User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Can this tool forecast peanut allergies?
Pediatricians may have a new aid to better predict peanut allergies among infants with atopic dermatitis.
Their study of the implementation of the scorecard was presented at the Pediatric Academic Societies annual meeting.
Infants with atopic dermatitis or eczema are six times more likely to have an egg allergy and eleven times more likely to have a peanut allergy at age 12 months than are infants without atopic dermatitis.
The scorecard reflects recent directives from the National Institute of Allergy and Infectious Diseases to help combat the public health problem.
“When the NIAID prevention of peanut allergy guidelines first came out, it asked pediatricians to serve as frontline practitioners in implementing them by identifying children at risk for peanut allergy and guiding families on what to do next,” said Waheeda Samady, MD, professor of pediatrics at Northwestern University, Chicago. “The impetus for the study was to further support pediatricians in this role.”
Although pediatricians are trained to identify and even treat mild to moderate cases of atopic dermatitis, little emphasis has gone to categorizing the condition on the basis of severity and to correlating peanut allergy risk.
The predictive scorecard captures 14 images from one infant of mixed race, two White infants, two Black infants, and two Hispanic infants.
To create the card, two in-house pediatric dermatologists assessed 58 images from 13 children and categorized images from 0 (no signs of atopic dermatitis) to 4 (severe signs of atopic dermatitis). After a first pass on categorization, the doctors agreed on 84% of images.
Of 189 pediatricians who used the card, fewer than half reported that they “sometimes,” “very often,” or “always” used the scorecard for atopic dermatitis evaluation. A little fewer than three-quarters reported that their ability to diagnose and categorize atopic dermatitis improved.
“Severity staging of atopic dermatitis is not something that the general pediatrician necessarily performs on a day-to-day basis,” said Kawaljit Brar, MD, professor of pediatrics in the division of allergy and immunology at Hassenfeld Children’s Hospital in New York.
Dr. Brar explained that children who are identified as being at high risk are often referred to specialists such as her, who then perform allergy screenings and can determine whether introduction of food at home is safe or whether office feedings supervised by an allergist are necessary. Researchers have found that early introduction to peanuts for children with moderate to severe atopic dermatitis could prevent peanut allergy.
“This represents a wonderful initiative to educate pediatricians so that they understand which patients require screening for peanut allergy and which patients don’t and can just get introduced to peanuts at home,” Dr. Brar said.
The atopic dermatitis scorecard reflects a growing recognition that varying skin tones show levels of severity incongruously.
“Many of us in clinical practice have recognized that our education has not always been inclusive of patients with varying skin tones,” Dr. Samady said. “When we looked for photos of patients with different skin tones, we simply could not find any that we thought were appropriate. So we decided to take some ourselves, and we’re currently continuing to take photos in order to improve the scorecard we currently have.”
The study was funded by the National Institute of Health and Food Allergy Research and Education. Dr. Samady and Dr. Brar reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pediatricians may have a new aid to better predict peanut allergies among infants with atopic dermatitis.
Their study of the implementation of the scorecard was presented at the Pediatric Academic Societies annual meeting.
Infants with atopic dermatitis or eczema are six times more likely to have an egg allergy and eleven times more likely to have a peanut allergy at age 12 months than are infants without atopic dermatitis.
The scorecard reflects recent directives from the National Institute of Allergy and Infectious Diseases to help combat the public health problem.
“When the NIAID prevention of peanut allergy guidelines first came out, it asked pediatricians to serve as frontline practitioners in implementing them by identifying children at risk for peanut allergy and guiding families on what to do next,” said Waheeda Samady, MD, professor of pediatrics at Northwestern University, Chicago. “The impetus for the study was to further support pediatricians in this role.”
Although pediatricians are trained to identify and even treat mild to moderate cases of atopic dermatitis, little emphasis has gone to categorizing the condition on the basis of severity and to correlating peanut allergy risk.
The predictive scorecard captures 14 images from one infant of mixed race, two White infants, two Black infants, and two Hispanic infants.
To create the card, two in-house pediatric dermatologists assessed 58 images from 13 children and categorized images from 0 (no signs of atopic dermatitis) to 4 (severe signs of atopic dermatitis). After a first pass on categorization, the doctors agreed on 84% of images.
Of 189 pediatricians who used the card, fewer than half reported that they “sometimes,” “very often,” or “always” used the scorecard for atopic dermatitis evaluation. A little fewer than three-quarters reported that their ability to diagnose and categorize atopic dermatitis improved.
“Severity staging of atopic dermatitis is not something that the general pediatrician necessarily performs on a day-to-day basis,” said Kawaljit Brar, MD, professor of pediatrics in the division of allergy and immunology at Hassenfeld Children’s Hospital in New York.
Dr. Brar explained that children who are identified as being at high risk are often referred to specialists such as her, who then perform allergy screenings and can determine whether introduction of food at home is safe or whether office feedings supervised by an allergist are necessary. Researchers have found that early introduction to peanuts for children with moderate to severe atopic dermatitis could prevent peanut allergy.
“This represents a wonderful initiative to educate pediatricians so that they understand which patients require screening for peanut allergy and which patients don’t and can just get introduced to peanuts at home,” Dr. Brar said.
The atopic dermatitis scorecard reflects a growing recognition that varying skin tones show levels of severity incongruously.
“Many of us in clinical practice have recognized that our education has not always been inclusive of patients with varying skin tones,” Dr. Samady said. “When we looked for photos of patients with different skin tones, we simply could not find any that we thought were appropriate. So we decided to take some ourselves, and we’re currently continuing to take photos in order to improve the scorecard we currently have.”
The study was funded by the National Institute of Health and Food Allergy Research and Education. Dr. Samady and Dr. Brar reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pediatricians may have a new aid to better predict peanut allergies among infants with atopic dermatitis.
Their study of the implementation of the scorecard was presented at the Pediatric Academic Societies annual meeting.
Infants with atopic dermatitis or eczema are six times more likely to have an egg allergy and eleven times more likely to have a peanut allergy at age 12 months than are infants without atopic dermatitis.
The scorecard reflects recent directives from the National Institute of Allergy and Infectious Diseases to help combat the public health problem.
“When the NIAID prevention of peanut allergy guidelines first came out, it asked pediatricians to serve as frontline practitioners in implementing them by identifying children at risk for peanut allergy and guiding families on what to do next,” said Waheeda Samady, MD, professor of pediatrics at Northwestern University, Chicago. “The impetus for the study was to further support pediatricians in this role.”
Although pediatricians are trained to identify and even treat mild to moderate cases of atopic dermatitis, little emphasis has gone to categorizing the condition on the basis of severity and to correlating peanut allergy risk.
The predictive scorecard captures 14 images from one infant of mixed race, two White infants, two Black infants, and two Hispanic infants.
To create the card, two in-house pediatric dermatologists assessed 58 images from 13 children and categorized images from 0 (no signs of atopic dermatitis) to 4 (severe signs of atopic dermatitis). After a first pass on categorization, the doctors agreed on 84% of images.
Of 189 pediatricians who used the card, fewer than half reported that they “sometimes,” “very often,” or “always” used the scorecard for atopic dermatitis evaluation. A little fewer than three-quarters reported that their ability to diagnose and categorize atopic dermatitis improved.
“Severity staging of atopic dermatitis is not something that the general pediatrician necessarily performs on a day-to-day basis,” said Kawaljit Brar, MD, professor of pediatrics in the division of allergy and immunology at Hassenfeld Children’s Hospital in New York.
Dr. Brar explained that children who are identified as being at high risk are often referred to specialists such as her, who then perform allergy screenings and can determine whether introduction of food at home is safe or whether office feedings supervised by an allergist are necessary. Researchers have found that early introduction to peanuts for children with moderate to severe atopic dermatitis could prevent peanut allergy.
“This represents a wonderful initiative to educate pediatricians so that they understand which patients require screening for peanut allergy and which patients don’t and can just get introduced to peanuts at home,” Dr. Brar said.
The atopic dermatitis scorecard reflects a growing recognition that varying skin tones show levels of severity incongruously.
“Many of us in clinical practice have recognized that our education has not always been inclusive of patients with varying skin tones,” Dr. Samady said. “When we looked for photos of patients with different skin tones, we simply could not find any that we thought were appropriate. So we decided to take some ourselves, and we’re currently continuing to take photos in order to improve the scorecard we currently have.”
The study was funded by the National Institute of Health and Food Allergy Research and Education. Dr. Samady and Dr. Brar reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM PAS 2023
Two phase 3 trials show benefits of dupilumab for prurigo nodularis
The results, which were published online in Nature Medicine, were the basis for the FDA approval of dupilumab (Dupixent) for adults with PN in September 2022, the first treatment approved for treating PN in the United States.
“These positive studies support the involvement of type 2 cytokines in driving PN disease pathogenesis and the targeting of the [interleukin]-4/IL-13 axis as a novel therapeutic paradigm for patients with PN,” wrote the researchers, who were led by principal investigator Gil Yosipovitch, MD, professor of dermatology at the University of Miami, Fla. Dupilumab, an IL-4 receptor alpha antagonist, blocks the shared receptor component (IL-4R alpha) for IL-4 and IL-13.
For the two phase 3 trials, which were called LIBERTY-PN PRIME and PRIME2 and were sponsored by Sanofi and Regeneron Pharmaceuticals, researchers randomized adults with PN with 20 or more nodules and severe itch uncontrolled with topical therapies 1:1 to 300 mg dupilumab or placebo subcutaneously every 2 weeks for 24 weeks. The primary endpoint was pruritus improvement, which was measured by the proportion of patients with a 4-point or greater reduction in Worst Itch Numeric Rating Scale (WI-NRS) from baseline at week 24 (PRIME) or week 12 (PRIME2). Key secondary endpoints included a reduction in the number of nodules to 5 or fewer at week 24.
PRIME and PRIME2 enrolled 151 and 160 patients, respectively. In PRIME, 60% of patients in the dupilumab arm achieved a 4-point or greater reduction in the WI-NRS at week 24, compared with 18.4% of patients in the placebo arm (P < .001). In PRIME2, 37.2% of patients in the dupilumab arm achieved a 4-point or greater reduction in the WI-NRS at week 12, compared with 22% of patients in the placebo arm (P = .022).
The researchers also reported that, from an initial baseline of 20 to greater than 100 nodules, 32.0% of dupilumab-treated patients in PRIME and 25.6% in PRIME2 showed a reduction to 5 nodules or fewer, which corresponded to a response of “clear” or “almost clear” skin at week 12, compared with 11.8% and 12.2% of placebo-treated patients, respectively. This treatment effect on skin lesions continued to improve after week 12, with 48% of dupilumab-treated patients in PRIME and 44.9% in PRIME2 having five nodules or fewer at week 24, compared with 18.4% and 15.9% of placebo-treated patients, respectively. Safety was consistent with the known dupilumab safety profile.
“Validation is the first success of this paper,” said Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the study. “While both the safety and efficacy of dupilumab in these two phase 3 programs is the meat of the matter, nuanced highlights for me include the rigid nature of the exclusion criteria to ensure a study population that truly has PN as a stand-alone disease, rather than a secondary finding as we once believed to be the entire story. I think it’s important for us to recognize that it’s not one or the other, rather there is both ‘primary’ prurigo nodularis, and then there is secondary prurigo nodularis associated with something else [a wide range of underlying medical conditions], just like we divide primary and secondary hyperhidrosis.”
Dr. Yosipovitch reported having competing interests with several pharmaceutical companies, including Regeneron and Sanofi. Dr. Friedman disclosed that he is a consultant to and a speaker for Regeneron.
The results, which were published online in Nature Medicine, were the basis for the FDA approval of dupilumab (Dupixent) for adults with PN in September 2022, the first treatment approved for treating PN in the United States.
“These positive studies support the involvement of type 2 cytokines in driving PN disease pathogenesis and the targeting of the [interleukin]-4/IL-13 axis as a novel therapeutic paradigm for patients with PN,” wrote the researchers, who were led by principal investigator Gil Yosipovitch, MD, professor of dermatology at the University of Miami, Fla. Dupilumab, an IL-4 receptor alpha antagonist, blocks the shared receptor component (IL-4R alpha) for IL-4 and IL-13.
For the two phase 3 trials, which were called LIBERTY-PN PRIME and PRIME2 and were sponsored by Sanofi and Regeneron Pharmaceuticals, researchers randomized adults with PN with 20 or more nodules and severe itch uncontrolled with topical therapies 1:1 to 300 mg dupilumab or placebo subcutaneously every 2 weeks for 24 weeks. The primary endpoint was pruritus improvement, which was measured by the proportion of patients with a 4-point or greater reduction in Worst Itch Numeric Rating Scale (WI-NRS) from baseline at week 24 (PRIME) or week 12 (PRIME2). Key secondary endpoints included a reduction in the number of nodules to 5 or fewer at week 24.
PRIME and PRIME2 enrolled 151 and 160 patients, respectively. In PRIME, 60% of patients in the dupilumab arm achieved a 4-point or greater reduction in the WI-NRS at week 24, compared with 18.4% of patients in the placebo arm (P < .001). In PRIME2, 37.2% of patients in the dupilumab arm achieved a 4-point or greater reduction in the WI-NRS at week 12, compared with 22% of patients in the placebo arm (P = .022).
The researchers also reported that, from an initial baseline of 20 to greater than 100 nodules, 32.0% of dupilumab-treated patients in PRIME and 25.6% in PRIME2 showed a reduction to 5 nodules or fewer, which corresponded to a response of “clear” or “almost clear” skin at week 12, compared with 11.8% and 12.2% of placebo-treated patients, respectively. This treatment effect on skin lesions continued to improve after week 12, with 48% of dupilumab-treated patients in PRIME and 44.9% in PRIME2 having five nodules or fewer at week 24, compared with 18.4% and 15.9% of placebo-treated patients, respectively. Safety was consistent with the known dupilumab safety profile.
“Validation is the first success of this paper,” said Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the study. “While both the safety and efficacy of dupilumab in these two phase 3 programs is the meat of the matter, nuanced highlights for me include the rigid nature of the exclusion criteria to ensure a study population that truly has PN as a stand-alone disease, rather than a secondary finding as we once believed to be the entire story. I think it’s important for us to recognize that it’s not one or the other, rather there is both ‘primary’ prurigo nodularis, and then there is secondary prurigo nodularis associated with something else [a wide range of underlying medical conditions], just like we divide primary and secondary hyperhidrosis.”
Dr. Yosipovitch reported having competing interests with several pharmaceutical companies, including Regeneron and Sanofi. Dr. Friedman disclosed that he is a consultant to and a speaker for Regeneron.
The results, which were published online in Nature Medicine, were the basis for the FDA approval of dupilumab (Dupixent) for adults with PN in September 2022, the first treatment approved for treating PN in the United States.
“These positive studies support the involvement of type 2 cytokines in driving PN disease pathogenesis and the targeting of the [interleukin]-4/IL-13 axis as a novel therapeutic paradigm for patients with PN,” wrote the researchers, who were led by principal investigator Gil Yosipovitch, MD, professor of dermatology at the University of Miami, Fla. Dupilumab, an IL-4 receptor alpha antagonist, blocks the shared receptor component (IL-4R alpha) for IL-4 and IL-13.
For the two phase 3 trials, which were called LIBERTY-PN PRIME and PRIME2 and were sponsored by Sanofi and Regeneron Pharmaceuticals, researchers randomized adults with PN with 20 or more nodules and severe itch uncontrolled with topical therapies 1:1 to 300 mg dupilumab or placebo subcutaneously every 2 weeks for 24 weeks. The primary endpoint was pruritus improvement, which was measured by the proportion of patients with a 4-point or greater reduction in Worst Itch Numeric Rating Scale (WI-NRS) from baseline at week 24 (PRIME) or week 12 (PRIME2). Key secondary endpoints included a reduction in the number of nodules to 5 or fewer at week 24.
PRIME and PRIME2 enrolled 151 and 160 patients, respectively. In PRIME, 60% of patients in the dupilumab arm achieved a 4-point or greater reduction in the WI-NRS at week 24, compared with 18.4% of patients in the placebo arm (P < .001). In PRIME2, 37.2% of patients in the dupilumab arm achieved a 4-point or greater reduction in the WI-NRS at week 12, compared with 22% of patients in the placebo arm (P = .022).
The researchers also reported that, from an initial baseline of 20 to greater than 100 nodules, 32.0% of dupilumab-treated patients in PRIME and 25.6% in PRIME2 showed a reduction to 5 nodules or fewer, which corresponded to a response of “clear” or “almost clear” skin at week 12, compared with 11.8% and 12.2% of placebo-treated patients, respectively. This treatment effect on skin lesions continued to improve after week 12, with 48% of dupilumab-treated patients in PRIME and 44.9% in PRIME2 having five nodules or fewer at week 24, compared with 18.4% and 15.9% of placebo-treated patients, respectively. Safety was consistent with the known dupilumab safety profile.
“Validation is the first success of this paper,” said Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the study. “While both the safety and efficacy of dupilumab in these two phase 3 programs is the meat of the matter, nuanced highlights for me include the rigid nature of the exclusion criteria to ensure a study population that truly has PN as a stand-alone disease, rather than a secondary finding as we once believed to be the entire story. I think it’s important for us to recognize that it’s not one or the other, rather there is both ‘primary’ prurigo nodularis, and then there is secondary prurigo nodularis associated with something else [a wide range of underlying medical conditions], just like we divide primary and secondary hyperhidrosis.”
Dr. Yosipovitch reported having competing interests with several pharmaceutical companies, including Regeneron and Sanofi. Dr. Friedman disclosed that he is a consultant to and a speaker for Regeneron.
FROM NATURE MEDICINE
Bundled strategy increased preteen lipid screening
WASHINGTON – A bundled intervention combining point-of-care testing, electronic medical record support, and provider education significantly improved lipid screening rates in children aged 9-11 years, according to data from approximately 100 monthly visits over a 3-year period.
Guidelines from the National Heart, Lung, and Blood Institute currently recommend universal lipid screening for children aged 9-11 years, but screening rates in clinical practice remain low, according to Ruth E. Gardner, MD, of Penn State University, Hershey, and colleagues.
In a poster presented at the Pediatric Academic Societies annual meeting, Dr. Gardner and colleagues shared results of the implementation of a bundled testing protocol designed to improve screening.
The researchers reviewed data on lipid testing within 30 days for all 9- to 11-year-old well child visits at a single center between May 2019 and February 2022. The bundled intervention was introduced in May 2021.
The bundled protocol included in-office capillary testing and provider education. In addition, electronic medical record templates were modified to include prompts for lipid screening at relevant ages, and EMR orders were adjusted to include lipid testing. The researchers also collected targeted provider feedback on individualized screening rates in February 2022.
Screening rates were plotted monthly. For the period from May 2019 through May 2021, the rates averaged 6.5%. However, after the introduction of the bundled intervention, the rate increased to 29.9%. Following targeted provider feedback in February 2022, the researchers found an additional shift to 52.1% through March and April 2022.
The findings were limited by the use of data from a single center, and the researchers used an extended study period to account for disruptions to well-child care in the spring of 2020 related to the COVID-19 pandemic.
However, the results support the effectiveness of a bundled intervention for improving lipid screening rates in children aged 9-11 years, the researchers said, and targeted provider feedback and education could yield additional improvements, they concluded.
Preteen years are an optimal time for screening
“The current study is important because atherosclerosis begins in childhood, and screening at ages 9-11 is an optimal time to begin lifestyle changes to improve overall health and reduce risks of heart disease,” said Margaret Thew, DNP, FNP-BC, of the Medical College of Wisconsin, Milwaukee, in an interview.
Ms. Thew, who was not involved in the study, said, “The number of recommended and required screening items needed in pediatrics is vast, so many providers have to select which items to focus on for their health screenings with these ages.”
Overall, “I was impressed with the improvements that were made in this quality improvement study,” said Ms. Thew.
Barriers to lipid screening in this population include the reduced number of health screenings and immunizations recommended for this age group; the consequence is that access is limited to discuss preventive care opportunities, said Ms. Thew in an interview. Steps to overcome these barriers could include the use of many of the screening tools introduced in the current study, such as point-of-care testing in the office, use of the EMR to remind providers of testing, which can be done during well visits or school physicals, and educating providers about the current guidelines, she noted.
Other strategies to increase screening include moving the immunization series to provide more frequent appointments to children aged 9-11 years to offer education and preventive care, Ms. Thew added.
The study received no outside funding. The researchers had no financial conflicts to disclose. Ms. Thew had no financial conflicts to disclose and serves on the Editorial Advisory Board of Pediatric News.
WASHINGTON – A bundled intervention combining point-of-care testing, electronic medical record support, and provider education significantly improved lipid screening rates in children aged 9-11 years, according to data from approximately 100 monthly visits over a 3-year period.
Guidelines from the National Heart, Lung, and Blood Institute currently recommend universal lipid screening for children aged 9-11 years, but screening rates in clinical practice remain low, according to Ruth E. Gardner, MD, of Penn State University, Hershey, and colleagues.
In a poster presented at the Pediatric Academic Societies annual meeting, Dr. Gardner and colleagues shared results of the implementation of a bundled testing protocol designed to improve screening.
The researchers reviewed data on lipid testing within 30 days for all 9- to 11-year-old well child visits at a single center between May 2019 and February 2022. The bundled intervention was introduced in May 2021.
The bundled protocol included in-office capillary testing and provider education. In addition, electronic medical record templates were modified to include prompts for lipid screening at relevant ages, and EMR orders were adjusted to include lipid testing. The researchers also collected targeted provider feedback on individualized screening rates in February 2022.
Screening rates were plotted monthly. For the period from May 2019 through May 2021, the rates averaged 6.5%. However, after the introduction of the bundled intervention, the rate increased to 29.9%. Following targeted provider feedback in February 2022, the researchers found an additional shift to 52.1% through March and April 2022.
The findings were limited by the use of data from a single center, and the researchers used an extended study period to account for disruptions to well-child care in the spring of 2020 related to the COVID-19 pandemic.
However, the results support the effectiveness of a bundled intervention for improving lipid screening rates in children aged 9-11 years, the researchers said, and targeted provider feedback and education could yield additional improvements, they concluded.
Preteen years are an optimal time for screening
“The current study is important because atherosclerosis begins in childhood, and screening at ages 9-11 is an optimal time to begin lifestyle changes to improve overall health and reduce risks of heart disease,” said Margaret Thew, DNP, FNP-BC, of the Medical College of Wisconsin, Milwaukee, in an interview.
Ms. Thew, who was not involved in the study, said, “The number of recommended and required screening items needed in pediatrics is vast, so many providers have to select which items to focus on for their health screenings with these ages.”
Overall, “I was impressed with the improvements that were made in this quality improvement study,” said Ms. Thew.
Barriers to lipid screening in this population include the reduced number of health screenings and immunizations recommended for this age group; the consequence is that access is limited to discuss preventive care opportunities, said Ms. Thew in an interview. Steps to overcome these barriers could include the use of many of the screening tools introduced in the current study, such as point-of-care testing in the office, use of the EMR to remind providers of testing, which can be done during well visits or school physicals, and educating providers about the current guidelines, she noted.
Other strategies to increase screening include moving the immunization series to provide more frequent appointments to children aged 9-11 years to offer education and preventive care, Ms. Thew added.
The study received no outside funding. The researchers had no financial conflicts to disclose. Ms. Thew had no financial conflicts to disclose and serves on the Editorial Advisory Board of Pediatric News.
WASHINGTON – A bundled intervention combining point-of-care testing, electronic medical record support, and provider education significantly improved lipid screening rates in children aged 9-11 years, according to data from approximately 100 monthly visits over a 3-year period.
Guidelines from the National Heart, Lung, and Blood Institute currently recommend universal lipid screening for children aged 9-11 years, but screening rates in clinical practice remain low, according to Ruth E. Gardner, MD, of Penn State University, Hershey, and colleagues.
In a poster presented at the Pediatric Academic Societies annual meeting, Dr. Gardner and colleagues shared results of the implementation of a bundled testing protocol designed to improve screening.
The researchers reviewed data on lipid testing within 30 days for all 9- to 11-year-old well child visits at a single center between May 2019 and February 2022. The bundled intervention was introduced in May 2021.
The bundled protocol included in-office capillary testing and provider education. In addition, electronic medical record templates were modified to include prompts for lipid screening at relevant ages, and EMR orders were adjusted to include lipid testing. The researchers also collected targeted provider feedback on individualized screening rates in February 2022.
Screening rates were plotted monthly. For the period from May 2019 through May 2021, the rates averaged 6.5%. However, after the introduction of the bundled intervention, the rate increased to 29.9%. Following targeted provider feedback in February 2022, the researchers found an additional shift to 52.1% through March and April 2022.
The findings were limited by the use of data from a single center, and the researchers used an extended study period to account for disruptions to well-child care in the spring of 2020 related to the COVID-19 pandemic.
However, the results support the effectiveness of a bundled intervention for improving lipid screening rates in children aged 9-11 years, the researchers said, and targeted provider feedback and education could yield additional improvements, they concluded.
Preteen years are an optimal time for screening
“The current study is important because atherosclerosis begins in childhood, and screening at ages 9-11 is an optimal time to begin lifestyle changes to improve overall health and reduce risks of heart disease,” said Margaret Thew, DNP, FNP-BC, of the Medical College of Wisconsin, Milwaukee, in an interview.
Ms. Thew, who was not involved in the study, said, “The number of recommended and required screening items needed in pediatrics is vast, so many providers have to select which items to focus on for their health screenings with these ages.”
Overall, “I was impressed with the improvements that were made in this quality improvement study,” said Ms. Thew.
Barriers to lipid screening in this population include the reduced number of health screenings and immunizations recommended for this age group; the consequence is that access is limited to discuss preventive care opportunities, said Ms. Thew in an interview. Steps to overcome these barriers could include the use of many of the screening tools introduced in the current study, such as point-of-care testing in the office, use of the EMR to remind providers of testing, which can be done during well visits or school physicals, and educating providers about the current guidelines, she noted.
Other strategies to increase screening include moving the immunization series to provide more frequent appointments to children aged 9-11 years to offer education and preventive care, Ms. Thew added.
The study received no outside funding. The researchers had no financial conflicts to disclose. Ms. Thew had no financial conflicts to disclose and serves on the Editorial Advisory Board of Pediatric News.
FROM PAS 2023
Boys may carry the weight, or overweight, of adults’ infertility
Overweight boy, infertile man?
When it comes to causes of infertility, history and science have generally focused on women. A lot of the research overlooks men, but some previous studies have suggested that male infertility contributes to about half of the cases of couple infertility. The reason for much of that male infertility, however, has been a mystery. Until now.
A group of Italian investigators looked at the declining trend in sperm counts over the past 40 years and the increase of childhood obesity. Is there a correlation? The researchers think so. Childhood obesity can be linked to multiple causes, but the researchers zeroed in on the effect that obesity has on metabolic rates and, therefore, testicular growth.
Collecting data on testicular volume, body mass index (BMI), and insulin resistance from 268 boys aged 2-18 years, the researchers discovered that those with normal weight and normal insulin levels had testicular volumes 1.5 times higher than their overweight counterparts and 1.5-2 times higher than those with hyperinsulinemia, building a case for obesity being a factor for infertility later in life.
Since low testicular volume is associated with lower sperm count and production as an adult, putting two and two together makes a compelling argument for childhood obesity being a major male infertility culprit. It also creates even more urgency for the health care industry and community decision makers to focus on childhood obesity.
It sure would be nice to be able to take one of the many risk factors for future human survival off the table. Maybe by taking something, like cake, off the table.
Fecal transplantation moves to the kitchen
Fecal microbiota transplantation is an effective way to treat Clostridioides difficile infection, but, in the end, it’s still a transplantation procedure involving a nasogastric or colorectal tube or rather large oral capsules with a demanding (30-40 capsules over 2 days) dosage. Please, Science, tell us there’s a better way.
Science, in the form of investigators at the University of Geneva and Lausanne University Hospital in Switzerland, has spoken, and there may be a better way. Presenting fecal beads: All the bacterial goodness of donor stool without the tubal insertions or massive quantities of giant capsules.
We know you’re scoffing out there, but it’s true. All you need is a little alginate, which is a “biocompatible polysaccharide isolated from brown algae” of the Phaeophyceae family. The donor feces is microencapsulated by mixing it with the alginate, dropping that mixture into water containing calcium chloride, turning it into a gel, and then freeze-drying the gel into small (just 2 mm), solid beads.
Sounds plausible enough, but what do you do with them? “These brownish beads can be easily dispersed in a liquid or food that is pleasant to eat. They also have no taste,” senior author Eric Allémann, PhD, said in a statement released by the University of Geneva.
Pleasant to eat? No taste? So which is it? If you really want to know, watch fecal beads week on the new season of “The Great British Baking Show,” when Paul and Prue judge poop baked into crumpets, crepes, and crostatas. Yum.
We’re on the low-oxygen diet
Nine out of ten doctors agree: Oxygen is more important to your continued well-being than food. After all, a human can go weeks without food, but just minutes without oxygen. However, ten out of ten doctors agree that the United States has an obesity problem. They all also agree that previous research has shown soldiers who train at high altitudes lose more weight than those training at lower altitudes.
So, on the one hand, we have a country full of overweight people, and on the other, we have low oxygen levels causing weight loss. The solution, then, is obvious: Stop breathing.
More specifically (and somewhat less facetiously), researchers from Louisiana have launched the Low Oxygen and Weight Status trial and are currently recruiting individuals with BMIs of 30-40 to, uh, suffocate themselves. No, no, it’s okay, it’s just when they’re sleeping.
Fine, straight face. Participants in the LOWS trial will undergo an 8-week period when they will consume a controlled weight-loss diet and spend their nights in a hypoxic sealed tent, where they will sleep in an environment with an oxygen level equivalent to 8,500 feet above sea level (roughly equivalent to Aspen, Colo.). They will be compared with people on the same diet who sleep in a normal, sea-level oxygen environment.
The study’s goal is to determine whether or not spending time in a low-oxygen environment will suppress appetite, increase energy expenditure, and improve weight loss and insulin sensitivity. Excessive weight loss in high-altitude environments isn’t a good thing for soldiers – they kind of need their muscles and body weight to do the whole soldiering thing – but it could be great for people struggling to lose those last few pounds. And it also may prove LOTME’s previous thesis: Air is not good.
Overweight boy, infertile man?
When it comes to causes of infertility, history and science have generally focused on women. A lot of the research overlooks men, but some previous studies have suggested that male infertility contributes to about half of the cases of couple infertility. The reason for much of that male infertility, however, has been a mystery. Until now.
A group of Italian investigators looked at the declining trend in sperm counts over the past 40 years and the increase of childhood obesity. Is there a correlation? The researchers think so. Childhood obesity can be linked to multiple causes, but the researchers zeroed in on the effect that obesity has on metabolic rates and, therefore, testicular growth.
Collecting data on testicular volume, body mass index (BMI), and insulin resistance from 268 boys aged 2-18 years, the researchers discovered that those with normal weight and normal insulin levels had testicular volumes 1.5 times higher than their overweight counterparts and 1.5-2 times higher than those with hyperinsulinemia, building a case for obesity being a factor for infertility later in life.
Since low testicular volume is associated with lower sperm count and production as an adult, putting two and two together makes a compelling argument for childhood obesity being a major male infertility culprit. It also creates even more urgency for the health care industry and community decision makers to focus on childhood obesity.
It sure would be nice to be able to take one of the many risk factors for future human survival off the table. Maybe by taking something, like cake, off the table.
Fecal transplantation moves to the kitchen
Fecal microbiota transplantation is an effective way to treat Clostridioides difficile infection, but, in the end, it’s still a transplantation procedure involving a nasogastric or colorectal tube or rather large oral capsules with a demanding (30-40 capsules over 2 days) dosage. Please, Science, tell us there’s a better way.
Science, in the form of investigators at the University of Geneva and Lausanne University Hospital in Switzerland, has spoken, and there may be a better way. Presenting fecal beads: All the bacterial goodness of donor stool without the tubal insertions or massive quantities of giant capsules.
We know you’re scoffing out there, but it’s true. All you need is a little alginate, which is a “biocompatible polysaccharide isolated from brown algae” of the Phaeophyceae family. The donor feces is microencapsulated by mixing it with the alginate, dropping that mixture into water containing calcium chloride, turning it into a gel, and then freeze-drying the gel into small (just 2 mm), solid beads.
Sounds plausible enough, but what do you do with them? “These brownish beads can be easily dispersed in a liquid or food that is pleasant to eat. They also have no taste,” senior author Eric Allémann, PhD, said in a statement released by the University of Geneva.
Pleasant to eat? No taste? So which is it? If you really want to know, watch fecal beads week on the new season of “The Great British Baking Show,” when Paul and Prue judge poop baked into crumpets, crepes, and crostatas. Yum.
We’re on the low-oxygen diet
Nine out of ten doctors agree: Oxygen is more important to your continued well-being than food. After all, a human can go weeks without food, but just minutes without oxygen. However, ten out of ten doctors agree that the United States has an obesity problem. They all also agree that previous research has shown soldiers who train at high altitudes lose more weight than those training at lower altitudes.
So, on the one hand, we have a country full of overweight people, and on the other, we have low oxygen levels causing weight loss. The solution, then, is obvious: Stop breathing.
More specifically (and somewhat less facetiously), researchers from Louisiana have launched the Low Oxygen and Weight Status trial and are currently recruiting individuals with BMIs of 30-40 to, uh, suffocate themselves. No, no, it’s okay, it’s just when they’re sleeping.
Fine, straight face. Participants in the LOWS trial will undergo an 8-week period when they will consume a controlled weight-loss diet and spend their nights in a hypoxic sealed tent, where they will sleep in an environment with an oxygen level equivalent to 8,500 feet above sea level (roughly equivalent to Aspen, Colo.). They will be compared with people on the same diet who sleep in a normal, sea-level oxygen environment.
The study’s goal is to determine whether or not spending time in a low-oxygen environment will suppress appetite, increase energy expenditure, and improve weight loss and insulin sensitivity. Excessive weight loss in high-altitude environments isn’t a good thing for soldiers – they kind of need their muscles and body weight to do the whole soldiering thing – but it could be great for people struggling to lose those last few pounds. And it also may prove LOTME’s previous thesis: Air is not good.
Overweight boy, infertile man?
When it comes to causes of infertility, history and science have generally focused on women. A lot of the research overlooks men, but some previous studies have suggested that male infertility contributes to about half of the cases of couple infertility. The reason for much of that male infertility, however, has been a mystery. Until now.
A group of Italian investigators looked at the declining trend in sperm counts over the past 40 years and the increase of childhood obesity. Is there a correlation? The researchers think so. Childhood obesity can be linked to multiple causes, but the researchers zeroed in on the effect that obesity has on metabolic rates and, therefore, testicular growth.
Collecting data on testicular volume, body mass index (BMI), and insulin resistance from 268 boys aged 2-18 years, the researchers discovered that those with normal weight and normal insulin levels had testicular volumes 1.5 times higher than their overweight counterparts and 1.5-2 times higher than those with hyperinsulinemia, building a case for obesity being a factor for infertility later in life.
Since low testicular volume is associated with lower sperm count and production as an adult, putting two and two together makes a compelling argument for childhood obesity being a major male infertility culprit. It also creates even more urgency for the health care industry and community decision makers to focus on childhood obesity.
It sure would be nice to be able to take one of the many risk factors for future human survival off the table. Maybe by taking something, like cake, off the table.
Fecal transplantation moves to the kitchen
Fecal microbiota transplantation is an effective way to treat Clostridioides difficile infection, but, in the end, it’s still a transplantation procedure involving a nasogastric or colorectal tube or rather large oral capsules with a demanding (30-40 capsules over 2 days) dosage. Please, Science, tell us there’s a better way.
Science, in the form of investigators at the University of Geneva and Lausanne University Hospital in Switzerland, has spoken, and there may be a better way. Presenting fecal beads: All the bacterial goodness of donor stool without the tubal insertions or massive quantities of giant capsules.
We know you’re scoffing out there, but it’s true. All you need is a little alginate, which is a “biocompatible polysaccharide isolated from brown algae” of the Phaeophyceae family. The donor feces is microencapsulated by mixing it with the alginate, dropping that mixture into water containing calcium chloride, turning it into a gel, and then freeze-drying the gel into small (just 2 mm), solid beads.
Sounds plausible enough, but what do you do with them? “These brownish beads can be easily dispersed in a liquid or food that is pleasant to eat. They also have no taste,” senior author Eric Allémann, PhD, said in a statement released by the University of Geneva.
Pleasant to eat? No taste? So which is it? If you really want to know, watch fecal beads week on the new season of “The Great British Baking Show,” when Paul and Prue judge poop baked into crumpets, crepes, and crostatas. Yum.
We’re on the low-oxygen diet
Nine out of ten doctors agree: Oxygen is more important to your continued well-being than food. After all, a human can go weeks without food, but just minutes without oxygen. However, ten out of ten doctors agree that the United States has an obesity problem. They all also agree that previous research has shown soldiers who train at high altitudes lose more weight than those training at lower altitudes.
So, on the one hand, we have a country full of overweight people, and on the other, we have low oxygen levels causing weight loss. The solution, then, is obvious: Stop breathing.
More specifically (and somewhat less facetiously), researchers from Louisiana have launched the Low Oxygen and Weight Status trial and are currently recruiting individuals with BMIs of 30-40 to, uh, suffocate themselves. No, no, it’s okay, it’s just when they’re sleeping.
Fine, straight face. Participants in the LOWS trial will undergo an 8-week period when they will consume a controlled weight-loss diet and spend their nights in a hypoxic sealed tent, where they will sleep in an environment with an oxygen level equivalent to 8,500 feet above sea level (roughly equivalent to Aspen, Colo.). They will be compared with people on the same diet who sleep in a normal, sea-level oxygen environment.
The study’s goal is to determine whether or not spending time in a low-oxygen environment will suppress appetite, increase energy expenditure, and improve weight loss and insulin sensitivity. Excessive weight loss in high-altitude environments isn’t a good thing for soldiers – they kind of need their muscles and body weight to do the whole soldiering thing – but it could be great for people struggling to lose those last few pounds. And it also may prove LOTME’s previous thesis: Air is not good.
Itchy pustules over hair follicles
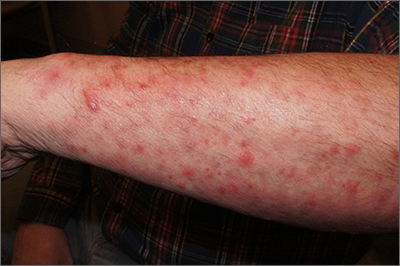
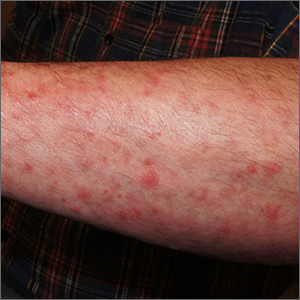
A potassium hydroxide (KOH) preparation of pus and dry superficial skin taken from 1 of the pustules revealed multiple hyphae and confirmed a diagnosis of nodular granulomatous perifolliculitis, also called Majocchi granuloma.
Majocchi granuloma is a reactive process of inflammation caused by infection of the follicular unit(s) by a dermatophyte—most often the same Trichophyton species responsible for more superficial tinea. On exam, there may be a solitary papule, pustule, or nodule. More often, there are multiple papules and pustules grouped within an annular plaque in hair-bearing areas on the head, trunk, or extremities. Majocchi granuloma can occur in patients who are healthy and those who are immunosuppressed.1 It can also occur when a topical steroid is applied to unsuspected tinea, as occurred here. In this case, the patient was accustomed to having multiple skin plaques of psoriasis and assumed this was a stubborn manifestation of that.
Because the infection penetrates deeper than most topical therapies can effectively reach at adequate concentrations, systemic medications are the treatments of choice. Terbinafine, itraconazole, and fluconazole are all effective options but need to be used for several weeks to be effective.
This patient received terbinafine 250 mg/d for 6 weeks and the pustules cleared completely. He continued with his other psoriasis medications throughout his treatment.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. İlkit M, Durdu M, Karakaş M. Majocchi’s granuloma: a symptom complex caused by fungal pathogens. Med Mycol. 2012;50:449-457. doi: 10.3109/13693786.2012.669503

A potassium hydroxide (KOH) preparation of pus and dry superficial skin taken from 1 of the pustules revealed multiple hyphae and confirmed a diagnosis of nodular granulomatous perifolliculitis, also called Majocchi granuloma.
Majocchi granuloma is a reactive process of inflammation caused by infection of the follicular unit(s) by a dermatophyte—most often the same Trichophyton species responsible for more superficial tinea. On exam, there may be a solitary papule, pustule, or nodule. More often, there are multiple papules and pustules grouped within an annular plaque in hair-bearing areas on the head, trunk, or extremities. Majocchi granuloma can occur in patients who are healthy and those who are immunosuppressed.1 It can also occur when a topical steroid is applied to unsuspected tinea, as occurred here. In this case, the patient was accustomed to having multiple skin plaques of psoriasis and assumed this was a stubborn manifestation of that.
Because the infection penetrates deeper than most topical therapies can effectively reach at adequate concentrations, systemic medications are the treatments of choice. Terbinafine, itraconazole, and fluconazole are all effective options but need to be used for several weeks to be effective.
This patient received terbinafine 250 mg/d for 6 weeks and the pustules cleared completely. He continued with his other psoriasis medications throughout his treatment.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

A potassium hydroxide (KOH) preparation of pus and dry superficial skin taken from 1 of the pustules revealed multiple hyphae and confirmed a diagnosis of nodular granulomatous perifolliculitis, also called Majocchi granuloma.
Majocchi granuloma is a reactive process of inflammation caused by infection of the follicular unit(s) by a dermatophyte—most often the same Trichophyton species responsible for more superficial tinea. On exam, there may be a solitary papule, pustule, or nodule. More often, there are multiple papules and pustules grouped within an annular plaque in hair-bearing areas on the head, trunk, or extremities. Majocchi granuloma can occur in patients who are healthy and those who are immunosuppressed.1 It can also occur when a topical steroid is applied to unsuspected tinea, as occurred here. In this case, the patient was accustomed to having multiple skin plaques of psoriasis and assumed this was a stubborn manifestation of that.
Because the infection penetrates deeper than most topical therapies can effectively reach at adequate concentrations, systemic medications are the treatments of choice. Terbinafine, itraconazole, and fluconazole are all effective options but need to be used for several weeks to be effective.
This patient received terbinafine 250 mg/d for 6 weeks and the pustules cleared completely. He continued with his other psoriasis medications throughout his treatment.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. İlkit M, Durdu M, Karakaş M. Majocchi’s granuloma: a symptom complex caused by fungal pathogens. Med Mycol. 2012;50:449-457. doi: 10.3109/13693786.2012.669503
1. İlkit M, Durdu M, Karakaş M. Majocchi’s granuloma: a symptom complex caused by fungal pathogens. Med Mycol. 2012;50:449-457. doi: 10.3109/13693786.2012.669503
Clinical trials: Top priority for long COVID
The Centers for Disease Control and Prevention and the U.S. Census Bureau estimate that 6.1% of the U.S. adult population is living with long COVID, with millions more debilitated worldwide. The demand for substantial treatment is enormous, but the urgency to fund and begin the necessary range of clinical trials has not met the severity of the problem.
While trials are slowly beginning to happen, the treatment choices and trial design require crucial nuances and understanding of viral-onset illnesses, and few research groups are creating strong trials that fully reflect the complexities of this landscape.
These recommendations recognize that roughly half of long COVID patients have new-onset myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and dysautonomia from COVID, which must be at the forefront of how trials are designed and conducted, and are additionally based on the current hypotheses about long COVID’s pathophysiologies.
1: Drugs proposed by experts in postviral fields should be prioritized
Upward of 50 drugs for viral-onset conditions like ME/CFS, dysautonomia, AIDS, and others have been waiting for years to go to trial, but have not had the funding to do so.
Treatments proposed by experts in viral-onset illnesses (such as ME/CFS and dysautonomia) should be prioritized (PM R. 2022 Oct;14[10]:1270-91), as outside researchers are not familiar with these fields and their potential treatment options.
2: Drugs targeting a wide range of mechanisms should be trialed
Treatments that should be trialed include anticoagulants/antiplatelets for clotting and vascular functioning, immunomodulators including JAK-STAT inhibitors, COVID-specific antivirals and antivirals against reactivated herpesviruses (Valcyte, Valacyclovir, EBV vaccine).
Other options include prescription mast cell stabilizers (ketotifen, cromolyn sodium), drugs that regulate microglial activation (low-dose naltrexone, low-dose aripiprazole), anti-CGRP medications, beta-blockers, and intravenous immunoglobulin.
Others include medications that target mitochondrial dysfunction; ivabradine; pyridostigmine;, DRP1 inhibitors; supplements showing success in patient communities including lactoferrin, ubiquinone, and nattokinase; and therapies targeting glymphatic/lymphatic dysfunction, microbiome therapies, and therapeutic peptides.
3: Use appropriate long COVID subtypes
Long COVID is an umbrella term that encompasses multiple new-onset and worsened conditions and symptoms after COVID. Roughly half of long COVID patients likely meet the criteria for ME/CFS and/or dysautonomia. Others may have new-onset diabetes, major clotting events, lung damage, neurological disorders, loss of smell or taste, and other manifestations.
Patients in different categories likely have different responses to treatments. It’s critical to identify appropriate subtypes for each trial, ideally performing detailed analyses to identify the treatments that work best, and don’t, for each subtype.
4: Behavioral treatments, especially those that have harmed similar populations, should not be trialed
Behavioral treatments including exercise, graded exercise therapy (GET), and cognitive-behavioral therapy (CBT) should not be trialed, let alone prioritized, for long COVID.
In patients with postexertional malaise (PEM), one of the most common long COVID symptoms, exercise is actively harmful and causes dysfunctional metabolic patterns, cardiac preload failure, impaired systemic oxygen extraction, and more. GET and CBT have failed similar populations , and exercise is explicitly contraindicated by the World Health Organization, the British National Institute for Health and Care Excellence, the CDC, and other organizations.
Resources should instead be put toward the wide range of medications that have not yet adequately undergone clinical trials.
5: PCR and antibody tests should not be used as inclusion criteria for trial participants
Only an estimated 1%-3% of cases in the first wave of COVID were documented, and the CDC estimates that only 25% of cases through September 2021 were documented. Similarly, antibody tests are unreliable to determine past infection, as roughly a third of patients don’t seroconvert, and a similar proportion serorevert within a few months. Using polymerase chain reaction (PCR) and antibody testing to determine who should be included in clinical trials limits who is eligible to participate in research, particularly those who have been ill for longer. Additionally, the majority of those who serorevert are women, so using antibody tests for inclusion introduces a selection bias and may miss mechanisms of immune system functioning that are part of long COVID.
PCR tests also have high false-negative rates and requiring them in research excludes people with lower viral loads with long COVID, which would confound findings.
These issues with testing also lead to COVID-infected people accidentally being included in control groups, which ruins the credibility of the research findings completely.
6: Include comparator groups
There are several common diagnoses that occur in people with long COVID, including ME/CFS, postural orthostatic tachycardia syndrome, small-fiber neuropathy, mast cell activation syndrome, and Ehlers-Danlos syndrome.
Identifying people with these conditions within the trial cohort improves research across all fields, benefiting all groups, and helps clarify what types of patients benefit most from certain medications.
7: Identify the right endpoints; avoid the wrong ones
Even though our understanding of the pathophysiology of long COVID is still evolving, it’s still possible to do clinical trials by identifying strong endpoints and outcome measures.
Several tools have been designed for viral-onset conditions and should be used alongside other endpoints. Postexertional malaise and autonomic symptoms, which are some of the most common symptoms of long COVID, can be measured with the validated DSQ-PEM and COMPASS-31, respectively. Tools for cognitive dysfunction trials should capture specific and common types of impairment, like processing speed.
Endpoints should be high-impact and aim for large improvements that have clinical significance over small improvements that do not have clinical significance.
Objective tests should be incorporated where possible; some to consider include natural killer cell functioning, cerebral blood flow, T-cell functioning, levels of reactivated herpesviruses, blood lactate levels, and microclots, as testing becomes available.
Mental health outcomes shouldn’t be primary endpoints, except where a trial is targeting a specific mental health condition because of COVID (for example, premenstrual dysphoric disorder).
If mental health conditions are tracked secondarily, it’s vital not to use questionnaires that include physical symptoms like fatigue, difficulty concentrating, difficulty sleeping, or palpitations, as these artificially increase depression and anxiety scores in chronically ill respondents. Tools that include physical symptoms (Patient Health Questionnaire–9, Beck Anxiety Inventory, Beck Depression Inventory) can be replaced with scales like the PHQ-2, General Anxiety Disorder–7, Hospital Anxiety and Depression Scale, or PROMIS-29 subscales.
Because certain cytokines and other inflammatory markers may naturally decrease over time without corresponding improvement in the ME/CFS subtype, caution should be taken when using cytokines as endpoints.
8: Consider enrollment and objectives carefully
A proportion of people with long COVID will recover in the early months after infection. Ideally, clinical trials will primarily study treatments in patients who have been ill 6 months or longer, as some natural recovery will happen before that can bias studies.
But where resources are abundant, it is ideal for trials to additionally look at whether the treatments can help patients in the early months recover and prevent progression to the later stage.
9: Tracking illness duration is crucial
Research from ME/CFS shows that there may be an immune change in the first few years of the illness, where cytokines decrease without any corresponding change in symptom improvement.
Because of this and the possibility that other markers follow the same pattern, disease duration should be a core feature of all analyses and trial designs. Trial outcomes should be designed to answer the question of whether the medication helps patients at different durations of illness.
10: Prioritize patient populations less likely to recover without intervention
Some long COVID phenotypes seem less likely to recover without intervention. Trials should take care to focus on these patient populations, which include those with neurologic symptoms and those meeting ME/CFS criteria.
11: Account for the relapsing/remitting nature
Outcome measures need to be assessed in a way that can distinguish a temporary remission, which is part of the natural course of the disease, from a permanent cure.
Factors that can contribute to the relapsing/remitting nature include physical and cognitive postexertional malaise, menstrual cycle changes, and seasonal changes.
12: Trial participants should reflect the diversity of the long COVID population
Certain demographics are more likely to be affected by acute and long COVID and need to be appropriately recruited and reflected in research, including in patient engagement.
Trials must include high numbers of Hispanic/Latinx, Black, and indigenous communities, queer and transgender populations, and women. Trial materials and design need to incorporate linguistic diversity in addition to racial/ethnic diversity.
Upward of 75% of long COVID cases happen after mild acute cases; clinical researchers should ensure that nonhospitalized patients make up the bulk of trial participants.
13: Utilize meaningful engagement of patients, especially in treatment selection and study design
Meaningful patient engagement means engaging multiple patients at every step of the trial process, from treatment selection to study design to analysis to communication of the results.
Patient experiences are extremely valuable and contain information that researchers may not be familiar with, including the nature and patterns of the illness, insights into possible treatments, and barriers to documentation and care that may also impact research. Tapping into those patient experiences will make trials stronger.
Overall, the landscape of long COVID clinical trials is ripe for discovery, and researchers choosing to go down this path will be deeply appreciated by the patient community.
Hannah Davis is a long COVID patient-researcher and cofounder of the Patient-Led Research Collaborative, an organization studying the long-term effects of COVID.
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention and the U.S. Census Bureau estimate that 6.1% of the U.S. adult population is living with long COVID, with millions more debilitated worldwide. The demand for substantial treatment is enormous, but the urgency to fund and begin the necessary range of clinical trials has not met the severity of the problem.
While trials are slowly beginning to happen, the treatment choices and trial design require crucial nuances and understanding of viral-onset illnesses, and few research groups are creating strong trials that fully reflect the complexities of this landscape.
These recommendations recognize that roughly half of long COVID patients have new-onset myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and dysautonomia from COVID, which must be at the forefront of how trials are designed and conducted, and are additionally based on the current hypotheses about long COVID’s pathophysiologies.
1: Drugs proposed by experts in postviral fields should be prioritized
Upward of 50 drugs for viral-onset conditions like ME/CFS, dysautonomia, AIDS, and others have been waiting for years to go to trial, but have not had the funding to do so.
Treatments proposed by experts in viral-onset illnesses (such as ME/CFS and dysautonomia) should be prioritized (PM R. 2022 Oct;14[10]:1270-91), as outside researchers are not familiar with these fields and their potential treatment options.
2: Drugs targeting a wide range of mechanisms should be trialed
Treatments that should be trialed include anticoagulants/antiplatelets for clotting and vascular functioning, immunomodulators including JAK-STAT inhibitors, COVID-specific antivirals and antivirals against reactivated herpesviruses (Valcyte, Valacyclovir, EBV vaccine).
Other options include prescription mast cell stabilizers (ketotifen, cromolyn sodium), drugs that regulate microglial activation (low-dose naltrexone, low-dose aripiprazole), anti-CGRP medications, beta-blockers, and intravenous immunoglobulin.
Others include medications that target mitochondrial dysfunction; ivabradine; pyridostigmine;, DRP1 inhibitors; supplements showing success in patient communities including lactoferrin, ubiquinone, and nattokinase; and therapies targeting glymphatic/lymphatic dysfunction, microbiome therapies, and therapeutic peptides.
3: Use appropriate long COVID subtypes
Long COVID is an umbrella term that encompasses multiple new-onset and worsened conditions and symptoms after COVID. Roughly half of long COVID patients likely meet the criteria for ME/CFS and/or dysautonomia. Others may have new-onset diabetes, major clotting events, lung damage, neurological disorders, loss of smell or taste, and other manifestations.
Patients in different categories likely have different responses to treatments. It’s critical to identify appropriate subtypes for each trial, ideally performing detailed analyses to identify the treatments that work best, and don’t, for each subtype.
4: Behavioral treatments, especially those that have harmed similar populations, should not be trialed
Behavioral treatments including exercise, graded exercise therapy (GET), and cognitive-behavioral therapy (CBT) should not be trialed, let alone prioritized, for long COVID.
In patients with postexertional malaise (PEM), one of the most common long COVID symptoms, exercise is actively harmful and causes dysfunctional metabolic patterns, cardiac preload failure, impaired systemic oxygen extraction, and more. GET and CBT have failed similar populations , and exercise is explicitly contraindicated by the World Health Organization, the British National Institute for Health and Care Excellence, the CDC, and other organizations.
Resources should instead be put toward the wide range of medications that have not yet adequately undergone clinical trials.
5: PCR and antibody tests should not be used as inclusion criteria for trial participants
Only an estimated 1%-3% of cases in the first wave of COVID were documented, and the CDC estimates that only 25% of cases through September 2021 were documented. Similarly, antibody tests are unreliable to determine past infection, as roughly a third of patients don’t seroconvert, and a similar proportion serorevert within a few months. Using polymerase chain reaction (PCR) and antibody testing to determine who should be included in clinical trials limits who is eligible to participate in research, particularly those who have been ill for longer. Additionally, the majority of those who serorevert are women, so using antibody tests for inclusion introduces a selection bias and may miss mechanisms of immune system functioning that are part of long COVID.
PCR tests also have high false-negative rates and requiring them in research excludes people with lower viral loads with long COVID, which would confound findings.
These issues with testing also lead to COVID-infected people accidentally being included in control groups, which ruins the credibility of the research findings completely.
6: Include comparator groups
There are several common diagnoses that occur in people with long COVID, including ME/CFS, postural orthostatic tachycardia syndrome, small-fiber neuropathy, mast cell activation syndrome, and Ehlers-Danlos syndrome.
Identifying people with these conditions within the trial cohort improves research across all fields, benefiting all groups, and helps clarify what types of patients benefit most from certain medications.
7: Identify the right endpoints; avoid the wrong ones
Even though our understanding of the pathophysiology of long COVID is still evolving, it’s still possible to do clinical trials by identifying strong endpoints and outcome measures.
Several tools have been designed for viral-onset conditions and should be used alongside other endpoints. Postexertional malaise and autonomic symptoms, which are some of the most common symptoms of long COVID, can be measured with the validated DSQ-PEM and COMPASS-31, respectively. Tools for cognitive dysfunction trials should capture specific and common types of impairment, like processing speed.
Endpoints should be high-impact and aim for large improvements that have clinical significance over small improvements that do not have clinical significance.
Objective tests should be incorporated where possible; some to consider include natural killer cell functioning, cerebral blood flow, T-cell functioning, levels of reactivated herpesviruses, blood lactate levels, and microclots, as testing becomes available.
Mental health outcomes shouldn’t be primary endpoints, except where a trial is targeting a specific mental health condition because of COVID (for example, premenstrual dysphoric disorder).
If mental health conditions are tracked secondarily, it’s vital not to use questionnaires that include physical symptoms like fatigue, difficulty concentrating, difficulty sleeping, or palpitations, as these artificially increase depression and anxiety scores in chronically ill respondents. Tools that include physical symptoms (Patient Health Questionnaire–9, Beck Anxiety Inventory, Beck Depression Inventory) can be replaced with scales like the PHQ-2, General Anxiety Disorder–7, Hospital Anxiety and Depression Scale, or PROMIS-29 subscales.
Because certain cytokines and other inflammatory markers may naturally decrease over time without corresponding improvement in the ME/CFS subtype, caution should be taken when using cytokines as endpoints.
8: Consider enrollment and objectives carefully
A proportion of people with long COVID will recover in the early months after infection. Ideally, clinical trials will primarily study treatments in patients who have been ill 6 months or longer, as some natural recovery will happen before that can bias studies.
But where resources are abundant, it is ideal for trials to additionally look at whether the treatments can help patients in the early months recover and prevent progression to the later stage.
9: Tracking illness duration is crucial
Research from ME/CFS shows that there may be an immune change in the first few years of the illness, where cytokines decrease without any corresponding change in symptom improvement.
Because of this and the possibility that other markers follow the same pattern, disease duration should be a core feature of all analyses and trial designs. Trial outcomes should be designed to answer the question of whether the medication helps patients at different durations of illness.
10: Prioritize patient populations less likely to recover without intervention
Some long COVID phenotypes seem less likely to recover without intervention. Trials should take care to focus on these patient populations, which include those with neurologic symptoms and those meeting ME/CFS criteria.
11: Account for the relapsing/remitting nature
Outcome measures need to be assessed in a way that can distinguish a temporary remission, which is part of the natural course of the disease, from a permanent cure.
Factors that can contribute to the relapsing/remitting nature include physical and cognitive postexertional malaise, menstrual cycle changes, and seasonal changes.
12: Trial participants should reflect the diversity of the long COVID population
Certain demographics are more likely to be affected by acute and long COVID and need to be appropriately recruited and reflected in research, including in patient engagement.
Trials must include high numbers of Hispanic/Latinx, Black, and indigenous communities, queer and transgender populations, and women. Trial materials and design need to incorporate linguistic diversity in addition to racial/ethnic diversity.
Upward of 75% of long COVID cases happen after mild acute cases; clinical researchers should ensure that nonhospitalized patients make up the bulk of trial participants.
13: Utilize meaningful engagement of patients, especially in treatment selection and study design
Meaningful patient engagement means engaging multiple patients at every step of the trial process, from treatment selection to study design to analysis to communication of the results.
Patient experiences are extremely valuable and contain information that researchers may not be familiar with, including the nature and patterns of the illness, insights into possible treatments, and barriers to documentation and care that may also impact research. Tapping into those patient experiences will make trials stronger.
Overall, the landscape of long COVID clinical trials is ripe for discovery, and researchers choosing to go down this path will be deeply appreciated by the patient community.
Hannah Davis is a long COVID patient-researcher and cofounder of the Patient-Led Research Collaborative, an organization studying the long-term effects of COVID.
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention and the U.S. Census Bureau estimate that 6.1% of the U.S. adult population is living with long COVID, with millions more debilitated worldwide. The demand for substantial treatment is enormous, but the urgency to fund and begin the necessary range of clinical trials has not met the severity of the problem.
While trials are slowly beginning to happen, the treatment choices and trial design require crucial nuances and understanding of viral-onset illnesses, and few research groups are creating strong trials that fully reflect the complexities of this landscape.
These recommendations recognize that roughly half of long COVID patients have new-onset myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and dysautonomia from COVID, which must be at the forefront of how trials are designed and conducted, and are additionally based on the current hypotheses about long COVID’s pathophysiologies.
1: Drugs proposed by experts in postviral fields should be prioritized
Upward of 50 drugs for viral-onset conditions like ME/CFS, dysautonomia, AIDS, and others have been waiting for years to go to trial, but have not had the funding to do so.
Treatments proposed by experts in viral-onset illnesses (such as ME/CFS and dysautonomia) should be prioritized (PM R. 2022 Oct;14[10]:1270-91), as outside researchers are not familiar with these fields and their potential treatment options.
2: Drugs targeting a wide range of mechanisms should be trialed
Treatments that should be trialed include anticoagulants/antiplatelets for clotting and vascular functioning, immunomodulators including JAK-STAT inhibitors, COVID-specific antivirals and antivirals against reactivated herpesviruses (Valcyte, Valacyclovir, EBV vaccine).
Other options include prescription mast cell stabilizers (ketotifen, cromolyn sodium), drugs that regulate microglial activation (low-dose naltrexone, low-dose aripiprazole), anti-CGRP medications, beta-blockers, and intravenous immunoglobulin.
Others include medications that target mitochondrial dysfunction; ivabradine; pyridostigmine;, DRP1 inhibitors; supplements showing success in patient communities including lactoferrin, ubiquinone, and nattokinase; and therapies targeting glymphatic/lymphatic dysfunction, microbiome therapies, and therapeutic peptides.
3: Use appropriate long COVID subtypes
Long COVID is an umbrella term that encompasses multiple new-onset and worsened conditions and symptoms after COVID. Roughly half of long COVID patients likely meet the criteria for ME/CFS and/or dysautonomia. Others may have new-onset diabetes, major clotting events, lung damage, neurological disorders, loss of smell or taste, and other manifestations.
Patients in different categories likely have different responses to treatments. It’s critical to identify appropriate subtypes for each trial, ideally performing detailed analyses to identify the treatments that work best, and don’t, for each subtype.
4: Behavioral treatments, especially those that have harmed similar populations, should not be trialed
Behavioral treatments including exercise, graded exercise therapy (GET), and cognitive-behavioral therapy (CBT) should not be trialed, let alone prioritized, for long COVID.
In patients with postexertional malaise (PEM), one of the most common long COVID symptoms, exercise is actively harmful and causes dysfunctional metabolic patterns, cardiac preload failure, impaired systemic oxygen extraction, and more. GET and CBT have failed similar populations , and exercise is explicitly contraindicated by the World Health Organization, the British National Institute for Health and Care Excellence, the CDC, and other organizations.
Resources should instead be put toward the wide range of medications that have not yet adequately undergone clinical trials.
5: PCR and antibody tests should not be used as inclusion criteria for trial participants
Only an estimated 1%-3% of cases in the first wave of COVID were documented, and the CDC estimates that only 25% of cases through September 2021 were documented. Similarly, antibody tests are unreliable to determine past infection, as roughly a third of patients don’t seroconvert, and a similar proportion serorevert within a few months. Using polymerase chain reaction (PCR) and antibody testing to determine who should be included in clinical trials limits who is eligible to participate in research, particularly those who have been ill for longer. Additionally, the majority of those who serorevert are women, so using antibody tests for inclusion introduces a selection bias and may miss mechanisms of immune system functioning that are part of long COVID.
PCR tests also have high false-negative rates and requiring them in research excludes people with lower viral loads with long COVID, which would confound findings.
These issues with testing also lead to COVID-infected people accidentally being included in control groups, which ruins the credibility of the research findings completely.
6: Include comparator groups
There are several common diagnoses that occur in people with long COVID, including ME/CFS, postural orthostatic tachycardia syndrome, small-fiber neuropathy, mast cell activation syndrome, and Ehlers-Danlos syndrome.
Identifying people with these conditions within the trial cohort improves research across all fields, benefiting all groups, and helps clarify what types of patients benefit most from certain medications.
7: Identify the right endpoints; avoid the wrong ones
Even though our understanding of the pathophysiology of long COVID is still evolving, it’s still possible to do clinical trials by identifying strong endpoints and outcome measures.
Several tools have been designed for viral-onset conditions and should be used alongside other endpoints. Postexertional malaise and autonomic symptoms, which are some of the most common symptoms of long COVID, can be measured with the validated DSQ-PEM and COMPASS-31, respectively. Tools for cognitive dysfunction trials should capture specific and common types of impairment, like processing speed.
Endpoints should be high-impact and aim for large improvements that have clinical significance over small improvements that do not have clinical significance.
Objective tests should be incorporated where possible; some to consider include natural killer cell functioning, cerebral blood flow, T-cell functioning, levels of reactivated herpesviruses, blood lactate levels, and microclots, as testing becomes available.
Mental health outcomes shouldn’t be primary endpoints, except where a trial is targeting a specific mental health condition because of COVID (for example, premenstrual dysphoric disorder).
If mental health conditions are tracked secondarily, it’s vital not to use questionnaires that include physical symptoms like fatigue, difficulty concentrating, difficulty sleeping, or palpitations, as these artificially increase depression and anxiety scores in chronically ill respondents. Tools that include physical symptoms (Patient Health Questionnaire–9, Beck Anxiety Inventory, Beck Depression Inventory) can be replaced with scales like the PHQ-2, General Anxiety Disorder–7, Hospital Anxiety and Depression Scale, or PROMIS-29 subscales.
Because certain cytokines and other inflammatory markers may naturally decrease over time without corresponding improvement in the ME/CFS subtype, caution should be taken when using cytokines as endpoints.
8: Consider enrollment and objectives carefully
A proportion of people with long COVID will recover in the early months after infection. Ideally, clinical trials will primarily study treatments in patients who have been ill 6 months or longer, as some natural recovery will happen before that can bias studies.
But where resources are abundant, it is ideal for trials to additionally look at whether the treatments can help patients in the early months recover and prevent progression to the later stage.
9: Tracking illness duration is crucial
Research from ME/CFS shows that there may be an immune change in the first few years of the illness, where cytokines decrease without any corresponding change in symptom improvement.
Because of this and the possibility that other markers follow the same pattern, disease duration should be a core feature of all analyses and trial designs. Trial outcomes should be designed to answer the question of whether the medication helps patients at different durations of illness.
10: Prioritize patient populations less likely to recover without intervention
Some long COVID phenotypes seem less likely to recover without intervention. Trials should take care to focus on these patient populations, which include those with neurologic symptoms and those meeting ME/CFS criteria.
11: Account for the relapsing/remitting nature
Outcome measures need to be assessed in a way that can distinguish a temporary remission, which is part of the natural course of the disease, from a permanent cure.
Factors that can contribute to the relapsing/remitting nature include physical and cognitive postexertional malaise, menstrual cycle changes, and seasonal changes.
12: Trial participants should reflect the diversity of the long COVID population
Certain demographics are more likely to be affected by acute and long COVID and need to be appropriately recruited and reflected in research, including in patient engagement.
Trials must include high numbers of Hispanic/Latinx, Black, and indigenous communities, queer and transgender populations, and women. Trial materials and design need to incorporate linguistic diversity in addition to racial/ethnic diversity.
Upward of 75% of long COVID cases happen after mild acute cases; clinical researchers should ensure that nonhospitalized patients make up the bulk of trial participants.
13: Utilize meaningful engagement of patients, especially in treatment selection and study design
Meaningful patient engagement means engaging multiple patients at every step of the trial process, from treatment selection to study design to analysis to communication of the results.
Patient experiences are extremely valuable and contain information that researchers may not be familiar with, including the nature and patterns of the illness, insights into possible treatments, and barriers to documentation and care that may also impact research. Tapping into those patient experiences will make trials stronger.
Overall, the landscape of long COVID clinical trials is ripe for discovery, and researchers choosing to go down this path will be deeply appreciated by the patient community.
Hannah Davis is a long COVID patient-researcher and cofounder of the Patient-Led Research Collaborative, an organization studying the long-term effects of COVID.
A version of this article first appeared on Medscape.com.
Colchicine’s 2010 price spike had major impact on gout care
A large price increase for colchicine in 2010 led to a significant falloff in its use for gout that persisted for the next decade while emergency and rheumatology visits for gout rose, suggesting poorer disease control, a retrospective cohort study reported.
The price of colchicine, commonly prescribed for acute gout attacks, climbed from $11.25 per prescription in 2009 to $190.49 in 2011, with the average out-of-pocket cost more than quadrupling, from $7.37 to $29.42, the study noted. Colchicine prescriptions for gout declined 27% over the next decade, according to adjusted analyses that the study authors performed.
“A roughly 16-fold increase in colchicine prices appeared to have lowered colchicine use over the next decade,” senior author Zirui Song, MD, PhD, an associate professor of health care policy and medicine at Harvard Medical School and an internist at Massachusetts General Hospital in Boston, told this news organization in written comments. “Over the same period, patients with gout used more of other medications that could treat gout. They also had more emergency department visits for gout and rheumatologist visits for gout, which potentially signals poorer disease control.”
The study, published online in JAMA Internal Medicine, examined MarketScan data from a longitudinal cohort of patients who had employer-sponsored health insurance and a diagnosis of gout from 2007 to 2019. MarketScan is an IBM database of medical and drug data from employers and health plans. The study examined more than 2.7 million patient-year observations over the 13-year period.
How the price increase happened
After 2011, a large percentage of patients shifted to less effective but more affordable drugs to treat gout. Prescriptions for allopurinol increased 32% (P < .001) and oral corticosteroids 8.3% over the decade. “These are imperfect substitutes,” Dr. Song said. “Allopurinol is used to prevent gout, while oral corticosteroids can be used to treat a gout flare.”
At the same time, visits for gout-related complaints to emergency departments and rheumatology offices increased through the ensuing years: 39.8% and 10.5% on an adjusted analysis, respectively (P < .001 for both).
Colchicine is actually a drug that predates the creation of the U.S. Food and Drug Administration in 1938 and had been grandfathered under its Unapproved Drug Initiative. Then in 2009, the FDA determined that colchicine was effective for treating arthritis-related gout flares after the manufacturer, URL Pharma, presented results of a randomized, controlled trial of 185 patients with gout.
The next year, the FDA granted URL Pharma 3 years of market exclusivity for the drug under the brand name Colcrys, now trademarked by Takeda Pharmaceuticals.
The latest study noted that longer-term analysis of the impact of the FDA’s decision had been lacking. The goal, said Dr. Song, was “to better understand the long-run implications of large drug price increases in the U.S. by studying the case of colchicine.”
He added, “For drugs that lack competition, large price increases can have large economic and clinical consequences over many years.”
Absorbing the cost
Lead author Dan P. Ly, MD, PhD, MPP, assistant professor at the University of California, Los Angeles, added, “Our study has large implications [for] when generic medications or other medications experience large price increases. Use of the medication in question drops or patients have to pay more out of pocket, and patient health can suffer as a result.”
The dropoff in colchicine use in this patient population could have been worse, Dr. Song said. “Despite colchicine use decreasing by 27% over nearly a decade, the fact that it did not decline more suggests that for patients with gout, the large price increase was mostly absorbed by their insurers, employers, or themselves – e.g., passed through to higher premiums, lower wages, or higher cost-sharing.”
Aaron Kesselheim, MD, JD, MPH, a professor at Harvard Medical School, Boston, reported previously on the price consequences of colchicine early on after the FDA granted the manufacturer market exclusivity.
“In our past research, we looked at how the massive increase in the price of colchicine increased spending on the drug and reduced use in a relatively short time period after the price hike,” said Dr. Kesselheim, who was not involved in this current study by Dr. Ly, Dr. Song, and Mia Giuriato, BBA, MA, from Harvard Medical School. “This study evaluated the experiences of patients with gout over multiple years and showed that the reductions in use persisted and were associated with increases in ED and rheumatology visits, suggesting worsening control of gout due to the relative inaccessibility of the drug at the new high price.”
The latest findings have public policy implications, Dr. Kesselheim said. “In the case of colchicine, the FDA made a bad pitch, leading to a home run for the manufacturer and a shutout for patients.”
“The FDA needs to make sure to take into account the quite predictable patient effects that can result from disruptions to competition when it considers taking steps like it did in the colchicine case to disrupt the market and create an artificial monopoly, even if the FDA acted in the best of intentions in this case,” Dr. Kesselheim added.
Dr. Song received funding for the study from the National Institutes of Health and Arnold Ventures. He also disclosed receiving personal fees from the Research Triangle Institute, Google Ventures, VBID Health, and the International Foundation of Employee Benefit Plans. Dr. Ly, Ms. Giuriato, and Dr. Kesselheim report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A large price increase for colchicine in 2010 led to a significant falloff in its use for gout that persisted for the next decade while emergency and rheumatology visits for gout rose, suggesting poorer disease control, a retrospective cohort study reported.
The price of colchicine, commonly prescribed for acute gout attacks, climbed from $11.25 per prescription in 2009 to $190.49 in 2011, with the average out-of-pocket cost more than quadrupling, from $7.37 to $29.42, the study noted. Colchicine prescriptions for gout declined 27% over the next decade, according to adjusted analyses that the study authors performed.
“A roughly 16-fold increase in colchicine prices appeared to have lowered colchicine use over the next decade,” senior author Zirui Song, MD, PhD, an associate professor of health care policy and medicine at Harvard Medical School and an internist at Massachusetts General Hospital in Boston, told this news organization in written comments. “Over the same period, patients with gout used more of other medications that could treat gout. They also had more emergency department visits for gout and rheumatologist visits for gout, which potentially signals poorer disease control.”
The study, published online in JAMA Internal Medicine, examined MarketScan data from a longitudinal cohort of patients who had employer-sponsored health insurance and a diagnosis of gout from 2007 to 2019. MarketScan is an IBM database of medical and drug data from employers and health plans. The study examined more than 2.7 million patient-year observations over the 13-year period.
How the price increase happened
After 2011, a large percentage of patients shifted to less effective but more affordable drugs to treat gout. Prescriptions for allopurinol increased 32% (P < .001) and oral corticosteroids 8.3% over the decade. “These are imperfect substitutes,” Dr. Song said. “Allopurinol is used to prevent gout, while oral corticosteroids can be used to treat a gout flare.”
At the same time, visits for gout-related complaints to emergency departments and rheumatology offices increased through the ensuing years: 39.8% and 10.5% on an adjusted analysis, respectively (P < .001 for both).
Colchicine is actually a drug that predates the creation of the U.S. Food and Drug Administration in 1938 and had been grandfathered under its Unapproved Drug Initiative. Then in 2009, the FDA determined that colchicine was effective for treating arthritis-related gout flares after the manufacturer, URL Pharma, presented results of a randomized, controlled trial of 185 patients with gout.
The next year, the FDA granted URL Pharma 3 years of market exclusivity for the drug under the brand name Colcrys, now trademarked by Takeda Pharmaceuticals.
The latest study noted that longer-term analysis of the impact of the FDA’s decision had been lacking. The goal, said Dr. Song, was “to better understand the long-run implications of large drug price increases in the U.S. by studying the case of colchicine.”
He added, “For drugs that lack competition, large price increases can have large economic and clinical consequences over many years.”
Absorbing the cost
Lead author Dan P. Ly, MD, PhD, MPP, assistant professor at the University of California, Los Angeles, added, “Our study has large implications [for] when generic medications or other medications experience large price increases. Use of the medication in question drops or patients have to pay more out of pocket, and patient health can suffer as a result.”
The dropoff in colchicine use in this patient population could have been worse, Dr. Song said. “Despite colchicine use decreasing by 27% over nearly a decade, the fact that it did not decline more suggests that for patients with gout, the large price increase was mostly absorbed by their insurers, employers, or themselves – e.g., passed through to higher premiums, lower wages, or higher cost-sharing.”
Aaron Kesselheim, MD, JD, MPH, a professor at Harvard Medical School, Boston, reported previously on the price consequences of colchicine early on after the FDA granted the manufacturer market exclusivity.
“In our past research, we looked at how the massive increase in the price of colchicine increased spending on the drug and reduced use in a relatively short time period after the price hike,” said Dr. Kesselheim, who was not involved in this current study by Dr. Ly, Dr. Song, and Mia Giuriato, BBA, MA, from Harvard Medical School. “This study evaluated the experiences of patients with gout over multiple years and showed that the reductions in use persisted and were associated with increases in ED and rheumatology visits, suggesting worsening control of gout due to the relative inaccessibility of the drug at the new high price.”
The latest findings have public policy implications, Dr. Kesselheim said. “In the case of colchicine, the FDA made a bad pitch, leading to a home run for the manufacturer and a shutout for patients.”
“The FDA needs to make sure to take into account the quite predictable patient effects that can result from disruptions to competition when it considers taking steps like it did in the colchicine case to disrupt the market and create an artificial monopoly, even if the FDA acted in the best of intentions in this case,” Dr. Kesselheim added.
Dr. Song received funding for the study from the National Institutes of Health and Arnold Ventures. He also disclosed receiving personal fees from the Research Triangle Institute, Google Ventures, VBID Health, and the International Foundation of Employee Benefit Plans. Dr. Ly, Ms. Giuriato, and Dr. Kesselheim report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A large price increase for colchicine in 2010 led to a significant falloff in its use for gout that persisted for the next decade while emergency and rheumatology visits for gout rose, suggesting poorer disease control, a retrospective cohort study reported.
The price of colchicine, commonly prescribed for acute gout attacks, climbed from $11.25 per prescription in 2009 to $190.49 in 2011, with the average out-of-pocket cost more than quadrupling, from $7.37 to $29.42, the study noted. Colchicine prescriptions for gout declined 27% over the next decade, according to adjusted analyses that the study authors performed.
“A roughly 16-fold increase in colchicine prices appeared to have lowered colchicine use over the next decade,” senior author Zirui Song, MD, PhD, an associate professor of health care policy and medicine at Harvard Medical School and an internist at Massachusetts General Hospital in Boston, told this news organization in written comments. “Over the same period, patients with gout used more of other medications that could treat gout. They also had more emergency department visits for gout and rheumatologist visits for gout, which potentially signals poorer disease control.”
The study, published online in JAMA Internal Medicine, examined MarketScan data from a longitudinal cohort of patients who had employer-sponsored health insurance and a diagnosis of gout from 2007 to 2019. MarketScan is an IBM database of medical and drug data from employers and health plans. The study examined more than 2.7 million patient-year observations over the 13-year period.
How the price increase happened
After 2011, a large percentage of patients shifted to less effective but more affordable drugs to treat gout. Prescriptions for allopurinol increased 32% (P < .001) and oral corticosteroids 8.3% over the decade. “These are imperfect substitutes,” Dr. Song said. “Allopurinol is used to prevent gout, while oral corticosteroids can be used to treat a gout flare.”
At the same time, visits for gout-related complaints to emergency departments and rheumatology offices increased through the ensuing years: 39.8% and 10.5% on an adjusted analysis, respectively (P < .001 for both).
Colchicine is actually a drug that predates the creation of the U.S. Food and Drug Administration in 1938 and had been grandfathered under its Unapproved Drug Initiative. Then in 2009, the FDA determined that colchicine was effective for treating arthritis-related gout flares after the manufacturer, URL Pharma, presented results of a randomized, controlled trial of 185 patients with gout.
The next year, the FDA granted URL Pharma 3 years of market exclusivity for the drug under the brand name Colcrys, now trademarked by Takeda Pharmaceuticals.
The latest study noted that longer-term analysis of the impact of the FDA’s decision had been lacking. The goal, said Dr. Song, was “to better understand the long-run implications of large drug price increases in the U.S. by studying the case of colchicine.”
He added, “For drugs that lack competition, large price increases can have large economic and clinical consequences over many years.”
Absorbing the cost
Lead author Dan P. Ly, MD, PhD, MPP, assistant professor at the University of California, Los Angeles, added, “Our study has large implications [for] when generic medications or other medications experience large price increases. Use of the medication in question drops or patients have to pay more out of pocket, and patient health can suffer as a result.”
The dropoff in colchicine use in this patient population could have been worse, Dr. Song said. “Despite colchicine use decreasing by 27% over nearly a decade, the fact that it did not decline more suggests that for patients with gout, the large price increase was mostly absorbed by their insurers, employers, or themselves – e.g., passed through to higher premiums, lower wages, or higher cost-sharing.”
Aaron Kesselheim, MD, JD, MPH, a professor at Harvard Medical School, Boston, reported previously on the price consequences of colchicine early on after the FDA granted the manufacturer market exclusivity.
“In our past research, we looked at how the massive increase in the price of colchicine increased spending on the drug and reduced use in a relatively short time period after the price hike,” said Dr. Kesselheim, who was not involved in this current study by Dr. Ly, Dr. Song, and Mia Giuriato, BBA, MA, from Harvard Medical School. “This study evaluated the experiences of patients with gout over multiple years and showed that the reductions in use persisted and were associated with increases in ED and rheumatology visits, suggesting worsening control of gout due to the relative inaccessibility of the drug at the new high price.”
The latest findings have public policy implications, Dr. Kesselheim said. “In the case of colchicine, the FDA made a bad pitch, leading to a home run for the manufacturer and a shutout for patients.”
“The FDA needs to make sure to take into account the quite predictable patient effects that can result from disruptions to competition when it considers taking steps like it did in the colchicine case to disrupt the market and create an artificial monopoly, even if the FDA acted in the best of intentions in this case,” Dr. Kesselheim added.
Dr. Song received funding for the study from the National Institutes of Health and Arnold Ventures. He also disclosed receiving personal fees from the Research Triangle Institute, Google Ventures, VBID Health, and the International Foundation of Employee Benefit Plans. Dr. Ly, Ms. Giuriato, and Dr. Kesselheim report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Study of hospitalizations in Canada quantifies benefit of COVID-19 vaccine to reduce death, ICU admissions
A cohort study of more than 1.5 million hospital admissions in Canada through the first 2 years of the COVID-19 pandemic has quantified the benefit of vaccinations. Unvaccinated patients were found to be up to 15 times more likely to die from COVID-19 than fully vaccinated patients.
Investigators analyzed 1.513 million admissions at 155 hospitals across Canada from March 15, 2020, to May 28, 2022. The study included 51,679 adult admissions and 4,035 pediatric admissions for COVID-19. Although the share of COVID-19 admissions increased in the fifth and sixth waves, from Dec. 26, 2021, to March 19, 2022 – after the full vaccine rollout – to 7.73% from 2.47% in the previous four waves, the proportion of adults admitted to the intensive care unit was significantly lower, at 8.7% versus 21.8% (odds ratio, 0.35; 95% confidence interval, 0.32-0.36).
“The good thing about waves five and six was we were able to show the COVID cases tended to be less severe, but on the other hand, because the disease in the community was so much higher, the demands on the health care system were much higher than the previous waves,” study author Charles Frenette, MD, director of infection prevention and control at McGill University, Montreal, and chair of the study’s adult subgroup, said in an interview. “But here we were able to show the benefit of vaccinations, particularly the boosting dose, in protecting against those severe outcomes.”
The study, published in JAMA Network Open, used the Canadian Nosocomial Infection Surveillance Program database, which collects hospital data across Canada. It was activated in March 2020 to collect details on all COVID-19 admissions, co-author Nisha Thampi, MD, chair of the study’s pediatric subgroup, told this news organization.
“We’re now over 3 years into the pandemic, and CNISP continues to monitor COVID-19 as well as other pathogens in near real time,” said Dr. Thampi, an associate professor and infectious disease specialist at Children’s Hospital of Eastern Ontario.
“That’s a particular strength of this surveillance program as well. We would see this data on a biweekly basis, and that allows for [us] to implement timely protection and action.”
Tracing trends over six waves
The study tracked COVID-19 hospitalizations during six waves. The first lasted from March 15 to August 31, 2020, and the second lasted from Sept. 1, 2020, to Feb. 28, 2021. The wild-type variant was dominant during both waves. The third wave lasted from March 1 to June 30, 2021, and was marked by the mixed Alpha, Beta, and Gamma variants. The fourth wave lasted from July 1 to Dec. 25, 2021, when the Alpha variant was dominant. The Omicron variant dominated during waves five (Dec. 26, 2021, to March 19, 2022) and six (March 20 to May 28, 2022).
Hospitalizations reached a peak of 14,461 in wave five. ICU admissions, however, peaked at 2,164 during wave four, and all-cause deaths peaked at 1,663 during wave two.
The investigators also analyzed how unvaccinated patients fared, compared with the fully vaccinated and the fully vaccinated-plus (that is, patients with one or more additional doses). During waves five and six, unvaccinated patients were 4.3 times more likely to end up in the ICU than fully vaccinated patients and were 12.2 times more likely than fully vaccinated-plus patients. Likewise, the rate for all-cause in-hospital death for unvaccinated patients was 3.9 times greater than that for fully vaccinated patients and 15.1 times greater than that for fully vaccinated-plus patients.
The effect of vaccines emerged in waves three and four, said Dr. Frenette. “We started to see really, really significant protection and benefit from the vaccine, not only in incidence of admission but also in the incidence of complications of ICU care, ventilation, and mortality.”
Results for pediatric patients were similar to those for adults, Dr. Thampi noted. During waves five and six, overall admissions peaked, but the share of ICU admissions decreased to 9.4% from 18.1%, which was the rate during the previous four waves (OR, 0.47).
“What’s important is how pediatric hospitalizations changed over the course of the various waves,” said Dr. Thampi.
“Where we saw the highest admissions during the early Omicron dominance, we actually had the lowest numbers of hospitalizations with death and admissions into ICUs.”
Doing more with the data
David Fisman, MD, MPH, a professor of epidemiology at the University of Toronto, said, “This is a study that shows us how tremendously dramatic the effects of the COVID-19 vaccine were in terms of saving lives during the pandemic.” Dr. Fisman was not involved in the study.
But CNISP, which receives funding from Public Health Agency of Canada, could do more with the data it collects to better protect the public from COVID-19 and other nosocomial infections, Dr. Fisman said.
“The first problematic thing about this paper is that Canadians are paying for a surveillance system that looks at risks of acquiring infections, including COVID-19 infections, in the hospital, but that data is not fed back to the people paying for its production,” he said.
“So, Canadians don’t have the ability to really understand in real time how much risk they’re experiencing via going to the hospital for some other reason.”
The study was independently supported. Dr. Frenette and Dr. Thampi report no relevant financial relationships. Dr. Fisman has disclosed financial relationships with Pfizer, AstraZeneca, Sanofi, Seqirus, Merck, the Ontario Nurses Association, and the Elementary Teachers’ Federation of Ontario.
A version of this article first appeared on Medscape.com.
A cohort study of more than 1.5 million hospital admissions in Canada through the first 2 years of the COVID-19 pandemic has quantified the benefit of vaccinations. Unvaccinated patients were found to be up to 15 times more likely to die from COVID-19 than fully vaccinated patients.
Investigators analyzed 1.513 million admissions at 155 hospitals across Canada from March 15, 2020, to May 28, 2022. The study included 51,679 adult admissions and 4,035 pediatric admissions for COVID-19. Although the share of COVID-19 admissions increased in the fifth and sixth waves, from Dec. 26, 2021, to March 19, 2022 – after the full vaccine rollout – to 7.73% from 2.47% in the previous four waves, the proportion of adults admitted to the intensive care unit was significantly lower, at 8.7% versus 21.8% (odds ratio, 0.35; 95% confidence interval, 0.32-0.36).
“The good thing about waves five and six was we were able to show the COVID cases tended to be less severe, but on the other hand, because the disease in the community was so much higher, the demands on the health care system were much higher than the previous waves,” study author Charles Frenette, MD, director of infection prevention and control at McGill University, Montreal, and chair of the study’s adult subgroup, said in an interview. “But here we were able to show the benefit of vaccinations, particularly the boosting dose, in protecting against those severe outcomes.”
The study, published in JAMA Network Open, used the Canadian Nosocomial Infection Surveillance Program database, which collects hospital data across Canada. It was activated in March 2020 to collect details on all COVID-19 admissions, co-author Nisha Thampi, MD, chair of the study’s pediatric subgroup, told this news organization.
“We’re now over 3 years into the pandemic, and CNISP continues to monitor COVID-19 as well as other pathogens in near real time,” said Dr. Thampi, an associate professor and infectious disease specialist at Children’s Hospital of Eastern Ontario.
“That’s a particular strength of this surveillance program as well. We would see this data on a biweekly basis, and that allows for [us] to implement timely protection and action.”
Tracing trends over six waves
The study tracked COVID-19 hospitalizations during six waves. The first lasted from March 15 to August 31, 2020, and the second lasted from Sept. 1, 2020, to Feb. 28, 2021. The wild-type variant was dominant during both waves. The third wave lasted from March 1 to June 30, 2021, and was marked by the mixed Alpha, Beta, and Gamma variants. The fourth wave lasted from July 1 to Dec. 25, 2021, when the Alpha variant was dominant. The Omicron variant dominated during waves five (Dec. 26, 2021, to March 19, 2022) and six (March 20 to May 28, 2022).
Hospitalizations reached a peak of 14,461 in wave five. ICU admissions, however, peaked at 2,164 during wave four, and all-cause deaths peaked at 1,663 during wave two.
The investigators also analyzed how unvaccinated patients fared, compared with the fully vaccinated and the fully vaccinated-plus (that is, patients with one or more additional doses). During waves five and six, unvaccinated patients were 4.3 times more likely to end up in the ICU than fully vaccinated patients and were 12.2 times more likely than fully vaccinated-plus patients. Likewise, the rate for all-cause in-hospital death for unvaccinated patients was 3.9 times greater than that for fully vaccinated patients and 15.1 times greater than that for fully vaccinated-plus patients.
The effect of vaccines emerged in waves three and four, said Dr. Frenette. “We started to see really, really significant protection and benefit from the vaccine, not only in incidence of admission but also in the incidence of complications of ICU care, ventilation, and mortality.”
Results for pediatric patients were similar to those for adults, Dr. Thampi noted. During waves five and six, overall admissions peaked, but the share of ICU admissions decreased to 9.4% from 18.1%, which was the rate during the previous four waves (OR, 0.47).
“What’s important is how pediatric hospitalizations changed over the course of the various waves,” said Dr. Thampi.
“Where we saw the highest admissions during the early Omicron dominance, we actually had the lowest numbers of hospitalizations with death and admissions into ICUs.”
Doing more with the data
David Fisman, MD, MPH, a professor of epidemiology at the University of Toronto, said, “This is a study that shows us how tremendously dramatic the effects of the COVID-19 vaccine were in terms of saving lives during the pandemic.” Dr. Fisman was not involved in the study.
But CNISP, which receives funding from Public Health Agency of Canada, could do more with the data it collects to better protect the public from COVID-19 and other nosocomial infections, Dr. Fisman said.
“The first problematic thing about this paper is that Canadians are paying for a surveillance system that looks at risks of acquiring infections, including COVID-19 infections, in the hospital, but that data is not fed back to the people paying for its production,” he said.
“So, Canadians don’t have the ability to really understand in real time how much risk they’re experiencing via going to the hospital for some other reason.”
The study was independently supported. Dr. Frenette and Dr. Thampi report no relevant financial relationships. Dr. Fisman has disclosed financial relationships with Pfizer, AstraZeneca, Sanofi, Seqirus, Merck, the Ontario Nurses Association, and the Elementary Teachers’ Federation of Ontario.
A version of this article first appeared on Medscape.com.
A cohort study of more than 1.5 million hospital admissions in Canada through the first 2 years of the COVID-19 pandemic has quantified the benefit of vaccinations. Unvaccinated patients were found to be up to 15 times more likely to die from COVID-19 than fully vaccinated patients.
Investigators analyzed 1.513 million admissions at 155 hospitals across Canada from March 15, 2020, to May 28, 2022. The study included 51,679 adult admissions and 4,035 pediatric admissions for COVID-19. Although the share of COVID-19 admissions increased in the fifth and sixth waves, from Dec. 26, 2021, to March 19, 2022 – after the full vaccine rollout – to 7.73% from 2.47% in the previous four waves, the proportion of adults admitted to the intensive care unit was significantly lower, at 8.7% versus 21.8% (odds ratio, 0.35; 95% confidence interval, 0.32-0.36).
“The good thing about waves five and six was we were able to show the COVID cases tended to be less severe, but on the other hand, because the disease in the community was so much higher, the demands on the health care system were much higher than the previous waves,” study author Charles Frenette, MD, director of infection prevention and control at McGill University, Montreal, and chair of the study’s adult subgroup, said in an interview. “But here we were able to show the benefit of vaccinations, particularly the boosting dose, in protecting against those severe outcomes.”
The study, published in JAMA Network Open, used the Canadian Nosocomial Infection Surveillance Program database, which collects hospital data across Canada. It was activated in March 2020 to collect details on all COVID-19 admissions, co-author Nisha Thampi, MD, chair of the study’s pediatric subgroup, told this news organization.
“We’re now over 3 years into the pandemic, and CNISP continues to monitor COVID-19 as well as other pathogens in near real time,” said Dr. Thampi, an associate professor and infectious disease specialist at Children’s Hospital of Eastern Ontario.
“That’s a particular strength of this surveillance program as well. We would see this data on a biweekly basis, and that allows for [us] to implement timely protection and action.”
Tracing trends over six waves
The study tracked COVID-19 hospitalizations during six waves. The first lasted from March 15 to August 31, 2020, and the second lasted from Sept. 1, 2020, to Feb. 28, 2021. The wild-type variant was dominant during both waves. The third wave lasted from March 1 to June 30, 2021, and was marked by the mixed Alpha, Beta, and Gamma variants. The fourth wave lasted from July 1 to Dec. 25, 2021, when the Alpha variant was dominant. The Omicron variant dominated during waves five (Dec. 26, 2021, to March 19, 2022) and six (March 20 to May 28, 2022).
Hospitalizations reached a peak of 14,461 in wave five. ICU admissions, however, peaked at 2,164 during wave four, and all-cause deaths peaked at 1,663 during wave two.
The investigators also analyzed how unvaccinated patients fared, compared with the fully vaccinated and the fully vaccinated-plus (that is, patients with one or more additional doses). During waves five and six, unvaccinated patients were 4.3 times more likely to end up in the ICU than fully vaccinated patients and were 12.2 times more likely than fully vaccinated-plus patients. Likewise, the rate for all-cause in-hospital death for unvaccinated patients was 3.9 times greater than that for fully vaccinated patients and 15.1 times greater than that for fully vaccinated-plus patients.
The effect of vaccines emerged in waves three and four, said Dr. Frenette. “We started to see really, really significant protection and benefit from the vaccine, not only in incidence of admission but also in the incidence of complications of ICU care, ventilation, and mortality.”
Results for pediatric patients were similar to those for adults, Dr. Thampi noted. During waves five and six, overall admissions peaked, but the share of ICU admissions decreased to 9.4% from 18.1%, which was the rate during the previous four waves (OR, 0.47).
“What’s important is how pediatric hospitalizations changed over the course of the various waves,” said Dr. Thampi.
“Where we saw the highest admissions during the early Omicron dominance, we actually had the lowest numbers of hospitalizations with death and admissions into ICUs.”
Doing more with the data
David Fisman, MD, MPH, a professor of epidemiology at the University of Toronto, said, “This is a study that shows us how tremendously dramatic the effects of the COVID-19 vaccine were in terms of saving lives during the pandemic.” Dr. Fisman was not involved in the study.
But CNISP, which receives funding from Public Health Agency of Canada, could do more with the data it collects to better protect the public from COVID-19 and other nosocomial infections, Dr. Fisman said.
“The first problematic thing about this paper is that Canadians are paying for a surveillance system that looks at risks of acquiring infections, including COVID-19 infections, in the hospital, but that data is not fed back to the people paying for its production,” he said.
“So, Canadians don’t have the ability to really understand in real time how much risk they’re experiencing via going to the hospital for some other reason.”
The study was independently supported. Dr. Frenette and Dr. Thampi report no relevant financial relationships. Dr. Fisman has disclosed financial relationships with Pfizer, AstraZeneca, Sanofi, Seqirus, Merck, the Ontario Nurses Association, and the Elementary Teachers’ Federation of Ontario.
A version of this article first appeared on Medscape.com.
FDA expands use of dapagliflozin to broader range of HF
– including HF with mildly reduced ejection fraction (HFmrEF) and with preserved ejection fraction (HFpEF).
The sodium-glucose cotransporter 2 (SGLT2) inhibitor was previously approved in the United States for adults with heart failure with reduced ejection fraction (HFrEF).
The expanded indication is based on data from the phase 3 DELIVER trial, which showed clear clinical benefits of the SGLT2 inhibitor for patients with HF regardless of left ventricular function.
In the trial, which included more than 6,200 patients, dapagliflozin led to a statistically significant and clinically meaningful early reduction in the primary composite endpoint of cardiovascular (CV) death or worsening HF for patients with HFmrEF or HFpEFF.
In addition, results of a pooled analysis of the DAPA-HF and DELIVER phase 3 trials showed a consistent benefit from dapagliflozin treatment in significantly reducing the combined endpoint of CV death or HF hospitalization across the range of LVEF.
The European Commission expanded the indication for dapagliflozin (Forxiga) to include HF across the full spectrum of LVEF in February.
The SGLT2 inhibitor is also approved for use by patients with chronic kidney disease. It was first approved in 2014 to improve glycemic control for patients with diabetes mellitus.
A version of this article first appeared on Medscape.com.
– including HF with mildly reduced ejection fraction (HFmrEF) and with preserved ejection fraction (HFpEF).
The sodium-glucose cotransporter 2 (SGLT2) inhibitor was previously approved in the United States for adults with heart failure with reduced ejection fraction (HFrEF).
The expanded indication is based on data from the phase 3 DELIVER trial, which showed clear clinical benefits of the SGLT2 inhibitor for patients with HF regardless of left ventricular function.
In the trial, which included more than 6,200 patients, dapagliflozin led to a statistically significant and clinically meaningful early reduction in the primary composite endpoint of cardiovascular (CV) death or worsening HF for patients with HFmrEF or HFpEFF.
In addition, results of a pooled analysis of the DAPA-HF and DELIVER phase 3 trials showed a consistent benefit from dapagliflozin treatment in significantly reducing the combined endpoint of CV death or HF hospitalization across the range of LVEF.
The European Commission expanded the indication for dapagliflozin (Forxiga) to include HF across the full spectrum of LVEF in February.
The SGLT2 inhibitor is also approved for use by patients with chronic kidney disease. It was first approved in 2014 to improve glycemic control for patients with diabetes mellitus.
A version of this article first appeared on Medscape.com.
– including HF with mildly reduced ejection fraction (HFmrEF) and with preserved ejection fraction (HFpEF).
The sodium-glucose cotransporter 2 (SGLT2) inhibitor was previously approved in the United States for adults with heart failure with reduced ejection fraction (HFrEF).
The expanded indication is based on data from the phase 3 DELIVER trial, which showed clear clinical benefits of the SGLT2 inhibitor for patients with HF regardless of left ventricular function.
In the trial, which included more than 6,200 patients, dapagliflozin led to a statistically significant and clinically meaningful early reduction in the primary composite endpoint of cardiovascular (CV) death or worsening HF for patients with HFmrEF or HFpEFF.
In addition, results of a pooled analysis of the DAPA-HF and DELIVER phase 3 trials showed a consistent benefit from dapagliflozin treatment in significantly reducing the combined endpoint of CV death or HF hospitalization across the range of LVEF.
The European Commission expanded the indication for dapagliflozin (Forxiga) to include HF across the full spectrum of LVEF in February.
The SGLT2 inhibitor is also approved for use by patients with chronic kidney disease. It was first approved in 2014 to improve glycemic control for patients with diabetes mellitus.
A version of this article first appeared on Medscape.com.
The 30th-birthday gift that could save a life
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
Milestone birthdays are always memorable – those ages when your life seems to fundamentally change somehow. Age 16: A license to drive. Age 18: You can vote to determine your own future and serve in the military. At 21, 3 years after adulthood, you are finally allowed to drink alcohol, for some reason. And then ... nothing much happens. At least until you turn 65 and become eligible for Medicare.
But imagine a future when turning 30 might be the biggest milestone birthday of all. Imagine a future when, at 30, you get your genome sequenced and doctors tell you what needs to be done to save your life.
That future may not be far off, as a new study shows us that
Getting your genome sequenced is a double-edged sword. Of course, there is the potential for substantial benefit; finding certain mutations allows for definitive therapy before it’s too late. That said, there are genetic diseases without a cure and without a treatment. Knowing about that destiny may do more harm than good.
Three conditions are described by the CDC as “Tier 1” conditions, genetic syndromes with a significant impact on life expectancy that also have definitive, effective therapies.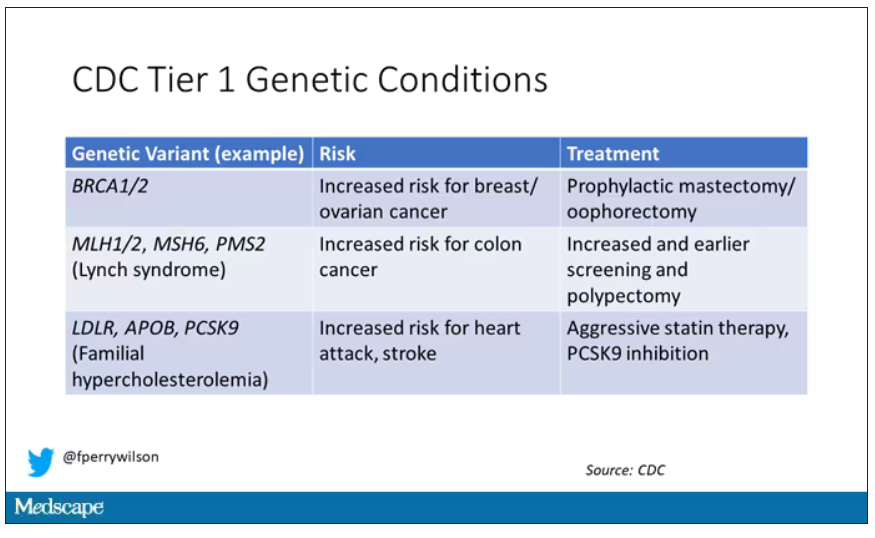
These include mutations like BRCA1/2, associated with a high risk for breast and ovarian cancer; mutations associated with Lynch syndrome, which confer an elevated risk for colon cancer; and mutations associated with familial hypercholesterolemia, which confer elevated risk for cardiovascular events.
In each of these cases, there is clear evidence that early intervention can save lives. Individuals at high risk for breast and ovarian cancer can get prophylactic mastectomy and salpingo-oophorectomy. Those with Lynch syndrome can get more frequent screening for colon cancer and polypectomy, and those with familial hypercholesterolemia can get aggressive lipid-lowering therapy.
I think most of us would probably want to know if we had one of these conditions. Most of us would use that information to take concrete steps to decrease our risk. But just because a rational person would choose to do something doesn’t mean it’s feasible. After all, we’re talking about tests and treatments that have significant costs.
In a recent issue of Annals of Internal Medicine, Josh Peterson and David Veenstra present a detailed accounting of the cost and benefit of a hypothetical nationwide, universal screening program for Tier 1 conditions. And in the end, it may actually be worth it.
Cost-benefit analyses work by comparing two independent policy choices: the status quo – in this case, a world in which some people get tested for these conditions, but generally only if they are at high risk based on strong family history; and an alternative policy – in this case, universal screening for these conditions starting at some age.
After that, it’s time to play the assumption game. Using the best available data, the authors estimated the percentage of the population that will have each condition, the percentage of those individuals who will definitively act on the information, and how effective those actions would be if taken.
The authors provide an example. First, they assume that the prevalence of mutations leading to a high risk for breast and ovarian cancer is around 0.7%, and that up to 40% of people who learn that they have one of these mutations would undergo prophylactic mastectomy, which would reduce the risk for breast cancer by around 94%. (I ran these numbers past my wife, a breast surgical oncologist, who agreed that they seem reasonable.)
Assumptions in place, it’s time to consider costs. The cost of the screening test itself: The authors use $250 as their average per-person cost. But we also have the cost of treatment – around $22,000 per person for a bilateral prophylactic mastectomy; the cost of statin therapy for those with familial hypercholesterolemia; or the cost of all of those colonoscopies for those with Lynch syndrome.
Finally, we assess quality of life. Obviously, living longer is generally considered better than living shorter, but marginal increases in life expectancy at the cost of quality of life might not be a rational choice.
You then churn these assumptions through a computer and see what comes out. How many dollars does it take to save one quality-adjusted life-year (QALY)? I’ll tell you right now that $50,000 per QALY used to be the unofficial standard for a “cost-effective” intervention in the United States. Researchers have more recently used $100,000 as that threshold.
Let’s look at some hard numbers.
If you screened 100,000 people at age 30 years, 1,500 would get news that something in their genetics was, more or less, a ticking time bomb. Some would choose to get definitive treatment and the authors estimate that the strategy would prevent 85 cases of cancer. You’d prevent nine heart attacks and five strokes by lowering cholesterol levels among those with familial hypercholesterolemia. Obviously, these aren’t huge numbers, but of course most people don’t have these hereditary risk factors. For your average 30-year-old, the genetic screening test will be completely uneventful, but for those 1,500 it will be life-changing, and potentially life-saving.
But is it worth it? The authors estimate that, at the midpoint of all their assumptions, the cost of this program would be $68,000 per QALY saved.
Of course, that depends on all those assumptions we talked about. Interestingly, the single factor that changes the cost-effectiveness the most in this analysis is the cost of the genetic test itself, which I guess makes sense, considering we’d be talking about testing a huge segment of the population. If the test cost $100 instead of $250, the cost per QALY would be $39,700 – well within the range that most policymakers would support. And given the rate at which the cost of genetic testing is decreasing, and the obvious economies of scale here, I think $100 per test is totally feasible.
The future will bring other changes as well. Right now, there are only three hereditary conditions designated as Tier 1 by the CDC. If conditions are added, that might also swing the calculation more heavily toward benefit.
This will represent a stark change from how we think about genetic testing currently, focusing on those whose pretest probability of an abnormal result is high due to family history or other risk factors. But for the 20-year-olds out there, I wouldn’t be surprised if your 30th birthday is a bit more significant than you have been anticipating.
Dr. Wilson is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator in New Haven, Conn. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
Milestone birthdays are always memorable – those ages when your life seems to fundamentally change somehow. Age 16: A license to drive. Age 18: You can vote to determine your own future and serve in the military. At 21, 3 years after adulthood, you are finally allowed to drink alcohol, for some reason. And then ... nothing much happens. At least until you turn 65 and become eligible for Medicare.
But imagine a future when turning 30 might be the biggest milestone birthday of all. Imagine a future when, at 30, you get your genome sequenced and doctors tell you what needs to be done to save your life.
That future may not be far off, as a new study shows us that
Getting your genome sequenced is a double-edged sword. Of course, there is the potential for substantial benefit; finding certain mutations allows for definitive therapy before it’s too late. That said, there are genetic diseases without a cure and without a treatment. Knowing about that destiny may do more harm than good.
Three conditions are described by the CDC as “Tier 1” conditions, genetic syndromes with a significant impact on life expectancy that also have definitive, effective therapies.
These include mutations like BRCA1/2, associated with a high risk for breast and ovarian cancer; mutations associated with Lynch syndrome, which confer an elevated risk for colon cancer; and mutations associated with familial hypercholesterolemia, which confer elevated risk for cardiovascular events.
In each of these cases, there is clear evidence that early intervention can save lives. Individuals at high risk for breast and ovarian cancer can get prophylactic mastectomy and salpingo-oophorectomy. Those with Lynch syndrome can get more frequent screening for colon cancer and polypectomy, and those with familial hypercholesterolemia can get aggressive lipid-lowering therapy.
I think most of us would probably want to know if we had one of these conditions. Most of us would use that information to take concrete steps to decrease our risk. But just because a rational person would choose to do something doesn’t mean it’s feasible. After all, we’re talking about tests and treatments that have significant costs.
In a recent issue of Annals of Internal Medicine, Josh Peterson and David Veenstra present a detailed accounting of the cost and benefit of a hypothetical nationwide, universal screening program for Tier 1 conditions. And in the end, it may actually be worth it.
Cost-benefit analyses work by comparing two independent policy choices: the status quo – in this case, a world in which some people get tested for these conditions, but generally only if they are at high risk based on strong family history; and an alternative policy – in this case, universal screening for these conditions starting at some age.
After that, it’s time to play the assumption game. Using the best available data, the authors estimated the percentage of the population that will have each condition, the percentage of those individuals who will definitively act on the information, and how effective those actions would be if taken.
The authors provide an example. First, they assume that the prevalence of mutations leading to a high risk for breast and ovarian cancer is around 0.7%, and that up to 40% of people who learn that they have one of these mutations would undergo prophylactic mastectomy, which would reduce the risk for breast cancer by around 94%. (I ran these numbers past my wife, a breast surgical oncologist, who agreed that they seem reasonable.)
Assumptions in place, it’s time to consider costs. The cost of the screening test itself: The authors use $250 as their average per-person cost. But we also have the cost of treatment – around $22,000 per person for a bilateral prophylactic mastectomy; the cost of statin therapy for those with familial hypercholesterolemia; or the cost of all of those colonoscopies for those with Lynch syndrome.
Finally, we assess quality of life. Obviously, living longer is generally considered better than living shorter, but marginal increases in life expectancy at the cost of quality of life might not be a rational choice.
You then churn these assumptions through a computer and see what comes out. How many dollars does it take to save one quality-adjusted life-year (QALY)? I’ll tell you right now that $50,000 per QALY used to be the unofficial standard for a “cost-effective” intervention in the United States. Researchers have more recently used $100,000 as that threshold.
Let’s look at some hard numbers.
If you screened 100,000 people at age 30 years, 1,500 would get news that something in their genetics was, more or less, a ticking time bomb. Some would choose to get definitive treatment and the authors estimate that the strategy would prevent 85 cases of cancer. You’d prevent nine heart attacks and five strokes by lowering cholesterol levels among those with familial hypercholesterolemia. Obviously, these aren’t huge numbers, but of course most people don’t have these hereditary risk factors. For your average 30-year-old, the genetic screening test will be completely uneventful, but for those 1,500 it will be life-changing, and potentially life-saving.
But is it worth it? The authors estimate that, at the midpoint of all their assumptions, the cost of this program would be $68,000 per QALY saved.
Of course, that depends on all those assumptions we talked about. Interestingly, the single factor that changes the cost-effectiveness the most in this analysis is the cost of the genetic test itself, which I guess makes sense, considering we’d be talking about testing a huge segment of the population. If the test cost $100 instead of $250, the cost per QALY would be $39,700 – well within the range that most policymakers would support. And given the rate at which the cost of genetic testing is decreasing, and the obvious economies of scale here, I think $100 per test is totally feasible.
The future will bring other changes as well. Right now, there are only three hereditary conditions designated as Tier 1 by the CDC. If conditions are added, that might also swing the calculation more heavily toward benefit.
This will represent a stark change from how we think about genetic testing currently, focusing on those whose pretest probability of an abnormal result is high due to family history or other risk factors. But for the 20-year-olds out there, I wouldn’t be surprised if your 30th birthday is a bit more significant than you have been anticipating.
Dr. Wilson is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator in New Haven, Conn. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
Milestone birthdays are always memorable – those ages when your life seems to fundamentally change somehow. Age 16: A license to drive. Age 18: You can vote to determine your own future and serve in the military. At 21, 3 years after adulthood, you are finally allowed to drink alcohol, for some reason. And then ... nothing much happens. At least until you turn 65 and become eligible for Medicare.
But imagine a future when turning 30 might be the biggest milestone birthday of all. Imagine a future when, at 30, you get your genome sequenced and doctors tell you what needs to be done to save your life.
That future may not be far off, as a new study shows us that
Getting your genome sequenced is a double-edged sword. Of course, there is the potential for substantial benefit; finding certain mutations allows for definitive therapy before it’s too late. That said, there are genetic diseases without a cure and without a treatment. Knowing about that destiny may do more harm than good.
Three conditions are described by the CDC as “Tier 1” conditions, genetic syndromes with a significant impact on life expectancy that also have definitive, effective therapies.
These include mutations like BRCA1/2, associated with a high risk for breast and ovarian cancer; mutations associated with Lynch syndrome, which confer an elevated risk for colon cancer; and mutations associated with familial hypercholesterolemia, which confer elevated risk for cardiovascular events.
In each of these cases, there is clear evidence that early intervention can save lives. Individuals at high risk for breast and ovarian cancer can get prophylactic mastectomy and salpingo-oophorectomy. Those with Lynch syndrome can get more frequent screening for colon cancer and polypectomy, and those with familial hypercholesterolemia can get aggressive lipid-lowering therapy.
I think most of us would probably want to know if we had one of these conditions. Most of us would use that information to take concrete steps to decrease our risk. But just because a rational person would choose to do something doesn’t mean it’s feasible. After all, we’re talking about tests and treatments that have significant costs.
In a recent issue of Annals of Internal Medicine, Josh Peterson and David Veenstra present a detailed accounting of the cost and benefit of a hypothetical nationwide, universal screening program for Tier 1 conditions. And in the end, it may actually be worth it.
Cost-benefit analyses work by comparing two independent policy choices: the status quo – in this case, a world in which some people get tested for these conditions, but generally only if they are at high risk based on strong family history; and an alternative policy – in this case, universal screening for these conditions starting at some age.
After that, it’s time to play the assumption game. Using the best available data, the authors estimated the percentage of the population that will have each condition, the percentage of those individuals who will definitively act on the information, and how effective those actions would be if taken.
The authors provide an example. First, they assume that the prevalence of mutations leading to a high risk for breast and ovarian cancer is around 0.7%, and that up to 40% of people who learn that they have one of these mutations would undergo prophylactic mastectomy, which would reduce the risk for breast cancer by around 94%. (I ran these numbers past my wife, a breast surgical oncologist, who agreed that they seem reasonable.)
Assumptions in place, it’s time to consider costs. The cost of the screening test itself: The authors use $250 as their average per-person cost. But we also have the cost of treatment – around $22,000 per person for a bilateral prophylactic mastectomy; the cost of statin therapy for those with familial hypercholesterolemia; or the cost of all of those colonoscopies for those with Lynch syndrome.
Finally, we assess quality of life. Obviously, living longer is generally considered better than living shorter, but marginal increases in life expectancy at the cost of quality of life might not be a rational choice.
You then churn these assumptions through a computer and see what comes out. How many dollars does it take to save one quality-adjusted life-year (QALY)? I’ll tell you right now that $50,000 per QALY used to be the unofficial standard for a “cost-effective” intervention in the United States. Researchers have more recently used $100,000 as that threshold.
Let’s look at some hard numbers.
If you screened 100,000 people at age 30 years, 1,500 would get news that something in their genetics was, more or less, a ticking time bomb. Some would choose to get definitive treatment and the authors estimate that the strategy would prevent 85 cases of cancer. You’d prevent nine heart attacks and five strokes by lowering cholesterol levels among those with familial hypercholesterolemia. Obviously, these aren’t huge numbers, but of course most people don’t have these hereditary risk factors. For your average 30-year-old, the genetic screening test will be completely uneventful, but for those 1,500 it will be life-changing, and potentially life-saving.
But is it worth it? The authors estimate that, at the midpoint of all their assumptions, the cost of this program would be $68,000 per QALY saved.
Of course, that depends on all those assumptions we talked about. Interestingly, the single factor that changes the cost-effectiveness the most in this analysis is the cost of the genetic test itself, which I guess makes sense, considering we’d be talking about testing a huge segment of the population. If the test cost $100 instead of $250, the cost per QALY would be $39,700 – well within the range that most policymakers would support. And given the rate at which the cost of genetic testing is decreasing, and the obvious economies of scale here, I think $100 per test is totally feasible.
The future will bring other changes as well. Right now, there are only three hereditary conditions designated as Tier 1 by the CDC. If conditions are added, that might also swing the calculation more heavily toward benefit.
This will represent a stark change from how we think about genetic testing currently, focusing on those whose pretest probability of an abnormal result is high due to family history or other risk factors. But for the 20-year-olds out there, I wouldn’t be surprised if your 30th birthday is a bit more significant than you have been anticipating.
Dr. Wilson is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator in New Haven, Conn. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.