User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
‘Substantial’ variation in responses to BP meds
A new study has shown a substantial variation in the blood pressure response to various antihypertensive medications between individuals, raising the possibility of future personalized therapy.
“We found that using the optimal antihypertensive drug for a particular patient resulted in an average of a 4.4 mm Hg greater reduction of blood pressure compared with a random choice of the other drugs. That is quite a substantial difference, and could be equivalent to adding in another drug,” lead author Johan Sundström, MD, Uppsala (Sweden) University Hospital, told this news organization.
“These preliminary findings suggest that some people may be better treated with one antihypertensive drug rather than another. This is opening up the field of hypertension for personalized medicine,” he added.
The study was published online in the Journal of the American Medical Association.
The authors noted that despite global access to multiple classes of highly effective blood pressure-lowering drugs, only one in four women and one in five men with hypertension reach treatment targets. While most hypertension guidelines advocate combination pharmacotherapy, many patients in routine care continue to be treated with monotherapy, with adverse effects and nonadherence being important clinical problems.
“One drug often does not give enough blood pressure reduction, but patients are often reluctant to up-titrate to two drugs,” Dr. Sundström said. “While we know that the four recommended classes of antihypertensives lower blood pressure equally well on average, we don’t know if their efficacy is the same in individual patients.
“We wondered whether there could be different optimal drugs for different people, and if we could identify the optimal drug for each person then maybe more patients could get to target levels with just one drug,” he said.
The researchers conducted a randomized, double-blind, repeated crossover trial at an outpatient research clinic in Sweden, studying 280 men and women with grade 1 hypertension at low risk for cardiovascular events.
Each participant was scheduled for 2 months’ treatment in random order with each of four different classes of antihypertensive drugs: an ACE inhibitor, lisinopril; an angiotensin II blocker, candesartan; a thiazide diuretic, hydrochlorothiazide; a calcium channel blocker, amlodipine.
There were then repeated treatment periods for two drug classes to try to account for any effect of a particular event that might have affected the blood pressure at one point in time. Ambulatory daytime systolic blood pressure was measured at the end of each treatment period.
Results showed that variation in systolic blood pressure was large between treatments on average, between participants on average, within participants taking the same treatment, and between treatments in the same participant.
Overall, personalized treatment using the optimal single-drug therapy led to a 4.4–mm Hg lower systolic blood pressure in the trial population than a random choice of any of the other drug classes.
Taking into consideration that lisinopril was found to be on average the most efficacious of the drugs at the selected doses, personalized treatment compared with lisinopril still led to a 3.1–mm Hg improvement in systolic blood pressure.
The researchers noted that the mean additional blood pressure reduction achievable by using the optimal agent was of a magnitude twice that achieved by doubling the dose of a first drug, and more than half that of adding a second drug on average.
While there were only small differences between certain drugs (e.g., candesartan vs. lisinopril; amlodipine vs. hydrochlorothiazide), for all other comparisons tested, the choice was important, with particularly large gains to be made by personalizing the choice between candesartan vs. amlodipine and between lisinopril vs. amlodipine.
In addition, some people showed very large differences in response to different drugs, whereas others did not have much difference at all.
How to identify the optimal drug?
“The million-dollar question is how we identify the best drug for each individual patient,” Dr. Sundström said. “This study has opened Pandora’s box. We now need to figure out how to go forward and how we tailor treatment in each patient.”
In the study, the researchers suggest that personalizing therapy could be achieved either by identifying the phenotypic characteristics that are associated with enhanced response to one treatment vs. another or by directly measuring the individual’s responses to a series of treatments to ascertain which is most effective.
Addressing the first scenario, Dr. Sundström explained: “We can analyze the characteristics of patients who did best on each drug. There are many variables we can look at here such as age, diet, baseline blood pressure, exercise levels, smoking status, race, body weight, salt intake, and findings from genetic tests. We are going to try to look into these to see if we can find any predictors of response to various different drugs.”
For the second strategy, he suggested that patients starting pharmacologic therapy could try a few different treatments. “For example, we could give patients two different drugs and ask them to alternate treatment periods with each of them and measure their blood pressure with a home monitoring kit and record adverse effects.”
Nonadherence “is such a big problem with antihypertensives,” he added. “This approach may allow patients to be more empowered when choosing the right treatment, which should help adherence in the longer term.”
‘Proof-of-principle’
Commenting on the study in an accompanying editorial, Robert M. Carey, MD, University of Virginia Health System, Charlottesville, wrote: “At this stage, the findings are more theoretical than immediately practical for the implementation of personalized antihypertensive drug therapy, but the study does provide proof-of-principle and the authors suggest a few scenarios in which a personalized approach could be used in the future.”
He said the practical ramifications of personally targeted therapy remain unclear, given that determination of an individual’s response to a series of short test treatments before selecting long-term therapy may be considered too cumbersome, and currently few phenotypic markers are currently available that would be likely to accurately predict the individual response to a particular therapy.
Dr. Carey concluded that the results of this study “encourage the further pursuit of larger randomized trials using similar repeated crossover designs to validate this concept and eventually in trials with longer follow-up data to determine whether there is improvement in long-term clinical outcomes compared with current strategies.”
He added that the results support the possibility that personalized medical treatment of hypertension “may ultimately supplement or even supplant the current method of antihypertensive drug decision-making in the future.”
This study was supported by the Swedish Research Council; Kjell and Märta Beijer Foundation; and Anders Wiklöf. Dr. Sundström reported owning stock in Symptoms Europe AB and Anagram Kommunikation AB. Coauthor Emil Hagström, MD, PhD, reported receiving grants from Pfizer and Amgen and personal fees from Amgen, Novo Nordisk, Bayer, AstraZeneca, Amarin, and Novartis. Coauthor Ollie Östlund, PhD, reported fees from Uppsala University paid to his institution, Uppsala Clinical Research Center, for its participation in the PHYSIC trial during the conduct of the study. Dr. Carey reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new study has shown a substantial variation in the blood pressure response to various antihypertensive medications between individuals, raising the possibility of future personalized therapy.
“We found that using the optimal antihypertensive drug for a particular patient resulted in an average of a 4.4 mm Hg greater reduction of blood pressure compared with a random choice of the other drugs. That is quite a substantial difference, and could be equivalent to adding in another drug,” lead author Johan Sundström, MD, Uppsala (Sweden) University Hospital, told this news organization.
“These preliminary findings suggest that some people may be better treated with one antihypertensive drug rather than another. This is opening up the field of hypertension for personalized medicine,” he added.
The study was published online in the Journal of the American Medical Association.
The authors noted that despite global access to multiple classes of highly effective blood pressure-lowering drugs, only one in four women and one in five men with hypertension reach treatment targets. While most hypertension guidelines advocate combination pharmacotherapy, many patients in routine care continue to be treated with monotherapy, with adverse effects and nonadherence being important clinical problems.
“One drug often does not give enough blood pressure reduction, but patients are often reluctant to up-titrate to two drugs,” Dr. Sundström said. “While we know that the four recommended classes of antihypertensives lower blood pressure equally well on average, we don’t know if their efficacy is the same in individual patients.
“We wondered whether there could be different optimal drugs for different people, and if we could identify the optimal drug for each person then maybe more patients could get to target levels with just one drug,” he said.
The researchers conducted a randomized, double-blind, repeated crossover trial at an outpatient research clinic in Sweden, studying 280 men and women with grade 1 hypertension at low risk for cardiovascular events.
Each participant was scheduled for 2 months’ treatment in random order with each of four different classes of antihypertensive drugs: an ACE inhibitor, lisinopril; an angiotensin II blocker, candesartan; a thiazide diuretic, hydrochlorothiazide; a calcium channel blocker, amlodipine.
There were then repeated treatment periods for two drug classes to try to account for any effect of a particular event that might have affected the blood pressure at one point in time. Ambulatory daytime systolic blood pressure was measured at the end of each treatment period.
Results showed that variation in systolic blood pressure was large between treatments on average, between participants on average, within participants taking the same treatment, and between treatments in the same participant.
Overall, personalized treatment using the optimal single-drug therapy led to a 4.4–mm Hg lower systolic blood pressure in the trial population than a random choice of any of the other drug classes.
Taking into consideration that lisinopril was found to be on average the most efficacious of the drugs at the selected doses, personalized treatment compared with lisinopril still led to a 3.1–mm Hg improvement in systolic blood pressure.
The researchers noted that the mean additional blood pressure reduction achievable by using the optimal agent was of a magnitude twice that achieved by doubling the dose of a first drug, and more than half that of adding a second drug on average.
While there were only small differences between certain drugs (e.g., candesartan vs. lisinopril; amlodipine vs. hydrochlorothiazide), for all other comparisons tested, the choice was important, with particularly large gains to be made by personalizing the choice between candesartan vs. amlodipine and between lisinopril vs. amlodipine.
In addition, some people showed very large differences in response to different drugs, whereas others did not have much difference at all.
How to identify the optimal drug?
“The million-dollar question is how we identify the best drug for each individual patient,” Dr. Sundström said. “This study has opened Pandora’s box. We now need to figure out how to go forward and how we tailor treatment in each patient.”
In the study, the researchers suggest that personalizing therapy could be achieved either by identifying the phenotypic characteristics that are associated with enhanced response to one treatment vs. another or by directly measuring the individual’s responses to a series of treatments to ascertain which is most effective.
Addressing the first scenario, Dr. Sundström explained: “We can analyze the characteristics of patients who did best on each drug. There are many variables we can look at here such as age, diet, baseline blood pressure, exercise levels, smoking status, race, body weight, salt intake, and findings from genetic tests. We are going to try to look into these to see if we can find any predictors of response to various different drugs.”
For the second strategy, he suggested that patients starting pharmacologic therapy could try a few different treatments. “For example, we could give patients two different drugs and ask them to alternate treatment periods with each of them and measure their blood pressure with a home monitoring kit and record adverse effects.”
Nonadherence “is such a big problem with antihypertensives,” he added. “This approach may allow patients to be more empowered when choosing the right treatment, which should help adherence in the longer term.”
‘Proof-of-principle’
Commenting on the study in an accompanying editorial, Robert M. Carey, MD, University of Virginia Health System, Charlottesville, wrote: “At this stage, the findings are more theoretical than immediately practical for the implementation of personalized antihypertensive drug therapy, but the study does provide proof-of-principle and the authors suggest a few scenarios in which a personalized approach could be used in the future.”
He said the practical ramifications of personally targeted therapy remain unclear, given that determination of an individual’s response to a series of short test treatments before selecting long-term therapy may be considered too cumbersome, and currently few phenotypic markers are currently available that would be likely to accurately predict the individual response to a particular therapy.
Dr. Carey concluded that the results of this study “encourage the further pursuit of larger randomized trials using similar repeated crossover designs to validate this concept and eventually in trials with longer follow-up data to determine whether there is improvement in long-term clinical outcomes compared with current strategies.”
He added that the results support the possibility that personalized medical treatment of hypertension “may ultimately supplement or even supplant the current method of antihypertensive drug decision-making in the future.”
This study was supported by the Swedish Research Council; Kjell and Märta Beijer Foundation; and Anders Wiklöf. Dr. Sundström reported owning stock in Symptoms Europe AB and Anagram Kommunikation AB. Coauthor Emil Hagström, MD, PhD, reported receiving grants from Pfizer and Amgen and personal fees from Amgen, Novo Nordisk, Bayer, AstraZeneca, Amarin, and Novartis. Coauthor Ollie Östlund, PhD, reported fees from Uppsala University paid to his institution, Uppsala Clinical Research Center, for its participation in the PHYSIC trial during the conduct of the study. Dr. Carey reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new study has shown a substantial variation in the blood pressure response to various antihypertensive medications between individuals, raising the possibility of future personalized therapy.
“We found that using the optimal antihypertensive drug for a particular patient resulted in an average of a 4.4 mm Hg greater reduction of blood pressure compared with a random choice of the other drugs. That is quite a substantial difference, and could be equivalent to adding in another drug,” lead author Johan Sundström, MD, Uppsala (Sweden) University Hospital, told this news organization.
“These preliminary findings suggest that some people may be better treated with one antihypertensive drug rather than another. This is opening up the field of hypertension for personalized medicine,” he added.
The study was published online in the Journal of the American Medical Association.
The authors noted that despite global access to multiple classes of highly effective blood pressure-lowering drugs, only one in four women and one in five men with hypertension reach treatment targets. While most hypertension guidelines advocate combination pharmacotherapy, many patients in routine care continue to be treated with monotherapy, with adverse effects and nonadherence being important clinical problems.
“One drug often does not give enough blood pressure reduction, but patients are often reluctant to up-titrate to two drugs,” Dr. Sundström said. “While we know that the four recommended classes of antihypertensives lower blood pressure equally well on average, we don’t know if their efficacy is the same in individual patients.
“We wondered whether there could be different optimal drugs for different people, and if we could identify the optimal drug for each person then maybe more patients could get to target levels with just one drug,” he said.
The researchers conducted a randomized, double-blind, repeated crossover trial at an outpatient research clinic in Sweden, studying 280 men and women with grade 1 hypertension at low risk for cardiovascular events.
Each participant was scheduled for 2 months’ treatment in random order with each of four different classes of antihypertensive drugs: an ACE inhibitor, lisinopril; an angiotensin II blocker, candesartan; a thiazide diuretic, hydrochlorothiazide; a calcium channel blocker, amlodipine.
There were then repeated treatment periods for two drug classes to try to account for any effect of a particular event that might have affected the blood pressure at one point in time. Ambulatory daytime systolic blood pressure was measured at the end of each treatment period.
Results showed that variation in systolic blood pressure was large between treatments on average, between participants on average, within participants taking the same treatment, and between treatments in the same participant.
Overall, personalized treatment using the optimal single-drug therapy led to a 4.4–mm Hg lower systolic blood pressure in the trial population than a random choice of any of the other drug classes.
Taking into consideration that lisinopril was found to be on average the most efficacious of the drugs at the selected doses, personalized treatment compared with lisinopril still led to a 3.1–mm Hg improvement in systolic blood pressure.
The researchers noted that the mean additional blood pressure reduction achievable by using the optimal agent was of a magnitude twice that achieved by doubling the dose of a first drug, and more than half that of adding a second drug on average.
While there were only small differences between certain drugs (e.g., candesartan vs. lisinopril; amlodipine vs. hydrochlorothiazide), for all other comparisons tested, the choice was important, with particularly large gains to be made by personalizing the choice between candesartan vs. amlodipine and between lisinopril vs. amlodipine.
In addition, some people showed very large differences in response to different drugs, whereas others did not have much difference at all.
How to identify the optimal drug?
“The million-dollar question is how we identify the best drug for each individual patient,” Dr. Sundström said. “This study has opened Pandora’s box. We now need to figure out how to go forward and how we tailor treatment in each patient.”
In the study, the researchers suggest that personalizing therapy could be achieved either by identifying the phenotypic characteristics that are associated with enhanced response to one treatment vs. another or by directly measuring the individual’s responses to a series of treatments to ascertain which is most effective.
Addressing the first scenario, Dr. Sundström explained: “We can analyze the characteristics of patients who did best on each drug. There are many variables we can look at here such as age, diet, baseline blood pressure, exercise levels, smoking status, race, body weight, salt intake, and findings from genetic tests. We are going to try to look into these to see if we can find any predictors of response to various different drugs.”
For the second strategy, he suggested that patients starting pharmacologic therapy could try a few different treatments. “For example, we could give patients two different drugs and ask them to alternate treatment periods with each of them and measure their blood pressure with a home monitoring kit and record adverse effects.”
Nonadherence “is such a big problem with antihypertensives,” he added. “This approach may allow patients to be more empowered when choosing the right treatment, which should help adherence in the longer term.”
‘Proof-of-principle’
Commenting on the study in an accompanying editorial, Robert M. Carey, MD, University of Virginia Health System, Charlottesville, wrote: “At this stage, the findings are more theoretical than immediately practical for the implementation of personalized antihypertensive drug therapy, but the study does provide proof-of-principle and the authors suggest a few scenarios in which a personalized approach could be used in the future.”
He said the practical ramifications of personally targeted therapy remain unclear, given that determination of an individual’s response to a series of short test treatments before selecting long-term therapy may be considered too cumbersome, and currently few phenotypic markers are currently available that would be likely to accurately predict the individual response to a particular therapy.
Dr. Carey concluded that the results of this study “encourage the further pursuit of larger randomized trials using similar repeated crossover designs to validate this concept and eventually in trials with longer follow-up data to determine whether there is improvement in long-term clinical outcomes compared with current strategies.”
He added that the results support the possibility that personalized medical treatment of hypertension “may ultimately supplement or even supplant the current method of antihypertensive drug decision-making in the future.”
This study was supported by the Swedish Research Council; Kjell and Märta Beijer Foundation; and Anders Wiklöf. Dr. Sundström reported owning stock in Symptoms Europe AB and Anagram Kommunikation AB. Coauthor Emil Hagström, MD, PhD, reported receiving grants from Pfizer and Amgen and personal fees from Amgen, Novo Nordisk, Bayer, AstraZeneca, Amarin, and Novartis. Coauthor Ollie Östlund, PhD, reported fees from Uppsala University paid to his institution, Uppsala Clinical Research Center, for its participation in the PHYSIC trial during the conduct of the study. Dr. Carey reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FDA clears first patch to treat axillary hyperhidrosis
The Food and Drug Administration on April 13 cleared the first patch to reduce excessive underarm sweating for adults with primary axillary hyperhidrosis.
The single-use, disposable, prescription-only patch will be marketed as Brella. It consists of a sodium sheet with an adhesive overlay. A health care provider applies it to the patient’s underarm for up to 3 minutes and then repeats the process on the other underarm.
The developer, Candesant Biomedical, says the patch uses the company’s patented targeted alkali thermolysis (TAT) technology, which was built on the principle that heat is generated when sodium reacts with water in sweat. “The thermal energy created by the sodium sheet is precisely localized, microtargeting sweat glands to significantly reduce sweat production,” according to the company’s press release announcing the FDA decision.
FDA clearance was based on data from the pivotal randomized, double-blind, multicenter SAHARA study, which indicated that the product is effective and well tolerated.
Patients experienced a reduction in sweat that was maintained for 3 months or longer, according to trial results.
The SAHARA trial results were reported in a late-breaking abstract at the annual meeting of the American Academy of Dermatology in March.
The trial enrolled 110 individuals with Hyperhidrosis Disease Severity Scale (HDSS) scores of 3 or 4 (indicating frequent sweating or sweating that always interferes with daily activities). Trial participants were randomly assigned to receive either an active TAT or a sham patch, which was applied for up to 3 minutes.
At the meeting, lead investigator David M. Pariser, MD, a dermatologist practicing in Norfolk, Va., reported that at 4 weeks, 63.6% of patients in the active patch group achieved an HDSS score of 1 or 2, compared with 44.2% of those in the sham treatment group (P = .0332). Also, 43.2% of those in the active-patch group achieved an improvement of 2 points or greater on the HDSS, as compared with 16.3% of those in the sham treatment group (P = .0107) .
In addition, 9.1% of those in the active-patch group achieved a 3-point improvement on the HDSS, compared with none in the sham group. “That’s an amazing improvement; you’re basically going from moderate or severe to none,” Dr. Pariser said at the meeting.
As for adverse events (AEs), 13 patients in the active-patch group experienced AEs at the treatment site. Six patients experienced erythema; four experienced erosion; two experienced burning, itching, or stinging; and one had underarm odor.
“The two procedure-related AEs in the TAT-treated group were compensatory sweating and irritant contact dermatitis due to the adhesive,” Dr. Pariser said. He noted that most AEs resolved in fewer than 2 weeks, and all AEs were mild to moderate.
According to the International Hyperhidrosis Society, about 1.3 million people in the United States have axillary hyperhidrosis, and about a third report that sweating is barely tolerable and frequently interferes with daily activities or is intolerable and always interferes with daily activities.
The patch will be available within months in select U.S. markets beginning in late summer. The company says the markets will be listed on its website.
A company representative told this news organization that because it is an in-office procedure, pricing will vary, depending on the practice. “With that said, Candesant expects doctors will charge about the same for one session of the Brella SweatControl Patch as they would for a high-end, in-office facial or chemical peel,” the representative said.
Dr. Pariser is a consultant or investigator for Bickel Biotechnology, Biofrontera AG, Bristol-Myers Squibb, the Celgene Corporation, Novartis Pharmaceuticals, Pfizer, Regeneron, and Sanofi.
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration on April 13 cleared the first patch to reduce excessive underarm sweating for adults with primary axillary hyperhidrosis.
The single-use, disposable, prescription-only patch will be marketed as Brella. It consists of a sodium sheet with an adhesive overlay. A health care provider applies it to the patient’s underarm for up to 3 minutes and then repeats the process on the other underarm.
The developer, Candesant Biomedical, says the patch uses the company’s patented targeted alkali thermolysis (TAT) technology, which was built on the principle that heat is generated when sodium reacts with water in sweat. “The thermal energy created by the sodium sheet is precisely localized, microtargeting sweat glands to significantly reduce sweat production,” according to the company’s press release announcing the FDA decision.
FDA clearance was based on data from the pivotal randomized, double-blind, multicenter SAHARA study, which indicated that the product is effective and well tolerated.
Patients experienced a reduction in sweat that was maintained for 3 months or longer, according to trial results.
The SAHARA trial results were reported in a late-breaking abstract at the annual meeting of the American Academy of Dermatology in March.
The trial enrolled 110 individuals with Hyperhidrosis Disease Severity Scale (HDSS) scores of 3 or 4 (indicating frequent sweating or sweating that always interferes with daily activities). Trial participants were randomly assigned to receive either an active TAT or a sham patch, which was applied for up to 3 minutes.
At the meeting, lead investigator David M. Pariser, MD, a dermatologist practicing in Norfolk, Va., reported that at 4 weeks, 63.6% of patients in the active patch group achieved an HDSS score of 1 or 2, compared with 44.2% of those in the sham treatment group (P = .0332). Also, 43.2% of those in the active-patch group achieved an improvement of 2 points or greater on the HDSS, as compared with 16.3% of those in the sham treatment group (P = .0107) .
In addition, 9.1% of those in the active-patch group achieved a 3-point improvement on the HDSS, compared with none in the sham group. “That’s an amazing improvement; you’re basically going from moderate or severe to none,” Dr. Pariser said at the meeting.
As for adverse events (AEs), 13 patients in the active-patch group experienced AEs at the treatment site. Six patients experienced erythema; four experienced erosion; two experienced burning, itching, or stinging; and one had underarm odor.
“The two procedure-related AEs in the TAT-treated group were compensatory sweating and irritant contact dermatitis due to the adhesive,” Dr. Pariser said. He noted that most AEs resolved in fewer than 2 weeks, and all AEs were mild to moderate.
According to the International Hyperhidrosis Society, about 1.3 million people in the United States have axillary hyperhidrosis, and about a third report that sweating is barely tolerable and frequently interferes with daily activities or is intolerable and always interferes with daily activities.
The patch will be available within months in select U.S. markets beginning in late summer. The company says the markets will be listed on its website.
A company representative told this news organization that because it is an in-office procedure, pricing will vary, depending on the practice. “With that said, Candesant expects doctors will charge about the same for one session of the Brella SweatControl Patch as they would for a high-end, in-office facial or chemical peel,” the representative said.
Dr. Pariser is a consultant or investigator for Bickel Biotechnology, Biofrontera AG, Bristol-Myers Squibb, the Celgene Corporation, Novartis Pharmaceuticals, Pfizer, Regeneron, and Sanofi.
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration on April 13 cleared the first patch to reduce excessive underarm sweating for adults with primary axillary hyperhidrosis.
The single-use, disposable, prescription-only patch will be marketed as Brella. It consists of a sodium sheet with an adhesive overlay. A health care provider applies it to the patient’s underarm for up to 3 minutes and then repeats the process on the other underarm.
The developer, Candesant Biomedical, says the patch uses the company’s patented targeted alkali thermolysis (TAT) technology, which was built on the principle that heat is generated when sodium reacts with water in sweat. “The thermal energy created by the sodium sheet is precisely localized, microtargeting sweat glands to significantly reduce sweat production,” according to the company’s press release announcing the FDA decision.
FDA clearance was based on data from the pivotal randomized, double-blind, multicenter SAHARA study, which indicated that the product is effective and well tolerated.
Patients experienced a reduction in sweat that was maintained for 3 months or longer, according to trial results.
The SAHARA trial results were reported in a late-breaking abstract at the annual meeting of the American Academy of Dermatology in March.
The trial enrolled 110 individuals with Hyperhidrosis Disease Severity Scale (HDSS) scores of 3 or 4 (indicating frequent sweating or sweating that always interferes with daily activities). Trial participants were randomly assigned to receive either an active TAT or a sham patch, which was applied for up to 3 minutes.
At the meeting, lead investigator David M. Pariser, MD, a dermatologist practicing in Norfolk, Va., reported that at 4 weeks, 63.6% of patients in the active patch group achieved an HDSS score of 1 or 2, compared with 44.2% of those in the sham treatment group (P = .0332). Also, 43.2% of those in the active-patch group achieved an improvement of 2 points or greater on the HDSS, as compared with 16.3% of those in the sham treatment group (P = .0107) .
In addition, 9.1% of those in the active-patch group achieved a 3-point improvement on the HDSS, compared with none in the sham group. “That’s an amazing improvement; you’re basically going from moderate or severe to none,” Dr. Pariser said at the meeting.
As for adverse events (AEs), 13 patients in the active-patch group experienced AEs at the treatment site. Six patients experienced erythema; four experienced erosion; two experienced burning, itching, or stinging; and one had underarm odor.
“The two procedure-related AEs in the TAT-treated group were compensatory sweating and irritant contact dermatitis due to the adhesive,” Dr. Pariser said. He noted that most AEs resolved in fewer than 2 weeks, and all AEs were mild to moderate.
According to the International Hyperhidrosis Society, about 1.3 million people in the United States have axillary hyperhidrosis, and about a third report that sweating is barely tolerable and frequently interferes with daily activities or is intolerable and always interferes with daily activities.
The patch will be available within months in select U.S. markets beginning in late summer. The company says the markets will be listed on its website.
A company representative told this news organization that because it is an in-office procedure, pricing will vary, depending on the practice. “With that said, Candesant expects doctors will charge about the same for one session of the Brella SweatControl Patch as they would for a high-end, in-office facial or chemical peel,” the representative said.
Dr. Pariser is a consultant or investigator for Bickel Biotechnology, Biofrontera AG, Bristol-Myers Squibb, the Celgene Corporation, Novartis Pharmaceuticals, Pfizer, Regeneron, and Sanofi.
A version of this article originally appeared on Medscape.com.
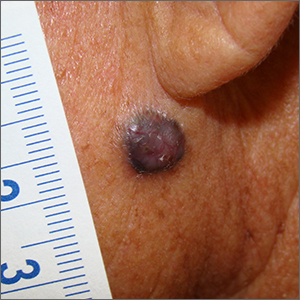
Dark facial lesion
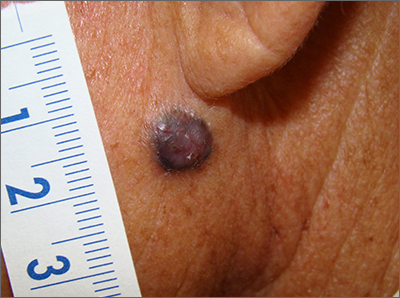
Although an elevated and pigmented lesion should be considered for possible melanoma, this one had prominent telangiectasias and was proven to be a basal cell carcinoma (BCC) on biopsy.
While the literature often focuses on light-colored skin types and the high risk of skin cancers, individuals with darker skin can also get melanoma and nonmelanoma skin cancer. Half of the BCCs in African American people are pigmented BCCs, compared to less than 10% for Caucasian individuals. Individuals who are Hispanic have twice the likelihood of pigmented BCCs as those who are Caucasian.1 Pigmented BCCs manifest as darker lesions, as occurred in this individual. Nonpigmented BCCs tend to be pink or pale in color.
Typically, superficial and very small, nodular BCCs can be successfully treated with 2 cycles of electrodesiccation and curettage. EDC should, however, be avoided in low-risk BCCs when these lesions occur in areas of secondary hair growth, such as the beard or scalp. This is because the epidermis follows the hair follicle, and in sites with deep hair follicles, EDC would have to get down to the subcutis to effectively clear the tumor.
For larger, nodular BCCs, full-thickness excision with adequate margins is warranted. For high-risk types, and those in high-risk areas near the nose, eyes, mouth, and ears, Mohs micrographic surgery is recommended to maximize the likelihood of complete excision while minimizing the loss of normal tissue.
Since the physician suspected this was a pigmented BCC, he performed a superficial shave biopsy on a small representative area of the lesion for diagnosis. This patient’s biopsy confirmed a nodular-type pigmented BCC. The lesion was removed in the office with 5-mm margins oriented along the resting skin tension lines with good closure and cosmetic results.
The patient was advised to have routine skin evaluations every 6 months due to the high risk of additional cancers. He was also advised to take oral niacinamide 500 mg twice daily, which can reduce the risk of actinic keratoses and nonmelanoma skin cancers by 15% and 23%, respectively, in those who have had lesions.2
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Higgins S, Nazemi A, Chow M, et al. Review of nonmelanoma skin cancer in African Americans, Hispanics, and Asians. Dermatol Surg. 2018;44:903-910. doi: 10.1097/DSS.0000000000001547
2. Starr P. Oral nicotinamide prevents common skin cancers in high-risk patients, reduces costs. Am Health Drug Benefits. 2015;8(spec issue):13-14.

Although an elevated and pigmented lesion should be considered for possible melanoma, this one had prominent telangiectasias and was proven to be a basal cell carcinoma (BCC) on biopsy.
While the literature often focuses on light-colored skin types and the high risk of skin cancers, individuals with darker skin can also get melanoma and nonmelanoma skin cancer. Half of the BCCs in African American people are pigmented BCCs, compared to less than 10% for Caucasian individuals. Individuals who are Hispanic have twice the likelihood of pigmented BCCs as those who are Caucasian.1 Pigmented BCCs manifest as darker lesions, as occurred in this individual. Nonpigmented BCCs tend to be pink or pale in color.
Typically, superficial and very small, nodular BCCs can be successfully treated with 2 cycles of electrodesiccation and curettage. EDC should, however, be avoided in low-risk BCCs when these lesions occur in areas of secondary hair growth, such as the beard or scalp. This is because the epidermis follows the hair follicle, and in sites with deep hair follicles, EDC would have to get down to the subcutis to effectively clear the tumor.
For larger, nodular BCCs, full-thickness excision with adequate margins is warranted. For high-risk types, and those in high-risk areas near the nose, eyes, mouth, and ears, Mohs micrographic surgery is recommended to maximize the likelihood of complete excision while minimizing the loss of normal tissue.
Since the physician suspected this was a pigmented BCC, he performed a superficial shave biopsy on a small representative area of the lesion for diagnosis. This patient’s biopsy confirmed a nodular-type pigmented BCC. The lesion was removed in the office with 5-mm margins oriented along the resting skin tension lines with good closure and cosmetic results.
The patient was advised to have routine skin evaluations every 6 months due to the high risk of additional cancers. He was also advised to take oral niacinamide 500 mg twice daily, which can reduce the risk of actinic keratoses and nonmelanoma skin cancers by 15% and 23%, respectively, in those who have had lesions.2
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.

Although an elevated and pigmented lesion should be considered for possible melanoma, this one had prominent telangiectasias and was proven to be a basal cell carcinoma (BCC) on biopsy.
While the literature often focuses on light-colored skin types and the high risk of skin cancers, individuals with darker skin can also get melanoma and nonmelanoma skin cancer. Half of the BCCs in African American people are pigmented BCCs, compared to less than 10% for Caucasian individuals. Individuals who are Hispanic have twice the likelihood of pigmented BCCs as those who are Caucasian.1 Pigmented BCCs manifest as darker lesions, as occurred in this individual. Nonpigmented BCCs tend to be pink or pale in color.
Typically, superficial and very small, nodular BCCs can be successfully treated with 2 cycles of electrodesiccation and curettage. EDC should, however, be avoided in low-risk BCCs when these lesions occur in areas of secondary hair growth, such as the beard or scalp. This is because the epidermis follows the hair follicle, and in sites with deep hair follicles, EDC would have to get down to the subcutis to effectively clear the tumor.
For larger, nodular BCCs, full-thickness excision with adequate margins is warranted. For high-risk types, and those in high-risk areas near the nose, eyes, mouth, and ears, Mohs micrographic surgery is recommended to maximize the likelihood of complete excision while minimizing the loss of normal tissue.
Since the physician suspected this was a pigmented BCC, he performed a superficial shave biopsy on a small representative area of the lesion for diagnosis. This patient’s biopsy confirmed a nodular-type pigmented BCC. The lesion was removed in the office with 5-mm margins oriented along the resting skin tension lines with good closure and cosmetic results.
The patient was advised to have routine skin evaluations every 6 months due to the high risk of additional cancers. He was also advised to take oral niacinamide 500 mg twice daily, which can reduce the risk of actinic keratoses and nonmelanoma skin cancers by 15% and 23%, respectively, in those who have had lesions.2
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Higgins S, Nazemi A, Chow M, et al. Review of nonmelanoma skin cancer in African Americans, Hispanics, and Asians. Dermatol Surg. 2018;44:903-910. doi: 10.1097/DSS.0000000000001547
2. Starr P. Oral nicotinamide prevents common skin cancers in high-risk patients, reduces costs. Am Health Drug Benefits. 2015;8(spec issue):13-14.
1. Higgins S, Nazemi A, Chow M, et al. Review of nonmelanoma skin cancer in African Americans, Hispanics, and Asians. Dermatol Surg. 2018;44:903-910. doi: 10.1097/DSS.0000000000001547
2. Starr P. Oral nicotinamide prevents common skin cancers in high-risk patients, reduces costs. Am Health Drug Benefits. 2015;8(spec issue):13-14.
Long COVID: ‘On par’ with heart disease, cancer, book says
Filmmaker Gez Medinger and immunologist Danny Altmann have been dubbed by British media as “COVID’s odd couple,” and they don’t mind at all. Discussing their recent book, The Long COVID Handbook, the authors lean into their animated roles: Medinger is a passionate patient-researcher and “guinea pig” (his words) in search of his own healing, and Altmann is a no-nonsense, data-driven scientist and “Professor Boring” (as he puts it).
And the message they have about the impact of long COVID is stunning.
“The clinical burden [of long COVID] is somewhere on par with the whole of heart disease all over again, or the whole of oncology all over again, which are our biggest clinical bills concurrently,” Altmann said.
The pair met early in the pandemic, after Medinger became infected during the first wave and interviewed Altmann for his YouTube channel, which has more than 5 million views.
“Danny was one of the first people from the medical establishment to sort of stand up on the parapet and wave a flag and say, ‘Hey, guys, there’s a problem here.’ And that was incredibly validating for 2 million people in the U.K. alone who were suffering with long COVID,” Medinger said.
Their relationship works, not just for publishing one of the first definitive guides to long COVID, but also as a model for how patients with lived experiences can lead the way in medicine – from giving the condition its name to driving the medical establishment for recognition, clinical research, and therapeutic answers.
With Altmann currently leading a major research project at Imperial College London on long COVID and Medinger’s social media platform and communication skills, they’re both advancing the world’s understanding of the disease in their own way.
“We’re now more than 3 years into this completely mysterious, uncharted disease process with a whole globe full of really desperate people,” said Altmann. “It’s a living, organic thing, and yet that also demands some kind of order and collation and pulling together into some kind of sense. So I was very pleased when Gez approached me to help him with the book.”
In it, they translate everything they’ve learned about the condition that’s “scattered in 100,000 places around the globe” into a digestible format. It tells two sides of the same story: the anecdotal experiences Medinger has undergone or observed in the long COVID community through more than a dozen of his own patient-led studies, as well the hard science and research that’s amassing in the medical world.
In an interview,
What are the book’s key takeaways for you?
Medinger: “I would say we put together an incredibly comprehensive couple of chapters on the hypotheses, big picture, what’s causing long COVID. And then the nitty-gritty research for everything that we’ve found out that is going on. ... And the other part of the book that I think is particularly important, beyond the tips for managing symptoms, is the content on mental health and the impact on your emotional state and your capacity and just how huge that is. ... That has been the most powerful thing for patients when they’ve read it. And they’ve said that they’ve just been crying all the way through those chapters because suddenly they feel heard and seen.”
Altmann: “Obviously, you’d expect me to say that the parts of the book that I love most are the kind of hard-nosed, medical, mechanistic bits. ... We’ve got 150 million-plus desperate people deciding or not deciding to go and see their general practitioner, getting a fair hearing or not getting a fair hearing. And the poor doctor has never learned this in medical school, has never read a textbook on it, and hasn’t a clue what’s coming through the door.
How are they expected to know what to do? So I think the least we can do in some of those chapters is feed into their knowledge of general medicine and give them some clues. ... I think if we can explain to people what might be going on in them, and to their doctors, what on earth they might do about it, what kind of tests they might order, that helps a bit.”
How did you balance the more controversial parts of the book, including the chapter about so-called “treatments”? For instance, the book recounts Gez’s harrowing experience with ivermectin as a frightening warning. But Danny, you were nervous about even mentioning unproven and potentially dangerous treatments as things people have tried and have looked into.
Medinger: “We had to try and work out how to handle the topic, how to handle those points of view, whilst at the same time still being informative. I think the book is stronger for that chapter, too. The other thing would certainly have been to just not address the subject, but it’s one of the things that people want to know the most about. And there’s also a lot of bad information floating around out there about certain treatments. Ivermectin, for example, and this is what happened to me when I tried it. ‘Don’t do it. It’s not recommended. Please don’t.’
I think it was also very important to include because that cautionary tale really applies to every single one of those treatments that people might be hearing about that hasn’t been backed up by efficacy and safety studies.”
Altmann: “I think Gez has been quite diplomatic. That chapter was, I think, a testament to the power of the book. And the biggest test of our marriage as ‘the odd couple.’ Because when I first read the first draft of what Gez had written, I said, ‘my name can’t even be on this book. Otherwise, I’ll be sacked.’
And we had to find marriage counseling after that, and a way back to write a version of that chapter that expressed both halves of those concerns in a way that did justice to those different viewpoints. And I think that makes it quite a strong chapter.”
What do you think are the most urgent next steps in the search for solving long COVID?
Medinger: “I would personally like to try and get some sort of answer on viral persistence. ... If there’s one thing that feels like it would be treatable in theory, and would make sense why we’re still getting all of these symptoms this whole time later, it’s that, so I would like to try and establish or eliminate viral persistence. So if you gave me Elon Musk’s wealth, that’s what I would throw a bunch of the money at, trying to either eliminate or establish that.
And then, you know, the other important thing is a diagnostic test. Danny always talks about how important it is. Once you have that, it helps you suddenly open the doors to all these other things that you can do. And treatment trials. Let’s throw some meds at this so that we have an educated guess at what might work and put them into high-powered, randomized, controlled trials and see if anything comes out because from the patient perspective, I don’t think any of us wants to wait for 5 years for that stuff to start happening.”
Altmann: “I completely agree. If you go to a website, like clinicaltrials.gov, you’ll find an immense number of clinical trials on COVID. There isn’t really a shortage of them, some of them better-powered to get an answer than others.”
How do you think public policy needs to adapt for long COVID, including social safety nets such as workers’ compensation and disability benefits?
Medinger: “In terms of public policy, what I would like would be some public acknowledgment that it’s real from government sources. Just the acknowledgment that it’s real and it remains a risk even now.”
Altmann: “Nobody in politics asks my opinion. I think they’d hate to hear it. Because if I went to see them and said, well, actually, if you thought the COVID pandemic was bad, wait till you see what’s on the table now. We’ve created a disabled population in our country of 2 million, at least a portion if not more of people who are not fully contributory to the workforce anymore ... [with] legal wrangles about retirement and health insurance and pensions, and a human right to adequate health care. Which means, ideally, a purpose-built clinic where they can have their respiratory opinion and their rheumatology opinion and their endocrine opinion and their neurology opinion, all under one roof.”
You’ve both shown so much optimism. Why is that?
Altmann: “I’ve been an immunologist for a long time now, and written all my decades of grant applications, where as a community we made what, at the time, were kind of wild promises and wildly optimistic projections of how our knowledge of tumor immunity would revolutionize cancer care, and how knowledge of autoimmunity would revolutionize care of all the autoimmune diseases.
And weirdly almost every word we wrote over those 25 or 30 years came true. Cancer immunotherapy was revolutionized, and biologics for diabetes, multiple sclerosis, and arthritis were revolutionized. So if I have faith that those things came true, I have complete faith in this as well.”
Medinger: “From the patient perspective, what I would say is that we are seeing people who’ve been ill for more than 2 years recover. People are suddenly turning the corner when they might not have expected to.
And while we don’t quite know exactly why yet, and it’s not everyone, every single time I hear the story of someone saying, ‘I’m pretty much back to where I was, I feel like I’ve recovered,’ I feel great. Even if I haven’t. Because I know that every single time I hear someone say that, that just increases the probability that I will, too.”
A version of this article first appeared on WebMD.com.
Filmmaker Gez Medinger and immunologist Danny Altmann have been dubbed by British media as “COVID’s odd couple,” and they don’t mind at all. Discussing their recent book, The Long COVID Handbook, the authors lean into their animated roles: Medinger is a passionate patient-researcher and “guinea pig” (his words) in search of his own healing, and Altmann is a no-nonsense, data-driven scientist and “Professor Boring” (as he puts it).
And the message they have about the impact of long COVID is stunning.
“The clinical burden [of long COVID] is somewhere on par with the whole of heart disease all over again, or the whole of oncology all over again, which are our biggest clinical bills concurrently,” Altmann said.
The pair met early in the pandemic, after Medinger became infected during the first wave and interviewed Altmann for his YouTube channel, which has more than 5 million views.
“Danny was one of the first people from the medical establishment to sort of stand up on the parapet and wave a flag and say, ‘Hey, guys, there’s a problem here.’ And that was incredibly validating for 2 million people in the U.K. alone who were suffering with long COVID,” Medinger said.
Their relationship works, not just for publishing one of the first definitive guides to long COVID, but also as a model for how patients with lived experiences can lead the way in medicine – from giving the condition its name to driving the medical establishment for recognition, clinical research, and therapeutic answers.
With Altmann currently leading a major research project at Imperial College London on long COVID and Medinger’s social media platform and communication skills, they’re both advancing the world’s understanding of the disease in their own way.
“We’re now more than 3 years into this completely mysterious, uncharted disease process with a whole globe full of really desperate people,” said Altmann. “It’s a living, organic thing, and yet that also demands some kind of order and collation and pulling together into some kind of sense. So I was very pleased when Gez approached me to help him with the book.”
In it, they translate everything they’ve learned about the condition that’s “scattered in 100,000 places around the globe” into a digestible format. It tells two sides of the same story: the anecdotal experiences Medinger has undergone or observed in the long COVID community through more than a dozen of his own patient-led studies, as well the hard science and research that’s amassing in the medical world.
In an interview,
What are the book’s key takeaways for you?
Medinger: “I would say we put together an incredibly comprehensive couple of chapters on the hypotheses, big picture, what’s causing long COVID. And then the nitty-gritty research for everything that we’ve found out that is going on. ... And the other part of the book that I think is particularly important, beyond the tips for managing symptoms, is the content on mental health and the impact on your emotional state and your capacity and just how huge that is. ... That has been the most powerful thing for patients when they’ve read it. And they’ve said that they’ve just been crying all the way through those chapters because suddenly they feel heard and seen.”
Altmann: “Obviously, you’d expect me to say that the parts of the book that I love most are the kind of hard-nosed, medical, mechanistic bits. ... We’ve got 150 million-plus desperate people deciding or not deciding to go and see their general practitioner, getting a fair hearing or not getting a fair hearing. And the poor doctor has never learned this in medical school, has never read a textbook on it, and hasn’t a clue what’s coming through the door.
How are they expected to know what to do? So I think the least we can do in some of those chapters is feed into their knowledge of general medicine and give them some clues. ... I think if we can explain to people what might be going on in them, and to their doctors, what on earth they might do about it, what kind of tests they might order, that helps a bit.”
How did you balance the more controversial parts of the book, including the chapter about so-called “treatments”? For instance, the book recounts Gez’s harrowing experience with ivermectin as a frightening warning. But Danny, you were nervous about even mentioning unproven and potentially dangerous treatments as things people have tried and have looked into.
Medinger: “We had to try and work out how to handle the topic, how to handle those points of view, whilst at the same time still being informative. I think the book is stronger for that chapter, too. The other thing would certainly have been to just not address the subject, but it’s one of the things that people want to know the most about. And there’s also a lot of bad information floating around out there about certain treatments. Ivermectin, for example, and this is what happened to me when I tried it. ‘Don’t do it. It’s not recommended. Please don’t.’
I think it was also very important to include because that cautionary tale really applies to every single one of those treatments that people might be hearing about that hasn’t been backed up by efficacy and safety studies.”
Altmann: “I think Gez has been quite diplomatic. That chapter was, I think, a testament to the power of the book. And the biggest test of our marriage as ‘the odd couple.’ Because when I first read the first draft of what Gez had written, I said, ‘my name can’t even be on this book. Otherwise, I’ll be sacked.’
And we had to find marriage counseling after that, and a way back to write a version of that chapter that expressed both halves of those concerns in a way that did justice to those different viewpoints. And I think that makes it quite a strong chapter.”
What do you think are the most urgent next steps in the search for solving long COVID?
Medinger: “I would personally like to try and get some sort of answer on viral persistence. ... If there’s one thing that feels like it would be treatable in theory, and would make sense why we’re still getting all of these symptoms this whole time later, it’s that, so I would like to try and establish or eliminate viral persistence. So if you gave me Elon Musk’s wealth, that’s what I would throw a bunch of the money at, trying to either eliminate or establish that.
And then, you know, the other important thing is a diagnostic test. Danny always talks about how important it is. Once you have that, it helps you suddenly open the doors to all these other things that you can do. And treatment trials. Let’s throw some meds at this so that we have an educated guess at what might work and put them into high-powered, randomized, controlled trials and see if anything comes out because from the patient perspective, I don’t think any of us wants to wait for 5 years for that stuff to start happening.”
Altmann: “I completely agree. If you go to a website, like clinicaltrials.gov, you’ll find an immense number of clinical trials on COVID. There isn’t really a shortage of them, some of them better-powered to get an answer than others.”
How do you think public policy needs to adapt for long COVID, including social safety nets such as workers’ compensation and disability benefits?
Medinger: “In terms of public policy, what I would like would be some public acknowledgment that it’s real from government sources. Just the acknowledgment that it’s real and it remains a risk even now.”
Altmann: “Nobody in politics asks my opinion. I think they’d hate to hear it. Because if I went to see them and said, well, actually, if you thought the COVID pandemic was bad, wait till you see what’s on the table now. We’ve created a disabled population in our country of 2 million, at least a portion if not more of people who are not fully contributory to the workforce anymore ... [with] legal wrangles about retirement and health insurance and pensions, and a human right to adequate health care. Which means, ideally, a purpose-built clinic where they can have their respiratory opinion and their rheumatology opinion and their endocrine opinion and their neurology opinion, all under one roof.”
You’ve both shown so much optimism. Why is that?
Altmann: “I’ve been an immunologist for a long time now, and written all my decades of grant applications, where as a community we made what, at the time, were kind of wild promises and wildly optimistic projections of how our knowledge of tumor immunity would revolutionize cancer care, and how knowledge of autoimmunity would revolutionize care of all the autoimmune diseases.
And weirdly almost every word we wrote over those 25 or 30 years came true. Cancer immunotherapy was revolutionized, and biologics for diabetes, multiple sclerosis, and arthritis were revolutionized. So if I have faith that those things came true, I have complete faith in this as well.”
Medinger: “From the patient perspective, what I would say is that we are seeing people who’ve been ill for more than 2 years recover. People are suddenly turning the corner when they might not have expected to.
And while we don’t quite know exactly why yet, and it’s not everyone, every single time I hear the story of someone saying, ‘I’m pretty much back to where I was, I feel like I’ve recovered,’ I feel great. Even if I haven’t. Because I know that every single time I hear someone say that, that just increases the probability that I will, too.”
A version of this article first appeared on WebMD.com.
Filmmaker Gez Medinger and immunologist Danny Altmann have been dubbed by British media as “COVID’s odd couple,” and they don’t mind at all. Discussing their recent book, The Long COVID Handbook, the authors lean into their animated roles: Medinger is a passionate patient-researcher and “guinea pig” (his words) in search of his own healing, and Altmann is a no-nonsense, data-driven scientist and “Professor Boring” (as he puts it).
And the message they have about the impact of long COVID is stunning.
“The clinical burden [of long COVID] is somewhere on par with the whole of heart disease all over again, or the whole of oncology all over again, which are our biggest clinical bills concurrently,” Altmann said.
The pair met early in the pandemic, after Medinger became infected during the first wave and interviewed Altmann for his YouTube channel, which has more than 5 million views.
“Danny was one of the first people from the medical establishment to sort of stand up on the parapet and wave a flag and say, ‘Hey, guys, there’s a problem here.’ And that was incredibly validating for 2 million people in the U.K. alone who were suffering with long COVID,” Medinger said.
Their relationship works, not just for publishing one of the first definitive guides to long COVID, but also as a model for how patients with lived experiences can lead the way in medicine – from giving the condition its name to driving the medical establishment for recognition, clinical research, and therapeutic answers.
With Altmann currently leading a major research project at Imperial College London on long COVID and Medinger’s social media platform and communication skills, they’re both advancing the world’s understanding of the disease in their own way.
“We’re now more than 3 years into this completely mysterious, uncharted disease process with a whole globe full of really desperate people,” said Altmann. “It’s a living, organic thing, and yet that also demands some kind of order and collation and pulling together into some kind of sense. So I was very pleased when Gez approached me to help him with the book.”
In it, they translate everything they’ve learned about the condition that’s “scattered in 100,000 places around the globe” into a digestible format. It tells two sides of the same story: the anecdotal experiences Medinger has undergone or observed in the long COVID community through more than a dozen of his own patient-led studies, as well the hard science and research that’s amassing in the medical world.
In an interview,
What are the book’s key takeaways for you?
Medinger: “I would say we put together an incredibly comprehensive couple of chapters on the hypotheses, big picture, what’s causing long COVID. And then the nitty-gritty research for everything that we’ve found out that is going on. ... And the other part of the book that I think is particularly important, beyond the tips for managing symptoms, is the content on mental health and the impact on your emotional state and your capacity and just how huge that is. ... That has been the most powerful thing for patients when they’ve read it. And they’ve said that they’ve just been crying all the way through those chapters because suddenly they feel heard and seen.”
Altmann: “Obviously, you’d expect me to say that the parts of the book that I love most are the kind of hard-nosed, medical, mechanistic bits. ... We’ve got 150 million-plus desperate people deciding or not deciding to go and see their general practitioner, getting a fair hearing or not getting a fair hearing. And the poor doctor has never learned this in medical school, has never read a textbook on it, and hasn’t a clue what’s coming through the door.
How are they expected to know what to do? So I think the least we can do in some of those chapters is feed into their knowledge of general medicine and give them some clues. ... I think if we can explain to people what might be going on in them, and to their doctors, what on earth they might do about it, what kind of tests they might order, that helps a bit.”
How did you balance the more controversial parts of the book, including the chapter about so-called “treatments”? For instance, the book recounts Gez’s harrowing experience with ivermectin as a frightening warning. But Danny, you were nervous about even mentioning unproven and potentially dangerous treatments as things people have tried and have looked into.
Medinger: “We had to try and work out how to handle the topic, how to handle those points of view, whilst at the same time still being informative. I think the book is stronger for that chapter, too. The other thing would certainly have been to just not address the subject, but it’s one of the things that people want to know the most about. And there’s also a lot of bad information floating around out there about certain treatments. Ivermectin, for example, and this is what happened to me when I tried it. ‘Don’t do it. It’s not recommended. Please don’t.’
I think it was also very important to include because that cautionary tale really applies to every single one of those treatments that people might be hearing about that hasn’t been backed up by efficacy and safety studies.”
Altmann: “I think Gez has been quite diplomatic. That chapter was, I think, a testament to the power of the book. And the biggest test of our marriage as ‘the odd couple.’ Because when I first read the first draft of what Gez had written, I said, ‘my name can’t even be on this book. Otherwise, I’ll be sacked.’
And we had to find marriage counseling after that, and a way back to write a version of that chapter that expressed both halves of those concerns in a way that did justice to those different viewpoints. And I think that makes it quite a strong chapter.”
What do you think are the most urgent next steps in the search for solving long COVID?
Medinger: “I would personally like to try and get some sort of answer on viral persistence. ... If there’s one thing that feels like it would be treatable in theory, and would make sense why we’re still getting all of these symptoms this whole time later, it’s that, so I would like to try and establish or eliminate viral persistence. So if you gave me Elon Musk’s wealth, that’s what I would throw a bunch of the money at, trying to either eliminate or establish that.
And then, you know, the other important thing is a diagnostic test. Danny always talks about how important it is. Once you have that, it helps you suddenly open the doors to all these other things that you can do. And treatment trials. Let’s throw some meds at this so that we have an educated guess at what might work and put them into high-powered, randomized, controlled trials and see if anything comes out because from the patient perspective, I don’t think any of us wants to wait for 5 years for that stuff to start happening.”
Altmann: “I completely agree. If you go to a website, like clinicaltrials.gov, you’ll find an immense number of clinical trials on COVID. There isn’t really a shortage of them, some of them better-powered to get an answer than others.”
How do you think public policy needs to adapt for long COVID, including social safety nets such as workers’ compensation and disability benefits?
Medinger: “In terms of public policy, what I would like would be some public acknowledgment that it’s real from government sources. Just the acknowledgment that it’s real and it remains a risk even now.”
Altmann: “Nobody in politics asks my opinion. I think they’d hate to hear it. Because if I went to see them and said, well, actually, if you thought the COVID pandemic was bad, wait till you see what’s on the table now. We’ve created a disabled population in our country of 2 million, at least a portion if not more of people who are not fully contributory to the workforce anymore ... [with] legal wrangles about retirement and health insurance and pensions, and a human right to adequate health care. Which means, ideally, a purpose-built clinic where they can have their respiratory opinion and their rheumatology opinion and their endocrine opinion and their neurology opinion, all under one roof.”
You’ve both shown so much optimism. Why is that?
Altmann: “I’ve been an immunologist for a long time now, and written all my decades of grant applications, where as a community we made what, at the time, were kind of wild promises and wildly optimistic projections of how our knowledge of tumor immunity would revolutionize cancer care, and how knowledge of autoimmunity would revolutionize care of all the autoimmune diseases.
And weirdly almost every word we wrote over those 25 or 30 years came true. Cancer immunotherapy was revolutionized, and biologics for diabetes, multiple sclerosis, and arthritis were revolutionized. So if I have faith that those things came true, I have complete faith in this as well.”
Medinger: “From the patient perspective, what I would say is that we are seeing people who’ve been ill for more than 2 years recover. People are suddenly turning the corner when they might not have expected to.
And while we don’t quite know exactly why yet, and it’s not everyone, every single time I hear the story of someone saying, ‘I’m pretty much back to where I was, I feel like I’ve recovered,’ I feel great. Even if I haven’t. Because I know that every single time I hear someone say that, that just increases the probability that I will, too.”
A version of this article first appeared on WebMD.com.
Cancer, heart disease vaccines may be ready by 2030, Moderna says
The announcement is yet another sign of what many are calling “the golden age” of vaccine development, which is largely credited to the pandemic’s use of mRNA technology to create COVID-19 vaccines.
“I think what we have learned in recent months is that if you ever thought that mRNA was just for infectious diseases, or just for COVID, the evidence now is that that’s absolutely not the case,” Moderna Chief Medical Officer Paul Burton, MD, PhD, told The Guardian. “It can be applied to all sorts of disease areas; we are in cancer, infectious disease, cardiovascular disease, autoimmune diseases, rare disease. We have studies in all of those areas, and they have all shown tremendous promise.”
The U.S. Food and Drug Administration recently designated two new Moderna vaccines as breakthrough therapies: a shot that prevents respiratory syncytial virus (RSV) in older people and a shot that helps prevent the recurrence of melanoma. The FDA’s breakthrough designation is given when a new treatment’s early trial results are substantially better than an existing therapy.
The mRNA vaccine technology that made headlines for its role in COVID-19 vaccines works by teaching the body how to make a specific protein to help the immune system prevent or target a certain disease.
Dr. Burton anticipates that mRNA technology will result in breakthroughs such as a cancer vaccine that can be personalized based on the features of a specific tumor.
“I think we will have mRNA-based therapies for rare diseases that were previously undruggable, and I think that 10 years from now, we will be approaching a world where you truly can identify the genetic cause of a disease and, with relative simplicity, go and edit that out and repair it using mRNA-based technology,” he said.
The Moderna executive made the statements before its annual update on its vaccine pipeline projects, which the company calls “Vaccines Day.” The Massachusetts-based drugmaker said it has given someone the first dose of a “next-generation” COVID-19 vaccine in a phase III trial, has made progress on a Lyme disease shot, and is developing a vaccine for the highly contagious norovirus.
In all, Moderna expects “six major vaccine product launches in the next few years,” the company said in a statement, adding that it expects the COVID-19 booster market alone to be valued at $15 billion.
A version of this article first appeared on WebMD.com.
The announcement is yet another sign of what many are calling “the golden age” of vaccine development, which is largely credited to the pandemic’s use of mRNA technology to create COVID-19 vaccines.
“I think what we have learned in recent months is that if you ever thought that mRNA was just for infectious diseases, or just for COVID, the evidence now is that that’s absolutely not the case,” Moderna Chief Medical Officer Paul Burton, MD, PhD, told The Guardian. “It can be applied to all sorts of disease areas; we are in cancer, infectious disease, cardiovascular disease, autoimmune diseases, rare disease. We have studies in all of those areas, and they have all shown tremendous promise.”
The U.S. Food and Drug Administration recently designated two new Moderna vaccines as breakthrough therapies: a shot that prevents respiratory syncytial virus (RSV) in older people and a shot that helps prevent the recurrence of melanoma. The FDA’s breakthrough designation is given when a new treatment’s early trial results are substantially better than an existing therapy.
The mRNA vaccine technology that made headlines for its role in COVID-19 vaccines works by teaching the body how to make a specific protein to help the immune system prevent or target a certain disease.
Dr. Burton anticipates that mRNA technology will result in breakthroughs such as a cancer vaccine that can be personalized based on the features of a specific tumor.
“I think we will have mRNA-based therapies for rare diseases that were previously undruggable, and I think that 10 years from now, we will be approaching a world where you truly can identify the genetic cause of a disease and, with relative simplicity, go and edit that out and repair it using mRNA-based technology,” he said.
The Moderna executive made the statements before its annual update on its vaccine pipeline projects, which the company calls “Vaccines Day.” The Massachusetts-based drugmaker said it has given someone the first dose of a “next-generation” COVID-19 vaccine in a phase III trial, has made progress on a Lyme disease shot, and is developing a vaccine for the highly contagious norovirus.
In all, Moderna expects “six major vaccine product launches in the next few years,” the company said in a statement, adding that it expects the COVID-19 booster market alone to be valued at $15 billion.
A version of this article first appeared on WebMD.com.
The announcement is yet another sign of what many are calling “the golden age” of vaccine development, which is largely credited to the pandemic’s use of mRNA technology to create COVID-19 vaccines.
“I think what we have learned in recent months is that if you ever thought that mRNA was just for infectious diseases, or just for COVID, the evidence now is that that’s absolutely not the case,” Moderna Chief Medical Officer Paul Burton, MD, PhD, told The Guardian. “It can be applied to all sorts of disease areas; we are in cancer, infectious disease, cardiovascular disease, autoimmune diseases, rare disease. We have studies in all of those areas, and they have all shown tremendous promise.”
The U.S. Food and Drug Administration recently designated two new Moderna vaccines as breakthrough therapies: a shot that prevents respiratory syncytial virus (RSV) in older people and a shot that helps prevent the recurrence of melanoma. The FDA’s breakthrough designation is given when a new treatment’s early trial results are substantially better than an existing therapy.
The mRNA vaccine technology that made headlines for its role in COVID-19 vaccines works by teaching the body how to make a specific protein to help the immune system prevent or target a certain disease.
Dr. Burton anticipates that mRNA technology will result in breakthroughs such as a cancer vaccine that can be personalized based on the features of a specific tumor.
“I think we will have mRNA-based therapies for rare diseases that were previously undruggable, and I think that 10 years from now, we will be approaching a world where you truly can identify the genetic cause of a disease and, with relative simplicity, go and edit that out and repair it using mRNA-based technology,” he said.
The Moderna executive made the statements before its annual update on its vaccine pipeline projects, which the company calls “Vaccines Day.” The Massachusetts-based drugmaker said it has given someone the first dose of a “next-generation” COVID-19 vaccine in a phase III trial, has made progress on a Lyme disease shot, and is developing a vaccine for the highly contagious norovirus.
In all, Moderna expects “six major vaccine product launches in the next few years,” the company said in a statement, adding that it expects the COVID-19 booster market alone to be valued at $15 billion.
A version of this article first appeared on WebMD.com.
New COVID variant on WHO’s radar causing itchy eyes in children
A new COVID-19 variant that recently landed on the World Health Organization’s radar may cause previously unseen symptoms in children, according to a new report.
While the variant, called “Arcturus,” hasn’t yet made the Centers for Disease Control and Prevention’s watchlist, , according to The Times of India.
The new itchy eye symptom is in addition to a high fever and cough, Vipin M. Vashishtha, MD, said on Twitter, noting that pediatric COVID cases have picked up there for the first time in 6 months.
The country has also seen a rise in adenovirus cases among children with similar symptoms. COVID and adenovirus cannot be distinguished without testing, and many parents don’t want to have their children tested because the swabs are uncomfortable, The Times of India reported. One doctor told the newspaper that among every 10 children with COVID-like symptoms, 2 or 3 of them had tested positive on a COVID test taken at home.
Health officials in India are doing mock drills to check how prepared the country’s hospitals are as India sees cases rise, the BBC reported. India struggled during a COVID-19 surge in 2021, at which time sickened people were seen lying on sidewalks outside overflowing hospitals, and reports surfaced of a black market for private citizens to buy oxygen.
Arcturus (formally, Omicron subvariant XBB.1.16) made news recently as it landed on the WHO’s radar after surfacing in India. A WHO official called it “one to watch.” The Times of India reported that 234 new cases of XBB.1.16 were included in the country’s latest 5,676 new infections, meaning the subvariant accounts for 4% of new COVID cases.
A version of this article originally appeared on WebMD.com.
A new COVID-19 variant that recently landed on the World Health Organization’s radar may cause previously unseen symptoms in children, according to a new report.
While the variant, called “Arcturus,” hasn’t yet made the Centers for Disease Control and Prevention’s watchlist, , according to The Times of India.
The new itchy eye symptom is in addition to a high fever and cough, Vipin M. Vashishtha, MD, said on Twitter, noting that pediatric COVID cases have picked up there for the first time in 6 months.
The country has also seen a rise in adenovirus cases among children with similar symptoms. COVID and adenovirus cannot be distinguished without testing, and many parents don’t want to have their children tested because the swabs are uncomfortable, The Times of India reported. One doctor told the newspaper that among every 10 children with COVID-like symptoms, 2 or 3 of them had tested positive on a COVID test taken at home.
Health officials in India are doing mock drills to check how prepared the country’s hospitals are as India sees cases rise, the BBC reported. India struggled during a COVID-19 surge in 2021, at which time sickened people were seen lying on sidewalks outside overflowing hospitals, and reports surfaced of a black market for private citizens to buy oxygen.
Arcturus (formally, Omicron subvariant XBB.1.16) made news recently as it landed on the WHO’s radar after surfacing in India. A WHO official called it “one to watch.” The Times of India reported that 234 new cases of XBB.1.16 were included in the country’s latest 5,676 new infections, meaning the subvariant accounts for 4% of new COVID cases.
A version of this article originally appeared on WebMD.com.
A new COVID-19 variant that recently landed on the World Health Organization’s radar may cause previously unseen symptoms in children, according to a new report.
While the variant, called “Arcturus,” hasn’t yet made the Centers for Disease Control and Prevention’s watchlist, , according to The Times of India.
The new itchy eye symptom is in addition to a high fever and cough, Vipin M. Vashishtha, MD, said on Twitter, noting that pediatric COVID cases have picked up there for the first time in 6 months.
The country has also seen a rise in adenovirus cases among children with similar symptoms. COVID and adenovirus cannot be distinguished without testing, and many parents don’t want to have their children tested because the swabs are uncomfortable, The Times of India reported. One doctor told the newspaper that among every 10 children with COVID-like symptoms, 2 or 3 of them had tested positive on a COVID test taken at home.
Health officials in India are doing mock drills to check how prepared the country’s hospitals are as India sees cases rise, the BBC reported. India struggled during a COVID-19 surge in 2021, at which time sickened people were seen lying on sidewalks outside overflowing hospitals, and reports surfaced of a black market for private citizens to buy oxygen.
Arcturus (formally, Omicron subvariant XBB.1.16) made news recently as it landed on the WHO’s radar after surfacing in India. A WHO official called it “one to watch.” The Times of India reported that 234 new cases of XBB.1.16 were included in the country’s latest 5,676 new infections, meaning the subvariant accounts for 4% of new COVID cases.
A version of this article originally appeared on WebMD.com.
AHA statement targets nuance in CVD risk assessment of women
In a new scientific statement, the American Heart Association highlighted the importance of incorporating nonbiological risk factors and social determinants of health in cardiovascular disease (CVD) risk assessment for women, particularly women from different racial and ethnic backgrounds.
CVD risk assessment in women is multifaceted and goes well beyond traditional risk factors to include sex-specific biological risk factors, as well as social, behavioral, and environmental factors, the writing group noted.
They said a greater focus on addressing all CVD risk factors among women from underrepresented races and ethnicities is warranted to avert future CVD.
The scientific statement was published online in Circulation.
Look beyond traditional risk factors
“Risk assessment is the first step in preventing heart disease, yet there are many limitations to traditional risk factors and their ability to comprehensively estimate a woman’s risk for cardiovascular disease,” Jennifer H. Mieres, MD, vice chair of the writing group and professor of cardiology at Hofstra University, Hempstead, N.Y., said in a news release.
“The delivery of equitable cardiovascular health care for women depends on improving the knowledge and awareness of all members of the healthcare team about the full spectrum of cardiovascular risk factors for women, including female-specific and female-predominant risk factors,” Dr. Mieres added.
Female-specific factors that should be included in CVD risk assessment include pregnancy-related conditions such as preeclampsia, preterm delivery, and gestational diabetes, the writing group said.
Other factors include menstrual cycle history; types of birth control and/or hormone replacement therapy used; polycystic ovarian syndrome (PCOS), which affects 10% of women of reproductive age and is associated with increased CVD risk; and autoimmune disorders, depression, and PTSD, all of which are more common in women and are also associated with higher risk for CVD.
The statement also highlights the key role that social determinants of health (SDOH) play in the development of CVD in women, particularly women from diverse racial and ethnic backgrounds. SDOH include education level, economic stability, neighborhood safety, working conditions, environmental hazards, and access to quality health care.
“It is critical that risk assessment be expanded to include [SDOH] as risk factors if we are to improve health outcomes in all women,” Laxmi Mehta, MD, chair of the writing group and director of preventative cardiology and women’s cardiovascular health at Ohio State University Wexner Medical Center, Columbus, said in the news release.
“It is also important for the health care team to consider [SDOH] when working with women on shared decisions about cardiovascular disease prevention and treatment,” Dr. Mehta noted.
No one-size-fits-all approach
The statement highlighted significant differences in CVD risk among women of different racial and ethnic backgrounds and provides detailed CV risk factor profiles for non-Hispanic Black, Hispanic/Latinx, Asian and American Indian/Alaska Native women.
It noted that language barriers, discrimination, acculturation, and health care access disproportionately affect women of underrepresented racial and ethnic groups. These factors result in a higher prevalence of CVD and significant challenges in CVD diagnosis and treatment.
“When customizing CVD prevention and treatment strategies to improve cardiovascular health for women, a one-size-fits-all approach is unlikely to be successful,” Dr. Mieres said.
“We must be cognizant of the complex interplay of sex, race and ethnicity, as well as social determinants of health, and how they impact the risk of cardiovascular disease and adverse outcomes in order to avert future CVD morbidity and mortality,” Dr. Mieres added.
Looking ahead, the writing group said future CVD prevention guidelines could be strengthened by including culturally-specific lifestyle recommendations.
They also said community-based approaches, faith-based community partnerships, and peer support to encourage a healthy lifestyle could play a key role in preventing CVD among all women.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA’s Cardiovascular Disease and Stroke in Women and Underrepresented Populations Committee of the Council on Clinical Cardiology, the Council on Cardiovascular and Stroke Nursing, the Council on Hypertension, the Council on Lifelong Congenital Heart Disease and Heart Health in the Young, the Council on Lifestyle and Cardiometabolic Health, the Council on Peripheral Vascular Disease, and the Stroke Council.
A version of this article first appeared on Medscape.com.
In a new scientific statement, the American Heart Association highlighted the importance of incorporating nonbiological risk factors and social determinants of health in cardiovascular disease (CVD) risk assessment for women, particularly women from different racial and ethnic backgrounds.
CVD risk assessment in women is multifaceted and goes well beyond traditional risk factors to include sex-specific biological risk factors, as well as social, behavioral, and environmental factors, the writing group noted.
They said a greater focus on addressing all CVD risk factors among women from underrepresented races and ethnicities is warranted to avert future CVD.
The scientific statement was published online in Circulation.
Look beyond traditional risk factors
“Risk assessment is the first step in preventing heart disease, yet there are many limitations to traditional risk factors and their ability to comprehensively estimate a woman’s risk for cardiovascular disease,” Jennifer H. Mieres, MD, vice chair of the writing group and professor of cardiology at Hofstra University, Hempstead, N.Y., said in a news release.
“The delivery of equitable cardiovascular health care for women depends on improving the knowledge and awareness of all members of the healthcare team about the full spectrum of cardiovascular risk factors for women, including female-specific and female-predominant risk factors,” Dr. Mieres added.
Female-specific factors that should be included in CVD risk assessment include pregnancy-related conditions such as preeclampsia, preterm delivery, and gestational diabetes, the writing group said.
Other factors include menstrual cycle history; types of birth control and/or hormone replacement therapy used; polycystic ovarian syndrome (PCOS), which affects 10% of women of reproductive age and is associated with increased CVD risk; and autoimmune disorders, depression, and PTSD, all of which are more common in women and are also associated with higher risk for CVD.
The statement also highlights the key role that social determinants of health (SDOH) play in the development of CVD in women, particularly women from diverse racial and ethnic backgrounds. SDOH include education level, economic stability, neighborhood safety, working conditions, environmental hazards, and access to quality health care.
“It is critical that risk assessment be expanded to include [SDOH] as risk factors if we are to improve health outcomes in all women,” Laxmi Mehta, MD, chair of the writing group and director of preventative cardiology and women’s cardiovascular health at Ohio State University Wexner Medical Center, Columbus, said in the news release.
“It is also important for the health care team to consider [SDOH] when working with women on shared decisions about cardiovascular disease prevention and treatment,” Dr. Mehta noted.
No one-size-fits-all approach
The statement highlighted significant differences in CVD risk among women of different racial and ethnic backgrounds and provides detailed CV risk factor profiles for non-Hispanic Black, Hispanic/Latinx, Asian and American Indian/Alaska Native women.
It noted that language barriers, discrimination, acculturation, and health care access disproportionately affect women of underrepresented racial and ethnic groups. These factors result in a higher prevalence of CVD and significant challenges in CVD diagnosis and treatment.
“When customizing CVD prevention and treatment strategies to improve cardiovascular health for women, a one-size-fits-all approach is unlikely to be successful,” Dr. Mieres said.
“We must be cognizant of the complex interplay of sex, race and ethnicity, as well as social determinants of health, and how they impact the risk of cardiovascular disease and adverse outcomes in order to avert future CVD morbidity and mortality,” Dr. Mieres added.
Looking ahead, the writing group said future CVD prevention guidelines could be strengthened by including culturally-specific lifestyle recommendations.
They also said community-based approaches, faith-based community partnerships, and peer support to encourage a healthy lifestyle could play a key role in preventing CVD among all women.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA’s Cardiovascular Disease and Stroke in Women and Underrepresented Populations Committee of the Council on Clinical Cardiology, the Council on Cardiovascular and Stroke Nursing, the Council on Hypertension, the Council on Lifelong Congenital Heart Disease and Heart Health in the Young, the Council on Lifestyle and Cardiometabolic Health, the Council on Peripheral Vascular Disease, and the Stroke Council.
A version of this article first appeared on Medscape.com.
In a new scientific statement, the American Heart Association highlighted the importance of incorporating nonbiological risk factors and social determinants of health in cardiovascular disease (CVD) risk assessment for women, particularly women from different racial and ethnic backgrounds.
CVD risk assessment in women is multifaceted and goes well beyond traditional risk factors to include sex-specific biological risk factors, as well as social, behavioral, and environmental factors, the writing group noted.
They said a greater focus on addressing all CVD risk factors among women from underrepresented races and ethnicities is warranted to avert future CVD.
The scientific statement was published online in Circulation.
Look beyond traditional risk factors
“Risk assessment is the first step in preventing heart disease, yet there are many limitations to traditional risk factors and their ability to comprehensively estimate a woman’s risk for cardiovascular disease,” Jennifer H. Mieres, MD, vice chair of the writing group and professor of cardiology at Hofstra University, Hempstead, N.Y., said in a news release.
“The delivery of equitable cardiovascular health care for women depends on improving the knowledge and awareness of all members of the healthcare team about the full spectrum of cardiovascular risk factors for women, including female-specific and female-predominant risk factors,” Dr. Mieres added.
Female-specific factors that should be included in CVD risk assessment include pregnancy-related conditions such as preeclampsia, preterm delivery, and gestational diabetes, the writing group said.
Other factors include menstrual cycle history; types of birth control and/or hormone replacement therapy used; polycystic ovarian syndrome (PCOS), which affects 10% of women of reproductive age and is associated with increased CVD risk; and autoimmune disorders, depression, and PTSD, all of which are more common in women and are also associated with higher risk for CVD.
The statement also highlights the key role that social determinants of health (SDOH) play in the development of CVD in women, particularly women from diverse racial and ethnic backgrounds. SDOH include education level, economic stability, neighborhood safety, working conditions, environmental hazards, and access to quality health care.
“It is critical that risk assessment be expanded to include [SDOH] as risk factors if we are to improve health outcomes in all women,” Laxmi Mehta, MD, chair of the writing group and director of preventative cardiology and women’s cardiovascular health at Ohio State University Wexner Medical Center, Columbus, said in the news release.
“It is also important for the health care team to consider [SDOH] when working with women on shared decisions about cardiovascular disease prevention and treatment,” Dr. Mehta noted.
No one-size-fits-all approach
The statement highlighted significant differences in CVD risk among women of different racial and ethnic backgrounds and provides detailed CV risk factor profiles for non-Hispanic Black, Hispanic/Latinx, Asian and American Indian/Alaska Native women.
It noted that language barriers, discrimination, acculturation, and health care access disproportionately affect women of underrepresented racial and ethnic groups. These factors result in a higher prevalence of CVD and significant challenges in CVD diagnosis and treatment.
“When customizing CVD prevention and treatment strategies to improve cardiovascular health for women, a one-size-fits-all approach is unlikely to be successful,” Dr. Mieres said.
“We must be cognizant of the complex interplay of sex, race and ethnicity, as well as social determinants of health, and how they impact the risk of cardiovascular disease and adverse outcomes in order to avert future CVD morbidity and mortality,” Dr. Mieres added.
Looking ahead, the writing group said future CVD prevention guidelines could be strengthened by including culturally-specific lifestyle recommendations.
They also said community-based approaches, faith-based community partnerships, and peer support to encourage a healthy lifestyle could play a key role in preventing CVD among all women.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA’s Cardiovascular Disease and Stroke in Women and Underrepresented Populations Committee of the Council on Clinical Cardiology, the Council on Cardiovascular and Stroke Nursing, the Council on Hypertension, the Council on Lifelong Congenital Heart Disease and Heart Health in the Young, the Council on Lifestyle and Cardiometabolic Health, the Council on Peripheral Vascular Disease, and the Stroke Council.
A version of this article first appeared on Medscape.com.
FROM CIRCULATION
NSAID use in diabetes may worsen risk for first HF hospitalization
suggests a prospective, controlled study.
Certain subgroups may account for much of the excess risk, the results suggest, including the very elderly, patients with uncontrolled diabetes, those prescribed an NSAID for the first time, and patients already taking both a renin-angiotensin system inhibitor (RASi) and a diuretic.
Such patients with a firm indication for NSAIDs potentially could “be the ones benefiting most from closer follow-up, reduced dosage, or other mitigation strategies,” Anders Holt, MD, said in an interview.
Dr. Holt, of Copenhagen University Hospital and Herlev-Gentofte Hospital in Hellerup, Denmark, is lead author on the analysis of Danish registry data published in the Journal of the American College of Cardiology. He presented essentially the same results in preliminary form at the 2022 annual congress of the European Society of Cardiology.
HF hospitalizations linked to NSAIDs, the published report notes, are often attributed to symptoms from temporary fluid overload, often without worsening cardiac function, that stem from the drugs’ renal effects.
“One could speculate,” Dr. Holt said, that such HF events might be less severe and even associated with better outcomes, compared with other forms of heart failure.
But the current analysis provides a hint to the contrary, he observed. The 5-year mortality was similar for patients with HF linked to NSAIDs and those with other forms of HF, “which could suggest that NSAID-associated heart failure is more than transient fluid overload.”
The drugs may promote HF through direct effects on the heart by any of several proposed mechanisms, including “induction of arrhythmias and heart fibrosis, vasoconstriction, subclinical inflammation, and blood pressure elevation,” Dr. Holt said.
The current study doesn’t determine whether NSAID-associated HF stems from transient fluid overload or direct cardiac effects, but it’s “most likely both.”
In other limitations, the analysis is unable to “reliably explore” whether promotion of HF is an NSAID class effect, a “clinically relevant” point given the drugs’ varying effects on cardiovascular risk, states an accompanying editorial. Nor was it able to determine whether the drugs exert a dose-response effect on HF risk, noted Hassan Khan, MD, PhD, Norton Healthcare, Louisville, Ky., and Setor K. Kunutsor, MD, PhD, University of Leicester (England).
Still, “given the well-established relationship between the use of NSAIDs and increased HF, these findings are not unexpected because type 2 diabetes is also a major risk factor for HF.”
But it may be “premature to issue guideline recommendations based on a single observational study,” the editorialists wrote. “Further robust clinical trial evidence is needed to replicate these results and investigate the relationship of the type and dose of NSAIDs with HF risk. However, it should be realized that short-term or long-term use of NSAIDs may be detrimental to cardiovascular health.”
The analysis covered 23,308 patients from throughout Denmark with a type 2 diabetes diagnosis and no HF history who experienced a first HF hospitalization; their age averaged 76 years and 39% were women.
They served as their own controls; their NSAID exposures at two 28-day periods preceding the HF event, the one immediately before and the other preceding it by 56 days, were compared as the index and control periods, respectively.
Exposure to NSAIDs was defined as obtaining a prescription for celecoxib, diclofenac, ibuprofen, or naproxen, “as these are NSAIDs used primarily in Denmark,” the report states.
The odds ratios for HF hospitalization associated with NSAID exposure within 28 days preceding the event were 1.43 (95% confidence interval, 1.27-1.63) overall, 1.41 (95% CI, 1.16-1.71) for an NSAID given on top of both RASi and diuretics, 1.68 (95% CI, 1.00-2.88) for patients with elevated hemoglobin A1c, 1.78 (95% CI, 1.39-2.28) for those 80 or older, and 2.71 (95% CI, 1.78-4.23) for those with prior NSAID use.
That NSAID use and diabetes are each associated with increased risk for HF is well established, Dr. Holt observed. Yet the drugs had been prescribed to 16% of patients in the study.
“One of the more surprising findings, to me, was the quite substantial use of prescribed NSAIDs in a population of patients with diabetes, a patient group with a well-established cardiovascular risk,” he said.
“This patient group is only growing, so emphasis on the possible associations between even short-term NSAID use and incident heart failure is probably timely and perhaps needed.”
Dr. Holt and the study were supported by grants from Ib Mogens Kristiansens Almene Fond, Helsefonden, Snedkermester Sophus Jacobsen og hustru Astrid Jacobsen Fond, Marie og M.B. Richters Fond, and the Dagmar Marshalls Fond. Dr. Khan and Dr. Kunutsor reported no relevant relationships.
A version of this article first appeared on Medscape.com.
suggests a prospective, controlled study.
Certain subgroups may account for much of the excess risk, the results suggest, including the very elderly, patients with uncontrolled diabetes, those prescribed an NSAID for the first time, and patients already taking both a renin-angiotensin system inhibitor (RASi) and a diuretic.
Such patients with a firm indication for NSAIDs potentially could “be the ones benefiting most from closer follow-up, reduced dosage, or other mitigation strategies,” Anders Holt, MD, said in an interview.
Dr. Holt, of Copenhagen University Hospital and Herlev-Gentofte Hospital in Hellerup, Denmark, is lead author on the analysis of Danish registry data published in the Journal of the American College of Cardiology. He presented essentially the same results in preliminary form at the 2022 annual congress of the European Society of Cardiology.
HF hospitalizations linked to NSAIDs, the published report notes, are often attributed to symptoms from temporary fluid overload, often without worsening cardiac function, that stem from the drugs’ renal effects.
“One could speculate,” Dr. Holt said, that such HF events might be less severe and even associated with better outcomes, compared with other forms of heart failure.
But the current analysis provides a hint to the contrary, he observed. The 5-year mortality was similar for patients with HF linked to NSAIDs and those with other forms of HF, “which could suggest that NSAID-associated heart failure is more than transient fluid overload.”
The drugs may promote HF through direct effects on the heart by any of several proposed mechanisms, including “induction of arrhythmias and heart fibrosis, vasoconstriction, subclinical inflammation, and blood pressure elevation,” Dr. Holt said.
The current study doesn’t determine whether NSAID-associated HF stems from transient fluid overload or direct cardiac effects, but it’s “most likely both.”
In other limitations, the analysis is unable to “reliably explore” whether promotion of HF is an NSAID class effect, a “clinically relevant” point given the drugs’ varying effects on cardiovascular risk, states an accompanying editorial. Nor was it able to determine whether the drugs exert a dose-response effect on HF risk, noted Hassan Khan, MD, PhD, Norton Healthcare, Louisville, Ky., and Setor K. Kunutsor, MD, PhD, University of Leicester (England).
Still, “given the well-established relationship between the use of NSAIDs and increased HF, these findings are not unexpected because type 2 diabetes is also a major risk factor for HF.”
But it may be “premature to issue guideline recommendations based on a single observational study,” the editorialists wrote. “Further robust clinical trial evidence is needed to replicate these results and investigate the relationship of the type and dose of NSAIDs with HF risk. However, it should be realized that short-term or long-term use of NSAIDs may be detrimental to cardiovascular health.”
The analysis covered 23,308 patients from throughout Denmark with a type 2 diabetes diagnosis and no HF history who experienced a first HF hospitalization; their age averaged 76 years and 39% were women.
They served as their own controls; their NSAID exposures at two 28-day periods preceding the HF event, the one immediately before and the other preceding it by 56 days, were compared as the index and control periods, respectively.
Exposure to NSAIDs was defined as obtaining a prescription for celecoxib, diclofenac, ibuprofen, or naproxen, “as these are NSAIDs used primarily in Denmark,” the report states.
The odds ratios for HF hospitalization associated with NSAID exposure within 28 days preceding the event were 1.43 (95% confidence interval, 1.27-1.63) overall, 1.41 (95% CI, 1.16-1.71) for an NSAID given on top of both RASi and diuretics, 1.68 (95% CI, 1.00-2.88) for patients with elevated hemoglobin A1c, 1.78 (95% CI, 1.39-2.28) for those 80 or older, and 2.71 (95% CI, 1.78-4.23) for those with prior NSAID use.
That NSAID use and diabetes are each associated with increased risk for HF is well established, Dr. Holt observed. Yet the drugs had been prescribed to 16% of patients in the study.
“One of the more surprising findings, to me, was the quite substantial use of prescribed NSAIDs in a population of patients with diabetes, a patient group with a well-established cardiovascular risk,” he said.
“This patient group is only growing, so emphasis on the possible associations between even short-term NSAID use and incident heart failure is probably timely and perhaps needed.”
Dr. Holt and the study were supported by grants from Ib Mogens Kristiansens Almene Fond, Helsefonden, Snedkermester Sophus Jacobsen og hustru Astrid Jacobsen Fond, Marie og M.B. Richters Fond, and the Dagmar Marshalls Fond. Dr. Khan and Dr. Kunutsor reported no relevant relationships.
A version of this article first appeared on Medscape.com.
suggests a prospective, controlled study.
Certain subgroups may account for much of the excess risk, the results suggest, including the very elderly, patients with uncontrolled diabetes, those prescribed an NSAID for the first time, and patients already taking both a renin-angiotensin system inhibitor (RASi) and a diuretic.
Such patients with a firm indication for NSAIDs potentially could “be the ones benefiting most from closer follow-up, reduced dosage, or other mitigation strategies,” Anders Holt, MD, said in an interview.
Dr. Holt, of Copenhagen University Hospital and Herlev-Gentofte Hospital in Hellerup, Denmark, is lead author on the analysis of Danish registry data published in the Journal of the American College of Cardiology. He presented essentially the same results in preliminary form at the 2022 annual congress of the European Society of Cardiology.
HF hospitalizations linked to NSAIDs, the published report notes, are often attributed to symptoms from temporary fluid overload, often without worsening cardiac function, that stem from the drugs’ renal effects.
“One could speculate,” Dr. Holt said, that such HF events might be less severe and even associated with better outcomes, compared with other forms of heart failure.
But the current analysis provides a hint to the contrary, he observed. The 5-year mortality was similar for patients with HF linked to NSAIDs and those with other forms of HF, “which could suggest that NSAID-associated heart failure is more than transient fluid overload.”
The drugs may promote HF through direct effects on the heart by any of several proposed mechanisms, including “induction of arrhythmias and heart fibrosis, vasoconstriction, subclinical inflammation, and blood pressure elevation,” Dr. Holt said.
The current study doesn’t determine whether NSAID-associated HF stems from transient fluid overload or direct cardiac effects, but it’s “most likely both.”
In other limitations, the analysis is unable to “reliably explore” whether promotion of HF is an NSAID class effect, a “clinically relevant” point given the drugs’ varying effects on cardiovascular risk, states an accompanying editorial. Nor was it able to determine whether the drugs exert a dose-response effect on HF risk, noted Hassan Khan, MD, PhD, Norton Healthcare, Louisville, Ky., and Setor K. Kunutsor, MD, PhD, University of Leicester (England).
Still, “given the well-established relationship between the use of NSAIDs and increased HF, these findings are not unexpected because type 2 diabetes is also a major risk factor for HF.”
But it may be “premature to issue guideline recommendations based on a single observational study,” the editorialists wrote. “Further robust clinical trial evidence is needed to replicate these results and investigate the relationship of the type and dose of NSAIDs with HF risk. However, it should be realized that short-term or long-term use of NSAIDs may be detrimental to cardiovascular health.”
The analysis covered 23,308 patients from throughout Denmark with a type 2 diabetes diagnosis and no HF history who experienced a first HF hospitalization; their age averaged 76 years and 39% were women.
They served as their own controls; their NSAID exposures at two 28-day periods preceding the HF event, the one immediately before and the other preceding it by 56 days, were compared as the index and control periods, respectively.
Exposure to NSAIDs was defined as obtaining a prescription for celecoxib, diclofenac, ibuprofen, or naproxen, “as these are NSAIDs used primarily in Denmark,” the report states.
The odds ratios for HF hospitalization associated with NSAID exposure within 28 days preceding the event were 1.43 (95% confidence interval, 1.27-1.63) overall, 1.41 (95% CI, 1.16-1.71) for an NSAID given on top of both RASi and diuretics, 1.68 (95% CI, 1.00-2.88) for patients with elevated hemoglobin A1c, 1.78 (95% CI, 1.39-2.28) for those 80 or older, and 2.71 (95% CI, 1.78-4.23) for those with prior NSAID use.
That NSAID use and diabetes are each associated with increased risk for HF is well established, Dr. Holt observed. Yet the drugs had been prescribed to 16% of patients in the study.
“One of the more surprising findings, to me, was the quite substantial use of prescribed NSAIDs in a population of patients with diabetes, a patient group with a well-established cardiovascular risk,” he said.
“This patient group is only growing, so emphasis on the possible associations between even short-term NSAID use and incident heart failure is probably timely and perhaps needed.”
Dr. Holt and the study were supported by grants from Ib Mogens Kristiansens Almene Fond, Helsefonden, Snedkermester Sophus Jacobsen og hustru Astrid Jacobsen Fond, Marie og M.B. Richters Fond, and the Dagmar Marshalls Fond. Dr. Khan and Dr. Kunutsor reported no relevant relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Parents of patients with rheumatic disease, MIS-C strongly hesitant of COVID vaccination
NEW ORLEANS – Parents’ concerns about vaccinating their children against COVID-19 remain a substantial barrier to immunizing children against the disease, whether those children have chronic rheumatologic conditions or a history of multisystem inflammatory syndrome in children (MIS-C), according to two studies presented at the Pediatric Rheumatology Symposium.
Parents of children who developed MIS-C after a SARS-CoV-2 infection were particularly hesitant to vaccinate, despite strong encouragement from health care professionals at Baylor College of Medicine, Houston, said the presenter of one of the studies.
“Unfortunately, it remains unclear who is susceptible and what the mechanisms are” when it comes to MIS-C, Mariana Sanchez Villa, MS, a research coordinator at Baylor, told attendees. “Because of this, there is much hesitancy to vaccinate children with a history of MIS-C against COVID-19 out of a fear that hyperinflammation may occur.”
Ms. Sanchez Villa reported findings on the vaccination rate among patients who had been hospitalized with MIS-C. The researchers included all 295 patients who presented at the hospital with MIS-C between May 2020 and October 2022. Overall, 5% of these patients had been vaccinated against COVID-19 before they were diagnosed with MIS-C. When all these patients and their families came to outpatient follow-up appointments after discharge, the subspecialist clinicians recommended the children receive the COVID-19 vaccine 3 months after discharge. The researchers then reviewed the patients’ charts to see who did and did not receive the vaccine, which they confirmed through the state’s immunization registry.
Among the 295 patients with MIS-C, 1 died, and 99 (34%) received at least one COVID-19 vaccine dose after their diagnosis, including 7 of the 15 who had also been vaccinated prior to their MIS-C diagnosis. Just over half of the vaccinated patients (58%) were male. They received their vaccine an average 8.8 months after their hospitalization, when they were an average 10 years old, and all but one of the vaccine doses they received were the Pfizer/BioNTech mRNA vaccine.
Only 9 of the 99 vaccinated patients are fully vaccinated, defined as receiving the primary series plus the recommended boosters. Of the other patients, 13 received only one dose of the vaccine, 60 received two doses, and 17 received at least three doses of the primary series doses but no bivalent boosters. Over a subsequent average 11 months of follow-up, none of the vaccinated patients returned to the hospital with a recurrence of MIS-C or any other hyperinflammatory condition. The seven patients who had been vaccinated both before and after their MIS-C diagnosis have also not had any recurrence of a hyperinflammatory condition.
“SARS-CoV-2 vaccination is well-tolerated by children with a history of MIS-C,” the researchers concluded. Ms. Sanchez Villa referenced two other studies, in The Pediatric Infectious Disease Journal and in JAMA Network Open, with similar findings on the safety of COVID-19 vaccination in patients who have had MIS-C. “This is reassuring as SARS-CoV-2 becomes endemic and annual vaccination against SARS-CoV-2 is considered.”
Dilan Dissanayake, MD, PhD, a rheumatologist at The Hospital for Sick Children in Toronto, who attended the presentation, told this news organization that data increasingly show a “synergistic protective effect” from COVID-19 infection and vaccination. That is, “having COVID or having MIS-C once doesn’t necessarily preclude you from having it again,” thereby supporting the importance of vaccination after an MIS-C diagnosis. In talking to parents about vaccinating, he has found it most helpful for them to hear about rheumatologists’ experience regarding COVID-19 vaccination.
“Particularly as the pandemic went on, being able to comfortably say that we have this large patient group, as well as collaborators across the world who have been monitoring for any safety issues, and that all the data has been reassuring” has been most useful for parents to hear, Dr. Dissanayake said.
The other study, led by Beth Rutstein, MD, MSCE, an attending rheumatologist at Children’s Hospital of Philadelphia, focused on the population of pediatric rheumatology patients by surveying pediatric rheumatologists who were members of the Childhood Arthritis and Rheumatology Research Alliance. The survey, conducted from March to May 2022, included questions about the rheumatologists’ COVID-19 vaccination practices as well as perceptions of the vaccine by the parents of their patients.
The 219 respondents included 74% pediatric rheumatologists and 21% fellows. Nearly all the respondents (98%) believed that any disease flares after COVID-19 vaccination would be mild and/or rare, and nearly all (98%) recommend their patients be vaccinated against COVID-19.
The primary finding from the study was that “we [rheumatologists] have different concerns from the families,” coauthor and presenter Vidya Sivaraman, MD, a pediatric rheumatologist at Nationwide Children’s Hospital and the Ohio State University in Columbus, told this news organization. “We’re more worried about the efficacy of the vaccine on immunosuppressive medications,” such as rituximab, which depletes B cells, Dr. Sivaraman said, but concerns about the vaccine’s immunogenicity or efficacy were very low among parents.
Just over half the clinicians surveyed (59%) were concerned about how effective the vaccine would be for their patients, especially those receiving immunosuppressive therapy. Health care professionals were most concerned about patients on rituximab – all clinicians reported concerns about the vaccine’s effectiveness in these patients – followed by patients taking systemic corticosteroids (86%), mycophenolate mofetil (59%), and Janus kinase inhibitors (46%).
Most clinicians (88%) reported that they had temporarily modified a patient’s immunosuppressive therapy to allow for vaccination, following guidelines by the American College of Rheumatology. Aside from a small proportion of health care professionals who checked patients’ post-vaccination serology primarily for research purposes, most clinicians (82%) did not collect this serology.
In regard to adverse events, the concern cited most often by respondents was myocarditis (76%), followed by development of new autoimmune conditions (29%) and thrombosis (22%), but the clinicians ranked these adverse events as low risk.
Meanwhile, the top three concerns about vaccination among parents, as reported to physicians, were worries about side effects, lack of long-term safety data on the vaccine, and misinformation they had heard, such as anxiety about changes to their child’s genetics or vaccination causing a COVID-19 infection. “They’re seeing things on social media from other parents [saying that COVID-19 vaccines are] going to affect their fertility, so they don’t want their daughters to get it,” Dr. Sivaraman said as another example of commonly cited misinformation.
Nearly half of the respondents (47%) said more than half of their families had concerns about side effects and the lack of data on long-term outcomes after vaccination. Only 8.5% of physicians said that fewer than 10% of their families were anxious about side effects. In addition, 39% of physicians said more than half of their families had concerns about misinformation they had heard, and only 16% of physicians had heard about misinformation concerns from fewer than 10% of their patients.
Other concerns cited by parents included their child’s disease flaring; lack of data on how well the vaccine would stimulate their child’s immune system; their child having already had COVID-19; and not believing COVID-19 was a major health risk to their child. Nearly every respondent (98%) said they had parents who turned down COVID-19 vaccination, and a majority (75%) reported that more than 10% of their patients had parents who were hesitant about COVID-19 vaccination.
No external funding was noted for either study. Ms. Sanchez Villa had no relevant financial relationships, but two abstract coauthors reported financial relationships with Pfizer and Moderna, and one reported a financial relationship with Novartis. Dr. Rutstein, Dr. Sivaraman, and Dr. Dissanayake had no relevant financial relationships.
A version of this article first appeared on Medscape.com.
NEW ORLEANS – Parents’ concerns about vaccinating their children against COVID-19 remain a substantial barrier to immunizing children against the disease, whether those children have chronic rheumatologic conditions or a history of multisystem inflammatory syndrome in children (MIS-C), according to two studies presented at the Pediatric Rheumatology Symposium.
Parents of children who developed MIS-C after a SARS-CoV-2 infection were particularly hesitant to vaccinate, despite strong encouragement from health care professionals at Baylor College of Medicine, Houston, said the presenter of one of the studies.
“Unfortunately, it remains unclear who is susceptible and what the mechanisms are” when it comes to MIS-C, Mariana Sanchez Villa, MS, a research coordinator at Baylor, told attendees. “Because of this, there is much hesitancy to vaccinate children with a history of MIS-C against COVID-19 out of a fear that hyperinflammation may occur.”
Ms. Sanchez Villa reported findings on the vaccination rate among patients who had been hospitalized with MIS-C. The researchers included all 295 patients who presented at the hospital with MIS-C between May 2020 and October 2022. Overall, 5% of these patients had been vaccinated against COVID-19 before they were diagnosed with MIS-C. When all these patients and their families came to outpatient follow-up appointments after discharge, the subspecialist clinicians recommended the children receive the COVID-19 vaccine 3 months after discharge. The researchers then reviewed the patients’ charts to see who did and did not receive the vaccine, which they confirmed through the state’s immunization registry.
Among the 295 patients with MIS-C, 1 died, and 99 (34%) received at least one COVID-19 vaccine dose after their diagnosis, including 7 of the 15 who had also been vaccinated prior to their MIS-C diagnosis. Just over half of the vaccinated patients (58%) were male. They received their vaccine an average 8.8 months after their hospitalization, when they were an average 10 years old, and all but one of the vaccine doses they received were the Pfizer/BioNTech mRNA vaccine.
Only 9 of the 99 vaccinated patients are fully vaccinated, defined as receiving the primary series plus the recommended boosters. Of the other patients, 13 received only one dose of the vaccine, 60 received two doses, and 17 received at least three doses of the primary series doses but no bivalent boosters. Over a subsequent average 11 months of follow-up, none of the vaccinated patients returned to the hospital with a recurrence of MIS-C or any other hyperinflammatory condition. The seven patients who had been vaccinated both before and after their MIS-C diagnosis have also not had any recurrence of a hyperinflammatory condition.
“SARS-CoV-2 vaccination is well-tolerated by children with a history of MIS-C,” the researchers concluded. Ms. Sanchez Villa referenced two other studies, in The Pediatric Infectious Disease Journal and in JAMA Network Open, with similar findings on the safety of COVID-19 vaccination in patients who have had MIS-C. “This is reassuring as SARS-CoV-2 becomes endemic and annual vaccination against SARS-CoV-2 is considered.”
Dilan Dissanayake, MD, PhD, a rheumatologist at The Hospital for Sick Children in Toronto, who attended the presentation, told this news organization that data increasingly show a “synergistic protective effect” from COVID-19 infection and vaccination. That is, “having COVID or having MIS-C once doesn’t necessarily preclude you from having it again,” thereby supporting the importance of vaccination after an MIS-C diagnosis. In talking to parents about vaccinating, he has found it most helpful for them to hear about rheumatologists’ experience regarding COVID-19 vaccination.
“Particularly as the pandemic went on, being able to comfortably say that we have this large patient group, as well as collaborators across the world who have been monitoring for any safety issues, and that all the data has been reassuring” has been most useful for parents to hear, Dr. Dissanayake said.
The other study, led by Beth Rutstein, MD, MSCE, an attending rheumatologist at Children’s Hospital of Philadelphia, focused on the population of pediatric rheumatology patients by surveying pediatric rheumatologists who were members of the Childhood Arthritis and Rheumatology Research Alliance. The survey, conducted from March to May 2022, included questions about the rheumatologists’ COVID-19 vaccination practices as well as perceptions of the vaccine by the parents of their patients.
The 219 respondents included 74% pediatric rheumatologists and 21% fellows. Nearly all the respondents (98%) believed that any disease flares after COVID-19 vaccination would be mild and/or rare, and nearly all (98%) recommend their patients be vaccinated against COVID-19.
The primary finding from the study was that “we [rheumatologists] have different concerns from the families,” coauthor and presenter Vidya Sivaraman, MD, a pediatric rheumatologist at Nationwide Children’s Hospital and the Ohio State University in Columbus, told this news organization. “We’re more worried about the efficacy of the vaccine on immunosuppressive medications,” such as rituximab, which depletes B cells, Dr. Sivaraman said, but concerns about the vaccine’s immunogenicity or efficacy were very low among parents.
Just over half the clinicians surveyed (59%) were concerned about how effective the vaccine would be for their patients, especially those receiving immunosuppressive therapy. Health care professionals were most concerned about patients on rituximab – all clinicians reported concerns about the vaccine’s effectiveness in these patients – followed by patients taking systemic corticosteroids (86%), mycophenolate mofetil (59%), and Janus kinase inhibitors (46%).
Most clinicians (88%) reported that they had temporarily modified a patient’s immunosuppressive therapy to allow for vaccination, following guidelines by the American College of Rheumatology. Aside from a small proportion of health care professionals who checked patients’ post-vaccination serology primarily for research purposes, most clinicians (82%) did not collect this serology.
In regard to adverse events, the concern cited most often by respondents was myocarditis (76%), followed by development of new autoimmune conditions (29%) and thrombosis (22%), but the clinicians ranked these adverse events as low risk.
Meanwhile, the top three concerns about vaccination among parents, as reported to physicians, were worries about side effects, lack of long-term safety data on the vaccine, and misinformation they had heard, such as anxiety about changes to their child’s genetics or vaccination causing a COVID-19 infection. “They’re seeing things on social media from other parents [saying that COVID-19 vaccines are] going to affect their fertility, so they don’t want their daughters to get it,” Dr. Sivaraman said as another example of commonly cited misinformation.
Nearly half of the respondents (47%) said more than half of their families had concerns about side effects and the lack of data on long-term outcomes after vaccination. Only 8.5% of physicians said that fewer than 10% of their families were anxious about side effects. In addition, 39% of physicians said more than half of their families had concerns about misinformation they had heard, and only 16% of physicians had heard about misinformation concerns from fewer than 10% of their patients.
Other concerns cited by parents included their child’s disease flaring; lack of data on how well the vaccine would stimulate their child’s immune system; their child having already had COVID-19; and not believing COVID-19 was a major health risk to their child. Nearly every respondent (98%) said they had parents who turned down COVID-19 vaccination, and a majority (75%) reported that more than 10% of their patients had parents who were hesitant about COVID-19 vaccination.
No external funding was noted for either study. Ms. Sanchez Villa had no relevant financial relationships, but two abstract coauthors reported financial relationships with Pfizer and Moderna, and one reported a financial relationship with Novartis. Dr. Rutstein, Dr. Sivaraman, and Dr. Dissanayake had no relevant financial relationships.
A version of this article first appeared on Medscape.com.
NEW ORLEANS – Parents’ concerns about vaccinating their children against COVID-19 remain a substantial barrier to immunizing children against the disease, whether those children have chronic rheumatologic conditions or a history of multisystem inflammatory syndrome in children (MIS-C), according to two studies presented at the Pediatric Rheumatology Symposium.
Parents of children who developed MIS-C after a SARS-CoV-2 infection were particularly hesitant to vaccinate, despite strong encouragement from health care professionals at Baylor College of Medicine, Houston, said the presenter of one of the studies.
“Unfortunately, it remains unclear who is susceptible and what the mechanisms are” when it comes to MIS-C, Mariana Sanchez Villa, MS, a research coordinator at Baylor, told attendees. “Because of this, there is much hesitancy to vaccinate children with a history of MIS-C against COVID-19 out of a fear that hyperinflammation may occur.”
Ms. Sanchez Villa reported findings on the vaccination rate among patients who had been hospitalized with MIS-C. The researchers included all 295 patients who presented at the hospital with MIS-C between May 2020 and October 2022. Overall, 5% of these patients had been vaccinated against COVID-19 before they were diagnosed with MIS-C. When all these patients and their families came to outpatient follow-up appointments after discharge, the subspecialist clinicians recommended the children receive the COVID-19 vaccine 3 months after discharge. The researchers then reviewed the patients’ charts to see who did and did not receive the vaccine, which they confirmed through the state’s immunization registry.
Among the 295 patients with MIS-C, 1 died, and 99 (34%) received at least one COVID-19 vaccine dose after their diagnosis, including 7 of the 15 who had also been vaccinated prior to their MIS-C diagnosis. Just over half of the vaccinated patients (58%) were male. They received their vaccine an average 8.8 months after their hospitalization, when they were an average 10 years old, and all but one of the vaccine doses they received were the Pfizer/BioNTech mRNA vaccine.
Only 9 of the 99 vaccinated patients are fully vaccinated, defined as receiving the primary series plus the recommended boosters. Of the other patients, 13 received only one dose of the vaccine, 60 received two doses, and 17 received at least three doses of the primary series doses but no bivalent boosters. Over a subsequent average 11 months of follow-up, none of the vaccinated patients returned to the hospital with a recurrence of MIS-C or any other hyperinflammatory condition. The seven patients who had been vaccinated both before and after their MIS-C diagnosis have also not had any recurrence of a hyperinflammatory condition.
“SARS-CoV-2 vaccination is well-tolerated by children with a history of MIS-C,” the researchers concluded. Ms. Sanchez Villa referenced two other studies, in The Pediatric Infectious Disease Journal and in JAMA Network Open, with similar findings on the safety of COVID-19 vaccination in patients who have had MIS-C. “This is reassuring as SARS-CoV-2 becomes endemic and annual vaccination against SARS-CoV-2 is considered.”
Dilan Dissanayake, MD, PhD, a rheumatologist at The Hospital for Sick Children in Toronto, who attended the presentation, told this news organization that data increasingly show a “synergistic protective effect” from COVID-19 infection and vaccination. That is, “having COVID or having MIS-C once doesn’t necessarily preclude you from having it again,” thereby supporting the importance of vaccination after an MIS-C diagnosis. In talking to parents about vaccinating, he has found it most helpful for them to hear about rheumatologists’ experience regarding COVID-19 vaccination.
“Particularly as the pandemic went on, being able to comfortably say that we have this large patient group, as well as collaborators across the world who have been monitoring for any safety issues, and that all the data has been reassuring” has been most useful for parents to hear, Dr. Dissanayake said.
The other study, led by Beth Rutstein, MD, MSCE, an attending rheumatologist at Children’s Hospital of Philadelphia, focused on the population of pediatric rheumatology patients by surveying pediatric rheumatologists who were members of the Childhood Arthritis and Rheumatology Research Alliance. The survey, conducted from March to May 2022, included questions about the rheumatologists’ COVID-19 vaccination practices as well as perceptions of the vaccine by the parents of their patients.
The 219 respondents included 74% pediatric rheumatologists and 21% fellows. Nearly all the respondents (98%) believed that any disease flares after COVID-19 vaccination would be mild and/or rare, and nearly all (98%) recommend their patients be vaccinated against COVID-19.
The primary finding from the study was that “we [rheumatologists] have different concerns from the families,” coauthor and presenter Vidya Sivaraman, MD, a pediatric rheumatologist at Nationwide Children’s Hospital and the Ohio State University in Columbus, told this news organization. “We’re more worried about the efficacy of the vaccine on immunosuppressive medications,” such as rituximab, which depletes B cells, Dr. Sivaraman said, but concerns about the vaccine’s immunogenicity or efficacy were very low among parents.
Just over half the clinicians surveyed (59%) were concerned about how effective the vaccine would be for their patients, especially those receiving immunosuppressive therapy. Health care professionals were most concerned about patients on rituximab – all clinicians reported concerns about the vaccine’s effectiveness in these patients – followed by patients taking systemic corticosteroids (86%), mycophenolate mofetil (59%), and Janus kinase inhibitors (46%).
Most clinicians (88%) reported that they had temporarily modified a patient’s immunosuppressive therapy to allow for vaccination, following guidelines by the American College of Rheumatology. Aside from a small proportion of health care professionals who checked patients’ post-vaccination serology primarily for research purposes, most clinicians (82%) did not collect this serology.
In regard to adverse events, the concern cited most often by respondents was myocarditis (76%), followed by development of new autoimmune conditions (29%) and thrombosis (22%), but the clinicians ranked these adverse events as low risk.
Meanwhile, the top three concerns about vaccination among parents, as reported to physicians, were worries about side effects, lack of long-term safety data on the vaccine, and misinformation they had heard, such as anxiety about changes to their child’s genetics or vaccination causing a COVID-19 infection. “They’re seeing things on social media from other parents [saying that COVID-19 vaccines are] going to affect their fertility, so they don’t want their daughters to get it,” Dr. Sivaraman said as another example of commonly cited misinformation.
Nearly half of the respondents (47%) said more than half of their families had concerns about side effects and the lack of data on long-term outcomes after vaccination. Only 8.5% of physicians said that fewer than 10% of their families were anxious about side effects. In addition, 39% of physicians said more than half of their families had concerns about misinformation they had heard, and only 16% of physicians had heard about misinformation concerns from fewer than 10% of their patients.
Other concerns cited by parents included their child’s disease flaring; lack of data on how well the vaccine would stimulate their child’s immune system; their child having already had COVID-19; and not believing COVID-19 was a major health risk to their child. Nearly every respondent (98%) said they had parents who turned down COVID-19 vaccination, and a majority (75%) reported that more than 10% of their patients had parents who were hesitant about COVID-19 vaccination.
No external funding was noted for either study. Ms. Sanchez Villa had no relevant financial relationships, but two abstract coauthors reported financial relationships with Pfizer and Moderna, and one reported a financial relationship with Novartis. Dr. Rutstein, Dr. Sivaraman, and Dr. Dissanayake had no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT PRSYM 2023
The earlier baricitinib for severe alopecia areata is started, the better
NEW ORLEANS – In the nearly 1 year .
“The journey to JAK inhibition in alopecia areata has been incredible,” Raj Chovatiya, MD, PhD, assistant professor of dermatology and director of the center for eczema and itch at Northwestern University, Chicago, said at the annual meeting of the American Academy of Dermatology. “JAK inhibitors are here to stay, and I think baricitinib offers an amazing opportunity for the right patients.”
The efficacy and safety of baricitinib (Olumiant) for AA was studied in two randomized, double-blind, placebo-controlled trials (BRAVE-AA1 and BRAVE-AA2) with patients who had at least 50% scalp hair loss as measured by the Severity of Alopecia Tool (SALT) for more than 6 months. Patients in these trials received either a placebo, 2 mg of baricitinib, or 4 mg of baricitinib every day. The primary measurement of efficacy for both trials was the proportion of patients who achieved a SALT score of 20 or less, or at least 80% scalp hair coverage at week 36. The researchers found that 36%-39% of individuals in the 4-mg arm achieved a SALT score of less than 20, compared with 19%-23% of individuals in the 2 mg arm. Similar outcomes were observed for eyebrow and eyelash hair loss.
Most adverse events observed in BRAVE-AA1 and BRAVE-AA2 were in the mild to moderate range, and the actual number of adverse events leading to permanent discontinuation was extremely low. The most common adverse events were upper respiratory tract infections, headache, nasopharyngitis, acne, urinary tract infections, and an increase in blood creatine kinase.
Baricitinib is not recommended for use in combination with other JAK inhibitors, biologic immunomodulators, or other potent immunosuppressants, Dr. Chovatiya said. Required lab evaluations include baseline testing for tuberculosis and viral hepatitis; CBC, hepatic function, and renal function at baseline and then as clinically indicated; and lipids after 12 weeks of therapy, then as clinically indicated. The recommended starting dose of baricitinib is 2 mg per day, which can be increased to 4 mg per day if the response is not adequate. “However, for patients with nearly complete or complete scalp hair loss, with or without substantial eyelash or eyebrow hair loss, 4 mg once daily is recommended,” he said. “Once an adequate response is achieved, it’s recommended to reduce from 4 to 2 mg daily.”
52-week, 76-week data
According to pooled data from BRAVE-AA1 and BRAVE-AA2 published online March 1, 2023, efficacy continues to increase out to 52 weeks. Specifically, by week 52, 39% of individuals in the 4 mg arm achieved a SALT score of 20 or less, compared with 22.6% of individuals in the 2 mg arm. “You see similar linear growth in the eyebrow and eyelash response loss as well,” Dr. Chovatiya said.
In other findings, patients in the 4 mg treatment arm who achieved a SALT score of 20 or less at week 52 were eligible for randomized down titration, provided that they had stayed on the same dose of baricitinib from initial randomization. According to data from baricitinib manufacturer Eli Lilly, 77.5% of patients who stepped down to the 2 mg dose from the 4 mg dose at week 52 achieved a SALT score of 20 or less at week 76, Dr. Chovatiya said. “If I can keep someone on 4 mg that’s great, but it looks like you can go to a lower dose and do a pretty good job,” he said.
Patients in the baricitinib arms who achieved a SALT score of 20 or less at week 52 were eligible for randomized withdrawal, provided that they had stayed on the same dose of the drug from initial randomization. According to Dr. Chovatiya, 89.4% of individuals who remained on the 4 mg dose to week 76 maintained a SALT score of 20 or less, compared with 33.3% of those who switched from the 4 mg to placebo. “The takeaway here is that clinically, longitudinal treatment looks to be required in this time period” for continued efficacy, he said. “However, what this looks like in the real world remains to be seen.”
A recently published integrated analysis of safety data from BRAVE-AA1 and BRAVE-AA2 reported that no deaths occurred and of the few reported serious infections, nearly half were COVID-19. There was a single case of multidermatomal herpes zoster and no cases of tuberculosis. One patient with risk factors for MI had an MI during a placebo-controlled period, and one study participant with a history of COVID-19 infection developed a pulmonary embolism at day 638. There was one case each of chronic lymphocytic leukemia, B-cell lymphoma, breast cancer, and appendicitis.
Baseline severity and treatment response
“Does treatment response vary with baseline disease status?” Dr. Chovatiya asked. “Yes. People with very severe hair loss [defined as a SALT score of 95 or higher] tended to do worse, while the rest of the study population did even better – an almost twofold difference. This means that you want to treat as early as you possibly can. It’s interesting to note that you don’t see this difference as much in the case of eyebrows and eyelashes. This makes sense, though. Eyebrows and eyelashes probably behave differently in terms of growth than the scalp does.”
Certain baseline characteristics of patients in BRAVE-AA1 and BRAVE-AA2 portended better outcomes. Women tended to fare better than men, but individuals who had longer histories of AA did not respond well. “People who had a shorter duration of their current episode of AA also did better than people who had a longer current episode, so we want to think about treating as soon as we possibly can,” Dr. Chovatiya said.
Dr. Chovatiya disclosed that he is a consultant to, a speaker for, investigator, and/or a member of the advisory board for several pharmaceutical companies, including Eli Lilly.
NEW ORLEANS – In the nearly 1 year .
“The journey to JAK inhibition in alopecia areata has been incredible,” Raj Chovatiya, MD, PhD, assistant professor of dermatology and director of the center for eczema and itch at Northwestern University, Chicago, said at the annual meeting of the American Academy of Dermatology. “JAK inhibitors are here to stay, and I think baricitinib offers an amazing opportunity for the right patients.”
The efficacy and safety of baricitinib (Olumiant) for AA was studied in two randomized, double-blind, placebo-controlled trials (BRAVE-AA1 and BRAVE-AA2) with patients who had at least 50% scalp hair loss as measured by the Severity of Alopecia Tool (SALT) for more than 6 months. Patients in these trials received either a placebo, 2 mg of baricitinib, or 4 mg of baricitinib every day. The primary measurement of efficacy for both trials was the proportion of patients who achieved a SALT score of 20 or less, or at least 80% scalp hair coverage at week 36. The researchers found that 36%-39% of individuals in the 4-mg arm achieved a SALT score of less than 20, compared with 19%-23% of individuals in the 2 mg arm. Similar outcomes were observed for eyebrow and eyelash hair loss.
Most adverse events observed in BRAVE-AA1 and BRAVE-AA2 were in the mild to moderate range, and the actual number of adverse events leading to permanent discontinuation was extremely low. The most common adverse events were upper respiratory tract infections, headache, nasopharyngitis, acne, urinary tract infections, and an increase in blood creatine kinase.
Baricitinib is not recommended for use in combination with other JAK inhibitors, biologic immunomodulators, or other potent immunosuppressants, Dr. Chovatiya said. Required lab evaluations include baseline testing for tuberculosis and viral hepatitis; CBC, hepatic function, and renal function at baseline and then as clinically indicated; and lipids after 12 weeks of therapy, then as clinically indicated. The recommended starting dose of baricitinib is 2 mg per day, which can be increased to 4 mg per day if the response is not adequate. “However, for patients with nearly complete or complete scalp hair loss, with or without substantial eyelash or eyebrow hair loss, 4 mg once daily is recommended,” he said. “Once an adequate response is achieved, it’s recommended to reduce from 4 to 2 mg daily.”
52-week, 76-week data
According to pooled data from BRAVE-AA1 and BRAVE-AA2 published online March 1, 2023, efficacy continues to increase out to 52 weeks. Specifically, by week 52, 39% of individuals in the 4 mg arm achieved a SALT score of 20 or less, compared with 22.6% of individuals in the 2 mg arm. “You see similar linear growth in the eyebrow and eyelash response loss as well,” Dr. Chovatiya said.
In other findings, patients in the 4 mg treatment arm who achieved a SALT score of 20 or less at week 52 were eligible for randomized down titration, provided that they had stayed on the same dose of baricitinib from initial randomization. According to data from baricitinib manufacturer Eli Lilly, 77.5% of patients who stepped down to the 2 mg dose from the 4 mg dose at week 52 achieved a SALT score of 20 or less at week 76, Dr. Chovatiya said. “If I can keep someone on 4 mg that’s great, but it looks like you can go to a lower dose and do a pretty good job,” he said.
Patients in the baricitinib arms who achieved a SALT score of 20 or less at week 52 were eligible for randomized withdrawal, provided that they had stayed on the same dose of the drug from initial randomization. According to Dr. Chovatiya, 89.4% of individuals who remained on the 4 mg dose to week 76 maintained a SALT score of 20 or less, compared with 33.3% of those who switched from the 4 mg to placebo. “The takeaway here is that clinically, longitudinal treatment looks to be required in this time period” for continued efficacy, he said. “However, what this looks like in the real world remains to be seen.”
A recently published integrated analysis of safety data from BRAVE-AA1 and BRAVE-AA2 reported that no deaths occurred and of the few reported serious infections, nearly half were COVID-19. There was a single case of multidermatomal herpes zoster and no cases of tuberculosis. One patient with risk factors for MI had an MI during a placebo-controlled period, and one study participant with a history of COVID-19 infection developed a pulmonary embolism at day 638. There was one case each of chronic lymphocytic leukemia, B-cell lymphoma, breast cancer, and appendicitis.
Baseline severity and treatment response
“Does treatment response vary with baseline disease status?” Dr. Chovatiya asked. “Yes. People with very severe hair loss [defined as a SALT score of 95 or higher] tended to do worse, while the rest of the study population did even better – an almost twofold difference. This means that you want to treat as early as you possibly can. It’s interesting to note that you don’t see this difference as much in the case of eyebrows and eyelashes. This makes sense, though. Eyebrows and eyelashes probably behave differently in terms of growth than the scalp does.”
Certain baseline characteristics of patients in BRAVE-AA1 and BRAVE-AA2 portended better outcomes. Women tended to fare better than men, but individuals who had longer histories of AA did not respond well. “People who had a shorter duration of their current episode of AA also did better than people who had a longer current episode, so we want to think about treating as soon as we possibly can,” Dr. Chovatiya said.
Dr. Chovatiya disclosed that he is a consultant to, a speaker for, investigator, and/or a member of the advisory board for several pharmaceutical companies, including Eli Lilly.
NEW ORLEANS – In the nearly 1 year .
“The journey to JAK inhibition in alopecia areata has been incredible,” Raj Chovatiya, MD, PhD, assistant professor of dermatology and director of the center for eczema and itch at Northwestern University, Chicago, said at the annual meeting of the American Academy of Dermatology. “JAK inhibitors are here to stay, and I think baricitinib offers an amazing opportunity for the right patients.”
The efficacy and safety of baricitinib (Olumiant) for AA was studied in two randomized, double-blind, placebo-controlled trials (BRAVE-AA1 and BRAVE-AA2) with patients who had at least 50% scalp hair loss as measured by the Severity of Alopecia Tool (SALT) for more than 6 months. Patients in these trials received either a placebo, 2 mg of baricitinib, or 4 mg of baricitinib every day. The primary measurement of efficacy for both trials was the proportion of patients who achieved a SALT score of 20 or less, or at least 80% scalp hair coverage at week 36. The researchers found that 36%-39% of individuals in the 4-mg arm achieved a SALT score of less than 20, compared with 19%-23% of individuals in the 2 mg arm. Similar outcomes were observed for eyebrow and eyelash hair loss.
Most adverse events observed in BRAVE-AA1 and BRAVE-AA2 were in the mild to moderate range, and the actual number of adverse events leading to permanent discontinuation was extremely low. The most common adverse events were upper respiratory tract infections, headache, nasopharyngitis, acne, urinary tract infections, and an increase in blood creatine kinase.
Baricitinib is not recommended for use in combination with other JAK inhibitors, biologic immunomodulators, or other potent immunosuppressants, Dr. Chovatiya said. Required lab evaluations include baseline testing for tuberculosis and viral hepatitis; CBC, hepatic function, and renal function at baseline and then as clinically indicated; and lipids after 12 weeks of therapy, then as clinically indicated. The recommended starting dose of baricitinib is 2 mg per day, which can be increased to 4 mg per day if the response is not adequate. “However, for patients with nearly complete or complete scalp hair loss, with or without substantial eyelash or eyebrow hair loss, 4 mg once daily is recommended,” he said. “Once an adequate response is achieved, it’s recommended to reduce from 4 to 2 mg daily.”
52-week, 76-week data
According to pooled data from BRAVE-AA1 and BRAVE-AA2 published online March 1, 2023, efficacy continues to increase out to 52 weeks. Specifically, by week 52, 39% of individuals in the 4 mg arm achieved a SALT score of 20 or less, compared with 22.6% of individuals in the 2 mg arm. “You see similar linear growth in the eyebrow and eyelash response loss as well,” Dr. Chovatiya said.
In other findings, patients in the 4 mg treatment arm who achieved a SALT score of 20 or less at week 52 were eligible for randomized down titration, provided that they had stayed on the same dose of baricitinib from initial randomization. According to data from baricitinib manufacturer Eli Lilly, 77.5% of patients who stepped down to the 2 mg dose from the 4 mg dose at week 52 achieved a SALT score of 20 or less at week 76, Dr. Chovatiya said. “If I can keep someone on 4 mg that’s great, but it looks like you can go to a lower dose and do a pretty good job,” he said.
Patients in the baricitinib arms who achieved a SALT score of 20 or less at week 52 were eligible for randomized withdrawal, provided that they had stayed on the same dose of the drug from initial randomization. According to Dr. Chovatiya, 89.4% of individuals who remained on the 4 mg dose to week 76 maintained a SALT score of 20 or less, compared with 33.3% of those who switched from the 4 mg to placebo. “The takeaway here is that clinically, longitudinal treatment looks to be required in this time period” for continued efficacy, he said. “However, what this looks like in the real world remains to be seen.”
A recently published integrated analysis of safety data from BRAVE-AA1 and BRAVE-AA2 reported that no deaths occurred and of the few reported serious infections, nearly half were COVID-19. There was a single case of multidermatomal herpes zoster and no cases of tuberculosis. One patient with risk factors for MI had an MI during a placebo-controlled period, and one study participant with a history of COVID-19 infection developed a pulmonary embolism at day 638. There was one case each of chronic lymphocytic leukemia, B-cell lymphoma, breast cancer, and appendicitis.
Baseline severity and treatment response
“Does treatment response vary with baseline disease status?” Dr. Chovatiya asked. “Yes. People with very severe hair loss [defined as a SALT score of 95 or higher] tended to do worse, while the rest of the study population did even better – an almost twofold difference. This means that you want to treat as early as you possibly can. It’s interesting to note that you don’t see this difference as much in the case of eyebrows and eyelashes. This makes sense, though. Eyebrows and eyelashes probably behave differently in terms of growth than the scalp does.”
Certain baseline characteristics of patients in BRAVE-AA1 and BRAVE-AA2 portended better outcomes. Women tended to fare better than men, but individuals who had longer histories of AA did not respond well. “People who had a shorter duration of their current episode of AA also did better than people who had a longer current episode, so we want to think about treating as soon as we possibly can,” Dr. Chovatiya said.
Dr. Chovatiya disclosed that he is a consultant to, a speaker for, investigator, and/or a member of the advisory board for several pharmaceutical companies, including Eli Lilly.
AT AAD 2023