User login
New guidelines update VTE treatment recommendations
Updated guidelines regarding the treatment of patients with venous thromboembolism advise abandoning the routine use of compression stockings for prevention of postthrombotic syndrome in patients who have had an acute deep vein thrombosis, according to Dr. Clive Kearon, lead author of the American College of Chest Physicians’ 10th edition of “Antithrombotic Therapy for VTE Disease” (Chest. 2015. doi: 10.1016/j.chest.2015.11.026).
The VTE guidelines include 12 recommendations. Two other key changes from the previous guidelines include new treatment recommendations about which patients with isolated subsegmental pulmonary embolism (PE) should, and should not, receive anticoagulant therapy, and as a recommendation for the use of non–vitamin K antagonist oral anticoagulants (NOACs) instead of warfarin for initial and long-term treatment of VTE in patients without cancer.
It is another of the group’s “living guidelines,” intended to be flexible, easy-to-update recommendations … based on the best available evidence, and to identify gaps in our knowledge and areas for future research,” Dr. Kearon of McMaster University, Hamilton, Ont., said in an interview.
“Clinicians and guideline developers would like clinician decisions to be supported by very strong, or almost irrefutable, evidence,” he said. ”It’s difficult to do studies that provide irrefutable evidence, however,” and most of the updated recommendations are not based on the highest level of study evidence – large, randomized controlled trials.
Nevertheless, “the quality of evidence that supports guidelines and clinical decision making is much better now than it was 20 or 30 years ago,” Dr. Kearon said, mainly because more recent studies are considerably larger and involve multiple clinical centers. Plus, “we’re continually improving our skills at doing high-quality studies and studies that have a low potential for bias.”
The old recommendation to use graduated compression stockings for 2 years after DVT to reduce the risk of postthrombotic syndrome was mainly based on findings of two small single-center randomized trials, published in the Lancet and Annals of Internal Medicine, in which patients and study personnel were not blinded to stocking use. Since then, a much larger multicenter, placebo-controlled trial found that routine use of graduated compression stockings did not reduce postthrombotic syndrome or have other important benefits in 410 patients with a first proximal DVT randomized to receive either active or placebo compression stockings. The incidence of postthrombotic syndrome was 14% in the active group and 13% in the placebo group – a nonsignificant difference. The same study also found that routine use of graduated compression stockings did not reduce leg pain during the 3 months after a DVT – although the stockings were still able to reduce acute symptoms of DVT, and chronic symptoms in patients with postthrombotic syndrome.
The recommendation to replace warfarin with NOACs is based on new data suggesting that the agents are associated with a lower risk of bleeding, and on observations that NOACs are much easier for patients and clinicians to use. Several of the studies upon which earlier guidelines were based have been reanalyzed, Dr. Kearon and his coauthors wrote. There are also now extensive data on the comparative safety of NOACs and warfarin.
“Based on less bleeding with NOACs and greater convenience for patients and health care providers, we now suggest that a NOAC is used in preference to VKA [vitamin K antagonist] for the initial and long-term treatment of VTE in patients without cancer,” they wrote.
The recommendation to employ watchful waiting over anticoagulation in some patients with subsegmental pulmonary embolism is based on a compendium of clinical evidence rather than on large studies. A true subsegmental PE is unlikely to need anticoagulation, because it will have arisen from a small clot and thus carry a small risk of progression or recurrence.
“There is, however, high-quality evidence for the efficacy and safety of anticoagulant therapy in patients with larger PE, and this is expected to apply similarly to patients with subsegmental PE,” the authors wrote. “Whether the risk of progressive or recurrent VTE is high enough to justify anticoagulation in patients with subsegmental PE is uncertain.”
If clinical assessment suggests that anticoagulation isn’t appropriate, these patients should have a confirmatory bilateral ultrasound to rule out proximal DVTs, especially in high-risk locations. If a DVT is detected, clinicians may choose to conduct subsequent ultrasounds to identify and treat any evolving proximal clots.
The guideline has been endorsed by the American Association for Clinical Chemistry, the American College of Clinical Pharmacy, the International Society for Thrombosis and Haemostasis, and the American Society of Health-System Pharmacists.
Dr. Kearon has been compensated for speaking engagements sponsored by Boehringer Ingelheim and Bayer Healthcare related to VTE therapy. Some of the other guideline authors also disclosed relationships with pharmaceutical companies.
Updated guidelines regarding the treatment of patients with venous thromboembolism advise abandoning the routine use of compression stockings for prevention of postthrombotic syndrome in patients who have had an acute deep vein thrombosis, according to Dr. Clive Kearon, lead author of the American College of Chest Physicians’ 10th edition of “Antithrombotic Therapy for VTE Disease” (Chest. 2015. doi: 10.1016/j.chest.2015.11.026).
The VTE guidelines include 12 recommendations. Two other key changes from the previous guidelines include new treatment recommendations about which patients with isolated subsegmental pulmonary embolism (PE) should, and should not, receive anticoagulant therapy, and as a recommendation for the use of non–vitamin K antagonist oral anticoagulants (NOACs) instead of warfarin for initial and long-term treatment of VTE in patients without cancer.
It is another of the group’s “living guidelines,” intended to be flexible, easy-to-update recommendations … based on the best available evidence, and to identify gaps in our knowledge and areas for future research,” Dr. Kearon of McMaster University, Hamilton, Ont., said in an interview.
“Clinicians and guideline developers would like clinician decisions to be supported by very strong, or almost irrefutable, evidence,” he said. ”It’s difficult to do studies that provide irrefutable evidence, however,” and most of the updated recommendations are not based on the highest level of study evidence – large, randomized controlled trials.
Nevertheless, “the quality of evidence that supports guidelines and clinical decision making is much better now than it was 20 or 30 years ago,” Dr. Kearon said, mainly because more recent studies are considerably larger and involve multiple clinical centers. Plus, “we’re continually improving our skills at doing high-quality studies and studies that have a low potential for bias.”
The old recommendation to use graduated compression stockings for 2 years after DVT to reduce the risk of postthrombotic syndrome was mainly based on findings of two small single-center randomized trials, published in the Lancet and Annals of Internal Medicine, in which patients and study personnel were not blinded to stocking use. Since then, a much larger multicenter, placebo-controlled trial found that routine use of graduated compression stockings did not reduce postthrombotic syndrome or have other important benefits in 410 patients with a first proximal DVT randomized to receive either active or placebo compression stockings. The incidence of postthrombotic syndrome was 14% in the active group and 13% in the placebo group – a nonsignificant difference. The same study also found that routine use of graduated compression stockings did not reduce leg pain during the 3 months after a DVT – although the stockings were still able to reduce acute symptoms of DVT, and chronic symptoms in patients with postthrombotic syndrome.
The recommendation to replace warfarin with NOACs is based on new data suggesting that the agents are associated with a lower risk of bleeding, and on observations that NOACs are much easier for patients and clinicians to use. Several of the studies upon which earlier guidelines were based have been reanalyzed, Dr. Kearon and his coauthors wrote. There are also now extensive data on the comparative safety of NOACs and warfarin.
“Based on less bleeding with NOACs and greater convenience for patients and health care providers, we now suggest that a NOAC is used in preference to VKA [vitamin K antagonist] for the initial and long-term treatment of VTE in patients without cancer,” they wrote.
The recommendation to employ watchful waiting over anticoagulation in some patients with subsegmental pulmonary embolism is based on a compendium of clinical evidence rather than on large studies. A true subsegmental PE is unlikely to need anticoagulation, because it will have arisen from a small clot and thus carry a small risk of progression or recurrence.
“There is, however, high-quality evidence for the efficacy and safety of anticoagulant therapy in patients with larger PE, and this is expected to apply similarly to patients with subsegmental PE,” the authors wrote. “Whether the risk of progressive or recurrent VTE is high enough to justify anticoagulation in patients with subsegmental PE is uncertain.”
If clinical assessment suggests that anticoagulation isn’t appropriate, these patients should have a confirmatory bilateral ultrasound to rule out proximal DVTs, especially in high-risk locations. If a DVT is detected, clinicians may choose to conduct subsequent ultrasounds to identify and treat any evolving proximal clots.
The guideline has been endorsed by the American Association for Clinical Chemistry, the American College of Clinical Pharmacy, the International Society for Thrombosis and Haemostasis, and the American Society of Health-System Pharmacists.
Dr. Kearon has been compensated for speaking engagements sponsored by Boehringer Ingelheim and Bayer Healthcare related to VTE therapy. Some of the other guideline authors also disclosed relationships with pharmaceutical companies.
Updated guidelines regarding the treatment of patients with venous thromboembolism advise abandoning the routine use of compression stockings for prevention of postthrombotic syndrome in patients who have had an acute deep vein thrombosis, according to Dr. Clive Kearon, lead author of the American College of Chest Physicians’ 10th edition of “Antithrombotic Therapy for VTE Disease” (Chest. 2015. doi: 10.1016/j.chest.2015.11.026).
The VTE guidelines include 12 recommendations. Two other key changes from the previous guidelines include new treatment recommendations about which patients with isolated subsegmental pulmonary embolism (PE) should, and should not, receive anticoagulant therapy, and as a recommendation for the use of non–vitamin K antagonist oral anticoagulants (NOACs) instead of warfarin for initial and long-term treatment of VTE in patients without cancer.
It is another of the group’s “living guidelines,” intended to be flexible, easy-to-update recommendations … based on the best available evidence, and to identify gaps in our knowledge and areas for future research,” Dr. Kearon of McMaster University, Hamilton, Ont., said in an interview.
“Clinicians and guideline developers would like clinician decisions to be supported by very strong, or almost irrefutable, evidence,” he said. ”It’s difficult to do studies that provide irrefutable evidence, however,” and most of the updated recommendations are not based on the highest level of study evidence – large, randomized controlled trials.
Nevertheless, “the quality of evidence that supports guidelines and clinical decision making is much better now than it was 20 or 30 years ago,” Dr. Kearon said, mainly because more recent studies are considerably larger and involve multiple clinical centers. Plus, “we’re continually improving our skills at doing high-quality studies and studies that have a low potential for bias.”
The old recommendation to use graduated compression stockings for 2 years after DVT to reduce the risk of postthrombotic syndrome was mainly based on findings of two small single-center randomized trials, published in the Lancet and Annals of Internal Medicine, in which patients and study personnel were not blinded to stocking use. Since then, a much larger multicenter, placebo-controlled trial found that routine use of graduated compression stockings did not reduce postthrombotic syndrome or have other important benefits in 410 patients with a first proximal DVT randomized to receive either active or placebo compression stockings. The incidence of postthrombotic syndrome was 14% in the active group and 13% in the placebo group – a nonsignificant difference. The same study also found that routine use of graduated compression stockings did not reduce leg pain during the 3 months after a DVT – although the stockings were still able to reduce acute symptoms of DVT, and chronic symptoms in patients with postthrombotic syndrome.
The recommendation to replace warfarin with NOACs is based on new data suggesting that the agents are associated with a lower risk of bleeding, and on observations that NOACs are much easier for patients and clinicians to use. Several of the studies upon which earlier guidelines were based have been reanalyzed, Dr. Kearon and his coauthors wrote. There are also now extensive data on the comparative safety of NOACs and warfarin.
“Based on less bleeding with NOACs and greater convenience for patients and health care providers, we now suggest that a NOAC is used in preference to VKA [vitamin K antagonist] for the initial and long-term treatment of VTE in patients without cancer,” they wrote.
The recommendation to employ watchful waiting over anticoagulation in some patients with subsegmental pulmonary embolism is based on a compendium of clinical evidence rather than on large studies. A true subsegmental PE is unlikely to need anticoagulation, because it will have arisen from a small clot and thus carry a small risk of progression or recurrence.
“There is, however, high-quality evidence for the efficacy and safety of anticoagulant therapy in patients with larger PE, and this is expected to apply similarly to patients with subsegmental PE,” the authors wrote. “Whether the risk of progressive or recurrent VTE is high enough to justify anticoagulation in patients with subsegmental PE is uncertain.”
If clinical assessment suggests that anticoagulation isn’t appropriate, these patients should have a confirmatory bilateral ultrasound to rule out proximal DVTs, especially in high-risk locations. If a DVT is detected, clinicians may choose to conduct subsequent ultrasounds to identify and treat any evolving proximal clots.
The guideline has been endorsed by the American Association for Clinical Chemistry, the American College of Clinical Pharmacy, the International Society for Thrombosis and Haemostasis, and the American Society of Health-System Pharmacists.
Dr. Kearon has been compensated for speaking engagements sponsored by Boehringer Ingelheim and Bayer Healthcare related to VTE therapy. Some of the other guideline authors also disclosed relationships with pharmaceutical companies.
FROM CHEST
USPSTF urges extra step before treating hypertension
Screening for and treating high blood pressure (HBP) to prevent cardiovascular and renal disease is a tried-and-true preventive intervention that is supported by strong evidence. And not surprisingly, when the US Preventive Services Task Force (USPSTF) recently updated its 2007 recommendation for blood pressure screening for adults, it once again gave an A recommendation for those ages 18 years and older. What is noteworthy, however, is that this update concentrates on the accuracy of blood pressure measurement methods and optimal frequency of screening.1
The most significant modification of past recommendations is that HBP found with office measurement of blood pressure (OMBP) should be confirmed with either ambulatory blood pressure monitoring (ABPM) or home blood pressure monitoring (HBPM) before starting treatment. (For its recommendation, the USPSTF used the HBP definition from the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure [TABLE 1].2,3)
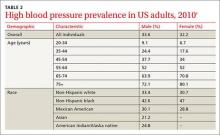
Ensuring accurate blood-pressure measurements. More than 30% of adults in the United States have HBP, with prevalence increasing with age (TABLE 2).2 Only about half of this population has HBP under control.4 This modifiable condition contributes to more than 360,000 deaths annually.2 However, while treatment of true HBP results in substantial benefits, it is important not to over-diagnose HBP and over-treat it.
Studies have shown that 15% to 30% of individuals diagnosed with HBP in a clinical setting will have blood pressure in the normal range when measurements are taken outside of the doctor’s office.1 This discrepancy can be due to measurement error, regression to the mean, recent caffeine ingestion by the patient, or isolated clinical hypertension wherein the stress and anxiety caused by clinic visits elevates blood pressure transiently.
With this in mind, the USPSTF recommends that OMBP-detected HBP be confirmed with either ABPM or HBPM. Of these 2 follow-up methods, ABPM is supported by stronger evidence and is preferred. The USPSTF includes HBPM as an alternative because ABPM equipment may not always be available—or affordable—and using the equipment may present logistical challenges.
Starting off on the right foot
Screening for HBP in a clinical setting is more accurate if conducted according to recommended procedures: use an appropriately sized cuff; take the measurement at least 5 minutes after the patient’s arrival while he or she is seated with legs uncrossed and the cuffed arm is at the level of the heart; and record the mean of 2 separate measurements. There appears to be no real difference in the accuracy of automated vs manual sphygmomanometers.
Optimal frequency of screening varies. While the USPSTF found little evidence to support any particular overall screening frequency, it recommends annual screening for those who are 40 years of age or older and those ages 18 to 39 who are obese or overweight, are African American, or who have high-normal blood pressure (TABLE 3).1 Screening every 3 to 5 years is recommended for individuals not in these categories.
Initial steps in treating HBP. The Task Force also commented on which medications to use when initiating HBP treatment (after lifestyle and dietary interventions). Non-African Americans should receive a thiazide diuretic, calcium channel blocker, angiotensin-converting enzyme inhibitor, or angiotensin-receptor blocker. African Americans should begin treatment with a thiazide diuretic or calcium channel blocker. These recommendations appear to have been adopted from the Eighth Joint National Committee, since the accompanying evidence report for the USPSTF’s update did not address this issue.5
Don't forget patient support
Patient support is key. As of June 2015, the Community Preventive Services Task Force (CPSTF) recommends self-measured blood pressure monitoring combined with additional support as a means of improving blood pressure control in those with HBP.4
Supportive measures include things such as patient counseling on medications and health behavior changes (eg, diet and exercise); education on HBP and blood pressure self-management; and use of secure electronic or Web-based tools such as text or e-mail reminders to measure blood pressure, show up for appointments, or communicate blood pressure readings to healthcare providers. Patients who participate in home self-measurement of blood pressure with additional support lower their systolic blood pressure, on average, 1.4 mm Hg more than those who do not.4
Remaining questions
The new USPSTF recommendation leaves several issues unaddressed. For one thing, the Affordable Care Act mandates that commercial health insurance plans provide services with an A or B Task Force recommendation to patients with no copayments. So does the new HBP recommendation mean payers have to make ABPM and HBPM available to patients at no charge?
There are other questions, too. If HBP detected by OMBP is not confirmed when ABPM is performed, should ABPM be repeated, and if so, at what interval? What is the role of emerging technologies that use devices other than arm cuffs to measure blood pressure?
Despite these uncertainties, the new USPSTF and CPSTF recommendations refine the longstanding in-office–only approach to diagnosing and monitoring HBP and advocate newer technologies that could help improve diagnostic accuracy, avoid over-diagnosis and over-treatment, and improve patient adherence to treatment goals.
1. US Preventive Services Task Force. High blood pressure in adults: screening. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/high-blood-pressure-in-adults-screening. Accessed November 24, 2015.
2. Piper MA, Evans CV, Burda BU, et al. Screening for high blood pressure in adults: a systematic evidence review for the U.S. Preventive Services Task Force. Available at: http://www.ncbi.nlm.nih.gov/books/NBK269495/. Accessed November 24, 2015.
3. US Department of Health and Human Services. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Available at: http://www.nhlbi.nih.gov/files/docs/guidelines/jnc7full.pdf. Accessed November 24, 2015.
4. Community Preventive Services Task Force. Cardiovascular disease prevention and control: self-measured blood pressure monitoring interventions for improved blood pressure control — when combined with additional support. Available at: http://www.thecommunityguide.org/cvd/SMBP-additional.html. Accessed November 24, 2015.
5. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC8). JAMA. 2014;311:507-520.
Screening for and treating high blood pressure (HBP) to prevent cardiovascular and renal disease is a tried-and-true preventive intervention that is supported by strong evidence. And not surprisingly, when the US Preventive Services Task Force (USPSTF) recently updated its 2007 recommendation for blood pressure screening for adults, it once again gave an A recommendation for those ages 18 years and older. What is noteworthy, however, is that this update concentrates on the accuracy of blood pressure measurement methods and optimal frequency of screening.1
The most significant modification of past recommendations is that HBP found with office measurement of blood pressure (OMBP) should be confirmed with either ambulatory blood pressure monitoring (ABPM) or home blood pressure monitoring (HBPM) before starting treatment. (For its recommendation, the USPSTF used the HBP definition from the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure [TABLE 1].2,3)
Ensuring accurate blood-pressure measurements. More than 30% of adults in the United States have HBP, with prevalence increasing with age (TABLE 2).2 Only about half of this population has HBP under control.4 This modifiable condition contributes to more than 360,000 deaths annually.2 However, while treatment of true HBP results in substantial benefits, it is important not to over-diagnose HBP and over-treat it.
Studies have shown that 15% to 30% of individuals diagnosed with HBP in a clinical setting will have blood pressure in the normal range when measurements are taken outside of the doctor’s office.1 This discrepancy can be due to measurement error, regression to the mean, recent caffeine ingestion by the patient, or isolated clinical hypertension wherein the stress and anxiety caused by clinic visits elevates blood pressure transiently.
With this in mind, the USPSTF recommends that OMBP-detected HBP be confirmed with either ABPM or HBPM. Of these 2 follow-up methods, ABPM is supported by stronger evidence and is preferred. The USPSTF includes HBPM as an alternative because ABPM equipment may not always be available—or affordable—and using the equipment may present logistical challenges.
Starting off on the right foot
Screening for HBP in a clinical setting is more accurate if conducted according to recommended procedures: use an appropriately sized cuff; take the measurement at least 5 minutes after the patient’s arrival while he or she is seated with legs uncrossed and the cuffed arm is at the level of the heart; and record the mean of 2 separate measurements. There appears to be no real difference in the accuracy of automated vs manual sphygmomanometers.
Optimal frequency of screening varies. While the USPSTF found little evidence to support any particular overall screening frequency, it recommends annual screening for those who are 40 years of age or older and those ages 18 to 39 who are obese or overweight, are African American, or who have high-normal blood pressure (TABLE 3).1 Screening every 3 to 5 years is recommended for individuals not in these categories.
Initial steps in treating HBP. The Task Force also commented on which medications to use when initiating HBP treatment (after lifestyle and dietary interventions). Non-African Americans should receive a thiazide diuretic, calcium channel blocker, angiotensin-converting enzyme inhibitor, or angiotensin-receptor blocker. African Americans should begin treatment with a thiazide diuretic or calcium channel blocker. These recommendations appear to have been adopted from the Eighth Joint National Committee, since the accompanying evidence report for the USPSTF’s update did not address this issue.5
Don't forget patient support
Patient support is key. As of June 2015, the Community Preventive Services Task Force (CPSTF) recommends self-measured blood pressure monitoring combined with additional support as a means of improving blood pressure control in those with HBP.4
Supportive measures include things such as patient counseling on medications and health behavior changes (eg, diet and exercise); education on HBP and blood pressure self-management; and use of secure electronic or Web-based tools such as text or e-mail reminders to measure blood pressure, show up for appointments, or communicate blood pressure readings to healthcare providers. Patients who participate in home self-measurement of blood pressure with additional support lower their systolic blood pressure, on average, 1.4 mm Hg more than those who do not.4
Remaining questions
The new USPSTF recommendation leaves several issues unaddressed. For one thing, the Affordable Care Act mandates that commercial health insurance plans provide services with an A or B Task Force recommendation to patients with no copayments. So does the new HBP recommendation mean payers have to make ABPM and HBPM available to patients at no charge?
There are other questions, too. If HBP detected by OMBP is not confirmed when ABPM is performed, should ABPM be repeated, and if so, at what interval? What is the role of emerging technologies that use devices other than arm cuffs to measure blood pressure?
Despite these uncertainties, the new USPSTF and CPSTF recommendations refine the longstanding in-office–only approach to diagnosing and monitoring HBP and advocate newer technologies that could help improve diagnostic accuracy, avoid over-diagnosis and over-treatment, and improve patient adherence to treatment goals.
Screening for and treating high blood pressure (HBP) to prevent cardiovascular and renal disease is a tried-and-true preventive intervention that is supported by strong evidence. And not surprisingly, when the US Preventive Services Task Force (USPSTF) recently updated its 2007 recommendation for blood pressure screening for adults, it once again gave an A recommendation for those ages 18 years and older. What is noteworthy, however, is that this update concentrates on the accuracy of blood pressure measurement methods and optimal frequency of screening.1
The most significant modification of past recommendations is that HBP found with office measurement of blood pressure (OMBP) should be confirmed with either ambulatory blood pressure monitoring (ABPM) or home blood pressure monitoring (HBPM) before starting treatment. (For its recommendation, the USPSTF used the HBP definition from the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure [TABLE 1].2,3)
Ensuring accurate blood-pressure measurements. More than 30% of adults in the United States have HBP, with prevalence increasing with age (TABLE 2).2 Only about half of this population has HBP under control.4 This modifiable condition contributes to more than 360,000 deaths annually.2 However, while treatment of true HBP results in substantial benefits, it is important not to over-diagnose HBP and over-treat it.
Studies have shown that 15% to 30% of individuals diagnosed with HBP in a clinical setting will have blood pressure in the normal range when measurements are taken outside of the doctor’s office.1 This discrepancy can be due to measurement error, regression to the mean, recent caffeine ingestion by the patient, or isolated clinical hypertension wherein the stress and anxiety caused by clinic visits elevates blood pressure transiently.
With this in mind, the USPSTF recommends that OMBP-detected HBP be confirmed with either ABPM or HBPM. Of these 2 follow-up methods, ABPM is supported by stronger evidence and is preferred. The USPSTF includes HBPM as an alternative because ABPM equipment may not always be available—or affordable—and using the equipment may present logistical challenges.
Starting off on the right foot
Screening for HBP in a clinical setting is more accurate if conducted according to recommended procedures: use an appropriately sized cuff; take the measurement at least 5 minutes after the patient’s arrival while he or she is seated with legs uncrossed and the cuffed arm is at the level of the heart; and record the mean of 2 separate measurements. There appears to be no real difference in the accuracy of automated vs manual sphygmomanometers.
Optimal frequency of screening varies. While the USPSTF found little evidence to support any particular overall screening frequency, it recommends annual screening for those who are 40 years of age or older and those ages 18 to 39 who are obese or overweight, are African American, or who have high-normal blood pressure (TABLE 3).1 Screening every 3 to 5 years is recommended for individuals not in these categories.
Initial steps in treating HBP. The Task Force also commented on which medications to use when initiating HBP treatment (after lifestyle and dietary interventions). Non-African Americans should receive a thiazide diuretic, calcium channel blocker, angiotensin-converting enzyme inhibitor, or angiotensin-receptor blocker. African Americans should begin treatment with a thiazide diuretic or calcium channel blocker. These recommendations appear to have been adopted from the Eighth Joint National Committee, since the accompanying evidence report for the USPSTF’s update did not address this issue.5
Don't forget patient support
Patient support is key. As of June 2015, the Community Preventive Services Task Force (CPSTF) recommends self-measured blood pressure monitoring combined with additional support as a means of improving blood pressure control in those with HBP.4
Supportive measures include things such as patient counseling on medications and health behavior changes (eg, diet and exercise); education on HBP and blood pressure self-management; and use of secure electronic or Web-based tools such as text or e-mail reminders to measure blood pressure, show up for appointments, or communicate blood pressure readings to healthcare providers. Patients who participate in home self-measurement of blood pressure with additional support lower their systolic blood pressure, on average, 1.4 mm Hg more than those who do not.4
Remaining questions
The new USPSTF recommendation leaves several issues unaddressed. For one thing, the Affordable Care Act mandates that commercial health insurance plans provide services with an A or B Task Force recommendation to patients with no copayments. So does the new HBP recommendation mean payers have to make ABPM and HBPM available to patients at no charge?
There are other questions, too. If HBP detected by OMBP is not confirmed when ABPM is performed, should ABPM be repeated, and if so, at what interval? What is the role of emerging technologies that use devices other than arm cuffs to measure blood pressure?
Despite these uncertainties, the new USPSTF and CPSTF recommendations refine the longstanding in-office–only approach to diagnosing and monitoring HBP and advocate newer technologies that could help improve diagnostic accuracy, avoid over-diagnosis and over-treatment, and improve patient adherence to treatment goals.
1. US Preventive Services Task Force. High blood pressure in adults: screening. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/high-blood-pressure-in-adults-screening. Accessed November 24, 2015.
2. Piper MA, Evans CV, Burda BU, et al. Screening for high blood pressure in adults: a systematic evidence review for the U.S. Preventive Services Task Force. Available at: http://www.ncbi.nlm.nih.gov/books/NBK269495/. Accessed November 24, 2015.
3. US Department of Health and Human Services. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Available at: http://www.nhlbi.nih.gov/files/docs/guidelines/jnc7full.pdf. Accessed November 24, 2015.
4. Community Preventive Services Task Force. Cardiovascular disease prevention and control: self-measured blood pressure monitoring interventions for improved blood pressure control — when combined with additional support. Available at: http://www.thecommunityguide.org/cvd/SMBP-additional.html. Accessed November 24, 2015.
5. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC8). JAMA. 2014;311:507-520.
1. US Preventive Services Task Force. High blood pressure in adults: screening. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/high-blood-pressure-in-adults-screening. Accessed November 24, 2015.
2. Piper MA, Evans CV, Burda BU, et al. Screening for high blood pressure in adults: a systematic evidence review for the U.S. Preventive Services Task Force. Available at: http://www.ncbi.nlm.nih.gov/books/NBK269495/. Accessed November 24, 2015.
3. US Department of Health and Human Services. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Available at: http://www.nhlbi.nih.gov/files/docs/guidelines/jnc7full.pdf. Accessed November 24, 2015.
4. Community Preventive Services Task Force. Cardiovascular disease prevention and control: self-measured blood pressure monitoring interventions for improved blood pressure control — when combined with additional support. Available at: http://www.thecommunityguide.org/cvd/SMBP-additional.html. Accessed November 24, 2015.
5. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC8). JAMA. 2014;311:507-520.
Status Report From the American Acne & Rosacea Society on Medical Management of Acne in Adult Women, Part 3: Oral Therapies
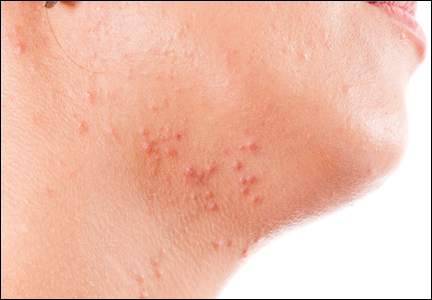
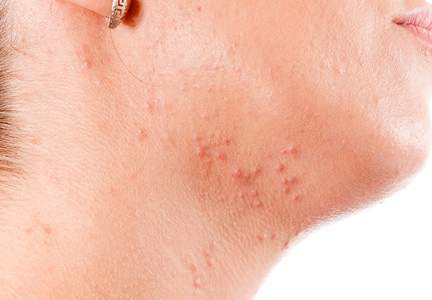
Selection of oral agents for treatment of AV in adult women is dependent on multiple factors including the patient’s age, medication history, child-bearing potential, clinical presentation, and treatment preference following a discussion of the anticipated benefits versus potential risks.1,2 In patients with the mixed inflammatory and comedonal clinical pattern of AV, oral antibiotics can be used concurrently with topical therapies when moderate to severe inflammatory lesions are noted.3,4 However, many adult women who had AV as teenagers have already utilized oral antibiotic therapies in the past and often are interested in alternative options, express concerns regarding antibiotic resistance, report a history of antibiotic-associated yeast infections or other side effects, and/or encounter issues related to drug-drug interactions.3,5-8 Oral hormonal therapies such as combination oral contraceptives (COCs) or spironolactone often are utilized to treat adult women with AV, sometimes in combination with each other or other agents. Combination oral contraceptives appear to be especially effective in the management of the U-shaped clinical pattern or predominantly inflammatory, late-onset AV.1,5,9,10 Potential warnings, contraindications, adverse effects, and drug-drug interactions are important to keep in mind when considering the use of oral hormonal therapies.8-10 Oral isotretinoin, which should be prescribed with strict adherence to the iPLEDGE™ program (https://www.ipledgeprogram.com/), remains a viable option for cases of severe nodular AV and selected cases of refractory inflammatory AV, especially when scarring and/or marked psychosocial distress are noted.1,2,5,11 Although it is recognized that adult women with AV typically present with either a mixed inflammatory and comedonal or U-shaped clinical pattern predominantly involving the lower face and anterolateral neck, the available data do not adequately differentiate the relative responsiveness of these clinical patterns to specific therapeutic agents.
Combination Oral Contraceptives
Combination oral contraceptives are commonly used to treat AV in adult women, including those without and those with measurable androgen excess (eg, polycystic ovary syndrome [PCOS]). Combination oral contraceptives contain ethinyl estradiol and a progestational agent (eg, progestin); the latter varies in terms of its nonselective receptor interactions and the relative magnitude or absence of androgenic effects.10,12,13 Although some COCs are approved by the US Food and Drug Administration (FDA) for AV, there is little data available to determine the comparative efficacy among these and other COCs.10,14 When choosing a COC for treatment of AV, it is best to select an agent whose effectiveness is supported by evidence from clinical studies.10,15
Mechanisms of Action
The reported mechanisms of action for COCs include inhibition of ovarian androgen production and ovulation through gonadotropin suppression; upregulated synthesis of sex hormone–binding globulin, which decreases free testosterone levels through receptor binding; and inhibition of 5α-reductase (by some progestins), which reduces conversion of testosterone to dihydrotestosterone, the active derivative that induces androgenic effects at peripheral target tissues.10,13,16,17
Therapeutic Benefits
Use of COCs to treat AV in adult women who do not have measurable androgen excess is most rational in patients who also desire a method of contraception. Multiple monotherapy studies have demonstrated the efficacy of COCs in the treatment of AV on the face and trunk.4,10,12,15,17,18 It may take a minimum of 3 monthly cycles of use before acne lesion counts begin to appreciably decrease.12,15,19-21 Initiating COC therapy during menstruation ensures the absence of pregnancy. Combination oral contraceptives may be used with other topical and oral therapies for AV.2,3,9,10 Potential ancillary benefits of COCs include normalization of the menstrual cycle; reduced premenstrual dysphoric disorder symptoms; and reduced risk of endometrial cancer (approximately 50%), ovarian cancer (approximately 40%), and colorectal cancer.22-24
Risks and Contraindications
It is important to consider the potential risks associated with the use of COCs, especially in women with AV who are not seeking a method of contraception. Side effects of COCs can include nausea, breast tenderness, breakthrough bleeding, and weight gain.25,26 Potential adverse associations of COCs are described in the Table. The major potential vascular associations include venous thromboembolism, myocardial infarction, and cerebrovascular accident, all of which are influenced by concurrent factors such as a history of smoking, age (≥35 years), and hypertension.27-32 It is recommended that blood pressure be measured before initiating COC therapy as part of the general examination.33
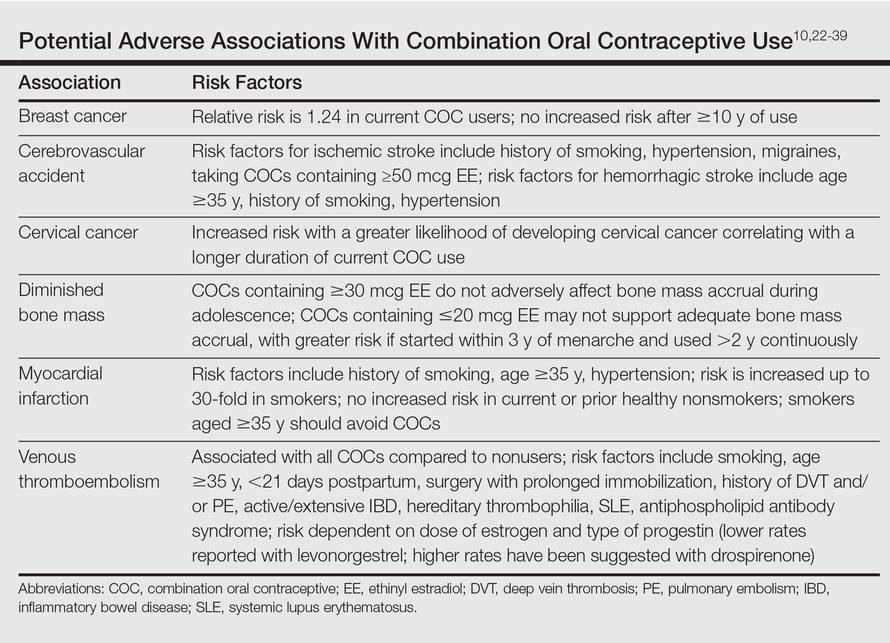
The potential increase in breast cancer risk appears to be low, while the cervical cancer risk is reported to increase relative to the duration of use.34-37 This latter observation may be due to the greater likelihood of unprotected sex in women using a COC and exposure to multiple sexual partners in some cases, which may increase the likelihood of oncogenic human papillomavirus infection of the cervix. If a dermatologist elects to prescribe a COC to treat AV, it has been suggested that the patient also consult with her general practitioner or gynecologist to undergo pelvic and breast examinations and a Papanicolaou test.33 The recommendation for initial screening for cervical cancer is within 3 years of initiation of sexual intercourse or by 21 years of age, whichever is first.33,38,39
Combination oral contraceptives are not ideal for all adult women with AV. Absolute contraindications are pregnancy and history of thromboembolic, cardiac, or hepatic disease; in women aged 35 years and older who smoke, relative contraindications include hypertension, diabetes, migraines, breastfeeding, and current breast or liver cancer.33 In adult women with AV who have relative contra-indications but are likely to benefit from the use of a COC when other options are limited or not viable, consultation with a gynecologist is prudent. Other than rifamycin antibiotics (eg, rifampin) and griseofulvin, there is no definitive evidence that oral antibiotics (eg, tetracycline) or oral antifungal agents reduce the contraceptive efficacy of COCs, although cautions remain in print within some approved package inserts.8
Spironolactone
Available since 1957, spironolactone is an oral aldos-terone antagonist and potassium-sparing diuretic used to treat hypertension and congestive heart failure.9 Recognition of its antiandrogenic effects led to its use in dermatology to treat certain dermatologic disorders in women (eg, hirsutism, alopecia, AV).1,4,5,9,10 Spironolactone is not approved for AV by the FDA; therefore, available data from multiple independent studies and retrospective analyses that have been collectively reviewed support its efficacy when used as both monotherapy or in combination with other agents in adult women with AV, especially those with a U-shaped pattern and/or late-onset AV.9,40-43
Mechanism of Action
Spironolactone inhibits sebaceous gland activity through peripheral androgen receptor blockade, inhibition of 5α-reductase, decrease in androgen production, and increase in sex hormone–binding globulin.9,10,40
Therapeutic Benefits
Good to excellent improvement of AV in women, many of whom are postadolescent, has ranged from 66% to 100% in published reports9,40-43; however, inclusion and exclusion criteria, dosing regimens, and concomitant therapies were not usually controlled. Spironolactone has been used to treat AV in adult women as monotherapy or in combination with topical agents, oral antibiotics, and COCs.9,40-42 Additionally, dose-ranging studies have not been completed with spironolactone for AV.9,40 The suggested dose range is 50 mg to 200 mg daily; however, it usually is best to start at 50 mg daily and increase to 100 mg daily if clinical response is not adequate after 2 to 3 months. The gastrointestinal (GI) absorption of spironolactone is increased when ingested with a high-fat meal.9,10
Once effective control of AV is achieved, it is optimal to use the lowest dose needed to continue reasonable suppression of new AV lesions. There is no defined end point for spironolactone use in AV, with or without concurrent PCOS, as many adult women usually continue treatment with low-dose therapy because they experience marked flaring shortly after the drug is stopped.9
Risks and Contraindications
Side effects associated with spironolactone are dose related and include increased diuresis, migraines, menstrual irregularities, breast tenderness, gynecomastia, fatigue, and dizziness.9,10,40-44 Side effects (particularly menstrual irregularities and breast tenderness) are more common at doses higher than 100 mg daily, especially when used as monotherapy without concurrent use of a COC.9,40
Spironolactone-associated hyperkalemia is most clinically relevant in patients on higher doses (eg, 100–200 mg daily), in those with renal impairment and/or congestive heart failure, and when used concurrently with certain other medications. In any patient on spironolactone, the risk of clinically relevant hyperkalemia may be increased by coingestion of potassium supplements, potassium-based salt substitutes, potassium-sparing diuretics (eg, amiloride, triamterene); aldosterone antagonists and angiotensin-converting enzyme inhibitors (eg, lisinopril, benazepril); angiotensin II receptor blockers (eg, losartan, valsartan); and tri-methoprim (with or without sulfamethoxazole).8,9,40,45 Spironolactone may also increase serum levels of lithium or digoxin.9,40,45,46 For management of AV, it is best that spironolactone be avoided in patients taking any of these medications.9
In healthy adult women with AV who are not on medications or supplements that interact adversely with spironolactone, there is no definitive recommendation regarding monitoring of serum potassium levels during treatment with spironolactone, and it has been suggested that monitoring serum potassium levels in this subgroup is not necessary.47 However, each clinician is advised to choose whether or not they wish to obtain baseline and/or periodic serum potassium levels when prescribing spironolactone for AV based on their degree of comfort and the patient’s history. Baseline and periodic blood testing to evaluate serum electrolytes and renal function are reasonable, especially as adult women with AV are usually treated with spironolactone over a prolonged period of time.9
The FDA black box warning for spironolactone states that it is tumorigenic in chronic toxicity studies in rats and refers to exposures 25- to 100-fold higher than those administered to humans.9,48 Although continued vigilance is warranted, evaluation of large populations of women treated with spironolactone do not suggest an association with increased risk of breast cancer.49,50
Spironolactone is a category C drug and thus should be avoided during pregnancy, primarily due to animal data suggesting risks of hypospadias and feminization in male fetuses.9 Importantly, there is an absence of reports linking exposure during pregnancy with congenital defects in humans, including in 2 known cases of high-dose exposures for maternal Bartter syndrome.9
The active metabolite, canrenone, is known to be present in breast milk at 0.2% of the maternal daily dose, but breastfeeding is generally believed to be safe with spironolactone based on evidence to date.9
Oral Antibiotics
Oral antibiotic therapy may be used in combination with a topical regimen to treat AV in adult women, keeping in mind some important caveats.1-7 For instance, monotherapy with oral antibiotics should be avoided, and concomitant use of benzoyl peroxide is suggested to reduce emergence of antibiotic-resistant Propionibacterium acnes strains.3,4 A therapeutic exit plan also is suggested when prescribing oral antibiotics to limit treatment to 3 to 4 months, if possible, to help mitigate the emergence of antibiotic-resistant bacteria (eg, staphylococci and streptococci).3-5,51
Tetracyclines, especially doxycycline and minocycline, are the most commonly prescribed agents. Doxycycline use warrants patient education on measures to limit the risks of esophageal and GI side effects and phototoxicity; enteric-coated and small tablet formulations have been shown to reduce GI side effects, especially when administered with food.3,52-55 In addition to vestibular side effects and hyperpigmentation, minocycline may be associated with rare but potentially severe adverse reactions such as drug hypersensitivity syndrome, autoimmune hepatitis, and lupus-like syndrome, which are reported more commonly in women.5,52,54 Vestibular side effects have been shown to decrease with use of extended-release tablets with weight-based dosing.53
Oral Isotretinoin
Oral isotretinoin is well established as highly effective for treatment of severe, recalcitrant AV, including nodular acne on the face and trunk.4,56 Currently available oral isotretinoins are branded generic formulations based on the pharmacokinetic profile of the original brand (Accutane [Roche Pharmaceuticals]) and with the use of Lidose Technology (Absorica [Cipher Pharmaceuticals]), which substantially increases GI absorption of isotretinoin in the absence of ingestion with a high-calorie, high-fat meal.57 The short- and long-term efficacy, dosing regimens, safety considerations, and serious teratogenic risks for oral isotretinoin are well published.4,56-58 Importantly, oral isotretinoin must be prescribed with strict adherence to the federally mandated iPLEDGE risk management program.
Low-dose oral isotretinoin therapy (<0.5 mg/kg–1 mg/kg daily) administered over several months longer than conventional regimens (ie, 16–20 weeks) has been suggested with demonstrated efficacy.57 However, this approach is not optimal due to the lack of established sustained clearance of AV after discontinuation of therapy and the greater potential for exposure to isotretinoin during pregnancy. Recurrences of AV do occur after completion of isotretinoin therapy, especially if cumulative systemic exposure to the drug during the initial course of treatment was inadequate.56,57
Oral isotretinoin has been shown to be effective in AV in adult women with or without PCOS with 0.5 mg/kg to 1 mg/kg daily and a total cumulative exposure of 120 mg/kg to 150 mg/kg.59 In one study, the presence of PCOS and greater number of nodules at baseline were predictive of a higher risk of relapse during the second year posttreatment.59
Conclusion
All oral therapies that are used to treat AV in adult women warrant individual consideration of possible benefits versus risks. Careful attention to possible side effects, patient-related risk factors, and potential drug-drug interactions is important. End points of therapy are not well established, with the exception of oral isotretinoin therapy. Clinicians must use their judgment in each case along with obtaining feedback from patients regarding the selection of therapy after a discussion of the available options.
- Holzmann R, Shakery K. Postadolescent acne in females. Skin Pharmacol Physiol. 2014;27(suppl 1):3-8.
- Villasenor J, Berson DS, Kroshinsky D. Treatment guidelines in adult women. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:198-207.
- Del Rosso JQ, Kim G. Optimizing use of oral antibiotics in acne vulgaris. Dermatol Clin. 2009;27:33-42.
- Gollnick H, Cunliffe W, Berson D, et al. Management of acne: report from a Global Alliance to Improve Outcomes in Acne. J Am Acad Dermatol. 2003;49(suppl 1):S1-S37.
- Fisk WA, Lev-Tov HA, Sivamani RK. Epidemiology and management of acne in adult women. Curr Derm Rep. 2014;3:29-39.
- Del Rosso JQ, Leyden JJ. Status report on antibiotic resistance: implications for the dermatologist. Dermatol Clin. 2007;25:127-132.
- Bowe WP, Leyden JJ. Clinical implications of antibiotic resistance: risk of systemic infection from Staphylococcus and Streptococcus. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:125-133.
- Del Rosso JQ. Oral antibiotic drug interactions of clinical significance to dermatologists. Dermatol Clin. 2009;27:91-94.
- Kim GK, Del Rosso JQ. Oral spironolactone in post-teenage female patients with acne vulgaris: practical considerations for the clinician based on current data and clinical experience. J Clin Aesthet Dermatol. 2012;5:37-50.
- Keri J, Berson DS, Thiboutot DM. Hormonal treatment of acne in women. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:146-155.
- American Academy of Dermatology. Position statement on isotretinoin. AAD Web site. https://www.aad.org /Forms/Policies/Uploads/PS/PS-Isotretinoin.pdf. Updated November 13, 2010. Accessed October 28, 2015.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. June 2012;7:CD004425.
- Sitruk-Ware R. Pharmacology of different progestogens: the special case of drospirenone. Climacteric. 2005;8 (suppl 3):4-12.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for the treatment of acne. Cochrane Database Syst Rev. July 2012;7:CD004425.
- Thiboutot D, Archer DF, Lemay A, et al. A randomized, controlled trial of a low-dose contraceptive containing 20 microg of ethinyl estradiol and 100 microg of levonogestrel for acne treatment. Fertil Steril. 2001;76:461-468.
- Koulianos GT. Treatment of acne with oral contraceptives: criteria for pill selection. Cutis. 2000;66:281-286.
- Rabe T, Kowald A, Ortmann J, et al. Inhibition of skin 5-alpha reductase by oral contraceptive progestins in vitro. Gynecol Endocrinol. 2000;14:223-230.
- Palli MB, Reyes-Habito CM, Lima XT, et al. A single-center, randomized double-blind, parallel-group study to examine the safety and efficacy of 3mg drospirenone/0.02mg ethinyl estradiol compared with placebo in the treatment of moderate truncal acne vulgaris. J Drugs Dermatol. 2013;12:633-637.
- Koltun W, Maloney JM, Marr J, et al. Treatment of moderate acne vulgaris using a combined oral contraceptive containing ethinylestradiol 20 μg plus drospirenone 3 mg administered in a 24/4 regimen: a pooled analysis. Eur J Obstet Gynecol Reprod Biol. 2011;155:171-175.
- Maloney JM, Dietze P, Watson D, et al. A randomized controlled trial of a low-dose combined oral contraceptive containing 3 mg drospirenone plus 20 μg ethinylestradiol in the treatment of acne vulgaris: lesion counts, investigator ratings and subject self-assessment. J Drugs Dermatol. 2009;8:837-844.
- Lucky AW, Koltun W, Thiboutot D, et al. A combined oral contraceptive containing 3-mg drospirenone/20-μg ethinyl estradiol in the treatment of acne vulgaris: a randomized, double-blind, placebo-controlled study evaluating lesion counts and participant self-assessment. Cutis. 2008;82:143-150.
- Burkman R, Schlesselman JJ, Zieman M. Safety concerns and health benefits associated with oral contraception. Am J Obstet Gynecol. 2004;190(suppl 4):S5-S22.
- Maguire K, Westhoff C. The state of hormonal contraception today: established and emerging noncontraceptive health benefits. Am J Obstet Gynecol. 2011;205 (suppl 4):S4-S8.
- Weiss NS, Sayvetz TA. Incidence of endometrial cancer in relation to the use of oral contraceptives. N Engl J Med. 1980;302:551-554.
- Tyler KH, Zirwas MJ. Contraception and the dermatologist. J Am Acad Dermatol. 2013;68:1022-1029.
- Gallo MF, Lopez LM, Grimes DA, et al. Combination contraceptives: effects on weight. Cochrane Database Syst Rev. 2008;4:CD003987.
- de Bastos M, Stegeman BH, Rosendaal FR, et al. Combined oral contraceptives: venous thrombosis. Cochrane Database Syst Rev. 2014;3:CD010813.
- Raymond EG, Burke AE, Espey E. Combined hormonal contraceptives and venous thromboembolism: putting the risks into perspective. Obstet Gynecol. 2012;119:1039-1044.
- Jick SS, Hernandez RK. Risk of non-fatal venous thromboembolism in women using oral contraceptives containing drospirenone compared with women using oral contraceptives containing levonorgestrel: case-control study using United States claims data. BMJ. 2011;342:d2151.
- US Food and Drug Administration Office of Surveillance and Epidemiology. Combined hormonal contraceptives (CHCs) and the risk of cardiovascular disease endpoints. US Food and Drug Administration Web site. http://www.fda.gov/downloads/Drugs /Drug Safety/UCM277384.pdf. Accessed October 28, 2015.
- The American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. Risk of venous thromboembolism among users of drospirenone-containing oral contraceptive pills. Obstet Gynecol. 2012;120:1239-1242.
- World Health Organization. Cardiovascular Disease and Steroid Hormone Contraception: Report of a WHO Scientific Group. Geneva, Switzerland: World Health Organization; 1998. Technical Report Series 877.
- Frangos JE, Alavian CN, Kimball AB. Acne and oral contraceptives: update on women’s health screening guidelines. J Am Acad Dermatol. 2008;58:781-786.
- Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53 297 women with breast cancer and 100 239 women without breast cancer from 54 epidemiological studies. Lancet. 1996;347:1713-1727.
- Gierisch JM, Coeytaux RR, Urrutia RP, et al. Oral contraceptive use and risk of breast, cervical, colorectal, and endometrial cancers: a systematic review. Cancer Epidemiol Biomarkers Prev. 2013;22:1931-1943.
- International Collaboration of Epidemiological Studies of Cervical Cancer. Cervical cancer and hormonal contraceptives: collaborative reanalysis of individual data for 16 573 women with cervical cancer and 35 509 women without cervical cancer from 24 epidemiological studies. Lancet. 2007;370:1609-1621.
- Agostino H, Di Meglio G. Low-dose oral contraceptives in adolescents: how low can you go? J Pediatr Adolesc Gynecol. 2010;23:195-201.
- Buzney E, Sheu J, Buzney C, et al. Polycystic ovary syndrome: a review for dermatologists: part II. Treatment. J Am Acad Dermatol. 2014;71:859.e1-859.e15.
- Stewart FH, Harper CC, Ellertson CE, et al. Clinical breast and pelvic examination requirements for hormonal contraception: current practice vs evidence. JAMA. 2001;285:2232-2239.
- Sawaya ME, Somani N. Antiandrogens and androgen inhibitors. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelpha, PA: Saunders; 2013:361-374.
- Muhlemann MF, Carter GD, Cream JJ, et al. Oral spironolactone: an effective treatment for acne vulgaris in women. Br J Dermatol. 1986;115:227-232.
- Shaw JC. Low-dose adjunctive spironolactone in the treatment of acne in women: a retrospective analysis of 85 consecutively treated patients. J Am Acad Dermatol. 2000;43:498-502.
- Sato K, Matsumoto D, Iizuka F, et al. Anti-androgenic therapy using oral spironolactone for acne vulgaris in Asians. Aesth Plast Surg. 2006;30:689-694.
- Shaw JC, White LE. Long-term safety of spironolactone in acne: results of an 8-year follow-up study. J Cutan Med Surg. 2002;6:541-545.
- Stockley I. Antihypertensive drug interactions. In: Stockley I, ed. Drug Interactions. 5th ed. London, United Kingdom: Pharmaceutical Press; 1999:335-347.
- Antoniou T, Gomes T, Mamdani MM, et al. Trimethoprim-sulfamethoxazole induced hyperkalaemia in elderly patients receiving spironolactone: nested case-control study. BMJ. 2011;343:d5228.
- Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944.
- Aldactone [package insert]. New York, NY: Pfizer Inc; 2008.
- Biggar RJ, Andersen EW, Wohlfahrt J, et al. Spironolactone use and the risk of breast and gynecologic cancers. Cancer Epidemiol. 2013;37:870-875.
- Mackenzie IS, Macdonald TM, Thompson A, et al. Spironolactone and risk of incident breast cancer in women older than 55 years: retrospective, matched cohort study. BMJ. 2012;345:e4447.
- Dreno B, Thiboutot D, Gollnick H, et al. Antibiotic stewardship in dermatology: limiting antibiotic use in acne. Eur J Dermatol. 2014;24:330-334.
- Kim S, Michaels BD, Kim GK, et al. Systemic antibacterial agents. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelpha, PA: Saunders; 2013:61-97.
- Leyden JJ, Del Rosso JQ. Oral antibiotic therapy for acne vulgaris: pharmacokinetic and pharmacodynamics perspectives. J Clin Aesthet Dermatol. 2011;4:40-47.
- Del Rosso JQ. Oral antibiotics. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:113-124.
- Del Rosso JQ. Oral doxycycline in the management of acne vulgaris: current perspectives on clinical use and recent findings with a new double-scored small tablet formulation. J Clin Aesthet Dermatol. 2015;8:19-26.
- Osofsky MG, Strauss JS. Isotretinoin. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:134-145.
- Leyden JJ, Del Rosso JQ, Baum EW. The use of isotretinoin in the treatment of acne vulgaris: clinical considerations and future directions. J Clin Aesthet Dermatol. 2014;7(suppl 2):S3-S21.
- Patton TJ, Ferris LK. Systemic retinoids. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelpha, PA: Saunders; 2013:252-268.
- Cakir GA, Erdogan FG, Gurler A. Isotretinoin treatment in nodulocystic acne with and without polycystic ovary syndrome: efficacy and determinants of relapse. Int J Dermatol. 2013;52:371-376.
Selection of oral agents for treatment of AV in adult women is dependent on multiple factors including the patient’s age, medication history, child-bearing potential, clinical presentation, and treatment preference following a discussion of the anticipated benefits versus potential risks.1,2 In patients with the mixed inflammatory and comedonal clinical pattern of AV, oral antibiotics can be used concurrently with topical therapies when moderate to severe inflammatory lesions are noted.3,4 However, many adult women who had AV as teenagers have already utilized oral antibiotic therapies in the past and often are interested in alternative options, express concerns regarding antibiotic resistance, report a history of antibiotic-associated yeast infections or other side effects, and/or encounter issues related to drug-drug interactions.3,5-8 Oral hormonal therapies such as combination oral contraceptives (COCs) or spironolactone often are utilized to treat adult women with AV, sometimes in combination with each other or other agents. Combination oral contraceptives appear to be especially effective in the management of the U-shaped clinical pattern or predominantly inflammatory, late-onset AV.1,5,9,10 Potential warnings, contraindications, adverse effects, and drug-drug interactions are important to keep in mind when considering the use of oral hormonal therapies.8-10 Oral isotretinoin, which should be prescribed with strict adherence to the iPLEDGE™ program (https://www.ipledgeprogram.com/), remains a viable option for cases of severe nodular AV and selected cases of refractory inflammatory AV, especially when scarring and/or marked psychosocial distress are noted.1,2,5,11 Although it is recognized that adult women with AV typically present with either a mixed inflammatory and comedonal or U-shaped clinical pattern predominantly involving the lower face and anterolateral neck, the available data do not adequately differentiate the relative responsiveness of these clinical patterns to specific therapeutic agents.
Combination Oral Contraceptives
Combination oral contraceptives are commonly used to treat AV in adult women, including those without and those with measurable androgen excess (eg, polycystic ovary syndrome [PCOS]). Combination oral contraceptives contain ethinyl estradiol and a progestational agent (eg, progestin); the latter varies in terms of its nonselective receptor interactions and the relative magnitude or absence of androgenic effects.10,12,13 Although some COCs are approved by the US Food and Drug Administration (FDA) for AV, there is little data available to determine the comparative efficacy among these and other COCs.10,14 When choosing a COC for treatment of AV, it is best to select an agent whose effectiveness is supported by evidence from clinical studies.10,15
Mechanisms of Action
The reported mechanisms of action for COCs include inhibition of ovarian androgen production and ovulation through gonadotropin suppression; upregulated synthesis of sex hormone–binding globulin, which decreases free testosterone levels through receptor binding; and inhibition of 5α-reductase (by some progestins), which reduces conversion of testosterone to dihydrotestosterone, the active derivative that induces androgenic effects at peripheral target tissues.10,13,16,17
Therapeutic Benefits
Use of COCs to treat AV in adult women who do not have measurable androgen excess is most rational in patients who also desire a method of contraception. Multiple monotherapy studies have demonstrated the efficacy of COCs in the treatment of AV on the face and trunk.4,10,12,15,17,18 It may take a minimum of 3 monthly cycles of use before acne lesion counts begin to appreciably decrease.12,15,19-21 Initiating COC therapy during menstruation ensures the absence of pregnancy. Combination oral contraceptives may be used with other topical and oral therapies for AV.2,3,9,10 Potential ancillary benefits of COCs include normalization of the menstrual cycle; reduced premenstrual dysphoric disorder symptoms; and reduced risk of endometrial cancer (approximately 50%), ovarian cancer (approximately 40%), and colorectal cancer.22-24
Risks and Contraindications
It is important to consider the potential risks associated with the use of COCs, especially in women with AV who are not seeking a method of contraception. Side effects of COCs can include nausea, breast tenderness, breakthrough bleeding, and weight gain.25,26 Potential adverse associations of COCs are described in the Table. The major potential vascular associations include venous thromboembolism, myocardial infarction, and cerebrovascular accident, all of which are influenced by concurrent factors such as a history of smoking, age (≥35 years), and hypertension.27-32 It is recommended that blood pressure be measured before initiating COC therapy as part of the general examination.33

The potential increase in breast cancer risk appears to be low, while the cervical cancer risk is reported to increase relative to the duration of use.34-37 This latter observation may be due to the greater likelihood of unprotected sex in women using a COC and exposure to multiple sexual partners in some cases, which may increase the likelihood of oncogenic human papillomavirus infection of the cervix. If a dermatologist elects to prescribe a COC to treat AV, it has been suggested that the patient also consult with her general practitioner or gynecologist to undergo pelvic and breast examinations and a Papanicolaou test.33 The recommendation for initial screening for cervical cancer is within 3 years of initiation of sexual intercourse or by 21 years of age, whichever is first.33,38,39
Combination oral contraceptives are not ideal for all adult women with AV. Absolute contraindications are pregnancy and history of thromboembolic, cardiac, or hepatic disease; in women aged 35 years and older who smoke, relative contraindications include hypertension, diabetes, migraines, breastfeeding, and current breast or liver cancer.33 In adult women with AV who have relative contra-indications but are likely to benefit from the use of a COC when other options are limited or not viable, consultation with a gynecologist is prudent. Other than rifamycin antibiotics (eg, rifampin) and griseofulvin, there is no definitive evidence that oral antibiotics (eg, tetracycline) or oral antifungal agents reduce the contraceptive efficacy of COCs, although cautions remain in print within some approved package inserts.8
Spironolactone
Available since 1957, spironolactone is an oral aldos-terone antagonist and potassium-sparing diuretic used to treat hypertension and congestive heart failure.9 Recognition of its antiandrogenic effects led to its use in dermatology to treat certain dermatologic disorders in women (eg, hirsutism, alopecia, AV).1,4,5,9,10 Spironolactone is not approved for AV by the FDA; therefore, available data from multiple independent studies and retrospective analyses that have been collectively reviewed support its efficacy when used as both monotherapy or in combination with other agents in adult women with AV, especially those with a U-shaped pattern and/or late-onset AV.9,40-43
Mechanism of Action
Spironolactone inhibits sebaceous gland activity through peripheral androgen receptor blockade, inhibition of 5α-reductase, decrease in androgen production, and increase in sex hormone–binding globulin.9,10,40
Therapeutic Benefits
Good to excellent improvement of AV in women, many of whom are postadolescent, has ranged from 66% to 100% in published reports9,40-43; however, inclusion and exclusion criteria, dosing regimens, and concomitant therapies were not usually controlled. Spironolactone has been used to treat AV in adult women as monotherapy or in combination with topical agents, oral antibiotics, and COCs.9,40-42 Additionally, dose-ranging studies have not been completed with spironolactone for AV.9,40 The suggested dose range is 50 mg to 200 mg daily; however, it usually is best to start at 50 mg daily and increase to 100 mg daily if clinical response is not adequate after 2 to 3 months. The gastrointestinal (GI) absorption of spironolactone is increased when ingested with a high-fat meal.9,10
Once effective control of AV is achieved, it is optimal to use the lowest dose needed to continue reasonable suppression of new AV lesions. There is no defined end point for spironolactone use in AV, with or without concurrent PCOS, as many adult women usually continue treatment with low-dose therapy because they experience marked flaring shortly after the drug is stopped.9
Risks and Contraindications
Side effects associated with spironolactone are dose related and include increased diuresis, migraines, menstrual irregularities, breast tenderness, gynecomastia, fatigue, and dizziness.9,10,40-44 Side effects (particularly menstrual irregularities and breast tenderness) are more common at doses higher than 100 mg daily, especially when used as monotherapy without concurrent use of a COC.9,40
Spironolactone-associated hyperkalemia is most clinically relevant in patients on higher doses (eg, 100–200 mg daily), in those with renal impairment and/or congestive heart failure, and when used concurrently with certain other medications. In any patient on spironolactone, the risk of clinically relevant hyperkalemia may be increased by coingestion of potassium supplements, potassium-based salt substitutes, potassium-sparing diuretics (eg, amiloride, triamterene); aldosterone antagonists and angiotensin-converting enzyme inhibitors (eg, lisinopril, benazepril); angiotensin II receptor blockers (eg, losartan, valsartan); and tri-methoprim (with or without sulfamethoxazole).8,9,40,45 Spironolactone may also increase serum levels of lithium or digoxin.9,40,45,46 For management of AV, it is best that spironolactone be avoided in patients taking any of these medications.9
In healthy adult women with AV who are not on medications or supplements that interact adversely with spironolactone, there is no definitive recommendation regarding monitoring of serum potassium levels during treatment with spironolactone, and it has been suggested that monitoring serum potassium levels in this subgroup is not necessary.47 However, each clinician is advised to choose whether or not they wish to obtain baseline and/or periodic serum potassium levels when prescribing spironolactone for AV based on their degree of comfort and the patient’s history. Baseline and periodic blood testing to evaluate serum electrolytes and renal function are reasonable, especially as adult women with AV are usually treated with spironolactone over a prolonged period of time.9
The FDA black box warning for spironolactone states that it is tumorigenic in chronic toxicity studies in rats and refers to exposures 25- to 100-fold higher than those administered to humans.9,48 Although continued vigilance is warranted, evaluation of large populations of women treated with spironolactone do not suggest an association with increased risk of breast cancer.49,50
Spironolactone is a category C drug and thus should be avoided during pregnancy, primarily due to animal data suggesting risks of hypospadias and feminization in male fetuses.9 Importantly, there is an absence of reports linking exposure during pregnancy with congenital defects in humans, including in 2 known cases of high-dose exposures for maternal Bartter syndrome.9
The active metabolite, canrenone, is known to be present in breast milk at 0.2% of the maternal daily dose, but breastfeeding is generally believed to be safe with spironolactone based on evidence to date.9
Oral Antibiotics
Oral antibiotic therapy may be used in combination with a topical regimen to treat AV in adult women, keeping in mind some important caveats.1-7 For instance, monotherapy with oral antibiotics should be avoided, and concomitant use of benzoyl peroxide is suggested to reduce emergence of antibiotic-resistant Propionibacterium acnes strains.3,4 A therapeutic exit plan also is suggested when prescribing oral antibiotics to limit treatment to 3 to 4 months, if possible, to help mitigate the emergence of antibiotic-resistant bacteria (eg, staphylococci and streptococci).3-5,51
Tetracyclines, especially doxycycline and minocycline, are the most commonly prescribed agents. Doxycycline use warrants patient education on measures to limit the risks of esophageal and GI side effects and phototoxicity; enteric-coated and small tablet formulations have been shown to reduce GI side effects, especially when administered with food.3,52-55 In addition to vestibular side effects and hyperpigmentation, minocycline may be associated with rare but potentially severe adverse reactions such as drug hypersensitivity syndrome, autoimmune hepatitis, and lupus-like syndrome, which are reported more commonly in women.5,52,54 Vestibular side effects have been shown to decrease with use of extended-release tablets with weight-based dosing.53
Oral Isotretinoin
Oral isotretinoin is well established as highly effective for treatment of severe, recalcitrant AV, including nodular acne on the face and trunk.4,56 Currently available oral isotretinoins are branded generic formulations based on the pharmacokinetic profile of the original brand (Accutane [Roche Pharmaceuticals]) and with the use of Lidose Technology (Absorica [Cipher Pharmaceuticals]), which substantially increases GI absorption of isotretinoin in the absence of ingestion with a high-calorie, high-fat meal.57 The short- and long-term efficacy, dosing regimens, safety considerations, and serious teratogenic risks for oral isotretinoin are well published.4,56-58 Importantly, oral isotretinoin must be prescribed with strict adherence to the federally mandated iPLEDGE risk management program.
Low-dose oral isotretinoin therapy (<0.5 mg/kg–1 mg/kg daily) administered over several months longer than conventional regimens (ie, 16–20 weeks) has been suggested with demonstrated efficacy.57 However, this approach is not optimal due to the lack of established sustained clearance of AV after discontinuation of therapy and the greater potential for exposure to isotretinoin during pregnancy. Recurrences of AV do occur after completion of isotretinoin therapy, especially if cumulative systemic exposure to the drug during the initial course of treatment was inadequate.56,57
Oral isotretinoin has been shown to be effective in AV in adult women with or without PCOS with 0.5 mg/kg to 1 mg/kg daily and a total cumulative exposure of 120 mg/kg to 150 mg/kg.59 In one study, the presence of PCOS and greater number of nodules at baseline were predictive of a higher risk of relapse during the second year posttreatment.59
Conclusion
All oral therapies that are used to treat AV in adult women warrant individual consideration of possible benefits versus risks. Careful attention to possible side effects, patient-related risk factors, and potential drug-drug interactions is important. End points of therapy are not well established, with the exception of oral isotretinoin therapy. Clinicians must use their judgment in each case along with obtaining feedback from patients regarding the selection of therapy after a discussion of the available options.
Selection of oral agents for treatment of AV in adult women is dependent on multiple factors including the patient’s age, medication history, child-bearing potential, clinical presentation, and treatment preference following a discussion of the anticipated benefits versus potential risks.1,2 In patients with the mixed inflammatory and comedonal clinical pattern of AV, oral antibiotics can be used concurrently with topical therapies when moderate to severe inflammatory lesions are noted.3,4 However, many adult women who had AV as teenagers have already utilized oral antibiotic therapies in the past and often are interested in alternative options, express concerns regarding antibiotic resistance, report a history of antibiotic-associated yeast infections or other side effects, and/or encounter issues related to drug-drug interactions.3,5-8 Oral hormonal therapies such as combination oral contraceptives (COCs) or spironolactone often are utilized to treat adult women with AV, sometimes in combination with each other or other agents. Combination oral contraceptives appear to be especially effective in the management of the U-shaped clinical pattern or predominantly inflammatory, late-onset AV.1,5,9,10 Potential warnings, contraindications, adverse effects, and drug-drug interactions are important to keep in mind when considering the use of oral hormonal therapies.8-10 Oral isotretinoin, which should be prescribed with strict adherence to the iPLEDGE™ program (https://www.ipledgeprogram.com/), remains a viable option for cases of severe nodular AV and selected cases of refractory inflammatory AV, especially when scarring and/or marked psychosocial distress are noted.1,2,5,11 Although it is recognized that adult women with AV typically present with either a mixed inflammatory and comedonal or U-shaped clinical pattern predominantly involving the lower face and anterolateral neck, the available data do not adequately differentiate the relative responsiveness of these clinical patterns to specific therapeutic agents.
Combination Oral Contraceptives
Combination oral contraceptives are commonly used to treat AV in adult women, including those without and those with measurable androgen excess (eg, polycystic ovary syndrome [PCOS]). Combination oral contraceptives contain ethinyl estradiol and a progestational agent (eg, progestin); the latter varies in terms of its nonselective receptor interactions and the relative magnitude or absence of androgenic effects.10,12,13 Although some COCs are approved by the US Food and Drug Administration (FDA) for AV, there is little data available to determine the comparative efficacy among these and other COCs.10,14 When choosing a COC for treatment of AV, it is best to select an agent whose effectiveness is supported by evidence from clinical studies.10,15
Mechanisms of Action
The reported mechanisms of action for COCs include inhibition of ovarian androgen production and ovulation through gonadotropin suppression; upregulated synthesis of sex hormone–binding globulin, which decreases free testosterone levels through receptor binding; and inhibition of 5α-reductase (by some progestins), which reduces conversion of testosterone to dihydrotestosterone, the active derivative that induces androgenic effects at peripheral target tissues.10,13,16,17
Therapeutic Benefits
Use of COCs to treat AV in adult women who do not have measurable androgen excess is most rational in patients who also desire a method of contraception. Multiple monotherapy studies have demonstrated the efficacy of COCs in the treatment of AV on the face and trunk.4,10,12,15,17,18 It may take a minimum of 3 monthly cycles of use before acne lesion counts begin to appreciably decrease.12,15,19-21 Initiating COC therapy during menstruation ensures the absence of pregnancy. Combination oral contraceptives may be used with other topical and oral therapies for AV.2,3,9,10 Potential ancillary benefits of COCs include normalization of the menstrual cycle; reduced premenstrual dysphoric disorder symptoms; and reduced risk of endometrial cancer (approximately 50%), ovarian cancer (approximately 40%), and colorectal cancer.22-24
Risks and Contraindications
It is important to consider the potential risks associated with the use of COCs, especially in women with AV who are not seeking a method of contraception. Side effects of COCs can include nausea, breast tenderness, breakthrough bleeding, and weight gain.25,26 Potential adverse associations of COCs are described in the Table. The major potential vascular associations include venous thromboembolism, myocardial infarction, and cerebrovascular accident, all of which are influenced by concurrent factors such as a history of smoking, age (≥35 years), and hypertension.27-32 It is recommended that blood pressure be measured before initiating COC therapy as part of the general examination.33

The potential increase in breast cancer risk appears to be low, while the cervical cancer risk is reported to increase relative to the duration of use.34-37 This latter observation may be due to the greater likelihood of unprotected sex in women using a COC and exposure to multiple sexual partners in some cases, which may increase the likelihood of oncogenic human papillomavirus infection of the cervix. If a dermatologist elects to prescribe a COC to treat AV, it has been suggested that the patient also consult with her general practitioner or gynecologist to undergo pelvic and breast examinations and a Papanicolaou test.33 The recommendation for initial screening for cervical cancer is within 3 years of initiation of sexual intercourse or by 21 years of age, whichever is first.33,38,39
Combination oral contraceptives are not ideal for all adult women with AV. Absolute contraindications are pregnancy and history of thromboembolic, cardiac, or hepatic disease; in women aged 35 years and older who smoke, relative contraindications include hypertension, diabetes, migraines, breastfeeding, and current breast or liver cancer.33 In adult women with AV who have relative contra-indications but are likely to benefit from the use of a COC when other options are limited or not viable, consultation with a gynecologist is prudent. Other than rifamycin antibiotics (eg, rifampin) and griseofulvin, there is no definitive evidence that oral antibiotics (eg, tetracycline) or oral antifungal agents reduce the contraceptive efficacy of COCs, although cautions remain in print within some approved package inserts.8
Spironolactone
Available since 1957, spironolactone is an oral aldos-terone antagonist and potassium-sparing diuretic used to treat hypertension and congestive heart failure.9 Recognition of its antiandrogenic effects led to its use in dermatology to treat certain dermatologic disorders in women (eg, hirsutism, alopecia, AV).1,4,5,9,10 Spironolactone is not approved for AV by the FDA; therefore, available data from multiple independent studies and retrospective analyses that have been collectively reviewed support its efficacy when used as both monotherapy or in combination with other agents in adult women with AV, especially those with a U-shaped pattern and/or late-onset AV.9,40-43
Mechanism of Action
Spironolactone inhibits sebaceous gland activity through peripheral androgen receptor blockade, inhibition of 5α-reductase, decrease in androgen production, and increase in sex hormone–binding globulin.9,10,40
Therapeutic Benefits
Good to excellent improvement of AV in women, many of whom are postadolescent, has ranged from 66% to 100% in published reports9,40-43; however, inclusion and exclusion criteria, dosing regimens, and concomitant therapies were not usually controlled. Spironolactone has been used to treat AV in adult women as monotherapy or in combination with topical agents, oral antibiotics, and COCs.9,40-42 Additionally, dose-ranging studies have not been completed with spironolactone for AV.9,40 The suggested dose range is 50 mg to 200 mg daily; however, it usually is best to start at 50 mg daily and increase to 100 mg daily if clinical response is not adequate after 2 to 3 months. The gastrointestinal (GI) absorption of spironolactone is increased when ingested with a high-fat meal.9,10
Once effective control of AV is achieved, it is optimal to use the lowest dose needed to continue reasonable suppression of new AV lesions. There is no defined end point for spironolactone use in AV, with or without concurrent PCOS, as many adult women usually continue treatment with low-dose therapy because they experience marked flaring shortly after the drug is stopped.9
Risks and Contraindications
Side effects associated with spironolactone are dose related and include increased diuresis, migraines, menstrual irregularities, breast tenderness, gynecomastia, fatigue, and dizziness.9,10,40-44 Side effects (particularly menstrual irregularities and breast tenderness) are more common at doses higher than 100 mg daily, especially when used as monotherapy without concurrent use of a COC.9,40
Spironolactone-associated hyperkalemia is most clinically relevant in patients on higher doses (eg, 100–200 mg daily), in those with renal impairment and/or congestive heart failure, and when used concurrently with certain other medications. In any patient on spironolactone, the risk of clinically relevant hyperkalemia may be increased by coingestion of potassium supplements, potassium-based salt substitutes, potassium-sparing diuretics (eg, amiloride, triamterene); aldosterone antagonists and angiotensin-converting enzyme inhibitors (eg, lisinopril, benazepril); angiotensin II receptor blockers (eg, losartan, valsartan); and tri-methoprim (with or without sulfamethoxazole).8,9,40,45 Spironolactone may also increase serum levels of lithium or digoxin.9,40,45,46 For management of AV, it is best that spironolactone be avoided in patients taking any of these medications.9
In healthy adult women with AV who are not on medications or supplements that interact adversely with spironolactone, there is no definitive recommendation regarding monitoring of serum potassium levels during treatment with spironolactone, and it has been suggested that monitoring serum potassium levels in this subgroup is not necessary.47 However, each clinician is advised to choose whether or not they wish to obtain baseline and/or periodic serum potassium levels when prescribing spironolactone for AV based on their degree of comfort and the patient’s history. Baseline and periodic blood testing to evaluate serum electrolytes and renal function are reasonable, especially as adult women with AV are usually treated with spironolactone over a prolonged period of time.9
The FDA black box warning for spironolactone states that it is tumorigenic in chronic toxicity studies in rats and refers to exposures 25- to 100-fold higher than those administered to humans.9,48 Although continued vigilance is warranted, evaluation of large populations of women treated with spironolactone do not suggest an association with increased risk of breast cancer.49,50
Spironolactone is a category C drug and thus should be avoided during pregnancy, primarily due to animal data suggesting risks of hypospadias and feminization in male fetuses.9 Importantly, there is an absence of reports linking exposure during pregnancy with congenital defects in humans, including in 2 known cases of high-dose exposures for maternal Bartter syndrome.9
The active metabolite, canrenone, is known to be present in breast milk at 0.2% of the maternal daily dose, but breastfeeding is generally believed to be safe with spironolactone based on evidence to date.9
Oral Antibiotics
Oral antibiotic therapy may be used in combination with a topical regimen to treat AV in adult women, keeping in mind some important caveats.1-7 For instance, monotherapy with oral antibiotics should be avoided, and concomitant use of benzoyl peroxide is suggested to reduce emergence of antibiotic-resistant Propionibacterium acnes strains.3,4 A therapeutic exit plan also is suggested when prescribing oral antibiotics to limit treatment to 3 to 4 months, if possible, to help mitigate the emergence of antibiotic-resistant bacteria (eg, staphylococci and streptococci).3-5,51
Tetracyclines, especially doxycycline and minocycline, are the most commonly prescribed agents. Doxycycline use warrants patient education on measures to limit the risks of esophageal and GI side effects and phototoxicity; enteric-coated and small tablet formulations have been shown to reduce GI side effects, especially when administered with food.3,52-55 In addition to vestibular side effects and hyperpigmentation, minocycline may be associated with rare but potentially severe adverse reactions such as drug hypersensitivity syndrome, autoimmune hepatitis, and lupus-like syndrome, which are reported more commonly in women.5,52,54 Vestibular side effects have been shown to decrease with use of extended-release tablets with weight-based dosing.53
Oral Isotretinoin
Oral isotretinoin is well established as highly effective for treatment of severe, recalcitrant AV, including nodular acne on the face and trunk.4,56 Currently available oral isotretinoins are branded generic formulations based on the pharmacokinetic profile of the original brand (Accutane [Roche Pharmaceuticals]) and with the use of Lidose Technology (Absorica [Cipher Pharmaceuticals]), which substantially increases GI absorption of isotretinoin in the absence of ingestion with a high-calorie, high-fat meal.57 The short- and long-term efficacy, dosing regimens, safety considerations, and serious teratogenic risks for oral isotretinoin are well published.4,56-58 Importantly, oral isotretinoin must be prescribed with strict adherence to the federally mandated iPLEDGE risk management program.
Low-dose oral isotretinoin therapy (<0.5 mg/kg–1 mg/kg daily) administered over several months longer than conventional regimens (ie, 16–20 weeks) has been suggested with demonstrated efficacy.57 However, this approach is not optimal due to the lack of established sustained clearance of AV after discontinuation of therapy and the greater potential for exposure to isotretinoin during pregnancy. Recurrences of AV do occur after completion of isotretinoin therapy, especially if cumulative systemic exposure to the drug during the initial course of treatment was inadequate.56,57
Oral isotretinoin has been shown to be effective in AV in adult women with or without PCOS with 0.5 mg/kg to 1 mg/kg daily and a total cumulative exposure of 120 mg/kg to 150 mg/kg.59 In one study, the presence of PCOS and greater number of nodules at baseline were predictive of a higher risk of relapse during the second year posttreatment.59
Conclusion
All oral therapies that are used to treat AV in adult women warrant individual consideration of possible benefits versus risks. Careful attention to possible side effects, patient-related risk factors, and potential drug-drug interactions is important. End points of therapy are not well established, with the exception of oral isotretinoin therapy. Clinicians must use their judgment in each case along with obtaining feedback from patients regarding the selection of therapy after a discussion of the available options.
- Holzmann R, Shakery K. Postadolescent acne in females. Skin Pharmacol Physiol. 2014;27(suppl 1):3-8.
- Villasenor J, Berson DS, Kroshinsky D. Treatment guidelines in adult women. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:198-207.
- Del Rosso JQ, Kim G. Optimizing use of oral antibiotics in acne vulgaris. Dermatol Clin. 2009;27:33-42.
- Gollnick H, Cunliffe W, Berson D, et al. Management of acne: report from a Global Alliance to Improve Outcomes in Acne. J Am Acad Dermatol. 2003;49(suppl 1):S1-S37.
- Fisk WA, Lev-Tov HA, Sivamani RK. Epidemiology and management of acne in adult women. Curr Derm Rep. 2014;3:29-39.
- Del Rosso JQ, Leyden JJ. Status report on antibiotic resistance: implications for the dermatologist. Dermatol Clin. 2007;25:127-132.
- Bowe WP, Leyden JJ. Clinical implications of antibiotic resistance: risk of systemic infection from Staphylococcus and Streptococcus. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:125-133.
- Del Rosso JQ. Oral antibiotic drug interactions of clinical significance to dermatologists. Dermatol Clin. 2009;27:91-94.
- Kim GK, Del Rosso JQ. Oral spironolactone in post-teenage female patients with acne vulgaris: practical considerations for the clinician based on current data and clinical experience. J Clin Aesthet Dermatol. 2012;5:37-50.
- Keri J, Berson DS, Thiboutot DM. Hormonal treatment of acne in women. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:146-155.
- American Academy of Dermatology. Position statement on isotretinoin. AAD Web site. https://www.aad.org /Forms/Policies/Uploads/PS/PS-Isotretinoin.pdf. Updated November 13, 2010. Accessed October 28, 2015.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. June 2012;7:CD004425.
- Sitruk-Ware R. Pharmacology of different progestogens: the special case of drospirenone. Climacteric. 2005;8 (suppl 3):4-12.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for the treatment of acne. Cochrane Database Syst Rev. July 2012;7:CD004425.
- Thiboutot D, Archer DF, Lemay A, et al. A randomized, controlled trial of a low-dose contraceptive containing 20 microg of ethinyl estradiol and 100 microg of levonogestrel for acne treatment. Fertil Steril. 2001;76:461-468.
- Koulianos GT. Treatment of acne with oral contraceptives: criteria for pill selection. Cutis. 2000;66:281-286.
- Rabe T, Kowald A, Ortmann J, et al. Inhibition of skin 5-alpha reductase by oral contraceptive progestins in vitro. Gynecol Endocrinol. 2000;14:223-230.
- Palli MB, Reyes-Habito CM, Lima XT, et al. A single-center, randomized double-blind, parallel-group study to examine the safety and efficacy of 3mg drospirenone/0.02mg ethinyl estradiol compared with placebo in the treatment of moderate truncal acne vulgaris. J Drugs Dermatol. 2013;12:633-637.
- Koltun W, Maloney JM, Marr J, et al. Treatment of moderate acne vulgaris using a combined oral contraceptive containing ethinylestradiol 20 μg plus drospirenone 3 mg administered in a 24/4 regimen: a pooled analysis. Eur J Obstet Gynecol Reprod Biol. 2011;155:171-175.
- Maloney JM, Dietze P, Watson D, et al. A randomized controlled trial of a low-dose combined oral contraceptive containing 3 mg drospirenone plus 20 μg ethinylestradiol in the treatment of acne vulgaris: lesion counts, investigator ratings and subject self-assessment. J Drugs Dermatol. 2009;8:837-844.
- Lucky AW, Koltun W, Thiboutot D, et al. A combined oral contraceptive containing 3-mg drospirenone/20-μg ethinyl estradiol in the treatment of acne vulgaris: a randomized, double-blind, placebo-controlled study evaluating lesion counts and participant self-assessment. Cutis. 2008;82:143-150.
- Burkman R, Schlesselman JJ, Zieman M. Safety concerns and health benefits associated with oral contraception. Am J Obstet Gynecol. 2004;190(suppl 4):S5-S22.
- Maguire K, Westhoff C. The state of hormonal contraception today: established and emerging noncontraceptive health benefits. Am J Obstet Gynecol. 2011;205 (suppl 4):S4-S8.
- Weiss NS, Sayvetz TA. Incidence of endometrial cancer in relation to the use of oral contraceptives. N Engl J Med. 1980;302:551-554.
- Tyler KH, Zirwas MJ. Contraception and the dermatologist. J Am Acad Dermatol. 2013;68:1022-1029.
- Gallo MF, Lopez LM, Grimes DA, et al. Combination contraceptives: effects on weight. Cochrane Database Syst Rev. 2008;4:CD003987.
- de Bastos M, Stegeman BH, Rosendaal FR, et al. Combined oral contraceptives: venous thrombosis. Cochrane Database Syst Rev. 2014;3:CD010813.
- Raymond EG, Burke AE, Espey E. Combined hormonal contraceptives and venous thromboembolism: putting the risks into perspective. Obstet Gynecol. 2012;119:1039-1044.
- Jick SS, Hernandez RK. Risk of non-fatal venous thromboembolism in women using oral contraceptives containing drospirenone compared with women using oral contraceptives containing levonorgestrel: case-control study using United States claims data. BMJ. 2011;342:d2151.
- US Food and Drug Administration Office of Surveillance and Epidemiology. Combined hormonal contraceptives (CHCs) and the risk of cardiovascular disease endpoints. US Food and Drug Administration Web site. http://www.fda.gov/downloads/Drugs /Drug Safety/UCM277384.pdf. Accessed October 28, 2015.
- The American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. Risk of venous thromboembolism among users of drospirenone-containing oral contraceptive pills. Obstet Gynecol. 2012;120:1239-1242.
- World Health Organization. Cardiovascular Disease and Steroid Hormone Contraception: Report of a WHO Scientific Group. Geneva, Switzerland: World Health Organization; 1998. Technical Report Series 877.
- Frangos JE, Alavian CN, Kimball AB. Acne and oral contraceptives: update on women’s health screening guidelines. J Am Acad Dermatol. 2008;58:781-786.
- Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53 297 women with breast cancer and 100 239 women without breast cancer from 54 epidemiological studies. Lancet. 1996;347:1713-1727.
- Gierisch JM, Coeytaux RR, Urrutia RP, et al. Oral contraceptive use and risk of breast, cervical, colorectal, and endometrial cancers: a systematic review. Cancer Epidemiol Biomarkers Prev. 2013;22:1931-1943.
- International Collaboration of Epidemiological Studies of Cervical Cancer. Cervical cancer and hormonal contraceptives: collaborative reanalysis of individual data for 16 573 women with cervical cancer and 35 509 women without cervical cancer from 24 epidemiological studies. Lancet. 2007;370:1609-1621.
- Agostino H, Di Meglio G. Low-dose oral contraceptives in adolescents: how low can you go? J Pediatr Adolesc Gynecol. 2010;23:195-201.
- Buzney E, Sheu J, Buzney C, et al. Polycystic ovary syndrome: a review for dermatologists: part II. Treatment. J Am Acad Dermatol. 2014;71:859.e1-859.e15.
- Stewart FH, Harper CC, Ellertson CE, et al. Clinical breast and pelvic examination requirements for hormonal contraception: current practice vs evidence. JAMA. 2001;285:2232-2239.
- Sawaya ME, Somani N. Antiandrogens and androgen inhibitors. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelpha, PA: Saunders; 2013:361-374.
- Muhlemann MF, Carter GD, Cream JJ, et al. Oral spironolactone: an effective treatment for acne vulgaris in women. Br J Dermatol. 1986;115:227-232.
- Shaw JC. Low-dose adjunctive spironolactone in the treatment of acne in women: a retrospective analysis of 85 consecutively treated patients. J Am Acad Dermatol. 2000;43:498-502.
- Sato K, Matsumoto D, Iizuka F, et al. Anti-androgenic therapy using oral spironolactone for acne vulgaris in Asians. Aesth Plast Surg. 2006;30:689-694.
- Shaw JC, White LE. Long-term safety of spironolactone in acne: results of an 8-year follow-up study. J Cutan Med Surg. 2002;6:541-545.
- Stockley I. Antihypertensive drug interactions. In: Stockley I, ed. Drug Interactions. 5th ed. London, United Kingdom: Pharmaceutical Press; 1999:335-347.
- Antoniou T, Gomes T, Mamdani MM, et al. Trimethoprim-sulfamethoxazole induced hyperkalaemia in elderly patients receiving spironolactone: nested case-control study. BMJ. 2011;343:d5228.
- Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944.
- Aldactone [package insert]. New York, NY: Pfizer Inc; 2008.
- Biggar RJ, Andersen EW, Wohlfahrt J, et al. Spironolactone use and the risk of breast and gynecologic cancers. Cancer Epidemiol. 2013;37:870-875.
- Mackenzie IS, Macdonald TM, Thompson A, et al. Spironolactone and risk of incident breast cancer in women older than 55 years: retrospective, matched cohort study. BMJ. 2012;345:e4447.
- Dreno B, Thiboutot D, Gollnick H, et al. Antibiotic stewardship in dermatology: limiting antibiotic use in acne. Eur J Dermatol. 2014;24:330-334.
- Kim S, Michaels BD, Kim GK, et al. Systemic antibacterial agents. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelpha, PA: Saunders; 2013:61-97.
- Leyden JJ, Del Rosso JQ. Oral antibiotic therapy for acne vulgaris: pharmacokinetic and pharmacodynamics perspectives. J Clin Aesthet Dermatol. 2011;4:40-47.
- Del Rosso JQ. Oral antibiotics. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:113-124.
- Del Rosso JQ. Oral doxycycline in the management of acne vulgaris: current perspectives on clinical use and recent findings with a new double-scored small tablet formulation. J Clin Aesthet Dermatol. 2015;8:19-26.
- Osofsky MG, Strauss JS. Isotretinoin. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:134-145.
- Leyden JJ, Del Rosso JQ, Baum EW. The use of isotretinoin in the treatment of acne vulgaris: clinical considerations and future directions. J Clin Aesthet Dermatol. 2014;7(suppl 2):S3-S21.
- Patton TJ, Ferris LK. Systemic retinoids. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelpha, PA: Saunders; 2013:252-268.
- Cakir GA, Erdogan FG, Gurler A. Isotretinoin treatment in nodulocystic acne with and without polycystic ovary syndrome: efficacy and determinants of relapse. Int J Dermatol. 2013;52:371-376.
- Holzmann R, Shakery K. Postadolescent acne in females. Skin Pharmacol Physiol. 2014;27(suppl 1):3-8.
- Villasenor J, Berson DS, Kroshinsky D. Treatment guidelines in adult women. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:198-207.
- Del Rosso JQ, Kim G. Optimizing use of oral antibiotics in acne vulgaris. Dermatol Clin. 2009;27:33-42.
- Gollnick H, Cunliffe W, Berson D, et al. Management of acne: report from a Global Alliance to Improve Outcomes in Acne. J Am Acad Dermatol. 2003;49(suppl 1):S1-S37.
- Fisk WA, Lev-Tov HA, Sivamani RK. Epidemiology and management of acne in adult women. Curr Derm Rep. 2014;3:29-39.
- Del Rosso JQ, Leyden JJ. Status report on antibiotic resistance: implications for the dermatologist. Dermatol Clin. 2007;25:127-132.
- Bowe WP, Leyden JJ. Clinical implications of antibiotic resistance: risk of systemic infection from Staphylococcus and Streptococcus. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:125-133.
- Del Rosso JQ. Oral antibiotic drug interactions of clinical significance to dermatologists. Dermatol Clin. 2009;27:91-94.
- Kim GK, Del Rosso JQ. Oral spironolactone in post-teenage female patients with acne vulgaris: practical considerations for the clinician based on current data and clinical experience. J Clin Aesthet Dermatol. 2012;5:37-50.
- Keri J, Berson DS, Thiboutot DM. Hormonal treatment of acne in women. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:146-155.
- American Academy of Dermatology. Position statement on isotretinoin. AAD Web site. https://www.aad.org /Forms/Policies/Uploads/PS/PS-Isotretinoin.pdf. Updated November 13, 2010. Accessed October 28, 2015.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. June 2012;7:CD004425.
- Sitruk-Ware R. Pharmacology of different progestogens: the special case of drospirenone. Climacteric. 2005;8 (suppl 3):4-12.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for the treatment of acne. Cochrane Database Syst Rev. July 2012;7:CD004425.
- Thiboutot D, Archer DF, Lemay A, et al. A randomized, controlled trial of a low-dose contraceptive containing 20 microg of ethinyl estradiol and 100 microg of levonogestrel for acne treatment. Fertil Steril. 2001;76:461-468.
- Koulianos GT. Treatment of acne with oral contraceptives: criteria for pill selection. Cutis. 2000;66:281-286.
- Rabe T, Kowald A, Ortmann J, et al. Inhibition of skin 5-alpha reductase by oral contraceptive progestins in vitro. Gynecol Endocrinol. 2000;14:223-230.
- Palli MB, Reyes-Habito CM, Lima XT, et al. A single-center, randomized double-blind, parallel-group study to examine the safety and efficacy of 3mg drospirenone/0.02mg ethinyl estradiol compared with placebo in the treatment of moderate truncal acne vulgaris. J Drugs Dermatol. 2013;12:633-637.
- Koltun W, Maloney JM, Marr J, et al. Treatment of moderate acne vulgaris using a combined oral contraceptive containing ethinylestradiol 20 μg plus drospirenone 3 mg administered in a 24/4 regimen: a pooled analysis. Eur J Obstet Gynecol Reprod Biol. 2011;155:171-175.
- Maloney JM, Dietze P, Watson D, et al. A randomized controlled trial of a low-dose combined oral contraceptive containing 3 mg drospirenone plus 20 μg ethinylestradiol in the treatment of acne vulgaris: lesion counts, investigator ratings and subject self-assessment. J Drugs Dermatol. 2009;8:837-844.
- Lucky AW, Koltun W, Thiboutot D, et al. A combined oral contraceptive containing 3-mg drospirenone/20-μg ethinyl estradiol in the treatment of acne vulgaris: a randomized, double-blind, placebo-controlled study evaluating lesion counts and participant self-assessment. Cutis. 2008;82:143-150.
- Burkman R, Schlesselman JJ, Zieman M. Safety concerns and health benefits associated with oral contraception. Am J Obstet Gynecol. 2004;190(suppl 4):S5-S22.
- Maguire K, Westhoff C. The state of hormonal contraception today: established and emerging noncontraceptive health benefits. Am J Obstet Gynecol. 2011;205 (suppl 4):S4-S8.
- Weiss NS, Sayvetz TA. Incidence of endometrial cancer in relation to the use of oral contraceptives. N Engl J Med. 1980;302:551-554.
- Tyler KH, Zirwas MJ. Contraception and the dermatologist. J Am Acad Dermatol. 2013;68:1022-1029.
- Gallo MF, Lopez LM, Grimes DA, et al. Combination contraceptives: effects on weight. Cochrane Database Syst Rev. 2008;4:CD003987.
- de Bastos M, Stegeman BH, Rosendaal FR, et al. Combined oral contraceptives: venous thrombosis. Cochrane Database Syst Rev. 2014;3:CD010813.
- Raymond EG, Burke AE, Espey E. Combined hormonal contraceptives and venous thromboembolism: putting the risks into perspective. Obstet Gynecol. 2012;119:1039-1044.
- Jick SS, Hernandez RK. Risk of non-fatal venous thromboembolism in women using oral contraceptives containing drospirenone compared with women using oral contraceptives containing levonorgestrel: case-control study using United States claims data. BMJ. 2011;342:d2151.
- US Food and Drug Administration Office of Surveillance and Epidemiology. Combined hormonal contraceptives (CHCs) and the risk of cardiovascular disease endpoints. US Food and Drug Administration Web site. http://www.fda.gov/downloads/Drugs /Drug Safety/UCM277384.pdf. Accessed October 28, 2015.
- The American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. Risk of venous thromboembolism among users of drospirenone-containing oral contraceptive pills. Obstet Gynecol. 2012;120:1239-1242.
- World Health Organization. Cardiovascular Disease and Steroid Hormone Contraception: Report of a WHO Scientific Group. Geneva, Switzerland: World Health Organization; 1998. Technical Report Series 877.
- Frangos JE, Alavian CN, Kimball AB. Acne and oral contraceptives: update on women’s health screening guidelines. J Am Acad Dermatol. 2008;58:781-786.
- Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53 297 women with breast cancer and 100 239 women without breast cancer from 54 epidemiological studies. Lancet. 1996;347:1713-1727.
- Gierisch JM, Coeytaux RR, Urrutia RP, et al. Oral contraceptive use and risk of breast, cervical, colorectal, and endometrial cancers: a systematic review. Cancer Epidemiol Biomarkers Prev. 2013;22:1931-1943.
- International Collaboration of Epidemiological Studies of Cervical Cancer. Cervical cancer and hormonal contraceptives: collaborative reanalysis of individual data for 16 573 women with cervical cancer and 35 509 women without cervical cancer from 24 epidemiological studies. Lancet. 2007;370:1609-1621.
- Agostino H, Di Meglio G. Low-dose oral contraceptives in adolescents: how low can you go? J Pediatr Adolesc Gynecol. 2010;23:195-201.
- Buzney E, Sheu J, Buzney C, et al. Polycystic ovary syndrome: a review for dermatologists: part II. Treatment. J Am Acad Dermatol. 2014;71:859.e1-859.e15.
- Stewart FH, Harper CC, Ellertson CE, et al. Clinical breast and pelvic examination requirements for hormonal contraception: current practice vs evidence. JAMA. 2001;285:2232-2239.
- Sawaya ME, Somani N. Antiandrogens and androgen inhibitors. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelpha, PA: Saunders; 2013:361-374.
- Muhlemann MF, Carter GD, Cream JJ, et al. Oral spironolactone: an effective treatment for acne vulgaris in women. Br J Dermatol. 1986;115:227-232.
- Shaw JC. Low-dose adjunctive spironolactone in the treatment of acne in women: a retrospective analysis of 85 consecutively treated patients. J Am Acad Dermatol. 2000;43:498-502.
- Sato K, Matsumoto D, Iizuka F, et al. Anti-androgenic therapy using oral spironolactone for acne vulgaris in Asians. Aesth Plast Surg. 2006;30:689-694.
- Shaw JC, White LE. Long-term safety of spironolactone in acne: results of an 8-year follow-up study. J Cutan Med Surg. 2002;6:541-545.
- Stockley I. Antihypertensive drug interactions. In: Stockley I, ed. Drug Interactions. 5th ed. London, United Kingdom: Pharmaceutical Press; 1999:335-347.
- Antoniou T, Gomes T, Mamdani MM, et al. Trimethoprim-sulfamethoxazole induced hyperkalaemia in elderly patients receiving spironolactone: nested case-control study. BMJ. 2011;343:d5228.
- Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944.
- Aldactone [package insert]. New York, NY: Pfizer Inc; 2008.
- Biggar RJ, Andersen EW, Wohlfahrt J, et al. Spironolactone use and the risk of breast and gynecologic cancers. Cancer Epidemiol. 2013;37:870-875.
- Mackenzie IS, Macdonald TM, Thompson A, et al. Spironolactone and risk of incident breast cancer in women older than 55 years: retrospective, matched cohort study. BMJ. 2012;345:e4447.
- Dreno B, Thiboutot D, Gollnick H, et al. Antibiotic stewardship in dermatology: limiting antibiotic use in acne. Eur J Dermatol. 2014;24:330-334.
- Kim S, Michaels BD, Kim GK, et al. Systemic antibacterial agents. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelpha, PA: Saunders; 2013:61-97.
- Leyden JJ, Del Rosso JQ. Oral antibiotic therapy for acne vulgaris: pharmacokinetic and pharmacodynamics perspectives. J Clin Aesthet Dermatol. 2011;4:40-47.
- Del Rosso JQ. Oral antibiotics. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:113-124.
- Del Rosso JQ. Oral doxycycline in the management of acne vulgaris: current perspectives on clinical use and recent findings with a new double-scored small tablet formulation. J Clin Aesthet Dermatol. 2015;8:19-26.
- Osofsky MG, Strauss JS. Isotretinoin. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:134-145.
- Leyden JJ, Del Rosso JQ, Baum EW. The use of isotretinoin in the treatment of acne vulgaris: clinical considerations and future directions. J Clin Aesthet Dermatol. 2014;7(suppl 2):S3-S21.
- Patton TJ, Ferris LK. Systemic retinoids. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelpha, PA: Saunders; 2013:252-268.
- Cakir GA, Erdogan FG, Gurler A. Isotretinoin treatment in nodulocystic acne with and without polycystic ovary syndrome: efficacy and determinants of relapse. Int J Dermatol. 2013;52:371-376.
Practice Points
- Use of combination oral contraceptives to treat acne vulgaris (AV) in adult women who do not have measurable androgen excess is most rational in patients who also desire a method of contraception.
- Spironolactone is widely accepted as an oral agent that can be effective in treating adult women with AV and may be used in combination with other therapies.
- Monotherapy with oral antibiotics should be avoided in the treatment of adult women with AV, and concomitant use of benzoyl peroxide is suggested to reduce emergence of antibiotic-resistant Propionibacterium acnes strains.
- Oral isotretinoin use in adult women with AV warrants strict adherence to pregnancy prevention measures and requirements set forth by the federally mandated iPLEDGE™ risk management program.
Status Report From the American Acne & Rosacea Society on Medical Management of Acne in Adult Women, Part 2: Topical Therapies
It seems intuitive that clinicians in dermatology would automatically recognize the importance of proper selection and integration of skin care products and techniques in the management of acne vulgaris (AV). However, an understanding of the fundamental importance of skin care in AV management and the scientific basis for maintaining epidermal barrier (EpB) function and repair cannot be assumed. In fact, there is limited scientific information about EpB dysfunction and AV or the adjunctive benefits of specific skin care products. However, some data have emerged that can be successfully applied by clinicians.1-9
In part 2 of this series, emphasis is placed on skin care and topical therapies for the treatment of AV in adult women. In addition to the plethora of cleanser and moisturizer formulations that exist in the marketplace, there are many over-the-counter (OTC) products marketed to treat AV that contain benzoyl peroxide (BP) and salicylic acid. Importantly, women tend to be selective about what they use to cleanse and moisturize their skin, and use of OTC products to treat AV is common among adult women.10,11
A thorough discussion of EpB impairment, related inflammatory cascades, and potential relevance to AV are beyond the scope of this article. In short, appropriate skin care products can reduce the inflammation and sensitivity associated with increased transepidermal water loss and reduced stratum corneum hydration and can mitigate EpB impairments induced by certain acne medications or vehicles.1,12 Available data support the adjunctive benefit of proper skin care in the management of AV by mitigating cutaneous irritation and potentially contributing to a reduction in AV lesions.2-4,7,13 Use of a formulation that also provides broad-spectrum photoprotection also is helpful.3,4
Another challenge is the myriad of cosmeceuticals that are heavily marketed to adult women with AV.13,14 Unfortunately, the scientific evidence supporting these products for treatment of AV is limited, resulting in the clinician’s inability to make specific recommendations. The core message is to incorporate skin care products that can reduce EpB impairment and mitigate cutaneous irritation associated with some AV therapies.1-4,7-9,12
OTC Topical Therapies
The marketplace is replete with several OTC products for treatment of AV, most of which contain BP and salicylic acid.15,16 There is a lack of efficacy data for OTC products for AV, including cleansers and topical medications, although some may be beneficial for milder cases. A variety of formulations are available to choose from, usually without the advice of a clinician. Additionally, heavy marketing is directed at adult women with AV, which may promote the use of therapies that may not be optimal for their respective AV severity or may cause facial skin irritation. Self-treatment may also cause delay in seeking dermatologic care, increasing the risk of persistent or permanent sequelae. Delay in adequate treatment is a major risk factor for the development of acne scars.17
Prescription Topical Therapies
Despite the high prevalence of AV in adult women, there is a paucity of studies evaluating topical therapies for AV in this subset.18-24 Reports in the literature on AV in adult women have focused on systemic hormonal agents (eg, oral contraceptives, spironolactone); however, more recent reports have addressed the use of topical therapies in this subpopulation.11,25-30 Published data on topical formulations are predominantly post hoc analyses from pivotal randomized controlled trials (RCTs) that included adolescents and adults of both genders with facial AV located above the jawline and predominantly moderate in severity.11,26,28,30 Participants in all of these studies presented with non-nodular, mixed inflammatory, and comedonal facial AV above the jawline, with inclusion criteria that required a minimum of 20 comedonal lesions and 20 papulopustular lesions at baseline. An important differentiating factor among these various post hoc analyses evaluating adult women versus adolescent girls with AV are the ages used to separate adults from adolescents. A dividing line of 18 years and older was used in some reports (eg, adapalene gel 0.3%, dapsone gel 5%), while other reports used 25 years and older to separate adolescent girls from adult women (ie, clindamycin phosphate [CP] 1.2%– BP 3.75% gel, adapalene 0.1%–BP 2.5% gel).11,26,28,30
Importantly, these studies included adult women with AV who presented with mixed comedonal and inflammatory AV (mixed pattern AV) similar to adolescents. None of the studies included women with a U-shaped AV pattern or lower facial AV characterized by deep inflammatory lesions that are often tender and few in number. Unfortunately, there is a lack of data evaluating topical therapies for these patterns of AV in adult women, including AV below the jawline and on the trunk. Although mixed pattern AV has been reported to affect 75% to 90% of adult women with AV, epidemiologic data quantifying the clinical AV patterns affecting adult women are limited.11,22,29,31,32 More well-designed studies are needed.
The treatment of AV in adult women may incorporate any of the topical therapies used to treat AV in adolescents, especially as studies encompass both the adolescent and adult age ranges. This is especially true with mixed pattern AV, which is the predominant presentation in participants enrolled in clinical trials with topical therapies, especially of moderate severity.
Herein we provide a summary of the topical therapies that have been evaluated by post hoc analyses of data from pivotal studies in adult women with AV.
Adapalene Gel 0.3%
Adapalene exhibits retinoid activity with efficacy in reducing inflammatory and comedonal AV lesions shown with both 0.1% and 0.3% concentrations.33-35 Post hoc analyses of 2 pivotal RCTs of patients with facial AV showed that adapalene gel 0.3% once daily (n=74; mean age, 27.2 years) was superior to vehicle once daily (n=43; mean age, 25.2 years) in both mean and median percentage reductions of comedonal, inflammatory, and total lesions in women 18 years and older who were treated for 12 weeks; the difference in mean percentage lesion reduction from vehicle for total AV lesions was statistically significant at 12 weeks (P=.045).26 Adapalene gel 0.3% produced a favorable skin tolerability profile similar to adapalene gel 0.1%, with the most common adverse reactions being discomfort and dryness.
Advantages of topical retinoid therapy in adult women with facial AV are reduction in postinflammatory hyperpigmentation and therapeutic modulation of chronic photodamage (eg, fine lines, rough texture, dyschromia).29,36,37 Disadvantages include signs and symptoms of cutaneous irritation, although this tends to occur less frequently on facial skin with adapalene gel 0.3% as compared to other topical retinoids that exhibit comparable efficacy.33-37 Topical retinoid therapy on the anterior neck and upper chest should be used cautiously, as these anatomic sites appear to be more prone to cutaneous irritation.
Dapsone Gel 5%
Dapsone is a sulfone antimicrobial and anti-inflammatory agent that has been shown to be effective, safe, and well tolerated in the treatment of AV in a topical 5% formulation.38,39 A post hoc analysis of pivotal 12-week trial data suggested that dapsone gel 5% twice daily produced greater AV reductions in females compared to males; no gender differences were noted in adverse effects, which were low in frequency.39 A separate subgroup analysis compared outcomes among adult women (≥18 years of age; n=434) and adolescent girls (12–17 years of age; n=347) treated with dapsone gel 5%.11 The proportion with no or minimal acne based on the Global Acne Assessment Score at week 12 was greater in adult women (53.5%) versus adolescent girls (45.3%, P=.022), with significantly greater percentage reductions in both noninflammatory (P<.0001) and total lesion counts (P=.0008) observed in the adult group. Percentage reductions in inflammatory lesions were similar in both groups. No major safety or tolerability issues or new safety signals were noted. Advantages of dapsone gel 5% are highly favorable tolerability and the perception of decreased oily skin in some participants.38,39
Clindamycin Phosphate 1.2%–Benzoyl Peroxide 3.75% Gel
The combination formulation of CP 1.2%– BP 3.75% gel applied once daily has been shown to be effective, well tolerated, and safe for the treatment of facial AV, with a gender analysis noting an apparent greater efficacy in females.40,41 A post hoc analysis from the 12-week pivotal study data in adult women aged 25 years and older showed a mean percentage change from baseline in inflammatory and noninflammatory lesion counts and the percentage of participants who achieved a 2-grade improvement by global assessment to be 68.7%, 60.4%, and 52.7% in actively treated participants (n=29), respectively, which was significantly superior to vehicle applied once daily (n=43; P=.019, P=.020, and P=.074, respectively).42 No relevant differences in tolerability were noted among treatment groups, and no participants discontinued therapy due to adverse events. Advantages of CP 1.2%–BP 3.75% gel are highly favorable skin tolerability and the perception of decreased oily skin in some participants.41-43
Adapalene 0.1%–Benzoyl Peroxide 2.5% Gel
A meta-analysis of pooled data from 3 RCTs evaluated use of adapalene 0.1%–BP 2.5% gel applied once daily in adult women aged 25 years and older with facial AV (n=130) versus vehicle gel applied once daily (n=124).30 The percentage of participants who achieved investigator global assessment ratings of clear or almost clear was 39.2% in actively treated participants versus 18.5% with vehicle (P<.001), and median percentage lesion reduction was approximately 30% greater in those treated with adapalene 0.1%–BP 2.5% gel versus vehicle gel. Tolerability and safety were favorable.
Other Agents
Topical azelaic acid (20% cream formulation, 15% gel formulation) has been suggested as a treatment option for adult women with AV, including patients with darker skin who are more prone to persistent hyperpigmentation.29
Conclusion
Proper skin care is an important component in the management of AV in adult women. Data for topical therapies in this subpopulation are limited; however, post hoc analyses provide some information regarding their efficacy in treating mixed pattern AV. More well-designed studies are needed to better evaluate the use of topical agents in adult women with AV. Although most topical AV therapies appear to be safe for use during pregnancy when properly used and limited to facial application, their use in women of childbearing potential and during pregnancy warrants individual consideration; topical retinoids are best avoided during pregnancy, especially tazarotene, which is rated category X.44 In part 3 of this series, oral therapies used to treat AV in adult women will be discussed.
1. Thiboutot D, Del Rosso JQ. Acne vulgaris and the epidermal barrier: is acne vulgaris associated with inherent epidermal abnormalities that cause impairment of barrier functions? do any topical acne therapies alter the structural and/or functional integrity of the epidermal barrier? J Clin Aesthet Dermatol. 2013;6:18-24.
2. Subramanyan K. Role of mild cleansing in the management of patient skin. Dermatol Ther. 2004;17(suppl 1):26-34.
3. Del Rosso JQ, Gold M, Rueda MJ, et al. Efficacy, safety, and subject satisfaction of a specified skin care regimen to cleanse, medicate, moisturize, and protect the skin of patients under treatment for acne vulgaris. J Clin Aesthet Dermatol. 2015;8:22-30.
4. Del Rosso JQ, Brandt S. The role of skin care as an integral component in the management of acne vulgaris: part 2: tolerability and performance of a designated skin care regimen using a foam wash and moisturizer SPF 30 in patients with acne vulgaris undergoing active treatment. J Clin Aesthet Dermatol. 2013;6:28-36.
5. Draelos ZD. Facial cosmetics for acne patients. Cosmetics in Dermatology. 2nd ed. New York, NY: Churchill Livingstone Inc; 1995:22-23.
6. Leyden JJ, Del Rosso JQ, Baum EW. The use of isotretinoin in the treatment of acne vulgaris: clinical considerations and future directions. J Clin Aesthet Dermatol. 2014;7(suppl 2):S3-S21.
7. Hayashi N, Kawashima M. Study of the usefulness of moisturizers on adherence of acne patients treated with adapalene. J Dermatol. 2014;41:592-597.
8. Isoda K, Seki T, Inoue Y, et al. Efficacy of the combined use of a facial cleanser and moisturizers for the care of mild acne patients with sensitive skin. J Dermatol. 2015;42:181-188.
9. Hensley D, Meckfessel MH. Tolerability of a skin care regimen formulated for acne-prone skin in children. Pediatr Dermatol. 2015;32:501-505.
10. Tanghetti EA, Kawata AK, Daniels SR, et al. Understanding the burden of adult female acne. J Clin Aesthet Dermatol. 2014;7:22-30.
11. Del Rosso JQ, Kircik L, Gallagher CJ. Comparative efficacy and tolerability of dapsone 5% gel in adult versus adolescent females with acne vulgaris. J Clin Aesthet Dermatol. 2015;8:31-37.
12. Del Rosso JQ, Levin J. The clinical relevance of maintaining the functional integrity of the stratum corneum in both healthy and disease-affected skin. J Clin Aesthet Dermatol. 2011;4:22-42.
13. Levin J, Momin SB. How much do we really know about our favorite cosmeceutical ingredients? J Clin Aesthet Dermatol. 2010;3:22-41.
14. Draelos ZD. Acne. In: Draelos ZD, ed. Cosmeceuticals. 2nd ed. Philadelphia, PA: Saunders-Elsevier; 2009:175-180.
15. Kircik LH, Gwazdauskas J, Butners V, et al. Evaluation of the efficacy, tolerability, and safety of an over-the-counter acne regimen containing benzoyl peroxide and salicylic acid in subjects with acne. J Drugs Dermatol. 2013;12:259-264.
16. Decker A, Graber EM. Over-the-counter acne treatments: a review. J Clin Aesthet Dermatol. 2012;5:32-40.
17. Layton AM, Henderson C, Cunliffe WJ. A clinical evaluation of acne scarring and its incidence. Clin Exp Dermatol. 1994;19:303-308.
18. Perkins AC, Maglione J, Hillebrand GG, et al. Acne vulgaris in women: prevalence across the life span. J Womens Health. 2012;21:223-230.
19. Poli F, Dreno B, Verschoore M. An epidemiological study of acne in female adults: results of a survey conducted in France. J Eur Acad Dermatol Venereol. 2001;15:541-545.
20. Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56-59.
21. Capitanio B, Sinagra JL, Bordignon V, et al. Underestimated clinical features of postadolescent acne. J Am Acad Dermatol. 2010;63:782-788.
22. Holzmann R, Shakery K. Postadolescent acne in females. Skin Pharmacol Physiol. 2014;27(suppl 1):3-8.
23. Williams C, Layton AM. Persistent acne in women: implications for the patient and for therapy. Am J Clin Dermatol. 2006;7:281-290.
24. Rendon MI, Rodriguez DA, Kawata AK, et al. Acne treatment patterns, expectations, and satisfaction among adult females of different races/ethnicities. Clin Cosmet Investig Dermatol. 2015;8:231-238.
25. Villasenor J, Berson DS, Kroshinsky D. Treatment guidelines in adult women. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:198-207.
26. Berson D, Alexis A. Adapalene 0.3% for the treatment of acne in women. J Clin Aesthet Dermatol. 2013;6:32-35.
27. Del Rosso JQ. Topical therapy for acne in women: is there a role for clindamycin phosphate–benzoyl peroxide gel? Cutis. 2014;94:177-182.
28. Zeichner JA. The efficacy and tolerability of a fixed combination (1.2%) and benzoyl peroxide (3.75%) aqueous gel in adult females with facial acne vulgaris. J Clin Aesthet Dermatol. 2015;8:21-25.
29. Fisk WA, Lev-Tov HA, Sivamani RK. Epidemiology and management of acne in adult women. Curr Derm Rep. 2014;3:29-39.
30. Stein-Gold L. Adapalene 0.1%-benzoyl peroxide 2.5% gel in adult female acne. Poster presented at: Winter Clinical Dermatology Conference; January 16–21, 2015; Maui, Hawaii.
31. Dréno B, Thiboutot D, Layton AM, et al. Large-scale international study enhances understanding of an emerging acne population: adult females. J Euro Acad Dermatol Venereol. 2015;29:1096-1106.
32. Dréno B, Layton AM, Zouboulis CC, et al. Adult female acne: a new paradigm. J Euro Acad Dermatol Venereol. 2013;27:1063-1070.
33. Shalita A, Weiss JS, Chalker DK, et al. A comparison of the efficacy and safety of adapalene gel 0.1% and tretinoin gel 0.025% in the treatment of acne vulgaris: a multicenter trial. J Am Acad Dermatol. 1996;34:482-485.
34. Pariser DM, Thiboutot DM, Clark SD, et al. The efficacy and safety of adapalene gel 0.3% in the treatment of acne vulgaris: a randomized, multicenter, investigator-blinded, controlled comparison study versus adapalene gel 0.1% and vehicle. Cutis. 2005;76:145-151.
35. Thiboutot D, Pariser DM, Egan N, et al. Adapalene gel 0.3% for the treatment of acne vulgaris: a multicenter, randomized, double-blind, controlled, phase III trial. J Am Acad Dermatol. 2006;54:242-250.
36. Tanghetti E, Dhawan S, Green L, et al. Randomized comparison of the safety and efficacy of tazarotene 0.1% cream and adapalene 0.3% gel in the treatment of patients with at least moderate facial acne vulgaris. J Drugs Dermatol. 2010;9:549-558.
37. Hui AM, Shalita AR. Topical retinoids. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:86-94.
38. Draelos Z, Carter E, Maloney JM, et al. Two randomized studies demonstrate the efficacy and safety of dapsone gel 5% for the treatment of acne vulgaris. J Am Acad Dermatol. 2007;56:439.e1-439.e10.
39. Tanghetti E, Harper JC, Oefelein MG. The efficacy and tolerability of dapsone 5% gel in female vs male patients with facial acne vulgaris: gender as a clinically relevant outcome variable. J Drugs Dermatol. 2012;11:1417-1421.
40. Pariser DM, Rich P, Cook-Bolden FE, et al. An aqueous gel fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 3.75% for the once-daily treatment of moderate to severe acne vulgaris. J Drugs Dermatol. 2014;13:1083-1089.
41. Harper JC. The efficacy and tolerability of a fixed combination clindamycin (1.2%) and benzoyl peroxide (3.75%) aqueous gel in patients with facial acne vulgaris: gender as a clinically relevant outcome variable. J Drugs Dermatol. 2015;14:381-384.
42. Zeichner JA. The efficacy and tolerability of a fixed combination clindamycin (1.2%) and benzoyl peroxide (3.75%) aqueous gel in adult female patients with facial acne vulgaris. J Clin Aesthet Dermatol. 2015;8:21-25.
43. Coley MK, Berson DS, Callendar VD. Overview of treatment principles for skin of color. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:70-85.
44. Ebede TL, Berson DS. Acne in pregnancy. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:177-181.
It seems intuitive that clinicians in dermatology would automatically recognize the importance of proper selection and integration of skin care products and techniques in the management of acne vulgaris (AV). However, an understanding of the fundamental importance of skin care in AV management and the scientific basis for maintaining epidermal barrier (EpB) function and repair cannot be assumed. In fact, there is limited scientific information about EpB dysfunction and AV or the adjunctive benefits of specific skin care products. However, some data have emerged that can be successfully applied by clinicians.1-9
In part 2 of this series, emphasis is placed on skin care and topical therapies for the treatment of AV in adult women. In addition to the plethora of cleanser and moisturizer formulations that exist in the marketplace, there are many over-the-counter (OTC) products marketed to treat AV that contain benzoyl peroxide (BP) and salicylic acid. Importantly, women tend to be selective about what they use to cleanse and moisturize their skin, and use of OTC products to treat AV is common among adult women.10,11
A thorough discussion of EpB impairment, related inflammatory cascades, and potential relevance to AV are beyond the scope of this article. In short, appropriate skin care products can reduce the inflammation and sensitivity associated with increased transepidermal water loss and reduced stratum corneum hydration and can mitigate EpB impairments induced by certain acne medications or vehicles.1,12 Available data support the adjunctive benefit of proper skin care in the management of AV by mitigating cutaneous irritation and potentially contributing to a reduction in AV lesions.2-4,7,13 Use of a formulation that also provides broad-spectrum photoprotection also is helpful.3,4
Another challenge is the myriad of cosmeceuticals that are heavily marketed to adult women with AV.13,14 Unfortunately, the scientific evidence supporting these products for treatment of AV is limited, resulting in the clinician’s inability to make specific recommendations. The core message is to incorporate skin care products that can reduce EpB impairment and mitigate cutaneous irritation associated with some AV therapies.1-4,7-9,12
OTC Topical Therapies
The marketplace is replete with several OTC products for treatment of AV, most of which contain BP and salicylic acid.15,16 There is a lack of efficacy data for OTC products for AV, including cleansers and topical medications, although some may be beneficial for milder cases. A variety of formulations are available to choose from, usually without the advice of a clinician. Additionally, heavy marketing is directed at adult women with AV, which may promote the use of therapies that may not be optimal for their respective AV severity or may cause facial skin irritation. Self-treatment may also cause delay in seeking dermatologic care, increasing the risk of persistent or permanent sequelae. Delay in adequate treatment is a major risk factor for the development of acne scars.17
Prescription Topical Therapies
Despite the high prevalence of AV in adult women, there is a paucity of studies evaluating topical therapies for AV in this subset.18-24 Reports in the literature on AV in adult women have focused on systemic hormonal agents (eg, oral contraceptives, spironolactone); however, more recent reports have addressed the use of topical therapies in this subpopulation.11,25-30 Published data on topical formulations are predominantly post hoc analyses from pivotal randomized controlled trials (RCTs) that included adolescents and adults of both genders with facial AV located above the jawline and predominantly moderate in severity.11,26,28,30 Participants in all of these studies presented with non-nodular, mixed inflammatory, and comedonal facial AV above the jawline, with inclusion criteria that required a minimum of 20 comedonal lesions and 20 papulopustular lesions at baseline. An important differentiating factor among these various post hoc analyses evaluating adult women versus adolescent girls with AV are the ages used to separate adults from adolescents. A dividing line of 18 years and older was used in some reports (eg, adapalene gel 0.3%, dapsone gel 5%), while other reports used 25 years and older to separate adolescent girls from adult women (ie, clindamycin phosphate [CP] 1.2%– BP 3.75% gel, adapalene 0.1%–BP 2.5% gel).11,26,28,30
Importantly, these studies included adult women with AV who presented with mixed comedonal and inflammatory AV (mixed pattern AV) similar to adolescents. None of the studies included women with a U-shaped AV pattern or lower facial AV characterized by deep inflammatory lesions that are often tender and few in number. Unfortunately, there is a lack of data evaluating topical therapies for these patterns of AV in adult women, including AV below the jawline and on the trunk. Although mixed pattern AV has been reported to affect 75% to 90% of adult women with AV, epidemiologic data quantifying the clinical AV patterns affecting adult women are limited.11,22,29,31,32 More well-designed studies are needed.
The treatment of AV in adult women may incorporate any of the topical therapies used to treat AV in adolescents, especially as studies encompass both the adolescent and adult age ranges. This is especially true with mixed pattern AV, which is the predominant presentation in participants enrolled in clinical trials with topical therapies, especially of moderate severity.
Herein we provide a summary of the topical therapies that have been evaluated by post hoc analyses of data from pivotal studies in adult women with AV.
Adapalene Gel 0.3%
Adapalene exhibits retinoid activity with efficacy in reducing inflammatory and comedonal AV lesions shown with both 0.1% and 0.3% concentrations.33-35 Post hoc analyses of 2 pivotal RCTs of patients with facial AV showed that adapalene gel 0.3% once daily (n=74; mean age, 27.2 years) was superior to vehicle once daily (n=43; mean age, 25.2 years) in both mean and median percentage reductions of comedonal, inflammatory, and total lesions in women 18 years and older who were treated for 12 weeks; the difference in mean percentage lesion reduction from vehicle for total AV lesions was statistically significant at 12 weeks (P=.045).26 Adapalene gel 0.3% produced a favorable skin tolerability profile similar to adapalene gel 0.1%, with the most common adverse reactions being discomfort and dryness.
Advantages of topical retinoid therapy in adult women with facial AV are reduction in postinflammatory hyperpigmentation and therapeutic modulation of chronic photodamage (eg, fine lines, rough texture, dyschromia).29,36,37 Disadvantages include signs and symptoms of cutaneous irritation, although this tends to occur less frequently on facial skin with adapalene gel 0.3% as compared to other topical retinoids that exhibit comparable efficacy.33-37 Topical retinoid therapy on the anterior neck and upper chest should be used cautiously, as these anatomic sites appear to be more prone to cutaneous irritation.
Dapsone Gel 5%
Dapsone is a sulfone antimicrobial and anti-inflammatory agent that has been shown to be effective, safe, and well tolerated in the treatment of AV in a topical 5% formulation.38,39 A post hoc analysis of pivotal 12-week trial data suggested that dapsone gel 5% twice daily produced greater AV reductions in females compared to males; no gender differences were noted in adverse effects, which were low in frequency.39 A separate subgroup analysis compared outcomes among adult women (≥18 years of age; n=434) and adolescent girls (12–17 years of age; n=347) treated with dapsone gel 5%.11 The proportion with no or minimal acne based on the Global Acne Assessment Score at week 12 was greater in adult women (53.5%) versus adolescent girls (45.3%, P=.022), with significantly greater percentage reductions in both noninflammatory (P<.0001) and total lesion counts (P=.0008) observed in the adult group. Percentage reductions in inflammatory lesions were similar in both groups. No major safety or tolerability issues or new safety signals were noted. Advantages of dapsone gel 5% are highly favorable tolerability and the perception of decreased oily skin in some participants.38,39
Clindamycin Phosphate 1.2%–Benzoyl Peroxide 3.75% Gel
The combination formulation of CP 1.2%– BP 3.75% gel applied once daily has been shown to be effective, well tolerated, and safe for the treatment of facial AV, with a gender analysis noting an apparent greater efficacy in females.40,41 A post hoc analysis from the 12-week pivotal study data in adult women aged 25 years and older showed a mean percentage change from baseline in inflammatory and noninflammatory lesion counts and the percentage of participants who achieved a 2-grade improvement by global assessment to be 68.7%, 60.4%, and 52.7% in actively treated participants (n=29), respectively, which was significantly superior to vehicle applied once daily (n=43; P=.019, P=.020, and P=.074, respectively).42 No relevant differences in tolerability were noted among treatment groups, and no participants discontinued therapy due to adverse events. Advantages of CP 1.2%–BP 3.75% gel are highly favorable skin tolerability and the perception of decreased oily skin in some participants.41-43
Adapalene 0.1%–Benzoyl Peroxide 2.5% Gel
A meta-analysis of pooled data from 3 RCTs evaluated use of adapalene 0.1%–BP 2.5% gel applied once daily in adult women aged 25 years and older with facial AV (n=130) versus vehicle gel applied once daily (n=124).30 The percentage of participants who achieved investigator global assessment ratings of clear or almost clear was 39.2% in actively treated participants versus 18.5% with vehicle (P<.001), and median percentage lesion reduction was approximately 30% greater in those treated with adapalene 0.1%–BP 2.5% gel versus vehicle gel. Tolerability and safety were favorable.
Other Agents
Topical azelaic acid (20% cream formulation, 15% gel formulation) has been suggested as a treatment option for adult women with AV, including patients with darker skin who are more prone to persistent hyperpigmentation.29
Conclusion
Proper skin care is an important component in the management of AV in adult women. Data for topical therapies in this subpopulation are limited; however, post hoc analyses provide some information regarding their efficacy in treating mixed pattern AV. More well-designed studies are needed to better evaluate the use of topical agents in adult women with AV. Although most topical AV therapies appear to be safe for use during pregnancy when properly used and limited to facial application, their use in women of childbearing potential and during pregnancy warrants individual consideration; topical retinoids are best avoided during pregnancy, especially tazarotene, which is rated category X.44 In part 3 of this series, oral therapies used to treat AV in adult women will be discussed.
It seems intuitive that clinicians in dermatology would automatically recognize the importance of proper selection and integration of skin care products and techniques in the management of acne vulgaris (AV). However, an understanding of the fundamental importance of skin care in AV management and the scientific basis for maintaining epidermal barrier (EpB) function and repair cannot be assumed. In fact, there is limited scientific information about EpB dysfunction and AV or the adjunctive benefits of specific skin care products. However, some data have emerged that can be successfully applied by clinicians.1-9
In part 2 of this series, emphasis is placed on skin care and topical therapies for the treatment of AV in adult women. In addition to the plethora of cleanser and moisturizer formulations that exist in the marketplace, there are many over-the-counter (OTC) products marketed to treat AV that contain benzoyl peroxide (BP) and salicylic acid. Importantly, women tend to be selective about what they use to cleanse and moisturize their skin, and use of OTC products to treat AV is common among adult women.10,11
A thorough discussion of EpB impairment, related inflammatory cascades, and potential relevance to AV are beyond the scope of this article. In short, appropriate skin care products can reduce the inflammation and sensitivity associated with increased transepidermal water loss and reduced stratum corneum hydration and can mitigate EpB impairments induced by certain acne medications or vehicles.1,12 Available data support the adjunctive benefit of proper skin care in the management of AV by mitigating cutaneous irritation and potentially contributing to a reduction in AV lesions.2-4,7,13 Use of a formulation that also provides broad-spectrum photoprotection also is helpful.3,4
Another challenge is the myriad of cosmeceuticals that are heavily marketed to adult women with AV.13,14 Unfortunately, the scientific evidence supporting these products for treatment of AV is limited, resulting in the clinician’s inability to make specific recommendations. The core message is to incorporate skin care products that can reduce EpB impairment and mitigate cutaneous irritation associated with some AV therapies.1-4,7-9,12
OTC Topical Therapies
The marketplace is replete with several OTC products for treatment of AV, most of which contain BP and salicylic acid.15,16 There is a lack of efficacy data for OTC products for AV, including cleansers and topical medications, although some may be beneficial for milder cases. A variety of formulations are available to choose from, usually without the advice of a clinician. Additionally, heavy marketing is directed at adult women with AV, which may promote the use of therapies that may not be optimal for their respective AV severity or may cause facial skin irritation. Self-treatment may also cause delay in seeking dermatologic care, increasing the risk of persistent or permanent sequelae. Delay in adequate treatment is a major risk factor for the development of acne scars.17
Prescription Topical Therapies
Despite the high prevalence of AV in adult women, there is a paucity of studies evaluating topical therapies for AV in this subset.18-24 Reports in the literature on AV in adult women have focused on systemic hormonal agents (eg, oral contraceptives, spironolactone); however, more recent reports have addressed the use of topical therapies in this subpopulation.11,25-30 Published data on topical formulations are predominantly post hoc analyses from pivotal randomized controlled trials (RCTs) that included adolescents and adults of both genders with facial AV located above the jawline and predominantly moderate in severity.11,26,28,30 Participants in all of these studies presented with non-nodular, mixed inflammatory, and comedonal facial AV above the jawline, with inclusion criteria that required a minimum of 20 comedonal lesions and 20 papulopustular lesions at baseline. An important differentiating factor among these various post hoc analyses evaluating adult women versus adolescent girls with AV are the ages used to separate adults from adolescents. A dividing line of 18 years and older was used in some reports (eg, adapalene gel 0.3%, dapsone gel 5%), while other reports used 25 years and older to separate adolescent girls from adult women (ie, clindamycin phosphate [CP] 1.2%– BP 3.75% gel, adapalene 0.1%–BP 2.5% gel).11,26,28,30
Importantly, these studies included adult women with AV who presented with mixed comedonal and inflammatory AV (mixed pattern AV) similar to adolescents. None of the studies included women with a U-shaped AV pattern or lower facial AV characterized by deep inflammatory lesions that are often tender and few in number. Unfortunately, there is a lack of data evaluating topical therapies for these patterns of AV in adult women, including AV below the jawline and on the trunk. Although mixed pattern AV has been reported to affect 75% to 90% of adult women with AV, epidemiologic data quantifying the clinical AV patterns affecting adult women are limited.11,22,29,31,32 More well-designed studies are needed.
The treatment of AV in adult women may incorporate any of the topical therapies used to treat AV in adolescents, especially as studies encompass both the adolescent and adult age ranges. This is especially true with mixed pattern AV, which is the predominant presentation in participants enrolled in clinical trials with topical therapies, especially of moderate severity.
Herein we provide a summary of the topical therapies that have been evaluated by post hoc analyses of data from pivotal studies in adult women with AV.
Adapalene Gel 0.3%
Adapalene exhibits retinoid activity with efficacy in reducing inflammatory and comedonal AV lesions shown with both 0.1% and 0.3% concentrations.33-35 Post hoc analyses of 2 pivotal RCTs of patients with facial AV showed that adapalene gel 0.3% once daily (n=74; mean age, 27.2 years) was superior to vehicle once daily (n=43; mean age, 25.2 years) in both mean and median percentage reductions of comedonal, inflammatory, and total lesions in women 18 years and older who were treated for 12 weeks; the difference in mean percentage lesion reduction from vehicle for total AV lesions was statistically significant at 12 weeks (P=.045).26 Adapalene gel 0.3% produced a favorable skin tolerability profile similar to adapalene gel 0.1%, with the most common adverse reactions being discomfort and dryness.
Advantages of topical retinoid therapy in adult women with facial AV are reduction in postinflammatory hyperpigmentation and therapeutic modulation of chronic photodamage (eg, fine lines, rough texture, dyschromia).29,36,37 Disadvantages include signs and symptoms of cutaneous irritation, although this tends to occur less frequently on facial skin with adapalene gel 0.3% as compared to other topical retinoids that exhibit comparable efficacy.33-37 Topical retinoid therapy on the anterior neck and upper chest should be used cautiously, as these anatomic sites appear to be more prone to cutaneous irritation.
Dapsone Gel 5%
Dapsone is a sulfone antimicrobial and anti-inflammatory agent that has been shown to be effective, safe, and well tolerated in the treatment of AV in a topical 5% formulation.38,39 A post hoc analysis of pivotal 12-week trial data suggested that dapsone gel 5% twice daily produced greater AV reductions in females compared to males; no gender differences were noted in adverse effects, which were low in frequency.39 A separate subgroup analysis compared outcomes among adult women (≥18 years of age; n=434) and adolescent girls (12–17 years of age; n=347) treated with dapsone gel 5%.11 The proportion with no or minimal acne based on the Global Acne Assessment Score at week 12 was greater in adult women (53.5%) versus adolescent girls (45.3%, P=.022), with significantly greater percentage reductions in both noninflammatory (P<.0001) and total lesion counts (P=.0008) observed in the adult group. Percentage reductions in inflammatory lesions were similar in both groups. No major safety or tolerability issues or new safety signals were noted. Advantages of dapsone gel 5% are highly favorable tolerability and the perception of decreased oily skin in some participants.38,39
Clindamycin Phosphate 1.2%–Benzoyl Peroxide 3.75% Gel
The combination formulation of CP 1.2%– BP 3.75% gel applied once daily has been shown to be effective, well tolerated, and safe for the treatment of facial AV, with a gender analysis noting an apparent greater efficacy in females.40,41 A post hoc analysis from the 12-week pivotal study data in adult women aged 25 years and older showed a mean percentage change from baseline in inflammatory and noninflammatory lesion counts and the percentage of participants who achieved a 2-grade improvement by global assessment to be 68.7%, 60.4%, and 52.7% in actively treated participants (n=29), respectively, which was significantly superior to vehicle applied once daily (n=43; P=.019, P=.020, and P=.074, respectively).42 No relevant differences in tolerability were noted among treatment groups, and no participants discontinued therapy due to adverse events. Advantages of CP 1.2%–BP 3.75% gel are highly favorable skin tolerability and the perception of decreased oily skin in some participants.41-43
Adapalene 0.1%–Benzoyl Peroxide 2.5% Gel
A meta-analysis of pooled data from 3 RCTs evaluated use of adapalene 0.1%–BP 2.5% gel applied once daily in adult women aged 25 years and older with facial AV (n=130) versus vehicle gel applied once daily (n=124).30 The percentage of participants who achieved investigator global assessment ratings of clear or almost clear was 39.2% in actively treated participants versus 18.5% with vehicle (P<.001), and median percentage lesion reduction was approximately 30% greater in those treated with adapalene 0.1%–BP 2.5% gel versus vehicle gel. Tolerability and safety were favorable.
Other Agents
Topical azelaic acid (20% cream formulation, 15% gel formulation) has been suggested as a treatment option for adult women with AV, including patients with darker skin who are more prone to persistent hyperpigmentation.29
Conclusion
Proper skin care is an important component in the management of AV in adult women. Data for topical therapies in this subpopulation are limited; however, post hoc analyses provide some information regarding their efficacy in treating mixed pattern AV. More well-designed studies are needed to better evaluate the use of topical agents in adult women with AV. Although most topical AV therapies appear to be safe for use during pregnancy when properly used and limited to facial application, their use in women of childbearing potential and during pregnancy warrants individual consideration; topical retinoids are best avoided during pregnancy, especially tazarotene, which is rated category X.44 In part 3 of this series, oral therapies used to treat AV in adult women will be discussed.
1. Thiboutot D, Del Rosso JQ. Acne vulgaris and the epidermal barrier: is acne vulgaris associated with inherent epidermal abnormalities that cause impairment of barrier functions? do any topical acne therapies alter the structural and/or functional integrity of the epidermal barrier? J Clin Aesthet Dermatol. 2013;6:18-24.
2. Subramanyan K. Role of mild cleansing in the management of patient skin. Dermatol Ther. 2004;17(suppl 1):26-34.
3. Del Rosso JQ, Gold M, Rueda MJ, et al. Efficacy, safety, and subject satisfaction of a specified skin care regimen to cleanse, medicate, moisturize, and protect the skin of patients under treatment for acne vulgaris. J Clin Aesthet Dermatol. 2015;8:22-30.
4. Del Rosso JQ, Brandt S. The role of skin care as an integral component in the management of acne vulgaris: part 2: tolerability and performance of a designated skin care regimen using a foam wash and moisturizer SPF 30 in patients with acne vulgaris undergoing active treatment. J Clin Aesthet Dermatol. 2013;6:28-36.
5. Draelos ZD. Facial cosmetics for acne patients. Cosmetics in Dermatology. 2nd ed. New York, NY: Churchill Livingstone Inc; 1995:22-23.
6. Leyden JJ, Del Rosso JQ, Baum EW. The use of isotretinoin in the treatment of acne vulgaris: clinical considerations and future directions. J Clin Aesthet Dermatol. 2014;7(suppl 2):S3-S21.
7. Hayashi N, Kawashima M. Study of the usefulness of moisturizers on adherence of acne patients treated with adapalene. J Dermatol. 2014;41:592-597.
8. Isoda K, Seki T, Inoue Y, et al. Efficacy of the combined use of a facial cleanser and moisturizers for the care of mild acne patients with sensitive skin. J Dermatol. 2015;42:181-188.
9. Hensley D, Meckfessel MH. Tolerability of a skin care regimen formulated for acne-prone skin in children. Pediatr Dermatol. 2015;32:501-505.
10. Tanghetti EA, Kawata AK, Daniels SR, et al. Understanding the burden of adult female acne. J Clin Aesthet Dermatol. 2014;7:22-30.
11. Del Rosso JQ, Kircik L, Gallagher CJ. Comparative efficacy and tolerability of dapsone 5% gel in adult versus adolescent females with acne vulgaris. J Clin Aesthet Dermatol. 2015;8:31-37.
12. Del Rosso JQ, Levin J. The clinical relevance of maintaining the functional integrity of the stratum corneum in both healthy and disease-affected skin. J Clin Aesthet Dermatol. 2011;4:22-42.
13. Levin J, Momin SB. How much do we really know about our favorite cosmeceutical ingredients? J Clin Aesthet Dermatol. 2010;3:22-41.
14. Draelos ZD. Acne. In: Draelos ZD, ed. Cosmeceuticals. 2nd ed. Philadelphia, PA: Saunders-Elsevier; 2009:175-180.
15. Kircik LH, Gwazdauskas J, Butners V, et al. Evaluation of the efficacy, tolerability, and safety of an over-the-counter acne regimen containing benzoyl peroxide and salicylic acid in subjects with acne. J Drugs Dermatol. 2013;12:259-264.
16. Decker A, Graber EM. Over-the-counter acne treatments: a review. J Clin Aesthet Dermatol. 2012;5:32-40.
17. Layton AM, Henderson C, Cunliffe WJ. A clinical evaluation of acne scarring and its incidence. Clin Exp Dermatol. 1994;19:303-308.
18. Perkins AC, Maglione J, Hillebrand GG, et al. Acne vulgaris in women: prevalence across the life span. J Womens Health. 2012;21:223-230.
19. Poli F, Dreno B, Verschoore M. An epidemiological study of acne in female adults: results of a survey conducted in France. J Eur Acad Dermatol Venereol. 2001;15:541-545.
20. Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56-59.
21. Capitanio B, Sinagra JL, Bordignon V, et al. Underestimated clinical features of postadolescent acne. J Am Acad Dermatol. 2010;63:782-788.
22. Holzmann R, Shakery K. Postadolescent acne in females. Skin Pharmacol Physiol. 2014;27(suppl 1):3-8.
23. Williams C, Layton AM. Persistent acne in women: implications for the patient and for therapy. Am J Clin Dermatol. 2006;7:281-290.
24. Rendon MI, Rodriguez DA, Kawata AK, et al. Acne treatment patterns, expectations, and satisfaction among adult females of different races/ethnicities. Clin Cosmet Investig Dermatol. 2015;8:231-238.
25. Villasenor J, Berson DS, Kroshinsky D. Treatment guidelines in adult women. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:198-207.
26. Berson D, Alexis A. Adapalene 0.3% for the treatment of acne in women. J Clin Aesthet Dermatol. 2013;6:32-35.
27. Del Rosso JQ. Topical therapy for acne in women: is there a role for clindamycin phosphate–benzoyl peroxide gel? Cutis. 2014;94:177-182.
28. Zeichner JA. The efficacy and tolerability of a fixed combination (1.2%) and benzoyl peroxide (3.75%) aqueous gel in adult females with facial acne vulgaris. J Clin Aesthet Dermatol. 2015;8:21-25.
29. Fisk WA, Lev-Tov HA, Sivamani RK. Epidemiology and management of acne in adult women. Curr Derm Rep. 2014;3:29-39.
30. Stein-Gold L. Adapalene 0.1%-benzoyl peroxide 2.5% gel in adult female acne. Poster presented at: Winter Clinical Dermatology Conference; January 16–21, 2015; Maui, Hawaii.
31. Dréno B, Thiboutot D, Layton AM, et al. Large-scale international study enhances understanding of an emerging acne population: adult females. J Euro Acad Dermatol Venereol. 2015;29:1096-1106.
32. Dréno B, Layton AM, Zouboulis CC, et al. Adult female acne: a new paradigm. J Euro Acad Dermatol Venereol. 2013;27:1063-1070.
33. Shalita A, Weiss JS, Chalker DK, et al. A comparison of the efficacy and safety of adapalene gel 0.1% and tretinoin gel 0.025% in the treatment of acne vulgaris: a multicenter trial. J Am Acad Dermatol. 1996;34:482-485.
34. Pariser DM, Thiboutot DM, Clark SD, et al. The efficacy and safety of adapalene gel 0.3% in the treatment of acne vulgaris: a randomized, multicenter, investigator-blinded, controlled comparison study versus adapalene gel 0.1% and vehicle. Cutis. 2005;76:145-151.
35. Thiboutot D, Pariser DM, Egan N, et al. Adapalene gel 0.3% for the treatment of acne vulgaris: a multicenter, randomized, double-blind, controlled, phase III trial. J Am Acad Dermatol. 2006;54:242-250.
36. Tanghetti E, Dhawan S, Green L, et al. Randomized comparison of the safety and efficacy of tazarotene 0.1% cream and adapalene 0.3% gel in the treatment of patients with at least moderate facial acne vulgaris. J Drugs Dermatol. 2010;9:549-558.
37. Hui AM, Shalita AR. Topical retinoids. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:86-94.
38. Draelos Z, Carter E, Maloney JM, et al. Two randomized studies demonstrate the efficacy and safety of dapsone gel 5% for the treatment of acne vulgaris. J Am Acad Dermatol. 2007;56:439.e1-439.e10.
39. Tanghetti E, Harper JC, Oefelein MG. The efficacy and tolerability of dapsone 5% gel in female vs male patients with facial acne vulgaris: gender as a clinically relevant outcome variable. J Drugs Dermatol. 2012;11:1417-1421.
40. Pariser DM, Rich P, Cook-Bolden FE, et al. An aqueous gel fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 3.75% for the once-daily treatment of moderate to severe acne vulgaris. J Drugs Dermatol. 2014;13:1083-1089.
41. Harper JC. The efficacy and tolerability of a fixed combination clindamycin (1.2%) and benzoyl peroxide (3.75%) aqueous gel in patients with facial acne vulgaris: gender as a clinically relevant outcome variable. J Drugs Dermatol. 2015;14:381-384.
42. Zeichner JA. The efficacy and tolerability of a fixed combination clindamycin (1.2%) and benzoyl peroxide (3.75%) aqueous gel in adult female patients with facial acne vulgaris. J Clin Aesthet Dermatol. 2015;8:21-25.
43. Coley MK, Berson DS, Callendar VD. Overview of treatment principles for skin of color. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:70-85.
44. Ebede TL, Berson DS. Acne in pregnancy. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:177-181.
1. Thiboutot D, Del Rosso JQ. Acne vulgaris and the epidermal barrier: is acne vulgaris associated with inherent epidermal abnormalities that cause impairment of barrier functions? do any topical acne therapies alter the structural and/or functional integrity of the epidermal barrier? J Clin Aesthet Dermatol. 2013;6:18-24.
2. Subramanyan K. Role of mild cleansing in the management of patient skin. Dermatol Ther. 2004;17(suppl 1):26-34.
3. Del Rosso JQ, Gold M, Rueda MJ, et al. Efficacy, safety, and subject satisfaction of a specified skin care regimen to cleanse, medicate, moisturize, and protect the skin of patients under treatment for acne vulgaris. J Clin Aesthet Dermatol. 2015;8:22-30.
4. Del Rosso JQ, Brandt S. The role of skin care as an integral component in the management of acne vulgaris: part 2: tolerability and performance of a designated skin care regimen using a foam wash and moisturizer SPF 30 in patients with acne vulgaris undergoing active treatment. J Clin Aesthet Dermatol. 2013;6:28-36.
5. Draelos ZD. Facial cosmetics for acne patients. Cosmetics in Dermatology. 2nd ed. New York, NY: Churchill Livingstone Inc; 1995:22-23.
6. Leyden JJ, Del Rosso JQ, Baum EW. The use of isotretinoin in the treatment of acne vulgaris: clinical considerations and future directions. J Clin Aesthet Dermatol. 2014;7(suppl 2):S3-S21.
7. Hayashi N, Kawashima M. Study of the usefulness of moisturizers on adherence of acne patients treated with adapalene. J Dermatol. 2014;41:592-597.
8. Isoda K, Seki T, Inoue Y, et al. Efficacy of the combined use of a facial cleanser and moisturizers for the care of mild acne patients with sensitive skin. J Dermatol. 2015;42:181-188.
9. Hensley D, Meckfessel MH. Tolerability of a skin care regimen formulated for acne-prone skin in children. Pediatr Dermatol. 2015;32:501-505.
10. Tanghetti EA, Kawata AK, Daniels SR, et al. Understanding the burden of adult female acne. J Clin Aesthet Dermatol. 2014;7:22-30.
11. Del Rosso JQ, Kircik L, Gallagher CJ. Comparative efficacy and tolerability of dapsone 5% gel in adult versus adolescent females with acne vulgaris. J Clin Aesthet Dermatol. 2015;8:31-37.
12. Del Rosso JQ, Levin J. The clinical relevance of maintaining the functional integrity of the stratum corneum in both healthy and disease-affected skin. J Clin Aesthet Dermatol. 2011;4:22-42.
13. Levin J, Momin SB. How much do we really know about our favorite cosmeceutical ingredients? J Clin Aesthet Dermatol. 2010;3:22-41.
14. Draelos ZD. Acne. In: Draelos ZD, ed. Cosmeceuticals. 2nd ed. Philadelphia, PA: Saunders-Elsevier; 2009:175-180.
15. Kircik LH, Gwazdauskas J, Butners V, et al. Evaluation of the efficacy, tolerability, and safety of an over-the-counter acne regimen containing benzoyl peroxide and salicylic acid in subjects with acne. J Drugs Dermatol. 2013;12:259-264.
16. Decker A, Graber EM. Over-the-counter acne treatments: a review. J Clin Aesthet Dermatol. 2012;5:32-40.
17. Layton AM, Henderson C, Cunliffe WJ. A clinical evaluation of acne scarring and its incidence. Clin Exp Dermatol. 1994;19:303-308.
18. Perkins AC, Maglione J, Hillebrand GG, et al. Acne vulgaris in women: prevalence across the life span. J Womens Health. 2012;21:223-230.
19. Poli F, Dreno B, Verschoore M. An epidemiological study of acne in female adults: results of a survey conducted in France. J Eur Acad Dermatol Venereol. 2001;15:541-545.
20. Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56-59.
21. Capitanio B, Sinagra JL, Bordignon V, et al. Underestimated clinical features of postadolescent acne. J Am Acad Dermatol. 2010;63:782-788.
22. Holzmann R, Shakery K. Postadolescent acne in females. Skin Pharmacol Physiol. 2014;27(suppl 1):3-8.
23. Williams C, Layton AM. Persistent acne in women: implications for the patient and for therapy. Am J Clin Dermatol. 2006;7:281-290.
24. Rendon MI, Rodriguez DA, Kawata AK, et al. Acne treatment patterns, expectations, and satisfaction among adult females of different races/ethnicities. Clin Cosmet Investig Dermatol. 2015;8:231-238.
25. Villasenor J, Berson DS, Kroshinsky D. Treatment guidelines in adult women. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:198-207.
26. Berson D, Alexis A. Adapalene 0.3% for the treatment of acne in women. J Clin Aesthet Dermatol. 2013;6:32-35.
27. Del Rosso JQ. Topical therapy for acne in women: is there a role for clindamycin phosphate–benzoyl peroxide gel? Cutis. 2014;94:177-182.
28. Zeichner JA. The efficacy and tolerability of a fixed combination (1.2%) and benzoyl peroxide (3.75%) aqueous gel in adult females with facial acne vulgaris. J Clin Aesthet Dermatol. 2015;8:21-25.
29. Fisk WA, Lev-Tov HA, Sivamani RK. Epidemiology and management of acne in adult women. Curr Derm Rep. 2014;3:29-39.
30. Stein-Gold L. Adapalene 0.1%-benzoyl peroxide 2.5% gel in adult female acne. Poster presented at: Winter Clinical Dermatology Conference; January 16–21, 2015; Maui, Hawaii.
31. Dréno B, Thiboutot D, Layton AM, et al. Large-scale international study enhances understanding of an emerging acne population: adult females. J Euro Acad Dermatol Venereol. 2015;29:1096-1106.
32. Dréno B, Layton AM, Zouboulis CC, et al. Adult female acne: a new paradigm. J Euro Acad Dermatol Venereol. 2013;27:1063-1070.
33. Shalita A, Weiss JS, Chalker DK, et al. A comparison of the efficacy and safety of adapalene gel 0.1% and tretinoin gel 0.025% in the treatment of acne vulgaris: a multicenter trial. J Am Acad Dermatol. 1996;34:482-485.
34. Pariser DM, Thiboutot DM, Clark SD, et al. The efficacy and safety of adapalene gel 0.3% in the treatment of acne vulgaris: a randomized, multicenter, investigator-blinded, controlled comparison study versus adapalene gel 0.1% and vehicle. Cutis. 2005;76:145-151.
35. Thiboutot D, Pariser DM, Egan N, et al. Adapalene gel 0.3% for the treatment of acne vulgaris: a multicenter, randomized, double-blind, controlled, phase III trial. J Am Acad Dermatol. 2006;54:242-250.
36. Tanghetti E, Dhawan S, Green L, et al. Randomized comparison of the safety and efficacy of tazarotene 0.1% cream and adapalene 0.3% gel in the treatment of patients with at least moderate facial acne vulgaris. J Drugs Dermatol. 2010;9:549-558.
37. Hui AM, Shalita AR. Topical retinoids. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:86-94.
38. Draelos Z, Carter E, Maloney JM, et al. Two randomized studies demonstrate the efficacy and safety of dapsone gel 5% for the treatment of acne vulgaris. J Am Acad Dermatol. 2007;56:439.e1-439.e10.
39. Tanghetti E, Harper JC, Oefelein MG. The efficacy and tolerability of dapsone 5% gel in female vs male patients with facial acne vulgaris: gender as a clinically relevant outcome variable. J Drugs Dermatol. 2012;11:1417-1421.
40. Pariser DM, Rich P, Cook-Bolden FE, et al. An aqueous gel fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 3.75% for the once-daily treatment of moderate to severe acne vulgaris. J Drugs Dermatol. 2014;13:1083-1089.
41. Harper JC. The efficacy and tolerability of a fixed combination clindamycin (1.2%) and benzoyl peroxide (3.75%) aqueous gel in patients with facial acne vulgaris: gender as a clinically relevant outcome variable. J Drugs Dermatol. 2015;14:381-384.
42. Zeichner JA. The efficacy and tolerability of a fixed combination clindamycin (1.2%) and benzoyl peroxide (3.75%) aqueous gel in adult female patients with facial acne vulgaris. J Clin Aesthet Dermatol. 2015;8:21-25.
43. Coley MK, Berson DS, Callendar VD. Overview of treatment principles for skin of color. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:70-85.
44. Ebede TL, Berson DS. Acne in pregnancy. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:177-181.
Practice Points
- Data from randomized controlled clinical trials (RCTs) of topical agents used for the treatment of acne in adult women has been gleaned through subanalyses of larger pivotal studies with adapalene gel 0.3%, dapsone gel 5%, clindamycin phosphate 1.2%–benzoyl peroxide 3.75% gel, and adapalene 0.1%–benzoyl peroxide 2.5% gel.
- Efficacy and tolerability/safety results from RCTs of these topical agents evaluated outcomes for the clinical pattern of mixed inflammatory, comedonal, and non-nodular acne located on the face above the jawline margin.
- More data are needed on the treatment of acne in adult women with topical agents, systemic agents, and combination regimens, including results for the full spectrum of clinical presentations.
Status Report From the American Acne & Rosacea Society on Medical Management of Acne in Adult Women, Part 1: Overview, Clinical Characteristics, and Laboratory Evaluation
It was not long ago that acne vulgaris (AV) was commonly considered to be a skin disease that affected teenagers with little attention given to preadolescent and postadolescent AV. This perspective has changed, with more attention being given to AV across a broad range of affected age groups, including preadolescent, adolescent, and postadolescent subgroups.1-5 Earlier onset of adrenarche has led to earlier development of AV in many young girls, with a higher range of dehydroepiandrosterone sulfate (DHEAS) levels observed overall in those with AV as compared to a normal age-matched population.3,4 At the other end of the age spectrum, AV is a common phenomenon in adult females, with at least half of women estimated to exhibit some form of AV.1,2,5-8 Based on a large survey of females and males (N=1013), the prevalence of AV in adult females has been reported to be 50.9%, 35.2%, 26.3%, and 15.3% among women aged 20 to 29 years, 30 to 39 years, 40 to 49 years, and 50 years and older, respectively.2 Acne vulgaris that persists beyond adolescence into adulthood is termed persistent acne, or early-onset acne, and the development of AV in women 25 years and older who have not previously been affected by AV has been termed late-onset acne.6,8,9 Publications on the management of AV in adult women have focused primarily on systemic hormonal therapies; however, topical therapies more recently have received greater attention in this subpopulation9-12 and will be discussed in part 2 of this series. Because data on AV in women are limited primarily to involvement of the face and neck region, this article does not address truncal AV unless otherwise specified. Table 1 depicts factors that can influence the management of AV in adult women.
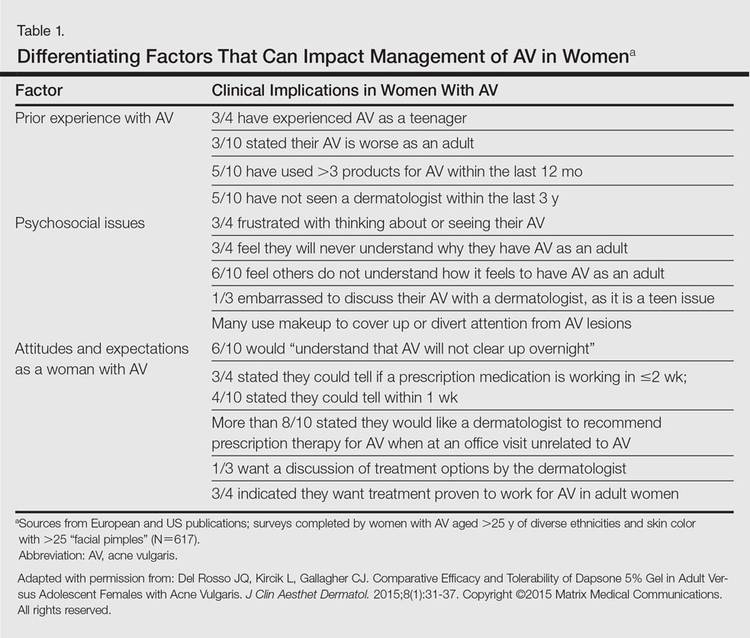
Visible Patterns and Considerations for Clinical Evaluation
Clinical Patterns
Although epidemiologic and demographic data are limited in the subpopulation of women with AV, it is reported that females account for up to 82% of adults with AV, with approximately 75% presenting with AV that is clinically similar to their disease course in adolescence.2,5,13 Among those women with persistent AV, some state that their AV is worse compared to adolescence, while others report it is not as severe. The pattern of AV often is similar to that seen in adolescence, presenting as mixed comedonal and inflammatory papular/pustular lesions diffusely distributed on the face; in other cases, a more selectively distributed U-shaped pattern is noted, characterized predominantly by inflammatory papules and/or nodules involving the lower cheeks and jawline margin, with lesions also commonly noted on the anterior and lateral neck.5,8,9,13-16 A U-shaped pattern is believed to be more common in late-onset AV, often with persistence into the mid-40s.1,15,17 It is important to emphasize the need for additional studies on the demographics and clinical characteristics of AV in adult females, especially correlations between onset, age, and clinical patterns of AV.
An international, prospective, observational study assessed the clinical characteristics of AV in adults (aged ≥25 years) at a dermatology visit for acne (N=374).16 Participants who were under management for their AV showed severity grades of mild (clear/almost clear) in 47.3% of cases. Involvement of multiple facial sites—cheeks, forehead, mandibular region, and temples—was noted in 89.8% of women, often with both inflammatory and comedonal lesions, which is a pattern similar to adolescent AV. Inflammatory lesions alone were observed in 6.4% of women, 17.1% had comedonal AV only, and truncal AV was present in 48.4%.16 Additional well-designed studies are needed to determine if this study reflects an accurate qualitative and quantitative depiction of the spectrum of AV in adult females.
Mandibular Pattern
In the observational study of AV in adults, AV localized to the mandibular area was noted in only 11.2% of participants.16 Women with localized mandibular AV were more likely than women without localized AV to be employed, noted greater daily stress levels, and tended to report more psychologically stressful jobs. Interestingly, the subgroup with mandibular acne alone was much less likely to exhibit a global severity grade of moderate or higher (7.1% vs 50.1%), truncal acne (19.0% vs 51.9%), postinflammatory hyperpigmentation (23.8% vs 51.9%), and erythema (19.0% vs 48.4%), suggesting a unique subset of AV presentation.16
Ethnicity/Skin Color
Women of all ethnicities and skin types may be affected by AV.1,18-20 Earlier age of onset of AV has been suggested in white women; however, earlier onset of adrenarche may be more frequent in black girls, which supports an earlier age of onset of AV in this subpopulation.15-17 Women with skin of color usually express greater concern with persistent dyschromia at sites where lesions have resolved, and presence of acne scars is a concern among women regardless of skin color, ethnicity, or race.18,20-22
Scarring
Acne scarring has been noted to affect up to three-fourths of adult women in one report17 and often is stated by patients to be a cause of concern and frustration.1,5,17
Perimenstrual Flaring
Flaring associated with menses is commonly reported in adult females with AV, with 56%, 17%, and 3% of women in one study (n=230) reporting worsening before, during, or after menses, respectively.21
External Factors
Comedogenic products used for skin care, cover-up makeup, or hair care may be important to consider in selected cases as potential etiologic or exacerbating factors in adult females with AV; they also may be used in the management of AV.23-25 Adult females often are perplexed and frustrated by the presence of AV after their teenaged years and anxiously wonder about or search for the potential causes. Many women use cosmetic products to cover up facial AV.5,23-25 Therefore, even if skin care or personal hygiene products or makeup are not believed to be an etiologic factor, many patients appreciate that their dermatologist addressed skin care and cosmetics as a component of AV management and provided appropriate recommendations.5,13
Ingestion of dietary supplements containing whey protein have been associated with precipitation of AV.26,27 Diets with specific content characteristics have been implicated as potential etiologic or exacerbating factors for AV; however, data are limited and specific recommendations remain elusive at present. Individual cases may warrant consideration of dietary factors, especially when treatment resistance is noted.28 Importantly, progestin-only contraceptives (ie, injectables, intrauterine devices) also can exacerbate or induce AV.29
Hyperandrogenism
Although most adult females with AV are reported to have normal serum androgen levels when tested, it is important to explore potential signs and symptoms that are suggestive of underlying hyperandrogenism through both the patient’s history and physical examination.9-11,21,29-33 Some investigators have suggested that underlying peripheral hyperandrogenism is the leading cause of AV in adult females, with or without concurrent polycystic ovarian syndrome (PCOS), though it is believed that most women with AV exhibit normal results when undergoing laboratory testing for androgen excess.10,11,21,29,30 Nevertheless, it is important to consider the possibility of underlying causes of androgen excess (Table 2), the most common being PCOS and late-onset congenital adrenal hyperplasia; an androgen-secreting tumor is less common.11,29-33 It is suggested that screening for underlying endocrinopathy should be conducted in women presenting with (1) AV recalcitrant to conventional treatment, (2) sudden emergence of severe AV, (3) concurrent signs/symptoms of androgen excess, and/or (4) AV relapse shortly after isotretinoin therapy.7,11,16,33

Hirsutism and acanthosis nigricans have been reported to be more reliable predictors of hyperandrogenism than androgenic alopecia.21 Although it may be subtle in some cases, acanthosis nigricans is harder to camouflage, so the clinician can usually detect it if a thorough physical examination is performed. However, a patient may not voluntarily report to the clinician and their staff that she has hair removed, so despite a thorough examination, the clinician may not detect hirsutism. Therefore, it is important to inquire directly about the presence of hairs (pigmented terminal vs “peach fuzz” hairs), their anatomic location, and any hair removal practices the patient has used. The absence of androgenic alopecia does not exclude underlying hyperandrogenism; however, its presence, especially in younger women, may serve as a clinical marker for underlying hyperandrogenism.5 Some women may camouflage more subtle alopecia through hairstyling, but obtaining this history usually is not problematic, as most women are distressed by any degree of hair loss.
Laboratory Evaluation—A relatively straightforward approach to the workup of androgen excess includes assessment of serum DHEAS, free testosterone, and total testosterone levels.10,30 Elevation of serum DHEAS levels indicates an adrenal source of androgen production. Elevation of testosterone is associated with excess androgens produced by the ovaries. Modest elevations of DHEAS are most commonly associated with late-onset congenital adrenal hyperplasia that may not have been previously diagnosed. Modest elevation of testosterone is most commonly associated with PCOS, which also can be accompanied by an elevated luteinizing hormone:follicle-stimulating hormone ratio of 2.5:1 to 3:1.10,30 Marked elevations of DHEAS or testosterone can be indicative of adrenal or ovarian tumors, respectively.30
In some cases, a woman might have elevated DHEAS and testosterone levels. A 17-hydroxyprogesterone test can help discriminate between an adrenal or ovarian source of androgen excess in these cases, as elevated 17-hydroxyprogesterone levels indicate that the androgens are coming from the adrenal gland.10,30
It is important that laboratory evaluation be performed when ovulation is not occurring. Blood tests can be drawn just prior to or during menses. It is important that a woman is not taking an oral contraceptive at the time of testing, which can mask an underlying endocrine abnormality.10,11,29,30 Generally, testing can be performed at least 4 to 6 weeks after stopping the oral contraceptive.
Psychosocial Impact
Facial AV exhibits a broad range of adverse psychological and social effects on many adult females.2,5,13,18 It can be associated with depression, anxiety, psychological stress, and suicidal ideation; therefore, thorough screening for these comorbidities may be warranted in some patients.2,18
Conclusion
The epidemiology, clinical presentation, and clinical and laboratory evaluation of AV in adult females was reviewed in part 1 of this 3-part series. It is important for the clinician to assess the clinical presentation, psychosocial effects, and the possibility of underlying causes of androgen excess. In part 2, skin care and topical management of AV in adult females will be discussed.
1. Perkins AC, Maglione J, Hillebrand GG, et al. Acne vulgaris in women: prevalence across the life span. J Womens Health (Larchmt). 2012;21: 223-230.
2. Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56-59.
3. Lucky AW, Biro FM, Huster GA, et al. Acne vulgaris in premenarchal girls. an early sign of puberty associated with rising levels of dehydroepiandrosterone. Arch Dermatol. 1994;130:308-314.
4. Mancini AJ, Baldwin HE, Eichenfield LF, et al. Acne life cycle: the spectrum of pediatric disease. Semin Cutan Med Surg. 2011;30(suppl 3):S2-S5.
5. Tanghetti EA, Kawata AK, Daniels SR, et al. Understanding the burden of adult female acne. J Clin Aesthet Dermatol. 2014;7:22-30.
6. Goulden V, Stables GI, Cunliffe WJ. Prevalence of facial acne in adults. J Am Acad Dermatol. 1999;41: 577-580.
7. Marks R. Acne and its management beyond the age of 35 years. Am J Clin Dermatol. 2004;5:459-462.
8. Preneau S, Dreno B. Female acne—a different subtype of teenager acne? J Eur Acad Dermatol Venereol. 2012;26:277-282.
9. Kim GK, Del Rosso JQ. Oral spironolactone in post-teenage female patients with acne vulgaris: practical considerations for the clinician based on current data and clinical experience. J Clin Aesthet Dermatol. 2012;5:37-50.
10. Thiboutot D, Chen W. Update and future of hormonal therapy in acne. Dermatology. 2003;206:57-67.
11. Villasenor J, Berson D, Kroshinsky D. Treatment guidelines in adult women. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:198-207.
12. Del Rosso JQ, Zeichner J. What’s new in the medicine cabinet? a panoramic review of clinically relevant information for the busy dermatologist. J Clin Aesthet Dermatol. 2014;7:26-30.
13. Del Rosso JQ, Kircik L, Gallagher CJ. Comparative efficacy and tolerability of dapsone 5% gel in adult versus adolescent females with acne vulgaris. J Clin Aesthet Dermatol. 2015;8:31-37.
14. Dreno B, Layton A, Zouboulis CC, et al. Adult female acne: a new paradigm. J Eur Acad Dermatol Venereol. 2013;27:1063-1070.
15. Choi CW, Lee DH, Kim HS, et al. The clinical features of late onset acne compared with early onset acne in women. J Eur Acad Dermatol Venereol. 2011;25:454-461.
16. Dréno B, Thiboutot D, Layton AM, et al; Global Alliance to Improve Outcomes in Acne. Large-scale international study enhances understanding of an emerging acne population: adult females. J Eur Acad Dermatol Venereol. 2015;29:1096-1106.
17. Kane A, Niang SO, Diagne AC, et al. Epidemiologic, clinical, and therapeutic features of acne in Dakar, Senegal. Int J Dermatol. 2007;46(suppl 1):36-38.
18. Callender VD, Alexis AF, Daniels SR, et al. Racial differences in clinical characteristics, perceptions and behaviors, and psychosocial impact of adult female acne. J Clin Aesthet Dermatol. 2014;7:19-31.
19. Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
20. Rendon MI, Rodriguez DA, Kawata AK, et al. Acne treatment patterns, expectations, and satisfaction among adult females of different races/ethnicities. Clin Cosmet Investig Dermatol. 2015;8:231-238.
21. Khunger N, Kumar C. A clinico-epidemiological study of adult acne: is it different from adolescent acne? Indian J Dermatol Venereol Leprol. 2012;78: 335-341.
22. Alexis AF. Acne vulgaris in skin of color: understanding nuances and optimizing treatment outcomes. J Drugs Dermatol. 2014;13(suppl 6):S61-S65.
23. Dall’oglio F, Tedeschi A, Fabbrocini G, et al. Cosmetics for acne: indications and recommendations for an evidence-based approach. G Ital Dermatol Venereol. 2015;150:1-11.
24. Draelos Z. Facial cosmetics for acne patients. In: Draelos Z. Cosmetics in Dermatology. 2nd Ed. New York, NY: Churchill Livingstone Inc; 1995:15-28.
25. Cunliffe WJ. Acne. London, United Kingdom: Martin Dunitz Ltd; 1989.
26. Simonart T. Acne and whey protein supplementation among bodybuilders. Dermatology. 2012;225:256-258.
27. Silverberg NB. Whey protein precipitating moderate to severe acne flares in 5 teenaged athletes. Cutis. 2012;90:70-72.
28. Bronsnick T, Murzaku EC, Rao BK. Diet in dermatology: part I. atopic dermatitis, acne, and nonmelanoma skin cancer. J Am Acad Dermatol. 2014;71:1039.
29. Keri J, Berson DS, Thiboutot DM. Hormonal treatment of acne in women. In: Shalita AR, Del Rosso J, Webster G, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:146-155.
30. Thiboutot D. Hormones and acne: pathophysiology, clinical evaluation and therapies. Sem Cutan Med Surg. 2001;20:144-153.
31. Borgia F, Cannavò S, Guarneri F, et al. Correlation between endocrinological parameters and acne severity in adult women. Acta Derm Venereol. 2004;84:201-204.
32. Clark CM, Rudolph J, Gerber DA, et al. Dermatologic manifestation of hyperandrogenism: a retrospective chart review. Skinmed. 2014;12:84-88.
33. Zeichner JA. Evaluating and treating the adult female patient with acne. J Drugs Dermatol. 2013;12:1416-1427.
It was not long ago that acne vulgaris (AV) was commonly considered to be a skin disease that affected teenagers with little attention given to preadolescent and postadolescent AV. This perspective has changed, with more attention being given to AV across a broad range of affected age groups, including preadolescent, adolescent, and postadolescent subgroups.1-5 Earlier onset of adrenarche has led to earlier development of AV in many young girls, with a higher range of dehydroepiandrosterone sulfate (DHEAS) levels observed overall in those with AV as compared to a normal age-matched population.3,4 At the other end of the age spectrum, AV is a common phenomenon in adult females, with at least half of women estimated to exhibit some form of AV.1,2,5-8 Based on a large survey of females and males (N=1013), the prevalence of AV in adult females has been reported to be 50.9%, 35.2%, 26.3%, and 15.3% among women aged 20 to 29 years, 30 to 39 years, 40 to 49 years, and 50 years and older, respectively.2 Acne vulgaris that persists beyond adolescence into adulthood is termed persistent acne, or early-onset acne, and the development of AV in women 25 years and older who have not previously been affected by AV has been termed late-onset acne.6,8,9 Publications on the management of AV in adult women have focused primarily on systemic hormonal therapies; however, topical therapies more recently have received greater attention in this subpopulation9-12 and will be discussed in part 2 of this series. Because data on AV in women are limited primarily to involvement of the face and neck region, this article does not address truncal AV unless otherwise specified. Table 1 depicts factors that can influence the management of AV in adult women.

Visible Patterns and Considerations for Clinical Evaluation
Clinical Patterns
Although epidemiologic and demographic data are limited in the subpopulation of women with AV, it is reported that females account for up to 82% of adults with AV, with approximately 75% presenting with AV that is clinically similar to their disease course in adolescence.2,5,13 Among those women with persistent AV, some state that their AV is worse compared to adolescence, while others report it is not as severe. The pattern of AV often is similar to that seen in adolescence, presenting as mixed comedonal and inflammatory papular/pustular lesions diffusely distributed on the face; in other cases, a more selectively distributed U-shaped pattern is noted, characterized predominantly by inflammatory papules and/or nodules involving the lower cheeks and jawline margin, with lesions also commonly noted on the anterior and lateral neck.5,8,9,13-16 A U-shaped pattern is believed to be more common in late-onset AV, often with persistence into the mid-40s.1,15,17 It is important to emphasize the need for additional studies on the demographics and clinical characteristics of AV in adult females, especially correlations between onset, age, and clinical patterns of AV.
An international, prospective, observational study assessed the clinical characteristics of AV in adults (aged ≥25 years) at a dermatology visit for acne (N=374).16 Participants who were under management for their AV showed severity grades of mild (clear/almost clear) in 47.3% of cases. Involvement of multiple facial sites—cheeks, forehead, mandibular region, and temples—was noted in 89.8% of women, often with both inflammatory and comedonal lesions, which is a pattern similar to adolescent AV. Inflammatory lesions alone were observed in 6.4% of women, 17.1% had comedonal AV only, and truncal AV was present in 48.4%.16 Additional well-designed studies are needed to determine if this study reflects an accurate qualitative and quantitative depiction of the spectrum of AV in adult females.
Mandibular Pattern
In the observational study of AV in adults, AV localized to the mandibular area was noted in only 11.2% of participants.16 Women with localized mandibular AV were more likely than women without localized AV to be employed, noted greater daily stress levels, and tended to report more psychologically stressful jobs. Interestingly, the subgroup with mandibular acne alone was much less likely to exhibit a global severity grade of moderate or higher (7.1% vs 50.1%), truncal acne (19.0% vs 51.9%), postinflammatory hyperpigmentation (23.8% vs 51.9%), and erythema (19.0% vs 48.4%), suggesting a unique subset of AV presentation.16
Ethnicity/Skin Color
Women of all ethnicities and skin types may be affected by AV.1,18-20 Earlier age of onset of AV has been suggested in white women; however, earlier onset of adrenarche may be more frequent in black girls, which supports an earlier age of onset of AV in this subpopulation.15-17 Women with skin of color usually express greater concern with persistent dyschromia at sites where lesions have resolved, and presence of acne scars is a concern among women regardless of skin color, ethnicity, or race.18,20-22
Scarring
Acne scarring has been noted to affect up to three-fourths of adult women in one report17 and often is stated by patients to be a cause of concern and frustration.1,5,17
Perimenstrual Flaring
Flaring associated with menses is commonly reported in adult females with AV, with 56%, 17%, and 3% of women in one study (n=230) reporting worsening before, during, or after menses, respectively.21
External Factors
Comedogenic products used for skin care, cover-up makeup, or hair care may be important to consider in selected cases as potential etiologic or exacerbating factors in adult females with AV; they also may be used in the management of AV.23-25 Adult females often are perplexed and frustrated by the presence of AV after their teenaged years and anxiously wonder about or search for the potential causes. Many women use cosmetic products to cover up facial AV.5,23-25 Therefore, even if skin care or personal hygiene products or makeup are not believed to be an etiologic factor, many patients appreciate that their dermatologist addressed skin care and cosmetics as a component of AV management and provided appropriate recommendations.5,13
Ingestion of dietary supplements containing whey protein have been associated with precipitation of AV.26,27 Diets with specific content characteristics have been implicated as potential etiologic or exacerbating factors for AV; however, data are limited and specific recommendations remain elusive at present. Individual cases may warrant consideration of dietary factors, especially when treatment resistance is noted.28 Importantly, progestin-only contraceptives (ie, injectables, intrauterine devices) also can exacerbate or induce AV.29
Hyperandrogenism
Although most adult females with AV are reported to have normal serum androgen levels when tested, it is important to explore potential signs and symptoms that are suggestive of underlying hyperandrogenism through both the patient’s history and physical examination.9-11,21,29-33 Some investigators have suggested that underlying peripheral hyperandrogenism is the leading cause of AV in adult females, with or without concurrent polycystic ovarian syndrome (PCOS), though it is believed that most women with AV exhibit normal results when undergoing laboratory testing for androgen excess.10,11,21,29,30 Nevertheless, it is important to consider the possibility of underlying causes of androgen excess (Table 2), the most common being PCOS and late-onset congenital adrenal hyperplasia; an androgen-secreting tumor is less common.11,29-33 It is suggested that screening for underlying endocrinopathy should be conducted in women presenting with (1) AV recalcitrant to conventional treatment, (2) sudden emergence of severe AV, (3) concurrent signs/symptoms of androgen excess, and/or (4) AV relapse shortly after isotretinoin therapy.7,11,16,33

Hirsutism and acanthosis nigricans have been reported to be more reliable predictors of hyperandrogenism than androgenic alopecia.21 Although it may be subtle in some cases, acanthosis nigricans is harder to camouflage, so the clinician can usually detect it if a thorough physical examination is performed. However, a patient may not voluntarily report to the clinician and their staff that she has hair removed, so despite a thorough examination, the clinician may not detect hirsutism. Therefore, it is important to inquire directly about the presence of hairs (pigmented terminal vs “peach fuzz” hairs), their anatomic location, and any hair removal practices the patient has used. The absence of androgenic alopecia does not exclude underlying hyperandrogenism; however, its presence, especially in younger women, may serve as a clinical marker for underlying hyperandrogenism.5 Some women may camouflage more subtle alopecia through hairstyling, but obtaining this history usually is not problematic, as most women are distressed by any degree of hair loss.
Laboratory Evaluation—A relatively straightforward approach to the workup of androgen excess includes assessment of serum DHEAS, free testosterone, and total testosterone levels.10,30 Elevation of serum DHEAS levels indicates an adrenal source of androgen production. Elevation of testosterone is associated with excess androgens produced by the ovaries. Modest elevations of DHEAS are most commonly associated with late-onset congenital adrenal hyperplasia that may not have been previously diagnosed. Modest elevation of testosterone is most commonly associated with PCOS, which also can be accompanied by an elevated luteinizing hormone:follicle-stimulating hormone ratio of 2.5:1 to 3:1.10,30 Marked elevations of DHEAS or testosterone can be indicative of adrenal or ovarian tumors, respectively.30
In some cases, a woman might have elevated DHEAS and testosterone levels. A 17-hydroxyprogesterone test can help discriminate between an adrenal or ovarian source of androgen excess in these cases, as elevated 17-hydroxyprogesterone levels indicate that the androgens are coming from the adrenal gland.10,30
It is important that laboratory evaluation be performed when ovulation is not occurring. Blood tests can be drawn just prior to or during menses. It is important that a woman is not taking an oral contraceptive at the time of testing, which can mask an underlying endocrine abnormality.10,11,29,30 Generally, testing can be performed at least 4 to 6 weeks after stopping the oral contraceptive.
Psychosocial Impact
Facial AV exhibits a broad range of adverse psychological and social effects on many adult females.2,5,13,18 It can be associated with depression, anxiety, psychological stress, and suicidal ideation; therefore, thorough screening for these comorbidities may be warranted in some patients.2,18
Conclusion
The epidemiology, clinical presentation, and clinical and laboratory evaluation of AV in adult females was reviewed in part 1 of this 3-part series. It is important for the clinician to assess the clinical presentation, psychosocial effects, and the possibility of underlying causes of androgen excess. In part 2, skin care and topical management of AV in adult females will be discussed.
It was not long ago that acne vulgaris (AV) was commonly considered to be a skin disease that affected teenagers with little attention given to preadolescent and postadolescent AV. This perspective has changed, with more attention being given to AV across a broad range of affected age groups, including preadolescent, adolescent, and postadolescent subgroups.1-5 Earlier onset of adrenarche has led to earlier development of AV in many young girls, with a higher range of dehydroepiandrosterone sulfate (DHEAS) levels observed overall in those with AV as compared to a normal age-matched population.3,4 At the other end of the age spectrum, AV is a common phenomenon in adult females, with at least half of women estimated to exhibit some form of AV.1,2,5-8 Based on a large survey of females and males (N=1013), the prevalence of AV in adult females has been reported to be 50.9%, 35.2%, 26.3%, and 15.3% among women aged 20 to 29 years, 30 to 39 years, 40 to 49 years, and 50 years and older, respectively.2 Acne vulgaris that persists beyond adolescence into adulthood is termed persistent acne, or early-onset acne, and the development of AV in women 25 years and older who have not previously been affected by AV has been termed late-onset acne.6,8,9 Publications on the management of AV in adult women have focused primarily on systemic hormonal therapies; however, topical therapies more recently have received greater attention in this subpopulation9-12 and will be discussed in part 2 of this series. Because data on AV in women are limited primarily to involvement of the face and neck region, this article does not address truncal AV unless otherwise specified. Table 1 depicts factors that can influence the management of AV in adult women.

Visible Patterns and Considerations for Clinical Evaluation
Clinical Patterns
Although epidemiologic and demographic data are limited in the subpopulation of women with AV, it is reported that females account for up to 82% of adults with AV, with approximately 75% presenting with AV that is clinically similar to their disease course in adolescence.2,5,13 Among those women with persistent AV, some state that their AV is worse compared to adolescence, while others report it is not as severe. The pattern of AV often is similar to that seen in adolescence, presenting as mixed comedonal and inflammatory papular/pustular lesions diffusely distributed on the face; in other cases, a more selectively distributed U-shaped pattern is noted, characterized predominantly by inflammatory papules and/or nodules involving the lower cheeks and jawline margin, with lesions also commonly noted on the anterior and lateral neck.5,8,9,13-16 A U-shaped pattern is believed to be more common in late-onset AV, often with persistence into the mid-40s.1,15,17 It is important to emphasize the need for additional studies on the demographics and clinical characteristics of AV in adult females, especially correlations between onset, age, and clinical patterns of AV.
An international, prospective, observational study assessed the clinical characteristics of AV in adults (aged ≥25 years) at a dermatology visit for acne (N=374).16 Participants who were under management for their AV showed severity grades of mild (clear/almost clear) in 47.3% of cases. Involvement of multiple facial sites—cheeks, forehead, mandibular region, and temples—was noted in 89.8% of women, often with both inflammatory and comedonal lesions, which is a pattern similar to adolescent AV. Inflammatory lesions alone were observed in 6.4% of women, 17.1% had comedonal AV only, and truncal AV was present in 48.4%.16 Additional well-designed studies are needed to determine if this study reflects an accurate qualitative and quantitative depiction of the spectrum of AV in adult females.
Mandibular Pattern
In the observational study of AV in adults, AV localized to the mandibular area was noted in only 11.2% of participants.16 Women with localized mandibular AV were more likely than women without localized AV to be employed, noted greater daily stress levels, and tended to report more psychologically stressful jobs. Interestingly, the subgroup with mandibular acne alone was much less likely to exhibit a global severity grade of moderate or higher (7.1% vs 50.1%), truncal acne (19.0% vs 51.9%), postinflammatory hyperpigmentation (23.8% vs 51.9%), and erythema (19.0% vs 48.4%), suggesting a unique subset of AV presentation.16
Ethnicity/Skin Color
Women of all ethnicities and skin types may be affected by AV.1,18-20 Earlier age of onset of AV has been suggested in white women; however, earlier onset of adrenarche may be more frequent in black girls, which supports an earlier age of onset of AV in this subpopulation.15-17 Women with skin of color usually express greater concern with persistent dyschromia at sites where lesions have resolved, and presence of acne scars is a concern among women regardless of skin color, ethnicity, or race.18,20-22
Scarring
Acne scarring has been noted to affect up to three-fourths of adult women in one report17 and often is stated by patients to be a cause of concern and frustration.1,5,17
Perimenstrual Flaring
Flaring associated with menses is commonly reported in adult females with AV, with 56%, 17%, and 3% of women in one study (n=230) reporting worsening before, during, or after menses, respectively.21
External Factors
Comedogenic products used for skin care, cover-up makeup, or hair care may be important to consider in selected cases as potential etiologic or exacerbating factors in adult females with AV; they also may be used in the management of AV.23-25 Adult females often are perplexed and frustrated by the presence of AV after their teenaged years and anxiously wonder about or search for the potential causes. Many women use cosmetic products to cover up facial AV.5,23-25 Therefore, even if skin care or personal hygiene products or makeup are not believed to be an etiologic factor, many patients appreciate that their dermatologist addressed skin care and cosmetics as a component of AV management and provided appropriate recommendations.5,13
Ingestion of dietary supplements containing whey protein have been associated with precipitation of AV.26,27 Diets with specific content characteristics have been implicated as potential etiologic or exacerbating factors for AV; however, data are limited and specific recommendations remain elusive at present. Individual cases may warrant consideration of dietary factors, especially when treatment resistance is noted.28 Importantly, progestin-only contraceptives (ie, injectables, intrauterine devices) also can exacerbate or induce AV.29
Hyperandrogenism
Although most adult females with AV are reported to have normal serum androgen levels when tested, it is important to explore potential signs and symptoms that are suggestive of underlying hyperandrogenism through both the patient’s history and physical examination.9-11,21,29-33 Some investigators have suggested that underlying peripheral hyperandrogenism is the leading cause of AV in adult females, with or without concurrent polycystic ovarian syndrome (PCOS), though it is believed that most women with AV exhibit normal results when undergoing laboratory testing for androgen excess.10,11,21,29,30 Nevertheless, it is important to consider the possibility of underlying causes of androgen excess (Table 2), the most common being PCOS and late-onset congenital adrenal hyperplasia; an androgen-secreting tumor is less common.11,29-33 It is suggested that screening for underlying endocrinopathy should be conducted in women presenting with (1) AV recalcitrant to conventional treatment, (2) sudden emergence of severe AV, (3) concurrent signs/symptoms of androgen excess, and/or (4) AV relapse shortly after isotretinoin therapy.7,11,16,33

Hirsutism and acanthosis nigricans have been reported to be more reliable predictors of hyperandrogenism than androgenic alopecia.21 Although it may be subtle in some cases, acanthosis nigricans is harder to camouflage, so the clinician can usually detect it if a thorough physical examination is performed. However, a patient may not voluntarily report to the clinician and their staff that she has hair removed, so despite a thorough examination, the clinician may not detect hirsutism. Therefore, it is important to inquire directly about the presence of hairs (pigmented terminal vs “peach fuzz” hairs), their anatomic location, and any hair removal practices the patient has used. The absence of androgenic alopecia does not exclude underlying hyperandrogenism; however, its presence, especially in younger women, may serve as a clinical marker for underlying hyperandrogenism.5 Some women may camouflage more subtle alopecia through hairstyling, but obtaining this history usually is not problematic, as most women are distressed by any degree of hair loss.
Laboratory Evaluation—A relatively straightforward approach to the workup of androgen excess includes assessment of serum DHEAS, free testosterone, and total testosterone levels.10,30 Elevation of serum DHEAS levels indicates an adrenal source of androgen production. Elevation of testosterone is associated with excess androgens produced by the ovaries. Modest elevations of DHEAS are most commonly associated with late-onset congenital adrenal hyperplasia that may not have been previously diagnosed. Modest elevation of testosterone is most commonly associated with PCOS, which also can be accompanied by an elevated luteinizing hormone:follicle-stimulating hormone ratio of 2.5:1 to 3:1.10,30 Marked elevations of DHEAS or testosterone can be indicative of adrenal or ovarian tumors, respectively.30
In some cases, a woman might have elevated DHEAS and testosterone levels. A 17-hydroxyprogesterone test can help discriminate between an adrenal or ovarian source of androgen excess in these cases, as elevated 17-hydroxyprogesterone levels indicate that the androgens are coming from the adrenal gland.10,30
It is important that laboratory evaluation be performed when ovulation is not occurring. Blood tests can be drawn just prior to or during menses. It is important that a woman is not taking an oral contraceptive at the time of testing, which can mask an underlying endocrine abnormality.10,11,29,30 Generally, testing can be performed at least 4 to 6 weeks after stopping the oral contraceptive.
Psychosocial Impact
Facial AV exhibits a broad range of adverse psychological and social effects on many adult females.2,5,13,18 It can be associated with depression, anxiety, psychological stress, and suicidal ideation; therefore, thorough screening for these comorbidities may be warranted in some patients.2,18
Conclusion
The epidemiology, clinical presentation, and clinical and laboratory evaluation of AV in adult females was reviewed in part 1 of this 3-part series. It is important for the clinician to assess the clinical presentation, psychosocial effects, and the possibility of underlying causes of androgen excess. In part 2, skin care and topical management of AV in adult females will be discussed.
1. Perkins AC, Maglione J, Hillebrand GG, et al. Acne vulgaris in women: prevalence across the life span. J Womens Health (Larchmt). 2012;21: 223-230.
2. Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56-59.
3. Lucky AW, Biro FM, Huster GA, et al. Acne vulgaris in premenarchal girls. an early sign of puberty associated with rising levels of dehydroepiandrosterone. Arch Dermatol. 1994;130:308-314.
4. Mancini AJ, Baldwin HE, Eichenfield LF, et al. Acne life cycle: the spectrum of pediatric disease. Semin Cutan Med Surg. 2011;30(suppl 3):S2-S5.
5. Tanghetti EA, Kawata AK, Daniels SR, et al. Understanding the burden of adult female acne. J Clin Aesthet Dermatol. 2014;7:22-30.
6. Goulden V, Stables GI, Cunliffe WJ. Prevalence of facial acne in adults. J Am Acad Dermatol. 1999;41: 577-580.
7. Marks R. Acne and its management beyond the age of 35 years. Am J Clin Dermatol. 2004;5:459-462.
8. Preneau S, Dreno B. Female acne—a different subtype of teenager acne? J Eur Acad Dermatol Venereol. 2012;26:277-282.
9. Kim GK, Del Rosso JQ. Oral spironolactone in post-teenage female patients with acne vulgaris: practical considerations for the clinician based on current data and clinical experience. J Clin Aesthet Dermatol. 2012;5:37-50.
10. Thiboutot D, Chen W. Update and future of hormonal therapy in acne. Dermatology. 2003;206:57-67.
11. Villasenor J, Berson D, Kroshinsky D. Treatment guidelines in adult women. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:198-207.
12. Del Rosso JQ, Zeichner J. What’s new in the medicine cabinet? a panoramic review of clinically relevant information for the busy dermatologist. J Clin Aesthet Dermatol. 2014;7:26-30.
13. Del Rosso JQ, Kircik L, Gallagher CJ. Comparative efficacy and tolerability of dapsone 5% gel in adult versus adolescent females with acne vulgaris. J Clin Aesthet Dermatol. 2015;8:31-37.
14. Dreno B, Layton A, Zouboulis CC, et al. Adult female acne: a new paradigm. J Eur Acad Dermatol Venereol. 2013;27:1063-1070.
15. Choi CW, Lee DH, Kim HS, et al. The clinical features of late onset acne compared with early onset acne in women. J Eur Acad Dermatol Venereol. 2011;25:454-461.
16. Dréno B, Thiboutot D, Layton AM, et al; Global Alliance to Improve Outcomes in Acne. Large-scale international study enhances understanding of an emerging acne population: adult females. J Eur Acad Dermatol Venereol. 2015;29:1096-1106.
17. Kane A, Niang SO, Diagne AC, et al. Epidemiologic, clinical, and therapeutic features of acne in Dakar, Senegal. Int J Dermatol. 2007;46(suppl 1):36-38.
18. Callender VD, Alexis AF, Daniels SR, et al. Racial differences in clinical characteristics, perceptions and behaviors, and psychosocial impact of adult female acne. J Clin Aesthet Dermatol. 2014;7:19-31.
19. Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
20. Rendon MI, Rodriguez DA, Kawata AK, et al. Acne treatment patterns, expectations, and satisfaction among adult females of different races/ethnicities. Clin Cosmet Investig Dermatol. 2015;8:231-238.
21. Khunger N, Kumar C. A clinico-epidemiological study of adult acne: is it different from adolescent acne? Indian J Dermatol Venereol Leprol. 2012;78: 335-341.
22. Alexis AF. Acne vulgaris in skin of color: understanding nuances and optimizing treatment outcomes. J Drugs Dermatol. 2014;13(suppl 6):S61-S65.
23. Dall’oglio F, Tedeschi A, Fabbrocini G, et al. Cosmetics for acne: indications and recommendations for an evidence-based approach. G Ital Dermatol Venereol. 2015;150:1-11.
24. Draelos Z. Facial cosmetics for acne patients. In: Draelos Z. Cosmetics in Dermatology. 2nd Ed. New York, NY: Churchill Livingstone Inc; 1995:15-28.
25. Cunliffe WJ. Acne. London, United Kingdom: Martin Dunitz Ltd; 1989.
26. Simonart T. Acne and whey protein supplementation among bodybuilders. Dermatology. 2012;225:256-258.
27. Silverberg NB. Whey protein precipitating moderate to severe acne flares in 5 teenaged athletes. Cutis. 2012;90:70-72.
28. Bronsnick T, Murzaku EC, Rao BK. Diet in dermatology: part I. atopic dermatitis, acne, and nonmelanoma skin cancer. J Am Acad Dermatol. 2014;71:1039.
29. Keri J, Berson DS, Thiboutot DM. Hormonal treatment of acne in women. In: Shalita AR, Del Rosso J, Webster G, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:146-155.
30. Thiboutot D. Hormones and acne: pathophysiology, clinical evaluation and therapies. Sem Cutan Med Surg. 2001;20:144-153.
31. Borgia F, Cannavò S, Guarneri F, et al. Correlation between endocrinological parameters and acne severity in adult women. Acta Derm Venereol. 2004;84:201-204.
32. Clark CM, Rudolph J, Gerber DA, et al. Dermatologic manifestation of hyperandrogenism: a retrospective chart review. Skinmed. 2014;12:84-88.
33. Zeichner JA. Evaluating and treating the adult female patient with acne. J Drugs Dermatol. 2013;12:1416-1427.
1. Perkins AC, Maglione J, Hillebrand GG, et al. Acne vulgaris in women: prevalence across the life span. J Womens Health (Larchmt). 2012;21: 223-230.
2. Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56-59.
3. Lucky AW, Biro FM, Huster GA, et al. Acne vulgaris in premenarchal girls. an early sign of puberty associated with rising levels of dehydroepiandrosterone. Arch Dermatol. 1994;130:308-314.
4. Mancini AJ, Baldwin HE, Eichenfield LF, et al. Acne life cycle: the spectrum of pediatric disease. Semin Cutan Med Surg. 2011;30(suppl 3):S2-S5.
5. Tanghetti EA, Kawata AK, Daniels SR, et al. Understanding the burden of adult female acne. J Clin Aesthet Dermatol. 2014;7:22-30.
6. Goulden V, Stables GI, Cunliffe WJ. Prevalence of facial acne in adults. J Am Acad Dermatol. 1999;41: 577-580.
7. Marks R. Acne and its management beyond the age of 35 years. Am J Clin Dermatol. 2004;5:459-462.
8. Preneau S, Dreno B. Female acne—a different subtype of teenager acne? J Eur Acad Dermatol Venereol. 2012;26:277-282.
9. Kim GK, Del Rosso JQ. Oral spironolactone in post-teenage female patients with acne vulgaris: practical considerations for the clinician based on current data and clinical experience. J Clin Aesthet Dermatol. 2012;5:37-50.
10. Thiboutot D, Chen W. Update and future of hormonal therapy in acne. Dermatology. 2003;206:57-67.
11. Villasenor J, Berson D, Kroshinsky D. Treatment guidelines in adult women. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:198-207.
12. Del Rosso JQ, Zeichner J. What’s new in the medicine cabinet? a panoramic review of clinically relevant information for the busy dermatologist. J Clin Aesthet Dermatol. 2014;7:26-30.
13. Del Rosso JQ, Kircik L, Gallagher CJ. Comparative efficacy and tolerability of dapsone 5% gel in adult versus adolescent females with acne vulgaris. J Clin Aesthet Dermatol. 2015;8:31-37.
14. Dreno B, Layton A, Zouboulis CC, et al. Adult female acne: a new paradigm. J Eur Acad Dermatol Venereol. 2013;27:1063-1070.
15. Choi CW, Lee DH, Kim HS, et al. The clinical features of late onset acne compared with early onset acne in women. J Eur Acad Dermatol Venereol. 2011;25:454-461.
16. Dréno B, Thiboutot D, Layton AM, et al; Global Alliance to Improve Outcomes in Acne. Large-scale international study enhances understanding of an emerging acne population: adult females. J Eur Acad Dermatol Venereol. 2015;29:1096-1106.
17. Kane A, Niang SO, Diagne AC, et al. Epidemiologic, clinical, and therapeutic features of acne in Dakar, Senegal. Int J Dermatol. 2007;46(suppl 1):36-38.
18. Callender VD, Alexis AF, Daniels SR, et al. Racial differences in clinical characteristics, perceptions and behaviors, and psychosocial impact of adult female acne. J Clin Aesthet Dermatol. 2014;7:19-31.
19. Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
20. Rendon MI, Rodriguez DA, Kawata AK, et al. Acne treatment patterns, expectations, and satisfaction among adult females of different races/ethnicities. Clin Cosmet Investig Dermatol. 2015;8:231-238.
21. Khunger N, Kumar C. A clinico-epidemiological study of adult acne: is it different from adolescent acne? Indian J Dermatol Venereol Leprol. 2012;78: 335-341.
22. Alexis AF. Acne vulgaris in skin of color: understanding nuances and optimizing treatment outcomes. J Drugs Dermatol. 2014;13(suppl 6):S61-S65.
23. Dall’oglio F, Tedeschi A, Fabbrocini G, et al. Cosmetics for acne: indications and recommendations for an evidence-based approach. G Ital Dermatol Venereol. 2015;150:1-11.
24. Draelos Z. Facial cosmetics for acne patients. In: Draelos Z. Cosmetics in Dermatology. 2nd Ed. New York, NY: Churchill Livingstone Inc; 1995:15-28.
25. Cunliffe WJ. Acne. London, United Kingdom: Martin Dunitz Ltd; 1989.
26. Simonart T. Acne and whey protein supplementation among bodybuilders. Dermatology. 2012;225:256-258.
27. Silverberg NB. Whey protein precipitating moderate to severe acne flares in 5 teenaged athletes. Cutis. 2012;90:70-72.
28. Bronsnick T, Murzaku EC, Rao BK. Diet in dermatology: part I. atopic dermatitis, acne, and nonmelanoma skin cancer. J Am Acad Dermatol. 2014;71:1039.
29. Keri J, Berson DS, Thiboutot DM. Hormonal treatment of acne in women. In: Shalita AR, Del Rosso J, Webster G, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:146-155.
30. Thiboutot D. Hormones and acne: pathophysiology, clinical evaluation and therapies. Sem Cutan Med Surg. 2001;20:144-153.
31. Borgia F, Cannavò S, Guarneri F, et al. Correlation between endocrinological parameters and acne severity in adult women. Acta Derm Venereol. 2004;84:201-204.
32. Clark CM, Rudolph J, Gerber DA, et al. Dermatologic manifestation of hyperandrogenism: a retrospective chart review. Skinmed. 2014;12:84-88.
33. Zeichner JA. Evaluating and treating the adult female patient with acne. J Drugs Dermatol. 2013;12:1416-1427.
Practice Points
- Acne in adult women is common and may persist beyond the adolescent years or may be late in onset with emergence usually during the early to mid-20s.
- Adult women with acne often are frustrated, as they perceive it as a disorder of teenagers and are perplexed by its presence later in life. They often are distressed by unpredictable flares as well as difficulty with covering lesions and associated dyschromia and scarring.
- Clinical patterns of acne in adult women are mixed inflammatory and comedonal facial acne or a U-shaped pattern of inflammatory lesions involving the lower face and neck.
- Laboratory testing is not considered mandatory in all cases. The clinician is encouraged to carefully evaluate each case and determine if further evaluation to detect a cause of androgen excess is warranted.
Study establishes protocol for perioperative dabigatran discontinuation
TORONTO – In atrial fibrillation (AF) patients who must discontinue dabigatran for elective surgery, the risk of both stroke and major bleeding can be reduced to low levels using a formalized strategy for stopping and then restarting anticoagulation, according to results of a prospective study presented at the International Society on Thrombosis and Haemostasis Congress.
Among key findings presented at a press conference at the ISTH 2015 Congress, no strokes were recorded in more than 500 patients managed with the protocol, and the major bleeding rate was less than 2%, reported the study’s principal investigator, Dr. Sam Schulman, professor of hematology and thromboembolism, McMaster University, Hamilton, Ont.
Data from this study (Circulation 2015) were reported at the press conference alongside a second study of perioperative warfarin management. Both studies are potentially practice changing, because they supply evidence-based guidance for anticoagulation in patients with AF.
Based on the findings from these two studies, “it is important to get this message out” that there are now data available on which to base clinical decisions, reported Dr. Schulman, who is also president of the ISTH 2015 Congress. His data were presented alongside a study that found no benefit from heparin bridging in AF patients when warfarin was stopped 5 days in advance of surgery.
In the study presented by Dr. Schulman, 542 patients with AF who were on dabigatran and scheduled for elective surgery were managed on a prespecified protocol for risk assessment. The protocol provided a time for stopping dabigatran before surgery based on such factors as renal function and procedure-related bleeding risk. Dabigatran was restarted after surgery on prespecified measures of surgery complexity and severity of consequences if bleeding occurred.
The primary outcome evaluated in the study was major bleeding in the first 30 days. Other outcomes of interest included thromboembolic complications, death and minor bleeding.
Major bleeding was observed in 1.8% of patients, a rate that Dr. Schulman characterized as “low and acceptable” in the context of expected background bleeding rates. There were four deaths, but all were unrelated to either bleeding or arterial thromboembolism. The only thromboembolic complication was a single transient ischemic attack. Minor bleeding occurred in 5.2%.
On the basis of the protocol, about half of the patients discontinued dabigatran 24 hours before surgery. No patient discontinued therapy more than 96 hours prior to surgery. The median time to resumption of dabigatran after surgery was 1 day, but the point at which it was restarted ranged between hours and 2 days. Bridging, which describes the injection of heparin for short-term anticoagulation, was not employed preoperatively but was used in 1.7% of cases postoperatively.
At the press conference, data also were reported from the BRIDGE study. That study, published online in the New England Journal of Medicine (2015 June 22; epub ahead of print ), found that bridging was not an effective strategy in AF patients who discontinue warfarin prior to elective surgery. In the press conference, Dr. Thomas L. Ortel, hematology/oncology division, Duke University Medical Center, Durham, N.C., agreed with Dr. Schulman that this is an area where evidence is needed to guide care.
In the absence of data, “physicians do whatever they think is best,” Dr. Schulman noted at the press conference. Referring to strategies for stopping anticoagulants for surgery in patients with AF, Dr. Schulman said, “some of them stop the blood thinner too early because they are afraid that the patient is going to bleed during surgery and instead the patient can have a stroke. Some stop too late, and the patient can have bleeding.”
The data presented at the meeting provide an evidence base for clinical decisions. Dr. Schulman suggested that these data are meaningful for guiding care.
Dr. Ortel disclosed grant/research support from Eisai and Pfizer. Dr. Schulman had no disclosures.
TORONTO – In atrial fibrillation (AF) patients who must discontinue dabigatran for elective surgery, the risk of both stroke and major bleeding can be reduced to low levels using a formalized strategy for stopping and then restarting anticoagulation, according to results of a prospective study presented at the International Society on Thrombosis and Haemostasis Congress.
Among key findings presented at a press conference at the ISTH 2015 Congress, no strokes were recorded in more than 500 patients managed with the protocol, and the major bleeding rate was less than 2%, reported the study’s principal investigator, Dr. Sam Schulman, professor of hematology and thromboembolism, McMaster University, Hamilton, Ont.
Data from this study (Circulation 2015) were reported at the press conference alongside a second study of perioperative warfarin management. Both studies are potentially practice changing, because they supply evidence-based guidance for anticoagulation in patients with AF.
Based on the findings from these two studies, “it is important to get this message out” that there are now data available on which to base clinical decisions, reported Dr. Schulman, who is also president of the ISTH 2015 Congress. His data were presented alongside a study that found no benefit from heparin bridging in AF patients when warfarin was stopped 5 days in advance of surgery.
In the study presented by Dr. Schulman, 542 patients with AF who were on dabigatran and scheduled for elective surgery were managed on a prespecified protocol for risk assessment. The protocol provided a time for stopping dabigatran before surgery based on such factors as renal function and procedure-related bleeding risk. Dabigatran was restarted after surgery on prespecified measures of surgery complexity and severity of consequences if bleeding occurred.
The primary outcome evaluated in the study was major bleeding in the first 30 days. Other outcomes of interest included thromboembolic complications, death and minor bleeding.
Major bleeding was observed in 1.8% of patients, a rate that Dr. Schulman characterized as “low and acceptable” in the context of expected background bleeding rates. There were four deaths, but all were unrelated to either bleeding or arterial thromboembolism. The only thromboembolic complication was a single transient ischemic attack. Minor bleeding occurred in 5.2%.
On the basis of the protocol, about half of the patients discontinued dabigatran 24 hours before surgery. No patient discontinued therapy more than 96 hours prior to surgery. The median time to resumption of dabigatran after surgery was 1 day, but the point at which it was restarted ranged between hours and 2 days. Bridging, which describes the injection of heparin for short-term anticoagulation, was not employed preoperatively but was used in 1.7% of cases postoperatively.
At the press conference, data also were reported from the BRIDGE study. That study, published online in the New England Journal of Medicine (2015 June 22; epub ahead of print ), found that bridging was not an effective strategy in AF patients who discontinue warfarin prior to elective surgery. In the press conference, Dr. Thomas L. Ortel, hematology/oncology division, Duke University Medical Center, Durham, N.C., agreed with Dr. Schulman that this is an area where evidence is needed to guide care.
In the absence of data, “physicians do whatever they think is best,” Dr. Schulman noted at the press conference. Referring to strategies for stopping anticoagulants for surgery in patients with AF, Dr. Schulman said, “some of them stop the blood thinner too early because they are afraid that the patient is going to bleed during surgery and instead the patient can have a stroke. Some stop too late, and the patient can have bleeding.”
The data presented at the meeting provide an evidence base for clinical decisions. Dr. Schulman suggested that these data are meaningful for guiding care.
Dr. Ortel disclosed grant/research support from Eisai and Pfizer. Dr. Schulman had no disclosures.
TORONTO – In atrial fibrillation (AF) patients who must discontinue dabigatran for elective surgery, the risk of both stroke and major bleeding can be reduced to low levels using a formalized strategy for stopping and then restarting anticoagulation, according to results of a prospective study presented at the International Society on Thrombosis and Haemostasis Congress.
Among key findings presented at a press conference at the ISTH 2015 Congress, no strokes were recorded in more than 500 patients managed with the protocol, and the major bleeding rate was less than 2%, reported the study’s principal investigator, Dr. Sam Schulman, professor of hematology and thromboembolism, McMaster University, Hamilton, Ont.
Data from this study (Circulation 2015) were reported at the press conference alongside a second study of perioperative warfarin management. Both studies are potentially practice changing, because they supply evidence-based guidance for anticoagulation in patients with AF.
Based on the findings from these two studies, “it is important to get this message out” that there are now data available on which to base clinical decisions, reported Dr. Schulman, who is also president of the ISTH 2015 Congress. His data were presented alongside a study that found no benefit from heparin bridging in AF patients when warfarin was stopped 5 days in advance of surgery.
In the study presented by Dr. Schulman, 542 patients with AF who were on dabigatran and scheduled for elective surgery were managed on a prespecified protocol for risk assessment. The protocol provided a time for stopping dabigatran before surgery based on such factors as renal function and procedure-related bleeding risk. Dabigatran was restarted after surgery on prespecified measures of surgery complexity and severity of consequences if bleeding occurred.
The primary outcome evaluated in the study was major bleeding in the first 30 days. Other outcomes of interest included thromboembolic complications, death and minor bleeding.
Major bleeding was observed in 1.8% of patients, a rate that Dr. Schulman characterized as “low and acceptable” in the context of expected background bleeding rates. There were four deaths, but all were unrelated to either bleeding or arterial thromboembolism. The only thromboembolic complication was a single transient ischemic attack. Minor bleeding occurred in 5.2%.
On the basis of the protocol, about half of the patients discontinued dabigatran 24 hours before surgery. No patient discontinued therapy more than 96 hours prior to surgery. The median time to resumption of dabigatran after surgery was 1 day, but the point at which it was restarted ranged between hours and 2 days. Bridging, which describes the injection of heparin for short-term anticoagulation, was not employed preoperatively but was used in 1.7% of cases postoperatively.
At the press conference, data also were reported from the BRIDGE study. That study, published online in the New England Journal of Medicine (2015 June 22; epub ahead of print ), found that bridging was not an effective strategy in AF patients who discontinue warfarin prior to elective surgery. In the press conference, Dr. Thomas L. Ortel, hematology/oncology division, Duke University Medical Center, Durham, N.C., agreed with Dr. Schulman that this is an area where evidence is needed to guide care.
In the absence of data, “physicians do whatever they think is best,” Dr. Schulman noted at the press conference. Referring to strategies for stopping anticoagulants for surgery in patients with AF, Dr. Schulman said, “some of them stop the blood thinner too early because they are afraid that the patient is going to bleed during surgery and instead the patient can have a stroke. Some stop too late, and the patient can have bleeding.”
The data presented at the meeting provide an evidence base for clinical decisions. Dr. Schulman suggested that these data are meaningful for guiding care.
Dr. Ortel disclosed grant/research support from Eisai and Pfizer. Dr. Schulman had no disclosures.
AT 2015 ISTH CONGRESS
Key clinical point: The risk of stroke and major bleeding can be reduced to low levels using a formalized strategy for stopping and then restarting dabigatran.
Major finding: The protocol developed provided a time for stopping dabigatran before surgery based on such factors as renal function and procedure-related bleeding risk. Dabigatran was restarted after surgery on prespecified measures of surgery complexity and severity of consequences if bleeding occurred.
Data source: 542 patients with AF who were on dabigatran and scheduled for elective surgery were managed on a prespecified protocol for risk assessment.
Disclosures: Dr. Ortel disclosed grant/research support from Eisai and Pfizer. Dr. Schulman had no disclosures.
AHA/ACC updates hypertension guidelines for CAD patients
The first update to U.S. guidelines for managing hypertension in adult patients with coronary artery disease in 8 years reset the target blood pressure for most of these patients to less than 140/90 mm Hg, and highlighted beta-blockers, renin-angiotensin-aldosterone system blockers, and thiazide diuretics as the mainstays of drug treatment for these patients.
The main messages in the new scientific statement from the American Heart Association, American College of Cardiology, and American Society of Hypertension, released on March 31 in an article published online (Hypertension 2015 [doi:10.116/HYP.0000000000000018]) are the blood pressure targets set for patients with coronary artery disease (CAD) and the designations of the preferred drugs to use to achieve the blood pressure goals when lifestyle measures alone prove inadequate, said Dr. Clive Rosendorff, chair of the panel that wrote the new statement.
But the statement also highlighted that a blood pressure target of less than 130/80 mm Hg “could be considered” and was reasonable for selected CAD patients whom physicians judge capable of achieving this lower blood pressure level safely and who are at especially high risk for cerebrovascular events.
“We felt the best evidence [to prevent future cardiovascular events] was to reduce pressure below 140/90 mm Hg, but a goal pressure of less than 130/80 mm Hg may be appropriate in some cases; we left it to the discretion of physicians to decide which blood pressure target to choose,” said Dr. Rosendorff, professor of medicine at Mount Sinai Hospital in New York.
The default blood pressure goal of less than 140/90 for most CAD patients represented an increase from the less than 130/80 mm Hg goal set by the prior edition of this guideline, issued in 2007 (Circulation 2007;115:2761-88). Current evidence for the lower blood pressure target of less than 130/80 mm Hg “was not as strong,” Dr. Rosendorff said in an interview. He suggested that physicians consider using the lower target for patients who are younger, reasonably healthy, able to tolerate a regimen that brings them to a lower blood pressure without an increase in angina or other significant effects caused by the drugs themselves, do not experience compromised renal function with reduced blood pressure, and have an increased risk for cerebrovascular events.
“These guidelines are not rigid, and should involve a discussion with the patient of the benefits and risks,” he said.
The new statement targets a blood pressure goal of less than 150/90 mm Hg for CAD patients who are more than 80 years old.
The new target for CAD patients represents something of a response to the blood pressure target of less than 150/90 mm Hg for people at least 60 years old recommended last year in recommendations made by the panel originally assembled as the Eighth Joint National Committee (JNC 8) (JAMA 2014;311:507-20). Although the JNC 8 recommendations aimed at the general population in a primary prevention setting, as opposed to CAD patients for whom secondary prevention is the goal, the target of less than 150/90 mm Hg became “highly controversial” and was a factor in composing the new recommendation, Dr. Rosendorff said. He also stressed that the AHA, ACC, and ASH have assembled a group that is formulating new recommendations for diagnosing and managing hypertension for the general population in a primary prevention setting that will come out sometime in the future.
The new hypertension guideline for CAD patients and the 2014 statement from the JNC 8 panel should be seen as distinct recommendations because they targeted different patient populations and because they were based on different ground rules for evidence, said Dr. Suzanne Oparil, one of three people who served on both writing groups. The JNC 8 group focused exclusively on findings from randomized, controlled trials that used hard cardiovascular disease endpoints. The writing committee for the new guidelines targeted specifically at CAD patients also considered evidence from epidemiologic studies. In addition, the new guidelines is targeted at primarily a cardiologist audience, while the 2014 JNC 8 guidelines were written primarily for primary care physicians, she said in an interview.
“I do not believe that the new CAD guidelines will change practice. They reflect what most cardiologists already do,” said Dr. Oparil, professor of medicine and director of the vascular biology and hypertension program at the University of Alabama, Birmingham.
Regarding antihypertensive drug selection the new statement endorses a focus on treating hypertensive patients with established CAD with a beta-blocker, a renin-angiotensin-aldosterone system blocker such as an ACE inhibitor or angiotensin-receptor blocker, and a thiazide or thiazide-like diuretic. Hypertensive patients with CAD should immediately start on all three drug classes, Dr. Rosendorff said.
“For patients with established CAD, a treatment with a beta-blocker moves from the limbo they are in for treating uncomplicated hypertension to center stage,” he said. The statement gives more detailed guidance on which specific drugs from the beta-blocker class have the best evidence for efficacy in various types of patients with CAD.
Dr. Rosendorff had no disclosures. Dr. Oparil has been a consultant to Bayer, Daiichi Sankyo, and Pfizer, and has received research grants from Medtronic, Merck, Novartis, and Takeda.
On Twitter @mitchelzoler
The new statement on treating hypertension in patients with established coronary artery disease clears up what had been a confusing situation for U.S. physicians during the past year.
In early 2014, the panel that had originally been assembled as the Eighth Joint National Committee (JNC 8) issued a statement that called for a blood pressure target of less than 150/90 mm Hg for people 60 years or older (JAMA 2014;311:507-20). People were very confused about that, and may have erroneously believed that this recommendation applied to patients with CAD. I and many of my colleagues believe that having a recommendation to treat to just less than 150/90 mm Hg potentially put millions of CAD patients at risk, especially at risk for stroke. The new statement highlights the high risk faced by CAD patients who need special attention to their blood pressure.

|
| Mitchel L. Zoler/Fronbtline Medical News Dr. Elliott M. Antman |
The epidemiologic evidence clearly shows that increased blood pressure relates to an increased risk for cardiovascular events across a blood pressure range from 115/75 mm Hg to 185/115 mm Hg.
The new recommendations for CAD patients also say that a target blood pressure of less than 130/80 mm Hg may be preferred for selected patients, although the statement does not offer clear steps on how to identify these patients. Physicians must use their clinical judgment.
In my practice, I make sure not to drop a patient’s creatinine clearance to an unacceptably low level, and I would especially consider the lower target for patients with a history of heart failure or left ventricular dilatation or hypertrophy. I believe that in the past, physicians have been too conservative about blood pressure reduction in CAD patients, in part out of a concern about reducing perfusion pressure too much. I believe that if a CAD patient can tolerate a lower blood pressure and the treatment it takes to achieve it, then it is better to be more aggressive.
We also must always remember that the lifestyle modifications, including less dietary sodium, weight loss, and exercise, are the first steps to reducing blood pressure.
Dr. Elliott M. Antman is professor of medicine at Harvard University in Boston and president of the American Heart Association. He had no relevant disclosures. He made these comments in an interview.
The new statement on treating hypertension in patients with established coronary artery disease clears up what had been a confusing situation for U.S. physicians during the past year.
In early 2014, the panel that had originally been assembled as the Eighth Joint National Committee (JNC 8) issued a statement that called for a blood pressure target of less than 150/90 mm Hg for people 60 years or older (JAMA 2014;311:507-20). People were very confused about that, and may have erroneously believed that this recommendation applied to patients with CAD. I and many of my colleagues believe that having a recommendation to treat to just less than 150/90 mm Hg potentially put millions of CAD patients at risk, especially at risk for stroke. The new statement highlights the high risk faced by CAD patients who need special attention to their blood pressure.

|
| Mitchel L. Zoler/Fronbtline Medical News Dr. Elliott M. Antman |
The epidemiologic evidence clearly shows that increased blood pressure relates to an increased risk for cardiovascular events across a blood pressure range from 115/75 mm Hg to 185/115 mm Hg.
The new recommendations for CAD patients also say that a target blood pressure of less than 130/80 mm Hg may be preferred for selected patients, although the statement does not offer clear steps on how to identify these patients. Physicians must use their clinical judgment.
In my practice, I make sure not to drop a patient’s creatinine clearance to an unacceptably low level, and I would especially consider the lower target for patients with a history of heart failure or left ventricular dilatation or hypertrophy. I believe that in the past, physicians have been too conservative about blood pressure reduction in CAD patients, in part out of a concern about reducing perfusion pressure too much. I believe that if a CAD patient can tolerate a lower blood pressure and the treatment it takes to achieve it, then it is better to be more aggressive.
We also must always remember that the lifestyle modifications, including less dietary sodium, weight loss, and exercise, are the first steps to reducing blood pressure.
Dr. Elliott M. Antman is professor of medicine at Harvard University in Boston and president of the American Heart Association. He had no relevant disclosures. He made these comments in an interview.
The new statement on treating hypertension in patients with established coronary artery disease clears up what had been a confusing situation for U.S. physicians during the past year.
In early 2014, the panel that had originally been assembled as the Eighth Joint National Committee (JNC 8) issued a statement that called for a blood pressure target of less than 150/90 mm Hg for people 60 years or older (JAMA 2014;311:507-20). People were very confused about that, and may have erroneously believed that this recommendation applied to patients with CAD. I and many of my colleagues believe that having a recommendation to treat to just less than 150/90 mm Hg potentially put millions of CAD patients at risk, especially at risk for stroke. The new statement highlights the high risk faced by CAD patients who need special attention to their blood pressure.

|
| Mitchel L. Zoler/Fronbtline Medical News Dr. Elliott M. Antman |
The epidemiologic evidence clearly shows that increased blood pressure relates to an increased risk for cardiovascular events across a blood pressure range from 115/75 mm Hg to 185/115 mm Hg.
The new recommendations for CAD patients also say that a target blood pressure of less than 130/80 mm Hg may be preferred for selected patients, although the statement does not offer clear steps on how to identify these patients. Physicians must use their clinical judgment.
In my practice, I make sure not to drop a patient’s creatinine clearance to an unacceptably low level, and I would especially consider the lower target for patients with a history of heart failure or left ventricular dilatation or hypertrophy. I believe that in the past, physicians have been too conservative about blood pressure reduction in CAD patients, in part out of a concern about reducing perfusion pressure too much. I believe that if a CAD patient can tolerate a lower blood pressure and the treatment it takes to achieve it, then it is better to be more aggressive.
We also must always remember that the lifestyle modifications, including less dietary sodium, weight loss, and exercise, are the first steps to reducing blood pressure.
Dr. Elliott M. Antman is professor of medicine at Harvard University in Boston and president of the American Heart Association. He had no relevant disclosures. He made these comments in an interview.
The first update to U.S. guidelines for managing hypertension in adult patients with coronary artery disease in 8 years reset the target blood pressure for most of these patients to less than 140/90 mm Hg, and highlighted beta-blockers, renin-angiotensin-aldosterone system blockers, and thiazide diuretics as the mainstays of drug treatment for these patients.
The main messages in the new scientific statement from the American Heart Association, American College of Cardiology, and American Society of Hypertension, released on March 31 in an article published online (Hypertension 2015 [doi:10.116/HYP.0000000000000018]) are the blood pressure targets set for patients with coronary artery disease (CAD) and the designations of the preferred drugs to use to achieve the blood pressure goals when lifestyle measures alone prove inadequate, said Dr. Clive Rosendorff, chair of the panel that wrote the new statement.
But the statement also highlighted that a blood pressure target of less than 130/80 mm Hg “could be considered” and was reasonable for selected CAD patients whom physicians judge capable of achieving this lower blood pressure level safely and who are at especially high risk for cerebrovascular events.
“We felt the best evidence [to prevent future cardiovascular events] was to reduce pressure below 140/90 mm Hg, but a goal pressure of less than 130/80 mm Hg may be appropriate in some cases; we left it to the discretion of physicians to decide which blood pressure target to choose,” said Dr. Rosendorff, professor of medicine at Mount Sinai Hospital in New York.
The default blood pressure goal of less than 140/90 for most CAD patients represented an increase from the less than 130/80 mm Hg goal set by the prior edition of this guideline, issued in 2007 (Circulation 2007;115:2761-88). Current evidence for the lower blood pressure target of less than 130/80 mm Hg “was not as strong,” Dr. Rosendorff said in an interview. He suggested that physicians consider using the lower target for patients who are younger, reasonably healthy, able to tolerate a regimen that brings them to a lower blood pressure without an increase in angina or other significant effects caused by the drugs themselves, do not experience compromised renal function with reduced blood pressure, and have an increased risk for cerebrovascular events.
“These guidelines are not rigid, and should involve a discussion with the patient of the benefits and risks,” he said.
The new statement targets a blood pressure goal of less than 150/90 mm Hg for CAD patients who are more than 80 years old.
The new target for CAD patients represents something of a response to the blood pressure target of less than 150/90 mm Hg for people at least 60 years old recommended last year in recommendations made by the panel originally assembled as the Eighth Joint National Committee (JNC 8) (JAMA 2014;311:507-20). Although the JNC 8 recommendations aimed at the general population in a primary prevention setting, as opposed to CAD patients for whom secondary prevention is the goal, the target of less than 150/90 mm Hg became “highly controversial” and was a factor in composing the new recommendation, Dr. Rosendorff said. He also stressed that the AHA, ACC, and ASH have assembled a group that is formulating new recommendations for diagnosing and managing hypertension for the general population in a primary prevention setting that will come out sometime in the future.
The new hypertension guideline for CAD patients and the 2014 statement from the JNC 8 panel should be seen as distinct recommendations because they targeted different patient populations and because they were based on different ground rules for evidence, said Dr. Suzanne Oparil, one of three people who served on both writing groups. The JNC 8 group focused exclusively on findings from randomized, controlled trials that used hard cardiovascular disease endpoints. The writing committee for the new guidelines targeted specifically at CAD patients also considered evidence from epidemiologic studies. In addition, the new guidelines is targeted at primarily a cardiologist audience, while the 2014 JNC 8 guidelines were written primarily for primary care physicians, she said in an interview.
“I do not believe that the new CAD guidelines will change practice. They reflect what most cardiologists already do,” said Dr. Oparil, professor of medicine and director of the vascular biology and hypertension program at the University of Alabama, Birmingham.
Regarding antihypertensive drug selection the new statement endorses a focus on treating hypertensive patients with established CAD with a beta-blocker, a renin-angiotensin-aldosterone system blocker such as an ACE inhibitor or angiotensin-receptor blocker, and a thiazide or thiazide-like diuretic. Hypertensive patients with CAD should immediately start on all three drug classes, Dr. Rosendorff said.
“For patients with established CAD, a treatment with a beta-blocker moves from the limbo they are in for treating uncomplicated hypertension to center stage,” he said. The statement gives more detailed guidance on which specific drugs from the beta-blocker class have the best evidence for efficacy in various types of patients with CAD.
Dr. Rosendorff had no disclosures. Dr. Oparil has been a consultant to Bayer, Daiichi Sankyo, and Pfizer, and has received research grants from Medtronic, Merck, Novartis, and Takeda.
On Twitter @mitchelzoler
The first update to U.S. guidelines for managing hypertension in adult patients with coronary artery disease in 8 years reset the target blood pressure for most of these patients to less than 140/90 mm Hg, and highlighted beta-blockers, renin-angiotensin-aldosterone system blockers, and thiazide diuretics as the mainstays of drug treatment for these patients.
The main messages in the new scientific statement from the American Heart Association, American College of Cardiology, and American Society of Hypertension, released on March 31 in an article published online (Hypertension 2015 [doi:10.116/HYP.0000000000000018]) are the blood pressure targets set for patients with coronary artery disease (CAD) and the designations of the preferred drugs to use to achieve the blood pressure goals when lifestyle measures alone prove inadequate, said Dr. Clive Rosendorff, chair of the panel that wrote the new statement.
But the statement also highlighted that a blood pressure target of less than 130/80 mm Hg “could be considered” and was reasonable for selected CAD patients whom physicians judge capable of achieving this lower blood pressure level safely and who are at especially high risk for cerebrovascular events.
“We felt the best evidence [to prevent future cardiovascular events] was to reduce pressure below 140/90 mm Hg, but a goal pressure of less than 130/80 mm Hg may be appropriate in some cases; we left it to the discretion of physicians to decide which blood pressure target to choose,” said Dr. Rosendorff, professor of medicine at Mount Sinai Hospital in New York.
The default blood pressure goal of less than 140/90 for most CAD patients represented an increase from the less than 130/80 mm Hg goal set by the prior edition of this guideline, issued in 2007 (Circulation 2007;115:2761-88). Current evidence for the lower blood pressure target of less than 130/80 mm Hg “was not as strong,” Dr. Rosendorff said in an interview. He suggested that physicians consider using the lower target for patients who are younger, reasonably healthy, able to tolerate a regimen that brings them to a lower blood pressure without an increase in angina or other significant effects caused by the drugs themselves, do not experience compromised renal function with reduced blood pressure, and have an increased risk for cerebrovascular events.
“These guidelines are not rigid, and should involve a discussion with the patient of the benefits and risks,” he said.
The new statement targets a blood pressure goal of less than 150/90 mm Hg for CAD patients who are more than 80 years old.
The new target for CAD patients represents something of a response to the blood pressure target of less than 150/90 mm Hg for people at least 60 years old recommended last year in recommendations made by the panel originally assembled as the Eighth Joint National Committee (JNC 8) (JAMA 2014;311:507-20). Although the JNC 8 recommendations aimed at the general population in a primary prevention setting, as opposed to CAD patients for whom secondary prevention is the goal, the target of less than 150/90 mm Hg became “highly controversial” and was a factor in composing the new recommendation, Dr. Rosendorff said. He also stressed that the AHA, ACC, and ASH have assembled a group that is formulating new recommendations for diagnosing and managing hypertension for the general population in a primary prevention setting that will come out sometime in the future.
The new hypertension guideline for CAD patients and the 2014 statement from the JNC 8 panel should be seen as distinct recommendations because they targeted different patient populations and because they were based on different ground rules for evidence, said Dr. Suzanne Oparil, one of three people who served on both writing groups. The JNC 8 group focused exclusively on findings from randomized, controlled trials that used hard cardiovascular disease endpoints. The writing committee for the new guidelines targeted specifically at CAD patients also considered evidence from epidemiologic studies. In addition, the new guidelines is targeted at primarily a cardiologist audience, while the 2014 JNC 8 guidelines were written primarily for primary care physicians, she said in an interview.
“I do not believe that the new CAD guidelines will change practice. They reflect what most cardiologists already do,” said Dr. Oparil, professor of medicine and director of the vascular biology and hypertension program at the University of Alabama, Birmingham.
Regarding antihypertensive drug selection the new statement endorses a focus on treating hypertensive patients with established CAD with a beta-blocker, a renin-angiotensin-aldosterone system blocker such as an ACE inhibitor or angiotensin-receptor blocker, and a thiazide or thiazide-like diuretic. Hypertensive patients with CAD should immediately start on all three drug classes, Dr. Rosendorff said.
“For patients with established CAD, a treatment with a beta-blocker moves from the limbo they are in for treating uncomplicated hypertension to center stage,” he said. The statement gives more detailed guidance on which specific drugs from the beta-blocker class have the best evidence for efficacy in various types of patients with CAD.
Dr. Rosendorff had no disclosures. Dr. Oparil has been a consultant to Bayer, Daiichi Sankyo, and Pfizer, and has received research grants from Medtronic, Merck, Novartis, and Takeda.
On Twitter @mitchelzoler
FROM HYPERTENSION
ACP: Avoid ECG, MPI cardiac screening in low-risk patients
Clinicians should not screen for cardiac disease in asymptomatic, low-risk adults using resting or stress electrocardiography, stress echocardiography, or stress myocardial perfusion imaging , according to new guidelines from the American College of Physicians.
“There is no evidence that cardiac screening of low-risk adults with resting or stress ECG, stress echocardiography, or stress MPI improves outcomes, but it is associated with increased costs and potential harms,” wrote the guideline’s author, Dr. Roger Chou, associate professor of medicine at Oregon Health & Science University, Portland.
The recommendation is based on a systematic literature review, recommendations from the U.S. Preventive Services Task Force, and American College of Cardiology guidelines. The new ACP clinical guideline was published March 17 in Annals of Internal Medicine (doi: 10.7326/M14-1225).
“What we are saying here is that, as physicians, we have responsibility to understand what the pretest probability is, and what the likelihood is that someone actually has disease – and if it’s low enough, then doing the screening test is going to cause a lot more false positives than true positives,” Dr. Robert Centor, regional dean of the Huntsville Medical Campus of the University of Alabama at Birmingham, explained in an interview.
“Even if it is a true positive, there is no evidence that we can find that finding that heart disease will do anything other than lead someone to do a procedure that we have no evidence will improve their outcomes,” added Dr. Centor, chair of the ACP Board of Regents.
Despite existing recommendations to the contrary, physicians are increasingly performing these tests on low-risk patients, the ACP cautioned.
For example, a Consumer Reports survey found that “39% of asymptomatic adults without high blood pressure or a high cholesterol level reported having ECG within the past 5 years, and 12% reported undergoing exercise ECG,” Dr. Chou wrote in his report on behalf of the ACP High Value Care Task Force. More than half of those patients said their physicians recommended the tests as part of their routine health care.
The rise in the use of such tests is likely the result of a combination of factors, Dr. Centor said. Those factors include money (patients see no out-of-pocket cost and thus don’t consider the cost of tests in their decision making), direct-to-consumer advertising, fear on behalf of physicians that they might miss a diagnosis, and a lack of understanding by patients on the adverse effects of screening if they are at low risk for heart disease.
Dr. Chou identified a number of potential harms related to unnecessary screenings, including sudden death or hospitalization during stress tests; adverse events from pharmacologics used to induce stress; radiation exposure from myocardial perfusion imaging; false positive results that, in turn, lead to anxiety by the patient and additional unnecessary tests and treatments; disease labeling; and downstream harms from follow-up testing and interventions.
“To be most effective, efforts to reduce the use of imaging should be multifocal and should address clinician behavior, patient expectations, direct-to-consumer screening programs, and financial incentives,” Dr. Chou explained.
In low-risk patients, physicians instead should “focus on treating modifiable risk factors (such as smoking, diabetes, hypertension, hyperlipidemia, and overweight) and encouraging healthy levels of exercise,” according to the guideline.
Clinicians should not screen for cardiac disease in asymptomatic, low-risk adults using resting or stress electrocardiography, stress echocardiography, or stress myocardial perfusion imaging , according to new guidelines from the American College of Physicians.
“There is no evidence that cardiac screening of low-risk adults with resting or stress ECG, stress echocardiography, or stress MPI improves outcomes, but it is associated with increased costs and potential harms,” wrote the guideline’s author, Dr. Roger Chou, associate professor of medicine at Oregon Health & Science University, Portland.
The recommendation is based on a systematic literature review, recommendations from the U.S. Preventive Services Task Force, and American College of Cardiology guidelines. The new ACP clinical guideline was published March 17 in Annals of Internal Medicine (doi: 10.7326/M14-1225).
“What we are saying here is that, as physicians, we have responsibility to understand what the pretest probability is, and what the likelihood is that someone actually has disease – and if it’s low enough, then doing the screening test is going to cause a lot more false positives than true positives,” Dr. Robert Centor, regional dean of the Huntsville Medical Campus of the University of Alabama at Birmingham, explained in an interview.
“Even if it is a true positive, there is no evidence that we can find that finding that heart disease will do anything other than lead someone to do a procedure that we have no evidence will improve their outcomes,” added Dr. Centor, chair of the ACP Board of Regents.
Despite existing recommendations to the contrary, physicians are increasingly performing these tests on low-risk patients, the ACP cautioned.
For example, a Consumer Reports survey found that “39% of asymptomatic adults without high blood pressure or a high cholesterol level reported having ECG within the past 5 years, and 12% reported undergoing exercise ECG,” Dr. Chou wrote in his report on behalf of the ACP High Value Care Task Force. More than half of those patients said their physicians recommended the tests as part of their routine health care.
The rise in the use of such tests is likely the result of a combination of factors, Dr. Centor said. Those factors include money (patients see no out-of-pocket cost and thus don’t consider the cost of tests in their decision making), direct-to-consumer advertising, fear on behalf of physicians that they might miss a diagnosis, and a lack of understanding by patients on the adverse effects of screening if they are at low risk for heart disease.
Dr. Chou identified a number of potential harms related to unnecessary screenings, including sudden death or hospitalization during stress tests; adverse events from pharmacologics used to induce stress; radiation exposure from myocardial perfusion imaging; false positive results that, in turn, lead to anxiety by the patient and additional unnecessary tests and treatments; disease labeling; and downstream harms from follow-up testing and interventions.
“To be most effective, efforts to reduce the use of imaging should be multifocal and should address clinician behavior, patient expectations, direct-to-consumer screening programs, and financial incentives,” Dr. Chou explained.
In low-risk patients, physicians instead should “focus on treating modifiable risk factors (such as smoking, diabetes, hypertension, hyperlipidemia, and overweight) and encouraging healthy levels of exercise,” according to the guideline.
Clinicians should not screen for cardiac disease in asymptomatic, low-risk adults using resting or stress electrocardiography, stress echocardiography, or stress myocardial perfusion imaging , according to new guidelines from the American College of Physicians.
“There is no evidence that cardiac screening of low-risk adults with resting or stress ECG, stress echocardiography, or stress MPI improves outcomes, but it is associated with increased costs and potential harms,” wrote the guideline’s author, Dr. Roger Chou, associate professor of medicine at Oregon Health & Science University, Portland.
The recommendation is based on a systematic literature review, recommendations from the U.S. Preventive Services Task Force, and American College of Cardiology guidelines. The new ACP clinical guideline was published March 17 in Annals of Internal Medicine (doi: 10.7326/M14-1225).
“What we are saying here is that, as physicians, we have responsibility to understand what the pretest probability is, and what the likelihood is that someone actually has disease – and if it’s low enough, then doing the screening test is going to cause a lot more false positives than true positives,” Dr. Robert Centor, regional dean of the Huntsville Medical Campus of the University of Alabama at Birmingham, explained in an interview.
“Even if it is a true positive, there is no evidence that we can find that finding that heart disease will do anything other than lead someone to do a procedure that we have no evidence will improve their outcomes,” added Dr. Centor, chair of the ACP Board of Regents.
Despite existing recommendations to the contrary, physicians are increasingly performing these tests on low-risk patients, the ACP cautioned.
For example, a Consumer Reports survey found that “39% of asymptomatic adults without high blood pressure or a high cholesterol level reported having ECG within the past 5 years, and 12% reported undergoing exercise ECG,” Dr. Chou wrote in his report on behalf of the ACP High Value Care Task Force. More than half of those patients said their physicians recommended the tests as part of their routine health care.
The rise in the use of such tests is likely the result of a combination of factors, Dr. Centor said. Those factors include money (patients see no out-of-pocket cost and thus don’t consider the cost of tests in their decision making), direct-to-consumer advertising, fear on behalf of physicians that they might miss a diagnosis, and a lack of understanding by patients on the adverse effects of screening if they are at low risk for heart disease.
Dr. Chou identified a number of potential harms related to unnecessary screenings, including sudden death or hospitalization during stress tests; adverse events from pharmacologics used to induce stress; radiation exposure from myocardial perfusion imaging; false positive results that, in turn, lead to anxiety by the patient and additional unnecessary tests and treatments; disease labeling; and downstream harms from follow-up testing and interventions.
“To be most effective, efforts to reduce the use of imaging should be multifocal and should address clinician behavior, patient expectations, direct-to-consumer screening programs, and financial incentives,” Dr. Chou explained.
In low-risk patients, physicians instead should “focus on treating modifiable risk factors (such as smoking, diabetes, hypertension, hyperlipidemia, and overweight) and encouraging healthy levels of exercise,” according to the guideline.
FROM ANNALS OF INTERNAL MEDICINE
FDA guidance focuses on infections with reusable devices, including duodenoscopes
Recommendations to manufacturers about improving the safety of reusable medical devices and an upcoming advisory committee meeting on duodenoscope-associated infections are two efforts recently announced by the Food and Drug Administration that address the risks associated with reusable devices.
A final guidance document for industry on reprocessing reusable medical devices includes recommendations “aimed at helping device manufacturers develop safer reusable devices, especially those devices that pose a greater risk of infection,” according to the March 12 announcement. Also included in the guidance are criteria that should be met in instructions for reprocessing reusable devices, “to ensure users understand and correctly follow the reprocessing instructions,” and recommendations that manufacturers should consider “reprocessing challenges” at the early stages of the design of such devices.
The same announcement said that in mid-May, the FDA was convening a 2-day meeting of the agency’s Gastroenterology and Urology Devices Panel to discuss the recent reports of infections associated with the use of duodenoscopes in endoscopic retrograde cholangiopancreatography (ERCP) procedures in U.S. hospitals.
The announcement was issued less than a month after the agency alerted health care professionals and the public about the association with duodenoscopes and the transmission of multidrug-resistant bacterial infections in patients who had undergone ERCP procedures, despite proper cleaning and disinfection of the devices. Between January 2013 and December 2014, the agency received 75 medical device adverse event reports for about 135 patients in the United States “relating to possible microbial transmission from reprocessed duodenoscopes,” according to the safety communication issued by the FDA on Feb. 19.
These reports and cases described in the medical literature have occurred even when manufacturer instructions for cleaning and sterilization were followed.
“Although the complex design of duodenoscopes improves the efficiency and effectiveness of ERCP, it causes challenges for cleaning and high-level disinfection,” according to the statement, which pointed out that it can be difficult to access some parts of the duodenoscopes when they are cleaned. Problems include the “elevator” mechanism at the tip of the duodenoscope, which should be manually brushed, but a brush may not be able to reach microscopic crevices in this mechanism and “residual body fluids and organic debris may remain in these crevices after cleaning and disinfection,” possibly exposing patients to serious infections if the fluids are contaminated with microbes.
The infections reported include carbapenem-resistant Enterobacteriaceae (CRE), according to the first FDA statement, which did not mention whether any of the reports were fatal.
But on Feb. 18, the UCLA Health System announced that CRE may have been transmitted to seven patients during ERCP procedures, and may have contributed to the death of two of the patients. The two devices implicated in these cases are no longer used and the medical center has started to use a decontamination process “that goes above and beyond manufacturer and national standards” for the devices, the statement said. More than 100 patients who had an ERCP between October 2014 and January 2015 at UCLA have been notified they may have been infected with CRE.
The FDA statement includes recommendations for facilities and staff that reprocess duodenoscopes, for patients, and for health care professionals. One recommendation is to take a duodenoscope out of service if there is any suspicion it may be linked to a multidrug-resistant infection in a patient who has undergone ERCP.
In early March, another outbreak was reported at Cedars-Sinai Medical Center in Los Angeles, which announced that four patients who had undergone an ERCP procedure between August 2014 and January 2015 with the same duodenoscope had been infected with CRE, “despite the fact that Cedars-Sinai meticulously followed the disinfection procedure for duodenoscopes recommended in instructions provided by the manufacturer (Olympus Corporation) and the FDA.” This duodenoscope was the TJF-Q180 V model, a Cedars-Sinai spokesperson confirmed.
This particular duodenoscope has not yet been cleared for marketing, but has been used commercially, according to an FDA statement March 4 updating the duodenoscope-associated infection issue. The statement said that there was “no evidence” that the lack of clearance was associated with infections, and that the reported infections were associated with duodenoscopes from all three manufacturers of the devices used in the United States. In addition, the FDA statement noted that if the TJF-Q180 V duodenoscope was removed from the market, there may not be enough duodenoscopes to meet “the clinical demand in the United States of approximately 500,000 procedures per year.”
At the advisory panel meeting May 14-15, the FDA will ask the expert panel to discuss and make recommendations on various issues, including approaches that ensure patient safety during ERCP procedures and the effectiveness of the cleaning, disinfection, and sterilization procedures for duodenoscopes.
The FDA is asking health care professionals to report any infections possibly related to ERCP duodenoscopes to the manufacturers and the FDA’s MedWatch program.
The Centers for Disease Control and Prevention has provided an interim protocol for health care facilities, with information on monitoring for bacterial contamination of duodenoscopes after reprocessing and other reprocessing issues.
Recommendations to manufacturers about improving the safety of reusable medical devices and an upcoming advisory committee meeting on duodenoscope-associated infections are two efforts recently announced by the Food and Drug Administration that address the risks associated with reusable devices.
A final guidance document for industry on reprocessing reusable medical devices includes recommendations “aimed at helping device manufacturers develop safer reusable devices, especially those devices that pose a greater risk of infection,” according to the March 12 announcement. Also included in the guidance are criteria that should be met in instructions for reprocessing reusable devices, “to ensure users understand and correctly follow the reprocessing instructions,” and recommendations that manufacturers should consider “reprocessing challenges” at the early stages of the design of such devices.
The same announcement said that in mid-May, the FDA was convening a 2-day meeting of the agency’s Gastroenterology and Urology Devices Panel to discuss the recent reports of infections associated with the use of duodenoscopes in endoscopic retrograde cholangiopancreatography (ERCP) procedures in U.S. hospitals.
The announcement was issued less than a month after the agency alerted health care professionals and the public about the association with duodenoscopes and the transmission of multidrug-resistant bacterial infections in patients who had undergone ERCP procedures, despite proper cleaning and disinfection of the devices. Between January 2013 and December 2014, the agency received 75 medical device adverse event reports for about 135 patients in the United States “relating to possible microbial transmission from reprocessed duodenoscopes,” according to the safety communication issued by the FDA on Feb. 19.
These reports and cases described in the medical literature have occurred even when manufacturer instructions for cleaning and sterilization were followed.
“Although the complex design of duodenoscopes improves the efficiency and effectiveness of ERCP, it causes challenges for cleaning and high-level disinfection,” according to the statement, which pointed out that it can be difficult to access some parts of the duodenoscopes when they are cleaned. Problems include the “elevator” mechanism at the tip of the duodenoscope, which should be manually brushed, but a brush may not be able to reach microscopic crevices in this mechanism and “residual body fluids and organic debris may remain in these crevices after cleaning and disinfection,” possibly exposing patients to serious infections if the fluids are contaminated with microbes.
The infections reported include carbapenem-resistant Enterobacteriaceae (CRE), according to the first FDA statement, which did not mention whether any of the reports were fatal.
But on Feb. 18, the UCLA Health System announced that CRE may have been transmitted to seven patients during ERCP procedures, and may have contributed to the death of two of the patients. The two devices implicated in these cases are no longer used and the medical center has started to use a decontamination process “that goes above and beyond manufacturer and national standards” for the devices, the statement said. More than 100 patients who had an ERCP between October 2014 and January 2015 at UCLA have been notified they may have been infected with CRE.
The FDA statement includes recommendations for facilities and staff that reprocess duodenoscopes, for patients, and for health care professionals. One recommendation is to take a duodenoscope out of service if there is any suspicion it may be linked to a multidrug-resistant infection in a patient who has undergone ERCP.
In early March, another outbreak was reported at Cedars-Sinai Medical Center in Los Angeles, which announced that four patients who had undergone an ERCP procedure between August 2014 and January 2015 with the same duodenoscope had been infected with CRE, “despite the fact that Cedars-Sinai meticulously followed the disinfection procedure for duodenoscopes recommended in instructions provided by the manufacturer (Olympus Corporation) and the FDA.” This duodenoscope was the TJF-Q180 V model, a Cedars-Sinai spokesperson confirmed.
This particular duodenoscope has not yet been cleared for marketing, but has been used commercially, according to an FDA statement March 4 updating the duodenoscope-associated infection issue. The statement said that there was “no evidence” that the lack of clearance was associated with infections, and that the reported infections were associated with duodenoscopes from all three manufacturers of the devices used in the United States. In addition, the FDA statement noted that if the TJF-Q180 V duodenoscope was removed from the market, there may not be enough duodenoscopes to meet “the clinical demand in the United States of approximately 500,000 procedures per year.”
At the advisory panel meeting May 14-15, the FDA will ask the expert panel to discuss and make recommendations on various issues, including approaches that ensure patient safety during ERCP procedures and the effectiveness of the cleaning, disinfection, and sterilization procedures for duodenoscopes.
The FDA is asking health care professionals to report any infections possibly related to ERCP duodenoscopes to the manufacturers and the FDA’s MedWatch program.
The Centers for Disease Control and Prevention has provided an interim protocol for health care facilities, with information on monitoring for bacterial contamination of duodenoscopes after reprocessing and other reprocessing issues.
Recommendations to manufacturers about improving the safety of reusable medical devices and an upcoming advisory committee meeting on duodenoscope-associated infections are two efforts recently announced by the Food and Drug Administration that address the risks associated with reusable devices.
A final guidance document for industry on reprocessing reusable medical devices includes recommendations “aimed at helping device manufacturers develop safer reusable devices, especially those devices that pose a greater risk of infection,” according to the March 12 announcement. Also included in the guidance are criteria that should be met in instructions for reprocessing reusable devices, “to ensure users understand and correctly follow the reprocessing instructions,” and recommendations that manufacturers should consider “reprocessing challenges” at the early stages of the design of such devices.
The same announcement said that in mid-May, the FDA was convening a 2-day meeting of the agency’s Gastroenterology and Urology Devices Panel to discuss the recent reports of infections associated with the use of duodenoscopes in endoscopic retrograde cholangiopancreatography (ERCP) procedures in U.S. hospitals.
The announcement was issued less than a month after the agency alerted health care professionals and the public about the association with duodenoscopes and the transmission of multidrug-resistant bacterial infections in patients who had undergone ERCP procedures, despite proper cleaning and disinfection of the devices. Between January 2013 and December 2014, the agency received 75 medical device adverse event reports for about 135 patients in the United States “relating to possible microbial transmission from reprocessed duodenoscopes,” according to the safety communication issued by the FDA on Feb. 19.
These reports and cases described in the medical literature have occurred even when manufacturer instructions for cleaning and sterilization were followed.
“Although the complex design of duodenoscopes improves the efficiency and effectiveness of ERCP, it causes challenges for cleaning and high-level disinfection,” according to the statement, which pointed out that it can be difficult to access some parts of the duodenoscopes when they are cleaned. Problems include the “elevator” mechanism at the tip of the duodenoscope, which should be manually brushed, but a brush may not be able to reach microscopic crevices in this mechanism and “residual body fluids and organic debris may remain in these crevices after cleaning and disinfection,” possibly exposing patients to serious infections if the fluids are contaminated with microbes.
The infections reported include carbapenem-resistant Enterobacteriaceae (CRE), according to the first FDA statement, which did not mention whether any of the reports were fatal.
But on Feb. 18, the UCLA Health System announced that CRE may have been transmitted to seven patients during ERCP procedures, and may have contributed to the death of two of the patients. The two devices implicated in these cases are no longer used and the medical center has started to use a decontamination process “that goes above and beyond manufacturer and national standards” for the devices, the statement said. More than 100 patients who had an ERCP between October 2014 and January 2015 at UCLA have been notified they may have been infected with CRE.
The FDA statement includes recommendations for facilities and staff that reprocess duodenoscopes, for patients, and for health care professionals. One recommendation is to take a duodenoscope out of service if there is any suspicion it may be linked to a multidrug-resistant infection in a patient who has undergone ERCP.
In early March, another outbreak was reported at Cedars-Sinai Medical Center in Los Angeles, which announced that four patients who had undergone an ERCP procedure between August 2014 and January 2015 with the same duodenoscope had been infected with CRE, “despite the fact that Cedars-Sinai meticulously followed the disinfection procedure for duodenoscopes recommended in instructions provided by the manufacturer (Olympus Corporation) and the FDA.” This duodenoscope was the TJF-Q180 V model, a Cedars-Sinai spokesperson confirmed.
This particular duodenoscope has not yet been cleared for marketing, but has been used commercially, according to an FDA statement March 4 updating the duodenoscope-associated infection issue. The statement said that there was “no evidence” that the lack of clearance was associated with infections, and that the reported infections were associated with duodenoscopes from all three manufacturers of the devices used in the United States. In addition, the FDA statement noted that if the TJF-Q180 V duodenoscope was removed from the market, there may not be enough duodenoscopes to meet “the clinical demand in the United States of approximately 500,000 procedures per year.”
At the advisory panel meeting May 14-15, the FDA will ask the expert panel to discuss and make recommendations on various issues, including approaches that ensure patient safety during ERCP procedures and the effectiveness of the cleaning, disinfection, and sterilization procedures for duodenoscopes.
The FDA is asking health care professionals to report any infections possibly related to ERCP duodenoscopes to the manufacturers and the FDA’s MedWatch program.
The Centers for Disease Control and Prevention has provided an interim protocol for health care facilities, with information on monitoring for bacterial contamination of duodenoscopes after reprocessing and other reprocessing issues.
Guideline recommends combination therapy for smoking cessation in cancer patients
The National Comprehensive Cancer Network has published a new guideline on smoking cessation for cancer patients that recommends combining pharmacologic therapy with counseling as the most effective approach, along with rigorous review and close follow-ups to prevent relapses.
“Although the medical community recognizes the importance of smoking cessation, supporting patients in ceasing to smoke is generally not done well. Our hope is that by addressing smoking cessation in a cancer patient population, we can make it easier for oncologists to effectively support their patients in achieving their smoking cessation goals,” Dr. Peter Shields, deputy director of the Ohio State University Comprehensive Cancer Center, said in a written statement. Of the estimated 590,000 cancer deaths in 2015, about 170,000, or nearly 30%, will be caused by tobacco smoking. Quitting tobacco improves cancer treatment effectiveness and reduces cancer recurrence, according to the NCCN.
Read the full statement on the NCCN website.
The National Comprehensive Cancer Network has published a new guideline on smoking cessation for cancer patients that recommends combining pharmacologic therapy with counseling as the most effective approach, along with rigorous review and close follow-ups to prevent relapses.
“Although the medical community recognizes the importance of smoking cessation, supporting patients in ceasing to smoke is generally not done well. Our hope is that by addressing smoking cessation in a cancer patient population, we can make it easier for oncologists to effectively support their patients in achieving their smoking cessation goals,” Dr. Peter Shields, deputy director of the Ohio State University Comprehensive Cancer Center, said in a written statement. Of the estimated 590,000 cancer deaths in 2015, about 170,000, or nearly 30%, will be caused by tobacco smoking. Quitting tobacco improves cancer treatment effectiveness and reduces cancer recurrence, according to the NCCN.
Read the full statement on the NCCN website.
The National Comprehensive Cancer Network has published a new guideline on smoking cessation for cancer patients that recommends combining pharmacologic therapy with counseling as the most effective approach, along with rigorous review and close follow-ups to prevent relapses.
“Although the medical community recognizes the importance of smoking cessation, supporting patients in ceasing to smoke is generally not done well. Our hope is that by addressing smoking cessation in a cancer patient population, we can make it easier for oncologists to effectively support their patients in achieving their smoking cessation goals,” Dr. Peter Shields, deputy director of the Ohio State University Comprehensive Cancer Center, said in a written statement. Of the estimated 590,000 cancer deaths in 2015, about 170,000, or nearly 30%, will be caused by tobacco smoking. Quitting tobacco improves cancer treatment effectiveness and reduces cancer recurrence, according to the NCCN.
Read the full statement on the NCCN website.