User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
New-Onset Pemphigoid Gestationis Following COVID-19 Vaccination
To the Editor:
Pemphigoid gestationis (PG), or gestational pemphigoid, is a rare autoimmune bullous disease (AIBD) occurring in 1 in 50,000 pregnancies. It is characterized by abrupt development of intensely pruritic papules and urticarial plaques, followed by an eruption of blisters.1 We present a case of new-onset PG that erupted 10 days following SARs-CoV-2 messenger RNA (mRNA) vaccination with BNT162b2 (Pfizer-BioNTech).
A 36-year-old pregnant woman (gravida 1, para 0, aborta 0) at 37 weeks’ gestation presented to our AIBD clinic with a pruritic dermatitis of 6 weeks’ duration that developed 10 days after receiving the second dose of BNT162b2. Multiple intensely pruritic, red bumps presented first on the forearms and within days spread to the thighs, hands, and abdomen, followed by progression to the ankles, feet, and back 2 weeks later. An initial biopsy was consistent with subacute spongiotic dermatitis with rare eosinophils. She found minimal relief from diphenhydramine or topical steroids. She denied oral, nasal, ocular, or genital involvement or history of any other skin disease. The pregnancy had been otherwise uneventful.
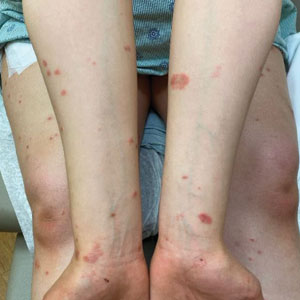
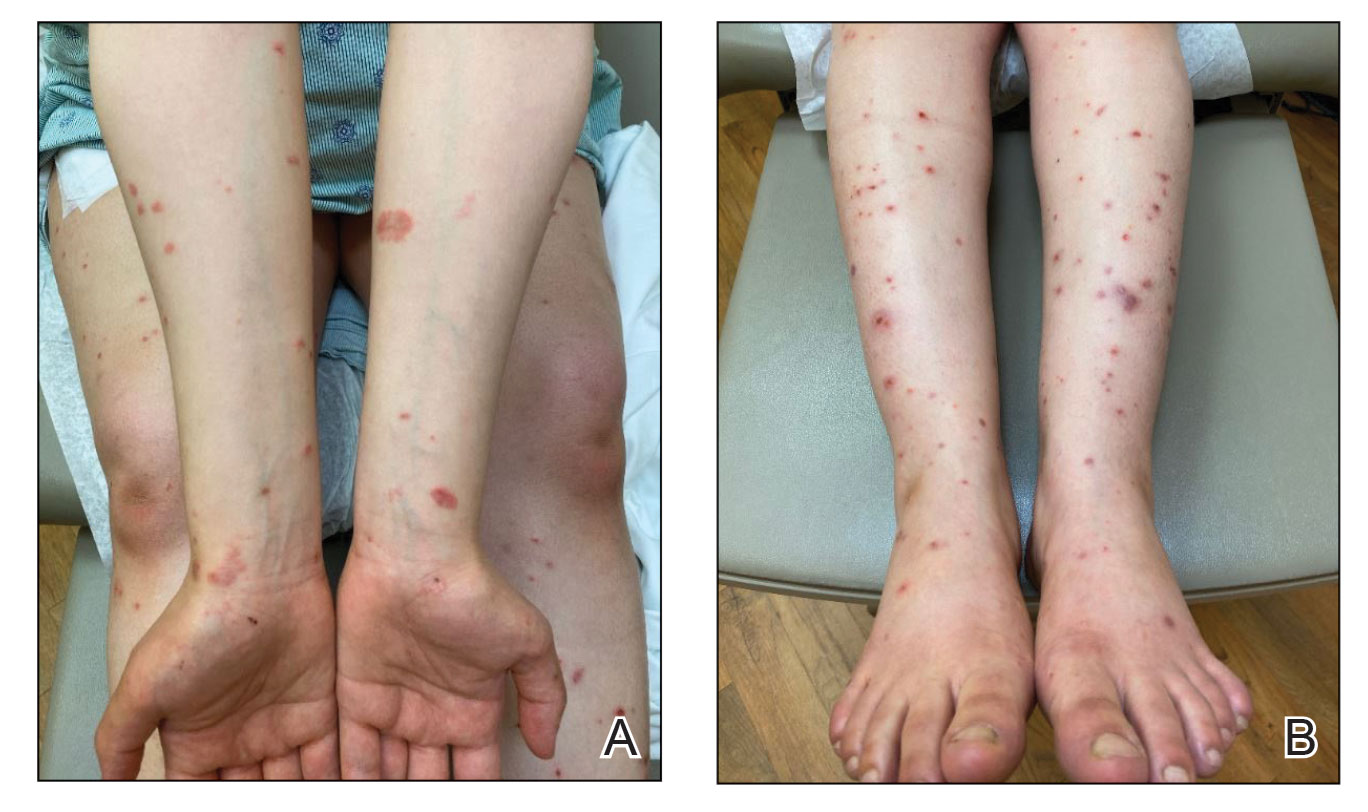
Physical examination revealed annular edematous plaques on the trunk and buttocks; excoriated and erythematous papules on the neck, trunk, arms, and legs; and scattered vesicles along the fingers, arms, hands, abdomen, back, legs, and feet (Figure 1). The Bullous Pemphigoid Disease Area Index (BPDAI) total skin activity score was 25.3, corresponding to moderate disease activity (validated at 20–56).2 The BPDAI total pruritus component score was 20. A repeat biopsy for direct immunofluorescence showed faint linear deposits of IgG and bright linear deposits of C3 along the basement membrane zone. Indirect immunofluorescence showed linear deposits of IgG localized to the blister roof of salt-split skin at a dilution of 1:40. An enzyme-linked immunosorbent assay for anti-BP180 was 62 U/mL (negative, <9 U/mL; positive, ≥9 U/mL), and anti-BP230 autoantibodies were less than 9 U/mL (negative <9 U/mL; positive, ≥9 U/mL). Given these clinical and histopathologic findings, PG was diagnosed.
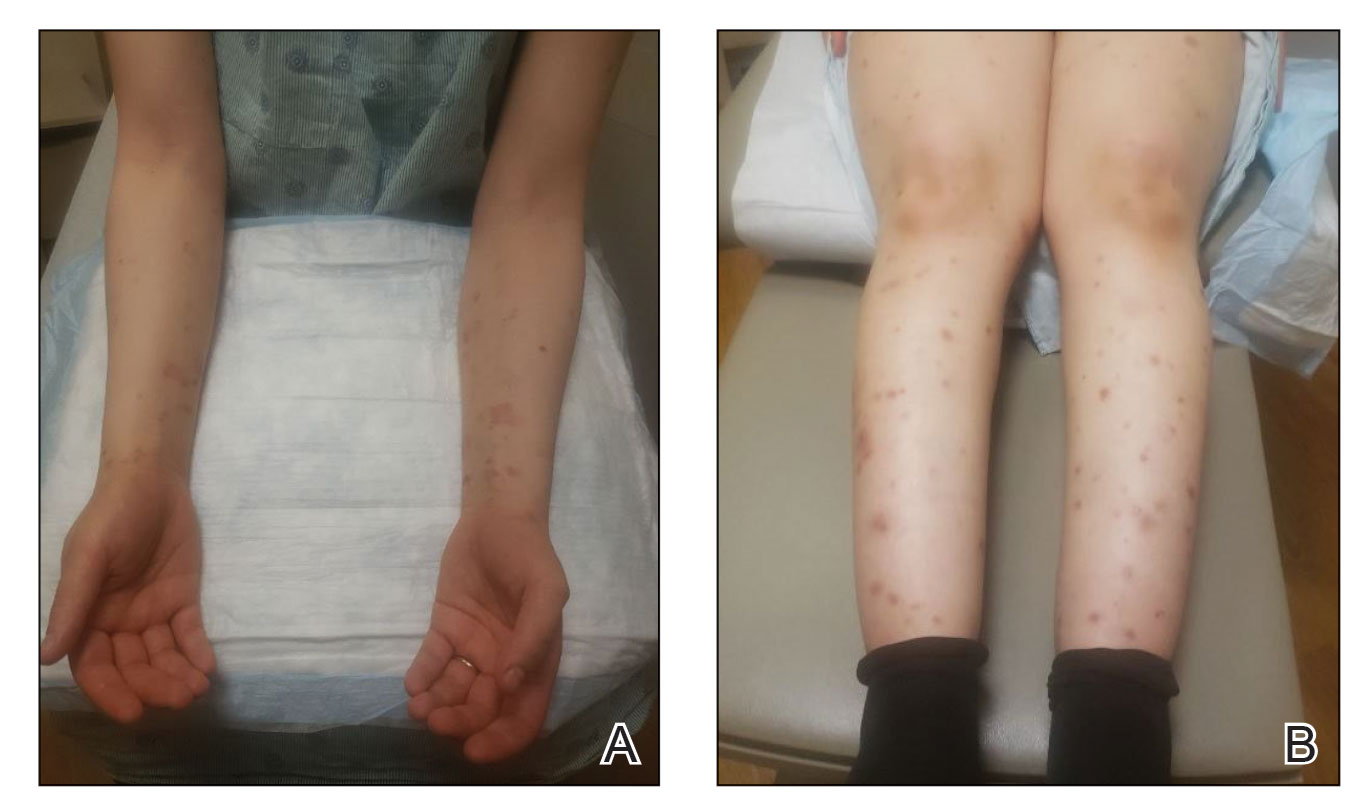
The patient was started on prednisone 20 mg and antihistamines while continuing topical steroids. Pruritus and blistering improved close to delivery. Fetal monitoring with regular biophysical profiles remained normal. The patient delivered a healthy neonate without skin lesions at 40 weeks’ gestation. The disease flared 2 days after delivery, and prednisone was increased to 40 mg and slowly tapered. Two months after delivery, the patient remained on prednisone 10 mg daily with ongoing but reduced blistering and pruritus (Figure 2). The BPDAI total skin activity and pruritus component scores remained elevated at 20.3 and 14, respectively, and anti-BP180 was 44 U/mL. After a discussion with the patient on safe systemic therapy while breastfeeding, intravenous immunoglobulin (IVIG) was initiated. The patient received 3 monthly infusions at 2 g/kg and was able to taper the prednisone to 5 mg every other day without new lesions. Four months after completion of IVIG therapy, she achieved complete remission off all therapy.
Management of PG begins with topical corticosteroids, but most patients require systemic steroid therapy.1 Remission commonly occurs close to delivery, and 75% of patients flare post partum, though the disease typically resolves 6 months following delivery.1,3,4 For persistent intrapartum cases requiring more than prednisone 20 mg daily, therapy can include dapsone, IVIG, azathioprine, rituximab, or plasmapheresis.4,5 Dapsone and IVIG are compatible with breastfeeding postpartum, but if dapsone is selected, the infant must be monitored for hemolytic anemia.5 Pemphigoid gestationis increases the risk for a premature or small-for-gestational-age neonate, necessitating regular fetal monitoring until delivery.1 Cutaneous lesions may affect the newborn, though this occurrence is rare and self-limiting.6 Pemphigoid gestationis may recur in subsequent pregnancies at a rate of 33% to 55%, with earlier and more severe presentations.4
Clinically and histologically, PG closely resembles bullous pemphigoid (BP), but the exact pathogenesis is not fully understood. Recently, another case of what was termed pseudo-PG has been described 3 days following administration of the second dose of the Pfizer-BioNTech COVID-19 vaccine.7 Since the introduction of COVID-19 mRNA vaccines, cases of postvaccination BP, BP-like eruptions, and pemphigus vulgaris have been described.8-11 Tomayko et al10 reported 12 cases of subepidermal eruptions, including BP, in which 7 patients developed blisters after the second dose of either the Pfizer-BioNTech or Moderna mRNA vaccine. Three patients who developed BP after the first dose of the vaccine and chose to receive the second dose tolerated it well, with a mild flare observed in 1 patient.10 Similarly, subsequent vaccine doses in reports of vaccine-associated AIBD resulted in increased disease activity in 21% of cases.12 COVID-19 vaccine–associated BP, similar to drug-induced BP, seemingly displays a milder course of disease compared to the classic form of BP.10,13 More follow-up is needed to better understand these reactions and inform appropriate discussions on the administration of booster doses. Currently, completion of the vaccination series against COVID-19 is advisable given the paucity of reports of postvaccination AIBD and the risk for COVID-19 infection, but careful discussions on a case-by-case basis are warranted related to the risk for disease exacerbation following subsequent vaccinations.
The clinical presentation and diagnostic evaluation of our patient’s rash were consistent with PG. The temporal relationship between vaccine administration and PG lesion onset suggests the mRNA vaccine triggered AIBD in our patient. Interestingly, AIBD associated with COVID-19 is not unique to only the vaccines and has been observed following infection with the virus itself.14 The high rate of vaccination against COVID-19 in contrast with the low number of reported cases of AIBD after vaccination supports the overall safety of COVID-19 vaccines but identifies a need for further understanding of the processes that lead to the development of autoimmune conditions in at-risk populations.
- Wiznia LE, Pomeranz MK. Skin changes and diseases in pregnancy. In: Kang S, Amagai M, Bruckner AL, et al, eds. Fitzpatrick’s Dermatology. 9th ed. McGraw-Hill Education; 2019.
- Masmoudi W, Vaillant M, Vassileva S, et al. International validation of the Bullous Pemphigoid Disease Area Index severity score and calculation of cut-off values for defining mild, moderate and severe types of bullous pemphigoid. Br J Dermatol. 2021;184:1106-1112. doi:10.1111/bjd.19611
- Semkova K, Black M. Pemphigoid gestationis: current insights into pathogenesis and treatment. Eur J Obstet Gynecol Reprod Biol. 2009;145:138-144.
- Savervall C, Sand FL, Thomsen SF. Pemphigoid gestationis: current perspectives. Clin Cosmet Investig Dermatol. 2017;10:441-449.
- Braunstein I, Werth V. Treatment of dermatologic connective tissue disease and autoimmune blistering disorders in pregnancy. Dermatol Ther. 2013;26:354-363.
- Lipozencic J, Ljubojevic S, Bukvic-Mokos Z. Pemphigoid gestationis. Clin Dermatol. 2012;30:51-55.
- de Lorenzi C, Kaya G, Toutous Trellu L. Pseudo-pemphigoid gestationis eruption following SARS-CoV-2 vaccination with mRNA vaccine. Dermatopathology (Basel). 2022;9:203-206. doi:10.3390/dermatopathology9030025
- McMahon DE, Kovarik CL, Damsky W, et al. Clinical and pathologic correlation of cutaneous COVID-19 vaccine reactions including V-REPP: a registry-based study. J Am Acad Dermatol. 2022;86:113-121.
- Solimani F, Mansour Y, Didona D, et al. Development of severe pemphigus vulgaris following SARS-CoV-2 vaccination with BNT162b2. J Eur Acad Dermatol Venereol. 2021;35:E649-E651.
- Tomayko MM, Damsky W, Fathy R, et al. Subepidermal blistering eruptions, including bullous pemphigoid, following COVID-19 vaccination. J Allergy Clin Immunol. 2021;148:750-751.
- Coto-Segura P, Fernandez-Prada M, Mir-Bonafe M, et al. Vesiculobullous skin reactions induced by COVID-19 mRNA vaccine: report of four cases and review of the literature. Clin Exp Dermatol. 2022;47:141-143.
- Kasperkiewicz M, Woodley DT. Association between vaccination and autoimmune bullous diseases: a systematic review. J Am Acad Dermatol. 2022;86:1160-1164.
- Stavropoulos PG, Soura E, Antoniou C. Drug-induced pemphigoid: a review of the literature. J Eur Acad Dermatol Venereol. 2014;28:1133-1140.
- Olson N, Eckhardt D, Delano A. New-onset bullous pemphigoid in a COVID-19 patient. Case Rep Dermatol Med. 2021;2021:5575111.
To the Editor:
Pemphigoid gestationis (PG), or gestational pemphigoid, is a rare autoimmune bullous disease (AIBD) occurring in 1 in 50,000 pregnancies. It is characterized by abrupt development of intensely pruritic papules and urticarial plaques, followed by an eruption of blisters.1 We present a case of new-onset PG that erupted 10 days following SARs-CoV-2 messenger RNA (mRNA) vaccination with BNT162b2 (Pfizer-BioNTech).
A 36-year-old pregnant woman (gravida 1, para 0, aborta 0) at 37 weeks’ gestation presented to our AIBD clinic with a pruritic dermatitis of 6 weeks’ duration that developed 10 days after receiving the second dose of BNT162b2. Multiple intensely pruritic, red bumps presented first on the forearms and within days spread to the thighs, hands, and abdomen, followed by progression to the ankles, feet, and back 2 weeks later. An initial biopsy was consistent with subacute spongiotic dermatitis with rare eosinophils. She found minimal relief from diphenhydramine or topical steroids. She denied oral, nasal, ocular, or genital involvement or history of any other skin disease. The pregnancy had been otherwise uneventful.
Physical examination revealed annular edematous plaques on the trunk and buttocks; excoriated and erythematous papules on the neck, trunk, arms, and legs; and scattered vesicles along the fingers, arms, hands, abdomen, back, legs, and feet (Figure 1). The Bullous Pemphigoid Disease Area Index (BPDAI) total skin activity score was 25.3, corresponding to moderate disease activity (validated at 20–56).2 The BPDAI total pruritus component score was 20. A repeat biopsy for direct immunofluorescence showed faint linear deposits of IgG and bright linear deposits of C3 along the basement membrane zone. Indirect immunofluorescence showed linear deposits of IgG localized to the blister roof of salt-split skin at a dilution of 1:40. An enzyme-linked immunosorbent assay for anti-BP180 was 62 U/mL (negative, <9 U/mL; positive, ≥9 U/mL), and anti-BP230 autoantibodies were less than 9 U/mL (negative <9 U/mL; positive, ≥9 U/mL). Given these clinical and histopathologic findings, PG was diagnosed.

The patient was started on prednisone 20 mg and antihistamines while continuing topical steroids. Pruritus and blistering improved close to delivery. Fetal monitoring with regular biophysical profiles remained normal. The patient delivered a healthy neonate without skin lesions at 40 weeks’ gestation. The disease flared 2 days after delivery, and prednisone was increased to 40 mg and slowly tapered. Two months after delivery, the patient remained on prednisone 10 mg daily with ongoing but reduced blistering and pruritus (Figure 2). The BPDAI total skin activity and pruritus component scores remained elevated at 20.3 and 14, respectively, and anti-BP180 was 44 U/mL. After a discussion with the patient on safe systemic therapy while breastfeeding, intravenous immunoglobulin (IVIG) was initiated. The patient received 3 monthly infusions at 2 g/kg and was able to taper the prednisone to 5 mg every other day without new lesions. Four months after completion of IVIG therapy, she achieved complete remission off all therapy.

Management of PG begins with topical corticosteroids, but most patients require systemic steroid therapy.1 Remission commonly occurs close to delivery, and 75% of patients flare post partum, though the disease typically resolves 6 months following delivery.1,3,4 For persistent intrapartum cases requiring more than prednisone 20 mg daily, therapy can include dapsone, IVIG, azathioprine, rituximab, or plasmapheresis.4,5 Dapsone and IVIG are compatible with breastfeeding postpartum, but if dapsone is selected, the infant must be monitored for hemolytic anemia.5 Pemphigoid gestationis increases the risk for a premature or small-for-gestational-age neonate, necessitating regular fetal monitoring until delivery.1 Cutaneous lesions may affect the newborn, though this occurrence is rare and self-limiting.6 Pemphigoid gestationis may recur in subsequent pregnancies at a rate of 33% to 55%, with earlier and more severe presentations.4
Clinically and histologically, PG closely resembles bullous pemphigoid (BP), but the exact pathogenesis is not fully understood. Recently, another case of what was termed pseudo-PG has been described 3 days following administration of the second dose of the Pfizer-BioNTech COVID-19 vaccine.7 Since the introduction of COVID-19 mRNA vaccines, cases of postvaccination BP, BP-like eruptions, and pemphigus vulgaris have been described.8-11 Tomayko et al10 reported 12 cases of subepidermal eruptions, including BP, in which 7 patients developed blisters after the second dose of either the Pfizer-BioNTech or Moderna mRNA vaccine. Three patients who developed BP after the first dose of the vaccine and chose to receive the second dose tolerated it well, with a mild flare observed in 1 patient.10 Similarly, subsequent vaccine doses in reports of vaccine-associated AIBD resulted in increased disease activity in 21% of cases.12 COVID-19 vaccine–associated BP, similar to drug-induced BP, seemingly displays a milder course of disease compared to the classic form of BP.10,13 More follow-up is needed to better understand these reactions and inform appropriate discussions on the administration of booster doses. Currently, completion of the vaccination series against COVID-19 is advisable given the paucity of reports of postvaccination AIBD and the risk for COVID-19 infection, but careful discussions on a case-by-case basis are warranted related to the risk for disease exacerbation following subsequent vaccinations.
The clinical presentation and diagnostic evaluation of our patient’s rash were consistent with PG. The temporal relationship between vaccine administration and PG lesion onset suggests the mRNA vaccine triggered AIBD in our patient. Interestingly, AIBD associated with COVID-19 is not unique to only the vaccines and has been observed following infection with the virus itself.14 The high rate of vaccination against COVID-19 in contrast with the low number of reported cases of AIBD after vaccination supports the overall safety of COVID-19 vaccines but identifies a need for further understanding of the processes that lead to the development of autoimmune conditions in at-risk populations.
To the Editor:
Pemphigoid gestationis (PG), or gestational pemphigoid, is a rare autoimmune bullous disease (AIBD) occurring in 1 in 50,000 pregnancies. It is characterized by abrupt development of intensely pruritic papules and urticarial plaques, followed by an eruption of blisters.1 We present a case of new-onset PG that erupted 10 days following SARs-CoV-2 messenger RNA (mRNA) vaccination with BNT162b2 (Pfizer-BioNTech).
A 36-year-old pregnant woman (gravida 1, para 0, aborta 0) at 37 weeks’ gestation presented to our AIBD clinic with a pruritic dermatitis of 6 weeks’ duration that developed 10 days after receiving the second dose of BNT162b2. Multiple intensely pruritic, red bumps presented first on the forearms and within days spread to the thighs, hands, and abdomen, followed by progression to the ankles, feet, and back 2 weeks later. An initial biopsy was consistent with subacute spongiotic dermatitis with rare eosinophils. She found minimal relief from diphenhydramine or topical steroids. She denied oral, nasal, ocular, or genital involvement or history of any other skin disease. The pregnancy had been otherwise uneventful.
Physical examination revealed annular edematous plaques on the trunk and buttocks; excoriated and erythematous papules on the neck, trunk, arms, and legs; and scattered vesicles along the fingers, arms, hands, abdomen, back, legs, and feet (Figure 1). The Bullous Pemphigoid Disease Area Index (BPDAI) total skin activity score was 25.3, corresponding to moderate disease activity (validated at 20–56).2 The BPDAI total pruritus component score was 20. A repeat biopsy for direct immunofluorescence showed faint linear deposits of IgG and bright linear deposits of C3 along the basement membrane zone. Indirect immunofluorescence showed linear deposits of IgG localized to the blister roof of salt-split skin at a dilution of 1:40. An enzyme-linked immunosorbent assay for anti-BP180 was 62 U/mL (negative, <9 U/mL; positive, ≥9 U/mL), and anti-BP230 autoantibodies were less than 9 U/mL (negative <9 U/mL; positive, ≥9 U/mL). Given these clinical and histopathologic findings, PG was diagnosed.

The patient was started on prednisone 20 mg and antihistamines while continuing topical steroids. Pruritus and blistering improved close to delivery. Fetal monitoring with regular biophysical profiles remained normal. The patient delivered a healthy neonate without skin lesions at 40 weeks’ gestation. The disease flared 2 days after delivery, and prednisone was increased to 40 mg and slowly tapered. Two months after delivery, the patient remained on prednisone 10 mg daily with ongoing but reduced blistering and pruritus (Figure 2). The BPDAI total skin activity and pruritus component scores remained elevated at 20.3 and 14, respectively, and anti-BP180 was 44 U/mL. After a discussion with the patient on safe systemic therapy while breastfeeding, intravenous immunoglobulin (IVIG) was initiated. The patient received 3 monthly infusions at 2 g/kg and was able to taper the prednisone to 5 mg every other day without new lesions. Four months after completion of IVIG therapy, she achieved complete remission off all therapy.

Management of PG begins with topical corticosteroids, but most patients require systemic steroid therapy.1 Remission commonly occurs close to delivery, and 75% of patients flare post partum, though the disease typically resolves 6 months following delivery.1,3,4 For persistent intrapartum cases requiring more than prednisone 20 mg daily, therapy can include dapsone, IVIG, azathioprine, rituximab, or plasmapheresis.4,5 Dapsone and IVIG are compatible with breastfeeding postpartum, but if dapsone is selected, the infant must be monitored for hemolytic anemia.5 Pemphigoid gestationis increases the risk for a premature or small-for-gestational-age neonate, necessitating regular fetal monitoring until delivery.1 Cutaneous lesions may affect the newborn, though this occurrence is rare and self-limiting.6 Pemphigoid gestationis may recur in subsequent pregnancies at a rate of 33% to 55%, with earlier and more severe presentations.4
Clinically and histologically, PG closely resembles bullous pemphigoid (BP), but the exact pathogenesis is not fully understood. Recently, another case of what was termed pseudo-PG has been described 3 days following administration of the second dose of the Pfizer-BioNTech COVID-19 vaccine.7 Since the introduction of COVID-19 mRNA vaccines, cases of postvaccination BP, BP-like eruptions, and pemphigus vulgaris have been described.8-11 Tomayko et al10 reported 12 cases of subepidermal eruptions, including BP, in which 7 patients developed blisters after the second dose of either the Pfizer-BioNTech or Moderna mRNA vaccine. Three patients who developed BP after the first dose of the vaccine and chose to receive the second dose tolerated it well, with a mild flare observed in 1 patient.10 Similarly, subsequent vaccine doses in reports of vaccine-associated AIBD resulted in increased disease activity in 21% of cases.12 COVID-19 vaccine–associated BP, similar to drug-induced BP, seemingly displays a milder course of disease compared to the classic form of BP.10,13 More follow-up is needed to better understand these reactions and inform appropriate discussions on the administration of booster doses. Currently, completion of the vaccination series against COVID-19 is advisable given the paucity of reports of postvaccination AIBD and the risk for COVID-19 infection, but careful discussions on a case-by-case basis are warranted related to the risk for disease exacerbation following subsequent vaccinations.
The clinical presentation and diagnostic evaluation of our patient’s rash were consistent with PG. The temporal relationship between vaccine administration and PG lesion onset suggests the mRNA vaccine triggered AIBD in our patient. Interestingly, AIBD associated with COVID-19 is not unique to only the vaccines and has been observed following infection with the virus itself.14 The high rate of vaccination against COVID-19 in contrast with the low number of reported cases of AIBD after vaccination supports the overall safety of COVID-19 vaccines but identifies a need for further understanding of the processes that lead to the development of autoimmune conditions in at-risk populations.
- Wiznia LE, Pomeranz MK. Skin changes and diseases in pregnancy. In: Kang S, Amagai M, Bruckner AL, et al, eds. Fitzpatrick’s Dermatology. 9th ed. McGraw-Hill Education; 2019.
- Masmoudi W, Vaillant M, Vassileva S, et al. International validation of the Bullous Pemphigoid Disease Area Index severity score and calculation of cut-off values for defining mild, moderate and severe types of bullous pemphigoid. Br J Dermatol. 2021;184:1106-1112. doi:10.1111/bjd.19611
- Semkova K, Black M. Pemphigoid gestationis: current insights into pathogenesis and treatment. Eur J Obstet Gynecol Reprod Biol. 2009;145:138-144.
- Savervall C, Sand FL, Thomsen SF. Pemphigoid gestationis: current perspectives. Clin Cosmet Investig Dermatol. 2017;10:441-449.
- Braunstein I, Werth V. Treatment of dermatologic connective tissue disease and autoimmune blistering disorders in pregnancy. Dermatol Ther. 2013;26:354-363.
- Lipozencic J, Ljubojevic S, Bukvic-Mokos Z. Pemphigoid gestationis. Clin Dermatol. 2012;30:51-55.
- de Lorenzi C, Kaya G, Toutous Trellu L. Pseudo-pemphigoid gestationis eruption following SARS-CoV-2 vaccination with mRNA vaccine. Dermatopathology (Basel). 2022;9:203-206. doi:10.3390/dermatopathology9030025
- McMahon DE, Kovarik CL, Damsky W, et al. Clinical and pathologic correlation of cutaneous COVID-19 vaccine reactions including V-REPP: a registry-based study. J Am Acad Dermatol. 2022;86:113-121.
- Solimani F, Mansour Y, Didona D, et al. Development of severe pemphigus vulgaris following SARS-CoV-2 vaccination with BNT162b2. J Eur Acad Dermatol Venereol. 2021;35:E649-E651.
- Tomayko MM, Damsky W, Fathy R, et al. Subepidermal blistering eruptions, including bullous pemphigoid, following COVID-19 vaccination. J Allergy Clin Immunol. 2021;148:750-751.
- Coto-Segura P, Fernandez-Prada M, Mir-Bonafe M, et al. Vesiculobullous skin reactions induced by COVID-19 mRNA vaccine: report of four cases and review of the literature. Clin Exp Dermatol. 2022;47:141-143.
- Kasperkiewicz M, Woodley DT. Association between vaccination and autoimmune bullous diseases: a systematic review. J Am Acad Dermatol. 2022;86:1160-1164.
- Stavropoulos PG, Soura E, Antoniou C. Drug-induced pemphigoid: a review of the literature. J Eur Acad Dermatol Venereol. 2014;28:1133-1140.
- Olson N, Eckhardt D, Delano A. New-onset bullous pemphigoid in a COVID-19 patient. Case Rep Dermatol Med. 2021;2021:5575111.
- Wiznia LE, Pomeranz MK. Skin changes and diseases in pregnancy. In: Kang S, Amagai M, Bruckner AL, et al, eds. Fitzpatrick’s Dermatology. 9th ed. McGraw-Hill Education; 2019.
- Masmoudi W, Vaillant M, Vassileva S, et al. International validation of the Bullous Pemphigoid Disease Area Index severity score and calculation of cut-off values for defining mild, moderate and severe types of bullous pemphigoid. Br J Dermatol. 2021;184:1106-1112. doi:10.1111/bjd.19611
- Semkova K, Black M. Pemphigoid gestationis: current insights into pathogenesis and treatment. Eur J Obstet Gynecol Reprod Biol. 2009;145:138-144.
- Savervall C, Sand FL, Thomsen SF. Pemphigoid gestationis: current perspectives. Clin Cosmet Investig Dermatol. 2017;10:441-449.
- Braunstein I, Werth V. Treatment of dermatologic connective tissue disease and autoimmune blistering disorders in pregnancy. Dermatol Ther. 2013;26:354-363.
- Lipozencic J, Ljubojevic S, Bukvic-Mokos Z. Pemphigoid gestationis. Clin Dermatol. 2012;30:51-55.
- de Lorenzi C, Kaya G, Toutous Trellu L. Pseudo-pemphigoid gestationis eruption following SARS-CoV-2 vaccination with mRNA vaccine. Dermatopathology (Basel). 2022;9:203-206. doi:10.3390/dermatopathology9030025
- McMahon DE, Kovarik CL, Damsky W, et al. Clinical and pathologic correlation of cutaneous COVID-19 vaccine reactions including V-REPP: a registry-based study. J Am Acad Dermatol. 2022;86:113-121.
- Solimani F, Mansour Y, Didona D, et al. Development of severe pemphigus vulgaris following SARS-CoV-2 vaccination with BNT162b2. J Eur Acad Dermatol Venereol. 2021;35:E649-E651.
- Tomayko MM, Damsky W, Fathy R, et al. Subepidermal blistering eruptions, including bullous pemphigoid, following COVID-19 vaccination. J Allergy Clin Immunol. 2021;148:750-751.
- Coto-Segura P, Fernandez-Prada M, Mir-Bonafe M, et al. Vesiculobullous skin reactions induced by COVID-19 mRNA vaccine: report of four cases and review of the literature. Clin Exp Dermatol. 2022;47:141-143.
- Kasperkiewicz M, Woodley DT. Association between vaccination and autoimmune bullous diseases: a systematic review. J Am Acad Dermatol. 2022;86:1160-1164.
- Stavropoulos PG, Soura E, Antoniou C. Drug-induced pemphigoid: a review of the literature. J Eur Acad Dermatol Venereol. 2014;28:1133-1140.
- Olson N, Eckhardt D, Delano A. New-onset bullous pemphigoid in a COVID-19 patient. Case Rep Dermatol Med. 2021;2021:5575111.
Practice Points
- Dermatologists should be aware that COVID-19 messenger RNA vaccinations may present with various cutaneous complications.
- Pemphigoid gestationis should be considered in a pregnant or postpartum woman with an unexplained eruption of persistent, pruritic, urticarial lesions and blisters occurring postvaccination. Treatments include high-potency topical steroids and frequently systemic corticosteroids, along with steroid-sparing agents in severe cases.
Papular Reticulated Rash
The Diagnosis: Prurigo Pigmentosa
Histopathology of the punch biopsy revealed subcorneal collections of neutrophils flanked by a spongiotic epidermis with neutrophil and eosinophil exocytosis. Rare dyskeratotic keratinocytes were identified at the dermoepidermal junction, and grampositive bacterial organisms were seen in a follicular infundibulum with purulent inflammation. The dermis demonstrated a mildly dense superficial perivascular and interstitial infiltrate composed of lymphocytes, histiocytes, scattered neutrophils, and eosinophils (Figure).
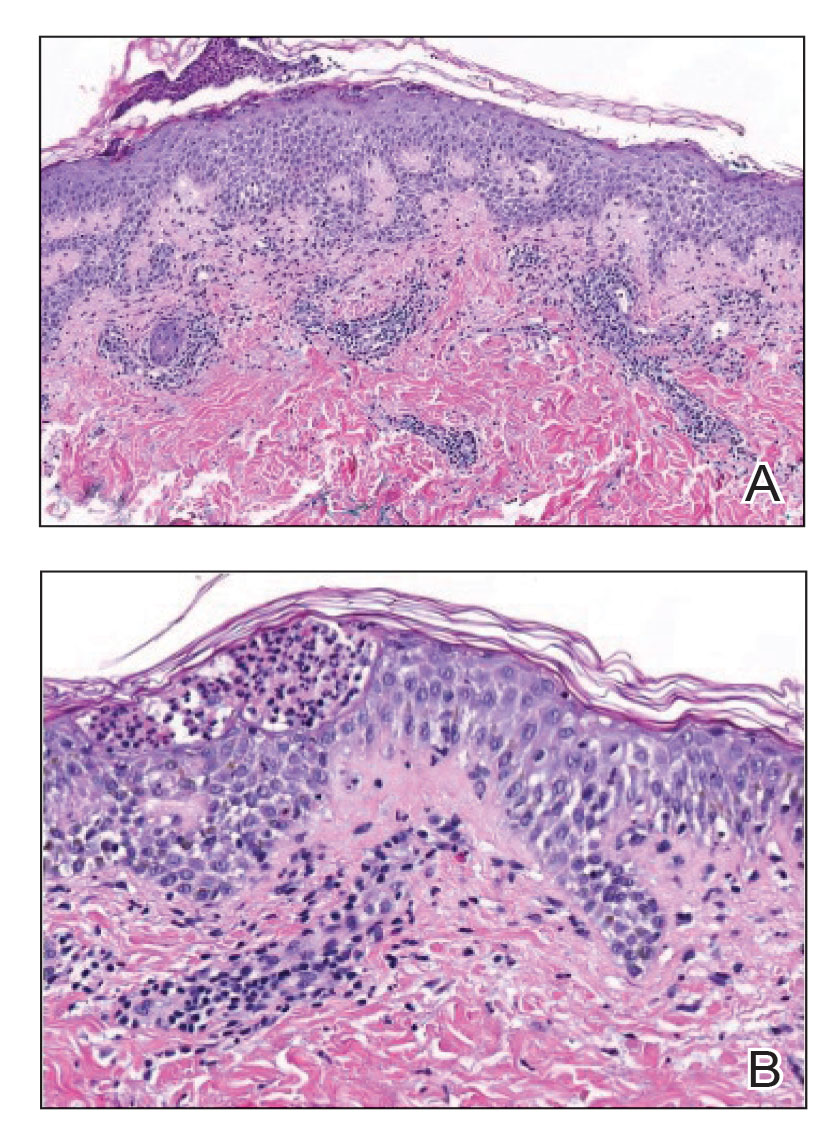
Given the combination of clinical and histologic findings, a diagnosis of prurigo pigmentosa (PP) was rendered and a urinalysis was ordered, which confirmed ketonuria. The patient was started on minocycline 100 mg twice daily and was advised to reintroduce carbohydrates into her diet. Resolution of the inflammatory component of the rash was achieved at 3-week follow-up, with residual reticulated postinflammatory hyperpigmentation.
Prurigo pigmentosa is a rare, albeit globally underrecognized, inflammatory dermatosis characterized by pruritic, symmetric, erythematous papules and plaques on the chest, back, neck, and rarely the arms and forehead that subsequently involute, leaving reticular postinflammatory hyperpigmentation.1 Prurigo pigmentosa is predominant in females (2.6:1 ratio). The mean age at presentation is 24.4 years, and it most commonly has been documented among populations in Asian countries, though it is unclear if a genetic predilection exists, as reports of PP are increasing globally with improved clinical awareness.1,2
The etiology of PP remains unknown; however, associations are well documented between PP and a ketogenic state secondary to uncontrolled diabetes, a low-carbohydrate diet, anorexia nervosa, or bariatric surgery.3 It is theorized that high serum ketones lead to perivascular ketone deposition, which induces neutrophil migration and chemotaxis,4 as substantiated by evidence of rash resolution with correction of the ketogenic state and improvement after administration of tetracyclines, a drug class known for neutrophil chemotaxis inhibition.5 Improvement of PP via these treatment mechanisms suggests that ketone bodies may play a role in the pathogenesis of PP.
Interestingly, Kafle et al6 reported that patients with PP commonly have bacterial colonies and associated inflammatory sequelae at the level of the hair follicles, which suggests that follicular involvement plays a role in the pathogenesis of PP. These findings are consistent with our patient’s histopathology consisting of gram-positive organisms and purulent inflammation at the infundibulum. The histopathologic features of PP are stage specific.1 Early stages are characterized by a superficial perivascular infiltrate of neutrophils that then spread to dermal papillae. Neutrophils then quickly sweep through the epidermis, causing spongiosis, ballooning, necrotic keratocytes, and consequent surface epithelium abscess formation. Over time, the dermal infiltrate assumes a lichenoid pattern as eosinophils and lymphocytes invade and predominate over neutrophils. Eventually, melanophages appear in the dermis as the epidermis undergoes hyperplasia, parakeratosis, and hyperpigmentation.1 The histologic differential diagnosis for PP is broad and varies based on the stage-specific progression of clinical and histopathologic findings.
Similar to PP, subacute cutaneous lupus erythematosus has a female predominance and resolves with subsequent dyspigmentation; however, it initially is characterized by annular plaques with central clearing or papulosquamous lesions restricted to sun-exposed skin. Photosensitivity is a prominent feature, and roughly 50% of patients meet diagnostic criteria for systemic lupus erythematosus.7 Histopathology shows interface changes with increased dermal mucin and a perivascular lymphoplasmacytic inflammatory infiltrate.
Papular pityriasis rosea can present as a pruritic papular rash on the back and chest; however, it most commonly is associated with a herald patch and typically follows a flulike prodrome.8 Biopsy reveals mounds of parakeratosis with mild spongiosis, perivascular inflammation, and extravasated erythrocytes.
Galli-Galli disease can present as a pruritic rash with follicular papules under the breasts and other flexural areas but histopathologically shows elongated rete ridges with dermal melanosis and acantholysis.9
Hailey-Hailey disease commonly presents in the third decade of life and can manifest as painful, pruritic, vesicular lesions on erythematous skin distributed on the back, neck, and inframammary region, as seen in our case; however, it is histopathologically associated with widespread epidermal acantholysis unlike the findings seen in our patient.10
First-line treatment of PP includes antibiotics such as minocycline, doxycycline, and dapsone due to their anti-inflammatory properties and ability to inhibit neutrophil chemotaxis. In patients with nutritional deficiencies or ketosis, reintroduction of carbohydrates alone has been effective.5,11
Prurigo pigmentosa is an underrecognized inflammatory dermatosis with a complex stage-dependent clinicopathologic presentation. Clinicians should be aware of the etiologic and histopathologic patterns of this unique dermatosis. Rash presentation in the context of a low-carbohydrate diet should prompt biopsy as well as treatment with antibiotics and dietary reintroduction of carbohydrates.
- Böer A, Misago N, Wolter M, et al. Prurigo pigmentosa: a distinctive inflammatory disease of the skin. Am J Dermatopathol. 2003;25:117-129. doi:10.1097/00000372-200304000-00005
- de Sousa Vargas TJ, Abreu Raposo CM, Lima RB, et al. Prurigo pigmentosa: report of 3 cases from Brazil and literature review. Am J Dermatopathol. 2017;39:267-274. doi:10.1097/DAD.0000000000000643
- Mufti A, Mirali S, Abduelmula A, et al. Clinical manifestations and treatment outcomes in prurigo pigmentosa (Nagashima disease): a systematic review of the literature. JAAD Int. 2021;3:79. doi:10.1016/J .JDIN.2021.03.003
- Beutler BD, Cohen PR, Lee RA. Prurigo pigmentosa: literature review. Am J Clin Dermatol. 2015;16:533-543. doi:10.1007/S40257-015-0154-4
- Chiam LYT, Goh BK, Lim KS, et al. Prurigo pigmentosa: a report of two cases that responded to minocycline. Clin Exp Dermatol. 2009;34. doi:10.1111/J.1365-2230.2009.03253.X
- Kafle SU, Swe SM, Hsiao PF, et al. Folliculitis in prurigo pigmentosa: a proposed pathogenesis based on clinical and pathological observation. J Cutan Pathol. 2017;44:20-27. doi:10.1111/CUP.12829
- Sontheimer RD. Subacute cutaneous lupus erythematosus: 25-year evolution of a prototypic subset (subphenotype) of lupus erythematosus defined by characteristic cutaneous, pathological, immunological, and genetic findings. Autoimmun Rev. 2005;4:253-263. doi:10.1016/J .AUTREV.2004.10.00
- Leung AKC, Lam JM, Leong KF, et al. Pityriasis rosea: an updated review. Curr Pediatr Rev. 2021;17:201-211. doi:10.2174/15733963166662 00923161330
- Sprecher E, Indelman M, Khamaysi Z, et al. Galli-Galli disease is an acantholytic variant of Dowling-Degos disease. Br J Dermatol. 2007;156:572-574. doi:10.1111/J.1365-2133.2006.07703.X
- Burge SM. Hailey-Hailey disease: the clinical features, response to treatment and prognosis. Br J Dermatol. 1992;126:275-282. doi:10.1111/J.1365-2133.1992.TB00658
- Lu L-Y, Chen C-B. Keto rash: ketoacidosis-induced prurigo pigmentosa. Mayo Clin Proc. 2022;97:20-21. doi:10.1016/j.mayocp.2021.11.019
The Diagnosis: Prurigo Pigmentosa
Histopathology of the punch biopsy revealed subcorneal collections of neutrophils flanked by a spongiotic epidermis with neutrophil and eosinophil exocytosis. Rare dyskeratotic keratinocytes were identified at the dermoepidermal junction, and grampositive bacterial organisms were seen in a follicular infundibulum with purulent inflammation. The dermis demonstrated a mildly dense superficial perivascular and interstitial infiltrate composed of lymphocytes, histiocytes, scattered neutrophils, and eosinophils (Figure).

Given the combination of clinical and histologic findings, a diagnosis of prurigo pigmentosa (PP) was rendered and a urinalysis was ordered, which confirmed ketonuria. The patient was started on minocycline 100 mg twice daily and was advised to reintroduce carbohydrates into her diet. Resolution of the inflammatory component of the rash was achieved at 3-week follow-up, with residual reticulated postinflammatory hyperpigmentation.
Prurigo pigmentosa is a rare, albeit globally underrecognized, inflammatory dermatosis characterized by pruritic, symmetric, erythematous papules and plaques on the chest, back, neck, and rarely the arms and forehead that subsequently involute, leaving reticular postinflammatory hyperpigmentation.1 Prurigo pigmentosa is predominant in females (2.6:1 ratio). The mean age at presentation is 24.4 years, and it most commonly has been documented among populations in Asian countries, though it is unclear if a genetic predilection exists, as reports of PP are increasing globally with improved clinical awareness.1,2
The etiology of PP remains unknown; however, associations are well documented between PP and a ketogenic state secondary to uncontrolled diabetes, a low-carbohydrate diet, anorexia nervosa, or bariatric surgery.3 It is theorized that high serum ketones lead to perivascular ketone deposition, which induces neutrophil migration and chemotaxis,4 as substantiated by evidence of rash resolution with correction of the ketogenic state and improvement after administration of tetracyclines, a drug class known for neutrophil chemotaxis inhibition.5 Improvement of PP via these treatment mechanisms suggests that ketone bodies may play a role in the pathogenesis of PP.
Interestingly, Kafle et al6 reported that patients with PP commonly have bacterial colonies and associated inflammatory sequelae at the level of the hair follicles, which suggests that follicular involvement plays a role in the pathogenesis of PP. These findings are consistent with our patient’s histopathology consisting of gram-positive organisms and purulent inflammation at the infundibulum. The histopathologic features of PP are stage specific.1 Early stages are characterized by a superficial perivascular infiltrate of neutrophils that then spread to dermal papillae. Neutrophils then quickly sweep through the epidermis, causing spongiosis, ballooning, necrotic keratocytes, and consequent surface epithelium abscess formation. Over time, the dermal infiltrate assumes a lichenoid pattern as eosinophils and lymphocytes invade and predominate over neutrophils. Eventually, melanophages appear in the dermis as the epidermis undergoes hyperplasia, parakeratosis, and hyperpigmentation.1 The histologic differential diagnosis for PP is broad and varies based on the stage-specific progression of clinical and histopathologic findings.
Similar to PP, subacute cutaneous lupus erythematosus has a female predominance and resolves with subsequent dyspigmentation; however, it initially is characterized by annular plaques with central clearing or papulosquamous lesions restricted to sun-exposed skin. Photosensitivity is a prominent feature, and roughly 50% of patients meet diagnostic criteria for systemic lupus erythematosus.7 Histopathology shows interface changes with increased dermal mucin and a perivascular lymphoplasmacytic inflammatory infiltrate.
Papular pityriasis rosea can present as a pruritic papular rash on the back and chest; however, it most commonly is associated with a herald patch and typically follows a flulike prodrome.8 Biopsy reveals mounds of parakeratosis with mild spongiosis, perivascular inflammation, and extravasated erythrocytes.
Galli-Galli disease can present as a pruritic rash with follicular papules under the breasts and other flexural areas but histopathologically shows elongated rete ridges with dermal melanosis and acantholysis.9
Hailey-Hailey disease commonly presents in the third decade of life and can manifest as painful, pruritic, vesicular lesions on erythematous skin distributed on the back, neck, and inframammary region, as seen in our case; however, it is histopathologically associated with widespread epidermal acantholysis unlike the findings seen in our patient.10
First-line treatment of PP includes antibiotics such as minocycline, doxycycline, and dapsone due to their anti-inflammatory properties and ability to inhibit neutrophil chemotaxis. In patients with nutritional deficiencies or ketosis, reintroduction of carbohydrates alone has been effective.5,11
Prurigo pigmentosa is an underrecognized inflammatory dermatosis with a complex stage-dependent clinicopathologic presentation. Clinicians should be aware of the etiologic and histopathologic patterns of this unique dermatosis. Rash presentation in the context of a low-carbohydrate diet should prompt biopsy as well as treatment with antibiotics and dietary reintroduction of carbohydrates.
The Diagnosis: Prurigo Pigmentosa
Histopathology of the punch biopsy revealed subcorneal collections of neutrophils flanked by a spongiotic epidermis with neutrophil and eosinophil exocytosis. Rare dyskeratotic keratinocytes were identified at the dermoepidermal junction, and grampositive bacterial organisms were seen in a follicular infundibulum with purulent inflammation. The dermis demonstrated a mildly dense superficial perivascular and interstitial infiltrate composed of lymphocytes, histiocytes, scattered neutrophils, and eosinophils (Figure).

Given the combination of clinical and histologic findings, a diagnosis of prurigo pigmentosa (PP) was rendered and a urinalysis was ordered, which confirmed ketonuria. The patient was started on minocycline 100 mg twice daily and was advised to reintroduce carbohydrates into her diet. Resolution of the inflammatory component of the rash was achieved at 3-week follow-up, with residual reticulated postinflammatory hyperpigmentation.
Prurigo pigmentosa is a rare, albeit globally underrecognized, inflammatory dermatosis characterized by pruritic, symmetric, erythematous papules and plaques on the chest, back, neck, and rarely the arms and forehead that subsequently involute, leaving reticular postinflammatory hyperpigmentation.1 Prurigo pigmentosa is predominant in females (2.6:1 ratio). The mean age at presentation is 24.4 years, and it most commonly has been documented among populations in Asian countries, though it is unclear if a genetic predilection exists, as reports of PP are increasing globally with improved clinical awareness.1,2
The etiology of PP remains unknown; however, associations are well documented between PP and a ketogenic state secondary to uncontrolled diabetes, a low-carbohydrate diet, anorexia nervosa, or bariatric surgery.3 It is theorized that high serum ketones lead to perivascular ketone deposition, which induces neutrophil migration and chemotaxis,4 as substantiated by evidence of rash resolution with correction of the ketogenic state and improvement after administration of tetracyclines, a drug class known for neutrophil chemotaxis inhibition.5 Improvement of PP via these treatment mechanisms suggests that ketone bodies may play a role in the pathogenesis of PP.
Interestingly, Kafle et al6 reported that patients with PP commonly have bacterial colonies and associated inflammatory sequelae at the level of the hair follicles, which suggests that follicular involvement plays a role in the pathogenesis of PP. These findings are consistent with our patient’s histopathology consisting of gram-positive organisms and purulent inflammation at the infundibulum. The histopathologic features of PP are stage specific.1 Early stages are characterized by a superficial perivascular infiltrate of neutrophils that then spread to dermal papillae. Neutrophils then quickly sweep through the epidermis, causing spongiosis, ballooning, necrotic keratocytes, and consequent surface epithelium abscess formation. Over time, the dermal infiltrate assumes a lichenoid pattern as eosinophils and lymphocytes invade and predominate over neutrophils. Eventually, melanophages appear in the dermis as the epidermis undergoes hyperplasia, parakeratosis, and hyperpigmentation.1 The histologic differential diagnosis for PP is broad and varies based on the stage-specific progression of clinical and histopathologic findings.
Similar to PP, subacute cutaneous lupus erythematosus has a female predominance and resolves with subsequent dyspigmentation; however, it initially is characterized by annular plaques with central clearing or papulosquamous lesions restricted to sun-exposed skin. Photosensitivity is a prominent feature, and roughly 50% of patients meet diagnostic criteria for systemic lupus erythematosus.7 Histopathology shows interface changes with increased dermal mucin and a perivascular lymphoplasmacytic inflammatory infiltrate.
Papular pityriasis rosea can present as a pruritic papular rash on the back and chest; however, it most commonly is associated with a herald patch and typically follows a flulike prodrome.8 Biopsy reveals mounds of parakeratosis with mild spongiosis, perivascular inflammation, and extravasated erythrocytes.
Galli-Galli disease can present as a pruritic rash with follicular papules under the breasts and other flexural areas but histopathologically shows elongated rete ridges with dermal melanosis and acantholysis.9
Hailey-Hailey disease commonly presents in the third decade of life and can manifest as painful, pruritic, vesicular lesions on erythematous skin distributed on the back, neck, and inframammary region, as seen in our case; however, it is histopathologically associated with widespread epidermal acantholysis unlike the findings seen in our patient.10
First-line treatment of PP includes antibiotics such as minocycline, doxycycline, and dapsone due to their anti-inflammatory properties and ability to inhibit neutrophil chemotaxis. In patients with nutritional deficiencies or ketosis, reintroduction of carbohydrates alone has been effective.5,11
Prurigo pigmentosa is an underrecognized inflammatory dermatosis with a complex stage-dependent clinicopathologic presentation. Clinicians should be aware of the etiologic and histopathologic patterns of this unique dermatosis. Rash presentation in the context of a low-carbohydrate diet should prompt biopsy as well as treatment with antibiotics and dietary reintroduction of carbohydrates.
- Böer A, Misago N, Wolter M, et al. Prurigo pigmentosa: a distinctive inflammatory disease of the skin. Am J Dermatopathol. 2003;25:117-129. doi:10.1097/00000372-200304000-00005
- de Sousa Vargas TJ, Abreu Raposo CM, Lima RB, et al. Prurigo pigmentosa: report of 3 cases from Brazil and literature review. Am J Dermatopathol. 2017;39:267-274. doi:10.1097/DAD.0000000000000643
- Mufti A, Mirali S, Abduelmula A, et al. Clinical manifestations and treatment outcomes in prurigo pigmentosa (Nagashima disease): a systematic review of the literature. JAAD Int. 2021;3:79. doi:10.1016/J .JDIN.2021.03.003
- Beutler BD, Cohen PR, Lee RA. Prurigo pigmentosa: literature review. Am J Clin Dermatol. 2015;16:533-543. doi:10.1007/S40257-015-0154-4
- Chiam LYT, Goh BK, Lim KS, et al. Prurigo pigmentosa: a report of two cases that responded to minocycline. Clin Exp Dermatol. 2009;34. doi:10.1111/J.1365-2230.2009.03253.X
- Kafle SU, Swe SM, Hsiao PF, et al. Folliculitis in prurigo pigmentosa: a proposed pathogenesis based on clinical and pathological observation. J Cutan Pathol. 2017;44:20-27. doi:10.1111/CUP.12829
- Sontheimer RD. Subacute cutaneous lupus erythematosus: 25-year evolution of a prototypic subset (subphenotype) of lupus erythematosus defined by characteristic cutaneous, pathological, immunological, and genetic findings. Autoimmun Rev. 2005;4:253-263. doi:10.1016/J .AUTREV.2004.10.00
- Leung AKC, Lam JM, Leong KF, et al. Pityriasis rosea: an updated review. Curr Pediatr Rev. 2021;17:201-211. doi:10.2174/15733963166662 00923161330
- Sprecher E, Indelman M, Khamaysi Z, et al. Galli-Galli disease is an acantholytic variant of Dowling-Degos disease. Br J Dermatol. 2007;156:572-574. doi:10.1111/J.1365-2133.2006.07703.X
- Burge SM. Hailey-Hailey disease: the clinical features, response to treatment and prognosis. Br J Dermatol. 1992;126:275-282. doi:10.1111/J.1365-2133.1992.TB00658
- Lu L-Y, Chen C-B. Keto rash: ketoacidosis-induced prurigo pigmentosa. Mayo Clin Proc. 2022;97:20-21. doi:10.1016/j.mayocp.2021.11.019
- Böer A, Misago N, Wolter M, et al. Prurigo pigmentosa: a distinctive inflammatory disease of the skin. Am J Dermatopathol. 2003;25:117-129. doi:10.1097/00000372-200304000-00005
- de Sousa Vargas TJ, Abreu Raposo CM, Lima RB, et al. Prurigo pigmentosa: report of 3 cases from Brazil and literature review. Am J Dermatopathol. 2017;39:267-274. doi:10.1097/DAD.0000000000000643
- Mufti A, Mirali S, Abduelmula A, et al. Clinical manifestations and treatment outcomes in prurigo pigmentosa (Nagashima disease): a systematic review of the literature. JAAD Int. 2021;3:79. doi:10.1016/J .JDIN.2021.03.003
- Beutler BD, Cohen PR, Lee RA. Prurigo pigmentosa: literature review. Am J Clin Dermatol. 2015;16:533-543. doi:10.1007/S40257-015-0154-4
- Chiam LYT, Goh BK, Lim KS, et al. Prurigo pigmentosa: a report of two cases that responded to minocycline. Clin Exp Dermatol. 2009;34. doi:10.1111/J.1365-2230.2009.03253.X
- Kafle SU, Swe SM, Hsiao PF, et al. Folliculitis in prurigo pigmentosa: a proposed pathogenesis based on clinical and pathological observation. J Cutan Pathol. 2017;44:20-27. doi:10.1111/CUP.12829
- Sontheimer RD. Subacute cutaneous lupus erythematosus: 25-year evolution of a prototypic subset (subphenotype) of lupus erythematosus defined by characteristic cutaneous, pathological, immunological, and genetic findings. Autoimmun Rev. 2005;4:253-263. doi:10.1016/J .AUTREV.2004.10.00
- Leung AKC, Lam JM, Leong KF, et al. Pityriasis rosea: an updated review. Curr Pediatr Rev. 2021;17:201-211. doi:10.2174/15733963166662 00923161330
- Sprecher E, Indelman M, Khamaysi Z, et al. Galli-Galli disease is an acantholytic variant of Dowling-Degos disease. Br J Dermatol. 2007;156:572-574. doi:10.1111/J.1365-2133.2006.07703.X
- Burge SM. Hailey-Hailey disease: the clinical features, response to treatment and prognosis. Br J Dermatol. 1992;126:275-282. doi:10.1111/J.1365-2133.1992.TB00658
- Lu L-Y, Chen C-B. Keto rash: ketoacidosis-induced prurigo pigmentosa. Mayo Clin Proc. 2022;97:20-21. doi:10.1016/j.mayocp.2021.11.019
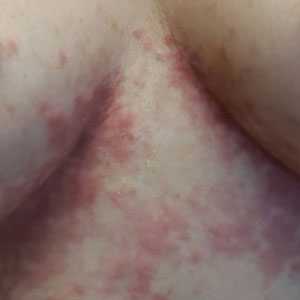
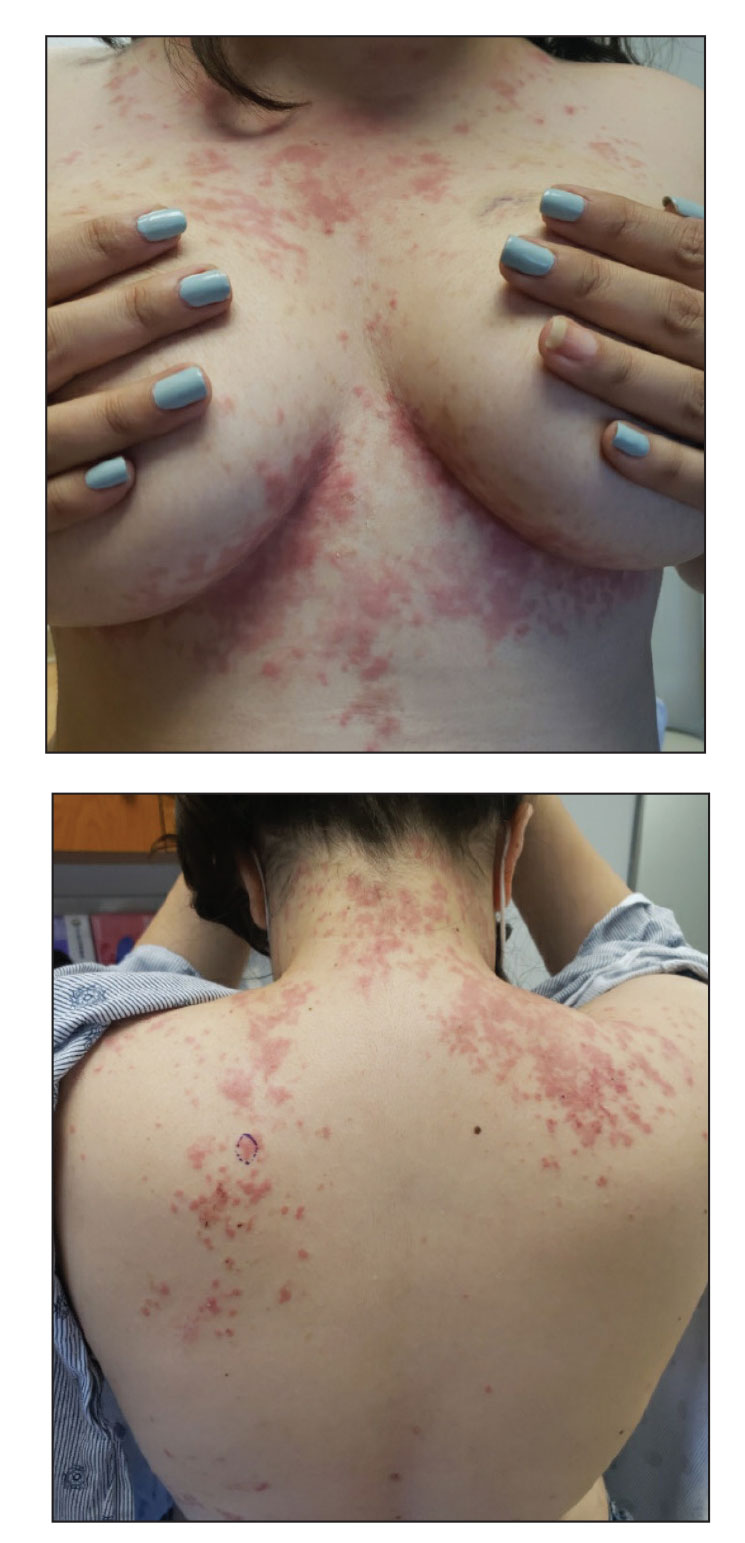
An otherwise healthy 22-year-old woman presented with a painful eruption with burning and pruritus that had been slowly worsening as it spread over the last 4 weeks. The rash first appeared on the lower chest and inframammary folds (top) and spread to the upper chest, neck, back (bottom), arms, and lower face. Physical examination revealed multiple illdefined, erythematous papules, patches, and plaques on the chest, back, neck, and upper abdomen. Individual lesions coalesced into plaques that displayed a reticular configuration. There were no lesions in the axillae. The patient had been following a low-carbohydrate diet for 4 months. A punch biopsy was performed.
Mohs surgery improves survival in early-stage Merkel cell carcinoma
SEATTLE – The use of
Compared with conventional wide local excision, survival was significantly improved among patients treated with Mohs, and a subgroup analysis showed that the survival benefit remained for patients with risk factors.
“At 10 years, overall survival was about 21% higher for those treated with Mohs surgery versus those treated with conventional surgery,” said lead author Shayan Cheraghlou, MD, a dermatology resident at the New York University School of Medicine. “On multivariable analysis, which controlled for tumor and patient factors, Mohs was associated with an over 40% reduction in the hazard for death.”
The findings were presented at the annual meeting of the American College of Mohs Surgery.
MCC is a rare, aggressive, neuroendocrine cutaneous malignancy that carries a high mortality rate. The estimated 5-year survival for patients with localized disease is about 50%, Dr. Cheraghlou noted. “That extrapolates to about 55% for T1 tumors and down to about 30% for T4 tumors.”
Although it’s considered to be a rare cancer, the incidence of MCC has been rapidly rising, and in fact it doubled during the period from the 1990s to the 2010s.
Most commonly treated with wide local excision with or without adjuvant radiation therapy, Mohs as monotherapy may offer an alternative treatment option for patients with MCC. It is generally accepted that the optimal treatment for tumors without regional lymph node involvement is surgical, but the data regarding the optimal surgical approach are mixed. Current National Comprehensive Cancer Network guidelines state that either Mohs surgery or wide local excision can be used.
“However, these guidelines do not indicate a preference for one modality over the other,” said Dr. Cheraghlou, “and present them as interchangeable treatment options.”
A growing body of literature supports Mohs surgery for many types of rare tumors, including MCC. For example, as previously reported at the 2021 ACMS meeting, one study found that Mohs surgery compared favorably with the standard treatment approach when it came to recurrence rates for patients with MCC. The 5-year disease-specific survival rate was 91.2% for patients with stage I disease and 68.6% for patients with stage IIa. These rates were comparable with rates for historical control patients who were treated with wide local excision, with or without radiation (81%-87% for stage I disease, and 63%-67% for stage II).
Study details
In the current study, Dr. Cheraghlou and colleagues sought to evaluate the association of the type of surgical approach with patient survival after excision of early-stage MCC. They conducted a retrospective cohort study using the National Cancer Database to identify cases of MCC with T1/T2 tumors. A total of 2,313 patients who were diagnosed from 2004 to 2018 with pathologically confirmed negative lymph node involvement and who were treated with Mohs surgery or wide lesion excision were included in the analysis.
“About 90% were T1 tumors, about 40% were located on the head and neck, and the vast majority – about 60% – were treated with wide local excision,” he explained. “Only about 5% received Mohs surgery for treatment of the primary tumor.”
But when the researchers assessed survival outcomes, they found that treatment with Mohs surgery was associated with significantly improved overall survival.
The unadjusted 3-, 5-, and 10-year survival rates for patients treated with Mohs was 87.4% (SE: 3.4%), 84.5% (SE: 3.9%), and 81.8% (SE: 4.6%), respectively, while for wide lesion excision, the rates were 86.1% (SE: 0.9%), 76.9% (SE: 1.2%), and 60.9% (SE: 2.0%), respectively.
For patients who underwent treatment with narrow margin excision, survival rates were similar as for those treated with wide lesion excision, with 3-, 5-, and 10-year survival rates of 84.8% (SE: 1.4%), 78.3% (SE: 1.7%), and 60.8% (SE: 3.6%), respectively.
On multivariable survival analysis, Mohs surgery was associated with significantly improved survival, compared with wide lesion excision (hazard ratio, 0.594; P = .038). This was also true after multivariable analysis for patients who had one or more NCCN risk factors, for whom improved survival was also seen with Mohs (HR, 0.530; P = .026).
The results did not differ after a sensitivity analysis that included T3 and T4 tumors.
Given that the use of Mohs was so infrequent, compared with standard surgery, the researchers investigated the factors that were associated with the use of Mohs. High-volume MCC centers were significantly more likely to utilize Mohs than wide lesion excision (odds ratio, 1.993; P < .001), compared with other facilities.
“This study has important implications going forward,” Dr. Cheraghlou concluded. “We think it’s important how few patients were treated with Mohs for Merkel cell, and it was slightly more likely to happen in a high-volume center.”
The reasoning for that may be that high-volume centers are more likely to have a surgeon trained to perform Mohs surgery for MCC. “Or perhaps they are more attuned to the benefits of this procedure,” he said. “We can’t tell that from our data, but its notable that it’s such a small proportion of patients – especially when we consider that it is associated with improved survival for the patients who receive it.”
He added that efforts to increase the utilization of Mohs may yield improved local control and overall survival for these patients. “And perhaps with more data, future versions of guidelines may indicate a preference for Mohs over conventional incisions.”
No changes to current practice
Asked to comment on the study, Anthony J. Olszanski, RPh, MD, associate professor, department of hematology/oncology, Fox Chase Cancer Center, Philadelphia, noted that while the results are intriguing, they must be interpreted with caution.
“This study was retrospective in nature, and unrecognized biases can influence results,” he said. “Additionally, given the relative rarity of Merkel cell carcinoma, the sample size is expectantly small.”
But importantly, Dr. Olszanski emphasized, Mohs may more often have been recommended for patients with lesions that appear less aggressive. “Many patients undergoing wide lesion excision may have been referred by Mohs surgeons secondary to features or characteristics of lesions which were worrisome,” he explained. “The results of this study do not opine on why Mohs would impact overall survival over wide lesion excision, a point worthy of consideration. Presently, both modalities can be considered for patients with T1/T2 MCC. The results of this study should not change current practice and would lend themselves to a more robust study.”
No external funding of the study was reported. Dr. Cheraghlou has disclosed no relevant financial relationships. Dr. Olszanski has received financial support from Merck and BMS for participated on advisory boards.
A version of this article originally appeared on Medscape.com.
SEATTLE – The use of
Compared with conventional wide local excision, survival was significantly improved among patients treated with Mohs, and a subgroup analysis showed that the survival benefit remained for patients with risk factors.
“At 10 years, overall survival was about 21% higher for those treated with Mohs surgery versus those treated with conventional surgery,” said lead author Shayan Cheraghlou, MD, a dermatology resident at the New York University School of Medicine. “On multivariable analysis, which controlled for tumor and patient factors, Mohs was associated with an over 40% reduction in the hazard for death.”
The findings were presented at the annual meeting of the American College of Mohs Surgery.
MCC is a rare, aggressive, neuroendocrine cutaneous malignancy that carries a high mortality rate. The estimated 5-year survival for patients with localized disease is about 50%, Dr. Cheraghlou noted. “That extrapolates to about 55% for T1 tumors and down to about 30% for T4 tumors.”
Although it’s considered to be a rare cancer, the incidence of MCC has been rapidly rising, and in fact it doubled during the period from the 1990s to the 2010s.
Most commonly treated with wide local excision with or without adjuvant radiation therapy, Mohs as monotherapy may offer an alternative treatment option for patients with MCC. It is generally accepted that the optimal treatment for tumors without regional lymph node involvement is surgical, but the data regarding the optimal surgical approach are mixed. Current National Comprehensive Cancer Network guidelines state that either Mohs surgery or wide local excision can be used.
“However, these guidelines do not indicate a preference for one modality over the other,” said Dr. Cheraghlou, “and present them as interchangeable treatment options.”
A growing body of literature supports Mohs surgery for many types of rare tumors, including MCC. For example, as previously reported at the 2021 ACMS meeting, one study found that Mohs surgery compared favorably with the standard treatment approach when it came to recurrence rates for patients with MCC. The 5-year disease-specific survival rate was 91.2% for patients with stage I disease and 68.6% for patients with stage IIa. These rates were comparable with rates for historical control patients who were treated with wide local excision, with or without radiation (81%-87% for stage I disease, and 63%-67% for stage II).
Study details
In the current study, Dr. Cheraghlou and colleagues sought to evaluate the association of the type of surgical approach with patient survival after excision of early-stage MCC. They conducted a retrospective cohort study using the National Cancer Database to identify cases of MCC with T1/T2 tumors. A total of 2,313 patients who were diagnosed from 2004 to 2018 with pathologically confirmed negative lymph node involvement and who were treated with Mohs surgery or wide lesion excision were included in the analysis.
“About 90% were T1 tumors, about 40% were located on the head and neck, and the vast majority – about 60% – were treated with wide local excision,” he explained. “Only about 5% received Mohs surgery for treatment of the primary tumor.”
But when the researchers assessed survival outcomes, they found that treatment with Mohs surgery was associated with significantly improved overall survival.
The unadjusted 3-, 5-, and 10-year survival rates for patients treated with Mohs was 87.4% (SE: 3.4%), 84.5% (SE: 3.9%), and 81.8% (SE: 4.6%), respectively, while for wide lesion excision, the rates were 86.1% (SE: 0.9%), 76.9% (SE: 1.2%), and 60.9% (SE: 2.0%), respectively.
For patients who underwent treatment with narrow margin excision, survival rates were similar as for those treated with wide lesion excision, with 3-, 5-, and 10-year survival rates of 84.8% (SE: 1.4%), 78.3% (SE: 1.7%), and 60.8% (SE: 3.6%), respectively.
On multivariable survival analysis, Mohs surgery was associated with significantly improved survival, compared with wide lesion excision (hazard ratio, 0.594; P = .038). This was also true after multivariable analysis for patients who had one or more NCCN risk factors, for whom improved survival was also seen with Mohs (HR, 0.530; P = .026).
The results did not differ after a sensitivity analysis that included T3 and T4 tumors.
Given that the use of Mohs was so infrequent, compared with standard surgery, the researchers investigated the factors that were associated with the use of Mohs. High-volume MCC centers were significantly more likely to utilize Mohs than wide lesion excision (odds ratio, 1.993; P < .001), compared with other facilities.
“This study has important implications going forward,” Dr. Cheraghlou concluded. “We think it’s important how few patients were treated with Mohs for Merkel cell, and it was slightly more likely to happen in a high-volume center.”
The reasoning for that may be that high-volume centers are more likely to have a surgeon trained to perform Mohs surgery for MCC. “Or perhaps they are more attuned to the benefits of this procedure,” he said. “We can’t tell that from our data, but its notable that it’s such a small proportion of patients – especially when we consider that it is associated with improved survival for the patients who receive it.”
He added that efforts to increase the utilization of Mohs may yield improved local control and overall survival for these patients. “And perhaps with more data, future versions of guidelines may indicate a preference for Mohs over conventional incisions.”
No changes to current practice
Asked to comment on the study, Anthony J. Olszanski, RPh, MD, associate professor, department of hematology/oncology, Fox Chase Cancer Center, Philadelphia, noted that while the results are intriguing, they must be interpreted with caution.
“This study was retrospective in nature, and unrecognized biases can influence results,” he said. “Additionally, given the relative rarity of Merkel cell carcinoma, the sample size is expectantly small.”
But importantly, Dr. Olszanski emphasized, Mohs may more often have been recommended for patients with lesions that appear less aggressive. “Many patients undergoing wide lesion excision may have been referred by Mohs surgeons secondary to features or characteristics of lesions which were worrisome,” he explained. “The results of this study do not opine on why Mohs would impact overall survival over wide lesion excision, a point worthy of consideration. Presently, both modalities can be considered for patients with T1/T2 MCC. The results of this study should not change current practice and would lend themselves to a more robust study.”
No external funding of the study was reported. Dr. Cheraghlou has disclosed no relevant financial relationships. Dr. Olszanski has received financial support from Merck and BMS for participated on advisory boards.
A version of this article originally appeared on Medscape.com.
SEATTLE – The use of
Compared with conventional wide local excision, survival was significantly improved among patients treated with Mohs, and a subgroup analysis showed that the survival benefit remained for patients with risk factors.
“At 10 years, overall survival was about 21% higher for those treated with Mohs surgery versus those treated with conventional surgery,” said lead author Shayan Cheraghlou, MD, a dermatology resident at the New York University School of Medicine. “On multivariable analysis, which controlled for tumor and patient factors, Mohs was associated with an over 40% reduction in the hazard for death.”
The findings were presented at the annual meeting of the American College of Mohs Surgery.
MCC is a rare, aggressive, neuroendocrine cutaneous malignancy that carries a high mortality rate. The estimated 5-year survival for patients with localized disease is about 50%, Dr. Cheraghlou noted. “That extrapolates to about 55% for T1 tumors and down to about 30% for T4 tumors.”
Although it’s considered to be a rare cancer, the incidence of MCC has been rapidly rising, and in fact it doubled during the period from the 1990s to the 2010s.
Most commonly treated with wide local excision with or without adjuvant radiation therapy, Mohs as monotherapy may offer an alternative treatment option for patients with MCC. It is generally accepted that the optimal treatment for tumors without regional lymph node involvement is surgical, but the data regarding the optimal surgical approach are mixed. Current National Comprehensive Cancer Network guidelines state that either Mohs surgery or wide local excision can be used.
“However, these guidelines do not indicate a preference for one modality over the other,” said Dr. Cheraghlou, “and present them as interchangeable treatment options.”
A growing body of literature supports Mohs surgery for many types of rare tumors, including MCC. For example, as previously reported at the 2021 ACMS meeting, one study found that Mohs surgery compared favorably with the standard treatment approach when it came to recurrence rates for patients with MCC. The 5-year disease-specific survival rate was 91.2% for patients with stage I disease and 68.6% for patients with stage IIa. These rates were comparable with rates for historical control patients who were treated with wide local excision, with or without radiation (81%-87% for stage I disease, and 63%-67% for stage II).
Study details
In the current study, Dr. Cheraghlou and colleagues sought to evaluate the association of the type of surgical approach with patient survival after excision of early-stage MCC. They conducted a retrospective cohort study using the National Cancer Database to identify cases of MCC with T1/T2 tumors. A total of 2,313 patients who were diagnosed from 2004 to 2018 with pathologically confirmed negative lymph node involvement and who were treated with Mohs surgery or wide lesion excision were included in the analysis.
“About 90% were T1 tumors, about 40% were located on the head and neck, and the vast majority – about 60% – were treated with wide local excision,” he explained. “Only about 5% received Mohs surgery for treatment of the primary tumor.”
But when the researchers assessed survival outcomes, they found that treatment with Mohs surgery was associated with significantly improved overall survival.
The unadjusted 3-, 5-, and 10-year survival rates for patients treated with Mohs was 87.4% (SE: 3.4%), 84.5% (SE: 3.9%), and 81.8% (SE: 4.6%), respectively, while for wide lesion excision, the rates were 86.1% (SE: 0.9%), 76.9% (SE: 1.2%), and 60.9% (SE: 2.0%), respectively.
For patients who underwent treatment with narrow margin excision, survival rates were similar as for those treated with wide lesion excision, with 3-, 5-, and 10-year survival rates of 84.8% (SE: 1.4%), 78.3% (SE: 1.7%), and 60.8% (SE: 3.6%), respectively.
On multivariable survival analysis, Mohs surgery was associated with significantly improved survival, compared with wide lesion excision (hazard ratio, 0.594; P = .038). This was also true after multivariable analysis for patients who had one or more NCCN risk factors, for whom improved survival was also seen with Mohs (HR, 0.530; P = .026).
The results did not differ after a sensitivity analysis that included T3 and T4 tumors.
Given that the use of Mohs was so infrequent, compared with standard surgery, the researchers investigated the factors that were associated with the use of Mohs. High-volume MCC centers were significantly more likely to utilize Mohs than wide lesion excision (odds ratio, 1.993; P < .001), compared with other facilities.
“This study has important implications going forward,” Dr. Cheraghlou concluded. “We think it’s important how few patients were treated with Mohs for Merkel cell, and it was slightly more likely to happen in a high-volume center.”
The reasoning for that may be that high-volume centers are more likely to have a surgeon trained to perform Mohs surgery for MCC. “Or perhaps they are more attuned to the benefits of this procedure,” he said. “We can’t tell that from our data, but its notable that it’s such a small proportion of patients – especially when we consider that it is associated with improved survival for the patients who receive it.”
He added that efforts to increase the utilization of Mohs may yield improved local control and overall survival for these patients. “And perhaps with more data, future versions of guidelines may indicate a preference for Mohs over conventional incisions.”
No changes to current practice
Asked to comment on the study, Anthony J. Olszanski, RPh, MD, associate professor, department of hematology/oncology, Fox Chase Cancer Center, Philadelphia, noted that while the results are intriguing, they must be interpreted with caution.
“This study was retrospective in nature, and unrecognized biases can influence results,” he said. “Additionally, given the relative rarity of Merkel cell carcinoma, the sample size is expectantly small.”
But importantly, Dr. Olszanski emphasized, Mohs may more often have been recommended for patients with lesions that appear less aggressive. “Many patients undergoing wide lesion excision may have been referred by Mohs surgeons secondary to features or characteristics of lesions which were worrisome,” he explained. “The results of this study do not opine on why Mohs would impact overall survival over wide lesion excision, a point worthy of consideration. Presently, both modalities can be considered for patients with T1/T2 MCC. The results of this study should not change current practice and would lend themselves to a more robust study.”
No external funding of the study was reported. Dr. Cheraghlou has disclosed no relevant financial relationships. Dr. Olszanski has received financial support from Merck and BMS for participated on advisory boards.
A version of this article originally appeared on Medscape.com.
AT ACMS 2023
Study shifts burden of IgG4-related disease to women
The incidence and prevalence of IgG4-related disease each rose considerably from 2015 to 2019 in the United States, and the risk of death in those with the immune-mediated condition is about 2.5 times higher than those who are not affected, based on an analysis of claims data from commercially insured adults.
The first population-based study of IgG4-RD incidence, prevalence, and mortality establishes “key benchmarks for informing the diagnosis and management of patients” with a condition “that causes fibrosing inflammatory lesions at nearly any anatomic site,” and wasn’t initially described until 2001, Zachary S. Wallace, MD, and associates said in Annals of the Rheumatic Diseases.
The increases in incidence and prevalence likely reflected increased disease awareness, they suggested. Overall U.S. incidence was 1.2 per 100,000 person-years for the 5-year period of 2015-2019, rising 86% from 0.78 per 100,000 person-years to 1.45 in 2018 before dropping to 1.39 in 2019. The change in prevalence was even greater, increasing 122% from 2.41 per 100,000 persons in 2015 to 5.34 per 100,000 in 2019, the investigators said.
Previous studies had indicated that the majority of patients with IgG4-RD were male, but the current study, using Optum’s Clinformatics Data Mart, which includes commercial health plan and Medicare Advantage members in all 50 states, showed that both incidence and prevalence (see graph) were higher among women, noted Dr. Wallace of Massachusetts General Hospital, Boston, and associates. They identified 524 patients (57.6% female) in the database who met the criteria for IgG4-RD from Jan. 1, 2010, to Dec. 31, 2019.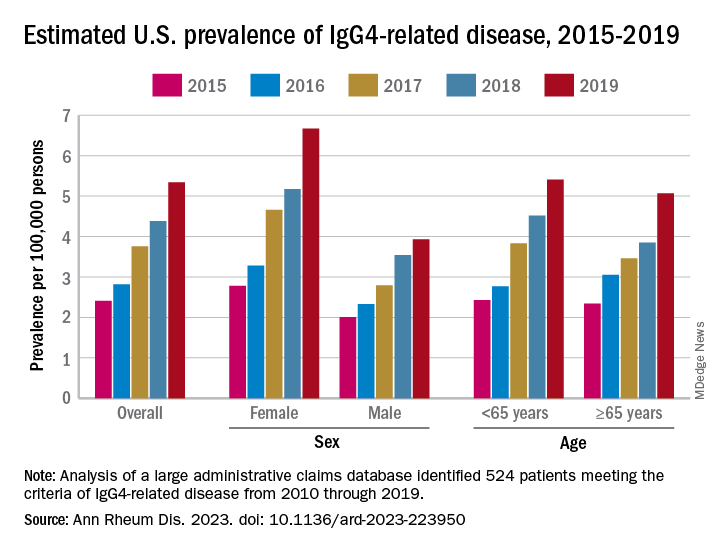
Incidence over the course of the study “was similar in patients identified as Asian or White but lower in those identified as Black or Hispanic,” they noted, adding that “the prevalence of IgG4-RD during this period reflected similar trends.” A jump in prevalence from 2018 to 2019, however, left White patients with a much higher rate (6.13 per 100,000 persons) than Asian patients (4.54 per 100,000), Black patients (3.42), and Hispanic patients (3.02).
For the mortality analysis, 516 patients with IgG4-RD were age-, sex-, and race-matched with 5,160 patients without IgG4-RD. Mortality was 3.42 and 1.46 per 100 person-years, respectively, over the 5.5 years of follow-up, so IgG4-RD was associated with a 2.5-fold higher risk of death. “The association of IgG4-RD with a higher risk of death was observed across the age spectrum and among both male and female patients,” the researchers said.
“Clinicians across specialties should be aware of IgG4-RD given the incidence, prevalence, and excess risk of death associated with this condition. ... Additional studies are urgently needed to define optimal management strategies to improve survival,” they wrote.
The study was supported by a grant to Massachusetts General Hospital from Sanofi, and Dr. Wallace received funding from the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the Rheumatology Research Foundation. He has received research support and consulting fees from several companies, and four coinvestigators are employees of Sanofi.
The incidence and prevalence of IgG4-related disease each rose considerably from 2015 to 2019 in the United States, and the risk of death in those with the immune-mediated condition is about 2.5 times higher than those who are not affected, based on an analysis of claims data from commercially insured adults.
The first population-based study of IgG4-RD incidence, prevalence, and mortality establishes “key benchmarks for informing the diagnosis and management of patients” with a condition “that causes fibrosing inflammatory lesions at nearly any anatomic site,” and wasn’t initially described until 2001, Zachary S. Wallace, MD, and associates said in Annals of the Rheumatic Diseases.
The increases in incidence and prevalence likely reflected increased disease awareness, they suggested. Overall U.S. incidence was 1.2 per 100,000 person-years for the 5-year period of 2015-2019, rising 86% from 0.78 per 100,000 person-years to 1.45 in 2018 before dropping to 1.39 in 2019. The change in prevalence was even greater, increasing 122% from 2.41 per 100,000 persons in 2015 to 5.34 per 100,000 in 2019, the investigators said.
Previous studies had indicated that the majority of patients with IgG4-RD were male, but the current study, using Optum’s Clinformatics Data Mart, which includes commercial health plan and Medicare Advantage members in all 50 states, showed that both incidence and prevalence (see graph) were higher among women, noted Dr. Wallace of Massachusetts General Hospital, Boston, and associates. They identified 524 patients (57.6% female) in the database who met the criteria for IgG4-RD from Jan. 1, 2010, to Dec. 31, 2019.
Incidence over the course of the study “was similar in patients identified as Asian or White but lower in those identified as Black or Hispanic,” they noted, adding that “the prevalence of IgG4-RD during this period reflected similar trends.” A jump in prevalence from 2018 to 2019, however, left White patients with a much higher rate (6.13 per 100,000 persons) than Asian patients (4.54 per 100,000), Black patients (3.42), and Hispanic patients (3.02).
For the mortality analysis, 516 patients with IgG4-RD were age-, sex-, and race-matched with 5,160 patients without IgG4-RD. Mortality was 3.42 and 1.46 per 100 person-years, respectively, over the 5.5 years of follow-up, so IgG4-RD was associated with a 2.5-fold higher risk of death. “The association of IgG4-RD with a higher risk of death was observed across the age spectrum and among both male and female patients,” the researchers said.
“Clinicians across specialties should be aware of IgG4-RD given the incidence, prevalence, and excess risk of death associated with this condition. ... Additional studies are urgently needed to define optimal management strategies to improve survival,” they wrote.
The study was supported by a grant to Massachusetts General Hospital from Sanofi, and Dr. Wallace received funding from the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the Rheumatology Research Foundation. He has received research support and consulting fees from several companies, and four coinvestigators are employees of Sanofi.
The incidence and prevalence of IgG4-related disease each rose considerably from 2015 to 2019 in the United States, and the risk of death in those with the immune-mediated condition is about 2.5 times higher than those who are not affected, based on an analysis of claims data from commercially insured adults.
The first population-based study of IgG4-RD incidence, prevalence, and mortality establishes “key benchmarks for informing the diagnosis and management of patients” with a condition “that causes fibrosing inflammatory lesions at nearly any anatomic site,” and wasn’t initially described until 2001, Zachary S. Wallace, MD, and associates said in Annals of the Rheumatic Diseases.
The increases in incidence and prevalence likely reflected increased disease awareness, they suggested. Overall U.S. incidence was 1.2 per 100,000 person-years for the 5-year period of 2015-2019, rising 86% from 0.78 per 100,000 person-years to 1.45 in 2018 before dropping to 1.39 in 2019. The change in prevalence was even greater, increasing 122% from 2.41 per 100,000 persons in 2015 to 5.34 per 100,000 in 2019, the investigators said.
Previous studies had indicated that the majority of patients with IgG4-RD were male, but the current study, using Optum’s Clinformatics Data Mart, which includes commercial health plan and Medicare Advantage members in all 50 states, showed that both incidence and prevalence (see graph) were higher among women, noted Dr. Wallace of Massachusetts General Hospital, Boston, and associates. They identified 524 patients (57.6% female) in the database who met the criteria for IgG4-RD from Jan. 1, 2010, to Dec. 31, 2019.
Incidence over the course of the study “was similar in patients identified as Asian or White but lower in those identified as Black or Hispanic,” they noted, adding that “the prevalence of IgG4-RD during this period reflected similar trends.” A jump in prevalence from 2018 to 2019, however, left White patients with a much higher rate (6.13 per 100,000 persons) than Asian patients (4.54 per 100,000), Black patients (3.42), and Hispanic patients (3.02).
For the mortality analysis, 516 patients with IgG4-RD were age-, sex-, and race-matched with 5,160 patients without IgG4-RD. Mortality was 3.42 and 1.46 per 100 person-years, respectively, over the 5.5 years of follow-up, so IgG4-RD was associated with a 2.5-fold higher risk of death. “The association of IgG4-RD with a higher risk of death was observed across the age spectrum and among both male and female patients,” the researchers said.
“Clinicians across specialties should be aware of IgG4-RD given the incidence, prevalence, and excess risk of death associated with this condition. ... Additional studies are urgently needed to define optimal management strategies to improve survival,” they wrote.
The study was supported by a grant to Massachusetts General Hospital from Sanofi, and Dr. Wallace received funding from the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the Rheumatology Research Foundation. He has received research support and consulting fees from several companies, and four coinvestigators are employees of Sanofi.
FROM ANNALS OF THE RHEUMATIC DISEASES
Axial spondyloarthritis versus axial psoriatic arthritis: Different entities?
Are there clinically significant differences between axial spondyloarthritis with psoriasis and psoriatic arthritis with axial symptoms? Does it matter?
It all depends on whom you ask, but right now the evidence seems to be tipping in favor of the “splitters” who cite evidence supporting their contention that axial spondyloarthritis (axSpA)/ankylosing spondylitis (AS) with psoriasis and psoriatic arthritis (PsA) with axial symptoms are distinct clinical entities that require more precise diagnosis and treatment.
“Lumpers,” in contrast, argue that they are different points on the same clinical spectrum.
The debate is not just of academic interest, but has real consequences for patients, say specialists on both sides of the aisle.
Overlapping features, different presentations
“Axial SpA and axPsA have overlapping features but also meaningful differences in genetics, clinical presentation, imaging, and immunophenotype. Efforts are underway to develop classification criteria for axPsA to aid research efforts as well as clinical diagnosis and management,” Philip J. Mease, MD, director of rheumatology research at Swedish Medical Center/Providence–St. Joseph Health in Seattle, and colleagues contend.
In an editorial published in the International Journal of Rheumatic Diseases, Dr. Mease and colleagues noted that, although HLA-B*27 is a genetic risk factor for both axPsA and axSpA, some HLA-B alleles are significantly associated with axPsA, whereas other alleles are associated with axSpA.
In addition, while genes in the interleukin-23 and IL-17 pathway are associated with increased risk for axSpA, genes in the IL-13 pathway have been identified as risk markers for axPsA, they noted.
Two cohorts better than one?
Dafna Gladman, MD, professor of medicine at the University of Toronto and senior scientist at the Schroeder Arthritis Institute at Toronto Western Hospital, and colleagues have a unique perspective on the similarities and differences between the disease entities.
Her group’s research uses data on cohorts of patients treated in two separate clinics at Toronto Western Hospital: one for patients with PsA, and one for patients with axial spondyloarthritis, including those with ankylosing spondylitis, nonradiographic axSpA, and spondylitis associated with inflammatory bowel disease.
“Our work has shown that there are differences, and one of the reasons that it’s now important is that the anti–IL-23 medications, both the IL-12/23 inhibitor ustekinumab [Stelara] and the IL-23 inhibitor guselkumab [Tremfya] work for psoriatic arthritis, whereas IL-23 did not work in ankylosing spondylitis, so that provided further impetus to look into the distinction between the two groups,” Dr. Gladman said in an interview.
Dr. Gladman and colleagues published a study in Rheumatology in which they compared clinical presentations and features of patients with AS with or without psoriasis with patients with axPsA.
They found that patients with AS with or without psoriasis tended to be younger, had a higher proportion of males to females, and were more likely to be positive for HLA-B*27. Patients with AS also had more back pain at presentation, worse axial disease activity scores, worse global assessments by physicians, and higher grades of sacroiliitis, and they were more likely to be taking biologic agents.
“What that showed, right off the top, that whether we’re looking at the total group or we’re looking specifically at those patients who have psoriasis or don’t have psoriasis, they are different from those with psoriatic arthritis with axial disease,” she said.
They concluded that “axPsA seems to be a distinct entity.”
Two clinics, same presentation
Because the aforementioned study included all patients with PsA with or without peripheral disease, the investigators decided to filter out some of the background noise and conduct a second study in which they compared patients who presented to the two clinics with the same presentation, either with spinal disease and psoriasis to the spondylitis clinic, or with psoriasis and isolated axial disease to the PsA clinic.
The results, published in Annals of the Rheumatic Diseases, showed that just 2.03% of patients with PsA had isolated axial disease, and an additional 29.38% had axial and peripheral disease.
In this study, “you can see that even in that group there are distinct differences. The patients that are labeled psoriatic spondylitis are different from those that are labeled ankylosing spondylitis with psoriasis,” Dr. Gladman said.
Isolated axial disease in patients with PsA was associated with HLA-B*27 positivity and lower Health Assessment Questionnaire scores. In addition, patients who were HLA-B*27 positive also had a nearly eightfold higher risk for developing peripheral disease over time.
Patients with isolated axial PsA were significantly more likely to be diagnosed at an older age (mean, 37.44 vs. 29.65 years), had higher Psoriasis Area Severity Index scores and a higher likelihood of having psoriatic nail lesions than patients with AS with isolated axial disease and psoriasis.
In contrast, patients with isolated axSpA with psoriasis were more likely to have inflammatory back pain, spinal pain, joint pain/swelling, and areas of localized tenderness, and they had greater severity of morning stiffness.
Dr. Gladman noted that, although AS and PsA are associated with the same gene that encodes for the IL-23 receptor, each condition is associated with a different single-nucleotide polymorphism.
Same disease, different flavors?
But as Mark Twain said, it is difference of opinion that makes horse races, and some specialists in rheumatology say that axSpA amd axPsA are just two sides of the same coin.
“There are always different schools of thought. I believe that they are not different diseases, but a spectrum of diseases,” said Shailendra Singh, MD, a rheumatologist at Unity Health Medical Center in Searcy, Ark., and past president of the Arkansas Rheumatology Association.
In an interview, Dr. Singh said that the spectrum ranges from diseases with primarily axial involvement, such as AS, to those with primarily peripheral involvement, such as reactive arthritis.
He pointed out that these conditions have overlapping symptoms, including enthesitis, dactylitis, and uveitis, and inflammatory arthritis.
Daniel Wendling, MD, PhD, from the Centre Hospitalier Régional Universitaire de Besançon (France), Université de Franche-Comté, and colleagues agreed.
“The criteria currently available for both SpA [ASAS (Assessment of Spondyloarthritis International Society) criteria] and PsA [CASPAR (Classification for Psoriatic Arthritis) criteria] are classification criteria, not diagnostic criteria. They are not very stringent and are not exclusive. Thus, the same patient can easily be classified simultaneously in both entities, making the distinction between axSpA with psoriasis and axPsA theoretical,” they wrote in an editorial published in Joint Bone Spine.
They cited as an example of the allegedly fuzzy criteria a prospective study conducted by the investigators in Bath, England, in which modified New York criteria for AS were met by 24% of patients with AS, and CASPAR criteria for PsA were met by an equal number of patients with AS.
Therapeutic implications
Dr. Wendling and colleagues acknowledge the differences cited in studies by Dr. Gladman, Dr. Mease, and others between patients with axPsA and those with axSpA, but argue that the differences are not that great and not so clear.
“It should also be emphasized that, although some differences between axPsA and axSpA reach statistical significance, they are mostly at the margin, with low odd ratios,” they wrote.
“It is also important to consider the variability in the definition of axPsA, sometimes simply ‘physician reported’ and elsewhere based on the modified New York radiographic criteria; the latter are only present late in the course of the disease, and this may induce bias,” they continued.
Dr. Singh agreed that, as noted by Dr. Gladman, some patients will respond to anti–IL-17, anti–IL-23, and anti–IL-12/23 agents, whereas others will have better responses with tumor necrosis factor (TNF) inhibitors, and still others, such as those with peripheral involvement in the hands and feet may fare better with nonbiologic disease-modifying antirheumatic drugs such as methotrexate.
Answers to come?
Dr. Gladman noted that the information available to date about the efficacy of IL-23 inhibition in axPsA is based on a post hoc analysis of the PSUMMIT 1 and 2 controlled trials in PsA, and is not definitive.
The randomized, controlled STAR trial, currently recruiting patients, is designed to see whether guselkumab can reduce axial symptoms and inflammation in patients with active axPsA.
“What I say is, there is a rationale for [anti–IL-23] to work in psoriatic arthritis, and not work in ankylosing spondylitis,” she said.
In contrast, IL-17 inhibitors, anti-TNF agents, and Janus kinase inhibitors show efficacy against both axPsA and AS. Rituximab is ineffective against PsA, but has shown efficacy against AS, especially in patients with neurologic complications from anti-TNF agents.
“There may be other medications that would work more specifically in axial psoriatic arthritis that don’t work in ankylosing spondylitis, but at least recognizing that there may be some differences, and that therefore a correct diagnosis should be obtained, might be important,” she said.
Ideally, the picture will become clearer with results from the ongoing Axial Involvement in Psoriatic Arthritis cohort, a joint project of ASAS and the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis. The multinational, cross-sectional study is designed “to systematically evaluate clinical and imaging manifestations indicative of axial involvement in patients with PsA and to develop classification criteria and a unified nomenclature for axial involvement in PsA that would allow defining a homogeneous subgroup of patients for research.”
Stay tuned.
Dr. Gladman’s research is supported by a grant from the Krembil Foundation. Dr. Singh disclosed research support from various companies. Funding sources and conflict of interest disclosures from other works cited are contained in their respective references.
Are there clinically significant differences between axial spondyloarthritis with psoriasis and psoriatic arthritis with axial symptoms? Does it matter?
It all depends on whom you ask, but right now the evidence seems to be tipping in favor of the “splitters” who cite evidence supporting their contention that axial spondyloarthritis (axSpA)/ankylosing spondylitis (AS) with psoriasis and psoriatic arthritis (PsA) with axial symptoms are distinct clinical entities that require more precise diagnosis and treatment.
“Lumpers,” in contrast, argue that they are different points on the same clinical spectrum.
The debate is not just of academic interest, but has real consequences for patients, say specialists on both sides of the aisle.
Overlapping features, different presentations
“Axial SpA and axPsA have overlapping features but also meaningful differences in genetics, clinical presentation, imaging, and immunophenotype. Efforts are underway to develop classification criteria for axPsA to aid research efforts as well as clinical diagnosis and management,” Philip J. Mease, MD, director of rheumatology research at Swedish Medical Center/Providence–St. Joseph Health in Seattle, and colleagues contend.
In an editorial published in the International Journal of Rheumatic Diseases, Dr. Mease and colleagues noted that, although HLA-B*27 is a genetic risk factor for both axPsA and axSpA, some HLA-B alleles are significantly associated with axPsA, whereas other alleles are associated with axSpA.
In addition, while genes in the interleukin-23 and IL-17 pathway are associated with increased risk for axSpA, genes in the IL-13 pathway have been identified as risk markers for axPsA, they noted.
Two cohorts better than one?
Dafna Gladman, MD, professor of medicine at the University of Toronto and senior scientist at the Schroeder Arthritis Institute at Toronto Western Hospital, and colleagues have a unique perspective on the similarities and differences between the disease entities.
Her group’s research uses data on cohorts of patients treated in two separate clinics at Toronto Western Hospital: one for patients with PsA, and one for patients with axial spondyloarthritis, including those with ankylosing spondylitis, nonradiographic axSpA, and spondylitis associated with inflammatory bowel disease.
“Our work has shown that there are differences, and one of the reasons that it’s now important is that the anti–IL-23 medications, both the IL-12/23 inhibitor ustekinumab [Stelara] and the IL-23 inhibitor guselkumab [Tremfya] work for psoriatic arthritis, whereas IL-23 did not work in ankylosing spondylitis, so that provided further impetus to look into the distinction between the two groups,” Dr. Gladman said in an interview.
Dr. Gladman and colleagues published a study in Rheumatology in which they compared clinical presentations and features of patients with AS with or without psoriasis with patients with axPsA.
They found that patients with AS with or without psoriasis tended to be younger, had a higher proportion of males to females, and were more likely to be positive for HLA-B*27. Patients with AS also had more back pain at presentation, worse axial disease activity scores, worse global assessments by physicians, and higher grades of sacroiliitis, and they were more likely to be taking biologic agents.
“What that showed, right off the top, that whether we’re looking at the total group or we’re looking specifically at those patients who have psoriasis or don’t have psoriasis, they are different from those with psoriatic arthritis with axial disease,” she said.
They concluded that “axPsA seems to be a distinct entity.”
Two clinics, same presentation
Because the aforementioned study included all patients with PsA with or without peripheral disease, the investigators decided to filter out some of the background noise and conduct a second study in which they compared patients who presented to the two clinics with the same presentation, either with spinal disease and psoriasis to the spondylitis clinic, or with psoriasis and isolated axial disease to the PsA clinic.
The results, published in Annals of the Rheumatic Diseases, showed that just 2.03% of patients with PsA had isolated axial disease, and an additional 29.38% had axial and peripheral disease.
In this study, “you can see that even in that group there are distinct differences. The patients that are labeled psoriatic spondylitis are different from those that are labeled ankylosing spondylitis with psoriasis,” Dr. Gladman said.
Isolated axial disease in patients with PsA was associated with HLA-B*27 positivity and lower Health Assessment Questionnaire scores. In addition, patients who were HLA-B*27 positive also had a nearly eightfold higher risk for developing peripheral disease over time.
Patients with isolated axial PsA were significantly more likely to be diagnosed at an older age (mean, 37.44 vs. 29.65 years), had higher Psoriasis Area Severity Index scores and a higher likelihood of having psoriatic nail lesions than patients with AS with isolated axial disease and psoriasis.
In contrast, patients with isolated axSpA with psoriasis were more likely to have inflammatory back pain, spinal pain, joint pain/swelling, and areas of localized tenderness, and they had greater severity of morning stiffness.
Dr. Gladman noted that, although AS and PsA are associated with the same gene that encodes for the IL-23 receptor, each condition is associated with a different single-nucleotide polymorphism.
Same disease, different flavors?
But as Mark Twain said, it is difference of opinion that makes horse races, and some specialists in rheumatology say that axSpA amd axPsA are just two sides of the same coin.
“There are always different schools of thought. I believe that they are not different diseases, but a spectrum of diseases,” said Shailendra Singh, MD, a rheumatologist at Unity Health Medical Center in Searcy, Ark., and past president of the Arkansas Rheumatology Association.
In an interview, Dr. Singh said that the spectrum ranges from diseases with primarily axial involvement, such as AS, to those with primarily peripheral involvement, such as reactive arthritis.
He pointed out that these conditions have overlapping symptoms, including enthesitis, dactylitis, and uveitis, and inflammatory arthritis.
Daniel Wendling, MD, PhD, from the Centre Hospitalier Régional Universitaire de Besançon (France), Université de Franche-Comté, and colleagues agreed.
“The criteria currently available for both SpA [ASAS (Assessment of Spondyloarthritis International Society) criteria] and PsA [CASPAR (Classification for Psoriatic Arthritis) criteria] are classification criteria, not diagnostic criteria. They are not very stringent and are not exclusive. Thus, the same patient can easily be classified simultaneously in both entities, making the distinction between axSpA with psoriasis and axPsA theoretical,” they wrote in an editorial published in Joint Bone Spine.
They cited as an example of the allegedly fuzzy criteria a prospective study conducted by the investigators in Bath, England, in which modified New York criteria for AS were met by 24% of patients with AS, and CASPAR criteria for PsA were met by an equal number of patients with AS.
Therapeutic implications
Dr. Wendling and colleagues acknowledge the differences cited in studies by Dr. Gladman, Dr. Mease, and others between patients with axPsA and those with axSpA, but argue that the differences are not that great and not so clear.
“It should also be emphasized that, although some differences between axPsA and axSpA reach statistical significance, they are mostly at the margin, with low odd ratios,” they wrote.
“It is also important to consider the variability in the definition of axPsA, sometimes simply ‘physician reported’ and elsewhere based on the modified New York radiographic criteria; the latter are only present late in the course of the disease, and this may induce bias,” they continued.
Dr. Singh agreed that, as noted by Dr. Gladman, some patients will respond to anti–IL-17, anti–IL-23, and anti–IL-12/23 agents, whereas others will have better responses with tumor necrosis factor (TNF) inhibitors, and still others, such as those with peripheral involvement in the hands and feet may fare better with nonbiologic disease-modifying antirheumatic drugs such as methotrexate.
Answers to come?
Dr. Gladman noted that the information available to date about the efficacy of IL-23 inhibition in axPsA is based on a post hoc analysis of the PSUMMIT 1 and 2 controlled trials in PsA, and is not definitive.
The randomized, controlled STAR trial, currently recruiting patients, is designed to see whether guselkumab can reduce axial symptoms and inflammation in patients with active axPsA.
“What I say is, there is a rationale for [anti–IL-23] to work in psoriatic arthritis, and not work in ankylosing spondylitis,” she said.
In contrast, IL-17 inhibitors, anti-TNF agents, and Janus kinase inhibitors show efficacy against both axPsA and AS. Rituximab is ineffective against PsA, but has shown efficacy against AS, especially in patients with neurologic complications from anti-TNF agents.
“There may be other medications that would work more specifically in axial psoriatic arthritis that don’t work in ankylosing spondylitis, but at least recognizing that there may be some differences, and that therefore a correct diagnosis should be obtained, might be important,” she said.
Ideally, the picture will become clearer with results from the ongoing Axial Involvement in Psoriatic Arthritis cohort, a joint project of ASAS and the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis. The multinational, cross-sectional study is designed “to systematically evaluate clinical and imaging manifestations indicative of axial involvement in patients with PsA and to develop classification criteria and a unified nomenclature for axial involvement in PsA that would allow defining a homogeneous subgroup of patients for research.”
Stay tuned.
Dr. Gladman’s research is supported by a grant from the Krembil Foundation. Dr. Singh disclosed research support from various companies. Funding sources and conflict of interest disclosures from other works cited are contained in their respective references.
Are there clinically significant differences between axial spondyloarthritis with psoriasis and psoriatic arthritis with axial symptoms? Does it matter?
It all depends on whom you ask, but right now the evidence seems to be tipping in favor of the “splitters” who cite evidence supporting their contention that axial spondyloarthritis (axSpA)/ankylosing spondylitis (AS) with psoriasis and psoriatic arthritis (PsA) with axial symptoms are distinct clinical entities that require more precise diagnosis and treatment.
“Lumpers,” in contrast, argue that they are different points on the same clinical spectrum.
The debate is not just of academic interest, but has real consequences for patients, say specialists on both sides of the aisle.
Overlapping features, different presentations
“Axial SpA and axPsA have overlapping features but also meaningful differences in genetics, clinical presentation, imaging, and immunophenotype. Efforts are underway to develop classification criteria for axPsA to aid research efforts as well as clinical diagnosis and management,” Philip J. Mease, MD, director of rheumatology research at Swedish Medical Center/Providence–St. Joseph Health in Seattle, and colleagues contend.
In an editorial published in the International Journal of Rheumatic Diseases, Dr. Mease and colleagues noted that, although HLA-B*27 is a genetic risk factor for both axPsA and axSpA, some HLA-B alleles are significantly associated with axPsA, whereas other alleles are associated with axSpA.
In addition, while genes in the interleukin-23 and IL-17 pathway are associated with increased risk for axSpA, genes in the IL-13 pathway have been identified as risk markers for axPsA, they noted.
Two cohorts better than one?
Dafna Gladman, MD, professor of medicine at the University of Toronto and senior scientist at the Schroeder Arthritis Institute at Toronto Western Hospital, and colleagues have a unique perspective on the similarities and differences between the disease entities.
Her group’s research uses data on cohorts of patients treated in two separate clinics at Toronto Western Hospital: one for patients with PsA, and one for patients with axial spondyloarthritis, including those with ankylosing spondylitis, nonradiographic axSpA, and spondylitis associated with inflammatory bowel disease.
“Our work has shown that there are differences, and one of the reasons that it’s now important is that the anti–IL-23 medications, both the IL-12/23 inhibitor ustekinumab [Stelara] and the IL-23 inhibitor guselkumab [Tremfya] work for psoriatic arthritis, whereas IL-23 did not work in ankylosing spondylitis, so that provided further impetus to look into the distinction between the two groups,” Dr. Gladman said in an interview.
Dr. Gladman and colleagues published a study in Rheumatology in which they compared clinical presentations and features of patients with AS with or without psoriasis with patients with axPsA.
They found that patients with AS with or without psoriasis tended to be younger, had a higher proportion of males to females, and were more likely to be positive for HLA-B*27. Patients with AS also had more back pain at presentation, worse axial disease activity scores, worse global assessments by physicians, and higher grades of sacroiliitis, and they were more likely to be taking biologic agents.
“What that showed, right off the top, that whether we’re looking at the total group or we’re looking specifically at those patients who have psoriasis or don’t have psoriasis, they are different from those with psoriatic arthritis with axial disease,” she said.
They concluded that “axPsA seems to be a distinct entity.”
Two clinics, same presentation
Because the aforementioned study included all patients with PsA with or without peripheral disease, the investigators decided to filter out some of the background noise and conduct a second study in which they compared patients who presented to the two clinics with the same presentation, either with spinal disease and psoriasis to the spondylitis clinic, or with psoriasis and isolated axial disease to the PsA clinic.
The results, published in Annals of the Rheumatic Diseases, showed that just 2.03% of patients with PsA had isolated axial disease, and an additional 29.38% had axial and peripheral disease.
In this study, “you can see that even in that group there are distinct differences. The patients that are labeled psoriatic spondylitis are different from those that are labeled ankylosing spondylitis with psoriasis,” Dr. Gladman said.
Isolated axial disease in patients with PsA was associated with HLA-B*27 positivity and lower Health Assessment Questionnaire scores. In addition, patients who were HLA-B*27 positive also had a nearly eightfold higher risk for developing peripheral disease over time.
Patients with isolated axial PsA were significantly more likely to be diagnosed at an older age (mean, 37.44 vs. 29.65 years), had higher Psoriasis Area Severity Index scores and a higher likelihood of having psoriatic nail lesions than patients with AS with isolated axial disease and psoriasis.
In contrast, patients with isolated axSpA with psoriasis were more likely to have inflammatory back pain, spinal pain, joint pain/swelling, and areas of localized tenderness, and they had greater severity of morning stiffness.
Dr. Gladman noted that, although AS and PsA are associated with the same gene that encodes for the IL-23 receptor, each condition is associated with a different single-nucleotide polymorphism.
Same disease, different flavors?
But as Mark Twain said, it is difference of opinion that makes horse races, and some specialists in rheumatology say that axSpA amd axPsA are just two sides of the same coin.
“There are always different schools of thought. I believe that they are not different diseases, but a spectrum of diseases,” said Shailendra Singh, MD, a rheumatologist at Unity Health Medical Center in Searcy, Ark., and past president of the Arkansas Rheumatology Association.
In an interview, Dr. Singh said that the spectrum ranges from diseases with primarily axial involvement, such as AS, to those with primarily peripheral involvement, such as reactive arthritis.
He pointed out that these conditions have overlapping symptoms, including enthesitis, dactylitis, and uveitis, and inflammatory arthritis.
Daniel Wendling, MD, PhD, from the Centre Hospitalier Régional Universitaire de Besançon (France), Université de Franche-Comté, and colleagues agreed.
“The criteria currently available for both SpA [ASAS (Assessment of Spondyloarthritis International Society) criteria] and PsA [CASPAR (Classification for Psoriatic Arthritis) criteria] are classification criteria, not diagnostic criteria. They are not very stringent and are not exclusive. Thus, the same patient can easily be classified simultaneously in both entities, making the distinction between axSpA with psoriasis and axPsA theoretical,” they wrote in an editorial published in Joint Bone Spine.
They cited as an example of the allegedly fuzzy criteria a prospective study conducted by the investigators in Bath, England, in which modified New York criteria for AS were met by 24% of patients with AS, and CASPAR criteria for PsA were met by an equal number of patients with AS.
Therapeutic implications
Dr. Wendling and colleagues acknowledge the differences cited in studies by Dr. Gladman, Dr. Mease, and others between patients with axPsA and those with axSpA, but argue that the differences are not that great and not so clear.
“It should also be emphasized that, although some differences between axPsA and axSpA reach statistical significance, they are mostly at the margin, with low odd ratios,” they wrote.
“It is also important to consider the variability in the definition of axPsA, sometimes simply ‘physician reported’ and elsewhere based on the modified New York radiographic criteria; the latter are only present late in the course of the disease, and this may induce bias,” they continued.
Dr. Singh agreed that, as noted by Dr. Gladman, some patients will respond to anti–IL-17, anti–IL-23, and anti–IL-12/23 agents, whereas others will have better responses with tumor necrosis factor (TNF) inhibitors, and still others, such as those with peripheral involvement in the hands and feet may fare better with nonbiologic disease-modifying antirheumatic drugs such as methotrexate.
Answers to come?
Dr. Gladman noted that the information available to date about the efficacy of IL-23 inhibition in axPsA is based on a post hoc analysis of the PSUMMIT 1 and 2 controlled trials in PsA, and is not definitive.
The randomized, controlled STAR trial, currently recruiting patients, is designed to see whether guselkumab can reduce axial symptoms and inflammation in patients with active axPsA.
“What I say is, there is a rationale for [anti–IL-23] to work in psoriatic arthritis, and not work in ankylosing spondylitis,” she said.
In contrast, IL-17 inhibitors, anti-TNF agents, and Janus kinase inhibitors show efficacy against both axPsA and AS. Rituximab is ineffective against PsA, but has shown efficacy against AS, especially in patients with neurologic complications from anti-TNF agents.
“There may be other medications that would work more specifically in axial psoriatic arthritis that don’t work in ankylosing spondylitis, but at least recognizing that there may be some differences, and that therefore a correct diagnosis should be obtained, might be important,” she said.
Ideally, the picture will become clearer with results from the ongoing Axial Involvement in Psoriatic Arthritis cohort, a joint project of ASAS and the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis. The multinational, cross-sectional study is designed “to systematically evaluate clinical and imaging manifestations indicative of axial involvement in patients with PsA and to develop classification criteria and a unified nomenclature for axial involvement in PsA that would allow defining a homogeneous subgroup of patients for research.”
Stay tuned.
Dr. Gladman’s research is supported by a grant from the Krembil Foundation. Dr. Singh disclosed research support from various companies. Funding sources and conflict of interest disclosures from other works cited are contained in their respective references.
1,726-nm lasers poised to revolutionize acne treatment, expert predicts
PHOENIX – When Jeffrey Dover, MD, addressed audience members gathered for a session on cutting-edge technologies at the annual conference of the American Society for Laser Medicine and Surgery, he reflected on a conversation he had with R. Rox Anderson, MD, almost 40 years ago, about eventually finding a cure for acne.
“Despite the fact that we have over-the-counter therapies, prescription therapies, and all kinds of devices available to treat acne, there are still barriers to care that get in the way of treatment,” said Dr. Dover, director of SkinCare Physicians in Chestnut Hill, Mass. “If we had a device based on innovative light science that could meet the needs of the acne patient to get rid of these barriers, wouldn’t that be something wonderful?”
The answer to this question, he said, is now “yes,” because of advances in lasers that target sebaceous glands.
In a seminal paper published in 2012, Fernanda H. Sakamoto, MD, PhD, Dr. Anderson, and colleagues demonstrated the potential for a free electron laser to target sebaceous glands . Following several years of refinement, Dr. Dover said.
“With the 1,726-nm laser, there is some selective absorption in sebum in skin, which beats out absorption in the other chromophores,” he said. “But it’s not a big difference like it is, for example, for pulsed-dye lasers and vascular targets. ... This means that the therapeutic window is relatively small and protecting the rest of the epidermis and dermis is crucial to be able to target these lesions or the sebaceous gland without unnecessary damage. If we can protect the epidermis and heat just the sebaceous glands, we should be able to get Accutane-like results if we get durability [by] shrinking sebaceous glands.”
Effective cooling, whether contact cooling, bulk cooling, or air cooling, is crucial to success, he continued. “It’s got to be robust and highly specific to protect the skin, so you don’t end up with side effects that are worse than the disease.”
The AviClear laser delivers seven 3-mm spots, which takes into account the thermal relaxation times of the sebaceous glands. The algorithm delivers a treatment imprint at roughly 0.3 Hz and a 1.5-mm depth of penetration, and the device relies on contact cooling. In pivotal data submitted to the FDA, 104 individuals with moderate to severe acne received three treatments with the AviClear 1 month apart, with follow-up at 1, 3, 6, and 12 months post treatment. They had no other treatment regimens, and the primary endpoint was the percentage of patients who achieved a 50% reduction in inflammatory lesion count 3 months after the final treatment. The secondary endpoint was an Investigator’s Global Assessment (IGA) improvement of 2 or greater.
Dr. Dover, who helped design the study, said that, at 3 months, 80% of those treated achieved a 50% or greater reduction in inflammatory lesion count (P < .001). As for secondary endpoints, 36% of individuals were assessed as having clear or almost clear skin; 47% achieved a 2-point or greater improvement in IGA score, compared with baseline, and 87% achieved a 1-point or greater improvement in IGA score, compared with baseline. By 6 months, 88% of individuals achieved a 50% or greater reduction in inflammatory lesion count; this improved to 92% by 12 months (P < .001).
“All of these procedures were done with no topical anesthetic, no intralesional anesthetic, and they tolerated these quite well,” he said. “There was no down time that required medical intervention after the treatments. All posttreatment erythema and swelling resolved quickly,” and 75% of the patients were “very satisfied” with the treatments.
The Accure Laser System features a proprietary technology that precisely controls thermal gradient depth. “So instead of guessing whether you are delivering the correct amount of heat, it actually tells you,” said Dr. Dover, a past president of the ASLMS and the American Society for Dermatologic Surgery. “It correlates surface and at-depth temperatures, and there’s an infrared camera for real-time accurate temperature monitoring.” The device features highly controlled air cooling and a pulsing pattern that ensures treatment of sebaceous glands of all sizes and at all depths. The clinical end marker is peak epidermal temperature.
In a study supported by Accure, the manufacturer, researchers evaluated the efficacy of the Accure Laser System in 35 subjects with types I to VI skin, who received four monthly treatments 30-45 minutes each, and were followed 12, 26, 39, and 52 weeks following their last treatment. To date, data out to 52 weeks is available for 17 study participants. According to Dr. Dover, the researchers found 80% clearance at 12 weeks following the last treatment, with continued improvement at 52 weeks. One hundred percent of subjects responded. Side effects included erythema, edema, crusting, blisters, and inflammatory papules. “None of these were medically significant,” he said.
As dermatologists begin to incorporate the AviClear and Accure devices into their practices, Dr. Dover said that he is reminded of the conversation he had some 40 years ago with Dr. Anderson about finding a cure for acne, and he feels a bit awestruck. “These 1,726-nm lasers are effective for treating acne. I personally think they are going to revolutionize the way we treat at least some of our patients with acne. They may both be effective for treating facial acne scars. Time will tell. Further study of both scarring and acne are needed to fully categorize the benefit and to optimize treatments.”
To date no direct clinical comparisons have been made between the AviClear and Accure devices.
Dr. Dover reported that he is a consultant for Cutera, the manufacturer for AviClear. He also performs research for the company.
PHOENIX – When Jeffrey Dover, MD, addressed audience members gathered for a session on cutting-edge technologies at the annual conference of the American Society for Laser Medicine and Surgery, he reflected on a conversation he had with R. Rox Anderson, MD, almost 40 years ago, about eventually finding a cure for acne.
“Despite the fact that we have over-the-counter therapies, prescription therapies, and all kinds of devices available to treat acne, there are still barriers to care that get in the way of treatment,” said Dr. Dover, director of SkinCare Physicians in Chestnut Hill, Mass. “If we had a device based on innovative light science that could meet the needs of the acne patient to get rid of these barriers, wouldn’t that be something wonderful?”
The answer to this question, he said, is now “yes,” because of advances in lasers that target sebaceous glands.
In a seminal paper published in 2012, Fernanda H. Sakamoto, MD, PhD, Dr. Anderson, and colleagues demonstrated the potential for a free electron laser to target sebaceous glands . Following several years of refinement, Dr. Dover said.
“With the 1,726-nm laser, there is some selective absorption in sebum in skin, which beats out absorption in the other chromophores,” he said. “But it’s not a big difference like it is, for example, for pulsed-dye lasers and vascular targets. ... This means that the therapeutic window is relatively small and protecting the rest of the epidermis and dermis is crucial to be able to target these lesions or the sebaceous gland without unnecessary damage. If we can protect the epidermis and heat just the sebaceous glands, we should be able to get Accutane-like results if we get durability [by] shrinking sebaceous glands.”
Effective cooling, whether contact cooling, bulk cooling, or air cooling, is crucial to success, he continued. “It’s got to be robust and highly specific to protect the skin, so you don’t end up with side effects that are worse than the disease.”
The AviClear laser delivers seven 3-mm spots, which takes into account the thermal relaxation times of the sebaceous glands. The algorithm delivers a treatment imprint at roughly 0.3 Hz and a 1.5-mm depth of penetration, and the device relies on contact cooling. In pivotal data submitted to the FDA, 104 individuals with moderate to severe acne received three treatments with the AviClear 1 month apart, with follow-up at 1, 3, 6, and 12 months post treatment. They had no other treatment regimens, and the primary endpoint was the percentage of patients who achieved a 50% reduction in inflammatory lesion count 3 months after the final treatment. The secondary endpoint was an Investigator’s Global Assessment (IGA) improvement of 2 or greater.
Dr. Dover, who helped design the study, said that, at 3 months, 80% of those treated achieved a 50% or greater reduction in inflammatory lesion count (P < .001). As for secondary endpoints, 36% of individuals were assessed as having clear or almost clear skin; 47% achieved a 2-point or greater improvement in IGA score, compared with baseline, and 87% achieved a 1-point or greater improvement in IGA score, compared with baseline. By 6 months, 88% of individuals achieved a 50% or greater reduction in inflammatory lesion count; this improved to 92% by 12 months (P < .001).
“All of these procedures were done with no topical anesthetic, no intralesional anesthetic, and they tolerated these quite well,” he said. “There was no down time that required medical intervention after the treatments. All posttreatment erythema and swelling resolved quickly,” and 75% of the patients were “very satisfied” with the treatments.
The Accure Laser System features a proprietary technology that precisely controls thermal gradient depth. “So instead of guessing whether you are delivering the correct amount of heat, it actually tells you,” said Dr. Dover, a past president of the ASLMS and the American Society for Dermatologic Surgery. “It correlates surface and at-depth temperatures, and there’s an infrared camera for real-time accurate temperature monitoring.” The device features highly controlled air cooling and a pulsing pattern that ensures treatment of sebaceous glands of all sizes and at all depths. The clinical end marker is peak epidermal temperature.
In a study supported by Accure, the manufacturer, researchers evaluated the efficacy of the Accure Laser System in 35 subjects with types I to VI skin, who received four monthly treatments 30-45 minutes each, and were followed 12, 26, 39, and 52 weeks following their last treatment. To date, data out to 52 weeks is available for 17 study participants. According to Dr. Dover, the researchers found 80% clearance at 12 weeks following the last treatment, with continued improvement at 52 weeks. One hundred percent of subjects responded. Side effects included erythema, edema, crusting, blisters, and inflammatory papules. “None of these were medically significant,” he said.
As dermatologists begin to incorporate the AviClear and Accure devices into their practices, Dr. Dover said that he is reminded of the conversation he had some 40 years ago with Dr. Anderson about finding a cure for acne, and he feels a bit awestruck. “These 1,726-nm lasers are effective for treating acne. I personally think they are going to revolutionize the way we treat at least some of our patients with acne. They may both be effective for treating facial acne scars. Time will tell. Further study of both scarring and acne are needed to fully categorize the benefit and to optimize treatments.”
To date no direct clinical comparisons have been made between the AviClear and Accure devices.
Dr. Dover reported that he is a consultant for Cutera, the manufacturer for AviClear. He also performs research for the company.
PHOENIX – When Jeffrey Dover, MD, addressed audience members gathered for a session on cutting-edge technologies at the annual conference of the American Society for Laser Medicine and Surgery, he reflected on a conversation he had with R. Rox Anderson, MD, almost 40 years ago, about eventually finding a cure for acne.
“Despite the fact that we have over-the-counter therapies, prescription therapies, and all kinds of devices available to treat acne, there are still barriers to care that get in the way of treatment,” said Dr. Dover, director of SkinCare Physicians in Chestnut Hill, Mass. “If we had a device based on innovative light science that could meet the needs of the acne patient to get rid of these barriers, wouldn’t that be something wonderful?”
The answer to this question, he said, is now “yes,” because of advances in lasers that target sebaceous glands.
In a seminal paper published in 2012, Fernanda H. Sakamoto, MD, PhD, Dr. Anderson, and colleagues demonstrated the potential for a free electron laser to target sebaceous glands . Following several years of refinement, Dr. Dover said.
“With the 1,726-nm laser, there is some selective absorption in sebum in skin, which beats out absorption in the other chromophores,” he said. “But it’s not a big difference like it is, for example, for pulsed-dye lasers and vascular targets. ... This means that the therapeutic window is relatively small and protecting the rest of the epidermis and dermis is crucial to be able to target these lesions or the sebaceous gland without unnecessary damage. If we can protect the epidermis and heat just the sebaceous glands, we should be able to get Accutane-like results if we get durability [by] shrinking sebaceous glands.”
Effective cooling, whether contact cooling, bulk cooling, or air cooling, is crucial to success, he continued. “It’s got to be robust and highly specific to protect the skin, so you don’t end up with side effects that are worse than the disease.”
The AviClear laser delivers seven 3-mm spots, which takes into account the thermal relaxation times of the sebaceous glands. The algorithm delivers a treatment imprint at roughly 0.3 Hz and a 1.5-mm depth of penetration, and the device relies on contact cooling. In pivotal data submitted to the FDA, 104 individuals with moderate to severe acne received three treatments with the AviClear 1 month apart, with follow-up at 1, 3, 6, and 12 months post treatment. They had no other treatment regimens, and the primary endpoint was the percentage of patients who achieved a 50% reduction in inflammatory lesion count 3 months after the final treatment. The secondary endpoint was an Investigator’s Global Assessment (IGA) improvement of 2 or greater.
Dr. Dover, who helped design the study, said that, at 3 months, 80% of those treated achieved a 50% or greater reduction in inflammatory lesion count (P < .001). As for secondary endpoints, 36% of individuals were assessed as having clear or almost clear skin; 47% achieved a 2-point or greater improvement in IGA score, compared with baseline, and 87% achieved a 1-point or greater improvement in IGA score, compared with baseline. By 6 months, 88% of individuals achieved a 50% or greater reduction in inflammatory lesion count; this improved to 92% by 12 months (P < .001).
“All of these procedures were done with no topical anesthetic, no intralesional anesthetic, and they tolerated these quite well,” he said. “There was no down time that required medical intervention after the treatments. All posttreatment erythema and swelling resolved quickly,” and 75% of the patients were “very satisfied” with the treatments.
The Accure Laser System features a proprietary technology that precisely controls thermal gradient depth. “So instead of guessing whether you are delivering the correct amount of heat, it actually tells you,” said Dr. Dover, a past president of the ASLMS and the American Society for Dermatologic Surgery. “It correlates surface and at-depth temperatures, and there’s an infrared camera for real-time accurate temperature monitoring.” The device features highly controlled air cooling and a pulsing pattern that ensures treatment of sebaceous glands of all sizes and at all depths. The clinical end marker is peak epidermal temperature.
In a study supported by Accure, the manufacturer, researchers evaluated the efficacy of the Accure Laser System in 35 subjects with types I to VI skin, who received four monthly treatments 30-45 minutes each, and were followed 12, 26, 39, and 52 weeks following their last treatment. To date, data out to 52 weeks is available for 17 study participants. According to Dr. Dover, the researchers found 80% clearance at 12 weeks following the last treatment, with continued improvement at 52 weeks. One hundred percent of subjects responded. Side effects included erythema, edema, crusting, blisters, and inflammatory papules. “None of these were medically significant,” he said.
As dermatologists begin to incorporate the AviClear and Accure devices into their practices, Dr. Dover said that he is reminded of the conversation he had some 40 years ago with Dr. Anderson about finding a cure for acne, and he feels a bit awestruck. “These 1,726-nm lasers are effective for treating acne. I personally think they are going to revolutionize the way we treat at least some of our patients with acne. They may both be effective for treating facial acne scars. Time will tell. Further study of both scarring and acne are needed to fully categorize the benefit and to optimize treatments.”
To date no direct clinical comparisons have been made between the AviClear and Accure devices.
Dr. Dover reported that he is a consultant for Cutera, the manufacturer for AviClear. He also performs research for the company.
AT ASLMS 2023
Scarred med student inspired by dermatologist who treated her
It’s not uncommon for a medical student to change specialty plans. For Jamie Harris, a second-year student at the University of Florida School of Medicine, Gainesville, that decision came as the result of a vicious dog and an empathetic doctor.
, a New York dermatologist whose approach involves early and aggressive treatment. After treating her, Dr. Bhanusali offered to have Ms. Harris shadow him.
She returned to school to shadow other dermatologists and to research the specialty before taking Dr. Bhanusali up on his offer. Ms. Harris sat in on procedures and meetings with patients and studied Dr. Bhanusali’s approach to the specialty. “I just fell in love with dermatology,” Ms. Harris told this news organization. “I knew that what I wanted for my own career was exactly how he runs his practice and how he treats patients.”
Life-changing injury
In 2020, Ms. Harris was a sophomore in the University of Florida’s medical honors program, an accelerated track that allows students to earn both a bachelor of science degree and a doctor of medicine degree in 7 years. She had finished studying at a friend’s apartment and was watching television when the rescue dog the friend adopted lunged at Ms. Harris, biting her on the face. “I was just cowering in the corner of the couch,” she recalls. “I didn’t go into fight-or-flight mode; I just went into hide mode.”
After receiving stitches in the emergency department, she visited several dermatologists and plastic surgeons for further treatment. There was scarring from her forehead to her chin, which was particularly severe on her upper cheek just under her eye. But because there was no infection or medical problems, the doctors turned her away. “They said, ‘OK, you look great.’ I did not look great,” she said.
Ms. Harris’ doctors advised her to wait a year before starting treatment for the scarring, a traditional approach. She was frustrated. “At the time, I was interested in becoming a pediatrician and thought, ‘No kid is going to want me as their doctor.’ ” But she accepted the medical advice – until her mother remembered a news story she’d seen.
Bridger Walker, a 6-year-old Wyoming boy, made headlines when he saved his younger sister from a dog that was attacking, but he was bitten multiple times as a result. Dr. Bhanusali treated the boy’s scarring.
Ms. Harris and her mother contacted the doctor, and after meeting via Zoom, Dr. Bhanusali agreed to treat her right away. He used lasers to resurface the skin, which created a suitable foundation for the scar cream, and he administered steroid injections to soften the scar tissue.
‘I see you’
Dr. Bhansali said he was impressed with the young student he treated. “There’s curiosity, and then there’s genuine passion. She has the latter,” he said in an interview. “Having gone through this, she will understand the value of research and keeping up with the literature and that just because something is being done a certain way today doesn’t mean it has to be that way tomorrow.”
Ms. Harris agrees that the experience will make her a better dermatologist. “One of the best parts about dermatology is that you can see your results in real time and really see what’s working and what’s not working. The potential for innovation is just amazing.”
But Ms. Harris believes she also gained empathy with dermatology patients. “I know exactly what it’s like to look in the mirror and not even recognize yourself, just have your eyes go straight to one thing and feel like the whole world is staring at you,” she said. “I’ll be able to reassure people that no matter what their concern is, whether it’s eczema or acne, whether it’s one pimple, I see you, and I know exactly how that feels.”
A version of this article first appeared on Medscape.com.
It’s not uncommon for a medical student to change specialty plans. For Jamie Harris, a second-year student at the University of Florida School of Medicine, Gainesville, that decision came as the result of a vicious dog and an empathetic doctor.
, a New York dermatologist whose approach involves early and aggressive treatment. After treating her, Dr. Bhanusali offered to have Ms. Harris shadow him.
She returned to school to shadow other dermatologists and to research the specialty before taking Dr. Bhanusali up on his offer. Ms. Harris sat in on procedures and meetings with patients and studied Dr. Bhanusali’s approach to the specialty. “I just fell in love with dermatology,” Ms. Harris told this news organization. “I knew that what I wanted for my own career was exactly how he runs his practice and how he treats patients.”

Life-changing injury
In 2020, Ms. Harris was a sophomore in the University of Florida’s medical honors program, an accelerated track that allows students to earn both a bachelor of science degree and a doctor of medicine degree in 7 years. She had finished studying at a friend’s apartment and was watching television when the rescue dog the friend adopted lunged at Ms. Harris, biting her on the face. “I was just cowering in the corner of the couch,” she recalls. “I didn’t go into fight-or-flight mode; I just went into hide mode.”
After receiving stitches in the emergency department, she visited several dermatologists and plastic surgeons for further treatment. There was scarring from her forehead to her chin, which was particularly severe on her upper cheek just under her eye. But because there was no infection or medical problems, the doctors turned her away. “They said, ‘OK, you look great.’ I did not look great,” she said.
Ms. Harris’ doctors advised her to wait a year before starting treatment for the scarring, a traditional approach. She was frustrated. “At the time, I was interested in becoming a pediatrician and thought, ‘No kid is going to want me as their doctor.’ ” But she accepted the medical advice – until her mother remembered a news story she’d seen.
Bridger Walker, a 6-year-old Wyoming boy, made headlines when he saved his younger sister from a dog that was attacking, but he was bitten multiple times as a result. Dr. Bhanusali treated the boy’s scarring.
Ms. Harris and her mother contacted the doctor, and after meeting via Zoom, Dr. Bhanusali agreed to treat her right away. He used lasers to resurface the skin, which created a suitable foundation for the scar cream, and he administered steroid injections to soften the scar tissue.
‘I see you’
Dr. Bhansali said he was impressed with the young student he treated. “There’s curiosity, and then there’s genuine passion. She has the latter,” he said in an interview. “Having gone through this, she will understand the value of research and keeping up with the literature and that just because something is being done a certain way today doesn’t mean it has to be that way tomorrow.”
Ms. Harris agrees that the experience will make her a better dermatologist. “One of the best parts about dermatology is that you can see your results in real time and really see what’s working and what’s not working. The potential for innovation is just amazing.”
But Ms. Harris believes she also gained empathy with dermatology patients. “I know exactly what it’s like to look in the mirror and not even recognize yourself, just have your eyes go straight to one thing and feel like the whole world is staring at you,” she said. “I’ll be able to reassure people that no matter what their concern is, whether it’s eczema or acne, whether it’s one pimple, I see you, and I know exactly how that feels.”
A version of this article first appeared on Medscape.com.
It’s not uncommon for a medical student to change specialty plans. For Jamie Harris, a second-year student at the University of Florida School of Medicine, Gainesville, that decision came as the result of a vicious dog and an empathetic doctor.
, a New York dermatologist whose approach involves early and aggressive treatment. After treating her, Dr. Bhanusali offered to have Ms. Harris shadow him.
She returned to school to shadow other dermatologists and to research the specialty before taking Dr. Bhanusali up on his offer. Ms. Harris sat in on procedures and meetings with patients and studied Dr. Bhanusali’s approach to the specialty. “I just fell in love with dermatology,” Ms. Harris told this news organization. “I knew that what I wanted for my own career was exactly how he runs his practice and how he treats patients.”

Life-changing injury
In 2020, Ms. Harris was a sophomore in the University of Florida’s medical honors program, an accelerated track that allows students to earn both a bachelor of science degree and a doctor of medicine degree in 7 years. She had finished studying at a friend’s apartment and was watching television when the rescue dog the friend adopted lunged at Ms. Harris, biting her on the face. “I was just cowering in the corner of the couch,” she recalls. “I didn’t go into fight-or-flight mode; I just went into hide mode.”
After receiving stitches in the emergency department, she visited several dermatologists and plastic surgeons for further treatment. There was scarring from her forehead to her chin, which was particularly severe on her upper cheek just under her eye. But because there was no infection or medical problems, the doctors turned her away. “They said, ‘OK, you look great.’ I did not look great,” she said.
Ms. Harris’ doctors advised her to wait a year before starting treatment for the scarring, a traditional approach. She was frustrated. “At the time, I was interested in becoming a pediatrician and thought, ‘No kid is going to want me as their doctor.’ ” But she accepted the medical advice – until her mother remembered a news story she’d seen.
Bridger Walker, a 6-year-old Wyoming boy, made headlines when he saved his younger sister from a dog that was attacking, but he was bitten multiple times as a result. Dr. Bhanusali treated the boy’s scarring.
Ms. Harris and her mother contacted the doctor, and after meeting via Zoom, Dr. Bhanusali agreed to treat her right away. He used lasers to resurface the skin, which created a suitable foundation for the scar cream, and he administered steroid injections to soften the scar tissue.
‘I see you’
Dr. Bhansali said he was impressed with the young student he treated. “There’s curiosity, and then there’s genuine passion. She has the latter,” he said in an interview. “Having gone through this, she will understand the value of research and keeping up with the literature and that just because something is being done a certain way today doesn’t mean it has to be that way tomorrow.”
Ms. Harris agrees that the experience will make her a better dermatologist. “One of the best parts about dermatology is that you can see your results in real time and really see what’s working and what’s not working. The potential for innovation is just amazing.”
But Ms. Harris believes she also gained empathy with dermatology patients. “I know exactly what it’s like to look in the mirror and not even recognize yourself, just have your eyes go straight to one thing and feel like the whole world is staring at you,” she said. “I’ll be able to reassure people that no matter what their concern is, whether it’s eczema or acne, whether it’s one pimple, I see you, and I know exactly how that feels.”
A version of this article first appeared on Medscape.com.
Contact allergens lurk in diabetes devices
in a presentation at the annual meeting of the American Contact Dermatitis Society.
Advanced technologies used for the management of diabetes fall into three main categories, said Dr. Chen, of the department of dermatology, Stanford University, Redwood City, Calif. Continuous glucose monitoring (CGM) devices, which are worn on the body, collect glucose measurements. Continuous subcutaneous insulin infusion (CSII) devices are attached to the body via an infusion set and are now available as tubing-free patch pumps that are attached directly to the skin via a catheter. Glucose-responsive insulin delivery systems combine the sensing and delivery features of the other two types of devices.
Once thought to be rare, reports of skin complications related to diabetes devices have been increasing in recent years, she said. Some reports suggest that at any given time, skin complications may affect as many as one quarter to one half of patients who use these devices, “so this is an important issue,” she emphasized. “Skin reactions are a major factor in device discontinuation, so we as clinicians need to be really proactive about treating these reactions.”
Risk factors for skin complications related to diabetes devices include sensitization to the adhesive used with the devices, as well as prolonged exposure to the device, Dr. Chen said. Younger age also appears to be a risk factor, as is a compromised skin barrier in the area where the device is used.
Unfortunately, obtaining details on the specific adhesives and the raw materials used in these devices, so as to customize patch testing, remains a challenge, she said. “Patch testing initially was often negative to commercially available allergens, even while patients were testing positive to pieces of device adhesive,” she noted.
Consider isobornyl acrylate
An article published in 2017 in Contact Dermatitis was “a major breakthrough” in that it identified isobornyl acrylate (IBOA) as an allergen in connection with the Freestyle Libre, a CGM device that was relatively new at the time. The finding was serendipitous, Dr. Chen said. A patient being treated for suspected allergic contact dermatitis in connection with use of a Freestyle Libre device was tested for IBOA accidentally, after the nurse administering the patch test thought that this was part of the standard acrylate series, she explained.
Subsequently, researchers identified 15 patients who had experienced reactions to the Freestyle Libre; 12 of 13 patients who were patch tested for IBOA tested positive. IBOA was found throughout the device, particularly where the top and bottom plastic components were connected, Dr. Chen said. This suggested that the IBOA was in the device housing and had diffused into the adhesive that attached the device to the skin.
An article published in 2018 in the Journal of Diabetes Science described three patients who developed severe allergic contact dermatitis from IBOA while using a CGM device, Dr. Chen said. The investigators confirmed that there were no reactions to the adhesive itself, again suggesting that IBOA had diffused into the adhesive from other parts of the device.
Although the authors were bound by a confidentiality agreement regarding the individual adhesive components, “the authors noted most of the acrylates in the adhesive were not present in commercially available acrylate series for patch testing,” she said.
IBOA, the ACDS’ Allergen of the Year in 2020, is common in sealants, glues, and adhesives, Dr. Chen said. Although IBOA had been reported infrequently as an allergen, it has now been identified as a “potential culprit” behind skin reactions in many diabetes devices, including CSII and CGM devices, she added.
In addition, N,N-dimethylacrylamide (DMAA) is an allergen that has been identified in several diabetes devices and often occurs with IBOA in medical-grade UV-cured adhesives, Dr. Chen noted. Other allergens identified in diabetes devices include colophony, which is present in many adhesives, as well as other acrylates and epoxy resin.
Diabetes devices are constantly evolving. IBOA is no longer found in Freestyle Libre devices. It is important that clinicians stay up to date with the medical literature and advocate for partnership with device manufacturers, she emphasized.
Patch testing
When diabetes devices are suspected as the source of allergic contact dermatitis, a minimum of a baseline series that contains colophony at a concentration of 20% in petrolatum should be carried out, Dr. Chen said. Commercialized patch test trays, which include plastics, glues, acrylates, epoxy resins/isocyanates, and colophony derivatives, should be ideal. “Personal-care products should be included if they are potentially relevant,” she added.
Dr. Chen shared tables published in Contact Dermatitis in 2021 with examples of screening test series. She said to consider including screening for other allergens more recently discovered in diabetes devices, including 2,2’-methylenebis(6-tert-butyl-4-methylphenol) monoacrylate (MBPA) 1.5% pet; dipropylene glycol diacrylate (DPGDA) 0.1% pet; and butylated hydroxytoluene (BHT) 2% pet.
Testing for monomethyl ether of hydroquinone should also be considered; this may be included in the test preparations for IBOA and DMAA.
Management strategies
For patients who experience skin reactions to their diabetes devices, consideration may given to relocating the device to another area of skin or changing sensors more frequently, according to Dr. Chen.
For some patients, the reaction can be managed with corticosteroid cream, ointment, solution, or nasal spray. Topical antibiotics or topical antihistamines can be helpful, as can barrier dressings, solutions, or sprays, she said. The best solution is to change to a device that does not have the culprit allergen, “but that is difficult, since we don’t know what is in these devices,” she added. Good alternatives include the Eversense CGM device or devices that have been demonstrated not to contain IBOA, such as the Freestyle Libre 2 or the newer version of the Omnipod, an insulin delivery system
Looking ahead, Dr. Chen said that “mandatory labeling is needed, as devices with the same name may have different compositions, depending on the date of manufacture.” Allergens relevant to people with diabetes are constantly evolving, and many are still unidentified, so clinicians and manufacturers need to work together to identify the culprit allergens and their sources, she said.
Dr. Chen has served as principal investigator or subinvestigator for Amgen, AbbVie, and Sanofi Regeneron and as a consultant for Purity Brands.
A version of this article first appeared on Medscape.com.
in a presentation at the annual meeting of the American Contact Dermatitis Society.
Advanced technologies used for the management of diabetes fall into three main categories, said Dr. Chen, of the department of dermatology, Stanford University, Redwood City, Calif. Continuous glucose monitoring (CGM) devices, which are worn on the body, collect glucose measurements. Continuous subcutaneous insulin infusion (CSII) devices are attached to the body via an infusion set and are now available as tubing-free patch pumps that are attached directly to the skin via a catheter. Glucose-responsive insulin delivery systems combine the sensing and delivery features of the other two types of devices.
Once thought to be rare, reports of skin complications related to diabetes devices have been increasing in recent years, she said. Some reports suggest that at any given time, skin complications may affect as many as one quarter to one half of patients who use these devices, “so this is an important issue,” she emphasized. “Skin reactions are a major factor in device discontinuation, so we as clinicians need to be really proactive about treating these reactions.”
Risk factors for skin complications related to diabetes devices include sensitization to the adhesive used with the devices, as well as prolonged exposure to the device, Dr. Chen said. Younger age also appears to be a risk factor, as is a compromised skin barrier in the area where the device is used.
Unfortunately, obtaining details on the specific adhesives and the raw materials used in these devices, so as to customize patch testing, remains a challenge, she said. “Patch testing initially was often negative to commercially available allergens, even while patients were testing positive to pieces of device adhesive,” she noted.
Consider isobornyl acrylate
An article published in 2017 in Contact Dermatitis was “a major breakthrough” in that it identified isobornyl acrylate (IBOA) as an allergen in connection with the Freestyle Libre, a CGM device that was relatively new at the time. The finding was serendipitous, Dr. Chen said. A patient being treated for suspected allergic contact dermatitis in connection with use of a Freestyle Libre device was tested for IBOA accidentally, after the nurse administering the patch test thought that this was part of the standard acrylate series, she explained.
Subsequently, researchers identified 15 patients who had experienced reactions to the Freestyle Libre; 12 of 13 patients who were patch tested for IBOA tested positive. IBOA was found throughout the device, particularly where the top and bottom plastic components were connected, Dr. Chen said. This suggested that the IBOA was in the device housing and had diffused into the adhesive that attached the device to the skin.
An article published in 2018 in the Journal of Diabetes Science described three patients who developed severe allergic contact dermatitis from IBOA while using a CGM device, Dr. Chen said. The investigators confirmed that there were no reactions to the adhesive itself, again suggesting that IBOA had diffused into the adhesive from other parts of the device.
Although the authors were bound by a confidentiality agreement regarding the individual adhesive components, “the authors noted most of the acrylates in the adhesive were not present in commercially available acrylate series for patch testing,” she said.
IBOA, the ACDS’ Allergen of the Year in 2020, is common in sealants, glues, and adhesives, Dr. Chen said. Although IBOA had been reported infrequently as an allergen, it has now been identified as a “potential culprit” behind skin reactions in many diabetes devices, including CSII and CGM devices, she added.
In addition, N,N-dimethylacrylamide (DMAA) is an allergen that has been identified in several diabetes devices and often occurs with IBOA in medical-grade UV-cured adhesives, Dr. Chen noted. Other allergens identified in diabetes devices include colophony, which is present in many adhesives, as well as other acrylates and epoxy resin.
Diabetes devices are constantly evolving. IBOA is no longer found in Freestyle Libre devices. It is important that clinicians stay up to date with the medical literature and advocate for partnership with device manufacturers, she emphasized.
Patch testing
When diabetes devices are suspected as the source of allergic contact dermatitis, a minimum of a baseline series that contains colophony at a concentration of 20% in petrolatum should be carried out, Dr. Chen said. Commercialized patch test trays, which include plastics, glues, acrylates, epoxy resins/isocyanates, and colophony derivatives, should be ideal. “Personal-care products should be included if they are potentially relevant,” she added.
Dr. Chen shared tables published in Contact Dermatitis in 2021 with examples of screening test series. She said to consider including screening for other allergens more recently discovered in diabetes devices, including 2,2’-methylenebis(6-tert-butyl-4-methylphenol) monoacrylate (MBPA) 1.5% pet; dipropylene glycol diacrylate (DPGDA) 0.1% pet; and butylated hydroxytoluene (BHT) 2% pet.
Testing for monomethyl ether of hydroquinone should also be considered; this may be included in the test preparations for IBOA and DMAA.
Management strategies
For patients who experience skin reactions to their diabetes devices, consideration may given to relocating the device to another area of skin or changing sensors more frequently, according to Dr. Chen.
For some patients, the reaction can be managed with corticosteroid cream, ointment, solution, or nasal spray. Topical antibiotics or topical antihistamines can be helpful, as can barrier dressings, solutions, or sprays, she said. The best solution is to change to a device that does not have the culprit allergen, “but that is difficult, since we don’t know what is in these devices,” she added. Good alternatives include the Eversense CGM device or devices that have been demonstrated not to contain IBOA, such as the Freestyle Libre 2 or the newer version of the Omnipod, an insulin delivery system
Looking ahead, Dr. Chen said that “mandatory labeling is needed, as devices with the same name may have different compositions, depending on the date of manufacture.” Allergens relevant to people with diabetes are constantly evolving, and many are still unidentified, so clinicians and manufacturers need to work together to identify the culprit allergens and their sources, she said.
Dr. Chen has served as principal investigator or subinvestigator for Amgen, AbbVie, and Sanofi Regeneron and as a consultant for Purity Brands.
A version of this article first appeared on Medscape.com.
in a presentation at the annual meeting of the American Contact Dermatitis Society.
Advanced technologies used for the management of diabetes fall into three main categories, said Dr. Chen, of the department of dermatology, Stanford University, Redwood City, Calif. Continuous glucose monitoring (CGM) devices, which are worn on the body, collect glucose measurements. Continuous subcutaneous insulin infusion (CSII) devices are attached to the body via an infusion set and are now available as tubing-free patch pumps that are attached directly to the skin via a catheter. Glucose-responsive insulin delivery systems combine the sensing and delivery features of the other two types of devices.
Once thought to be rare, reports of skin complications related to diabetes devices have been increasing in recent years, she said. Some reports suggest that at any given time, skin complications may affect as many as one quarter to one half of patients who use these devices, “so this is an important issue,” she emphasized. “Skin reactions are a major factor in device discontinuation, so we as clinicians need to be really proactive about treating these reactions.”
Risk factors for skin complications related to diabetes devices include sensitization to the adhesive used with the devices, as well as prolonged exposure to the device, Dr. Chen said. Younger age also appears to be a risk factor, as is a compromised skin barrier in the area where the device is used.
Unfortunately, obtaining details on the specific adhesives and the raw materials used in these devices, so as to customize patch testing, remains a challenge, she said. “Patch testing initially was often negative to commercially available allergens, even while patients were testing positive to pieces of device adhesive,” she noted.
Consider isobornyl acrylate
An article published in 2017 in Contact Dermatitis was “a major breakthrough” in that it identified isobornyl acrylate (IBOA) as an allergen in connection with the Freestyle Libre, a CGM device that was relatively new at the time. The finding was serendipitous, Dr. Chen said. A patient being treated for suspected allergic contact dermatitis in connection with use of a Freestyle Libre device was tested for IBOA accidentally, after the nurse administering the patch test thought that this was part of the standard acrylate series, she explained.
Subsequently, researchers identified 15 patients who had experienced reactions to the Freestyle Libre; 12 of 13 patients who were patch tested for IBOA tested positive. IBOA was found throughout the device, particularly where the top and bottom plastic components were connected, Dr. Chen said. This suggested that the IBOA was in the device housing and had diffused into the adhesive that attached the device to the skin.
An article published in 2018 in the Journal of Diabetes Science described three patients who developed severe allergic contact dermatitis from IBOA while using a CGM device, Dr. Chen said. The investigators confirmed that there were no reactions to the adhesive itself, again suggesting that IBOA had diffused into the adhesive from other parts of the device.
Although the authors were bound by a confidentiality agreement regarding the individual adhesive components, “the authors noted most of the acrylates in the adhesive were not present in commercially available acrylate series for patch testing,” she said.
IBOA, the ACDS’ Allergen of the Year in 2020, is common in sealants, glues, and adhesives, Dr. Chen said. Although IBOA had been reported infrequently as an allergen, it has now been identified as a “potential culprit” behind skin reactions in many diabetes devices, including CSII and CGM devices, she added.
In addition, N,N-dimethylacrylamide (DMAA) is an allergen that has been identified in several diabetes devices and often occurs with IBOA in medical-grade UV-cured adhesives, Dr. Chen noted. Other allergens identified in diabetes devices include colophony, which is present in many adhesives, as well as other acrylates and epoxy resin.
Diabetes devices are constantly evolving. IBOA is no longer found in Freestyle Libre devices. It is important that clinicians stay up to date with the medical literature and advocate for partnership with device manufacturers, she emphasized.
Patch testing
When diabetes devices are suspected as the source of allergic contact dermatitis, a minimum of a baseline series that contains colophony at a concentration of 20% in petrolatum should be carried out, Dr. Chen said. Commercialized patch test trays, which include plastics, glues, acrylates, epoxy resins/isocyanates, and colophony derivatives, should be ideal. “Personal-care products should be included if they are potentially relevant,” she added.
Dr. Chen shared tables published in Contact Dermatitis in 2021 with examples of screening test series. She said to consider including screening for other allergens more recently discovered in diabetes devices, including 2,2’-methylenebis(6-tert-butyl-4-methylphenol) monoacrylate (MBPA) 1.5% pet; dipropylene glycol diacrylate (DPGDA) 0.1% pet; and butylated hydroxytoluene (BHT) 2% pet.
Testing for monomethyl ether of hydroquinone should also be considered; this may be included in the test preparations for IBOA and DMAA.
Management strategies
For patients who experience skin reactions to their diabetes devices, consideration may given to relocating the device to another area of skin or changing sensors more frequently, according to Dr. Chen.
For some patients, the reaction can be managed with corticosteroid cream, ointment, solution, or nasal spray. Topical antibiotics or topical antihistamines can be helpful, as can barrier dressings, solutions, or sprays, she said. The best solution is to change to a device that does not have the culprit allergen, “but that is difficult, since we don’t know what is in these devices,” she added. Good alternatives include the Eversense CGM device or devices that have been demonstrated not to contain IBOA, such as the Freestyle Libre 2 or the newer version of the Omnipod, an insulin delivery system
Looking ahead, Dr. Chen said that “mandatory labeling is needed, as devices with the same name may have different compositions, depending on the date of manufacture.” Allergens relevant to people with diabetes are constantly evolving, and many are still unidentified, so clinicians and manufacturers need to work together to identify the culprit allergens and their sources, she said.
Dr. Chen has served as principal investigator or subinvestigator for Amgen, AbbVie, and Sanofi Regeneron and as a consultant for Purity Brands.
A version of this article first appeared on Medscape.com.
FROM ACDS 2023
Beware the hidden allergens in nutritional supplements
, Alison Ehrlich, MD, said at the annual meeting of the American Contact Dermatitis Society.
Allergens may be hidden in a range of supplement products, from colorings in vitamin C powders to some vitamins used in hair products and other products.
“In general, our patients do not tell us what supplements they are taking,” said Dr. Ehrlich, a dermatologist who practices in Washington, D.C. Antiaging, sleep, and weight loss/weight control supplements are among the most popular, she said.
Surveys have shown that many patients do not discuss supplement use with their health care providers, in part because they believe their providers would disapprove of supplement use, and patients are not educated about supplements, she said. “This is definitely an area that we should try to learn more about,” she added.
Current regulations regarding dietary supplements stem from the Dietary Supplement Health and Education Act of 1994, which defined dietary supplements as distinct from meals but regulated them as a category of food, not as medications. Dietary supplements can be vitamins, minerals, herbs, and extracts, Dr. Ehrlich said.
“There is not a lot of safety wrapped around how supplements come onto the market,” she explained. “It is not the manufacturer’s responsibility to test these products and make sure they are safe. When they get pulled off the market, it is because safety reports are getting back to the FDA.”
Consequently, a detailed history of supplement use is important, as it may reveal possible allergens as the cause of previously unidentified reactions, she said.
Dr. Ehrlich shared a case involving a patient who claimed to have had a reaction to a “Prevage-like” product that was labeled as a crepe repair cream. Listed among the product’s ingredients was idebenone, a synthetic version of the popular antioxidant known as Coenzyme Q.
Be wary of vitamins
Another potential source of allergy is vitamin C supplements, which became especially popular during the pandemic as people sought additional immune system support, Dr. Ehrlich noted. “What kind of vitamin C product our patients are taking is important,” she said. For example, some vitamin C powders contain coloring agents, such as carmine. Some also contain gelatin, which may cause an allergic reaction in individuals with alpha-gal syndrome, she added.
In general, water-soluble vitamins such as vitamins B1 to B9, B12, and C are more likely to cause an immediate reaction, Dr. Ehrlich said. Fat-soluble vitamins, such as vitamins A, D, E, and K, are more likely to cause a delayed reaction of allergic contact dermatitis.
Dr. Ehrlich described some unusual reactions to vitamins that have been reported, including a systemic allergy associated with vitamin B1 (thiamine), burning mouth syndrome associated with vitamin B3 (nicotinate), contact urticaria associated with vitamin B5 (panthenol), systemic allergy and generalized ACD associated with vitamin E (tocopherol), and erythema multiforme–like ACD associated with vitamin K1.
Notably, vitamin B5 has been associated with ACD as an ingredient in hair products, moisturizers, and wound care products, as well as B-complex vitamins and fortified foods, Dr. Ehrlich said.
Herbs and spices can act as allergens as well. Turmeric is a spice that has become a popular supplement ingredient, she said. Turmeric and curcumin (found in turmeric) can be used as a dye for its yellow color as well as a flavoring but has been associated with allergic reactions. Another popular herbal supplement, ginkgo biloba, has been marketed as a product that improves memory and cognition. It is available in pill form and in herbal teas.
“It’s really important to think about what herbal products our patients are taking, and not just in pill form,” Dr. Ehrlich said. “We need to expand our thoughts on what the herbs are in.”
Consider food additives as allergens
Food additives, in the form of colorants, preservatives, or flavoring agents, can cause allergic reactions, Dr. Ehrlich noted.
The question of whether food-additive contact sensitivity has a role in the occurrence of atopic dermatitis (AD) in children remains unclear, she said. However, a study published in 2020 found that 62% of children with AD had positive patch test reactions to at least one food-additive allergen, compared with 20% of children without AD. The additives responsible for the most reactions were azorubine (24.4%); formic acid (15.6%); and carmine, cochineal red, and amaranth (13.3% for each).
Common colorant culprits in allergic reactions include carmine, annatto, tartrazine, and spices (such as paprika and saffron), Dr. Ehrlich said. Carmine is used in meat to prevent photo-oxidation and to preserve a red color, and it has other uses as well, she said. Carmine has been associated with ACD, AD flares, and immediate hypersensitivity. Annatto is used in foods, including processed foods, butter, and cheese, to provide a yellow color. It is also found in some lipsticks and has been associated with urticaria and angioedema, she noted.
Food preservatives that have been associated with allergic reactions include butylated hydroxyanisole and sulfites, Dr. Ehrlich said. Sulfites are used to prevent food from turning brown, and it may be present in dried fruit, fruit juice, molasses, pickled foods, vinegar, and wine.
Reports of ACD in response to sodium metabisulfite have been increasing, she noted. Other sulfite reactions may occur with exposure to other products, such as cosmetics, body washes, and swimming pool water, she said.
Awareness of allergens in supplements is important “because the number of our patients taking supplements for different reasons is increasing” and allergens in supplements could account for flares, Dr. Ehrlich said. Clinicians should encourage patients to tell them what supplements they use. Clinicians should review the ingredients in these supplements with their patients to identify potential allergens that may be causing reactions, she advised.
Dr. Ehrlich has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, Alison Ehrlich, MD, said at the annual meeting of the American Contact Dermatitis Society.
Allergens may be hidden in a range of supplement products, from colorings in vitamin C powders to some vitamins used in hair products and other products.
“In general, our patients do not tell us what supplements they are taking,” said Dr. Ehrlich, a dermatologist who practices in Washington, D.C. Antiaging, sleep, and weight loss/weight control supplements are among the most popular, she said.
Surveys have shown that many patients do not discuss supplement use with their health care providers, in part because they believe their providers would disapprove of supplement use, and patients are not educated about supplements, she said. “This is definitely an area that we should try to learn more about,” she added.
Current regulations regarding dietary supplements stem from the Dietary Supplement Health and Education Act of 1994, which defined dietary supplements as distinct from meals but regulated them as a category of food, not as medications. Dietary supplements can be vitamins, minerals, herbs, and extracts, Dr. Ehrlich said.
“There is not a lot of safety wrapped around how supplements come onto the market,” she explained. “It is not the manufacturer’s responsibility to test these products and make sure they are safe. When they get pulled off the market, it is because safety reports are getting back to the FDA.”
Consequently, a detailed history of supplement use is important, as it may reveal possible allergens as the cause of previously unidentified reactions, she said.
Dr. Ehrlich shared a case involving a patient who claimed to have had a reaction to a “Prevage-like” product that was labeled as a crepe repair cream. Listed among the product’s ingredients was idebenone, a synthetic version of the popular antioxidant known as Coenzyme Q.
Be wary of vitamins
Another potential source of allergy is vitamin C supplements, which became especially popular during the pandemic as people sought additional immune system support, Dr. Ehrlich noted. “What kind of vitamin C product our patients are taking is important,” she said. For example, some vitamin C powders contain coloring agents, such as carmine. Some also contain gelatin, which may cause an allergic reaction in individuals with alpha-gal syndrome, she added.
In general, water-soluble vitamins such as vitamins B1 to B9, B12, and C are more likely to cause an immediate reaction, Dr. Ehrlich said. Fat-soluble vitamins, such as vitamins A, D, E, and K, are more likely to cause a delayed reaction of allergic contact dermatitis.
Dr. Ehrlich described some unusual reactions to vitamins that have been reported, including a systemic allergy associated with vitamin B1 (thiamine), burning mouth syndrome associated with vitamin B3 (nicotinate), contact urticaria associated with vitamin B5 (panthenol), systemic allergy and generalized ACD associated with vitamin E (tocopherol), and erythema multiforme–like ACD associated with vitamin K1.
Notably, vitamin B5 has been associated with ACD as an ingredient in hair products, moisturizers, and wound care products, as well as B-complex vitamins and fortified foods, Dr. Ehrlich said.
Herbs and spices can act as allergens as well. Turmeric is a spice that has become a popular supplement ingredient, she said. Turmeric and curcumin (found in turmeric) can be used as a dye for its yellow color as well as a flavoring but has been associated with allergic reactions. Another popular herbal supplement, ginkgo biloba, has been marketed as a product that improves memory and cognition. It is available in pill form and in herbal teas.
“It’s really important to think about what herbal products our patients are taking, and not just in pill form,” Dr. Ehrlich said. “We need to expand our thoughts on what the herbs are in.”
Consider food additives as allergens
Food additives, in the form of colorants, preservatives, or flavoring agents, can cause allergic reactions, Dr. Ehrlich noted.
The question of whether food-additive contact sensitivity has a role in the occurrence of atopic dermatitis (AD) in children remains unclear, she said. However, a study published in 2020 found that 62% of children with AD had positive patch test reactions to at least one food-additive allergen, compared with 20% of children without AD. The additives responsible for the most reactions were azorubine (24.4%); formic acid (15.6%); and carmine, cochineal red, and amaranth (13.3% for each).
Common colorant culprits in allergic reactions include carmine, annatto, tartrazine, and spices (such as paprika and saffron), Dr. Ehrlich said. Carmine is used in meat to prevent photo-oxidation and to preserve a red color, and it has other uses as well, she said. Carmine has been associated with ACD, AD flares, and immediate hypersensitivity. Annatto is used in foods, including processed foods, butter, and cheese, to provide a yellow color. It is also found in some lipsticks and has been associated with urticaria and angioedema, she noted.
Food preservatives that have been associated with allergic reactions include butylated hydroxyanisole and sulfites, Dr. Ehrlich said. Sulfites are used to prevent food from turning brown, and it may be present in dried fruit, fruit juice, molasses, pickled foods, vinegar, and wine.
Reports of ACD in response to sodium metabisulfite have been increasing, she noted. Other sulfite reactions may occur with exposure to other products, such as cosmetics, body washes, and swimming pool water, she said.
Awareness of allergens in supplements is important “because the number of our patients taking supplements for different reasons is increasing” and allergens in supplements could account for flares, Dr. Ehrlich said. Clinicians should encourage patients to tell them what supplements they use. Clinicians should review the ingredients in these supplements with their patients to identify potential allergens that may be causing reactions, she advised.
Dr. Ehrlich has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, Alison Ehrlich, MD, said at the annual meeting of the American Contact Dermatitis Society.
Allergens may be hidden in a range of supplement products, from colorings in vitamin C powders to some vitamins used in hair products and other products.
“In general, our patients do not tell us what supplements they are taking,” said Dr. Ehrlich, a dermatologist who practices in Washington, D.C. Antiaging, sleep, and weight loss/weight control supplements are among the most popular, she said.
Surveys have shown that many patients do not discuss supplement use with their health care providers, in part because they believe their providers would disapprove of supplement use, and patients are not educated about supplements, she said. “This is definitely an area that we should try to learn more about,” she added.
Current regulations regarding dietary supplements stem from the Dietary Supplement Health and Education Act of 1994, which defined dietary supplements as distinct from meals but regulated them as a category of food, not as medications. Dietary supplements can be vitamins, minerals, herbs, and extracts, Dr. Ehrlich said.
“There is not a lot of safety wrapped around how supplements come onto the market,” she explained. “It is not the manufacturer’s responsibility to test these products and make sure they are safe. When they get pulled off the market, it is because safety reports are getting back to the FDA.”
Consequently, a detailed history of supplement use is important, as it may reveal possible allergens as the cause of previously unidentified reactions, she said.
Dr. Ehrlich shared a case involving a patient who claimed to have had a reaction to a “Prevage-like” product that was labeled as a crepe repair cream. Listed among the product’s ingredients was idebenone, a synthetic version of the popular antioxidant known as Coenzyme Q.
Be wary of vitamins
Another potential source of allergy is vitamin C supplements, which became especially popular during the pandemic as people sought additional immune system support, Dr. Ehrlich noted. “What kind of vitamin C product our patients are taking is important,” she said. For example, some vitamin C powders contain coloring agents, such as carmine. Some also contain gelatin, which may cause an allergic reaction in individuals with alpha-gal syndrome, she added.
In general, water-soluble vitamins such as vitamins B1 to B9, B12, and C are more likely to cause an immediate reaction, Dr. Ehrlich said. Fat-soluble vitamins, such as vitamins A, D, E, and K, are more likely to cause a delayed reaction of allergic contact dermatitis.
Dr. Ehrlich described some unusual reactions to vitamins that have been reported, including a systemic allergy associated with vitamin B1 (thiamine), burning mouth syndrome associated with vitamin B3 (nicotinate), contact urticaria associated with vitamin B5 (panthenol), systemic allergy and generalized ACD associated with vitamin E (tocopherol), and erythema multiforme–like ACD associated with vitamin K1.
Notably, vitamin B5 has been associated with ACD as an ingredient in hair products, moisturizers, and wound care products, as well as B-complex vitamins and fortified foods, Dr. Ehrlich said.
Herbs and spices can act as allergens as well. Turmeric is a spice that has become a popular supplement ingredient, she said. Turmeric and curcumin (found in turmeric) can be used as a dye for its yellow color as well as a flavoring but has been associated with allergic reactions. Another popular herbal supplement, ginkgo biloba, has been marketed as a product that improves memory and cognition. It is available in pill form and in herbal teas.
“It’s really important to think about what herbal products our patients are taking, and not just in pill form,” Dr. Ehrlich said. “We need to expand our thoughts on what the herbs are in.”
Consider food additives as allergens
Food additives, in the form of colorants, preservatives, or flavoring agents, can cause allergic reactions, Dr. Ehrlich noted.
The question of whether food-additive contact sensitivity has a role in the occurrence of atopic dermatitis (AD) in children remains unclear, she said. However, a study published in 2020 found that 62% of children with AD had positive patch test reactions to at least one food-additive allergen, compared with 20% of children without AD. The additives responsible for the most reactions were azorubine (24.4%); formic acid (15.6%); and carmine, cochineal red, and amaranth (13.3% for each).
Common colorant culprits in allergic reactions include carmine, annatto, tartrazine, and spices (such as paprika and saffron), Dr. Ehrlich said. Carmine is used in meat to prevent photo-oxidation and to preserve a red color, and it has other uses as well, she said. Carmine has been associated with ACD, AD flares, and immediate hypersensitivity. Annatto is used in foods, including processed foods, butter, and cheese, to provide a yellow color. It is also found in some lipsticks and has been associated with urticaria and angioedema, she noted.
Food preservatives that have been associated with allergic reactions include butylated hydroxyanisole and sulfites, Dr. Ehrlich said. Sulfites are used to prevent food from turning brown, and it may be present in dried fruit, fruit juice, molasses, pickled foods, vinegar, and wine.
Reports of ACD in response to sodium metabisulfite have been increasing, she noted. Other sulfite reactions may occur with exposure to other products, such as cosmetics, body washes, and swimming pool water, she said.
Awareness of allergens in supplements is important “because the number of our patients taking supplements for different reasons is increasing” and allergens in supplements could account for flares, Dr. Ehrlich said. Clinicians should encourage patients to tell them what supplements they use. Clinicians should review the ingredients in these supplements with their patients to identify potential allergens that may be causing reactions, she advised.
Dr. Ehrlich has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ACDS 2023
Controlled hyperthermia: Novel treatment of BCCs without surgery continues to be refined
PHOENIX – .
“For 2,000 years, it’s been known that heat can kill cancers,” an apoptotic reaction “rather than a destructive reaction coming from excessive heat,” Christopher B. Zachary, MD, said at the annual conference of the American Society for Laser Medicine and Surgery, where the study was presented during an abstract session.
Dr. Zachary, professor and chair emeritus of the department of dermatology at the University of California, Irvine, and colleagues, evaluated a novel, noninvasive technique of controlled hyperthermia and mapping protocol (CHAMP) designed to help clinicians with margin assessment and treatment of superficial and nodular BCCs. For this prospective study, which was first described at the 2022 ASLMS annual conference and is being conducted at three centers, 73 patients with biopsy-proven superficial and nodular BCCs have been scanned with the VivoSight Dx optical coherence tomography (OCT) device to map BCC tumor margins.
The BCCs were treated with the Sciton 1,064-nm Er:YAG laser equipped with a 4-mm beam diameter scan pattern with no overlap and an 8-millisecond pulse duration, randomized to either standard 120-140 J/cm2 pulses until tissue graying and contraction was observed, or the CHAMP controlled hyperthermia technique using repeated 25 J/cm2 pulses under thermal camera imaging to maintain a consistent temperature of 55º C for 60 seconds. Patients were rescanned by OCT at 3 to 12 months for any signs of residual tumor and if positive, were retreated. Finally, lesions were excised for evidence of histological clearance.
To date, 48 patients have completed the study. Among the 26 patients treated with the CHAMP method, 22 (84.6%) were histologically clear, as were 19 of the 22 (86.4%) in the standard treatment group. Ulceration was uncommon with the CHAMP method, and patients healed with modest erythema, Dr. Zachary said.
Pretreatment OCT mapping of BCCs indicated that tumors extended beyond their 5-mm clinical margins in 11 cases (15%). “This will be of interest to those who treat BCCs by Mohs or standard excision,” he said. Increased vascularity measured by dynamic OCT was noted in most CHAMP patients immediately after irradiation, which suggests that apoptosis was the primary mechanism of tumor response instead of vascular destruction.
“The traditional technique for using the long pulsed 1,064-nm Er:YAG laser to cause damage and destruction of BCC is 120-140 J/cm2 at one or two passes until you get to an endpoint of graying and contraction of tissue,” Dr. Zachary said. “That’s opposed to the ‘Low and Slow’ approach [where you use] multiple pulses at 25 J/cm2 until you achieve an optimal time and temperature. If you treat above 60º C, you tend to get epidermal blistering, prolonged healing, and interestingly, absence of pain. I think that’s because you kill off the nerve fibers. With the low fluence multiple scan technique, you’re going for an even flat-top heating.”
Currently, he and his colleagues consider 55 degrees at 60 seconds as “the optimal parameters,” he said, but “it could be 45 degrees at 90 seconds or two minutes. We don’t know yet.”
In an interview at the meeting, one of the abstract session moderators, Mathew M. Avram, MD, JD, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital, Boston, said that he was encouraged by the study results as investigations into effective, noninvasive treatment of BCC continue to move forward. “Details matter such as the temperature [of energy delivery] and noninvasive imaging to delineate the appropriate margins,” said Dr. Avram, who has conducted research on the 1,064-nm long-pulsed Nd:YAG laser as an alternative treatment for nonfacial BCCs in patients who are poor surgical candidates.
“Hopefully, at some point,” he said, such approaches will “become the standard of care for many BCCs that we are now treating surgically. I don’t think this will happen in the next 3 years, but I think in the long term, it will emerge as the treatment of choice.”
The study is being funded by Michelson Diagnostics. Sciton provided the long-pulsed 1,064-nm lasers devices being used in the trial. Dr. Zachary reported having no relevant disclosures. Dr. Avram disclosed that he has received consulting fees from Sciton.
PHOENIX – .
“For 2,000 years, it’s been known that heat can kill cancers,” an apoptotic reaction “rather than a destructive reaction coming from excessive heat,” Christopher B. Zachary, MD, said at the annual conference of the American Society for Laser Medicine and Surgery, where the study was presented during an abstract session.
Dr. Zachary, professor and chair emeritus of the department of dermatology at the University of California, Irvine, and colleagues, evaluated a novel, noninvasive technique of controlled hyperthermia and mapping protocol (CHAMP) designed to help clinicians with margin assessment and treatment of superficial and nodular BCCs. For this prospective study, which was first described at the 2022 ASLMS annual conference and is being conducted at three centers, 73 patients with biopsy-proven superficial and nodular BCCs have been scanned with the VivoSight Dx optical coherence tomography (OCT) device to map BCC tumor margins.
The BCCs were treated with the Sciton 1,064-nm Er:YAG laser equipped with a 4-mm beam diameter scan pattern with no overlap and an 8-millisecond pulse duration, randomized to either standard 120-140 J/cm2 pulses until tissue graying and contraction was observed, or the CHAMP controlled hyperthermia technique using repeated 25 J/cm2 pulses under thermal camera imaging to maintain a consistent temperature of 55º C for 60 seconds. Patients were rescanned by OCT at 3 to 12 months for any signs of residual tumor and if positive, were retreated. Finally, lesions were excised for evidence of histological clearance.
To date, 48 patients have completed the study. Among the 26 patients treated with the CHAMP method, 22 (84.6%) were histologically clear, as were 19 of the 22 (86.4%) in the standard treatment group. Ulceration was uncommon with the CHAMP method, and patients healed with modest erythema, Dr. Zachary said.
Pretreatment OCT mapping of BCCs indicated that tumors extended beyond their 5-mm clinical margins in 11 cases (15%). “This will be of interest to those who treat BCCs by Mohs or standard excision,” he said. Increased vascularity measured by dynamic OCT was noted in most CHAMP patients immediately after irradiation, which suggests that apoptosis was the primary mechanism of tumor response instead of vascular destruction.
“The traditional technique for using the long pulsed 1,064-nm Er:YAG laser to cause damage and destruction of BCC is 120-140 J/cm2 at one or two passes until you get to an endpoint of graying and contraction of tissue,” Dr. Zachary said. “That’s opposed to the ‘Low and Slow’ approach [where you use] multiple pulses at 25 J/cm2 until you achieve an optimal time and temperature. If you treat above 60º C, you tend to get epidermal blistering, prolonged healing, and interestingly, absence of pain. I think that’s because you kill off the nerve fibers. With the low fluence multiple scan technique, you’re going for an even flat-top heating.”
Currently, he and his colleagues consider 55 degrees at 60 seconds as “the optimal parameters,” he said, but “it could be 45 degrees at 90 seconds or two minutes. We don’t know yet.”
In an interview at the meeting, one of the abstract session moderators, Mathew M. Avram, MD, JD, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital, Boston, said that he was encouraged by the study results as investigations into effective, noninvasive treatment of BCC continue to move forward. “Details matter such as the temperature [of energy delivery] and noninvasive imaging to delineate the appropriate margins,” said Dr. Avram, who has conducted research on the 1,064-nm long-pulsed Nd:YAG laser as an alternative treatment for nonfacial BCCs in patients who are poor surgical candidates.
“Hopefully, at some point,” he said, such approaches will “become the standard of care for many BCCs that we are now treating surgically. I don’t think this will happen in the next 3 years, but I think in the long term, it will emerge as the treatment of choice.”
The study is being funded by Michelson Diagnostics. Sciton provided the long-pulsed 1,064-nm lasers devices being used in the trial. Dr. Zachary reported having no relevant disclosures. Dr. Avram disclosed that he has received consulting fees from Sciton.
PHOENIX – .
“For 2,000 years, it’s been known that heat can kill cancers,” an apoptotic reaction “rather than a destructive reaction coming from excessive heat,” Christopher B. Zachary, MD, said at the annual conference of the American Society for Laser Medicine and Surgery, where the study was presented during an abstract session.
Dr. Zachary, professor and chair emeritus of the department of dermatology at the University of California, Irvine, and colleagues, evaluated a novel, noninvasive technique of controlled hyperthermia and mapping protocol (CHAMP) designed to help clinicians with margin assessment and treatment of superficial and nodular BCCs. For this prospective study, which was first described at the 2022 ASLMS annual conference and is being conducted at three centers, 73 patients with biopsy-proven superficial and nodular BCCs have been scanned with the VivoSight Dx optical coherence tomography (OCT) device to map BCC tumor margins.
The BCCs were treated with the Sciton 1,064-nm Er:YAG laser equipped with a 4-mm beam diameter scan pattern with no overlap and an 8-millisecond pulse duration, randomized to either standard 120-140 J/cm2 pulses until tissue graying and contraction was observed, or the CHAMP controlled hyperthermia technique using repeated 25 J/cm2 pulses under thermal camera imaging to maintain a consistent temperature of 55º C for 60 seconds. Patients were rescanned by OCT at 3 to 12 months for any signs of residual tumor and if positive, were retreated. Finally, lesions were excised for evidence of histological clearance.
To date, 48 patients have completed the study. Among the 26 patients treated with the CHAMP method, 22 (84.6%) were histologically clear, as were 19 of the 22 (86.4%) in the standard treatment group. Ulceration was uncommon with the CHAMP method, and patients healed with modest erythema, Dr. Zachary said.
Pretreatment OCT mapping of BCCs indicated that tumors extended beyond their 5-mm clinical margins in 11 cases (15%). “This will be of interest to those who treat BCCs by Mohs or standard excision,” he said. Increased vascularity measured by dynamic OCT was noted in most CHAMP patients immediately after irradiation, which suggests that apoptosis was the primary mechanism of tumor response instead of vascular destruction.
“The traditional technique for using the long pulsed 1,064-nm Er:YAG laser to cause damage and destruction of BCC is 120-140 J/cm2 at one or two passes until you get to an endpoint of graying and contraction of tissue,” Dr. Zachary said. “That’s opposed to the ‘Low and Slow’ approach [where you use] multiple pulses at 25 J/cm2 until you achieve an optimal time and temperature. If you treat above 60º C, you tend to get epidermal blistering, prolonged healing, and interestingly, absence of pain. I think that’s because you kill off the nerve fibers. With the low fluence multiple scan technique, you’re going for an even flat-top heating.”
Currently, he and his colleagues consider 55 degrees at 60 seconds as “the optimal parameters,” he said, but “it could be 45 degrees at 90 seconds or two minutes. We don’t know yet.”
In an interview at the meeting, one of the abstract session moderators, Mathew M. Avram, MD, JD, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital, Boston, said that he was encouraged by the study results as investigations into effective, noninvasive treatment of BCC continue to move forward. “Details matter such as the temperature [of energy delivery] and noninvasive imaging to delineate the appropriate margins,” said Dr. Avram, who has conducted research on the 1,064-nm long-pulsed Nd:YAG laser as an alternative treatment for nonfacial BCCs in patients who are poor surgical candidates.
“Hopefully, at some point,” he said, such approaches will “become the standard of care for many BCCs that we are now treating surgically. I don’t think this will happen in the next 3 years, but I think in the long term, it will emerge as the treatment of choice.”
The study is being funded by Michelson Diagnostics. Sciton provided the long-pulsed 1,064-nm lasers devices being used in the trial. Dr. Zachary reported having no relevant disclosures. Dr. Avram disclosed that he has received consulting fees from Sciton.
AT ASLMS 2023