User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
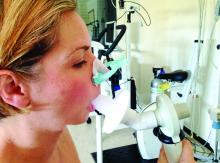
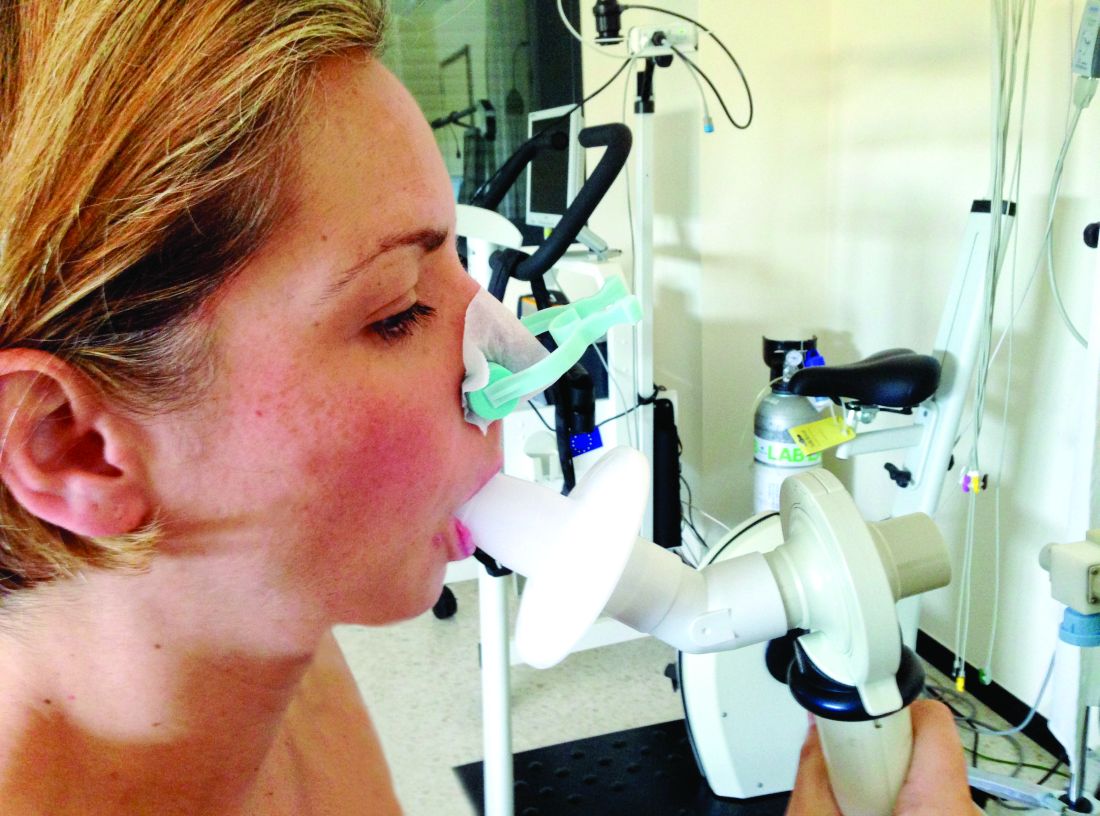
Race-specific spirometry may miss emphysema diagnoses
An overreliance on spirometry to identify emphysema led to missed cases in Black individuals, particularly men, based on a secondary data analysis of 2,674 people.
“Over the last few years, there has been growing debate around the use of race adjustment in diagnostic algorithms and equations commonly used in medicine,” lead author Gabrielle Yi-Hui Liu, MD, said in an interview. “Whereas, previously it was common to accept racial or ethnic differences in clinical measures and outcomes as inherent differences among populations, there is now more recognition of how racism, socioeconomic status, and environmental exposures can cause these racial differences. Our initial interest in this study was to examine how the use of race-specific spirometry reference equations, and the use of spirometry in general, may be contributing to racial disparities.”
“Previous studies have suggested that the use of race-specific equations in spirometry can exacerbate racial inequities in healthcare outcomes by under-recognition of early disease in Black adults, and this study adds to that evidence,” said Suman Pal, MBBS, of the University of New Mexico, Albuquerque, in an interview.
“By examining the crucial ways in which systemic factors in medicine, such as race-specific equations, exacerbate racial inequities in healthcare, this study is a timely analysis in a moment of national reckoning of structural racism,” said Dr. Pal, who was not involved in the study.
In a study published in Annals of Internal Medicine, Dr. Liu and colleagues at Northwestern University, Chicago, conducted a secondary analysis of data from the CARDIA Lung study (Coronary Artery Risk Development In Young Adults).
The primary outcome of the study was the prevalence of emphysema among participants with various measures of normal spirometry results, stratified by sex and race. The normal results included an forced expiratory volume in 1 second (FEV1)–forced vital capacity (FVC) ratio greater than or equal to 0.7 or greater than or equal to the lower limit of normal. The participants also were stratified by FEV1 percent predicted, using race-specific reference equations, for FEV1 between 80% and 99% of predicted, or an FEV1 between 100% and 120% of predicted.
The study population included 485 Black men, 762 Black women, 659 White men, and 768 White women who received both a CT scan (in 2010-2011) and spirometry (obtained in 2015-2016) in the CARDIA study. The mean age of the participants at the spirometry exam was 55 years.
A total of 5.3% of the participants had emphysema after stratifying by FEV1-FVC ratio. The prevalence was significantly higher for Black men, compared with White men (12.3% vs. 4.0%; relative risk, 3.0), and for Black women, compared with White women (5.0% vs. 2.6%; RR, 1.9).
The association between Black race and emphysema risk persisted but decreased when the researchers used a race-neutral estimate.
When the participants were stratified by race-specific FEV1 percent predicted, 6.5% of individuals with a race-specific FEV1 between 80% and 99% had emphysema. After controlling for factors including age and smoking, emphysema was significantly more prevalent in Black men versus White men (15.5% vs. 4.0%) and in Black women, compared with White women (6.6% vs. 3.4%).
The racial difference persisted in men with a race-specific FEV1 between 100% and 120% of predicted. Of these, 4.0% had emphysema. The prevalence was significantly higher in Black men, compared with White men (13.9% vs. 2.2%), but similar between Black women and White women (2.6% vs. 2.0%).
The use of race-neutral equations reduced, but did not eliminate, these disparities, the researchers said.
The findings were limited by the lack of CT imaging data from the same visit as the final spirometry collection, the researchers noted. “Given that imaging was obtained 5 years before spirometry and emphysema is an irreversible finding, this may have led to an overall underestimation of the prevalence of emphysema.”
Spirometry alone misses cases
“We were surprised by the substantial rates of emphysema we saw among Black men in our cohort with normal spirometry,” Dr. Liu said in an interview. “We did not expect to find than more than one in eight Black men with an FEV1 between 100% and 120% predicted would have emphysema – a rate more than six times higher than White men with the same range of FEV1.”
“One takeaway is that we are likely missing a lot of people with impaired respiratory health or true lung disease by only using spirometry to diagnose COPD,” said Dr. Liu. In clinical practice, “physicians should consider ordering CT scans on patients with normal spirometry who have respiratory symptoms such as cough or shortness of breath. If emphysema is found, physicians should discuss mitigating any potential risk factors and consider the use of COPD medications such as inhalers.
“Our findings also support using race-neutral reference equations to interpret spirometry instead of race-specific equations. Racial disparities in rates of emphysema among those with ‘normal’ FEV1 [between 80% and 120% predicted], were attenuated or eliminated when race-neutral equations were used to calculate FEV1. This suggests that race-specific equations are normalizing worse lung health in Black adults,” Dr. Liu explained.
“We need to continue research into additional tools that can be used to assess respiratory health and diagnose COPD, while keeping in mind how these tools may affect racial disparities,” said Dr. Liu. “Our study suggests that our reliance on spirometry measures such as FEV1/FVC ratio and FEV1 is missing a number of people with respiratory symptoms and CT evidence of lung disease, and that this is disproportionately affecting Black adults in the United States.” Looking ahead, “it is important to find better tools to identify people with impaired respiratory health or early manifestations of disease so we can intercept chronic lung disease before it becomes clinically apparent and patients have sustained significant lung damage.”
The CARDIA study was supported by the National Heart, Lung, and Blood Institute in collaboration with the University of Alabama at Birmingham, Northwestern University, the University of Minnesota, and the Kaiser Foundation Research Institute. Dr. Liu was supported by a grant from the National Institutes of Health. The researchers had no financial conflicts to disclose. Dr. Pal had no financial conflicts to disclose.
*This article was updated 7/22/2022.
An overreliance on spirometry to identify emphysema led to missed cases in Black individuals, particularly men, based on a secondary data analysis of 2,674 people.
“Over the last few years, there has been growing debate around the use of race adjustment in diagnostic algorithms and equations commonly used in medicine,” lead author Gabrielle Yi-Hui Liu, MD, said in an interview. “Whereas, previously it was common to accept racial or ethnic differences in clinical measures and outcomes as inherent differences among populations, there is now more recognition of how racism, socioeconomic status, and environmental exposures can cause these racial differences. Our initial interest in this study was to examine how the use of race-specific spirometry reference equations, and the use of spirometry in general, may be contributing to racial disparities.”
“Previous studies have suggested that the use of race-specific equations in spirometry can exacerbate racial inequities in healthcare outcomes by under-recognition of early disease in Black adults, and this study adds to that evidence,” said Suman Pal, MBBS, of the University of New Mexico, Albuquerque, in an interview.
“By examining the crucial ways in which systemic factors in medicine, such as race-specific equations, exacerbate racial inequities in healthcare, this study is a timely analysis in a moment of national reckoning of structural racism,” said Dr. Pal, who was not involved in the study.
In a study published in Annals of Internal Medicine, Dr. Liu and colleagues at Northwestern University, Chicago, conducted a secondary analysis of data from the CARDIA Lung study (Coronary Artery Risk Development In Young Adults).
The primary outcome of the study was the prevalence of emphysema among participants with various measures of normal spirometry results, stratified by sex and race. The normal results included an forced expiratory volume in 1 second (FEV1)–forced vital capacity (FVC) ratio greater than or equal to 0.7 or greater than or equal to the lower limit of normal. The participants also were stratified by FEV1 percent predicted, using race-specific reference equations, for FEV1 between 80% and 99% of predicted, or an FEV1 between 100% and 120% of predicted.
The study population included 485 Black men, 762 Black women, 659 White men, and 768 White women who received both a CT scan (in 2010-2011) and spirometry (obtained in 2015-2016) in the CARDIA study. The mean age of the participants at the spirometry exam was 55 years.
A total of 5.3% of the participants had emphysema after stratifying by FEV1-FVC ratio. The prevalence was significantly higher for Black men, compared with White men (12.3% vs. 4.0%; relative risk, 3.0), and for Black women, compared with White women (5.0% vs. 2.6%; RR, 1.9).
The association between Black race and emphysema risk persisted but decreased when the researchers used a race-neutral estimate.
When the participants were stratified by race-specific FEV1 percent predicted, 6.5% of individuals with a race-specific FEV1 between 80% and 99% had emphysema. After controlling for factors including age and smoking, emphysema was significantly more prevalent in Black men versus White men (15.5% vs. 4.0%) and in Black women, compared with White women (6.6% vs. 3.4%).
The racial difference persisted in men with a race-specific FEV1 between 100% and 120% of predicted. Of these, 4.0% had emphysema. The prevalence was significantly higher in Black men, compared with White men (13.9% vs. 2.2%), but similar between Black women and White women (2.6% vs. 2.0%).
The use of race-neutral equations reduced, but did not eliminate, these disparities, the researchers said.
The findings were limited by the lack of CT imaging data from the same visit as the final spirometry collection, the researchers noted. “Given that imaging was obtained 5 years before spirometry and emphysema is an irreversible finding, this may have led to an overall underestimation of the prevalence of emphysema.”
Spirometry alone misses cases
“We were surprised by the substantial rates of emphysema we saw among Black men in our cohort with normal spirometry,” Dr. Liu said in an interview. “We did not expect to find than more than one in eight Black men with an FEV1 between 100% and 120% predicted would have emphysema – a rate more than six times higher than White men with the same range of FEV1.”
“One takeaway is that we are likely missing a lot of people with impaired respiratory health or true lung disease by only using spirometry to diagnose COPD,” said Dr. Liu. In clinical practice, “physicians should consider ordering CT scans on patients with normal spirometry who have respiratory symptoms such as cough or shortness of breath. If emphysema is found, physicians should discuss mitigating any potential risk factors and consider the use of COPD medications such as inhalers.
“Our findings also support using race-neutral reference equations to interpret spirometry instead of race-specific equations. Racial disparities in rates of emphysema among those with ‘normal’ FEV1 [between 80% and 120% predicted], were attenuated or eliminated when race-neutral equations were used to calculate FEV1. This suggests that race-specific equations are normalizing worse lung health in Black adults,” Dr. Liu explained.
“We need to continue research into additional tools that can be used to assess respiratory health and diagnose COPD, while keeping in mind how these tools may affect racial disparities,” said Dr. Liu. “Our study suggests that our reliance on spirometry measures such as FEV1/FVC ratio and FEV1 is missing a number of people with respiratory symptoms and CT evidence of lung disease, and that this is disproportionately affecting Black adults in the United States.” Looking ahead, “it is important to find better tools to identify people with impaired respiratory health or early manifestations of disease so we can intercept chronic lung disease before it becomes clinically apparent and patients have sustained significant lung damage.”
The CARDIA study was supported by the National Heart, Lung, and Blood Institute in collaboration with the University of Alabama at Birmingham, Northwestern University, the University of Minnesota, and the Kaiser Foundation Research Institute. Dr. Liu was supported by a grant from the National Institutes of Health. The researchers had no financial conflicts to disclose. Dr. Pal had no financial conflicts to disclose.
*This article was updated 7/22/2022.
An overreliance on spirometry to identify emphysema led to missed cases in Black individuals, particularly men, based on a secondary data analysis of 2,674 people.
“Over the last few years, there has been growing debate around the use of race adjustment in diagnostic algorithms and equations commonly used in medicine,” lead author Gabrielle Yi-Hui Liu, MD, said in an interview. “Whereas, previously it was common to accept racial or ethnic differences in clinical measures and outcomes as inherent differences among populations, there is now more recognition of how racism, socioeconomic status, and environmental exposures can cause these racial differences. Our initial interest in this study was to examine how the use of race-specific spirometry reference equations, and the use of spirometry in general, may be contributing to racial disparities.”
“Previous studies have suggested that the use of race-specific equations in spirometry can exacerbate racial inequities in healthcare outcomes by under-recognition of early disease in Black adults, and this study adds to that evidence,” said Suman Pal, MBBS, of the University of New Mexico, Albuquerque, in an interview.
“By examining the crucial ways in which systemic factors in medicine, such as race-specific equations, exacerbate racial inequities in healthcare, this study is a timely analysis in a moment of national reckoning of structural racism,” said Dr. Pal, who was not involved in the study.
In a study published in Annals of Internal Medicine, Dr. Liu and colleagues at Northwestern University, Chicago, conducted a secondary analysis of data from the CARDIA Lung study (Coronary Artery Risk Development In Young Adults).
The primary outcome of the study was the prevalence of emphysema among participants with various measures of normal spirometry results, stratified by sex and race. The normal results included an forced expiratory volume in 1 second (FEV1)–forced vital capacity (FVC) ratio greater than or equal to 0.7 or greater than or equal to the lower limit of normal. The participants also were stratified by FEV1 percent predicted, using race-specific reference equations, for FEV1 between 80% and 99% of predicted, or an FEV1 between 100% and 120% of predicted.
The study population included 485 Black men, 762 Black women, 659 White men, and 768 White women who received both a CT scan (in 2010-2011) and spirometry (obtained in 2015-2016) in the CARDIA study. The mean age of the participants at the spirometry exam was 55 years.
A total of 5.3% of the participants had emphysema after stratifying by FEV1-FVC ratio. The prevalence was significantly higher for Black men, compared with White men (12.3% vs. 4.0%; relative risk, 3.0), and for Black women, compared with White women (5.0% vs. 2.6%; RR, 1.9).
The association between Black race and emphysema risk persisted but decreased when the researchers used a race-neutral estimate.
When the participants were stratified by race-specific FEV1 percent predicted, 6.5% of individuals with a race-specific FEV1 between 80% and 99% had emphysema. After controlling for factors including age and smoking, emphysema was significantly more prevalent in Black men versus White men (15.5% vs. 4.0%) and in Black women, compared with White women (6.6% vs. 3.4%).
The racial difference persisted in men with a race-specific FEV1 between 100% and 120% of predicted. Of these, 4.0% had emphysema. The prevalence was significantly higher in Black men, compared with White men (13.9% vs. 2.2%), but similar between Black women and White women (2.6% vs. 2.0%).
The use of race-neutral equations reduced, but did not eliminate, these disparities, the researchers said.
The findings were limited by the lack of CT imaging data from the same visit as the final spirometry collection, the researchers noted. “Given that imaging was obtained 5 years before spirometry and emphysema is an irreversible finding, this may have led to an overall underestimation of the prevalence of emphysema.”
Spirometry alone misses cases
“We were surprised by the substantial rates of emphysema we saw among Black men in our cohort with normal spirometry,” Dr. Liu said in an interview. “We did not expect to find than more than one in eight Black men with an FEV1 between 100% and 120% predicted would have emphysema – a rate more than six times higher than White men with the same range of FEV1.”
“One takeaway is that we are likely missing a lot of people with impaired respiratory health or true lung disease by only using spirometry to diagnose COPD,” said Dr. Liu. In clinical practice, “physicians should consider ordering CT scans on patients with normal spirometry who have respiratory symptoms such as cough or shortness of breath. If emphysema is found, physicians should discuss mitigating any potential risk factors and consider the use of COPD medications such as inhalers.
“Our findings also support using race-neutral reference equations to interpret spirometry instead of race-specific equations. Racial disparities in rates of emphysema among those with ‘normal’ FEV1 [between 80% and 120% predicted], were attenuated or eliminated when race-neutral equations were used to calculate FEV1. This suggests that race-specific equations are normalizing worse lung health in Black adults,” Dr. Liu explained.
“We need to continue research into additional tools that can be used to assess respiratory health and diagnose COPD, while keeping in mind how these tools may affect racial disparities,” said Dr. Liu. “Our study suggests that our reliance on spirometry measures such as FEV1/FVC ratio and FEV1 is missing a number of people with respiratory symptoms and CT evidence of lung disease, and that this is disproportionately affecting Black adults in the United States.” Looking ahead, “it is important to find better tools to identify people with impaired respiratory health or early manifestations of disease so we can intercept chronic lung disease before it becomes clinically apparent and patients have sustained significant lung damage.”
The CARDIA study was supported by the National Heart, Lung, and Blood Institute in collaboration with the University of Alabama at Birmingham, Northwestern University, the University of Minnesota, and the Kaiser Foundation Research Institute. Dr. Liu was supported by a grant from the National Institutes of Health. The researchers had no financial conflicts to disclose. Dr. Pal had no financial conflicts to disclose.
*This article was updated 7/22/2022.
FROM ANNALS OF INTERNAL MEDICINE
Nurses’ cohort study: Endometriosis elevates stroke risk
Women who’ve had endometriosis carry an elevated risk of stroke with them for the rest of their lives, with the greatest risk found in women who’ve had a hysterectomy with an oophorectomy, according to a cohort study of the Nurses’ Health Study.
“This is yet additional evidence that those girls and women with endometriosis are having effects across their lives and in multiple aspects of their health and well-being,” senior study author Stacey A. Missmer, ScD, of the Michigan State University, East Lansing, said in an interview. “This is not, in quotes ‘just a gynecologic condition,’ ” Dr. Missmer added. “It is not strictly about the pelvic pain or infertility, but it really is about the whole health across the life course.”
The study included 112,056 women in the NHSII cohort study who were followed from 1989 to June 2017, documenting 893 incident cases of stroke among them – an incidence of less than 1%. Endometriosis was reported in 5,244 women, and 93% of the cohort were White.
Multivariate adjusted models showed that women who had laparoscopically confirmed endometriosis had a 34% greater risk of stroke than women without a history of endometriosis. Leslie V. Farland, ScD, of the University of Arizona, Tucson, was lead author of the study.
While previous studies have demonstrated an increased risk of cardiovascular disease, heart attack, angina, and atherosclerosis in women who’ve had endometriosis, this is the first study that has confirmed an additional increased risk of stroke, Dr. Missmer said.
Another novel finding, Dr. Missmer said, is that while the CVD risks for these women “seem to peak at an earlier age,” the study found no age differences for stroke risk. “That also reinforces that these stroke events are often happening in an age range typical for stroke, which is further removed from when women are thinking about their gynecologic health specifically.”
These findings don’t translate into a significantly greater risk for stroke overall in women who’ve had endometriosis, Dr. Missmer said. She characterized the risk as “not negligible, but it’s not a huge increased risk.” The absolute risk is still fairly low, she said.
“We don’t want to give the impression that all women with endometriosis need to be panicked or fearful about stroke, she said. “Rather, the messaging is that this yet another bit of evidence that whole health care for those with endometriosis is important.”
Women who’ve had endometriosis and their primary care providers need to be attuned to stroke risk, she said. “This is a critical condition that primary care physicians need to engage around, and perhaps if symptoms related to cardiovascular and cerebrovascular disease emerge in their patients, they need to be engaging cardiology and similar types of support. This is not just about the gynecologists.”
The study also explored other factors that may contribute to stroke risk, with the most significant being hysterectomy with bilateral oophorectomy, Dr. Missmer said.
This study was unique because it used laparoscopically confirmed rather than self-reported endometriosis, said Louise D. McCullough, MD, neurology chair at the University of Texas Health Science Center, Houston. Another strength of the study she noted was its longitudinal design, although the cohort study design yielded a low number of stroke patients.
“Regardless, I do think it was a very important study because we have a growing recognition about how women’s health and factors such as pregnancy, infertility, parity, complications, and gonadal hormones such as estrogen can influence a woman’s stroke risk much later in life,” Dr. McCullough said in an interview.
Future studies into the relationship between endometriosis and CVD and stroke risk should focus on the mechanism behind the inflammation that occurs in endometriosis, Dr. McCullough said. “Part of it is probably the loss of hormones if a patient has to have an oophorectomy, but part of it is just what do these diseases do for a woman’s later risk – and for primary care physicians, ob.gyns., and stroke neurologists to recognize that these are questions we should ask: Have you ever had eclampsia or preeclampsia? Did you have endometriosis? Have you had miscarriages?”
The study received funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute for Neurological Disorders and Stroke. Dr. Missmer disclosed relationships with Shanghai Huilun Biotechnology, Roche, and AbbVie. Dr. McCullough has no relevant disclosures.
Women who’ve had endometriosis carry an elevated risk of stroke with them for the rest of their lives, with the greatest risk found in women who’ve had a hysterectomy with an oophorectomy, according to a cohort study of the Nurses’ Health Study.
“This is yet additional evidence that those girls and women with endometriosis are having effects across their lives and in multiple aspects of their health and well-being,” senior study author Stacey A. Missmer, ScD, of the Michigan State University, East Lansing, said in an interview. “This is not, in quotes ‘just a gynecologic condition,’ ” Dr. Missmer added. “It is not strictly about the pelvic pain or infertility, but it really is about the whole health across the life course.”
The study included 112,056 women in the NHSII cohort study who were followed from 1989 to June 2017, documenting 893 incident cases of stroke among them – an incidence of less than 1%. Endometriosis was reported in 5,244 women, and 93% of the cohort were White.
Multivariate adjusted models showed that women who had laparoscopically confirmed endometriosis had a 34% greater risk of stroke than women without a history of endometriosis. Leslie V. Farland, ScD, of the University of Arizona, Tucson, was lead author of the study.
While previous studies have demonstrated an increased risk of cardiovascular disease, heart attack, angina, and atherosclerosis in women who’ve had endometriosis, this is the first study that has confirmed an additional increased risk of stroke, Dr. Missmer said.
Another novel finding, Dr. Missmer said, is that while the CVD risks for these women “seem to peak at an earlier age,” the study found no age differences for stroke risk. “That also reinforces that these stroke events are often happening in an age range typical for stroke, which is further removed from when women are thinking about their gynecologic health specifically.”
These findings don’t translate into a significantly greater risk for stroke overall in women who’ve had endometriosis, Dr. Missmer said. She characterized the risk as “not negligible, but it’s not a huge increased risk.” The absolute risk is still fairly low, she said.
“We don’t want to give the impression that all women with endometriosis need to be panicked or fearful about stroke, she said. “Rather, the messaging is that this yet another bit of evidence that whole health care for those with endometriosis is important.”
Women who’ve had endometriosis and their primary care providers need to be attuned to stroke risk, she said. “This is a critical condition that primary care physicians need to engage around, and perhaps if symptoms related to cardiovascular and cerebrovascular disease emerge in their patients, they need to be engaging cardiology and similar types of support. This is not just about the gynecologists.”
The study also explored other factors that may contribute to stroke risk, with the most significant being hysterectomy with bilateral oophorectomy, Dr. Missmer said.
This study was unique because it used laparoscopically confirmed rather than self-reported endometriosis, said Louise D. McCullough, MD, neurology chair at the University of Texas Health Science Center, Houston. Another strength of the study she noted was its longitudinal design, although the cohort study design yielded a low number of stroke patients.
“Regardless, I do think it was a very important study because we have a growing recognition about how women’s health and factors such as pregnancy, infertility, parity, complications, and gonadal hormones such as estrogen can influence a woman’s stroke risk much later in life,” Dr. McCullough said in an interview.
Future studies into the relationship between endometriosis and CVD and stroke risk should focus on the mechanism behind the inflammation that occurs in endometriosis, Dr. McCullough said. “Part of it is probably the loss of hormones if a patient has to have an oophorectomy, but part of it is just what do these diseases do for a woman’s later risk – and for primary care physicians, ob.gyns., and stroke neurologists to recognize that these are questions we should ask: Have you ever had eclampsia or preeclampsia? Did you have endometriosis? Have you had miscarriages?”
The study received funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute for Neurological Disorders and Stroke. Dr. Missmer disclosed relationships with Shanghai Huilun Biotechnology, Roche, and AbbVie. Dr. McCullough has no relevant disclosures.
Women who’ve had endometriosis carry an elevated risk of stroke with them for the rest of their lives, with the greatest risk found in women who’ve had a hysterectomy with an oophorectomy, according to a cohort study of the Nurses’ Health Study.
“This is yet additional evidence that those girls and women with endometriosis are having effects across their lives and in multiple aspects of their health and well-being,” senior study author Stacey A. Missmer, ScD, of the Michigan State University, East Lansing, said in an interview. “This is not, in quotes ‘just a gynecologic condition,’ ” Dr. Missmer added. “It is not strictly about the pelvic pain or infertility, but it really is about the whole health across the life course.”
The study included 112,056 women in the NHSII cohort study who were followed from 1989 to June 2017, documenting 893 incident cases of stroke among them – an incidence of less than 1%. Endometriosis was reported in 5,244 women, and 93% of the cohort were White.
Multivariate adjusted models showed that women who had laparoscopically confirmed endometriosis had a 34% greater risk of stroke than women without a history of endometriosis. Leslie V. Farland, ScD, of the University of Arizona, Tucson, was lead author of the study.
While previous studies have demonstrated an increased risk of cardiovascular disease, heart attack, angina, and atherosclerosis in women who’ve had endometriosis, this is the first study that has confirmed an additional increased risk of stroke, Dr. Missmer said.
Another novel finding, Dr. Missmer said, is that while the CVD risks for these women “seem to peak at an earlier age,” the study found no age differences for stroke risk. “That also reinforces that these stroke events are often happening in an age range typical for stroke, which is further removed from when women are thinking about their gynecologic health specifically.”
These findings don’t translate into a significantly greater risk for stroke overall in women who’ve had endometriosis, Dr. Missmer said. She characterized the risk as “not negligible, but it’s not a huge increased risk.” The absolute risk is still fairly low, she said.
“We don’t want to give the impression that all women with endometriosis need to be panicked or fearful about stroke, she said. “Rather, the messaging is that this yet another bit of evidence that whole health care for those with endometriosis is important.”
Women who’ve had endometriosis and their primary care providers need to be attuned to stroke risk, she said. “This is a critical condition that primary care physicians need to engage around, and perhaps if symptoms related to cardiovascular and cerebrovascular disease emerge in their patients, they need to be engaging cardiology and similar types of support. This is not just about the gynecologists.”
The study also explored other factors that may contribute to stroke risk, with the most significant being hysterectomy with bilateral oophorectomy, Dr. Missmer said.
This study was unique because it used laparoscopically confirmed rather than self-reported endometriosis, said Louise D. McCullough, MD, neurology chair at the University of Texas Health Science Center, Houston. Another strength of the study she noted was its longitudinal design, although the cohort study design yielded a low number of stroke patients.
“Regardless, I do think it was a very important study because we have a growing recognition about how women’s health and factors such as pregnancy, infertility, parity, complications, and gonadal hormones such as estrogen can influence a woman’s stroke risk much later in life,” Dr. McCullough said in an interview.
Future studies into the relationship between endometriosis and CVD and stroke risk should focus on the mechanism behind the inflammation that occurs in endometriosis, Dr. McCullough said. “Part of it is probably the loss of hormones if a patient has to have an oophorectomy, but part of it is just what do these diseases do for a woman’s later risk – and for primary care physicians, ob.gyns., and stroke neurologists to recognize that these are questions we should ask: Have you ever had eclampsia or preeclampsia? Did you have endometriosis? Have you had miscarriages?”
The study received funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute for Neurological Disorders and Stroke. Dr. Missmer disclosed relationships with Shanghai Huilun Biotechnology, Roche, and AbbVie. Dr. McCullough has no relevant disclosures.
FROM STROKE
Overly tight sodium restriction may worsen HFpEF outcomes
Cutting out almost all salt when preparing meals was associated with a worse prognosis in patients with heart failure with preserved ejection fraction (HFpEF), according to the results of a new study.
Results from a post hoc analysis of the TOPCAT trial show that those with a cooking salt score of zero were at significantly higher risk of the primary outcome of cardiovascular (CV) death, HF hospitalization, and aborted cardiac arrest than those whose score was above zero. Survival was similar in both groups.
“Some patients restrict dietary salt intake as least as possible according to their physicians’ words or their own understanding. However, the present study found that, in patients with heart failure with preserved ejection fraction, overstrict salt restriction could lead to poor prognosis – mainly heart failure hospitalization,” explained professor Chen Liu, MD, and Weihao Liang, MD, Sun Yat-sen University First Affiliated Hospital, Guangzhou, Guangdong, China.
“Thus, when giving salt restriction advice to patients with heart failure with preserved ejection fraction, physicians should be careful instead of just saying “as least as possible,” they said in an email to this news organization.
The study was published in the journal Heart.
The authors note that HF guidelines recommend reduced salt intake, but there’s a lack of high-quality evidence to support those recommendations and no consensus on how low to go.
Previous studies have shown that reduced dietary sodium intake was associated with worse survival and higher readmission rate in patients with HF, whereas the SODIUM HF trial reported earlier this year that dietary sodium intake of less than 100 mmol (1,500 mg) per day did not improve 1-year clinical outcomes but moderately improved quality of life and New York Heart Association functional class.
“In daily clinical practice, we noticed that some physicians advised patients with heart failure to take salt as least as possible, but it could lead to hyponatremia and loss of appetite, which has been frequently reported to be associated with poor prognosis. Thus, we wanted to investigate the potential effect of overstrict salt restriction,” Dr. Liu and Dr. Liang explained.
The investigators examined data from 1,713 participants aged 50 and older with HFpEF (left ventricular ejection fraction 45% or greater) in the phase 3 TOPCAT trial, excluding those from Russia and Georgia. Patients self-reported how much salt they added to cooking staples, such as rice, pasta, potatoes, soup, meat, and vegetables, and were scored as 0 (none), 1 (⅛ teaspoon), 2 (¼ teaspoon), and 3 (½ teaspoon or more) points. Median follow-up was 2.9 years.
TOPCAT failed to show that spironolactone improved CV outcomes over placebo, but regional differences in data from Russia/Georgia and the Americas have raised concerns about its validity.
In the present analysis, almost half the participants (816) had a cooking salt score of 0, 56.4% were male, and 80.8% were White. They were more likely than participants with a salt score greater than zero to have a previous HF hospitalization, diabetes, poor renal function, and a lower ejection fraction (57% vs. 60%). Half were randomly assigned to spironolactone.
Compared with patients with a cooking salt score of 0, patients with a cooking salt score greater than 0 had significantly lower risks of the primary outcome (hazard ratio, 0.760; P = .002) and HF hospitalization (HR, 0.737; P = .003) but not all-cause (HR, 0.838) or CV (HR, 0.782) death.
The findings were consistent after full adjustment, with hazard ratios of 0.834 (P = .046), 0.791 (P = .024), 0.944, and 0.872, respectively.
Results of subgroup analyses suggested that patients aged 70 years or younger (HR, 0.644) and those of Black and other ethnicities (HR, 0.574) were at greater risk of the primary outcome from aggressive restriction of cooking salt.
“It was an interesting but unproved finding,” Dr. Liu and Dr. Liang observed. “One possible explanation is the difference in RAAS [renin-angiotensin-aldosterone system] physiology and its response to salt restriction among races, and the other is the difference in accustomed food, because the cooking salt score only accounted for sodium added during cooking but not sodium from ingredients.”
Spearman correlation analyses showed that the cooking salt score correlated significantly with systolic and diastolic blood pressure, serum sodium, and chloronium levels but not with plasma volume status, suggesting that low sodium intake did not have an intravascular volume contraction effect on patients with HFpEF.
The authors pointed out that the salt score was self-reported, hemodynamic parameters were seldom acquired in TOPCAT, and that reverse causation between low dietary sodium intake and worse HF might still exist, despite a propensity score-matching sensitivity analysis.
Reached for comment, Mary Norine Walsh, MD, the medical director of heart failure and cardiac transplantation, Ascension St. Vincent Heart Center, Indianapolis, said in an email that the authors appropriately excluded patients enrolled from Russia and Georgia because of concerns about the representativeness of patients with HFpEF in these two countries, which has been previously demonstrated.
“What limits the importance of the authors’ findings, which they acknowledge, is that the sodium intake for each patient was self-reported,” she said. “No confirmatory testing was done and recall bias could clearly have played a role.”
“Last, many patients with HFpEF have significant volume overload and dyspnea and appropriate sodium restriction is needed to help address symptoms and achieve a euvolemic state,” added Dr. Walsh, a past president of the American College of Cardiology.
Future trials are needed to determine an optimal salt restriction range for patients with heart failure, Dr. Liu and Dr. Liang suggested. “A randomized controlled trial may be hard to achieve because it is difficult to set a perfect control group. Therefore, an analysis using real-world data with a dose-response curve could be ideal.”
The study was funded by the National Natural Science Foundation of China, Guangdong Natural Science Foundation, and China Postdoctoral Science Foundation. The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cutting out almost all salt when preparing meals was associated with a worse prognosis in patients with heart failure with preserved ejection fraction (HFpEF), according to the results of a new study.
Results from a post hoc analysis of the TOPCAT trial show that those with a cooking salt score of zero were at significantly higher risk of the primary outcome of cardiovascular (CV) death, HF hospitalization, and aborted cardiac arrest than those whose score was above zero. Survival was similar in both groups.
“Some patients restrict dietary salt intake as least as possible according to their physicians’ words or their own understanding. However, the present study found that, in patients with heart failure with preserved ejection fraction, overstrict salt restriction could lead to poor prognosis – mainly heart failure hospitalization,” explained professor Chen Liu, MD, and Weihao Liang, MD, Sun Yat-sen University First Affiliated Hospital, Guangzhou, Guangdong, China.
“Thus, when giving salt restriction advice to patients with heart failure with preserved ejection fraction, physicians should be careful instead of just saying “as least as possible,” they said in an email to this news organization.
The study was published in the journal Heart.
The authors note that HF guidelines recommend reduced salt intake, but there’s a lack of high-quality evidence to support those recommendations and no consensus on how low to go.
Previous studies have shown that reduced dietary sodium intake was associated with worse survival and higher readmission rate in patients with HF, whereas the SODIUM HF trial reported earlier this year that dietary sodium intake of less than 100 mmol (1,500 mg) per day did not improve 1-year clinical outcomes but moderately improved quality of life and New York Heart Association functional class.
“In daily clinical practice, we noticed that some physicians advised patients with heart failure to take salt as least as possible, but it could lead to hyponatremia and loss of appetite, which has been frequently reported to be associated with poor prognosis. Thus, we wanted to investigate the potential effect of overstrict salt restriction,” Dr. Liu and Dr. Liang explained.
The investigators examined data from 1,713 participants aged 50 and older with HFpEF (left ventricular ejection fraction 45% or greater) in the phase 3 TOPCAT trial, excluding those from Russia and Georgia. Patients self-reported how much salt they added to cooking staples, such as rice, pasta, potatoes, soup, meat, and vegetables, and were scored as 0 (none), 1 (⅛ teaspoon), 2 (¼ teaspoon), and 3 (½ teaspoon or more) points. Median follow-up was 2.9 years.
TOPCAT failed to show that spironolactone improved CV outcomes over placebo, but regional differences in data from Russia/Georgia and the Americas have raised concerns about its validity.
In the present analysis, almost half the participants (816) had a cooking salt score of 0, 56.4% were male, and 80.8% were White. They were more likely than participants with a salt score greater than zero to have a previous HF hospitalization, diabetes, poor renal function, and a lower ejection fraction (57% vs. 60%). Half were randomly assigned to spironolactone.
Compared with patients with a cooking salt score of 0, patients with a cooking salt score greater than 0 had significantly lower risks of the primary outcome (hazard ratio, 0.760; P = .002) and HF hospitalization (HR, 0.737; P = .003) but not all-cause (HR, 0.838) or CV (HR, 0.782) death.
The findings were consistent after full adjustment, with hazard ratios of 0.834 (P = .046), 0.791 (P = .024), 0.944, and 0.872, respectively.
Results of subgroup analyses suggested that patients aged 70 years or younger (HR, 0.644) and those of Black and other ethnicities (HR, 0.574) were at greater risk of the primary outcome from aggressive restriction of cooking salt.
“It was an interesting but unproved finding,” Dr. Liu and Dr. Liang observed. “One possible explanation is the difference in RAAS [renin-angiotensin-aldosterone system] physiology and its response to salt restriction among races, and the other is the difference in accustomed food, because the cooking salt score only accounted for sodium added during cooking but not sodium from ingredients.”
Spearman correlation analyses showed that the cooking salt score correlated significantly with systolic and diastolic blood pressure, serum sodium, and chloronium levels but not with plasma volume status, suggesting that low sodium intake did not have an intravascular volume contraction effect on patients with HFpEF.
The authors pointed out that the salt score was self-reported, hemodynamic parameters were seldom acquired in TOPCAT, and that reverse causation between low dietary sodium intake and worse HF might still exist, despite a propensity score-matching sensitivity analysis.
Reached for comment, Mary Norine Walsh, MD, the medical director of heart failure and cardiac transplantation, Ascension St. Vincent Heart Center, Indianapolis, said in an email that the authors appropriately excluded patients enrolled from Russia and Georgia because of concerns about the representativeness of patients with HFpEF in these two countries, which has been previously demonstrated.
“What limits the importance of the authors’ findings, which they acknowledge, is that the sodium intake for each patient was self-reported,” she said. “No confirmatory testing was done and recall bias could clearly have played a role.”
“Last, many patients with HFpEF have significant volume overload and dyspnea and appropriate sodium restriction is needed to help address symptoms and achieve a euvolemic state,” added Dr. Walsh, a past president of the American College of Cardiology.
Future trials are needed to determine an optimal salt restriction range for patients with heart failure, Dr. Liu and Dr. Liang suggested. “A randomized controlled trial may be hard to achieve because it is difficult to set a perfect control group. Therefore, an analysis using real-world data with a dose-response curve could be ideal.”
The study was funded by the National Natural Science Foundation of China, Guangdong Natural Science Foundation, and China Postdoctoral Science Foundation. The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cutting out almost all salt when preparing meals was associated with a worse prognosis in patients with heart failure with preserved ejection fraction (HFpEF), according to the results of a new study.
Results from a post hoc analysis of the TOPCAT trial show that those with a cooking salt score of zero were at significantly higher risk of the primary outcome of cardiovascular (CV) death, HF hospitalization, and aborted cardiac arrest than those whose score was above zero. Survival was similar in both groups.
“Some patients restrict dietary salt intake as least as possible according to their physicians’ words or their own understanding. However, the present study found that, in patients with heart failure with preserved ejection fraction, overstrict salt restriction could lead to poor prognosis – mainly heart failure hospitalization,” explained professor Chen Liu, MD, and Weihao Liang, MD, Sun Yat-sen University First Affiliated Hospital, Guangzhou, Guangdong, China.
“Thus, when giving salt restriction advice to patients with heart failure with preserved ejection fraction, physicians should be careful instead of just saying “as least as possible,” they said in an email to this news organization.
The study was published in the journal Heart.
The authors note that HF guidelines recommend reduced salt intake, but there’s a lack of high-quality evidence to support those recommendations and no consensus on how low to go.
Previous studies have shown that reduced dietary sodium intake was associated with worse survival and higher readmission rate in patients with HF, whereas the SODIUM HF trial reported earlier this year that dietary sodium intake of less than 100 mmol (1,500 mg) per day did not improve 1-year clinical outcomes but moderately improved quality of life and New York Heart Association functional class.
“In daily clinical practice, we noticed that some physicians advised patients with heart failure to take salt as least as possible, but it could lead to hyponatremia and loss of appetite, which has been frequently reported to be associated with poor prognosis. Thus, we wanted to investigate the potential effect of overstrict salt restriction,” Dr. Liu and Dr. Liang explained.
The investigators examined data from 1,713 participants aged 50 and older with HFpEF (left ventricular ejection fraction 45% or greater) in the phase 3 TOPCAT trial, excluding those from Russia and Georgia. Patients self-reported how much salt they added to cooking staples, such as rice, pasta, potatoes, soup, meat, and vegetables, and were scored as 0 (none), 1 (⅛ teaspoon), 2 (¼ teaspoon), and 3 (½ teaspoon or more) points. Median follow-up was 2.9 years.
TOPCAT failed to show that spironolactone improved CV outcomes over placebo, but regional differences in data from Russia/Georgia and the Americas have raised concerns about its validity.
In the present analysis, almost half the participants (816) had a cooking salt score of 0, 56.4% were male, and 80.8% were White. They were more likely than participants with a salt score greater than zero to have a previous HF hospitalization, diabetes, poor renal function, and a lower ejection fraction (57% vs. 60%). Half were randomly assigned to spironolactone.
Compared with patients with a cooking salt score of 0, patients with a cooking salt score greater than 0 had significantly lower risks of the primary outcome (hazard ratio, 0.760; P = .002) and HF hospitalization (HR, 0.737; P = .003) but not all-cause (HR, 0.838) or CV (HR, 0.782) death.
The findings were consistent after full adjustment, with hazard ratios of 0.834 (P = .046), 0.791 (P = .024), 0.944, and 0.872, respectively.
Results of subgroup analyses suggested that patients aged 70 years or younger (HR, 0.644) and those of Black and other ethnicities (HR, 0.574) were at greater risk of the primary outcome from aggressive restriction of cooking salt.
“It was an interesting but unproved finding,” Dr. Liu and Dr. Liang observed. “One possible explanation is the difference in RAAS [renin-angiotensin-aldosterone system] physiology and its response to salt restriction among races, and the other is the difference in accustomed food, because the cooking salt score only accounted for sodium added during cooking but not sodium from ingredients.”
Spearman correlation analyses showed that the cooking salt score correlated significantly with systolic and diastolic blood pressure, serum sodium, and chloronium levels but not with plasma volume status, suggesting that low sodium intake did not have an intravascular volume contraction effect on patients with HFpEF.
The authors pointed out that the salt score was self-reported, hemodynamic parameters were seldom acquired in TOPCAT, and that reverse causation between low dietary sodium intake and worse HF might still exist, despite a propensity score-matching sensitivity analysis.
Reached for comment, Mary Norine Walsh, MD, the medical director of heart failure and cardiac transplantation, Ascension St. Vincent Heart Center, Indianapolis, said in an email that the authors appropriately excluded patients enrolled from Russia and Georgia because of concerns about the representativeness of patients with HFpEF in these two countries, which has been previously demonstrated.
“What limits the importance of the authors’ findings, which they acknowledge, is that the sodium intake for each patient was self-reported,” she said. “No confirmatory testing was done and recall bias could clearly have played a role.”
“Last, many patients with HFpEF have significant volume overload and dyspnea and appropriate sodium restriction is needed to help address symptoms and achieve a euvolemic state,” added Dr. Walsh, a past president of the American College of Cardiology.
Future trials are needed to determine an optimal salt restriction range for patients with heart failure, Dr. Liu and Dr. Liang suggested. “A randomized controlled trial may be hard to achieve because it is difficult to set a perfect control group. Therefore, an analysis using real-world data with a dose-response curve could be ideal.”
The study was funded by the National Natural Science Foundation of China, Guangdong Natural Science Foundation, and China Postdoctoral Science Foundation. The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM HEART
Biden tests positive for COVID-19: White House
Biden, 79, is experiencing “very mild” symptoms, White House Press Secretary Karine Jean-Pierre said in a statement. The president is fully vaccinated and has been boosted twice and has started taking the antiviral Paxlovid since testing positive, Ms. Jean-Pierre said.
President Biden plans to isolate at the White House and “will continue to carry out all of his duties fully during that time,” the statement said.
“He has been in contact with members of the White House staff by phone this morning, and will participate in his planned meetings at the White House this morning via phone and Zoom from the residence.”
President Biden will return to in-person work after he tests negative.
This is a developing story. Please check back for updates. A version of this article first appeared on WebMD.com .
Biden, 79, is experiencing “very mild” symptoms, White House Press Secretary Karine Jean-Pierre said in a statement. The president is fully vaccinated and has been boosted twice and has started taking the antiviral Paxlovid since testing positive, Ms. Jean-Pierre said.
President Biden plans to isolate at the White House and “will continue to carry out all of his duties fully during that time,” the statement said.
“He has been in contact with members of the White House staff by phone this morning, and will participate in his planned meetings at the White House this morning via phone and Zoom from the residence.”
President Biden will return to in-person work after he tests negative.
This is a developing story. Please check back for updates. A version of this article first appeared on WebMD.com .
Biden, 79, is experiencing “very mild” symptoms, White House Press Secretary Karine Jean-Pierre said in a statement. The president is fully vaccinated and has been boosted twice and has started taking the antiviral Paxlovid since testing positive, Ms. Jean-Pierre said.
President Biden plans to isolate at the White House and “will continue to carry out all of his duties fully during that time,” the statement said.
“He has been in contact with members of the White House staff by phone this morning, and will participate in his planned meetings at the White House this morning via phone and Zoom from the residence.”
President Biden will return to in-person work after he tests negative.
This is a developing story. Please check back for updates. A version of this article first appeared on WebMD.com .
Hormone therapy didn’t increase recurrence or mortality in women treated for breast cancer
Hormone therapy did not increase mortality in postmenopausal women treated for early-stage estrogen receptor–positive breast cancer, but, in longitudinal data from Denmark, there was a recurrence risk with vaginal estrogen therapy among those treated with aromatase inhibitors.
Genitourinary syndrome of menopause (GSM) – including vaginal dryness, burning, and urinary incontinence – is common in women treated for breast cancer. Adjuvant endocrine therapy, particularly aromatase inhibitors, can aggravate these symptoms. Both local and systemic estrogen therapy are recommended for alleviating GSM symptoms in healthy women, but concerns have been raised about their use in women with breast cancer. Previous studies examining this have suggested possible risks for breast cancer recurrence, but those studies have had several limitations including small samples and short follow-up, particularly for vaginal estrogen therapy.
In the new study, from a national Danish cohort of 8,461 postmenopausal women diagnosed between 1997 and 2004 and treated for early-stage invasive estrogen receptor–positive nonmetastatic breast cancer, neither systemic menopausal hormone therapy (MHT) nor local vaginal estrogen therapy (VET) were associated with an overall increased risk for either breast cancer recurrence or mortality. However, in the subset who had received an aromatase inhibitor – with or without tamoxifen – there was a statistically significant increased risk for breast cancer recurrence, but not mortality.
The results were published in the Journal of the National Cancer Institute.
“The data are reassuring for the majority of women with no adjuvant therapy or tamoxifen. But for those using adjuvant aromatase inhibitors, there might be a small risk,” study lead author Søren Cold, MD, PhD, senior oncologist in the department of oncology at Odense (Denmark) University Hospital, Odense, said in an interview.
Moreover, Dr. Cold noted, while this study didn’t find an increased recurrence risk with MHT for women taking aromatase inhibitors, other studies have. One in particular was stopped because of harm. The reason for the difference here is likely that the previous sample was small – just 133 women.
“Our study is mainly focusing on the use of vaginal estrogen. We had so few patients using systemic menopausal hormone therapy, those data don’t mean much. ... The risk with systemic therapy has been established. The vaginal use hasn’t been thoroughly studied before,” he noted.
Breast cancer recurrence elevated with VET and aromatase inhibitors
The study pool was 9,710 women who underwent complete resection for estrogen-positive breast cancer and were all allocated to 5 years of adjuvant endocrine treatment or no adjuvant treatment, according to guidelines. Overall, 3,112 received no adjuvant endocrine treatment, 2,007 were treated with tamoxifen only, 403 with an aromatase inhibitor, and 2,939 with a sequence of tamoxifen and an aromatase inhibitor.
After exclusion of 1,249 who had received VET or MHT prior to breast cancer diagnosis, there were 6,391 not prescribed any estrogen hormonal treatment, 1,957 prescribed VET, and 133 prescribed MHT with or without VET.
During an estimated median 9.8 years’ follow-up, 1,333 women (16%) had a breast cancer recurrence. Of those, 111 had received VET, 16 MHT, and 1,206 neither. Compared with those receiving no hormonal treatment, the adjusted risk of recurrence was similar for the VET users (hazard ratio, 1.08; 95% confidence interval, 0.89-1.32).
However, there was an increased risk for recurrence associated with initiating VET during aromatase inhibitor treatment (HR, 1.39, 95% CI, 1.04-1.85). For women receiving MHT, the adjusted relative risk of recurrence with aromatase inhibitors wasn’t significant (HR, 1.05; 95% CI, 0.62-1.78).
Overall, compared with women who never used hormonal treatment, the absolute 10-year breast cancer recurrence risk was 19.2% for never-users of VET or MHT, 15.4% in VET users, and 17.1% in MHT users.
No differences found for mortality
Of the 8,461 women in the study, 40% (3,370) died during an estimated median follow-up of 15.2 years. Of those, 497 had received VET, 47 MHT, and 2,826 neither. Compared with the never-users of estrogen therapy, the adjusted HR for overall survival in VET users was 0.78 (95% CI, 0.71-0.87). The analysis stratified by adjuvant endocrine therapy didn’t show an increase in VET users by use of aromatase inhibitors (aHR, 0.94, 95% CI, 0.70-1.26). The same was found for women prescribed MHT, compared with never-users (aHR, 0.94; 95% CI, 0.70-1.26).
Never-users of VET or MHT had an absolute 10-year overall survival of 73.8% versus 79.5% and 80.5% among the women who used VET or MHT, respectively.
Asked to comment, Nanette Santoro, MD, professor and E. Stewart Taylor Chair of Obstetrics & Gynecology at the University of Colorado at Denver, Aurora, said in an interview: “It is important to look at this issue. These findings raise but don’t answer the question that vaginal estradiol may not be as safe as we hope it is for women with breast cancer using an aromatase inhibitor.”
However, she also pointed out that “the overall increase in risk is not enormous; mortality risk was not increased. Women need to consider that there may be some risk associated with this option in their decision making about taking it. Having a satisfying sex life is also important for many women! It is really compassionate use for quality of life, so there is always that unknown element of risk in the discussion. That unknown risk has to be balanced against the benefit that the estrogen provides.”
And, Dr. Santoro also noted that the use of prescription data poses limitations. “It cannot tell us what was going on in the minds of the patient and the prescriber. There may be differences in the prescriber’s impression of the patient’s risk of recurrence that influenced the decision to provide a prescription. ... Women using AIs [aromatase inhibitors] often get pretty severe vaginal dryness symptoms and may need more estrogen to be comfortable with intercourse, but we really cannot tell this from what is in this paper.”
Indeed, Dr. Cold said: “We admit it’s not a randomized study, but we’ve done what was possible to take [confounding] factors into account, including age, tumor size, nodal status, histology, and comorbidities.”
He suggested that a potential therapeutic approach to reducing the recurrence risk might be to switch VET-treated women to tamoxifen after 2-3 years of aromatase inhibitors.
This work was supported by Breast Friends, a part of the Danish Cancer Society. Dr. Cold received support from Breast Friends for the current study. Some of the other coauthors have pharmaceutical company disclosures. Dr. Santoro is a member of the scientific advisory boards for Astellas, Menogenix, Que Oncology, and Amazon Ember, and is a consultant for Ansh Labs.
Hormone therapy did not increase mortality in postmenopausal women treated for early-stage estrogen receptor–positive breast cancer, but, in longitudinal data from Denmark, there was a recurrence risk with vaginal estrogen therapy among those treated with aromatase inhibitors.
Genitourinary syndrome of menopause (GSM) – including vaginal dryness, burning, and urinary incontinence – is common in women treated for breast cancer. Adjuvant endocrine therapy, particularly aromatase inhibitors, can aggravate these symptoms. Both local and systemic estrogen therapy are recommended for alleviating GSM symptoms in healthy women, but concerns have been raised about their use in women with breast cancer. Previous studies examining this have suggested possible risks for breast cancer recurrence, but those studies have had several limitations including small samples and short follow-up, particularly for vaginal estrogen therapy.
In the new study, from a national Danish cohort of 8,461 postmenopausal women diagnosed between 1997 and 2004 and treated for early-stage invasive estrogen receptor–positive nonmetastatic breast cancer, neither systemic menopausal hormone therapy (MHT) nor local vaginal estrogen therapy (VET) were associated with an overall increased risk for either breast cancer recurrence or mortality. However, in the subset who had received an aromatase inhibitor – with or without tamoxifen – there was a statistically significant increased risk for breast cancer recurrence, but not mortality.
The results were published in the Journal of the National Cancer Institute.
“The data are reassuring for the majority of women with no adjuvant therapy or tamoxifen. But for those using adjuvant aromatase inhibitors, there might be a small risk,” study lead author Søren Cold, MD, PhD, senior oncologist in the department of oncology at Odense (Denmark) University Hospital, Odense, said in an interview.
Moreover, Dr. Cold noted, while this study didn’t find an increased recurrence risk with MHT for women taking aromatase inhibitors, other studies have. One in particular was stopped because of harm. The reason for the difference here is likely that the previous sample was small – just 133 women.
“Our study is mainly focusing on the use of vaginal estrogen. We had so few patients using systemic menopausal hormone therapy, those data don’t mean much. ... The risk with systemic therapy has been established. The vaginal use hasn’t been thoroughly studied before,” he noted.
Breast cancer recurrence elevated with VET and aromatase inhibitors
The study pool was 9,710 women who underwent complete resection for estrogen-positive breast cancer and were all allocated to 5 years of adjuvant endocrine treatment or no adjuvant treatment, according to guidelines. Overall, 3,112 received no adjuvant endocrine treatment, 2,007 were treated with tamoxifen only, 403 with an aromatase inhibitor, and 2,939 with a sequence of tamoxifen and an aromatase inhibitor.
After exclusion of 1,249 who had received VET or MHT prior to breast cancer diagnosis, there were 6,391 not prescribed any estrogen hormonal treatment, 1,957 prescribed VET, and 133 prescribed MHT with or without VET.
During an estimated median 9.8 years’ follow-up, 1,333 women (16%) had a breast cancer recurrence. Of those, 111 had received VET, 16 MHT, and 1,206 neither. Compared with those receiving no hormonal treatment, the adjusted risk of recurrence was similar for the VET users (hazard ratio, 1.08; 95% confidence interval, 0.89-1.32).
However, there was an increased risk for recurrence associated with initiating VET during aromatase inhibitor treatment (HR, 1.39, 95% CI, 1.04-1.85). For women receiving MHT, the adjusted relative risk of recurrence with aromatase inhibitors wasn’t significant (HR, 1.05; 95% CI, 0.62-1.78).
Overall, compared with women who never used hormonal treatment, the absolute 10-year breast cancer recurrence risk was 19.2% for never-users of VET or MHT, 15.4% in VET users, and 17.1% in MHT users.
No differences found for mortality
Of the 8,461 women in the study, 40% (3,370) died during an estimated median follow-up of 15.2 years. Of those, 497 had received VET, 47 MHT, and 2,826 neither. Compared with the never-users of estrogen therapy, the adjusted HR for overall survival in VET users was 0.78 (95% CI, 0.71-0.87). The analysis stratified by adjuvant endocrine therapy didn’t show an increase in VET users by use of aromatase inhibitors (aHR, 0.94, 95% CI, 0.70-1.26). The same was found for women prescribed MHT, compared with never-users (aHR, 0.94; 95% CI, 0.70-1.26).
Never-users of VET or MHT had an absolute 10-year overall survival of 73.8% versus 79.5% and 80.5% among the women who used VET or MHT, respectively.
Asked to comment, Nanette Santoro, MD, professor and E. Stewart Taylor Chair of Obstetrics & Gynecology at the University of Colorado at Denver, Aurora, said in an interview: “It is important to look at this issue. These findings raise but don’t answer the question that vaginal estradiol may not be as safe as we hope it is for women with breast cancer using an aromatase inhibitor.”
However, she also pointed out that “the overall increase in risk is not enormous; mortality risk was not increased. Women need to consider that there may be some risk associated with this option in their decision making about taking it. Having a satisfying sex life is also important for many women! It is really compassionate use for quality of life, so there is always that unknown element of risk in the discussion. That unknown risk has to be balanced against the benefit that the estrogen provides.”
And, Dr. Santoro also noted that the use of prescription data poses limitations. “It cannot tell us what was going on in the minds of the patient and the prescriber. There may be differences in the prescriber’s impression of the patient’s risk of recurrence that influenced the decision to provide a prescription. ... Women using AIs [aromatase inhibitors] often get pretty severe vaginal dryness symptoms and may need more estrogen to be comfortable with intercourse, but we really cannot tell this from what is in this paper.”
Indeed, Dr. Cold said: “We admit it’s not a randomized study, but we’ve done what was possible to take [confounding] factors into account, including age, tumor size, nodal status, histology, and comorbidities.”
He suggested that a potential therapeutic approach to reducing the recurrence risk might be to switch VET-treated women to tamoxifen after 2-3 years of aromatase inhibitors.
This work was supported by Breast Friends, a part of the Danish Cancer Society. Dr. Cold received support from Breast Friends for the current study. Some of the other coauthors have pharmaceutical company disclosures. Dr. Santoro is a member of the scientific advisory boards for Astellas, Menogenix, Que Oncology, and Amazon Ember, and is a consultant for Ansh Labs.
Hormone therapy did not increase mortality in postmenopausal women treated for early-stage estrogen receptor–positive breast cancer, but, in longitudinal data from Denmark, there was a recurrence risk with vaginal estrogen therapy among those treated with aromatase inhibitors.
Genitourinary syndrome of menopause (GSM) – including vaginal dryness, burning, and urinary incontinence – is common in women treated for breast cancer. Adjuvant endocrine therapy, particularly aromatase inhibitors, can aggravate these symptoms. Both local and systemic estrogen therapy are recommended for alleviating GSM symptoms in healthy women, but concerns have been raised about their use in women with breast cancer. Previous studies examining this have suggested possible risks for breast cancer recurrence, but those studies have had several limitations including small samples and short follow-up, particularly for vaginal estrogen therapy.
In the new study, from a national Danish cohort of 8,461 postmenopausal women diagnosed between 1997 and 2004 and treated for early-stage invasive estrogen receptor–positive nonmetastatic breast cancer, neither systemic menopausal hormone therapy (MHT) nor local vaginal estrogen therapy (VET) were associated with an overall increased risk for either breast cancer recurrence or mortality. However, in the subset who had received an aromatase inhibitor – with or without tamoxifen – there was a statistically significant increased risk for breast cancer recurrence, but not mortality.
The results were published in the Journal of the National Cancer Institute.
“The data are reassuring for the majority of women with no adjuvant therapy or tamoxifen. But for those using adjuvant aromatase inhibitors, there might be a small risk,” study lead author Søren Cold, MD, PhD, senior oncologist in the department of oncology at Odense (Denmark) University Hospital, Odense, said in an interview.
Moreover, Dr. Cold noted, while this study didn’t find an increased recurrence risk with MHT for women taking aromatase inhibitors, other studies have. One in particular was stopped because of harm. The reason for the difference here is likely that the previous sample was small – just 133 women.
“Our study is mainly focusing on the use of vaginal estrogen. We had so few patients using systemic menopausal hormone therapy, those data don’t mean much. ... The risk with systemic therapy has been established. The vaginal use hasn’t been thoroughly studied before,” he noted.
Breast cancer recurrence elevated with VET and aromatase inhibitors
The study pool was 9,710 women who underwent complete resection for estrogen-positive breast cancer and were all allocated to 5 years of adjuvant endocrine treatment or no adjuvant treatment, according to guidelines. Overall, 3,112 received no adjuvant endocrine treatment, 2,007 were treated with tamoxifen only, 403 with an aromatase inhibitor, and 2,939 with a sequence of tamoxifen and an aromatase inhibitor.
After exclusion of 1,249 who had received VET or MHT prior to breast cancer diagnosis, there were 6,391 not prescribed any estrogen hormonal treatment, 1,957 prescribed VET, and 133 prescribed MHT with or without VET.
During an estimated median 9.8 years’ follow-up, 1,333 women (16%) had a breast cancer recurrence. Of those, 111 had received VET, 16 MHT, and 1,206 neither. Compared with those receiving no hormonal treatment, the adjusted risk of recurrence was similar for the VET users (hazard ratio, 1.08; 95% confidence interval, 0.89-1.32).
However, there was an increased risk for recurrence associated with initiating VET during aromatase inhibitor treatment (HR, 1.39, 95% CI, 1.04-1.85). For women receiving MHT, the adjusted relative risk of recurrence with aromatase inhibitors wasn’t significant (HR, 1.05; 95% CI, 0.62-1.78).
Overall, compared with women who never used hormonal treatment, the absolute 10-year breast cancer recurrence risk was 19.2% for never-users of VET or MHT, 15.4% in VET users, and 17.1% in MHT users.
No differences found for mortality
Of the 8,461 women in the study, 40% (3,370) died during an estimated median follow-up of 15.2 years. Of those, 497 had received VET, 47 MHT, and 2,826 neither. Compared with the never-users of estrogen therapy, the adjusted HR for overall survival in VET users was 0.78 (95% CI, 0.71-0.87). The analysis stratified by adjuvant endocrine therapy didn’t show an increase in VET users by use of aromatase inhibitors (aHR, 0.94, 95% CI, 0.70-1.26). The same was found for women prescribed MHT, compared with never-users (aHR, 0.94; 95% CI, 0.70-1.26).
Never-users of VET or MHT had an absolute 10-year overall survival of 73.8% versus 79.5% and 80.5% among the women who used VET or MHT, respectively.
Asked to comment, Nanette Santoro, MD, professor and E. Stewart Taylor Chair of Obstetrics & Gynecology at the University of Colorado at Denver, Aurora, said in an interview: “It is important to look at this issue. These findings raise but don’t answer the question that vaginal estradiol may not be as safe as we hope it is for women with breast cancer using an aromatase inhibitor.”
However, she also pointed out that “the overall increase in risk is not enormous; mortality risk was not increased. Women need to consider that there may be some risk associated with this option in their decision making about taking it. Having a satisfying sex life is also important for many women! It is really compassionate use for quality of life, so there is always that unknown element of risk in the discussion. That unknown risk has to be balanced against the benefit that the estrogen provides.”
And, Dr. Santoro also noted that the use of prescription data poses limitations. “It cannot tell us what was going on in the minds of the patient and the prescriber. There may be differences in the prescriber’s impression of the patient’s risk of recurrence that influenced the decision to provide a prescription. ... Women using AIs [aromatase inhibitors] often get pretty severe vaginal dryness symptoms and may need more estrogen to be comfortable with intercourse, but we really cannot tell this from what is in this paper.”
Indeed, Dr. Cold said: “We admit it’s not a randomized study, but we’ve done what was possible to take [confounding] factors into account, including age, tumor size, nodal status, histology, and comorbidities.”
He suggested that a potential therapeutic approach to reducing the recurrence risk might be to switch VET-treated women to tamoxifen after 2-3 years of aromatase inhibitors.
This work was supported by Breast Friends, a part of the Danish Cancer Society. Dr. Cold received support from Breast Friends for the current study. Some of the other coauthors have pharmaceutical company disclosures. Dr. Santoro is a member of the scientific advisory boards for Astellas, Menogenix, Que Oncology, and Amazon Ember, and is a consultant for Ansh Labs.
FROM THE JOURNAL OF THE NATIONAL CANCER INSTITUTE
Former nurses of historic Black hospital sue to preserve its legacy
A training facility for Black doctors and nurses in St. Louis, which was the only public hospital for Black community from the late 1930s through the mid-1950s, has been at the center of many contentious community protests over the years and is facing another.
A federal lawsuit was filed recently by the nurses’ alumni of Homer G. Phillips Hospital against a St. Louis developer who is using the hospital’s name for a small for-profit urgent care health facility.
Homer G. Phillips was a St. Louis attorney and civic leader who joined with other Black leaders in 1922 to gain money for a hospital that would serve the Black community, according to online sources. He didn’t live to see the hospital named in his honor completed in 1937.
The former Homer G. Phillips Hospital closed in 1979 despite the community’s outcry at that time, according to The Missouri Independent. The building sat vacant for many years before being converted into a senior center, Yvonne Jones, alumni president, said in an interview.
She said of the new health center, which hasn’t opened yet, “We are not against the facility; we want to protect the name and legacy” of the original hospital, which remains at the heart of the historic St. Louis Black community.
At press time, the developer and his attorneys had not returned this news organization’s request for comment.
Having a new center with the name of the iconic hospital would mean that “the goodwill and the pride it represents has been usurped,” said Zenobia Thompson, who served as head nurse of Homer G. Phillips and is now the co-chair of the Change the Name Coalition. It formed last year after Ms. Thompson and others noticed a sign posted at the site of the new health center that lists it as the Homer G. Phillips Hospital, with a trademark symbol that the nurses say it doesn’t have a right to.
The coalition, which meets weekly, sponsored a petition and has been protesting at the site of the new center twice a month, Ms. Thompson said.
“We wrote a letter to [developer] Paul McKee that the legacy not be trivialized for commercial reasons,” Ms. Thompson said.
Richard Voytas, attorney for the alumni group, said in an interview that the developer did not ask permission from the nurses to use the trademark and he didn’t know if the nurses will grant that permission now. “If they [the developers] use the name, it is very important that they honor the Homer G. Phillips legacy,” Mr. Voytas said.
Honoring a legacy or taking advantage of a name?
In her new book, Climbing the Ladder, Chasing the Dream: A History of Homer G. Phillips Hospital, author Candace O’Connor cites the importance of the hospital’s heritage.
“Several nurses came from rural, impoverished backgrounds and went on to get jobs all across the country,” Ms. O’Connor wrote in the book. “Because all you had to do was say, ‘I’m from Homer Phillips,’ and they would say ‘you’re hired.’ It didn’t just change the nurse. It created opportunities for whole families.”
The area where the hospital remains once boasted a grocery store, high school, college, ice cream shop, and renowned Black churches, some of which still exist as historical sites. “They built up the area for Blacks who couldn’t go anywhere else,” Ms. Jones said.
In the suit, the alumni group describes itself as a 100-year-old philanthropic organization that brought healthcare to St. Louis’ historically underserved Black community and remains very active in the area today in fundraising and community outreach efforts. The group has been fighting with the developers since learning in 2019 about the proposed use of the name that is “confusingly similar” to the trademark and immediately voiced its objections via lawsuit, demanding that another name be chosen, stating:
“…in its name and efforts to market its for-profit urgent care facility immediately within plaintiff’s primary market to directly compete with plaintiff for name recognition and goodwill, only increases the likelihood of consumer confusion and, upon information and belief, represents an effort by defendants’ to pass off their products and services as those offered by plaintiff and its members.”
“Defendants stated purpose in using the mark, or a phrase confusingly similar to the mark, for its name is to ‘honor’ the name of Homer G. Phillips and to ‘emulate his spirit andtenacity in serving the health care needs of North St. Louis,’” the suit continues.
The St. Louis Board of Aldermen passed a resolution in December calling the use of the name for the new health center an “inappropriate cultural appropriation.” Mayor Tishaura Jones and Congresswoman Cori Bush followed that with a joint statement: “Profiting off of Homer G. Phillips’ name on a small 3-bed facility that will fail to meet the needs of the most vulnerable in our communities is an insult to Homer G. Phillips’ legacy and the Black community.”
The alumni group is requesting a jury trial and damages to be determined at trial, three times the defendant’s profits or plaintiffs’ damages, whichever is greater, along with attorneys’ fees and interest.
A version of this article first appeared on Medscape.com.
A training facility for Black doctors and nurses in St. Louis, which was the only public hospital for Black community from the late 1930s through the mid-1950s, has been at the center of many contentious community protests over the years and is facing another.
A federal lawsuit was filed recently by the nurses’ alumni of Homer G. Phillips Hospital against a St. Louis developer who is using the hospital’s name for a small for-profit urgent care health facility.
Homer G. Phillips was a St. Louis attorney and civic leader who joined with other Black leaders in 1922 to gain money for a hospital that would serve the Black community, according to online sources. He didn’t live to see the hospital named in his honor completed in 1937.
The former Homer G. Phillips Hospital closed in 1979 despite the community’s outcry at that time, according to The Missouri Independent. The building sat vacant for many years before being converted into a senior center, Yvonne Jones, alumni president, said in an interview.
She said of the new health center, which hasn’t opened yet, “We are not against the facility; we want to protect the name and legacy” of the original hospital, which remains at the heart of the historic St. Louis Black community.
At press time, the developer and his attorneys had not returned this news organization’s request for comment.
Having a new center with the name of the iconic hospital would mean that “the goodwill and the pride it represents has been usurped,” said Zenobia Thompson, who served as head nurse of Homer G. Phillips and is now the co-chair of the Change the Name Coalition. It formed last year after Ms. Thompson and others noticed a sign posted at the site of the new health center that lists it as the Homer G. Phillips Hospital, with a trademark symbol that the nurses say it doesn’t have a right to.
The coalition, which meets weekly, sponsored a petition and has been protesting at the site of the new center twice a month, Ms. Thompson said.
“We wrote a letter to [developer] Paul McKee that the legacy not be trivialized for commercial reasons,” Ms. Thompson said.
Richard Voytas, attorney for the alumni group, said in an interview that the developer did not ask permission from the nurses to use the trademark and he didn’t know if the nurses will grant that permission now. “If they [the developers] use the name, it is very important that they honor the Homer G. Phillips legacy,” Mr. Voytas said.
Honoring a legacy or taking advantage of a name?
In her new book, Climbing the Ladder, Chasing the Dream: A History of Homer G. Phillips Hospital, author Candace O’Connor cites the importance of the hospital’s heritage.
“Several nurses came from rural, impoverished backgrounds and went on to get jobs all across the country,” Ms. O’Connor wrote in the book. “Because all you had to do was say, ‘I’m from Homer Phillips,’ and they would say ‘you’re hired.’ It didn’t just change the nurse. It created opportunities for whole families.”
The area where the hospital remains once boasted a grocery store, high school, college, ice cream shop, and renowned Black churches, some of which still exist as historical sites. “They built up the area for Blacks who couldn’t go anywhere else,” Ms. Jones said.
In the suit, the alumni group describes itself as a 100-year-old philanthropic organization that brought healthcare to St. Louis’ historically underserved Black community and remains very active in the area today in fundraising and community outreach efforts. The group has been fighting with the developers since learning in 2019 about the proposed use of the name that is “confusingly similar” to the trademark and immediately voiced its objections via lawsuit, demanding that another name be chosen, stating:
“…in its name and efforts to market its for-profit urgent care facility immediately within plaintiff’s primary market to directly compete with plaintiff for name recognition and goodwill, only increases the likelihood of consumer confusion and, upon information and belief, represents an effort by defendants’ to pass off their products and services as those offered by plaintiff and its members.”
“Defendants stated purpose in using the mark, or a phrase confusingly similar to the mark, for its name is to ‘honor’ the name of Homer G. Phillips and to ‘emulate his spirit andtenacity in serving the health care needs of North St. Louis,’” the suit continues.
The St. Louis Board of Aldermen passed a resolution in December calling the use of the name for the new health center an “inappropriate cultural appropriation.” Mayor Tishaura Jones and Congresswoman Cori Bush followed that with a joint statement: “Profiting off of Homer G. Phillips’ name on a small 3-bed facility that will fail to meet the needs of the most vulnerable in our communities is an insult to Homer G. Phillips’ legacy and the Black community.”
The alumni group is requesting a jury trial and damages to be determined at trial, three times the defendant’s profits or plaintiffs’ damages, whichever is greater, along with attorneys’ fees and interest.
A version of this article first appeared on Medscape.com.
A training facility for Black doctors and nurses in St. Louis, which was the only public hospital for Black community from the late 1930s through the mid-1950s, has been at the center of many contentious community protests over the years and is facing another.
A federal lawsuit was filed recently by the nurses’ alumni of Homer G. Phillips Hospital against a St. Louis developer who is using the hospital’s name for a small for-profit urgent care health facility.
Homer G. Phillips was a St. Louis attorney and civic leader who joined with other Black leaders in 1922 to gain money for a hospital that would serve the Black community, according to online sources. He didn’t live to see the hospital named in his honor completed in 1937.
The former Homer G. Phillips Hospital closed in 1979 despite the community’s outcry at that time, according to The Missouri Independent. The building sat vacant for many years before being converted into a senior center, Yvonne Jones, alumni president, said in an interview.
She said of the new health center, which hasn’t opened yet, “We are not against the facility; we want to protect the name and legacy” of the original hospital, which remains at the heart of the historic St. Louis Black community.
At press time, the developer and his attorneys had not returned this news organization’s request for comment.
Having a new center with the name of the iconic hospital would mean that “the goodwill and the pride it represents has been usurped,” said Zenobia Thompson, who served as head nurse of Homer G. Phillips and is now the co-chair of the Change the Name Coalition. It formed last year after Ms. Thompson and others noticed a sign posted at the site of the new health center that lists it as the Homer G. Phillips Hospital, with a trademark symbol that the nurses say it doesn’t have a right to.
The coalition, which meets weekly, sponsored a petition and has been protesting at the site of the new center twice a month, Ms. Thompson said.
“We wrote a letter to [developer] Paul McKee that the legacy not be trivialized for commercial reasons,” Ms. Thompson said.
Richard Voytas, attorney for the alumni group, said in an interview that the developer did not ask permission from the nurses to use the trademark and he didn’t know if the nurses will grant that permission now. “If they [the developers] use the name, it is very important that they honor the Homer G. Phillips legacy,” Mr. Voytas said.
Honoring a legacy or taking advantage of a name?
In her new book, Climbing the Ladder, Chasing the Dream: A History of Homer G. Phillips Hospital, author Candace O’Connor cites the importance of the hospital’s heritage.
“Several nurses came from rural, impoverished backgrounds and went on to get jobs all across the country,” Ms. O’Connor wrote in the book. “Because all you had to do was say, ‘I’m from Homer Phillips,’ and they would say ‘you’re hired.’ It didn’t just change the nurse. It created opportunities for whole families.”
The area where the hospital remains once boasted a grocery store, high school, college, ice cream shop, and renowned Black churches, some of which still exist as historical sites. “They built up the area for Blacks who couldn’t go anywhere else,” Ms. Jones said.
In the suit, the alumni group describes itself as a 100-year-old philanthropic organization that brought healthcare to St. Louis’ historically underserved Black community and remains very active in the area today in fundraising and community outreach efforts. The group has been fighting with the developers since learning in 2019 about the proposed use of the name that is “confusingly similar” to the trademark and immediately voiced its objections via lawsuit, demanding that another name be chosen, stating:
“…in its name and efforts to market its for-profit urgent care facility immediately within plaintiff’s primary market to directly compete with plaintiff for name recognition and goodwill, only increases the likelihood of consumer confusion and, upon information and belief, represents an effort by defendants’ to pass off their products and services as those offered by plaintiff and its members.”
“Defendants stated purpose in using the mark, or a phrase confusingly similar to the mark, for its name is to ‘honor’ the name of Homer G. Phillips and to ‘emulate his spirit andtenacity in serving the health care needs of North St. Louis,’” the suit continues.
The St. Louis Board of Aldermen passed a resolution in December calling the use of the name for the new health center an “inappropriate cultural appropriation.” Mayor Tishaura Jones and Congresswoman Cori Bush followed that with a joint statement: “Profiting off of Homer G. Phillips’ name on a small 3-bed facility that will fail to meet the needs of the most vulnerable in our communities is an insult to Homer G. Phillips’ legacy and the Black community.”
The alumni group is requesting a jury trial and damages to be determined at trial, three times the defendant’s profits or plaintiffs’ damages, whichever is greater, along with attorneys’ fees and interest.
A version of this article first appeared on Medscape.com.
Leg lesions
A 4-mm punch biopsy performed on the central portion of a lesion revealed thickening of the epidermis and altered collagen in the dermis consistent with acquired reactive perforating collagenosis (ARPC).
ARPC is strongly associated with diabetes, renal disease, and malignancy. ARPC manifests as an eruption of intensely pruritic papules to small plaques (with a central plug or firm dry depression) on the trunk, or more commonly, on the extremities. The etiology is unclear but altered collagen from systemic disease, trauma, or cold exposure may trigger collagen elimination.1 Secondary infection may occur due to the intensity of itching. ARPC develops in adulthood; epidemiologic data are lacking and prevalence has not been systematically assessed.2
Treatment approaches are based on small case reports and case series. Common antipruritic therapies, such as topical and intralesional steroids, oral antihistamines, and vitamin-D analogues, have had mixed success. UV therapy is effective for nephrogenic pruritus; case reports suggest it has also been helpful for ARPC. Similarly, keratolytics and topical and systemic retinoids have shown promise. Allopurinol, which reduces free radicals, has also demonstrated its utility.3
This patient was started on topical triamcinolone 0.1% cream bid and narrowband UV-B phototherapy 3 times weekly with marked improvement in her itching. Lesions decreased in number over 3 months of follow-up but did not completely resolve.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Zhang X, Yang Y, Shao S. Acquired reactive perforating collagenosis: a case report and review of the literature. Medicine (Baltimore). 2020;99:e20391. doi: 10.1097/MD.0000000000020391
2. Karpouzis A, Giatromanolaki A, Sivridis E, et al. Acquired reactive perforating collagenosis: current status. J Dermatol. 2010;37:585-592. doi: 10.1111/j.1346-8138.2010.00918.x
3. Lukács J, Schliemann S, Elsner P. Treatment of acquired reactive perforating dermatosis - a systematic review. J Dtsch Dermatol Ges. 2018;16:825-842. doi: 10.1111/ddg.13561
A 4-mm punch biopsy performed on the central portion of a lesion revealed thickening of the epidermis and altered collagen in the dermis consistent with acquired reactive perforating collagenosis (ARPC).
ARPC is strongly associated with diabetes, renal disease, and malignancy. ARPC manifests as an eruption of intensely pruritic papules to small plaques (with a central plug or firm dry depression) on the trunk, or more commonly, on the extremities. The etiology is unclear but altered collagen from systemic disease, trauma, or cold exposure may trigger collagen elimination.1 Secondary infection may occur due to the intensity of itching. ARPC develops in adulthood; epidemiologic data are lacking and prevalence has not been systematically assessed.2
Treatment approaches are based on small case reports and case series. Common antipruritic therapies, such as topical and intralesional steroids, oral antihistamines, and vitamin-D analogues, have had mixed success. UV therapy is effective for nephrogenic pruritus; case reports suggest it has also been helpful for ARPC. Similarly, keratolytics and topical and systemic retinoids have shown promise. Allopurinol, which reduces free radicals, has also demonstrated its utility.3
This patient was started on topical triamcinolone 0.1% cream bid and narrowband UV-B phototherapy 3 times weekly with marked improvement in her itching. Lesions decreased in number over 3 months of follow-up but did not completely resolve.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
A 4-mm punch biopsy performed on the central portion of a lesion revealed thickening of the epidermis and altered collagen in the dermis consistent with acquired reactive perforating collagenosis (ARPC).
ARPC is strongly associated with diabetes, renal disease, and malignancy. ARPC manifests as an eruption of intensely pruritic papules to small plaques (with a central plug or firm dry depression) on the trunk, or more commonly, on the extremities. The etiology is unclear but altered collagen from systemic disease, trauma, or cold exposure may trigger collagen elimination.1 Secondary infection may occur due to the intensity of itching. ARPC develops in adulthood; epidemiologic data are lacking and prevalence has not been systematically assessed.2
Treatment approaches are based on small case reports and case series. Common antipruritic therapies, such as topical and intralesional steroids, oral antihistamines, and vitamin-D analogues, have had mixed success. UV therapy is effective for nephrogenic pruritus; case reports suggest it has also been helpful for ARPC. Similarly, keratolytics and topical and systemic retinoids have shown promise. Allopurinol, which reduces free radicals, has also demonstrated its utility.3
This patient was started on topical triamcinolone 0.1% cream bid and narrowband UV-B phototherapy 3 times weekly with marked improvement in her itching. Lesions decreased in number over 3 months of follow-up but did not completely resolve.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Zhang X, Yang Y, Shao S. Acquired reactive perforating collagenosis: a case report and review of the literature. Medicine (Baltimore). 2020;99:e20391. doi: 10.1097/MD.0000000000020391
2. Karpouzis A, Giatromanolaki A, Sivridis E, et al. Acquired reactive perforating collagenosis: current status. J Dermatol. 2010;37:585-592. doi: 10.1111/j.1346-8138.2010.00918.x
3. Lukács J, Schliemann S, Elsner P. Treatment of acquired reactive perforating dermatosis - a systematic review. J Dtsch Dermatol Ges. 2018;16:825-842. doi: 10.1111/ddg.13561
1. Zhang X, Yang Y, Shao S. Acquired reactive perforating collagenosis: a case report and review of the literature. Medicine (Baltimore). 2020;99:e20391. doi: 10.1097/MD.0000000000020391
2. Karpouzis A, Giatromanolaki A, Sivridis E, et al. Acquired reactive perforating collagenosis: current status. J Dermatol. 2010;37:585-592. doi: 10.1111/j.1346-8138.2010.00918.x
3. Lukács J, Schliemann S, Elsner P. Treatment of acquired reactive perforating dermatosis - a systematic review. J Dtsch Dermatol Ges. 2018;16:825-842. doi: 10.1111/ddg.13561
NAFLD strongly correlated with psoriasis, PsA; risk linked to severity
NEW YORK – – and probably in those with psoriatic arthritis (PsA) as well, according to a systematic review and meta-analysis presented at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
“Our findings imply that psoriatic patients should be screened with an ultrasonographic exam in cases where there are metabolic features that are associated with NAFLD,” reported Francesco Bellinato, MD, a researcher in the section of dermatology and venereology, University of Verona (Italy).
The data are strong. Of 76 nonduplicate publications found in the literature, the 11 observational studies included in the meta-analysis met stringent criteria, including a diagnosis of psoriasis and PsA based on objective criteria, NAFLD confirmed with liver biopsy or imaging, and odds rates calculated with 95% confidence intervals.
From these 11 studies, aggregate data were available for 249,333 psoriatic patients, of which 49% had NAFLD, and 1,491,402 were healthy controls. Among the controls, 36% had NAFLD. Four of the studies were from North America, four from Europe, and three from Asia.
In the pooled data, the risk of NAFLD among those with psoriasis relative to healthy controls fell just short of a twofold increase (odds ratio, 1.96; 95% CI, 1.70-2.26; P < .001). When stratified by studies that confirmed NAFLD by biopsy relative to ultrasonography, there was no significant heterogeneity.
Eight of the studies included an analysis of relative risk in the context of skin lesion severity defined by Psoriasis Area and Severity Index (PASI) score. Relative to those without NAFLD, psoriatic patients with NAFLD had a significant greater mean PASI score on a pooled weighted mean difference analysis (OR, 3.93; 95% CI, 2.01-5.84; P < .0001).
For PsA relative to no PsA in the five studies that compared risk between these two groups, the risk of NAFLD was again nearly twofold higher. This fell short of conventional definition of statistical significance, but it was associated with a strong trend (OR, 1.83; 95% CI, 0.98-3.43; P = .06).
The risk of NAFLD among patients with psoriasis was not found to vary significantly when assessed by univariable meta-regressions across numerous characteristics, such as sex and body mass index.
In one of the largest of the observational studies included in the meta-analysis by Alexis Ogdie, MD, associate professor of medicine and epidemiology at the University of Pennsylvania, Philadelphia, and colleagues, data were analyzed in more than 1.5 million patients, which included 54,251 patients with rheumatoid arthritis. While the hazard ratio of NAFLD was increased for both psoriasis (HR, 2.23) and PsA (HR, 2.11), it was not elevated in those with RA (HR, 0.96).
Risk by severity, possible mechanisms
This study also included an analysis of NAFLD risk according to psoriasis severity. While risk was still significant among those with mild disease (HR, 1.18; 95% CI, 1.07-1.30), it was almost twofold greater in those with moderate to severe psoriasis (HR, 2.23; 95% CI, 1.73-2.87).
Dr. Bellinato conceded that the mechanisms underlying the association between psoriasis and NAFLD are unknown, but he said “metaflammation” is suspected.
“The secretion of proinflammatory, prothrombotic, and oxidative stress mediators in both psoriatic skin and adipose tissue might act systemically and promote insulin resistance and other metabolic derangements that promote the development and progression of NAFLD,” Dr. Bellinato explained.
He thinks that noninvasive screening methods, such as currently used methods to calculate fibrosis score, might be useful for evaluating patients with psoriasis for NAFLD and referring them to a hepatologist when appropriate.
Given the strong association with NAFLD, Dr. Bellinato suggested that “the findings of this meta-analysis pave the way for novel, large, prospective, and histologically based studies.”
The association between psoriasis and NAFLD is clinically relevant, agreed Joel M. Gelfand, MD, vice-chair of clinical research and medical director of the clinical studies unit, department of dermatology, University of Pennsylvania, Philadelphia.
“It is not clear if psoriasis causes fatty liver disease or vice versa, but clinicians should be aware of this association,” he said in an interview. Dr. Gelfand was a coauthor of the study by Dr. Ogdie and colleagues and led another more recent population-based study that implicated methotrexate as a factor in psoriasis-related hepatotoxicity.
If NAFLD is identified in a patient with psoriasis, treatments are limited, but Dr. Gelfand suggested that patients should be made aware of the risk. “Clinicians should encourage patients with psoriasis to take measures to protect their liver, such as avoiding drinking alcohol to excess and trying to maintain a healthy body weight,” he said.
Dr. Bellinato reported no conflicts of interest. Dr. Gelfand has financial relationships with more than 10 pharmaceutical companies, including those that make therapies for psoriasis.
NEW YORK – – and probably in those with psoriatic arthritis (PsA) as well, according to a systematic review and meta-analysis presented at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
“Our findings imply that psoriatic patients should be screened with an ultrasonographic exam in cases where there are metabolic features that are associated with NAFLD,” reported Francesco Bellinato, MD, a researcher in the section of dermatology and venereology, University of Verona (Italy).
The data are strong. Of 76 nonduplicate publications found in the literature, the 11 observational studies included in the meta-analysis met stringent criteria, including a diagnosis of psoriasis and PsA based on objective criteria, NAFLD confirmed with liver biopsy or imaging, and odds rates calculated with 95% confidence intervals.
From these 11 studies, aggregate data were available for 249,333 psoriatic patients, of which 49% had NAFLD, and 1,491,402 were healthy controls. Among the controls, 36% had NAFLD. Four of the studies were from North America, four from Europe, and three from Asia.
In the pooled data, the risk of NAFLD among those with psoriasis relative to healthy controls fell just short of a twofold increase (odds ratio, 1.96; 95% CI, 1.70-2.26; P < .001). When stratified by studies that confirmed NAFLD by biopsy relative to ultrasonography, there was no significant heterogeneity.
Eight of the studies included an analysis of relative risk in the context of skin lesion severity defined by Psoriasis Area and Severity Index (PASI) score. Relative to those without NAFLD, psoriatic patients with NAFLD had a significant greater mean PASI score on a pooled weighted mean difference analysis (OR, 3.93; 95% CI, 2.01-5.84; P < .0001).
For PsA relative to no PsA in the five studies that compared risk between these two groups, the risk of NAFLD was again nearly twofold higher. This fell short of conventional definition of statistical significance, but it was associated with a strong trend (OR, 1.83; 95% CI, 0.98-3.43; P = .06).
The risk of NAFLD among patients with psoriasis was not found to vary significantly when assessed by univariable meta-regressions across numerous characteristics, such as sex and body mass index.
In one of the largest of the observational studies included in the meta-analysis by Alexis Ogdie, MD, associate professor of medicine and epidemiology at the University of Pennsylvania, Philadelphia, and colleagues, data were analyzed in more than 1.5 million patients, which included 54,251 patients with rheumatoid arthritis. While the hazard ratio of NAFLD was increased for both psoriasis (HR, 2.23) and PsA (HR, 2.11), it was not elevated in those with RA (HR, 0.96).
Risk by severity, possible mechanisms
This study also included an analysis of NAFLD risk according to psoriasis severity. While risk was still significant among those with mild disease (HR, 1.18; 95% CI, 1.07-1.30), it was almost twofold greater in those with moderate to severe psoriasis (HR, 2.23; 95% CI, 1.73-2.87).
Dr. Bellinato conceded that the mechanisms underlying the association between psoriasis and NAFLD are unknown, but he said “metaflammation” is suspected.
“The secretion of proinflammatory, prothrombotic, and oxidative stress mediators in both psoriatic skin and adipose tissue might act systemically and promote insulin resistance and other metabolic derangements that promote the development and progression of NAFLD,” Dr. Bellinato explained.
He thinks that noninvasive screening methods, such as currently used methods to calculate fibrosis score, might be useful for evaluating patients with psoriasis for NAFLD and referring them to a hepatologist when appropriate.
Given the strong association with NAFLD, Dr. Bellinato suggested that “the findings of this meta-analysis pave the way for novel, large, prospective, and histologically based studies.”
The association between psoriasis and NAFLD is clinically relevant, agreed Joel M. Gelfand, MD, vice-chair of clinical research and medical director of the clinical studies unit, department of dermatology, University of Pennsylvania, Philadelphia.
“It is not clear if psoriasis causes fatty liver disease or vice versa, but clinicians should be aware of this association,” he said in an interview. Dr. Gelfand was a coauthor of the study by Dr. Ogdie and colleagues and led another more recent population-based study that implicated methotrexate as a factor in psoriasis-related hepatotoxicity.
If NAFLD is identified in a patient with psoriasis, treatments are limited, but Dr. Gelfand suggested that patients should be made aware of the risk. “Clinicians should encourage patients with psoriasis to take measures to protect their liver, such as avoiding drinking alcohol to excess and trying to maintain a healthy body weight,” he said.
Dr. Bellinato reported no conflicts of interest. Dr. Gelfand has financial relationships with more than 10 pharmaceutical companies, including those that make therapies for psoriasis.
NEW YORK – – and probably in those with psoriatic arthritis (PsA) as well, according to a systematic review and meta-analysis presented at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
“Our findings imply that psoriatic patients should be screened with an ultrasonographic exam in cases where there are metabolic features that are associated with NAFLD,” reported Francesco Bellinato, MD, a researcher in the section of dermatology and venereology, University of Verona (Italy).
The data are strong. Of 76 nonduplicate publications found in the literature, the 11 observational studies included in the meta-analysis met stringent criteria, including a diagnosis of psoriasis and PsA based on objective criteria, NAFLD confirmed with liver biopsy or imaging, and odds rates calculated with 95% confidence intervals.
From these 11 studies, aggregate data were available for 249,333 psoriatic patients, of which 49% had NAFLD, and 1,491,402 were healthy controls. Among the controls, 36% had NAFLD. Four of the studies were from North America, four from Europe, and three from Asia.
In the pooled data, the risk of NAFLD among those with psoriasis relative to healthy controls fell just short of a twofold increase (odds ratio, 1.96; 95% CI, 1.70-2.26; P < .001). When stratified by studies that confirmed NAFLD by biopsy relative to ultrasonography, there was no significant heterogeneity.
Eight of the studies included an analysis of relative risk in the context of skin lesion severity defined by Psoriasis Area and Severity Index (PASI) score. Relative to those without NAFLD, psoriatic patients with NAFLD had a significant greater mean PASI score on a pooled weighted mean difference analysis (OR, 3.93; 95% CI, 2.01-5.84; P < .0001).
For PsA relative to no PsA in the five studies that compared risk between these two groups, the risk of NAFLD was again nearly twofold higher. This fell short of conventional definition of statistical significance, but it was associated with a strong trend (OR, 1.83; 95% CI, 0.98-3.43; P = .06).
The risk of NAFLD among patients with psoriasis was not found to vary significantly when assessed by univariable meta-regressions across numerous characteristics, such as sex and body mass index.
In one of the largest of the observational studies included in the meta-analysis by Alexis Ogdie, MD, associate professor of medicine and epidemiology at the University of Pennsylvania, Philadelphia, and colleagues, data were analyzed in more than 1.5 million patients, which included 54,251 patients with rheumatoid arthritis. While the hazard ratio of NAFLD was increased for both psoriasis (HR, 2.23) and PsA (HR, 2.11), it was not elevated in those with RA (HR, 0.96).
Risk by severity, possible mechanisms
This study also included an analysis of NAFLD risk according to psoriasis severity. While risk was still significant among those with mild disease (HR, 1.18; 95% CI, 1.07-1.30), it was almost twofold greater in those with moderate to severe psoriasis (HR, 2.23; 95% CI, 1.73-2.87).
Dr. Bellinato conceded that the mechanisms underlying the association between psoriasis and NAFLD are unknown, but he said “metaflammation” is suspected.
“The secretion of proinflammatory, prothrombotic, and oxidative stress mediators in both psoriatic skin and adipose tissue might act systemically and promote insulin resistance and other metabolic derangements that promote the development and progression of NAFLD,” Dr. Bellinato explained.
He thinks that noninvasive screening methods, such as currently used methods to calculate fibrosis score, might be useful for evaluating patients with psoriasis for NAFLD and referring them to a hepatologist when appropriate.
Given the strong association with NAFLD, Dr. Bellinato suggested that “the findings of this meta-analysis pave the way for novel, large, prospective, and histologically based studies.”
The association between psoriasis and NAFLD is clinically relevant, agreed Joel M. Gelfand, MD, vice-chair of clinical research and medical director of the clinical studies unit, department of dermatology, University of Pennsylvania, Philadelphia.
“It is not clear if psoriasis causes fatty liver disease or vice versa, but clinicians should be aware of this association,” he said in an interview. Dr. Gelfand was a coauthor of the study by Dr. Ogdie and colleagues and led another more recent population-based study that implicated methotrexate as a factor in psoriasis-related hepatotoxicity.
If NAFLD is identified in a patient with psoriasis, treatments are limited, but Dr. Gelfand suggested that patients should be made aware of the risk. “Clinicians should encourage patients with psoriasis to take measures to protect their liver, such as avoiding drinking alcohol to excess and trying to maintain a healthy body weight,” he said.
Dr. Bellinato reported no conflicts of interest. Dr. Gelfand has financial relationships with more than 10 pharmaceutical companies, including those that make therapies for psoriasis.
AT GRAPPA 2022
Statins linked to lower diabetes risk after acute pancreatitis
Use of cholesterol-lowering statins was linked to a lower risk of developing a subtype of diabetes that occurs after acute pancreatitis, according to a new report.
The benefits of statins depended on the consistency of usage, with regular users having a lower risk of developing postpancreatitis diabetes than irregular users. The results were similar with low, moderate, and high statin doses, as well as in cases of both mild and severe acute pancreatitis.
“About 15% of patients with acute pancreatitis will develop diabetes mellitus in the next 5 years, and although we can monitor for it, we can’t do anything to prevent it,” Nikhil Thiruvengadam, MD, the lead study author and a gastroenterologist at Loma Linda (Calif.) University, told this news organization.
“This could push you as a clinician to prescribe [a statin if you have a reason to] because it could provide two benefits instead of just one,” he said.
The study was published online in Clinical Gastroenterology and Hepatology.
Steady use mattered, not dose
Patients with acute pancreatitis face at least a twofold increased risk of developing postpancreatitis diabetes, the study authors write. Although previous studies have shown that statins can lower the incidence and severity of acute pancreatitis, they haven’t been studied for the prevention of postpancreatitis diabetes.
In a collaborative study with several other universities, Dr. Thiruvengadam and colleagues examined commercial insurance claims from the Optum Clinformatics database to assess the impact of statins on 118,479 patients without preexisting diabetes admitted for a first episode of acute pancreatitis between 2008 and 2020.
They compared patients who consistently used statins with irregular users and nonusers. Regular statin usage was defined as patients who had statin prescriptions filled for at least 80% of the year prior to their acute pancreatitis diagnosis. The analysis included 9,048 patients (7.6%) who used statins regularly, 27,272 (23%) who used statins irregularly, and 82,159 (69.3%) nonusers.
With a median follow-up of 3.5 years, the 5-year cumulative incidence of postpancreatitis diabetes was 7.5% among regular statin users and 12.7% among nonusers. Regular statin users had a 42% lower risk of developing postpancreatitis diabetes, compared with nonusers. Irregular statin users had a 15% lower risk of postpancreatitis diabetes.
In addition, the 5-year cumulative incidence of insulin-dependent postpancreatitis diabetes was 2.4% among regular statin users and 6.6% among nonusers. Regular statin users had a 52% lower risk of developing insulin-dependent diabetes as compared with nonusers.
Daily dosage didn’t demonstrate a linear dose-response relationship. That means high-dose statins may not be more effective in preventing diabetes as compared with lower doses, the study authors write.
Statin usage was effective across additional analyses, including sex, etiologies of pancreatitis, and in both mild and severe acute pancreatitis. According to the study authors, this suggests that a broad population of these patients may benefit from statins.
“We were pleasantly surprised by the variety of findings,” Dr. Thiruvengadam said. “We’re seeing strong signals, especially with consistency of usage.”
Ongoing studies
The results may seem paradoxical, the study authors write, given an epidemiologic association with a slight increase in new-onset diabetes with statin initiation. But, as other researchers have reported, postpancreatitis diabetes and type 2 diabetes have different clinical features and underlying pathophysiology. For example, patients with postpancreatitis diabetes have much higher rates of requiring insulin, hospitalization, and all-cause mortality, the study authors write.
In fact, postpancreatitis diabetes is thought to be driven by chronic low-grade inflammation attributable to interleukin-6 and tumor necrosis factor–alpha. Statins have been shown to reduce tumor necrosis factor–alpha secretion and the production of C-reactive protein in response to circulating interleukin-6 in hepatocytes, they write.
The results should inform long-term prospective studies of acute pancreatitis, the study authors write, as well as randomized controlled trials of statins.
In the meantime, gastroenterologists and primary care physicians who see outpatients after hospitalization for acute pancreatitis may consider using statins, particularly in those who may have another possible indication for statin therapy, such as mild hyperlipidemia.
“There appears to be a low-dose benefit, which is another reason why providers may consider using statins, though it’s not for everyone with pancreatitis,” Dr. Thiruvengadam said. “This could be an exploratory pathway and suggested for use in the right setting.”
The Type 1 Diabetes in Acute Pancreatitis Consortium, sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases, is conducting an observational cohort study at more than a dozen locations across the country to investigate the incidence, etiology, and pathophysiology of diabetes after acute pancreatitis.
“Diabetes is surprisingly common after even a single attack of acute pancreatitis,” Chris Forsmark, MD, professor of medicine and chief of the division of gastroenterology, hepatology, and nutrition at the University of Florida, Gainesville, told this news organization.
Dr. Forsmark, who wasn’t involved with this study, is a member of T1DAPC and one of the principal investigators in Florida.
“The reduction of risk by 42% is quite substantial,” he said. “Like all such studies, there is risk of bias and confounding in determining the actual risk. Nonetheless, the results provide a strong reason for confirmation in other datasets and for further study.”
The study didn’t report funding support. Dr. Thiruvengadam and Dr. Forsmark report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Use of cholesterol-lowering statins was linked to a lower risk of developing a subtype of diabetes that occurs after acute pancreatitis, according to a new report.
The benefits of statins depended on the consistency of usage, with regular users having a lower risk of developing postpancreatitis diabetes than irregular users. The results were similar with low, moderate, and high statin doses, as well as in cases of both mild and severe acute pancreatitis.
“About 15% of patients with acute pancreatitis will develop diabetes mellitus in the next 5 years, and although we can monitor for it, we can’t do anything to prevent it,” Nikhil Thiruvengadam, MD, the lead study author and a gastroenterologist at Loma Linda (Calif.) University, told this news organization.
“This could push you as a clinician to prescribe [a statin if you have a reason to] because it could provide two benefits instead of just one,” he said.
The study was published online in Clinical Gastroenterology and Hepatology.
Steady use mattered, not dose
Patients with acute pancreatitis face at least a twofold increased risk of developing postpancreatitis diabetes, the study authors write. Although previous studies have shown that statins can lower the incidence and severity of acute pancreatitis, they haven’t been studied for the prevention of postpancreatitis diabetes.
In a collaborative study with several other universities, Dr. Thiruvengadam and colleagues examined commercial insurance claims from the Optum Clinformatics database to assess the impact of statins on 118,479 patients without preexisting diabetes admitted for a first episode of acute pancreatitis between 2008 and 2020.
They compared patients who consistently used statins with irregular users and nonusers. Regular statin usage was defined as patients who had statin prescriptions filled for at least 80% of the year prior to their acute pancreatitis diagnosis. The analysis included 9,048 patients (7.6%) who used statins regularly, 27,272 (23%) who used statins irregularly, and 82,159 (69.3%) nonusers.
With a median follow-up of 3.5 years, the 5-year cumulative incidence of postpancreatitis diabetes was 7.5% among regular statin users and 12.7% among nonusers. Regular statin users had a 42% lower risk of developing postpancreatitis diabetes, compared with nonusers. Irregular statin users had a 15% lower risk of postpancreatitis diabetes.
In addition, the 5-year cumulative incidence of insulin-dependent postpancreatitis diabetes was 2.4% among regular statin users and 6.6% among nonusers. Regular statin users had a 52% lower risk of developing insulin-dependent diabetes as compared with nonusers.
Daily dosage didn’t demonstrate a linear dose-response relationship. That means high-dose statins may not be more effective in preventing diabetes as compared with lower doses, the study authors write.
Statin usage was effective across additional analyses, including sex, etiologies of pancreatitis, and in both mild and severe acute pancreatitis. According to the study authors, this suggests that a broad population of these patients may benefit from statins.
“We were pleasantly surprised by the variety of findings,” Dr. Thiruvengadam said. “We’re seeing strong signals, especially with consistency of usage.”
Ongoing studies
The results may seem paradoxical, the study authors write, given an epidemiologic association with a slight increase in new-onset diabetes with statin initiation. But, as other researchers have reported, postpancreatitis diabetes and type 2 diabetes have different clinical features and underlying pathophysiology. For example, patients with postpancreatitis diabetes have much higher rates of requiring insulin, hospitalization, and all-cause mortality, the study authors write.
In fact, postpancreatitis diabetes is thought to be driven by chronic low-grade inflammation attributable to interleukin-6 and tumor necrosis factor–alpha. Statins have been shown to reduce tumor necrosis factor–alpha secretion and the production of C-reactive protein in response to circulating interleukin-6 in hepatocytes, they write.
The results should inform long-term prospective studies of acute pancreatitis, the study authors write, as well as randomized controlled trials of statins.
In the meantime, gastroenterologists and primary care physicians who see outpatients after hospitalization for acute pancreatitis may consider using statins, particularly in those who may have another possible indication for statin therapy, such as mild hyperlipidemia.
“There appears to be a low-dose benefit, which is another reason why providers may consider using statins, though it’s not for everyone with pancreatitis,” Dr. Thiruvengadam said. “This could be an exploratory pathway and suggested for use in the right setting.”
The Type 1 Diabetes in Acute Pancreatitis Consortium, sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases, is conducting an observational cohort study at more than a dozen locations across the country to investigate the incidence, etiology, and pathophysiology of diabetes after acute pancreatitis.
“Diabetes is surprisingly common after even a single attack of acute pancreatitis,” Chris Forsmark, MD, professor of medicine and chief of the division of gastroenterology, hepatology, and nutrition at the University of Florida, Gainesville, told this news organization.
Dr. Forsmark, who wasn’t involved with this study, is a member of T1DAPC and one of the principal investigators in Florida.
“The reduction of risk by 42% is quite substantial,” he said. “Like all such studies, there is risk of bias and confounding in determining the actual risk. Nonetheless, the results provide a strong reason for confirmation in other datasets and for further study.”
The study didn’t report funding support. Dr. Thiruvengadam and Dr. Forsmark report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Use of cholesterol-lowering statins was linked to a lower risk of developing a subtype of diabetes that occurs after acute pancreatitis, according to a new report.
The benefits of statins depended on the consistency of usage, with regular users having a lower risk of developing postpancreatitis diabetes than irregular users. The results were similar with low, moderate, and high statin doses, as well as in cases of both mild and severe acute pancreatitis.
“About 15% of patients with acute pancreatitis will develop diabetes mellitus in the next 5 years, and although we can monitor for it, we can’t do anything to prevent it,” Nikhil Thiruvengadam, MD, the lead study author and a gastroenterologist at Loma Linda (Calif.) University, told this news organization.
“This could push you as a clinician to prescribe [a statin if you have a reason to] because it could provide two benefits instead of just one,” he said.
The study was published online in Clinical Gastroenterology and Hepatology.
Steady use mattered, not dose
Patients with acute pancreatitis face at least a twofold increased risk of developing postpancreatitis diabetes, the study authors write. Although previous studies have shown that statins can lower the incidence and severity of acute pancreatitis, they haven’t been studied for the prevention of postpancreatitis diabetes.
In a collaborative study with several other universities, Dr. Thiruvengadam and colleagues examined commercial insurance claims from the Optum Clinformatics database to assess the impact of statins on 118,479 patients without preexisting diabetes admitted for a first episode of acute pancreatitis between 2008 and 2020.
They compared patients who consistently used statins with irregular users and nonusers. Regular statin usage was defined as patients who had statin prescriptions filled for at least 80% of the year prior to their acute pancreatitis diagnosis. The analysis included 9,048 patients (7.6%) who used statins regularly, 27,272 (23%) who used statins irregularly, and 82,159 (69.3%) nonusers.
With a median follow-up of 3.5 years, the 5-year cumulative incidence of postpancreatitis diabetes was 7.5% among regular statin users and 12.7% among nonusers. Regular statin users had a 42% lower risk of developing postpancreatitis diabetes, compared with nonusers. Irregular statin users had a 15% lower risk of postpancreatitis diabetes.
In addition, the 5-year cumulative incidence of insulin-dependent postpancreatitis diabetes was 2.4% among regular statin users and 6.6% among nonusers. Regular statin users had a 52% lower risk of developing insulin-dependent diabetes as compared with nonusers.
Daily dosage didn’t demonstrate a linear dose-response relationship. That means high-dose statins may not be more effective in preventing diabetes as compared with lower doses, the study authors write.
Statin usage was effective across additional analyses, including sex, etiologies of pancreatitis, and in both mild and severe acute pancreatitis. According to the study authors, this suggests that a broad population of these patients may benefit from statins.
“We were pleasantly surprised by the variety of findings,” Dr. Thiruvengadam said. “We’re seeing strong signals, especially with consistency of usage.”
Ongoing studies
The results may seem paradoxical, the study authors write, given an epidemiologic association with a slight increase in new-onset diabetes with statin initiation. But, as other researchers have reported, postpancreatitis diabetes and type 2 diabetes have different clinical features and underlying pathophysiology. For example, patients with postpancreatitis diabetes have much higher rates of requiring insulin, hospitalization, and all-cause mortality, the study authors write.
In fact, postpancreatitis diabetes is thought to be driven by chronic low-grade inflammation attributable to interleukin-6 and tumor necrosis factor–alpha. Statins have been shown to reduce tumor necrosis factor–alpha secretion and the production of C-reactive protein in response to circulating interleukin-6 in hepatocytes, they write.
The results should inform long-term prospective studies of acute pancreatitis, the study authors write, as well as randomized controlled trials of statins.
In the meantime, gastroenterologists and primary care physicians who see outpatients after hospitalization for acute pancreatitis may consider using statins, particularly in those who may have another possible indication for statin therapy, such as mild hyperlipidemia.
“There appears to be a low-dose benefit, which is another reason why providers may consider using statins, though it’s not for everyone with pancreatitis,” Dr. Thiruvengadam said. “This could be an exploratory pathway and suggested for use in the right setting.”
The Type 1 Diabetes in Acute Pancreatitis Consortium, sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases, is conducting an observational cohort study at more than a dozen locations across the country to investigate the incidence, etiology, and pathophysiology of diabetes after acute pancreatitis.
“Diabetes is surprisingly common after even a single attack of acute pancreatitis,” Chris Forsmark, MD, professor of medicine and chief of the division of gastroenterology, hepatology, and nutrition at the University of Florida, Gainesville, told this news organization.
Dr. Forsmark, who wasn’t involved with this study, is a member of T1DAPC and one of the principal investigators in Florida.
“The reduction of risk by 42% is quite substantial,” he said. “Like all such studies, there is risk of bias and confounding in determining the actual risk. Nonetheless, the results provide a strong reason for confirmation in other datasets and for further study.”
The study didn’t report funding support. Dr. Thiruvengadam and Dr. Forsmark report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
COVID-19 infection late in pregnancy linked to sevenfold risk of preterm birth
Pregnant women who get infected with SARS-CoV-2 in their third trimester are almost three times as likely to have a preterm birth, while infection after 34 weeks’ gestation raises this risk sevenfold, based on the largest matched population-based cohort study published to date.
These findings support previous studies, underscoring the need for pregnant women and their families to take preventive measures against infection, lead author Noga Fallach, MA, of the Kahn-Sagol-Maccabi Research and Innovation Center, Tel Aviv, and colleagues reported.
Past research has suggested that COVID-19 may cause low birth weights and preterm birth in pregnant women, but those studies didn’t report outcomes for each trimester, the investigators wrote in PLoS ONE, noting that “timing of viral infection during fetal development may affect birth and other health outcomes.”
To address this knowledge gap, the investigators looked back at data from 2,703 pregnant women in Israel who tested positive for SARS-CoV-2 from Feb. 21, 2020, to July 2, 2021. Pregnancy outcomes in these women were compared with outcomes in an equal number of uninfected pregnant women. Vaccination status was not reported.
Comparing the two groups showed that catching COVID-19 in the third trimester was linked with nearly triple the risk of preterm birth (odds ratio, 2.76; 95% confidence interval, 1.63-4.67), and more than quadruple the risk if COVID-19 symptoms were present (OR, 4.28; 95% CI, 1.94-9.41). Women who tested positive for SARS-CoV-2 after 34 weeks’ gestation were seven times more likely than uninfected women to deliver early (OR, 7.10; 95% CI, 2.44-20.61).
Pregnant women who caught COVID-19 in the first two trimesters were not significantly more likely to have a preterm birth. Infection was not associated with abnormally low birth rates, or pregnancy loss, in any trimester.
Tal Patalon, MD, coauthor and head of the Kahn-Sagol-Maccabi Research and Innovation Center, focused on these more optimistic findings in an interview.
“The results are encouraging, and reassuring that COVID-19 infection during pregnancy is not associated with any type of pregnancy loss,” Dr. Patalon said.
She also pointed out that the women in the study were infected with SARS-CoV-2 variants that are no longer common.
“It should be remembered that the research group tested the COVID-19 pre-Delta variants, and does not refer to the dominant variant today, which is Omicron,” Dr. Patalon said.
Still, the investigators concluded that the “results underline the importance of preventive measures taken against SARS-CoV-2 infection among pregnant women and their families.”
Sonja A. Rasmussen, MD, of the University of Florida, Gainesville, said that the issue with out-of-date variants in published research has been one of the “real challenges” in studying the ever-evolving COVID-19 pandemic; however, it’s not a good enough reason to dismiss this study.
“I think at this point, we need to assume that it applies to Omicron too,” Dr. Rasmussen said, noting that other respiratory viruses, like influenza, have also been shown to increase the risk of preterm birth when contracted in late pregnancy.
While the present findings highlight the risk of infection in the third trimester, Dr. Rasmussen advised women in all stages of pregnancy to protect themselves against COVID-19, based on the knowledge that illness in a mother can affect normal growth and development in a fetus, even if it doesn’t lead to preterm birth.
“A mom getting sick during pregnancy is not good for the baby,” Dr. Rasmussen said. “The baby’s really dependent on the mom. So you want that baby to have good nutrition throughout the pregnancy. It’s just as important earlier on as later. And you want that baby to get good oxygenation no matter what time [in the pregnancy]. I know that people want a little bit of a break [from preventive measures]. But I would emphasize that if you’re pregnant, we do all sorts of things during pregnancy to make sure that our babies are safe and healthy, and I would continue that for the whole pregnancy.”
Specifically, Dr. Rasmussen advised social distancing, use of an N95 mask, and vaccination. Getting vaccinated during pregnancy helps newborns fight off infection until 6 months of age, she added, when they become eligible for vaccination themselves. This added benefit was recently reported in a study published in the New England Journal of Medicine , for which Dr. Rasmussen cowrote an editorial .
“Vaccines have been approved for 6 months and older,” Dr. Rasmussen said. “But what do you do in those first 6 months of life? That’s a high-risk time for kids.”
Despite these risks, convincing pregnant women to get vaccinated remains a key challenge for health care providers, according to Dr. Rasmussen, even with an abundance of safety data. “Early on [in the pandemic], we said we didn’t know a lot about risks. We knew that other vaccines were safe during pregnancy, but we didn’t have a lot of information about a COVID-19 vaccine. But now we have a lot of data on safety during pregnancy, and these vaccines appear to be completely safe, based on the information we have. There have been many, many pregnant women vaccinated in the United States and in other countries.”
For reluctant expecting mothers, Dr. Rasmussen offered some words of advice: “I know that you worry about anything you do when you’re pregnant. But this is something that you can do to help your baby – now, to make a preterm birth less likely, and later, after the baby is born.
“The most important thing is for the pregnant person to hear this [vaccine recommendation] from their doctor,” she added. “If they’re going to listen to anybody, they’re going to listen to their physician. That’s what the data have shown for a long time.”
The investigators and Dr. Rasmussen disclosed no conflicts of interest.
Pregnant women who get infected with SARS-CoV-2 in their third trimester are almost three times as likely to have a preterm birth, while infection after 34 weeks’ gestation raises this risk sevenfold, based on the largest matched population-based cohort study published to date.
These findings support previous studies, underscoring the need for pregnant women and their families to take preventive measures against infection, lead author Noga Fallach, MA, of the Kahn-Sagol-Maccabi Research and Innovation Center, Tel Aviv, and colleagues reported.
Past research has suggested that COVID-19 may cause low birth weights and preterm birth in pregnant women, but those studies didn’t report outcomes for each trimester, the investigators wrote in PLoS ONE, noting that “timing of viral infection during fetal development may affect birth and other health outcomes.”
To address this knowledge gap, the investigators looked back at data from 2,703 pregnant women in Israel who tested positive for SARS-CoV-2 from Feb. 21, 2020, to July 2, 2021. Pregnancy outcomes in these women were compared with outcomes in an equal number of uninfected pregnant women. Vaccination status was not reported.
Comparing the two groups showed that catching COVID-19 in the third trimester was linked with nearly triple the risk of preterm birth (odds ratio, 2.76; 95% confidence interval, 1.63-4.67), and more than quadruple the risk if COVID-19 symptoms were present (OR, 4.28; 95% CI, 1.94-9.41). Women who tested positive for SARS-CoV-2 after 34 weeks’ gestation were seven times more likely than uninfected women to deliver early (OR, 7.10; 95% CI, 2.44-20.61).
Pregnant women who caught COVID-19 in the first two trimesters were not significantly more likely to have a preterm birth. Infection was not associated with abnormally low birth rates, or pregnancy loss, in any trimester.
Tal Patalon, MD, coauthor and head of the Kahn-Sagol-Maccabi Research and Innovation Center, focused on these more optimistic findings in an interview.
“The results are encouraging, and reassuring that COVID-19 infection during pregnancy is not associated with any type of pregnancy loss,” Dr. Patalon said.
She also pointed out that the women in the study were infected with SARS-CoV-2 variants that are no longer common.
“It should be remembered that the research group tested the COVID-19 pre-Delta variants, and does not refer to the dominant variant today, which is Omicron,” Dr. Patalon said.
Still, the investigators concluded that the “results underline the importance of preventive measures taken against SARS-CoV-2 infection among pregnant women and their families.”
Sonja A. Rasmussen, MD, of the University of Florida, Gainesville, said that the issue with out-of-date variants in published research has been one of the “real challenges” in studying the ever-evolving COVID-19 pandemic; however, it’s not a good enough reason to dismiss this study.
“I think at this point, we need to assume that it applies to Omicron too,” Dr. Rasmussen said, noting that other respiratory viruses, like influenza, have also been shown to increase the risk of preterm birth when contracted in late pregnancy.
While the present findings highlight the risk of infection in the third trimester, Dr. Rasmussen advised women in all stages of pregnancy to protect themselves against COVID-19, based on the knowledge that illness in a mother can affect normal growth and development in a fetus, even if it doesn’t lead to preterm birth.
“A mom getting sick during pregnancy is not good for the baby,” Dr. Rasmussen said. “The baby’s really dependent on the mom. So you want that baby to have good nutrition throughout the pregnancy. It’s just as important earlier on as later. And you want that baby to get good oxygenation no matter what time [in the pregnancy]. I know that people want a little bit of a break [from preventive measures]. But I would emphasize that if you’re pregnant, we do all sorts of things during pregnancy to make sure that our babies are safe and healthy, and I would continue that for the whole pregnancy.”
Specifically, Dr. Rasmussen advised social distancing, use of an N95 mask, and vaccination. Getting vaccinated during pregnancy helps newborns fight off infection until 6 months of age, she added, when they become eligible for vaccination themselves. This added benefit was recently reported in a study published in the New England Journal of Medicine , for which Dr. Rasmussen cowrote an editorial .
“Vaccines have been approved for 6 months and older,” Dr. Rasmussen said. “But what do you do in those first 6 months of life? That’s a high-risk time for kids.”
Despite these risks, convincing pregnant women to get vaccinated remains a key challenge for health care providers, according to Dr. Rasmussen, even with an abundance of safety data. “Early on [in the pandemic], we said we didn’t know a lot about risks. We knew that other vaccines were safe during pregnancy, but we didn’t have a lot of information about a COVID-19 vaccine. But now we have a lot of data on safety during pregnancy, and these vaccines appear to be completely safe, based on the information we have. There have been many, many pregnant women vaccinated in the United States and in other countries.”
For reluctant expecting mothers, Dr. Rasmussen offered some words of advice: “I know that you worry about anything you do when you’re pregnant. But this is something that you can do to help your baby – now, to make a preterm birth less likely, and later, after the baby is born.
“The most important thing is for the pregnant person to hear this [vaccine recommendation] from their doctor,” she added. “If they’re going to listen to anybody, they’re going to listen to their physician. That’s what the data have shown for a long time.”
The investigators and Dr. Rasmussen disclosed no conflicts of interest.
Pregnant women who get infected with SARS-CoV-2 in their third trimester are almost three times as likely to have a preterm birth, while infection after 34 weeks’ gestation raises this risk sevenfold, based on the largest matched population-based cohort study published to date.
These findings support previous studies, underscoring the need for pregnant women and their families to take preventive measures against infection, lead author Noga Fallach, MA, of the Kahn-Sagol-Maccabi Research and Innovation Center, Tel Aviv, and colleagues reported.
Past research has suggested that COVID-19 may cause low birth weights and preterm birth in pregnant women, but those studies didn’t report outcomes for each trimester, the investigators wrote in PLoS ONE, noting that “timing of viral infection during fetal development may affect birth and other health outcomes.”
To address this knowledge gap, the investigators looked back at data from 2,703 pregnant women in Israel who tested positive for SARS-CoV-2 from Feb. 21, 2020, to July 2, 2021. Pregnancy outcomes in these women were compared with outcomes in an equal number of uninfected pregnant women. Vaccination status was not reported.
Comparing the two groups showed that catching COVID-19 in the third trimester was linked with nearly triple the risk of preterm birth (odds ratio, 2.76; 95% confidence interval, 1.63-4.67), and more than quadruple the risk if COVID-19 symptoms were present (OR, 4.28; 95% CI, 1.94-9.41). Women who tested positive for SARS-CoV-2 after 34 weeks’ gestation were seven times more likely than uninfected women to deliver early (OR, 7.10; 95% CI, 2.44-20.61).
Pregnant women who caught COVID-19 in the first two trimesters were not significantly more likely to have a preterm birth. Infection was not associated with abnormally low birth rates, or pregnancy loss, in any trimester.
Tal Patalon, MD, coauthor and head of the Kahn-Sagol-Maccabi Research and Innovation Center, focused on these more optimistic findings in an interview.
“The results are encouraging, and reassuring that COVID-19 infection during pregnancy is not associated with any type of pregnancy loss,” Dr. Patalon said.
She also pointed out that the women in the study were infected with SARS-CoV-2 variants that are no longer common.
“It should be remembered that the research group tested the COVID-19 pre-Delta variants, and does not refer to the dominant variant today, which is Omicron,” Dr. Patalon said.
Still, the investigators concluded that the “results underline the importance of preventive measures taken against SARS-CoV-2 infection among pregnant women and their families.”
Sonja A. Rasmussen, MD, of the University of Florida, Gainesville, said that the issue with out-of-date variants in published research has been one of the “real challenges” in studying the ever-evolving COVID-19 pandemic; however, it’s not a good enough reason to dismiss this study.
“I think at this point, we need to assume that it applies to Omicron too,” Dr. Rasmussen said, noting that other respiratory viruses, like influenza, have also been shown to increase the risk of preterm birth when contracted in late pregnancy.
While the present findings highlight the risk of infection in the third trimester, Dr. Rasmussen advised women in all stages of pregnancy to protect themselves against COVID-19, based on the knowledge that illness in a mother can affect normal growth and development in a fetus, even if it doesn’t lead to preterm birth.
“A mom getting sick during pregnancy is not good for the baby,” Dr. Rasmussen said. “The baby’s really dependent on the mom. So you want that baby to have good nutrition throughout the pregnancy. It’s just as important earlier on as later. And you want that baby to get good oxygenation no matter what time [in the pregnancy]. I know that people want a little bit of a break [from preventive measures]. But I would emphasize that if you’re pregnant, we do all sorts of things during pregnancy to make sure that our babies are safe and healthy, and I would continue that for the whole pregnancy.”
Specifically, Dr. Rasmussen advised social distancing, use of an N95 mask, and vaccination. Getting vaccinated during pregnancy helps newborns fight off infection until 6 months of age, she added, when they become eligible for vaccination themselves. This added benefit was recently reported in a study published in the New England Journal of Medicine , for which Dr. Rasmussen cowrote an editorial .
“Vaccines have been approved for 6 months and older,” Dr. Rasmussen said. “But what do you do in those first 6 months of life? That’s a high-risk time for kids.”
Despite these risks, convincing pregnant women to get vaccinated remains a key challenge for health care providers, according to Dr. Rasmussen, even with an abundance of safety data. “Early on [in the pandemic], we said we didn’t know a lot about risks. We knew that other vaccines were safe during pregnancy, but we didn’t have a lot of information about a COVID-19 vaccine. But now we have a lot of data on safety during pregnancy, and these vaccines appear to be completely safe, based on the information we have. There have been many, many pregnant women vaccinated in the United States and in other countries.”
For reluctant expecting mothers, Dr. Rasmussen offered some words of advice: “I know that you worry about anything you do when you’re pregnant. But this is something that you can do to help your baby – now, to make a preterm birth less likely, and later, after the baby is born.
“The most important thing is for the pregnant person to hear this [vaccine recommendation] from their doctor,” she added. “If they’re going to listen to anybody, they’re going to listen to their physician. That’s what the data have shown for a long time.”
The investigators and Dr. Rasmussen disclosed no conflicts of interest.
FROM PLOS ONE