User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Conspiracy theories
It ain’t what you don’t know that gets you into trouble. It’s what you know for sure that just ain’t so. – Josh Billings
and intends to use COVID vaccinations as a devious way to implant microchips in us. He will then, of course, use the new 5G towers to track us all (although what Gates will do with the information that I was shopping at a Trader Joe’s yesterday is yet unknown).
It’s easy to dismiss patients with these beliefs as nuts or dumb or both. They’re neither, they’re just human. Conspiracy theories have been shared from the first time two humans met. They are, after all, simply hypotheses to explain an experience that’s difficult to understand. Making up a story to explain things feels safer than living with the unknown, and so we do. Our natural tendency to be suspicious makes conspiracy hypotheses more salient and more likely to spread. The pandemic itself is exacerbating this problem: People are alone and afraid, and dependent on social media for connection. Add a compelling story about a nefarious robber baron plotting to exploit us and you’ve got the conditions for conspiracy theories to explode like wind-driven wildfires. Astonishingly, a Pew Research poll showed 36% of Americans surveyed who have heard something about it say the Bill Gates cabal theory is “probably” or “definitely” true.
That many patients fervently believe conspiracy theories poses several problems for us. First, when a vaccine does become available, some patients will refuse to be vaccinated. The consequences to their health and the health of the community are grave. Secondly, whenever patients have cause to distrust doctors, it makes our jobs more challenging. If they don’t trust us on vaccines, it can spread to not trusting us about wearing masks or sunscreens or taking statins. Lastly, it’s near impossible to have a friendly conversation with a patient carrying forth on why Bill Gates is not in jail or how I’m part of the medical-industrial complex enabling him. Sheesh.
It isn’t their fault. The underpinning of these beliefs can be understood as a cognitive bias. In this case, an idea that is easy to imagine or recall is believed to be true more than an idea that is complex and difficult. Understanding viral replication and R0 numbers or viral vectors and protein subunit vaccines is hard. Imagining a chip being injected into your arm is easy. And, as behavioral economist Daniel Kahneman opined, we humans possess an almost unlimited ability to ignore our ignorance. We physicians can help in a way that friends and family members can’t. Here are ways you can help patients who believe in conspiracy theories:
Approach this problem like any other infirmity, with compassion. No one wants to drink too much and knock out their teeth falling off a bike. It was a mistake. Similarly, when people are steeped in self-delusion, it’s not a misdeed, it’s a lapse. Be kind and respectful.
Meet them where they are. It might be helpful to state with sincerity: So you feel that there is a government plot to use COVID to track us? Have you considered that might not be true?
Have the conversation in private. Harder even than being wrong is being publicly wrong.
Try the Socratic method. (We’re pretty good at this from teaching students and residents.) Conspiracy-believing patients have the illusion of knowledge, yet, like students, it’s often easy to show them their gaps. Do so gently by leading them to discover for themselves.
Stop when you stall. You cannot change someone’s mind by dint of force. However, you surely can damage your relationship if you keep pushing them.
Don’t worry if you fail to break through; you might yet have moved them a bit. This might make it possible for them to discover the truth later. Or, you could simply switch to explain what holds up the ground we walk upon. There’s rumor we’re supported on the backs of turtles, all the way down. Maybe Bill Gates is feeding them.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
It ain’t what you don’t know that gets you into trouble. It’s what you know for sure that just ain’t so. – Josh Billings
and intends to use COVID vaccinations as a devious way to implant microchips in us. He will then, of course, use the new 5G towers to track us all (although what Gates will do with the information that I was shopping at a Trader Joe’s yesterday is yet unknown).
It’s easy to dismiss patients with these beliefs as nuts or dumb or both. They’re neither, they’re just human. Conspiracy theories have been shared from the first time two humans met. They are, after all, simply hypotheses to explain an experience that’s difficult to understand. Making up a story to explain things feels safer than living with the unknown, and so we do. Our natural tendency to be suspicious makes conspiracy hypotheses more salient and more likely to spread. The pandemic itself is exacerbating this problem: People are alone and afraid, and dependent on social media for connection. Add a compelling story about a nefarious robber baron plotting to exploit us and you’ve got the conditions for conspiracy theories to explode like wind-driven wildfires. Astonishingly, a Pew Research poll showed 36% of Americans surveyed who have heard something about it say the Bill Gates cabal theory is “probably” or “definitely” true.
That many patients fervently believe conspiracy theories poses several problems for us. First, when a vaccine does become available, some patients will refuse to be vaccinated. The consequences to their health and the health of the community are grave. Secondly, whenever patients have cause to distrust doctors, it makes our jobs more challenging. If they don’t trust us on vaccines, it can spread to not trusting us about wearing masks or sunscreens or taking statins. Lastly, it’s near impossible to have a friendly conversation with a patient carrying forth on why Bill Gates is not in jail or how I’m part of the medical-industrial complex enabling him. Sheesh.
It isn’t their fault. The underpinning of these beliefs can be understood as a cognitive bias. In this case, an idea that is easy to imagine or recall is believed to be true more than an idea that is complex and difficult. Understanding viral replication and R0 numbers or viral vectors and protein subunit vaccines is hard. Imagining a chip being injected into your arm is easy. And, as behavioral economist Daniel Kahneman opined, we humans possess an almost unlimited ability to ignore our ignorance. We physicians can help in a way that friends and family members can’t. Here are ways you can help patients who believe in conspiracy theories:
Approach this problem like any other infirmity, with compassion. No one wants to drink too much and knock out their teeth falling off a bike. It was a mistake. Similarly, when people are steeped in self-delusion, it’s not a misdeed, it’s a lapse. Be kind and respectful.
Meet them where they are. It might be helpful to state with sincerity: So you feel that there is a government plot to use COVID to track us? Have you considered that might not be true?
Have the conversation in private. Harder even than being wrong is being publicly wrong.
Try the Socratic method. (We’re pretty good at this from teaching students and residents.) Conspiracy-believing patients have the illusion of knowledge, yet, like students, it’s often easy to show them their gaps. Do so gently by leading them to discover for themselves.
Stop when you stall. You cannot change someone’s mind by dint of force. However, you surely can damage your relationship if you keep pushing them.
Don’t worry if you fail to break through; you might yet have moved them a bit. This might make it possible for them to discover the truth later. Or, you could simply switch to explain what holds up the ground we walk upon. There’s rumor we’re supported on the backs of turtles, all the way down. Maybe Bill Gates is feeding them.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
It ain’t what you don’t know that gets you into trouble. It’s what you know for sure that just ain’t so. – Josh Billings
and intends to use COVID vaccinations as a devious way to implant microchips in us. He will then, of course, use the new 5G towers to track us all (although what Gates will do with the information that I was shopping at a Trader Joe’s yesterday is yet unknown).
It’s easy to dismiss patients with these beliefs as nuts or dumb or both. They’re neither, they’re just human. Conspiracy theories have been shared from the first time two humans met. They are, after all, simply hypotheses to explain an experience that’s difficult to understand. Making up a story to explain things feels safer than living with the unknown, and so we do. Our natural tendency to be suspicious makes conspiracy hypotheses more salient and more likely to spread. The pandemic itself is exacerbating this problem: People are alone and afraid, and dependent on social media for connection. Add a compelling story about a nefarious robber baron plotting to exploit us and you’ve got the conditions for conspiracy theories to explode like wind-driven wildfires. Astonishingly, a Pew Research poll showed 36% of Americans surveyed who have heard something about it say the Bill Gates cabal theory is “probably” or “definitely” true.
That many patients fervently believe conspiracy theories poses several problems for us. First, when a vaccine does become available, some patients will refuse to be vaccinated. The consequences to their health and the health of the community are grave. Secondly, whenever patients have cause to distrust doctors, it makes our jobs more challenging. If they don’t trust us on vaccines, it can spread to not trusting us about wearing masks or sunscreens or taking statins. Lastly, it’s near impossible to have a friendly conversation with a patient carrying forth on why Bill Gates is not in jail or how I’m part of the medical-industrial complex enabling him. Sheesh.
It isn’t their fault. The underpinning of these beliefs can be understood as a cognitive bias. In this case, an idea that is easy to imagine or recall is believed to be true more than an idea that is complex and difficult. Understanding viral replication and R0 numbers or viral vectors and protein subunit vaccines is hard. Imagining a chip being injected into your arm is easy. And, as behavioral economist Daniel Kahneman opined, we humans possess an almost unlimited ability to ignore our ignorance. We physicians can help in a way that friends and family members can’t. Here are ways you can help patients who believe in conspiracy theories:
Approach this problem like any other infirmity, with compassion. No one wants to drink too much and knock out their teeth falling off a bike. It was a mistake. Similarly, when people are steeped in self-delusion, it’s not a misdeed, it’s a lapse. Be kind and respectful.
Meet them where they are. It might be helpful to state with sincerity: So you feel that there is a government plot to use COVID to track us? Have you considered that might not be true?
Have the conversation in private. Harder even than being wrong is being publicly wrong.
Try the Socratic method. (We’re pretty good at this from teaching students and residents.) Conspiracy-believing patients have the illusion of knowledge, yet, like students, it’s often easy to show them their gaps. Do so gently by leading them to discover for themselves.
Stop when you stall. You cannot change someone’s mind by dint of force. However, you surely can damage your relationship if you keep pushing them.
Don’t worry if you fail to break through; you might yet have moved them a bit. This might make it possible for them to discover the truth later. Or, you could simply switch to explain what holds up the ground we walk upon. There’s rumor we’re supported on the backs of turtles, all the way down. Maybe Bill Gates is feeding them.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
Diffuse idiopathic skeletal hyperostosis heart risk higher than expected
More people with diffuse idiopathic skeletal hyperostosis (DISH) develop cardiovascular disease (CVD) than is predicted by the Framingham Risk Score, results of an observational study have shown.
Notably, a higher rate of myocardial infarction (MI) was seen in those with DISH than in those without DISH over the 10-year follow-up period (24.4% vs. 4.3%; P = .0055).
“We propose more scrutiny is warranted in evaluating CV risk in these patients, more demanding treatment target goals should be established, and as a result, earlier and more aggressive preventive medical interventions instituted,” corresponding author Reuven Mader, MD, and associates wrote in Arthritis Research & Therapy.
“What Mader’s study is pointing out is that it’s worth the radiologist reporting [DISH],” Elizabeth A. Regan, MD, PhD, from the National Jewish Health Center in Denver, said in an interview.
DISH on a chest x-ray or CT scan should be another “red flag to be even more attentive to cardiovascular risk,” she added, particularly because studies have shown that people with DISH tend to be obese, have metabolic syndrome, or diabetes – all of which independently increase their risk for cardiovascular disease.
An old condition often found by accident
Physicians have known about DISH for many years, Dr. Mader of Ha’Emek Medical Center in Afula, Israel, observed in an interview. Historical evidence suggests it was present more than a thousand years ago, but it wasn’t until the 1950s that it gained scientific interest. Originally coined Forestier’s disease, it was renamed DISH in the late 1960s following the realization that it was not limited to the spine.
“It is a condition which is characterized by new bone formation,” Dr. Mader explained. This new bone formation has some predilection for the entheses – the tendons, ligaments, or joint capsules, that attach to the bone.
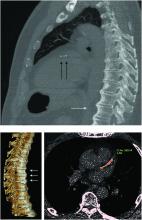
“Diagnosis of the disease is based mainly on radiographs, especially of the thoracic spine, and it requires the formation of bridges that connect at least four contiguous vertebra,” he continued.
“The bridges are usually right-sided and usually the intervertebral spaces are spared. Classically there is no involvement of the sacroiliac joints, although there are some changes that might involve the sacroiliac joints but in a different manner than in inflammatory sacroiliitis.”
DISH was originally thought to be a pain syndrome, which has “not played out,” Dr. Regan noted in her interview. While there may be people who experience pain as a result of DISH, most cases are asymptomatic and usually picked up incidentally on a chest x-ray or CT scan.
“It’s something that’s not obvious,” she said. One of the main problems it can cause is stiffness and lack of mobility in the spine and this can lead to quite severe fractures in some cases, such as during a car accident. Hence spinal surgeons and other orthopedic specialists, such as Dr. Regan, have also taken an interest in the condition.
“Apart from the thoracic spine, DISH may also involve the cervical spine; there have been many reports about difficulty in swallowing, breathing, and in the lumbar spine, spinal stenosis and so forth,” Dr. Mader said. The differential diagnosis includes ankylosing spondylitis, although there is some evidence that the two can coexist.
“The diagnosis depends on the alertness of the examining physician,” he added, noting that rheumatologists and other specialists would be “very aware of this condition” and “sensitive to changes that we see when we examine these patients.”
DISH and heightened cardiovascular risk
Previous work by Dr. Mader and associates has shown that people with DISH are more often affected by the metabolic syndrome than are those without DISH. The cross-sectional study had excluded those with preexisting CVD and found that people with DISH had a significantly higher Framingham Risk Score, compared with a control group of people with osteoarthritis and no DISH (P = .004), which in turn meant they had a significantly (P = .007) higher 10-year risk for developing CVD.
The aim of their most recent study was to compare the actual rate of CV events in 2016 versus those predicted by the Framingham Risk Score in 2006. To do this, they compared the available electronic medical records of 45 individuals with DISH and 47 without it.
The results showed that almost 39% of people with DISH had developed CVD, whereas the Framingham Risk Score had estimated that just under 27% would develop CVD.
For every 1% increase in the CVD risk calculated by the Framingham Risk Score, the odds of CVD increased by 4% in the DISH group versus the control group (P = .02).
While there was a significant (P < .003) difference in the Framingham Risk Score between the DISH and control groups in 2006 (28.6% vs. 17.8%), there was no overall statistical difference (P = .2) in the composite CVD outcome (38.8% vs. 25.5%) 10 years later, as calculated by the revised Framingham Risk Score, which included MI, cerebrovascular accident, transient ischemic attack, peripheral artery disease, and heart failure with preserved ejection fraction.
“We are dealing with patients who are in their 70s. So, it is expected that this group of patients will be more often affected by cardiovascular disease” than younger individuals, Dr. Mader observed. That said, the study’s findings “confirm the theory that patients with DISH have a high likelihood of developing cardiovascular disease,” he added, acknowledging that it was only the risk for MI that was statistically significantly higher in people with DISH than in the controls.
DISH and coronary artery calcification
“It might be even more interesting to have a different control population that had no osteoarthritis,” Dr. Regan observed.
As the associate director of the COPDGene study, Dr. Regan has access to data collected from a large cohort of people with chronic obstructive pulmonary disease (COPD; n = 2,728), around 13% of whom were identified as having DISH in one recent study.
In that study, the presence of DISH versus no DISH was associated with a 37% higher risk for having coronary artery calcification (CAC) – a marker for atherosclerosis and cardiovascular disease. Two-thirds of people with DISH had CAC, compared with 46.9% of those without DISH (P < .001). The prevalence of DISH was 8.8% in those without CAC, 12.8% in those with a CAC score of 1-100, 20% in those with a CAC score of 100-400, and 24.7% in those with a CAC score of more than 400, which is associated with a very high risk for coronary artery disease.
Dr. Regan observed that information on heart attacks and strokes were collected within the COPDGene study, so it would be possible to look at cardiovascular risk in their patients with DISH and confirm the findings of Mader and colleagues.
“I think the most important thing is recognizing that there are things going on in the spine that are important to people’s general health,” Dr. Regan said.
Dr. Mader noted: “It makes sense that patients with DISH should be more meticulously followed for at least the traditional risk factors and better treated because they are at a higher risk for these events.”
The study received no financial support. Neither Dr. Mader nor Dr. Regan had any conflicts of interest to disclose.
SOURCE: Glick K et al. Arthritis Res Ther. 2020. doi: 10.1186/s13075-020-02278-w.
More people with diffuse idiopathic skeletal hyperostosis (DISH) develop cardiovascular disease (CVD) than is predicted by the Framingham Risk Score, results of an observational study have shown.
Notably, a higher rate of myocardial infarction (MI) was seen in those with DISH than in those without DISH over the 10-year follow-up period (24.4% vs. 4.3%; P = .0055).
“We propose more scrutiny is warranted in evaluating CV risk in these patients, more demanding treatment target goals should be established, and as a result, earlier and more aggressive preventive medical interventions instituted,” corresponding author Reuven Mader, MD, and associates wrote in Arthritis Research & Therapy.
“What Mader’s study is pointing out is that it’s worth the radiologist reporting [DISH],” Elizabeth A. Regan, MD, PhD, from the National Jewish Health Center in Denver, said in an interview.
DISH on a chest x-ray or CT scan should be another “red flag to be even more attentive to cardiovascular risk,” she added, particularly because studies have shown that people with DISH tend to be obese, have metabolic syndrome, or diabetes – all of which independently increase their risk for cardiovascular disease.
An old condition often found by accident
Physicians have known about DISH for many years, Dr. Mader of Ha’Emek Medical Center in Afula, Israel, observed in an interview. Historical evidence suggests it was present more than a thousand years ago, but it wasn’t until the 1950s that it gained scientific interest. Originally coined Forestier’s disease, it was renamed DISH in the late 1960s following the realization that it was not limited to the spine.
“It is a condition which is characterized by new bone formation,” Dr. Mader explained. This new bone formation has some predilection for the entheses – the tendons, ligaments, or joint capsules, that attach to the bone.
“Diagnosis of the disease is based mainly on radiographs, especially of the thoracic spine, and it requires the formation of bridges that connect at least four contiguous vertebra,” he continued.
“The bridges are usually right-sided and usually the intervertebral spaces are spared. Classically there is no involvement of the sacroiliac joints, although there are some changes that might involve the sacroiliac joints but in a different manner than in inflammatory sacroiliitis.”
DISH was originally thought to be a pain syndrome, which has “not played out,” Dr. Regan noted in her interview. While there may be people who experience pain as a result of DISH, most cases are asymptomatic and usually picked up incidentally on a chest x-ray or CT scan.
“It’s something that’s not obvious,” she said. One of the main problems it can cause is stiffness and lack of mobility in the spine and this can lead to quite severe fractures in some cases, such as during a car accident. Hence spinal surgeons and other orthopedic specialists, such as Dr. Regan, have also taken an interest in the condition.
“Apart from the thoracic spine, DISH may also involve the cervical spine; there have been many reports about difficulty in swallowing, breathing, and in the lumbar spine, spinal stenosis and so forth,” Dr. Mader said. The differential diagnosis includes ankylosing spondylitis, although there is some evidence that the two can coexist.
“The diagnosis depends on the alertness of the examining physician,” he added, noting that rheumatologists and other specialists would be “very aware of this condition” and “sensitive to changes that we see when we examine these patients.”
DISH and heightened cardiovascular risk
Previous work by Dr. Mader and associates has shown that people with DISH are more often affected by the metabolic syndrome than are those without DISH. The cross-sectional study had excluded those with preexisting CVD and found that people with DISH had a significantly higher Framingham Risk Score, compared with a control group of people with osteoarthritis and no DISH (P = .004), which in turn meant they had a significantly (P = .007) higher 10-year risk for developing CVD.
The aim of their most recent study was to compare the actual rate of CV events in 2016 versus those predicted by the Framingham Risk Score in 2006. To do this, they compared the available electronic medical records of 45 individuals with DISH and 47 without it.
The results showed that almost 39% of people with DISH had developed CVD, whereas the Framingham Risk Score had estimated that just under 27% would develop CVD.
For every 1% increase in the CVD risk calculated by the Framingham Risk Score, the odds of CVD increased by 4% in the DISH group versus the control group (P = .02).
While there was a significant (P < .003) difference in the Framingham Risk Score between the DISH and control groups in 2006 (28.6% vs. 17.8%), there was no overall statistical difference (P = .2) in the composite CVD outcome (38.8% vs. 25.5%) 10 years later, as calculated by the revised Framingham Risk Score, which included MI, cerebrovascular accident, transient ischemic attack, peripheral artery disease, and heart failure with preserved ejection fraction.
“We are dealing with patients who are in their 70s. So, it is expected that this group of patients will be more often affected by cardiovascular disease” than younger individuals, Dr. Mader observed. That said, the study’s findings “confirm the theory that patients with DISH have a high likelihood of developing cardiovascular disease,” he added, acknowledging that it was only the risk for MI that was statistically significantly higher in people with DISH than in the controls.
DISH and coronary artery calcification
“It might be even more interesting to have a different control population that had no osteoarthritis,” Dr. Regan observed.
As the associate director of the COPDGene study, Dr. Regan has access to data collected from a large cohort of people with chronic obstructive pulmonary disease (COPD; n = 2,728), around 13% of whom were identified as having DISH in one recent study.
In that study, the presence of DISH versus no DISH was associated with a 37% higher risk for having coronary artery calcification (CAC) – a marker for atherosclerosis and cardiovascular disease. Two-thirds of people with DISH had CAC, compared with 46.9% of those without DISH (P < .001). The prevalence of DISH was 8.8% in those without CAC, 12.8% in those with a CAC score of 1-100, 20% in those with a CAC score of 100-400, and 24.7% in those with a CAC score of more than 400, which is associated with a very high risk for coronary artery disease.
Dr. Regan observed that information on heart attacks and strokes were collected within the COPDGene study, so it would be possible to look at cardiovascular risk in their patients with DISH and confirm the findings of Mader and colleagues.
“I think the most important thing is recognizing that there are things going on in the spine that are important to people’s general health,” Dr. Regan said.
Dr. Mader noted: “It makes sense that patients with DISH should be more meticulously followed for at least the traditional risk factors and better treated because they are at a higher risk for these events.”
The study received no financial support. Neither Dr. Mader nor Dr. Regan had any conflicts of interest to disclose.
SOURCE: Glick K et al. Arthritis Res Ther. 2020. doi: 10.1186/s13075-020-02278-w.
More people with diffuse idiopathic skeletal hyperostosis (DISH) develop cardiovascular disease (CVD) than is predicted by the Framingham Risk Score, results of an observational study have shown.
Notably, a higher rate of myocardial infarction (MI) was seen in those with DISH than in those without DISH over the 10-year follow-up period (24.4% vs. 4.3%; P = .0055).
“We propose more scrutiny is warranted in evaluating CV risk in these patients, more demanding treatment target goals should be established, and as a result, earlier and more aggressive preventive medical interventions instituted,” corresponding author Reuven Mader, MD, and associates wrote in Arthritis Research & Therapy.
“What Mader’s study is pointing out is that it’s worth the radiologist reporting [DISH],” Elizabeth A. Regan, MD, PhD, from the National Jewish Health Center in Denver, said in an interview.
DISH on a chest x-ray or CT scan should be another “red flag to be even more attentive to cardiovascular risk,” she added, particularly because studies have shown that people with DISH tend to be obese, have metabolic syndrome, or diabetes – all of which independently increase their risk for cardiovascular disease.
An old condition often found by accident
Physicians have known about DISH for many years, Dr. Mader of Ha’Emek Medical Center in Afula, Israel, observed in an interview. Historical evidence suggests it was present more than a thousand years ago, but it wasn’t until the 1950s that it gained scientific interest. Originally coined Forestier’s disease, it was renamed DISH in the late 1960s following the realization that it was not limited to the spine.
“It is a condition which is characterized by new bone formation,” Dr. Mader explained. This new bone formation has some predilection for the entheses – the tendons, ligaments, or joint capsules, that attach to the bone.
“Diagnosis of the disease is based mainly on radiographs, especially of the thoracic spine, and it requires the formation of bridges that connect at least four contiguous vertebra,” he continued.
“The bridges are usually right-sided and usually the intervertebral spaces are spared. Classically there is no involvement of the sacroiliac joints, although there are some changes that might involve the sacroiliac joints but in a different manner than in inflammatory sacroiliitis.”
DISH was originally thought to be a pain syndrome, which has “not played out,” Dr. Regan noted in her interview. While there may be people who experience pain as a result of DISH, most cases are asymptomatic and usually picked up incidentally on a chest x-ray or CT scan.
“It’s something that’s not obvious,” she said. One of the main problems it can cause is stiffness and lack of mobility in the spine and this can lead to quite severe fractures in some cases, such as during a car accident. Hence spinal surgeons and other orthopedic specialists, such as Dr. Regan, have also taken an interest in the condition.
“Apart from the thoracic spine, DISH may also involve the cervical spine; there have been many reports about difficulty in swallowing, breathing, and in the lumbar spine, spinal stenosis and so forth,” Dr. Mader said. The differential diagnosis includes ankylosing spondylitis, although there is some evidence that the two can coexist.
“The diagnosis depends on the alertness of the examining physician,” he added, noting that rheumatologists and other specialists would be “very aware of this condition” and “sensitive to changes that we see when we examine these patients.”
DISH and heightened cardiovascular risk
Previous work by Dr. Mader and associates has shown that people with DISH are more often affected by the metabolic syndrome than are those without DISH. The cross-sectional study had excluded those with preexisting CVD and found that people with DISH had a significantly higher Framingham Risk Score, compared with a control group of people with osteoarthritis and no DISH (P = .004), which in turn meant they had a significantly (P = .007) higher 10-year risk for developing CVD.
The aim of their most recent study was to compare the actual rate of CV events in 2016 versus those predicted by the Framingham Risk Score in 2006. To do this, they compared the available electronic medical records of 45 individuals with DISH and 47 without it.
The results showed that almost 39% of people with DISH had developed CVD, whereas the Framingham Risk Score had estimated that just under 27% would develop CVD.
For every 1% increase in the CVD risk calculated by the Framingham Risk Score, the odds of CVD increased by 4% in the DISH group versus the control group (P = .02).
While there was a significant (P < .003) difference in the Framingham Risk Score between the DISH and control groups in 2006 (28.6% vs. 17.8%), there was no overall statistical difference (P = .2) in the composite CVD outcome (38.8% vs. 25.5%) 10 years later, as calculated by the revised Framingham Risk Score, which included MI, cerebrovascular accident, transient ischemic attack, peripheral artery disease, and heart failure with preserved ejection fraction.
“We are dealing with patients who are in their 70s. So, it is expected that this group of patients will be more often affected by cardiovascular disease” than younger individuals, Dr. Mader observed. That said, the study’s findings “confirm the theory that patients with DISH have a high likelihood of developing cardiovascular disease,” he added, acknowledging that it was only the risk for MI that was statistically significantly higher in people with DISH than in the controls.
DISH and coronary artery calcification
“It might be even more interesting to have a different control population that had no osteoarthritis,” Dr. Regan observed.
As the associate director of the COPDGene study, Dr. Regan has access to data collected from a large cohort of people with chronic obstructive pulmonary disease (COPD; n = 2,728), around 13% of whom were identified as having DISH in one recent study.
In that study, the presence of DISH versus no DISH was associated with a 37% higher risk for having coronary artery calcification (CAC) – a marker for atherosclerosis and cardiovascular disease. Two-thirds of people with DISH had CAC, compared with 46.9% of those without DISH (P < .001). The prevalence of DISH was 8.8% in those without CAC, 12.8% in those with a CAC score of 1-100, 20% in those with a CAC score of 100-400, and 24.7% in those with a CAC score of more than 400, which is associated with a very high risk for coronary artery disease.
Dr. Regan observed that information on heart attacks and strokes were collected within the COPDGene study, so it would be possible to look at cardiovascular risk in their patients with DISH and confirm the findings of Mader and colleagues.
“I think the most important thing is recognizing that there are things going on in the spine that are important to people’s general health,” Dr. Regan said.
Dr. Mader noted: “It makes sense that patients with DISH should be more meticulously followed for at least the traditional risk factors and better treated because they are at a higher risk for these events.”
The study received no financial support. Neither Dr. Mader nor Dr. Regan had any conflicts of interest to disclose.
SOURCE: Glick K et al. Arthritis Res Ther. 2020. doi: 10.1186/s13075-020-02278-w.
FROM ARTHRITIS RESEARCH & THERAPY
COVID-19 outcomes no worse in patients on TNF inhibitors or methotrexate
Continued use of tumor necrosis factor inhibitors or methotrexate is acceptable in most patients who acquire COVID-19, results of a recent cohort study suggest.
Among patients on tumor necrosis factor inhibitors (TNFi) or methotrexate who developed COVID-19, death and hospitalization rates were similar to matched COVID-19 patients not on those medications, according to authors of the multicenter research network study.
Reassuringly, likelihood of hospitalization and mortality were not significantly different between 214 patients with COVID-19 taking TNFi or methotrexate and 31,862 matched COVID-19 patients not on those medications, according to the investigators, whose findings were published recently in the Journal of the American Academy of Dermatology.
Zachary Zinn, MD, corresponding author on the study, said in an interview that the findings suggest these medicines can be safely continued in the majority of patients taking them during the COVID-19 pandemic.
“If you’re a prescribing physician who’s giving patients TNF inhibitors or methotrexate or both, I think you can comfortably tell your patients there is good data that these do not lead to worse outcomes if you get COVID-19,” said Dr. Zinn, associate professor in the department of dermatology at West Virginia University, Morgantown.
The findings from these researchers corroborate a growing body of evidence suggesting that immunosuppressive treatments can be continued in patients with dermatologic and rheumatic conditions.
In recent guidance from the National Psoriasis Foundation, released Sept. 4, an expert consensus panel cited 15 studies that they said suggested that treatments for psoriasis or psoriatic arthritis “do not meaningfully alter the risk of acquiring SARS-CoV-2 infection or having worse COVID-19 outcomes.”
That said, the data to date are mainly from small case series and registry studies based on spontaneously reported COVID-19 cases, which suggests a continued need for shared decision making. In addition, chronic systemic corticosteroids should be avoided for management of psoriatic arthritis, the guidance states, based on rheumatology and gastroenterology literature suggesting this treatment is linked to worse COVID-19 outcomes.
In the interview, Dr. Zinn noted that some previous studies of immunosuppressive treatments in patients who acquire COVID-19 have aggregated data on numerous classes of biologic medications, lessening the strength of data for each specific medication.
“By focusing specifically on TNF inhibitors and methotrexate, this study gives better guidance to prescribers of these medications,” he said.
To see whether TNFi or methotrexate increased risk of worsened COVID-19 outcomes, Dr. Zinn and coinvestigators evaluated data from TriNetX, a research network that includes approximately 53 million unique patient records, predominantly in the United States.
They identified 32,076 adult patients with COVID-19, of whom 214 had recent exposure to TNFi or methotrexate. The patients in the TNFi/methotrexate group were similar in age to those without exposure to those drugs, at 55.1 versus 53.2 years, respectively. However, patients in the drug exposure group were more frequently White, female, and had substantially more comorbidities, including diabetes and obesity, according to the investigators.
Nevertheless, the likelihood of hospitalization was not statistically different in the TNFi/methotrexate group versus the non-TNFi/methotrexate group, with a risk ratio of 0.91 (95% confidence interval, 0.68-1.22; P = .5260).
Likewise, the likelihood of death was not different between groups, with a RR of 0.87 (95% CI, 0.42-1.78; P = .6958). Looking at subgroups of patients exposed to TNFi or methotrexate only didn’t change the results, the investigators added.
Taken together, the findings argue against interruption of these treatments because of the fear of the possibly worse COVID-19 outcomes, the investigators concluded, although they emphasized the need for more research.
“Because the COVID-19 pandemic is ongoing, there is a desperate need for evidence-based data on biologic and immunomodulator exposure in the setting of COVID-19 infection,” they wrote.
Dr. Zinn and coauthors reported no conflicts of interest and no funding sources related to the study.
SOURCE: Zinn Z et al. J Am Acad Dermatol. 2020 Sep 11. doi: 10.1016/j.jaad.2020.09.009.
Continued use of tumor necrosis factor inhibitors or methotrexate is acceptable in most patients who acquire COVID-19, results of a recent cohort study suggest.
Among patients on tumor necrosis factor inhibitors (TNFi) or methotrexate who developed COVID-19, death and hospitalization rates were similar to matched COVID-19 patients not on those medications, according to authors of the multicenter research network study.
Reassuringly, likelihood of hospitalization and mortality were not significantly different between 214 patients with COVID-19 taking TNFi or methotrexate and 31,862 matched COVID-19 patients not on those medications, according to the investigators, whose findings were published recently in the Journal of the American Academy of Dermatology.
Zachary Zinn, MD, corresponding author on the study, said in an interview that the findings suggest these medicines can be safely continued in the majority of patients taking them during the COVID-19 pandemic.
“If you’re a prescribing physician who’s giving patients TNF inhibitors or methotrexate or both, I think you can comfortably tell your patients there is good data that these do not lead to worse outcomes if you get COVID-19,” said Dr. Zinn, associate professor in the department of dermatology at West Virginia University, Morgantown.
The findings from these researchers corroborate a growing body of evidence suggesting that immunosuppressive treatments can be continued in patients with dermatologic and rheumatic conditions.
In recent guidance from the National Psoriasis Foundation, released Sept. 4, an expert consensus panel cited 15 studies that they said suggested that treatments for psoriasis or psoriatic arthritis “do not meaningfully alter the risk of acquiring SARS-CoV-2 infection or having worse COVID-19 outcomes.”
That said, the data to date are mainly from small case series and registry studies based on spontaneously reported COVID-19 cases, which suggests a continued need for shared decision making. In addition, chronic systemic corticosteroids should be avoided for management of psoriatic arthritis, the guidance states, based on rheumatology and gastroenterology literature suggesting this treatment is linked to worse COVID-19 outcomes.
In the interview, Dr. Zinn noted that some previous studies of immunosuppressive treatments in patients who acquire COVID-19 have aggregated data on numerous classes of biologic medications, lessening the strength of data for each specific medication.
“By focusing specifically on TNF inhibitors and methotrexate, this study gives better guidance to prescribers of these medications,” he said.
To see whether TNFi or methotrexate increased risk of worsened COVID-19 outcomes, Dr. Zinn and coinvestigators evaluated data from TriNetX, a research network that includes approximately 53 million unique patient records, predominantly in the United States.
They identified 32,076 adult patients with COVID-19, of whom 214 had recent exposure to TNFi or methotrexate. The patients in the TNFi/methotrexate group were similar in age to those without exposure to those drugs, at 55.1 versus 53.2 years, respectively. However, patients in the drug exposure group were more frequently White, female, and had substantially more comorbidities, including diabetes and obesity, according to the investigators.
Nevertheless, the likelihood of hospitalization was not statistically different in the TNFi/methotrexate group versus the non-TNFi/methotrexate group, with a risk ratio of 0.91 (95% confidence interval, 0.68-1.22; P = .5260).
Likewise, the likelihood of death was not different between groups, with a RR of 0.87 (95% CI, 0.42-1.78; P = .6958). Looking at subgroups of patients exposed to TNFi or methotrexate only didn’t change the results, the investigators added.
Taken together, the findings argue against interruption of these treatments because of the fear of the possibly worse COVID-19 outcomes, the investigators concluded, although they emphasized the need for more research.
“Because the COVID-19 pandemic is ongoing, there is a desperate need for evidence-based data on biologic and immunomodulator exposure in the setting of COVID-19 infection,” they wrote.
Dr. Zinn and coauthors reported no conflicts of interest and no funding sources related to the study.
SOURCE: Zinn Z et al. J Am Acad Dermatol. 2020 Sep 11. doi: 10.1016/j.jaad.2020.09.009.
Continued use of tumor necrosis factor inhibitors or methotrexate is acceptable in most patients who acquire COVID-19, results of a recent cohort study suggest.
Among patients on tumor necrosis factor inhibitors (TNFi) or methotrexate who developed COVID-19, death and hospitalization rates were similar to matched COVID-19 patients not on those medications, according to authors of the multicenter research network study.
Reassuringly, likelihood of hospitalization and mortality were not significantly different between 214 patients with COVID-19 taking TNFi or methotrexate and 31,862 matched COVID-19 patients not on those medications, according to the investigators, whose findings were published recently in the Journal of the American Academy of Dermatology.
Zachary Zinn, MD, corresponding author on the study, said in an interview that the findings suggest these medicines can be safely continued in the majority of patients taking them during the COVID-19 pandemic.
“If you’re a prescribing physician who’s giving patients TNF inhibitors or methotrexate or both, I think you can comfortably tell your patients there is good data that these do not lead to worse outcomes if you get COVID-19,” said Dr. Zinn, associate professor in the department of dermatology at West Virginia University, Morgantown.
The findings from these researchers corroborate a growing body of evidence suggesting that immunosuppressive treatments can be continued in patients with dermatologic and rheumatic conditions.
In recent guidance from the National Psoriasis Foundation, released Sept. 4, an expert consensus panel cited 15 studies that they said suggested that treatments for psoriasis or psoriatic arthritis “do not meaningfully alter the risk of acquiring SARS-CoV-2 infection or having worse COVID-19 outcomes.”
That said, the data to date are mainly from small case series and registry studies based on spontaneously reported COVID-19 cases, which suggests a continued need for shared decision making. In addition, chronic systemic corticosteroids should be avoided for management of psoriatic arthritis, the guidance states, based on rheumatology and gastroenterology literature suggesting this treatment is linked to worse COVID-19 outcomes.
In the interview, Dr. Zinn noted that some previous studies of immunosuppressive treatments in patients who acquire COVID-19 have aggregated data on numerous classes of biologic medications, lessening the strength of data for each specific medication.
“By focusing specifically on TNF inhibitors and methotrexate, this study gives better guidance to prescribers of these medications,” he said.
To see whether TNFi or methotrexate increased risk of worsened COVID-19 outcomes, Dr. Zinn and coinvestigators evaluated data from TriNetX, a research network that includes approximately 53 million unique patient records, predominantly in the United States.
They identified 32,076 adult patients with COVID-19, of whom 214 had recent exposure to TNFi or methotrexate. The patients in the TNFi/methotrexate group were similar in age to those without exposure to those drugs, at 55.1 versus 53.2 years, respectively. However, patients in the drug exposure group were more frequently White, female, and had substantially more comorbidities, including diabetes and obesity, according to the investigators.
Nevertheless, the likelihood of hospitalization was not statistically different in the TNFi/methotrexate group versus the non-TNFi/methotrexate group, with a risk ratio of 0.91 (95% confidence interval, 0.68-1.22; P = .5260).
Likewise, the likelihood of death was not different between groups, with a RR of 0.87 (95% CI, 0.42-1.78; P = .6958). Looking at subgroups of patients exposed to TNFi or methotrexate only didn’t change the results, the investigators added.
Taken together, the findings argue against interruption of these treatments because of the fear of the possibly worse COVID-19 outcomes, the investigators concluded, although they emphasized the need for more research.
“Because the COVID-19 pandemic is ongoing, there is a desperate need for evidence-based data on biologic and immunomodulator exposure in the setting of COVID-19 infection,” they wrote.
Dr. Zinn and coauthors reported no conflicts of interest and no funding sources related to the study.
SOURCE: Zinn Z et al. J Am Acad Dermatol. 2020 Sep 11. doi: 10.1016/j.jaad.2020.09.009.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Novel calculator predicts cancer risk in patients with CVD
Individualized 10-year and lifetime risks of cancer can now for the first time be estimated in patients with established cardiovascular disease, Cilie C. van ’t Klooster, MD, reported at the virtual annual congress of the European Society of Cardiology.
She and her coinvestigators have developed an easy-to-use predictive model that generates individualized risk estimates for total cancer, lung cancer, and colorectal cancer. The tool relies on nine readily available clinical variables: age, sex, smoking, weight, height, alcohol use, diabetes, antiplatelet drug use, and C-reactive protein level. The cancer risk calculator factors in an individual’s competing risk of death because of cardiovascular disease (CVD).
The risk calculator was developed using data on 7,280 patients with established CVD enrolled in the ongoing long-term Dutch UCC-SMART (Utrecht Cardiovascular Cohort – Second Manifestations of Arterial Disease) study, then independently validated in 9,322 patients in the double-blind CANTOS (Canakinumab Anti-Inflammatory Thrombosis Outcomes) trial, explained Dr. van ’t Klooster of Utrecht (the Netherlands) University.
Several other prediction models estimate the risk of a specific type of cancer, most commonly breast cancer or lung cancer. But the new Utrecht prediction tool is the first one to estimate total cancer risk. It’s also the first to apply specifically to patients with known CVD, thus filling an unmet need, because patients with established CVD are known to be on average at 19% increased risk of total cancer and 56% greater risk for lung cancer, compared with the general population. This is thought to be caused mainly by shared risk factors, including smoking, obesity, and low-grade systemic inflammation.
As the Utrecht/CANTOS analysis shows, however, that 19% increased relative risk for cancer in patients with CVD doesn’t tell the whole story. While the median lifetime and 10-year risks of total cancer in CANTOS were 26% and 10%, respectively, the individual patient risks for total cancer estimated using the Dutch prediction model ranged from 1% to 52% for lifetime and from 1% to 31% for 10-year risk. The same was true for lung cancer risk: median 5% lifetime and 2% 10-year risks, with individual patient risks ranging from 0% to 37% and from 0% to 24%. Likewise for colorectal cancer: a median 4% lifetime risk, ranging from 0% to 6%, and a median 2% risk over the next 10 years, with personalized risks ranging as high as 13% for lifetime risk and 6% for 10-year colorectal cancer risk.
The risk calculator performed “reasonably well,” according to Dr. van ’t Klooster. She pointed to a C-statistic of 0.74 for lung cancer, 0.63 for total cancer, and 0.64 for colorectal cancer. It’s possible the risk predictor’s performance could be further enhanced by incorporation of several potentially important factors that weren’t available in the UCC-SMART derivation cohort, including race, education level, and socioeconomic status, she added.
Potential applications for the risk calculator in clinical practice require further study, but include using the lifetime risk prediction for cancer as a motivational aid in conversations with patients about the importance of behavioral change in support of a healthier lifestyle. Also, a high predicted 10-year lung cancer risk could potentially be used to lower the threshold for a screening chest CT, resulting in earlier detection and treatment of lung cancer, Dr. van ’t Klooster noted.
In an interview, Bonnie Ky, MD, MSCE, praised the risk prediction study as rigorously executed, topical, and clinically significant.
“This paper signifies the overlap between our two disciplines of cancer and cardiovascular disease in terms of the risks that we face together when we care for this patient population,” said Dr. Ky, a cardiologist at the University of Pennsylvania, Philadelphia.
“Many of us in medicine believe in the importance of risk prediction: identifying who’s at high risk and doing everything we can to mitigate that risk. This paper speaks to that and moves us one step closer to accomplishing that aim,” added Dr. Ky, who is editor in chief of JACC: CardioOncology, which published the study simultaneously with Dr. van ’t Klooster’s presentation at ESC 2020. The paper provides direct access to the risk calculator.
Dr. van ’t Klooster reported having no financial conflicts regarding her study. UCC-SMART is funded by a Utrecht University grant, and CANTOS was funded by Novartis.
SOURCE: van ’t Klooster CC. ESC 2020 and JACC CardioOncol. 2020 Aug. doi: 10.1016/j.jaccao.2020.07.001.
Individualized 10-year and lifetime risks of cancer can now for the first time be estimated in patients with established cardiovascular disease, Cilie C. van ’t Klooster, MD, reported at the virtual annual congress of the European Society of Cardiology.
She and her coinvestigators have developed an easy-to-use predictive model that generates individualized risk estimates for total cancer, lung cancer, and colorectal cancer. The tool relies on nine readily available clinical variables: age, sex, smoking, weight, height, alcohol use, diabetes, antiplatelet drug use, and C-reactive protein level. The cancer risk calculator factors in an individual’s competing risk of death because of cardiovascular disease (CVD).
The risk calculator was developed using data on 7,280 patients with established CVD enrolled in the ongoing long-term Dutch UCC-SMART (Utrecht Cardiovascular Cohort – Second Manifestations of Arterial Disease) study, then independently validated in 9,322 patients in the double-blind CANTOS (Canakinumab Anti-Inflammatory Thrombosis Outcomes) trial, explained Dr. van ’t Klooster of Utrecht (the Netherlands) University.
Several other prediction models estimate the risk of a specific type of cancer, most commonly breast cancer or lung cancer. But the new Utrecht prediction tool is the first one to estimate total cancer risk. It’s also the first to apply specifically to patients with known CVD, thus filling an unmet need, because patients with established CVD are known to be on average at 19% increased risk of total cancer and 56% greater risk for lung cancer, compared with the general population. This is thought to be caused mainly by shared risk factors, including smoking, obesity, and low-grade systemic inflammation.
As the Utrecht/CANTOS analysis shows, however, that 19% increased relative risk for cancer in patients with CVD doesn’t tell the whole story. While the median lifetime and 10-year risks of total cancer in CANTOS were 26% and 10%, respectively, the individual patient risks for total cancer estimated using the Dutch prediction model ranged from 1% to 52% for lifetime and from 1% to 31% for 10-year risk. The same was true for lung cancer risk: median 5% lifetime and 2% 10-year risks, with individual patient risks ranging from 0% to 37% and from 0% to 24%. Likewise for colorectal cancer: a median 4% lifetime risk, ranging from 0% to 6%, and a median 2% risk over the next 10 years, with personalized risks ranging as high as 13% for lifetime risk and 6% for 10-year colorectal cancer risk.
The risk calculator performed “reasonably well,” according to Dr. van ’t Klooster. She pointed to a C-statistic of 0.74 for lung cancer, 0.63 for total cancer, and 0.64 for colorectal cancer. It’s possible the risk predictor’s performance could be further enhanced by incorporation of several potentially important factors that weren’t available in the UCC-SMART derivation cohort, including race, education level, and socioeconomic status, she added.
Potential applications for the risk calculator in clinical practice require further study, but include using the lifetime risk prediction for cancer as a motivational aid in conversations with patients about the importance of behavioral change in support of a healthier lifestyle. Also, a high predicted 10-year lung cancer risk could potentially be used to lower the threshold for a screening chest CT, resulting in earlier detection and treatment of lung cancer, Dr. van ’t Klooster noted.
In an interview, Bonnie Ky, MD, MSCE, praised the risk prediction study as rigorously executed, topical, and clinically significant.
“This paper signifies the overlap between our two disciplines of cancer and cardiovascular disease in terms of the risks that we face together when we care for this patient population,” said Dr. Ky, a cardiologist at the University of Pennsylvania, Philadelphia.
“Many of us in medicine believe in the importance of risk prediction: identifying who’s at high risk and doing everything we can to mitigate that risk. This paper speaks to that and moves us one step closer to accomplishing that aim,” added Dr. Ky, who is editor in chief of JACC: CardioOncology, which published the study simultaneously with Dr. van ’t Klooster’s presentation at ESC 2020. The paper provides direct access to the risk calculator.
Dr. van ’t Klooster reported having no financial conflicts regarding her study. UCC-SMART is funded by a Utrecht University grant, and CANTOS was funded by Novartis.
SOURCE: van ’t Klooster CC. ESC 2020 and JACC CardioOncol. 2020 Aug. doi: 10.1016/j.jaccao.2020.07.001.
Individualized 10-year and lifetime risks of cancer can now for the first time be estimated in patients with established cardiovascular disease, Cilie C. van ’t Klooster, MD, reported at the virtual annual congress of the European Society of Cardiology.
She and her coinvestigators have developed an easy-to-use predictive model that generates individualized risk estimates for total cancer, lung cancer, and colorectal cancer. The tool relies on nine readily available clinical variables: age, sex, smoking, weight, height, alcohol use, diabetes, antiplatelet drug use, and C-reactive protein level. The cancer risk calculator factors in an individual’s competing risk of death because of cardiovascular disease (CVD).
The risk calculator was developed using data on 7,280 patients with established CVD enrolled in the ongoing long-term Dutch UCC-SMART (Utrecht Cardiovascular Cohort – Second Manifestations of Arterial Disease) study, then independently validated in 9,322 patients in the double-blind CANTOS (Canakinumab Anti-Inflammatory Thrombosis Outcomes) trial, explained Dr. van ’t Klooster of Utrecht (the Netherlands) University.
Several other prediction models estimate the risk of a specific type of cancer, most commonly breast cancer or lung cancer. But the new Utrecht prediction tool is the first one to estimate total cancer risk. It’s also the first to apply specifically to patients with known CVD, thus filling an unmet need, because patients with established CVD are known to be on average at 19% increased risk of total cancer and 56% greater risk for lung cancer, compared with the general population. This is thought to be caused mainly by shared risk factors, including smoking, obesity, and low-grade systemic inflammation.
As the Utrecht/CANTOS analysis shows, however, that 19% increased relative risk for cancer in patients with CVD doesn’t tell the whole story. While the median lifetime and 10-year risks of total cancer in CANTOS were 26% and 10%, respectively, the individual patient risks for total cancer estimated using the Dutch prediction model ranged from 1% to 52% for lifetime and from 1% to 31% for 10-year risk. The same was true for lung cancer risk: median 5% lifetime and 2% 10-year risks, with individual patient risks ranging from 0% to 37% and from 0% to 24%. Likewise for colorectal cancer: a median 4% lifetime risk, ranging from 0% to 6%, and a median 2% risk over the next 10 years, with personalized risks ranging as high as 13% for lifetime risk and 6% for 10-year colorectal cancer risk.
The risk calculator performed “reasonably well,” according to Dr. van ’t Klooster. She pointed to a C-statistic of 0.74 for lung cancer, 0.63 for total cancer, and 0.64 for colorectal cancer. It’s possible the risk predictor’s performance could be further enhanced by incorporation of several potentially important factors that weren’t available in the UCC-SMART derivation cohort, including race, education level, and socioeconomic status, she added.
Potential applications for the risk calculator in clinical practice require further study, but include using the lifetime risk prediction for cancer as a motivational aid in conversations with patients about the importance of behavioral change in support of a healthier lifestyle. Also, a high predicted 10-year lung cancer risk could potentially be used to lower the threshold for a screening chest CT, resulting in earlier detection and treatment of lung cancer, Dr. van ’t Klooster noted.
In an interview, Bonnie Ky, MD, MSCE, praised the risk prediction study as rigorously executed, topical, and clinically significant.
“This paper signifies the overlap between our two disciplines of cancer and cardiovascular disease in terms of the risks that we face together when we care for this patient population,” said Dr. Ky, a cardiologist at the University of Pennsylvania, Philadelphia.
“Many of us in medicine believe in the importance of risk prediction: identifying who’s at high risk and doing everything we can to mitigate that risk. This paper speaks to that and moves us one step closer to accomplishing that aim,” added Dr. Ky, who is editor in chief of JACC: CardioOncology, which published the study simultaneously with Dr. van ’t Klooster’s presentation at ESC 2020. The paper provides direct access to the risk calculator.
Dr. van ’t Klooster reported having no financial conflicts regarding her study. UCC-SMART is funded by a Utrecht University grant, and CANTOS was funded by Novartis.
SOURCE: van ’t Klooster CC. ESC 2020 and JACC CardioOncol. 2020 Aug. doi: 10.1016/j.jaccao.2020.07.001.
FROM ESC CONGRESS 2020
Physician income drops, burnout spikes globally in pandemic
according to the results of a Medscape survey.
More than 7,500 physicians – nearly 5,000 in the United States, and others in Brazil, France, Germany, Mexico, Portugal, Spain, and the United Kingdom – responded to questions about their struggles to save patients and how the pandemic has changed their income and their lives at home and at work.
The pain was evident in this response from an emergency medicine physician in Spain: “It has been the worst time in my life ever, in both my personal and professional life.”
Conversely, some reported positive effects.
An internist in Brazil wrote: “I feel more proud of my career than ever before.”
One quarter of U.S. physicians considering earlier retirement
Physicians in the United States were asked what career changes, if any, they were considering in light of their experience with COVID-19. Although a little more than half (51%) said they were not planning any changes, 25% answered, “retiring earlier than previously planned,” and 12% answered, “a career change away from medicine.”
The number of physicians reporting an income drop was highest in Brazil (63% reported a drop), followed by the United States (62%), Mexico (56%), Portugal (49%), Germany (42%), France (41%), and Spain (31%). The question was not asked in the United Kingdom survey.
In the United States, the size of the drop has been substantial: 9% lost 76%-100% of their income; 14% lost 51%-75%; 28% lost 26%-50%; 33% lost 11%-25%; and 15% lost 1%-10%.
The U.S. specialists with the largest drop in income were ophthalmologists, who lost 51%, followed by allergists (46%), plastic surgeons (46%), and otolaryngologists (45%).
“I’m looking for a new profession due to economic impact,” an otolaryngologist in the United States said. “We are at risk while essentially using our private savings to keep our practice solvent.”
More than half of U.S. physicians (54%) have personally treated patients with COVID-19. Percentages were higher in France, Spain, and the United Kingdom (percentages ranged from 60%-68%).
The United States led all eight countries in treating patients with COVID-19 via telemedicine, at 26%. Germany had the lowest telemedicine percentage, at 10%.
Burnout intensifies
About two thirds of US physicians (64%) said that burnout had intensified during the crisis (70% of female physicians and 61% of male physicians said it had).
Many factors are feeding the burnout.
A critical care physician in the United States responded, “It is terrible to see people arriving at their rooms and assuming they were going to die soon; to see people saying goodbye to their families before dying or before being intubated.”
In all eight countries, a substantial percentage of physicians reported they “sometimes, often or always” treated patients with COVID-19 without the proper personal protective equipment. Spain had by far the largest percentage who answered that way (67%), followed by France (45%), Mexico (40%), the United Kingdom (34%), Brazil and Germany (28% each); and the United States and Portugal (23% each).
A U.S. rheumatologist wrote: “The fact that we were sent to take care of infectious patients without proper protection equipment made me feel we were betrayed in this fight.”
Sense of duty to volunteer to treat COVID-19 patients varied substantially among countries, from 69% who felt that way in Spain to 40% in Brazil. Half (50%) in the United States felt that way.
“Altruism must take second place where a real and present threat exists to my own personal existence,” one U.S. internist wrote.
Numbers personally infected
One fifth of physicians in Spain and the United Kingdom had personally been infected with the virus. Brazil, France, and Mexico had the next highest numbers, with 13%-15% of physicians infected; 5%-6% in the United States, Germany, and Portugal said they had been infected.
The percentage of physicians who reported that immediate family members had been infected ranged from 25% in Spain to 6% in Portugal. Among US physicians, 9% reported that family members had been diagnosed with COVID-19.
In the United States, 44% of respondents who had family living with them at home during the pandemic reported that relationships at home were more stressed because of stay-at-home guidelines and social distancing. Almost half (47%) said there had been no change, and 9% said relationships were less stressed.
Eating is coping mechanism of choice
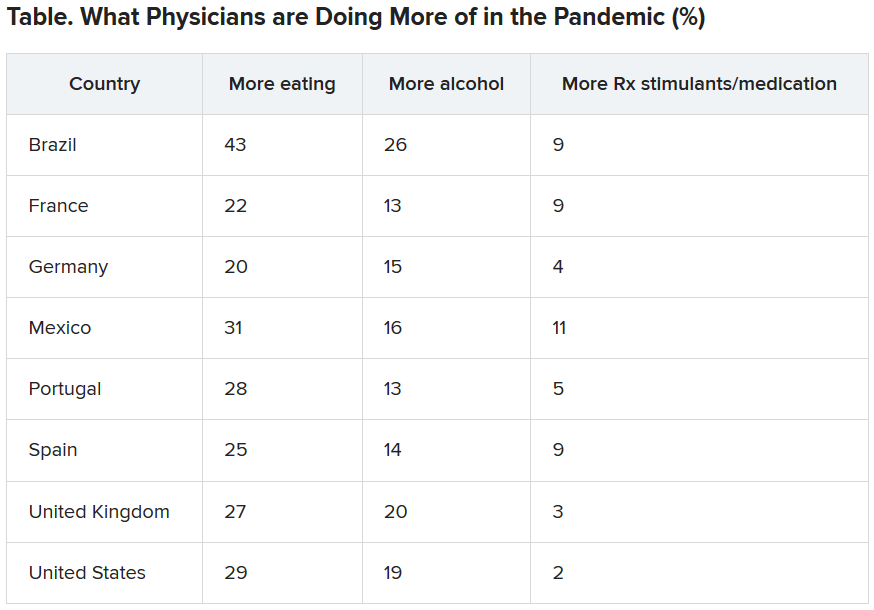
Physicians were asked what they were doing more of during the pandemic, and food seemed to be the top source of comfort in all eight countries.
Loneliness reports differ across globe
Portugal had the highest percentage (51%) of physicians reporting increased loneliness. Next were Brazil (48%), the United States (46%), the United Kingdom (42%), France (41%), Spain and Mexico (40% each), and Germany (32%).
All eight countries lacked workplace activities to help physicians with grief. More than half (55%) of U.K. physicians reported having such activities available at their workplace, whereas only 25% of physicians in Germany did; 12%-24% of respondents across the countries were unsure about the offerings.
This article first appeared on Medscape.com.
according to the results of a Medscape survey.
More than 7,500 physicians – nearly 5,000 in the United States, and others in Brazil, France, Germany, Mexico, Portugal, Spain, and the United Kingdom – responded to questions about their struggles to save patients and how the pandemic has changed their income and their lives at home and at work.
The pain was evident in this response from an emergency medicine physician in Spain: “It has been the worst time in my life ever, in both my personal and professional life.”
Conversely, some reported positive effects.
An internist in Brazil wrote: “I feel more proud of my career than ever before.”
One quarter of U.S. physicians considering earlier retirement
Physicians in the United States were asked what career changes, if any, they were considering in light of their experience with COVID-19. Although a little more than half (51%) said they were not planning any changes, 25% answered, “retiring earlier than previously planned,” and 12% answered, “a career change away from medicine.”
The number of physicians reporting an income drop was highest in Brazil (63% reported a drop), followed by the United States (62%), Mexico (56%), Portugal (49%), Germany (42%), France (41%), and Spain (31%). The question was not asked in the United Kingdom survey.
In the United States, the size of the drop has been substantial: 9% lost 76%-100% of their income; 14% lost 51%-75%; 28% lost 26%-50%; 33% lost 11%-25%; and 15% lost 1%-10%.
The U.S. specialists with the largest drop in income were ophthalmologists, who lost 51%, followed by allergists (46%), plastic surgeons (46%), and otolaryngologists (45%).
“I’m looking for a new profession due to economic impact,” an otolaryngologist in the United States said. “We are at risk while essentially using our private savings to keep our practice solvent.”
More than half of U.S. physicians (54%) have personally treated patients with COVID-19. Percentages were higher in France, Spain, and the United Kingdom (percentages ranged from 60%-68%).
The United States led all eight countries in treating patients with COVID-19 via telemedicine, at 26%. Germany had the lowest telemedicine percentage, at 10%.
Burnout intensifies
About two thirds of US physicians (64%) said that burnout had intensified during the crisis (70% of female physicians and 61% of male physicians said it had).
Many factors are feeding the burnout.
A critical care physician in the United States responded, “It is terrible to see people arriving at their rooms and assuming they were going to die soon; to see people saying goodbye to their families before dying or before being intubated.”
In all eight countries, a substantial percentage of physicians reported they “sometimes, often or always” treated patients with COVID-19 without the proper personal protective equipment. Spain had by far the largest percentage who answered that way (67%), followed by France (45%), Mexico (40%), the United Kingdom (34%), Brazil and Germany (28% each); and the United States and Portugal (23% each).
A U.S. rheumatologist wrote: “The fact that we were sent to take care of infectious patients without proper protection equipment made me feel we were betrayed in this fight.”
Sense of duty to volunteer to treat COVID-19 patients varied substantially among countries, from 69% who felt that way in Spain to 40% in Brazil. Half (50%) in the United States felt that way.
“Altruism must take second place where a real and present threat exists to my own personal existence,” one U.S. internist wrote.
Numbers personally infected
One fifth of physicians in Spain and the United Kingdom had personally been infected with the virus. Brazil, France, and Mexico had the next highest numbers, with 13%-15% of physicians infected; 5%-6% in the United States, Germany, and Portugal said they had been infected.
The percentage of physicians who reported that immediate family members had been infected ranged from 25% in Spain to 6% in Portugal. Among US physicians, 9% reported that family members had been diagnosed with COVID-19.
In the United States, 44% of respondents who had family living with them at home during the pandemic reported that relationships at home were more stressed because of stay-at-home guidelines and social distancing. Almost half (47%) said there had been no change, and 9% said relationships were less stressed.
Eating is coping mechanism of choice
Physicians were asked what they were doing more of during the pandemic, and food seemed to be the top source of comfort in all eight countries.
Loneliness reports differ across globe
Portugal had the highest percentage (51%) of physicians reporting increased loneliness. Next were Brazil (48%), the United States (46%), the United Kingdom (42%), France (41%), Spain and Mexico (40% each), and Germany (32%).
All eight countries lacked workplace activities to help physicians with grief. More than half (55%) of U.K. physicians reported having such activities available at their workplace, whereas only 25% of physicians in Germany did; 12%-24% of respondents across the countries were unsure about the offerings.
This article first appeared on Medscape.com.
according to the results of a Medscape survey.
More than 7,500 physicians – nearly 5,000 in the United States, and others in Brazil, France, Germany, Mexico, Portugal, Spain, and the United Kingdom – responded to questions about their struggles to save patients and how the pandemic has changed their income and their lives at home and at work.
The pain was evident in this response from an emergency medicine physician in Spain: “It has been the worst time in my life ever, in both my personal and professional life.”
Conversely, some reported positive effects.
An internist in Brazil wrote: “I feel more proud of my career than ever before.”
One quarter of U.S. physicians considering earlier retirement
Physicians in the United States were asked what career changes, if any, they were considering in light of their experience with COVID-19. Although a little more than half (51%) said they were not planning any changes, 25% answered, “retiring earlier than previously planned,” and 12% answered, “a career change away from medicine.”
The number of physicians reporting an income drop was highest in Brazil (63% reported a drop), followed by the United States (62%), Mexico (56%), Portugal (49%), Germany (42%), France (41%), and Spain (31%). The question was not asked in the United Kingdom survey.
In the United States, the size of the drop has been substantial: 9% lost 76%-100% of their income; 14% lost 51%-75%; 28% lost 26%-50%; 33% lost 11%-25%; and 15% lost 1%-10%.
The U.S. specialists with the largest drop in income were ophthalmologists, who lost 51%, followed by allergists (46%), plastic surgeons (46%), and otolaryngologists (45%).
“I’m looking for a new profession due to economic impact,” an otolaryngologist in the United States said. “We are at risk while essentially using our private savings to keep our practice solvent.”
More than half of U.S. physicians (54%) have personally treated patients with COVID-19. Percentages were higher in France, Spain, and the United Kingdom (percentages ranged from 60%-68%).
The United States led all eight countries in treating patients with COVID-19 via telemedicine, at 26%. Germany had the lowest telemedicine percentage, at 10%.
Burnout intensifies
About two thirds of US physicians (64%) said that burnout had intensified during the crisis (70% of female physicians and 61% of male physicians said it had).
Many factors are feeding the burnout.
A critical care physician in the United States responded, “It is terrible to see people arriving at their rooms and assuming they were going to die soon; to see people saying goodbye to their families before dying or before being intubated.”
In all eight countries, a substantial percentage of physicians reported they “sometimes, often or always” treated patients with COVID-19 without the proper personal protective equipment. Spain had by far the largest percentage who answered that way (67%), followed by France (45%), Mexico (40%), the United Kingdom (34%), Brazil and Germany (28% each); and the United States and Portugal (23% each).
A U.S. rheumatologist wrote: “The fact that we were sent to take care of infectious patients without proper protection equipment made me feel we were betrayed in this fight.”
Sense of duty to volunteer to treat COVID-19 patients varied substantially among countries, from 69% who felt that way in Spain to 40% in Brazil. Half (50%) in the United States felt that way.
“Altruism must take second place where a real and present threat exists to my own personal existence,” one U.S. internist wrote.
Numbers personally infected
One fifth of physicians in Spain and the United Kingdom had personally been infected with the virus. Brazil, France, and Mexico had the next highest numbers, with 13%-15% of physicians infected; 5%-6% in the United States, Germany, and Portugal said they had been infected.
The percentage of physicians who reported that immediate family members had been infected ranged from 25% in Spain to 6% in Portugal. Among US physicians, 9% reported that family members had been diagnosed with COVID-19.
In the United States, 44% of respondents who had family living with them at home during the pandemic reported that relationships at home were more stressed because of stay-at-home guidelines and social distancing. Almost half (47%) said there had been no change, and 9% said relationships were less stressed.
Eating is coping mechanism of choice
Physicians were asked what they were doing more of during the pandemic, and food seemed to be the top source of comfort in all eight countries.
Loneliness reports differ across globe
Portugal had the highest percentage (51%) of physicians reporting increased loneliness. Next were Brazil (48%), the United States (46%), the United Kingdom (42%), France (41%), Spain and Mexico (40% each), and Germany (32%).
All eight countries lacked workplace activities to help physicians with grief. More than half (55%) of U.K. physicians reported having such activities available at their workplace, whereas only 25% of physicians in Germany did; 12%-24% of respondents across the countries were unsure about the offerings.
This article first appeared on Medscape.com.
Infectious COVID-19 can persist in gut for weeks
Stool tests were positive among people with no GI symptoms, and in some cases up to 6 days after nasopharyngeal swabs yielded negative results.
The small pilot study suggests a quiescent but active infection in the gut. Stool testing revealed genomic evidence of active infection in 7 of the 15 participants tested in one of two hospitals in Hong Kong.
“We found active and prolonged SARS-CoV-2 infection in the stool of patients with COVID-19, even after recovery, suggesting that coronavirus could remain in the gut of asymptomatic carriers,” senior author Siew C. Ng, MBBS, PhD, told Medscape Medical News.
“Due to the potential threat of fecal-oral transmission, it is important to maintain long-term coronavirus and health surveillance,” said Ng, Associate Director of the Centre for Gut Microbiota Research at the Chinese University of Hong Kong (CUHK).
“Discharged patients and their caretakers should remain vigilant and observe strict personal and toileting hygiene,” she added.
The prospective, observational study was published online July 20 in Gut.
Ramping up COVID-19 testing
As a follow-up to these and other findings – including the testing of more than 2,000 stool samples in children and the needy arriving at Hong Kong airports starting March 29 – the same investigators are establishing a CUHK Coronavirus Testing Center.
As of Aug. 31, the detection rate in tested children was 0.28%. The Center plans to offer as many as 2,000 COVID-19 tests daily going forward to help identify asymptomatic carriers, the investigators announced in a Sept. 7 news release.
In contrast to nasopharyngeal sampling, stool specimens are “more convenient, safe and non-invasive to collect in the pediatric population,” professor Paul Chan, chairman of the Department of Microbiology, CU Medicine, said in the release. “This makes the stool test a better option for COVID-19 screening in babies, young children and those whose respiratory samples are difficult to collect.”
Even though previous researchers identified SARS-CoV-2 in the stool, the activity and infectivity of the virus in the gastrointestinal tract during and after COVID-19 respiratory positivity remained largely unknown.
Active infection detected in stool
This prospective study involved 15 people hospitalized with COVID-19 in March and April. Participants were a median 55 years old (range, 22-71 years) and all presented with respiratory symptoms. Only one patient had concurrent GI symptoms at admission. Median length of stay was 21 days.
Investigators collected fecal samples serially until discharge. They extracted viral DNA to test for transcriptional genetic evidence of active infection, which they detected in 7 of 15 patients. The patient with GI symptoms was not in this positive group.
The findings suggest a “quiescent but active GI infection,” the researchers note.
Three of the seven patients continued to test positive for active infection in their stool up to 6 days after respiratory clearance of SARS-CoV-2.
Microbiome matters
The investigators also extracted, amplified, and sequenced DNA from the stool samples. Their “metagenomic” profile revealed the type and amounts of bacterial strains in each patient’s gut microbiome.
Interestingly, bacterial strains differed between people with high SARS-CoV-2 infectivity versus participants with low to no evidence of active infection.
“Stool with high viral activity had higher abundance of pathogenic bacteria,” Ng said. In contrast, people with low or no infectivity had more beneficial bacterial strains, including bacteria that play critical roles in boosting host immunity.
Each patient’s microbiome composition changed during the course of the study. Whether the microbiome alters the course of COVID-19 or COVID-19 alters the composition of the microbiome requires further study, the authors note.
The U.S. Food and Drug Administration and officials in other countries have contacted the Hong Kong investigators for more details on their stool testing strategy, professor Francis K.L. Chan, dean of the faculty of medicine and director of the Centre for Gut Microbiota Research at CUHK, stated in the news release.
Further research into revealing the infectivity and pathogenesis of SARS-CoV-2 in the GI tract is warranted. The value of modulating the human gut microbiome in this patient population could be worthwhile to investigate as well, the researchers said.
Novel finding
“Some of it is not-so-new news and some is new,” David A. Johnson, MD, told Medscape Medical News when asked to comment on the study.
For example, previous researchers have detected SARS-CoV-2 virus in the stool. However, this study takes it a step further and shows that the virus present in stool can remain infectious on the basis of metagenomic signatures.
Furthermore, the virus can remain infectious in the gut even after a patient tests negative for COVID-19 through nasopharyngeal sampling – in this report up to 6 days later, said Johnson, professor of medicine, chief of gastroenterology, Eastern Virginia Medical School in Norfolk, Va.
The study carries important implications for people who currently test negative following active COVID-19 infection, he added. Centers for Disease Control and Prevention criteria clear a person as negative after two nasopharyngeal swabs at least 24 hours apart.
People in this category could believe they are no longer infectious and might return to a setting where they could infect others, Johnson said.
One potential means for spreading SARS-CoV-2 from the gut is from a toilet plume, as Johnson previously highlighted in a video report for Medscape Medical News.
The study authors disclosed no relevant financial relationships. Johnson serves as an adviser to WebMD/Medscape.
This article first appeared on Medscape.com.
Stool tests were positive among people with no GI symptoms, and in some cases up to 6 days after nasopharyngeal swabs yielded negative results.
The small pilot study suggests a quiescent but active infection in the gut. Stool testing revealed genomic evidence of active infection in 7 of the 15 participants tested in one of two hospitals in Hong Kong.
“We found active and prolonged SARS-CoV-2 infection in the stool of patients with COVID-19, even after recovery, suggesting that coronavirus could remain in the gut of asymptomatic carriers,” senior author Siew C. Ng, MBBS, PhD, told Medscape Medical News.
“Due to the potential threat of fecal-oral transmission, it is important to maintain long-term coronavirus and health surveillance,” said Ng, Associate Director of the Centre for Gut Microbiota Research at the Chinese University of Hong Kong (CUHK).
“Discharged patients and their caretakers should remain vigilant and observe strict personal and toileting hygiene,” she added.
The prospective, observational study was published online July 20 in Gut.
Ramping up COVID-19 testing
As a follow-up to these and other findings – including the testing of more than 2,000 stool samples in children and the needy arriving at Hong Kong airports starting March 29 – the same investigators are establishing a CUHK Coronavirus Testing Center.
As of Aug. 31, the detection rate in tested children was 0.28%. The Center plans to offer as many as 2,000 COVID-19 tests daily going forward to help identify asymptomatic carriers, the investigators announced in a Sept. 7 news release.
In contrast to nasopharyngeal sampling, stool specimens are “more convenient, safe and non-invasive to collect in the pediatric population,” professor Paul Chan, chairman of the Department of Microbiology, CU Medicine, said in the release. “This makes the stool test a better option for COVID-19 screening in babies, young children and those whose respiratory samples are difficult to collect.”
Even though previous researchers identified SARS-CoV-2 in the stool, the activity and infectivity of the virus in the gastrointestinal tract during and after COVID-19 respiratory positivity remained largely unknown.
Active infection detected in stool
This prospective study involved 15 people hospitalized with COVID-19 in March and April. Participants were a median 55 years old (range, 22-71 years) and all presented with respiratory symptoms. Only one patient had concurrent GI symptoms at admission. Median length of stay was 21 days.
Investigators collected fecal samples serially until discharge. They extracted viral DNA to test for transcriptional genetic evidence of active infection, which they detected in 7 of 15 patients. The patient with GI symptoms was not in this positive group.
The findings suggest a “quiescent but active GI infection,” the researchers note.
Three of the seven patients continued to test positive for active infection in their stool up to 6 days after respiratory clearance of SARS-CoV-2.
Microbiome matters
The investigators also extracted, amplified, and sequenced DNA from the stool samples. Their “metagenomic” profile revealed the type and amounts of bacterial strains in each patient’s gut microbiome.
Interestingly, bacterial strains differed between people with high SARS-CoV-2 infectivity versus participants with low to no evidence of active infection.
“Stool with high viral activity had higher abundance of pathogenic bacteria,” Ng said. In contrast, people with low or no infectivity had more beneficial bacterial strains, including bacteria that play critical roles in boosting host immunity.
Each patient’s microbiome composition changed during the course of the study. Whether the microbiome alters the course of COVID-19 or COVID-19 alters the composition of the microbiome requires further study, the authors note.
The U.S. Food and Drug Administration and officials in other countries have contacted the Hong Kong investigators for more details on their stool testing strategy, professor Francis K.L. Chan, dean of the faculty of medicine and director of the Centre for Gut Microbiota Research at CUHK, stated in the news release.
Further research into revealing the infectivity and pathogenesis of SARS-CoV-2 in the GI tract is warranted. The value of modulating the human gut microbiome in this patient population could be worthwhile to investigate as well, the researchers said.
Novel finding
“Some of it is not-so-new news and some is new,” David A. Johnson, MD, told Medscape Medical News when asked to comment on the study.
For example, previous researchers have detected SARS-CoV-2 virus in the stool. However, this study takes it a step further and shows that the virus present in stool can remain infectious on the basis of metagenomic signatures.
Furthermore, the virus can remain infectious in the gut even after a patient tests negative for COVID-19 through nasopharyngeal sampling – in this report up to 6 days later, said Johnson, professor of medicine, chief of gastroenterology, Eastern Virginia Medical School in Norfolk, Va.
The study carries important implications for people who currently test negative following active COVID-19 infection, he added. Centers for Disease Control and Prevention criteria clear a person as negative after two nasopharyngeal swabs at least 24 hours apart.
People in this category could believe they are no longer infectious and might return to a setting where they could infect others, Johnson said.
One potential means for spreading SARS-CoV-2 from the gut is from a toilet plume, as Johnson previously highlighted in a video report for Medscape Medical News.
The study authors disclosed no relevant financial relationships. Johnson serves as an adviser to WebMD/Medscape.
This article first appeared on Medscape.com.
Stool tests were positive among people with no GI symptoms, and in some cases up to 6 days after nasopharyngeal swabs yielded negative results.
The small pilot study suggests a quiescent but active infection in the gut. Stool testing revealed genomic evidence of active infection in 7 of the 15 participants tested in one of two hospitals in Hong Kong.
“We found active and prolonged SARS-CoV-2 infection in the stool of patients with COVID-19, even after recovery, suggesting that coronavirus could remain in the gut of asymptomatic carriers,” senior author Siew C. Ng, MBBS, PhD, told Medscape Medical News.
“Due to the potential threat of fecal-oral transmission, it is important to maintain long-term coronavirus and health surveillance,” said Ng, Associate Director of the Centre for Gut Microbiota Research at the Chinese University of Hong Kong (CUHK).
“Discharged patients and their caretakers should remain vigilant and observe strict personal and toileting hygiene,” she added.
The prospective, observational study was published online July 20 in Gut.
Ramping up COVID-19 testing
As a follow-up to these and other findings – including the testing of more than 2,000 stool samples in children and the needy arriving at Hong Kong airports starting March 29 – the same investigators are establishing a CUHK Coronavirus Testing Center.
As of Aug. 31, the detection rate in tested children was 0.28%. The Center plans to offer as many as 2,000 COVID-19 tests daily going forward to help identify asymptomatic carriers, the investigators announced in a Sept. 7 news release.
In contrast to nasopharyngeal sampling, stool specimens are “more convenient, safe and non-invasive to collect in the pediatric population,” professor Paul Chan, chairman of the Department of Microbiology, CU Medicine, said in the release. “This makes the stool test a better option for COVID-19 screening in babies, young children and those whose respiratory samples are difficult to collect.”
Even though previous researchers identified SARS-CoV-2 in the stool, the activity and infectivity of the virus in the gastrointestinal tract during and after COVID-19 respiratory positivity remained largely unknown.
Active infection detected in stool
This prospective study involved 15 people hospitalized with COVID-19 in March and April. Participants were a median 55 years old (range, 22-71 years) and all presented with respiratory symptoms. Only one patient had concurrent GI symptoms at admission. Median length of stay was 21 days.
Investigators collected fecal samples serially until discharge. They extracted viral DNA to test for transcriptional genetic evidence of active infection, which they detected in 7 of 15 patients. The patient with GI symptoms was not in this positive group.
The findings suggest a “quiescent but active GI infection,” the researchers note.
Three of the seven patients continued to test positive for active infection in their stool up to 6 days after respiratory clearance of SARS-CoV-2.
Microbiome matters
The investigators also extracted, amplified, and sequenced DNA from the stool samples. Their “metagenomic” profile revealed the type and amounts of bacterial strains in each patient’s gut microbiome.
Interestingly, bacterial strains differed between people with high SARS-CoV-2 infectivity versus participants with low to no evidence of active infection.
“Stool with high viral activity had higher abundance of pathogenic bacteria,” Ng said. In contrast, people with low or no infectivity had more beneficial bacterial strains, including bacteria that play critical roles in boosting host immunity.
Each patient’s microbiome composition changed during the course of the study. Whether the microbiome alters the course of COVID-19 or COVID-19 alters the composition of the microbiome requires further study, the authors note.
The U.S. Food and Drug Administration and officials in other countries have contacted the Hong Kong investigators for more details on their stool testing strategy, professor Francis K.L. Chan, dean of the faculty of medicine and director of the Centre for Gut Microbiota Research at CUHK, stated in the news release.
Further research into revealing the infectivity and pathogenesis of SARS-CoV-2 in the GI tract is warranted. The value of modulating the human gut microbiome in this patient population could be worthwhile to investigate as well, the researchers said.
Novel finding
“Some of it is not-so-new news and some is new,” David A. Johnson, MD, told Medscape Medical News when asked to comment on the study.
For example, previous researchers have detected SARS-CoV-2 virus in the stool. However, this study takes it a step further and shows that the virus present in stool can remain infectious on the basis of metagenomic signatures.
Furthermore, the virus can remain infectious in the gut even after a patient tests negative for COVID-19 through nasopharyngeal sampling – in this report up to 6 days later, said Johnson, professor of medicine, chief of gastroenterology, Eastern Virginia Medical School in Norfolk, Va.
The study carries important implications for people who currently test negative following active COVID-19 infection, he added. Centers for Disease Control and Prevention criteria clear a person as negative after two nasopharyngeal swabs at least 24 hours apart.
People in this category could believe they are no longer infectious and might return to a setting where they could infect others, Johnson said.
One potential means for spreading SARS-CoV-2 from the gut is from a toilet plume, as Johnson previously highlighted in a video report for Medscape Medical News.
The study authors disclosed no relevant financial relationships. Johnson serves as an adviser to WebMD/Medscape.
This article first appeared on Medscape.com.
Worry over family, friends the main driver of COVID-19 stress
Individuals are more worried about family members becoming ill with COVID-19 or about unknowingly transmitting the disease to family members than they are about contracting it themselves, results of a new survey show.
Investigators surveyed over 3,000 adults, using an online questionnaire. Of the respondents, about 20% were health care workers, and most were living in locations with active stay-at-home orders at the time of the survey.
Close to half of participants were worried about family members contracting the virus, one third were worried about unknowingly infecting others, and 20% were worried about contracting the virus themselves.
“We were a little surprised to see that people were more concerned about others than about themselves, specifically worrying about whether a family member would contract COVID-19 and whether they might unintentionally infect others,” lead author Ran Barzilay, MD, PhD, child and adolescent psychiatrist at the Children’s Hospital of Philadelphia (CHOP), told Medscape Medical News.
The study was published online August 20 in Translational Psychiatry.
Interactive platform
“The pandemic has provided a unique opportunity to study resilience in healthcare professionals and others,” said Barzilay, assistant professor at the Lifespan Brain Institute, a collaboration between CHOP and the University of Pennsylvania, under the directorship of Raquel Gur, MD, PhD.
“After the pandemic broke out in March, we launched a website in early April where we surveyed people for levels of resilience, mental health, and well-being during the outbreak,” he added.
Survey participants then shared it with their contacts.
“To date, over 7000 people have completed it – mostly from the US but also from Israel,” Barzilay said.
The survey was anonymous, but participants could choose to have follow-up contact. The survey included an interactive 21-item resilience questionnaire and an assessment of COVID-19-related items related to worries concerning the following: contracting, dying from, or currently having the illness; having a family member contract the illness; unknowingly infecting others; and experiencing significant financial burden.
A total of 1350 participants took a second survey on anxiety and depression that utilized the Generalized Anxiety Disorder–7 and the Patient Health Questionnaire–2.
“What makes the survey unique is that it’s not just a means of collecting data but also an interactive platform that gives participants immediate personalized feedback, based on their responses to the resilience and well-being surveys, with practical tips and recommendations for stress management and ways of boosting resilience,” Barzilay said.
Tend and befriend
Ten days into the survey, data were available on 3,042 participants (64% women, 54% with advanced education, 20.5% health care providers), who ranged in age from 18 to 70 years (mean [SD], 38.9 [11.9] years).
After accounting for covariates, the researchers found that participants reported more distress about family members contracting COVID-19 and about unknowingly infecting others than about getting COVID-19 themselves (48.5% and 36% vs. 19.9%, respectively; P < .0005).
Increased COVID-19-related worries were associated with 22% higher anxiety and 16.1% higher depression scores; women had higher scores than men on both.
Each 1-SD increase in the composite score of COVID-19 worries was associated with over twice the increased probability of generalized anxiety and depression (odds ratio, 2.23; 95% confidence interval, 1.88-2.65; and OR, 1.67; 95% CI, 1.41-1.98, respectively; for both, P < .001).
On the other hand, for every 1-SD increase in the resilience score, there was a 64.9% decrease in the possibility of screening positive for generalized anxiety disorder and a 69.3% decrease in the possibility of screening positive for depression (for both, P < .0001).
Compared to participants from Israel, US participants were “more stressed” about contracting, dying from, and currently having COVID-19 themselves. Overall, Israeli participants scored higher than US participants on the resilience scale.
Rates of anxiety and depression did not differ significantly between healthcare providers and others. Health care providers worried more about contracting COVID-19 themselves and worried less about finances after COVID-19.
The authors propose that survey participants were more worried about others than about themselves because of “prosocial behavior under stress” and “tend-and-befriend,” whereby, “in response to threat, humans tend to protect their close ones (tending) and seek out their social group for mutual defense (befriending).”
This type of altruistic behavior has been “described in acute situations throughout history” and has been “linked to mechanisms of resilience for overcoming adversity,” the authors indicate.
Demographic biases
Commenting on the findings for Medscape Medical News, Golnaz Tabibnia, PhD, a neuroscientist at the University of California, Irvine, who was not involved in the research, suggested that although higher resilience scores were associated with lower COVID-related worries, it is possible, “as the authors suggest, that having more resilience resources makes you less worried, but the causality could go the other direction as well, and less worry/rumination may lead to more resilience.”
Also commenting on the study for Medscape Medical News, Christiaan Vinkers, MD, PhD, a psychiatrist at the Amsterdam University Medical Center, Amsterdam, the Netherlands, said it was noteworthy that healthcare providers reported similar levels of mood and anxiety symptoms, compared to others.
“This is encouraging, as it suggests adequate resilience levels in professionals who work in the front lines of the COVID-19 pandemic,” he said.
Resilience occurs not only at the individual level but also at the community level, which may help explain the striking differences in COVID-19-related worries and anxiety between participants from the United States and Israel, Vinkers added.
E. Alison Holman, PhD, professor, Sue and Bill Gross School of Nursing, University of California, Irvine, noted that respondents were predominantly white, female, and had relatively high incomes, “suggesting strong demographic biases in those who chose to participate.”
Holman, who was not involved with the study, told Medscape Medical News that the “findings do not address the real impact of COVID-19 on the hardest-hit communities in America – poor, Black, and Latinx communities, where a large proportion of essential workers live.”
Barzilay acknowledged that, “unfortunately, because of the way the study was circulated, it did not reach minorities, which is one of the things we want to improve.”
The study is ongoing and has been translated into Spanish, French, and Hebrew. The team plans to collect data on diverse populations.
The study was supported by grants from the National Institute of Mental Health, the Lifespan Brain Institute of Children’s Hospital of Philadelphia, Penn Medicine, the University of Pennsylvania, and in part by the Zuckerman STEM Leadership Program. Barzilay serves on the scientific board and reports stock ownership in Taliaz Health. The other authors, Golnaz, Vinkers, and Holman have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Individuals are more worried about family members becoming ill with COVID-19 or about unknowingly transmitting the disease to family members than they are about contracting it themselves, results of a new survey show.
Investigators surveyed over 3,000 adults, using an online questionnaire. Of the respondents, about 20% were health care workers, and most were living in locations with active stay-at-home orders at the time of the survey.
Close to half of participants were worried about family members contracting the virus, one third were worried about unknowingly infecting others, and 20% were worried about contracting the virus themselves.
“We were a little surprised to see that people were more concerned about others than about themselves, specifically worrying about whether a family member would contract COVID-19 and whether they might unintentionally infect others,” lead author Ran Barzilay, MD, PhD, child and adolescent psychiatrist at the Children’s Hospital of Philadelphia (CHOP), told Medscape Medical News.
The study was published online August 20 in Translational Psychiatry.
Interactive platform
“The pandemic has provided a unique opportunity to study resilience in healthcare professionals and others,” said Barzilay, assistant professor at the Lifespan Brain Institute, a collaboration between CHOP and the University of Pennsylvania, under the directorship of Raquel Gur, MD, PhD.
“After the pandemic broke out in March, we launched a website in early April where we surveyed people for levels of resilience, mental health, and well-being during the outbreak,” he added.
Survey participants then shared it with their contacts.
“To date, over 7000 people have completed it – mostly from the US but also from Israel,” Barzilay said.
The survey was anonymous, but participants could choose to have follow-up contact. The survey included an interactive 21-item resilience questionnaire and an assessment of COVID-19-related items related to worries concerning the following: contracting, dying from, or currently having the illness; having a family member contract the illness; unknowingly infecting others; and experiencing significant financial burden.
A total of 1350 participants took a second survey on anxiety and depression that utilized the Generalized Anxiety Disorder–7 and the Patient Health Questionnaire–2.
“What makes the survey unique is that it’s not just a means of collecting data but also an interactive platform that gives participants immediate personalized feedback, based on their responses to the resilience and well-being surveys, with practical tips and recommendations for stress management and ways of boosting resilience,” Barzilay said.
Tend and befriend
Ten days into the survey, data were available on 3,042 participants (64% women, 54% with advanced education, 20.5% health care providers), who ranged in age from 18 to 70 years (mean [SD], 38.9 [11.9] years).
After accounting for covariates, the researchers found that participants reported more distress about family members contracting COVID-19 and about unknowingly infecting others than about getting COVID-19 themselves (48.5% and 36% vs. 19.9%, respectively; P < .0005).
Increased COVID-19-related worries were associated with 22% higher anxiety and 16.1% higher depression scores; women had higher scores than men on both.
Each 1-SD increase in the composite score of COVID-19 worries was associated with over twice the increased probability of generalized anxiety and depression (odds ratio, 2.23; 95% confidence interval, 1.88-2.65; and OR, 1.67; 95% CI, 1.41-1.98, respectively; for both, P < .001).
On the other hand, for every 1-SD increase in the resilience score, there was a 64.9% decrease in the possibility of screening positive for generalized anxiety disorder and a 69.3% decrease in the possibility of screening positive for depression (for both, P < .0001).
Compared to participants from Israel, US participants were “more stressed” about contracting, dying from, and currently having COVID-19 themselves. Overall, Israeli participants scored higher than US participants on the resilience scale.
Rates of anxiety and depression did not differ significantly between healthcare providers and others. Health care providers worried more about contracting COVID-19 themselves and worried less about finances after COVID-19.
The authors propose that survey participants were more worried about others than about themselves because of “prosocial behavior under stress” and “tend-and-befriend,” whereby, “in response to threat, humans tend to protect their close ones (tending) and seek out their social group for mutual defense (befriending).”
This type of altruistic behavior has been “described in acute situations throughout history” and has been “linked to mechanisms of resilience for overcoming adversity,” the authors indicate.
Demographic biases
Commenting on the findings for Medscape Medical News, Golnaz Tabibnia, PhD, a neuroscientist at the University of California, Irvine, who was not involved in the research, suggested that although higher resilience scores were associated with lower COVID-related worries, it is possible, “as the authors suggest, that having more resilience resources makes you less worried, but the causality could go the other direction as well, and less worry/rumination may lead to more resilience.”
Also commenting on the study for Medscape Medical News, Christiaan Vinkers, MD, PhD, a psychiatrist at the Amsterdam University Medical Center, Amsterdam, the Netherlands, said it was noteworthy that healthcare providers reported similar levels of mood and anxiety symptoms, compared to others.
“This is encouraging, as it suggests adequate resilience levels in professionals who work in the front lines of the COVID-19 pandemic,” he said.
Resilience occurs not only at the individual level but also at the community level, which may help explain the striking differences in COVID-19-related worries and anxiety between participants from the United States and Israel, Vinkers added.
E. Alison Holman, PhD, professor, Sue and Bill Gross School of Nursing, University of California, Irvine, noted that respondents were predominantly white, female, and had relatively high incomes, “suggesting strong demographic biases in those who chose to participate.”
Holman, who was not involved with the study, told Medscape Medical News that the “findings do not address the real impact of COVID-19 on the hardest-hit communities in America – poor, Black, and Latinx communities, where a large proportion of essential workers live.”
Barzilay acknowledged that, “unfortunately, because of the way the study was circulated, it did not reach minorities, which is one of the things we want to improve.”
The study is ongoing and has been translated into Spanish, French, and Hebrew. The team plans to collect data on diverse populations.
The study was supported by grants from the National Institute of Mental Health, the Lifespan Brain Institute of Children’s Hospital of Philadelphia, Penn Medicine, the University of Pennsylvania, and in part by the Zuckerman STEM Leadership Program. Barzilay serves on the scientific board and reports stock ownership in Taliaz Health. The other authors, Golnaz, Vinkers, and Holman have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Individuals are more worried about family members becoming ill with COVID-19 or about unknowingly transmitting the disease to family members than they are about contracting it themselves, results of a new survey show.
Investigators surveyed over 3,000 adults, using an online questionnaire. Of the respondents, about 20% were health care workers, and most were living in locations with active stay-at-home orders at the time of the survey.
Close to half of participants were worried about family members contracting the virus, one third were worried about unknowingly infecting others, and 20% were worried about contracting the virus themselves.
“We were a little surprised to see that people were more concerned about others than about themselves, specifically worrying about whether a family member would contract COVID-19 and whether they might unintentionally infect others,” lead author Ran Barzilay, MD, PhD, child and adolescent psychiatrist at the Children’s Hospital of Philadelphia (CHOP), told Medscape Medical News.
The study was published online August 20 in Translational Psychiatry.
Interactive platform
“The pandemic has provided a unique opportunity to study resilience in healthcare professionals and others,” said Barzilay, assistant professor at the Lifespan Brain Institute, a collaboration between CHOP and the University of Pennsylvania, under the directorship of Raquel Gur, MD, PhD.
“After the pandemic broke out in March, we launched a website in early April where we surveyed people for levels of resilience, mental health, and well-being during the outbreak,” he added.
Survey participants then shared it with their contacts.
“To date, over 7000 people have completed it – mostly from the US but also from Israel,” Barzilay said.
The survey was anonymous, but participants could choose to have follow-up contact. The survey included an interactive 21-item resilience questionnaire and an assessment of COVID-19-related items related to worries concerning the following: contracting, dying from, or currently having the illness; having a family member contract the illness; unknowingly infecting others; and experiencing significant financial burden.
A total of 1350 participants took a second survey on anxiety and depression that utilized the Generalized Anxiety Disorder–7 and the Patient Health Questionnaire–2.
“What makes the survey unique is that it’s not just a means of collecting data but also an interactive platform that gives participants immediate personalized feedback, based on their responses to the resilience and well-being surveys, with practical tips and recommendations for stress management and ways of boosting resilience,” Barzilay said.
Tend and befriend
Ten days into the survey, data were available on 3,042 participants (64% women, 54% with advanced education, 20.5% health care providers), who ranged in age from 18 to 70 years (mean [SD], 38.9 [11.9] years).
After accounting for covariates, the researchers found that participants reported more distress about family members contracting COVID-19 and about unknowingly infecting others than about getting COVID-19 themselves (48.5% and 36% vs. 19.9%, respectively; P < .0005).
Increased COVID-19-related worries were associated with 22% higher anxiety and 16.1% higher depression scores; women had higher scores than men on both.
Each 1-SD increase in the composite score of COVID-19 worries was associated with over twice the increased probability of generalized anxiety and depression (odds ratio, 2.23; 95% confidence interval, 1.88-2.65; and OR, 1.67; 95% CI, 1.41-1.98, respectively; for both, P < .001).
On the other hand, for every 1-SD increase in the resilience score, there was a 64.9% decrease in the possibility of screening positive for generalized anxiety disorder and a 69.3% decrease in the possibility of screening positive for depression (for both, P < .0001).
Compared to participants from Israel, US participants were “more stressed” about contracting, dying from, and currently having COVID-19 themselves. Overall, Israeli participants scored higher than US participants on the resilience scale.
Rates of anxiety and depression did not differ significantly between healthcare providers and others. Health care providers worried more about contracting COVID-19 themselves and worried less about finances after COVID-19.
The authors propose that survey participants were more worried about others than about themselves because of “prosocial behavior under stress” and “tend-and-befriend,” whereby, “in response to threat, humans tend to protect their close ones (tending) and seek out their social group for mutual defense (befriending).”
This type of altruistic behavior has been “described in acute situations throughout history” and has been “linked to mechanisms of resilience for overcoming adversity,” the authors indicate.
Demographic biases
Commenting on the findings for Medscape Medical News, Golnaz Tabibnia, PhD, a neuroscientist at the University of California, Irvine, who was not involved in the research, suggested that although higher resilience scores were associated with lower COVID-related worries, it is possible, “as the authors suggest, that having more resilience resources makes you less worried, but the causality could go the other direction as well, and less worry/rumination may lead to more resilience.”
Also commenting on the study for Medscape Medical News, Christiaan Vinkers, MD, PhD, a psychiatrist at the Amsterdam University Medical Center, Amsterdam, the Netherlands, said it was noteworthy that healthcare providers reported similar levels of mood and anxiety symptoms, compared to others.
“This is encouraging, as it suggests adequate resilience levels in professionals who work in the front lines of the COVID-19 pandemic,” he said.
Resilience occurs not only at the individual level but also at the community level, which may help explain the striking differences in COVID-19-related worries and anxiety between participants from the United States and Israel, Vinkers added.
E. Alison Holman, PhD, professor, Sue and Bill Gross School of Nursing, University of California, Irvine, noted that respondents were predominantly white, female, and had relatively high incomes, “suggesting strong demographic biases in those who chose to participate.”
Holman, who was not involved with the study, told Medscape Medical News that the “findings do not address the real impact of COVID-19 on the hardest-hit communities in America – poor, Black, and Latinx communities, where a large proportion of essential workers live.”
Barzilay acknowledged that, “unfortunately, because of the way the study was circulated, it did not reach minorities, which is one of the things we want to improve.”
The study is ongoing and has been translated into Spanish, French, and Hebrew. The team plans to collect data on diverse populations.
The study was supported by grants from the National Institute of Mental Health, the Lifespan Brain Institute of Children’s Hospital of Philadelphia, Penn Medicine, the University of Pennsylvania, and in part by the Zuckerman STEM Leadership Program. Barzilay serves on the scientific board and reports stock ownership in Taliaz Health. The other authors, Golnaz, Vinkers, and Holman have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
The earlier the better for colchicine post-MI: COLCOT
The earlier the anti-inflammatory drug colchicine is initiated after a myocardial infarction (MI) the greater the benefit, a new COLCOT analysis suggests.
The parent trial was conducted in patients with a recent MI because of the intense inflammation present at that time, and added colchicine 0.5 mg daily to standard care within 30 days following MI.
As previously reported, colchicine significantly reduced the risk of the primary end point – a composite of cardiovascular (CV) death, resuscitated cardiac arrest, MI, stroke, or urgent hospitalization for angina requiring revascularization – by 23% compared with placebo.
This new analysis shows the risk was reduced by 48% in patients receiving colchicine within 3 days of an MI (4.3% vs. 8.3%; adjusted hazard ratio, 0.52; 95% confidence interval, 0.32-0.84, P = .007).
Risk of a secondary efficacy end point – CV death, resuscitated cardiac arrest, MI, or stroke – was reduced by 45% over an average follow up of 22.7 months (3.3% vs 6.1%; adjusted HR, 0.55; 95% CI, 0.32-0.95, P = .031).
“We believe that our results support an early, in-hospital initiation of adjunctive colchicine for post-MI prevention,” Nadia Bouabdallaoui, MD, Montreal Heart Institute, Quebec, Canada, said during an online session devoted to colchicine at the European Society of Cardiology Congress 2020.
Session moderator Massimo Imazio, MD, professor of cardiology at the University of Turin, Italy, said the improved outcomes suggest that earlier treatment is better – a finding that parallels his own experience using colchicine in patients with pericarditis.
“This substudy is very important because this is probably also the year in cardiovascular applications [that] early use of the drug could improve outcomes,” he said.
Positive data have been accumulating for colchicine from COLCOT, LoDoCo, and, most recently, the LoDoCo2 trial, even as another anti-inflammatory drug, methotrexate, flamed out as secondary prevention in the CIRT trial.
The new COLCOT substudy included 4,661 of the 4,745 original patients and examined treatment initiation using three strata: within 0-3 days (n = 1,193), 4-7 days (n = 720), and 8-30 days (n = 2,748). Patients who received treatment within 3 days were slightly younger, more likely to be smokers, and to have a shorter time from MI to randomization (2.1 days vs 5.1 days vs. 20.8 days, respectively).
In the subset receiving treatment within 3 days, those assigned to colchicine had the same number of cardiac deaths as those given placebo (2 vs. 2) but fewer resuscitated cardiac arrests (1 vs. 3), MIs (17 vs. 29), strokes (1 vs. 5), and urgent hospitalizations for angina requiring revascularization (6 vs. 17).
“A larger trial might have allowed for a better assessment of individual endpoints and subgroups,” observed Bouabdallaoui.
Although there is growing support for colchicine, experts caution that the drug many not be for everyone. In COLCOT, 1 in 10 patients were unable to tolerate the drug, largely because of gastrointestinal (GI) issues.
Pharmacogenomics substudy
A second COLCOT substudy aimed to identify genetic markers predictive of colchicine response and to gain insights into the mechanisms behind this response. It included 767 patients treated with colchicine and another 755 treated with placebo – or about one-third the patients in the original trial.
A genome-wide association study did not find a significant association for the primary CV endpoint, although a prespecified subgroup analysis in men identified an interesting region on chromosome 9 (variant: rs10811106), which just missed reaching genomewide significance, said Marie-Pierre Dubé, PhD, director of the Université de Montréal Beaulieu-Saucier Pharmacogenomics Centre at the Montreal Heart Institute.
In addition, the genomewide analysis found two significant regions for GI events: one on chromosome 6 (variant: rs6916345) and one on chromosome 10 (variant: rs74795203).
For each of the identified regions, the researchers then tested the effect of the allele in the placebo group and the interaction between the genetic variant and treatment with colchicine. For the chromosome 9 region in males, there was no effect in the placebo group and a significant interaction in the colchicine group.
For the significant GI event findings, there was a small effect for the chromosome 6 region in the placebo group and a very significant interaction with colchicine, Dubé said. Similarly, there was no effect for the chromosome 10 region in the placebo group and a significant interaction with colchicine.
Additional analyses in stratified patient populations showed that males with the protective allele (CC) for the chromosome 9 region represented 83% of the population. The primary CV endpoint occurred in 3.2% of these men treated with colchicine and 6.3% treated with placebo (HR, 0.46; 95% CI, 0.24 - 0.86).
For the gastrointestinal events, 25% of patients carried the risk allele (AA) for the chromosome 6 region and 36.9% of these had GI events when treated with colchicine versus 18.6% when treated with placebo (HR, 2.42; 95% CI, 1.57-3.72).
Similarly, 13% of individuals carried one or two copies of the risk allele (AG+GG) for the chromosome 10 region and the risk of GI events in these was nearly four times higher with colchicine (47.1% vs. 18.9%; HR, 3.98; 95% CI 2.24-7.07).
Functional genomic analyses of the identified regions were also performed and showed that the chromosome 9 locus overlaps with the SAXO1 gene, a stabilizer of axonemal microtubules 1.
“The leading variant at this locus (rs10811106 C allele) correlated with the expression of the HAUS6 gene, which is involved in microtubule generation from existing microtubules, and may interact with the effect of colchicine, which is known to inhibit microtubule formation,” observed Dubé.
Also, the chromosome 6 locus associated with gastrointestinal events was colocalizing with the Crohn’s disease locus, adding further support for this region.
“The results support potential personalized approaches to inflammation reduction for cardiovascular prevention,” Dubé said.
This is a post hoc subgroup analysis, however, and replication is necessary, ideally in prospective randomized trials, she noted.
The substudy is important because it provides further insights into the link between colchicine and microtubule polymerization, affecting the activation of the inflammasome, session moderator Imazio said.
“Second, it is important because pharmacogenomics can help us to better understand the optimal responder to colchicine and colchicine resistance,” he said. “So it can be useful for personalized medicine, leading to the proper use of the drug for the proper patient.”
COLCOT was supported by the government of Quebec, the Canadian Institutes of Health Research, and philanthropic foundations. Bouabdallaoui has disclosed no relevant financial relationships. Dubé reported grants from the government of Quebec; personal fees from DalCor and GlaxoSmithKline; research support from AstraZeneca, Pfizer, Servier, Sanofi; and minor equity interest in DalCor. Dubé is also coauthor of patents on pharmacogenomics-guided CETP inhibition, and pharmacogenomics markers of response to colchicine.
This article first appeared on Medscape.com.
The earlier the anti-inflammatory drug colchicine is initiated after a myocardial infarction (MI) the greater the benefit, a new COLCOT analysis suggests.
The parent trial was conducted in patients with a recent MI because of the intense inflammation present at that time, and added colchicine 0.5 mg daily to standard care within 30 days following MI.
As previously reported, colchicine significantly reduced the risk of the primary end point – a composite of cardiovascular (CV) death, resuscitated cardiac arrest, MI, stroke, or urgent hospitalization for angina requiring revascularization – by 23% compared with placebo.
This new analysis shows the risk was reduced by 48% in patients receiving colchicine within 3 days of an MI (4.3% vs. 8.3%; adjusted hazard ratio, 0.52; 95% confidence interval, 0.32-0.84, P = .007).
Risk of a secondary efficacy end point – CV death, resuscitated cardiac arrest, MI, or stroke – was reduced by 45% over an average follow up of 22.7 months (3.3% vs 6.1%; adjusted HR, 0.55; 95% CI, 0.32-0.95, P = .031).
“We believe that our results support an early, in-hospital initiation of adjunctive colchicine for post-MI prevention,” Nadia Bouabdallaoui, MD, Montreal Heart Institute, Quebec, Canada, said during an online session devoted to colchicine at the European Society of Cardiology Congress 2020.
Session moderator Massimo Imazio, MD, professor of cardiology at the University of Turin, Italy, said the improved outcomes suggest that earlier treatment is better – a finding that parallels his own experience using colchicine in patients with pericarditis.
“This substudy is very important because this is probably also the year in cardiovascular applications [that] early use of the drug could improve outcomes,” he said.
Positive data have been accumulating for colchicine from COLCOT, LoDoCo, and, most recently, the LoDoCo2 trial, even as another anti-inflammatory drug, methotrexate, flamed out as secondary prevention in the CIRT trial.
The new COLCOT substudy included 4,661 of the 4,745 original patients and examined treatment initiation using three strata: within 0-3 days (n = 1,193), 4-7 days (n = 720), and 8-30 days (n = 2,748). Patients who received treatment within 3 days were slightly younger, more likely to be smokers, and to have a shorter time from MI to randomization (2.1 days vs 5.1 days vs. 20.8 days, respectively).
In the subset receiving treatment within 3 days, those assigned to colchicine had the same number of cardiac deaths as those given placebo (2 vs. 2) but fewer resuscitated cardiac arrests (1 vs. 3), MIs (17 vs. 29), strokes (1 vs. 5), and urgent hospitalizations for angina requiring revascularization (6 vs. 17).
“A larger trial might have allowed for a better assessment of individual endpoints and subgroups,” observed Bouabdallaoui.
Although there is growing support for colchicine, experts caution that the drug many not be for everyone. In COLCOT, 1 in 10 patients were unable to tolerate the drug, largely because of gastrointestinal (GI) issues.
Pharmacogenomics substudy
A second COLCOT substudy aimed to identify genetic markers predictive of colchicine response and to gain insights into the mechanisms behind this response. It included 767 patients treated with colchicine and another 755 treated with placebo – or about one-third the patients in the original trial.
A genome-wide association study did not find a significant association for the primary CV endpoint, although a prespecified subgroup analysis in men identified an interesting region on chromosome 9 (variant: rs10811106), which just missed reaching genomewide significance, said Marie-Pierre Dubé, PhD, director of the Université de Montréal Beaulieu-Saucier Pharmacogenomics Centre at the Montreal Heart Institute.
In addition, the genomewide analysis found two significant regions for GI events: one on chromosome 6 (variant: rs6916345) and one on chromosome 10 (variant: rs74795203).
For each of the identified regions, the researchers then tested the effect of the allele in the placebo group and the interaction between the genetic variant and treatment with colchicine. For the chromosome 9 region in males, there was no effect in the placebo group and a significant interaction in the colchicine group.
For the significant GI event findings, there was a small effect for the chromosome 6 region in the placebo group and a very significant interaction with colchicine, Dubé said. Similarly, there was no effect for the chromosome 10 region in the placebo group and a significant interaction with colchicine.
Additional analyses in stratified patient populations showed that males with the protective allele (CC) for the chromosome 9 region represented 83% of the population. The primary CV endpoint occurred in 3.2% of these men treated with colchicine and 6.3% treated with placebo (HR, 0.46; 95% CI, 0.24 - 0.86).
For the gastrointestinal events, 25% of patients carried the risk allele (AA) for the chromosome 6 region and 36.9% of these had GI events when treated with colchicine versus 18.6% when treated with placebo (HR, 2.42; 95% CI, 1.57-3.72).
Similarly, 13% of individuals carried one or two copies of the risk allele (AG+GG) for the chromosome 10 region and the risk of GI events in these was nearly four times higher with colchicine (47.1% vs. 18.9%; HR, 3.98; 95% CI 2.24-7.07).
Functional genomic analyses of the identified regions were also performed and showed that the chromosome 9 locus overlaps with the SAXO1 gene, a stabilizer of axonemal microtubules 1.
“The leading variant at this locus (rs10811106 C allele) correlated with the expression of the HAUS6 gene, which is involved in microtubule generation from existing microtubules, and may interact with the effect of colchicine, which is known to inhibit microtubule formation,” observed Dubé.
Also, the chromosome 6 locus associated with gastrointestinal events was colocalizing with the Crohn’s disease locus, adding further support for this region.
“The results support potential personalized approaches to inflammation reduction for cardiovascular prevention,” Dubé said.
This is a post hoc subgroup analysis, however, and replication is necessary, ideally in prospective randomized trials, she noted.
The substudy is important because it provides further insights into the link between colchicine and microtubule polymerization, affecting the activation of the inflammasome, session moderator Imazio said.
“Second, it is important because pharmacogenomics can help us to better understand the optimal responder to colchicine and colchicine resistance,” he said. “So it can be useful for personalized medicine, leading to the proper use of the drug for the proper patient.”
COLCOT was supported by the government of Quebec, the Canadian Institutes of Health Research, and philanthropic foundations. Bouabdallaoui has disclosed no relevant financial relationships. Dubé reported grants from the government of Quebec; personal fees from DalCor and GlaxoSmithKline; research support from AstraZeneca, Pfizer, Servier, Sanofi; and minor equity interest in DalCor. Dubé is also coauthor of patents on pharmacogenomics-guided CETP inhibition, and pharmacogenomics markers of response to colchicine.
This article first appeared on Medscape.com.
The earlier the anti-inflammatory drug colchicine is initiated after a myocardial infarction (MI) the greater the benefit, a new COLCOT analysis suggests.
The parent trial was conducted in patients with a recent MI because of the intense inflammation present at that time, and added colchicine 0.5 mg daily to standard care within 30 days following MI.
As previously reported, colchicine significantly reduced the risk of the primary end point – a composite of cardiovascular (CV) death, resuscitated cardiac arrest, MI, stroke, or urgent hospitalization for angina requiring revascularization – by 23% compared with placebo.
This new analysis shows the risk was reduced by 48% in patients receiving colchicine within 3 days of an MI (4.3% vs. 8.3%; adjusted hazard ratio, 0.52; 95% confidence interval, 0.32-0.84, P = .007).
Risk of a secondary efficacy end point – CV death, resuscitated cardiac arrest, MI, or stroke – was reduced by 45% over an average follow up of 22.7 months (3.3% vs 6.1%; adjusted HR, 0.55; 95% CI, 0.32-0.95, P = .031).
“We believe that our results support an early, in-hospital initiation of adjunctive colchicine for post-MI prevention,” Nadia Bouabdallaoui, MD, Montreal Heart Institute, Quebec, Canada, said during an online session devoted to colchicine at the European Society of Cardiology Congress 2020.
Session moderator Massimo Imazio, MD, professor of cardiology at the University of Turin, Italy, said the improved outcomes suggest that earlier treatment is better – a finding that parallels his own experience using colchicine in patients with pericarditis.
“This substudy is very important because this is probably also the year in cardiovascular applications [that] early use of the drug could improve outcomes,” he said.
Positive data have been accumulating for colchicine from COLCOT, LoDoCo, and, most recently, the LoDoCo2 trial, even as another anti-inflammatory drug, methotrexate, flamed out as secondary prevention in the CIRT trial.
The new COLCOT substudy included 4,661 of the 4,745 original patients and examined treatment initiation using three strata: within 0-3 days (n = 1,193), 4-7 days (n = 720), and 8-30 days (n = 2,748). Patients who received treatment within 3 days were slightly younger, more likely to be smokers, and to have a shorter time from MI to randomization (2.1 days vs 5.1 days vs. 20.8 days, respectively).
In the subset receiving treatment within 3 days, those assigned to colchicine had the same number of cardiac deaths as those given placebo (2 vs. 2) but fewer resuscitated cardiac arrests (1 vs. 3), MIs (17 vs. 29), strokes (1 vs. 5), and urgent hospitalizations for angina requiring revascularization (6 vs. 17).
“A larger trial might have allowed for a better assessment of individual endpoints and subgroups,” observed Bouabdallaoui.
Although there is growing support for colchicine, experts caution that the drug many not be for everyone. In COLCOT, 1 in 10 patients were unable to tolerate the drug, largely because of gastrointestinal (GI) issues.
Pharmacogenomics substudy
A second COLCOT substudy aimed to identify genetic markers predictive of colchicine response and to gain insights into the mechanisms behind this response. It included 767 patients treated with colchicine and another 755 treated with placebo – or about one-third the patients in the original trial.
A genome-wide association study did not find a significant association for the primary CV endpoint, although a prespecified subgroup analysis in men identified an interesting region on chromosome 9 (variant: rs10811106), which just missed reaching genomewide significance, said Marie-Pierre Dubé, PhD, director of the Université de Montréal Beaulieu-Saucier Pharmacogenomics Centre at the Montreal Heart Institute.
In addition, the genomewide analysis found two significant regions for GI events: one on chromosome 6 (variant: rs6916345) and one on chromosome 10 (variant: rs74795203).
For each of the identified regions, the researchers then tested the effect of the allele in the placebo group and the interaction between the genetic variant and treatment with colchicine. For the chromosome 9 region in males, there was no effect in the placebo group and a significant interaction in the colchicine group.
For the significant GI event findings, there was a small effect for the chromosome 6 region in the placebo group and a very significant interaction with colchicine, Dubé said. Similarly, there was no effect for the chromosome 10 region in the placebo group and a significant interaction with colchicine.
Additional analyses in stratified patient populations showed that males with the protective allele (CC) for the chromosome 9 region represented 83% of the population. The primary CV endpoint occurred in 3.2% of these men treated with colchicine and 6.3% treated with placebo (HR, 0.46; 95% CI, 0.24 - 0.86).
For the gastrointestinal events, 25% of patients carried the risk allele (AA) for the chromosome 6 region and 36.9% of these had GI events when treated with colchicine versus 18.6% when treated with placebo (HR, 2.42; 95% CI, 1.57-3.72).
Similarly, 13% of individuals carried one or two copies of the risk allele (AG+GG) for the chromosome 10 region and the risk of GI events in these was nearly four times higher with colchicine (47.1% vs. 18.9%; HR, 3.98; 95% CI 2.24-7.07).
Functional genomic analyses of the identified regions were also performed and showed that the chromosome 9 locus overlaps with the SAXO1 gene, a stabilizer of axonemal microtubules 1.
“The leading variant at this locus (rs10811106 C allele) correlated with the expression of the HAUS6 gene, which is involved in microtubule generation from existing microtubules, and may interact with the effect of colchicine, which is known to inhibit microtubule formation,” observed Dubé.
Also, the chromosome 6 locus associated with gastrointestinal events was colocalizing with the Crohn’s disease locus, adding further support for this region.
“The results support potential personalized approaches to inflammation reduction for cardiovascular prevention,” Dubé said.
This is a post hoc subgroup analysis, however, and replication is necessary, ideally in prospective randomized trials, she noted.
The substudy is important because it provides further insights into the link between colchicine and microtubule polymerization, affecting the activation of the inflammasome, session moderator Imazio said.
“Second, it is important because pharmacogenomics can help us to better understand the optimal responder to colchicine and colchicine resistance,” he said. “So it can be useful for personalized medicine, leading to the proper use of the drug for the proper patient.”
COLCOT was supported by the government of Quebec, the Canadian Institutes of Health Research, and philanthropic foundations. Bouabdallaoui has disclosed no relevant financial relationships. Dubé reported grants from the government of Quebec; personal fees from DalCor and GlaxoSmithKline; research support from AstraZeneca, Pfizer, Servier, Sanofi; and minor equity interest in DalCor. Dubé is also coauthor of patents on pharmacogenomics-guided CETP inhibition, and pharmacogenomics markers of response to colchicine.
This article first appeared on Medscape.com.
ISCHEMIA trial revisited: Some MAY benefit from invasive strategy
The landmark ISCHEMIA trial rattled the cardiology world with its message that clinical outcomes weren’t significantly better with a routine initial invasive strategy than with medical therapy alone in patients with stable coronary artery disease and moderate or severe myocardial ischemia on noninvasive testing. But
ISCHEMIA participants in the sweet spot for an initial invasive strategy were the ones with a baseline history of mild to moderate heart failure symptoms and a left ventricular ejection fraction (LVEF) of 35%-45%, Renato Lopes, MD, PhD, reported at the virtual annual congress of the European Society of Cardiology.
“An invasive approach may be beneficial in this subgroup of high-risk patients with moderate to severe ischemia and a history of heart failure and left ventricular dysfunction,” declared Dr. Lopes, professor of medicine at Duke University, Durham, N.C.
He was quick to add, however, that this finding from ISCHEMIA should be considered hypothesis generating in light of the small sample size in the subgroup analysis.
The ISCHEMIA trial randomized 5,179 patients with stable CAD and at least moderate myocardial ischemia on noninvasive testing to a routine invasive or conservative management strategy. At 4 years of follow-up there was no significant between-group difference in cardiovascular outcomes (N Engl J Med. 2020 Apr 9;382[15]:1395-407).
Patients with a baseline LVEF below 35% weren’t eligible for enrollment in ISCHEMIA. That’s because the prior Surgical Treatment for Ischemic Heart Failure Extension Study (STICHES) showed patients with ischemic cardiomyopathy and an LVEF below 35% had a significantly lower cardiovascular death rate with surgical revascularization plus medical therapy than optimal medical therapy alone at 10 years of follow-up (N Engl J Med. 2016 Apr 21;374[16]:1511-20).
But what about the impact of immediate revascularization as compared with medical management alone in patients with milder impairment of LVEF in the 35%-45% range and/or a history of symptomatic heart failure? Theoretically, the improved blood flow to ischemic myocardium obtained via revascularization in such patients might activate hibernating myocardium and reduce ventricular dysfunction, thereby reducing the risk of cardiovascular events. The ISCHEMIA trial provided a unique opportunity to prospectively examine this question in 398 affected study participants, Dr. Lopes explained.
This 398-patient subgroup with a baseline history of heart failure and/or left ventricular dysfunction (HF/LVD) was at higher risk than patients without those features. Indeed, the primary outcome in ISCHEMIA – a composite of cardiovascular death, nonfatal MI, or hospitalization for unstable angina, heart failure, or resuscitated cardiac arrest – occurred in 22.7% of the HF/LVD group at 4 years of follow-up, compared with 13.8% of the much larger group without HF/LVD. The HF/LVD group also had significantly higher rates of the secondary composite endpoints of cardiovascular death or MI (19.7% vs. 12.3%) and all-cause mortality or heart failure (15% vs. 6.9%).
The provocative central finding in this new subanalysis was that patients in the HF/LVD subgroup fared significantly better in terms of cardiovascular events if randomized to the initial invasive approach. Indeed, their 4-year rate of the primary outcome was 17.2%, compared with 29.3% with an initial conservative approach. The various secondary outcomes followed suit. In contrast, the primary outcome occurred in 13% of patients without HF/LVD who were randomized to the invasive strategy, not significantly different from the 14.6% rate with conservative management.
Drilling deeper into the data, Dr. Lopes and coinvestigators found that the enhanced event-free survival benefit of an initial invasive strategy was restricted to the 28 patients having both a baseline history of symptomatic heart failure and an LVEF of 35%-45%. There was no significant difference in outcomes with an invasive versus conservative strategy in the 177 patients with a history of heart failure whose LVEF was greater than 50% – that is, patients with heart failure with preserved ejection fraction – nor in the 193 participants with an LVEF of 35%-45% but no history of symptomatic heart failure.
In an interview, Mark H. Drazner, MD, commented, “this is an interesting hypothesis, for sure, that warrants further study to confirm whether it’s valid. And if it is valid, there could be real implications. If this is true, I think there could be a decent number of patients out there that this would have implications for.
“The ISCHEMIA trial was a heroic effort. While there are certainly logistical hurdles involved in anybody doing an ISCHEMIA 2 trial based on this small subgroup analysis, other people could start looking at retrospective datasets and see if they can confirm these findings to build momentum to study this further,” said Dr. Drazner, professor of medicine and chief of clinical cardiology at the University of Texas Southwestern Medical Center, Dallas, as well as an associate editor at Circulation.
Dr. Lopes reported receiving research grants from Amgen, Bristol-Myers Squibb, GlaxoSmithKline, Medtronic, Pfizer, and Sanofi-Aventis as well as serving as a consultant to a handful of pharmaceutical companies, none relevant to his presentation.
Simultaneous with his presentation at ESC Congress 2020, Dr. Lopes’ study was published online in Circulation.
SOURCE: Lopes R et al. Circulation. 2020 Aug 29. doi: 10.1161/CIRCULATIONAHA.120.050304.
The landmark ISCHEMIA trial rattled the cardiology world with its message that clinical outcomes weren’t significantly better with a routine initial invasive strategy than with medical therapy alone in patients with stable coronary artery disease and moderate or severe myocardial ischemia on noninvasive testing. But
ISCHEMIA participants in the sweet spot for an initial invasive strategy were the ones with a baseline history of mild to moderate heart failure symptoms and a left ventricular ejection fraction (LVEF) of 35%-45%, Renato Lopes, MD, PhD, reported at the virtual annual congress of the European Society of Cardiology.
“An invasive approach may be beneficial in this subgroup of high-risk patients with moderate to severe ischemia and a history of heart failure and left ventricular dysfunction,” declared Dr. Lopes, professor of medicine at Duke University, Durham, N.C.
He was quick to add, however, that this finding from ISCHEMIA should be considered hypothesis generating in light of the small sample size in the subgroup analysis.
The ISCHEMIA trial randomized 5,179 patients with stable CAD and at least moderate myocardial ischemia on noninvasive testing to a routine invasive or conservative management strategy. At 4 years of follow-up there was no significant between-group difference in cardiovascular outcomes (N Engl J Med. 2020 Apr 9;382[15]:1395-407).
Patients with a baseline LVEF below 35% weren’t eligible for enrollment in ISCHEMIA. That’s because the prior Surgical Treatment for Ischemic Heart Failure Extension Study (STICHES) showed patients with ischemic cardiomyopathy and an LVEF below 35% had a significantly lower cardiovascular death rate with surgical revascularization plus medical therapy than optimal medical therapy alone at 10 years of follow-up (N Engl J Med. 2016 Apr 21;374[16]:1511-20).
But what about the impact of immediate revascularization as compared with medical management alone in patients with milder impairment of LVEF in the 35%-45% range and/or a history of symptomatic heart failure? Theoretically, the improved blood flow to ischemic myocardium obtained via revascularization in such patients might activate hibernating myocardium and reduce ventricular dysfunction, thereby reducing the risk of cardiovascular events. The ISCHEMIA trial provided a unique opportunity to prospectively examine this question in 398 affected study participants, Dr. Lopes explained.
This 398-patient subgroup with a baseline history of heart failure and/or left ventricular dysfunction (HF/LVD) was at higher risk than patients without those features. Indeed, the primary outcome in ISCHEMIA – a composite of cardiovascular death, nonfatal MI, or hospitalization for unstable angina, heart failure, or resuscitated cardiac arrest – occurred in 22.7% of the HF/LVD group at 4 years of follow-up, compared with 13.8% of the much larger group without HF/LVD. The HF/LVD group also had significantly higher rates of the secondary composite endpoints of cardiovascular death or MI (19.7% vs. 12.3%) and all-cause mortality or heart failure (15% vs. 6.9%).
The provocative central finding in this new subanalysis was that patients in the HF/LVD subgroup fared significantly better in terms of cardiovascular events if randomized to the initial invasive approach. Indeed, their 4-year rate of the primary outcome was 17.2%, compared with 29.3% with an initial conservative approach. The various secondary outcomes followed suit. In contrast, the primary outcome occurred in 13% of patients without HF/LVD who were randomized to the invasive strategy, not significantly different from the 14.6% rate with conservative management.
Drilling deeper into the data, Dr. Lopes and coinvestigators found that the enhanced event-free survival benefit of an initial invasive strategy was restricted to the 28 patients having both a baseline history of symptomatic heart failure and an LVEF of 35%-45%. There was no significant difference in outcomes with an invasive versus conservative strategy in the 177 patients with a history of heart failure whose LVEF was greater than 50% – that is, patients with heart failure with preserved ejection fraction – nor in the 193 participants with an LVEF of 35%-45% but no history of symptomatic heart failure.
In an interview, Mark H. Drazner, MD, commented, “this is an interesting hypothesis, for sure, that warrants further study to confirm whether it’s valid. And if it is valid, there could be real implications. If this is true, I think there could be a decent number of patients out there that this would have implications for.
“The ISCHEMIA trial was a heroic effort. While there are certainly logistical hurdles involved in anybody doing an ISCHEMIA 2 trial based on this small subgroup analysis, other people could start looking at retrospective datasets and see if they can confirm these findings to build momentum to study this further,” said Dr. Drazner, professor of medicine and chief of clinical cardiology at the University of Texas Southwestern Medical Center, Dallas, as well as an associate editor at Circulation.
Dr. Lopes reported receiving research grants from Amgen, Bristol-Myers Squibb, GlaxoSmithKline, Medtronic, Pfizer, and Sanofi-Aventis as well as serving as a consultant to a handful of pharmaceutical companies, none relevant to his presentation.
Simultaneous with his presentation at ESC Congress 2020, Dr. Lopes’ study was published online in Circulation.
SOURCE: Lopes R et al. Circulation. 2020 Aug 29. doi: 10.1161/CIRCULATIONAHA.120.050304.
The landmark ISCHEMIA trial rattled the cardiology world with its message that clinical outcomes weren’t significantly better with a routine initial invasive strategy than with medical therapy alone in patients with stable coronary artery disease and moderate or severe myocardial ischemia on noninvasive testing. But
ISCHEMIA participants in the sweet spot for an initial invasive strategy were the ones with a baseline history of mild to moderate heart failure symptoms and a left ventricular ejection fraction (LVEF) of 35%-45%, Renato Lopes, MD, PhD, reported at the virtual annual congress of the European Society of Cardiology.
“An invasive approach may be beneficial in this subgroup of high-risk patients with moderate to severe ischemia and a history of heart failure and left ventricular dysfunction,” declared Dr. Lopes, professor of medicine at Duke University, Durham, N.C.
He was quick to add, however, that this finding from ISCHEMIA should be considered hypothesis generating in light of the small sample size in the subgroup analysis.
The ISCHEMIA trial randomized 5,179 patients with stable CAD and at least moderate myocardial ischemia on noninvasive testing to a routine invasive or conservative management strategy. At 4 years of follow-up there was no significant between-group difference in cardiovascular outcomes (N Engl J Med. 2020 Apr 9;382[15]:1395-407).
Patients with a baseline LVEF below 35% weren’t eligible for enrollment in ISCHEMIA. That’s because the prior Surgical Treatment for Ischemic Heart Failure Extension Study (STICHES) showed patients with ischemic cardiomyopathy and an LVEF below 35% had a significantly lower cardiovascular death rate with surgical revascularization plus medical therapy than optimal medical therapy alone at 10 years of follow-up (N Engl J Med. 2016 Apr 21;374[16]:1511-20).
But what about the impact of immediate revascularization as compared with medical management alone in patients with milder impairment of LVEF in the 35%-45% range and/or a history of symptomatic heart failure? Theoretically, the improved blood flow to ischemic myocardium obtained via revascularization in such patients might activate hibernating myocardium and reduce ventricular dysfunction, thereby reducing the risk of cardiovascular events. The ISCHEMIA trial provided a unique opportunity to prospectively examine this question in 398 affected study participants, Dr. Lopes explained.
This 398-patient subgroup with a baseline history of heart failure and/or left ventricular dysfunction (HF/LVD) was at higher risk than patients without those features. Indeed, the primary outcome in ISCHEMIA – a composite of cardiovascular death, nonfatal MI, or hospitalization for unstable angina, heart failure, or resuscitated cardiac arrest – occurred in 22.7% of the HF/LVD group at 4 years of follow-up, compared with 13.8% of the much larger group without HF/LVD. The HF/LVD group also had significantly higher rates of the secondary composite endpoints of cardiovascular death or MI (19.7% vs. 12.3%) and all-cause mortality or heart failure (15% vs. 6.9%).
The provocative central finding in this new subanalysis was that patients in the HF/LVD subgroup fared significantly better in terms of cardiovascular events if randomized to the initial invasive approach. Indeed, their 4-year rate of the primary outcome was 17.2%, compared with 29.3% with an initial conservative approach. The various secondary outcomes followed suit. In contrast, the primary outcome occurred in 13% of patients without HF/LVD who were randomized to the invasive strategy, not significantly different from the 14.6% rate with conservative management.
Drilling deeper into the data, Dr. Lopes and coinvestigators found that the enhanced event-free survival benefit of an initial invasive strategy was restricted to the 28 patients having both a baseline history of symptomatic heart failure and an LVEF of 35%-45%. There was no significant difference in outcomes with an invasive versus conservative strategy in the 177 patients with a history of heart failure whose LVEF was greater than 50% – that is, patients with heart failure with preserved ejection fraction – nor in the 193 participants with an LVEF of 35%-45% but no history of symptomatic heart failure.
In an interview, Mark H. Drazner, MD, commented, “this is an interesting hypothesis, for sure, that warrants further study to confirm whether it’s valid. And if it is valid, there could be real implications. If this is true, I think there could be a decent number of patients out there that this would have implications for.
“The ISCHEMIA trial was a heroic effort. While there are certainly logistical hurdles involved in anybody doing an ISCHEMIA 2 trial based on this small subgroup analysis, other people could start looking at retrospective datasets and see if they can confirm these findings to build momentum to study this further,” said Dr. Drazner, professor of medicine and chief of clinical cardiology at the University of Texas Southwestern Medical Center, Dallas, as well as an associate editor at Circulation.
Dr. Lopes reported receiving research grants from Amgen, Bristol-Myers Squibb, GlaxoSmithKline, Medtronic, Pfizer, and Sanofi-Aventis as well as serving as a consultant to a handful of pharmaceutical companies, none relevant to his presentation.
Simultaneous with his presentation at ESC Congress 2020, Dr. Lopes’ study was published online in Circulation.
SOURCE: Lopes R et al. Circulation. 2020 Aug 29. doi: 10.1161/CIRCULATIONAHA.120.050304.
FROM ESC CONGRESS 2020
Cardiovascular risk factors linked to brain atrophy in MS
The presence of cardiovascular risk factors in patients with multiple sclerosis (MS) is associated with a greater degree of brain atrophy even in young patients who are unlikely to have small vessel disease, a new study has shown.
The results were presented by Raffaello Bonacchi, MD, Vita-Salute San Raffaele University, Milan, Italy, at at the Joint European Committee for Treatment and Research in Multiple Sclerosis–Americas Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS–ACTRIMS) 2020, this year known as MSVirtual2020. .
“Our results suggest that even low levels of exposure to cardiovascular risk factors are important in MS and might affect brain atrophy—and therefore long-term disability—even in young patients,” Dr. Bonacchi said.
“It is not only smoking,” he added. “Other cardiovascular risk factors also appear to be implicated. We found a synergistic effect of the different risk factors.”
These are only preliminary data and need to be confirmed in other studies,” he said, “but it does suggest that MS neurologists need to pay attention to comprehensive care—not just MS disease activity.
“They also need to be discussing lifestyle with their patients, evaluating their cardiovascular risk factors, and giving advice on stopping smoking, lowering blood pressure, cholesterol, etc.”
Brain changes
Dr. Bonacchi explained that previous studies have suggested a relationship between cardiovascular risk factors and changes on magnetic resonance imaging (MRI) and clinical outcomes in patients with MS that may be mediated by small vessel disease and/or inflammation.
“Small vessel disease is widespread in the population over 50 years of age, but in this study we wanted to look at the impact of cardiovascular risk factors in younger patients with MS who are not likely to have much small vessel disease to try and see whether there is still a relationship with brain atrophy or white/gray matter lesions,” he said.
Previous studies have not set an age limit for examining this relationship and they have also assessed the presence versus absence of cardiovascular risk factors, without attempting to grade the strength of exposure, he noted.
For the current study, the researchers examined several cardiovascular risk factors and in addition to just being present or absent. They also graded each risk factor as being stringent or not depending on a certain threshold.
For example, smoking was defined as a threshold of 5 pack-years (smoking 5 cigarettes a day for 20 years or 20 cigarettes a day for 5 years). And the more stringent definition was 10 pack-years.
For hypertension, the stringent definition was consistently high blood pressure levels and use of antihypertensive medication, with similar definitions used for cholesterol and diabetes.
This was a cross-sectional observational study in 124 patients with MS and 95 healthy controls. The researchers examined MRI scans and neurological exams and investigated whether the amount of cardiovascular risk factors a patient was exposed to was associated with degree of brain atrophy and white matter/gray matter volume. Results were adjusted for age, sex, disease duration, phenotype (relapsing-remitting versus progressive MS) and treatment.
Results showed no significant difference if patients were exposed to at least one classical risk factor versus no risk factors. But if a patient had at least two classical risk factors, significant differences were found in gray matter, white matter, and total brain volume.
Patients with MS and no risk factors had a mean brain volume of 1524 mL versus 1481 mL in those with at least two risk factors, a difference that was significant (P = 0.003). Mean gray matter volume was 856 mL in MS patients without cardiovascular risk factors and 836 mL in those with at least two risk factors (P = 0.01) Mean white matter volume was 668 mL in MS patients without cardiovascular risk factors and 845 mL in those with at least two risk factors (P = 0.03).
“This is one of the first studies to have graded degrees of risk factors and we found one stringent risk factor was associated with the same effects on brain atrophy as two less stringent risk factors,” Dr. Bonacchi reported.
Healthy controls showed no differences in any of the brain volume outcomes in those with or without cardiovascular risk factors.
“As our population was under aged 50 years, who are unlikely to have much small vessel disease, our results suggest that the influence of cardiovascular risk factors on brain atrophy in MS is not just mediated through small vessel disease and is probably also mediated by increased inflammation,” Dr. Bonacchi suggested.
Impact of CV risk factors
Commenting on the study, Dalia Rotstein, MD, assistant professor, department of neurology, University of Toronto, Ontario, Canada, session cochair, said: “This is an interesting study that captures the impact of cardiovascular risk factors on various measures of brain atrophy in MS.”
The cohort was quite young, under age 50, and the effect on brain atrophy was increased with more severe cardiovascular risk factors, she noted.
“The investigators compared these effects to a population of healthy controls and did not observe as substantial an effect in controls. However, they were likely underpowered for the analysis in the healthy controls because of a relatively small number of subjects with cardiovascular risk factors in this group,” Dr. Rotstein noted.
“More research is needed to determine whether the observed relationship is unique to MS and whether treating cardiovascular risk factors may help protect against neurodegeneration in MS,” she added.
Dr. Bonacchi has reported no relevant financial relationships. Dr. Rotstein has reported acting as a consultant for Roche, Alexion, Novartis, EMD Serono, and Sanofi Aventis.
SOURCE: Bonacchi R. et al. MSVirtual2020. Session PS04.05.
This article originally appeared on Medscape.com .
The presence of cardiovascular risk factors in patients with multiple sclerosis (MS) is associated with a greater degree of brain atrophy even in young patients who are unlikely to have small vessel disease, a new study has shown.
The results were presented by Raffaello Bonacchi, MD, Vita-Salute San Raffaele University, Milan, Italy, at at the Joint European Committee for Treatment and Research in Multiple Sclerosis–Americas Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS–ACTRIMS) 2020, this year known as MSVirtual2020. .
“Our results suggest that even low levels of exposure to cardiovascular risk factors are important in MS and might affect brain atrophy—and therefore long-term disability—even in young patients,” Dr. Bonacchi said.
“It is not only smoking,” he added. “Other cardiovascular risk factors also appear to be implicated. We found a synergistic effect of the different risk factors.”
These are only preliminary data and need to be confirmed in other studies,” he said, “but it does suggest that MS neurologists need to pay attention to comprehensive care—not just MS disease activity.
“They also need to be discussing lifestyle with their patients, evaluating their cardiovascular risk factors, and giving advice on stopping smoking, lowering blood pressure, cholesterol, etc.”
Brain changes
Dr. Bonacchi explained that previous studies have suggested a relationship between cardiovascular risk factors and changes on magnetic resonance imaging (MRI) and clinical outcomes in patients with MS that may be mediated by small vessel disease and/or inflammation.
“Small vessel disease is widespread in the population over 50 years of age, but in this study we wanted to look at the impact of cardiovascular risk factors in younger patients with MS who are not likely to have much small vessel disease to try and see whether there is still a relationship with brain atrophy or white/gray matter lesions,” he said.
Previous studies have not set an age limit for examining this relationship and they have also assessed the presence versus absence of cardiovascular risk factors, without attempting to grade the strength of exposure, he noted.
For the current study, the researchers examined several cardiovascular risk factors and in addition to just being present or absent. They also graded each risk factor as being stringent or not depending on a certain threshold.
For example, smoking was defined as a threshold of 5 pack-years (smoking 5 cigarettes a day for 20 years or 20 cigarettes a day for 5 years). And the more stringent definition was 10 pack-years.
For hypertension, the stringent definition was consistently high blood pressure levels and use of antihypertensive medication, with similar definitions used for cholesterol and diabetes.
This was a cross-sectional observational study in 124 patients with MS and 95 healthy controls. The researchers examined MRI scans and neurological exams and investigated whether the amount of cardiovascular risk factors a patient was exposed to was associated with degree of brain atrophy and white matter/gray matter volume. Results were adjusted for age, sex, disease duration, phenotype (relapsing-remitting versus progressive MS) and treatment.
Results showed no significant difference if patients were exposed to at least one classical risk factor versus no risk factors. But if a patient had at least two classical risk factors, significant differences were found in gray matter, white matter, and total brain volume.
Patients with MS and no risk factors had a mean brain volume of 1524 mL versus 1481 mL in those with at least two risk factors, a difference that was significant (P = 0.003). Mean gray matter volume was 856 mL in MS patients without cardiovascular risk factors and 836 mL in those with at least two risk factors (P = 0.01) Mean white matter volume was 668 mL in MS patients without cardiovascular risk factors and 845 mL in those with at least two risk factors (P = 0.03).
“This is one of the first studies to have graded degrees of risk factors and we found one stringent risk factor was associated with the same effects on brain atrophy as two less stringent risk factors,” Dr. Bonacchi reported.
Healthy controls showed no differences in any of the brain volume outcomes in those with or without cardiovascular risk factors.
“As our population was under aged 50 years, who are unlikely to have much small vessel disease, our results suggest that the influence of cardiovascular risk factors on brain atrophy in MS is not just mediated through small vessel disease and is probably also mediated by increased inflammation,” Dr. Bonacchi suggested.
Impact of CV risk factors
Commenting on the study, Dalia Rotstein, MD, assistant professor, department of neurology, University of Toronto, Ontario, Canada, session cochair, said: “This is an interesting study that captures the impact of cardiovascular risk factors on various measures of brain atrophy in MS.”
The cohort was quite young, under age 50, and the effect on brain atrophy was increased with more severe cardiovascular risk factors, she noted.
“The investigators compared these effects to a population of healthy controls and did not observe as substantial an effect in controls. However, they were likely underpowered for the analysis in the healthy controls because of a relatively small number of subjects with cardiovascular risk factors in this group,” Dr. Rotstein noted.
“More research is needed to determine whether the observed relationship is unique to MS and whether treating cardiovascular risk factors may help protect against neurodegeneration in MS,” she added.
Dr. Bonacchi has reported no relevant financial relationships. Dr. Rotstein has reported acting as a consultant for Roche, Alexion, Novartis, EMD Serono, and Sanofi Aventis.
SOURCE: Bonacchi R. et al. MSVirtual2020. Session PS04.05.
This article originally appeared on Medscape.com .
The presence of cardiovascular risk factors in patients with multiple sclerosis (MS) is associated with a greater degree of brain atrophy even in young patients who are unlikely to have small vessel disease, a new study has shown.
The results were presented by Raffaello Bonacchi, MD, Vita-Salute San Raffaele University, Milan, Italy, at at the Joint European Committee for Treatment and Research in Multiple Sclerosis–Americas Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS–ACTRIMS) 2020, this year known as MSVirtual2020. .
“Our results suggest that even low levels of exposure to cardiovascular risk factors are important in MS and might affect brain atrophy—and therefore long-term disability—even in young patients,” Dr. Bonacchi said.
“It is not only smoking,” he added. “Other cardiovascular risk factors also appear to be implicated. We found a synergistic effect of the different risk factors.”
These are only preliminary data and need to be confirmed in other studies,” he said, “but it does suggest that MS neurologists need to pay attention to comprehensive care—not just MS disease activity.
“They also need to be discussing lifestyle with their patients, evaluating their cardiovascular risk factors, and giving advice on stopping smoking, lowering blood pressure, cholesterol, etc.”
Brain changes
Dr. Bonacchi explained that previous studies have suggested a relationship between cardiovascular risk factors and changes on magnetic resonance imaging (MRI) and clinical outcomes in patients with MS that may be mediated by small vessel disease and/or inflammation.
“Small vessel disease is widespread in the population over 50 years of age, but in this study we wanted to look at the impact of cardiovascular risk factors in younger patients with MS who are not likely to have much small vessel disease to try and see whether there is still a relationship with brain atrophy or white/gray matter lesions,” he said.
Previous studies have not set an age limit for examining this relationship and they have also assessed the presence versus absence of cardiovascular risk factors, without attempting to grade the strength of exposure, he noted.
For the current study, the researchers examined several cardiovascular risk factors and in addition to just being present or absent. They also graded each risk factor as being stringent or not depending on a certain threshold.
For example, smoking was defined as a threshold of 5 pack-years (smoking 5 cigarettes a day for 20 years or 20 cigarettes a day for 5 years). And the more stringent definition was 10 pack-years.
For hypertension, the stringent definition was consistently high blood pressure levels and use of antihypertensive medication, with similar definitions used for cholesterol and diabetes.
This was a cross-sectional observational study in 124 patients with MS and 95 healthy controls. The researchers examined MRI scans and neurological exams and investigated whether the amount of cardiovascular risk factors a patient was exposed to was associated with degree of brain atrophy and white matter/gray matter volume. Results were adjusted for age, sex, disease duration, phenotype (relapsing-remitting versus progressive MS) and treatment.
Results showed no significant difference if patients were exposed to at least one classical risk factor versus no risk factors. But if a patient had at least two classical risk factors, significant differences were found in gray matter, white matter, and total brain volume.
Patients with MS and no risk factors had a mean brain volume of 1524 mL versus 1481 mL in those with at least two risk factors, a difference that was significant (P = 0.003). Mean gray matter volume was 856 mL in MS patients without cardiovascular risk factors and 836 mL in those with at least two risk factors (P = 0.01) Mean white matter volume was 668 mL in MS patients without cardiovascular risk factors and 845 mL in those with at least two risk factors (P = 0.03).
“This is one of the first studies to have graded degrees of risk factors and we found one stringent risk factor was associated with the same effects on brain atrophy as two less stringent risk factors,” Dr. Bonacchi reported.
Healthy controls showed no differences in any of the brain volume outcomes in those with or without cardiovascular risk factors.
“As our population was under aged 50 years, who are unlikely to have much small vessel disease, our results suggest that the influence of cardiovascular risk factors on brain atrophy in MS is not just mediated through small vessel disease and is probably also mediated by increased inflammation,” Dr. Bonacchi suggested.
Impact of CV risk factors
Commenting on the study, Dalia Rotstein, MD, assistant professor, department of neurology, University of Toronto, Ontario, Canada, session cochair, said: “This is an interesting study that captures the impact of cardiovascular risk factors on various measures of brain atrophy in MS.”
The cohort was quite young, under age 50, and the effect on brain atrophy was increased with more severe cardiovascular risk factors, she noted.
“The investigators compared these effects to a population of healthy controls and did not observe as substantial an effect in controls. However, they were likely underpowered for the analysis in the healthy controls because of a relatively small number of subjects with cardiovascular risk factors in this group,” Dr. Rotstein noted.
“More research is needed to determine whether the observed relationship is unique to MS and whether treating cardiovascular risk factors may help protect against neurodegeneration in MS,” she added.
Dr. Bonacchi has reported no relevant financial relationships. Dr. Rotstein has reported acting as a consultant for Roche, Alexion, Novartis, EMD Serono, and Sanofi Aventis.
SOURCE: Bonacchi R. et al. MSVirtual2020. Session PS04.05.
This article originally appeared on Medscape.com .
FROM MSVirtual2020