User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
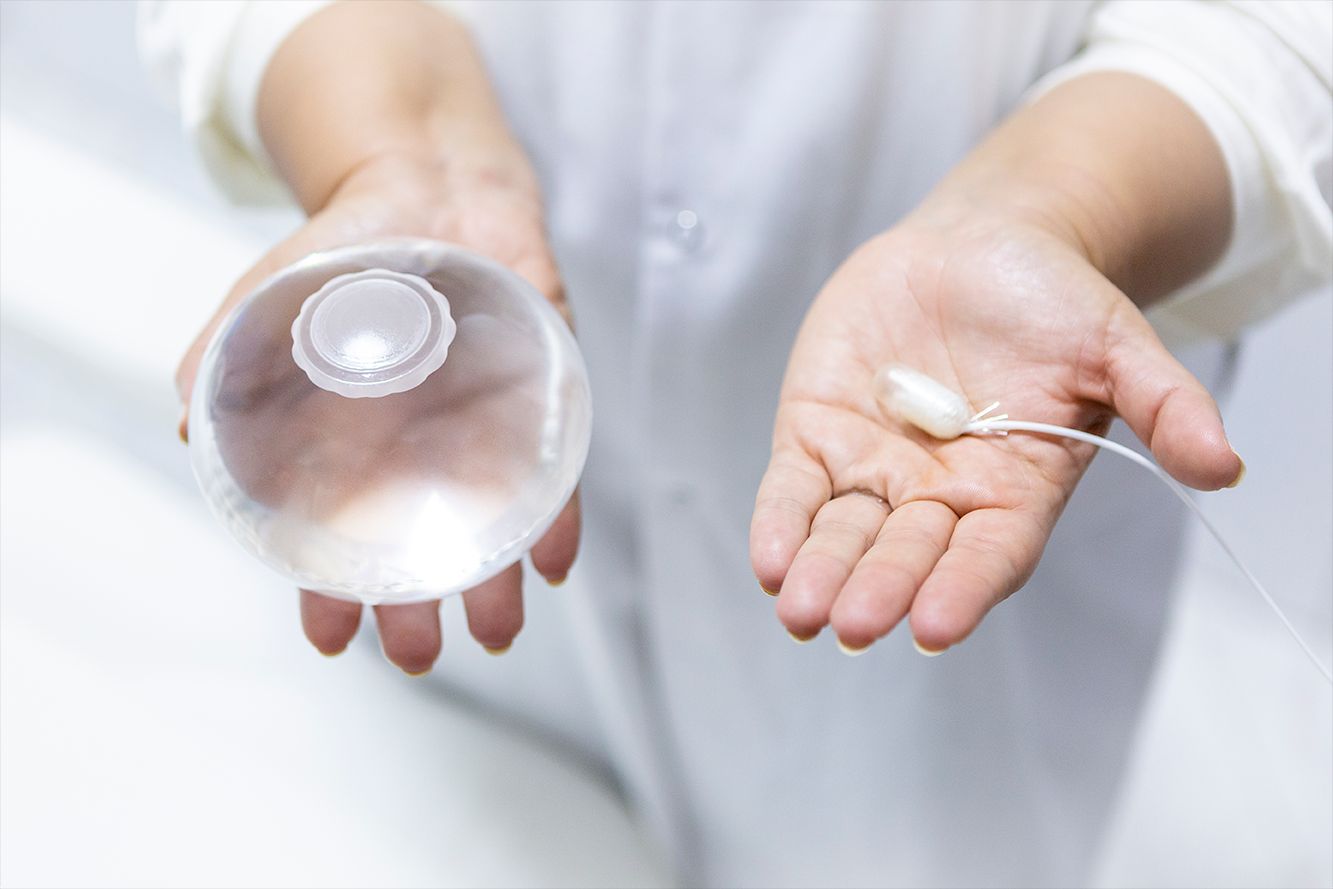
FDA clears iLet bionic pancreas insulin delivery system
Working together with a previously cleared integrated continuous glucose monitor (CGM), the entire new system is called the iLet Bionic Pancreas. It differs from current automated insulin delivery (AID) systems in its increased level of automation. The adaptive algorithm is initialized using only the patient’s body weight, without other insulin dosing parameters. Rather than entering specific carbohydrate counts, users only input whether the carbohydrate amount in the meal is “small,” “medium,” or “large.” The algorithm adapts over time to users’ individual 24/7 insulin needs.
Pivotal data for the system were presented in June 2022 at the annual scientific sessions of the American Diabetes Association.
In the 16-center trial involving 440 adults and children 6 years and older with type 1 diabetes, the system reduced hemoglobin A1c by 0.5 percentage points by 13 weeks, without increased hypoglycemia. They spent an average of 2.6 hours more time in range, compared with standard of care (either currently available AIDs, stand-alone pump and CGM devices, or multiple daily injections plus CGM).
The FDA had granted the iLet a breakthrough device designation in December 2019.
Anne L. Peters, MD, a professor of medicine at the University of Southern California, Los Angeles, and director of the USC clinical diabetes program, commented on the pivotal study and the system in June 2022. She called the study “cool” because it enrolled more than 25% minority individuals “who aren’t routinely studied in these insulin device trials” and also that it included people with a range of baseline A1c levels, with more than 30% greater than 8%.
Regarding the system’s algorithm, she pointed out that it “doesn’t allow for the individual using the pump to fidget with it. They can’t override the system and they can’t put in other insulin doses. The system is just there to take care of their diabetes.”
That might represent a limitation for some with type 1 diabetes, study coprincipal investigator Roy W. Beck, MD, PhD, said in an interview during the ADA meeting. “The iLet could dramatically reduce type 1 diabetes management burden for many patients, but it might not suit everyone. For example, somebody who’s very compulsive and has an A1c of 6.5% and is used to manipulating what they do, this is probably not a good system for them because the system is kind of taking over.”
On the other hand, Dr. Peters said, “I think what’s important about this system is that it may allow for greater use of automated insulin delivery systems. It may allow primary care providers to use these systems without needing all sorts of support, and patients may be able to use these devices more simply than a device where they have to do carb counting and adjusting in ways that I think tend to be pretty complicated and require higher numeracy and literacy skills.”
The “bionic pancreas” was originally conceived as a dual-hormone system including glucagon delivery as well as insulin. Beta Bionics is continuing to work with the FDA on that front.
A version of this article first appeared on Medscape.com.
Working together with a previously cleared integrated continuous glucose monitor (CGM), the entire new system is called the iLet Bionic Pancreas. It differs from current automated insulin delivery (AID) systems in its increased level of automation. The adaptive algorithm is initialized using only the patient’s body weight, without other insulin dosing parameters. Rather than entering specific carbohydrate counts, users only input whether the carbohydrate amount in the meal is “small,” “medium,” or “large.” The algorithm adapts over time to users’ individual 24/7 insulin needs.
Pivotal data for the system were presented in June 2022 at the annual scientific sessions of the American Diabetes Association.
In the 16-center trial involving 440 adults and children 6 years and older with type 1 diabetes, the system reduced hemoglobin A1c by 0.5 percentage points by 13 weeks, without increased hypoglycemia. They spent an average of 2.6 hours more time in range, compared with standard of care (either currently available AIDs, stand-alone pump and CGM devices, or multiple daily injections plus CGM).
The FDA had granted the iLet a breakthrough device designation in December 2019.
Anne L. Peters, MD, a professor of medicine at the University of Southern California, Los Angeles, and director of the USC clinical diabetes program, commented on the pivotal study and the system in June 2022. She called the study “cool” because it enrolled more than 25% minority individuals “who aren’t routinely studied in these insulin device trials” and also that it included people with a range of baseline A1c levels, with more than 30% greater than 8%.
Regarding the system’s algorithm, she pointed out that it “doesn’t allow for the individual using the pump to fidget with it. They can’t override the system and they can’t put in other insulin doses. The system is just there to take care of their diabetes.”
That might represent a limitation for some with type 1 diabetes, study coprincipal investigator Roy W. Beck, MD, PhD, said in an interview during the ADA meeting. “The iLet could dramatically reduce type 1 diabetes management burden for many patients, but it might not suit everyone. For example, somebody who’s very compulsive and has an A1c of 6.5% and is used to manipulating what they do, this is probably not a good system for them because the system is kind of taking over.”
On the other hand, Dr. Peters said, “I think what’s important about this system is that it may allow for greater use of automated insulin delivery systems. It may allow primary care providers to use these systems without needing all sorts of support, and patients may be able to use these devices more simply than a device where they have to do carb counting and adjusting in ways that I think tend to be pretty complicated and require higher numeracy and literacy skills.”
The “bionic pancreas” was originally conceived as a dual-hormone system including glucagon delivery as well as insulin. Beta Bionics is continuing to work with the FDA on that front.
A version of this article first appeared on Medscape.com.
Working together with a previously cleared integrated continuous glucose monitor (CGM), the entire new system is called the iLet Bionic Pancreas. It differs from current automated insulin delivery (AID) systems in its increased level of automation. The adaptive algorithm is initialized using only the patient’s body weight, without other insulin dosing parameters. Rather than entering specific carbohydrate counts, users only input whether the carbohydrate amount in the meal is “small,” “medium,” or “large.” The algorithm adapts over time to users’ individual 24/7 insulin needs.
Pivotal data for the system were presented in June 2022 at the annual scientific sessions of the American Diabetes Association.
In the 16-center trial involving 440 adults and children 6 years and older with type 1 diabetes, the system reduced hemoglobin A1c by 0.5 percentage points by 13 weeks, without increased hypoglycemia. They spent an average of 2.6 hours more time in range, compared with standard of care (either currently available AIDs, stand-alone pump and CGM devices, or multiple daily injections plus CGM).
The FDA had granted the iLet a breakthrough device designation in December 2019.
Anne L. Peters, MD, a professor of medicine at the University of Southern California, Los Angeles, and director of the USC clinical diabetes program, commented on the pivotal study and the system in June 2022. She called the study “cool” because it enrolled more than 25% minority individuals “who aren’t routinely studied in these insulin device trials” and also that it included people with a range of baseline A1c levels, with more than 30% greater than 8%.
Regarding the system’s algorithm, she pointed out that it “doesn’t allow for the individual using the pump to fidget with it. They can’t override the system and they can’t put in other insulin doses. The system is just there to take care of their diabetes.”
That might represent a limitation for some with type 1 diabetes, study coprincipal investigator Roy W. Beck, MD, PhD, said in an interview during the ADA meeting. “The iLet could dramatically reduce type 1 diabetes management burden for many patients, but it might not suit everyone. For example, somebody who’s very compulsive and has an A1c of 6.5% and is used to manipulating what they do, this is probably not a good system for them because the system is kind of taking over.”
On the other hand, Dr. Peters said, “I think what’s important about this system is that it may allow for greater use of automated insulin delivery systems. It may allow primary care providers to use these systems without needing all sorts of support, and patients may be able to use these devices more simply than a device where they have to do carb counting and adjusting in ways that I think tend to be pretty complicated and require higher numeracy and literacy skills.”
The “bionic pancreas” was originally conceived as a dual-hormone system including glucagon delivery as well as insulin. Beta Bionics is continuing to work with the FDA on that front.
A version of this article first appeared on Medscape.com.
‘Staggering’ weight loss and benefits in body composition with tirzepatide
DUBLIN – , according to the latest results of the SURMOUNT-1 study.
The new analysis showed that up to 63% of participants achieved a reduction in body weight of at least 20%, and all three tirzepatide doses (5 mg, 10 mg, and 15 mg) led to substantial, clinically meaningful, and sustained body-weight reduction, compared with placebo at 72 weeks of follow-up.
Mean weight loss was –16.0%, –21.4%, and –22.5% with tirzepatide 5 mg, 10 mg, and 15 mg, compared with –2.4% for placebo (all P < .001 vs. placebo). And among participants taking the highest 15-mg dose of tirzepatide, 96%, 90%, and 78% of patients achieved weight reductions of at least 5%, 10%, and 15%.
Tirzepatide is approved in the United States and the European Union for the treatment of type 2 diabetes but is not yet approved for obesity in any country. The manufacturer of tirzepatide, Eli Lilly, intends to seek approval for the drug as an obesity treatment from the U.S. Food and Drug Administration, European Medicines Agency, and in other territories beginning in 2023.
Regardless of baseline BMI category, 9 out of 10 people achieved the greater than or equal to 5% body weight reduction threshold across all doses of tirzepatide, and at the higher doses, over one-third achieved weight loss of 25% or more.
“Similar to lifestyle and surgical treatments, participants on tirzepatide had around a threefold greater percent reduction in fat mass, compared with lean mass, resulting in an overall improvement in body composition,” reported SURMOUNT-1 co-investigator Louis Aronne, MD, Comprehensive Weight Control Center, Weill Cornell Medicine, New York.
“This is staggering weight loss,” remarked Dr. Aronne. “To put it in perspective, mean weight loss in people having Lap-Band surgery is 17%, mean weight loss for sleeve gastrectomy is 25%, and gastric bypass is 33%, which puts the effects of tirzepatide squarely in the realm of bariatric surgery.”
“Something we have sought for decades, we have finally been able to achieve,” he asserted. “I still remember exactly where I was when I saw these results for the first time last April. I knew something big was happening,” declared Dr. Aronne when presenting the latest analyses at the 2023 European Congress on Obesity. Full study results were published in the New England Journal of Medicine.
Moderator Gabriella Lieberman, MD, endocrinologist and head of the Israeli Center for Weight Management, Sheba Medical Center, Ramat-Gan, Israel, welcomed the study but also expressed caution. “It’s very potent, but as we see generally with potent therapies, I think it will change how we look at nutritional advice and the role of the dietician will change. I’m a bit worried the drug is running fast and the support, which is crucial with these treatments, is not keeping up, and we’ll have to deal with some effects later, such as sarcopenia,” she pointed out in an interview.
“We have to treat these drugs as if they are bariatric surgery. I see patients on these types of drugs in clinic and their appetite is so suppressed that they think they can afford to eat things that are unhealthy because they lose weight, and that’s what they want. There has to be a responsible adult looking at what they’re eating, and not just clapping their hands for the weight loss, but ensuring they are not deprived of anything,” she said.
Weight loss and body composition explored
Tirzepatide is a novel glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist that works to activate the GIP and GLP-1 receptors, respectively, found in areas of the brain important for appetite regulation, decreasing food intake, and modulating fat utilization.
The phase 3, double-blind, randomized, controlled trial included data from 2,539 adults with a BMI greater than or equal to 30 kg/m2 (class I, II, III obesity) or greater than or equal to 27 kg/m2 (overweight) with one or more weight-related complications, excluding diabetes. At baseline, mean body weight was 104.8 kg, mean BMI was 38.0 kg/m2, and 94.5% of participants had BMI greater than or equal to 30 kg/m2.
Patients were randomized to once-weekly subcutaneous tirzepatide (5 mg, 10 mg, or 15 mg) or placebo for 72 weeks. The primary objective was to show that tirzepatide was superior to placebo in terms of percentage change in body weight and proportion of participants with body-weight reduction of greater than or equal to 5%. The percentage change from baseline body weight and proportion of participants with body weight reduction greater than or equal to 5% were also assessed across BMI categories of greater than or equal to 27 to less than 30 kg/m2, greater than or equal to 30 to less than 35 kg/m2 (class 1 obesity), greater than or equal to 35 to less than 40 kg/m2 (class 2 obesity), and greater than or equal to 40 kg/m2 (class 3 obesity).
In addition, in a retrospective subanalysis, body composition was evaluated in a subpopulation that underwent dual-energy x-ray absorptiometry, assessing change from baseline body composition within age subgroups less than 50 years (n = 99), 50-64.9 years (n = 41), and greater than or equal to 65 years (n = 20).
The average weight reduction over the 72 weeks of follow-up was –16.0%, –21.4%, and –22.5% with tirzepatide 5 mg, 10 mg, and 15 mg, compared with –2.4% for participants taking placebo (all P < .001 vs. placebo).
The percentages of participants reaching target weight reductions of greater than or equal to 5%, greater than or equal to 10%, greater than or equal to 15%, greater than or equal to 20%, and greater than or equal to 25% were recorded. Over 90% achieved greater than or equal to 5% weight loss, irrespective of BMI and tirzepatide dose, while 55.5% and 62.9% in the 10-mg and 15-mg groups achieved greater than or equal to 20% weight loss, and 35.0% and 39.7% in the 10-mg and 15-mg groups achieved greater than or equal to 25% weight loss, respectively.
By increasing BMI category, in the 10-mg group, weight loss was –18.2 kg, –21.9 kg, –22.0, and –20.7 kg; and in the 15-mg group, weight loss was –18.1kg, –21.2 kg, –24.5 kg, and –22.8 kg. Weight loss in the 5-mg group ranged from –16.6 kg to –15.9 kg from lowest to highest BMI category.
“In the lower-weight categories, there is less weight to lose, so we see a flattening of the curve [with a] maximum of around 18%, so it may be that as we learn more about a drug that is so potent, we recognize that we don’t need to use such a high dose in people with BMI 27-30 kg/m2,” he explained. “It’s the higher BMI categories where we need the higher dose.”
As with lifestyle and surgical treatments, participants taking tirzepatide had around a three times greater percentage reduction in fat mass than lean mass, resulting in an overall improvement in body composition, reported Dr. Aronne.
“We want loss of fat, not lean mass, and we know that we lose around one part lean to three parts fat mass when on a diet and exercise regimen,” he went on to explain. “We see exactly this [balance of lean-to-fat-mass loss] here with 33.9% total fat mass reduction in the treatment group, compared with 8.2% in the placebo group.”
Visceral fat mass reduction was 40% in the treatment group, compared with 7.3% with placebo. “It’s good to see there’s more loss of visceral fat,” said Dr. Aronne. Lean mass loss was 10.9%. “So around three times greater reduction in fat over lean mass loss, resulting in overall improvement of body composition,” he reported.
Also, in older people (≥ 65 years) there was approximately no difference in fat versus lean mass loss, compared with younger people, despite older people being more likely to lose more lean mass.
With respect to patient-reported outcomes based on the 36-item Short-Form Health Survey (SF-36), Dr. Aronne said that physical functioning scores significantly improved at 72 weeks, compared with placebo, particularly in participants with physical function limitations at baseline.
“In an interesting subanalysis, those with physical limitations at baseline showed a significant improvement versus placebo of over 5% difference [considered significant],” he added.
Safety and tolerability were previously reported in the NEJM article. The most common adverse events with tirzepatide were gastrointestinal, and adverse events causing treatment discontinuation occurred in 4.3%, 7.1%, 6.2%, and 2.6% of participants receiving 5-mg, 10-mg, and 15-mg doses or placebo, respectively.
“A revolution is coming in the treatment of obesity and cardiometabolic disease, and most physicians cannot grasp this. We’re finally getting the efficacy we’ve been looking for that will produce benefits in every realm,” concluded Dr. Aronne. “These data show that we are now hitting all the secondary endpoints and making our patients better.”
“I think this bodes well. I always envisioned a time when the treatment of obesity would come first before the treatment of cardiometabolic complications of obesity, and I think we’re on the verge of that era with semaglutide, tirzepatide, and the very exciting treatments to come.”
The SURMOUNT-1 trial was sponsored by Lilly. Dr. Aronne is cofounder, chief scientific advisor, and a member of the board of directors for Intellihealth. He is also a paid scientific advisory board member for Eli Lilly.
A version of this article first appeared on Medscape.com.
DUBLIN – , according to the latest results of the SURMOUNT-1 study.
The new analysis showed that up to 63% of participants achieved a reduction in body weight of at least 20%, and all three tirzepatide doses (5 mg, 10 mg, and 15 mg) led to substantial, clinically meaningful, and sustained body-weight reduction, compared with placebo at 72 weeks of follow-up.
Mean weight loss was –16.0%, –21.4%, and –22.5% with tirzepatide 5 mg, 10 mg, and 15 mg, compared with –2.4% for placebo (all P < .001 vs. placebo). And among participants taking the highest 15-mg dose of tirzepatide, 96%, 90%, and 78% of patients achieved weight reductions of at least 5%, 10%, and 15%.
Tirzepatide is approved in the United States and the European Union for the treatment of type 2 diabetes but is not yet approved for obesity in any country. The manufacturer of tirzepatide, Eli Lilly, intends to seek approval for the drug as an obesity treatment from the U.S. Food and Drug Administration, European Medicines Agency, and in other territories beginning in 2023.
Regardless of baseline BMI category, 9 out of 10 people achieved the greater than or equal to 5% body weight reduction threshold across all doses of tirzepatide, and at the higher doses, over one-third achieved weight loss of 25% or more.
“Similar to lifestyle and surgical treatments, participants on tirzepatide had around a threefold greater percent reduction in fat mass, compared with lean mass, resulting in an overall improvement in body composition,” reported SURMOUNT-1 co-investigator Louis Aronne, MD, Comprehensive Weight Control Center, Weill Cornell Medicine, New York.
“This is staggering weight loss,” remarked Dr. Aronne. “To put it in perspective, mean weight loss in people having Lap-Band surgery is 17%, mean weight loss for sleeve gastrectomy is 25%, and gastric bypass is 33%, which puts the effects of tirzepatide squarely in the realm of bariatric surgery.”
“Something we have sought for decades, we have finally been able to achieve,” he asserted. “I still remember exactly where I was when I saw these results for the first time last April. I knew something big was happening,” declared Dr. Aronne when presenting the latest analyses at the 2023 European Congress on Obesity. Full study results were published in the New England Journal of Medicine.
Moderator Gabriella Lieberman, MD, endocrinologist and head of the Israeli Center for Weight Management, Sheba Medical Center, Ramat-Gan, Israel, welcomed the study but also expressed caution. “It’s very potent, but as we see generally with potent therapies, I think it will change how we look at nutritional advice and the role of the dietician will change. I’m a bit worried the drug is running fast and the support, which is crucial with these treatments, is not keeping up, and we’ll have to deal with some effects later, such as sarcopenia,” she pointed out in an interview.
“We have to treat these drugs as if they are bariatric surgery. I see patients on these types of drugs in clinic and their appetite is so suppressed that they think they can afford to eat things that are unhealthy because they lose weight, and that’s what they want. There has to be a responsible adult looking at what they’re eating, and not just clapping their hands for the weight loss, but ensuring they are not deprived of anything,” she said.
Weight loss and body composition explored
Tirzepatide is a novel glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist that works to activate the GIP and GLP-1 receptors, respectively, found in areas of the brain important for appetite regulation, decreasing food intake, and modulating fat utilization.
The phase 3, double-blind, randomized, controlled trial included data from 2,539 adults with a BMI greater than or equal to 30 kg/m2 (class I, II, III obesity) or greater than or equal to 27 kg/m2 (overweight) with one or more weight-related complications, excluding diabetes. At baseline, mean body weight was 104.8 kg, mean BMI was 38.0 kg/m2, and 94.5% of participants had BMI greater than or equal to 30 kg/m2.
Patients were randomized to once-weekly subcutaneous tirzepatide (5 mg, 10 mg, or 15 mg) or placebo for 72 weeks. The primary objective was to show that tirzepatide was superior to placebo in terms of percentage change in body weight and proportion of participants with body-weight reduction of greater than or equal to 5%. The percentage change from baseline body weight and proportion of participants with body weight reduction greater than or equal to 5% were also assessed across BMI categories of greater than or equal to 27 to less than 30 kg/m2, greater than or equal to 30 to less than 35 kg/m2 (class 1 obesity), greater than or equal to 35 to less than 40 kg/m2 (class 2 obesity), and greater than or equal to 40 kg/m2 (class 3 obesity).
In addition, in a retrospective subanalysis, body composition was evaluated in a subpopulation that underwent dual-energy x-ray absorptiometry, assessing change from baseline body composition within age subgroups less than 50 years (n = 99), 50-64.9 years (n = 41), and greater than or equal to 65 years (n = 20).
The average weight reduction over the 72 weeks of follow-up was –16.0%, –21.4%, and –22.5% with tirzepatide 5 mg, 10 mg, and 15 mg, compared with –2.4% for participants taking placebo (all P < .001 vs. placebo).
The percentages of participants reaching target weight reductions of greater than or equal to 5%, greater than or equal to 10%, greater than or equal to 15%, greater than or equal to 20%, and greater than or equal to 25% were recorded. Over 90% achieved greater than or equal to 5% weight loss, irrespective of BMI and tirzepatide dose, while 55.5% and 62.9% in the 10-mg and 15-mg groups achieved greater than or equal to 20% weight loss, and 35.0% and 39.7% in the 10-mg and 15-mg groups achieved greater than or equal to 25% weight loss, respectively.
By increasing BMI category, in the 10-mg group, weight loss was –18.2 kg, –21.9 kg, –22.0, and –20.7 kg; and in the 15-mg group, weight loss was –18.1kg, –21.2 kg, –24.5 kg, and –22.8 kg. Weight loss in the 5-mg group ranged from –16.6 kg to –15.9 kg from lowest to highest BMI category.
“In the lower-weight categories, there is less weight to lose, so we see a flattening of the curve [with a] maximum of around 18%, so it may be that as we learn more about a drug that is so potent, we recognize that we don’t need to use such a high dose in people with BMI 27-30 kg/m2,” he explained. “It’s the higher BMI categories where we need the higher dose.”
As with lifestyle and surgical treatments, participants taking tirzepatide had around a three times greater percentage reduction in fat mass than lean mass, resulting in an overall improvement in body composition, reported Dr. Aronne.
“We want loss of fat, not lean mass, and we know that we lose around one part lean to three parts fat mass when on a diet and exercise regimen,” he went on to explain. “We see exactly this [balance of lean-to-fat-mass loss] here with 33.9% total fat mass reduction in the treatment group, compared with 8.2% in the placebo group.”
Visceral fat mass reduction was 40% in the treatment group, compared with 7.3% with placebo. “It’s good to see there’s more loss of visceral fat,” said Dr. Aronne. Lean mass loss was 10.9%. “So around three times greater reduction in fat over lean mass loss, resulting in overall improvement of body composition,” he reported.
Also, in older people (≥ 65 years) there was approximately no difference in fat versus lean mass loss, compared with younger people, despite older people being more likely to lose more lean mass.
With respect to patient-reported outcomes based on the 36-item Short-Form Health Survey (SF-36), Dr. Aronne said that physical functioning scores significantly improved at 72 weeks, compared with placebo, particularly in participants with physical function limitations at baseline.
“In an interesting subanalysis, those with physical limitations at baseline showed a significant improvement versus placebo of over 5% difference [considered significant],” he added.
Safety and tolerability were previously reported in the NEJM article. The most common adverse events with tirzepatide were gastrointestinal, and adverse events causing treatment discontinuation occurred in 4.3%, 7.1%, 6.2%, and 2.6% of participants receiving 5-mg, 10-mg, and 15-mg doses or placebo, respectively.
“A revolution is coming in the treatment of obesity and cardiometabolic disease, and most physicians cannot grasp this. We’re finally getting the efficacy we’ve been looking for that will produce benefits in every realm,” concluded Dr. Aronne. “These data show that we are now hitting all the secondary endpoints and making our patients better.”
“I think this bodes well. I always envisioned a time when the treatment of obesity would come first before the treatment of cardiometabolic complications of obesity, and I think we’re on the verge of that era with semaglutide, tirzepatide, and the very exciting treatments to come.”
The SURMOUNT-1 trial was sponsored by Lilly. Dr. Aronne is cofounder, chief scientific advisor, and a member of the board of directors for Intellihealth. He is also a paid scientific advisory board member for Eli Lilly.
A version of this article first appeared on Medscape.com.
DUBLIN – , according to the latest results of the SURMOUNT-1 study.
The new analysis showed that up to 63% of participants achieved a reduction in body weight of at least 20%, and all three tirzepatide doses (5 mg, 10 mg, and 15 mg) led to substantial, clinically meaningful, and sustained body-weight reduction, compared with placebo at 72 weeks of follow-up.
Mean weight loss was –16.0%, –21.4%, and –22.5% with tirzepatide 5 mg, 10 mg, and 15 mg, compared with –2.4% for placebo (all P < .001 vs. placebo). And among participants taking the highest 15-mg dose of tirzepatide, 96%, 90%, and 78% of patients achieved weight reductions of at least 5%, 10%, and 15%.
Tirzepatide is approved in the United States and the European Union for the treatment of type 2 diabetes but is not yet approved for obesity in any country. The manufacturer of tirzepatide, Eli Lilly, intends to seek approval for the drug as an obesity treatment from the U.S. Food and Drug Administration, European Medicines Agency, and in other territories beginning in 2023.
Regardless of baseline BMI category, 9 out of 10 people achieved the greater than or equal to 5% body weight reduction threshold across all doses of tirzepatide, and at the higher doses, over one-third achieved weight loss of 25% or more.
“Similar to lifestyle and surgical treatments, participants on tirzepatide had around a threefold greater percent reduction in fat mass, compared with lean mass, resulting in an overall improvement in body composition,” reported SURMOUNT-1 co-investigator Louis Aronne, MD, Comprehensive Weight Control Center, Weill Cornell Medicine, New York.
“This is staggering weight loss,” remarked Dr. Aronne. “To put it in perspective, mean weight loss in people having Lap-Band surgery is 17%, mean weight loss for sleeve gastrectomy is 25%, and gastric bypass is 33%, which puts the effects of tirzepatide squarely in the realm of bariatric surgery.”
“Something we have sought for decades, we have finally been able to achieve,” he asserted. “I still remember exactly where I was when I saw these results for the first time last April. I knew something big was happening,” declared Dr. Aronne when presenting the latest analyses at the 2023 European Congress on Obesity. Full study results were published in the New England Journal of Medicine.
Moderator Gabriella Lieberman, MD, endocrinologist and head of the Israeli Center for Weight Management, Sheba Medical Center, Ramat-Gan, Israel, welcomed the study but also expressed caution. “It’s very potent, but as we see generally with potent therapies, I think it will change how we look at nutritional advice and the role of the dietician will change. I’m a bit worried the drug is running fast and the support, which is crucial with these treatments, is not keeping up, and we’ll have to deal with some effects later, such as sarcopenia,” she pointed out in an interview.
“We have to treat these drugs as if they are bariatric surgery. I see patients on these types of drugs in clinic and their appetite is so suppressed that they think they can afford to eat things that are unhealthy because they lose weight, and that’s what they want. There has to be a responsible adult looking at what they’re eating, and not just clapping their hands for the weight loss, but ensuring they are not deprived of anything,” she said.
Weight loss and body composition explored
Tirzepatide is a novel glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist that works to activate the GIP and GLP-1 receptors, respectively, found in areas of the brain important for appetite regulation, decreasing food intake, and modulating fat utilization.
The phase 3, double-blind, randomized, controlled trial included data from 2,539 adults with a BMI greater than or equal to 30 kg/m2 (class I, II, III obesity) or greater than or equal to 27 kg/m2 (overweight) with one or more weight-related complications, excluding diabetes. At baseline, mean body weight was 104.8 kg, mean BMI was 38.0 kg/m2, and 94.5% of participants had BMI greater than or equal to 30 kg/m2.
Patients were randomized to once-weekly subcutaneous tirzepatide (5 mg, 10 mg, or 15 mg) or placebo for 72 weeks. The primary objective was to show that tirzepatide was superior to placebo in terms of percentage change in body weight and proportion of participants with body-weight reduction of greater than or equal to 5%. The percentage change from baseline body weight and proportion of participants with body weight reduction greater than or equal to 5% were also assessed across BMI categories of greater than or equal to 27 to less than 30 kg/m2, greater than or equal to 30 to less than 35 kg/m2 (class 1 obesity), greater than or equal to 35 to less than 40 kg/m2 (class 2 obesity), and greater than or equal to 40 kg/m2 (class 3 obesity).
In addition, in a retrospective subanalysis, body composition was evaluated in a subpopulation that underwent dual-energy x-ray absorptiometry, assessing change from baseline body composition within age subgroups less than 50 years (n = 99), 50-64.9 years (n = 41), and greater than or equal to 65 years (n = 20).
The average weight reduction over the 72 weeks of follow-up was –16.0%, –21.4%, and –22.5% with tirzepatide 5 mg, 10 mg, and 15 mg, compared with –2.4% for participants taking placebo (all P < .001 vs. placebo).
The percentages of participants reaching target weight reductions of greater than or equal to 5%, greater than or equal to 10%, greater than or equal to 15%, greater than or equal to 20%, and greater than or equal to 25% were recorded. Over 90% achieved greater than or equal to 5% weight loss, irrespective of BMI and tirzepatide dose, while 55.5% and 62.9% in the 10-mg and 15-mg groups achieved greater than or equal to 20% weight loss, and 35.0% and 39.7% in the 10-mg and 15-mg groups achieved greater than or equal to 25% weight loss, respectively.
By increasing BMI category, in the 10-mg group, weight loss was –18.2 kg, –21.9 kg, –22.0, and –20.7 kg; and in the 15-mg group, weight loss was –18.1kg, –21.2 kg, –24.5 kg, and –22.8 kg. Weight loss in the 5-mg group ranged from –16.6 kg to –15.9 kg from lowest to highest BMI category.
“In the lower-weight categories, there is less weight to lose, so we see a flattening of the curve [with a] maximum of around 18%, so it may be that as we learn more about a drug that is so potent, we recognize that we don’t need to use such a high dose in people with BMI 27-30 kg/m2,” he explained. “It’s the higher BMI categories where we need the higher dose.”
As with lifestyle and surgical treatments, participants taking tirzepatide had around a three times greater percentage reduction in fat mass than lean mass, resulting in an overall improvement in body composition, reported Dr. Aronne.
“We want loss of fat, not lean mass, and we know that we lose around one part lean to three parts fat mass when on a diet and exercise regimen,” he went on to explain. “We see exactly this [balance of lean-to-fat-mass loss] here with 33.9% total fat mass reduction in the treatment group, compared with 8.2% in the placebo group.”
Visceral fat mass reduction was 40% in the treatment group, compared with 7.3% with placebo. “It’s good to see there’s more loss of visceral fat,” said Dr. Aronne. Lean mass loss was 10.9%. “So around three times greater reduction in fat over lean mass loss, resulting in overall improvement of body composition,” he reported.
Also, in older people (≥ 65 years) there was approximately no difference in fat versus lean mass loss, compared with younger people, despite older people being more likely to lose more lean mass.
With respect to patient-reported outcomes based on the 36-item Short-Form Health Survey (SF-36), Dr. Aronne said that physical functioning scores significantly improved at 72 weeks, compared with placebo, particularly in participants with physical function limitations at baseline.
“In an interesting subanalysis, those with physical limitations at baseline showed a significant improvement versus placebo of over 5% difference [considered significant],” he added.
Safety and tolerability were previously reported in the NEJM article. The most common adverse events with tirzepatide were gastrointestinal, and adverse events causing treatment discontinuation occurred in 4.3%, 7.1%, 6.2%, and 2.6% of participants receiving 5-mg, 10-mg, and 15-mg doses or placebo, respectively.
“A revolution is coming in the treatment of obesity and cardiometabolic disease, and most physicians cannot grasp this. We’re finally getting the efficacy we’ve been looking for that will produce benefits in every realm,” concluded Dr. Aronne. “These data show that we are now hitting all the secondary endpoints and making our patients better.”
“I think this bodes well. I always envisioned a time when the treatment of obesity would come first before the treatment of cardiometabolic complications of obesity, and I think we’re on the verge of that era with semaglutide, tirzepatide, and the very exciting treatments to come.”
The SURMOUNT-1 trial was sponsored by Lilly. Dr. Aronne is cofounder, chief scientific advisor, and a member of the board of directors for Intellihealth. He is also a paid scientific advisory board member for Eli Lilly.
A version of this article first appeared on Medscape.com.
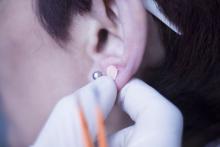
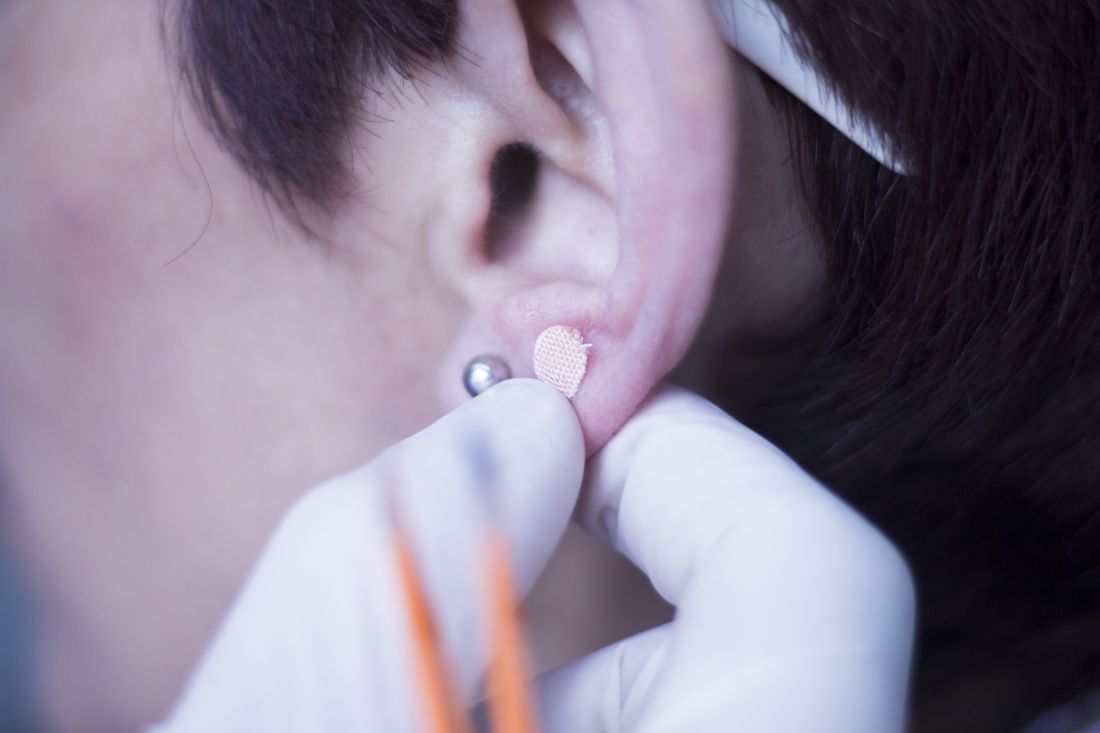
Ear acupuncture with diet aids weight loss
DUBLIN – with high levels of visceral fat and overweight/obesity.
Three months of auricular acupuncture stimulation and dietary restriction led to a mean weight loss of nearly 9 kg plus a drop in waist circumference of more than 10 cm.
According to the researchers, acupuncture beads, used in Japan to augment weight loss for more than 30 years, are thought to stimulate nerves and organs that regulate appetite, satiety, hunger, and food cravings.
Findings of the observational study were presented by Takahiro Fujimoto, MD, PhD, Clinic F, Tokyo, at this year’s European Congress on Obesity.
Together with a prior study using the same intervention in women, Dr. Fujimoto and colleagues have now gathered data in more than 1,000 individuals, he said. “We wanted a method that was simple and noninvasive that would serve as a support to exercise and dietary therapy,” Dr. Fujimoto said in an interview.
“We believe there is an effect,” he asserted. “Acupuncture’s effect lies in stimulating the satiety center with benefits in helping individuals to control their food cravings and intake when reducing meals,” he said, pointing out that similar techniques have been used in patients undergoing withdrawal from drug addiction and in smoking cessation. He explained that acupuncture beads are believed to help individuals change their lifestyle habits, and added that “the relapse rate after 6 months is addressed in another paper, and it is very low.”
Professor Jason C.G. Halford, PhD, head of school at the University of Leeds, England, and president of the European Association for the Study of Obesity, commented on the findings. “There is no control group here receiving everything but the acupuncture,” he noted. “As such, it could be other elements of the intervention driving this [effect] including the act of keeping a food diary increasing awareness of one’s diet. A randomized controlled trial would be the next step.”
In women, the technique led to significantly more weight loss than in those who were untreated, and weight loss was maintained for 6 months after the end of treatment.
The researchers added that acupuncture stimulation with beads was a simpler method than traditional use of intradermal needles requiring expert acupuncturists. The stimulation is applied with 1.5-mm metal ear beads on 6 points of the outer ear (shen men, food pipe, upper stomach opening, stomach, lungs, and endocrine system) that correspond to meridian lines, and as such, restores the flow of qi by resolving any blockages or disruption. This may help with a variety of health conditions, according to the researchers. Placed on both ears, surgical tape was used to keep the beads in place to ensure participants continuously received uniform pressure on each of the six acupuncture points.
Dietary guidance was provided to participants to help reduce food intake by half, and nutritional supplements were given to compensate for any deficiencies. Participants attended twice-weekly clinic visits for bead sticking and diet progress monitoring. Body weight, body fat percentage, fat mass, lean mass, muscle mass, body mass index (BMI), and abdominal fat were assessed at the start and end of the study period.
“Since these tiny metal beads are attached to six points on the outer ear that stimulate nerves and organs which regulate appetite, satiety, and hunger, this type of acupuncture does not require complex knowledge or skill,” explained Dr. Fujimoto.
The results of the latest study, in men only, build on a prior study of more than 1,300 women who also received auricular acupuncture stimulation with beads as well as a halving of their food intake. In women, the weight loss program led to total body weight loss of 11.2% over 3 months.
At baseline, the 81 male participants, ages 21-78 years, had a mean BMI of 28.4 kg/m2 and mean waist circumference of 98.4 cm. Body fat percentage was 28.2%.
After 3 months, participants lost a mean of 8.6 kg (P < .001), decreased waist circumference by a mean of 10.4 cm (P < .001), and lost a mean of 4.0% of total body fat (P < .001). Visceral fat levels also fell by 2.2 points (P < .001), from 15.2 points at baseline to 13.0 points after 3 months. (A healthy visceral fat rating is between 1 and 12 points.) BMI decreased by almost 3 kg/m2 (from 28.4 at baseline to 25.5 at 3 months; P < .001).
Improvement in muscle-to-fat ratio was greater in men than women, whereas women had a greater decrease in percentage body fat than men.
“Whilst receiving ear acupuncture, the investigators asked participants to cut their food intake by half. It’s not unreasonable to expect that this major dietary change was the main reason participants lost weight,” remarked Graham Wheeler, PhD, statistical ambassador at the Royal Statistical Society, United Kingdom.
He also commented on the lack of a control group: “This study does not show us the impact of ear acupuncture on weight loss.”
Dr. Fujimoto and Dr. Halford have reported no relevant financial relationships. Dr. Wheeler is a statistical ambassador for the Royal Statistical Society, is employed by GSK, and holds an honorary senior lecturer post at Imperial College London.
A version of this article first appeared on Medscape.com.
DUBLIN – with high levels of visceral fat and overweight/obesity.
Three months of auricular acupuncture stimulation and dietary restriction led to a mean weight loss of nearly 9 kg plus a drop in waist circumference of more than 10 cm.
According to the researchers, acupuncture beads, used in Japan to augment weight loss for more than 30 years, are thought to stimulate nerves and organs that regulate appetite, satiety, hunger, and food cravings.
Findings of the observational study were presented by Takahiro Fujimoto, MD, PhD, Clinic F, Tokyo, at this year’s European Congress on Obesity.
Together with a prior study using the same intervention in women, Dr. Fujimoto and colleagues have now gathered data in more than 1,000 individuals, he said. “We wanted a method that was simple and noninvasive that would serve as a support to exercise and dietary therapy,” Dr. Fujimoto said in an interview.
“We believe there is an effect,” he asserted. “Acupuncture’s effect lies in stimulating the satiety center with benefits in helping individuals to control their food cravings and intake when reducing meals,” he said, pointing out that similar techniques have been used in patients undergoing withdrawal from drug addiction and in smoking cessation. He explained that acupuncture beads are believed to help individuals change their lifestyle habits, and added that “the relapse rate after 6 months is addressed in another paper, and it is very low.”
Professor Jason C.G. Halford, PhD, head of school at the University of Leeds, England, and president of the European Association for the Study of Obesity, commented on the findings. “There is no control group here receiving everything but the acupuncture,” he noted. “As such, it could be other elements of the intervention driving this [effect] including the act of keeping a food diary increasing awareness of one’s diet. A randomized controlled trial would be the next step.”
In women, the technique led to significantly more weight loss than in those who were untreated, and weight loss was maintained for 6 months after the end of treatment.
The researchers added that acupuncture stimulation with beads was a simpler method than traditional use of intradermal needles requiring expert acupuncturists. The stimulation is applied with 1.5-mm metal ear beads on 6 points of the outer ear (shen men, food pipe, upper stomach opening, stomach, lungs, and endocrine system) that correspond to meridian lines, and as such, restores the flow of qi by resolving any blockages or disruption. This may help with a variety of health conditions, according to the researchers. Placed on both ears, surgical tape was used to keep the beads in place to ensure participants continuously received uniform pressure on each of the six acupuncture points.
Dietary guidance was provided to participants to help reduce food intake by half, and nutritional supplements were given to compensate for any deficiencies. Participants attended twice-weekly clinic visits for bead sticking and diet progress monitoring. Body weight, body fat percentage, fat mass, lean mass, muscle mass, body mass index (BMI), and abdominal fat were assessed at the start and end of the study period.
“Since these tiny metal beads are attached to six points on the outer ear that stimulate nerves and organs which regulate appetite, satiety, and hunger, this type of acupuncture does not require complex knowledge or skill,” explained Dr. Fujimoto.
The results of the latest study, in men only, build on a prior study of more than 1,300 women who also received auricular acupuncture stimulation with beads as well as a halving of their food intake. In women, the weight loss program led to total body weight loss of 11.2% over 3 months.
At baseline, the 81 male participants, ages 21-78 years, had a mean BMI of 28.4 kg/m2 and mean waist circumference of 98.4 cm. Body fat percentage was 28.2%.
After 3 months, participants lost a mean of 8.6 kg (P < .001), decreased waist circumference by a mean of 10.4 cm (P < .001), and lost a mean of 4.0% of total body fat (P < .001). Visceral fat levels also fell by 2.2 points (P < .001), from 15.2 points at baseline to 13.0 points after 3 months. (A healthy visceral fat rating is between 1 and 12 points.) BMI decreased by almost 3 kg/m2 (from 28.4 at baseline to 25.5 at 3 months; P < .001).
Improvement in muscle-to-fat ratio was greater in men than women, whereas women had a greater decrease in percentage body fat than men.
“Whilst receiving ear acupuncture, the investigators asked participants to cut their food intake by half. It’s not unreasonable to expect that this major dietary change was the main reason participants lost weight,” remarked Graham Wheeler, PhD, statistical ambassador at the Royal Statistical Society, United Kingdom.
He also commented on the lack of a control group: “This study does not show us the impact of ear acupuncture on weight loss.”
Dr. Fujimoto and Dr. Halford have reported no relevant financial relationships. Dr. Wheeler is a statistical ambassador for the Royal Statistical Society, is employed by GSK, and holds an honorary senior lecturer post at Imperial College London.
A version of this article first appeared on Medscape.com.
DUBLIN – with high levels of visceral fat and overweight/obesity.
Three months of auricular acupuncture stimulation and dietary restriction led to a mean weight loss of nearly 9 kg plus a drop in waist circumference of more than 10 cm.
According to the researchers, acupuncture beads, used in Japan to augment weight loss for more than 30 years, are thought to stimulate nerves and organs that regulate appetite, satiety, hunger, and food cravings.
Findings of the observational study were presented by Takahiro Fujimoto, MD, PhD, Clinic F, Tokyo, at this year’s European Congress on Obesity.
Together with a prior study using the same intervention in women, Dr. Fujimoto and colleagues have now gathered data in more than 1,000 individuals, he said. “We wanted a method that was simple and noninvasive that would serve as a support to exercise and dietary therapy,” Dr. Fujimoto said in an interview.
“We believe there is an effect,” he asserted. “Acupuncture’s effect lies in stimulating the satiety center with benefits in helping individuals to control their food cravings and intake when reducing meals,” he said, pointing out that similar techniques have been used in patients undergoing withdrawal from drug addiction and in smoking cessation. He explained that acupuncture beads are believed to help individuals change their lifestyle habits, and added that “the relapse rate after 6 months is addressed in another paper, and it is very low.”
Professor Jason C.G. Halford, PhD, head of school at the University of Leeds, England, and president of the European Association for the Study of Obesity, commented on the findings. “There is no control group here receiving everything but the acupuncture,” he noted. “As such, it could be other elements of the intervention driving this [effect] including the act of keeping a food diary increasing awareness of one’s diet. A randomized controlled trial would be the next step.”
In women, the technique led to significantly more weight loss than in those who were untreated, and weight loss was maintained for 6 months after the end of treatment.
The researchers added that acupuncture stimulation with beads was a simpler method than traditional use of intradermal needles requiring expert acupuncturists. The stimulation is applied with 1.5-mm metal ear beads on 6 points of the outer ear (shen men, food pipe, upper stomach opening, stomach, lungs, and endocrine system) that correspond to meridian lines, and as such, restores the flow of qi by resolving any blockages or disruption. This may help with a variety of health conditions, according to the researchers. Placed on both ears, surgical tape was used to keep the beads in place to ensure participants continuously received uniform pressure on each of the six acupuncture points.
Dietary guidance was provided to participants to help reduce food intake by half, and nutritional supplements were given to compensate for any deficiencies. Participants attended twice-weekly clinic visits for bead sticking and diet progress monitoring. Body weight, body fat percentage, fat mass, lean mass, muscle mass, body mass index (BMI), and abdominal fat were assessed at the start and end of the study period.
“Since these tiny metal beads are attached to six points on the outer ear that stimulate nerves and organs which regulate appetite, satiety, and hunger, this type of acupuncture does not require complex knowledge or skill,” explained Dr. Fujimoto.
The results of the latest study, in men only, build on a prior study of more than 1,300 women who also received auricular acupuncture stimulation with beads as well as a halving of their food intake. In women, the weight loss program led to total body weight loss of 11.2% over 3 months.
At baseline, the 81 male participants, ages 21-78 years, had a mean BMI of 28.4 kg/m2 and mean waist circumference of 98.4 cm. Body fat percentage was 28.2%.
After 3 months, participants lost a mean of 8.6 kg (P < .001), decreased waist circumference by a mean of 10.4 cm (P < .001), and lost a mean of 4.0% of total body fat (P < .001). Visceral fat levels also fell by 2.2 points (P < .001), from 15.2 points at baseline to 13.0 points after 3 months. (A healthy visceral fat rating is between 1 and 12 points.) BMI decreased by almost 3 kg/m2 (from 28.4 at baseline to 25.5 at 3 months; P < .001).
Improvement in muscle-to-fat ratio was greater in men than women, whereas women had a greater decrease in percentage body fat than men.
“Whilst receiving ear acupuncture, the investigators asked participants to cut their food intake by half. It’s not unreasonable to expect that this major dietary change was the main reason participants lost weight,” remarked Graham Wheeler, PhD, statistical ambassador at the Royal Statistical Society, United Kingdom.
He also commented on the lack of a control group: “This study does not show us the impact of ear acupuncture on weight loss.”
Dr. Fujimoto and Dr. Halford have reported no relevant financial relationships. Dr. Wheeler is a statistical ambassador for the Royal Statistical Society, is employed by GSK, and holds an honorary senior lecturer post at Imperial College London.
A version of this article first appeared on Medscape.com.
AT ECO 2023
HFpEF: New guidelines are pertinent for primary care
This transcript has been edited for clarity.
I’m Dr. Neil Skolnik. The incidence of HFpEF is increasing, yet it’s underrecognized. Now that there are evidence-based treatment approaches that improve outcomes, we’ve started to look for this condition and are diagnosing it more often. HFpEF is commonly encountered in primary care.
We should be thinking about HFpEF when we see adults with shortness of breath and/or fatigue and reduced exercise capacity, particularly in the settings of obesity, hypertension, or diabetes. It may not be simple deconditioning; it could be HFpEF.
I’ll organize this discussion into three topics: when to think about HFpEF, how to diagnosis it. and how to treat it.
When to think about HFpEF. When we see a person with risk factors (e.g., older age, obesity, diabetes, hypertension) experiencing dyspnea or fatigue with physical activity, their symptoms are not always from simple deconditioning. HFpEF should be on our differential as well as chronic obstructive pulmonary disease (COPD).
Making the diagnosis. HFpEF is defined as a clinical diagnosis of HR with left ventricular EF (LVEF) greater than 50%. Remember, in HF with reduced EF (HFrEF), the EF is less than 40%, and the EF in midrange HF is 40%-50%. See this recent HF review for more details on reduced and midrange ejection fractions.
For practical purposes, to diagnose HFpEF, check for an elevated N-terminal pro B-type natriuretic peptide (NT-proBNP) (> 125 pg/mL) and evidence of diastolic dysfunction on echocardiogram. Be aware that patients with obesity and HFpEF have lower BNP concentrations than those without obesity, and one professional society has suggested that a 50% reduction in BNP cutoff values should be used when making the diagnosis in patients with obesity.
Of course, we evaluate for other causes of dyspnea and/or edema including lung (most commonly COPD), liver, or kidney disease. When the diagnosis of HFpEF is made, consider whether further evaluation is warranted for specific underlying causes of HFpEF, such as amyloidosis, sarcoid, hemochromatosis, or hypertrophic cardiomyopathy.
Treatment. The evolution of the management of HFpEF has been intriguing. I recommend that people take a look at the guidelines and read the supporting trials. Finding effective therapies has taken longer than it did for HFrEF, but finally, an effective therapy for HFpEF is available.
To quote the guidelines, diuretics should be used “judiciously as needed” to reduce pulmonary congestion and improve symptoms. But here’s the big deal. The mainstays of treatment for HFpEF are the sodium-glucose cotransporter 2 (SGLT2) inhibitors on the basis of the findings of two trials: DELIVER (dapagliflozin) and EMPEROR-Preserved (empagliflozin), both of which have shown very impressive levels of benefit.
Both trials lasted a little over 2 years and found a statistically significant approximately 30% decline in HF hospitalizations and a numerical reduction of about 10% in cardiovascular death, which was statistically significant in meta-analysis. That’s over 2 years! That’s a large level of effect. They also showed improvements in symptoms and health status. Therefore, SGLT2 inhibitors are first-line treatment for all individuals with HFpEF, currently graded as a Class 2a (moderate) recommendation, but likely soon to be upgraded to Class 1 (strong) recommendation.
After the SGLT2 inhibitors, treatment is based on evidence which is not as strong and the recommendations are graded as Class 2b (weak) recommendations. In men with an LVEF less than 55%-60% and for women with any EF, use of a mineralocorticoid antagonist (MRA), an angiotensin receptor-neprilysin inhibitor, or if an ARN inhibitor is not feasible, an angiotensin receptor blocker (ARB) may be considered.
Nonpharmacologic management is also important. Exercise and weight loss (if the patient is overweight) can improve symptoms and quality of life. A new intervention, an implantable ambulatory pulmonary artery sensor, called CardioMEMS, has been evaluated in two trials, showing a decrease in HF hospitalizations. This may be considered for those who experience hospitalizations for HF and continue to experience New York Heart Association functional Class 3 symptoms despite optimal guideline-directed medical therapy or those who have lability in volume status or other medical problems (such as obesity or COPD) that make it difficult to tell whether their symptoms are from HFpEF or a comorbid condition.
In summary:
- Have a low threshold to evaluate for HFpEF in any patients who have shortness of breath, fatigue with exertion, or fluid overload.
- Initially evaluate with an NT-proBNP level and an echocardiogram.
- First-line treatment is an evidence-based SGLT2 inhibitor along with exercise and perhaps weight loss if needed. A loop diuretic can be used as needed to control volume status. Then you can consider, based on symptoms and details discussed above, an MRA, ARN inhibitor, or ARB.
This is important information for a diagnosis that is common in primary care, HFpEF, and for which we now have impressive, effective treatment.
Dr. Skolnik is a professor in the department of family medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the department of family medicine at Abington (Pa.) Jefferson Health. He disclosed conflicts of interest with AstraZeneca, Teva, Eli Lilly, Boehringer Ingelheim; Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck, and Bayer.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I’m Dr. Neil Skolnik. The incidence of HFpEF is increasing, yet it’s underrecognized. Now that there are evidence-based treatment approaches that improve outcomes, we’ve started to look for this condition and are diagnosing it more often. HFpEF is commonly encountered in primary care.
We should be thinking about HFpEF when we see adults with shortness of breath and/or fatigue and reduced exercise capacity, particularly in the settings of obesity, hypertension, or diabetes. It may not be simple deconditioning; it could be HFpEF.
I’ll organize this discussion into three topics: when to think about HFpEF, how to diagnosis it. and how to treat it.
When to think about HFpEF. When we see a person with risk factors (e.g., older age, obesity, diabetes, hypertension) experiencing dyspnea or fatigue with physical activity, their symptoms are not always from simple deconditioning. HFpEF should be on our differential as well as chronic obstructive pulmonary disease (COPD).
Making the diagnosis. HFpEF is defined as a clinical diagnosis of HR with left ventricular EF (LVEF) greater than 50%. Remember, in HF with reduced EF (HFrEF), the EF is less than 40%, and the EF in midrange HF is 40%-50%. See this recent HF review for more details on reduced and midrange ejection fractions.
For practical purposes, to diagnose HFpEF, check for an elevated N-terminal pro B-type natriuretic peptide (NT-proBNP) (> 125 pg/mL) and evidence of diastolic dysfunction on echocardiogram. Be aware that patients with obesity and HFpEF have lower BNP concentrations than those without obesity, and one professional society has suggested that a 50% reduction in BNP cutoff values should be used when making the diagnosis in patients with obesity.
Of course, we evaluate for other causes of dyspnea and/or edema including lung (most commonly COPD), liver, or kidney disease. When the diagnosis of HFpEF is made, consider whether further evaluation is warranted for specific underlying causes of HFpEF, such as amyloidosis, sarcoid, hemochromatosis, or hypertrophic cardiomyopathy.
Treatment. The evolution of the management of HFpEF has been intriguing. I recommend that people take a look at the guidelines and read the supporting trials. Finding effective therapies has taken longer than it did for HFrEF, but finally, an effective therapy for HFpEF is available.
To quote the guidelines, diuretics should be used “judiciously as needed” to reduce pulmonary congestion and improve symptoms. But here’s the big deal. The mainstays of treatment for HFpEF are the sodium-glucose cotransporter 2 (SGLT2) inhibitors on the basis of the findings of two trials: DELIVER (dapagliflozin) and EMPEROR-Preserved (empagliflozin), both of which have shown very impressive levels of benefit.
Both trials lasted a little over 2 years and found a statistically significant approximately 30% decline in HF hospitalizations and a numerical reduction of about 10% in cardiovascular death, which was statistically significant in meta-analysis. That’s over 2 years! That’s a large level of effect. They also showed improvements in symptoms and health status. Therefore, SGLT2 inhibitors are first-line treatment for all individuals with HFpEF, currently graded as a Class 2a (moderate) recommendation, but likely soon to be upgraded to Class 1 (strong) recommendation.
After the SGLT2 inhibitors, treatment is based on evidence which is not as strong and the recommendations are graded as Class 2b (weak) recommendations. In men with an LVEF less than 55%-60% and for women with any EF, use of a mineralocorticoid antagonist (MRA), an angiotensin receptor-neprilysin inhibitor, or if an ARN inhibitor is not feasible, an angiotensin receptor blocker (ARB) may be considered.
Nonpharmacologic management is also important. Exercise and weight loss (if the patient is overweight) can improve symptoms and quality of life. A new intervention, an implantable ambulatory pulmonary artery sensor, called CardioMEMS, has been evaluated in two trials, showing a decrease in HF hospitalizations. This may be considered for those who experience hospitalizations for HF and continue to experience New York Heart Association functional Class 3 symptoms despite optimal guideline-directed medical therapy or those who have lability in volume status or other medical problems (such as obesity or COPD) that make it difficult to tell whether their symptoms are from HFpEF or a comorbid condition.
In summary:
- Have a low threshold to evaluate for HFpEF in any patients who have shortness of breath, fatigue with exertion, or fluid overload.
- Initially evaluate with an NT-proBNP level and an echocardiogram.
- First-line treatment is an evidence-based SGLT2 inhibitor along with exercise and perhaps weight loss if needed. A loop diuretic can be used as needed to control volume status. Then you can consider, based on symptoms and details discussed above, an MRA, ARN inhibitor, or ARB.
This is important information for a diagnosis that is common in primary care, HFpEF, and for which we now have impressive, effective treatment.
Dr. Skolnik is a professor in the department of family medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the department of family medicine at Abington (Pa.) Jefferson Health. He disclosed conflicts of interest with AstraZeneca, Teva, Eli Lilly, Boehringer Ingelheim; Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck, and Bayer.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I’m Dr. Neil Skolnik. The incidence of HFpEF is increasing, yet it’s underrecognized. Now that there are evidence-based treatment approaches that improve outcomes, we’ve started to look for this condition and are diagnosing it more often. HFpEF is commonly encountered in primary care.
We should be thinking about HFpEF when we see adults with shortness of breath and/or fatigue and reduced exercise capacity, particularly in the settings of obesity, hypertension, or diabetes. It may not be simple deconditioning; it could be HFpEF.
I’ll organize this discussion into three topics: when to think about HFpEF, how to diagnosis it. and how to treat it.
When to think about HFpEF. When we see a person with risk factors (e.g., older age, obesity, diabetes, hypertension) experiencing dyspnea or fatigue with physical activity, their symptoms are not always from simple deconditioning. HFpEF should be on our differential as well as chronic obstructive pulmonary disease (COPD).
Making the diagnosis. HFpEF is defined as a clinical diagnosis of HR with left ventricular EF (LVEF) greater than 50%. Remember, in HF with reduced EF (HFrEF), the EF is less than 40%, and the EF in midrange HF is 40%-50%. See this recent HF review for more details on reduced and midrange ejection fractions.
For practical purposes, to diagnose HFpEF, check for an elevated N-terminal pro B-type natriuretic peptide (NT-proBNP) (> 125 pg/mL) and evidence of diastolic dysfunction on echocardiogram. Be aware that patients with obesity and HFpEF have lower BNP concentrations than those without obesity, and one professional society has suggested that a 50% reduction in BNP cutoff values should be used when making the diagnosis in patients with obesity.
Of course, we evaluate for other causes of dyspnea and/or edema including lung (most commonly COPD), liver, or kidney disease. When the diagnosis of HFpEF is made, consider whether further evaluation is warranted for specific underlying causes of HFpEF, such as amyloidosis, sarcoid, hemochromatosis, or hypertrophic cardiomyopathy.
Treatment. The evolution of the management of HFpEF has been intriguing. I recommend that people take a look at the guidelines and read the supporting trials. Finding effective therapies has taken longer than it did for HFrEF, but finally, an effective therapy for HFpEF is available.
To quote the guidelines, diuretics should be used “judiciously as needed” to reduce pulmonary congestion and improve symptoms. But here’s the big deal. The mainstays of treatment for HFpEF are the sodium-glucose cotransporter 2 (SGLT2) inhibitors on the basis of the findings of two trials: DELIVER (dapagliflozin) and EMPEROR-Preserved (empagliflozin), both of which have shown very impressive levels of benefit.
Both trials lasted a little over 2 years and found a statistically significant approximately 30% decline in HF hospitalizations and a numerical reduction of about 10% in cardiovascular death, which was statistically significant in meta-analysis. That’s over 2 years! That’s a large level of effect. They also showed improvements in symptoms and health status. Therefore, SGLT2 inhibitors are first-line treatment for all individuals with HFpEF, currently graded as a Class 2a (moderate) recommendation, but likely soon to be upgraded to Class 1 (strong) recommendation.
After the SGLT2 inhibitors, treatment is based on evidence which is not as strong and the recommendations are graded as Class 2b (weak) recommendations. In men with an LVEF less than 55%-60% and for women with any EF, use of a mineralocorticoid antagonist (MRA), an angiotensin receptor-neprilysin inhibitor, or if an ARN inhibitor is not feasible, an angiotensin receptor blocker (ARB) may be considered.
Nonpharmacologic management is also important. Exercise and weight loss (if the patient is overweight) can improve symptoms and quality of life. A new intervention, an implantable ambulatory pulmonary artery sensor, called CardioMEMS, has been evaluated in two trials, showing a decrease in HF hospitalizations. This may be considered for those who experience hospitalizations for HF and continue to experience New York Heart Association functional Class 3 symptoms despite optimal guideline-directed medical therapy or those who have lability in volume status or other medical problems (such as obesity or COPD) that make it difficult to tell whether their symptoms are from HFpEF or a comorbid condition.
In summary:
- Have a low threshold to evaluate for HFpEF in any patients who have shortness of breath, fatigue with exertion, or fluid overload.
- Initially evaluate with an NT-proBNP level and an echocardiogram.
- First-line treatment is an evidence-based SGLT2 inhibitor along with exercise and perhaps weight loss if needed. A loop diuretic can be used as needed to control volume status. Then you can consider, based on symptoms and details discussed above, an MRA, ARN inhibitor, or ARB.
This is important information for a diagnosis that is common in primary care, HFpEF, and for which we now have impressive, effective treatment.
Dr. Skolnik is a professor in the department of family medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the department of family medicine at Abington (Pa.) Jefferson Health. He disclosed conflicts of interest with AstraZeneca, Teva, Eli Lilly, Boehringer Ingelheim; Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck, and Bayer.
A version of this article first appeared on Medscape.com.
No expiration date for sex
For health professionals, the thought that our parents and grandparents don’t have sex – or didn’t – might be comforting.
The reality is that, for a significant proportion of our older patients, sex has no use-by date. Humans are sexual beings throughout their lives, yet the culture has concealed that fact.
According to Rome, the purpose of sex is to make children. According to Hollywood, sex is only for the young, the healthy, and the beautiful. For the medical profession, sex consists mainly of risks or dysfunctions.
The results of these biases? Sexuality and intimacy are essential elements for quality of life, with clear physical, emotional, and relational benefits.
Let’s look at the data when researchers dared to ask seniors about their sexuality.
We start with the 2015 U.K. national research on sexuality. The study found a link between age and a decline in various aspects of sexual activity – but not a zeroing-out. For example, among men aged 70-79, 59% reported having had sex in the past year, with 19% having intercourse at least twice a month and 18% masturbating at least that often. Above age 80, those numbers dropped to 39%, 6%, and 5%, respectively. The reason behind the declines? A combination of taboo, fear of disease, use of medications or other interventions that disrupt sexual function or cause disfigurement, and a little bit of age itself.
What about women? Among women ages 70-79, 39% said they’d had sex in the past year, with 6% having intercourse at least twice per month and 5% masturbating two times or more monthly. Above age 80, those numbers were 10%, 4.5%, and 1%, respectively. Driving the falloff in women were the same factors as for men, plus the sad reality that many heterosexual women become widowed because their older male partners die earlier.
The male-female difference also reflects lower levels of testosterone in women. And, because women say they value intimacy more than performance, we have two explanations for their lower frequency of masturbation. After all, a lot of intimacy occurs without either intercourse or masturbation.
Surprising and relevant is the amount of distress – or rather, their relative lack thereof – older patients report because of sexual problems. At age 18-44, 11% of U.S. women indicated sexual distress; at age 45-64, the figure was 15%; and at age 65 and up, 9%.
For clinicians, those figures should prompt us to look more closely at alternative forms of sexual expression – those not involving intercourse or masturbation – in the aged, a field physicians typically do not consider.
Although dyspareunia or erectile problems affect many in long-standing relationships, neither is a reason to abstain from sexual pleasure. Indeed, in many couples, oral sex will replace vaginal intercourse, and if urinary, fecal, or flatal incontinence intrude, couples often waive oral sex in favor of more cuddling, kissing, digital stimulation, and other forms of sexual pleasure.
What about the expiry date for sex?
Fascinating research from Nils Beckman, PhD, and colleagues found that the sex drive persists even as people (and men in particular) reach their 100th year. Dr. Beckman’s group interviewed 269 Swedish seniors, all without dementia, at age 97. Sexual desire was affirmed by 27% of men and 5% of women in the survey. Among the men, 32% said they still had sexual thoughts, compared with 18% of women. Meanwhile, 26% of the men and 15% of the women said they missed sexual activity.
What should clinicians do with this information? First, we could start talking about sex with our older patients. According to the 97-year-old Swedes, most want us to! More than 8 in 10 of both women and men in the survey expressed positive views about questions on sexuality. And please don’t be scared to address the subject in the single senior. They, too, can have a sexual or relationship issue and are happy when we raise the subject. They’re not scared to talk about masturbation, either.
When caring for those with chronic diseases, cancer, in the course of physical rehabilitation, and even in the last phase of life, the clinical experience indicates that our patients are happy when we address sexuality and intimacy. Doing so can open the door to the admission of a problem and a corresponding solution, a lubricant or a PDE5 inhibitor.
But sometimes the solution is the conversation itself: Roughly 25% of patients are sufficiently helped simply by talking about sex. Addressing the importance of sexual pleasure is nearly always valuable.
Here are a few ice-breakers I find helpful:
- Did taking this medication change aspects of sexuality? If so, does that bother you?
- Knowing that continuing intimacy is healthy, do you mind if I address that subject?
- We know that aspects of sexuality and intimacy are healthy. Without a partner, some people become sexually isolated. Would you like to talk about that?’
If addressing sexuality has benefits, what about sex itself?
We are gradually learning more about the many short-, intermediate-, and long-term health benefits of solo and joint sexual activity. Short-term benefits include muscle relaxation, pain relief (even, perhaps ironically, for headaches), and better sleep – all pretty valuable for older adults. Examples of intermediate-term benefits include stress relief and less depression. Research from the United States has found that hugging can reduce the concentrations of proinflammatory cytokines, and kissing positively influences cholesterol levels.
Finally, while the long-term benefits of sex might be less relevant for seniors, they do exist.
Among them are delayed onset of dementia and a substantial reduction in cardiovascular and cerebrovascular problems in men. More sex has been linked to longevity, with men benefiting a bit more than women from going through the entire process, including an orgasm, whereas women appear to gain from having a “satisfying” sex life, which does not always require an orgasm.
Let us not forget that these benefits apply to both patients and clinicians alike. Addressing intimacy and sexuality can ease eventual sexual concerns and potentially create a stronger clinician-patient relationship.
Dr. Gianotten, MD is emeritus senior lecturer in medical sexology, Erasmus University Medical Centre, Rotterdam, the Netherlands. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
For health professionals, the thought that our parents and grandparents don’t have sex – or didn’t – might be comforting.
The reality is that, for a significant proportion of our older patients, sex has no use-by date. Humans are sexual beings throughout their lives, yet the culture has concealed that fact.
According to Rome, the purpose of sex is to make children. According to Hollywood, sex is only for the young, the healthy, and the beautiful. For the medical profession, sex consists mainly of risks or dysfunctions.
The results of these biases? Sexuality and intimacy are essential elements for quality of life, with clear physical, emotional, and relational benefits.
Let’s look at the data when researchers dared to ask seniors about their sexuality.
We start with the 2015 U.K. national research on sexuality. The study found a link between age and a decline in various aspects of sexual activity – but not a zeroing-out. For example, among men aged 70-79, 59% reported having had sex in the past year, with 19% having intercourse at least twice a month and 18% masturbating at least that often. Above age 80, those numbers dropped to 39%, 6%, and 5%, respectively. The reason behind the declines? A combination of taboo, fear of disease, use of medications or other interventions that disrupt sexual function or cause disfigurement, and a little bit of age itself.
What about women? Among women ages 70-79, 39% said they’d had sex in the past year, with 6% having intercourse at least twice per month and 5% masturbating two times or more monthly. Above age 80, those numbers were 10%, 4.5%, and 1%, respectively. Driving the falloff in women were the same factors as for men, plus the sad reality that many heterosexual women become widowed because their older male partners die earlier.
The male-female difference also reflects lower levels of testosterone in women. And, because women say they value intimacy more than performance, we have two explanations for their lower frequency of masturbation. After all, a lot of intimacy occurs without either intercourse or masturbation.
Surprising and relevant is the amount of distress – or rather, their relative lack thereof – older patients report because of sexual problems. At age 18-44, 11% of U.S. women indicated sexual distress; at age 45-64, the figure was 15%; and at age 65 and up, 9%.
For clinicians, those figures should prompt us to look more closely at alternative forms of sexual expression – those not involving intercourse or masturbation – in the aged, a field physicians typically do not consider.
Although dyspareunia or erectile problems affect many in long-standing relationships, neither is a reason to abstain from sexual pleasure. Indeed, in many couples, oral sex will replace vaginal intercourse, and if urinary, fecal, or flatal incontinence intrude, couples often waive oral sex in favor of more cuddling, kissing, digital stimulation, and other forms of sexual pleasure.
What about the expiry date for sex?
Fascinating research from Nils Beckman, PhD, and colleagues found that the sex drive persists even as people (and men in particular) reach their 100th year. Dr. Beckman’s group interviewed 269 Swedish seniors, all without dementia, at age 97. Sexual desire was affirmed by 27% of men and 5% of women in the survey. Among the men, 32% said they still had sexual thoughts, compared with 18% of women. Meanwhile, 26% of the men and 15% of the women said they missed sexual activity.
What should clinicians do with this information? First, we could start talking about sex with our older patients. According to the 97-year-old Swedes, most want us to! More than 8 in 10 of both women and men in the survey expressed positive views about questions on sexuality. And please don’t be scared to address the subject in the single senior. They, too, can have a sexual or relationship issue and are happy when we raise the subject. They’re not scared to talk about masturbation, either.
When caring for those with chronic diseases, cancer, in the course of physical rehabilitation, and even in the last phase of life, the clinical experience indicates that our patients are happy when we address sexuality and intimacy. Doing so can open the door to the admission of a problem and a corresponding solution, a lubricant or a PDE5 inhibitor.
But sometimes the solution is the conversation itself: Roughly 25% of patients are sufficiently helped simply by talking about sex. Addressing the importance of sexual pleasure is nearly always valuable.
Here are a few ice-breakers I find helpful:
- Did taking this medication change aspects of sexuality? If so, does that bother you?
- Knowing that continuing intimacy is healthy, do you mind if I address that subject?
- We know that aspects of sexuality and intimacy are healthy. Without a partner, some people become sexually isolated. Would you like to talk about that?’
If addressing sexuality has benefits, what about sex itself?
We are gradually learning more about the many short-, intermediate-, and long-term health benefits of solo and joint sexual activity. Short-term benefits include muscle relaxation, pain relief (even, perhaps ironically, for headaches), and better sleep – all pretty valuable for older adults. Examples of intermediate-term benefits include stress relief and less depression. Research from the United States has found that hugging can reduce the concentrations of proinflammatory cytokines, and kissing positively influences cholesterol levels.
Finally, while the long-term benefits of sex might be less relevant for seniors, they do exist.
Among them are delayed onset of dementia and a substantial reduction in cardiovascular and cerebrovascular problems in men. More sex has been linked to longevity, with men benefiting a bit more than women from going through the entire process, including an orgasm, whereas women appear to gain from having a “satisfying” sex life, which does not always require an orgasm.
Let us not forget that these benefits apply to both patients and clinicians alike. Addressing intimacy and sexuality can ease eventual sexual concerns and potentially create a stronger clinician-patient relationship.
Dr. Gianotten, MD is emeritus senior lecturer in medical sexology, Erasmus University Medical Centre, Rotterdam, the Netherlands. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
For health professionals, the thought that our parents and grandparents don’t have sex – or didn’t – might be comforting.
The reality is that, for a significant proportion of our older patients, sex has no use-by date. Humans are sexual beings throughout their lives, yet the culture has concealed that fact.
According to Rome, the purpose of sex is to make children. According to Hollywood, sex is only for the young, the healthy, and the beautiful. For the medical profession, sex consists mainly of risks or dysfunctions.
The results of these biases? Sexuality and intimacy are essential elements for quality of life, with clear physical, emotional, and relational benefits.
Let’s look at the data when researchers dared to ask seniors about their sexuality.
We start with the 2015 U.K. national research on sexuality. The study found a link between age and a decline in various aspects of sexual activity – but not a zeroing-out. For example, among men aged 70-79, 59% reported having had sex in the past year, with 19% having intercourse at least twice a month and 18% masturbating at least that often. Above age 80, those numbers dropped to 39%, 6%, and 5%, respectively. The reason behind the declines? A combination of taboo, fear of disease, use of medications or other interventions that disrupt sexual function or cause disfigurement, and a little bit of age itself.
What about women? Among women ages 70-79, 39% said they’d had sex in the past year, with 6% having intercourse at least twice per month and 5% masturbating two times or more monthly. Above age 80, those numbers were 10%, 4.5%, and 1%, respectively. Driving the falloff in women were the same factors as for men, plus the sad reality that many heterosexual women become widowed because their older male partners die earlier.
The male-female difference also reflects lower levels of testosterone in women. And, because women say they value intimacy more than performance, we have two explanations for their lower frequency of masturbation. After all, a lot of intimacy occurs without either intercourse or masturbation.
Surprising and relevant is the amount of distress – or rather, their relative lack thereof – older patients report because of sexual problems. At age 18-44, 11% of U.S. women indicated sexual distress; at age 45-64, the figure was 15%; and at age 65 and up, 9%.
For clinicians, those figures should prompt us to look more closely at alternative forms of sexual expression – those not involving intercourse or masturbation – in the aged, a field physicians typically do not consider.
Although dyspareunia or erectile problems affect many in long-standing relationships, neither is a reason to abstain from sexual pleasure. Indeed, in many couples, oral sex will replace vaginal intercourse, and if urinary, fecal, or flatal incontinence intrude, couples often waive oral sex in favor of more cuddling, kissing, digital stimulation, and other forms of sexual pleasure.
What about the expiry date for sex?
Fascinating research from Nils Beckman, PhD, and colleagues found that the sex drive persists even as people (and men in particular) reach their 100th year. Dr. Beckman’s group interviewed 269 Swedish seniors, all without dementia, at age 97. Sexual desire was affirmed by 27% of men and 5% of women in the survey. Among the men, 32% said they still had sexual thoughts, compared with 18% of women. Meanwhile, 26% of the men and 15% of the women said they missed sexual activity.
What should clinicians do with this information? First, we could start talking about sex with our older patients. According to the 97-year-old Swedes, most want us to! More than 8 in 10 of both women and men in the survey expressed positive views about questions on sexuality. And please don’t be scared to address the subject in the single senior. They, too, can have a sexual or relationship issue and are happy when we raise the subject. They’re not scared to talk about masturbation, either.
When caring for those with chronic diseases, cancer, in the course of physical rehabilitation, and even in the last phase of life, the clinical experience indicates that our patients are happy when we address sexuality and intimacy. Doing so can open the door to the admission of a problem and a corresponding solution, a lubricant or a PDE5 inhibitor.
But sometimes the solution is the conversation itself: Roughly 25% of patients are sufficiently helped simply by talking about sex. Addressing the importance of sexual pleasure is nearly always valuable.
Here are a few ice-breakers I find helpful:
- Did taking this medication change aspects of sexuality? If so, does that bother you?
- Knowing that continuing intimacy is healthy, do you mind if I address that subject?
- We know that aspects of sexuality and intimacy are healthy. Without a partner, some people become sexually isolated. Would you like to talk about that?’
If addressing sexuality has benefits, what about sex itself?
We are gradually learning more about the many short-, intermediate-, and long-term health benefits of solo and joint sexual activity. Short-term benefits include muscle relaxation, pain relief (even, perhaps ironically, for headaches), and better sleep – all pretty valuable for older adults. Examples of intermediate-term benefits include stress relief and less depression. Research from the United States has found that hugging can reduce the concentrations of proinflammatory cytokines, and kissing positively influences cholesterol levels.
Finally, while the long-term benefits of sex might be less relevant for seniors, they do exist.
Among them are delayed onset of dementia and a substantial reduction in cardiovascular and cerebrovascular problems in men. More sex has been linked to longevity, with men benefiting a bit more than women from going through the entire process, including an orgasm, whereas women appear to gain from having a “satisfying” sex life, which does not always require an orgasm.
Let us not forget that these benefits apply to both patients and clinicians alike. Addressing intimacy and sexuality can ease eventual sexual concerns and potentially create a stronger clinician-patient relationship.
Dr. Gianotten, MD is emeritus senior lecturer in medical sexology, Erasmus University Medical Centre, Rotterdam, the Netherlands. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Obesity drug with swallowable balloon boosts weight loss
DUBLIN – A swallowable gastric balloon (Allurion Balloon, formerly known as Elipse) combined with daily subcutaneous injections of the glucagonlike peptide 1 (GLP-1) agonist liraglutide (Saxenda, Novo Nordisk), leads to a significant average total body weight loss of 19% (18 kg or 40 lb) after around 4 months in people with obesity.
said Roberta Ienca, MD, from the Clinica Nuova Villa Claudia, Rome, who presented the findings at this year’s European Congress on Obesity.
“Despite both the balloon and liraglutide working on the early satiety feeling, the introduction of liraglutide around 1 month after [swallowing the balloon] or more frequently after 3-4 months, could sustain these feelings for a longer period of time,” she said in an interview.
“The addition of the GLP-1 agonist therapy (liraglutide) to patients treated with the Allurion program [gastric balloon] is feasible, safe, and effective in those who need additional weight loss,” she emphasized.
The balloon stayed inside participants’ stomachs for an average of 16 weeks and liraglutide was continued for an average of 4 months, resulting in a mean reduction in body mass index (BMI) of 6.4 kg/m2.
The Allurion is the world’s first and only swallowable gastric balloon placed without surgery, endoscopy, or anesthesia, and is excreted naturally after around 16 weeks.
The Allurion program delivered “excellent weight loss in individuals with overweight and obesity without going under the knife, and liraglutide has the potential to further safely enhance weight loss in cases of suboptimal adherence with the program,” Dr. Ienca said. “These two treatment approaches appear to have complementary mechanisms of action in a geographically and demographically diverse population.”
Adelardo Caballero, MD, director of the Institute of Obesity, Madrid, said that he had over 6 years of experience with the Allurion balloon in around 2,500 cases. “Over the last 3 years, we have been using Allurion balloons in combination with GLP-1 agonists. In Europe, use of the swallowable gastric balloon is common, the results are good, and it is a safe tool.”
“Using liraglutide daily in subcutaneous form is authorized in Europe and is useful in overweight and mild obesity, while use in the combination [with the balloon] is also very popular,” he explained. “In the future, the combined use of semaglutide once-weekly GLP-1 agonist or the use of dual GLP-1/gastric inhibitory polypeptide agonists [such as tirzepatide] with the swallowable intragastric balloon Allurion program or endoscopic sleeve gastroplasty will improve results,” he added.
Average 40-lb weight loss with balloon and liraglutide
For the current study, data from three international multidisciplinary obesity centers (in Italy, Spain, and Egypt) were retrospectively analyzed. All 181 patients received the combination of the Allurion balloon and liraglutide, with the latter added 4-16 weeks after swallowing the balloon.
During a 20-minute outpatient visit, participants swallowed the balloon, which was filled with liquid after reaching the stomach, and placement was confirmed by x-ray. The balloon remained inserted for around 15-17 weeks (mean 16 weeks) before natural excretion. All patients received liraglutide once daily for 1-6 months (mean 4 months). After excreting the balloon, patients started the Mediterranean diet for weight maintenance and were followed for at least 6 months.
Patients were monitored for weight loss, percentage total body weight loss, percentage excess weight loss, and BMI reduction. The timing of combining drug therapy with the Allurion program, metabolic results, and adverse event data were collected. However, Dr. Ienca explained that “the study was preliminary and aimed to evaluate feasibility and results of a combined treatment, so we didn’t collect long-term data.”
Liraglutide was mostly added in cases of unsatisfactory weight loss to boost weight reduction in patients with high BMIs, to sustain weight maintenance, and to aid diabetes control in patients with satisfactory weight loss. There were no criteria for time of onset of drug therapy in terms of a time point or percentage weight loss.
Before treatment, mean weight was 94.8 ± 21 kg and mean BMI was 33.7 ± 6.2 kg/m2. After 4 months of balloon treatment, weight loss, percentage total body weight loss, percentage excess weight loss, and decrease in BMI were 13.1 ± 7 kg, 13.9% ± 7.7%, 74.3% ± 57.1%, and 4.5 ±1.4 kg/m2 respectively.
After a mean duration of 4 months of liraglutide treatment (in addition to the gastric balloon), participants lost on average 18.1 ± 12.1 kg overall and 18.7% ± 12% of their initial total body weight. They shed 99.4% ± 84.9% of excess weight and reduced BMI by 6.4 ± 5.9 kg/m2.
Dr. Ienca explained that the study did not explore the separate contributions of the balloon or drug therapy to weight loss. “However, existing literature shows that the Allurion program leads to a weight loss of approximately 14% of total body weight after 4 months, while liraglutide studies report 12% of total body weight loss at 1 year,” he noted.
When describing the mechanism of action, Dr. Ienca said the Allurion balloon induces satiety and delays gastric emptying but the feeling of satiety starts to decrease after the first month. “For a few patients, this feeling of satiety decreases more rapidly or they have more difficulty putting in place new alimentary habits. In these patients, the addition of liraglutide gives an additional boost to support this behavioral change.”
Liraglutide-related adverse events included nausea (16.5%), diarrhea (3.3%), constipation (2.2%), and headache (1.7%), as well as drug discontinuation due to tachycardia/chest pain (1.1%) and gastrointestinal symptoms (1.1%).
Balloon removal because of intolerance occurred in 1.1% of patients, gastric dilation in 0.5%, and early balloon deflation in 0.5%. Other expected balloon-related adverse events included nausea, vomiting, and abdominal cramps.
The researchers note that the Allurion program offers a more acceptable option to balloon placement by endoscopy.
“The ease of use, low rate of adverse events, and potentially lower cost of the Allurion Program could enable much wider application of this critical intervention, and ultimately, help the millions who struggle with obesity and its associated health complications.”
A version of this article originally appeared on Medscape.com.
DUBLIN – A swallowable gastric balloon (Allurion Balloon, formerly known as Elipse) combined with daily subcutaneous injections of the glucagonlike peptide 1 (GLP-1) agonist liraglutide (Saxenda, Novo Nordisk), leads to a significant average total body weight loss of 19% (18 kg or 40 lb) after around 4 months in people with obesity.
said Roberta Ienca, MD, from the Clinica Nuova Villa Claudia, Rome, who presented the findings at this year’s European Congress on Obesity.
“Despite both the balloon and liraglutide working on the early satiety feeling, the introduction of liraglutide around 1 month after [swallowing the balloon] or more frequently after 3-4 months, could sustain these feelings for a longer period of time,” she said in an interview.
“The addition of the GLP-1 agonist therapy (liraglutide) to patients treated with the Allurion program [gastric balloon] is feasible, safe, and effective in those who need additional weight loss,” she emphasized.
The balloon stayed inside participants’ stomachs for an average of 16 weeks and liraglutide was continued for an average of 4 months, resulting in a mean reduction in body mass index (BMI) of 6.4 kg/m2.
The Allurion is the world’s first and only swallowable gastric balloon placed without surgery, endoscopy, or anesthesia, and is excreted naturally after around 16 weeks.
The Allurion program delivered “excellent weight loss in individuals with overweight and obesity without going under the knife, and liraglutide has the potential to further safely enhance weight loss in cases of suboptimal adherence with the program,” Dr. Ienca said. “These two treatment approaches appear to have complementary mechanisms of action in a geographically and demographically diverse population.”
Adelardo Caballero, MD, director of the Institute of Obesity, Madrid, said that he had over 6 years of experience with the Allurion balloon in around 2,500 cases. “Over the last 3 years, we have been using Allurion balloons in combination with GLP-1 agonists. In Europe, use of the swallowable gastric balloon is common, the results are good, and it is a safe tool.”
“Using liraglutide daily in subcutaneous form is authorized in Europe and is useful in overweight and mild obesity, while use in the combination [with the balloon] is also very popular,” he explained. “In the future, the combined use of semaglutide once-weekly GLP-1 agonist or the use of dual GLP-1/gastric inhibitory polypeptide agonists [such as tirzepatide] with the swallowable intragastric balloon Allurion program or endoscopic sleeve gastroplasty will improve results,” he added.
Average 40-lb weight loss with balloon and liraglutide
For the current study, data from three international multidisciplinary obesity centers (in Italy, Spain, and Egypt) were retrospectively analyzed. All 181 patients received the combination of the Allurion balloon and liraglutide, with the latter added 4-16 weeks after swallowing the balloon.
During a 20-minute outpatient visit, participants swallowed the balloon, which was filled with liquid after reaching the stomach, and placement was confirmed by x-ray. The balloon remained inserted for around 15-17 weeks (mean 16 weeks) before natural excretion. All patients received liraglutide once daily for 1-6 months (mean 4 months). After excreting the balloon, patients started the Mediterranean diet for weight maintenance and were followed for at least 6 months.
Patients were monitored for weight loss, percentage total body weight loss, percentage excess weight loss, and BMI reduction. The timing of combining drug therapy with the Allurion program, metabolic results, and adverse event data were collected. However, Dr. Ienca explained that “the study was preliminary and aimed to evaluate feasibility and results of a combined treatment, so we didn’t collect long-term data.”
Liraglutide was mostly added in cases of unsatisfactory weight loss to boost weight reduction in patients with high BMIs, to sustain weight maintenance, and to aid diabetes control in patients with satisfactory weight loss. There were no criteria for time of onset of drug therapy in terms of a time point or percentage weight loss.
Before treatment, mean weight was 94.8 ± 21 kg and mean BMI was 33.7 ± 6.2 kg/m2. After 4 months of balloon treatment, weight loss, percentage total body weight loss, percentage excess weight loss, and decrease in BMI were 13.1 ± 7 kg, 13.9% ± 7.7%, 74.3% ± 57.1%, and 4.5 ±1.4 kg/m2 respectively.
After a mean duration of 4 months of liraglutide treatment (in addition to the gastric balloon), participants lost on average 18.1 ± 12.1 kg overall and 18.7% ± 12% of their initial total body weight. They shed 99.4% ± 84.9% of excess weight and reduced BMI by 6.4 ± 5.9 kg/m2.
Dr. Ienca explained that the study did not explore the separate contributions of the balloon or drug therapy to weight loss. “However, existing literature shows that the Allurion program leads to a weight loss of approximately 14% of total body weight after 4 months, while liraglutide studies report 12% of total body weight loss at 1 year,” he noted.
When describing the mechanism of action, Dr. Ienca said the Allurion balloon induces satiety and delays gastric emptying but the feeling of satiety starts to decrease after the first month. “For a few patients, this feeling of satiety decreases more rapidly or they have more difficulty putting in place new alimentary habits. In these patients, the addition of liraglutide gives an additional boost to support this behavioral change.”
Liraglutide-related adverse events included nausea (16.5%), diarrhea (3.3%), constipation (2.2%), and headache (1.7%), as well as drug discontinuation due to tachycardia/chest pain (1.1%) and gastrointestinal symptoms (1.1%).
Balloon removal because of intolerance occurred in 1.1% of patients, gastric dilation in 0.5%, and early balloon deflation in 0.5%. Other expected balloon-related adverse events included nausea, vomiting, and abdominal cramps.
The researchers note that the Allurion program offers a more acceptable option to balloon placement by endoscopy.
“The ease of use, low rate of adverse events, and potentially lower cost of the Allurion Program could enable much wider application of this critical intervention, and ultimately, help the millions who struggle with obesity and its associated health complications.”
A version of this article originally appeared on Medscape.com.
DUBLIN – A swallowable gastric balloon (Allurion Balloon, formerly known as Elipse) combined with daily subcutaneous injections of the glucagonlike peptide 1 (GLP-1) agonist liraglutide (Saxenda, Novo Nordisk), leads to a significant average total body weight loss of 19% (18 kg or 40 lb) after around 4 months in people with obesity.
said Roberta Ienca, MD, from the Clinica Nuova Villa Claudia, Rome, who presented the findings at this year’s European Congress on Obesity.
“Despite both the balloon and liraglutide working on the early satiety feeling, the introduction of liraglutide around 1 month after [swallowing the balloon] or more frequently after 3-4 months, could sustain these feelings for a longer period of time,” she said in an interview.
“The addition of the GLP-1 agonist therapy (liraglutide) to patients treated with the Allurion program [gastric balloon] is feasible, safe, and effective in those who need additional weight loss,” she emphasized.
The balloon stayed inside participants’ stomachs for an average of 16 weeks and liraglutide was continued for an average of 4 months, resulting in a mean reduction in body mass index (BMI) of 6.4 kg/m2.
The Allurion is the world’s first and only swallowable gastric balloon placed without surgery, endoscopy, or anesthesia, and is excreted naturally after around 16 weeks.
The Allurion program delivered “excellent weight loss in individuals with overweight and obesity without going under the knife, and liraglutide has the potential to further safely enhance weight loss in cases of suboptimal adherence with the program,” Dr. Ienca said. “These two treatment approaches appear to have complementary mechanisms of action in a geographically and demographically diverse population.”
Adelardo Caballero, MD, director of the Institute of Obesity, Madrid, said that he had over 6 years of experience with the Allurion balloon in around 2,500 cases. “Over the last 3 years, we have been using Allurion balloons in combination with GLP-1 agonists. In Europe, use of the swallowable gastric balloon is common, the results are good, and it is a safe tool.”
“Using liraglutide daily in subcutaneous form is authorized in Europe and is useful in overweight and mild obesity, while use in the combination [with the balloon] is also very popular,” he explained. “In the future, the combined use of semaglutide once-weekly GLP-1 agonist or the use of dual GLP-1/gastric inhibitory polypeptide agonists [such as tirzepatide] with the swallowable intragastric balloon Allurion program or endoscopic sleeve gastroplasty will improve results,” he added.
Average 40-lb weight loss with balloon and liraglutide
For the current study, data from three international multidisciplinary obesity centers (in Italy, Spain, and Egypt) were retrospectively analyzed. All 181 patients received the combination of the Allurion balloon and liraglutide, with the latter added 4-16 weeks after swallowing the balloon.
During a 20-minute outpatient visit, participants swallowed the balloon, which was filled with liquid after reaching the stomach, and placement was confirmed by x-ray. The balloon remained inserted for around 15-17 weeks (mean 16 weeks) before natural excretion. All patients received liraglutide once daily for 1-6 months (mean 4 months). After excreting the balloon, patients started the Mediterranean diet for weight maintenance and were followed for at least 6 months.
Patients were monitored for weight loss, percentage total body weight loss, percentage excess weight loss, and BMI reduction. The timing of combining drug therapy with the Allurion program, metabolic results, and adverse event data were collected. However, Dr. Ienca explained that “the study was preliminary and aimed to evaluate feasibility and results of a combined treatment, so we didn’t collect long-term data.”
Liraglutide was mostly added in cases of unsatisfactory weight loss to boost weight reduction in patients with high BMIs, to sustain weight maintenance, and to aid diabetes control in patients with satisfactory weight loss. There were no criteria for time of onset of drug therapy in terms of a time point or percentage weight loss.
Before treatment, mean weight was 94.8 ± 21 kg and mean BMI was 33.7 ± 6.2 kg/m2. After 4 months of balloon treatment, weight loss, percentage total body weight loss, percentage excess weight loss, and decrease in BMI were 13.1 ± 7 kg, 13.9% ± 7.7%, 74.3% ± 57.1%, and 4.5 ±1.4 kg/m2 respectively.
After a mean duration of 4 months of liraglutide treatment (in addition to the gastric balloon), participants lost on average 18.1 ± 12.1 kg overall and 18.7% ± 12% of their initial total body weight. They shed 99.4% ± 84.9% of excess weight and reduced BMI by 6.4 ± 5.9 kg/m2.
Dr. Ienca explained that the study did not explore the separate contributions of the balloon or drug therapy to weight loss. “However, existing literature shows that the Allurion program leads to a weight loss of approximately 14% of total body weight after 4 months, while liraglutide studies report 12% of total body weight loss at 1 year,” he noted.
When describing the mechanism of action, Dr. Ienca said the Allurion balloon induces satiety and delays gastric emptying but the feeling of satiety starts to decrease after the first month. “For a few patients, this feeling of satiety decreases more rapidly or they have more difficulty putting in place new alimentary habits. In these patients, the addition of liraglutide gives an additional boost to support this behavioral change.”
Liraglutide-related adverse events included nausea (16.5%), diarrhea (3.3%), constipation (2.2%), and headache (1.7%), as well as drug discontinuation due to tachycardia/chest pain (1.1%) and gastrointestinal symptoms (1.1%).
Balloon removal because of intolerance occurred in 1.1% of patients, gastric dilation in 0.5%, and early balloon deflation in 0.5%. Other expected balloon-related adverse events included nausea, vomiting, and abdominal cramps.
The researchers note that the Allurion program offers a more acceptable option to balloon placement by endoscopy.
“The ease of use, low rate of adverse events, and potentially lower cost of the Allurion Program could enable much wider application of this critical intervention, and ultimately, help the millions who struggle with obesity and its associated health complications.”
A version of this article originally appeared on Medscape.com.
AT ECO 2023
Internet use a modifiable dementia risk factor in older adults?
Investigators followed more than 18,000 older individuals and found that regular Internet use was associated with about a 50% reduction in dementia risk, compared with their counterparts who did not use the Internet regularly.
They also found that longer duration of regular Internet use was associated with a reduced risk of dementia, although excessive daily Internet usage appeared to adversely affect dementia risk.
“Online engagement can develop and maintain cognitive reserve – resiliency against physiological damage to the brain – and increased cognitive reserve can, in turn, compensate for brain aging and reduce the risk of dementia,” study investigator Gawon Cho, a doctoral candidate at New York University School of Global Public Health, said in an interview.
The study was published online in the Journal of the American Geriatrics Society.
Unexamined benefits
Prior research has shown that older adult Internet users have “better overall cognitive performance, verbal reasoning, and memory,” compared with nonusers, the authors note.
However, because this body of research consists of cross-sectional analyses and longitudinal studies with brief follow-up periods, the long-term cognitive benefits of Internet usage remain “unexamined.”
In addition, despite “extensive evidence of a disproportionately high burden of dementia in people of color, individuals without higher education, and adults who experienced other socioeconomic hardships, little is known about whether the Internet has exacerbated population-level disparities in cognitive health,” the investigators add.
Another question concerns whether excessive Internet usage may actually be detrimental to neurocognitive outcomes. However, “existing evidence on the adverse effects of Internet usage is concentrated in younger populations whose brains are still undergoing maturation.”
Ms. Cho said the motivation for the study was the lack of longitudinal studies on this topic, especially those with sufficient follow-up periods. In addition, she said, there is insufficient evidence about how changes in Internet usage in older age are associated with prospective dementia risk.
For the study, investigators turned to participants in the Health and Retirement Study, an ongoing longitudinal survey of a nationally representative sample of U.S.-based older adults (aged ≥ 50 years).
All participants (n = 18,154; 47.36% male; median age, 55.17 years) were dementia-free, community-dwelling older adults who completed a 2002 baseline cognitive assessment and were asked about Internet usage every 2 years thereafter.
Participants were followed from 2002 to 2018 for a maximum of 17.1 years (median, 7.9 years), which is the longest follow-up period to date. Of the total sample, 64.76% were regular Internet users.
The study’s primary outcome was incident dementia, based on performance on the Modified Telephone Interview for Cognitive Status (TICS-M), which was administered every 2 years.
The exposure examined in the study was cumulative Internet usage in late adulthood, defined as “the number of biennial waves where participants used the Internet regularly during the first three waves.”
In addition, participants were asked how many hours they spent using the Internet during the past week for activities other than viewing television shows or movies.
The researchers also investigated whether the link between Internet usage and dementia risk varied by educational attainment, race-ethnicity, sex, and generational cohort.
Covariates included baseline TICS-M score, health, age, household income, marital status, and region of residence.
U-shaped curve
More than half of the sample (52.96%) showed no changes in Internet use from baseline during the study period, while one-fifth (20.54%) did show changes in use.
Investigators found a robust link between Internet usage and lower dementia risk (cause-specific hazard ratio, 0.57 [95% CI, 0.46-0.71]) – a finding that remained even after adjusting for self-selection into baseline usage (csHR, 0.54 [0.41-0.72]) and signs of cognitive decline at baseline (csHR, 0.62 [0.46-0.85]).
Each additional wave of regular Internet usage was associated with a 21% decrease in the risk of dementia (95% CI, 13%-29%), wherein additional regular periods were associated with reduced dementia risk (csHR, 0.80 [95% CI, 0.68-0.95]).
“The difference in risk between regular and nonregular users did not vary by educational attainment, race-ethnicity, sex, and generation,” the investigators note.
A U-shaped association was found between daily hours of online engagement, wherein the lowest risk was observed in those with 0.1-2 hours of usage (compared with 0 hours of usage). The risk increased in a “monotonic fashion” after 2 hours, with 6.1-8 hours of usage showing the highest risk.
This finding was not considered statistically significant, but the “consistent U-shaped trend offers a preliminary suggestion that excessive online engagement may have adverse cognitive effects on older adults,” the investigators note.
“Among older adults, regular Internet users may experience a lower risk of dementia compared to nonregular users, and longer periods of regular Internet usage in late adulthood may help reduce the risks of subsequent dementia incidence,” said Ms. Cho. “Nonetheless, using the Internet excessively daily may negatively affect the risk of dementia in older adults.”
Bidirectional relationship?
Commenting for this article, Claire Sexton, DPhil, Alzheimer’s Association senior director of scientific programs and outreach, noted that some risk factors for Alzheimer’s or other dementias can’t be changed, while others are modifiable, “either at a personal or a population level.”
She called the current research “important” because it “identifies a potentially modifiable factor that may influence dementia risk.”
However, cautioned Dr. Sexton, who was not involved with the study, the findings cannot establish cause and effect. In fact, the relationship may be bidirectional.
“It may be that regular Internet usage is associated with increased cognitive stimulation, and in turn reduced risk of dementia; or it may be that individuals with lower risk of dementia are more likely to engage in regular Internet usage,” she said. Thus, “interventional studies are able to shed more light on causation.”
The Health and Retirement Study is sponsored by the National Institute on Aging and is conducted by the University of Michigan, Ann Arbor. Ms. Cho, her coauthors, and Dr. Sexton have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Investigators followed more than 18,000 older individuals and found that regular Internet use was associated with about a 50% reduction in dementia risk, compared with their counterparts who did not use the Internet regularly.
They also found that longer duration of regular Internet use was associated with a reduced risk of dementia, although excessive daily Internet usage appeared to adversely affect dementia risk.
“Online engagement can develop and maintain cognitive reserve – resiliency against physiological damage to the brain – and increased cognitive reserve can, in turn, compensate for brain aging and reduce the risk of dementia,” study investigator Gawon Cho, a doctoral candidate at New York University School of Global Public Health, said in an interview.
The study was published online in the Journal of the American Geriatrics Society.
Unexamined benefits
Prior research has shown that older adult Internet users have “better overall cognitive performance, verbal reasoning, and memory,” compared with nonusers, the authors note.
However, because this body of research consists of cross-sectional analyses and longitudinal studies with brief follow-up periods, the long-term cognitive benefits of Internet usage remain “unexamined.”
In addition, despite “extensive evidence of a disproportionately high burden of dementia in people of color, individuals without higher education, and adults who experienced other socioeconomic hardships, little is known about whether the Internet has exacerbated population-level disparities in cognitive health,” the investigators add.
Another question concerns whether excessive Internet usage may actually be detrimental to neurocognitive outcomes. However, “existing evidence on the adverse effects of Internet usage is concentrated in younger populations whose brains are still undergoing maturation.”
Ms. Cho said the motivation for the study was the lack of longitudinal studies on this topic, especially those with sufficient follow-up periods. In addition, she said, there is insufficient evidence about how changes in Internet usage in older age are associated with prospective dementia risk.
For the study, investigators turned to participants in the Health and Retirement Study, an ongoing longitudinal survey of a nationally representative sample of U.S.-based older adults (aged ≥ 50 years).
All participants (n = 18,154; 47.36% male; median age, 55.17 years) were dementia-free, community-dwelling older adults who completed a 2002 baseline cognitive assessment and were asked about Internet usage every 2 years thereafter.
Participants were followed from 2002 to 2018 for a maximum of 17.1 years (median, 7.9 years), which is the longest follow-up period to date. Of the total sample, 64.76% were regular Internet users.
The study’s primary outcome was incident dementia, based on performance on the Modified Telephone Interview for Cognitive Status (TICS-M), which was administered every 2 years.
The exposure examined in the study was cumulative Internet usage in late adulthood, defined as “the number of biennial waves where participants used the Internet regularly during the first three waves.”
In addition, participants were asked how many hours they spent using the Internet during the past week for activities other than viewing television shows or movies.
The researchers also investigated whether the link between Internet usage and dementia risk varied by educational attainment, race-ethnicity, sex, and generational cohort.
Covariates included baseline TICS-M score, health, age, household income, marital status, and region of residence.
U-shaped curve
More than half of the sample (52.96%) showed no changes in Internet use from baseline during the study period, while one-fifth (20.54%) did show changes in use.
Investigators found a robust link between Internet usage and lower dementia risk (cause-specific hazard ratio, 0.57 [95% CI, 0.46-0.71]) – a finding that remained even after adjusting for self-selection into baseline usage (csHR, 0.54 [0.41-0.72]) and signs of cognitive decline at baseline (csHR, 0.62 [0.46-0.85]).
Each additional wave of regular Internet usage was associated with a 21% decrease in the risk of dementia (95% CI, 13%-29%), wherein additional regular periods were associated with reduced dementia risk (csHR, 0.80 [95% CI, 0.68-0.95]).
“The difference in risk between regular and nonregular users did not vary by educational attainment, race-ethnicity, sex, and generation,” the investigators note.
A U-shaped association was found between daily hours of online engagement, wherein the lowest risk was observed in those with 0.1-2 hours of usage (compared with 0 hours of usage). The risk increased in a “monotonic fashion” after 2 hours, with 6.1-8 hours of usage showing the highest risk.
This finding was not considered statistically significant, but the “consistent U-shaped trend offers a preliminary suggestion that excessive online engagement may have adverse cognitive effects on older adults,” the investigators note.
“Among older adults, regular Internet users may experience a lower risk of dementia compared to nonregular users, and longer periods of regular Internet usage in late adulthood may help reduce the risks of subsequent dementia incidence,” said Ms. Cho. “Nonetheless, using the Internet excessively daily may negatively affect the risk of dementia in older adults.”
Bidirectional relationship?
Commenting for this article, Claire Sexton, DPhil, Alzheimer’s Association senior director of scientific programs and outreach, noted that some risk factors for Alzheimer’s or other dementias can’t be changed, while others are modifiable, “either at a personal or a population level.”
She called the current research “important” because it “identifies a potentially modifiable factor that may influence dementia risk.”
However, cautioned Dr. Sexton, who was not involved with the study, the findings cannot establish cause and effect. In fact, the relationship may be bidirectional.
“It may be that regular Internet usage is associated with increased cognitive stimulation, and in turn reduced risk of dementia; or it may be that individuals with lower risk of dementia are more likely to engage in regular Internet usage,” she said. Thus, “interventional studies are able to shed more light on causation.”
The Health and Retirement Study is sponsored by the National Institute on Aging and is conducted by the University of Michigan, Ann Arbor. Ms. Cho, her coauthors, and Dr. Sexton have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Investigators followed more than 18,000 older individuals and found that regular Internet use was associated with about a 50% reduction in dementia risk, compared with their counterparts who did not use the Internet regularly.
They also found that longer duration of regular Internet use was associated with a reduced risk of dementia, although excessive daily Internet usage appeared to adversely affect dementia risk.
“Online engagement can develop and maintain cognitive reserve – resiliency against physiological damage to the brain – and increased cognitive reserve can, in turn, compensate for brain aging and reduce the risk of dementia,” study investigator Gawon Cho, a doctoral candidate at New York University School of Global Public Health, said in an interview.
The study was published online in the Journal of the American Geriatrics Society.
Unexamined benefits
Prior research has shown that older adult Internet users have “better overall cognitive performance, verbal reasoning, and memory,” compared with nonusers, the authors note.
However, because this body of research consists of cross-sectional analyses and longitudinal studies with brief follow-up periods, the long-term cognitive benefits of Internet usage remain “unexamined.”
In addition, despite “extensive evidence of a disproportionately high burden of dementia in people of color, individuals without higher education, and adults who experienced other socioeconomic hardships, little is known about whether the Internet has exacerbated population-level disparities in cognitive health,” the investigators add.
Another question concerns whether excessive Internet usage may actually be detrimental to neurocognitive outcomes. However, “existing evidence on the adverse effects of Internet usage is concentrated in younger populations whose brains are still undergoing maturation.”
Ms. Cho said the motivation for the study was the lack of longitudinal studies on this topic, especially those with sufficient follow-up periods. In addition, she said, there is insufficient evidence about how changes in Internet usage in older age are associated with prospective dementia risk.
For the study, investigators turned to participants in the Health and Retirement Study, an ongoing longitudinal survey of a nationally representative sample of U.S.-based older adults (aged ≥ 50 years).
All participants (n = 18,154; 47.36% male; median age, 55.17 years) were dementia-free, community-dwelling older adults who completed a 2002 baseline cognitive assessment and were asked about Internet usage every 2 years thereafter.
Participants were followed from 2002 to 2018 for a maximum of 17.1 years (median, 7.9 years), which is the longest follow-up period to date. Of the total sample, 64.76% were regular Internet users.
The study’s primary outcome was incident dementia, based on performance on the Modified Telephone Interview for Cognitive Status (TICS-M), which was administered every 2 years.
The exposure examined in the study was cumulative Internet usage in late adulthood, defined as “the number of biennial waves where participants used the Internet regularly during the first three waves.”
In addition, participants were asked how many hours they spent using the Internet during the past week for activities other than viewing television shows or movies.
The researchers also investigated whether the link between Internet usage and dementia risk varied by educational attainment, race-ethnicity, sex, and generational cohort.
Covariates included baseline TICS-M score, health, age, household income, marital status, and region of residence.
U-shaped curve
More than half of the sample (52.96%) showed no changes in Internet use from baseline during the study period, while one-fifth (20.54%) did show changes in use.
Investigators found a robust link between Internet usage and lower dementia risk (cause-specific hazard ratio, 0.57 [95% CI, 0.46-0.71]) – a finding that remained even after adjusting for self-selection into baseline usage (csHR, 0.54 [0.41-0.72]) and signs of cognitive decline at baseline (csHR, 0.62 [0.46-0.85]).
Each additional wave of regular Internet usage was associated with a 21% decrease in the risk of dementia (95% CI, 13%-29%), wherein additional regular periods were associated with reduced dementia risk (csHR, 0.80 [95% CI, 0.68-0.95]).
“The difference in risk between regular and nonregular users did not vary by educational attainment, race-ethnicity, sex, and generation,” the investigators note.
A U-shaped association was found between daily hours of online engagement, wherein the lowest risk was observed in those with 0.1-2 hours of usage (compared with 0 hours of usage). The risk increased in a “monotonic fashion” after 2 hours, with 6.1-8 hours of usage showing the highest risk.
This finding was not considered statistically significant, but the “consistent U-shaped trend offers a preliminary suggestion that excessive online engagement may have adverse cognitive effects on older adults,” the investigators note.
“Among older adults, regular Internet users may experience a lower risk of dementia compared to nonregular users, and longer periods of regular Internet usage in late adulthood may help reduce the risks of subsequent dementia incidence,” said Ms. Cho. “Nonetheless, using the Internet excessively daily may negatively affect the risk of dementia in older adults.”
Bidirectional relationship?
Commenting for this article, Claire Sexton, DPhil, Alzheimer’s Association senior director of scientific programs and outreach, noted that some risk factors for Alzheimer’s or other dementias can’t be changed, while others are modifiable, “either at a personal or a population level.”
She called the current research “important” because it “identifies a potentially modifiable factor that may influence dementia risk.”
However, cautioned Dr. Sexton, who was not involved with the study, the findings cannot establish cause and effect. In fact, the relationship may be bidirectional.
“It may be that regular Internet usage is associated with increased cognitive stimulation, and in turn reduced risk of dementia; or it may be that individuals with lower risk of dementia are more likely to engage in regular Internet usage,” she said. Thus, “interventional studies are able to shed more light on causation.”
The Health and Retirement Study is sponsored by the National Institute on Aging and is conducted by the University of Michigan, Ann Arbor. Ms. Cho, her coauthors, and Dr. Sexton have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN GERIATRICS SOCIETY
Review supports continued mask-wearing in health care visits
A new study urges people to continue wearing protective masks in medical settings, even though the U.S. public health emergency declaration around COVID-19 has expired.
Masks continue to lower the risk of catching the virus during medical visits, according to the study, published in Annals of Internal Medicine. And there was not much difference between wearing surgical masks and N95 respirators in health care settings.
The researchers reviewed 3 randomized trials and 21 observational studies to compare the effectiveness of those and cloth masks in reducing COVID-19 transmission.
Tara N. Palmore, MD, of George Washington University, Washington, and David K. Henderson, MD, of the National Institutes of Health, Bethesda, Md., wrote in an opinion article accompanying the study.
“In our enthusiasm to return to the appearance and feeling of normalcy, and as institutions decide which mitigation strategies to discontinue, we strongly advocate not discarding this important lesson learned for the sake of our patients’ safety,” Dr. Palmore and Dr. Henderson wrote.
Surgical masks limit the spread of aerosols and droplets from people who have the flu, coronaviruses or other respiratory viruses, CNN reported. And while masks are not 100% effective, they substantially lower the amount of virus put into the air via coughing and talking.
The study said one reason people should wear masks to medical settings is because “health care personnel are notorious for coming to work while ill.” Transmission from patient to staff and staff to patient is still possible, but rare, when both are masked.
The review authors reported no conflicts of interest. Dr. Palmore has received grants from the NIH, Rigel, Gilead, and AbbVie, and Dr. Henderson is a past president of the Society for Healthcare Epidemiology of America.
A version of this article first appeared on WebMD.com.
A new study urges people to continue wearing protective masks in medical settings, even though the U.S. public health emergency declaration around COVID-19 has expired.
Masks continue to lower the risk of catching the virus during medical visits, according to the study, published in Annals of Internal Medicine. And there was not much difference between wearing surgical masks and N95 respirators in health care settings.
The researchers reviewed 3 randomized trials and 21 observational studies to compare the effectiveness of those and cloth masks in reducing COVID-19 transmission.
Tara N. Palmore, MD, of George Washington University, Washington, and David K. Henderson, MD, of the National Institutes of Health, Bethesda, Md., wrote in an opinion article accompanying the study.
“In our enthusiasm to return to the appearance and feeling of normalcy, and as institutions decide which mitigation strategies to discontinue, we strongly advocate not discarding this important lesson learned for the sake of our patients’ safety,” Dr. Palmore and Dr. Henderson wrote.
Surgical masks limit the spread of aerosols and droplets from people who have the flu, coronaviruses or other respiratory viruses, CNN reported. And while masks are not 100% effective, they substantially lower the amount of virus put into the air via coughing and talking.
The study said one reason people should wear masks to medical settings is because “health care personnel are notorious for coming to work while ill.” Transmission from patient to staff and staff to patient is still possible, but rare, when both are masked.
The review authors reported no conflicts of interest. Dr. Palmore has received grants from the NIH, Rigel, Gilead, and AbbVie, and Dr. Henderson is a past president of the Society for Healthcare Epidemiology of America.
A version of this article first appeared on WebMD.com.
A new study urges people to continue wearing protective masks in medical settings, even though the U.S. public health emergency declaration around COVID-19 has expired.
Masks continue to lower the risk of catching the virus during medical visits, according to the study, published in Annals of Internal Medicine. And there was not much difference between wearing surgical masks and N95 respirators in health care settings.
The researchers reviewed 3 randomized trials and 21 observational studies to compare the effectiveness of those and cloth masks in reducing COVID-19 transmission.
Tara N. Palmore, MD, of George Washington University, Washington, and David K. Henderson, MD, of the National Institutes of Health, Bethesda, Md., wrote in an opinion article accompanying the study.
“In our enthusiasm to return to the appearance and feeling of normalcy, and as institutions decide which mitigation strategies to discontinue, we strongly advocate not discarding this important lesson learned for the sake of our patients’ safety,” Dr. Palmore and Dr. Henderson wrote.
Surgical masks limit the spread of aerosols and droplets from people who have the flu, coronaviruses or other respiratory viruses, CNN reported. And while masks are not 100% effective, they substantially lower the amount of virus put into the air via coughing and talking.
The study said one reason people should wear masks to medical settings is because “health care personnel are notorious for coming to work while ill.” Transmission from patient to staff and staff to patient is still possible, but rare, when both are masked.
The review authors reported no conflicts of interest. Dr. Palmore has received grants from the NIH, Rigel, Gilead, and AbbVie, and Dr. Henderson is a past president of the Society for Healthcare Epidemiology of America.
A version of this article first appeared on WebMD.com.
FROM ANNALS OF INTERNAL MEDICINE
COVID emergency over, but hundreds are still dying weekly
Traci Sikes’s older sister Debbie had survived several health setbacks in life – a heart attack, a cancer diagnosis, and a couple of botched surgeries for a bad back. But by early 2023, the 68-year-old from Brownwood, Tex., was in remission from lymphoma, feeling stronger, and celebrating a birthday for one of her 11 beloved grandchildren.
Then Debbie caught COVID-19. Less than 2 months later, in March, she died of severe lung damage caused by the coronavirus.
Traci was able to make the trip from her home in Washington state to Texas to be with Debbie before she died. She was grateful that she arrived while her sister was still lucid and to hear her sister’s last word – “love” – spoken to one of her grandchildren before she took her final breath.
“My sister was wonderful,” Sikes said. “And she shouldn’t be gone.”
Just 6 months after President Joe Biden declared last fall that “the pandemic is over,” Just as both the World Health Organization and U.S. government recently ended the 3-year-old coronavirus public health emergency, COVID is still killing more than 100 people every day in the U.S., according to the CDC, and amid widespread efforts to move on and drop protective measures, the country’s most vulnerable people are still at significant risk.
The prevailing attitude that we need to learn to live with the current level of risk feels like a “slap in the face,” for COVID grievers who have already paid the price,” said Sabila Khan, who cofounded a Facebook group for COVID loss support, which now has more than 14,000 members.
It also minimizes the continuing loss of life and that so many people are still dying traumatic and unnecessary deaths, she said.
“It feels like it’s been brushed aside,” she said. “Like, ‘It’s business as usual. It’s over. Take off your mask.’ My family and I are still masked, and we’re probably the only ones masked in any given room.”
The abandoning of protective measures also fails to recognize the ongoing and catastrophic risks of long COVID and the experiences of an estimated 26 million people in the U.S. living with long COVID.
“It’s been drummed into us that death is the only serious outcome [of the virus] and we still haven’t made enough space for the idea that long COVID is a very serious outcome,” said David Putrino, PhD, director of rehabilitation innovation for the Mount Sinai Health System in New York, who has helped care for thousands of patients with long COVID.
Historic drop in life expectancy
More than 1.1 million Americans have died from COVID over the past 3 years, and experts say the official numbers are likely underestimated because of errors in death certificate reporting. Although deaths have waned from earlier in the pandemic, the disease has become the fourth leading cause of death in the United States after heart disease, cancer, and “unintentional injury” such as drug overdoses.
What makes these deaths all the more tragic is that COVID is a preventable disease, said Carla Sevin, MD, a critical care doctor and director of the Pulmonary Patient Care Center at Vanderbilt University Medical Center in Nashville, Tenn. Masking, available vaccines, and social distancing have all been shown to significantly lower the risk of spreading and catching the virus. New drugs have also made it possible for infected people to survive COVID.
“It’s possible to not spread COVID,” she said. “It’s possible to protect yourself against COVID. It’s possible to treat COVID. And we’re doing all of those things imperfectly.”
By the end of 2021, Americans overall were dying 3 years sooner, on average, than they were before the pandemic, with life expectancy dropping from 79 years to 76 years, the largest decline in a century.
Globally, the COVID death toll is nearing 7 million. Across all ages, on average, each person who died passed away 10 years younger than they otherwise would have. That’s tens of millions of years wiped away.
As U.S. surgeon and health researcher Atul Gawande, MD, put it in a New York Times essay about the pandemic response: “Human development has been pushed into reverse.”
What is an acceptable threshold of death?
In the United States, more than 80% of deaths from the disease have been in people age 65 and older. Underlying medical conditions and disabilities also raise the risk of severe illness and dying from COVID.
The virus is also disproportionately killing Black, Hispanic, and Indigenous people and those with less access to health care. Racialized groups are dying from COVID at younger ages. COVID advocates and Americans who’ve lost loved ones to the disease say our willingness to accept these facts and the current mortality rate amounts to health-based discrimination.
“Would politicians be approaching this differently had it mostly affected rich white people?” Ms. Khan said.
Ms. Khan’s dad, Shafqat, was an advocate and community organizer for Pakistani immigrants. After contracting COVID, he was rushed to a hospital near his daughter’s Jersey City, N.J., home from a rehab facility where he was being treated for an aggressive form of Parkinson’s disease. For the 8 days her father was in the hospital, she and other family members couldn’t visit him, and he wasn’t even well enough to talk on the phone. He died from COVID in April 2020.
“My father was an extraordinary person who did so much good and he died alone, terrified in a hospital,” she said. “I can’t even wrap my head around that and how he deserved more. No one deserves that.”
At Vanderbilt University Medical Center, where she works as a critical care doctor, COVID deaths are now different from those in the early days of the pandemic, Dr. Sevin said. Most patients now in the intensive care unit are older and immunocompromised – and they tend to blend in more with others in the intensive care unit. That makes the impact of COVID even more hidden and easily ignored.
“It’s easy not to value somebody who’s an invisible number you don’t know,” she said. “You don’t see them writing their will and talking to their best friend. You don’t see the tears rolling down their face because they know what’s going to happen to them and they’re going to asphyxiate to death.”
One COVID patient who died recently in Dr. Sevin’s ICU ward was an older woman who had no living relatives. “She was very, very lonely, and we would always stand outside the door on rounds, and she would motion for us to come in, but we had to then all gown up,” Dr. Sevin said. “It just breaks your heart that people are still having to go through it.”
Dr. Sevin finds it frustrating that so many of the measures that public health officials fought so hard for over the last 3 years – including masking guidelines, government-funded vaccine clinics, and access to potentially life-saving antiviral medications – are now going away because of the lifting of the pandemic emergency declaration.
What makes matters worse, she said, is that public consciousness about taking precautions to protect others is starting to disappear in favor of an “all or nothing attitude” about the continued risks.
“Like either I’m going to stay home and be a hermit, or I’m going to just throw caution to the wind and go to bars and let people yell in my face,” she said. “We learned some hard lessons, and I wish we could hold onto those.”
Americans like Traci Sikes who’ve lost loved ones and health care workers on the front lines say it is particularly frustrating that so many people are framing the current response to the risks of COVID as “personal choice” over responsibility to others, as well as a sense of fatalism and lack of urgent care.
“Why does nobody seem to be angry about this?” Ms. Sikes said. “People talk about COVID like it’s just another thing to die from. But my sister didn’t have to die from it at all.”
A version of this article first appeared on WebMD.com.
Traci Sikes’s older sister Debbie had survived several health setbacks in life – a heart attack, a cancer diagnosis, and a couple of botched surgeries for a bad back. But by early 2023, the 68-year-old from Brownwood, Tex., was in remission from lymphoma, feeling stronger, and celebrating a birthday for one of her 11 beloved grandchildren.
Then Debbie caught COVID-19. Less than 2 months later, in March, she died of severe lung damage caused by the coronavirus.
Traci was able to make the trip from her home in Washington state to Texas to be with Debbie before she died. She was grateful that she arrived while her sister was still lucid and to hear her sister’s last word – “love” – spoken to one of her grandchildren before she took her final breath.
“My sister was wonderful,” Sikes said. “And she shouldn’t be gone.”
Just 6 months after President Joe Biden declared last fall that “the pandemic is over,” Just as both the World Health Organization and U.S. government recently ended the 3-year-old coronavirus public health emergency, COVID is still killing more than 100 people every day in the U.S., according to the CDC, and amid widespread efforts to move on and drop protective measures, the country’s most vulnerable people are still at significant risk.
The prevailing attitude that we need to learn to live with the current level of risk feels like a “slap in the face,” for COVID grievers who have already paid the price,” said Sabila Khan, who cofounded a Facebook group for COVID loss support, which now has more than 14,000 members.
It also minimizes the continuing loss of life and that so many people are still dying traumatic and unnecessary deaths, she said.
“It feels like it’s been brushed aside,” she said. “Like, ‘It’s business as usual. It’s over. Take off your mask.’ My family and I are still masked, and we’re probably the only ones masked in any given room.”
The abandoning of protective measures also fails to recognize the ongoing and catastrophic risks of long COVID and the experiences of an estimated 26 million people in the U.S. living with long COVID.
“It’s been drummed into us that death is the only serious outcome [of the virus] and we still haven’t made enough space for the idea that long COVID is a very serious outcome,” said David Putrino, PhD, director of rehabilitation innovation for the Mount Sinai Health System in New York, who has helped care for thousands of patients with long COVID.
Historic drop in life expectancy
More than 1.1 million Americans have died from COVID over the past 3 years, and experts say the official numbers are likely underestimated because of errors in death certificate reporting. Although deaths have waned from earlier in the pandemic, the disease has become the fourth leading cause of death in the United States after heart disease, cancer, and “unintentional injury” such as drug overdoses.
What makes these deaths all the more tragic is that COVID is a preventable disease, said Carla Sevin, MD, a critical care doctor and director of the Pulmonary Patient Care Center at Vanderbilt University Medical Center in Nashville, Tenn. Masking, available vaccines, and social distancing have all been shown to significantly lower the risk of spreading and catching the virus. New drugs have also made it possible for infected people to survive COVID.
“It’s possible to not spread COVID,” she said. “It’s possible to protect yourself against COVID. It’s possible to treat COVID. And we’re doing all of those things imperfectly.”
By the end of 2021, Americans overall were dying 3 years sooner, on average, than they were before the pandemic, with life expectancy dropping from 79 years to 76 years, the largest decline in a century.
Globally, the COVID death toll is nearing 7 million. Across all ages, on average, each person who died passed away 10 years younger than they otherwise would have. That’s tens of millions of years wiped away.
As U.S. surgeon and health researcher Atul Gawande, MD, put it in a New York Times essay about the pandemic response: “Human development has been pushed into reverse.”
What is an acceptable threshold of death?
In the United States, more than 80% of deaths from the disease have been in people age 65 and older. Underlying medical conditions and disabilities also raise the risk of severe illness and dying from COVID.
The virus is also disproportionately killing Black, Hispanic, and Indigenous people and those with less access to health care. Racialized groups are dying from COVID at younger ages. COVID advocates and Americans who’ve lost loved ones to the disease say our willingness to accept these facts and the current mortality rate amounts to health-based discrimination.
“Would politicians be approaching this differently had it mostly affected rich white people?” Ms. Khan said.
Ms. Khan’s dad, Shafqat, was an advocate and community organizer for Pakistani immigrants. After contracting COVID, he was rushed to a hospital near his daughter’s Jersey City, N.J., home from a rehab facility where he was being treated for an aggressive form of Parkinson’s disease. For the 8 days her father was in the hospital, she and other family members couldn’t visit him, and he wasn’t even well enough to talk on the phone. He died from COVID in April 2020.
“My father was an extraordinary person who did so much good and he died alone, terrified in a hospital,” she said. “I can’t even wrap my head around that and how he deserved more. No one deserves that.”
At Vanderbilt University Medical Center, where she works as a critical care doctor, COVID deaths are now different from those in the early days of the pandemic, Dr. Sevin said. Most patients now in the intensive care unit are older and immunocompromised – and they tend to blend in more with others in the intensive care unit. That makes the impact of COVID even more hidden and easily ignored.
“It’s easy not to value somebody who’s an invisible number you don’t know,” she said. “You don’t see them writing their will and talking to their best friend. You don’t see the tears rolling down their face because they know what’s going to happen to them and they’re going to asphyxiate to death.”
One COVID patient who died recently in Dr. Sevin’s ICU ward was an older woman who had no living relatives. “She was very, very lonely, and we would always stand outside the door on rounds, and she would motion for us to come in, but we had to then all gown up,” Dr. Sevin said. “It just breaks your heart that people are still having to go through it.”
Dr. Sevin finds it frustrating that so many of the measures that public health officials fought so hard for over the last 3 years – including masking guidelines, government-funded vaccine clinics, and access to potentially life-saving antiviral medications – are now going away because of the lifting of the pandemic emergency declaration.
What makes matters worse, she said, is that public consciousness about taking precautions to protect others is starting to disappear in favor of an “all or nothing attitude” about the continued risks.
“Like either I’m going to stay home and be a hermit, or I’m going to just throw caution to the wind and go to bars and let people yell in my face,” she said. “We learned some hard lessons, and I wish we could hold onto those.”
Americans like Traci Sikes who’ve lost loved ones and health care workers on the front lines say it is particularly frustrating that so many people are framing the current response to the risks of COVID as “personal choice” over responsibility to others, as well as a sense of fatalism and lack of urgent care.
“Why does nobody seem to be angry about this?” Ms. Sikes said. “People talk about COVID like it’s just another thing to die from. But my sister didn’t have to die from it at all.”
A version of this article first appeared on WebMD.com.
Traci Sikes’s older sister Debbie had survived several health setbacks in life – a heart attack, a cancer diagnosis, and a couple of botched surgeries for a bad back. But by early 2023, the 68-year-old from Brownwood, Tex., was in remission from lymphoma, feeling stronger, and celebrating a birthday for one of her 11 beloved grandchildren.
Then Debbie caught COVID-19. Less than 2 months later, in March, she died of severe lung damage caused by the coronavirus.
Traci was able to make the trip from her home in Washington state to Texas to be with Debbie before she died. She was grateful that she arrived while her sister was still lucid and to hear her sister’s last word – “love” – spoken to one of her grandchildren before she took her final breath.
“My sister was wonderful,” Sikes said. “And she shouldn’t be gone.”
Just 6 months after President Joe Biden declared last fall that “the pandemic is over,” Just as both the World Health Organization and U.S. government recently ended the 3-year-old coronavirus public health emergency, COVID is still killing more than 100 people every day in the U.S., according to the CDC, and amid widespread efforts to move on and drop protective measures, the country’s most vulnerable people are still at significant risk.
The prevailing attitude that we need to learn to live with the current level of risk feels like a “slap in the face,” for COVID grievers who have already paid the price,” said Sabila Khan, who cofounded a Facebook group for COVID loss support, which now has more than 14,000 members.
It also minimizes the continuing loss of life and that so many people are still dying traumatic and unnecessary deaths, she said.
“It feels like it’s been brushed aside,” she said. “Like, ‘It’s business as usual. It’s over. Take off your mask.’ My family and I are still masked, and we’re probably the only ones masked in any given room.”
The abandoning of protective measures also fails to recognize the ongoing and catastrophic risks of long COVID and the experiences of an estimated 26 million people in the U.S. living with long COVID.
“It’s been drummed into us that death is the only serious outcome [of the virus] and we still haven’t made enough space for the idea that long COVID is a very serious outcome,” said David Putrino, PhD, director of rehabilitation innovation for the Mount Sinai Health System in New York, who has helped care for thousands of patients with long COVID.
Historic drop in life expectancy
More than 1.1 million Americans have died from COVID over the past 3 years, and experts say the official numbers are likely underestimated because of errors in death certificate reporting. Although deaths have waned from earlier in the pandemic, the disease has become the fourth leading cause of death in the United States after heart disease, cancer, and “unintentional injury” such as drug overdoses.
What makes these deaths all the more tragic is that COVID is a preventable disease, said Carla Sevin, MD, a critical care doctor and director of the Pulmonary Patient Care Center at Vanderbilt University Medical Center in Nashville, Tenn. Masking, available vaccines, and social distancing have all been shown to significantly lower the risk of spreading and catching the virus. New drugs have also made it possible for infected people to survive COVID.
“It’s possible to not spread COVID,” she said. “It’s possible to protect yourself against COVID. It’s possible to treat COVID. And we’re doing all of those things imperfectly.”
By the end of 2021, Americans overall were dying 3 years sooner, on average, than they were before the pandemic, with life expectancy dropping from 79 years to 76 years, the largest decline in a century.
Globally, the COVID death toll is nearing 7 million. Across all ages, on average, each person who died passed away 10 years younger than they otherwise would have. That’s tens of millions of years wiped away.
As U.S. surgeon and health researcher Atul Gawande, MD, put it in a New York Times essay about the pandemic response: “Human development has been pushed into reverse.”
What is an acceptable threshold of death?
In the United States, more than 80% of deaths from the disease have been in people age 65 and older. Underlying medical conditions and disabilities also raise the risk of severe illness and dying from COVID.
The virus is also disproportionately killing Black, Hispanic, and Indigenous people and those with less access to health care. Racialized groups are dying from COVID at younger ages. COVID advocates and Americans who’ve lost loved ones to the disease say our willingness to accept these facts and the current mortality rate amounts to health-based discrimination.
“Would politicians be approaching this differently had it mostly affected rich white people?” Ms. Khan said.
Ms. Khan’s dad, Shafqat, was an advocate and community organizer for Pakistani immigrants. After contracting COVID, he was rushed to a hospital near his daughter’s Jersey City, N.J., home from a rehab facility where he was being treated for an aggressive form of Parkinson’s disease. For the 8 days her father was in the hospital, she and other family members couldn’t visit him, and he wasn’t even well enough to talk on the phone. He died from COVID in April 2020.
“My father was an extraordinary person who did so much good and he died alone, terrified in a hospital,” she said. “I can’t even wrap my head around that and how he deserved more. No one deserves that.”
At Vanderbilt University Medical Center, where she works as a critical care doctor, COVID deaths are now different from those in the early days of the pandemic, Dr. Sevin said. Most patients now in the intensive care unit are older and immunocompromised – and they tend to blend in more with others in the intensive care unit. That makes the impact of COVID even more hidden and easily ignored.
“It’s easy not to value somebody who’s an invisible number you don’t know,” she said. “You don’t see them writing their will and talking to their best friend. You don’t see the tears rolling down their face because they know what’s going to happen to them and they’re going to asphyxiate to death.”
One COVID patient who died recently in Dr. Sevin’s ICU ward was an older woman who had no living relatives. “She was very, very lonely, and we would always stand outside the door on rounds, and she would motion for us to come in, but we had to then all gown up,” Dr. Sevin said. “It just breaks your heart that people are still having to go through it.”
Dr. Sevin finds it frustrating that so many of the measures that public health officials fought so hard for over the last 3 years – including masking guidelines, government-funded vaccine clinics, and access to potentially life-saving antiviral medications – are now going away because of the lifting of the pandemic emergency declaration.
What makes matters worse, she said, is that public consciousness about taking precautions to protect others is starting to disappear in favor of an “all or nothing attitude” about the continued risks.
“Like either I’m going to stay home and be a hermit, or I’m going to just throw caution to the wind and go to bars and let people yell in my face,” she said. “We learned some hard lessons, and I wish we could hold onto those.”
Americans like Traci Sikes who’ve lost loved ones and health care workers on the front lines say it is particularly frustrating that so many people are framing the current response to the risks of COVID as “personal choice” over responsibility to others, as well as a sense of fatalism and lack of urgent care.
“Why does nobody seem to be angry about this?” Ms. Sikes said. “People talk about COVID like it’s just another thing to die from. But my sister didn’t have to die from it at all.”
A version of this article first appeared on WebMD.com.
CDC cuts back hospital data reporting on COVID
When the federal government’s public health emergency (PHE) ended on May 11, the Centers for Disease Control and Prevention scaled back the amount of COVID-related data that it had required hospitals to collect and report during the previous 3 years. The CDC had to do this, an agency spokesman said in an interview, because “CDC’s authorizations to collect certain types of public health data” expired with the PHE.
The CDC insists that it will have enough data to keep up with the virus, which repeatedly defied scientists’ expectations during the course of the pandemic. But some experts have doubts about whether this will turn out to be the case.
While the COVID pandemic is subsiding and transitioning to an endemic phase, many things about the coronavirus are still not understood, noted Marisa Eisenberg, PhD, associate professor of epidemiology at the University of Michigan, Ann Arbor.
“COVID is here to stay, and it ebbs and flows but is staying at fairly consistent levels across the country,” she said in an interview. “Meanwhile, we haven’t established a regular seasonality for COVID that we see for most other respiratory illnesses. We’re still seeing pretty rapidly invading new waves of variants. With flu and other respiratory illnesses, you often see a particular variant in each season. There’s an established pattern. For COVID, that’s still shifting.”
Similarly, Sam Scarpino, PhD, a public health expert at Northeastern University, Boston, told the New York Times: “The CDC is shuffling COVID into the deck of infectious diseases that we’re satisfied living with. One thousand deaths a week is just unacceptable.”
William Schaffner, MD, a professor of preventive medicine and health policy at Vanderbilt University Medical Center, Nashville, Tenn., said in an interview that “how we deal with influenza is something of a template or a model for what the CDC is trying to get to with COVID.” It’s not practical for physicians and hospitals to report every flu case, and the same is now true for COVID. However, “we’re still asking for data on people who are hospitalized with COVID to be reported. That will give us a measure of the major public health impact.”
Dr. Eisenberg doesn’t fully subscribe to this notion. “COVID and influenza are both respiratory illnesses, and our initial pandemic response was based on playbooks that we’d built for potential flu pandemics. But COVID is not the flu. We still have to grapple with the fact that it’s killing a lot more people than the flu does. So maybe it’s a template, but not a perfect one.”
What data is being deleted
The CDC is now requiring hospitals to submit COVID-related data weekly, rather than daily, as it previously had. In addition, the agency has cut the number of data elements that hospitals must report from 62 to 44. Among the data fields that are now optional for hospitals to report are the numbers of hospitalized children with suspected or lab-confirmed COVID; hospitalized and ventilated COVID patients; adults in the ICU with suspected or lab-confirmed COVID; adult and pediatric admissions with suspected COVID; COVID-related emergency department visits; and inpatients with hospital-acquired COVID.
Although widely feared by health care workers and the public, hospital-acquired COVID has never been a major factor in the pandemic, Dr. Schaffner said. “So why ask for something that’s actually not so critical? Let’s keep the emphasis on rapid, accurate reporting of people who are hospitalized because of this disease.”
Akin Demehin, senior director for quality and patient safety policy for the American Hospital Association, agreed that the rate of hospital-acquired COVID cases “has been very low throughout the pandemic.” That was one reason why CDC made this measure optional.
Dr. Eisenberg concurred with this view. “We worried about [hospital-acquired COVID] a lot, and then, because people were very careful, it wasn’t as much of a problem as we feared it would be.” But she added a note of caution: “Masking and other [preventive guidelines] are shifting in hospitals, so it will be interesting to see whether that affects things.”
CDC justifies its new policy
To put the hospital data reporting changes in context, it’s important to know that CDC will no longer directly track community levels of COVID and the percentage of tests that come back positive for COVID, which until now were used to measure transmission rates. (Laboratories no longer have to report these test data, whether they are in hospitals or in the community.) To track death rates, CDC will rely on the National Vital Statistics System, which is accurate but lags other kinds of surveillance by 2-3 weeks, according to the New York Times.
In a recent MMWR report, CDC defended its new COVID surveillance system, saying: “Weekly COVID-19 hospital admission levels and the percentage of all COVID-19–associated deaths will be primary surveillance indicators. Emergency department visits and percentage of positive SARS-CoV-2 laboratory test results will help detect early changes in trends. Genomic surveillance will continue to help identify and monitor SARS-CoV-2 variants.”
Clarifying the latter point, CDC said that national genomic surveillance, along with wastewater surveillance, will continue to be used to estimate COVID variant proportions. Dr. Eisenberg stressed the importance of genomic surveillance at the hundreds of sites that CDC now maintains across the country. But currently, many of these sites are only monitoring the level of COVID.
CDC also observed that COVID-19 hospital admission levels have been shown to be “concordant” with community levels of SARS-CoV-2 infection. Therefore, rates of COVID-associated admissions and the percentages of positive test results, COVID ED visits, and COVID deaths are “suitable and timely indicators of trends in COVID-19 activity and severity.”
Ready to shift to voluntary reporting?
In a news release, AHA praised the “streamlining” of CDC requirements for data reporting but said that it hoped that mandatory reporting would be phased out as soon as possible.
The association noted that this would require action by the Centers for Medicare & Medicaid Services. CMS now enforces the CDC requirements with a “condition of participation” (COP) provision, by which noncompliant hospitals could be excluded from Medicare. CMS has extended this COP to April 30, 2024, although it could choose to ask the Secretary of Health and Human Services to terminate it earlier.
If mandatory reporting were repealed, would most hospitals still report on the key COVID metrics? Mr. Demehin noted that before CMS implemented its COP, hospitals reported COVID data voluntarily, “and the participation rate was well over 90%. So setting up a mechanism similar to that is something we’ve encouraged CMS to consider.”
Dr. Eisenberg is skeptical. While bigger hospitals with more resources might continue reporting voluntarily, she said, safety-net hospitals in underserved areas might not, because they are especially short staffed. “Then you have disparities in which hospitals will report.”
Vaccinations: The sleeping dragon
COVID continues to ravage the nation. According to CDC statistics, there were 1,109 deaths from COVID in the U.S. in the week ending May 6, and total deaths have hit 1.13 million. There were 1,333 new COVID-related hospital admissions, and 7,261 people were in the hospital because of COVID.
Another eye-catching number: Only 16.9% of the U.S. population has received an updated COVID vaccine booster. Dr. Schaffner thinks that this is what we should really keep our eye on. While the combination of vaccinations and widespread SARS-CoV-2 infections has conferred herd immunity on most Americans, he said it’s temporary. “Whether your immunity comes from the virus and recovery from disease or from the vaccines, that immunity will wane over time. Unless we keep our vaccination rate up, we may see more future cases. We’ll have to see how that works out. But I’m nervous about that, because people do appear to be nonchalant.”
A version of this article first appeared on Medscape.com.
When the federal government’s public health emergency (PHE) ended on May 11, the Centers for Disease Control and Prevention scaled back the amount of COVID-related data that it had required hospitals to collect and report during the previous 3 years. The CDC had to do this, an agency spokesman said in an interview, because “CDC’s authorizations to collect certain types of public health data” expired with the PHE.
The CDC insists that it will have enough data to keep up with the virus, which repeatedly defied scientists’ expectations during the course of the pandemic. But some experts have doubts about whether this will turn out to be the case.
While the COVID pandemic is subsiding and transitioning to an endemic phase, many things about the coronavirus are still not understood, noted Marisa Eisenberg, PhD, associate professor of epidemiology at the University of Michigan, Ann Arbor.
“COVID is here to stay, and it ebbs and flows but is staying at fairly consistent levels across the country,” she said in an interview. “Meanwhile, we haven’t established a regular seasonality for COVID that we see for most other respiratory illnesses. We’re still seeing pretty rapidly invading new waves of variants. With flu and other respiratory illnesses, you often see a particular variant in each season. There’s an established pattern. For COVID, that’s still shifting.”
Similarly, Sam Scarpino, PhD, a public health expert at Northeastern University, Boston, told the New York Times: “The CDC is shuffling COVID into the deck of infectious diseases that we’re satisfied living with. One thousand deaths a week is just unacceptable.”
William Schaffner, MD, a professor of preventive medicine and health policy at Vanderbilt University Medical Center, Nashville, Tenn., said in an interview that “how we deal with influenza is something of a template or a model for what the CDC is trying to get to with COVID.” It’s not practical for physicians and hospitals to report every flu case, and the same is now true for COVID. However, “we’re still asking for data on people who are hospitalized with COVID to be reported. That will give us a measure of the major public health impact.”
Dr. Eisenberg doesn’t fully subscribe to this notion. “COVID and influenza are both respiratory illnesses, and our initial pandemic response was based on playbooks that we’d built for potential flu pandemics. But COVID is not the flu. We still have to grapple with the fact that it’s killing a lot more people than the flu does. So maybe it’s a template, but not a perfect one.”
What data is being deleted
The CDC is now requiring hospitals to submit COVID-related data weekly, rather than daily, as it previously had. In addition, the agency has cut the number of data elements that hospitals must report from 62 to 44. Among the data fields that are now optional for hospitals to report are the numbers of hospitalized children with suspected or lab-confirmed COVID; hospitalized and ventilated COVID patients; adults in the ICU with suspected or lab-confirmed COVID; adult and pediatric admissions with suspected COVID; COVID-related emergency department visits; and inpatients with hospital-acquired COVID.
Although widely feared by health care workers and the public, hospital-acquired COVID has never been a major factor in the pandemic, Dr. Schaffner said. “So why ask for something that’s actually not so critical? Let’s keep the emphasis on rapid, accurate reporting of people who are hospitalized because of this disease.”
Akin Demehin, senior director for quality and patient safety policy for the American Hospital Association, agreed that the rate of hospital-acquired COVID cases “has been very low throughout the pandemic.” That was one reason why CDC made this measure optional.
Dr. Eisenberg concurred with this view. “We worried about [hospital-acquired COVID] a lot, and then, because people were very careful, it wasn’t as much of a problem as we feared it would be.” But she added a note of caution: “Masking and other [preventive guidelines] are shifting in hospitals, so it will be interesting to see whether that affects things.”
CDC justifies its new policy
To put the hospital data reporting changes in context, it’s important to know that CDC will no longer directly track community levels of COVID and the percentage of tests that come back positive for COVID, which until now were used to measure transmission rates. (Laboratories no longer have to report these test data, whether they are in hospitals or in the community.) To track death rates, CDC will rely on the National Vital Statistics System, which is accurate but lags other kinds of surveillance by 2-3 weeks, according to the New York Times.
In a recent MMWR report, CDC defended its new COVID surveillance system, saying: “Weekly COVID-19 hospital admission levels and the percentage of all COVID-19–associated deaths will be primary surveillance indicators. Emergency department visits and percentage of positive SARS-CoV-2 laboratory test results will help detect early changes in trends. Genomic surveillance will continue to help identify and monitor SARS-CoV-2 variants.”
Clarifying the latter point, CDC said that national genomic surveillance, along with wastewater surveillance, will continue to be used to estimate COVID variant proportions. Dr. Eisenberg stressed the importance of genomic surveillance at the hundreds of sites that CDC now maintains across the country. But currently, many of these sites are only monitoring the level of COVID.
CDC also observed that COVID-19 hospital admission levels have been shown to be “concordant” with community levels of SARS-CoV-2 infection. Therefore, rates of COVID-associated admissions and the percentages of positive test results, COVID ED visits, and COVID deaths are “suitable and timely indicators of trends in COVID-19 activity and severity.”
Ready to shift to voluntary reporting?
In a news release, AHA praised the “streamlining” of CDC requirements for data reporting but said that it hoped that mandatory reporting would be phased out as soon as possible.
The association noted that this would require action by the Centers for Medicare & Medicaid Services. CMS now enforces the CDC requirements with a “condition of participation” (COP) provision, by which noncompliant hospitals could be excluded from Medicare. CMS has extended this COP to April 30, 2024, although it could choose to ask the Secretary of Health and Human Services to terminate it earlier.
If mandatory reporting were repealed, would most hospitals still report on the key COVID metrics? Mr. Demehin noted that before CMS implemented its COP, hospitals reported COVID data voluntarily, “and the participation rate was well over 90%. So setting up a mechanism similar to that is something we’ve encouraged CMS to consider.”
Dr. Eisenberg is skeptical. While bigger hospitals with more resources might continue reporting voluntarily, she said, safety-net hospitals in underserved areas might not, because they are especially short staffed. “Then you have disparities in which hospitals will report.”
Vaccinations: The sleeping dragon
COVID continues to ravage the nation. According to CDC statistics, there were 1,109 deaths from COVID in the U.S. in the week ending May 6, and total deaths have hit 1.13 million. There were 1,333 new COVID-related hospital admissions, and 7,261 people were in the hospital because of COVID.
Another eye-catching number: Only 16.9% of the U.S. population has received an updated COVID vaccine booster. Dr. Schaffner thinks that this is what we should really keep our eye on. While the combination of vaccinations and widespread SARS-CoV-2 infections has conferred herd immunity on most Americans, he said it’s temporary. “Whether your immunity comes from the virus and recovery from disease or from the vaccines, that immunity will wane over time. Unless we keep our vaccination rate up, we may see more future cases. We’ll have to see how that works out. But I’m nervous about that, because people do appear to be nonchalant.”
A version of this article first appeared on Medscape.com.
When the federal government’s public health emergency (PHE) ended on May 11, the Centers for Disease Control and Prevention scaled back the amount of COVID-related data that it had required hospitals to collect and report during the previous 3 years. The CDC had to do this, an agency spokesman said in an interview, because “CDC’s authorizations to collect certain types of public health data” expired with the PHE.
The CDC insists that it will have enough data to keep up with the virus, which repeatedly defied scientists’ expectations during the course of the pandemic. But some experts have doubts about whether this will turn out to be the case.
While the COVID pandemic is subsiding and transitioning to an endemic phase, many things about the coronavirus are still not understood, noted Marisa Eisenberg, PhD, associate professor of epidemiology at the University of Michigan, Ann Arbor.
“COVID is here to stay, and it ebbs and flows but is staying at fairly consistent levels across the country,” she said in an interview. “Meanwhile, we haven’t established a regular seasonality for COVID that we see for most other respiratory illnesses. We’re still seeing pretty rapidly invading new waves of variants. With flu and other respiratory illnesses, you often see a particular variant in each season. There’s an established pattern. For COVID, that’s still shifting.”
Similarly, Sam Scarpino, PhD, a public health expert at Northeastern University, Boston, told the New York Times: “The CDC is shuffling COVID into the deck of infectious diseases that we’re satisfied living with. One thousand deaths a week is just unacceptable.”
William Schaffner, MD, a professor of preventive medicine and health policy at Vanderbilt University Medical Center, Nashville, Tenn., said in an interview that “how we deal with influenza is something of a template or a model for what the CDC is trying to get to with COVID.” It’s not practical for physicians and hospitals to report every flu case, and the same is now true for COVID. However, “we’re still asking for data on people who are hospitalized with COVID to be reported. That will give us a measure of the major public health impact.”
Dr. Eisenberg doesn’t fully subscribe to this notion. “COVID and influenza are both respiratory illnesses, and our initial pandemic response was based on playbooks that we’d built for potential flu pandemics. But COVID is not the flu. We still have to grapple with the fact that it’s killing a lot more people than the flu does. So maybe it’s a template, but not a perfect one.”
What data is being deleted
The CDC is now requiring hospitals to submit COVID-related data weekly, rather than daily, as it previously had. In addition, the agency has cut the number of data elements that hospitals must report from 62 to 44. Among the data fields that are now optional for hospitals to report are the numbers of hospitalized children with suspected or lab-confirmed COVID; hospitalized and ventilated COVID patients; adults in the ICU with suspected or lab-confirmed COVID; adult and pediatric admissions with suspected COVID; COVID-related emergency department visits; and inpatients with hospital-acquired COVID.
Although widely feared by health care workers and the public, hospital-acquired COVID has never been a major factor in the pandemic, Dr. Schaffner said. “So why ask for something that’s actually not so critical? Let’s keep the emphasis on rapid, accurate reporting of people who are hospitalized because of this disease.”
Akin Demehin, senior director for quality and patient safety policy for the American Hospital Association, agreed that the rate of hospital-acquired COVID cases “has been very low throughout the pandemic.” That was one reason why CDC made this measure optional.
Dr. Eisenberg concurred with this view. “We worried about [hospital-acquired COVID] a lot, and then, because people were very careful, it wasn’t as much of a problem as we feared it would be.” But she added a note of caution: “Masking and other [preventive guidelines] are shifting in hospitals, so it will be interesting to see whether that affects things.”
CDC justifies its new policy
To put the hospital data reporting changes in context, it’s important to know that CDC will no longer directly track community levels of COVID and the percentage of tests that come back positive for COVID, which until now were used to measure transmission rates. (Laboratories no longer have to report these test data, whether they are in hospitals or in the community.) To track death rates, CDC will rely on the National Vital Statistics System, which is accurate but lags other kinds of surveillance by 2-3 weeks, according to the New York Times.
In a recent MMWR report, CDC defended its new COVID surveillance system, saying: “Weekly COVID-19 hospital admission levels and the percentage of all COVID-19–associated deaths will be primary surveillance indicators. Emergency department visits and percentage of positive SARS-CoV-2 laboratory test results will help detect early changes in trends. Genomic surveillance will continue to help identify and monitor SARS-CoV-2 variants.”
Clarifying the latter point, CDC said that national genomic surveillance, along with wastewater surveillance, will continue to be used to estimate COVID variant proportions. Dr. Eisenberg stressed the importance of genomic surveillance at the hundreds of sites that CDC now maintains across the country. But currently, many of these sites are only monitoring the level of COVID.
CDC also observed that COVID-19 hospital admission levels have been shown to be “concordant” with community levels of SARS-CoV-2 infection. Therefore, rates of COVID-associated admissions and the percentages of positive test results, COVID ED visits, and COVID deaths are “suitable and timely indicators of trends in COVID-19 activity and severity.”
Ready to shift to voluntary reporting?
In a news release, AHA praised the “streamlining” of CDC requirements for data reporting but said that it hoped that mandatory reporting would be phased out as soon as possible.
The association noted that this would require action by the Centers for Medicare & Medicaid Services. CMS now enforces the CDC requirements with a “condition of participation” (COP) provision, by which noncompliant hospitals could be excluded from Medicare. CMS has extended this COP to April 30, 2024, although it could choose to ask the Secretary of Health and Human Services to terminate it earlier.
If mandatory reporting were repealed, would most hospitals still report on the key COVID metrics? Mr. Demehin noted that before CMS implemented its COP, hospitals reported COVID data voluntarily, “and the participation rate was well over 90%. So setting up a mechanism similar to that is something we’ve encouraged CMS to consider.”
Dr. Eisenberg is skeptical. While bigger hospitals with more resources might continue reporting voluntarily, she said, safety-net hospitals in underserved areas might not, because they are especially short staffed. “Then you have disparities in which hospitals will report.”
Vaccinations: The sleeping dragon
COVID continues to ravage the nation. According to CDC statistics, there were 1,109 deaths from COVID in the U.S. in the week ending May 6, and total deaths have hit 1.13 million. There were 1,333 new COVID-related hospital admissions, and 7,261 people were in the hospital because of COVID.
Another eye-catching number: Only 16.9% of the U.S. population has received an updated COVID vaccine booster. Dr. Schaffner thinks that this is what we should really keep our eye on. While the combination of vaccinations and widespread SARS-CoV-2 infections has conferred herd immunity on most Americans, he said it’s temporary. “Whether your immunity comes from the virus and recovery from disease or from the vaccines, that immunity will wane over time. Unless we keep our vaccination rate up, we may see more future cases. We’ll have to see how that works out. But I’m nervous about that, because people do appear to be nonchalant.”
A version of this article first appeared on Medscape.com.