User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Mixing Paxlovid With Specific Immunosuppressants Risks Serious Adverse Reactions
The Pharmacovigilance Risk Assessment Committee (PRAC) of the European Medicines Agency (EMA) has issued a reminder to healthcare professionals regarding the potential serious adverse reactions associated with Paxlovid when administered in combination with specific immunosuppressants.
These immunosuppressants, encompassing calcineurin inhibitors (tacrolimus and ciclosporin) and mTOR inhibitors (everolimus and sirolimus), possess a narrow safe dosage range. They are recognized for their role in diminishing the activity of the immune system and are typically prescribed for autoimmune conditions and organ transplant recipients.
The highlighted risk arises due to drug-drug interactions, which can compromise the body’s ability to eliminate these medicines effectively.
Paxlovid, also known as nirmatrelvir with ritonavir, is an antiviral medication used to treat COVID-19 in adults who do not require supplemental oxygen and who are at an increased risk of progressing to severe COVID-19. It should be administered as soon as possible after a diagnosis of COVID-19 has been made and within 5 days of symptom onset.
Conditional marketing authorization for Paxlovid was granted across the European Union (EU) on January 28, 2022, and subsequently transitioned to full marketing authorization on February 24, 2023.
Developed by Pfizer, Paxlovid exhibited an 89% reduction in the risk for hospitalization or death among unvaccinated individuals in a phase 2-3 clinical trial. This led the National Institutes of Health to prioritize Paxlovid over other COVID-19 treatments. Subsequent real-world studies have affirmed its effectiveness, even among the vaccinated.
When combining Paxlovid with tacrolimus, ciclosporin, everolimus, or sirolimus, healthcare professionals need to actively monitor their blood levels. This proactive approach is essential to mitigate the risk for drug-drug interactions and potential serious reactions. They should collaborate with a multidisciplinary team of specialists to navigate the complexities of administering these medications concurrently.
Further, Paxlovid must not be coadministered with medications highly reliant on CYP3A liver enzymes for elimination, such as the immunosuppressant voclosporin. When administered together, there is a risk for these drugs interfering with each other’s metabolism, potentially leading to altered blood levels, reduced effectiveness, or an increased risk for adverse reactions.
After a thorough review, PRAC has highlighted potential serious adverse reactions, including fatal cases, due to drug interactions between Paxlovid and specified immunosuppressants. Thus, it issued a direct healthcare professional communication (DHPC) to emphasize the recognized risk for these interactions, as previously outlined in Paxlovid’s product information.
The DHPC for Paxlovid will undergo further evaluation by EMA’s Committee for Medicinal Products for Human Use and, upon adoption, will be disseminated to healthcare professionals. The communication plan will include publication on the DHPCs page and in national registers across EU Member States.
A version of this article appeared on Medscape.com.
The Pharmacovigilance Risk Assessment Committee (PRAC) of the European Medicines Agency (EMA) has issued a reminder to healthcare professionals regarding the potential serious adverse reactions associated with Paxlovid when administered in combination with specific immunosuppressants.
These immunosuppressants, encompassing calcineurin inhibitors (tacrolimus and ciclosporin) and mTOR inhibitors (everolimus and sirolimus), possess a narrow safe dosage range. They are recognized for their role in diminishing the activity of the immune system and are typically prescribed for autoimmune conditions and organ transplant recipients.
The highlighted risk arises due to drug-drug interactions, which can compromise the body’s ability to eliminate these medicines effectively.
Paxlovid, also known as nirmatrelvir with ritonavir, is an antiviral medication used to treat COVID-19 in adults who do not require supplemental oxygen and who are at an increased risk of progressing to severe COVID-19. It should be administered as soon as possible after a diagnosis of COVID-19 has been made and within 5 days of symptom onset.
Conditional marketing authorization for Paxlovid was granted across the European Union (EU) on January 28, 2022, and subsequently transitioned to full marketing authorization on February 24, 2023.
Developed by Pfizer, Paxlovid exhibited an 89% reduction in the risk for hospitalization or death among unvaccinated individuals in a phase 2-3 clinical trial. This led the National Institutes of Health to prioritize Paxlovid over other COVID-19 treatments. Subsequent real-world studies have affirmed its effectiveness, even among the vaccinated.
When combining Paxlovid with tacrolimus, ciclosporin, everolimus, or sirolimus, healthcare professionals need to actively monitor their blood levels. This proactive approach is essential to mitigate the risk for drug-drug interactions and potential serious reactions. They should collaborate with a multidisciplinary team of specialists to navigate the complexities of administering these medications concurrently.
Further, Paxlovid must not be coadministered with medications highly reliant on CYP3A liver enzymes for elimination, such as the immunosuppressant voclosporin. When administered together, there is a risk for these drugs interfering with each other’s metabolism, potentially leading to altered blood levels, reduced effectiveness, or an increased risk for adverse reactions.
After a thorough review, PRAC has highlighted potential serious adverse reactions, including fatal cases, due to drug interactions between Paxlovid and specified immunosuppressants. Thus, it issued a direct healthcare professional communication (DHPC) to emphasize the recognized risk for these interactions, as previously outlined in Paxlovid’s product information.
The DHPC for Paxlovid will undergo further evaluation by EMA’s Committee for Medicinal Products for Human Use and, upon adoption, will be disseminated to healthcare professionals. The communication plan will include publication on the DHPCs page and in national registers across EU Member States.
A version of this article appeared on Medscape.com.
The Pharmacovigilance Risk Assessment Committee (PRAC) of the European Medicines Agency (EMA) has issued a reminder to healthcare professionals regarding the potential serious adverse reactions associated with Paxlovid when administered in combination with specific immunosuppressants.
These immunosuppressants, encompassing calcineurin inhibitors (tacrolimus and ciclosporin) and mTOR inhibitors (everolimus and sirolimus), possess a narrow safe dosage range. They are recognized for their role in diminishing the activity of the immune system and are typically prescribed for autoimmune conditions and organ transplant recipients.
The highlighted risk arises due to drug-drug interactions, which can compromise the body’s ability to eliminate these medicines effectively.
Paxlovid, also known as nirmatrelvir with ritonavir, is an antiviral medication used to treat COVID-19 in adults who do not require supplemental oxygen and who are at an increased risk of progressing to severe COVID-19. It should be administered as soon as possible after a diagnosis of COVID-19 has been made and within 5 days of symptom onset.
Conditional marketing authorization for Paxlovid was granted across the European Union (EU) on January 28, 2022, and subsequently transitioned to full marketing authorization on February 24, 2023.
Developed by Pfizer, Paxlovid exhibited an 89% reduction in the risk for hospitalization or death among unvaccinated individuals in a phase 2-3 clinical trial. This led the National Institutes of Health to prioritize Paxlovid over other COVID-19 treatments. Subsequent real-world studies have affirmed its effectiveness, even among the vaccinated.
When combining Paxlovid with tacrolimus, ciclosporin, everolimus, or sirolimus, healthcare professionals need to actively monitor their blood levels. This proactive approach is essential to mitigate the risk for drug-drug interactions and potential serious reactions. They should collaborate with a multidisciplinary team of specialists to navigate the complexities of administering these medications concurrently.
Further, Paxlovid must not be coadministered with medications highly reliant on CYP3A liver enzymes for elimination, such as the immunosuppressant voclosporin. When administered together, there is a risk for these drugs interfering with each other’s metabolism, potentially leading to altered blood levels, reduced effectiveness, or an increased risk for adverse reactions.
After a thorough review, PRAC has highlighted potential serious adverse reactions, including fatal cases, due to drug interactions between Paxlovid and specified immunosuppressants. Thus, it issued a direct healthcare professional communication (DHPC) to emphasize the recognized risk for these interactions, as previously outlined in Paxlovid’s product information.
The DHPC for Paxlovid will undergo further evaluation by EMA’s Committee for Medicinal Products for Human Use and, upon adoption, will be disseminated to healthcare professionals. The communication plan will include publication on the DHPCs page and in national registers across EU Member States.
A version of this article appeared on Medscape.com.
Virus and Booster Apathy Could Be Fueling Long COVID
A celebrity makeup artist, the 55-year-old New Yorker had been boosted and vaccinated at every opportunity since vaccines were approved at the end of 2020, until the fall of 2023, when she skipped the shot.
“I really started subscribing to the mindset that you have an immune system and your immune system is supposed to work for you,” she said. “That was the stupidest thing I’ve ever done.”
Maio was not the only person to skip the latest booster: A recent study reported that while nearly 80% of adults in the United States said they’d received their first series of vaccines, barely 20% were up to date on boosters. Nor was Maio alone in getting long COVID 4 years after the start of the deadliest pandemic in a century.
It’s tempting, this far out from the shutdowns of 2020, to think the virus is over, that we’re immune, and nobody’s going to get sick anymore. But while fewer people are getting COVID, it is still very much a part of our lives. And as Maio and others are learning the hard way, long COVID is, too — and it can be deadly.
For those who have recently contracted long COVID, it can feel as if the whole world has moved on from the pandemic, and they are being left behind.
Too Easy to Let Our Guard Down
“It’s really difficult to prevent exposure to COVID no matter how careful you are and no matter how many times you are vaccinated,” said Akiko Iwasaki, an immunology professor at Yale School of Medicine, New Haven, Connecticut, and pioneer in long COVID research. Iwasaki was quick to point out that “we should never blame anybody for getting long COVID because there is no bulletproof way of preventing long COVID from happening” — although research shows you can increase your protection through vaccination, masking, and increasing ventilation indoors.
Also, just because you didn’t get long COVID after catching the virus once, doesn’t mean you’ll dodge the bullet if you get sick again, as Maio has now learned twice. She had long COVID in 2022 after her second bout with the virus, with breathing problems and brain fog that lasted for several months.
Subsequent long COVID experiences won’t necessarily mimic previous ones. Although Maio developed brain fog again, this time she didn’t have the breathing problems that plagued her in 2022. Instead, she had headaches so excruciating she thought she was dying of a brain aneurysm.
A Journal of the American Medical Association study released in May identified the 37 most common symptoms of long COVID, including symptom subgroups that occurred in 80% of the nearly 10,000 study participants. But the symptoms that patients with long COVID are experiencing now are slightly different from earlier in the pandemic or at least that’s what doctors are finding at the Post-COVID Recovery Clinic affiliated with the University of Pittsburgh Medical Center.
Michael Risbano, MD, the clinic’s codirector, said fewer patients have pulmonary or lung damage now than in the past, but a steady stream report problems with brain fog, forgetfulness, exercise intolerance (shortness of breath and fatigue with exercise and difficulty performing any kind of exertional activity), and post-exertional malaise (feeling wiped out or fatigued for hours or even days after physical or mental activity).
Long COVID Treatments Showing Improvement — Slowly
“There still isn’t a great way to treat any of this,” said Risbano, whose clinic is involved with the National Institute of Health’s RECOVER-VITAL trial, which is evaluating potential treatments including Paxlovid and exercise to treat autonomic dysfunction with similarities to myalgic encephalomyelitis/chronic fatigue syndrome and POTS, exercise intolerance, and neurocognitive effects such as brain fog.
Risbano and colleagues have found that physical therapy and exercise training have helped patients with exercise intolerance and neurocognitive problems. “It’s not a quick thing where they go through one visit and are better the next day,” he stressed. “It takes a little bit of time, a little bit of effort, a little bit of homework — there are no silver bullets, no magic medications.”
A quick fix was definitely not in the cards for Dean Jones, PhD, who could barely move when he developed long COVID in May 2023. A 74-year-old biochemist and professor of medicine at Emory University in Atlanta, Georgia, he’d recovered fully the first time he had COVID, in August 2022, but had a completely different experience the second time. He had been vaccinated four times when he began experiencing chronic fatigue, intense exertion-induced migraines, severe airway congestion, brain fog, and shortness of breath. The symptoms began after Memorial Day and worsened over the next month.
His resting heart rate began racing even when he was sleeping, jumping from 53 to 70 beats per minute. “It was almost as though the virus had hit my heart rather than the lungs alone,” he said.
Doctors prescribed multiple inhalers and glucocorticoids to calm Jones’s immune system. The worst symptoms began to abate after a few weeks. The bad ones continued for fully 2 months, severely limiting Jones’s activity. Although he no longer slept all day, just walking from one room to another was exhausting. A dedicated scientist who typically worked 10-15 hours a day before getting sick, he was lucky to focus on work-related tasks for a fraction of that time.
Although the migraines went away early on, the headaches remained until well into the fall. Jones’s energy level gradually returned, and by Christmas, he was beginning to feel as healthy as he had before getting COVID in May.
Still, he’s not complaining that it took so long to get better. “At 74, there’s a lot of colleagues who have already passed away,” he said. “I respect the realities of my age. There are so many people who died from COVID that I’m thankful I had those vaccines. I’m thankful that I pulled through it and was able to rebound.”
Time Helps Healing — But Prompt Care Still Needed
Recovery is the case for most patients with long COVID, said Lisa Sanders, MD, medical director of the Yale New Haven Health Systems Long COVID Consultation Clinic, which opened in March 2023. Although the clinic has a small segment of patients who have had the condition since 2020, “people who recover, who are most people, move on,” she said. “Even the patients who sometimes have to wait a month or so to see me, some of them say, ‘I’m already starting to get better. I wasn’t sure I should come.’”
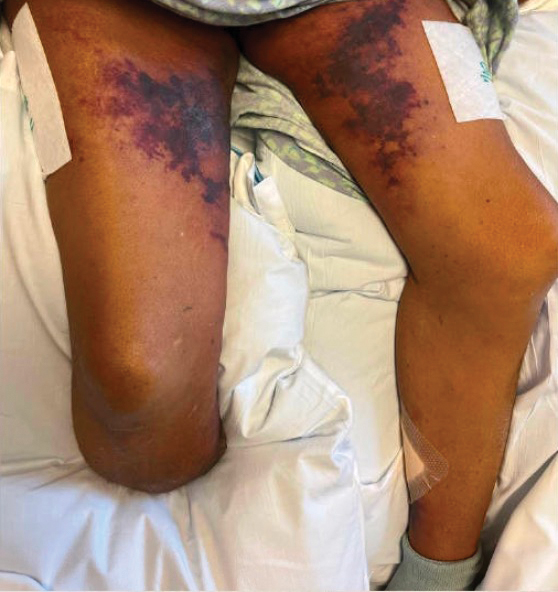
Maio, too, is recovering but only after multiple visits to the emergency room and a neurologist in late December and early January. The third emergency room trip was prompted after a brief episode in which she lost the feeling in her legs, which began convulsing. A CAT scan showed severely constricted blood vessels in her brain, leading the medical team to speculate she might have reversible cerebral vasoconstriction syndrome (RCVS), which can trigger the thunderclap headaches that had been causing her such misery.
After her third such headache prompted a fourth emergency room visit, further tests confirmed RCVS, which doctors said was related to inflammation caused by COVID. Maio was then admitted to the hospital, where she spent 4 days starting on a regimen of blood pressure medication, magnesium for the headaches, and oxycodone for pain management.
The TV show Maio works on went back into production after the holidays. She went back at the end of January. She’s still having headaches, though they’re less intense, and she’s still taking medication. She was scheduled for another test to look at her blood vessels in February.
Maio has yet to forgive herself for skipping the last booster, even though there’s no guarantee it would have prevented her from getting sick. Her message for others: it’s better to be safe than to be as sorry as she is.
“I’ll never, ever be persuaded by people who don’t believe in vaccines because I believe in science, and I believe in vaccines — that’s why people don’t die at the age of 30 anymore,” she said. “I really think that people need to know about this and what to expect. Because it is horrendous. It is very painful. I would never want anyone to go through this. Ever.”
A version of this article appeared on Medscape.com.
A celebrity makeup artist, the 55-year-old New Yorker had been boosted and vaccinated at every opportunity since vaccines were approved at the end of 2020, until the fall of 2023, when she skipped the shot.
“I really started subscribing to the mindset that you have an immune system and your immune system is supposed to work for you,” she said. “That was the stupidest thing I’ve ever done.”
Maio was not the only person to skip the latest booster: A recent study reported that while nearly 80% of adults in the United States said they’d received their first series of vaccines, barely 20% were up to date on boosters. Nor was Maio alone in getting long COVID 4 years after the start of the deadliest pandemic in a century.
It’s tempting, this far out from the shutdowns of 2020, to think the virus is over, that we’re immune, and nobody’s going to get sick anymore. But while fewer people are getting COVID, it is still very much a part of our lives. And as Maio and others are learning the hard way, long COVID is, too — and it can be deadly.
For those who have recently contracted long COVID, it can feel as if the whole world has moved on from the pandemic, and they are being left behind.
Too Easy to Let Our Guard Down
“It’s really difficult to prevent exposure to COVID no matter how careful you are and no matter how many times you are vaccinated,” said Akiko Iwasaki, an immunology professor at Yale School of Medicine, New Haven, Connecticut, and pioneer in long COVID research. Iwasaki was quick to point out that “we should never blame anybody for getting long COVID because there is no bulletproof way of preventing long COVID from happening” — although research shows you can increase your protection through vaccination, masking, and increasing ventilation indoors.
Also, just because you didn’t get long COVID after catching the virus once, doesn’t mean you’ll dodge the bullet if you get sick again, as Maio has now learned twice. She had long COVID in 2022 after her second bout with the virus, with breathing problems and brain fog that lasted for several months.
Subsequent long COVID experiences won’t necessarily mimic previous ones. Although Maio developed brain fog again, this time she didn’t have the breathing problems that plagued her in 2022. Instead, she had headaches so excruciating she thought she was dying of a brain aneurysm.
A Journal of the American Medical Association study released in May identified the 37 most common symptoms of long COVID, including symptom subgroups that occurred in 80% of the nearly 10,000 study participants. But the symptoms that patients with long COVID are experiencing now are slightly different from earlier in the pandemic or at least that’s what doctors are finding at the Post-COVID Recovery Clinic affiliated with the University of Pittsburgh Medical Center.
Michael Risbano, MD, the clinic’s codirector, said fewer patients have pulmonary or lung damage now than in the past, but a steady stream report problems with brain fog, forgetfulness, exercise intolerance (shortness of breath and fatigue with exercise and difficulty performing any kind of exertional activity), and post-exertional malaise (feeling wiped out or fatigued for hours or even days after physical or mental activity).
Long COVID Treatments Showing Improvement — Slowly
“There still isn’t a great way to treat any of this,” said Risbano, whose clinic is involved with the National Institute of Health’s RECOVER-VITAL trial, which is evaluating potential treatments including Paxlovid and exercise to treat autonomic dysfunction with similarities to myalgic encephalomyelitis/chronic fatigue syndrome and POTS, exercise intolerance, and neurocognitive effects such as brain fog.
Risbano and colleagues have found that physical therapy and exercise training have helped patients with exercise intolerance and neurocognitive problems. “It’s not a quick thing where they go through one visit and are better the next day,” he stressed. “It takes a little bit of time, a little bit of effort, a little bit of homework — there are no silver bullets, no magic medications.”
A quick fix was definitely not in the cards for Dean Jones, PhD, who could barely move when he developed long COVID in May 2023. A 74-year-old biochemist and professor of medicine at Emory University in Atlanta, Georgia, he’d recovered fully the first time he had COVID, in August 2022, but had a completely different experience the second time. He had been vaccinated four times when he began experiencing chronic fatigue, intense exertion-induced migraines, severe airway congestion, brain fog, and shortness of breath. The symptoms began after Memorial Day and worsened over the next month.
His resting heart rate began racing even when he was sleeping, jumping from 53 to 70 beats per minute. “It was almost as though the virus had hit my heart rather than the lungs alone,” he said.
Doctors prescribed multiple inhalers and glucocorticoids to calm Jones’s immune system. The worst symptoms began to abate after a few weeks. The bad ones continued for fully 2 months, severely limiting Jones’s activity. Although he no longer slept all day, just walking from one room to another was exhausting. A dedicated scientist who typically worked 10-15 hours a day before getting sick, he was lucky to focus on work-related tasks for a fraction of that time.
Although the migraines went away early on, the headaches remained until well into the fall. Jones’s energy level gradually returned, and by Christmas, he was beginning to feel as healthy as he had before getting COVID in May.
Still, he’s not complaining that it took so long to get better. “At 74, there’s a lot of colleagues who have already passed away,” he said. “I respect the realities of my age. There are so many people who died from COVID that I’m thankful I had those vaccines. I’m thankful that I pulled through it and was able to rebound.”
Time Helps Healing — But Prompt Care Still Needed
Recovery is the case for most patients with long COVID, said Lisa Sanders, MD, medical director of the Yale New Haven Health Systems Long COVID Consultation Clinic, which opened in March 2023. Although the clinic has a small segment of patients who have had the condition since 2020, “people who recover, who are most people, move on,” she said. “Even the patients who sometimes have to wait a month or so to see me, some of them say, ‘I’m already starting to get better. I wasn’t sure I should come.’”
Maio, too, is recovering but only after multiple visits to the emergency room and a neurologist in late December and early January. The third emergency room trip was prompted after a brief episode in which she lost the feeling in her legs, which began convulsing. A CAT scan showed severely constricted blood vessels in her brain, leading the medical team to speculate she might have reversible cerebral vasoconstriction syndrome (RCVS), which can trigger the thunderclap headaches that had been causing her such misery.
After her third such headache prompted a fourth emergency room visit, further tests confirmed RCVS, which doctors said was related to inflammation caused by COVID. Maio was then admitted to the hospital, where she spent 4 days starting on a regimen of blood pressure medication, magnesium for the headaches, and oxycodone for pain management.
The TV show Maio works on went back into production after the holidays. She went back at the end of January. She’s still having headaches, though they’re less intense, and she’s still taking medication. She was scheduled for another test to look at her blood vessels in February.
Maio has yet to forgive herself for skipping the last booster, even though there’s no guarantee it would have prevented her from getting sick. Her message for others: it’s better to be safe than to be as sorry as she is.
“I’ll never, ever be persuaded by people who don’t believe in vaccines because I believe in science, and I believe in vaccines — that’s why people don’t die at the age of 30 anymore,” she said. “I really think that people need to know about this and what to expect. Because it is horrendous. It is very painful. I would never want anyone to go through this. Ever.”
A version of this article appeared on Medscape.com.
A celebrity makeup artist, the 55-year-old New Yorker had been boosted and vaccinated at every opportunity since vaccines were approved at the end of 2020, until the fall of 2023, when she skipped the shot.
“I really started subscribing to the mindset that you have an immune system and your immune system is supposed to work for you,” she said. “That was the stupidest thing I’ve ever done.”
Maio was not the only person to skip the latest booster: A recent study reported that while nearly 80% of adults in the United States said they’d received their first series of vaccines, barely 20% were up to date on boosters. Nor was Maio alone in getting long COVID 4 years after the start of the deadliest pandemic in a century.
It’s tempting, this far out from the shutdowns of 2020, to think the virus is over, that we’re immune, and nobody’s going to get sick anymore. But while fewer people are getting COVID, it is still very much a part of our lives. And as Maio and others are learning the hard way, long COVID is, too — and it can be deadly.
For those who have recently contracted long COVID, it can feel as if the whole world has moved on from the pandemic, and they are being left behind.
Too Easy to Let Our Guard Down
“It’s really difficult to prevent exposure to COVID no matter how careful you are and no matter how many times you are vaccinated,” said Akiko Iwasaki, an immunology professor at Yale School of Medicine, New Haven, Connecticut, and pioneer in long COVID research. Iwasaki was quick to point out that “we should never blame anybody for getting long COVID because there is no bulletproof way of preventing long COVID from happening” — although research shows you can increase your protection through vaccination, masking, and increasing ventilation indoors.
Also, just because you didn’t get long COVID after catching the virus once, doesn’t mean you’ll dodge the bullet if you get sick again, as Maio has now learned twice. She had long COVID in 2022 after her second bout with the virus, with breathing problems and brain fog that lasted for several months.
Subsequent long COVID experiences won’t necessarily mimic previous ones. Although Maio developed brain fog again, this time she didn’t have the breathing problems that plagued her in 2022. Instead, she had headaches so excruciating she thought she was dying of a brain aneurysm.
A Journal of the American Medical Association study released in May identified the 37 most common symptoms of long COVID, including symptom subgroups that occurred in 80% of the nearly 10,000 study participants. But the symptoms that patients with long COVID are experiencing now are slightly different from earlier in the pandemic or at least that’s what doctors are finding at the Post-COVID Recovery Clinic affiliated with the University of Pittsburgh Medical Center.
Michael Risbano, MD, the clinic’s codirector, said fewer patients have pulmonary or lung damage now than in the past, but a steady stream report problems with brain fog, forgetfulness, exercise intolerance (shortness of breath and fatigue with exercise and difficulty performing any kind of exertional activity), and post-exertional malaise (feeling wiped out or fatigued for hours or even days after physical or mental activity).
Long COVID Treatments Showing Improvement — Slowly
“There still isn’t a great way to treat any of this,” said Risbano, whose clinic is involved with the National Institute of Health’s RECOVER-VITAL trial, which is evaluating potential treatments including Paxlovid and exercise to treat autonomic dysfunction with similarities to myalgic encephalomyelitis/chronic fatigue syndrome and POTS, exercise intolerance, and neurocognitive effects such as brain fog.
Risbano and colleagues have found that physical therapy and exercise training have helped patients with exercise intolerance and neurocognitive problems. “It’s not a quick thing where they go through one visit and are better the next day,” he stressed. “It takes a little bit of time, a little bit of effort, a little bit of homework — there are no silver bullets, no magic medications.”
A quick fix was definitely not in the cards for Dean Jones, PhD, who could barely move when he developed long COVID in May 2023. A 74-year-old biochemist and professor of medicine at Emory University in Atlanta, Georgia, he’d recovered fully the first time he had COVID, in August 2022, but had a completely different experience the second time. He had been vaccinated four times when he began experiencing chronic fatigue, intense exertion-induced migraines, severe airway congestion, brain fog, and shortness of breath. The symptoms began after Memorial Day and worsened over the next month.
His resting heart rate began racing even when he was sleeping, jumping from 53 to 70 beats per minute. “It was almost as though the virus had hit my heart rather than the lungs alone,” he said.
Doctors prescribed multiple inhalers and glucocorticoids to calm Jones’s immune system. The worst symptoms began to abate after a few weeks. The bad ones continued for fully 2 months, severely limiting Jones’s activity. Although he no longer slept all day, just walking from one room to another was exhausting. A dedicated scientist who typically worked 10-15 hours a day before getting sick, he was lucky to focus on work-related tasks for a fraction of that time.
Although the migraines went away early on, the headaches remained until well into the fall. Jones’s energy level gradually returned, and by Christmas, he was beginning to feel as healthy as he had before getting COVID in May.
Still, he’s not complaining that it took so long to get better. “At 74, there’s a lot of colleagues who have already passed away,” he said. “I respect the realities of my age. There are so many people who died from COVID that I’m thankful I had those vaccines. I’m thankful that I pulled through it and was able to rebound.”
Time Helps Healing — But Prompt Care Still Needed
Recovery is the case for most patients with long COVID, said Lisa Sanders, MD, medical director of the Yale New Haven Health Systems Long COVID Consultation Clinic, which opened in March 2023. Although the clinic has a small segment of patients who have had the condition since 2020, “people who recover, who are most people, move on,” she said. “Even the patients who sometimes have to wait a month or so to see me, some of them say, ‘I’m already starting to get better. I wasn’t sure I should come.’”
Maio, too, is recovering but only after multiple visits to the emergency room and a neurologist in late December and early January. The third emergency room trip was prompted after a brief episode in which she lost the feeling in her legs, which began convulsing. A CAT scan showed severely constricted blood vessels in her brain, leading the medical team to speculate she might have reversible cerebral vasoconstriction syndrome (RCVS), which can trigger the thunderclap headaches that had been causing her such misery.
After her third such headache prompted a fourth emergency room visit, further tests confirmed RCVS, which doctors said was related to inflammation caused by COVID. Maio was then admitted to the hospital, where she spent 4 days starting on a regimen of blood pressure medication, magnesium for the headaches, and oxycodone for pain management.
The TV show Maio works on went back into production after the holidays. She went back at the end of January. She’s still having headaches, though they’re less intense, and she’s still taking medication. She was scheduled for another test to look at her blood vessels in February.
Maio has yet to forgive herself for skipping the last booster, even though there’s no guarantee it would have prevented her from getting sick. Her message for others: it’s better to be safe than to be as sorry as she is.
“I’ll never, ever be persuaded by people who don’t believe in vaccines because I believe in science, and I believe in vaccines — that’s why people don’t die at the age of 30 anymore,” she said. “I really think that people need to know about this and what to expect. Because it is horrendous. It is very painful. I would never want anyone to go through this. Ever.”
A version of this article appeared on Medscape.com.
Expert Hopes to Expand Ohio Model of Melanoma Case Reporting
SAN DIEGO – Soon after Brett M. Coldiron, MD, launched his Cincinnati-based dermatology and Mohs surgery practice more than 20 years ago, he reported his first three cases of thin melanomas to the Ohio Department of Health, as mandated by state law.
“I got sent reams of paperwork to fill out that I did not understand,” Dr. Coldiron, a past president of the American College of Mohs Surgery and the American Academy of Dermatology, recalled at the annual Cutaneous Malignancy Update. “Then, I got chewed out for not reporting sooner and threatened with thousands of dollars in fines if I did not promptly report the forms in the future. It was an obnoxious experience.”
About 15 years later, while testifying at the Ohio Legislature on medical reasons to restrict the use of tanning beds, a lobbyist for the tanning bed industry told him that the melanoma rates had been stable in Ohio for the previous 5 years. “It turns out they were cherry picking certain segments of data to fit their narrative,” Dr. Coldiron said. “I was stunned and it kind of deflated me. I thought about this for a long time, and thought, ‘how do we solve this issue of reporting melanoma cases without adding work to existing staff if you’re a small practice and without spending significant amounts of money? Let’s make this easier.’ ”
In addition to reducing the use of tanning beds, proper reporting of melanoma cases is important for reasons that include efforts to increase sunscreen use and to be counted in ongoing research efforts to obtain a realistic snapshot of melanoma prevalence and incidence, he said.
Quality of melanoma case reporting relies on the Centers for Disease Control and Prevention’s National Program of Cancer Registries (NPCR), and the National Cancer Institute’s Surveillance Epidemiology and End Results (SEER) Program, which collects data on the incidence, treatment, staging, and survival for 28% of the US population. All 50 states and US territories require melanoma to be reported to the NPCR, but while most hospital systems have reporting protocols and dedicated data registrars, private practices may not.
Also, many dermatopathology practices operate independently and do not have dedicated registrars and may not report cases. “Melanoma is unique in that it is often completely managed in outpatient settings and these melanomas may never be reported,” said Dr. Coldiron, current president of the Ohio Dermatological Foundation. “That’s the practice gap.” One study published in 2018 found that only 49% of dermatologists knew that melanoma was a reportable disease and only 34% routinely reported newly diagnosed cases to their state’s cancer registry. He characterized melanoma reporting as an unfunded mandate.
“Hospitals are doing the most of them, because they have a registrar,” he said. “Small practices have to assign someone to do this, and it can be difficult to train that person. It’s time consuming. The first time we did it, it took an hour,” but, he said, taking a 2-hour tutorial from the Ohio Department of Health helped.
He noted that there is a lack of awareness and clinicians think it’s the dermatopathologist’s job to report cases, “while the dermatopathologist thinks it’s the clinician’s job,” and many of the entry fields are not applicable to thinner melanomas.
There is also a “patchwork” of ways that state departments of health accept the information, not all electronically, he continued. For example, those in Arizona, Montana, West Virginia, Delaware, Vermont, and Maine accept paper copies only, “meaning you have to download a PDF, fill it out, and fax it back to them,” Dr. Coldiron said at the meeting, which was hosted by Scripps Cancer Center.
“We have them sign a HIPAA form and take the two-hour online tutorial,” he said. They download data that Ohio dermatologists have faxed to a dedicated secure HIPAA-compliant cloud-based fax line that Dr. Coldiron has set up, and the cases are then sent to the Ohio Department of Health.
Dr. Coldiron and colleagues have also partnered with the University of Cincinnati Clermont, which offers a National Cancer Registries Association–accredited certificate program — one of several nationwide. Students in this program are trained to become cancer registrars. “The university staff are gung-ho about it because they are looking for easy cases to train the students on. Also, the Ohio Department of Health staff are keen to help train the students and even help them find jobs or hire them after they complete the degree. Staff from the department of health and college faculty are fully engaged and supervising. It’s a win-win for all.”
According to Dr. Coldiron, in 2023, 8 Ohio dermatology practices were sending their reports to the fax line he set up and 7 more have signed up in recent months, making 15 practices to date. “It’s self-perpetuating at this point,” he said. “The Ohio Department of Health and the University of Cincinnati are invested in this program long-term.” The fax service costs Dr. Coldiron $42 per month — a small price to pay, he said, for being a clearinghouse for private Ohio dermatology practices looking for a practical way to report their melanoma cases. The model has increased melanoma reporting in Ohio by 2.8% in the last 2 years, “which doesn’t seem like that many, but if there are 6500 cases of melanoma, and you can increase reporting by a couple hundred cases, that’s a lot,” he said.
His goal is to expand this model to more states. “Dermatologists, surgical oncologists, and cancer center administrators should embrace this opportunity to make their practices a clearinghouse for their state,” he said. “This is an opportunity to improve state health, quality improvement projects, help providers, and gain recognition as a center of excellence. The increase in incidence of melanoma will lend great clout to public and legislative requests for prevention, treatment, and research dollars.”
In an interview, Hugh Greenway, MD, the head of Mohs and dermatologic surgery at Scripps Clinic in San Diego, also noted that cutaneous melanoma is significantly underreported in spite of individual state requirements. “As Dr. Coldiron reminds us, the main reason is that in many cases the pathology diagnosis and report come from the dermatologist’s/dermatopathologist’s office,” Dr. Greenway said. “With no hospital or large multispecialty laboratory involved, the reporting may be incomplete or not done. This is not the case with almost all other cancers where a hospital laboratory is involved.”
If widespread adoption of Dr. Coldiron’s model can occur, he added, “then we will have much better melanoma reporting data on which to both help our patients and specialty. He is to be applauded for producing a workable solution to the problem of underreporting.”
Dr. Coldiron reported having no relevant disclosures. Dr. Greenway reported that he conducts research for Castle Biosciences. He is also course director of the annual Cutaneous Malignancy Update.
SAN DIEGO – Soon after Brett M. Coldiron, MD, launched his Cincinnati-based dermatology and Mohs surgery practice more than 20 years ago, he reported his first three cases of thin melanomas to the Ohio Department of Health, as mandated by state law.
“I got sent reams of paperwork to fill out that I did not understand,” Dr. Coldiron, a past president of the American College of Mohs Surgery and the American Academy of Dermatology, recalled at the annual Cutaneous Malignancy Update. “Then, I got chewed out for not reporting sooner and threatened with thousands of dollars in fines if I did not promptly report the forms in the future. It was an obnoxious experience.”
About 15 years later, while testifying at the Ohio Legislature on medical reasons to restrict the use of tanning beds, a lobbyist for the tanning bed industry told him that the melanoma rates had been stable in Ohio for the previous 5 years. “It turns out they were cherry picking certain segments of data to fit their narrative,” Dr. Coldiron said. “I was stunned and it kind of deflated me. I thought about this for a long time, and thought, ‘how do we solve this issue of reporting melanoma cases without adding work to existing staff if you’re a small practice and without spending significant amounts of money? Let’s make this easier.’ ”
In addition to reducing the use of tanning beds, proper reporting of melanoma cases is important for reasons that include efforts to increase sunscreen use and to be counted in ongoing research efforts to obtain a realistic snapshot of melanoma prevalence and incidence, he said.
Quality of melanoma case reporting relies on the Centers for Disease Control and Prevention’s National Program of Cancer Registries (NPCR), and the National Cancer Institute’s Surveillance Epidemiology and End Results (SEER) Program, which collects data on the incidence, treatment, staging, and survival for 28% of the US population. All 50 states and US territories require melanoma to be reported to the NPCR, but while most hospital systems have reporting protocols and dedicated data registrars, private practices may not.
Also, many dermatopathology practices operate independently and do not have dedicated registrars and may not report cases. “Melanoma is unique in that it is often completely managed in outpatient settings and these melanomas may never be reported,” said Dr. Coldiron, current president of the Ohio Dermatological Foundation. “That’s the practice gap.” One study published in 2018 found that only 49% of dermatologists knew that melanoma was a reportable disease and only 34% routinely reported newly diagnosed cases to their state’s cancer registry. He characterized melanoma reporting as an unfunded mandate.
“Hospitals are doing the most of them, because they have a registrar,” he said. “Small practices have to assign someone to do this, and it can be difficult to train that person. It’s time consuming. The first time we did it, it took an hour,” but, he said, taking a 2-hour tutorial from the Ohio Department of Health helped.
He noted that there is a lack of awareness and clinicians think it’s the dermatopathologist’s job to report cases, “while the dermatopathologist thinks it’s the clinician’s job,” and many of the entry fields are not applicable to thinner melanomas.
There is also a “patchwork” of ways that state departments of health accept the information, not all electronically, he continued. For example, those in Arizona, Montana, West Virginia, Delaware, Vermont, and Maine accept paper copies only, “meaning you have to download a PDF, fill it out, and fax it back to them,” Dr. Coldiron said at the meeting, which was hosted by Scripps Cancer Center.
“We have them sign a HIPAA form and take the two-hour online tutorial,” he said. They download data that Ohio dermatologists have faxed to a dedicated secure HIPAA-compliant cloud-based fax line that Dr. Coldiron has set up, and the cases are then sent to the Ohio Department of Health.
Dr. Coldiron and colleagues have also partnered with the University of Cincinnati Clermont, which offers a National Cancer Registries Association–accredited certificate program — one of several nationwide. Students in this program are trained to become cancer registrars. “The university staff are gung-ho about it because they are looking for easy cases to train the students on. Also, the Ohio Department of Health staff are keen to help train the students and even help them find jobs or hire them after they complete the degree. Staff from the department of health and college faculty are fully engaged and supervising. It’s a win-win for all.”
According to Dr. Coldiron, in 2023, 8 Ohio dermatology practices were sending their reports to the fax line he set up and 7 more have signed up in recent months, making 15 practices to date. “It’s self-perpetuating at this point,” he said. “The Ohio Department of Health and the University of Cincinnati are invested in this program long-term.” The fax service costs Dr. Coldiron $42 per month — a small price to pay, he said, for being a clearinghouse for private Ohio dermatology practices looking for a practical way to report their melanoma cases. The model has increased melanoma reporting in Ohio by 2.8% in the last 2 years, “which doesn’t seem like that many, but if there are 6500 cases of melanoma, and you can increase reporting by a couple hundred cases, that’s a lot,” he said.
His goal is to expand this model to more states. “Dermatologists, surgical oncologists, and cancer center administrators should embrace this opportunity to make their practices a clearinghouse for their state,” he said. “This is an opportunity to improve state health, quality improvement projects, help providers, and gain recognition as a center of excellence. The increase in incidence of melanoma will lend great clout to public and legislative requests for prevention, treatment, and research dollars.”
In an interview, Hugh Greenway, MD, the head of Mohs and dermatologic surgery at Scripps Clinic in San Diego, also noted that cutaneous melanoma is significantly underreported in spite of individual state requirements. “As Dr. Coldiron reminds us, the main reason is that in many cases the pathology diagnosis and report come from the dermatologist’s/dermatopathologist’s office,” Dr. Greenway said. “With no hospital or large multispecialty laboratory involved, the reporting may be incomplete or not done. This is not the case with almost all other cancers where a hospital laboratory is involved.”
If widespread adoption of Dr. Coldiron’s model can occur, he added, “then we will have much better melanoma reporting data on which to both help our patients and specialty. He is to be applauded for producing a workable solution to the problem of underreporting.”
Dr. Coldiron reported having no relevant disclosures. Dr. Greenway reported that he conducts research for Castle Biosciences. He is also course director of the annual Cutaneous Malignancy Update.
SAN DIEGO – Soon after Brett M. Coldiron, MD, launched his Cincinnati-based dermatology and Mohs surgery practice more than 20 years ago, he reported his first three cases of thin melanomas to the Ohio Department of Health, as mandated by state law.
“I got sent reams of paperwork to fill out that I did not understand,” Dr. Coldiron, a past president of the American College of Mohs Surgery and the American Academy of Dermatology, recalled at the annual Cutaneous Malignancy Update. “Then, I got chewed out for not reporting sooner and threatened with thousands of dollars in fines if I did not promptly report the forms in the future. It was an obnoxious experience.”
About 15 years later, while testifying at the Ohio Legislature on medical reasons to restrict the use of tanning beds, a lobbyist for the tanning bed industry told him that the melanoma rates had been stable in Ohio for the previous 5 years. “It turns out they were cherry picking certain segments of data to fit their narrative,” Dr. Coldiron said. “I was stunned and it kind of deflated me. I thought about this for a long time, and thought, ‘how do we solve this issue of reporting melanoma cases without adding work to existing staff if you’re a small practice and without spending significant amounts of money? Let’s make this easier.’ ”
In addition to reducing the use of tanning beds, proper reporting of melanoma cases is important for reasons that include efforts to increase sunscreen use and to be counted in ongoing research efforts to obtain a realistic snapshot of melanoma prevalence and incidence, he said.
Quality of melanoma case reporting relies on the Centers for Disease Control and Prevention’s National Program of Cancer Registries (NPCR), and the National Cancer Institute’s Surveillance Epidemiology and End Results (SEER) Program, which collects data on the incidence, treatment, staging, and survival for 28% of the US population. All 50 states and US territories require melanoma to be reported to the NPCR, but while most hospital systems have reporting protocols and dedicated data registrars, private practices may not.
Also, many dermatopathology practices operate independently and do not have dedicated registrars and may not report cases. “Melanoma is unique in that it is often completely managed in outpatient settings and these melanomas may never be reported,” said Dr. Coldiron, current president of the Ohio Dermatological Foundation. “That’s the practice gap.” One study published in 2018 found that only 49% of dermatologists knew that melanoma was a reportable disease and only 34% routinely reported newly diagnosed cases to their state’s cancer registry. He characterized melanoma reporting as an unfunded mandate.
“Hospitals are doing the most of them, because they have a registrar,” he said. “Small practices have to assign someone to do this, and it can be difficult to train that person. It’s time consuming. The first time we did it, it took an hour,” but, he said, taking a 2-hour tutorial from the Ohio Department of Health helped.
He noted that there is a lack of awareness and clinicians think it’s the dermatopathologist’s job to report cases, “while the dermatopathologist thinks it’s the clinician’s job,” and many of the entry fields are not applicable to thinner melanomas.
There is also a “patchwork” of ways that state departments of health accept the information, not all electronically, he continued. For example, those in Arizona, Montana, West Virginia, Delaware, Vermont, and Maine accept paper copies only, “meaning you have to download a PDF, fill it out, and fax it back to them,” Dr. Coldiron said at the meeting, which was hosted by Scripps Cancer Center.
“We have them sign a HIPAA form and take the two-hour online tutorial,” he said. They download data that Ohio dermatologists have faxed to a dedicated secure HIPAA-compliant cloud-based fax line that Dr. Coldiron has set up, and the cases are then sent to the Ohio Department of Health.
Dr. Coldiron and colleagues have also partnered with the University of Cincinnati Clermont, which offers a National Cancer Registries Association–accredited certificate program — one of several nationwide. Students in this program are trained to become cancer registrars. “The university staff are gung-ho about it because they are looking for easy cases to train the students on. Also, the Ohio Department of Health staff are keen to help train the students and even help them find jobs or hire them after they complete the degree. Staff from the department of health and college faculty are fully engaged and supervising. It’s a win-win for all.”
According to Dr. Coldiron, in 2023, 8 Ohio dermatology practices were sending their reports to the fax line he set up and 7 more have signed up in recent months, making 15 practices to date. “It’s self-perpetuating at this point,” he said. “The Ohio Department of Health and the University of Cincinnati are invested in this program long-term.” The fax service costs Dr. Coldiron $42 per month — a small price to pay, he said, for being a clearinghouse for private Ohio dermatology practices looking for a practical way to report their melanoma cases. The model has increased melanoma reporting in Ohio by 2.8% in the last 2 years, “which doesn’t seem like that many, but if there are 6500 cases of melanoma, and you can increase reporting by a couple hundred cases, that’s a lot,” he said.
His goal is to expand this model to more states. “Dermatologists, surgical oncologists, and cancer center administrators should embrace this opportunity to make their practices a clearinghouse for their state,” he said. “This is an opportunity to improve state health, quality improvement projects, help providers, and gain recognition as a center of excellence. The increase in incidence of melanoma will lend great clout to public and legislative requests for prevention, treatment, and research dollars.”
In an interview, Hugh Greenway, MD, the head of Mohs and dermatologic surgery at Scripps Clinic in San Diego, also noted that cutaneous melanoma is significantly underreported in spite of individual state requirements. “As Dr. Coldiron reminds us, the main reason is that in many cases the pathology diagnosis and report come from the dermatologist’s/dermatopathologist’s office,” Dr. Greenway said. “With no hospital or large multispecialty laboratory involved, the reporting may be incomplete or not done. This is not the case with almost all other cancers where a hospital laboratory is involved.”
If widespread adoption of Dr. Coldiron’s model can occur, he added, “then we will have much better melanoma reporting data on which to both help our patients and specialty. He is to be applauded for producing a workable solution to the problem of underreporting.”
Dr. Coldiron reported having no relevant disclosures. Dr. Greenway reported that he conducts research for Castle Biosciences. He is also course director of the annual Cutaneous Malignancy Update.
FROM MELANOMA 2024
Diabetes Tech Falls Short as Hypoglycemic Challenges Persist
TOPLINE:
Despite diabetes technology, many with type 1 diabetes (T1D) miss glycemic targets and experience severe hypoglycemia and impaired awareness of hypoglycemia (IAH).
METHODOLOGY:
- The clinical management of T1D through technology is now recognized as the standard of care, but its real-world impact on glycemic targets and severe hypoglycemic events and IAH is unclear.
- Researchers assessed the self-reported prevalence of glycemic metrics, severe hypoglycemia, and hypoglycemia awareness according to the use of continuous glucose monitoring (CGM) and automated insulin delivery (AID) systems.
- They enrolled 2044 individuals diagnosed with T1D for at least 2 years (mean age, 43.0 years; 72.1% women; 95.4% White) from the T1D Exchange Registry and online communities who filled an online survey.
- Participants were stratified on the basis of the presence or absence of CGM and different insulin delivery methods (multiple daily injections, conventional pumps, or AID systems).
- The primary outcome was the proportion of participants who achieved glycemic targets (self-reported A1c), had severe hypoglycemia (any low glucose incidence in 12 months), and/or IAH (a modified Gold score on a seven-point Likert scale).
TAKEAWAY:
- Most participants (91.7%) used CGM, and 50.8% of CGM users used an AID system.
- Despite advanced interventions, only 59.6% (95% CI, 57.3%-61.8%) of CGM users met the glycemic target (A1c < 7%), while nearly 40% of CGM users and 35.6% of AID users didn’t reach the target.
- At least one event of severe hypoglycemia in the previous 12 months was reported in 10.8% of CGM users and 16.6% of those using an AID system.
- IAH prevalence was seen in 31.1% (95% CI, 29.0%-33.2%) and 30.3% (95% CI, 17.5%-33.3%) of participants using CGM and CGM + AID, respectively.
IN PRACTICE:
“Educational initiatives continue to be important for all individuals with type 1 diabetes, and the development of novel therapeutic options and strategies, including bihormonal AID systems and beta-cell replacement, will be required to enable more of these individuals to meet treatment goals,” the authors wrote.
SOURCE:
The study, published online in Diabetes Care, was led by Jennifer L. Sherr, MD, PhD, Yale School of Medicine, New Haven, Connecticut.
LIMITATIONS:
The survey participants in this study were from the T1D Exchange online community, who tend to be highly involved, have technology experience, and are more likely to achieve glycemic targets. The data reported as part of the survey were based on self-reports by participants and may be subject to recall bias. Notably, severe hypoglycemic events may be overreported by individuals using CGM and AID systems due to sensor alarms.
DISCLOSURES:
The study was funded by Vertex Pharmaceuticals. Several authors disclosed financial relationships, including grants, consulting fees, honoraria, stock ownership, and employment with pharmaceutical and device companies and other entities.
A version of this article appeared on Medscape.com.
TOPLINE:
Despite diabetes technology, many with type 1 diabetes (T1D) miss glycemic targets and experience severe hypoglycemia and impaired awareness of hypoglycemia (IAH).
METHODOLOGY:
- The clinical management of T1D through technology is now recognized as the standard of care, but its real-world impact on glycemic targets and severe hypoglycemic events and IAH is unclear.
- Researchers assessed the self-reported prevalence of glycemic metrics, severe hypoglycemia, and hypoglycemia awareness according to the use of continuous glucose monitoring (CGM) and automated insulin delivery (AID) systems.
- They enrolled 2044 individuals diagnosed with T1D for at least 2 years (mean age, 43.0 years; 72.1% women; 95.4% White) from the T1D Exchange Registry and online communities who filled an online survey.
- Participants were stratified on the basis of the presence or absence of CGM and different insulin delivery methods (multiple daily injections, conventional pumps, or AID systems).
- The primary outcome was the proportion of participants who achieved glycemic targets (self-reported A1c), had severe hypoglycemia (any low glucose incidence in 12 months), and/or IAH (a modified Gold score on a seven-point Likert scale).
TAKEAWAY:
- Most participants (91.7%) used CGM, and 50.8% of CGM users used an AID system.
- Despite advanced interventions, only 59.6% (95% CI, 57.3%-61.8%) of CGM users met the glycemic target (A1c < 7%), while nearly 40% of CGM users and 35.6% of AID users didn’t reach the target.
- At least one event of severe hypoglycemia in the previous 12 months was reported in 10.8% of CGM users and 16.6% of those using an AID system.
- IAH prevalence was seen in 31.1% (95% CI, 29.0%-33.2%) and 30.3% (95% CI, 17.5%-33.3%) of participants using CGM and CGM + AID, respectively.
IN PRACTICE:
“Educational initiatives continue to be important for all individuals with type 1 diabetes, and the development of novel therapeutic options and strategies, including bihormonal AID systems and beta-cell replacement, will be required to enable more of these individuals to meet treatment goals,” the authors wrote.
SOURCE:
The study, published online in Diabetes Care, was led by Jennifer L. Sherr, MD, PhD, Yale School of Medicine, New Haven, Connecticut.
LIMITATIONS:
The survey participants in this study were from the T1D Exchange online community, who tend to be highly involved, have technology experience, and are more likely to achieve glycemic targets. The data reported as part of the survey were based on self-reports by participants and may be subject to recall bias. Notably, severe hypoglycemic events may be overreported by individuals using CGM and AID systems due to sensor alarms.
DISCLOSURES:
The study was funded by Vertex Pharmaceuticals. Several authors disclosed financial relationships, including grants, consulting fees, honoraria, stock ownership, and employment with pharmaceutical and device companies and other entities.
A version of this article appeared on Medscape.com.
TOPLINE:
Despite diabetes technology, many with type 1 diabetes (T1D) miss glycemic targets and experience severe hypoglycemia and impaired awareness of hypoglycemia (IAH).
METHODOLOGY:
- The clinical management of T1D through technology is now recognized as the standard of care, but its real-world impact on glycemic targets and severe hypoglycemic events and IAH is unclear.
- Researchers assessed the self-reported prevalence of glycemic metrics, severe hypoglycemia, and hypoglycemia awareness according to the use of continuous glucose monitoring (CGM) and automated insulin delivery (AID) systems.
- They enrolled 2044 individuals diagnosed with T1D for at least 2 years (mean age, 43.0 years; 72.1% women; 95.4% White) from the T1D Exchange Registry and online communities who filled an online survey.
- Participants were stratified on the basis of the presence or absence of CGM and different insulin delivery methods (multiple daily injections, conventional pumps, or AID systems).
- The primary outcome was the proportion of participants who achieved glycemic targets (self-reported A1c), had severe hypoglycemia (any low glucose incidence in 12 months), and/or IAH (a modified Gold score on a seven-point Likert scale).
TAKEAWAY:
- Most participants (91.7%) used CGM, and 50.8% of CGM users used an AID system.
- Despite advanced interventions, only 59.6% (95% CI, 57.3%-61.8%) of CGM users met the glycemic target (A1c < 7%), while nearly 40% of CGM users and 35.6% of AID users didn’t reach the target.
- At least one event of severe hypoglycemia in the previous 12 months was reported in 10.8% of CGM users and 16.6% of those using an AID system.
- IAH prevalence was seen in 31.1% (95% CI, 29.0%-33.2%) and 30.3% (95% CI, 17.5%-33.3%) of participants using CGM and CGM + AID, respectively.
IN PRACTICE:
“Educational initiatives continue to be important for all individuals with type 1 diabetes, and the development of novel therapeutic options and strategies, including bihormonal AID systems and beta-cell replacement, will be required to enable more of these individuals to meet treatment goals,” the authors wrote.
SOURCE:
The study, published online in Diabetes Care, was led by Jennifer L. Sherr, MD, PhD, Yale School of Medicine, New Haven, Connecticut.
LIMITATIONS:
The survey participants in this study were from the T1D Exchange online community, who tend to be highly involved, have technology experience, and are more likely to achieve glycemic targets. The data reported as part of the survey were based on self-reports by participants and may be subject to recall bias. Notably, severe hypoglycemic events may be overreported by individuals using CGM and AID systems due to sensor alarms.
DISCLOSURES:
The study was funded by Vertex Pharmaceuticals. Several authors disclosed financial relationships, including grants, consulting fees, honoraria, stock ownership, and employment with pharmaceutical and device companies and other entities.
A version of this article appeared on Medscape.com.
Premeal Stomach-Filling Capsule Effective for Weight Loss
TOPLINE:
Oral intragastric expandable capsules taken twice daily before meals reduce body weight in adults with overweight or obesity compared with placebo, with mild gastrointestinal adverse events.
METHODOLOGY:
- Numerous anti-obesity pharmacotherapies have demonstrated effectiveness in reducing weight, but they may lead to side effects.
- This 24-week phase 3 randomized placebo-controlled study evaluated 2.24 g oral intragastric expandable capsules for weight loss in 280 adults (ages 18-60 years) with overweight or obesity (body mass index ≥ 24 kg/m2).
- One capsule, taken before lunch and dinner with water, expands to fill about one quarter of average stomach volume and then passes through the body, similar to the US Food and Drug Administration–cleared device Plenity.
- Primary endpoints were the percentage change in body weight from baseline and the weight loss response rate (weight loss of at least 5% of baseline body weight) at week 24.
- Researchers analyzed efficacy outcomes in two ways: Intention to treat (a full analysis based on groups to which they were randomly assigned) and per protocol (based on data from participants who follow the protocol).
TAKEAWAY:
- At 24 weeks, the change in mean body weight was higher with intragastric expandable capsules than with placebo using the per protocol set (estimated treatment difference [ETD], −3.6%; P < .001), with similar results using the full analysis set.
- The weight loss response rate at 24 weeks was higher with intragastric expandable capsules than with placebo using the per protocol set (ETD, 29.6%; P < .001), with similar results using the full analysis set.
- Reduction in fasting insulin levels was higher with intragastric expandable capsules than with placebo (P = .008), while improvements in the lipid profile, fasting plasma glucose levels, and heart rate were similar between the groups.
- Gastrointestinal disorders were reported in 25.0% of participants in the intragastric expandable capsule group compared with 21.9% in the placebo group, with most being transient and mild in severity.
IN PRACTICE:
“As a mild and safe anti-obesity medication, intragastric expandable capsules provide a new therapeutic choice for individuals with overweight or obesity, helping them to enhance and maintain the effect of diet restriction,” wrote the authors.
SOURCE:
Difei Lu, MD, Department of Endocrinology, Peking University First Hospital, Beijing, China, led the study, which was published online in Diabetes, Obesity and Metabolism.
LIMITATIONS:
The study included individuals who were overweight or obese, who might have been more willing to lose weight than the general population. Moreover, only 3.25% of the participants had type 2 diabetes, and participants were relatively young. This may have reduced the potential to discover metabolic or cardiovascular improvement by the product.
DISCLOSURES:
This study was funded by Xiamen Junde Pharmaceutical Technology. The authors disclosed no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
Oral intragastric expandable capsules taken twice daily before meals reduce body weight in adults with overweight or obesity compared with placebo, with mild gastrointestinal adverse events.
METHODOLOGY:
- Numerous anti-obesity pharmacotherapies have demonstrated effectiveness in reducing weight, but they may lead to side effects.
- This 24-week phase 3 randomized placebo-controlled study evaluated 2.24 g oral intragastric expandable capsules for weight loss in 280 adults (ages 18-60 years) with overweight or obesity (body mass index ≥ 24 kg/m2).
- One capsule, taken before lunch and dinner with water, expands to fill about one quarter of average stomach volume and then passes through the body, similar to the US Food and Drug Administration–cleared device Plenity.
- Primary endpoints were the percentage change in body weight from baseline and the weight loss response rate (weight loss of at least 5% of baseline body weight) at week 24.
- Researchers analyzed efficacy outcomes in two ways: Intention to treat (a full analysis based on groups to which they were randomly assigned) and per protocol (based on data from participants who follow the protocol).
TAKEAWAY:
- At 24 weeks, the change in mean body weight was higher with intragastric expandable capsules than with placebo using the per protocol set (estimated treatment difference [ETD], −3.6%; P < .001), with similar results using the full analysis set.
- The weight loss response rate at 24 weeks was higher with intragastric expandable capsules than with placebo using the per protocol set (ETD, 29.6%; P < .001), with similar results using the full analysis set.
- Reduction in fasting insulin levels was higher with intragastric expandable capsules than with placebo (P = .008), while improvements in the lipid profile, fasting plasma glucose levels, and heart rate were similar between the groups.
- Gastrointestinal disorders were reported in 25.0% of participants in the intragastric expandable capsule group compared with 21.9% in the placebo group, with most being transient and mild in severity.
IN PRACTICE:
“As a mild and safe anti-obesity medication, intragastric expandable capsules provide a new therapeutic choice for individuals with overweight or obesity, helping them to enhance and maintain the effect of diet restriction,” wrote the authors.
SOURCE:
Difei Lu, MD, Department of Endocrinology, Peking University First Hospital, Beijing, China, led the study, which was published online in Diabetes, Obesity and Metabolism.
LIMITATIONS:
The study included individuals who were overweight or obese, who might have been more willing to lose weight than the general population. Moreover, only 3.25% of the participants had type 2 diabetes, and participants were relatively young. This may have reduced the potential to discover metabolic or cardiovascular improvement by the product.
DISCLOSURES:
This study was funded by Xiamen Junde Pharmaceutical Technology. The authors disclosed no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
Oral intragastric expandable capsules taken twice daily before meals reduce body weight in adults with overweight or obesity compared with placebo, with mild gastrointestinal adverse events.
METHODOLOGY:
- Numerous anti-obesity pharmacotherapies have demonstrated effectiveness in reducing weight, but they may lead to side effects.
- This 24-week phase 3 randomized placebo-controlled study evaluated 2.24 g oral intragastric expandable capsules for weight loss in 280 adults (ages 18-60 years) with overweight or obesity (body mass index ≥ 24 kg/m2).
- One capsule, taken before lunch and dinner with water, expands to fill about one quarter of average stomach volume and then passes through the body, similar to the US Food and Drug Administration–cleared device Plenity.
- Primary endpoints were the percentage change in body weight from baseline and the weight loss response rate (weight loss of at least 5% of baseline body weight) at week 24.
- Researchers analyzed efficacy outcomes in two ways: Intention to treat (a full analysis based on groups to which they were randomly assigned) and per protocol (based on data from participants who follow the protocol).
TAKEAWAY:
- At 24 weeks, the change in mean body weight was higher with intragastric expandable capsules than with placebo using the per protocol set (estimated treatment difference [ETD], −3.6%; P < .001), with similar results using the full analysis set.
- The weight loss response rate at 24 weeks was higher with intragastric expandable capsules than with placebo using the per protocol set (ETD, 29.6%; P < .001), with similar results using the full analysis set.
- Reduction in fasting insulin levels was higher with intragastric expandable capsules than with placebo (P = .008), while improvements in the lipid profile, fasting plasma glucose levels, and heart rate were similar between the groups.
- Gastrointestinal disorders were reported in 25.0% of participants in the intragastric expandable capsule group compared with 21.9% in the placebo group, with most being transient and mild in severity.
IN PRACTICE:
“As a mild and safe anti-obesity medication, intragastric expandable capsules provide a new therapeutic choice for individuals with overweight or obesity, helping them to enhance and maintain the effect of diet restriction,” wrote the authors.
SOURCE:
Difei Lu, MD, Department of Endocrinology, Peking University First Hospital, Beijing, China, led the study, which was published online in Diabetes, Obesity and Metabolism.
LIMITATIONS:
The study included individuals who were overweight or obese, who might have been more willing to lose weight than the general population. Moreover, only 3.25% of the participants had type 2 diabetes, and participants were relatively young. This may have reduced the potential to discover metabolic or cardiovascular improvement by the product.
DISCLOSURES:
This study was funded by Xiamen Junde Pharmaceutical Technology. The authors disclosed no conflicts of interest.
A version of this article appeared on Medscape.com.
Hypertriglyceridemia in Young Adults Raises Red Flag
TOPLINE:
Persistent hypertriglyceridemia is linked to an increased risk for type 2 diabetes (T2D) in young adults, independent of lifestyle factors.
METHODOLOGY:
- This prospective study analyzed the data of 1,840,251 individuals aged 20-39 years from the South Korean National Health Insurance Service database (mean age 34 years, 71% male).
- The individuals had undergone four consecutive annual health checkups between 2009 and 2012 and had no history of T2D.
- The individuals were sorted into five groups indicating the number of hypertriglyceridemia diagnoses over four consecutive years: 0, 1, 2, 3, and 4, defined as serum fasting triglyceride levels of 150 mg/dL or higher.
- Data on lifestyle-related risk factors, such as smoking status and heavy alcohol consumption, were collected through self-reported questionnaires.
- The primary outcome was newly diagnosed cases of T2D. Over a mean follow-up of 6.53 years, a total of 40,286 individuals developed T2D.
TAKEAWAY:
- The cumulative incidence of T2D increased with an increase in exposure scores for hypertriglyceridemia (log-rank test, P < .001), independent of lifestyle-related factors.
- The incidence rate per 1000 person-years was 1.25 for participants with an exposure score of 0 and 11.55 for those with a score of 4.
- For individuals with exposure scores of 1, 2, 3, and 4, the adjusted hazard ratios for incident diabetes were 1.674 (95% CI, 1.619-1.732), 2.192 (2.117-2.269), 2.637 (2.548-2.73), and 3.715 (3.6-3.834), respectively, vs those with an exposure score of 0.
- Exploratory subgroup analyses suggested the risk for T2D in persistent hypertriglyceridemia were more pronounced among people in their 20s than in their 30s and in women.
IN PRACTICE:
“Identification of individuals at higher risk based on triglyceride levels and management strategies for persistent hypertriglyceridemia in young adults could potentially reduce the burden of young-onset type 2 diabetes and enhance long-term health outcomes,” the authors wrote.
SOURCE:
The study, led by Min-Kyung Lee, Division of Endocrinology and Metabolism, Department of Internal Medicine, Myongji Hospital, Hanyang University College of Medicine, Seoul, Republic of Korea, was published online in Diabetes Research and Clinical Practice.
LIMITATIONS:
The scoring system based on fasting triglyceride levels of ≥ 150 mg/dL may have limitations, as the cumulative incidence of T2D also varied significantly for mean triglyceride levels. Moreover, relying on a single annual health checkup for hypertriglyceridemia diagnosis might not capture short-term fluctuations. Despite sufficient cases and a high follow-up rate, the study might have underestimated the incidence of T2D.
DISCLOSURES:
This work was supported by the National Research Foundation of Korea grant funded by the Korean Government and the faculty grant of Myongji Hospital. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
Persistent hypertriglyceridemia is linked to an increased risk for type 2 diabetes (T2D) in young adults, independent of lifestyle factors.
METHODOLOGY:
- This prospective study analyzed the data of 1,840,251 individuals aged 20-39 years from the South Korean National Health Insurance Service database (mean age 34 years, 71% male).
- The individuals had undergone four consecutive annual health checkups between 2009 and 2012 and had no history of T2D.
- The individuals were sorted into five groups indicating the number of hypertriglyceridemia diagnoses over four consecutive years: 0, 1, 2, 3, and 4, defined as serum fasting triglyceride levels of 150 mg/dL or higher.
- Data on lifestyle-related risk factors, such as smoking status and heavy alcohol consumption, were collected through self-reported questionnaires.
- The primary outcome was newly diagnosed cases of T2D. Over a mean follow-up of 6.53 years, a total of 40,286 individuals developed T2D.
TAKEAWAY:
- The cumulative incidence of T2D increased with an increase in exposure scores for hypertriglyceridemia (log-rank test, P < .001), independent of lifestyle-related factors.
- The incidence rate per 1000 person-years was 1.25 for participants with an exposure score of 0 and 11.55 for those with a score of 4.
- For individuals with exposure scores of 1, 2, 3, and 4, the adjusted hazard ratios for incident diabetes were 1.674 (95% CI, 1.619-1.732), 2.192 (2.117-2.269), 2.637 (2.548-2.73), and 3.715 (3.6-3.834), respectively, vs those with an exposure score of 0.
- Exploratory subgroup analyses suggested the risk for T2D in persistent hypertriglyceridemia were more pronounced among people in their 20s than in their 30s and in women.
IN PRACTICE:
“Identification of individuals at higher risk based on triglyceride levels and management strategies for persistent hypertriglyceridemia in young adults could potentially reduce the burden of young-onset type 2 diabetes and enhance long-term health outcomes,” the authors wrote.
SOURCE:
The study, led by Min-Kyung Lee, Division of Endocrinology and Metabolism, Department of Internal Medicine, Myongji Hospital, Hanyang University College of Medicine, Seoul, Republic of Korea, was published online in Diabetes Research and Clinical Practice.
LIMITATIONS:
The scoring system based on fasting triglyceride levels of ≥ 150 mg/dL may have limitations, as the cumulative incidence of T2D also varied significantly for mean triglyceride levels. Moreover, relying on a single annual health checkup for hypertriglyceridemia diagnosis might not capture short-term fluctuations. Despite sufficient cases and a high follow-up rate, the study might have underestimated the incidence of T2D.
DISCLOSURES:
This work was supported by the National Research Foundation of Korea grant funded by the Korean Government and the faculty grant of Myongji Hospital. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
Persistent hypertriglyceridemia is linked to an increased risk for type 2 diabetes (T2D) in young adults, independent of lifestyle factors.
METHODOLOGY:
- This prospective study analyzed the data of 1,840,251 individuals aged 20-39 years from the South Korean National Health Insurance Service database (mean age 34 years, 71% male).
- The individuals had undergone four consecutive annual health checkups between 2009 and 2012 and had no history of T2D.
- The individuals were sorted into five groups indicating the number of hypertriglyceridemia diagnoses over four consecutive years: 0, 1, 2, 3, and 4, defined as serum fasting triglyceride levels of 150 mg/dL or higher.
- Data on lifestyle-related risk factors, such as smoking status and heavy alcohol consumption, were collected through self-reported questionnaires.
- The primary outcome was newly diagnosed cases of T2D. Over a mean follow-up of 6.53 years, a total of 40,286 individuals developed T2D.
TAKEAWAY:
- The cumulative incidence of T2D increased with an increase in exposure scores for hypertriglyceridemia (log-rank test, P < .001), independent of lifestyle-related factors.
- The incidence rate per 1000 person-years was 1.25 for participants with an exposure score of 0 and 11.55 for those with a score of 4.
- For individuals with exposure scores of 1, 2, 3, and 4, the adjusted hazard ratios for incident diabetes were 1.674 (95% CI, 1.619-1.732), 2.192 (2.117-2.269), 2.637 (2.548-2.73), and 3.715 (3.6-3.834), respectively, vs those with an exposure score of 0.
- Exploratory subgroup analyses suggested the risk for T2D in persistent hypertriglyceridemia were more pronounced among people in their 20s than in their 30s and in women.
IN PRACTICE:
“Identification of individuals at higher risk based on triglyceride levels and management strategies for persistent hypertriglyceridemia in young adults could potentially reduce the burden of young-onset type 2 diabetes and enhance long-term health outcomes,” the authors wrote.
SOURCE:
The study, led by Min-Kyung Lee, Division of Endocrinology and Metabolism, Department of Internal Medicine, Myongji Hospital, Hanyang University College of Medicine, Seoul, Republic of Korea, was published online in Diabetes Research and Clinical Practice.
LIMITATIONS:
The scoring system based on fasting triglyceride levels of ≥ 150 mg/dL may have limitations, as the cumulative incidence of T2D also varied significantly for mean triglyceride levels. Moreover, relying on a single annual health checkup for hypertriglyceridemia diagnosis might not capture short-term fluctuations. Despite sufficient cases and a high follow-up rate, the study might have underestimated the incidence of T2D.
DISCLOSURES:
This work was supported by the National Research Foundation of Korea grant funded by the Korean Government and the faculty grant of Myongji Hospital. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
Universal CVD Risk Prediction Model Shows Good Performance
TOPLINE:
A universal cardiovascular disease (CVD) prediction tool performs well in patients with and without atherosclerotic CVD (ASCVD), a new study showed, suggesting this model could facilitate transition from primary to secondary prevention by streamlining risk classification.
METHODOLOGY:
- Researchers used different models to evaluate whether established CVD predictors, including age, sex, race, diabetes, systolic blood pressure, or smoking, are associated with major adverse cardiovascular events (MACEs), including myocardial infarction (MI), stroke, and heart failure (HF), among 9138 patients, mean age 63.8 years, in the Atherosclerosis Risk in Communities (ARIC) study.
- Of these, 609 had ASCVD (history of MI, ischemic stroke, or symptomatic peripheral artery disease) and 8529 did not.
- They extended their exploration to other predictors available in clinical practice, including family history of premature ASCVD, high-sensitivity C-reactive protein, lipoprotein(a), triglycerides, and apolipoprotein B, as well as predictors of HF such as body mass index and heart rate and blood-based cardiac biomarkers.
- An external validation analysis included 5322 participants in the Multi-Ethnic Study of Atherosclerosis (MESA).
- Over a median follow-up of 18.9 years, 3209 ARIC participants (35%) developed MACE for an incidence rate per 1000 person-years of 21.3 for MACE, 12.6 for MI/stroke, and 13.8 for HF.
TAKEAWAY:
- Of all candidate predictors, 10 variables (including established predictors and cardiac biomarkers) were included in the universal prediction model, which demonstrated good calibration in both those with ASCVD (hazard ratio [HR] C-statistic, 0.692; 95% CI, 0.650-0.735) and without ASCVD (HR C-statistic, 0.748; 95% CI, 0.726-0.770).
- As anticipated, the risk for MACE was generally lower in those with no prior ASCVD, but the 5-year risk in the highest quintile of predicted risk in those without ASCVD was higher than that in the lowest two quintiles of the ASCVD group.
- The universal risk prediction model was validated in the MESA community–based cohort; over a median follow-up of 13.7 years, 12% of participants with and without prior ASCVD developed MACE for an incidence rate per 1000 person-years of 10.2 for MACE, 7.4 for MI/stroke, and 4.3 for HF.
- The results were generally similar when examining individual outcomes (MI/stroke and HF) and for both no ASCVD and ASCVD groups across demographic subgroups by age, sex, and race.
IN PRACTICE:
The findings “support the importance of established predictors for classifying long-term CVD risk in both primary and secondary prevention settings,” the authors wrote, adding an advantage to this risk prediction approach could be to help providers and patients “further personalize secondary prevention.”
In an accompanying editorial, Pier Sergio Saba, MD, PhD, Clinical and Interventional Cardiology, Sassari University Hospital, Sassari, Italy, and others said the universal risk assessment approach “is conceptually promising” but noted patients with ASCVD represented only 7% of the study population, and this population was relatively young, potentially limiting the applicability of this risk model in older individuals. Before the risk model can be used in clinical settings, results need to be validated and given incorporation of cardiac biomarkers, “careful cost-benefit analyses may also be needed,” the editorial writers added.
SOURCE:
The study was conducted by Yejin Mok, PHD, MPH, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, and colleagues. It was published online on January 29, 2024, in the Journal of the American College of Cardiology (JACC).
LIMITATIONS:
The somewhat limited number of study participants with prior ASCVD precluded researchers from quantifying the prognostic impact of ASCVD subtypes (eg, history of MI vs stroke vs peripheral artery disease). The study didn’t have data on some predictors recognized in guidelines (eg, coronary artery calcium and left ventricular ejection fraction). The ARIC analysis included only Black and White participants, and although models were validated in MESA, which included Chinese and Hispanic adults, extrapolation of results to more racially/ethnically diverse populations should be done with care.
DISCLOSURES:
The ARIC study received funding from the National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health, and Department of Health and Human Services. The MESA study was supported by the NHLBI and National Center for Advancing Translational Sciences. The study authors and editorial writers had no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
A universal cardiovascular disease (CVD) prediction tool performs well in patients with and without atherosclerotic CVD (ASCVD), a new study showed, suggesting this model could facilitate transition from primary to secondary prevention by streamlining risk classification.
METHODOLOGY:
- Researchers used different models to evaluate whether established CVD predictors, including age, sex, race, diabetes, systolic blood pressure, or smoking, are associated with major adverse cardiovascular events (MACEs), including myocardial infarction (MI), stroke, and heart failure (HF), among 9138 patients, mean age 63.8 years, in the Atherosclerosis Risk in Communities (ARIC) study.
- Of these, 609 had ASCVD (history of MI, ischemic stroke, or symptomatic peripheral artery disease) and 8529 did not.
- They extended their exploration to other predictors available in clinical practice, including family history of premature ASCVD, high-sensitivity C-reactive protein, lipoprotein(a), triglycerides, and apolipoprotein B, as well as predictors of HF such as body mass index and heart rate and blood-based cardiac biomarkers.
- An external validation analysis included 5322 participants in the Multi-Ethnic Study of Atherosclerosis (MESA).
- Over a median follow-up of 18.9 years, 3209 ARIC participants (35%) developed MACE for an incidence rate per 1000 person-years of 21.3 for MACE, 12.6 for MI/stroke, and 13.8 for HF.
TAKEAWAY:
- Of all candidate predictors, 10 variables (including established predictors and cardiac biomarkers) were included in the universal prediction model, which demonstrated good calibration in both those with ASCVD (hazard ratio [HR] C-statistic, 0.692; 95% CI, 0.650-0.735) and without ASCVD (HR C-statistic, 0.748; 95% CI, 0.726-0.770).
- As anticipated, the risk for MACE was generally lower in those with no prior ASCVD, but the 5-year risk in the highest quintile of predicted risk in those without ASCVD was higher than that in the lowest two quintiles of the ASCVD group.
- The universal risk prediction model was validated in the MESA community–based cohort; over a median follow-up of 13.7 years, 12% of participants with and without prior ASCVD developed MACE for an incidence rate per 1000 person-years of 10.2 for MACE, 7.4 for MI/stroke, and 4.3 for HF.
- The results were generally similar when examining individual outcomes (MI/stroke and HF) and for both no ASCVD and ASCVD groups across demographic subgroups by age, sex, and race.
IN PRACTICE:
The findings “support the importance of established predictors for classifying long-term CVD risk in both primary and secondary prevention settings,” the authors wrote, adding an advantage to this risk prediction approach could be to help providers and patients “further personalize secondary prevention.”
In an accompanying editorial, Pier Sergio Saba, MD, PhD, Clinical and Interventional Cardiology, Sassari University Hospital, Sassari, Italy, and others said the universal risk assessment approach “is conceptually promising” but noted patients with ASCVD represented only 7% of the study population, and this population was relatively young, potentially limiting the applicability of this risk model in older individuals. Before the risk model can be used in clinical settings, results need to be validated and given incorporation of cardiac biomarkers, “careful cost-benefit analyses may also be needed,” the editorial writers added.
SOURCE:
The study was conducted by Yejin Mok, PHD, MPH, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, and colleagues. It was published online on January 29, 2024, in the Journal of the American College of Cardiology (JACC).
LIMITATIONS:
The somewhat limited number of study participants with prior ASCVD precluded researchers from quantifying the prognostic impact of ASCVD subtypes (eg, history of MI vs stroke vs peripheral artery disease). The study didn’t have data on some predictors recognized in guidelines (eg, coronary artery calcium and left ventricular ejection fraction). The ARIC analysis included only Black and White participants, and although models were validated in MESA, which included Chinese and Hispanic adults, extrapolation of results to more racially/ethnically diverse populations should be done with care.
DISCLOSURES:
The ARIC study received funding from the National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health, and Department of Health and Human Services. The MESA study was supported by the NHLBI and National Center for Advancing Translational Sciences. The study authors and editorial writers had no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
A universal cardiovascular disease (CVD) prediction tool performs well in patients with and without atherosclerotic CVD (ASCVD), a new study showed, suggesting this model could facilitate transition from primary to secondary prevention by streamlining risk classification.
METHODOLOGY:
- Researchers used different models to evaluate whether established CVD predictors, including age, sex, race, diabetes, systolic blood pressure, or smoking, are associated with major adverse cardiovascular events (MACEs), including myocardial infarction (MI), stroke, and heart failure (HF), among 9138 patients, mean age 63.8 years, in the Atherosclerosis Risk in Communities (ARIC) study.
- Of these, 609 had ASCVD (history of MI, ischemic stroke, or symptomatic peripheral artery disease) and 8529 did not.
- They extended their exploration to other predictors available in clinical practice, including family history of premature ASCVD, high-sensitivity C-reactive protein, lipoprotein(a), triglycerides, and apolipoprotein B, as well as predictors of HF such as body mass index and heart rate and blood-based cardiac biomarkers.
- An external validation analysis included 5322 participants in the Multi-Ethnic Study of Atherosclerosis (MESA).
- Over a median follow-up of 18.9 years, 3209 ARIC participants (35%) developed MACE for an incidence rate per 1000 person-years of 21.3 for MACE, 12.6 for MI/stroke, and 13.8 for HF.
TAKEAWAY:
- Of all candidate predictors, 10 variables (including established predictors and cardiac biomarkers) were included in the universal prediction model, which demonstrated good calibration in both those with ASCVD (hazard ratio [HR] C-statistic, 0.692; 95% CI, 0.650-0.735) and without ASCVD (HR C-statistic, 0.748; 95% CI, 0.726-0.770).
- As anticipated, the risk for MACE was generally lower in those with no prior ASCVD, but the 5-year risk in the highest quintile of predicted risk in those without ASCVD was higher than that in the lowest two quintiles of the ASCVD group.
- The universal risk prediction model was validated in the MESA community–based cohort; over a median follow-up of 13.7 years, 12% of participants with and without prior ASCVD developed MACE for an incidence rate per 1000 person-years of 10.2 for MACE, 7.4 for MI/stroke, and 4.3 for HF.
- The results were generally similar when examining individual outcomes (MI/stroke and HF) and for both no ASCVD and ASCVD groups across demographic subgroups by age, sex, and race.
IN PRACTICE:
The findings “support the importance of established predictors for classifying long-term CVD risk in both primary and secondary prevention settings,” the authors wrote, adding an advantage to this risk prediction approach could be to help providers and patients “further personalize secondary prevention.”
In an accompanying editorial, Pier Sergio Saba, MD, PhD, Clinical and Interventional Cardiology, Sassari University Hospital, Sassari, Italy, and others said the universal risk assessment approach “is conceptually promising” but noted patients with ASCVD represented only 7% of the study population, and this population was relatively young, potentially limiting the applicability of this risk model in older individuals. Before the risk model can be used in clinical settings, results need to be validated and given incorporation of cardiac biomarkers, “careful cost-benefit analyses may also be needed,” the editorial writers added.
SOURCE:
The study was conducted by Yejin Mok, PHD, MPH, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, and colleagues. It was published online on January 29, 2024, in the Journal of the American College of Cardiology (JACC).
LIMITATIONS:
The somewhat limited number of study participants with prior ASCVD precluded researchers from quantifying the prognostic impact of ASCVD subtypes (eg, history of MI vs stroke vs peripheral artery disease). The study didn’t have data on some predictors recognized in guidelines (eg, coronary artery calcium and left ventricular ejection fraction). The ARIC analysis included only Black and White participants, and although models were validated in MESA, which included Chinese and Hispanic adults, extrapolation of results to more racially/ethnically diverse populations should be done with care.
DISCLOSURES:
The ARIC study received funding from the National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health, and Department of Health and Human Services. The MESA study was supported by the NHLBI and National Center for Advancing Translational Sciences. The study authors and editorial writers had no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Salt Substitute Reduces Risk for New Hypertension
Replacing regular salt with a salt substitute reduced the incidence of new hypertension compared with a usual salt group, without provoking hypotension, new data showed.
Among a group of older adults with normal blood pressure (BP), those who swapped table salt for a salt substitute — consisting of 62.5% sodium chloride, 25% potassium chloride, and 12.5% flavorings — were 40% less apt to develop hypertension over 2 years than were peers who continued with regular salt.
“From a public health perspective, our study results indicate that everyone in the whole population, either hypertensive or normotensive, can benefit from replacing regular salt with potassium-enriched salt substitute,” lead author Yangfeng Wu, MD, PhD, professor and executive associate director, Peking University Clinical Research Institute, Beijing, China, told this news organization.
“Thus, ,” Dr. Wu said.
The study was published online on February 12 in the Journal of the American College of Cardiology.
“Considering the failing strategy to reduce the intake of salt worldwide, salt substitution is an attractive alternative. The food industry and authorities should prepare strategies for wide-scale implementation of salt substitutes,” Rik Olde Engberink, MD, PhD, with Amsterdam University Medical Center, wrote in a linked editorial.
Population Strategy for Hypertension Prevention
The DECIDE-Salt clinical trial was a cluster-randomized trial conducted in 48 residential elderly care facilities in China with 1612 participants (1230 men and 382 women) aged 55 years or older. The trial assessed the effect of two sodium reduction strategies in lowering BP — replacing salt with a salt substitute and progressive restriction of the salt supply.
In the original study, the salt substitute intervention lowered systolic/diastolic BP significantly by 7.1/1.9 mm Hg vs the usual salt group. The progressive restriction of salt had no impact on BP vs usual salt or salt substitute groups.
This post hoc analysis of DECIDE-Salt focused on 609 participants (mean age, 71 years; 74% men) who were normotensive at baseline (mean BP, 122/74 mm Hg), with 298 in the usual salt group and 313 in the salt substitute group.
Compared with the usual salt group, the salt substitute group had a lower incidence of hypertension over 2 years (adjusted hazard ratio [HR], 0.60; 95% CI, 0.39-0.92; P = .02), with no increase in episodes of hypotension (P = .76).
From baseline to 2 years, there was no change in mean systolic/diastolic BP in the salt substitution group, whereas the usual salt group experienced a significant increase in systolic/diastolic BP (mean, 7.0/2.1 mm Hg).
The post hoc results from DECIDE-Salt are in line with a previous study from Peru, which also investigated mostly normotensive participants and reported a 51% lower risk of developing hypertension in the salt substitute group, as reported previously by this news organization.
“Although the study involved only participants aged 55 years and above, the epidemic of hypertension and its relations with sodium and potassium intake are not limited to older adults. Thus, we believe the salt substitution should also be beneficial to younger adults,” Dr. Wu said.
Notable Analysis
Reached for comment, Ankur Shah, MD, Division of Kidney Disease and Hypertension, Warren Alpert Medical School of Brown University, Providence, Rhode Island, said the study is “notable due to the limited and conflicting reports on the effects of salt substitution in individuals with normal blood pressure.”
“There is a growing body of literature on the impact of salt substitution in controlling hypertension, but less is known about prevention,” Dr. Shah, who was not involved in the study, told this news organization.
“The study certainly has population-level implications, as the design of a cluster-randomized trial at the facility level makes for a clear path to implementation — sodium substitution in elderly care facilities. That said, this is also the greatest limitation — extrapolating to the general population may not be accurate,” Dr. Shah noted.
There is also a potential concern with salt substitutes in patients with kidney disease, who typically are advised to lower potassium intake.
“Supplementing potassium could result in hyperkalemia, which can be life-threatening if severe, and patients taking medications that interfere with the kidney’s ability to excrete potassium should be cautious as well,” Dr. Shah said.
This research was supported by a grant from the National Key Research and Development Program, Ministry of Science and Technology of China. China Salt General Company at Yulin provided the usual salt and salt substitute used in the study free of charge. Dr. Wu, Dr. Engberink, and Dr. Shah had no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
Replacing regular salt with a salt substitute reduced the incidence of new hypertension compared with a usual salt group, without provoking hypotension, new data showed.
Among a group of older adults with normal blood pressure (BP), those who swapped table salt for a salt substitute — consisting of 62.5% sodium chloride, 25% potassium chloride, and 12.5% flavorings — were 40% less apt to develop hypertension over 2 years than were peers who continued with regular salt.
“From a public health perspective, our study results indicate that everyone in the whole population, either hypertensive or normotensive, can benefit from replacing regular salt with potassium-enriched salt substitute,” lead author Yangfeng Wu, MD, PhD, professor and executive associate director, Peking University Clinical Research Institute, Beijing, China, told this news organization.
“Thus, ,” Dr. Wu said.
The study was published online on February 12 in the Journal of the American College of Cardiology.
“Considering the failing strategy to reduce the intake of salt worldwide, salt substitution is an attractive alternative. The food industry and authorities should prepare strategies for wide-scale implementation of salt substitutes,” Rik Olde Engberink, MD, PhD, with Amsterdam University Medical Center, wrote in a linked editorial.
Population Strategy for Hypertension Prevention
The DECIDE-Salt clinical trial was a cluster-randomized trial conducted in 48 residential elderly care facilities in China with 1612 participants (1230 men and 382 women) aged 55 years or older. The trial assessed the effect of two sodium reduction strategies in lowering BP — replacing salt with a salt substitute and progressive restriction of the salt supply.
In the original study, the salt substitute intervention lowered systolic/diastolic BP significantly by 7.1/1.9 mm Hg vs the usual salt group. The progressive restriction of salt had no impact on BP vs usual salt or salt substitute groups.
This post hoc analysis of DECIDE-Salt focused on 609 participants (mean age, 71 years; 74% men) who were normotensive at baseline (mean BP, 122/74 mm Hg), with 298 in the usual salt group and 313 in the salt substitute group.
Compared with the usual salt group, the salt substitute group had a lower incidence of hypertension over 2 years (adjusted hazard ratio [HR], 0.60; 95% CI, 0.39-0.92; P = .02), with no increase in episodes of hypotension (P = .76).
From baseline to 2 years, there was no change in mean systolic/diastolic BP in the salt substitution group, whereas the usual salt group experienced a significant increase in systolic/diastolic BP (mean, 7.0/2.1 mm Hg).
The post hoc results from DECIDE-Salt are in line with a previous study from Peru, which also investigated mostly normotensive participants and reported a 51% lower risk of developing hypertension in the salt substitute group, as reported previously by this news organization.
“Although the study involved only participants aged 55 years and above, the epidemic of hypertension and its relations with sodium and potassium intake are not limited to older adults. Thus, we believe the salt substitution should also be beneficial to younger adults,” Dr. Wu said.
Notable Analysis
Reached for comment, Ankur Shah, MD, Division of Kidney Disease and Hypertension, Warren Alpert Medical School of Brown University, Providence, Rhode Island, said the study is “notable due to the limited and conflicting reports on the effects of salt substitution in individuals with normal blood pressure.”
“There is a growing body of literature on the impact of salt substitution in controlling hypertension, but less is known about prevention,” Dr. Shah, who was not involved in the study, told this news organization.
“The study certainly has population-level implications, as the design of a cluster-randomized trial at the facility level makes for a clear path to implementation — sodium substitution in elderly care facilities. That said, this is also the greatest limitation — extrapolating to the general population may not be accurate,” Dr. Shah noted.
There is also a potential concern with salt substitutes in patients with kidney disease, who typically are advised to lower potassium intake.
“Supplementing potassium could result in hyperkalemia, which can be life-threatening if severe, and patients taking medications that interfere with the kidney’s ability to excrete potassium should be cautious as well,” Dr. Shah said.
This research was supported by a grant from the National Key Research and Development Program, Ministry of Science and Technology of China. China Salt General Company at Yulin provided the usual salt and salt substitute used in the study free of charge. Dr. Wu, Dr. Engberink, and Dr. Shah had no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
Replacing regular salt with a salt substitute reduced the incidence of new hypertension compared with a usual salt group, without provoking hypotension, new data showed.
Among a group of older adults with normal blood pressure (BP), those who swapped table salt for a salt substitute — consisting of 62.5% sodium chloride, 25% potassium chloride, and 12.5% flavorings — were 40% less apt to develop hypertension over 2 years than were peers who continued with regular salt.
“From a public health perspective, our study results indicate that everyone in the whole population, either hypertensive or normotensive, can benefit from replacing regular salt with potassium-enriched salt substitute,” lead author Yangfeng Wu, MD, PhD, professor and executive associate director, Peking University Clinical Research Institute, Beijing, China, told this news organization.
“Thus, ,” Dr. Wu said.
The study was published online on February 12 in the Journal of the American College of Cardiology.
“Considering the failing strategy to reduce the intake of salt worldwide, salt substitution is an attractive alternative. The food industry and authorities should prepare strategies for wide-scale implementation of salt substitutes,” Rik Olde Engberink, MD, PhD, with Amsterdam University Medical Center, wrote in a linked editorial.
Population Strategy for Hypertension Prevention
The DECIDE-Salt clinical trial was a cluster-randomized trial conducted in 48 residential elderly care facilities in China with 1612 participants (1230 men and 382 women) aged 55 years or older. The trial assessed the effect of two sodium reduction strategies in lowering BP — replacing salt with a salt substitute and progressive restriction of the salt supply.
In the original study, the salt substitute intervention lowered systolic/diastolic BP significantly by 7.1/1.9 mm Hg vs the usual salt group. The progressive restriction of salt had no impact on BP vs usual salt or salt substitute groups.
This post hoc analysis of DECIDE-Salt focused on 609 participants (mean age, 71 years; 74% men) who were normotensive at baseline (mean BP, 122/74 mm Hg), with 298 in the usual salt group and 313 in the salt substitute group.
Compared with the usual salt group, the salt substitute group had a lower incidence of hypertension over 2 years (adjusted hazard ratio [HR], 0.60; 95% CI, 0.39-0.92; P = .02), with no increase in episodes of hypotension (P = .76).
From baseline to 2 years, there was no change in mean systolic/diastolic BP in the salt substitution group, whereas the usual salt group experienced a significant increase in systolic/diastolic BP (mean, 7.0/2.1 mm Hg).
The post hoc results from DECIDE-Salt are in line with a previous study from Peru, which also investigated mostly normotensive participants and reported a 51% lower risk of developing hypertension in the salt substitute group, as reported previously by this news organization.
“Although the study involved only participants aged 55 years and above, the epidemic of hypertension and its relations with sodium and potassium intake are not limited to older adults. Thus, we believe the salt substitution should also be beneficial to younger adults,” Dr. Wu said.
Notable Analysis
Reached for comment, Ankur Shah, MD, Division of Kidney Disease and Hypertension, Warren Alpert Medical School of Brown University, Providence, Rhode Island, said the study is “notable due to the limited and conflicting reports on the effects of salt substitution in individuals with normal blood pressure.”
“There is a growing body of literature on the impact of salt substitution in controlling hypertension, but less is known about prevention,” Dr. Shah, who was not involved in the study, told this news organization.
“The study certainly has population-level implications, as the design of a cluster-randomized trial at the facility level makes for a clear path to implementation — sodium substitution in elderly care facilities. That said, this is also the greatest limitation — extrapolating to the general population may not be accurate,” Dr. Shah noted.
There is also a potential concern with salt substitutes in patients with kidney disease, who typically are advised to lower potassium intake.
“Supplementing potassium could result in hyperkalemia, which can be life-threatening if severe, and patients taking medications that interfere with the kidney’s ability to excrete potassium should be cautious as well,” Dr. Shah said.
This research was supported by a grant from the National Key Research and Development Program, Ministry of Science and Technology of China. China Salt General Company at Yulin provided the usual salt and salt substitute used in the study free of charge. Dr. Wu, Dr. Engberink, and Dr. Shah had no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
Spinal Cord Injury Tied to Greater Risk for Heart Disease
TOPLINE:
Spinal cord injury (SCI) is associated with a significantly greater risk for heart disease than that of the general non-SCI population, especially among those with severe disability, new observational data suggest.
METHODOLOGY:
- Researchers analyzed data from Korea’s National Health Insurance Service on 5083 patients with cervical, thoracic, or lumbar SCI (mean age, 58; 75% men) and 1:3 age- and sex-matched non-SCI controls.
- The study endpoint was new-onset myocardial infarction (MI), heart failure (HF), or atrial fibrillation (AF) during a mean follow-up of 4.3 years.
- Covariates included low income, living in an urban or rural area, alcohol consumption, smoking status, physical activity engagement, body mass index, and blood pressure; comorbidities included hypertension, type 2 diabetes, and dyslipidemia.
TAKEAWAY:
- A total of 169 MI events (7.3 per 1000 person-years), 426 HF events (18.8 per 1000 person-years), and 158 AF events (6.8 per 1000 person-years) occurred among SCI survivors.
- After adjustment, SCI survivors had a higher risk for MI (adjusted hazard ratio [aHR], 2.41), HF (aHR, 2.24), and AF (aHR, 1.84) than that of controls.
- Cervical and lumbar SCI survivors had an increased risk for heart disease compared with controls regardless of disability, and the risk was slightly higher for those with a disability; for cervical SCI survivors with a disability, aHRs for MI, HF, and AF, respectively, were 2.30, 2.05, and 1.73; for lumbar SCI survivors with a disability, aHRs were 2.79, 2.35, and 2.47.
- Thoracic SCI survivors with disability had a higher risk for MI (aHR, 5.62) and HF (aHR, 3.31) than controls.
IN PRACTICE:
“[T]he recognition and treatment of modifiable cardiovascular risk factors must be reinforced in the SCI population, [and] proper rehabilitation and education should be considered to prevent autonomic dysreflexia or orthostatic hypotension,” the authors wrote.
In an accompanying editorial, Christopher R. West, PhD, and Jacquelyn J. Cragg, PhD, both of the University of British Columbia, Vancouver, Canada, noted that clinical guidelines for cardiovascular and cardiometabolic disease after SCI don’t include approaches to help mitigate the risk for cardiac events such as those reported in the study; therefore, they wrote, the findings “should act as ‘call-to-arms’ to researchers and clinicians to shift gears from tradition and begin studying the clinical efficacy of neuraxial therapies that could help restore autonomic balance [in SCI], such as targeted neuromodulation.”
SOURCE:
The study was led by Jung Eun Yoo, MD, PhD of Seoul National University College of Medicine, Seoul, South Korea, and published online on February 12 in the Journal of the American College of Cardiology.
LIMITATIONS:
The database was not designed for the SCI population, so data are incomplete. The incidence of thoracic SCI was particularly low. Because SCI survivors may have impaired perception of chest pain in ischemic heart disease, those with asymptomatic or silent heart disease may not have been captured during follow-up. All study participants were Korean, so the findings may not be generalizable to other ethnicities.
DISCLOSURES:
This research was partially supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health and Welfare, South Korea. The study authors and the editorialists had no relevant relationships to disclose.
A version of this article appeared on Medscape.com.
TOPLINE:
Spinal cord injury (SCI) is associated with a significantly greater risk for heart disease than that of the general non-SCI population, especially among those with severe disability, new observational data suggest.
METHODOLOGY:
- Researchers analyzed data from Korea’s National Health Insurance Service on 5083 patients with cervical, thoracic, or lumbar SCI (mean age, 58; 75% men) and 1:3 age- and sex-matched non-SCI controls.
- The study endpoint was new-onset myocardial infarction (MI), heart failure (HF), or atrial fibrillation (AF) during a mean follow-up of 4.3 years.
- Covariates included low income, living in an urban or rural area, alcohol consumption, smoking status, physical activity engagement, body mass index, and blood pressure; comorbidities included hypertension, type 2 diabetes, and dyslipidemia.
TAKEAWAY:
- A total of 169 MI events (7.3 per 1000 person-years), 426 HF events (18.8 per 1000 person-years), and 158 AF events (6.8 per 1000 person-years) occurred among SCI survivors.
- After adjustment, SCI survivors had a higher risk for MI (adjusted hazard ratio [aHR], 2.41), HF (aHR, 2.24), and AF (aHR, 1.84) than that of controls.
- Cervical and lumbar SCI survivors had an increased risk for heart disease compared with controls regardless of disability, and the risk was slightly higher for those with a disability; for cervical SCI survivors with a disability, aHRs for MI, HF, and AF, respectively, were 2.30, 2.05, and 1.73; for lumbar SCI survivors with a disability, aHRs were 2.79, 2.35, and 2.47.
- Thoracic SCI survivors with disability had a higher risk for MI (aHR, 5.62) and HF (aHR, 3.31) than controls.
IN PRACTICE:
“[T]he recognition and treatment of modifiable cardiovascular risk factors must be reinforced in the SCI population, [and] proper rehabilitation and education should be considered to prevent autonomic dysreflexia or orthostatic hypotension,” the authors wrote.
In an accompanying editorial, Christopher R. West, PhD, and Jacquelyn J. Cragg, PhD, both of the University of British Columbia, Vancouver, Canada, noted that clinical guidelines for cardiovascular and cardiometabolic disease after SCI don’t include approaches to help mitigate the risk for cardiac events such as those reported in the study; therefore, they wrote, the findings “should act as ‘call-to-arms’ to researchers and clinicians to shift gears from tradition and begin studying the clinical efficacy of neuraxial therapies that could help restore autonomic balance [in SCI], such as targeted neuromodulation.”
SOURCE:
The study was led by Jung Eun Yoo, MD, PhD of Seoul National University College of Medicine, Seoul, South Korea, and published online on February 12 in the Journal of the American College of Cardiology.
LIMITATIONS:
The database was not designed for the SCI population, so data are incomplete. The incidence of thoracic SCI was particularly low. Because SCI survivors may have impaired perception of chest pain in ischemic heart disease, those with asymptomatic or silent heart disease may not have been captured during follow-up. All study participants were Korean, so the findings may not be generalizable to other ethnicities.
DISCLOSURES:
This research was partially supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health and Welfare, South Korea. The study authors and the editorialists had no relevant relationships to disclose.
A version of this article appeared on Medscape.com.
TOPLINE:
Spinal cord injury (SCI) is associated with a significantly greater risk for heart disease than that of the general non-SCI population, especially among those with severe disability, new observational data suggest.
METHODOLOGY:
- Researchers analyzed data from Korea’s National Health Insurance Service on 5083 patients with cervical, thoracic, or lumbar SCI (mean age, 58; 75% men) and 1:3 age- and sex-matched non-SCI controls.
- The study endpoint was new-onset myocardial infarction (MI), heart failure (HF), or atrial fibrillation (AF) during a mean follow-up of 4.3 years.
- Covariates included low income, living in an urban or rural area, alcohol consumption, smoking status, physical activity engagement, body mass index, and blood pressure; comorbidities included hypertension, type 2 diabetes, and dyslipidemia.
TAKEAWAY:
- A total of 169 MI events (7.3 per 1000 person-years), 426 HF events (18.8 per 1000 person-years), and 158 AF events (6.8 per 1000 person-years) occurred among SCI survivors.
- After adjustment, SCI survivors had a higher risk for MI (adjusted hazard ratio [aHR], 2.41), HF (aHR, 2.24), and AF (aHR, 1.84) than that of controls.
- Cervical and lumbar SCI survivors had an increased risk for heart disease compared with controls regardless of disability, and the risk was slightly higher for those with a disability; for cervical SCI survivors with a disability, aHRs for MI, HF, and AF, respectively, were 2.30, 2.05, and 1.73; for lumbar SCI survivors with a disability, aHRs were 2.79, 2.35, and 2.47.
- Thoracic SCI survivors with disability had a higher risk for MI (aHR, 5.62) and HF (aHR, 3.31) than controls.
IN PRACTICE:
“[T]he recognition and treatment of modifiable cardiovascular risk factors must be reinforced in the SCI population, [and] proper rehabilitation and education should be considered to prevent autonomic dysreflexia or orthostatic hypotension,” the authors wrote.
In an accompanying editorial, Christopher R. West, PhD, and Jacquelyn J. Cragg, PhD, both of the University of British Columbia, Vancouver, Canada, noted that clinical guidelines for cardiovascular and cardiometabolic disease after SCI don’t include approaches to help mitigate the risk for cardiac events such as those reported in the study; therefore, they wrote, the findings “should act as ‘call-to-arms’ to researchers and clinicians to shift gears from tradition and begin studying the clinical efficacy of neuraxial therapies that could help restore autonomic balance [in SCI], such as targeted neuromodulation.”
SOURCE:
The study was led by Jung Eun Yoo, MD, PhD of Seoul National University College of Medicine, Seoul, South Korea, and published online on February 12 in the Journal of the American College of Cardiology.
LIMITATIONS:
The database was not designed for the SCI population, so data are incomplete. The incidence of thoracic SCI was particularly low. Because SCI survivors may have impaired perception of chest pain in ischemic heart disease, those with asymptomatic or silent heart disease may not have been captured during follow-up. All study participants were Korean, so the findings may not be generalizable to other ethnicities.
DISCLOSURES:
This research was partially supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health and Welfare, South Korea. The study authors and the editorialists had no relevant relationships to disclose.
A version of this article appeared on Medscape.com.
Painful Retiform Purpura in a Peritoneal Dialysis Patient
The Diagnosis: Calcific Uremic Arteriolopathy
Computed tomography of the abdomen and pelvis with contrast revealed a right complex renal cyst with peripheral calcification; computed tomography of the head without contrast revealed atherosclerotic changes with calcification of the intracranial arteries, vertebral basilar arteries, and bilateral branches of the ophthalmic artery. Histopathology revealed occlusive vasculopathy with epidermal ischemic changes as well as dermal and subcutaneous vascular congestion and small thrombi. Within the subcutis, there were tiny stippled calcium deposits within very small vascular lumina (Figure). The combination of clinical and histological findings was highly suggestive of calcific uremic arteriolopathy, and the patient was transitioned to hemodialysis against a low-calcium bath to avoid hypercalcemia. Unfortunately, she developed complications related to sepsis and experienced worsening mentation. After a discussion with palliative care, the patient was transitioned to comfort measures and discharged home on hospice 1 week after the biopsy at her family’s request.
Calcific uremic arteriolopathy (also known as calciphylaxis) is a rare, life-threatening syndrome of widespread vascular calcification leading to microvascular occlusion within the dermis and subcutaneous tissues.1 Clinically, it typically manifests as severely painful, purpuric skin lesions that evolve through phases of blistering, ulceration, and ultimately visible skin necrosis.2 The pain likely is a consequence of ischemia and nociceptive activation and often may precede any visibly apparent skin lesions.3 Risk factors associated with the development of this condition include female sex; history of diabetes mellitus, obesity, rapid weight loss, or end-stage renal disease; abnormalities in calcium and phosphorus homeostasis; and vitamin K deficiency.1,3 It is more prevalent in patients on peritoneal dialysis compared to hemodialysis.4
Calciphylaxis is diagnosed with combined clinical and histopathological evidence. Laboratory test abnormalities are not specific for disease; therefore, skin biopsy is the standard confirmatory test, though its practice is contentious due to the risk for nonhealing ulceration and increasing risk for infection.1 Findings suggestive of disease include focal to diffuse calcification (intravascular, extravascular, or perieccrine), superficial fat calcium deposition, mid panniculus calcium deposition, mid panniculus vascular thrombi, and focal to diffuse angioplasia.5 The hallmark feature is diffuse calcification of small capillaries in adipose tissue.6
The mortality rate associated with this disease is high—a 6-month mortality rate of 27% to 43% has been reported from the time of diagnosis7-9—which often is related to subsequent superimposed infections patients acquire from necrotic skin tissue.2 The disease also carries high morbidity, with patients experiencing frequent hospitalizations related to pain, infections, and nonhealing wounds.6 There is no standard treatment, and trials have been limited to small sample sizes. A multidisciplinary treatment approach is essential to maximize outcomes, which includes wound care, risk factor modification, analgesia, and symptomatic management strategies.1,2,6
Some pharmacologic agents have received noteworthy attention in treating calciphylaxis, including sodium thiosulfate (STS), bisphosphonates, and vitamin K supplementation.1 The strongest evidence supporting the use of STS comes from 2 trials involving 53 and 27 dialysis patients, with complete remission in 14 (26%) and 14 (52%) patients, respectively.10,11 However, these trials did not include control groups to compare outcomes, and mortality rates were similarly high among partial responders and nonresponders compared with patients not treated with STS. A 2018 systematic review failed to assess the efficacy of STS alone for the treatment of calciphylaxis but suggested there may be a future role for it, with 251 of 358 patients (70.1%) responding to therapy.12
Erythema ab igne is a cutaneous reaction related to long-term heat exposure, often from electronic devices such as laptops, heating pads, space heaters, or hot-water bottles.13,14 Clinically, this rash appears as an erythematous, purpuric, or hyperpigmented reticular dermatosis that is below the clinical threshold to define a thermal burn.13 Lesions often are seen on the anterior thighs or across the abdomen.15 There usually are no long-term clinical sequelae; however, rare malignant transformation has been documented in cases of atrophy or nonhealing ulceration.16 Treatment is supportive with removal of the offending agent, but hyperpigmentation may persist for months to years.14
Livedo reticularis is a cutaneous pattern of mottled violaceous or hyperpigmented changes that often signifies underlying vascular dermal changes.17 It can be seen in various pathologic states, including vasculitis, autoimmune disease, connective tissue disease, neurologic disease, infection, or malignancy, or it can be drug induced.18 There are no pathognomonic microscopic changes, as the histology will drastically differ based on the etiology. Workup can be extensive; cues to the underlying pathology should be sought based on the patient’s history and concurrent presenting symptoms. Livedo reticularis is the most common dermatologic finding in patients with antiphospholipid syndrome, and workup should include antiphospholipid antibodies (eg, lupus anticoagulant, anticardiolipin, anti–beta-2-glycoproteins) as well as lupus testing (eg, antinuclear antibodies, anti– double-stranded DNA).19 Treatment is targeted at the underlying disease process.
Cryoglobulinemia is a disease characterized by abnormal serum immunoglobulins that precipitate at cold temperatures and is further subcategorized by the type of complexes that are deposited.20 Type I represents purely monoclonal cryoglobulins, type III purely polyclonal, and type II a mixed picture. Clinical manifestations arise from excessive deposition of these proteins in the skin, joints, peripheral vasculature, and kidneys leading to purpuric skin lesions, chronic ulceration, arthralgia, and glomerulonephritis. Cutaneous findings may include erythematous to purpuric macular or papular changes with or without the presence of ulceration, infarction, or hemorrhagic crusting.21 Systemic disease often underlies a diagnosis, and further investigation for hepatitis C virus, connective tissue disease, and hematologic malignancies should be considered.20 Treatment is targeted at underlying systemic disease, such as antiviral treatment for hepatitis or chemotherapeutic regimens for hematologic disease.22
Polyarteritis nodosa is a systemic necrotizing vasculitis that typically involves small- to medium-sized arteries. Cutaneous manifestations often include subcutaneous nodules, livedo reticularis, and ulcerations most found on the lower extremities.23 Systemic symptoms including fever, myalgia, arthralgia, and neuropathy often are present. Characteristic histopathology findings include inflammation and destruction of medium-sized arteries at the junctional zone of the dermis and subcutis along with microaneurysms along the vessels.24 Treatment is based on the severity of disease, with localized cutaneous disease often being controlled with topical steroids and anti-inflammatory agents, while more widespread disease requires immunosuppression with systemic steroids, hydroxychloroquine, azathioprine, methotrexate, mycophenolate mofetil, or intravenous immunoglobulins.23
- Nigwekar SU, Thadhani R, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378:1704-1714. doi:10.1056/NEJMra1505292
- Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146. doi:10.1053/j.ajkd.2015.01.034
- Chang JJ. Calciphylaxis: diagnosis, pathogenesis, and treatment. Adv Skin Wound Care. 2019;32:205-215. doi:10.1097/01 .ASW.0000554443.14002.13
- Zhang Y, Corapi KM, Luongo M, et al. Calciphylaxis in peritoneal dialysis patients: a single center cohort study. Int J Nephrol Renovasc Dis. 2016;9:235-241. doi:10.2147/ijnrd.S115701
- Chen TY, Lehman JS, Gibson LE, et al. Histopathology of calciphylaxis: cohort study with clinical correlations. Am J Dermatopathol. 2017;39:795-802. doi:10.1097/DAD.0000000000000824
- Kodumudi V, Jeha GM, Mydlo N, et al. Management of cutaneous calciphylaxis. Adv Ther. 2020;37:4797-4807. doi:10.1007 /s12325-020-01504-w
- Nigwekar SU, Zhao S, Wenger J, et al. A nationally representative study of calcific uremic arteriolopathy risk factors. J Am Soc Nephrol. 2016;27:3421-3429. doi:10.1681/asn.2015091065
- McCarthy JT, El-Azhary RA, Patzelt MT, et al. Survival, risk factors, and effect of treatment in 101 patients with calciphylaxis. Mayo Clin Proc. 2016;91:1384-1394. doi:10.1016/j.mayocp.2016.06.025
- Fine A, Zacharias J. Calciphylaxis is usually non-ulcerating: risk factors, outcome and therapy. Kidney Int. 2002;61:2210-2217. doi:10.1046/j.1523-1755.2002.00375.x
- Nigwekar SU, Brunelli SM, Meade D, et al. Sodium thiosulfate therapy for calcific uremic arteriolopathy. Clin J Am Soc Nephrol. 2013;8:1162-1170. doi:10.2215/cjn.09880912
- Zitt E, König M, Vychytil A, et al. Use of sodium thiosulphate in a multi-interventional setting for the treatment of calciphylaxis in dialysis patients. Nephrol Dial Transplant. 2013;28:1232-1240. doi:10.1093/ndt/gfs548
- Peng T, Zhuo L, Wang Y, et al. Systematic review of sodium thiosulfate in treating calciphylaxis in chronic kidney disease patients. Nephrology (Carlton). 2018;23:669-675. doi:10.1111/nep.13081
- Miller K, Hunt R, Chu J, et al. Erythema ab igne. Dermatol Online J. 2011;17:28.
- Kettelhut EA, Traylor J, Sathe NC, et al. Erythema ab igne. StatPearls. StatPearls Publishing; 2022.
- Knöpfel N, Weibel L. Erythema Ab Igne. JAMA Dermatol. 2021;157: 106. doi:10.1001/jamadermatol.2020.3995
- Sigmon JR, Cantrell J, Teague D, et al. Poorly differentiated carcinoma arising in the setting of erythema ab igne. Am J Dermatopathol. 2013;35:676-678. doi:10.1097/DAD.0b013e3182871648
- Rose AE, Sagger V, Boyd KP, et al. Livedo reticularis. Dermatol Online J. 2013;19:20705.
- Sajjan VV, Lunge S, Swamy MB, et al. Livedo reticularis: a review of the literature. Indian Dermatol Online J. 2015;6:315-321. doi:10.4103/2229-5178.164493
- Uthman IW, Khamashta MA. Livedo racemosa: a striking dermatological sign for the antiphospholipid syndrome. J Rheumatol. 2006;33:2379-2382.
- Desbois AC, Cacoub P, Saadoun D. Cryoglobulinemia: an update in 2019. Joint Bone Spine. 2019;86:707-713. doi:10.1016/j .jbspin.2019.01.016
- Cohen SJ, Pittelkow MR, Su WP. Cutaneous manifestations of cryoglobulinemia: clinical and histopathologic study of seventy-two patients. J Am Acad Dermatol. 1991;25(1, pt 1):21-27. doi:10.1016 /0190-9622(91)70168-2
- Takada S, Shimizu T, Hadano Y, et al. Cryoglobulinemia (review). Mol Med Rep. 2012;6:3-8. doi:10.3892/mmr.2012.861
- Turska M, Parada-Turska J. Cutaneous polyarteritis nodosa. Wiad Lek. 2018;71(1, pt 1):73-77.
- De Virgilio A, Greco A, Magliulo G, et al. Polyarteritis nodosa: a contemporary overview. Autoimmun Rev. 2016;15:564-570. doi:10.1016/j.autrev.2016.02.015
The Diagnosis: Calcific Uremic Arteriolopathy
Computed tomography of the abdomen and pelvis with contrast revealed a right complex renal cyst with peripheral calcification; computed tomography of the head without contrast revealed atherosclerotic changes with calcification of the intracranial arteries, vertebral basilar arteries, and bilateral branches of the ophthalmic artery. Histopathology revealed occlusive vasculopathy with epidermal ischemic changes as well as dermal and subcutaneous vascular congestion and small thrombi. Within the subcutis, there were tiny stippled calcium deposits within very small vascular lumina (Figure). The combination of clinical and histological findings was highly suggestive of calcific uremic arteriolopathy, and the patient was transitioned to hemodialysis against a low-calcium bath to avoid hypercalcemia. Unfortunately, she developed complications related to sepsis and experienced worsening mentation. After a discussion with palliative care, the patient was transitioned to comfort measures and discharged home on hospice 1 week after the biopsy at her family’s request.

Calcific uremic arteriolopathy (also known as calciphylaxis) is a rare, life-threatening syndrome of widespread vascular calcification leading to microvascular occlusion within the dermis and subcutaneous tissues.1 Clinically, it typically manifests as severely painful, purpuric skin lesions that evolve through phases of blistering, ulceration, and ultimately visible skin necrosis.2 The pain likely is a consequence of ischemia and nociceptive activation and often may precede any visibly apparent skin lesions.3 Risk factors associated with the development of this condition include female sex; history of diabetes mellitus, obesity, rapid weight loss, or end-stage renal disease; abnormalities in calcium and phosphorus homeostasis; and vitamin K deficiency.1,3 It is more prevalent in patients on peritoneal dialysis compared to hemodialysis.4
Calciphylaxis is diagnosed with combined clinical and histopathological evidence. Laboratory test abnormalities are not specific for disease; therefore, skin biopsy is the standard confirmatory test, though its practice is contentious due to the risk for nonhealing ulceration and increasing risk for infection.1 Findings suggestive of disease include focal to diffuse calcification (intravascular, extravascular, or perieccrine), superficial fat calcium deposition, mid panniculus calcium deposition, mid panniculus vascular thrombi, and focal to diffuse angioplasia.5 The hallmark feature is diffuse calcification of small capillaries in adipose tissue.6
The mortality rate associated with this disease is high—a 6-month mortality rate of 27% to 43% has been reported from the time of diagnosis7-9—which often is related to subsequent superimposed infections patients acquire from necrotic skin tissue.2 The disease also carries high morbidity, with patients experiencing frequent hospitalizations related to pain, infections, and nonhealing wounds.6 There is no standard treatment, and trials have been limited to small sample sizes. A multidisciplinary treatment approach is essential to maximize outcomes, which includes wound care, risk factor modification, analgesia, and symptomatic management strategies.1,2,6
Some pharmacologic agents have received noteworthy attention in treating calciphylaxis, including sodium thiosulfate (STS), bisphosphonates, and vitamin K supplementation.1 The strongest evidence supporting the use of STS comes from 2 trials involving 53 and 27 dialysis patients, with complete remission in 14 (26%) and 14 (52%) patients, respectively.10,11 However, these trials did not include control groups to compare outcomes, and mortality rates were similarly high among partial responders and nonresponders compared with patients not treated with STS. A 2018 systematic review failed to assess the efficacy of STS alone for the treatment of calciphylaxis but suggested there may be a future role for it, with 251 of 358 patients (70.1%) responding to therapy.12
Erythema ab igne is a cutaneous reaction related to long-term heat exposure, often from electronic devices such as laptops, heating pads, space heaters, or hot-water bottles.13,14 Clinically, this rash appears as an erythematous, purpuric, or hyperpigmented reticular dermatosis that is below the clinical threshold to define a thermal burn.13 Lesions often are seen on the anterior thighs or across the abdomen.15 There usually are no long-term clinical sequelae; however, rare malignant transformation has been documented in cases of atrophy or nonhealing ulceration.16 Treatment is supportive with removal of the offending agent, but hyperpigmentation may persist for months to years.14
Livedo reticularis is a cutaneous pattern of mottled violaceous or hyperpigmented changes that often signifies underlying vascular dermal changes.17 It can be seen in various pathologic states, including vasculitis, autoimmune disease, connective tissue disease, neurologic disease, infection, or malignancy, or it can be drug induced.18 There are no pathognomonic microscopic changes, as the histology will drastically differ based on the etiology. Workup can be extensive; cues to the underlying pathology should be sought based on the patient’s history and concurrent presenting symptoms. Livedo reticularis is the most common dermatologic finding in patients with antiphospholipid syndrome, and workup should include antiphospholipid antibodies (eg, lupus anticoagulant, anticardiolipin, anti–beta-2-glycoproteins) as well as lupus testing (eg, antinuclear antibodies, anti– double-stranded DNA).19 Treatment is targeted at the underlying disease process.
Cryoglobulinemia is a disease characterized by abnormal serum immunoglobulins that precipitate at cold temperatures and is further subcategorized by the type of complexes that are deposited.20 Type I represents purely monoclonal cryoglobulins, type III purely polyclonal, and type II a mixed picture. Clinical manifestations arise from excessive deposition of these proteins in the skin, joints, peripheral vasculature, and kidneys leading to purpuric skin lesions, chronic ulceration, arthralgia, and glomerulonephritis. Cutaneous findings may include erythematous to purpuric macular or papular changes with or without the presence of ulceration, infarction, or hemorrhagic crusting.21 Systemic disease often underlies a diagnosis, and further investigation for hepatitis C virus, connective tissue disease, and hematologic malignancies should be considered.20 Treatment is targeted at underlying systemic disease, such as antiviral treatment for hepatitis or chemotherapeutic regimens for hematologic disease.22
Polyarteritis nodosa is a systemic necrotizing vasculitis that typically involves small- to medium-sized arteries. Cutaneous manifestations often include subcutaneous nodules, livedo reticularis, and ulcerations most found on the lower extremities.23 Systemic symptoms including fever, myalgia, arthralgia, and neuropathy often are present. Characteristic histopathology findings include inflammation and destruction of medium-sized arteries at the junctional zone of the dermis and subcutis along with microaneurysms along the vessels.24 Treatment is based on the severity of disease, with localized cutaneous disease often being controlled with topical steroids and anti-inflammatory agents, while more widespread disease requires immunosuppression with systemic steroids, hydroxychloroquine, azathioprine, methotrexate, mycophenolate mofetil, or intravenous immunoglobulins.23
The Diagnosis: Calcific Uremic Arteriolopathy
Computed tomography of the abdomen and pelvis with contrast revealed a right complex renal cyst with peripheral calcification; computed tomography of the head without contrast revealed atherosclerotic changes with calcification of the intracranial arteries, vertebral basilar arteries, and bilateral branches of the ophthalmic artery. Histopathology revealed occlusive vasculopathy with epidermal ischemic changes as well as dermal and subcutaneous vascular congestion and small thrombi. Within the subcutis, there were tiny stippled calcium deposits within very small vascular lumina (Figure). The combination of clinical and histological findings was highly suggestive of calcific uremic arteriolopathy, and the patient was transitioned to hemodialysis against a low-calcium bath to avoid hypercalcemia. Unfortunately, she developed complications related to sepsis and experienced worsening mentation. After a discussion with palliative care, the patient was transitioned to comfort measures and discharged home on hospice 1 week after the biopsy at her family’s request.

Calcific uremic arteriolopathy (also known as calciphylaxis) is a rare, life-threatening syndrome of widespread vascular calcification leading to microvascular occlusion within the dermis and subcutaneous tissues.1 Clinically, it typically manifests as severely painful, purpuric skin lesions that evolve through phases of blistering, ulceration, and ultimately visible skin necrosis.2 The pain likely is a consequence of ischemia and nociceptive activation and often may precede any visibly apparent skin lesions.3 Risk factors associated with the development of this condition include female sex; history of diabetes mellitus, obesity, rapid weight loss, or end-stage renal disease; abnormalities in calcium and phosphorus homeostasis; and vitamin K deficiency.1,3 It is more prevalent in patients on peritoneal dialysis compared to hemodialysis.4
Calciphylaxis is diagnosed with combined clinical and histopathological evidence. Laboratory test abnormalities are not specific for disease; therefore, skin biopsy is the standard confirmatory test, though its practice is contentious due to the risk for nonhealing ulceration and increasing risk for infection.1 Findings suggestive of disease include focal to diffuse calcification (intravascular, extravascular, or perieccrine), superficial fat calcium deposition, mid panniculus calcium deposition, mid panniculus vascular thrombi, and focal to diffuse angioplasia.5 The hallmark feature is diffuse calcification of small capillaries in adipose tissue.6
The mortality rate associated with this disease is high—a 6-month mortality rate of 27% to 43% has been reported from the time of diagnosis7-9—which often is related to subsequent superimposed infections patients acquire from necrotic skin tissue.2 The disease also carries high morbidity, with patients experiencing frequent hospitalizations related to pain, infections, and nonhealing wounds.6 There is no standard treatment, and trials have been limited to small sample sizes. A multidisciplinary treatment approach is essential to maximize outcomes, which includes wound care, risk factor modification, analgesia, and symptomatic management strategies.1,2,6
Some pharmacologic agents have received noteworthy attention in treating calciphylaxis, including sodium thiosulfate (STS), bisphosphonates, and vitamin K supplementation.1 The strongest evidence supporting the use of STS comes from 2 trials involving 53 and 27 dialysis patients, with complete remission in 14 (26%) and 14 (52%) patients, respectively.10,11 However, these trials did not include control groups to compare outcomes, and mortality rates were similarly high among partial responders and nonresponders compared with patients not treated with STS. A 2018 systematic review failed to assess the efficacy of STS alone for the treatment of calciphylaxis but suggested there may be a future role for it, with 251 of 358 patients (70.1%) responding to therapy.12
Erythema ab igne is a cutaneous reaction related to long-term heat exposure, often from electronic devices such as laptops, heating pads, space heaters, or hot-water bottles.13,14 Clinically, this rash appears as an erythematous, purpuric, or hyperpigmented reticular dermatosis that is below the clinical threshold to define a thermal burn.13 Lesions often are seen on the anterior thighs or across the abdomen.15 There usually are no long-term clinical sequelae; however, rare malignant transformation has been documented in cases of atrophy or nonhealing ulceration.16 Treatment is supportive with removal of the offending agent, but hyperpigmentation may persist for months to years.14
Livedo reticularis is a cutaneous pattern of mottled violaceous or hyperpigmented changes that often signifies underlying vascular dermal changes.17 It can be seen in various pathologic states, including vasculitis, autoimmune disease, connective tissue disease, neurologic disease, infection, or malignancy, or it can be drug induced.18 There are no pathognomonic microscopic changes, as the histology will drastically differ based on the etiology. Workup can be extensive; cues to the underlying pathology should be sought based on the patient’s history and concurrent presenting symptoms. Livedo reticularis is the most common dermatologic finding in patients with antiphospholipid syndrome, and workup should include antiphospholipid antibodies (eg, lupus anticoagulant, anticardiolipin, anti–beta-2-glycoproteins) as well as lupus testing (eg, antinuclear antibodies, anti– double-stranded DNA).19 Treatment is targeted at the underlying disease process.
Cryoglobulinemia is a disease characterized by abnormal serum immunoglobulins that precipitate at cold temperatures and is further subcategorized by the type of complexes that are deposited.20 Type I represents purely monoclonal cryoglobulins, type III purely polyclonal, and type II a mixed picture. Clinical manifestations arise from excessive deposition of these proteins in the skin, joints, peripheral vasculature, and kidneys leading to purpuric skin lesions, chronic ulceration, arthralgia, and glomerulonephritis. Cutaneous findings may include erythematous to purpuric macular or papular changes with or without the presence of ulceration, infarction, or hemorrhagic crusting.21 Systemic disease often underlies a diagnosis, and further investigation for hepatitis C virus, connective tissue disease, and hematologic malignancies should be considered.20 Treatment is targeted at underlying systemic disease, such as antiviral treatment for hepatitis or chemotherapeutic regimens for hematologic disease.22
Polyarteritis nodosa is a systemic necrotizing vasculitis that typically involves small- to medium-sized arteries. Cutaneous manifestations often include subcutaneous nodules, livedo reticularis, and ulcerations most found on the lower extremities.23 Systemic symptoms including fever, myalgia, arthralgia, and neuropathy often are present. Characteristic histopathology findings include inflammation and destruction of medium-sized arteries at the junctional zone of the dermis and subcutis along with microaneurysms along the vessels.24 Treatment is based on the severity of disease, with localized cutaneous disease often being controlled with topical steroids and anti-inflammatory agents, while more widespread disease requires immunosuppression with systemic steroids, hydroxychloroquine, azathioprine, methotrexate, mycophenolate mofetil, or intravenous immunoglobulins.23
- Nigwekar SU, Thadhani R, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378:1704-1714. doi:10.1056/NEJMra1505292
- Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146. doi:10.1053/j.ajkd.2015.01.034
- Chang JJ. Calciphylaxis: diagnosis, pathogenesis, and treatment. Adv Skin Wound Care. 2019;32:205-215. doi:10.1097/01 .ASW.0000554443.14002.13
- Zhang Y, Corapi KM, Luongo M, et al. Calciphylaxis in peritoneal dialysis patients: a single center cohort study. Int J Nephrol Renovasc Dis. 2016;9:235-241. doi:10.2147/ijnrd.S115701
- Chen TY, Lehman JS, Gibson LE, et al. Histopathology of calciphylaxis: cohort study with clinical correlations. Am J Dermatopathol. 2017;39:795-802. doi:10.1097/DAD.0000000000000824
- Kodumudi V, Jeha GM, Mydlo N, et al. Management of cutaneous calciphylaxis. Adv Ther. 2020;37:4797-4807. doi:10.1007 /s12325-020-01504-w
- Nigwekar SU, Zhao S, Wenger J, et al. A nationally representative study of calcific uremic arteriolopathy risk factors. J Am Soc Nephrol. 2016;27:3421-3429. doi:10.1681/asn.2015091065
- McCarthy JT, El-Azhary RA, Patzelt MT, et al. Survival, risk factors, and effect of treatment in 101 patients with calciphylaxis. Mayo Clin Proc. 2016;91:1384-1394. doi:10.1016/j.mayocp.2016.06.025
- Fine A, Zacharias J. Calciphylaxis is usually non-ulcerating: risk factors, outcome and therapy. Kidney Int. 2002;61:2210-2217. doi:10.1046/j.1523-1755.2002.00375.x
- Nigwekar SU, Brunelli SM, Meade D, et al. Sodium thiosulfate therapy for calcific uremic arteriolopathy. Clin J Am Soc Nephrol. 2013;8:1162-1170. doi:10.2215/cjn.09880912
- Zitt E, König M, Vychytil A, et al. Use of sodium thiosulphate in a multi-interventional setting for the treatment of calciphylaxis in dialysis patients. Nephrol Dial Transplant. 2013;28:1232-1240. doi:10.1093/ndt/gfs548
- Peng T, Zhuo L, Wang Y, et al. Systematic review of sodium thiosulfate in treating calciphylaxis in chronic kidney disease patients. Nephrology (Carlton). 2018;23:669-675. doi:10.1111/nep.13081
- Miller K, Hunt R, Chu J, et al. Erythema ab igne. Dermatol Online J. 2011;17:28.
- Kettelhut EA, Traylor J, Sathe NC, et al. Erythema ab igne. StatPearls. StatPearls Publishing; 2022.
- Knöpfel N, Weibel L. Erythema Ab Igne. JAMA Dermatol. 2021;157: 106. doi:10.1001/jamadermatol.2020.3995
- Sigmon JR, Cantrell J, Teague D, et al. Poorly differentiated carcinoma arising in the setting of erythema ab igne. Am J Dermatopathol. 2013;35:676-678. doi:10.1097/DAD.0b013e3182871648
- Rose AE, Sagger V, Boyd KP, et al. Livedo reticularis. Dermatol Online J. 2013;19:20705.
- Sajjan VV, Lunge S, Swamy MB, et al. Livedo reticularis: a review of the literature. Indian Dermatol Online J. 2015;6:315-321. doi:10.4103/2229-5178.164493
- Uthman IW, Khamashta MA. Livedo racemosa: a striking dermatological sign for the antiphospholipid syndrome. J Rheumatol. 2006;33:2379-2382.
- Desbois AC, Cacoub P, Saadoun D. Cryoglobulinemia: an update in 2019. Joint Bone Spine. 2019;86:707-713. doi:10.1016/j .jbspin.2019.01.016
- Cohen SJ, Pittelkow MR, Su WP. Cutaneous manifestations of cryoglobulinemia: clinical and histopathologic study of seventy-two patients. J Am Acad Dermatol. 1991;25(1, pt 1):21-27. doi:10.1016 /0190-9622(91)70168-2
- Takada S, Shimizu T, Hadano Y, et al. Cryoglobulinemia (review). Mol Med Rep. 2012;6:3-8. doi:10.3892/mmr.2012.861
- Turska M, Parada-Turska J. Cutaneous polyarteritis nodosa. Wiad Lek. 2018;71(1, pt 1):73-77.
- De Virgilio A, Greco A, Magliulo G, et al. Polyarteritis nodosa: a contemporary overview. Autoimmun Rev. 2016;15:564-570. doi:10.1016/j.autrev.2016.02.015
- Nigwekar SU, Thadhani R, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378:1704-1714. doi:10.1056/NEJMra1505292
- Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146. doi:10.1053/j.ajkd.2015.01.034
- Chang JJ. Calciphylaxis: diagnosis, pathogenesis, and treatment. Adv Skin Wound Care. 2019;32:205-215. doi:10.1097/01 .ASW.0000554443.14002.13
- Zhang Y, Corapi KM, Luongo M, et al. Calciphylaxis in peritoneal dialysis patients: a single center cohort study. Int J Nephrol Renovasc Dis. 2016;9:235-241. doi:10.2147/ijnrd.S115701
- Chen TY, Lehman JS, Gibson LE, et al. Histopathology of calciphylaxis: cohort study with clinical correlations. Am J Dermatopathol. 2017;39:795-802. doi:10.1097/DAD.0000000000000824
- Kodumudi V, Jeha GM, Mydlo N, et al. Management of cutaneous calciphylaxis. Adv Ther. 2020;37:4797-4807. doi:10.1007 /s12325-020-01504-w
- Nigwekar SU, Zhao S, Wenger J, et al. A nationally representative study of calcific uremic arteriolopathy risk factors. J Am Soc Nephrol. 2016;27:3421-3429. doi:10.1681/asn.2015091065
- McCarthy JT, El-Azhary RA, Patzelt MT, et al. Survival, risk factors, and effect of treatment in 101 patients with calciphylaxis. Mayo Clin Proc. 2016;91:1384-1394. doi:10.1016/j.mayocp.2016.06.025
- Fine A, Zacharias J. Calciphylaxis is usually non-ulcerating: risk factors, outcome and therapy. Kidney Int. 2002;61:2210-2217. doi:10.1046/j.1523-1755.2002.00375.x
- Nigwekar SU, Brunelli SM, Meade D, et al. Sodium thiosulfate therapy for calcific uremic arteriolopathy. Clin J Am Soc Nephrol. 2013;8:1162-1170. doi:10.2215/cjn.09880912
- Zitt E, König M, Vychytil A, et al. Use of sodium thiosulphate in a multi-interventional setting for the treatment of calciphylaxis in dialysis patients. Nephrol Dial Transplant. 2013;28:1232-1240. doi:10.1093/ndt/gfs548
- Peng T, Zhuo L, Wang Y, et al. Systematic review of sodium thiosulfate in treating calciphylaxis in chronic kidney disease patients. Nephrology (Carlton). 2018;23:669-675. doi:10.1111/nep.13081
- Miller K, Hunt R, Chu J, et al. Erythema ab igne. Dermatol Online J. 2011;17:28.
- Kettelhut EA, Traylor J, Sathe NC, et al. Erythema ab igne. StatPearls. StatPearls Publishing; 2022.
- Knöpfel N, Weibel L. Erythema Ab Igne. JAMA Dermatol. 2021;157: 106. doi:10.1001/jamadermatol.2020.3995
- Sigmon JR, Cantrell J, Teague D, et al. Poorly differentiated carcinoma arising in the setting of erythema ab igne. Am J Dermatopathol. 2013;35:676-678. doi:10.1097/DAD.0b013e3182871648
- Rose AE, Sagger V, Boyd KP, et al. Livedo reticularis. Dermatol Online J. 2013;19:20705.
- Sajjan VV, Lunge S, Swamy MB, et al. Livedo reticularis: a review of the literature. Indian Dermatol Online J. 2015;6:315-321. doi:10.4103/2229-5178.164493
- Uthman IW, Khamashta MA. Livedo racemosa: a striking dermatological sign for the antiphospholipid syndrome. J Rheumatol. 2006;33:2379-2382.
- Desbois AC, Cacoub P, Saadoun D. Cryoglobulinemia: an update in 2019. Joint Bone Spine. 2019;86:707-713. doi:10.1016/j .jbspin.2019.01.016
- Cohen SJ, Pittelkow MR, Su WP. Cutaneous manifestations of cryoglobulinemia: clinical and histopathologic study of seventy-two patients. J Am Acad Dermatol. 1991;25(1, pt 1):21-27. doi:10.1016 /0190-9622(91)70168-2
- Takada S, Shimizu T, Hadano Y, et al. Cryoglobulinemia (review). Mol Med Rep. 2012;6:3-8. doi:10.3892/mmr.2012.861
- Turska M, Parada-Turska J. Cutaneous polyarteritis nodosa. Wiad Lek. 2018;71(1, pt 1):73-77.
- De Virgilio A, Greco A, Magliulo G, et al. Polyarteritis nodosa: a contemporary overview. Autoimmun Rev. 2016;15:564-570. doi:10.1016/j.autrev.2016.02.015
A 72-year-old woman presented to the emergency department with concerns of confusion and lethargy during a session of peritoneal dialysis, which she had been receiving for the last 2 years for end-stage renal disease. She had a history of type 2 diabetes mellitus, diabetic retinopathy, hypertension, coronary artery disease, and peripheral vascular disease preceding a recent right below-knee amputation. A review of systems was positive for a rash on the thighs of several weeks’ duration that was preceded by several days of burning pain in the same distribution. Physical examination revealed retiform purpura with irregular contours and interspersed white stellate patterns scattered across the superomedial thighs, right lower back, and left lower abdomen. An initial laboratory workup revealed an elevated creatinine level of 5.03 mg/dL (reference range, 0.6–1.1 mg/dL; baseline level, 3.0 mg/dL) and mild leukocytosis (12.5 cells/mm3 [reference range, 4.5–11.0 cells/mm3]). Dermatology was consulted, and a 4-mm punch biopsy was obtained from the left medial thigh. Nephrology, infectious disease, and wound care consultations also were placed.