User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Employment vs. private practice: Who’s happier?
Alexandra Kharazi, MD, a California-based cardiothoracic surgeon, previously worked as an employed physician and is now in private practice. Though she appreciates that there are some trade-offs to working with her small group of three surgeons, Dr. Kharazi has no qualms about her choice.
“For me, it’s an issue of autonomy,” she said. “While I have to work a lot of hours, I don’t have to adhere to a strict schedule. I also don’t have to follow specific policies and rules.”
In contrast, Cassandra Boduch, MD, an employed psychiatrist with PsychPlus in Houston, is very satisfied with working as an employee. “I looked into private practice, but no one really prepares you for the complications that come with it,” she said. “There’s a lot more that goes into it than people realize.”
By hanging up her own shingle, Dr. Kharazi may be living a rapidly shrinking dream. According to the American Medical Association, between 2012 and 2022, the share of physicians working in private practice fell from 60% to 47%. The share of physicians working in hospitals as direct employees or contractors increased from about 6% to about 10% during the same time period.
, according to the AMA.
Though the traditional dream of owning your own practice may be slipping away, are employed physicians less happy than are their self-employed peers? By many measures, the answer is no.
In Medscape’s Employed Physicians Report 2023, doctors weighed in on the pros and cons of their jobs.
When asked what they like most about their jobs, employed physician respondents reported “not having to run a business” as their number-one benefit, followed closely by a stable income. The fact that employers pay for malpractice insurance ranked third, followed by work-life balance.
“We get no business classes in medical school or residency,” said one employed physician. “Having a good salary feels good,” said another. Yet another respondent chimed in: “Running a practice as a small business has become undoable over the past 10-12 years.”
And 50% of employed physicians said that they were “very satisfied/satisfied” with their degree of autonomy.
Still, employed physicians also had plenty to say about the downsides of their jobs.
Many pointed to “feeling like a cog in the machine,” and one doctor pointed to the hassle of dealing with bureaucracy. Others complained about the fact that nonphysicians ran the business and lacked an understanding of what physicians really need from their jobs. When asked whether administrative rules made sense, 63% of physician respondents said that yes, the rules make sense for the business; but, only 52% said that the rules make sense for the doctors themselves.
Other complaints included the requirement to reach high productivity targets and too low an income potential. In the 9 years since Medscape’s 2104 Employed Physicians Report, the share of employed doctors paid on a straight salary has declined from 46% to 31%. Those compensated on a base salary plus productivity targets and other performance metrics rose from 13% in 2014 to 32% now.
“Many doctors go into private practice because of the freedom it brings and the potential financial incentives,” added Dr. Boduch. “I know that many doctors have a dream of working for themselves, and in many cases, that works out great for them.”
Dr. Boduch noted that in her job as chief medical officer at PsychPlus, she still has flexibility plus the perks of working with a bigger practice. In this scenario, Dr. Boduch said, the company can negotiate with insurance companies, allowing her the financial rewards of private practice.
What’s right for you?
“I think it might be somewhat generational,” said Cody Futch, senior recruiting executive at AMN Healthcare. “It used to be that fewer hospitals offered employment, so private practice was the way to go. Now, there are fewer privates because hospitals and corporations are buying them up.”
This reality has potentially shaped the way younger generations approach their workplace. Also, Gen Z tends to have less intention to stay with a current employer for the long term than did their parents. “Older physicians were trained to expect they’d run their own business and build it over the years,” said Mr. Futch. “The younger generations look at it as a job, something they may want to switch in a few years. It’s a combination of candidates wanting more options, and also the fact that there are more options to be employed.”
Along those lines, younger generations in general tend to place work-life balance as a higher priority than do older generations, and employed physicians place this equation high on the list as well. In the Employed Physicians Report 2023, 54% said that they are satisfied or better with their work-life balance, up from 51% in the 2022 report.
With that in mind, Dr. Kharazi noted that flexibility is one of the chief reasons why she likes private practice. “If my kid has an event I want to attend, I don’t have to adhere to a strict schedule,” she said.
Satisfaction as an employee vs. employed doctor sometimes changes based on the type of medicine you practice too. With specialties that tend to be primarily outpatient, such as dermatology and allergy, private practice may be the best option regardless. “Hospitals don’t seek out those specialists as much and the specialists can operate successfully without a hospital,” said Mr. Futch.
Hospitals try to incentivize doctors with perks like hefty sign-on bonuses, student loan forgiveness, plenty of vacation time, and more. They also put money into marketing their doctors, a time-consuming and expensive aspect that is tough to shoulder in private practice, especially in the early years. Mr. Futch adds that many doctors view employment as a more stable option. “As the government changes reimbursement policies, the income from private practice fluctuates,” he said. “So many doctors worry that if they buy into a private practice, it is a risky endeavor.”
Hospitals aren’t always a sure bet in that regard, either: They go through tough financial times, lay off staff, or make salary cuts. Historically, however, employment tends to be the safer route, which can make it an attractive option.
Ultimately, the pros and cons of each scenario are individual. It’s up to physicians to do their own math and balance sheet before making a decision.
A version of this article first appeared on Medscape.com.
Alexandra Kharazi, MD, a California-based cardiothoracic surgeon, previously worked as an employed physician and is now in private practice. Though she appreciates that there are some trade-offs to working with her small group of three surgeons, Dr. Kharazi has no qualms about her choice.
“For me, it’s an issue of autonomy,” she said. “While I have to work a lot of hours, I don’t have to adhere to a strict schedule. I also don’t have to follow specific policies and rules.”
In contrast, Cassandra Boduch, MD, an employed psychiatrist with PsychPlus in Houston, is very satisfied with working as an employee. “I looked into private practice, but no one really prepares you for the complications that come with it,” she said. “There’s a lot more that goes into it than people realize.”
By hanging up her own shingle, Dr. Kharazi may be living a rapidly shrinking dream. According to the American Medical Association, between 2012 and 2022, the share of physicians working in private practice fell from 60% to 47%. The share of physicians working in hospitals as direct employees or contractors increased from about 6% to about 10% during the same time period.
, according to the AMA.
Though the traditional dream of owning your own practice may be slipping away, are employed physicians less happy than are their self-employed peers? By many measures, the answer is no.
In Medscape’s Employed Physicians Report 2023, doctors weighed in on the pros and cons of their jobs.
When asked what they like most about their jobs, employed physician respondents reported “not having to run a business” as their number-one benefit, followed closely by a stable income. The fact that employers pay for malpractice insurance ranked third, followed by work-life balance.
“We get no business classes in medical school or residency,” said one employed physician. “Having a good salary feels good,” said another. Yet another respondent chimed in: “Running a practice as a small business has become undoable over the past 10-12 years.”
And 50% of employed physicians said that they were “very satisfied/satisfied” with their degree of autonomy.
Still, employed physicians also had plenty to say about the downsides of their jobs.
Many pointed to “feeling like a cog in the machine,” and one doctor pointed to the hassle of dealing with bureaucracy. Others complained about the fact that nonphysicians ran the business and lacked an understanding of what physicians really need from their jobs. When asked whether administrative rules made sense, 63% of physician respondents said that yes, the rules make sense for the business; but, only 52% said that the rules make sense for the doctors themselves.
Other complaints included the requirement to reach high productivity targets and too low an income potential. In the 9 years since Medscape’s 2104 Employed Physicians Report, the share of employed doctors paid on a straight salary has declined from 46% to 31%. Those compensated on a base salary plus productivity targets and other performance metrics rose from 13% in 2014 to 32% now.
“Many doctors go into private practice because of the freedom it brings and the potential financial incentives,” added Dr. Boduch. “I know that many doctors have a dream of working for themselves, and in many cases, that works out great for them.”
Dr. Boduch noted that in her job as chief medical officer at PsychPlus, she still has flexibility plus the perks of working with a bigger practice. In this scenario, Dr. Boduch said, the company can negotiate with insurance companies, allowing her the financial rewards of private practice.
What’s right for you?
“I think it might be somewhat generational,” said Cody Futch, senior recruiting executive at AMN Healthcare. “It used to be that fewer hospitals offered employment, so private practice was the way to go. Now, there are fewer privates because hospitals and corporations are buying them up.”
This reality has potentially shaped the way younger generations approach their workplace. Also, Gen Z tends to have less intention to stay with a current employer for the long term than did their parents. “Older physicians were trained to expect they’d run their own business and build it over the years,” said Mr. Futch. “The younger generations look at it as a job, something they may want to switch in a few years. It’s a combination of candidates wanting more options, and also the fact that there are more options to be employed.”
Along those lines, younger generations in general tend to place work-life balance as a higher priority than do older generations, and employed physicians place this equation high on the list as well. In the Employed Physicians Report 2023, 54% said that they are satisfied or better with their work-life balance, up from 51% in the 2022 report.
With that in mind, Dr. Kharazi noted that flexibility is one of the chief reasons why she likes private practice. “If my kid has an event I want to attend, I don’t have to adhere to a strict schedule,” she said.
Satisfaction as an employee vs. employed doctor sometimes changes based on the type of medicine you practice too. With specialties that tend to be primarily outpatient, such as dermatology and allergy, private practice may be the best option regardless. “Hospitals don’t seek out those specialists as much and the specialists can operate successfully without a hospital,” said Mr. Futch.
Hospitals try to incentivize doctors with perks like hefty sign-on bonuses, student loan forgiveness, plenty of vacation time, and more. They also put money into marketing their doctors, a time-consuming and expensive aspect that is tough to shoulder in private practice, especially in the early years. Mr. Futch adds that many doctors view employment as a more stable option. “As the government changes reimbursement policies, the income from private practice fluctuates,” he said. “So many doctors worry that if they buy into a private practice, it is a risky endeavor.”
Hospitals aren’t always a sure bet in that regard, either: They go through tough financial times, lay off staff, or make salary cuts. Historically, however, employment tends to be the safer route, which can make it an attractive option.
Ultimately, the pros and cons of each scenario are individual. It’s up to physicians to do their own math and balance sheet before making a decision.
A version of this article first appeared on Medscape.com.
Alexandra Kharazi, MD, a California-based cardiothoracic surgeon, previously worked as an employed physician and is now in private practice. Though she appreciates that there are some trade-offs to working with her small group of three surgeons, Dr. Kharazi has no qualms about her choice.
“For me, it’s an issue of autonomy,” she said. “While I have to work a lot of hours, I don’t have to adhere to a strict schedule. I also don’t have to follow specific policies and rules.”
In contrast, Cassandra Boduch, MD, an employed psychiatrist with PsychPlus in Houston, is very satisfied with working as an employee. “I looked into private practice, but no one really prepares you for the complications that come with it,” she said. “There’s a lot more that goes into it than people realize.”
By hanging up her own shingle, Dr. Kharazi may be living a rapidly shrinking dream. According to the American Medical Association, between 2012 and 2022, the share of physicians working in private practice fell from 60% to 47%. The share of physicians working in hospitals as direct employees or contractors increased from about 6% to about 10% during the same time period.
, according to the AMA.
Though the traditional dream of owning your own practice may be slipping away, are employed physicians less happy than are their self-employed peers? By many measures, the answer is no.
In Medscape’s Employed Physicians Report 2023, doctors weighed in on the pros and cons of their jobs.
When asked what they like most about their jobs, employed physician respondents reported “not having to run a business” as their number-one benefit, followed closely by a stable income. The fact that employers pay for malpractice insurance ranked third, followed by work-life balance.
“We get no business classes in medical school or residency,” said one employed physician. “Having a good salary feels good,” said another. Yet another respondent chimed in: “Running a practice as a small business has become undoable over the past 10-12 years.”
And 50% of employed physicians said that they were “very satisfied/satisfied” with their degree of autonomy.
Still, employed physicians also had plenty to say about the downsides of their jobs.
Many pointed to “feeling like a cog in the machine,” and one doctor pointed to the hassle of dealing with bureaucracy. Others complained about the fact that nonphysicians ran the business and lacked an understanding of what physicians really need from their jobs. When asked whether administrative rules made sense, 63% of physician respondents said that yes, the rules make sense for the business; but, only 52% said that the rules make sense for the doctors themselves.
Other complaints included the requirement to reach high productivity targets and too low an income potential. In the 9 years since Medscape’s 2104 Employed Physicians Report, the share of employed doctors paid on a straight salary has declined from 46% to 31%. Those compensated on a base salary plus productivity targets and other performance metrics rose from 13% in 2014 to 32% now.
“Many doctors go into private practice because of the freedom it brings and the potential financial incentives,” added Dr. Boduch. “I know that many doctors have a dream of working for themselves, and in many cases, that works out great for them.”
Dr. Boduch noted that in her job as chief medical officer at PsychPlus, she still has flexibility plus the perks of working with a bigger practice. In this scenario, Dr. Boduch said, the company can negotiate with insurance companies, allowing her the financial rewards of private practice.
What’s right for you?
“I think it might be somewhat generational,” said Cody Futch, senior recruiting executive at AMN Healthcare. “It used to be that fewer hospitals offered employment, so private practice was the way to go. Now, there are fewer privates because hospitals and corporations are buying them up.”
This reality has potentially shaped the way younger generations approach their workplace. Also, Gen Z tends to have less intention to stay with a current employer for the long term than did their parents. “Older physicians were trained to expect they’d run their own business and build it over the years,” said Mr. Futch. “The younger generations look at it as a job, something they may want to switch in a few years. It’s a combination of candidates wanting more options, and also the fact that there are more options to be employed.”
Along those lines, younger generations in general tend to place work-life balance as a higher priority than do older generations, and employed physicians place this equation high on the list as well. In the Employed Physicians Report 2023, 54% said that they are satisfied or better with their work-life balance, up from 51% in the 2022 report.
With that in mind, Dr. Kharazi noted that flexibility is one of the chief reasons why she likes private practice. “If my kid has an event I want to attend, I don’t have to adhere to a strict schedule,” she said.
Satisfaction as an employee vs. employed doctor sometimes changes based on the type of medicine you practice too. With specialties that tend to be primarily outpatient, such as dermatology and allergy, private practice may be the best option regardless. “Hospitals don’t seek out those specialists as much and the specialists can operate successfully without a hospital,” said Mr. Futch.
Hospitals try to incentivize doctors with perks like hefty sign-on bonuses, student loan forgiveness, plenty of vacation time, and more. They also put money into marketing their doctors, a time-consuming and expensive aspect that is tough to shoulder in private practice, especially in the early years. Mr. Futch adds that many doctors view employment as a more stable option. “As the government changes reimbursement policies, the income from private practice fluctuates,” he said. “So many doctors worry that if they buy into a private practice, it is a risky endeavor.”
Hospitals aren’t always a sure bet in that regard, either: They go through tough financial times, lay off staff, or make salary cuts. Historically, however, employment tends to be the safer route, which can make it an attractive option.
Ultimately, the pros and cons of each scenario are individual. It’s up to physicians to do their own math and balance sheet before making a decision.
A version of this article first appeared on Medscape.com.
Teledermatology model takes hold with grants to underserved areas
, according to a press release from the university.
Four institutions will receive grants to implement the George Washington University model, which involved partnering with a local organization to provide an entry point for individuals in areas with limited access to medical care, with support from Pfizer Global Medical Grants.
“Targeting those who lack access to quality-based care for inflammatory dermatologic conditions, including atopic dermatitis (AD) and others, the grants will reach communities in Miami-Dade County, Fla., Los Angeles County, Calif., rural communities in Oregon, and downtown Philadelphia,” according to the announcement. GW’s Teledermatology Free Clinic was conceived in the wake of the COVID-19 pandemic, which further highlighted disparities in access to dermatologic care, Adam Friedman, M.D., professor and chair of dermatology at George Washington University, said in the press release.
GW implemented its clinic for residents in underserved areas of Washington, D.C., in partnership with the Rodham Institute and the Temple of Praise Church. “We set up a free clinic at the church through which patients were integrated into the GW medical records system, provided instruction on telemedicine best practices, exposed to comprehensive education about AD and underwent a free telemedicine visit with a member of the department of dermatology,” Dr. Friedman explained.
Most participants – 70% – did not have a dermatologist, 94% were extremely satisfied with the experience, and 90% reported that the clinic had a significant impact on the management of their AD, according to the results of a recently published postengagement survey.
The following are the recipients of the “Quality Improvement Initiative: Bridging the Inflammatory Dermatosis Care Divide with Teledermatology Grant Program”:
- Scott Elman, MD, assistant professor of clinical dermatology and medical director of outpatient dermatology at the University of Miami and his team will create a clinic in partnership with Lotus House, a resource center and residential facility serving homeless women and infants, with focus on interventions in both English and Spanish.
- Nada Elbuluk, MD, associate professor of clinical dermatology and director of the Skin of Color and Pigmentary Disorders Program, at the University of Southern California, will lead a team to expand the role of two programs she created, Derm RISES, which targets inner city students, and Dermmunity, a community-based program that provides dermatology education to underserved communities in the Los Angeles area.
- Alex Ortega-Loayza, MD, associate professor of dermatology at Oregon Health & Science University and his team will partner with the Oregon Rural Practice-based Research Network to implement their teledermatology program at five clinics that serve different portions of rural and underserved communities across Oregon.
- Jules Lipoff, MD, clinical associate professor of dermatology, Temple University, Philadelphia, will lead a pilot program to establish a telemedicine dermatology clinic with Philadelphia FIGHT, a federally qualified health center in downtown Philadelphia where many patients lack high-speed Internet, and patients will be allowed direct access to telemedicine dermatology appointments within the primary care facility. The clinic’s patient population includes patients living with HIV, people who identify as LGBTQ+ and those who identify as trans or with a gender not matching their sex assigned at birth.
All four projects will complete postassessment surveys and quality assessment initiatives.
The GW clinic is ongoing, with plans for expansion and the establishment of additional programs with community partners in the Washington area, Dr. Friedman said in an interview.
“While these partnerships are in their infancy, I have high hopes that we will be able to impact even more individuals afflicted with dermatologic diseases and gain more insights into best practices for community engagement,” he added. “Many individuals who have come through our free clinic have followed up, by telehealth and/or in person at GW, depending on the clinical need to maintain continuity of care. In numerous cases, my impression is that this first point of contact is the key to ongoing treatment success, because it enables the access that may have been missing and engenders trust and confidence.”
, according to a press release from the university.
Four institutions will receive grants to implement the George Washington University model, which involved partnering with a local organization to provide an entry point for individuals in areas with limited access to medical care, with support from Pfizer Global Medical Grants.
“Targeting those who lack access to quality-based care for inflammatory dermatologic conditions, including atopic dermatitis (AD) and others, the grants will reach communities in Miami-Dade County, Fla., Los Angeles County, Calif., rural communities in Oregon, and downtown Philadelphia,” according to the announcement. GW’s Teledermatology Free Clinic was conceived in the wake of the COVID-19 pandemic, which further highlighted disparities in access to dermatologic care, Adam Friedman, M.D., professor and chair of dermatology at George Washington University, said in the press release.
GW implemented its clinic for residents in underserved areas of Washington, D.C., in partnership with the Rodham Institute and the Temple of Praise Church. “We set up a free clinic at the church through which patients were integrated into the GW medical records system, provided instruction on telemedicine best practices, exposed to comprehensive education about AD and underwent a free telemedicine visit with a member of the department of dermatology,” Dr. Friedman explained.
Most participants – 70% – did not have a dermatologist, 94% were extremely satisfied with the experience, and 90% reported that the clinic had a significant impact on the management of their AD, according to the results of a recently published postengagement survey.
The following are the recipients of the “Quality Improvement Initiative: Bridging the Inflammatory Dermatosis Care Divide with Teledermatology Grant Program”:
- Scott Elman, MD, assistant professor of clinical dermatology and medical director of outpatient dermatology at the University of Miami and his team will create a clinic in partnership with Lotus House, a resource center and residential facility serving homeless women and infants, with focus on interventions in both English and Spanish.
- Nada Elbuluk, MD, associate professor of clinical dermatology and director of the Skin of Color and Pigmentary Disorders Program, at the University of Southern California, will lead a team to expand the role of two programs she created, Derm RISES, which targets inner city students, and Dermmunity, a community-based program that provides dermatology education to underserved communities in the Los Angeles area.
- Alex Ortega-Loayza, MD, associate professor of dermatology at Oregon Health & Science University and his team will partner with the Oregon Rural Practice-based Research Network to implement their teledermatology program at five clinics that serve different portions of rural and underserved communities across Oregon.
- Jules Lipoff, MD, clinical associate professor of dermatology, Temple University, Philadelphia, will lead a pilot program to establish a telemedicine dermatology clinic with Philadelphia FIGHT, a federally qualified health center in downtown Philadelphia where many patients lack high-speed Internet, and patients will be allowed direct access to telemedicine dermatology appointments within the primary care facility. The clinic’s patient population includes patients living with HIV, people who identify as LGBTQ+ and those who identify as trans or with a gender not matching their sex assigned at birth.
All four projects will complete postassessment surveys and quality assessment initiatives.
The GW clinic is ongoing, with plans for expansion and the establishment of additional programs with community partners in the Washington area, Dr. Friedman said in an interview.
“While these partnerships are in their infancy, I have high hopes that we will be able to impact even more individuals afflicted with dermatologic diseases and gain more insights into best practices for community engagement,” he added. “Many individuals who have come through our free clinic have followed up, by telehealth and/or in person at GW, depending on the clinical need to maintain continuity of care. In numerous cases, my impression is that this first point of contact is the key to ongoing treatment success, because it enables the access that may have been missing and engenders trust and confidence.”
, according to a press release from the university.
Four institutions will receive grants to implement the George Washington University model, which involved partnering with a local organization to provide an entry point for individuals in areas with limited access to medical care, with support from Pfizer Global Medical Grants.
“Targeting those who lack access to quality-based care for inflammatory dermatologic conditions, including atopic dermatitis (AD) and others, the grants will reach communities in Miami-Dade County, Fla., Los Angeles County, Calif., rural communities in Oregon, and downtown Philadelphia,” according to the announcement. GW’s Teledermatology Free Clinic was conceived in the wake of the COVID-19 pandemic, which further highlighted disparities in access to dermatologic care, Adam Friedman, M.D., professor and chair of dermatology at George Washington University, said in the press release.
GW implemented its clinic for residents in underserved areas of Washington, D.C., in partnership with the Rodham Institute and the Temple of Praise Church. “We set up a free clinic at the church through which patients were integrated into the GW medical records system, provided instruction on telemedicine best practices, exposed to comprehensive education about AD and underwent a free telemedicine visit with a member of the department of dermatology,” Dr. Friedman explained.
Most participants – 70% – did not have a dermatologist, 94% were extremely satisfied with the experience, and 90% reported that the clinic had a significant impact on the management of their AD, according to the results of a recently published postengagement survey.
The following are the recipients of the “Quality Improvement Initiative: Bridging the Inflammatory Dermatosis Care Divide with Teledermatology Grant Program”:
- Scott Elman, MD, assistant professor of clinical dermatology and medical director of outpatient dermatology at the University of Miami and his team will create a clinic in partnership with Lotus House, a resource center and residential facility serving homeless women and infants, with focus on interventions in both English and Spanish.
- Nada Elbuluk, MD, associate professor of clinical dermatology and director of the Skin of Color and Pigmentary Disorders Program, at the University of Southern California, will lead a team to expand the role of two programs she created, Derm RISES, which targets inner city students, and Dermmunity, a community-based program that provides dermatology education to underserved communities in the Los Angeles area.
- Alex Ortega-Loayza, MD, associate professor of dermatology at Oregon Health & Science University and his team will partner with the Oregon Rural Practice-based Research Network to implement their teledermatology program at five clinics that serve different portions of rural and underserved communities across Oregon.
- Jules Lipoff, MD, clinical associate professor of dermatology, Temple University, Philadelphia, will lead a pilot program to establish a telemedicine dermatology clinic with Philadelphia FIGHT, a federally qualified health center in downtown Philadelphia where many patients lack high-speed Internet, and patients will be allowed direct access to telemedicine dermatology appointments within the primary care facility. The clinic’s patient population includes patients living with HIV, people who identify as LGBTQ+ and those who identify as trans or with a gender not matching their sex assigned at birth.
All four projects will complete postassessment surveys and quality assessment initiatives.
The GW clinic is ongoing, with plans for expansion and the establishment of additional programs with community partners in the Washington area, Dr. Friedman said in an interview.
“While these partnerships are in their infancy, I have high hopes that we will be able to impact even more individuals afflicted with dermatologic diseases and gain more insights into best practices for community engagement,” he added. “Many individuals who have come through our free clinic have followed up, by telehealth and/or in person at GW, depending on the clinical need to maintain continuity of care. In numerous cases, my impression is that this first point of contact is the key to ongoing treatment success, because it enables the access that may have been missing and engenders trust and confidence.”
FDA proposes ban on hair straightener ingredients
The
The proposal specifies that formaldehyde would be banned, as well as other chemicals that release formaldehyde, such as methylene glycol. Using hair smoothing products containing formaldehyde and formaldehyde-releasing chemicals “is linked to short-term adverse health effects, such as sensitization reactions and breathing problems, and long-term adverse health effects, including an increased risk of certain cancers,” the proposal states.
One study published last year showed that repeated use of hair straightening products, also called relaxers, could more than double the risk of uterine cancer. Although that study didn’t find that the uterine cancer risk varied based on a person’s race, the researchers noted that women who are Black are among the most likely to use the products and tend to start using them at younger ages, compared with people of other races and ethnicities.
Hair straightening products have also been linked to elevated risks of hormone-sensitive cancers, such as breast cancer and ovarian cancer.
Rep. Ayanna Pressley (D-Mass.) and Rep. Shontel Brown (D-Ohio) applauded the proposed rule in a statement issued jointly on Oct. 6. “The FDA’s proposal to ban these harmful chemicals in hair straighteners and relaxers is a win for public health – especially the health of Black women who are disproportionately put at risk by these products as a result of systemic racism and anti–Black hair sentiment,” Rep. Pressley said The two congresswomen wrote a letter to the FDA earlier this year requesting the topic be investigated.
“Regardless of how we wear our hair, we should be allowed to show up in the world without putting our health at risk. I applaud the FDA for being responsive to our calls and advancing a rule that will help prevent manufacturers from making a profit at the expense of our health,” Rep. Pressley said in the statement. “The administration should finalize this rule without delay.”
A version of this article appeared on WebMD.com
The
The proposal specifies that formaldehyde would be banned, as well as other chemicals that release formaldehyde, such as methylene glycol. Using hair smoothing products containing formaldehyde and formaldehyde-releasing chemicals “is linked to short-term adverse health effects, such as sensitization reactions and breathing problems, and long-term adverse health effects, including an increased risk of certain cancers,” the proposal states.
One study published last year showed that repeated use of hair straightening products, also called relaxers, could more than double the risk of uterine cancer. Although that study didn’t find that the uterine cancer risk varied based on a person’s race, the researchers noted that women who are Black are among the most likely to use the products and tend to start using them at younger ages, compared with people of other races and ethnicities.
Hair straightening products have also been linked to elevated risks of hormone-sensitive cancers, such as breast cancer and ovarian cancer.
Rep. Ayanna Pressley (D-Mass.) and Rep. Shontel Brown (D-Ohio) applauded the proposed rule in a statement issued jointly on Oct. 6. “The FDA’s proposal to ban these harmful chemicals in hair straighteners and relaxers is a win for public health – especially the health of Black women who are disproportionately put at risk by these products as a result of systemic racism and anti–Black hair sentiment,” Rep. Pressley said The two congresswomen wrote a letter to the FDA earlier this year requesting the topic be investigated.
“Regardless of how we wear our hair, we should be allowed to show up in the world without putting our health at risk. I applaud the FDA for being responsive to our calls and advancing a rule that will help prevent manufacturers from making a profit at the expense of our health,” Rep. Pressley said in the statement. “The administration should finalize this rule without delay.”
A version of this article appeared on WebMD.com
The
The proposal specifies that formaldehyde would be banned, as well as other chemicals that release formaldehyde, such as methylene glycol. Using hair smoothing products containing formaldehyde and formaldehyde-releasing chemicals “is linked to short-term adverse health effects, such as sensitization reactions and breathing problems, and long-term adverse health effects, including an increased risk of certain cancers,” the proposal states.
One study published last year showed that repeated use of hair straightening products, also called relaxers, could more than double the risk of uterine cancer. Although that study didn’t find that the uterine cancer risk varied based on a person’s race, the researchers noted that women who are Black are among the most likely to use the products and tend to start using them at younger ages, compared with people of other races and ethnicities.
Hair straightening products have also been linked to elevated risks of hormone-sensitive cancers, such as breast cancer and ovarian cancer.
Rep. Ayanna Pressley (D-Mass.) and Rep. Shontel Brown (D-Ohio) applauded the proposed rule in a statement issued jointly on Oct. 6. “The FDA’s proposal to ban these harmful chemicals in hair straighteners and relaxers is a win for public health – especially the health of Black women who are disproportionately put at risk by these products as a result of systemic racism and anti–Black hair sentiment,” Rep. Pressley said The two congresswomen wrote a letter to the FDA earlier this year requesting the topic be investigated.
“Regardless of how we wear our hair, we should be allowed to show up in the world without putting our health at risk. I applaud the FDA for being responsive to our calls and advancing a rule that will help prevent manufacturers from making a profit at the expense of our health,” Rep. Pressley said in the statement. “The administration should finalize this rule without delay.”
A version of this article appeared on WebMD.com
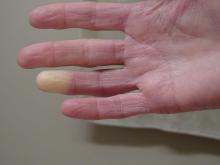
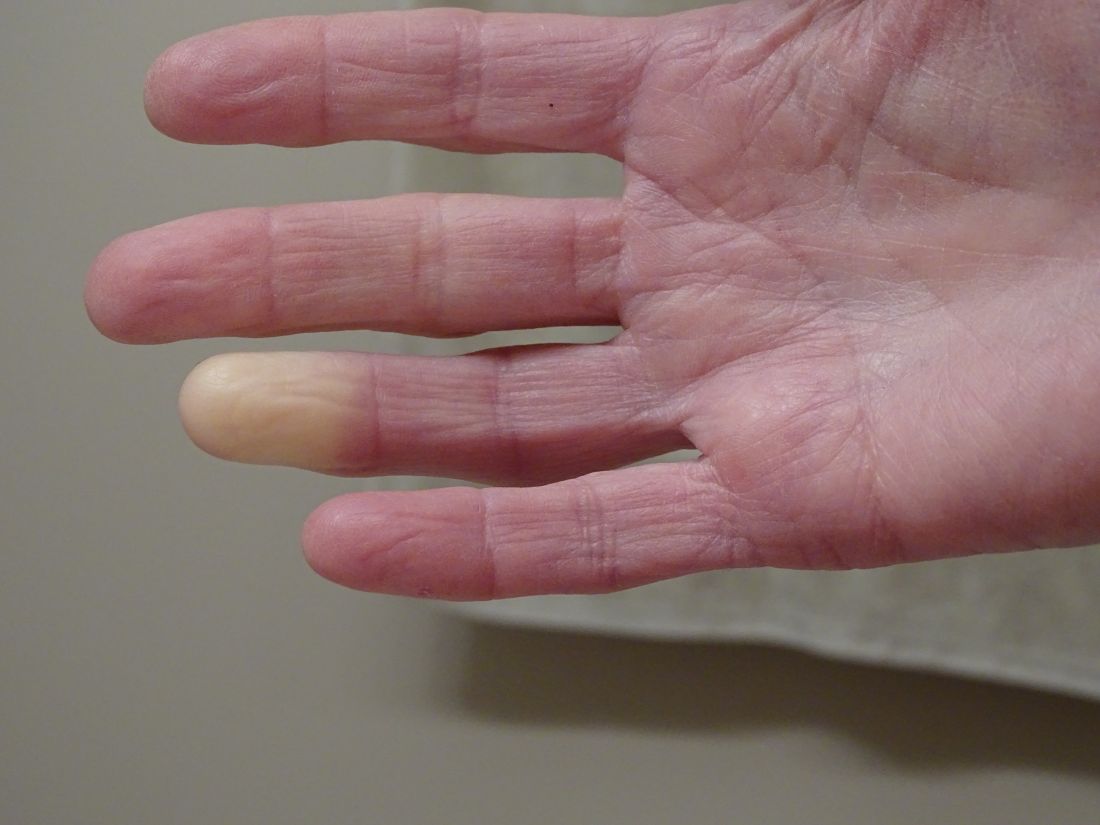
Researchers link two genes to Raynaud’s disease
Researchers have identified two genes that may contribute to Raynaud’s phenomenon, a condition where blood vessels in the extremities constrict and limit blood flow.
Raynaud’s is a relatively common condition, affecting 2%-5% of the general population. Though Raynaud’s can be an annoyance for some, it can also cause severe pain and can require medication.
These newly identified genes will hopefully lead to new therapeutic options, said Maik Pietzner, PhD, chair in health data modeling at Queen Mary University of London’s Precision Healthcare University Research Institute (PHURI) and group leader in the Computational Medicine Group at the Berlin Institute of Health at Charité – Universitätsmedizin Berlin, Germany.
Dr. Pietzner led the research along with Claudia Langenberg, MD, PhD, director of PHURI.
The study was published in Nature Communications.
Largest genomic study of Raynaud’s to date
The researchers looked through electronic medical records from the UK Biobank, a large-scale database that contains genetic and health information on half a million participants. They identified more than 5,100 individuals with Raynaud’s, of which 68% had primary Raynaud’s. These participants were compared with more than 439,000 controls who did not have Raynaud’s.
In a secondary analysis, the team also used health records from the Queen Mary University of London Genes & Health Study, which contains health information on individuals of South Asian ancestry.
The researchers identified two genes that are likely involved with Raynaud’s. The first, ADRA2A, encodes for the alpha-2A adrenergic receptor that can cause vasoconstriction of small blood vessels in response to stress hormones. Researchers have long suspected that this type of receptor could be involved with Raynaud’s, but there was debate over which receptor subtype was responsible.
“Our finding of alpha-2A receptors is quite interesting because the focus has always been on alpha-2C receptors,” said Dr. Pietzner. “It’s only a letter, but it’s a massive difference in terms of biology and physiology,” he said, and could be why therapies targeting 2C receptors have been ineffective.
The second strongest association was for the transcription factor IRX1. Less is known about this gene, but the data we do have suggest that it is involved with regulating the dilation of blood vessels, Dr. Pietzner noted.
“There might be balance between the ADRA2A finding being responsible for constriction and the IRX1 finding indirectly linked to the dilation of those vessels following constrictions. Having both may explain why these prolonged episodes of vasoconstriction lead to a loss of oxygen to the tissues,” so they turn white and then blue, he said.
Because the Biobank cohort was European-centric, Dr. Pietzner and colleagues also identified 400 cases of Raynaud’s in British individuals of Bangladeshi and Pakistani ancestry and were able to replicate the association between IRX1 and Raynaud’s. Data on ADRA2A were unavailable.
The genes identified are associated with primary Raynaud’s. Secondary Raynaud’s is a rarer type of the condition that occurs along with autoimmune disorders, such as scleroderma, and is generally more severe.
It’s long been suspected that Raynaud’s had some genetic component, because half of patients with Raynaud’s have another family member with the same condition, said Laura Hummers, MD, who codirects the John Hopkins Scleroderma Center in Baltimore. She was not involved with the study.
This is “the largest study of this kind that’s been done,” she said, and the first to show a potential mechanism behind this genetic association.
The main gene finding, ADRA2A, “points to a receptor on the cells that regulate the tone of these blood vessels,” she continued. “It suggests maybe there’s too many of these receptors or they’re overly sensitive; something about them is different that makes patients more susceptible to these cold triggers. Knowing that is potentially really important, because it could give you a direct way to intervene, if true.”
New therapeutic avenues
The first-line treatment for primary Raynaud’s is behavioral interventions, such as maintaining body and extremity warmth and avoiding certain vasoconstricting drugs, said Kimberly Lakin, MD, a rheumatologist at the Hospital for Special Surgery in New York, who not involved in the research. These drugs could include over-the-counter decongestants and certain medications for attention-deficit/hyperactivity disorder.
If these behavioral interventions are not enough, clinicians most commonly prescribe calcium channel blockers. These medications are vasodilators but can be a concern for people with normal or already low blood pressure, Dr. Lakin said. They can also cause symptoms such as headache, leg swelling, constipation, and other gastrointestinal symptoms.
Other medications, such as fluoxetine, may also be considered as a later-line therapy, “but the effectiveness is fairly limited in Raynaud’s,” she said. “Certainly, other medication options that would be helpful and driven by the mechanisms of Raynaud’s would add to our ability to help patients.”
As it turns out, one of the genes identified in the study, ADRA2A, “is actually one of the most commonly targeted genes by drugs,” said Dr. Pietzner. Because the findings suggest that ADRA2A is overexpressed in Raynaud, a selective inhibitor like the antidepressant mirtazapine could be a promising candidate to repurpose for treating Raynaud’s, he said.
Limitations to electronic medical record analyses
Both Dr. Hummers and Dr. Lakin noted that research using diagnostic codes from medical records to identify cases has some limitations. The study may have included patients misdiagnosed with Raynaud’s when perhaps they had another condition. Patients with milder Raynaud’s who have not sought medical attention for the condition would not be represented in the study, Dr. Lakin said.
The UK Biobank includes individuals of mostly European descent, so an analysis confirming these findings in a more diverse population would be helpful, she said.
However, both Dr. Lakin and Dr. Hummers agreed that the study contributes to the understanding of the mechanisms behind Raynaud’s. Although the two identified genes were tied to primary Raynaud’s, the study’s findings could potentially apply to secondary Raynaud’s as well, Dr. Hummers said.
“Anything we learn about primary Raynaud’s may have implication for Raynaud’s more broadly,” she noted.
Dr. Hummers and Dr. Lakin disclosed no relevant financial relationships. Dr. Pietzner has received partnership funding for the MRC Clinical Pharmacology Training Scheme (cofunded by MRC and Roche, UCB, Eli Lilly, and Novartis) and a PhD studentship jointly funded by the UK Engineering and Physical Sciences Research Council and AstraZeneca. Dr. Pietzner also has unrestricted educational grant support for the UK Pharmacogenetics and Stratified Medicine Network from Bristol-Myers Squibb.
A version of this article appeared on Medscape.com.
Researchers have identified two genes that may contribute to Raynaud’s phenomenon, a condition where blood vessels in the extremities constrict and limit blood flow.
Raynaud’s is a relatively common condition, affecting 2%-5% of the general population. Though Raynaud’s can be an annoyance for some, it can also cause severe pain and can require medication.
These newly identified genes will hopefully lead to new therapeutic options, said Maik Pietzner, PhD, chair in health data modeling at Queen Mary University of London’s Precision Healthcare University Research Institute (PHURI) and group leader in the Computational Medicine Group at the Berlin Institute of Health at Charité – Universitätsmedizin Berlin, Germany.
Dr. Pietzner led the research along with Claudia Langenberg, MD, PhD, director of PHURI.
The study was published in Nature Communications.
Largest genomic study of Raynaud’s to date
The researchers looked through electronic medical records from the UK Biobank, a large-scale database that contains genetic and health information on half a million participants. They identified more than 5,100 individuals with Raynaud’s, of which 68% had primary Raynaud’s. These participants were compared with more than 439,000 controls who did not have Raynaud’s.
In a secondary analysis, the team also used health records from the Queen Mary University of London Genes & Health Study, which contains health information on individuals of South Asian ancestry.
The researchers identified two genes that are likely involved with Raynaud’s. The first, ADRA2A, encodes for the alpha-2A adrenergic receptor that can cause vasoconstriction of small blood vessels in response to stress hormones. Researchers have long suspected that this type of receptor could be involved with Raynaud’s, but there was debate over which receptor subtype was responsible.
“Our finding of alpha-2A receptors is quite interesting because the focus has always been on alpha-2C receptors,” said Dr. Pietzner. “It’s only a letter, but it’s a massive difference in terms of biology and physiology,” he said, and could be why therapies targeting 2C receptors have been ineffective.
The second strongest association was for the transcription factor IRX1. Less is known about this gene, but the data we do have suggest that it is involved with regulating the dilation of blood vessels, Dr. Pietzner noted.
“There might be balance between the ADRA2A finding being responsible for constriction and the IRX1 finding indirectly linked to the dilation of those vessels following constrictions. Having both may explain why these prolonged episodes of vasoconstriction lead to a loss of oxygen to the tissues,” so they turn white and then blue, he said.
Because the Biobank cohort was European-centric, Dr. Pietzner and colleagues also identified 400 cases of Raynaud’s in British individuals of Bangladeshi and Pakistani ancestry and were able to replicate the association between IRX1 and Raynaud’s. Data on ADRA2A were unavailable.
The genes identified are associated with primary Raynaud’s. Secondary Raynaud’s is a rarer type of the condition that occurs along with autoimmune disorders, such as scleroderma, and is generally more severe.
It’s long been suspected that Raynaud’s had some genetic component, because half of patients with Raynaud’s have another family member with the same condition, said Laura Hummers, MD, who codirects the John Hopkins Scleroderma Center in Baltimore. She was not involved with the study.
This is “the largest study of this kind that’s been done,” she said, and the first to show a potential mechanism behind this genetic association.
The main gene finding, ADRA2A, “points to a receptor on the cells that regulate the tone of these blood vessels,” she continued. “It suggests maybe there’s too many of these receptors or they’re overly sensitive; something about them is different that makes patients more susceptible to these cold triggers. Knowing that is potentially really important, because it could give you a direct way to intervene, if true.”
New therapeutic avenues
The first-line treatment for primary Raynaud’s is behavioral interventions, such as maintaining body and extremity warmth and avoiding certain vasoconstricting drugs, said Kimberly Lakin, MD, a rheumatologist at the Hospital for Special Surgery in New York, who not involved in the research. These drugs could include over-the-counter decongestants and certain medications for attention-deficit/hyperactivity disorder.
If these behavioral interventions are not enough, clinicians most commonly prescribe calcium channel blockers. These medications are vasodilators but can be a concern for people with normal or already low blood pressure, Dr. Lakin said. They can also cause symptoms such as headache, leg swelling, constipation, and other gastrointestinal symptoms.
Other medications, such as fluoxetine, may also be considered as a later-line therapy, “but the effectiveness is fairly limited in Raynaud’s,” she said. “Certainly, other medication options that would be helpful and driven by the mechanisms of Raynaud’s would add to our ability to help patients.”
As it turns out, one of the genes identified in the study, ADRA2A, “is actually one of the most commonly targeted genes by drugs,” said Dr. Pietzner. Because the findings suggest that ADRA2A is overexpressed in Raynaud, a selective inhibitor like the antidepressant mirtazapine could be a promising candidate to repurpose for treating Raynaud’s, he said.
Limitations to electronic medical record analyses
Both Dr. Hummers and Dr. Lakin noted that research using diagnostic codes from medical records to identify cases has some limitations. The study may have included patients misdiagnosed with Raynaud’s when perhaps they had another condition. Patients with milder Raynaud’s who have not sought medical attention for the condition would not be represented in the study, Dr. Lakin said.
The UK Biobank includes individuals of mostly European descent, so an analysis confirming these findings in a more diverse population would be helpful, she said.
However, both Dr. Lakin and Dr. Hummers agreed that the study contributes to the understanding of the mechanisms behind Raynaud’s. Although the two identified genes were tied to primary Raynaud’s, the study’s findings could potentially apply to secondary Raynaud’s as well, Dr. Hummers said.
“Anything we learn about primary Raynaud’s may have implication for Raynaud’s more broadly,” she noted.
Dr. Hummers and Dr. Lakin disclosed no relevant financial relationships. Dr. Pietzner has received partnership funding for the MRC Clinical Pharmacology Training Scheme (cofunded by MRC and Roche, UCB, Eli Lilly, and Novartis) and a PhD studentship jointly funded by the UK Engineering and Physical Sciences Research Council and AstraZeneca. Dr. Pietzner also has unrestricted educational grant support for the UK Pharmacogenetics and Stratified Medicine Network from Bristol-Myers Squibb.
A version of this article appeared on Medscape.com.
Researchers have identified two genes that may contribute to Raynaud’s phenomenon, a condition where blood vessels in the extremities constrict and limit blood flow.
Raynaud’s is a relatively common condition, affecting 2%-5% of the general population. Though Raynaud’s can be an annoyance for some, it can also cause severe pain and can require medication.
These newly identified genes will hopefully lead to new therapeutic options, said Maik Pietzner, PhD, chair in health data modeling at Queen Mary University of London’s Precision Healthcare University Research Institute (PHURI) and group leader in the Computational Medicine Group at the Berlin Institute of Health at Charité – Universitätsmedizin Berlin, Germany.
Dr. Pietzner led the research along with Claudia Langenberg, MD, PhD, director of PHURI.
The study was published in Nature Communications.
Largest genomic study of Raynaud’s to date
The researchers looked through electronic medical records from the UK Biobank, a large-scale database that contains genetic and health information on half a million participants. They identified more than 5,100 individuals with Raynaud’s, of which 68% had primary Raynaud’s. These participants were compared with more than 439,000 controls who did not have Raynaud’s.
In a secondary analysis, the team also used health records from the Queen Mary University of London Genes & Health Study, which contains health information on individuals of South Asian ancestry.
The researchers identified two genes that are likely involved with Raynaud’s. The first, ADRA2A, encodes for the alpha-2A adrenergic receptor that can cause vasoconstriction of small blood vessels in response to stress hormones. Researchers have long suspected that this type of receptor could be involved with Raynaud’s, but there was debate over which receptor subtype was responsible.
“Our finding of alpha-2A receptors is quite interesting because the focus has always been on alpha-2C receptors,” said Dr. Pietzner. “It’s only a letter, but it’s a massive difference in terms of biology and physiology,” he said, and could be why therapies targeting 2C receptors have been ineffective.
The second strongest association was for the transcription factor IRX1. Less is known about this gene, but the data we do have suggest that it is involved with regulating the dilation of blood vessels, Dr. Pietzner noted.
“There might be balance between the ADRA2A finding being responsible for constriction and the IRX1 finding indirectly linked to the dilation of those vessels following constrictions. Having both may explain why these prolonged episodes of vasoconstriction lead to a loss of oxygen to the tissues,” so they turn white and then blue, he said.
Because the Biobank cohort was European-centric, Dr. Pietzner and colleagues also identified 400 cases of Raynaud’s in British individuals of Bangladeshi and Pakistani ancestry and were able to replicate the association between IRX1 and Raynaud’s. Data on ADRA2A were unavailable.
The genes identified are associated with primary Raynaud’s. Secondary Raynaud’s is a rarer type of the condition that occurs along with autoimmune disorders, such as scleroderma, and is generally more severe.
It’s long been suspected that Raynaud’s had some genetic component, because half of patients with Raynaud’s have another family member with the same condition, said Laura Hummers, MD, who codirects the John Hopkins Scleroderma Center in Baltimore. She was not involved with the study.
This is “the largest study of this kind that’s been done,” she said, and the first to show a potential mechanism behind this genetic association.
The main gene finding, ADRA2A, “points to a receptor on the cells that regulate the tone of these blood vessels,” she continued. “It suggests maybe there’s too many of these receptors or they’re overly sensitive; something about them is different that makes patients more susceptible to these cold triggers. Knowing that is potentially really important, because it could give you a direct way to intervene, if true.”
New therapeutic avenues
The first-line treatment for primary Raynaud’s is behavioral interventions, such as maintaining body and extremity warmth and avoiding certain vasoconstricting drugs, said Kimberly Lakin, MD, a rheumatologist at the Hospital for Special Surgery in New York, who not involved in the research. These drugs could include over-the-counter decongestants and certain medications for attention-deficit/hyperactivity disorder.
If these behavioral interventions are not enough, clinicians most commonly prescribe calcium channel blockers. These medications are vasodilators but can be a concern for people with normal or already low blood pressure, Dr. Lakin said. They can also cause symptoms such as headache, leg swelling, constipation, and other gastrointestinal symptoms.
Other medications, such as fluoxetine, may also be considered as a later-line therapy, “but the effectiveness is fairly limited in Raynaud’s,” she said. “Certainly, other medication options that would be helpful and driven by the mechanisms of Raynaud’s would add to our ability to help patients.”
As it turns out, one of the genes identified in the study, ADRA2A, “is actually one of the most commonly targeted genes by drugs,” said Dr. Pietzner. Because the findings suggest that ADRA2A is overexpressed in Raynaud, a selective inhibitor like the antidepressant mirtazapine could be a promising candidate to repurpose for treating Raynaud’s, he said.
Limitations to electronic medical record analyses
Both Dr. Hummers and Dr. Lakin noted that research using diagnostic codes from medical records to identify cases has some limitations. The study may have included patients misdiagnosed with Raynaud’s when perhaps they had another condition. Patients with milder Raynaud’s who have not sought medical attention for the condition would not be represented in the study, Dr. Lakin said.
The UK Biobank includes individuals of mostly European descent, so an analysis confirming these findings in a more diverse population would be helpful, she said.
However, both Dr. Lakin and Dr. Hummers agreed that the study contributes to the understanding of the mechanisms behind Raynaud’s. Although the two identified genes were tied to primary Raynaud’s, the study’s findings could potentially apply to secondary Raynaud’s as well, Dr. Hummers said.
“Anything we learn about primary Raynaud’s may have implication for Raynaud’s more broadly,” she noted.
Dr. Hummers and Dr. Lakin disclosed no relevant financial relationships. Dr. Pietzner has received partnership funding for the MRC Clinical Pharmacology Training Scheme (cofunded by MRC and Roche, UCB, Eli Lilly, and Novartis) and a PhD studentship jointly funded by the UK Engineering and Physical Sciences Research Council and AstraZeneca. Dr. Pietzner also has unrestricted educational grant support for the UK Pharmacogenetics and Stratified Medicine Network from Bristol-Myers Squibb.
A version of this article appeared on Medscape.com.
FROM NATURE COMMUNICATIONS
Employed physicians: A survival guide
The strike by health care workers at Kaiser Permanente may not involve physicians (yet). But as more doctors in the United States are finding themselves working as salaried employees, physicians can – and probably will – become a powerful force for change in a health care system that has shown itself to be increasingly hostile to employee concerns over issues involving patient care, wages and benefits, safety, and well-being.
Salaried employment has its challenges. Physician-employees may have less autonomy and voice in decision-making that affects patients. They may splinter into fragmented work groups; feel isolated; and have different imperatives based on who they are, what they want, and where they work. They may feel more removed from their patients and struggle to build strong relationships, with their employers in the way.
Yet important opportunities exist for doctors when embracing their employee side. Examples of these interests include adequate compensation, wellness, job security, patient and worker safety, health care quality, reasonable workloads and schedules, and fair treatment by employers, including the need to exhibit a strong collective voice in organizational decision-making.
Some believe that physician-employees must be unionized to maximize their rights and power as employees. Many expect physician unionization to take hold more fully over time. Medical residents, the doctors of tomorrow, are already considering unionization in greater numbers. Some are also doing it in the same employment setting alongside other health professionals, such as nurses.
Having studied doctors and their employment situations for years, I am convinced that whether through unionization or another approach, physicians must also change how they think about control; train and learn alongside other health care workers who share similar interests; and elevate at an early career stage their knowledge of the business side of health care.
Adopt a more pragmatic definition of autonomy
Doctors must embrace an updated definition of autonomy – one that matches their status as highly paid labor.
When I have spoken to physicians in my research about what autonomy means to them, many seem unable to reconceptualize it from a vague and absolute form of their profession’s strategic control over their economic fates and technical skills toward an individualized control that is situation-specific, one centered on winning the daily fights about workplace bread-and-butter issues such as those mentioned above.
But a more pragmatic definition of autonomy could get doctors focused on influencing important issues of the patient-care day and enhance their negotiating power with employers. It would allow physicians to break out of what often seems a paralysis of inaction – waiting for employers, insurers, or the government to reinstate the profession’s idealized version of control by handing it back the keys to the health care system through major regulatory, structural, and reimbursement-related changes. This fantasy is unlikely to become reality.
Physician-employees I’ve talked to over the years understand their everyday challenges. But when it comes to engaging in localized and sustained action to overcome them, they often perform less well, leading to feelings of helplessness and burnout. Valuing tactical control over their jobs and work setting will yield smaller but more impactful wins as employees intent on making their everyday work lives better.
Train alongside other health care professionals
Physicians must accept that how they are trained no longer prepares them for the employee world into which most are dropped. For instance, unless doctors are trained collaboratively alongside other health care professionals – such as nurses – they are less likely to identify closely with these colleagues once in practice. There is strength in numbers, so this mutual identification empowers both groups of employees. Yet, medical education remains largely the same: training young medical students in isolation for the first couple of years, then placing them into clerkships and residencies where true interprofessional care opportunities remain stunted and secondary to the “physician as captain of the team” mantra.
Unfortunately, the “hidden curriculum” of medicine helps convince medical students and residents early in their careers that they are the unquestioned leaders in patient care settings. This hierarchy encourages some doctors to keep their psychological distance from other members of the health care team and to resist sharing power, concerns, or insights with less skilled health care workers. This socialization harms the ability of physicians to act in a unified fashion alongside these other workers. Having physicians learn and train alongside other health professionals yields positive benefits for collective advocacy, including a shared sense of purpose, positive views on collaboration with others in the health setting, and greater development of bonds with nonphysician coworkers.
Integrate business with medical training in real time
Medical students and residents generally lack exposure to the everyday business realities of the U.S. health care system. This gap hinders their ability to understand the employee world and push for the types of changes and work conditions that benefit all health care workers. Formal business and management training should be a required part of every U.S. medical school and residency curriculum from day one. If you see it at all in medical schools now, it is mostly by accident, or given separate treatment in the form of standalone MBA or MPH degrees that rarely integrate organically and in real time with actual medical training. Not every doctor needs an MBA or MPH degree. However, all of them require a stronger contextual understanding of how the medicine they wish to practice is shaped by the economic and fiscal circumstances surrounding it – circumstances they do not control.
This is another reason why young doctors are unhappy and burned out. They cannot push for specific changes or properly critique the pros and cons of how their work is structured because they have not been made aware, in real time as they learn clinical practice, how their jobs are shaped by realities such as insurance coverage and reimbursement, the fragmentation of the care delivery system, their employer’s financial health , and the socioeconomic circumstances of their patients. They aren’t given the methods and tools related to process and quality improvement, budgeting, negotiation, risk management, leadership, and talent management that might help them navigate these undermining forces. They also get little advance exposure in their training to important workplace “soft” skills in such areas as how to work in teams, networking, communication and listening, empathy, and problem-solving – all necessary foci for bringing them closer to other health care workers and advocating alongside them effectively with health care employers.
Now is the time for physicians to embrace their identity as employees. Doing so is in their own best interest as professionals. It will help others in the health care workforce as well as patients. Moreover, it provides a needed counterbalance to the powerful corporate ethos now ascendant in U.S. health care.
Timothy Hoff, PhD, is a professor of management and healthcare systems at Northeastern University, Boston, and an associate fellow at the University of Oxford, England. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
The strike by health care workers at Kaiser Permanente may not involve physicians (yet). But as more doctors in the United States are finding themselves working as salaried employees, physicians can – and probably will – become a powerful force for change in a health care system that has shown itself to be increasingly hostile to employee concerns over issues involving patient care, wages and benefits, safety, and well-being.
Salaried employment has its challenges. Physician-employees may have less autonomy and voice in decision-making that affects patients. They may splinter into fragmented work groups; feel isolated; and have different imperatives based on who they are, what they want, and where they work. They may feel more removed from their patients and struggle to build strong relationships, with their employers in the way.
Yet important opportunities exist for doctors when embracing their employee side. Examples of these interests include adequate compensation, wellness, job security, patient and worker safety, health care quality, reasonable workloads and schedules, and fair treatment by employers, including the need to exhibit a strong collective voice in organizational decision-making.
Some believe that physician-employees must be unionized to maximize their rights and power as employees. Many expect physician unionization to take hold more fully over time. Medical residents, the doctors of tomorrow, are already considering unionization in greater numbers. Some are also doing it in the same employment setting alongside other health professionals, such as nurses.
Having studied doctors and their employment situations for years, I am convinced that whether through unionization or another approach, physicians must also change how they think about control; train and learn alongside other health care workers who share similar interests; and elevate at an early career stage their knowledge of the business side of health care.
Adopt a more pragmatic definition of autonomy
Doctors must embrace an updated definition of autonomy – one that matches their status as highly paid labor.
When I have spoken to physicians in my research about what autonomy means to them, many seem unable to reconceptualize it from a vague and absolute form of their profession’s strategic control over their economic fates and technical skills toward an individualized control that is situation-specific, one centered on winning the daily fights about workplace bread-and-butter issues such as those mentioned above.
But a more pragmatic definition of autonomy could get doctors focused on influencing important issues of the patient-care day and enhance their negotiating power with employers. It would allow physicians to break out of what often seems a paralysis of inaction – waiting for employers, insurers, or the government to reinstate the profession’s idealized version of control by handing it back the keys to the health care system through major regulatory, structural, and reimbursement-related changes. This fantasy is unlikely to become reality.
Physician-employees I’ve talked to over the years understand their everyday challenges. But when it comes to engaging in localized and sustained action to overcome them, they often perform less well, leading to feelings of helplessness and burnout. Valuing tactical control over their jobs and work setting will yield smaller but more impactful wins as employees intent on making their everyday work lives better.
Train alongside other health care professionals
Physicians must accept that how they are trained no longer prepares them for the employee world into which most are dropped. For instance, unless doctors are trained collaboratively alongside other health care professionals – such as nurses – they are less likely to identify closely with these colleagues once in practice. There is strength in numbers, so this mutual identification empowers both groups of employees. Yet, medical education remains largely the same: training young medical students in isolation for the first couple of years, then placing them into clerkships and residencies where true interprofessional care opportunities remain stunted and secondary to the “physician as captain of the team” mantra.
Unfortunately, the “hidden curriculum” of medicine helps convince medical students and residents early in their careers that they are the unquestioned leaders in patient care settings. This hierarchy encourages some doctors to keep their psychological distance from other members of the health care team and to resist sharing power, concerns, or insights with less skilled health care workers. This socialization harms the ability of physicians to act in a unified fashion alongside these other workers. Having physicians learn and train alongside other health professionals yields positive benefits for collective advocacy, including a shared sense of purpose, positive views on collaboration with others in the health setting, and greater development of bonds with nonphysician coworkers.
Integrate business with medical training in real time
Medical students and residents generally lack exposure to the everyday business realities of the U.S. health care system. This gap hinders their ability to understand the employee world and push for the types of changes and work conditions that benefit all health care workers. Formal business and management training should be a required part of every U.S. medical school and residency curriculum from day one. If you see it at all in medical schools now, it is mostly by accident, or given separate treatment in the form of standalone MBA or MPH degrees that rarely integrate organically and in real time with actual medical training. Not every doctor needs an MBA or MPH degree. However, all of them require a stronger contextual understanding of how the medicine they wish to practice is shaped by the economic and fiscal circumstances surrounding it – circumstances they do not control.
This is another reason why young doctors are unhappy and burned out. They cannot push for specific changes or properly critique the pros and cons of how their work is structured because they have not been made aware, in real time as they learn clinical practice, how their jobs are shaped by realities such as insurance coverage and reimbursement, the fragmentation of the care delivery system, their employer’s financial health , and the socioeconomic circumstances of their patients. They aren’t given the methods and tools related to process and quality improvement, budgeting, negotiation, risk management, leadership, and talent management that might help them navigate these undermining forces. They also get little advance exposure in their training to important workplace “soft” skills in such areas as how to work in teams, networking, communication and listening, empathy, and problem-solving – all necessary foci for bringing them closer to other health care workers and advocating alongside them effectively with health care employers.
Now is the time for physicians to embrace their identity as employees. Doing so is in their own best interest as professionals. It will help others in the health care workforce as well as patients. Moreover, it provides a needed counterbalance to the powerful corporate ethos now ascendant in U.S. health care.
Timothy Hoff, PhD, is a professor of management and healthcare systems at Northeastern University, Boston, and an associate fellow at the University of Oxford, England. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
The strike by health care workers at Kaiser Permanente may not involve physicians (yet). But as more doctors in the United States are finding themselves working as salaried employees, physicians can – and probably will – become a powerful force for change in a health care system that has shown itself to be increasingly hostile to employee concerns over issues involving patient care, wages and benefits, safety, and well-being.
Salaried employment has its challenges. Physician-employees may have less autonomy and voice in decision-making that affects patients. They may splinter into fragmented work groups; feel isolated; and have different imperatives based on who they are, what they want, and where they work. They may feel more removed from their patients and struggle to build strong relationships, with their employers in the way.
Yet important opportunities exist for doctors when embracing their employee side. Examples of these interests include adequate compensation, wellness, job security, patient and worker safety, health care quality, reasonable workloads and schedules, and fair treatment by employers, including the need to exhibit a strong collective voice in organizational decision-making.
Some believe that physician-employees must be unionized to maximize their rights and power as employees. Many expect physician unionization to take hold more fully over time. Medical residents, the doctors of tomorrow, are already considering unionization in greater numbers. Some are also doing it in the same employment setting alongside other health professionals, such as nurses.
Having studied doctors and their employment situations for years, I am convinced that whether through unionization or another approach, physicians must also change how they think about control; train and learn alongside other health care workers who share similar interests; and elevate at an early career stage their knowledge of the business side of health care.
Adopt a more pragmatic definition of autonomy
Doctors must embrace an updated definition of autonomy – one that matches their status as highly paid labor.
When I have spoken to physicians in my research about what autonomy means to them, many seem unable to reconceptualize it from a vague and absolute form of their profession’s strategic control over their economic fates and technical skills toward an individualized control that is situation-specific, one centered on winning the daily fights about workplace bread-and-butter issues such as those mentioned above.
But a more pragmatic definition of autonomy could get doctors focused on influencing important issues of the patient-care day and enhance their negotiating power with employers. It would allow physicians to break out of what often seems a paralysis of inaction – waiting for employers, insurers, or the government to reinstate the profession’s idealized version of control by handing it back the keys to the health care system through major regulatory, structural, and reimbursement-related changes. This fantasy is unlikely to become reality.
Physician-employees I’ve talked to over the years understand their everyday challenges. But when it comes to engaging in localized and sustained action to overcome them, they often perform less well, leading to feelings of helplessness and burnout. Valuing tactical control over their jobs and work setting will yield smaller but more impactful wins as employees intent on making their everyday work lives better.
Train alongside other health care professionals
Physicians must accept that how they are trained no longer prepares them for the employee world into which most are dropped. For instance, unless doctors are trained collaboratively alongside other health care professionals – such as nurses – they are less likely to identify closely with these colleagues once in practice. There is strength in numbers, so this mutual identification empowers both groups of employees. Yet, medical education remains largely the same: training young medical students in isolation for the first couple of years, then placing them into clerkships and residencies where true interprofessional care opportunities remain stunted and secondary to the “physician as captain of the team” mantra.
Unfortunately, the “hidden curriculum” of medicine helps convince medical students and residents early in their careers that they are the unquestioned leaders in patient care settings. This hierarchy encourages some doctors to keep their psychological distance from other members of the health care team and to resist sharing power, concerns, or insights with less skilled health care workers. This socialization harms the ability of physicians to act in a unified fashion alongside these other workers. Having physicians learn and train alongside other health professionals yields positive benefits for collective advocacy, including a shared sense of purpose, positive views on collaboration with others in the health setting, and greater development of bonds with nonphysician coworkers.
Integrate business with medical training in real time
Medical students and residents generally lack exposure to the everyday business realities of the U.S. health care system. This gap hinders their ability to understand the employee world and push for the types of changes and work conditions that benefit all health care workers. Formal business and management training should be a required part of every U.S. medical school and residency curriculum from day one. If you see it at all in medical schools now, it is mostly by accident, or given separate treatment in the form of standalone MBA or MPH degrees that rarely integrate organically and in real time with actual medical training. Not every doctor needs an MBA or MPH degree. However, all of them require a stronger contextual understanding of how the medicine they wish to practice is shaped by the economic and fiscal circumstances surrounding it – circumstances they do not control.
This is another reason why young doctors are unhappy and burned out. They cannot push for specific changes or properly critique the pros and cons of how their work is structured because they have not been made aware, in real time as they learn clinical practice, how their jobs are shaped by realities such as insurance coverage and reimbursement, the fragmentation of the care delivery system, their employer’s financial health , and the socioeconomic circumstances of their patients. They aren’t given the methods and tools related to process and quality improvement, budgeting, negotiation, risk management, leadership, and talent management that might help them navigate these undermining forces. They also get little advance exposure in their training to important workplace “soft” skills in such areas as how to work in teams, networking, communication and listening, empathy, and problem-solving – all necessary foci for bringing them closer to other health care workers and advocating alongside them effectively with health care employers.
Now is the time for physicians to embrace their identity as employees. Doing so is in their own best interest as professionals. It will help others in the health care workforce as well as patients. Moreover, it provides a needed counterbalance to the powerful corporate ethos now ascendant in U.S. health care.
Timothy Hoff, PhD, is a professor of management and healthcare systems at Northeastern University, Boston, and an associate fellow at the University of Oxford, England. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
FDA approves bimekizumab for moderate to severe plaque psoriasis in adults
, the manufacturer announced in a press release.
The indication is for adults who are candidates for systemic therapy or phototherapy.
With this approval, bimekizumab becomes the only interleukin (IL)-17A and IL-17F inhibitor approved for the treatment of these patients. Psoriasis affects more than 7.5 million U.S. adults, according to the National Psoriasis Foundation.
“We have been eagerly awaiting bimekizumab,” Mark Lebwohl, MD, bimekizumab investigator and dean for clinical therapeutics at the Icahn School of Medicine at Mount Sinai, New York City, said in the press release.
Dr. Lebwohl states that bimekizumab “achieved superior levels of skin clearance at week 16 compared to placebo and three existing biologics for psoriasis, with responses being rapid and lasting up to a year. Long-term data have also shown that the majority of patients maintained high levels of clinical response through three years.”
The most common adverse reactions (occurring in at least 1% of patients) are upper respiratory infections, oral candidiasis, headache, tinea infections, gastroenteritis, herpes simplex infections, acne, folliculitis, other Candida infections, fatigue, and injection site reactions, according to the company, UCB.
Available in about 1 month in U.S.
Bimekizumab can be administered by a health care provider or it can be self-injected by a patient after training. It is available as a single-dose prefilled autoinjector and a single-dose prefilled syringe and will be available in the United States in about 1 month.
The recommended dosage of bimekizumab for patients with psoriasis is 320 mg (two subcutaneous injections of 160 mg each) at baseline, then on weeks 4, 8, 12, and 16, then every 8 weeks thereafter. For patients weighing at least 120 kg (about 265 lb), a dosage of 320 mg every 4 weeks after week 16 may be considered, the company states.
Three phase 3 trials
Approval was based on three phase 3 multicenter, randomized, placebo and/or active comparator-controlled trials: bimekizumab versus placebo and ustekinumab (BE VIVID); versus placebo (BE READY); and versus adalimumab (BE SURE).
“All studies met their co-primary endpoints and all ranked secondary endpoints,” the company reports. Secondary endpoints included the Psoriasis Area and Severity Index (PASI) 75 at week 4 and PASI 100 (complete skin clearance) at week 16.
Highlights from the trials include the following results, according to UCB:
- Clear or almost clear skin: More than 8 out of 10 patients achieved a 90% or greater reduction from baseline in the PASI 90 and an Investigator’s Global Assessment score of 0/1 at week 16.
- Complete skin clearance: About 60% of patients achieved PASI 100 at week 16.
- Time to response: More than 70% of patients achieved PASI 75 at week 4 following one 320-mg dose.
Safety information
The safety information includes the statement that bimekizumab may increase the risk for suicidal ideation and behavior, though a causal association has not been established. Prescribers should advise patients, caregivers, and families “to monitor for emergence or worsening of depression, suicidal ideation, or other mood changes,” according to the prescribing information.
Bimekizumab is being studied for other conditions, including hidradenitis suppurativa. In the European Union, it was approved for the treatment of psoriasis in 2021 and for the treatment of psoriatic arthritis and ankylosing spondylitis in June 2023.
Dr. Lebwohl is an investigator for UCB. He has not accepted any consulting payments from UCB.
A version of this article first appeared on Medscape.com.
, the manufacturer announced in a press release.
The indication is for adults who are candidates for systemic therapy or phototherapy.
With this approval, bimekizumab becomes the only interleukin (IL)-17A and IL-17F inhibitor approved for the treatment of these patients. Psoriasis affects more than 7.5 million U.S. adults, according to the National Psoriasis Foundation.
“We have been eagerly awaiting bimekizumab,” Mark Lebwohl, MD, bimekizumab investigator and dean for clinical therapeutics at the Icahn School of Medicine at Mount Sinai, New York City, said in the press release.
Dr. Lebwohl states that bimekizumab “achieved superior levels of skin clearance at week 16 compared to placebo and three existing biologics for psoriasis, with responses being rapid and lasting up to a year. Long-term data have also shown that the majority of patients maintained high levels of clinical response through three years.”
The most common adverse reactions (occurring in at least 1% of patients) are upper respiratory infections, oral candidiasis, headache, tinea infections, gastroenteritis, herpes simplex infections, acne, folliculitis, other Candida infections, fatigue, and injection site reactions, according to the company, UCB.
Available in about 1 month in U.S.
Bimekizumab can be administered by a health care provider or it can be self-injected by a patient after training. It is available as a single-dose prefilled autoinjector and a single-dose prefilled syringe and will be available in the United States in about 1 month.
The recommended dosage of bimekizumab for patients with psoriasis is 320 mg (two subcutaneous injections of 160 mg each) at baseline, then on weeks 4, 8, 12, and 16, then every 8 weeks thereafter. For patients weighing at least 120 kg (about 265 lb), a dosage of 320 mg every 4 weeks after week 16 may be considered, the company states.
Three phase 3 trials
Approval was based on three phase 3 multicenter, randomized, placebo and/or active comparator-controlled trials: bimekizumab versus placebo and ustekinumab (BE VIVID); versus placebo (BE READY); and versus adalimumab (BE SURE).
“All studies met their co-primary endpoints and all ranked secondary endpoints,” the company reports. Secondary endpoints included the Psoriasis Area and Severity Index (PASI) 75 at week 4 and PASI 100 (complete skin clearance) at week 16.
Highlights from the trials include the following results, according to UCB:
- Clear or almost clear skin: More than 8 out of 10 patients achieved a 90% or greater reduction from baseline in the PASI 90 and an Investigator’s Global Assessment score of 0/1 at week 16.
- Complete skin clearance: About 60% of patients achieved PASI 100 at week 16.
- Time to response: More than 70% of patients achieved PASI 75 at week 4 following one 320-mg dose.
Safety information
The safety information includes the statement that bimekizumab may increase the risk for suicidal ideation and behavior, though a causal association has not been established. Prescribers should advise patients, caregivers, and families “to monitor for emergence or worsening of depression, suicidal ideation, or other mood changes,” according to the prescribing information.
Bimekizumab is being studied for other conditions, including hidradenitis suppurativa. In the European Union, it was approved for the treatment of psoriasis in 2021 and for the treatment of psoriatic arthritis and ankylosing spondylitis in June 2023.
Dr. Lebwohl is an investigator for UCB. He has not accepted any consulting payments from UCB.
A version of this article first appeared on Medscape.com.
, the manufacturer announced in a press release.
The indication is for adults who are candidates for systemic therapy or phototherapy.
With this approval, bimekizumab becomes the only interleukin (IL)-17A and IL-17F inhibitor approved for the treatment of these patients. Psoriasis affects more than 7.5 million U.S. adults, according to the National Psoriasis Foundation.
“We have been eagerly awaiting bimekizumab,” Mark Lebwohl, MD, bimekizumab investigator and dean for clinical therapeutics at the Icahn School of Medicine at Mount Sinai, New York City, said in the press release.
Dr. Lebwohl states that bimekizumab “achieved superior levels of skin clearance at week 16 compared to placebo and three existing biologics for psoriasis, with responses being rapid and lasting up to a year. Long-term data have also shown that the majority of patients maintained high levels of clinical response through three years.”
The most common adverse reactions (occurring in at least 1% of patients) are upper respiratory infections, oral candidiasis, headache, tinea infections, gastroenteritis, herpes simplex infections, acne, folliculitis, other Candida infections, fatigue, and injection site reactions, according to the company, UCB.
Available in about 1 month in U.S.
Bimekizumab can be administered by a health care provider or it can be self-injected by a patient after training. It is available as a single-dose prefilled autoinjector and a single-dose prefilled syringe and will be available in the United States in about 1 month.
The recommended dosage of bimekizumab for patients with psoriasis is 320 mg (two subcutaneous injections of 160 mg each) at baseline, then on weeks 4, 8, 12, and 16, then every 8 weeks thereafter. For patients weighing at least 120 kg (about 265 lb), a dosage of 320 mg every 4 weeks after week 16 may be considered, the company states.
Three phase 3 trials
Approval was based on three phase 3 multicenter, randomized, placebo and/or active comparator-controlled trials: bimekizumab versus placebo and ustekinumab (BE VIVID); versus placebo (BE READY); and versus adalimumab (BE SURE).
“All studies met their co-primary endpoints and all ranked secondary endpoints,” the company reports. Secondary endpoints included the Psoriasis Area and Severity Index (PASI) 75 at week 4 and PASI 100 (complete skin clearance) at week 16.
Highlights from the trials include the following results, according to UCB:
- Clear or almost clear skin: More than 8 out of 10 patients achieved a 90% or greater reduction from baseline in the PASI 90 and an Investigator’s Global Assessment score of 0/1 at week 16.
- Complete skin clearance: About 60% of patients achieved PASI 100 at week 16.
- Time to response: More than 70% of patients achieved PASI 75 at week 4 following one 320-mg dose.
Safety information
The safety information includes the statement that bimekizumab may increase the risk for suicidal ideation and behavior, though a causal association has not been established. Prescribers should advise patients, caregivers, and families “to monitor for emergence or worsening of depression, suicidal ideation, or other mood changes,” according to the prescribing information.
Bimekizumab is being studied for other conditions, including hidradenitis suppurativa. In the European Union, it was approved for the treatment of psoriasis in 2021 and for the treatment of psoriatic arthritis and ankylosing spondylitis in June 2023.
Dr. Lebwohl is an investigator for UCB. He has not accepted any consulting payments from UCB.
A version of this article first appeared on Medscape.com.
Once-weekly topical therapy shows promise for moderate to severe acne
TOPLINE:
METHODOLOGY:
- Poor patient compliance with topical acne therapies is a common clinical challenge.
- In a 12-week, randomized, controlled, phase 2b trial of 181 patients 12 years of age and older, researchers investigated the safety, tolerability, and efficacy of DMT310, a powdered mixture of Spongilla lacustris for treating moderate to severe acne. (In vitro studies have found that components of S. lacustris, a freshwater sponge, have effects that include antimicrobial activity against Cutibacterium acnes and anti-inflammatory activity in human keratinocytes).
- The study’s primary efficacy endpoint was the absolute change in inflammatory lesion count from baseline to week 12.
- Endpoint success was defined as an Investigator Global Assessment (IGA) score of 0 or 1 and at least a two-grade improvement from baseline at week 12.
TAKEAWAY:
- Of the 181 patients, 91 received DMT310 (applied once a week to the face and washed off after 10-15 minutes), and 90 received placebo.
- Patients in the DMT310 arm showed a significantly greater mean reduction in the number of inflammatory lesions at week 12, compared with those in the placebo arm (–15.64 vs. –10.84, respectively; P < .001).
- Similarly, patients in the DMT310 arm showed a significantly greater mean reduction in the number of noninflammatory lesions at week 12, compared with those in the placebo arm (–18.26 vs. –12.41, respectively; P < .001).
- At week 12, endpoint success based on IGA scores also significantly favored patients in the DMT310 arm, compared with those in the placebo arm (44.40% vs. 17.78%; P < .001).
IN PRACTICE:
This study is too preliminary to have practice application. The researchers concluded that the findings “support further study of DMT310 in larger, confirmatory phase 3 trials.”
SOURCE:
Lawrence F. Eichenfield, MD, professor of dermatology and pediatrics at the University of California, San Diego, led the research. The study was published online June 7 in the Journal of the American Academy of Dermatology.
LIMITATIONS:
The analysis did not include an active comparator group and it enrolled a limited number of Asian patients.
DISCLOSURES:
Dr. Eichenfield disclosed that he is a consultant to Dermata, which is developing DMT310, as were three other authors of the study. One author is a company employee. The remaining authors disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Poor patient compliance with topical acne therapies is a common clinical challenge.
- In a 12-week, randomized, controlled, phase 2b trial of 181 patients 12 years of age and older, researchers investigated the safety, tolerability, and efficacy of DMT310, a powdered mixture of Spongilla lacustris for treating moderate to severe acne. (In vitro studies have found that components of S. lacustris, a freshwater sponge, have effects that include antimicrobial activity against Cutibacterium acnes and anti-inflammatory activity in human keratinocytes).
- The study’s primary efficacy endpoint was the absolute change in inflammatory lesion count from baseline to week 12.
- Endpoint success was defined as an Investigator Global Assessment (IGA) score of 0 or 1 and at least a two-grade improvement from baseline at week 12.
TAKEAWAY:
- Of the 181 patients, 91 received DMT310 (applied once a week to the face and washed off after 10-15 minutes), and 90 received placebo.
- Patients in the DMT310 arm showed a significantly greater mean reduction in the number of inflammatory lesions at week 12, compared with those in the placebo arm (–15.64 vs. –10.84, respectively; P < .001).
- Similarly, patients in the DMT310 arm showed a significantly greater mean reduction in the number of noninflammatory lesions at week 12, compared with those in the placebo arm (–18.26 vs. –12.41, respectively; P < .001).
- At week 12, endpoint success based on IGA scores also significantly favored patients in the DMT310 arm, compared with those in the placebo arm (44.40% vs. 17.78%; P < .001).
IN PRACTICE:
This study is too preliminary to have practice application. The researchers concluded that the findings “support further study of DMT310 in larger, confirmatory phase 3 trials.”
SOURCE:
Lawrence F. Eichenfield, MD, professor of dermatology and pediatrics at the University of California, San Diego, led the research. The study was published online June 7 in the Journal of the American Academy of Dermatology.
LIMITATIONS:
The analysis did not include an active comparator group and it enrolled a limited number of Asian patients.
DISCLOSURES:
Dr. Eichenfield disclosed that he is a consultant to Dermata, which is developing DMT310, as were three other authors of the study. One author is a company employee. The remaining authors disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Poor patient compliance with topical acne therapies is a common clinical challenge.
- In a 12-week, randomized, controlled, phase 2b trial of 181 patients 12 years of age and older, researchers investigated the safety, tolerability, and efficacy of DMT310, a powdered mixture of Spongilla lacustris for treating moderate to severe acne. (In vitro studies have found that components of S. lacustris, a freshwater sponge, have effects that include antimicrobial activity against Cutibacterium acnes and anti-inflammatory activity in human keratinocytes).
- The study’s primary efficacy endpoint was the absolute change in inflammatory lesion count from baseline to week 12.
- Endpoint success was defined as an Investigator Global Assessment (IGA) score of 0 or 1 and at least a two-grade improvement from baseline at week 12.
TAKEAWAY:
- Of the 181 patients, 91 received DMT310 (applied once a week to the face and washed off after 10-15 minutes), and 90 received placebo.
- Patients in the DMT310 arm showed a significantly greater mean reduction in the number of inflammatory lesions at week 12, compared with those in the placebo arm (–15.64 vs. –10.84, respectively; P < .001).
- Similarly, patients in the DMT310 arm showed a significantly greater mean reduction in the number of noninflammatory lesions at week 12, compared with those in the placebo arm (–18.26 vs. –12.41, respectively; P < .001).
- At week 12, endpoint success based on IGA scores also significantly favored patients in the DMT310 arm, compared with those in the placebo arm (44.40% vs. 17.78%; P < .001).
IN PRACTICE:
This study is too preliminary to have practice application. The researchers concluded that the findings “support further study of DMT310 in larger, confirmatory phase 3 trials.”
SOURCE:
Lawrence F. Eichenfield, MD, professor of dermatology and pediatrics at the University of California, San Diego, led the research. The study was published online June 7 in the Journal of the American Academy of Dermatology.
LIMITATIONS:
The analysis did not include an active comparator group and it enrolled a limited number of Asian patients.
DISCLOSURES:
Dr. Eichenfield disclosed that he is a consultant to Dermata, which is developing DMT310, as were three other authors of the study. One author is a company employee. The remaining authors disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
FDA approves nivolumab for resected stage IIB/C melanoma
The , expanding the melanoma indication for the programmed death receptor-1 (PD-1) inhibitor.
Nivolumab, developed by Bristol-Myers Squibb, was previously approved as a single agent or in combination with ipilimumab for patients aged 12 years and older with unresectable or metastatic melanoma and for the adjuvant treatment of those aged 12 and older with completely resected stage III or IV melanoma.
The new approval was based on findings from the phase 3 CHECKMATE-76K trial, which randomly assigned 790 patients in a 2:1 ratio to receive nivolumab 480 mg or placebo by intravenous infusion. All patients in the trial had good performance status, had undergone complete resection of the primary melanoma with negative margins, and had tested negative on sentinel lymph node assessment within 12 weeks prior to randomization. Patients received treatment every 4 weeks for up to 1 year or until disease recurrence or unacceptable toxicity occurred.
Nivolumab reduced the risk of recurrence or death by 58% compared with placebo (hazard ratio, 0.42). Recurrence-free survival at 1 year was 89% with treatment, vs 79.4% with placebo. Median recurrence-free survival at 5 years was not reached in either arm.
Adverse reactions that were reported in at least 20% of patients included fatigue, musculoskeletal pain, rash, diarrhea, and pruritus.
The recommended nivolumab dose for patients weighing 40 kg or more is 480 mg every 4 weeks or 240 mg every 2 weeks until disease recurrence or unacceptable toxicity for up to 1 year. For pediatric patients who weigh less than 40 kg, the recommended dose is 3 mg/kg every 2 weeks or 6 mg/kg every 4 weeks until disease recurrence or unacceptable toxicity for up to 1 year.
Bristol-Myers Squibb’s application for approval led to the agent’s being granted orphan drug designation, allowing expedited review.
A version of this article appeared on Medscape.com.
The , expanding the melanoma indication for the programmed death receptor-1 (PD-1) inhibitor.
Nivolumab, developed by Bristol-Myers Squibb, was previously approved as a single agent or in combination with ipilimumab for patients aged 12 years and older with unresectable or metastatic melanoma and for the adjuvant treatment of those aged 12 and older with completely resected stage III or IV melanoma.
The new approval was based on findings from the phase 3 CHECKMATE-76K trial, which randomly assigned 790 patients in a 2:1 ratio to receive nivolumab 480 mg or placebo by intravenous infusion. All patients in the trial had good performance status, had undergone complete resection of the primary melanoma with negative margins, and had tested negative on sentinel lymph node assessment within 12 weeks prior to randomization. Patients received treatment every 4 weeks for up to 1 year or until disease recurrence or unacceptable toxicity occurred.
Nivolumab reduced the risk of recurrence or death by 58% compared with placebo (hazard ratio, 0.42). Recurrence-free survival at 1 year was 89% with treatment, vs 79.4% with placebo. Median recurrence-free survival at 5 years was not reached in either arm.
Adverse reactions that were reported in at least 20% of patients included fatigue, musculoskeletal pain, rash, diarrhea, and pruritus.
The recommended nivolumab dose for patients weighing 40 kg or more is 480 mg every 4 weeks or 240 mg every 2 weeks until disease recurrence or unacceptable toxicity for up to 1 year. For pediatric patients who weigh less than 40 kg, the recommended dose is 3 mg/kg every 2 weeks or 6 mg/kg every 4 weeks until disease recurrence or unacceptable toxicity for up to 1 year.
Bristol-Myers Squibb’s application for approval led to the agent’s being granted orphan drug designation, allowing expedited review.
A version of this article appeared on Medscape.com.
The , expanding the melanoma indication for the programmed death receptor-1 (PD-1) inhibitor.
Nivolumab, developed by Bristol-Myers Squibb, was previously approved as a single agent or in combination with ipilimumab for patients aged 12 years and older with unresectable or metastatic melanoma and for the adjuvant treatment of those aged 12 and older with completely resected stage III or IV melanoma.
The new approval was based on findings from the phase 3 CHECKMATE-76K trial, which randomly assigned 790 patients in a 2:1 ratio to receive nivolumab 480 mg or placebo by intravenous infusion. All patients in the trial had good performance status, had undergone complete resection of the primary melanoma with negative margins, and had tested negative on sentinel lymph node assessment within 12 weeks prior to randomization. Patients received treatment every 4 weeks for up to 1 year or until disease recurrence or unacceptable toxicity occurred.
Nivolumab reduced the risk of recurrence or death by 58% compared with placebo (hazard ratio, 0.42). Recurrence-free survival at 1 year was 89% with treatment, vs 79.4% with placebo. Median recurrence-free survival at 5 years was not reached in either arm.
Adverse reactions that were reported in at least 20% of patients included fatigue, musculoskeletal pain, rash, diarrhea, and pruritus.
The recommended nivolumab dose for patients weighing 40 kg or more is 480 mg every 4 weeks or 240 mg every 2 weeks until disease recurrence or unacceptable toxicity for up to 1 year. For pediatric patients who weigh less than 40 kg, the recommended dose is 3 mg/kg every 2 weeks or 6 mg/kg every 4 weeks until disease recurrence or unacceptable toxicity for up to 1 year.
Bristol-Myers Squibb’s application for approval led to the agent’s being granted orphan drug designation, allowing expedited review.
A version of this article appeared on Medscape.com.
Artificial intelligence in the office: Part 2
In the year since generative artificial intelligence (AI) software first began to emerge for use, the staggering pace and breadth of development has condensed years of growth and change into months and weeks. Among the settings where these tools may find the greatest straight-line relevance is private medical practice.
. A multitude of generative AI products with potential medical applications are now available, with new ones appearing almost weekly. (As always, I have no financial interest in any product or service mentioned in this column.)
Last month, I discussed ChatGPT, the best-known AI algorithm, and some of its applications in clinical practice, such as generating website, video, and blog content. ChatGPT can also provide rapid and concise answers to general medical questions, like a search engine – but with more natural language processing and contextual understanding. Additionally, the algorithm can draft generic medical documents, including templates for after-visit summaries, postprocedure instructions, referrals, prior authorization appeal letters, and educational handouts.
Another useful feature of ChatGPT is its ability to provide accurate and conversational language translations, thus serving as an interpreter during clinic visits in situations where a human translator is not available. It also has potential uses in clinical research by finding resources, formulating hypotheses, drafting study protocols, and collecting large amounts of data in short periods of time. Other possibilities include survey administration, clinical trial recruitment, and automatic medication monitoring.
GPT-4, the latest version of ChatGPT, is reported to have greater problem-solving abilities and an even broader knowledge base. Among its claimed skills are the ability to find the latest literature in a given area, write a discharge summary for a patient following an uncomplicated surgery, and an image analysis feature to identify objects in photos. GPT-4 has been praised as having “the potential to help drive medical innovation, from aiding with patient discharge notes, summarizing recent clinical trials, providing information on ethical guidelines, and much more.”
Bard, an AI “chat bot” introduced by Google earlier this year, intends to leverage Google’s enormous database to compete with ChatGPT in providing answers to medical questions. Bard also hopes to play a pivotal role in expanding telemedicine and remote care via Google’s secure connections and access to patient records and medical history, and “facilitate seamless communication through appointment scheduling, messaging, and sharing medical images,” according to PackT, a website for IT professionals. The company claims that Bard’s integration of AI and machine learning capabilities will serve to elevate health care efficiency and patient outcomes, PackT says, and “the platform’s AI system quickly and accurately analyzes patient records, identifies patterns and trends, and aids medical professionals in developing effective treatment plans.”
Doximity has introduced an AI engine called DocsGPT, an encrypted, HIPAA-compliant writing assistant that, the company says, can draft any form of professional correspondence, including prior authorization letters, insurance appeals, patient support letters, and patient education materials. The service is available at no charge to all U.S. physicians and medical students through their Doximity accounts.
Microsoft has introduced several AI products. BioGPT is a language model specifically designed for health care. Compared with GPT models that are trained on more general text data, BioGPT is purported to have a deeper understanding of the language used in biomedical research and can generate more accurate and relevant outputs for biomedical tasks, such as drug discovery, disease classification, and clinical decision support. Fabric is another health care–specific data and analytics platform the company described in an announcement in May. It can combine data from sources such as electronic health records, images, lab systems, medical devices, and claims systems so hospitals and offices can standardize it and access it in the same place. Microsoft said the new tools will help eliminate the “time-consuming” process of searching through these sources one by one. Microsoft will also offer a new generative AI chatbot called the Azure Health Bot, which can pull information from a health organization’s own internal data as well as reputable external sources such as the Food and Drug Administration and the National Institutes of Health.
Several other AI products are available for clinicians. Tana served as an administrative aid and a clinical helper during the height of the COVID-19 pandemic, answering frequently asked questions, facilitating appointment management, and gathering preliminary medical information prior to teleconsultations. Dougall GPT is another AI chatbot tailored for health care professionals. It provides clinicians with AI-tuned answers to their queries, augmented by links to relevant, up-to-date, authoritative resources. It also assists in drafting patient instructions, consultation summaries, speeches, and professional correspondence. Wang has created Clinical Camel, an open-source health care–focused chatbot that assembles medical data with a combination of user-shared conversations and synthetic conversations derived from curated clinical articles. The Chinese company Baidu has rolled out Ernie as a potential rival to ChatGPT. You get the idea.
Of course, the inherent drawbacks of AI, such as producing false or biased information, perpetuating harmful stereotypes, and presenting information that has since been proven inaccurate or out-of-date, must always be kept in mind. All AI algorithms have been criticized for giving wrong answers, as their datasets are generally culled from information published in 2021 or earlier. Several of them have been shown to fabricate information – a phenomenon labeled “artificial hallucinations” in one article. “The scientific community must be vigilant in verifying the accuracy and reliability of the information provided by AI tools,” wrote the authors of that paper. “Researchers should use AI as an aid rather than a replacement for critical thinking and fact-checking.”
In the year since generative artificial intelligence (AI) software first began to emerge for use, the staggering pace and breadth of development has condensed years of growth and change into months and weeks. Among the settings where these tools may find the greatest straight-line relevance is private medical practice.
. A multitude of generative AI products with potential medical applications are now available, with new ones appearing almost weekly. (As always, I have no financial interest in any product or service mentioned in this column.)
Last month, I discussed ChatGPT, the best-known AI algorithm, and some of its applications in clinical practice, such as generating website, video, and blog content. ChatGPT can also provide rapid and concise answers to general medical questions, like a search engine – but with more natural language processing and contextual understanding. Additionally, the algorithm can draft generic medical documents, including templates for after-visit summaries, postprocedure instructions, referrals, prior authorization appeal letters, and educational handouts.
Another useful feature of ChatGPT is its ability to provide accurate and conversational language translations, thus serving as an interpreter during clinic visits in situations where a human translator is not available. It also has potential uses in clinical research by finding resources, formulating hypotheses, drafting study protocols, and collecting large amounts of data in short periods of time. Other possibilities include survey administration, clinical trial recruitment, and automatic medication monitoring.
GPT-4, the latest version of ChatGPT, is reported to have greater problem-solving abilities and an even broader knowledge base. Among its claimed skills are the ability to find the latest literature in a given area, write a discharge summary for a patient following an uncomplicated surgery, and an image analysis feature to identify objects in photos. GPT-4 has been praised as having “the potential to help drive medical innovation, from aiding with patient discharge notes, summarizing recent clinical trials, providing information on ethical guidelines, and much more.”
Bard, an AI “chat bot” introduced by Google earlier this year, intends to leverage Google’s enormous database to compete with ChatGPT in providing answers to medical questions. Bard also hopes to play a pivotal role in expanding telemedicine and remote care via Google’s secure connections and access to patient records and medical history, and “facilitate seamless communication through appointment scheduling, messaging, and sharing medical images,” according to PackT, a website for IT professionals. The company claims that Bard’s integration of AI and machine learning capabilities will serve to elevate health care efficiency and patient outcomes, PackT says, and “the platform’s AI system quickly and accurately analyzes patient records, identifies patterns and trends, and aids medical professionals in developing effective treatment plans.”
Doximity has introduced an AI engine called DocsGPT, an encrypted, HIPAA-compliant writing assistant that, the company says, can draft any form of professional correspondence, including prior authorization letters, insurance appeals, patient support letters, and patient education materials. The service is available at no charge to all U.S. physicians and medical students through their Doximity accounts.
Microsoft has introduced several AI products. BioGPT is a language model specifically designed for health care. Compared with GPT models that are trained on more general text data, BioGPT is purported to have a deeper understanding of the language used in biomedical research and can generate more accurate and relevant outputs for biomedical tasks, such as drug discovery, disease classification, and clinical decision support. Fabric is another health care–specific data and analytics platform the company described in an announcement in May. It can combine data from sources such as electronic health records, images, lab systems, medical devices, and claims systems so hospitals and offices can standardize it and access it in the same place. Microsoft said the new tools will help eliminate the “time-consuming” process of searching through these sources one by one. Microsoft will also offer a new generative AI chatbot called the Azure Health Bot, which can pull information from a health organization’s own internal data as well as reputable external sources such as the Food and Drug Administration and the National Institutes of Health.
Several other AI products are available for clinicians. Tana served as an administrative aid and a clinical helper during the height of the COVID-19 pandemic, answering frequently asked questions, facilitating appointment management, and gathering preliminary medical information prior to teleconsultations. Dougall GPT is another AI chatbot tailored for health care professionals. It provides clinicians with AI-tuned answers to their queries, augmented by links to relevant, up-to-date, authoritative resources. It also assists in drafting patient instructions, consultation summaries, speeches, and professional correspondence. Wang has created Clinical Camel, an open-source health care–focused chatbot that assembles medical data with a combination of user-shared conversations and synthetic conversations derived from curated clinical articles. The Chinese company Baidu has rolled out Ernie as a potential rival to ChatGPT. You get the idea.
Of course, the inherent drawbacks of AI, such as producing false or biased information, perpetuating harmful stereotypes, and presenting information that has since been proven inaccurate or out-of-date, must always be kept in mind. All AI algorithms have been criticized for giving wrong answers, as their datasets are generally culled from information published in 2021 or earlier. Several of them have been shown to fabricate information – a phenomenon labeled “artificial hallucinations” in one article. “The scientific community must be vigilant in verifying the accuracy and reliability of the information provided by AI tools,” wrote the authors of that paper. “Researchers should use AI as an aid rather than a replacement for critical thinking and fact-checking.”
In the year since generative artificial intelligence (AI) software first began to emerge for use, the staggering pace and breadth of development has condensed years of growth and change into months and weeks. Among the settings where these tools may find the greatest straight-line relevance is private medical practice.
. A multitude of generative AI products with potential medical applications are now available, with new ones appearing almost weekly. (As always, I have no financial interest in any product or service mentioned in this column.)
Last month, I discussed ChatGPT, the best-known AI algorithm, and some of its applications in clinical practice, such as generating website, video, and blog content. ChatGPT can also provide rapid and concise answers to general medical questions, like a search engine – but with more natural language processing and contextual understanding. Additionally, the algorithm can draft generic medical documents, including templates for after-visit summaries, postprocedure instructions, referrals, prior authorization appeal letters, and educational handouts.
Another useful feature of ChatGPT is its ability to provide accurate and conversational language translations, thus serving as an interpreter during clinic visits in situations where a human translator is not available. It also has potential uses in clinical research by finding resources, formulating hypotheses, drafting study protocols, and collecting large amounts of data in short periods of time. Other possibilities include survey administration, clinical trial recruitment, and automatic medication monitoring.
GPT-4, the latest version of ChatGPT, is reported to have greater problem-solving abilities and an even broader knowledge base. Among its claimed skills are the ability to find the latest literature in a given area, write a discharge summary for a patient following an uncomplicated surgery, and an image analysis feature to identify objects in photos. GPT-4 has been praised as having “the potential to help drive medical innovation, from aiding with patient discharge notes, summarizing recent clinical trials, providing information on ethical guidelines, and much more.”
Bard, an AI “chat bot” introduced by Google earlier this year, intends to leverage Google’s enormous database to compete with ChatGPT in providing answers to medical questions. Bard also hopes to play a pivotal role in expanding telemedicine and remote care via Google’s secure connections and access to patient records and medical history, and “facilitate seamless communication through appointment scheduling, messaging, and sharing medical images,” according to PackT, a website for IT professionals. The company claims that Bard’s integration of AI and machine learning capabilities will serve to elevate health care efficiency and patient outcomes, PackT says, and “the platform’s AI system quickly and accurately analyzes patient records, identifies patterns and trends, and aids medical professionals in developing effective treatment plans.”
Doximity has introduced an AI engine called DocsGPT, an encrypted, HIPAA-compliant writing assistant that, the company says, can draft any form of professional correspondence, including prior authorization letters, insurance appeals, patient support letters, and patient education materials. The service is available at no charge to all U.S. physicians and medical students through their Doximity accounts.
Microsoft has introduced several AI products. BioGPT is a language model specifically designed for health care. Compared with GPT models that are trained on more general text data, BioGPT is purported to have a deeper understanding of the language used in biomedical research and can generate more accurate and relevant outputs for biomedical tasks, such as drug discovery, disease classification, and clinical decision support. Fabric is another health care–specific data and analytics platform the company described in an announcement in May. It can combine data from sources such as electronic health records, images, lab systems, medical devices, and claims systems so hospitals and offices can standardize it and access it in the same place. Microsoft said the new tools will help eliminate the “time-consuming” process of searching through these sources one by one. Microsoft will also offer a new generative AI chatbot called the Azure Health Bot, which can pull information from a health organization’s own internal data as well as reputable external sources such as the Food and Drug Administration and the National Institutes of Health.
Several other AI products are available for clinicians. Tana served as an administrative aid and a clinical helper during the height of the COVID-19 pandemic, answering frequently asked questions, facilitating appointment management, and gathering preliminary medical information prior to teleconsultations. Dougall GPT is another AI chatbot tailored for health care professionals. It provides clinicians with AI-tuned answers to their queries, augmented by links to relevant, up-to-date, authoritative resources. It also assists in drafting patient instructions, consultation summaries, speeches, and professional correspondence. Wang has created Clinical Camel, an open-source health care–focused chatbot that assembles medical data with a combination of user-shared conversations and synthetic conversations derived from curated clinical articles. The Chinese company Baidu has rolled out Ernie as a potential rival to ChatGPT. You get the idea.
Of course, the inherent drawbacks of AI, such as producing false or biased information, perpetuating harmful stereotypes, and presenting information that has since been proven inaccurate or out-of-date, must always be kept in mind. All AI algorithms have been criticized for giving wrong answers, as their datasets are generally culled from information published in 2021 or earlier. Several of them have been shown to fabricate information – a phenomenon labeled “artificial hallucinations” in one article. “The scientific community must be vigilant in verifying the accuracy and reliability of the information provided by AI tools,” wrote the authors of that paper. “Researchers should use AI as an aid rather than a replacement for critical thinking and fact-checking.”
AI chatbot ‘hallucinates’ faulty medical intelligence
Artificial intelligence (AI) models are typically a year out of date and have this “charming problem of hallucinating made-up data and saying it with all the certainty of an attending on rounds,” Isaac Kohane, MD, PhD, Harvard Medical School, Boston, told a packed audience at plenary at an annual scientific meeting on infectious diseases.
Dr. Kohane, chair of the department of biomedical informatics, says the future intersection between AI and health care is “muddy.”
Echoing questions about the accuracy of new AI tools, researchers at the meeting presented the results of their new test of ChatGPT.
To test the accuracy of ChatGPT’s version 3.5, the researchers asked it if there are any boxed warnings on the U.S. Food and Drug Administration’s label for common antibiotics, and if so, what they are.
ChatGPT provided correct answers about FDA boxed warnings for only 12 of the 41 antibiotics queried – a matching rate of just 29%.
For the other 29 antibiotics, ChatGPT either “incorrectly reported that there was an FDA boxed warning when there was not, or inaccurately or incorrectly reported the boxed warning,” Rebecca Linfield, MD, infectious diseases fellow, Stanford (Calif.) University, said in an interview.
Uncritical AI use risky
Nine of the 41 antibiotics included in the query have boxed warnings. And ChatGPT correctly identified all nine, but only three were the matching adverse event (33%). For the 32 antibiotics without an FDA boxed warning, ChatGPT correctly reported that 28% (9 of 32) do not have a boxed warning.
For example, ChatGPT stated that the antibiotic fidaxomicin has a boxed warning for increased risk for Clostridioides difficile, “but it is the first-line antibiotic used to treat C. difficile,” Dr. Linfield pointed out.
ChatGPT also reported that cefepime increased the risk for death in those with pneumonia and fabricated a study supporting that assertion. “However, cefepime is a first-line drug for those with hospital-acquired pneumonia,” Dr. Linfield explained.
“I can imagine a worried family member finding this through ChatGPT, and needing to have extensive reassurances from the patient’s physicians about why this antibiotic was chosen,” she said.
ChatGPT also incorrectly stated that aztreonam has a boxed warning for increased mortality.
“The risk is that both physicians and the public uncritically use ChatGPT as an easily accessible, readable source of clinically validated information, when these large language models are meant to generate fluid text, and not necessarily accurate information,” Dr. Linfield told this news organization.
Dr. Linfield said that the next step is to compare the ChatGPT 3.5 used in this analysis with ChatGPT 4, as well as with Google’s Med-PaLM 2 after it is released to the public.
Advancing fast
At plenary, Dr. Kohane pointed out that AI is a quick learner and improvements in tools are coming fast.
As an example, just 3 years ago, the best AI tool could score about as well as the worst student taking the medical boards, he told the audience. “Three years later, the leading large language models are scoring better than 90% of all the candidates. What’s it going to be doing next year?” he asked.
“I don’t know,” Dr. Kohane said, “but it will be better than this year.” AI will “transform health care.”
A version of this article first appeared on Medscape.com.
Artificial intelligence (AI) models are typically a year out of date and have this “charming problem of hallucinating made-up data and saying it with all the certainty of an attending on rounds,” Isaac Kohane, MD, PhD, Harvard Medical School, Boston, told a packed audience at plenary at an annual scientific meeting on infectious diseases.
Dr. Kohane, chair of the department of biomedical informatics, says the future intersection between AI and health care is “muddy.”
Echoing questions about the accuracy of new AI tools, researchers at the meeting presented the results of their new test of ChatGPT.
To test the accuracy of ChatGPT’s version 3.5, the researchers asked it if there are any boxed warnings on the U.S. Food and Drug Administration’s label for common antibiotics, and if so, what they are.
ChatGPT provided correct answers about FDA boxed warnings for only 12 of the 41 antibiotics queried – a matching rate of just 29%.
For the other 29 antibiotics, ChatGPT either “incorrectly reported that there was an FDA boxed warning when there was not, or inaccurately or incorrectly reported the boxed warning,” Rebecca Linfield, MD, infectious diseases fellow, Stanford (Calif.) University, said in an interview.
Uncritical AI use risky
Nine of the 41 antibiotics included in the query have boxed warnings. And ChatGPT correctly identified all nine, but only three were the matching adverse event (33%). For the 32 antibiotics without an FDA boxed warning, ChatGPT correctly reported that 28% (9 of 32) do not have a boxed warning.
For example, ChatGPT stated that the antibiotic fidaxomicin has a boxed warning for increased risk for Clostridioides difficile, “but it is the first-line antibiotic used to treat C. difficile,” Dr. Linfield pointed out.
ChatGPT also reported that cefepime increased the risk for death in those with pneumonia and fabricated a study supporting that assertion. “However, cefepime is a first-line drug for those with hospital-acquired pneumonia,” Dr. Linfield explained.
“I can imagine a worried family member finding this through ChatGPT, and needing to have extensive reassurances from the patient’s physicians about why this antibiotic was chosen,” she said.
ChatGPT also incorrectly stated that aztreonam has a boxed warning for increased mortality.
“The risk is that both physicians and the public uncritically use ChatGPT as an easily accessible, readable source of clinically validated information, when these large language models are meant to generate fluid text, and not necessarily accurate information,” Dr. Linfield told this news organization.
Dr. Linfield said that the next step is to compare the ChatGPT 3.5 used in this analysis with ChatGPT 4, as well as with Google’s Med-PaLM 2 after it is released to the public.
Advancing fast
At plenary, Dr. Kohane pointed out that AI is a quick learner and improvements in tools are coming fast.
As an example, just 3 years ago, the best AI tool could score about as well as the worst student taking the medical boards, he told the audience. “Three years later, the leading large language models are scoring better than 90% of all the candidates. What’s it going to be doing next year?” he asked.
“I don’t know,” Dr. Kohane said, “but it will be better than this year.” AI will “transform health care.”
A version of this article first appeared on Medscape.com.
Artificial intelligence (AI) models are typically a year out of date and have this “charming problem of hallucinating made-up data and saying it with all the certainty of an attending on rounds,” Isaac Kohane, MD, PhD, Harvard Medical School, Boston, told a packed audience at plenary at an annual scientific meeting on infectious diseases.
Dr. Kohane, chair of the department of biomedical informatics, says the future intersection between AI and health care is “muddy.”
Echoing questions about the accuracy of new AI tools, researchers at the meeting presented the results of their new test of ChatGPT.
To test the accuracy of ChatGPT’s version 3.5, the researchers asked it if there are any boxed warnings on the U.S. Food and Drug Administration’s label for common antibiotics, and if so, what they are.
ChatGPT provided correct answers about FDA boxed warnings for only 12 of the 41 antibiotics queried – a matching rate of just 29%.
For the other 29 antibiotics, ChatGPT either “incorrectly reported that there was an FDA boxed warning when there was not, or inaccurately or incorrectly reported the boxed warning,” Rebecca Linfield, MD, infectious diseases fellow, Stanford (Calif.) University, said in an interview.
Uncritical AI use risky
Nine of the 41 antibiotics included in the query have boxed warnings. And ChatGPT correctly identified all nine, but only three were the matching adverse event (33%). For the 32 antibiotics without an FDA boxed warning, ChatGPT correctly reported that 28% (9 of 32) do not have a boxed warning.
For example, ChatGPT stated that the antibiotic fidaxomicin has a boxed warning for increased risk for Clostridioides difficile, “but it is the first-line antibiotic used to treat C. difficile,” Dr. Linfield pointed out.
ChatGPT also reported that cefepime increased the risk for death in those with pneumonia and fabricated a study supporting that assertion. “However, cefepime is a first-line drug for those with hospital-acquired pneumonia,” Dr. Linfield explained.
“I can imagine a worried family member finding this through ChatGPT, and needing to have extensive reassurances from the patient’s physicians about why this antibiotic was chosen,” she said.
ChatGPT also incorrectly stated that aztreonam has a boxed warning for increased mortality.
“The risk is that both physicians and the public uncritically use ChatGPT as an easily accessible, readable source of clinically validated information, when these large language models are meant to generate fluid text, and not necessarily accurate information,” Dr. Linfield told this news organization.
Dr. Linfield said that the next step is to compare the ChatGPT 3.5 used in this analysis with ChatGPT 4, as well as with Google’s Med-PaLM 2 after it is released to the public.
Advancing fast
At plenary, Dr. Kohane pointed out that AI is a quick learner and improvements in tools are coming fast.
As an example, just 3 years ago, the best AI tool could score about as well as the worst student taking the medical boards, he told the audience. “Three years later, the leading large language models are scoring better than 90% of all the candidates. What’s it going to be doing next year?” he asked.
“I don’t know,” Dr. Kohane said, “but it will be better than this year.” AI will “transform health care.”
A version of this article first appeared on Medscape.com.
FROM IDWEEK 2023