User login
What’s Eating You? Cheyletiella Mites
Identifying Characteristics and Disease Transmission
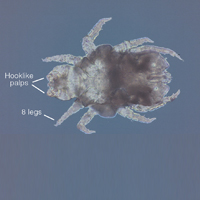
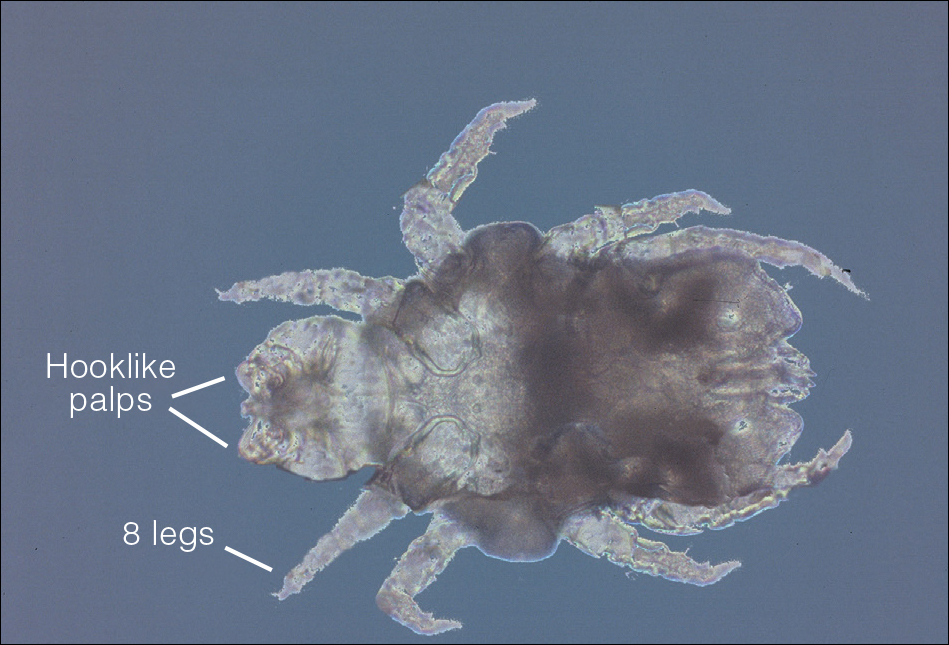
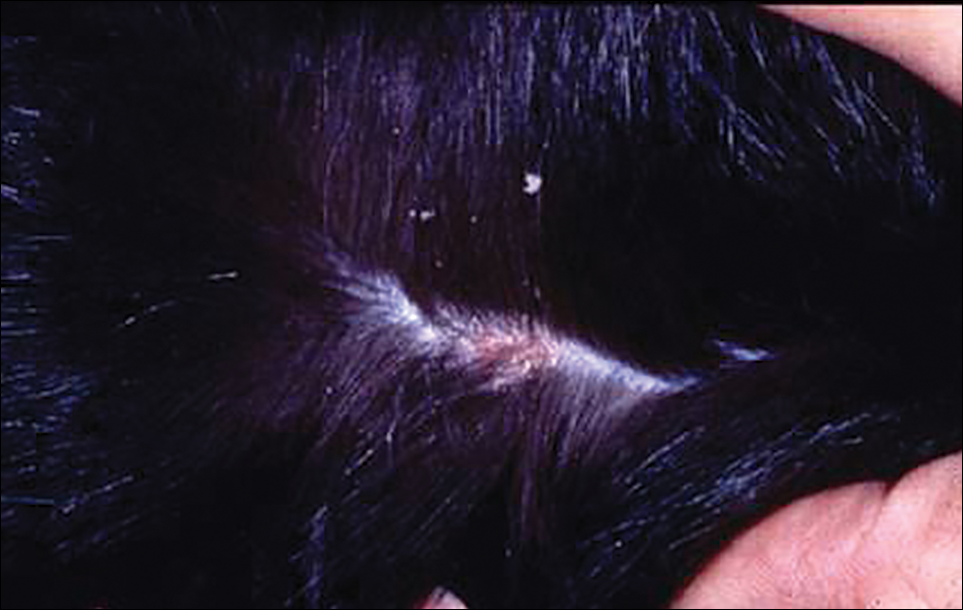
Cheyletiella are nonburrowing mites characterized by hooklike anterior palps (Figure 1) that have a worldwide distribution. Human dermatitis is the result of contact with an affected animal and may present as papular or bullous lesions. Cheyletiella blakei affects cats, Cheyletiella parasitovorax is found on rabbits, and Cheyletiella yasguri is found on dogs. The mites live in the outer layer of the epidermis of the host animal and feed on surface debris and tissue fluids.1 They complete an entire 35-day life cycle on a single animal host. The larval, nymph, and adult male mites die within 48 hours of separation from a host. The female mite and possibly the eggs can live up to 10 days off the host, which makes environmental decontamination a critical part of pest control.2 In animals, the mite often produces a subtle dermatitis sometimes called walking dandruff (Figure 2).3 Affected animals also can be asymptomatic, and up to 50% of rabbits in commercial colonies may harbor Cheyletiella or other mites.4
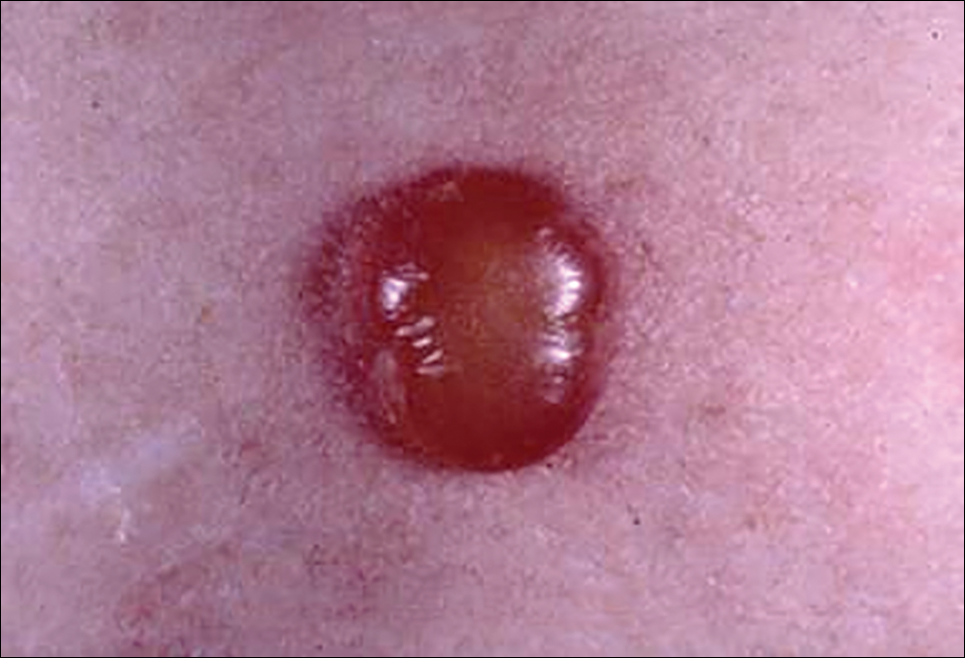
The typical human patient with Cheyletiella-associated dermatitis is a female 40 years or younger who presents with grouped pruritic papules.5 Although papules usually are grouped on exposed areas, they also may be widespread.6,7 Bullous eruptions caused by Cheyletiella mites may mimic those found in immunobullous diseases (Figure 3).8 Children may experience widespread dermatitis after taking a nap where a dog has slept.9 Pet owners, farmers, and veterinarians frequently present with zoonotic mite-induced dermatitis.10 Arthralgia and peripheral eosinophilia caused by Cheyletiella infestation also has been reported.11
Management of Affected Pets
In a case of human infestation resulting from an affected pet, the implicated pet should be evaluated by a qualified veterinarian. Various diagnostic techniques for animals have been used, including adhesive tape preparations.12 A rapid knockdown insecticidal spray marketed for use on animals has been used to facilitate collection of mites, but some pets may be susceptible to toxicity from insecticides. The scaly area should be carefully brushed with a toothbrush or fine-tooth comb, and all scales, crust, and hair collected should be placed in a resealable plastic storage bag. When alcohol is added to the bag, most contents will sink, but the mites tend to float. Vacuum cleaners fitted with in-line filters also have been used to collect mites. The filter samples can be treated with hot potassium hydroxide, then floated in a concentrated sugar solution to collect the ectoparasites.13 Often, a straightforward approach using a #10 blade to provide a skin scraping from the animal in question is effective.14
Various treatment modalities may be employed by the veterinarian, including dips or shampoos, as well as fipronil.15,16 A single application of fipronil 10% has been shown to be highly effective in the elimination of mites after a single application in cats.17 Oral ivermectin and topical amitraz also have been used.18,19 A veterinarian should treat the animals, as some are more susceptible to toxicity from topical or systemic agents.
Treatment in Humans
Cheyletiella infestations in humans usually are self-limited and resolve within a few weeks after treatment of the source animal. Symptomatic treatment with antipruritic medications and topical steroids may be of use while awaiting resolution. Identification and treatment of the vector is key to eliminating the infestation and preventing recurrence.
- Angarano DW, Parish LC. Comparative dermatology: parasitic disorders. Clin Dermatol. 1994;12:543-550.
- Kunkle GA, Miller WH Jr. Cheyletiella infestation in humans. Arch Dermatol. 1980;116:1345.
- Rivers JK, Martin J, Pukay B. Walking dandruff and Cheyletiella dermatitis. J Am Acad Dermatol. 1986;15:1130-1133.
- Flatt RE, Wiemers J. A survey of fur mites in domestic rabbits. Lab Animal Sci. 1976;26:758-761.
- Lee BW. Cheyletiella dermatitis: a report of fourteen cases. Cutis. 1991;47:111-114.
- Cohen SR. Cheyletiella dermatitis. A mite infestation of rabbit, cat, dog and man. Arch Dermatol. 1980;116:435-437.
- Bradrup F, Andersen KE, Kristensen S. Infection in man and dog with the mite, Cheyletiella yasguri Smiley [in German]. Hautarzt. 1979;30:497-500.
- Cvancara JL, Elston DM. Bullous eruption in a patient with systemic lupus erythematosus: mite dermatitis caused by Cheyletiella blakei. J Am Acad Dermatol. 1997;37:265-267.
- Shelley ED, Shelley WB, Pula JF, et al. The diagnostic challenge of nonburrowing mite bites. Cheyletiella yasguri. JAMA. 1984;251:2690-2691.
- Beck W. Farm animals as disease vectors of parasitic epizoonoses and zoophilic dermatophytes and their importance in dermatology [in German]. Hautartz. 1999;50:621-628.
- Dobrosavljevic DD, Popovic ND, Radovanovic SS. Systemic manifestations of Cheyletiella infestation in man. Int J Dermatol. 2007;46:397-399.
- Ottenschot TR, Gil D. Cheyletiellosis in long-haired cats. Tijdschr Diergeneeskd. 1978;103:1104-1108.
- Klayman E, Schillhorn van Veen TW. Diagnosis of ectoparasitism. Mod Vet Pract. 1981;62:767-771.
- Milley C, Dryden M, Rosenkrantz W, et al. Comparison of parasitic mite retrieval methods in a population of community cats [published online Jun 3, 2016]. J Feline Med Surg. pii:1098612X16650717.
- McKeever PJ, Allen SK. Dermatitis associated with Cheyletiella infestation in cats. J Am Vet Med Assoc. 1979;174:718-720.
- Chadwick AJ. Use of a 0.25 per cent fipronil pump spray formulation to treat canine cheyletiellosis. J Small Anim Pract. 1997;38:261-262.
- Scarampella F, Pollmeier M, Visser M, et al. Efficacy of fipronil in the treatment of feline cheyletiellosis. Vet Parasitol. 2005;129:333-339.
- Folz SD, Kakuk TJ, Henke CL, et al. Clinical evaluation of amitraz for treatment of canine scabies. Mod Vet Pract. 1984;65:597-600.
- Dourmishev AL, Dourmishev LA, Schwartz RA. Ivermectin: pharmacology and application in dermatology. Int J Dermatol. 2005;44:981-988.
Identifying Characteristics and Disease Transmission
Cheyletiella are nonburrowing mites characterized by hooklike anterior palps (Figure 1) that have a worldwide distribution. Human dermatitis is the result of contact with an affected animal and may present as papular or bullous lesions. Cheyletiella blakei affects cats, Cheyletiella parasitovorax is found on rabbits, and Cheyletiella yasguri is found on dogs. The mites live in the outer layer of the epidermis of the host animal and feed on surface debris and tissue fluids.1 They complete an entire 35-day life cycle on a single animal host. The larval, nymph, and adult male mites die within 48 hours of separation from a host. The female mite and possibly the eggs can live up to 10 days off the host, which makes environmental decontamination a critical part of pest control.2 In animals, the mite often produces a subtle dermatitis sometimes called walking dandruff (Figure 2).3 Affected animals also can be asymptomatic, and up to 50% of rabbits in commercial colonies may harbor Cheyletiella or other mites.4


The typical human patient with Cheyletiella-associated dermatitis is a female 40 years or younger who presents with grouped pruritic papules.5 Although papules usually are grouped on exposed areas, they also may be widespread.6,7 Bullous eruptions caused by Cheyletiella mites may mimic those found in immunobullous diseases (Figure 3).8 Children may experience widespread dermatitis after taking a nap where a dog has slept.9 Pet owners, farmers, and veterinarians frequently present with zoonotic mite-induced dermatitis.10 Arthralgia and peripheral eosinophilia caused by Cheyletiella infestation also has been reported.11

Management of Affected Pets
In a case of human infestation resulting from an affected pet, the implicated pet should be evaluated by a qualified veterinarian. Various diagnostic techniques for animals have been used, including adhesive tape preparations.12 A rapid knockdown insecticidal spray marketed for use on animals has been used to facilitate collection of mites, but some pets may be susceptible to toxicity from insecticides. The scaly area should be carefully brushed with a toothbrush or fine-tooth comb, and all scales, crust, and hair collected should be placed in a resealable plastic storage bag. When alcohol is added to the bag, most contents will sink, but the mites tend to float. Vacuum cleaners fitted with in-line filters also have been used to collect mites. The filter samples can be treated with hot potassium hydroxide, then floated in a concentrated sugar solution to collect the ectoparasites.13 Often, a straightforward approach using a #10 blade to provide a skin scraping from the animal in question is effective.14
Various treatment modalities may be employed by the veterinarian, including dips or shampoos, as well as fipronil.15,16 A single application of fipronil 10% has been shown to be highly effective in the elimination of mites after a single application in cats.17 Oral ivermectin and topical amitraz also have been used.18,19 A veterinarian should treat the animals, as some are more susceptible to toxicity from topical or systemic agents.
Treatment in Humans
Cheyletiella infestations in humans usually are self-limited and resolve within a few weeks after treatment of the source animal. Symptomatic treatment with antipruritic medications and topical steroids may be of use while awaiting resolution. Identification and treatment of the vector is key to eliminating the infestation and preventing recurrence.
Identifying Characteristics and Disease Transmission
Cheyletiella are nonburrowing mites characterized by hooklike anterior palps (Figure 1) that have a worldwide distribution. Human dermatitis is the result of contact with an affected animal and may present as papular or bullous lesions. Cheyletiella blakei affects cats, Cheyletiella parasitovorax is found on rabbits, and Cheyletiella yasguri is found on dogs. The mites live in the outer layer of the epidermis of the host animal and feed on surface debris and tissue fluids.1 They complete an entire 35-day life cycle on a single animal host. The larval, nymph, and adult male mites die within 48 hours of separation from a host. The female mite and possibly the eggs can live up to 10 days off the host, which makes environmental decontamination a critical part of pest control.2 In animals, the mite often produces a subtle dermatitis sometimes called walking dandruff (Figure 2).3 Affected animals also can be asymptomatic, and up to 50% of rabbits in commercial colonies may harbor Cheyletiella or other mites.4


The typical human patient with Cheyletiella-associated dermatitis is a female 40 years or younger who presents with grouped pruritic papules.5 Although papules usually are grouped on exposed areas, they also may be widespread.6,7 Bullous eruptions caused by Cheyletiella mites may mimic those found in immunobullous diseases (Figure 3).8 Children may experience widespread dermatitis after taking a nap where a dog has slept.9 Pet owners, farmers, and veterinarians frequently present with zoonotic mite-induced dermatitis.10 Arthralgia and peripheral eosinophilia caused by Cheyletiella infestation also has been reported.11

Management of Affected Pets
In a case of human infestation resulting from an affected pet, the implicated pet should be evaluated by a qualified veterinarian. Various diagnostic techniques for animals have been used, including adhesive tape preparations.12 A rapid knockdown insecticidal spray marketed for use on animals has been used to facilitate collection of mites, but some pets may be susceptible to toxicity from insecticides. The scaly area should be carefully brushed with a toothbrush or fine-tooth comb, and all scales, crust, and hair collected should be placed in a resealable plastic storage bag. When alcohol is added to the bag, most contents will sink, but the mites tend to float. Vacuum cleaners fitted with in-line filters also have been used to collect mites. The filter samples can be treated with hot potassium hydroxide, then floated in a concentrated sugar solution to collect the ectoparasites.13 Often, a straightforward approach using a #10 blade to provide a skin scraping from the animal in question is effective.14
Various treatment modalities may be employed by the veterinarian, including dips or shampoos, as well as fipronil.15,16 A single application of fipronil 10% has been shown to be highly effective in the elimination of mites after a single application in cats.17 Oral ivermectin and topical amitraz also have been used.18,19 A veterinarian should treat the animals, as some are more susceptible to toxicity from topical or systemic agents.
Treatment in Humans
Cheyletiella infestations in humans usually are self-limited and resolve within a few weeks after treatment of the source animal. Symptomatic treatment with antipruritic medications and topical steroids may be of use while awaiting resolution. Identification and treatment of the vector is key to eliminating the infestation and preventing recurrence.
- Angarano DW, Parish LC. Comparative dermatology: parasitic disorders. Clin Dermatol. 1994;12:543-550.
- Kunkle GA, Miller WH Jr. Cheyletiella infestation in humans. Arch Dermatol. 1980;116:1345.
- Rivers JK, Martin J, Pukay B. Walking dandruff and Cheyletiella dermatitis. J Am Acad Dermatol. 1986;15:1130-1133.
- Flatt RE, Wiemers J. A survey of fur mites in domestic rabbits. Lab Animal Sci. 1976;26:758-761.
- Lee BW. Cheyletiella dermatitis: a report of fourteen cases. Cutis. 1991;47:111-114.
- Cohen SR. Cheyletiella dermatitis. A mite infestation of rabbit, cat, dog and man. Arch Dermatol. 1980;116:435-437.
- Bradrup F, Andersen KE, Kristensen S. Infection in man and dog with the mite, Cheyletiella yasguri Smiley [in German]. Hautarzt. 1979;30:497-500.
- Cvancara JL, Elston DM. Bullous eruption in a patient with systemic lupus erythematosus: mite dermatitis caused by Cheyletiella blakei. J Am Acad Dermatol. 1997;37:265-267.
- Shelley ED, Shelley WB, Pula JF, et al. The diagnostic challenge of nonburrowing mite bites. Cheyletiella yasguri. JAMA. 1984;251:2690-2691.
- Beck W. Farm animals as disease vectors of parasitic epizoonoses and zoophilic dermatophytes and their importance in dermatology [in German]. Hautartz. 1999;50:621-628.
- Dobrosavljevic DD, Popovic ND, Radovanovic SS. Systemic manifestations of Cheyletiella infestation in man. Int J Dermatol. 2007;46:397-399.
- Ottenschot TR, Gil D. Cheyletiellosis in long-haired cats. Tijdschr Diergeneeskd. 1978;103:1104-1108.
- Klayman E, Schillhorn van Veen TW. Diagnosis of ectoparasitism. Mod Vet Pract. 1981;62:767-771.
- Milley C, Dryden M, Rosenkrantz W, et al. Comparison of parasitic mite retrieval methods in a population of community cats [published online Jun 3, 2016]. J Feline Med Surg. pii:1098612X16650717.
- McKeever PJ, Allen SK. Dermatitis associated with Cheyletiella infestation in cats. J Am Vet Med Assoc. 1979;174:718-720.
- Chadwick AJ. Use of a 0.25 per cent fipronil pump spray formulation to treat canine cheyletiellosis. J Small Anim Pract. 1997;38:261-262.
- Scarampella F, Pollmeier M, Visser M, et al. Efficacy of fipronil in the treatment of feline cheyletiellosis. Vet Parasitol. 2005;129:333-339.
- Folz SD, Kakuk TJ, Henke CL, et al. Clinical evaluation of amitraz for treatment of canine scabies. Mod Vet Pract. 1984;65:597-600.
- Dourmishev AL, Dourmishev LA, Schwartz RA. Ivermectin: pharmacology and application in dermatology. Int J Dermatol. 2005;44:981-988.
- Angarano DW, Parish LC. Comparative dermatology: parasitic disorders. Clin Dermatol. 1994;12:543-550.
- Kunkle GA, Miller WH Jr. Cheyletiella infestation in humans. Arch Dermatol. 1980;116:1345.
- Rivers JK, Martin J, Pukay B. Walking dandruff and Cheyletiella dermatitis. J Am Acad Dermatol. 1986;15:1130-1133.
- Flatt RE, Wiemers J. A survey of fur mites in domestic rabbits. Lab Animal Sci. 1976;26:758-761.
- Lee BW. Cheyletiella dermatitis: a report of fourteen cases. Cutis. 1991;47:111-114.
- Cohen SR. Cheyletiella dermatitis. A mite infestation of rabbit, cat, dog and man. Arch Dermatol. 1980;116:435-437.
- Bradrup F, Andersen KE, Kristensen S. Infection in man and dog with the mite, Cheyletiella yasguri Smiley [in German]. Hautarzt. 1979;30:497-500.
- Cvancara JL, Elston DM. Bullous eruption in a patient with systemic lupus erythematosus: mite dermatitis caused by Cheyletiella blakei. J Am Acad Dermatol. 1997;37:265-267.
- Shelley ED, Shelley WB, Pula JF, et al. The diagnostic challenge of nonburrowing mite bites. Cheyletiella yasguri. JAMA. 1984;251:2690-2691.
- Beck W. Farm animals as disease vectors of parasitic epizoonoses and zoophilic dermatophytes and their importance in dermatology [in German]. Hautartz. 1999;50:621-628.
- Dobrosavljevic DD, Popovic ND, Radovanovic SS. Systemic manifestations of Cheyletiella infestation in man. Int J Dermatol. 2007;46:397-399.
- Ottenschot TR, Gil D. Cheyletiellosis in long-haired cats. Tijdschr Diergeneeskd. 1978;103:1104-1108.
- Klayman E, Schillhorn van Veen TW. Diagnosis of ectoparasitism. Mod Vet Pract. 1981;62:767-771.
- Milley C, Dryden M, Rosenkrantz W, et al. Comparison of parasitic mite retrieval methods in a population of community cats [published online Jun 3, 2016]. J Feline Med Surg. pii:1098612X16650717.
- McKeever PJ, Allen SK. Dermatitis associated with Cheyletiella infestation in cats. J Am Vet Med Assoc. 1979;174:718-720.
- Chadwick AJ. Use of a 0.25 per cent fipronil pump spray formulation to treat canine cheyletiellosis. J Small Anim Pract. 1997;38:261-262.
- Scarampella F, Pollmeier M, Visser M, et al. Efficacy of fipronil in the treatment of feline cheyletiellosis. Vet Parasitol. 2005;129:333-339.
- Folz SD, Kakuk TJ, Henke CL, et al. Clinical evaluation of amitraz for treatment of canine scabies. Mod Vet Pract. 1984;65:597-600.
- Dourmishev AL, Dourmishev LA, Schwartz RA. Ivermectin: pharmacology and application in dermatology. Int J Dermatol. 2005;44:981-988.
Practice Points
- Cheyletiella mites can cause a range of cutaneous and systemic symptoms in affected individuals.
- Diagnosis can be difficult and requires a high level of suspicion, with inquiries directed at animal exposures.
- Identification of the animal vector and treatment by a knowledgeable veterinarian is necessary to prevent recurrence in humans.
Flesh-Colored Nodule With Underlying Sclerotic Plaque
The Diagnosis: Collision Tumor
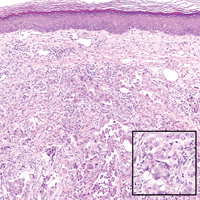
Excisional biopsy and histopathological examination demonstrated a collision tumor composed of a benign intradermal melanocytic nevus, tumor of follicular infundibulum, and an underlying sclerosing epithelial neoplasm, with a differential diagnosis of desmoplastic trichoepithelioma, morpheaform basal cell carcinoma, and microcystic adnexal carcinoma (Figure).
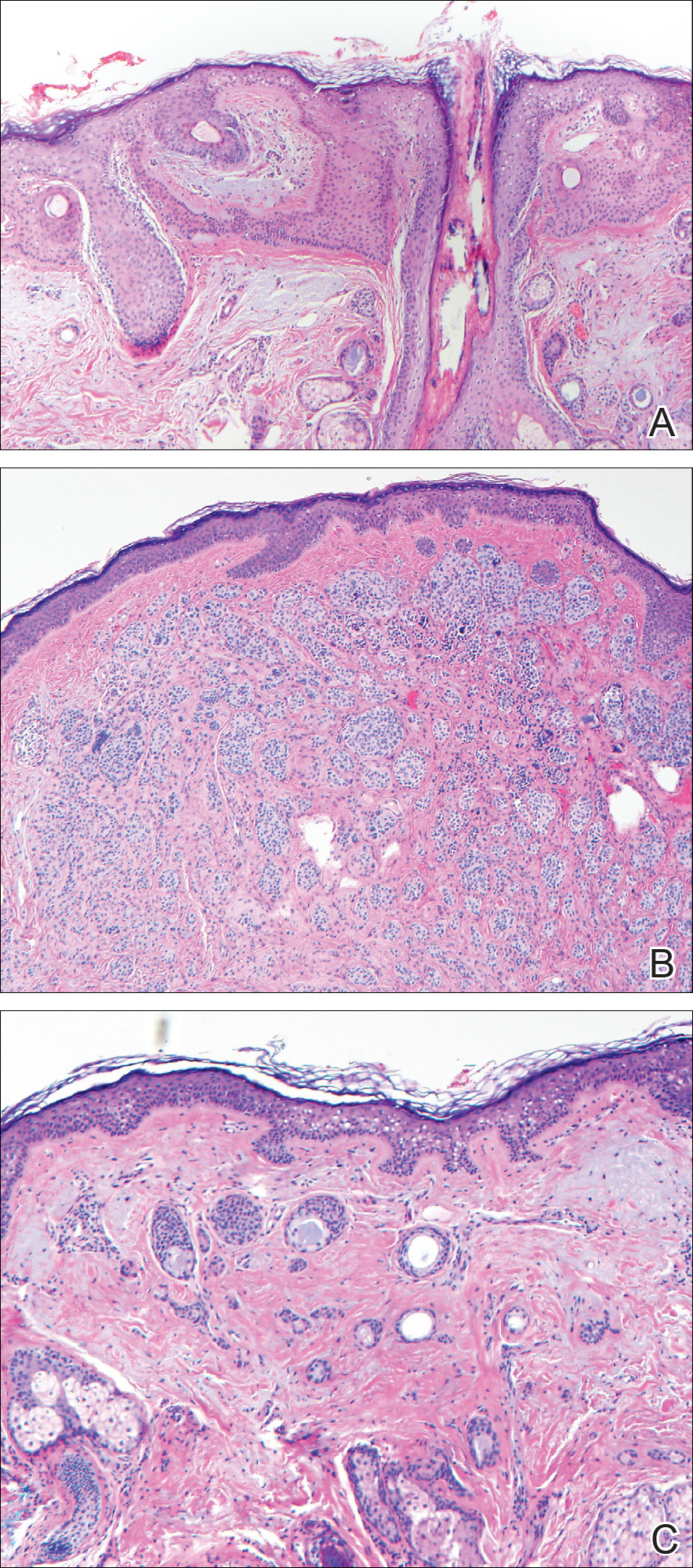
Common acquired melanocytic nevus presents clinically as a macule, papule, or nodule with smooth regular borders. The pigmented variant displays an evenly distributed pigment on the lesion. Intradermal melanocytic nevus often presents as a flesh-colored nodule, as in our case. Histopathologically, benign intradermal nevus typically is composed of a proliferation of melanocytes that exhibit dispersion as they go deeper in the dermis and maturation that manifests as melanocytes becoming smaller and more spindled in the deeper portions of the lesion.1 These 2 characteristics plus the bland cytology seen in the present case confirm the benign characteristic of this lesion (Figure, B).
In addition to the benign intradermal melanocytic nevus, an adjacent tumor of follicular infundibulum was noted. Tumor of follicular infundibulum is a rare adnexal tumor. It occurs frequently on the head and neck and shows some female predominance.2,3 Multiple lesions and eruptive lesions are rare forms that also have been reported.4 Histopathologically, the tumor demonstrates an epithelial plate that is present in the papillary dermis and is connected to the epidermis at multiple points with attachment to the follicular outer root sheath. Peripheral palisading is characteristically present above an eosinophilic basement membrane (Figure, A). Rare reports have documented sebaceous and eccrine differentiation.5,6
Tumor of follicular infundibulum has been reported to be associated with other tumors. Organoid nevus (nevus sebaceous), trichilemmal tumor, and fibroma have been reported to occur as a collision tumor with tumor of follicular infundibulum. An association with Cowden disease also has been described.7 Biopsies that represent partial samples should be interpreted cautiously, as step sections can reveal basal cell carcinoma.
The term sclerosing epithelial neoplasm describes tumors that share a paisley tielike epithelial pattern and sclerotic stroma. Small specimens often require clinicopathologic correlation (Figure, C). The differential diagnosis includes morpheaform basal cell carcinoma, desmoplastic trichoepithelioma, and microcystic adnexal carcinoma. A panel of stains using Ber-EP4, PHLDA1, cytokeratin 15, and cytokeratin 19 has been proposed to help differentiate these entities.8 CD34 and cytokeratin 20 also have been used with varying success in small specimens.9,10
- Ferringer T, Peckham S, Ko CJ, et al. Melanocytic neoplasms. In: Elston DM, Ferringer T, eds. Dermatopathology. 2nd ed. Philadelphia, PA: Elsevier Saunders; 2014:105-109.
- Headington JT. Tumors of the hair follicle. Am J Pathol. 1976;85:480-505.
- Davis DA, Cohen PR. Hair follicle nevus: case report and review of the literature. Pediatr Dermatol. 1996;13:135-138.
- Ikeda S, Kawada J, Yaguchi H, et al. A case of unilateral, systematized linear hair follicle nevi associated with epidermal nevus-like lesions. Dermatology. 2003;206:172-174.
- Mehregan AH. Hair follicle tumors of the skin. J Cutan Pathol. 1985;12:189-195.
- Mahalingam M, Bhawan J, Finn R, et al. Tumor of the follicular infundibulum with sebaceous differentiation. J Cutan Pathol. 2001;28:314-317.
- Cribier B, Grosshans E. Tumor of the follicular infundibulum: a clinicopathologic study. J Am Acad Dermatol. 1995;33:979-984.
- Sellheyer K, Nelson P, Kutzner H, et al. The immunohistochemical differential diagnosis of microcystic adnexal carcinoma, desmoplastic trichoepithelioma and morpheaform basal cell carcinoma using BerEP4 and stem cell markers. J Cutan Pathol. 2013;40:363-370.
- Abesamis-Cubillan E, El-Shabrawi-Caelen L, LeBoit PE. Merkel cells and sclerosing epithelial neoplasms. Am J Dermatopathol. 2000;22:311-315.
- Smith KJ, Williams J, Corbett D, et al. Microcystic adnexal carcinoma: an immunohistochemical study including markers of proliferation and apoptosis. Am J Surg Pathol. 2001;25:464-471.
The Diagnosis: Collision Tumor
Excisional biopsy and histopathological examination demonstrated a collision tumor composed of a benign intradermal melanocytic nevus, tumor of follicular infundibulum, and an underlying sclerosing epithelial neoplasm, with a differential diagnosis of desmoplastic trichoepithelioma, morpheaform basal cell carcinoma, and microcystic adnexal carcinoma (Figure).

Common acquired melanocytic nevus presents clinically as a macule, papule, or nodule with smooth regular borders. The pigmented variant displays an evenly distributed pigment on the lesion. Intradermal melanocytic nevus often presents as a flesh-colored nodule, as in our case. Histopathologically, benign intradermal nevus typically is composed of a proliferation of melanocytes that exhibit dispersion as they go deeper in the dermis and maturation that manifests as melanocytes becoming smaller and more spindled in the deeper portions of the lesion.1 These 2 characteristics plus the bland cytology seen in the present case confirm the benign characteristic of this lesion (Figure, B).
In addition to the benign intradermal melanocytic nevus, an adjacent tumor of follicular infundibulum was noted. Tumor of follicular infundibulum is a rare adnexal tumor. It occurs frequently on the head and neck and shows some female predominance.2,3 Multiple lesions and eruptive lesions are rare forms that also have been reported.4 Histopathologically, the tumor demonstrates an epithelial plate that is present in the papillary dermis and is connected to the epidermis at multiple points with attachment to the follicular outer root sheath. Peripheral palisading is characteristically present above an eosinophilic basement membrane (Figure, A). Rare reports have documented sebaceous and eccrine differentiation.5,6
Tumor of follicular infundibulum has been reported to be associated with other tumors. Organoid nevus (nevus sebaceous), trichilemmal tumor, and fibroma have been reported to occur as a collision tumor with tumor of follicular infundibulum. An association with Cowden disease also has been described.7 Biopsies that represent partial samples should be interpreted cautiously, as step sections can reveal basal cell carcinoma.
The term sclerosing epithelial neoplasm describes tumors that share a paisley tielike epithelial pattern and sclerotic stroma. Small specimens often require clinicopathologic correlation (Figure, C). The differential diagnosis includes morpheaform basal cell carcinoma, desmoplastic trichoepithelioma, and microcystic adnexal carcinoma. A panel of stains using Ber-EP4, PHLDA1, cytokeratin 15, and cytokeratin 19 has been proposed to help differentiate these entities.8 CD34 and cytokeratin 20 also have been used with varying success in small specimens.9,10
The Diagnosis: Collision Tumor
Excisional biopsy and histopathological examination demonstrated a collision tumor composed of a benign intradermal melanocytic nevus, tumor of follicular infundibulum, and an underlying sclerosing epithelial neoplasm, with a differential diagnosis of desmoplastic trichoepithelioma, morpheaform basal cell carcinoma, and microcystic adnexal carcinoma (Figure).

Common acquired melanocytic nevus presents clinically as a macule, papule, or nodule with smooth regular borders. The pigmented variant displays an evenly distributed pigment on the lesion. Intradermal melanocytic nevus often presents as a flesh-colored nodule, as in our case. Histopathologically, benign intradermal nevus typically is composed of a proliferation of melanocytes that exhibit dispersion as they go deeper in the dermis and maturation that manifests as melanocytes becoming smaller and more spindled in the deeper portions of the lesion.1 These 2 characteristics plus the bland cytology seen in the present case confirm the benign characteristic of this lesion (Figure, B).
In addition to the benign intradermal melanocytic nevus, an adjacent tumor of follicular infundibulum was noted. Tumor of follicular infundibulum is a rare adnexal tumor. It occurs frequently on the head and neck and shows some female predominance.2,3 Multiple lesions and eruptive lesions are rare forms that also have been reported.4 Histopathologically, the tumor demonstrates an epithelial plate that is present in the papillary dermis and is connected to the epidermis at multiple points with attachment to the follicular outer root sheath. Peripheral palisading is characteristically present above an eosinophilic basement membrane (Figure, A). Rare reports have documented sebaceous and eccrine differentiation.5,6
Tumor of follicular infundibulum has been reported to be associated with other tumors. Organoid nevus (nevus sebaceous), trichilemmal tumor, and fibroma have been reported to occur as a collision tumor with tumor of follicular infundibulum. An association with Cowden disease also has been described.7 Biopsies that represent partial samples should be interpreted cautiously, as step sections can reveal basal cell carcinoma.
The term sclerosing epithelial neoplasm describes tumors that share a paisley tielike epithelial pattern and sclerotic stroma. Small specimens often require clinicopathologic correlation (Figure, C). The differential diagnosis includes morpheaform basal cell carcinoma, desmoplastic trichoepithelioma, and microcystic adnexal carcinoma. A panel of stains using Ber-EP4, PHLDA1, cytokeratin 15, and cytokeratin 19 has been proposed to help differentiate these entities.8 CD34 and cytokeratin 20 also have been used with varying success in small specimens.9,10
- Ferringer T, Peckham S, Ko CJ, et al. Melanocytic neoplasms. In: Elston DM, Ferringer T, eds. Dermatopathology. 2nd ed. Philadelphia, PA: Elsevier Saunders; 2014:105-109.
- Headington JT. Tumors of the hair follicle. Am J Pathol. 1976;85:480-505.
- Davis DA, Cohen PR. Hair follicle nevus: case report and review of the literature. Pediatr Dermatol. 1996;13:135-138.
- Ikeda S, Kawada J, Yaguchi H, et al. A case of unilateral, systematized linear hair follicle nevi associated with epidermal nevus-like lesions. Dermatology. 2003;206:172-174.
- Mehregan AH. Hair follicle tumors of the skin. J Cutan Pathol. 1985;12:189-195.
- Mahalingam M, Bhawan J, Finn R, et al. Tumor of the follicular infundibulum with sebaceous differentiation. J Cutan Pathol. 2001;28:314-317.
- Cribier B, Grosshans E. Tumor of the follicular infundibulum: a clinicopathologic study. J Am Acad Dermatol. 1995;33:979-984.
- Sellheyer K, Nelson P, Kutzner H, et al. The immunohistochemical differential diagnosis of microcystic adnexal carcinoma, desmoplastic trichoepithelioma and morpheaform basal cell carcinoma using BerEP4 and stem cell markers. J Cutan Pathol. 2013;40:363-370.
- Abesamis-Cubillan E, El-Shabrawi-Caelen L, LeBoit PE. Merkel cells and sclerosing epithelial neoplasms. Am J Dermatopathol. 2000;22:311-315.
- Smith KJ, Williams J, Corbett D, et al. Microcystic adnexal carcinoma: an immunohistochemical study including markers of proliferation and apoptosis. Am J Surg Pathol. 2001;25:464-471.
- Ferringer T, Peckham S, Ko CJ, et al. Melanocytic neoplasms. In: Elston DM, Ferringer T, eds. Dermatopathology. 2nd ed. Philadelphia, PA: Elsevier Saunders; 2014:105-109.
- Headington JT. Tumors of the hair follicle. Am J Pathol. 1976;85:480-505.
- Davis DA, Cohen PR. Hair follicle nevus: case report and review of the literature. Pediatr Dermatol. 1996;13:135-138.
- Ikeda S, Kawada J, Yaguchi H, et al. A case of unilateral, systematized linear hair follicle nevi associated with epidermal nevus-like lesions. Dermatology. 2003;206:172-174.
- Mehregan AH. Hair follicle tumors of the skin. J Cutan Pathol. 1985;12:189-195.
- Mahalingam M, Bhawan J, Finn R, et al. Tumor of the follicular infundibulum with sebaceous differentiation. J Cutan Pathol. 2001;28:314-317.
- Cribier B, Grosshans E. Tumor of the follicular infundibulum: a clinicopathologic study. J Am Acad Dermatol. 1995;33:979-984.
- Sellheyer K, Nelson P, Kutzner H, et al. The immunohistochemical differential diagnosis of microcystic adnexal carcinoma, desmoplastic trichoepithelioma and morpheaform basal cell carcinoma using BerEP4 and stem cell markers. J Cutan Pathol. 2013;40:363-370.
- Abesamis-Cubillan E, El-Shabrawi-Caelen L, LeBoit PE. Merkel cells and sclerosing epithelial neoplasms. Am J Dermatopathol. 2000;22:311-315.
- Smith KJ, Williams J, Corbett D, et al. Microcystic adnexal carcinoma: an immunohistochemical study including markers of proliferation and apoptosis. Am J Surg Pathol. 2001;25:464-471.
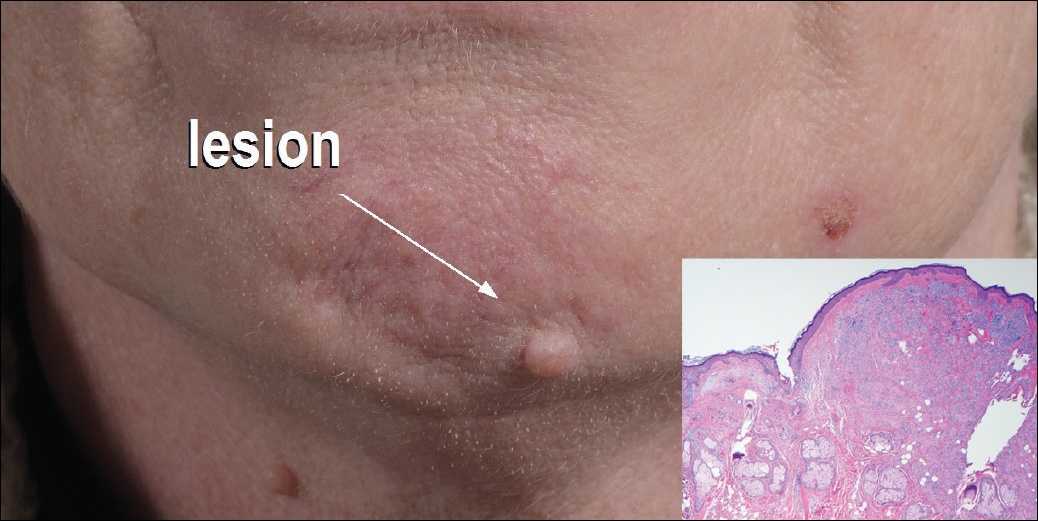
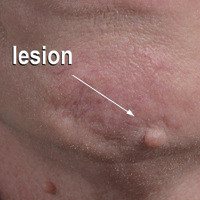
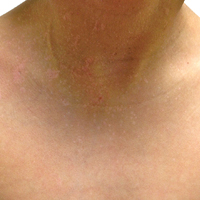
A 54-year-old man presented with a flesh-colored lesion on the chin. The nodule measured 0.6 cm in diameter. There was an underlying sclerotic plaque with indistinct borders.
Bluish Gray Hyperpigmentation on the Face and Neck
The Diagnosis: Erythema Dyschromicum Perstans
Erythema dyschromicum perstans (EDP), also referred to as ashy dermatosis, was first described by Ramirez1 in 1957 who labeled the patients los cenicientos (the ashen ones). It preferentially affects women in the second decade of life; however, patients of all ages can be affected, with reported cases occurring in children as young as 2 years of age.2 Most patients have Fitzpatrick skin type IV, mainly Amerindian, Hispanic South Asian, and Southwest Asian; however, there are cases reported worldwide.3 A genetic predisposition is proposed, as major histocompatibility complex genes associated with HLA-DR4⁎0407 are frequent in Mexican patients with ashy dermatosis and in the Amerindian population.4
The etiology of EDP is unknown. Various contributing factors have been reported including alimentary, occupational, and climatic factors,5,6 yet none have been conclusively demonstrated. High expression of CD36 (thrombospondin receptor not found in normal skin) in spinous and granular layers, CD94 (cytotoxic cell marker) in the basal cell layer and in the inflammatory dermal infiltrate,7 and focal keratinocytic expression of intercellular adhesion molecule I (CD54) in the active lesions of EDP, as well as the absence of these findings in normal skin, suggests an immunologic role in the development of the disease.8
Erythema dyschromicum perstans presents clinically with blue-gray hyperpigmented macules varying in size and shape and developing symmetrically in both sun-exposed and sun-protected areas of the face, neck, trunk, arms, and sometimes the dorsal hands (Figures 1 and 2). Notable sparing of the palms, soles, scalp, and mucous membranes occurs.
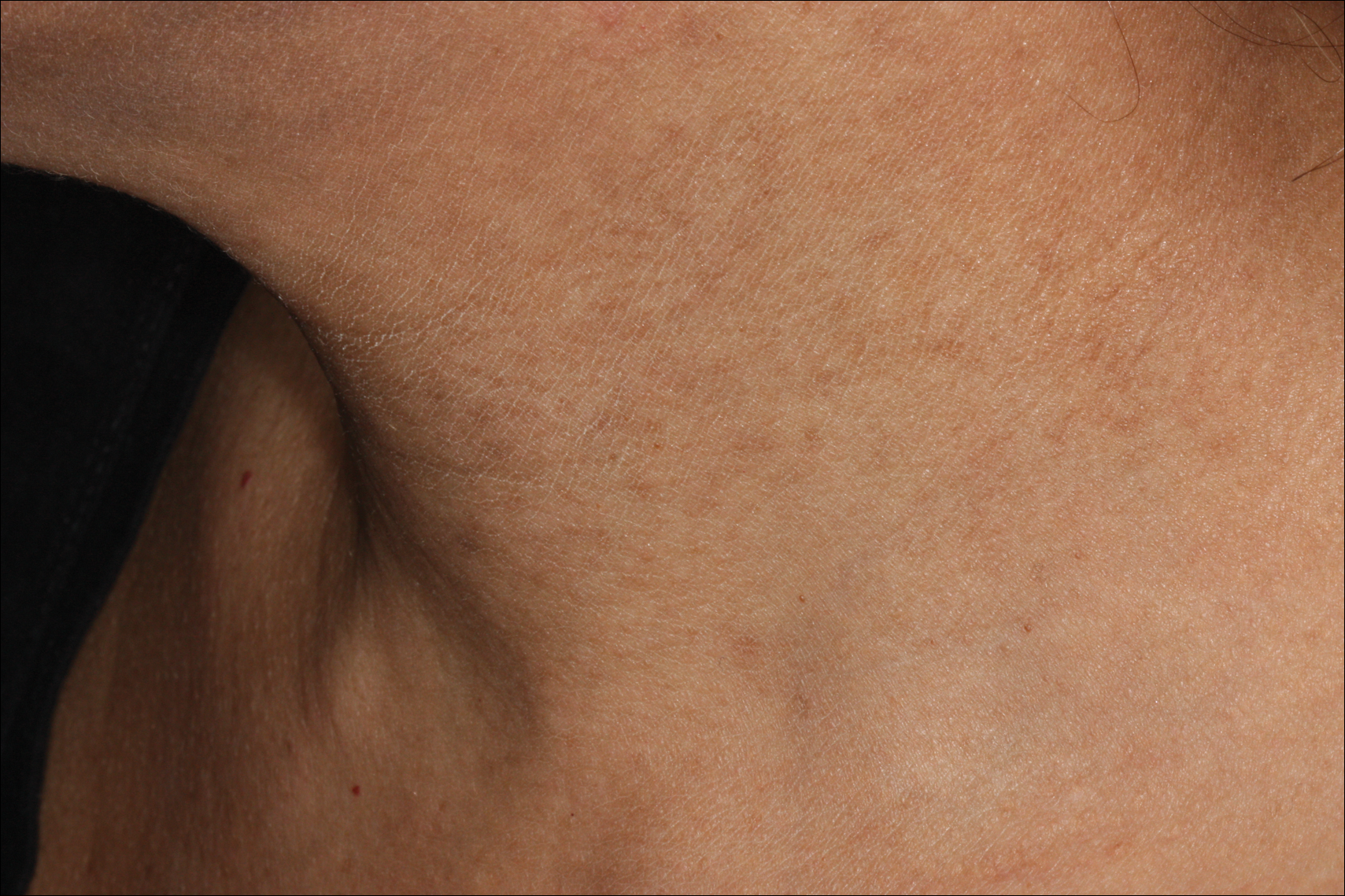
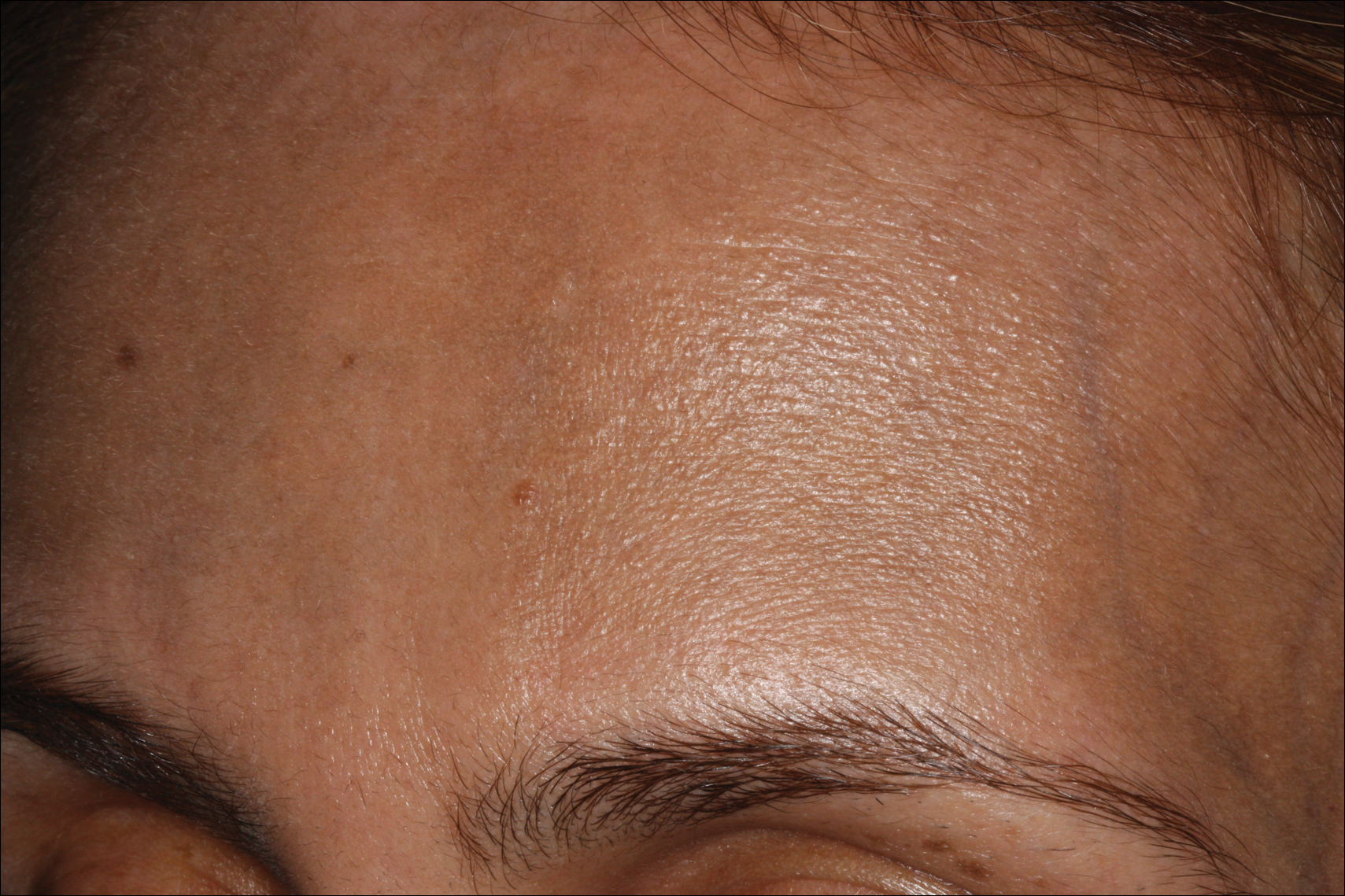
Occasionally, in the early active stage of the disease, elevated erythematous borders are noted surrounding the hyperpigmented macules. Eventually a hypopigmented halo develops after a prolonged duration of disease.9 The eruption typically is chronic and asymptomatic, though some cases may be pruritic.10
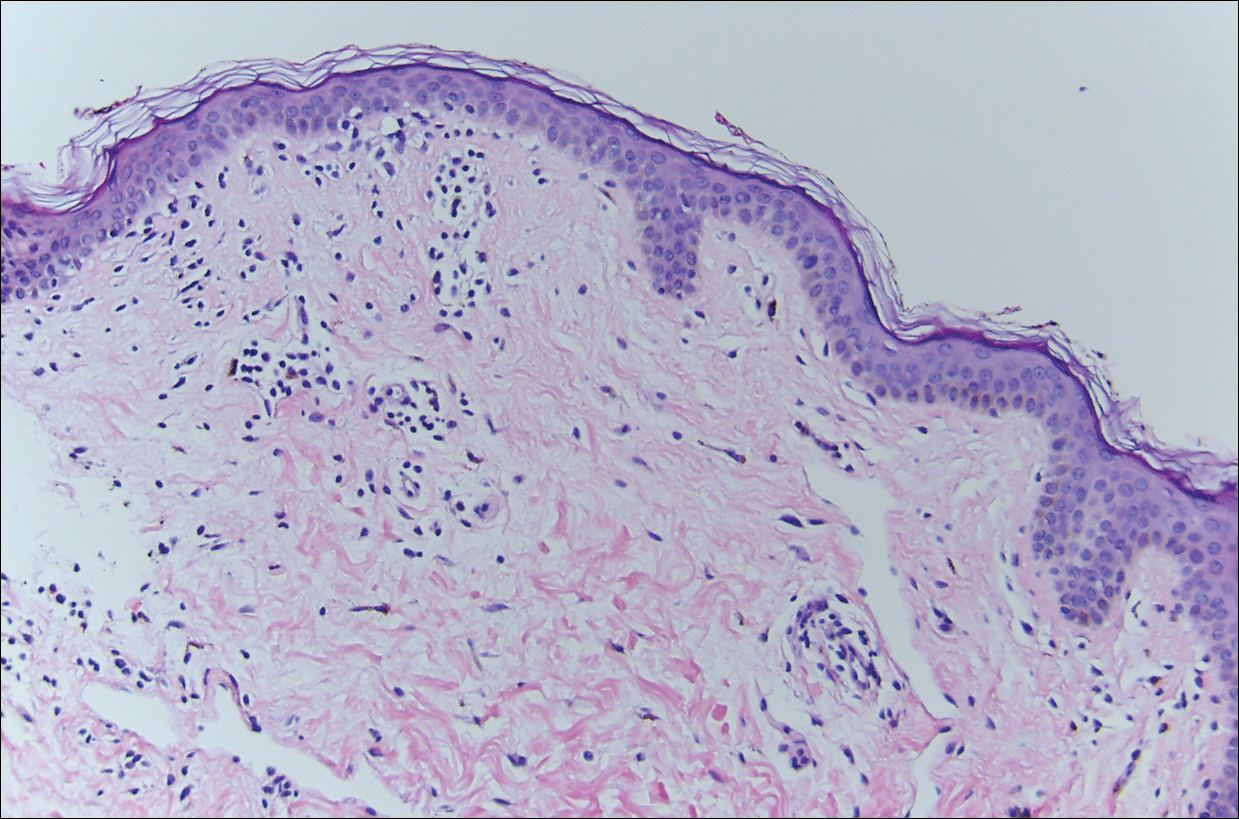
Histopathologically, the early lesions of EDP with an erythematous active border reveal lichenoid dermatitis with basal vacuolar change and occasional Civatte bodies. A mild to moderate perivascular lymphohistiocytic infiltrate admixed with melanophages can be seen in the papillary dermis (Figure 3). In older lesions, the inflammatory infiltrate is sparse, and pigment incontinence consistent with postinflammatory pigmentation is prominent, though melanophages extending deep into the reticular dermis may aid in distinguishing EDP from other causes of postinflammatory pigment alteration.7,11
Erythema dyschromicum perstans and lichen planus pigmentosus (LPP) may be indistinguishable histopathologically and may both be variants of lichen planus actinicus. Lichen planus pigmentosus often differs from EDP in that it presents with brown-black macules and patches often on the face and flexural areas. A subset of cases of LPP also may have mucous membrane involvement. The erythematous border that characterizes the active lesion of EDP is characteristically absent in LPP. In addition, pruritus often is reported with LPP. Direct immunofluorescence is not a beneficial tool in distinguishing the entities.12
Other differential diagnoses of predominantly facial hyperpigmentation include a lichenoid drug eruption; drug-induced hyperpigmentation (deposition disorder); postinflammatory hyperpigmentation following atopic dermatitis; contact dermatitis or photosensitivity reaction; early pinta; and cutaneous findings of systemic diseases manifesting with diffuse hyperpigmentation such as lupus erythematosus, dermatomyositis, hemochromatosis, and Addison disease. A detailed history including medication use, thorough clinical examination, and careful histopathologic evaluation will help distinguish these conditions.
Chrysiasis is a rare bluish to slate gray discoloration of the skin that predominantly occurs in sun-exposed areas. It is caused by chronic use of gold salts, which have been used to treat rheumatoid arthritis. UV light may contribute to induce the uptake of gold and subsequently stimulate tyrosinase activity.13 Histologic features of chrysiasis include dermal and perivascular gold deposition within the macrophages and endothelial cells as well as extracellular granules. It demonstrates an orange-red birefringence on fluorescent microscopy.14,15
Minocycline-induced hyperpigmentation is a well-recognized side effect of this drug. It is dose dependent and appears as a blue-black pigmentation that most frequently affects the shins, ankles, and arms.16 Three distinct types were documented: abnormal discoloration of the skin that has been linked to deposition of pigmented metabolites of minocycline producing blue-black pigmentation at the site of scarring or prior inflammation (type 1); blue-gray pigmentation affecting normal skin, mainly the legs (type 2); and elevated levels of melanin on the sun-exposed areas producing dirty skin syndrome (type 3).17,18
Topical and systemic corticosteroids, UV light therapy, oral dapsone, griseofulvin, retinoids, and clofazimine are reported as treatment options for ashy dermatosis, though results typically are disappointing.7
- Ramirez CO. Los cenicientos: problema clinica. In: Memoria del Primer Congresso Centroamericano de Dermatologica, December 5-8, 1957. San Salvador, El Salvador; 1957:122-130.
- Lee SJ, Chung KY. Erythema dyschromicum perstans in early childhood. J Dermatol. 1999;26:119-121.
- Homez-Chacin, Barroso C. On the etiopathogenic of the erythema dyschromicum perstans: possibility of a melanosis neurocutaneous. Dermatol Venez. 1996;4:149-151.
- Correa MC, Memije EV, Vargas-Alarcon G, et al. HLA-DR association with the genetic susceptibility to develop ashy dermatosis in Mexican Mestizo patients [published online November 20, 2006]. J Am Acad Dermatol. 2007;56:617-620.
- Jablonska S. Ingestion of ammonium nitrate as a possible cause of erythema dyschromicum perstans (ashy dermatosis). Dermatologica. 1975;150:287-291.
- Stevenson JR, Miura M. Erythema dyschromicum perstans (ashy dermatosis). Arch Dermatol. 1966;94:196-199.
- Baranda L, Torres-Alvarez B, Cortes-Franco R, et al. Involvement of cell adhesion and activation molecules in the pathogenesis of erythema dyschromicum perstans (ashy dermatitis). the effect of clofazimine therapy. Arch Dermatol. 1997;133:325-329.
- Vasquez-Ochoa LA, Isaza-Guzman DM, Orozco-Mora B, et al. Immunopathologic study of erythema dyschromicum perstans (ashy dermatosis). Int J Dermatol. 2006;45:937-941.
- Convit J, Kerdel-Vegas F, Roderiguez G. Erythema dyschromicum perstans: a hiltherto undescribed skin disease. J Invest Dermatol. 1961;36:457-462.
- Ono S, Miyachi Y, Kabashima K. Ashy dermatosis with prior pruritic and scaling skin lesions. J Dermatol. 2012;39:1103-1104.
- Sanchez NP, Pathak MA, Sato SS, et al. Circumscribed dermal melaninoses: classification, light, histochemical, and electron microscopic studies on three patients with the erythema dyschromicum perstans type. Int J Dermatol. 1982;21:25-32.
- Vega ME, Waxtein L, Arenas R, et al. Ashy dermatosis and lichen planus pigmentosus: a clinicopathologic study of 31 cases. Int J Dermatol. 1992;31:90-94.
- Ahmed SV, Sajjan R. Chrysiasis: a gold "curse!" [published online May 21, 2009]. BMJ Case Rep. 2009;2009.
- Fiscus V, Hankinson A, Alweis R. Minocycline-induced hyperpigmentation. J Community Hosp Intern Med Perspect. 2014;4. doi:10.3402/jchimp.v4.24063.
- Cox AJ, Marich KW. Gold in the dermis following gold therapy for rheumatoid arthritis. Arch Dermatol. 1973;108:655-657.
- al-Talib RK, Wright DH, Theaker JM. Orange-red birefringence of gold particles in paraffin wax embedded sections: an aid to the diagnosis of chrysiasis. Histopathology. 1994;24:176-178.
- Meyer AJ, Nahass GT. Hyperpigmented patches on the dorsa of the feet. minocycline pigmentation. Arch Dermatol. 1995;131:1447-1450.
- Bayne-Poorman M, Shubrook J. Bluish pigmentation of face and sclera. J Fam Pract. 2010;59:519-522.
The Diagnosis: Erythema Dyschromicum Perstans
Erythema dyschromicum perstans (EDP), also referred to as ashy dermatosis, was first described by Ramirez1 in 1957 who labeled the patients los cenicientos (the ashen ones). It preferentially affects women in the second decade of life; however, patients of all ages can be affected, with reported cases occurring in children as young as 2 years of age.2 Most patients have Fitzpatrick skin type IV, mainly Amerindian, Hispanic South Asian, and Southwest Asian; however, there are cases reported worldwide.3 A genetic predisposition is proposed, as major histocompatibility complex genes associated with HLA-DR4⁎0407 are frequent in Mexican patients with ashy dermatosis and in the Amerindian population.4
The etiology of EDP is unknown. Various contributing factors have been reported including alimentary, occupational, and climatic factors,5,6 yet none have been conclusively demonstrated. High expression of CD36 (thrombospondin receptor not found in normal skin) in spinous and granular layers, CD94 (cytotoxic cell marker) in the basal cell layer and in the inflammatory dermal infiltrate,7 and focal keratinocytic expression of intercellular adhesion molecule I (CD54) in the active lesions of EDP, as well as the absence of these findings in normal skin, suggests an immunologic role in the development of the disease.8
Erythema dyschromicum perstans presents clinically with blue-gray hyperpigmented macules varying in size and shape and developing symmetrically in both sun-exposed and sun-protected areas of the face, neck, trunk, arms, and sometimes the dorsal hands (Figures 1 and 2). Notable sparing of the palms, soles, scalp, and mucous membranes occurs.


Occasionally, in the early active stage of the disease, elevated erythematous borders are noted surrounding the hyperpigmented macules. Eventually a hypopigmented halo develops after a prolonged duration of disease.9 The eruption typically is chronic and asymptomatic, though some cases may be pruritic.10
Histopathologically, the early lesions of EDP with an erythematous active border reveal lichenoid dermatitis with basal vacuolar change and occasional Civatte bodies. A mild to moderate perivascular lymphohistiocytic infiltrate admixed with melanophages can be seen in the papillary dermis (Figure 3). In older lesions, the inflammatory infiltrate is sparse, and pigment incontinence consistent with postinflammatory pigmentation is prominent, though melanophages extending deep into the reticular dermis may aid in distinguishing EDP from other causes of postinflammatory pigment alteration.7,11

Erythema dyschromicum perstans and lichen planus pigmentosus (LPP) may be indistinguishable histopathologically and may both be variants of lichen planus actinicus. Lichen planus pigmentosus often differs from EDP in that it presents with brown-black macules and patches often on the face and flexural areas. A subset of cases of LPP also may have mucous membrane involvement. The erythematous border that characterizes the active lesion of EDP is characteristically absent in LPP. In addition, pruritus often is reported with LPP. Direct immunofluorescence is not a beneficial tool in distinguishing the entities.12
Other differential diagnoses of predominantly facial hyperpigmentation include a lichenoid drug eruption; drug-induced hyperpigmentation (deposition disorder); postinflammatory hyperpigmentation following atopic dermatitis; contact dermatitis or photosensitivity reaction; early pinta; and cutaneous findings of systemic diseases manifesting with diffuse hyperpigmentation such as lupus erythematosus, dermatomyositis, hemochromatosis, and Addison disease. A detailed history including medication use, thorough clinical examination, and careful histopathologic evaluation will help distinguish these conditions.
Chrysiasis is a rare bluish to slate gray discoloration of the skin that predominantly occurs in sun-exposed areas. It is caused by chronic use of gold salts, which have been used to treat rheumatoid arthritis. UV light may contribute to induce the uptake of gold and subsequently stimulate tyrosinase activity.13 Histologic features of chrysiasis include dermal and perivascular gold deposition within the macrophages and endothelial cells as well as extracellular granules. It demonstrates an orange-red birefringence on fluorescent microscopy.14,15
Minocycline-induced hyperpigmentation is a well-recognized side effect of this drug. It is dose dependent and appears as a blue-black pigmentation that most frequently affects the shins, ankles, and arms.16 Three distinct types were documented: abnormal discoloration of the skin that has been linked to deposition of pigmented metabolites of minocycline producing blue-black pigmentation at the site of scarring or prior inflammation (type 1); blue-gray pigmentation affecting normal skin, mainly the legs (type 2); and elevated levels of melanin on the sun-exposed areas producing dirty skin syndrome (type 3).17,18
Topical and systemic corticosteroids, UV light therapy, oral dapsone, griseofulvin, retinoids, and clofazimine are reported as treatment options for ashy dermatosis, though results typically are disappointing.7
The Diagnosis: Erythema Dyschromicum Perstans
Erythema dyschromicum perstans (EDP), also referred to as ashy dermatosis, was first described by Ramirez1 in 1957 who labeled the patients los cenicientos (the ashen ones). It preferentially affects women in the second decade of life; however, patients of all ages can be affected, with reported cases occurring in children as young as 2 years of age.2 Most patients have Fitzpatrick skin type IV, mainly Amerindian, Hispanic South Asian, and Southwest Asian; however, there are cases reported worldwide.3 A genetic predisposition is proposed, as major histocompatibility complex genes associated with HLA-DR4⁎0407 are frequent in Mexican patients with ashy dermatosis and in the Amerindian population.4
The etiology of EDP is unknown. Various contributing factors have been reported including alimentary, occupational, and climatic factors,5,6 yet none have been conclusively demonstrated. High expression of CD36 (thrombospondin receptor not found in normal skin) in spinous and granular layers, CD94 (cytotoxic cell marker) in the basal cell layer and in the inflammatory dermal infiltrate,7 and focal keratinocytic expression of intercellular adhesion molecule I (CD54) in the active lesions of EDP, as well as the absence of these findings in normal skin, suggests an immunologic role in the development of the disease.8
Erythema dyschromicum perstans presents clinically with blue-gray hyperpigmented macules varying in size and shape and developing symmetrically in both sun-exposed and sun-protected areas of the face, neck, trunk, arms, and sometimes the dorsal hands (Figures 1 and 2). Notable sparing of the palms, soles, scalp, and mucous membranes occurs.


Occasionally, in the early active stage of the disease, elevated erythematous borders are noted surrounding the hyperpigmented macules. Eventually a hypopigmented halo develops after a prolonged duration of disease.9 The eruption typically is chronic and asymptomatic, though some cases may be pruritic.10
Histopathologically, the early lesions of EDP with an erythematous active border reveal lichenoid dermatitis with basal vacuolar change and occasional Civatte bodies. A mild to moderate perivascular lymphohistiocytic infiltrate admixed with melanophages can be seen in the papillary dermis (Figure 3). In older lesions, the inflammatory infiltrate is sparse, and pigment incontinence consistent with postinflammatory pigmentation is prominent, though melanophages extending deep into the reticular dermis may aid in distinguishing EDP from other causes of postinflammatory pigment alteration.7,11

Erythema dyschromicum perstans and lichen planus pigmentosus (LPP) may be indistinguishable histopathologically and may both be variants of lichen planus actinicus. Lichen planus pigmentosus often differs from EDP in that it presents with brown-black macules and patches often on the face and flexural areas. A subset of cases of LPP also may have mucous membrane involvement. The erythematous border that characterizes the active lesion of EDP is characteristically absent in LPP. In addition, pruritus often is reported with LPP. Direct immunofluorescence is not a beneficial tool in distinguishing the entities.12
Other differential diagnoses of predominantly facial hyperpigmentation include a lichenoid drug eruption; drug-induced hyperpigmentation (deposition disorder); postinflammatory hyperpigmentation following atopic dermatitis; contact dermatitis or photosensitivity reaction; early pinta; and cutaneous findings of systemic diseases manifesting with diffuse hyperpigmentation such as lupus erythematosus, dermatomyositis, hemochromatosis, and Addison disease. A detailed history including medication use, thorough clinical examination, and careful histopathologic evaluation will help distinguish these conditions.
Chrysiasis is a rare bluish to slate gray discoloration of the skin that predominantly occurs in sun-exposed areas. It is caused by chronic use of gold salts, which have been used to treat rheumatoid arthritis. UV light may contribute to induce the uptake of gold and subsequently stimulate tyrosinase activity.13 Histologic features of chrysiasis include dermal and perivascular gold deposition within the macrophages and endothelial cells as well as extracellular granules. It demonstrates an orange-red birefringence on fluorescent microscopy.14,15
Minocycline-induced hyperpigmentation is a well-recognized side effect of this drug. It is dose dependent and appears as a blue-black pigmentation that most frequently affects the shins, ankles, and arms.16 Three distinct types were documented: abnormal discoloration of the skin that has been linked to deposition of pigmented metabolites of minocycline producing blue-black pigmentation at the site of scarring or prior inflammation (type 1); blue-gray pigmentation affecting normal skin, mainly the legs (type 2); and elevated levels of melanin on the sun-exposed areas producing dirty skin syndrome (type 3).17,18
Topical and systemic corticosteroids, UV light therapy, oral dapsone, griseofulvin, retinoids, and clofazimine are reported as treatment options for ashy dermatosis, though results typically are disappointing.7
- Ramirez CO. Los cenicientos: problema clinica. In: Memoria del Primer Congresso Centroamericano de Dermatologica, December 5-8, 1957. San Salvador, El Salvador; 1957:122-130.
- Lee SJ, Chung KY. Erythema dyschromicum perstans in early childhood. J Dermatol. 1999;26:119-121.
- Homez-Chacin, Barroso C. On the etiopathogenic of the erythema dyschromicum perstans: possibility of a melanosis neurocutaneous. Dermatol Venez. 1996;4:149-151.
- Correa MC, Memije EV, Vargas-Alarcon G, et al. HLA-DR association with the genetic susceptibility to develop ashy dermatosis in Mexican Mestizo patients [published online November 20, 2006]. J Am Acad Dermatol. 2007;56:617-620.
- Jablonska S. Ingestion of ammonium nitrate as a possible cause of erythema dyschromicum perstans (ashy dermatosis). Dermatologica. 1975;150:287-291.
- Stevenson JR, Miura M. Erythema dyschromicum perstans (ashy dermatosis). Arch Dermatol. 1966;94:196-199.
- Baranda L, Torres-Alvarez B, Cortes-Franco R, et al. Involvement of cell adhesion and activation molecules in the pathogenesis of erythema dyschromicum perstans (ashy dermatitis). the effect of clofazimine therapy. Arch Dermatol. 1997;133:325-329.
- Vasquez-Ochoa LA, Isaza-Guzman DM, Orozco-Mora B, et al. Immunopathologic study of erythema dyschromicum perstans (ashy dermatosis). Int J Dermatol. 2006;45:937-941.
- Convit J, Kerdel-Vegas F, Roderiguez G. Erythema dyschromicum perstans: a hiltherto undescribed skin disease. J Invest Dermatol. 1961;36:457-462.
- Ono S, Miyachi Y, Kabashima K. Ashy dermatosis with prior pruritic and scaling skin lesions. J Dermatol. 2012;39:1103-1104.
- Sanchez NP, Pathak MA, Sato SS, et al. Circumscribed dermal melaninoses: classification, light, histochemical, and electron microscopic studies on three patients with the erythema dyschromicum perstans type. Int J Dermatol. 1982;21:25-32.
- Vega ME, Waxtein L, Arenas R, et al. Ashy dermatosis and lichen planus pigmentosus: a clinicopathologic study of 31 cases. Int J Dermatol. 1992;31:90-94.
- Ahmed SV, Sajjan R. Chrysiasis: a gold "curse!" [published online May 21, 2009]. BMJ Case Rep. 2009;2009.
- Fiscus V, Hankinson A, Alweis R. Minocycline-induced hyperpigmentation. J Community Hosp Intern Med Perspect. 2014;4. doi:10.3402/jchimp.v4.24063.
- Cox AJ, Marich KW. Gold in the dermis following gold therapy for rheumatoid arthritis. Arch Dermatol. 1973;108:655-657.
- al-Talib RK, Wright DH, Theaker JM. Orange-red birefringence of gold particles in paraffin wax embedded sections: an aid to the diagnosis of chrysiasis. Histopathology. 1994;24:176-178.
- Meyer AJ, Nahass GT. Hyperpigmented patches on the dorsa of the feet. minocycline pigmentation. Arch Dermatol. 1995;131:1447-1450.
- Bayne-Poorman M, Shubrook J. Bluish pigmentation of face and sclera. J Fam Pract. 2010;59:519-522.
- Ramirez CO. Los cenicientos: problema clinica. In: Memoria del Primer Congresso Centroamericano de Dermatologica, December 5-8, 1957. San Salvador, El Salvador; 1957:122-130.
- Lee SJ, Chung KY. Erythema dyschromicum perstans in early childhood. J Dermatol. 1999;26:119-121.
- Homez-Chacin, Barroso C. On the etiopathogenic of the erythema dyschromicum perstans: possibility of a melanosis neurocutaneous. Dermatol Venez. 1996;4:149-151.
- Correa MC, Memije EV, Vargas-Alarcon G, et al. HLA-DR association with the genetic susceptibility to develop ashy dermatosis in Mexican Mestizo patients [published online November 20, 2006]. J Am Acad Dermatol. 2007;56:617-620.
- Jablonska S. Ingestion of ammonium nitrate as a possible cause of erythema dyschromicum perstans (ashy dermatosis). Dermatologica. 1975;150:287-291.
- Stevenson JR, Miura M. Erythema dyschromicum perstans (ashy dermatosis). Arch Dermatol. 1966;94:196-199.
- Baranda L, Torres-Alvarez B, Cortes-Franco R, et al. Involvement of cell adhesion and activation molecules in the pathogenesis of erythema dyschromicum perstans (ashy dermatitis). the effect of clofazimine therapy. Arch Dermatol. 1997;133:325-329.
- Vasquez-Ochoa LA, Isaza-Guzman DM, Orozco-Mora B, et al. Immunopathologic study of erythema dyschromicum perstans (ashy dermatosis). Int J Dermatol. 2006;45:937-941.
- Convit J, Kerdel-Vegas F, Roderiguez G. Erythema dyschromicum perstans: a hiltherto undescribed skin disease. J Invest Dermatol. 1961;36:457-462.
- Ono S, Miyachi Y, Kabashima K. Ashy dermatosis with prior pruritic and scaling skin lesions. J Dermatol. 2012;39:1103-1104.
- Sanchez NP, Pathak MA, Sato SS, et al. Circumscribed dermal melaninoses: classification, light, histochemical, and electron microscopic studies on three patients with the erythema dyschromicum perstans type. Int J Dermatol. 1982;21:25-32.
- Vega ME, Waxtein L, Arenas R, et al. Ashy dermatosis and lichen planus pigmentosus: a clinicopathologic study of 31 cases. Int J Dermatol. 1992;31:90-94.
- Ahmed SV, Sajjan R. Chrysiasis: a gold "curse!" [published online May 21, 2009]. BMJ Case Rep. 2009;2009.
- Fiscus V, Hankinson A, Alweis R. Minocycline-induced hyperpigmentation. J Community Hosp Intern Med Perspect. 2014;4. doi:10.3402/jchimp.v4.24063.
- Cox AJ, Marich KW. Gold in the dermis following gold therapy for rheumatoid arthritis. Arch Dermatol. 1973;108:655-657.
- al-Talib RK, Wright DH, Theaker JM. Orange-red birefringence of gold particles in paraffin wax embedded sections: an aid to the diagnosis of chrysiasis. Histopathology. 1994;24:176-178.
- Meyer AJ, Nahass GT. Hyperpigmented patches on the dorsa of the feet. minocycline pigmentation. Arch Dermatol. 1995;131:1447-1450.
- Bayne-Poorman M, Shubrook J. Bluish pigmentation of face and sclera. J Fam Pract. 2010;59:519-522.
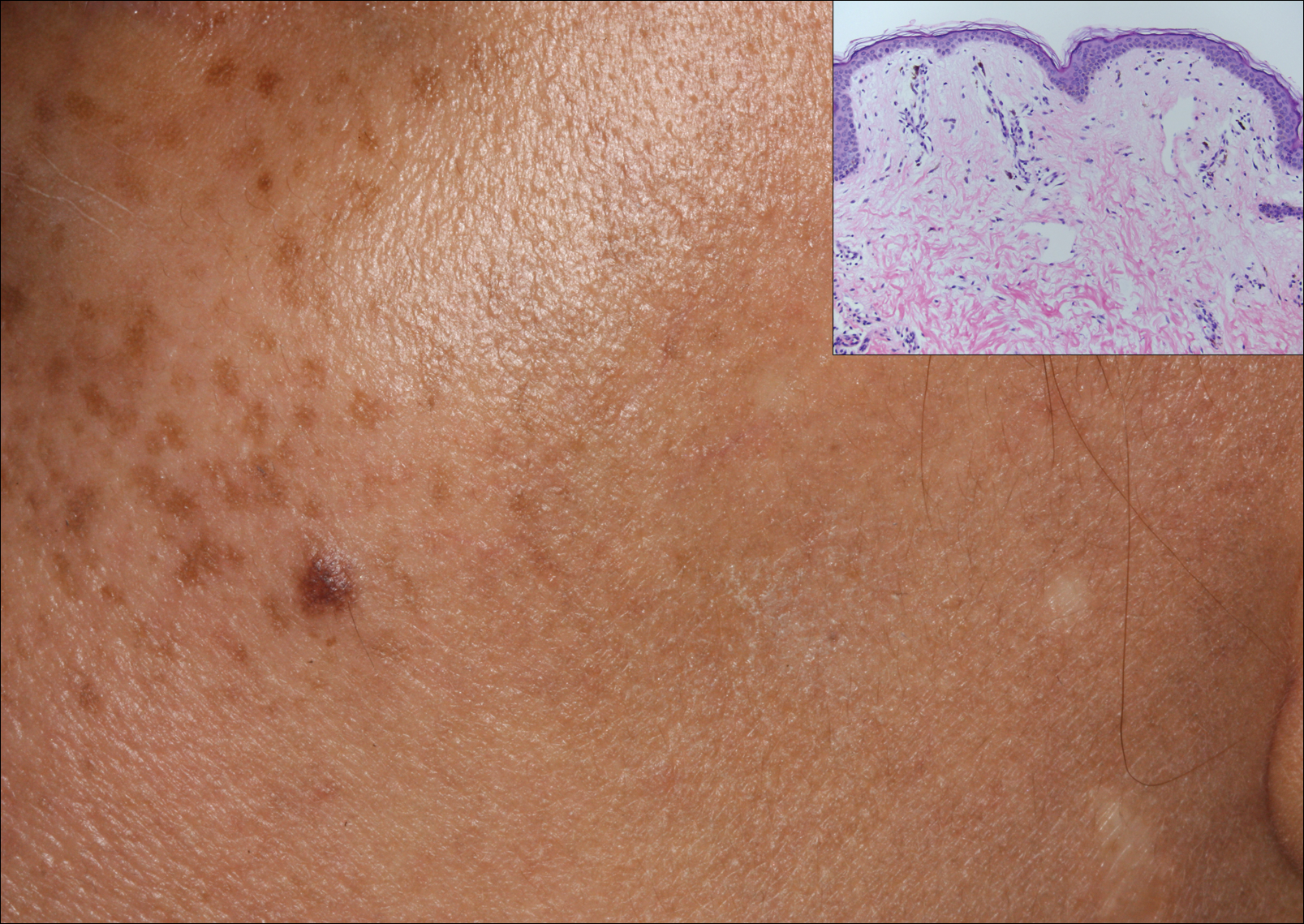
A middle-aged woman with Fitzpatrick skin type IV was evaluated for progressive hyperpigmentation of several months' duration involving the neck, jawline, both sides of the face, and forehead. The lesions were mildly pruritic. She denied contact with any new substance and there was no history of an eruption preceding the hyperpigmentation. Medical history included chronic anemia that was managed with iron supplementation. On physical examination, blue-gray nonscaly macules and patches were observed distributed symmetrically on the neck, jawline, sides of the face, and forehead. Microscopic examination of 2 shave biopsies revealed subtle vacuolar interface dermatitis with mild perivascular lymphocytic infiltrate and dermal melanophages (inset).
What’s Eating You? Lone Star Tick (Amblyomma americanum)
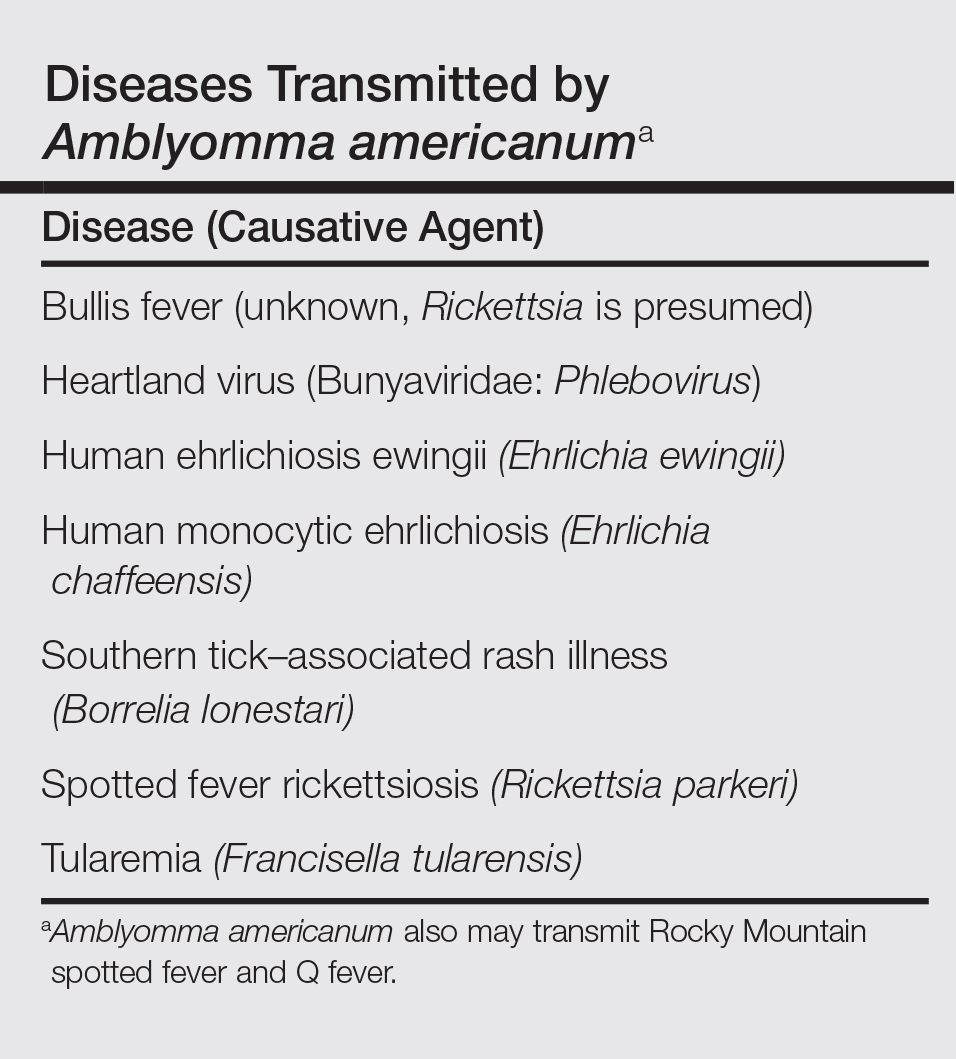
The lone star tick (Amblyomma americanum) is distributed throughout much of the eastern United States. It serves as a vector for species of Rickettsia, Ehrlichia, and Borrelia that are an important cause of tick-borne illness (Table). In addition, the bite of the lone star tick can cause impressive local and systemic reactions. Delayed anaphylaxis to ingestion of red meat has been attributed to the bite of A americanum.1 Herein, we discuss human disease associated with the lone star tick as well as potential tick-control measures.
Tick Characteristics
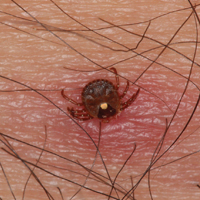
Lone star ticks are characterized by long anterior mouthparts and an ornate scutum (hard dorsal plate). Widely spaced eyes and posterior festoons also are present. In contrast to some other ticks, adanal plates are absent on the ventral surface in male lone star ticks. Amblyomma americanum demonstrates a single white spot on the female’s scutum (Figure 1). The male has inverted horseshoe markings on the posterior scutum. The female’s scutum often covers only a portion of the body to allow room for engorgement.
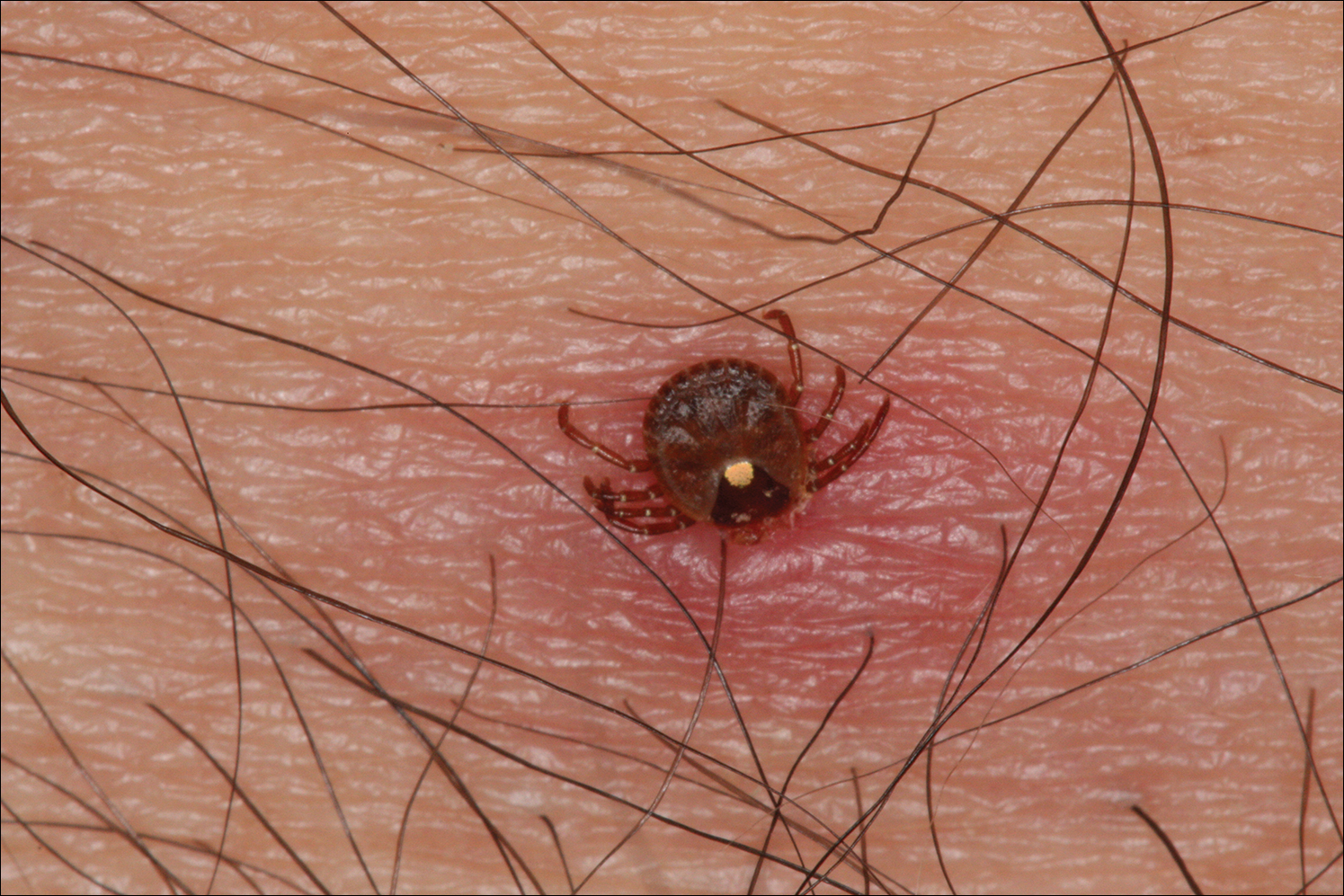
Patients usually become aware of tick bites while the tick is still attached to the skin, which provides the physician with an opportunity to identify the tick and discuss tick-control measures as well as symptoms of tick-borne disease. Once the tick has been removed, delayed-type hypersensitivity to the tick antigens continues at the attachment site. Erythema and pruritus can be dramatic. Nodules with a pseudolymphomatous histology can occur. Milder reactions respond to application of topical corticosteroids. More intense reactions may require intralesional corticosteroid injection or even surgical excision.
Most hard ticks have a 3-host life cycle, meaning they attach for one long blood meal during each phase of the life cycle. Because they search for a new host for each blood meal, they are efficient disease vectors. The larval ticks, so-called seed ticks, have 6 legs and feed on small animals. Nymphs and adults feed on larger animals. Nymphs resemble small adult ticks with 8 legs but are sexually immature.
Distribution
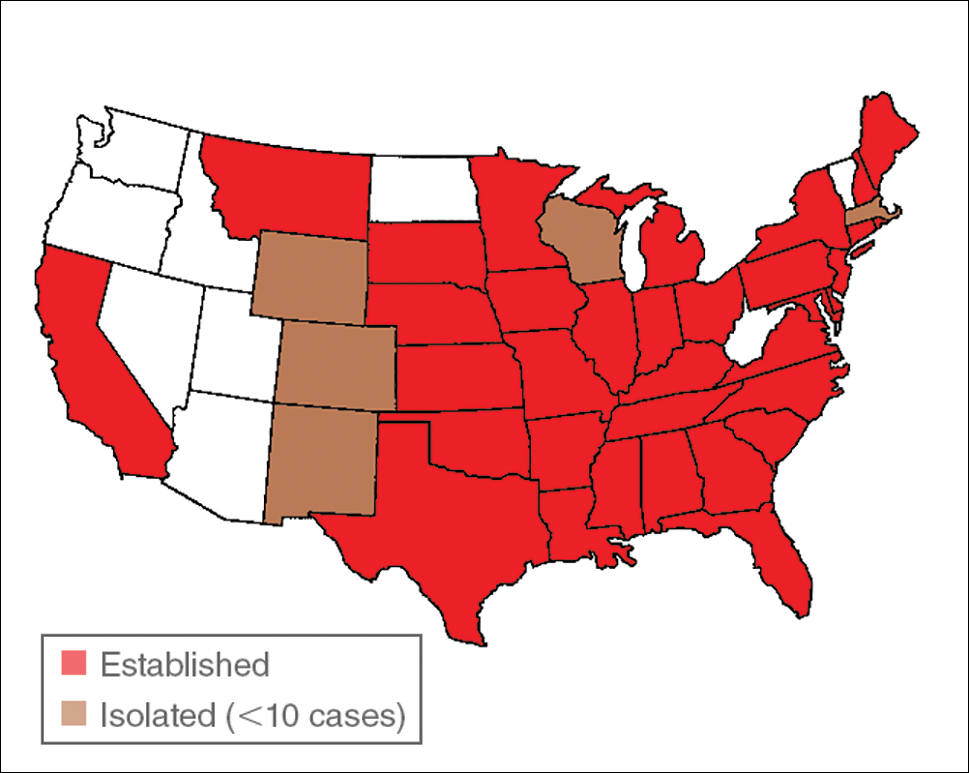
Amblyomma americanum has a wide distribution in the United States from Texas to Iowa and as far north as Maine (Figure 2).2 Tick attachments often are seen in individuals who work outdoors, especially in areas where new commercial or residential development disrupts the environment and the tick’s usual hosts move out of the area. Hungry ticks are left behind in search of a host.
Disease Transmission
Lone star ticks have been implicated as vectors of Ehrlichia chaffeensis, the agent of human monocytic ehrlichiosis (HME),3 which has been documented from the mid-Atlantic to south-central United States. It may present as a somewhat milder Rocky Mountain spotted fever–like illness with fever and headache or as a life-threatening systemic illness with organ failure. Prompt diagnosis and treatment with a tetracycline has been correlated with a better prognosis.4 Immunofluorescent antibody testing and polymerase chain reaction can be used to establish the diagnosis.5 Two tick species—A americanum and Dermacentor variabilis—have been implicated as vectors, but A americanum appears to be the major vector.6,7
The lone star tick also is a vector for Erlichia ewingii, the cause of human ehrlichiosis ewingii. Human ehrlichiosis ewingii is a rare disease that presents similar to HME, with most reported cases occurring in immunocompromised hosts.8
A novel member of the Phlebovirus genus, the Heartland virus, was first described in 2 Missouri farmers who presented with symptoms similar to HME but did not respond to doxycycline treatment.9 The virus has since been isolated from A americanum adult ticks, implicating them as the major vectors of the disease.10
Rickettsia parkeri, a cause of spotted fever rickettsiosis, is responsible for an eschar-associated illness in affected individuals.11 The organism has been detected in A americanum ticks collected from the wild. Experiments show the tick is capable of transmitting R parkeri to animals in the laboratory. It is unclear, however, what role A americanum plays in the natural transmission of the disease.12
In Missouri, strains of Borrelia have been isolated from A americanum ticks that feed on cottontail rabbits, but it seems unlikely that the tick plays any role in transmission of true Lyme disease13,14; Borrelia has been shown to have poor survival in the saliva of A americanum beyond 24 hours.15 Southern tick–associated rash illness is a Lyme disease–like illness with several reported cases due to A americanum.16 Patients generally present with an erythema migrans–like rash and may have headache, fever, arthralgia, or myalgia.16 The causative organism remains unclear, though Borrelia lonestari has been implicated.17 Lone star ticks also transmit tularemia and may transmit Rocky Mountain spotted fever and Q fever.13
Bullis fever (first reported at Camp Bullis near San Antonio, Texas) affected huge numbers of military personnel from 1942 to 1943.18 The causative organism appears to be rickettsial. During one outbreak of Bullis fever, it was noted that A americanum was so numerous that more than 4000 adult ticks were collected under a single juniper tree and more than 1000 ticks were removed from a single soldier who sat in a thicket for 2 hours.12 No cases of Bullis fever have been reported in recent years,12 which probably relates to the introduction of fire ants.
Disease Hosts
At Little Rock Air Force Base in Arkansas, A americanum has been a source of Ehrlichia infection. During one outbreak, deer in the area were found to have as many as 2550 ticks per ear,19 which demonstrates the magnitude of tick infestation in some areas of the United States. Tick infestation is not merely of concern to the US military. Ticks are ubiquitous and can be found on neatly trimmed suburban lawns as well as in rough thickets.
More recently, bites from A americanum have been found to induce allergies to red meat in some patients.1 IgE antibodies directed against galactose-alpha-1,3-galactose (alpha gal) have been implicated as the cause of this reaction. These antibodies cause delayed-onset anaphylaxis occurring 3 to 6 hours after ingestion of red meat. Tick bites appear to be the most important and perhaps the only cause of IgE antibodies to alpha gal in the United States.1
Wild white-tailed deer serve as reservoir hosts for several diseases transmitted by A americanum, including HME, human ehrlichiosis ewingii, and Southern tick–associated rash illness.12,20 Communities located close to wildlife reserves may have higher rates of infection.21 Application of acaricides to corn contained in deer feeders has been shown to be an effective method of decreasing local tick populations, which is a potential method for disease control in at-risk areas, though it is costly and time consuming.22
Tick-Control Measures
Hard ticks produce little urine. Instead, excess water is eliminated via salivation back into the host. Loss of water also occurs through spiracles. Absorption of water from the atmosphere is important for the tick to maintain hydration. The tick produces intensely hygroscopic saliva that absorbs water from surrounding moist air. The humidified saliva is then reingested by the tick. In hot climates, ticks are prone to dehydration unless they can find a source of moist air, usually within a layer of leaf debris.23 When the leaf debris is stirred by a human walking through the area, the tick can make contact with the human. Therefore, removal of leaf debris is a critical part of tick-control efforts, as it reduces tick numbers by means of dehydration. Tick eggs also require sufficient humidity to hatch. Leaf removal increases the effectiveness of insecticide applications, which would otherwise do little harm to the ticks below if sprayed on top of leaf debris.
Some lone star ticks attach to birds and disseminate widely. Attachments to animal hosts with long-range migration patterns complicate tick-control efforts.24 Animal migration may contribute to the spread of disease from one geographic region to another.
Imported fire ants are voracious eaters that gather and consume ticks eggs. Fire ants provide an excellent natural means of tick control. Tick numbers in places such as Camp Bullis have declined dramatically since the introduction of imported fire ants.25
- Commins SP, Platts-Mills TA. Tick bites and red meat allergy. Curr Opin Allergy Clin Immunol. 2013;13:354-359.
- Springer YP, Eisen L, Beati L, et al. Spatial distribution of counties in the continental United States with records of occurrence of Amblyomma americanum (Ixodida: Ixodidae). J Med Entomol. 2014;51:342-351.
- Yu X, Piesman JF, Olson JG, et al. Geographic distribution of different genetic types of Ehrlichia chaffeensis. Am J Trop Med Hyg. 1997;56:679-680.
- Dumler JS, Bakken JS. Human ehrlichiosis: newly recognized infections transmitted by ticks. An Rev Med. 1998;49:201-213.
- Dumler JS, Madigan JE, Pusterla N, et al. Ehrlichioses in humans: epidemiology, clinical presentation, diagnosis, and treatment. Clin Infect Dis. 2007;45(suppl 1):S45-S51.
- Lockhart JM, Davidson WR, Stallknecht DE, et al. Natural history of Ehrlichia chaffeensis (Ricketsiales: Ehrlichiea) in the piedmont physiographic province of Georgia. J Parasitol. 1997;83:887-894.
- Centers for Disease Control and Prevention (CDC). Human ehrlichiosis—Maryland, 1994. MMWR Morb Mortal Wkly Rep. 1996;45:798-802.
- Ismail N, Bloch KC, McBride JW. Human ehrlichiosis and anaplasmosis. Clin Lab Med. 2010;30:261-292.
- McMullan LK, Folk SM, Kelly AJ, et al. A new phlebovirus associated with severe febrile illness in Missouri. N Engl J Med. 2012;367:834-841.
- Savage HM, Godsey MS Jr, Panella NA, et al. Surveillance for heartland virus (Bunyaviridae: Phlebovirus) in Missouri during 2013: first detection of virus in adults of Amblyomma americanum (Acari: Ixodidae) [published online March 30, 2016]. J Med Entomol. pii:tjw028.
- Cragun WC, Bartlett BL, Ellis MW, et al. The expanding spectrum of eschar-associated rickettsioses in the United States. Arch Dermatol. 2010;146:641-648.
- Paddock CD, Sumner JW, Comer JA, et al. Rickettsia parkeri: a newly recognized cause of spotted fever rickettsiosis in the United States. Clin Infect Dis. 2004;38:805-811.
- Goddard J, Varela-Stokes AS. Role of the lone star tick, Amblyomma americanum (L.) in human and animal diseases. Vet Parasitol. 2009;160:1-12.
- Oliver JH, Kollars TM, Chandler FW, et al. First isolation and cultivation of Borrelia burgdorferi sensu lato from Missouri. J Clin Microbiol. 1998;36:1-5.
- Ledin KE, Zeidner NS, Ribeiro JM, et al. Borreliacidal activity of saliva of the tick Amblyomma americanum. Med Vet Entomol. 2005;19:90-95.
- Feder HM Jr, Hoss DM, Zemel L, et al. Southern tick-associated rash illness (STARI) in the North: STARI following a tick bite in Long Island, New York. Clin Infect Dis. 2011;53:e142-e146.
- Varela AS, Luttrell MP, Howerth EW, et al. First culture isolation of Borrelia lonestari, putative agent of southern tick-associated rash illness. J Clin Microbiol. 2004;42:1163-1169.
- Livesay HR, Pollard M. Laboratory report on a clinical syndrome referred to as “Bullis Fever.” Am J Trop Med. 1943;23:475-479.
- Goddard J. Ticks and tickborne diseases affecting military personnel. US Air Force School of Aerospace Medicine USAFSAM-SR-89-2. http://www.dtic.mil/dtic/tr/fulltext/u2/a221956.pdf. Published September 1989. Accessed January 19, 2017.
- Lockhart JM, Davidson WR, Stallkneeckt DE, et al. Isolation of Ehrlichia chaffeensis from wild white tailed deer (Odocoileus virginianus) confirms their role as natural reservoir hosts. J Clin Microbiol. 1997;35:1681-1686.
- Standaert SM, Dawson JE, Schaffner W, et al. Ehrlichiosis in a golf-oriented retirement community. N Engl J Med. 1995;333:420-425.
- Schulze TL, Jordan RA, Hung RW, et al. Effectiveness of the 4-Poster passive topical treatment device in the control of Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae) in New Jersey. Vector Borne Zoonotic Dis. 2009;9:389-400.
- Strey OF, Teel PD, Longnecker MT, et al. Survival and water-balance characteristics of unfed Amblyomma cajennense (Acari: Ixodidae). J Med Entomol. 1996;33:63-73.
- Popham TW, Garris GI, Barre N. Development of a computer model of the population dynamics of Amblyomma variegatum and simulations of eradication strategies for use in the Caribbean. Ann New York Acad Sci. 1996;791:452-465.
- Burns EC, Melancon DG. Effect of important fire ant (Hymenoptera: Formicidae) invasion on lone star tick (Acarina: Ixodidae) populations. J Med Entomol. 1977;14:247-249.
The lone star tick (Amblyomma americanum) is distributed throughout much of the eastern United States. It serves as a vector for species of Rickettsia, Ehrlichia, and Borrelia that are an important cause of tick-borne illness (Table). In addition, the bite of the lone star tick can cause impressive local and systemic reactions. Delayed anaphylaxis to ingestion of red meat has been attributed to the bite of A americanum.1 Herein, we discuss human disease associated with the lone star tick as well as potential tick-control measures.

Tick Characteristics
Lone star ticks are characterized by long anterior mouthparts and an ornate scutum (hard dorsal plate). Widely spaced eyes and posterior festoons also are present. In contrast to some other ticks, adanal plates are absent on the ventral surface in male lone star ticks. Amblyomma americanum demonstrates a single white spot on the female’s scutum (Figure 1). The male has inverted horseshoe markings on the posterior scutum. The female’s scutum often covers only a portion of the body to allow room for engorgement.

Patients usually become aware of tick bites while the tick is still attached to the skin, which provides the physician with an opportunity to identify the tick and discuss tick-control measures as well as symptoms of tick-borne disease. Once the tick has been removed, delayed-type hypersensitivity to the tick antigens continues at the attachment site. Erythema and pruritus can be dramatic. Nodules with a pseudolymphomatous histology can occur. Milder reactions respond to application of topical corticosteroids. More intense reactions may require intralesional corticosteroid injection or even surgical excision.
Most hard ticks have a 3-host life cycle, meaning they attach for one long blood meal during each phase of the life cycle. Because they search for a new host for each blood meal, they are efficient disease vectors. The larval ticks, so-called seed ticks, have 6 legs and feed on small animals. Nymphs and adults feed on larger animals. Nymphs resemble small adult ticks with 8 legs but are sexually immature.
Distribution
Amblyomma americanum has a wide distribution in the United States from Texas to Iowa and as far north as Maine (Figure 2).2 Tick attachments often are seen in individuals who work outdoors, especially in areas where new commercial or residential development disrupts the environment and the tick’s usual hosts move out of the area. Hungry ticks are left behind in search of a host.

Disease Transmission
Lone star ticks have been implicated as vectors of Ehrlichia chaffeensis, the agent of human monocytic ehrlichiosis (HME),3 which has been documented from the mid-Atlantic to south-central United States. It may present as a somewhat milder Rocky Mountain spotted fever–like illness with fever and headache or as a life-threatening systemic illness with organ failure. Prompt diagnosis and treatment with a tetracycline has been correlated with a better prognosis.4 Immunofluorescent antibody testing and polymerase chain reaction can be used to establish the diagnosis.5 Two tick species—A americanum and Dermacentor variabilis—have been implicated as vectors, but A americanum appears to be the major vector.6,7
The lone star tick also is a vector for Erlichia ewingii, the cause of human ehrlichiosis ewingii. Human ehrlichiosis ewingii is a rare disease that presents similar to HME, with most reported cases occurring in immunocompromised hosts.8
A novel member of the Phlebovirus genus, the Heartland virus, was first described in 2 Missouri farmers who presented with symptoms similar to HME but did not respond to doxycycline treatment.9 The virus has since been isolated from A americanum adult ticks, implicating them as the major vectors of the disease.10
Rickettsia parkeri, a cause of spotted fever rickettsiosis, is responsible for an eschar-associated illness in affected individuals.11 The organism has been detected in A americanum ticks collected from the wild. Experiments show the tick is capable of transmitting R parkeri to animals in the laboratory. It is unclear, however, what role A americanum plays in the natural transmission of the disease.12
In Missouri, strains of Borrelia have been isolated from A americanum ticks that feed on cottontail rabbits, but it seems unlikely that the tick plays any role in transmission of true Lyme disease13,14; Borrelia has been shown to have poor survival in the saliva of A americanum beyond 24 hours.15 Southern tick–associated rash illness is a Lyme disease–like illness with several reported cases due to A americanum.16 Patients generally present with an erythema migrans–like rash and may have headache, fever, arthralgia, or myalgia.16 The causative organism remains unclear, though Borrelia lonestari has been implicated.17 Lone star ticks also transmit tularemia and may transmit Rocky Mountain spotted fever and Q fever.13
Bullis fever (first reported at Camp Bullis near San Antonio, Texas) affected huge numbers of military personnel from 1942 to 1943.18 The causative organism appears to be rickettsial. During one outbreak of Bullis fever, it was noted that A americanum was so numerous that more than 4000 adult ticks were collected under a single juniper tree and more than 1000 ticks were removed from a single soldier who sat in a thicket for 2 hours.12 No cases of Bullis fever have been reported in recent years,12 which probably relates to the introduction of fire ants.
Disease Hosts
At Little Rock Air Force Base in Arkansas, A americanum has been a source of Ehrlichia infection. During one outbreak, deer in the area were found to have as many as 2550 ticks per ear,19 which demonstrates the magnitude of tick infestation in some areas of the United States. Tick infestation is not merely of concern to the US military. Ticks are ubiquitous and can be found on neatly trimmed suburban lawns as well as in rough thickets.
More recently, bites from A americanum have been found to induce allergies to red meat in some patients.1 IgE antibodies directed against galactose-alpha-1,3-galactose (alpha gal) have been implicated as the cause of this reaction. These antibodies cause delayed-onset anaphylaxis occurring 3 to 6 hours after ingestion of red meat. Tick bites appear to be the most important and perhaps the only cause of IgE antibodies to alpha gal in the United States.1
Wild white-tailed deer serve as reservoir hosts for several diseases transmitted by A americanum, including HME, human ehrlichiosis ewingii, and Southern tick–associated rash illness.12,20 Communities located close to wildlife reserves may have higher rates of infection.21 Application of acaricides to corn contained in deer feeders has been shown to be an effective method of decreasing local tick populations, which is a potential method for disease control in at-risk areas, though it is costly and time consuming.22
Tick-Control Measures
Hard ticks produce little urine. Instead, excess water is eliminated via salivation back into the host. Loss of water also occurs through spiracles. Absorption of water from the atmosphere is important for the tick to maintain hydration. The tick produces intensely hygroscopic saliva that absorbs water from surrounding moist air. The humidified saliva is then reingested by the tick. In hot climates, ticks are prone to dehydration unless they can find a source of moist air, usually within a layer of leaf debris.23 When the leaf debris is stirred by a human walking through the area, the tick can make contact with the human. Therefore, removal of leaf debris is a critical part of tick-control efforts, as it reduces tick numbers by means of dehydration. Tick eggs also require sufficient humidity to hatch. Leaf removal increases the effectiveness of insecticide applications, which would otherwise do little harm to the ticks below if sprayed on top of leaf debris.
Some lone star ticks attach to birds and disseminate widely. Attachments to animal hosts with long-range migration patterns complicate tick-control efforts.24 Animal migration may contribute to the spread of disease from one geographic region to another.
Imported fire ants are voracious eaters that gather and consume ticks eggs. Fire ants provide an excellent natural means of tick control. Tick numbers in places such as Camp Bullis have declined dramatically since the introduction of imported fire ants.25
The lone star tick (Amblyomma americanum) is distributed throughout much of the eastern United States. It serves as a vector for species of Rickettsia, Ehrlichia, and Borrelia that are an important cause of tick-borne illness (Table). In addition, the bite of the lone star tick can cause impressive local and systemic reactions. Delayed anaphylaxis to ingestion of red meat has been attributed to the bite of A americanum.1 Herein, we discuss human disease associated with the lone star tick as well as potential tick-control measures.

Tick Characteristics
Lone star ticks are characterized by long anterior mouthparts and an ornate scutum (hard dorsal plate). Widely spaced eyes and posterior festoons also are present. In contrast to some other ticks, adanal plates are absent on the ventral surface in male lone star ticks. Amblyomma americanum demonstrates a single white spot on the female’s scutum (Figure 1). The male has inverted horseshoe markings on the posterior scutum. The female’s scutum often covers only a portion of the body to allow room for engorgement.

Patients usually become aware of tick bites while the tick is still attached to the skin, which provides the physician with an opportunity to identify the tick and discuss tick-control measures as well as symptoms of tick-borne disease. Once the tick has been removed, delayed-type hypersensitivity to the tick antigens continues at the attachment site. Erythema and pruritus can be dramatic. Nodules with a pseudolymphomatous histology can occur. Milder reactions respond to application of topical corticosteroids. More intense reactions may require intralesional corticosteroid injection or even surgical excision.
Most hard ticks have a 3-host life cycle, meaning they attach for one long blood meal during each phase of the life cycle. Because they search for a new host for each blood meal, they are efficient disease vectors. The larval ticks, so-called seed ticks, have 6 legs and feed on small animals. Nymphs and adults feed on larger animals. Nymphs resemble small adult ticks with 8 legs but are sexually immature.
Distribution
Amblyomma americanum has a wide distribution in the United States from Texas to Iowa and as far north as Maine (Figure 2).2 Tick attachments often are seen in individuals who work outdoors, especially in areas where new commercial or residential development disrupts the environment and the tick’s usual hosts move out of the area. Hungry ticks are left behind in search of a host.

Disease Transmission
Lone star ticks have been implicated as vectors of Ehrlichia chaffeensis, the agent of human monocytic ehrlichiosis (HME),3 which has been documented from the mid-Atlantic to south-central United States. It may present as a somewhat milder Rocky Mountain spotted fever–like illness with fever and headache or as a life-threatening systemic illness with organ failure. Prompt diagnosis and treatment with a tetracycline has been correlated with a better prognosis.4 Immunofluorescent antibody testing and polymerase chain reaction can be used to establish the diagnosis.5 Two tick species—A americanum and Dermacentor variabilis—have been implicated as vectors, but A americanum appears to be the major vector.6,7
The lone star tick also is a vector for Erlichia ewingii, the cause of human ehrlichiosis ewingii. Human ehrlichiosis ewingii is a rare disease that presents similar to HME, with most reported cases occurring in immunocompromised hosts.8
A novel member of the Phlebovirus genus, the Heartland virus, was first described in 2 Missouri farmers who presented with symptoms similar to HME but did not respond to doxycycline treatment.9 The virus has since been isolated from A americanum adult ticks, implicating them as the major vectors of the disease.10
Rickettsia parkeri, a cause of spotted fever rickettsiosis, is responsible for an eschar-associated illness in affected individuals.11 The organism has been detected in A americanum ticks collected from the wild. Experiments show the tick is capable of transmitting R parkeri to animals in the laboratory. It is unclear, however, what role A americanum plays in the natural transmission of the disease.12
In Missouri, strains of Borrelia have been isolated from A americanum ticks that feed on cottontail rabbits, but it seems unlikely that the tick plays any role in transmission of true Lyme disease13,14; Borrelia has been shown to have poor survival in the saliva of A americanum beyond 24 hours.15 Southern tick–associated rash illness is a Lyme disease–like illness with several reported cases due to A americanum.16 Patients generally present with an erythema migrans–like rash and may have headache, fever, arthralgia, or myalgia.16 The causative organism remains unclear, though Borrelia lonestari has been implicated.17 Lone star ticks also transmit tularemia and may transmit Rocky Mountain spotted fever and Q fever.13
Bullis fever (first reported at Camp Bullis near San Antonio, Texas) affected huge numbers of military personnel from 1942 to 1943.18 The causative organism appears to be rickettsial. During one outbreak of Bullis fever, it was noted that A americanum was so numerous that more than 4000 adult ticks were collected under a single juniper tree and more than 1000 ticks were removed from a single soldier who sat in a thicket for 2 hours.12 No cases of Bullis fever have been reported in recent years,12 which probably relates to the introduction of fire ants.
Disease Hosts
At Little Rock Air Force Base in Arkansas, A americanum has been a source of Ehrlichia infection. During one outbreak, deer in the area were found to have as many as 2550 ticks per ear,19 which demonstrates the magnitude of tick infestation in some areas of the United States. Tick infestation is not merely of concern to the US military. Ticks are ubiquitous and can be found on neatly trimmed suburban lawns as well as in rough thickets.
More recently, bites from A americanum have been found to induce allergies to red meat in some patients.1 IgE antibodies directed against galactose-alpha-1,3-galactose (alpha gal) have been implicated as the cause of this reaction. These antibodies cause delayed-onset anaphylaxis occurring 3 to 6 hours after ingestion of red meat. Tick bites appear to be the most important and perhaps the only cause of IgE antibodies to alpha gal in the United States.1
Wild white-tailed deer serve as reservoir hosts for several diseases transmitted by A americanum, including HME, human ehrlichiosis ewingii, and Southern tick–associated rash illness.12,20 Communities located close to wildlife reserves may have higher rates of infection.21 Application of acaricides to corn contained in deer feeders has been shown to be an effective method of decreasing local tick populations, which is a potential method for disease control in at-risk areas, though it is costly and time consuming.22
Tick-Control Measures
Hard ticks produce little urine. Instead, excess water is eliminated via salivation back into the host. Loss of water also occurs through spiracles. Absorption of water from the atmosphere is important for the tick to maintain hydration. The tick produces intensely hygroscopic saliva that absorbs water from surrounding moist air. The humidified saliva is then reingested by the tick. In hot climates, ticks are prone to dehydration unless they can find a source of moist air, usually within a layer of leaf debris.23 When the leaf debris is stirred by a human walking through the area, the tick can make contact with the human. Therefore, removal of leaf debris is a critical part of tick-control efforts, as it reduces tick numbers by means of dehydration. Tick eggs also require sufficient humidity to hatch. Leaf removal increases the effectiveness of insecticide applications, which would otherwise do little harm to the ticks below if sprayed on top of leaf debris.
Some lone star ticks attach to birds and disseminate widely. Attachments to animal hosts with long-range migration patterns complicate tick-control efforts.24 Animal migration may contribute to the spread of disease from one geographic region to another.
Imported fire ants are voracious eaters that gather and consume ticks eggs. Fire ants provide an excellent natural means of tick control. Tick numbers in places such as Camp Bullis have declined dramatically since the introduction of imported fire ants.25
- Commins SP, Platts-Mills TA. Tick bites and red meat allergy. Curr Opin Allergy Clin Immunol. 2013;13:354-359.
- Springer YP, Eisen L, Beati L, et al. Spatial distribution of counties in the continental United States with records of occurrence of Amblyomma americanum (Ixodida: Ixodidae). J Med Entomol. 2014;51:342-351.
- Yu X, Piesman JF, Olson JG, et al. Geographic distribution of different genetic types of Ehrlichia chaffeensis. Am J Trop Med Hyg. 1997;56:679-680.
- Dumler JS, Bakken JS. Human ehrlichiosis: newly recognized infections transmitted by ticks. An Rev Med. 1998;49:201-213.
- Dumler JS, Madigan JE, Pusterla N, et al. Ehrlichioses in humans: epidemiology, clinical presentation, diagnosis, and treatment. Clin Infect Dis. 2007;45(suppl 1):S45-S51.
- Lockhart JM, Davidson WR, Stallknecht DE, et al. Natural history of Ehrlichia chaffeensis (Ricketsiales: Ehrlichiea) in the piedmont physiographic province of Georgia. J Parasitol. 1997;83:887-894.
- Centers for Disease Control and Prevention (CDC). Human ehrlichiosis—Maryland, 1994. MMWR Morb Mortal Wkly Rep. 1996;45:798-802.
- Ismail N, Bloch KC, McBride JW. Human ehrlichiosis and anaplasmosis. Clin Lab Med. 2010;30:261-292.
- McMullan LK, Folk SM, Kelly AJ, et al. A new phlebovirus associated with severe febrile illness in Missouri. N Engl J Med. 2012;367:834-841.
- Savage HM, Godsey MS Jr, Panella NA, et al. Surveillance for heartland virus (Bunyaviridae: Phlebovirus) in Missouri during 2013: first detection of virus in adults of Amblyomma americanum (Acari: Ixodidae) [published online March 30, 2016]. J Med Entomol. pii:tjw028.
- Cragun WC, Bartlett BL, Ellis MW, et al. The expanding spectrum of eschar-associated rickettsioses in the United States. Arch Dermatol. 2010;146:641-648.
- Paddock CD, Sumner JW, Comer JA, et al. Rickettsia parkeri: a newly recognized cause of spotted fever rickettsiosis in the United States. Clin Infect Dis. 2004;38:805-811.
- Goddard J, Varela-Stokes AS. Role of the lone star tick, Amblyomma americanum (L.) in human and animal diseases. Vet Parasitol. 2009;160:1-12.
- Oliver JH, Kollars TM, Chandler FW, et al. First isolation and cultivation of Borrelia burgdorferi sensu lato from Missouri. J Clin Microbiol. 1998;36:1-5.
- Ledin KE, Zeidner NS, Ribeiro JM, et al. Borreliacidal activity of saliva of the tick Amblyomma americanum. Med Vet Entomol. 2005;19:90-95.
- Feder HM Jr, Hoss DM, Zemel L, et al. Southern tick-associated rash illness (STARI) in the North: STARI following a tick bite in Long Island, New York. Clin Infect Dis. 2011;53:e142-e146.
- Varela AS, Luttrell MP, Howerth EW, et al. First culture isolation of Borrelia lonestari, putative agent of southern tick-associated rash illness. J Clin Microbiol. 2004;42:1163-1169.
- Livesay HR, Pollard M. Laboratory report on a clinical syndrome referred to as “Bullis Fever.” Am J Trop Med. 1943;23:475-479.
- Goddard J. Ticks and tickborne diseases affecting military personnel. US Air Force School of Aerospace Medicine USAFSAM-SR-89-2. http://www.dtic.mil/dtic/tr/fulltext/u2/a221956.pdf. Published September 1989. Accessed January 19, 2017.
- Lockhart JM, Davidson WR, Stallkneeckt DE, et al. Isolation of Ehrlichia chaffeensis from wild white tailed deer (Odocoileus virginianus) confirms their role as natural reservoir hosts. J Clin Microbiol. 1997;35:1681-1686.
- Standaert SM, Dawson JE, Schaffner W, et al. Ehrlichiosis in a golf-oriented retirement community. N Engl J Med. 1995;333:420-425.
- Schulze TL, Jordan RA, Hung RW, et al. Effectiveness of the 4-Poster passive topical treatment device in the control of Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae) in New Jersey. Vector Borne Zoonotic Dis. 2009;9:389-400.
- Strey OF, Teel PD, Longnecker MT, et al. Survival and water-balance characteristics of unfed Amblyomma cajennense (Acari: Ixodidae). J Med Entomol. 1996;33:63-73.
- Popham TW, Garris GI, Barre N. Development of a computer model of the population dynamics of Amblyomma variegatum and simulations of eradication strategies for use in the Caribbean. Ann New York Acad Sci. 1996;791:452-465.
- Burns EC, Melancon DG. Effect of important fire ant (Hymenoptera: Formicidae) invasion on lone star tick (Acarina: Ixodidae) populations. J Med Entomol. 1977;14:247-249.
- Commins SP, Platts-Mills TA. Tick bites and red meat allergy. Curr Opin Allergy Clin Immunol. 2013;13:354-359.
- Springer YP, Eisen L, Beati L, et al. Spatial distribution of counties in the continental United States with records of occurrence of Amblyomma americanum (Ixodida: Ixodidae). J Med Entomol. 2014;51:342-351.
- Yu X, Piesman JF, Olson JG, et al. Geographic distribution of different genetic types of Ehrlichia chaffeensis. Am J Trop Med Hyg. 1997;56:679-680.
- Dumler JS, Bakken JS. Human ehrlichiosis: newly recognized infections transmitted by ticks. An Rev Med. 1998;49:201-213.
- Dumler JS, Madigan JE, Pusterla N, et al. Ehrlichioses in humans: epidemiology, clinical presentation, diagnosis, and treatment. Clin Infect Dis. 2007;45(suppl 1):S45-S51.
- Lockhart JM, Davidson WR, Stallknecht DE, et al. Natural history of Ehrlichia chaffeensis (Ricketsiales: Ehrlichiea) in the piedmont physiographic province of Georgia. J Parasitol. 1997;83:887-894.
- Centers for Disease Control and Prevention (CDC). Human ehrlichiosis—Maryland, 1994. MMWR Morb Mortal Wkly Rep. 1996;45:798-802.
- Ismail N, Bloch KC, McBride JW. Human ehrlichiosis and anaplasmosis. Clin Lab Med. 2010;30:261-292.
- McMullan LK, Folk SM, Kelly AJ, et al. A new phlebovirus associated with severe febrile illness in Missouri. N Engl J Med. 2012;367:834-841.
- Savage HM, Godsey MS Jr, Panella NA, et al. Surveillance for heartland virus (Bunyaviridae: Phlebovirus) in Missouri during 2013: first detection of virus in adults of Amblyomma americanum (Acari: Ixodidae) [published online March 30, 2016]. J Med Entomol. pii:tjw028.
- Cragun WC, Bartlett BL, Ellis MW, et al. The expanding spectrum of eschar-associated rickettsioses in the United States. Arch Dermatol. 2010;146:641-648.
- Paddock CD, Sumner JW, Comer JA, et al. Rickettsia parkeri: a newly recognized cause of spotted fever rickettsiosis in the United States. Clin Infect Dis. 2004;38:805-811.
- Goddard J, Varela-Stokes AS. Role of the lone star tick, Amblyomma americanum (L.) in human and animal diseases. Vet Parasitol. 2009;160:1-12.
- Oliver JH, Kollars TM, Chandler FW, et al. First isolation and cultivation of Borrelia burgdorferi sensu lato from Missouri. J Clin Microbiol. 1998;36:1-5.
- Ledin KE, Zeidner NS, Ribeiro JM, et al. Borreliacidal activity of saliva of the tick Amblyomma americanum. Med Vet Entomol. 2005;19:90-95.
- Feder HM Jr, Hoss DM, Zemel L, et al. Southern tick-associated rash illness (STARI) in the North: STARI following a tick bite in Long Island, New York. Clin Infect Dis. 2011;53:e142-e146.
- Varela AS, Luttrell MP, Howerth EW, et al. First culture isolation of Borrelia lonestari, putative agent of southern tick-associated rash illness. J Clin Microbiol. 2004;42:1163-1169.
- Livesay HR, Pollard M. Laboratory report on a clinical syndrome referred to as “Bullis Fever.” Am J Trop Med. 1943;23:475-479.
- Goddard J. Ticks and tickborne diseases affecting military personnel. US Air Force School of Aerospace Medicine USAFSAM-SR-89-2. http://www.dtic.mil/dtic/tr/fulltext/u2/a221956.pdf. Published September 1989. Accessed January 19, 2017.
- Lockhart JM, Davidson WR, Stallkneeckt DE, et al. Isolation of Ehrlichia chaffeensis from wild white tailed deer (Odocoileus virginianus) confirms their role as natural reservoir hosts. J Clin Microbiol. 1997;35:1681-1686.
- Standaert SM, Dawson JE, Schaffner W, et al. Ehrlichiosis in a golf-oriented retirement community. N Engl J Med. 1995;333:420-425.
- Schulze TL, Jordan RA, Hung RW, et al. Effectiveness of the 4-Poster passive topical treatment device in the control of Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae) in New Jersey. Vector Borne Zoonotic Dis. 2009;9:389-400.
- Strey OF, Teel PD, Longnecker MT, et al. Survival and water-balance characteristics of unfed Amblyomma cajennense (Acari: Ixodidae). J Med Entomol. 1996;33:63-73.
- Popham TW, Garris GI, Barre N. Development of a computer model of the population dynamics of Amblyomma variegatum and simulations of eradication strategies for use in the Caribbean. Ann New York Acad Sci. 1996;791:452-465.
- Burns EC, Melancon DG. Effect of important fire ant (Hymenoptera: Formicidae) invasion on lone star tick (Acarina: Ixodidae) populations. J Med Entomol. 1977;14:247-249.
Practice Points
- Amblyomma americanum (lone star tick) is widely distributed throughout the United States and is an important cause of several tick-borne illnesses.
- Prompt diagnosis and treatment of tick-borne disease improves patient outcomes.
- In some cases, tick bites may cause the human host to develop certain IgE antibodies that result in a delayed-onset anaphylaxis after ingestion of red meat.
Clinicians Should Retain the Ability to Choose a Pathologist
As employers search for ways to reduce the cost of providing health care to their employees, there is a growing trend toward narrowed provider networks and exclusive laboratory contracts. In the case of clinical pathology, some of these choices make sense from the employer’s perspective. A complete blood cell count or comprehensive metabolic panel is done on a machine and the result is much the same regardless of the laboratory. So why not have all laboratory tests performed by the lowest bidder?
Laboratories vary in quality and anatomic pathology services are different from blood tests. Each slide must be interpreted by a physician and skill in the interpretation of skin specimens varies widely. Dermatopathology was one of the first subspecialties to be recognized within pathology, as it requires a high level of expertise. Clinicopathological correlation often is key to the accurate interpretation of a specimen. The stakes are high, and a delay in diagnosis of melanoma remains one of the most serious errors in medicine and one of the most common causes for litigation in dermatology.
The accurate interpretation of skin biopsy specimens becomes especially difficult when inadequate or misleading clinical information accompanies the specimen. A study of 589 biopsies submitted by primary care physicians and reported by general pathologists demonstrated a 6.5% error rate. False-negative errors were the most common, but false-positives also were observed.1 A study of pigmented lesions referred to the University of California, San Francisco, demonstrated a discordance rate of 14.3%.2 The degree of discordance would be expected to vary based on the range of diagnoses included in each study.
Board-certified dermatopathologists have varying areas of expertise and there is notable subjectivity in the interpretation of biopsy specimens. In the case of problematic pigmented lesions such as atypical Spitz nevi, there can be low interobserver agreement even among the experts in categorizing lesions as malignant versus nonmalignant (κ=0.30).3 The low concordance among expert dermatopathologists demonstrates that light microscopic features alone often are inadequate for diagnosis. Advanced studies, including immunohistochemical stains, can help to clarify the diagnosis. In the case of atypical Spitz tumors, the contribution of special stains to the final diagnosis is statistically similar to that of hematoxylin and eosin sections and age, suggesting that nothing alone is sufficiently reliable to establish a definitive diagnosis in every case.4 Although helpful, these studies are costly, and savings obtained by sending cases to the lowest bidder can evaporate quickly. Costs are higher when factoring in molecular studies, which can run upwards of $3000 per slide; the cost of litigation related to incorrect diagnoses; or the human costs of an incorrect diagnosis.
As a group, dermatopathologists are highly skilled in the interpretation of skin specimens, but challenging lesions are common in the routine practice of dermatopathology. A study of 1249 pigmented melanocytic lesions demonstrated substantial agreement among expert dermatopathologists for less problematic lesions, though agreement was greater for patients 40 years and older (κ=0.67) than for younger patients (κ=0.49). Agreement was lower for patients with atypical mole syndrome (κ=0.31).5 These discrepancies occur despite the fact that there is good interobserver reproducibility for grading of individual histological features such as asymmetry, circumscription, irregular confluent nests, single melanocytes predominating, absence of maturation, suprabasal melanocytes, symmetrical melanin, deep melanin, cytological atypia, mitoses, dermal lymphocytic infiltrate, and necrosis.6 These results indicate that accurate diagnoses cannot be reliably established simply by grading a list of histological features. Accurate diagnosis requires complex pattern recognition and integration of findings. Conflicting criteria often are present and an accurate interpretation requires considerable judgment as to which features are significant and which are not.
Separation of sebaceous adenoma, sebaceoma, and well-differentiated sebaceous carcinoma is another challenging area, and interobserver consensus can be as low as 11%,7 which suggests notable subjectivity in the criteria for diagnosis of nonmelanocytic tumors and emphasizes the importance of communication between the dermatopathologist and clinician when determining how to manage an ambiguous lesion. The interpretation of inflammatory skin diseases, alopecia, and lymphoid proliferations also can be problematic, and expert consultation often is required.
All dermatologists receive substantial training in dermatopathology, which puts them in an excellent position to interpret ambiguous findings in the context of the clinical presentation. Sometimes the dermatologist who has seen the clinical presentation can be in the best position to make the diagnosis. All clinicians must be wary of bias and an objective set of eyes often can be helpful. Communication is crucial to ensure appropriate care for each patient, and policies that restrict the choice of pathologist can be damaging.
- Trotter MJ, Bruecks AK. Interpretation of skin biopsies by general pathologists: diagnostic discrepancy rate measured by blinded review. Arch Pathol Lab Med. 2003;127:1489-1492.
- Shoo BA, Sagebiel RW, Kashani-Sabet M. Discordance in the histopathologic diagnosis of melanoma at a melanoma referral center [published online March 19, 2010]. J Am Acad Dermatol. 2010;62:751-756.
- Gerami P, Busam K, Cochran A, et al. Histomorphologic assessment and interobserver diagnostic reproducibility of atypical spitzoid melanocytic neoplasms with long-term follow-up. Am J Surg Pathol. 2014;38:934-940.
- Puri PK, Ferringer TC, Tyler WB, et al. Statistical analysis of the concordance of immunohistochemical stains with the final diagnosis in spitzoid neoplasms. Am J Dermatopathol. 2011;33:72-77.
- Braun RP, Gutkowicz-Krusin D, Rabinovitz H, et al. Agreement of dermatopathologists in the evaluation of clinically difficult melanocytic lesions: how golden is the ‘gold standard’? Dermatology. 2012;224:51-58.
- Urso C, Rongioletti F, Innocenzi D, et al. Interobserver reproducibility of histological features in cutaneous malignant melanoma. J Clin Pathol. 2005;58:1194-1198.
- Harvey NT, Budgeon CA, Leecy T, et al. Interobserver variability in the diagnosis of circumscribed sebaceous neoplasms of the skin. Pathology. 2013;45:581-586.
As employers search for ways to reduce the cost of providing health care to their employees, there is a growing trend toward narrowed provider networks and exclusive laboratory contracts. In the case of clinical pathology, some of these choices make sense from the employer’s perspective. A complete blood cell count or comprehensive metabolic panel is done on a machine and the result is much the same regardless of the laboratory. So why not have all laboratory tests performed by the lowest bidder?
Laboratories vary in quality and anatomic pathology services are different from blood tests. Each slide must be interpreted by a physician and skill in the interpretation of skin specimens varies widely. Dermatopathology was one of the first subspecialties to be recognized within pathology, as it requires a high level of expertise. Clinicopathological correlation often is key to the accurate interpretation of a specimen. The stakes are high, and a delay in diagnosis of melanoma remains one of the most serious errors in medicine and one of the most common causes for litigation in dermatology.
The accurate interpretation of skin biopsy specimens becomes especially difficult when inadequate or misleading clinical information accompanies the specimen. A study of 589 biopsies submitted by primary care physicians and reported by general pathologists demonstrated a 6.5% error rate. False-negative errors were the most common, but false-positives also were observed.1 A study of pigmented lesions referred to the University of California, San Francisco, demonstrated a discordance rate of 14.3%.2 The degree of discordance would be expected to vary based on the range of diagnoses included in each study.
Board-certified dermatopathologists have varying areas of expertise and there is notable subjectivity in the interpretation of biopsy specimens. In the case of problematic pigmented lesions such as atypical Spitz nevi, there can be low interobserver agreement even among the experts in categorizing lesions as malignant versus nonmalignant (κ=0.30).3 The low concordance among expert dermatopathologists demonstrates that light microscopic features alone often are inadequate for diagnosis. Advanced studies, including immunohistochemical stains, can help to clarify the diagnosis. In the case of atypical Spitz tumors, the contribution of special stains to the final diagnosis is statistically similar to that of hematoxylin and eosin sections and age, suggesting that nothing alone is sufficiently reliable to establish a definitive diagnosis in every case.4 Although helpful, these studies are costly, and savings obtained by sending cases to the lowest bidder can evaporate quickly. Costs are higher when factoring in molecular studies, which can run upwards of $3000 per slide; the cost of litigation related to incorrect diagnoses; or the human costs of an incorrect diagnosis.
As a group, dermatopathologists are highly skilled in the interpretation of skin specimens, but challenging lesions are common in the routine practice of dermatopathology. A study of 1249 pigmented melanocytic lesions demonstrated substantial agreement among expert dermatopathologists for less problematic lesions, though agreement was greater for patients 40 years and older (κ=0.67) than for younger patients (κ=0.49). Agreement was lower for patients with atypical mole syndrome (κ=0.31).5 These discrepancies occur despite the fact that there is good interobserver reproducibility for grading of individual histological features such as asymmetry, circumscription, irregular confluent nests, single melanocytes predominating, absence of maturation, suprabasal melanocytes, symmetrical melanin, deep melanin, cytological atypia, mitoses, dermal lymphocytic infiltrate, and necrosis.6 These results indicate that accurate diagnoses cannot be reliably established simply by grading a list of histological features. Accurate diagnosis requires complex pattern recognition and integration of findings. Conflicting criteria often are present and an accurate interpretation requires considerable judgment as to which features are significant and which are not.
Separation of sebaceous adenoma, sebaceoma, and well-differentiated sebaceous carcinoma is another challenging area, and interobserver consensus can be as low as 11%,7 which suggests notable subjectivity in the criteria for diagnosis of nonmelanocytic tumors and emphasizes the importance of communication between the dermatopathologist and clinician when determining how to manage an ambiguous lesion. The interpretation of inflammatory skin diseases, alopecia, and lymphoid proliferations also can be problematic, and expert consultation often is required.
All dermatologists receive substantial training in dermatopathology, which puts them in an excellent position to interpret ambiguous findings in the context of the clinical presentation. Sometimes the dermatologist who has seen the clinical presentation can be in the best position to make the diagnosis. All clinicians must be wary of bias and an objective set of eyes often can be helpful. Communication is crucial to ensure appropriate care for each patient, and policies that restrict the choice of pathologist can be damaging.
As employers search for ways to reduce the cost of providing health care to their employees, there is a growing trend toward narrowed provider networks and exclusive laboratory contracts. In the case of clinical pathology, some of these choices make sense from the employer’s perspective. A complete blood cell count or comprehensive metabolic panel is done on a machine and the result is much the same regardless of the laboratory. So why not have all laboratory tests performed by the lowest bidder?
Laboratories vary in quality and anatomic pathology services are different from blood tests. Each slide must be interpreted by a physician and skill in the interpretation of skin specimens varies widely. Dermatopathology was one of the first subspecialties to be recognized within pathology, as it requires a high level of expertise. Clinicopathological correlation often is key to the accurate interpretation of a specimen. The stakes are high, and a delay in diagnosis of melanoma remains one of the most serious errors in medicine and one of the most common causes for litigation in dermatology.
The accurate interpretation of skin biopsy specimens becomes especially difficult when inadequate or misleading clinical information accompanies the specimen. A study of 589 biopsies submitted by primary care physicians and reported by general pathologists demonstrated a 6.5% error rate. False-negative errors were the most common, but false-positives also were observed.1 A study of pigmented lesions referred to the University of California, San Francisco, demonstrated a discordance rate of 14.3%.2 The degree of discordance would be expected to vary based on the range of diagnoses included in each study.
Board-certified dermatopathologists have varying areas of expertise and there is notable subjectivity in the interpretation of biopsy specimens. In the case of problematic pigmented lesions such as atypical Spitz nevi, there can be low interobserver agreement even among the experts in categorizing lesions as malignant versus nonmalignant (κ=0.30).3 The low concordance among expert dermatopathologists demonstrates that light microscopic features alone often are inadequate for diagnosis. Advanced studies, including immunohistochemical stains, can help to clarify the diagnosis. In the case of atypical Spitz tumors, the contribution of special stains to the final diagnosis is statistically similar to that of hematoxylin and eosin sections and age, suggesting that nothing alone is sufficiently reliable to establish a definitive diagnosis in every case.4 Although helpful, these studies are costly, and savings obtained by sending cases to the lowest bidder can evaporate quickly. Costs are higher when factoring in molecular studies, which can run upwards of $3000 per slide; the cost of litigation related to incorrect diagnoses; or the human costs of an incorrect diagnosis.
As a group, dermatopathologists are highly skilled in the interpretation of skin specimens, but challenging lesions are common in the routine practice of dermatopathology. A study of 1249 pigmented melanocytic lesions demonstrated substantial agreement among expert dermatopathologists for less problematic lesions, though agreement was greater for patients 40 years and older (κ=0.67) than for younger patients (κ=0.49). Agreement was lower for patients with atypical mole syndrome (κ=0.31).5 These discrepancies occur despite the fact that there is good interobserver reproducibility for grading of individual histological features such as asymmetry, circumscription, irregular confluent nests, single melanocytes predominating, absence of maturation, suprabasal melanocytes, symmetrical melanin, deep melanin, cytological atypia, mitoses, dermal lymphocytic infiltrate, and necrosis.6 These results indicate that accurate diagnoses cannot be reliably established simply by grading a list of histological features. Accurate diagnosis requires complex pattern recognition and integration of findings. Conflicting criteria often are present and an accurate interpretation requires considerable judgment as to which features are significant and which are not.
Separation of sebaceous adenoma, sebaceoma, and well-differentiated sebaceous carcinoma is another challenging area, and interobserver consensus can be as low as 11%,7 which suggests notable subjectivity in the criteria for diagnosis of nonmelanocytic tumors and emphasizes the importance of communication between the dermatopathologist and clinician when determining how to manage an ambiguous lesion. The interpretation of inflammatory skin diseases, alopecia, and lymphoid proliferations also can be problematic, and expert consultation often is required.
All dermatologists receive substantial training in dermatopathology, which puts them in an excellent position to interpret ambiguous findings in the context of the clinical presentation. Sometimes the dermatologist who has seen the clinical presentation can be in the best position to make the diagnosis. All clinicians must be wary of bias and an objective set of eyes often can be helpful. Communication is crucial to ensure appropriate care for each patient, and policies that restrict the choice of pathologist can be damaging.
- Trotter MJ, Bruecks AK. Interpretation of skin biopsies by general pathologists: diagnostic discrepancy rate measured by blinded review. Arch Pathol Lab Med. 2003;127:1489-1492.
- Shoo BA, Sagebiel RW, Kashani-Sabet M. Discordance in the histopathologic diagnosis of melanoma at a melanoma referral center [published online March 19, 2010]. J Am Acad Dermatol. 2010;62:751-756.
- Gerami P, Busam K, Cochran A, et al. Histomorphologic assessment and interobserver diagnostic reproducibility of atypical spitzoid melanocytic neoplasms with long-term follow-up. Am J Surg Pathol. 2014;38:934-940.
- Puri PK, Ferringer TC, Tyler WB, et al. Statistical analysis of the concordance of immunohistochemical stains with the final diagnosis in spitzoid neoplasms. Am J Dermatopathol. 2011;33:72-77.
- Braun RP, Gutkowicz-Krusin D, Rabinovitz H, et al. Agreement of dermatopathologists in the evaluation of clinically difficult melanocytic lesions: how golden is the ‘gold standard’? Dermatology. 2012;224:51-58.
- Urso C, Rongioletti F, Innocenzi D, et al. Interobserver reproducibility of histological features in cutaneous malignant melanoma. J Clin Pathol. 2005;58:1194-1198.
- Harvey NT, Budgeon CA, Leecy T, et al. Interobserver variability in the diagnosis of circumscribed sebaceous neoplasms of the skin. Pathology. 2013;45:581-586.
- Trotter MJ, Bruecks AK. Interpretation of skin biopsies by general pathologists: diagnostic discrepancy rate measured by blinded review. Arch Pathol Lab Med. 2003;127:1489-1492.
- Shoo BA, Sagebiel RW, Kashani-Sabet M. Discordance in the histopathologic diagnosis of melanoma at a melanoma referral center [published online March 19, 2010]. J Am Acad Dermatol. 2010;62:751-756.
- Gerami P, Busam K, Cochran A, et al. Histomorphologic assessment and interobserver diagnostic reproducibility of atypical spitzoid melanocytic neoplasms with long-term follow-up. Am J Surg Pathol. 2014;38:934-940.
- Puri PK, Ferringer TC, Tyler WB, et al. Statistical analysis of the concordance of immunohistochemical stains with the final diagnosis in spitzoid neoplasms. Am J Dermatopathol. 2011;33:72-77.
- Braun RP, Gutkowicz-Krusin D, Rabinovitz H, et al. Agreement of dermatopathologists in the evaluation of clinically difficult melanocytic lesions: how golden is the ‘gold standard’? Dermatology. 2012;224:51-58.
- Urso C, Rongioletti F, Innocenzi D, et al. Interobserver reproducibility of histological features in cutaneous malignant melanoma. J Clin Pathol. 2005;58:1194-1198.
- Harvey NT, Budgeon CA, Leecy T, et al. Interobserver variability in the diagnosis of circumscribed sebaceous neoplasms of the skin. Pathology. 2013;45:581-586.
Epidermodysplasia Verruciformis and the Risk for Malignancy
To the Editor:
Epidermodysplasia verruciformis (EV) is a rare autosomal-recessive genodermatosis characterized by widespread infection with specific strains of human papillomavirus (HPV). Patients with EV have a unique susceptibility to acquire HPV due to defects in cellular immunity to the presenting antigens.1 These defects may be related to mutations of the EVER genes or due to acquisition of an immunosuppressive condition.2,3 Infections with HPV-3 and HPV-10 do not lead to the development of malignancies. However, infection with HPV-5, HPV-8, and HPV-14 can lead to the development of nonmelanoma skin cancers, usually squamous cell carcinomas (SCCs), in approximately 60% of patients.3,4 This viral condition lasts throughout the patient’s lifetime and presents as tinea versicolor–like macules and patches. These lesions may be confused with seborrheic keratosis or verruca plana.5 Lesions typically are hypopigmented but occasionally may be hyperpigmented or erythematous. They often are found on the trunk, but lesions on the face, arms, palms, legs, and soles have been reported.5 Mucous membranes are always spared. Epidermodysplasia verruciformis often presents in childhood, except in cases related to acquired immunosuppression. The condition has no sex or racial predilection and no geographical preference.5
A 7-year-old boy (Fitzpatrick skin type V) presented with an asymptomatic rash on the trunk (Figure 1), dorsal aspect of the hands, and forehead. The lesions first appeared 5 years prior on the upper back and upper chest and had recently spread to the forehead and frontal aspect of the scalp. The patient had a history of myelomeningocele, which was corrected at birth with surgical placement of a ventriculoperitoneal shunt. The patient was otherwise healthy and met all appropriate developmental milestones for his age group. Family history revealed consanguinity of the patient’s paternal grandparents who were first cousins. The patient’s mother denied any other family member having similar rashes or lesions.
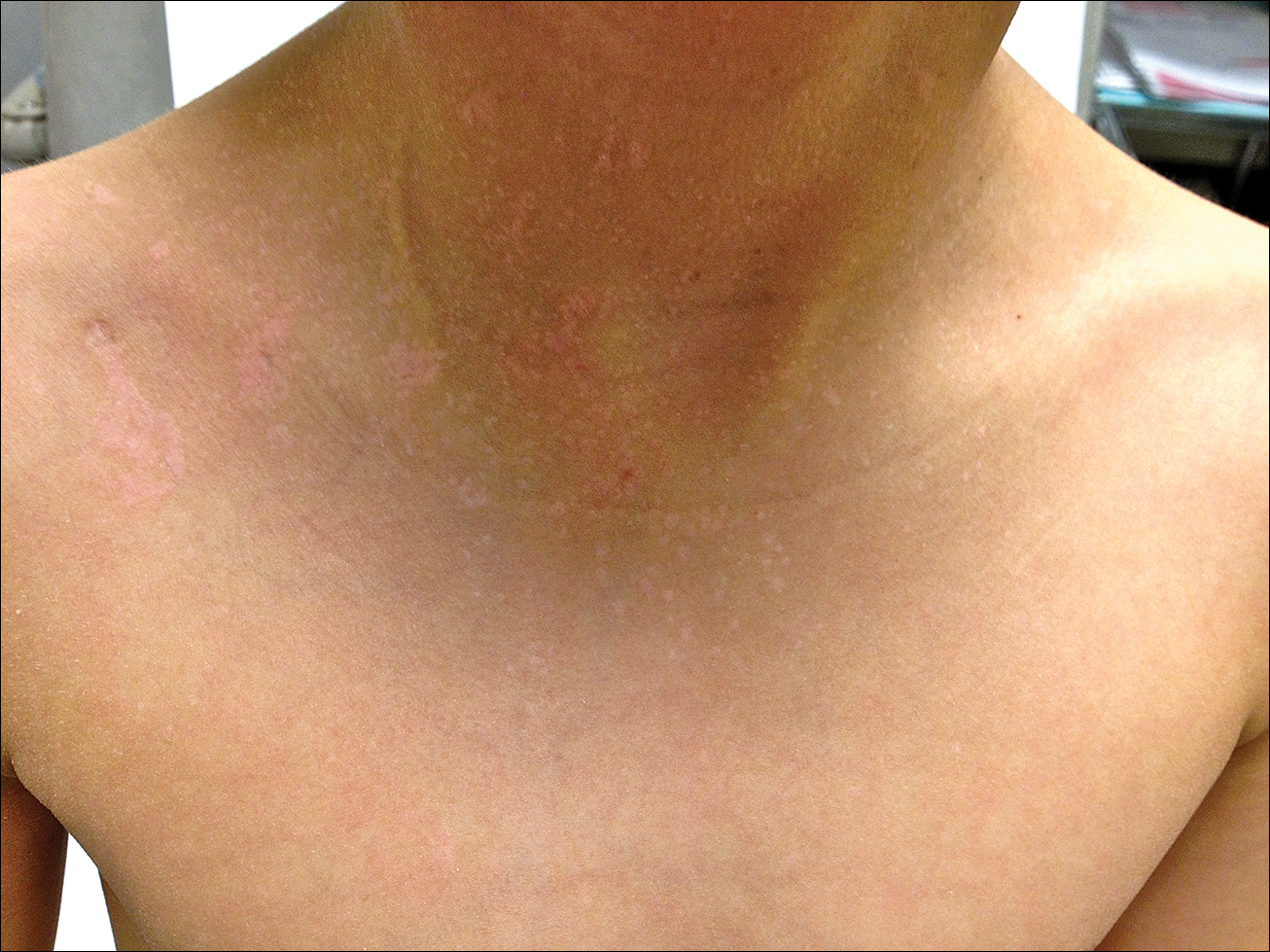
The patient had been treated for pityriasis versicolor on and off for 2 years by another dermatologist. His mother reported faithfully applying ketoconazole cream twice daily for several months with no improvement. She also reported using topical steroids, which did not provide any benefit. The patient and mother denied any associated pruritus, bleeding, burning, or physical discomfort.
Skin examination revealed diffuse, flat, polymorphous, hypopigmented and salmon-colored hyperkeratotic macules and patches with mild scaling on the upper region of the anterior aspect of the chest and upper back (Figure 2A). Additionally, the patient had an extensive number of lesions on the forehead and frontal aspect of the scalp (Figure 2B).

A shave biopsy demonstrated a thick basket weave stratum corneum, koilocytes, and large pale keratinocytes with characteristic blue cytoplasm. These findings were characteristic for EV.
At the patient’s 3-month follow-up visit, he again denied any symptoms associated with the lesions and reported that the appearance was diminishing in severity. On examination there was no evidence of SCC. The mother was advised to discontinue all topical treatments for the patient and return to the office every 3 to 6 months for regular skin surveillance. The mother was further advised to protect the patient from UV radiation with sunscreen and sun-protective clothing.
Epidermodysplasia verruciformis was first reported by Lewandowsky and Lutz6 in 1922. This rare condition often presents in childhood and is characterized by a persistent HPV infection and an autosomal-recessive inheritance pattern. Reports in the literature frequently involve kindreds. Often, patients with EV have a family history of first-degree or second-degree consanguinity.7
The clinical presentation of EV often resembles a pityriasis versicolor–like eruption. However, pityriasis versicolor is less commonly seen in childhood and is more prevalent in patients aged 21 to 30 years, likely due to increased sebum production and changing hormone levels. Furthermore, it is unusual to see pityriasis versicolor affect the face and scalp.8 Lesions of EV vary from hypopigmented and pinkish red macules to confluent patches and hyperkeratotic verrucalike lesions.3 Clinical characteristics also may include dyschromic patches; lesions that resemble flat warts on the trunk, face, and distal arms; and/or lesions that appear similar to seborrheic keratoses on the dorsal aspect of the hands.9,10
Mutations of the EVER gene downregulate a cell’s ability to adequately attack the HPV antigens.11 Although some patients with EV are found to have mutations of the EVER1 and EVER2 genes, a notable portion of patients with EV lack these mutations. Three other causes of EV include acquisition of immunosuppressive conditions including lymphoma, solid organ transplant, and human immunodeficiency virus. If one suspects autosomal-recessive inheritance of EV, genetic testing such as polymerase chain reaction DNA fragment analysis can be performed to determine if there are mutations on the EVER1 or EVER2 genes.12
The inability of patients with EV to mount an immune response to multiple types of HPV increases the risk for developing cutaneous malignancies.7 Additionally, it is known that UV radiation diminishes skin cell immunity, and the combination of EV and UV radiation further increases the risk for developing SCCs.11 The development of nonmelanoma skin cancers usually occurs on sun-exposed skin 20 to 30 years after the onset of lesions, with the highest occurrence of SCCs presenting in the fourth decade of life.1
Protection from UV light exposure is critical to reduce the risk for malignancy. Treatment options for EV lesions have included topical imiquimod 5%, 5-fluorouracil, oral isotretinoin, and intralesional interferon alfa, but patients are often refractory to these interventions. Curettage, surgical excision, electrosurgery, and laser ablation can be effective for individual lesions but carry a greater risk for scarring.1 Photodynamic therapy with aminolevulinic acid and blue light represents a promising option that deserves further study.
Epidermodysplasia verruciformis should be considered as a differential diagnosis in all patients presenting with disseminated lesions resembling pityriasis versicolor that are unresponsive to treatment. A biopsy will help to establish the diagnosis. Patients should minimize sun exposure and report any skin lesions that are changing in appearance.
- Hoffner MV, Camacho FM. Surgical treatment of epidermodysplasia verruciformis. Dermatol Surg. 2010;36:363-367.
- McDermott D, Gammon B, Snijders P. Autosomal dominant epidermodysplasia verruciformis lacking a known EVER1 or EVER2 mutation. Pediatr Dermatol. 2009;26:306-310.
- Patel T, Morrison K, Rady P, et al. Epidermodysplasia verruciformis and susceptibility to HPV. Dis Markers. 2010;29:199-206.
- Hultgren TL, Srinivasan SK, DiMaio DJ. Epidermodysplasia verruciformis occurring in a patient with human immunodeficiency virus: a case report. Cutis. 2007;79:308-311.
- Oliveira W, Netu C, Rady P, et al. Clinical aspects of epidermodysplasia verruciformis. J Eur Acad Dermatol Venereol. 2003;17:394-398.
- Lewandowsky F, Lutz W. Ein Fall einer bisher nicht beschriebenen Hauterkrankung (epidermodysplasia verruciformis). Arch Dermatol Syphilol. 1922;141:193-203.
- Prystowsky S, Herndon J, Freeman R, et al. Epidermodysplasia verruciformis. Am J Dis Child. 1976;130:437-440.
- Kyriakis KP, Terzoudi S, Palamaras I, et al. Pityriasis versicolor prevalence by age and gender. Mycoses. 2006;49:517-518.
- Nuovo G, Ishag M. The histologic spectrum of epidermodysplasia verruciformis. Am J Surg Pathol. 2000;24:1400-1406.
- Jacobelli S, Laude H, Carlotti A, et al. Epidermodysplasia verruciformis in human immunodeficiency virus-infected patients: a marker of human papillomavirus-related disorders not affected by antiretroviral therapy. Arch Dermatol. 2011;147:590-596.
- Rogers HD, MacGregor JL, Nord KM, et al. Acquired epidermodysplasia verruciformis. J Am Acad Dermatol. 2009;60:316-320.
- Gober MD, Rady PL, He Q, et al. Novel homozygous frameshift mutation of EVER1 gene in an epidermodysplasia verruciformis patient. J Invest Dermatol. 2007;127:817-820.
To the Editor:
Epidermodysplasia verruciformis (EV) is a rare autosomal-recessive genodermatosis characterized by widespread infection with specific strains of human papillomavirus (HPV). Patients with EV have a unique susceptibility to acquire HPV due to defects in cellular immunity to the presenting antigens.1 These defects may be related to mutations of the EVER genes or due to acquisition of an immunosuppressive condition.2,3 Infections with HPV-3 and HPV-10 do not lead to the development of malignancies. However, infection with HPV-5, HPV-8, and HPV-14 can lead to the development of nonmelanoma skin cancers, usually squamous cell carcinomas (SCCs), in approximately 60% of patients.3,4 This viral condition lasts throughout the patient’s lifetime and presents as tinea versicolor–like macules and patches. These lesions may be confused with seborrheic keratosis or verruca plana.5 Lesions typically are hypopigmented but occasionally may be hyperpigmented or erythematous. They often are found on the trunk, but lesions on the face, arms, palms, legs, and soles have been reported.5 Mucous membranes are always spared. Epidermodysplasia verruciformis often presents in childhood, except in cases related to acquired immunosuppression. The condition has no sex or racial predilection and no geographical preference.5
A 7-year-old boy (Fitzpatrick skin type V) presented with an asymptomatic rash on the trunk (Figure 1), dorsal aspect of the hands, and forehead. The lesions first appeared 5 years prior on the upper back and upper chest and had recently spread to the forehead and frontal aspect of the scalp. The patient had a history of myelomeningocele, which was corrected at birth with surgical placement of a ventriculoperitoneal shunt. The patient was otherwise healthy and met all appropriate developmental milestones for his age group. Family history revealed consanguinity of the patient’s paternal grandparents who were first cousins. The patient’s mother denied any other family member having similar rashes or lesions.

The patient had been treated for pityriasis versicolor on and off for 2 years by another dermatologist. His mother reported faithfully applying ketoconazole cream twice daily for several months with no improvement. She also reported using topical steroids, which did not provide any benefit. The patient and mother denied any associated pruritus, bleeding, burning, or physical discomfort.
Skin examination revealed diffuse, flat, polymorphous, hypopigmented and salmon-colored hyperkeratotic macules and patches with mild scaling on the upper region of the anterior aspect of the chest and upper back (Figure 2A). Additionally, the patient had an extensive number of lesions on the forehead and frontal aspect of the scalp (Figure 2B).

A shave biopsy demonstrated a thick basket weave stratum corneum, koilocytes, and large pale keratinocytes with characteristic blue cytoplasm. These findings were characteristic for EV.
At the patient’s 3-month follow-up visit, he again denied any symptoms associated with the lesions and reported that the appearance was diminishing in severity. On examination there was no evidence of SCC. The mother was advised to discontinue all topical treatments for the patient and return to the office every 3 to 6 months for regular skin surveillance. The mother was further advised to protect the patient from UV radiation with sunscreen and sun-protective clothing.
Epidermodysplasia verruciformis was first reported by Lewandowsky and Lutz6 in 1922. This rare condition often presents in childhood and is characterized by a persistent HPV infection and an autosomal-recessive inheritance pattern. Reports in the literature frequently involve kindreds. Often, patients with EV have a family history of first-degree or second-degree consanguinity.7
The clinical presentation of EV often resembles a pityriasis versicolor–like eruption. However, pityriasis versicolor is less commonly seen in childhood and is more prevalent in patients aged 21 to 30 years, likely due to increased sebum production and changing hormone levels. Furthermore, it is unusual to see pityriasis versicolor affect the face and scalp.8 Lesions of EV vary from hypopigmented and pinkish red macules to confluent patches and hyperkeratotic verrucalike lesions.3 Clinical characteristics also may include dyschromic patches; lesions that resemble flat warts on the trunk, face, and distal arms; and/or lesions that appear similar to seborrheic keratoses on the dorsal aspect of the hands.9,10
Mutations of the EVER gene downregulate a cell’s ability to adequately attack the HPV antigens.11 Although some patients with EV are found to have mutations of the EVER1 and EVER2 genes, a notable portion of patients with EV lack these mutations. Three other causes of EV include acquisition of immunosuppressive conditions including lymphoma, solid organ transplant, and human immunodeficiency virus. If one suspects autosomal-recessive inheritance of EV, genetic testing such as polymerase chain reaction DNA fragment analysis can be performed to determine if there are mutations on the EVER1 or EVER2 genes.12
The inability of patients with EV to mount an immune response to multiple types of HPV increases the risk for developing cutaneous malignancies.7 Additionally, it is known that UV radiation diminishes skin cell immunity, and the combination of EV and UV radiation further increases the risk for developing SCCs.11 The development of nonmelanoma skin cancers usually occurs on sun-exposed skin 20 to 30 years after the onset of lesions, with the highest occurrence of SCCs presenting in the fourth decade of life.1
Protection from UV light exposure is critical to reduce the risk for malignancy. Treatment options for EV lesions have included topical imiquimod 5%, 5-fluorouracil, oral isotretinoin, and intralesional interferon alfa, but patients are often refractory to these interventions. Curettage, surgical excision, electrosurgery, and laser ablation can be effective for individual lesions but carry a greater risk for scarring.1 Photodynamic therapy with aminolevulinic acid and blue light represents a promising option that deserves further study.
Epidermodysplasia verruciformis should be considered as a differential diagnosis in all patients presenting with disseminated lesions resembling pityriasis versicolor that are unresponsive to treatment. A biopsy will help to establish the diagnosis. Patients should minimize sun exposure and report any skin lesions that are changing in appearance.
To the Editor:
Epidermodysplasia verruciformis (EV) is a rare autosomal-recessive genodermatosis characterized by widespread infection with specific strains of human papillomavirus (HPV). Patients with EV have a unique susceptibility to acquire HPV due to defects in cellular immunity to the presenting antigens.1 These defects may be related to mutations of the EVER genes or due to acquisition of an immunosuppressive condition.2,3 Infections with HPV-3 and HPV-10 do not lead to the development of malignancies. However, infection with HPV-5, HPV-8, and HPV-14 can lead to the development of nonmelanoma skin cancers, usually squamous cell carcinomas (SCCs), in approximately 60% of patients.3,4 This viral condition lasts throughout the patient’s lifetime and presents as tinea versicolor–like macules and patches. These lesions may be confused with seborrheic keratosis or verruca plana.5 Lesions typically are hypopigmented but occasionally may be hyperpigmented or erythematous. They often are found on the trunk, but lesions on the face, arms, palms, legs, and soles have been reported.5 Mucous membranes are always spared. Epidermodysplasia verruciformis often presents in childhood, except in cases related to acquired immunosuppression. The condition has no sex or racial predilection and no geographical preference.5
A 7-year-old boy (Fitzpatrick skin type V) presented with an asymptomatic rash on the trunk (Figure 1), dorsal aspect of the hands, and forehead. The lesions first appeared 5 years prior on the upper back and upper chest and had recently spread to the forehead and frontal aspect of the scalp. The patient had a history of myelomeningocele, which was corrected at birth with surgical placement of a ventriculoperitoneal shunt. The patient was otherwise healthy and met all appropriate developmental milestones for his age group. Family history revealed consanguinity of the patient’s paternal grandparents who were first cousins. The patient’s mother denied any other family member having similar rashes or lesions.

The patient had been treated for pityriasis versicolor on and off for 2 years by another dermatologist. His mother reported faithfully applying ketoconazole cream twice daily for several months with no improvement. She also reported using topical steroids, which did not provide any benefit. The patient and mother denied any associated pruritus, bleeding, burning, or physical discomfort.
Skin examination revealed diffuse, flat, polymorphous, hypopigmented and salmon-colored hyperkeratotic macules and patches with mild scaling on the upper region of the anterior aspect of the chest and upper back (Figure 2A). Additionally, the patient had an extensive number of lesions on the forehead and frontal aspect of the scalp (Figure 2B).

A shave biopsy demonstrated a thick basket weave stratum corneum, koilocytes, and large pale keratinocytes with characteristic blue cytoplasm. These findings were characteristic for EV.
At the patient’s 3-month follow-up visit, he again denied any symptoms associated with the lesions and reported that the appearance was diminishing in severity. On examination there was no evidence of SCC. The mother was advised to discontinue all topical treatments for the patient and return to the office every 3 to 6 months for regular skin surveillance. The mother was further advised to protect the patient from UV radiation with sunscreen and sun-protective clothing.
Epidermodysplasia verruciformis was first reported by Lewandowsky and Lutz6 in 1922. This rare condition often presents in childhood and is characterized by a persistent HPV infection and an autosomal-recessive inheritance pattern. Reports in the literature frequently involve kindreds. Often, patients with EV have a family history of first-degree or second-degree consanguinity.7
The clinical presentation of EV often resembles a pityriasis versicolor–like eruption. However, pityriasis versicolor is less commonly seen in childhood and is more prevalent in patients aged 21 to 30 years, likely due to increased sebum production and changing hormone levels. Furthermore, it is unusual to see pityriasis versicolor affect the face and scalp.8 Lesions of EV vary from hypopigmented and pinkish red macules to confluent patches and hyperkeratotic verrucalike lesions.3 Clinical characteristics also may include dyschromic patches; lesions that resemble flat warts on the trunk, face, and distal arms; and/or lesions that appear similar to seborrheic keratoses on the dorsal aspect of the hands.9,10
Mutations of the EVER gene downregulate a cell’s ability to adequately attack the HPV antigens.11 Although some patients with EV are found to have mutations of the EVER1 and EVER2 genes, a notable portion of patients with EV lack these mutations. Three other causes of EV include acquisition of immunosuppressive conditions including lymphoma, solid organ transplant, and human immunodeficiency virus. If one suspects autosomal-recessive inheritance of EV, genetic testing such as polymerase chain reaction DNA fragment analysis can be performed to determine if there are mutations on the EVER1 or EVER2 genes.12
The inability of patients with EV to mount an immune response to multiple types of HPV increases the risk for developing cutaneous malignancies.7 Additionally, it is known that UV radiation diminishes skin cell immunity, and the combination of EV and UV radiation further increases the risk for developing SCCs.11 The development of nonmelanoma skin cancers usually occurs on sun-exposed skin 20 to 30 years after the onset of lesions, with the highest occurrence of SCCs presenting in the fourth decade of life.1
Protection from UV light exposure is critical to reduce the risk for malignancy. Treatment options for EV lesions have included topical imiquimod 5%, 5-fluorouracil, oral isotretinoin, and intralesional interferon alfa, but patients are often refractory to these interventions. Curettage, surgical excision, electrosurgery, and laser ablation can be effective for individual lesions but carry a greater risk for scarring.1 Photodynamic therapy with aminolevulinic acid and blue light represents a promising option that deserves further study.
Epidermodysplasia verruciformis should be considered as a differential diagnosis in all patients presenting with disseminated lesions resembling pityriasis versicolor that are unresponsive to treatment. A biopsy will help to establish the diagnosis. Patients should minimize sun exposure and report any skin lesions that are changing in appearance.
- Hoffner MV, Camacho FM. Surgical treatment of epidermodysplasia verruciformis. Dermatol Surg. 2010;36:363-367.
- McDermott D, Gammon B, Snijders P. Autosomal dominant epidermodysplasia verruciformis lacking a known EVER1 or EVER2 mutation. Pediatr Dermatol. 2009;26:306-310.
- Patel T, Morrison K, Rady P, et al. Epidermodysplasia verruciformis and susceptibility to HPV. Dis Markers. 2010;29:199-206.
- Hultgren TL, Srinivasan SK, DiMaio DJ. Epidermodysplasia verruciformis occurring in a patient with human immunodeficiency virus: a case report. Cutis. 2007;79:308-311.
- Oliveira W, Netu C, Rady P, et al. Clinical aspects of epidermodysplasia verruciformis. J Eur Acad Dermatol Venereol. 2003;17:394-398.
- Lewandowsky F, Lutz W. Ein Fall einer bisher nicht beschriebenen Hauterkrankung (epidermodysplasia verruciformis). Arch Dermatol Syphilol. 1922;141:193-203.
- Prystowsky S, Herndon J, Freeman R, et al. Epidermodysplasia verruciformis. Am J Dis Child. 1976;130:437-440.
- Kyriakis KP, Terzoudi S, Palamaras I, et al. Pityriasis versicolor prevalence by age and gender. Mycoses. 2006;49:517-518.
- Nuovo G, Ishag M. The histologic spectrum of epidermodysplasia verruciformis. Am J Surg Pathol. 2000;24:1400-1406.
- Jacobelli S, Laude H, Carlotti A, et al. Epidermodysplasia verruciformis in human immunodeficiency virus-infected patients: a marker of human papillomavirus-related disorders not affected by antiretroviral therapy. Arch Dermatol. 2011;147:590-596.
- Rogers HD, MacGregor JL, Nord KM, et al. Acquired epidermodysplasia verruciformis. J Am Acad Dermatol. 2009;60:316-320.
- Gober MD, Rady PL, He Q, et al. Novel homozygous frameshift mutation of EVER1 gene in an epidermodysplasia verruciformis patient. J Invest Dermatol. 2007;127:817-820.
- Hoffner MV, Camacho FM. Surgical treatment of epidermodysplasia verruciformis. Dermatol Surg. 2010;36:363-367.
- McDermott D, Gammon B, Snijders P. Autosomal dominant epidermodysplasia verruciformis lacking a known EVER1 or EVER2 mutation. Pediatr Dermatol. 2009;26:306-310.
- Patel T, Morrison K, Rady P, et al. Epidermodysplasia verruciformis and susceptibility to HPV. Dis Markers. 2010;29:199-206.
- Hultgren TL, Srinivasan SK, DiMaio DJ. Epidermodysplasia verruciformis occurring in a patient with human immunodeficiency virus: a case report. Cutis. 2007;79:308-311.
- Oliveira W, Netu C, Rady P, et al. Clinical aspects of epidermodysplasia verruciformis. J Eur Acad Dermatol Venereol. 2003;17:394-398.
- Lewandowsky F, Lutz W. Ein Fall einer bisher nicht beschriebenen Hauterkrankung (epidermodysplasia verruciformis). Arch Dermatol Syphilol. 1922;141:193-203.
- Prystowsky S, Herndon J, Freeman R, et al. Epidermodysplasia verruciformis. Am J Dis Child. 1976;130:437-440.
- Kyriakis KP, Terzoudi S, Palamaras I, et al. Pityriasis versicolor prevalence by age and gender. Mycoses. 2006;49:517-518.
- Nuovo G, Ishag M. The histologic spectrum of epidermodysplasia verruciformis. Am J Surg Pathol. 2000;24:1400-1406.
- Jacobelli S, Laude H, Carlotti A, et al. Epidermodysplasia verruciformis in human immunodeficiency virus-infected patients: a marker of human papillomavirus-related disorders not affected by antiretroviral therapy. Arch Dermatol. 2011;147:590-596.
- Rogers HD, MacGregor JL, Nord KM, et al. Acquired epidermodysplasia verruciformis. J Am Acad Dermatol. 2009;60:316-320.
- Gober MD, Rady PL, He Q, et al. Novel homozygous frameshift mutation of EVER1 gene in an epidermodysplasia verruciformis patient. J Invest Dermatol. 2007;127:817-820.
Practice Points
- Epidermodysplasia verruciformis (EV) is a rare genodermatosis that usually presents in early childhood and presents as verrucous papules and plaques most commonly on the skin of the head, neck, and upper extremities. It often is misdiagnosed at pityriasis versicolor.
- Mutations of the EVER1 and EVER2 genes have been identified as a source for developing EV.
- Epidermodysplasia verruciformis produces wartlike lesions in individuals who have a unique susceptibility to acquiring the human papillomavirus and early onset of nonmelanoma skin cancers, most commonly squamous cell carcinomas related to viral oncogenesis.
- Avoidance and protection from UV exposure is a critical component of treatment plans for patients with EV.
Rapidly Growing Scalp Nodule
Cutaneous Metastasis of Pulmonary Adenocarcinoma
Cutaneous metastasis of pulmonary adenocarcinoma (CMPA) is a rare phenomenon with an overall survival rate of less than 5 months.1,2 Often, CMPA can be the heralding feature of an aggressive systemic malignancy in 2.8% to 22% of reported cases.2-4 Clinically, CMPAs often present as fixed, violaceous, ulcerated nodules on the chest wall, scalp, or site of a prior procedure.3,5,6 Other clinical presentations have been described including zosteriform and inflammatory carcinomalike CMPA and CMPA on the tip of the nose.7 Histologically, CMPA presents as a subdermal collection of atypical glands arranged as clustered aggregates of infiltrative glands penetrating the dermal stroma (quiz image). The atypical glands have large oval nuclei with high nuclear to cytoplasm ratios with scant pale cytoplasm.
Cutaneous metastasis of pulmonary adenocarcinoma is difficult to distinguish from other metastatic or primary glandular malignancies based on histology alone. Immunohistochemical analysis can aid in the diagnosis of the primary tumor. Pulmonary adenocarcinomas are positive for cytokeratin (CK) 7 and thyroid transcription factor 1 (TTF-1), and they are negative for CK5/6 and CK20.7 The differential diagnosis for CMPA includes other internal malignancies such as invasive ductal adenocarcinoma of the breast and gastrointestinal adenocarcinomas (eg, gastric or colorectal carcinoma [CRC]). Additionally, endometriosis and primary sebaceous carcinomas can mimic cutaneous metastatic adenocarcinomas.
Endometriosis can mimic adenocarcinoma, especially when presenting as a subdermal nodule. However, the scattered dermal glands are cytologically banal and are surrounded by uterine-type stroma and extravasated hemorrhage, a classic presentation of endometriosis (Figure 1).
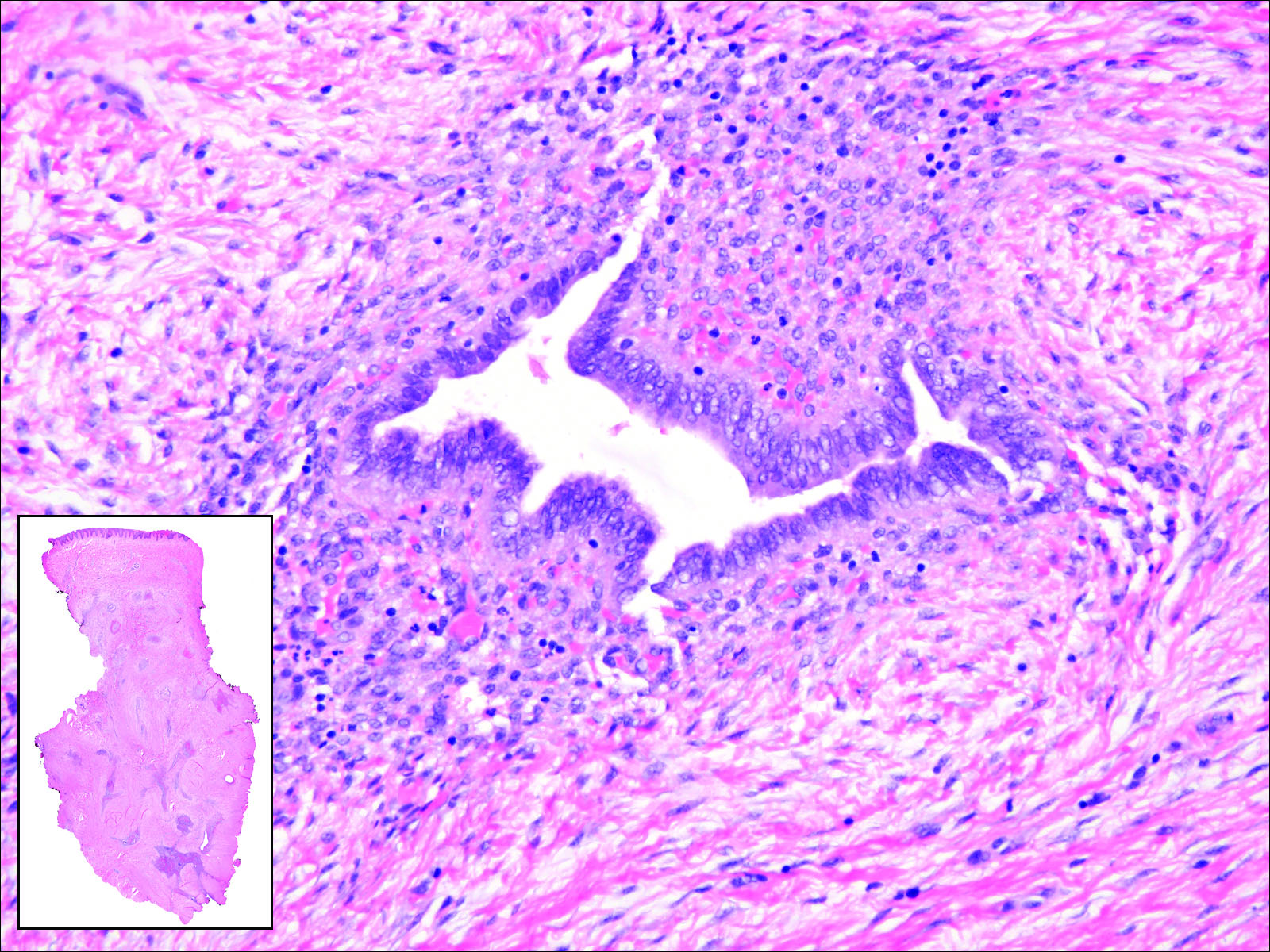
Invasive ductal carcinoma of the breast is one of the most common cutaneous metastases of internal malignancy.3 Clinically, these lesions present on the chest wall or abdomen as flesh-colored nodules. Histopathology generally reveals either tubular or single tumor cells infiltrating the dermis with surrounding desmoplastic fibrosis (Figure 2). Immunohistochemistry typically is positive for CK7, estrogen receptor, and mammaglobin, and negative for CK20, CK5/6, and TTF-1.

Gastrointestinal adenocarcinomas encompass a variety of primary sites that can metastasize to the skin including CRC. Clinically, cutaneous metastases of CRC present as multiple nodules on the trunk, abdomen, or umbilicus (also known as Sister Mary Joseph nodule).7,8 Distinguishing CRC as the primary site of origin can be difficult; however, there are subtle differences depending on the histologic subtype. In well-differentiated CRCs, well-defined atypical glands are haphazardly arranged within the dermis (Figure 3), while poorly differentiated lesions can present as single cells or with a signet ring-like morphology (Figure 4). For perianal lesions, extramammary Paget disease should be considered when biopsies show large, amphophilic, intraepithelial cells. These lesions often present with mucin and CK20 expression and are frequently associated with colorectal malignancies.9 Another characteristic feature of CRC is central necrosis with karyorrhectic debris, known as dirty necrosis. Immunohistochemical analysis typically shows expression of caudal type homeobox 2 and CK20 with infrequent expression of CK7 and no expression of TTF-1; however, additional clinical history (eg, history of colorectal adenocarcinoma, positive fecal occult blood test) often is the best distinguishing feature.
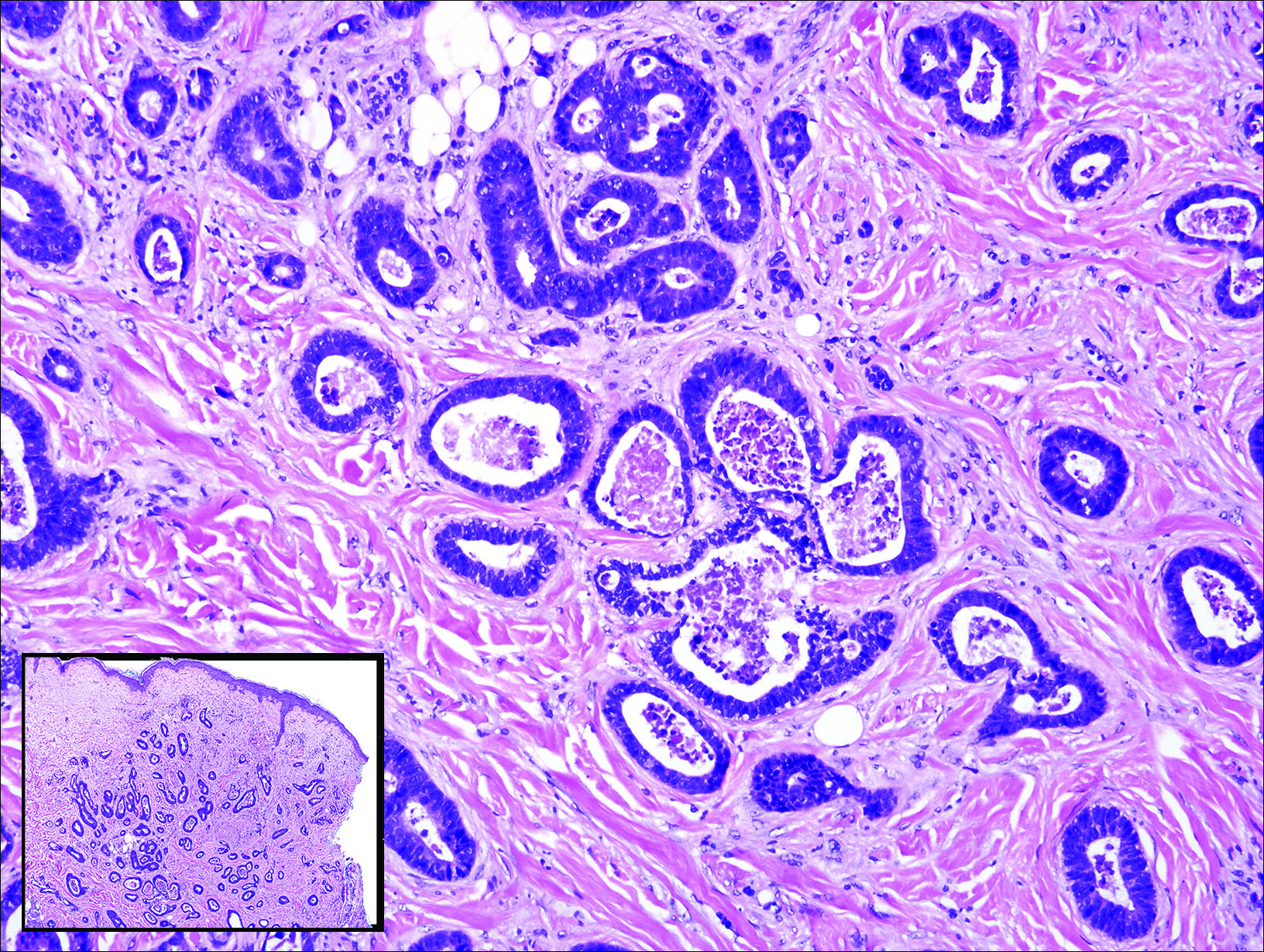
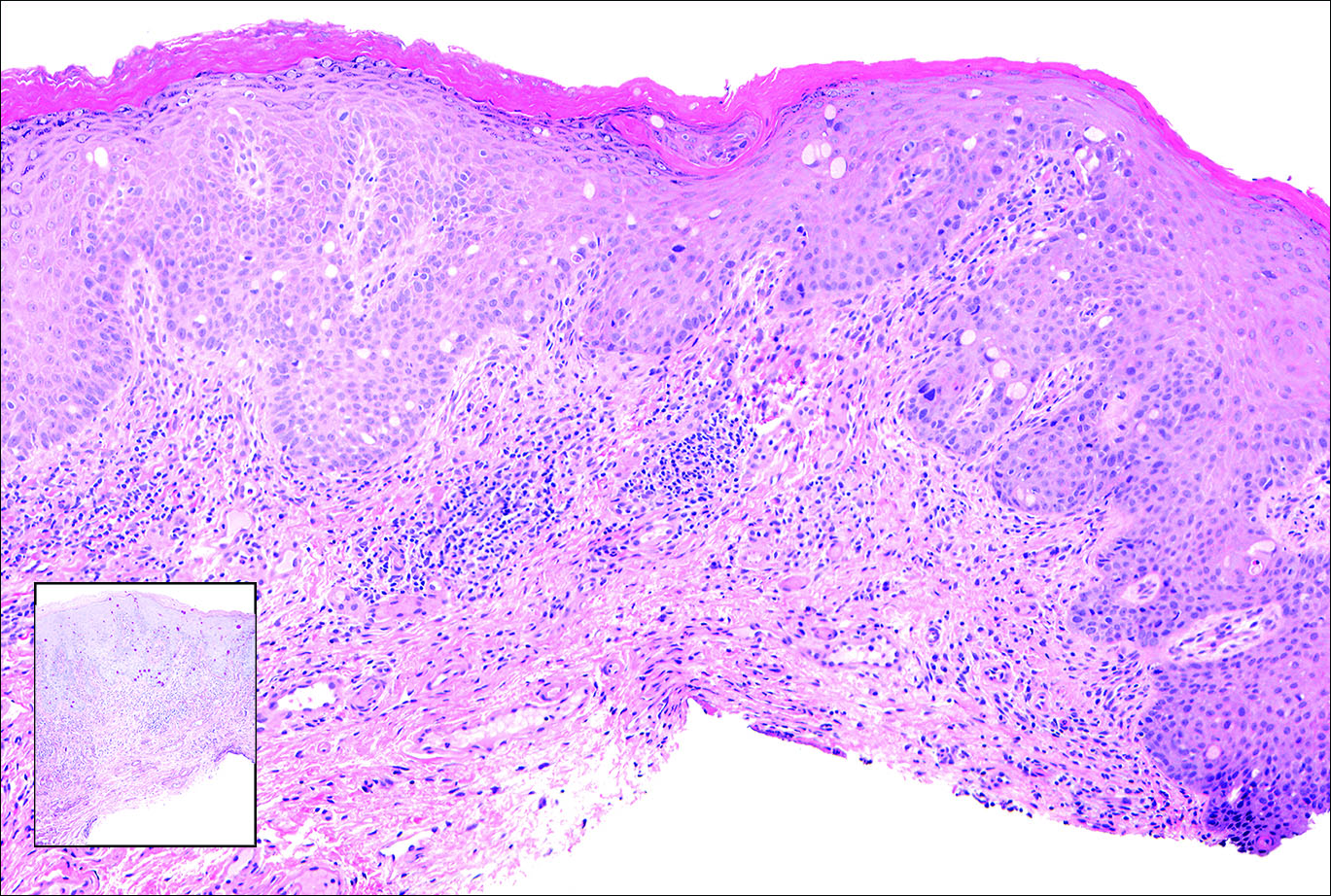
Primary sebaceous carcinoma also can mimic metastatic adenocarcinoma within the skin and is histologically similar to metastatic adenocarcinomas. The most distinguishing feature is sebaceous differentiation characterized by sebocytes, which have a vacuolated cytoplasm giving the nucleus a scalloped appearance, frequently with adjacent ductlike structures (Figure 5). Epidermotropism sometimes is present in sebaceous carcinomas but cannot be relied on as a distinguishing feature. Immunohistochemical analysis also is a helpful tool; these tumors typically are positive for p63 and podoplanin, distinguishing them from negative-staining metastatic adenocarcinomas.10,11
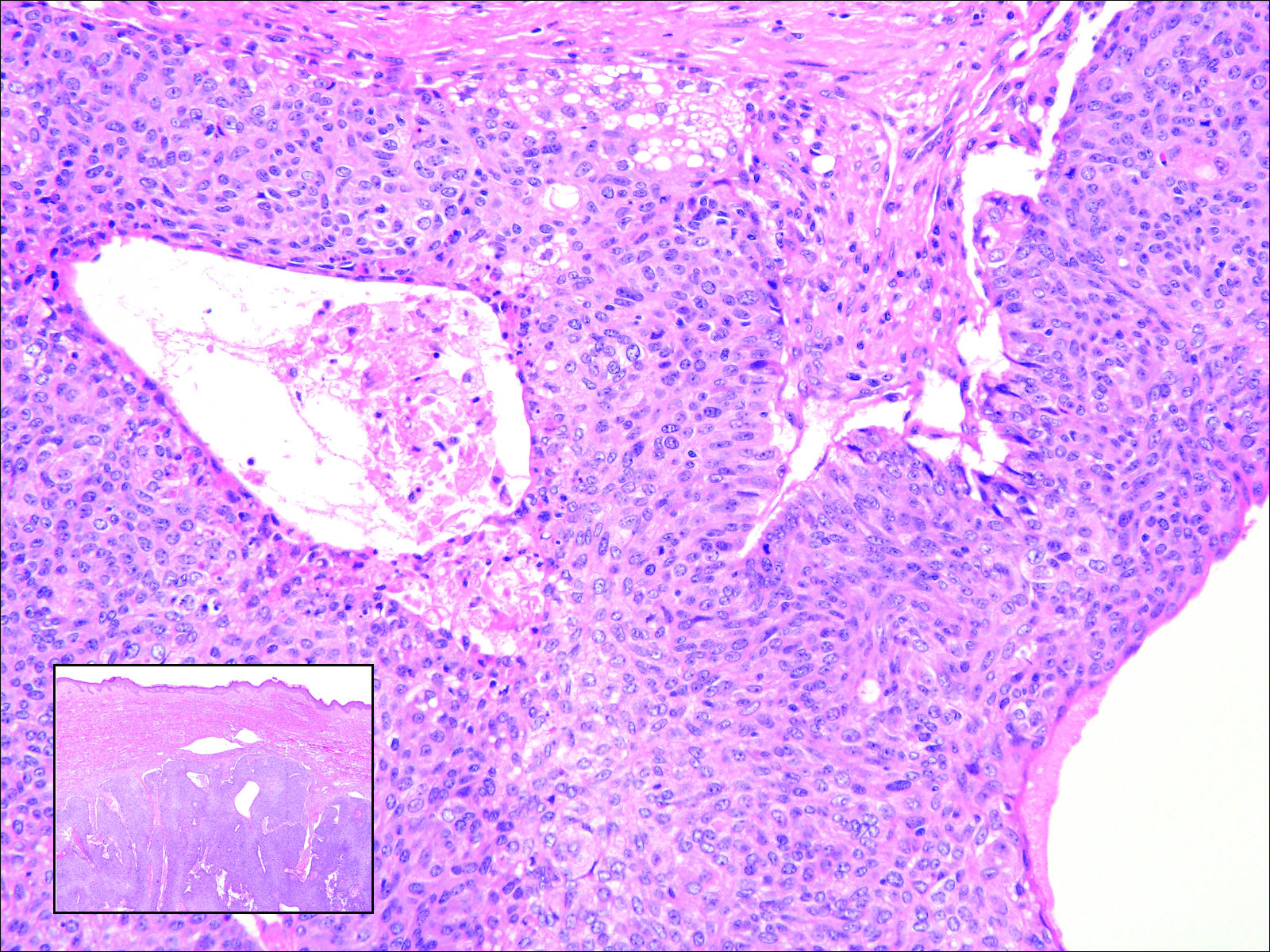
- Terashima T, Kanazawa M. Lung cancer with skin metastasis. Chest. 1994;106:1448-1450.
- Song Z, Lin B, Shao L, et al. Cutaneous metastasis as a initial presentation in advanced non-small cell lung cancer and its poor survival prognosis. J Cancer Res Clin Oncol. 2012;138:1613-1617.
- Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29(2, pt 1):228-236.
- Saeed S, Keehn CA, Morgan MB. Cutaneous metastasis: a clinical, pathological, and immunohistochemical appraisal. J Cutan Pathol. 2004;31:419-430.
- Chang SE, Choi JC, Moon KC. A papillary carcinoma: cutaneous metastases from lung cancer. J Dermatol. 2001;28:110-111.
- Snow S, Madjar D, Reizner G, et al. Renal cell carcinoma metastatic to the scalp: case report and review of the literature. Dermatol Surg. 2001;27:192-194.
- Alcaraz I, Cerroni L, Rutten A, et al. Cutaneous metastases from internal malignancies: a clinicopathologic and immunohistochemical review. Am J Dermatopathol. 2012;34:347-393.
- Schwartz IS. Sister (Mary?) Joseph's nodule. N Engl J Med. 1987;316:1348-1349.
- Goldblum J, Hart W. Perianal Paget's disease: a histologic and immunohistochemical study of 11 cases with and without associated rectal adenocarcinoma. Am J Surg Pathol. 1998;22:170-179.
- Ivan D, Nash J, Preito V, et al. Use of p63 expression in distinguishing primary and metastatic cutaneous adnexal neoplasms from metastatic adenocarcinoma to skin. J Cutan Pathol. 2006;34:474-480.
- Liang H, Wu H, Giorgadze T, et al. Podoplanin is a highly sensitive and specific marker to distinguish primary skin adnexal carcinomas from adenocarcinomas metastatic to skin. Am J Surg Pathol. 2007;31:304-310.
Cutaneous Metastasis of Pulmonary Adenocarcinoma
Cutaneous metastasis of pulmonary adenocarcinoma (CMPA) is a rare phenomenon with an overall survival rate of less than 5 months.1,2 Often, CMPA can be the heralding feature of an aggressive systemic malignancy in 2.8% to 22% of reported cases.2-4 Clinically, CMPAs often present as fixed, violaceous, ulcerated nodules on the chest wall, scalp, or site of a prior procedure.3,5,6 Other clinical presentations have been described including zosteriform and inflammatory carcinomalike CMPA and CMPA on the tip of the nose.7 Histologically, CMPA presents as a subdermal collection of atypical glands arranged as clustered aggregates of infiltrative glands penetrating the dermal stroma (quiz image). The atypical glands have large oval nuclei with high nuclear to cytoplasm ratios with scant pale cytoplasm.
Cutaneous metastasis of pulmonary adenocarcinoma is difficult to distinguish from other metastatic or primary glandular malignancies based on histology alone. Immunohistochemical analysis can aid in the diagnosis of the primary tumor. Pulmonary adenocarcinomas are positive for cytokeratin (CK) 7 and thyroid transcription factor 1 (TTF-1), and they are negative for CK5/6 and CK20.7 The differential diagnosis for CMPA includes other internal malignancies such as invasive ductal adenocarcinoma of the breast and gastrointestinal adenocarcinomas (eg, gastric or colorectal carcinoma [CRC]). Additionally, endometriosis and primary sebaceous carcinomas can mimic cutaneous metastatic adenocarcinomas.
Endometriosis can mimic adenocarcinoma, especially when presenting as a subdermal nodule. However, the scattered dermal glands are cytologically banal and are surrounded by uterine-type stroma and extravasated hemorrhage, a classic presentation of endometriosis (Figure 1).

Invasive ductal carcinoma of the breast is one of the most common cutaneous metastases of internal malignancy.3 Clinically, these lesions present on the chest wall or abdomen as flesh-colored nodules. Histopathology generally reveals either tubular or single tumor cells infiltrating the dermis with surrounding desmoplastic fibrosis (Figure 2). Immunohistochemistry typically is positive for CK7, estrogen receptor, and mammaglobin, and negative for CK20, CK5/6, and TTF-1.

Gastrointestinal adenocarcinomas encompass a variety of primary sites that can metastasize to the skin including CRC. Clinically, cutaneous metastases of CRC present as multiple nodules on the trunk, abdomen, or umbilicus (also known as Sister Mary Joseph nodule).7,8 Distinguishing CRC as the primary site of origin can be difficult; however, there are subtle differences depending on the histologic subtype. In well-differentiated CRCs, well-defined atypical glands are haphazardly arranged within the dermis (Figure 3), while poorly differentiated lesions can present as single cells or with a signet ring-like morphology (Figure 4). For perianal lesions, extramammary Paget disease should be considered when biopsies show large, amphophilic, intraepithelial cells. These lesions often present with mucin and CK20 expression and are frequently associated with colorectal malignancies.9 Another characteristic feature of CRC is central necrosis with karyorrhectic debris, known as dirty necrosis. Immunohistochemical analysis typically shows expression of caudal type homeobox 2 and CK20 with infrequent expression of CK7 and no expression of TTF-1; however, additional clinical history (eg, history of colorectal adenocarcinoma, positive fecal occult blood test) often is the best distinguishing feature.


Primary sebaceous carcinoma also can mimic metastatic adenocarcinoma within the skin and is histologically similar to metastatic adenocarcinomas. The most distinguishing feature is sebaceous differentiation characterized by sebocytes, which have a vacuolated cytoplasm giving the nucleus a scalloped appearance, frequently with adjacent ductlike structures (Figure 5). Epidermotropism sometimes is present in sebaceous carcinomas but cannot be relied on as a distinguishing feature. Immunohistochemical analysis also is a helpful tool; these tumors typically are positive for p63 and podoplanin, distinguishing them from negative-staining metastatic adenocarcinomas.10,11

Cutaneous Metastasis of Pulmonary Adenocarcinoma
Cutaneous metastasis of pulmonary adenocarcinoma (CMPA) is a rare phenomenon with an overall survival rate of less than 5 months.1,2 Often, CMPA can be the heralding feature of an aggressive systemic malignancy in 2.8% to 22% of reported cases.2-4 Clinically, CMPAs often present as fixed, violaceous, ulcerated nodules on the chest wall, scalp, or site of a prior procedure.3,5,6 Other clinical presentations have been described including zosteriform and inflammatory carcinomalike CMPA and CMPA on the tip of the nose.7 Histologically, CMPA presents as a subdermal collection of atypical glands arranged as clustered aggregates of infiltrative glands penetrating the dermal stroma (quiz image). The atypical glands have large oval nuclei with high nuclear to cytoplasm ratios with scant pale cytoplasm.
Cutaneous metastasis of pulmonary adenocarcinoma is difficult to distinguish from other metastatic or primary glandular malignancies based on histology alone. Immunohistochemical analysis can aid in the diagnosis of the primary tumor. Pulmonary adenocarcinomas are positive for cytokeratin (CK) 7 and thyroid transcription factor 1 (TTF-1), and they are negative for CK5/6 and CK20.7 The differential diagnosis for CMPA includes other internal malignancies such as invasive ductal adenocarcinoma of the breast and gastrointestinal adenocarcinomas (eg, gastric or colorectal carcinoma [CRC]). Additionally, endometriosis and primary sebaceous carcinomas can mimic cutaneous metastatic adenocarcinomas.
Endometriosis can mimic adenocarcinoma, especially when presenting as a subdermal nodule. However, the scattered dermal glands are cytologically banal and are surrounded by uterine-type stroma and extravasated hemorrhage, a classic presentation of endometriosis (Figure 1).

Invasive ductal carcinoma of the breast is one of the most common cutaneous metastases of internal malignancy.3 Clinically, these lesions present on the chest wall or abdomen as flesh-colored nodules. Histopathology generally reveals either tubular or single tumor cells infiltrating the dermis with surrounding desmoplastic fibrosis (Figure 2). Immunohistochemistry typically is positive for CK7, estrogen receptor, and mammaglobin, and negative for CK20, CK5/6, and TTF-1.

Gastrointestinal adenocarcinomas encompass a variety of primary sites that can metastasize to the skin including CRC. Clinically, cutaneous metastases of CRC present as multiple nodules on the trunk, abdomen, or umbilicus (also known as Sister Mary Joseph nodule).7,8 Distinguishing CRC as the primary site of origin can be difficult; however, there are subtle differences depending on the histologic subtype. In well-differentiated CRCs, well-defined atypical glands are haphazardly arranged within the dermis (Figure 3), while poorly differentiated lesions can present as single cells or with a signet ring-like morphology (Figure 4). For perianal lesions, extramammary Paget disease should be considered when biopsies show large, amphophilic, intraepithelial cells. These lesions often present with mucin and CK20 expression and are frequently associated with colorectal malignancies.9 Another characteristic feature of CRC is central necrosis with karyorrhectic debris, known as dirty necrosis. Immunohistochemical analysis typically shows expression of caudal type homeobox 2 and CK20 with infrequent expression of CK7 and no expression of TTF-1; however, additional clinical history (eg, history of colorectal adenocarcinoma, positive fecal occult blood test) often is the best distinguishing feature.


Primary sebaceous carcinoma also can mimic metastatic adenocarcinoma within the skin and is histologically similar to metastatic adenocarcinomas. The most distinguishing feature is sebaceous differentiation characterized by sebocytes, which have a vacuolated cytoplasm giving the nucleus a scalloped appearance, frequently with adjacent ductlike structures (Figure 5). Epidermotropism sometimes is present in sebaceous carcinomas but cannot be relied on as a distinguishing feature. Immunohistochemical analysis also is a helpful tool; these tumors typically are positive for p63 and podoplanin, distinguishing them from negative-staining metastatic adenocarcinomas.10,11

- Terashima T, Kanazawa M. Lung cancer with skin metastasis. Chest. 1994;106:1448-1450.
- Song Z, Lin B, Shao L, et al. Cutaneous metastasis as a initial presentation in advanced non-small cell lung cancer and its poor survival prognosis. J Cancer Res Clin Oncol. 2012;138:1613-1617.
- Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29(2, pt 1):228-236.
- Saeed S, Keehn CA, Morgan MB. Cutaneous metastasis: a clinical, pathological, and immunohistochemical appraisal. J Cutan Pathol. 2004;31:419-430.
- Chang SE, Choi JC, Moon KC. A papillary carcinoma: cutaneous metastases from lung cancer. J Dermatol. 2001;28:110-111.
- Snow S, Madjar D, Reizner G, et al. Renal cell carcinoma metastatic to the scalp: case report and review of the literature. Dermatol Surg. 2001;27:192-194.
- Alcaraz I, Cerroni L, Rutten A, et al. Cutaneous metastases from internal malignancies: a clinicopathologic and immunohistochemical review. Am J Dermatopathol. 2012;34:347-393.
- Schwartz IS. Sister (Mary?) Joseph's nodule. N Engl J Med. 1987;316:1348-1349.
- Goldblum J, Hart W. Perianal Paget's disease: a histologic and immunohistochemical study of 11 cases with and without associated rectal adenocarcinoma. Am J Surg Pathol. 1998;22:170-179.
- Ivan D, Nash J, Preito V, et al. Use of p63 expression in distinguishing primary and metastatic cutaneous adnexal neoplasms from metastatic adenocarcinoma to skin. J Cutan Pathol. 2006;34:474-480.
- Liang H, Wu H, Giorgadze T, et al. Podoplanin is a highly sensitive and specific marker to distinguish primary skin adnexal carcinomas from adenocarcinomas metastatic to skin. Am J Surg Pathol. 2007;31:304-310.
- Terashima T, Kanazawa M. Lung cancer with skin metastasis. Chest. 1994;106:1448-1450.
- Song Z, Lin B, Shao L, et al. Cutaneous metastasis as a initial presentation in advanced non-small cell lung cancer and its poor survival prognosis. J Cancer Res Clin Oncol. 2012;138:1613-1617.
- Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29(2, pt 1):228-236.
- Saeed S, Keehn CA, Morgan MB. Cutaneous metastasis: a clinical, pathological, and immunohistochemical appraisal. J Cutan Pathol. 2004;31:419-430.
- Chang SE, Choi JC, Moon KC. A papillary carcinoma: cutaneous metastases from lung cancer. J Dermatol. 2001;28:110-111.
- Snow S, Madjar D, Reizner G, et al. Renal cell carcinoma metastatic to the scalp: case report and review of the literature. Dermatol Surg. 2001;27:192-194.
- Alcaraz I, Cerroni L, Rutten A, et al. Cutaneous metastases from internal malignancies: a clinicopathologic and immunohistochemical review. Am J Dermatopathol. 2012;34:347-393.
- Schwartz IS. Sister (Mary?) Joseph's nodule. N Engl J Med. 1987;316:1348-1349.
- Goldblum J, Hart W. Perianal Paget's disease: a histologic and immunohistochemical study of 11 cases with and without associated rectal adenocarcinoma. Am J Surg Pathol. 1998;22:170-179.
- Ivan D, Nash J, Preito V, et al. Use of p63 expression in distinguishing primary and metastatic cutaneous adnexal neoplasms from metastatic adenocarcinoma to skin. J Cutan Pathol. 2006;34:474-480.
- Liang H, Wu H, Giorgadze T, et al. Podoplanin is a highly sensitive and specific marker to distinguish primary skin adnexal carcinomas from adenocarcinomas metastatic to skin. Am J Surg Pathol. 2007;31:304-310.
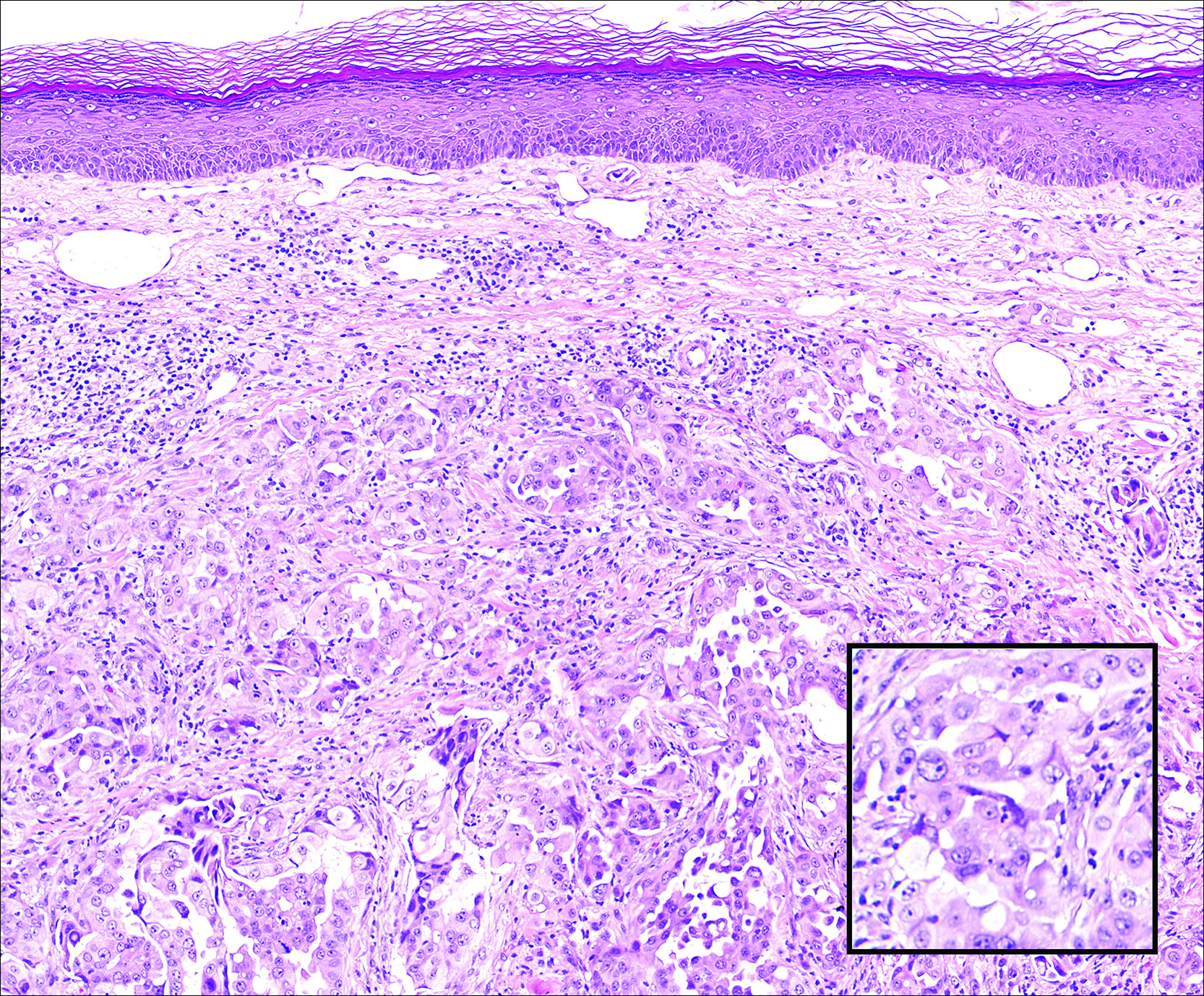
A 67-year-old woman with no history of malignancy presented with a scalp nodule. The photomicrograph showed atypical glands forming a subepidermal nodule with pleomorphic cells characterized by scant eosinophilic cytoplasm and large prominent nucleoli. Immunohistochemical analysis revealed diffuse thyroid transcription factor 1 and cytokeratin 7 positivity.
Managing Patients With Alopecia
What does the patient need to know at the first visit?
When I communicate with alopecia patients at the first visit, I make sure they know that I’m there to help them—that I won’t minimize their concerns and that I understand how important their condition is to them. Alopecia can be frustrating for both the patient and the physician, and there often is a confounding background of psychosocial stress and/or a history of physicians who have dismissed the patient’s concerns about his or her hair loss as trivial. Establishing an effective doctor-patient relationship is key in treating alopecia. Physicians sometimes may be left feeling like the patient wants to keep them in the room until his or her hair regrows, but in reality you simply need to reassure the patient that you are comfortable with the evaluation and treatment of alopecia and that several steps will be required but you will get started today.
How do you use punch biopsies to determine the best treatment options?
My most important tips regarding alopecia diagnosis relate to scalp biopsies, which usually are required in distinguishing chronic cutaneous lupus erythematosus from other scarring alopecias. First, an absorbable gelatin compressed sponge is your best friend. A small strip inserted into the punch biopsy wound results in prompt hemostasis without the need for sutures, and the resulting scar often looks as good or better than that produced by suturing. Next, don’t biopsy the active advancing borders of an alopecia patch, as the findings usually are nonspecific. Instead, biopsy a well-established portion that has been present for at least 4 to 6 months but is still active. In inconclusive cases, a biopsy of a scarred area stained with Verhoeff elastic stain can demonstrate characteristic patterns of elastic tissue loss and often establish a diagnosis. It is important to distinguish chronic cutaneous lupus erythematosus from other forms of scarring alopecia, as it is more likely to respond to antimalarials.
What are your go-to treatments? Are your recommendations anecdotal or evidence based?
There isn’t an extensive arsenal of evidence-based therapy for refractory scarring alopecia, but that doesn’t mean we don’t have effective therapies; it simply means that our treatments are based on experience without accompanying randomized controlled trials. We need to produce more evidence, but patients with severe disease still need to be treated in the meantime. It’s important to remember that therapeutic complacency can result in permanent irreversible scarring. The presence of easily extractable anagen hairs is a sign of active disease. This simple test is helpful to monitor therapeutic progress.
Topical and intralesional corticosteroids can be extremely useful and often are underused. In general, the risk of scarring and atrophy from untreated disease is much greater than that from the corticosteroid. On the scalp, atrophy often presents as erythema, which should not be confused with erythema related to active disease. Dermoscopy is useful to demonstrate that the redness represents dermal atrophy with prominence of the subpapillary plexus of vessels.
When systemic therapy is required, antimalarials, retinoids, dapsone, thalidomide, sulfasalazine, mycophenolate mofetil, and methotrexate have all been used successfully in the setting of cutaneous lupus erythematosus, while topical tazarotene and topical calcineurin inhibitors are generally disappointing.
For the treatment of lichen planopilaris, intralesional corticosteroids, oral retinoids, and excimer laser can be effective. In contrast, antimalarials usually are not effective in preventing disease progression. The peroxisome proliferator-activated receptor-γ agonist pioglitazone can be effective, but reported results vary widely. In my experience, mycophenolate mofetil is generally reliable in patients with refractory disease. Dutasteride can be effective as a first-line therapy in the setting of frontal fibrosing alopecia, although some of the noted improvement may relate to the nonscarring portion of the disease in patients with a background of pattern alopecia.
How do you keep patients compliant with treatment?
Again, the key to treatment compliance is to establish an effective doctor-patient relationship. Whenever possible, begin with adequately potent therapy to give patients an early response. Don’t hesitate to use prednisone initially for inflammatory scarring alopecia. Patients need to see signs of progress in order to stay compliant with treatment, and long trials of ineffective therapies destroy trust. Adequate doses of intralesional or oral corticosteroids often are appropriate to ensure an early response with subsequent transition to steroid-sparing agents.
What do you do if they refuse treatment?
Try to find out why—often it’s simply fear of side effects. Patient education is key, and it can help tremendously to share with them the number of patients you have treated safely with the medication in question and assure them that you know how to monitor for the important side effects.
What resources do you recommend to patients for more information?
It is helpful to keep a handy list of patient advocacy Web sites. Well-established support groups such as the National Alopecia Areata Foundation (https://www.naaf.org) and the Cicatricial Alopecia Research Foundation (http://www.carfintl.org) provide excellent information for patients and help to support research to improve outcomes for these difficult disorders.
What does the patient need to know at the first visit?
When I communicate with alopecia patients at the first visit, I make sure they know that I’m there to help them—that I won’t minimize their concerns and that I understand how important their condition is to them. Alopecia can be frustrating for both the patient and the physician, and there often is a confounding background of psychosocial stress and/or a history of physicians who have dismissed the patient’s concerns about his or her hair loss as trivial. Establishing an effective doctor-patient relationship is key in treating alopecia. Physicians sometimes may be left feeling like the patient wants to keep them in the room until his or her hair regrows, but in reality you simply need to reassure the patient that you are comfortable with the evaluation and treatment of alopecia and that several steps will be required but you will get started today.
How do you use punch biopsies to determine the best treatment options?
My most important tips regarding alopecia diagnosis relate to scalp biopsies, which usually are required in distinguishing chronic cutaneous lupus erythematosus from other scarring alopecias. First, an absorbable gelatin compressed sponge is your best friend. A small strip inserted into the punch biopsy wound results in prompt hemostasis without the need for sutures, and the resulting scar often looks as good or better than that produced by suturing. Next, don’t biopsy the active advancing borders of an alopecia patch, as the findings usually are nonspecific. Instead, biopsy a well-established portion that has been present for at least 4 to 6 months but is still active. In inconclusive cases, a biopsy of a scarred area stained with Verhoeff elastic stain can demonstrate characteristic patterns of elastic tissue loss and often establish a diagnosis. It is important to distinguish chronic cutaneous lupus erythematosus from other forms of scarring alopecia, as it is more likely to respond to antimalarials.
What are your go-to treatments? Are your recommendations anecdotal or evidence based?
There isn’t an extensive arsenal of evidence-based therapy for refractory scarring alopecia, but that doesn’t mean we don’t have effective therapies; it simply means that our treatments are based on experience without accompanying randomized controlled trials. We need to produce more evidence, but patients with severe disease still need to be treated in the meantime. It’s important to remember that therapeutic complacency can result in permanent irreversible scarring. The presence of easily extractable anagen hairs is a sign of active disease. This simple test is helpful to monitor therapeutic progress.
Topical and intralesional corticosteroids can be extremely useful and often are underused. In general, the risk of scarring and atrophy from untreated disease is much greater than that from the corticosteroid. On the scalp, atrophy often presents as erythema, which should not be confused with erythema related to active disease. Dermoscopy is useful to demonstrate that the redness represents dermal atrophy with prominence of the subpapillary plexus of vessels.
When systemic therapy is required, antimalarials, retinoids, dapsone, thalidomide, sulfasalazine, mycophenolate mofetil, and methotrexate have all been used successfully in the setting of cutaneous lupus erythematosus, while topical tazarotene and topical calcineurin inhibitors are generally disappointing.
For the treatment of lichen planopilaris, intralesional corticosteroids, oral retinoids, and excimer laser can be effective. In contrast, antimalarials usually are not effective in preventing disease progression. The peroxisome proliferator-activated receptor-γ agonist pioglitazone can be effective, but reported results vary widely. In my experience, mycophenolate mofetil is generally reliable in patients with refractory disease. Dutasteride can be effective as a first-line therapy in the setting of frontal fibrosing alopecia, although some of the noted improvement may relate to the nonscarring portion of the disease in patients with a background of pattern alopecia.
How do you keep patients compliant with treatment?
Again, the key to treatment compliance is to establish an effective doctor-patient relationship. Whenever possible, begin with adequately potent therapy to give patients an early response. Don’t hesitate to use prednisone initially for inflammatory scarring alopecia. Patients need to see signs of progress in order to stay compliant with treatment, and long trials of ineffective therapies destroy trust. Adequate doses of intralesional or oral corticosteroids often are appropriate to ensure an early response with subsequent transition to steroid-sparing agents.
What do you do if they refuse treatment?
Try to find out why—often it’s simply fear of side effects. Patient education is key, and it can help tremendously to share with them the number of patients you have treated safely with the medication in question and assure them that you know how to monitor for the important side effects.
What resources do you recommend to patients for more information?
It is helpful to keep a handy list of patient advocacy Web sites. Well-established support groups such as the National Alopecia Areata Foundation (https://www.naaf.org) and the Cicatricial Alopecia Research Foundation (http://www.carfintl.org) provide excellent information for patients and help to support research to improve outcomes for these difficult disorders.
What does the patient need to know at the first visit?
When I communicate with alopecia patients at the first visit, I make sure they know that I’m there to help them—that I won’t minimize their concerns and that I understand how important their condition is to them. Alopecia can be frustrating for both the patient and the physician, and there often is a confounding background of psychosocial stress and/or a history of physicians who have dismissed the patient’s concerns about his or her hair loss as trivial. Establishing an effective doctor-patient relationship is key in treating alopecia. Physicians sometimes may be left feeling like the patient wants to keep them in the room until his or her hair regrows, but in reality you simply need to reassure the patient that you are comfortable with the evaluation and treatment of alopecia and that several steps will be required but you will get started today.
How do you use punch biopsies to determine the best treatment options?
My most important tips regarding alopecia diagnosis relate to scalp biopsies, which usually are required in distinguishing chronic cutaneous lupus erythematosus from other scarring alopecias. First, an absorbable gelatin compressed sponge is your best friend. A small strip inserted into the punch biopsy wound results in prompt hemostasis without the need for sutures, and the resulting scar often looks as good or better than that produced by suturing. Next, don’t biopsy the active advancing borders of an alopecia patch, as the findings usually are nonspecific. Instead, biopsy a well-established portion that has been present for at least 4 to 6 months but is still active. In inconclusive cases, a biopsy of a scarred area stained with Verhoeff elastic stain can demonstrate characteristic patterns of elastic tissue loss and often establish a diagnosis. It is important to distinguish chronic cutaneous lupus erythematosus from other forms of scarring alopecia, as it is more likely to respond to antimalarials.
What are your go-to treatments? Are your recommendations anecdotal or evidence based?
There isn’t an extensive arsenal of evidence-based therapy for refractory scarring alopecia, but that doesn’t mean we don’t have effective therapies; it simply means that our treatments are based on experience without accompanying randomized controlled trials. We need to produce more evidence, but patients with severe disease still need to be treated in the meantime. It’s important to remember that therapeutic complacency can result in permanent irreversible scarring. The presence of easily extractable anagen hairs is a sign of active disease. This simple test is helpful to monitor therapeutic progress.
Topical and intralesional corticosteroids can be extremely useful and often are underused. In general, the risk of scarring and atrophy from untreated disease is much greater than that from the corticosteroid. On the scalp, atrophy often presents as erythema, which should not be confused with erythema related to active disease. Dermoscopy is useful to demonstrate that the redness represents dermal atrophy with prominence of the subpapillary plexus of vessels.
When systemic therapy is required, antimalarials, retinoids, dapsone, thalidomide, sulfasalazine, mycophenolate mofetil, and methotrexate have all been used successfully in the setting of cutaneous lupus erythematosus, while topical tazarotene and topical calcineurin inhibitors are generally disappointing.
For the treatment of lichen planopilaris, intralesional corticosteroids, oral retinoids, and excimer laser can be effective. In contrast, antimalarials usually are not effective in preventing disease progression. The peroxisome proliferator-activated receptor-γ agonist pioglitazone can be effective, but reported results vary widely. In my experience, mycophenolate mofetil is generally reliable in patients with refractory disease. Dutasteride can be effective as a first-line therapy in the setting of frontal fibrosing alopecia, although some of the noted improvement may relate to the nonscarring portion of the disease in patients with a background of pattern alopecia.
How do you keep patients compliant with treatment?
Again, the key to treatment compliance is to establish an effective doctor-patient relationship. Whenever possible, begin with adequately potent therapy to give patients an early response. Don’t hesitate to use prednisone initially for inflammatory scarring alopecia. Patients need to see signs of progress in order to stay compliant with treatment, and long trials of ineffective therapies destroy trust. Adequate doses of intralesional or oral corticosteroids often are appropriate to ensure an early response with subsequent transition to steroid-sparing agents.
What do you do if they refuse treatment?
Try to find out why—often it’s simply fear of side effects. Patient education is key, and it can help tremendously to share with them the number of patients you have treated safely with the medication in question and assure them that you know how to monitor for the important side effects.
What resources do you recommend to patients for more information?
It is helpful to keep a handy list of patient advocacy Web sites. Well-established support groups such as the National Alopecia Areata Foundation (https://www.naaf.org) and the Cicatricial Alopecia Research Foundation (http://www.carfintl.org) provide excellent information for patients and help to support research to improve outcomes for these difficult disorders.
What’s Eating You? Ant-Induced Alopecia (Pheidole)
Case Report
An 18-year-old Iranian man presented to the dermatology clinic with hair loss of 1 night’s duration. He denied pruritus, pain, discharge, or flaking. The patient had no notable personal, family, or surgical history and was not currently taking any medications. He denied recent travel. The patient reported that he found hair on his pillow upon waking up in the morning prior to coming to the clinic. On physical examination, 2 ants (Figure 1) were found on the scalp and alopecia with a vertical linear distribution was noted (Figure 2). Hairs of various lengths were found on the scalp within the distribution of the alopecia. No excoriations, crusting, seborrhea, or other areas of hair loss were detected. Wood lamp examination was negative. Based on these findings, which were concordant with similar findings from prior reports,1-4 a diagnosis of ant-induced alopecia was made. Hair regrowth was noted within 1 week with full appearance of normal-length hair within 2.5 weeks.


Comment
Ant-induced alopecia is a form of localized hair loss caused by the Pheidole genus, the second largest genus of ants in the world.5 These ants can be found worldwide, but most cases of ant-induced alopecia have been from Iran, with at least 1 reported case from Turkey.1-4,6 An early case series of ant-induced alopecia was reported in 1999,6 but the causative species was not described at that time.
The majority of reported cases of ant-induced alopecia are attributed to the barber ant (Pheidole pallidula). This type of alopecia is caused by worker ants within the species hierarchy.1,4,6 The P pallidula worker ants are dimorphic and are classified as major and minor workers.7 Major workers have body lengths ranging up to 6 mm, whereas minor workers have body lengths ranging up to 4 mm. Major workers have larger heads and mandibles than minor workers and also have up to 2 pairs of denticles on the cranium.5 The minor workers are foragers and mainly collect food, whereas the major workers defend the nest and store food.8 These ants have widespread habitats with the ability to live in indoor and outdoor environments.
The presentation of hair loss caused by these ants is acute. Hair loss usually is confined to one specific area. Some patients may report pruritus or may present with erythematous lesions from ant stings or manual scratching.5 None of these signs or symptoms were seen in our patient. Some investigators have suggested that the barber ant is attracted to the hair of individuals with seborrheic dermatitis,1 but our patient had no medical history of seborrheic dermatitis. Most likely, ants are attracted to excess sebum on the scalp in select individuals in their search for food and cause localized hair destruction.
Localized hair loss, as depicted in our case, should warrant a thorough evaluation for alopecia areata, trichotillomania, and tinea capitis.9 Alopecia areata should be considered in individuals with multiple focal patches of hair loss that have a positive hair pull test from peripheral sites of active lesions. Tinea capitis usually has localized sites of hair loss with underlying scaling, crusting, pruritus, erythema, and discharge from lesions, with positive potassium hydroxide preparations or fungal cultures. Trichotillomania typically presents with a spared peripheral fringe of hair. Remaining hairs may be thick and hyperpigmented as a response to repeated pulling, and biopsy often demonstrates fracture or degeneration of the hair shaft. A psychiatric evaluation may be warranted in cases of trichotillomania. Other cases of arthropod-induced hair loss include tick bite alopecia10,11 and hair loss induced by numerous honeybee stings,12 and these diagnoses should be suspected in patients with a history of ants on their pillow or in those from endemic areas.
No specific treatment is indicated in cases of ant-induced alopecia because hair usually regrows to its normal length without intervention.
- Shamsadini S. Localized scalp hair shedding caused by Pheidole ants and overview of similar case reports. Dermatol Online J. 2003;9:12.
- Aghaei S, Sodaifi M. Circumscribed scalp hair loss following multiple hair-cutter ant invasion. Dermatol Online J. 2004;10:14.
- Mortazavi M, Mansouri P. Ant-induced alopecia: report of 2 cases and review of the literature. Dermatol Online J. 2004;10:19.
- Kapdağli S, Seçkin D, Baba M, et al. Localized hair breakage caused by ants. Pediatr Dermatol. 2006;23:519-520.
- Ogata K. Toxonomy and biology of the genus Pheidole of Japan. Nature and Insects. 1981;16:17-22.
- Radmanesh M, Mousavipour M. Alopecia induced by ants. Trans R Soc Trop Med Hyg. 1999;93:427.
- Hölldobler B, Wilson EO. The Ants. Cambridge, MA: Harvard University Press; 1990.
- Wilson EO. Pheidole in the New World: A Dominant Hyperdiverse Ant Genus. Cambridge MA: Harvard University Press; 2003.
- Veraldi S, Lunardon L, Francia C, et al. Alopecia caused by the “barber ant” Pheidole pallidula. Int J Dermatol. 2008;47:1329-1330.
- Marshall J. Alopecia after tick bite. S Afr Med J. 1966;40: 555-556.
- Heyl T. Tick bite alopecia. Clin Exp Dermatol. 1982;7: 537-542.
- Sharma AK, Sharma RC, Sharma NL. Diffuse hair loss following multiple honeybee stings. Dermatology. 1997;195:305.
Case Report
An 18-year-old Iranian man presented to the dermatology clinic with hair loss of 1 night’s duration. He denied pruritus, pain, discharge, or flaking. The patient had no notable personal, family, or surgical history and was not currently taking any medications. He denied recent travel. The patient reported that he found hair on his pillow upon waking up in the morning prior to coming to the clinic. On physical examination, 2 ants (Figure 1) were found on the scalp and alopecia with a vertical linear distribution was noted (Figure 2). Hairs of various lengths were found on the scalp within the distribution of the alopecia. No excoriations, crusting, seborrhea, or other areas of hair loss were detected. Wood lamp examination was negative. Based on these findings, which were concordant with similar findings from prior reports,1-4 a diagnosis of ant-induced alopecia was made. Hair regrowth was noted within 1 week with full appearance of normal-length hair within 2.5 weeks.


Comment
Ant-induced alopecia is a form of localized hair loss caused by the Pheidole genus, the second largest genus of ants in the world.5 These ants can be found worldwide, but most cases of ant-induced alopecia have been from Iran, with at least 1 reported case from Turkey.1-4,6 An early case series of ant-induced alopecia was reported in 1999,6 but the causative species was not described at that time.
The majority of reported cases of ant-induced alopecia are attributed to the barber ant (Pheidole pallidula). This type of alopecia is caused by worker ants within the species hierarchy.1,4,6 The P pallidula worker ants are dimorphic and are classified as major and minor workers.7 Major workers have body lengths ranging up to 6 mm, whereas minor workers have body lengths ranging up to 4 mm. Major workers have larger heads and mandibles than minor workers and also have up to 2 pairs of denticles on the cranium.5 The minor workers are foragers and mainly collect food, whereas the major workers defend the nest and store food.8 These ants have widespread habitats with the ability to live in indoor and outdoor environments.
The presentation of hair loss caused by these ants is acute. Hair loss usually is confined to one specific area. Some patients may report pruritus or may present with erythematous lesions from ant stings or manual scratching.5 None of these signs or symptoms were seen in our patient. Some investigators have suggested that the barber ant is attracted to the hair of individuals with seborrheic dermatitis,1 but our patient had no medical history of seborrheic dermatitis. Most likely, ants are attracted to excess sebum on the scalp in select individuals in their search for food and cause localized hair destruction.
Localized hair loss, as depicted in our case, should warrant a thorough evaluation for alopecia areata, trichotillomania, and tinea capitis.9 Alopecia areata should be considered in individuals with multiple focal patches of hair loss that have a positive hair pull test from peripheral sites of active lesions. Tinea capitis usually has localized sites of hair loss with underlying scaling, crusting, pruritus, erythema, and discharge from lesions, with positive potassium hydroxide preparations or fungal cultures. Trichotillomania typically presents with a spared peripheral fringe of hair. Remaining hairs may be thick and hyperpigmented as a response to repeated pulling, and biopsy often demonstrates fracture or degeneration of the hair shaft. A psychiatric evaluation may be warranted in cases of trichotillomania. Other cases of arthropod-induced hair loss include tick bite alopecia10,11 and hair loss induced by numerous honeybee stings,12 and these diagnoses should be suspected in patients with a history of ants on their pillow or in those from endemic areas.
No specific treatment is indicated in cases of ant-induced alopecia because hair usually regrows to its normal length without intervention.
Case Report
An 18-year-old Iranian man presented to the dermatology clinic with hair loss of 1 night’s duration. He denied pruritus, pain, discharge, or flaking. The patient had no notable personal, family, or surgical history and was not currently taking any medications. He denied recent travel. The patient reported that he found hair on his pillow upon waking up in the morning prior to coming to the clinic. On physical examination, 2 ants (Figure 1) were found on the scalp and alopecia with a vertical linear distribution was noted (Figure 2). Hairs of various lengths were found on the scalp within the distribution of the alopecia. No excoriations, crusting, seborrhea, or other areas of hair loss were detected. Wood lamp examination was negative. Based on these findings, which were concordant with similar findings from prior reports,1-4 a diagnosis of ant-induced alopecia was made. Hair regrowth was noted within 1 week with full appearance of normal-length hair within 2.5 weeks.


Comment
Ant-induced alopecia is a form of localized hair loss caused by the Pheidole genus, the second largest genus of ants in the world.5 These ants can be found worldwide, but most cases of ant-induced alopecia have been from Iran, with at least 1 reported case from Turkey.1-4,6 An early case series of ant-induced alopecia was reported in 1999,6 but the causative species was not described at that time.
The majority of reported cases of ant-induced alopecia are attributed to the barber ant (Pheidole pallidula). This type of alopecia is caused by worker ants within the species hierarchy.1,4,6 The P pallidula worker ants are dimorphic and are classified as major and minor workers.7 Major workers have body lengths ranging up to 6 mm, whereas minor workers have body lengths ranging up to 4 mm. Major workers have larger heads and mandibles than minor workers and also have up to 2 pairs of denticles on the cranium.5 The minor workers are foragers and mainly collect food, whereas the major workers defend the nest and store food.8 These ants have widespread habitats with the ability to live in indoor and outdoor environments.
The presentation of hair loss caused by these ants is acute. Hair loss usually is confined to one specific area. Some patients may report pruritus or may present with erythematous lesions from ant stings or manual scratching.5 None of these signs or symptoms were seen in our patient. Some investigators have suggested that the barber ant is attracted to the hair of individuals with seborrheic dermatitis,1 but our patient had no medical history of seborrheic dermatitis. Most likely, ants are attracted to excess sebum on the scalp in select individuals in their search for food and cause localized hair destruction.
Localized hair loss, as depicted in our case, should warrant a thorough evaluation for alopecia areata, trichotillomania, and tinea capitis.9 Alopecia areata should be considered in individuals with multiple focal patches of hair loss that have a positive hair pull test from peripheral sites of active lesions. Tinea capitis usually has localized sites of hair loss with underlying scaling, crusting, pruritus, erythema, and discharge from lesions, with positive potassium hydroxide preparations or fungal cultures. Trichotillomania typically presents with a spared peripheral fringe of hair. Remaining hairs may be thick and hyperpigmented as a response to repeated pulling, and biopsy often demonstrates fracture or degeneration of the hair shaft. A psychiatric evaluation may be warranted in cases of trichotillomania. Other cases of arthropod-induced hair loss include tick bite alopecia10,11 and hair loss induced by numerous honeybee stings,12 and these diagnoses should be suspected in patients with a history of ants on their pillow or in those from endemic areas.
No specific treatment is indicated in cases of ant-induced alopecia because hair usually regrows to its normal length without intervention.
- Shamsadini S. Localized scalp hair shedding caused by Pheidole ants and overview of similar case reports. Dermatol Online J. 2003;9:12.
- Aghaei S, Sodaifi M. Circumscribed scalp hair loss following multiple hair-cutter ant invasion. Dermatol Online J. 2004;10:14.
- Mortazavi M, Mansouri P. Ant-induced alopecia: report of 2 cases and review of the literature. Dermatol Online J. 2004;10:19.
- Kapdağli S, Seçkin D, Baba M, et al. Localized hair breakage caused by ants. Pediatr Dermatol. 2006;23:519-520.
- Ogata K. Toxonomy and biology of the genus Pheidole of Japan. Nature and Insects. 1981;16:17-22.
- Radmanesh M, Mousavipour M. Alopecia induced by ants. Trans R Soc Trop Med Hyg. 1999;93:427.
- Hölldobler B, Wilson EO. The Ants. Cambridge, MA: Harvard University Press; 1990.
- Wilson EO. Pheidole in the New World: A Dominant Hyperdiverse Ant Genus. Cambridge MA: Harvard University Press; 2003.
- Veraldi S, Lunardon L, Francia C, et al. Alopecia caused by the “barber ant” Pheidole pallidula. Int J Dermatol. 2008;47:1329-1330.
- Marshall J. Alopecia after tick bite. S Afr Med J. 1966;40: 555-556.
- Heyl T. Tick bite alopecia. Clin Exp Dermatol. 1982;7: 537-542.
- Sharma AK, Sharma RC, Sharma NL. Diffuse hair loss following multiple honeybee stings. Dermatology. 1997;195:305.
- Shamsadini S. Localized scalp hair shedding caused by Pheidole ants and overview of similar case reports. Dermatol Online J. 2003;9:12.
- Aghaei S, Sodaifi M. Circumscribed scalp hair loss following multiple hair-cutter ant invasion. Dermatol Online J. 2004;10:14.
- Mortazavi M, Mansouri P. Ant-induced alopecia: report of 2 cases and review of the literature. Dermatol Online J. 2004;10:19.
- Kapdağli S, Seçkin D, Baba M, et al. Localized hair breakage caused by ants. Pediatr Dermatol. 2006;23:519-520.
- Ogata K. Toxonomy and biology of the genus Pheidole of Japan. Nature and Insects. 1981;16:17-22.
- Radmanesh M, Mousavipour M. Alopecia induced by ants. Trans R Soc Trop Med Hyg. 1999;93:427.
- Hölldobler B, Wilson EO. The Ants. Cambridge, MA: Harvard University Press; 1990.
- Wilson EO. Pheidole in the New World: A Dominant Hyperdiverse Ant Genus. Cambridge MA: Harvard University Press; 2003.
- Veraldi S, Lunardon L, Francia C, et al. Alopecia caused by the “barber ant” Pheidole pallidula. Int J Dermatol. 2008;47:1329-1330.
- Marshall J. Alopecia after tick bite. S Afr Med J. 1966;40: 555-556.
- Heyl T. Tick bite alopecia. Clin Exp Dermatol. 1982;7: 537-542.
- Sharma AK, Sharma RC, Sharma NL. Diffuse hair loss following multiple honeybee stings. Dermatology. 1997;195:305.
Practice Points
- Ant-induced alopecia should be considered in the differential diagnosis for patients from endemic regions (eg, Iran, Turkey) with new-onset localized hair loss or in patients recently visiting those areas with a concordant history.
- Ant-induced alopecia is thought to result from mechanical and/or chemical breakage, most commonly caused by Pheidole ants, leaving follicles intact and allowing for hair regrowth without treatment through the normal hair cycle.
What Is Your Diagnosis? Idiopathic Guttate Hypomelanosis
The Diagnosis: Idiopathic Guttate Hypomelanosis
A biopsy of the largest lesion from the left leg superior to the lateral malleolus was performed. Histopathologic examination revealed solar elastosis, diminished number of focal melanocytes and pigment within keratinocytes compared to uninvolved skin, and presence of hyperkeratosis with flattening of rete ridges. The clinical presentation along with histopathologic analysis confirmed a diagnosis of idiopathic guttate hypomelanosis (IGH). The lesions were treated with short-exposure cryotherapy, which resulted in partial repigmentation after several treatments.
Idiopathic guttate hypomelanosis is a common but underreported condition in elderly patients that usually presents with small, discrete, asymptomatic, hypopigmented macules. The frequency of IGH increases with age.1 Frequency of the condition is much lower in patients aged 21 to 30 years and does not exceed 7%. Lesions of IGH have a predilection for sun-exposed areas such as the arms and legs but rarely can be seen on the face and trunk. Facial lesions of IGH are more frequently reported in women.1 The size of lesions can be up to 1.5 cm in diameter. The condition generally is self-limited, but some patients may express aesthetic concerns. Rare cases of IGH in children have been associated with prolonged sun exposure.2
The etiology of IGH is unknown but an association with sun exposure has been noted. Patients with IGH frequently show other signs of photoaging, such as numerous seborrheic keratoses, solar lentigines, xeroses, freckles, and actinic keratoses.1 Short-term exposure to UVB radiation and psoralen plus UVA therapy has been shown to cause IGH in patients with chronic diseases such as mycosis fungoides.3-5 One small study that examined renal transplant recipients determined an association between HLA-DQ3 antigens and IGH, whereas HLA-DR8 antigens were not identified in any patients with IGH, indicating it may have some advantage in preventing the development of IGH.6 Shin et al1 reported that IGH was prevalent among patients who regularly traumatized their skin by scrubbing.
Clinically, IGH should be differentiated from other conditions characterized by hypopigmentation, such as pityriasis alba, pityriasis versicolor, postinflammatory hypopigmentation, progressive macular hypomelanosis, and vitiligo. Aside from clinical examination, histopathologic studies are helpful in making a definitive diagnosis. The differential diagnosis of IGH is presented in the Table.
Histopathology of IGH lesions usually reveals slight atrophy of the epidermis with flattening of rete ridges and concomitant hyperkeratosis. A thickened stratum granulosum also has been noted in lesions of IGH.2 The diminished number of melanocytes and melanin pigment granules along with hyperkeratosis both appear to contribute to the hypopigmentation noted in IGH.7 Ultrastructural studies of lesions of IGH can confirm melanocytic degeneration and a decreased number of melanosomes in melanocytes and keratinocytes.2,8
There is no uniformly effective treatment of IGH. Topical application of tacrolimus and tretinoin have shown efficacy in repigmenting IGH lesions.8,9 Short-exposure cryotherapy with a duration of 3 to 5 seconds, localized chemical peels, and/or local dermabrasion can be helpful.10-12 CO2 lasers also have demonstrated promising results.13
- Shin MK, Jeong KH, Oh IH, et al. Clinical features of idiopathic guttate hypomelanosis in 646 subjects and association with other aspects of photoaging. Int J Dermatol. 2011;50:798-805.
- Kim SK, Kim EH, Kang HY, et al. Comprehensive understanding of idiopathic guttate hypomelanosis: clinical and histopathological correlation. Int J Dermatol. 2010;49:162-166.
- Friedland R, David M, Feinmesser M, et al. Idiopathic guttate hypomelanosis-like lesions in patients with mycosis fungoides: a new adverse effect of phototherapy. J Eur Acad Dermatol Venereol. 2010;24:1026-1030.
- Kaya TI, Yazici AC, Tursen U, et al. Idiopathic guttate hypomelanosis: idiopathic or ultraviolet induced? Photodermatol Photoimmunol Photomed. 2005;21:270-271.
- Loquai C, Metze D, Nashan D, et al. Confetti-like lesions with hyperkeratosis: a novel ultraviolet-induced hypomelanotic disorder? Br J Dermatol. 2005;153:190-193.
- Arrunategui A, Trujillo RA, Marulanda MP, et al. HLA-DQ3 is associated with idiopathic guttate hypomelanosis, whereas HLA-DR8 is not, in a group of renal transplant patients. Int J Dermatol. 2002;41:744-747.
- Wallace ML, Grichnik JM, Prieto VG, et al. Numbers and differentiation status of melanocytes in idiopathic guttate hypomelanosis. J Cutan Pathol. 1998;25:375-379.
- Ortonne JP, Perrot H. Idiopathic guttate hypomelanosis. ultrastructural study. Arch Dermatol. 1980;116:664-668.
- Rerknimitr P, Disphanurat W, Achariyakul M. Topical tacrolimus significantly promotes repigmentation in idiopathic guttate hypomelanosis: a double-blind, randomized, placebo-controlled study. J Eur Acad Dermatol Venereol. 2013;27:460-464.
- Pagnoni A, Kligman AM, Sadiq I, et al. Hypopigmented macules of photodamaged skin and their treatment with topical tretinoin. Acta Derm Venereol. 1999;79:305-310.
- Kumarasinghe SP. 3-5 second cryotherapy is effective in idiopathic guttate hypomelanosis. J Dermatol. 2004;31:457-459.
- Hexsel DM. Treatment of idiopathic guttate hypomelanosis by localized superficial dermabrasion. Dermatol Surg. 1999;25:917-918.
- Shin J, Kim M, Park SH, et al. The effect of fractional carbon dioxide lasers on idiopathic guttate hypomelanosis: a preliminary study. J Eur Acad Dermatol Venereol. 2013;27:e243-e246.
The Diagnosis: Idiopathic Guttate Hypomelanosis
A biopsy of the largest lesion from the left leg superior to the lateral malleolus was performed. Histopathologic examination revealed solar elastosis, diminished number of focal melanocytes and pigment within keratinocytes compared to uninvolved skin, and presence of hyperkeratosis with flattening of rete ridges. The clinical presentation along with histopathologic analysis confirmed a diagnosis of idiopathic guttate hypomelanosis (IGH). The lesions were treated with short-exposure cryotherapy, which resulted in partial repigmentation after several treatments.
Idiopathic guttate hypomelanosis is a common but underreported condition in elderly patients that usually presents with small, discrete, asymptomatic, hypopigmented macules. The frequency of IGH increases with age.1 Frequency of the condition is much lower in patients aged 21 to 30 years and does not exceed 7%. Lesions of IGH have a predilection for sun-exposed areas such as the arms and legs but rarely can be seen on the face and trunk. Facial lesions of IGH are more frequently reported in women.1 The size of lesions can be up to 1.5 cm in diameter. The condition generally is self-limited, but some patients may express aesthetic concerns. Rare cases of IGH in children have been associated with prolonged sun exposure.2
The etiology of IGH is unknown but an association with sun exposure has been noted. Patients with IGH frequently show other signs of photoaging, such as numerous seborrheic keratoses, solar lentigines, xeroses, freckles, and actinic keratoses.1 Short-term exposure to UVB radiation and psoralen plus UVA therapy has been shown to cause IGH in patients with chronic diseases such as mycosis fungoides.3-5 One small study that examined renal transplant recipients determined an association between HLA-DQ3 antigens and IGH, whereas HLA-DR8 antigens were not identified in any patients with IGH, indicating it may have some advantage in preventing the development of IGH.6 Shin et al1 reported that IGH was prevalent among patients who regularly traumatized their skin by scrubbing.
Clinically, IGH should be differentiated from other conditions characterized by hypopigmentation, such as pityriasis alba, pityriasis versicolor, postinflammatory hypopigmentation, progressive macular hypomelanosis, and vitiligo. Aside from clinical examination, histopathologic studies are helpful in making a definitive diagnosis. The differential diagnosis of IGH is presented in the Table.


Histopathology of IGH lesions usually reveals slight atrophy of the epidermis with flattening of rete ridges and concomitant hyperkeratosis. A thickened stratum granulosum also has been noted in lesions of IGH.2 The diminished number of melanocytes and melanin pigment granules along with hyperkeratosis both appear to contribute to the hypopigmentation noted in IGH.7 Ultrastructural studies of lesions of IGH can confirm melanocytic degeneration and a decreased number of melanosomes in melanocytes and keratinocytes.2,8
There is no uniformly effective treatment of IGH. Topical application of tacrolimus and tretinoin have shown efficacy in repigmenting IGH lesions.8,9 Short-exposure cryotherapy with a duration of 3 to 5 seconds, localized chemical peels, and/or local dermabrasion can be helpful.10-12 CO2 lasers also have demonstrated promising results.13
The Diagnosis: Idiopathic Guttate Hypomelanosis
A biopsy of the largest lesion from the left leg superior to the lateral malleolus was performed. Histopathologic examination revealed solar elastosis, diminished number of focal melanocytes and pigment within keratinocytes compared to uninvolved skin, and presence of hyperkeratosis with flattening of rete ridges. The clinical presentation along with histopathologic analysis confirmed a diagnosis of idiopathic guttate hypomelanosis (IGH). The lesions were treated with short-exposure cryotherapy, which resulted in partial repigmentation after several treatments.
Idiopathic guttate hypomelanosis is a common but underreported condition in elderly patients that usually presents with small, discrete, asymptomatic, hypopigmented macules. The frequency of IGH increases with age.1 Frequency of the condition is much lower in patients aged 21 to 30 years and does not exceed 7%. Lesions of IGH have a predilection for sun-exposed areas such as the arms and legs but rarely can be seen on the face and trunk. Facial lesions of IGH are more frequently reported in women.1 The size of lesions can be up to 1.5 cm in diameter. The condition generally is self-limited, but some patients may express aesthetic concerns. Rare cases of IGH in children have been associated with prolonged sun exposure.2
The etiology of IGH is unknown but an association with sun exposure has been noted. Patients with IGH frequently show other signs of photoaging, such as numerous seborrheic keratoses, solar lentigines, xeroses, freckles, and actinic keratoses.1 Short-term exposure to UVB radiation and psoralen plus UVA therapy has been shown to cause IGH in patients with chronic diseases such as mycosis fungoides.3-5 One small study that examined renal transplant recipients determined an association between HLA-DQ3 antigens and IGH, whereas HLA-DR8 antigens were not identified in any patients with IGH, indicating it may have some advantage in preventing the development of IGH.6 Shin et al1 reported that IGH was prevalent among patients who regularly traumatized their skin by scrubbing.
Clinically, IGH should be differentiated from other conditions characterized by hypopigmentation, such as pityriasis alba, pityriasis versicolor, postinflammatory hypopigmentation, progressive macular hypomelanosis, and vitiligo. Aside from clinical examination, histopathologic studies are helpful in making a definitive diagnosis. The differential diagnosis of IGH is presented in the Table.


Histopathology of IGH lesions usually reveals slight atrophy of the epidermis with flattening of rete ridges and concomitant hyperkeratosis. A thickened stratum granulosum also has been noted in lesions of IGH.2 The diminished number of melanocytes and melanin pigment granules along with hyperkeratosis both appear to contribute to the hypopigmentation noted in IGH.7 Ultrastructural studies of lesions of IGH can confirm melanocytic degeneration and a decreased number of melanosomes in melanocytes and keratinocytes.2,8
There is no uniformly effective treatment of IGH. Topical application of tacrolimus and tretinoin have shown efficacy in repigmenting IGH lesions.8,9 Short-exposure cryotherapy with a duration of 3 to 5 seconds, localized chemical peels, and/or local dermabrasion can be helpful.10-12 CO2 lasers also have demonstrated promising results.13
- Shin MK, Jeong KH, Oh IH, et al. Clinical features of idiopathic guttate hypomelanosis in 646 subjects and association with other aspects of photoaging. Int J Dermatol. 2011;50:798-805.
- Kim SK, Kim EH, Kang HY, et al. Comprehensive understanding of idiopathic guttate hypomelanosis: clinical and histopathological correlation. Int J Dermatol. 2010;49:162-166.
- Friedland R, David M, Feinmesser M, et al. Idiopathic guttate hypomelanosis-like lesions in patients with mycosis fungoides: a new adverse effect of phototherapy. J Eur Acad Dermatol Venereol. 2010;24:1026-1030.
- Kaya TI, Yazici AC, Tursen U, et al. Idiopathic guttate hypomelanosis: idiopathic or ultraviolet induced? Photodermatol Photoimmunol Photomed. 2005;21:270-271.
- Loquai C, Metze D, Nashan D, et al. Confetti-like lesions with hyperkeratosis: a novel ultraviolet-induced hypomelanotic disorder? Br J Dermatol. 2005;153:190-193.
- Arrunategui A, Trujillo RA, Marulanda MP, et al. HLA-DQ3 is associated with idiopathic guttate hypomelanosis, whereas HLA-DR8 is not, in a group of renal transplant patients. Int J Dermatol. 2002;41:744-747.
- Wallace ML, Grichnik JM, Prieto VG, et al. Numbers and differentiation status of melanocytes in idiopathic guttate hypomelanosis. J Cutan Pathol. 1998;25:375-379.
- Ortonne JP, Perrot H. Idiopathic guttate hypomelanosis. ultrastructural study. Arch Dermatol. 1980;116:664-668.
- Rerknimitr P, Disphanurat W, Achariyakul M. Topical tacrolimus significantly promotes repigmentation in idiopathic guttate hypomelanosis: a double-blind, randomized, placebo-controlled study. J Eur Acad Dermatol Venereol. 2013;27:460-464.
- Pagnoni A, Kligman AM, Sadiq I, et al. Hypopigmented macules of photodamaged skin and their treatment with topical tretinoin. Acta Derm Venereol. 1999;79:305-310.
- Kumarasinghe SP. 3-5 second cryotherapy is effective in idiopathic guttate hypomelanosis. J Dermatol. 2004;31:457-459.
- Hexsel DM. Treatment of idiopathic guttate hypomelanosis by localized superficial dermabrasion. Dermatol Surg. 1999;25:917-918.
- Shin J, Kim M, Park SH, et al. The effect of fractional carbon dioxide lasers on idiopathic guttate hypomelanosis: a preliminary study. J Eur Acad Dermatol Venereol. 2013;27:e243-e246.
- Shin MK, Jeong KH, Oh IH, et al. Clinical features of idiopathic guttate hypomelanosis in 646 subjects and association with other aspects of photoaging. Int J Dermatol. 2011;50:798-805.
- Kim SK, Kim EH, Kang HY, et al. Comprehensive understanding of idiopathic guttate hypomelanosis: clinical and histopathological correlation. Int J Dermatol. 2010;49:162-166.
- Friedland R, David M, Feinmesser M, et al. Idiopathic guttate hypomelanosis-like lesions in patients with mycosis fungoides: a new adverse effect of phototherapy. J Eur Acad Dermatol Venereol. 2010;24:1026-1030.
- Kaya TI, Yazici AC, Tursen U, et al. Idiopathic guttate hypomelanosis: idiopathic or ultraviolet induced? Photodermatol Photoimmunol Photomed. 2005;21:270-271.
- Loquai C, Metze D, Nashan D, et al. Confetti-like lesions with hyperkeratosis: a novel ultraviolet-induced hypomelanotic disorder? Br J Dermatol. 2005;153:190-193.
- Arrunategui A, Trujillo RA, Marulanda MP, et al. HLA-DQ3 is associated with idiopathic guttate hypomelanosis, whereas HLA-DR8 is not, in a group of renal transplant patients. Int J Dermatol. 2002;41:744-747.
- Wallace ML, Grichnik JM, Prieto VG, et al. Numbers and differentiation status of melanocytes in idiopathic guttate hypomelanosis. J Cutan Pathol. 1998;25:375-379.
- Ortonne JP, Perrot H. Idiopathic guttate hypomelanosis. ultrastructural study. Arch Dermatol. 1980;116:664-668.
- Rerknimitr P, Disphanurat W, Achariyakul M. Topical tacrolimus significantly promotes repigmentation in idiopathic guttate hypomelanosis: a double-blind, randomized, placebo-controlled study. J Eur Acad Dermatol Venereol. 2013;27:460-464.
- Pagnoni A, Kligman AM, Sadiq I, et al. Hypopigmented macules of photodamaged skin and their treatment with topical tretinoin. Acta Derm Venereol. 1999;79:305-310.
- Kumarasinghe SP. 3-5 second cryotherapy is effective in idiopathic guttate hypomelanosis. J Dermatol. 2004;31:457-459.
- Hexsel DM. Treatment of idiopathic guttate hypomelanosis by localized superficial dermabrasion. Dermatol Surg. 1999;25:917-918.
- Shin J, Kim M, Park SH, et al. The effect of fractional carbon dioxide lasers on idiopathic guttate hypomelanosis: a preliminary study. J Eur Acad Dermatol Venereol. 2013;27:e243-e246.
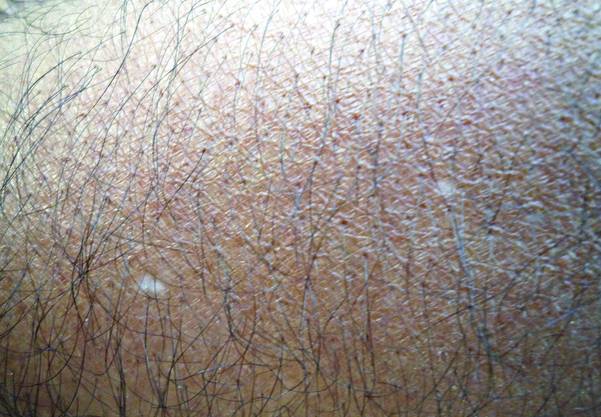
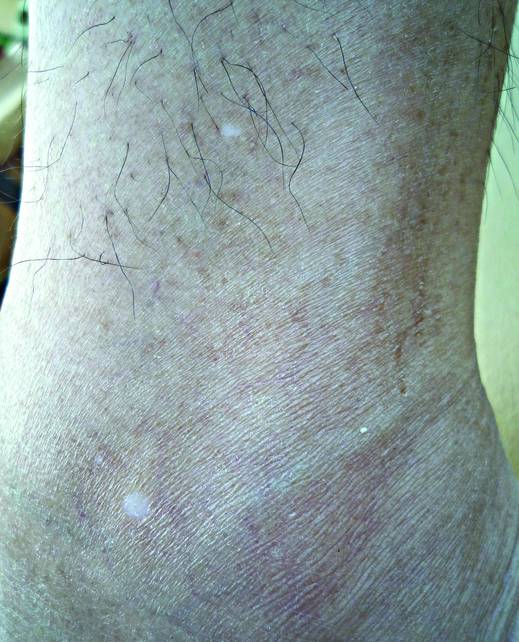
A 58-year-old man presented with disseminated, hypopigmented, asymptomatic lesions on the right arm (top) and left leg (bottom) that had been present for approximately 6 years. The patient reported that the lesions had become more visible and greater in number within the last year. Multiple circular hypopigmented macules of various sizes ranging from 1 to 3 mm in diameter were identified. No scaling was seen. Physical examination was otherwise unremarkable.