User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Inside the flawed White House testing scheme that did not protect Trump
The president has said others are tested before getting close to him, appearing to hold it as an iron shield of safety. He has largely eschewed mask-wearing and social distancing in meetings, travel and public events, while holding rallies for thousands of often maskless supporters.
The Trump administration has increasingly pinned its coronavirus testing strategy for the nation on antigen tests, which do not need a traditional lab for processing and quickly return results to patients. But the results are less accurate than those of the slower PCR tests.
An early antigen test used by the White House was woefully inaccurate. But the new antigen test the White House is using has not been independently evaluated for accuracy and reliability. Moreover, this is the kit the Trump administration is pushing out to thousands of nursing homes to test residents and staff.
Testing “isn’t a ‘get out of jail free card,’” said Dr. Alan Wells, medical director of clinical labs at the University of Pittsburgh Medical Center and creator of its test for the novel coronavirus. In general, antigen tests can miss up to half the cases that are detected by polymerase chain reaction tests, depending on the population of patients tested, he said.
The White House said the president’s diagnosis was confirmed with a PCR test but declined to say which test delivered his initial result. The White House has been using a new antigen test from Abbott Laboratories to screen its staff for COVID-19, according to two administration officials.
The test, known as BinaxNOW, received an emergency use authorization from the Food and Drug Administration in August. It produces results in 15 minutes. Yet little is independently known about how effective it is. According to the company, the test is 97% accurate in detecting positives and 98.5% accurate in identifying those without disease. Abbott’s stated performance of its antigen test was based on examining people within 7 days of COVID symptoms appearing.
The president and first lady have both had symptoms, according to White House chief of staff Mark Meadows and the first lady’s Twitter account. The president was admitted to Walter Reed National Military Medical Center on Friday evening “out of an abundance of caution,” White House press secretary Kayleigh McEnany said in a statement.
Vice President Mike Pence is also tested daily for the virus and tested negative, spokesperson Devin O’Malley said Friday, but he did not respond to a follow-up question about which test was used.
Trump heavily promoted another Abbott rapid testing device, the ID NOW, earlier this year. But that test relies on different technology than the newer Abbott antigen test.
“I have not seen any independent evaluation of the Binax assay in the literature or in the blogs,” Wells said. “It is an unknown.”
The Department of Health and Human Services announced in August that it had signed a $760 million contract with Abbott for 150 million BinaxNOW antigen tests, which are now being distributed to nursing homes and historically black colleges and universities, as well as to governors to help inform decisions about opening and closing schools. The Big Ten football conference has also pinned playing hopes on the deployment of antigen tests following Trump’s political pressure.
However, even senior federal officials concede that a test alone isn’t likely to stop the spread of a virus that has sickened more than 7 million Americans.
“Testing does not substitute for avoiding crowded indoor spaces, washing hands, or wearing a mask when you can’t physically distance; further, a negative test today does not mean that you won’t be positive tomorrow,” Adm. Brett Giroir, the senior HHS official helming the administration’s testing effort, said in a statement at the time.
Trump could be part of a “super-spreading event,” said Dr. Michael Osterholm, director of the Center for Infectious Disease Research and Policy at the University of Minnesota.
Given the timing of Trump’s positive test — which he announced on Twitter early Friday – his infection “likely happened 5 or more days ago,” Osterholm said. “If so, then he was widely infectious as early as Tuesday,” the day of the first presidential debate in Cleveland.
At least seven people who attended a Rose Garden announcement last Saturday, when Trump announced his nomination of Judge Amy Coney Barrett to the Supreme Court, have since tested positive for the coronavirus. They include Trump’s former adviser Kellyanne Conway, Republican Sens. Mike Lee and Thom Tillis, and the president of the University of Notre Dame, the Rev. John Jenkins.
“Having that many infected people there all at one time, we’re still going to see transmission coming off that event for a couple days,” Osterholm said.
Osterholm notes that about 20% of infected people lead to 80% of COVID-19 cases, because “super spreaders” can infect so many people at once.
He notes that participants and audience members at Tuesday’s debate were separated by at least 6 feet. But 6 feet isn’t always enough to prevent infection, he said.
While many COVID-19 infections appear to be spread by respiratory droplets, which usually fall to the ground within 6 feet, people who are singing or speaking loudly can project virus much further. Evidence also suggests that the novel coronavirus can spread through aerosols, floating in the air like a speck of dust.
“I wonder how much virus was floating in that room that night,” Osterholm said.
Other experts say it’s too soon to say whether Trump was infected in a super-spreader event. “The president and his wife have had many exposures to many people in enclosed venues without protection,” so they could have been infected at any number of places, said Dr. William Schaffner, an infectious disease specialist at the Vanderbilt University School of Medicine.
Although Democratic presidential candidate and former Vice President Joe Biden tested negative for the virus with a PCR test Friday, experts note that false-negative results are common in the first few days after infection. Test results over the next several days will yield more useful information.
It can take more than a week for the virus to reproduce enough to be detected, Wells said: “You are probably not detectable for 3, 5, 7, even 10 days after you’re exposed.”
In Minnesota, where Trump held an outdoor campaign rally in Duluth with hundreds of attendees Wednesday, health officials warned that a 14-day quarantine is necessary, regardless of test results.
“Anyone who was a direct contact of President Trump or known COVID-19 cases needs to quarantine and should get tested,” the Minnesota Department of Health said.
Ongoing lapses in test result reporting could hamper efforts to track and isolate sick people. As of Sept. 10, 21 states and the District of Columbia were not reporting all antigen test results, according to a KHN investigation, a lapse in reporting that officials say leaves them blind to disease spread. Since then, public health departments in Arizona, North Carolina and South Dakota all have announced plans to add antigen testing to their case reporting.
Requests for comment to the D.C. Department of Health were referred to Mayor Muriel Bowser’s office, which did not respond. District health officials told KHN in early September that the White House does not report antigen test results to them – a potential violation of federal law under the CARES Act, which says any institution performing tests to diagnose COVID-19 must report all results to local or state public health departments.
Dr. Amesh Adalja, a senior scholar at the Johns Hopkins University Center for Health Security, said it’s not surprising that Trump tested positive, given that so many of his close associates – including his national security adviser and Secret Service officers – have also been infected by the virus.
“When you look at the number of social contacts and travel schedules, it’s not surprising,” Adalja said.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
The president has said others are tested before getting close to him, appearing to hold it as an iron shield of safety. He has largely eschewed mask-wearing and social distancing in meetings, travel and public events, while holding rallies for thousands of often maskless supporters.
The Trump administration has increasingly pinned its coronavirus testing strategy for the nation on antigen tests, which do not need a traditional lab for processing and quickly return results to patients. But the results are less accurate than those of the slower PCR tests.
An early antigen test used by the White House was woefully inaccurate. But the new antigen test the White House is using has not been independently evaluated for accuracy and reliability. Moreover, this is the kit the Trump administration is pushing out to thousands of nursing homes to test residents and staff.
Testing “isn’t a ‘get out of jail free card,’” said Dr. Alan Wells, medical director of clinical labs at the University of Pittsburgh Medical Center and creator of its test for the novel coronavirus. In general, antigen tests can miss up to half the cases that are detected by polymerase chain reaction tests, depending on the population of patients tested, he said.
The White House said the president’s diagnosis was confirmed with a PCR test but declined to say which test delivered his initial result. The White House has been using a new antigen test from Abbott Laboratories to screen its staff for COVID-19, according to two administration officials.
The test, known as BinaxNOW, received an emergency use authorization from the Food and Drug Administration in August. It produces results in 15 minutes. Yet little is independently known about how effective it is. According to the company, the test is 97% accurate in detecting positives and 98.5% accurate in identifying those without disease. Abbott’s stated performance of its antigen test was based on examining people within 7 days of COVID symptoms appearing.
The president and first lady have both had symptoms, according to White House chief of staff Mark Meadows and the first lady’s Twitter account. The president was admitted to Walter Reed National Military Medical Center on Friday evening “out of an abundance of caution,” White House press secretary Kayleigh McEnany said in a statement.
Vice President Mike Pence is also tested daily for the virus and tested negative, spokesperson Devin O’Malley said Friday, but he did not respond to a follow-up question about which test was used.
Trump heavily promoted another Abbott rapid testing device, the ID NOW, earlier this year. But that test relies on different technology than the newer Abbott antigen test.
“I have not seen any independent evaluation of the Binax assay in the literature or in the blogs,” Wells said. “It is an unknown.”
The Department of Health and Human Services announced in August that it had signed a $760 million contract with Abbott for 150 million BinaxNOW antigen tests, which are now being distributed to nursing homes and historically black colleges and universities, as well as to governors to help inform decisions about opening and closing schools. The Big Ten football conference has also pinned playing hopes on the deployment of antigen tests following Trump’s political pressure.
However, even senior federal officials concede that a test alone isn’t likely to stop the spread of a virus that has sickened more than 7 million Americans.
“Testing does not substitute for avoiding crowded indoor spaces, washing hands, or wearing a mask when you can’t physically distance; further, a negative test today does not mean that you won’t be positive tomorrow,” Adm. Brett Giroir, the senior HHS official helming the administration’s testing effort, said in a statement at the time.
Trump could be part of a “super-spreading event,” said Dr. Michael Osterholm, director of the Center for Infectious Disease Research and Policy at the University of Minnesota.
Given the timing of Trump’s positive test — which he announced on Twitter early Friday – his infection “likely happened 5 or more days ago,” Osterholm said. “If so, then he was widely infectious as early as Tuesday,” the day of the first presidential debate in Cleveland.
At least seven people who attended a Rose Garden announcement last Saturday, when Trump announced his nomination of Judge Amy Coney Barrett to the Supreme Court, have since tested positive for the coronavirus. They include Trump’s former adviser Kellyanne Conway, Republican Sens. Mike Lee and Thom Tillis, and the president of the University of Notre Dame, the Rev. John Jenkins.
“Having that many infected people there all at one time, we’re still going to see transmission coming off that event for a couple days,” Osterholm said.
Osterholm notes that about 20% of infected people lead to 80% of COVID-19 cases, because “super spreaders” can infect so many people at once.
He notes that participants and audience members at Tuesday’s debate were separated by at least 6 feet. But 6 feet isn’t always enough to prevent infection, he said.
While many COVID-19 infections appear to be spread by respiratory droplets, which usually fall to the ground within 6 feet, people who are singing or speaking loudly can project virus much further. Evidence also suggests that the novel coronavirus can spread through aerosols, floating in the air like a speck of dust.
“I wonder how much virus was floating in that room that night,” Osterholm said.
Other experts say it’s too soon to say whether Trump was infected in a super-spreader event. “The president and his wife have had many exposures to many people in enclosed venues without protection,” so they could have been infected at any number of places, said Dr. William Schaffner, an infectious disease specialist at the Vanderbilt University School of Medicine.
Although Democratic presidential candidate and former Vice President Joe Biden tested negative for the virus with a PCR test Friday, experts note that false-negative results are common in the first few days after infection. Test results over the next several days will yield more useful information.
It can take more than a week for the virus to reproduce enough to be detected, Wells said: “You are probably not detectable for 3, 5, 7, even 10 days after you’re exposed.”
In Minnesota, where Trump held an outdoor campaign rally in Duluth with hundreds of attendees Wednesday, health officials warned that a 14-day quarantine is necessary, regardless of test results.
“Anyone who was a direct contact of President Trump or known COVID-19 cases needs to quarantine and should get tested,” the Minnesota Department of Health said.
Ongoing lapses in test result reporting could hamper efforts to track and isolate sick people. As of Sept. 10, 21 states and the District of Columbia were not reporting all antigen test results, according to a KHN investigation, a lapse in reporting that officials say leaves them blind to disease spread. Since then, public health departments in Arizona, North Carolina and South Dakota all have announced plans to add antigen testing to their case reporting.
Requests for comment to the D.C. Department of Health were referred to Mayor Muriel Bowser’s office, which did not respond. District health officials told KHN in early September that the White House does not report antigen test results to them – a potential violation of federal law under the CARES Act, which says any institution performing tests to diagnose COVID-19 must report all results to local or state public health departments.
Dr. Amesh Adalja, a senior scholar at the Johns Hopkins University Center for Health Security, said it’s not surprising that Trump tested positive, given that so many of his close associates – including his national security adviser and Secret Service officers – have also been infected by the virus.
“When you look at the number of social contacts and travel schedules, it’s not surprising,” Adalja said.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
The president has said others are tested before getting close to him, appearing to hold it as an iron shield of safety. He has largely eschewed mask-wearing and social distancing in meetings, travel and public events, while holding rallies for thousands of often maskless supporters.
The Trump administration has increasingly pinned its coronavirus testing strategy for the nation on antigen tests, which do not need a traditional lab for processing and quickly return results to patients. But the results are less accurate than those of the slower PCR tests.
An early antigen test used by the White House was woefully inaccurate. But the new antigen test the White House is using has not been independently evaluated for accuracy and reliability. Moreover, this is the kit the Trump administration is pushing out to thousands of nursing homes to test residents and staff.
Testing “isn’t a ‘get out of jail free card,’” said Dr. Alan Wells, medical director of clinical labs at the University of Pittsburgh Medical Center and creator of its test for the novel coronavirus. In general, antigen tests can miss up to half the cases that are detected by polymerase chain reaction tests, depending on the population of patients tested, he said.
The White House said the president’s diagnosis was confirmed with a PCR test but declined to say which test delivered his initial result. The White House has been using a new antigen test from Abbott Laboratories to screen its staff for COVID-19, according to two administration officials.
The test, known as BinaxNOW, received an emergency use authorization from the Food and Drug Administration in August. It produces results in 15 minutes. Yet little is independently known about how effective it is. According to the company, the test is 97% accurate in detecting positives and 98.5% accurate in identifying those without disease. Abbott’s stated performance of its antigen test was based on examining people within 7 days of COVID symptoms appearing.
The president and first lady have both had symptoms, according to White House chief of staff Mark Meadows and the first lady’s Twitter account. The president was admitted to Walter Reed National Military Medical Center on Friday evening “out of an abundance of caution,” White House press secretary Kayleigh McEnany said in a statement.
Vice President Mike Pence is also tested daily for the virus and tested negative, spokesperson Devin O’Malley said Friday, but he did not respond to a follow-up question about which test was used.
Trump heavily promoted another Abbott rapid testing device, the ID NOW, earlier this year. But that test relies on different technology than the newer Abbott antigen test.
“I have not seen any independent evaluation of the Binax assay in the literature or in the blogs,” Wells said. “It is an unknown.”
The Department of Health and Human Services announced in August that it had signed a $760 million contract with Abbott for 150 million BinaxNOW antigen tests, which are now being distributed to nursing homes and historically black colleges and universities, as well as to governors to help inform decisions about opening and closing schools. The Big Ten football conference has also pinned playing hopes on the deployment of antigen tests following Trump’s political pressure.
However, even senior federal officials concede that a test alone isn’t likely to stop the spread of a virus that has sickened more than 7 million Americans.
“Testing does not substitute for avoiding crowded indoor spaces, washing hands, or wearing a mask when you can’t physically distance; further, a negative test today does not mean that you won’t be positive tomorrow,” Adm. Brett Giroir, the senior HHS official helming the administration’s testing effort, said in a statement at the time.
Trump could be part of a “super-spreading event,” said Dr. Michael Osterholm, director of the Center for Infectious Disease Research and Policy at the University of Minnesota.
Given the timing of Trump’s positive test — which he announced on Twitter early Friday – his infection “likely happened 5 or more days ago,” Osterholm said. “If so, then he was widely infectious as early as Tuesday,” the day of the first presidential debate in Cleveland.
At least seven people who attended a Rose Garden announcement last Saturday, when Trump announced his nomination of Judge Amy Coney Barrett to the Supreme Court, have since tested positive for the coronavirus. They include Trump’s former adviser Kellyanne Conway, Republican Sens. Mike Lee and Thom Tillis, and the president of the University of Notre Dame, the Rev. John Jenkins.
“Having that many infected people there all at one time, we’re still going to see transmission coming off that event for a couple days,” Osterholm said.
Osterholm notes that about 20% of infected people lead to 80% of COVID-19 cases, because “super spreaders” can infect so many people at once.
He notes that participants and audience members at Tuesday’s debate were separated by at least 6 feet. But 6 feet isn’t always enough to prevent infection, he said.
While many COVID-19 infections appear to be spread by respiratory droplets, which usually fall to the ground within 6 feet, people who are singing or speaking loudly can project virus much further. Evidence also suggests that the novel coronavirus can spread through aerosols, floating in the air like a speck of dust.
“I wonder how much virus was floating in that room that night,” Osterholm said.
Other experts say it’s too soon to say whether Trump was infected in a super-spreader event. “The president and his wife have had many exposures to many people in enclosed venues without protection,” so they could have been infected at any number of places, said Dr. William Schaffner, an infectious disease specialist at the Vanderbilt University School of Medicine.
Although Democratic presidential candidate and former Vice President Joe Biden tested negative for the virus with a PCR test Friday, experts note that false-negative results are common in the first few days after infection. Test results over the next several days will yield more useful information.
It can take more than a week for the virus to reproduce enough to be detected, Wells said: “You are probably not detectable for 3, 5, 7, even 10 days after you’re exposed.”
In Minnesota, where Trump held an outdoor campaign rally in Duluth with hundreds of attendees Wednesday, health officials warned that a 14-day quarantine is necessary, regardless of test results.
“Anyone who was a direct contact of President Trump or known COVID-19 cases needs to quarantine and should get tested,” the Minnesota Department of Health said.
Ongoing lapses in test result reporting could hamper efforts to track and isolate sick people. As of Sept. 10, 21 states and the District of Columbia were not reporting all antigen test results, according to a KHN investigation, a lapse in reporting that officials say leaves them blind to disease spread. Since then, public health departments in Arizona, North Carolina and South Dakota all have announced plans to add antigen testing to their case reporting.
Requests for comment to the D.C. Department of Health were referred to Mayor Muriel Bowser’s office, which did not respond. District health officials told KHN in early September that the White House does not report antigen test results to them – a potential violation of federal law under the CARES Act, which says any institution performing tests to diagnose COVID-19 must report all results to local or state public health departments.
Dr. Amesh Adalja, a senior scholar at the Johns Hopkins University Center for Health Security, said it’s not surprising that Trump tested positive, given that so many of his close associates – including his national security adviser and Secret Service officers – have also been infected by the virus.
“When you look at the number of social contacts and travel schedules, it’s not surprising,” Adalja said.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Time to screen for liver disease in type 2 diabetes?
With high rates of fatty liver disease known to occur among people with type 2 diabetes, is it time to introduce routine liver screening into daily diabetes practice? The answer depends on whom you ask, and then there are still some important caveats.
From the hepatologist’s perspective, there is no excuse not to consider liver surveillance now that noninvasive screening methods are available, suggested Michael Trauner, MD, of the Medical University of Vienna.
“From a practical standpoint, I think every type 2 diabetic over 50 years of age is at high risk,” and consequently should be screened at diagnosis, Dr. Trauner said during a debate at the virtual annual meeting of the European Association for the Study of Diabetes. “I would screen at diagnosis and then decide on recall depending on noninvasive fibrosis markers.”
“It’s a rising problem that we are facing these days,” observed Michael Roden, MD, chair and professor of internal medicine, endocrinology and metabolic diseases at Heinrich-Heine University in Düsseldorf, Germany, and who cochaired the session. Not only do people with type 2 diabetes have an increased risk for developing liver diseases, but also there’s a higher risk for those with fatty liver diseases developing type 2 diabetes.
A meta-analysis published in Gut in just last week illustrates just how big a problem this is – nonalcoholic fatty liver disease (NAFLD) “doubled the risk of type 2 diabetes,” said Dr Rosen, who is also the director of the division of endocrinology and diabetology at University Clinics Düsseldorf. That analysis was based on more than 500,000 people, almost 28,000 of whom had incident diabetes over a 5-year period.
Screening tools scarce
This makes liver screening in type 2 diabetes patients “a formidable challenge,” cautioned Gianluca Perseghin, MD, professor of endocrinology at the Monza (Italy) Polyclinic and the University of Milano-Bicocca in Milan.
“Hepatologists generally see only the most severe cases,” Dr. Perseghin said. Diabetologists and endocrinologists would be likely to see huge numbers of patients that could potentially be at risk for liver disease and following the recommendations set out in the joint European Association for the Study of the Liver/EASD/European Association for the Study of Obesity guidelines would result in a huge number of patients being identified and potentially needing referral, he argued.
“At this stage, we need to build friendly, reliable and cost-effective screening process to be applied in the health systems,” Dr. Perseghin suggested. He proposed that liver surveillance would need to be not only personalized on a patient level, but also at the infrastructure level. Measuring liver enzymes, for example, was going to be less accurate in picking up liver disease but blood tests were widely available, whereas imaging methods were not going to be something all diabetes clinics would have immediate access to.
“There are clearly a lot of provocative decisions still to be made,” acknowledged Philip Newsome, PhD, FRCPE, an honorary consultant hepatologist at the University of Birmingham (England) and who cochaired the debate.
“We need to demonstrate that looking for the presence of liver disease in this cohort changes their outcomes in a way that is cost effective,” Dr. Newsome, who is also the secretary general of EASL.
“Tests are evolving, but more importantly, treatments are evolving. So, the decision around cost effectiveness will clearly change,” he added.
NAFLD therapies unclar
“There are still a lot of questions,” Dr. Newsome said during a Novo-Nordisk–sponsored “Meet the Expert” session discussing EASL-EASD-EASO guidelines. “We don’t have any licensed therapies at the moment. But there’s been a huge amount of investment, looking at all sorts of different approaches.”
Dr. Newsome added: “We also don’t know how to monitor these patients. Most of the noninvasive are very useful for staging patients, but we don’t really understand how useful they are for monitoring changes in fibrosis.”
Diabetologist Hannele Yki-Järvinen, MD, PhD, of the University of Helsinki, gave her thoughts on the topic during the same session.
“We should add FIB-4 [Fibrosis-4 index] to the annual exam and ask the lab to calculate FIB-4 automatically,” Dr. Yki-Järvinen said. FIB-4is calculated using the patients age and the results of readily available blood tests that measure the AST/ALT ratio and the platelet count.
Dr. Trauner has received advisory fees and grant support from various companies with an interest in developing liver-directed therapies, and is also a coinventor of 24-norursodeoxycholic acid under development for cholestatic liver disease and potentially NAFLD. Dr. Perseghin has received honoraria and grant support from various pharmaceutical companies with an interest in diabetes care. Dr. Roden did not provide any disclosures. Dr. Newsome has received research grants from Boehringer Ingelheim and Novo Nordisk and acted as a consultant to many pharmaceutical companies. Dr. Yki-Järvinen disclosed receiving consultancy fees from Eli Lilly, MSD, and Novo Nordisk.
SOURCE: Trauner M; Persghin G. EASD 2020, Session S27.
With high rates of fatty liver disease known to occur among people with type 2 diabetes, is it time to introduce routine liver screening into daily diabetes practice? The answer depends on whom you ask, and then there are still some important caveats.
From the hepatologist’s perspective, there is no excuse not to consider liver surveillance now that noninvasive screening methods are available, suggested Michael Trauner, MD, of the Medical University of Vienna.
“From a practical standpoint, I think every type 2 diabetic over 50 years of age is at high risk,” and consequently should be screened at diagnosis, Dr. Trauner said during a debate at the virtual annual meeting of the European Association for the Study of Diabetes. “I would screen at diagnosis and then decide on recall depending on noninvasive fibrosis markers.”
“It’s a rising problem that we are facing these days,” observed Michael Roden, MD, chair and professor of internal medicine, endocrinology and metabolic diseases at Heinrich-Heine University in Düsseldorf, Germany, and who cochaired the session. Not only do people with type 2 diabetes have an increased risk for developing liver diseases, but also there’s a higher risk for those with fatty liver diseases developing type 2 diabetes.
A meta-analysis published in Gut in just last week illustrates just how big a problem this is – nonalcoholic fatty liver disease (NAFLD) “doubled the risk of type 2 diabetes,” said Dr Rosen, who is also the director of the division of endocrinology and diabetology at University Clinics Düsseldorf. That analysis was based on more than 500,000 people, almost 28,000 of whom had incident diabetes over a 5-year period.
Screening tools scarce
This makes liver screening in type 2 diabetes patients “a formidable challenge,” cautioned Gianluca Perseghin, MD, professor of endocrinology at the Monza (Italy) Polyclinic and the University of Milano-Bicocca in Milan.
“Hepatologists generally see only the most severe cases,” Dr. Perseghin said. Diabetologists and endocrinologists would be likely to see huge numbers of patients that could potentially be at risk for liver disease and following the recommendations set out in the joint European Association for the Study of the Liver/EASD/European Association for the Study of Obesity guidelines would result in a huge number of patients being identified and potentially needing referral, he argued.
“At this stage, we need to build friendly, reliable and cost-effective screening process to be applied in the health systems,” Dr. Perseghin suggested. He proposed that liver surveillance would need to be not only personalized on a patient level, but also at the infrastructure level. Measuring liver enzymes, for example, was going to be less accurate in picking up liver disease but blood tests were widely available, whereas imaging methods were not going to be something all diabetes clinics would have immediate access to.
“There are clearly a lot of provocative decisions still to be made,” acknowledged Philip Newsome, PhD, FRCPE, an honorary consultant hepatologist at the University of Birmingham (England) and who cochaired the debate.
“We need to demonstrate that looking for the presence of liver disease in this cohort changes their outcomes in a way that is cost effective,” Dr. Newsome, who is also the secretary general of EASL.
“Tests are evolving, but more importantly, treatments are evolving. So, the decision around cost effectiveness will clearly change,” he added.
NAFLD therapies unclar
“There are still a lot of questions,” Dr. Newsome said during a Novo-Nordisk–sponsored “Meet the Expert” session discussing EASL-EASD-EASO guidelines. “We don’t have any licensed therapies at the moment. But there’s been a huge amount of investment, looking at all sorts of different approaches.”
Dr. Newsome added: “We also don’t know how to monitor these patients. Most of the noninvasive are very useful for staging patients, but we don’t really understand how useful they are for monitoring changes in fibrosis.”
Diabetologist Hannele Yki-Järvinen, MD, PhD, of the University of Helsinki, gave her thoughts on the topic during the same session.
“We should add FIB-4 [Fibrosis-4 index] to the annual exam and ask the lab to calculate FIB-4 automatically,” Dr. Yki-Järvinen said. FIB-4is calculated using the patients age and the results of readily available blood tests that measure the AST/ALT ratio and the platelet count.
Dr. Trauner has received advisory fees and grant support from various companies with an interest in developing liver-directed therapies, and is also a coinventor of 24-norursodeoxycholic acid under development for cholestatic liver disease and potentially NAFLD. Dr. Perseghin has received honoraria and grant support from various pharmaceutical companies with an interest in diabetes care. Dr. Roden did not provide any disclosures. Dr. Newsome has received research grants from Boehringer Ingelheim and Novo Nordisk and acted as a consultant to many pharmaceutical companies. Dr. Yki-Järvinen disclosed receiving consultancy fees from Eli Lilly, MSD, and Novo Nordisk.
SOURCE: Trauner M; Persghin G. EASD 2020, Session S27.
With high rates of fatty liver disease known to occur among people with type 2 diabetes, is it time to introduce routine liver screening into daily diabetes practice? The answer depends on whom you ask, and then there are still some important caveats.
From the hepatologist’s perspective, there is no excuse not to consider liver surveillance now that noninvasive screening methods are available, suggested Michael Trauner, MD, of the Medical University of Vienna.
“From a practical standpoint, I think every type 2 diabetic over 50 years of age is at high risk,” and consequently should be screened at diagnosis, Dr. Trauner said during a debate at the virtual annual meeting of the European Association for the Study of Diabetes. “I would screen at diagnosis and then decide on recall depending on noninvasive fibrosis markers.”
“It’s a rising problem that we are facing these days,” observed Michael Roden, MD, chair and professor of internal medicine, endocrinology and metabolic diseases at Heinrich-Heine University in Düsseldorf, Germany, and who cochaired the session. Not only do people with type 2 diabetes have an increased risk for developing liver diseases, but also there’s a higher risk for those with fatty liver diseases developing type 2 diabetes.
A meta-analysis published in Gut in just last week illustrates just how big a problem this is – nonalcoholic fatty liver disease (NAFLD) “doubled the risk of type 2 diabetes,” said Dr Rosen, who is also the director of the division of endocrinology and diabetology at University Clinics Düsseldorf. That analysis was based on more than 500,000 people, almost 28,000 of whom had incident diabetes over a 5-year period.
Screening tools scarce
This makes liver screening in type 2 diabetes patients “a formidable challenge,” cautioned Gianluca Perseghin, MD, professor of endocrinology at the Monza (Italy) Polyclinic and the University of Milano-Bicocca in Milan.
“Hepatologists generally see only the most severe cases,” Dr. Perseghin said. Diabetologists and endocrinologists would be likely to see huge numbers of patients that could potentially be at risk for liver disease and following the recommendations set out in the joint European Association for the Study of the Liver/EASD/European Association for the Study of Obesity guidelines would result in a huge number of patients being identified and potentially needing referral, he argued.
“At this stage, we need to build friendly, reliable and cost-effective screening process to be applied in the health systems,” Dr. Perseghin suggested. He proposed that liver surveillance would need to be not only personalized on a patient level, but also at the infrastructure level. Measuring liver enzymes, for example, was going to be less accurate in picking up liver disease but blood tests were widely available, whereas imaging methods were not going to be something all diabetes clinics would have immediate access to.
“There are clearly a lot of provocative decisions still to be made,” acknowledged Philip Newsome, PhD, FRCPE, an honorary consultant hepatologist at the University of Birmingham (England) and who cochaired the debate.
“We need to demonstrate that looking for the presence of liver disease in this cohort changes their outcomes in a way that is cost effective,” Dr. Newsome, who is also the secretary general of EASL.
“Tests are evolving, but more importantly, treatments are evolving. So, the decision around cost effectiveness will clearly change,” he added.
NAFLD therapies unclar
“There are still a lot of questions,” Dr. Newsome said during a Novo-Nordisk–sponsored “Meet the Expert” session discussing EASL-EASD-EASO guidelines. “We don’t have any licensed therapies at the moment. But there’s been a huge amount of investment, looking at all sorts of different approaches.”
Dr. Newsome added: “We also don’t know how to monitor these patients. Most of the noninvasive are very useful for staging patients, but we don’t really understand how useful they are for monitoring changes in fibrosis.”
Diabetologist Hannele Yki-Järvinen, MD, PhD, of the University of Helsinki, gave her thoughts on the topic during the same session.
“We should add FIB-4 [Fibrosis-4 index] to the annual exam and ask the lab to calculate FIB-4 automatically,” Dr. Yki-Järvinen said. FIB-4is calculated using the patients age and the results of readily available blood tests that measure the AST/ALT ratio and the platelet count.
Dr. Trauner has received advisory fees and grant support from various companies with an interest in developing liver-directed therapies, and is also a coinventor of 24-norursodeoxycholic acid under development for cholestatic liver disease and potentially NAFLD. Dr. Perseghin has received honoraria and grant support from various pharmaceutical companies with an interest in diabetes care. Dr. Roden did not provide any disclosures. Dr. Newsome has received research grants from Boehringer Ingelheim and Novo Nordisk and acted as a consultant to many pharmaceutical companies. Dr. Yki-Järvinen disclosed receiving consultancy fees from Eli Lilly, MSD, and Novo Nordisk.
SOURCE: Trauner M; Persghin G. EASD 2020, Session S27.
REPORTING FROM EASD 2020
Dapagliflozin’s CKD performance sends heart failure messages
The DAPA-CKD trial results, which proved dapagliflozin’s efficacy for slowing chronic kidney disease progression in patients selected for signs of worsening renal function, also have important messages for cardiologists, especially heart failure physicians.
Those messages include findings that were “consistent” with the results of the earlier DAPA-HF trial, which tested the same sodium-glucose transporter 2 (SGLT2) inhibitor in patients selected for having heart failure with reduced ejection fraction (HFrEF). In addition, a specific action of dapagliflozin (Farxiga) on the patients in DAPA-CKD, which enrolled patients based on markers of chronic kidney disease (CKD), was prevention of first and recurrent heart failure hospitalizations, John J.V. McMurray, MD, said at the virtual annual scientific meeting of the Heart Failure Society of America, further highlighting the role that dapagliflozin has in reducing both heart failure and renal events.
What DAPA-CKD means for heart failure
The main findings from the DAPA-CKD trial, published in September in the New England Journal of Medicine, included as a secondary outcome the combined rate of death from cardiovascular causes or hospitalization for heart failure (HHF). Treatment with dapagliflozin linked with a significant 29% relative reduction in this endpoint, compared with placebo-treated patients. At the HFSA meeting, Dr. McMurray reported for the first time the specific HHF numbers, a prespecified secondary endpoint for the study.
Patients on dapagliflozin had 37 total HHF events (1.7%), including both first-time and subsequent hospitalizations, while patients in the placebo arm had a total of 71 HHF events (3.3%) during the study’s median 2.4 years of follow-up, an absolute reduction of 1.6% that translated into a relative risk reduction of 49%.
The HHF findings from DAPA-CKD importantly showed that SGLT2 inhibition in patients with signs of renal dysfunction “will not only slow progression of kidney disease but will also reduce the risk of developing heart failure, crucially in patients with or without type 2 diabetes,” explained Dr. McMurray in an interview. “Cardiologists often consult in the kidney wards and advise on management of patients with chronic kidney disease, even those without heart failure.”
The DAPA-CKD findings carry another important message for heart failure management regarding the minimum level of renal function a patient can have and still safely receive dapagliflozin or possibly another agent from the same SGLT2 inhibitor class. In DAPA-CKD, patients safely received dapagliflozin with an estimated glomerular filtration rate (eGFR) as low as 25 mL/min per 1.73 m2; 14% of enrolled patients had an eGFR of 25-29 mL/min per 1.73 m2.
“Typically, about 40%-50% of patients with heart failure have chronic kidney disease,” which makes this safety finding important to clinicians who care for heart failure patients, but it’s also important for any patient who might be a candidate for dapagliflozin or another drug from its class. “We had no strong evidence before this trial that SGLT2 inhibition could reduce hard renal endpoints,” specifically need for chronic dialysis, renal transplant, or renal death, “in patients with or without diabetes,” Dr. McMurray said.
DAPA-CKD grows the pool of eligible heart failure patients
A further consequence of the DAPA-CKD findings is that when, as expected, regulatory bodies give dapagliflozin an indication for treating the types of CKD patients enrolled in the trial, it will functionally expand this treatment to an even larger swath of heart failure patients who currently don’t qualify for this treatment, specifically patients with CKD who also have heart failure with preserved ejection fraction (HFpEF). On Oct. 2, 2020, the Food and Drug Administration fast-tracked dapagliflozin for the CKD indication by granting it Breakthrough Therapy Designation based on the DAPA-CKD results.
Results first reported in 2019 from the DAPA-HF trial led to dapagliflozin receiving a labeled indication for treating HFrEF, the types of heart failure patients enrolled in the trial. Direct evidence on the efficacy of SGLT2 inhibitors for patients with HFpEF will not be available until results from a few trials now in progress become available during the next 12 months.
In the meantime, nearly half of patients with HFpEF also have CKD, noted Dr. McMurray, and another large portion of HFpEF patients have type 2 diabetes and hence qualify for SGLT2 inhibitor treatment that way. “Obviously, we would like to know specifically about heart failure outcomes in patients with HFpEF” on SGLT2 inhibitor treatment, he acknowledged. But the recent approval of dapagliflozin for patients with HFrEF and the likely indication coming soon for treating CKD means that the number of patients with heart failure who are not eligible for SGLT2 inhibitor treatment is dwindling down to some extent.
New DAPA-HF results show no drug, device interactions
In a separate session at the HFSA virtual meeting, Dr. McMurray and several collaborators on the DAPA-HF trial presented results from some new analyses. Dr. McMurray looked at the impact of dapagliflozin treatment on the primary endpoint when patients were stratified by the diuretic dosage they received at study entry. The results showed that “the benefits from dapagliflozin were irrespective of the use of background diuretic therapy or the diuretic dose,” he reported. Study findings also showed that roughly three-quarters of patients in the study had no change in their diuretic dosage during the course of the trial, that the fraction of patients who had an increase in their dosage was about the same as those whose diuretic dosage decreased, and that this pattern was similar in both the patients on dapagliflozin and in those randomized to placebo.
Another set of new analyses from DAPA-HF looked at the impact on dapagliflozin efficacy of background medical and device therapies for heart failure, as well as background diabetes therapies. The findings showed no signal of an interaction with background therapies. “The effects of dapagliflozin are incremental and complimentary to conventional therapies for HFrEF,” concluded Lars Kober, MD, a professor and heart failure physician at Copenhagen University Hospital.
DAPA-CKD was funded by AstraZeneca, the company that markets dapagliflozin (Farxiga). Dr. McMurray’s employer, Glasgow University, has received payments from AstraZeneca and several other companies to compensate for his time overseeing various clinical trials. Dr. Kober has received honoraria for speaking on behalf of several companies including AstraZeneca.
The DAPA-CKD trial results, which proved dapagliflozin’s efficacy for slowing chronic kidney disease progression in patients selected for signs of worsening renal function, also have important messages for cardiologists, especially heart failure physicians.
Those messages include findings that were “consistent” with the results of the earlier DAPA-HF trial, which tested the same sodium-glucose transporter 2 (SGLT2) inhibitor in patients selected for having heart failure with reduced ejection fraction (HFrEF). In addition, a specific action of dapagliflozin (Farxiga) on the patients in DAPA-CKD, which enrolled patients based on markers of chronic kidney disease (CKD), was prevention of first and recurrent heart failure hospitalizations, John J.V. McMurray, MD, said at the virtual annual scientific meeting of the Heart Failure Society of America, further highlighting the role that dapagliflozin has in reducing both heart failure and renal events.
What DAPA-CKD means for heart failure
The main findings from the DAPA-CKD trial, published in September in the New England Journal of Medicine, included as a secondary outcome the combined rate of death from cardiovascular causes or hospitalization for heart failure (HHF). Treatment with dapagliflozin linked with a significant 29% relative reduction in this endpoint, compared with placebo-treated patients. At the HFSA meeting, Dr. McMurray reported for the first time the specific HHF numbers, a prespecified secondary endpoint for the study.
Patients on dapagliflozin had 37 total HHF events (1.7%), including both first-time and subsequent hospitalizations, while patients in the placebo arm had a total of 71 HHF events (3.3%) during the study’s median 2.4 years of follow-up, an absolute reduction of 1.6% that translated into a relative risk reduction of 49%.
The HHF findings from DAPA-CKD importantly showed that SGLT2 inhibition in patients with signs of renal dysfunction “will not only slow progression of kidney disease but will also reduce the risk of developing heart failure, crucially in patients with or without type 2 diabetes,” explained Dr. McMurray in an interview. “Cardiologists often consult in the kidney wards and advise on management of patients with chronic kidney disease, even those without heart failure.”
The DAPA-CKD findings carry another important message for heart failure management regarding the minimum level of renal function a patient can have and still safely receive dapagliflozin or possibly another agent from the same SGLT2 inhibitor class. In DAPA-CKD, patients safely received dapagliflozin with an estimated glomerular filtration rate (eGFR) as low as 25 mL/min per 1.73 m2; 14% of enrolled patients had an eGFR of 25-29 mL/min per 1.73 m2.
“Typically, about 40%-50% of patients with heart failure have chronic kidney disease,” which makes this safety finding important to clinicians who care for heart failure patients, but it’s also important for any patient who might be a candidate for dapagliflozin or another drug from its class. “We had no strong evidence before this trial that SGLT2 inhibition could reduce hard renal endpoints,” specifically need for chronic dialysis, renal transplant, or renal death, “in patients with or without diabetes,” Dr. McMurray said.
DAPA-CKD grows the pool of eligible heart failure patients
A further consequence of the DAPA-CKD findings is that when, as expected, regulatory bodies give dapagliflozin an indication for treating the types of CKD patients enrolled in the trial, it will functionally expand this treatment to an even larger swath of heart failure patients who currently don’t qualify for this treatment, specifically patients with CKD who also have heart failure with preserved ejection fraction (HFpEF). On Oct. 2, 2020, the Food and Drug Administration fast-tracked dapagliflozin for the CKD indication by granting it Breakthrough Therapy Designation based on the DAPA-CKD results.
Results first reported in 2019 from the DAPA-HF trial led to dapagliflozin receiving a labeled indication for treating HFrEF, the types of heart failure patients enrolled in the trial. Direct evidence on the efficacy of SGLT2 inhibitors for patients with HFpEF will not be available until results from a few trials now in progress become available during the next 12 months.
In the meantime, nearly half of patients with HFpEF also have CKD, noted Dr. McMurray, and another large portion of HFpEF patients have type 2 diabetes and hence qualify for SGLT2 inhibitor treatment that way. “Obviously, we would like to know specifically about heart failure outcomes in patients with HFpEF” on SGLT2 inhibitor treatment, he acknowledged. But the recent approval of dapagliflozin for patients with HFrEF and the likely indication coming soon for treating CKD means that the number of patients with heart failure who are not eligible for SGLT2 inhibitor treatment is dwindling down to some extent.
New DAPA-HF results show no drug, device interactions
In a separate session at the HFSA virtual meeting, Dr. McMurray and several collaborators on the DAPA-HF trial presented results from some new analyses. Dr. McMurray looked at the impact of dapagliflozin treatment on the primary endpoint when patients were stratified by the diuretic dosage they received at study entry. The results showed that “the benefits from dapagliflozin were irrespective of the use of background diuretic therapy or the diuretic dose,” he reported. Study findings also showed that roughly three-quarters of patients in the study had no change in their diuretic dosage during the course of the trial, that the fraction of patients who had an increase in their dosage was about the same as those whose diuretic dosage decreased, and that this pattern was similar in both the patients on dapagliflozin and in those randomized to placebo.
Another set of new analyses from DAPA-HF looked at the impact on dapagliflozin efficacy of background medical and device therapies for heart failure, as well as background diabetes therapies. The findings showed no signal of an interaction with background therapies. “The effects of dapagliflozin are incremental and complimentary to conventional therapies for HFrEF,” concluded Lars Kober, MD, a professor and heart failure physician at Copenhagen University Hospital.
DAPA-CKD was funded by AstraZeneca, the company that markets dapagliflozin (Farxiga). Dr. McMurray’s employer, Glasgow University, has received payments from AstraZeneca and several other companies to compensate for his time overseeing various clinical trials. Dr. Kober has received honoraria for speaking on behalf of several companies including AstraZeneca.
The DAPA-CKD trial results, which proved dapagliflozin’s efficacy for slowing chronic kidney disease progression in patients selected for signs of worsening renal function, also have important messages for cardiologists, especially heart failure physicians.
Those messages include findings that were “consistent” with the results of the earlier DAPA-HF trial, which tested the same sodium-glucose transporter 2 (SGLT2) inhibitor in patients selected for having heart failure with reduced ejection fraction (HFrEF). In addition, a specific action of dapagliflozin (Farxiga) on the patients in DAPA-CKD, which enrolled patients based on markers of chronic kidney disease (CKD), was prevention of first and recurrent heart failure hospitalizations, John J.V. McMurray, MD, said at the virtual annual scientific meeting of the Heart Failure Society of America, further highlighting the role that dapagliflozin has in reducing both heart failure and renal events.
What DAPA-CKD means for heart failure
The main findings from the DAPA-CKD trial, published in September in the New England Journal of Medicine, included as a secondary outcome the combined rate of death from cardiovascular causes or hospitalization for heart failure (HHF). Treatment with dapagliflozin linked with a significant 29% relative reduction in this endpoint, compared with placebo-treated patients. At the HFSA meeting, Dr. McMurray reported for the first time the specific HHF numbers, a prespecified secondary endpoint for the study.
Patients on dapagliflozin had 37 total HHF events (1.7%), including both first-time and subsequent hospitalizations, while patients in the placebo arm had a total of 71 HHF events (3.3%) during the study’s median 2.4 years of follow-up, an absolute reduction of 1.6% that translated into a relative risk reduction of 49%.
The HHF findings from DAPA-CKD importantly showed that SGLT2 inhibition in patients with signs of renal dysfunction “will not only slow progression of kidney disease but will also reduce the risk of developing heart failure, crucially in patients with or without type 2 diabetes,” explained Dr. McMurray in an interview. “Cardiologists often consult in the kidney wards and advise on management of patients with chronic kidney disease, even those without heart failure.”
The DAPA-CKD findings carry another important message for heart failure management regarding the minimum level of renal function a patient can have and still safely receive dapagliflozin or possibly another agent from the same SGLT2 inhibitor class. In DAPA-CKD, patients safely received dapagliflozin with an estimated glomerular filtration rate (eGFR) as low as 25 mL/min per 1.73 m2; 14% of enrolled patients had an eGFR of 25-29 mL/min per 1.73 m2.
“Typically, about 40%-50% of patients with heart failure have chronic kidney disease,” which makes this safety finding important to clinicians who care for heart failure patients, but it’s also important for any patient who might be a candidate for dapagliflozin or another drug from its class. “We had no strong evidence before this trial that SGLT2 inhibition could reduce hard renal endpoints,” specifically need for chronic dialysis, renal transplant, or renal death, “in patients with or without diabetes,” Dr. McMurray said.
DAPA-CKD grows the pool of eligible heart failure patients
A further consequence of the DAPA-CKD findings is that when, as expected, regulatory bodies give dapagliflozin an indication for treating the types of CKD patients enrolled in the trial, it will functionally expand this treatment to an even larger swath of heart failure patients who currently don’t qualify for this treatment, specifically patients with CKD who also have heart failure with preserved ejection fraction (HFpEF). On Oct. 2, 2020, the Food and Drug Administration fast-tracked dapagliflozin for the CKD indication by granting it Breakthrough Therapy Designation based on the DAPA-CKD results.
Results first reported in 2019 from the DAPA-HF trial led to dapagliflozin receiving a labeled indication for treating HFrEF, the types of heart failure patients enrolled in the trial. Direct evidence on the efficacy of SGLT2 inhibitors for patients with HFpEF will not be available until results from a few trials now in progress become available during the next 12 months.
In the meantime, nearly half of patients with HFpEF also have CKD, noted Dr. McMurray, and another large portion of HFpEF patients have type 2 diabetes and hence qualify for SGLT2 inhibitor treatment that way. “Obviously, we would like to know specifically about heart failure outcomes in patients with HFpEF” on SGLT2 inhibitor treatment, he acknowledged. But the recent approval of dapagliflozin for patients with HFrEF and the likely indication coming soon for treating CKD means that the number of patients with heart failure who are not eligible for SGLT2 inhibitor treatment is dwindling down to some extent.
New DAPA-HF results show no drug, device interactions
In a separate session at the HFSA virtual meeting, Dr. McMurray and several collaborators on the DAPA-HF trial presented results from some new analyses. Dr. McMurray looked at the impact of dapagliflozin treatment on the primary endpoint when patients were stratified by the diuretic dosage they received at study entry. The results showed that “the benefits from dapagliflozin were irrespective of the use of background diuretic therapy or the diuretic dose,” he reported. Study findings also showed that roughly three-quarters of patients in the study had no change in their diuretic dosage during the course of the trial, that the fraction of patients who had an increase in their dosage was about the same as those whose diuretic dosage decreased, and that this pattern was similar in both the patients on dapagliflozin and in those randomized to placebo.
Another set of new analyses from DAPA-HF looked at the impact on dapagliflozin efficacy of background medical and device therapies for heart failure, as well as background diabetes therapies. The findings showed no signal of an interaction with background therapies. “The effects of dapagliflozin are incremental and complimentary to conventional therapies for HFrEF,” concluded Lars Kober, MD, a professor and heart failure physician at Copenhagen University Hospital.
DAPA-CKD was funded by AstraZeneca, the company that markets dapagliflozin (Farxiga). Dr. McMurray’s employer, Glasgow University, has received payments from AstraZeneca and several other companies to compensate for his time overseeing various clinical trials. Dr. Kober has received honoraria for speaking on behalf of several companies including AstraZeneca.
FROM HFSA 2020
‘Celebration’ will be ‘short-lived’ if COVID vaccine rushed: Experts
on Wednesday.
The career staff of the Food and Drug Administration can be counted on to appropriately weigh whether a vaccine should be cleared for use in preventing COVID-19, witnesses, including Paul A. Offit, MD, of Children’s Hospital of Philadelphia, told the House Energy and Commerce Committee’s oversight and investigations panel.
FDA staffers would object to attempts by the Trump administration to rush a vaccine to the public without proper vetting, as would veteran federal researchers, including National Institutes of Health Director Francis S. Collins, MD, PhD, and Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, Offit said.
“If COVID-19 vaccines are released before they’re ready to be released, you will hear from these people, and you will also hear from people like Dr. Francis Collins and Tony Fauci, both of whom are trusted by the American public, as well as many other academicians and researchers who wouldn’t stand for this,” he said.
“The public is already nervous about these vaccines,” said Offit, who serves on key FDA and Centers for Disease Control and Prevention committees overseeing vaccine policy. “If trusted health officials stand up and decry a premature release, the celebration by the administration will be short-lived.”
Overly optimistic estimates about a potential approval can only serve to erode the public’s trust in these crucial vaccines, said another witness, Ashish K. Jha, MD, MPH, the dean of Brown University’s School of Public Health, in Providence, Rhode Island.
“All political leaders need to stop talking about things like time lines,” Jha told the lawmakers.
President Donald Trump has several times suggested that a COVID vaccine might be approved ahead of the November 3 election, where he faces a significant challenge from his Democratic rival, former Vice President Joe Biden.
In a Tuesday night debate with Biden, Trump again raised the idea of a quick approval. “Now we’re weeks away from a vaccine,” Trump said during the debate.
Trump’s estimates, though, are not in line with those offered by most firms involved with making vaccines. The most optimistic projections have come from Pfizer Inc. The drugmaker’s chief executive, Albert Bourla, has spoken about his company possibly having data to present to the FDA as early as late October about the safety and effectiveness of a vaccine.
In a September 8 interview with the Today show, Bourla said there was a 60% chance his company would meet that goal. In response to a question, he made it clear his comments applied to a potential Pfizer application, not an approval or release of a vaccine by that time.
In response to concerns about political pressures, the FDA in June issued guidance outlining what its staff would require for approval of a COVID-19 vaccine.
Pushback on politics
Another witness at the Wednesday hearing, Mark McClellan, MD, PhD, a former FDA commissioner (2002 – 2004), pushed back on objections to a potential release of further guidance from the agency.
“Some recent statements from the White House have implied that FDA’s plan to release additional written guidance on its expectations for emergency use authorization of a vaccine is unnecessarily raising the bar on regulatory standards for authorization,” said McClellan in his testimony for the House panel. “That is not the case.”
Instead, further FDA guidance would be a welcome form of feedback for the firms trying to develop COVID-19 vaccines, according to McClellan, who also serves on the board of directors for Johnson & Johnson. Johnson & Johnson is among the firms that have advanced a COVID-19 vaccine candidate to phase 3 testing. In his role as a director, he serves on the board’s regulatory compliance committee.
Along with politics, recent stumbles at FDA with emergency use authorizations (EUAs) of treatments for COVID-19 have eroded the public’s confidence in the agency, Jha told the House panel. The FDA approved hydroxychloroquine, a medicine promoted by Trump for use in COVID, under an EUA in March and then revoked this clearance in June.
Jha said the FDA’s most serious misstep was its handling of convalescent plasma, which was approved through an EUA on August 23 “in a highly advertised and widely televised announcement including the president.
“The announcement solidified in the public conversation the impression that, increasingly with this administration, politics are taking over trusted, nonpartisan scientific institutions,” he said in his testimony.
Approving a COVID-19 vaccine on the limited evidence through an EUA would mark a serious departure from FDA policy, according to Jha.
“While we sometimes accept a certain level of potential harm in experimental treatments for those who are severely ill, vaccines are given to healthy people and therefore need to have a substantially higher measure of safety and effectiveness,” he explained.
Jha said the FDA has only once before used this EUA approach for a vaccine. That was for a vaccine against inhaled anthrax and was mostly distributed to high-risk soldiers and civilians in war zones.
COVID-19, in contrast, is an infection that has changed lives around the world. The virus has contributed to more than 1 million deaths, including more than 200,000 in the United States, according to the World Health Organization.
Scientists are hoping vaccines will help curb this infection, although much of the future success of vaccines depends on how widely they are used, witnesses told the House panel.
Debate on approaches for vaccine effectiveness
In his testimony, Jha also noted concerns about COVID-19 vaccine trials. He included a reference to a Sept. 22 opinion article titled, “These Coronavirus Trials Don›t Answer the One Question We Need to Know,” which was written by Peter Doshi, PhD, of the University of Maryland School of Pharmacy, in Baltimore, and Eric Topol, MD, a professor of molecular medicine at Scripps Research in La Jolla, Calif. Topol is also editor in chief of Medscape.
Topol and Doshi questioned why the firms Moderna, Pfizer, and AstraZeneca structured their competing trials such that “a vaccine could meet the companies’ benchmark for success if it lowered the risk of mild Covid-19, but was never shown to reduce moderate or severe forms of the disease, or the risk of hospitalization, admissions to the intensive care unit or death.”
“To say a vaccine works should mean that most people no longer run the risk of getting seriously sick,” Topol and Doshi wrote. “That’s not what these trials will determine.”
There was disagreement about this point at the hearing. U.S. Representative Morgan Griffith (R-Va.) read the section of the Doshi-Topol article quoted above and asked one witness, Offit, to weigh in.
“Do you agree with those concerns? And either way, tell me why,” Griffith asked.
“I don’t agree,” Offit responded.
“I think it’s actually much harder to prevent asymptomatic infection or mildly symptomatic infection,” he said. “If you can prevent that, you are much more likely to prevent moderate to severe disease. So I think they have it backwards.”
But other researchers also question the approaches used with the current crop of COVID-19 vaccines.
“With the current protocols, it is conceivable that a vaccine might be considered effective – and eventually approved – based primarily on its ability to prevent mild cases alone,” wrote William Haseltine, PhD, president of the nonprofit ACCESS Health International, in a September 22 opinion article in the Washington Post titled: “Beware of COVID-19 Vaccine Trials Designed to Succeed From the Start.”
In an interview with Medscape Medical News on Wednesday, Haseltine said he maintains these concerns about the tests. Earlier in his career, he was a leader in HIV research through his lab at Harvard University in Cambridge, Massachusetts, and he subsequently led a biotech company, Human Genome Sciences.
He fears consumers will not get what they might expect from the vaccines being tested.
“What people care about is if this is going to keep them out of the hospital and will it keep them alive. And that’s not even part of this protocol,” Haseltine said.
This article first appeared on Medscape.com.
on Wednesday.
The career staff of the Food and Drug Administration can be counted on to appropriately weigh whether a vaccine should be cleared for use in preventing COVID-19, witnesses, including Paul A. Offit, MD, of Children’s Hospital of Philadelphia, told the House Energy and Commerce Committee’s oversight and investigations panel.
FDA staffers would object to attempts by the Trump administration to rush a vaccine to the public without proper vetting, as would veteran federal researchers, including National Institutes of Health Director Francis S. Collins, MD, PhD, and Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, Offit said.
“If COVID-19 vaccines are released before they’re ready to be released, you will hear from these people, and you will also hear from people like Dr. Francis Collins and Tony Fauci, both of whom are trusted by the American public, as well as many other academicians and researchers who wouldn’t stand for this,” he said.
“The public is already nervous about these vaccines,” said Offit, who serves on key FDA and Centers for Disease Control and Prevention committees overseeing vaccine policy. “If trusted health officials stand up and decry a premature release, the celebration by the administration will be short-lived.”
Overly optimistic estimates about a potential approval can only serve to erode the public’s trust in these crucial vaccines, said another witness, Ashish K. Jha, MD, MPH, the dean of Brown University’s School of Public Health, in Providence, Rhode Island.
“All political leaders need to stop talking about things like time lines,” Jha told the lawmakers.
President Donald Trump has several times suggested that a COVID vaccine might be approved ahead of the November 3 election, where he faces a significant challenge from his Democratic rival, former Vice President Joe Biden.
In a Tuesday night debate with Biden, Trump again raised the idea of a quick approval. “Now we’re weeks away from a vaccine,” Trump said during the debate.
Trump’s estimates, though, are not in line with those offered by most firms involved with making vaccines. The most optimistic projections have come from Pfizer Inc. The drugmaker’s chief executive, Albert Bourla, has spoken about his company possibly having data to present to the FDA as early as late October about the safety and effectiveness of a vaccine.
In a September 8 interview with the Today show, Bourla said there was a 60% chance his company would meet that goal. In response to a question, he made it clear his comments applied to a potential Pfizer application, not an approval or release of a vaccine by that time.
In response to concerns about political pressures, the FDA in June issued guidance outlining what its staff would require for approval of a COVID-19 vaccine.
Pushback on politics
Another witness at the Wednesday hearing, Mark McClellan, MD, PhD, a former FDA commissioner (2002 – 2004), pushed back on objections to a potential release of further guidance from the agency.
“Some recent statements from the White House have implied that FDA’s plan to release additional written guidance on its expectations for emergency use authorization of a vaccine is unnecessarily raising the bar on regulatory standards for authorization,” said McClellan in his testimony for the House panel. “That is not the case.”
Instead, further FDA guidance would be a welcome form of feedback for the firms trying to develop COVID-19 vaccines, according to McClellan, who also serves on the board of directors for Johnson & Johnson. Johnson & Johnson is among the firms that have advanced a COVID-19 vaccine candidate to phase 3 testing. In his role as a director, he serves on the board’s regulatory compliance committee.
Along with politics, recent stumbles at FDA with emergency use authorizations (EUAs) of treatments for COVID-19 have eroded the public’s confidence in the agency, Jha told the House panel. The FDA approved hydroxychloroquine, a medicine promoted by Trump for use in COVID, under an EUA in March and then revoked this clearance in June.
Jha said the FDA’s most serious misstep was its handling of convalescent plasma, which was approved through an EUA on August 23 “in a highly advertised and widely televised announcement including the president.
“The announcement solidified in the public conversation the impression that, increasingly with this administration, politics are taking over trusted, nonpartisan scientific institutions,” he said in his testimony.
Approving a COVID-19 vaccine on the limited evidence through an EUA would mark a serious departure from FDA policy, according to Jha.
“While we sometimes accept a certain level of potential harm in experimental treatments for those who are severely ill, vaccines are given to healthy people and therefore need to have a substantially higher measure of safety and effectiveness,” he explained.
Jha said the FDA has only once before used this EUA approach for a vaccine. That was for a vaccine against inhaled anthrax and was mostly distributed to high-risk soldiers and civilians in war zones.
COVID-19, in contrast, is an infection that has changed lives around the world. The virus has contributed to more than 1 million deaths, including more than 200,000 in the United States, according to the World Health Organization.
Scientists are hoping vaccines will help curb this infection, although much of the future success of vaccines depends on how widely they are used, witnesses told the House panel.
Debate on approaches for vaccine effectiveness
In his testimony, Jha also noted concerns about COVID-19 vaccine trials. He included a reference to a Sept. 22 opinion article titled, “These Coronavirus Trials Don›t Answer the One Question We Need to Know,” which was written by Peter Doshi, PhD, of the University of Maryland School of Pharmacy, in Baltimore, and Eric Topol, MD, a professor of molecular medicine at Scripps Research in La Jolla, Calif. Topol is also editor in chief of Medscape.
Topol and Doshi questioned why the firms Moderna, Pfizer, and AstraZeneca structured their competing trials such that “a vaccine could meet the companies’ benchmark for success if it lowered the risk of mild Covid-19, but was never shown to reduce moderate or severe forms of the disease, or the risk of hospitalization, admissions to the intensive care unit or death.”
“To say a vaccine works should mean that most people no longer run the risk of getting seriously sick,” Topol and Doshi wrote. “That’s not what these trials will determine.”
There was disagreement about this point at the hearing. U.S. Representative Morgan Griffith (R-Va.) read the section of the Doshi-Topol article quoted above and asked one witness, Offit, to weigh in.
“Do you agree with those concerns? And either way, tell me why,” Griffith asked.
“I don’t agree,” Offit responded.
“I think it’s actually much harder to prevent asymptomatic infection or mildly symptomatic infection,” he said. “If you can prevent that, you are much more likely to prevent moderate to severe disease. So I think they have it backwards.”
But other researchers also question the approaches used with the current crop of COVID-19 vaccines.
“With the current protocols, it is conceivable that a vaccine might be considered effective – and eventually approved – based primarily on its ability to prevent mild cases alone,” wrote William Haseltine, PhD, president of the nonprofit ACCESS Health International, in a September 22 opinion article in the Washington Post titled: “Beware of COVID-19 Vaccine Trials Designed to Succeed From the Start.”
In an interview with Medscape Medical News on Wednesday, Haseltine said he maintains these concerns about the tests. Earlier in his career, he was a leader in HIV research through his lab at Harvard University in Cambridge, Massachusetts, and he subsequently led a biotech company, Human Genome Sciences.
He fears consumers will not get what they might expect from the vaccines being tested.
“What people care about is if this is going to keep them out of the hospital and will it keep them alive. And that’s not even part of this protocol,” Haseltine said.
This article first appeared on Medscape.com.
on Wednesday.
The career staff of the Food and Drug Administration can be counted on to appropriately weigh whether a vaccine should be cleared for use in preventing COVID-19, witnesses, including Paul A. Offit, MD, of Children’s Hospital of Philadelphia, told the House Energy and Commerce Committee’s oversight and investigations panel.
FDA staffers would object to attempts by the Trump administration to rush a vaccine to the public without proper vetting, as would veteran federal researchers, including National Institutes of Health Director Francis S. Collins, MD, PhD, and Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, Offit said.
“If COVID-19 vaccines are released before they’re ready to be released, you will hear from these people, and you will also hear from people like Dr. Francis Collins and Tony Fauci, both of whom are trusted by the American public, as well as many other academicians and researchers who wouldn’t stand for this,” he said.
“The public is already nervous about these vaccines,” said Offit, who serves on key FDA and Centers for Disease Control and Prevention committees overseeing vaccine policy. “If trusted health officials stand up and decry a premature release, the celebration by the administration will be short-lived.”
Overly optimistic estimates about a potential approval can only serve to erode the public’s trust in these crucial vaccines, said another witness, Ashish K. Jha, MD, MPH, the dean of Brown University’s School of Public Health, in Providence, Rhode Island.
“All political leaders need to stop talking about things like time lines,” Jha told the lawmakers.
President Donald Trump has several times suggested that a COVID vaccine might be approved ahead of the November 3 election, where he faces a significant challenge from his Democratic rival, former Vice President Joe Biden.
In a Tuesday night debate with Biden, Trump again raised the idea of a quick approval. “Now we’re weeks away from a vaccine,” Trump said during the debate.
Trump’s estimates, though, are not in line with those offered by most firms involved with making vaccines. The most optimistic projections have come from Pfizer Inc. The drugmaker’s chief executive, Albert Bourla, has spoken about his company possibly having data to present to the FDA as early as late October about the safety and effectiveness of a vaccine.
In a September 8 interview with the Today show, Bourla said there was a 60% chance his company would meet that goal. In response to a question, he made it clear his comments applied to a potential Pfizer application, not an approval or release of a vaccine by that time.
In response to concerns about political pressures, the FDA in June issued guidance outlining what its staff would require for approval of a COVID-19 vaccine.
Pushback on politics
Another witness at the Wednesday hearing, Mark McClellan, MD, PhD, a former FDA commissioner (2002 – 2004), pushed back on objections to a potential release of further guidance from the agency.
“Some recent statements from the White House have implied that FDA’s plan to release additional written guidance on its expectations for emergency use authorization of a vaccine is unnecessarily raising the bar on regulatory standards for authorization,” said McClellan in his testimony for the House panel. “That is not the case.”
Instead, further FDA guidance would be a welcome form of feedback for the firms trying to develop COVID-19 vaccines, according to McClellan, who also serves on the board of directors for Johnson & Johnson. Johnson & Johnson is among the firms that have advanced a COVID-19 vaccine candidate to phase 3 testing. In his role as a director, he serves on the board’s regulatory compliance committee.
Along with politics, recent stumbles at FDA with emergency use authorizations (EUAs) of treatments for COVID-19 have eroded the public’s confidence in the agency, Jha told the House panel. The FDA approved hydroxychloroquine, a medicine promoted by Trump for use in COVID, under an EUA in March and then revoked this clearance in June.
Jha said the FDA’s most serious misstep was its handling of convalescent plasma, which was approved through an EUA on August 23 “in a highly advertised and widely televised announcement including the president.
“The announcement solidified in the public conversation the impression that, increasingly with this administration, politics are taking over trusted, nonpartisan scientific institutions,” he said in his testimony.
Approving a COVID-19 vaccine on the limited evidence through an EUA would mark a serious departure from FDA policy, according to Jha.
“While we sometimes accept a certain level of potential harm in experimental treatments for those who are severely ill, vaccines are given to healthy people and therefore need to have a substantially higher measure of safety and effectiveness,” he explained.
Jha said the FDA has only once before used this EUA approach for a vaccine. That was for a vaccine against inhaled anthrax and was mostly distributed to high-risk soldiers and civilians in war zones.
COVID-19, in contrast, is an infection that has changed lives around the world. The virus has contributed to more than 1 million deaths, including more than 200,000 in the United States, according to the World Health Organization.
Scientists are hoping vaccines will help curb this infection, although much of the future success of vaccines depends on how widely they are used, witnesses told the House panel.
Debate on approaches for vaccine effectiveness
In his testimony, Jha also noted concerns about COVID-19 vaccine trials. He included a reference to a Sept. 22 opinion article titled, “These Coronavirus Trials Don›t Answer the One Question We Need to Know,” which was written by Peter Doshi, PhD, of the University of Maryland School of Pharmacy, in Baltimore, and Eric Topol, MD, a professor of molecular medicine at Scripps Research in La Jolla, Calif. Topol is also editor in chief of Medscape.
Topol and Doshi questioned why the firms Moderna, Pfizer, and AstraZeneca structured their competing trials such that “a vaccine could meet the companies’ benchmark for success if it lowered the risk of mild Covid-19, but was never shown to reduce moderate or severe forms of the disease, or the risk of hospitalization, admissions to the intensive care unit or death.”
“To say a vaccine works should mean that most people no longer run the risk of getting seriously sick,” Topol and Doshi wrote. “That’s not what these trials will determine.”
There was disagreement about this point at the hearing. U.S. Representative Morgan Griffith (R-Va.) read the section of the Doshi-Topol article quoted above and asked one witness, Offit, to weigh in.
“Do you agree with those concerns? And either way, tell me why,” Griffith asked.
“I don’t agree,” Offit responded.
“I think it’s actually much harder to prevent asymptomatic infection or mildly symptomatic infection,” he said. “If you can prevent that, you are much more likely to prevent moderate to severe disease. So I think they have it backwards.”
But other researchers also question the approaches used with the current crop of COVID-19 vaccines.
“With the current protocols, it is conceivable that a vaccine might be considered effective – and eventually approved – based primarily on its ability to prevent mild cases alone,” wrote William Haseltine, PhD, president of the nonprofit ACCESS Health International, in a September 22 opinion article in the Washington Post titled: “Beware of COVID-19 Vaccine Trials Designed to Succeed From the Start.”
In an interview with Medscape Medical News on Wednesday, Haseltine said he maintains these concerns about the tests. Earlier in his career, he was a leader in HIV research through his lab at Harvard University in Cambridge, Massachusetts, and he subsequently led a biotech company, Human Genome Sciences.
He fears consumers will not get what they might expect from the vaccines being tested.
“What people care about is if this is going to keep them out of the hospital and will it keep them alive. And that’s not even part of this protocol,” Haseltine said.
This article first appeared on Medscape.com.
Nerve damage linked to prone positioning in COVID-19
A new case series describes peripheral nerve injuries associated with this type of positioning and suggests ways to minimize the potential damage.
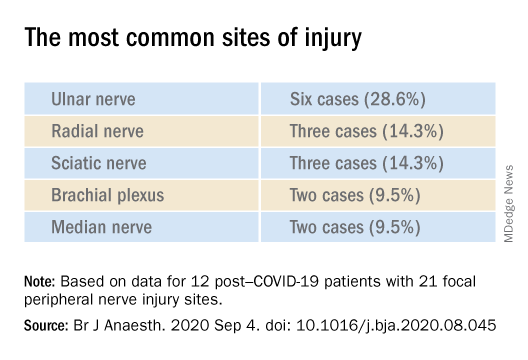
“Physicians should remain aware of increased susceptibility to peripheral nerve damage in patients with severe COVID-19 after prone positioning, since it is surprisingly common among these patients, and should refine standard protocols accordingly to reduce that risk,” said senior author Colin Franz, MD, PhD, director of the Electrodiagnostic Laboratory, Shirley Ryan AbilityLab, Chicago.
The article was published online Sept. 4 in the British Journal of Anaesthesiology.
Unique type of nerve injury
Many patients who are admitted to the intensive care unit with COVID-19 undergo invasive mechanical ventilation because of acute respiratory distress syndrome (ARDS). Clinical guidelines recommend that such patients lie in the prone position 12-16 hours per day.
“Prone positioning for up to 16 hours is a therapy we use for patients with more severe forms of ARDS, and high-level evidence points to mortality benefit in patients with moderate to severe ARDS if [mechanical] ventilation occurs,” said study coauthor James McCauley Walter, MD, of the pulmonary division at Northwestern University, Chicago.
With a “significant number of COVID-19 patients flooding the ICU, we quickly started to prone a lot of them, but if you are in a specific position for multiple hours a day, coupled with the neurotoxic effects of the SARS-CoV-2 virus itself, you may be exposed to a unique type of nerve injury,” he said.
Dr. Walter said that the “incidence of asymmetric neuropathies seems out of proportion to what has been reported in non–COVID-19 settings, which is what caught our attention.”
Many of these patients are discharged to rehabilitation hospitals, and “what we noticed, which was unique about COVID-19 patients coming to our rehab hospital, was that, compared with other patients who had been critically ill with a long hospital stay, there was a significantly higher percentage of COVID-19 patients who had peripheral nerve damage,” Dr. Franz said.
The authors described 12 of these patients who were admitted between April 24 and June 30, 2020 (mean age, 60.3 years; range, 23-80 years). The sample included White, Black, and Hispanic individuals. Eleven of the 12 post–COVID-19 patients with peripheral nerve damage had experienced prone positioning during acute management.
The average number of days patients received mechanical ventilation was 33.6 (range, 12-62 days). The average number of proning sessions was 4.5 (range, 1-16) with an average of 81.2 hours (range, 16-252 hours) spent prone.
A major contributor
Dr. Franz suggested that prone positioning is likely not the only cause of peripheral nerve damage but “may play a big role in these patients who are vulnerable because of viral infection and the critical illness that causes damage and nerve injuries.”
“The first component of lifesaving care for the critically ill in the ICU is intravenous fluids, mechanical ventilation, steroids, and antibiotics for infection,” said Dr. Walter.
“We are trying to come up with ways to place patients in prone position in safer ways, to pay attention to pressure points and areas of injury that we have seen and try to offload them, to see if we can decrease the rate of these injuries,” he added.
The researchers’ article includes a heat map diagram as a “template for where to focus the most efforts, in terms of decreasing pressure,” Dr. Walter said.
“The nerves are accepting too much force for gravely ill COVID-19 patients to handle, so we suggest using the template to determine where extra padding might be needed, or a protocol that might include changes in positioning,” he added.
Dr. Franz described the interventions used for COVID-19 patients with prone positioning–related peripheral nerve damage. “The first step is trying to address the problems one by one, either trying to solve them through exercise or teaching new skills, new ways to compensate, beginning with basic activities, such as getting out of bed and self-care,” he said.
Long-term recovery of nerve injuries depends on how severe the injuries are. Some nerves can slowly regenerate – possibly at the rate of 1 inch per month – which can be a long process, taking between a year and 18 months.
Dr. Franz said that therapies for this condition are “extrapolated from clinical trial work” on promoting nerve regeneration after surgery using electrical stimulation to enable nerves to regrow at a faster rate.
“Regeneration is not only slow, but it may not happen completely, leaving the patient with permanent nerve damage – in fact, based on our experience and what has been reported, the percentage of patients with full recovery is only 10%,” he said.
The most common symptomatic complaint other than lack of movement or feeling is neuropathic pain, “which may require medication to take the edge off the pain,” Dr. Franz added.
Irreversible damage?
Commenting on the study, Tae Chung, MD, of the departments of physical medicine, rehabilitation, and neurology, Johns Hopkins University, Baltimore, said the study “provides one of the first and the largest description of peripheral nerve injury associated with prone positioning for management of ARDS from COVID-19.”
Dr. Chung, who was not involved in the research, noted that “various neurological complications from COVID-19 have been reported, and some of them may result in irreversible neurological damage or delay the recovery from COVID-19 infection,” so “accurate and timely diagnosis of such neurological complications is critical for rehabilitation of the COVID-19 survivors.”
The study received no funding. Dr. Franz, Dr. Walter, study coauthors, and Dr. Chung report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A new case series describes peripheral nerve injuries associated with this type of positioning and suggests ways to minimize the potential damage.

“Physicians should remain aware of increased susceptibility to peripheral nerve damage in patients with severe COVID-19 after prone positioning, since it is surprisingly common among these patients, and should refine standard protocols accordingly to reduce that risk,” said senior author Colin Franz, MD, PhD, director of the Electrodiagnostic Laboratory, Shirley Ryan AbilityLab, Chicago.
The article was published online Sept. 4 in the British Journal of Anaesthesiology.
Unique type of nerve injury
Many patients who are admitted to the intensive care unit with COVID-19 undergo invasive mechanical ventilation because of acute respiratory distress syndrome (ARDS). Clinical guidelines recommend that such patients lie in the prone position 12-16 hours per day.
“Prone positioning for up to 16 hours is a therapy we use for patients with more severe forms of ARDS, and high-level evidence points to mortality benefit in patients with moderate to severe ARDS if [mechanical] ventilation occurs,” said study coauthor James McCauley Walter, MD, of the pulmonary division at Northwestern University, Chicago.
With a “significant number of COVID-19 patients flooding the ICU, we quickly started to prone a lot of them, but if you are in a specific position for multiple hours a day, coupled with the neurotoxic effects of the SARS-CoV-2 virus itself, you may be exposed to a unique type of nerve injury,” he said.
Dr. Walter said that the “incidence of asymmetric neuropathies seems out of proportion to what has been reported in non–COVID-19 settings, which is what caught our attention.”
Many of these patients are discharged to rehabilitation hospitals, and “what we noticed, which was unique about COVID-19 patients coming to our rehab hospital, was that, compared with other patients who had been critically ill with a long hospital stay, there was a significantly higher percentage of COVID-19 patients who had peripheral nerve damage,” Dr. Franz said.
The authors described 12 of these patients who were admitted between April 24 and June 30, 2020 (mean age, 60.3 years; range, 23-80 years). The sample included White, Black, and Hispanic individuals. Eleven of the 12 post–COVID-19 patients with peripheral nerve damage had experienced prone positioning during acute management.
The average number of days patients received mechanical ventilation was 33.6 (range, 12-62 days). The average number of proning sessions was 4.5 (range, 1-16) with an average of 81.2 hours (range, 16-252 hours) spent prone.
A major contributor
Dr. Franz suggested that prone positioning is likely not the only cause of peripheral nerve damage but “may play a big role in these patients who are vulnerable because of viral infection and the critical illness that causes damage and nerve injuries.”
“The first component of lifesaving care for the critically ill in the ICU is intravenous fluids, mechanical ventilation, steroids, and antibiotics for infection,” said Dr. Walter.
“We are trying to come up with ways to place patients in prone position in safer ways, to pay attention to pressure points and areas of injury that we have seen and try to offload them, to see if we can decrease the rate of these injuries,” he added.
The researchers’ article includes a heat map diagram as a “template for where to focus the most efforts, in terms of decreasing pressure,” Dr. Walter said.
“The nerves are accepting too much force for gravely ill COVID-19 patients to handle, so we suggest using the template to determine where extra padding might be needed, or a protocol that might include changes in positioning,” he added.
Dr. Franz described the interventions used for COVID-19 patients with prone positioning–related peripheral nerve damage. “The first step is trying to address the problems one by one, either trying to solve them through exercise or teaching new skills, new ways to compensate, beginning with basic activities, such as getting out of bed and self-care,” he said.
Long-term recovery of nerve injuries depends on how severe the injuries are. Some nerves can slowly regenerate – possibly at the rate of 1 inch per month – which can be a long process, taking between a year and 18 months.
Dr. Franz said that therapies for this condition are “extrapolated from clinical trial work” on promoting nerve regeneration after surgery using electrical stimulation to enable nerves to regrow at a faster rate.
“Regeneration is not only slow, but it may not happen completely, leaving the patient with permanent nerve damage – in fact, based on our experience and what has been reported, the percentage of patients with full recovery is only 10%,” he said.
The most common symptomatic complaint other than lack of movement or feeling is neuropathic pain, “which may require medication to take the edge off the pain,” Dr. Franz added.
Irreversible damage?
Commenting on the study, Tae Chung, MD, of the departments of physical medicine, rehabilitation, and neurology, Johns Hopkins University, Baltimore, said the study “provides one of the first and the largest description of peripheral nerve injury associated with prone positioning for management of ARDS from COVID-19.”
Dr. Chung, who was not involved in the research, noted that “various neurological complications from COVID-19 have been reported, and some of them may result in irreversible neurological damage or delay the recovery from COVID-19 infection,” so “accurate and timely diagnosis of such neurological complications is critical for rehabilitation of the COVID-19 survivors.”
The study received no funding. Dr. Franz, Dr. Walter, study coauthors, and Dr. Chung report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A new case series describes peripheral nerve injuries associated with this type of positioning and suggests ways to minimize the potential damage.

“Physicians should remain aware of increased susceptibility to peripheral nerve damage in patients with severe COVID-19 after prone positioning, since it is surprisingly common among these patients, and should refine standard protocols accordingly to reduce that risk,” said senior author Colin Franz, MD, PhD, director of the Electrodiagnostic Laboratory, Shirley Ryan AbilityLab, Chicago.
The article was published online Sept. 4 in the British Journal of Anaesthesiology.
Unique type of nerve injury
Many patients who are admitted to the intensive care unit with COVID-19 undergo invasive mechanical ventilation because of acute respiratory distress syndrome (ARDS). Clinical guidelines recommend that such patients lie in the prone position 12-16 hours per day.
“Prone positioning for up to 16 hours is a therapy we use for patients with more severe forms of ARDS, and high-level evidence points to mortality benefit in patients with moderate to severe ARDS if [mechanical] ventilation occurs,” said study coauthor James McCauley Walter, MD, of the pulmonary division at Northwestern University, Chicago.
With a “significant number of COVID-19 patients flooding the ICU, we quickly started to prone a lot of them, but if you are in a specific position for multiple hours a day, coupled with the neurotoxic effects of the SARS-CoV-2 virus itself, you may be exposed to a unique type of nerve injury,” he said.
Dr. Walter said that the “incidence of asymmetric neuropathies seems out of proportion to what has been reported in non–COVID-19 settings, which is what caught our attention.”
Many of these patients are discharged to rehabilitation hospitals, and “what we noticed, which was unique about COVID-19 patients coming to our rehab hospital, was that, compared with other patients who had been critically ill with a long hospital stay, there was a significantly higher percentage of COVID-19 patients who had peripheral nerve damage,” Dr. Franz said.
The authors described 12 of these patients who were admitted between April 24 and June 30, 2020 (mean age, 60.3 years; range, 23-80 years). The sample included White, Black, and Hispanic individuals. Eleven of the 12 post–COVID-19 patients with peripheral nerve damage had experienced prone positioning during acute management.
The average number of days patients received mechanical ventilation was 33.6 (range, 12-62 days). The average number of proning sessions was 4.5 (range, 1-16) with an average of 81.2 hours (range, 16-252 hours) spent prone.
A major contributor
Dr. Franz suggested that prone positioning is likely not the only cause of peripheral nerve damage but “may play a big role in these patients who are vulnerable because of viral infection and the critical illness that causes damage and nerve injuries.”
“The first component of lifesaving care for the critically ill in the ICU is intravenous fluids, mechanical ventilation, steroids, and antibiotics for infection,” said Dr. Walter.
“We are trying to come up with ways to place patients in prone position in safer ways, to pay attention to pressure points and areas of injury that we have seen and try to offload them, to see if we can decrease the rate of these injuries,” he added.
The researchers’ article includes a heat map diagram as a “template for where to focus the most efforts, in terms of decreasing pressure,” Dr. Walter said.
“The nerves are accepting too much force for gravely ill COVID-19 patients to handle, so we suggest using the template to determine where extra padding might be needed, or a protocol that might include changes in positioning,” he added.
Dr. Franz described the interventions used for COVID-19 patients with prone positioning–related peripheral nerve damage. “The first step is trying to address the problems one by one, either trying to solve them through exercise or teaching new skills, new ways to compensate, beginning with basic activities, such as getting out of bed and self-care,” he said.
Long-term recovery of nerve injuries depends on how severe the injuries are. Some nerves can slowly regenerate – possibly at the rate of 1 inch per month – which can be a long process, taking between a year and 18 months.
Dr. Franz said that therapies for this condition are “extrapolated from clinical trial work” on promoting nerve regeneration after surgery using electrical stimulation to enable nerves to regrow at a faster rate.
“Regeneration is not only slow, but it may not happen completely, leaving the patient with permanent nerve damage – in fact, based on our experience and what has been reported, the percentage of patients with full recovery is only 10%,” he said.
The most common symptomatic complaint other than lack of movement or feeling is neuropathic pain, “which may require medication to take the edge off the pain,” Dr. Franz added.
Irreversible damage?
Commenting on the study, Tae Chung, MD, of the departments of physical medicine, rehabilitation, and neurology, Johns Hopkins University, Baltimore, said the study “provides one of the first and the largest description of peripheral nerve injury associated with prone positioning for management of ARDS from COVID-19.”
Dr. Chung, who was not involved in the research, noted that “various neurological complications from COVID-19 have been reported, and some of them may result in irreversible neurological damage or delay the recovery from COVID-19 infection,” so “accurate and timely diagnosis of such neurological complications is critical for rehabilitation of the COVID-19 survivors.”
The study received no funding. Dr. Franz, Dr. Walter, study coauthors, and Dr. Chung report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM THE BRITISH JOURNAL OF ANAESTHESIOLOGY
British protocol allows insulin-treated pilots to fly safely
A protocol developed in the United Kingdom that allows commercial pilots with insulin-treated diabetes to fly airplanes has resulted in precise glycemic control during flight and no safety issues, new research finds.
The results are believed to be the largest-ever dataset for people with insulin-treated diabetes in “safety-critical” occupations, said Gillian L. Garden, MD, who presented the findings this week at the virtual annual meeting of the European Association for the Study of Diabetes.
The protocol, which involves multiple glucose measurements before and throughout flights and corrective action for out-of-range values, resulted in 98% of glucose values in target range with no pilot incapacitation. The results were also published in Diabetes Care earlier this year, noted Dr. Garden, a clinical fellow in diabetes and endocrinology at the Royal Surrey NHS Foundation Trust, Guildford, England.
“There were no safety concerns at all and certainly no episodes of pilot incapacitation throughout the [7.5] years of the study. Our study proves that the protocol is feasible, is practical to implement, and is easily understood by both pilots and copilots,” she observed.
Dr. Garden foresees wider use of this approach: “We believe the study is of international importance and this protocol could be adopted by other aviation authorities to allow more insulin-treated pilots worldwide to be able to fly commercial aircraft.”
“With proper oversight and a defined protocol such as the one that we’ve been working to produce it is possible for anybody with insulin-treated diabetes to, in fact, adequately perform other safety-critical occupations as well, and it would be good to see fewer people being discriminated against on the basis of their diabetes,” she emphasized.
‘Impressive’ study of highly motivated individuals
Historically, insulin-treated patients – with both types of diabetes – had been barred from many “safety-critical” occupations, including commercial airline piloting. This was out of concern both for the potential immediate effects of hypoglycemia, including cognitive impairment and slowing of reaction times, as well as the long-term effects of diabetes, including vision loss and nerve damage, Dr. Garden explained.
However, “with advances in diabetes management, including different insulin types, methods of delivery, and glucose-monitoring systems, it’s now possible for individuals to have excellent glycemic control. This, along with the implementation of legislation against discrimination, has allowed insulin-treated people to no longer be debarred from certain employments,” she explained during an EASD press briefing on Sept. 24.
An expert panel convened in 2010 by the U.K. Civil Aviation Authority developed the protocol, and in 2012, the CAA began issuing class 1 medical certificates to insulin-treated pilots. The protocol was subsequently adopted by Ireland in 2015 and by Austria in 2016.
Initial results from nearly 9,000 glucose readings of 26 U.K. pilots who received a certificate between 2012 and 2015 were reported at the EASD 2016 Annual Meeting and published in 2017.
The current study is far larger, with 38,621 glucose readings from 49 pilots from the United Kingdom, Ireland, and Austria who have been using the protocol since 2012.
Mark Evans, MD, of Addenbrookes Hospital, Cambridge, England, said in an interview that “I thought this was a fascinating paper. ... I was deeply impressed by the data.”
Dr. Evans, who chairs the U.K. Department of Transport advisory panel on driving and diabetes, also noted: “The group of people with insulin-treated diabetes flying planes are a phenomenally motivated group who are prepared to do things that probably most drivers of motor vehicles would find oppressive or very difficult to do.”
“I thought the outcomes were really impressive in terms of the amount of time they were able to maintain themselves within glucose target ranges.”
Indeed, Dr. Garden said, “pilots are typically very organized and used to dealing with strict protocols with regard to all of the processes they have to follow before they fly and the safety checks they have to do. They adapted to this additional safety measure really well.”
Traffic light protocol keeps pilots in range
The protocol requires pilots to perform fingerstick glucose checks 30 minutes prior to flight, every hour during flight, and 30 minutes before landing. They must also attend clinical reviews every 6 months.
A traffic light system is used to denote acceptable pre- and in-flight glucose levels, with green meaning acceptable (5.0-15.0 mmol/L [90-270 mg/dL]), amber indicating caution for low (4.0-4.9 mmol/L [72-88 mg/dL]) or high (15.1-20.0 mmol/L [272-360 mg/dL]) blood glucose. Red requires immediate action (low blood glucose <4 mmol/L [72 mg/dL] and high >20 mmol/L [>360 mg/dL]).
Low amber values require the pilot to ingest 10-15 fast-acting carbohydrates and retest after 30 minutes. Low red values indicate the pilot must hand over the controls to the copilot. High readings of >15.0 mmol/L (>270 mg/dL) require an insulin dosing review. A high red value also requires the pilot to hand over the controls.
Of the 49 pilots, 84% had type 1 diabetes and 16% had insulin-treated type 2 diabetes. Most (61%) had class 1 medical certificates (required to validate a commercial pilot license) and 39% had class 2 medical certificates (required to validate a private pilot’s license). Median diabetes duration was 10.9 years.
Of note, all had become pilots prior to diabetes onset. As of now, the EU Aviation Safety Agency doesn’t allow people with preexisting insulin-treated diabetes to become pilots.
“We are fighting to change that, but with the U.K. leaving the EU, the Civil Aviation Authority might pursue it [separately]. We don’t know how that will pan out,” Dr. Garden noted during the briefing.
Over the 7.5 years, 97.7% of readings were within the green range, while just 1.42% were in the low amber range and 0.75% in the high amber range. Just 48 readings (0.12%) were in the low red range and 6 (0.02%) in the high red range. Of the 48 low reds, just 14 were recorded during flight. Of the six high reds, only two occurred during flight.
There were no instances of pilot incapacitation or changes in average hemoglobin A1c.
The results should alleviate concerns expressed after a prior report that pilots’ overall glycemic control could worsen if they pushed too hard to avoid lows, Dr. Garden noted.
The proportion of out-of-range values declined from 5.7% in 2013 to 1.2% in 2019. Low red values didn’t change (0.2% in 2013 and 0.1% in 2019) but high red values had completely disappeared by 2017.
What about CGM?
In response to a question during the briefing about use of continuous glucose monitoring, Dr. Garden said that some of the pilots were using CGM in addition to following the fingerstick protocol.
At the time the protocol was developed a decade ago, CGM wasn’t considered accurate enough and there wasn’t evidence for its use at high altitude.
But there has been a great deal more data since then, she said, noting “we believe it would be safer to use now because of how good that equipment is. ... Certainly, there’s a good number [of pilots] using CGM, and hopefully that will increase and the protocol will change to allow them all to use CGM if they want to.
“I think we’ll probably see CGM in the protocol within the next year to 2 years. Hopefully, that will make things a lot easier, so pilots won’t have to prick their fingers while they’re flying.”
Her group is currently conducting a study (DEXFLY) on use of the Dexcom G6 in addition to fingersticks in commercial pilots with insulin-treated diabetes. Results are expected by the end of the year.
Dr. Evans commented: “I think it’s a no-brainer that CGM will become the gold standard. I understand why they’re going to want to be cautious about this, but if they can generate data to show it will be a low-risk change, I think it will come.”
He also noted that it was only a couple of years ago that U.K. law was changed to allow car drivers with insulin-treated diabetes to use CGM as part of their glucose-testing requirements (before driving and every 2 hours). CGM still isn’t approved for use by drivers of trucks or other large vehicles, but “I think at some point in the future it will become more accepted,” Dr. Evans commented.
Dr. Garden reported no relevant financial relationships. Dr. Evans has reported being an advisory board member of, speaker for, and/or grant recipient from Novo Nordisk, Dexcom, Medtronic, Abbott, Eli Lilly, and Roche.
A version of this article originally appeared on Medscape.com.
A protocol developed in the United Kingdom that allows commercial pilots with insulin-treated diabetes to fly airplanes has resulted in precise glycemic control during flight and no safety issues, new research finds.
The results are believed to be the largest-ever dataset for people with insulin-treated diabetes in “safety-critical” occupations, said Gillian L. Garden, MD, who presented the findings this week at the virtual annual meeting of the European Association for the Study of Diabetes.
The protocol, which involves multiple glucose measurements before and throughout flights and corrective action for out-of-range values, resulted in 98% of glucose values in target range with no pilot incapacitation. The results were also published in Diabetes Care earlier this year, noted Dr. Garden, a clinical fellow in diabetes and endocrinology at the Royal Surrey NHS Foundation Trust, Guildford, England.
“There were no safety concerns at all and certainly no episodes of pilot incapacitation throughout the [7.5] years of the study. Our study proves that the protocol is feasible, is practical to implement, and is easily understood by both pilots and copilots,” she observed.
Dr. Garden foresees wider use of this approach: “We believe the study is of international importance and this protocol could be adopted by other aviation authorities to allow more insulin-treated pilots worldwide to be able to fly commercial aircraft.”
“With proper oversight and a defined protocol such as the one that we’ve been working to produce it is possible for anybody with insulin-treated diabetes to, in fact, adequately perform other safety-critical occupations as well, and it would be good to see fewer people being discriminated against on the basis of their diabetes,” she emphasized.
‘Impressive’ study of highly motivated individuals
Historically, insulin-treated patients – with both types of diabetes – had been barred from many “safety-critical” occupations, including commercial airline piloting. This was out of concern both for the potential immediate effects of hypoglycemia, including cognitive impairment and slowing of reaction times, as well as the long-term effects of diabetes, including vision loss and nerve damage, Dr. Garden explained.
However, “with advances in diabetes management, including different insulin types, methods of delivery, and glucose-monitoring systems, it’s now possible for individuals to have excellent glycemic control. This, along with the implementation of legislation against discrimination, has allowed insulin-treated people to no longer be debarred from certain employments,” she explained during an EASD press briefing on Sept. 24.
An expert panel convened in 2010 by the U.K. Civil Aviation Authority developed the protocol, and in 2012, the CAA began issuing class 1 medical certificates to insulin-treated pilots. The protocol was subsequently adopted by Ireland in 2015 and by Austria in 2016.
Initial results from nearly 9,000 glucose readings of 26 U.K. pilots who received a certificate between 2012 and 2015 were reported at the EASD 2016 Annual Meeting and published in 2017.
The current study is far larger, with 38,621 glucose readings from 49 pilots from the United Kingdom, Ireland, and Austria who have been using the protocol since 2012.
Mark Evans, MD, of Addenbrookes Hospital, Cambridge, England, said in an interview that “I thought this was a fascinating paper. ... I was deeply impressed by the data.”
Dr. Evans, who chairs the U.K. Department of Transport advisory panel on driving and diabetes, also noted: “The group of people with insulin-treated diabetes flying planes are a phenomenally motivated group who are prepared to do things that probably most drivers of motor vehicles would find oppressive or very difficult to do.”
“I thought the outcomes were really impressive in terms of the amount of time they were able to maintain themselves within glucose target ranges.”
Indeed, Dr. Garden said, “pilots are typically very organized and used to dealing with strict protocols with regard to all of the processes they have to follow before they fly and the safety checks they have to do. They adapted to this additional safety measure really well.”
Traffic light protocol keeps pilots in range
The protocol requires pilots to perform fingerstick glucose checks 30 minutes prior to flight, every hour during flight, and 30 minutes before landing. They must also attend clinical reviews every 6 months.
A traffic light system is used to denote acceptable pre- and in-flight glucose levels, with green meaning acceptable (5.0-15.0 mmol/L [90-270 mg/dL]), amber indicating caution for low (4.0-4.9 mmol/L [72-88 mg/dL]) or high (15.1-20.0 mmol/L [272-360 mg/dL]) blood glucose. Red requires immediate action (low blood glucose <4 mmol/L [72 mg/dL] and high >20 mmol/L [>360 mg/dL]).
Low amber values require the pilot to ingest 10-15 fast-acting carbohydrates and retest after 30 minutes. Low red values indicate the pilot must hand over the controls to the copilot. High readings of >15.0 mmol/L (>270 mg/dL) require an insulin dosing review. A high red value also requires the pilot to hand over the controls.
Of the 49 pilots, 84% had type 1 diabetes and 16% had insulin-treated type 2 diabetes. Most (61%) had class 1 medical certificates (required to validate a commercial pilot license) and 39% had class 2 medical certificates (required to validate a private pilot’s license). Median diabetes duration was 10.9 years.
Of note, all had become pilots prior to diabetes onset. As of now, the EU Aviation Safety Agency doesn’t allow people with preexisting insulin-treated diabetes to become pilots.
“We are fighting to change that, but with the U.K. leaving the EU, the Civil Aviation Authority might pursue it [separately]. We don’t know how that will pan out,” Dr. Garden noted during the briefing.
Over the 7.5 years, 97.7% of readings were within the green range, while just 1.42% were in the low amber range and 0.75% in the high amber range. Just 48 readings (0.12%) were in the low red range and 6 (0.02%) in the high red range. Of the 48 low reds, just 14 were recorded during flight. Of the six high reds, only two occurred during flight.
There were no instances of pilot incapacitation or changes in average hemoglobin A1c.
The results should alleviate concerns expressed after a prior report that pilots’ overall glycemic control could worsen if they pushed too hard to avoid lows, Dr. Garden noted.
The proportion of out-of-range values declined from 5.7% in 2013 to 1.2% in 2019. Low red values didn’t change (0.2% in 2013 and 0.1% in 2019) but high red values had completely disappeared by 2017.
What about CGM?
In response to a question during the briefing about use of continuous glucose monitoring, Dr. Garden said that some of the pilots were using CGM in addition to following the fingerstick protocol.
At the time the protocol was developed a decade ago, CGM wasn’t considered accurate enough and there wasn’t evidence for its use at high altitude.
But there has been a great deal more data since then, she said, noting “we believe it would be safer to use now because of how good that equipment is. ... Certainly, there’s a good number [of pilots] using CGM, and hopefully that will increase and the protocol will change to allow them all to use CGM if they want to.
“I think we’ll probably see CGM in the protocol within the next year to 2 years. Hopefully, that will make things a lot easier, so pilots won’t have to prick their fingers while they’re flying.”
Her group is currently conducting a study (DEXFLY) on use of the Dexcom G6 in addition to fingersticks in commercial pilots with insulin-treated diabetes. Results are expected by the end of the year.
Dr. Evans commented: “I think it’s a no-brainer that CGM will become the gold standard. I understand why they’re going to want to be cautious about this, but if they can generate data to show it will be a low-risk change, I think it will come.”
He also noted that it was only a couple of years ago that U.K. law was changed to allow car drivers with insulin-treated diabetes to use CGM as part of their glucose-testing requirements (before driving and every 2 hours). CGM still isn’t approved for use by drivers of trucks or other large vehicles, but “I think at some point in the future it will become more accepted,” Dr. Evans commented.
Dr. Garden reported no relevant financial relationships. Dr. Evans has reported being an advisory board member of, speaker for, and/or grant recipient from Novo Nordisk, Dexcom, Medtronic, Abbott, Eli Lilly, and Roche.
A version of this article originally appeared on Medscape.com.
A protocol developed in the United Kingdom that allows commercial pilots with insulin-treated diabetes to fly airplanes has resulted in precise glycemic control during flight and no safety issues, new research finds.
The results are believed to be the largest-ever dataset for people with insulin-treated diabetes in “safety-critical” occupations, said Gillian L. Garden, MD, who presented the findings this week at the virtual annual meeting of the European Association for the Study of Diabetes.
The protocol, which involves multiple glucose measurements before and throughout flights and corrective action for out-of-range values, resulted in 98% of glucose values in target range with no pilot incapacitation. The results were also published in Diabetes Care earlier this year, noted Dr. Garden, a clinical fellow in diabetes and endocrinology at the Royal Surrey NHS Foundation Trust, Guildford, England.
“There were no safety concerns at all and certainly no episodes of pilot incapacitation throughout the [7.5] years of the study. Our study proves that the protocol is feasible, is practical to implement, and is easily understood by both pilots and copilots,” she observed.
Dr. Garden foresees wider use of this approach: “We believe the study is of international importance and this protocol could be adopted by other aviation authorities to allow more insulin-treated pilots worldwide to be able to fly commercial aircraft.”
“With proper oversight and a defined protocol such as the one that we’ve been working to produce it is possible for anybody with insulin-treated diabetes to, in fact, adequately perform other safety-critical occupations as well, and it would be good to see fewer people being discriminated against on the basis of their diabetes,” she emphasized.
‘Impressive’ study of highly motivated individuals
Historically, insulin-treated patients – with both types of diabetes – had been barred from many “safety-critical” occupations, including commercial airline piloting. This was out of concern both for the potential immediate effects of hypoglycemia, including cognitive impairment and slowing of reaction times, as well as the long-term effects of diabetes, including vision loss and nerve damage, Dr. Garden explained.
However, “with advances in diabetes management, including different insulin types, methods of delivery, and glucose-monitoring systems, it’s now possible for individuals to have excellent glycemic control. This, along with the implementation of legislation against discrimination, has allowed insulin-treated people to no longer be debarred from certain employments,” she explained during an EASD press briefing on Sept. 24.
An expert panel convened in 2010 by the U.K. Civil Aviation Authority developed the protocol, and in 2012, the CAA began issuing class 1 medical certificates to insulin-treated pilots. The protocol was subsequently adopted by Ireland in 2015 and by Austria in 2016.
Initial results from nearly 9,000 glucose readings of 26 U.K. pilots who received a certificate between 2012 and 2015 were reported at the EASD 2016 Annual Meeting and published in 2017.
The current study is far larger, with 38,621 glucose readings from 49 pilots from the United Kingdom, Ireland, and Austria who have been using the protocol since 2012.
Mark Evans, MD, of Addenbrookes Hospital, Cambridge, England, said in an interview that “I thought this was a fascinating paper. ... I was deeply impressed by the data.”
Dr. Evans, who chairs the U.K. Department of Transport advisory panel on driving and diabetes, also noted: “The group of people with insulin-treated diabetes flying planes are a phenomenally motivated group who are prepared to do things that probably most drivers of motor vehicles would find oppressive or very difficult to do.”
“I thought the outcomes were really impressive in terms of the amount of time they were able to maintain themselves within glucose target ranges.”
Indeed, Dr. Garden said, “pilots are typically very organized and used to dealing with strict protocols with regard to all of the processes they have to follow before they fly and the safety checks they have to do. They adapted to this additional safety measure really well.”
Traffic light protocol keeps pilots in range
The protocol requires pilots to perform fingerstick glucose checks 30 minutes prior to flight, every hour during flight, and 30 minutes before landing. They must also attend clinical reviews every 6 months.
A traffic light system is used to denote acceptable pre- and in-flight glucose levels, with green meaning acceptable (5.0-15.0 mmol/L [90-270 mg/dL]), amber indicating caution for low (4.0-4.9 mmol/L [72-88 mg/dL]) or high (15.1-20.0 mmol/L [272-360 mg/dL]) blood glucose. Red requires immediate action (low blood glucose <4 mmol/L [72 mg/dL] and high >20 mmol/L [>360 mg/dL]).
Low amber values require the pilot to ingest 10-15 fast-acting carbohydrates and retest after 30 minutes. Low red values indicate the pilot must hand over the controls to the copilot. High readings of >15.0 mmol/L (>270 mg/dL) require an insulin dosing review. A high red value also requires the pilot to hand over the controls.
Of the 49 pilots, 84% had type 1 diabetes and 16% had insulin-treated type 2 diabetes. Most (61%) had class 1 medical certificates (required to validate a commercial pilot license) and 39% had class 2 medical certificates (required to validate a private pilot’s license). Median diabetes duration was 10.9 years.
Of note, all had become pilots prior to diabetes onset. As of now, the EU Aviation Safety Agency doesn’t allow people with preexisting insulin-treated diabetes to become pilots.
“We are fighting to change that, but with the U.K. leaving the EU, the Civil Aviation Authority might pursue it [separately]. We don’t know how that will pan out,” Dr. Garden noted during the briefing.
Over the 7.5 years, 97.7% of readings were within the green range, while just 1.42% were in the low amber range and 0.75% in the high amber range. Just 48 readings (0.12%) were in the low red range and 6 (0.02%) in the high red range. Of the 48 low reds, just 14 were recorded during flight. Of the six high reds, only two occurred during flight.
There were no instances of pilot incapacitation or changes in average hemoglobin A1c.
The results should alleviate concerns expressed after a prior report that pilots’ overall glycemic control could worsen if they pushed too hard to avoid lows, Dr. Garden noted.
The proportion of out-of-range values declined from 5.7% in 2013 to 1.2% in 2019. Low red values didn’t change (0.2% in 2013 and 0.1% in 2019) but high red values had completely disappeared by 2017.
What about CGM?
In response to a question during the briefing about use of continuous glucose monitoring, Dr. Garden said that some of the pilots were using CGM in addition to following the fingerstick protocol.
At the time the protocol was developed a decade ago, CGM wasn’t considered accurate enough and there wasn’t evidence for its use at high altitude.
But there has been a great deal more data since then, she said, noting “we believe it would be safer to use now because of how good that equipment is. ... Certainly, there’s a good number [of pilots] using CGM, and hopefully that will increase and the protocol will change to allow them all to use CGM if they want to.
“I think we’ll probably see CGM in the protocol within the next year to 2 years. Hopefully, that will make things a lot easier, so pilots won’t have to prick their fingers while they’re flying.”
Her group is currently conducting a study (DEXFLY) on use of the Dexcom G6 in addition to fingersticks in commercial pilots with insulin-treated diabetes. Results are expected by the end of the year.
Dr. Evans commented: “I think it’s a no-brainer that CGM will become the gold standard. I understand why they’re going to want to be cautious about this, but if they can generate data to show it will be a low-risk change, I think it will come.”
He also noted that it was only a couple of years ago that U.K. law was changed to allow car drivers with insulin-treated diabetes to use CGM as part of their glucose-testing requirements (before driving and every 2 hours). CGM still isn’t approved for use by drivers of trucks or other large vehicles, but “I think at some point in the future it will become more accepted,” Dr. Evans commented.
Dr. Garden reported no relevant financial relationships. Dr. Evans has reported being an advisory board member of, speaker for, and/or grant recipient from Novo Nordisk, Dexcom, Medtronic, Abbott, Eli Lilly, and Roche.
A version of this article originally appeared on Medscape.com.
FROM EASD 2020
Trump signs Medicare loan relief bill delaying repayments
President Trump on Oct. 1 signed a bill to keep the federal government running through December 11. This “continuing resolution” (CR), which was approved by the Senate Wednesday on an 84-10 vote, according to The New York Times, includes provisions to delay repayment by physicians of pandemic-related Medicare loans and to reduce the loans’ interest rate.
In an earlier news release, the American Medical Association reported that Congress and the White House had agreed to include the provisions on Medicare loans in the CR.
Under the Medicare Accelerated and Advance Payments (AAP) program, the Centers for Medicare & Medicaid Services advanced money to physicians who were financially impacted by the pandemic. The program, created in March, was suspended in late April.
Physicians who received the Medicare loans were supposed to start paying them back 120 days after they were made. CMS planned to recoup the advances by offsetting them against Medicare claims payments due to physicians. Practices had up to 210 days (7 months) to repay the loans through this process before being asked to repay them directly with interest of 10.25%.
For the practices that received these advances, that meant their Medicare cash flow was scheduled to dry up, starting in August. However, CMS quietly abstained from collecting these payments when they came due, according to Modern Healthcare.
New terms
The amount to be recouped from each claim is reduced from 100% to 25% of the claim for the first 11 months and to 50% of claims withheld for an additional 6 months. If the loan is not repaid in full by then, the provider must pay the balance with interest of 4%.
More than 80% of the $100 billion that CMS loaned to healthcare providers through May 2 went to hospitals, Modern Healthcare calculated. Of the remainder, specialty or multispecialty practices received $3.5 billion, internal medicine specialists got $24 million, family physicians were loaned $15 million, and federally qualified health centers received $20 million.
In the AMA’s news release, AMA President Susan Bailey, MD, who assumed the post in June, called the original loan repayment plan an “economic sword hanging over physician practices.”
This article first appeared on Medscape.com.
President Trump on Oct. 1 signed a bill to keep the federal government running through December 11. This “continuing resolution” (CR), which was approved by the Senate Wednesday on an 84-10 vote, according to The New York Times, includes provisions to delay repayment by physicians of pandemic-related Medicare loans and to reduce the loans’ interest rate.
In an earlier news release, the American Medical Association reported that Congress and the White House had agreed to include the provisions on Medicare loans in the CR.
Under the Medicare Accelerated and Advance Payments (AAP) program, the Centers for Medicare & Medicaid Services advanced money to physicians who were financially impacted by the pandemic. The program, created in March, was suspended in late April.
Physicians who received the Medicare loans were supposed to start paying them back 120 days after they were made. CMS planned to recoup the advances by offsetting them against Medicare claims payments due to physicians. Practices had up to 210 days (7 months) to repay the loans through this process before being asked to repay them directly with interest of 10.25%.
For the practices that received these advances, that meant their Medicare cash flow was scheduled to dry up, starting in August. However, CMS quietly abstained from collecting these payments when they came due, according to Modern Healthcare.
New terms
The amount to be recouped from each claim is reduced from 100% to 25% of the claim for the first 11 months and to 50% of claims withheld for an additional 6 months. If the loan is not repaid in full by then, the provider must pay the balance with interest of 4%.
More than 80% of the $100 billion that CMS loaned to healthcare providers through May 2 went to hospitals, Modern Healthcare calculated. Of the remainder, specialty or multispecialty practices received $3.5 billion, internal medicine specialists got $24 million, family physicians were loaned $15 million, and federally qualified health centers received $20 million.
In the AMA’s news release, AMA President Susan Bailey, MD, who assumed the post in June, called the original loan repayment plan an “economic sword hanging over physician practices.”
This article first appeared on Medscape.com.
President Trump on Oct. 1 signed a bill to keep the federal government running through December 11. This “continuing resolution” (CR), which was approved by the Senate Wednesday on an 84-10 vote, according to The New York Times, includes provisions to delay repayment by physicians of pandemic-related Medicare loans and to reduce the loans’ interest rate.
In an earlier news release, the American Medical Association reported that Congress and the White House had agreed to include the provisions on Medicare loans in the CR.
Under the Medicare Accelerated and Advance Payments (AAP) program, the Centers for Medicare & Medicaid Services advanced money to physicians who were financially impacted by the pandemic. The program, created in March, was suspended in late April.
Physicians who received the Medicare loans were supposed to start paying them back 120 days after they were made. CMS planned to recoup the advances by offsetting them against Medicare claims payments due to physicians. Practices had up to 210 days (7 months) to repay the loans through this process before being asked to repay them directly with interest of 10.25%.
For the practices that received these advances, that meant their Medicare cash flow was scheduled to dry up, starting in August. However, CMS quietly abstained from collecting these payments when they came due, according to Modern Healthcare.
New terms
The amount to be recouped from each claim is reduced from 100% to 25% of the claim for the first 11 months and to 50% of claims withheld for an additional 6 months. If the loan is not repaid in full by then, the provider must pay the balance with interest of 4%.
More than 80% of the $100 billion that CMS loaned to healthcare providers through May 2 went to hospitals, Modern Healthcare calculated. Of the remainder, specialty or multispecialty practices received $3.5 billion, internal medicine specialists got $24 million, family physicians were loaned $15 million, and federally qualified health centers received $20 million.
In the AMA’s news release, AMA President Susan Bailey, MD, who assumed the post in June, called the original loan repayment plan an “economic sword hanging over physician practices.”
This article first appeared on Medscape.com.
AHA scientific statement highlights cardiorenal benefit of new diabetes drugs
To protect the heart and kidneys, sodium-glucose transporter 2 (SGLT2) inhibitors and glucagonlike peptide–1 (GLP-1) receptor agonists should be considered for people with type 2 diabetes and chronic kidney disease (CKD), the American Heart Association advised in a new scientific statement.
Taken together, the results of relevant clinical trials indicate that SGLT2 inhibitors and GLP-1 receptor agonists safely and significantly reduce the risk for cardiovascular (CV) events, death, and the slow progression of CKD to end-stage kidney disease, including the risks for dialysis, transplantation, and death, the writing group says.
The scientific statement was published online Sept. 28 in Circulation.
“There has been rapid reporting of high-quality data in the cardio-renal-metabolic space with significant heart and kidney benefits, particularly with these two newer classes of antihyperglycemic agents,” Janani Rangaswami, MD, who chaired the writing group, said in an interview.
“More recent data show benefits in chronic kidney disease and heart failure even in patients without diabetes,” said Dr. Rangaswami, Einstein Medical Center and Sidney Kimmel Medical College, both in Philadelphia.
“These data are practice-changing in both cardiology and nephrology, and usher in a new era of disease-modifying therapies in heart and kidney disease,” Dr. Rangaswami added.
Recommendations at a glance
- Provide early and ongoing assessment of risks for CVD and CKD to patients who may benefit from SGLT2 inhibitors of GLP-1 receptor agonists.
- Tailor medication choices that meet the needs of individual patients. Realize that, given “consistent class-wide effects,” the choice of a specific SGLT2 inhibitor or GLP-1 receptor agonist may be dictated by affordability, coverage, and formulary considerations.
- Adjust all medications in tandem with these medicines and consider the burden of polypharmacy, which is common among people with type 2 diabetes. Adjust concomitant therapies and deprescribe where possible.
- Identify risks for hypoglycemia and educate patients on the signs so they can seek treatment quickly.
- Monitor and control high blood pressure.
- Counsel patients about the risks for and symptoms of euglycemic diabetic ketoacidosis when taking SGLT2 inhibitors, as well as classic DKA, which can be fatal.
- Regularly screen and counsel patients about foot care to prevent foot ulcers or blisters that can quickly become infected and lead to amputation.
The writing group identified two additional patient subgroups that may benefit from SGLT2 inhibitors and GLP-1 receptor agonists: those with heart failure with reduced ejection fraction with or without diabetes; and those with CKD who do not have diabetes. They say more data are anticipated to validate the use of SGLT2 inhibitors and GLP-1 receptor agonists in these “at-risk” patients.
Collaborative care model
The writing group proposed a collaborative care model, bridging cardiologists, nephrologists, endocrinologists, and primary care physicians, to help facilitate the “prompt and appropriate” integration of these new classes of medications in the management of patients with type 2 diabetes and CKD.
There is “an unmet need for a cardio-renal-metabolic care model that incorporates best practices in the real world to help align these therapies, especially with vulnerable high-risk patients with cardiorenal disease, and to overcome barriers toward uptake of these agents. Hopefully this statement provides some guidance to the cardiology and nephrology communities in that area,” Dr. Rangaswami said in an interview.
But old habits die hard, as research continues to show the slow adoption of these newer medications in the real world.
For example, a large observational study published last year showed a “striking” discordance between evidence-based, guideline-recommended use of SGLT2 inhibitors for the treatment of type 2 diabetes and their actual uptake in clinical practice.
Paradoxically, patients with CVD, heart failure, hypertension, CKD, and those at risk for hypoglycemia were less apt to receive an SGLT2 inhibitor than other patients.
“The relatively slow uptake of these agents is multifactorial,” Dr. Rangaswami said. “Cardiologists and nephrologists may suffer from some level of ‘therapeutic inertia’ when using new agents they are unfamiliar with and originally branded as ‘antidiabetic’ agents, with the perception of these agents being outside the scope of their practice.”
Two other factors are also at play. “The current health care system is based on ‘specialty silos,’ where specialists tend to stick to the traditional scope of their specialty and are reluctant to view these agents as part of their therapeutic armamentarium. Finally, insurance coverage barriers and affordability also limit the use on a widespread basis,” Dr. Rangaswami said.
A version of this article originally appeared on Medscape.com .
To protect the heart and kidneys, sodium-glucose transporter 2 (SGLT2) inhibitors and glucagonlike peptide–1 (GLP-1) receptor agonists should be considered for people with type 2 diabetes and chronic kidney disease (CKD), the American Heart Association advised in a new scientific statement.
Taken together, the results of relevant clinical trials indicate that SGLT2 inhibitors and GLP-1 receptor agonists safely and significantly reduce the risk for cardiovascular (CV) events, death, and the slow progression of CKD to end-stage kidney disease, including the risks for dialysis, transplantation, and death, the writing group says.
The scientific statement was published online Sept. 28 in Circulation.
“There has been rapid reporting of high-quality data in the cardio-renal-metabolic space with significant heart and kidney benefits, particularly with these two newer classes of antihyperglycemic agents,” Janani Rangaswami, MD, who chaired the writing group, said in an interview.
“More recent data show benefits in chronic kidney disease and heart failure even in patients without diabetes,” said Dr. Rangaswami, Einstein Medical Center and Sidney Kimmel Medical College, both in Philadelphia.
“These data are practice-changing in both cardiology and nephrology, and usher in a new era of disease-modifying therapies in heart and kidney disease,” Dr. Rangaswami added.
Recommendations at a glance
- Provide early and ongoing assessment of risks for CVD and CKD to patients who may benefit from SGLT2 inhibitors of GLP-1 receptor agonists.
- Tailor medication choices that meet the needs of individual patients. Realize that, given “consistent class-wide effects,” the choice of a specific SGLT2 inhibitor or GLP-1 receptor agonist may be dictated by affordability, coverage, and formulary considerations.
- Adjust all medications in tandem with these medicines and consider the burden of polypharmacy, which is common among people with type 2 diabetes. Adjust concomitant therapies and deprescribe where possible.
- Identify risks for hypoglycemia and educate patients on the signs so they can seek treatment quickly.
- Monitor and control high blood pressure.
- Counsel patients about the risks for and symptoms of euglycemic diabetic ketoacidosis when taking SGLT2 inhibitors, as well as classic DKA, which can be fatal.
- Regularly screen and counsel patients about foot care to prevent foot ulcers or blisters that can quickly become infected and lead to amputation.
The writing group identified two additional patient subgroups that may benefit from SGLT2 inhibitors and GLP-1 receptor agonists: those with heart failure with reduced ejection fraction with or without diabetes; and those with CKD who do not have diabetes. They say more data are anticipated to validate the use of SGLT2 inhibitors and GLP-1 receptor agonists in these “at-risk” patients.
Collaborative care model
The writing group proposed a collaborative care model, bridging cardiologists, nephrologists, endocrinologists, and primary care physicians, to help facilitate the “prompt and appropriate” integration of these new classes of medications in the management of patients with type 2 diabetes and CKD.
There is “an unmet need for a cardio-renal-metabolic care model that incorporates best practices in the real world to help align these therapies, especially with vulnerable high-risk patients with cardiorenal disease, and to overcome barriers toward uptake of these agents. Hopefully this statement provides some guidance to the cardiology and nephrology communities in that area,” Dr. Rangaswami said in an interview.
But old habits die hard, as research continues to show the slow adoption of these newer medications in the real world.
For example, a large observational study published last year showed a “striking” discordance between evidence-based, guideline-recommended use of SGLT2 inhibitors for the treatment of type 2 diabetes and their actual uptake in clinical practice.
Paradoxically, patients with CVD, heart failure, hypertension, CKD, and those at risk for hypoglycemia were less apt to receive an SGLT2 inhibitor than other patients.
“The relatively slow uptake of these agents is multifactorial,” Dr. Rangaswami said. “Cardiologists and nephrologists may suffer from some level of ‘therapeutic inertia’ when using new agents they are unfamiliar with and originally branded as ‘antidiabetic’ agents, with the perception of these agents being outside the scope of their practice.”
Two other factors are also at play. “The current health care system is based on ‘specialty silos,’ where specialists tend to stick to the traditional scope of their specialty and are reluctant to view these agents as part of their therapeutic armamentarium. Finally, insurance coverage barriers and affordability also limit the use on a widespread basis,” Dr. Rangaswami said.
A version of this article originally appeared on Medscape.com .
To protect the heart and kidneys, sodium-glucose transporter 2 (SGLT2) inhibitors and glucagonlike peptide–1 (GLP-1) receptor agonists should be considered for people with type 2 diabetes and chronic kidney disease (CKD), the American Heart Association advised in a new scientific statement.
Taken together, the results of relevant clinical trials indicate that SGLT2 inhibitors and GLP-1 receptor agonists safely and significantly reduce the risk for cardiovascular (CV) events, death, and the slow progression of CKD to end-stage kidney disease, including the risks for dialysis, transplantation, and death, the writing group says.
The scientific statement was published online Sept. 28 in Circulation.
“There has been rapid reporting of high-quality data in the cardio-renal-metabolic space with significant heart and kidney benefits, particularly with these two newer classes of antihyperglycemic agents,” Janani Rangaswami, MD, who chaired the writing group, said in an interview.
“More recent data show benefits in chronic kidney disease and heart failure even in patients without diabetes,” said Dr. Rangaswami, Einstein Medical Center and Sidney Kimmel Medical College, both in Philadelphia.
“These data are practice-changing in both cardiology and nephrology, and usher in a new era of disease-modifying therapies in heart and kidney disease,” Dr. Rangaswami added.
Recommendations at a glance
- Provide early and ongoing assessment of risks for CVD and CKD to patients who may benefit from SGLT2 inhibitors of GLP-1 receptor agonists.
- Tailor medication choices that meet the needs of individual patients. Realize that, given “consistent class-wide effects,” the choice of a specific SGLT2 inhibitor or GLP-1 receptor agonist may be dictated by affordability, coverage, and formulary considerations.
- Adjust all medications in tandem with these medicines and consider the burden of polypharmacy, which is common among people with type 2 diabetes. Adjust concomitant therapies and deprescribe where possible.
- Identify risks for hypoglycemia and educate patients on the signs so they can seek treatment quickly.
- Monitor and control high blood pressure.
- Counsel patients about the risks for and symptoms of euglycemic diabetic ketoacidosis when taking SGLT2 inhibitors, as well as classic DKA, which can be fatal.
- Regularly screen and counsel patients about foot care to prevent foot ulcers or blisters that can quickly become infected and lead to amputation.
The writing group identified two additional patient subgroups that may benefit from SGLT2 inhibitors and GLP-1 receptor agonists: those with heart failure with reduced ejection fraction with or without diabetes; and those with CKD who do not have diabetes. They say more data are anticipated to validate the use of SGLT2 inhibitors and GLP-1 receptor agonists in these “at-risk” patients.
Collaborative care model
The writing group proposed a collaborative care model, bridging cardiologists, nephrologists, endocrinologists, and primary care physicians, to help facilitate the “prompt and appropriate” integration of these new classes of medications in the management of patients with type 2 diabetes and CKD.
There is “an unmet need for a cardio-renal-metabolic care model that incorporates best practices in the real world to help align these therapies, especially with vulnerable high-risk patients with cardiorenal disease, and to overcome barriers toward uptake of these agents. Hopefully this statement provides some guidance to the cardiology and nephrology communities in that area,” Dr. Rangaswami said in an interview.
But old habits die hard, as research continues to show the slow adoption of these newer medications in the real world.
For example, a large observational study published last year showed a “striking” discordance between evidence-based, guideline-recommended use of SGLT2 inhibitors for the treatment of type 2 diabetes and their actual uptake in clinical practice.
Paradoxically, patients with CVD, heart failure, hypertension, CKD, and those at risk for hypoglycemia were less apt to receive an SGLT2 inhibitor than other patients.
“The relatively slow uptake of these agents is multifactorial,” Dr. Rangaswami said. “Cardiologists and nephrologists may suffer from some level of ‘therapeutic inertia’ when using new agents they are unfamiliar with and originally branded as ‘antidiabetic’ agents, with the perception of these agents being outside the scope of their practice.”
Two other factors are also at play. “The current health care system is based on ‘specialty silos,’ where specialists tend to stick to the traditional scope of their specialty and are reluctant to view these agents as part of their therapeutic armamentarium. Finally, insurance coverage barriers and affordability also limit the use on a widespread basis,” Dr. Rangaswami said.
A version of this article originally appeared on Medscape.com .
Geriatric patients: My three rules for them
I have been in practice for 31 years, so many of my patients are now in their 80s and 90s. Practices age with us, and I have been seeing many of these patients for 25-30 years.
Absolutely, positively make sure you move!
Our older patients often have many reasons not to move, including pain from arthritis, deconditioning, muscle weakness, fatigue, and depression. “Keeping moving” is probably the most important thing a patient can do for their health.
Holme and Anderssen studied a large cohort of men for cardiovascular risk in 1972 and again in 2000. The surviving men were followed over an additional 12 years.1 They found that 30 minutes of physical activity 6 days a week was associated with a 40% reduction in mortality. Sedentary men had a reduced life expectancy of about 5 years, compared with men who were moderately to vigorously physically active.
Stewart etal. studied the benefit of physical activity in people with stable coronary disease.2 They concluded that, in patients with stable coronary heart disease, more physical activity was associated with lower mortality, and the largest benefit occurred in the sedentary patient groups and the highest cardiac risk groups.
Saint-Maurice et al. studied the effects of total daily step count and step intensity on mortality risk.3 They found that the risk of all-cause mortality decreases as the total number of daily steps increases, but that the speed of those steps did not make a difference. This is very encouraging data for our elderly patients. Moving is the secret, even if it may not be moving at a fast pace!
Never, ever get on a ladder!
This one should be part of every geriatric’s assessment and every Medicare wellness exam. I first experienced the horror of what can happen when elderly people climb when a 96-year-old healthy patient of mine fell off his roof and died. I never thought to tell him climbing on the roof was an awful idea.
Akland et al. looked at the epidemiology and outcomes of ladder-related falls that required ICU admission.4 Hospital mortality was 26%, and almost all of the mortalities occurred in older males in domestic falls, who died as a result of traumatic brain injury. Fewer than half of the survivors were living independently 1 year after the fall.
Valmuur et al. studied ladder related falls in Australia.5 They found that rates of ladder related falls requiring hospitalization rose from about 20/100,000 for men ages 15-29 years to 78/100,000 for men aged over 60 years. Of those who died from fall-related injury, 82% were over the age of 60, with more than 70% dying from head injuries.
Schaffarczyk et al. looked at the impact of nonoccupational falls from ladders in men aged over 50 years.6 The mean age of the patients in the study was 64 years (range, 50-85), with 27% suffering severe trauma. There was a striking impact on long-term function occurring in over half the study patients. The authors did interviews with patients in follow-up long after the falls and found that most never thought of themselves at risk for a fall, and after the experience of a bad fall, would never consider going on a ladder again. I think it is important for health care professionals to discuss the dangers of ladder use with our older patients, pointing out the higher risk of falling and the potential for the fall to be a life-changing or life-ending event.
Let them eat!
Many patients have a reduced appetite as they age. We work hard with our patients to choose a healthy diet throughout their lives, to help ward off obesity, treat hypertension, prevent or control diabetes, or provide heart health. Many patients just stop being interested in food, reduce intake, and may lose weight and muscle mass. When my patients pass the age of 85, I change my focus to encouraging them to eat for calories, socialization, and joy. I think the marginal benefits of more restrictive diets are small, compared with the benefits of helping your patients enjoy eating again. I ask patients what their very favorite foods are and encourage them to have them.
Pearl
Keep your patients eating and moving, except not onto a ladder!
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Holme I, Anderssen SA. Increases in physical activity is as important as smoking cessation for reduction in total mortality in elderly men: 12 years of follow-up of the Oslo II study. Br J Sports Med. 2015; 49:743-8.
2. Stewart RAH et al. Physical activity and mortality in patients with stable coronary heart disease. J Am Coll Cardiol. 2017 Oct 3;70(14):1689-1700..
3. Saint-Maurice PF et al. Association of daily step count and step intensity with mortality among U.S. adults. JAMA 2020;323:1151-60.
4. Ackland HM et al. Danger at every rung: Epidemiology and outcomes of ICU-admitted ladder-related trauma. Injury. 2016;47:1109-117.
5. Vallmuur K et al. Falls from ladders in Australia: comparing occupational and nonoccupational injuries across age groups. Aust N Z J Public Health. 2016 Dec;40(6):559-63.
6. Schaffarczyk K et al. Nonoccupational falls from ladders in men 50 years and over: Contributing factors and impact. Injury. 2020 Aug;51(8):1798-1804.
I have been in practice for 31 years, so many of my patients are now in their 80s and 90s. Practices age with us, and I have been seeing many of these patients for 25-30 years.
Absolutely, positively make sure you move!
Our older patients often have many reasons not to move, including pain from arthritis, deconditioning, muscle weakness, fatigue, and depression. “Keeping moving” is probably the most important thing a patient can do for their health.
Holme and Anderssen studied a large cohort of men for cardiovascular risk in 1972 and again in 2000. The surviving men were followed over an additional 12 years.1 They found that 30 minutes of physical activity 6 days a week was associated with a 40% reduction in mortality. Sedentary men had a reduced life expectancy of about 5 years, compared with men who were moderately to vigorously physically active.
Stewart etal. studied the benefit of physical activity in people with stable coronary disease.2 They concluded that, in patients with stable coronary heart disease, more physical activity was associated with lower mortality, and the largest benefit occurred in the sedentary patient groups and the highest cardiac risk groups.
Saint-Maurice et al. studied the effects of total daily step count and step intensity on mortality risk.3 They found that the risk of all-cause mortality decreases as the total number of daily steps increases, but that the speed of those steps did not make a difference. This is very encouraging data for our elderly patients. Moving is the secret, even if it may not be moving at a fast pace!
Never, ever get on a ladder!
This one should be part of every geriatric’s assessment and every Medicare wellness exam. I first experienced the horror of what can happen when elderly people climb when a 96-year-old healthy patient of mine fell off his roof and died. I never thought to tell him climbing on the roof was an awful idea.
Akland et al. looked at the epidemiology and outcomes of ladder-related falls that required ICU admission.4 Hospital mortality was 26%, and almost all of the mortalities occurred in older males in domestic falls, who died as a result of traumatic brain injury. Fewer than half of the survivors were living independently 1 year after the fall.
Valmuur et al. studied ladder related falls in Australia.5 They found that rates of ladder related falls requiring hospitalization rose from about 20/100,000 for men ages 15-29 years to 78/100,000 for men aged over 60 years. Of those who died from fall-related injury, 82% were over the age of 60, with more than 70% dying from head injuries.
Schaffarczyk et al. looked at the impact of nonoccupational falls from ladders in men aged over 50 years.6 The mean age of the patients in the study was 64 years (range, 50-85), with 27% suffering severe trauma. There was a striking impact on long-term function occurring in over half the study patients. The authors did interviews with patients in follow-up long after the falls and found that most never thought of themselves at risk for a fall, and after the experience of a bad fall, would never consider going on a ladder again. I think it is important for health care professionals to discuss the dangers of ladder use with our older patients, pointing out the higher risk of falling and the potential for the fall to be a life-changing or life-ending event.
Let them eat!
Many patients have a reduced appetite as they age. We work hard with our patients to choose a healthy diet throughout their lives, to help ward off obesity, treat hypertension, prevent or control diabetes, or provide heart health. Many patients just stop being interested in food, reduce intake, and may lose weight and muscle mass. When my patients pass the age of 85, I change my focus to encouraging them to eat for calories, socialization, and joy. I think the marginal benefits of more restrictive diets are small, compared with the benefits of helping your patients enjoy eating again. I ask patients what their very favorite foods are and encourage them to have them.
Pearl
Keep your patients eating and moving, except not onto a ladder!
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Holme I, Anderssen SA. Increases in physical activity is as important as smoking cessation for reduction in total mortality in elderly men: 12 years of follow-up of the Oslo II study. Br J Sports Med. 2015; 49:743-8.
2. Stewart RAH et al. Physical activity and mortality in patients with stable coronary heart disease. J Am Coll Cardiol. 2017 Oct 3;70(14):1689-1700..
3. Saint-Maurice PF et al. Association of daily step count and step intensity with mortality among U.S. adults. JAMA 2020;323:1151-60.
4. Ackland HM et al. Danger at every rung: Epidemiology and outcomes of ICU-admitted ladder-related trauma. Injury. 2016;47:1109-117.
5. Vallmuur K et al. Falls from ladders in Australia: comparing occupational and nonoccupational injuries across age groups. Aust N Z J Public Health. 2016 Dec;40(6):559-63.
6. Schaffarczyk K et al. Nonoccupational falls from ladders in men 50 years and over: Contributing factors and impact. Injury. 2020 Aug;51(8):1798-1804.
I have been in practice for 31 years, so many of my patients are now in their 80s and 90s. Practices age with us, and I have been seeing many of these patients for 25-30 years.
Absolutely, positively make sure you move!
Our older patients often have many reasons not to move, including pain from arthritis, deconditioning, muscle weakness, fatigue, and depression. “Keeping moving” is probably the most important thing a patient can do for their health.
Holme and Anderssen studied a large cohort of men for cardiovascular risk in 1972 and again in 2000. The surviving men were followed over an additional 12 years.1 They found that 30 minutes of physical activity 6 days a week was associated with a 40% reduction in mortality. Sedentary men had a reduced life expectancy of about 5 years, compared with men who were moderately to vigorously physically active.
Stewart etal. studied the benefit of physical activity in people with stable coronary disease.2 They concluded that, in patients with stable coronary heart disease, more physical activity was associated with lower mortality, and the largest benefit occurred in the sedentary patient groups and the highest cardiac risk groups.
Saint-Maurice et al. studied the effects of total daily step count and step intensity on mortality risk.3 They found that the risk of all-cause mortality decreases as the total number of daily steps increases, but that the speed of those steps did not make a difference. This is very encouraging data for our elderly patients. Moving is the secret, even if it may not be moving at a fast pace!
Never, ever get on a ladder!
This one should be part of every geriatric’s assessment and every Medicare wellness exam. I first experienced the horror of what can happen when elderly people climb when a 96-year-old healthy patient of mine fell off his roof and died. I never thought to tell him climbing on the roof was an awful idea.
Akland et al. looked at the epidemiology and outcomes of ladder-related falls that required ICU admission.4 Hospital mortality was 26%, and almost all of the mortalities occurred in older males in domestic falls, who died as a result of traumatic brain injury. Fewer than half of the survivors were living independently 1 year after the fall.
Valmuur et al. studied ladder related falls in Australia.5 They found that rates of ladder related falls requiring hospitalization rose from about 20/100,000 for men ages 15-29 years to 78/100,000 for men aged over 60 years. Of those who died from fall-related injury, 82% were over the age of 60, with more than 70% dying from head injuries.
Schaffarczyk et al. looked at the impact of nonoccupational falls from ladders in men aged over 50 years.6 The mean age of the patients in the study was 64 years (range, 50-85), with 27% suffering severe trauma. There was a striking impact on long-term function occurring in over half the study patients. The authors did interviews with patients in follow-up long after the falls and found that most never thought of themselves at risk for a fall, and after the experience of a bad fall, would never consider going on a ladder again. I think it is important for health care professionals to discuss the dangers of ladder use with our older patients, pointing out the higher risk of falling and the potential for the fall to be a life-changing or life-ending event.
Let them eat!
Many patients have a reduced appetite as they age. We work hard with our patients to choose a healthy diet throughout their lives, to help ward off obesity, treat hypertension, prevent or control diabetes, or provide heart health. Many patients just stop being interested in food, reduce intake, and may lose weight and muscle mass. When my patients pass the age of 85, I change my focus to encouraging them to eat for calories, socialization, and joy. I think the marginal benefits of more restrictive diets are small, compared with the benefits of helping your patients enjoy eating again. I ask patients what their very favorite foods are and encourage them to have them.
Pearl
Keep your patients eating and moving, except not onto a ladder!
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Holme I, Anderssen SA. Increases in physical activity is as important as smoking cessation for reduction in total mortality in elderly men: 12 years of follow-up of the Oslo II study. Br J Sports Med. 2015; 49:743-8.
2. Stewart RAH et al. Physical activity and mortality in patients with stable coronary heart disease. J Am Coll Cardiol. 2017 Oct 3;70(14):1689-1700..
3. Saint-Maurice PF et al. Association of daily step count and step intensity with mortality among U.S. adults. JAMA 2020;323:1151-60.
4. Ackland HM et al. Danger at every rung: Epidemiology and outcomes of ICU-admitted ladder-related trauma. Injury. 2016;47:1109-117.
5. Vallmuur K et al. Falls from ladders in Australia: comparing occupational and nonoccupational injuries across age groups. Aust N Z J Public Health. 2016 Dec;40(6):559-63.
6. Schaffarczyk K et al. Nonoccupational falls from ladders in men 50 years and over: Contributing factors and impact. Injury. 2020 Aug;51(8):1798-1804.
New data challenge primary care’s inattention to aldosterone in hypertension
Jun Yang, MBBS, had watched as her father, who had battled hypertension for decades, ended up on four medications that still couldn’t bring his blood pressure to a healthy level. The cardiovascular endocrinologist then ran some tests, and soon thereafter her father had his blood pressure optimized on just one targeted medication.
Dr. Yang’s father was found to have a hormonal condition known as primary aldosteronism (PA) as the cause of his hypertension.
It turns out that PA is not as rare as once thought.
An eye-catching report in Annals of Internal Medicine this spring of an unexpectedly high prevalence of primary aldosteronism among a diverse cross section of U.S. patients with hypertension has raised issues that could dramatically change the way doctors in America, and elsewhere, assess and manage high blood pressure.
Foremost is the question of whether primary care physicians – the clinicians at the front line for diagnosing and initially treating most patients with hypertension – will absorb and act on this new evidence. For them, aldosteronism doesn’t automatically come to mind when they see high numbers on a BP monitor, and yet this latest research found that up to a third of all 726 patients in the study who were diagnosed with hypertension and with high urinary salt levels had PA.
That translates to a roughly three- to fivefold increase over standard prevalence estimates, and is a ”game changer” for how clinicians should approach hypertension management and PA diagnosis going forward, said John W. Funder, MD, in an editorial accompanying the Annals study.
Long considered relatively uncommon, hypertension driven by an excess of the hormone aldosterone, often because of an adenoma on the adrenal gland, is not the same as conventional “essential” hypertension. The former benefits from early diagnosis because its treatment is completely different – close to half of all PA patients can be treated definitively and quickly with surgical removal of an adenoma from one side of the adrenal gland.
For other PA patients, who have bilateral adrenal hyperplasia that is impossible to resolve surgically, treatment with drugs called mineralocorticoid receptor antagonists (MRAs), such as spironolactone, is needed because they target the hormonal cause of the high BP.
But what usually happens is that a patient with PA is mistakenly diagnosed with essential hypertension, in which the classic approach to treatment is to start with one regular antihypertensive drug, and add on further ones from different drug classes if blood pressure is not adequately controlled. When patients are taking three drugs, without adequate control, they are labeled as having “resistant hypertension.”
But in the case of PA, none of these conventional antihypertensives work, and the process of continuing to monitor and add different drugs wastes time, during which patients deteriorate.
“We need to change the culture of waiting for hypertension to be resistant and have patients riddled with end-organ damage,” due to years of persistently high BP and excess aldosterone “before we look for a secondary cause” like PA, declared Dr. Yang, of Hudson Institute of Medical Research and Monash University in Melbourne, during an interview.
So early diagnosis and prompt treatment of PA is key.
In addition to boosting the public health importance of early PA detection in hypertensive patients, the new up-sized PA prevalence numbers throw a spotlight on primary care physicians (PCPs) as key players who will need to apply the findings to practice on a public health scale.
These novel results create a need for “new guidelines, and a radically revised game plan with the key role of PCPs” emphasized in future management of patients with hypertension, said Dr. Funder, a professor of medicine at Monash University, in a second recent editorial in Hypertension.
“Buy-in by PCPs is essential,” agrees Robert M. Carey, MD, a cardiovascular endocrinologist and professor of medicine at the University of Virginia in Charlottesville, and a coauthor of the new study.
But he too acknowledges that this presents a major challenge. PCPs and internists, who diagnose a lot of hypertension, are “not used to thinking about aldosterone,” he said in an interview, encapsulating the key problem faced by proponents of earlier and more widespread PA assessment.
This dilemma looms as a “huge public health issue,” Dr. Carey warned.
‘We’re a long way from getting’ PCPs to buy in to PA screening
Will PCPs grow more comfortable with screening patients for PA themselves, or might they become more willing to refer hypertensive individuals for assessment at an expert center?
One skeptic is Ross D. Feldman, MD, a hypertension-management researcher and professor of medicine at the University of Manitoba in Winnipeg. The finding about high PA prevalence in patients with hypertension “is brand new, [and] the message needs to get to PCPs,” he said. But, “We’re a long way from getting it” to them. “I don’t know how to do that. It will be a tough sell.”
In addition, repositioning MRAs as an earlier option for many hypertensive patients won’t be easy either, because “we’ll never have outcome-trial data for MRAs,” given that they are now generic drugs, he noted.
“No clinical trial data show [MRAs] are first-line drugs,” said Dr. Feldman, who explained that, instead, MRAs are considered “go-to drugs” for patients with treatment-resistant hypertension, a niche therapeutic area. Results from the PATHWAY-2 trial published 5 years ago in Lancet showed “spironolactone was clearly the most effective treatment for the condition,” according to the report authors.
But even among patients with resistant hypertension, screening for PA dramatically lags despite being enshrined in guidelines.
“PCPs should start checking aldosterone-to-renin ratios [a widely used PA screen] in all patients with resistant hypertension or hypertension with hypokalemia, and then refer patients to specialists for testing and management,” said Jordana B. Cohen, MD, a nephrologist and hypertension researcher at the University of Pennsylvania in Philadelphia.
But recent studies of U.S. patient populations with clinical characteristics that meet existing criteria for PA screening showed that just 1%-2% of these individuals underwent an initial PA assessment, she noted, citing reports in the journals Surgery and Hypertension.
“We need to prioritize improving screening in these high-risk patients,” she stressed in an interview.
This illustrates that, in some respects, the new prevalence numbers are beside the point, because PA has been going unscreened and overlooked far too often even in the context of historical, lower prevalence rates, said Dr. Yang.
“The key point is that approximately 1 in 10 people with hypertension, and even more with resistant hypertension, have a form of the disease that is worse than essential hypertension but is routinely missed at present” and is also highly treatable.
“Evidence for the need for increased awareness of PA has been building for 2 decades,” stressed Dr. Yang, who has coauthored several commentaries and reviews that have bemoaned PA’s underappreciated status.
Interest in partnering with PCPs on guidance grows
One potential solution is to have endocrinologists and hypertension specialists’ partner with PCPs to come up with diagnostic and management recommendations. Both Dr. Funder and Dr. Carey are opinion leaders regarding the role of aldosterone in hypertension, and both were coauthors of the 2016 Endocrine Society guideline for PA assessment and management published in the Journal of Clinical Endocrinology & Metabolism , with Dr. Funder chairing the writing panel.
Now approaching its fifth year in effect, this guideline is “due for revision,” and “my hope is that we’ll be able to partner with one or more PCP organizations to come up with a version of the guideline targeted to PCPs,” Dr. Carey said.
He voiced interest in working on this with the American College of Physicians, which represents U.S. internal medicine physicians, and the American Academy of Family Physicians.
“We definitely need a partnership and educational efforts to get the word out from these organizations and not from a specialty society,” said Dr. Carey.
Dr. Funder said he has submitted a proposal to the Endocrine Society for a guidelines update he would chair with Dr. Carey’s assistance and with a diverse writing group that includes PCPs. Dr. Carey said that ideally this panel would write and release a revised guideline in 2021.
“Several of us are chomping at the bit to get this done,” he noted.
But participation by the ACP and AAFP remain uncertain as of September 2020. When approached about this, an ACP spokesperson said the organization had no comment. A spokesperson for the AAFP said, “It’s too early to tell if we will partner with any other organizations to develop guidelines specific to excess aldosterone, and how such guidelines might be received by our members.”
Recent history shows little cooperation between ACP, AAFP, and what might be termed the U.S. hypertension “establishment.” For example, when the American College of Cardiology and the American Heart Association released their most recent essential hypertension management guidelines in Hypertension in 2018, it was never adopted by ACP or AAFP.
The latter two organizations continue to endorse a higher BP threshold for diagnosing hypertension, and higher treatment targets set by alternative expert panels to those of the AHA/ACC.
Collaboration feasible, although PCPs overworked
Dr. Carey hopes that this episode will not preclude agreement over PA screening.
“I think it is still possible to partner with [the ACP and AAFP],” he observed, adding that he believes high PA prevalence among hypertensive patients and its consequences when unrecognized is “noncontentious.”
But he acknowledges that other, substantial hurdles also exist, notably the “overwhelming workload” that American PCPs already face.
David O’Gurek, MD, a family and community medicine physician at the Lewis Katz School of Medicine of Temple University in Philadelphia, agrees that a revamped approach to PA screening developed cooperatively between PCPs and specialists is an important goal and potentially feasible despite prior disagreements. “There has to be room for collaboration,” he said, but also emphasized the need for developing policies based on a systematic evidence review and a focus on patient-centered outcomes.
“We’re certainly missing patients with PA, but there needs to be greater clarity and standardization about the most appropriate screening approach and cutoff level” for flagging patients who need specialized assessment, Dr. O’Gurek said in an interview.
The current endocrinology literature also shows that experts remain divided on how best to accomplish this.
And some hypertension specialists question whether existing evidence is conclusive enough to warrant revised guidelines.
Dr. Cohen, the nephrologist and hypertension researcher, said that, while the recent prevalence report in Annals of Internal Medicine is “intriguing, hypothesis-generating information that suggests we are missing many cases of hyperaldosteronism in routine care,” she nevertheless believes that “we need additional data to be able to truly understand the breadth and implications of the findings.”
William C. Cushman, MD, a hypertension management specialist at the University of Tennessee Health Science Center in Memphis, agrees.
Changing existing practice guidelines “really needs randomized, controlled trials demonstrating a difference in long-term outcomes, ideally major cardiovascular outcomes,” that result from broader PA screening, he said.
Dr. Carey concurs that more evidence is needed to confirm the Annals report, but is confident this evidence will be in hand by the time a guideline-revision panel meets in 2021.
Australian model of PCPs screening for PA could be implemented in United States
An example of what might be possible when PCPs, endocrinologists, and hypertension specialists work together to make PA screening more accessible can be found in Melbourne, at the Endocrine Hypertension Service of Monash Health, in association with the Hudson Institute of Medical Research.
This began operating in July 2016, cofounded by Dr. Yang, whose experiences with her own father made her sensitive to the issue.
The service’s aim is to “address the underdiagnosis of PA, and to offer a streamlined diagnostic service for patients with hypertension,” with an “extensive outreach program” targeted to regional PCPs that, among other messages, encourages them to screen patients for PA when blood pressures exceed 140/90 mm Hg.
During its first 3 years of operation, the service saw 267 patients, with PA diagnosed in 135 and ruled out in 73 patients.
Notably, the proportion of these patients referred from PCPs jumped from 21% of 70 patients during the first year of operation to 47% of 70 patients during year 2, and 52% of 127 patients during the third year, ending in July 2019, said Dr. Yang, who continues to help run the service.
During the first year, a scant 3% of referred patients had recently diagnosed hypertension, but this rose to 14% during the second year, and to 19% during the most recent year with data available.
The median duration of diagnosed hypertension among referred patients fell from 11 years during year 1, to 7 years during year 3.
Service clinicians diagnosed 37 patients with unilateral adenomas, and removed them from 23 patients with four more awaiting surgery and the remaining 10 opting instead for medical management. Another 95 patients went on therapy with a MRA, and during the most recent year studied all patients who began a MRA regimen had a partial or complete clinical response.
Dr. Carey said the “creative program represents a model for implementation in U.S. practice.
Dr. Funder, Dr. Carey, Dr. Feldman, Dr. Yang, Dr. Cohen, and Dr. O’Gurek had no relevant disclosures. Dr. Cushman has been a consultant to Novartis, received personal fees from Sanofi, and research funding from Eli Lilly.
Jun Yang, MBBS, had watched as her father, who had battled hypertension for decades, ended up on four medications that still couldn’t bring his blood pressure to a healthy level. The cardiovascular endocrinologist then ran some tests, and soon thereafter her father had his blood pressure optimized on just one targeted medication.
Dr. Yang’s father was found to have a hormonal condition known as primary aldosteronism (PA) as the cause of his hypertension.
It turns out that PA is not as rare as once thought.
An eye-catching report in Annals of Internal Medicine this spring of an unexpectedly high prevalence of primary aldosteronism among a diverse cross section of U.S. patients with hypertension has raised issues that could dramatically change the way doctors in America, and elsewhere, assess and manage high blood pressure.
Foremost is the question of whether primary care physicians – the clinicians at the front line for diagnosing and initially treating most patients with hypertension – will absorb and act on this new evidence. For them, aldosteronism doesn’t automatically come to mind when they see high numbers on a BP monitor, and yet this latest research found that up to a third of all 726 patients in the study who were diagnosed with hypertension and with high urinary salt levels had PA.
That translates to a roughly three- to fivefold increase over standard prevalence estimates, and is a ”game changer” for how clinicians should approach hypertension management and PA diagnosis going forward, said John W. Funder, MD, in an editorial accompanying the Annals study.
Long considered relatively uncommon, hypertension driven by an excess of the hormone aldosterone, often because of an adenoma on the adrenal gland, is not the same as conventional “essential” hypertension. The former benefits from early diagnosis because its treatment is completely different – close to half of all PA patients can be treated definitively and quickly with surgical removal of an adenoma from one side of the adrenal gland.
For other PA patients, who have bilateral adrenal hyperplasia that is impossible to resolve surgically, treatment with drugs called mineralocorticoid receptor antagonists (MRAs), such as spironolactone, is needed because they target the hormonal cause of the high BP.
But what usually happens is that a patient with PA is mistakenly diagnosed with essential hypertension, in which the classic approach to treatment is to start with one regular antihypertensive drug, and add on further ones from different drug classes if blood pressure is not adequately controlled. When patients are taking three drugs, without adequate control, they are labeled as having “resistant hypertension.”
But in the case of PA, none of these conventional antihypertensives work, and the process of continuing to monitor and add different drugs wastes time, during which patients deteriorate.
“We need to change the culture of waiting for hypertension to be resistant and have patients riddled with end-organ damage,” due to years of persistently high BP and excess aldosterone “before we look for a secondary cause” like PA, declared Dr. Yang, of Hudson Institute of Medical Research and Monash University in Melbourne, during an interview.
So early diagnosis and prompt treatment of PA is key.
In addition to boosting the public health importance of early PA detection in hypertensive patients, the new up-sized PA prevalence numbers throw a spotlight on primary care physicians (PCPs) as key players who will need to apply the findings to practice on a public health scale.
These novel results create a need for “new guidelines, and a radically revised game plan with the key role of PCPs” emphasized in future management of patients with hypertension, said Dr. Funder, a professor of medicine at Monash University, in a second recent editorial in Hypertension.
“Buy-in by PCPs is essential,” agrees Robert M. Carey, MD, a cardiovascular endocrinologist and professor of medicine at the University of Virginia in Charlottesville, and a coauthor of the new study.
But he too acknowledges that this presents a major challenge. PCPs and internists, who diagnose a lot of hypertension, are “not used to thinking about aldosterone,” he said in an interview, encapsulating the key problem faced by proponents of earlier and more widespread PA assessment.
This dilemma looms as a “huge public health issue,” Dr. Carey warned.
‘We’re a long way from getting’ PCPs to buy in to PA screening
Will PCPs grow more comfortable with screening patients for PA themselves, or might they become more willing to refer hypertensive individuals for assessment at an expert center?
One skeptic is Ross D. Feldman, MD, a hypertension-management researcher and professor of medicine at the University of Manitoba in Winnipeg. The finding about high PA prevalence in patients with hypertension “is brand new, [and] the message needs to get to PCPs,” he said. But, “We’re a long way from getting it” to them. “I don’t know how to do that. It will be a tough sell.”
In addition, repositioning MRAs as an earlier option for many hypertensive patients won’t be easy either, because “we’ll never have outcome-trial data for MRAs,” given that they are now generic drugs, he noted.
“No clinical trial data show [MRAs] are first-line drugs,” said Dr. Feldman, who explained that, instead, MRAs are considered “go-to drugs” for patients with treatment-resistant hypertension, a niche therapeutic area. Results from the PATHWAY-2 trial published 5 years ago in Lancet showed “spironolactone was clearly the most effective treatment for the condition,” according to the report authors.
But even among patients with resistant hypertension, screening for PA dramatically lags despite being enshrined in guidelines.
“PCPs should start checking aldosterone-to-renin ratios [a widely used PA screen] in all patients with resistant hypertension or hypertension with hypokalemia, and then refer patients to specialists for testing and management,” said Jordana B. Cohen, MD, a nephrologist and hypertension researcher at the University of Pennsylvania in Philadelphia.
But recent studies of U.S. patient populations with clinical characteristics that meet existing criteria for PA screening showed that just 1%-2% of these individuals underwent an initial PA assessment, she noted, citing reports in the journals Surgery and Hypertension.
“We need to prioritize improving screening in these high-risk patients,” she stressed in an interview.
This illustrates that, in some respects, the new prevalence numbers are beside the point, because PA has been going unscreened and overlooked far too often even in the context of historical, lower prevalence rates, said Dr. Yang.
“The key point is that approximately 1 in 10 people with hypertension, and even more with resistant hypertension, have a form of the disease that is worse than essential hypertension but is routinely missed at present” and is also highly treatable.
“Evidence for the need for increased awareness of PA has been building for 2 decades,” stressed Dr. Yang, who has coauthored several commentaries and reviews that have bemoaned PA’s underappreciated status.
Interest in partnering with PCPs on guidance grows
One potential solution is to have endocrinologists and hypertension specialists’ partner with PCPs to come up with diagnostic and management recommendations. Both Dr. Funder and Dr. Carey are opinion leaders regarding the role of aldosterone in hypertension, and both were coauthors of the 2016 Endocrine Society guideline for PA assessment and management published in the Journal of Clinical Endocrinology & Metabolism , with Dr. Funder chairing the writing panel.
Now approaching its fifth year in effect, this guideline is “due for revision,” and “my hope is that we’ll be able to partner with one or more PCP organizations to come up with a version of the guideline targeted to PCPs,” Dr. Carey said.
He voiced interest in working on this with the American College of Physicians, which represents U.S. internal medicine physicians, and the American Academy of Family Physicians.
“We definitely need a partnership and educational efforts to get the word out from these organizations and not from a specialty society,” said Dr. Carey.
Dr. Funder said he has submitted a proposal to the Endocrine Society for a guidelines update he would chair with Dr. Carey’s assistance and with a diverse writing group that includes PCPs. Dr. Carey said that ideally this panel would write and release a revised guideline in 2021.
“Several of us are chomping at the bit to get this done,” he noted.
But participation by the ACP and AAFP remain uncertain as of September 2020. When approached about this, an ACP spokesperson said the organization had no comment. A spokesperson for the AAFP said, “It’s too early to tell if we will partner with any other organizations to develop guidelines specific to excess aldosterone, and how such guidelines might be received by our members.”
Recent history shows little cooperation between ACP, AAFP, and what might be termed the U.S. hypertension “establishment.” For example, when the American College of Cardiology and the American Heart Association released their most recent essential hypertension management guidelines in Hypertension in 2018, it was never adopted by ACP or AAFP.
The latter two organizations continue to endorse a higher BP threshold for diagnosing hypertension, and higher treatment targets set by alternative expert panels to those of the AHA/ACC.
Collaboration feasible, although PCPs overworked
Dr. Carey hopes that this episode will not preclude agreement over PA screening.
“I think it is still possible to partner with [the ACP and AAFP],” he observed, adding that he believes high PA prevalence among hypertensive patients and its consequences when unrecognized is “noncontentious.”
But he acknowledges that other, substantial hurdles also exist, notably the “overwhelming workload” that American PCPs already face.
David O’Gurek, MD, a family and community medicine physician at the Lewis Katz School of Medicine of Temple University in Philadelphia, agrees that a revamped approach to PA screening developed cooperatively between PCPs and specialists is an important goal and potentially feasible despite prior disagreements. “There has to be room for collaboration,” he said, but also emphasized the need for developing policies based on a systematic evidence review and a focus on patient-centered outcomes.
“We’re certainly missing patients with PA, but there needs to be greater clarity and standardization about the most appropriate screening approach and cutoff level” for flagging patients who need specialized assessment, Dr. O’Gurek said in an interview.
The current endocrinology literature also shows that experts remain divided on how best to accomplish this.
And some hypertension specialists question whether existing evidence is conclusive enough to warrant revised guidelines.
Dr. Cohen, the nephrologist and hypertension researcher, said that, while the recent prevalence report in Annals of Internal Medicine is “intriguing, hypothesis-generating information that suggests we are missing many cases of hyperaldosteronism in routine care,” she nevertheless believes that “we need additional data to be able to truly understand the breadth and implications of the findings.”
William C. Cushman, MD, a hypertension management specialist at the University of Tennessee Health Science Center in Memphis, agrees.
Changing existing practice guidelines “really needs randomized, controlled trials demonstrating a difference in long-term outcomes, ideally major cardiovascular outcomes,” that result from broader PA screening, he said.
Dr. Carey concurs that more evidence is needed to confirm the Annals report, but is confident this evidence will be in hand by the time a guideline-revision panel meets in 2021.
Australian model of PCPs screening for PA could be implemented in United States
An example of what might be possible when PCPs, endocrinologists, and hypertension specialists work together to make PA screening more accessible can be found in Melbourne, at the Endocrine Hypertension Service of Monash Health, in association with the Hudson Institute of Medical Research.
This began operating in July 2016, cofounded by Dr. Yang, whose experiences with her own father made her sensitive to the issue.
The service’s aim is to “address the underdiagnosis of PA, and to offer a streamlined diagnostic service for patients with hypertension,” with an “extensive outreach program” targeted to regional PCPs that, among other messages, encourages them to screen patients for PA when blood pressures exceed 140/90 mm Hg.
During its first 3 years of operation, the service saw 267 patients, with PA diagnosed in 135 and ruled out in 73 patients.
Notably, the proportion of these patients referred from PCPs jumped from 21% of 70 patients during the first year of operation to 47% of 70 patients during year 2, and 52% of 127 patients during the third year, ending in July 2019, said Dr. Yang, who continues to help run the service.
During the first year, a scant 3% of referred patients had recently diagnosed hypertension, but this rose to 14% during the second year, and to 19% during the most recent year with data available.
The median duration of diagnosed hypertension among referred patients fell from 11 years during year 1, to 7 years during year 3.
Service clinicians diagnosed 37 patients with unilateral adenomas, and removed them from 23 patients with four more awaiting surgery and the remaining 10 opting instead for medical management. Another 95 patients went on therapy with a MRA, and during the most recent year studied all patients who began a MRA regimen had a partial or complete clinical response.
Dr. Carey said the “creative program represents a model for implementation in U.S. practice.
Dr. Funder, Dr. Carey, Dr. Feldman, Dr. Yang, Dr. Cohen, and Dr. O’Gurek had no relevant disclosures. Dr. Cushman has been a consultant to Novartis, received personal fees from Sanofi, and research funding from Eli Lilly.
Jun Yang, MBBS, had watched as her father, who had battled hypertension for decades, ended up on four medications that still couldn’t bring his blood pressure to a healthy level. The cardiovascular endocrinologist then ran some tests, and soon thereafter her father had his blood pressure optimized on just one targeted medication.
Dr. Yang’s father was found to have a hormonal condition known as primary aldosteronism (PA) as the cause of his hypertension.
It turns out that PA is not as rare as once thought.
An eye-catching report in Annals of Internal Medicine this spring of an unexpectedly high prevalence of primary aldosteronism among a diverse cross section of U.S. patients with hypertension has raised issues that could dramatically change the way doctors in America, and elsewhere, assess and manage high blood pressure.
Foremost is the question of whether primary care physicians – the clinicians at the front line for diagnosing and initially treating most patients with hypertension – will absorb and act on this new evidence. For them, aldosteronism doesn’t automatically come to mind when they see high numbers on a BP monitor, and yet this latest research found that up to a third of all 726 patients in the study who were diagnosed with hypertension and with high urinary salt levels had PA.
That translates to a roughly three- to fivefold increase over standard prevalence estimates, and is a ”game changer” for how clinicians should approach hypertension management and PA diagnosis going forward, said John W. Funder, MD, in an editorial accompanying the Annals study.
Long considered relatively uncommon, hypertension driven by an excess of the hormone aldosterone, often because of an adenoma on the adrenal gland, is not the same as conventional “essential” hypertension. The former benefits from early diagnosis because its treatment is completely different – close to half of all PA patients can be treated definitively and quickly with surgical removal of an adenoma from one side of the adrenal gland.
For other PA patients, who have bilateral adrenal hyperplasia that is impossible to resolve surgically, treatment with drugs called mineralocorticoid receptor antagonists (MRAs), such as spironolactone, is needed because they target the hormonal cause of the high BP.
But what usually happens is that a patient with PA is mistakenly diagnosed with essential hypertension, in which the classic approach to treatment is to start with one regular antihypertensive drug, and add on further ones from different drug classes if blood pressure is not adequately controlled. When patients are taking three drugs, without adequate control, they are labeled as having “resistant hypertension.”
But in the case of PA, none of these conventional antihypertensives work, and the process of continuing to monitor and add different drugs wastes time, during which patients deteriorate.
“We need to change the culture of waiting for hypertension to be resistant and have patients riddled with end-organ damage,” due to years of persistently high BP and excess aldosterone “before we look for a secondary cause” like PA, declared Dr. Yang, of Hudson Institute of Medical Research and Monash University in Melbourne, during an interview.
So early diagnosis and prompt treatment of PA is key.
In addition to boosting the public health importance of early PA detection in hypertensive patients, the new up-sized PA prevalence numbers throw a spotlight on primary care physicians (PCPs) as key players who will need to apply the findings to practice on a public health scale.
These novel results create a need for “new guidelines, and a radically revised game plan with the key role of PCPs” emphasized in future management of patients with hypertension, said Dr. Funder, a professor of medicine at Monash University, in a second recent editorial in Hypertension.
“Buy-in by PCPs is essential,” agrees Robert M. Carey, MD, a cardiovascular endocrinologist and professor of medicine at the University of Virginia in Charlottesville, and a coauthor of the new study.
But he too acknowledges that this presents a major challenge. PCPs and internists, who diagnose a lot of hypertension, are “not used to thinking about aldosterone,” he said in an interview, encapsulating the key problem faced by proponents of earlier and more widespread PA assessment.
This dilemma looms as a “huge public health issue,” Dr. Carey warned.
‘We’re a long way from getting’ PCPs to buy in to PA screening
Will PCPs grow more comfortable with screening patients for PA themselves, or might they become more willing to refer hypertensive individuals for assessment at an expert center?
One skeptic is Ross D. Feldman, MD, a hypertension-management researcher and professor of medicine at the University of Manitoba in Winnipeg. The finding about high PA prevalence in patients with hypertension “is brand new, [and] the message needs to get to PCPs,” he said. But, “We’re a long way from getting it” to them. “I don’t know how to do that. It will be a tough sell.”
In addition, repositioning MRAs as an earlier option for many hypertensive patients won’t be easy either, because “we’ll never have outcome-trial data for MRAs,” given that they are now generic drugs, he noted.
“No clinical trial data show [MRAs] are first-line drugs,” said Dr. Feldman, who explained that, instead, MRAs are considered “go-to drugs” for patients with treatment-resistant hypertension, a niche therapeutic area. Results from the PATHWAY-2 trial published 5 years ago in Lancet showed “spironolactone was clearly the most effective treatment for the condition,” according to the report authors.
But even among patients with resistant hypertension, screening for PA dramatically lags despite being enshrined in guidelines.
“PCPs should start checking aldosterone-to-renin ratios [a widely used PA screen] in all patients with resistant hypertension or hypertension with hypokalemia, and then refer patients to specialists for testing and management,” said Jordana B. Cohen, MD, a nephrologist and hypertension researcher at the University of Pennsylvania in Philadelphia.
But recent studies of U.S. patient populations with clinical characteristics that meet existing criteria for PA screening showed that just 1%-2% of these individuals underwent an initial PA assessment, she noted, citing reports in the journals Surgery and Hypertension.
“We need to prioritize improving screening in these high-risk patients,” she stressed in an interview.
This illustrates that, in some respects, the new prevalence numbers are beside the point, because PA has been going unscreened and overlooked far too often even in the context of historical, lower prevalence rates, said Dr. Yang.
“The key point is that approximately 1 in 10 people with hypertension, and even more with resistant hypertension, have a form of the disease that is worse than essential hypertension but is routinely missed at present” and is also highly treatable.
“Evidence for the need for increased awareness of PA has been building for 2 decades,” stressed Dr. Yang, who has coauthored several commentaries and reviews that have bemoaned PA’s underappreciated status.
Interest in partnering with PCPs on guidance grows
One potential solution is to have endocrinologists and hypertension specialists’ partner with PCPs to come up with diagnostic and management recommendations. Both Dr. Funder and Dr. Carey are opinion leaders regarding the role of aldosterone in hypertension, and both were coauthors of the 2016 Endocrine Society guideline for PA assessment and management published in the Journal of Clinical Endocrinology & Metabolism , with Dr. Funder chairing the writing panel.
Now approaching its fifth year in effect, this guideline is “due for revision,” and “my hope is that we’ll be able to partner with one or more PCP organizations to come up with a version of the guideline targeted to PCPs,” Dr. Carey said.
He voiced interest in working on this with the American College of Physicians, which represents U.S. internal medicine physicians, and the American Academy of Family Physicians.
“We definitely need a partnership and educational efforts to get the word out from these organizations and not from a specialty society,” said Dr. Carey.
Dr. Funder said he has submitted a proposal to the Endocrine Society for a guidelines update he would chair with Dr. Carey’s assistance and with a diverse writing group that includes PCPs. Dr. Carey said that ideally this panel would write and release a revised guideline in 2021.
“Several of us are chomping at the bit to get this done,” he noted.
But participation by the ACP and AAFP remain uncertain as of September 2020. When approached about this, an ACP spokesperson said the organization had no comment. A spokesperson for the AAFP said, “It’s too early to tell if we will partner with any other organizations to develop guidelines specific to excess aldosterone, and how such guidelines might be received by our members.”
Recent history shows little cooperation between ACP, AAFP, and what might be termed the U.S. hypertension “establishment.” For example, when the American College of Cardiology and the American Heart Association released their most recent essential hypertension management guidelines in Hypertension in 2018, it was never adopted by ACP or AAFP.
The latter two organizations continue to endorse a higher BP threshold for diagnosing hypertension, and higher treatment targets set by alternative expert panels to those of the AHA/ACC.
Collaboration feasible, although PCPs overworked
Dr. Carey hopes that this episode will not preclude agreement over PA screening.
“I think it is still possible to partner with [the ACP and AAFP],” he observed, adding that he believes high PA prevalence among hypertensive patients and its consequences when unrecognized is “noncontentious.”
But he acknowledges that other, substantial hurdles also exist, notably the “overwhelming workload” that American PCPs already face.
David O’Gurek, MD, a family and community medicine physician at the Lewis Katz School of Medicine of Temple University in Philadelphia, agrees that a revamped approach to PA screening developed cooperatively between PCPs and specialists is an important goal and potentially feasible despite prior disagreements. “There has to be room for collaboration,” he said, but also emphasized the need for developing policies based on a systematic evidence review and a focus on patient-centered outcomes.
“We’re certainly missing patients with PA, but there needs to be greater clarity and standardization about the most appropriate screening approach and cutoff level” for flagging patients who need specialized assessment, Dr. O’Gurek said in an interview.
The current endocrinology literature also shows that experts remain divided on how best to accomplish this.
And some hypertension specialists question whether existing evidence is conclusive enough to warrant revised guidelines.
Dr. Cohen, the nephrologist and hypertension researcher, said that, while the recent prevalence report in Annals of Internal Medicine is “intriguing, hypothesis-generating information that suggests we are missing many cases of hyperaldosteronism in routine care,” she nevertheless believes that “we need additional data to be able to truly understand the breadth and implications of the findings.”
William C. Cushman, MD, a hypertension management specialist at the University of Tennessee Health Science Center in Memphis, agrees.
Changing existing practice guidelines “really needs randomized, controlled trials demonstrating a difference in long-term outcomes, ideally major cardiovascular outcomes,” that result from broader PA screening, he said.
Dr. Carey concurs that more evidence is needed to confirm the Annals report, but is confident this evidence will be in hand by the time a guideline-revision panel meets in 2021.
Australian model of PCPs screening for PA could be implemented in United States
An example of what might be possible when PCPs, endocrinologists, and hypertension specialists work together to make PA screening more accessible can be found in Melbourne, at the Endocrine Hypertension Service of Monash Health, in association with the Hudson Institute of Medical Research.
This began operating in July 2016, cofounded by Dr. Yang, whose experiences with her own father made her sensitive to the issue.
The service’s aim is to “address the underdiagnosis of PA, and to offer a streamlined diagnostic service for patients with hypertension,” with an “extensive outreach program” targeted to regional PCPs that, among other messages, encourages them to screen patients for PA when blood pressures exceed 140/90 mm Hg.
During its first 3 years of operation, the service saw 267 patients, with PA diagnosed in 135 and ruled out in 73 patients.
Notably, the proportion of these patients referred from PCPs jumped from 21% of 70 patients during the first year of operation to 47% of 70 patients during year 2, and 52% of 127 patients during the third year, ending in July 2019, said Dr. Yang, who continues to help run the service.
During the first year, a scant 3% of referred patients had recently diagnosed hypertension, but this rose to 14% during the second year, and to 19% during the most recent year with data available.
The median duration of diagnosed hypertension among referred patients fell from 11 years during year 1, to 7 years during year 3.
Service clinicians diagnosed 37 patients with unilateral adenomas, and removed them from 23 patients with four more awaiting surgery and the remaining 10 opting instead for medical management. Another 95 patients went on therapy with a MRA, and during the most recent year studied all patients who began a MRA regimen had a partial or complete clinical response.
Dr. Carey said the “creative program represents a model for implementation in U.S. practice.
Dr. Funder, Dr. Carey, Dr. Feldman, Dr. Yang, Dr. Cohen, and Dr. O’Gurek had no relevant disclosures. Dr. Cushman has been a consultant to Novartis, received personal fees from Sanofi, and research funding from Eli Lilly.