User login
Nail Changes Associated With Thyroid Disease
The major classifications of thyroid disease include hyperthyroidism, which is seen in Graves disease, and hypothyroidism due to iodine deficiency and Hashimoto thyroiditis, which have potentially devastating health consequences. The prevalence of hyperthyroidism ranges from 0.2% to 1.3% in iodine-sufficient parts of the world, and the prevalence of hypothyroidism in the general population is 5.3% in Europe and 3.7% in the United States.1 Thyroid hormones physiologically potentiate α- and β-adrenergic receptors by increasing their sensitivity to catecholamines. Excess thyroid hormones manifest as tachycardia, increased cardiac output, increased body temperature, hyperhidrosis, and warm moist skin. Reduced sensitivity of adrenergic receptors to catecholamines from insufficient thyroid hormones results in a lower metabolic rate and decreases response to the sympathetic nervous system.2 Nail changes in thyroid patients have not been well studied.3 Our objectives were to characterize nail findings in patients with thyroid disease. Early diagnosis of thyroid disease and prompt referral for treatment may be instrumental in preventing serious morbidities and permanent sequelae.
Methods
PubMed, Scopus, Web of Science, and Google Scholar were searched for the terms nail + thyroid, nail + hyperthyroid, nail + hypothyroid, nail + Graves, and nail + Hashimoto on June 10, 2020, and then updated on November 18, 2020. All English-language articles were included. Non–English-language articles and those that did not describe clinical trials of nail changes in patients with thyroid disease were excluded. One study that utilized survey-based data for nail changes without corroboration with physical examination findings was excluded. Hypothyroidism/hyperthyroidism was defined by all authors as measurement of serum thyroid hormones triiodothyronine, thyroxine, and thyroid-stimulating hormone outside of the normal range. Eight studies were included in the final analysis. Patient demographics, thyroid disease type, physical examination findings, nail clinical findings, age at diagnosis, age at onset of nail changes, treatments/medications, and comorbidities were recorded and analyzed.
Results
Nail changes in patients with thyroid disease were reported in 8 studies (7 cross-sectional, 1 retrospective cohort) and are summarized in the Table.4-11 The mean age was 41.2 years (range, 5–80 years), with a higher representation of females (range, 70%–94% female). The most common nail changes in thyroid patients were koilonychia, clubbing, and nail brittleness. Other changes included onycholysis, thin nails, dryness, and changes in nail growth rate. Frequent physical findings were xerosis, pruritus, and alopecia.
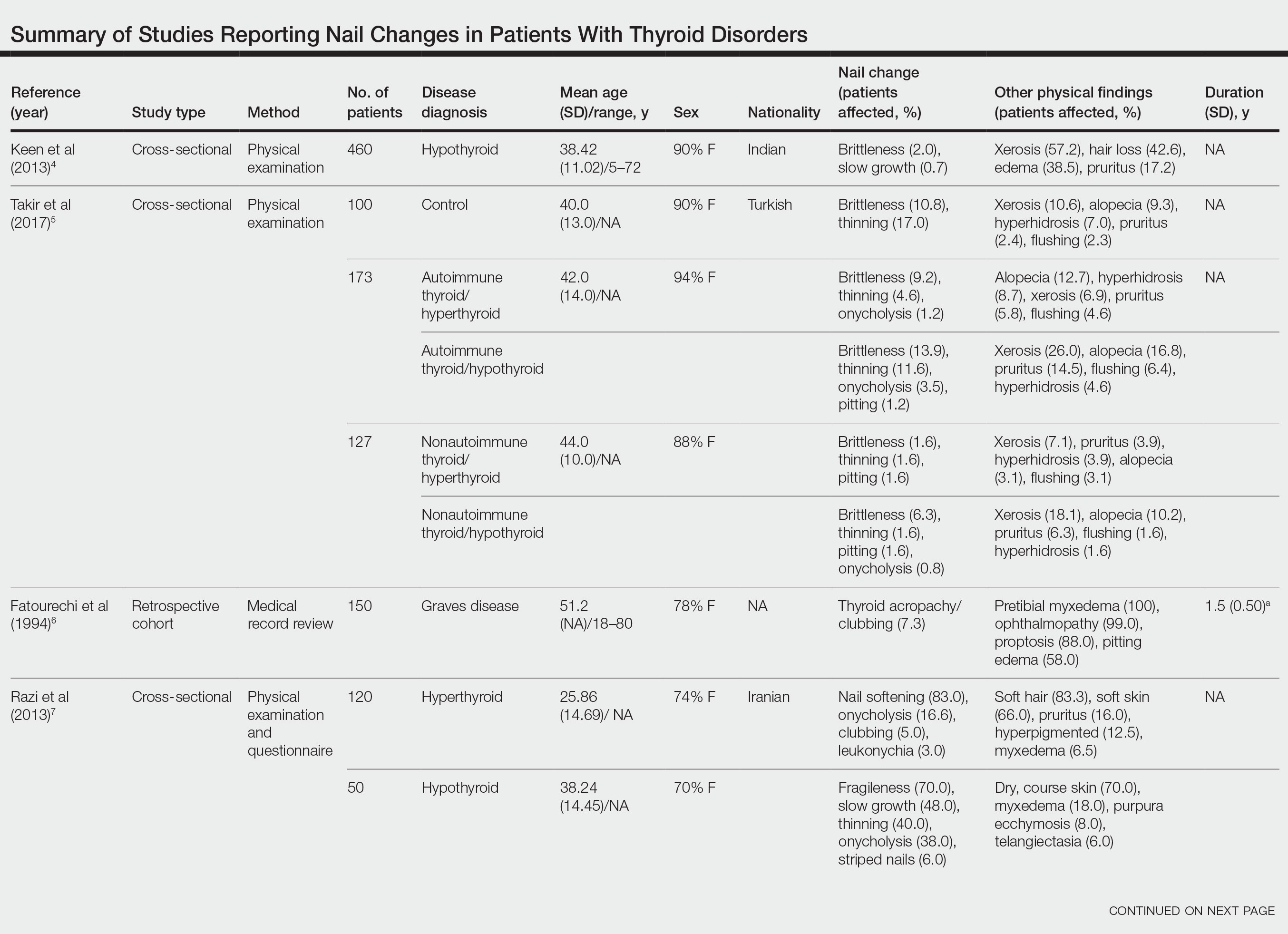
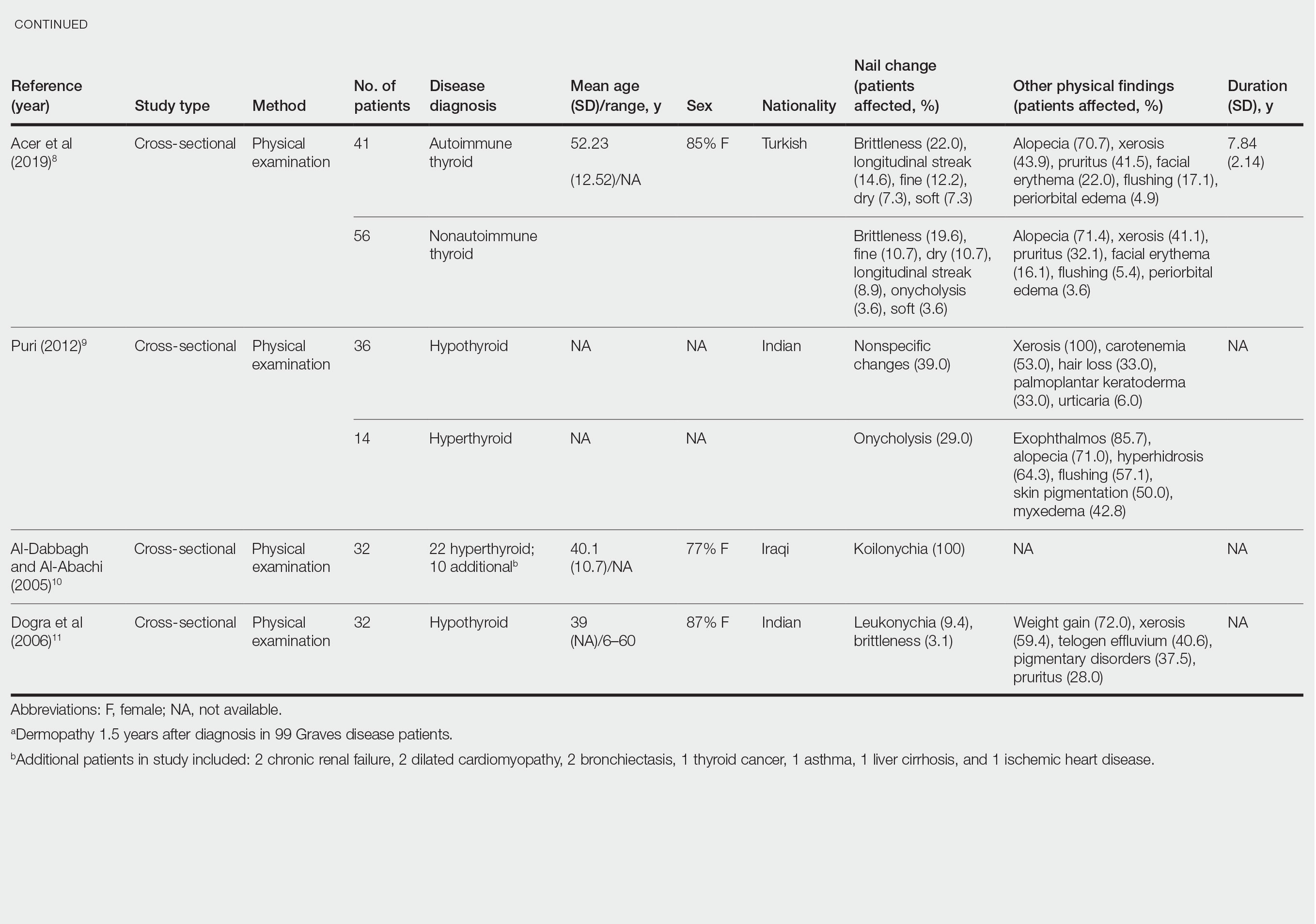
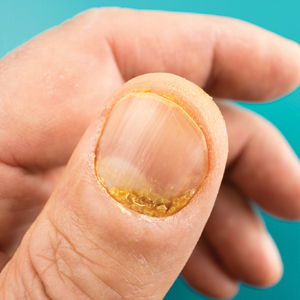
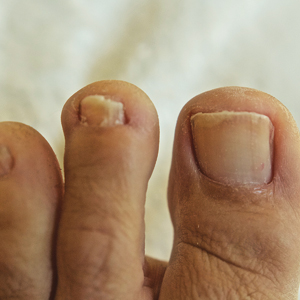
Both koilonychia and clubbing were reported in patients with hyperthyroidism. In a study of 32 patients with koilonychia, 22 (68.8%) were diagnosed with hyperthyroidism.10 Nail clubbing affected 7.3% of Graves disease patients (n=150)6 and 5.0% of hyperthyroid patients (n=120).7 Dermopathy presented more than 1 year after diagnosis of Graves disease in 99 (66%) of 150 patients as a late manifestation of thyrotoxicosis.6 Additional physical features in patients with Graves disease (n=150) were pretibial myxedema (100%), ophthalmopathy (99.0%), and proptosis (88.0%). Non–Graves hyperthyroid patients showed physical features of soft hair (83.3%) and soft skin (66.0%).7
Nail brittleness was a frequently reported nail change in thyroid patients (4/8 studies, 50%), most often seen in 22% of autoimmune patients, 19.6% of nonautoimmune patients, 13.9% of hypothyroid patients, and 9.2% of hyperthyroid patients.5,8 For comparison, brittle nails presented in 10.8% of participants in a control group.5 Brittle nails in thyroid patients often are accompanied by other nail findings such as thinning, onycholysis, and pitting.
Among hypothyroid patients, nail changes included fragility (70%; n=50), slow growth (48%; n=50), thinning (40%; n=50), onycholysis (38%; n=50),7 and brittleness (13.9%; n=173).5 Less common nail changes in hypothyroid patients were leukonychia (9.4%; n=32), striped nails (6%; n=50), and pitting (1.2%; n=173).5,7,11 Among hyperthyroid patients, the most common nail changes were koilonychia (100%; n=22), softening (83%; n=120), onycholysis (29%; n=14), and brittleness (9.2%; n=173).5,7,9,10 Less common nail changes in hyperthyroid patients were clubbing (5%; n=120), thinning (4.6%; n=173), and leukonychia (3%; n=120).5,7
Additional cutaneous findings of thyroid disorder included xerosis, alopecia, pruritus, and weight change. Xerosis was most common in hypothyroid disease (57.2%; n=460).4 In 2 studies,8,9 alopecia affected approximately 70% of autoimmune, nonautoimmune, and hyperthyroid patients. Hair loss was reported in 42.6% (n=460)4 and 33.0% (n=36)9 of hypothyroid patients. Additionally, pruritus affected up to 28% (n=32)11 of hypothyroid and 16.0% (n=120)7 of hyperthyroid patients and was more common in autoimmune (41%) vs nonautoimmune (32%) thyroid patients.8 Weight gain was seen in 72% of hypothyroid patients (n=32),11 and soft hair and skin were reported in 83.3% and 66% of hyperthyroid patients (n=120), respectively.7 Flushing was a less common physical finding in thyroid patients (usually affecting <10%); however, it also was reported in 17.1% of autoimmune and 57.1% of hyperthyroid patients from 2 separate studies.8,9
Comment
There are limited data describing nail changes with thyroid disease. Singal and Arora3 reported in their clinical review of nail changes in systemic disease that koilonychia, onycholysis, and melanonychia are associated with thyroid disorders. We similarly found that koilonychia and onycholysis are associated with thyroid disorders without an association with melanonychia.
In his clinical review of thyroid hormone action on the skin, Safer12 described hypothyroid patients having coarse, dull, thin, and brittle nails, whereas in thyrotoxicosis, patients had shiny, soft, and concave nails with onycholysis; however, the author commented that there were limited data on the clinical findings in thyroid disorders. These nail findings are consistent with our results, but onycholysis was more common in hypothyroid patients than in hyperthyroid patients in our review. Fox13 reported on 30 cases of onycholysis, stating that it affected patients with hypothyroidism and improved with thyroid treatment. In a clinical review of 8 commonly seen nail abnormalities, Fowler et al14 reported that hyperthyroidism was associated with nail findings in 5% of cases and may result in onycholysis of the fourth and fifth nails or all nails. They also reported that onychorrhexis may be seen in patients with hypothyroidism, a finding that differed from our results.14
The mechanism of nail changes in thyroid disease has not been well studied. A protein/amino acid–deficiency state may contribute to the development of koilonychia. Hyperthyroid patients, who have high metabolic activity, may have hypoalbuminemia, leading to koilonychia.15 Hypothyroidism causes hypothermia from decreased metabolic rate and secondary compensatory vasoconstriction. Vasoconstriction decreases blood flow of nutrients and oxygen to cutaneous structures and may cause slow-growing, brittle nails. In hyperthyroidism, vasodilation alternatively may contribute to the fast-growing nails. Anti–thyroid-stimulating hormone receptor antibodies in Graves disease may increase the synthesis of hyaluronic acid and glycosaminoglycans from fibroblasts, keratinocytes, adipocytes, or endothelial cells in the dermis and may contribute to development of clubbing.16
Our review is subject to several limitations. We recorded nail findings as they were described in the original studies; however, we could not confirm the accuracy of these descriptions. In addition, some specific nail changes were not described in sufficient detail. In all but 1 study, dermatologists performed the physical examination. In the study by Al-Dabbagh and Al-Abachi,10 the physical examinations were performed by general medicine physicians, but they selected only for patients with koilonychia and did not assess for other skin findings. Fragile nails and brittle nails were described in hypothyroid and hyperthyroid patients, but these nail changes were not described in detail. There also were studies describing nail changes in thyroid patients; some studies had small numbers of patients, and many did not have a control group.
Conclusion
Nail changes may be early clinical presenting signs of thyroid disorders and may be the clue to prompt diagnosis of thyroid disease. Dermatologists should be mindful that fragile, slow-growing, thin nails and onycholysis are associated with hypothyroidism and that koilonychia, softening, onycholysis, and brittle nail changes may be seen in hyperthyroidism. Our review aimed to describe nail changes associated with thyroid disease to guide dermatologists on diagnosis and promote future research on dermatologic manifestations of thyroid disease. Future research is necessary to explore the association between koilonychia and hyperthyroidism as well as the association of nail changes with thyroid disease duration and severity.
- Taylor PN, Albrecht D, Scholz A, et al. Global epidemiology of hyperthyroidism and hypothyroidism. Nat Rev Endocrinol. 2018;14:301-316.
- Lause M, Kamboj A, Faith EF. Dermatologic manifestations of endocrine disorders. Transl Pediatr. 2017;6:300-312.
- Singal A, Arora R. Nail as a window of systemic diseases. Indian Dermatol Online J. 2015;6:67-74.
- Keen MA, Hassan I, Bhat MH. A clinical study of the cutaneous manifestations of hypothyroidism in Kashmir Valley. Indian J Dermatol. 2013;58:326.
- Takir M, Özlü E, Köstek O, et al. Skin findings in autoimmune and nonautoimmune thyroid disease with respect to thyroid functional status and healthy controls. Turk J Med Sci. 2017;47:764-770.
- Fatourechi V, Pajouhi M, Fransway AF. Dermopathy of Graves disease (pretibial myxedema). review of 150 cases. Medicine (Baltimore). 1994;73:1-7.
- Razi A, Golforoushan F, Nejad AB, et al. Evaluation of dermal symptoms in hypothyroidism and hyperthyroidism. Pak J Biol Sci. 2013;16:541-544.
- Acer E, Ag˘aog˘lu E, Yorulmaz G, et al. Evaluation of cutaneous manifestations in patients under treatment with thyroid disease. Turkderm-Turk Arch Dermatol Venereol. 2019;54:46-50.
- Puri N. A study on cutaneous manifestations of thyroid disease. Indian J Dermatol. 2012;57:247-248.
- Al-Dabbagh TQ, Al-Abachi KG. Nutritional koilonychia in 32 Iraqi subjects. Ann Saudi Med. 2005;25:154-157.
- Dogra A, Dua A, Singh P. Thyroid and skin. Indian J Dermatol. 2006;51:96-99.
- Safer JD. Thyroid hormone action on skin. Dermatoendocrinol. 2011;3:211-215.
- Fox EC. Diseases of the nails: report of cases of onycholysis. Arch Derm Syphilol. 1940;41:98-112.
- Fowler JR, Stern E, English JC 3rd, et al. A hand surgeon’s guide to common onychodystrophies. Hand (N Y). 2014;9:24-28.
- Truswell AS. Nutritional factors in disease. In: Edwards CRW, Bouchier IAD, Haslett C, et al, eds. Davidson’s Principles and Practice of Medicine. 17th ed. Churchill Livingstone; 1995:554.
- Heymann WR. Cutaneous manifestations of thyroid disease. J Am Acad Dermatol. 1992;26:885-902.
The major classifications of thyroid disease include hyperthyroidism, which is seen in Graves disease, and hypothyroidism due to iodine deficiency and Hashimoto thyroiditis, which have potentially devastating health consequences. The prevalence of hyperthyroidism ranges from 0.2% to 1.3% in iodine-sufficient parts of the world, and the prevalence of hypothyroidism in the general population is 5.3% in Europe and 3.7% in the United States.1 Thyroid hormones physiologically potentiate α- and β-adrenergic receptors by increasing their sensitivity to catecholamines. Excess thyroid hormones manifest as tachycardia, increased cardiac output, increased body temperature, hyperhidrosis, and warm moist skin. Reduced sensitivity of adrenergic receptors to catecholamines from insufficient thyroid hormones results in a lower metabolic rate and decreases response to the sympathetic nervous system.2 Nail changes in thyroid patients have not been well studied.3 Our objectives were to characterize nail findings in patients with thyroid disease. Early diagnosis of thyroid disease and prompt referral for treatment may be instrumental in preventing serious morbidities and permanent sequelae.
Methods
PubMed, Scopus, Web of Science, and Google Scholar were searched for the terms nail + thyroid, nail + hyperthyroid, nail + hypothyroid, nail + Graves, and nail + Hashimoto on June 10, 2020, and then updated on November 18, 2020. All English-language articles were included. Non–English-language articles and those that did not describe clinical trials of nail changes in patients with thyroid disease were excluded. One study that utilized survey-based data for nail changes without corroboration with physical examination findings was excluded. Hypothyroidism/hyperthyroidism was defined by all authors as measurement of serum thyroid hormones triiodothyronine, thyroxine, and thyroid-stimulating hormone outside of the normal range. Eight studies were included in the final analysis. Patient demographics, thyroid disease type, physical examination findings, nail clinical findings, age at diagnosis, age at onset of nail changes, treatments/medications, and comorbidities were recorded and analyzed.
Results
Nail changes in patients with thyroid disease were reported in 8 studies (7 cross-sectional, 1 retrospective cohort) and are summarized in the Table.4-11 The mean age was 41.2 years (range, 5–80 years), with a higher representation of females (range, 70%–94% female). The most common nail changes in thyroid patients were koilonychia, clubbing, and nail brittleness. Other changes included onycholysis, thin nails, dryness, and changes in nail growth rate. Frequent physical findings were xerosis, pruritus, and alopecia.


Both koilonychia and clubbing were reported in patients with hyperthyroidism. In a study of 32 patients with koilonychia, 22 (68.8%) were diagnosed with hyperthyroidism.10 Nail clubbing affected 7.3% of Graves disease patients (n=150)6 and 5.0% of hyperthyroid patients (n=120).7 Dermopathy presented more than 1 year after diagnosis of Graves disease in 99 (66%) of 150 patients as a late manifestation of thyrotoxicosis.6 Additional physical features in patients with Graves disease (n=150) were pretibial myxedema (100%), ophthalmopathy (99.0%), and proptosis (88.0%). Non–Graves hyperthyroid patients showed physical features of soft hair (83.3%) and soft skin (66.0%).7
Nail brittleness was a frequently reported nail change in thyroid patients (4/8 studies, 50%), most often seen in 22% of autoimmune patients, 19.6% of nonautoimmune patients, 13.9% of hypothyroid patients, and 9.2% of hyperthyroid patients.5,8 For comparison, brittle nails presented in 10.8% of participants in a control group.5 Brittle nails in thyroid patients often are accompanied by other nail findings such as thinning, onycholysis, and pitting.
Among hypothyroid patients, nail changes included fragility (70%; n=50), slow growth (48%; n=50), thinning (40%; n=50), onycholysis (38%; n=50),7 and brittleness (13.9%; n=173).5 Less common nail changes in hypothyroid patients were leukonychia (9.4%; n=32), striped nails (6%; n=50), and pitting (1.2%; n=173).5,7,11 Among hyperthyroid patients, the most common nail changes were koilonychia (100%; n=22), softening (83%; n=120), onycholysis (29%; n=14), and brittleness (9.2%; n=173).5,7,9,10 Less common nail changes in hyperthyroid patients were clubbing (5%; n=120), thinning (4.6%; n=173), and leukonychia (3%; n=120).5,7
Additional cutaneous findings of thyroid disorder included xerosis, alopecia, pruritus, and weight change. Xerosis was most common in hypothyroid disease (57.2%; n=460).4 In 2 studies,8,9 alopecia affected approximately 70% of autoimmune, nonautoimmune, and hyperthyroid patients. Hair loss was reported in 42.6% (n=460)4 and 33.0% (n=36)9 of hypothyroid patients. Additionally, pruritus affected up to 28% (n=32)11 of hypothyroid and 16.0% (n=120)7 of hyperthyroid patients and was more common in autoimmune (41%) vs nonautoimmune (32%) thyroid patients.8 Weight gain was seen in 72% of hypothyroid patients (n=32),11 and soft hair and skin were reported in 83.3% and 66% of hyperthyroid patients (n=120), respectively.7 Flushing was a less common physical finding in thyroid patients (usually affecting <10%); however, it also was reported in 17.1% of autoimmune and 57.1% of hyperthyroid patients from 2 separate studies.8,9
Comment
There are limited data describing nail changes with thyroid disease. Singal and Arora3 reported in their clinical review of nail changes in systemic disease that koilonychia, onycholysis, and melanonychia are associated with thyroid disorders. We similarly found that koilonychia and onycholysis are associated with thyroid disorders without an association with melanonychia.
In his clinical review of thyroid hormone action on the skin, Safer12 described hypothyroid patients having coarse, dull, thin, and brittle nails, whereas in thyrotoxicosis, patients had shiny, soft, and concave nails with onycholysis; however, the author commented that there were limited data on the clinical findings in thyroid disorders. These nail findings are consistent with our results, but onycholysis was more common in hypothyroid patients than in hyperthyroid patients in our review. Fox13 reported on 30 cases of onycholysis, stating that it affected patients with hypothyroidism and improved with thyroid treatment. In a clinical review of 8 commonly seen nail abnormalities, Fowler et al14 reported that hyperthyroidism was associated with nail findings in 5% of cases and may result in onycholysis of the fourth and fifth nails or all nails. They also reported that onychorrhexis may be seen in patients with hypothyroidism, a finding that differed from our results.14
The mechanism of nail changes in thyroid disease has not been well studied. A protein/amino acid–deficiency state may contribute to the development of koilonychia. Hyperthyroid patients, who have high metabolic activity, may have hypoalbuminemia, leading to koilonychia.15 Hypothyroidism causes hypothermia from decreased metabolic rate and secondary compensatory vasoconstriction. Vasoconstriction decreases blood flow of nutrients and oxygen to cutaneous structures and may cause slow-growing, brittle nails. In hyperthyroidism, vasodilation alternatively may contribute to the fast-growing nails. Anti–thyroid-stimulating hormone receptor antibodies in Graves disease may increase the synthesis of hyaluronic acid and glycosaminoglycans from fibroblasts, keratinocytes, adipocytes, or endothelial cells in the dermis and may contribute to development of clubbing.16
Our review is subject to several limitations. We recorded nail findings as they were described in the original studies; however, we could not confirm the accuracy of these descriptions. In addition, some specific nail changes were not described in sufficient detail. In all but 1 study, dermatologists performed the physical examination. In the study by Al-Dabbagh and Al-Abachi,10 the physical examinations were performed by general medicine physicians, but they selected only for patients with koilonychia and did not assess for other skin findings. Fragile nails and brittle nails were described in hypothyroid and hyperthyroid patients, but these nail changes were not described in detail. There also were studies describing nail changes in thyroid patients; some studies had small numbers of patients, and many did not have a control group.
Conclusion
Nail changes may be early clinical presenting signs of thyroid disorders and may be the clue to prompt diagnosis of thyroid disease. Dermatologists should be mindful that fragile, slow-growing, thin nails and onycholysis are associated with hypothyroidism and that koilonychia, softening, onycholysis, and brittle nail changes may be seen in hyperthyroidism. Our review aimed to describe nail changes associated with thyroid disease to guide dermatologists on diagnosis and promote future research on dermatologic manifestations of thyroid disease. Future research is necessary to explore the association between koilonychia and hyperthyroidism as well as the association of nail changes with thyroid disease duration and severity.
The major classifications of thyroid disease include hyperthyroidism, which is seen in Graves disease, and hypothyroidism due to iodine deficiency and Hashimoto thyroiditis, which have potentially devastating health consequences. The prevalence of hyperthyroidism ranges from 0.2% to 1.3% in iodine-sufficient parts of the world, and the prevalence of hypothyroidism in the general population is 5.3% in Europe and 3.7% in the United States.1 Thyroid hormones physiologically potentiate α- and β-adrenergic receptors by increasing their sensitivity to catecholamines. Excess thyroid hormones manifest as tachycardia, increased cardiac output, increased body temperature, hyperhidrosis, and warm moist skin. Reduced sensitivity of adrenergic receptors to catecholamines from insufficient thyroid hormones results in a lower metabolic rate and decreases response to the sympathetic nervous system.2 Nail changes in thyroid patients have not been well studied.3 Our objectives were to characterize nail findings in patients with thyroid disease. Early diagnosis of thyroid disease and prompt referral for treatment may be instrumental in preventing serious morbidities and permanent sequelae.
Methods
PubMed, Scopus, Web of Science, and Google Scholar were searched for the terms nail + thyroid, nail + hyperthyroid, nail + hypothyroid, nail + Graves, and nail + Hashimoto on June 10, 2020, and then updated on November 18, 2020. All English-language articles were included. Non–English-language articles and those that did not describe clinical trials of nail changes in patients with thyroid disease were excluded. One study that utilized survey-based data for nail changes without corroboration with physical examination findings was excluded. Hypothyroidism/hyperthyroidism was defined by all authors as measurement of serum thyroid hormones triiodothyronine, thyroxine, and thyroid-stimulating hormone outside of the normal range. Eight studies were included in the final analysis. Patient demographics, thyroid disease type, physical examination findings, nail clinical findings, age at diagnosis, age at onset of nail changes, treatments/medications, and comorbidities were recorded and analyzed.
Results
Nail changes in patients with thyroid disease were reported in 8 studies (7 cross-sectional, 1 retrospective cohort) and are summarized in the Table.4-11 The mean age was 41.2 years (range, 5–80 years), with a higher representation of females (range, 70%–94% female). The most common nail changes in thyroid patients were koilonychia, clubbing, and nail brittleness. Other changes included onycholysis, thin nails, dryness, and changes in nail growth rate. Frequent physical findings were xerosis, pruritus, and alopecia.


Both koilonychia and clubbing were reported in patients with hyperthyroidism. In a study of 32 patients with koilonychia, 22 (68.8%) were diagnosed with hyperthyroidism.10 Nail clubbing affected 7.3% of Graves disease patients (n=150)6 and 5.0% of hyperthyroid patients (n=120).7 Dermopathy presented more than 1 year after diagnosis of Graves disease in 99 (66%) of 150 patients as a late manifestation of thyrotoxicosis.6 Additional physical features in patients with Graves disease (n=150) were pretibial myxedema (100%), ophthalmopathy (99.0%), and proptosis (88.0%). Non–Graves hyperthyroid patients showed physical features of soft hair (83.3%) and soft skin (66.0%).7
Nail brittleness was a frequently reported nail change in thyroid patients (4/8 studies, 50%), most often seen in 22% of autoimmune patients, 19.6% of nonautoimmune patients, 13.9% of hypothyroid patients, and 9.2% of hyperthyroid patients.5,8 For comparison, brittle nails presented in 10.8% of participants in a control group.5 Brittle nails in thyroid patients often are accompanied by other nail findings such as thinning, onycholysis, and pitting.
Among hypothyroid patients, nail changes included fragility (70%; n=50), slow growth (48%; n=50), thinning (40%; n=50), onycholysis (38%; n=50),7 and brittleness (13.9%; n=173).5 Less common nail changes in hypothyroid patients were leukonychia (9.4%; n=32), striped nails (6%; n=50), and pitting (1.2%; n=173).5,7,11 Among hyperthyroid patients, the most common nail changes were koilonychia (100%; n=22), softening (83%; n=120), onycholysis (29%; n=14), and brittleness (9.2%; n=173).5,7,9,10 Less common nail changes in hyperthyroid patients were clubbing (5%; n=120), thinning (4.6%; n=173), and leukonychia (3%; n=120).5,7
Additional cutaneous findings of thyroid disorder included xerosis, alopecia, pruritus, and weight change. Xerosis was most common in hypothyroid disease (57.2%; n=460).4 In 2 studies,8,9 alopecia affected approximately 70% of autoimmune, nonautoimmune, and hyperthyroid patients. Hair loss was reported in 42.6% (n=460)4 and 33.0% (n=36)9 of hypothyroid patients. Additionally, pruritus affected up to 28% (n=32)11 of hypothyroid and 16.0% (n=120)7 of hyperthyroid patients and was more common in autoimmune (41%) vs nonautoimmune (32%) thyroid patients.8 Weight gain was seen in 72% of hypothyroid patients (n=32),11 and soft hair and skin were reported in 83.3% and 66% of hyperthyroid patients (n=120), respectively.7 Flushing was a less common physical finding in thyroid patients (usually affecting <10%); however, it also was reported in 17.1% of autoimmune and 57.1% of hyperthyroid patients from 2 separate studies.8,9
Comment
There are limited data describing nail changes with thyroid disease. Singal and Arora3 reported in their clinical review of nail changes in systemic disease that koilonychia, onycholysis, and melanonychia are associated with thyroid disorders. We similarly found that koilonychia and onycholysis are associated with thyroid disorders without an association with melanonychia.
In his clinical review of thyroid hormone action on the skin, Safer12 described hypothyroid patients having coarse, dull, thin, and brittle nails, whereas in thyrotoxicosis, patients had shiny, soft, and concave nails with onycholysis; however, the author commented that there were limited data on the clinical findings in thyroid disorders. These nail findings are consistent with our results, but onycholysis was more common in hypothyroid patients than in hyperthyroid patients in our review. Fox13 reported on 30 cases of onycholysis, stating that it affected patients with hypothyroidism and improved with thyroid treatment. In a clinical review of 8 commonly seen nail abnormalities, Fowler et al14 reported that hyperthyroidism was associated with nail findings in 5% of cases and may result in onycholysis of the fourth and fifth nails or all nails. They also reported that onychorrhexis may be seen in patients with hypothyroidism, a finding that differed from our results.14
The mechanism of nail changes in thyroid disease has not been well studied. A protein/amino acid–deficiency state may contribute to the development of koilonychia. Hyperthyroid patients, who have high metabolic activity, may have hypoalbuminemia, leading to koilonychia.15 Hypothyroidism causes hypothermia from decreased metabolic rate and secondary compensatory vasoconstriction. Vasoconstriction decreases blood flow of nutrients and oxygen to cutaneous structures and may cause slow-growing, brittle nails. In hyperthyroidism, vasodilation alternatively may contribute to the fast-growing nails. Anti–thyroid-stimulating hormone receptor antibodies in Graves disease may increase the synthesis of hyaluronic acid and glycosaminoglycans from fibroblasts, keratinocytes, adipocytes, or endothelial cells in the dermis and may contribute to development of clubbing.16
Our review is subject to several limitations. We recorded nail findings as they were described in the original studies; however, we could not confirm the accuracy of these descriptions. In addition, some specific nail changes were not described in sufficient detail. In all but 1 study, dermatologists performed the physical examination. In the study by Al-Dabbagh and Al-Abachi,10 the physical examinations were performed by general medicine physicians, but they selected only for patients with koilonychia and did not assess for other skin findings. Fragile nails and brittle nails were described in hypothyroid and hyperthyroid patients, but these nail changes were not described in detail. There also were studies describing nail changes in thyroid patients; some studies had small numbers of patients, and many did not have a control group.
Conclusion
Nail changes may be early clinical presenting signs of thyroid disorders and may be the clue to prompt diagnosis of thyroid disease. Dermatologists should be mindful that fragile, slow-growing, thin nails and onycholysis are associated with hypothyroidism and that koilonychia, softening, onycholysis, and brittle nail changes may be seen in hyperthyroidism. Our review aimed to describe nail changes associated with thyroid disease to guide dermatologists on diagnosis and promote future research on dermatologic manifestations of thyroid disease. Future research is necessary to explore the association between koilonychia and hyperthyroidism as well as the association of nail changes with thyroid disease duration and severity.
- Taylor PN, Albrecht D, Scholz A, et al. Global epidemiology of hyperthyroidism and hypothyroidism. Nat Rev Endocrinol. 2018;14:301-316.
- Lause M, Kamboj A, Faith EF. Dermatologic manifestations of endocrine disorders. Transl Pediatr. 2017;6:300-312.
- Singal A, Arora R. Nail as a window of systemic diseases. Indian Dermatol Online J. 2015;6:67-74.
- Keen MA, Hassan I, Bhat MH. A clinical study of the cutaneous manifestations of hypothyroidism in Kashmir Valley. Indian J Dermatol. 2013;58:326.
- Takir M, Özlü E, Köstek O, et al. Skin findings in autoimmune and nonautoimmune thyroid disease with respect to thyroid functional status and healthy controls. Turk J Med Sci. 2017;47:764-770.
- Fatourechi V, Pajouhi M, Fransway AF. Dermopathy of Graves disease (pretibial myxedema). review of 150 cases. Medicine (Baltimore). 1994;73:1-7.
- Razi A, Golforoushan F, Nejad AB, et al. Evaluation of dermal symptoms in hypothyroidism and hyperthyroidism. Pak J Biol Sci. 2013;16:541-544.
- Acer E, Ag˘aog˘lu E, Yorulmaz G, et al. Evaluation of cutaneous manifestations in patients under treatment with thyroid disease. Turkderm-Turk Arch Dermatol Venereol. 2019;54:46-50.
- Puri N. A study on cutaneous manifestations of thyroid disease. Indian J Dermatol. 2012;57:247-248.
- Al-Dabbagh TQ, Al-Abachi KG. Nutritional koilonychia in 32 Iraqi subjects. Ann Saudi Med. 2005;25:154-157.
- Dogra A, Dua A, Singh P. Thyroid and skin. Indian J Dermatol. 2006;51:96-99.
- Safer JD. Thyroid hormone action on skin. Dermatoendocrinol. 2011;3:211-215.
- Fox EC. Diseases of the nails: report of cases of onycholysis. Arch Derm Syphilol. 1940;41:98-112.
- Fowler JR, Stern E, English JC 3rd, et al. A hand surgeon’s guide to common onychodystrophies. Hand (N Y). 2014;9:24-28.
- Truswell AS. Nutritional factors in disease. In: Edwards CRW, Bouchier IAD, Haslett C, et al, eds. Davidson’s Principles and Practice of Medicine. 17th ed. Churchill Livingstone; 1995:554.
- Heymann WR. Cutaneous manifestations of thyroid disease. J Am Acad Dermatol. 1992;26:885-902.
- Taylor PN, Albrecht D, Scholz A, et al. Global epidemiology of hyperthyroidism and hypothyroidism. Nat Rev Endocrinol. 2018;14:301-316.
- Lause M, Kamboj A, Faith EF. Dermatologic manifestations of endocrine disorders. Transl Pediatr. 2017;6:300-312.
- Singal A, Arora R. Nail as a window of systemic diseases. Indian Dermatol Online J. 2015;6:67-74.
- Keen MA, Hassan I, Bhat MH. A clinical study of the cutaneous manifestations of hypothyroidism in Kashmir Valley. Indian J Dermatol. 2013;58:326.
- Takir M, Özlü E, Köstek O, et al. Skin findings in autoimmune and nonautoimmune thyroid disease with respect to thyroid functional status and healthy controls. Turk J Med Sci. 2017;47:764-770.
- Fatourechi V, Pajouhi M, Fransway AF. Dermopathy of Graves disease (pretibial myxedema). review of 150 cases. Medicine (Baltimore). 1994;73:1-7.
- Razi A, Golforoushan F, Nejad AB, et al. Evaluation of dermal symptoms in hypothyroidism and hyperthyroidism. Pak J Biol Sci. 2013;16:541-544.
- Acer E, Ag˘aog˘lu E, Yorulmaz G, et al. Evaluation of cutaneous manifestations in patients under treatment with thyroid disease. Turkderm-Turk Arch Dermatol Venereol. 2019;54:46-50.
- Puri N. A study on cutaneous manifestations of thyroid disease. Indian J Dermatol. 2012;57:247-248.
- Al-Dabbagh TQ, Al-Abachi KG. Nutritional koilonychia in 32 Iraqi subjects. Ann Saudi Med. 2005;25:154-157.
- Dogra A, Dua A, Singh P. Thyroid and skin. Indian J Dermatol. 2006;51:96-99.
- Safer JD. Thyroid hormone action on skin. Dermatoendocrinol. 2011;3:211-215.
- Fox EC. Diseases of the nails: report of cases of onycholysis. Arch Derm Syphilol. 1940;41:98-112.
- Fowler JR, Stern E, English JC 3rd, et al. A hand surgeon’s guide to common onychodystrophies. Hand (N Y). 2014;9:24-28.
- Truswell AS. Nutritional factors in disease. In: Edwards CRW, Bouchier IAD, Haslett C, et al, eds. Davidson’s Principles and Practice of Medicine. 17th ed. Churchill Livingstone; 1995:554.
- Heymann WR. Cutaneous manifestations of thyroid disease. J Am Acad Dermatol. 1992;26:885-902.
Practice Points
- Koilonychia is associated with hyperthyroidism.
- Clubbing is a manifestation of thyroid acropachy in Graves disease and also affects other patients with hyperthyroidism.
- Onycholysis improves in patients with hypothyroidism treated with thyroid hormone replacement therapy.
Online Information About Hydroquinone: An Assessment of Accuracy and Readability
To the Editor:
The internet is a popular resource for patients seeking information about dermatologic treatments. Hydroquinone (HQ) cream 4% is approved by the US Food and Drug Administration for skin hyperpigmentation.1 The agency enforced the CARES (Coronavirus Aid, Relief, and Economic Security) Act and OTC (over-the-counter) Monograph Reform on September 25, 2020, to restrict distribution of OTC HQ.2 Exogenous ochronosis is listed as a potential adverse effect in the prescribing information for HQ.1
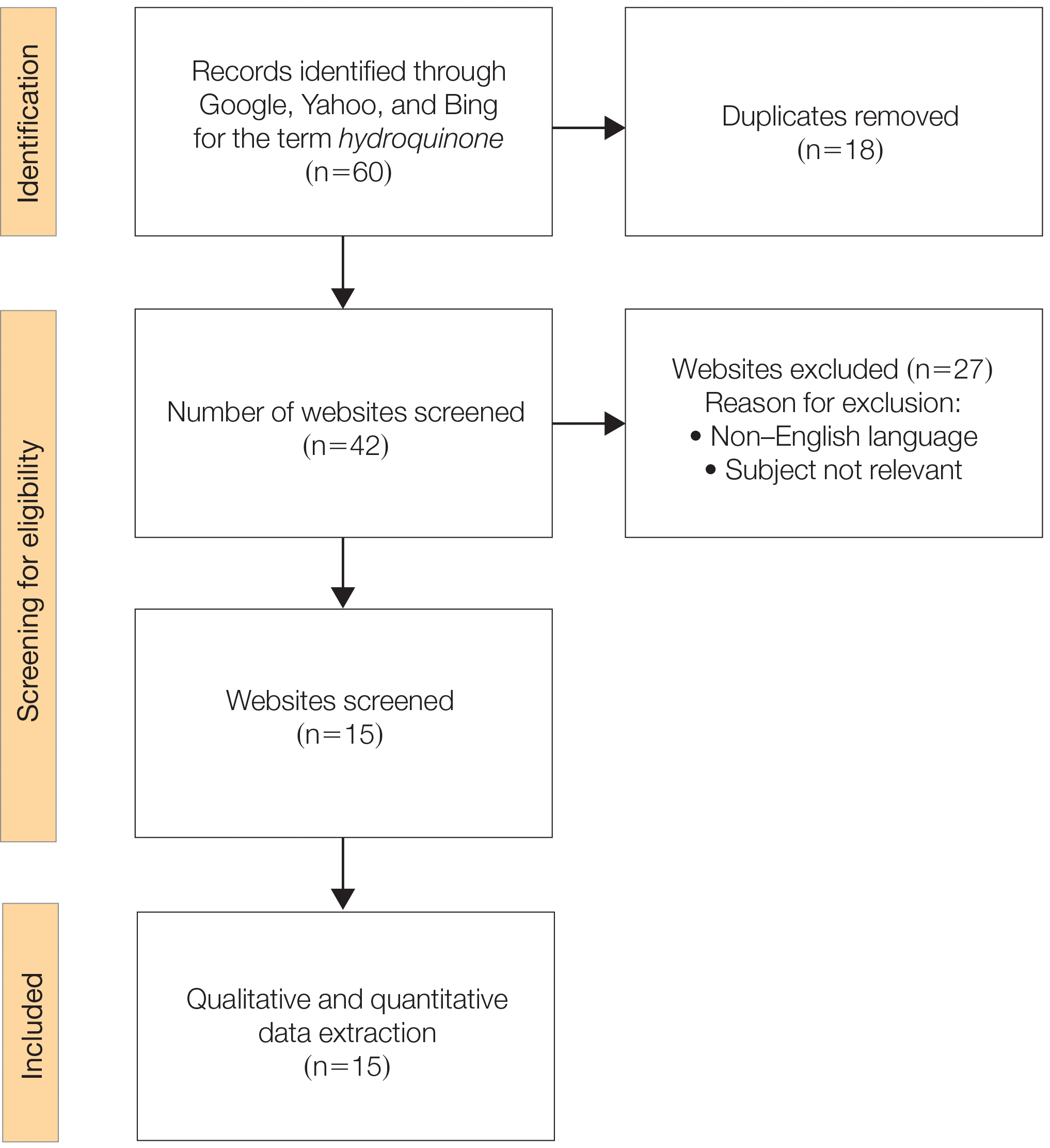
We sought to assess online resources on HQ for accuracy of information, including the recent OTC ban, as well as readability. The word hydroquinone was searched on 3 internet search engines—Google, Yahoo, and Bing—on December 12, 2020, each for the first 20 URLs (ie, websites)(total of 60 URLs). Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA)(Figure) reporting guidelines were used to assess a list of relevant websites to include in the final analysis. Website data were reviewed by both authors. Eighteen duplicates and 27 irrelevant and non–English-language URLs were excluded. The remaining 15 websites were analyzed. Based on a previously published and validated tool, a pro forma was designed to evaluate information on HQ for each website based on accountability, quality, readability, display, support, and transparency (Table).1,3

Scores for all 15 websites are listed in eTable 1. The mean overall (total) score was
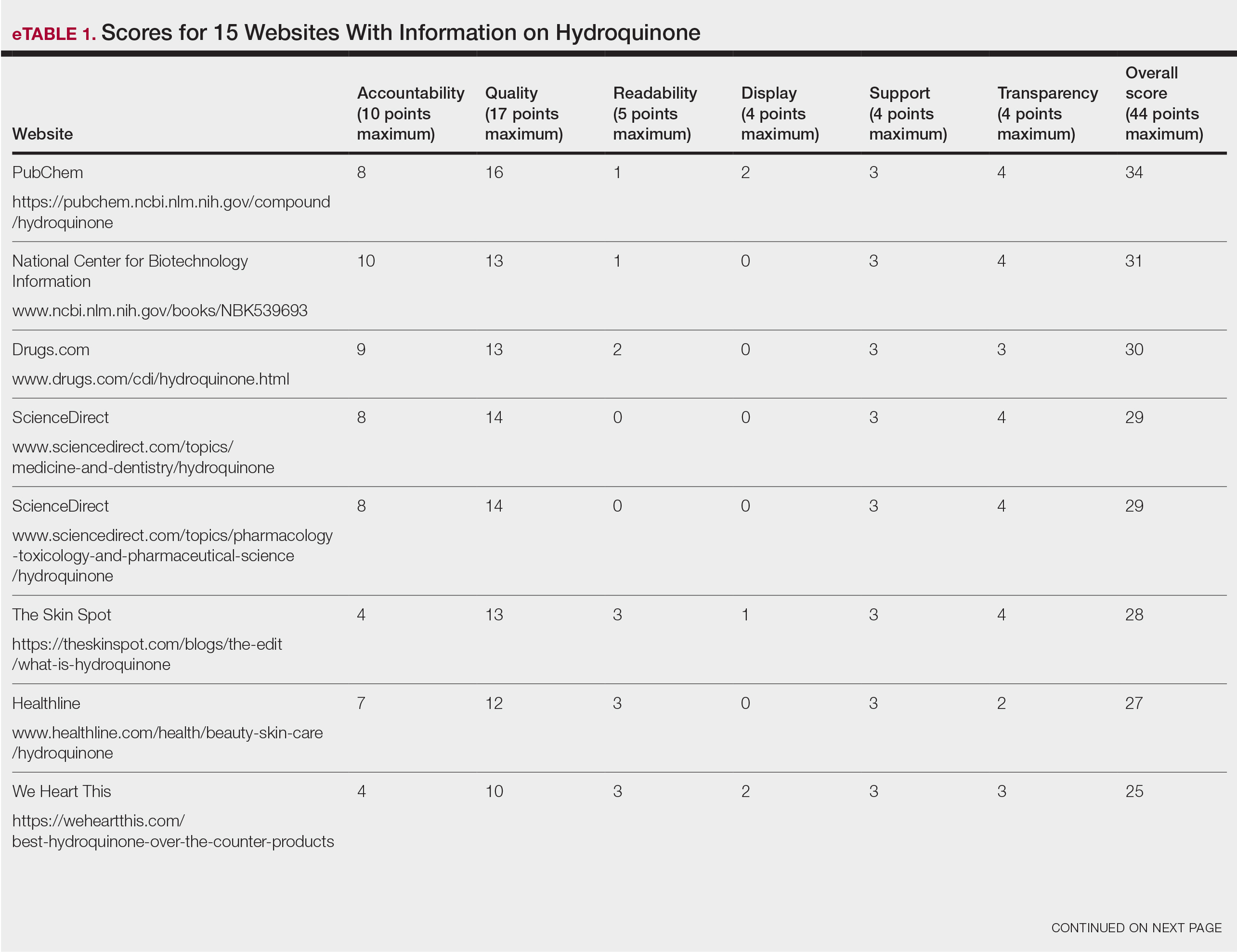
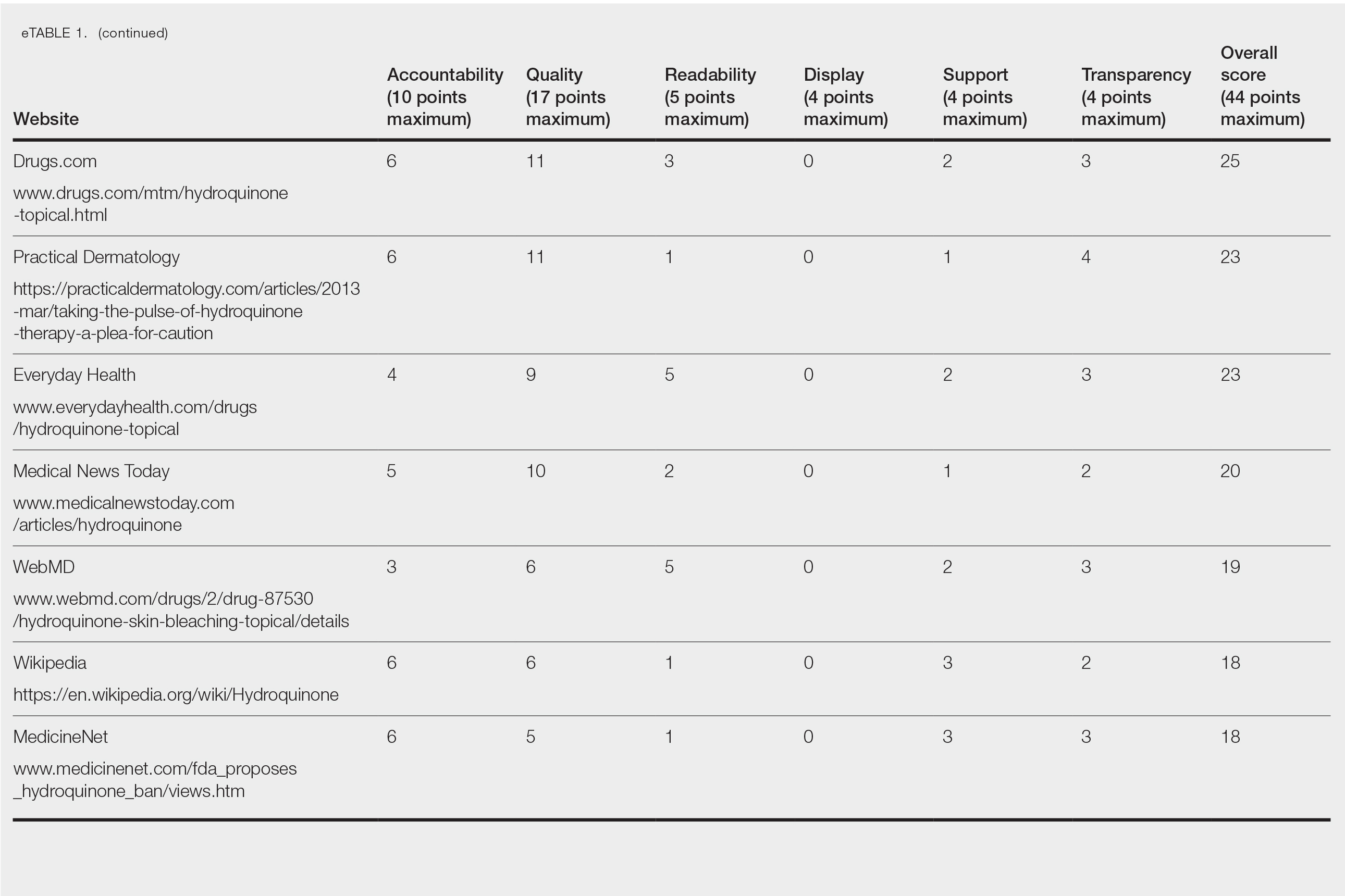
The mean display score was 0.3 (of a possible 4; range, 0–2); 66.7% of websites (10/15) had advertisements or irrelevant material. Only 6.7% and 13.3% of websites included relevant videos or images, respectively, on applying HQ (eTable 2). We identified only 3 photographs—across all 15 websites—that depicted skin, all of which were Fitzpatrick skin types II or III. Therefore, none of the websites included a diversity of images to indicate broad ethnic relatability.
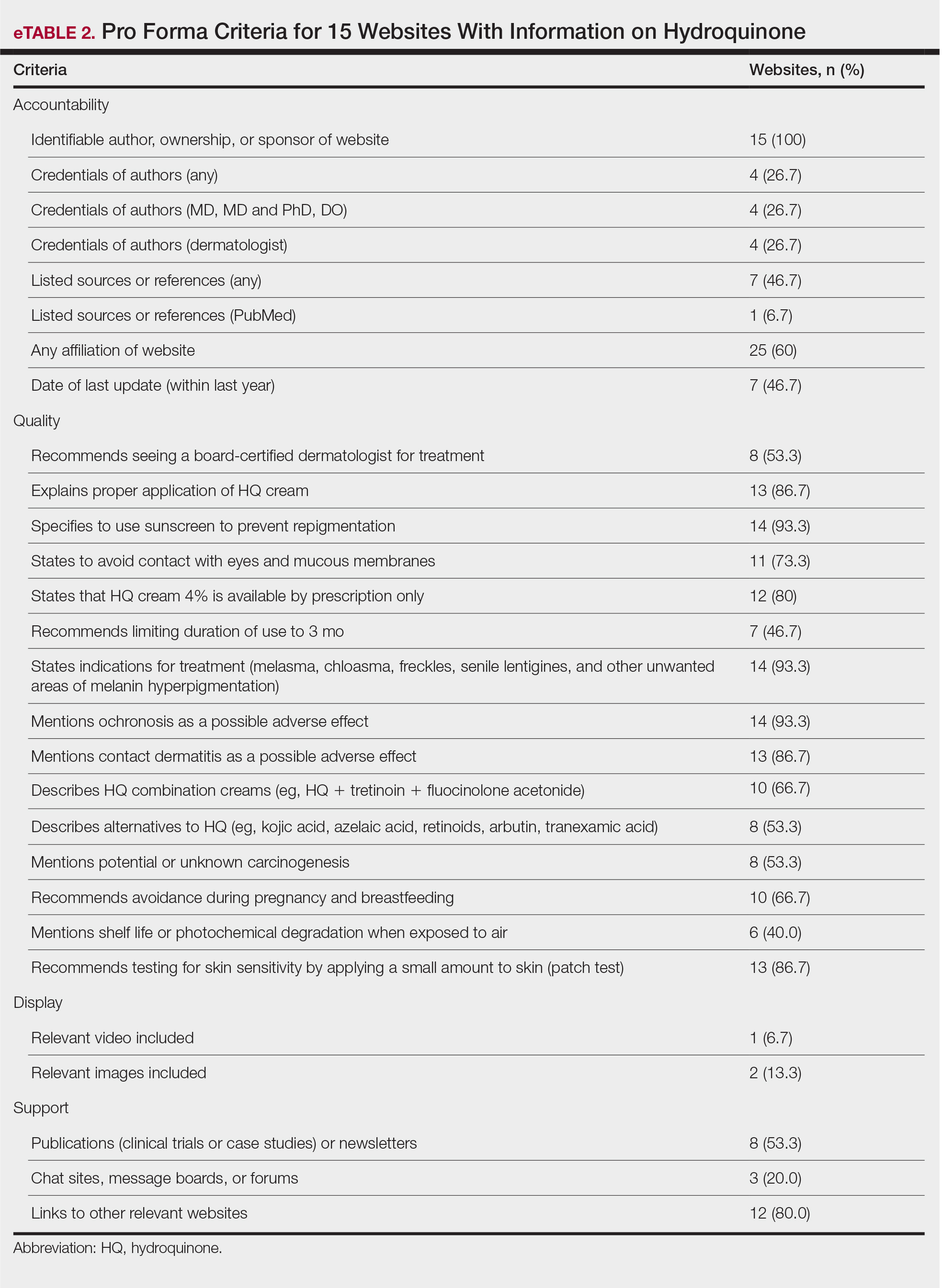
The average support score was 2.5 (of a possible 4; range, 1–3); 20% (3/15) of URLs included chat sites, message boards, or forums, and approximately half (8/15 [53.3%]) included references. Only 7 URLs (46.7%) had been updated in the last 12 months. Only 4 (26.7%) were written by a board-certified dermatologist (eTable 2). Most (60%) websites contained advertising, though none were sponsored by a pharmaceutical company that manufactures HQ.
Only 46.7% (7/15) of websites recommended limiting a course of HQ treatment to 3 months; only 40% (6/15) mentioned shelf life or photochemical degradation when exposed to air. Although 93.3% (14/15) of URLs mentioned ochronosis, a clinical description of the condition was provided in only 33.3% (5/15)—none with images.
Only 2 sites (13.3%; Everyday Health and WebMD) met the accepted 7th-grade reading level for online patient education material; those sites scored lower on quality (9 of 17 and 6 of 17, respectively) than sites with higher overall scores.
None of the 15 websites studied, therefore, demonstrated optimal features on combined measures of accountability, quality, readability, display, support, and transparency regarding HQ. Notably, the American Academy of Dermatology website (www.aad.org) was not among the 15 websites studied; the AAD website mentions HQ in a section on melasma, but only minimal detail is provided.
Limitations of this study include the small number of websites analyzed and possible selection bias because only 3 internet search engines were used to identify websites for study and analysis.
Previously, we analyzed content about HQ on the video-sharing and social media platform YouTube.4 The most viewed YouTube videos on HQ had poor-quality information (ie, only 20% mentioned ochronosis and only 28.6% recommended sunscreen [N=70]). However, average reading level of these videos was 7th grade.4,5 Therefore, YouTube HQ content, though comprehensible, generally is of poor quality.
By conducting a search for website content about HQ, we found that the most popular URLs had either accurate information with poor readability or lower-quality educational material that was more comprehensible. We conclude that there is a need to develop online patient education materials on HQ that are characterized by high-quality, up-to-date medical information; have been written by board-certified dermatologists; are comprehensible (ie, no more than approximately 1200 words and written at a 7th-grade reading level); and contain relevant clinical images and references. We encourage dermatologists to recognize the limitations of online patient education resources on HQ and educate patients on the proper use of the drug as well as its potential adverse effects
- US National Library of Medicine. Label: hydroquinone cream. DailyMed website. Updated November 24, 2020. Accessed May 19, 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=dc72c0b2-4505-4dcf-8a69-889cd9f41693
- US Congress. H.R.748 - CARES Act. 116th Congress (2019-2020). Updated March 27, 2020. Accessed May 19, 2022. https://www.congress.gov/bill/116th-congress/house-bill/748/text?fbclid=IwAR3ZxGP6AKUl6ce-dlWSU6D5MfCLD576nWNBV5YTE7R2a0IdLY4Usw4oOv4
- Kang R, Lipner S. Evaluation of onychomycosis information on the internet. J Drugs Dermatol. 2019;18:484-487.
- Ishack S, Lipner SR. Assessing the impact and educational value of YouTube as a source of information on hydroquinone: a content-quality and readability analysis. J Dermatolog Treat. 2020:1-3. doi:10.1080/09546634.2020.1782318
- Weiss BD. Health Literacy: A Manual for Clinicians. American Medical Association Foundation and American Medical Association; 2003. Accessed May 19, 2022. http://lib.ncfh.org/pdfs/6617.pdf
To the Editor:
The internet is a popular resource for patients seeking information about dermatologic treatments. Hydroquinone (HQ) cream 4% is approved by the US Food and Drug Administration for skin hyperpigmentation.1 The agency enforced the CARES (Coronavirus Aid, Relief, and Economic Security) Act and OTC (over-the-counter) Monograph Reform on September 25, 2020, to restrict distribution of OTC HQ.2 Exogenous ochronosis is listed as a potential adverse effect in the prescribing information for HQ.1

We sought to assess online resources on HQ for accuracy of information, including the recent OTC ban, as well as readability. The word hydroquinone was searched on 3 internet search engines—Google, Yahoo, and Bing—on December 12, 2020, each for the first 20 URLs (ie, websites)(total of 60 URLs). Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA)(Figure) reporting guidelines were used to assess a list of relevant websites to include in the final analysis. Website data were reviewed by both authors. Eighteen duplicates and 27 irrelevant and non–English-language URLs were excluded. The remaining 15 websites were analyzed. Based on a previously published and validated tool, a pro forma was designed to evaluate information on HQ for each website based on accountability, quality, readability, display, support, and transparency (Table).1,3

Scores for all 15 websites are listed in eTable 1. The mean overall (total) score was


The mean display score was 0.3 (of a possible 4; range, 0–2); 66.7% of websites (10/15) had advertisements or irrelevant material. Only 6.7% and 13.3% of websites included relevant videos or images, respectively, on applying HQ (eTable 2). We identified only 3 photographs—across all 15 websites—that depicted skin, all of which were Fitzpatrick skin types II or III. Therefore, none of the websites included a diversity of images to indicate broad ethnic relatability.

The average support score was 2.5 (of a possible 4; range, 1–3); 20% (3/15) of URLs included chat sites, message boards, or forums, and approximately half (8/15 [53.3%]) included references. Only 7 URLs (46.7%) had been updated in the last 12 months. Only 4 (26.7%) were written by a board-certified dermatologist (eTable 2). Most (60%) websites contained advertising, though none were sponsored by a pharmaceutical company that manufactures HQ.
Only 46.7% (7/15) of websites recommended limiting a course of HQ treatment to 3 months; only 40% (6/15) mentioned shelf life or photochemical degradation when exposed to air. Although 93.3% (14/15) of URLs mentioned ochronosis, a clinical description of the condition was provided in only 33.3% (5/15)—none with images.
Only 2 sites (13.3%; Everyday Health and WebMD) met the accepted 7th-grade reading level for online patient education material; those sites scored lower on quality (9 of 17 and 6 of 17, respectively) than sites with higher overall scores.
None of the 15 websites studied, therefore, demonstrated optimal features on combined measures of accountability, quality, readability, display, support, and transparency regarding HQ. Notably, the American Academy of Dermatology website (www.aad.org) was not among the 15 websites studied; the AAD website mentions HQ in a section on melasma, but only minimal detail is provided.
Limitations of this study include the small number of websites analyzed and possible selection bias because only 3 internet search engines were used to identify websites for study and analysis.
Previously, we analyzed content about HQ on the video-sharing and social media platform YouTube.4 The most viewed YouTube videos on HQ had poor-quality information (ie, only 20% mentioned ochronosis and only 28.6% recommended sunscreen [N=70]). However, average reading level of these videos was 7th grade.4,5 Therefore, YouTube HQ content, though comprehensible, generally is of poor quality.
By conducting a search for website content about HQ, we found that the most popular URLs had either accurate information with poor readability or lower-quality educational material that was more comprehensible. We conclude that there is a need to develop online patient education materials on HQ that are characterized by high-quality, up-to-date medical information; have been written by board-certified dermatologists; are comprehensible (ie, no more than approximately 1200 words and written at a 7th-grade reading level); and contain relevant clinical images and references. We encourage dermatologists to recognize the limitations of online patient education resources on HQ and educate patients on the proper use of the drug as well as its potential adverse effects
To the Editor:
The internet is a popular resource for patients seeking information about dermatologic treatments. Hydroquinone (HQ) cream 4% is approved by the US Food and Drug Administration for skin hyperpigmentation.1 The agency enforced the CARES (Coronavirus Aid, Relief, and Economic Security) Act and OTC (over-the-counter) Monograph Reform on September 25, 2020, to restrict distribution of OTC HQ.2 Exogenous ochronosis is listed as a potential adverse effect in the prescribing information for HQ.1

We sought to assess online resources on HQ for accuracy of information, including the recent OTC ban, as well as readability. The word hydroquinone was searched on 3 internet search engines—Google, Yahoo, and Bing—on December 12, 2020, each for the first 20 URLs (ie, websites)(total of 60 URLs). Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA)(Figure) reporting guidelines were used to assess a list of relevant websites to include in the final analysis. Website data were reviewed by both authors. Eighteen duplicates and 27 irrelevant and non–English-language URLs were excluded. The remaining 15 websites were analyzed. Based on a previously published and validated tool, a pro forma was designed to evaluate information on HQ for each website based on accountability, quality, readability, display, support, and transparency (Table).1,3

Scores for all 15 websites are listed in eTable 1. The mean overall (total) score was


The mean display score was 0.3 (of a possible 4; range, 0–2); 66.7% of websites (10/15) had advertisements or irrelevant material. Only 6.7% and 13.3% of websites included relevant videos or images, respectively, on applying HQ (eTable 2). We identified only 3 photographs—across all 15 websites—that depicted skin, all of which were Fitzpatrick skin types II or III. Therefore, none of the websites included a diversity of images to indicate broad ethnic relatability.

The average support score was 2.5 (of a possible 4; range, 1–3); 20% (3/15) of URLs included chat sites, message boards, or forums, and approximately half (8/15 [53.3%]) included references. Only 7 URLs (46.7%) had been updated in the last 12 months. Only 4 (26.7%) were written by a board-certified dermatologist (eTable 2). Most (60%) websites contained advertising, though none were sponsored by a pharmaceutical company that manufactures HQ.
Only 46.7% (7/15) of websites recommended limiting a course of HQ treatment to 3 months; only 40% (6/15) mentioned shelf life or photochemical degradation when exposed to air. Although 93.3% (14/15) of URLs mentioned ochronosis, a clinical description of the condition was provided in only 33.3% (5/15)—none with images.
Only 2 sites (13.3%; Everyday Health and WebMD) met the accepted 7th-grade reading level for online patient education material; those sites scored lower on quality (9 of 17 and 6 of 17, respectively) than sites with higher overall scores.
None of the 15 websites studied, therefore, demonstrated optimal features on combined measures of accountability, quality, readability, display, support, and transparency regarding HQ. Notably, the American Academy of Dermatology website (www.aad.org) was not among the 15 websites studied; the AAD website mentions HQ in a section on melasma, but only minimal detail is provided.
Limitations of this study include the small number of websites analyzed and possible selection bias because only 3 internet search engines were used to identify websites for study and analysis.
Previously, we analyzed content about HQ on the video-sharing and social media platform YouTube.4 The most viewed YouTube videos on HQ had poor-quality information (ie, only 20% mentioned ochronosis and only 28.6% recommended sunscreen [N=70]). However, average reading level of these videos was 7th grade.4,5 Therefore, YouTube HQ content, though comprehensible, generally is of poor quality.
By conducting a search for website content about HQ, we found that the most popular URLs had either accurate information with poor readability or lower-quality educational material that was more comprehensible. We conclude that there is a need to develop online patient education materials on HQ that are characterized by high-quality, up-to-date medical information; have been written by board-certified dermatologists; are comprehensible (ie, no more than approximately 1200 words and written at a 7th-grade reading level); and contain relevant clinical images and references. We encourage dermatologists to recognize the limitations of online patient education resources on HQ and educate patients on the proper use of the drug as well as its potential adverse effects
- US National Library of Medicine. Label: hydroquinone cream. DailyMed website. Updated November 24, 2020. Accessed May 19, 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=dc72c0b2-4505-4dcf-8a69-889cd9f41693
- US Congress. H.R.748 - CARES Act. 116th Congress (2019-2020). Updated March 27, 2020. Accessed May 19, 2022. https://www.congress.gov/bill/116th-congress/house-bill/748/text?fbclid=IwAR3ZxGP6AKUl6ce-dlWSU6D5MfCLD576nWNBV5YTE7R2a0IdLY4Usw4oOv4
- Kang R, Lipner S. Evaluation of onychomycosis information on the internet. J Drugs Dermatol. 2019;18:484-487.
- Ishack S, Lipner SR. Assessing the impact and educational value of YouTube as a source of information on hydroquinone: a content-quality and readability analysis. J Dermatolog Treat. 2020:1-3. doi:10.1080/09546634.2020.1782318
- Weiss BD. Health Literacy: A Manual for Clinicians. American Medical Association Foundation and American Medical Association; 2003. Accessed May 19, 2022. http://lib.ncfh.org/pdfs/6617.pdf
- US National Library of Medicine. Label: hydroquinone cream. DailyMed website. Updated November 24, 2020. Accessed May 19, 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=dc72c0b2-4505-4dcf-8a69-889cd9f41693
- US Congress. H.R.748 - CARES Act. 116th Congress (2019-2020). Updated March 27, 2020. Accessed May 19, 2022. https://www.congress.gov/bill/116th-congress/house-bill/748/text?fbclid=IwAR3ZxGP6AKUl6ce-dlWSU6D5MfCLD576nWNBV5YTE7R2a0IdLY4Usw4oOv4
- Kang R, Lipner S. Evaluation of onychomycosis information on the internet. J Drugs Dermatol. 2019;18:484-487.
- Ishack S, Lipner SR. Assessing the impact and educational value of YouTube as a source of information on hydroquinone: a content-quality and readability analysis. J Dermatolog Treat. 2020:1-3. doi:10.1080/09546634.2020.1782318
- Weiss BD. Health Literacy: A Manual for Clinicians. American Medical Association Foundation and American Medical Association; 2003. Accessed May 19, 2022. http://lib.ncfh.org/pdfs/6617.pdf
Practice Points
- Hydroquinone (HQ) 4% is US Food and Drug Administration (FDA) approved for skin hyperpigmentation including melasma.
- In September 2020, the FDA enforced the CARES (Coronavirus Aid, Relief, and Economic Security) Act and OTC (over-the-counter) Monograph Reform, announcing that HQ is not classified as Category II/not generally recognized as safe and effective, thus prohibiting the distribution of OTC HQ products.
- Exogenous ochronosis is a potential side effect associated with HQ.
- There is a need for dermatologists to develop online patient education materials on HQ that are characterized by high-quality and up-to-date medical information.
A Contrasting Dark Background for Nail Sampling
Practice Gap
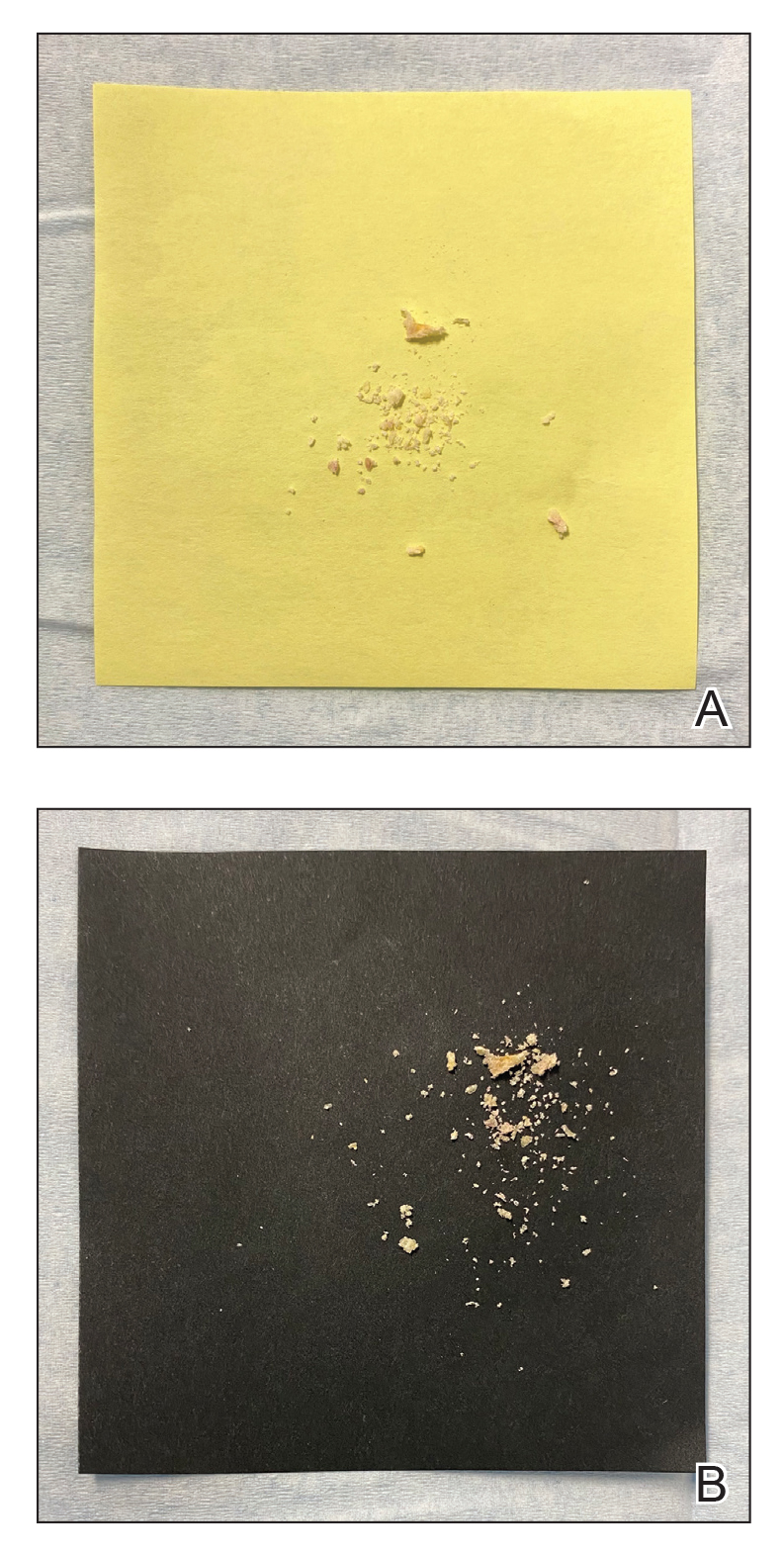
Mycologic testing is necessary and cost-effective1 for appropriate diagnosis and treatment of onychomycosis. Empiric treatment of onychodystrophy for presumed onychomycosis can result in misdiagnosis, treatment failure, or potential adverse effects caused by medications.2 Collection of ample subungual debris facilitates the sensitivity and specificity of fungal culture and fungal polymerase chain reaction. However, the naturally pale hue of subungual debris makes specimen estimation challenging, particularly when using a similarly light-colored gauze or piece of paper for collection (Figure, A).
The Technique
A sheet from a black sticky notepad (widely available and cost-effective) can be adapted for making a diagnosis of onychomycosis (Figure, B).
Practical Implication
Use of a dark background that contrasts with light-hued nail debris is valuable to ensure an adequate specimen for fungal culture and polymerase chain reaction.
- Gupta AK, Versteeg SG, Shear NH. Confirmatory testing prior to initiating onychomycosis therapy is cost effective. J Cutan Med Surg. 2018;22:129-141. doi:10.1177/1203475417733461
- Lipner SR, Scher RK. Onychomycosis—a small step for quality of care. Curr Med Res Opin. 2016;32:865-867. doi:10.1185/03007995.2016.1147026
Practice Gap
Mycologic testing is necessary and cost-effective1 for appropriate diagnosis and treatment of onychomycosis. Empiric treatment of onychodystrophy for presumed onychomycosis can result in misdiagnosis, treatment failure, or potential adverse effects caused by medications.2 Collection of ample subungual debris facilitates the sensitivity and specificity of fungal culture and fungal polymerase chain reaction. However, the naturally pale hue of subungual debris makes specimen estimation challenging, particularly when using a similarly light-colored gauze or piece of paper for collection (Figure, A).

The Technique
A sheet from a black sticky notepad (widely available and cost-effective) can be adapted for making a diagnosis of onychomycosis (Figure, B).
Practical Implication
Use of a dark background that contrasts with light-hued nail debris is valuable to ensure an adequate specimen for fungal culture and polymerase chain reaction.
Practice Gap
Mycologic testing is necessary and cost-effective1 for appropriate diagnosis and treatment of onychomycosis. Empiric treatment of onychodystrophy for presumed onychomycosis can result in misdiagnosis, treatment failure, or potential adverse effects caused by medications.2 Collection of ample subungual debris facilitates the sensitivity and specificity of fungal culture and fungal polymerase chain reaction. However, the naturally pale hue of subungual debris makes specimen estimation challenging, particularly when using a similarly light-colored gauze or piece of paper for collection (Figure, A).

The Technique
A sheet from a black sticky notepad (widely available and cost-effective) can be adapted for making a diagnosis of onychomycosis (Figure, B).
Practical Implication
Use of a dark background that contrasts with light-hued nail debris is valuable to ensure an adequate specimen for fungal culture and polymerase chain reaction.
- Gupta AK, Versteeg SG, Shear NH. Confirmatory testing prior to initiating onychomycosis therapy is cost effective. J Cutan Med Surg. 2018;22:129-141. doi:10.1177/1203475417733461
- Lipner SR, Scher RK. Onychomycosis—a small step for quality of care. Curr Med Res Opin. 2016;32:865-867. doi:10.1185/03007995.2016.1147026
- Gupta AK, Versteeg SG, Shear NH. Confirmatory testing prior to initiating onychomycosis therapy is cost effective. J Cutan Med Surg. 2018;22:129-141. doi:10.1177/1203475417733461
- Lipner SR, Scher RK. Onychomycosis—a small step for quality of care. Curr Med Res Opin. 2016;32:865-867. doi:10.1185/03007995.2016.1147026
The Top 100 Most-Cited Articles on Nail Psoriasis: A Bibliometric Analysis
To the Editor:
Nail psoriasis is highly prevalent in patients with cutaneous psoriasis and also may present as an isolated finding. There is a strong association between nail psoriasis and development of psoriatic arthritis (PsA). However, publications on nail psoriasis are sparse compared with articles describing cutaneous psoriasis.1 Our objectives were to analyze the nail psoriasis literature for content, citations, and media attention.
The Web of Science database was searched for the term nail psoriasis on April 27, 2020, and publications by year, subject, and article type were compiled. Total and average yearly citations were calculated to create a list of the top 100 most-cited articles (eTable). First and last authors, sex, and Altmetric Attention Scores were then recorded. The Wilcoxon rank sum test was calculated to compare the relationship of Altmetric scores between nail psoriasis–specific references and others on the list.
In our data set, the average total number of citations was 134.09 (range, 42–1617), with average yearly citations ranging from 2 to 108. Altmetric scores—measures of media attention of scholarly work—were available for 58 of 100 papers (58%), with an average score of 33.2 (range, 1–509).
Of the top 100 most-cited articles using the search term nail psoriasis, only 20% focused on nail psoriasis, with the remainder concentrating on psoriasis/PsA. Only 32% and 24% of first and last authors, respectively, were female. Fifty-two percent and 31% of the articles were published in dermatology and arthritis/rheumatology journals, respectively. There was no statistically significant difference in Altmetric scores between nail psoriasis–specific and other articles in our data set (P=.7551).
For the nail psoriasis–specific articles, all 20 highlighted a lack of nail clinical trials, a positive association with PsA, and a correlation of increased cutaneous psoriasis body surface area with increased onychodystrophy likelihood.2 Three of 20 (15%) articles stated that nail psoriasis often is overlooked, despite the negative impact on quality of life,1 and emphasized the importance of patient compliance owing to the chronic nature of the disease. Only 1 of 20 (5%) articles focused on nail psoriasis treatments.3 There was no overlap between the 100 most-cited psoriasis articles from 1970 to 2012 and our top 100 articles on nail psoriasis.4
Treatment recommendations for nail psoriasis by consensus were published by a nail expert group in 2019.5 For 3 or fewer nails involved, suggested first-line treatment is intralesional matrix injections with triamcinolone acetonide. For more than 3 affected nails, systemic treatment with oral or biologic therapy is recommended.5 Although this article is likely to change clinical practice, it did not qualify for our list because it did not garner sufficient citations in the brief period between its publication date and our search (July 2019–April 2020).
This study is subject to several limitations. Only the Web of Science database was utilized, and only the term nail psoriasis was searched, potentially excluding relevant articles. Using total citations biases toward older articles.
Our bibliometric analysis highlights a lack of publications on nail psoriasis, with most articles focusing on psoriasis and PsA. This deficiency in highly cited nail psoriasis references is likely to be a barrier to physicians in managing patients with nail disease. There is a need for controlled clinical trials and better mechanisms to disseminate information on management of nail psoriasis to practicing physicians.
- Williamson L, Dalbeth N, Dockerty JL, et al. Extended report: nail disease in psoriatic arthritis—clinically important, potentially treatable and often overlooked. Rheumatology (Oxford). 2004;43:790-794. doi:10.1093/rheumatology/keh198
- Reich K. Approach to managing patients with nail psoriasis. J Eur Acad Dermatol Venereol. 2009;23(suppl 1):15-21. doi:10.1111/j.1468-3083.2009.03364.x
- de Berker D. Management of nail psoriasis. Clin Exp Dermatol. 2000;25:357-362. doi:10.1046/j.1365-2230.2000.00663.x
- Wu JJ, Choi YM, Marczynski W. The 100 most cited psoriasis articles in clinical dermatologic journals, 1970 to 2012. J Clin Aesthet Dermatol. 2014;7:10-19.
- Rigopoulos D, Baran R, Chiheb S, et al. Recommendations for the definition, evaluation, and treatment of nail psoriasis in adult patients with no or mild skin psoriasis: a dermatologist and nail expert group consensus. J Am Acad Dermatol. 2019;81:228-240. doi:10.1016/j.jaad.2019.01.072
To the Editor:
Nail psoriasis is highly prevalent in patients with cutaneous psoriasis and also may present as an isolated finding. There is a strong association between nail psoriasis and development of psoriatic arthritis (PsA). However, publications on nail psoriasis are sparse compared with articles describing cutaneous psoriasis.1 Our objectives were to analyze the nail psoriasis literature for content, citations, and media attention.
The Web of Science database was searched for the term nail psoriasis on April 27, 2020, and publications by year, subject, and article type were compiled. Total and average yearly citations were calculated to create a list of the top 100 most-cited articles (eTable). First and last authors, sex, and Altmetric Attention Scores were then recorded. The Wilcoxon rank sum test was calculated to compare the relationship of Altmetric scores between nail psoriasis–specific references and others on the list.
In our data set, the average total number of citations was 134.09 (range, 42–1617), with average yearly citations ranging from 2 to 108. Altmetric scores—measures of media attention of scholarly work—were available for 58 of 100 papers (58%), with an average score of 33.2 (range, 1–509).
Of the top 100 most-cited articles using the search term nail psoriasis, only 20% focused on nail psoriasis, with the remainder concentrating on psoriasis/PsA. Only 32% and 24% of first and last authors, respectively, were female. Fifty-two percent and 31% of the articles were published in dermatology and arthritis/rheumatology journals, respectively. There was no statistically significant difference in Altmetric scores between nail psoriasis–specific and other articles in our data set (P=.7551).
For the nail psoriasis–specific articles, all 20 highlighted a lack of nail clinical trials, a positive association with PsA, and a correlation of increased cutaneous psoriasis body surface area with increased onychodystrophy likelihood.2 Three of 20 (15%) articles stated that nail psoriasis often is overlooked, despite the negative impact on quality of life,1 and emphasized the importance of patient compliance owing to the chronic nature of the disease. Only 1 of 20 (5%) articles focused on nail psoriasis treatments.3 There was no overlap between the 100 most-cited psoriasis articles from 1970 to 2012 and our top 100 articles on nail psoriasis.4
Treatment recommendations for nail psoriasis by consensus were published by a nail expert group in 2019.5 For 3 or fewer nails involved, suggested first-line treatment is intralesional matrix injections with triamcinolone acetonide. For more than 3 affected nails, systemic treatment with oral or biologic therapy is recommended.5 Although this article is likely to change clinical practice, it did not qualify for our list because it did not garner sufficient citations in the brief period between its publication date and our search (July 2019–April 2020).
This study is subject to several limitations. Only the Web of Science database was utilized, and only the term nail psoriasis was searched, potentially excluding relevant articles. Using total citations biases toward older articles.
Our bibliometric analysis highlights a lack of publications on nail psoriasis, with most articles focusing on psoriasis and PsA. This deficiency in highly cited nail psoriasis references is likely to be a barrier to physicians in managing patients with nail disease. There is a need for controlled clinical trials and better mechanisms to disseminate information on management of nail psoriasis to practicing physicians.
To the Editor:
Nail psoriasis is highly prevalent in patients with cutaneous psoriasis and also may present as an isolated finding. There is a strong association between nail psoriasis and development of psoriatic arthritis (PsA). However, publications on nail psoriasis are sparse compared with articles describing cutaneous psoriasis.1 Our objectives were to analyze the nail psoriasis literature for content, citations, and media attention.
The Web of Science database was searched for the term nail psoriasis on April 27, 2020, and publications by year, subject, and article type were compiled. Total and average yearly citations were calculated to create a list of the top 100 most-cited articles (eTable). First and last authors, sex, and Altmetric Attention Scores were then recorded. The Wilcoxon rank sum test was calculated to compare the relationship of Altmetric scores between nail psoriasis–specific references and others on the list.
In our data set, the average total number of citations was 134.09 (range, 42–1617), with average yearly citations ranging from 2 to 108. Altmetric scores—measures of media attention of scholarly work—were available for 58 of 100 papers (58%), with an average score of 33.2 (range, 1–509).
Of the top 100 most-cited articles using the search term nail psoriasis, only 20% focused on nail psoriasis, with the remainder concentrating on psoriasis/PsA. Only 32% and 24% of first and last authors, respectively, were female. Fifty-two percent and 31% of the articles were published in dermatology and arthritis/rheumatology journals, respectively. There was no statistically significant difference in Altmetric scores between nail psoriasis–specific and other articles in our data set (P=.7551).
For the nail psoriasis–specific articles, all 20 highlighted a lack of nail clinical trials, a positive association with PsA, and a correlation of increased cutaneous psoriasis body surface area with increased onychodystrophy likelihood.2 Three of 20 (15%) articles stated that nail psoriasis often is overlooked, despite the negative impact on quality of life,1 and emphasized the importance of patient compliance owing to the chronic nature of the disease. Only 1 of 20 (5%) articles focused on nail psoriasis treatments.3 There was no overlap between the 100 most-cited psoriasis articles from 1970 to 2012 and our top 100 articles on nail psoriasis.4
Treatment recommendations for nail psoriasis by consensus were published by a nail expert group in 2019.5 For 3 or fewer nails involved, suggested first-line treatment is intralesional matrix injections with triamcinolone acetonide. For more than 3 affected nails, systemic treatment with oral or biologic therapy is recommended.5 Although this article is likely to change clinical practice, it did not qualify for our list because it did not garner sufficient citations in the brief period between its publication date and our search (July 2019–April 2020).
This study is subject to several limitations. Only the Web of Science database was utilized, and only the term nail psoriasis was searched, potentially excluding relevant articles. Using total citations biases toward older articles.
Our bibliometric analysis highlights a lack of publications on nail psoriasis, with most articles focusing on psoriasis and PsA. This deficiency in highly cited nail psoriasis references is likely to be a barrier to physicians in managing patients with nail disease. There is a need for controlled clinical trials and better mechanisms to disseminate information on management of nail psoriasis to practicing physicians.
- Williamson L, Dalbeth N, Dockerty JL, et al. Extended report: nail disease in psoriatic arthritis—clinically important, potentially treatable and often overlooked. Rheumatology (Oxford). 2004;43:790-794. doi:10.1093/rheumatology/keh198
- Reich K. Approach to managing patients with nail psoriasis. J Eur Acad Dermatol Venereol. 2009;23(suppl 1):15-21. doi:10.1111/j.1468-3083.2009.03364.x
- de Berker D. Management of nail psoriasis. Clin Exp Dermatol. 2000;25:357-362. doi:10.1046/j.1365-2230.2000.00663.x
- Wu JJ, Choi YM, Marczynski W. The 100 most cited psoriasis articles in clinical dermatologic journals, 1970 to 2012. J Clin Aesthet Dermatol. 2014;7:10-19.
- Rigopoulos D, Baran R, Chiheb S, et al. Recommendations for the definition, evaluation, and treatment of nail psoriasis in adult patients with no or mild skin psoriasis: a dermatologist and nail expert group consensus. J Am Acad Dermatol. 2019;81:228-240. doi:10.1016/j.jaad.2019.01.072
- Williamson L, Dalbeth N, Dockerty JL, et al. Extended report: nail disease in psoriatic arthritis—clinically important, potentially treatable and often overlooked. Rheumatology (Oxford). 2004;43:790-794. doi:10.1093/rheumatology/keh198
- Reich K. Approach to managing patients with nail psoriasis. J Eur Acad Dermatol Venereol. 2009;23(suppl 1):15-21. doi:10.1111/j.1468-3083.2009.03364.x
- de Berker D. Management of nail psoriasis. Clin Exp Dermatol. 2000;25:357-362. doi:10.1046/j.1365-2230.2000.00663.x
- Wu JJ, Choi YM, Marczynski W. The 100 most cited psoriasis articles in clinical dermatologic journals, 1970 to 2012. J Clin Aesthet Dermatol. 2014;7:10-19.
- Rigopoulos D, Baran R, Chiheb S, et al. Recommendations for the definition, evaluation, and treatment of nail psoriasis in adult patients with no or mild skin psoriasis: a dermatologist and nail expert group consensus. J Am Acad Dermatol. 2019;81:228-240. doi:10.1016/j.jaad.2019.01.072
Utilizing a Sleep Mask to Reduce Patient Anxiety During Nail Surgery
Practice Gap
Perioperative anxiety is common in patients undergoing nail surgery. Patients might worry about seeing blood; about the procedure itself, including nail avulsion; and about associated pain and disfigurement. Nail surgery causes a high level of anxiety that correlates positively with postoperative pain1 and overall patient dissatisfaction. Furthermore, surgery-related anxiety is a predictor of increased postoperative analgesic use2 and delayed recovery.3
Therefore, implementing strategies that reduce perioperative anxiety may help minimize postoperative pain. Squeezing a stress ball, hand-holding, virtual reality, and music are tools that have been studied to reduce anxiety in the context of Mohs micrographic surgery; these strategies have not been studied for nail surgery.
The Technique
Using a sleep mask is a practical solution to reduce patient anxiety during nail surgery. A minority of patients will choose to watch their surgical procedure; most become unnerved observing their nail surgery. Using a sleep mask diverts visual attention from the surgical field without physically interfering with the nail surgeon. Utilizing a sleep mask is cost-effective, with disposable sleep masks available online for less than $0.30 each. Patients can bring their own mask, or a mask can be offered prior to surgery.
If desired, patients are instructed to wear the sleep mask during the entirety of the procedure, starting from anesthetic infiltration until wound closure and dressing application. Any adjustments can be made with the patient’s free hand. The sleep mask can be offered to patients of all ages undergoing nail surgery under local anesthesia, except babies and young children, who require general anesthesia.
Practical Implications
Distraction is an important strategy to reduce anxiety and pain in patients undergoing surgical procedures. In an observational study of 3087 surgical patients, 36% reported that self-distraction was the most helpful strategy for coping with preoperative anxiety.4 In a randomized, open-label clinical trial of 72 patients undergoing peripheral venous catheterization, asking the patients simple questions during the procedure was more effective than local anesthesia in reducing the perception of pain.5
It is crucial to implement strategies to reduce anxiety in patients undergoing nail surgery. Using a sleep mask impedes direct visualization of the surgical field, thus distracting the patient’s sight and attention from the procedure. Furthermore, this technique is safe and cost-effective.
Controlled clinical trials are necessary to assess the efficacy of this method in reducing nail surgery–related anxiety in comparison to other techniques.
- Navarro-Gastón D, Munuera-Martínez PV. Prevalence of preoperative anxiety and its relationship with postoperative pain in foot nail surgery: a cross-sectional study. Int J Environ Res Public Health. 2020;17:4481. doi:10.3390/ijerph17124481
- Ip HYV, Abrishami A, Peng PWH, et al. Predictors of postoperative pain and analgesic consumption: a qualitative systematic review. Anesthesiology. 2009;111:657-677. doi:10.1097/ALN.0b013e3181aae87a
- Mavros MN, Athanasiou S, Gkegkes ID, et al. Do psychological variables affect early surgical recovery? PLoS One. 2011;6:E20306. doi:10.1371/journal.pone.0020306
- Aust H, Rüsch D, Schuster M, et al. Coping strategies in anxious surgical patients. BMC Health Serv Res. 2016;16:250. doi:10.1186/s12913-016-1492-5
- Balanyuk I, Ledonne G, Provenzano M, et al. Distraction technique for pain reduction in peripheral venous catheterization: randomized, controlled trial. Acta Biomed. 2018;89(suppl 4):55-63. doi:10.23750/abmv89i4-S.7115
Practice Gap
Perioperative anxiety is common in patients undergoing nail surgery. Patients might worry about seeing blood; about the procedure itself, including nail avulsion; and about associated pain and disfigurement. Nail surgery causes a high level of anxiety that correlates positively with postoperative pain1 and overall patient dissatisfaction. Furthermore, surgery-related anxiety is a predictor of increased postoperative analgesic use2 and delayed recovery.3
Therefore, implementing strategies that reduce perioperative anxiety may help minimize postoperative pain. Squeezing a stress ball, hand-holding, virtual reality, and music are tools that have been studied to reduce anxiety in the context of Mohs micrographic surgery; these strategies have not been studied for nail surgery.
The Technique
Using a sleep mask is a practical solution to reduce patient anxiety during nail surgery. A minority of patients will choose to watch their surgical procedure; most become unnerved observing their nail surgery. Using a sleep mask diverts visual attention from the surgical field without physically interfering with the nail surgeon. Utilizing a sleep mask is cost-effective, with disposable sleep masks available online for less than $0.30 each. Patients can bring their own mask, or a mask can be offered prior to surgery.
If desired, patients are instructed to wear the sleep mask during the entirety of the procedure, starting from anesthetic infiltration until wound closure and dressing application. Any adjustments can be made with the patient’s free hand. The sleep mask can be offered to patients of all ages undergoing nail surgery under local anesthesia, except babies and young children, who require general anesthesia.
Practical Implications
Distraction is an important strategy to reduce anxiety and pain in patients undergoing surgical procedures. In an observational study of 3087 surgical patients, 36% reported that self-distraction was the most helpful strategy for coping with preoperative anxiety.4 In a randomized, open-label clinical trial of 72 patients undergoing peripheral venous catheterization, asking the patients simple questions during the procedure was more effective than local anesthesia in reducing the perception of pain.5
It is crucial to implement strategies to reduce anxiety in patients undergoing nail surgery. Using a sleep mask impedes direct visualization of the surgical field, thus distracting the patient’s sight and attention from the procedure. Furthermore, this technique is safe and cost-effective.
Controlled clinical trials are necessary to assess the efficacy of this method in reducing nail surgery–related anxiety in comparison to other techniques.
Practice Gap
Perioperative anxiety is common in patients undergoing nail surgery. Patients might worry about seeing blood; about the procedure itself, including nail avulsion; and about associated pain and disfigurement. Nail surgery causes a high level of anxiety that correlates positively with postoperative pain1 and overall patient dissatisfaction. Furthermore, surgery-related anxiety is a predictor of increased postoperative analgesic use2 and delayed recovery.3
Therefore, implementing strategies that reduce perioperative anxiety may help minimize postoperative pain. Squeezing a stress ball, hand-holding, virtual reality, and music are tools that have been studied to reduce anxiety in the context of Mohs micrographic surgery; these strategies have not been studied for nail surgery.
The Technique
Using a sleep mask is a practical solution to reduce patient anxiety during nail surgery. A minority of patients will choose to watch their surgical procedure; most become unnerved observing their nail surgery. Using a sleep mask diverts visual attention from the surgical field without physically interfering with the nail surgeon. Utilizing a sleep mask is cost-effective, with disposable sleep masks available online for less than $0.30 each. Patients can bring their own mask, or a mask can be offered prior to surgery.
If desired, patients are instructed to wear the sleep mask during the entirety of the procedure, starting from anesthetic infiltration until wound closure and dressing application. Any adjustments can be made with the patient’s free hand. The sleep mask can be offered to patients of all ages undergoing nail surgery under local anesthesia, except babies and young children, who require general anesthesia.
Practical Implications
Distraction is an important strategy to reduce anxiety and pain in patients undergoing surgical procedures. In an observational study of 3087 surgical patients, 36% reported that self-distraction was the most helpful strategy for coping with preoperative anxiety.4 In a randomized, open-label clinical trial of 72 patients undergoing peripheral venous catheterization, asking the patients simple questions during the procedure was more effective than local anesthesia in reducing the perception of pain.5
It is crucial to implement strategies to reduce anxiety in patients undergoing nail surgery. Using a sleep mask impedes direct visualization of the surgical field, thus distracting the patient’s sight and attention from the procedure. Furthermore, this technique is safe and cost-effective.
Controlled clinical trials are necessary to assess the efficacy of this method in reducing nail surgery–related anxiety in comparison to other techniques.
- Navarro-Gastón D, Munuera-Martínez PV. Prevalence of preoperative anxiety and its relationship with postoperative pain in foot nail surgery: a cross-sectional study. Int J Environ Res Public Health. 2020;17:4481. doi:10.3390/ijerph17124481
- Ip HYV, Abrishami A, Peng PWH, et al. Predictors of postoperative pain and analgesic consumption: a qualitative systematic review. Anesthesiology. 2009;111:657-677. doi:10.1097/ALN.0b013e3181aae87a
- Mavros MN, Athanasiou S, Gkegkes ID, et al. Do psychological variables affect early surgical recovery? PLoS One. 2011;6:E20306. doi:10.1371/journal.pone.0020306
- Aust H, Rüsch D, Schuster M, et al. Coping strategies in anxious surgical patients. BMC Health Serv Res. 2016;16:250. doi:10.1186/s12913-016-1492-5
- Balanyuk I, Ledonne G, Provenzano M, et al. Distraction technique for pain reduction in peripheral venous catheterization: randomized, controlled trial. Acta Biomed. 2018;89(suppl 4):55-63. doi:10.23750/abmv89i4-S.7115
- Navarro-Gastón D, Munuera-Martínez PV. Prevalence of preoperative anxiety and its relationship with postoperative pain in foot nail surgery: a cross-sectional study. Int J Environ Res Public Health. 2020;17:4481. doi:10.3390/ijerph17124481
- Ip HYV, Abrishami A, Peng PWH, et al. Predictors of postoperative pain and analgesic consumption: a qualitative systematic review. Anesthesiology. 2009;111:657-677. doi:10.1097/ALN.0b013e3181aae87a
- Mavros MN, Athanasiou S, Gkegkes ID, et al. Do psychological variables affect early surgical recovery? PLoS One. 2011;6:E20306. doi:10.1371/journal.pone.0020306
- Aust H, Rüsch D, Schuster M, et al. Coping strategies in anxious surgical patients. BMC Health Serv Res. 2016;16:250. doi:10.1186/s12913-016-1492-5
- Balanyuk I, Ledonne G, Provenzano M, et al. Distraction technique for pain reduction in peripheral venous catheterization: randomized, controlled trial. Acta Biomed. 2018;89(suppl 4):55-63. doi:10.23750/abmv89i4-S.7115
Recommendations for Pregnant Members of Dermatology Health Care Teams During the COVID-19 Pandemic
Information is scarce regarding the impact of COVID-19 on pregnant women and newborns; health care workers (HCWs), particularly pregnant women,1 who are caring for patients during the pandemic might experience concern and uncertainty. The American College of Obstetricians and Gynecologists (ACOG) released recommendations, based on expert consensus, regarding pregnant HCWs on December 14, 2020.2 We propose an appropriation of the ACOG recommendations for dermatologists and their teams caring for patients during the COVID-19 pandemic.
Risks to Pregnant HCWs
Worldwide, viral pneumonia is a leading cause of death during pregnancy,3 with higher mortality documented among pregnant patients during the 1918 influenza pandemic and the 2003 severe acute respiratory syndrome–associated coronavirus pandemic,3 and an increased rate of hospital admission documented among these patients compared to the general population during the 2009 H1N1 influenza pandemic.4
Data from the Centers for Disease Control and Prevention (CDC) suggest that pregnant women with symptomatic COVID-19 (n=30,415) are at increased risk for the following (compared to nonpregnant women with symptomatic COVID-19 [n=431,410])5:
• Admission to the intensive care unit (10.5 of every 1000 cases vs 3.9 of every 1000 cases; adjusted risk ratio [aRR]=3.0; 95% CI, 2.6-3.4)
• Receipt of invasive ventilation (2.9 of every 1000 cases vs 1.1 of every 1000 cases; aRR=2.9; 95% CI, 2.2-3.8)
• Receipt of extracorporeal membrane oxygenation (0.7 of every 1000 cases vs 0.3 of every 1000 cases; aRR=2.4; 95% CI, 1.5-4.0)
• Death (1.5 of every 1000 cases vs 1.2 of every 1000 cases; aRR=1.7; 95% CI, 1.2-2.4).
Although the absolute risk of severe COVID-19–related outcomes is low, the CDC includes pregnant women in its increased risk category for COVID-19. Furthermore, in a systematic review of 61 studies comprising 790 COVID-19–positive pregnant women and 548 newborns, the rates of cesarean delivery, premature birth, low birth weight, and adverse pregnancy events (the latter comprising preterm birth, death or stillbirth, and early termination of pregnancy) were estimated to be 72%, 23%, 7%, and 27%, respectively.6 In a systematic review of 39 studies (case series and cohort studies), comprising 936 SARS-CoV-2–tested newborns of mothers with COVID-19, mother-to-fetus transmission of SARS-CoV-2 occurred during the third trimester in approximately 3.2% of infected mothers.7
In pregnant women with COVID-19 who develop cytokine storm syndrome, a fetal inflammatory response syndrome can ensue, which has been shown to cause ventricular expansion and bleeding in animal models.8 In addition, underlying conditions, such as cardiovascular disease, diabetes mellitus, pre-existing lung disease, and obesity, which are well-established risks factors for severe COVID-19 in nonpregnant patients, can increase the severity of COVID-19 in pregnant women.5,9-11
Recommendations From ACOG for Pregnant HCWs
The American College of Obstetricians and Gynecologists recommends that health care facilities consider limiting the exposure of pregnant HCWs to patients with confirmed or suspected COVID-19. They also recommend that pregnant women continue to work in patient-facing roles if they want to, if recommended personal protective equipment (PPE) is available for them to wear.2 The US Food and Drug Administration issued an Emergency Use Authorization for 2 messenger RNA COVID-19 vaccines. Although these vaccines have not been tested in pregnant women, ACOG recommends that COVID-19 vaccines not be withheld from pregnant women who fulfill the criteria for vaccination; pregnant women who decline vaccination should be supported in their decision.12 In dermatology, telemedicine is an effective alternative to face-to-face visits, reducing the risk of transmitting SARS-CoV-2 to physicians and patients.
Ideally, pregnant dermatology attending physicians and residents can continue to provide care through teledermatology. They also can continue to provide in-person care, if they choose to; however, higher-risk procedures should be avoided.12 In dermatology, that might include ablative laser procedures to the face, prolonged surgery, such as hair transplantation, and intraoral or intranasal procedures. Alternatively, pregnant dermatology residents can be allocated to clinical rotations in which face-to-face contact with patients is not required such as dermatopathology and a research rotation. Likewise, telework options can be encouraged for other pregnant members of dermatology teams, including front-desk staff, nurses, medical assistants, and remaining ancillary staff.
Guidance on Face Masks for Pregnant HCWs
Universal masking of HCWs has been shown to reduce the rate of health care–related acquisition of SARS-CoV-2.13 However, extended use or reuse of N95 respirators might contribute to SARS-CoV-2 transmission.14 The American College of Obstetricians and Gynecologists recommends that all HCWs wear a face mask at all times while working in a health care facility, even if patients are wearing a face covering or face mask.2 Based on CDC guidelines,15 HCWs in regions where community transmission is moderate or substantial should wear eye protection in addition to a face mask, and they should wear an N95, N95-equivalent, or higher-level respirator instead of a face mask when performing aerosol-generating procedures and surgical procedures. If working in a patient-facing role caring for patients with suspected or confirmed COVID-19, HCWs should wear an N95, N95-equivalent, or higher-level respirator; gown; gloves; and eye protection (goggles or a disposable face shield).15
Final Thoughts
COVID-19 has brought about acute and likely permanent changes to the US health care system. Dermatologists are integral members of that system and are essential to the treatment of patients with skin, hair, and nail disorders. Pregnant dermatologists and residents should refrain from patient-facing roles when feasible; however, when all recommended PPE are available, they may continue to work in patient-facing roles until they give birth if they desire to do so. Alternatively, teledermatology and non–face-to-face rotations should be encouraged. Higher-risk and aerosol-generating procedures are of particular concern regarding the risk for transmitting SARS-CoV-2 and should be avoided. Correct and universal use of PPE is paramount; when all recommended PPE is not available, pregnant HCWs should avoid exposure to patients with suspected or confirmed COVID-19. These recommendations will help safeguard pregnant members of dermatology teams during the COVID-19 pandemic while maximizing patient care.
- Rashidi Fakari F, Simbar M. Coronavirus pandemic and worries during pregnancy; a letter to editor. Arch Acad Emerg Med. 2020;8:E21.
- The American College of Obstetricians and Gynecologists. COVID-19 FAQs for obstetrician-gynecologists, obstetrics. 2020. Accessed April 21, 2021. https://www.acog.org/clinical-information/physician-faqs/covid-19-faqs-for-ob-gyns-obstetrics
- Schwartz DA, Graham AL. Potential maternal and infant outcomes from (Wuhan) coronavirus 2019-nCoV infecting pregnant women: lessons from SARS, MERS, and other human coronavirus infections. Viruses. 2020;12:194. doi:10.3390/v12020194
- Yan J, Guo J, Fan C, et al. Coronavirus disease 2019 in pregnant women: a report based on 116 cases. Am J Obstet Gynecol. 2020;223:111.e1-111.e14. doi:10.1016/j.ajog.2020.04.014
- Zambrano LD, Ellington S, Strid P, et al; . Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status—United States, January 22–October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1641-1647. doi:10.15585/mmwr.mm6944e3
- Dubey P, Reddy SY, Manuel S, et al. Maternal and neonatal characteristics and outcomes among COVID-19 infected women: an updated systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2020;252:490-501. doi:10.1016/j.ejogrb.2020.07.034
- Kotlyar AM, Grechukhina O, Chen A, et al. Vertical transmission of coronavirus disease 2019: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020;224:35-53.e3. doi:10.1016/j.ajog.2020.07.049
- Mitchell T, MacDonald JW, Srinouanpranchanh S, et al. Evidence of cardiac involvement in the fetal inflammatory response syndrome: disruption of gene networks programming cardiac development in nonhuman primates. Am J Obstet Gynecol. 2018;218:438.e1-438.e16. doi:10.1016/j.ajog.2018.01.009
- Ellington S, Strid P, Tong VT, et al. Characteristics of women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status—United States, January 22–June 7, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:769-775. doi:10.15585/mmwr.mm6925a1
- Panagiotakopoulos L, Myers TR, Gee J, et al. SARS-CoV-2 infection among hospitalized pregnant women: reasons for admission and pregnancy characteristics—eight U.S. health care centers, March 1–May 30, 2020. 2020. doi:10.15585/mmwr.mm6938e2
- Knight M, Bunch K, Vousden N, et al; . Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: national population based cohort study. BMJ. 2020;369:m2107. doi:10.1136/bmj.m2107
- The American College of Obstetricians and Gynecologists. Vaccinating pregnant and lactating patients against COVID-19. December 2020. Updated March 24, 2021. Accessed April 28, 2021. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/12/vaccinating-pregnant-and-lactating-patients-against-covid-19
- Seidelman JL, Lewis SS, Advani SD, et al. Universal masking is an effective strategy to flatten the severe acute respiratory coronavirus virus 2 (SARS-CoV-2) healthcare worker epidemiologic curve. Infect Control Hosp Epidemiol. 2020;41:1466-1467. doi:10.1017/ice.2020.31314.
- Degesys NF, Wang RC, Kwan E, et al. Correlation between N95 extended use and reuse and fit failure in an emergency department. JAMA. 2020;324:94-96. doi:10.1001/jama.2020.9843
- Centers for Disease Control and Prevention. Interim infection prevention and control recommendations for healthcare personnel during the coronavirus disease 2019 (COVID-19) pandemic 2020. Updated February 23, 2021. Accessed April 21, 2021. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Finfection-control%2Fcontrol-recommendations.html
Information is scarce regarding the impact of COVID-19 on pregnant women and newborns; health care workers (HCWs), particularly pregnant women,1 who are caring for patients during the pandemic might experience concern and uncertainty. The American College of Obstetricians and Gynecologists (ACOG) released recommendations, based on expert consensus, regarding pregnant HCWs on December 14, 2020.2 We propose an appropriation of the ACOG recommendations for dermatologists and their teams caring for patients during the COVID-19 pandemic.
Risks to Pregnant HCWs
Worldwide, viral pneumonia is a leading cause of death during pregnancy,3 with higher mortality documented among pregnant patients during the 1918 influenza pandemic and the 2003 severe acute respiratory syndrome–associated coronavirus pandemic,3 and an increased rate of hospital admission documented among these patients compared to the general population during the 2009 H1N1 influenza pandemic.4
Data from the Centers for Disease Control and Prevention (CDC) suggest that pregnant women with symptomatic COVID-19 (n=30,415) are at increased risk for the following (compared to nonpregnant women with symptomatic COVID-19 [n=431,410])5:
• Admission to the intensive care unit (10.5 of every 1000 cases vs 3.9 of every 1000 cases; adjusted risk ratio [aRR]=3.0; 95% CI, 2.6-3.4)
• Receipt of invasive ventilation (2.9 of every 1000 cases vs 1.1 of every 1000 cases; aRR=2.9; 95% CI, 2.2-3.8)
• Receipt of extracorporeal membrane oxygenation (0.7 of every 1000 cases vs 0.3 of every 1000 cases; aRR=2.4; 95% CI, 1.5-4.0)
• Death (1.5 of every 1000 cases vs 1.2 of every 1000 cases; aRR=1.7; 95% CI, 1.2-2.4).
Although the absolute risk of severe COVID-19–related outcomes is low, the CDC includes pregnant women in its increased risk category for COVID-19. Furthermore, in a systematic review of 61 studies comprising 790 COVID-19–positive pregnant women and 548 newborns, the rates of cesarean delivery, premature birth, low birth weight, and adverse pregnancy events (the latter comprising preterm birth, death or stillbirth, and early termination of pregnancy) were estimated to be 72%, 23%, 7%, and 27%, respectively.6 In a systematic review of 39 studies (case series and cohort studies), comprising 936 SARS-CoV-2–tested newborns of mothers with COVID-19, mother-to-fetus transmission of SARS-CoV-2 occurred during the third trimester in approximately 3.2% of infected mothers.7
In pregnant women with COVID-19 who develop cytokine storm syndrome, a fetal inflammatory response syndrome can ensue, which has been shown to cause ventricular expansion and bleeding in animal models.8 In addition, underlying conditions, such as cardiovascular disease, diabetes mellitus, pre-existing lung disease, and obesity, which are well-established risks factors for severe COVID-19 in nonpregnant patients, can increase the severity of COVID-19 in pregnant women.5,9-11
Recommendations From ACOG for Pregnant HCWs
The American College of Obstetricians and Gynecologists recommends that health care facilities consider limiting the exposure of pregnant HCWs to patients with confirmed or suspected COVID-19. They also recommend that pregnant women continue to work in patient-facing roles if they want to, if recommended personal protective equipment (PPE) is available for them to wear.2 The US Food and Drug Administration issued an Emergency Use Authorization for 2 messenger RNA COVID-19 vaccines. Although these vaccines have not been tested in pregnant women, ACOG recommends that COVID-19 vaccines not be withheld from pregnant women who fulfill the criteria for vaccination; pregnant women who decline vaccination should be supported in their decision.12 In dermatology, telemedicine is an effective alternative to face-to-face visits, reducing the risk of transmitting SARS-CoV-2 to physicians and patients.
Ideally, pregnant dermatology attending physicians and residents can continue to provide care through teledermatology. They also can continue to provide in-person care, if they choose to; however, higher-risk procedures should be avoided.12 In dermatology, that might include ablative laser procedures to the face, prolonged surgery, such as hair transplantation, and intraoral or intranasal procedures. Alternatively, pregnant dermatology residents can be allocated to clinical rotations in which face-to-face contact with patients is not required such as dermatopathology and a research rotation. Likewise, telework options can be encouraged for other pregnant members of dermatology teams, including front-desk staff, nurses, medical assistants, and remaining ancillary staff.
Guidance on Face Masks for Pregnant HCWs
Universal masking of HCWs has been shown to reduce the rate of health care–related acquisition of SARS-CoV-2.13 However, extended use or reuse of N95 respirators might contribute to SARS-CoV-2 transmission.14 The American College of Obstetricians and Gynecologists recommends that all HCWs wear a face mask at all times while working in a health care facility, even if patients are wearing a face covering or face mask.2 Based on CDC guidelines,15 HCWs in regions where community transmission is moderate or substantial should wear eye protection in addition to a face mask, and they should wear an N95, N95-equivalent, or higher-level respirator instead of a face mask when performing aerosol-generating procedures and surgical procedures. If working in a patient-facing role caring for patients with suspected or confirmed COVID-19, HCWs should wear an N95, N95-equivalent, or higher-level respirator; gown; gloves; and eye protection (goggles or a disposable face shield).15
Final Thoughts
COVID-19 has brought about acute and likely permanent changes to the US health care system. Dermatologists are integral members of that system and are essential to the treatment of patients with skin, hair, and nail disorders. Pregnant dermatologists and residents should refrain from patient-facing roles when feasible; however, when all recommended PPE are available, they may continue to work in patient-facing roles until they give birth if they desire to do so. Alternatively, teledermatology and non–face-to-face rotations should be encouraged. Higher-risk and aerosol-generating procedures are of particular concern regarding the risk for transmitting SARS-CoV-2 and should be avoided. Correct and universal use of PPE is paramount; when all recommended PPE is not available, pregnant HCWs should avoid exposure to patients with suspected or confirmed COVID-19. These recommendations will help safeguard pregnant members of dermatology teams during the COVID-19 pandemic while maximizing patient care.
Information is scarce regarding the impact of COVID-19 on pregnant women and newborns; health care workers (HCWs), particularly pregnant women,1 who are caring for patients during the pandemic might experience concern and uncertainty. The American College of Obstetricians and Gynecologists (ACOG) released recommendations, based on expert consensus, regarding pregnant HCWs on December 14, 2020.2 We propose an appropriation of the ACOG recommendations for dermatologists and their teams caring for patients during the COVID-19 pandemic.
Risks to Pregnant HCWs
Worldwide, viral pneumonia is a leading cause of death during pregnancy,3 with higher mortality documented among pregnant patients during the 1918 influenza pandemic and the 2003 severe acute respiratory syndrome–associated coronavirus pandemic,3 and an increased rate of hospital admission documented among these patients compared to the general population during the 2009 H1N1 influenza pandemic.4
Data from the Centers for Disease Control and Prevention (CDC) suggest that pregnant women with symptomatic COVID-19 (n=30,415) are at increased risk for the following (compared to nonpregnant women with symptomatic COVID-19 [n=431,410])5:
• Admission to the intensive care unit (10.5 of every 1000 cases vs 3.9 of every 1000 cases; adjusted risk ratio [aRR]=3.0; 95% CI, 2.6-3.4)
• Receipt of invasive ventilation (2.9 of every 1000 cases vs 1.1 of every 1000 cases; aRR=2.9; 95% CI, 2.2-3.8)
• Receipt of extracorporeal membrane oxygenation (0.7 of every 1000 cases vs 0.3 of every 1000 cases; aRR=2.4; 95% CI, 1.5-4.0)
• Death (1.5 of every 1000 cases vs 1.2 of every 1000 cases; aRR=1.7; 95% CI, 1.2-2.4).
Although the absolute risk of severe COVID-19–related outcomes is low, the CDC includes pregnant women in its increased risk category for COVID-19. Furthermore, in a systematic review of 61 studies comprising 790 COVID-19–positive pregnant women and 548 newborns, the rates of cesarean delivery, premature birth, low birth weight, and adverse pregnancy events (the latter comprising preterm birth, death or stillbirth, and early termination of pregnancy) were estimated to be 72%, 23%, 7%, and 27%, respectively.6 In a systematic review of 39 studies (case series and cohort studies), comprising 936 SARS-CoV-2–tested newborns of mothers with COVID-19, mother-to-fetus transmission of SARS-CoV-2 occurred during the third trimester in approximately 3.2% of infected mothers.7
In pregnant women with COVID-19 who develop cytokine storm syndrome, a fetal inflammatory response syndrome can ensue, which has been shown to cause ventricular expansion and bleeding in animal models.8 In addition, underlying conditions, such as cardiovascular disease, diabetes mellitus, pre-existing lung disease, and obesity, which are well-established risks factors for severe COVID-19 in nonpregnant patients, can increase the severity of COVID-19 in pregnant women.5,9-11
Recommendations From ACOG for Pregnant HCWs
The American College of Obstetricians and Gynecologists recommends that health care facilities consider limiting the exposure of pregnant HCWs to patients with confirmed or suspected COVID-19. They also recommend that pregnant women continue to work in patient-facing roles if they want to, if recommended personal protective equipment (PPE) is available for them to wear.2 The US Food and Drug Administration issued an Emergency Use Authorization for 2 messenger RNA COVID-19 vaccines. Although these vaccines have not been tested in pregnant women, ACOG recommends that COVID-19 vaccines not be withheld from pregnant women who fulfill the criteria for vaccination; pregnant women who decline vaccination should be supported in their decision.12 In dermatology, telemedicine is an effective alternative to face-to-face visits, reducing the risk of transmitting SARS-CoV-2 to physicians and patients.
Ideally, pregnant dermatology attending physicians and residents can continue to provide care through teledermatology. They also can continue to provide in-person care, if they choose to; however, higher-risk procedures should be avoided.12 In dermatology, that might include ablative laser procedures to the face, prolonged surgery, such as hair transplantation, and intraoral or intranasal procedures. Alternatively, pregnant dermatology residents can be allocated to clinical rotations in which face-to-face contact with patients is not required such as dermatopathology and a research rotation. Likewise, telework options can be encouraged for other pregnant members of dermatology teams, including front-desk staff, nurses, medical assistants, and remaining ancillary staff.
Guidance on Face Masks for Pregnant HCWs
Universal masking of HCWs has been shown to reduce the rate of health care–related acquisition of SARS-CoV-2.13 However, extended use or reuse of N95 respirators might contribute to SARS-CoV-2 transmission.14 The American College of Obstetricians and Gynecologists recommends that all HCWs wear a face mask at all times while working in a health care facility, even if patients are wearing a face covering or face mask.2 Based on CDC guidelines,15 HCWs in regions where community transmission is moderate or substantial should wear eye protection in addition to a face mask, and they should wear an N95, N95-equivalent, or higher-level respirator instead of a face mask when performing aerosol-generating procedures and surgical procedures. If working in a patient-facing role caring for patients with suspected or confirmed COVID-19, HCWs should wear an N95, N95-equivalent, or higher-level respirator; gown; gloves; and eye protection (goggles or a disposable face shield).15
Final Thoughts
COVID-19 has brought about acute and likely permanent changes to the US health care system. Dermatologists are integral members of that system and are essential to the treatment of patients with skin, hair, and nail disorders. Pregnant dermatologists and residents should refrain from patient-facing roles when feasible; however, when all recommended PPE are available, they may continue to work in patient-facing roles until they give birth if they desire to do so. Alternatively, teledermatology and non–face-to-face rotations should be encouraged. Higher-risk and aerosol-generating procedures are of particular concern regarding the risk for transmitting SARS-CoV-2 and should be avoided. Correct and universal use of PPE is paramount; when all recommended PPE is not available, pregnant HCWs should avoid exposure to patients with suspected or confirmed COVID-19. These recommendations will help safeguard pregnant members of dermatology teams during the COVID-19 pandemic while maximizing patient care.
- Rashidi Fakari F, Simbar M. Coronavirus pandemic and worries during pregnancy; a letter to editor. Arch Acad Emerg Med. 2020;8:E21.
- The American College of Obstetricians and Gynecologists. COVID-19 FAQs for obstetrician-gynecologists, obstetrics. 2020. Accessed April 21, 2021. https://www.acog.org/clinical-information/physician-faqs/covid-19-faqs-for-ob-gyns-obstetrics
- Schwartz DA, Graham AL. Potential maternal and infant outcomes from (Wuhan) coronavirus 2019-nCoV infecting pregnant women: lessons from SARS, MERS, and other human coronavirus infections. Viruses. 2020;12:194. doi:10.3390/v12020194
- Yan J, Guo J, Fan C, et al. Coronavirus disease 2019 in pregnant women: a report based on 116 cases. Am J Obstet Gynecol. 2020;223:111.e1-111.e14. doi:10.1016/j.ajog.2020.04.014
- Zambrano LD, Ellington S, Strid P, et al; . Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status—United States, January 22–October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1641-1647. doi:10.15585/mmwr.mm6944e3
- Dubey P, Reddy SY, Manuel S, et al. Maternal and neonatal characteristics and outcomes among COVID-19 infected women: an updated systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2020;252:490-501. doi:10.1016/j.ejogrb.2020.07.034
- Kotlyar AM, Grechukhina O, Chen A, et al. Vertical transmission of coronavirus disease 2019: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020;224:35-53.e3. doi:10.1016/j.ajog.2020.07.049
- Mitchell T, MacDonald JW, Srinouanpranchanh S, et al. Evidence of cardiac involvement in the fetal inflammatory response syndrome: disruption of gene networks programming cardiac development in nonhuman primates. Am J Obstet Gynecol. 2018;218:438.e1-438.e16. doi:10.1016/j.ajog.2018.01.009
- Ellington S, Strid P, Tong VT, et al. Characteristics of women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status—United States, January 22–June 7, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:769-775. doi:10.15585/mmwr.mm6925a1
- Panagiotakopoulos L, Myers TR, Gee J, et al. SARS-CoV-2 infection among hospitalized pregnant women: reasons for admission and pregnancy characteristics—eight U.S. health care centers, March 1–May 30, 2020. 2020. doi:10.15585/mmwr.mm6938e2
- Knight M, Bunch K, Vousden N, et al; . Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: national population based cohort study. BMJ. 2020;369:m2107. doi:10.1136/bmj.m2107
- The American College of Obstetricians and Gynecologists. Vaccinating pregnant and lactating patients against COVID-19. December 2020. Updated March 24, 2021. Accessed April 28, 2021. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/12/vaccinating-pregnant-and-lactating-patients-against-covid-19
- Seidelman JL, Lewis SS, Advani SD, et al. Universal masking is an effective strategy to flatten the severe acute respiratory coronavirus virus 2 (SARS-CoV-2) healthcare worker epidemiologic curve. Infect Control Hosp Epidemiol. 2020;41:1466-1467. doi:10.1017/ice.2020.31314.
- Degesys NF, Wang RC, Kwan E, et al. Correlation between N95 extended use and reuse and fit failure in an emergency department. JAMA. 2020;324:94-96. doi:10.1001/jama.2020.9843
- Centers for Disease Control and Prevention. Interim infection prevention and control recommendations for healthcare personnel during the coronavirus disease 2019 (COVID-19) pandemic 2020. Updated February 23, 2021. Accessed April 21, 2021. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Finfection-control%2Fcontrol-recommendations.html
- Rashidi Fakari F, Simbar M. Coronavirus pandemic and worries during pregnancy; a letter to editor. Arch Acad Emerg Med. 2020;8:E21.
- The American College of Obstetricians and Gynecologists. COVID-19 FAQs for obstetrician-gynecologists, obstetrics. 2020. Accessed April 21, 2021. https://www.acog.org/clinical-information/physician-faqs/covid-19-faqs-for-ob-gyns-obstetrics
- Schwartz DA, Graham AL. Potential maternal and infant outcomes from (Wuhan) coronavirus 2019-nCoV infecting pregnant women: lessons from SARS, MERS, and other human coronavirus infections. Viruses. 2020;12:194. doi:10.3390/v12020194
- Yan J, Guo J, Fan C, et al. Coronavirus disease 2019 in pregnant women: a report based on 116 cases. Am J Obstet Gynecol. 2020;223:111.e1-111.e14. doi:10.1016/j.ajog.2020.04.014
- Zambrano LD, Ellington S, Strid P, et al; . Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status—United States, January 22–October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1641-1647. doi:10.15585/mmwr.mm6944e3
- Dubey P, Reddy SY, Manuel S, et al. Maternal and neonatal characteristics and outcomes among COVID-19 infected women: an updated systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2020;252:490-501. doi:10.1016/j.ejogrb.2020.07.034
- Kotlyar AM, Grechukhina O, Chen A, et al. Vertical transmission of coronavirus disease 2019: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020;224:35-53.e3. doi:10.1016/j.ajog.2020.07.049
- Mitchell T, MacDonald JW, Srinouanpranchanh S, et al. Evidence of cardiac involvement in the fetal inflammatory response syndrome: disruption of gene networks programming cardiac development in nonhuman primates. Am J Obstet Gynecol. 2018;218:438.e1-438.e16. doi:10.1016/j.ajog.2018.01.009
- Ellington S, Strid P, Tong VT, et al. Characteristics of women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status—United States, January 22–June 7, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:769-775. doi:10.15585/mmwr.mm6925a1
- Panagiotakopoulos L, Myers TR, Gee J, et al. SARS-CoV-2 infection among hospitalized pregnant women: reasons for admission and pregnancy characteristics—eight U.S. health care centers, March 1–May 30, 2020. 2020. doi:10.15585/mmwr.mm6938e2
- Knight M, Bunch K, Vousden N, et al; . Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: national population based cohort study. BMJ. 2020;369:m2107. doi:10.1136/bmj.m2107
- The American College of Obstetricians and Gynecologists. Vaccinating pregnant and lactating patients against COVID-19. December 2020. Updated March 24, 2021. Accessed April 28, 2021. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/12/vaccinating-pregnant-and-lactating-patients-against-covid-19
- Seidelman JL, Lewis SS, Advani SD, et al. Universal masking is an effective strategy to flatten the severe acute respiratory coronavirus virus 2 (SARS-CoV-2) healthcare worker epidemiologic curve. Infect Control Hosp Epidemiol. 2020;41:1466-1467. doi:10.1017/ice.2020.31314.
- Degesys NF, Wang RC, Kwan E, et al. Correlation between N95 extended use and reuse and fit failure in an emergency department. JAMA. 2020;324:94-96. doi:10.1001/jama.2020.9843
- Centers for Disease Control and Prevention. Interim infection prevention and control recommendations for healthcare personnel during the coronavirus disease 2019 (COVID-19) pandemic 2020. Updated February 23, 2021. Accessed April 21, 2021. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Finfection-control%2Fcontrol-recommendations.html
Practice Points
- Pregnant women are at an increased risk for severe illness due to COVID-19 compared with nonpregnant women; therefore, it is important to protect pregnant health care workers who are caring for patients during the current pandemic.
- Although currently available COVID-19 vaccines have not been tested in pregnant women, they should not be withheld from pregnant individuals.
- Pregnant attending physicians and residents in dermatology can continue to provide care through telemedicine; if they choose to, and if all recommended personal protective equipment (PPE) are available, they can continue to provide in-person care.
- Correct and comprehensive use of PPE by pregnant health care workers is paramount to minimizing exposure to SARS-CoV-2.
Head to Toe: Recommendations for Physician Head and Shoe Coverings to Limit COVID-19 Transmission
Personal protective equipment (PPE) is an important component in limiting transmission of SARS-CoV-2. The World Health Organization and Centers for Disease Control and Prevention issued guidelines for appropriate PPE use, but recommendations for head and shoe coverings are lacking. In this article, we analyze the literature on pathogen transmission via hair and shoes and make evidence-based recommendations for PPE selection during the COVID-19 pandemic.
Pathogens on Shoes and Hair
Hair and shoes may act as vehicles for pathogen transmission. In a study that simulated contamination of uncovered skin in health care workers after intubating manikins in respiratory distress, 8 (100%) had fluorescent markers on the hair, 6 (75%) on the neck, and 4 (50%) on the shoes.1 In another study of postsurgical operating room (OR) surfaces (517 cultures), uncovered shoe tops and reusable hair coverings had 10-times more bacterial colony–forming units compared to other surfaces. On average, disposable shoe covers/head coverings had less than one-third bacterial colony–forming units compared with uncovered shoes/reusable hair coverings.2
Hair characteristics and coverings may affect pathogen transmission. Exposed hair may collect bacteria, as Staphylococcus aureus and Staphylococcus epidermidis attach to both scalp and facial hair. In one case, β-hemolytic streptococci cultured from the scalp of a perioperative nurse was linked to postsurgical infections in 20 patients.3 Hair coverings include bouffant caps and skullcaps. The bouffant cap is similar to a shower cap; it is relatively loose and secured around the head with elastic. The skullcap, or scrub cap, is tighter but leaves the neck nape and sideburns exposed. In a study comparing disposable bouffant caps, disposable skullcaps, and home-laundered cloth skullcaps worn by 2 teams of 5 surgeons, the disposable bouffant caps had the highest permeability, penetration, and microbial shed of airborne particles.4
Physicians’ shoes may act as fomites for transmission of pathogens to patients. In a study of 41 physicians and nurses in an acute care hospital, shoe soles were positive for at least one pathogen in 12 (29.3%) participants; methicillin-resistant Staphylococcus aureus was most common. Additionally, 98% (49/50) of shoes worn outdoors showed positive bacterial cultures compared to 56% (28/50) of shoes reserved for the OR only.5 In a study examining ventilation effects on airborne pathogens in the OR, 15% of OR airborne bacteria originated from OR floors, and higher bacterial counts correlated with a higher number of steps in the OR.2 In another study designed to evaluate SARS-CoV-2 distribution on hospital floors, 70% (7/10) of quantitative polymerase chain reaction assays performed on floor samples from intensive care units were positive. In addition, 100% (3/3) of swabs taken from hospital pharmacy floors with no COVID-19 patients were positive for SARS-CoV-2, meaning contaminated shoes likely served as vectors.6 Middle East respiratory syndrome, SARS-CoV-2, and influenza viruses may survive on porous and nonporous materials for hours to days.7Enterococcus, Candida, and Aspergillus may survive on textiles for up to 90 days.3
Recommendations for Hair and Shoe Coverings
We recommend that physicians utilize disposable skullcaps to cover the hair and consider a hooded gown or coverall for neck/ear coverage. We also recommend that physicians designate shoes that remain in the workplace and can be easily washed or disinfected at least weekly; physicians may choose to wash or disinfect shoes more often if they frequently are performing procedures that generate aerosols. Additionally, physicians should always wear shoe coverings when caring for patients (Table 1).
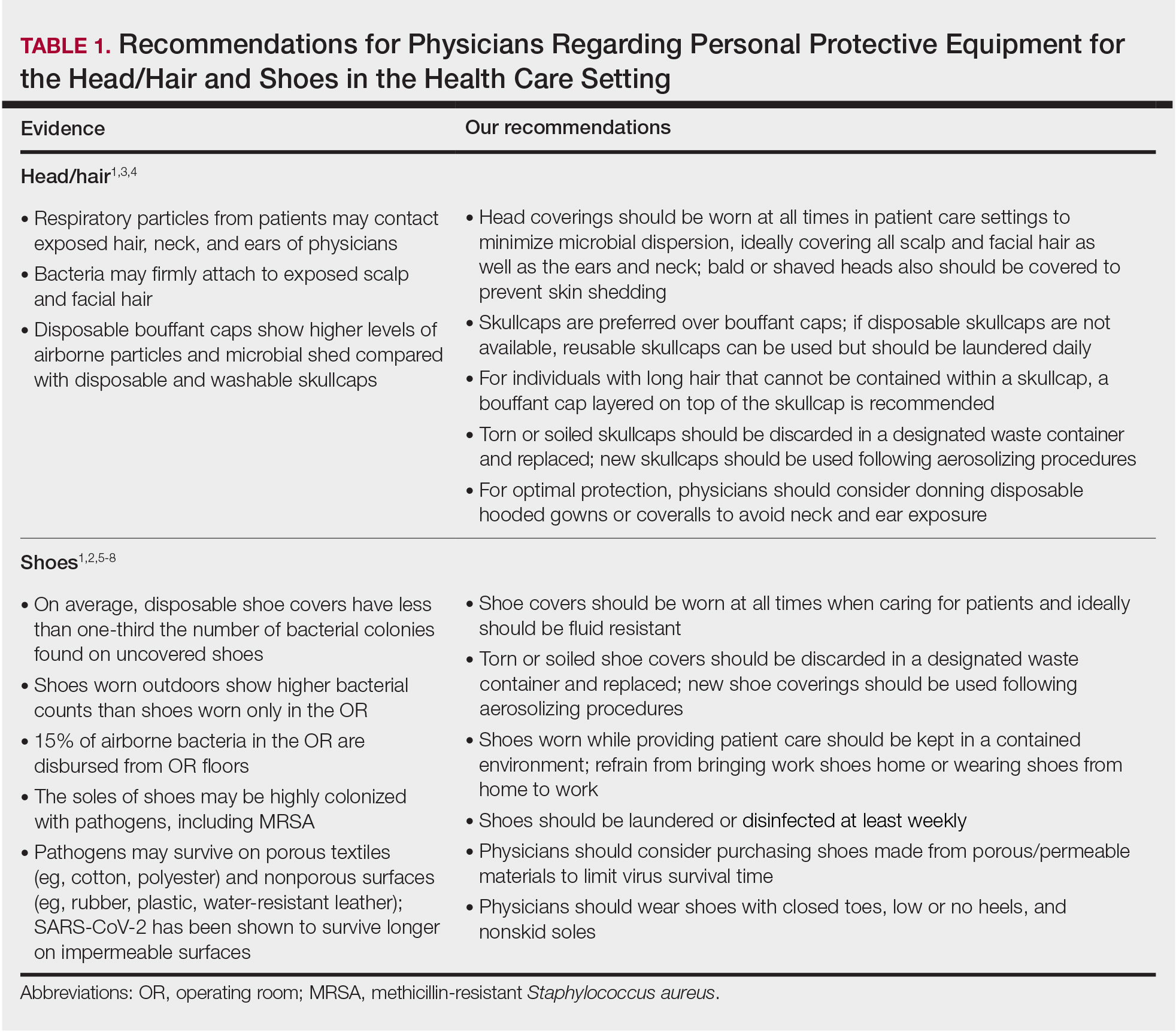
Our hair and shoe covering recommendations may serve to protect dermatologists when caring for patients. These protocols may be particularly important for dermatologists performing high-risk procedures, including facial surgery, intraoral/intranasal procedures, and treatment with ablative lasers and facial injectables, especially when the patient is unmasked. These recommendations may limit viral transmission to dermatologists and also protect individuals living in their households. Additional established guidelines by the American Academy of Dermatology, American Society for Dermatologic Surgery, and World Health Organization are listed in Table 2.8-10
Current PPE recommendations that do not include hair and shoe coverings may be inadequate for limiting SARS-CoV-2 exposure between and among physicians and patients. Adherence to head covering and shoe recommendations may aid in reducing unwanted SARS-CoV-2 transmission in the health care setting, even as the pandemic continues.
- Feldman O, Meir M, Shavit D, et al. Exposure to a surrogate measure of contamination from simulated patients by emergency department personnel wearing personal protective equipment. JAMA. 2020;323:2091-2093. doi:10.1001/jama.2020.6633
- Alexander JW, Van Sweringen H, Vanoss K, et al. Surveillance of bacterial colonization in operating rooms. Surg Infect (Larchmt). 2013;14:345-351. doi:10.1089/sur.2012.134
- Blanchard J. Clinical issues—August 2010. AORN Journal. 2010;92:228-232. doi:10.1016/j.aorn.2010.06.001
- Markel TA, Gormley T, Greeley D, et al. Hats off: a study of different operating room headgear assessed by environmental quality indicators. J Am Coll Surg. 2017;225:573-581. doi:10.1016/j.jamcollsurg.2017.08.014
- Kanwar A, Thakur M, Wazzan M, et al. Clothing and shoes of personnel as potential vectors for transfer of health care-associated pathogens to the community. Am J Infect Control. 2019;47:577-579. doi:10.1016/j.ajic.2019.01.028
- Guo ZD, Wang ZY, Zhang SF, et al. Aerosol and surface distribution of severe acute respiratory syndrome coronavirus 2 in hospital wards, Wuhan, China, 2020. Emerg Infect Dis. 2020;26:1583-1591. doi:10.3201/eid2607.200885
- Otter JA, Donskey C, Yezli S, et al. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: the possible role of dry surface contamination. J Hosp Infect. 2016;92:235-250. doi:10.1016/j.jhin.2015.08.027
- Centers for Disease Control and Prevention. Science Brief: SARS-CoV-2 and Surface (Fomite) Transmission for Indoor Community Environments. https://www.cdc.gov/coronavirus/2019-ncov/more/science-and-research/surface-transmission.html#ref10
- American Academy of Dermatology. Clinical guidance for COVID-19. Accessed March 15, 2021. https://www.aad.org/member/practice/coronavirus/clinical-guidance
- Narla S, Alam M, Ozog DM, et al. American Society of Dermatologic Surgery Association (ASDSA) and American Society for Laser Medicine & Surgery (ASLMS) guidance for cosmetic dermatology practices during COVID-19. Updated January 11, 2021. Accessed March 15, 2021. https://www.asds.net/Portals/0/PDF/asdsa/asdsa-aslms-cosmetic-reopening-guidance.pdf
- World Health Organization. Country & technical guidance—coronavirus disease (COVID-19). Accessed March 15, 2021. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance-publications
Personal protective equipment (PPE) is an important component in limiting transmission of SARS-CoV-2. The World Health Organization and Centers for Disease Control and Prevention issued guidelines for appropriate PPE use, but recommendations for head and shoe coverings are lacking. In this article, we analyze the literature on pathogen transmission via hair and shoes and make evidence-based recommendations for PPE selection during the COVID-19 pandemic.
Pathogens on Shoes and Hair
Hair and shoes may act as vehicles for pathogen transmission. In a study that simulated contamination of uncovered skin in health care workers after intubating manikins in respiratory distress, 8 (100%) had fluorescent markers on the hair, 6 (75%) on the neck, and 4 (50%) on the shoes.1 In another study of postsurgical operating room (OR) surfaces (517 cultures), uncovered shoe tops and reusable hair coverings had 10-times more bacterial colony–forming units compared to other surfaces. On average, disposable shoe covers/head coverings had less than one-third bacterial colony–forming units compared with uncovered shoes/reusable hair coverings.2
Hair characteristics and coverings may affect pathogen transmission. Exposed hair may collect bacteria, as Staphylococcus aureus and Staphylococcus epidermidis attach to both scalp and facial hair. In one case, β-hemolytic streptococci cultured from the scalp of a perioperative nurse was linked to postsurgical infections in 20 patients.3 Hair coverings include bouffant caps and skullcaps. The bouffant cap is similar to a shower cap; it is relatively loose and secured around the head with elastic. The skullcap, or scrub cap, is tighter but leaves the neck nape and sideburns exposed. In a study comparing disposable bouffant caps, disposable skullcaps, and home-laundered cloth skullcaps worn by 2 teams of 5 surgeons, the disposable bouffant caps had the highest permeability, penetration, and microbial shed of airborne particles.4
Physicians’ shoes may act as fomites for transmission of pathogens to patients. In a study of 41 physicians and nurses in an acute care hospital, shoe soles were positive for at least one pathogen in 12 (29.3%) participants; methicillin-resistant Staphylococcus aureus was most common. Additionally, 98% (49/50) of shoes worn outdoors showed positive bacterial cultures compared to 56% (28/50) of shoes reserved for the OR only.5 In a study examining ventilation effects on airborne pathogens in the OR, 15% of OR airborne bacteria originated from OR floors, and higher bacterial counts correlated with a higher number of steps in the OR.2 In another study designed to evaluate SARS-CoV-2 distribution on hospital floors, 70% (7/10) of quantitative polymerase chain reaction assays performed on floor samples from intensive care units were positive. In addition, 100% (3/3) of swabs taken from hospital pharmacy floors with no COVID-19 patients were positive for SARS-CoV-2, meaning contaminated shoes likely served as vectors.6 Middle East respiratory syndrome, SARS-CoV-2, and influenza viruses may survive on porous and nonporous materials for hours to days.7Enterococcus, Candida, and Aspergillus may survive on textiles for up to 90 days.3
Recommendations for Hair and Shoe Coverings
We recommend that physicians utilize disposable skullcaps to cover the hair and consider a hooded gown or coverall for neck/ear coverage. We also recommend that physicians designate shoes that remain in the workplace and can be easily washed or disinfected at least weekly; physicians may choose to wash or disinfect shoes more often if they frequently are performing procedures that generate aerosols. Additionally, physicians should always wear shoe coverings when caring for patients (Table 1).

Our hair and shoe covering recommendations may serve to protect dermatologists when caring for patients. These protocols may be particularly important for dermatologists performing high-risk procedures, including facial surgery, intraoral/intranasal procedures, and treatment with ablative lasers and facial injectables, especially when the patient is unmasked. These recommendations may limit viral transmission to dermatologists and also protect individuals living in their households. Additional established guidelines by the American Academy of Dermatology, American Society for Dermatologic Surgery, and World Health Organization are listed in Table 2.8-10
Current PPE recommendations that do not include hair and shoe coverings may be inadequate for limiting SARS-CoV-2 exposure between and among physicians and patients. Adherence to head covering and shoe recommendations may aid in reducing unwanted SARS-CoV-2 transmission in the health care setting, even as the pandemic continues.

Personal protective equipment (PPE) is an important component in limiting transmission of SARS-CoV-2. The World Health Organization and Centers for Disease Control and Prevention issued guidelines for appropriate PPE use, but recommendations for head and shoe coverings are lacking. In this article, we analyze the literature on pathogen transmission via hair and shoes and make evidence-based recommendations for PPE selection during the COVID-19 pandemic.
Pathogens on Shoes and Hair
Hair and shoes may act as vehicles for pathogen transmission. In a study that simulated contamination of uncovered skin in health care workers after intubating manikins in respiratory distress, 8 (100%) had fluorescent markers on the hair, 6 (75%) on the neck, and 4 (50%) on the shoes.1 In another study of postsurgical operating room (OR) surfaces (517 cultures), uncovered shoe tops and reusable hair coverings had 10-times more bacterial colony–forming units compared to other surfaces. On average, disposable shoe covers/head coverings had less than one-third bacterial colony–forming units compared with uncovered shoes/reusable hair coverings.2
Hair characteristics and coverings may affect pathogen transmission. Exposed hair may collect bacteria, as Staphylococcus aureus and Staphylococcus epidermidis attach to both scalp and facial hair. In one case, β-hemolytic streptococci cultured from the scalp of a perioperative nurse was linked to postsurgical infections in 20 patients.3 Hair coverings include bouffant caps and skullcaps. The bouffant cap is similar to a shower cap; it is relatively loose and secured around the head with elastic. The skullcap, or scrub cap, is tighter but leaves the neck nape and sideburns exposed. In a study comparing disposable bouffant caps, disposable skullcaps, and home-laundered cloth skullcaps worn by 2 teams of 5 surgeons, the disposable bouffant caps had the highest permeability, penetration, and microbial shed of airborne particles.4
Physicians’ shoes may act as fomites for transmission of pathogens to patients. In a study of 41 physicians and nurses in an acute care hospital, shoe soles were positive for at least one pathogen in 12 (29.3%) participants; methicillin-resistant Staphylococcus aureus was most common. Additionally, 98% (49/50) of shoes worn outdoors showed positive bacterial cultures compared to 56% (28/50) of shoes reserved for the OR only.5 In a study examining ventilation effects on airborne pathogens in the OR, 15% of OR airborne bacteria originated from OR floors, and higher bacterial counts correlated with a higher number of steps in the OR.2 In another study designed to evaluate SARS-CoV-2 distribution on hospital floors, 70% (7/10) of quantitative polymerase chain reaction assays performed on floor samples from intensive care units were positive. In addition, 100% (3/3) of swabs taken from hospital pharmacy floors with no COVID-19 patients were positive for SARS-CoV-2, meaning contaminated shoes likely served as vectors.6 Middle East respiratory syndrome, SARS-CoV-2, and influenza viruses may survive on porous and nonporous materials for hours to days.7Enterococcus, Candida, and Aspergillus may survive on textiles for up to 90 days.3
Recommendations for Hair and Shoe Coverings
We recommend that physicians utilize disposable skullcaps to cover the hair and consider a hooded gown or coverall for neck/ear coverage. We also recommend that physicians designate shoes that remain in the workplace and can be easily washed or disinfected at least weekly; physicians may choose to wash or disinfect shoes more often if they frequently are performing procedures that generate aerosols. Additionally, physicians should always wear shoe coverings when caring for patients (Table 1).

Our hair and shoe covering recommendations may serve to protect dermatologists when caring for patients. These protocols may be particularly important for dermatologists performing high-risk procedures, including facial surgery, intraoral/intranasal procedures, and treatment with ablative lasers and facial injectables, especially when the patient is unmasked. These recommendations may limit viral transmission to dermatologists and also protect individuals living in their households. Additional established guidelines by the American Academy of Dermatology, American Society for Dermatologic Surgery, and World Health Organization are listed in Table 2.8-10
Current PPE recommendations that do not include hair and shoe coverings may be inadequate for limiting SARS-CoV-2 exposure between and among physicians and patients. Adherence to head covering and shoe recommendations may aid in reducing unwanted SARS-CoV-2 transmission in the health care setting, even as the pandemic continues.

- Feldman O, Meir M, Shavit D, et al. Exposure to a surrogate measure of contamination from simulated patients by emergency department personnel wearing personal protective equipment. JAMA. 2020;323:2091-2093. doi:10.1001/jama.2020.6633
- Alexander JW, Van Sweringen H, Vanoss K, et al. Surveillance of bacterial colonization in operating rooms. Surg Infect (Larchmt). 2013;14:345-351. doi:10.1089/sur.2012.134
- Blanchard J. Clinical issues—August 2010. AORN Journal. 2010;92:228-232. doi:10.1016/j.aorn.2010.06.001
- Markel TA, Gormley T, Greeley D, et al. Hats off: a study of different operating room headgear assessed by environmental quality indicators. J Am Coll Surg. 2017;225:573-581. doi:10.1016/j.jamcollsurg.2017.08.014
- Kanwar A, Thakur M, Wazzan M, et al. Clothing and shoes of personnel as potential vectors for transfer of health care-associated pathogens to the community. Am J Infect Control. 2019;47:577-579. doi:10.1016/j.ajic.2019.01.028
- Guo ZD, Wang ZY, Zhang SF, et al. Aerosol and surface distribution of severe acute respiratory syndrome coronavirus 2 in hospital wards, Wuhan, China, 2020. Emerg Infect Dis. 2020;26:1583-1591. doi:10.3201/eid2607.200885
- Otter JA, Donskey C, Yezli S, et al. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: the possible role of dry surface contamination. J Hosp Infect. 2016;92:235-250. doi:10.1016/j.jhin.2015.08.027
- Centers for Disease Control and Prevention. Science Brief: SARS-CoV-2 and Surface (Fomite) Transmission for Indoor Community Environments. https://www.cdc.gov/coronavirus/2019-ncov/more/science-and-research/surface-transmission.html#ref10
- American Academy of Dermatology. Clinical guidance for COVID-19. Accessed March 15, 2021. https://www.aad.org/member/practice/coronavirus/clinical-guidance
- Narla S, Alam M, Ozog DM, et al. American Society of Dermatologic Surgery Association (ASDSA) and American Society for Laser Medicine & Surgery (ASLMS) guidance for cosmetic dermatology practices during COVID-19. Updated January 11, 2021. Accessed March 15, 2021. https://www.asds.net/Portals/0/PDF/asdsa/asdsa-aslms-cosmetic-reopening-guidance.pdf
- World Health Organization. Country & technical guidance—coronavirus disease (COVID-19). Accessed March 15, 2021. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance-publications
- Feldman O, Meir M, Shavit D, et al. Exposure to a surrogate measure of contamination from simulated patients by emergency department personnel wearing personal protective equipment. JAMA. 2020;323:2091-2093. doi:10.1001/jama.2020.6633
- Alexander JW, Van Sweringen H, Vanoss K, et al. Surveillance of bacterial colonization in operating rooms. Surg Infect (Larchmt). 2013;14:345-351. doi:10.1089/sur.2012.134
- Blanchard J. Clinical issues—August 2010. AORN Journal. 2010;92:228-232. doi:10.1016/j.aorn.2010.06.001
- Markel TA, Gormley T, Greeley D, et al. Hats off: a study of different operating room headgear assessed by environmental quality indicators. J Am Coll Surg. 2017;225:573-581. doi:10.1016/j.jamcollsurg.2017.08.014
- Kanwar A, Thakur M, Wazzan M, et al. Clothing and shoes of personnel as potential vectors for transfer of health care-associated pathogens to the community. Am J Infect Control. 2019;47:577-579. doi:10.1016/j.ajic.2019.01.028
- Guo ZD, Wang ZY, Zhang SF, et al. Aerosol and surface distribution of severe acute respiratory syndrome coronavirus 2 in hospital wards, Wuhan, China, 2020. Emerg Infect Dis. 2020;26:1583-1591. doi:10.3201/eid2607.200885
- Otter JA, Donskey C, Yezli S, et al. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: the possible role of dry surface contamination. J Hosp Infect. 2016;92:235-250. doi:10.1016/j.jhin.2015.08.027
- Centers for Disease Control and Prevention. Science Brief: SARS-CoV-2 and Surface (Fomite) Transmission for Indoor Community Environments. https://www.cdc.gov/coronavirus/2019-ncov/more/science-and-research/surface-transmission.html#ref10
- American Academy of Dermatology. Clinical guidance for COVID-19. Accessed March 15, 2021. https://www.aad.org/member/practice/coronavirus/clinical-guidance
- Narla S, Alam M, Ozog DM, et al. American Society of Dermatologic Surgery Association (ASDSA) and American Society for Laser Medicine & Surgery (ASLMS) guidance for cosmetic dermatology practices during COVID-19. Updated January 11, 2021. Accessed March 15, 2021. https://www.asds.net/Portals/0/PDF/asdsa/asdsa-aslms-cosmetic-reopening-guidance.pdf
- World Health Organization. Country & technical guidance—coronavirus disease (COVID-19). Accessed March 15, 2021. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance-publications
Practice Points
- Consistent use of personal protective equipment, including masks, face shields, goggles, and gloves, may limit transmission of SARS-CoV-2.
- Hair and shoes also may transmit SARS-CoV-2, but recommendations for hair and shoe coverings to prevent SARS-CoV-2 are lacking.
Comparison of Dermatologist Ratings on Health Care–Specific and General Consumer Websites
Health care–specific (eg, Healthgrades, Zocdoc, Vitals, WebMD) and general consumer websites (eg, Google, Yelp) are popular platforms for patients to find physicians, schedule appointments, and review physician experiences. Patients find ratings on these websites more trustworthy than standardized surveys distributed by hospitals, but many physicians do not trust the reviews on these sites. For example, in a survey of both physicians (n=828) and patients (n=494), 36% of physicians trusted online reviews compared to 57% of patients.1 The objective of this study was to determine if health care–specific or general consumer websites more accurately reflect overall patient sentiment. This knowledge can help physicians who are seeking to improve the patient experience understand which websites have more accurate and trustworthy reviews.
Methods
A list of dermatologists from the top 10 most and least dermatologist–dense areas in the United States was compiled to examine different physician populations.2 Equal numbers of male and female dermatologists were randomly selected from the most dense areas. All physicians were included from the least dense areas because of limited sample size. Ratings were collected from websites most likely to appear on the first page of a Google search for a physician name, as these are most likely to be seen by patients. Descriptive statistics were generated to describe the study population; mean and median physician rating (using a scale of 1–5); SD; and minimum, maximum, and interquartile ranges. Spearman correlation coefficients were generated to examine the strength of association between ratings from website pairs. P<.05 was considered statistically significant, with analyses performed in R (3.6.2) for Windows (the R Foundation).
Results
A total of 167 representative physicians were included in this analysis; 141 from the most dense areas, and 26 from the least dense areas. The lowest average ratings for the entire sample and most dermatologist–dense areas were found on Yelp (3.61 and 3.60, respectively), and the lowest ratings in the least dermatologist–dense areas were found on Google (3.45)(Table 1). Correlation coefficient values were lowest for Zocdoc and Healthgrades (0.263) and highest for Vitals and WebMD (0.963)(Table 2). The health care–specific sites were closer to the overall average (4.06) than the general consumer sites (eFigure).
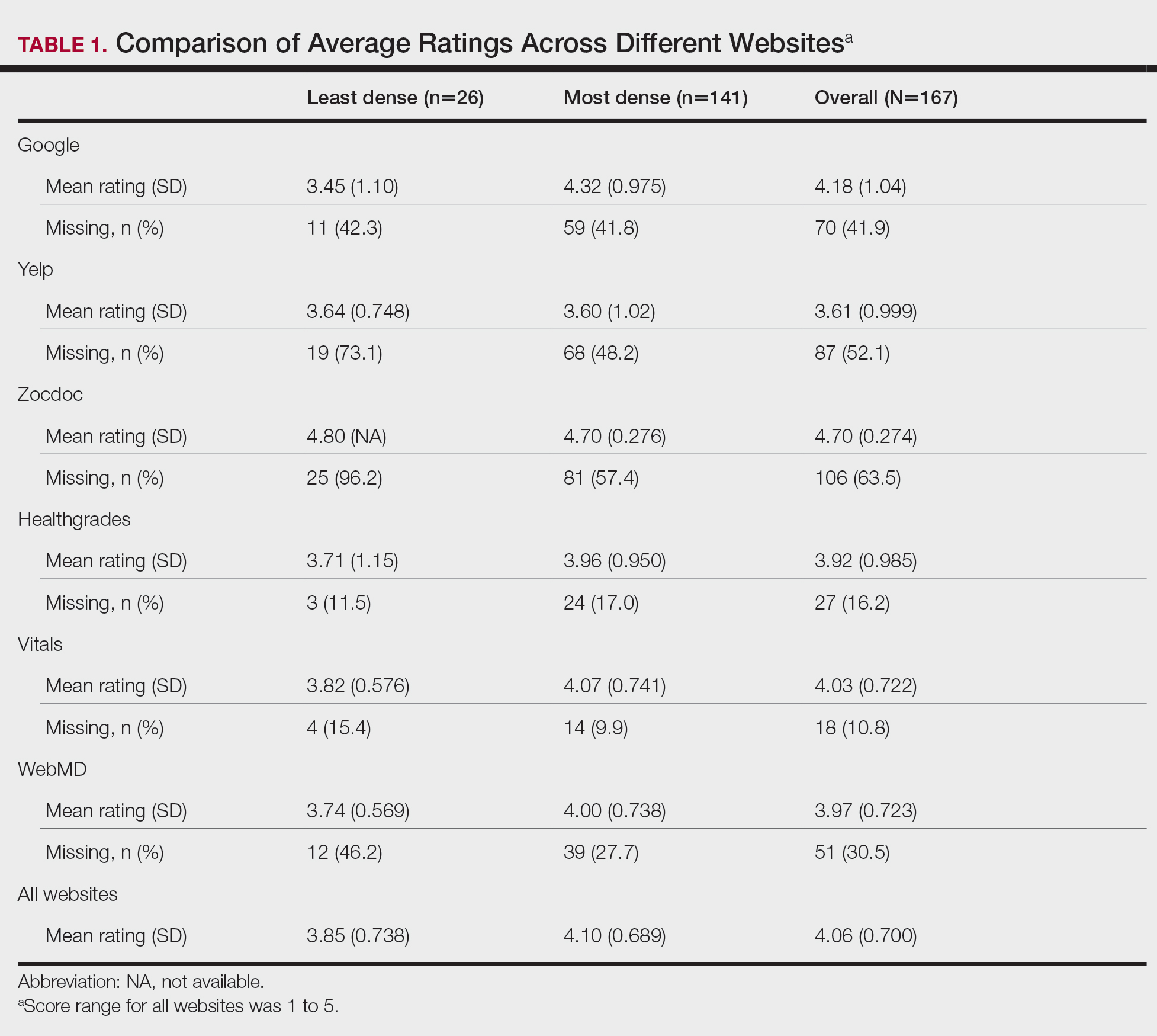
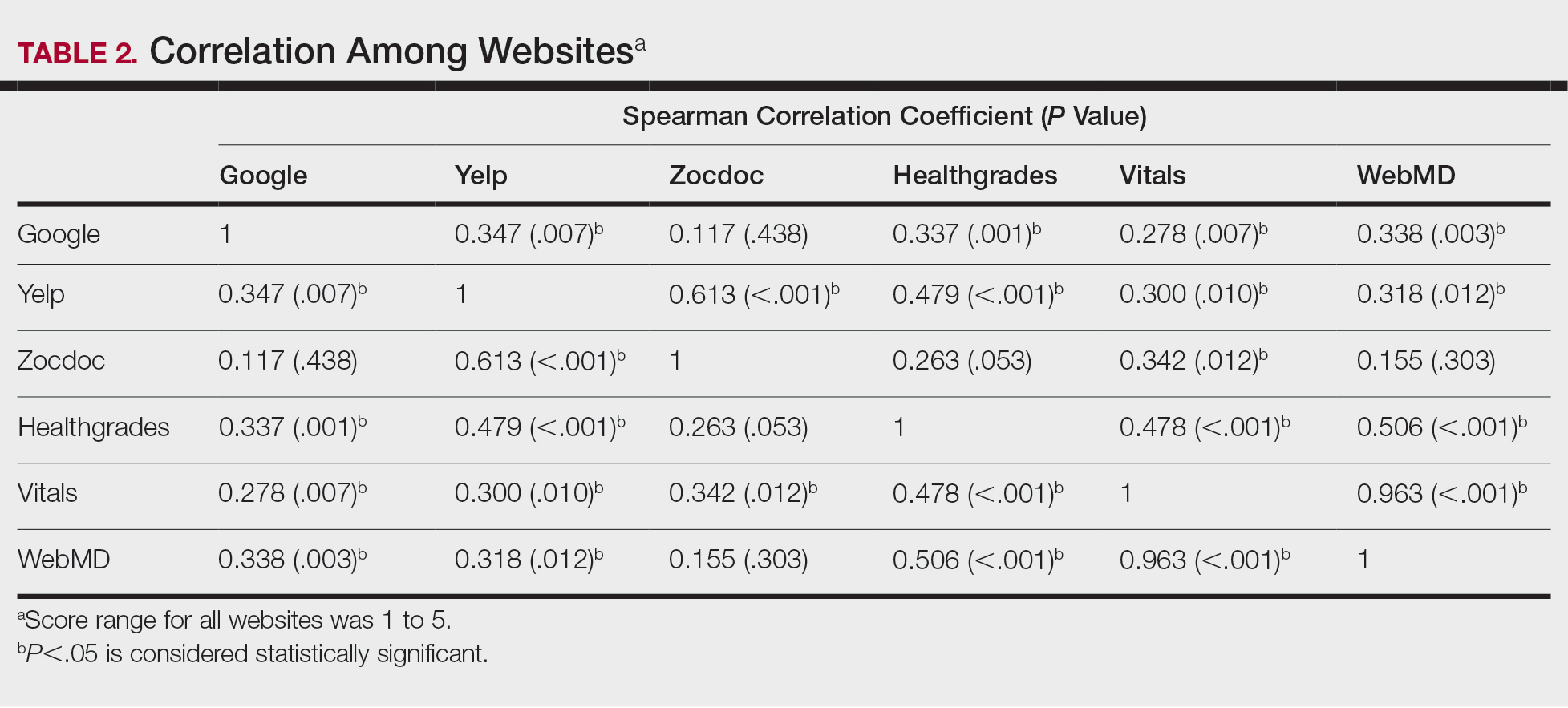
Comment
Although dermatologist ratings on each site had a broad range, we found that patients typically expressed negative interactions on general consumer websites rather than health care–specific websites. When comparing the ratings of the same group of dermatologists across different sites, ratings on health care–specific sites had a higher degree of correlation, with physician ratings more similar between 2 health care–specific sites and less similar between a health care–specific and a general consumer website. This pattern was consistent in both dermatologist-dense and dermatologist-poor areas, despite patients having varying levels of access to dermatologic care and medical resources and potentially different regional preferences of consumer websites. Taken together, these findings imply that health care–specific websites more consistently reflect overall patient sentiment.
Although one 2016 study comparing reviews of dermatology practices on Zocdoc and Yelp also demonstrated lower average ratings on Yelp,3 our study suggests that this trend is not isolated to these 2 sites but can be seen when comparing many health care–specific sites vs general consumer sites.
Our study compared ratings of dermatologists among popular websites to understand those that are most representative of patient attitudes toward physicians. These findings are important because online reviews reflect the entire patient experience, not just the patient-physician interaction, which may explain why physician scores on standardized questionnaires, such as Press Ganey surveys, do not correlate well with their online reviews.4 In a study comparing 98 physicians with negative online ratings to 82 physicians in similar departments with positive ratings, there was no significant difference in scores on patient-physician interaction questions on the Press Ganey survey.5 However, physicians who received negative online reviews scored lower on Press Ganey questions related to nonphysician interactions (eg, office cleanliness, interactions with staff).
The current study was subject to several limitations. Our analysis included all physicians in our random selection without accounting for those physicians with a greater online presence who might be more cognizant of these ratings and try to manipulate them through a reputation-management company or public relations consultant.
Conclusion
Our study suggests that consumer websites are not primarily used by disgruntled patients wishing to express grievances; instead, on average, most physicians received positive reviews. Furthermore, health care–specific websites show a higher degree of concordance than and may more accurately reflect overall patient attitudes toward their physicians than general consumer sites. Reviews from these health care–specific sites may be more helpful than general consumer websites in allowing physicians to understand patient sentiment and improve patient experiences.
- Frost C, Mesfin A. Online reviews of orthopedic surgeons: an emerging trend. Orthopedics. 2015;38:e257-e262. doi:10.3928/01477447-20150402-52
- Waqas B, Cooley V, Lipner SR. Association of sex, location, and experience with online patient ratings of dermatologists. J Am Acad Dermatol. 2020;83:954-955.
- Smith RJ, Lipoff JB. Evaluation of dermatology practice online reviews: lessons from qualitative analysis. JAMA Dermatol. 2016;152:153-157. doi:10.1001/jamadermatol.2015.3950
- Chen J, Presson A, Zhang C, et al. Online physician review websites poorly correlate to a validated metric of patient satisfaction. J Surg Res. 2018;227:1-6.
- Widmer RJ, Maurer MJ, Nayar VR, et al. Online physician reviews do not reflect patient satisfaction survey responses. Mayo Clinic Proc. 2018;93:453-457.
Health care–specific (eg, Healthgrades, Zocdoc, Vitals, WebMD) and general consumer websites (eg, Google, Yelp) are popular platforms for patients to find physicians, schedule appointments, and review physician experiences. Patients find ratings on these websites more trustworthy than standardized surveys distributed by hospitals, but many physicians do not trust the reviews on these sites. For example, in a survey of both physicians (n=828) and patients (n=494), 36% of physicians trusted online reviews compared to 57% of patients.1 The objective of this study was to determine if health care–specific or general consumer websites more accurately reflect overall patient sentiment. This knowledge can help physicians who are seeking to improve the patient experience understand which websites have more accurate and trustworthy reviews.
Methods
A list of dermatologists from the top 10 most and least dermatologist–dense areas in the United States was compiled to examine different physician populations.2 Equal numbers of male and female dermatologists were randomly selected from the most dense areas. All physicians were included from the least dense areas because of limited sample size. Ratings were collected from websites most likely to appear on the first page of a Google search for a physician name, as these are most likely to be seen by patients. Descriptive statistics were generated to describe the study population; mean and median physician rating (using a scale of 1–5); SD; and minimum, maximum, and interquartile ranges. Spearman correlation coefficients were generated to examine the strength of association between ratings from website pairs. P<.05 was considered statistically significant, with analyses performed in R (3.6.2) for Windows (the R Foundation).
Results
A total of 167 representative physicians were included in this analysis; 141 from the most dense areas, and 26 from the least dense areas. The lowest average ratings for the entire sample and most dermatologist–dense areas were found on Yelp (3.61 and 3.60, respectively), and the lowest ratings in the least dermatologist–dense areas were found on Google (3.45)(Table 1). Correlation coefficient values were lowest for Zocdoc and Healthgrades (0.263) and highest for Vitals and WebMD (0.963)(Table 2). The health care–specific sites were closer to the overall average (4.06) than the general consumer sites (eFigure).



Comment
Although dermatologist ratings on each site had a broad range, we found that patients typically expressed negative interactions on general consumer websites rather than health care–specific websites. When comparing the ratings of the same group of dermatologists across different sites, ratings on health care–specific sites had a higher degree of correlation, with physician ratings more similar between 2 health care–specific sites and less similar between a health care–specific and a general consumer website. This pattern was consistent in both dermatologist-dense and dermatologist-poor areas, despite patients having varying levels of access to dermatologic care and medical resources and potentially different regional preferences of consumer websites. Taken together, these findings imply that health care–specific websites more consistently reflect overall patient sentiment.
Although one 2016 study comparing reviews of dermatology practices on Zocdoc and Yelp also demonstrated lower average ratings on Yelp,3 our study suggests that this trend is not isolated to these 2 sites but can be seen when comparing many health care–specific sites vs general consumer sites.
Our study compared ratings of dermatologists among popular websites to understand those that are most representative of patient attitudes toward physicians. These findings are important because online reviews reflect the entire patient experience, not just the patient-physician interaction, which may explain why physician scores on standardized questionnaires, such as Press Ganey surveys, do not correlate well with their online reviews.4 In a study comparing 98 physicians with negative online ratings to 82 physicians in similar departments with positive ratings, there was no significant difference in scores on patient-physician interaction questions on the Press Ganey survey.5 However, physicians who received negative online reviews scored lower on Press Ganey questions related to nonphysician interactions (eg, office cleanliness, interactions with staff).
The current study was subject to several limitations. Our analysis included all physicians in our random selection without accounting for those physicians with a greater online presence who might be more cognizant of these ratings and try to manipulate them through a reputation-management company or public relations consultant.
Conclusion
Our study suggests that consumer websites are not primarily used by disgruntled patients wishing to express grievances; instead, on average, most physicians received positive reviews. Furthermore, health care–specific websites show a higher degree of concordance than and may more accurately reflect overall patient attitudes toward their physicians than general consumer sites. Reviews from these health care–specific sites may be more helpful than general consumer websites in allowing physicians to understand patient sentiment and improve patient experiences.
Health care–specific (eg, Healthgrades, Zocdoc, Vitals, WebMD) and general consumer websites (eg, Google, Yelp) are popular platforms for patients to find physicians, schedule appointments, and review physician experiences. Patients find ratings on these websites more trustworthy than standardized surveys distributed by hospitals, but many physicians do not trust the reviews on these sites. For example, in a survey of both physicians (n=828) and patients (n=494), 36% of physicians trusted online reviews compared to 57% of patients.1 The objective of this study was to determine if health care–specific or general consumer websites more accurately reflect overall patient sentiment. This knowledge can help physicians who are seeking to improve the patient experience understand which websites have more accurate and trustworthy reviews.
Methods
A list of dermatologists from the top 10 most and least dermatologist–dense areas in the United States was compiled to examine different physician populations.2 Equal numbers of male and female dermatologists were randomly selected from the most dense areas. All physicians were included from the least dense areas because of limited sample size. Ratings were collected from websites most likely to appear on the first page of a Google search for a physician name, as these are most likely to be seen by patients. Descriptive statistics were generated to describe the study population; mean and median physician rating (using a scale of 1–5); SD; and minimum, maximum, and interquartile ranges. Spearman correlation coefficients were generated to examine the strength of association between ratings from website pairs. P<.05 was considered statistically significant, with analyses performed in R (3.6.2) for Windows (the R Foundation).
Results
A total of 167 representative physicians were included in this analysis; 141 from the most dense areas, and 26 from the least dense areas. The lowest average ratings for the entire sample and most dermatologist–dense areas were found on Yelp (3.61 and 3.60, respectively), and the lowest ratings in the least dermatologist–dense areas were found on Google (3.45)(Table 1). Correlation coefficient values were lowest for Zocdoc and Healthgrades (0.263) and highest for Vitals and WebMD (0.963)(Table 2). The health care–specific sites were closer to the overall average (4.06) than the general consumer sites (eFigure).



Comment
Although dermatologist ratings on each site had a broad range, we found that patients typically expressed negative interactions on general consumer websites rather than health care–specific websites. When comparing the ratings of the same group of dermatologists across different sites, ratings on health care–specific sites had a higher degree of correlation, with physician ratings more similar between 2 health care–specific sites and less similar between a health care–specific and a general consumer website. This pattern was consistent in both dermatologist-dense and dermatologist-poor areas, despite patients having varying levels of access to dermatologic care and medical resources and potentially different regional preferences of consumer websites. Taken together, these findings imply that health care–specific websites more consistently reflect overall patient sentiment.
Although one 2016 study comparing reviews of dermatology practices on Zocdoc and Yelp also demonstrated lower average ratings on Yelp,3 our study suggests that this trend is not isolated to these 2 sites but can be seen when comparing many health care–specific sites vs general consumer sites.
Our study compared ratings of dermatologists among popular websites to understand those that are most representative of patient attitudes toward physicians. These findings are important because online reviews reflect the entire patient experience, not just the patient-physician interaction, which may explain why physician scores on standardized questionnaires, such as Press Ganey surveys, do not correlate well with their online reviews.4 In a study comparing 98 physicians with negative online ratings to 82 physicians in similar departments with positive ratings, there was no significant difference in scores on patient-physician interaction questions on the Press Ganey survey.5 However, physicians who received negative online reviews scored lower on Press Ganey questions related to nonphysician interactions (eg, office cleanliness, interactions with staff).
The current study was subject to several limitations. Our analysis included all physicians in our random selection without accounting for those physicians with a greater online presence who might be more cognizant of these ratings and try to manipulate them through a reputation-management company or public relations consultant.
Conclusion
Our study suggests that consumer websites are not primarily used by disgruntled patients wishing to express grievances; instead, on average, most physicians received positive reviews. Furthermore, health care–specific websites show a higher degree of concordance than and may more accurately reflect overall patient attitudes toward their physicians than general consumer sites. Reviews from these health care–specific sites may be more helpful than general consumer websites in allowing physicians to understand patient sentiment and improve patient experiences.
- Frost C, Mesfin A. Online reviews of orthopedic surgeons: an emerging trend. Orthopedics. 2015;38:e257-e262. doi:10.3928/01477447-20150402-52
- Waqas B, Cooley V, Lipner SR. Association of sex, location, and experience with online patient ratings of dermatologists. J Am Acad Dermatol. 2020;83:954-955.
- Smith RJ, Lipoff JB. Evaluation of dermatology practice online reviews: lessons from qualitative analysis. JAMA Dermatol. 2016;152:153-157. doi:10.1001/jamadermatol.2015.3950
- Chen J, Presson A, Zhang C, et al. Online physician review websites poorly correlate to a validated metric of patient satisfaction. J Surg Res. 2018;227:1-6.
- Widmer RJ, Maurer MJ, Nayar VR, et al. Online physician reviews do not reflect patient satisfaction survey responses. Mayo Clinic Proc. 2018;93:453-457.
- Frost C, Mesfin A. Online reviews of orthopedic surgeons: an emerging trend. Orthopedics. 2015;38:e257-e262. doi:10.3928/01477447-20150402-52
- Waqas B, Cooley V, Lipner SR. Association of sex, location, and experience with online patient ratings of dermatologists. J Am Acad Dermatol. 2020;83:954-955.
- Smith RJ, Lipoff JB. Evaluation of dermatology practice online reviews: lessons from qualitative analysis. JAMA Dermatol. 2016;152:153-157. doi:10.1001/jamadermatol.2015.3950
- Chen J, Presson A, Zhang C, et al. Online physician review websites poorly correlate to a validated metric of patient satisfaction. J Surg Res. 2018;227:1-6.
- Widmer RJ, Maurer MJ, Nayar VR, et al. Online physician reviews do not reflect patient satisfaction survey responses. Mayo Clinic Proc. 2018;93:453-457.
Practice Points
- Online physician-rating websites are commonly used by patients to find physicians and review experiences.
- Health care–specific sites may more accurately reflect patient sentiment than general consumer sites.
- Dermatologists can use health care–specific sites to understand patient sentiment and learn how to improve patient experiences.
Are There Mobile Applications Related to Nail Disorders?
The use of mobile devices in health care settings has enhanced clinical practice through real-time communication and direct patient monitoring.1 With advancements in technology, improving the accessibility and quality of patient care using mobile devices is a hot topic. In 2018, 261.34 million people worldwide used smartphones compared to 280.54 million in 2021—a 7.3% increase.2 Revenue from sales of mobile applications (apps) is projected to reach $693 billion in 2021.3
A range of apps targeted to patients is available for acne, melanoma, and teledermatology.4-6 Nail disorders are a common concern, representing 21.1 million outpatient visits in 2007 to 2016,7 but, to date, the availability of apps related to nail disorders has not been explored. In this study, we investigated iOS (Apple’s iPhone Operating System) and Android apps to determine the types of nail health apps that are available, using psoriasis and hair loss apps as comparator groups.
Methods
A standard app analytics and market data tool (App Annie; https://www.appannie.com/en/) was utilized to search for iOS and Android nail mobile apps.4,5 The analysis was performed on a single day (March 23, 2020), given that app searches can change on a daily basis. Our search included the following keywords:
Results
Nail-Related Apps
Using keywords for nail-related terms on iOS and Android platforms, our search returned few specific and informative apps related to nail disorders (Table 1). When the terms brittle nails, nail, nail health, nail squamous cell carcinoma, and nail tumor were searched, all available nail apps were either nail games or virtual nail salons for entertainment purposes. For the terms nail melanoma and subungual melanoma, there were no specific nail apps that appeared in the search results; rather, the App Annie search yielded only general dermatology and melanoma apps. The terms onycholysis and paronychia both yielded 0 hits for iOS and Android.
The only search terms that returned specific nail apps were onychomycosis and toenail fungus. Initially, when onychomycosis was searched, only 1 Google Play Medical category app was found: “Nail fungal infection (model onychomycosis).” Although this app recently was removed from the app store, it previously allowed the user to upload a nail photograph, with which a computing algorithm assessed whether the presentation was a fungal nail infection. Toenail fungus returned 1 iOS Medical category app and 5 Android Health & Fitness category apps with reference material for patients. Neither of the 2 medical apps for onychomycosis and toenail fungus referenced a physician involved in the app development.
Psoriasis Comparator
On the contrary, a search for
Hair Loss Comparator
Search terms related to hair conditions—specifically, alopecia—yielded 0 apps for iOS and 10 for Android platforms (Table 2). Using the search term hair loss, 12 apps for iOS and 50 apps for Android were found within the Health & Fitness, Medical, and Beauty categories. The search terms hair health and hair loss resulted in 2 and 12 apps in both iOS and Android, respectively. In addition, the search term scalp was associated with 6 related apps in iOS and 7 in Android, both in the Health & Fitness and Medical categories.
Other Findings
Most apps for psoriasis and hair health were identified as patient focused. Although iOS and Android are different operating systems, some health apps overlapped: subungual melanoma and toenail fungus had a 20% overlap; psoriasis, 19%; chronic skin disease, 2%; alopecia, 0%; hair loss and hair health, 10%; and scalp, 18%. iOS and Android nail entertainment games had approximately a 30% overlap. Tables 1 and 2 also compare the number of free and paid apps; most available apps were free.
Comment
With continued growth in mobile device ownership and app development has been parallel growth in the creation and use of apps to enhance medical care.1 In a study analyzing the most popular dermatology apps, 62% (18/29) and 38% (11/29) of apps targeted patients and physicians, respectively.6 Our study showed that (1) there are few nail disorder apps available for patient education and (2) there is no evidence that a physician was consulted for content input. Because patients who can effectively communicate their health concerns before and after seeing a physician have better self-reported clinical outcomes,9 it is important to have nail disorder apps available to patients for referencing. The nail health app options differ notably from psoriasis and hair loss apps, with apps for the latter 2 topics found in Medical and Health & Fitness categories—targeting patients who seek immediate access to health care and education.
Although there are several general dermatology apps that contain reference information for patients pertaining to nail conditions,6 using any of those apps would require a patient to have prior knowledge that dermatologists specialize in nail disorders and necessitate several steps to find nail-relevant information. For example, the patient would have to search dermatology in the iOS and Android app stores, select the available app (eg, Dermatology Database), and then search within that app for nail disorders. Therefore, a patient who is concerned about a possible subungual melanoma would not be able to easily find clinical images and explanations using an app.
Study Limitations
This study was subject to several limitations. Android and iOS app stores have undisclosed computing algorithms that might have filtered apps based on specific word inquiry. Also, our queries were based on specific relevant keywords for nail conditions, psoriasis, and hair loss; use of different keywords might have yielded different results. Additionally, app options change on a daily basis, so a search today (ie, any given day) might yield slightly different results than it did on March 23, 2020.
Conclusion
Specific nail disorder apps available for patient reference are limited. App developers should consider accessibility (ie, clear language, ease of use, cost-effectiveness, usability on iOS- and Android-operated devices) and content (accurate medical information from experts) when considering new apps. A solution to this problem is for established medical organizations to create nail disorder apps specifically for patients.10 For example, the American Academy of Dermatology has iOS and Android apps that are relevant to physicians (MyDermPath+, Dialogues in Dermatology, Mohs Surgery Appropriate Use Criteria) but no comparable apps for patients; patient-appropriate nail apps are necessary.11 In addition, it would be beneficial to patients if established app companies consulted with dermatologists on pertinent nail content.
In sum, we found few available nail health apps on the iOS or Android platforms that provided accessible and timely information to patients regarding nail disorders. There is an immediate need to produce apps related to nail health for appropriate patient education.
- Wallace S, Clark M, White J. ‘It’s on my iPhone’: attitudes to the use of mobile computing devices in medical education, a mixed-methods study. BMJ Open. 2012;2:e001099.
- O’Dea S. Number of smartphone users in the United States from 2018 to 2024 (in millions). Statista website. April 21, 2020. Accessed February 19, 2021. https://www.statista.com/statistics/201182/forecast-of-smartphone-users-in-the-us/
- Clement J. Worldwide mobile app revenues in 2014 to 2023. Statista website. Published February 4, 2021. Accessed February 19, 2021.https://www.statista.com/statistics/269025/worldwide-mobile-app-revenue-forecast/
- Poushter J, Bishop C, Chwe H. Social media use continues to rise in developing countries but plateaus across developed ones. Pew Research Center Washington DC. Published June 19, 2018. Accessed February 19, 2021. https://www.pewresearch.org/global/2018/06/19/social-media-use-continues-to-rise-in-developing-countries-but-plateaus-across-developed-ones/
- Flaten HK, St Claire C, Schlager E, et al. Growth of mobile applications in dermatology—2017 update. Dermatol Online J. 2018 February;24:1-4. Accessed February 19, 2021. https://escholarship.org/uc/item/3hs7n9z6
- Tongdee E, Markowitz O. Mobile app rankings in dermatology. Cutis. 2018;102:252-256.
- Lipner SR, Hancock J, Fleischer AB. The ambulatory care burden of nail conditions in the United States [published online October 21, 2019]. J Dermatol Treat. doi:10.1080/09546634.2019
- Gu L, Lipner SR. Analysis of education on nail conditions at the American Academy of Dermatology annual meetings. Cutis. 2020;105:259-260.
- King A, Hoppe RB. “Best practice” for patient-centered communication: a narrative review. J Grad Med Educ. 2013;3:385-393.
- Larson RS. A path to better-quality mHealth apps. JMIR Mhealth Uhealth. 2018;6:E10414.
- Academy apps. American Academy of Dermatology website. Accessed February 19, 2021. https://www.aad.org/member/publications/apps
The use of mobile devices in health care settings has enhanced clinical practice through real-time communication and direct patient monitoring.1 With advancements in technology, improving the accessibility and quality of patient care using mobile devices is a hot topic. In 2018, 261.34 million people worldwide used smartphones compared to 280.54 million in 2021—a 7.3% increase.2 Revenue from sales of mobile applications (apps) is projected to reach $693 billion in 2021.3
A range of apps targeted to patients is available for acne, melanoma, and teledermatology.4-6 Nail disorders are a common concern, representing 21.1 million outpatient visits in 2007 to 2016,7 but, to date, the availability of apps related to nail disorders has not been explored. In this study, we investigated iOS (Apple’s iPhone Operating System) and Android apps to determine the types of nail health apps that are available, using psoriasis and hair loss apps as comparator groups.
Methods
A standard app analytics and market data tool (App Annie; https://www.appannie.com/en/) was utilized to search for iOS and Android nail mobile apps.4,5 The analysis was performed on a single day (March 23, 2020), given that app searches can change on a daily basis. Our search included the following keywords:
Results
Nail-Related Apps
Using keywords for nail-related terms on iOS and Android platforms, our search returned few specific and informative apps related to nail disorders (Table 1). When the terms brittle nails, nail, nail health, nail squamous cell carcinoma, and nail tumor were searched, all available nail apps were either nail games or virtual nail salons for entertainment purposes. For the terms nail melanoma and subungual melanoma, there were no specific nail apps that appeared in the search results; rather, the App Annie search yielded only general dermatology and melanoma apps. The terms onycholysis and paronychia both yielded 0 hits for iOS and Android.

The only search terms that returned specific nail apps were onychomycosis and toenail fungus. Initially, when onychomycosis was searched, only 1 Google Play Medical category app was found: “Nail fungal infection (model onychomycosis).” Although this app recently was removed from the app store, it previously allowed the user to upload a nail photograph, with which a computing algorithm assessed whether the presentation was a fungal nail infection. Toenail fungus returned 1 iOS Medical category app and 5 Android Health & Fitness category apps with reference material for patients. Neither of the 2 medical apps for onychomycosis and toenail fungus referenced a physician involved in the app development.
Psoriasis Comparator
On the contrary, a search for

Hair Loss Comparator
Search terms related to hair conditions—specifically, alopecia—yielded 0 apps for iOS and 10 for Android platforms (Table 2). Using the search term hair loss, 12 apps for iOS and 50 apps for Android were found within the Health & Fitness, Medical, and Beauty categories. The search terms hair health and hair loss resulted in 2 and 12 apps in both iOS and Android, respectively. In addition, the search term scalp was associated with 6 related apps in iOS and 7 in Android, both in the Health & Fitness and Medical categories.
Other Findings
Most apps for psoriasis and hair health were identified as patient focused. Although iOS and Android are different operating systems, some health apps overlapped: subungual melanoma and toenail fungus had a 20% overlap; psoriasis, 19%; chronic skin disease, 2%; alopecia, 0%; hair loss and hair health, 10%; and scalp, 18%. iOS and Android nail entertainment games had approximately a 30% overlap. Tables 1 and 2 also compare the number of free and paid apps; most available apps were free.
Comment
With continued growth in mobile device ownership and app development has been parallel growth in the creation and use of apps to enhance medical care.1 In a study analyzing the most popular dermatology apps, 62% (18/29) and 38% (11/29) of apps targeted patients and physicians, respectively.6 Our study showed that (1) there are few nail disorder apps available for patient education and (2) there is no evidence that a physician was consulted for content input. Because patients who can effectively communicate their health concerns before and after seeing a physician have better self-reported clinical outcomes,9 it is important to have nail disorder apps available to patients for referencing. The nail health app options differ notably from psoriasis and hair loss apps, with apps for the latter 2 topics found in Medical and Health & Fitness categories—targeting patients who seek immediate access to health care and education.
Although there are several general dermatology apps that contain reference information for patients pertaining to nail conditions,6 using any of those apps would require a patient to have prior knowledge that dermatologists specialize in nail disorders and necessitate several steps to find nail-relevant information. For example, the patient would have to search dermatology in the iOS and Android app stores, select the available app (eg, Dermatology Database), and then search within that app for nail disorders. Therefore, a patient who is concerned about a possible subungual melanoma would not be able to easily find clinical images and explanations using an app.
Study Limitations
This study was subject to several limitations. Android and iOS app stores have undisclosed computing algorithms that might have filtered apps based on specific word inquiry. Also, our queries were based on specific relevant keywords for nail conditions, psoriasis, and hair loss; use of different keywords might have yielded different results. Additionally, app options change on a daily basis, so a search today (ie, any given day) might yield slightly different results than it did on March 23, 2020.
Conclusion
Specific nail disorder apps available for patient reference are limited. App developers should consider accessibility (ie, clear language, ease of use, cost-effectiveness, usability on iOS- and Android-operated devices) and content (accurate medical information from experts) when considering new apps. A solution to this problem is for established medical organizations to create nail disorder apps specifically for patients.10 For example, the American Academy of Dermatology has iOS and Android apps that are relevant to physicians (MyDermPath+, Dialogues in Dermatology, Mohs Surgery Appropriate Use Criteria) but no comparable apps for patients; patient-appropriate nail apps are necessary.11 In addition, it would be beneficial to patients if established app companies consulted with dermatologists on pertinent nail content.
In sum, we found few available nail health apps on the iOS or Android platforms that provided accessible and timely information to patients regarding nail disorders. There is an immediate need to produce apps related to nail health for appropriate patient education.
The use of mobile devices in health care settings has enhanced clinical practice through real-time communication and direct patient monitoring.1 With advancements in technology, improving the accessibility and quality of patient care using mobile devices is a hot topic. In 2018, 261.34 million people worldwide used smartphones compared to 280.54 million in 2021—a 7.3% increase.2 Revenue from sales of mobile applications (apps) is projected to reach $693 billion in 2021.3
A range of apps targeted to patients is available for acne, melanoma, and teledermatology.4-6 Nail disorders are a common concern, representing 21.1 million outpatient visits in 2007 to 2016,7 but, to date, the availability of apps related to nail disorders has not been explored. In this study, we investigated iOS (Apple’s iPhone Operating System) and Android apps to determine the types of nail health apps that are available, using psoriasis and hair loss apps as comparator groups.
Methods
A standard app analytics and market data tool (App Annie; https://www.appannie.com/en/) was utilized to search for iOS and Android nail mobile apps.4,5 The analysis was performed on a single day (March 23, 2020), given that app searches can change on a daily basis. Our search included the following keywords:
Results
Nail-Related Apps
Using keywords for nail-related terms on iOS and Android platforms, our search returned few specific and informative apps related to nail disorders (Table 1). When the terms brittle nails, nail, nail health, nail squamous cell carcinoma, and nail tumor were searched, all available nail apps were either nail games or virtual nail salons for entertainment purposes. For the terms nail melanoma and subungual melanoma, there were no specific nail apps that appeared in the search results; rather, the App Annie search yielded only general dermatology and melanoma apps. The terms onycholysis and paronychia both yielded 0 hits for iOS and Android.

The only search terms that returned specific nail apps were onychomycosis and toenail fungus. Initially, when onychomycosis was searched, only 1 Google Play Medical category app was found: “Nail fungal infection (model onychomycosis).” Although this app recently was removed from the app store, it previously allowed the user to upload a nail photograph, with which a computing algorithm assessed whether the presentation was a fungal nail infection. Toenail fungus returned 1 iOS Medical category app and 5 Android Health & Fitness category apps with reference material for patients. Neither of the 2 medical apps for onychomycosis and toenail fungus referenced a physician involved in the app development.
Psoriasis Comparator
On the contrary, a search for

Hair Loss Comparator
Search terms related to hair conditions—specifically, alopecia—yielded 0 apps for iOS and 10 for Android platforms (Table 2). Using the search term hair loss, 12 apps for iOS and 50 apps for Android were found within the Health & Fitness, Medical, and Beauty categories. The search terms hair health and hair loss resulted in 2 and 12 apps in both iOS and Android, respectively. In addition, the search term scalp was associated with 6 related apps in iOS and 7 in Android, both in the Health & Fitness and Medical categories.
Other Findings
Most apps for psoriasis and hair health were identified as patient focused. Although iOS and Android are different operating systems, some health apps overlapped: subungual melanoma and toenail fungus had a 20% overlap; psoriasis, 19%; chronic skin disease, 2%; alopecia, 0%; hair loss and hair health, 10%; and scalp, 18%. iOS and Android nail entertainment games had approximately a 30% overlap. Tables 1 and 2 also compare the number of free and paid apps; most available apps were free.
Comment
With continued growth in mobile device ownership and app development has been parallel growth in the creation and use of apps to enhance medical care.1 In a study analyzing the most popular dermatology apps, 62% (18/29) and 38% (11/29) of apps targeted patients and physicians, respectively.6 Our study showed that (1) there are few nail disorder apps available for patient education and (2) there is no evidence that a physician was consulted for content input. Because patients who can effectively communicate their health concerns before and after seeing a physician have better self-reported clinical outcomes,9 it is important to have nail disorder apps available to patients for referencing. The nail health app options differ notably from psoriasis and hair loss apps, with apps for the latter 2 topics found in Medical and Health & Fitness categories—targeting patients who seek immediate access to health care and education.
Although there are several general dermatology apps that contain reference information for patients pertaining to nail conditions,6 using any of those apps would require a patient to have prior knowledge that dermatologists specialize in nail disorders and necessitate several steps to find nail-relevant information. For example, the patient would have to search dermatology in the iOS and Android app stores, select the available app (eg, Dermatology Database), and then search within that app for nail disorders. Therefore, a patient who is concerned about a possible subungual melanoma would not be able to easily find clinical images and explanations using an app.
Study Limitations
This study was subject to several limitations. Android and iOS app stores have undisclosed computing algorithms that might have filtered apps based on specific word inquiry. Also, our queries were based on specific relevant keywords for nail conditions, psoriasis, and hair loss; use of different keywords might have yielded different results. Additionally, app options change on a daily basis, so a search today (ie, any given day) might yield slightly different results than it did on March 23, 2020.
Conclusion
Specific nail disorder apps available for patient reference are limited. App developers should consider accessibility (ie, clear language, ease of use, cost-effectiveness, usability on iOS- and Android-operated devices) and content (accurate medical information from experts) when considering new apps. A solution to this problem is for established medical organizations to create nail disorder apps specifically for patients.10 For example, the American Academy of Dermatology has iOS and Android apps that are relevant to physicians (MyDermPath+, Dialogues in Dermatology, Mohs Surgery Appropriate Use Criteria) but no comparable apps for patients; patient-appropriate nail apps are necessary.11 In addition, it would be beneficial to patients if established app companies consulted with dermatologists on pertinent nail content.
In sum, we found few available nail health apps on the iOS or Android platforms that provided accessible and timely information to patients regarding nail disorders. There is an immediate need to produce apps related to nail health for appropriate patient education.
- Wallace S, Clark M, White J. ‘It’s on my iPhone’: attitudes to the use of mobile computing devices in medical education, a mixed-methods study. BMJ Open. 2012;2:e001099.
- O’Dea S. Number of smartphone users in the United States from 2018 to 2024 (in millions). Statista website. April 21, 2020. Accessed February 19, 2021. https://www.statista.com/statistics/201182/forecast-of-smartphone-users-in-the-us/
- Clement J. Worldwide mobile app revenues in 2014 to 2023. Statista website. Published February 4, 2021. Accessed February 19, 2021.https://www.statista.com/statistics/269025/worldwide-mobile-app-revenue-forecast/
- Poushter J, Bishop C, Chwe H. Social media use continues to rise in developing countries but plateaus across developed ones. Pew Research Center Washington DC. Published June 19, 2018. Accessed February 19, 2021. https://www.pewresearch.org/global/2018/06/19/social-media-use-continues-to-rise-in-developing-countries-but-plateaus-across-developed-ones/
- Flaten HK, St Claire C, Schlager E, et al. Growth of mobile applications in dermatology—2017 update. Dermatol Online J. 2018 February;24:1-4. Accessed February 19, 2021. https://escholarship.org/uc/item/3hs7n9z6
- Tongdee E, Markowitz O. Mobile app rankings in dermatology. Cutis. 2018;102:252-256.
- Lipner SR, Hancock J, Fleischer AB. The ambulatory care burden of nail conditions in the United States [published online October 21, 2019]. J Dermatol Treat. doi:10.1080/09546634.2019
- Gu L, Lipner SR. Analysis of education on nail conditions at the American Academy of Dermatology annual meetings. Cutis. 2020;105:259-260.
- King A, Hoppe RB. “Best practice” for patient-centered communication: a narrative review. J Grad Med Educ. 2013;3:385-393.
- Larson RS. A path to better-quality mHealth apps. JMIR Mhealth Uhealth. 2018;6:E10414.
- Academy apps. American Academy of Dermatology website. Accessed February 19, 2021. https://www.aad.org/member/publications/apps
- Wallace S, Clark M, White J. ‘It’s on my iPhone’: attitudes to the use of mobile computing devices in medical education, a mixed-methods study. BMJ Open. 2012;2:e001099.
- O’Dea S. Number of smartphone users in the United States from 2018 to 2024 (in millions). Statista website. April 21, 2020. Accessed February 19, 2021. https://www.statista.com/statistics/201182/forecast-of-smartphone-users-in-the-us/
- Clement J. Worldwide mobile app revenues in 2014 to 2023. Statista website. Published February 4, 2021. Accessed February 19, 2021.https://www.statista.com/statistics/269025/worldwide-mobile-app-revenue-forecast/
- Poushter J, Bishop C, Chwe H. Social media use continues to rise in developing countries but plateaus across developed ones. Pew Research Center Washington DC. Published June 19, 2018. Accessed February 19, 2021. https://www.pewresearch.org/global/2018/06/19/social-media-use-continues-to-rise-in-developing-countries-but-plateaus-across-developed-ones/
- Flaten HK, St Claire C, Schlager E, et al. Growth of mobile applications in dermatology—2017 update. Dermatol Online J. 2018 February;24:1-4. Accessed February 19, 2021. https://escholarship.org/uc/item/3hs7n9z6
- Tongdee E, Markowitz O. Mobile app rankings in dermatology. Cutis. 2018;102:252-256.
- Lipner SR, Hancock J, Fleischer AB. The ambulatory care burden of nail conditions in the United States [published online October 21, 2019]. J Dermatol Treat. doi:10.1080/09546634.2019
- Gu L, Lipner SR. Analysis of education on nail conditions at the American Academy of Dermatology annual meetings. Cutis. 2020;105:259-260.
- King A, Hoppe RB. “Best practice” for patient-centered communication: a narrative review. J Grad Med Educ. 2013;3:385-393.
- Larson RS. A path to better-quality mHealth apps. JMIR Mhealth Uhealth. 2018;6:E10414.
- Academy apps. American Academy of Dermatology website. Accessed February 19, 2021. https://www.aad.org/member/publications/apps
Practice Points
- Patient-targeted mobile applications (apps) might aid with clinical referencing and education.
- There are patient-directed psoriasis and hair loss apps on iOS and Android platforms, but informative apps related to nail disorders are limited.
- There is a need to develop apps related to nail health for patient education.
Onychomycosis: New Developments in Diagnosis, Treatment, and Antifungal Medication Safety
Onychomycosis is the most prevalent nail condition worldwide and has a significant impact on quality of life.1 There were 10 million physician visits for nail fungal infections in the National Ambulatory Medical Care Survey from 2007 to 2016, which was more than double the number of all other nail diagnoses combined.2 Therefore, it is important for dermatologists to be familiar with the most current data on diagnosis and treatment of this extremely common nail disease as well as antifungal medication safety.
Onychomycosis Diagnosis
Diagnosis of onychomycosis using clinical examination alone has poor sensitivity and specificity and may lead to progression of disease and unwanted side effects from inappropriate therapy.3,4 Dermoscopy is a useful adjunct but diagnostically is still inferior compared to mycologic testing.5 Classical methods of diagnosis include potassium hydroxide staining with microscopy, fungal culture, and histopathology. Polymerase chain reaction is a newer technique with wide accessibility and excellent sensitivity and specificity.6 Although these techniques have excellent diagnostic accuracy both alone and in combination, the ideal test would have 100% sensitivity and specificity and would not require nail sampling. Artificial intelligence recently has been studied for the diagnosis of onychomycosis. In a prospective study of 90 patients with onychodystrophy who had photographs of the nails taken by nonphysicians, deep neural networks showed comparable sensitivity (70.2% vs 73.0%) and specificity (72.7% vs 49.7%) for diagnosis of onychomycosis vs clinical examination by dermatologists with a mean of 5.6 years of experience.7 Therefore, artificial intelligence may be considered as a supplement to clinical examination for dermatology residents and junior attending dermatologists and may be superior to clinical examination by nondermatologists, but mycologic confirmation is still necessary before initiating onychomycosis treatment.
Treatment of Onychomycosis
There are 3 topical therapies (ciclopirox lacquer 8%, efinaconazole solution 10%, and tavaborole solution 5%) and 3 oral therapies (terbinafine, itraconazole, and griseofulvin) that are approved by the US Food and Drug Administration for onychomycosis therapy. Griseofulvin rarely is used due to the availability of more efficacious treatment options. Fluconazole is an off-label treatment that often is used in the United States.8
There are new data on the efficacy and safety of topical onychomycosis treatments in children. A phase 4 open‐label study of efinaconazole solution 10% applied once daily for 48 weeks was performed in children aged 6 to 16 years with distal lateral subungual onychomycosis (N=62).9,10 The medication was both well tolerated and safe in children. The only treatment-related adverse event was onychocryptosis, which was reported by 2 patients. At week 52, mycologic cure was 65% and complete cure was 40% (N=50). In a pharmacokinetic assessment performed in a subset of 17 patients aged 12 to 16 years, efinaconazole was measured at very low levels in plasma.9
A phase 4 open-label study also was performed to evaluate the safety, pharmacokinetics, and efficacy of tavaborole for treatment of distal lateral subungual onychomycosis in children aged 6 years to under 17 years (N=55).11 Tavaborole solution 5% was applied once daily for 48 weeks; at week 52, mycologic and complete cures were 36.2% and 8.5%, respectively (N=47). Systemic exposure was low (Cmax=5.9 ng/mL [day 29]) in a subset of patients aged 12 years to under 17 years (N=37), and the medication demonstrated good safety and tolerability.11
Fosravuconazole was approved for treatment of onychomycosis in Japan in 2018. In a randomized, double-blind, phase 3 trial of oral fosravuconazole 100 mg once daily (n=101) vs placebo (n=52) for 12 weeks in patients with onychomycosis (mean age, 58.4 years), the complete cure rate at 48 weeks was 59.4%.12 In a small trial of 37 elderly patients (mean age, 78.1 years), complete cure rates were 5.0% in patients with a nail plate thickness of 3 mm or greater and 58.8% in those with a thickness lessthan 3 mm, and there were no severe adverse events.13 In addition to excellent efficacy and proven safety in elderly adults, the main advantage of fosravuconazole is less-potent inhibition of cytochrome P450 3A compared to other triazole antifungals, with no contraindicated drugs listed.
Safety of Antifungals
There are new data describing the safety of oral terbinafine in pregnant women and immunosuppressed patients. In a nationwide cohort study conducted in Denmark (1,650,649 pregnancies [942 oral terbinafine exposed, 9420 unexposed matched cohorts]), there was no association between oral or topical terbinafine exposure during pregnancy and risk of preterm birth, small-for-gestational-age birth weight, low birth weight, or stillbirth.14 In a small study of 13 kidney transplant recipients taking oral tacrolimus, cyclosporine, or everolimus who were treated with oral terbinafine, there were no severe drug interactions and no clinical consequences in renal grafts.15
There also is new information on laboratory abnormalities in adults, children, and patients with comorbidities who are taking oral terbinafine. In a retrospective study of 944 adult patients without pre-existing hepatic or hematologic conditions who were prescribed 3 months of oral terbinafine for onychomycosis, abnormal monitoring liver function tests (LFTs) and complete blood cell counts (CBCs) were uncommon (2.4% and 2.8%, respectively) and mild and resolved after treatment completion. In addition, patients with laboratory abnormalities were an average of 14.8 years older and approximately 3-times more likely to be 65 years or older compared to the overall study population.16 There were similar findings in a retrospective study of 134 children 18 years or younger who were prescribed oral terbinafine for superficial fungal infections. Abnormal monitoring LFTs and CBCs were uncommon (1.7% and 4.4%, respectively) and mild, resolving after after treatment completion.17 Finally, in a study of 255 patients with a pre-existing liver or hematologic condition who were prescribed oral terbinafine for onychomycosis, worsening of LFT or CBC values were rare, and all resolved after treatment completion or medication discontinuation.18
Final Thoughts
Mycologic confirmation is still necessary before treatment despite encouraging data on use of artificial intelligence for diagnosis of onychomycosis. Efinaconazole solution 10% and tavaborole solution 5% have shown good safety, tolerability, and efficacy in children with onychomycosis. Recent data suggest the safety of oral terbinafine in pregnant women and kidney transplant recipients, but these findings must be corroborated before its use in these populations. Fosravuconazole is a promising systemic treatment for onychomycosis with no drug-drug interactions reported to date. While baseline laboratory testing is recommended before prescribing terbinafine, interval laboratory monitoring may not be necessary in healthy adults.19 Prospective studies are necessary to corroborate these findings before formal recommendations can be made for prescribing terbinafine in the special populations discussed above, including children, and for interval laboratory monitoring.
- Stewart CR, Algu L, Kamran R, et al. Effect of onychomycosis and treatment on patient-reported quality-of-life outcomes: a systematic review [published online June 2, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.05.143
- Lipner SR, Hancock JE, Fleischer AB. The ambulatory care burden of nail conditions in the United States [published online October 21, 2019]. J Dermatolog Treat. doi:10.1080/09546634.2019.1679337
- Lipner SR, Scher RK. Onychomycosis--a small step for quality of care. Curr Med Res Opin. 2016;32:865-867.
- Lipner SR, Scher RK. Confirmatory testing for onychomycosis. JAMA Dermatol. 2016;152:847.
- Piraccini BM, Balestri R, Starace M, et al. Nail digital dermoscopy (onychoscopy) in the diagnosis of onychomycosis. J Eur Acad Dermatol Venereol. 2013;27:509-513.
- Lipner SR, Scher RK. Onychomycosis: clinical overview and diagnosis. J Am Acad Dermatol. 2019;80:835-851.
- Kim YJ, Han SS, Yang HJ, et al. Prospective, comparative evaluation of a deep neural network and dermoscopy in the diagnosis of onychomycosis. PLoS One. 2020;15:e0234334.
- Lipner SR, Scher RK. Onychomycosis: treatment and prevention of recurrence. J Am Acad Dermatol. 2019;80:853-867.
- Eichenfield LF, Elewski B, Sugarman JL, et al. Efinaconazole 10% topical solution for the treatment of onychomycosis in pediatric patients: open-label phase 4 study [published online July 2, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.06.1004
- Eichenfield LF, Elewski B, Sugarman JL, et al. Safety, pharmacokinetics, and efficacy of efinaconazole 10% topical solution for onychomycosis treatment in pediatric patients. J Drugs Dermatol. 2020;19:867-872.
- Rich P, Spellman M, Purohit V, et al. Tavaborole 5% topical solution for the treatment of toenail onychomycosis in pediatric patients: results from a phase 4 open-label study. J Drugs Dermatol. 2019;18:190-195.
- Watanabe S, Tsubouchi I, Okubo A. Efficacy and safety of fosravuconazole L-lysine ethanolate, a novel oral triazole antifungal agent, for the treatment of onychomycosis: a multicenter, double-blind, randomized phase III study. J Dermatol. 2018;45:1151-1159.
- Noguchi H, Matsumoto T, Kimura U, et al. Fosravuconazole to treat severe onychomycosis in the elderly [published online October 25, 2020]. J Dermatol. doi:10.1111/1346-8138.15651
- Andersson NW, Thomsen SF, Andersen JT. Exposure to terbinafine in pregnancy and risk of preterm birth, small for gestational age, low birth weight, and stillbirth: a nationwide cohort study [published online October 22, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.10.034
- Moreno-Sabater A, Ouali N, Chasset F, et al. Severe onychomycosis management with oral terbinafine in a kidney transplantation setting: clinical follow-up by image analysis [published online November 27, 2020]. Mycoses. doi:10.1111/myc.13220
- Wang Y, Geizhals S, Lipner SR. Retrospective analysis of laboratory abnormalities in patients prescribed terbinafine for onychomycosis. J Am Acad Dermatol. 2021;84:497-499.
- Wang Y, Lipner SR. Retrospective analysis of laboratory abnormalities in pediatric patients prescribed terbinafine for superficial fungal infections [published online January 27, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.01.073
- Wang Y, Lipner SR. Retrospective analysis of laboratory abnormalities in patients with preexisting liver and hematologic diseases prescribed terbinafine for onychomycosis. J Am Acad Dermatol. 2021;84:220-221.
- Lamisil. Prescribing information. Novartis Pharmaceuticals Corporation; 2010. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/022071s003lbl.pdf
Onychomycosis is the most prevalent nail condition worldwide and has a significant impact on quality of life.1 There were 10 million physician visits for nail fungal infections in the National Ambulatory Medical Care Survey from 2007 to 2016, which was more than double the number of all other nail diagnoses combined.2 Therefore, it is important for dermatologists to be familiar with the most current data on diagnosis and treatment of this extremely common nail disease as well as antifungal medication safety.
Onychomycosis Diagnosis
Diagnosis of onychomycosis using clinical examination alone has poor sensitivity and specificity and may lead to progression of disease and unwanted side effects from inappropriate therapy.3,4 Dermoscopy is a useful adjunct but diagnostically is still inferior compared to mycologic testing.5 Classical methods of diagnosis include potassium hydroxide staining with microscopy, fungal culture, and histopathology. Polymerase chain reaction is a newer technique with wide accessibility and excellent sensitivity and specificity.6 Although these techniques have excellent diagnostic accuracy both alone and in combination, the ideal test would have 100% sensitivity and specificity and would not require nail sampling. Artificial intelligence recently has been studied for the diagnosis of onychomycosis. In a prospective study of 90 patients with onychodystrophy who had photographs of the nails taken by nonphysicians, deep neural networks showed comparable sensitivity (70.2% vs 73.0%) and specificity (72.7% vs 49.7%) for diagnosis of onychomycosis vs clinical examination by dermatologists with a mean of 5.6 years of experience.7 Therefore, artificial intelligence may be considered as a supplement to clinical examination for dermatology residents and junior attending dermatologists and may be superior to clinical examination by nondermatologists, but mycologic confirmation is still necessary before initiating onychomycosis treatment.
Treatment of Onychomycosis
There are 3 topical therapies (ciclopirox lacquer 8%, efinaconazole solution 10%, and tavaborole solution 5%) and 3 oral therapies (terbinafine, itraconazole, and griseofulvin) that are approved by the US Food and Drug Administration for onychomycosis therapy. Griseofulvin rarely is used due to the availability of more efficacious treatment options. Fluconazole is an off-label treatment that often is used in the United States.8
There are new data on the efficacy and safety of topical onychomycosis treatments in children. A phase 4 open‐label study of efinaconazole solution 10% applied once daily for 48 weeks was performed in children aged 6 to 16 years with distal lateral subungual onychomycosis (N=62).9,10 The medication was both well tolerated and safe in children. The only treatment-related adverse event was onychocryptosis, which was reported by 2 patients. At week 52, mycologic cure was 65% and complete cure was 40% (N=50). In a pharmacokinetic assessment performed in a subset of 17 patients aged 12 to 16 years, efinaconazole was measured at very low levels in plasma.9
A phase 4 open-label study also was performed to evaluate the safety, pharmacokinetics, and efficacy of tavaborole for treatment of distal lateral subungual onychomycosis in children aged 6 years to under 17 years (N=55).11 Tavaborole solution 5% was applied once daily for 48 weeks; at week 52, mycologic and complete cures were 36.2% and 8.5%, respectively (N=47). Systemic exposure was low (Cmax=5.9 ng/mL [day 29]) in a subset of patients aged 12 years to under 17 years (N=37), and the medication demonstrated good safety and tolerability.11
Fosravuconazole was approved for treatment of onychomycosis in Japan in 2018. In a randomized, double-blind, phase 3 trial of oral fosravuconazole 100 mg once daily (n=101) vs placebo (n=52) for 12 weeks in patients with onychomycosis (mean age, 58.4 years), the complete cure rate at 48 weeks was 59.4%.12 In a small trial of 37 elderly patients (mean age, 78.1 years), complete cure rates were 5.0% in patients with a nail plate thickness of 3 mm or greater and 58.8% in those with a thickness lessthan 3 mm, and there were no severe adverse events.13 In addition to excellent efficacy and proven safety in elderly adults, the main advantage of fosravuconazole is less-potent inhibition of cytochrome P450 3A compared to other triazole antifungals, with no contraindicated drugs listed.
Safety of Antifungals
There are new data describing the safety of oral terbinafine in pregnant women and immunosuppressed patients. In a nationwide cohort study conducted in Denmark (1,650,649 pregnancies [942 oral terbinafine exposed, 9420 unexposed matched cohorts]), there was no association between oral or topical terbinafine exposure during pregnancy and risk of preterm birth, small-for-gestational-age birth weight, low birth weight, or stillbirth.14 In a small study of 13 kidney transplant recipients taking oral tacrolimus, cyclosporine, or everolimus who were treated with oral terbinafine, there were no severe drug interactions and no clinical consequences in renal grafts.15
There also is new information on laboratory abnormalities in adults, children, and patients with comorbidities who are taking oral terbinafine. In a retrospective study of 944 adult patients without pre-existing hepatic or hematologic conditions who were prescribed 3 months of oral terbinafine for onychomycosis, abnormal monitoring liver function tests (LFTs) and complete blood cell counts (CBCs) were uncommon (2.4% and 2.8%, respectively) and mild and resolved after treatment completion. In addition, patients with laboratory abnormalities were an average of 14.8 years older and approximately 3-times more likely to be 65 years or older compared to the overall study population.16 There were similar findings in a retrospective study of 134 children 18 years or younger who were prescribed oral terbinafine for superficial fungal infections. Abnormal monitoring LFTs and CBCs were uncommon (1.7% and 4.4%, respectively) and mild, resolving after after treatment completion.17 Finally, in a study of 255 patients with a pre-existing liver or hematologic condition who were prescribed oral terbinafine for onychomycosis, worsening of LFT or CBC values were rare, and all resolved after treatment completion or medication discontinuation.18
Final Thoughts
Mycologic confirmation is still necessary before treatment despite encouraging data on use of artificial intelligence for diagnosis of onychomycosis. Efinaconazole solution 10% and tavaborole solution 5% have shown good safety, tolerability, and efficacy in children with onychomycosis. Recent data suggest the safety of oral terbinafine in pregnant women and kidney transplant recipients, but these findings must be corroborated before its use in these populations. Fosravuconazole is a promising systemic treatment for onychomycosis with no drug-drug interactions reported to date. While baseline laboratory testing is recommended before prescribing terbinafine, interval laboratory monitoring may not be necessary in healthy adults.19 Prospective studies are necessary to corroborate these findings before formal recommendations can be made for prescribing terbinafine in the special populations discussed above, including children, and for interval laboratory monitoring.
Onychomycosis is the most prevalent nail condition worldwide and has a significant impact on quality of life.1 There were 10 million physician visits for nail fungal infections in the National Ambulatory Medical Care Survey from 2007 to 2016, which was more than double the number of all other nail diagnoses combined.2 Therefore, it is important for dermatologists to be familiar with the most current data on diagnosis and treatment of this extremely common nail disease as well as antifungal medication safety.
Onychomycosis Diagnosis
Diagnosis of onychomycosis using clinical examination alone has poor sensitivity and specificity and may lead to progression of disease and unwanted side effects from inappropriate therapy.3,4 Dermoscopy is a useful adjunct but diagnostically is still inferior compared to mycologic testing.5 Classical methods of diagnosis include potassium hydroxide staining with microscopy, fungal culture, and histopathology. Polymerase chain reaction is a newer technique with wide accessibility and excellent sensitivity and specificity.6 Although these techniques have excellent diagnostic accuracy both alone and in combination, the ideal test would have 100% sensitivity and specificity and would not require nail sampling. Artificial intelligence recently has been studied for the diagnosis of onychomycosis. In a prospective study of 90 patients with onychodystrophy who had photographs of the nails taken by nonphysicians, deep neural networks showed comparable sensitivity (70.2% vs 73.0%) and specificity (72.7% vs 49.7%) for diagnosis of onychomycosis vs clinical examination by dermatologists with a mean of 5.6 years of experience.7 Therefore, artificial intelligence may be considered as a supplement to clinical examination for dermatology residents and junior attending dermatologists and may be superior to clinical examination by nondermatologists, but mycologic confirmation is still necessary before initiating onychomycosis treatment.
Treatment of Onychomycosis
There are 3 topical therapies (ciclopirox lacquer 8%, efinaconazole solution 10%, and tavaborole solution 5%) and 3 oral therapies (terbinafine, itraconazole, and griseofulvin) that are approved by the US Food and Drug Administration for onychomycosis therapy. Griseofulvin rarely is used due to the availability of more efficacious treatment options. Fluconazole is an off-label treatment that often is used in the United States.8
There are new data on the efficacy and safety of topical onychomycosis treatments in children. A phase 4 open‐label study of efinaconazole solution 10% applied once daily for 48 weeks was performed in children aged 6 to 16 years with distal lateral subungual onychomycosis (N=62).9,10 The medication was both well tolerated and safe in children. The only treatment-related adverse event was onychocryptosis, which was reported by 2 patients. At week 52, mycologic cure was 65% and complete cure was 40% (N=50). In a pharmacokinetic assessment performed in a subset of 17 patients aged 12 to 16 years, efinaconazole was measured at very low levels in plasma.9
A phase 4 open-label study also was performed to evaluate the safety, pharmacokinetics, and efficacy of tavaborole for treatment of distal lateral subungual onychomycosis in children aged 6 years to under 17 years (N=55).11 Tavaborole solution 5% was applied once daily for 48 weeks; at week 52, mycologic and complete cures were 36.2% and 8.5%, respectively (N=47). Systemic exposure was low (Cmax=5.9 ng/mL [day 29]) in a subset of patients aged 12 years to under 17 years (N=37), and the medication demonstrated good safety and tolerability.11
Fosravuconazole was approved for treatment of onychomycosis in Japan in 2018. In a randomized, double-blind, phase 3 trial of oral fosravuconazole 100 mg once daily (n=101) vs placebo (n=52) for 12 weeks in patients with onychomycosis (mean age, 58.4 years), the complete cure rate at 48 weeks was 59.4%.12 In a small trial of 37 elderly patients (mean age, 78.1 years), complete cure rates were 5.0% in patients with a nail plate thickness of 3 mm or greater and 58.8% in those with a thickness lessthan 3 mm, and there were no severe adverse events.13 In addition to excellent efficacy and proven safety in elderly adults, the main advantage of fosravuconazole is less-potent inhibition of cytochrome P450 3A compared to other triazole antifungals, with no contraindicated drugs listed.
Safety of Antifungals
There are new data describing the safety of oral terbinafine in pregnant women and immunosuppressed patients. In a nationwide cohort study conducted in Denmark (1,650,649 pregnancies [942 oral terbinafine exposed, 9420 unexposed matched cohorts]), there was no association between oral or topical terbinafine exposure during pregnancy and risk of preterm birth, small-for-gestational-age birth weight, low birth weight, or stillbirth.14 In a small study of 13 kidney transplant recipients taking oral tacrolimus, cyclosporine, or everolimus who were treated with oral terbinafine, there were no severe drug interactions and no clinical consequences in renal grafts.15
There also is new information on laboratory abnormalities in adults, children, and patients with comorbidities who are taking oral terbinafine. In a retrospective study of 944 adult patients without pre-existing hepatic or hematologic conditions who were prescribed 3 months of oral terbinafine for onychomycosis, abnormal monitoring liver function tests (LFTs) and complete blood cell counts (CBCs) were uncommon (2.4% and 2.8%, respectively) and mild and resolved after treatment completion. In addition, patients with laboratory abnormalities were an average of 14.8 years older and approximately 3-times more likely to be 65 years or older compared to the overall study population.16 There were similar findings in a retrospective study of 134 children 18 years or younger who were prescribed oral terbinafine for superficial fungal infections. Abnormal monitoring LFTs and CBCs were uncommon (1.7% and 4.4%, respectively) and mild, resolving after after treatment completion.17 Finally, in a study of 255 patients with a pre-existing liver or hematologic condition who were prescribed oral terbinafine for onychomycosis, worsening of LFT or CBC values were rare, and all resolved after treatment completion or medication discontinuation.18
Final Thoughts
Mycologic confirmation is still necessary before treatment despite encouraging data on use of artificial intelligence for diagnosis of onychomycosis. Efinaconazole solution 10% and tavaborole solution 5% have shown good safety, tolerability, and efficacy in children with onychomycosis. Recent data suggest the safety of oral terbinafine in pregnant women and kidney transplant recipients, but these findings must be corroborated before its use in these populations. Fosravuconazole is a promising systemic treatment for onychomycosis with no drug-drug interactions reported to date. While baseline laboratory testing is recommended before prescribing terbinafine, interval laboratory monitoring may not be necessary in healthy adults.19 Prospective studies are necessary to corroborate these findings before formal recommendations can be made for prescribing terbinafine in the special populations discussed above, including children, and for interval laboratory monitoring.
- Stewart CR, Algu L, Kamran R, et al. Effect of onychomycosis and treatment on patient-reported quality-of-life outcomes: a systematic review [published online June 2, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.05.143
- Lipner SR, Hancock JE, Fleischer AB. The ambulatory care burden of nail conditions in the United States [published online October 21, 2019]. J Dermatolog Treat. doi:10.1080/09546634.2019.1679337
- Lipner SR, Scher RK. Onychomycosis--a small step for quality of care. Curr Med Res Opin. 2016;32:865-867.
- Lipner SR, Scher RK. Confirmatory testing for onychomycosis. JAMA Dermatol. 2016;152:847.
- Piraccini BM, Balestri R, Starace M, et al. Nail digital dermoscopy (onychoscopy) in the diagnosis of onychomycosis. J Eur Acad Dermatol Venereol. 2013;27:509-513.
- Lipner SR, Scher RK. Onychomycosis: clinical overview and diagnosis. J Am Acad Dermatol. 2019;80:835-851.
- Kim YJ, Han SS, Yang HJ, et al. Prospective, comparative evaluation of a deep neural network and dermoscopy in the diagnosis of onychomycosis. PLoS One. 2020;15:e0234334.
- Lipner SR, Scher RK. Onychomycosis: treatment and prevention of recurrence. J Am Acad Dermatol. 2019;80:853-867.
- Eichenfield LF, Elewski B, Sugarman JL, et al. Efinaconazole 10% topical solution for the treatment of onychomycosis in pediatric patients: open-label phase 4 study [published online July 2, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.06.1004
- Eichenfield LF, Elewski B, Sugarman JL, et al. Safety, pharmacokinetics, and efficacy of efinaconazole 10% topical solution for onychomycosis treatment in pediatric patients. J Drugs Dermatol. 2020;19:867-872.
- Rich P, Spellman M, Purohit V, et al. Tavaborole 5% topical solution for the treatment of toenail onychomycosis in pediatric patients: results from a phase 4 open-label study. J Drugs Dermatol. 2019;18:190-195.
- Watanabe S, Tsubouchi I, Okubo A. Efficacy and safety of fosravuconazole L-lysine ethanolate, a novel oral triazole antifungal agent, for the treatment of onychomycosis: a multicenter, double-blind, randomized phase III study. J Dermatol. 2018;45:1151-1159.
- Noguchi H, Matsumoto T, Kimura U, et al. Fosravuconazole to treat severe onychomycosis in the elderly [published online October 25, 2020]. J Dermatol. doi:10.1111/1346-8138.15651
- Andersson NW, Thomsen SF, Andersen JT. Exposure to terbinafine in pregnancy and risk of preterm birth, small for gestational age, low birth weight, and stillbirth: a nationwide cohort study [published online October 22, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.10.034
- Moreno-Sabater A, Ouali N, Chasset F, et al. Severe onychomycosis management with oral terbinafine in a kidney transplantation setting: clinical follow-up by image analysis [published online November 27, 2020]. Mycoses. doi:10.1111/myc.13220
- Wang Y, Geizhals S, Lipner SR. Retrospective analysis of laboratory abnormalities in patients prescribed terbinafine for onychomycosis. J Am Acad Dermatol. 2021;84:497-499.
- Wang Y, Lipner SR. Retrospective analysis of laboratory abnormalities in pediatric patients prescribed terbinafine for superficial fungal infections [published online January 27, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.01.073
- Wang Y, Lipner SR. Retrospective analysis of laboratory abnormalities in patients with preexisting liver and hematologic diseases prescribed terbinafine for onychomycosis. J Am Acad Dermatol. 2021;84:220-221.
- Lamisil. Prescribing information. Novartis Pharmaceuticals Corporation; 2010. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/022071s003lbl.pdf
- Stewart CR, Algu L, Kamran R, et al. Effect of onychomycosis and treatment on patient-reported quality-of-life outcomes: a systematic review [published online June 2, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.05.143
- Lipner SR, Hancock JE, Fleischer AB. The ambulatory care burden of nail conditions in the United States [published online October 21, 2019]. J Dermatolog Treat. doi:10.1080/09546634.2019.1679337
- Lipner SR, Scher RK. Onychomycosis--a small step for quality of care. Curr Med Res Opin. 2016;32:865-867.
- Lipner SR, Scher RK. Confirmatory testing for onychomycosis. JAMA Dermatol. 2016;152:847.
- Piraccini BM, Balestri R, Starace M, et al. Nail digital dermoscopy (onychoscopy) in the diagnosis of onychomycosis. J Eur Acad Dermatol Venereol. 2013;27:509-513.
- Lipner SR, Scher RK. Onychomycosis: clinical overview and diagnosis. J Am Acad Dermatol. 2019;80:835-851.
- Kim YJ, Han SS, Yang HJ, et al. Prospective, comparative evaluation of a deep neural network and dermoscopy in the diagnosis of onychomycosis. PLoS One. 2020;15:e0234334.
- Lipner SR, Scher RK. Onychomycosis: treatment and prevention of recurrence. J Am Acad Dermatol. 2019;80:853-867.
- Eichenfield LF, Elewski B, Sugarman JL, et al. Efinaconazole 10% topical solution for the treatment of onychomycosis in pediatric patients: open-label phase 4 study [published online July 2, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.06.1004
- Eichenfield LF, Elewski B, Sugarman JL, et al. Safety, pharmacokinetics, and efficacy of efinaconazole 10% topical solution for onychomycosis treatment in pediatric patients. J Drugs Dermatol. 2020;19:867-872.
- Rich P, Spellman M, Purohit V, et al. Tavaborole 5% topical solution for the treatment of toenail onychomycosis in pediatric patients: results from a phase 4 open-label study. J Drugs Dermatol. 2019;18:190-195.
- Watanabe S, Tsubouchi I, Okubo A. Efficacy and safety of fosravuconazole L-lysine ethanolate, a novel oral triazole antifungal agent, for the treatment of onychomycosis: a multicenter, double-blind, randomized phase III study. J Dermatol. 2018;45:1151-1159.
- Noguchi H, Matsumoto T, Kimura U, et al. Fosravuconazole to treat severe onychomycosis in the elderly [published online October 25, 2020]. J Dermatol. doi:10.1111/1346-8138.15651
- Andersson NW, Thomsen SF, Andersen JT. Exposure to terbinafine in pregnancy and risk of preterm birth, small for gestational age, low birth weight, and stillbirth: a nationwide cohort study [published online October 22, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.10.034
- Moreno-Sabater A, Ouali N, Chasset F, et al. Severe onychomycosis management with oral terbinafine in a kidney transplantation setting: clinical follow-up by image analysis [published online November 27, 2020]. Mycoses. doi:10.1111/myc.13220
- Wang Y, Geizhals S, Lipner SR. Retrospective analysis of laboratory abnormalities in patients prescribed terbinafine for onychomycosis. J Am Acad Dermatol. 2021;84:497-499.
- Wang Y, Lipner SR. Retrospective analysis of laboratory abnormalities in pediatric patients prescribed terbinafine for superficial fungal infections [published online January 27, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.01.073
- Wang Y, Lipner SR. Retrospective analysis of laboratory abnormalities in patients with preexisting liver and hematologic diseases prescribed terbinafine for onychomycosis. J Am Acad Dermatol. 2021;84:220-221.
- Lamisil. Prescribing information. Novartis Pharmaceuticals Corporation; 2010. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/022071s003lbl.pdf