User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
When is an allergic reaction to raw plant food due to tree pollen?
A new guideline aims to help primary care doctors differentiate pollen food syndrome (PFS) – a cross-reactive allergic reaction to certain raw, but not cooked, plant foods – from other food allergies.
The guideline from the British Society of Allergy and Clinical Immunology (BSACI) focuses on birch tree pollen, the major sensitizing PFS allergen in Northern Europe. Providers may be able to diagnose PFS related to birch pollen from clinical history alone, including the foods involved and the rapidity of symptom onset, write lead author Isabel J. Skypala, PhD, RD, of Imperial College London, and her colleagues.
The new BSACI guideline for diagnosis and management of PFS was published in Clinical & Experimental Allergy.
PFS is common and increasingly prevalent
PFS – also called oral allergy syndrome and pollen food allergy syndrome – is common and increasingly prevalent. PFS can begin at any age but usually starts in pollen-sensitized school-age children and adults with seasonal allergic rhinitis.
Symptoms from similar proteins in food
Mild to moderate allergic symptoms develop quickly when people sensitized to birch pollen eat raw plant foods that contain proteins similar to those in the pollen, such as pathogenesis-related protein PR-10. The allergens are broken down by cooking or processing.
Symptoms usually occur immediately or within 15 minutes of eating. Patients may have tingling; itching or soreness in the mouth, throat, or ears; mild lip and oral mucosa angioedema; itchy hands, sneezing, or eye symptoms; tongue or pharynx angioedema; perioral rash; cough; abdominal pain; nausea; and/or worsening of eczema. In children, itch and rash may predominate.
Triggers depend on pollen type
PFS triggers vary depending on a person’s pollen sensitization, which is affected by their geographic area and local dietary habits. In the United Kingdom, almost 70% of birch-allergic adults and more than 40% of birch-allergic children have PFS, the authors write.
Typical triggers include eating apples, stone fruits, kiwis, carrots, celery, hazelnuts, almonds, walnuts, soymilk, and peanuts, as well as peeling potatoes or other root vegetables. Freshly prepared vegetable or fruit smoothies or juices, celery, soymilk, raw nuts, large quantities of roasted nuts, and concentrated nut products can cause more severe reactions.
Diagnostic clinical history
If a patient answers yes to these questions, they almost certainly have PFS, the authors write:
- Are symptoms caused by raw fruits, nuts, carrots, or celery?
- Are the same trigger foods tolerated when they’re cooked well or roasted?
- Do symptoms come immediately or within a few minutes of eating?
- Do symptoms occur in the oropharynx and include tingling, itching, or swelling?
- Does the patient have seasonal allergic rhinitis or sensitization to pollen?
Testing needed for some cases
Allergy tests may be needed for people who report atypical or severe reactions or who also react to cooked or processed plant foods, such as roasted nuts, nuts in foods, fruits or vegetables in juices and smoothies, and soy products other than milk. Tests may also be needed for people who react to foods that are not linked with PFS, such as cashews, pistachios, macadamias, sesame seeds, beans, lentils, and chickpeas.
Whether PFS reactions also occur to roasted hazelnuts, almonds, walnuts, Brazil nuts, or peanuts, either alone or in composite foods such as chocolates, spreads, desserts, and snacks, is unclear.
An oral food challenge to confirm PFS is needed only if the history and diagnostic tests are inconclusive or if the patient is avoiding multiple foods.
Dietary management
PFS is managed by excluding known trigger foods. This becomes challenging for patients with preexisting food allergies and for vegetarians and vegans.
Personalized dietary advice is needed to avoid nutritional imbalance, minimize anxiety and unnecessary food restrictions, and improve quality of life. Reactions after accidental exposure often resolve without medication, and if antihistamines are needed, they rarely require self-injectable devices.
Guideline helpful beyond the United Kingdom and birch pollen
Allyson S. Larkin, MD, associate professor of pediatrics at the University of Pittsburgh School of Medicine, told this news organization in an email that the guideline summarizes in great detail the pathophysiology behind PFS and highlights how component testing may help diagnose patients and manage the condition.
“Patients worry very much about the progression and severity of allergic reactions,” said Dr. Larkin, who was not involved in the guideline development.
“As the authors note, recognizing the nutritional consequences of dietary restrictions is important, and nutrition consults and suitable alternative suggestions are very helpful for these patients, especially for those with food allergy or who are vegetarian or vegan.”
Jill A. Poole, MD, professor of medicine and chief of the Division of Allergy and Immunology at the University of Nebraska College of Medicine, Omaha, noted that PFS, although common, is underrecognized by the public and by health care providers.
“People are not allergic to the specific food, but they are allergic to a seasonal allergen, such as birch tree, that cross-reacts with the food protein, which is typically changed with cooking,” she explained in an email.
“This differs from reactions by those who have moderate to severe allergic food-specific reactions that may include systemic reactions like anaphylaxis from eating certain foods,” she said.
“Importantly, the number of cross-reacting foods with seasonal pollens continues to grow, and the extent of testing has expanded in recent years,” advised Dr. Poole, who also was not involved in the guideline development.
The authors recommend further related research into food immunotherapy and other novel PFS treatments. They also want to raise awareness of factors affecting PFS prevalence, such as increased spread and allergenicity of pollen due to climate change, pollution, the global consumption of previously local traditional foods, and the increase in vegetarian and vegan diets.
The authors, Dr. Larkin, and Dr. Poole report no relevant financial relationships involving this guideline. The guideline was not funded.
A version of this article first appeared on Medscape.com.
A new guideline aims to help primary care doctors differentiate pollen food syndrome (PFS) – a cross-reactive allergic reaction to certain raw, but not cooked, plant foods – from other food allergies.
The guideline from the British Society of Allergy and Clinical Immunology (BSACI) focuses on birch tree pollen, the major sensitizing PFS allergen in Northern Europe. Providers may be able to diagnose PFS related to birch pollen from clinical history alone, including the foods involved and the rapidity of symptom onset, write lead author Isabel J. Skypala, PhD, RD, of Imperial College London, and her colleagues.
The new BSACI guideline for diagnosis and management of PFS was published in Clinical & Experimental Allergy.
PFS is common and increasingly prevalent
PFS – also called oral allergy syndrome and pollen food allergy syndrome – is common and increasingly prevalent. PFS can begin at any age but usually starts in pollen-sensitized school-age children and adults with seasonal allergic rhinitis.
Symptoms from similar proteins in food
Mild to moderate allergic symptoms develop quickly when people sensitized to birch pollen eat raw plant foods that contain proteins similar to those in the pollen, such as pathogenesis-related protein PR-10. The allergens are broken down by cooking or processing.
Symptoms usually occur immediately or within 15 minutes of eating. Patients may have tingling; itching or soreness in the mouth, throat, or ears; mild lip and oral mucosa angioedema; itchy hands, sneezing, or eye symptoms; tongue or pharynx angioedema; perioral rash; cough; abdominal pain; nausea; and/or worsening of eczema. In children, itch and rash may predominate.
Triggers depend on pollen type
PFS triggers vary depending on a person’s pollen sensitization, which is affected by their geographic area and local dietary habits. In the United Kingdom, almost 70% of birch-allergic adults and more than 40% of birch-allergic children have PFS, the authors write.
Typical triggers include eating apples, stone fruits, kiwis, carrots, celery, hazelnuts, almonds, walnuts, soymilk, and peanuts, as well as peeling potatoes or other root vegetables. Freshly prepared vegetable or fruit smoothies or juices, celery, soymilk, raw nuts, large quantities of roasted nuts, and concentrated nut products can cause more severe reactions.
Diagnostic clinical history
If a patient answers yes to these questions, they almost certainly have PFS, the authors write:
- Are symptoms caused by raw fruits, nuts, carrots, or celery?
- Are the same trigger foods tolerated when they’re cooked well or roasted?
- Do symptoms come immediately or within a few minutes of eating?
- Do symptoms occur in the oropharynx and include tingling, itching, or swelling?
- Does the patient have seasonal allergic rhinitis or sensitization to pollen?
Testing needed for some cases
Allergy tests may be needed for people who report atypical or severe reactions or who also react to cooked or processed plant foods, such as roasted nuts, nuts in foods, fruits or vegetables in juices and smoothies, and soy products other than milk. Tests may also be needed for people who react to foods that are not linked with PFS, such as cashews, pistachios, macadamias, sesame seeds, beans, lentils, and chickpeas.
Whether PFS reactions also occur to roasted hazelnuts, almonds, walnuts, Brazil nuts, or peanuts, either alone or in composite foods such as chocolates, spreads, desserts, and snacks, is unclear.
An oral food challenge to confirm PFS is needed only if the history and diagnostic tests are inconclusive or if the patient is avoiding multiple foods.
Dietary management
PFS is managed by excluding known trigger foods. This becomes challenging for patients with preexisting food allergies and for vegetarians and vegans.
Personalized dietary advice is needed to avoid nutritional imbalance, minimize anxiety and unnecessary food restrictions, and improve quality of life. Reactions after accidental exposure often resolve without medication, and if antihistamines are needed, they rarely require self-injectable devices.
Guideline helpful beyond the United Kingdom and birch pollen
Allyson S. Larkin, MD, associate professor of pediatrics at the University of Pittsburgh School of Medicine, told this news organization in an email that the guideline summarizes in great detail the pathophysiology behind PFS and highlights how component testing may help diagnose patients and manage the condition.
“Patients worry very much about the progression and severity of allergic reactions,” said Dr. Larkin, who was not involved in the guideline development.
“As the authors note, recognizing the nutritional consequences of dietary restrictions is important, and nutrition consults and suitable alternative suggestions are very helpful for these patients, especially for those with food allergy or who are vegetarian or vegan.”
Jill A. Poole, MD, professor of medicine and chief of the Division of Allergy and Immunology at the University of Nebraska College of Medicine, Omaha, noted that PFS, although common, is underrecognized by the public and by health care providers.
“People are not allergic to the specific food, but they are allergic to a seasonal allergen, such as birch tree, that cross-reacts with the food protein, which is typically changed with cooking,” she explained in an email.
“This differs from reactions by those who have moderate to severe allergic food-specific reactions that may include systemic reactions like anaphylaxis from eating certain foods,” she said.
“Importantly, the number of cross-reacting foods with seasonal pollens continues to grow, and the extent of testing has expanded in recent years,” advised Dr. Poole, who also was not involved in the guideline development.
The authors recommend further related research into food immunotherapy and other novel PFS treatments. They also want to raise awareness of factors affecting PFS prevalence, such as increased spread and allergenicity of pollen due to climate change, pollution, the global consumption of previously local traditional foods, and the increase in vegetarian and vegan diets.
The authors, Dr. Larkin, and Dr. Poole report no relevant financial relationships involving this guideline. The guideline was not funded.
A version of this article first appeared on Medscape.com.
A new guideline aims to help primary care doctors differentiate pollen food syndrome (PFS) – a cross-reactive allergic reaction to certain raw, but not cooked, plant foods – from other food allergies.
The guideline from the British Society of Allergy and Clinical Immunology (BSACI) focuses on birch tree pollen, the major sensitizing PFS allergen in Northern Europe. Providers may be able to diagnose PFS related to birch pollen from clinical history alone, including the foods involved and the rapidity of symptom onset, write lead author Isabel J. Skypala, PhD, RD, of Imperial College London, and her colleagues.
The new BSACI guideline for diagnosis and management of PFS was published in Clinical & Experimental Allergy.
PFS is common and increasingly prevalent
PFS – also called oral allergy syndrome and pollen food allergy syndrome – is common and increasingly prevalent. PFS can begin at any age but usually starts in pollen-sensitized school-age children and adults with seasonal allergic rhinitis.
Symptoms from similar proteins in food
Mild to moderate allergic symptoms develop quickly when people sensitized to birch pollen eat raw plant foods that contain proteins similar to those in the pollen, such as pathogenesis-related protein PR-10. The allergens are broken down by cooking or processing.
Symptoms usually occur immediately or within 15 minutes of eating. Patients may have tingling; itching or soreness in the mouth, throat, or ears; mild lip and oral mucosa angioedema; itchy hands, sneezing, or eye symptoms; tongue or pharynx angioedema; perioral rash; cough; abdominal pain; nausea; and/or worsening of eczema. In children, itch and rash may predominate.
Triggers depend on pollen type
PFS triggers vary depending on a person’s pollen sensitization, which is affected by their geographic area and local dietary habits. In the United Kingdom, almost 70% of birch-allergic adults and more than 40% of birch-allergic children have PFS, the authors write.
Typical triggers include eating apples, stone fruits, kiwis, carrots, celery, hazelnuts, almonds, walnuts, soymilk, and peanuts, as well as peeling potatoes or other root vegetables. Freshly prepared vegetable or fruit smoothies or juices, celery, soymilk, raw nuts, large quantities of roasted nuts, and concentrated nut products can cause more severe reactions.
Diagnostic clinical history
If a patient answers yes to these questions, they almost certainly have PFS, the authors write:
- Are symptoms caused by raw fruits, nuts, carrots, or celery?
- Are the same trigger foods tolerated when they’re cooked well or roasted?
- Do symptoms come immediately or within a few minutes of eating?
- Do symptoms occur in the oropharynx and include tingling, itching, or swelling?
- Does the patient have seasonal allergic rhinitis or sensitization to pollen?
Testing needed for some cases
Allergy tests may be needed for people who report atypical or severe reactions or who also react to cooked or processed plant foods, such as roasted nuts, nuts in foods, fruits or vegetables in juices and smoothies, and soy products other than milk. Tests may also be needed for people who react to foods that are not linked with PFS, such as cashews, pistachios, macadamias, sesame seeds, beans, lentils, and chickpeas.
Whether PFS reactions also occur to roasted hazelnuts, almonds, walnuts, Brazil nuts, or peanuts, either alone or in composite foods such as chocolates, spreads, desserts, and snacks, is unclear.
An oral food challenge to confirm PFS is needed only if the history and diagnostic tests are inconclusive or if the patient is avoiding multiple foods.
Dietary management
PFS is managed by excluding known trigger foods. This becomes challenging for patients with preexisting food allergies and for vegetarians and vegans.
Personalized dietary advice is needed to avoid nutritional imbalance, minimize anxiety and unnecessary food restrictions, and improve quality of life. Reactions after accidental exposure often resolve without medication, and if antihistamines are needed, they rarely require self-injectable devices.
Guideline helpful beyond the United Kingdom and birch pollen
Allyson S. Larkin, MD, associate professor of pediatrics at the University of Pittsburgh School of Medicine, told this news organization in an email that the guideline summarizes in great detail the pathophysiology behind PFS and highlights how component testing may help diagnose patients and manage the condition.
“Patients worry very much about the progression and severity of allergic reactions,” said Dr. Larkin, who was not involved in the guideline development.
“As the authors note, recognizing the nutritional consequences of dietary restrictions is important, and nutrition consults and suitable alternative suggestions are very helpful for these patients, especially for those with food allergy or who are vegetarian or vegan.”
Jill A. Poole, MD, professor of medicine and chief of the Division of Allergy and Immunology at the University of Nebraska College of Medicine, Omaha, noted that PFS, although common, is underrecognized by the public and by health care providers.
“People are not allergic to the specific food, but they are allergic to a seasonal allergen, such as birch tree, that cross-reacts with the food protein, which is typically changed with cooking,” she explained in an email.
“This differs from reactions by those who have moderate to severe allergic food-specific reactions that may include systemic reactions like anaphylaxis from eating certain foods,” she said.
“Importantly, the number of cross-reacting foods with seasonal pollens continues to grow, and the extent of testing has expanded in recent years,” advised Dr. Poole, who also was not involved in the guideline development.
The authors recommend further related research into food immunotherapy and other novel PFS treatments. They also want to raise awareness of factors affecting PFS prevalence, such as increased spread and allergenicity of pollen due to climate change, pollution, the global consumption of previously local traditional foods, and the increase in vegetarian and vegan diets.
The authors, Dr. Larkin, and Dr. Poole report no relevant financial relationships involving this guideline. The guideline was not funded.
A version of this article first appeared on Medscape.com.
COVID vaccination does not appear to worsen symptoms of Parkinson’s disease
Nonmotor symptoms seemed to improve after SARS-CoV-2 vaccination, although the investigators could not verify a causal relationship.
Vaccination programs should continue for patients with Parkinson’s disease, they said, reporting their clinical results at the International Congress of Parkinson’s Disease and Movement Disorders.
The International Parkinson and Movement Disorder Society has recommended vaccining patients with Parkinson’s disease. “All approved mRNA-based and viral vector vaccines are not expected to interact with Parkinson’s disease, but patients [still] report concern with regard to the benefits, risks, and safeness in Parkinson’s disease,” Mayela Rodríguez-Violante, MD, MSc, and colleagues wrote in an abstract of their findings.
Social isolation may be contributing to these beliefs and concerns, though this is inconclusive.
Investigators from Mexico City conducted a retrospective study of patients with Parkinson’s disease to see how COVID-19 vaccination affected motor and nonmotor symptoms. They enlisted 60 patients (66.7% were male; aged 65.7 ± 11.35 years) who received either a vector-viral vaccine (Vaxzevria Coronavirus) or an mRNA vaccine (BNT162b2).
A Wilcoxon signed-rank test assessed scale differences before and after vaccination, measuring motor involvement (Unified Parkinson’s Disease Rating Scale), nonmotor involvement (Non-Motor Rating Scale [NMSS]), cognitive impairment (Montreal Cognitive Assessment), and quality of life (8-item Parkinson’s Disease Questionnaire index).
Investigators found no significant difference between scales, although they did notice a marked improvement in non-motor symptoms.
“The main takeaway is that vaccination against COVID-19 does not appear to worsen motor or nonmotor symptoms in persons with Parkinson’s disease. The benefits outweigh the risks,” said Dr. Rodríguez-Violante, the study’s lead author and a movement disorder specialist at the National Institute of Neurology and Neurosurgery, Mexico City.
Next steps are to increase the sample size to see if it’s possible to have a similar number in terms of type of vaccine, said Dr. Rodríguez-Violante. “Also, the data presented refers to primary series doses so booster effects will also be studied.”
Few studies have looked at vaccines and their possible effects on this patient population. However, a 2021 study of 181 patients with Parkinson’s disease reported that 2 (1.1%) had adverse effects after receiving the BNT162b2 mRNA vaccine. One of the patients, a 61-year-old woman with a decade-long history of Parkinson’s disease, developed severe, continuous, generalized dyskinesia 6 hours after a first dose of vaccine. The second patient was 79 years old and had Parkinson’s disease for 5 years. She developed fever, confusion, delusions, and continuous severe dyskinesia for 3 days following her vaccination.
“This highlights that there is a variability in the response triggered by the vaccine that might likely depend on individual immunological profiles … clinicians should be aware of this possibility and monitor their patients after they receive their vaccination,” Roberto Erro, MD, PhD and colleagues wrote in the Movement Disorders journal.
Nonmotor symptoms seemed to improve after SARS-CoV-2 vaccination, although the investigators could not verify a causal relationship.
Vaccination programs should continue for patients with Parkinson’s disease, they said, reporting their clinical results at the International Congress of Parkinson’s Disease and Movement Disorders.
The International Parkinson and Movement Disorder Society has recommended vaccining patients with Parkinson’s disease. “All approved mRNA-based and viral vector vaccines are not expected to interact with Parkinson’s disease, but patients [still] report concern with regard to the benefits, risks, and safeness in Parkinson’s disease,” Mayela Rodríguez-Violante, MD, MSc, and colleagues wrote in an abstract of their findings.
Social isolation may be contributing to these beliefs and concerns, though this is inconclusive.
Investigators from Mexico City conducted a retrospective study of patients with Parkinson’s disease to see how COVID-19 vaccination affected motor and nonmotor symptoms. They enlisted 60 patients (66.7% were male; aged 65.7 ± 11.35 years) who received either a vector-viral vaccine (Vaxzevria Coronavirus) or an mRNA vaccine (BNT162b2).
A Wilcoxon signed-rank test assessed scale differences before and after vaccination, measuring motor involvement (Unified Parkinson’s Disease Rating Scale), nonmotor involvement (Non-Motor Rating Scale [NMSS]), cognitive impairment (Montreal Cognitive Assessment), and quality of life (8-item Parkinson’s Disease Questionnaire index).
Investigators found no significant difference between scales, although they did notice a marked improvement in non-motor symptoms.
“The main takeaway is that vaccination against COVID-19 does not appear to worsen motor or nonmotor symptoms in persons with Parkinson’s disease. The benefits outweigh the risks,” said Dr. Rodríguez-Violante, the study’s lead author and a movement disorder specialist at the National Institute of Neurology and Neurosurgery, Mexico City.
Next steps are to increase the sample size to see if it’s possible to have a similar number in terms of type of vaccine, said Dr. Rodríguez-Violante. “Also, the data presented refers to primary series doses so booster effects will also be studied.”
Few studies have looked at vaccines and their possible effects on this patient population. However, a 2021 study of 181 patients with Parkinson’s disease reported that 2 (1.1%) had adverse effects after receiving the BNT162b2 mRNA vaccine. One of the patients, a 61-year-old woman with a decade-long history of Parkinson’s disease, developed severe, continuous, generalized dyskinesia 6 hours after a first dose of vaccine. The second patient was 79 years old and had Parkinson’s disease for 5 years. She developed fever, confusion, delusions, and continuous severe dyskinesia for 3 days following her vaccination.
“This highlights that there is a variability in the response triggered by the vaccine that might likely depend on individual immunological profiles … clinicians should be aware of this possibility and monitor their patients after they receive their vaccination,” Roberto Erro, MD, PhD and colleagues wrote in the Movement Disorders journal.
Nonmotor symptoms seemed to improve after SARS-CoV-2 vaccination, although the investigators could not verify a causal relationship.
Vaccination programs should continue for patients with Parkinson’s disease, they said, reporting their clinical results at the International Congress of Parkinson’s Disease and Movement Disorders.
The International Parkinson and Movement Disorder Society has recommended vaccining patients with Parkinson’s disease. “All approved mRNA-based and viral vector vaccines are not expected to interact with Parkinson’s disease, but patients [still] report concern with regard to the benefits, risks, and safeness in Parkinson’s disease,” Mayela Rodríguez-Violante, MD, MSc, and colleagues wrote in an abstract of their findings.
Social isolation may be contributing to these beliefs and concerns, though this is inconclusive.
Investigators from Mexico City conducted a retrospective study of patients with Parkinson’s disease to see how COVID-19 vaccination affected motor and nonmotor symptoms. They enlisted 60 patients (66.7% were male; aged 65.7 ± 11.35 years) who received either a vector-viral vaccine (Vaxzevria Coronavirus) or an mRNA vaccine (BNT162b2).
A Wilcoxon signed-rank test assessed scale differences before and after vaccination, measuring motor involvement (Unified Parkinson’s Disease Rating Scale), nonmotor involvement (Non-Motor Rating Scale [NMSS]), cognitive impairment (Montreal Cognitive Assessment), and quality of life (8-item Parkinson’s Disease Questionnaire index).
Investigators found no significant difference between scales, although they did notice a marked improvement in non-motor symptoms.
“The main takeaway is that vaccination against COVID-19 does not appear to worsen motor or nonmotor symptoms in persons with Parkinson’s disease. The benefits outweigh the risks,” said Dr. Rodríguez-Violante, the study’s lead author and a movement disorder specialist at the National Institute of Neurology and Neurosurgery, Mexico City.
Next steps are to increase the sample size to see if it’s possible to have a similar number in terms of type of vaccine, said Dr. Rodríguez-Violante. “Also, the data presented refers to primary series doses so booster effects will also be studied.”
Few studies have looked at vaccines and their possible effects on this patient population. However, a 2021 study of 181 patients with Parkinson’s disease reported that 2 (1.1%) had adverse effects after receiving the BNT162b2 mRNA vaccine. One of the patients, a 61-year-old woman with a decade-long history of Parkinson’s disease, developed severe, continuous, generalized dyskinesia 6 hours after a first dose of vaccine. The second patient was 79 years old and had Parkinson’s disease for 5 years. She developed fever, confusion, delusions, and continuous severe dyskinesia for 3 days following her vaccination.
“This highlights that there is a variability in the response triggered by the vaccine that might likely depend on individual immunological profiles … clinicians should be aware of this possibility and monitor their patients after they receive their vaccination,” Roberto Erro, MD, PhD and colleagues wrote in the Movement Disorders journal.
FROM MDS 2022
FDA okays terlipressin (Terlivaz) injection for hepatorenal syndrome
The Food and Drug Administration has approved terlipressin (Terlivaz), the first and only drug approved for patients with hepatorenal syndrome (HRS).
HRS is characterized by progressive deterioration in kidney function in people with advanced liver disease.
Terlipressin is an injectable synthetic vasopressin analogue indicated for patients with HRS who are experiencing rapid deterioration of kidney function (type 1 HRS). The condition affects an estimated 35,000 Americans annually.
The safety and efficacy of terlipressin for type 1 HRS was assessed in the phase 3 CONFIRM trial, which involved 300 patients in the United States and Canada.
Patients received an injection of terlipressin (0.85 mg) or placebo every 6 hours for a maximum of 14 days. The dose was adjusted on the basis of changes in kidney function.
Twenty-nine percent of patients in the terlipressin group experienced improvement in kidney function, vs. 16% in the placebo group.
The CONFIRM trial met its primary endpoint of verified HRS reversal, defined as renal function improvement, avoidance of dialysis, and short-term survival (P = .012).
To achieve this endpoint, patients had to have two consecutive serum creatinine (SCr) values of ≤ 1.5 mg/dL at least 2 hours apart by day 14 or be discharged from the hospital.
The most commonly observed adverse reactions that occurred in at least 4% of patients treated with terlipressin were abdominal pain (19.5%), nausea (16%), respiratory failure (15.5%), diarrhea (13%), and dyspnea (12.5%).
Results of the CONFIRM trial were published in The New England Journal of Medicine.
“Diagnosing and treating HRS can be challenging, and every minute counts when managing patients who have it,” said Steven Romano, MD, executive vice president and chief scientific officer at Mallinckrodt, which makes the drug.
“Terlivaz gives physicians the first FDA-approved option for treating HRS patients with rapid reduction in kidney function that may help them improve kidney function and lessen the associated need for renal replacement therapy, such as dialysis,” Dr. Romano said.
The company plans to launch the product in the coming weeks.
The application for terlipressin for HRS was granted priority review and fast-track status, as well as orphan drug designation, which provides incentives to assist and encourage the development of drugs for rare diseases.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved terlipressin (Terlivaz), the first and only drug approved for patients with hepatorenal syndrome (HRS).
HRS is characterized by progressive deterioration in kidney function in people with advanced liver disease.
Terlipressin is an injectable synthetic vasopressin analogue indicated for patients with HRS who are experiencing rapid deterioration of kidney function (type 1 HRS). The condition affects an estimated 35,000 Americans annually.
The safety and efficacy of terlipressin for type 1 HRS was assessed in the phase 3 CONFIRM trial, which involved 300 patients in the United States and Canada.
Patients received an injection of terlipressin (0.85 mg) or placebo every 6 hours for a maximum of 14 days. The dose was adjusted on the basis of changes in kidney function.
Twenty-nine percent of patients in the terlipressin group experienced improvement in kidney function, vs. 16% in the placebo group.
The CONFIRM trial met its primary endpoint of verified HRS reversal, defined as renal function improvement, avoidance of dialysis, and short-term survival (P = .012).
To achieve this endpoint, patients had to have two consecutive serum creatinine (SCr) values of ≤ 1.5 mg/dL at least 2 hours apart by day 14 or be discharged from the hospital.
The most commonly observed adverse reactions that occurred in at least 4% of patients treated with terlipressin were abdominal pain (19.5%), nausea (16%), respiratory failure (15.5%), diarrhea (13%), and dyspnea (12.5%).
Results of the CONFIRM trial were published in The New England Journal of Medicine.
“Diagnosing and treating HRS can be challenging, and every minute counts when managing patients who have it,” said Steven Romano, MD, executive vice president and chief scientific officer at Mallinckrodt, which makes the drug.
“Terlivaz gives physicians the first FDA-approved option for treating HRS patients with rapid reduction in kidney function that may help them improve kidney function and lessen the associated need for renal replacement therapy, such as dialysis,” Dr. Romano said.
The company plans to launch the product in the coming weeks.
The application for terlipressin for HRS was granted priority review and fast-track status, as well as orphan drug designation, which provides incentives to assist and encourage the development of drugs for rare diseases.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved terlipressin (Terlivaz), the first and only drug approved for patients with hepatorenal syndrome (HRS).
HRS is characterized by progressive deterioration in kidney function in people with advanced liver disease.
Terlipressin is an injectable synthetic vasopressin analogue indicated for patients with HRS who are experiencing rapid deterioration of kidney function (type 1 HRS). The condition affects an estimated 35,000 Americans annually.
The safety and efficacy of terlipressin for type 1 HRS was assessed in the phase 3 CONFIRM trial, which involved 300 patients in the United States and Canada.
Patients received an injection of terlipressin (0.85 mg) or placebo every 6 hours for a maximum of 14 days. The dose was adjusted on the basis of changes in kidney function.
Twenty-nine percent of patients in the terlipressin group experienced improvement in kidney function, vs. 16% in the placebo group.
The CONFIRM trial met its primary endpoint of verified HRS reversal, defined as renal function improvement, avoidance of dialysis, and short-term survival (P = .012).
To achieve this endpoint, patients had to have two consecutive serum creatinine (SCr) values of ≤ 1.5 mg/dL at least 2 hours apart by day 14 or be discharged from the hospital.
The most commonly observed adverse reactions that occurred in at least 4% of patients treated with terlipressin were abdominal pain (19.5%), nausea (16%), respiratory failure (15.5%), diarrhea (13%), and dyspnea (12.5%).
Results of the CONFIRM trial were published in The New England Journal of Medicine.
“Diagnosing and treating HRS can be challenging, and every minute counts when managing patients who have it,” said Steven Romano, MD, executive vice president and chief scientific officer at Mallinckrodt, which makes the drug.
“Terlivaz gives physicians the first FDA-approved option for treating HRS patients with rapid reduction in kidney function that may help them improve kidney function and lessen the associated need for renal replacement therapy, such as dialysis,” Dr. Romano said.
The company plans to launch the product in the coming weeks.
The application for terlipressin for HRS was granted priority review and fast-track status, as well as orphan drug designation, which provides incentives to assist and encourage the development of drugs for rare diseases.
A version of this article first appeared on Medscape.com.
Could cold exposure, especially shivering, combat type 2 diabetes?
STOCKHOLM – Shivering upon repeated short exposures to cold improves glucose tolerance, decreases fasting blood glucose and lipid levels, and markedly reduces blood pressure, show new study results in adults with obesity and overweight.
Presenting the preliminary findings at the annual meeting of the European Association for the Study of Diabetes, Adam Sellers, a PhD student from Maastricht (the Netherlands) University, said: “The results are highly promising and may eventually suggest an alternative treatment or preventative measure for type 2 diabetes.”
Dr. Sellers found that 10 daily 1-hour sessions of shivering at 10° C led to 85% of participants showing a drop in fasting glucose, and a 32% drop in lipid levels, as well as a blood pressure drop of around 8% overall.
Although cold exposure is known to increase brown fat, Dr. Sellers doesn’t believe this explains his findings. “This research, in addition to two other prior studies, suggest that shivering and skeletal muscle may play a more important role than brown fat,” he said.
“Muscle can contract mechanically – [the concept of the] shivers – thereby generating heat, and there is considerably more muscle than brown fat in a human, so shivering can burn more calories and produce more heat,” he explained.
He added that, in the future, “in a similar way to saunas and steam rooms, there might be cold rooms where people go and sit in the cold room and shiver, or possibly patients attend hospital and shivering is induced.”
Audience member Anna Krook, PhD, professor of integrative physiology at the Karolinska Institute, Stockholm, commented on the work, saying the results are “potent” and demonstrate the metabolic effect of shivering. “One thing that struck me was, given the time the subject had to spend – 1 hour shivering over 10 days, I wonder if 1 hour of exercise would show similarly potent effects, and perhaps for those people who cannot perform exercise for whatever reason this might be a good alternative.”
She pointed out that, in terms of translation into practice, it “really depends on how tolerable this is. It also shows how important our muscle is in regulating metabolism. The study showed that you had to be shivering, and it wasn’t just enough to be cold, which has implications for the role of brown fat, especially when we consider the small amount of brown fat we have compared to muscle, which can be half of body weight.”
And Denis P. Blondin, PhD, said: “The reality is that we know it can be difficult and even painful for individuals with obesity to perform exercise, and therefore, cold exposure offers a passive way of improving our metabolic profile and cardiovascular health.”
“Some will argue that it is unrealistic to propose cold exposure as a therapy, but people overlook the fact that cold exposure [mostly through cold-water immersion] has increased in popularity over the past 5 years and has also been a cultural staple for many Nordic countries, albeit often performed with heat exposure as well [see the use of saunas and cold-water swimming in Finland and other Nordic countries],” added Dr. Blondin, of the faculty of medicine and health sciences, University of Sherbrooke (Que.)
“While it can certainly be uncomfortable at first (like starting an exercise program), we adapt very quickly,” he added.
1 hour in a cold-water suit to induce shivering
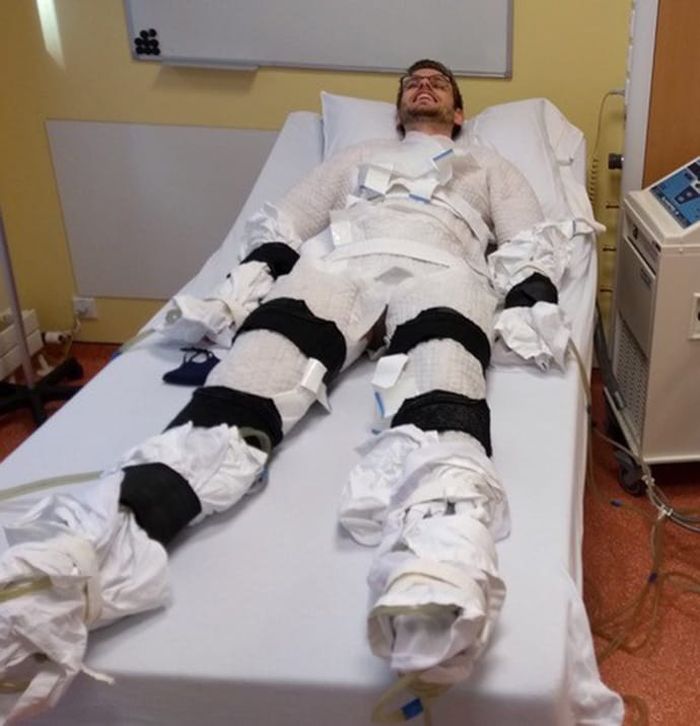
In the current study, Dr. Sellers exposed adults (aged 40-75 years; 11 men and 4 postmenopausal women) with overweight/obesity (body mass index, 27-35 kg/m2) to 10 consecutive cold exposures of at least 1 hour of shivering per cold exposure.
“The shivering in this new research was more intense [than in prior studies] and was induced with a different cold exposure method – a 10° C water-perfused suit [compared with a prior study of 14-15° C, 6 hours/day]. This facilitated a shorter cold exposure duration, deemed feasible for the participants,” explained Dr. Sellers.
“At baseline, participants had glucose and A1c levels at the upper end of the normal criteria [5.5 mmol/l and 5.4%, respectively],” he said, referring to measurements that were suggestive of possible progression to type 2 diabetes.
He explained how the cold exposure was applied. “We induced the cold with a water-perfused suit worn by the participant, through which water flows at 10° C, and this cools the participant. Eventually, the participant starts to shiver, and does so for at least 1 hour every morning for 10 days.”
Participants’ shivering-induced heat production was measured via surface electromyography and visual observation to confirm the presence of shivering. Both before and after the 10-day course of shivering, physiological measurements were taken in the morning while participants were at rest in an overnight fasted state, and under thermoneutral conditions. Blood pressure and fasting blood glucose were measured.
A 2-hour oral glucose tolerance test (OGTT) was conducted twice for each participant: on the morning before the 10-day course of shivering and again on the morning after the final 10th day of shivering.
The primary endpoint was change from before to after the 10-day shivering intervention, as represented by the total area under the curve of glucose levels over time during the OGTT.
“This provides a measure of the glucose concentrations in the blood before and after the 10 shivering sessions over the 10 days.”
Fasting glucose and blood lipids fall, glucose tolerance improves
After 10 shivering sessions, mean fasting plasma glucose decreased significantly in 13 out of the 15 participants, compared with before the first session (from 5.84 mmol/L to 5.67 mmol/L; P = .013).
Glucose tolerance during the OGTT improved by 6% (P = .041). “We can see that this was not due to a change in their insulin concentrations in the blood,” remarked Dr. Sellers, referring to the finding that plasma insulin concentrations at baseline and during the OGTT did not change.
Fasting plasma triglyceride and free-fatty acid concentrations also decreased significantly by 32% (P = .001) and 11% (P = .036), respectively.
“This is important because free-fatty acids are involved in the role of insulin resistance,” said Dr. Sellers. “In addition, the large reduction in serum triglycerides could have implications for atherosclerosis, which may also be beneficial.”
Dr. Sellers also found that systolic blood pressure decreased by 10 mm Hg or 7.4% (P < .001), while diastolic blood pressure decreased by 7 mm Hg or 8.1% (P < .001) on average. This lowering was seen in all participants.
“Again, quite strikingly, all participants showed” a reduction in blood pressure, said Dr. Sellers, which he noted relates to a decrease in resting heart rate (P = .062).
Brown fat or skeletal muscle contraction?
Dr. Sellers pointed out that, despite nonshivering thermogenesis being involved in mild cold acclimation, the data so far suggest that some level of mild muscle activity or shivering appears crucial in provoking the beneficial metabolic effects of cold acclimation.
“Brown fat is a metabolic heating system inside our bodies, burning calories”, explained Dr. Sellers. “This generates heat and prevents calories from being deposited as normal white fat. Brown fat is activated during cold and when we eat, but its activity is less in older adults and in individuals with obesity and diabetes.”
“Going forward, we might investigate the effects of shorter duration – so more intense shivering – to try and elucidate more precisely the optimum duration and intensity of shivering needed,” said Dr. Sellers.
“Our findings are promising and may have important health implications. In future studies, we plan to assess the effect of shivering in adults with type 2 diabetes,” he concluded.
Dr. Seller and Dr. Krook have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
STOCKHOLM – Shivering upon repeated short exposures to cold improves glucose tolerance, decreases fasting blood glucose and lipid levels, and markedly reduces blood pressure, show new study results in adults with obesity and overweight.
Presenting the preliminary findings at the annual meeting of the European Association for the Study of Diabetes, Adam Sellers, a PhD student from Maastricht (the Netherlands) University, said: “The results are highly promising and may eventually suggest an alternative treatment or preventative measure for type 2 diabetes.”
Dr. Sellers found that 10 daily 1-hour sessions of shivering at 10° C led to 85% of participants showing a drop in fasting glucose, and a 32% drop in lipid levels, as well as a blood pressure drop of around 8% overall.
Although cold exposure is known to increase brown fat, Dr. Sellers doesn’t believe this explains his findings. “This research, in addition to two other prior studies, suggest that shivering and skeletal muscle may play a more important role than brown fat,” he said.
“Muscle can contract mechanically – [the concept of the] shivers – thereby generating heat, and there is considerably more muscle than brown fat in a human, so shivering can burn more calories and produce more heat,” he explained.
He added that, in the future, “in a similar way to saunas and steam rooms, there might be cold rooms where people go and sit in the cold room and shiver, or possibly patients attend hospital and shivering is induced.”
Audience member Anna Krook, PhD, professor of integrative physiology at the Karolinska Institute, Stockholm, commented on the work, saying the results are “potent” and demonstrate the metabolic effect of shivering. “One thing that struck me was, given the time the subject had to spend – 1 hour shivering over 10 days, I wonder if 1 hour of exercise would show similarly potent effects, and perhaps for those people who cannot perform exercise for whatever reason this might be a good alternative.”
She pointed out that, in terms of translation into practice, it “really depends on how tolerable this is. It also shows how important our muscle is in regulating metabolism. The study showed that you had to be shivering, and it wasn’t just enough to be cold, which has implications for the role of brown fat, especially when we consider the small amount of brown fat we have compared to muscle, which can be half of body weight.”
And Denis P. Blondin, PhD, said: “The reality is that we know it can be difficult and even painful for individuals with obesity to perform exercise, and therefore, cold exposure offers a passive way of improving our metabolic profile and cardiovascular health.”
“Some will argue that it is unrealistic to propose cold exposure as a therapy, but people overlook the fact that cold exposure [mostly through cold-water immersion] has increased in popularity over the past 5 years and has also been a cultural staple for many Nordic countries, albeit often performed with heat exposure as well [see the use of saunas and cold-water swimming in Finland and other Nordic countries],” added Dr. Blondin, of the faculty of medicine and health sciences, University of Sherbrooke (Que.)
“While it can certainly be uncomfortable at first (like starting an exercise program), we adapt very quickly,” he added.
1 hour in a cold-water suit to induce shivering
In the current study, Dr. Sellers exposed adults (aged 40-75 years; 11 men and 4 postmenopausal women) with overweight/obesity (body mass index, 27-35 kg/m2) to 10 consecutive cold exposures of at least 1 hour of shivering per cold exposure.
“The shivering in this new research was more intense [than in prior studies] and was induced with a different cold exposure method – a 10° C water-perfused suit [compared with a prior study of 14-15° C, 6 hours/day]. This facilitated a shorter cold exposure duration, deemed feasible for the participants,” explained Dr. Sellers.
“At baseline, participants had glucose and A1c levels at the upper end of the normal criteria [5.5 mmol/l and 5.4%, respectively],” he said, referring to measurements that were suggestive of possible progression to type 2 diabetes.
He explained how the cold exposure was applied. “We induced the cold with a water-perfused suit worn by the participant, through which water flows at 10° C, and this cools the participant. Eventually, the participant starts to shiver, and does so for at least 1 hour every morning for 10 days.”
Participants’ shivering-induced heat production was measured via surface electromyography and visual observation to confirm the presence of shivering. Both before and after the 10-day course of shivering, physiological measurements were taken in the morning while participants were at rest in an overnight fasted state, and under thermoneutral conditions. Blood pressure and fasting blood glucose were measured.
A 2-hour oral glucose tolerance test (OGTT) was conducted twice for each participant: on the morning before the 10-day course of shivering and again on the morning after the final 10th day of shivering.
The primary endpoint was change from before to after the 10-day shivering intervention, as represented by the total area under the curve of glucose levels over time during the OGTT.
“This provides a measure of the glucose concentrations in the blood before and after the 10 shivering sessions over the 10 days.”
Fasting glucose and blood lipids fall, glucose tolerance improves
After 10 shivering sessions, mean fasting plasma glucose decreased significantly in 13 out of the 15 participants, compared with before the first session (from 5.84 mmol/L to 5.67 mmol/L; P = .013).
Glucose tolerance during the OGTT improved by 6% (P = .041). “We can see that this was not due to a change in their insulin concentrations in the blood,” remarked Dr. Sellers, referring to the finding that plasma insulin concentrations at baseline and during the OGTT did not change.
Fasting plasma triglyceride and free-fatty acid concentrations also decreased significantly by 32% (P = .001) and 11% (P = .036), respectively.
“This is important because free-fatty acids are involved in the role of insulin resistance,” said Dr. Sellers. “In addition, the large reduction in serum triglycerides could have implications for atherosclerosis, which may also be beneficial.”
Dr. Sellers also found that systolic blood pressure decreased by 10 mm Hg or 7.4% (P < .001), while diastolic blood pressure decreased by 7 mm Hg or 8.1% (P < .001) on average. This lowering was seen in all participants.
“Again, quite strikingly, all participants showed” a reduction in blood pressure, said Dr. Sellers, which he noted relates to a decrease in resting heart rate (P = .062).
Brown fat or skeletal muscle contraction?
Dr. Sellers pointed out that, despite nonshivering thermogenesis being involved in mild cold acclimation, the data so far suggest that some level of mild muscle activity or shivering appears crucial in provoking the beneficial metabolic effects of cold acclimation.
“Brown fat is a metabolic heating system inside our bodies, burning calories”, explained Dr. Sellers. “This generates heat and prevents calories from being deposited as normal white fat. Brown fat is activated during cold and when we eat, but its activity is less in older adults and in individuals with obesity and diabetes.”
“Going forward, we might investigate the effects of shorter duration – so more intense shivering – to try and elucidate more precisely the optimum duration and intensity of shivering needed,” said Dr. Sellers.
“Our findings are promising and may have important health implications. In future studies, we plan to assess the effect of shivering in adults with type 2 diabetes,” he concluded.
Dr. Seller and Dr. Krook have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
STOCKHOLM – Shivering upon repeated short exposures to cold improves glucose tolerance, decreases fasting blood glucose and lipid levels, and markedly reduces blood pressure, show new study results in adults with obesity and overweight.
Presenting the preliminary findings at the annual meeting of the European Association for the Study of Diabetes, Adam Sellers, a PhD student from Maastricht (the Netherlands) University, said: “The results are highly promising and may eventually suggest an alternative treatment or preventative measure for type 2 diabetes.”
Dr. Sellers found that 10 daily 1-hour sessions of shivering at 10° C led to 85% of participants showing a drop in fasting glucose, and a 32% drop in lipid levels, as well as a blood pressure drop of around 8% overall.
Although cold exposure is known to increase brown fat, Dr. Sellers doesn’t believe this explains his findings. “This research, in addition to two other prior studies, suggest that shivering and skeletal muscle may play a more important role than brown fat,” he said.
“Muscle can contract mechanically – [the concept of the] shivers – thereby generating heat, and there is considerably more muscle than brown fat in a human, so shivering can burn more calories and produce more heat,” he explained.
He added that, in the future, “in a similar way to saunas and steam rooms, there might be cold rooms where people go and sit in the cold room and shiver, or possibly patients attend hospital and shivering is induced.”
Audience member Anna Krook, PhD, professor of integrative physiology at the Karolinska Institute, Stockholm, commented on the work, saying the results are “potent” and demonstrate the metabolic effect of shivering. “One thing that struck me was, given the time the subject had to spend – 1 hour shivering over 10 days, I wonder if 1 hour of exercise would show similarly potent effects, and perhaps for those people who cannot perform exercise for whatever reason this might be a good alternative.”
She pointed out that, in terms of translation into practice, it “really depends on how tolerable this is. It also shows how important our muscle is in regulating metabolism. The study showed that you had to be shivering, and it wasn’t just enough to be cold, which has implications for the role of brown fat, especially when we consider the small amount of brown fat we have compared to muscle, which can be half of body weight.”
And Denis P. Blondin, PhD, said: “The reality is that we know it can be difficult and even painful for individuals with obesity to perform exercise, and therefore, cold exposure offers a passive way of improving our metabolic profile and cardiovascular health.”
“Some will argue that it is unrealistic to propose cold exposure as a therapy, but people overlook the fact that cold exposure [mostly through cold-water immersion] has increased in popularity over the past 5 years and has also been a cultural staple for many Nordic countries, albeit often performed with heat exposure as well [see the use of saunas and cold-water swimming in Finland and other Nordic countries],” added Dr. Blondin, of the faculty of medicine and health sciences, University of Sherbrooke (Que.)
“While it can certainly be uncomfortable at first (like starting an exercise program), we adapt very quickly,” he added.
1 hour in a cold-water suit to induce shivering
In the current study, Dr. Sellers exposed adults (aged 40-75 years; 11 men and 4 postmenopausal women) with overweight/obesity (body mass index, 27-35 kg/m2) to 10 consecutive cold exposures of at least 1 hour of shivering per cold exposure.
“The shivering in this new research was more intense [than in prior studies] and was induced with a different cold exposure method – a 10° C water-perfused suit [compared with a prior study of 14-15° C, 6 hours/day]. This facilitated a shorter cold exposure duration, deemed feasible for the participants,” explained Dr. Sellers.
“At baseline, participants had glucose and A1c levels at the upper end of the normal criteria [5.5 mmol/l and 5.4%, respectively],” he said, referring to measurements that were suggestive of possible progression to type 2 diabetes.
He explained how the cold exposure was applied. “We induced the cold with a water-perfused suit worn by the participant, through which water flows at 10° C, and this cools the participant. Eventually, the participant starts to shiver, and does so for at least 1 hour every morning for 10 days.”
Participants’ shivering-induced heat production was measured via surface electromyography and visual observation to confirm the presence of shivering. Both before and after the 10-day course of shivering, physiological measurements were taken in the morning while participants were at rest in an overnight fasted state, and under thermoneutral conditions. Blood pressure and fasting blood glucose were measured.
A 2-hour oral glucose tolerance test (OGTT) was conducted twice for each participant: on the morning before the 10-day course of shivering and again on the morning after the final 10th day of shivering.
The primary endpoint was change from before to after the 10-day shivering intervention, as represented by the total area under the curve of glucose levels over time during the OGTT.
“This provides a measure of the glucose concentrations in the blood before and after the 10 shivering sessions over the 10 days.”
Fasting glucose and blood lipids fall, glucose tolerance improves
After 10 shivering sessions, mean fasting plasma glucose decreased significantly in 13 out of the 15 participants, compared with before the first session (from 5.84 mmol/L to 5.67 mmol/L; P = .013).
Glucose tolerance during the OGTT improved by 6% (P = .041). “We can see that this was not due to a change in their insulin concentrations in the blood,” remarked Dr. Sellers, referring to the finding that plasma insulin concentrations at baseline and during the OGTT did not change.
Fasting plasma triglyceride and free-fatty acid concentrations also decreased significantly by 32% (P = .001) and 11% (P = .036), respectively.
“This is important because free-fatty acids are involved in the role of insulin resistance,” said Dr. Sellers. “In addition, the large reduction in serum triglycerides could have implications for atherosclerosis, which may also be beneficial.”
Dr. Sellers also found that systolic blood pressure decreased by 10 mm Hg or 7.4% (P < .001), while diastolic blood pressure decreased by 7 mm Hg or 8.1% (P < .001) on average. This lowering was seen in all participants.
“Again, quite strikingly, all participants showed” a reduction in blood pressure, said Dr. Sellers, which he noted relates to a decrease in resting heart rate (P = .062).
Brown fat or skeletal muscle contraction?
Dr. Sellers pointed out that, despite nonshivering thermogenesis being involved in mild cold acclimation, the data so far suggest that some level of mild muscle activity or shivering appears crucial in provoking the beneficial metabolic effects of cold acclimation.
“Brown fat is a metabolic heating system inside our bodies, burning calories”, explained Dr. Sellers. “This generates heat and prevents calories from being deposited as normal white fat. Brown fat is activated during cold and when we eat, but its activity is less in older adults and in individuals with obesity and diabetes.”
“Going forward, we might investigate the effects of shorter duration – so more intense shivering – to try and elucidate more precisely the optimum duration and intensity of shivering needed,” said Dr. Sellers.
“Our findings are promising and may have important health implications. In future studies, we plan to assess the effect of shivering in adults with type 2 diabetes,” he concluded.
Dr. Seller and Dr. Krook have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT EASD 2022
Mothers’ diabetes linked to ADHD in their children
Children born to women who develop diabetes either before or during their pregnancy could be at risk for developing attention-deficit/hyperactivity disorder, data from a large multinational cohort study appear to show.
Considering more than 4.5 million mother-child pairs, it was found that children whose mothers had diabetes around the time of their pregnancy were 16% more likely to have ADHD diagnosed than were those whose mothers did not.
An increased risk was seen regardless of the type of diabetes, and regardless of whether or not the diabetes was present before or appeared during the pregnancy.
“We found a small increased risk of ADHD in children born to mothers with diabetes, including pregestational diabetes and gestational diabetes,” Carolyn Cesta, PhD, reported at the annual meeting of the European Association for the Study of Diabetes.
Dr. Cesta, a postdoctoral researcher in the Centre for Pharmacoepidemiology at the Karolinska Institutet in Stockholm noted that the effect sizes seen were lower than had been reported previously.
“This may be because we adjusted for a large number of covariates, including maternal ADHD and psychiatric disorders,” Dr. Cesta said.
ADHD and diabetes
“Previous studies have reported an increase in the risk of ADHD in children born to mothers with diabetes,” explained Dr. Cesta.
However, “these studies have been limited by the use of self-reported data, small sample sizes, lack of adjustment for important confounders, and they’re often limited to [White] populations,” she added. “There’s a lot of heterogeneity between these studies,” she said.
To try to iron out the differences seen in the prior studies, Dr. Cesta and associates looked at data from several databases based in Hong Kong (Clinical Data Analysis and Reporting System), four Nordic countries (Population Health Registers for Finland, Iceland, Norway, and Sweden), and Taiwan (National Health Insurance Database).
To create the matched mother-child pairs, the databases were searched to find women who had children born between 2001 and 2018, and who had follow-up data available up to 2020 on not only their diabetes status and child’s ADHD status, but also other parameters, such as other maternal diagnoses, maternal medications, and a host of sociodemographic factors.
More than 24 potentially confounding or covariates were considered in the analysis, which used Cox proportional hazard regression modeling and propensity score analysis to calculate hazard ratios with 95% confidence intervals.
“We looked at whether [mothers] had a diagnosis of ADHD themselves, or other psychiatric disorders, because there is high heritability for these disorders,” Dr. Cesta said, indicating that all bases had endeavored to be covered.
Main findings
Results showed some differences in the prevalence of diabetes and ADHD between the three cohorts used in the analysis. The prevalence of any maternal diabetes ranged from 8.8% in the Hong Kong cohort to 3.3% in the Taiwan cohort, with a prevalence of 6.8% for the Nordic cohort.
Rates of pregestational diabetes were lowest in the Taiwan and Hong Kong cohorts, at 0.2% and 0.5%, respectively, and 2.2% in the Nordic cohort. Gestational diabetes rates were a respective 3.1%, 7.8%, and 4.6%.
The highest rate of ADHD in children was seen in the Taiwan cohort, at 9.6%, followed by 4.2% for the Hong Kong cohort, and 2.6% for the Nordic cohort.
The hazard ratio for having childhood ADHD was 1.16 when comparing any maternal diabetes to no maternal diabetes, 1.40 comparing mothers with and without pregestational diabetes, and a respective 1.36 and 1.37 comparing those with and without type 1 diabetes, and those with and without type 2 diabetes.
The HR for childhood ADHD comparing mothers with and without gestational diabetes was 1.13.
“Within the analysis for gestational diabetes, we had enough numbers to look at siblings that are discordant for maternal gestational diabetes,” Dr. Cesta said. Essentially “we’re comparing two siblings from the same mother, one that was exposed to gestational diabetes, one that wasn’t,” she explained.
Interestingly there was no association between ADHD and maternal gestational diabetes in the sibling analysis (HR, 1.0).
“When it comes to gestational diabetes, the evidence from our sibling analysis indicate that the association may actually be confounded by shared genetics and environmental factors,” said Dr. Cesta.
“So, future studies should explore the role of specific genetic factors in glycemic control during pregnancy and the relationship between maternal diabetes and ADHD.”
Answering long-standing questions
These data will help a lot in answering questions that clinicians have been asking themselves a long time, commented Jardena Puder, MD, who chaired the session.
“It still remains a bit puzzling that genetic and environmental factors could be responsible, if you see the same effect in type 1 [diabetes], and in type 2 [diabetes], and gestational diabetes,” said Dr. Puder, who is an endocrinologist and diabetologist at the woman-mother-child department at the Vaud University Hospital Center, Lausanne, Switzerland.
Type 1 and type 2 are “very distinct” in terms of the genetic and environmental factors involved, “so, the fact that you see [the effect] in both remains a bit puzzling,” said Dr. Puder.
“I wish we had the numbers to be able to do the sibling analysis for type 1 and type 2, just to see if we could tease anything out,” said Dr. Cesta.
“I do think this is part of the bigger question of what the relationship is between, like, metabolic disorders and psychiatric disorders, because even outside of pregnancy, we see that there’s often a comorbidity with them. So, it’s a good point.”
The next step is to look at the role of treatment and what effects glycemic control might have on the small, but still apparent, association between maternal diabetes and ADHD.
The study had multiple funders including the Hong Kong Research Grant Council, NordForsk, the Research Council of Norway, the Norwegian ADHD Research Network, the Hong Kong Innovation and Technology Commission, and European Horizon 2020.
Dr. Cesta had no conflicts of interest to disclose. Dr. Puder chaired the session in which the findings were presented and made no specific disclosures.
Children born to women who develop diabetes either before or during their pregnancy could be at risk for developing attention-deficit/hyperactivity disorder, data from a large multinational cohort study appear to show.
Considering more than 4.5 million mother-child pairs, it was found that children whose mothers had diabetes around the time of their pregnancy were 16% more likely to have ADHD diagnosed than were those whose mothers did not.
An increased risk was seen regardless of the type of diabetes, and regardless of whether or not the diabetes was present before or appeared during the pregnancy.
“We found a small increased risk of ADHD in children born to mothers with diabetes, including pregestational diabetes and gestational diabetes,” Carolyn Cesta, PhD, reported at the annual meeting of the European Association for the Study of Diabetes.
Dr. Cesta, a postdoctoral researcher in the Centre for Pharmacoepidemiology at the Karolinska Institutet in Stockholm noted that the effect sizes seen were lower than had been reported previously.
“This may be because we adjusted for a large number of covariates, including maternal ADHD and psychiatric disorders,” Dr. Cesta said.
ADHD and diabetes
“Previous studies have reported an increase in the risk of ADHD in children born to mothers with diabetes,” explained Dr. Cesta.
However, “these studies have been limited by the use of self-reported data, small sample sizes, lack of adjustment for important confounders, and they’re often limited to [White] populations,” she added. “There’s a lot of heterogeneity between these studies,” she said.
To try to iron out the differences seen in the prior studies, Dr. Cesta and associates looked at data from several databases based in Hong Kong (Clinical Data Analysis and Reporting System), four Nordic countries (Population Health Registers for Finland, Iceland, Norway, and Sweden), and Taiwan (National Health Insurance Database).
To create the matched mother-child pairs, the databases were searched to find women who had children born between 2001 and 2018, and who had follow-up data available up to 2020 on not only their diabetes status and child’s ADHD status, but also other parameters, such as other maternal diagnoses, maternal medications, and a host of sociodemographic factors.
More than 24 potentially confounding or covariates were considered in the analysis, which used Cox proportional hazard regression modeling and propensity score analysis to calculate hazard ratios with 95% confidence intervals.
“We looked at whether [mothers] had a diagnosis of ADHD themselves, or other psychiatric disorders, because there is high heritability for these disorders,” Dr. Cesta said, indicating that all bases had endeavored to be covered.
Main findings
Results showed some differences in the prevalence of diabetes and ADHD between the three cohorts used in the analysis. The prevalence of any maternal diabetes ranged from 8.8% in the Hong Kong cohort to 3.3% in the Taiwan cohort, with a prevalence of 6.8% for the Nordic cohort.
Rates of pregestational diabetes were lowest in the Taiwan and Hong Kong cohorts, at 0.2% and 0.5%, respectively, and 2.2% in the Nordic cohort. Gestational diabetes rates were a respective 3.1%, 7.8%, and 4.6%.
The highest rate of ADHD in children was seen in the Taiwan cohort, at 9.6%, followed by 4.2% for the Hong Kong cohort, and 2.6% for the Nordic cohort.
The hazard ratio for having childhood ADHD was 1.16 when comparing any maternal diabetes to no maternal diabetes, 1.40 comparing mothers with and without pregestational diabetes, and a respective 1.36 and 1.37 comparing those with and without type 1 diabetes, and those with and without type 2 diabetes.
The HR for childhood ADHD comparing mothers with and without gestational diabetes was 1.13.
“Within the analysis for gestational diabetes, we had enough numbers to look at siblings that are discordant for maternal gestational diabetes,” Dr. Cesta said. Essentially “we’re comparing two siblings from the same mother, one that was exposed to gestational diabetes, one that wasn’t,” she explained.
Interestingly there was no association between ADHD and maternal gestational diabetes in the sibling analysis (HR, 1.0).
“When it comes to gestational diabetes, the evidence from our sibling analysis indicate that the association may actually be confounded by shared genetics and environmental factors,” said Dr. Cesta.
“So, future studies should explore the role of specific genetic factors in glycemic control during pregnancy and the relationship between maternal diabetes and ADHD.”
Answering long-standing questions
These data will help a lot in answering questions that clinicians have been asking themselves a long time, commented Jardena Puder, MD, who chaired the session.
“It still remains a bit puzzling that genetic and environmental factors could be responsible, if you see the same effect in type 1 [diabetes], and in type 2 [diabetes], and gestational diabetes,” said Dr. Puder, who is an endocrinologist and diabetologist at the woman-mother-child department at the Vaud University Hospital Center, Lausanne, Switzerland.
Type 1 and type 2 are “very distinct” in terms of the genetic and environmental factors involved, “so, the fact that you see [the effect] in both remains a bit puzzling,” said Dr. Puder.
“I wish we had the numbers to be able to do the sibling analysis for type 1 and type 2, just to see if we could tease anything out,” said Dr. Cesta.
“I do think this is part of the bigger question of what the relationship is between, like, metabolic disorders and psychiatric disorders, because even outside of pregnancy, we see that there’s often a comorbidity with them. So, it’s a good point.”
The next step is to look at the role of treatment and what effects glycemic control might have on the small, but still apparent, association between maternal diabetes and ADHD.
The study had multiple funders including the Hong Kong Research Grant Council, NordForsk, the Research Council of Norway, the Norwegian ADHD Research Network, the Hong Kong Innovation and Technology Commission, and European Horizon 2020.
Dr. Cesta had no conflicts of interest to disclose. Dr. Puder chaired the session in which the findings were presented and made no specific disclosures.
Children born to women who develop diabetes either before or during their pregnancy could be at risk for developing attention-deficit/hyperactivity disorder, data from a large multinational cohort study appear to show.
Considering more than 4.5 million mother-child pairs, it was found that children whose mothers had diabetes around the time of their pregnancy were 16% more likely to have ADHD diagnosed than were those whose mothers did not.
An increased risk was seen regardless of the type of diabetes, and regardless of whether or not the diabetes was present before or appeared during the pregnancy.
“We found a small increased risk of ADHD in children born to mothers with diabetes, including pregestational diabetes and gestational diabetes,” Carolyn Cesta, PhD, reported at the annual meeting of the European Association for the Study of Diabetes.
Dr. Cesta, a postdoctoral researcher in the Centre for Pharmacoepidemiology at the Karolinska Institutet in Stockholm noted that the effect sizes seen were lower than had been reported previously.
“This may be because we adjusted for a large number of covariates, including maternal ADHD and psychiatric disorders,” Dr. Cesta said.
ADHD and diabetes
“Previous studies have reported an increase in the risk of ADHD in children born to mothers with diabetes,” explained Dr. Cesta.
However, “these studies have been limited by the use of self-reported data, small sample sizes, lack of adjustment for important confounders, and they’re often limited to [White] populations,” she added. “There’s a lot of heterogeneity between these studies,” she said.
To try to iron out the differences seen in the prior studies, Dr. Cesta and associates looked at data from several databases based in Hong Kong (Clinical Data Analysis and Reporting System), four Nordic countries (Population Health Registers for Finland, Iceland, Norway, and Sweden), and Taiwan (National Health Insurance Database).
To create the matched mother-child pairs, the databases were searched to find women who had children born between 2001 and 2018, and who had follow-up data available up to 2020 on not only their diabetes status and child’s ADHD status, but also other parameters, such as other maternal diagnoses, maternal medications, and a host of sociodemographic factors.
More than 24 potentially confounding or covariates were considered in the analysis, which used Cox proportional hazard regression modeling and propensity score analysis to calculate hazard ratios with 95% confidence intervals.
“We looked at whether [mothers] had a diagnosis of ADHD themselves, or other psychiatric disorders, because there is high heritability for these disorders,” Dr. Cesta said, indicating that all bases had endeavored to be covered.
Main findings
Results showed some differences in the prevalence of diabetes and ADHD between the three cohorts used in the analysis. The prevalence of any maternal diabetes ranged from 8.8% in the Hong Kong cohort to 3.3% in the Taiwan cohort, with a prevalence of 6.8% for the Nordic cohort.
Rates of pregestational diabetes were lowest in the Taiwan and Hong Kong cohorts, at 0.2% and 0.5%, respectively, and 2.2% in the Nordic cohort. Gestational diabetes rates were a respective 3.1%, 7.8%, and 4.6%.
The highest rate of ADHD in children was seen in the Taiwan cohort, at 9.6%, followed by 4.2% for the Hong Kong cohort, and 2.6% for the Nordic cohort.
The hazard ratio for having childhood ADHD was 1.16 when comparing any maternal diabetes to no maternal diabetes, 1.40 comparing mothers with and without pregestational diabetes, and a respective 1.36 and 1.37 comparing those with and without type 1 diabetes, and those with and without type 2 diabetes.
The HR for childhood ADHD comparing mothers with and without gestational diabetes was 1.13.
“Within the analysis for gestational diabetes, we had enough numbers to look at siblings that are discordant for maternal gestational diabetes,” Dr. Cesta said. Essentially “we’re comparing two siblings from the same mother, one that was exposed to gestational diabetes, one that wasn’t,” she explained.
Interestingly there was no association between ADHD and maternal gestational diabetes in the sibling analysis (HR, 1.0).
“When it comes to gestational diabetes, the evidence from our sibling analysis indicate that the association may actually be confounded by shared genetics and environmental factors,” said Dr. Cesta.
“So, future studies should explore the role of specific genetic factors in glycemic control during pregnancy and the relationship between maternal diabetes and ADHD.”
Answering long-standing questions
These data will help a lot in answering questions that clinicians have been asking themselves a long time, commented Jardena Puder, MD, who chaired the session.
“It still remains a bit puzzling that genetic and environmental factors could be responsible, if you see the same effect in type 1 [diabetes], and in type 2 [diabetes], and gestational diabetes,” said Dr. Puder, who is an endocrinologist and diabetologist at the woman-mother-child department at the Vaud University Hospital Center, Lausanne, Switzerland.
Type 1 and type 2 are “very distinct” in terms of the genetic and environmental factors involved, “so, the fact that you see [the effect] in both remains a bit puzzling,” said Dr. Puder.
“I wish we had the numbers to be able to do the sibling analysis for type 1 and type 2, just to see if we could tease anything out,” said Dr. Cesta.
“I do think this is part of the bigger question of what the relationship is between, like, metabolic disorders and psychiatric disorders, because even outside of pregnancy, we see that there’s often a comorbidity with them. So, it’s a good point.”
The next step is to look at the role of treatment and what effects glycemic control might have on the small, but still apparent, association between maternal diabetes and ADHD.
The study had multiple funders including the Hong Kong Research Grant Council, NordForsk, the Research Council of Norway, the Norwegian ADHD Research Network, the Hong Kong Innovation and Technology Commission, and European Horizon 2020.
Dr. Cesta had no conflicts of interest to disclose. Dr. Puder chaired the session in which the findings were presented and made no specific disclosures.
FROM EASD 2022
Scalp plaque
A punch biopsy was performed, and the results were consistent with pityriasis amiantacea arising from psoriasis. In an older patient, a keratinaceous horn would be worrisome for a squamous cell carcinoma. In a younger patient, like this one, it is more likely an atypical manifestation of a more common dermatosis.
Pityriasis amiantacea is an unusual disorder in which thick adherent scales form on the scalp; it is most common in children, adolescents, and young adults. There is no racial predilection. With this condition, patients complain of a fixed plaque that may shed scale but not as quickly as it accumulates. It can be an isolated finding, but more often it is a secondary manifestation of an underlying case of psoriasis, seborrheic dermatitis, tinea capitis, or atopic dermatitis.1
A punch biopsy performed on the scalp should include the skin underlying the compact keratin scale. However, to avoid excessive bleeding, use lidocaine with epinephrine. Allow 15 minutes for the anesthesia to take effect before beginning the procedure.
Treatment depends on the underlying cause but includes debridement of the aggregated scale with a topical keratolytic (such as salicylic acid or topical fluocinolone oil 0.01% applied) at night and washed out 7 to 10 hours later.
The patient was advised to use over-the-counter 2% salicylic acid shampoo daily and to apply topical clobetasol 0.05% solution nightly for 4 weeks and once weekly after clearance for another 3 months. At the 3-month follow-up, the patient’s scalp was clear.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Ettler J, Wetter DA, Pittelkow MR. Pityriasis amiantacea: a distinctive presentation of psoriasis associated with tumour necrosis factor-α inhibitor therapy. Clin Exp Dermatol. 2012;37:639-641. doi: 10.1111/j.1365-2230.2011.04286.x

A punch biopsy was performed, and the results were consistent with pityriasis amiantacea arising from psoriasis. In an older patient, a keratinaceous horn would be worrisome for a squamous cell carcinoma. In a younger patient, like this one, it is more likely an atypical manifestation of a more common dermatosis.
Pityriasis amiantacea is an unusual disorder in which thick adherent scales form on the scalp; it is most common in children, adolescents, and young adults. There is no racial predilection. With this condition, patients complain of a fixed plaque that may shed scale but not as quickly as it accumulates. It can be an isolated finding, but more often it is a secondary manifestation of an underlying case of psoriasis, seborrheic dermatitis, tinea capitis, or atopic dermatitis.1
A punch biopsy performed on the scalp should include the skin underlying the compact keratin scale. However, to avoid excessive bleeding, use lidocaine with epinephrine. Allow 15 minutes for the anesthesia to take effect before beginning the procedure.
Treatment depends on the underlying cause but includes debridement of the aggregated scale with a topical keratolytic (such as salicylic acid or topical fluocinolone oil 0.01% applied) at night and washed out 7 to 10 hours later.
The patient was advised to use over-the-counter 2% salicylic acid shampoo daily and to apply topical clobetasol 0.05% solution nightly for 4 weeks and once weekly after clearance for another 3 months. At the 3-month follow-up, the patient’s scalp was clear.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

A punch biopsy was performed, and the results were consistent with pityriasis amiantacea arising from psoriasis. In an older patient, a keratinaceous horn would be worrisome for a squamous cell carcinoma. In a younger patient, like this one, it is more likely an atypical manifestation of a more common dermatosis.
Pityriasis amiantacea is an unusual disorder in which thick adherent scales form on the scalp; it is most common in children, adolescents, and young adults. There is no racial predilection. With this condition, patients complain of a fixed plaque that may shed scale but not as quickly as it accumulates. It can be an isolated finding, but more often it is a secondary manifestation of an underlying case of psoriasis, seborrheic dermatitis, tinea capitis, or atopic dermatitis.1
A punch biopsy performed on the scalp should include the skin underlying the compact keratin scale. However, to avoid excessive bleeding, use lidocaine with epinephrine. Allow 15 minutes for the anesthesia to take effect before beginning the procedure.
Treatment depends on the underlying cause but includes debridement of the aggregated scale with a topical keratolytic (such as salicylic acid or topical fluocinolone oil 0.01% applied) at night and washed out 7 to 10 hours later.
The patient was advised to use over-the-counter 2% salicylic acid shampoo daily and to apply topical clobetasol 0.05% solution nightly for 4 weeks and once weekly after clearance for another 3 months. At the 3-month follow-up, the patient’s scalp was clear.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Ettler J, Wetter DA, Pittelkow MR. Pityriasis amiantacea: a distinctive presentation of psoriasis associated with tumour necrosis factor-α inhibitor therapy. Clin Exp Dermatol. 2012;37:639-641. doi: 10.1111/j.1365-2230.2011.04286.x
1. Ettler J, Wetter DA, Pittelkow MR. Pityriasis amiantacea: a distinctive presentation of psoriasis associated with tumour necrosis factor-α inhibitor therapy. Clin Exp Dermatol. 2012;37:639-641. doi: 10.1111/j.1365-2230.2011.04286.x
Night owls may have greater risks of T2D and CVD
In the study involving 51 people, night owls metabolized fat less efficiently, showed less insulin sensitivity, and demonstrated lower physical fitness than early birds, lead author Steven K. Malin, PhD, of Rutgers University, New Brunswick, N.J., and colleagues reported.
Prior publications have suggested that night owls, formally known as “late chronotypes,” have an increased risk of obesity, type 2 diabetes, and cardiovascular disease, Dr. Malin said in an interview. But no previous research involved the gold-standard measurement tools used in this study, including euglycemic clamp and indirect calorimetry to quantify fat metabolism.
Dr. Malin also noted that this is the first study of its kind to characterize metabolism during both rest and exercise.
The study, published in Experimental Physiology, involved 24 early birds and 27 night owls classified by the Morning-Eveningness Questionnaire. All participants were sedentary, reporting less than one hour of structured exercise per week, and had metabolic syndrome according to Adult Treatment Panel III report criteria. Groups were otherwise demographically similar, with average ages in each group of approximately 54-55 years.
Compared with night owls, early birds were more physically active during the morning into midday. During exercise, they metabolized more fat and demonstrated greater physical fitness based on VO2max readings. At rest, early birds also came out ahead – they had higher fat oxidation and non–oxidative glucose disposal, suggesting more sensitivity to insulin.
“Collectively, this work highlights and supports chronotype as a potential risk factor related to type 2 diabetes and cardiovascular disease risk,” the investigators concluded.
Night owls have less metabolic control
Jed Friedman, PhD, director of OU Health Harold Hamm Diabetes Center at the University of Oklahoma Health Sciences Center, Oklahoma City, praised the study for the size of the groups the researchers compared with each other and how well matched those groups were, as well as the “state-of-the-art” measurement tools employed.
The findings show that night owls have “less metabolic control,” Dr. Friedman said in an interview.
“That’s a term that’s frequently invoked in [regard to] prediabetes,” he said. “Blood sugar goes up, because when you’re eating a high carbohydrate diet, your cells aren’t metabolizing sugar properly. That tends to raise your risk for a lot of diseases.”
Dr. Friedman added that the findings align with those of previous studies that have linked less sleep with changes in brain biology, and therefore behavior, especially in dietary choices.
“When you’re tired, the mechanisms for appetite control go haywire,” Dr. Friedman explained. “The evidence suggests that sugar is the primary driver for what people eat when they’re tired. That obviously has implications for diabetes and metabolic syndrome. So sleeping more really can help you control cravings.”
Dr. Friedman also noted that people who are tired tend to engage in less physical activity, further increasing their risk of metabolic issues. To control this risk, he advised people to return to their circadian rhythms, which could mean forgetting the midnight snack.
“Having a daily pattern that’s in sync with chronicity, or these daily rhythms, is associated with greater health,” Dr. Friedman said. “We’re not really made to eat at night. I think this [study] kind of reinforces that.”
Can a night owl become an early bird?
When asked if a person’s natural circadian rhythm can be later, Dr. Malin responded that chronotypes may be dictated by genetics and age, as well as external drivers like work schedule. For these reasons, it’s “tricky” to answer whether night owls can turn into early birds and reap the potential health benefits of making that shift.
“Given that so many life factors can influence what our routine entails, it’s hard to know if we [can] truly change our chronotype or if rather we [can] learn to manage,” Dr. Malin said. “In either case, there is some work that suggests people can adopt earlier bedtimes and waketimes through practical recommendations.”
Specifically, he suggested increasing physical activity during the day, and adjusting bedtimes gradually by 15-minute increments.
“Go to bed 15 minutes earlier then wake up 15 minutes earlier,” Dr. Malin said. “In time, and depending on how things are going, this can expand to another 15-minute window. Then, during the earlier time waking up, a person can engage in light physical activity to help with promoting general fitness. If they can get outside with sunlight, that would be great too, as the natural sunlight would provide cues to the circadian system to adjust.”
The study was supported by the National Institutes of Health. The investigators and Dr. Friedman disclosed no conflicts of interest.
In the study involving 51 people, night owls metabolized fat less efficiently, showed less insulin sensitivity, and demonstrated lower physical fitness than early birds, lead author Steven K. Malin, PhD, of Rutgers University, New Brunswick, N.J., and colleagues reported.
Prior publications have suggested that night owls, formally known as “late chronotypes,” have an increased risk of obesity, type 2 diabetes, and cardiovascular disease, Dr. Malin said in an interview. But no previous research involved the gold-standard measurement tools used in this study, including euglycemic clamp and indirect calorimetry to quantify fat metabolism.
Dr. Malin also noted that this is the first study of its kind to characterize metabolism during both rest and exercise.
The study, published in Experimental Physiology, involved 24 early birds and 27 night owls classified by the Morning-Eveningness Questionnaire. All participants were sedentary, reporting less than one hour of structured exercise per week, and had metabolic syndrome according to Adult Treatment Panel III report criteria. Groups were otherwise demographically similar, with average ages in each group of approximately 54-55 years.
Compared with night owls, early birds were more physically active during the morning into midday. During exercise, they metabolized more fat and demonstrated greater physical fitness based on VO2max readings. At rest, early birds also came out ahead – they had higher fat oxidation and non–oxidative glucose disposal, suggesting more sensitivity to insulin.
“Collectively, this work highlights and supports chronotype as a potential risk factor related to type 2 diabetes and cardiovascular disease risk,” the investigators concluded.
Night owls have less metabolic control
Jed Friedman, PhD, director of OU Health Harold Hamm Diabetes Center at the University of Oklahoma Health Sciences Center, Oklahoma City, praised the study for the size of the groups the researchers compared with each other and how well matched those groups were, as well as the “state-of-the-art” measurement tools employed.
The findings show that night owls have “less metabolic control,” Dr. Friedman said in an interview.
“That’s a term that’s frequently invoked in [regard to] prediabetes,” he said. “Blood sugar goes up, because when you’re eating a high carbohydrate diet, your cells aren’t metabolizing sugar properly. That tends to raise your risk for a lot of diseases.”
Dr. Friedman added that the findings align with those of previous studies that have linked less sleep with changes in brain biology, and therefore behavior, especially in dietary choices.
“When you’re tired, the mechanisms for appetite control go haywire,” Dr. Friedman explained. “The evidence suggests that sugar is the primary driver for what people eat when they’re tired. That obviously has implications for diabetes and metabolic syndrome. So sleeping more really can help you control cravings.”
Dr. Friedman also noted that people who are tired tend to engage in less physical activity, further increasing their risk of metabolic issues. To control this risk, he advised people to return to their circadian rhythms, which could mean forgetting the midnight snack.
“Having a daily pattern that’s in sync with chronicity, or these daily rhythms, is associated with greater health,” Dr. Friedman said. “We’re not really made to eat at night. I think this [study] kind of reinforces that.”
Can a night owl become an early bird?
When asked if a person’s natural circadian rhythm can be later, Dr. Malin responded that chronotypes may be dictated by genetics and age, as well as external drivers like work schedule. For these reasons, it’s “tricky” to answer whether night owls can turn into early birds and reap the potential health benefits of making that shift.
“Given that so many life factors can influence what our routine entails, it’s hard to know if we [can] truly change our chronotype or if rather we [can] learn to manage,” Dr. Malin said. “In either case, there is some work that suggests people can adopt earlier bedtimes and waketimes through practical recommendations.”
Specifically, he suggested increasing physical activity during the day, and adjusting bedtimes gradually by 15-minute increments.
“Go to bed 15 minutes earlier then wake up 15 minutes earlier,” Dr. Malin said. “In time, and depending on how things are going, this can expand to another 15-minute window. Then, during the earlier time waking up, a person can engage in light physical activity to help with promoting general fitness. If they can get outside with sunlight, that would be great too, as the natural sunlight would provide cues to the circadian system to adjust.”
The study was supported by the National Institutes of Health. The investigators and Dr. Friedman disclosed no conflicts of interest.
In the study involving 51 people, night owls metabolized fat less efficiently, showed less insulin sensitivity, and demonstrated lower physical fitness than early birds, lead author Steven K. Malin, PhD, of Rutgers University, New Brunswick, N.J., and colleagues reported.
Prior publications have suggested that night owls, formally known as “late chronotypes,” have an increased risk of obesity, type 2 diabetes, and cardiovascular disease, Dr. Malin said in an interview. But no previous research involved the gold-standard measurement tools used in this study, including euglycemic clamp and indirect calorimetry to quantify fat metabolism.
Dr. Malin also noted that this is the first study of its kind to characterize metabolism during both rest and exercise.
The study, published in Experimental Physiology, involved 24 early birds and 27 night owls classified by the Morning-Eveningness Questionnaire. All participants were sedentary, reporting less than one hour of structured exercise per week, and had metabolic syndrome according to Adult Treatment Panel III report criteria. Groups were otherwise demographically similar, with average ages in each group of approximately 54-55 years.
Compared with night owls, early birds were more physically active during the morning into midday. During exercise, they metabolized more fat and demonstrated greater physical fitness based on VO2max readings. At rest, early birds also came out ahead – they had higher fat oxidation and non–oxidative glucose disposal, suggesting more sensitivity to insulin.
“Collectively, this work highlights and supports chronotype as a potential risk factor related to type 2 diabetes and cardiovascular disease risk,” the investigators concluded.
Night owls have less metabolic control
Jed Friedman, PhD, director of OU Health Harold Hamm Diabetes Center at the University of Oklahoma Health Sciences Center, Oklahoma City, praised the study for the size of the groups the researchers compared with each other and how well matched those groups were, as well as the “state-of-the-art” measurement tools employed.
The findings show that night owls have “less metabolic control,” Dr. Friedman said in an interview.
“That’s a term that’s frequently invoked in [regard to] prediabetes,” he said. “Blood sugar goes up, because when you’re eating a high carbohydrate diet, your cells aren’t metabolizing sugar properly. That tends to raise your risk for a lot of diseases.”
Dr. Friedman added that the findings align with those of previous studies that have linked less sleep with changes in brain biology, and therefore behavior, especially in dietary choices.
“When you’re tired, the mechanisms for appetite control go haywire,” Dr. Friedman explained. “The evidence suggests that sugar is the primary driver for what people eat when they’re tired. That obviously has implications for diabetes and metabolic syndrome. So sleeping more really can help you control cravings.”
Dr. Friedman also noted that people who are tired tend to engage in less physical activity, further increasing their risk of metabolic issues. To control this risk, he advised people to return to their circadian rhythms, which could mean forgetting the midnight snack.
“Having a daily pattern that’s in sync with chronicity, or these daily rhythms, is associated with greater health,” Dr. Friedman said. “We’re not really made to eat at night. I think this [study] kind of reinforces that.”
Can a night owl become an early bird?
When asked if a person’s natural circadian rhythm can be later, Dr. Malin responded that chronotypes may be dictated by genetics and age, as well as external drivers like work schedule. For these reasons, it’s “tricky” to answer whether night owls can turn into early birds and reap the potential health benefits of making that shift.
“Given that so many life factors can influence what our routine entails, it’s hard to know if we [can] truly change our chronotype or if rather we [can] learn to manage,” Dr. Malin said. “In either case, there is some work that suggests people can adopt earlier bedtimes and waketimes through practical recommendations.”
Specifically, he suggested increasing physical activity during the day, and adjusting bedtimes gradually by 15-minute increments.
“Go to bed 15 minutes earlier then wake up 15 minutes earlier,” Dr. Malin said. “In time, and depending on how things are going, this can expand to another 15-minute window. Then, during the earlier time waking up, a person can engage in light physical activity to help with promoting general fitness. If they can get outside with sunlight, that would be great too, as the natural sunlight would provide cues to the circadian system to adjust.”
The study was supported by the National Institutes of Health. The investigators and Dr. Friedman disclosed no conflicts of interest.
FROM EXPERIMENTAL PHYSIOLOGY
‘Amazing’ data for cheap beta-blocker gel for diabetic foot ulcers
STOCKHOLM – Esmolol hydrochloride gel (Galnobax, NovoLead) appears to be a safe and effective novel topical treatment option for diabetic foot ulcers, according to results from a new trial of the drug, which is widely available as a generic and is inexpensive.
Of note, the proportion of participants achieving target ulcer closure at 12 weeks with esmolol (plus standard of care) was around 60% compared with just over 40% in patients who received standard of care alone.
Presenting the findings at this year’s annual meeting of the European Association for the Study of Diabetes was Ashu Rastogi, MD, a professor of endocrinology at the Postgraduate Institute of Medical Education and Research in Chandigarh, India.
“Esmolol can be given topically as a 14% gel and is a novel treatment option in diabetic foot ulcer,” said Dr. Rastogi.
Esmolol, a short-acting beta-adrenergic blocker, is currently approved by the U.S. Food and Drug Administration for cardiac indications only, such as short-term use for controlling supraventricular tachycardia. Beta-blockers are also used to treat hypertension.
However, esmolol has also been repurposed and formulated as a topical gel for the treatment of hard-to-heal diabetic foot ulcers (mainly neuropathic grade 1).
Audience member Ketan Dhatariya, MBBS, MD, PhD, a National Health Service consultant in diabetes, endocrinology, and general medicine and honorary senior lecturer at Norfolk and Norwich University Hospitals, England, enthused about the findings.
“This is an amazing study. I’m part of a working group looking at the updating of a guideline for the International Working Group of the Diabetic Foot, reviewing all the studies on wound healing, specifically pharmacological interventions. This is way beyond anything shown to date in terms of medical intervention. [The authors] should be congratulated; this is really astounding,” he told this news organization.
“Right now, there is very little out there in terms of pharmacological interventions that have shown benefit,” he added. “Once this study has been peer-reviewed and is published properly, it is potentially game-changing because it is a generic, worldwide, cheap, and freely available medication.”
Study across 27 sites in India
Prior phase 1/2 data have shown that 60% of ulcers completely closed with esmolol (14% gel) compared with 39% with standard of care. Encouraged by these findings, a phase 3 randomized, double-blind placebo-controlled study was conducted across 27 sites in India.
Patients were a mean age of 56 years, and had a body mass index (BMI) of 25-26 kg/m2 and mean hemoglobin A1c of 8.4%-8.7%. Around 70% of participants were men. Mean ulcer area was approximately 460-500 mm2, two-thirds of the ulcers were plantar, and mean ulcer duration was 40-50 weeks.
After screening and discontinuations (39 participants), a 12-week treatment phase began with patients randomized to one of three groups: esmolol (14% gel) along with standard of care administered twice daily (57 completers); standard of care only (63 completers); or vehicle gel (placebo) along with standard of care administered twice daily (17 completers).
Standard of care comprised wound cleaning, debridement, maintenance of moist wound environment, twice-daily fresh bandages, and off-loading footwear as needed, and was provided to all participants irrespective of study group.
The 12-week treatment period was followed by an observation period of 12 weeks up to the 24-week study endpoint.
The primary efficacy endpoint was the proportion of participants achieving target ulcer closure (100% re-epithelialization without drainage or dressing requirement) within the 12-week treatment phase.
Secondary endpoints included time to target ulcer closure during the 12-week treatment phase and proportion of participants achieving target ulcer closure by 24 weeks (end of study). Investigators were blinded throughout.
Subanalyses were conducted based on ulcer location, size, and age, as well as estimated glomerular filtration rate less than 90 mL/min and ankle-brachial index under 0.9 but greater than 0.7.
50% more patients on esmolol had complete ulcer closure
The proportion of participants with complete ulcer closure at 12 weeks was 60.3% in the esmolol plus standard of care group, compared with 41.7% with standard of care only, a difference of 18.6% (odds ratio, 2.13; P = .0276).
“The 24-week end-of-study data show what happened in the 12 weeks following end of treatment,” said Dr. Rastogi, turning to results showing that by 24 weeks the proportion of participants with complete ulcer closure was 77.2% versus 55.6%, respectively, with a difference of 21.6% (OR, 2.71; P = .013).
Time to ulcer closure (a secondary endpoint) was similar between the esmolol plus standard of care vs. standard of care groups (74.3 vs. 72.5 days).
The impact of ulcer location on complete ulcer closure, a subanalysis, showed a higher proportion of patients experienced complete ulcer closure with esmolol plus standard of care versus standard of care. For example, in plantar-based ulcers, esmolol led to complete closure in 58.7% vs. 43.1%, while for nonplantar ulcers, complete closure was found in 63.6% vs. 38.1%.
In wounds less than 5 cm2, the proportion of complete closures was 66.0% vs. 50.0% for esmolol compared with standard of care alone, while in wounds over 5 cm2, these proportions were 47.6% vs. 26.9%.
Subanalyses also showed that esmolol was substantially better in patients with BMI greater than 25, ulcer duration over 12 weeks, and A1c above 8%.
Also, a subanalysis stratified by “real-life” situations favored esmolol, showing a 50.9% difference in the proportion of patients with diabetic foot ulcer healing in those with a history of hypertension and a 31.8% difference favoring esmolol in those with an abnormal electrocardiogram.
Overall, the proportions of patients who had an adverse event were 13.2%, 18.4%, and 37.5% in the esmolol plus standard of care, standard of care alone, and vehicle plus standard of care groups, respectively, and the vast majority were unrelated to study drug. There were no serious adverse events in the esmolol plus standard of care group.
A class effect of beta blockers?
The proposed mechanism of action of esmolol relates to a sequence of reducing inflammation (via vasodilation, fibroblast migration, and cytokine reduction); proliferation by beta-blockade (improves keratinocyte migration and epithelialization); and remodeling (increases collagen turnover).
Asked by an audience member if the observations were a class effect and systemic effect of beta-blockers, Dr. Rastogi said he could not say for sure that it was a class effect, but they deliberately used a beta-1 adrenergic receptor antagonist.
“It may not be a systemic effect because we have some patients who use beta-blockers systemically and they still have diabetic foot ulcers,” he said.
Dr. Rastogi and Dr. Dhatariya have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
STOCKHOLM – Esmolol hydrochloride gel (Galnobax, NovoLead) appears to be a safe and effective novel topical treatment option for diabetic foot ulcers, according to results from a new trial of the drug, which is widely available as a generic and is inexpensive.
Of note, the proportion of participants achieving target ulcer closure at 12 weeks with esmolol (plus standard of care) was around 60% compared with just over 40% in patients who received standard of care alone.
Presenting the findings at this year’s annual meeting of the European Association for the Study of Diabetes was Ashu Rastogi, MD, a professor of endocrinology at the Postgraduate Institute of Medical Education and Research in Chandigarh, India.
“Esmolol can be given topically as a 14% gel and is a novel treatment option in diabetic foot ulcer,” said Dr. Rastogi.
Esmolol, a short-acting beta-adrenergic blocker, is currently approved by the U.S. Food and Drug Administration for cardiac indications only, such as short-term use for controlling supraventricular tachycardia. Beta-blockers are also used to treat hypertension.
However, esmolol has also been repurposed and formulated as a topical gel for the treatment of hard-to-heal diabetic foot ulcers (mainly neuropathic grade 1).
Audience member Ketan Dhatariya, MBBS, MD, PhD, a National Health Service consultant in diabetes, endocrinology, and general medicine and honorary senior lecturer at Norfolk and Norwich University Hospitals, England, enthused about the findings.
“This is an amazing study. I’m part of a working group looking at the updating of a guideline for the International Working Group of the Diabetic Foot, reviewing all the studies on wound healing, specifically pharmacological interventions. This is way beyond anything shown to date in terms of medical intervention. [The authors] should be congratulated; this is really astounding,” he told this news organization.
“Right now, there is very little out there in terms of pharmacological interventions that have shown benefit,” he added. “Once this study has been peer-reviewed and is published properly, it is potentially game-changing because it is a generic, worldwide, cheap, and freely available medication.”
Study across 27 sites in India
Prior phase 1/2 data have shown that 60% of ulcers completely closed with esmolol (14% gel) compared with 39% with standard of care. Encouraged by these findings, a phase 3 randomized, double-blind placebo-controlled study was conducted across 27 sites in India.
Patients were a mean age of 56 years, and had a body mass index (BMI) of 25-26 kg/m2 and mean hemoglobin A1c of 8.4%-8.7%. Around 70% of participants were men. Mean ulcer area was approximately 460-500 mm2, two-thirds of the ulcers were plantar, and mean ulcer duration was 40-50 weeks.
After screening and discontinuations (39 participants), a 12-week treatment phase began with patients randomized to one of three groups: esmolol (14% gel) along with standard of care administered twice daily (57 completers); standard of care only (63 completers); or vehicle gel (placebo) along with standard of care administered twice daily (17 completers).
Standard of care comprised wound cleaning, debridement, maintenance of moist wound environment, twice-daily fresh bandages, and off-loading footwear as needed, and was provided to all participants irrespective of study group.
The 12-week treatment period was followed by an observation period of 12 weeks up to the 24-week study endpoint.
The primary efficacy endpoint was the proportion of participants achieving target ulcer closure (100% re-epithelialization without drainage or dressing requirement) within the 12-week treatment phase.
Secondary endpoints included time to target ulcer closure during the 12-week treatment phase and proportion of participants achieving target ulcer closure by 24 weeks (end of study). Investigators were blinded throughout.
Subanalyses were conducted based on ulcer location, size, and age, as well as estimated glomerular filtration rate less than 90 mL/min and ankle-brachial index under 0.9 but greater than 0.7.
50% more patients on esmolol had complete ulcer closure
The proportion of participants with complete ulcer closure at 12 weeks was 60.3% in the esmolol plus standard of care group, compared with 41.7% with standard of care only, a difference of 18.6% (odds ratio, 2.13; P = .0276).
“The 24-week end-of-study data show what happened in the 12 weeks following end of treatment,” said Dr. Rastogi, turning to results showing that by 24 weeks the proportion of participants with complete ulcer closure was 77.2% versus 55.6%, respectively, with a difference of 21.6% (OR, 2.71; P = .013).
Time to ulcer closure (a secondary endpoint) was similar between the esmolol plus standard of care vs. standard of care groups (74.3 vs. 72.5 days).
The impact of ulcer location on complete ulcer closure, a subanalysis, showed a higher proportion of patients experienced complete ulcer closure with esmolol plus standard of care versus standard of care. For example, in plantar-based ulcers, esmolol led to complete closure in 58.7% vs. 43.1%, while for nonplantar ulcers, complete closure was found in 63.6% vs. 38.1%.
In wounds less than 5 cm2, the proportion of complete closures was 66.0% vs. 50.0% for esmolol compared with standard of care alone, while in wounds over 5 cm2, these proportions were 47.6% vs. 26.9%.
Subanalyses also showed that esmolol was substantially better in patients with BMI greater than 25, ulcer duration over 12 weeks, and A1c above 8%.
Also, a subanalysis stratified by “real-life” situations favored esmolol, showing a 50.9% difference in the proportion of patients with diabetic foot ulcer healing in those with a history of hypertension and a 31.8% difference favoring esmolol in those with an abnormal electrocardiogram.
Overall, the proportions of patients who had an adverse event were 13.2%, 18.4%, and 37.5% in the esmolol plus standard of care, standard of care alone, and vehicle plus standard of care groups, respectively, and the vast majority were unrelated to study drug. There were no serious adverse events in the esmolol plus standard of care group.
A class effect of beta blockers?
The proposed mechanism of action of esmolol relates to a sequence of reducing inflammation (via vasodilation, fibroblast migration, and cytokine reduction); proliferation by beta-blockade (improves keratinocyte migration and epithelialization); and remodeling (increases collagen turnover).
Asked by an audience member if the observations were a class effect and systemic effect of beta-blockers, Dr. Rastogi said he could not say for sure that it was a class effect, but they deliberately used a beta-1 adrenergic receptor antagonist.
“It may not be a systemic effect because we have some patients who use beta-blockers systemically and they still have diabetic foot ulcers,” he said.
Dr. Rastogi and Dr. Dhatariya have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
STOCKHOLM – Esmolol hydrochloride gel (Galnobax, NovoLead) appears to be a safe and effective novel topical treatment option for diabetic foot ulcers, according to results from a new trial of the drug, which is widely available as a generic and is inexpensive.
Of note, the proportion of participants achieving target ulcer closure at 12 weeks with esmolol (plus standard of care) was around 60% compared with just over 40% in patients who received standard of care alone.
Presenting the findings at this year’s annual meeting of the European Association for the Study of Diabetes was Ashu Rastogi, MD, a professor of endocrinology at the Postgraduate Institute of Medical Education and Research in Chandigarh, India.
“Esmolol can be given topically as a 14% gel and is a novel treatment option in diabetic foot ulcer,” said Dr. Rastogi.
Esmolol, a short-acting beta-adrenergic blocker, is currently approved by the U.S. Food and Drug Administration for cardiac indications only, such as short-term use for controlling supraventricular tachycardia. Beta-blockers are also used to treat hypertension.
However, esmolol has also been repurposed and formulated as a topical gel for the treatment of hard-to-heal diabetic foot ulcers (mainly neuropathic grade 1).
Audience member Ketan Dhatariya, MBBS, MD, PhD, a National Health Service consultant in diabetes, endocrinology, and general medicine and honorary senior lecturer at Norfolk and Norwich University Hospitals, England, enthused about the findings.
“This is an amazing study. I’m part of a working group looking at the updating of a guideline for the International Working Group of the Diabetic Foot, reviewing all the studies on wound healing, specifically pharmacological interventions. This is way beyond anything shown to date in terms of medical intervention. [The authors] should be congratulated; this is really astounding,” he told this news organization.
“Right now, there is very little out there in terms of pharmacological interventions that have shown benefit,” he added. “Once this study has been peer-reviewed and is published properly, it is potentially game-changing because it is a generic, worldwide, cheap, and freely available medication.”
Study across 27 sites in India
Prior phase 1/2 data have shown that 60% of ulcers completely closed with esmolol (14% gel) compared with 39% with standard of care. Encouraged by these findings, a phase 3 randomized, double-blind placebo-controlled study was conducted across 27 sites in India.
Patients were a mean age of 56 years, and had a body mass index (BMI) of 25-26 kg/m2 and mean hemoglobin A1c of 8.4%-8.7%. Around 70% of participants were men. Mean ulcer area was approximately 460-500 mm2, two-thirds of the ulcers were plantar, and mean ulcer duration was 40-50 weeks.
After screening and discontinuations (39 participants), a 12-week treatment phase began with patients randomized to one of three groups: esmolol (14% gel) along with standard of care administered twice daily (57 completers); standard of care only (63 completers); or vehicle gel (placebo) along with standard of care administered twice daily (17 completers).
Standard of care comprised wound cleaning, debridement, maintenance of moist wound environment, twice-daily fresh bandages, and off-loading footwear as needed, and was provided to all participants irrespective of study group.
The 12-week treatment period was followed by an observation period of 12 weeks up to the 24-week study endpoint.
The primary efficacy endpoint was the proportion of participants achieving target ulcer closure (100% re-epithelialization without drainage or dressing requirement) within the 12-week treatment phase.
Secondary endpoints included time to target ulcer closure during the 12-week treatment phase and proportion of participants achieving target ulcer closure by 24 weeks (end of study). Investigators were blinded throughout.
Subanalyses were conducted based on ulcer location, size, and age, as well as estimated glomerular filtration rate less than 90 mL/min and ankle-brachial index under 0.9 but greater than 0.7.
50% more patients on esmolol had complete ulcer closure
The proportion of participants with complete ulcer closure at 12 weeks was 60.3% in the esmolol plus standard of care group, compared with 41.7% with standard of care only, a difference of 18.6% (odds ratio, 2.13; P = .0276).
“The 24-week end-of-study data show what happened in the 12 weeks following end of treatment,” said Dr. Rastogi, turning to results showing that by 24 weeks the proportion of participants with complete ulcer closure was 77.2% versus 55.6%, respectively, with a difference of 21.6% (OR, 2.71; P = .013).
Time to ulcer closure (a secondary endpoint) was similar between the esmolol plus standard of care vs. standard of care groups (74.3 vs. 72.5 days).
The impact of ulcer location on complete ulcer closure, a subanalysis, showed a higher proportion of patients experienced complete ulcer closure with esmolol plus standard of care versus standard of care. For example, in plantar-based ulcers, esmolol led to complete closure in 58.7% vs. 43.1%, while for nonplantar ulcers, complete closure was found in 63.6% vs. 38.1%.
In wounds less than 5 cm2, the proportion of complete closures was 66.0% vs. 50.0% for esmolol compared with standard of care alone, while in wounds over 5 cm2, these proportions were 47.6% vs. 26.9%.
Subanalyses also showed that esmolol was substantially better in patients with BMI greater than 25, ulcer duration over 12 weeks, and A1c above 8%.
Also, a subanalysis stratified by “real-life” situations favored esmolol, showing a 50.9% difference in the proportion of patients with diabetic foot ulcer healing in those with a history of hypertension and a 31.8% difference favoring esmolol in those with an abnormal electrocardiogram.
Overall, the proportions of patients who had an adverse event were 13.2%, 18.4%, and 37.5% in the esmolol plus standard of care, standard of care alone, and vehicle plus standard of care groups, respectively, and the vast majority were unrelated to study drug. There were no serious adverse events in the esmolol plus standard of care group.
A class effect of beta blockers?
The proposed mechanism of action of esmolol relates to a sequence of reducing inflammation (via vasodilation, fibroblast migration, and cytokine reduction); proliferation by beta-blockade (improves keratinocyte migration and epithelialization); and remodeling (increases collagen turnover).
Asked by an audience member if the observations were a class effect and systemic effect of beta-blockers, Dr. Rastogi said he could not say for sure that it was a class effect, but they deliberately used a beta-1 adrenergic receptor antagonist.
“It may not be a systemic effect because we have some patients who use beta-blockers systemically and they still have diabetic foot ulcers,” he said.
Dr. Rastogi and Dr. Dhatariya have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM EASD 2022
Formula may be right for infants, but experts warn that toddlers don’t need it
Formulas for toddlers are a burgeoning business in the United States: Sales of the drinks more than doubled in recent years as companies convinced parents that their little ones needed the liquid boost. But many experts warn that these products, designed for children ages 1-3, fill no nutritional needs beyond what is available in a typical toddler diet, are subject to less regulation than infant formula, and are expensive.
In addition, some parents feed the toddler versions to infants even though they do not meet federal standards for infant formula and may not provide babies with adequate nutrients to sustain their growth.
Pediatricians and federal health officials say that when most children turn 1, they can begin drinking cow milk or an unsweetened plant-based milk substitute. In a 2019 “consensus” statement, the American Academy of Pediatrics and other health and nutrition organizations recommended against using toddler formulas, saying “they offer no unique nutritional value beyond what could be obtained with healthy foods; furthermore, they may contribute added sugars to the diet.” The toddler formulas often contain sweeteners and fats that add calories.
Some of the same companies that produce infant formula – including Enfamil, Gerber, and Similac – also make toddler formulas, as do some smaller, boutique brands that advertise that they have organic or other special qualities. Toddler formulas are available nearly everywhere infant formulas are sold and are marketed as providing extra nutrients to help children’s brain, immune system, and eye development, among other benefits. They are different from medical formulas prescribed for children with specific needs.
A 2020 study found that sales of toddler formula in the United States rose to $92 million in 2015 from $39 million in 2006.
Parents are often confused by the marketing for the formulas, according to a study led by Jennifer Harris, PhD, a marketing and public health researcher at the University of Connecticut, Hartford. She found that 60% of caregivers falsely believed toddler formulas have nutrients that toddlers can’t get from other foods.
Anthony Porto, MD, MPH, a pediatric gastroenterologist and pediatrics professor at Yale University, New Haven, Conn., said he is concerned these products could be giving toddlers more nutrients and calories than they need. Unlike what’s designed for infants, toddler formula has no nutritional regulations: Experts say standardizing a supplement to toddlers’ diets is impossible because no two children are alike.
In focus groups, Dr. Harris said, parents report feeding their children toddler formula to fill nutritional gaps when a child isn’t eating enough, a common concern among parents.
“Infants are often voracious eaters,” said Stephen Daniels, MD, chair of pediatrics at Children’s Hospital Colorado, Aurora. But at around a year of age, children’s growth plateaus, he said, and “they’re suddenly not hungry in the way they used to be anymore.” That can worry parents, he added, but “it’s a completely normal phenomenon.”
If parents have concerns about their children’s diet, Dr. Daniels said, they should consult a pediatrician or family doctor.
Blanche Lincoln, president of the Infant Nutrition Council of America, which represents the makers of Enfamil, Gerber, Similac, and store brands, said in an email that the toddler formulas can be helpful because they can fill “nutritional gaps during this period of transition to table foods.” Ms. Lincoln, a former U.S. senator from Arkansas, said the drinks “help contribute to the specific nutritional needs of toddlers by providing energy and important nutrients, as well as essential vitamins and minerals during this important period of growth and development.”
But toddler formula isn’t being ingested by toddlers alone – it’s also being fed to infants. In a recent study, Dr. Porto and colleagues found that 5% of infants’ parents reported giving their babies drinks marketed for the older age group. And Dr. Harris’ research indicated that 22% of parents of infants older than 6 months had fed their babies toddler formula in the previous month. Both studies were conducted before the recent infant formula shortage, which may have exacerbated the problem.
“Infant formulas and toddler formulas tend to be next to each other in the supermarket,” Dr. Harris said. “They look similar, but the toddler formulas are cheaper than the infant formulas. So people confuse them, and they grab the wrong one. Or they think: ‘Oh, this one is less expensive. I’ll get this one instead.’ ”
According to an email from Food and Drug Administration spokesperson Lindsay Haake, toddler drinks do not meet the definition of infant formula, so they are not subject to the same requirements. That means they do not have to undergo the clinical trials and pathogen safety testing that the infant versions do. “Unlike infant formulas, toddler formulas are not necessary to meet the nutritional needs of their intended consumers,” Ms. Haake said.
In a statement to KHN, the Infant Nutrition Council of America said: “Toddler drinks have a distinctive use and nutritional makeup from infant formula; the two are not interchangeable. The labeling of toddler nutritional drinks explicitly identifies the product as a toddler drink intended for children 12 months and older on the front of the package label.”
However, several expensive toddler formula brands made by smaller companies – often advertised as being made from goat milk, A2 whole milk (which lacks one common milk protein), or vegan ingredients that aren’t soy – do meet nutritional requirements for infants, and some advertise that.
Dr. Harris argued that this confuses parents, too, and shouldn’t be allowed. Just because a toddler formula has the nutritional ingredients required by the FDA for infant formula doesn’t mean it has met the other tests required of infant formula.
Federal regulators have not forced any of the companies to withdraw those products. In an email, FDA spokesperson Marianna Naum said: “The FDA does not comment on potential compliance actions.”
One company, Nature’s One, whose toddler formulas are named “Baby’s Only,” received warning letters a decade ago from the FDA about marketing them for infants. That case was closed in 2016. The company’s website says that Baby’s Only formula “meets nutrient requirements for infant” and that “Baby’s Only Organic® can be served up to 3 years of age.” Critics say that language implies the formula is fine for babies younger than 1. The company’s website and its Instagram account feature customer testimonials from parents who report feeding the formula to their infants, as well as pictures of infants drinking it.
Jay Highman, CEO and president of Nature’s One, said that Baby’s Only is clearly labeled as a toddler formula and that the back of the can states that “Baby’s Only is intended for a toddler 1 year of age or older OR when directed by a health care professional.” He also said that since the company launched in 1999, its formulas have met all the nutritional, manufacturing, and safety standards required of infant formula even though they don’t have to. “We behaved like we are an infant formula, but we were selling it as a toddler formula.”
He said that the clinical trials required by the FDA are a huge barrier to bringing a new infant formula to market and that many other countries don’t require a clinical trial. Baby’s Only recently completed a clinical trial, and the company expects to be able to sell it as an infant formula soon.
Yet pediatricians and nutritional experts continue to caution parents about using the toddler drinks. “There’s no question that infant formula is very important in the first year of life,” Dr. Daniels said. But he doesn’t recommend the toddler version “because it’s not that useful, because it’s confusing, because it’s expensive.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Formulas for toddlers are a burgeoning business in the United States: Sales of the drinks more than doubled in recent years as companies convinced parents that their little ones needed the liquid boost. But many experts warn that these products, designed for children ages 1-3, fill no nutritional needs beyond what is available in a typical toddler diet, are subject to less regulation than infant formula, and are expensive.
In addition, some parents feed the toddler versions to infants even though they do not meet federal standards for infant formula and may not provide babies with adequate nutrients to sustain their growth.
Pediatricians and federal health officials say that when most children turn 1, they can begin drinking cow milk or an unsweetened plant-based milk substitute. In a 2019 “consensus” statement, the American Academy of Pediatrics and other health and nutrition organizations recommended against using toddler formulas, saying “they offer no unique nutritional value beyond what could be obtained with healthy foods; furthermore, they may contribute added sugars to the diet.” The toddler formulas often contain sweeteners and fats that add calories.
Some of the same companies that produce infant formula – including Enfamil, Gerber, and Similac – also make toddler formulas, as do some smaller, boutique brands that advertise that they have organic or other special qualities. Toddler formulas are available nearly everywhere infant formulas are sold and are marketed as providing extra nutrients to help children’s brain, immune system, and eye development, among other benefits. They are different from medical formulas prescribed for children with specific needs.
A 2020 study found that sales of toddler formula in the United States rose to $92 million in 2015 from $39 million in 2006.
Parents are often confused by the marketing for the formulas, according to a study led by Jennifer Harris, PhD, a marketing and public health researcher at the University of Connecticut, Hartford. She found that 60% of caregivers falsely believed toddler formulas have nutrients that toddlers can’t get from other foods.
Anthony Porto, MD, MPH, a pediatric gastroenterologist and pediatrics professor at Yale University, New Haven, Conn., said he is concerned these products could be giving toddlers more nutrients and calories than they need. Unlike what’s designed for infants, toddler formula has no nutritional regulations: Experts say standardizing a supplement to toddlers’ diets is impossible because no two children are alike.
In focus groups, Dr. Harris said, parents report feeding their children toddler formula to fill nutritional gaps when a child isn’t eating enough, a common concern among parents.
“Infants are often voracious eaters,” said Stephen Daniels, MD, chair of pediatrics at Children’s Hospital Colorado, Aurora. But at around a year of age, children’s growth plateaus, he said, and “they’re suddenly not hungry in the way they used to be anymore.” That can worry parents, he added, but “it’s a completely normal phenomenon.”
If parents have concerns about their children’s diet, Dr. Daniels said, they should consult a pediatrician or family doctor.
Blanche Lincoln, president of the Infant Nutrition Council of America, which represents the makers of Enfamil, Gerber, Similac, and store brands, said in an email that the toddler formulas can be helpful because they can fill “nutritional gaps during this period of transition to table foods.” Ms. Lincoln, a former U.S. senator from Arkansas, said the drinks “help contribute to the specific nutritional needs of toddlers by providing energy and important nutrients, as well as essential vitamins and minerals during this important period of growth and development.”
But toddler formula isn’t being ingested by toddlers alone – it’s also being fed to infants. In a recent study, Dr. Porto and colleagues found that 5% of infants’ parents reported giving their babies drinks marketed for the older age group. And Dr. Harris’ research indicated that 22% of parents of infants older than 6 months had fed their babies toddler formula in the previous month. Both studies were conducted before the recent infant formula shortage, which may have exacerbated the problem.
“Infant formulas and toddler formulas tend to be next to each other in the supermarket,” Dr. Harris said. “They look similar, but the toddler formulas are cheaper than the infant formulas. So people confuse them, and they grab the wrong one. Or they think: ‘Oh, this one is less expensive. I’ll get this one instead.’ ”
According to an email from Food and Drug Administration spokesperson Lindsay Haake, toddler drinks do not meet the definition of infant formula, so they are not subject to the same requirements. That means they do not have to undergo the clinical trials and pathogen safety testing that the infant versions do. “Unlike infant formulas, toddler formulas are not necessary to meet the nutritional needs of their intended consumers,” Ms. Haake said.
In a statement to KHN, the Infant Nutrition Council of America said: “Toddler drinks have a distinctive use and nutritional makeup from infant formula; the two are not interchangeable. The labeling of toddler nutritional drinks explicitly identifies the product as a toddler drink intended for children 12 months and older on the front of the package label.”
However, several expensive toddler formula brands made by smaller companies – often advertised as being made from goat milk, A2 whole milk (which lacks one common milk protein), or vegan ingredients that aren’t soy – do meet nutritional requirements for infants, and some advertise that.
Dr. Harris argued that this confuses parents, too, and shouldn’t be allowed. Just because a toddler formula has the nutritional ingredients required by the FDA for infant formula doesn’t mean it has met the other tests required of infant formula.
Federal regulators have not forced any of the companies to withdraw those products. In an email, FDA spokesperson Marianna Naum said: “The FDA does not comment on potential compliance actions.”
One company, Nature’s One, whose toddler formulas are named “Baby’s Only,” received warning letters a decade ago from the FDA about marketing them for infants. That case was closed in 2016. The company’s website says that Baby’s Only formula “meets nutrient requirements for infant” and that “Baby’s Only Organic® can be served up to 3 years of age.” Critics say that language implies the formula is fine for babies younger than 1. The company’s website and its Instagram account feature customer testimonials from parents who report feeding the formula to their infants, as well as pictures of infants drinking it.
Jay Highman, CEO and president of Nature’s One, said that Baby’s Only is clearly labeled as a toddler formula and that the back of the can states that “Baby’s Only is intended for a toddler 1 year of age or older OR when directed by a health care professional.” He also said that since the company launched in 1999, its formulas have met all the nutritional, manufacturing, and safety standards required of infant formula even though they don’t have to. “We behaved like we are an infant formula, but we were selling it as a toddler formula.”
He said that the clinical trials required by the FDA are a huge barrier to bringing a new infant formula to market and that many other countries don’t require a clinical trial. Baby’s Only recently completed a clinical trial, and the company expects to be able to sell it as an infant formula soon.
Yet pediatricians and nutritional experts continue to caution parents about using the toddler drinks. “There’s no question that infant formula is very important in the first year of life,” Dr. Daniels said. But he doesn’t recommend the toddler version “because it’s not that useful, because it’s confusing, because it’s expensive.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Formulas for toddlers are a burgeoning business in the United States: Sales of the drinks more than doubled in recent years as companies convinced parents that their little ones needed the liquid boost. But many experts warn that these products, designed for children ages 1-3, fill no nutritional needs beyond what is available in a typical toddler diet, are subject to less regulation than infant formula, and are expensive.
In addition, some parents feed the toddler versions to infants even though they do not meet federal standards for infant formula and may not provide babies with adequate nutrients to sustain their growth.
Pediatricians and federal health officials say that when most children turn 1, they can begin drinking cow milk or an unsweetened plant-based milk substitute. In a 2019 “consensus” statement, the American Academy of Pediatrics and other health and nutrition organizations recommended against using toddler formulas, saying “they offer no unique nutritional value beyond what could be obtained with healthy foods; furthermore, they may contribute added sugars to the diet.” The toddler formulas often contain sweeteners and fats that add calories.
Some of the same companies that produce infant formula – including Enfamil, Gerber, and Similac – also make toddler formulas, as do some smaller, boutique brands that advertise that they have organic or other special qualities. Toddler formulas are available nearly everywhere infant formulas are sold and are marketed as providing extra nutrients to help children’s brain, immune system, and eye development, among other benefits. They are different from medical formulas prescribed for children with specific needs.
A 2020 study found that sales of toddler formula in the United States rose to $92 million in 2015 from $39 million in 2006.
Parents are often confused by the marketing for the formulas, according to a study led by Jennifer Harris, PhD, a marketing and public health researcher at the University of Connecticut, Hartford. She found that 60% of caregivers falsely believed toddler formulas have nutrients that toddlers can’t get from other foods.
Anthony Porto, MD, MPH, a pediatric gastroenterologist and pediatrics professor at Yale University, New Haven, Conn., said he is concerned these products could be giving toddlers more nutrients and calories than they need. Unlike what’s designed for infants, toddler formula has no nutritional regulations: Experts say standardizing a supplement to toddlers’ diets is impossible because no two children are alike.
In focus groups, Dr. Harris said, parents report feeding their children toddler formula to fill nutritional gaps when a child isn’t eating enough, a common concern among parents.
“Infants are often voracious eaters,” said Stephen Daniels, MD, chair of pediatrics at Children’s Hospital Colorado, Aurora. But at around a year of age, children’s growth plateaus, he said, and “they’re suddenly not hungry in the way they used to be anymore.” That can worry parents, he added, but “it’s a completely normal phenomenon.”
If parents have concerns about their children’s diet, Dr. Daniels said, they should consult a pediatrician or family doctor.
Blanche Lincoln, president of the Infant Nutrition Council of America, which represents the makers of Enfamil, Gerber, Similac, and store brands, said in an email that the toddler formulas can be helpful because they can fill “nutritional gaps during this period of transition to table foods.” Ms. Lincoln, a former U.S. senator from Arkansas, said the drinks “help contribute to the specific nutritional needs of toddlers by providing energy and important nutrients, as well as essential vitamins and minerals during this important period of growth and development.”
But toddler formula isn’t being ingested by toddlers alone – it’s also being fed to infants. In a recent study, Dr. Porto and colleagues found that 5% of infants’ parents reported giving their babies drinks marketed for the older age group. And Dr. Harris’ research indicated that 22% of parents of infants older than 6 months had fed their babies toddler formula in the previous month. Both studies were conducted before the recent infant formula shortage, which may have exacerbated the problem.
“Infant formulas and toddler formulas tend to be next to each other in the supermarket,” Dr. Harris said. “They look similar, but the toddler formulas are cheaper than the infant formulas. So people confuse them, and they grab the wrong one. Or they think: ‘Oh, this one is less expensive. I’ll get this one instead.’ ”
According to an email from Food and Drug Administration spokesperson Lindsay Haake, toddler drinks do not meet the definition of infant formula, so they are not subject to the same requirements. That means they do not have to undergo the clinical trials and pathogen safety testing that the infant versions do. “Unlike infant formulas, toddler formulas are not necessary to meet the nutritional needs of their intended consumers,” Ms. Haake said.
In a statement to KHN, the Infant Nutrition Council of America said: “Toddler drinks have a distinctive use and nutritional makeup from infant formula; the two are not interchangeable. The labeling of toddler nutritional drinks explicitly identifies the product as a toddler drink intended for children 12 months and older on the front of the package label.”
However, several expensive toddler formula brands made by smaller companies – often advertised as being made from goat milk, A2 whole milk (which lacks one common milk protein), or vegan ingredients that aren’t soy – do meet nutritional requirements for infants, and some advertise that.
Dr. Harris argued that this confuses parents, too, and shouldn’t be allowed. Just because a toddler formula has the nutritional ingredients required by the FDA for infant formula doesn’t mean it has met the other tests required of infant formula.
Federal regulators have not forced any of the companies to withdraw those products. In an email, FDA spokesperson Marianna Naum said: “The FDA does not comment on potential compliance actions.”
One company, Nature’s One, whose toddler formulas are named “Baby’s Only,” received warning letters a decade ago from the FDA about marketing them for infants. That case was closed in 2016. The company’s website says that Baby’s Only formula “meets nutrient requirements for infant” and that “Baby’s Only Organic® can be served up to 3 years of age.” Critics say that language implies the formula is fine for babies younger than 1. The company’s website and its Instagram account feature customer testimonials from parents who report feeding the formula to their infants, as well as pictures of infants drinking it.
Jay Highman, CEO and president of Nature’s One, said that Baby’s Only is clearly labeled as a toddler formula and that the back of the can states that “Baby’s Only is intended for a toddler 1 year of age or older OR when directed by a health care professional.” He also said that since the company launched in 1999, its formulas have met all the nutritional, manufacturing, and safety standards required of infant formula even though they don’t have to. “We behaved like we are an infant formula, but we were selling it as a toddler formula.”
He said that the clinical trials required by the FDA are a huge barrier to bringing a new infant formula to market and that many other countries don’t require a clinical trial. Baby’s Only recently completed a clinical trial, and the company expects to be able to sell it as an infant formula soon.
Yet pediatricians and nutritional experts continue to caution parents about using the toddler drinks. “There’s no question that infant formula is very important in the first year of life,” Dr. Daniels said. But he doesn’t recommend the toddler version “because it’s not that useful, because it’s confusing, because it’s expensive.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Could exercise improve bone health in youth with type 1 diabetes?
In a small cross-sectional study of 10- to 16-year-old girls with and without type 1 diabetes, both groups were equally physically active, based on their replies to the bone-specific physical activity questionnaire (BPAQ).
However, among the more sedentary girls (with BPAQ scores below the median), those with type 1 diabetes had worse markers of bone health in imaging tests compared with the girls without diabetes.
the researchers summarize in a poster presented at the annual meeting of the American Society of Bone and Mineral Research.
However, this is early research and further study is needed, the group cautions.
“Ongoing studies with objective measures of physical activity as well as interventional studies will clarify whether increasing physical activity may improve bone health and reduce fracture risk in this vulnerable group,” they conclude.
“If you look at the sedentary kids, there’s a big discrepancy between the kids who have diabetes and the control kids, and that’s if we’re looking at radius or tibia or trabecular bone density or estimated failure load,” senior author Deborah M. Mitchell, MD, said in an interview at the poster session.
However, “when we look at the kids who are more physically active, we’re really not seeing as much difference [in bone health] between the kids with and without diabetes,” said Dr. Mitchell, a pediatric endocrinologist at Massachusetts General Hospital and assistant professor at Harvard Medical School, Boston.
But she also acknowledged, “There’s all sorts of caveats, including that this is retrospective questionnaire data.”
However, if further, rigorous studies confirm these findings, “physical activity is potentially a really effective means of improving bone quality in kids with type 1 diabetes.”
“This study suggests that bone-loading physical activity can substantially improve skeletal health in children with [type 1 diabetes] and should provide hope for patients and their families that they can take some action to prevent or mitigate the effects of diabetes on bone,” coauthor and incoming ASBMR President Mary L. Bouxsein, PhD, told this news organization in an email.
“We interpret these data as an important reason to advocate for increased time in moderate to vigorous bone-loading activity,” said Dr. Bouxsein, professor, department of orthopedic surgery, Harvard Medical School, Boston, “though the ‘dose’ in terms of hours per day or episodes per week to promote optimal bone health is still to be determined.”
“Ongoing debate,” “need stronger proof”
Asked for comment, Laura K. Bachrach, MD, who was not involved with the research, noted: “Activity benefits the development of bone strength through effects on bone geometry more than ‘density,’ and conversely, lack of physical activity can compromise gains in cortical bone diameter and thickness.”
However, “there is ongoing debate about the impact of type 1 diabetes on bone health and the factor(s) determining risk,” Dr. Bachrach, a pediatric endocrinologist at Stanford Children’s Health, Palo Alto, Calif., told this news organization in an email.
The current findings suggest “that physical activity in adolescent girls provided protection against potential adverse effects of type 1 diabetes,” said Dr. Bachrach, who spoke about bone fragility in childhood in a video commentary in 2021.
Study strengths, she noted, “include the rigor and expertise of the investigators, use of multiple surrogate measures that capture bone geometry/microarchitecture, as well as the inclusion of healthy local controls.”
“The study is limited by the cross-sectional design and subjects who opted, or not, to be active,” she added. “Stronger proof of the protective effects of activity on bone health in type 1 diabetes would require a randomized longitudinal intervention study, as alluded to by the authors of the study.”
Hypothesis: Those with type 1 diabetes acquire less bone mass in early 20s
The excess fracture risk in children with type 1 diabetes has been previously reported and is 14%-35% higher than the fracture risk in children without diabetes, Dr. Bouxsein explained. And “between 30% to 50% of kids [with type 1 diabetes] will have a fracture before the age of 18, so the excess fracture risk in diabetes is not clinically obvious,” she added.
However, “several lines of evidence strongly suggest that bone mass and microarchitecture at the time of peak bone mass (early 20s) is a major determinant of fracture risk throughout the lifespan,” she noted.
“Our hypothesis,” Dr. Bouxsein said, “is that the metabolic disruptions of diabetes, when they are present during the acquisition of peak bone mass, interfere with optimal bone development, and therefore may contribute to increased fracture risk later in life.”
Dr. Bachrach agreed that “peak bone strength is achieved by early adulthood, making childhood and adolescence important times to optimize bone health,” and that “peak bone strength is a predictor of lifetime risk of osteoporosis.”
“The diagnosis of pediatric osteoporosis is made when a child or teen sustains a vertebral fracture or femur fracture with minimal or no trauma,” she explained. “The diagnosis can also be made in a pediatric patient with low BMD [bone mineral density] for age in combination with a history of several long-bone fractures.”
Dr. Mitchell noted that type 1 diabetes is associated with a higher risk of fractures, which is sixfold in adults. In another study, she said, the group showed that in 10- to 16-year-old girls who’ve only had diabetes for a few years, “trabecular bone density is lower, they have lower estimated failure load, and longitudinally when we follow them, at least at the radius, we’re seeing bone loss at a relatively young age when we wouldn’t be expecting to see bone loss.”
80 girls enrolled, half had type 1 diabetes
Researchers enrolled 36 girls with type 1 diabetes and 44 girls without type 1 diabetes (controls) who were a mean age of 14.7 years and most (92%) were White. The girls with and without diabetes had similar rates of previous fractures (44% and 51%).
Those with diabetes had been diagnosed at a mean age of 9 years and had had diabetes for a mean of 4.6 years.
Researchers calculated participants’ total BPAQ scores based on type, duration, and frequency of bone-loading activities.
Participants had dual-energy X-ray absorptiometry scans to determine areal bone mineral density (BMD) at the total hip, femoral neck, lumbar spine, and whole body less head.
They also had high-resolution peripheral quantitative computed tomography at the distal tibia and radius to determine volumetric BMD, bone microarchitecture, and estimated bone strength (calculated using microfinite element analysis).
The two groups had similar total BPAQ scores (57.3 and 64.6), with a median score of 49.
BPAQ scores were positively associated with areal BMD at all sites (whole body, lumbar spine, total hip, femoral neck, and 1/3 radius) and with trabecular BMD and estimated failure load at the distal radius and tibia (P < .05 for all, adjusted for bone age).
Among participants with low physical activity (BPAQ below the median), compared with controls, those with type 1 diabetes had 6.6% lower aerial BMD at the lumbar spine (0.868 vs. 0.929 g/cm3; P = .04), 8% lower trabecular volumetric BMD at the distal radius (128.5 vs. 156.8 mg/cm3; P = .01), and 12% lower estimated failure load. Results at the distal tibia were similar.
Next steps
“More observational studies in males and females across a broader age spectrum would be helpful,” Dr. Bachrach noted. “The ‘gold standard’ model would be a long-term randomized controlled activity intervention study.”
“Further studies are underway [in girls and boys] using objective measures of activity including accelerometry and longitudinal observation to help confirm the findings from the current study,” Dr. Bouxsein said. “Ultimately, trials of activity interventions in children with [type 1 diabetes] will be the gold standard to determine to what extent physical activity can mitigate bone disease in [type 1 diabetes],” she agreed.
The study authors and Dr. Bachrach have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a small cross-sectional study of 10- to 16-year-old girls with and without type 1 diabetes, both groups were equally physically active, based on their replies to the bone-specific physical activity questionnaire (BPAQ).
However, among the more sedentary girls (with BPAQ scores below the median), those with type 1 diabetes had worse markers of bone health in imaging tests compared with the girls without diabetes.
the researchers summarize in a poster presented at the annual meeting of the American Society of Bone and Mineral Research.
However, this is early research and further study is needed, the group cautions.
“Ongoing studies with objective measures of physical activity as well as interventional studies will clarify whether increasing physical activity may improve bone health and reduce fracture risk in this vulnerable group,” they conclude.
“If you look at the sedentary kids, there’s a big discrepancy between the kids who have diabetes and the control kids, and that’s if we’re looking at radius or tibia or trabecular bone density or estimated failure load,” senior author Deborah M. Mitchell, MD, said in an interview at the poster session.
However, “when we look at the kids who are more physically active, we’re really not seeing as much difference [in bone health] between the kids with and without diabetes,” said Dr. Mitchell, a pediatric endocrinologist at Massachusetts General Hospital and assistant professor at Harvard Medical School, Boston.
But she also acknowledged, “There’s all sorts of caveats, including that this is retrospective questionnaire data.”
However, if further, rigorous studies confirm these findings, “physical activity is potentially a really effective means of improving bone quality in kids with type 1 diabetes.”
“This study suggests that bone-loading physical activity can substantially improve skeletal health in children with [type 1 diabetes] and should provide hope for patients and their families that they can take some action to prevent or mitigate the effects of diabetes on bone,” coauthor and incoming ASBMR President Mary L. Bouxsein, PhD, told this news organization in an email.
“We interpret these data as an important reason to advocate for increased time in moderate to vigorous bone-loading activity,” said Dr. Bouxsein, professor, department of orthopedic surgery, Harvard Medical School, Boston, “though the ‘dose’ in terms of hours per day or episodes per week to promote optimal bone health is still to be determined.”
“Ongoing debate,” “need stronger proof”
Asked for comment, Laura K. Bachrach, MD, who was not involved with the research, noted: “Activity benefits the development of bone strength through effects on bone geometry more than ‘density,’ and conversely, lack of physical activity can compromise gains in cortical bone diameter and thickness.”
However, “there is ongoing debate about the impact of type 1 diabetes on bone health and the factor(s) determining risk,” Dr. Bachrach, a pediatric endocrinologist at Stanford Children’s Health, Palo Alto, Calif., told this news organization in an email.
The current findings suggest “that physical activity in adolescent girls provided protection against potential adverse effects of type 1 diabetes,” said Dr. Bachrach, who spoke about bone fragility in childhood in a video commentary in 2021.
Study strengths, she noted, “include the rigor and expertise of the investigators, use of multiple surrogate measures that capture bone geometry/microarchitecture, as well as the inclusion of healthy local controls.”
“The study is limited by the cross-sectional design and subjects who opted, or not, to be active,” she added. “Stronger proof of the protective effects of activity on bone health in type 1 diabetes would require a randomized longitudinal intervention study, as alluded to by the authors of the study.”
Hypothesis: Those with type 1 diabetes acquire less bone mass in early 20s
The excess fracture risk in children with type 1 diabetes has been previously reported and is 14%-35% higher than the fracture risk in children without diabetes, Dr. Bouxsein explained. And “between 30% to 50% of kids [with type 1 diabetes] will have a fracture before the age of 18, so the excess fracture risk in diabetes is not clinically obvious,” she added.
However, “several lines of evidence strongly suggest that bone mass and microarchitecture at the time of peak bone mass (early 20s) is a major determinant of fracture risk throughout the lifespan,” she noted.
“Our hypothesis,” Dr. Bouxsein said, “is that the metabolic disruptions of diabetes, when they are present during the acquisition of peak bone mass, interfere with optimal bone development, and therefore may contribute to increased fracture risk later in life.”
Dr. Bachrach agreed that “peak bone strength is achieved by early adulthood, making childhood and adolescence important times to optimize bone health,” and that “peak bone strength is a predictor of lifetime risk of osteoporosis.”
“The diagnosis of pediatric osteoporosis is made when a child or teen sustains a vertebral fracture or femur fracture with minimal or no trauma,” she explained. “The diagnosis can also be made in a pediatric patient with low BMD [bone mineral density] for age in combination with a history of several long-bone fractures.”
Dr. Mitchell noted that type 1 diabetes is associated with a higher risk of fractures, which is sixfold in adults. In another study, she said, the group showed that in 10- to 16-year-old girls who’ve only had diabetes for a few years, “trabecular bone density is lower, they have lower estimated failure load, and longitudinally when we follow them, at least at the radius, we’re seeing bone loss at a relatively young age when we wouldn’t be expecting to see bone loss.”
80 girls enrolled, half had type 1 diabetes
Researchers enrolled 36 girls with type 1 diabetes and 44 girls without type 1 diabetes (controls) who were a mean age of 14.7 years and most (92%) were White. The girls with and without diabetes had similar rates of previous fractures (44% and 51%).
Those with diabetes had been diagnosed at a mean age of 9 years and had had diabetes for a mean of 4.6 years.
Researchers calculated participants’ total BPAQ scores based on type, duration, and frequency of bone-loading activities.
Participants had dual-energy X-ray absorptiometry scans to determine areal bone mineral density (BMD) at the total hip, femoral neck, lumbar spine, and whole body less head.
They also had high-resolution peripheral quantitative computed tomography at the distal tibia and radius to determine volumetric BMD, bone microarchitecture, and estimated bone strength (calculated using microfinite element analysis).
The two groups had similar total BPAQ scores (57.3 and 64.6), with a median score of 49.
BPAQ scores were positively associated with areal BMD at all sites (whole body, lumbar spine, total hip, femoral neck, and 1/3 radius) and with trabecular BMD and estimated failure load at the distal radius and tibia (P < .05 for all, adjusted for bone age).
Among participants with low physical activity (BPAQ below the median), compared with controls, those with type 1 diabetes had 6.6% lower aerial BMD at the lumbar spine (0.868 vs. 0.929 g/cm3; P = .04), 8% lower trabecular volumetric BMD at the distal radius (128.5 vs. 156.8 mg/cm3; P = .01), and 12% lower estimated failure load. Results at the distal tibia were similar.
Next steps
“More observational studies in males and females across a broader age spectrum would be helpful,” Dr. Bachrach noted. “The ‘gold standard’ model would be a long-term randomized controlled activity intervention study.”
“Further studies are underway [in girls and boys] using objective measures of activity including accelerometry and longitudinal observation to help confirm the findings from the current study,” Dr. Bouxsein said. “Ultimately, trials of activity interventions in children with [type 1 diabetes] will be the gold standard to determine to what extent physical activity can mitigate bone disease in [type 1 diabetes],” she agreed.
The study authors and Dr. Bachrach have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a small cross-sectional study of 10- to 16-year-old girls with and without type 1 diabetes, both groups were equally physically active, based on their replies to the bone-specific physical activity questionnaire (BPAQ).
However, among the more sedentary girls (with BPAQ scores below the median), those with type 1 diabetes had worse markers of bone health in imaging tests compared with the girls without diabetes.
the researchers summarize in a poster presented at the annual meeting of the American Society of Bone and Mineral Research.
However, this is early research and further study is needed, the group cautions.
“Ongoing studies with objective measures of physical activity as well as interventional studies will clarify whether increasing physical activity may improve bone health and reduce fracture risk in this vulnerable group,” they conclude.
“If you look at the sedentary kids, there’s a big discrepancy between the kids who have diabetes and the control kids, and that’s if we’re looking at radius or tibia or trabecular bone density or estimated failure load,” senior author Deborah M. Mitchell, MD, said in an interview at the poster session.
However, “when we look at the kids who are more physically active, we’re really not seeing as much difference [in bone health] between the kids with and without diabetes,” said Dr. Mitchell, a pediatric endocrinologist at Massachusetts General Hospital and assistant professor at Harvard Medical School, Boston.
But she also acknowledged, “There’s all sorts of caveats, including that this is retrospective questionnaire data.”
However, if further, rigorous studies confirm these findings, “physical activity is potentially a really effective means of improving bone quality in kids with type 1 diabetes.”
“This study suggests that bone-loading physical activity can substantially improve skeletal health in children with [type 1 diabetes] and should provide hope for patients and their families that they can take some action to prevent or mitigate the effects of diabetes on bone,” coauthor and incoming ASBMR President Mary L. Bouxsein, PhD, told this news organization in an email.
“We interpret these data as an important reason to advocate for increased time in moderate to vigorous bone-loading activity,” said Dr. Bouxsein, professor, department of orthopedic surgery, Harvard Medical School, Boston, “though the ‘dose’ in terms of hours per day or episodes per week to promote optimal bone health is still to be determined.”
“Ongoing debate,” “need stronger proof”
Asked for comment, Laura K. Bachrach, MD, who was not involved with the research, noted: “Activity benefits the development of bone strength through effects on bone geometry more than ‘density,’ and conversely, lack of physical activity can compromise gains in cortical bone diameter and thickness.”
However, “there is ongoing debate about the impact of type 1 diabetes on bone health and the factor(s) determining risk,” Dr. Bachrach, a pediatric endocrinologist at Stanford Children’s Health, Palo Alto, Calif., told this news organization in an email.
The current findings suggest “that physical activity in adolescent girls provided protection against potential adverse effects of type 1 diabetes,” said Dr. Bachrach, who spoke about bone fragility in childhood in a video commentary in 2021.
Study strengths, she noted, “include the rigor and expertise of the investigators, use of multiple surrogate measures that capture bone geometry/microarchitecture, as well as the inclusion of healthy local controls.”
“The study is limited by the cross-sectional design and subjects who opted, or not, to be active,” she added. “Stronger proof of the protective effects of activity on bone health in type 1 diabetes would require a randomized longitudinal intervention study, as alluded to by the authors of the study.”
Hypothesis: Those with type 1 diabetes acquire less bone mass in early 20s
The excess fracture risk in children with type 1 diabetes has been previously reported and is 14%-35% higher than the fracture risk in children without diabetes, Dr. Bouxsein explained. And “between 30% to 50% of kids [with type 1 diabetes] will have a fracture before the age of 18, so the excess fracture risk in diabetes is not clinically obvious,” she added.
However, “several lines of evidence strongly suggest that bone mass and microarchitecture at the time of peak bone mass (early 20s) is a major determinant of fracture risk throughout the lifespan,” she noted.
“Our hypothesis,” Dr. Bouxsein said, “is that the metabolic disruptions of diabetes, when they are present during the acquisition of peak bone mass, interfere with optimal bone development, and therefore may contribute to increased fracture risk later in life.”
Dr. Bachrach agreed that “peak bone strength is achieved by early adulthood, making childhood and adolescence important times to optimize bone health,” and that “peak bone strength is a predictor of lifetime risk of osteoporosis.”
“The diagnosis of pediatric osteoporosis is made when a child or teen sustains a vertebral fracture or femur fracture with minimal or no trauma,” she explained. “The diagnosis can also be made in a pediatric patient with low BMD [bone mineral density] for age in combination with a history of several long-bone fractures.”
Dr. Mitchell noted that type 1 diabetes is associated with a higher risk of fractures, which is sixfold in adults. In another study, she said, the group showed that in 10- to 16-year-old girls who’ve only had diabetes for a few years, “trabecular bone density is lower, they have lower estimated failure load, and longitudinally when we follow them, at least at the radius, we’re seeing bone loss at a relatively young age when we wouldn’t be expecting to see bone loss.”
80 girls enrolled, half had type 1 diabetes
Researchers enrolled 36 girls with type 1 diabetes and 44 girls without type 1 diabetes (controls) who were a mean age of 14.7 years and most (92%) were White. The girls with and without diabetes had similar rates of previous fractures (44% and 51%).
Those with diabetes had been diagnosed at a mean age of 9 years and had had diabetes for a mean of 4.6 years.
Researchers calculated participants’ total BPAQ scores based on type, duration, and frequency of bone-loading activities.
Participants had dual-energy X-ray absorptiometry scans to determine areal bone mineral density (BMD) at the total hip, femoral neck, lumbar spine, and whole body less head.
They also had high-resolution peripheral quantitative computed tomography at the distal tibia and radius to determine volumetric BMD, bone microarchitecture, and estimated bone strength (calculated using microfinite element analysis).
The two groups had similar total BPAQ scores (57.3 and 64.6), with a median score of 49.
BPAQ scores were positively associated with areal BMD at all sites (whole body, lumbar spine, total hip, femoral neck, and 1/3 radius) and with trabecular BMD and estimated failure load at the distal radius and tibia (P < .05 for all, adjusted for bone age).
Among participants with low physical activity (BPAQ below the median), compared with controls, those with type 1 diabetes had 6.6% lower aerial BMD at the lumbar spine (0.868 vs. 0.929 g/cm3; P = .04), 8% lower trabecular volumetric BMD at the distal radius (128.5 vs. 156.8 mg/cm3; P = .01), and 12% lower estimated failure load. Results at the distal tibia were similar.
Next steps
“More observational studies in males and females across a broader age spectrum would be helpful,” Dr. Bachrach noted. “The ‘gold standard’ model would be a long-term randomized controlled activity intervention study.”
“Further studies are underway [in girls and boys] using objective measures of activity including accelerometry and longitudinal observation to help confirm the findings from the current study,” Dr. Bouxsein said. “Ultimately, trials of activity interventions in children with [type 1 diabetes] will be the gold standard to determine to what extent physical activity can mitigate bone disease in [type 1 diabetes],” she agreed.
The study authors and Dr. Bachrach have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ASBMR 2022