User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
In one state, pandemic tamped down lice and scabies cases
.
When COVID-19 was declared a public health emergency by the World Health Organization in March 2020, many countries including the United States enacted lockdown and isolation measures to help contain the spread of the disease. Since scabies and lice are both spread by direct contact, “we hypothesized that the nationwide lockdown would influence the transmission of these two conditions among individuals,” wrote Marianne Bonanno, MD, of the University of North Carolina, Chapel Hill, and colleagues.
“The pandemic created a unique opportunity for real-life observations following physical distancing measures being put in place,” coauthor Christopher Sayed, MD, associate professor of dermatology at UNC, said in an interview. “It makes intuitive sense that since lice and scabies spread by cost physical contact that rates would decrease with school closures and other physical distancing measures. Reports from other countries in which extended families more often live together and were forced to spend more time in close quarters saw increased rates so it was interesting to see this contrast,” he noted.
In the study, the researchers reviewed data from 1,858 cases of adult scabies, 893 cases of pediatric scabies, and 804 cases of pediatric lice reported in North Carolina between March 2017 and February 2021. They compared monthly cases of scabies and lice, and prescriptions during the period before the pandemic (March 2017 to February 2020), and during the pandemic (March 2020 to February 2021).
Pediatric lice cases decreased by 60.6% over the study period (P < .001). Significant decreases also occurred in adult scabies (31.1%, P < .001) and pediatric scabies (39%, P < .01).
The number of prescriptions for lice and scabies also decreased significantly (P < .01) during the study period, although these numbers differed from the actual cases. Prescriptions decreased by 41.4%, 29.9%, and 69.3% for pediatric scabies, adult scabies, and pediatric lice, respectively.
Both pediatric scabies and pediatric lice showed a greater drop in prescriptions than in cases, while the drop in prescriptions for adult scabies was slightly less than the drop in cases.
The difference in the decreased numbers between cases and prescriptions may stem from the decrease in close contacts during the pandemic, which decreased the need for multiple prescriptions, but other potential explanations could be examined in future studies, the researchers wrote in their discussion.
The study findings were limited by several factors including the cross-sectional design and potential underdiagnosis and underreporting, as well as the focus only on a population in a single state, which may limit generalizability, the researchers noted.
However, the results offer preliminary insights on the impact of COVID-19 restrictions on scabies and lice, and suggest the potential value of physical distancing to reduce transmission of both conditions, especially in settings such as schools and prisons, to help contain future outbreaks, they concluded.
The study findings reinforce physical contact as the likely route of disease transmission, for lice and scabies, Dr. Sayed said in the interview. “It’s possible distancing measures on a small scale could be considered for outbreaks in institutional settings, though the risks of these infestations are much lower than with COVID-19,” he said. “It will be interesting to observe trends as physical distancing measures end to see if cases rebound in the next few years,” he added.
Drop in cases likely temporary
“Examining the epidemiology of different infectious diseases over time is an interesting and important area of study,” said Sheilagh Maguiness, MD, associate professor of dermatology and pediatrics at the University of Minnesota, Minneapolis, who was asked to comment on the results.
“The pandemic dramatically altered the daily lives of adults and children across the globe, and we can learn a lot from studying how social distancing and prolonged masking has made an impact on the incidence and prevalence of different infectious illnesses in the country and across the world,” she said in an interview.
Dr. Maguiness said she was not surprised by the study findings. “In fact, other countries have published similar studies documenting a reduction in both head lice and scabies infestations during the time of the pandemic,” she said. “In France, it was noted that during March to December 2020, there was a reduction in sales for topical head lice and scabies treatments of 44% and 14%, respectively. Similarly, a study from Argentina documented a decline in head lice infestations by about 25% among children,” she said.
“I personally noted a marked decrease in both of these diagnoses among children in my own clinic,” she added.
“Since both of these conditions are spread through close physical contact with others, it makes sense that there would be a steep decline in ectoparasitic infections during times of social distancing. However, anecdotally we are now diagnosing and treating these infestations again more regularly in our clinic,” said Dr. Maguiness. “As social distancing relaxes, I would expect that the incidence of both head lice and scabies will again increase.”
The study received no outside funding. The researchers and Dr. Maguiness had no financial conflicts to disclose.
.
When COVID-19 was declared a public health emergency by the World Health Organization in March 2020, many countries including the United States enacted lockdown and isolation measures to help contain the spread of the disease. Since scabies and lice are both spread by direct contact, “we hypothesized that the nationwide lockdown would influence the transmission of these two conditions among individuals,” wrote Marianne Bonanno, MD, of the University of North Carolina, Chapel Hill, and colleagues.
“The pandemic created a unique opportunity for real-life observations following physical distancing measures being put in place,” coauthor Christopher Sayed, MD, associate professor of dermatology at UNC, said in an interview. “It makes intuitive sense that since lice and scabies spread by cost physical contact that rates would decrease with school closures and other physical distancing measures. Reports from other countries in which extended families more often live together and were forced to spend more time in close quarters saw increased rates so it was interesting to see this contrast,” he noted.
In the study, the researchers reviewed data from 1,858 cases of adult scabies, 893 cases of pediatric scabies, and 804 cases of pediatric lice reported in North Carolina between March 2017 and February 2021. They compared monthly cases of scabies and lice, and prescriptions during the period before the pandemic (March 2017 to February 2020), and during the pandemic (March 2020 to February 2021).
Pediatric lice cases decreased by 60.6% over the study period (P < .001). Significant decreases also occurred in adult scabies (31.1%, P < .001) and pediatric scabies (39%, P < .01).
The number of prescriptions for lice and scabies also decreased significantly (P < .01) during the study period, although these numbers differed from the actual cases. Prescriptions decreased by 41.4%, 29.9%, and 69.3% for pediatric scabies, adult scabies, and pediatric lice, respectively.
Both pediatric scabies and pediatric lice showed a greater drop in prescriptions than in cases, while the drop in prescriptions for adult scabies was slightly less than the drop in cases.
The difference in the decreased numbers between cases and prescriptions may stem from the decrease in close contacts during the pandemic, which decreased the need for multiple prescriptions, but other potential explanations could be examined in future studies, the researchers wrote in their discussion.
The study findings were limited by several factors including the cross-sectional design and potential underdiagnosis and underreporting, as well as the focus only on a population in a single state, which may limit generalizability, the researchers noted.
However, the results offer preliminary insights on the impact of COVID-19 restrictions on scabies and lice, and suggest the potential value of physical distancing to reduce transmission of both conditions, especially in settings such as schools and prisons, to help contain future outbreaks, they concluded.
The study findings reinforce physical contact as the likely route of disease transmission, for lice and scabies, Dr. Sayed said in the interview. “It’s possible distancing measures on a small scale could be considered for outbreaks in institutional settings, though the risks of these infestations are much lower than with COVID-19,” he said. “It will be interesting to observe trends as physical distancing measures end to see if cases rebound in the next few years,” he added.
Drop in cases likely temporary
“Examining the epidemiology of different infectious diseases over time is an interesting and important area of study,” said Sheilagh Maguiness, MD, associate professor of dermatology and pediatrics at the University of Minnesota, Minneapolis, who was asked to comment on the results.
“The pandemic dramatically altered the daily lives of adults and children across the globe, and we can learn a lot from studying how social distancing and prolonged masking has made an impact on the incidence and prevalence of different infectious illnesses in the country and across the world,” she said in an interview.
Dr. Maguiness said she was not surprised by the study findings. “In fact, other countries have published similar studies documenting a reduction in both head lice and scabies infestations during the time of the pandemic,” she said. “In France, it was noted that during March to December 2020, there was a reduction in sales for topical head lice and scabies treatments of 44% and 14%, respectively. Similarly, a study from Argentina documented a decline in head lice infestations by about 25% among children,” she said.
“I personally noted a marked decrease in both of these diagnoses among children in my own clinic,” she added.
“Since both of these conditions are spread through close physical contact with others, it makes sense that there would be a steep decline in ectoparasitic infections during times of social distancing. However, anecdotally we are now diagnosing and treating these infestations again more regularly in our clinic,” said Dr. Maguiness. “As social distancing relaxes, I would expect that the incidence of both head lice and scabies will again increase.”
The study received no outside funding. The researchers and Dr. Maguiness had no financial conflicts to disclose.
.
When COVID-19 was declared a public health emergency by the World Health Organization in March 2020, many countries including the United States enacted lockdown and isolation measures to help contain the spread of the disease. Since scabies and lice are both spread by direct contact, “we hypothesized that the nationwide lockdown would influence the transmission of these two conditions among individuals,” wrote Marianne Bonanno, MD, of the University of North Carolina, Chapel Hill, and colleagues.
“The pandemic created a unique opportunity for real-life observations following physical distancing measures being put in place,” coauthor Christopher Sayed, MD, associate professor of dermatology at UNC, said in an interview. “It makes intuitive sense that since lice and scabies spread by cost physical contact that rates would decrease with school closures and other physical distancing measures. Reports from other countries in which extended families more often live together and were forced to spend more time in close quarters saw increased rates so it was interesting to see this contrast,” he noted.
In the study, the researchers reviewed data from 1,858 cases of adult scabies, 893 cases of pediatric scabies, and 804 cases of pediatric lice reported in North Carolina between March 2017 and February 2021. They compared monthly cases of scabies and lice, and prescriptions during the period before the pandemic (March 2017 to February 2020), and during the pandemic (March 2020 to February 2021).
Pediatric lice cases decreased by 60.6% over the study period (P < .001). Significant decreases also occurred in adult scabies (31.1%, P < .001) and pediatric scabies (39%, P < .01).
The number of prescriptions for lice and scabies also decreased significantly (P < .01) during the study period, although these numbers differed from the actual cases. Prescriptions decreased by 41.4%, 29.9%, and 69.3% for pediatric scabies, adult scabies, and pediatric lice, respectively.
Both pediatric scabies and pediatric lice showed a greater drop in prescriptions than in cases, while the drop in prescriptions for adult scabies was slightly less than the drop in cases.
The difference in the decreased numbers between cases and prescriptions may stem from the decrease in close contacts during the pandemic, which decreased the need for multiple prescriptions, but other potential explanations could be examined in future studies, the researchers wrote in their discussion.
The study findings were limited by several factors including the cross-sectional design and potential underdiagnosis and underreporting, as well as the focus only on a population in a single state, which may limit generalizability, the researchers noted.
However, the results offer preliminary insights on the impact of COVID-19 restrictions on scabies and lice, and suggest the potential value of physical distancing to reduce transmission of both conditions, especially in settings such as schools and prisons, to help contain future outbreaks, they concluded.
The study findings reinforce physical contact as the likely route of disease transmission, for lice and scabies, Dr. Sayed said in the interview. “It’s possible distancing measures on a small scale could be considered for outbreaks in institutional settings, though the risks of these infestations are much lower than with COVID-19,” he said. “It will be interesting to observe trends as physical distancing measures end to see if cases rebound in the next few years,” he added.
Drop in cases likely temporary
“Examining the epidemiology of different infectious diseases over time is an interesting and important area of study,” said Sheilagh Maguiness, MD, associate professor of dermatology and pediatrics at the University of Minnesota, Minneapolis, who was asked to comment on the results.
“The pandemic dramatically altered the daily lives of adults and children across the globe, and we can learn a lot from studying how social distancing and prolonged masking has made an impact on the incidence and prevalence of different infectious illnesses in the country and across the world,” she said in an interview.
Dr. Maguiness said she was not surprised by the study findings. “In fact, other countries have published similar studies documenting a reduction in both head lice and scabies infestations during the time of the pandemic,” she said. “In France, it was noted that during March to December 2020, there was a reduction in sales for topical head lice and scabies treatments of 44% and 14%, respectively. Similarly, a study from Argentina documented a decline in head lice infestations by about 25% among children,” she said.
“I personally noted a marked decrease in both of these diagnoses among children in my own clinic,” she added.
“Since both of these conditions are spread through close physical contact with others, it makes sense that there would be a steep decline in ectoparasitic infections during times of social distancing. However, anecdotally we are now diagnosing and treating these infestations again more regularly in our clinic,” said Dr. Maguiness. “As social distancing relaxes, I would expect that the incidence of both head lice and scabies will again increase.”
The study received no outside funding. The researchers and Dr. Maguiness had no financial conflicts to disclose.
FROM PEDIATRIC DERMATOLOGY
CT scan changes indicate increased mortality risk in ever-smokers
Longitudinal progression of parenchymal changes on CT images — also referred to as quantitative interstitial abnormalities (QIA) – is independently associated with decreased lung function and an increased all-cause mortality risk, an analysis of two cohorts of ever-smokers indicates. And among the main risk factors for QIA progression is smoking.
“These abnormalities have gone by a few different names but fundamentally, they are high density findings of chest CT that in some cases represent early or subtle evidence of pulmonary fibrosis,” Samuel Ash, MD, MPH, assistant professor of medicine, Brigham and Women’s Hospital, Boston, told this news organization.
So when I see someone with visual evidence of this type of change on their chest CT, I make sure to emphasize that while they don’t have interstitial lung disease [ILD] yet, these findings suggest they may be susceptible to lung injury from tobacco smoke and that if they don’t stop smoking now, they are at risk for a disease like interstitial pulmonary fibrosis [IPF] which is a highly morbid disease with a high mortality risk,” he added.
The study was published online in the journal CHEST.
Ever-smoking cohorts
Analysis of QIA progression on CT chest scans was carried out on participants from the Genetic Epidemiology of COPD (COPDGene) study as well as those from the Pittsburgh Lung Screening Study (PLuSS). COPDGene was a prospective cohort of over 10,300 ever-smokers with at least a 10–pack-year smoking history between the ages of 45 and 80. Participants underwent a series of tests including chest CT scans at baseline between 2006 and 2011 and again approximately 5 years later.
Patients with a postbronchodilator forced expiratory volume in 1 second (FEV1) of 80% or more of predicted and a FEV1-to-FVC (forced vital capacity) ratio of at least 0.7 were defined to have GOLD stage 0 disease while those with a postbronchodilator FEV1 of 80% or less than predicted and a FEV1-to-FVC ratio of at least 0.7 were defined to have preserved ratio impaired spirometry (PRISm) disease.
PLuSS involved 3,642 ever-smokers between the ages of 50 years and 79 years with at least a 12.5–pack-year history with no prior history of lung cancer. Participants again underwent a series of tests including a CT scan on visit 1 between 2002 and 2005 and then a second CT scan at a second visit almost 9 years later. “In the COPDGene cohort, 4,635 participants had complete clinical data, CT scans and spirometry from visits 1 and 2 for analysis,” the authors reported.
At visit 1 almost 48% of participants were current smokers and the mean pack-year history of the cohort was 41.9 years. The mean time between visits 1 and 2 was 5.6 years. Both the mean prebronchodilator FEV1 as well as the mean FVC decreased between visits 1 and 2. For example, the mean prebronchodilator FEV1 dropped from 2.2 liters to 2.0 liters between visits 1 and 2 while the mean prebronchodilator FVC decreased from 3.2 liters to 3.0 liters between the first and second visits.
In the PLuSS cohort, 1,307 participants had complete imaging and spirometry data available for visits 1 and 2 for analysis. The mean time between visits 1 and 2 was 8.6 years. Over 59% of the cohort were current smokers with a mean pack-year history of 65. Again, the mean prebronchodilator FEV1 and FVC both dropped between visit 1 and 2, as the authors note.
The mean prebronchodilator FEV1, for example, decreased from 2.5 liters to 2.1 liters between visits 1 and 2 while the mean prebronchodilator FVC dropped from 3.6 liters to 3.2 liters during the same interval. Looking at risk factors associated with QIA progression, investigators note that each additional year of baseline age was associated with a higher annual increase in QIA by 0.01% per year (95% confidence interval, 0.01%-0.02%; P < .001) in the COPDGene cohort and a 0.02% increase (95% CI, 0.01%-0.02%; P < .001) in the PLuSS cohort.
Female sex in turn was associated with a 0.07% per year (95% CI, 0.02%-0.12%; P = .003) higher increase in the QIA, compared with men in the COPDGene cohort and a 0.14% (95% CI, 0.02%-0.26%; P = .025) per year higher increase in the QIA in the PLuSS cohort. Current smoking status was only associated with a higher rate of QIA progression in the COPDGene cohort at a rate of 0.10% per year (95% CI, 0.06%-0.15%; P < .001).
Lastly, every copy of the minor allele of the MUIC5B promoter polymorphism was associated with a 0.12% per year (95% CI, 0.07%-0.16%; P < .0001) increase in QIA in the COPDGene cohort as well.
Smoking cessation
Smoking cessation is the obvious first step for patients with evidence of QIA progression but physicians can probably do more for these patients sooner, Dr. Ash said. “If we use heart disease as an analogy, we don’t want to start treating someone until they have a heart attack or are in heart failure, we start by checking their cholesterol and blood pressure and treating them with medications to prevent progression.”
Similarly, physicians need to start thinking about IPF and other lung diseases in the same way. For IPF, medications such as pirfenidone (Esbriet) and nintedanib (Ofev) do not reverse prior lung damage but they do slow disease progression and physicians need to initiate treatment before patients are short of breath, not after. Meantime, Dr. Ash advised physicians that, if they have a patient who is getting a CT scan for whatever reason, they should keep a close eye on whether or not patients have any of these interstitial changes and, if they do, then if the changes are getting worse.
“These patients are likely to be the ones who are going to develop IPF and who may benefit from ongoing imaging surveillance,” he said. And while clinicians may not yet be ready to use a quantitative tool at the bedside, “this tool – or one like it – is coming and we have to start thinking about how to incorporate these types of devices into our clinical practice.”
Temporal changes
Asked to comment on the findings, Surya Bhatt, MD, associate professor of medicine at the University of Alabama at Birmingham, said that the study advances the community’s understanding of the relationship between temporal changes in objectively measured interstitial lung abnormalities and several important clinical outcomes, including lung function decline and mortality. “Several risk factors for progression were also identified,” he noted.
“And these results make a case for initiating clinical trials to determine whether early treatment with existing antifibrotic medications in these high risk individuals can decrease the perpetuation of these permanent lung changes,” Dr. Bhatt said.
The COPDGene study was supported in part by contributions made by an industry advisory board. Dr. Ash was supported in part by Quantitative Imaging Solutions. Dr. Bhatt declared that he has receiving consulting fees or has service on advisory boards for Boehringer Ingelheim and Sanofi/Regeneron. He ha also received fee for CME from IntegrityCE.
Longitudinal progression of parenchymal changes on CT images — also referred to as quantitative interstitial abnormalities (QIA) – is independently associated with decreased lung function and an increased all-cause mortality risk, an analysis of two cohorts of ever-smokers indicates. And among the main risk factors for QIA progression is smoking.
“These abnormalities have gone by a few different names but fundamentally, they are high density findings of chest CT that in some cases represent early or subtle evidence of pulmonary fibrosis,” Samuel Ash, MD, MPH, assistant professor of medicine, Brigham and Women’s Hospital, Boston, told this news organization.
So when I see someone with visual evidence of this type of change on their chest CT, I make sure to emphasize that while they don’t have interstitial lung disease [ILD] yet, these findings suggest they may be susceptible to lung injury from tobacco smoke and that if they don’t stop smoking now, they are at risk for a disease like interstitial pulmonary fibrosis [IPF] which is a highly morbid disease with a high mortality risk,” he added.
The study was published online in the journal CHEST.
Ever-smoking cohorts
Analysis of QIA progression on CT chest scans was carried out on participants from the Genetic Epidemiology of COPD (COPDGene) study as well as those from the Pittsburgh Lung Screening Study (PLuSS). COPDGene was a prospective cohort of over 10,300 ever-smokers with at least a 10–pack-year smoking history between the ages of 45 and 80. Participants underwent a series of tests including chest CT scans at baseline between 2006 and 2011 and again approximately 5 years later.
Patients with a postbronchodilator forced expiratory volume in 1 second (FEV1) of 80% or more of predicted and a FEV1-to-FVC (forced vital capacity) ratio of at least 0.7 were defined to have GOLD stage 0 disease while those with a postbronchodilator FEV1 of 80% or less than predicted and a FEV1-to-FVC ratio of at least 0.7 were defined to have preserved ratio impaired spirometry (PRISm) disease.
PLuSS involved 3,642 ever-smokers between the ages of 50 years and 79 years with at least a 12.5–pack-year history with no prior history of lung cancer. Participants again underwent a series of tests including a CT scan on visit 1 between 2002 and 2005 and then a second CT scan at a second visit almost 9 years later. “In the COPDGene cohort, 4,635 participants had complete clinical data, CT scans and spirometry from visits 1 and 2 for analysis,” the authors reported.
At visit 1 almost 48% of participants were current smokers and the mean pack-year history of the cohort was 41.9 years. The mean time between visits 1 and 2 was 5.6 years. Both the mean prebronchodilator FEV1 as well as the mean FVC decreased between visits 1 and 2. For example, the mean prebronchodilator FEV1 dropped from 2.2 liters to 2.0 liters between visits 1 and 2 while the mean prebronchodilator FVC decreased from 3.2 liters to 3.0 liters between the first and second visits.
In the PLuSS cohort, 1,307 participants had complete imaging and spirometry data available for visits 1 and 2 for analysis. The mean time between visits 1 and 2 was 8.6 years. Over 59% of the cohort were current smokers with a mean pack-year history of 65. Again, the mean prebronchodilator FEV1 and FVC both dropped between visit 1 and 2, as the authors note.
The mean prebronchodilator FEV1, for example, decreased from 2.5 liters to 2.1 liters between visits 1 and 2 while the mean prebronchodilator FVC dropped from 3.6 liters to 3.2 liters during the same interval. Looking at risk factors associated with QIA progression, investigators note that each additional year of baseline age was associated with a higher annual increase in QIA by 0.01% per year (95% confidence interval, 0.01%-0.02%; P < .001) in the COPDGene cohort and a 0.02% increase (95% CI, 0.01%-0.02%; P < .001) in the PLuSS cohort.
Female sex in turn was associated with a 0.07% per year (95% CI, 0.02%-0.12%; P = .003) higher increase in the QIA, compared with men in the COPDGene cohort and a 0.14% (95% CI, 0.02%-0.26%; P = .025) per year higher increase in the QIA in the PLuSS cohort. Current smoking status was only associated with a higher rate of QIA progression in the COPDGene cohort at a rate of 0.10% per year (95% CI, 0.06%-0.15%; P < .001).
Lastly, every copy of the minor allele of the MUIC5B promoter polymorphism was associated with a 0.12% per year (95% CI, 0.07%-0.16%; P < .0001) increase in QIA in the COPDGene cohort as well.
Smoking cessation
Smoking cessation is the obvious first step for patients with evidence of QIA progression but physicians can probably do more for these patients sooner, Dr. Ash said. “If we use heart disease as an analogy, we don’t want to start treating someone until they have a heart attack or are in heart failure, we start by checking their cholesterol and blood pressure and treating them with medications to prevent progression.”
Similarly, physicians need to start thinking about IPF and other lung diseases in the same way. For IPF, medications such as pirfenidone (Esbriet) and nintedanib (Ofev) do not reverse prior lung damage but they do slow disease progression and physicians need to initiate treatment before patients are short of breath, not after. Meantime, Dr. Ash advised physicians that, if they have a patient who is getting a CT scan for whatever reason, they should keep a close eye on whether or not patients have any of these interstitial changes and, if they do, then if the changes are getting worse.
“These patients are likely to be the ones who are going to develop IPF and who may benefit from ongoing imaging surveillance,” he said. And while clinicians may not yet be ready to use a quantitative tool at the bedside, “this tool – or one like it – is coming and we have to start thinking about how to incorporate these types of devices into our clinical practice.”
Temporal changes
Asked to comment on the findings, Surya Bhatt, MD, associate professor of medicine at the University of Alabama at Birmingham, said that the study advances the community’s understanding of the relationship between temporal changes in objectively measured interstitial lung abnormalities and several important clinical outcomes, including lung function decline and mortality. “Several risk factors for progression were also identified,” he noted.
“And these results make a case for initiating clinical trials to determine whether early treatment with existing antifibrotic medications in these high risk individuals can decrease the perpetuation of these permanent lung changes,” Dr. Bhatt said.
The COPDGene study was supported in part by contributions made by an industry advisory board. Dr. Ash was supported in part by Quantitative Imaging Solutions. Dr. Bhatt declared that he has receiving consulting fees or has service on advisory boards for Boehringer Ingelheim and Sanofi/Regeneron. He ha also received fee for CME from IntegrityCE.
Longitudinal progression of parenchymal changes on CT images — also referred to as quantitative interstitial abnormalities (QIA) – is independently associated with decreased lung function and an increased all-cause mortality risk, an analysis of two cohorts of ever-smokers indicates. And among the main risk factors for QIA progression is smoking.
“These abnormalities have gone by a few different names but fundamentally, they are high density findings of chest CT that in some cases represent early or subtle evidence of pulmonary fibrosis,” Samuel Ash, MD, MPH, assistant professor of medicine, Brigham and Women’s Hospital, Boston, told this news organization.
So when I see someone with visual evidence of this type of change on their chest CT, I make sure to emphasize that while they don’t have interstitial lung disease [ILD] yet, these findings suggest they may be susceptible to lung injury from tobacco smoke and that if they don’t stop smoking now, they are at risk for a disease like interstitial pulmonary fibrosis [IPF] which is a highly morbid disease with a high mortality risk,” he added.
The study was published online in the journal CHEST.
Ever-smoking cohorts
Analysis of QIA progression on CT chest scans was carried out on participants from the Genetic Epidemiology of COPD (COPDGene) study as well as those from the Pittsburgh Lung Screening Study (PLuSS). COPDGene was a prospective cohort of over 10,300 ever-smokers with at least a 10–pack-year smoking history between the ages of 45 and 80. Participants underwent a series of tests including chest CT scans at baseline between 2006 and 2011 and again approximately 5 years later.
Patients with a postbronchodilator forced expiratory volume in 1 second (FEV1) of 80% or more of predicted and a FEV1-to-FVC (forced vital capacity) ratio of at least 0.7 were defined to have GOLD stage 0 disease while those with a postbronchodilator FEV1 of 80% or less than predicted and a FEV1-to-FVC ratio of at least 0.7 were defined to have preserved ratio impaired spirometry (PRISm) disease.
PLuSS involved 3,642 ever-smokers between the ages of 50 years and 79 years with at least a 12.5–pack-year history with no prior history of lung cancer. Participants again underwent a series of tests including a CT scan on visit 1 between 2002 and 2005 and then a second CT scan at a second visit almost 9 years later. “In the COPDGene cohort, 4,635 participants had complete clinical data, CT scans and spirometry from visits 1 and 2 for analysis,” the authors reported.
At visit 1 almost 48% of participants were current smokers and the mean pack-year history of the cohort was 41.9 years. The mean time between visits 1 and 2 was 5.6 years. Both the mean prebronchodilator FEV1 as well as the mean FVC decreased between visits 1 and 2. For example, the mean prebronchodilator FEV1 dropped from 2.2 liters to 2.0 liters between visits 1 and 2 while the mean prebronchodilator FVC decreased from 3.2 liters to 3.0 liters between the first and second visits.
In the PLuSS cohort, 1,307 participants had complete imaging and spirometry data available for visits 1 and 2 for analysis. The mean time between visits 1 and 2 was 8.6 years. Over 59% of the cohort were current smokers with a mean pack-year history of 65. Again, the mean prebronchodilator FEV1 and FVC both dropped between visit 1 and 2, as the authors note.
The mean prebronchodilator FEV1, for example, decreased from 2.5 liters to 2.1 liters between visits 1 and 2 while the mean prebronchodilator FVC dropped from 3.6 liters to 3.2 liters during the same interval. Looking at risk factors associated with QIA progression, investigators note that each additional year of baseline age was associated with a higher annual increase in QIA by 0.01% per year (95% confidence interval, 0.01%-0.02%; P < .001) in the COPDGene cohort and a 0.02% increase (95% CI, 0.01%-0.02%; P < .001) in the PLuSS cohort.
Female sex in turn was associated with a 0.07% per year (95% CI, 0.02%-0.12%; P = .003) higher increase in the QIA, compared with men in the COPDGene cohort and a 0.14% (95% CI, 0.02%-0.26%; P = .025) per year higher increase in the QIA in the PLuSS cohort. Current smoking status was only associated with a higher rate of QIA progression in the COPDGene cohort at a rate of 0.10% per year (95% CI, 0.06%-0.15%; P < .001).
Lastly, every copy of the minor allele of the MUIC5B promoter polymorphism was associated with a 0.12% per year (95% CI, 0.07%-0.16%; P < .0001) increase in QIA in the COPDGene cohort as well.
Smoking cessation
Smoking cessation is the obvious first step for patients with evidence of QIA progression but physicians can probably do more for these patients sooner, Dr. Ash said. “If we use heart disease as an analogy, we don’t want to start treating someone until they have a heart attack or are in heart failure, we start by checking their cholesterol and blood pressure and treating them with medications to prevent progression.”
Similarly, physicians need to start thinking about IPF and other lung diseases in the same way. For IPF, medications such as pirfenidone (Esbriet) and nintedanib (Ofev) do not reverse prior lung damage but they do slow disease progression and physicians need to initiate treatment before patients are short of breath, not after. Meantime, Dr. Ash advised physicians that, if they have a patient who is getting a CT scan for whatever reason, they should keep a close eye on whether or not patients have any of these interstitial changes and, if they do, then if the changes are getting worse.
“These patients are likely to be the ones who are going to develop IPF and who may benefit from ongoing imaging surveillance,” he said. And while clinicians may not yet be ready to use a quantitative tool at the bedside, “this tool – or one like it – is coming and we have to start thinking about how to incorporate these types of devices into our clinical practice.”
Temporal changes
Asked to comment on the findings, Surya Bhatt, MD, associate professor of medicine at the University of Alabama at Birmingham, said that the study advances the community’s understanding of the relationship between temporal changes in objectively measured interstitial lung abnormalities and several important clinical outcomes, including lung function decline and mortality. “Several risk factors for progression were also identified,” he noted.
“And these results make a case for initiating clinical trials to determine whether early treatment with existing antifibrotic medications in these high risk individuals can decrease the perpetuation of these permanent lung changes,” Dr. Bhatt said.
The COPDGene study was supported in part by contributions made by an industry advisory board. Dr. Ash was supported in part by Quantitative Imaging Solutions. Dr. Bhatt declared that he has receiving consulting fees or has service on advisory boards for Boehringer Ingelheim and Sanofi/Regeneron. He ha also received fee for CME from IntegrityCE.
FROM CHEST
Children and COVID: Severe illness rising as vaccination effort stalls
, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
After new child cases jumped by 22% during the week of July 15-21, the two successive weeks have produced increases of 3.9% (July 22-29) and 1.2% (July 30-Aug. 4). The latest weekly count from all states and territories still reporting was 96,599, the AAP and CHA said in their weekly COVID report, noting that several states have stopped reporting child cases and that others are reporting every other week.
The deceleration in new cases, however, does not apply to emergency department visits and hospital admissions. The proportion of ED visits with diagnosed COVID rose steadily throughout June and July, as 7-day averages went from 2.6% on June 1 to 6.3% on July 31 for children aged 0-11 years, from 2.1% to 3.1% for children aged 12-15, and from 2.4% to 3.5% for 16- to 17-year-olds, according to data from the Centers for Disease Control and Prevention.
The rate of new admissions with confirmed COVID, which reached 0.46 per 100,000 population for children aged 0-17 years on July 30, has more than tripled since early April, when it had fallen to 0.13 per 100,000 in the wake of the Omicron surge, the CDC reported on its COVID Data Tracker.
A smaller but more detailed sample of children from the COVID-19–Associated Hospitalization Network (COVID-NET), which covers nearly 100 counties in 14 states, indicates that the increase in new admissions is occurring almost entirely among children aged 0-4 years, who had a rate of 5.6 per 100,000 for the week of July 17-23, compared with 0.8 per 100,000 for 5- to 11-year-olds and 1.5 per 100,000 for those aged 12-17, the CDC said.
Vaccine’s summer rollout gets lukewarm reception
As a group, children aged 0-4 years have not exactly flocked to the COVID-19 vaccine. As of Aug. 2 – about 6 weeks since the vaccine was authorized for children aged 6 months to 4 years – just 3.8% of those eligible had received at least one dose. Among children aged 5-11 the corresponding number on Aug. 2 was 37.4%, and for those aged 12-17 years it was 70.3%, the CDC data show.
That 3.8% of children aged less than 5 years represents almost 756,000 initial doses. That compares with over 6 million children aged 5-11 years who had received at least one dose through the first 6 weeks of their vaccination experience and over 5 million children aged 12-15, according to the COVID Data Tracker.
, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
After new child cases jumped by 22% during the week of July 15-21, the two successive weeks have produced increases of 3.9% (July 22-29) and 1.2% (July 30-Aug. 4). The latest weekly count from all states and territories still reporting was 96,599, the AAP and CHA said in their weekly COVID report, noting that several states have stopped reporting child cases and that others are reporting every other week.
The deceleration in new cases, however, does not apply to emergency department visits and hospital admissions. The proportion of ED visits with diagnosed COVID rose steadily throughout June and July, as 7-day averages went from 2.6% on June 1 to 6.3% on July 31 for children aged 0-11 years, from 2.1% to 3.1% for children aged 12-15, and from 2.4% to 3.5% for 16- to 17-year-olds, according to data from the Centers for Disease Control and Prevention.
The rate of new admissions with confirmed COVID, which reached 0.46 per 100,000 population for children aged 0-17 years on July 30, has more than tripled since early April, when it had fallen to 0.13 per 100,000 in the wake of the Omicron surge, the CDC reported on its COVID Data Tracker.
A smaller but more detailed sample of children from the COVID-19–Associated Hospitalization Network (COVID-NET), which covers nearly 100 counties in 14 states, indicates that the increase in new admissions is occurring almost entirely among children aged 0-4 years, who had a rate of 5.6 per 100,000 for the week of July 17-23, compared with 0.8 per 100,000 for 5- to 11-year-olds and 1.5 per 100,000 for those aged 12-17, the CDC said.
Vaccine’s summer rollout gets lukewarm reception
As a group, children aged 0-4 years have not exactly flocked to the COVID-19 vaccine. As of Aug. 2 – about 6 weeks since the vaccine was authorized for children aged 6 months to 4 years – just 3.8% of those eligible had received at least one dose. Among children aged 5-11 the corresponding number on Aug. 2 was 37.4%, and for those aged 12-17 years it was 70.3%, the CDC data show.
That 3.8% of children aged less than 5 years represents almost 756,000 initial doses. That compares with over 6 million children aged 5-11 years who had received at least one dose through the first 6 weeks of their vaccination experience and over 5 million children aged 12-15, according to the COVID Data Tracker.
, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
After new child cases jumped by 22% during the week of July 15-21, the two successive weeks have produced increases of 3.9% (July 22-29) and 1.2% (July 30-Aug. 4). The latest weekly count from all states and territories still reporting was 96,599, the AAP and CHA said in their weekly COVID report, noting that several states have stopped reporting child cases and that others are reporting every other week.
The deceleration in new cases, however, does not apply to emergency department visits and hospital admissions. The proportion of ED visits with diagnosed COVID rose steadily throughout June and July, as 7-day averages went from 2.6% on June 1 to 6.3% on July 31 for children aged 0-11 years, from 2.1% to 3.1% for children aged 12-15, and from 2.4% to 3.5% for 16- to 17-year-olds, according to data from the Centers for Disease Control and Prevention.
The rate of new admissions with confirmed COVID, which reached 0.46 per 100,000 population for children aged 0-17 years on July 30, has more than tripled since early April, when it had fallen to 0.13 per 100,000 in the wake of the Omicron surge, the CDC reported on its COVID Data Tracker.
A smaller but more detailed sample of children from the COVID-19–Associated Hospitalization Network (COVID-NET), which covers nearly 100 counties in 14 states, indicates that the increase in new admissions is occurring almost entirely among children aged 0-4 years, who had a rate of 5.6 per 100,000 for the week of July 17-23, compared with 0.8 per 100,000 for 5- to 11-year-olds and 1.5 per 100,000 for those aged 12-17, the CDC said.
Vaccine’s summer rollout gets lukewarm reception
As a group, children aged 0-4 years have not exactly flocked to the COVID-19 vaccine. As of Aug. 2 – about 6 weeks since the vaccine was authorized for children aged 6 months to 4 years – just 3.8% of those eligible had received at least one dose. Among children aged 5-11 the corresponding number on Aug. 2 was 37.4%, and for those aged 12-17 years it was 70.3%, the CDC data show.
That 3.8% of children aged less than 5 years represents almost 756,000 initial doses. That compares with over 6 million children aged 5-11 years who had received at least one dose through the first 6 weeks of their vaccination experience and over 5 million children aged 12-15, according to the COVID Data Tracker.
Jury out on synbiotics for kids with GI disorders
That’s the conclusion of a position paper from the European Society for Pediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) special interest group on gut microbiota and modifications and its working group for pre- and probiotics.
Based on their review of available data, the group could not offer a recommendation on use of any specific synbiotic preparation for treatment of acute gastroenteritis, Helicobacter pylori (H. pylori) infection, inflammatory bowel disease (IBD), infantile colic, functional abdominal pain, irritable bowel syndrome (IBS), or constipation.
No recommendation can be formulated on their use in the prevention of food allergies, the group also says.
The same goes for prevention of necrotizing enterocolitis (NEC) in preterm infants and newborns with cyanotic congenital heart disease, as well as prevention of food allergies.
The position paper was published online in the Journal of Pediatric Gastroenterology and Nutrition.
Few studies, major limitations
A synbiotic mixture comprises probiotics and prebiotics selectively utilized by host microorganisms that confers a health benefit on the host.
While the number of studies evaluating the effect of different synbiotics is increasing, the results to date are “ambiguous,” report first author Iva Hojsak, with Children’s Hospital Zagreb, Croatia, and colleagues. Well-designed studies using the same outcome measures for specific clinical indications are needed to allow comparison between studies, they write.
To gauge their effect on pediatric GI disorders, the group searched the literature for studies in English that compared the use of synbiotics, in all delivery vehicles and formulations, at any dose, with no synbiotic (placebo or no treatment or other interventions).
They found very few studies that addressed the specific indications of interest, ranging from two randomized controlled trials (RCTs) each for infantile colic and IBD to five RCTs for acute gastroenteritis.
There were only two indications (acute gastroenteritis and H. pylori) where two synbiotic preparations were tested.
The studies often included a limited number of participants, had significant methodological biases, scarcely reported on side effects or adverse events, and reported different outcomes, making inter-study comparisons tough.
“Comparison of studies was further limited by the synbiotic preparation used, where dose effect was not assessed,” the group writes. Also, few studies used the same synbiotic preparation for a specific clinical indication or the same amount of live bacteria and prebiotic in the preparation.
The authors made note of the newly stringent recommendations for direct evidence proposed by the International Scientific Association for Probiotics and Prebiotics, which state RCTs need to compare the synergistic synbiotic combination, the substrate alone, the live microorganisms alone, and a control.
Outside experts weigh in
Offering outside perspective, Gail Cresci, PhD, microbiome researcher, department of pediatric gastroenterology, hepatology, and nutrition, Cleveland Clinic Children’s Hospital, said what’s “most notable with this review is that there is an issue with studies that incorporate a prebiotic and probiotic, in that there is much heterogeneity with the probiotic strains and prebiotic substrates that are investigated.”
Dr. Cresci also noted that “both pre- and probiotics have specific mechanisms of action based on the substrate and strain, respectively, so to pull the trials together and analyze as a ‘synbiotic’ treating all the combinations the same is not accurate [and] also limits the ability to do meta-analyses and make recommendations in a position paper.”
Geoffrey Preidis, MD, PhD, spokesperson for the American Gastroenterological Association (AGA), also reviewed the paper for this news organization.
He noted that few studies tested the exact same synbiotic preparation for a given clinical indication.
“For the majority of GI disorders examined in this review, the total number of studies testing a particular synbiotic formulation is exactly one. Clinical recommendations rarely can be made based on the results of a single trial,” said Dr. Preidis, a pediatric gastroenterologist with Texas Children’s Hospital, Houston.
“Perhaps most importantly, most studies do not report safety data as rigorously as these data are reported in pharmaceutical trials, so the risk of side effects could be higher than we think,” he noted.
“Millions of Americans take probiotics. They are the third most commonly used dietary supplement behind vitamins and minerals,” Dr. Preidis added. “Prebiotics and synbiotics also are increasing in popularity. They can be found almost everywhere – in supermarkets, drugstores, health food stores, and online – in pill or powder form and in some foods and beverages.
None of these products have been approved by the [U.S. Food and Drug Administration] to treat, cure, or prevent any disease. In most circumstances, there is not enough clinical evidence to suggest a clear value to be gained for most consumers,” he said.
Dr. Preidis said he agrees with the conclusions of this “thoughtfully written position paper” on whether synbiotics have a role in the management of GI disorders in children.
“Synbiotics should not be given routinely to infants or children to manage GI disorders at this time,” he said in an interview. “Potential beneficial effects are not yet confirmed in multiple well-designed, adequately powered trials that test the same synbiotic combination and dose, measure the same outcomes, and rigorously document all adverse effects.”
This research had no specific funding. Dr. Hojsak received payment/honorarium for lectures from BioGaia, Nutricia, Biocodex, AbelaPharm, Nestle, Abbott, Sandoz, Oktal Pharma, and Takeda. Dr. Cresci and Dr. Preidis report no relevant disclosures.
A version of this article first appeared on Medscape.com.
That’s the conclusion of a position paper from the European Society for Pediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) special interest group on gut microbiota and modifications and its working group for pre- and probiotics.
Based on their review of available data, the group could not offer a recommendation on use of any specific synbiotic preparation for treatment of acute gastroenteritis, Helicobacter pylori (H. pylori) infection, inflammatory bowel disease (IBD), infantile colic, functional abdominal pain, irritable bowel syndrome (IBS), or constipation.
No recommendation can be formulated on their use in the prevention of food allergies, the group also says.
The same goes for prevention of necrotizing enterocolitis (NEC) in preterm infants and newborns with cyanotic congenital heart disease, as well as prevention of food allergies.
The position paper was published online in the Journal of Pediatric Gastroenterology and Nutrition.
Few studies, major limitations
A synbiotic mixture comprises probiotics and prebiotics selectively utilized by host microorganisms that confers a health benefit on the host.
While the number of studies evaluating the effect of different synbiotics is increasing, the results to date are “ambiguous,” report first author Iva Hojsak, with Children’s Hospital Zagreb, Croatia, and colleagues. Well-designed studies using the same outcome measures for specific clinical indications are needed to allow comparison between studies, they write.
To gauge their effect on pediatric GI disorders, the group searched the literature for studies in English that compared the use of synbiotics, in all delivery vehicles and formulations, at any dose, with no synbiotic (placebo or no treatment or other interventions).
They found very few studies that addressed the specific indications of interest, ranging from two randomized controlled trials (RCTs) each for infantile colic and IBD to five RCTs for acute gastroenteritis.
There were only two indications (acute gastroenteritis and H. pylori) where two synbiotic preparations were tested.
The studies often included a limited number of participants, had significant methodological biases, scarcely reported on side effects or adverse events, and reported different outcomes, making inter-study comparisons tough.
“Comparison of studies was further limited by the synbiotic preparation used, where dose effect was not assessed,” the group writes. Also, few studies used the same synbiotic preparation for a specific clinical indication or the same amount of live bacteria and prebiotic in the preparation.
The authors made note of the newly stringent recommendations for direct evidence proposed by the International Scientific Association for Probiotics and Prebiotics, which state RCTs need to compare the synergistic synbiotic combination, the substrate alone, the live microorganisms alone, and a control.
Outside experts weigh in
Offering outside perspective, Gail Cresci, PhD, microbiome researcher, department of pediatric gastroenterology, hepatology, and nutrition, Cleveland Clinic Children’s Hospital, said what’s “most notable with this review is that there is an issue with studies that incorporate a prebiotic and probiotic, in that there is much heterogeneity with the probiotic strains and prebiotic substrates that are investigated.”
Dr. Cresci also noted that “both pre- and probiotics have specific mechanisms of action based on the substrate and strain, respectively, so to pull the trials together and analyze as a ‘synbiotic’ treating all the combinations the same is not accurate [and] also limits the ability to do meta-analyses and make recommendations in a position paper.”
Geoffrey Preidis, MD, PhD, spokesperson for the American Gastroenterological Association (AGA), also reviewed the paper for this news organization.
He noted that few studies tested the exact same synbiotic preparation for a given clinical indication.
“For the majority of GI disorders examined in this review, the total number of studies testing a particular synbiotic formulation is exactly one. Clinical recommendations rarely can be made based on the results of a single trial,” said Dr. Preidis, a pediatric gastroenterologist with Texas Children’s Hospital, Houston.
“Perhaps most importantly, most studies do not report safety data as rigorously as these data are reported in pharmaceutical trials, so the risk of side effects could be higher than we think,” he noted.
“Millions of Americans take probiotics. They are the third most commonly used dietary supplement behind vitamins and minerals,” Dr. Preidis added. “Prebiotics and synbiotics also are increasing in popularity. They can be found almost everywhere – in supermarkets, drugstores, health food stores, and online – in pill or powder form and in some foods and beverages.
None of these products have been approved by the [U.S. Food and Drug Administration] to treat, cure, or prevent any disease. In most circumstances, there is not enough clinical evidence to suggest a clear value to be gained for most consumers,” he said.
Dr. Preidis said he agrees with the conclusions of this “thoughtfully written position paper” on whether synbiotics have a role in the management of GI disorders in children.
“Synbiotics should not be given routinely to infants or children to manage GI disorders at this time,” he said in an interview. “Potential beneficial effects are not yet confirmed in multiple well-designed, adequately powered trials that test the same synbiotic combination and dose, measure the same outcomes, and rigorously document all adverse effects.”
This research had no specific funding. Dr. Hojsak received payment/honorarium for lectures from BioGaia, Nutricia, Biocodex, AbelaPharm, Nestle, Abbott, Sandoz, Oktal Pharma, and Takeda. Dr. Cresci and Dr. Preidis report no relevant disclosures.
A version of this article first appeared on Medscape.com.
That’s the conclusion of a position paper from the European Society for Pediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) special interest group on gut microbiota and modifications and its working group for pre- and probiotics.
Based on their review of available data, the group could not offer a recommendation on use of any specific synbiotic preparation for treatment of acute gastroenteritis, Helicobacter pylori (H. pylori) infection, inflammatory bowel disease (IBD), infantile colic, functional abdominal pain, irritable bowel syndrome (IBS), or constipation.
No recommendation can be formulated on their use in the prevention of food allergies, the group also says.
The same goes for prevention of necrotizing enterocolitis (NEC) in preterm infants and newborns with cyanotic congenital heart disease, as well as prevention of food allergies.
The position paper was published online in the Journal of Pediatric Gastroenterology and Nutrition.
Few studies, major limitations
A synbiotic mixture comprises probiotics and prebiotics selectively utilized by host microorganisms that confers a health benefit on the host.
While the number of studies evaluating the effect of different synbiotics is increasing, the results to date are “ambiguous,” report first author Iva Hojsak, with Children’s Hospital Zagreb, Croatia, and colleagues. Well-designed studies using the same outcome measures for specific clinical indications are needed to allow comparison between studies, they write.
To gauge their effect on pediatric GI disorders, the group searched the literature for studies in English that compared the use of synbiotics, in all delivery vehicles and formulations, at any dose, with no synbiotic (placebo or no treatment or other interventions).
They found very few studies that addressed the specific indications of interest, ranging from two randomized controlled trials (RCTs) each for infantile colic and IBD to five RCTs for acute gastroenteritis.
There were only two indications (acute gastroenteritis and H. pylori) where two synbiotic preparations were tested.
The studies often included a limited number of participants, had significant methodological biases, scarcely reported on side effects or adverse events, and reported different outcomes, making inter-study comparisons tough.
“Comparison of studies was further limited by the synbiotic preparation used, where dose effect was not assessed,” the group writes. Also, few studies used the same synbiotic preparation for a specific clinical indication or the same amount of live bacteria and prebiotic in the preparation.
The authors made note of the newly stringent recommendations for direct evidence proposed by the International Scientific Association for Probiotics and Prebiotics, which state RCTs need to compare the synergistic synbiotic combination, the substrate alone, the live microorganisms alone, and a control.
Outside experts weigh in
Offering outside perspective, Gail Cresci, PhD, microbiome researcher, department of pediatric gastroenterology, hepatology, and nutrition, Cleveland Clinic Children’s Hospital, said what’s “most notable with this review is that there is an issue with studies that incorporate a prebiotic and probiotic, in that there is much heterogeneity with the probiotic strains and prebiotic substrates that are investigated.”
Dr. Cresci also noted that “both pre- and probiotics have specific mechanisms of action based on the substrate and strain, respectively, so to pull the trials together and analyze as a ‘synbiotic’ treating all the combinations the same is not accurate [and] also limits the ability to do meta-analyses and make recommendations in a position paper.”
Geoffrey Preidis, MD, PhD, spokesperson for the American Gastroenterological Association (AGA), also reviewed the paper for this news organization.
He noted that few studies tested the exact same synbiotic preparation for a given clinical indication.
“For the majority of GI disorders examined in this review, the total number of studies testing a particular synbiotic formulation is exactly one. Clinical recommendations rarely can be made based on the results of a single trial,” said Dr. Preidis, a pediatric gastroenterologist with Texas Children’s Hospital, Houston.
“Perhaps most importantly, most studies do not report safety data as rigorously as these data are reported in pharmaceutical trials, so the risk of side effects could be higher than we think,” he noted.
“Millions of Americans take probiotics. They are the third most commonly used dietary supplement behind vitamins and minerals,” Dr. Preidis added. “Prebiotics and synbiotics also are increasing in popularity. They can be found almost everywhere – in supermarkets, drugstores, health food stores, and online – in pill or powder form and in some foods and beverages.
None of these products have been approved by the [U.S. Food and Drug Administration] to treat, cure, or prevent any disease. In most circumstances, there is not enough clinical evidence to suggest a clear value to be gained for most consumers,” he said.
Dr. Preidis said he agrees with the conclusions of this “thoughtfully written position paper” on whether synbiotics have a role in the management of GI disorders in children.
“Synbiotics should not be given routinely to infants or children to manage GI disorders at this time,” he said in an interview. “Potential beneficial effects are not yet confirmed in multiple well-designed, adequately powered trials that test the same synbiotic combination and dose, measure the same outcomes, and rigorously document all adverse effects.”
This research had no specific funding. Dr. Hojsak received payment/honorarium for lectures from BioGaia, Nutricia, Biocodex, AbelaPharm, Nestle, Abbott, Sandoz, Oktal Pharma, and Takeda. Dr. Cresci and Dr. Preidis report no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM JOURNAL OF PEDIATRIC GASTROENTEROLOGY AND NUTRITION
Updates on treatment/prevention of VTE in cancer patients
Updated clinical practice guidelines for the treatment and prevention of venous thromboembolism for patients with cancer, including those with cancer and COVID-19, have been released by the International Initiative on Thrombosis and Cancer (ITAC), an academic working group of VTE experts.
“Because patients with cancer have a baseline increased risk of VTE, compared with patients without cancer, the combination of both COVID-19 and cancer – and its effect on VTE risk and treatment – is of concern,” said the authors, led by Dominique Farge, MD, PhD, Nord Universite de Paris.
they added.
The new guidelines were published online in The Lancet Oncology.
“Cancer-associated VTE remains an important clinical problem, associated with increased morbidity and mortality,” Dr. Farge and colleagues observed.
“The ITAC guidelines’ companion free web-based mobile application will assist the practicing clinician with decision making at various levels to provide optimal care of patients with cancer to treat and prevent VTE,” they emphasized. More information is available at itaccme.com.
Cancer patients with COVID
The new section of the guidelines notes that the treatment and prevention of VTE for cancer patients infected with SARS-CoV-2 remain the same as for patients without COVID.
Whether or not cancer patients with COVID-19 are hospitalized, have been discharged, or are ambulatory, they should be assessed for the risk of VTE, as should any other patient. For cancer patients with COVID-19 who are hospitalized, pharmacologic prophylaxis should be given at the same dose and anticoagulant type as for hospitalized cancer patients who do not have COVID-19.
Following discharge, VTE prophylaxis is not advised for cancer patients infected with SARS-CoV-2, and routine primary pharmacologic prophylaxis of VTE for ambulatory patients with COVID-19 is also not recommended, the authors noted.
Initial treatment of established VTE
Initial treatment of established VTE for up to 10 days of anticoagulation should include low-molecular-weight heparin (LMWH) when creatinine clearance is at least 30 mL/min.
“A regimen of LMWH, taken once per day, is recommended unless a twice-per-day regimen is required because of patients’ characteristics,” the authors noted. These characteristics include a high risk of bleeding, moderate renal failure, and the need for technical intervention, including surgery.
If a twice-a-day regimen is required, only enoxaparin at a dose of 1 mg/kg twice daily can be used, the authors cautioned.
For patients with a low risk of gastrointestinal or genitourinary bleeding, rivaroxaban (Xarelto) or apixaban (Eliquis) can be given in the first 10 days, as well as edoxaban (Lixiana). The latter should be started after at least 5 days of parenteral anticoagulation, provided creatinine clearance is at least 30 mL/min.
“Unfractionated heparin as well as fondaparinux (GlaxoSmithKline) can be also used for the initial treatment of established VTE when LMWH or direct oral anticoagulants are contraindicated,” Dr. Farge and colleagues wrote.
Thrombolysis can be considered on a case-by-case basis, although physicians must pay attention to specific contraindications, especially bleeding risk.
“In the initial treatment of VTE, inferior vena cava filters might be considered when anticoagulant treatment is contraindicated or, in the case of pulmonary embolism, when recurrence occurs under optimal anticoagulation,” the authors noted.
Maintenance VTE treatment
For maintenance therapy, which the authors define as early maintenance for up to 6 months and long-term maintenance beyond 6 months, they point out that LMWHs are preferred over vitamin K antagonists for the treatment of VTE when the creatinine clearance is again at least 30 mL/min.
Any of the direct oral anticoagulants (DOAs) – edoxaban, rivaroxaban, or apixaban – is also recommended for the same patients, provided there is no risk of inducing a strong drug-drug interaction or GI absorption is impaired.
However, the DOAs should be used with caution for patients with GI malignancies, especially upper GI cancers, because data show there is an increased risk of GI bleeding with both edoxaban and rivaroxaban.
“LMWH or direct oral anticoagulants should be used for a minimum of 6 months to treat established VTE in patients with cancer,” the authors wrote.
“After 6 months, termination or continuation of anticoagulation (LMWH, direct oral anticoagulants, or vitamin K antagonists) should be based on individual evaluation of the benefit-risk ratio,” they added.
Treatment of VTE recurrence
The guideline authors explain that three options can be considered in the event of VTE recurrence. These include an increase in the LMWH dose by 20%-25%, or a switch to a DOA, or, if patients are taking a DOA, a switch to an LMWH. If the patient is taking a vitamin K antagonist, it can be switched to either an LMWH or a DOA.
For treatment of catheter-related thrombosis, anticoagulant treatment is recommended for a minimum of 3 months and as long as the central venous catheter is in place. In this setting, the LMWHs are recommended.
The central venous catheter can be kept in place if it is functional, well positioned, and is not infected, provided there is good resolution of symptoms under close surveillance while anticoagulants are being administered.
In surgically treated patients, the LMWH, given once a day, to patients with a serum creatinine concentration of at least 30 mL/min can be used to prevent VTE. Alternatively, VTE can be prevented by the use low-dose unfractionated heparin, given three times a day.
“Pharmacological prophylaxis should be started 2-12 h preoperatively and continued for at least 7–10 days,” Dr. Farge and colleagues advised. In this setting, there is insufficient evidence to support the use of fondaparinux or a DOA as an alternative to an LMWH for the prophylaxis of postoperative VTE. “Use of the highest prophylactic dose of LMWH to prevent postoperative VTE in patients with cancer is recommended,” the authors advised.
Furthermore, extended prophylaxis of at least 4 weeks with LMWH is advised to prevent postoperative VTE after major abdominal or pelvic surgery. Mechanical methods are not recommended except when pharmacologic methods are contraindicated. Inferior vena cava filters are also not recommended for routine prophylaxis.
Patients with reduced mobility
For medically treated hospitalized patients with cancer whose mobility is reduced, the authors recommend prophylaxis with either an LMWH or fondaparinux, provided their creatinine clearance is at least 30 mL/min. These patients can also be treated with unfractionated heparin, they add.
In contrast, DOAs are not recommended – at least not routinely – in this setting, the authors cautioned. Primary pharmacologic prophylaxis of VTE with either LMWH or DOAs – either rivaroxaban or apixaban – is indicated in ambulatory patients with locally advanced or metastatic pancreatic cancer who are receiving systemic anticancer therapy, provided they are at low risk of bleeding.
However, primary pharmacologic prophylaxis with LMWH is not recommended outside of a clinical trial for patients with locally advanced or metastatic lung cancer who are undergoing systemic anticancer therapy, even for patients who are at low risk of bleeding.
For ambulatory patients who are receiving systemic anticancer therapy and who are at intermediate risk of VTE, primary prophylaxis with rivaroxaban or apixaban is recommended for those with myeloma who are receiving immunomodulatory therapy plus steroids or other systemic therapies.
In this setting, oral anticoagulants should consist of a vitamin K antagonist, given at low or therapeutic doses, or apixaban, given at prophylactic doses. Alternatively, LMWH, given at prophylactic doses, or low-dose aspirin, given at a dose of 100 mg/day, can be used.
Catheter-related thrombosis
Use of anticoagulation for routine prophylaxis of catheter-related thrombosis is not recommended. Catheters should be inserted on the right side in the jugular vein, and the distal extremity of the central catheter should be located at the junction of the superior vena cava and the right atrium. “In patients requiring central venous catheters, we suggest the use of implanted ports over peripheral inserted central catheter lines,” the authors noted.
The authors described a number of unique situations regarding the treatment of VTE. These situations include patients with a brain tumor, for whom treatment of established VTE should favor either LMWH or a DOA. The authors also recommended the use of LMWH or unfractionated heparin, started postoperatively, for the prevention of VTE for patients undergoing neurosurgery.
In contrast, pharmacologic prophylaxis of VTE in medically treated patients with a brain tumor who are not undergoing neurosurgery is not recommended. “In the presence of severe renal failure...we suggest using unfractionated heparin followed by early vitamin K antagonists (possibly from day 1) or LMWH adjusted to anti-Xa concentration of the treatment of established VTE,” Dr. Farge and colleagues wrote.
Anticoagulant treatment is also recommended for a minimum of 3 months for children with symptomatic catheter-related thrombosis and as long as the central venous catheter is in place. For children with acute lymphoblastic leukemia who are undergoing induction chemotherapy, LMWH is also recommended as thromboprophylaxis.
For children who require a central venous catheter, the authors suggested that physicians use implanted ports over peripherally inserted central lines.
A version of this article first appeared on Medscape.com.
Updated clinical practice guidelines for the treatment and prevention of venous thromboembolism for patients with cancer, including those with cancer and COVID-19, have been released by the International Initiative on Thrombosis and Cancer (ITAC), an academic working group of VTE experts.
“Because patients with cancer have a baseline increased risk of VTE, compared with patients without cancer, the combination of both COVID-19 and cancer – and its effect on VTE risk and treatment – is of concern,” said the authors, led by Dominique Farge, MD, PhD, Nord Universite de Paris.
they added.
The new guidelines were published online in The Lancet Oncology.
“Cancer-associated VTE remains an important clinical problem, associated with increased morbidity and mortality,” Dr. Farge and colleagues observed.
“The ITAC guidelines’ companion free web-based mobile application will assist the practicing clinician with decision making at various levels to provide optimal care of patients with cancer to treat and prevent VTE,” they emphasized. More information is available at itaccme.com.
Cancer patients with COVID
The new section of the guidelines notes that the treatment and prevention of VTE for cancer patients infected with SARS-CoV-2 remain the same as for patients without COVID.
Whether or not cancer patients with COVID-19 are hospitalized, have been discharged, or are ambulatory, they should be assessed for the risk of VTE, as should any other patient. For cancer patients with COVID-19 who are hospitalized, pharmacologic prophylaxis should be given at the same dose and anticoagulant type as for hospitalized cancer patients who do not have COVID-19.
Following discharge, VTE prophylaxis is not advised for cancer patients infected with SARS-CoV-2, and routine primary pharmacologic prophylaxis of VTE for ambulatory patients with COVID-19 is also not recommended, the authors noted.
Initial treatment of established VTE
Initial treatment of established VTE for up to 10 days of anticoagulation should include low-molecular-weight heparin (LMWH) when creatinine clearance is at least 30 mL/min.
“A regimen of LMWH, taken once per day, is recommended unless a twice-per-day regimen is required because of patients’ characteristics,” the authors noted. These characteristics include a high risk of bleeding, moderate renal failure, and the need for technical intervention, including surgery.
If a twice-a-day regimen is required, only enoxaparin at a dose of 1 mg/kg twice daily can be used, the authors cautioned.
For patients with a low risk of gastrointestinal or genitourinary bleeding, rivaroxaban (Xarelto) or apixaban (Eliquis) can be given in the first 10 days, as well as edoxaban (Lixiana). The latter should be started after at least 5 days of parenteral anticoagulation, provided creatinine clearance is at least 30 mL/min.
“Unfractionated heparin as well as fondaparinux (GlaxoSmithKline) can be also used for the initial treatment of established VTE when LMWH or direct oral anticoagulants are contraindicated,” Dr. Farge and colleagues wrote.
Thrombolysis can be considered on a case-by-case basis, although physicians must pay attention to specific contraindications, especially bleeding risk.
“In the initial treatment of VTE, inferior vena cava filters might be considered when anticoagulant treatment is contraindicated or, in the case of pulmonary embolism, when recurrence occurs under optimal anticoagulation,” the authors noted.
Maintenance VTE treatment
For maintenance therapy, which the authors define as early maintenance for up to 6 months and long-term maintenance beyond 6 months, they point out that LMWHs are preferred over vitamin K antagonists for the treatment of VTE when the creatinine clearance is again at least 30 mL/min.
Any of the direct oral anticoagulants (DOAs) – edoxaban, rivaroxaban, or apixaban – is also recommended for the same patients, provided there is no risk of inducing a strong drug-drug interaction or GI absorption is impaired.
However, the DOAs should be used with caution for patients with GI malignancies, especially upper GI cancers, because data show there is an increased risk of GI bleeding with both edoxaban and rivaroxaban.
“LMWH or direct oral anticoagulants should be used for a minimum of 6 months to treat established VTE in patients with cancer,” the authors wrote.
“After 6 months, termination or continuation of anticoagulation (LMWH, direct oral anticoagulants, or vitamin K antagonists) should be based on individual evaluation of the benefit-risk ratio,” they added.
Treatment of VTE recurrence
The guideline authors explain that three options can be considered in the event of VTE recurrence. These include an increase in the LMWH dose by 20%-25%, or a switch to a DOA, or, if patients are taking a DOA, a switch to an LMWH. If the patient is taking a vitamin K antagonist, it can be switched to either an LMWH or a DOA.
For treatment of catheter-related thrombosis, anticoagulant treatment is recommended for a minimum of 3 months and as long as the central venous catheter is in place. In this setting, the LMWHs are recommended.
The central venous catheter can be kept in place if it is functional, well positioned, and is not infected, provided there is good resolution of symptoms under close surveillance while anticoagulants are being administered.
In surgically treated patients, the LMWH, given once a day, to patients with a serum creatinine concentration of at least 30 mL/min can be used to prevent VTE. Alternatively, VTE can be prevented by the use low-dose unfractionated heparin, given three times a day.
“Pharmacological prophylaxis should be started 2-12 h preoperatively and continued for at least 7–10 days,” Dr. Farge and colleagues advised. In this setting, there is insufficient evidence to support the use of fondaparinux or a DOA as an alternative to an LMWH for the prophylaxis of postoperative VTE. “Use of the highest prophylactic dose of LMWH to prevent postoperative VTE in patients with cancer is recommended,” the authors advised.
Furthermore, extended prophylaxis of at least 4 weeks with LMWH is advised to prevent postoperative VTE after major abdominal or pelvic surgery. Mechanical methods are not recommended except when pharmacologic methods are contraindicated. Inferior vena cava filters are also not recommended for routine prophylaxis.
Patients with reduced mobility
For medically treated hospitalized patients with cancer whose mobility is reduced, the authors recommend prophylaxis with either an LMWH or fondaparinux, provided their creatinine clearance is at least 30 mL/min. These patients can also be treated with unfractionated heparin, they add.
In contrast, DOAs are not recommended – at least not routinely – in this setting, the authors cautioned. Primary pharmacologic prophylaxis of VTE with either LMWH or DOAs – either rivaroxaban or apixaban – is indicated in ambulatory patients with locally advanced or metastatic pancreatic cancer who are receiving systemic anticancer therapy, provided they are at low risk of bleeding.
However, primary pharmacologic prophylaxis with LMWH is not recommended outside of a clinical trial for patients with locally advanced or metastatic lung cancer who are undergoing systemic anticancer therapy, even for patients who are at low risk of bleeding.
For ambulatory patients who are receiving systemic anticancer therapy and who are at intermediate risk of VTE, primary prophylaxis with rivaroxaban or apixaban is recommended for those with myeloma who are receiving immunomodulatory therapy plus steroids or other systemic therapies.
In this setting, oral anticoagulants should consist of a vitamin K antagonist, given at low or therapeutic doses, or apixaban, given at prophylactic doses. Alternatively, LMWH, given at prophylactic doses, or low-dose aspirin, given at a dose of 100 mg/day, can be used.
Catheter-related thrombosis
Use of anticoagulation for routine prophylaxis of catheter-related thrombosis is not recommended. Catheters should be inserted on the right side in the jugular vein, and the distal extremity of the central catheter should be located at the junction of the superior vena cava and the right atrium. “In patients requiring central venous catheters, we suggest the use of implanted ports over peripheral inserted central catheter lines,” the authors noted.
The authors described a number of unique situations regarding the treatment of VTE. These situations include patients with a brain tumor, for whom treatment of established VTE should favor either LMWH or a DOA. The authors also recommended the use of LMWH or unfractionated heparin, started postoperatively, for the prevention of VTE for patients undergoing neurosurgery.
In contrast, pharmacologic prophylaxis of VTE in medically treated patients with a brain tumor who are not undergoing neurosurgery is not recommended. “In the presence of severe renal failure...we suggest using unfractionated heparin followed by early vitamin K antagonists (possibly from day 1) or LMWH adjusted to anti-Xa concentration of the treatment of established VTE,” Dr. Farge and colleagues wrote.
Anticoagulant treatment is also recommended for a minimum of 3 months for children with symptomatic catheter-related thrombosis and as long as the central venous catheter is in place. For children with acute lymphoblastic leukemia who are undergoing induction chemotherapy, LMWH is also recommended as thromboprophylaxis.
For children who require a central venous catheter, the authors suggested that physicians use implanted ports over peripherally inserted central lines.
A version of this article first appeared on Medscape.com.
Updated clinical practice guidelines for the treatment and prevention of venous thromboembolism for patients with cancer, including those with cancer and COVID-19, have been released by the International Initiative on Thrombosis and Cancer (ITAC), an academic working group of VTE experts.
“Because patients with cancer have a baseline increased risk of VTE, compared with patients without cancer, the combination of both COVID-19 and cancer – and its effect on VTE risk and treatment – is of concern,” said the authors, led by Dominique Farge, MD, PhD, Nord Universite de Paris.
they added.
The new guidelines were published online in The Lancet Oncology.
“Cancer-associated VTE remains an important clinical problem, associated with increased morbidity and mortality,” Dr. Farge and colleagues observed.
“The ITAC guidelines’ companion free web-based mobile application will assist the practicing clinician with decision making at various levels to provide optimal care of patients with cancer to treat and prevent VTE,” they emphasized. More information is available at itaccme.com.
Cancer patients with COVID
The new section of the guidelines notes that the treatment and prevention of VTE for cancer patients infected with SARS-CoV-2 remain the same as for patients without COVID.
Whether or not cancer patients with COVID-19 are hospitalized, have been discharged, or are ambulatory, they should be assessed for the risk of VTE, as should any other patient. For cancer patients with COVID-19 who are hospitalized, pharmacologic prophylaxis should be given at the same dose and anticoagulant type as for hospitalized cancer patients who do not have COVID-19.
Following discharge, VTE prophylaxis is not advised for cancer patients infected with SARS-CoV-2, and routine primary pharmacologic prophylaxis of VTE for ambulatory patients with COVID-19 is also not recommended, the authors noted.
Initial treatment of established VTE
Initial treatment of established VTE for up to 10 days of anticoagulation should include low-molecular-weight heparin (LMWH) when creatinine clearance is at least 30 mL/min.
“A regimen of LMWH, taken once per day, is recommended unless a twice-per-day regimen is required because of patients’ characteristics,” the authors noted. These characteristics include a high risk of bleeding, moderate renal failure, and the need for technical intervention, including surgery.
If a twice-a-day regimen is required, only enoxaparin at a dose of 1 mg/kg twice daily can be used, the authors cautioned.
For patients with a low risk of gastrointestinal or genitourinary bleeding, rivaroxaban (Xarelto) or apixaban (Eliquis) can be given in the first 10 days, as well as edoxaban (Lixiana). The latter should be started after at least 5 days of parenteral anticoagulation, provided creatinine clearance is at least 30 mL/min.
“Unfractionated heparin as well as fondaparinux (GlaxoSmithKline) can be also used for the initial treatment of established VTE when LMWH or direct oral anticoagulants are contraindicated,” Dr. Farge and colleagues wrote.
Thrombolysis can be considered on a case-by-case basis, although physicians must pay attention to specific contraindications, especially bleeding risk.
“In the initial treatment of VTE, inferior vena cava filters might be considered when anticoagulant treatment is contraindicated or, in the case of pulmonary embolism, when recurrence occurs under optimal anticoagulation,” the authors noted.
Maintenance VTE treatment
For maintenance therapy, which the authors define as early maintenance for up to 6 months and long-term maintenance beyond 6 months, they point out that LMWHs are preferred over vitamin K antagonists for the treatment of VTE when the creatinine clearance is again at least 30 mL/min.
Any of the direct oral anticoagulants (DOAs) – edoxaban, rivaroxaban, or apixaban – is also recommended for the same patients, provided there is no risk of inducing a strong drug-drug interaction or GI absorption is impaired.
However, the DOAs should be used with caution for patients with GI malignancies, especially upper GI cancers, because data show there is an increased risk of GI bleeding with both edoxaban and rivaroxaban.
“LMWH or direct oral anticoagulants should be used for a minimum of 6 months to treat established VTE in patients with cancer,” the authors wrote.
“After 6 months, termination or continuation of anticoagulation (LMWH, direct oral anticoagulants, or vitamin K antagonists) should be based on individual evaluation of the benefit-risk ratio,” they added.
Treatment of VTE recurrence
The guideline authors explain that three options can be considered in the event of VTE recurrence. These include an increase in the LMWH dose by 20%-25%, or a switch to a DOA, or, if patients are taking a DOA, a switch to an LMWH. If the patient is taking a vitamin K antagonist, it can be switched to either an LMWH or a DOA.
For treatment of catheter-related thrombosis, anticoagulant treatment is recommended for a minimum of 3 months and as long as the central venous catheter is in place. In this setting, the LMWHs are recommended.
The central venous catheter can be kept in place if it is functional, well positioned, and is not infected, provided there is good resolution of symptoms under close surveillance while anticoagulants are being administered.
In surgically treated patients, the LMWH, given once a day, to patients with a serum creatinine concentration of at least 30 mL/min can be used to prevent VTE. Alternatively, VTE can be prevented by the use low-dose unfractionated heparin, given three times a day.
“Pharmacological prophylaxis should be started 2-12 h preoperatively and continued for at least 7–10 days,” Dr. Farge and colleagues advised. In this setting, there is insufficient evidence to support the use of fondaparinux or a DOA as an alternative to an LMWH for the prophylaxis of postoperative VTE. “Use of the highest prophylactic dose of LMWH to prevent postoperative VTE in patients with cancer is recommended,” the authors advised.
Furthermore, extended prophylaxis of at least 4 weeks with LMWH is advised to prevent postoperative VTE after major abdominal or pelvic surgery. Mechanical methods are not recommended except when pharmacologic methods are contraindicated. Inferior vena cava filters are also not recommended for routine prophylaxis.
Patients with reduced mobility
For medically treated hospitalized patients with cancer whose mobility is reduced, the authors recommend prophylaxis with either an LMWH or fondaparinux, provided their creatinine clearance is at least 30 mL/min. These patients can also be treated with unfractionated heparin, they add.
In contrast, DOAs are not recommended – at least not routinely – in this setting, the authors cautioned. Primary pharmacologic prophylaxis of VTE with either LMWH or DOAs – either rivaroxaban or apixaban – is indicated in ambulatory patients with locally advanced or metastatic pancreatic cancer who are receiving systemic anticancer therapy, provided they are at low risk of bleeding.
However, primary pharmacologic prophylaxis with LMWH is not recommended outside of a clinical trial for patients with locally advanced or metastatic lung cancer who are undergoing systemic anticancer therapy, even for patients who are at low risk of bleeding.
For ambulatory patients who are receiving systemic anticancer therapy and who are at intermediate risk of VTE, primary prophylaxis with rivaroxaban or apixaban is recommended for those with myeloma who are receiving immunomodulatory therapy plus steroids or other systemic therapies.
In this setting, oral anticoagulants should consist of a vitamin K antagonist, given at low or therapeutic doses, or apixaban, given at prophylactic doses. Alternatively, LMWH, given at prophylactic doses, or low-dose aspirin, given at a dose of 100 mg/day, can be used.
Catheter-related thrombosis
Use of anticoagulation for routine prophylaxis of catheter-related thrombosis is not recommended. Catheters should be inserted on the right side in the jugular vein, and the distal extremity of the central catheter should be located at the junction of the superior vena cava and the right atrium. “In patients requiring central venous catheters, we suggest the use of implanted ports over peripheral inserted central catheter lines,” the authors noted.
The authors described a number of unique situations regarding the treatment of VTE. These situations include patients with a brain tumor, for whom treatment of established VTE should favor either LMWH or a DOA. The authors also recommended the use of LMWH or unfractionated heparin, started postoperatively, for the prevention of VTE for patients undergoing neurosurgery.
In contrast, pharmacologic prophylaxis of VTE in medically treated patients with a brain tumor who are not undergoing neurosurgery is not recommended. “In the presence of severe renal failure...we suggest using unfractionated heparin followed by early vitamin K antagonists (possibly from day 1) or LMWH adjusted to anti-Xa concentration of the treatment of established VTE,” Dr. Farge and colleagues wrote.
Anticoagulant treatment is also recommended for a minimum of 3 months for children with symptomatic catheter-related thrombosis and as long as the central venous catheter is in place. For children with acute lymphoblastic leukemia who are undergoing induction chemotherapy, LMWH is also recommended as thromboprophylaxis.
For children who require a central venous catheter, the authors suggested that physicians use implanted ports over peripherally inserted central lines.
A version of this article first appeared on Medscape.com.
FROM THE LANCET ONCOLOGY
Mysterious cases of illness with an unusual cause
So begins the search for evidence.
No relations or common journeys
Between March and July 2021, cases of the bacterial infectious disease sprang up in Georgia, Kansas, Minnesota, and Texas, with the disease being fatal for two of those affected. Usually, cases of melioidosis occur in the United States after traveling to regions where the pathogen is prevalent. However, none of the patients had undertaken any previous international travel.
When the genomes of the bacterial strains (Burkholderia pseudomallei) were sequenced, they showed a high level of concordance, suggesting a common source of infection. The bacterial strain is similar to those that are found in Southeast Asia above all. An imported product from there was taken into consideration as the trigger.
The Centers for Disease Control and Prevention examined blood samples from the patients, as well as samples from the soil, water, food, and household items around their homes.
Aroma spray as a trigger
In October, the cause of the melioidosis was finally identified in the house of the patient from Georgia: an aromatherapy spray. The genetic fingerprint of the bacterial strain matched with that from the other patients. The common trigger was thus discovered.
The contaminated spray, with a lavender-chamomile scent for room fragrancing, was sold between February and October in some branches of Walmart, as well as in their online store. The product was therefore recalled and it was checked whether the ingredients were also being used in other products.
The CDC requested physicians to also take melioidosis into account if they were presented with acute bacterial infections that did not respond to normal antibiotics and to inquire whether the affected room spray had been used.
More information about melioidosis
Melioidosis is an infectious disease affecting humans and animals. The trigger is the bacteria B pseudomallei. The disease appears predominantly in tropical regions, especially in Southeast Asia and northern Australia.
Transmission
The bacteria can be found in contaminated water and soil. It is disseminated between humans and animals through direct contact with the infectious source, such as through inhaling dust particles or water droplets, or through consuming contaminated water or food. Human-to-human transmission is extremely rare. Recently however, tropical saltwater fish were identified as potential carriers.
Symptoms
Melioidosis has a wide range of symptoms, which can lead to its being confused with other diseases such as tuberculosis or other forms of pneumonia. There are different forms of the disease, each with different symptoms.
- Localized infection: localized pain and swelling, fever, ulceration, and abscess.
- Pulmonary infection: cough, chest pain, high fever, headaches, and loss of appetite
- Bacteremia: fever, headaches, breathing problems, stomach discomfort, joint pain, and disorientation.
- Disseminated infection: fever, weight loss, stomach or chest pain, muscle or joint pain, headaches, central nervous system infections, and epileptic seizures.
The incubation time is not clearly defined and can be from 1 day to several years; however, the symptoms mostly emerge 2-4 weeks after exposure. The risk factors include diabetes, high alcohol consumption, chronic pulmonary or kidney disease, and immunodeficiencies.
Diagnosis based on the symptoms is often difficult since the clinical picture is similar to other, more common conditions.
Therapy
If the melioidosis is identified as such, it can be treated with only mildly effective antibiotics, since it has a natural resistance to many commonly used antibiotics. The type of infection and the course of treatment also affects the long-term outcome. Without treatment, 90% of the infections have a fatal outcome. With appropriate treatment, the mortality rate still lies at 40%.
Therapy generally begins with intravenous antibiotic therapy for at least 2-8 weeks (ceftazidime or meropenem). Oral antibiotic therapy then follows for 3-6 months (trimethoprim-sulfamethoxazole or amoxicillin/clavulanic acid). If the patient is allergic to penicillin, alternative antibiotics can be used.
Use as a bioweapon
The CDC classifies B. pseudomallei as a potential pathogen for biological attack (class-B candidate). The agency lists the potential reasons for use as a bioweapon as:
- The pathogen can be found naturally in certain regions.
- The triggered disease can take a serious course and ultimately be fatal without appropriate therapy.
- In the past, the United States has used similar pathogens in wars as bioweapons.
In a potential attack, the pathogen could be spread through air, water, or food, and by doing so, many people would be exposed. Any contact with the bacteria can result in melioidosis. As the bacteria cannot be seen, smelled, or tasted, the biological attack would not be recognized for some time. A certain amount of time can also pass until the pathogen is identified, once fever and respiratory diseases have developed.
In such an emergency, the CDC would collaborate with other federal and local authorities to supply specialized testing laboratories and provide the public with information.
This content was translated from Coliquio. A version appeared on Medscape.com.
So begins the search for evidence.
No relations or common journeys
Between March and July 2021, cases of the bacterial infectious disease sprang up in Georgia, Kansas, Minnesota, and Texas, with the disease being fatal for two of those affected. Usually, cases of melioidosis occur in the United States after traveling to regions where the pathogen is prevalent. However, none of the patients had undertaken any previous international travel.
When the genomes of the bacterial strains (Burkholderia pseudomallei) were sequenced, they showed a high level of concordance, suggesting a common source of infection. The bacterial strain is similar to those that are found in Southeast Asia above all. An imported product from there was taken into consideration as the trigger.
The Centers for Disease Control and Prevention examined blood samples from the patients, as well as samples from the soil, water, food, and household items around their homes.
Aroma spray as a trigger
In October, the cause of the melioidosis was finally identified in the house of the patient from Georgia: an aromatherapy spray. The genetic fingerprint of the bacterial strain matched with that from the other patients. The common trigger was thus discovered.
The contaminated spray, with a lavender-chamomile scent for room fragrancing, was sold between February and October in some branches of Walmart, as well as in their online store. The product was therefore recalled and it was checked whether the ingredients were also being used in other products.
The CDC requested physicians to also take melioidosis into account if they were presented with acute bacterial infections that did not respond to normal antibiotics and to inquire whether the affected room spray had been used.
More information about melioidosis
Melioidosis is an infectious disease affecting humans and animals. The trigger is the bacteria B pseudomallei. The disease appears predominantly in tropical regions, especially in Southeast Asia and northern Australia.
Transmission
The bacteria can be found in contaminated water and soil. It is disseminated between humans and animals through direct contact with the infectious source, such as through inhaling dust particles or water droplets, or through consuming contaminated water or food. Human-to-human transmission is extremely rare. Recently however, tropical saltwater fish were identified as potential carriers.
Symptoms
Melioidosis has a wide range of symptoms, which can lead to its being confused with other diseases such as tuberculosis or other forms of pneumonia. There are different forms of the disease, each with different symptoms.
- Localized infection: localized pain and swelling, fever, ulceration, and abscess.
- Pulmonary infection: cough, chest pain, high fever, headaches, and loss of appetite
- Bacteremia: fever, headaches, breathing problems, stomach discomfort, joint pain, and disorientation.
- Disseminated infection: fever, weight loss, stomach or chest pain, muscle or joint pain, headaches, central nervous system infections, and epileptic seizures.
The incubation time is not clearly defined and can be from 1 day to several years; however, the symptoms mostly emerge 2-4 weeks after exposure. The risk factors include diabetes, high alcohol consumption, chronic pulmonary or kidney disease, and immunodeficiencies.
Diagnosis based on the symptoms is often difficult since the clinical picture is similar to other, more common conditions.
Therapy
If the melioidosis is identified as such, it can be treated with only mildly effective antibiotics, since it has a natural resistance to many commonly used antibiotics. The type of infection and the course of treatment also affects the long-term outcome. Without treatment, 90% of the infections have a fatal outcome. With appropriate treatment, the mortality rate still lies at 40%.
Therapy generally begins with intravenous antibiotic therapy for at least 2-8 weeks (ceftazidime or meropenem). Oral antibiotic therapy then follows for 3-6 months (trimethoprim-sulfamethoxazole or amoxicillin/clavulanic acid). If the patient is allergic to penicillin, alternative antibiotics can be used.
Use as a bioweapon
The CDC classifies B. pseudomallei as a potential pathogen for biological attack (class-B candidate). The agency lists the potential reasons for use as a bioweapon as:
- The pathogen can be found naturally in certain regions.
- The triggered disease can take a serious course and ultimately be fatal without appropriate therapy.
- In the past, the United States has used similar pathogens in wars as bioweapons.
In a potential attack, the pathogen could be spread through air, water, or food, and by doing so, many people would be exposed. Any contact with the bacteria can result in melioidosis. As the bacteria cannot be seen, smelled, or tasted, the biological attack would not be recognized for some time. A certain amount of time can also pass until the pathogen is identified, once fever and respiratory diseases have developed.
In such an emergency, the CDC would collaborate with other federal and local authorities to supply specialized testing laboratories and provide the public with information.
This content was translated from Coliquio. A version appeared on Medscape.com.
So begins the search for evidence.
No relations or common journeys
Between March and July 2021, cases of the bacterial infectious disease sprang up in Georgia, Kansas, Minnesota, and Texas, with the disease being fatal for two of those affected. Usually, cases of melioidosis occur in the United States after traveling to regions where the pathogen is prevalent. However, none of the patients had undertaken any previous international travel.
When the genomes of the bacterial strains (Burkholderia pseudomallei) were sequenced, they showed a high level of concordance, suggesting a common source of infection. The bacterial strain is similar to those that are found in Southeast Asia above all. An imported product from there was taken into consideration as the trigger.
The Centers for Disease Control and Prevention examined blood samples from the patients, as well as samples from the soil, water, food, and household items around their homes.
Aroma spray as a trigger
In October, the cause of the melioidosis was finally identified in the house of the patient from Georgia: an aromatherapy spray. The genetic fingerprint of the bacterial strain matched with that from the other patients. The common trigger was thus discovered.
The contaminated spray, with a lavender-chamomile scent for room fragrancing, was sold between February and October in some branches of Walmart, as well as in their online store. The product was therefore recalled and it was checked whether the ingredients were also being used in other products.
The CDC requested physicians to also take melioidosis into account if they were presented with acute bacterial infections that did not respond to normal antibiotics and to inquire whether the affected room spray had been used.
More information about melioidosis
Melioidosis is an infectious disease affecting humans and animals. The trigger is the bacteria B pseudomallei. The disease appears predominantly in tropical regions, especially in Southeast Asia and northern Australia.
Transmission
The bacteria can be found in contaminated water and soil. It is disseminated between humans and animals through direct contact with the infectious source, such as through inhaling dust particles or water droplets, or through consuming contaminated water or food. Human-to-human transmission is extremely rare. Recently however, tropical saltwater fish were identified as potential carriers.
Symptoms
Melioidosis has a wide range of symptoms, which can lead to its being confused with other diseases such as tuberculosis or other forms of pneumonia. There are different forms of the disease, each with different symptoms.
- Localized infection: localized pain and swelling, fever, ulceration, and abscess.
- Pulmonary infection: cough, chest pain, high fever, headaches, and loss of appetite
- Bacteremia: fever, headaches, breathing problems, stomach discomfort, joint pain, and disorientation.
- Disseminated infection: fever, weight loss, stomach or chest pain, muscle or joint pain, headaches, central nervous system infections, and epileptic seizures.
The incubation time is not clearly defined and can be from 1 day to several years; however, the symptoms mostly emerge 2-4 weeks after exposure. The risk factors include diabetes, high alcohol consumption, chronic pulmonary or kidney disease, and immunodeficiencies.
Diagnosis based on the symptoms is often difficult since the clinical picture is similar to other, more common conditions.
Therapy
If the melioidosis is identified as such, it can be treated with only mildly effective antibiotics, since it has a natural resistance to many commonly used antibiotics. The type of infection and the course of treatment also affects the long-term outcome. Without treatment, 90% of the infections have a fatal outcome. With appropriate treatment, the mortality rate still lies at 40%.
Therapy generally begins with intravenous antibiotic therapy for at least 2-8 weeks (ceftazidime or meropenem). Oral antibiotic therapy then follows for 3-6 months (trimethoprim-sulfamethoxazole or amoxicillin/clavulanic acid). If the patient is allergic to penicillin, alternative antibiotics can be used.
Use as a bioweapon
The CDC classifies B. pseudomallei as a potential pathogen for biological attack (class-B candidate). The agency lists the potential reasons for use as a bioweapon as:
- The pathogen can be found naturally in certain regions.
- The triggered disease can take a serious course and ultimately be fatal without appropriate therapy.
- In the past, the United States has used similar pathogens in wars as bioweapons.
In a potential attack, the pathogen could be spread through air, water, or food, and by doing so, many people would be exposed. Any contact with the bacteria can result in melioidosis. As the bacteria cannot be seen, smelled, or tasted, the biological attack would not be recognized for some time. A certain amount of time can also pass until the pathogen is identified, once fever and respiratory diseases have developed.
In such an emergency, the CDC would collaborate with other federal and local authorities to supply specialized testing laboratories and provide the public with information.
This content was translated from Coliquio. A version appeared on Medscape.com.
How well do vaccines protect against long COVID?
New York City veterinarian Erin Kulick used to be a weekend warrior. Only 2½ years ago, the 38-year-old new mother played ultimate Frisbee and flag football with friends. She went for regular 30-minute runs to burn off stress.
Now, Dr. Kulick is usually so exhausted, she can’t walk nonstop for 15 minutes. She recently tried to take her 4-year-old son, Cooper, to the American Museum of Natural History for his first visit, but ended up on a bench outside the museum, sobbing in the rain, because she couldn’t even get through the first hurdle of standing in line. “I just wanted to be there with my kid,” she said.
Dr. Kulick got sick with COVID-19 at the start of the pandemic in March 2020, 9 months before the first vaccine would be approved. Now she is among the estimated one in five infected Americans, or 19%, whose symptoms developed into long COVID.
Dr. Kulick also is now vaccinated and boosted. Had a vaccine been available sooner, could it have protected her from long COVID?
Evidence is starting to show it’s likely.
“The best way not to have long COVID is not to have COVID at all,” said Leora Horwitz, MD, a professor of population health and medicine at New York University. “To the extent that vaccination can prevent you from getting COVID at all, then it helps to reduce long COVID.”
And People with more serious initial illness appear more likely to have prolonged symptoms, but those with milder disease can certainly get it, too.
“You’re more likely to have long COVID with more severe disease, and we have ample evidence that vaccination reduces the severity of disease,” Dr. Horwitz said. “We also now have quite a lot of evidence that vaccination does reduce your risk of long COVID – probably because it reduces your risk of severe disease.”
There is little consensus about how much vaccines can lower the risk of long-term COVID symptoms, but several studies suggest that number lies anywhere from 15% to more than 80%.
That might seem like a big variation, but infectious disease experts argue that trying to interpret the gap isn’t as important as noticing what’s consistent across all these studies: “Vaccines do offer some protection, but it’s incomplete,” said Ziyad Al-Aly, MD, chief of research and development at the Veterans Affairs St. Louis Health Care System. Dr. Al-Aly, who has led several large studies on long COVID, said focusing on the fact that vaccines do offer some protection is a much better public health message than looking at the different levels of risk.
“Vaccines do a miraculous job for what they were designed to do,” said Dr. Al-Aly. “Vaccines were designed to reduce the risk of hospitalization ... and for that, vaccines are still holding up, even with all the changes in the virus.”
Still, Elena Azzolini, MD, PhD, head of the Humanitas Research Hospital’s vaccination center in Milan, thinks some studies may have underestimated the level of long COVID protection from vaccines because of limits in the study methods, such as not including enough women, who are more affected by long COVID. Her recent study, which looked at 2,560 health care professionals working in nine Italian centers from March 2020 to April 2022, focused on the risk for healthy women and men in their 20s to their 70s.
In the paper, Dr. Azzolini and associates reported that two or three doses of vaccine reduced the risk of hospitalization from COVID-19 from 42% among those who are unvaccinated to 16%-17%. In other words, they found unvaccinated people in the study were nearly three times as likely to have serious symptoms for longer than 4 weeks.
But Dr. Azzolini and Dr. Al-Aly still say that, even for the vaccinated, as long as COVID is around, masks are necessary. That’s because current vaccines don’t do enough to reduce transmission, said Dr. Al-Aly. “The only way that can really help [stop] transmission is covering our nose and mouth with a mask.”
How vaccinations affect people who already have long COVID
Some long COVID patients have said they got better after they get boosted, while some say they’re getting worse, said Dr. Horwitz, who is also a lead investigator at the National Institutes of Health’s flagship RECOVER program, a 4-year research project to study long COVID across the United States. (The NIH is still recruiting volunteers for these studies, which are also open to people who have never had COVID.)
One study published in the British Medical Journal analyzed survey data of more than 28,000 people infected with COVID in the United Kingdom and found a 13% reduction in long-term symptoms after a first dose of the vaccine, although it was unclear from the data if the improvement was sustained.
A second dose was associated with another 8% improvement over a 2-month period. “It’s reassuring that we see an average modest improvement in symptoms, not an average worsening in symptoms,” said Daniel Ayoubkhani, principal statistician at the U.K. Office for National Statistics and lead author of the study. Of course, the experience will differ among different people.
“It doesn’t appear that vaccination is the silver bullet that’s going to eradicate long COVID,” he said, but evidence from multiple studies suggests vaccines may help people with long-term symptoms.
Akiko Iwasaki, PhD, an immunobiologist at Yale University, New Haven, Conn., told a White House summit in July that one of the best ways to prevent long COVID is to develop the next generation of vaccines that also prevent milder cases by blocking transmission in the first place.
Back in New York, Dr. Kulick is now triple vaccinated. She’s due for a fourth dose soon but admits she’s “terrified every time” that she’s going to get sicker.
In her Facebook support group for long COVID, she reads that most people with prolonged symptoms handle it well. She has also noticed some of her symptoms eased after her first two doses of vaccine.
Since being diagnosed, Dr. Kulick learned she has a genetic condition, Ehlers-Danlos syndrome, which affects connective tissues that support skin, joints, organs, and blood vessels, and which her doctors say may have made her more prone to long COVID. She’s also being screened for autoimmune diseases, but for now, the only relief she has found has come from long COVID physical therapy, changes to her diet, and integrative medicine.
Dr. Kulick is still trying to figure out how she can get better while keeping her long hours at her veterinary job – and her health benefits. She is thankful her husband is a devoted caregiver to their son and a professional jazz musician with a schedule that allows for some flexibility.
“But it’s really hard when every week feels like I’ve run a marathon,” she said. “I can barely make it through.”
A version of this article first appeared on WebMD.com.
New York City veterinarian Erin Kulick used to be a weekend warrior. Only 2½ years ago, the 38-year-old new mother played ultimate Frisbee and flag football with friends. She went for regular 30-minute runs to burn off stress.
Now, Dr. Kulick is usually so exhausted, she can’t walk nonstop for 15 minutes. She recently tried to take her 4-year-old son, Cooper, to the American Museum of Natural History for his first visit, but ended up on a bench outside the museum, sobbing in the rain, because she couldn’t even get through the first hurdle of standing in line. “I just wanted to be there with my kid,” she said.
Dr. Kulick got sick with COVID-19 at the start of the pandemic in March 2020, 9 months before the first vaccine would be approved. Now she is among the estimated one in five infected Americans, or 19%, whose symptoms developed into long COVID.
Dr. Kulick also is now vaccinated and boosted. Had a vaccine been available sooner, could it have protected her from long COVID?
Evidence is starting to show it’s likely.
“The best way not to have long COVID is not to have COVID at all,” said Leora Horwitz, MD, a professor of population health and medicine at New York University. “To the extent that vaccination can prevent you from getting COVID at all, then it helps to reduce long COVID.”
And People with more serious initial illness appear more likely to have prolonged symptoms, but those with milder disease can certainly get it, too.
“You’re more likely to have long COVID with more severe disease, and we have ample evidence that vaccination reduces the severity of disease,” Dr. Horwitz said. “We also now have quite a lot of evidence that vaccination does reduce your risk of long COVID – probably because it reduces your risk of severe disease.”
There is little consensus about how much vaccines can lower the risk of long-term COVID symptoms, but several studies suggest that number lies anywhere from 15% to more than 80%.
That might seem like a big variation, but infectious disease experts argue that trying to interpret the gap isn’t as important as noticing what’s consistent across all these studies: “Vaccines do offer some protection, but it’s incomplete,” said Ziyad Al-Aly, MD, chief of research and development at the Veterans Affairs St. Louis Health Care System. Dr. Al-Aly, who has led several large studies on long COVID, said focusing on the fact that vaccines do offer some protection is a much better public health message than looking at the different levels of risk.
“Vaccines do a miraculous job for what they were designed to do,” said Dr. Al-Aly. “Vaccines were designed to reduce the risk of hospitalization ... and for that, vaccines are still holding up, even with all the changes in the virus.”
Still, Elena Azzolini, MD, PhD, head of the Humanitas Research Hospital’s vaccination center in Milan, thinks some studies may have underestimated the level of long COVID protection from vaccines because of limits in the study methods, such as not including enough women, who are more affected by long COVID. Her recent study, which looked at 2,560 health care professionals working in nine Italian centers from March 2020 to April 2022, focused on the risk for healthy women and men in their 20s to their 70s.
In the paper, Dr. Azzolini and associates reported that two or three doses of vaccine reduced the risk of hospitalization from COVID-19 from 42% among those who are unvaccinated to 16%-17%. In other words, they found unvaccinated people in the study were nearly three times as likely to have serious symptoms for longer than 4 weeks.
But Dr. Azzolini and Dr. Al-Aly still say that, even for the vaccinated, as long as COVID is around, masks are necessary. That’s because current vaccines don’t do enough to reduce transmission, said Dr. Al-Aly. “The only way that can really help [stop] transmission is covering our nose and mouth with a mask.”
How vaccinations affect people who already have long COVID
Some long COVID patients have said they got better after they get boosted, while some say they’re getting worse, said Dr. Horwitz, who is also a lead investigator at the National Institutes of Health’s flagship RECOVER program, a 4-year research project to study long COVID across the United States. (The NIH is still recruiting volunteers for these studies, which are also open to people who have never had COVID.)
One study published in the British Medical Journal analyzed survey data of more than 28,000 people infected with COVID in the United Kingdom and found a 13% reduction in long-term symptoms after a first dose of the vaccine, although it was unclear from the data if the improvement was sustained.
A second dose was associated with another 8% improvement over a 2-month period. “It’s reassuring that we see an average modest improvement in symptoms, not an average worsening in symptoms,” said Daniel Ayoubkhani, principal statistician at the U.K. Office for National Statistics and lead author of the study. Of course, the experience will differ among different people.
“It doesn’t appear that vaccination is the silver bullet that’s going to eradicate long COVID,” he said, but evidence from multiple studies suggests vaccines may help people with long-term symptoms.
Akiko Iwasaki, PhD, an immunobiologist at Yale University, New Haven, Conn., told a White House summit in July that one of the best ways to prevent long COVID is to develop the next generation of vaccines that also prevent milder cases by blocking transmission in the first place.
Back in New York, Dr. Kulick is now triple vaccinated. She’s due for a fourth dose soon but admits she’s “terrified every time” that she’s going to get sicker.
In her Facebook support group for long COVID, she reads that most people with prolonged symptoms handle it well. She has also noticed some of her symptoms eased after her first two doses of vaccine.
Since being diagnosed, Dr. Kulick learned she has a genetic condition, Ehlers-Danlos syndrome, which affects connective tissues that support skin, joints, organs, and blood vessels, and which her doctors say may have made her more prone to long COVID. She’s also being screened for autoimmune diseases, but for now, the only relief she has found has come from long COVID physical therapy, changes to her diet, and integrative medicine.
Dr. Kulick is still trying to figure out how she can get better while keeping her long hours at her veterinary job – and her health benefits. She is thankful her husband is a devoted caregiver to their son and a professional jazz musician with a schedule that allows for some flexibility.
“But it’s really hard when every week feels like I’ve run a marathon,” she said. “I can barely make it through.”
A version of this article first appeared on WebMD.com.
New York City veterinarian Erin Kulick used to be a weekend warrior. Only 2½ years ago, the 38-year-old new mother played ultimate Frisbee and flag football with friends. She went for regular 30-minute runs to burn off stress.
Now, Dr. Kulick is usually so exhausted, she can’t walk nonstop for 15 minutes. She recently tried to take her 4-year-old son, Cooper, to the American Museum of Natural History for his first visit, but ended up on a bench outside the museum, sobbing in the rain, because she couldn’t even get through the first hurdle of standing in line. “I just wanted to be there with my kid,” she said.
Dr. Kulick got sick with COVID-19 at the start of the pandemic in March 2020, 9 months before the first vaccine would be approved. Now she is among the estimated one in five infected Americans, or 19%, whose symptoms developed into long COVID.
Dr. Kulick also is now vaccinated and boosted. Had a vaccine been available sooner, could it have protected her from long COVID?
Evidence is starting to show it’s likely.
“The best way not to have long COVID is not to have COVID at all,” said Leora Horwitz, MD, a professor of population health and medicine at New York University. “To the extent that vaccination can prevent you from getting COVID at all, then it helps to reduce long COVID.”
And People with more serious initial illness appear more likely to have prolonged symptoms, but those with milder disease can certainly get it, too.
“You’re more likely to have long COVID with more severe disease, and we have ample evidence that vaccination reduces the severity of disease,” Dr. Horwitz said. “We also now have quite a lot of evidence that vaccination does reduce your risk of long COVID – probably because it reduces your risk of severe disease.”
There is little consensus about how much vaccines can lower the risk of long-term COVID symptoms, but several studies suggest that number lies anywhere from 15% to more than 80%.
That might seem like a big variation, but infectious disease experts argue that trying to interpret the gap isn’t as important as noticing what’s consistent across all these studies: “Vaccines do offer some protection, but it’s incomplete,” said Ziyad Al-Aly, MD, chief of research and development at the Veterans Affairs St. Louis Health Care System. Dr. Al-Aly, who has led several large studies on long COVID, said focusing on the fact that vaccines do offer some protection is a much better public health message than looking at the different levels of risk.
“Vaccines do a miraculous job for what they were designed to do,” said Dr. Al-Aly. “Vaccines were designed to reduce the risk of hospitalization ... and for that, vaccines are still holding up, even with all the changes in the virus.”
Still, Elena Azzolini, MD, PhD, head of the Humanitas Research Hospital’s vaccination center in Milan, thinks some studies may have underestimated the level of long COVID protection from vaccines because of limits in the study methods, such as not including enough women, who are more affected by long COVID. Her recent study, which looked at 2,560 health care professionals working in nine Italian centers from March 2020 to April 2022, focused on the risk for healthy women and men in their 20s to their 70s.
In the paper, Dr. Azzolini and associates reported that two or three doses of vaccine reduced the risk of hospitalization from COVID-19 from 42% among those who are unvaccinated to 16%-17%. In other words, they found unvaccinated people in the study were nearly three times as likely to have serious symptoms for longer than 4 weeks.
But Dr. Azzolini and Dr. Al-Aly still say that, even for the vaccinated, as long as COVID is around, masks are necessary. That’s because current vaccines don’t do enough to reduce transmission, said Dr. Al-Aly. “The only way that can really help [stop] transmission is covering our nose and mouth with a mask.”
How vaccinations affect people who already have long COVID
Some long COVID patients have said they got better after they get boosted, while some say they’re getting worse, said Dr. Horwitz, who is also a lead investigator at the National Institutes of Health’s flagship RECOVER program, a 4-year research project to study long COVID across the United States. (The NIH is still recruiting volunteers for these studies, which are also open to people who have never had COVID.)
One study published in the British Medical Journal analyzed survey data of more than 28,000 people infected with COVID in the United Kingdom and found a 13% reduction in long-term symptoms after a first dose of the vaccine, although it was unclear from the data if the improvement was sustained.
A second dose was associated with another 8% improvement over a 2-month period. “It’s reassuring that we see an average modest improvement in symptoms, not an average worsening in symptoms,” said Daniel Ayoubkhani, principal statistician at the U.K. Office for National Statistics and lead author of the study. Of course, the experience will differ among different people.
“It doesn’t appear that vaccination is the silver bullet that’s going to eradicate long COVID,” he said, but evidence from multiple studies suggests vaccines may help people with long-term symptoms.
Akiko Iwasaki, PhD, an immunobiologist at Yale University, New Haven, Conn., told a White House summit in July that one of the best ways to prevent long COVID is to develop the next generation of vaccines that also prevent milder cases by blocking transmission in the first place.
Back in New York, Dr. Kulick is now triple vaccinated. She’s due for a fourth dose soon but admits she’s “terrified every time” that she’s going to get sicker.
In her Facebook support group for long COVID, she reads that most people with prolonged symptoms handle it well. She has also noticed some of her symptoms eased after her first two doses of vaccine.
Since being diagnosed, Dr. Kulick learned she has a genetic condition, Ehlers-Danlos syndrome, which affects connective tissues that support skin, joints, organs, and blood vessels, and which her doctors say may have made her more prone to long COVID. She’s also being screened for autoimmune diseases, but for now, the only relief she has found has come from long COVID physical therapy, changes to her diet, and integrative medicine.
Dr. Kulick is still trying to figure out how she can get better while keeping her long hours at her veterinary job – and her health benefits. She is thankful her husband is a devoted caregiver to their son and a professional jazz musician with a schedule that allows for some flexibility.
“But it’s really hard when every week feels like I’ve run a marathon,” she said. “I can barely make it through.”
A version of this article first appeared on WebMD.com.
Early time-restricted eating ups weight loss, but jury still out
, new findings suggest.
Previous studies have produced mixed results regarding the weight-loss potential for intermittent fasting, the practice of alternating eating with extended fasting, and the “time-restricted eating” format, where eating is restricted to a specific, often 10-hour, time window during the day.
In a new randomized clinical trial of 90 people with obesity in which that time window was 7 AM through 3 PM, so 8 hours long, researchers report that “eTRE was more effective for losing weight and lowering diastolic blood pressure than was eating over a period of 12 or more hours at 14 weeks. The eTRE intervention may therefore be an effective treatment for both obesity and hypertension.” The study, by Humaira Jamshed, PhD, of the department of nutrition sciences, University of Alabama at Birmingham, and colleagues, was published in JAMA Internal Medicine.
In an accompanying invited commentary, Shalender Bhasin, MBBS, points out that the study findings differ from those of a previous trial published in April of 139 adults conducted in China, which did not find a significant weight loss benefit with TRE versus ad lib eating.
“The scientific premise and the preclinical data of the effects of TRE are promising, but the inconsistency among studies renders it difficult to draw strong inferences from these well-conducted but relatively small trials,” notes Dr. Bhasin, of Harvard Medical School, Boston.
Need for larger and longer trials of TRE
Dr. Bhasin says – and the study authors also acknowledge – that much larger randomized clinical trials of longer duration are needed “to comprehensively evaluate the hypothesized benefits and risks of long-term TRE of calorically restricted diets in adults.”
Commenting on the study for the U.K. Science Media Centre, Simon Steenson, PhD, nutrition scientist, British Nutrition Foundation, said “one of the strengths of this new study is the trial design and the number of people who were recruited compared to many of the previous trials to date.”
However, Dr. Steenson also pointed to the prior Chinese research as evidence that the inconsistencies across studies highlight the need for larger and longer trials, with cardiovascular as well as weight-loss endpoints.
Still, Dr. Steenson said, “For individuals who may find that this pattern of eating fits better with their lifestyle and preferences, time-restricted feeding is one option for reducing overall calorie intake that might be a suitable approach for some. Ultimately, it is about finding the best approach to moderate calorie intake that works for each person, as successful and sustained weight loss is about ensuring the diet is feasible to follow in the long-term.”
Differences in weight loss, diastolic BP, but not all measures
The study population included 90 adults seen at the Weight Loss Medicine clinic at the University of Alabama at Birmingham between August 2018 and December 2019. Participants had a body mass index of 30-60 kg/m2, and none had diabetes.
They were randomized to eTRE with the 7 AM to PM eating window or a control schedule with eating across 12 hours or more, mimicking U.S. median mealtimes, at least 6 days a week. All participants received 30-minute weight-loss counseling sessions at baseline and at weeks 2, 6, and 10 and were advised to follow a diet of 500 kcal/day below their resting energy expenditure and exercise 75-150 minutes per week.
The eTRE group adhered with their schedule a mean of 6 days per week, lower than the 6.3 days among controls (P = .03), and adherence declined by about 0.4 days per week in the eTRE group over the 14 weeks (P = .001).
At 14 weeks, both the eTRE group and controls had lost clinically meaningful amounts of weight, at –6.3 kg and –4.0 kg, respectively, but the –2.3 kg difference was significant (P = .002).
However, there was no difference in absolute fat loss (P = .09) or ratio of fat loss to weight loss (P = .43). There were also no significant differences in changes in other body composition parameters, including visceral fat and waist circumference.
Diastolic blood pressure was lowered by an additional 4 mmHg in the eTRE group, compared with controls at 14 weeks (P = .04), but there were no significant differences in systolic blood pressure, heart rate, glucose, A1c levels, insulin levels, measures of insulin resistance, or plasma lipids.
There were no differences between the two groups in self-reported physical activity, energy intake, or dietary macronutrient composition either. However, weight-loss modeling in 77 participants with at least two weight measurements indicated that the eTRE group reduced their intake by about 214 kcal/day, compared with controls (P = .04).
Those in the eTRE group also showed greater improvements in measures of mood disturbance, vigor-activity, fatigue-inertia, and depression-dejection. Other mood and sleep endpoints were similar between groups.
In a secondary analysis of just the 59 participants who completed the study, eTRE was also more effective at reducing body fat (P = .047) and trunk fat (P = .03).
About 41% of the eTRE completers planned to continue the practice after the study concluded.
The study was supported by grants from the National Center for Advancing Translational Sciences of the National Institutes of Health and the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Jamshed has reported no relevant financial relationships. Dr. Bhasin has reported receiving grants to his institution for research on which Dr. Bhasin is the principal investigator from AbbVie and MIB, receiving personal fees from OPKO and Aditum and holding equity interest in FPT and XYOne. Dr. Steenson has declared funding in support of the British Nutrition Foundation that comes from a range of sources.
A version of this article first appeared on Medscape.com.
, new findings suggest.
Previous studies have produced mixed results regarding the weight-loss potential for intermittent fasting, the practice of alternating eating with extended fasting, and the “time-restricted eating” format, where eating is restricted to a specific, often 10-hour, time window during the day.
In a new randomized clinical trial of 90 people with obesity in which that time window was 7 AM through 3 PM, so 8 hours long, researchers report that “eTRE was more effective for losing weight and lowering diastolic blood pressure than was eating over a period of 12 or more hours at 14 weeks. The eTRE intervention may therefore be an effective treatment for both obesity and hypertension.” The study, by Humaira Jamshed, PhD, of the department of nutrition sciences, University of Alabama at Birmingham, and colleagues, was published in JAMA Internal Medicine.
In an accompanying invited commentary, Shalender Bhasin, MBBS, points out that the study findings differ from those of a previous trial published in April of 139 adults conducted in China, which did not find a significant weight loss benefit with TRE versus ad lib eating.
“The scientific premise and the preclinical data of the effects of TRE are promising, but the inconsistency among studies renders it difficult to draw strong inferences from these well-conducted but relatively small trials,” notes Dr. Bhasin, of Harvard Medical School, Boston.
Need for larger and longer trials of TRE
Dr. Bhasin says – and the study authors also acknowledge – that much larger randomized clinical trials of longer duration are needed “to comprehensively evaluate the hypothesized benefits and risks of long-term TRE of calorically restricted diets in adults.”
Commenting on the study for the U.K. Science Media Centre, Simon Steenson, PhD, nutrition scientist, British Nutrition Foundation, said “one of the strengths of this new study is the trial design and the number of people who were recruited compared to many of the previous trials to date.”
However, Dr. Steenson also pointed to the prior Chinese research as evidence that the inconsistencies across studies highlight the need for larger and longer trials, with cardiovascular as well as weight-loss endpoints.
Still, Dr. Steenson said, “For individuals who may find that this pattern of eating fits better with their lifestyle and preferences, time-restricted feeding is one option for reducing overall calorie intake that might be a suitable approach for some. Ultimately, it is about finding the best approach to moderate calorie intake that works for each person, as successful and sustained weight loss is about ensuring the diet is feasible to follow in the long-term.”
Differences in weight loss, diastolic BP, but not all measures
The study population included 90 adults seen at the Weight Loss Medicine clinic at the University of Alabama at Birmingham between August 2018 and December 2019. Participants had a body mass index of 30-60 kg/m2, and none had diabetes.
They were randomized to eTRE with the 7 AM to PM eating window or a control schedule with eating across 12 hours or more, mimicking U.S. median mealtimes, at least 6 days a week. All participants received 30-minute weight-loss counseling sessions at baseline and at weeks 2, 6, and 10 and were advised to follow a diet of 500 kcal/day below their resting energy expenditure and exercise 75-150 minutes per week.
The eTRE group adhered with their schedule a mean of 6 days per week, lower than the 6.3 days among controls (P = .03), and adherence declined by about 0.4 days per week in the eTRE group over the 14 weeks (P = .001).
At 14 weeks, both the eTRE group and controls had lost clinically meaningful amounts of weight, at –6.3 kg and –4.0 kg, respectively, but the –2.3 kg difference was significant (P = .002).
However, there was no difference in absolute fat loss (P = .09) or ratio of fat loss to weight loss (P = .43). There were also no significant differences in changes in other body composition parameters, including visceral fat and waist circumference.
Diastolic blood pressure was lowered by an additional 4 mmHg in the eTRE group, compared with controls at 14 weeks (P = .04), but there were no significant differences in systolic blood pressure, heart rate, glucose, A1c levels, insulin levels, measures of insulin resistance, or plasma lipids.
There were no differences between the two groups in self-reported physical activity, energy intake, or dietary macronutrient composition either. However, weight-loss modeling in 77 participants with at least two weight measurements indicated that the eTRE group reduced their intake by about 214 kcal/day, compared with controls (P = .04).
Those in the eTRE group also showed greater improvements in measures of mood disturbance, vigor-activity, fatigue-inertia, and depression-dejection. Other mood and sleep endpoints were similar between groups.
In a secondary analysis of just the 59 participants who completed the study, eTRE was also more effective at reducing body fat (P = .047) and trunk fat (P = .03).
About 41% of the eTRE completers planned to continue the practice after the study concluded.
The study was supported by grants from the National Center for Advancing Translational Sciences of the National Institutes of Health and the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Jamshed has reported no relevant financial relationships. Dr. Bhasin has reported receiving grants to his institution for research on which Dr. Bhasin is the principal investigator from AbbVie and MIB, receiving personal fees from OPKO and Aditum and holding equity interest in FPT and XYOne. Dr. Steenson has declared funding in support of the British Nutrition Foundation that comes from a range of sources.
A version of this article first appeared on Medscape.com.
, new findings suggest.
Previous studies have produced mixed results regarding the weight-loss potential for intermittent fasting, the practice of alternating eating with extended fasting, and the “time-restricted eating” format, where eating is restricted to a specific, often 10-hour, time window during the day.
In a new randomized clinical trial of 90 people with obesity in which that time window was 7 AM through 3 PM, so 8 hours long, researchers report that “eTRE was more effective for losing weight and lowering diastolic blood pressure than was eating over a period of 12 or more hours at 14 weeks. The eTRE intervention may therefore be an effective treatment for both obesity and hypertension.” The study, by Humaira Jamshed, PhD, of the department of nutrition sciences, University of Alabama at Birmingham, and colleagues, was published in JAMA Internal Medicine.
In an accompanying invited commentary, Shalender Bhasin, MBBS, points out that the study findings differ from those of a previous trial published in April of 139 adults conducted in China, which did not find a significant weight loss benefit with TRE versus ad lib eating.
“The scientific premise and the preclinical data of the effects of TRE are promising, but the inconsistency among studies renders it difficult to draw strong inferences from these well-conducted but relatively small trials,” notes Dr. Bhasin, of Harvard Medical School, Boston.
Need for larger and longer trials of TRE
Dr. Bhasin says – and the study authors also acknowledge – that much larger randomized clinical trials of longer duration are needed “to comprehensively evaluate the hypothesized benefits and risks of long-term TRE of calorically restricted diets in adults.”
Commenting on the study for the U.K. Science Media Centre, Simon Steenson, PhD, nutrition scientist, British Nutrition Foundation, said “one of the strengths of this new study is the trial design and the number of people who were recruited compared to many of the previous trials to date.”
However, Dr. Steenson also pointed to the prior Chinese research as evidence that the inconsistencies across studies highlight the need for larger and longer trials, with cardiovascular as well as weight-loss endpoints.
Still, Dr. Steenson said, “For individuals who may find that this pattern of eating fits better with their lifestyle and preferences, time-restricted feeding is one option for reducing overall calorie intake that might be a suitable approach for some. Ultimately, it is about finding the best approach to moderate calorie intake that works for each person, as successful and sustained weight loss is about ensuring the diet is feasible to follow in the long-term.”
Differences in weight loss, diastolic BP, but not all measures
The study population included 90 adults seen at the Weight Loss Medicine clinic at the University of Alabama at Birmingham between August 2018 and December 2019. Participants had a body mass index of 30-60 kg/m2, and none had diabetes.
They were randomized to eTRE with the 7 AM to PM eating window or a control schedule with eating across 12 hours or more, mimicking U.S. median mealtimes, at least 6 days a week. All participants received 30-minute weight-loss counseling sessions at baseline and at weeks 2, 6, and 10 and were advised to follow a diet of 500 kcal/day below their resting energy expenditure and exercise 75-150 minutes per week.
The eTRE group adhered with their schedule a mean of 6 days per week, lower than the 6.3 days among controls (P = .03), and adherence declined by about 0.4 days per week in the eTRE group over the 14 weeks (P = .001).
At 14 weeks, both the eTRE group and controls had lost clinically meaningful amounts of weight, at –6.3 kg and –4.0 kg, respectively, but the –2.3 kg difference was significant (P = .002).
However, there was no difference in absolute fat loss (P = .09) or ratio of fat loss to weight loss (P = .43). There were also no significant differences in changes in other body composition parameters, including visceral fat and waist circumference.
Diastolic blood pressure was lowered by an additional 4 mmHg in the eTRE group, compared with controls at 14 weeks (P = .04), but there were no significant differences in systolic blood pressure, heart rate, glucose, A1c levels, insulin levels, measures of insulin resistance, or plasma lipids.
There were no differences between the two groups in self-reported physical activity, energy intake, or dietary macronutrient composition either. However, weight-loss modeling in 77 participants with at least two weight measurements indicated that the eTRE group reduced their intake by about 214 kcal/day, compared with controls (P = .04).
Those in the eTRE group also showed greater improvements in measures of mood disturbance, vigor-activity, fatigue-inertia, and depression-dejection. Other mood and sleep endpoints were similar between groups.
In a secondary analysis of just the 59 participants who completed the study, eTRE was also more effective at reducing body fat (P = .047) and trunk fat (P = .03).
About 41% of the eTRE completers planned to continue the practice after the study concluded.
The study was supported by grants from the National Center for Advancing Translational Sciences of the National Institutes of Health and the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Jamshed has reported no relevant financial relationships. Dr. Bhasin has reported receiving grants to his institution for research on which Dr. Bhasin is the principal investigator from AbbVie and MIB, receiving personal fees from OPKO and Aditum and holding equity interest in FPT and XYOne. Dr. Steenson has declared funding in support of the British Nutrition Foundation that comes from a range of sources.
A version of this article first appeared on Medscape.com.
FROM JAMA INTERNAL MEDICINE
Pigmented Papules on the Face, Neck, and Chest
The Diagnosis: Syringoma
Syringomas are benign adnexal tumors with distinct histopathologic features, including the characteristic comma- or tadpole-shaped tail comprised of dilated cystic eccrine ducts. Clinically, syringomas typically present predominantly in the periorbital region in adolescent girls. They may present as solitary or multiple lesions, and sites such as the genital area, palms, scalp, and chest rarely can be involved.1 Eruptive syringoma is a clinical subtype of syringoma that is seen on the face, neck, chest, and axillae that predominantly occurs in females with skin of color in countries such as Asia and Africa before or during puberty.2,3 Lesions appear as small, flesh-colored or slightly pigmented, flat-topped papules.3 The condition can be cosmetically disfiguring and difficult to treat, especially in patients with darker skin.
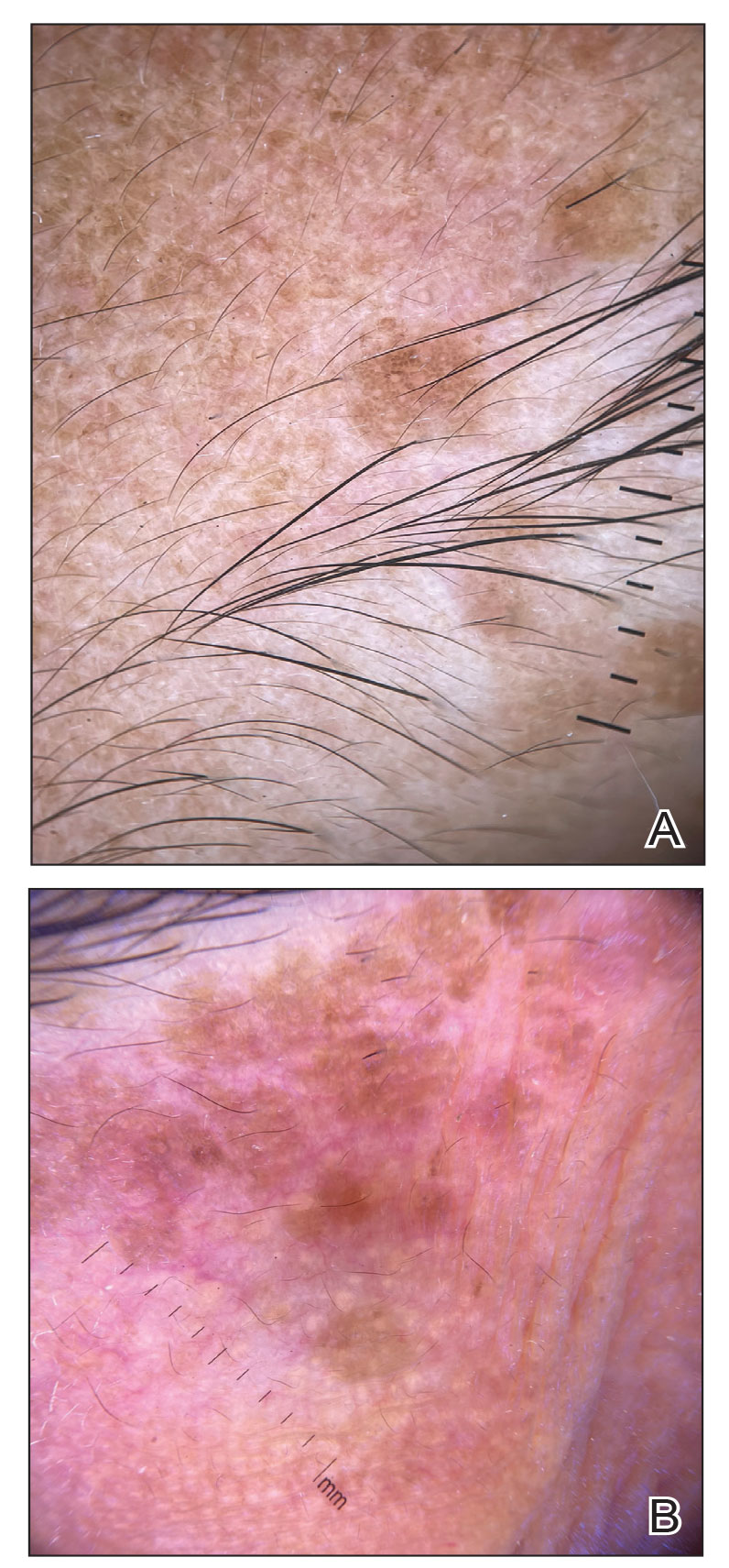
In our patient, dermoscopic evaluation revealed reticular light brown lines, structureless light brown areas, clustered brown dots, globules, and reticular vessels on a faint background (Figure 1A). Glittering yellow-whitish round structures over a fading pink-brown background also were seen at some sites (Figure 1B). Histologic examination of a neck lesion revealed an epidermis with focal acanthosis; the upper dermis had tumor islands and ducts with cells with round to vesicular nuclei and eosinophilic cytoplasm. A well-circumscribed tumor in the dermis composed of tubules of varying sizes lined by cuboidal cells was seen, consistent with syringoma (Figure 2).
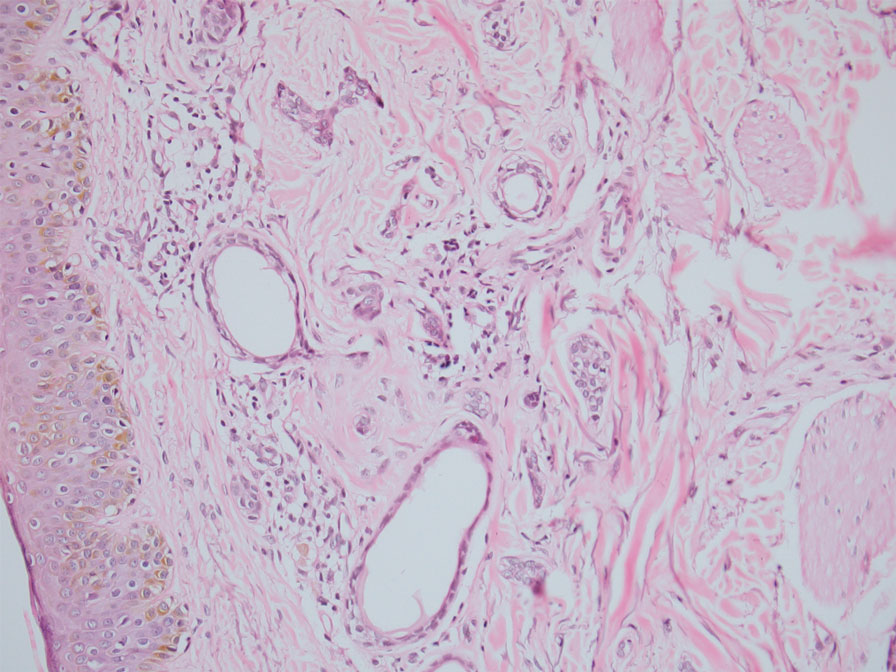
Dermoscopic features of syringomas have not been widely studied. Hayashi et al4 reported the dermoscopic features of unilateral linear syringomas as a delicate and faint reticular pigmentation network and multiple hypopigmented areas. Sakiyama et al5 also defined an incomplete pigment network with faint erythema in 2 eruptive syringoma cases.
Treatment of this condition is for cosmetic reasons only, and there are no reports of long-term morbidity associated with the disease.6,7 Multiple therapeutic options are available but are associated with complications such as hyperpigmentation and sclerosis in patients with skin of color due to the dermal location of these syringomas. Management of syringomas includes topical and surgical methods, including topical retinoids such as tretinoin and atropine solution 1%; surgical methods include dermabrasion, excision, cryotherapy, electrocautery, electrofulguration, laser therapy, and chemical cautery. However, there is a substantial risk for recurrence with these treatment options. In a case series of 5 patients with periorbital syringomas, treatment using radiofrequency and a CO2 laser was performed with favorable outcomes, highlighting the use of combination therapies for treatment.8 Seo et al9 reported a retrospective case series of 92 patients with periorbital syringomas in which they treated one group with CO2 laser and the other with botulinum toxin A injection; CO2 laser combined with botulinum toxin A showed a greater effect than laser treatment alone. The differential diagnosis includes pigmented plane warts, sebaceous hyperplasia, eruptive xanthomas, and hidrocystomas. Pigmented plane warts characteristically present as flat-topped papules with small hemorrhagic dots or tiny pinpoint vessels on dermoscopy. In sebaceous hyperplasia, yellowish umbilicated papular lesions are seen with crown vessels on dermoscopy. Eruptive xanthomas usually are erythematous to yellow, dome-shaped papules that appear mainly over the extensor aspects of the extremities. Hidrocystoma presents as a solitary translucent larger syringomalike lesion commonly seen in the periorbital region and/or on the cheeks.
We report a case of widespread syringomas with multiple close mimickers such as pigmented plane warts; however, dermoscopy of the lesions helped to arrive at the diagnosis. Dermatologists should be aware of this condition and its benign nature to ensure correct diagnosis and appropriate treatment.
- Williams K, Shinkai K. Evaluation and management of the patient with multiple syringomas: a systematic review of the literature. J Am Acad Dermatol. 2016;74:1234.e9-1240.e9.
- Tsunemi Y, Ihn H, Saeki H, et al. Generalized eruptive syringoma. Pediatr Dermatol. 2005;22:492-493.
- Singh S, Tewari R, Gupta S. An unusual case of generalised eruptive syringoma in an adult male. Med J Armed Forces India. 2014;70:389-391.
- Hayashi Y, Tanaka M, Nakajima S, et al. Unilateral linear syringoma in a Japanese female: dermoscopic differentiation from lichen lanus linearis. Dermatol Rep. 2011;3:E42.
- Sakiyama M, Maeda M, Fujimoto N, et al. Eruptive syringoma localized in intertriginous areas. J Dtsch Dermatol Ges. 2014;12:72-73.
- Wang JI, Roenigk HH Jr. Treatment of multiple facial syringomas with the carbon dioxide (CO2) laser. Dermatol Surg. 1999;25:136-139.
- Tsunemi Y, Ihn H, Saeki H, et al. Generalized eruptive syringoma. Pediatr Dermatol. 2005;22:492-493.
- Hasson A, Farias MM, Nicklas C, et al. Periorbital syringoma treated with radiofrequency and carbon dioxide (CO2) laser in 5 patients. J Drugs Dermatol. 2012;11:879-880.
- Seo HM, Choi JY, Min J, et al. Carbon dioxide laser combined with botulinum toxin A for patients with periorbital syringomas [published online March 31, 2016]. J Cosmet Laser Ther. 2016;18:149-153.
The Diagnosis: Syringoma
Syringomas are benign adnexal tumors with distinct histopathologic features, including the characteristic comma- or tadpole-shaped tail comprised of dilated cystic eccrine ducts. Clinically, syringomas typically present predominantly in the periorbital region in adolescent girls. They may present as solitary or multiple lesions, and sites such as the genital area, palms, scalp, and chest rarely can be involved.1 Eruptive syringoma is a clinical subtype of syringoma that is seen on the face, neck, chest, and axillae that predominantly occurs in females with skin of color in countries such as Asia and Africa before or during puberty.2,3 Lesions appear as small, flesh-colored or slightly pigmented, flat-topped papules.3 The condition can be cosmetically disfiguring and difficult to treat, especially in patients with darker skin.

In our patient, dermoscopic evaluation revealed reticular light brown lines, structureless light brown areas, clustered brown dots, globules, and reticular vessels on a faint background (Figure 1A). Glittering yellow-whitish round structures over a fading pink-brown background also were seen at some sites (Figure 1B). Histologic examination of a neck lesion revealed an epidermis with focal acanthosis; the upper dermis had tumor islands and ducts with cells with round to vesicular nuclei and eosinophilic cytoplasm. A well-circumscribed tumor in the dermis composed of tubules of varying sizes lined by cuboidal cells was seen, consistent with syringoma (Figure 2).

Dermoscopic features of syringomas have not been widely studied. Hayashi et al4 reported the dermoscopic features of unilateral linear syringomas as a delicate and faint reticular pigmentation network and multiple hypopigmented areas. Sakiyama et al5 also defined an incomplete pigment network with faint erythema in 2 eruptive syringoma cases.
Treatment of this condition is for cosmetic reasons only, and there are no reports of long-term morbidity associated with the disease.6,7 Multiple therapeutic options are available but are associated with complications such as hyperpigmentation and sclerosis in patients with skin of color due to the dermal location of these syringomas. Management of syringomas includes topical and surgical methods, including topical retinoids such as tretinoin and atropine solution 1%; surgical methods include dermabrasion, excision, cryotherapy, electrocautery, electrofulguration, laser therapy, and chemical cautery. However, there is a substantial risk for recurrence with these treatment options. In a case series of 5 patients with periorbital syringomas, treatment using radiofrequency and a CO2 laser was performed with favorable outcomes, highlighting the use of combination therapies for treatment.8 Seo et al9 reported a retrospective case series of 92 patients with periorbital syringomas in which they treated one group with CO2 laser and the other with botulinum toxin A injection; CO2 laser combined with botulinum toxin A showed a greater effect than laser treatment alone. The differential diagnosis includes pigmented plane warts, sebaceous hyperplasia, eruptive xanthomas, and hidrocystomas. Pigmented plane warts characteristically present as flat-topped papules with small hemorrhagic dots or tiny pinpoint vessels on dermoscopy. In sebaceous hyperplasia, yellowish umbilicated papular lesions are seen with crown vessels on dermoscopy. Eruptive xanthomas usually are erythematous to yellow, dome-shaped papules that appear mainly over the extensor aspects of the extremities. Hidrocystoma presents as a solitary translucent larger syringomalike lesion commonly seen in the periorbital region and/or on the cheeks.
We report a case of widespread syringomas with multiple close mimickers such as pigmented plane warts; however, dermoscopy of the lesions helped to arrive at the diagnosis. Dermatologists should be aware of this condition and its benign nature to ensure correct diagnosis and appropriate treatment.
The Diagnosis: Syringoma
Syringomas are benign adnexal tumors with distinct histopathologic features, including the characteristic comma- or tadpole-shaped tail comprised of dilated cystic eccrine ducts. Clinically, syringomas typically present predominantly in the periorbital region in adolescent girls. They may present as solitary or multiple lesions, and sites such as the genital area, palms, scalp, and chest rarely can be involved.1 Eruptive syringoma is a clinical subtype of syringoma that is seen on the face, neck, chest, and axillae that predominantly occurs in females with skin of color in countries such as Asia and Africa before or during puberty.2,3 Lesions appear as small, flesh-colored or slightly pigmented, flat-topped papules.3 The condition can be cosmetically disfiguring and difficult to treat, especially in patients with darker skin.

In our patient, dermoscopic evaluation revealed reticular light brown lines, structureless light brown areas, clustered brown dots, globules, and reticular vessels on a faint background (Figure 1A). Glittering yellow-whitish round structures over a fading pink-brown background also were seen at some sites (Figure 1B). Histologic examination of a neck lesion revealed an epidermis with focal acanthosis; the upper dermis had tumor islands and ducts with cells with round to vesicular nuclei and eosinophilic cytoplasm. A well-circumscribed tumor in the dermis composed of tubules of varying sizes lined by cuboidal cells was seen, consistent with syringoma (Figure 2).

Dermoscopic features of syringomas have not been widely studied. Hayashi et al4 reported the dermoscopic features of unilateral linear syringomas as a delicate and faint reticular pigmentation network and multiple hypopigmented areas. Sakiyama et al5 also defined an incomplete pigment network with faint erythema in 2 eruptive syringoma cases.
Treatment of this condition is for cosmetic reasons only, and there are no reports of long-term morbidity associated with the disease.6,7 Multiple therapeutic options are available but are associated with complications such as hyperpigmentation and sclerosis in patients with skin of color due to the dermal location of these syringomas. Management of syringomas includes topical and surgical methods, including topical retinoids such as tretinoin and atropine solution 1%; surgical methods include dermabrasion, excision, cryotherapy, electrocautery, electrofulguration, laser therapy, and chemical cautery. However, there is a substantial risk for recurrence with these treatment options. In a case series of 5 patients with periorbital syringomas, treatment using radiofrequency and a CO2 laser was performed with favorable outcomes, highlighting the use of combination therapies for treatment.8 Seo et al9 reported a retrospective case series of 92 patients with periorbital syringomas in which they treated one group with CO2 laser and the other with botulinum toxin A injection; CO2 laser combined with botulinum toxin A showed a greater effect than laser treatment alone. The differential diagnosis includes pigmented plane warts, sebaceous hyperplasia, eruptive xanthomas, and hidrocystomas. Pigmented plane warts characteristically present as flat-topped papules with small hemorrhagic dots or tiny pinpoint vessels on dermoscopy. In sebaceous hyperplasia, yellowish umbilicated papular lesions are seen with crown vessels on dermoscopy. Eruptive xanthomas usually are erythematous to yellow, dome-shaped papules that appear mainly over the extensor aspects of the extremities. Hidrocystoma presents as a solitary translucent larger syringomalike lesion commonly seen in the periorbital region and/or on the cheeks.
We report a case of widespread syringomas with multiple close mimickers such as pigmented plane warts; however, dermoscopy of the lesions helped to arrive at the diagnosis. Dermatologists should be aware of this condition and its benign nature to ensure correct diagnosis and appropriate treatment.
- Williams K, Shinkai K. Evaluation and management of the patient with multiple syringomas: a systematic review of the literature. J Am Acad Dermatol. 2016;74:1234.e9-1240.e9.
- Tsunemi Y, Ihn H, Saeki H, et al. Generalized eruptive syringoma. Pediatr Dermatol. 2005;22:492-493.
- Singh S, Tewari R, Gupta S. An unusual case of generalised eruptive syringoma in an adult male. Med J Armed Forces India. 2014;70:389-391.
- Hayashi Y, Tanaka M, Nakajima S, et al. Unilateral linear syringoma in a Japanese female: dermoscopic differentiation from lichen lanus linearis. Dermatol Rep. 2011;3:E42.
- Sakiyama M, Maeda M, Fujimoto N, et al. Eruptive syringoma localized in intertriginous areas. J Dtsch Dermatol Ges. 2014;12:72-73.
- Wang JI, Roenigk HH Jr. Treatment of multiple facial syringomas with the carbon dioxide (CO2) laser. Dermatol Surg. 1999;25:136-139.
- Tsunemi Y, Ihn H, Saeki H, et al. Generalized eruptive syringoma. Pediatr Dermatol. 2005;22:492-493.
- Hasson A, Farias MM, Nicklas C, et al. Periorbital syringoma treated with radiofrequency and carbon dioxide (CO2) laser in 5 patients. J Drugs Dermatol. 2012;11:879-880.
- Seo HM, Choi JY, Min J, et al. Carbon dioxide laser combined with botulinum toxin A for patients with periorbital syringomas [published online March 31, 2016]. J Cosmet Laser Ther. 2016;18:149-153.
- Williams K, Shinkai K. Evaluation and management of the patient with multiple syringomas: a systematic review of the literature. J Am Acad Dermatol. 2016;74:1234.e9-1240.e9.
- Tsunemi Y, Ihn H, Saeki H, et al. Generalized eruptive syringoma. Pediatr Dermatol. 2005;22:492-493.
- Singh S, Tewari R, Gupta S. An unusual case of generalised eruptive syringoma in an adult male. Med J Armed Forces India. 2014;70:389-391.
- Hayashi Y, Tanaka M, Nakajima S, et al. Unilateral linear syringoma in a Japanese female: dermoscopic differentiation from lichen lanus linearis. Dermatol Rep. 2011;3:E42.
- Sakiyama M, Maeda M, Fujimoto N, et al. Eruptive syringoma localized in intertriginous areas. J Dtsch Dermatol Ges. 2014;12:72-73.
- Wang JI, Roenigk HH Jr. Treatment of multiple facial syringomas with the carbon dioxide (CO2) laser. Dermatol Surg. 1999;25:136-139.
- Tsunemi Y, Ihn H, Saeki H, et al. Generalized eruptive syringoma. Pediatr Dermatol. 2005;22:492-493.
- Hasson A, Farias MM, Nicklas C, et al. Periorbital syringoma treated with radiofrequency and carbon dioxide (CO2) laser in 5 patients. J Drugs Dermatol. 2012;11:879-880.
- Seo HM, Choi JY, Min J, et al. Carbon dioxide laser combined with botulinum toxin A for patients with periorbital syringomas [published online March 31, 2016]. J Cosmet Laser Ther. 2016;18:149-153.
A 46-year-old woman presented with multiple asymptomatic, flesh-colored, hyperpigmented papules on the face of 5 to 6 months’ duration that were progressively increasing in number. The lesions first appeared near the eyebrows and cheeks (top) and subsequently spread to involve the neck. She had no notable medical history. Cutaneous examination revealed multiple tan to brown papules over the periorbital, malar, and neck regions ranging in size from 1 to 5 mm. The lesions over the periorbital region were arranged in a linear pattern (bottom). Similar lesions also were present on the chest and arms. No other sites were involved, and systemic examination was normal.
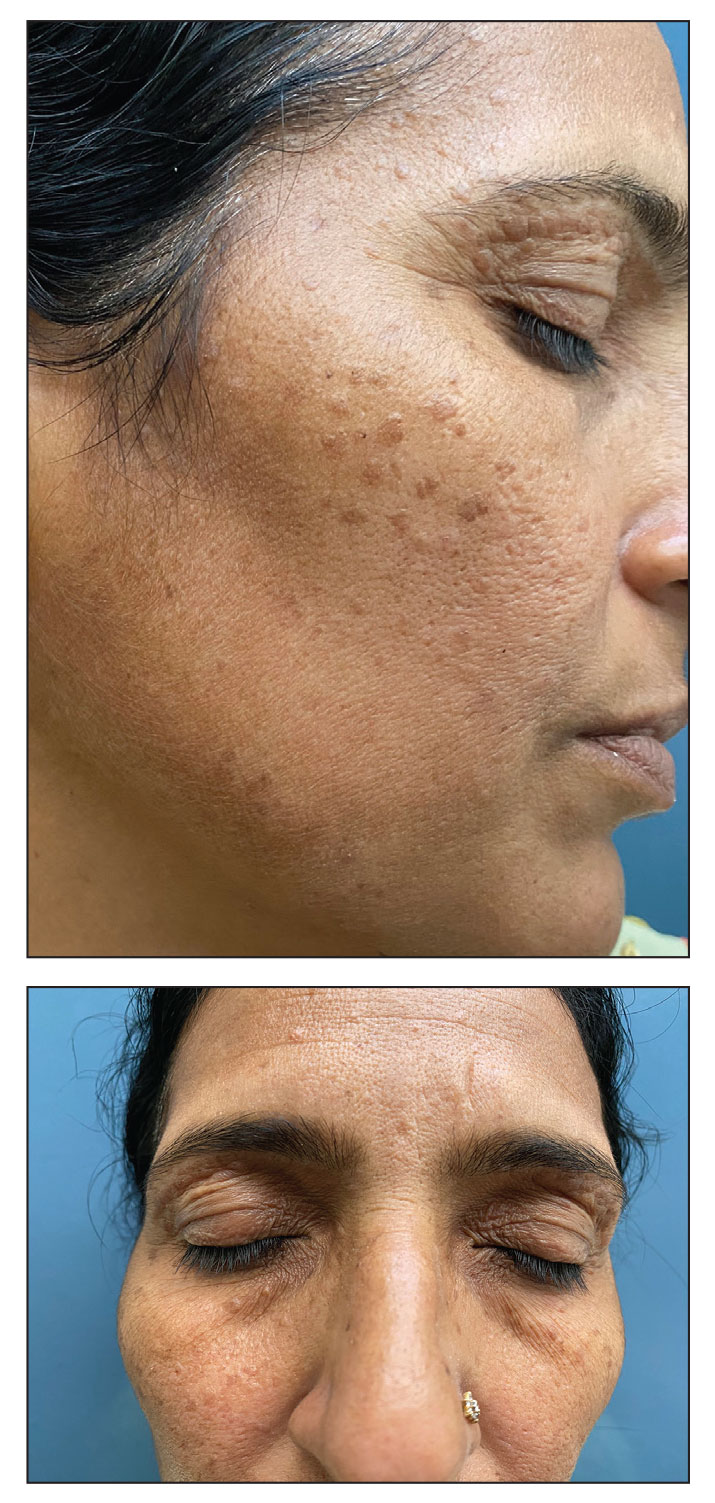
HCV reinfection uncommon among people who inject drugs
The findings, which are based on prospective data from 13 countries, including the United States, and were published in Annals of Internal Medicine (2022 Aug 8. doi: 10.7326/M21-4119), should encourage physicians to treat HCV in people with a history of injection drug use, said lead author Jason Grebely, PhD. They should also pressure payers to lift reimbursement restrictions on the same population.
“Direct-acting antiviral medications for HCV infection are safe and effective among people receiving OAT and people with recent injecting-drug use,” the investigators wrote. “Concerns remain, however, that HCV reinfection may reduce the benefits of cure among people who inject drugs and compromise HCV elimination efforts.”
They explored these concerns through a 3-year extension of the phase 3 CO-STAR trial that evaluated elbasvir and grazoprevir in people consistently taking OAT. Participants in the CO-STAR trial, which had a 96% sustained virologic response rate among those who completed therapy, could elect to participate in the present study, offering a prospective look at long-term reinfection.
Out of 296 participants in the CO-STAR trial, 286 were evaluable for reinfection and 199 enrolled in the present extension. The majority were White (79.4%) and male (75.9%), with most taking methadone (79%), followed by buprenorphine (20%). At 6 months, 40 out of 191 respondents (21%) reported injection-drug use in the previous month. At the 3-year mark, 26 out of 142 respondents (18%) disclosed injection-drug use in the previous month.
For all participants in the CO-STAR trial, the overall rate of reinfection at 3 years was 1.7 per 100 person-years (95% confidence interval, 0.8-3.0), which is lower than the rate reported in systematic reviews (3.8 per 100 person-years), according to the investigators.
In the extension analysis, the 3-year reinfection rate was lower still, at 1.2 per 100 person-years. The rate was slightly higher among people who reported injection-drug use in the previous month (1.9 per 100 person-years), and slightly lower among those who did not report injection-drug use in the prior month (0.5 per 100 person-years). More pronounced differences in reinfection were observed among participants who shared needles (6.4 per 100 person-years), versus those who didn’t share needles (1.5 per 100 person years).
Low reinfection rate may help facilitate removal of reimbursement restrictions
“Most of the reinfections in this study occurred within 24 weeks of completing treatment, suggesting that this is a key period for optimizing treatment of opioid use disorder and for providing access to needle and syringe programs that have documented benefits in preventing HCV transmission,” the investigators wrote.
This is one of the largest observational studies of its kind to date, bolstered by “excellent study retention” and a “well-characterized cohort,” with findings that should prompt real-world action, said Dr. Grebely, who is head of the hepatitis C and drug use group in the viral hepatitis clinical research program at the Kirby Institute, University of New South Wales, Sydney.
“Given that reinfection has often been cited ... by some providers as a reason for not offering treatment to people receiving OAT, the low reinfection rate in this study will be incredibly important for guiding practice and ensuring therapy is not withheld from this group,” Dr. Grebely said in an interview. “In terms of policy implications, these data may also help to facilitate the removal of reimbursement restrictions based on recent drug/alcohol use criteria that are in place among many payers in the United States.”
More research needed to determine optimal intervention strategies
Carl Latkin, PhD, professor and vice chair of the department of health, behavior, and society at Johns Hopkins University, Baltimore, called the present publication a “great article and well-done study with long-term follow-up.”
Dr. Latkin, who investigates biobehavioral interventions for disadvantaged communities, said the reported rate of reinfection is “very low among a group of current and former injectors.”
Affirming Dr. Grebely’s call for supportive practices by physicians and payers, Dr. Latkin said: “The study highlights the importance of improving access to medication for opioid use disorder. This level of treatment adherence in this group is much higher than for many other medications. Given these data, it would be difficult for payers to have a rational reason for blanket restrictions for HCV treatment among people who use drugs.”
Dr. Latkin explained that “it isn’t simply injection drug use per se” that drives HCV reinfection; instead, he cited social factors, such as lack of housing, as well as withdrawal symptoms, especially among those without access to medications for opioid use disorder (MOUD).
Dr. Latkin and Grebely also agreed that more research is needed to determine optimal intervention strategies.
Dr. Grebely called for one to enhance HCV testing and linkage to care, a topic he covered in a recent review article (Lancet Gastroenterol Hepatol. 2022 May;7[5]:426-45.).
Dr. Latkin said that, while it’s clear that “syringe services programs, accessible HCV treatment, and MOUD are needed,” it is unclear how much coverage is necessary for a given population.
Findings support critical nature of needle and syringe exchange programs
Sarah M. Kattakuzhy, MD, an associate professor in the division of clinical care & research at the Institute of Human Virology, University of Maryland, Baltimore, agreed that the findings “support the critical nature of needle and syringe exchange programs.”
“As most cities in the United States fall well below the high coverage needle and syringe program threshold required to maximally prevent disease transmission, the study serves as a push toward an evidence-based harm reduction policy,” she said.
Dr. Kattakuzhy he added that the study “supports the need to longitudinally engage individuals after HCV treatment to monitor reinfection risk behaviors and test for reinfection,” she continued.
When it came to translating all the data to populations in the United States, she offered a more guarded view.
“Critically, the study population included only individuals who were engaged with OAT and adherent for 3 or more months, selecting to a population of individuals with high adherence and engagement in care,” Dr. Kattakuzhy said in an interview. “As such, the study findings are not applicable to other cross sections of the drug-using community, including individuals not engaged in OAT, and cohorts with higher rates of ongoing injection drug use. Furthermore, there are known genetic impacts on spontaneous clearance, and emerging data on the immunology of reinfection.
“Studies with a focus on less engaged, higher-risk, and minority populations with active drug use are required to answer the remaining questions in HCV reinfection,” she said.
The study was supported by Merck, the Australian Government Department of Health, and the Australian National Health and Medical Research Council. Dr. Grebely disclosed receiving funding from Cepheid, the manufacturer of the Xpert HCV assay. The other investigators disclosed additional relationships with Gilead, AbbVie, Cepheid, and others. Dr. Latkin and Dr. Kattakuzhy disclosed no relevant conflicts of interest.
The findings, which are based on prospective data from 13 countries, including the United States, and were published in Annals of Internal Medicine (2022 Aug 8. doi: 10.7326/M21-4119), should encourage physicians to treat HCV in people with a history of injection drug use, said lead author Jason Grebely, PhD. They should also pressure payers to lift reimbursement restrictions on the same population.
“Direct-acting antiviral medications for HCV infection are safe and effective among people receiving OAT and people with recent injecting-drug use,” the investigators wrote. “Concerns remain, however, that HCV reinfection may reduce the benefits of cure among people who inject drugs and compromise HCV elimination efforts.”
They explored these concerns through a 3-year extension of the phase 3 CO-STAR trial that evaluated elbasvir and grazoprevir in people consistently taking OAT. Participants in the CO-STAR trial, which had a 96% sustained virologic response rate among those who completed therapy, could elect to participate in the present study, offering a prospective look at long-term reinfection.
Out of 296 participants in the CO-STAR trial, 286 were evaluable for reinfection and 199 enrolled in the present extension. The majority were White (79.4%) and male (75.9%), with most taking methadone (79%), followed by buprenorphine (20%). At 6 months, 40 out of 191 respondents (21%) reported injection-drug use in the previous month. At the 3-year mark, 26 out of 142 respondents (18%) disclosed injection-drug use in the previous month.
For all participants in the CO-STAR trial, the overall rate of reinfection at 3 years was 1.7 per 100 person-years (95% confidence interval, 0.8-3.0), which is lower than the rate reported in systematic reviews (3.8 per 100 person-years), according to the investigators.
In the extension analysis, the 3-year reinfection rate was lower still, at 1.2 per 100 person-years. The rate was slightly higher among people who reported injection-drug use in the previous month (1.9 per 100 person-years), and slightly lower among those who did not report injection-drug use in the prior month (0.5 per 100 person-years). More pronounced differences in reinfection were observed among participants who shared needles (6.4 per 100 person-years), versus those who didn’t share needles (1.5 per 100 person years).
Low reinfection rate may help facilitate removal of reimbursement restrictions
“Most of the reinfections in this study occurred within 24 weeks of completing treatment, suggesting that this is a key period for optimizing treatment of opioid use disorder and for providing access to needle and syringe programs that have documented benefits in preventing HCV transmission,” the investigators wrote.
This is one of the largest observational studies of its kind to date, bolstered by “excellent study retention” and a “well-characterized cohort,” with findings that should prompt real-world action, said Dr. Grebely, who is head of the hepatitis C and drug use group in the viral hepatitis clinical research program at the Kirby Institute, University of New South Wales, Sydney.
“Given that reinfection has often been cited ... by some providers as a reason for not offering treatment to people receiving OAT, the low reinfection rate in this study will be incredibly important for guiding practice and ensuring therapy is not withheld from this group,” Dr. Grebely said in an interview. “In terms of policy implications, these data may also help to facilitate the removal of reimbursement restrictions based on recent drug/alcohol use criteria that are in place among many payers in the United States.”
More research needed to determine optimal intervention strategies
Carl Latkin, PhD, professor and vice chair of the department of health, behavior, and society at Johns Hopkins University, Baltimore, called the present publication a “great article and well-done study with long-term follow-up.”
Dr. Latkin, who investigates biobehavioral interventions for disadvantaged communities, said the reported rate of reinfection is “very low among a group of current and former injectors.”
Affirming Dr. Grebely’s call for supportive practices by physicians and payers, Dr. Latkin said: “The study highlights the importance of improving access to medication for opioid use disorder. This level of treatment adherence in this group is much higher than for many other medications. Given these data, it would be difficult for payers to have a rational reason for blanket restrictions for HCV treatment among people who use drugs.”
Dr. Latkin explained that “it isn’t simply injection drug use per se” that drives HCV reinfection; instead, he cited social factors, such as lack of housing, as well as withdrawal symptoms, especially among those without access to medications for opioid use disorder (MOUD).
Dr. Latkin and Grebely also agreed that more research is needed to determine optimal intervention strategies.
Dr. Grebely called for one to enhance HCV testing and linkage to care, a topic he covered in a recent review article (Lancet Gastroenterol Hepatol. 2022 May;7[5]:426-45.).
Dr. Latkin said that, while it’s clear that “syringe services programs, accessible HCV treatment, and MOUD are needed,” it is unclear how much coverage is necessary for a given population.
Findings support critical nature of needle and syringe exchange programs
Sarah M. Kattakuzhy, MD, an associate professor in the division of clinical care & research at the Institute of Human Virology, University of Maryland, Baltimore, agreed that the findings “support the critical nature of needle and syringe exchange programs.”
“As most cities in the United States fall well below the high coverage needle and syringe program threshold required to maximally prevent disease transmission, the study serves as a push toward an evidence-based harm reduction policy,” she said.
Dr. Kattakuzhy he added that the study “supports the need to longitudinally engage individuals after HCV treatment to monitor reinfection risk behaviors and test for reinfection,” she continued.
When it came to translating all the data to populations in the United States, she offered a more guarded view.
“Critically, the study population included only individuals who were engaged with OAT and adherent for 3 or more months, selecting to a population of individuals with high adherence and engagement in care,” Dr. Kattakuzhy said in an interview. “As such, the study findings are not applicable to other cross sections of the drug-using community, including individuals not engaged in OAT, and cohorts with higher rates of ongoing injection drug use. Furthermore, there are known genetic impacts on spontaneous clearance, and emerging data on the immunology of reinfection.
“Studies with a focus on less engaged, higher-risk, and minority populations with active drug use are required to answer the remaining questions in HCV reinfection,” she said.
The study was supported by Merck, the Australian Government Department of Health, and the Australian National Health and Medical Research Council. Dr. Grebely disclosed receiving funding from Cepheid, the manufacturer of the Xpert HCV assay. The other investigators disclosed additional relationships with Gilead, AbbVie, Cepheid, and others. Dr. Latkin and Dr. Kattakuzhy disclosed no relevant conflicts of interest.
The findings, which are based on prospective data from 13 countries, including the United States, and were published in Annals of Internal Medicine (2022 Aug 8. doi: 10.7326/M21-4119), should encourage physicians to treat HCV in people with a history of injection drug use, said lead author Jason Grebely, PhD. They should also pressure payers to lift reimbursement restrictions on the same population.
“Direct-acting antiviral medications for HCV infection are safe and effective among people receiving OAT and people with recent injecting-drug use,” the investigators wrote. “Concerns remain, however, that HCV reinfection may reduce the benefits of cure among people who inject drugs and compromise HCV elimination efforts.”
They explored these concerns through a 3-year extension of the phase 3 CO-STAR trial that evaluated elbasvir and grazoprevir in people consistently taking OAT. Participants in the CO-STAR trial, which had a 96% sustained virologic response rate among those who completed therapy, could elect to participate in the present study, offering a prospective look at long-term reinfection.
Out of 296 participants in the CO-STAR trial, 286 were evaluable for reinfection and 199 enrolled in the present extension. The majority were White (79.4%) and male (75.9%), with most taking methadone (79%), followed by buprenorphine (20%). At 6 months, 40 out of 191 respondents (21%) reported injection-drug use in the previous month. At the 3-year mark, 26 out of 142 respondents (18%) disclosed injection-drug use in the previous month.
For all participants in the CO-STAR trial, the overall rate of reinfection at 3 years was 1.7 per 100 person-years (95% confidence interval, 0.8-3.0), which is lower than the rate reported in systematic reviews (3.8 per 100 person-years), according to the investigators.
In the extension analysis, the 3-year reinfection rate was lower still, at 1.2 per 100 person-years. The rate was slightly higher among people who reported injection-drug use in the previous month (1.9 per 100 person-years), and slightly lower among those who did not report injection-drug use in the prior month (0.5 per 100 person-years). More pronounced differences in reinfection were observed among participants who shared needles (6.4 per 100 person-years), versus those who didn’t share needles (1.5 per 100 person years).
Low reinfection rate may help facilitate removal of reimbursement restrictions
“Most of the reinfections in this study occurred within 24 weeks of completing treatment, suggesting that this is a key period for optimizing treatment of opioid use disorder and for providing access to needle and syringe programs that have documented benefits in preventing HCV transmission,” the investigators wrote.
This is one of the largest observational studies of its kind to date, bolstered by “excellent study retention” and a “well-characterized cohort,” with findings that should prompt real-world action, said Dr. Grebely, who is head of the hepatitis C and drug use group in the viral hepatitis clinical research program at the Kirby Institute, University of New South Wales, Sydney.
“Given that reinfection has often been cited ... by some providers as a reason for not offering treatment to people receiving OAT, the low reinfection rate in this study will be incredibly important for guiding practice and ensuring therapy is not withheld from this group,” Dr. Grebely said in an interview. “In terms of policy implications, these data may also help to facilitate the removal of reimbursement restrictions based on recent drug/alcohol use criteria that are in place among many payers in the United States.”
More research needed to determine optimal intervention strategies
Carl Latkin, PhD, professor and vice chair of the department of health, behavior, and society at Johns Hopkins University, Baltimore, called the present publication a “great article and well-done study with long-term follow-up.”
Dr. Latkin, who investigates biobehavioral interventions for disadvantaged communities, said the reported rate of reinfection is “very low among a group of current and former injectors.”
Affirming Dr. Grebely’s call for supportive practices by physicians and payers, Dr. Latkin said: “The study highlights the importance of improving access to medication for opioid use disorder. This level of treatment adherence in this group is much higher than for many other medications. Given these data, it would be difficult for payers to have a rational reason for blanket restrictions for HCV treatment among people who use drugs.”
Dr. Latkin explained that “it isn’t simply injection drug use per se” that drives HCV reinfection; instead, he cited social factors, such as lack of housing, as well as withdrawal symptoms, especially among those without access to medications for opioid use disorder (MOUD).
Dr. Latkin and Grebely also agreed that more research is needed to determine optimal intervention strategies.
Dr. Grebely called for one to enhance HCV testing and linkage to care, a topic he covered in a recent review article (Lancet Gastroenterol Hepatol. 2022 May;7[5]:426-45.).
Dr. Latkin said that, while it’s clear that “syringe services programs, accessible HCV treatment, and MOUD are needed,” it is unclear how much coverage is necessary for a given population.
Findings support critical nature of needle and syringe exchange programs
Sarah M. Kattakuzhy, MD, an associate professor in the division of clinical care & research at the Institute of Human Virology, University of Maryland, Baltimore, agreed that the findings “support the critical nature of needle and syringe exchange programs.”
“As most cities in the United States fall well below the high coverage needle and syringe program threshold required to maximally prevent disease transmission, the study serves as a push toward an evidence-based harm reduction policy,” she said.
Dr. Kattakuzhy he added that the study “supports the need to longitudinally engage individuals after HCV treatment to monitor reinfection risk behaviors and test for reinfection,” she continued.
When it came to translating all the data to populations in the United States, she offered a more guarded view.
“Critically, the study population included only individuals who were engaged with OAT and adherent for 3 or more months, selecting to a population of individuals with high adherence and engagement in care,” Dr. Kattakuzhy said in an interview. “As such, the study findings are not applicable to other cross sections of the drug-using community, including individuals not engaged in OAT, and cohorts with higher rates of ongoing injection drug use. Furthermore, there are known genetic impacts on spontaneous clearance, and emerging data on the immunology of reinfection.
“Studies with a focus on less engaged, higher-risk, and minority populations with active drug use are required to answer the remaining questions in HCV reinfection,” she said.
The study was supported by Merck, the Australian Government Department of Health, and the Australian National Health and Medical Research Council. Dr. Grebely disclosed receiving funding from Cepheid, the manufacturer of the Xpert HCV assay. The other investigators disclosed additional relationships with Gilead, AbbVie, Cepheid, and others. Dr. Latkin and Dr. Kattakuzhy disclosed no relevant conflicts of interest.
FROM ANNALS OF INTERNAL MEDICINE