User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
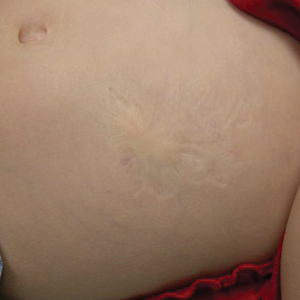
Atrophic Lesion on the Abdomen
The Diagnosis: Anetoderma of Prematurity
Anetoderma is a rare benign cutaneous disorder characterized by atrophic patches of skin due to dermal thinning. The term anetoderma is derived from the Greek words anetos (relaxed) and derma (skin).1 The physical appearance of the skin is associated with a reduction or loss of elastic tissue in the dermal layer, as seen on histolopathology.2
Two forms of anetoderma have been described. Primary anetoderma is an idiopathic form with no preceding inflammatory lesions. Secondary anetoderma is a reactive process linked to a known preceding inflammatory, infectious, autoimmune, or drug-induced condition.3 On histopathology, both primary and secondary anetoderma are characterized by a loss of elastic tissue or elastin fibers in the superficial to mid dermis.2
Anetoderma of prematurity was first described in 1996 by Prizant et al4 in 9 extremely premature (24-29 weeks' gestation) infants in neonatal intensive care units (NICUs). Although the exact mechanism behind anetoderma of prematurity is still unknown, Prizant et al4 and other investigators5 postulated that application of adhesive monitoring leads in the NICU played a role in the development of the lesions.
Iatrogenic anetoderma of prematurity is clinically characterized by circumscribed areas of either wrinkled macular depression or pouchlike herniations, ranging from flesh-colored to violaceous hues. Lesion size varies from a few millimeters to several centimeters in diameter, and they often are oval or round in shape.2 Although not common, it is possible for the atrophic patches to be preceded by an area of ecchymosis without necrosis or atrophy and, if present, they usually evolve within a few days to the characteristic appearance of anetoderma.3 They are found at discrete sites where monitoring leads or other medical devices are commonly placed, such as the forehead, abdomen, chest, and proximal limbs.
Lesions of anetoderma of prematurity are not present at birth, which distinguishes them from congenital anetoderma.6 It is unclear if the lesions are associated with the degree of prematurity, extremely low birth weight, or other associated factors of preterm birth. Although often clinically diagnosed, the diagnosis can be confirmed by a loss of elastic fibers on histopathology when stained with Verhoeff-van Gieson stain.1 Over time, the atrophic patches have the potential to evolve into herniated forms of anetoderma. Self-healing or improvement of the lesions often does not occur. Although the lesion is benign, it often requires surgical correction later in life for cosmesis.
Infants in the NICU are at risk for iatrogenic cutaneous injuries, which rarely may include anetoderma. Anetoderma of prematurity has been linked to the use of monitoring leads, adhesive tape, and other medical devices placed on the skin. Prizant et al4 postulated that the cause of anetoderma in these infants was irritants such as skin cleansers, urine, or sweat that may be trapped under the electrodes. Other hypotheses include local hypoxemia due to prolonged pressure from the electrodes on immature skin or excessive traction used when removing adhesive tape from the skin.7,8 Premature infants may be more susceptible to these lesions because of the reduced epidermal thickness of premature skin; immaturity of skin structure; or functional immaturity of elastin deposition regulators, such as elastase, lysyl oxidase, the complement system, and decay-accelerating factor.3 The diagnosis should be differentiated from congenital anetoderma, which also has been described in premature neonates but is characterized by lesions that are present at birth. Its origins are still unclear, despite having histopathologic features similar to iatrogenic anetoderma.9
Focal dermal hypoplasia (FDH) is the hallmark cutaneous finding in Goltz syndrome, a rare set of congenital abnormalities of the skin, oral structures, musculoskeletal system, and central nervous system. Similar to congenital anetoderma, FDH also is characterized by atrophic cutaneous lesions; however, the cutaneous lesions in FDH appear as linear, streaky atrophic lesions often with telangiectasias that follow Blaschko lines.10 The cutaneous lesions in FDH often are associated with other noncutaneous signs such as polydactyly or asymmetric limbs.10 Cutis laxa is caused by an abnormality in the elastic tissue resulting in a loose sagging appearance of the skin and frequently results in an aged facial appearance. There are both acquired and inherited forms that can be either solely cutaneous or present with extracutaneous features, such as cardiac abnormalities or emphysema.11
In contrast to the atrophic appearance of anetodermas, connective tissue nevi and nevus lipomatosus superficialis present as hamartomas that either can be present at birth or arise in infancy. Connective tissue nevi are hamartomas of dermal connective tissue that consist of excessive production of collagen, elastin, or glycosaminoglycans and appear as slightly elevated, flesh-colored to yellow nodules or plaques.12 Connective tissue nevi often are described in association with other diseases, most commonly tuberous sclerosis (shagreen patches) or familial cutaneous collagenoma. Nevus lipomatosus superficialis is an asymptomatic connective tissue hamartoma composed of mature adipocytes in the dermis. The lesions consist of clusters of flesh-colored to yellow, soft, rubbery papules or nodules with a smooth or verrucoid surface that do not cross the midline and may follow Blaschko lines.11
With advances in neonatal infant medical care, survival of extremely premature infants is increasing, and it is possible that this rare cutaneous disorder may become more prevalent. Care should be taken to avoid unnecessary pressure on surfaces where electrodes are placed and tightly applied adhesive tape. When electrodes are placed on the ventral side, the child should be placed supine; similarly, place electrodes on the dorsal side when the child is lying prone.5 A diagnosis of anetoderma of prematurity later in childhood may be difficult, so knowledge and awareness can help guide pediatricians and dermatologists to a correct diagnosis and prevent unnecessary evaluations and/or concerns.
- Misch KJ, Rhodes EL, Allen J, et al. Anetoderma of Jadassohn. J R Soc Med.1988;81:734-736.
- Venencie PY, Winkelmann RK. Histopathologic findings in anetoderma. Arch Dermatol. 1984;120:1040-1044.
- Maffeis L, Pugni L, Pietrasanta C, et al. Case report iatrogenic anetoderma of prematurity: a case report and review of the literature. 2014;2014:781493.
- Prizant TL, Lucky AW, Frieden IJ, et al. Spontaneous atrophic patches in extremely premature infants: anetoderma of prematurity. Arch Dermatol. 1996;132:671-674.
- Goujon E, Beer F, Gay S, et al. Anetoderma of prematurity: an iatrogenic consequence of neonatal intensive care anetoderma of prematurity from NICU. Arch Dermatol. 2010;146:565-567.
- Wain EM, Mellerio JE, Robson A, et al. Congenital anetoderma in a preterm infant. Pediatr Dermatol. 2008;25:626-629.
- Colditz PB, Dunster KR, Joy GJ, et al. Anetoderma of prematurity in association with electrocardiographic electrodes. J Am Acad Dermatol. 1999;41:479-481.
- Goujan E, Beer F, Gay S, et al. Study supervision. Arch Dermatol. 2010;146:565-567.
- Aberer E, Weissenbacher G. Congenital anetoderma induced by intrauterine infection? Arch Dermatol. 1997;133:526-527.
- Mallory SB, Krafchik BR, Moore DJ, et al. Goltz syndrome. Pediatr Dermatol. 1989;6:251-253.
- Bolognia J, Schaffer J, Cerroni L. Dermatology. Elsevier Saunders; 2017.
- Uitto J, Santa Cruz DJ, Eisen AZ. Connective tissue nevi of the skin. clinical, genetic, and histopathologic classification of hamartomas of the collagen, elastin, and proteoglycan type. J Am Acad Dermatol. 1980;3:441-461.
The Diagnosis: Anetoderma of Prematurity
Anetoderma is a rare benign cutaneous disorder characterized by atrophic patches of skin due to dermal thinning. The term anetoderma is derived from the Greek words anetos (relaxed) and derma (skin).1 The physical appearance of the skin is associated with a reduction or loss of elastic tissue in the dermal layer, as seen on histolopathology.2
Two forms of anetoderma have been described. Primary anetoderma is an idiopathic form with no preceding inflammatory lesions. Secondary anetoderma is a reactive process linked to a known preceding inflammatory, infectious, autoimmune, or drug-induced condition.3 On histopathology, both primary and secondary anetoderma are characterized by a loss of elastic tissue or elastin fibers in the superficial to mid dermis.2
Anetoderma of prematurity was first described in 1996 by Prizant et al4 in 9 extremely premature (24-29 weeks' gestation) infants in neonatal intensive care units (NICUs). Although the exact mechanism behind anetoderma of prematurity is still unknown, Prizant et al4 and other investigators5 postulated that application of adhesive monitoring leads in the NICU played a role in the development of the lesions.
Iatrogenic anetoderma of prematurity is clinically characterized by circumscribed areas of either wrinkled macular depression or pouchlike herniations, ranging from flesh-colored to violaceous hues. Lesion size varies from a few millimeters to several centimeters in diameter, and they often are oval or round in shape.2 Although not common, it is possible for the atrophic patches to be preceded by an area of ecchymosis without necrosis or atrophy and, if present, they usually evolve within a few days to the characteristic appearance of anetoderma.3 They are found at discrete sites where monitoring leads or other medical devices are commonly placed, such as the forehead, abdomen, chest, and proximal limbs.
Lesions of anetoderma of prematurity are not present at birth, which distinguishes them from congenital anetoderma.6 It is unclear if the lesions are associated with the degree of prematurity, extremely low birth weight, or other associated factors of preterm birth. Although often clinically diagnosed, the diagnosis can be confirmed by a loss of elastic fibers on histopathology when stained with Verhoeff-van Gieson stain.1 Over time, the atrophic patches have the potential to evolve into herniated forms of anetoderma. Self-healing or improvement of the lesions often does not occur. Although the lesion is benign, it often requires surgical correction later in life for cosmesis.
Infants in the NICU are at risk for iatrogenic cutaneous injuries, which rarely may include anetoderma. Anetoderma of prematurity has been linked to the use of monitoring leads, adhesive tape, and other medical devices placed on the skin. Prizant et al4 postulated that the cause of anetoderma in these infants was irritants such as skin cleansers, urine, or sweat that may be trapped under the electrodes. Other hypotheses include local hypoxemia due to prolonged pressure from the electrodes on immature skin or excessive traction used when removing adhesive tape from the skin.7,8 Premature infants may be more susceptible to these lesions because of the reduced epidermal thickness of premature skin; immaturity of skin structure; or functional immaturity of elastin deposition regulators, such as elastase, lysyl oxidase, the complement system, and decay-accelerating factor.3 The diagnosis should be differentiated from congenital anetoderma, which also has been described in premature neonates but is characterized by lesions that are present at birth. Its origins are still unclear, despite having histopathologic features similar to iatrogenic anetoderma.9
Focal dermal hypoplasia (FDH) is the hallmark cutaneous finding in Goltz syndrome, a rare set of congenital abnormalities of the skin, oral structures, musculoskeletal system, and central nervous system. Similar to congenital anetoderma, FDH also is characterized by atrophic cutaneous lesions; however, the cutaneous lesions in FDH appear as linear, streaky atrophic lesions often with telangiectasias that follow Blaschko lines.10 The cutaneous lesions in FDH often are associated with other noncutaneous signs such as polydactyly or asymmetric limbs.10 Cutis laxa is caused by an abnormality in the elastic tissue resulting in a loose sagging appearance of the skin and frequently results in an aged facial appearance. There are both acquired and inherited forms that can be either solely cutaneous or present with extracutaneous features, such as cardiac abnormalities or emphysema.11
In contrast to the atrophic appearance of anetodermas, connective tissue nevi and nevus lipomatosus superficialis present as hamartomas that either can be present at birth or arise in infancy. Connective tissue nevi are hamartomas of dermal connective tissue that consist of excessive production of collagen, elastin, or glycosaminoglycans and appear as slightly elevated, flesh-colored to yellow nodules or plaques.12 Connective tissue nevi often are described in association with other diseases, most commonly tuberous sclerosis (shagreen patches) or familial cutaneous collagenoma. Nevus lipomatosus superficialis is an asymptomatic connective tissue hamartoma composed of mature adipocytes in the dermis. The lesions consist of clusters of flesh-colored to yellow, soft, rubbery papules or nodules with a smooth or verrucoid surface that do not cross the midline and may follow Blaschko lines.11
With advances in neonatal infant medical care, survival of extremely premature infants is increasing, and it is possible that this rare cutaneous disorder may become more prevalent. Care should be taken to avoid unnecessary pressure on surfaces where electrodes are placed and tightly applied adhesive tape. When electrodes are placed on the ventral side, the child should be placed supine; similarly, place electrodes on the dorsal side when the child is lying prone.5 A diagnosis of anetoderma of prematurity later in childhood may be difficult, so knowledge and awareness can help guide pediatricians and dermatologists to a correct diagnosis and prevent unnecessary evaluations and/or concerns.
The Diagnosis: Anetoderma of Prematurity
Anetoderma is a rare benign cutaneous disorder characterized by atrophic patches of skin due to dermal thinning. The term anetoderma is derived from the Greek words anetos (relaxed) and derma (skin).1 The physical appearance of the skin is associated with a reduction or loss of elastic tissue in the dermal layer, as seen on histolopathology.2
Two forms of anetoderma have been described. Primary anetoderma is an idiopathic form with no preceding inflammatory lesions. Secondary anetoderma is a reactive process linked to a known preceding inflammatory, infectious, autoimmune, or drug-induced condition.3 On histopathology, both primary and secondary anetoderma are characterized by a loss of elastic tissue or elastin fibers in the superficial to mid dermis.2
Anetoderma of prematurity was first described in 1996 by Prizant et al4 in 9 extremely premature (24-29 weeks' gestation) infants in neonatal intensive care units (NICUs). Although the exact mechanism behind anetoderma of prematurity is still unknown, Prizant et al4 and other investigators5 postulated that application of adhesive monitoring leads in the NICU played a role in the development of the lesions.
Iatrogenic anetoderma of prematurity is clinically characterized by circumscribed areas of either wrinkled macular depression or pouchlike herniations, ranging from flesh-colored to violaceous hues. Lesion size varies from a few millimeters to several centimeters in diameter, and they often are oval or round in shape.2 Although not common, it is possible for the atrophic patches to be preceded by an area of ecchymosis without necrosis or atrophy and, if present, they usually evolve within a few days to the characteristic appearance of anetoderma.3 They are found at discrete sites where monitoring leads or other medical devices are commonly placed, such as the forehead, abdomen, chest, and proximal limbs.
Lesions of anetoderma of prematurity are not present at birth, which distinguishes them from congenital anetoderma.6 It is unclear if the lesions are associated with the degree of prematurity, extremely low birth weight, or other associated factors of preterm birth. Although often clinically diagnosed, the diagnosis can be confirmed by a loss of elastic fibers on histopathology when stained with Verhoeff-van Gieson stain.1 Over time, the atrophic patches have the potential to evolve into herniated forms of anetoderma. Self-healing or improvement of the lesions often does not occur. Although the lesion is benign, it often requires surgical correction later in life for cosmesis.
Infants in the NICU are at risk for iatrogenic cutaneous injuries, which rarely may include anetoderma. Anetoderma of prematurity has been linked to the use of monitoring leads, adhesive tape, and other medical devices placed on the skin. Prizant et al4 postulated that the cause of anetoderma in these infants was irritants such as skin cleansers, urine, or sweat that may be trapped under the electrodes. Other hypotheses include local hypoxemia due to prolonged pressure from the electrodes on immature skin or excessive traction used when removing adhesive tape from the skin.7,8 Premature infants may be more susceptible to these lesions because of the reduced epidermal thickness of premature skin; immaturity of skin structure; or functional immaturity of elastin deposition regulators, such as elastase, lysyl oxidase, the complement system, and decay-accelerating factor.3 The diagnosis should be differentiated from congenital anetoderma, which also has been described in premature neonates but is characterized by lesions that are present at birth. Its origins are still unclear, despite having histopathologic features similar to iatrogenic anetoderma.9
Focal dermal hypoplasia (FDH) is the hallmark cutaneous finding in Goltz syndrome, a rare set of congenital abnormalities of the skin, oral structures, musculoskeletal system, and central nervous system. Similar to congenital anetoderma, FDH also is characterized by atrophic cutaneous lesions; however, the cutaneous lesions in FDH appear as linear, streaky atrophic lesions often with telangiectasias that follow Blaschko lines.10 The cutaneous lesions in FDH often are associated with other noncutaneous signs such as polydactyly or asymmetric limbs.10 Cutis laxa is caused by an abnormality in the elastic tissue resulting in a loose sagging appearance of the skin and frequently results in an aged facial appearance. There are both acquired and inherited forms that can be either solely cutaneous or present with extracutaneous features, such as cardiac abnormalities or emphysema.11
In contrast to the atrophic appearance of anetodermas, connective tissue nevi and nevus lipomatosus superficialis present as hamartomas that either can be present at birth or arise in infancy. Connective tissue nevi are hamartomas of dermal connective tissue that consist of excessive production of collagen, elastin, or glycosaminoglycans and appear as slightly elevated, flesh-colored to yellow nodules or plaques.12 Connective tissue nevi often are described in association with other diseases, most commonly tuberous sclerosis (shagreen patches) or familial cutaneous collagenoma. Nevus lipomatosus superficialis is an asymptomatic connective tissue hamartoma composed of mature adipocytes in the dermis. The lesions consist of clusters of flesh-colored to yellow, soft, rubbery papules or nodules with a smooth or verrucoid surface that do not cross the midline and may follow Blaschko lines.11
With advances in neonatal infant medical care, survival of extremely premature infants is increasing, and it is possible that this rare cutaneous disorder may become more prevalent. Care should be taken to avoid unnecessary pressure on surfaces where electrodes are placed and tightly applied adhesive tape. When electrodes are placed on the ventral side, the child should be placed supine; similarly, place electrodes on the dorsal side when the child is lying prone.5 A diagnosis of anetoderma of prematurity later in childhood may be difficult, so knowledge and awareness can help guide pediatricians and dermatologists to a correct diagnosis and prevent unnecessary evaluations and/or concerns.
- Misch KJ, Rhodes EL, Allen J, et al. Anetoderma of Jadassohn. J R Soc Med.1988;81:734-736.
- Venencie PY, Winkelmann RK. Histopathologic findings in anetoderma. Arch Dermatol. 1984;120:1040-1044.
- Maffeis L, Pugni L, Pietrasanta C, et al. Case report iatrogenic anetoderma of prematurity: a case report and review of the literature. 2014;2014:781493.
- Prizant TL, Lucky AW, Frieden IJ, et al. Spontaneous atrophic patches in extremely premature infants: anetoderma of prematurity. Arch Dermatol. 1996;132:671-674.
- Goujon E, Beer F, Gay S, et al. Anetoderma of prematurity: an iatrogenic consequence of neonatal intensive care anetoderma of prematurity from NICU. Arch Dermatol. 2010;146:565-567.
- Wain EM, Mellerio JE, Robson A, et al. Congenital anetoderma in a preterm infant. Pediatr Dermatol. 2008;25:626-629.
- Colditz PB, Dunster KR, Joy GJ, et al. Anetoderma of prematurity in association with electrocardiographic electrodes. J Am Acad Dermatol. 1999;41:479-481.
- Goujan E, Beer F, Gay S, et al. Study supervision. Arch Dermatol. 2010;146:565-567.
- Aberer E, Weissenbacher G. Congenital anetoderma induced by intrauterine infection? Arch Dermatol. 1997;133:526-527.
- Mallory SB, Krafchik BR, Moore DJ, et al. Goltz syndrome. Pediatr Dermatol. 1989;6:251-253.
- Bolognia J, Schaffer J, Cerroni L. Dermatology. Elsevier Saunders; 2017.
- Uitto J, Santa Cruz DJ, Eisen AZ. Connective tissue nevi of the skin. clinical, genetic, and histopathologic classification of hamartomas of the collagen, elastin, and proteoglycan type. J Am Acad Dermatol. 1980;3:441-461.
- Misch KJ, Rhodes EL, Allen J, et al. Anetoderma of Jadassohn. J R Soc Med.1988;81:734-736.
- Venencie PY, Winkelmann RK. Histopathologic findings in anetoderma. Arch Dermatol. 1984;120:1040-1044.
- Maffeis L, Pugni L, Pietrasanta C, et al. Case report iatrogenic anetoderma of prematurity: a case report and review of the literature. 2014;2014:781493.
- Prizant TL, Lucky AW, Frieden IJ, et al. Spontaneous atrophic patches in extremely premature infants: anetoderma of prematurity. Arch Dermatol. 1996;132:671-674.
- Goujon E, Beer F, Gay S, et al. Anetoderma of prematurity: an iatrogenic consequence of neonatal intensive care anetoderma of prematurity from NICU. Arch Dermatol. 2010;146:565-567.
- Wain EM, Mellerio JE, Robson A, et al. Congenital anetoderma in a preterm infant. Pediatr Dermatol. 2008;25:626-629.
- Colditz PB, Dunster KR, Joy GJ, et al. Anetoderma of prematurity in association with electrocardiographic electrodes. J Am Acad Dermatol. 1999;41:479-481.
- Goujan E, Beer F, Gay S, et al. Study supervision. Arch Dermatol. 2010;146:565-567.
- Aberer E, Weissenbacher G. Congenital anetoderma induced by intrauterine infection? Arch Dermatol. 1997;133:526-527.
- Mallory SB, Krafchik BR, Moore DJ, et al. Goltz syndrome. Pediatr Dermatol. 1989;6:251-253.
- Bolognia J, Schaffer J, Cerroni L. Dermatology. Elsevier Saunders; 2017.
- Uitto J, Santa Cruz DJ, Eisen AZ. Connective tissue nevi of the skin. clinical, genetic, and histopathologic classification of hamartomas of the collagen, elastin, and proteoglycan type. J Am Acad Dermatol. 1980;3:441-461.

An 18-month-old child presented with a 4-cm, atrophic, flesh-colored plaque on the left lateral aspect of the abdomen with overlying wrinkling of the skin. There was no outpouching of the skin or pain associated with the lesion. No other skin abnormalities were noted. The child was born premature at 30 weeks’ gestation (birth weight, 1400 g). The postnatal course was complicated by respiratory distress syndrome requiring prolonged ventilator support. The infant was in the neonatal intensive care unit for 5 months. The atrophic lesion first developed at 5 months of life and remained stable. Although the lesion was not present at birth, the parents noted that it was preceded by an ecchymotic lesion without necrosis that was first noticed at 2 months of life while the patient was in the neonatal intensive care unit.
CRC risk in young adults: Not as high as previously reported
Implications for CRC screening.
New estimates for the risk of CRC in young adults, which differentiate colorectal adenocarcinoma from other types, are reported in a study published Dec. 15, 2020, in Annals of Internal Medicine.
They are important because this finding has implications for CRC screening, say a trio of experts in an accompanying editorial.
Reports of an increase in the incidence of CRC in younger adults have led to changes in screening for this cancer in the United States. The age for starting CRC screening has been lowered to 45 years (instead of 50 years) in recommendations issued in 2018 by the American Cancer Society, and also more recently in preliminary recommendations from the U.S. Preventive Services Task Force.
However, that 2018 ACS recommendation to lower the starting age to 45 years was based to a large extent on a report of a higher incidence of CRC in younger adults from a 2017 study that used the SEER (Surveillance, Epidemiology, and End Results) database).
But that SEER-based study considered “colorectal cancer” as a homogeneous group defined by topology, the editorialists pointed out.
The new study, the editorialists said, uses that same SEER database but has “disentangled colorectal adenocarcinoma, the target for screening, from other histologic CRC types, including neuroendocrine (carcinoid) tumors, for which screening is not recommended.”
The study authors explained that adenocarcinoma is a target for prevention through screening because it arises from precancerous polyps. Those growths can be detected and removed before cancer develops. That doesn’t apply to carcinoid tumors, which are frequently incidental findings on flexible sigmoidoscopy or colonoscopy.
These carcinoid tumors typically are indolent, with a better prognosis than most other cancer types, the editorialists added. “Most likely, the majority of carcinoid tumors identified by screening represent incidental findings with little health benefit from detection. In fact, many may be characterized as overdiagnosed tumors, which by definition increase the burden and harms of screening without the balance of additional benefit.”
This new analysis showed that 4%-20% of the lesions previously described as CRC were not adenocarcinoma but carcinoid tumors, the editorialists pointed out.
This figure rose even higher in the subgroup of findings pertaining to the rectum, the colonic segment with the largest reported increase in early-onset CRC. Here, up to 34% of lesions (depending on patient age) were carcinoid tumors rather than adenocarcinoma, they noted.
The three editorialists – Michael Bretthauer, MD, PhD, and Mette Kalager, MD, PhD, both of the University of Oslo, and David Weinberg, MD, MSc, of Fox Chase Cancer Center, Philadelphia – call for action based on the new findings.
“The ACS’s 2018 estimate of about 7,000 new CRC cases among persons aged 45-49 years in the United States (the justification for screening) needs to be adjusted downward on the basis of the new evidence,” the trio wrote.
They conclude that “caution is warranted when promoting the benefits of CRC screening for persons younger than 50 years.”
However, the senior author of the new study, Jordan Karlitz, MD, of Tulane University, New Orleans, strongly disagreed.
Contrary to the editorialists, Dr. Karlitz said in an interview that he and his colleagues firmly believe that colorectal cancer screening for average-risk patients should begin at age 45 and that their new research, despite its clarification about carcinoid tumors, provides evidence for that.
“There are a number of other studies that support screening at age 45 as well,” he said. “This [new] finding supports the presence of a large preclinical colorectal cancer case burden in patients in their 40s that is ultimately uncovered with screening initiation at age 50. Many of these cancers could be prevented or diagnosed at an earlier stage with screening at age 45.”
“This is the first study to analyze early-onset colorectal cancer by specific histologic subtype,” Dr. Karlitz also pointed out.
“Although colorectal carcinoids are increasing at a faster rate than adenocarcinomas, adenocarcinomas constitute the overwhelming majority of colorectal cancers in people in their 40s and are also steadily increasing, which has implications for beginning screening at age 45,” he said.
Adenocarcinomas also make up the “overwhelming majority” of colorectal cancers in patients under 50 overall and “are the main driving force behind the increased colorectal cancer burden we are seeing in young patients,” Dr. Karlitz added.
Furthermore, “modeling studies on which the USPSTF screening recommendations were based [which recommended starting at age 45] were confined to adenocarcinoma, thus excluding carcinoids from their analysis,” he said.
Steepest changes in adenocarcinomas in younger groups
In their study, Dr. Karlitz and colleagues assessed the incidence rates of early colorectal cancer, using SEER data from 2000 to 2016, and stratifying the data by histologic subtype (primarily adenocarcinoma and carcinoid tumors), age group (20-29, 30-39, 40-49, and 50-54 years), and subsite.
A total of 123,143 CRC cases were identified in 119,624 patients between the ages of 20-54 years during that time period.
The absolute incidence rates in the younger age groups (20-29 and 30-39 years) were very low, compared with those aged 40-49 and 50-54 years.
The greatest 3-year average annual incident rate changes in adenocarcinoma (2000-2002 vs. 2014-2016) for any age group or subsite were for rectal-only cases in the 20-29 years group (+39%), as well as rectal-only cases in those aged 30-39 years (+39%), and colon-only cases in the age 30-39 group (+20%).
There was also significant increase in rectal-only adenocarcinoma in individuals aged 50-54 years (+10%). A statistically significant increase in the annual percentage change for adenocarcinomas was observed for all age groups, except for colon-only cases in the 20-29 years group (0.7%) and for both colorectal (0.2%) and colon-only cases (–0.1%) in those aged 50-54 years.
Even though the absolute carcinoid tumor incidence rates were lower than for adenocarcinoma in all age groups and subsites, a statistically significant increase was observed in the 3-year average annual incidence rate of combined-site colorectal carcinoid tumors in all age groups from 2000–2002 and 2014–2016. This increase was largely the result of increases in rectal carcinoid tumors, the authors note.
The authors also highlighted the results in the 40- to 49-year age group “because of differing opinions on whether to begin average-risk screening at age 45 or 50 years.”
They reported that rates of rectal and colon adenocarcinoma are increasing “substantially,” whether measured by changes in 3-year average annual incidence rate or by annual percentage changes. The change in average annual incidence rate of colon-only adenocarcinoma for persons aged 40-49 years was 13% (12.21 to 13.85 per 100,000), and that of rectal adenocarcinoma was 16% (7.50 to 8.72 per 100,000). Corresponding annual percentage changes were 0.8% and 1.2%, respectively. “These significant increases in adenocarcinoma incident rates add to the debate over earlier screening at age 45 years,” they commented.
Calls for next steps
The editorialists emphasize restraint when promoting the benefits of colorectal screening for persons younger than 50 years.
They point out that the USPSTF released a provisional update of its CRC screening recommendations about lowering the age to initiate screening to 45 years, as reported by this news organization.
“No new empirical evidence has been found since the USPSTF update in 2016 to inform the effectiveness of screening in persons younger than 50 years,” they write, adding that similar to the American Cancer Society in 2018, the task force has relied exclusively on modeling studies.
This new data from Dr. Karlitz and colleagues “should prompt the modelers to recalculate their estimates of benefits and harms of screening,” they suggested. “Revisiting the model would also allow competing forms of CRC screening to be compared in light of new risk assumptions.
“Previous assumptions that screening tests are equally effective in younger and older patients and that screening adherence will approach 100% may also be reconsidered,” the editorialist commented.
The study authors concluded somewhat differently.
“In conclusion, adenocarcinoma rates increased in many early-onset subgroups but showed no significant increase in others, including colon-only cases in persons aged 20-29 and 50-54 years,” the investigators wrote.
They also observed that “rectal carcinoid tumors are increasing in young patients and may have a substantial impact on overall CRC incident rates.”
Those findings on rectal carcinoid tumors “underscore the importance of assessing histologic CRC subtypes independently,” the researchers said.
This new approach, of which the current study is a first effort, “may lead to a better understanding of the drivers of temporal changes in overall CRC incidence and a more accurate measurement of the outcomes of adenocarcinoma risk reduction efforts, and can guide future research.”
The study had no outside funding. Dr. Karlitz reported personal fees from Exact Sciences, personal fees from Myriad Genetics, and other fees from Gastro Girl and GI OnDEMAND, outside the submitted work. Dr. Bretthauer reports grants from Norwegian Research Council, grants from Norwegian Cancer Society for research in colorectal cancer screening. Dr. Weinberg and Dr. Kalager have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Implications for CRC screening.
Implications for CRC screening.
New estimates for the risk of CRC in young adults, which differentiate colorectal adenocarcinoma from other types, are reported in a study published Dec. 15, 2020, in Annals of Internal Medicine.
They are important because this finding has implications for CRC screening, say a trio of experts in an accompanying editorial.
Reports of an increase in the incidence of CRC in younger adults have led to changes in screening for this cancer in the United States. The age for starting CRC screening has been lowered to 45 years (instead of 50 years) in recommendations issued in 2018 by the American Cancer Society, and also more recently in preliminary recommendations from the U.S. Preventive Services Task Force.
However, that 2018 ACS recommendation to lower the starting age to 45 years was based to a large extent on a report of a higher incidence of CRC in younger adults from a 2017 study that used the SEER (Surveillance, Epidemiology, and End Results) database).
But that SEER-based study considered “colorectal cancer” as a homogeneous group defined by topology, the editorialists pointed out.
The new study, the editorialists said, uses that same SEER database but has “disentangled colorectal adenocarcinoma, the target for screening, from other histologic CRC types, including neuroendocrine (carcinoid) tumors, for which screening is not recommended.”
The study authors explained that adenocarcinoma is a target for prevention through screening because it arises from precancerous polyps. Those growths can be detected and removed before cancer develops. That doesn’t apply to carcinoid tumors, which are frequently incidental findings on flexible sigmoidoscopy or colonoscopy.
These carcinoid tumors typically are indolent, with a better prognosis than most other cancer types, the editorialists added. “Most likely, the majority of carcinoid tumors identified by screening represent incidental findings with little health benefit from detection. In fact, many may be characterized as overdiagnosed tumors, which by definition increase the burden and harms of screening without the balance of additional benefit.”
This new analysis showed that 4%-20% of the lesions previously described as CRC were not adenocarcinoma but carcinoid tumors, the editorialists pointed out.
This figure rose even higher in the subgroup of findings pertaining to the rectum, the colonic segment with the largest reported increase in early-onset CRC. Here, up to 34% of lesions (depending on patient age) were carcinoid tumors rather than adenocarcinoma, they noted.
The three editorialists – Michael Bretthauer, MD, PhD, and Mette Kalager, MD, PhD, both of the University of Oslo, and David Weinberg, MD, MSc, of Fox Chase Cancer Center, Philadelphia – call for action based on the new findings.
“The ACS’s 2018 estimate of about 7,000 new CRC cases among persons aged 45-49 years in the United States (the justification for screening) needs to be adjusted downward on the basis of the new evidence,” the trio wrote.
They conclude that “caution is warranted when promoting the benefits of CRC screening for persons younger than 50 years.”
However, the senior author of the new study, Jordan Karlitz, MD, of Tulane University, New Orleans, strongly disagreed.
Contrary to the editorialists, Dr. Karlitz said in an interview that he and his colleagues firmly believe that colorectal cancer screening for average-risk patients should begin at age 45 and that their new research, despite its clarification about carcinoid tumors, provides evidence for that.
“There are a number of other studies that support screening at age 45 as well,” he said. “This [new] finding supports the presence of a large preclinical colorectal cancer case burden in patients in their 40s that is ultimately uncovered with screening initiation at age 50. Many of these cancers could be prevented or diagnosed at an earlier stage with screening at age 45.”
“This is the first study to analyze early-onset colorectal cancer by specific histologic subtype,” Dr. Karlitz also pointed out.
“Although colorectal carcinoids are increasing at a faster rate than adenocarcinomas, adenocarcinomas constitute the overwhelming majority of colorectal cancers in people in their 40s and are also steadily increasing, which has implications for beginning screening at age 45,” he said.
Adenocarcinomas also make up the “overwhelming majority” of colorectal cancers in patients under 50 overall and “are the main driving force behind the increased colorectal cancer burden we are seeing in young patients,” Dr. Karlitz added.
Furthermore, “modeling studies on which the USPSTF screening recommendations were based [which recommended starting at age 45] were confined to adenocarcinoma, thus excluding carcinoids from their analysis,” he said.
Steepest changes in adenocarcinomas in younger groups
In their study, Dr. Karlitz and colleagues assessed the incidence rates of early colorectal cancer, using SEER data from 2000 to 2016, and stratifying the data by histologic subtype (primarily adenocarcinoma and carcinoid tumors), age group (20-29, 30-39, 40-49, and 50-54 years), and subsite.
A total of 123,143 CRC cases were identified in 119,624 patients between the ages of 20-54 years during that time period.
The absolute incidence rates in the younger age groups (20-29 and 30-39 years) were very low, compared with those aged 40-49 and 50-54 years.
The greatest 3-year average annual incident rate changes in adenocarcinoma (2000-2002 vs. 2014-2016) for any age group or subsite were for rectal-only cases in the 20-29 years group (+39%), as well as rectal-only cases in those aged 30-39 years (+39%), and colon-only cases in the age 30-39 group (+20%).
There was also significant increase in rectal-only adenocarcinoma in individuals aged 50-54 years (+10%). A statistically significant increase in the annual percentage change for adenocarcinomas was observed for all age groups, except for colon-only cases in the 20-29 years group (0.7%) and for both colorectal (0.2%) and colon-only cases (–0.1%) in those aged 50-54 years.
Even though the absolute carcinoid tumor incidence rates were lower than for adenocarcinoma in all age groups and subsites, a statistically significant increase was observed in the 3-year average annual incidence rate of combined-site colorectal carcinoid tumors in all age groups from 2000–2002 and 2014–2016. This increase was largely the result of increases in rectal carcinoid tumors, the authors note.
The authors also highlighted the results in the 40- to 49-year age group “because of differing opinions on whether to begin average-risk screening at age 45 or 50 years.”
They reported that rates of rectal and colon adenocarcinoma are increasing “substantially,” whether measured by changes in 3-year average annual incidence rate or by annual percentage changes. The change in average annual incidence rate of colon-only adenocarcinoma for persons aged 40-49 years was 13% (12.21 to 13.85 per 100,000), and that of rectal adenocarcinoma was 16% (7.50 to 8.72 per 100,000). Corresponding annual percentage changes were 0.8% and 1.2%, respectively. “These significant increases in adenocarcinoma incident rates add to the debate over earlier screening at age 45 years,” they commented.
Calls for next steps
The editorialists emphasize restraint when promoting the benefits of colorectal screening for persons younger than 50 years.
They point out that the USPSTF released a provisional update of its CRC screening recommendations about lowering the age to initiate screening to 45 years, as reported by this news organization.
“No new empirical evidence has been found since the USPSTF update in 2016 to inform the effectiveness of screening in persons younger than 50 years,” they write, adding that similar to the American Cancer Society in 2018, the task force has relied exclusively on modeling studies.
This new data from Dr. Karlitz and colleagues “should prompt the modelers to recalculate their estimates of benefits and harms of screening,” they suggested. “Revisiting the model would also allow competing forms of CRC screening to be compared in light of new risk assumptions.
“Previous assumptions that screening tests are equally effective in younger and older patients and that screening adherence will approach 100% may also be reconsidered,” the editorialist commented.
The study authors concluded somewhat differently.
“In conclusion, adenocarcinoma rates increased in many early-onset subgroups but showed no significant increase in others, including colon-only cases in persons aged 20-29 and 50-54 years,” the investigators wrote.
They also observed that “rectal carcinoid tumors are increasing in young patients and may have a substantial impact on overall CRC incident rates.”
Those findings on rectal carcinoid tumors “underscore the importance of assessing histologic CRC subtypes independently,” the researchers said.
This new approach, of which the current study is a first effort, “may lead to a better understanding of the drivers of temporal changes in overall CRC incidence and a more accurate measurement of the outcomes of adenocarcinoma risk reduction efforts, and can guide future research.”
The study had no outside funding. Dr. Karlitz reported personal fees from Exact Sciences, personal fees from Myriad Genetics, and other fees from Gastro Girl and GI OnDEMAND, outside the submitted work. Dr. Bretthauer reports grants from Norwegian Research Council, grants from Norwegian Cancer Society for research in colorectal cancer screening. Dr. Weinberg and Dr. Kalager have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New estimates for the risk of CRC in young adults, which differentiate colorectal adenocarcinoma from other types, are reported in a study published Dec. 15, 2020, in Annals of Internal Medicine.
They are important because this finding has implications for CRC screening, say a trio of experts in an accompanying editorial.
Reports of an increase in the incidence of CRC in younger adults have led to changes in screening for this cancer in the United States. The age for starting CRC screening has been lowered to 45 years (instead of 50 years) in recommendations issued in 2018 by the American Cancer Society, and also more recently in preliminary recommendations from the U.S. Preventive Services Task Force.
However, that 2018 ACS recommendation to lower the starting age to 45 years was based to a large extent on a report of a higher incidence of CRC in younger adults from a 2017 study that used the SEER (Surveillance, Epidemiology, and End Results) database).
But that SEER-based study considered “colorectal cancer” as a homogeneous group defined by topology, the editorialists pointed out.
The new study, the editorialists said, uses that same SEER database but has “disentangled colorectal adenocarcinoma, the target for screening, from other histologic CRC types, including neuroendocrine (carcinoid) tumors, for which screening is not recommended.”
The study authors explained that adenocarcinoma is a target for prevention through screening because it arises from precancerous polyps. Those growths can be detected and removed before cancer develops. That doesn’t apply to carcinoid tumors, which are frequently incidental findings on flexible sigmoidoscopy or colonoscopy.
These carcinoid tumors typically are indolent, with a better prognosis than most other cancer types, the editorialists added. “Most likely, the majority of carcinoid tumors identified by screening represent incidental findings with little health benefit from detection. In fact, many may be characterized as overdiagnosed tumors, which by definition increase the burden and harms of screening without the balance of additional benefit.”
This new analysis showed that 4%-20% of the lesions previously described as CRC were not adenocarcinoma but carcinoid tumors, the editorialists pointed out.
This figure rose even higher in the subgroup of findings pertaining to the rectum, the colonic segment with the largest reported increase in early-onset CRC. Here, up to 34% of lesions (depending on patient age) were carcinoid tumors rather than adenocarcinoma, they noted.
The three editorialists – Michael Bretthauer, MD, PhD, and Mette Kalager, MD, PhD, both of the University of Oslo, and David Weinberg, MD, MSc, of Fox Chase Cancer Center, Philadelphia – call for action based on the new findings.
“The ACS’s 2018 estimate of about 7,000 new CRC cases among persons aged 45-49 years in the United States (the justification for screening) needs to be adjusted downward on the basis of the new evidence,” the trio wrote.
They conclude that “caution is warranted when promoting the benefits of CRC screening for persons younger than 50 years.”
However, the senior author of the new study, Jordan Karlitz, MD, of Tulane University, New Orleans, strongly disagreed.
Contrary to the editorialists, Dr. Karlitz said in an interview that he and his colleagues firmly believe that colorectal cancer screening for average-risk patients should begin at age 45 and that their new research, despite its clarification about carcinoid tumors, provides evidence for that.
“There are a number of other studies that support screening at age 45 as well,” he said. “This [new] finding supports the presence of a large preclinical colorectal cancer case burden in patients in their 40s that is ultimately uncovered with screening initiation at age 50. Many of these cancers could be prevented or diagnosed at an earlier stage with screening at age 45.”
“This is the first study to analyze early-onset colorectal cancer by specific histologic subtype,” Dr. Karlitz also pointed out.
“Although colorectal carcinoids are increasing at a faster rate than adenocarcinomas, adenocarcinomas constitute the overwhelming majority of colorectal cancers in people in their 40s and are also steadily increasing, which has implications for beginning screening at age 45,” he said.
Adenocarcinomas also make up the “overwhelming majority” of colorectal cancers in patients under 50 overall and “are the main driving force behind the increased colorectal cancer burden we are seeing in young patients,” Dr. Karlitz added.
Furthermore, “modeling studies on which the USPSTF screening recommendations were based [which recommended starting at age 45] were confined to adenocarcinoma, thus excluding carcinoids from their analysis,” he said.
Steepest changes in adenocarcinomas in younger groups
In their study, Dr. Karlitz and colleagues assessed the incidence rates of early colorectal cancer, using SEER data from 2000 to 2016, and stratifying the data by histologic subtype (primarily adenocarcinoma and carcinoid tumors), age group (20-29, 30-39, 40-49, and 50-54 years), and subsite.
A total of 123,143 CRC cases were identified in 119,624 patients between the ages of 20-54 years during that time period.
The absolute incidence rates in the younger age groups (20-29 and 30-39 years) were very low, compared with those aged 40-49 and 50-54 years.
The greatest 3-year average annual incident rate changes in adenocarcinoma (2000-2002 vs. 2014-2016) for any age group or subsite were for rectal-only cases in the 20-29 years group (+39%), as well as rectal-only cases in those aged 30-39 years (+39%), and colon-only cases in the age 30-39 group (+20%).
There was also significant increase in rectal-only adenocarcinoma in individuals aged 50-54 years (+10%). A statistically significant increase in the annual percentage change for adenocarcinomas was observed for all age groups, except for colon-only cases in the 20-29 years group (0.7%) and for both colorectal (0.2%) and colon-only cases (–0.1%) in those aged 50-54 years.
Even though the absolute carcinoid tumor incidence rates were lower than for adenocarcinoma in all age groups and subsites, a statistically significant increase was observed in the 3-year average annual incidence rate of combined-site colorectal carcinoid tumors in all age groups from 2000–2002 and 2014–2016. This increase was largely the result of increases in rectal carcinoid tumors, the authors note.
The authors also highlighted the results in the 40- to 49-year age group “because of differing opinions on whether to begin average-risk screening at age 45 or 50 years.”
They reported that rates of rectal and colon adenocarcinoma are increasing “substantially,” whether measured by changes in 3-year average annual incidence rate or by annual percentage changes. The change in average annual incidence rate of colon-only adenocarcinoma for persons aged 40-49 years was 13% (12.21 to 13.85 per 100,000), and that of rectal adenocarcinoma was 16% (7.50 to 8.72 per 100,000). Corresponding annual percentage changes were 0.8% and 1.2%, respectively. “These significant increases in adenocarcinoma incident rates add to the debate over earlier screening at age 45 years,” they commented.
Calls for next steps
The editorialists emphasize restraint when promoting the benefits of colorectal screening for persons younger than 50 years.
They point out that the USPSTF released a provisional update of its CRC screening recommendations about lowering the age to initiate screening to 45 years, as reported by this news organization.
“No new empirical evidence has been found since the USPSTF update in 2016 to inform the effectiveness of screening in persons younger than 50 years,” they write, adding that similar to the American Cancer Society in 2018, the task force has relied exclusively on modeling studies.
This new data from Dr. Karlitz and colleagues “should prompt the modelers to recalculate their estimates of benefits and harms of screening,” they suggested. “Revisiting the model would also allow competing forms of CRC screening to be compared in light of new risk assumptions.
“Previous assumptions that screening tests are equally effective in younger and older patients and that screening adherence will approach 100% may also be reconsidered,” the editorialist commented.
The study authors concluded somewhat differently.
“In conclusion, adenocarcinoma rates increased in many early-onset subgroups but showed no significant increase in others, including colon-only cases in persons aged 20-29 and 50-54 years,” the investigators wrote.
They also observed that “rectal carcinoid tumors are increasing in young patients and may have a substantial impact on overall CRC incident rates.”
Those findings on rectal carcinoid tumors “underscore the importance of assessing histologic CRC subtypes independently,” the researchers said.
This new approach, of which the current study is a first effort, “may lead to a better understanding of the drivers of temporal changes in overall CRC incidence and a more accurate measurement of the outcomes of adenocarcinoma risk reduction efforts, and can guide future research.”
The study had no outside funding. Dr. Karlitz reported personal fees from Exact Sciences, personal fees from Myriad Genetics, and other fees from Gastro Girl and GI OnDEMAND, outside the submitted work. Dr. Bretthauer reports grants from Norwegian Research Council, grants from Norwegian Cancer Society for research in colorectal cancer screening. Dr. Weinberg and Dr. Kalager have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Baseline body surface area may drive optimal baricitinib responses
results from an analysis of phase 3 data showed.
“This proposed clinical tailoring approach for baricitinib 2 mg allows for treatment of patients who are more likely to respond to therapy and rapid decision on discontinuation of treatment for those who are not likely to benefit from baricitinib 2 mg,” Eric L. Simpson, MD, said during a late-breaking abstract session at the Revolutionizing Atopic Dermatitis virtual symposium.
Baricitinib is an oral, reversible and selective Janus kinase 1/JAK2 inhibitor that is approved in Europe for the treatment of moderate to severe AD in adults who are candidates for systemic therapy. In the United States, it is approved for treating rheumatoid arthritis, and is currently under Food and Drug Administration review in the United States for AD.
For the current analysis, Dr. Simpson, professor of dermatology at Oregon Health & Science University, Portland, and colleagues set out to identify responders to baricitinib 2 mg using a tailored approach based on baseline BSA affected and early clinical improvement in the phase 3 monotherapy trial BREEZE-AD5. The trial enrolled 440 patients: 147 to placebo, 147 to baricitinib 1 mg once daily, and 146 to baricitinib 2 mg once daily. The primary endpoint was Eczema Area and Severity Index (EASI)–75 at week 16.
“Understanding which patients can benefit most from this treatment was our goal,” Dr. Simpson said. “By tailoring your therapy, you can significantly improve the patient experience, increase the cost-effectiveness of a therapy, and you can ensure that only patients who are likely to benefit are exposed to a drug.”
The researchers used a classification and regression tree algorithm that identified baseline BSA as the strongest predictor of EASI-75 response at week 16. A BSA cutoff of 50% was established as the optimal cutoff for sensitivity and negative predictive value. Results for EASI-75 and Validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD) scores of 0 or 1 were confirmed using a BSA of 10%-50% at baseline to predict response, compared with a BSA or greater than 50% at baseline.
Sensitivity analyses revealed that about 90% of patients with an EASI-75 response were in the BSA 10%-50% group. Conversely, among patients with a BSA greater than 50%, the negative predictive value was greater than 90%, “so there’s a 90% chance you’re not going to hit that EASI-75 at week 16 if your BSA is greater than 50%,” Dr. Simpson explained. “The same holds true for vIGA-AD, so that 50% cutoff is important for understanding whether someone is going to respond or not.”
On the EASI-75, 38% of patients in the BSA 10%-50% group responded to baricitinib at week 16, compared with 10% in the BSA greater than 50% group. A similar association was observed on the vIGA-AD, where 32% of patients in the BSA 10%-50% group responded to baricitinib at week 16, compared with 5% in the BSA greater than 50% group.
When stratified by early response assessed at week 4, based on a 4-point improvement or greater on the Itch Numeric Rating Scale, 55% of those patients became EASI-75 responders, compared with 17% who were not. A similar association was observed by early response assessed at week 8.
“Due to the rapid onset of response, clinical assessment of patients after 4-8 weeks of initiation of baricitinib 2 mg treatment provided a positive feedback to patients who are likely to benefit from long-term therapy,” Dr. Simpson said. “This analysis may allow for a precision-medicine approach to therapy in moderate to severe AD.”
The study was supported by Eli Lilly, and was under license from Incyte. Dr. Simpson reported serving as an investigator for and consultant to numerous pharmaceutical companies.
results from an analysis of phase 3 data showed.
“This proposed clinical tailoring approach for baricitinib 2 mg allows for treatment of patients who are more likely to respond to therapy and rapid decision on discontinuation of treatment for those who are not likely to benefit from baricitinib 2 mg,” Eric L. Simpson, MD, said during a late-breaking abstract session at the Revolutionizing Atopic Dermatitis virtual symposium.
Baricitinib is an oral, reversible and selective Janus kinase 1/JAK2 inhibitor that is approved in Europe for the treatment of moderate to severe AD in adults who are candidates for systemic therapy. In the United States, it is approved for treating rheumatoid arthritis, and is currently under Food and Drug Administration review in the United States for AD.
For the current analysis, Dr. Simpson, professor of dermatology at Oregon Health & Science University, Portland, and colleagues set out to identify responders to baricitinib 2 mg using a tailored approach based on baseline BSA affected and early clinical improvement in the phase 3 monotherapy trial BREEZE-AD5. The trial enrolled 440 patients: 147 to placebo, 147 to baricitinib 1 mg once daily, and 146 to baricitinib 2 mg once daily. The primary endpoint was Eczema Area and Severity Index (EASI)–75 at week 16.
“Understanding which patients can benefit most from this treatment was our goal,” Dr. Simpson said. “By tailoring your therapy, you can significantly improve the patient experience, increase the cost-effectiveness of a therapy, and you can ensure that only patients who are likely to benefit are exposed to a drug.”
The researchers used a classification and regression tree algorithm that identified baseline BSA as the strongest predictor of EASI-75 response at week 16. A BSA cutoff of 50% was established as the optimal cutoff for sensitivity and negative predictive value. Results for EASI-75 and Validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD) scores of 0 or 1 were confirmed using a BSA of 10%-50% at baseline to predict response, compared with a BSA or greater than 50% at baseline.
Sensitivity analyses revealed that about 90% of patients with an EASI-75 response were in the BSA 10%-50% group. Conversely, among patients with a BSA greater than 50%, the negative predictive value was greater than 90%, “so there’s a 90% chance you’re not going to hit that EASI-75 at week 16 if your BSA is greater than 50%,” Dr. Simpson explained. “The same holds true for vIGA-AD, so that 50% cutoff is important for understanding whether someone is going to respond or not.”
On the EASI-75, 38% of patients in the BSA 10%-50% group responded to baricitinib at week 16, compared with 10% in the BSA greater than 50% group. A similar association was observed on the vIGA-AD, where 32% of patients in the BSA 10%-50% group responded to baricitinib at week 16, compared with 5% in the BSA greater than 50% group.
When stratified by early response assessed at week 4, based on a 4-point improvement or greater on the Itch Numeric Rating Scale, 55% of those patients became EASI-75 responders, compared with 17% who were not. A similar association was observed by early response assessed at week 8.
“Due to the rapid onset of response, clinical assessment of patients after 4-8 weeks of initiation of baricitinib 2 mg treatment provided a positive feedback to patients who are likely to benefit from long-term therapy,” Dr. Simpson said. “This analysis may allow for a precision-medicine approach to therapy in moderate to severe AD.”
The study was supported by Eli Lilly, and was under license from Incyte. Dr. Simpson reported serving as an investigator for and consultant to numerous pharmaceutical companies.
results from an analysis of phase 3 data showed.
“This proposed clinical tailoring approach for baricitinib 2 mg allows for treatment of patients who are more likely to respond to therapy and rapid decision on discontinuation of treatment for those who are not likely to benefit from baricitinib 2 mg,” Eric L. Simpson, MD, said during a late-breaking abstract session at the Revolutionizing Atopic Dermatitis virtual symposium.
Baricitinib is an oral, reversible and selective Janus kinase 1/JAK2 inhibitor that is approved in Europe for the treatment of moderate to severe AD in adults who are candidates for systemic therapy. In the United States, it is approved for treating rheumatoid arthritis, and is currently under Food and Drug Administration review in the United States for AD.
For the current analysis, Dr. Simpson, professor of dermatology at Oregon Health & Science University, Portland, and colleagues set out to identify responders to baricitinib 2 mg using a tailored approach based on baseline BSA affected and early clinical improvement in the phase 3 monotherapy trial BREEZE-AD5. The trial enrolled 440 patients: 147 to placebo, 147 to baricitinib 1 mg once daily, and 146 to baricitinib 2 mg once daily. The primary endpoint was Eczema Area and Severity Index (EASI)–75 at week 16.
“Understanding which patients can benefit most from this treatment was our goal,” Dr. Simpson said. “By tailoring your therapy, you can significantly improve the patient experience, increase the cost-effectiveness of a therapy, and you can ensure that only patients who are likely to benefit are exposed to a drug.”
The researchers used a classification and regression tree algorithm that identified baseline BSA as the strongest predictor of EASI-75 response at week 16. A BSA cutoff of 50% was established as the optimal cutoff for sensitivity and negative predictive value. Results for EASI-75 and Validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD) scores of 0 or 1 were confirmed using a BSA of 10%-50% at baseline to predict response, compared with a BSA or greater than 50% at baseline.
Sensitivity analyses revealed that about 90% of patients with an EASI-75 response were in the BSA 10%-50% group. Conversely, among patients with a BSA greater than 50%, the negative predictive value was greater than 90%, “so there’s a 90% chance you’re not going to hit that EASI-75 at week 16 if your BSA is greater than 50%,” Dr. Simpson explained. “The same holds true for vIGA-AD, so that 50% cutoff is important for understanding whether someone is going to respond or not.”
On the EASI-75, 38% of patients in the BSA 10%-50% group responded to baricitinib at week 16, compared with 10% in the BSA greater than 50% group. A similar association was observed on the vIGA-AD, where 32% of patients in the BSA 10%-50% group responded to baricitinib at week 16, compared with 5% in the BSA greater than 50% group.
When stratified by early response assessed at week 4, based on a 4-point improvement or greater on the Itch Numeric Rating Scale, 55% of those patients became EASI-75 responders, compared with 17% who were not. A similar association was observed by early response assessed at week 8.
“Due to the rapid onset of response, clinical assessment of patients after 4-8 weeks of initiation of baricitinib 2 mg treatment provided a positive feedback to patients who are likely to benefit from long-term therapy,” Dr. Simpson said. “This analysis may allow for a precision-medicine approach to therapy in moderate to severe AD.”
The study was supported by Eli Lilly, and was under license from Incyte. Dr. Simpson reported serving as an investigator for and consultant to numerous pharmaceutical companies.
FROM REVOLUTIONIZING AD 2020
Avoiding atopic dermatitis triggers easier said than done
“Guidelines on trigger avoidance are written as if it’s easy to do,” Jonathan I. Silverberg, MD, PhD, MPH, said during the Revolutionizing Atopic Dermatitis virtual symposium. “It turns out that trigger avoidance is really complicated.”
He and his colleagues conducted a study of most common triggers for itch based on a prospective dermatology practice–based study of 587 adults with AD . About two-thirds (65%) reported one or more itch trigger in the past week and 36% had three or more itch triggers in the past week. The two most common triggers were stress (35%) and sweat (31%).
“To me, this is provocative, because this is not how I was trained in residency,” said Dr. Silverberg, director of clinical research in the division of dermatology at George Washington University, Washington. “I was trained that it’s all about excess showering, dry air, or cold temperature. Those are important, but the most common triggers are stress and sweat.”
AD triggers are also commonly linked to seasonality. “If you ask patients when their AD is worse, sometimes it’s winter,” he said. “Sometimes it’s spring. Sometimes it’s summer. It turns out that there is a distinct set of triggers that are associated with AD seasonality.” Wintertime worsening of disease is associated with cold temperature and weather change, he continued, while springtime worsening of disease is often linked to weather change and dry air. Common summertime triggers for flares include hot temperature, heat, sweat, weather change, sunlight, humid air, and dry air. “In the fall, the weather change again comes up as a trigger. Humid air does as well.”
In their prospective study, Dr. Silverberg and colleagues found that 90% of those who had at least three itch triggers reported 3 months or less of AD remission in the past year, “meaning that 90% are reporting persistent disease when they have multiple itch triggers,” he said. In addition, 78% reported two or more flares per year and 61% reported that AD is worse during certain seasons.
Potential mitigation strategies for stress include stress management, biofeedback, meditation, relaxation training, and mindfulness. “These don’t necessarily require expensive psychotherapy,” he said. Freely available iPhone apps can be incorporated into daily practice, such as Calm, Relax with Andrew Johnson, Nature Sounds Relax and Sleep, Breathe2Relax, and Headspace.
Many AD patients are sedentary and avoid vigorous physical activity owing to heat and sweat as triggers. Simple solutions include exercising in a cooler temperature environment, “not just using fans,” he said. “Take a quick shower right after working out and consider pre- and/or post treatment with topical medication.”
High temperature and sweating can be problematic at bedtime, he continued. Even if the indoor temperature is 70° F, that might jump to 85° F or 90° F under a thick blanket. “That heat can trigger itch and may cause sweating, which can trigger itch,” said Dr. Silverberg, who has AD and is director of patch testing at George Washington University. Potential solutions include using a lighter blanket, lowering the indoor temperature, and wearing breathable pajamas.
Dryness, another common AD trigger, can be secondary to a combination of low outdoor and/or indoor humidity. “Lower outdoor humidity is a particular problem in the wintertime, because cold air doesn’t hold moisture as well,” he said. “That’s why the air feels much dryer in the wintertime. There’s also a problem of indoor heating and cooling. Sometimes central air systems can lower humidity to the point where it’s bone dry.”
In an effort to determine the impact of specific climatic factors on the U.S. prevalence of AD, Dr. Silverberg and colleagues conducted a study using a merged analysis of the 2007 National Survey of Children’s Health from a representative sample of 91,642 children aged 0-17 years and 2006-2007 measurements from the National Climate Data Center and Weather Service. They found that childhood AD prevalence was increased in geographical areas that use more indoor heat and cooling and had lower outdoor humidity. “So, we see that there’s a direct correlate of this dryness issue that is leading to more AD throughout the U.S.,” he said.
Practical solutions to mitigate the effect of dry air on AD include opening windows to allow entry of moist air, “which can be particularly helpful in residences that are overheated,” he said. “I deal with this a lot in patients who live in dormitories. Use humidifiers to add moisture back into the air. Aim for 40%-50% indoor humidity to avoid mold and dust mites. It’s better to use demineralized water to reduce bacterial growth. This can be helpful for aeroallergies. Of note, there are really no well-done studies that have examined the efficacy of humidifiers in AD, but based on our anecdotal experience, this is a good way to go.”
Cold temperatures and trigger intense itch, even in the setting of high humidity. “For me personally, this is one of my most brutal triggers,” Dr. Silverberg said. “When I’m in a place with extremes of cold, I get a rapid onset of itch, a mix of itch and pain, particularly on the dorsal hands. For solutions, you can encourage patients to avoid extremely low temperatures, to bundle up, and to potentially use hand warmers or other heating devices.”
Clothing can be a trigger as well, especially tight-fitting clothes, hot and nonbreathable clothes, and large-diameter wool, which has been shown to induce itching and irritation. Mitigation strategies include wearing loose-fitting, lightweight, nonirritating fabric. “Traditional cotton and silk fabrics have mixed evidence in improving AD but are generally safe,” he said. “Ultra- or superfine merino wool has been shown to be nonpruritic. There is sparse evidence to support chemically treated/coated clothing for AD, but this may be an emerging area.”
Dr. Silverberg pointed out variability of cultural perspectives and preferences for bathing practices, including temperature, duration, frequency, optimal bathing products, and the use of loofahs and other scrubbing products. “This stems from different perceptions of what it means to be clean, and how dry our skin should feel after a shower,” he said. “Many clinicians and patients were taught that regular bathing is harmful in AD. It turns out that’s not true.”
In a recently published systematic review and meta-analysis of 13 studies, he and his colleagues examined efficacy outcomes of different bathing/showering regimens in AD. All 13 studies showed numerically reduced AD severity with any bathing regimen in at least one time point. Numerical decreases over time were observed for body surface area (BSA), Eczema Area and Severity Index (EASI), and/or SCORAD measures for daily and less than daily bathing, with or without application of emollients or topical corticosteroids. In random effects regression models, taking baths more than or less than seven times per week were not associated with significant differences of Cohen’s D scores for EASI, SCORAD, or BSA. “The take-home message here is, let your AD patients bathe,” Dr. Silverberg said. “Bathing is good. It can be channeled to help the eczema, but it has to be done the right way.”
Patients should be counseled to use nonirritating cleansers and shampoos, avoid excessively long baths/showers, avoid excessively hot baths/showers, avoid excessive rubbing or scrubbing of skin, and to apply emollients and/or topical corticosteroids immediately after the bath/shower.
PROMIS Itch-Triggers is a simple and feasible checklist to screen for the most common itch triggers in AD in clinical practice (patients are asked to check off which of the following have caused their itch in the previous 7 days: cold temperature, hot temperature, heat, sweat, tight clothing, fragrances, boredom, talking about itch, stress, weather change, sunlight, humid air, dry air). “It takes less than 1 minute to complete,” he said. “Additional testing with skin patch and/or prick testing may be warranted to identify allergenic triggers.”
Dr. Silverberg reported that he is a consultant to and/or an advisory board member for several pharmaceutical companies. He is also a speaker for Regeneron and Sanofi and has received a grant from Galderma.
“Guidelines on trigger avoidance are written as if it’s easy to do,” Jonathan I. Silverberg, MD, PhD, MPH, said during the Revolutionizing Atopic Dermatitis virtual symposium. “It turns out that trigger avoidance is really complicated.”
He and his colleagues conducted a study of most common triggers for itch based on a prospective dermatology practice–based study of 587 adults with AD . About two-thirds (65%) reported one or more itch trigger in the past week and 36% had three or more itch triggers in the past week. The two most common triggers were stress (35%) and sweat (31%).
“To me, this is provocative, because this is not how I was trained in residency,” said Dr. Silverberg, director of clinical research in the division of dermatology at George Washington University, Washington. “I was trained that it’s all about excess showering, dry air, or cold temperature. Those are important, but the most common triggers are stress and sweat.”
AD triggers are also commonly linked to seasonality. “If you ask patients when their AD is worse, sometimes it’s winter,” he said. “Sometimes it’s spring. Sometimes it’s summer. It turns out that there is a distinct set of triggers that are associated with AD seasonality.” Wintertime worsening of disease is associated with cold temperature and weather change, he continued, while springtime worsening of disease is often linked to weather change and dry air. Common summertime triggers for flares include hot temperature, heat, sweat, weather change, sunlight, humid air, and dry air. “In the fall, the weather change again comes up as a trigger. Humid air does as well.”
In their prospective study, Dr. Silverberg and colleagues found that 90% of those who had at least three itch triggers reported 3 months or less of AD remission in the past year, “meaning that 90% are reporting persistent disease when they have multiple itch triggers,” he said. In addition, 78% reported two or more flares per year and 61% reported that AD is worse during certain seasons.
Potential mitigation strategies for stress include stress management, biofeedback, meditation, relaxation training, and mindfulness. “These don’t necessarily require expensive psychotherapy,” he said. Freely available iPhone apps can be incorporated into daily practice, such as Calm, Relax with Andrew Johnson, Nature Sounds Relax and Sleep, Breathe2Relax, and Headspace.
Many AD patients are sedentary and avoid vigorous physical activity owing to heat and sweat as triggers. Simple solutions include exercising in a cooler temperature environment, “not just using fans,” he said. “Take a quick shower right after working out and consider pre- and/or post treatment with topical medication.”
High temperature and sweating can be problematic at bedtime, he continued. Even if the indoor temperature is 70° F, that might jump to 85° F or 90° F under a thick blanket. “That heat can trigger itch and may cause sweating, which can trigger itch,” said Dr. Silverberg, who has AD and is director of patch testing at George Washington University. Potential solutions include using a lighter blanket, lowering the indoor temperature, and wearing breathable pajamas.
Dryness, another common AD trigger, can be secondary to a combination of low outdoor and/or indoor humidity. “Lower outdoor humidity is a particular problem in the wintertime, because cold air doesn’t hold moisture as well,” he said. “That’s why the air feels much dryer in the wintertime. There’s also a problem of indoor heating and cooling. Sometimes central air systems can lower humidity to the point where it’s bone dry.”
In an effort to determine the impact of specific climatic factors on the U.S. prevalence of AD, Dr. Silverberg and colleagues conducted a study using a merged analysis of the 2007 National Survey of Children’s Health from a representative sample of 91,642 children aged 0-17 years and 2006-2007 measurements from the National Climate Data Center and Weather Service. They found that childhood AD prevalence was increased in geographical areas that use more indoor heat and cooling and had lower outdoor humidity. “So, we see that there’s a direct correlate of this dryness issue that is leading to more AD throughout the U.S.,” he said.
Practical solutions to mitigate the effect of dry air on AD include opening windows to allow entry of moist air, “which can be particularly helpful in residences that are overheated,” he said. “I deal with this a lot in patients who live in dormitories. Use humidifiers to add moisture back into the air. Aim for 40%-50% indoor humidity to avoid mold and dust mites. It’s better to use demineralized water to reduce bacterial growth. This can be helpful for aeroallergies. Of note, there are really no well-done studies that have examined the efficacy of humidifiers in AD, but based on our anecdotal experience, this is a good way to go.”
Cold temperatures and trigger intense itch, even in the setting of high humidity. “For me personally, this is one of my most brutal triggers,” Dr. Silverberg said. “When I’m in a place with extremes of cold, I get a rapid onset of itch, a mix of itch and pain, particularly on the dorsal hands. For solutions, you can encourage patients to avoid extremely low temperatures, to bundle up, and to potentially use hand warmers or other heating devices.”
Clothing can be a trigger as well, especially tight-fitting clothes, hot and nonbreathable clothes, and large-diameter wool, which has been shown to induce itching and irritation. Mitigation strategies include wearing loose-fitting, lightweight, nonirritating fabric. “Traditional cotton and silk fabrics have mixed evidence in improving AD but are generally safe,” he said. “Ultra- or superfine merino wool has been shown to be nonpruritic. There is sparse evidence to support chemically treated/coated clothing for AD, but this may be an emerging area.”
Dr. Silverberg pointed out variability of cultural perspectives and preferences for bathing practices, including temperature, duration, frequency, optimal bathing products, and the use of loofahs and other scrubbing products. “This stems from different perceptions of what it means to be clean, and how dry our skin should feel after a shower,” he said. “Many clinicians and patients were taught that regular bathing is harmful in AD. It turns out that’s not true.”
In a recently published systematic review and meta-analysis of 13 studies, he and his colleagues examined efficacy outcomes of different bathing/showering regimens in AD. All 13 studies showed numerically reduced AD severity with any bathing regimen in at least one time point. Numerical decreases over time were observed for body surface area (BSA), Eczema Area and Severity Index (EASI), and/or SCORAD measures for daily and less than daily bathing, with or without application of emollients or topical corticosteroids. In random effects regression models, taking baths more than or less than seven times per week were not associated with significant differences of Cohen’s D scores for EASI, SCORAD, or BSA. “The take-home message here is, let your AD patients bathe,” Dr. Silverberg said. “Bathing is good. It can be channeled to help the eczema, but it has to be done the right way.”
Patients should be counseled to use nonirritating cleansers and shampoos, avoid excessively long baths/showers, avoid excessively hot baths/showers, avoid excessive rubbing or scrubbing of skin, and to apply emollients and/or topical corticosteroids immediately after the bath/shower.
PROMIS Itch-Triggers is a simple and feasible checklist to screen for the most common itch triggers in AD in clinical practice (patients are asked to check off which of the following have caused their itch in the previous 7 days: cold temperature, hot temperature, heat, sweat, tight clothing, fragrances, boredom, talking about itch, stress, weather change, sunlight, humid air, dry air). “It takes less than 1 minute to complete,” he said. “Additional testing with skin patch and/or prick testing may be warranted to identify allergenic triggers.”
Dr. Silverberg reported that he is a consultant to and/or an advisory board member for several pharmaceutical companies. He is also a speaker for Regeneron and Sanofi and has received a grant from Galderma.
“Guidelines on trigger avoidance are written as if it’s easy to do,” Jonathan I. Silverberg, MD, PhD, MPH, said during the Revolutionizing Atopic Dermatitis virtual symposium. “It turns out that trigger avoidance is really complicated.”
He and his colleagues conducted a study of most common triggers for itch based on a prospective dermatology practice–based study of 587 adults with AD . About two-thirds (65%) reported one or more itch trigger in the past week and 36% had three or more itch triggers in the past week. The two most common triggers were stress (35%) and sweat (31%).
“To me, this is provocative, because this is not how I was trained in residency,” said Dr. Silverberg, director of clinical research in the division of dermatology at George Washington University, Washington. “I was trained that it’s all about excess showering, dry air, or cold temperature. Those are important, but the most common triggers are stress and sweat.”
AD triggers are also commonly linked to seasonality. “If you ask patients when their AD is worse, sometimes it’s winter,” he said. “Sometimes it’s spring. Sometimes it’s summer. It turns out that there is a distinct set of triggers that are associated with AD seasonality.” Wintertime worsening of disease is associated with cold temperature and weather change, he continued, while springtime worsening of disease is often linked to weather change and dry air. Common summertime triggers for flares include hot temperature, heat, sweat, weather change, sunlight, humid air, and dry air. “In the fall, the weather change again comes up as a trigger. Humid air does as well.”
In their prospective study, Dr. Silverberg and colleagues found that 90% of those who had at least three itch triggers reported 3 months or less of AD remission in the past year, “meaning that 90% are reporting persistent disease when they have multiple itch triggers,” he said. In addition, 78% reported two or more flares per year and 61% reported that AD is worse during certain seasons.
Potential mitigation strategies for stress include stress management, biofeedback, meditation, relaxation training, and mindfulness. “These don’t necessarily require expensive psychotherapy,” he said. Freely available iPhone apps can be incorporated into daily practice, such as Calm, Relax with Andrew Johnson, Nature Sounds Relax and Sleep, Breathe2Relax, and Headspace.
Many AD patients are sedentary and avoid vigorous physical activity owing to heat and sweat as triggers. Simple solutions include exercising in a cooler temperature environment, “not just using fans,” he said. “Take a quick shower right after working out and consider pre- and/or post treatment with topical medication.”
High temperature and sweating can be problematic at bedtime, he continued. Even if the indoor temperature is 70° F, that might jump to 85° F or 90° F under a thick blanket. “That heat can trigger itch and may cause sweating, which can trigger itch,” said Dr. Silverberg, who has AD and is director of patch testing at George Washington University. Potential solutions include using a lighter blanket, lowering the indoor temperature, and wearing breathable pajamas.
Dryness, another common AD trigger, can be secondary to a combination of low outdoor and/or indoor humidity. “Lower outdoor humidity is a particular problem in the wintertime, because cold air doesn’t hold moisture as well,” he said. “That’s why the air feels much dryer in the wintertime. There’s also a problem of indoor heating and cooling. Sometimes central air systems can lower humidity to the point where it’s bone dry.”
In an effort to determine the impact of specific climatic factors on the U.S. prevalence of AD, Dr. Silverberg and colleagues conducted a study using a merged analysis of the 2007 National Survey of Children’s Health from a representative sample of 91,642 children aged 0-17 years and 2006-2007 measurements from the National Climate Data Center and Weather Service. They found that childhood AD prevalence was increased in geographical areas that use more indoor heat and cooling and had lower outdoor humidity. “So, we see that there’s a direct correlate of this dryness issue that is leading to more AD throughout the U.S.,” he said.
Practical solutions to mitigate the effect of dry air on AD include opening windows to allow entry of moist air, “which can be particularly helpful in residences that are overheated,” he said. “I deal with this a lot in patients who live in dormitories. Use humidifiers to add moisture back into the air. Aim for 40%-50% indoor humidity to avoid mold and dust mites. It’s better to use demineralized water to reduce bacterial growth. This can be helpful for aeroallergies. Of note, there are really no well-done studies that have examined the efficacy of humidifiers in AD, but based on our anecdotal experience, this is a good way to go.”
Cold temperatures and trigger intense itch, even in the setting of high humidity. “For me personally, this is one of my most brutal triggers,” Dr. Silverberg said. “When I’m in a place with extremes of cold, I get a rapid onset of itch, a mix of itch and pain, particularly on the dorsal hands. For solutions, you can encourage patients to avoid extremely low temperatures, to bundle up, and to potentially use hand warmers or other heating devices.”
Clothing can be a trigger as well, especially tight-fitting clothes, hot and nonbreathable clothes, and large-diameter wool, which has been shown to induce itching and irritation. Mitigation strategies include wearing loose-fitting, lightweight, nonirritating fabric. “Traditional cotton and silk fabrics have mixed evidence in improving AD but are generally safe,” he said. “Ultra- or superfine merino wool has been shown to be nonpruritic. There is sparse evidence to support chemically treated/coated clothing for AD, but this may be an emerging area.”
Dr. Silverberg pointed out variability of cultural perspectives and preferences for bathing practices, including temperature, duration, frequency, optimal bathing products, and the use of loofahs and other scrubbing products. “This stems from different perceptions of what it means to be clean, and how dry our skin should feel after a shower,” he said. “Many clinicians and patients were taught that regular bathing is harmful in AD. It turns out that’s not true.”
In a recently published systematic review and meta-analysis of 13 studies, he and his colleagues examined efficacy outcomes of different bathing/showering regimens in AD. All 13 studies showed numerically reduced AD severity with any bathing regimen in at least one time point. Numerical decreases over time were observed for body surface area (BSA), Eczema Area and Severity Index (EASI), and/or SCORAD measures for daily and less than daily bathing, with or without application of emollients or topical corticosteroids. In random effects regression models, taking baths more than or less than seven times per week were not associated with significant differences of Cohen’s D scores for EASI, SCORAD, or BSA. “The take-home message here is, let your AD patients bathe,” Dr. Silverberg said. “Bathing is good. It can be channeled to help the eczema, but it has to be done the right way.”
Patients should be counseled to use nonirritating cleansers and shampoos, avoid excessively long baths/showers, avoid excessively hot baths/showers, avoid excessive rubbing or scrubbing of skin, and to apply emollients and/or topical corticosteroids immediately after the bath/shower.
PROMIS Itch-Triggers is a simple and feasible checklist to screen for the most common itch triggers in AD in clinical practice (patients are asked to check off which of the following have caused their itch in the previous 7 days: cold temperature, hot temperature, heat, sweat, tight clothing, fragrances, boredom, talking about itch, stress, weather change, sunlight, humid air, dry air). “It takes less than 1 minute to complete,” he said. “Additional testing with skin patch and/or prick testing may be warranted to identify allergenic triggers.”
Dr. Silverberg reported that he is a consultant to and/or an advisory board member for several pharmaceutical companies. He is also a speaker for Regeneron and Sanofi and has received a grant from Galderma.
FROM REVOLUTIONIZING AD 2020
Bezafibrate eased pruritus in patients with fibrosing cholangiopathies
Once-daily treatment with the lipid-lowering agent bezafibrate significantly reduced moderate to severe pruritis among patients with cholestasis, according to the findings of a multicenter, double-blind, randomized, placebo-controlled study (Fibrates for Itch, or FITCH).
Two weeks after completing treatment, 45% of bezafibrate recipients met the primary endpoint, reporting at least a 50% decrease in itch on a 10-point visual analog scale (VAS), compared with 11% of patients in the placebo group (P = .003). There was also a statistically significant decrease in serum alkaline phosphatase (ALP) levels from baseline (35% vs. 6%, respectively; P = .03) that corresponded with improved pruritus, and bezafibrate significantly improved both morning and evening pruritus. Bezafibrate was not associated with myalgia, rhabdomyolysis, or serum alanine transaminase elevations but did lead to a 3% increase in serum creatinine that “was not different from the placebo group,” wrote Elsemieke de Vries, MD, PhD, of the department of gastroenterology & hepatology at Tytgat Institute for Liver and Intestinal Research, Amsterdam, and of department of gastroenterology & metabolism at Amsterdam University Medical Centers, and associates. Their report is in Gastroenterology.
Up to 70% of patients with cholangitis experience pruritus. Current guidelines recommend management with cholestyramine, rifampin, naltrexone, or sertraline, but efficacy and tolerability are subpar, the investigators wrote. In a recent study, a selective inhibitor of an ileal bile acid transporter (GSK2330672) reduced pruritus in primary biliary cholangitis but frequently was associated with diarrhea. In another recent study of patients with primary biliary cholangitis who had responded inadequately to ursodeoxycholic acid (BEZURSO), bezafibrate induced biochemical responses that correlated with improvements in pruritis (a secondary endpoint).
Lysophosphatidic acid (LPA) has been implicated in cholangiopathy-associated pruritus but is not found in bile. However, biliary drainage rapidly improves severe itch in patients with primary biliary cholangitis. Therefore, Dr. de Vries and associates hypothesized that an as-yet unknown factor in bile contributes to pruritus in fibrosing cholangiopathies and that bezafibrate reduces itch by “alleviating hepatobiliary cholestasis and injury and, thereby, reducing formation and biliary secretion of this biliary factor X.”
The FITCH study, which was conducted at seven academic hospitals in the Netherlands and one in Spain, enrolled 74 patients 18 years and older with primary biliary cholangitis or primary or secondary sclerosing cholangitis who reported having pruritus with an intensity of at least 5 on the 10-point VAS at baseline (with 10 indicating “worst itch possible”; median, 7; interquartile range, 7-8). Patients with hepatocellular cholestasis caused by medications or pregnancy were excluded. Ages among most participants ranged from 30s to 50s, and approximately two-thirds were female. None had received another pruritus treatment within 10 days of enrollment, and prior treatment with bezafibrate was not allowed. Patients received once-daily bezafibrate (400 mg) or placebo tablets for 21 days, with visits to the outpatient clinic on days 0, 21, and 35.
There were no serious adverse events or new safety signals. One event of oral pain was considered possibly related to bezafibrate, and itch and jaundice worsened in two patients after completing treatment. As in the 24-month BEZURSO study, increases in serum creatinine were modest and similar between groups (3% with bezafibrate and 5% with placebo). Myalgia and increases in serum alanine transaminase were observed in BEZURSO but not in FITCH. However, the short treatment duration provides “no judgment on long-term safety [of bezafibrate] in complex diseases such as primary sclerosing cholangitis or primary biliary cholangitis,” the investigators wrote.
Four patients discontinued treatment – three stopped placebo because of “unbearable pruritus,” and one stopped bezafibrate after developing acute bacterial cholangitis that required emergency treatment. Although FITCH excluded patients whose estimated glomerular filtration rate was less than 60 mL/min per 1.73 m2, one such patient was accidentally enrolled. Her serum creatinine, measured in mmol/L, rose from 121 at baseline to 148 on day 21, and then dropped to 134 after 2 weeks off treatment.
The trial was supported by patient donations, the Netherlands Society of Gastroenterology, and Instituto de Salud Carlos III. The investigators reported having no conflicts of interest.
SOURCE: de Vries E et al. Gastroenterology. 2020 Oct 5. doi: 10.1053/j.gastro.2020.10.001.
Itch really matters to patients with cholestatic liver diseases, and effective treatment can make a significant difference to life quality. Although therapies exist for cholestatic itch (such as cholestyramine, rifampin, and naltrexone) recent data from the United Kingdom and United States suggest that therapy in practice is poor. It is likely that this results, at least in part, from the limitations of the existing treatments which can be unpleasant to take (cholestyramine) or difficult to use because of monitoring needs and side-effects (rifampin and naltrexone). Itch has therefore been identified as an area of real unmet need in cholestatic disease and there are a number of trials in progress or in set-up. This is extremely positive for patients.
The FITCH trial is one of the first of these “new generation” cholestatic itch trials to report and explore the efficacy of the PPAR-agonist bezafibrate in a mixed cholestatic population. Clear benefit was seen with around 50% of all disease groups meeting the primary endpoint and good drug tolerance. Is bezafibrate therefore the answer to cholestatic itch? The cautious answer is ... possibly, but more experience is needed. The trial duration was only 21 days, which means that long-term safety and efficacy remain to be explored. Bezafibrate is now being used in practice to treat cholestatic itch with effects similar to those reported in the trial. It is therefore clearly an important new option. Where it ultimately ends up in the treatment pathway only time and experience will tell.
David Jones, BM, BCh, PhD, is a professor of liver immunology at Newcastle University, Newcastle Upon Tyne, England. He reported having no disclosures relevant to this commentary.
Itch really matters to patients with cholestatic liver diseases, and effective treatment can make a significant difference to life quality. Although therapies exist for cholestatic itch (such as cholestyramine, rifampin, and naltrexone) recent data from the United Kingdom and United States suggest that therapy in practice is poor. It is likely that this results, at least in part, from the limitations of the existing treatments which can be unpleasant to take (cholestyramine) or difficult to use because of monitoring needs and side-effects (rifampin and naltrexone). Itch has therefore been identified as an area of real unmet need in cholestatic disease and there are a number of trials in progress or in set-up. This is extremely positive for patients.
The FITCH trial is one of the first of these “new generation” cholestatic itch trials to report and explore the efficacy of the PPAR-agonist bezafibrate in a mixed cholestatic population. Clear benefit was seen with around 50% of all disease groups meeting the primary endpoint and good drug tolerance. Is bezafibrate therefore the answer to cholestatic itch? The cautious answer is ... possibly, but more experience is needed. The trial duration was only 21 days, which means that long-term safety and efficacy remain to be explored. Bezafibrate is now being used in practice to treat cholestatic itch with effects similar to those reported in the trial. It is therefore clearly an important new option. Where it ultimately ends up in the treatment pathway only time and experience will tell.
David Jones, BM, BCh, PhD, is a professor of liver immunology at Newcastle University, Newcastle Upon Tyne, England. He reported having no disclosures relevant to this commentary.
Itch really matters to patients with cholestatic liver diseases, and effective treatment can make a significant difference to life quality. Although therapies exist for cholestatic itch (such as cholestyramine, rifampin, and naltrexone) recent data from the United Kingdom and United States suggest that therapy in practice is poor. It is likely that this results, at least in part, from the limitations of the existing treatments which can be unpleasant to take (cholestyramine) or difficult to use because of monitoring needs and side-effects (rifampin and naltrexone). Itch has therefore been identified as an area of real unmet need in cholestatic disease and there are a number of trials in progress or in set-up. This is extremely positive for patients.
The FITCH trial is one of the first of these “new generation” cholestatic itch trials to report and explore the efficacy of the PPAR-agonist bezafibrate in a mixed cholestatic population. Clear benefit was seen with around 50% of all disease groups meeting the primary endpoint and good drug tolerance. Is bezafibrate therefore the answer to cholestatic itch? The cautious answer is ... possibly, but more experience is needed. The trial duration was only 21 days, which means that long-term safety and efficacy remain to be explored. Bezafibrate is now being used in practice to treat cholestatic itch with effects similar to those reported in the trial. It is therefore clearly an important new option. Where it ultimately ends up in the treatment pathway only time and experience will tell.
David Jones, BM, BCh, PhD, is a professor of liver immunology at Newcastle University, Newcastle Upon Tyne, England. He reported having no disclosures relevant to this commentary.
Once-daily treatment with the lipid-lowering agent bezafibrate significantly reduced moderate to severe pruritis among patients with cholestasis, according to the findings of a multicenter, double-blind, randomized, placebo-controlled study (Fibrates for Itch, or FITCH).
Two weeks after completing treatment, 45% of bezafibrate recipients met the primary endpoint, reporting at least a 50% decrease in itch on a 10-point visual analog scale (VAS), compared with 11% of patients in the placebo group (P = .003). There was also a statistically significant decrease in serum alkaline phosphatase (ALP) levels from baseline (35% vs. 6%, respectively; P = .03) that corresponded with improved pruritus, and bezafibrate significantly improved both morning and evening pruritus. Bezafibrate was not associated with myalgia, rhabdomyolysis, or serum alanine transaminase elevations but did lead to a 3% increase in serum creatinine that “was not different from the placebo group,” wrote Elsemieke de Vries, MD, PhD, of the department of gastroenterology & hepatology at Tytgat Institute for Liver and Intestinal Research, Amsterdam, and of department of gastroenterology & metabolism at Amsterdam University Medical Centers, and associates. Their report is in Gastroenterology.
Up to 70% of patients with cholangitis experience pruritus. Current guidelines recommend management with cholestyramine, rifampin, naltrexone, or sertraline, but efficacy and tolerability are subpar, the investigators wrote. In a recent study, a selective inhibitor of an ileal bile acid transporter (GSK2330672) reduced pruritus in primary biliary cholangitis but frequently was associated with diarrhea. In another recent study of patients with primary biliary cholangitis who had responded inadequately to ursodeoxycholic acid (BEZURSO), bezafibrate induced biochemical responses that correlated with improvements in pruritis (a secondary endpoint).
Lysophosphatidic acid (LPA) has been implicated in cholangiopathy-associated pruritus but is not found in bile. However, biliary drainage rapidly improves severe itch in patients with primary biliary cholangitis. Therefore, Dr. de Vries and associates hypothesized that an as-yet unknown factor in bile contributes to pruritus in fibrosing cholangiopathies and that bezafibrate reduces itch by “alleviating hepatobiliary cholestasis and injury and, thereby, reducing formation and biliary secretion of this biliary factor X.”
The FITCH study, which was conducted at seven academic hospitals in the Netherlands and one in Spain, enrolled 74 patients 18 years and older with primary biliary cholangitis or primary or secondary sclerosing cholangitis who reported having pruritus with an intensity of at least 5 on the 10-point VAS at baseline (with 10 indicating “worst itch possible”; median, 7; interquartile range, 7-8). Patients with hepatocellular cholestasis caused by medications or pregnancy were excluded. Ages among most participants ranged from 30s to 50s, and approximately two-thirds were female. None had received another pruritus treatment within 10 days of enrollment, and prior treatment with bezafibrate was not allowed. Patients received once-daily bezafibrate (400 mg) or placebo tablets for 21 days, with visits to the outpatient clinic on days 0, 21, and 35.
There were no serious adverse events or new safety signals. One event of oral pain was considered possibly related to bezafibrate, and itch and jaundice worsened in two patients after completing treatment. As in the 24-month BEZURSO study, increases in serum creatinine were modest and similar between groups (3% with bezafibrate and 5% with placebo). Myalgia and increases in serum alanine transaminase were observed in BEZURSO but not in FITCH. However, the short treatment duration provides “no judgment on long-term safety [of bezafibrate] in complex diseases such as primary sclerosing cholangitis or primary biliary cholangitis,” the investigators wrote.
Four patients discontinued treatment – three stopped placebo because of “unbearable pruritus,” and one stopped bezafibrate after developing acute bacterial cholangitis that required emergency treatment. Although FITCH excluded patients whose estimated glomerular filtration rate was less than 60 mL/min per 1.73 m2, one such patient was accidentally enrolled. Her serum creatinine, measured in mmol/L, rose from 121 at baseline to 148 on day 21, and then dropped to 134 after 2 weeks off treatment.
The trial was supported by patient donations, the Netherlands Society of Gastroenterology, and Instituto de Salud Carlos III. The investigators reported having no conflicts of interest.
SOURCE: de Vries E et al. Gastroenterology. 2020 Oct 5. doi: 10.1053/j.gastro.2020.10.001.
Once-daily treatment with the lipid-lowering agent bezafibrate significantly reduced moderate to severe pruritis among patients with cholestasis, according to the findings of a multicenter, double-blind, randomized, placebo-controlled study (Fibrates for Itch, or FITCH).
Two weeks after completing treatment, 45% of bezafibrate recipients met the primary endpoint, reporting at least a 50% decrease in itch on a 10-point visual analog scale (VAS), compared with 11% of patients in the placebo group (P = .003). There was also a statistically significant decrease in serum alkaline phosphatase (ALP) levels from baseline (35% vs. 6%, respectively; P = .03) that corresponded with improved pruritus, and bezafibrate significantly improved both morning and evening pruritus. Bezafibrate was not associated with myalgia, rhabdomyolysis, or serum alanine transaminase elevations but did lead to a 3% increase in serum creatinine that “was not different from the placebo group,” wrote Elsemieke de Vries, MD, PhD, of the department of gastroenterology & hepatology at Tytgat Institute for Liver and Intestinal Research, Amsterdam, and of department of gastroenterology & metabolism at Amsterdam University Medical Centers, and associates. Their report is in Gastroenterology.
Up to 70% of patients with cholangitis experience pruritus. Current guidelines recommend management with cholestyramine, rifampin, naltrexone, or sertraline, but efficacy and tolerability are subpar, the investigators wrote. In a recent study, a selective inhibitor of an ileal bile acid transporter (GSK2330672) reduced pruritus in primary biliary cholangitis but frequently was associated with diarrhea. In another recent study of patients with primary biliary cholangitis who had responded inadequately to ursodeoxycholic acid (BEZURSO), bezafibrate induced biochemical responses that correlated with improvements in pruritis (a secondary endpoint).
Lysophosphatidic acid (LPA) has been implicated in cholangiopathy-associated pruritus but is not found in bile. However, biliary drainage rapidly improves severe itch in patients with primary biliary cholangitis. Therefore, Dr. de Vries and associates hypothesized that an as-yet unknown factor in bile contributes to pruritus in fibrosing cholangiopathies and that bezafibrate reduces itch by “alleviating hepatobiliary cholestasis and injury and, thereby, reducing formation and biliary secretion of this biliary factor X.”
The FITCH study, which was conducted at seven academic hospitals in the Netherlands and one in Spain, enrolled 74 patients 18 years and older with primary biliary cholangitis or primary or secondary sclerosing cholangitis who reported having pruritus with an intensity of at least 5 on the 10-point VAS at baseline (with 10 indicating “worst itch possible”; median, 7; interquartile range, 7-8). Patients with hepatocellular cholestasis caused by medications or pregnancy were excluded. Ages among most participants ranged from 30s to 50s, and approximately two-thirds were female. None had received another pruritus treatment within 10 days of enrollment, and prior treatment with bezafibrate was not allowed. Patients received once-daily bezafibrate (400 mg) or placebo tablets for 21 days, with visits to the outpatient clinic on days 0, 21, and 35.
There were no serious adverse events or new safety signals. One event of oral pain was considered possibly related to bezafibrate, and itch and jaundice worsened in two patients after completing treatment. As in the 24-month BEZURSO study, increases in serum creatinine were modest and similar between groups (3% with bezafibrate and 5% with placebo). Myalgia and increases in serum alanine transaminase were observed in BEZURSO but not in FITCH. However, the short treatment duration provides “no judgment on long-term safety [of bezafibrate] in complex diseases such as primary sclerosing cholangitis or primary biliary cholangitis,” the investigators wrote.
Four patients discontinued treatment – three stopped placebo because of “unbearable pruritus,” and one stopped bezafibrate after developing acute bacterial cholangitis that required emergency treatment. Although FITCH excluded patients whose estimated glomerular filtration rate was less than 60 mL/min per 1.73 m2, one such patient was accidentally enrolled. Her serum creatinine, measured in mmol/L, rose from 121 at baseline to 148 on day 21, and then dropped to 134 after 2 weeks off treatment.
The trial was supported by patient donations, the Netherlands Society of Gastroenterology, and Instituto de Salud Carlos III. The investigators reported having no conflicts of interest.
SOURCE: de Vries E et al. Gastroenterology. 2020 Oct 5. doi: 10.1053/j.gastro.2020.10.001.
FROM GASTROENTEROLOGY
Expanding Verrucous Plaque on the Face
The Diagnosis: Blastomycosis
Histopathologic examination of 3 punch biopsies from the left side of the upper lip showed pseudoepitheliomatous hyperplasia with intraepidermal microabscesses and dermal suppurative granulomatous inflammation (Figure 1A). Stains were negative for periodic acid-Schiff, herpes simplex virus, and varicella-zoster virus. Direct and indirect immunofluorescence for skin autoantibodies were negative. Two separate tissue culture specimens showed no bacterial, fungal, or mycobacterial growth. Leishmania polymerase chain reaction and DNA sequencing were negative. An additional punch biopsy revealed yeast forms with broad-based budding and refractile walls (Figures 1B and 1C) that were highlighted with Grocott-Gomori methenamine-silver stain of the tissue (Figure 2). Chest radiography demonstrated no pulmonary involvement. In collaboration with an infectious disease specialist, the patient was started on itraconazole 200 mg twice daily for a total of 6 months.
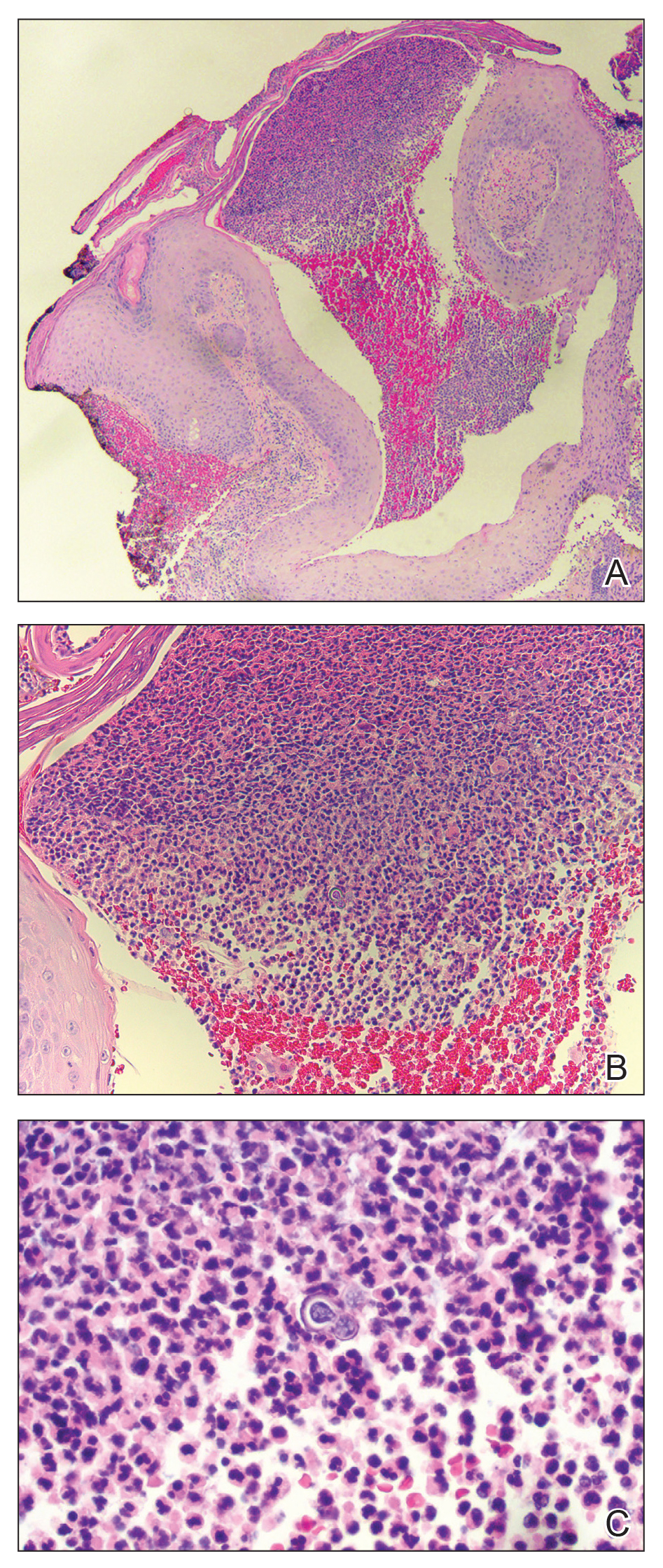
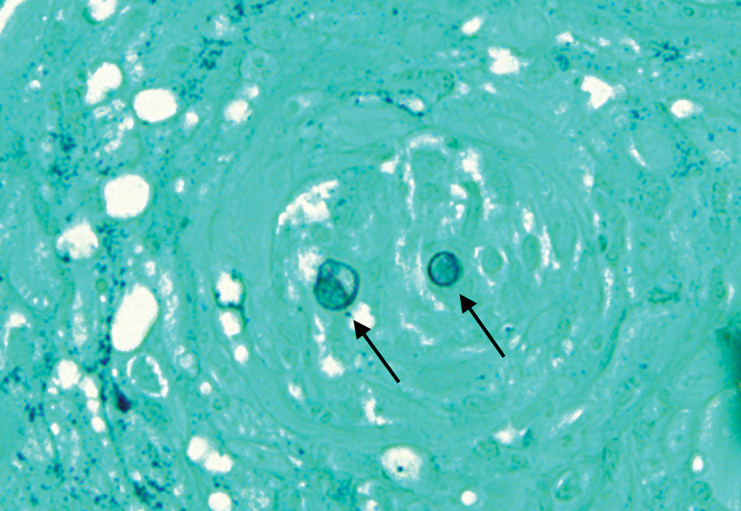
Blastomycosis is a fungal infection caused by Blastomyces dermatitidis, a thermally dimorphic fungus endemic in the soils of the Ohio and Mississippi River valleys and southeastern United States.1 It most commonly manifests as a pulmonary infection following inhalation of spores that are transformed into thick-walled yeasts capable of evading the host's immune system. Unlike other deep fungal infections, blastomycosis occurs in both immunocompetent and immunocompromised hosts. Extrapulmonary disease after hematogenous dissemination from the lungs occurs in approximately 25% to 30% of patients, with the skin as the most common site of dissemination.2 Clinically, cutaneous blastomycosis typically starts as papules that evolve into crusted vegetative plaques, often with central clearing or ulceration. Primary cutaneous blastomycosis is rare and occurs due to direct inoculation after trauma to the skin via an infected animal bite, direct inoculation in laboratory settings, or due to injury during outdoor activities involving contact with soil.3 Given our patient's horticultural hobbies, lack of pulmonary symptoms, and negative radiologic examination, primary cutaneous blastomycosis infection due to direct inoculation from contaminated soil was a possibility; however, definite confirmation was difficult, as the primary pulmonary infection of blastomycosis can be asymptomatic and therefore often goes undetected.
Cutaneous blastomycosis can be mistaken for pemphigus vegetans, leishmaniasis, herpes vegetans, bacterial pyoderma, and other deep fungal infections that also display pseudoepitheliomatous hyperplasia with pyogranulomatous inflammation on histopathology. Direct visualization of the characteristic yeast forms in a histologic specimen or the growth of fungus in culture is essential for a definitive diagnosis. The yeasts are 8 to 15 µm in diameter with thick, double-contoured walls and characteristically display broad-based budding.4 This budding pattern aids in differentiating blastomycosis from other entities with a similar histopathologic appearance. Chromoblastomycosis would show brown, thick-walled fungal cells inside giant cells, while coccidioidomycosis displays large spherules containing endospores, and leishmaniasis demonstrates amastigotes (small oval organisms with a bar-shaped kinetoplast) highlighted with Giemsa staining. Pemphigus vegetans would show intercellular deposition of IgG on direct immunofluorescence. Blastomyces dermatitidis can be difficult to visualize with routine hematoxylin and eosin stains, and it is important to note that a negative result does not exclude the possibility of blastomycosis, as demonstrated in our case.4 Special stains including Grocott-Gomori methenamine-silver and periodic acid-Schiff can aid in examining tissue for the presence of fungal elements, which typically can be found within histiocytes or abscesses in the dermis. Culture is the most sensitive method for detecting and diagnosing blastomycosis. Growth typically is detected in 5 to 10 days but can take up to 30 days if few organisms are present in the specimen.1
Although spontaneous remission can occur, it is recommended that all patients with cutaneous blastomycosis be treated to avoid dissemination and recurrence. Itraconazole currently is the treatment of choice.5 Doses typically are 200 to 400 mg/d for 8 to 12 months.6 Itraconazole-related side effects experienced by our patient during his 6-month treatment course included leg edema, 20-lb weight gain, gastrointestinal upset, blurred vision, and a transient increase in blood pressure, all resolving once the medication was discontinued. Complete resolution of the lesion was noted at the completion of the treatment course. At a 6-month posttreatment follow-up, residual scarring and alopecia were noted in parts of the previously affected areas of the upper cutaneous lip and nasolabial fold.
- Saccente M, Woods GL. Clinical and laboratory update on blastomycosis. Clin Microbiol Rev. 2010;23:367-831.
- Chapman SW, Lin AC, Hendricks KA, et al. Endemic blastomycosis in Mississippi: epidemiological and clinical studies. Semin Respir Infect. 1997;12:219-228.
- Gray NA, Baddour LM. Cutaneous inoculation blastomycosis. Clin Infect Dis. 2002;34:E44-E49.
- Patel AJ, Gattuso P, Reddy VB. Diagnosis of blastomycosis in surgical pathology and cytopathology: correlation with microbiologic culture. Am J Surg Pathol. 2010;34:256-261.
- Chapman SW, Dismukes WE, Proia LA, et al. Clinical practice guidelines for the management of blastomycosis: 2008 update by the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:1801-1812.
- Lomaestro, BM, Piatek MA. Update on drug interactions with azole antifungal agents. Ann Pharmacother. 1998;32:915-928.
The Diagnosis: Blastomycosis
Histopathologic examination of 3 punch biopsies from the left side of the upper lip showed pseudoepitheliomatous hyperplasia with intraepidermal microabscesses and dermal suppurative granulomatous inflammation (Figure 1A). Stains were negative for periodic acid-Schiff, herpes simplex virus, and varicella-zoster virus. Direct and indirect immunofluorescence for skin autoantibodies were negative. Two separate tissue culture specimens showed no bacterial, fungal, or mycobacterial growth. Leishmania polymerase chain reaction and DNA sequencing were negative. An additional punch biopsy revealed yeast forms with broad-based budding and refractile walls (Figures 1B and 1C) that were highlighted with Grocott-Gomori methenamine-silver stain of the tissue (Figure 2). Chest radiography demonstrated no pulmonary involvement. In collaboration with an infectious disease specialist, the patient was started on itraconazole 200 mg twice daily for a total of 6 months.


Blastomycosis is a fungal infection caused by Blastomyces dermatitidis, a thermally dimorphic fungus endemic in the soils of the Ohio and Mississippi River valleys and southeastern United States.1 It most commonly manifests as a pulmonary infection following inhalation of spores that are transformed into thick-walled yeasts capable of evading the host's immune system. Unlike other deep fungal infections, blastomycosis occurs in both immunocompetent and immunocompromised hosts. Extrapulmonary disease after hematogenous dissemination from the lungs occurs in approximately 25% to 30% of patients, with the skin as the most common site of dissemination.2 Clinically, cutaneous blastomycosis typically starts as papules that evolve into crusted vegetative plaques, often with central clearing or ulceration. Primary cutaneous blastomycosis is rare and occurs due to direct inoculation after trauma to the skin via an infected animal bite, direct inoculation in laboratory settings, or due to injury during outdoor activities involving contact with soil.3 Given our patient's horticultural hobbies, lack of pulmonary symptoms, and negative radiologic examination, primary cutaneous blastomycosis infection due to direct inoculation from contaminated soil was a possibility; however, definite confirmation was difficult, as the primary pulmonary infection of blastomycosis can be asymptomatic and therefore often goes undetected.
Cutaneous blastomycosis can be mistaken for pemphigus vegetans, leishmaniasis, herpes vegetans, bacterial pyoderma, and other deep fungal infections that also display pseudoepitheliomatous hyperplasia with pyogranulomatous inflammation on histopathology. Direct visualization of the characteristic yeast forms in a histologic specimen or the growth of fungus in culture is essential for a definitive diagnosis. The yeasts are 8 to 15 µm in diameter with thick, double-contoured walls and characteristically display broad-based budding.4 This budding pattern aids in differentiating blastomycosis from other entities with a similar histopathologic appearance. Chromoblastomycosis would show brown, thick-walled fungal cells inside giant cells, while coccidioidomycosis displays large spherules containing endospores, and leishmaniasis demonstrates amastigotes (small oval organisms with a bar-shaped kinetoplast) highlighted with Giemsa staining. Pemphigus vegetans would show intercellular deposition of IgG on direct immunofluorescence. Blastomyces dermatitidis can be difficult to visualize with routine hematoxylin and eosin stains, and it is important to note that a negative result does not exclude the possibility of blastomycosis, as demonstrated in our case.4 Special stains including Grocott-Gomori methenamine-silver and periodic acid-Schiff can aid in examining tissue for the presence of fungal elements, which typically can be found within histiocytes or abscesses in the dermis. Culture is the most sensitive method for detecting and diagnosing blastomycosis. Growth typically is detected in 5 to 10 days but can take up to 30 days if few organisms are present in the specimen.1
Although spontaneous remission can occur, it is recommended that all patients with cutaneous blastomycosis be treated to avoid dissemination and recurrence. Itraconazole currently is the treatment of choice.5 Doses typically are 200 to 400 mg/d for 8 to 12 months.6 Itraconazole-related side effects experienced by our patient during his 6-month treatment course included leg edema, 20-lb weight gain, gastrointestinal upset, blurred vision, and a transient increase in blood pressure, all resolving once the medication was discontinued. Complete resolution of the lesion was noted at the completion of the treatment course. At a 6-month posttreatment follow-up, residual scarring and alopecia were noted in parts of the previously affected areas of the upper cutaneous lip and nasolabial fold.
The Diagnosis: Blastomycosis
Histopathologic examination of 3 punch biopsies from the left side of the upper lip showed pseudoepitheliomatous hyperplasia with intraepidermal microabscesses and dermal suppurative granulomatous inflammation (Figure 1A). Stains were negative for periodic acid-Schiff, herpes simplex virus, and varicella-zoster virus. Direct and indirect immunofluorescence for skin autoantibodies were negative. Two separate tissue culture specimens showed no bacterial, fungal, or mycobacterial growth. Leishmania polymerase chain reaction and DNA sequencing were negative. An additional punch biopsy revealed yeast forms with broad-based budding and refractile walls (Figures 1B and 1C) that were highlighted with Grocott-Gomori methenamine-silver stain of the tissue (Figure 2). Chest radiography demonstrated no pulmonary involvement. In collaboration with an infectious disease specialist, the patient was started on itraconazole 200 mg twice daily for a total of 6 months.


Blastomycosis is a fungal infection caused by Blastomyces dermatitidis, a thermally dimorphic fungus endemic in the soils of the Ohio and Mississippi River valleys and southeastern United States.1 It most commonly manifests as a pulmonary infection following inhalation of spores that are transformed into thick-walled yeasts capable of evading the host's immune system. Unlike other deep fungal infections, blastomycosis occurs in both immunocompetent and immunocompromised hosts. Extrapulmonary disease after hematogenous dissemination from the lungs occurs in approximately 25% to 30% of patients, with the skin as the most common site of dissemination.2 Clinically, cutaneous blastomycosis typically starts as papules that evolve into crusted vegetative plaques, often with central clearing or ulceration. Primary cutaneous blastomycosis is rare and occurs due to direct inoculation after trauma to the skin via an infected animal bite, direct inoculation in laboratory settings, or due to injury during outdoor activities involving contact with soil.3 Given our patient's horticultural hobbies, lack of pulmonary symptoms, and negative radiologic examination, primary cutaneous blastomycosis infection due to direct inoculation from contaminated soil was a possibility; however, definite confirmation was difficult, as the primary pulmonary infection of blastomycosis can be asymptomatic and therefore often goes undetected.
Cutaneous blastomycosis can be mistaken for pemphigus vegetans, leishmaniasis, herpes vegetans, bacterial pyoderma, and other deep fungal infections that also display pseudoepitheliomatous hyperplasia with pyogranulomatous inflammation on histopathology. Direct visualization of the characteristic yeast forms in a histologic specimen or the growth of fungus in culture is essential for a definitive diagnosis. The yeasts are 8 to 15 µm in diameter with thick, double-contoured walls and characteristically display broad-based budding.4 This budding pattern aids in differentiating blastomycosis from other entities with a similar histopathologic appearance. Chromoblastomycosis would show brown, thick-walled fungal cells inside giant cells, while coccidioidomycosis displays large spherules containing endospores, and leishmaniasis demonstrates amastigotes (small oval organisms with a bar-shaped kinetoplast) highlighted with Giemsa staining. Pemphigus vegetans would show intercellular deposition of IgG on direct immunofluorescence. Blastomyces dermatitidis can be difficult to visualize with routine hematoxylin and eosin stains, and it is important to note that a negative result does not exclude the possibility of blastomycosis, as demonstrated in our case.4 Special stains including Grocott-Gomori methenamine-silver and periodic acid-Schiff can aid in examining tissue for the presence of fungal elements, which typically can be found within histiocytes or abscesses in the dermis. Culture is the most sensitive method for detecting and diagnosing blastomycosis. Growth typically is detected in 5 to 10 days but can take up to 30 days if few organisms are present in the specimen.1
Although spontaneous remission can occur, it is recommended that all patients with cutaneous blastomycosis be treated to avoid dissemination and recurrence. Itraconazole currently is the treatment of choice.5 Doses typically are 200 to 400 mg/d for 8 to 12 months.6 Itraconazole-related side effects experienced by our patient during his 6-month treatment course included leg edema, 20-lb weight gain, gastrointestinal upset, blurred vision, and a transient increase in blood pressure, all resolving once the medication was discontinued. Complete resolution of the lesion was noted at the completion of the treatment course. At a 6-month posttreatment follow-up, residual scarring and alopecia were noted in parts of the previously affected areas of the upper cutaneous lip and nasolabial fold.
- Saccente M, Woods GL. Clinical and laboratory update on blastomycosis. Clin Microbiol Rev. 2010;23:367-831.
- Chapman SW, Lin AC, Hendricks KA, et al. Endemic blastomycosis in Mississippi: epidemiological and clinical studies. Semin Respir Infect. 1997;12:219-228.
- Gray NA, Baddour LM. Cutaneous inoculation blastomycosis. Clin Infect Dis. 2002;34:E44-E49.
- Patel AJ, Gattuso P, Reddy VB. Diagnosis of blastomycosis in surgical pathology and cytopathology: correlation with microbiologic culture. Am J Surg Pathol. 2010;34:256-261.
- Chapman SW, Dismukes WE, Proia LA, et al. Clinical practice guidelines for the management of blastomycosis: 2008 update by the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:1801-1812.
- Lomaestro, BM, Piatek MA. Update on drug interactions with azole antifungal agents. Ann Pharmacother. 1998;32:915-928.
- Saccente M, Woods GL. Clinical and laboratory update on blastomycosis. Clin Microbiol Rev. 2010;23:367-831.
- Chapman SW, Lin AC, Hendricks KA, et al. Endemic blastomycosis in Mississippi: epidemiological and clinical studies. Semin Respir Infect. 1997;12:219-228.
- Gray NA, Baddour LM. Cutaneous inoculation blastomycosis. Clin Infect Dis. 2002;34:E44-E49.
- Patel AJ, Gattuso P, Reddy VB. Diagnosis of blastomycosis in surgical pathology and cytopathology: correlation with microbiologic culture. Am J Surg Pathol. 2010;34:256-261.
- Chapman SW, Dismukes WE, Proia LA, et al. Clinical practice guidelines for the management of blastomycosis: 2008 update by the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:1801-1812.
- Lomaestro, BM, Piatek MA. Update on drug interactions with azole antifungal agents. Ann Pharmacother. 1998;32:915-928.
A 69-year-old man presented with a slowly expanding, verrucous plaque on the left side of the upper cutaneous lip of 4 months’ duration. The lesion reportedly began as an abscess and had undergone incision and drainage followed by multiple courses of oral antibiotics that were unsuccessful prior to presentation to our clinic. The patient’s hobbies included gardening near his summer home in the mountains of western North Carolina, where he resided when the lesion appeared. Physical examination revealed an approximately 6×4-cm verrucous plaque with central ulceration on the left side of the upper cutaneous and vermilion lip extending to the nasolabial fold. A review of systems was negative for any systemic symptoms. Routine laboratory tests and computed tomography of the head and neck were normal.
Study confirms key COVID-19 risk factors in children
Children and adolescents who receive positive COVID-19 test results are not only more likely to have been in close contact with someone with a confirmed case of the virus but also are less likely to have reported consistent mask use among students and staff inside the school they attended, reported Charlotte V. Hobbs, MD, and colleagues at the University of Mississippi, Jackson.
In partnership with the Centers for Disease Control and Prevention’s COVID-19 Response Team, Dr. Hobbs and colleagues conducted a case-control study of 397 children and adolescents under 18 years of age to assess school, community, and close contact exposures associated with pediatric COVID-19. Patients tested for COVID-19 at outpatient health centers or emergency departments affiliated with the University of Mississippi Medical Center between Sept. 1 and Nov. 5, 2020, were included in the study.
Nearly two-thirds reported that exposure came from family members
Of the total study participants observed, 82 (21%) were under 4 years of age; 214 (54%) were female; 217 (55%) were non-Hispanic black, and 145 (37%) were non-Hispanic white. More than half (53%) sought testing because of COVID-19 symptoms. Of those who tested positive, 66% reported having come into close contact with a COVID-19 case, and 64% reported that those contacts were family members, compared with 15% of contacts who were schoolmates and 27% who were child care classmates.
All participants completed in-person school or child care attendance less than 14 days before testing positive for the virus, including 62% of patients testing positive and 68% of those testing negative. The authors noted that school attendance itself was not found to be associated with any positive test results. In fact, parents in 64% of positive cases and 76% of negative cases reported mask wearing among children and staff inside places of learning.
Of those study participants testing positive who did come into close contact with someone with COVID-19, the contacts were more likely to be family members than school or child care classmates. Specifically, they were more likely, in the 2-week period preceding testing, to have attended gatherings with individuals outside their immediate households, including social events and activities with other children. Parents of students testing positive were also less likely to report consistent indoor mask use among their children older than 2 years and school staff members.
School attendance was not found to increase likelihood of testing positive
Attending in-person school or child care during the 2 weeks before the SARS-CoV-2 test was not associated with greater likelihood of testing positive, the study authors noted, adding that the majority of study respondents reported universal mask use inside school and child care facilities, consistent with Mississippi State Department of Health recommended guidelines.
Dr. Hobbs and colleagues reported at least four limitations of the study. They noted that the study participants may not be representative of youth in other geographic regions of the country. They considered the possibility of unmeasured confounding of participant behaviors that may not have been factored into the study. No attempt was made to verify parent claims of mask use at schools and child care programs. Lastly, they acknowledged that “case or control status might be subject to misclassification because of imperfect sensitivity or specificity of PCR-based testing.
As of Dec. 14, 2020, the CDC reported that 10.2% of all COVID-19 cases in the United States were in children and adolescents under the age of 18.
“Continued efforts to prevent transmission at schools and child care programs are important, as are assessments of various types of activities and exposures to identify risk factors for COVID-19 as children engage in classroom and social interactions.” Promoting behaviors to reduce exposures to the virus among youth in the household, the community, schools, and child care programs is important to preventing outbreaks of the virus at schools, the authors cautioned.
In a separate interview with this news organization, Karalyn Kinsella, MD, general pediatrician in a small group private practice in Cheshire, Conn., said, “What this report tells me is that COVID cases are more common when mask use is inconsistent in schools and at home and in schools that don’t properly adhere to CDC guidelines. Overall, so long as social distancing guidelines are followed, schools are pretty safe places for kids during this pandemic.”
This finding is important, since many families are keeping their children out of school over fears of contracting the virus, she added. Some of the consequences these children are suffering include a lack of social connection and structure, which in some cases is leading to worsening anxiety and depression, and for those with disabilities, such as those who receive physical therapy, occupational therapy, speech or have IEPs, they’re not getting the full benefit of the services that they would otherwise receive in person, she observed.
“I don’t think families really understand the risks of getting together with family or friends “in their bubble” or the risk of continuing sports participation. This is where the majority of COVID cases are coming from,” she said, adding that it is important to discuss this risk with them at appointments. So, when families ask us what we think of in-person learning, I think we should feel fairly confident that the benefit may outweigh the risk.”
Dr. Hobbs and colleagues, and Dr. Kinsella, had no conflicts of interest to report.
SOURCE: MMWR Morb Mortal Wkly Rep. 2020;69:1925-9. doi: 10.15585/mmwr.mm6950e3.
Children and adolescents who receive positive COVID-19 test results are not only more likely to have been in close contact with someone with a confirmed case of the virus but also are less likely to have reported consistent mask use among students and staff inside the school they attended, reported Charlotte V. Hobbs, MD, and colleagues at the University of Mississippi, Jackson.
In partnership with the Centers for Disease Control and Prevention’s COVID-19 Response Team, Dr. Hobbs and colleagues conducted a case-control study of 397 children and adolescents under 18 years of age to assess school, community, and close contact exposures associated with pediatric COVID-19. Patients tested for COVID-19 at outpatient health centers or emergency departments affiliated with the University of Mississippi Medical Center between Sept. 1 and Nov. 5, 2020, were included in the study.
Nearly two-thirds reported that exposure came from family members
Of the total study participants observed, 82 (21%) were under 4 years of age; 214 (54%) were female; 217 (55%) were non-Hispanic black, and 145 (37%) were non-Hispanic white. More than half (53%) sought testing because of COVID-19 symptoms. Of those who tested positive, 66% reported having come into close contact with a COVID-19 case, and 64% reported that those contacts were family members, compared with 15% of contacts who were schoolmates and 27% who were child care classmates.
All participants completed in-person school or child care attendance less than 14 days before testing positive for the virus, including 62% of patients testing positive and 68% of those testing negative. The authors noted that school attendance itself was not found to be associated with any positive test results. In fact, parents in 64% of positive cases and 76% of negative cases reported mask wearing among children and staff inside places of learning.
Of those study participants testing positive who did come into close contact with someone with COVID-19, the contacts were more likely to be family members than school or child care classmates. Specifically, they were more likely, in the 2-week period preceding testing, to have attended gatherings with individuals outside their immediate households, including social events and activities with other children. Parents of students testing positive were also less likely to report consistent indoor mask use among their children older than 2 years and school staff members.
School attendance was not found to increase likelihood of testing positive
Attending in-person school or child care during the 2 weeks before the SARS-CoV-2 test was not associated with greater likelihood of testing positive, the study authors noted, adding that the majority of study respondents reported universal mask use inside school and child care facilities, consistent with Mississippi State Department of Health recommended guidelines.
Dr. Hobbs and colleagues reported at least four limitations of the study. They noted that the study participants may not be representative of youth in other geographic regions of the country. They considered the possibility of unmeasured confounding of participant behaviors that may not have been factored into the study. No attempt was made to verify parent claims of mask use at schools and child care programs. Lastly, they acknowledged that “case or control status might be subject to misclassification because of imperfect sensitivity or specificity of PCR-based testing.
As of Dec. 14, 2020, the CDC reported that 10.2% of all COVID-19 cases in the United States were in children and adolescents under the age of 18.
“Continued efforts to prevent transmission at schools and child care programs are important, as are assessments of various types of activities and exposures to identify risk factors for COVID-19 as children engage in classroom and social interactions.” Promoting behaviors to reduce exposures to the virus among youth in the household, the community, schools, and child care programs is important to preventing outbreaks of the virus at schools, the authors cautioned.
In a separate interview with this news organization, Karalyn Kinsella, MD, general pediatrician in a small group private practice in Cheshire, Conn., said, “What this report tells me is that COVID cases are more common when mask use is inconsistent in schools and at home and in schools that don’t properly adhere to CDC guidelines. Overall, so long as social distancing guidelines are followed, schools are pretty safe places for kids during this pandemic.”
This finding is important, since many families are keeping their children out of school over fears of contracting the virus, she added. Some of the consequences these children are suffering include a lack of social connection and structure, which in some cases is leading to worsening anxiety and depression, and for those with disabilities, such as those who receive physical therapy, occupational therapy, speech or have IEPs, they’re not getting the full benefit of the services that they would otherwise receive in person, she observed.
“I don’t think families really understand the risks of getting together with family or friends “in their bubble” or the risk of continuing sports participation. This is where the majority of COVID cases are coming from,” she said, adding that it is important to discuss this risk with them at appointments. So, when families ask us what we think of in-person learning, I think we should feel fairly confident that the benefit may outweigh the risk.”
Dr. Hobbs and colleagues, and Dr. Kinsella, had no conflicts of interest to report.
SOURCE: MMWR Morb Mortal Wkly Rep. 2020;69:1925-9. doi: 10.15585/mmwr.mm6950e3.
Children and adolescents who receive positive COVID-19 test results are not only more likely to have been in close contact with someone with a confirmed case of the virus but also are less likely to have reported consistent mask use among students and staff inside the school they attended, reported Charlotte V. Hobbs, MD, and colleagues at the University of Mississippi, Jackson.
In partnership with the Centers for Disease Control and Prevention’s COVID-19 Response Team, Dr. Hobbs and colleagues conducted a case-control study of 397 children and adolescents under 18 years of age to assess school, community, and close contact exposures associated with pediatric COVID-19. Patients tested for COVID-19 at outpatient health centers or emergency departments affiliated with the University of Mississippi Medical Center between Sept. 1 and Nov. 5, 2020, were included in the study.
Nearly two-thirds reported that exposure came from family members
Of the total study participants observed, 82 (21%) were under 4 years of age; 214 (54%) were female; 217 (55%) were non-Hispanic black, and 145 (37%) were non-Hispanic white. More than half (53%) sought testing because of COVID-19 symptoms. Of those who tested positive, 66% reported having come into close contact with a COVID-19 case, and 64% reported that those contacts were family members, compared with 15% of contacts who were schoolmates and 27% who were child care classmates.
All participants completed in-person school or child care attendance less than 14 days before testing positive for the virus, including 62% of patients testing positive and 68% of those testing negative. The authors noted that school attendance itself was not found to be associated with any positive test results. In fact, parents in 64% of positive cases and 76% of negative cases reported mask wearing among children and staff inside places of learning.
Of those study participants testing positive who did come into close contact with someone with COVID-19, the contacts were more likely to be family members than school or child care classmates. Specifically, they were more likely, in the 2-week period preceding testing, to have attended gatherings with individuals outside their immediate households, including social events and activities with other children. Parents of students testing positive were also less likely to report consistent indoor mask use among their children older than 2 years and school staff members.
School attendance was not found to increase likelihood of testing positive
Attending in-person school or child care during the 2 weeks before the SARS-CoV-2 test was not associated with greater likelihood of testing positive, the study authors noted, adding that the majority of study respondents reported universal mask use inside school and child care facilities, consistent with Mississippi State Department of Health recommended guidelines.
Dr. Hobbs and colleagues reported at least four limitations of the study. They noted that the study participants may not be representative of youth in other geographic regions of the country. They considered the possibility of unmeasured confounding of participant behaviors that may not have been factored into the study. No attempt was made to verify parent claims of mask use at schools and child care programs. Lastly, they acknowledged that “case or control status might be subject to misclassification because of imperfect sensitivity or specificity of PCR-based testing.
As of Dec. 14, 2020, the CDC reported that 10.2% of all COVID-19 cases in the United States were in children and adolescents under the age of 18.
“Continued efforts to prevent transmission at schools and child care programs are important, as are assessments of various types of activities and exposures to identify risk factors for COVID-19 as children engage in classroom and social interactions.” Promoting behaviors to reduce exposures to the virus among youth in the household, the community, schools, and child care programs is important to preventing outbreaks of the virus at schools, the authors cautioned.
In a separate interview with this news organization, Karalyn Kinsella, MD, general pediatrician in a small group private practice in Cheshire, Conn., said, “What this report tells me is that COVID cases are more common when mask use is inconsistent in schools and at home and in schools that don’t properly adhere to CDC guidelines. Overall, so long as social distancing guidelines are followed, schools are pretty safe places for kids during this pandemic.”
This finding is important, since many families are keeping their children out of school over fears of contracting the virus, she added. Some of the consequences these children are suffering include a lack of social connection and structure, which in some cases is leading to worsening anxiety and depression, and for those with disabilities, such as those who receive physical therapy, occupational therapy, speech or have IEPs, they’re not getting the full benefit of the services that they would otherwise receive in person, she observed.
“I don’t think families really understand the risks of getting together with family or friends “in their bubble” or the risk of continuing sports participation. This is where the majority of COVID cases are coming from,” she said, adding that it is important to discuss this risk with them at appointments. So, when families ask us what we think of in-person learning, I think we should feel fairly confident that the benefit may outweigh the risk.”
Dr. Hobbs and colleagues, and Dr. Kinsella, had no conflicts of interest to report.
SOURCE: MMWR Morb Mortal Wkly Rep. 2020;69:1925-9. doi: 10.15585/mmwr.mm6950e3.
FROM MORBIDITY AND MORTALITY WEEKLY REPORT
Younger adults present with more advanced esophageal adenocarcinoma
The incidence of esophageal adenocarcinoma in adults aged younger than 50 years increased threefold between 1975 and 2015, based on data from more than 34,000 cases.
Esophageal carcinoma rates overall have risen in the United States over the past 4 decades, but the average patient is in their 60s, wrote Don C. Codipilly, MD, of the Mayo Clinic, Rochester, Minn., and colleagues. Therefore, “data on the incidence, stage distribution, and outcomes of this segment of patients [younger than 50 years] with esophageal adenocarcinoma are relatively limited.”
In a study published in Cancer Epidemiology, Biomarkers & Prevention, the researchers identified 34,443 cases of esophageal adenocarcinoma using the Surveillance, Epidemiology, and End Results (SEER) database for the periods of 1975-1989, 1990-1999, and 2000-2015. The cases were limited to histologically confirmed cases and were stratified according to age at diagnosis: younger than 50 years, 50-69 years, and 70 years and older
Overall, the annual incidence of esophageal adenocarcinoma among individuals younger than 50 years increased from 0.08 per 100,000 persons in 1975 to 0.27 per 100,000 persons in 2015.
Younger patients show more advanced illness
Although the incidence rose across all three age groups during the study period, the largest increase was seen in those aged 70 years and older. However, the younger group was significantly more likely to present at more-advanced stages, the researchers pointed out: Between 2000 and 2015, localized disease represented only 15.1% of cases in those younger than 50 years, compared with 22.4% in patients aged 50-69 years and 32.2% in those 70 years and older. The incidence of regional/distant disease among younger patients has increased over time, with 81.8% in 1975-1989, 75.5% in 1990-1999, and 84.9% in 2000-2015 (P < .01), and this increase has been faster than among older groups, the researches noted. For comparison, during 2000-2015 only 77.6% of patients aged 50-69 years and 67.8% of patients 70 years and older had regional/distant disease.
In addition, the majority of cases of young-onset esophageal adenocarcinoma occurred in men in a trend that persisted across the study periods; 90% of patients younger than 50 years were male in 1975, and 86% of the younger patients in 2015 were male.
“There is no clear explanation for the higher proportion of advanced disease in younger patients, and further study is required to identify biologic, genetic, and environmental factors that may underlie this observation,” the researchers wrote. “A potential hypothesis is that ‘young-onset esophageal adenocarcinoma’ may involve rapid transition from intestinal metaplasia to esophageal adenocarcinoma, driven by an increase in signaling molecules that are active in the intestine,” they suggested.
The study findings were limited by several factors including the inability to review individual case records to confirm disease stage and to compare outcomes across ethnicities, and the lack of data on comorbidities in the SEER database, the researchers noted.
However, the results were strengthened by overall quality of the SEER database and use of multivariate analysis, they added. The evidence of increased incidence and increased odds of advanced disease in younger adults suggest that “reevaluation of our diagnostic and treatment strategies in this age group might need to be considered.”
Reasons for increase remain unclear
“While esophageal adenocarcinoma is uncommon overall in younger patients, this study importantly highlights that not only has the incidence of esophageal adenocarcinoma increased more than threefold in patients under the age of 50 over the last 4 decades, but that younger patients are presenting with more advanced disease and have overall poorer survival, compared to older patients,” Rahul A. Shimpi, MD, of Duke University, Durham, N.C., said in an interview.
“The reasons for these findings are unclear, but the authors propose a number of potential factors that could explain them. These include differences in tumor biology, rising rates of obesity and [gastroesophageal reflux disease] in younger patients, decreased endoscopic screening for and surveillance of Barrett’s esophagus in this age group, and differing therapeutic approaches to management,” Dr. Shimpi said.
“The findings from this study underscore that, while uncommon, clinicians need to be aware of the rising incidence of esophageal cancer in younger patients. It is important that even younger patients presenting with esophageal symptoms, such as dysphagia, undergo investigation,” he emphasized.
“I would like to see further study into the potential factors driving the findings in this study, including whether trends in differential treatment modalities account for some of the survival differences found in different age groups,” Dr. Shimpi added. “Finally, further research will ideally clarify optimal Barrett’s screening and surveillance approaches in patients younger than age 50 in order to determine whether new strategies might impact esophageal adenocarcinoma incidence and outcomes in this group.”
The study was funded in part by the National Cancer Institute and the National Center for Advancing Translational Sciences. Two authors disclosed relationships outside the submitted work, but Dr. Codipilly and the remaining authors had no financial conflicts to disclose. Dr. Shimpi had no financial conflicts to disclose.
SOURCE: Codipilly DC et al. Cancer Epidemiol Biomarkers Prev. 2020 Dec 11. doi: 10.1158/1055-9965.EPI-20-0944.
The incidence of esophageal adenocarcinoma in adults aged younger than 50 years increased threefold between 1975 and 2015, based on data from more than 34,000 cases.
Esophageal carcinoma rates overall have risen in the United States over the past 4 decades, but the average patient is in their 60s, wrote Don C. Codipilly, MD, of the Mayo Clinic, Rochester, Minn., and colleagues. Therefore, “data on the incidence, stage distribution, and outcomes of this segment of patients [younger than 50 years] with esophageal adenocarcinoma are relatively limited.”
In a study published in Cancer Epidemiology, Biomarkers & Prevention, the researchers identified 34,443 cases of esophageal adenocarcinoma using the Surveillance, Epidemiology, and End Results (SEER) database for the periods of 1975-1989, 1990-1999, and 2000-2015. The cases were limited to histologically confirmed cases and were stratified according to age at diagnosis: younger than 50 years, 50-69 years, and 70 years and older
Overall, the annual incidence of esophageal adenocarcinoma among individuals younger than 50 years increased from 0.08 per 100,000 persons in 1975 to 0.27 per 100,000 persons in 2015.
Younger patients show more advanced illness
Although the incidence rose across all three age groups during the study period, the largest increase was seen in those aged 70 years and older. However, the younger group was significantly more likely to present at more-advanced stages, the researchers pointed out: Between 2000 and 2015, localized disease represented only 15.1% of cases in those younger than 50 years, compared with 22.4% in patients aged 50-69 years and 32.2% in those 70 years and older. The incidence of regional/distant disease among younger patients has increased over time, with 81.8% in 1975-1989, 75.5% in 1990-1999, and 84.9% in 2000-2015 (P < .01), and this increase has been faster than among older groups, the researches noted. For comparison, during 2000-2015 only 77.6% of patients aged 50-69 years and 67.8% of patients 70 years and older had regional/distant disease.
In addition, the majority of cases of young-onset esophageal adenocarcinoma occurred in men in a trend that persisted across the study periods; 90% of patients younger than 50 years were male in 1975, and 86% of the younger patients in 2015 were male.
“There is no clear explanation for the higher proportion of advanced disease in younger patients, and further study is required to identify biologic, genetic, and environmental factors that may underlie this observation,” the researchers wrote. “A potential hypothesis is that ‘young-onset esophageal adenocarcinoma’ may involve rapid transition from intestinal metaplasia to esophageal adenocarcinoma, driven by an increase in signaling molecules that are active in the intestine,” they suggested.
The study findings were limited by several factors including the inability to review individual case records to confirm disease stage and to compare outcomes across ethnicities, and the lack of data on comorbidities in the SEER database, the researchers noted.
However, the results were strengthened by overall quality of the SEER database and use of multivariate analysis, they added. The evidence of increased incidence and increased odds of advanced disease in younger adults suggest that “reevaluation of our diagnostic and treatment strategies in this age group might need to be considered.”
Reasons for increase remain unclear
“While esophageal adenocarcinoma is uncommon overall in younger patients, this study importantly highlights that not only has the incidence of esophageal adenocarcinoma increased more than threefold in patients under the age of 50 over the last 4 decades, but that younger patients are presenting with more advanced disease and have overall poorer survival, compared to older patients,” Rahul A. Shimpi, MD, of Duke University, Durham, N.C., said in an interview.
“The reasons for these findings are unclear, but the authors propose a number of potential factors that could explain them. These include differences in tumor biology, rising rates of obesity and [gastroesophageal reflux disease] in younger patients, decreased endoscopic screening for and surveillance of Barrett’s esophagus in this age group, and differing therapeutic approaches to management,” Dr. Shimpi said.
“The findings from this study underscore that, while uncommon, clinicians need to be aware of the rising incidence of esophageal cancer in younger patients. It is important that even younger patients presenting with esophageal symptoms, such as dysphagia, undergo investigation,” he emphasized.
“I would like to see further study into the potential factors driving the findings in this study, including whether trends in differential treatment modalities account for some of the survival differences found in different age groups,” Dr. Shimpi added. “Finally, further research will ideally clarify optimal Barrett’s screening and surveillance approaches in patients younger than age 50 in order to determine whether new strategies might impact esophageal adenocarcinoma incidence and outcomes in this group.”
The study was funded in part by the National Cancer Institute and the National Center for Advancing Translational Sciences. Two authors disclosed relationships outside the submitted work, but Dr. Codipilly and the remaining authors had no financial conflicts to disclose. Dr. Shimpi had no financial conflicts to disclose.
SOURCE: Codipilly DC et al. Cancer Epidemiol Biomarkers Prev. 2020 Dec 11. doi: 10.1158/1055-9965.EPI-20-0944.
The incidence of esophageal adenocarcinoma in adults aged younger than 50 years increased threefold between 1975 and 2015, based on data from more than 34,000 cases.
Esophageal carcinoma rates overall have risen in the United States over the past 4 decades, but the average patient is in their 60s, wrote Don C. Codipilly, MD, of the Mayo Clinic, Rochester, Minn., and colleagues. Therefore, “data on the incidence, stage distribution, and outcomes of this segment of patients [younger than 50 years] with esophageal adenocarcinoma are relatively limited.”
In a study published in Cancer Epidemiology, Biomarkers & Prevention, the researchers identified 34,443 cases of esophageal adenocarcinoma using the Surveillance, Epidemiology, and End Results (SEER) database for the periods of 1975-1989, 1990-1999, and 2000-2015. The cases were limited to histologically confirmed cases and were stratified according to age at diagnosis: younger than 50 years, 50-69 years, and 70 years and older
Overall, the annual incidence of esophageal adenocarcinoma among individuals younger than 50 years increased from 0.08 per 100,000 persons in 1975 to 0.27 per 100,000 persons in 2015.
Younger patients show more advanced illness
Although the incidence rose across all three age groups during the study period, the largest increase was seen in those aged 70 years and older. However, the younger group was significantly more likely to present at more-advanced stages, the researchers pointed out: Between 2000 and 2015, localized disease represented only 15.1% of cases in those younger than 50 years, compared with 22.4% in patients aged 50-69 years and 32.2% in those 70 years and older. The incidence of regional/distant disease among younger patients has increased over time, with 81.8% in 1975-1989, 75.5% in 1990-1999, and 84.9% in 2000-2015 (P < .01), and this increase has been faster than among older groups, the researches noted. For comparison, during 2000-2015 only 77.6% of patients aged 50-69 years and 67.8% of patients 70 years and older had regional/distant disease.
In addition, the majority of cases of young-onset esophageal adenocarcinoma occurred in men in a trend that persisted across the study periods; 90% of patients younger than 50 years were male in 1975, and 86% of the younger patients in 2015 were male.
“There is no clear explanation for the higher proportion of advanced disease in younger patients, and further study is required to identify biologic, genetic, and environmental factors that may underlie this observation,” the researchers wrote. “A potential hypothesis is that ‘young-onset esophageal adenocarcinoma’ may involve rapid transition from intestinal metaplasia to esophageal adenocarcinoma, driven by an increase in signaling molecules that are active in the intestine,” they suggested.
The study findings were limited by several factors including the inability to review individual case records to confirm disease stage and to compare outcomes across ethnicities, and the lack of data on comorbidities in the SEER database, the researchers noted.
However, the results were strengthened by overall quality of the SEER database and use of multivariate analysis, they added. The evidence of increased incidence and increased odds of advanced disease in younger adults suggest that “reevaluation of our diagnostic and treatment strategies in this age group might need to be considered.”
Reasons for increase remain unclear
“While esophageal adenocarcinoma is uncommon overall in younger patients, this study importantly highlights that not only has the incidence of esophageal adenocarcinoma increased more than threefold in patients under the age of 50 over the last 4 decades, but that younger patients are presenting with more advanced disease and have overall poorer survival, compared to older patients,” Rahul A. Shimpi, MD, of Duke University, Durham, N.C., said in an interview.
“The reasons for these findings are unclear, but the authors propose a number of potential factors that could explain them. These include differences in tumor biology, rising rates of obesity and [gastroesophageal reflux disease] in younger patients, decreased endoscopic screening for and surveillance of Barrett’s esophagus in this age group, and differing therapeutic approaches to management,” Dr. Shimpi said.
“The findings from this study underscore that, while uncommon, clinicians need to be aware of the rising incidence of esophageal cancer in younger patients. It is important that even younger patients presenting with esophageal symptoms, such as dysphagia, undergo investigation,” he emphasized.
“I would like to see further study into the potential factors driving the findings in this study, including whether trends in differential treatment modalities account for some of the survival differences found in different age groups,” Dr. Shimpi added. “Finally, further research will ideally clarify optimal Barrett’s screening and surveillance approaches in patients younger than age 50 in order to determine whether new strategies might impact esophageal adenocarcinoma incidence and outcomes in this group.”
The study was funded in part by the National Cancer Institute and the National Center for Advancing Translational Sciences. Two authors disclosed relationships outside the submitted work, but Dr. Codipilly and the remaining authors had no financial conflicts to disclose. Dr. Shimpi had no financial conflicts to disclose.
SOURCE: Codipilly DC et al. Cancer Epidemiol Biomarkers Prev. 2020 Dec 11. doi: 10.1158/1055-9965.EPI-20-0944.
FROM CANCER EPIDEMIOLOGY, BIOMARKERS & PREVENTION
Osteoporosis prevalence in PsA similar to general population
The rates of osteopenia and osteoporosis among individuals with psoriatic arthritis are comparable to those seen in the general population, research suggests.
The cohort study, published in Arthritis Care & Research, also found that clinicians are likely to refer patients for bone mineral density (BMD) testing based on osteoporosis risk factors or psoriatic arthritis disease severity markers.
Timothy S.H. Kwok, MD, of the University of Toronto, and coauthors wrote that previous research suggested a possible link between psoriatic arthritis and osteoporosis or osteopenia. However, no cohort studies appear to have examined this association.
The study involved 201 individuals with psoriatic arthritis attending a single specialist clinic, who were enrolled in a longitudinal study of psoriatic arthritis (PsA) and who were also referred for BMD testing with dual-energy x-ray absorptiometry.
Of these participants, 13% had a BMD in the osteoporotic range, 45% were in the osteopenic range, and 42% were in the normal range for BMD. The prevalence of osteoporosis observed in the general population aged 50 or above, observed in an earlier large prospective study, ranged from 7% to 16%, and osteopenia ranged from 27% to 46%.
“Our study suggests that patients with PsA have similar BMDs compared to the general population,” the authors wrote.
Researchers did note the suggestion that patients with polyarthritis had lower BMDs over time. Because of the small number of events, this did not achieve statistical significance, but “this relationship warrants further research, given that multiple cohort studies have independently demonstrated polyarticular onset of disease predicting clinical deformities and erosive disease in PsA,” they wrote.
They also saw that patients with increased body mass index had a significant 21% lower odds of having a BMD in the osteoporotic range, while those using biologics had a significant 83% lower odds.
Among participants with BMD scores in the osteopenic or osteoporotic range, these scores were seen in the lumbar spine in 63% of measurements, the femoral neck in 88%, and the total hip in 39%. Mean T-scores for the lumbar spine were –0.30±0.32, and for the femoral neck were –1.10±1.04 and the total hip, –0.45±0.42.
The study also examined what factors were associated with referral for BMD testing. They found that increasing age, menopause, elevated acute phase reactants, or use of biologics, methotrexate, and systemic glucocorticoids were associated with a higher likelihood of undergoing BMD testing.
Noting that the latest Canadian clinical practice guidelines on BMD testing advise that age, menopause, and use of systemic glucocorticoids use are risk factors that should prompt testing, the authors suggested clinicians were using a combination of traditional osteoporosis risk factors and markers of psoriatic disease severity to underpin their decision to refer.
However, they commented that none of the factors associated with a higher likelihood of having a BMD test were actually associated with lower BMD scores.
“This suggests that clinicians may be over-screening patients with PsA for osteopenia/osteoporosis, as they do not appear to be at baseline higher risk for lower BMD scores than the general population,” they wrote. “This is of importance, as there are currently no formal recommendations with regards to the optimal interval or time to commence BMD testing within the recent major PsA guidelines.”
The study was supported by a grant from the Krembil Foundation. No conflicts of interest were declared.
SOURCE: Kwok TSH et al. Arthritis Care Res. 2020 Dec 16. doi: 10.1002/acr.24538.
The rates of osteopenia and osteoporosis among individuals with psoriatic arthritis are comparable to those seen in the general population, research suggests.
The cohort study, published in Arthritis Care & Research, also found that clinicians are likely to refer patients for bone mineral density (BMD) testing based on osteoporosis risk factors or psoriatic arthritis disease severity markers.
Timothy S.H. Kwok, MD, of the University of Toronto, and coauthors wrote that previous research suggested a possible link between psoriatic arthritis and osteoporosis or osteopenia. However, no cohort studies appear to have examined this association.
The study involved 201 individuals with psoriatic arthritis attending a single specialist clinic, who were enrolled in a longitudinal study of psoriatic arthritis (PsA) and who were also referred for BMD testing with dual-energy x-ray absorptiometry.
Of these participants, 13% had a BMD in the osteoporotic range, 45% were in the osteopenic range, and 42% were in the normal range for BMD. The prevalence of osteoporosis observed in the general population aged 50 or above, observed in an earlier large prospective study, ranged from 7% to 16%, and osteopenia ranged from 27% to 46%.
“Our study suggests that patients with PsA have similar BMDs compared to the general population,” the authors wrote.
Researchers did note the suggestion that patients with polyarthritis had lower BMDs over time. Because of the small number of events, this did not achieve statistical significance, but “this relationship warrants further research, given that multiple cohort studies have independently demonstrated polyarticular onset of disease predicting clinical deformities and erosive disease in PsA,” they wrote.
They also saw that patients with increased body mass index had a significant 21% lower odds of having a BMD in the osteoporotic range, while those using biologics had a significant 83% lower odds.
Among participants with BMD scores in the osteopenic or osteoporotic range, these scores were seen in the lumbar spine in 63% of measurements, the femoral neck in 88%, and the total hip in 39%. Mean T-scores for the lumbar spine were –0.30±0.32, and for the femoral neck were –1.10±1.04 and the total hip, –0.45±0.42.
The study also examined what factors were associated with referral for BMD testing. They found that increasing age, menopause, elevated acute phase reactants, or use of biologics, methotrexate, and systemic glucocorticoids were associated with a higher likelihood of undergoing BMD testing.
Noting that the latest Canadian clinical practice guidelines on BMD testing advise that age, menopause, and use of systemic glucocorticoids use are risk factors that should prompt testing, the authors suggested clinicians were using a combination of traditional osteoporosis risk factors and markers of psoriatic disease severity to underpin their decision to refer.
However, they commented that none of the factors associated with a higher likelihood of having a BMD test were actually associated with lower BMD scores.
“This suggests that clinicians may be over-screening patients with PsA for osteopenia/osteoporosis, as they do not appear to be at baseline higher risk for lower BMD scores than the general population,” they wrote. “This is of importance, as there are currently no formal recommendations with regards to the optimal interval or time to commence BMD testing within the recent major PsA guidelines.”
The study was supported by a grant from the Krembil Foundation. No conflicts of interest were declared.
SOURCE: Kwok TSH et al. Arthritis Care Res. 2020 Dec 16. doi: 10.1002/acr.24538.
The rates of osteopenia and osteoporosis among individuals with psoriatic arthritis are comparable to those seen in the general population, research suggests.
The cohort study, published in Arthritis Care & Research, also found that clinicians are likely to refer patients for bone mineral density (BMD) testing based on osteoporosis risk factors or psoriatic arthritis disease severity markers.
Timothy S.H. Kwok, MD, of the University of Toronto, and coauthors wrote that previous research suggested a possible link between psoriatic arthritis and osteoporosis or osteopenia. However, no cohort studies appear to have examined this association.
The study involved 201 individuals with psoriatic arthritis attending a single specialist clinic, who were enrolled in a longitudinal study of psoriatic arthritis (PsA) and who were also referred for BMD testing with dual-energy x-ray absorptiometry.
Of these participants, 13% had a BMD in the osteoporotic range, 45% were in the osteopenic range, and 42% were in the normal range for BMD. The prevalence of osteoporosis observed in the general population aged 50 or above, observed in an earlier large prospective study, ranged from 7% to 16%, and osteopenia ranged from 27% to 46%.
“Our study suggests that patients with PsA have similar BMDs compared to the general population,” the authors wrote.
Researchers did note the suggestion that patients with polyarthritis had lower BMDs over time. Because of the small number of events, this did not achieve statistical significance, but “this relationship warrants further research, given that multiple cohort studies have independently demonstrated polyarticular onset of disease predicting clinical deformities and erosive disease in PsA,” they wrote.
They also saw that patients with increased body mass index had a significant 21% lower odds of having a BMD in the osteoporotic range, while those using biologics had a significant 83% lower odds.
Among participants with BMD scores in the osteopenic or osteoporotic range, these scores were seen in the lumbar spine in 63% of measurements, the femoral neck in 88%, and the total hip in 39%. Mean T-scores for the lumbar spine were –0.30±0.32, and for the femoral neck were –1.10±1.04 and the total hip, –0.45±0.42.
The study also examined what factors were associated with referral for BMD testing. They found that increasing age, menopause, elevated acute phase reactants, or use of biologics, methotrexate, and systemic glucocorticoids were associated with a higher likelihood of undergoing BMD testing.
Noting that the latest Canadian clinical practice guidelines on BMD testing advise that age, menopause, and use of systemic glucocorticoids use are risk factors that should prompt testing, the authors suggested clinicians were using a combination of traditional osteoporosis risk factors and markers of psoriatic disease severity to underpin their decision to refer.
However, they commented that none of the factors associated with a higher likelihood of having a BMD test were actually associated with lower BMD scores.
“This suggests that clinicians may be over-screening patients with PsA for osteopenia/osteoporosis, as they do not appear to be at baseline higher risk for lower BMD scores than the general population,” they wrote. “This is of importance, as there are currently no formal recommendations with regards to the optimal interval or time to commence BMD testing within the recent major PsA guidelines.”
The study was supported by a grant from the Krembil Foundation. No conflicts of interest were declared.
SOURCE: Kwok TSH et al. Arthritis Care Res. 2020 Dec 16. doi: 10.1002/acr.24538.
FROM ARTHRITIS CARE & RESEARCH
Annual WCC visits significantly limit asthma worsening
There is a significant association between routine attendance at annual well-child care visits and a reduction in both total asthma exacerbations and severe exacerbations, Jason E. Lang, MD, MPH, of Duke University, Durham, N.C. reported in a study published in Pediatrics.
In a retrospective cohort study of 5,656 pediatric asthma patients under care at the Duke University Health System, Dr. Lang and colleagues sought to determine the effect yearly well-child care (WCC) visits have on the hazard rate of asthma exacerbations occurring during the following year. Patients included in the study were aged 5-17 years and had been receiving care between Jan. 1, 2014, and Dec. 31, 2019.
WCC visits demonstrate reduced exacerbations and hospitalizations
Nearly one-third of patients were found to have full WCC visit attendance, half were partially compliant, and 14% did not attend at all. A total of 2,974 asthma exacerbations were reported during the study period. Of those with a WCC visit during the previous year, exacerbations were reduced by 10% and asthma hospitalizations were lowered by 47%. Children with recent WCC visits were also more likely to be prescribed daily preventive medication and to experience an exacerbation in ambulatory care, which could play a crucial role in preventing further progression of the disease.
Of the WCC visits reported, 9.9% represented prescribing of new or changed asthma medication, 28.2% represented delivery of seasonal influenza vaccine, and 11% addressed assessment or management of asthma-related comorbidities. There was no observed difference in attendance between younger and older children.
Given that pediatric WCC visit attendance is “far from optimal,” with attendance improving from 46% in 1996-1998 to almost 60% in 2007-2008, “improving access to and attendance of WCC visits (especially from previously low-adhering families) may be an important public health intervention to reduce the problems of severe exacerbations and outcome disparities,” observed Dr. Lang and colleagues. The Abdus study also found that low WCC attendance appeared to be more common in those with lower income, lower parental education, and African American race.
Continuity of care providers across WCC visits plays a crucial role
Primary care pediatricians play a key role in successful management of chronic asthma, as evidenced in several studies showing the importance of continuity of care with the same provider for WCC. Such continuity encourages ongoing dialogue about asthma, and as the researchers speculated, may even reduce asthma hospitalization through better parental understanding of disease management, prevention, and management of comorbid conditions.
Although the study did not include measures of health literacy, the authors did conclude that pediatric asthma patients seen annually are more likely to be more knowledgeable about asthma and in a better position to recognize symptom exacerbation so they can seek timely care. In the past, lower health literacy has demonstrated both lower WCC visit attendance and increased emergency care visits and hospitalizations.
Because the study was conducted in a single university-based health system, the researchers were not able to capture fragmented care data. They also acknowledged the possible omission of confounding factors, especially those related to parental influence behaviors affecting daily disease management. One strength of the study was the ability researchers had to abstract granular data from their EHR system to document the time-varying effects that insurance status, obesity status, and WCC visits may have played. Given that they were able to assess effects according to sociodemographic factors, such as race and insurance status, the results should prove very helpful to other cities and health systems aiming to improve pediatric asthma control, observed Dr. Lang and colleagues.
Future studies should seek to further evaluate the role of WCC visits in promoting asthma control. Making WCC visits a renewed public health priority offers the possibility to limit severe asthma exacerbations, the researchers advised.
In a separate interview, Sydney Leibel, MD, MPH, a pediatric allergist/immunologist at Rady Children’s Hospital, San Diego, noted: “The outcomes of this study shine a light on the importance of regular primary care pediatrician follow-up in decreasing asthma-related health care utilization. Childhood asthma is a dynamic condition and follow-up with the pediatrician allows for modification of the treatment plan and reinforcement of good inhaler technique. It also allows for patients to express their concerns and gives the opportunity for subspecialty referral, if symptoms remain uncontrolled.
“This article also highlights the health disparities that exist in pediatric asthma in the United States. In our experience, treating children from lower-socioeconomic communities with difficult-to-control and severe asthma, case management has been very important in making sure our patient population understands our instructions, pick up their medications, and make their scheduled follow-up appointments,” Dr. Leibel continued.
“Regardless of the patient’s background, efforts to improve attendance of WCC visits, where good asthma control can be promoted, would be in our patient’s best interest and could go a long way in preventing unnecessary asthma exacerbations that require an ED visit or hospitalization,” the specialist concluded.
The study was funded by a grant from the National Heart, Lung, and Blood Institute, Duke Children’s Health & Discovery Initiative, and the National Institutes of Health. Dr. Lang and colleagues had no conflicts of interest and no relevant financial disclosures. Dr. Leibel said he had no relevant financial disclosures.
SOURCE: Lang JE et al. Pediatrics. 2020. doi: 10.1542/peds.2020-1023.
There is a significant association between routine attendance at annual well-child care visits and a reduction in both total asthma exacerbations and severe exacerbations, Jason E. Lang, MD, MPH, of Duke University, Durham, N.C. reported in a study published in Pediatrics.
In a retrospective cohort study of 5,656 pediatric asthma patients under care at the Duke University Health System, Dr. Lang and colleagues sought to determine the effect yearly well-child care (WCC) visits have on the hazard rate of asthma exacerbations occurring during the following year. Patients included in the study were aged 5-17 years and had been receiving care between Jan. 1, 2014, and Dec. 31, 2019.
WCC visits demonstrate reduced exacerbations and hospitalizations
Nearly one-third of patients were found to have full WCC visit attendance, half were partially compliant, and 14% did not attend at all. A total of 2,974 asthma exacerbations were reported during the study period. Of those with a WCC visit during the previous year, exacerbations were reduced by 10% and asthma hospitalizations were lowered by 47%. Children with recent WCC visits were also more likely to be prescribed daily preventive medication and to experience an exacerbation in ambulatory care, which could play a crucial role in preventing further progression of the disease.
Of the WCC visits reported, 9.9% represented prescribing of new or changed asthma medication, 28.2% represented delivery of seasonal influenza vaccine, and 11% addressed assessment or management of asthma-related comorbidities. There was no observed difference in attendance between younger and older children.
Given that pediatric WCC visit attendance is “far from optimal,” with attendance improving from 46% in 1996-1998 to almost 60% in 2007-2008, “improving access to and attendance of WCC visits (especially from previously low-adhering families) may be an important public health intervention to reduce the problems of severe exacerbations and outcome disparities,” observed Dr. Lang and colleagues. The Abdus study also found that low WCC attendance appeared to be more common in those with lower income, lower parental education, and African American race.
Continuity of care providers across WCC visits plays a crucial role
Primary care pediatricians play a key role in successful management of chronic asthma, as evidenced in several studies showing the importance of continuity of care with the same provider for WCC. Such continuity encourages ongoing dialogue about asthma, and as the researchers speculated, may even reduce asthma hospitalization through better parental understanding of disease management, prevention, and management of comorbid conditions.
Although the study did not include measures of health literacy, the authors did conclude that pediatric asthma patients seen annually are more likely to be more knowledgeable about asthma and in a better position to recognize symptom exacerbation so they can seek timely care. In the past, lower health literacy has demonstrated both lower WCC visit attendance and increased emergency care visits and hospitalizations.
Because the study was conducted in a single university-based health system, the researchers were not able to capture fragmented care data. They also acknowledged the possible omission of confounding factors, especially those related to parental influence behaviors affecting daily disease management. One strength of the study was the ability researchers had to abstract granular data from their EHR system to document the time-varying effects that insurance status, obesity status, and WCC visits may have played. Given that they were able to assess effects according to sociodemographic factors, such as race and insurance status, the results should prove very helpful to other cities and health systems aiming to improve pediatric asthma control, observed Dr. Lang and colleagues.
Future studies should seek to further evaluate the role of WCC visits in promoting asthma control. Making WCC visits a renewed public health priority offers the possibility to limit severe asthma exacerbations, the researchers advised.
In a separate interview, Sydney Leibel, MD, MPH, a pediatric allergist/immunologist at Rady Children’s Hospital, San Diego, noted: “The outcomes of this study shine a light on the importance of regular primary care pediatrician follow-up in decreasing asthma-related health care utilization. Childhood asthma is a dynamic condition and follow-up with the pediatrician allows for modification of the treatment plan and reinforcement of good inhaler technique. It also allows for patients to express their concerns and gives the opportunity for subspecialty referral, if symptoms remain uncontrolled.
“This article also highlights the health disparities that exist in pediatric asthma in the United States. In our experience, treating children from lower-socioeconomic communities with difficult-to-control and severe asthma, case management has been very important in making sure our patient population understands our instructions, pick up their medications, and make their scheduled follow-up appointments,” Dr. Leibel continued.
“Regardless of the patient’s background, efforts to improve attendance of WCC visits, where good asthma control can be promoted, would be in our patient’s best interest and could go a long way in preventing unnecessary asthma exacerbations that require an ED visit or hospitalization,” the specialist concluded.
The study was funded by a grant from the National Heart, Lung, and Blood Institute, Duke Children’s Health & Discovery Initiative, and the National Institutes of Health. Dr. Lang and colleagues had no conflicts of interest and no relevant financial disclosures. Dr. Leibel said he had no relevant financial disclosures.
SOURCE: Lang JE et al. Pediatrics. 2020. doi: 10.1542/peds.2020-1023.
There is a significant association between routine attendance at annual well-child care visits and a reduction in both total asthma exacerbations and severe exacerbations, Jason E. Lang, MD, MPH, of Duke University, Durham, N.C. reported in a study published in Pediatrics.
In a retrospective cohort study of 5,656 pediatric asthma patients under care at the Duke University Health System, Dr. Lang and colleagues sought to determine the effect yearly well-child care (WCC) visits have on the hazard rate of asthma exacerbations occurring during the following year. Patients included in the study were aged 5-17 years and had been receiving care between Jan. 1, 2014, and Dec. 31, 2019.
WCC visits demonstrate reduced exacerbations and hospitalizations
Nearly one-third of patients were found to have full WCC visit attendance, half were partially compliant, and 14% did not attend at all. A total of 2,974 asthma exacerbations were reported during the study period. Of those with a WCC visit during the previous year, exacerbations were reduced by 10% and asthma hospitalizations were lowered by 47%. Children with recent WCC visits were also more likely to be prescribed daily preventive medication and to experience an exacerbation in ambulatory care, which could play a crucial role in preventing further progression of the disease.
Of the WCC visits reported, 9.9% represented prescribing of new or changed asthma medication, 28.2% represented delivery of seasonal influenza vaccine, and 11% addressed assessment or management of asthma-related comorbidities. There was no observed difference in attendance between younger and older children.
Given that pediatric WCC visit attendance is “far from optimal,” with attendance improving from 46% in 1996-1998 to almost 60% in 2007-2008, “improving access to and attendance of WCC visits (especially from previously low-adhering families) may be an important public health intervention to reduce the problems of severe exacerbations and outcome disparities,” observed Dr. Lang and colleagues. The Abdus study also found that low WCC attendance appeared to be more common in those with lower income, lower parental education, and African American race.
Continuity of care providers across WCC visits plays a crucial role
Primary care pediatricians play a key role in successful management of chronic asthma, as evidenced in several studies showing the importance of continuity of care with the same provider for WCC. Such continuity encourages ongoing dialogue about asthma, and as the researchers speculated, may even reduce asthma hospitalization through better parental understanding of disease management, prevention, and management of comorbid conditions.
Although the study did not include measures of health literacy, the authors did conclude that pediatric asthma patients seen annually are more likely to be more knowledgeable about asthma and in a better position to recognize symptom exacerbation so they can seek timely care. In the past, lower health literacy has demonstrated both lower WCC visit attendance and increased emergency care visits and hospitalizations.
Because the study was conducted in a single university-based health system, the researchers were not able to capture fragmented care data. They also acknowledged the possible omission of confounding factors, especially those related to parental influence behaviors affecting daily disease management. One strength of the study was the ability researchers had to abstract granular data from their EHR system to document the time-varying effects that insurance status, obesity status, and WCC visits may have played. Given that they were able to assess effects according to sociodemographic factors, such as race and insurance status, the results should prove very helpful to other cities and health systems aiming to improve pediatric asthma control, observed Dr. Lang and colleagues.
Future studies should seek to further evaluate the role of WCC visits in promoting asthma control. Making WCC visits a renewed public health priority offers the possibility to limit severe asthma exacerbations, the researchers advised.
In a separate interview, Sydney Leibel, MD, MPH, a pediatric allergist/immunologist at Rady Children’s Hospital, San Diego, noted: “The outcomes of this study shine a light on the importance of regular primary care pediatrician follow-up in decreasing asthma-related health care utilization. Childhood asthma is a dynamic condition and follow-up with the pediatrician allows for modification of the treatment plan and reinforcement of good inhaler technique. It also allows for patients to express their concerns and gives the opportunity for subspecialty referral, if symptoms remain uncontrolled.
“This article also highlights the health disparities that exist in pediatric asthma in the United States. In our experience, treating children from lower-socioeconomic communities with difficult-to-control and severe asthma, case management has been very important in making sure our patient population understands our instructions, pick up their medications, and make their scheduled follow-up appointments,” Dr. Leibel continued.
“Regardless of the patient’s background, efforts to improve attendance of WCC visits, where good asthma control can be promoted, would be in our patient’s best interest and could go a long way in preventing unnecessary asthma exacerbations that require an ED visit or hospitalization,” the specialist concluded.
The study was funded by a grant from the National Heart, Lung, and Blood Institute, Duke Children’s Health & Discovery Initiative, and the National Institutes of Health. Dr. Lang and colleagues had no conflicts of interest and no relevant financial disclosures. Dr. Leibel said he had no relevant financial disclosures.
SOURCE: Lang JE et al. Pediatrics. 2020. doi: 10.1542/peds.2020-1023.
FROM PEDIATRICS