User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Debate: Should biologics be used for milder cases of psoriasis?
The issue was tackled in a debate at the American Academy of Dermatology Virtual Meeting Experience.
Taking the con side, Kenneth Gordon, MD, professor and chair of dermatology at the Medical College of Wisconsin, Milwaukee, argued that, with the high cost of biologics, availability of many alternatives, and other issues, “we should just say no. ... There is no good reason that we need to expand the use of biologics in patients with limited disease.”
On the pro side, Richard Langley, MD, professor of dermatology at Dalhousie University Halifax, N.S, argued for a nuanced approach. He noted that patients with smaller patches of disease can be just as miserable as patients who hit traditional benchmarks of increased severity, such as high body surface area involvement – especially if those small areas are in sensitive locations like the scalp, palms, or genitals.
The decision to use a biologic should hinge on how badly patients and their quality of life are affected, not on “some artificial and limiting definition” of severity, Dr. Langley said.
Dr. Gordon didn’t disagree, noting that current use criteria include objective measures as well as disease in sensitive areas and failure of alternative treatments.
Rather, he was concerned about “expanding the definition of who is eligible beyond these criteria ... to chase every last bit of” disease. “I don’t think we have” a good rationale for that approach, he said.
Cost is the most important issue, Dr. Gordon said.
With more biologics on the way and prices continuing to go up, “there is going to a be a huge challenge to our use of these expensive medicines over the next few years” from payers. “It is important that we use them smartly in order to make sure we are able to use them for people with severe disease” who really need them. If “we start using biologics for all our patients with psoriasis,” it will be a “cost disaster,” Dr. Gordon said.
In addition, topicals and home phototherapy can be effective as long as patients adhere to them, as can alternative systemic agents, such as methotrexate and apremilast.
Often with biologics, “the issue is mainly convenience” rather than a fundamental problem with the alternatives, and despite the good safety record in trials, “chasing the last bit” of psoriasis with a biologic “is not necessarily” without risk for the patient, Dr. Gordon said.
Still, there can be a “pretty significant disconnect” between how patients perceive their psoriasis and “what physicians are thinking and prescribing” for it based on objective measures, Dr. Langley noted. Sometimes patients who have limited disease but are in significant distress aren’t even receiving treatment or are only given another cream to add to their collection of ones that haven’t worked.
One problem with traditional severity classifications is that they don’t generally take patients’ subjective experience into account, he added. There’s also been a lack of standardization to the point that dermatologists, researchers, and payers can sometimes disagree over severity in a given patient.
There’s movement toward better incorporation of patient experience into severity considerations, but for now at least, a designation of mild psoriasis can underestimate the true severity of disease, Dr. Langley said.
Dr. Gordon and Dr. Langley reported receiving honoraria and/or research support from many pharmaceutical companies, including AbbVie, Pfizer, and Lilly.
A version of this article first appeared on Medscape.com.
The issue was tackled in a debate at the American Academy of Dermatology Virtual Meeting Experience.
Taking the con side, Kenneth Gordon, MD, professor and chair of dermatology at the Medical College of Wisconsin, Milwaukee, argued that, with the high cost of biologics, availability of many alternatives, and other issues, “we should just say no. ... There is no good reason that we need to expand the use of biologics in patients with limited disease.”
On the pro side, Richard Langley, MD, professor of dermatology at Dalhousie University Halifax, N.S, argued for a nuanced approach. He noted that patients with smaller patches of disease can be just as miserable as patients who hit traditional benchmarks of increased severity, such as high body surface area involvement – especially if those small areas are in sensitive locations like the scalp, palms, or genitals.
The decision to use a biologic should hinge on how badly patients and their quality of life are affected, not on “some artificial and limiting definition” of severity, Dr. Langley said.
Dr. Gordon didn’t disagree, noting that current use criteria include objective measures as well as disease in sensitive areas and failure of alternative treatments.
Rather, he was concerned about “expanding the definition of who is eligible beyond these criteria ... to chase every last bit of” disease. “I don’t think we have” a good rationale for that approach, he said.
Cost is the most important issue, Dr. Gordon said.
With more biologics on the way and prices continuing to go up, “there is going to a be a huge challenge to our use of these expensive medicines over the next few years” from payers. “It is important that we use them smartly in order to make sure we are able to use them for people with severe disease” who really need them. If “we start using biologics for all our patients with psoriasis,” it will be a “cost disaster,” Dr. Gordon said.
In addition, topicals and home phototherapy can be effective as long as patients adhere to them, as can alternative systemic agents, such as methotrexate and apremilast.
Often with biologics, “the issue is mainly convenience” rather than a fundamental problem with the alternatives, and despite the good safety record in trials, “chasing the last bit” of psoriasis with a biologic “is not necessarily” without risk for the patient, Dr. Gordon said.
Still, there can be a “pretty significant disconnect” between how patients perceive their psoriasis and “what physicians are thinking and prescribing” for it based on objective measures, Dr. Langley noted. Sometimes patients who have limited disease but are in significant distress aren’t even receiving treatment or are only given another cream to add to their collection of ones that haven’t worked.
One problem with traditional severity classifications is that they don’t generally take patients’ subjective experience into account, he added. There’s also been a lack of standardization to the point that dermatologists, researchers, and payers can sometimes disagree over severity in a given patient.
There’s movement toward better incorporation of patient experience into severity considerations, but for now at least, a designation of mild psoriasis can underestimate the true severity of disease, Dr. Langley said.
Dr. Gordon and Dr. Langley reported receiving honoraria and/or research support from many pharmaceutical companies, including AbbVie, Pfizer, and Lilly.
A version of this article first appeared on Medscape.com.
The issue was tackled in a debate at the American Academy of Dermatology Virtual Meeting Experience.
Taking the con side, Kenneth Gordon, MD, professor and chair of dermatology at the Medical College of Wisconsin, Milwaukee, argued that, with the high cost of biologics, availability of many alternatives, and other issues, “we should just say no. ... There is no good reason that we need to expand the use of biologics in patients with limited disease.”
On the pro side, Richard Langley, MD, professor of dermatology at Dalhousie University Halifax, N.S, argued for a nuanced approach. He noted that patients with smaller patches of disease can be just as miserable as patients who hit traditional benchmarks of increased severity, such as high body surface area involvement – especially if those small areas are in sensitive locations like the scalp, palms, or genitals.
The decision to use a biologic should hinge on how badly patients and their quality of life are affected, not on “some artificial and limiting definition” of severity, Dr. Langley said.
Dr. Gordon didn’t disagree, noting that current use criteria include objective measures as well as disease in sensitive areas and failure of alternative treatments.
Rather, he was concerned about “expanding the definition of who is eligible beyond these criteria ... to chase every last bit of” disease. “I don’t think we have” a good rationale for that approach, he said.
Cost is the most important issue, Dr. Gordon said.
With more biologics on the way and prices continuing to go up, “there is going to a be a huge challenge to our use of these expensive medicines over the next few years” from payers. “It is important that we use them smartly in order to make sure we are able to use them for people with severe disease” who really need them. If “we start using biologics for all our patients with psoriasis,” it will be a “cost disaster,” Dr. Gordon said.
In addition, topicals and home phototherapy can be effective as long as patients adhere to them, as can alternative systemic agents, such as methotrexate and apremilast.
Often with biologics, “the issue is mainly convenience” rather than a fundamental problem with the alternatives, and despite the good safety record in trials, “chasing the last bit” of psoriasis with a biologic “is not necessarily” without risk for the patient, Dr. Gordon said.
Still, there can be a “pretty significant disconnect” between how patients perceive their psoriasis and “what physicians are thinking and prescribing” for it based on objective measures, Dr. Langley noted. Sometimes patients who have limited disease but are in significant distress aren’t even receiving treatment or are only given another cream to add to their collection of ones that haven’t worked.
One problem with traditional severity classifications is that they don’t generally take patients’ subjective experience into account, he added. There’s also been a lack of standardization to the point that dermatologists, researchers, and payers can sometimes disagree over severity in a given patient.
There’s movement toward better incorporation of patient experience into severity considerations, but for now at least, a designation of mild psoriasis can underestimate the true severity of disease, Dr. Langley said.
Dr. Gordon and Dr. Langley reported receiving honoraria and/or research support from many pharmaceutical companies, including AbbVie, Pfizer, and Lilly.
A version of this article first appeared on Medscape.com.
Nurses or physicians: Who are at highest suicide risk?
Female nurses are at significantly greater risk of dying by suicide than physicians in findings that contradict previous research suggesting doctors are at greatest risk.
Results of a large retrospective cohort study show that nurses of both sexes were 18% more likely to die by suicide, compared with individuals in the general population. In addition, compared with female physicians, the suicide risk among female nurses was 70% higher.
“The main takeaway is that the risk of suicide among nurses is twice that of the general population and even higher than that among physicians, a population known to be at high risk,” lead author Matthew Davis, MPH, PhD, associate professor, department of systems, populations, and leadership, University of Michigan, Ann Arbor, said in an interview.
The study was published online April 14, 2021, in JAMA Psychiatry.
Focus on physicians
Compared with the general public, health care workers are at higher risk for suicide, but most studies of suicide have focused on physicians, Dr. Davis said.
Although “there were several older studies hinting that there might be a difference in suicide risk among nurses,” the data were insufficient to “make an overall conclusion,” he noted.
For that reason, his group “set out to make the best estimates possible” by using a large dataset from the National Violent Death Reporting System of the Centers for Disease Control and Prevention spanning the years 2007-2018 and focusing on suicides by individuals aged 30 years and older (n = 159,372 suicides).
Additional workforce data were acquired from the Bureau of Labor Statistics and the Association of Medical Colleges State Physician Workforce Data.
An important area of focus was method of suicide.
“ and know how to use them to overdose, which also increases their risk,” Dr. Davis said in a press release.
Enormous job strain
The researchers identified 2,374 suicides among nurses, 857 suicides among physicians, and 156,141 suicides in the general population.
Compared with the general population, nurses who died by suicide were more likely to be women, less racially diverse (non-Hispanic White), and more likely to have been married.
Rates of suicide were higher among nurses than among the general population, with a sex-adjusted incidence for 2017-2018 of 23.8 per 100,000 versus 20.1 per 100,000 (relative risk, 1.18; 95% confidence interval, 1.03-1.36).
The difference between suicide rates among female nurses and among women in the general population was even more striking: In 2017-2018, the suicide incidence among nurses was 17.1 per 100,000 versus 8.6 per 100,000 in the population at large (RR, 1.99; 95% CI, 1.82-2.18).
“In absolute terms, being a female nurse was associated with an additional 8.5 suicides per 100,000 (7.0-10.0), compared with the general population,” the authors reported.
In contrast, overall physician suicide rates were not statistically different from those of the general population (RR, 1.01; 95% CI, 0.79-1.30) except during the period 2011-2012 (11.7 per 100,000; 95% CI, 6.6-16.8 vs. 7.5 per 100,000; 95% CI, 7.2-7.7).
Clinicians of both sexes were more likely to use poisoning and less likely to use a firearm, compared with individuals in the general population who died by suicide. For example, 24.9% (23.5%-26.4%) of nurse suicides involved poisoning, compared to 16.8% (16.6%-17.0%) of suicides in the general population.
Toxicology reports showed that the presence of antidepressants, benzodiazepines, barbiturates, and opiates was more common in clinician suicides than suicides in the general population.
Dr. Davis suggested the higher risk for suicide among nurses, compared with physicians, might be attributed to “high job demands – for example, nurses provide the majority of bedside care, work long shifts in stressful environments, and have less autonomy.
“Health care workers and friends and family of health care workers need to be aware of mental health issues and suicide risk that can be associated with the job and, most importantly, recognize those who may be struggling and encourage them to get help by calling the National Suicide Prevention Lifeline,” he said.
Other potential contributors include “avoidance of mental health services due to stigma and greater access to the means to commit suicide via medication,” Dr. Davis noted.
Benchmark research
Commenting on the study, Constance Guille, MD, MSCR, professor in the department of psychiatry and behavioral science, Medical University of South Carolina, Charleston, noted that nurses are “predominantly female” and that women tend to be twice as likely as men to experience depression, which is a major risk factor for suicide. Thus, this population is particularly vulnerable.
One reason the investigators did not find that suicide rates were higher among physicians is that the health care professionals whom the researchers studied were older than 30 years. Thus, the study “excludes younger physicians in early practice or training, who likely do have higher suicide rates than the general population,” she suggested.
Dr. Guille, who is the author of an accompanying editorial and was not involved with the study, recommended “taking a public health approach, implementing preventative interventions, identifying people at high risk, providing treatment for health care professionals struggling with mental health problems, and destigmatizing help seeking.”
She encouraged clinicians to “reach out to colleagues who are struggling in a way to help them seek services and check in with them because it’s helpful when peers reach out.”
Dr. Davis noted that these disturbing trends will likely increase in the aftermath of the COVID-19 pandemic. “The pandemic has placed enormous strain on the health care workforce, and we fear this may have made the situation even worse.”
The current findings “will serve as a benchmark for future comparisons,” he said.
No source of funding for the study was reported. Dr. Davis has received consulting fees as a statistical reviewer for the journal Regional Anesthesia and Pain Medicine. His coauthors disclosed no relevant financial relationships. Dr. Guille has received grants from the National Institute on Drug Abuse, the American Foundation on Suicide Prevention, and the Duke Endowment and serves on the advisory board and speakers bureau of Sage Therapeutics.
A version of this article first appeared on Medscape.com.
Female nurses are at significantly greater risk of dying by suicide than physicians in findings that contradict previous research suggesting doctors are at greatest risk.
Results of a large retrospective cohort study show that nurses of both sexes were 18% more likely to die by suicide, compared with individuals in the general population. In addition, compared with female physicians, the suicide risk among female nurses was 70% higher.
“The main takeaway is that the risk of suicide among nurses is twice that of the general population and even higher than that among physicians, a population known to be at high risk,” lead author Matthew Davis, MPH, PhD, associate professor, department of systems, populations, and leadership, University of Michigan, Ann Arbor, said in an interview.
The study was published online April 14, 2021, in JAMA Psychiatry.
Focus on physicians
Compared with the general public, health care workers are at higher risk for suicide, but most studies of suicide have focused on physicians, Dr. Davis said.
Although “there were several older studies hinting that there might be a difference in suicide risk among nurses,” the data were insufficient to “make an overall conclusion,” he noted.
For that reason, his group “set out to make the best estimates possible” by using a large dataset from the National Violent Death Reporting System of the Centers for Disease Control and Prevention spanning the years 2007-2018 and focusing on suicides by individuals aged 30 years and older (n = 159,372 suicides).
Additional workforce data were acquired from the Bureau of Labor Statistics and the Association of Medical Colleges State Physician Workforce Data.
An important area of focus was method of suicide.
“ and know how to use them to overdose, which also increases their risk,” Dr. Davis said in a press release.
Enormous job strain
The researchers identified 2,374 suicides among nurses, 857 suicides among physicians, and 156,141 suicides in the general population.
Compared with the general population, nurses who died by suicide were more likely to be women, less racially diverse (non-Hispanic White), and more likely to have been married.
Rates of suicide were higher among nurses than among the general population, with a sex-adjusted incidence for 2017-2018 of 23.8 per 100,000 versus 20.1 per 100,000 (relative risk, 1.18; 95% confidence interval, 1.03-1.36).
The difference between suicide rates among female nurses and among women in the general population was even more striking: In 2017-2018, the suicide incidence among nurses was 17.1 per 100,000 versus 8.6 per 100,000 in the population at large (RR, 1.99; 95% CI, 1.82-2.18).
“In absolute terms, being a female nurse was associated with an additional 8.5 suicides per 100,000 (7.0-10.0), compared with the general population,” the authors reported.
In contrast, overall physician suicide rates were not statistically different from those of the general population (RR, 1.01; 95% CI, 0.79-1.30) except during the period 2011-2012 (11.7 per 100,000; 95% CI, 6.6-16.8 vs. 7.5 per 100,000; 95% CI, 7.2-7.7).
Clinicians of both sexes were more likely to use poisoning and less likely to use a firearm, compared with individuals in the general population who died by suicide. For example, 24.9% (23.5%-26.4%) of nurse suicides involved poisoning, compared to 16.8% (16.6%-17.0%) of suicides in the general population.
Toxicology reports showed that the presence of antidepressants, benzodiazepines, barbiturates, and opiates was more common in clinician suicides than suicides in the general population.
Dr. Davis suggested the higher risk for suicide among nurses, compared with physicians, might be attributed to “high job demands – for example, nurses provide the majority of bedside care, work long shifts in stressful environments, and have less autonomy.
“Health care workers and friends and family of health care workers need to be aware of mental health issues and suicide risk that can be associated with the job and, most importantly, recognize those who may be struggling and encourage them to get help by calling the National Suicide Prevention Lifeline,” he said.
Other potential contributors include “avoidance of mental health services due to stigma and greater access to the means to commit suicide via medication,” Dr. Davis noted.
Benchmark research
Commenting on the study, Constance Guille, MD, MSCR, professor in the department of psychiatry and behavioral science, Medical University of South Carolina, Charleston, noted that nurses are “predominantly female” and that women tend to be twice as likely as men to experience depression, which is a major risk factor for suicide. Thus, this population is particularly vulnerable.
One reason the investigators did not find that suicide rates were higher among physicians is that the health care professionals whom the researchers studied were older than 30 years. Thus, the study “excludes younger physicians in early practice or training, who likely do have higher suicide rates than the general population,” she suggested.
Dr. Guille, who is the author of an accompanying editorial and was not involved with the study, recommended “taking a public health approach, implementing preventative interventions, identifying people at high risk, providing treatment for health care professionals struggling with mental health problems, and destigmatizing help seeking.”
She encouraged clinicians to “reach out to colleagues who are struggling in a way to help them seek services and check in with them because it’s helpful when peers reach out.”
Dr. Davis noted that these disturbing trends will likely increase in the aftermath of the COVID-19 pandemic. “The pandemic has placed enormous strain on the health care workforce, and we fear this may have made the situation even worse.”
The current findings “will serve as a benchmark for future comparisons,” he said.
No source of funding for the study was reported. Dr. Davis has received consulting fees as a statistical reviewer for the journal Regional Anesthesia and Pain Medicine. His coauthors disclosed no relevant financial relationships. Dr. Guille has received grants from the National Institute on Drug Abuse, the American Foundation on Suicide Prevention, and the Duke Endowment and serves on the advisory board and speakers bureau of Sage Therapeutics.
A version of this article first appeared on Medscape.com.
Female nurses are at significantly greater risk of dying by suicide than physicians in findings that contradict previous research suggesting doctors are at greatest risk.
Results of a large retrospective cohort study show that nurses of both sexes were 18% more likely to die by suicide, compared with individuals in the general population. In addition, compared with female physicians, the suicide risk among female nurses was 70% higher.
“The main takeaway is that the risk of suicide among nurses is twice that of the general population and even higher than that among physicians, a population known to be at high risk,” lead author Matthew Davis, MPH, PhD, associate professor, department of systems, populations, and leadership, University of Michigan, Ann Arbor, said in an interview.
The study was published online April 14, 2021, in JAMA Psychiatry.
Focus on physicians
Compared with the general public, health care workers are at higher risk for suicide, but most studies of suicide have focused on physicians, Dr. Davis said.
Although “there were several older studies hinting that there might be a difference in suicide risk among nurses,” the data were insufficient to “make an overall conclusion,” he noted.
For that reason, his group “set out to make the best estimates possible” by using a large dataset from the National Violent Death Reporting System of the Centers for Disease Control and Prevention spanning the years 2007-2018 and focusing on suicides by individuals aged 30 years and older (n = 159,372 suicides).
Additional workforce data were acquired from the Bureau of Labor Statistics and the Association of Medical Colleges State Physician Workforce Data.
An important area of focus was method of suicide.
“ and know how to use them to overdose, which also increases their risk,” Dr. Davis said in a press release.
Enormous job strain
The researchers identified 2,374 suicides among nurses, 857 suicides among physicians, and 156,141 suicides in the general population.
Compared with the general population, nurses who died by suicide were more likely to be women, less racially diverse (non-Hispanic White), and more likely to have been married.
Rates of suicide were higher among nurses than among the general population, with a sex-adjusted incidence for 2017-2018 of 23.8 per 100,000 versus 20.1 per 100,000 (relative risk, 1.18; 95% confidence interval, 1.03-1.36).
The difference between suicide rates among female nurses and among women in the general population was even more striking: In 2017-2018, the suicide incidence among nurses was 17.1 per 100,000 versus 8.6 per 100,000 in the population at large (RR, 1.99; 95% CI, 1.82-2.18).
“In absolute terms, being a female nurse was associated with an additional 8.5 suicides per 100,000 (7.0-10.0), compared with the general population,” the authors reported.
In contrast, overall physician suicide rates were not statistically different from those of the general population (RR, 1.01; 95% CI, 0.79-1.30) except during the period 2011-2012 (11.7 per 100,000; 95% CI, 6.6-16.8 vs. 7.5 per 100,000; 95% CI, 7.2-7.7).
Clinicians of both sexes were more likely to use poisoning and less likely to use a firearm, compared with individuals in the general population who died by suicide. For example, 24.9% (23.5%-26.4%) of nurse suicides involved poisoning, compared to 16.8% (16.6%-17.0%) of suicides in the general population.
Toxicology reports showed that the presence of antidepressants, benzodiazepines, barbiturates, and opiates was more common in clinician suicides than suicides in the general population.
Dr. Davis suggested the higher risk for suicide among nurses, compared with physicians, might be attributed to “high job demands – for example, nurses provide the majority of bedside care, work long shifts in stressful environments, and have less autonomy.
“Health care workers and friends and family of health care workers need to be aware of mental health issues and suicide risk that can be associated with the job and, most importantly, recognize those who may be struggling and encourage them to get help by calling the National Suicide Prevention Lifeline,” he said.
Other potential contributors include “avoidance of mental health services due to stigma and greater access to the means to commit suicide via medication,” Dr. Davis noted.
Benchmark research
Commenting on the study, Constance Guille, MD, MSCR, professor in the department of psychiatry and behavioral science, Medical University of South Carolina, Charleston, noted that nurses are “predominantly female” and that women tend to be twice as likely as men to experience depression, which is a major risk factor for suicide. Thus, this population is particularly vulnerable.
One reason the investigators did not find that suicide rates were higher among physicians is that the health care professionals whom the researchers studied were older than 30 years. Thus, the study “excludes younger physicians in early practice or training, who likely do have higher suicide rates than the general population,” she suggested.
Dr. Guille, who is the author of an accompanying editorial and was not involved with the study, recommended “taking a public health approach, implementing preventative interventions, identifying people at high risk, providing treatment for health care professionals struggling with mental health problems, and destigmatizing help seeking.”
She encouraged clinicians to “reach out to colleagues who are struggling in a way to help them seek services and check in with them because it’s helpful when peers reach out.”
Dr. Davis noted that these disturbing trends will likely increase in the aftermath of the COVID-19 pandemic. “The pandemic has placed enormous strain on the health care workforce, and we fear this may have made the situation even worse.”
The current findings “will serve as a benchmark for future comparisons,” he said.
No source of funding for the study was reported. Dr. Davis has received consulting fees as a statistical reviewer for the journal Regional Anesthesia and Pain Medicine. His coauthors disclosed no relevant financial relationships. Dr. Guille has received grants from the National Institute on Drug Abuse, the American Foundation on Suicide Prevention, and the Duke Endowment and serves on the advisory board and speakers bureau of Sage Therapeutics.
A version of this article first appeared on Medscape.com.
Hispanic diabetes patients receive less guideline-based care
based on data from more than 7,000 individuals.
Racial and ethnic disparities in diabetes care remain a pervasive health problem, and minorities including non-Hispanic Blacks and Hispanics experience higher rates of complications, including retinopathy and neuropathy, compared with other groups, Felippe Ottoni Marcondes, MD, of Massachusetts General Hospital, Boston, and colleagues noted in a poster presented at the annual meeting of the Society for General Internal Medicine.
Data from previous studies have shown that diabetes patients who receive guideline-directed preventive care soon after diagnosis can reduce their risk of complications, they said.
To identify disparities in the provision of guideline-directed preventive care, the researchers analyzed data from 7,341 individuals who participated in the National Health Interview Survey from 2011 to 2017. They reviewed associations between race/ethnicity and visits to an eye specialist, a foot specialist, and checks of blood pressure and cholesterol in the past year among individuals diagnosed with diabetes within the past 5 years.
Overall, Hispanics had significantly lower rates of insurance coverage (75.9%), compared with non-Hispanic Whites (93.2%) and non-Hispanic Blacks (88.1%; P < .001).
Hispanics also were significantly less likely than Whites to have had a prior year eye exam (odds ratio, 0.80) and blood pressure check (OR, 0.45), after controlling for variables including age, sex, socioeconomic status, health insurance, general health status, U.S. region, marital status, body mass index, and various comorbidities.
Although insurance coverage mediated 42.8% of the total effect of race/ethnicity on annual eye specialist visits for Hispanics as compared with Whites, there was no significant effect for Blacks, compared with Whites.
COVID concerns impact diabetes disparities
“As the diabetes epidemic continues in the U.S., it is important to bring to the front of the diabetes care conversation racial/ethnic disparities that persisted or have been only partially addressed,” Dr. Marcondes said in an interview. “It is also important to emphasize that patients with diabetes are at higher risk for COVID-19 hospitalizations, complications, and death, and COVID-19 has disproportionately affected racial/ethnic minorities, so racial/ethnic minorities with diabetes have compounded risk of complications not only from diabetes but also from COVID-19.
“Importantly, our study highlights disparities in health care that are likely the product of systemic inequalities in access to care and insurance coverage at a moment when conversations about the race/racism and their health impact are fresh in the minds of public and health policy officials and the general public,” he emphasized.
“Unfortunately, I cannot say that I am surprised by our findings,” Dr. Marcondes said. “We expected to see some differences in the receipt of care for racial/ethnic minorities compared to white individuals for those recently diagnosed with diabetes, and that is exactly what our findings show.”
However, “what was perhaps intriguing is that disparities in the receipt of guideline-directed care were greater for Hispanic compared to White individuals than for Black compared to White individuals,” said Dr. Marcondes. “The causes of these differences are many. Hispanic individuals are less likely than White and Black persons to have insurance coverage.” Other unmeasured factors include language barriers that Hispanic individuals may face, as well as the bias and discrimination experienced by Hispanic and Black individuals alike.
Focus on equitable early intervention
“There is plenty of evidence in the medical literature that Black and Hispanic individuals with diabetes, as well as other minorities, have higher risk of complications of diabetes such as retinopathy, nephropathy, as well as cardiovascular risk factors such as high blood pressure and cholesterol,” Dr. Marcondes said. “Yet, complications in the time that immediately follows the diagnosis of diabetes are likely to be low.”
To reduce the risk of complications in the future, “physicians and health providers need to focus on providing equitable, guideline-directed treatment for their minority patients recently diagnosed with diabetes,” Dr. Marcondes emphasized. “Intervening early in the disease course will hopefully lead to a decrease in the rate of complications for racial/ethnic minorities. Clinicians, especially primary care physicians and providers, need to be aware that they are often the first encounter of many patients with the health care system. Effective communication and unbiased language on the part of clinicians will lead to stronger patient-physician relationships that foster opportunity to discuss disease prevention.
“Additional research is needed to evaluate the attitudes and biases of primary care providers and access the impact of patient navigation resources when treating minority patients with diabetes,” he concluded.
Digging Deeper into Disparities
“In diabetes, there are known racial and ethnic disparities such that minorities receive suboptimal screening and treatment, and have worse outcomes,” said Scott J. Pilla, MD, of The Johns Hopkins University School of Medicine, Baltimore, in an interview.
“This study examines disparities in diabetes preventive measures in the U.S. using a national survey (NHIS) over the past decade. They took the important step of stratifying their analyses by health insurance and socioeconomic status which, in addition to race, may have a large impact,” said Dr. Pilla. However, “One critique of the poster is that it is unclear whether the researchers weighted their analyses to account for the nationally representative sampling of the NHIS survey,” he noted.
Dr. Pilla said the finding that Hispanic patients had fewer diabetes preventive measures lines up with previous research in this area.
“I was surprised that the disparities did not extend to black patients, who have been found to also receive suboptimal care compared to white patients in other studies,” he noted.
The message for clinical practice: “Minorities with diabetes are at a higher risk of adverse diabetes outcomes and may need extra support and resources to achieve their evidence-based diabetes prevention,” Dr. Pilla said.
“More research is needed to understand the root cause of racial and ethnic disparities in diabetes management to tease apart possible contributors including health insurance coverage, socioeconomic factors, cultural and community factors, and systemic racism. This will help inform targeted approaches to reducing disparities in diabetes care,” he emphasized.
The researchers had no relevant financial conflicts to disclose. Dr. Pilla had no financial conflicts to disclose.
based on data from more than 7,000 individuals.
Racial and ethnic disparities in diabetes care remain a pervasive health problem, and minorities including non-Hispanic Blacks and Hispanics experience higher rates of complications, including retinopathy and neuropathy, compared with other groups, Felippe Ottoni Marcondes, MD, of Massachusetts General Hospital, Boston, and colleagues noted in a poster presented at the annual meeting of the Society for General Internal Medicine.
Data from previous studies have shown that diabetes patients who receive guideline-directed preventive care soon after diagnosis can reduce their risk of complications, they said.
To identify disparities in the provision of guideline-directed preventive care, the researchers analyzed data from 7,341 individuals who participated in the National Health Interview Survey from 2011 to 2017. They reviewed associations between race/ethnicity and visits to an eye specialist, a foot specialist, and checks of blood pressure and cholesterol in the past year among individuals diagnosed with diabetes within the past 5 years.
Overall, Hispanics had significantly lower rates of insurance coverage (75.9%), compared with non-Hispanic Whites (93.2%) and non-Hispanic Blacks (88.1%; P < .001).
Hispanics also were significantly less likely than Whites to have had a prior year eye exam (odds ratio, 0.80) and blood pressure check (OR, 0.45), after controlling for variables including age, sex, socioeconomic status, health insurance, general health status, U.S. region, marital status, body mass index, and various comorbidities.
Although insurance coverage mediated 42.8% of the total effect of race/ethnicity on annual eye specialist visits for Hispanics as compared with Whites, there was no significant effect for Blacks, compared with Whites.
COVID concerns impact diabetes disparities
“As the diabetes epidemic continues in the U.S., it is important to bring to the front of the diabetes care conversation racial/ethnic disparities that persisted or have been only partially addressed,” Dr. Marcondes said in an interview. “It is also important to emphasize that patients with diabetes are at higher risk for COVID-19 hospitalizations, complications, and death, and COVID-19 has disproportionately affected racial/ethnic minorities, so racial/ethnic minorities with diabetes have compounded risk of complications not only from diabetes but also from COVID-19.
“Importantly, our study highlights disparities in health care that are likely the product of systemic inequalities in access to care and insurance coverage at a moment when conversations about the race/racism and their health impact are fresh in the minds of public and health policy officials and the general public,” he emphasized.
“Unfortunately, I cannot say that I am surprised by our findings,” Dr. Marcondes said. “We expected to see some differences in the receipt of care for racial/ethnic minorities compared to white individuals for those recently diagnosed with diabetes, and that is exactly what our findings show.”
However, “what was perhaps intriguing is that disparities in the receipt of guideline-directed care were greater for Hispanic compared to White individuals than for Black compared to White individuals,” said Dr. Marcondes. “The causes of these differences are many. Hispanic individuals are less likely than White and Black persons to have insurance coverage.” Other unmeasured factors include language barriers that Hispanic individuals may face, as well as the bias and discrimination experienced by Hispanic and Black individuals alike.
Focus on equitable early intervention
“There is plenty of evidence in the medical literature that Black and Hispanic individuals with diabetes, as well as other minorities, have higher risk of complications of diabetes such as retinopathy, nephropathy, as well as cardiovascular risk factors such as high blood pressure and cholesterol,” Dr. Marcondes said. “Yet, complications in the time that immediately follows the diagnosis of diabetes are likely to be low.”
To reduce the risk of complications in the future, “physicians and health providers need to focus on providing equitable, guideline-directed treatment for their minority patients recently diagnosed with diabetes,” Dr. Marcondes emphasized. “Intervening early in the disease course will hopefully lead to a decrease in the rate of complications for racial/ethnic minorities. Clinicians, especially primary care physicians and providers, need to be aware that they are often the first encounter of many patients with the health care system. Effective communication and unbiased language on the part of clinicians will lead to stronger patient-physician relationships that foster opportunity to discuss disease prevention.
“Additional research is needed to evaluate the attitudes and biases of primary care providers and access the impact of patient navigation resources when treating minority patients with diabetes,” he concluded.
Digging Deeper into Disparities
“In diabetes, there are known racial and ethnic disparities such that minorities receive suboptimal screening and treatment, and have worse outcomes,” said Scott J. Pilla, MD, of The Johns Hopkins University School of Medicine, Baltimore, in an interview.
“This study examines disparities in diabetes preventive measures in the U.S. using a national survey (NHIS) over the past decade. They took the important step of stratifying their analyses by health insurance and socioeconomic status which, in addition to race, may have a large impact,” said Dr. Pilla. However, “One critique of the poster is that it is unclear whether the researchers weighted their analyses to account for the nationally representative sampling of the NHIS survey,” he noted.
Dr. Pilla said the finding that Hispanic patients had fewer diabetes preventive measures lines up with previous research in this area.
“I was surprised that the disparities did not extend to black patients, who have been found to also receive suboptimal care compared to white patients in other studies,” he noted.
The message for clinical practice: “Minorities with diabetes are at a higher risk of adverse diabetes outcomes and may need extra support and resources to achieve their evidence-based diabetes prevention,” Dr. Pilla said.
“More research is needed to understand the root cause of racial and ethnic disparities in diabetes management to tease apart possible contributors including health insurance coverage, socioeconomic factors, cultural and community factors, and systemic racism. This will help inform targeted approaches to reducing disparities in diabetes care,” he emphasized.
The researchers had no relevant financial conflicts to disclose. Dr. Pilla had no financial conflicts to disclose.
based on data from more than 7,000 individuals.
Racial and ethnic disparities in diabetes care remain a pervasive health problem, and minorities including non-Hispanic Blacks and Hispanics experience higher rates of complications, including retinopathy and neuropathy, compared with other groups, Felippe Ottoni Marcondes, MD, of Massachusetts General Hospital, Boston, and colleagues noted in a poster presented at the annual meeting of the Society for General Internal Medicine.
Data from previous studies have shown that diabetes patients who receive guideline-directed preventive care soon after diagnosis can reduce their risk of complications, they said.
To identify disparities in the provision of guideline-directed preventive care, the researchers analyzed data from 7,341 individuals who participated in the National Health Interview Survey from 2011 to 2017. They reviewed associations between race/ethnicity and visits to an eye specialist, a foot specialist, and checks of blood pressure and cholesterol in the past year among individuals diagnosed with diabetes within the past 5 years.
Overall, Hispanics had significantly lower rates of insurance coverage (75.9%), compared with non-Hispanic Whites (93.2%) and non-Hispanic Blacks (88.1%; P < .001).
Hispanics also were significantly less likely than Whites to have had a prior year eye exam (odds ratio, 0.80) and blood pressure check (OR, 0.45), after controlling for variables including age, sex, socioeconomic status, health insurance, general health status, U.S. region, marital status, body mass index, and various comorbidities.
Although insurance coverage mediated 42.8% of the total effect of race/ethnicity on annual eye specialist visits for Hispanics as compared with Whites, there was no significant effect for Blacks, compared with Whites.
COVID concerns impact diabetes disparities
“As the diabetes epidemic continues in the U.S., it is important to bring to the front of the diabetes care conversation racial/ethnic disparities that persisted or have been only partially addressed,” Dr. Marcondes said in an interview. “It is also important to emphasize that patients with diabetes are at higher risk for COVID-19 hospitalizations, complications, and death, and COVID-19 has disproportionately affected racial/ethnic minorities, so racial/ethnic minorities with diabetes have compounded risk of complications not only from diabetes but also from COVID-19.
“Importantly, our study highlights disparities in health care that are likely the product of systemic inequalities in access to care and insurance coverage at a moment when conversations about the race/racism and their health impact are fresh in the minds of public and health policy officials and the general public,” he emphasized.
“Unfortunately, I cannot say that I am surprised by our findings,” Dr. Marcondes said. “We expected to see some differences in the receipt of care for racial/ethnic minorities compared to white individuals for those recently diagnosed with diabetes, and that is exactly what our findings show.”
However, “what was perhaps intriguing is that disparities in the receipt of guideline-directed care were greater for Hispanic compared to White individuals than for Black compared to White individuals,” said Dr. Marcondes. “The causes of these differences are many. Hispanic individuals are less likely than White and Black persons to have insurance coverage.” Other unmeasured factors include language barriers that Hispanic individuals may face, as well as the bias and discrimination experienced by Hispanic and Black individuals alike.
Focus on equitable early intervention
“There is plenty of evidence in the medical literature that Black and Hispanic individuals with diabetes, as well as other minorities, have higher risk of complications of diabetes such as retinopathy, nephropathy, as well as cardiovascular risk factors such as high blood pressure and cholesterol,” Dr. Marcondes said. “Yet, complications in the time that immediately follows the diagnosis of diabetes are likely to be low.”
To reduce the risk of complications in the future, “physicians and health providers need to focus on providing equitable, guideline-directed treatment for their minority patients recently diagnosed with diabetes,” Dr. Marcondes emphasized. “Intervening early in the disease course will hopefully lead to a decrease in the rate of complications for racial/ethnic minorities. Clinicians, especially primary care physicians and providers, need to be aware that they are often the first encounter of many patients with the health care system. Effective communication and unbiased language on the part of clinicians will lead to stronger patient-physician relationships that foster opportunity to discuss disease prevention.
“Additional research is needed to evaluate the attitudes and biases of primary care providers and access the impact of patient navigation resources when treating minority patients with diabetes,” he concluded.
Digging Deeper into Disparities
“In diabetes, there are known racial and ethnic disparities such that minorities receive suboptimal screening and treatment, and have worse outcomes,” said Scott J. Pilla, MD, of The Johns Hopkins University School of Medicine, Baltimore, in an interview.
“This study examines disparities in diabetes preventive measures in the U.S. using a national survey (NHIS) over the past decade. They took the important step of stratifying their analyses by health insurance and socioeconomic status which, in addition to race, may have a large impact,” said Dr. Pilla. However, “One critique of the poster is that it is unclear whether the researchers weighted their analyses to account for the nationally representative sampling of the NHIS survey,” he noted.
Dr. Pilla said the finding that Hispanic patients had fewer diabetes preventive measures lines up with previous research in this area.
“I was surprised that the disparities did not extend to black patients, who have been found to also receive suboptimal care compared to white patients in other studies,” he noted.
The message for clinical practice: “Minorities with diabetes are at a higher risk of adverse diabetes outcomes and may need extra support and resources to achieve their evidence-based diabetes prevention,” Dr. Pilla said.
“More research is needed to understand the root cause of racial and ethnic disparities in diabetes management to tease apart possible contributors including health insurance coverage, socioeconomic factors, cultural and community factors, and systemic racism. This will help inform targeted approaches to reducing disparities in diabetes care,” he emphasized.
The researchers had no relevant financial conflicts to disclose. Dr. Pilla had no financial conflicts to disclose.
FROM SGIM 2021
Pros and cons of proposed recommendation for prediabetes and T2D screening
. If accepted as written, the new recommendation will be to “screen all asymptomatic adults ages 35 to 70 years who are overweight or obese.” Upon diagnosis of prediabetes, the recommendation is to offer or refer patients to preventive interventions.
This new recommendation would replace the one from 2015, which recommended screening adults aged 40-70 who are overweight or obese, lowering the age at which screening begins by 5 years. It would also replace the recommendation of referral to intensive behavioral counseling to promote a healthy diet and exercise.1
The American Diabetes Association (ADA) identifies A1c, fasting plasma glucose, or oral glucose tolerance tests as appropriate tests for the diagnosis of prediabetes and type 2 DM, and the new draft recommendation does not provide a preference for method of screening.2
The USPSTF’s draft recommendation could expand screening with the hope of identifying patients with prediabetes, or those with diabetes who are asymptomatic, with the intent of beginning treatment before there are serious complications.
Unknown diabetes or prediabetes diagnosis common
It has been estimated by the Centers for Disease Control and Prevention that 12% of U.S. adults had DM as of 2015, though nearly 24% were not aware that they had it. Also, according to the CDC, the prevalence of DM increases with age and is higher in those with less than a high school education. The same report indicates that more than 30% of U.S. adults have prediabetes, and with less than 12% of those individuals are aware of it.3 A possible explanation for a patient’s being unaware of a diagnosis could be that it has been documented in a chart but the patient does not know such information is in his or her health record. According to the evidence provided for the updated recommendation, earlier diagnosis may have an important benefit in preventing serious complications.
A modeling study compared simulated screening strategies and found that the most optimal screening strategy from a cost-effectiveness perspective begins between the ages of 30 and 45, with rescreening every 3-5 years. Further models have led researchers to conclude that early diagnosis can lead to decreased cardiovascular events as well as an opportunity for multifactorial treatment.1 For this reason, it makes sense to expand the ages of screening for obese and overweight individuals.
Treatment recommendations are more flexible
The change in treatment recommendations for a new diagnosis of prediabetes is potentially more useful. It may not be feasible or reasonable for physicians to always provide or refer their patients for intensive behavior interventions. The updated recommendation would allow for the inclusion of not only behavioral counseling and health education, but also potential medication options that are currently available but not approved, or that may be available in the future. The evidence review seemed to be mixed in outcome in this area, so the increased flexibility will likely allow for future opportunities.
Screening criteria may be too narrow
This recommendation, does not, however, provide any guidance on screening of individuals who have other risk factors besides a body mass index consistent with overweight or obesity. It seems that this may be a missed opportunity.
The draft statement clearly indicates that there are other factors associated with increased risk of developing DM, but does not consider these factors in determining which patients should be screened. Both the ADA and the American Association of Clinical Endocrinology (AACE) have recommendations for universal screening for all adults 45 and older, acknowledging that incidence of DM increases with age. The ADA also recommends screening individuals who are overweight or obese and have an additional risk factor regardless of age. The AACE recommends screening all individuals for risk factors regardless of age.
The current and draft recommendations by the USPSTF do not address other risk factors and indicate only that further research is needed to understand the risk associated with DM and the natural history of pre-DM and who may progress to DM or revert to normoglycemia. Without comment on other risk factors or universal screening with age, the USPSTF recommendation potentially would not be sensitive enough to capture all those who may meet criteria for prediabetes or DM.2,4
In addition to not addressing other risk factors and screening for those of normal and underweight BMI, the USPSTF recommendation does not address frequency of screening. The recommendations from both the ADA and the AACE indicate screening at 3-year intervals for those who are eligible – for any reason. The supporting evidence review did not seem to address this aspect, and so it is understandable that there was no comment. However, I feel this will lead physicians to turn to the other guidelines for guidance where there is disagreement in other aspects.
Ultimately, the draft updated recommendation will provide physicians with the opportunity to identify more patients with prediabetes and DM. This will be wonderful in terms of being able to offer treatments and lifestyle interventions to decrease the morbidity patients would face were these conditions not diagnosed. I hope that future recommendations will also address risk factors in addition to BMI as well as frequency of screening for those who remain at increased risk but initially screen negative.
Dr. Wheat is a family physician at Erie Family Health Center in Chicago. She is program director of Northwestern’s McGaw Family Medicine residency program at Humboldt Park, Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at [email protected].
References
1. Screening for prediabetes and type 2 diabetes mellitus. U.S. Preventive Services Task Force. 2021 Mar 16.
2. Classification and diagnosis of diabetes: Standards of medical care in diabetes – 2020. American Diabetes Association. Diabetes Care. 2020 Jan. doi: 10.2337/dc20-S002.
3. National Diabetes Statistics Report, 2020. Centers for Disease Control and Prevention.
4. American Association of Clinical Endocrinologists and American College of Endocrinology – clinical practice guidelines for developing a diabetes mellitus comprehensive care plan. Hadelsman Y et al. Endocr Pract. 2015 Apr. 1-87. doi: 10.4158/EP15672.GL.
. If accepted as written, the new recommendation will be to “screen all asymptomatic adults ages 35 to 70 years who are overweight or obese.” Upon diagnosis of prediabetes, the recommendation is to offer or refer patients to preventive interventions.
This new recommendation would replace the one from 2015, which recommended screening adults aged 40-70 who are overweight or obese, lowering the age at which screening begins by 5 years. It would also replace the recommendation of referral to intensive behavioral counseling to promote a healthy diet and exercise.1
The American Diabetes Association (ADA) identifies A1c, fasting plasma glucose, or oral glucose tolerance tests as appropriate tests for the diagnosis of prediabetes and type 2 DM, and the new draft recommendation does not provide a preference for method of screening.2
The USPSTF’s draft recommendation could expand screening with the hope of identifying patients with prediabetes, or those with diabetes who are asymptomatic, with the intent of beginning treatment before there are serious complications.
Unknown diabetes or prediabetes diagnosis common
It has been estimated by the Centers for Disease Control and Prevention that 12% of U.S. adults had DM as of 2015, though nearly 24% were not aware that they had it. Also, according to the CDC, the prevalence of DM increases with age and is higher in those with less than a high school education. The same report indicates that more than 30% of U.S. adults have prediabetes, and with less than 12% of those individuals are aware of it.3 A possible explanation for a patient’s being unaware of a diagnosis could be that it has been documented in a chart but the patient does not know such information is in his or her health record. According to the evidence provided for the updated recommendation, earlier diagnosis may have an important benefit in preventing serious complications.
A modeling study compared simulated screening strategies and found that the most optimal screening strategy from a cost-effectiveness perspective begins between the ages of 30 and 45, with rescreening every 3-5 years. Further models have led researchers to conclude that early diagnosis can lead to decreased cardiovascular events as well as an opportunity for multifactorial treatment.1 For this reason, it makes sense to expand the ages of screening for obese and overweight individuals.
Treatment recommendations are more flexible
The change in treatment recommendations for a new diagnosis of prediabetes is potentially more useful. It may not be feasible or reasonable for physicians to always provide or refer their patients for intensive behavior interventions. The updated recommendation would allow for the inclusion of not only behavioral counseling and health education, but also potential medication options that are currently available but not approved, or that may be available in the future. The evidence review seemed to be mixed in outcome in this area, so the increased flexibility will likely allow for future opportunities.
Screening criteria may be too narrow
This recommendation, does not, however, provide any guidance on screening of individuals who have other risk factors besides a body mass index consistent with overweight or obesity. It seems that this may be a missed opportunity.
The draft statement clearly indicates that there are other factors associated with increased risk of developing DM, but does not consider these factors in determining which patients should be screened. Both the ADA and the American Association of Clinical Endocrinology (AACE) have recommendations for universal screening for all adults 45 and older, acknowledging that incidence of DM increases with age. The ADA also recommends screening individuals who are overweight or obese and have an additional risk factor regardless of age. The AACE recommends screening all individuals for risk factors regardless of age.
The current and draft recommendations by the USPSTF do not address other risk factors and indicate only that further research is needed to understand the risk associated with DM and the natural history of pre-DM and who may progress to DM or revert to normoglycemia. Without comment on other risk factors or universal screening with age, the USPSTF recommendation potentially would not be sensitive enough to capture all those who may meet criteria for prediabetes or DM.2,4
In addition to not addressing other risk factors and screening for those of normal and underweight BMI, the USPSTF recommendation does not address frequency of screening. The recommendations from both the ADA and the AACE indicate screening at 3-year intervals for those who are eligible – for any reason. The supporting evidence review did not seem to address this aspect, and so it is understandable that there was no comment. However, I feel this will lead physicians to turn to the other guidelines for guidance where there is disagreement in other aspects.
Ultimately, the draft updated recommendation will provide physicians with the opportunity to identify more patients with prediabetes and DM. This will be wonderful in terms of being able to offer treatments and lifestyle interventions to decrease the morbidity patients would face were these conditions not diagnosed. I hope that future recommendations will also address risk factors in addition to BMI as well as frequency of screening for those who remain at increased risk but initially screen negative.
Dr. Wheat is a family physician at Erie Family Health Center in Chicago. She is program director of Northwestern’s McGaw Family Medicine residency program at Humboldt Park, Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at [email protected].
References
1. Screening for prediabetes and type 2 diabetes mellitus. U.S. Preventive Services Task Force. 2021 Mar 16.
2. Classification and diagnosis of diabetes: Standards of medical care in diabetes – 2020. American Diabetes Association. Diabetes Care. 2020 Jan. doi: 10.2337/dc20-S002.
3. National Diabetes Statistics Report, 2020. Centers for Disease Control and Prevention.
4. American Association of Clinical Endocrinologists and American College of Endocrinology – clinical practice guidelines for developing a diabetes mellitus comprehensive care plan. Hadelsman Y et al. Endocr Pract. 2015 Apr. 1-87. doi: 10.4158/EP15672.GL.
. If accepted as written, the new recommendation will be to “screen all asymptomatic adults ages 35 to 70 years who are overweight or obese.” Upon diagnosis of prediabetes, the recommendation is to offer or refer patients to preventive interventions.
This new recommendation would replace the one from 2015, which recommended screening adults aged 40-70 who are overweight or obese, lowering the age at which screening begins by 5 years. It would also replace the recommendation of referral to intensive behavioral counseling to promote a healthy diet and exercise.1
The American Diabetes Association (ADA) identifies A1c, fasting plasma glucose, or oral glucose tolerance tests as appropriate tests for the diagnosis of prediabetes and type 2 DM, and the new draft recommendation does not provide a preference for method of screening.2
The USPSTF’s draft recommendation could expand screening with the hope of identifying patients with prediabetes, or those with diabetes who are asymptomatic, with the intent of beginning treatment before there are serious complications.
Unknown diabetes or prediabetes diagnosis common
It has been estimated by the Centers for Disease Control and Prevention that 12% of U.S. adults had DM as of 2015, though nearly 24% were not aware that they had it. Also, according to the CDC, the prevalence of DM increases with age and is higher in those with less than a high school education. The same report indicates that more than 30% of U.S. adults have prediabetes, and with less than 12% of those individuals are aware of it.3 A possible explanation for a patient’s being unaware of a diagnosis could be that it has been documented in a chart but the patient does not know such information is in his or her health record. According to the evidence provided for the updated recommendation, earlier diagnosis may have an important benefit in preventing serious complications.
A modeling study compared simulated screening strategies and found that the most optimal screening strategy from a cost-effectiveness perspective begins between the ages of 30 and 45, with rescreening every 3-5 years. Further models have led researchers to conclude that early diagnosis can lead to decreased cardiovascular events as well as an opportunity for multifactorial treatment.1 For this reason, it makes sense to expand the ages of screening for obese and overweight individuals.
Treatment recommendations are more flexible
The change in treatment recommendations for a new diagnosis of prediabetes is potentially more useful. It may not be feasible or reasonable for physicians to always provide or refer their patients for intensive behavior interventions. The updated recommendation would allow for the inclusion of not only behavioral counseling and health education, but also potential medication options that are currently available but not approved, or that may be available in the future. The evidence review seemed to be mixed in outcome in this area, so the increased flexibility will likely allow for future opportunities.
Screening criteria may be too narrow
This recommendation, does not, however, provide any guidance on screening of individuals who have other risk factors besides a body mass index consistent with overweight or obesity. It seems that this may be a missed opportunity.
The draft statement clearly indicates that there are other factors associated with increased risk of developing DM, but does not consider these factors in determining which patients should be screened. Both the ADA and the American Association of Clinical Endocrinology (AACE) have recommendations for universal screening for all adults 45 and older, acknowledging that incidence of DM increases with age. The ADA also recommends screening individuals who are overweight or obese and have an additional risk factor regardless of age. The AACE recommends screening all individuals for risk factors regardless of age.
The current and draft recommendations by the USPSTF do not address other risk factors and indicate only that further research is needed to understand the risk associated with DM and the natural history of pre-DM and who may progress to DM or revert to normoglycemia. Without comment on other risk factors or universal screening with age, the USPSTF recommendation potentially would not be sensitive enough to capture all those who may meet criteria for prediabetes or DM.2,4
In addition to not addressing other risk factors and screening for those of normal and underweight BMI, the USPSTF recommendation does not address frequency of screening. The recommendations from both the ADA and the AACE indicate screening at 3-year intervals for those who are eligible – for any reason. The supporting evidence review did not seem to address this aspect, and so it is understandable that there was no comment. However, I feel this will lead physicians to turn to the other guidelines for guidance where there is disagreement in other aspects.
Ultimately, the draft updated recommendation will provide physicians with the opportunity to identify more patients with prediabetes and DM. This will be wonderful in terms of being able to offer treatments and lifestyle interventions to decrease the morbidity patients would face were these conditions not diagnosed. I hope that future recommendations will also address risk factors in addition to BMI as well as frequency of screening for those who remain at increased risk but initially screen negative.
Dr. Wheat is a family physician at Erie Family Health Center in Chicago. She is program director of Northwestern’s McGaw Family Medicine residency program at Humboldt Park, Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at [email protected].
References
1. Screening for prediabetes and type 2 diabetes mellitus. U.S. Preventive Services Task Force. 2021 Mar 16.
2. Classification and diagnosis of diabetes: Standards of medical care in diabetes – 2020. American Diabetes Association. Diabetes Care. 2020 Jan. doi: 10.2337/dc20-S002.
3. National Diabetes Statistics Report, 2020. Centers for Disease Control and Prevention.
4. American Association of Clinical Endocrinologists and American College of Endocrinology – clinical practice guidelines for developing a diabetes mellitus comprehensive care plan. Hadelsman Y et al. Endocr Pract. 2015 Apr. 1-87. doi: 10.4158/EP15672.GL.
More signs COVID shots are safe for pregnant women
As the U.S. races to vaccinate millions of people against the coronavirus, pregnant women face the extra challenge of not knowing whether the vaccines are safe for them or their unborn babies.
None of the recent COVID-19 vaccine trials, including those for Pfizer, Moderna, and Johnson & Johnson, enrolled pregnant or breastfeeding women because they consider them a high-risk group.
That was despite the Society for Maternal-Fetal Medicine and the American College of Obstetricians and Gynecologists asking that pregnant and breastfeeding women be included in trials. The Food and Drug Administration even included pregnant women in the COVID-19 vaccine emergency use authorization (EUA) because of their higher risk of having a more severe disease.
Despite that lack of clinical trial data, more and more smaller studies are suggesting that the vaccines are safe for both mother and child.
Pfizer is now studying its two-dose vaccine in 4,000 pregnant and breastfeeding women to see how safe, tolerated, and robust their immune response is. Researchers will also look at how safe the vaccine is for infants and whether mothers pass along antibodies to children. But the preliminary results won’t be available until the end of the year, a Pfizer spokesperson says.
Without that information, pregnant women are less likely to get vaccinated, according to a large international survey. Less than 45% of pregnant women in the United States said they intended to get vaccinated even when they were told the vaccine was safe and 90% effective. That figure rises to 52% of pregnant women in 16 countries, including the United States, compared with 74% of nonpregnant women willing to be vaccinated. The findings were published online March 1, 2021, in the European Journal of Epidemiology.
The vaccine-hesitant pregnant women in the international study were most concerned that the COVID-19 vaccine could harm their developing fetuses, a worry related to the lack of clinical evidence in pregnant women, said lead researcher Julia Wu, ScD, an epidemiologist at the Harvard School of Public Health’s Human Immunomics Initiative in Boston.
The information vacuum also increases the chances that “people will fall victim to misinformation campaigns like the one on social media that claims that the COVID-19 vaccine causes infertility,” Dr. Wu said. This unfounded claim has deterred some women of childbearing age from getting the vaccine.
Deciding to get vaccinated
Frontline health care professionals were in the first group eligible to receive the vaccine in December 2020. “All of us who were pregnant ... had to decide whether to wait for the data, because we don’t know what the risks are, or go ahead and get it [the vaccine]. We had been dealing with the pandemic for months and were afraid of being exposed to the virus and infecting family members,” said Jacqueline Parchem, MD, a maternal-fetal medicine specialist at the University of Texas Health Science Center, Houston.
Given the lack of safety data, the CDC guidance to pregnant women has been to consult with their doctors and that it’s a personal choice. The Center for Disease Control and Prevention’s latest vaccine guidance said that “there is no evidence that antibodies formed from COVID-19 vaccination cause any problem with pregnancy, including the development of the placenta.”
The CDC is monitoring vaccinated people through its v-safe program and reported on April 12 that more than 86,000 v-safe participants said they were pregnant when they were vaccinated.
Health care workers who were nursing their infants when they were eligible for the vaccine faced a similar dilemma as pregnant women – they lacked the data on them to make a truly informed decision.
“I was nervous about the vaccine side effects for myself and whether my son Bennett, who was about a year old, would experience any of these himself,” said Christa Carrig, a labor and delivery nurse at Massachusetts General Hospital in Boston, who was breastfeeding at the time.
She and Dr. Parchem know that pregnant women with COVID-19 are more likely to have severe illness and complications such as high blood pressure and preterm delivery. “Pregnancy takes a toll on the body. When a woman gets COVID-19 and that insult is added, women who were otherwise young and healthy get much sicker than you would expect,” said Ms. Carrig.
“As a high-risk pregnancy specialist, I know that, with COVID, that babies don’t do well when moms are sick,” said Dr. Parchem.
Pregnant women accounted for more than 84,629 cases of COVID-19 and 95 deaths in the United States between Jan. 22 last year and April 12 this year, according to the CDC COVID data tracker.
Dr. Parchem and Ms. Carrig decided to get vaccinated because of their high risk of exposure to COVID-19 at work. After the second dose, Ms. Carrig reported chills but Bennett had no side effects from breastfeeding. Dr. Parchem, who delivered a healthy baby boy in February, reported no side effects other than a sore arm.
“There’s also a psychological benefit to returning to some sense of normalcy,” said Dr. Parchem. “My mother was finally able to visit us to see the new baby after we were all vaccinated. This was the first visit in more than a year.”
New study results
Ms. Carrig was one of 131 vaccinated hospital workers in the Boston area who took part in the first study to profile the immune response in pregnant and breastfeeding women and compare it with both nonpregnant and pregnant women who had COVID-19.
The study was not designed to evaluate the safety of the vaccines or whether they prevent COVID-19 illness and hospitalizations. That is the role of the large vaccine trials, the authors said.
The participants were aged 18-45 years and received both doses of either Pfizer or Moderna vaccines during one of their trimesters. They provided blood and/or breast milk samples after each vaccine dose, 2-6 weeks after the last dose, and at delivery for the 10 who gave birth during the study.
The vaccines produced a similar strong antibody response among the pregnant/breastfeeding women and nonpregnant women. Their antibody levels were much higher than those found in the pregnant women who had COVID-19, the researchers reported on March 25, 2021, in the American Journal of Obstetrics and Gynecology.
“This is important because a lot of people tend to think once they’ve had COVID-19, they are protected from the virus. This finding suggests that the vaccines produce a stronger antibody response than the infection itself, and this might be important for long-lasting protection against COVID-19,” said Dr. Parchem.
The study also addressed whether newborns benefit from the antibodies produced by their mothers. “In the 10 women who delivered, we detected antibodies in their umbilical cords and breast milk,” says Andrea Edlow, MD, lead researcher and a maternal-fetal medicine specialist at Massachusetts General Hospital.
Newborns are particularly vulnerable to respiratory infections because they have small airways and their immune systems are underdeveloped. These infections can be lethal early in life.
“The public health strategy is to vaccinate mothers against respiratory viruses, bacteria, and parasites that neonates up to 6 months are exposed to. Influenza and pertussis (whooping cough) are two examples of vaccines that we give mothers that we know transfer [antibodies] across the umbilical cord,” said Dr. Edlow.
But this “passive transfer immunity” is different from active immunity, when the body produces its own antibody immune response, she explains.
A different study, also published in March, confirmed that antibodies were transferred from 27 vaccinated pregnant mothers to their infants when they delivered. A new finding was that the women who were vaccinated with both doses and earlier in their third semester passed on more antibodies than the women who were vaccinated later or with only one dose.
Impact of the studies
The Society for Maternal-Fetal Medicine updated its guidance on counseling pregnant and lactating patients about the COVID-19 vaccines to include Dr. Edlow’s study.
“We were struck by how much pregnant and breastfeeding women want to participate in research and to help others in the same situation make decisions. I hope this will be an example to drug companies doing research on new vaccines in the future – that they should not be left behind and can make decisions themselves whether to participate after weighing the risks and benefits,” said Dr. Edlow.
She continues to enroll more vaccinated women in her study in the Boston area, including non–health care workers who have asked to take part.
“It was worth getting vaccinated and participating in the study. I know that I have antibodies and it worked and that I passed them on to Bennett. Also, I know that all the information is available for other women who are questioning whether to get vaccinated or not,” said Ms. Carrig.
Dr. Parchem is also taking part in the CDC’s v-safe pregnancy registry, which is collecting health and safety data on vaccinated pregnant women.
Before she was vaccinated, Dr. Parchem said, “my advice was very measured because we lacked data either saying that it definitely works or showing that it was unsafe. Now that we have this data supporting the benefits, I feel more confident in recommending the vaccines.”
A version of this article first appeared on Medscape.com.
As the U.S. races to vaccinate millions of people against the coronavirus, pregnant women face the extra challenge of not knowing whether the vaccines are safe for them or their unborn babies.
None of the recent COVID-19 vaccine trials, including those for Pfizer, Moderna, and Johnson & Johnson, enrolled pregnant or breastfeeding women because they consider them a high-risk group.
That was despite the Society for Maternal-Fetal Medicine and the American College of Obstetricians and Gynecologists asking that pregnant and breastfeeding women be included in trials. The Food and Drug Administration even included pregnant women in the COVID-19 vaccine emergency use authorization (EUA) because of their higher risk of having a more severe disease.
Despite that lack of clinical trial data, more and more smaller studies are suggesting that the vaccines are safe for both mother and child.
Pfizer is now studying its two-dose vaccine in 4,000 pregnant and breastfeeding women to see how safe, tolerated, and robust their immune response is. Researchers will also look at how safe the vaccine is for infants and whether mothers pass along antibodies to children. But the preliminary results won’t be available until the end of the year, a Pfizer spokesperson says.
Without that information, pregnant women are less likely to get vaccinated, according to a large international survey. Less than 45% of pregnant women in the United States said they intended to get vaccinated even when they were told the vaccine was safe and 90% effective. That figure rises to 52% of pregnant women in 16 countries, including the United States, compared with 74% of nonpregnant women willing to be vaccinated. The findings were published online March 1, 2021, in the European Journal of Epidemiology.
The vaccine-hesitant pregnant women in the international study were most concerned that the COVID-19 vaccine could harm their developing fetuses, a worry related to the lack of clinical evidence in pregnant women, said lead researcher Julia Wu, ScD, an epidemiologist at the Harvard School of Public Health’s Human Immunomics Initiative in Boston.
The information vacuum also increases the chances that “people will fall victim to misinformation campaigns like the one on social media that claims that the COVID-19 vaccine causes infertility,” Dr. Wu said. This unfounded claim has deterred some women of childbearing age from getting the vaccine.
Deciding to get vaccinated
Frontline health care professionals were in the first group eligible to receive the vaccine in December 2020. “All of us who were pregnant ... had to decide whether to wait for the data, because we don’t know what the risks are, or go ahead and get it [the vaccine]. We had been dealing with the pandemic for months and were afraid of being exposed to the virus and infecting family members,” said Jacqueline Parchem, MD, a maternal-fetal medicine specialist at the University of Texas Health Science Center, Houston.
Given the lack of safety data, the CDC guidance to pregnant women has been to consult with their doctors and that it’s a personal choice. The Center for Disease Control and Prevention’s latest vaccine guidance said that “there is no evidence that antibodies formed from COVID-19 vaccination cause any problem with pregnancy, including the development of the placenta.”
The CDC is monitoring vaccinated people through its v-safe program and reported on April 12 that more than 86,000 v-safe participants said they were pregnant when they were vaccinated.
Health care workers who were nursing their infants when they were eligible for the vaccine faced a similar dilemma as pregnant women – they lacked the data on them to make a truly informed decision.
“I was nervous about the vaccine side effects for myself and whether my son Bennett, who was about a year old, would experience any of these himself,” said Christa Carrig, a labor and delivery nurse at Massachusetts General Hospital in Boston, who was breastfeeding at the time.
She and Dr. Parchem know that pregnant women with COVID-19 are more likely to have severe illness and complications such as high blood pressure and preterm delivery. “Pregnancy takes a toll on the body. When a woman gets COVID-19 and that insult is added, women who were otherwise young and healthy get much sicker than you would expect,” said Ms. Carrig.
“As a high-risk pregnancy specialist, I know that, with COVID, that babies don’t do well when moms are sick,” said Dr. Parchem.
Pregnant women accounted for more than 84,629 cases of COVID-19 and 95 deaths in the United States between Jan. 22 last year and April 12 this year, according to the CDC COVID data tracker.
Dr. Parchem and Ms. Carrig decided to get vaccinated because of their high risk of exposure to COVID-19 at work. After the second dose, Ms. Carrig reported chills but Bennett had no side effects from breastfeeding. Dr. Parchem, who delivered a healthy baby boy in February, reported no side effects other than a sore arm.
“There’s also a psychological benefit to returning to some sense of normalcy,” said Dr. Parchem. “My mother was finally able to visit us to see the new baby after we were all vaccinated. This was the first visit in more than a year.”
New study results
Ms. Carrig was one of 131 vaccinated hospital workers in the Boston area who took part in the first study to profile the immune response in pregnant and breastfeeding women and compare it with both nonpregnant and pregnant women who had COVID-19.
The study was not designed to evaluate the safety of the vaccines or whether they prevent COVID-19 illness and hospitalizations. That is the role of the large vaccine trials, the authors said.
The participants were aged 18-45 years and received both doses of either Pfizer or Moderna vaccines during one of their trimesters. They provided blood and/or breast milk samples after each vaccine dose, 2-6 weeks after the last dose, and at delivery for the 10 who gave birth during the study.
The vaccines produced a similar strong antibody response among the pregnant/breastfeeding women and nonpregnant women. Their antibody levels were much higher than those found in the pregnant women who had COVID-19, the researchers reported on March 25, 2021, in the American Journal of Obstetrics and Gynecology.
“This is important because a lot of people tend to think once they’ve had COVID-19, they are protected from the virus. This finding suggests that the vaccines produce a stronger antibody response than the infection itself, and this might be important for long-lasting protection against COVID-19,” said Dr. Parchem.
The study also addressed whether newborns benefit from the antibodies produced by their mothers. “In the 10 women who delivered, we detected antibodies in their umbilical cords and breast milk,” says Andrea Edlow, MD, lead researcher and a maternal-fetal medicine specialist at Massachusetts General Hospital.
Newborns are particularly vulnerable to respiratory infections because they have small airways and their immune systems are underdeveloped. These infections can be lethal early in life.
“The public health strategy is to vaccinate mothers against respiratory viruses, bacteria, and parasites that neonates up to 6 months are exposed to. Influenza and pertussis (whooping cough) are two examples of vaccines that we give mothers that we know transfer [antibodies] across the umbilical cord,” said Dr. Edlow.
But this “passive transfer immunity” is different from active immunity, when the body produces its own antibody immune response, she explains.
A different study, also published in March, confirmed that antibodies were transferred from 27 vaccinated pregnant mothers to their infants when they delivered. A new finding was that the women who were vaccinated with both doses and earlier in their third semester passed on more antibodies than the women who were vaccinated later or with only one dose.
Impact of the studies
The Society for Maternal-Fetal Medicine updated its guidance on counseling pregnant and lactating patients about the COVID-19 vaccines to include Dr. Edlow’s study.
“We were struck by how much pregnant and breastfeeding women want to participate in research and to help others in the same situation make decisions. I hope this will be an example to drug companies doing research on new vaccines in the future – that they should not be left behind and can make decisions themselves whether to participate after weighing the risks and benefits,” said Dr. Edlow.
She continues to enroll more vaccinated women in her study in the Boston area, including non–health care workers who have asked to take part.
“It was worth getting vaccinated and participating in the study. I know that I have antibodies and it worked and that I passed them on to Bennett. Also, I know that all the information is available for other women who are questioning whether to get vaccinated or not,” said Ms. Carrig.
Dr. Parchem is also taking part in the CDC’s v-safe pregnancy registry, which is collecting health and safety data on vaccinated pregnant women.
Before she was vaccinated, Dr. Parchem said, “my advice was very measured because we lacked data either saying that it definitely works or showing that it was unsafe. Now that we have this data supporting the benefits, I feel more confident in recommending the vaccines.”
A version of this article first appeared on Medscape.com.
As the U.S. races to vaccinate millions of people against the coronavirus, pregnant women face the extra challenge of not knowing whether the vaccines are safe for them or their unborn babies.
None of the recent COVID-19 vaccine trials, including those for Pfizer, Moderna, and Johnson & Johnson, enrolled pregnant or breastfeeding women because they consider them a high-risk group.
That was despite the Society for Maternal-Fetal Medicine and the American College of Obstetricians and Gynecologists asking that pregnant and breastfeeding women be included in trials. The Food and Drug Administration even included pregnant women in the COVID-19 vaccine emergency use authorization (EUA) because of their higher risk of having a more severe disease.
Despite that lack of clinical trial data, more and more smaller studies are suggesting that the vaccines are safe for both mother and child.
Pfizer is now studying its two-dose vaccine in 4,000 pregnant and breastfeeding women to see how safe, tolerated, and robust their immune response is. Researchers will also look at how safe the vaccine is for infants and whether mothers pass along antibodies to children. But the preliminary results won’t be available until the end of the year, a Pfizer spokesperson says.
Without that information, pregnant women are less likely to get vaccinated, according to a large international survey. Less than 45% of pregnant women in the United States said they intended to get vaccinated even when they were told the vaccine was safe and 90% effective. That figure rises to 52% of pregnant women in 16 countries, including the United States, compared with 74% of nonpregnant women willing to be vaccinated. The findings were published online March 1, 2021, in the European Journal of Epidemiology.
The vaccine-hesitant pregnant women in the international study were most concerned that the COVID-19 vaccine could harm their developing fetuses, a worry related to the lack of clinical evidence in pregnant women, said lead researcher Julia Wu, ScD, an epidemiologist at the Harvard School of Public Health’s Human Immunomics Initiative in Boston.
The information vacuum also increases the chances that “people will fall victim to misinformation campaigns like the one on social media that claims that the COVID-19 vaccine causes infertility,” Dr. Wu said. This unfounded claim has deterred some women of childbearing age from getting the vaccine.
Deciding to get vaccinated
Frontline health care professionals were in the first group eligible to receive the vaccine in December 2020. “All of us who were pregnant ... had to decide whether to wait for the data, because we don’t know what the risks are, or go ahead and get it [the vaccine]. We had been dealing with the pandemic for months and were afraid of being exposed to the virus and infecting family members,” said Jacqueline Parchem, MD, a maternal-fetal medicine specialist at the University of Texas Health Science Center, Houston.
Given the lack of safety data, the CDC guidance to pregnant women has been to consult with their doctors and that it’s a personal choice. The Center for Disease Control and Prevention’s latest vaccine guidance said that “there is no evidence that antibodies formed from COVID-19 vaccination cause any problem with pregnancy, including the development of the placenta.”
The CDC is monitoring vaccinated people through its v-safe program and reported on April 12 that more than 86,000 v-safe participants said they were pregnant when they were vaccinated.
Health care workers who were nursing their infants when they were eligible for the vaccine faced a similar dilemma as pregnant women – they lacked the data on them to make a truly informed decision.
“I was nervous about the vaccine side effects for myself and whether my son Bennett, who was about a year old, would experience any of these himself,” said Christa Carrig, a labor and delivery nurse at Massachusetts General Hospital in Boston, who was breastfeeding at the time.
She and Dr. Parchem know that pregnant women with COVID-19 are more likely to have severe illness and complications such as high blood pressure and preterm delivery. “Pregnancy takes a toll on the body. When a woman gets COVID-19 and that insult is added, women who were otherwise young and healthy get much sicker than you would expect,” said Ms. Carrig.
“As a high-risk pregnancy specialist, I know that, with COVID, that babies don’t do well when moms are sick,” said Dr. Parchem.
Pregnant women accounted for more than 84,629 cases of COVID-19 and 95 deaths in the United States between Jan. 22 last year and April 12 this year, according to the CDC COVID data tracker.
Dr. Parchem and Ms. Carrig decided to get vaccinated because of their high risk of exposure to COVID-19 at work. After the second dose, Ms. Carrig reported chills but Bennett had no side effects from breastfeeding. Dr. Parchem, who delivered a healthy baby boy in February, reported no side effects other than a sore arm.
“There’s also a psychological benefit to returning to some sense of normalcy,” said Dr. Parchem. “My mother was finally able to visit us to see the new baby after we were all vaccinated. This was the first visit in more than a year.”
New study results
Ms. Carrig was one of 131 vaccinated hospital workers in the Boston area who took part in the first study to profile the immune response in pregnant and breastfeeding women and compare it with both nonpregnant and pregnant women who had COVID-19.
The study was not designed to evaluate the safety of the vaccines or whether they prevent COVID-19 illness and hospitalizations. That is the role of the large vaccine trials, the authors said.
The participants were aged 18-45 years and received both doses of either Pfizer or Moderna vaccines during one of their trimesters. They provided blood and/or breast milk samples after each vaccine dose, 2-6 weeks after the last dose, and at delivery for the 10 who gave birth during the study.
The vaccines produced a similar strong antibody response among the pregnant/breastfeeding women and nonpregnant women. Their antibody levels were much higher than those found in the pregnant women who had COVID-19, the researchers reported on March 25, 2021, in the American Journal of Obstetrics and Gynecology.
“This is important because a lot of people tend to think once they’ve had COVID-19, they are protected from the virus. This finding suggests that the vaccines produce a stronger antibody response than the infection itself, and this might be important for long-lasting protection against COVID-19,” said Dr. Parchem.
The study also addressed whether newborns benefit from the antibodies produced by their mothers. “In the 10 women who delivered, we detected antibodies in their umbilical cords and breast milk,” says Andrea Edlow, MD, lead researcher and a maternal-fetal medicine specialist at Massachusetts General Hospital.
Newborns are particularly vulnerable to respiratory infections because they have small airways and their immune systems are underdeveloped. These infections can be lethal early in life.
“The public health strategy is to vaccinate mothers against respiratory viruses, bacteria, and parasites that neonates up to 6 months are exposed to. Influenza and pertussis (whooping cough) are two examples of vaccines that we give mothers that we know transfer [antibodies] across the umbilical cord,” said Dr. Edlow.
But this “passive transfer immunity” is different from active immunity, when the body produces its own antibody immune response, she explains.
A different study, also published in March, confirmed that antibodies were transferred from 27 vaccinated pregnant mothers to their infants when they delivered. A new finding was that the women who were vaccinated with both doses and earlier in their third semester passed on more antibodies than the women who were vaccinated later or with only one dose.
Impact of the studies
The Society for Maternal-Fetal Medicine updated its guidance on counseling pregnant and lactating patients about the COVID-19 vaccines to include Dr. Edlow’s study.
“We were struck by how much pregnant and breastfeeding women want to participate in research and to help others in the same situation make decisions. I hope this will be an example to drug companies doing research on new vaccines in the future – that they should not be left behind and can make decisions themselves whether to participate after weighing the risks and benefits,” said Dr. Edlow.
She continues to enroll more vaccinated women in her study in the Boston area, including non–health care workers who have asked to take part.
“It was worth getting vaccinated and participating in the study. I know that I have antibodies and it worked and that I passed them on to Bennett. Also, I know that all the information is available for other women who are questioning whether to get vaccinated or not,” said Ms. Carrig.
Dr. Parchem is also taking part in the CDC’s v-safe pregnancy registry, which is collecting health and safety data on vaccinated pregnant women.
Before she was vaccinated, Dr. Parchem said, “my advice was very measured because we lacked data either saying that it definitely works or showing that it was unsafe. Now that we have this data supporting the benefits, I feel more confident in recommending the vaccines.”
A version of this article first appeared on Medscape.com.
A Rash Against the Grain
ANSWER
The correct answer is dermatitis herpetiformis (DH; choice “b”).
DISCUSSION
DH is unusual, although not rare, and it is often overlooked in the investigation of itchy rashes—even by dermatologists. As is so often the case, the biopsy was the missing piece of the puzzle, since it clearly showed changes consistent with this condition.
The history of DH has some interesting overlap with that of biopsy. It was Rudolph Virchow, an 18th century Austrian pathologist, who first connected clinical disease to specific arrangements of disordered tissue seen microscopically—at that time, a giant leap for medicine. An American dermatologist, Louis Adolphus Duhring, had the good fortune to study under Virchow and his successors in Vienna and brought this cutting-edge knowledge back to this country, where he began the process of categorizing diseases by their histologic patterns as well as by their presentation and clinical course. This was when he discovered the basis for the condition that became known as Duhring disease, also known as dermatitis herpetiformis.
It was much later (1967) that a connection was made between DH and the ingestion of gluten. Our patient experienced a rapid decline in his itching and rash as soon as he started a gluten-free diet. However, since many patients find such a diet difficult to maintain, there are pharmacologic options as well: dapsone, colchicine, sulfa drugs, drugs from the tetracycline family, and nicotinamide. All have potential adverse effects with which the prescriber needs to become familiar.
With a combination of medication and reduced gluten intake, it’s entirely possible that the patient’s DH will permanently resolve, although he will remain at increased risk for other autoimmune conditions.
ANSWER
The correct answer is dermatitis herpetiformis (DH; choice “b”).
DISCUSSION
DH is unusual, although not rare, and it is often overlooked in the investigation of itchy rashes—even by dermatologists. As is so often the case, the biopsy was the missing piece of the puzzle, since it clearly showed changes consistent with this condition.
The history of DH has some interesting overlap with that of biopsy. It was Rudolph Virchow, an 18th century Austrian pathologist, who first connected clinical disease to specific arrangements of disordered tissue seen microscopically—at that time, a giant leap for medicine. An American dermatologist, Louis Adolphus Duhring, had the good fortune to study under Virchow and his successors in Vienna and brought this cutting-edge knowledge back to this country, where he began the process of categorizing diseases by their histologic patterns as well as by their presentation and clinical course. This was when he discovered the basis for the condition that became known as Duhring disease, also known as dermatitis herpetiformis.
It was much later (1967) that a connection was made between DH and the ingestion of gluten. Our patient experienced a rapid decline in his itching and rash as soon as he started a gluten-free diet. However, since many patients find such a diet difficult to maintain, there are pharmacologic options as well: dapsone, colchicine, sulfa drugs, drugs from the tetracycline family, and nicotinamide. All have potential adverse effects with which the prescriber needs to become familiar.
With a combination of medication and reduced gluten intake, it’s entirely possible that the patient’s DH will permanently resolve, although he will remain at increased risk for other autoimmune conditions.
ANSWER
The correct answer is dermatitis herpetiformis (DH; choice “b”).
DISCUSSION
DH is unusual, although not rare, and it is often overlooked in the investigation of itchy rashes—even by dermatologists. As is so often the case, the biopsy was the missing piece of the puzzle, since it clearly showed changes consistent with this condition.
The history of DH has some interesting overlap with that of biopsy. It was Rudolph Virchow, an 18th century Austrian pathologist, who first connected clinical disease to specific arrangements of disordered tissue seen microscopically—at that time, a giant leap for medicine. An American dermatologist, Louis Adolphus Duhring, had the good fortune to study under Virchow and his successors in Vienna and brought this cutting-edge knowledge back to this country, where he began the process of categorizing diseases by their histologic patterns as well as by their presentation and clinical course. This was when he discovered the basis for the condition that became known as Duhring disease, also known as dermatitis herpetiformis.
It was much later (1967) that a connection was made between DH and the ingestion of gluten. Our patient experienced a rapid decline in his itching and rash as soon as he started a gluten-free diet. However, since many patients find such a diet difficult to maintain, there are pharmacologic options as well: dapsone, colchicine, sulfa drugs, drugs from the tetracycline family, and nicotinamide. All have potential adverse effects with which the prescriber needs to become familiar.
With a combination of medication and reduced gluten intake, it’s entirely possible that the patient’s DH will permanently resolve, although he will remain at increased risk for other autoimmune conditions.
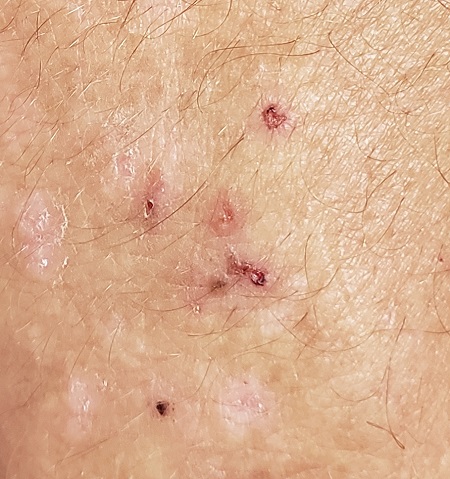
A 56-year-old man began to itch almost 10 years ago, when lesions appeared on his extensor forearms; they later branched out to his knees, scalp, and waistline. He has continued to suffer despite seeing numerous providers, including dermatologists.
An allergist pronounced him free of any significant allergies. Another provider was certain the patient had scabies, although his household was unaffected and the prescribed treatment—permethrin lotion and oral ivermectin—had no impact on the rash or the symptoms. Most of the other consulted providers diagnosed eczema, despite a complete lack of atopy in the patient or his family of origin. Furthermore, treatment with topical, oral, and intramuscular steroids had offered minimal and very short-lived relief. No one took a scraping or performed a biopsy of the rash.
The patient claims to be otherwise healthy, with no gastrointestinal symptoms and no new medications. Examination reveals a slightly overweight man in no distress.
The lesions in question are patches of excoriated papulovesicular lesions. Microscopic examination of a KOH prep shows no scabetic elements. His volar wrists, the sides of his fingers, and his genitals are free of lesions.
A shave biopsy is performed under local anesthetic. The pathology report indicates subepidermal collections of eosinophils.
Eating more fat may boost borderline low testosterone
Low-fat diets appear to decrease testosterone levels in men, but further randomized, controlled trials are needed to confirm this effect, the authors of a meta-analysis of six small intervention studies concluded.
A total of 206 healthy men with normal testosterone received a high-fat diet followed by a low-fat diet (or vice versa), and their mean total testosterone levels were 10%-15% lower (but still in the normal range) during the low-fat diet.
The study by registered nutritionist Joseph Whittaker, MSc, University of Worcester (England), and statistician Kexin Wu, MSc, University of Warwick, Coventry, England, was published online in the Journal of Steroid Biochemistry and Molecular Biology.
“I think our results are consistent and fairly strong, but they are not strong enough to give blanket recommendations,” Mr. Whittaker said in an interview.
However, “if somebody has low testosterone, particularly borderline, they could try increasing their fat intake, maybe on a Mediterranean diet,” he said, and see if that works to increase their testosterone by 60 ng/dL, the weighted mean difference in total testosterone levels between the low-fat versus high-fat diet interventions in this meta-analysis.
“A Mediterranean diet is a good way to increase ‘healthy fats,’ mono- and polyunsaturated fatty acids, which will likely decrease cardiovascular disease risk, and boost testosterone at the same time,” Mr. Whittaker noted.
Olive oil has been shown to boost testosterone more than butter, and it also reduces CVD, he continued. Nuts are high in “healthy fats” and consistently decrease CVD and mortality and may boost testosterone. Other sources of “good fat” in a healthy diet include avocado, and red meat and poultry in moderation.
“It is controversial, but our results also indicate that foods with saturated fatty acids may boost testosterone,” he added, noting however that such foods are also associated with an increase in cholesterol.
Is waning testosterone explained by leaner diet?
Men need healthy testosterone levels for good physical performance, mental health, and sexual health, and low levels are associated with a higher risk of heart disease, diabetes, and Alzheimer’s disease, according to a statement about this research issued by the University of Worcester.
Although testosterone levels do decline with advancing age, there has also been an additional age-independent and persistent decline in testosterone levels that began roughly after nutrition guidelines began recommending a lower-fat diet in 1965.
Fat consumption dropped from 45% of the diet in 1965 to 35% of the diet in 1991, and stayed around that lower level through to 2011.
However, it is not clear if this decrease in dietary fat intake might explain part of the concurrent decline in men’s testosterone levels.
Mr. Whittaker and Mr. Wu conducted a systematic literature review and identified six crossover intervention studies that compared testosterone levels during low-fat versus high-fat diets – Dorgan 1996, Wang 2005, Hamalainen 1984, Hill 1980, Reed 1987, and Hill 1979 – and then they combined these studies in a meta-analysis.
Five studies each enrolled 6-43 healthy men from North America, the United Kingdom, and Scandinavia, and the sixth study (Hill 1980) enrolled 34 healthy men from North America and 39 farm laborers from South Africa.
Overall, on average, the men were aged 34-54 years and slightly overweight (a mean body mass index of roughly 27 kg/m2) with normal testosterone (i.e., >300 ng/dL, based on the 2018 American Urological Association guidelines criteria).
Most men received a high-fat diet (40% of calories from fat) first, followed by a low-fat diet (on average 20% of calories from fat; range, 7%-25%), but the subgroup of men from South Africa received the low-fat diet first.
To put this into context, U.K. guidelines recommend a fat intake of less than 35% of daily calories, and U.S. guidelines recommend a fat intake of 20%-35% of daily calories.
The low-and high-fat interventions ranged from 2 to 10 weeks.
Lowest testosterone levels with low-fat vegetarian diets
Overall, on average, the men’s total testosterone was 475 mg/dL when they were consuming a low-fat diet and 532 mg/dL when they were consuming a high-fat diet.
However, the South African men had higher testosterone levels when they consumed a low-fat diet. This suggests that “men with European ancestry may experience a greater decrease in testosterone in response to a low-fat diet,” the researchers wrote.
The decrease in total testosterone in the low-fat versus high-fat diet was largest (26%) in the two studies of men who consumed a vegetarian diet (Hill 1979 and Hill 1980). These diets may have been low in zinc, since a marginal zinc deficiency has been shown to decrease total testosterone, Mr. Whittaker and Mr. Wu speculated.
The meta-analysis also showed that levels of free testosterone, urinary testosterone, and dihydrotestosterone declined during the low-fat diet, whereas levels of luteinizing hormone or sex hormone binding globulin were similar with both diets.
Men with low testosterone and overweight, obesity
What nutritional advice should practitioners give to men who have low testosterone and overweight/obesity?
“If you are very overweight, losing weight is going to dramatically improve your testosterone,” Mr. Whittaker said.
However, proponents of various diets are often in stark disagreement about the merits of a low-fat versus low-carbohydrate diet to lose weight.
“In general,” he continued, “the literature shows low-carb (high-fat) diets are better for weight loss [although many will disagree with that statement].”
Although nutrition guidelines have stressed the importance of limiting fat intake, fat in the diet is also associated with lower triglyceride levels and blood pressure and higher HDL cholesterol levels, and now in this study, higher testosterone levels.
More research needed
The researchers acknowledge study limitations: The meta-analysis included just a few small studies with heterogeneous designs and findings, and there was possible bias from confounding variables.
“Ideally, we would like to see a few more studies to confirm our results,” Mr. Whittaker said in the statement. “However, these studies may never come; normally researchers want to find new results, not replicate old ones. In the meantime, men with low testosterone would be wise to avoid low-fat diets.”
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Low-fat diets appear to decrease testosterone levels in men, but further randomized, controlled trials are needed to confirm this effect, the authors of a meta-analysis of six small intervention studies concluded.
A total of 206 healthy men with normal testosterone received a high-fat diet followed by a low-fat diet (or vice versa), and their mean total testosterone levels were 10%-15% lower (but still in the normal range) during the low-fat diet.
The study by registered nutritionist Joseph Whittaker, MSc, University of Worcester (England), and statistician Kexin Wu, MSc, University of Warwick, Coventry, England, was published online in the Journal of Steroid Biochemistry and Molecular Biology.
“I think our results are consistent and fairly strong, but they are not strong enough to give blanket recommendations,” Mr. Whittaker said in an interview.
However, “if somebody has low testosterone, particularly borderline, they could try increasing their fat intake, maybe on a Mediterranean diet,” he said, and see if that works to increase their testosterone by 60 ng/dL, the weighted mean difference in total testosterone levels between the low-fat versus high-fat diet interventions in this meta-analysis.
“A Mediterranean diet is a good way to increase ‘healthy fats,’ mono- and polyunsaturated fatty acids, which will likely decrease cardiovascular disease risk, and boost testosterone at the same time,” Mr. Whittaker noted.
Olive oil has been shown to boost testosterone more than butter, and it also reduces CVD, he continued. Nuts are high in “healthy fats” and consistently decrease CVD and mortality and may boost testosterone. Other sources of “good fat” in a healthy diet include avocado, and red meat and poultry in moderation.
“It is controversial, but our results also indicate that foods with saturated fatty acids may boost testosterone,” he added, noting however that such foods are also associated with an increase in cholesterol.
Is waning testosterone explained by leaner diet?
Men need healthy testosterone levels for good physical performance, mental health, and sexual health, and low levels are associated with a higher risk of heart disease, diabetes, and Alzheimer’s disease, according to a statement about this research issued by the University of Worcester.
Although testosterone levels do decline with advancing age, there has also been an additional age-independent and persistent decline in testosterone levels that began roughly after nutrition guidelines began recommending a lower-fat diet in 1965.
Fat consumption dropped from 45% of the diet in 1965 to 35% of the diet in 1991, and stayed around that lower level through to 2011.
However, it is not clear if this decrease in dietary fat intake might explain part of the concurrent decline in men’s testosterone levels.
Mr. Whittaker and Mr. Wu conducted a systematic literature review and identified six crossover intervention studies that compared testosterone levels during low-fat versus high-fat diets – Dorgan 1996, Wang 2005, Hamalainen 1984, Hill 1980, Reed 1987, and Hill 1979 – and then they combined these studies in a meta-analysis.
Five studies each enrolled 6-43 healthy men from North America, the United Kingdom, and Scandinavia, and the sixth study (Hill 1980) enrolled 34 healthy men from North America and 39 farm laborers from South Africa.
Overall, on average, the men were aged 34-54 years and slightly overweight (a mean body mass index of roughly 27 kg/m2) with normal testosterone (i.e., >300 ng/dL, based on the 2018 American Urological Association guidelines criteria).
Most men received a high-fat diet (40% of calories from fat) first, followed by a low-fat diet (on average 20% of calories from fat; range, 7%-25%), but the subgroup of men from South Africa received the low-fat diet first.
To put this into context, U.K. guidelines recommend a fat intake of less than 35% of daily calories, and U.S. guidelines recommend a fat intake of 20%-35% of daily calories.
The low-and high-fat interventions ranged from 2 to 10 weeks.
Lowest testosterone levels with low-fat vegetarian diets
Overall, on average, the men’s total testosterone was 475 mg/dL when they were consuming a low-fat diet and 532 mg/dL when they were consuming a high-fat diet.
However, the South African men had higher testosterone levels when they consumed a low-fat diet. This suggests that “men with European ancestry may experience a greater decrease in testosterone in response to a low-fat diet,” the researchers wrote.
The decrease in total testosterone in the low-fat versus high-fat diet was largest (26%) in the two studies of men who consumed a vegetarian diet (Hill 1979 and Hill 1980). These diets may have been low in zinc, since a marginal zinc deficiency has been shown to decrease total testosterone, Mr. Whittaker and Mr. Wu speculated.
The meta-analysis also showed that levels of free testosterone, urinary testosterone, and dihydrotestosterone declined during the low-fat diet, whereas levels of luteinizing hormone or sex hormone binding globulin were similar with both diets.
Men with low testosterone and overweight, obesity
What nutritional advice should practitioners give to men who have low testosterone and overweight/obesity?
“If you are very overweight, losing weight is going to dramatically improve your testosterone,” Mr. Whittaker said.
However, proponents of various diets are often in stark disagreement about the merits of a low-fat versus low-carbohydrate diet to lose weight.
“In general,” he continued, “the literature shows low-carb (high-fat) diets are better for weight loss [although many will disagree with that statement].”
Although nutrition guidelines have stressed the importance of limiting fat intake, fat in the diet is also associated with lower triglyceride levels and blood pressure and higher HDL cholesterol levels, and now in this study, higher testosterone levels.
More research needed
The researchers acknowledge study limitations: The meta-analysis included just a few small studies with heterogeneous designs and findings, and there was possible bias from confounding variables.
“Ideally, we would like to see a few more studies to confirm our results,” Mr. Whittaker said in the statement. “However, these studies may never come; normally researchers want to find new results, not replicate old ones. In the meantime, men with low testosterone would be wise to avoid low-fat diets.”
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Low-fat diets appear to decrease testosterone levels in men, but further randomized, controlled trials are needed to confirm this effect, the authors of a meta-analysis of six small intervention studies concluded.
A total of 206 healthy men with normal testosterone received a high-fat diet followed by a low-fat diet (or vice versa), and their mean total testosterone levels were 10%-15% lower (but still in the normal range) during the low-fat diet.
The study by registered nutritionist Joseph Whittaker, MSc, University of Worcester (England), and statistician Kexin Wu, MSc, University of Warwick, Coventry, England, was published online in the Journal of Steroid Biochemistry and Molecular Biology.
“I think our results are consistent and fairly strong, but they are not strong enough to give blanket recommendations,” Mr. Whittaker said in an interview.
However, “if somebody has low testosterone, particularly borderline, they could try increasing their fat intake, maybe on a Mediterranean diet,” he said, and see if that works to increase their testosterone by 60 ng/dL, the weighted mean difference in total testosterone levels between the low-fat versus high-fat diet interventions in this meta-analysis.
“A Mediterranean diet is a good way to increase ‘healthy fats,’ mono- and polyunsaturated fatty acids, which will likely decrease cardiovascular disease risk, and boost testosterone at the same time,” Mr. Whittaker noted.
Olive oil has been shown to boost testosterone more than butter, and it also reduces CVD, he continued. Nuts are high in “healthy fats” and consistently decrease CVD and mortality and may boost testosterone. Other sources of “good fat” in a healthy diet include avocado, and red meat and poultry in moderation.
“It is controversial, but our results also indicate that foods with saturated fatty acids may boost testosterone,” he added, noting however that such foods are also associated with an increase in cholesterol.
Is waning testosterone explained by leaner diet?
Men need healthy testosterone levels for good physical performance, mental health, and sexual health, and low levels are associated with a higher risk of heart disease, diabetes, and Alzheimer’s disease, according to a statement about this research issued by the University of Worcester.
Although testosterone levels do decline with advancing age, there has also been an additional age-independent and persistent decline in testosterone levels that began roughly after nutrition guidelines began recommending a lower-fat diet in 1965.
Fat consumption dropped from 45% of the diet in 1965 to 35% of the diet in 1991, and stayed around that lower level through to 2011.
However, it is not clear if this decrease in dietary fat intake might explain part of the concurrent decline in men’s testosterone levels.
Mr. Whittaker and Mr. Wu conducted a systematic literature review and identified six crossover intervention studies that compared testosterone levels during low-fat versus high-fat diets – Dorgan 1996, Wang 2005, Hamalainen 1984, Hill 1980, Reed 1987, and Hill 1979 – and then they combined these studies in a meta-analysis.
Five studies each enrolled 6-43 healthy men from North America, the United Kingdom, and Scandinavia, and the sixth study (Hill 1980) enrolled 34 healthy men from North America and 39 farm laborers from South Africa.
Overall, on average, the men were aged 34-54 years and slightly overweight (a mean body mass index of roughly 27 kg/m2) with normal testosterone (i.e., >300 ng/dL, based on the 2018 American Urological Association guidelines criteria).
Most men received a high-fat diet (40% of calories from fat) first, followed by a low-fat diet (on average 20% of calories from fat; range, 7%-25%), but the subgroup of men from South Africa received the low-fat diet first.
To put this into context, U.K. guidelines recommend a fat intake of less than 35% of daily calories, and U.S. guidelines recommend a fat intake of 20%-35% of daily calories.
The low-and high-fat interventions ranged from 2 to 10 weeks.
Lowest testosterone levels with low-fat vegetarian diets
Overall, on average, the men’s total testosterone was 475 mg/dL when they were consuming a low-fat diet and 532 mg/dL when they were consuming a high-fat diet.
However, the South African men had higher testosterone levels when they consumed a low-fat diet. This suggests that “men with European ancestry may experience a greater decrease in testosterone in response to a low-fat diet,” the researchers wrote.
The decrease in total testosterone in the low-fat versus high-fat diet was largest (26%) in the two studies of men who consumed a vegetarian diet (Hill 1979 and Hill 1980). These diets may have been low in zinc, since a marginal zinc deficiency has been shown to decrease total testosterone, Mr. Whittaker and Mr. Wu speculated.
The meta-analysis also showed that levels of free testosterone, urinary testosterone, and dihydrotestosterone declined during the low-fat diet, whereas levels of luteinizing hormone or sex hormone binding globulin were similar with both diets.
Men with low testosterone and overweight, obesity
What nutritional advice should practitioners give to men who have low testosterone and overweight/obesity?
“If you are very overweight, losing weight is going to dramatically improve your testosterone,” Mr. Whittaker said.
However, proponents of various diets are often in stark disagreement about the merits of a low-fat versus low-carbohydrate diet to lose weight.
“In general,” he continued, “the literature shows low-carb (high-fat) diets are better for weight loss [although many will disagree with that statement].”
Although nutrition guidelines have stressed the importance of limiting fat intake, fat in the diet is also associated with lower triglyceride levels and blood pressure and higher HDL cholesterol levels, and now in this study, higher testosterone levels.
More research needed
The researchers acknowledge study limitations: The meta-analysis included just a few small studies with heterogeneous designs and findings, and there was possible bias from confounding variables.
“Ideally, we would like to see a few more studies to confirm our results,” Mr. Whittaker said in the statement. “However, these studies may never come; normally researchers want to find new results, not replicate old ones. In the meantime, men with low testosterone would be wise to avoid low-fat diets.”
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID-19 vaccine response lower in kidney dialysis patients
the first study of its kind shows.
“It is well known that patients on dialysis may have a reduced response to vaccination,” Ayelet Grupper, MD, of Tel Aviv Medical Center, and colleagues observe. Their study was published online April 6 in the Clinical Journal of the American Society of Nephrology.
“I believe our findings should encourage patients with kidney failure treated with dialysis to be vaccinated as soon as vaccination becomes available for them, while we as caregivers should explore ways to enhance its efficacy in our patients,” senior author Moshe Shashar, MD, noted in a statement from the American Society of Nephrology.
Asked to comment, Peter Blake, MD, professor of medicine, University of Western Ontario, London, pointed out that COVID-19 is very common among hemodialysis patients and that the likelihood of these patients dying from it is very high. Indeed, 1.5% of approximately 12,500 patients receiving dialysis in the province of Ontario have died of COVID-19 – “a horrifying statistic and one that only long-term care home residents can compare with,” he told this news organization.
In the Israeli study, almost all dialysis patients mounted a serologic response to the Pfizer-BioNTech vaccine, which is “good news” overall, Dr. Blake said.
Also commenting on the study, Anushree Shirali, MD, of Yale University, New Haven, Conn., said she was impressed by the fact that most of the dialysis patients in the study mounted at least some IgG response to vaccination, which she said was good “in and of itself,” because that is not always the case with other vaccines.
Study compared dialysis patients with health care workers
The Israeli study included 56 patients who were receiving maintenance hemodialysis and 95 health care workers, who served as control persons.
“All participants had been previously vaccinated with the [Pfizer-BioNTech] vaccine, with the recommended dosing interval of 21 days between the first and second doses,” the investigators note. Immunogenicity was assessed using a dedicated immunoassay to quantify the level of IgG antibodies from participants’ plasma.
A cutoff for a positive antibody response was greater than or equal to 50 arbitrary units per milliliter (AU/mL). “All subjects in the control group developed a positive antibody response (≥50 AU/mL) as compared with 96% (54 of 56) in the dialysis group,” Dr. Shashar and colleagues report.
The median IgG level in the dialysis group was 2,900 AU/mL, which is significantly lower than the median of 7,401 AU/mL in the control group (P < .001), they report.
The investigators also observed a significant inverse correlation between older age and antibody levels in both groups.
The odds of being in the lower quartile were significantly higher for older individuals (odds ratio, 1.11 per year of age; P = .004) and for the dialysis group compared with the control group (OR, 2.7; P = .05).
Among the dialysis patients, older age and lower lymphocyte count were associated with antibody response in the lower quartile (OR, 1.22 per 1 year older; P = .03; and OR, 0.83 per 10-e3/mL-higher lymphocyte count; P = .05).
Among recipients older than 70 years, there was little difference in antibody response between the dialysis patients and the control group. Thus, age is clearly an important contributor to a robust humoral response, the authors observe.
For more than 90% of the patients receiving dialysis, the antibody response was well above 50 AU/mL, which was the cutoff for having a positive response.
Nevertheless, the authors suggest that their findings should prompt clinicians to consider either changing the dose or the schedule of COVID-19 vaccination for dialysis patients, as was done, for example, with the hepatitis B vaccine Engerix-B.
Dialysis patients now receive double doses of the hepatitis B vaccine, which is given in a four-series vaccine schedule rather than a three-series vaccine schedule, as is given to healthy individuals.
The authors also call for studies to assess the longevity of vaccine efficacy for dialysis patients and whether current vaccines are effective against variant strains among patients undergoing dialysis.
Some suggestion COVID-19 vaccines also elicit T-cell responses
Dr. Shirali said the news regarding the COVID-19 vaccine for dialysis patients is good, given the fact that such patients exhibit a poor response to the hepatitis B vaccine.
“There isn’t a large percentage of dialysis patients who mount a humoral response to the hepatitis B vaccine, even with the change in dosing that we use that is different than it is for the general population,” she told this news organization.
Dr. Shirali also noted that preliminary evidence suggests that COVID-19 vaccines elicit nonantibody and antibody T-cell responses and that such immunity is going to be just as important for protecting dialysis patients against COVID-19 as it is for protecting patients who are not receiving dialysis.
“Antibody responses are just one arm of vaccination,” she explained. “People can form memory T-cell responses with vaccination, and while this has not been well studied with COVID-19, there are preliminary data to suggest that T-cell responses are likely to be effective in the fight against COVID-19.” There is also the possibility that this type of response “may even be more durable than antibody responses,” she said.
The study received no funding. The authors, Dr. Blake and Dr. Shirali, have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
the first study of its kind shows.
“It is well known that patients on dialysis may have a reduced response to vaccination,” Ayelet Grupper, MD, of Tel Aviv Medical Center, and colleagues observe. Their study was published online April 6 in the Clinical Journal of the American Society of Nephrology.
“I believe our findings should encourage patients with kidney failure treated with dialysis to be vaccinated as soon as vaccination becomes available for them, while we as caregivers should explore ways to enhance its efficacy in our patients,” senior author Moshe Shashar, MD, noted in a statement from the American Society of Nephrology.
Asked to comment, Peter Blake, MD, professor of medicine, University of Western Ontario, London, pointed out that COVID-19 is very common among hemodialysis patients and that the likelihood of these patients dying from it is very high. Indeed, 1.5% of approximately 12,500 patients receiving dialysis in the province of Ontario have died of COVID-19 – “a horrifying statistic and one that only long-term care home residents can compare with,” he told this news organization.
In the Israeli study, almost all dialysis patients mounted a serologic response to the Pfizer-BioNTech vaccine, which is “good news” overall, Dr. Blake said.
Also commenting on the study, Anushree Shirali, MD, of Yale University, New Haven, Conn., said she was impressed by the fact that most of the dialysis patients in the study mounted at least some IgG response to vaccination, which she said was good “in and of itself,” because that is not always the case with other vaccines.
Study compared dialysis patients with health care workers
The Israeli study included 56 patients who were receiving maintenance hemodialysis and 95 health care workers, who served as control persons.
“All participants had been previously vaccinated with the [Pfizer-BioNTech] vaccine, with the recommended dosing interval of 21 days between the first and second doses,” the investigators note. Immunogenicity was assessed using a dedicated immunoassay to quantify the level of IgG antibodies from participants’ plasma.
A cutoff for a positive antibody response was greater than or equal to 50 arbitrary units per milliliter (AU/mL). “All subjects in the control group developed a positive antibody response (≥50 AU/mL) as compared with 96% (54 of 56) in the dialysis group,” Dr. Shashar and colleagues report.
The median IgG level in the dialysis group was 2,900 AU/mL, which is significantly lower than the median of 7,401 AU/mL in the control group (P < .001), they report.
The investigators also observed a significant inverse correlation between older age and antibody levels in both groups.
The odds of being in the lower quartile were significantly higher for older individuals (odds ratio, 1.11 per year of age; P = .004) and for the dialysis group compared with the control group (OR, 2.7; P = .05).
Among the dialysis patients, older age and lower lymphocyte count were associated with antibody response in the lower quartile (OR, 1.22 per 1 year older; P = .03; and OR, 0.83 per 10-e3/mL-higher lymphocyte count; P = .05).
Among recipients older than 70 years, there was little difference in antibody response between the dialysis patients and the control group. Thus, age is clearly an important contributor to a robust humoral response, the authors observe.
For more than 90% of the patients receiving dialysis, the antibody response was well above 50 AU/mL, which was the cutoff for having a positive response.
Nevertheless, the authors suggest that their findings should prompt clinicians to consider either changing the dose or the schedule of COVID-19 vaccination for dialysis patients, as was done, for example, with the hepatitis B vaccine Engerix-B.
Dialysis patients now receive double doses of the hepatitis B vaccine, which is given in a four-series vaccine schedule rather than a three-series vaccine schedule, as is given to healthy individuals.
The authors also call for studies to assess the longevity of vaccine efficacy for dialysis patients and whether current vaccines are effective against variant strains among patients undergoing dialysis.
Some suggestion COVID-19 vaccines also elicit T-cell responses
Dr. Shirali said the news regarding the COVID-19 vaccine for dialysis patients is good, given the fact that such patients exhibit a poor response to the hepatitis B vaccine.
“There isn’t a large percentage of dialysis patients who mount a humoral response to the hepatitis B vaccine, even with the change in dosing that we use that is different than it is for the general population,” she told this news organization.
Dr. Shirali also noted that preliminary evidence suggests that COVID-19 vaccines elicit nonantibody and antibody T-cell responses and that such immunity is going to be just as important for protecting dialysis patients against COVID-19 as it is for protecting patients who are not receiving dialysis.
“Antibody responses are just one arm of vaccination,” she explained. “People can form memory T-cell responses with vaccination, and while this has not been well studied with COVID-19, there are preliminary data to suggest that T-cell responses are likely to be effective in the fight against COVID-19.” There is also the possibility that this type of response “may even be more durable than antibody responses,” she said.
The study received no funding. The authors, Dr. Blake and Dr. Shirali, have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
the first study of its kind shows.
“It is well known that patients on dialysis may have a reduced response to vaccination,” Ayelet Grupper, MD, of Tel Aviv Medical Center, and colleagues observe. Their study was published online April 6 in the Clinical Journal of the American Society of Nephrology.
“I believe our findings should encourage patients with kidney failure treated with dialysis to be vaccinated as soon as vaccination becomes available for them, while we as caregivers should explore ways to enhance its efficacy in our patients,” senior author Moshe Shashar, MD, noted in a statement from the American Society of Nephrology.
Asked to comment, Peter Blake, MD, professor of medicine, University of Western Ontario, London, pointed out that COVID-19 is very common among hemodialysis patients and that the likelihood of these patients dying from it is very high. Indeed, 1.5% of approximately 12,500 patients receiving dialysis in the province of Ontario have died of COVID-19 – “a horrifying statistic and one that only long-term care home residents can compare with,” he told this news organization.
In the Israeli study, almost all dialysis patients mounted a serologic response to the Pfizer-BioNTech vaccine, which is “good news” overall, Dr. Blake said.
Also commenting on the study, Anushree Shirali, MD, of Yale University, New Haven, Conn., said she was impressed by the fact that most of the dialysis patients in the study mounted at least some IgG response to vaccination, which she said was good “in and of itself,” because that is not always the case with other vaccines.
Study compared dialysis patients with health care workers
The Israeli study included 56 patients who were receiving maintenance hemodialysis and 95 health care workers, who served as control persons.
“All participants had been previously vaccinated with the [Pfizer-BioNTech] vaccine, with the recommended dosing interval of 21 days between the first and second doses,” the investigators note. Immunogenicity was assessed using a dedicated immunoassay to quantify the level of IgG antibodies from participants’ plasma.
A cutoff for a positive antibody response was greater than or equal to 50 arbitrary units per milliliter (AU/mL). “All subjects in the control group developed a positive antibody response (≥50 AU/mL) as compared with 96% (54 of 56) in the dialysis group,” Dr. Shashar and colleagues report.
The median IgG level in the dialysis group was 2,900 AU/mL, which is significantly lower than the median of 7,401 AU/mL in the control group (P < .001), they report.
The investigators also observed a significant inverse correlation between older age and antibody levels in both groups.
The odds of being in the lower quartile were significantly higher for older individuals (odds ratio, 1.11 per year of age; P = .004) and for the dialysis group compared with the control group (OR, 2.7; P = .05).
Among the dialysis patients, older age and lower lymphocyte count were associated with antibody response in the lower quartile (OR, 1.22 per 1 year older; P = .03; and OR, 0.83 per 10-e3/mL-higher lymphocyte count; P = .05).
Among recipients older than 70 years, there was little difference in antibody response between the dialysis patients and the control group. Thus, age is clearly an important contributor to a robust humoral response, the authors observe.
For more than 90% of the patients receiving dialysis, the antibody response was well above 50 AU/mL, which was the cutoff for having a positive response.
Nevertheless, the authors suggest that their findings should prompt clinicians to consider either changing the dose or the schedule of COVID-19 vaccination for dialysis patients, as was done, for example, with the hepatitis B vaccine Engerix-B.
Dialysis patients now receive double doses of the hepatitis B vaccine, which is given in a four-series vaccine schedule rather than a three-series vaccine schedule, as is given to healthy individuals.
The authors also call for studies to assess the longevity of vaccine efficacy for dialysis patients and whether current vaccines are effective against variant strains among patients undergoing dialysis.
Some suggestion COVID-19 vaccines also elicit T-cell responses
Dr. Shirali said the news regarding the COVID-19 vaccine for dialysis patients is good, given the fact that such patients exhibit a poor response to the hepatitis B vaccine.
“There isn’t a large percentage of dialysis patients who mount a humoral response to the hepatitis B vaccine, even with the change in dosing that we use that is different than it is for the general population,” she told this news organization.
Dr. Shirali also noted that preliminary evidence suggests that COVID-19 vaccines elicit nonantibody and antibody T-cell responses and that such immunity is going to be just as important for protecting dialysis patients against COVID-19 as it is for protecting patients who are not receiving dialysis.
“Antibody responses are just one arm of vaccination,” she explained. “People can form memory T-cell responses with vaccination, and while this has not been well studied with COVID-19, there are preliminary data to suggest that T-cell responses are likely to be effective in the fight against COVID-19.” There is also the possibility that this type of response “may even be more durable than antibody responses,” she said.
The study received no funding. The authors, Dr. Blake and Dr. Shirali, have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Don’t screen for vitamin D in general population, says USPSTF
Seven years after concluding that evidence was insufficient to recommend screening for vitamin D deficiency in the general population, the United States Preventive Services Task Force (USPSTF) has revisited the issue – and come up with the same conclusion.
Overall, “the current evidence is inadequate to determine whether screening for and treatment of asymptomatic low 25(OH)D levels improve clinical outcomes in community dwelling adults,” the task force concluded in its statement, recommending an “I” for insufficient.
The statement was published online April 13 in JAMA.
In the absence of screening recommendations, clinicians may be best advised to instead focus on diet and supplementation for those considered at risk, said Anne R. Cappola, MD, of the University of Pennsylvania, Philadelphia.
“Rather than posing the question of screening the general population for vitamin D deficiency, let’s focus on ensuring that everyone consumes the age-based recommended daily allowance of vitamin D instead,” Dr. Cappola, a coauthor of the accompanying editorial, said in an interview.
No studies have directly evaluated benefits of screening
The latest USPSTF recommendation is based on a systematic review of the benefits and harms of screening and early treatment for vitamin D deficiency in asymptomatic, community-dwelling nonpregnant adults aged 18 or older in the primary care setting with no signs or symptoms of deficiency.
The review found no studies that directly evaluated the benefits of screening for vitamin D deficiency.
However, 26 randomized clinical trials and one nested case-control study evaluated the effectiveness of treatment of vitamin D deficiency with supplementation.
And while observational studies have linked lower vitamin D levels with a multitude of conditions and risks, evidence of any benefit was inconsistent, with none identified for most major outcomes in asymptomatic adults – the focus of the Task Force recommendation.
“Among asymptomatic, community-dwelling populations with low vitamin D levels, the evidence suggests that treatment with vitamin D has no effect on mortality or the incidence of fractures, falls, depression, diabetes, cardiovascular disease, cancer, or adverse events,” the review authors stress.
“The evidence is inconclusive about the effect of treatment on physical functioning and infection.”
One in four are vitamin D deficient
In terms of the further question of the potential harms of vitamin D screening of asymptomatic individuals, a key concern is the potential for misclassification and over- or underdiagnosis due to inconsistent cutoffs and variability of different screening assays, the review concluded.
However, with the rare exception of vitamin D toxicity from supplementation well above sufficient levels, treatment with vitamin D supplementation appears relatively safe.
With a lack of consensus even over the basic cutoff for vitamin D deficiency, the National Academy of Medicine determined in 2011 that hydroxyvitamin D (25[OH]D) levels below 20 ng/mL are deficient for bone health, with no evidence of different thresholds for any other health condition.
Based on that cutoff, the National Health and Nutrition Examination Survey (NHANES), reported in 2014 that 25% of the U.S. population over the age of 1 was vitamin D deficient, with 18% of the population having 25(OH)D levels of 12-19 ng/mL and 5% having very low levels (< 12 ng/mL).
More work needed to determine groups at risk
While the task force report did not delve into testing or treatment recommendations for symptomatic adults, key established risk factors that may help clinicians identify those who are vitamin D deficient include obesity, receiving little or no UVB light exposure, and older age.
In general, obesity is associated with a 1.3- to 2-fold risk of being vitamin D deficient based on the criteria used, while non-Hispanic Blacks are 2-10 times more likely to be deficient compared with non-Hispanic White patients, the task force noted.
However, the implications of vitamin D deficiency in certain populations can vary. For instance, non-Hispanic Black people, despite having a higher prevalence of lower vitamin D levels compared with White people, in fact, have lower reported rates of fractures.
To address the various issues and gain a better understanding of the complexities of vitamin D deficiency, the task force calls for further research in key areas.
“More research is needed to determine whether total serum 25(OH)D levels are the best measure of vitamin D deficiency and whether the best measure of vitamin D deficiency varies by subgroups defined by race, ethnicity, or sex,” the authors indicated.
Furthermore, “more research is needed to determine the cutoff that defines vitamin D deficiency and whether that cutoff varies by specific clinical outcome or by subgroups defined by race, ethnicity, or sex.”
No support for population-based screening in guidelines
With the lack of conclusive evidence, no organizations currently recommend population-based screening for vitamin D deficiency in asymptomatic patients, and the American Society for Clinical Pathology endorses this stance.
The Endocrine Society and the American Association of Clinical Endocrinologists meanwhile do recommend screening for vitamin D deficiency in patients considered at risk.
Data show there was as much as an 80-fold increase in Medicare reimbursement volumes for vitamin D testing among clinicians from 2000 to 2010; however, that rate may have leveled off after the National Academy of Medicine reported on set deficiency levels, said Sherri-Ann M. Burnett-Bowie, MD, MPH, Dr. Cappola’s editorial coauthor.
Dr. Burnett-Bowie noted that she regularly tests her patients’ vitamin D levels, however most of her patients have osteoporosis or fractures.
“I do screen them for vitamin D deficiency since optimizing their vitamin D will improve calcium absorption, which is important for treating their osteoporosis,” Dr. Burnett-Bowie, of the endocrine division, department of medicine, Massachusetts General Hospital, Boston, said in an interview.
In terms of broader testing of asymptomatic patients in the general population, however, any changes in screening will likely be contingent on developments in the effects of treatment, she said.
“Given the challenge in finding benefits of vitamin D supplementation in those who are deficient, it will likely be more challenging to find benefits from wider screening,” she concluded.
The USPSTF and editorialists reported having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Seven years after concluding that evidence was insufficient to recommend screening for vitamin D deficiency in the general population, the United States Preventive Services Task Force (USPSTF) has revisited the issue – and come up with the same conclusion.
Overall, “the current evidence is inadequate to determine whether screening for and treatment of asymptomatic low 25(OH)D levels improve clinical outcomes in community dwelling adults,” the task force concluded in its statement, recommending an “I” for insufficient.
The statement was published online April 13 in JAMA.
In the absence of screening recommendations, clinicians may be best advised to instead focus on diet and supplementation for those considered at risk, said Anne R. Cappola, MD, of the University of Pennsylvania, Philadelphia.
“Rather than posing the question of screening the general population for vitamin D deficiency, let’s focus on ensuring that everyone consumes the age-based recommended daily allowance of vitamin D instead,” Dr. Cappola, a coauthor of the accompanying editorial, said in an interview.
No studies have directly evaluated benefits of screening
The latest USPSTF recommendation is based on a systematic review of the benefits and harms of screening and early treatment for vitamin D deficiency in asymptomatic, community-dwelling nonpregnant adults aged 18 or older in the primary care setting with no signs or symptoms of deficiency.
The review found no studies that directly evaluated the benefits of screening for vitamin D deficiency.
However, 26 randomized clinical trials and one nested case-control study evaluated the effectiveness of treatment of vitamin D deficiency with supplementation.
And while observational studies have linked lower vitamin D levels with a multitude of conditions and risks, evidence of any benefit was inconsistent, with none identified for most major outcomes in asymptomatic adults – the focus of the Task Force recommendation.
“Among asymptomatic, community-dwelling populations with low vitamin D levels, the evidence suggests that treatment with vitamin D has no effect on mortality or the incidence of fractures, falls, depression, diabetes, cardiovascular disease, cancer, or adverse events,” the review authors stress.
“The evidence is inconclusive about the effect of treatment on physical functioning and infection.”
One in four are vitamin D deficient
In terms of the further question of the potential harms of vitamin D screening of asymptomatic individuals, a key concern is the potential for misclassification and over- or underdiagnosis due to inconsistent cutoffs and variability of different screening assays, the review concluded.
However, with the rare exception of vitamin D toxicity from supplementation well above sufficient levels, treatment with vitamin D supplementation appears relatively safe.
With a lack of consensus even over the basic cutoff for vitamin D deficiency, the National Academy of Medicine determined in 2011 that hydroxyvitamin D (25[OH]D) levels below 20 ng/mL are deficient for bone health, with no evidence of different thresholds for any other health condition.
Based on that cutoff, the National Health and Nutrition Examination Survey (NHANES), reported in 2014 that 25% of the U.S. population over the age of 1 was vitamin D deficient, with 18% of the population having 25(OH)D levels of 12-19 ng/mL and 5% having very low levels (< 12 ng/mL).
More work needed to determine groups at risk
While the task force report did not delve into testing or treatment recommendations for symptomatic adults, key established risk factors that may help clinicians identify those who are vitamin D deficient include obesity, receiving little or no UVB light exposure, and older age.
In general, obesity is associated with a 1.3- to 2-fold risk of being vitamin D deficient based on the criteria used, while non-Hispanic Blacks are 2-10 times more likely to be deficient compared with non-Hispanic White patients, the task force noted.
However, the implications of vitamin D deficiency in certain populations can vary. For instance, non-Hispanic Black people, despite having a higher prevalence of lower vitamin D levels compared with White people, in fact, have lower reported rates of fractures.
To address the various issues and gain a better understanding of the complexities of vitamin D deficiency, the task force calls for further research in key areas.
“More research is needed to determine whether total serum 25(OH)D levels are the best measure of vitamin D deficiency and whether the best measure of vitamin D deficiency varies by subgroups defined by race, ethnicity, or sex,” the authors indicated.
Furthermore, “more research is needed to determine the cutoff that defines vitamin D deficiency and whether that cutoff varies by specific clinical outcome or by subgroups defined by race, ethnicity, or sex.”
No support for population-based screening in guidelines
With the lack of conclusive evidence, no organizations currently recommend population-based screening for vitamin D deficiency in asymptomatic patients, and the American Society for Clinical Pathology endorses this stance.
The Endocrine Society and the American Association of Clinical Endocrinologists meanwhile do recommend screening for vitamin D deficiency in patients considered at risk.
Data show there was as much as an 80-fold increase in Medicare reimbursement volumes for vitamin D testing among clinicians from 2000 to 2010; however, that rate may have leveled off after the National Academy of Medicine reported on set deficiency levels, said Sherri-Ann M. Burnett-Bowie, MD, MPH, Dr. Cappola’s editorial coauthor.
Dr. Burnett-Bowie noted that she regularly tests her patients’ vitamin D levels, however most of her patients have osteoporosis or fractures.
“I do screen them for vitamin D deficiency since optimizing their vitamin D will improve calcium absorption, which is important for treating their osteoporosis,” Dr. Burnett-Bowie, of the endocrine division, department of medicine, Massachusetts General Hospital, Boston, said in an interview.
In terms of broader testing of asymptomatic patients in the general population, however, any changes in screening will likely be contingent on developments in the effects of treatment, she said.
“Given the challenge in finding benefits of vitamin D supplementation in those who are deficient, it will likely be more challenging to find benefits from wider screening,” she concluded.
The USPSTF and editorialists reported having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Seven years after concluding that evidence was insufficient to recommend screening for vitamin D deficiency in the general population, the United States Preventive Services Task Force (USPSTF) has revisited the issue – and come up with the same conclusion.
Overall, “the current evidence is inadequate to determine whether screening for and treatment of asymptomatic low 25(OH)D levels improve clinical outcomes in community dwelling adults,” the task force concluded in its statement, recommending an “I” for insufficient.
The statement was published online April 13 in JAMA.
In the absence of screening recommendations, clinicians may be best advised to instead focus on diet and supplementation for those considered at risk, said Anne R. Cappola, MD, of the University of Pennsylvania, Philadelphia.
“Rather than posing the question of screening the general population for vitamin D deficiency, let’s focus on ensuring that everyone consumes the age-based recommended daily allowance of vitamin D instead,” Dr. Cappola, a coauthor of the accompanying editorial, said in an interview.
No studies have directly evaluated benefits of screening
The latest USPSTF recommendation is based on a systematic review of the benefits and harms of screening and early treatment for vitamin D deficiency in asymptomatic, community-dwelling nonpregnant adults aged 18 or older in the primary care setting with no signs or symptoms of deficiency.
The review found no studies that directly evaluated the benefits of screening for vitamin D deficiency.
However, 26 randomized clinical trials and one nested case-control study evaluated the effectiveness of treatment of vitamin D deficiency with supplementation.
And while observational studies have linked lower vitamin D levels with a multitude of conditions and risks, evidence of any benefit was inconsistent, with none identified for most major outcomes in asymptomatic adults – the focus of the Task Force recommendation.
“Among asymptomatic, community-dwelling populations with low vitamin D levels, the evidence suggests that treatment with vitamin D has no effect on mortality or the incidence of fractures, falls, depression, diabetes, cardiovascular disease, cancer, or adverse events,” the review authors stress.
“The evidence is inconclusive about the effect of treatment on physical functioning and infection.”
One in four are vitamin D deficient
In terms of the further question of the potential harms of vitamin D screening of asymptomatic individuals, a key concern is the potential for misclassification and over- or underdiagnosis due to inconsistent cutoffs and variability of different screening assays, the review concluded.
However, with the rare exception of vitamin D toxicity from supplementation well above sufficient levels, treatment with vitamin D supplementation appears relatively safe.
With a lack of consensus even over the basic cutoff for vitamin D deficiency, the National Academy of Medicine determined in 2011 that hydroxyvitamin D (25[OH]D) levels below 20 ng/mL are deficient for bone health, with no evidence of different thresholds for any other health condition.
Based on that cutoff, the National Health and Nutrition Examination Survey (NHANES), reported in 2014 that 25% of the U.S. population over the age of 1 was vitamin D deficient, with 18% of the population having 25(OH)D levels of 12-19 ng/mL and 5% having very low levels (< 12 ng/mL).
More work needed to determine groups at risk
While the task force report did not delve into testing or treatment recommendations for symptomatic adults, key established risk factors that may help clinicians identify those who are vitamin D deficient include obesity, receiving little or no UVB light exposure, and older age.
In general, obesity is associated with a 1.3- to 2-fold risk of being vitamin D deficient based on the criteria used, while non-Hispanic Blacks are 2-10 times more likely to be deficient compared with non-Hispanic White patients, the task force noted.
However, the implications of vitamin D deficiency in certain populations can vary. For instance, non-Hispanic Black people, despite having a higher prevalence of lower vitamin D levels compared with White people, in fact, have lower reported rates of fractures.
To address the various issues and gain a better understanding of the complexities of vitamin D deficiency, the task force calls for further research in key areas.
“More research is needed to determine whether total serum 25(OH)D levels are the best measure of vitamin D deficiency and whether the best measure of vitamin D deficiency varies by subgroups defined by race, ethnicity, or sex,” the authors indicated.
Furthermore, “more research is needed to determine the cutoff that defines vitamin D deficiency and whether that cutoff varies by specific clinical outcome or by subgroups defined by race, ethnicity, or sex.”
No support for population-based screening in guidelines
With the lack of conclusive evidence, no organizations currently recommend population-based screening for vitamin D deficiency in asymptomatic patients, and the American Society for Clinical Pathology endorses this stance.
The Endocrine Society and the American Association of Clinical Endocrinologists meanwhile do recommend screening for vitamin D deficiency in patients considered at risk.
Data show there was as much as an 80-fold increase in Medicare reimbursement volumes for vitamin D testing among clinicians from 2000 to 2010; however, that rate may have leveled off after the National Academy of Medicine reported on set deficiency levels, said Sherri-Ann M. Burnett-Bowie, MD, MPH, Dr. Cappola’s editorial coauthor.
Dr. Burnett-Bowie noted that she regularly tests her patients’ vitamin D levels, however most of her patients have osteoporosis or fractures.
“I do screen them for vitamin D deficiency since optimizing their vitamin D will improve calcium absorption, which is important for treating their osteoporosis,” Dr. Burnett-Bowie, of the endocrine division, department of medicine, Massachusetts General Hospital, Boston, said in an interview.
In terms of broader testing of asymptomatic patients in the general population, however, any changes in screening will likely be contingent on developments in the effects of treatment, she said.
“Given the challenge in finding benefits of vitamin D supplementation in those who are deficient, it will likely be more challenging to find benefits from wider screening,” she concluded.
The USPSTF and editorialists reported having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Hyperpigmentation on the Head and Neck
The Diagnosis: Frontal Fibrosing Alopecia Overlapping With Lichen Planus Pigmentosus
Microscopic examination revealed focal dermal pigmentation, papillary fibrosis, and epidermal atrophy. These clinical and histologic findings indicated a diagnosis of fully developed lichen planus pigmentosus (LPP) overlapping with frontal fibrosing alopecia (FFA). Other cases have demonstrated an association between LPP and FFA.1,2
Lichen planus pigmentosus is considered an uncommon variant of lichen planus, as it has similar histopathologic findings and occasional coexistence.3,4 It is characterized by hyperpigmented macules primarily located in sun-exposed and flexural areas of the skin. First described in India,5 this disease has a predilection for darker skin (Fitzpatrick skin types III-V),6,7 and it has been reported in other racial and ethnic groups including Latin Americans, Middle Eastern populations, Japanese, and Koreans.4,8 Typically, lesions initially appear as ill-defined, blue-grey, round to oval macules that coalesce into hyperpigmented patches. Involvement most commonly begins at the forehead and temples, which are affected in nearly all patients. Infrequently, LPP can be generalized or affect the oral mucosa; involvement of the palms, soles, and nails does not occur. Patients may be asymptomatic, but some experience mild pruritus and burning. The disease course is chronic and insidious, with new lesions appearing over time and old lesions progressively darkening and expanding.6,7,9
Although the pathogenesis of LPP is unknown, several exposures have been implicated, such as amla oil, mustard oil, henna, hair dye, and environmental pollutants.7 Because lesions characteristically occur in sun-exposed areas, UV light also may be involved. In addition, studies have suggested that LPP is associated with endocrinopathies such as diabetes mellitus and dyslipidemias, as in our patient, as well as autoimmune conditions such as vitiligo and systemic lupus erythematosus.10,11
Histopathologic findings are characterized by vacuolar degeneration of the basal layer in the epidermis as well as perivascular lymphohistiocytic infiltration and the presence of melanophages in the dermis.3,9 Lichen planus pigmentosus is difficult to treat, as no consistently effective modality has been established. Topical tacrolimus, topical corticosteroids, oral retinoids, lasers, and sun protection have been implemented with underwhelming results.12
Frontal fibrosing alopecia is a variant of lichen planopilaris that predominantly affects postmenopausal women and presents with frontotemporal hair loss in a bandlike distribution.5,13 Both terminal and vellus hairs are affected. Involvement of multiple hair-bearing sites of the skin have been reported, including the entire scalp, eyebrows, and eyelashes. Affected areas may display hypopigmentation and be accompanied by pruritus and trichodynia.14,15 The pathogenesis currently is under investigation, with studies demonstrating autoimmune, genetic, and possibly even endocrine predispositions.16-18 Biopsies of lesions are indistinguishable from lichen planopilaris, which shows follicular lymphocytic infiltration, perifollicular fibrosis, interface dermatitis of the follicular infundibulum and isthmus, and vertical fibrous tracks.5 Patients with FFA have demonstrated variable responses to treatments, with one study showing improvement with oral finasteride or dutasteride.14 Topical and intralesional corticosteroids have yielded suboptimal effects. Other modalities include hydroxychloroquine and mycophenolate mofetil.15,19
Co-occurrence of LPP and FFA primarily is seen in postmenopausal women with darker skin,14,15 as in our patient, though premenopausal cases have been reported. Lichen planus pigmentosus may serve as a harbinger in most patients.1,2 In a similar fashion, our patient presented with hyperpigmented macular lesions prior to the onset of frontotemporal hair loss.
Our patient was started on finasteride 2.5 mg daily, minoxidil foam 5%, clobetasol solution 0.05%, triamcinolone ointment 0.1%, and hydrocortisone ointment 2.5%. She was instructed to commence treatment and follow up in 6 months.
The differential diagnosis includes dermatologic conditions that mimic both LPP and FFA. Postinflammatory hyperpigmentation and fixed drug reaction were unlikely based on the patient's history. The lesions of ashy dermatosis are characteristically gray erythematous macules on the trunk and limbs. Riehl melanosis is a rare pigmented contact dermatitis that is associated with a history of repeated contact with sensitizing allergens. Although Hori nevus is characterized by small, blue-gray or brown macules on the face, lesions predominantly occur on the bony prominences of the cheeks. Melasma also presents with dark to gray macules that affect the face and less commonly the neck, as in our patient.2
Early discoid lupus erythematosus presents with round erythematous plaques with overlying scale extending into the hair follicles. In pseudopalade of Brocq, an idiopathic cicatricial alopecia, lesions typically are flesh colored. Biopsy also shows epidermal atrophy with additional dermal sclerosis and fibrosis. Folliculitis decalvans is a scarring form of alopecia associated with erythema and pustules, findings that were not present in our patient. Keratosis follicularis spinulosa decalvans is a rare, X-linked inherited ichthyosis manifesting as scarring alopecia with follicular depressions and papules on the scalp in younger males. Photophobia and other manifestations may be present. Alopecia mucinosa is a nonscarring alopecia with grouped follicular erythematous patches or plaques. Mucin sometimes can be squeezed from affected areas, and histopathologic examination shows mucin accumulation.4
- Dlova NC. Frontal fibrosing alopecia and lichen planus pigmentosus: is there a link? Br J Dermatol. 2013;168:439-442.
- Pirmez R, Duque-Estrada B, Donati A, et al. Clinical and dermoscopic features of lichen planus pigmentosus in 37 patients with frontal fibrosing alopecia. Br J Dermatol. 2016;175:1387-1390.
- Rieder E, Kaplan J, Kamino H, et al. Lichen planus pigmentosus. Dermatol Online J. 2013;19:20713.
- Kashima A, Tajiri A, Yamashita A, et al. Two Japanese cases of lichen planus pigmentosus-inversus. Int J Dermatol. 2007;46:740-742.
- Bhutani L, Bedi T, Pandhi R. Lichen planus pigmentosus. Dermatologica. 1974;149:43-50.
- Ross EK, Tan E, Shapiro J. Update on primary cicatricial alopecias. J Am Acad Dermatol. 2005;53:1-37.
- Kanwa AJ, Dogra S, Handa S, et al. A study of 124 Indian patients with lichen planus pigmentosus. Clin Exp Dermatol. 2003;28:481-485.
- Al-Mutairi N, El-Khalawany M. Clinicopathological characteristics of lichen planus pigmentosus and its response to tacrolimus ointment: an open label, non-randomized, prospective study. J Eur Acad Dermatol Venereol. 2010;24:535-540.
- Vega ME, Waxtein L, Arenas R, et al. Ashy dermatosis and lichen planus pigmentosus: a clinicopathologic study of 31 cases. Int J Dermatol. 1992;31:90-94.
- Robles-Méndez JC, Rizo-Frías P, Herz-Ruelas ME, et al. Lichen planus pigmentosus and its variants: review and update. Int J Dermatol. 2018;57:505-514.
- Torres J, Guadalupe A, Reyes E, et al. Lichen planus pigmentosus in patients with endocrinopathies and hepatitis C. J Am Acad Dermatol. 2013;68:AB139.
- Kim JE, Won CH, Chang S, et al. Linear lichen planus pigmentosus of the forehead treated by neodymium:yttrium-aluminum-garnet laser and topical tacrolimus. J Dermatol. 2012;39:189-191.
- Kossard S. Postmenopausal frontal fibrosing alopecia: scarring alopecia in a pattern distribution. Arch Dermatol. 1994;130:770-774.
- Vano-Galvan S, Molina-Ruiz AM, Serrano-Falcon C, et al. Frontal fibrosing alopecia: a multicenter review of 355 patients. J Am Acad Dermatol. 2014;70:670-678.
- MacDonald A, Clark C, Holmes S. Frontal fibrosing alopecia: a review of 60 cases. J Am Acad Dermatol. 2012;67:955-961.
- Harries MJ, Meyer K, Chaudhry I, et al. Lichen planopilaris is characterized by immune privilege collapse of the hair follicle's epithelial stem cell niche. J Pathol. 2013;231:236-247.
- Karnik P, Tekeste Z, McCormick TS, et al. Hair follicle stem cell-specific PPARgamma deletion causes scarring alopecia. J Invest Dermatol. 2009;129:1243-1257.
- Rodriguez-Bayona B, Ruchaud S, Rodriguez C, et al. Autoantibodies against the chromosomal passenger protein INCENP found in a patient with Graham Little-Piccardi-Lassueur syndrome. J Autoimmune Dis. 2007;4:1.
- Rácz E, Gho C, Moorman PW, et al. Treatment of frontal fibrosing alopecia and lichen planopilaris: a systematic review. J Eur Acad Dermatol Venereol. 2013;27:1461-1470.
The Diagnosis: Frontal Fibrosing Alopecia Overlapping With Lichen Planus Pigmentosus
Microscopic examination revealed focal dermal pigmentation, papillary fibrosis, and epidermal atrophy. These clinical and histologic findings indicated a diagnosis of fully developed lichen planus pigmentosus (LPP) overlapping with frontal fibrosing alopecia (FFA). Other cases have demonstrated an association between LPP and FFA.1,2
Lichen planus pigmentosus is considered an uncommon variant of lichen planus, as it has similar histopathologic findings and occasional coexistence.3,4 It is characterized by hyperpigmented macules primarily located in sun-exposed and flexural areas of the skin. First described in India,5 this disease has a predilection for darker skin (Fitzpatrick skin types III-V),6,7 and it has been reported in other racial and ethnic groups including Latin Americans, Middle Eastern populations, Japanese, and Koreans.4,8 Typically, lesions initially appear as ill-defined, blue-grey, round to oval macules that coalesce into hyperpigmented patches. Involvement most commonly begins at the forehead and temples, which are affected in nearly all patients. Infrequently, LPP can be generalized or affect the oral mucosa; involvement of the palms, soles, and nails does not occur. Patients may be asymptomatic, but some experience mild pruritus and burning. The disease course is chronic and insidious, with new lesions appearing over time and old lesions progressively darkening and expanding.6,7,9
Although the pathogenesis of LPP is unknown, several exposures have been implicated, such as amla oil, mustard oil, henna, hair dye, and environmental pollutants.7 Because lesions characteristically occur in sun-exposed areas, UV light also may be involved. In addition, studies have suggested that LPP is associated with endocrinopathies such as diabetes mellitus and dyslipidemias, as in our patient, as well as autoimmune conditions such as vitiligo and systemic lupus erythematosus.10,11
Histopathologic findings are characterized by vacuolar degeneration of the basal layer in the epidermis as well as perivascular lymphohistiocytic infiltration and the presence of melanophages in the dermis.3,9 Lichen planus pigmentosus is difficult to treat, as no consistently effective modality has been established. Topical tacrolimus, topical corticosteroids, oral retinoids, lasers, and sun protection have been implemented with underwhelming results.12
Frontal fibrosing alopecia is a variant of lichen planopilaris that predominantly affects postmenopausal women and presents with frontotemporal hair loss in a bandlike distribution.5,13 Both terminal and vellus hairs are affected. Involvement of multiple hair-bearing sites of the skin have been reported, including the entire scalp, eyebrows, and eyelashes. Affected areas may display hypopigmentation and be accompanied by pruritus and trichodynia.14,15 The pathogenesis currently is under investigation, with studies demonstrating autoimmune, genetic, and possibly even endocrine predispositions.16-18 Biopsies of lesions are indistinguishable from lichen planopilaris, which shows follicular lymphocytic infiltration, perifollicular fibrosis, interface dermatitis of the follicular infundibulum and isthmus, and vertical fibrous tracks.5 Patients with FFA have demonstrated variable responses to treatments, with one study showing improvement with oral finasteride or dutasteride.14 Topical and intralesional corticosteroids have yielded suboptimal effects. Other modalities include hydroxychloroquine and mycophenolate mofetil.15,19
Co-occurrence of LPP and FFA primarily is seen in postmenopausal women with darker skin,14,15 as in our patient, though premenopausal cases have been reported. Lichen planus pigmentosus may serve as a harbinger in most patients.1,2 In a similar fashion, our patient presented with hyperpigmented macular lesions prior to the onset of frontotemporal hair loss.
Our patient was started on finasteride 2.5 mg daily, minoxidil foam 5%, clobetasol solution 0.05%, triamcinolone ointment 0.1%, and hydrocortisone ointment 2.5%. She was instructed to commence treatment and follow up in 6 months.
The differential diagnosis includes dermatologic conditions that mimic both LPP and FFA. Postinflammatory hyperpigmentation and fixed drug reaction were unlikely based on the patient's history. The lesions of ashy dermatosis are characteristically gray erythematous macules on the trunk and limbs. Riehl melanosis is a rare pigmented contact dermatitis that is associated with a history of repeated contact with sensitizing allergens. Although Hori nevus is characterized by small, blue-gray or brown macules on the face, lesions predominantly occur on the bony prominences of the cheeks. Melasma also presents with dark to gray macules that affect the face and less commonly the neck, as in our patient.2
Early discoid lupus erythematosus presents with round erythematous plaques with overlying scale extending into the hair follicles. In pseudopalade of Brocq, an idiopathic cicatricial alopecia, lesions typically are flesh colored. Biopsy also shows epidermal atrophy with additional dermal sclerosis and fibrosis. Folliculitis decalvans is a scarring form of alopecia associated with erythema and pustules, findings that were not present in our patient. Keratosis follicularis spinulosa decalvans is a rare, X-linked inherited ichthyosis manifesting as scarring alopecia with follicular depressions and papules on the scalp in younger males. Photophobia and other manifestations may be present. Alopecia mucinosa is a nonscarring alopecia with grouped follicular erythematous patches or plaques. Mucin sometimes can be squeezed from affected areas, and histopathologic examination shows mucin accumulation.4
The Diagnosis: Frontal Fibrosing Alopecia Overlapping With Lichen Planus Pigmentosus
Microscopic examination revealed focal dermal pigmentation, papillary fibrosis, and epidermal atrophy. These clinical and histologic findings indicated a diagnosis of fully developed lichen planus pigmentosus (LPP) overlapping with frontal fibrosing alopecia (FFA). Other cases have demonstrated an association between LPP and FFA.1,2
Lichen planus pigmentosus is considered an uncommon variant of lichen planus, as it has similar histopathologic findings and occasional coexistence.3,4 It is characterized by hyperpigmented macules primarily located in sun-exposed and flexural areas of the skin. First described in India,5 this disease has a predilection for darker skin (Fitzpatrick skin types III-V),6,7 and it has been reported in other racial and ethnic groups including Latin Americans, Middle Eastern populations, Japanese, and Koreans.4,8 Typically, lesions initially appear as ill-defined, blue-grey, round to oval macules that coalesce into hyperpigmented patches. Involvement most commonly begins at the forehead and temples, which are affected in nearly all patients. Infrequently, LPP can be generalized or affect the oral mucosa; involvement of the palms, soles, and nails does not occur. Patients may be asymptomatic, but some experience mild pruritus and burning. The disease course is chronic and insidious, with new lesions appearing over time and old lesions progressively darkening and expanding.6,7,9
Although the pathogenesis of LPP is unknown, several exposures have been implicated, such as amla oil, mustard oil, henna, hair dye, and environmental pollutants.7 Because lesions characteristically occur in sun-exposed areas, UV light also may be involved. In addition, studies have suggested that LPP is associated with endocrinopathies such as diabetes mellitus and dyslipidemias, as in our patient, as well as autoimmune conditions such as vitiligo and systemic lupus erythematosus.10,11
Histopathologic findings are characterized by vacuolar degeneration of the basal layer in the epidermis as well as perivascular lymphohistiocytic infiltration and the presence of melanophages in the dermis.3,9 Lichen planus pigmentosus is difficult to treat, as no consistently effective modality has been established. Topical tacrolimus, topical corticosteroids, oral retinoids, lasers, and sun protection have been implemented with underwhelming results.12
Frontal fibrosing alopecia is a variant of lichen planopilaris that predominantly affects postmenopausal women and presents with frontotemporal hair loss in a bandlike distribution.5,13 Both terminal and vellus hairs are affected. Involvement of multiple hair-bearing sites of the skin have been reported, including the entire scalp, eyebrows, and eyelashes. Affected areas may display hypopigmentation and be accompanied by pruritus and trichodynia.14,15 The pathogenesis currently is under investigation, with studies demonstrating autoimmune, genetic, and possibly even endocrine predispositions.16-18 Biopsies of lesions are indistinguishable from lichen planopilaris, which shows follicular lymphocytic infiltration, perifollicular fibrosis, interface dermatitis of the follicular infundibulum and isthmus, and vertical fibrous tracks.5 Patients with FFA have demonstrated variable responses to treatments, with one study showing improvement with oral finasteride or dutasteride.14 Topical and intralesional corticosteroids have yielded suboptimal effects. Other modalities include hydroxychloroquine and mycophenolate mofetil.15,19
Co-occurrence of LPP and FFA primarily is seen in postmenopausal women with darker skin,14,15 as in our patient, though premenopausal cases have been reported. Lichen planus pigmentosus may serve as a harbinger in most patients.1,2 In a similar fashion, our patient presented with hyperpigmented macular lesions prior to the onset of frontotemporal hair loss.
Our patient was started on finasteride 2.5 mg daily, minoxidil foam 5%, clobetasol solution 0.05%, triamcinolone ointment 0.1%, and hydrocortisone ointment 2.5%. She was instructed to commence treatment and follow up in 6 months.
The differential diagnosis includes dermatologic conditions that mimic both LPP and FFA. Postinflammatory hyperpigmentation and fixed drug reaction were unlikely based on the patient's history. The lesions of ashy dermatosis are characteristically gray erythematous macules on the trunk and limbs. Riehl melanosis is a rare pigmented contact dermatitis that is associated with a history of repeated contact with sensitizing allergens. Although Hori nevus is characterized by small, blue-gray or brown macules on the face, lesions predominantly occur on the bony prominences of the cheeks. Melasma also presents with dark to gray macules that affect the face and less commonly the neck, as in our patient.2
Early discoid lupus erythematosus presents with round erythematous plaques with overlying scale extending into the hair follicles. In pseudopalade of Brocq, an idiopathic cicatricial alopecia, lesions typically are flesh colored. Biopsy also shows epidermal atrophy with additional dermal sclerosis and fibrosis. Folliculitis decalvans is a scarring form of alopecia associated with erythema and pustules, findings that were not present in our patient. Keratosis follicularis spinulosa decalvans is a rare, X-linked inherited ichthyosis manifesting as scarring alopecia with follicular depressions and papules on the scalp in younger males. Photophobia and other manifestations may be present. Alopecia mucinosa is a nonscarring alopecia with grouped follicular erythematous patches or plaques. Mucin sometimes can be squeezed from affected areas, and histopathologic examination shows mucin accumulation.4
- Dlova NC. Frontal fibrosing alopecia and lichen planus pigmentosus: is there a link? Br J Dermatol. 2013;168:439-442.
- Pirmez R, Duque-Estrada B, Donati A, et al. Clinical and dermoscopic features of lichen planus pigmentosus in 37 patients with frontal fibrosing alopecia. Br J Dermatol. 2016;175:1387-1390.
- Rieder E, Kaplan J, Kamino H, et al. Lichen planus pigmentosus. Dermatol Online J. 2013;19:20713.
- Kashima A, Tajiri A, Yamashita A, et al. Two Japanese cases of lichen planus pigmentosus-inversus. Int J Dermatol. 2007;46:740-742.
- Bhutani L, Bedi T, Pandhi R. Lichen planus pigmentosus. Dermatologica. 1974;149:43-50.
- Ross EK, Tan E, Shapiro J. Update on primary cicatricial alopecias. J Am Acad Dermatol. 2005;53:1-37.
- Kanwa AJ, Dogra S, Handa S, et al. A study of 124 Indian patients with lichen planus pigmentosus. Clin Exp Dermatol. 2003;28:481-485.
- Al-Mutairi N, El-Khalawany M. Clinicopathological characteristics of lichen planus pigmentosus and its response to tacrolimus ointment: an open label, non-randomized, prospective study. J Eur Acad Dermatol Venereol. 2010;24:535-540.
- Vega ME, Waxtein L, Arenas R, et al. Ashy dermatosis and lichen planus pigmentosus: a clinicopathologic study of 31 cases. Int J Dermatol. 1992;31:90-94.
- Robles-Méndez JC, Rizo-Frías P, Herz-Ruelas ME, et al. Lichen planus pigmentosus and its variants: review and update. Int J Dermatol. 2018;57:505-514.
- Torres J, Guadalupe A, Reyes E, et al. Lichen planus pigmentosus in patients with endocrinopathies and hepatitis C. J Am Acad Dermatol. 2013;68:AB139.
- Kim JE, Won CH, Chang S, et al. Linear lichen planus pigmentosus of the forehead treated by neodymium:yttrium-aluminum-garnet laser and topical tacrolimus. J Dermatol. 2012;39:189-191.
- Kossard S. Postmenopausal frontal fibrosing alopecia: scarring alopecia in a pattern distribution. Arch Dermatol. 1994;130:770-774.
- Vano-Galvan S, Molina-Ruiz AM, Serrano-Falcon C, et al. Frontal fibrosing alopecia: a multicenter review of 355 patients. J Am Acad Dermatol. 2014;70:670-678.
- MacDonald A, Clark C, Holmes S. Frontal fibrosing alopecia: a review of 60 cases. J Am Acad Dermatol. 2012;67:955-961.
- Harries MJ, Meyer K, Chaudhry I, et al. Lichen planopilaris is characterized by immune privilege collapse of the hair follicle's epithelial stem cell niche. J Pathol. 2013;231:236-247.
- Karnik P, Tekeste Z, McCormick TS, et al. Hair follicle stem cell-specific PPARgamma deletion causes scarring alopecia. J Invest Dermatol. 2009;129:1243-1257.
- Rodriguez-Bayona B, Ruchaud S, Rodriguez C, et al. Autoantibodies against the chromosomal passenger protein INCENP found in a patient with Graham Little-Piccardi-Lassueur syndrome. J Autoimmune Dis. 2007;4:1.
- Rácz E, Gho C, Moorman PW, et al. Treatment of frontal fibrosing alopecia and lichen planopilaris: a systematic review. J Eur Acad Dermatol Venereol. 2013;27:1461-1470.
- Dlova NC. Frontal fibrosing alopecia and lichen planus pigmentosus: is there a link? Br J Dermatol. 2013;168:439-442.
- Pirmez R, Duque-Estrada B, Donati A, et al. Clinical and dermoscopic features of lichen planus pigmentosus in 37 patients with frontal fibrosing alopecia. Br J Dermatol. 2016;175:1387-1390.
- Rieder E, Kaplan J, Kamino H, et al. Lichen planus pigmentosus. Dermatol Online J. 2013;19:20713.
- Kashima A, Tajiri A, Yamashita A, et al. Two Japanese cases of lichen planus pigmentosus-inversus. Int J Dermatol. 2007;46:740-742.
- Bhutani L, Bedi T, Pandhi R. Lichen planus pigmentosus. Dermatologica. 1974;149:43-50.
- Ross EK, Tan E, Shapiro J. Update on primary cicatricial alopecias. J Am Acad Dermatol. 2005;53:1-37.
- Kanwa AJ, Dogra S, Handa S, et al. A study of 124 Indian patients with lichen planus pigmentosus. Clin Exp Dermatol. 2003;28:481-485.
- Al-Mutairi N, El-Khalawany M. Clinicopathological characteristics of lichen planus pigmentosus and its response to tacrolimus ointment: an open label, non-randomized, prospective study. J Eur Acad Dermatol Venereol. 2010;24:535-540.
- Vega ME, Waxtein L, Arenas R, et al. Ashy dermatosis and lichen planus pigmentosus: a clinicopathologic study of 31 cases. Int J Dermatol. 1992;31:90-94.
- Robles-Méndez JC, Rizo-Frías P, Herz-Ruelas ME, et al. Lichen planus pigmentosus and its variants: review and update. Int J Dermatol. 2018;57:505-514.
- Torres J, Guadalupe A, Reyes E, et al. Lichen planus pigmentosus in patients with endocrinopathies and hepatitis C. J Am Acad Dermatol. 2013;68:AB139.
- Kim JE, Won CH, Chang S, et al. Linear lichen planus pigmentosus of the forehead treated by neodymium:yttrium-aluminum-garnet laser and topical tacrolimus. J Dermatol. 2012;39:189-191.
- Kossard S. Postmenopausal frontal fibrosing alopecia: scarring alopecia in a pattern distribution. Arch Dermatol. 1994;130:770-774.
- Vano-Galvan S, Molina-Ruiz AM, Serrano-Falcon C, et al. Frontal fibrosing alopecia: a multicenter review of 355 patients. J Am Acad Dermatol. 2014;70:670-678.
- MacDonald A, Clark C, Holmes S. Frontal fibrosing alopecia: a review of 60 cases. J Am Acad Dermatol. 2012;67:955-961.
- Harries MJ, Meyer K, Chaudhry I, et al. Lichen planopilaris is characterized by immune privilege collapse of the hair follicle's epithelial stem cell niche. J Pathol. 2013;231:236-247.
- Karnik P, Tekeste Z, McCormick TS, et al. Hair follicle stem cell-specific PPARgamma deletion causes scarring alopecia. J Invest Dermatol. 2009;129:1243-1257.
- Rodriguez-Bayona B, Ruchaud S, Rodriguez C, et al. Autoantibodies against the chromosomal passenger protein INCENP found in a patient with Graham Little-Piccardi-Lassueur syndrome. J Autoimmune Dis. 2007;4:1.
- Rácz E, Gho C, Moorman PW, et al. Treatment of frontal fibrosing alopecia and lichen planopilaris: a systematic review. J Eur Acad Dermatol Venereol. 2013;27:1461-1470.
A 78-year-old Asian woman presented to the dermatology clinic with progressively worsening dark spots on the forehead and neck of 3 months’ duration. She noted mild pruritis and hair loss involving the eyebrows and anterior scalp. Her medical history was notable for type 2 diabetes mellitus. She denied any new medical conditions or medications and had no prior history of similar symptoms. Physical examination showed hyperpigmented brown macules and patches on the forehead (top) and anterior neck (bottom) with sparing of the posterior neck and lower face. Alopecia with areas of perifollicular erythema and hyperpigmentation with reduced follicular openings were present on the eyebrows and anterior forehead. Two punch biopsies of head and neck lesions were performed.