User login
More tools for the COVID toolbox
I was recently asked to see a 16-year-old, unvaccinated (against COVID-19) adolescent with hypothyroidism and obesity (body mass index 37 kg/m2) seen in the pediatric emergency department with tachycardia, O2 saturation 96%, urinary tract infection, poor appetite, and nausea. Her chest x-ray had low lung volumes but no infiltrates. She was noted to be dehydrated. Testing for COVID-19 was PCR positive.1
She was observed overnight, tolerated oral rehydration, and was being readied for discharge. Pediatric Infectious Diseases was called about prescribing remdesivir.
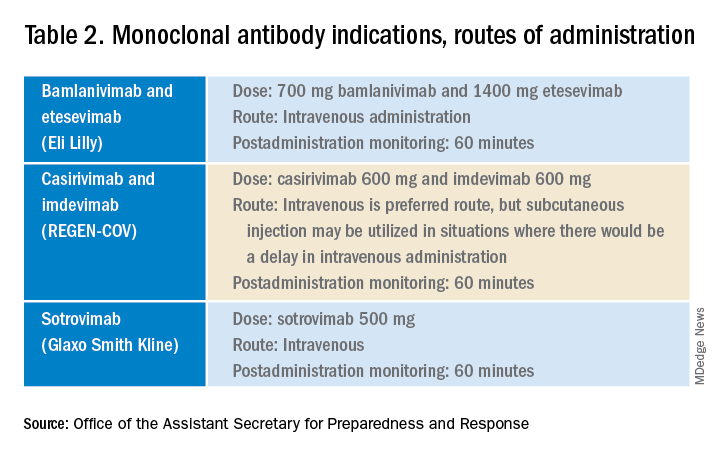
Remdesivir was not indicated as its current use is limited to inpatients with oxygen desaturations less than 94%. Infectious Diseases Society of America guidelines do recommend the use of monoclonal antibodies against the SARS-CoV-2 spike protein for prevention of COVID disease progression in high-risk individuals. Specifically, the IDSA guidelines say, “Among ambulatory patients with mild to moderate COVID-19 at high risk for progression to severe disease, bamlanivimab/etesevimab, casirivimab/imdevimab, or sotrovimab rather than no neutralizing antibody treatment.”
The Food and Drug Administration’s Emergency Use Authorization (EUA) allowed use of specific monoclonal antibodies (casirivimab/imdevimab in combination, bamlanivimab/etesevimab in combination, and sotrovimab alone) for individuals 12 years and above with a minimum weight of 40 kg with high-risk conditions, describing the evidence as moderate certainty.2
Several questions have arisen regarding their use. Which children qualify under the EUA? Are the available monoclonal antibodies effective for SARS-CoV-2 variants? What adverse events were observed? Are there implementation hurdles?
Unlike the EUA for prophylactic use, which targeted unvaccinated individuals and those unlikely to have a good antibody response to vaccine, use of monoclonal antibody for prevention of progression does not have such restrictions. Effectiveness may vary by local variant susceptibility and should be considered in the choice of the most appropriate monoclonal antibody therapy. Reductions in hospitalization and progression to critical disease status were reported from phase 3 studies; reductions were also observed in mortality in some, but not all, studies. Enhanced viral clearance on day 7 was observed with few subjects having persistent high viral load.
Which children qualify under the EUA? Adolescents 12 years and older and over 40 kg are eligible if a high risk condition is present. High-risk conditions include body mass index at the 85th percentile or higher, immunosuppressive disease, or receipt of immunosuppressive therapies, or baseline (pre-COVID infection) medical-related technological dependence such as tracheostomy or positive pressure ventilation. Additional high-risk conditions are neurodevelopmental disorders, sickle cell disease, congenital or acquired heart disease, asthma, or reactive airway or other chronic respiratory disease that requires daily medication for control, diabetes, chronic kidney disease, or pregnancy.3
Are the available monoclonal antibodies effective for SARS-CoV-2 variants? Of course, this is a critical question and relies on knowledge of the dominant variant in a specific geographic location. The CDC data on which variants are susceptible to which monoclonal therapies were updated as of Oct. 21 online (see Table 1). Local departments of public health often will have current data on the dominant variant in the community. Currently, the dominant variant in the United States is Delta and it is anticipated to be susceptible to the three monoclonal treatments authorized under the EUA based on in vitro neutralizing assays.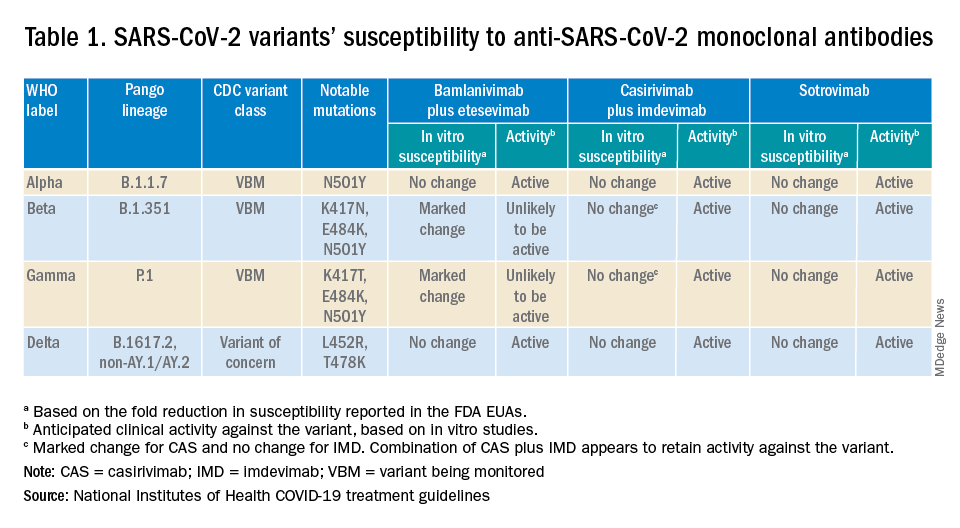
What adverse events were observed? Monoclonal antibody infusions are in general safe but anaphylaxis has been reported. Other infusion-related adverse events include urticaria, pruritis, flushing, pyrexia, shortness of breath, chest tightness, nausea, vomiting, and rash. Nearly all events were grade 1, mild, or grade 2, moderate. For nonsevere infusion-related reactions, consider slowing the infusion; if necessary, the infusion should be stopped.
Implementation challenges
The first challenge is finding a location to infuse the monoclonal antibodies. Although they can be given subcutaneously, the dose is large and little, if any, time is saved as the recommendation is for observation post administration for 1 hour. The challenge we and other centers may face is that the patients are COVID PCR+ and therefore our usual infusion program, which often is occupied by individuals already compromised and at high risk for severe COVID, is an undesirable location. We are planning to use the emergency department to accommodate such patients currently, but even that solution creates challenges for a busy, urban medical center.
Summary
Anti–SARS-CoV-2 monoclonal antibodies are an important part of the therapeutic approach to minimizing disease severity. Clinicians should review high-risk conditions in adolescents who are PCR+ for SARS-CoV-2 and have mild to moderate symptoms. Medical care systems should implement programs to make monoclonal infusions available for such high-risk adolescents.4 Obesity and asthma reactive airways or requiring daily medication for control are the two most common conditions that place adolescents with COVID-19 at risk for progression to hospitalization and severe disease in addition to the more traditional immune-compromising conditions and medical fragility.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University schools of medicine and public health and senior attending physician in pediatric infectious diseases, Boston Medical Center. Email him at [email protected].
References
1. Federal Response to COVID-19: Monoclonal Antibody Clinical Implementation Guide. U.S. Department of Health and Human Services. 2021 Sep 2.
2. Bhimraj A et al. IDSA Guidelines on the Treatment and Management of Patients with COVID-19. Last updated 2021 Nov 9.
3. Anti-SARS-CoV-2 Monoclonal Antibodies. National Institutes of Health’s COVID 19 Treatment Guidelines. Last updated 2021 Oct 19.
4. Spreading the Word on the Benefits of Monoclonal Antibodies for COVID-19, by Hannah R. Buchdahl. CDC Foundation, 2021 Jul 2.
I was recently asked to see a 16-year-old, unvaccinated (against COVID-19) adolescent with hypothyroidism and obesity (body mass index 37 kg/m2) seen in the pediatric emergency department with tachycardia, O2 saturation 96%, urinary tract infection, poor appetite, and nausea. Her chest x-ray had low lung volumes but no infiltrates. She was noted to be dehydrated. Testing for COVID-19 was PCR positive.1
She was observed overnight, tolerated oral rehydration, and was being readied for discharge. Pediatric Infectious Diseases was called about prescribing remdesivir.
Remdesivir was not indicated as its current use is limited to inpatients with oxygen desaturations less than 94%. Infectious Diseases Society of America guidelines do recommend the use of monoclonal antibodies against the SARS-CoV-2 spike protein for prevention of COVID disease progression in high-risk individuals. Specifically, the IDSA guidelines say, “Among ambulatory patients with mild to moderate COVID-19 at high risk for progression to severe disease, bamlanivimab/etesevimab, casirivimab/imdevimab, or sotrovimab rather than no neutralizing antibody treatment.”
The Food and Drug Administration’s Emergency Use Authorization (EUA) allowed use of specific monoclonal antibodies (casirivimab/imdevimab in combination, bamlanivimab/etesevimab in combination, and sotrovimab alone) for individuals 12 years and above with a minimum weight of 40 kg with high-risk conditions, describing the evidence as moderate certainty.2
Several questions have arisen regarding their use. Which children qualify under the EUA? Are the available monoclonal antibodies effective for SARS-CoV-2 variants? What adverse events were observed? Are there implementation hurdles?
Unlike the EUA for prophylactic use, which targeted unvaccinated individuals and those unlikely to have a good antibody response to vaccine, use of monoclonal antibody for prevention of progression does not have such restrictions. Effectiveness may vary by local variant susceptibility and should be considered in the choice of the most appropriate monoclonal antibody therapy. Reductions in hospitalization and progression to critical disease status were reported from phase 3 studies; reductions were also observed in mortality in some, but not all, studies. Enhanced viral clearance on day 7 was observed with few subjects having persistent high viral load.
Which children qualify under the EUA? Adolescents 12 years and older and over 40 kg are eligible if a high risk condition is present. High-risk conditions include body mass index at the 85th percentile or higher, immunosuppressive disease, or receipt of immunosuppressive therapies, or baseline (pre-COVID infection) medical-related technological dependence such as tracheostomy or positive pressure ventilation. Additional high-risk conditions are neurodevelopmental disorders, sickle cell disease, congenital or acquired heart disease, asthma, or reactive airway or other chronic respiratory disease that requires daily medication for control, diabetes, chronic kidney disease, or pregnancy.3
Are the available monoclonal antibodies effective for SARS-CoV-2 variants? Of course, this is a critical question and relies on knowledge of the dominant variant in a specific geographic location. The CDC data on which variants are susceptible to which monoclonal therapies were updated as of Oct. 21 online (see Table 1). Local departments of public health often will have current data on the dominant variant in the community. Currently, the dominant variant in the United States is Delta and it is anticipated to be susceptible to the three monoclonal treatments authorized under the EUA based on in vitro neutralizing assays.
What adverse events were observed? Monoclonal antibody infusions are in general safe but anaphylaxis has been reported. Other infusion-related adverse events include urticaria, pruritis, flushing, pyrexia, shortness of breath, chest tightness, nausea, vomiting, and rash. Nearly all events were grade 1, mild, or grade 2, moderate. For nonsevere infusion-related reactions, consider slowing the infusion; if necessary, the infusion should be stopped.
Implementation challenges
The first challenge is finding a location to infuse the monoclonal antibodies. Although they can be given subcutaneously, the dose is large and little, if any, time is saved as the recommendation is for observation post administration for 1 hour. The challenge we and other centers may face is that the patients are COVID PCR+ and therefore our usual infusion program, which often is occupied by individuals already compromised and at high risk for severe COVID, is an undesirable location. We are planning to use the emergency department to accommodate such patients currently, but even that solution creates challenges for a busy, urban medical center.

Summary
Anti–SARS-CoV-2 monoclonal antibodies are an important part of the therapeutic approach to minimizing disease severity. Clinicians should review high-risk conditions in adolescents who are PCR+ for SARS-CoV-2 and have mild to moderate symptoms. Medical care systems should implement programs to make monoclonal infusions available for such high-risk adolescents.4 Obesity and asthma reactive airways or requiring daily medication for control are the two most common conditions that place adolescents with COVID-19 at risk for progression to hospitalization and severe disease in addition to the more traditional immune-compromising conditions and medical fragility.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University schools of medicine and public health and senior attending physician in pediatric infectious diseases, Boston Medical Center. Email him at [email protected].
References
1. Federal Response to COVID-19: Monoclonal Antibody Clinical Implementation Guide. U.S. Department of Health and Human Services. 2021 Sep 2.
2. Bhimraj A et al. IDSA Guidelines on the Treatment and Management of Patients with COVID-19. Last updated 2021 Nov 9.
3. Anti-SARS-CoV-2 Monoclonal Antibodies. National Institutes of Health’s COVID 19 Treatment Guidelines. Last updated 2021 Oct 19.
4. Spreading the Word on the Benefits of Monoclonal Antibodies for COVID-19, by Hannah R. Buchdahl. CDC Foundation, 2021 Jul 2.
I was recently asked to see a 16-year-old, unvaccinated (against COVID-19) adolescent with hypothyroidism and obesity (body mass index 37 kg/m2) seen in the pediatric emergency department with tachycardia, O2 saturation 96%, urinary tract infection, poor appetite, and nausea. Her chest x-ray had low lung volumes but no infiltrates. She was noted to be dehydrated. Testing for COVID-19 was PCR positive.1
She was observed overnight, tolerated oral rehydration, and was being readied for discharge. Pediatric Infectious Diseases was called about prescribing remdesivir.
Remdesivir was not indicated as its current use is limited to inpatients with oxygen desaturations less than 94%. Infectious Diseases Society of America guidelines do recommend the use of monoclonal antibodies against the SARS-CoV-2 spike protein for prevention of COVID disease progression in high-risk individuals. Specifically, the IDSA guidelines say, “Among ambulatory patients with mild to moderate COVID-19 at high risk for progression to severe disease, bamlanivimab/etesevimab, casirivimab/imdevimab, or sotrovimab rather than no neutralizing antibody treatment.”
The Food and Drug Administration’s Emergency Use Authorization (EUA) allowed use of specific monoclonal antibodies (casirivimab/imdevimab in combination, bamlanivimab/etesevimab in combination, and sotrovimab alone) for individuals 12 years and above with a minimum weight of 40 kg with high-risk conditions, describing the evidence as moderate certainty.2
Several questions have arisen regarding their use. Which children qualify under the EUA? Are the available monoclonal antibodies effective for SARS-CoV-2 variants? What adverse events were observed? Are there implementation hurdles?
Unlike the EUA for prophylactic use, which targeted unvaccinated individuals and those unlikely to have a good antibody response to vaccine, use of monoclonal antibody for prevention of progression does not have such restrictions. Effectiveness may vary by local variant susceptibility and should be considered in the choice of the most appropriate monoclonal antibody therapy. Reductions in hospitalization and progression to critical disease status were reported from phase 3 studies; reductions were also observed in mortality in some, but not all, studies. Enhanced viral clearance on day 7 was observed with few subjects having persistent high viral load.
Which children qualify under the EUA? Adolescents 12 years and older and over 40 kg are eligible if a high risk condition is present. High-risk conditions include body mass index at the 85th percentile or higher, immunosuppressive disease, or receipt of immunosuppressive therapies, or baseline (pre-COVID infection) medical-related technological dependence such as tracheostomy or positive pressure ventilation. Additional high-risk conditions are neurodevelopmental disorders, sickle cell disease, congenital or acquired heart disease, asthma, or reactive airway or other chronic respiratory disease that requires daily medication for control, diabetes, chronic kidney disease, or pregnancy.3
Are the available monoclonal antibodies effective for SARS-CoV-2 variants? Of course, this is a critical question and relies on knowledge of the dominant variant in a specific geographic location. The CDC data on which variants are susceptible to which monoclonal therapies were updated as of Oct. 21 online (see Table 1). Local departments of public health often will have current data on the dominant variant in the community. Currently, the dominant variant in the United States is Delta and it is anticipated to be susceptible to the three monoclonal treatments authorized under the EUA based on in vitro neutralizing assays.
What adverse events were observed? Monoclonal antibody infusions are in general safe but anaphylaxis has been reported. Other infusion-related adverse events include urticaria, pruritis, flushing, pyrexia, shortness of breath, chest tightness, nausea, vomiting, and rash. Nearly all events were grade 1, mild, or grade 2, moderate. For nonsevere infusion-related reactions, consider slowing the infusion; if necessary, the infusion should be stopped.
Implementation challenges
The first challenge is finding a location to infuse the monoclonal antibodies. Although they can be given subcutaneously, the dose is large and little, if any, time is saved as the recommendation is for observation post administration for 1 hour. The challenge we and other centers may face is that the patients are COVID PCR+ and therefore our usual infusion program, which often is occupied by individuals already compromised and at high risk for severe COVID, is an undesirable location. We are planning to use the emergency department to accommodate such patients currently, but even that solution creates challenges for a busy, urban medical center.

Summary
Anti–SARS-CoV-2 monoclonal antibodies are an important part of the therapeutic approach to minimizing disease severity. Clinicians should review high-risk conditions in adolescents who are PCR+ for SARS-CoV-2 and have mild to moderate symptoms. Medical care systems should implement programs to make monoclonal infusions available for such high-risk adolescents.4 Obesity and asthma reactive airways or requiring daily medication for control are the two most common conditions that place adolescents with COVID-19 at risk for progression to hospitalization and severe disease in addition to the more traditional immune-compromising conditions and medical fragility.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University schools of medicine and public health and senior attending physician in pediatric infectious diseases, Boston Medical Center. Email him at [email protected].
References
1. Federal Response to COVID-19: Monoclonal Antibody Clinical Implementation Guide. U.S. Department of Health and Human Services. 2021 Sep 2.
2. Bhimraj A et al. IDSA Guidelines on the Treatment and Management of Patients with COVID-19. Last updated 2021 Nov 9.
3. Anti-SARS-CoV-2 Monoclonal Antibodies. National Institutes of Health’s COVID 19 Treatment Guidelines. Last updated 2021 Oct 19.
4. Spreading the Word on the Benefits of Monoclonal Antibodies for COVID-19, by Hannah R. Buchdahl. CDC Foundation, 2021 Jul 2.
COVID-19 in children and adolescents: Disease burden and severity
My first thought on this column was maybe Pediatric News has written sufficiently about SARS-CoV-2 infection, and it is time to move on. However, the agenda for the May 12th Advisory Committee on Immunization Practice includes a review of the Pfizer-BioNTech COVID-19 vaccine safety and immunogenicity data for the 12- to 15-year-old age cohort that suggests the potential for vaccine availability and roll out for early adolescents in the near future and the need for up-to-date knowledge about the incidence, severity, and long-term outcome of COVID-19 in the pediatric population.
Updating and summarizing the pediatric experience for the pediatric community on what children and adolescents have experienced because of SARS-CoV-2 infection is critical to address the myriad of questions that will come from colleagues, parents, and adolescents themselves. A great resource, published weekly, is the joint report from the American Academy of Pediatrics and the Children’s Hospital Association.1 As of April 29, 2021, 3,782,724 total child COVID-19 cases have been reported from 49 states, New York City (NYC), the District of Columbia, Guam, and Puerto Rico. Children represent approximately 14% of cases in the United States and not surprisingly are an increasing proportion of total cases as vaccine impact reduces cases among older age groups. Nearly 5% of the pediatric population has already been infected with SARS-CoV-2. Fortunately, compared with adults, hospitalization, severe disease, and mortality remain far lower both in number and proportion than in the adult population. Cumulative hospitalizations from 24 states and NYC total 15,456 (0.8%) among those infected, with 303 deaths reported (from 43 states, NYC, Guam, and Puerto Rico). Case fatality rate approximates 0.01% in the most recent summary of state reports. One of the limitations of this report is that each state decides how to report the age distribution of COVID-19 cases resulting in variation in age range; another is the data are limited to those details individual states chose to make publicly available.
Although children do not commonly develop severe disease, and the case fatality is low, there are still insights to be learned from understanding risk features for severe disease. Preston et al. reviewed discharge data from 869 medical facilities to describe patients 18 years or younger who had an inpatient or emergency department encounter with a primary or secondary COVID-19 discharge diagnosis from March 1 through October 31, 2020.2 They reported that approximately 2,430 (11.7%) children were hospitalized and 746, nearly 31% of those hospitalized, had severe COVID disease. Those at greatest risk for severe disease were children with comorbid conditions and those less than 12 years, compared with the 12- to 18-year age group. They did not identify race as a risk for severe disease in this study. Moreira et al. described risk factors for morbidity and death from COVID in children less than 18 years of age3 using CDC COVID-NET, the Centers for Disease Control and Prevention COVID-19–associated hospitalization surveillance network. They reported a hospitalization rate of 4.7% among 27,045 cases. They identified three risk factors for hospitalization – age, race/ethnicity, and comorbid conditions. Thirty-nine children (0.19%) died; children who were black, non-Hispanic, and those with an underlying medical condition had a significantly increased risk of death. Thirty-three (85%) children who died had a comorbidity, and 27 (69%) were African American or Hispanic/Latino. The U.S. experience in children is also consistent with reports from the United Kingdom, Italy, Spain, Germany, France, and South Korea.4 Deaths from COVID-19 were uncommon but relatively more frequent in older children, compared with younger age groups among children less than 18 years of age in these countries.
Acute COVID-19 and multisystem inflammatory syndrome in children (MIS-C) do not predominantly target the neurologic systems; however, neurologic complications have been reported, some of which appear to result in long-lasting disability. LaRovere et al. identified 354 (22%) of 1,695 patients less than 21 years of age with acute COVID or MIS-C who had neurologic signs or symptoms during their illness. Among those with neurologic involvement, most children had prior neurologic deficits, mild symptoms, that resolved by the time of discharge. Forty-three (12%) were considered life threatening and included severe encephalopathy, stroke, central nervous system infection/demyelination, Guillain-Barre syndrome or variant, or acute cerebral edema. Several children, including some who were previously healthy prior to COVID, had persistent neurologic deficits at discharge. In addition to neurologic morbidity, long COVID – a syndrome of persistent symptoms following acute COVID that lasts for more than 12 weeks without alternative diagnosis – has also been described in children. Buonsenso et al. assessed 129 children diagnosed with COVID-19 between March and November 2020 in Rome, Italy.5 Persisting symptoms after 120 days were reported by more than 50%. Symptoms like fatigue, muscle and joint pain, headache, insomnia, respiratory problems, and palpitations were most common. Clearly, further follow-up of the long-term outcomes is necessary to understand the full spectrum of morbidity resulting from COVID-19 disease in children and its natural history.
The current picture of COVID infection in children younger than 18 reinforces that children are part of the pandemic. Although deaths in children have now exceeded 300 cases, severe disease remains uncommon in both the United States and western Europe. Risk factors for severe disease include comorbid illness and race/ethnicity with a disproportionate number of severe cases in children with underlying comorbidity and in African American and Hispanic/Latino children. Ongoing surveillance is critical as changes are likely to be observed over time as viral evolution affects disease burden and characteristics.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University schools of medicine and public health and senior attending physician in pediatric infectious diseases, Boston Medical Center. Email him at [email protected].
References
1. Children and COVID-19: State-Level Data Report. Services AAP.org.
2. Preston LE et al. JAMA Network Open. 2021;4(4):e215298. doi:10.1001/jamanetworkopen.2021.5298
3. Moreira A et al. Eur J Pediatr. 2021;180:1659-63.
4. SS Bhopal et al. Lancet 2021. doi: 10.1016/ S2352-4642(21)00066-3.
5. Buonsenso D et al. medRxiv preprint. doi: 10.1101/2021.01.23.21250375.
My first thought on this column was maybe Pediatric News has written sufficiently about SARS-CoV-2 infection, and it is time to move on. However, the agenda for the May 12th Advisory Committee on Immunization Practice includes a review of the Pfizer-BioNTech COVID-19 vaccine safety and immunogenicity data for the 12- to 15-year-old age cohort that suggests the potential for vaccine availability and roll out for early adolescents in the near future and the need for up-to-date knowledge about the incidence, severity, and long-term outcome of COVID-19 in the pediatric population.
Updating and summarizing the pediatric experience for the pediatric community on what children and adolescents have experienced because of SARS-CoV-2 infection is critical to address the myriad of questions that will come from colleagues, parents, and adolescents themselves. A great resource, published weekly, is the joint report from the American Academy of Pediatrics and the Children’s Hospital Association.1 As of April 29, 2021, 3,782,724 total child COVID-19 cases have been reported from 49 states, New York City (NYC), the District of Columbia, Guam, and Puerto Rico. Children represent approximately 14% of cases in the United States and not surprisingly are an increasing proportion of total cases as vaccine impact reduces cases among older age groups. Nearly 5% of the pediatric population has already been infected with SARS-CoV-2. Fortunately, compared with adults, hospitalization, severe disease, and mortality remain far lower both in number and proportion than in the adult population. Cumulative hospitalizations from 24 states and NYC total 15,456 (0.8%) among those infected, with 303 deaths reported (from 43 states, NYC, Guam, and Puerto Rico). Case fatality rate approximates 0.01% in the most recent summary of state reports. One of the limitations of this report is that each state decides how to report the age distribution of COVID-19 cases resulting in variation in age range; another is the data are limited to those details individual states chose to make publicly available.
Although children do not commonly develop severe disease, and the case fatality is low, there are still insights to be learned from understanding risk features for severe disease. Preston et al. reviewed discharge data from 869 medical facilities to describe patients 18 years or younger who had an inpatient or emergency department encounter with a primary or secondary COVID-19 discharge diagnosis from March 1 through October 31, 2020.2 They reported that approximately 2,430 (11.7%) children were hospitalized and 746, nearly 31% of those hospitalized, had severe COVID disease. Those at greatest risk for severe disease were children with comorbid conditions and those less than 12 years, compared with the 12- to 18-year age group. They did not identify race as a risk for severe disease in this study. Moreira et al. described risk factors for morbidity and death from COVID in children less than 18 years of age3 using CDC COVID-NET, the Centers for Disease Control and Prevention COVID-19–associated hospitalization surveillance network. They reported a hospitalization rate of 4.7% among 27,045 cases. They identified three risk factors for hospitalization – age, race/ethnicity, and comorbid conditions. Thirty-nine children (0.19%) died; children who were black, non-Hispanic, and those with an underlying medical condition had a significantly increased risk of death. Thirty-three (85%) children who died had a comorbidity, and 27 (69%) were African American or Hispanic/Latino. The U.S. experience in children is also consistent with reports from the United Kingdom, Italy, Spain, Germany, France, and South Korea.4 Deaths from COVID-19 were uncommon but relatively more frequent in older children, compared with younger age groups among children less than 18 years of age in these countries.
Acute COVID-19 and multisystem inflammatory syndrome in children (MIS-C) do not predominantly target the neurologic systems; however, neurologic complications have been reported, some of which appear to result in long-lasting disability. LaRovere et al. identified 354 (22%) of 1,695 patients less than 21 years of age with acute COVID or MIS-C who had neurologic signs or symptoms during their illness. Among those with neurologic involvement, most children had prior neurologic deficits, mild symptoms, that resolved by the time of discharge. Forty-three (12%) were considered life threatening and included severe encephalopathy, stroke, central nervous system infection/demyelination, Guillain-Barre syndrome or variant, or acute cerebral edema. Several children, including some who were previously healthy prior to COVID, had persistent neurologic deficits at discharge. In addition to neurologic morbidity, long COVID – a syndrome of persistent symptoms following acute COVID that lasts for more than 12 weeks without alternative diagnosis – has also been described in children. Buonsenso et al. assessed 129 children diagnosed with COVID-19 between March and November 2020 in Rome, Italy.5 Persisting symptoms after 120 days were reported by more than 50%. Symptoms like fatigue, muscle and joint pain, headache, insomnia, respiratory problems, and palpitations were most common. Clearly, further follow-up of the long-term outcomes is necessary to understand the full spectrum of morbidity resulting from COVID-19 disease in children and its natural history.
The current picture of COVID infection in children younger than 18 reinforces that children are part of the pandemic. Although deaths in children have now exceeded 300 cases, severe disease remains uncommon in both the United States and western Europe. Risk factors for severe disease include comorbid illness and race/ethnicity with a disproportionate number of severe cases in children with underlying comorbidity and in African American and Hispanic/Latino children. Ongoing surveillance is critical as changes are likely to be observed over time as viral evolution affects disease burden and characteristics.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University schools of medicine and public health and senior attending physician in pediatric infectious diseases, Boston Medical Center. Email him at [email protected].
References
1. Children and COVID-19: State-Level Data Report. Services AAP.org.
2. Preston LE et al. JAMA Network Open. 2021;4(4):e215298. doi:10.1001/jamanetworkopen.2021.5298
3. Moreira A et al. Eur J Pediatr. 2021;180:1659-63.
4. SS Bhopal et al. Lancet 2021. doi: 10.1016/ S2352-4642(21)00066-3.
5. Buonsenso D et al. medRxiv preprint. doi: 10.1101/2021.01.23.21250375.
My first thought on this column was maybe Pediatric News has written sufficiently about SARS-CoV-2 infection, and it is time to move on. However, the agenda for the May 12th Advisory Committee on Immunization Practice includes a review of the Pfizer-BioNTech COVID-19 vaccine safety and immunogenicity data for the 12- to 15-year-old age cohort that suggests the potential for vaccine availability and roll out for early adolescents in the near future and the need for up-to-date knowledge about the incidence, severity, and long-term outcome of COVID-19 in the pediatric population.
Updating and summarizing the pediatric experience for the pediatric community on what children and adolescents have experienced because of SARS-CoV-2 infection is critical to address the myriad of questions that will come from colleagues, parents, and adolescents themselves. A great resource, published weekly, is the joint report from the American Academy of Pediatrics and the Children’s Hospital Association.1 As of April 29, 2021, 3,782,724 total child COVID-19 cases have been reported from 49 states, New York City (NYC), the District of Columbia, Guam, and Puerto Rico. Children represent approximately 14% of cases in the United States and not surprisingly are an increasing proportion of total cases as vaccine impact reduces cases among older age groups. Nearly 5% of the pediatric population has already been infected with SARS-CoV-2. Fortunately, compared with adults, hospitalization, severe disease, and mortality remain far lower both in number and proportion than in the adult population. Cumulative hospitalizations from 24 states and NYC total 15,456 (0.8%) among those infected, with 303 deaths reported (from 43 states, NYC, Guam, and Puerto Rico). Case fatality rate approximates 0.01% in the most recent summary of state reports. One of the limitations of this report is that each state decides how to report the age distribution of COVID-19 cases resulting in variation in age range; another is the data are limited to those details individual states chose to make publicly available.
Although children do not commonly develop severe disease, and the case fatality is low, there are still insights to be learned from understanding risk features for severe disease. Preston et al. reviewed discharge data from 869 medical facilities to describe patients 18 years or younger who had an inpatient or emergency department encounter with a primary or secondary COVID-19 discharge diagnosis from March 1 through October 31, 2020.2 They reported that approximately 2,430 (11.7%) children were hospitalized and 746, nearly 31% of those hospitalized, had severe COVID disease. Those at greatest risk for severe disease were children with comorbid conditions and those less than 12 years, compared with the 12- to 18-year age group. They did not identify race as a risk for severe disease in this study. Moreira et al. described risk factors for morbidity and death from COVID in children less than 18 years of age3 using CDC COVID-NET, the Centers for Disease Control and Prevention COVID-19–associated hospitalization surveillance network. They reported a hospitalization rate of 4.7% among 27,045 cases. They identified three risk factors for hospitalization – age, race/ethnicity, and comorbid conditions. Thirty-nine children (0.19%) died; children who were black, non-Hispanic, and those with an underlying medical condition had a significantly increased risk of death. Thirty-three (85%) children who died had a comorbidity, and 27 (69%) were African American or Hispanic/Latino. The U.S. experience in children is also consistent with reports from the United Kingdom, Italy, Spain, Germany, France, and South Korea.4 Deaths from COVID-19 were uncommon but relatively more frequent in older children, compared with younger age groups among children less than 18 years of age in these countries.
Acute COVID-19 and multisystem inflammatory syndrome in children (MIS-C) do not predominantly target the neurologic systems; however, neurologic complications have been reported, some of which appear to result in long-lasting disability. LaRovere et al. identified 354 (22%) of 1,695 patients less than 21 years of age with acute COVID or MIS-C who had neurologic signs or symptoms during their illness. Among those with neurologic involvement, most children had prior neurologic deficits, mild symptoms, that resolved by the time of discharge. Forty-three (12%) were considered life threatening and included severe encephalopathy, stroke, central nervous system infection/demyelination, Guillain-Barre syndrome or variant, or acute cerebral edema. Several children, including some who were previously healthy prior to COVID, had persistent neurologic deficits at discharge. In addition to neurologic morbidity, long COVID – a syndrome of persistent symptoms following acute COVID that lasts for more than 12 weeks without alternative diagnosis – has also been described in children. Buonsenso et al. assessed 129 children diagnosed with COVID-19 between March and November 2020 in Rome, Italy.5 Persisting symptoms after 120 days were reported by more than 50%. Symptoms like fatigue, muscle and joint pain, headache, insomnia, respiratory problems, and palpitations were most common. Clearly, further follow-up of the long-term outcomes is necessary to understand the full spectrum of morbidity resulting from COVID-19 disease in children and its natural history.
The current picture of COVID infection in children younger than 18 reinforces that children are part of the pandemic. Although deaths in children have now exceeded 300 cases, severe disease remains uncommon in both the United States and western Europe. Risk factors for severe disease include comorbid illness and race/ethnicity with a disproportionate number of severe cases in children with underlying comorbidity and in African American and Hispanic/Latino children. Ongoing surveillance is critical as changes are likely to be observed over time as viral evolution affects disease burden and characteristics.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University schools of medicine and public health and senior attending physician in pediatric infectious diseases, Boston Medical Center. Email him at [email protected].
References
1. Children and COVID-19: State-Level Data Report. Services AAP.org.
2. Preston LE et al. JAMA Network Open. 2021;4(4):e215298. doi:10.1001/jamanetworkopen.2021.5298
3. Moreira A et al. Eur J Pediatr. 2021;180:1659-63.
4. SS Bhopal et al. Lancet 2021. doi: 10.1016/ S2352-4642(21)00066-3.
5. Buonsenso D et al. medRxiv preprint. doi: 10.1101/2021.01.23.21250375.
Direct-acting agents cure hepatitis C in children
Between 23,000 and 46,000 U.S. children live with chronic hepatitis C virus with a prevalence of 0.17% anti–hepatitis C virus (HCV) antibody positivity in those aged 6-11 years and 0.39% among children aged 12-19 years. In the United States, genotype 1 is most frequent, followed by genotypes 2 and 3. About 99% of cases result from vertical transmission; transfusion-related cases have not been observed in recent decades.Only viremic mothers are at risk of transmission as those who have spontaneously cleared HCV viremia or have been treated successfully do not risk transmission. Maternal HCV viral load appears to be a risk factor for HCV transmission, however transmission is reported at all levels of viremia.
In conjunction with the opioid epidemics, the prevalence of HCV infection has increased over the last decade. The Centers for Disease Control and Prevention reported that, between 2009 and 2014, the prevalence of HCV infection increased from 1.8 to 3.4 per 1,000 live births. They identified substantial state-to-state variation with the highest rate in West Virginia (22.6 per 1,000 live births), and the lowest in Hawaii (0.7 per 1,000 live births). The implications are clear that increasing numbers of newborns are exposed to HCV and, if transmission rates are between 1% and 5%, 80-400 U.S. infants each year acquire HCV infection.
HCV in children
HCV in children is almost always associated with persistent transaminitis. Chronic infection is defined as the persistence of HCV RNA for at least 6 months, and clearance of HCV infection is determined by the persistent disappearance of HCV RNA. Regardless of infection status, an infant may have detectable maternal anti-HCV antibody in serum until 18 months of age, resulting from passive transfer. In addition, prolonged infection can lead to cirrhosis, hepatocellular carcinoma, or decompensated liver disease. Potential extrahepatic manifestations including reduced physical and psychosocial health also are linked to chronic HCV. Autoimmune disease also has been reported in children with HCV. As well, the stigma of HCV elicits fear in school and child care settings that is a result of public misunderstanding regarding routes of hepatitis C transmission. No restriction of regular childhood activities is required in the daily life of HCV-infected children.
Taken together, increasing rates of HCV infection in pregnant women, increasing numbers of exposed and infected infants annually, potential for both short- and long-term morbidity, and curative nontoxic treatment,
Screening for HCV
There is considerable discussion about which strategy for screening of at-risk infants is more appropriate. Some groups advocate for HCV-RNA testing within the first year of life. Proponents argue the use of a highly sensitive RNA assay early in life has potential to increase detection of infected infants while a negative result allows the conclusion the infant is not infected. Advocates hypothesize that early identification has potential to improve continued follow-up.
Opponents argue that early testing does not change the need for repeat testing after 18 months to confirm diagnosis. They also argue that HCV RNA is more expensive than an antibody-based testing; and treatment will not begin prior to age 3 as there is still opportunity for viremia to spontaneously clear.
Direct acting agents licensed
Ledipasvir/sofosbuvir (Harvoni) was initially demonstrated as curative for genotype 1, 4, 5, or 6 infection in a phase 2, multicenter, open-label study of 100 adolescents with genotype 1 treated for 12 weeks. Sustained virologic response (SVR) was documented in 98% of participants.The regimen was safe and well tolerated in this population, and the adult dosage formulation resulted in pharmacokinetic characteristics similar to those observed in adults. Two clinical trials supported the efficacy of ledipasvir/sofosbuvir in the pediatric population aged 3-11 years. This regimen also is recommended for interferon-experienced (± ribavirin, with or without an HCV protease inhibitor) children and adolescents aged 3 years or older with genotype 1 or 4. A 12-week course is recommended for patients without cirrhosis; 24 weeks is recommended for those with compensated cirrhosis. The combination of ledipasvir/sofosbuvir is the only treatment option for children aged 3-6 years with genotype 1, 4, 5, or 6 infection.
The efficacy of sofosbuvir/velpatasvir (Epclusa) once daily for 12 weeks was first evaluated in an open-label trial among children aged 6 years and older with genotype 1, 2, 3, 4, or 6 infection, without cirrhosis or with compensated cirrhosis. Subsequently, the “cocktail” was evaluated in children aged 6-12 years, with 76% genotype 1, 3% genotype 2, 15% genotype 3, and 6% genotype 4. SVR12 rates were 93% (50/54) in children with genotype 1, 91% (10/11) in those with genotype 3, and 100% in participants with genotype 2 (2/2) or genotype 4 (4/4). Sofosbuvir/velpatasvir was approved in March 2020 by the Food and Drug Administration for pediatric patients aged 6 years and older. Given its pangenotypic activity, safety, and efficacy, sofosbuvir/velpatasvir is currently recommended as a first choice for HCV treatment in children and adolescents aged at least 6 years.
The daily fixed-dose combination of glecaprevir/pibrentasvir (Mavyret) was approved in April 2019 for adolescents aged 12-17 years, and weighing at least 45 kg.Treatment is for 8 weeks, and includes treatment-naive patients without cirrhosis or those with compensated cirrhosis. SVR12 rates for Mavyret have ranged from 91% to 100 % across clinic trials. FDA approval and HCV guideline treatment recommendations for direct-acting antiviral (DAA)–experienced adolescents are based on clinical trial data from adults. Given its pangenotypic activity, safety, and efficacy record in adult patients, glecaprevir/pibrentasvir is recommended as a first choice for adolescent HCV treatment. Glecaprevir/pibrentasvir once approved for children less than 3 years of age will be safe and efficacious as a pangenotypic treatment option in children with chronic HCV infection.
Current recommendations
Tools for identifying HCV infected infants as early as a few months of age are available, yet studies demonstrate that a minority of at-risk children are tested for HCV using either an HCV polymerase chain reaction strategy early in life or an anti-HCV antibody strategy after 18 months of age.
Therapy with direct-acting agents is now licensed to those aged 3 years and offers the potential for cure, eliminating concern for possible progression after prolonged infection. Such therapy offers the potential to eliminate the stigma faced by many children as well as the hepatic and extrahepatic manifestations observed in children. Medication formulation and the child’s abilities to take the medication needs to be considered when prescribing DAAs. It is important to assess if the child can successfully swallow pills. Currently, Harvoni is the only medication that comes in both pellet and pill formulations. The dose is based on weight. The pellets need to be given in a small amount of nonacidic food; they cannot be chewed.
All children with chronic HCV infection are candidates for treatment. When significant fibrosis and/or cirrhosis is present treatment should not be delayed once the child is age 3 years; when only transaminitis is present, treatment can be delayed. In our experience, parents are eager to complete treatment before starting kindergarten.
Liver biopsy for obtaining liver tissue for histopathologic examination is not routinely indicated in children with chronic HCV infection but should be evaluated case by case. Noninvasive tests of hepatic fibrosis have been used in children, these include serologic markers (i.e., FibroSure) and radiologic tests such as ultrasound-based transient elastography (i.e., Fibroscan). Validation for pediatric patients is variable for the different serologic tests. Studies have shown that Fibroscan using the M probe is feasible for a wide range of ages, but poor patient cooperation may make measurement difficult.
Further details regarding dosing and choice of formulation is available at https://www.hcvguidelines.org/unique-populations/children.
Dr. Sabharwal is assistant professor of pediatrics at Boston University and attending physician in pediatric infectious diseases at Boston Medical Center. Ms. Moloney is an instructor in pediatrics at Boston University and a pediatric nurse practitioner in pediatric infectious diseases at Boston Medicine Center. Dr. Pelton is professor of pediatrics and epidemiology at Boston University and public health and senior attending physician at Boston Medical Center. Boston Medical Center received funding from AbbVie for study of Harvoni in Children 3 years of age and older. Email them at [email protected].
References
MMWR Morb Mortal Wkly Rep. 2017 May 12;66(18):470-3. Hepatol Commun. 2017 March 23. doi: 10.1002/hep4.1028. Hepatology. 2020 Feb;71(2):422-30. Lancet Gastroenterol Hepatol. 2019 Apr 11. doi: 10.1016/S2468-1253(19)30046-9. Arch Dis Child. 2006 Sep;91(9):781-5. J Pediatr Gastroenterol Nutr. 2010 Feb;50(2):123-31.
Between 23,000 and 46,000 U.S. children live with chronic hepatitis C virus with a prevalence of 0.17% anti–hepatitis C virus (HCV) antibody positivity in those aged 6-11 years and 0.39% among children aged 12-19 years. In the United States, genotype 1 is most frequent, followed by genotypes 2 and 3. About 99% of cases result from vertical transmission; transfusion-related cases have not been observed in recent decades.Only viremic mothers are at risk of transmission as those who have spontaneously cleared HCV viremia or have been treated successfully do not risk transmission. Maternal HCV viral load appears to be a risk factor for HCV transmission, however transmission is reported at all levels of viremia.
In conjunction with the opioid epidemics, the prevalence of HCV infection has increased over the last decade. The Centers for Disease Control and Prevention reported that, between 2009 and 2014, the prevalence of HCV infection increased from 1.8 to 3.4 per 1,000 live births. They identified substantial state-to-state variation with the highest rate in West Virginia (22.6 per 1,000 live births), and the lowest in Hawaii (0.7 per 1,000 live births). The implications are clear that increasing numbers of newborns are exposed to HCV and, if transmission rates are between 1% and 5%, 80-400 U.S. infants each year acquire HCV infection.
HCV in children
HCV in children is almost always associated with persistent transaminitis. Chronic infection is defined as the persistence of HCV RNA for at least 6 months, and clearance of HCV infection is determined by the persistent disappearance of HCV RNA. Regardless of infection status, an infant may have detectable maternal anti-HCV antibody in serum until 18 months of age, resulting from passive transfer. In addition, prolonged infection can lead to cirrhosis, hepatocellular carcinoma, or decompensated liver disease. Potential extrahepatic manifestations including reduced physical and psychosocial health also are linked to chronic HCV. Autoimmune disease also has been reported in children with HCV. As well, the stigma of HCV elicits fear in school and child care settings that is a result of public misunderstanding regarding routes of hepatitis C transmission. No restriction of regular childhood activities is required in the daily life of HCV-infected children.
Taken together, increasing rates of HCV infection in pregnant women, increasing numbers of exposed and infected infants annually, potential for both short- and long-term morbidity, and curative nontoxic treatment,
Screening for HCV
There is considerable discussion about which strategy for screening of at-risk infants is more appropriate. Some groups advocate for HCV-RNA testing within the first year of life. Proponents argue the use of a highly sensitive RNA assay early in life has potential to increase detection of infected infants while a negative result allows the conclusion the infant is not infected. Advocates hypothesize that early identification has potential to improve continued follow-up.
Opponents argue that early testing does not change the need for repeat testing after 18 months to confirm diagnosis. They also argue that HCV RNA is more expensive than an antibody-based testing; and treatment will not begin prior to age 3 as there is still opportunity for viremia to spontaneously clear.
Direct acting agents licensed
Ledipasvir/sofosbuvir (Harvoni) was initially demonstrated as curative for genotype 1, 4, 5, or 6 infection in a phase 2, multicenter, open-label study of 100 adolescents with genotype 1 treated for 12 weeks. Sustained virologic response (SVR) was documented in 98% of participants.The regimen was safe and well tolerated in this population, and the adult dosage formulation resulted in pharmacokinetic characteristics similar to those observed in adults. Two clinical trials supported the efficacy of ledipasvir/sofosbuvir in the pediatric population aged 3-11 years. This regimen also is recommended for interferon-experienced (± ribavirin, with or without an HCV protease inhibitor) children and adolescents aged 3 years or older with genotype 1 or 4. A 12-week course is recommended for patients without cirrhosis; 24 weeks is recommended for those with compensated cirrhosis. The combination of ledipasvir/sofosbuvir is the only treatment option for children aged 3-6 years with genotype 1, 4, 5, or 6 infection.
The efficacy of sofosbuvir/velpatasvir (Epclusa) once daily for 12 weeks was first evaluated in an open-label trial among children aged 6 years and older with genotype 1, 2, 3, 4, or 6 infection, without cirrhosis or with compensated cirrhosis. Subsequently, the “cocktail” was evaluated in children aged 6-12 years, with 76% genotype 1, 3% genotype 2, 15% genotype 3, and 6% genotype 4. SVR12 rates were 93% (50/54) in children with genotype 1, 91% (10/11) in those with genotype 3, and 100% in participants with genotype 2 (2/2) or genotype 4 (4/4). Sofosbuvir/velpatasvir was approved in March 2020 by the Food and Drug Administration for pediatric patients aged 6 years and older. Given its pangenotypic activity, safety, and efficacy, sofosbuvir/velpatasvir is currently recommended as a first choice for HCV treatment in children and adolescents aged at least 6 years.
The daily fixed-dose combination of glecaprevir/pibrentasvir (Mavyret) was approved in April 2019 for adolescents aged 12-17 years, and weighing at least 45 kg.Treatment is for 8 weeks, and includes treatment-naive patients without cirrhosis or those with compensated cirrhosis. SVR12 rates for Mavyret have ranged from 91% to 100 % across clinic trials. FDA approval and HCV guideline treatment recommendations for direct-acting antiviral (DAA)–experienced adolescents are based on clinical trial data from adults. Given its pangenotypic activity, safety, and efficacy record in adult patients, glecaprevir/pibrentasvir is recommended as a first choice for adolescent HCV treatment. Glecaprevir/pibrentasvir once approved for children less than 3 years of age will be safe and efficacious as a pangenotypic treatment option in children with chronic HCV infection.
Current recommendations
Tools for identifying HCV infected infants as early as a few months of age are available, yet studies demonstrate that a minority of at-risk children are tested for HCV using either an HCV polymerase chain reaction strategy early in life or an anti-HCV antibody strategy after 18 months of age.
Therapy with direct-acting agents is now licensed to those aged 3 years and offers the potential for cure, eliminating concern for possible progression after prolonged infection. Such therapy offers the potential to eliminate the stigma faced by many children as well as the hepatic and extrahepatic manifestations observed in children. Medication formulation and the child’s abilities to take the medication needs to be considered when prescribing DAAs. It is important to assess if the child can successfully swallow pills. Currently, Harvoni is the only medication that comes in both pellet and pill formulations. The dose is based on weight. The pellets need to be given in a small amount of nonacidic food; they cannot be chewed.
All children with chronic HCV infection are candidates for treatment. When significant fibrosis and/or cirrhosis is present treatment should not be delayed once the child is age 3 years; when only transaminitis is present, treatment can be delayed. In our experience, parents are eager to complete treatment before starting kindergarten.
Liver biopsy for obtaining liver tissue for histopathologic examination is not routinely indicated in children with chronic HCV infection but should be evaluated case by case. Noninvasive tests of hepatic fibrosis have been used in children, these include serologic markers (i.e., FibroSure) and radiologic tests such as ultrasound-based transient elastography (i.e., Fibroscan). Validation for pediatric patients is variable for the different serologic tests. Studies have shown that Fibroscan using the M probe is feasible for a wide range of ages, but poor patient cooperation may make measurement difficult.
Further details regarding dosing and choice of formulation is available at https://www.hcvguidelines.org/unique-populations/children.
Dr. Sabharwal is assistant professor of pediatrics at Boston University and attending physician in pediatric infectious diseases at Boston Medical Center. Ms. Moloney is an instructor in pediatrics at Boston University and a pediatric nurse practitioner in pediatric infectious diseases at Boston Medicine Center. Dr. Pelton is professor of pediatrics and epidemiology at Boston University and public health and senior attending physician at Boston Medical Center. Boston Medical Center received funding from AbbVie for study of Harvoni in Children 3 years of age and older. Email them at [email protected].
References
MMWR Morb Mortal Wkly Rep. 2017 May 12;66(18):470-3. Hepatol Commun. 2017 March 23. doi: 10.1002/hep4.1028. Hepatology. 2020 Feb;71(2):422-30. Lancet Gastroenterol Hepatol. 2019 Apr 11. doi: 10.1016/S2468-1253(19)30046-9. Arch Dis Child. 2006 Sep;91(9):781-5. J Pediatr Gastroenterol Nutr. 2010 Feb;50(2):123-31.
Between 23,000 and 46,000 U.S. children live with chronic hepatitis C virus with a prevalence of 0.17% anti–hepatitis C virus (HCV) antibody positivity in those aged 6-11 years and 0.39% among children aged 12-19 years. In the United States, genotype 1 is most frequent, followed by genotypes 2 and 3. About 99% of cases result from vertical transmission; transfusion-related cases have not been observed in recent decades.Only viremic mothers are at risk of transmission as those who have spontaneously cleared HCV viremia or have been treated successfully do not risk transmission. Maternal HCV viral load appears to be a risk factor for HCV transmission, however transmission is reported at all levels of viremia.
In conjunction with the opioid epidemics, the prevalence of HCV infection has increased over the last decade. The Centers for Disease Control and Prevention reported that, between 2009 and 2014, the prevalence of HCV infection increased from 1.8 to 3.4 per 1,000 live births. They identified substantial state-to-state variation with the highest rate in West Virginia (22.6 per 1,000 live births), and the lowest in Hawaii (0.7 per 1,000 live births). The implications are clear that increasing numbers of newborns are exposed to HCV and, if transmission rates are between 1% and 5%, 80-400 U.S. infants each year acquire HCV infection.
HCV in children
HCV in children is almost always associated with persistent transaminitis. Chronic infection is defined as the persistence of HCV RNA for at least 6 months, and clearance of HCV infection is determined by the persistent disappearance of HCV RNA. Regardless of infection status, an infant may have detectable maternal anti-HCV antibody in serum until 18 months of age, resulting from passive transfer. In addition, prolonged infection can lead to cirrhosis, hepatocellular carcinoma, or decompensated liver disease. Potential extrahepatic manifestations including reduced physical and psychosocial health also are linked to chronic HCV. Autoimmune disease also has been reported in children with HCV. As well, the stigma of HCV elicits fear in school and child care settings that is a result of public misunderstanding regarding routes of hepatitis C transmission. No restriction of regular childhood activities is required in the daily life of HCV-infected children.
Taken together, increasing rates of HCV infection in pregnant women, increasing numbers of exposed and infected infants annually, potential for both short- and long-term morbidity, and curative nontoxic treatment,
Screening for HCV
There is considerable discussion about which strategy for screening of at-risk infants is more appropriate. Some groups advocate for HCV-RNA testing within the first year of life. Proponents argue the use of a highly sensitive RNA assay early in life has potential to increase detection of infected infants while a negative result allows the conclusion the infant is not infected. Advocates hypothesize that early identification has potential to improve continued follow-up.
Opponents argue that early testing does not change the need for repeat testing after 18 months to confirm diagnosis. They also argue that HCV RNA is more expensive than an antibody-based testing; and treatment will not begin prior to age 3 as there is still opportunity for viremia to spontaneously clear.
Direct acting agents licensed
Ledipasvir/sofosbuvir (Harvoni) was initially demonstrated as curative for genotype 1, 4, 5, or 6 infection in a phase 2, multicenter, open-label study of 100 adolescents with genotype 1 treated for 12 weeks. Sustained virologic response (SVR) was documented in 98% of participants.The regimen was safe and well tolerated in this population, and the adult dosage formulation resulted in pharmacokinetic characteristics similar to those observed in adults. Two clinical trials supported the efficacy of ledipasvir/sofosbuvir in the pediatric population aged 3-11 years. This regimen also is recommended for interferon-experienced (± ribavirin, with or without an HCV protease inhibitor) children and adolescents aged 3 years or older with genotype 1 or 4. A 12-week course is recommended for patients without cirrhosis; 24 weeks is recommended for those with compensated cirrhosis. The combination of ledipasvir/sofosbuvir is the only treatment option for children aged 3-6 years with genotype 1, 4, 5, or 6 infection.
The efficacy of sofosbuvir/velpatasvir (Epclusa) once daily for 12 weeks was first evaluated in an open-label trial among children aged 6 years and older with genotype 1, 2, 3, 4, or 6 infection, without cirrhosis or with compensated cirrhosis. Subsequently, the “cocktail” was evaluated in children aged 6-12 years, with 76% genotype 1, 3% genotype 2, 15% genotype 3, and 6% genotype 4. SVR12 rates were 93% (50/54) in children with genotype 1, 91% (10/11) in those with genotype 3, and 100% in participants with genotype 2 (2/2) or genotype 4 (4/4). Sofosbuvir/velpatasvir was approved in March 2020 by the Food and Drug Administration for pediatric patients aged 6 years and older. Given its pangenotypic activity, safety, and efficacy, sofosbuvir/velpatasvir is currently recommended as a first choice for HCV treatment in children and adolescents aged at least 6 years.
The daily fixed-dose combination of glecaprevir/pibrentasvir (Mavyret) was approved in April 2019 for adolescents aged 12-17 years, and weighing at least 45 kg.Treatment is for 8 weeks, and includes treatment-naive patients without cirrhosis or those with compensated cirrhosis. SVR12 rates for Mavyret have ranged from 91% to 100 % across clinic trials. FDA approval and HCV guideline treatment recommendations for direct-acting antiviral (DAA)–experienced adolescents are based on clinical trial data from adults. Given its pangenotypic activity, safety, and efficacy record in adult patients, glecaprevir/pibrentasvir is recommended as a first choice for adolescent HCV treatment. Glecaprevir/pibrentasvir once approved for children less than 3 years of age will be safe and efficacious as a pangenotypic treatment option in children with chronic HCV infection.
Current recommendations
Tools for identifying HCV infected infants as early as a few months of age are available, yet studies demonstrate that a minority of at-risk children are tested for HCV using either an HCV polymerase chain reaction strategy early in life or an anti-HCV antibody strategy after 18 months of age.
Therapy with direct-acting agents is now licensed to those aged 3 years and offers the potential for cure, eliminating concern for possible progression after prolonged infection. Such therapy offers the potential to eliminate the stigma faced by many children as well as the hepatic and extrahepatic manifestations observed in children. Medication formulation and the child’s abilities to take the medication needs to be considered when prescribing DAAs. It is important to assess if the child can successfully swallow pills. Currently, Harvoni is the only medication that comes in both pellet and pill formulations. The dose is based on weight. The pellets need to be given in a small amount of nonacidic food; they cannot be chewed.
All children with chronic HCV infection are candidates for treatment. When significant fibrosis and/or cirrhosis is present treatment should not be delayed once the child is age 3 years; when only transaminitis is present, treatment can be delayed. In our experience, parents are eager to complete treatment before starting kindergarten.
Liver biopsy for obtaining liver tissue for histopathologic examination is not routinely indicated in children with chronic HCV infection but should be evaluated case by case. Noninvasive tests of hepatic fibrosis have been used in children, these include serologic markers (i.e., FibroSure) and radiologic tests such as ultrasound-based transient elastography (i.e., Fibroscan). Validation for pediatric patients is variable for the different serologic tests. Studies have shown that Fibroscan using the M probe is feasible for a wide range of ages, but poor patient cooperation may make measurement difficult.
Further details regarding dosing and choice of formulation is available at https://www.hcvguidelines.org/unique-populations/children.
Dr. Sabharwal is assistant professor of pediatrics at Boston University and attending physician in pediatric infectious diseases at Boston Medical Center. Ms. Moloney is an instructor in pediatrics at Boston University and a pediatric nurse practitioner in pediatric infectious diseases at Boston Medicine Center. Dr. Pelton is professor of pediatrics and epidemiology at Boston University and public health and senior attending physician at Boston Medical Center. Boston Medical Center received funding from AbbVie for study of Harvoni in Children 3 years of age and older. Email them at [email protected].
References
MMWR Morb Mortal Wkly Rep. 2017 May 12;66(18):470-3. Hepatol Commun. 2017 March 23. doi: 10.1002/hep4.1028. Hepatology. 2020 Feb;71(2):422-30. Lancet Gastroenterol Hepatol. 2019 Apr 11. doi: 10.1016/S2468-1253(19)30046-9. Arch Dis Child. 2006 Sep;91(9):781-5. J Pediatr Gastroenterol Nutr. 2010 Feb;50(2):123-31.
Consider COVID-19–associated multisystem hyperinflammatory syndrome
A 21-year-old young adult presented to the ED with a 1-week history of high fever, vomiting, diarrhea, and abdominal pain. His mother was SARS-CoV-2 positive by polymerase chain reaction approximately 3 weeks prior; his PCR was negative for SARS-CoV-2.
Following admission, he became hypotensive and tachycardic with evidence of myocarditis. His chest x-ray was normal and his O2 saturation was 100% on room air. His clinical presentation was initially suggestive of toxic shock syndrome without a rash, but despite aggressive fluid resuscitation and broad-spectrum antibiotics, he continued to clinically deteriorate with persistent high fever and increasing cardiac stress. Echocardiography revealed biventricular dysfunction. His laboratory abnormalities included rising inflammatory markers and troponin I and B-type natriuretic peptide (BNP). A repeat PCR for SARS-CoV-2 was negative on day 2 of illness. He was diagnosed as likely having macrophage-activation syndrome (MAS) despite the atypical features (myocarditis), and he received Anakinra with no apparent response. He also was given intravenous immunoglobulin (IVIg) for his myocarditis and subsequently high-dose steroids. He became afebrile, his blood pressure stabilized, his inflammatory markers declined, and over several days he returned to normal. His COVID-19 antibody test IgG was positive on day 4 of illness.
This case challenged us for several reasons. First, the PCR from his nasopharynx was negative on two occasions, which raises the issue of how sensitive and accurate these PCR tests are for SARS-CoV-2 or are patients with COVID-19–associated hyperinflammatory syndrome still PCR positive? Second, although we have seen many adult cases with a cytokine storm picture similar to this patient, nearly all of the prior cases had chest x-ray abnormalities and hypoxia. Third, the severity of the myocardial dysfunction and rising troponin and BNP also was unusual in our experience with COVID-19 infection. Lastly, the use of antibody detection to SARS-CoV-2 enabled us to confirm recent COIVD-19 disease and see his illness as part of the likely spectrum of clinical syndromes seen with this virus.
The Lancet reported eight children, aged 4-14 years, with a hyperinflammatory shock-like syndrome in early May.1 The cases had features similar to atypical Kawasaki disease, KD shock syndrome, and toxic shock syndrome. Each case had high fever for multiple days; diarrhea and abdominal pain was present in even children; elevated ferritin, C-reactive protein, d-dimer, increased troponins, and ventricular dysfunction also was present in seven. Most patients had no pulmonary involvement, and most tested negative for SARS-CoV-2 despite four of the eight having direct contact with a COVID-positive family member. All received IVIg and antibiotics; six received aspirin. Seven of the eight made a full recovery; one child died from a large cerebrovascular infarct.
Also in early May, the New York Times described a “mysterious” hyperinflammatory syndrome in children thought to be linked to COVID-19. A total of 76 suspected cases in children had been reported in New York state, three of whom died. The syndrome has been given the name pediatric multisystem inflammatory syndrome. The syndrome can resemble KD shock syndrome with rash; fever; conjunctivitis; hypotension; and redness in the lips, tongue and mucous membranes . It also can resemble toxic shock syndrome with abdominal pain, vomiting, and diarrhea. However, the degree of cardiac inflammation and dysfunction is substantial in many cases and usually beyond that seen in KD or toxic shock.
The syndrome is not limited to the United States. The Royal College of Pediatrics and Child Health has created a case definition:2
- A child presenting with persistent fever, inflammation (elevated C-reactive protein, neutrophilia, and lymphopenia) and evidence of single or multiorgan dysfunction (shock, cardiac, respiratory, renal, gastrointestinal, or neurologic) with additional features.
- Exclusion of any other microbial causes such as bacterial sepsis or staphylococcal or streptococcal shock syndromes, infections known to be associated with myocarditis (such as enterovirus).
- SARS-CoV-2 testing may or may not be positive.
As with our young adult, treatment is supportive, nonspecific, and aimed at quieting the inflammatory response. The current thinking is the syndrome is seen as antibody to SARS-CoV-2 appears and frequently the nasopharyngeal PCR is negative. It is hypothesized that the syndrome occurs in genetically predisposed hosts and potentially is a late-onset inflammatory process or potentially an antibody-triggered inflammatory process. The negative PCR from nasopharyngeal specimens reflects that the onset is later in the course of disease; whether fecal samples would be COVID positive is unknown. As with our case, antibody testing for IgG against SARS-CoV-2 is appropriate to confirm COVID-19 disease and may be positive as early as day 7.
The approach needs to be team oriented and include cardiology, rheumatology, infectious diseases, and intensive care specialists working collaboratively. Such cases should be considered COVID positive despite negative PCR tests, and full personal protective equipment should be used as we do not as yet know if live virus could be found in stool. We initiated treatment with Anakinra (an interleukin-1 type-1 receptor inhibitor) as part of our treatment protocol for MAS; we did not appreciate a response. He then received IVIg and high-dose steroids, and he recovered over several days with improved cardiac function and stable blood pressure.
What is the pathogenesis? Is SARS-CoV-2 causative or just an associated finding? Who are the at-risk children, adolescents, and adults? Is there a genetic predisposition? What therapies work best? The eight cases described in London all received IVIg, as did our case, and all but one improved and survived. In adults we have seen substantial inflammation with elevated C-reactive protein (often as high as 300), ferritin, lactate dehydrogenase, triglycerides, fibrinogen, and d-dimers, but nearly all have extensive pulmonary disease, hypoxia, and are SARS-CoV-2 positive by PCR. Influenza is also associated with a cytokine storm syndrome in adolescents and young adults.3 The mechanisms influenza virus uses to initiate a cytokine storm and strategies for immunomodulatory treatment may provide insights into COVID-19–associated multisystem hyperinflammatory syndrome.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University and public health and senior attending physician in pediatric infectious diseases at Boston Medical Center. Dr. Camelo is a senior fellow in pediatric infectious diseases at Boston Medical Center. They have no relevant financial disclosures. Email them at [email protected].
References
1. Riphagen S et al. Lancet. 2020 May 6. doi: 10.1016/S0140-6736(20)31094-1.
2. Royal College of Paediatrics and Child Health Guidance: Paediatric multisystem inflammatory syndrome temporally associated with COVID-19.
3. Liu Q et al.Cell Mol Immunol. 2016 Jan;13(1):3-10.
A 21-year-old young adult presented to the ED with a 1-week history of high fever, vomiting, diarrhea, and abdominal pain. His mother was SARS-CoV-2 positive by polymerase chain reaction approximately 3 weeks prior; his PCR was negative for SARS-CoV-2.
Following admission, he became hypotensive and tachycardic with evidence of myocarditis. His chest x-ray was normal and his O2 saturation was 100% on room air. His clinical presentation was initially suggestive of toxic shock syndrome without a rash, but despite aggressive fluid resuscitation and broad-spectrum antibiotics, he continued to clinically deteriorate with persistent high fever and increasing cardiac stress. Echocardiography revealed biventricular dysfunction. His laboratory abnormalities included rising inflammatory markers and troponin I and B-type natriuretic peptide (BNP). A repeat PCR for SARS-CoV-2 was negative on day 2 of illness. He was diagnosed as likely having macrophage-activation syndrome (MAS) despite the atypical features (myocarditis), and he received Anakinra with no apparent response. He also was given intravenous immunoglobulin (IVIg) for his myocarditis and subsequently high-dose steroids. He became afebrile, his blood pressure stabilized, his inflammatory markers declined, and over several days he returned to normal. His COVID-19 antibody test IgG was positive on day 4 of illness.
This case challenged us for several reasons. First, the PCR from his nasopharynx was negative on two occasions, which raises the issue of how sensitive and accurate these PCR tests are for SARS-CoV-2 or are patients with COVID-19–associated hyperinflammatory syndrome still PCR positive? Second, although we have seen many adult cases with a cytokine storm picture similar to this patient, nearly all of the prior cases had chest x-ray abnormalities and hypoxia. Third, the severity of the myocardial dysfunction and rising troponin and BNP also was unusual in our experience with COVID-19 infection. Lastly, the use of antibody detection to SARS-CoV-2 enabled us to confirm recent COIVD-19 disease and see his illness as part of the likely spectrum of clinical syndromes seen with this virus.
The Lancet reported eight children, aged 4-14 years, with a hyperinflammatory shock-like syndrome in early May.1 The cases had features similar to atypical Kawasaki disease, KD shock syndrome, and toxic shock syndrome. Each case had high fever for multiple days; diarrhea and abdominal pain was present in even children; elevated ferritin, C-reactive protein, d-dimer, increased troponins, and ventricular dysfunction also was present in seven. Most patients had no pulmonary involvement, and most tested negative for SARS-CoV-2 despite four of the eight having direct contact with a COVID-positive family member. All received IVIg and antibiotics; six received aspirin. Seven of the eight made a full recovery; one child died from a large cerebrovascular infarct.
Also in early May, the New York Times described a “mysterious” hyperinflammatory syndrome in children thought to be linked to COVID-19. A total of 76 suspected cases in children had been reported in New York state, three of whom died. The syndrome has been given the name pediatric multisystem inflammatory syndrome. The syndrome can resemble KD shock syndrome with rash; fever; conjunctivitis; hypotension; and redness in the lips, tongue and mucous membranes . It also can resemble toxic shock syndrome with abdominal pain, vomiting, and diarrhea. However, the degree of cardiac inflammation and dysfunction is substantial in many cases and usually beyond that seen in KD or toxic shock.
The syndrome is not limited to the United States. The Royal College of Pediatrics and Child Health has created a case definition:2
- A child presenting with persistent fever, inflammation (elevated C-reactive protein, neutrophilia, and lymphopenia) and evidence of single or multiorgan dysfunction (shock, cardiac, respiratory, renal, gastrointestinal, or neurologic) with additional features.
- Exclusion of any other microbial causes such as bacterial sepsis or staphylococcal or streptococcal shock syndromes, infections known to be associated with myocarditis (such as enterovirus).
- SARS-CoV-2 testing may or may not be positive.
As with our young adult, treatment is supportive, nonspecific, and aimed at quieting the inflammatory response. The current thinking is the syndrome is seen as antibody to SARS-CoV-2 appears and frequently the nasopharyngeal PCR is negative. It is hypothesized that the syndrome occurs in genetically predisposed hosts and potentially is a late-onset inflammatory process or potentially an antibody-triggered inflammatory process. The negative PCR from nasopharyngeal specimens reflects that the onset is later in the course of disease; whether fecal samples would be COVID positive is unknown. As with our case, antibody testing for IgG against SARS-CoV-2 is appropriate to confirm COVID-19 disease and may be positive as early as day 7.
The approach needs to be team oriented and include cardiology, rheumatology, infectious diseases, and intensive care specialists working collaboratively. Such cases should be considered COVID positive despite negative PCR tests, and full personal protective equipment should be used as we do not as yet know if live virus could be found in stool. We initiated treatment with Anakinra (an interleukin-1 type-1 receptor inhibitor) as part of our treatment protocol for MAS; we did not appreciate a response. He then received IVIg and high-dose steroids, and he recovered over several days with improved cardiac function and stable blood pressure.
What is the pathogenesis? Is SARS-CoV-2 causative or just an associated finding? Who are the at-risk children, adolescents, and adults? Is there a genetic predisposition? What therapies work best? The eight cases described in London all received IVIg, as did our case, and all but one improved and survived. In adults we have seen substantial inflammation with elevated C-reactive protein (often as high as 300), ferritin, lactate dehydrogenase, triglycerides, fibrinogen, and d-dimers, but nearly all have extensive pulmonary disease, hypoxia, and are SARS-CoV-2 positive by PCR. Influenza is also associated with a cytokine storm syndrome in adolescents and young adults.3 The mechanisms influenza virus uses to initiate a cytokine storm and strategies for immunomodulatory treatment may provide insights into COVID-19–associated multisystem hyperinflammatory syndrome.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University and public health and senior attending physician in pediatric infectious diseases at Boston Medical Center. Dr. Camelo is a senior fellow in pediatric infectious diseases at Boston Medical Center. They have no relevant financial disclosures. Email them at [email protected].
References
1. Riphagen S et al. Lancet. 2020 May 6. doi: 10.1016/S0140-6736(20)31094-1.
2. Royal College of Paediatrics and Child Health Guidance: Paediatric multisystem inflammatory syndrome temporally associated with COVID-19.
3. Liu Q et al.Cell Mol Immunol. 2016 Jan;13(1):3-10.
A 21-year-old young adult presented to the ED with a 1-week history of high fever, vomiting, diarrhea, and abdominal pain. His mother was SARS-CoV-2 positive by polymerase chain reaction approximately 3 weeks prior; his PCR was negative for SARS-CoV-2.
Following admission, he became hypotensive and tachycardic with evidence of myocarditis. His chest x-ray was normal and his O2 saturation was 100% on room air. His clinical presentation was initially suggestive of toxic shock syndrome without a rash, but despite aggressive fluid resuscitation and broad-spectrum antibiotics, he continued to clinically deteriorate with persistent high fever and increasing cardiac stress. Echocardiography revealed biventricular dysfunction. His laboratory abnormalities included rising inflammatory markers and troponin I and B-type natriuretic peptide (BNP). A repeat PCR for SARS-CoV-2 was negative on day 2 of illness. He was diagnosed as likely having macrophage-activation syndrome (MAS) despite the atypical features (myocarditis), and he received Anakinra with no apparent response. He also was given intravenous immunoglobulin (IVIg) for his myocarditis and subsequently high-dose steroids. He became afebrile, his blood pressure stabilized, his inflammatory markers declined, and over several days he returned to normal. His COVID-19 antibody test IgG was positive on day 4 of illness.
This case challenged us for several reasons. First, the PCR from his nasopharynx was negative on two occasions, which raises the issue of how sensitive and accurate these PCR tests are for SARS-CoV-2 or are patients with COVID-19–associated hyperinflammatory syndrome still PCR positive? Second, although we have seen many adult cases with a cytokine storm picture similar to this patient, nearly all of the prior cases had chest x-ray abnormalities and hypoxia. Third, the severity of the myocardial dysfunction and rising troponin and BNP also was unusual in our experience with COVID-19 infection. Lastly, the use of antibody detection to SARS-CoV-2 enabled us to confirm recent COIVD-19 disease and see his illness as part of the likely spectrum of clinical syndromes seen with this virus.
The Lancet reported eight children, aged 4-14 years, with a hyperinflammatory shock-like syndrome in early May.1 The cases had features similar to atypical Kawasaki disease, KD shock syndrome, and toxic shock syndrome. Each case had high fever for multiple days; diarrhea and abdominal pain was present in even children; elevated ferritin, C-reactive protein, d-dimer, increased troponins, and ventricular dysfunction also was present in seven. Most patients had no pulmonary involvement, and most tested negative for SARS-CoV-2 despite four of the eight having direct contact with a COVID-positive family member. All received IVIg and antibiotics; six received aspirin. Seven of the eight made a full recovery; one child died from a large cerebrovascular infarct.
Also in early May, the New York Times described a “mysterious” hyperinflammatory syndrome in children thought to be linked to COVID-19. A total of 76 suspected cases in children had been reported in New York state, three of whom died. The syndrome has been given the name pediatric multisystem inflammatory syndrome. The syndrome can resemble KD shock syndrome with rash; fever; conjunctivitis; hypotension; and redness in the lips, tongue and mucous membranes . It also can resemble toxic shock syndrome with abdominal pain, vomiting, and diarrhea. However, the degree of cardiac inflammation and dysfunction is substantial in many cases and usually beyond that seen in KD or toxic shock.
The syndrome is not limited to the United States. The Royal College of Pediatrics and Child Health has created a case definition:2
- A child presenting with persistent fever, inflammation (elevated C-reactive protein, neutrophilia, and lymphopenia) and evidence of single or multiorgan dysfunction (shock, cardiac, respiratory, renal, gastrointestinal, or neurologic) with additional features.
- Exclusion of any other microbial causes such as bacterial sepsis or staphylococcal or streptococcal shock syndromes, infections known to be associated with myocarditis (such as enterovirus).
- SARS-CoV-2 testing may or may not be positive.
As with our young adult, treatment is supportive, nonspecific, and aimed at quieting the inflammatory response. The current thinking is the syndrome is seen as antibody to SARS-CoV-2 appears and frequently the nasopharyngeal PCR is negative. It is hypothesized that the syndrome occurs in genetically predisposed hosts and potentially is a late-onset inflammatory process or potentially an antibody-triggered inflammatory process. The negative PCR from nasopharyngeal specimens reflects that the onset is later in the course of disease; whether fecal samples would be COVID positive is unknown. As with our case, antibody testing for IgG against SARS-CoV-2 is appropriate to confirm COVID-19 disease and may be positive as early as day 7.
The approach needs to be team oriented and include cardiology, rheumatology, infectious diseases, and intensive care specialists working collaboratively. Such cases should be considered COVID positive despite negative PCR tests, and full personal protective equipment should be used as we do not as yet know if live virus could be found in stool. We initiated treatment with Anakinra (an interleukin-1 type-1 receptor inhibitor) as part of our treatment protocol for MAS; we did not appreciate a response. He then received IVIg and high-dose steroids, and he recovered over several days with improved cardiac function and stable blood pressure.
What is the pathogenesis? Is SARS-CoV-2 causative or just an associated finding? Who are the at-risk children, adolescents, and adults? Is there a genetic predisposition? What therapies work best? The eight cases described in London all received IVIg, as did our case, and all but one improved and survived. In adults we have seen substantial inflammation with elevated C-reactive protein (often as high as 300), ferritin, lactate dehydrogenase, triglycerides, fibrinogen, and d-dimers, but nearly all have extensive pulmonary disease, hypoxia, and are SARS-CoV-2 positive by PCR. Influenza is also associated with a cytokine storm syndrome in adolescents and young adults.3 The mechanisms influenza virus uses to initiate a cytokine storm and strategies for immunomodulatory treatment may provide insights into COVID-19–associated multisystem hyperinflammatory syndrome.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University and public health and senior attending physician in pediatric infectious diseases at Boston Medical Center. Dr. Camelo is a senior fellow in pediatric infectious diseases at Boston Medical Center. They have no relevant financial disclosures. Email them at [email protected].
References
1. Riphagen S et al. Lancet. 2020 May 6. doi: 10.1016/S0140-6736(20)31094-1.
2. Royal College of Paediatrics and Child Health Guidance: Paediatric multisystem inflammatory syndrome temporally associated with COVID-19.
3. Liu Q et al.Cell Mol Immunol. 2016 Jan;13(1):3-10.
COVID-19 in children, pregnant women: What do we know?
A novel coronavirus, the causative agent of the current pandemic of viral respiratory illness and pneumonia, was first identified in Wuhan, Hubei, China. The disease has been given the name, coronavirus disease 2019 (COVID-19). The virus at last report has spread to more than 100 countries. Much of what we suspect about this virus comes from work on other severe coronavirus respiratory disease outbreaks – Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS). MERS-CoV was a viral respiratory disease, first reported in Saudi Arabia, that was identified in more than 27 additional countries. The disease was characterized by severe acute respiratory illness, including fever, cough, and shortness of breath. Among 2,499 cases, only two patients tested positive for MERS-CoV in the United States. SARS-CoV also caused a severe viral respiratory illness. SARS was first recognized in Asia in 2003 and was subsequently reported in approximately 25 countries. The last case reported was in 2004.
As of March 13, there are 137,066 cases worldwide of COVID-19 and 1,701 in the United States, according to the John Hopkins University Coronavirus COVID-19 resource center.
What about children?
The remarkable observation is how few seriously ill children have been identified in the face of global spread. Unlike the H1N1 influenza epidemic of 2009, where older adults were relatively spared and children were a major target population, COVID-19 appears to be relatively infrequent in children or too mild to come to diagnosis, to date. Specifically, among China’s first approximately 44,000 cases, less than 2% were identified in children less than 20 years of age, and severe disease was uncommon with no deaths in children less than 10 years of age reported. One child, 13 months of age, with acute respiratory distress syndrome and septic shock was reported in China. According to the Centers for Disease Control and Prevention webcast , children present with fever in about 50% of cases, cough, fatigue, and subsequently some (3%-30%) progress to shortness of breath. Some children and adults have presented with gastrointestinal disease initially. Viral RNA has been detected in respiratory secretions, blood, and stool of affected children; however, the samples were not cultured for virus so whether stool is a potential source for transmission is unclear. In adults, the disease appears to be most severe – with development of pneumonia – in the second week of illness. In both children and adults, the chest x-ray findings are an interstitial pneumonitis, ground glass appearance, and/or patchy infiltrates.

Are some children at greater risk? Are children the source of community transmission? Will children become a greater part of the disease pattern as further cases are identified and further testing is available? We cannot answer many of these questions about COVID-19 in children as yet, but as you are aware, data are accumulating daily, and the Centers for Disease Control and Prevention and the National Institutes of Health are providing regular updates.
A report from China gave us some idea about community transmission and infection risk for children. The Shenzhen CDC identified 391 COVID-19 cases and 1,286 close contacts. Household contacts and those persons traveling with a case of the virus were at highest risk of acquisition. The secondary attack rates within households was 15%; children were as likely to become infected as adults (medRxiv preprint. 2020. doi: 10.1101/2020.03.03.20028423).
What about pregnant women?
The data on pregnant women are even more limited. The concern about COVID-19 during pregnancy comes from our knowledge of adverse outcomes from other respiratory viral infections. For example, respiratory viral infections such as influenza have been associated with increased maternal risk of severe disease, and adverse neonatal outcomes, including low birth weight and preterm birth. The experience with SARS also is concerning for excess adverse maternal and neonatal complications such as spontaneous miscarriage, preterm delivery, intrauterine growth restriction, admission to the ICU, renal failure, and disseminated intravascular coagulopathy all were reported as complications of SARS infection during pregnancy.
Two studies on COVID-19 in pregnancy have been reported to date. In nine pregnant women reported by Chen et al., COVID-19 pneumonia was identified in the third trimester. The women presented with fever, cough, myalgia, sore throat, and/or malaise. Fetal distress was reported in two; all nine infants were born alive. Apgar scores were 8-10 at 1 minute. Five were found to have lymphopenia; three had increases in hepatic enzymes. None of the infants developed severe COVID-19 pneumonia. Amniotic fluid, cord blood, neonatal throat swab, and breast milk samples from six of the nine patients were tested for the novel coronavirus 2019, and all results were negative (Lancet. 2020 Feb 12. doi: 10.1016/S0140-6736[20]30360-3)https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30360-3/fulltext.
In a study by Zhu et al., nine pregnant women with confirmed COVID-19 infection were identified during Jan. 20-Feb. 5, 2020. The onset of clinical symptoms in these women occurred before delivery in four cases, on the day of delivery in two cases, and after delivery in three cases. Of the 10 neonates (one set of twins) many had clinical symptoms, but none were proven to be COVID-19 positive in their pharyngeal swabs. Shortness of breath was observed in six, fever in two, tachycardia in one. GI symptoms such as feeding intolerance, bloating, GI bleed, and vomiting also were observed. Chest radiography showed abnormalities in seven neonates at admission. Thrombocytopenia and/or disseminated intravascular coagulopathy also was reported. Five neonates recovered and were discharged, one died, and four neonates remained in hospital in a stable condition. It is unclear if the illness in these infants was related to COVID-19 (Transl Pediatrics. 2020 Feb. doi: 10.21037/tp.2020.02.06)http://tp.amegroups.com/article/view/35919/28274.
In the limited experience to date, no evidence of virus has been found in the breast milk of women with COVID-19, which is consistent with the SARS experience. Current recommendations are to separate the infant from known COVID-19 infected mothers either in a different room or in the mother’s room using a six foot rule, a barrier curtain of some type, and mask and hand washing prior to any contact between mother and infant. If the mother desires to breastfeed her child, the same precautions – mask and hand washing – should be in place.
What about treatment?
There are no proven effective therapies and supportive care has been the mainstay to date. Clinical trials of remdesivir have been initiated both by Gilead (compassionate use, open label) and by the National Institutes of Health (randomized remdesivirhttps://www.drugs.com/history/remdesivir.html vs. placebo) in adults based on in vitro data suggesting activity again COVID-19. Lopinavir/ritonavir (combination protease inhibitors) also have been administered off label, but no results are available as yet.
Keeping up
I suggest several valuable resources to keep yourself abreast of the rapidly changing COVID-19 story. First the CDC website or your local Department of Health. These are being updated frequently and include advisories on personal protective equipment, clusters of cases in your local community, and current recommendations for mitigation of the epidemic. I have listened to Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, and Robert R. Redfield, MD, the director of the CDC almost daily. I trust their viewpoints and transparency about what is and what is not known, as well as the why and wherefore of their guidance, remembering that each day brings new information and new guidance.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University and public health and senior attending physician at Boston Medical Center. He has no relevant financial disclosures. Email him at [email protected].
A novel coronavirus, the causative agent of the current pandemic of viral respiratory illness and pneumonia, was first identified in Wuhan, Hubei, China. The disease has been given the name, coronavirus disease 2019 (COVID-19). The virus at last report has spread to more than 100 countries. Much of what we suspect about this virus comes from work on other severe coronavirus respiratory disease outbreaks – Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS). MERS-CoV was a viral respiratory disease, first reported in Saudi Arabia, that was identified in more than 27 additional countries. The disease was characterized by severe acute respiratory illness, including fever, cough, and shortness of breath. Among 2,499 cases, only two patients tested positive for MERS-CoV in the United States. SARS-CoV also caused a severe viral respiratory illness. SARS was first recognized in Asia in 2003 and was subsequently reported in approximately 25 countries. The last case reported was in 2004.
As of March 13, there are 137,066 cases worldwide of COVID-19 and 1,701 in the United States, according to the John Hopkins University Coronavirus COVID-19 resource center.
What about children?
The remarkable observation is how few seriously ill children have been identified in the face of global spread. Unlike the H1N1 influenza epidemic of 2009, where older adults were relatively spared and children were a major target population, COVID-19 appears to be relatively infrequent in children or too mild to come to diagnosis, to date. Specifically, among China’s first approximately 44,000 cases, less than 2% were identified in children less than 20 years of age, and severe disease was uncommon with no deaths in children less than 10 years of age reported. One child, 13 months of age, with acute respiratory distress syndrome and septic shock was reported in China. According to the Centers for Disease Control and Prevention webcast , children present with fever in about 50% of cases, cough, fatigue, and subsequently some (3%-30%) progress to shortness of breath. Some children and adults have presented with gastrointestinal disease initially. Viral RNA has been detected in respiratory secretions, blood, and stool of affected children; however, the samples were not cultured for virus so whether stool is a potential source for transmission is unclear. In adults, the disease appears to be most severe – with development of pneumonia – in the second week of illness. In both children and adults, the chest x-ray findings are an interstitial pneumonitis, ground glass appearance, and/or patchy infiltrates.

Are some children at greater risk? Are children the source of community transmission? Will children become a greater part of the disease pattern as further cases are identified and further testing is available? We cannot answer many of these questions about COVID-19 in children as yet, but as you are aware, data are accumulating daily, and the Centers for Disease Control and Prevention and the National Institutes of Health are providing regular updates.
A report from China gave us some idea about community transmission and infection risk for children. The Shenzhen CDC identified 391 COVID-19 cases and 1,286 close contacts. Household contacts and those persons traveling with a case of the virus were at highest risk of acquisition. The secondary attack rates within households was 15%; children were as likely to become infected as adults (medRxiv preprint. 2020. doi: 10.1101/2020.03.03.20028423).
What about pregnant women?
The data on pregnant women are even more limited. The concern about COVID-19 during pregnancy comes from our knowledge of adverse outcomes from other respiratory viral infections. For example, respiratory viral infections such as influenza have been associated with increased maternal risk of severe disease, and adverse neonatal outcomes, including low birth weight and preterm birth. The experience with SARS also is concerning for excess adverse maternal and neonatal complications such as spontaneous miscarriage, preterm delivery, intrauterine growth restriction, admission to the ICU, renal failure, and disseminated intravascular coagulopathy all were reported as complications of SARS infection during pregnancy.
Two studies on COVID-19 in pregnancy have been reported to date. In nine pregnant women reported by Chen et al., COVID-19 pneumonia was identified in the third trimester. The women presented with fever, cough, myalgia, sore throat, and/or malaise. Fetal distress was reported in two; all nine infants were born alive. Apgar scores were 8-10 at 1 minute. Five were found to have lymphopenia; three had increases in hepatic enzymes. None of the infants developed severe COVID-19 pneumonia. Amniotic fluid, cord blood, neonatal throat swab, and breast milk samples from six of the nine patients were tested for the novel coronavirus 2019, and all results were negative (Lancet. 2020 Feb 12. doi: 10.1016/S0140-6736[20]30360-3)https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30360-3/fulltext.
In a study by Zhu et al., nine pregnant women with confirmed COVID-19 infection were identified during Jan. 20-Feb. 5, 2020. The onset of clinical symptoms in these women occurred before delivery in four cases, on the day of delivery in two cases, and after delivery in three cases. Of the 10 neonates (one set of twins) many had clinical symptoms, but none were proven to be COVID-19 positive in their pharyngeal swabs. Shortness of breath was observed in six, fever in two, tachycardia in one. GI symptoms such as feeding intolerance, bloating, GI bleed, and vomiting also were observed. Chest radiography showed abnormalities in seven neonates at admission. Thrombocytopenia and/or disseminated intravascular coagulopathy also was reported. Five neonates recovered and were discharged, one died, and four neonates remained in hospital in a stable condition. It is unclear if the illness in these infants was related to COVID-19 (Transl Pediatrics. 2020 Feb. doi: 10.21037/tp.2020.02.06)http://tp.amegroups.com/article/view/35919/28274.
In the limited experience to date, no evidence of virus has been found in the breast milk of women with COVID-19, which is consistent with the SARS experience. Current recommendations are to separate the infant from known COVID-19 infected mothers either in a different room or in the mother’s room using a six foot rule, a barrier curtain of some type, and mask and hand washing prior to any contact between mother and infant. If the mother desires to breastfeed her child, the same precautions – mask and hand washing – should be in place.
What about treatment?
There are no proven effective therapies and supportive care has been the mainstay to date. Clinical trials of remdesivir have been initiated both by Gilead (compassionate use, open label) and by the National Institutes of Health (randomized remdesivirhttps://www.drugs.com/history/remdesivir.html vs. placebo) in adults based on in vitro data suggesting activity again COVID-19. Lopinavir/ritonavir (combination protease inhibitors) also have been administered off label, but no results are available as yet.
Keeping up
I suggest several valuable resources to keep yourself abreast of the rapidly changing COVID-19 story. First the CDC website or your local Department of Health. These are being updated frequently and include advisories on personal protective equipment, clusters of cases in your local community, and current recommendations for mitigation of the epidemic. I have listened to Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, and Robert R. Redfield, MD, the director of the CDC almost daily. I trust their viewpoints and transparency about what is and what is not known, as well as the why and wherefore of their guidance, remembering that each day brings new information and new guidance.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University and public health and senior attending physician at Boston Medical Center. He has no relevant financial disclosures. Email him at [email protected].
A novel coronavirus, the causative agent of the current pandemic of viral respiratory illness and pneumonia, was first identified in Wuhan, Hubei, China. The disease has been given the name, coronavirus disease 2019 (COVID-19). The virus at last report has spread to more than 100 countries. Much of what we suspect about this virus comes from work on other severe coronavirus respiratory disease outbreaks – Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS). MERS-CoV was a viral respiratory disease, first reported in Saudi Arabia, that was identified in more than 27 additional countries. The disease was characterized by severe acute respiratory illness, including fever, cough, and shortness of breath. Among 2,499 cases, only two patients tested positive for MERS-CoV in the United States. SARS-CoV also caused a severe viral respiratory illness. SARS was first recognized in Asia in 2003 and was subsequently reported in approximately 25 countries. The last case reported was in 2004.
As of March 13, there are 137,066 cases worldwide of COVID-19 and 1,701 in the United States, according to the John Hopkins University Coronavirus COVID-19 resource center.
What about children?
The remarkable observation is how few seriously ill children have been identified in the face of global spread. Unlike the H1N1 influenza epidemic of 2009, where older adults were relatively spared and children were a major target population, COVID-19 appears to be relatively infrequent in children or too mild to come to diagnosis, to date. Specifically, among China’s first approximately 44,000 cases, less than 2% were identified in children less than 20 years of age, and severe disease was uncommon with no deaths in children less than 10 years of age reported. One child, 13 months of age, with acute respiratory distress syndrome and septic shock was reported in China. According to the Centers for Disease Control and Prevention webcast , children present with fever in about 50% of cases, cough, fatigue, and subsequently some (3%-30%) progress to shortness of breath. Some children and adults have presented with gastrointestinal disease initially. Viral RNA has been detected in respiratory secretions, blood, and stool of affected children; however, the samples were not cultured for virus so whether stool is a potential source for transmission is unclear. In adults, the disease appears to be most severe – with development of pneumonia – in the second week of illness. In both children and adults, the chest x-ray findings are an interstitial pneumonitis, ground glass appearance, and/or patchy infiltrates.

Are some children at greater risk? Are children the source of community transmission? Will children become a greater part of the disease pattern as further cases are identified and further testing is available? We cannot answer many of these questions about COVID-19 in children as yet, but as you are aware, data are accumulating daily, and the Centers for Disease Control and Prevention and the National Institutes of Health are providing regular updates.
A report from China gave us some idea about community transmission and infection risk for children. The Shenzhen CDC identified 391 COVID-19 cases and 1,286 close contacts. Household contacts and those persons traveling with a case of the virus were at highest risk of acquisition. The secondary attack rates within households was 15%; children were as likely to become infected as adults (medRxiv preprint. 2020. doi: 10.1101/2020.03.03.20028423).
What about pregnant women?
The data on pregnant women are even more limited. The concern about COVID-19 during pregnancy comes from our knowledge of adverse outcomes from other respiratory viral infections. For example, respiratory viral infections such as influenza have been associated with increased maternal risk of severe disease, and adverse neonatal outcomes, including low birth weight and preterm birth. The experience with SARS also is concerning for excess adverse maternal and neonatal complications such as spontaneous miscarriage, preterm delivery, intrauterine growth restriction, admission to the ICU, renal failure, and disseminated intravascular coagulopathy all were reported as complications of SARS infection during pregnancy.
Two studies on COVID-19 in pregnancy have been reported to date. In nine pregnant women reported by Chen et al., COVID-19 pneumonia was identified in the third trimester. The women presented with fever, cough, myalgia, sore throat, and/or malaise. Fetal distress was reported in two; all nine infants were born alive. Apgar scores were 8-10 at 1 minute. Five were found to have lymphopenia; three had increases in hepatic enzymes. None of the infants developed severe COVID-19 pneumonia. Amniotic fluid, cord blood, neonatal throat swab, and breast milk samples from six of the nine patients were tested for the novel coronavirus 2019, and all results were negative (Lancet. 2020 Feb 12. doi: 10.1016/S0140-6736[20]30360-3)https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30360-3/fulltext.
In a study by Zhu et al., nine pregnant women with confirmed COVID-19 infection were identified during Jan. 20-Feb. 5, 2020. The onset of clinical symptoms in these women occurred before delivery in four cases, on the day of delivery in two cases, and after delivery in three cases. Of the 10 neonates (one set of twins) many had clinical symptoms, but none were proven to be COVID-19 positive in their pharyngeal swabs. Shortness of breath was observed in six, fever in two, tachycardia in one. GI symptoms such as feeding intolerance, bloating, GI bleed, and vomiting also were observed. Chest radiography showed abnormalities in seven neonates at admission. Thrombocytopenia and/or disseminated intravascular coagulopathy also was reported. Five neonates recovered and were discharged, one died, and four neonates remained in hospital in a stable condition. It is unclear if the illness in these infants was related to COVID-19 (Transl Pediatrics. 2020 Feb. doi: 10.21037/tp.2020.02.06)http://tp.amegroups.com/article/view/35919/28274.
In the limited experience to date, no evidence of virus has been found in the breast milk of women with COVID-19, which is consistent with the SARS experience. Current recommendations are to separate the infant from known COVID-19 infected mothers either in a different room or in the mother’s room using a six foot rule, a barrier curtain of some type, and mask and hand washing prior to any contact between mother and infant. If the mother desires to breastfeed her child, the same precautions – mask and hand washing – should be in place.
What about treatment?
There are no proven effective therapies and supportive care has been the mainstay to date. Clinical trials of remdesivir have been initiated both by Gilead (compassionate use, open label) and by the National Institutes of Health (randomized remdesivirhttps://www.drugs.com/history/remdesivir.html vs. placebo) in adults based on in vitro data suggesting activity again COVID-19. Lopinavir/ritonavir (combination protease inhibitors) also have been administered off label, but no results are available as yet.
Keeping up
I suggest several valuable resources to keep yourself abreast of the rapidly changing COVID-19 story. First the CDC website or your local Department of Health. These are being updated frequently and include advisories on personal protective equipment, clusters of cases in your local community, and current recommendations for mitigation of the epidemic. I have listened to Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, and Robert R. Redfield, MD, the director of the CDC almost daily. I trust their viewpoints and transparency about what is and what is not known, as well as the why and wherefore of their guidance, remembering that each day brings new information and new guidance.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University and public health and senior attending physician at Boston Medical Center. He has no relevant financial disclosures. Email him at [email protected].
Flu vaccine: Larger impact on influenza burden than you thought?
ID Week, the annual meeting of the Infectious Disease Society of America, provided valuable insights into past season’s endemic influenza burden and the effectiveness of prevention strategies. Each year, there are from 9million to 49 million influenza cases in the United States, 140,000-960,000 hospitalized cases, and 12,000-70,000 deaths directly attributable to influenza infection. The burden disproportionately falls on infants and adults 65 years of age and older; 11,000-48,000 children are hospitalized, and as many as several hundred children may die from influenza and related complications. School age children (aged 5-19 years) and adults (aged 30-39 years) are a major part of the transmission cycle. Influenza vaccine underlies the prevention strategy for limiting the burden of disease in U.S. populations. ID Week provided new insights into critical questions about influenza vaccines.
1. What is the effectiveness of influenza vaccine against severe disease (hospitalization) in children? Does it vary by age? By type or subtype?
Angela P. Campbell, MD, MPH, of the Centers for Disease Control and Prevention, and associates presented data on influenza vaccine effectiveness from the New Vaccine Surveillance Network in children for the 2016-2017 and 2017-2018 season (ID Week session 99; Abstract 899). During both 2016-2017 and 2017-2018, H3N2 was the dominant virus and influenza B represented about one-third of cases, and H1N1 was a greater percentage of cases in 2017-2018. Influenza positivity among children younger than 18 years of age admitted to hospital with respiratory disease was 14% among unvaccinated and 8% among vaccinated children; effectiveness again hospitalization was 50%. Vaccine effectiveness (VE) was not statistically different between children younger than 8 years of age and those older that 8 years but did differ by vaccine type. VE was 76% against H1N1 disease, 59% again B disease, and only 33% against H3N2 disease.
Clearly, vaccination with influenza vaccine prevents serious respiratory disease. However, the impact of vaccine will vary by season and by which influenza stains are circulating in the community. The authors concluded that further understanding of the lower VE against H3N2 disease is needed.
2. Does the priming dose of influenza vaccine improve vaccine effectiveness?
Current recommendations call for a two-dose series for influenza vaccine in children aged 6 months through 8 years who have not had prior influenza vaccine. The recommendation is based on evidence demonstrating higher antibody responses in children receiving two doses, compared with a single dose. Using data from the U.S. Influenza Vaccine Effectiveness Network, Jessie R. Chung, MPH, of the CDC, and associates compared VE in children younger than 2 years receiving two doses in the first year of flu immunization (fully immunized), compared with those who received only one dose (partially immunized) (ID Week session 99; Abstract 900). VE was 53% for fully immunized and 23% for partially immunized children. Receipt of a single dose did not provide statistically significant protection against influenza. Surprisingly (to me), of 5,355 children aged 6 months to less than 2 years with no prior influenza vaccine, 1,870 (35%) received only one dose in the season.
The data strongly support the current recommendations for a priming dose, especially in young children, in the first season of influenza vaccine and warrants increased efforts to increase the update of second doses during the season. Hopefully we can do better in 2019!
3. Should we wait to vaccinate with influenza vaccine?
Some evidence suggests that waning immunity to influenza vaccine, primarily in those aged 65 years and older, may explain increased disease activity toward the end of influenza season. Other explanations include increasing viral diversity throughout the season, resulting in reduced effectiveness. Do such concerns warrant delaying immunization? The onset and peak of influenza season varies by year; in October 2019, 3% of tests performed on patients with respiratory illness were influenza positive. The trade-offs for delaying immunization until October are the unpredictability of onset of influenza season, the requirement for two doses in infants, the need for 2 weeks to achieve peak antibody concentrations, and the potential that fewer individuals will be vaccinated. Kathy Neuzil, MD, MPH, from the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, reviewed recent modeling (for adults aged 65 years and older) and reported that delaying vaccine programs until October is associated with greater burden of hospitalization if 14% fewer individuals (who would be vaccinated in August/September) are vaccinated (ID Week; Session 940).
In response to these concerns, the CDC recommendations for 2019 are that, in children aged 6 months through 8 years who need two doses, start early so that you can achieve both doses before influenza season (MMWR 2019 Aug 23;68[3]:1-21).In older children and adults, who need only a single dose, early vaccination (August and early September) may lead to reduced protection late in the influenza season?
4. How can we optimize vaccine impact?
Vaccine impact refers to the affect on a population level and not at an individual level. Meagan C. Fitzpatrick, PhD, from the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, evaluated the benefits of our moderately effective influenza vaccines (VE 40%-60%) to the population beyond those who are vaccinated. Her conclusions were that even a modestly effective vaccine prevents 21 million cases of influenza, 129,000 hospitalizations, and 62,000 deaths. And that two-thirds of the deaths prevented are from herd benefit (or indirect effects). Although both coverage and vaccine effectiveness are important, she reported that population impact was most sensitive to coverage, compared with vaccine effectiveness. Dr. Fitzpatrick found that targeting school-age children 6-19 years of age and adults 30-39 years of age maximizes the public health benefits (herd effects) of influenza vaccine. In 2018 season, influenza coverage was 63% for at least one dose in children aged 6 months through 17 years and 45% in adults aged 18 years and older; in the two target age groups 5-17 and 30-39 years, coverage was 59% and approximately 35%, respectively (ID Week; Session 939).
Clearly, even our modestly effective influenza vaccines have significant public health benefit in protecting the U.S. populations from serious disease and death. Efforts to increase vaccine uptake in school-age children, both those with and without comorbidity, and the 30- to 39-year-old adult cohort would likely further reduce the burden of serious disease from influenza.
In summary, despite a vaccine that is only moderately effective, there is clear evidence to support current recommendations of universal immunization beginning at 6 months of age. Delaying until October 1 is a good idea only if the same number of individuals will receive influenza vaccine, otherwise the hypothetical benefit is lost.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University schools of medicine and public health and is senior attending physician, Boston Medical Center. Dr. Pelton has investigator-initiated research awards to Boston Medical Center from Pfizer and Merck Vaccines. He also received honorarium as an advisory board member, participation in symposium and consultation from Seqirus and Merck Vaccine, Pfizer, and Sanofi Pasteur. Email him at [email protected].
ID Week, the annual meeting of the Infectious Disease Society of America, provided valuable insights into past season’s endemic influenza burden and the effectiveness of prevention strategies. Each year, there are from 9million to 49 million influenza cases in the United States, 140,000-960,000 hospitalized cases, and 12,000-70,000 deaths directly attributable to influenza infection. The burden disproportionately falls on infants and adults 65 years of age and older; 11,000-48,000 children are hospitalized, and as many as several hundred children may die from influenza and related complications. School age children (aged 5-19 years) and adults (aged 30-39 years) are a major part of the transmission cycle. Influenza vaccine underlies the prevention strategy for limiting the burden of disease in U.S. populations. ID Week provided new insights into critical questions about influenza vaccines.
1. What is the effectiveness of influenza vaccine against severe disease (hospitalization) in children? Does it vary by age? By type or subtype?
Angela P. Campbell, MD, MPH, of the Centers for Disease Control and Prevention, and associates presented data on influenza vaccine effectiveness from the New Vaccine Surveillance Network in children for the 2016-2017 and 2017-2018 season (ID Week session 99; Abstract 899). During both 2016-2017 and 2017-2018, H3N2 was the dominant virus and influenza B represented about one-third of cases, and H1N1 was a greater percentage of cases in 2017-2018. Influenza positivity among children younger than 18 years of age admitted to hospital with respiratory disease was 14% among unvaccinated and 8% among vaccinated children; effectiveness again hospitalization was 50%. Vaccine effectiveness (VE) was not statistically different between children younger than 8 years of age and those older that 8 years but did differ by vaccine type. VE was 76% against H1N1 disease, 59% again B disease, and only 33% against H3N2 disease.
Clearly, vaccination with influenza vaccine prevents serious respiratory disease. However, the impact of vaccine will vary by season and by which influenza stains are circulating in the community. The authors concluded that further understanding of the lower VE against H3N2 disease is needed.
2. Does the priming dose of influenza vaccine improve vaccine effectiveness?
Current recommendations call for a two-dose series for influenza vaccine in children aged 6 months through 8 years who have not had prior influenza vaccine. The recommendation is based on evidence demonstrating higher antibody responses in children receiving two doses, compared with a single dose. Using data from the U.S. Influenza Vaccine Effectiveness Network, Jessie R. Chung, MPH, of the CDC, and associates compared VE in children younger than 2 years receiving two doses in the first year of flu immunization (fully immunized), compared with those who received only one dose (partially immunized) (ID Week session 99; Abstract 900). VE was 53% for fully immunized and 23% for partially immunized children. Receipt of a single dose did not provide statistically significant protection against influenza. Surprisingly (to me), of 5,355 children aged 6 months to less than 2 years with no prior influenza vaccine, 1,870 (35%) received only one dose in the season.
The data strongly support the current recommendations for a priming dose, especially in young children, in the first season of influenza vaccine and warrants increased efforts to increase the update of second doses during the season. Hopefully we can do better in 2019!
3. Should we wait to vaccinate with influenza vaccine?
Some evidence suggests that waning immunity to influenza vaccine, primarily in those aged 65 years and older, may explain increased disease activity toward the end of influenza season. Other explanations include increasing viral diversity throughout the season, resulting in reduced effectiveness. Do such concerns warrant delaying immunization? The onset and peak of influenza season varies by year; in October 2019, 3% of tests performed on patients with respiratory illness were influenza positive. The trade-offs for delaying immunization until October are the unpredictability of onset of influenza season, the requirement for two doses in infants, the need for 2 weeks to achieve peak antibody concentrations, and the potential that fewer individuals will be vaccinated. Kathy Neuzil, MD, MPH, from the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, reviewed recent modeling (for adults aged 65 years and older) and reported that delaying vaccine programs until October is associated with greater burden of hospitalization if 14% fewer individuals (who would be vaccinated in August/September) are vaccinated (ID Week; Session 940).
In response to these concerns, the CDC recommendations for 2019 are that, in children aged 6 months through 8 years who need two doses, start early so that you can achieve both doses before influenza season (MMWR 2019 Aug 23;68[3]:1-21).In older children and adults, who need only a single dose, early vaccination (August and early September) may lead to reduced protection late in the influenza season?
4. How can we optimize vaccine impact?
Vaccine impact refers to the affect on a population level and not at an individual level. Meagan C. Fitzpatrick, PhD, from the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, evaluated the benefits of our moderately effective influenza vaccines (VE 40%-60%) to the population beyond those who are vaccinated. Her conclusions were that even a modestly effective vaccine prevents 21 million cases of influenza, 129,000 hospitalizations, and 62,000 deaths. And that two-thirds of the deaths prevented are from herd benefit (or indirect effects). Although both coverage and vaccine effectiveness are important, she reported that population impact was most sensitive to coverage, compared with vaccine effectiveness. Dr. Fitzpatrick found that targeting school-age children 6-19 years of age and adults 30-39 years of age maximizes the public health benefits (herd effects) of influenza vaccine. In 2018 season, influenza coverage was 63% for at least one dose in children aged 6 months through 17 years and 45% in adults aged 18 years and older; in the two target age groups 5-17 and 30-39 years, coverage was 59% and approximately 35%, respectively (ID Week; Session 939).
Clearly, even our modestly effective influenza vaccines have significant public health benefit in protecting the U.S. populations from serious disease and death. Efforts to increase vaccine uptake in school-age children, both those with and without comorbidity, and the 30- to 39-year-old adult cohort would likely further reduce the burden of serious disease from influenza.
In summary, despite a vaccine that is only moderately effective, there is clear evidence to support current recommendations of universal immunization beginning at 6 months of age. Delaying until October 1 is a good idea only if the same number of individuals will receive influenza vaccine, otherwise the hypothetical benefit is lost.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University schools of medicine and public health and is senior attending physician, Boston Medical Center. Dr. Pelton has investigator-initiated research awards to Boston Medical Center from Pfizer and Merck Vaccines. He also received honorarium as an advisory board member, participation in symposium and consultation from Seqirus and Merck Vaccine, Pfizer, and Sanofi Pasteur. Email him at [email protected].
ID Week, the annual meeting of the Infectious Disease Society of America, provided valuable insights into past season’s endemic influenza burden and the effectiveness of prevention strategies. Each year, there are from 9million to 49 million influenza cases in the United States, 140,000-960,000 hospitalized cases, and 12,000-70,000 deaths directly attributable to influenza infection. The burden disproportionately falls on infants and adults 65 years of age and older; 11,000-48,000 children are hospitalized, and as many as several hundred children may die from influenza and related complications. School age children (aged 5-19 years) and adults (aged 30-39 years) are a major part of the transmission cycle. Influenza vaccine underlies the prevention strategy for limiting the burden of disease in U.S. populations. ID Week provided new insights into critical questions about influenza vaccines.
1. What is the effectiveness of influenza vaccine against severe disease (hospitalization) in children? Does it vary by age? By type or subtype?
Angela P. Campbell, MD, MPH, of the Centers for Disease Control and Prevention, and associates presented data on influenza vaccine effectiveness from the New Vaccine Surveillance Network in children for the 2016-2017 and 2017-2018 season (ID Week session 99; Abstract 899). During both 2016-2017 and 2017-2018, H3N2 was the dominant virus and influenza B represented about one-third of cases, and H1N1 was a greater percentage of cases in 2017-2018. Influenza positivity among children younger than 18 years of age admitted to hospital with respiratory disease was 14% among unvaccinated and 8% among vaccinated children; effectiveness again hospitalization was 50%. Vaccine effectiveness (VE) was not statistically different between children younger than 8 years of age and those older that 8 years but did differ by vaccine type. VE was 76% against H1N1 disease, 59% again B disease, and only 33% against H3N2 disease.
Clearly, vaccination with influenza vaccine prevents serious respiratory disease. However, the impact of vaccine will vary by season and by which influenza stains are circulating in the community. The authors concluded that further understanding of the lower VE against H3N2 disease is needed.
2. Does the priming dose of influenza vaccine improve vaccine effectiveness?
Current recommendations call for a two-dose series for influenza vaccine in children aged 6 months through 8 years who have not had prior influenza vaccine. The recommendation is based on evidence demonstrating higher antibody responses in children receiving two doses, compared with a single dose. Using data from the U.S. Influenza Vaccine Effectiveness Network, Jessie R. Chung, MPH, of the CDC, and associates compared VE in children younger than 2 years receiving two doses in the first year of flu immunization (fully immunized), compared with those who received only one dose (partially immunized) (ID Week session 99; Abstract 900). VE was 53% for fully immunized and 23% for partially immunized children. Receipt of a single dose did not provide statistically significant protection against influenza. Surprisingly (to me), of 5,355 children aged 6 months to less than 2 years with no prior influenza vaccine, 1,870 (35%) received only one dose in the season.
The data strongly support the current recommendations for a priming dose, especially in young children, in the first season of influenza vaccine and warrants increased efforts to increase the update of second doses during the season. Hopefully we can do better in 2019!
3. Should we wait to vaccinate with influenza vaccine?
Some evidence suggests that waning immunity to influenza vaccine, primarily in those aged 65 years and older, may explain increased disease activity toward the end of influenza season. Other explanations include increasing viral diversity throughout the season, resulting in reduced effectiveness. Do such concerns warrant delaying immunization? The onset and peak of influenza season varies by year; in October 2019, 3% of tests performed on patients with respiratory illness were influenza positive. The trade-offs for delaying immunization until October are the unpredictability of onset of influenza season, the requirement for two doses in infants, the need for 2 weeks to achieve peak antibody concentrations, and the potential that fewer individuals will be vaccinated. Kathy Neuzil, MD, MPH, from the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, reviewed recent modeling (for adults aged 65 years and older) and reported that delaying vaccine programs until October is associated with greater burden of hospitalization if 14% fewer individuals (who would be vaccinated in August/September) are vaccinated (ID Week; Session 940).
In response to these concerns, the CDC recommendations for 2019 are that, in children aged 6 months through 8 years who need two doses, start early so that you can achieve both doses before influenza season (MMWR 2019 Aug 23;68[3]:1-21).In older children and adults, who need only a single dose, early vaccination (August and early September) may lead to reduced protection late in the influenza season?
4. How can we optimize vaccine impact?
Vaccine impact refers to the affect on a population level and not at an individual level. Meagan C. Fitzpatrick, PhD, from the Center for Vaccine Development and Global Health, University of Maryland School of Medicine, evaluated the benefits of our moderately effective influenza vaccines (VE 40%-60%) to the population beyond those who are vaccinated. Her conclusions were that even a modestly effective vaccine prevents 21 million cases of influenza, 129,000 hospitalizations, and 62,000 deaths. And that two-thirds of the deaths prevented are from herd benefit (or indirect effects). Although both coverage and vaccine effectiveness are important, she reported that population impact was most sensitive to coverage, compared with vaccine effectiveness. Dr. Fitzpatrick found that targeting school-age children 6-19 years of age and adults 30-39 years of age maximizes the public health benefits (herd effects) of influenza vaccine. In 2018 season, influenza coverage was 63% for at least one dose in children aged 6 months through 17 years and 45% in adults aged 18 years and older; in the two target age groups 5-17 and 30-39 years, coverage was 59% and approximately 35%, respectively (ID Week; Session 939).
Clearly, even our modestly effective influenza vaccines have significant public health benefit in protecting the U.S. populations from serious disease and death. Efforts to increase vaccine uptake in school-age children, both those with and without comorbidity, and the 30- to 39-year-old adult cohort would likely further reduce the burden of serious disease from influenza.
In summary, despite a vaccine that is only moderately effective, there is clear evidence to support current recommendations of universal immunization beginning at 6 months of age. Delaying until October 1 is a good idea only if the same number of individuals will receive influenza vaccine, otherwise the hypothetical benefit is lost.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University schools of medicine and public health and is senior attending physician, Boston Medical Center. Dr. Pelton has investigator-initiated research awards to Boston Medical Center from Pfizer and Merck Vaccines. He also received honorarium as an advisory board member, participation in symposium and consultation from Seqirus and Merck Vaccine, Pfizer, and Sanofi Pasteur. Email him at [email protected].
Identifying CMV infection in asymptomatic newborns – one step closer?
Cytomegalovirus (CMV) infection is the most common congenital viral infection in U.S. children, with a frequency between 0.5% and 1% of newborn infants resulting in approximately 30,000 infected children annually. A small minority (approximately 10%) can be identified in the neonatal period as symptomatic with jaundice (from direct hyperbilirubinemia), petechiae (from thrombocytopenia), hepatosplenomegaly, microcephaly, or other manifestations. The vast majority are asymptomatic at birth, yet 15% will have or develop sensorineural hearing loss (SNHL) during the first few years of life; others (1%-2%) will develop vision loss associated with retinal scars. Congenital CMV accounts for 20% of those with SNHL detected at birth and 25% of children with SNHL at 4 years of age.
Screening for congenital CMV has been an ongoing subject of debate. The challenges of implementing screening programs are related both to the diagnostics (collecting urine samples on newborns) as well as with the question of whether we have treatment and interventions to offer babies diagnosed with congenital CMV across the complete spectrum of clinical presentations.
Current screening programs implemented in some hospitals, called “targeted screening,” in which babies who fail newborn screening programs are tested for CMV, are not sufficient to achieve the goal of identifying babies who will need follow-up for early detection of SNHL or vision abnormalities, or possibly early antiviral therapy (Valcyte; valganciclovir), because only a small portion of those who eventually develop SNHL are currently identified by the targeted screening programs.1
However, its availability only has added to the debate as to whether the time has arrived for universal screening.
Vertical transmission of CMV occurs in utero (during any of the trimesters), at birth by passage through the birth canal, or postnatally by ingestion of breast milk. Neonatal infection (in utero and postnatal) occurs in both mothers with primary CMV infection during gestation and in those with recurrent infection (from a different viral strain) or reactivation of infection. Severe clinically symptomatic disease and sequelae is associated with primary maternal infection and early transmission to the fetus. However, it is estimated that nonprimary maternal infection accounts for 75% of neonatal infections. Transmission by breast milk to full-term, healthy infants does not appear to be associated with clinical illness or sequelae; however, preterm infants or those with birth weights less than 1,500 g have a small risk of developing clinical disease.
The polymerase chain reaction–based saliva CMV test (Alethia CMV Assay Test System) was licensed by the Food and Drug Administration in November 2018 after studies demonstrated high sensitivity and specificity, compared with viral culture (the gold standard). In one study, 17,327 infants were screened with the liquid-saliva PCR assay, and 0.5% tested positive for CMV on both the saliva test and culture. Sensitivity and specificity of the liquid-saliva PCR assay were 100% and 99.9%, respectively.2 The availability of an approved saliva-based assay that is both highly sensitive and specific overcomes the challenge of collecting urine, which has been a limiting factor in development of pragmatic universal screening programs. To date, most of the focus in identification of congenital CMV infection has been linking newborn hearing testing programs with CMV testing. For some, these have been labeled “targeted screening programs for CMV.” To us, these appear to be best practice for medical evaluations of an infant with identified SNHL. The availability of saliva-based CMV testing should enable virtually all children who fail newborn screening to be tested for CMV. In multiple studies,3,4 6% of infants with confirmed hearing screen failure tested positive for CMV. A recent study5 identified only 1 infant among the 171 infants who failed newborn screening, however only approximately 15% of the infants were eventually confirmed as hearing impaired at audiology follow-up, suggesting that programmatically testing for CMV might be limited to those with confirmed hearing loss if such can be accomplished within a narrow window of time.
The major challenge with linking CMV testing with newborn hearing screening is whether treatment with valganciclovir would be of value in congenital CMV infection and isolated hearing loss. Studies of children with symptomatic central nervous system congenital CMV disease provide evidence of improvement (or lack of progression) in hearing loss in those treated with valganciclovir. Few, if any of these children had isolated hearing loss in this pivotal study.6 An observational study reported improved outcomes in 55 of 59 (93%) children with congenital CMV and isolated SNHL treated with valganciclovir between birth to 12 weeks of life.7 Hearing improved in nearly 70% of ears, 27% showed no change, and only 3% demonstrated progression of hearing loss; most of the improved ears returned to normal hearing. Currently, a National Institutes of Health study (ValEAR) is recruiting CMV-infected infants with isolated SNHL and randomizing them to treatment with valganciclovir or placebo. The goal is to determine if infants treated with valganciclovir will have better hearing and language outcomes.
Linking CMV testing to those who fail newborn hearing screening programs is an important step, as it appears such children are at least five times more likely to be infected with CMV than is the overall birth cohort. However, such strategies fall short of identifying the majority of newborns with congenital CMV infection, who are completely asymptomatic yet are at risk for development of complications that potentially have substantial impact on their quality of life. Although the availability of sensitive and specific PCR testing in saliva provides a pragmatic approach to identify infected children, many questions remain. First, would a confirmatory test be necessary, such as urine PCR (now considered the gold standard by many CMV experts)? Second, once identified, what regimen for follow-up testing would be indicated to identify those with early SNHL or retinopathy, and until what age? Third, is there a role for treatment in asymptomatic infection? Would that treatment be prophylactic, prior to the development of clinical signs, or implemented once early evidence of SNHL or retinopathy is present?
The Valgan Toddler study – sponsored by NIH and the University of Alabama as part of the Collaborative Antiviral Study Group – will enroll children who are aged 1 month through 3 years and who had a recent diagnosis of hearing loss (within the prior 12 weeks) and evidence of congenital CMV infection. The purpose of this study is to compare the effect on hearing and neurologic outcomes in infants aged 1 month through 4 years with recent onset SNHL who receive 6 weeks of valganciclovir versus children who do not receive this drug. The results of such studies will be critical for the development of best practices.
In summary, the licensure of a rapid PCR-based tool for diagnosis of CMV infection from saliva adds to our ability to develop screening programs to detect asymptomatic infants with congenital CMV infection. The ability to link newborns who fail hearing screening programs with CMV testing will lead to more detection of CMV-infected neonates, both with isolated hearing loss, and subsequently with no signs or symptoms of infection. There is an urgent need for evidence from randomized clinical trials to enable the development of best practices for such infants.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University and senior attending physician at Boston Medical Center. Dr. Lapidot is a senior fellow in pediatric infectious diseases, Boston Medical Center. Neither Dr. Pelton nor Dr. Lapidot have any relevant financial disclosures. Email them at [email protected].
References
1. J Pediatric Infect Dis Soc. 2019 Mar 28;8(1):55-9.
2. N Engl J Med 2011 Jun 2; 364:2111-8.
3. Pediatrics. 2008 May;121(5):970-5
4. J Clin Virol. 2018 May;102:110-5.
5. J Pediatric Infect Dis Soc. 2019 Mar;8(1):55-9.
6. J Pediatr. 2003 Jul;143(1):16-25.
7. J Pediatr. 2018 Aug;199:166-70.
Cytomegalovirus (CMV) infection is the most common congenital viral infection in U.S. children, with a frequency between 0.5% and 1% of newborn infants resulting in approximately 30,000 infected children annually. A small minority (approximately 10%) can be identified in the neonatal period as symptomatic with jaundice (from direct hyperbilirubinemia), petechiae (from thrombocytopenia), hepatosplenomegaly, microcephaly, or other manifestations. The vast majority are asymptomatic at birth, yet 15% will have or develop sensorineural hearing loss (SNHL) during the first few years of life; others (1%-2%) will develop vision loss associated with retinal scars. Congenital CMV accounts for 20% of those with SNHL detected at birth and 25% of children with SNHL at 4 years of age.
Screening for congenital CMV has been an ongoing subject of debate. The challenges of implementing screening programs are related both to the diagnostics (collecting urine samples on newborns) as well as with the question of whether we have treatment and interventions to offer babies diagnosed with congenital CMV across the complete spectrum of clinical presentations.
Current screening programs implemented in some hospitals, called “targeted screening,” in which babies who fail newborn screening programs are tested for CMV, are not sufficient to achieve the goal of identifying babies who will need follow-up for early detection of SNHL or vision abnormalities, or possibly early antiviral therapy (Valcyte; valganciclovir), because only a small portion of those who eventually develop SNHL are currently identified by the targeted screening programs.1
However, its availability only has added to the debate as to whether the time has arrived for universal screening.
Vertical transmission of CMV occurs in utero (during any of the trimesters), at birth by passage through the birth canal, or postnatally by ingestion of breast milk. Neonatal infection (in utero and postnatal) occurs in both mothers with primary CMV infection during gestation and in those with recurrent infection (from a different viral strain) or reactivation of infection. Severe clinically symptomatic disease and sequelae is associated with primary maternal infection and early transmission to the fetus. However, it is estimated that nonprimary maternal infection accounts for 75% of neonatal infections. Transmission by breast milk to full-term, healthy infants does not appear to be associated with clinical illness or sequelae; however, preterm infants or those with birth weights less than 1,500 g have a small risk of developing clinical disease.
The polymerase chain reaction–based saliva CMV test (Alethia CMV Assay Test System) was licensed by the Food and Drug Administration in November 2018 after studies demonstrated high sensitivity and specificity, compared with viral culture (the gold standard). In one study, 17,327 infants were screened with the liquid-saliva PCR assay, and 0.5% tested positive for CMV on both the saliva test and culture. Sensitivity and specificity of the liquid-saliva PCR assay were 100% and 99.9%, respectively.2 The availability of an approved saliva-based assay that is both highly sensitive and specific overcomes the challenge of collecting urine, which has been a limiting factor in development of pragmatic universal screening programs. To date, most of the focus in identification of congenital CMV infection has been linking newborn hearing testing programs with CMV testing. For some, these have been labeled “targeted screening programs for CMV.” To us, these appear to be best practice for medical evaluations of an infant with identified SNHL. The availability of saliva-based CMV testing should enable virtually all children who fail newborn screening to be tested for CMV. In multiple studies,3,4 6% of infants with confirmed hearing screen failure tested positive for CMV. A recent study5 identified only 1 infant among the 171 infants who failed newborn screening, however only approximately 15% of the infants were eventually confirmed as hearing impaired at audiology follow-up, suggesting that programmatically testing for CMV might be limited to those with confirmed hearing loss if such can be accomplished within a narrow window of time.
The major challenge with linking CMV testing with newborn hearing screening is whether treatment with valganciclovir would be of value in congenital CMV infection and isolated hearing loss. Studies of children with symptomatic central nervous system congenital CMV disease provide evidence of improvement (or lack of progression) in hearing loss in those treated with valganciclovir. Few, if any of these children had isolated hearing loss in this pivotal study.6 An observational study reported improved outcomes in 55 of 59 (93%) children with congenital CMV and isolated SNHL treated with valganciclovir between birth to 12 weeks of life.7 Hearing improved in nearly 70% of ears, 27% showed no change, and only 3% demonstrated progression of hearing loss; most of the improved ears returned to normal hearing. Currently, a National Institutes of Health study (ValEAR) is recruiting CMV-infected infants with isolated SNHL and randomizing them to treatment with valganciclovir or placebo. The goal is to determine if infants treated with valganciclovir will have better hearing and language outcomes.
Linking CMV testing to those who fail newborn hearing screening programs is an important step, as it appears such children are at least five times more likely to be infected with CMV than is the overall birth cohort. However, such strategies fall short of identifying the majority of newborns with congenital CMV infection, who are completely asymptomatic yet are at risk for development of complications that potentially have substantial impact on their quality of life. Although the availability of sensitive and specific PCR testing in saliva provides a pragmatic approach to identify infected children, many questions remain. First, would a confirmatory test be necessary, such as urine PCR (now considered the gold standard by many CMV experts)? Second, once identified, what regimen for follow-up testing would be indicated to identify those with early SNHL or retinopathy, and until what age? Third, is there a role for treatment in asymptomatic infection? Would that treatment be prophylactic, prior to the development of clinical signs, or implemented once early evidence of SNHL or retinopathy is present?
The Valgan Toddler study – sponsored by NIH and the University of Alabama as part of the Collaborative Antiviral Study Group – will enroll children who are aged 1 month through 3 years and who had a recent diagnosis of hearing loss (within the prior 12 weeks) and evidence of congenital CMV infection. The purpose of this study is to compare the effect on hearing and neurologic outcomes in infants aged 1 month through 4 years with recent onset SNHL who receive 6 weeks of valganciclovir versus children who do not receive this drug. The results of such studies will be critical for the development of best practices.
In summary, the licensure of a rapid PCR-based tool for diagnosis of CMV infection from saliva adds to our ability to develop screening programs to detect asymptomatic infants with congenital CMV infection. The ability to link newborns who fail hearing screening programs with CMV testing will lead to more detection of CMV-infected neonates, both with isolated hearing loss, and subsequently with no signs or symptoms of infection. There is an urgent need for evidence from randomized clinical trials to enable the development of best practices for such infants.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University and senior attending physician at Boston Medical Center. Dr. Lapidot is a senior fellow in pediatric infectious diseases, Boston Medical Center. Neither Dr. Pelton nor Dr. Lapidot have any relevant financial disclosures. Email them at [email protected].
References
1. J Pediatric Infect Dis Soc. 2019 Mar 28;8(1):55-9.
2. N Engl J Med 2011 Jun 2; 364:2111-8.
3. Pediatrics. 2008 May;121(5):970-5
4. J Clin Virol. 2018 May;102:110-5.
5. J Pediatric Infect Dis Soc. 2019 Mar;8(1):55-9.
6. J Pediatr. 2003 Jul;143(1):16-25.
7. J Pediatr. 2018 Aug;199:166-70.
Cytomegalovirus (CMV) infection is the most common congenital viral infection in U.S. children, with a frequency between 0.5% and 1% of newborn infants resulting in approximately 30,000 infected children annually. A small minority (approximately 10%) can be identified in the neonatal period as symptomatic with jaundice (from direct hyperbilirubinemia), petechiae (from thrombocytopenia), hepatosplenomegaly, microcephaly, or other manifestations. The vast majority are asymptomatic at birth, yet 15% will have or develop sensorineural hearing loss (SNHL) during the first few years of life; others (1%-2%) will develop vision loss associated with retinal scars. Congenital CMV accounts for 20% of those with SNHL detected at birth and 25% of children with SNHL at 4 years of age.
Screening for congenital CMV has been an ongoing subject of debate. The challenges of implementing screening programs are related both to the diagnostics (collecting urine samples on newborns) as well as with the question of whether we have treatment and interventions to offer babies diagnosed with congenital CMV across the complete spectrum of clinical presentations.
Current screening programs implemented in some hospitals, called “targeted screening,” in which babies who fail newborn screening programs are tested for CMV, are not sufficient to achieve the goal of identifying babies who will need follow-up for early detection of SNHL or vision abnormalities, or possibly early antiviral therapy (Valcyte; valganciclovir), because only a small portion of those who eventually develop SNHL are currently identified by the targeted screening programs.1
However, its availability only has added to the debate as to whether the time has arrived for universal screening.
Vertical transmission of CMV occurs in utero (during any of the trimesters), at birth by passage through the birth canal, or postnatally by ingestion of breast milk. Neonatal infection (in utero and postnatal) occurs in both mothers with primary CMV infection during gestation and in those with recurrent infection (from a different viral strain) or reactivation of infection. Severe clinically symptomatic disease and sequelae is associated with primary maternal infection and early transmission to the fetus. However, it is estimated that nonprimary maternal infection accounts for 75% of neonatal infections. Transmission by breast milk to full-term, healthy infants does not appear to be associated with clinical illness or sequelae; however, preterm infants or those with birth weights less than 1,500 g have a small risk of developing clinical disease.
The polymerase chain reaction–based saliva CMV test (Alethia CMV Assay Test System) was licensed by the Food and Drug Administration in November 2018 after studies demonstrated high sensitivity and specificity, compared with viral culture (the gold standard). In one study, 17,327 infants were screened with the liquid-saliva PCR assay, and 0.5% tested positive for CMV on both the saliva test and culture. Sensitivity and specificity of the liquid-saliva PCR assay were 100% and 99.9%, respectively.2 The availability of an approved saliva-based assay that is both highly sensitive and specific overcomes the challenge of collecting urine, which has been a limiting factor in development of pragmatic universal screening programs. To date, most of the focus in identification of congenital CMV infection has been linking newborn hearing testing programs with CMV testing. For some, these have been labeled “targeted screening programs for CMV.” To us, these appear to be best practice for medical evaluations of an infant with identified SNHL. The availability of saliva-based CMV testing should enable virtually all children who fail newborn screening to be tested for CMV. In multiple studies,3,4 6% of infants with confirmed hearing screen failure tested positive for CMV. A recent study5 identified only 1 infant among the 171 infants who failed newborn screening, however only approximately 15% of the infants were eventually confirmed as hearing impaired at audiology follow-up, suggesting that programmatically testing for CMV might be limited to those with confirmed hearing loss if such can be accomplished within a narrow window of time.
The major challenge with linking CMV testing with newborn hearing screening is whether treatment with valganciclovir would be of value in congenital CMV infection and isolated hearing loss. Studies of children with symptomatic central nervous system congenital CMV disease provide evidence of improvement (or lack of progression) in hearing loss in those treated with valganciclovir. Few, if any of these children had isolated hearing loss in this pivotal study.6 An observational study reported improved outcomes in 55 of 59 (93%) children with congenital CMV and isolated SNHL treated with valganciclovir between birth to 12 weeks of life.7 Hearing improved in nearly 70% of ears, 27% showed no change, and only 3% demonstrated progression of hearing loss; most of the improved ears returned to normal hearing. Currently, a National Institutes of Health study (ValEAR) is recruiting CMV-infected infants with isolated SNHL and randomizing them to treatment with valganciclovir or placebo. The goal is to determine if infants treated with valganciclovir will have better hearing and language outcomes.
Linking CMV testing to those who fail newborn hearing screening programs is an important step, as it appears such children are at least five times more likely to be infected with CMV than is the overall birth cohort. However, such strategies fall short of identifying the majority of newborns with congenital CMV infection, who are completely asymptomatic yet are at risk for development of complications that potentially have substantial impact on their quality of life. Although the availability of sensitive and specific PCR testing in saliva provides a pragmatic approach to identify infected children, many questions remain. First, would a confirmatory test be necessary, such as urine PCR (now considered the gold standard by many CMV experts)? Second, once identified, what regimen for follow-up testing would be indicated to identify those with early SNHL or retinopathy, and until what age? Third, is there a role for treatment in asymptomatic infection? Would that treatment be prophylactic, prior to the development of clinical signs, or implemented once early evidence of SNHL or retinopathy is present?
The Valgan Toddler study – sponsored by NIH and the University of Alabama as part of the Collaborative Antiviral Study Group – will enroll children who are aged 1 month through 3 years and who had a recent diagnosis of hearing loss (within the prior 12 weeks) and evidence of congenital CMV infection. The purpose of this study is to compare the effect on hearing and neurologic outcomes in infants aged 1 month through 4 years with recent onset SNHL who receive 6 weeks of valganciclovir versus children who do not receive this drug. The results of such studies will be critical for the development of best practices.
In summary, the licensure of a rapid PCR-based tool for diagnosis of CMV infection from saliva adds to our ability to develop screening programs to detect asymptomatic infants with congenital CMV infection. The ability to link newborns who fail hearing screening programs with CMV testing will lead to more detection of CMV-infected neonates, both with isolated hearing loss, and subsequently with no signs or symptoms of infection. There is an urgent need for evidence from randomized clinical trials to enable the development of best practices for such infants.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University and senior attending physician at Boston Medical Center. Dr. Lapidot is a senior fellow in pediatric infectious diseases, Boston Medical Center. Neither Dr. Pelton nor Dr. Lapidot have any relevant financial disclosures. Email them at [email protected].
References
1. J Pediatric Infect Dis Soc. 2019 Mar 28;8(1):55-9.
2. N Engl J Med 2011 Jun 2; 364:2111-8.
3. Pediatrics. 2008 May;121(5):970-5
4. J Clin Virol. 2018 May;102:110-5.
5. J Pediatric Infect Dis Soc. 2019 Mar;8(1):55-9.
6. J Pediatr. 2003 Jul;143(1):16-25.
7. J Pediatr. 2018 Aug;199:166-70.
Hand, foot, and mouth disease: From self-limited to fatal
Hand, foot, and mouth disease (HFMD) has become a global challenge since its first description in 1957 in New Zealand and Canada. Clinicians readily recognize the characteristic syndrome in young children: fever associated with a papulovesicular rash affecting the palms or soles, or both, usually in spring, summer, or fall. In most cases the disease is self-limited, and brief. However, aseptic meningitis, brainstem encephalitis, acute flaccid paralysis or autonomic nervous system deregulation or cardiorespiratory failure may complicate the clinical course.
HFMD is caused by enterovirus A (formerly called human enterovirus A) which consists of 25 serotypes including multiple coxsackie A viruses, multiple enteroviruses, simian enteroviruses, and baboon enterovirus A13. The clinical spectrum spans from herpangina, characterized by fever and painful mouth ulcers most prominent in the posterior oral cavity (uvula, tonsils, soft plates, and anterior pharyngeal folds), to HFMD with papulovesicular rash on palms and soles with or without mouth ulcers/vesicles.1 In atypical cases, the rash may be maculopapular and may include the buttocks, knees or elbows.
In the United States, the predominant cause is coxsackie A16. However, coxsackie A6 appears to be emerging; often more than one HFMD causing virus is circulating concurrently and clinically indistinguishable. Globally, especially, in Asia, enterovirus 1 is a major cause of HFMD and more often associated with prominent central nervous system involvement. Disease can be sporadic or epidemic. An outbreak is usually defined as two or more cases within a defined geographic area; global epidemics as large as 1.5 million cases (Taiwan, 1998) have been reported, and outbreaks in China involving tens of thousands with multiple deaths have been reported.
In 2015, an outbreak of HFMD occurred during basic military training at Lackland Air Force Base, Bexar County, Texas, due to coxsackie A6.2 The illness was characterized by prodromal symptoms of fever and malaise followed by erosive stomatitis and a rash that began on the palms and soles. The rate of infection among trainees was 4.7% (50 of 1,054 persons).
The differential diagnosis includes aphthous ulcers and herpetic gingivostomatitis.3 Aphthous ulcers are seen more commonly in older children and adolescents, are often recurrent, are not seasonal, and are not associated with rash. Herpes simplex virus gingivostomatitis usually has a febrile prodrome, perioral lesions are frequent in addition to gum and tongue involvement, and gingival bleeding is common. HFMD usually has an incubation period of 3-5 days and fever, malaise, and myalgia prodrome followed by onset of oral and dermatologic manifestations in sequence. The skin rash has features that may overlap with varicella, erythema multiforme (EM) or drug eruption. Varicella usually involves the face before spreading to the extremities, and the lesions are characterized by umbilication and subsequent crusting. EM is characterized by target lesions and drug eruptions are morbilliform or maculopapular. The majority of cases of HFMD are diagnosed clinically; polymerase chain reaction testing is available and best performed on throat or vesicle specimens. Serologic testing for A16 and enterovirus 71 (IgM) is available. Infected patients shed virus for 2-4 weeks and virus is stable in the environment resulting in fecal-oral or oral-oral transmission.
Atypical features of HFMD include occurrence in the winter (outbreak in Alabama in 2011/2012) or an atypical distribution of rash involving the antecubital and popliteal fossae distribution of rash, or “eczema coxsackium” – the accentuation of rash in areas previously affected by atopic dermatitis. Additional features may include nail dystrophies that manifest as Beau lines (deep grooved lines that run from side to side on the fingernail or the toenail) and nail shedding.
A spectrum of neurologic complications has been observed, more frequently with EV71 and more frequently in Asia. The spectrum includes aseptic meningitis and brainstem encephalitis. Progressive cardiopulmonary failure also can be observed in severe cases. The hallmark of severe disease is often presentation with high fever, sweating, mottled skin, and tachycardia. Early signs of CNS involvement include myoclonic jerks, ataxia, and “wandering eyes.”3 Elevated white blood count and/or hyperglycemia may distinguish children with severe disease from benign disease. Anecdotal reports of response to treatment with high-dose methylprednisolone and intravenous immune globulin suggest that the neurologic disease may be an autoimmune phenomenon.
The clinician’s primary role is to accurately diagnose HFMD, provide supportive care for fever and dehydration, and identify those with early signs or laboratory features heralding a more severe course of disease.3 The Centers for Disease Control and Prevention recommends frequent hand washing after toileting and changing diapers, disinfecting surfaces such as toys, avoiding close contact with infected individuals or sharing of personal items for all affected patients. No antiviral treatment is available although improvement following early treatment with acyclovir has been reported anecdotally. Intravenous immunoglobulin has been used in severe cases in Asia with retrospective data analysis suggesting a potential for improvement when administered prior to cardiopulmonary arrest.1
Dr. Pelton is professor of pediatrics and epidemiology at Boston University. Dr. Pelton said he had no relevant financial disclosures. Email him at [email protected].
References
1. Cleveland Clinic Journal of Medicine 2014;81(9):537-43.
2. Morbidity and Mortality Weekly Report MMWR. 2016 Jul 8;65(26);678-80.
3. A Guide to clinical management and public health response for hand, foot and mouth disease (HFMD).
Hand, foot, and mouth disease (HFMD) has become a global challenge since its first description in 1957 in New Zealand and Canada. Clinicians readily recognize the characteristic syndrome in young children: fever associated with a papulovesicular rash affecting the palms or soles, or both, usually in spring, summer, or fall. In most cases the disease is self-limited, and brief. However, aseptic meningitis, brainstem encephalitis, acute flaccid paralysis or autonomic nervous system deregulation or cardiorespiratory failure may complicate the clinical course.
HFMD is caused by enterovirus A (formerly called human enterovirus A) which consists of 25 serotypes including multiple coxsackie A viruses, multiple enteroviruses, simian enteroviruses, and baboon enterovirus A13. The clinical spectrum spans from herpangina, characterized by fever and painful mouth ulcers most prominent in the posterior oral cavity (uvula, tonsils, soft plates, and anterior pharyngeal folds), to HFMD with papulovesicular rash on palms and soles with or without mouth ulcers/vesicles.1 In atypical cases, the rash may be maculopapular and may include the buttocks, knees or elbows.
In the United States, the predominant cause is coxsackie A16. However, coxsackie A6 appears to be emerging; often more than one HFMD causing virus is circulating concurrently and clinically indistinguishable. Globally, especially, in Asia, enterovirus 1 is a major cause of HFMD and more often associated with prominent central nervous system involvement. Disease can be sporadic or epidemic. An outbreak is usually defined as two or more cases within a defined geographic area; global epidemics as large as 1.5 million cases (Taiwan, 1998) have been reported, and outbreaks in China involving tens of thousands with multiple deaths have been reported.
In 2015, an outbreak of HFMD occurred during basic military training at Lackland Air Force Base, Bexar County, Texas, due to coxsackie A6.2 The illness was characterized by prodromal symptoms of fever and malaise followed by erosive stomatitis and a rash that began on the palms and soles. The rate of infection among trainees was 4.7% (50 of 1,054 persons).
The differential diagnosis includes aphthous ulcers and herpetic gingivostomatitis.3 Aphthous ulcers are seen more commonly in older children and adolescents, are often recurrent, are not seasonal, and are not associated with rash. Herpes simplex virus gingivostomatitis usually has a febrile prodrome, perioral lesions are frequent in addition to gum and tongue involvement, and gingival bleeding is common. HFMD usually has an incubation period of 3-5 days and fever, malaise, and myalgia prodrome followed by onset of oral and dermatologic manifestations in sequence. The skin rash has features that may overlap with varicella, erythema multiforme (EM) or drug eruption. Varicella usually involves the face before spreading to the extremities, and the lesions are characterized by umbilication and subsequent crusting. EM is characterized by target lesions and drug eruptions are morbilliform or maculopapular. The majority of cases of HFMD are diagnosed clinically; polymerase chain reaction testing is available and best performed on throat or vesicle specimens. Serologic testing for A16 and enterovirus 71 (IgM) is available. Infected patients shed virus for 2-4 weeks and virus is stable in the environment resulting in fecal-oral or oral-oral transmission.
Atypical features of HFMD include occurrence in the winter (outbreak in Alabama in 2011/2012) or an atypical distribution of rash involving the antecubital and popliteal fossae distribution of rash, or “eczema coxsackium” – the accentuation of rash in areas previously affected by atopic dermatitis. Additional features may include nail dystrophies that manifest as Beau lines (deep grooved lines that run from side to side on the fingernail or the toenail) and nail shedding.
A spectrum of neurologic complications has been observed, more frequently with EV71 and more frequently in Asia. The spectrum includes aseptic meningitis and brainstem encephalitis. Progressive cardiopulmonary failure also can be observed in severe cases. The hallmark of severe disease is often presentation with high fever, sweating, mottled skin, and tachycardia. Early signs of CNS involvement include myoclonic jerks, ataxia, and “wandering eyes.”3 Elevated white blood count and/or hyperglycemia may distinguish children with severe disease from benign disease. Anecdotal reports of response to treatment with high-dose methylprednisolone and intravenous immune globulin suggest that the neurologic disease may be an autoimmune phenomenon.
The clinician’s primary role is to accurately diagnose HFMD, provide supportive care for fever and dehydration, and identify those with early signs or laboratory features heralding a more severe course of disease.3 The Centers for Disease Control and Prevention recommends frequent hand washing after toileting and changing diapers, disinfecting surfaces such as toys, avoiding close contact with infected individuals or sharing of personal items for all affected patients. No antiviral treatment is available although improvement following early treatment with acyclovir has been reported anecdotally. Intravenous immunoglobulin has been used in severe cases in Asia with retrospective data analysis suggesting a potential for improvement when administered prior to cardiopulmonary arrest.1
Dr. Pelton is professor of pediatrics and epidemiology at Boston University. Dr. Pelton said he had no relevant financial disclosures. Email him at [email protected].
References
1. Cleveland Clinic Journal of Medicine 2014;81(9):537-43.
2. Morbidity and Mortality Weekly Report MMWR. 2016 Jul 8;65(26);678-80.
3. A Guide to clinical management and public health response for hand, foot and mouth disease (HFMD).
Hand, foot, and mouth disease (HFMD) has become a global challenge since its first description in 1957 in New Zealand and Canada. Clinicians readily recognize the characteristic syndrome in young children: fever associated with a papulovesicular rash affecting the palms or soles, or both, usually in spring, summer, or fall. In most cases the disease is self-limited, and brief. However, aseptic meningitis, brainstem encephalitis, acute flaccid paralysis or autonomic nervous system deregulation or cardiorespiratory failure may complicate the clinical course.
HFMD is caused by enterovirus A (formerly called human enterovirus A) which consists of 25 serotypes including multiple coxsackie A viruses, multiple enteroviruses, simian enteroviruses, and baboon enterovirus A13. The clinical spectrum spans from herpangina, characterized by fever and painful mouth ulcers most prominent in the posterior oral cavity (uvula, tonsils, soft plates, and anterior pharyngeal folds), to HFMD with papulovesicular rash on palms and soles with or without mouth ulcers/vesicles.1 In atypical cases, the rash may be maculopapular and may include the buttocks, knees or elbows.
In the United States, the predominant cause is coxsackie A16. However, coxsackie A6 appears to be emerging; often more than one HFMD causing virus is circulating concurrently and clinically indistinguishable. Globally, especially, in Asia, enterovirus 1 is a major cause of HFMD and more often associated with prominent central nervous system involvement. Disease can be sporadic or epidemic. An outbreak is usually defined as two or more cases within a defined geographic area; global epidemics as large as 1.5 million cases (Taiwan, 1998) have been reported, and outbreaks in China involving tens of thousands with multiple deaths have been reported.
In 2015, an outbreak of HFMD occurred during basic military training at Lackland Air Force Base, Bexar County, Texas, due to coxsackie A6.2 The illness was characterized by prodromal symptoms of fever and malaise followed by erosive stomatitis and a rash that began on the palms and soles. The rate of infection among trainees was 4.7% (50 of 1,054 persons).
The differential diagnosis includes aphthous ulcers and herpetic gingivostomatitis.3 Aphthous ulcers are seen more commonly in older children and adolescents, are often recurrent, are not seasonal, and are not associated with rash. Herpes simplex virus gingivostomatitis usually has a febrile prodrome, perioral lesions are frequent in addition to gum and tongue involvement, and gingival bleeding is common. HFMD usually has an incubation period of 3-5 days and fever, malaise, and myalgia prodrome followed by onset of oral and dermatologic manifestations in sequence. The skin rash has features that may overlap with varicella, erythema multiforme (EM) or drug eruption. Varicella usually involves the face before spreading to the extremities, and the lesions are characterized by umbilication and subsequent crusting. EM is characterized by target lesions and drug eruptions are morbilliform or maculopapular. The majority of cases of HFMD are diagnosed clinically; polymerase chain reaction testing is available and best performed on throat or vesicle specimens. Serologic testing for A16 and enterovirus 71 (IgM) is available. Infected patients shed virus for 2-4 weeks and virus is stable in the environment resulting in fecal-oral or oral-oral transmission.
Atypical features of HFMD include occurrence in the winter (outbreak in Alabama in 2011/2012) or an atypical distribution of rash involving the antecubital and popliteal fossae distribution of rash, or “eczema coxsackium” – the accentuation of rash in areas previously affected by atopic dermatitis. Additional features may include nail dystrophies that manifest as Beau lines (deep grooved lines that run from side to side on the fingernail or the toenail) and nail shedding.
A spectrum of neurologic complications has been observed, more frequently with EV71 and more frequently in Asia. The spectrum includes aseptic meningitis and brainstem encephalitis. Progressive cardiopulmonary failure also can be observed in severe cases. The hallmark of severe disease is often presentation with high fever, sweating, mottled skin, and tachycardia. Early signs of CNS involvement include myoclonic jerks, ataxia, and “wandering eyes.”3 Elevated white blood count and/or hyperglycemia may distinguish children with severe disease from benign disease. Anecdotal reports of response to treatment with high-dose methylprednisolone and intravenous immune globulin suggest that the neurologic disease may be an autoimmune phenomenon.
The clinician’s primary role is to accurately diagnose HFMD, provide supportive care for fever and dehydration, and identify those with early signs or laboratory features heralding a more severe course of disease.3 The Centers for Disease Control and Prevention recommends frequent hand washing after toileting and changing diapers, disinfecting surfaces such as toys, avoiding close contact with infected individuals or sharing of personal items for all affected patients. No antiviral treatment is available although improvement following early treatment with acyclovir has been reported anecdotally. Intravenous immunoglobulin has been used in severe cases in Asia with retrospective data analysis suggesting a potential for improvement when administered prior to cardiopulmonary arrest.1
Dr. Pelton is professor of pediatrics and epidemiology at Boston University. Dr. Pelton said he had no relevant financial disclosures. Email him at [email protected].
References
1. Cleveland Clinic Journal of Medicine 2014;81(9):537-43.
2. Morbidity and Mortality Weekly Report MMWR. 2016 Jul 8;65(26);678-80.
3. A Guide to clinical management and public health response for hand, foot and mouth disease (HFMD).
Evaluating fever in the first 90 days of life
Fever in the youngest of infants creates a challenge for the pediatric clinician. Fever is a common presentation for serious bacterial infection (SBI) although most fevers are due to viral infection. However, the clinical presentation does not necessarily differ, and the risk for a poor outcome in this age group is substantial.
In the early stages of my pediatric career, most febrile infants less than 90 days of age were evaluated for sepsis, admitted, and treated with antibiotics pending culture results. Group B streptococcal sepsis or Escherichia coli sepsis were common in the first month of life, and Haemophilus influenza type B or Streptococcus pneumoniae in the second and third months of life. The approach to fever in the first 90 days has changed following both the introduction of haemophilus and pneumococcal conjugate vaccines, the experience with risk stratification criteria for identifying infants at low risk for SBI, and the recognition of urinary tract infection (UTI) as a common source of infection in this age group as well as development of criteria for diagnosis.
A further nuance was subsequently added with the introduction of rapid diagnostics for viral infection. Byington et al. found that the majority of febrile infants less than 90 days of age had viral infection with enterovirus, respiratory syncytial virus (RSV), influenza or rotavirus.1 Using the Rochester risk stratification and the presence or absence of viral infection, she demonstrated that the risk of SBI was reduced in both high- and low-risk infants in the presence of viral infection; in low risk infants with viral infection, SBI was identified in 1.8%, compared with 3.1% in those without viral infection, and in high-risk infants. 5.5% has SBI when viral infection was found, compared to 16.7% in the absence of viral infection. She also proposed risk features to identify those infected with herpes simplex virus; age less than 42 days, vesicular rash, elevated alanine transaminase (ALT) and aspartate aminotransferase (AST), CSF pleocytosis, and seizure or twitching.
Greenhow et al. reported on the experience with “serious” bacterial infection in infants less than 90 days of age receiving care at Northern California Kaiser Permanente during the period 2005-2011.2 As pictured, the majority of children have UTI, and smaller numbers have bacteremia or meningitis. A small group of children with UTI have urosepsis as well; those with urosepsis can be differentiated from those with only UTI by age (less than 21 days), clinical exam (ill appearing), and elevated C reactive protein (greater than 20 mg/L) or elevated procalcitonin (greater than 0.5 ng/mL).3 Further evaluation of procalcitonin by other groups appears to validate its role in identifying children at low risk of SBI (procalcitonin less than 0.3 ng/mL).4
Currently, studies of febrile infants less than 90 days of age demonstrate that E. coli dominates in bacteremia, UTI, and meningitis, with Group B streptococcus as the next most frequent pathogen identified.2 Increasingly ampicillin resistance has been reported among E. coli isolates from both early- and late-onset disease as well as rare isolates that are resistant to third generation cephalosporins or gentamicin. Surveillance to identify changes in antimicrobial susceptibility will need to be ongoing to ensure that current approaches for initial therapy in high-risk infants aligns with current susceptibility patterns.
Dr. Pelton is chief of the section of pediatric infectious diseases and coordinator of the maternal-child HIV program at Boston Medical Center. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Pediatrics. 2004 Jun;113(6):1662-6.
2. Pediatr Infect Dis J. 2014 Jun;33(6):595-9.
3. Pediatr Infect Dis J. 2015 Jan;34(1):17-21.
4. JAMA Pediatr. 2016;170(1):17-18.
5. “AAP Proposes Update to Evaluating, Managing Febrile Infants Guideline,” The Hospitalist, 2016.
Fever in the youngest of infants creates a challenge for the pediatric clinician. Fever is a common presentation for serious bacterial infection (SBI) although most fevers are due to viral infection. However, the clinical presentation does not necessarily differ, and the risk for a poor outcome in this age group is substantial.
In the early stages of my pediatric career, most febrile infants less than 90 days of age were evaluated for sepsis, admitted, and treated with antibiotics pending culture results. Group B streptococcal sepsis or Escherichia coli sepsis were common in the first month of life, and Haemophilus influenza type B or Streptococcus pneumoniae in the second and third months of life. The approach to fever in the first 90 days has changed following both the introduction of haemophilus and pneumococcal conjugate vaccines, the experience with risk stratification criteria for identifying infants at low risk for SBI, and the recognition of urinary tract infection (UTI) as a common source of infection in this age group as well as development of criteria for diagnosis.
A further nuance was subsequently added with the introduction of rapid diagnostics for viral infection. Byington et al. found that the majority of febrile infants less than 90 days of age had viral infection with enterovirus, respiratory syncytial virus (RSV), influenza or rotavirus.1 Using the Rochester risk stratification and the presence or absence of viral infection, she demonstrated that the risk of SBI was reduced in both high- and low-risk infants in the presence of viral infection; in low risk infants with viral infection, SBI was identified in 1.8%, compared with 3.1% in those without viral infection, and in high-risk infants. 5.5% has SBI when viral infection was found, compared to 16.7% in the absence of viral infection. She also proposed risk features to identify those infected with herpes simplex virus; age less than 42 days, vesicular rash, elevated alanine transaminase (ALT) and aspartate aminotransferase (AST), CSF pleocytosis, and seizure or twitching.
Greenhow et al. reported on the experience with “serious” bacterial infection in infants less than 90 days of age receiving care at Northern California Kaiser Permanente during the period 2005-2011.2 As pictured, the majority of children have UTI, and smaller numbers have bacteremia or meningitis. A small group of children with UTI have urosepsis as well; those with urosepsis can be differentiated from those with only UTI by age (less than 21 days), clinical exam (ill appearing), and elevated C reactive protein (greater than 20 mg/L) or elevated procalcitonin (greater than 0.5 ng/mL).3 Further evaluation of procalcitonin by other groups appears to validate its role in identifying children at low risk of SBI (procalcitonin less than 0.3 ng/mL).4
Currently, studies of febrile infants less than 90 days of age demonstrate that E. coli dominates in bacteremia, UTI, and meningitis, with Group B streptococcus as the next most frequent pathogen identified.2 Increasingly ampicillin resistance has been reported among E. coli isolates from both early- and late-onset disease as well as rare isolates that are resistant to third generation cephalosporins or gentamicin. Surveillance to identify changes in antimicrobial susceptibility will need to be ongoing to ensure that current approaches for initial therapy in high-risk infants aligns with current susceptibility patterns.
Dr. Pelton is chief of the section of pediatric infectious diseases and coordinator of the maternal-child HIV program at Boston Medical Center. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Pediatrics. 2004 Jun;113(6):1662-6.
2. Pediatr Infect Dis J. 2014 Jun;33(6):595-9.
3. Pediatr Infect Dis J. 2015 Jan;34(1):17-21.
4. JAMA Pediatr. 2016;170(1):17-18.
5. “AAP Proposes Update to Evaluating, Managing Febrile Infants Guideline,” The Hospitalist, 2016.
Fever in the youngest of infants creates a challenge for the pediatric clinician. Fever is a common presentation for serious bacterial infection (SBI) although most fevers are due to viral infection. However, the clinical presentation does not necessarily differ, and the risk for a poor outcome in this age group is substantial.
In the early stages of my pediatric career, most febrile infants less than 90 days of age were evaluated for sepsis, admitted, and treated with antibiotics pending culture results. Group B streptococcal sepsis or Escherichia coli sepsis were common in the first month of life, and Haemophilus influenza type B or Streptococcus pneumoniae in the second and third months of life. The approach to fever in the first 90 days has changed following both the introduction of haemophilus and pneumococcal conjugate vaccines, the experience with risk stratification criteria for identifying infants at low risk for SBI, and the recognition of urinary tract infection (UTI) as a common source of infection in this age group as well as development of criteria for diagnosis.
A further nuance was subsequently added with the introduction of rapid diagnostics for viral infection. Byington et al. found that the majority of febrile infants less than 90 days of age had viral infection with enterovirus, respiratory syncytial virus (RSV), influenza or rotavirus.1 Using the Rochester risk stratification and the presence or absence of viral infection, she demonstrated that the risk of SBI was reduced in both high- and low-risk infants in the presence of viral infection; in low risk infants with viral infection, SBI was identified in 1.8%, compared with 3.1% in those without viral infection, and in high-risk infants. 5.5% has SBI when viral infection was found, compared to 16.7% in the absence of viral infection. She also proposed risk features to identify those infected with herpes simplex virus; age less than 42 days, vesicular rash, elevated alanine transaminase (ALT) and aspartate aminotransferase (AST), CSF pleocytosis, and seizure or twitching.
Greenhow et al. reported on the experience with “serious” bacterial infection in infants less than 90 days of age receiving care at Northern California Kaiser Permanente during the period 2005-2011.2 As pictured, the majority of children have UTI, and smaller numbers have bacteremia or meningitis. A small group of children with UTI have urosepsis as well; those with urosepsis can be differentiated from those with only UTI by age (less than 21 days), clinical exam (ill appearing), and elevated C reactive protein (greater than 20 mg/L) or elevated procalcitonin (greater than 0.5 ng/mL).3 Further evaluation of procalcitonin by other groups appears to validate its role in identifying children at low risk of SBI (procalcitonin less than 0.3 ng/mL).4
Currently, studies of febrile infants less than 90 days of age demonstrate that E. coli dominates in bacteremia, UTI, and meningitis, with Group B streptococcus as the next most frequent pathogen identified.2 Increasingly ampicillin resistance has been reported among E. coli isolates from both early- and late-onset disease as well as rare isolates that are resistant to third generation cephalosporins or gentamicin. Surveillance to identify changes in antimicrobial susceptibility will need to be ongoing to ensure that current approaches for initial therapy in high-risk infants aligns with current susceptibility patterns.
Dr. Pelton is chief of the section of pediatric infectious diseases and coordinator of the maternal-child HIV program at Boston Medical Center. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Pediatrics. 2004 Jun;113(6):1662-6.
2. Pediatr Infect Dis J. 2014 Jun;33(6):595-9.
3. Pediatr Infect Dis J. 2015 Jan;34(1):17-21.
4. JAMA Pediatr. 2016;170(1):17-18.
5. “AAP Proposes Update to Evaluating, Managing Febrile Infants Guideline,” The Hospitalist, 2016.
A ‘game changer’ for pediatric HIV
In memory of Anne Marie Regan, CPNP, senior research coordinator, Pediatric HIV Program, Boston City Hospital
Our first child with perinatal HIV presented in 1985 at age 4 weeks with failure to thrive, vomiting, diarrhea, and thrush. Over the next several years, the number of HIV-infected infants grew exponentially, and by 1991, we were caring for more than 50 infants and children at Boston City Hospital.
Antiretrovirals were marginally effective for HIV-infected infants and children at this time. Subsequently, we embarked on a national effort to prevent vertical transmission. We participated first in the study of pharmacokinetics of zidovudine (AZT) in newborns. We enrolled patients in ACTG 076 to test the hypothesis that treatment with AZT during pregnancy and labor, and in the infant, would reduce the risk of vertical transmission. Fifty U.S. and nine French sites enrolled 473 women between April 1991 and December 20, 1993. The results were spectacular; 8 of 100 infants in the AZT treatment group, compared with 25 out of 100 infants in the control group, developed HIV. By 1995, HIV testing was offered to all women at Boston Medical Center (formerly Boston City Hospital), and the promise of prevention of vertical transmission was reaching fruition. Between 1996 and 2016, approximately 500 HIV-infected women delivered at Boston Medical Center with vertical transmission identified in only 6 (1.2%) infants; without ACTG 076, we would have expected 125! In 2013, the Centers for Disease Control and Prevention reported that 70% of pregnant HIV-infected women received the complete 076 regimen, and 93% of mothers or infants received some part of the regimen. In 1992, 900 HIV-infected infants were diagnosed in the United States, and as many as 2,000 newborns were estimated to have been born infected with HIV; in 2015, 86 vertical transmissions were identified. This was, and remains, a remarkable accomplishment.
Dr. Pelton is chief of pediatric infectious diseases and coordinator of the maternal-child HIV program at Boston Medical Center. Ms. Moloney is a certified pediatric nurse practitioner in the division of pediatric infectious diseases. Dr. Pelton said he had no relevant financial disclosures, and Ms. Moloney is a speaker (on vaccines) for Sanofi Pasteur. Email them at [email protected].
In memory of Anne Marie Regan, CPNP, senior research coordinator, Pediatric HIV Program, Boston City Hospital
Our first child with perinatal HIV presented in 1985 at age 4 weeks with failure to thrive, vomiting, diarrhea, and thrush. Over the next several years, the number of HIV-infected infants grew exponentially, and by 1991, we were caring for more than 50 infants and children at Boston City Hospital.
Antiretrovirals were marginally effective for HIV-infected infants and children at this time. Subsequently, we embarked on a national effort to prevent vertical transmission. We participated first in the study of pharmacokinetics of zidovudine (AZT) in newborns. We enrolled patients in ACTG 076 to test the hypothesis that treatment with AZT during pregnancy and labor, and in the infant, would reduce the risk of vertical transmission. Fifty U.S. and nine French sites enrolled 473 women between April 1991 and December 20, 1993. The results were spectacular; 8 of 100 infants in the AZT treatment group, compared with 25 out of 100 infants in the control group, developed HIV. By 1995, HIV testing was offered to all women at Boston Medical Center (formerly Boston City Hospital), and the promise of prevention of vertical transmission was reaching fruition. Between 1996 and 2016, approximately 500 HIV-infected women delivered at Boston Medical Center with vertical transmission identified in only 6 (1.2%) infants; without ACTG 076, we would have expected 125! In 2013, the Centers for Disease Control and Prevention reported that 70% of pregnant HIV-infected women received the complete 076 regimen, and 93% of mothers or infants received some part of the regimen. In 1992, 900 HIV-infected infants were diagnosed in the United States, and as many as 2,000 newborns were estimated to have been born infected with HIV; in 2015, 86 vertical transmissions were identified. This was, and remains, a remarkable accomplishment.
Dr. Pelton is chief of pediatric infectious diseases and coordinator of the maternal-child HIV program at Boston Medical Center. Ms. Moloney is a certified pediatric nurse practitioner in the division of pediatric infectious diseases. Dr. Pelton said he had no relevant financial disclosures, and Ms. Moloney is a speaker (on vaccines) for Sanofi Pasteur. Email them at [email protected].
In memory of Anne Marie Regan, CPNP, senior research coordinator, Pediatric HIV Program, Boston City Hospital
Our first child with perinatal HIV presented in 1985 at age 4 weeks with failure to thrive, vomiting, diarrhea, and thrush. Over the next several years, the number of HIV-infected infants grew exponentially, and by 1991, we were caring for more than 50 infants and children at Boston City Hospital.
Antiretrovirals were marginally effective for HIV-infected infants and children at this time. Subsequently, we embarked on a national effort to prevent vertical transmission. We participated first in the study of pharmacokinetics of zidovudine (AZT) in newborns. We enrolled patients in ACTG 076 to test the hypothesis that treatment with AZT during pregnancy and labor, and in the infant, would reduce the risk of vertical transmission. Fifty U.S. and nine French sites enrolled 473 women between April 1991 and December 20, 1993. The results were spectacular; 8 of 100 infants in the AZT treatment group, compared with 25 out of 100 infants in the control group, developed HIV. By 1995, HIV testing was offered to all women at Boston Medical Center (formerly Boston City Hospital), and the promise of prevention of vertical transmission was reaching fruition. Between 1996 and 2016, approximately 500 HIV-infected women delivered at Boston Medical Center with vertical transmission identified in only 6 (1.2%) infants; without ACTG 076, we would have expected 125! In 2013, the Centers for Disease Control and Prevention reported that 70% of pregnant HIV-infected women received the complete 076 regimen, and 93% of mothers or infants received some part of the regimen. In 1992, 900 HIV-infected infants were diagnosed in the United States, and as many as 2,000 newborns were estimated to have been born infected with HIV; in 2015, 86 vertical transmissions were identified. This was, and remains, a remarkable accomplishment.
Dr. Pelton is chief of pediatric infectious diseases and coordinator of the maternal-child HIV program at Boston Medical Center. Ms. Moloney is a certified pediatric nurse practitioner in the division of pediatric infectious diseases. Dr. Pelton said he had no relevant financial disclosures, and Ms. Moloney is a speaker (on vaccines) for Sanofi Pasteur. Email them at [email protected].