension pneumocephalus were rare.
Perhaps the most concerning risk of HBOT for patients with TBI is the potential waste of human and financial resources.
Desperate physicians and patients
As a neurologist who regularly treats patients with TBI, I share the review authors’ frustration regarding the limited efficacy of available treatments. However, the suboptimal efficacy of currently available therapy is insufficient justification to recommend HBOT.
With respect to chronic TBI, it is difficult to imagine how HBOT could reverse brain injury that has been present for months or years. No other therapy exists that reliably encourages neuronal regeneration or prevents the development of posttraumatic epilepsy.
Frank Conidi, MD, a board-certified sports neurologist and headache specialist, shared his thoughts via email. He agrees that HBOT may have a role in TBI, but after reviewing Hadanny and colleagues’ paper, he concluded that there is insufficient evidence for the use of HBOT in all forms of TBI. He would like to see large multicenter, well-designed studies with standardized pressures and duration and a standard definition of the various types of head injury.
Ongoing research
There are at least five ongoing trials on HBOT for TBI or postconcussive syndrome, including the well-designed placebo-controlled HOT-POCS study. The latter has a novel placebo gas system that addresses Hadanny and colleagues’ contention that even low-dose HBOT might be effective.
The placebo arm in HOT-POCS mimics the HBO environment but provides only 0.21 ATA of oxygen, the same as room air. The active arm provides 100% oxygen at 2.0 ATA. If patients in both arms improve, the benefit will be caused by a placebo response, not HBOT.
Conflict of interest
Another concern with the review is that all three authors are affiliated with Aviv Scientific. This company has an exclusive partnership with the world’s largest hyperbaric medicine and research facility, the Sagol Center at Shamir Medical Center in Be’er Ya’akov, Israel.
This conflict of interest does not a priori invalidate their conclusions. However, official HBOT guidelines from a leading organization like the Undersea and Hyperbaric Medicine Society or the American Academy of Neurology would be preferable.
Conclusion
There is an urgent unmet need for more effective treatments for postconcussive syndrome and chronic TBI.
The review authors’ recommendations for HBOT seem premature. They are arguably a disservice to the many desperate patients and their families who will be tempted to expend valuable resources of time and money for an appealing but unproven therapy. Appropriately designed placebo-controlled studies such as HOT-POCS will help separate fact from wishful thinking.
Dr. Wilner is associate professor of neurology at University of Tennessee Health Science Center, Memphis. He reported a conflict of interest with Accordant Health Services.
A version of this article first appeared on Medscape.com.
A recent review by Hadanny and colleagues recommends hyperbaric oxygen therapy (HBOT) for acute moderate to severe traumatic brain injury (TBI) and selected patients with prolonged postconcussive syndrome.
This article piqued my curiosity because I trained in HBOT more than 20 years ago. As a passionate scuba diver, my motivation was to master treatment for air embolism and decompression illness. Thankfully, these diving accidents are rare. However, I used HBOT for nonhealing wounds, and its efficacy was sometimes remarkable.
Paradoxical results with oxygen therapy
Although it may seem self-evident that “more oxygen is better” for medical illness, this is not necessarily true. I recently interviewed Ola Didrik Saugstad, MD, who demonstrated that the traditional practice of resuscitating newborns with 100% oxygen was more toxic than resuscitation with air (which contains 21% oxygen). His counterintuitive discovery led to a lifesaving change in the international newborn resuscitation guidelines.
The Food and Drug Administration has approved HBOT for a wide variety of conditions, but some practitioners enthusiastically promote it for off-label indications. These include antiaging, autism, multiple sclerosis, and the aforementioned TBI.
More than 50 years ago, HBOT was proposed for stroke, another disorder where the brain has been deprived of oxygen. Despite obvious logic, clinical trials have been unconvincing. The FDA has not approved HBOT for stroke.
HBOT in practice
During HBOT, the patient breathes 100% oxygen while the whole body is pressurized within a hyperbaric chamber. The chamber’s construction allows pressures above normal sea level of 1.0 atmosphere absolute (ATA). For example, The U.S. Navy Treatment Table for decompression sickness recommends 100% oxygen at 2.8 ATA. Chambers may hold one or more patients at a time.
The frequency of therapy varies but often consists of 20-60 sessions lasting 90-120 minutes. For off-label use like TBI, patients usually pay out of pocket. Given the multiple treatments, costs can add up.
Inconsistent evidence and sham controls
The unwieldy 33-page evidence review by Hadanny and colleagues cites multiple studies supporting HBOT for TBI. However, many, if not all, suffer from methodological flaws. These include vague inclusion criteria, lack of a control group, small patient numbers, treatment at different times since injury, poorly defined or varying HBOT protocols, varying outcome measures, and superficial results analysis.
A sham or control arm is essential for HBOT research trials, given the potential placebo effect of placing a human being inside a large, high-tech, sealed tube for an hour or more. In some sham-controlled studies, which consisted of low-pressure oxygen (that is, 1.3 ATA as sham vs. 2.4 ATA as treatment), all groups experienced symptom improvement. The review authors argue that the low-dose HBOT sham arms were biologically active and that the improvements seen mean that both high- and low-dose HBOT is therapeutic. The alternative explanation is that the placebo effect accounted for improvement in both groups.
The late Michael Bennett, a world authority on hyperbaric and underwater medicine, doubted that conventional HBOT sham controls could genuinely have a therapeutic effect, and I agree. The upcoming HOT-POCS trial (discussed below) should answer the question more definitively.
Mechanisms of action and safety
Mechanisms of benefit for HBOT include increased oxygen availability and angiogenesis. Animal research suggests that it may reduce secondary cell death from TBI, through stabilization of the blood-brain barrier and inflammation reduction.
HBOT is generally safe and well tolerated. A retrospective analysis of 1.5 million outpatient hyperbaric treatments revealed that less than 1% were associated with adverse events. The most common were ear and sinus barotrauma. Because HBOT uses increased air pressure, patients must equalize their ears and sinuses. Those who cannot because of altered consciousness, anatomical defects, or congestion must undergo myringotomy or terminate therapy. Claustrophobia was the second most common adverse effect. Convulsions and tension pneumocephalus were rare.
Perhaps the most concerning risk of HBOT for patients with TBI is the potential waste of human and financial resources.
Desperate physicians and patients
As a neurologist who regularly treats patients with TBI, I share the review authors’ frustration regarding the limited efficacy of available treatments. However, the suboptimal efficacy of currently available therapy is insufficient justification to recommend HBOT.
With respect to chronic TBI, it is difficult to imagine how HBOT could reverse brain injury that has been present for months or years. No other therapy exists that reliably encourages neuronal regeneration or prevents the development of posttraumatic epilepsy.
Frank Conidi, MD, a board-certified sports neurologist and headache specialist, shared his thoughts via email. He agrees that HBOT may have a role in TBI, but after reviewing Hadanny and colleagues’ paper, he concluded that there is insufficient evidence for the use of HBOT in all forms of TBI. He would like to see large multicenter, well-designed studies with standardized pressures and duration and a standard definition of the various types of head injury.
Ongoing research
There are at least five ongoing trials on HBOT for TBI or postconcussive syndrome, including the well-designed placebo-controlled HOT-POCS study. The latter has a novel placebo gas system that addresses Hadanny and colleagues’ contention that even low-dose HBOT might be effective.
The placebo arm in HOT-POCS mimics the HBO environment but provides only 0.21 ATA of oxygen, the same as room air. The active arm provides 100% oxygen at 2.0 ATA. If patients in both arms improve, the benefit will be caused by a placebo response, not HBOT.
Conflict of interest
Another concern with the review is that all three authors are affiliated with Aviv Scientific. This company has an exclusive partnership with the world’s largest hyperbaric medicine and research facility, the Sagol Center at Shamir Medical Center in Be’er Ya’akov, Israel.
This conflict of interest does not a priori invalidate their conclusions. However, official HBOT guidelines from a leading organization like the Undersea and Hyperbaric Medicine Society or the American Academy of Neurology would be preferable.
Conclusion
There is an urgent unmet need for more effective treatments for postconcussive syndrome and chronic TBI.
The review authors’ recommendations for HBOT seem premature. They are arguably a disservice to the many desperate patients and their families who will be tempted to expend valuable resources of time and money for an appealing but unproven therapy. Appropriately designed placebo-controlled studies such as HOT-POCS will help separate fact from wishful thinking.
Dr. Wilner is associate professor of neurology at University of Tennessee Health Science Center, Memphis. He reported a conflict of interest with Accordant Health Services.
A version of this article first appeared on Medscape.com.
The future for the primary care physician?
“The doctor won’t see you now.”
The editor of the alumni magazine had succeeded in getting my attention. The shock value of the headline hooked me and I was drawn in to chase down the research. A study by a team of researchers at Harvard Medical School has published a study in the British Medical Journal revealing that “from 2013 to 2019 the share of U.S. health care visits delivered by nonphysicians such as nurse practitioners or physician assistants increased from 14% to 26%.” In other words, at more than a quarter of the health care visits in this country the patient is not seen by a physician. The percent seen by advanced-practice providers varied by complaint and specialty. For example, 47% of patients with a respiratory complaint saw a nurse practitioner or physicians assistant, while for an eye condition only 13% were seen by an advanced-practice provider. However, overall the increase was dramatic.
It doesn’t require much deep thinking to come up with some explanations for this shift in provider involvement. It boils down to supply and demand. Compared with other similar nations, we have fewer physicians. Physicians are leaving the profession for a variety of reasons, including disappointment with their work-life balance and a sense that too much of their day is devoted to meaningless work with user unfriendly computer systems.
The number of nurse practitioners and physician assistants is growing faster than that of physicians. In fact it has been predicted that over the next 2 decades advanced-practice providers will increase by more than 50% while the physician pool will grow by less than 5%.
We can mull over the how-we-got-here ad infinitum, but this recent study suggests that we had better hustle and invest some time rethinking the role of a physician and how we should adapt our education system to better prepare for those choosing the path through medical school. This mirror gazing is particularly critical for those of us doing primary care.
While in years past I often heard a discontented grumble from patients that “I was ‘only’ seen by the nurse practitioner,” this complaint has become much less frequent as patients have gained more experience with advanced-practice providers and have begun to accept the new reality and see the change as inevitable.
When someone tells me that their daughter or nephew or second cousin is planning on becoming a doctor, I pause and listen patiently as they go on proudly about it before asking if the young person has considered becoming a physician assistant. I say, “Ya know, if I were 60 years younger I think I would bypass medical school and become a nurse practitioner because they get to do all the cool things that I enjoyed about seeing patients. Sure, my mother wouldn’t have been able to introduce me as her son the doctor. But, my parents and I would have spent less time and money on my training, and I would have had less administrative hassle heaped on me once I went into practice.”
The essence of good primary care is Availability, Continuity, and Expertise. The first two attributes aren’t taught in medical school and rely on commitment and having enough bodies to deliver the care. When it comes to expertise, how important is the broad and deep education of traditional medical school when the provider is seeing the relatively narrow spectrum of bread-and-butter everyday complaints that fill the day for most primary care providers? Particularly, when the population has already been preselected by age, geography, and socioeconomic factors.
The usual argument against my assertion is that a higher-priced and more arduous education pathway better provides the physician with the tools to deal with the outliers, the diagnostic enigmas. My reply is that any good provider regardless of his or her years spent in training is taught to first admit what they don’t know. When faced with an enigma, call a consultant or, in the near future, access a Chatbot.
If the natural market and economic forces continue to drive the growth of advanced-practice providers, what role(s) remains for the medical school–trained primary care provider? Does she or he remain the leader of a team of providers? Does she or he still see patients and somehow remain the first among equals?
While patients seem to be warming to the notion of seeing a nonphysician provider, I often still hear the complaint but “I see a different provider every time I go to the office.” Continuity is one of those three keystones of quality primary care. It is not incompatible with a team concept of care delivery, but it does require a commitment to the concept and creative scheduling that allows the patient to see the same provider at almost every visit. I’m not sure where having a first-among-equals provider fits into that scheme. Is it just one more “different” provider?
Maybe the medical school–trained provider becomes a consultant physician, much as the British and Canadian systems seem to work. She or he would see patients only after the advanced primary care provider has done an evaluation and is unsure of the next step. Would this be at the same site or electronically? Is there a time lag? In my old-school interpretation, if the visit is not the same day or maybe the next day then it doesn’t satisfy my Availability requirement of primary care.
Primarily an educator who generally doesn’t see patients but instead trains advanced primary care providers, organizes them into teams that function to provide care in a timely fashion that emphasizes Continuity, and then performs ongoing, real time assessments to assure that care provided is at the highest level of Expertise.
It sounds like an interesting and challenging job description requiring a deep and broad education. Just not one that appeals to me. I would rather be a nurse practitioner or physician’s assistant who is on the front line and hands on.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected]
“The doctor won’t see you now.”
The editor of the alumni magazine had succeeded in getting my attention. The shock value of the headline hooked me and I was drawn in to chase down the research. A study by a team of researchers at Harvard Medical School has published a study in the British Medical Journal revealing that “from 2013 to 2019 the share of U.S. health care visits delivered by nonphysicians such as nurse practitioners or physician assistants increased from 14% to 26%.” In other words, at more than a quarter of the health care visits in this country the patient is not seen by a physician. The percent seen by advanced-practice providers varied by complaint and specialty. For example, 47% of patients with a respiratory complaint saw a nurse practitioner or physicians assistant, while for an eye condition only 13% were seen by an advanced-practice provider. However, overall the increase was dramatic.
It doesn’t require much deep thinking to come up with some explanations for this shift in provider involvement. It boils down to supply and demand. Compared with other similar nations, we have fewer physicians. Physicians are leaving the profession for a variety of reasons, including disappointment with their work-life balance and a sense that too much of their day is devoted to meaningless work with user unfriendly computer systems.
The number of nurse practitioners and physician assistants is growing faster than that of physicians. In fact it has been predicted that over the next 2 decades advanced-practice providers will increase by more than 50% while the physician pool will grow by less than 5%.
We can mull over the how-we-got-here ad infinitum, but this recent study suggests that we had better hustle and invest some time rethinking the role of a physician and how we should adapt our education system to better prepare for those choosing the path through medical school. This mirror gazing is particularly critical for those of us doing primary care.
While in years past I often heard a discontented grumble from patients that “I was ‘only’ seen by the nurse practitioner,” this complaint has become much less frequent as patients have gained more experience with advanced-practice providers and have begun to accept the new reality and see the change as inevitable.
When someone tells me that their daughter or nephew or second cousin is planning on becoming a doctor, I pause and listen patiently as they go on proudly about it before asking if the young person has considered becoming a physician assistant. I say, “Ya know, if I were 60 years younger I think I would bypass medical school and become a nurse practitioner because they get to do all the cool things that I enjoyed about seeing patients. Sure, my mother wouldn’t have been able to introduce me as her son the doctor. But, my parents and I would have spent less time and money on my training, and I would have had less administrative hassle heaped on me once I went into practice.”
The essence of good primary care is Availability, Continuity, and Expertise. The first two attributes aren’t taught in medical school and rely on commitment and having enough bodies to deliver the care. When it comes to expertise, how important is the broad and deep education of traditional medical school when the provider is seeing the relatively narrow spectrum of bread-and-butter everyday complaints that fill the day for most primary care providers? Particularly, when the population has already been preselected by age, geography, and socioeconomic factors.
The usual argument against my assertion is that a higher-priced and more arduous education pathway better provides the physician with the tools to deal with the outliers, the diagnostic enigmas. My reply is that any good provider regardless of his or her years spent in training is taught to first admit what they don’t know. When faced with an enigma, call a consultant or, in the near future, access a Chatbot.
If the natural market and economic forces continue to drive the growth of advanced-practice providers, what role(s) remains for the medical school–trained primary care provider? Does she or he remain the leader of a team of providers? Does she or he still see patients and somehow remain the first among equals?
While patients seem to be warming to the notion of seeing a nonphysician provider, I often still hear the complaint but “I see a different provider every time I go to the office.” Continuity is one of those three keystones of quality primary care. It is not incompatible with a team concept of care delivery, but it does require a commitment to the concept and creative scheduling that allows the patient to see the same provider at almost every visit. I’m not sure where having a first-among-equals provider fits into that scheme. Is it just one more “different” provider?
Maybe the medical school–trained provider becomes a consultant physician, much as the British and Canadian systems seem to work. She or he would see patients only after the advanced primary care provider has done an evaluation and is unsure of the next step. Would this be at the same site or electronically? Is there a time lag? In my old-school interpretation, if the visit is not the same day or maybe the next day then it doesn’t satisfy my Availability requirement of primary care.
Primarily an educator who generally doesn’t see patients but instead trains advanced primary care providers, organizes them into teams that function to provide care in a timely fashion that emphasizes Continuity, and then performs ongoing, real time assessments to assure that care provided is at the highest level of Expertise.
It sounds like an interesting and challenging job description requiring a deep and broad education. Just not one that appeals to me. I would rather be a nurse practitioner or physician’s assistant who is on the front line and hands on.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected]
“The doctor won’t see you now.”
The editor of the alumni magazine had succeeded in getting my attention. The shock value of the headline hooked me and I was drawn in to chase down the research. A study by a team of researchers at Harvard Medical School has published a study in the British Medical Journal revealing that “from 2013 to 2019 the share of U.S. health care visits delivered by nonphysicians such as nurse practitioners or physician assistants increased from 14% to 26%.” In other words, at more than a quarter of the health care visits in this country the patient is not seen by a physician. The percent seen by advanced-practice providers varied by complaint and specialty. For example, 47% of patients with a respiratory complaint saw a nurse practitioner or physicians assistant, while for an eye condition only 13% were seen by an advanced-practice provider. However, overall the increase was dramatic.
It doesn’t require much deep thinking to come up with some explanations for this shift in provider involvement. It boils down to supply and demand. Compared with other similar nations, we have fewer physicians. Physicians are leaving the profession for a variety of reasons, including disappointment with their work-life balance and a sense that too much of their day is devoted to meaningless work with user unfriendly computer systems.
The number of nurse practitioners and physician assistants is growing faster than that of physicians. In fact it has been predicted that over the next 2 decades advanced-practice providers will increase by more than 50% while the physician pool will grow by less than 5%.
We can mull over the how-we-got-here ad infinitum, but this recent study suggests that we had better hustle and invest some time rethinking the role of a physician and how we should adapt our education system to better prepare for those choosing the path through medical school. This mirror gazing is particularly critical for those of us doing primary care.
While in years past I often heard a discontented grumble from patients that “I was ‘only’ seen by the nurse practitioner,” this complaint has become much less frequent as patients have gained more experience with advanced-practice providers and have begun to accept the new reality and see the change as inevitable.
When someone tells me that their daughter or nephew or second cousin is planning on becoming a doctor, I pause and listen patiently as they go on proudly about it before asking if the young person has considered becoming a physician assistant. I say, “Ya know, if I were 60 years younger I think I would bypass medical school and become a nurse practitioner because they get to do all the cool things that I enjoyed about seeing patients. Sure, my mother wouldn’t have been able to introduce me as her son the doctor. But, my parents and I would have spent less time and money on my training, and I would have had less administrative hassle heaped on me once I went into practice.”
The essence of good primary care is Availability, Continuity, and Expertise. The first two attributes aren’t taught in medical school and rely on commitment and having enough bodies to deliver the care. When it comes to expertise, how important is the broad and deep education of traditional medical school when the provider is seeing the relatively narrow spectrum of bread-and-butter everyday complaints that fill the day for most primary care providers? Particularly, when the population has already been preselected by age, geography, and socioeconomic factors.
The usual argument against my assertion is that a higher-priced and more arduous education pathway better provides the physician with the tools to deal with the outliers, the diagnostic enigmas. My reply is that any good provider regardless of his or her years spent in training is taught to first admit what they don’t know. When faced with an enigma, call a consultant or, in the near future, access a Chatbot.
If the natural market and economic forces continue to drive the growth of advanced-practice providers, what role(s) remains for the medical school–trained primary care provider? Does she or he remain the leader of a team of providers? Does she or he still see patients and somehow remain the first among equals?
While patients seem to be warming to the notion of seeing a nonphysician provider, I often still hear the complaint but “I see a different provider every time I go to the office.” Continuity is one of those three keystones of quality primary care. It is not incompatible with a team concept of care delivery, but it does require a commitment to the concept and creative scheduling that allows the patient to see the same provider at almost every visit. I’m not sure where having a first-among-equals provider fits into that scheme. Is it just one more “different” provider?
Maybe the medical school–trained provider becomes a consultant physician, much as the British and Canadian systems seem to work. She or he would see patients only after the advanced primary care provider has done an evaluation and is unsure of the next step. Would this be at the same site or electronically? Is there a time lag? In my old-school interpretation, if the visit is not the same day or maybe the next day then it doesn’t satisfy my Availability requirement of primary care.
Primarily an educator who generally doesn’t see patients but instead trains advanced primary care providers, organizes them into teams that function to provide care in a timely fashion that emphasizes Continuity, and then performs ongoing, real time assessments to assure that care provided is at the highest level of Expertise.
It sounds like an interesting and challenging job description requiring a deep and broad education. Just not one that appeals to me. I would rather be a nurse practitioner or physician’s assistant who is on the front line and hands on.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected]
Rheumatoid arthritis: Five things to know
Rheumatoid arthritis (RA) is a chronic systemic inflammatory disease that typically presents as a symmetric inflammatory polyarthritis (synovitis) primarily affecting the hands and feet. Any joint lined by a synovial membrane may be involved; however, extraarticular involvement of various organs can be significant. RA is theorized to develop when a genetically susceptible individual experiences an external trigger (e.g., cigarette smoking, infection, trauma) that precipitates an autoimmune reaction.
Rheumatology is a rapidly advancing, but relatively young, subspecialty. An understanding of the pathophysiology, treatment, and classification of RA is still emerging. Here are five things to know about RA.
1. A healthier lifestyle is associated with a reduced risk of developing RA.
Large epidemiologic studies have identified several factors that increase the incidence of RA; these include an unhealthy diet, smoking, adiposity, low educational level, and low socioeconomic status. A patient’s response to antirheumatic medications can be affected by certain lifestyle habits, which can be associated with worse treatment outcomes; such habits include smoking, insufficient physical activity, and obesity, among others. Although methodologic problems may impede making firm conclusions regarding a causal role for these factors in the disease course and risk of developing RA, current evidence is sufficient to recommend quitting smoking, adopting a healthy diet, preventing obesity, and maintaining a high level of physical activity to support the effectiveness of current antirheumatic drugs.
In the Nurses’ Health Study, biennial questionnaires were used to collect lifestyle and medical information to determine which modifiable risk factors are associated with the risk for RA in women. Patient medical records were used to confirm incident RA and serostatus. The healthy lifestyle index score (HLIS), which includes five modifiable risk factors (smoking, alcohol consumption, body mass index, physical activity, and diet), was used to assess risk. Cox regression, which was adjusted for confounders, was used to model associations between HLIS and the incidence of RA. The study concluded that a healthier lifestyle was associated with a lower risk of developing RA, and a significant number of RA cases may be preventable if patients adopted four or more healthy lifestyle factors.
The Mediterranean diet is one current popular dietary option that appears to have promising evidence in many disease processes, including RA.
2. In pregnant women with RA, the course of the disease can change throughout pregnancy.
The course of RA often changes during pregnancy. About half of pregnant women with RA have low disease activity, and 20%-40% achieve remission by the third trimester; however, nearly 20% have worse or moderate to high disease activity during pregnancy that may require further therapeutic intervention. Postpartum flares of RA also may occur, with studies reporting rates of 39%-90%.
No specific guidelines address obstetric monitoring in patients with RA. Because few data suggest a significantly increased risk for preterm birth, preeclampsia, or fetal growth restriction, no special obstetric monitoring is indicated beyond what is performed for usual obstetric care.
Medications considered to be low risk in pregnancy include low-dose corticosteroids, antimalarial agents, sulfasalazine, and azathioprine. Certain tumor necrosis factor inhibitors are also thought to be relatively safe.
3. A well-designed exercise program can be beneficial in RA.
Regular physical activity has replaced bed rest as the recommended response to the stiffness and pain associated with RA. However, many patients who have RA do not really believe this. Lack of conviction and motivation appear to be the major factors that deter nearly half of patients with RA from moving about enough to help their situation. There is ample evidence about the benefits of physical activity in RA, but little research into why few patients with RA take advantage of it. The extreme physical inactivity of patients with RA becomes a vicious cycle in terms of health and disease progression. Thus, encouraging physical activity is an essential part of the overall treatment of RA.
Findings from randomized controlled trials show that exercise is fundamentally beneficial for patients with RA. The benefits of properly designed physical exercise programs include improved cardiorespiratory fitness and cardiovascular health, increased muscle mass, reduced adiposity, increased strength, and improved physical functioning, all achieved without exacerbation of disease activity or joint damage.
The American College of Rheumatology (ACR) has released recommendations for exercise interventions for RA. The ACR strongly recommends consistent engagement in an exercise program over no exercise. The type of exercise is open to interpretation. An exercise program for patients with rheumatic diseases aims to preserve or restore the range of motion of affected joints, increase muscle strength and endurance, and improve mood and decrease health risks associated with a sedentary lifestyle.
4. RA is a systemic disease that affects multiple organ systems.
Although synovitis is the pathologic hallmark of RA, extraarticular manifestations and comorbidities occur presumably owing to the complex, chronic, inflammatory, and autoimmune features of RA.
The most common cause of death in patients with RA is cardiovascular disease. Compared with the general population, patients with RA have two times the risk of having a myocardial infarction, and they have up to 50% greater cardiovascular mortality risk. Factors identified to play important roles in atherosclerotic damage and incident cardiovascular disease include severe and prolonged disease activity, inflammation (e.g., C-reactive protein, anti–citrullinated protein antibodies (ACPAs), cytokines, matrix-degrading enzymes), and genetics.
Respiratory disease is the second major cause of death in patients with RA; this occurs in 30%-40% of patients with RA. The lung interstitium, airways, and pleurae can all be affected by RA, but pulmonary vascular involvement is less common.
Central and peripheral nervous system involvement is typically attributed to RA-associated small-vessel vasculitis, joint damage, and/or drug toxicity. Evidence also suggests that systemic inflammation causes microvascular cerebral damage that is associated with the development of vascular dementia and Alzheimer’s disease. Finally, some observational studies have suggested that drugs commonly used to treat RA – disease-modifying antirheumatic drugs (DMARDs) and biologics – may reduce the incidence of dementia.
5. As treatment options for RA improve, many controversies have arisen.
In patients without RA symptoms but with biomarkers, experts debate whether early treatment with DMARDs could prevent irreversible joint damage.
There is no clear definition of pre-RA, but it could be defined as having positive markers for RA (e.g., positive rheumatoid factor and anti–cyclic citrullinated peptides) or having joint pain with abnormal ultrasonography findings but not having positive biomarkers. However, not all patients who have positive biomarkers progress to clinical RA, so what exactly determines this progression is unclear. Nevertheless, some clinicians do treat pre-RA. This was a major debate at the ACR’s 2022 meeting.
Studies have shown that early treatment of RA, including during the preclinical phase, can lead to better long-term outcomes. It can help reduce joint inflammation, control disease activity, and prevent or minimize irreversible joint damage. Early treatment also increases the likelihood of achieving remission or low disease activity, which improves quality of life for patients. Lifestyle interventions in these patients, including exercise, weight control, and cardiovascular health, may not prevent disease but may delay the onset of full-blown clinical RA.
The discovery of pre-RA has also underpinned the development of several clinical prevention trials in RA; specifically, the PRAIRI study demonstrated that a single dose of rituximab can delay the onset of clinically apparent RA in at-risk individuals. Additional studies are evaluating the ability of drugs, including abatacept, hydroxychloroquine, and methotrexate, to prevent or delay future RA.
Dual biologics target different pathways – ostensibly boosting efficacy – but unknowns, concerns over safety, and lack of evidence make the practice controversial.
Several randomized controlled trials have assessed the safety and efficacy of dual-biologic treatment of RA, but the results have been mixed, which has raised safety concerns. Overall, there is a paucity of data concerning the safety of the simultaneous use of more than one biologic. Dual therapy may constitute an efficacious and safe add-on treatment to biologic therapy, but properly conducted clinical investigations are needed. In the meantime, dual biologic therapy used at physicians’ discretion requires close monitoring of patients, with an emphasis on the safety profile.
Large language models (artificial intelligence [AI]) are rapidly taking hold in medicine. Many argue that they can enrich patient care, but they come with liability risks.
Large language models, such as AI chatbots or ChatGPT, can increase access to information, help with patient education, and support decision-making. Limitations include lack of personalization, clinical experience, and emotional connection. The use of large language models in health care is fraught with ethical and legal concerns.
Liability issues can arise if errors, inaccuracies, or adverse outcomes result from the use of AI chatbots. Determining liability may involve assessing factors such as the design and development of the AI system, training and deployment of the model, the communication of limitations and disclaimers to users, and the involvement of human healthcare professionals in the decision-making process.
To mitigate liability risks, AI chatbots in rheumatology must comply with applicable regulations and guidelines. Transparency in the capabilities and limitations of the system, clear communication of the boundaries of its advice, and the presence of human oversight are essential. Collaborating with legal experts and following best practices in the development and deployment of AI technologies can help to minimize liability concerns.
The benefits and risks associated with tapering DMARD therapy in patients with RA who have sustained remission of disease should be considered.
Although some patients with well-controlled RA have relapse after tapering or discontinuing DMARDs, some do not, making this treatment strategy a personal decision undertaken with a rheumatologist.
In the RETRO study, German researchers examined the effects of tapering or stopping DMARDs in patients whose RA was in sustained remission. In the phase 3 trial (n = 316), investigators randomized 303 patients with remission for ≥ 6 months who were on stable conventional synthetic or biologic DMARD treatment into three groups: (1) continuation on 100% DMARD dose, (2) tapering to 50% of the DMARD dose, and (3) 50% tapering followed by DMARD withdrawal. The proportion of patients who continued in remission at 1 year was 81.2% in group 1, 58.6% in group 2, and 43.3% in group 3. Predictors for flare-ups were female sex, longer disease duration, rheumatoid factor or ACPA positivity, and higher disease activity scores at baseline.
The abrupt cessation or reduction of DMARDs without medical supervision and guidance can exacerbate symptoms and result in disease flares.
The interplay between long COVID and RA is a recent phenomenon that needs to be considered.
RA shares similar symptoms with long COVID. Patients with a history of RA and a previous diagnosis of COVID-19 who have developed persistent joint or muscle symptoms pose a significant challenge to clinicians. Such patients may be experiencing long COVID or a flare-up of their preexisting rheumatic disease.
Immunosuppressive medications can potentially increase the risk for COVID-19, but it is not clear how they affect disease severity risk. Individuals with RA and long COVID need careful evaluation to balance the management of disease activity while considering the risks associated with immunosuppression and potential susceptibility to viral infections.
Dr. Dombrosky is a staff physician in rheumatology at Central Virginia VA Health Care System in Richmond. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Rheumatoid arthritis (RA) is a chronic systemic inflammatory disease that typically presents as a symmetric inflammatory polyarthritis (synovitis) primarily affecting the hands and feet. Any joint lined by a synovial membrane may be involved; however, extraarticular involvement of various organs can be significant. RA is theorized to develop when a genetically susceptible individual experiences an external trigger (e.g., cigarette smoking, infection, trauma) that precipitates an autoimmune reaction.
Rheumatology is a rapidly advancing, but relatively young, subspecialty. An understanding of the pathophysiology, treatment, and classification of RA is still emerging. Here are five things to know about RA.
1. A healthier lifestyle is associated with a reduced risk of developing RA.
Large epidemiologic studies have identified several factors that increase the incidence of RA; these include an unhealthy diet, smoking, adiposity, low educational level, and low socioeconomic status. A patient’s response to antirheumatic medications can be affected by certain lifestyle habits, which can be associated with worse treatment outcomes; such habits include smoking, insufficient physical activity, and obesity, among others. Although methodologic problems may impede making firm conclusions regarding a causal role for these factors in the disease course and risk of developing RA, current evidence is sufficient to recommend quitting smoking, adopting a healthy diet, preventing obesity, and maintaining a high level of physical activity to support the effectiveness of current antirheumatic drugs.
In the Nurses’ Health Study, biennial questionnaires were used to collect lifestyle and medical information to determine which modifiable risk factors are associated with the risk for RA in women. Patient medical records were used to confirm incident RA and serostatus. The healthy lifestyle index score (HLIS), which includes five modifiable risk factors (smoking, alcohol consumption, body mass index, physical activity, and diet), was used to assess risk. Cox regression, which was adjusted for confounders, was used to model associations between HLIS and the incidence of RA. The study concluded that a healthier lifestyle was associated with a lower risk of developing RA, and a significant number of RA cases may be preventable if patients adopted four or more healthy lifestyle factors.
The Mediterranean diet is one current popular dietary option that appears to have promising evidence in many disease processes, including RA.
2. In pregnant women with RA, the course of the disease can change throughout pregnancy.
The course of RA often changes during pregnancy. About half of pregnant women with RA have low disease activity, and 20%-40% achieve remission by the third trimester; however, nearly 20% have worse or moderate to high disease activity during pregnancy that may require further therapeutic intervention. Postpartum flares of RA also may occur, with studies reporting rates of 39%-90%.
No specific guidelines address obstetric monitoring in patients with RA. Because few data suggest a significantly increased risk for preterm birth, preeclampsia, or fetal growth restriction, no special obstetric monitoring is indicated beyond what is performed for usual obstetric care.
Medications considered to be low risk in pregnancy include low-dose corticosteroids, antimalarial agents, sulfasalazine, and azathioprine. Certain tumor necrosis factor inhibitors are also thought to be relatively safe.
3. A well-designed exercise program can be beneficial in RA.
Regular physical activity has replaced bed rest as the recommended response to the stiffness and pain associated with RA. However, many patients who have RA do not really believe this. Lack of conviction and motivation appear to be the major factors that deter nearly half of patients with RA from moving about enough to help their situation. There is ample evidence about the benefits of physical activity in RA, but little research into why few patients with RA take advantage of it. The extreme physical inactivity of patients with RA becomes a vicious cycle in terms of health and disease progression. Thus, encouraging physical activity is an essential part of the overall treatment of RA.
Findings from randomized controlled trials show that exercise is fundamentally beneficial for patients with RA. The benefits of properly designed physical exercise programs include improved cardiorespiratory fitness and cardiovascular health, increased muscle mass, reduced adiposity, increased strength, and improved physical functioning, all achieved without exacerbation of disease activity or joint damage.
The American College of Rheumatology (ACR) has released recommendations for exercise interventions for RA. The ACR strongly recommends consistent engagement in an exercise program over no exercise. The type of exercise is open to interpretation. An exercise program for patients with rheumatic diseases aims to preserve or restore the range of motion of affected joints, increase muscle strength and endurance, and improve mood and decrease health risks associated with a sedentary lifestyle.
4. RA is a systemic disease that affects multiple organ systems.
Although synovitis is the pathologic hallmark of RA, extraarticular manifestations and comorbidities occur presumably owing to the complex, chronic, inflammatory, and autoimmune features of RA.
The most common cause of death in patients with RA is cardiovascular disease. Compared with the general population, patients with RA have two times the risk of having a myocardial infarction, and they have up to 50% greater cardiovascular mortality risk. Factors identified to play important roles in atherosclerotic damage and incident cardiovascular disease include severe and prolonged disease activity, inflammation (e.g., C-reactive protein, anti–citrullinated protein antibodies (ACPAs), cytokines, matrix-degrading enzymes), and genetics.
Respiratory disease is the second major cause of death in patients with RA; this occurs in 30%-40% of patients with RA. The lung interstitium, airways, and pleurae can all be affected by RA, but pulmonary vascular involvement is less common.
Central and peripheral nervous system involvement is typically attributed to RA-associated small-vessel vasculitis, joint damage, and/or drug toxicity. Evidence also suggests that systemic inflammation causes microvascular cerebral damage that is associated with the development of vascular dementia and Alzheimer’s disease. Finally, some observational studies have suggested that drugs commonly used to treat RA – disease-modifying antirheumatic drugs (DMARDs) and biologics – may reduce the incidence of dementia.
5. As treatment options for RA improve, many controversies have arisen.
In patients without RA symptoms but with biomarkers, experts debate whether early treatment with DMARDs could prevent irreversible joint damage.
There is no clear definition of pre-RA, but it could be defined as having positive markers for RA (e.g., positive rheumatoid factor and anti–cyclic citrullinated peptides) or having joint pain with abnormal ultrasonography findings but not having positive biomarkers. However, not all patients who have positive biomarkers progress to clinical RA, so what exactly determines this progression is unclear. Nevertheless, some clinicians do treat pre-RA. This was a major debate at the ACR’s 2022 meeting.
Studies have shown that early treatment of RA, including during the preclinical phase, can lead to better long-term outcomes. It can help reduce joint inflammation, control disease activity, and prevent or minimize irreversible joint damage. Early treatment also increases the likelihood of achieving remission or low disease activity, which improves quality of life for patients. Lifestyle interventions in these patients, including exercise, weight control, and cardiovascular health, may not prevent disease but may delay the onset of full-blown clinical RA.
The discovery of pre-RA has also underpinned the development of several clinical prevention trials in RA; specifically, the PRAIRI study demonstrated that a single dose of rituximab can delay the onset of clinically apparent RA in at-risk individuals. Additional studies are evaluating the ability of drugs, including abatacept, hydroxychloroquine, and methotrexate, to prevent or delay future RA.
Dual biologics target different pathways – ostensibly boosting efficacy – but unknowns, concerns over safety, and lack of evidence make the practice controversial.
Several randomized controlled trials have assessed the safety and efficacy of dual-biologic treatment of RA, but the results have been mixed, which has raised safety concerns. Overall, there is a paucity of data concerning the safety of the simultaneous use of more than one biologic. Dual therapy may constitute an efficacious and safe add-on treatment to biologic therapy, but properly conducted clinical investigations are needed. In the meantime, dual biologic therapy used at physicians’ discretion requires close monitoring of patients, with an emphasis on the safety profile.
Large language models (artificial intelligence [AI]) are rapidly taking hold in medicine. Many argue that they can enrich patient care, but they come with liability risks.
Large language models, such as AI chatbots or ChatGPT, can increase access to information, help with patient education, and support decision-making. Limitations include lack of personalization, clinical experience, and emotional connection. The use of large language models in health care is fraught with ethical and legal concerns.
Liability issues can arise if errors, inaccuracies, or adverse outcomes result from the use of AI chatbots. Determining liability may involve assessing factors such as the design and development of the AI system, training and deployment of the model, the communication of limitations and disclaimers to users, and the involvement of human healthcare professionals in the decision-making process.
To mitigate liability risks, AI chatbots in rheumatology must comply with applicable regulations and guidelines. Transparency in the capabilities and limitations of the system, clear communication of the boundaries of its advice, and the presence of human oversight are essential. Collaborating with legal experts and following best practices in the development and deployment of AI technologies can help to minimize liability concerns.
The benefits and risks associated with tapering DMARD therapy in patients with RA who have sustained remission of disease should be considered.
Although some patients with well-controlled RA have relapse after tapering or discontinuing DMARDs, some do not, making this treatment strategy a personal decision undertaken with a rheumatologist.
In the RETRO study, German researchers examined the effects of tapering or stopping DMARDs in patients whose RA was in sustained remission. In the phase 3 trial (n = 316), investigators randomized 303 patients with remission for ≥ 6 months who were on stable conventional synthetic or biologic DMARD treatment into three groups: (1) continuation on 100% DMARD dose, (2) tapering to 50% of the DMARD dose, and (3) 50% tapering followed by DMARD withdrawal. The proportion of patients who continued in remission at 1 year was 81.2% in group 1, 58.6% in group 2, and 43.3% in group 3. Predictors for flare-ups were female sex, longer disease duration, rheumatoid factor or ACPA positivity, and higher disease activity scores at baseline.
The abrupt cessation or reduction of DMARDs without medical supervision and guidance can exacerbate symptoms and result in disease flares.
The interplay between long COVID and RA is a recent phenomenon that needs to be considered.
RA shares similar symptoms with long COVID. Patients with a history of RA and a previous diagnosis of COVID-19 who have developed persistent joint or muscle symptoms pose a significant challenge to clinicians. Such patients may be experiencing long COVID or a flare-up of their preexisting rheumatic disease.
Immunosuppressive medications can potentially increase the risk for COVID-19, but it is not clear how they affect disease severity risk. Individuals with RA and long COVID need careful evaluation to balance the management of disease activity while considering the risks associated with immunosuppression and potential susceptibility to viral infections.
Dr. Dombrosky is a staff physician in rheumatology at Central Virginia VA Health Care System in Richmond. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Rheumatoid arthritis (RA) is a chronic systemic inflammatory disease that typically presents as a symmetric inflammatory polyarthritis (synovitis) primarily affecting the hands and feet. Any joint lined by a synovial membrane may be involved; however, extraarticular involvement of various organs can be significant. RA is theorized to develop when a genetically susceptible individual experiences an external trigger (e.g., cigarette smoking, infection, trauma) that precipitates an autoimmune reaction.
Rheumatology is a rapidly advancing, but relatively young, subspecialty. An understanding of the pathophysiology, treatment, and classification of RA is still emerging. Here are five things to know about RA.
1. A healthier lifestyle is associated with a reduced risk of developing RA.
Large epidemiologic studies have identified several factors that increase the incidence of RA; these include an unhealthy diet, smoking, adiposity, low educational level, and low socioeconomic status. A patient’s response to antirheumatic medications can be affected by certain lifestyle habits, which can be associated with worse treatment outcomes; such habits include smoking, insufficient physical activity, and obesity, among others. Although methodologic problems may impede making firm conclusions regarding a causal role for these factors in the disease course and risk of developing RA, current evidence is sufficient to recommend quitting smoking, adopting a healthy diet, preventing obesity, and maintaining a high level of physical activity to support the effectiveness of current antirheumatic drugs.
In the Nurses’ Health Study, biennial questionnaires were used to collect lifestyle and medical information to determine which modifiable risk factors are associated with the risk for RA in women. Patient medical records were used to confirm incident RA and serostatus. The healthy lifestyle index score (HLIS), which includes five modifiable risk factors (smoking, alcohol consumption, body mass index, physical activity, and diet), was used to assess risk. Cox regression, which was adjusted for confounders, was used to model associations between HLIS and the incidence of RA. The study concluded that a healthier lifestyle was associated with a lower risk of developing RA, and a significant number of RA cases may be preventable if patients adopted four or more healthy lifestyle factors.
The Mediterranean diet is one current popular dietary option that appears to have promising evidence in many disease processes, including RA.
2. In pregnant women with RA, the course of the disease can change throughout pregnancy.
The course of RA often changes during pregnancy. About half of pregnant women with RA have low disease activity, and 20%-40% achieve remission by the third trimester; however, nearly 20% have worse or moderate to high disease activity during pregnancy that may require further therapeutic intervention. Postpartum flares of RA also may occur, with studies reporting rates of 39%-90%.
No specific guidelines address obstetric monitoring in patients with RA. Because few data suggest a significantly increased risk for preterm birth, preeclampsia, or fetal growth restriction, no special obstetric monitoring is indicated beyond what is performed for usual obstetric care.
Medications considered to be low risk in pregnancy include low-dose corticosteroids, antimalarial agents, sulfasalazine, and azathioprine. Certain tumor necrosis factor inhibitors are also thought to be relatively safe.
3. A well-designed exercise program can be beneficial in RA.
Regular physical activity has replaced bed rest as the recommended response to the stiffness and pain associated with RA. However, many patients who have RA do not really believe this. Lack of conviction and motivation appear to be the major factors that deter nearly half of patients with RA from moving about enough to help their situation. There is ample evidence about the benefits of physical activity in RA, but little research into why few patients with RA take advantage of it. The extreme physical inactivity of patients with RA becomes a vicious cycle in terms of health and disease progression. Thus, encouraging physical activity is an essential part of the overall treatment of RA.
Findings from randomized controlled trials show that exercise is fundamentally beneficial for patients with RA. The benefits of properly designed physical exercise programs include improved cardiorespiratory fitness and cardiovascular health, increased muscle mass, reduced adiposity, increased strength, and improved physical functioning, all achieved without exacerbation of disease activity or joint damage.
The American College of Rheumatology (ACR) has released recommendations for exercise interventions for RA. The ACR strongly recommends consistent engagement in an exercise program over no exercise. The type of exercise is open to interpretation. An exercise program for patients with rheumatic diseases aims to preserve or restore the range of motion of affected joints, increase muscle strength and endurance, and improve mood and decrease health risks associated with a sedentary lifestyle.
4. RA is a systemic disease that affects multiple organ systems.
Although synovitis is the pathologic hallmark of RA, extraarticular manifestations and comorbidities occur presumably owing to the complex, chronic, inflammatory, and autoimmune features of RA.
The most common cause of death in patients with RA is cardiovascular disease. Compared with the general population, patients with RA have two times the risk of having a myocardial infarction, and they have up to 50% greater cardiovascular mortality risk. Factors identified to play important roles in atherosclerotic damage and incident cardiovascular disease include severe and prolonged disease activity, inflammation (e.g., C-reactive protein, anti–citrullinated protein antibodies (ACPAs), cytokines, matrix-degrading enzymes), and genetics.
Respiratory disease is the second major cause of death in patients with RA; this occurs in 30%-40% of patients with RA. The lung interstitium, airways, and pleurae can all be affected by RA, but pulmonary vascular involvement is less common.
Central and peripheral nervous system involvement is typically attributed to RA-associated small-vessel vasculitis, joint damage, and/or drug toxicity. Evidence also suggests that systemic inflammation causes microvascular cerebral damage that is associated with the development of vascular dementia and Alzheimer’s disease. Finally, some observational studies have suggested that drugs commonly used to treat RA – disease-modifying antirheumatic drugs (DMARDs) and biologics – may reduce the incidence of dementia.
5. As treatment options for RA improve, many controversies have arisen.
In patients without RA symptoms but with biomarkers, experts debate whether early treatment with DMARDs could prevent irreversible joint damage.
There is no clear definition of pre-RA, but it could be defined as having positive markers for RA (e.g., positive rheumatoid factor and anti–cyclic citrullinated peptides) or having joint pain with abnormal ultrasonography findings but not having positive biomarkers. However, not all patients who have positive biomarkers progress to clinical RA, so what exactly determines this progression is unclear. Nevertheless, some clinicians do treat pre-RA. This was a major debate at the ACR’s 2022 meeting.
Studies have shown that early treatment of RA, including during the preclinical phase, can lead to better long-term outcomes. It can help reduce joint inflammation, control disease activity, and prevent or minimize irreversible joint damage. Early treatment also increases the likelihood of achieving remission or low disease activity, which improves quality of life for patients. Lifestyle interventions in these patients, including exercise, weight control, and cardiovascular health, may not prevent disease but may delay the onset of full-blown clinical RA.
The discovery of pre-RA has also underpinned the development of several clinical prevention trials in RA; specifically, the PRAIRI study demonstrated that a single dose of rituximab can delay the onset of clinically apparent RA in at-risk individuals. Additional studies are evaluating the ability of drugs, including abatacept, hydroxychloroquine, and methotrexate, to prevent or delay future RA.
Dual biologics target different pathways – ostensibly boosting efficacy – but unknowns, concerns over safety, and lack of evidence make the practice controversial.
Several randomized controlled trials have assessed the safety and efficacy of dual-biologic treatment of RA, but the results have been mixed, which has raised safety concerns. Overall, there is a paucity of data concerning the safety of the simultaneous use of more than one biologic. Dual therapy may constitute an efficacious and safe add-on treatment to biologic therapy, but properly conducted clinical investigations are needed. In the meantime, dual biologic therapy used at physicians’ discretion requires close monitoring of patients, with an emphasis on the safety profile.
Large language models (artificial intelligence [AI]) are rapidly taking hold in medicine. Many argue that they can enrich patient care, but they come with liability risks.
Large language models, such as AI chatbots or ChatGPT, can increase access to information, help with patient education, and support decision-making. Limitations include lack of personalization, clinical experience, and emotional connection. The use of large language models in health care is fraught with ethical and legal concerns.
Liability issues can arise if errors, inaccuracies, or adverse outcomes result from the use of AI chatbots. Determining liability may involve assessing factors such as the design and development of the AI system, training and deployment of the model, the communication of limitations and disclaimers to users, and the involvement of human healthcare professionals in the decision-making process.
To mitigate liability risks, AI chatbots in rheumatology must comply with applicable regulations and guidelines. Transparency in the capabilities and limitations of the system, clear communication of the boundaries of its advice, and the presence of human oversight are essential. Collaborating with legal experts and following best practices in the development and deployment of AI technologies can help to minimize liability concerns.
The benefits and risks associated with tapering DMARD therapy in patients with RA who have sustained remission of disease should be considered.
Although some patients with well-controlled RA have relapse after tapering or discontinuing DMARDs, some do not, making this treatment strategy a personal decision undertaken with a rheumatologist.
In the RETRO study, German researchers examined the effects of tapering or stopping DMARDs in patients whose RA was in sustained remission. In the phase 3 trial (n = 316), investigators randomized 303 patients with remission for ≥ 6 months who were on stable conventional synthetic or biologic DMARD treatment into three groups: (1) continuation on 100% DMARD dose, (2) tapering to 50% of the DMARD dose, and (3) 50% tapering followed by DMARD withdrawal. The proportion of patients who continued in remission at 1 year was 81.2% in group 1, 58.6% in group 2, and 43.3% in group 3. Predictors for flare-ups were female sex, longer disease duration, rheumatoid factor or ACPA positivity, and higher disease activity scores at baseline.
The abrupt cessation or reduction of DMARDs without medical supervision and guidance can exacerbate symptoms and result in disease flares.
The interplay between long COVID and RA is a recent phenomenon that needs to be considered.
RA shares similar symptoms with long COVID. Patients with a history of RA and a previous diagnosis of COVID-19 who have developed persistent joint or muscle symptoms pose a significant challenge to clinicians. Such patients may be experiencing long COVID or a flare-up of their preexisting rheumatic disease.
Immunosuppressive medications can potentially increase the risk for COVID-19, but it is not clear how they affect disease severity risk. Individuals with RA and long COVID need careful evaluation to balance the management of disease activity while considering the risks associated with immunosuppression and potential susceptibility to viral infections.
Dr. Dombrosky is a staff physician in rheumatology at Central Virginia VA Health Care System in Richmond. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Training more doctors should be our first priority, says ethicist
This transcript has been edited for clarity.
Recently, the Supreme Court of the United States struck down the use of affirmative action in admissions to colleges, universities, medical schools, and nursing schools. This has led to an enormous amount of worry and concern, particularly in medical school admissions in the world I’m in, where people start to say that diversity matters. Diversity is important.
I know many deans of medical schools immediately sent out messages of reassurance to their students, saying New York University or Stanford or Harvard or Minnesota or Case Western is still deeply concerned about diversity, and we’re going to do what we can to preserve attention to diversity.
I’ve served on admissions at a number of schools over the years for med school. I understand – and have been told – that diversity is important, and according to the Supreme Court, not explicitly by race. There are obviously many variables to take into account when trying to keep diversity at the forefront of admissions.
At the schools I’ve been at, including Columbia, NYU, University of Pittsburgh, University of Minnesota, and University of Pennsylvania, there are plenty of qualified students. Happily, we’ve always been engaged in some effort to try and whittle down the class to the size that we can manage and accept, and many qualified students don’t get admitted.
The first order of business for me is not to worry about how to maintain diversity. It’s to recognize that we need more doctors, nurses, and mental health care providers. I will, in a second, say a few words about diversity and where it fits into admissions, but I want to make the point clearly that what we should be doing is trying to expand the pool of students who are going to become doctors, nurses, mental health care providers, and social workers.
There are too many early retirements. We don’t have the person power we need to manage the health care challenges of an aging population. Let’s not get lost in arguing about what characteristics ought to get you into the finest medical schools. Let’s realize that we have to expand the number of schools we have.
We better be working pretty hard to expand our physician assistant programs, to make sure that we give full authority to qualified dentists and nurses who can help deliver some clinical care. We need more folks. That’s really where the battle ought to be: How do we get that done and how do we get it done quickly, not arguing about who’s in, who’s out, and why.
That said, diversity to me has never meant just race. I’m always interested in gender orientation, disability, and geographic input. Sometimes in decisions that you’re looking at, when I have students in front of me, they tell me they play a musical instrument or about the obstacles they had to overcome to get to medical school. Some of them will say they were involved in 4-H and did rodeo in high school or junior high school, which makes them a diverse potential student with characteristics that maybe some others don’t bring.
I’m not against diversity. I think having a rich set of experiences in any class – medicine, nursing, whatever it’s going to be – is beneficial to the students. They learn from each other. It is sometimes said that it’s also good for patients. I’m a little less excited about that, because I think our training goal should be to make every medical student and nursing student qualified to treat anybody.
I don’t think that, just because you’re Latinx or gay, that’s going to make a gay patient feel better. I think we should teach our students how to give care to everybody that they encounter. They shouldn’t have to match up characteristics to feel like they’re going to get quality care. That isn’t the right reason.
When you have a diverse set of providers, they can call that out and be on the alert for it, and that’s very important.
I also believe that we should think widely and broadly about diversity. Maybe race is out, but certainly other experiences related to income, background, struggle that got you to the point where you’re applying to medical school, motivation, the kinds of experiences you might have had caring for an elderly person, dealing with a disability or learning disability, and trying to overcome, let’s say, going to school in a poor area with not such a wonderful school, really help in terms of forming professionalism, empathy, and a caring point of view.
To me, the main goal is to expand our workforce. The secondary goal is to stay diverse, because we get better providers when we do so.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Recently, the Supreme Court of the United States struck down the use of affirmative action in admissions to colleges, universities, medical schools, and nursing schools. This has led to an enormous amount of worry and concern, particularly in medical school admissions in the world I’m in, where people start to say that diversity matters. Diversity is important.
I know many deans of medical schools immediately sent out messages of reassurance to their students, saying New York University or Stanford or Harvard or Minnesota or Case Western is still deeply concerned about diversity, and we’re going to do what we can to preserve attention to diversity.
I’ve served on admissions at a number of schools over the years for med school. I understand – and have been told – that diversity is important, and according to the Supreme Court, not explicitly by race. There are obviously many variables to take into account when trying to keep diversity at the forefront of admissions.
At the schools I’ve been at, including Columbia, NYU, University of Pittsburgh, University of Minnesota, and University of Pennsylvania, there are plenty of qualified students. Happily, we’ve always been engaged in some effort to try and whittle down the class to the size that we can manage and accept, and many qualified students don’t get admitted.
The first order of business for me is not to worry about how to maintain diversity. It’s to recognize that we need more doctors, nurses, and mental health care providers. I will, in a second, say a few words about diversity and where it fits into admissions, but I want to make the point clearly that what we should be doing is trying to expand the pool of students who are going to become doctors, nurses, mental health care providers, and social workers.
There are too many early retirements. We don’t have the person power we need to manage the health care challenges of an aging population. Let’s not get lost in arguing about what characteristics ought to get you into the finest medical schools. Let’s realize that we have to expand the number of schools we have.
We better be working pretty hard to expand our physician assistant programs, to make sure that we give full authority to qualified dentists and nurses who can help deliver some clinical care. We need more folks. That’s really where the battle ought to be: How do we get that done and how do we get it done quickly, not arguing about who’s in, who’s out, and why.
That said, diversity to me has never meant just race. I’m always interested in gender orientation, disability, and geographic input. Sometimes in decisions that you’re looking at, when I have students in front of me, they tell me they play a musical instrument or about the obstacles they had to overcome to get to medical school. Some of them will say they were involved in 4-H and did rodeo in high school or junior high school, which makes them a diverse potential student with characteristics that maybe some others don’t bring.
I’m not against diversity. I think having a rich set of experiences in any class – medicine, nursing, whatever it’s going to be – is beneficial to the students. They learn from each other. It is sometimes said that it’s also good for patients. I’m a little less excited about that, because I think our training goal should be to make every medical student and nursing student qualified to treat anybody.
I don’t think that, just because you’re Latinx or gay, that’s going to make a gay patient feel better. I think we should teach our students how to give care to everybody that they encounter. They shouldn’t have to match up characteristics to feel like they’re going to get quality care. That isn’t the right reason.
When you have a diverse set of providers, they can call that out and be on the alert for it, and that’s very important.
I also believe that we should think widely and broadly about diversity. Maybe race is out, but certainly other experiences related to income, background, struggle that got you to the point where you’re applying to medical school, motivation, the kinds of experiences you might have had caring for an elderly person, dealing with a disability or learning disability, and trying to overcome, let’s say, going to school in a poor area with not such a wonderful school, really help in terms of forming professionalism, empathy, and a caring point of view.
To me, the main goal is to expand our workforce. The secondary goal is to stay diverse, because we get better providers when we do so.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Recently, the Supreme Court of the United States struck down the use of affirmative action in admissions to colleges, universities, medical schools, and nursing schools. This has led to an enormous amount of worry and concern, particularly in medical school admissions in the world I’m in, where people start to say that diversity matters. Diversity is important.
I know many deans of medical schools immediately sent out messages of reassurance to their students, saying New York University or Stanford or Harvard or Minnesota or Case Western is still deeply concerned about diversity, and we’re going to do what we can to preserve attention to diversity.
I’ve served on admissions at a number of schools over the years for med school. I understand – and have been told – that diversity is important, and according to the Supreme Court, not explicitly by race. There are obviously many variables to take into account when trying to keep diversity at the forefront of admissions.
At the schools I’ve been at, including Columbia, NYU, University of Pittsburgh, University of Minnesota, and University of Pennsylvania, there are plenty of qualified students. Happily, we’ve always been engaged in some effort to try and whittle down the class to the size that we can manage and accept, and many qualified students don’t get admitted.
The first order of business for me is not to worry about how to maintain diversity. It’s to recognize that we need more doctors, nurses, and mental health care providers. I will, in a second, say a few words about diversity and where it fits into admissions, but I want to make the point clearly that what we should be doing is trying to expand the pool of students who are going to become doctors, nurses, mental health care providers, and social workers.
There are too many early retirements. We don’t have the person power we need to manage the health care challenges of an aging population. Let’s not get lost in arguing about what characteristics ought to get you into the finest medical schools. Let’s realize that we have to expand the number of schools we have.
We better be working pretty hard to expand our physician assistant programs, to make sure that we give full authority to qualified dentists and nurses who can help deliver some clinical care. We need more folks. That’s really where the battle ought to be: How do we get that done and how do we get it done quickly, not arguing about who’s in, who’s out, and why.
That said, diversity to me has never meant just race. I’m always interested in gender orientation, disability, and geographic input. Sometimes in decisions that you’re looking at, when I have students in front of me, they tell me they play a musical instrument or about the obstacles they had to overcome to get to medical school. Some of them will say they were involved in 4-H and did rodeo in high school or junior high school, which makes them a diverse potential student with characteristics that maybe some others don’t bring.
I’m not against diversity. I think having a rich set of experiences in any class – medicine, nursing, whatever it’s going to be – is beneficial to the students. They learn from each other. It is sometimes said that it’s also good for patients. I’m a little less excited about that, because I think our training goal should be to make every medical student and nursing student qualified to treat anybody.
I don’t think that, just because you’re Latinx or gay, that’s going to make a gay patient feel better. I think we should teach our students how to give care to everybody that they encounter. They shouldn’t have to match up characteristics to feel like they’re going to get quality care. That isn’t the right reason.
When you have a diverse set of providers, they can call that out and be on the alert for it, and that’s very important.
I also believe that we should think widely and broadly about diversity. Maybe race is out, but certainly other experiences related to income, background, struggle that got you to the point where you’re applying to medical school, motivation, the kinds of experiences you might have had caring for an elderly person, dealing with a disability or learning disability, and trying to overcome, let’s say, going to school in a poor area with not such a wonderful school, really help in terms of forming professionalism, empathy, and a caring point of view.
To me, the main goal is to expand our workforce. The secondary goal is to stay diverse, because we get better providers when we do so.
A version of this article first appeared on Medscape.com.
The surprising link between loneliness and Parkinson’s disease
This transcript has been edited for clarity.
On May 3, 2023, Surgeon General Vivek Murthy issued an advisory raising an alarm about what he called an “epidemic of loneliness” in the United States.
Now, I am not saying that Vivek Murthy read my book, “How Medicine Works and When It Doesn’t” – released in January and available in bookstores now – where, in chapter 11, I call attention to the problem of loneliness and its relationship to the exponential rise in deaths of despair. But Vivek, if you did, let me know. I could use the publicity.
No, of course the idea that loneliness is a public health issue is not new, but I’m glad to see it finally getting attention. At this point, studies have linked loneliness to heart disease, stroke, dementia, and premature death.
The UK Biobank is really a treasure trove of data for epidemiologists. I must see three to four studies a week coming out of this mega-dataset. This one, appearing in JAMA Neurology, caught my eye for its focus specifically on loneliness as a risk factor – something I’m hoping to see more of in the future.
The study examines data from just under 500,000 individuals in the United Kingdom who answered a survey including the question “Do you often feel lonely?” between 2006 and 2010; 18.4% of people answered yes. Individuals’ electronic health record data were then monitored over time to see who would get a new diagnosis code consistent with Parkinson’s disease. Through 2021, 2822 people did – that’s just over half a percent.
So, now we do the statistics thing. Of the nonlonely folks, 2,273 went on to develop Parkinson’s disease. Of those who said they often feel lonely, 549 people did. The raw numbers here, to be honest, aren’t that compelling. Lonely people had an absolute risk for Parkinson’s disease about 0.03% higher than that of nonlonely people. Put another way, you’d need to take over 3,000 lonely souls and make them not lonely to prevent 1 case of Parkinson’s disease.
Still, the costs of loneliness are not measured exclusively in Parkinson’s disease, and I would argue that the real risks here come from other sources: alcohol abuse, drug abuse, and suicide. Nevertheless, the weak but significant association with Parkinson’s disease reminds us that loneliness is a neurologic phenomenon. There is something about social connection that affects our brain in a way that is not just spiritual; it is actually biological.
Of course, people who say they are often lonely are different in other ways from people who report not being lonely. Lonely people, in this dataset, were younger, more likely to be female, less likely to have a college degree, in worse physical health, and engaged in more high-risk health behaviors like smoking.
The authors adjusted for all of these factors and found that, on the relative scale, lonely people were still about 20%-30% more likely to develop Parkinson’s disease.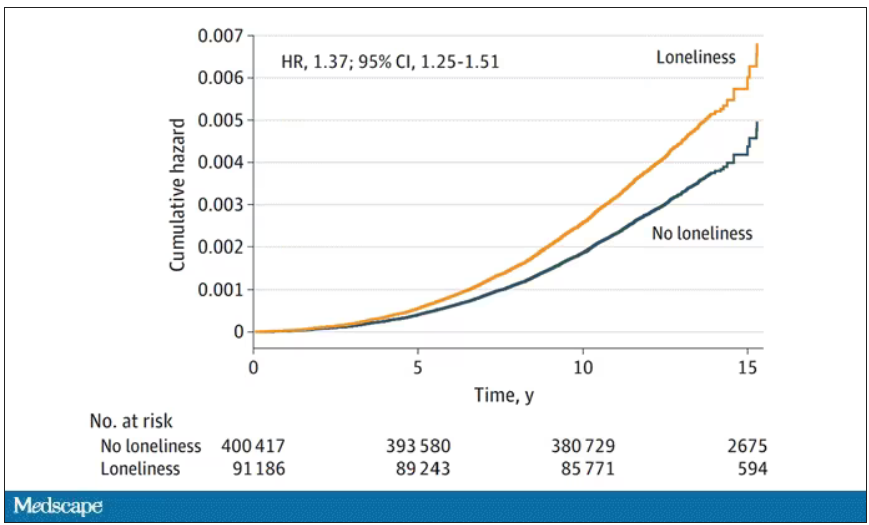
So, what do we do about this? There is no pill for loneliness, and God help us if there ever is. Recognizing the problem is a good start. But there are some policy things we can do to reduce loneliness. We can invest in public spaces that bring people together – parks, museums, libraries – and public transportation. We can deal with tech companies that are so optimized at capturing our attention that we cease to engage with other humans. And, individually, we can just reach out a bit more. We’ve spent the past few pandemic years with our attention focused sharply inward. It’s time to look out again.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale University’s Clinical and Translational Research Accelerator in New Haven, Conn. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
On May 3, 2023, Surgeon General Vivek Murthy issued an advisory raising an alarm about what he called an “epidemic of loneliness” in the United States.
Now, I am not saying that Vivek Murthy read my book, “How Medicine Works and When It Doesn’t” – released in January and available in bookstores now – where, in chapter 11, I call attention to the problem of loneliness and its relationship to the exponential rise in deaths of despair. But Vivek, if you did, let me know. I could use the publicity.
No, of course the idea that loneliness is a public health issue is not new, but I’m glad to see it finally getting attention. At this point, studies have linked loneliness to heart disease, stroke, dementia, and premature death.
The UK Biobank is really a treasure trove of data for epidemiologists. I must see three to four studies a week coming out of this mega-dataset. This one, appearing in JAMA Neurology, caught my eye for its focus specifically on loneliness as a risk factor – something I’m hoping to see more of in the future.
The study examines data from just under 500,000 individuals in the United Kingdom who answered a survey including the question “Do you often feel lonely?” between 2006 and 2010; 18.4% of people answered yes. Individuals’ electronic health record data were then monitored over time to see who would get a new diagnosis code consistent with Parkinson’s disease. Through 2021, 2822 people did – that’s just over half a percent.
So, now we do the statistics thing. Of the nonlonely folks, 2,273 went on to develop Parkinson’s disease. Of those who said they often feel lonely, 549 people did. The raw numbers here, to be honest, aren’t that compelling. Lonely people had an absolute risk for Parkinson’s disease about 0.03% higher than that of nonlonely people. Put another way, you’d need to take over 3,000 lonely souls and make them not lonely to prevent 1 case of Parkinson’s disease.
Still, the costs of loneliness are not measured exclusively in Parkinson’s disease, and I would argue that the real risks here come from other sources: alcohol abuse, drug abuse, and suicide. Nevertheless, the weak but significant association with Parkinson’s disease reminds us that loneliness is a neurologic phenomenon. There is something about social connection that affects our brain in a way that is not just spiritual; it is actually biological.
Of course, people who say they are often lonely are different in other ways from people who report not being lonely. Lonely people, in this dataset, were younger, more likely to be female, less likely to have a college degree, in worse physical health, and engaged in more high-risk health behaviors like smoking.
The authors adjusted for all of these factors and found that, on the relative scale, lonely people were still about 20%-30% more likely to develop Parkinson’s disease.
So, what do we do about this? There is no pill for loneliness, and God help us if there ever is. Recognizing the problem is a good start. But there are some policy things we can do to reduce loneliness. We can invest in public spaces that bring people together – parks, museums, libraries – and public transportation. We can deal with tech companies that are so optimized at capturing our attention that we cease to engage with other humans. And, individually, we can just reach out a bit more. We’ve spent the past few pandemic years with our attention focused sharply inward. It’s time to look out again.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale University’s Clinical and Translational Research Accelerator in New Haven, Conn. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
On May 3, 2023, Surgeon General Vivek Murthy issued an advisory raising an alarm about what he called an “epidemic of loneliness” in the United States.
Now, I am not saying that Vivek Murthy read my book, “How Medicine Works and When It Doesn’t” – released in January and available in bookstores now – where, in chapter 11, I call attention to the problem of loneliness and its relationship to the exponential rise in deaths of despair. But Vivek, if you did, let me know. I could use the publicity.
No, of course the idea that loneliness is a public health issue is not new, but I’m glad to see it finally getting attention. At this point, studies have linked loneliness to heart disease, stroke, dementia, and premature death.
The UK Biobank is really a treasure trove of data for epidemiologists. I must see three to four studies a week coming out of this mega-dataset. This one, appearing in JAMA Neurology, caught my eye for its focus specifically on loneliness as a risk factor – something I’m hoping to see more of in the future.
The study examines data from just under 500,000 individuals in the United Kingdom who answered a survey including the question “Do you often feel lonely?” between 2006 and 2010; 18.4% of people answered yes. Individuals’ electronic health record data were then monitored over time to see who would get a new diagnosis code consistent with Parkinson’s disease. Through 2021, 2822 people did – that’s just over half a percent.
So, now we do the statistics thing. Of the nonlonely folks, 2,273 went on to develop Parkinson’s disease. Of those who said they often feel lonely, 549 people did. The raw numbers here, to be honest, aren’t that compelling. Lonely people had an absolute risk for Parkinson’s disease about 0.03% higher than that of nonlonely people. Put another way, you’d need to take over 3,000 lonely souls and make them not lonely to prevent 1 case of Parkinson’s disease.
Still, the costs of loneliness are not measured exclusively in Parkinson’s disease, and I would argue that the real risks here come from other sources: alcohol abuse, drug abuse, and suicide. Nevertheless, the weak but significant association with Parkinson’s disease reminds us that loneliness is a neurologic phenomenon. There is something about social connection that affects our brain in a way that is not just spiritual; it is actually biological.
Of course, people who say they are often lonely are different in other ways from people who report not being lonely. Lonely people, in this dataset, were younger, more likely to be female, less likely to have a college degree, in worse physical health, and engaged in more high-risk health behaviors like smoking.
The authors adjusted for all of these factors and found that, on the relative scale, lonely people were still about 20%-30% more likely to develop Parkinson’s disease.
So, what do we do about this? There is no pill for loneliness, and God help us if there ever is. Recognizing the problem is a good start. But there are some policy things we can do to reduce loneliness. We can invest in public spaces that bring people together – parks, museums, libraries – and public transportation. We can deal with tech companies that are so optimized at capturing our attention that we cease to engage with other humans. And, individually, we can just reach out a bit more. We’ve spent the past few pandemic years with our attention focused sharply inward. It’s time to look out again.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale University’s Clinical and Translational Research Accelerator in New Haven, Conn. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Multivitamins and dementia: Untangling the COSMOS study web
I have written before about the COSMOS study and its finding that multivitamins (and chocolate) did not improve brain or cardiovascular health. So I was surprised to read that a “new” study found that vitamins can forestall dementia and age-related cognitive decline.
Upon closer look, the new data are neither new nor convincing, at least to me.
Chocolate and multivitamins for CVD and cancer prevention
The large randomized COSMOS trial was supposed to be the definitive study on chocolate that would establish its heart-health benefits without a doubt. Or, rather, the benefits of a cocoa bean extract in pill form given to healthy, older volunteers. The COSMOS study was negative. Chocolate, or the cocoa bean extract they used, did not reduce cardiovascular events.
And yet for all the prepublication importance attached to COSMOS, it is scarcely mentioned. Had it been positive, rest assured that Mars, the candy bar company that cofunded the research, and other interested parties would have been shouting it from the rooftops. As it is, they’re already spinning it.
Which brings us to the multivitamin component. COSMOS actually had a 2 × 2 design. In other words, there were four groups in this study: chocolate plus multivitamin, chocolate plus placebo, placebo plus multivitamin, and placebo plus placebo. This type of study design allows you to study two different interventions simultaneously, provided that they are independent and do not interact with each other. In addition to the primary cardiovascular endpoint, they also studied a cancer endpoint.
The multivitamin supplement didn’t reduce cardiovascular events either. Nor did it affect cancer outcomes. The main COSMOS study was negative and reinforced what countless other studies have proven: Taking a daily multivitamin does not reduce your risk of having a heart attack or developing cancer.
But wait, there’s more: COSMOS-Mind
But no researcher worth his salt studies just one or two endpoints in a study. The participants also underwent neurologic and memory testing. These results were reported separately in the COSMOS-Mind study.
COSMOS-Mind is often described as a separate (or “new”) study. In reality, it included the same participants from the original COSMOS trial and measured yet another primary outcome of cognitive performance on a series of tests administered by telephone. Although there is nothing inherently wrong with studying multiple outcomes in your patient population (after all, that salami isn’t going to slice itself), they cannot all be primary outcomes. Some, by necessity, must be secondary hypothesis–generating outcomes. If you test enough endpoints, multiple hypothesis testing dictates that eventually you will get a positive result simply by chance.
There was a time when the neurocognitive outcomes of COSMOS would have been reported in the same paper as the cardiovascular outcomes, but that time seems to have passed us by. Researchers live or die by the number of their publications, and there is an inherent advantage to squeezing as many publications as possible from the same dataset. Though, to be fair, the journal would probably have asked them to split up the paper as well.
In brief, the cocoa extract again fell short in COSMOS-Mind, but the multivitamin arm did better on the composite cognitive outcome. It was a fairly small difference – a 0.07-point improvement on the z-score at the 3-year mark (the z-score is the mean divided by the standard deviation). Much was also made of the fact that the improvement seemed to vary by prior history of cardiovascular disease (CVD). Those with a history of CVD had a 0.11-point improvement, whereas those without had a 0.06-point improvement. The authors couldn’t offer a definitive explanation for these findings. Any argument that multivitamins improve cardiovascular health and therefore prevent vascular dementia has to contend with the fact that the main COSMOS study didn’t show a cardiovascular benefit for vitamins. Speculation that you are treating nutritional deficiencies is exactly that: speculation.
A more salient question is: What does a 0.07-point improvement on the z-score mean clinically? This study didn’t assess whether a multivitamin supplement prevented dementia or allowed people to live independently for longer. In fairness, that would have been exceptionally difficult to do and would have required a much longer study.
Their one attempt to quantify the cognitive benefit clinically was a calculation about normal age-related decline. Test scores were 0.045 points lower for every 1-year increase in age among participants (their mean age was 73 years). So the authors contend that a 0.07-point increase, or the 0.083-point increase that they found at year 3, corresponds to 1.8 years of age-related decline forestalled. Whether this is an appropriate assumption, I leave for the reader to decide.
COSMOS-Web and replication
The results of COSMOS-Mind were seemingly bolstered by the recent publication of COSMOS-Web. Although I’ve seen this study described as having replicated the results of COSMOS-Mind, that description is a bit misleading. This was yet another ancillary COSMOS study; more than half of the 2,262 participants in COSMOS-Mind were also included in COSMOS-Web. Replicating results in the same people isn’t true replication.
The main difference between COSMOS-Mind and COSMOS-Web is that the former used a telephone interview to administer the cognitive tests and the latter used the Internet. They also had different endpoints, with COSMOS-Web looking at immediate recall rather than a global test composite.
COSMOS-Web was a positive study in that patients getting the multivitamin supplement did better on the test for immediate memory recall (remembering a list of 20 words), though they didn’t improve on tests of memory retention, executive function, or novel object recognition (basically a test where subjects have to identify matching geometric patterns and then recall them later). They were able to remember an additional 0.71 word on average, compared with 0.44 word in the placebo group. (For the record, it found no benefit for the cocoa extract).
Everybody does better on memory tests the second time around because practice makes perfect, hence the improvement in the placebo group. This benefit at 1 year did not survive to the end of follow-up at 3 years, in contrast to COSMOS-Mind, where the benefit was not apparent at 1 year and seen only at year 3. A history of cardiovascular disease didn’t seem to affect the results in COSMOS-Web as it did in COSMOS-Mind. As far as replications go, COSMOS-Web has some very non-negligible differences, compared with COSMOS-Mind. This incongruity, especially given the overlap in the patient populations is hard to reconcile. If COSMOS-Web was supposed to assuage any doubts that persisted after COSMOS-Mind, it hasn’t for me.
One of these studies is not like the others
Finally, although the COSMOS trial and all its ancillary study analyses suggest a neurocognitive benefit to multivitamin supplementation, it’s not the first study to test the matter. The Age-Related Eye Disease Study looked at vitamin C, vitamin E, beta-carotene, zinc, and copper. There was no benefit on any of the six cognitive tests administered to patients. The Women’s Health Study, the Women’s Antioxidant Cardiovascular Study and PREADViSE have all failed to show any benefit to the various vitamins and minerals they studied. A meta-analysis of 11 trials found no benefit to B vitamins in slowing cognitive aging.
The claim that COSMOS is the “first” study to test the hypothesis hinges on some careful wordplay. Prior studies tested specific vitamins, not a multivitamin. In the discussion of the paper, these other studies are critiqued for being short term. But the Physicians’ Health Study II did in fact study a multivitamin and assessed cognitive performance on average 2.5 years after randomization. It found no benefit. The authors of COSMOS-Web critiqued the 2.5-year wait to perform cognitive testing, saying it would have missed any short-term benefits. Although, given that they simultaneously praised their 3 years of follow-up, the criticism is hard to fully accept or even understand.
Whether follow-up is short or long, uses individual vitamins or a multivitamin, the results excluding COSMOS are uniformly negative.
Do enough tests in the same population, and something will rise above the noise just by chance. When you get a positive result in your research, it’s always exciting. But when a slew of studies that came before you are negative, you aren’t groundbreaking. You’re an outlier.
Dr. Labos is a cardiologist at Hôpital Notre-Dame, Montreal. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
I have written before about the COSMOS study and its finding that multivitamins (and chocolate) did not improve brain or cardiovascular health. So I was surprised to read that a “new” study found that vitamins can forestall dementia and age-related cognitive decline.
Upon closer look, the new data are neither new nor convincing, at least to me.
Chocolate and multivitamins for CVD and cancer prevention
The large randomized COSMOS trial was supposed to be the definitive study on chocolate that would establish its heart-health benefits without a doubt. Or, rather, the benefits of a cocoa bean extract in pill form given to healthy, older volunteers. The COSMOS study was negative. Chocolate, or the cocoa bean extract they used, did not reduce cardiovascular events.
And yet for all the prepublication importance attached to COSMOS, it is scarcely mentioned. Had it been positive, rest assured that Mars, the candy bar company that cofunded the research, and other interested parties would have been shouting it from the rooftops. As it is, they’re already spinning it.
Which brings us to the multivitamin component. COSMOS actually had a 2 × 2 design. In other words, there were four groups in this study: chocolate plus multivitamin, chocolate plus placebo, placebo plus multivitamin, and placebo plus placebo. This type of study design allows you to study two different interventions simultaneously, provided that they are independent and do not interact with each other. In addition to the primary cardiovascular endpoint, they also studied a cancer endpoint.
The multivitamin supplement didn’t reduce cardiovascular events either. Nor did it affect cancer outcomes. The main COSMOS study was negative and reinforced what countless other studies have proven: Taking a daily multivitamin does not reduce your risk of having a heart attack or developing cancer.
But wait, there’s more: COSMOS-Mind
But no researcher worth his salt studies just one or two endpoints in a study. The participants also underwent neurologic and memory testing. These results were reported separately in the COSMOS-Mind study.
COSMOS-Mind is often described as a separate (or “new”) study. In reality, it included the same participants from the original COSMOS trial and measured yet another primary outcome of cognitive performance on a series of tests administered by telephone. Although there is nothing inherently wrong with studying multiple outcomes in your patient population (after all, that salami isn’t going to slice itself), they cannot all be primary outcomes. Some, by necessity, must be secondary hypothesis–generating outcomes. If you test enough endpoints, multiple hypothesis testing dictates that eventually you will get a positive result simply by chance.
There was a time when the neurocognitive outcomes of COSMOS would have been reported in the same paper as the cardiovascular outcomes, but that time seems to have passed us by. Researchers live or die by the number of their publications, and there is an inherent advantage to squeezing as many publications as possible from the same dataset. Though, to be fair, the journal would probably have asked them to split up the paper as well.
In brief, the cocoa extract again fell short in COSMOS-Mind, but the multivitamin arm did better on the composite cognitive outcome. It was a fairly small difference – a 0.07-point improvement on the z-score at the 3-year mark (the z-score is the mean divided by the standard deviation). Much was also made of the fact that the improvement seemed to vary by prior history of cardiovascular disease (CVD). Those with a history of CVD had a 0.11-point improvement, whereas those without had a 0.06-point improvement. The authors couldn’t offer a definitive explanation for these findings. Any argument that multivitamins improve cardiovascular health and therefore prevent vascular dementia has to contend with the fact that the main COSMOS study didn’t show a cardiovascular benefit for vitamins. Speculation that you are treating nutritional deficiencies is exactly that: speculation.
A more salient question is: What does a 0.07-point improvement on the z-score mean clinically? This study didn’t assess whether a multivitamin supplement prevented dementia or allowed people to live independently for longer. In fairness, that would have been exceptionally difficult to do and would have required a much longer study.
Their one attempt to quantify the cognitive benefit clinically was a calculation about normal age-related decline. Test scores were 0.045 points lower for every 1-year increase in age among participants (their mean age was 73 years). So the authors contend that a 0.07-point increase, or the 0.083-point increase that they found at year 3, corresponds to 1.8 years of age-related decline forestalled. Whether this is an appropriate assumption, I leave for the reader to decide.
COSMOS-Web and replication
The results of COSMOS-Mind were seemingly bolstered by the recent publication of COSMOS-Web. Although I’ve seen this study described as having replicated the results of COSMOS-Mind, that description is a bit misleading. This was yet another ancillary COSMOS study; more than half of the 2,262 participants in COSMOS-Mind were also included in COSMOS-Web. Replicating results in the same people isn’t true replication.
The main difference between COSMOS-Mind and COSMOS-Web is that the former used a telephone interview to administer the cognitive tests and the latter used the Internet. They also had different endpoints, with COSMOS-Web looking at immediate recall rather than a global test composite.
COSMOS-Web was a positive study in that patients getting the multivitamin supplement did better on the test for immediate memory recall (remembering a list of 20 words), though they didn’t improve on tests of memory retention, executive function, or novel object recognition (basically a test where subjects have to identify matching geometric patterns and then recall them later). They were able to remember an additional 0.71 word on average, compared with 0.44 word in the placebo group. (For the record, it found no benefit for the cocoa extract).
Everybody does better on memory tests the second time around because practice makes perfect, hence the improvement in the placebo group. This benefit at 1 year did not survive to the end of follow-up at 3 years, in contrast to COSMOS-Mind, where the benefit was not apparent at 1 year and seen only at year 3. A history of cardiovascular disease didn’t seem to affect the results in COSMOS-Web as it did in COSMOS-Mind. As far as replications go, COSMOS-Web has some very non-negligible differences, compared with COSMOS-Mind. This incongruity, especially given the overlap in the patient populations is hard to reconcile. If COSMOS-Web was supposed to assuage any doubts that persisted after COSMOS-Mind, it hasn’t for me.
One of these studies is not like the others
Finally, although the COSMOS trial and all its ancillary study analyses suggest a neurocognitive benefit to multivitamin supplementation, it’s not the first study to test the matter. The Age-Related Eye Disease Study looked at vitamin C, vitamin E, beta-carotene, zinc, and copper. There was no benefit on any of the six cognitive tests administered to patients. The Women’s Health Study, the Women’s Antioxidant Cardiovascular Study and PREADViSE have all failed to show any benefit to the various vitamins and minerals they studied. A meta-analysis of 11 trials found no benefit to B vitamins in slowing cognitive aging.
The claim that COSMOS is the “first” study to test the hypothesis hinges on some careful wordplay. Prior studies tested specific vitamins, not a multivitamin. In the discussion of the paper, these other studies are critiqued for being short term. But the Physicians’ Health Study II did in fact study a multivitamin and assessed cognitive performance on average 2.5 years after randomization. It found no benefit. The authors of COSMOS-Web critiqued the 2.5-year wait to perform cognitive testing, saying it would have missed any short-term benefits. Although, given that they simultaneously praised their 3 years of follow-up, the criticism is hard to fully accept or even understand.
Whether follow-up is short or long, uses individual vitamins or a multivitamin, the results excluding COSMOS are uniformly negative.
Do enough tests in the same population, and something will rise above the noise just by chance. When you get a positive result in your research, it’s always exciting. But when a slew of studies that came before you are negative, you aren’t groundbreaking. You’re an outlier.
Dr. Labos is a cardiologist at Hôpital Notre-Dame, Montreal. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
I have written before about the COSMOS study and its finding that multivitamins (and chocolate) did not improve brain or cardiovascular health. So I was surprised to read that a “new” study found that vitamins can forestall dementia and age-related cognitive decline.
Upon closer look, the new data are neither new nor convincing, at least to me.
Chocolate and multivitamins for CVD and cancer prevention
The large randomized COSMOS trial was supposed to be the definitive study on chocolate that would establish its heart-health benefits without a doubt. Or, rather, the benefits of a cocoa bean extract in pill form given to healthy, older volunteers. The COSMOS study was negative. Chocolate, or the cocoa bean extract they used, did not reduce cardiovascular events.
And yet for all the prepublication importance attached to COSMOS, it is scarcely mentioned. Had it been positive, rest assured that Mars, the candy bar company that cofunded the research, and other interested parties would have been shouting it from the rooftops. As it is, they’re already spinning it.
Which brings us to the multivitamin component. COSMOS actually had a 2 × 2 design. In other words, there were four groups in this study: chocolate plus multivitamin, chocolate plus placebo, placebo plus multivitamin, and placebo plus placebo. This type of study design allows you to study two different interventions simultaneously, provided that they are independent and do not interact with each other. In addition to the primary cardiovascular endpoint, they also studied a cancer endpoint.
The multivitamin supplement didn’t reduce cardiovascular events either. Nor did it affect cancer outcomes. The main COSMOS study was negative and reinforced what countless other studies have proven: Taking a daily multivitamin does not reduce your risk of having a heart attack or developing cancer.
But wait, there’s more: COSMOS-Mind
But no researcher worth his salt studies just one or two endpoints in a study. The participants also underwent neurologic and memory testing. These results were reported separately in the COSMOS-Mind study.
COSMOS-Mind is often described as a separate (or “new”) study. In reality, it included the same participants from the original COSMOS trial and measured yet another primary outcome of cognitive performance on a series of tests administered by telephone. Although there is nothing inherently wrong with studying multiple outcomes in your patient population (after all, that salami isn’t going to slice itself), they cannot all be primary outcomes. Some, by necessity, must be secondary hypothesis–generating outcomes. If you test enough endpoints, multiple hypothesis testing dictates that eventually you will get a positive result simply by chance.
There was a time when the neurocognitive outcomes of COSMOS would have been reported in the same paper as the cardiovascular outcomes, but that time seems to have passed us by. Researchers live or die by the number of their publications, and there is an inherent advantage to squeezing as many publications as possible from the same dataset. Though, to be fair, the journal would probably have asked them to split up the paper as well.
In brief, the cocoa extract again fell short in COSMOS-Mind, but the multivitamin arm did better on the composite cognitive outcome. It was a fairly small difference – a 0.07-point improvement on the z-score at the 3-year mark (the z-score is the mean divided by the standard deviation). Much was also made of the fact that the improvement seemed to vary by prior history of cardiovascular disease (CVD). Those with a history of CVD had a 0.11-point improvement, whereas those without had a 0.06-point improvement. The authors couldn’t offer a definitive explanation for these findings. Any argument that multivitamins improve cardiovascular health and therefore prevent vascular dementia has to contend with the fact that the main COSMOS study didn’t show a cardiovascular benefit for vitamins. Speculation that you are treating nutritional deficiencies is exactly that: speculation.
A more salient question is: What does a 0.07-point improvement on the z-score mean clinically? This study didn’t assess whether a multivitamin supplement prevented dementia or allowed people to live independently for longer. In fairness, that would have been exceptionally difficult to do and would have required a much longer study.
Their one attempt to quantify the cognitive benefit clinically was a calculation about normal age-related decline. Test scores were 0.045 points lower for every 1-year increase in age among participants (their mean age was 73 years). So the authors contend that a 0.07-point increase, or the 0.083-point increase that they found at year 3, corresponds to 1.8 years of age-related decline forestalled. Whether this is an appropriate assumption, I leave for the reader to decide.
COSMOS-Web and replication
The results of COSMOS-Mind were seemingly bolstered by the recent publication of COSMOS-Web. Although I’ve seen this study described as having replicated the results of COSMOS-Mind, that description is a bit misleading. This was yet another ancillary COSMOS study; more than half of the 2,262 participants in COSMOS-Mind were also included in COSMOS-Web. Replicating results in the same people isn’t true replication.
The main difference between COSMOS-Mind and COSMOS-Web is that the former used a telephone interview to administer the cognitive tests and the latter used the Internet. They also had different endpoints, with COSMOS-Web looking at immediate recall rather than a global test composite.
COSMOS-Web was a positive study in that patients getting the multivitamin supplement did better on the test for immediate memory recall (remembering a list of 20 words), though they didn’t improve on tests of memory retention, executive function, or novel object recognition (basically a test where subjects have to identify matching geometric patterns and then recall them later). They were able to remember an additional 0.71 word on average, compared with 0.44 word in the placebo group. (For the record, it found no benefit for the cocoa extract).
Everybody does better on memory tests the second time around because practice makes perfect, hence the improvement in the placebo group. This benefit at 1 year did not survive to the end of follow-up at 3 years, in contrast to COSMOS-Mind, where the benefit was not apparent at 1 year and seen only at year 3. A history of cardiovascular disease didn’t seem to affect the results in COSMOS-Web as it did in COSMOS-Mind. As far as replications go, COSMOS-Web has some very non-negligible differences, compared with COSMOS-Mind. This incongruity, especially given the overlap in the patient populations is hard to reconcile. If COSMOS-Web was supposed to assuage any doubts that persisted after COSMOS-Mind, it hasn’t for me.
One of these studies is not like the others
Finally, although the COSMOS trial and all its ancillary study analyses suggest a neurocognitive benefit to multivitamin supplementation, it’s not the first study to test the matter. The Age-Related Eye Disease Study looked at vitamin C, vitamin E, beta-carotene, zinc, and copper. There was no benefit on any of the six cognitive tests administered to patients. The Women’s Health Study, the Women’s Antioxidant Cardiovascular Study and PREADViSE have all failed to show any benefit to the various vitamins and minerals they studied. A meta-analysis of 11 trials found no benefit to B vitamins in slowing cognitive aging.
The claim that COSMOS is the “first” study to test the hypothesis hinges on some careful wordplay. Prior studies tested specific vitamins, not a multivitamin. In the discussion of the paper, these other studies are critiqued for being short term. But the Physicians’ Health Study II did in fact study a multivitamin and assessed cognitive performance on average 2.5 years after randomization. It found no benefit. The authors of COSMOS-Web critiqued the 2.5-year wait to perform cognitive testing, saying it would have missed any short-term benefits. Although, given that they simultaneously praised their 3 years of follow-up, the criticism is hard to fully accept or even understand.
Whether follow-up is short or long, uses individual vitamins or a multivitamin, the results excluding COSMOS are uniformly negative.
Do enough tests in the same population, and something will rise above the noise just by chance. When you get a positive result in your research, it’s always exciting. But when a slew of studies that came before you are negative, you aren’t groundbreaking. You’re an outlier.
Dr. Labos is a cardiologist at Hôpital Notre-Dame, Montreal. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.